User login
Cancer-related clinical pearls from pediatric dermatology
KAUAI, HAWAII – Every child diagnosed with medulloblastoma deserves a careful dermatologic evaluation for possible comorbid basal cell nevus syndrome, according to Jennifer Huang, MD, a pediatric dermatologist at Boston Children’s Hospital and Harvard Medical School.
“Medulloblastoma occurs in 10%-20% of patients with basal cell nevus syndrome and can be the presenting sign. So if a patient with basal cell nevus syndrome gets medulloblastoma, it usually occurs within the first year of life – and it can be the first thing you see,” she said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dr. Huang presented a series of pediatric dermatology clinical pearls focused not only on basal cell nevus syndrome (BCNS) and medulloblastoma, but also on the implications of skin-limited Langerhans cell histiocytosis, how to recognize and treat drug-induced follicular eruptions in pediatric patients on targeted anticancer therapies, and when to suspect Demodex folliculitis in immunosuppressed patients.
Skin-limited Langerhans cell histiocytosis
Around 10%-20% of patients with Langerhans cell histiocytosis (LCH) have the skin-limited form of the malignancy. These are patients who, after a thorough workup, have a normal CBC, skeletal survey, and liver function tests; essentially, no evidence of multisystem disease.
“It’s very rare for patients who present with skin-limited LCH alone to develop multisystem disease and to require chemotherapy or other more aggressive treatment,” Dr. Huang said. “I think that skin-limited LCH is probably a separate entity with its own natural history distinct from multisystem disease. We can see that with current genomic testing: in multisystem LCH, BRAF mutations are identified in at least half of patients, but very few with skin-limited disease express those mutations.
“The clinical pearl here is if you have a patient with skin-limited LCH it very rarely progresses to multisystem involvement. It’s associated with a good prognosis. That doesn’t mean you shouldn’t monitor them, but I think it can be reassuring information for the family,” she said.
Basal cell nevus syndrome and medulloblastoma
“Half of cases of medulloblastoma are associated with mutations in the sonic hedgehog pathway – and a subset of that group has basal cell nevus syndrome,” Dr. Huang said.
BCNS is not a diagnosis frequently made by oncologists, who typically dismiss the multitude of lesions as skin tags, which they often mimic in both appearance and location, particularly on the neck and intertriginous areas. So it’s useful for dermatologists to establish a good referral relationship with their local oncologists.
“As dermatologists it’s really important to recognize not only the major features of basal cell nevus syndrome, but also the associated findings because we can really help in making this diagnosis early,” Dr. Huang stressed.
Early diagnosis of BCNS is a high priority for two reasons: to start treatment aimed at reducing development of basal cell carcinomas, and because radiation therapy for their medulloblastoma is contraindicated in patients with BCNS because it boosts their skin cancer burden.
BCNS is caused by mutations in the PTCH (Patched) gene found on chromosome arm 9q. The major features of BCNS include odontogenic keratocysts, palmoplantar pits, ectopic calcification, and, of course, basal cell carcinomas. The associated findings in BCNS, in addition to medulloblastoma, include macrocephaly and dysmorphic features such as cleft lip or palate, frontal bossing, and hypertelorism.
“I’ve treated a hundred at a time. It’s incredibly successful. It’s locally destructive. It leaves a little bit of hypopigmentation but no scar, which the CO2 laser will do in this instance. It’s actually a pretty cool modality,” said Dr. Eichenfield, professor of dermatology and pediatrics at Rady Children’s Hospital and the University of California, San Diego.
Follicular eruptions in cancer patients on MAPK inhibitors
Cutaneous reactions to anticancer drugs aimed at inhibiting the key MAPK (mitogen-activated protein kinase) pathway in children are common and diverse. Dr. Huang focused on the most common one: follicular eruptions, which occur in up to 80% of pediatric cancer patients on targeted therapy. These eruptions can express themselves in a variety of ways and are easily mistaken for comedonal acne, varicella zoster infection, herpes simplex, or bacterial folliculitis.
The key clues are highly suggestive that a follicular eruption in a child on targeted anticancer therapy is caused by the drug and not something else are the eruption’s symmetric distribution, that it’s truly follicular upon close inspection, and the timing: The eruption typically begins 2-3 weeks after initiation of therapy or within a week after a dose escalation.
Anti-inflammatory agents are the treatment mainstay. Treatment of the cutaneous eruption often is successful without need to discontinue the patient’s MAPK inhibitor.
“Even though some of these eruptions look comedonal, they’re not. It’s not a follicular plugging disorder, it’s an inflammatory condition. Topical steroids, oral tetracyclines, and dilute bleach baths all work pretty well. I haven’t had good experiences with keratolytics like tretinoin cream and benzoyl peroxide; they’re less effective. Dose reduction is the last resort for these patients. Often they are very sick. They need the drug and I think the last thing we want to do is take them off it,” Dr. Huang said.
She has observed that prepubertal children are more likely to have an eczematous reaction to their targeted anticancer therapy than a follicular eruption.
D. folliculitis in immunocompromised patients
“The clinical pearl here is to strongly consider the diagnosis of Demodex folliculitis in an immunosuppresed patient with an itchy acneiform eruption,” Dr. Huang said.
Demodex is a human mite which is part of the normal skin flora. She called it “a great mimicker”: It can cause dermatoses mistaken for rosacea, acne, seborrheic dermatitis, perioral facial dermatitis, blepharitis, and acute graft-versus-host disease.
In the setting of a young, immunosuppressed patient who develops an acneiform eruption, the differential diagnosis is lengthy and includes steroid-induced acne, a cutaneous reaction to targeted anticancer therapy, gram-negative folliculitis secondary to long-term antibiotic therapy, and Pityrosporum folliculitis, as well as D. folliculitis.
Demodex and P. folliculitis are the two acneiform dermatoses where itch figures prominently. A couple of clues are helpful in differentiating the two conditions: P. folliculitis often involves the chest and back, while D. folliculitis generally spares the trunk and is focused on the face and neck. And D. folliculitis typically arises when immunosuppression is weaned. Overgrowth of the mites occurs during immunosuppression, then as the immunosuppression is lifted a prominent inflammatory response with an acne-like appearance occurs.
Dr. Huang usually sticks with topical therapies for D. folliculitis. These include topical sulfur 5%, permethrin 5%, metronidazole, and/or ivermectin. If a young patient is unresponsive to this panoply of topical agents, she resorts to a single dose of oral ivermectin at 0.2 mg/kg, usually with good effect.
Dr. Huang reported having no financial conflicts of interest regarding her presentation.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Every child diagnosed with medulloblastoma deserves a careful dermatologic evaluation for possible comorbid basal cell nevus syndrome, according to Jennifer Huang, MD, a pediatric dermatologist at Boston Children’s Hospital and Harvard Medical School.
“Medulloblastoma occurs in 10%-20% of patients with basal cell nevus syndrome and can be the presenting sign. So if a patient with basal cell nevus syndrome gets medulloblastoma, it usually occurs within the first year of life – and it can be the first thing you see,” she said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dr. Huang presented a series of pediatric dermatology clinical pearls focused not only on basal cell nevus syndrome (BCNS) and medulloblastoma, but also on the implications of skin-limited Langerhans cell histiocytosis, how to recognize and treat drug-induced follicular eruptions in pediatric patients on targeted anticancer therapies, and when to suspect Demodex folliculitis in immunosuppressed patients.
Skin-limited Langerhans cell histiocytosis
Around 10%-20% of patients with Langerhans cell histiocytosis (LCH) have the skin-limited form of the malignancy. These are patients who, after a thorough workup, have a normal CBC, skeletal survey, and liver function tests; essentially, no evidence of multisystem disease.
“It’s very rare for patients who present with skin-limited LCH alone to develop multisystem disease and to require chemotherapy or other more aggressive treatment,” Dr. Huang said. “I think that skin-limited LCH is probably a separate entity with its own natural history distinct from multisystem disease. We can see that with current genomic testing: in multisystem LCH, BRAF mutations are identified in at least half of patients, but very few with skin-limited disease express those mutations.
“The clinical pearl here is if you have a patient with skin-limited LCH it very rarely progresses to multisystem involvement. It’s associated with a good prognosis. That doesn’t mean you shouldn’t monitor them, but I think it can be reassuring information for the family,” she said.
Basal cell nevus syndrome and medulloblastoma
“Half of cases of medulloblastoma are associated with mutations in the sonic hedgehog pathway – and a subset of that group has basal cell nevus syndrome,” Dr. Huang said.
BCNS is not a diagnosis frequently made by oncologists, who typically dismiss the multitude of lesions as skin tags, which they often mimic in both appearance and location, particularly on the neck and intertriginous areas. So it’s useful for dermatologists to establish a good referral relationship with their local oncologists.
“As dermatologists it’s really important to recognize not only the major features of basal cell nevus syndrome, but also the associated findings because we can really help in making this diagnosis early,” Dr. Huang stressed.
Early diagnosis of BCNS is a high priority for two reasons: to start treatment aimed at reducing development of basal cell carcinomas, and because radiation therapy for their medulloblastoma is contraindicated in patients with BCNS because it boosts their skin cancer burden.
BCNS is caused by mutations in the PTCH (Patched) gene found on chromosome arm 9q. The major features of BCNS include odontogenic keratocysts, palmoplantar pits, ectopic calcification, and, of course, basal cell carcinomas. The associated findings in BCNS, in addition to medulloblastoma, include macrocephaly and dysmorphic features such as cleft lip or palate, frontal bossing, and hypertelorism.
“I’ve treated a hundred at a time. It’s incredibly successful. It’s locally destructive. It leaves a little bit of hypopigmentation but no scar, which the CO2 laser will do in this instance. It’s actually a pretty cool modality,” said Dr. Eichenfield, professor of dermatology and pediatrics at Rady Children’s Hospital and the University of California, San Diego.
Follicular eruptions in cancer patients on MAPK inhibitors
Cutaneous reactions to anticancer drugs aimed at inhibiting the key MAPK (mitogen-activated protein kinase) pathway in children are common and diverse. Dr. Huang focused on the most common one: follicular eruptions, which occur in up to 80% of pediatric cancer patients on targeted therapy. These eruptions can express themselves in a variety of ways and are easily mistaken for comedonal acne, varicella zoster infection, herpes simplex, or bacterial folliculitis.
The key clues are highly suggestive that a follicular eruption in a child on targeted anticancer therapy is caused by the drug and not something else are the eruption’s symmetric distribution, that it’s truly follicular upon close inspection, and the timing: The eruption typically begins 2-3 weeks after initiation of therapy or within a week after a dose escalation.
Anti-inflammatory agents are the treatment mainstay. Treatment of the cutaneous eruption often is successful without need to discontinue the patient’s MAPK inhibitor.
“Even though some of these eruptions look comedonal, they’re not. It’s not a follicular plugging disorder, it’s an inflammatory condition. Topical steroids, oral tetracyclines, and dilute bleach baths all work pretty well. I haven’t had good experiences with keratolytics like tretinoin cream and benzoyl peroxide; they’re less effective. Dose reduction is the last resort for these patients. Often they are very sick. They need the drug and I think the last thing we want to do is take them off it,” Dr. Huang said.
She has observed that prepubertal children are more likely to have an eczematous reaction to their targeted anticancer therapy than a follicular eruption.
D. folliculitis in immunocompromised patients
“The clinical pearl here is to strongly consider the diagnosis of Demodex folliculitis in an immunosuppresed patient with an itchy acneiform eruption,” Dr. Huang said.
Demodex is a human mite which is part of the normal skin flora. She called it “a great mimicker”: It can cause dermatoses mistaken for rosacea, acne, seborrheic dermatitis, perioral facial dermatitis, blepharitis, and acute graft-versus-host disease.
In the setting of a young, immunosuppressed patient who develops an acneiform eruption, the differential diagnosis is lengthy and includes steroid-induced acne, a cutaneous reaction to targeted anticancer therapy, gram-negative folliculitis secondary to long-term antibiotic therapy, and Pityrosporum folliculitis, as well as D. folliculitis.
Demodex and P. folliculitis are the two acneiform dermatoses where itch figures prominently. A couple of clues are helpful in differentiating the two conditions: P. folliculitis often involves the chest and back, while D. folliculitis generally spares the trunk and is focused on the face and neck. And D. folliculitis typically arises when immunosuppression is weaned. Overgrowth of the mites occurs during immunosuppression, then as the immunosuppression is lifted a prominent inflammatory response with an acne-like appearance occurs.
Dr. Huang usually sticks with topical therapies for D. folliculitis. These include topical sulfur 5%, permethrin 5%, metronidazole, and/or ivermectin. If a young patient is unresponsive to this panoply of topical agents, she resorts to a single dose of oral ivermectin at 0.2 mg/kg, usually with good effect.
Dr. Huang reported having no financial conflicts of interest regarding her presentation.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Every child diagnosed with medulloblastoma deserves a careful dermatologic evaluation for possible comorbid basal cell nevus syndrome, according to Jennifer Huang, MD, a pediatric dermatologist at Boston Children’s Hospital and Harvard Medical School.
“Medulloblastoma occurs in 10%-20% of patients with basal cell nevus syndrome and can be the presenting sign. So if a patient with basal cell nevus syndrome gets medulloblastoma, it usually occurs within the first year of life – and it can be the first thing you see,” she said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dr. Huang presented a series of pediatric dermatology clinical pearls focused not only on basal cell nevus syndrome (BCNS) and medulloblastoma, but also on the implications of skin-limited Langerhans cell histiocytosis, how to recognize and treat drug-induced follicular eruptions in pediatric patients on targeted anticancer therapies, and when to suspect Demodex folliculitis in immunosuppressed patients.
Skin-limited Langerhans cell histiocytosis
Around 10%-20% of patients with Langerhans cell histiocytosis (LCH) have the skin-limited form of the malignancy. These are patients who, after a thorough workup, have a normal CBC, skeletal survey, and liver function tests; essentially, no evidence of multisystem disease.
“It’s very rare for patients who present with skin-limited LCH alone to develop multisystem disease and to require chemotherapy or other more aggressive treatment,” Dr. Huang said. “I think that skin-limited LCH is probably a separate entity with its own natural history distinct from multisystem disease. We can see that with current genomic testing: in multisystem LCH, BRAF mutations are identified in at least half of patients, but very few with skin-limited disease express those mutations.
“The clinical pearl here is if you have a patient with skin-limited LCH it very rarely progresses to multisystem involvement. It’s associated with a good prognosis. That doesn’t mean you shouldn’t monitor them, but I think it can be reassuring information for the family,” she said.
Basal cell nevus syndrome and medulloblastoma
“Half of cases of medulloblastoma are associated with mutations in the sonic hedgehog pathway – and a subset of that group has basal cell nevus syndrome,” Dr. Huang said.
BCNS is not a diagnosis frequently made by oncologists, who typically dismiss the multitude of lesions as skin tags, which they often mimic in both appearance and location, particularly on the neck and intertriginous areas. So it’s useful for dermatologists to establish a good referral relationship with their local oncologists.
“As dermatologists it’s really important to recognize not only the major features of basal cell nevus syndrome, but also the associated findings because we can really help in making this diagnosis early,” Dr. Huang stressed.
Early diagnosis of BCNS is a high priority for two reasons: to start treatment aimed at reducing development of basal cell carcinomas, and because radiation therapy for their medulloblastoma is contraindicated in patients with BCNS because it boosts their skin cancer burden.
BCNS is caused by mutations in the PTCH (Patched) gene found on chromosome arm 9q. The major features of BCNS include odontogenic keratocysts, palmoplantar pits, ectopic calcification, and, of course, basal cell carcinomas. The associated findings in BCNS, in addition to medulloblastoma, include macrocephaly and dysmorphic features such as cleft lip or palate, frontal bossing, and hypertelorism.
“I’ve treated a hundred at a time. It’s incredibly successful. It’s locally destructive. It leaves a little bit of hypopigmentation but no scar, which the CO2 laser will do in this instance. It’s actually a pretty cool modality,” said Dr. Eichenfield, professor of dermatology and pediatrics at Rady Children’s Hospital and the University of California, San Diego.
Follicular eruptions in cancer patients on MAPK inhibitors
Cutaneous reactions to anticancer drugs aimed at inhibiting the key MAPK (mitogen-activated protein kinase) pathway in children are common and diverse. Dr. Huang focused on the most common one: follicular eruptions, which occur in up to 80% of pediatric cancer patients on targeted therapy. These eruptions can express themselves in a variety of ways and are easily mistaken for comedonal acne, varicella zoster infection, herpes simplex, or bacterial folliculitis.
The key clues are highly suggestive that a follicular eruption in a child on targeted anticancer therapy is caused by the drug and not something else are the eruption’s symmetric distribution, that it’s truly follicular upon close inspection, and the timing: The eruption typically begins 2-3 weeks after initiation of therapy or within a week after a dose escalation.
Anti-inflammatory agents are the treatment mainstay. Treatment of the cutaneous eruption often is successful without need to discontinue the patient’s MAPK inhibitor.
“Even though some of these eruptions look comedonal, they’re not. It’s not a follicular plugging disorder, it’s an inflammatory condition. Topical steroids, oral tetracyclines, and dilute bleach baths all work pretty well. I haven’t had good experiences with keratolytics like tretinoin cream and benzoyl peroxide; they’re less effective. Dose reduction is the last resort for these patients. Often they are very sick. They need the drug and I think the last thing we want to do is take them off it,” Dr. Huang said.
She has observed that prepubertal children are more likely to have an eczematous reaction to their targeted anticancer therapy than a follicular eruption.
D. folliculitis in immunocompromised patients
“The clinical pearl here is to strongly consider the diagnosis of Demodex folliculitis in an immunosuppresed patient with an itchy acneiform eruption,” Dr. Huang said.
Demodex is a human mite which is part of the normal skin flora. She called it “a great mimicker”: It can cause dermatoses mistaken for rosacea, acne, seborrheic dermatitis, perioral facial dermatitis, blepharitis, and acute graft-versus-host disease.
In the setting of a young, immunosuppressed patient who develops an acneiform eruption, the differential diagnosis is lengthy and includes steroid-induced acne, a cutaneous reaction to targeted anticancer therapy, gram-negative folliculitis secondary to long-term antibiotic therapy, and Pityrosporum folliculitis, as well as D. folliculitis.
Demodex and P. folliculitis are the two acneiform dermatoses where itch figures prominently. A couple of clues are helpful in differentiating the two conditions: P. folliculitis often involves the chest and back, while D. folliculitis generally spares the trunk and is focused on the face and neck. And D. folliculitis typically arises when immunosuppression is weaned. Overgrowth of the mites occurs during immunosuppression, then as the immunosuppression is lifted a prominent inflammatory response with an acne-like appearance occurs.
Dr. Huang usually sticks with topical therapies for D. folliculitis. These include topical sulfur 5%, permethrin 5%, metronidazole, and/or ivermectin. If a young patient is unresponsive to this panoply of topical agents, she resorts to a single dose of oral ivermectin at 0.2 mg/kg, usually with good effect.
Dr. Huang reported having no financial conflicts of interest regarding her presentation.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
EAGLES: Smoking cessation therapy did not up cardiovascular risk
among stable adult smokers with up to one year of follow-up.
“In what we believe to be the largest smoking cessation clinical trial and the only trial comparing NRT, bupropion, and varenicline [with] placebo, we found no signal that smoking cessation pharmacotherapy increases the risk of serious cardiovascular disease or cardiovascular adverse events in a general population of smokers,” concluded Neal L. Benowitz, MD, of the University of California, San Francisco, and his associates. “While the number of events was small, the incidence of serious cardiovascular events was low, suggesting that any absolute increase in risk that we might have missed would be low and not clinically meaningful.” The findings were reported online April 9 in JAMA Internal Medicine.
In this double-blind, multicenter, triple-dummy trial (EAGLES), Dr. Benowitz and his associates randomly assigned 8,058 adult smokers, who did not have acute or unstable cardiovascular disease, to receive bupropion (150 mg twice daily), varenicline (1 mg twice daily), NRT (21-mg/day patch with tapering), or placebo for 12 weeks, followed by 12 weeks of follow-up. A total of 4,595 patients agreed to be followed for another 28 weeks during an extension phase of the trial. More than half of the patients were women and the average age of a participant was 47 years. The primary endpoint was time to major adverse cardiovascular event (MACE), including cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke. The researchers selected time to MACE as their primary endpoint to better detect differences among groups. One of the secondary end points was the occurrence of MACEs over the same 3 time intervals. Additionally, cardiovascular deaths, nonfatal MI, and nonfatal stroke (the components of MACE) were evaluated individually, as were hospitalizations for congestive heart failure and serious arrhythmias.
Differences in time to onset of MACE between all four patient groups, were not significant. The overall incidence of MACEs was less than 0.5% during all observation periods. There were also no significant differences in rates of the individual types of MACE, coronary revascularization, hospitalization for unstable angina, or new or worsening peripheral vascular disease requiring treatment among groups. Changes in body weight, blood pressure, and heart rate also were similar across patients.
There were five cardiovascular deaths, including one in the varenicline group, two in the bupropion group and two in the placebo group, according to the researchers. Overall the trial results “are consistent with and support previously published findings from meta-analyses and small clinical trials in smokers with known [cardiovascular disease],” they wrote.
GlaxoSmithKline and Pfizer, who make and market smoking cessation therapies, sponsored the study. Dr. Benowitz disclosed a consulting relationship with Pfizer and other pharmaceutical companies. He also has been a paid expert witness in litigation against tobacco companies. Eight coinvestigators disclosed ties to Pfizer, GlaxoSmithKline, and other companies.
SOURCE: Benowitz NL et al. JAMA Intern Med. 2018 Apr 9. doi: 10.1001/jamainternmed.2018.0397)
among stable adult smokers with up to one year of follow-up.
“In what we believe to be the largest smoking cessation clinical trial and the only trial comparing NRT, bupropion, and varenicline [with] placebo, we found no signal that smoking cessation pharmacotherapy increases the risk of serious cardiovascular disease or cardiovascular adverse events in a general population of smokers,” concluded Neal L. Benowitz, MD, of the University of California, San Francisco, and his associates. “While the number of events was small, the incidence of serious cardiovascular events was low, suggesting that any absolute increase in risk that we might have missed would be low and not clinically meaningful.” The findings were reported online April 9 in JAMA Internal Medicine.
In this double-blind, multicenter, triple-dummy trial (EAGLES), Dr. Benowitz and his associates randomly assigned 8,058 adult smokers, who did not have acute or unstable cardiovascular disease, to receive bupropion (150 mg twice daily), varenicline (1 mg twice daily), NRT (21-mg/day patch with tapering), or placebo for 12 weeks, followed by 12 weeks of follow-up. A total of 4,595 patients agreed to be followed for another 28 weeks during an extension phase of the trial. More than half of the patients were women and the average age of a participant was 47 years. The primary endpoint was time to major adverse cardiovascular event (MACE), including cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke. The researchers selected time to MACE as their primary endpoint to better detect differences among groups. One of the secondary end points was the occurrence of MACEs over the same 3 time intervals. Additionally, cardiovascular deaths, nonfatal MI, and nonfatal stroke (the components of MACE) were evaluated individually, as were hospitalizations for congestive heart failure and serious arrhythmias.
Differences in time to onset of MACE between all four patient groups, were not significant. The overall incidence of MACEs was less than 0.5% during all observation periods. There were also no significant differences in rates of the individual types of MACE, coronary revascularization, hospitalization for unstable angina, or new or worsening peripheral vascular disease requiring treatment among groups. Changes in body weight, blood pressure, and heart rate also were similar across patients.
There were five cardiovascular deaths, including one in the varenicline group, two in the bupropion group and two in the placebo group, according to the researchers. Overall the trial results “are consistent with and support previously published findings from meta-analyses and small clinical trials in smokers with known [cardiovascular disease],” they wrote.
GlaxoSmithKline and Pfizer, who make and market smoking cessation therapies, sponsored the study. Dr. Benowitz disclosed a consulting relationship with Pfizer and other pharmaceutical companies. He also has been a paid expert witness in litigation against tobacco companies. Eight coinvestigators disclosed ties to Pfizer, GlaxoSmithKline, and other companies.
SOURCE: Benowitz NL et al. JAMA Intern Med. 2018 Apr 9. doi: 10.1001/jamainternmed.2018.0397)
among stable adult smokers with up to one year of follow-up.
“In what we believe to be the largest smoking cessation clinical trial and the only trial comparing NRT, bupropion, and varenicline [with] placebo, we found no signal that smoking cessation pharmacotherapy increases the risk of serious cardiovascular disease or cardiovascular adverse events in a general population of smokers,” concluded Neal L. Benowitz, MD, of the University of California, San Francisco, and his associates. “While the number of events was small, the incidence of serious cardiovascular events was low, suggesting that any absolute increase in risk that we might have missed would be low and not clinically meaningful.” The findings were reported online April 9 in JAMA Internal Medicine.
In this double-blind, multicenter, triple-dummy trial (EAGLES), Dr. Benowitz and his associates randomly assigned 8,058 adult smokers, who did not have acute or unstable cardiovascular disease, to receive bupropion (150 mg twice daily), varenicline (1 mg twice daily), NRT (21-mg/day patch with tapering), or placebo for 12 weeks, followed by 12 weeks of follow-up. A total of 4,595 patients agreed to be followed for another 28 weeks during an extension phase of the trial. More than half of the patients were women and the average age of a participant was 47 years. The primary endpoint was time to major adverse cardiovascular event (MACE), including cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke. The researchers selected time to MACE as their primary endpoint to better detect differences among groups. One of the secondary end points was the occurrence of MACEs over the same 3 time intervals. Additionally, cardiovascular deaths, nonfatal MI, and nonfatal stroke (the components of MACE) were evaluated individually, as were hospitalizations for congestive heart failure and serious arrhythmias.
Differences in time to onset of MACE between all four patient groups, were not significant. The overall incidence of MACEs was less than 0.5% during all observation periods. There were also no significant differences in rates of the individual types of MACE, coronary revascularization, hospitalization for unstable angina, or new or worsening peripheral vascular disease requiring treatment among groups. Changes in body weight, blood pressure, and heart rate also were similar across patients.
There were five cardiovascular deaths, including one in the varenicline group, two in the bupropion group and two in the placebo group, according to the researchers. Overall the trial results “are consistent with and support previously published findings from meta-analyses and small clinical trials in smokers with known [cardiovascular disease],” they wrote.
GlaxoSmithKline and Pfizer, who make and market smoking cessation therapies, sponsored the study. Dr. Benowitz disclosed a consulting relationship with Pfizer and other pharmaceutical companies. He also has been a paid expert witness in litigation against tobacco companies. Eight coinvestigators disclosed ties to Pfizer, GlaxoSmithKline, and other companies.
SOURCE: Benowitz NL et al. JAMA Intern Med. 2018 Apr 9. doi: 10.1001/jamainternmed.2018.0397)
FROM JAMA INTERNAL MEDICINE
Key clinical point: The use of smoking cessation therapy did not increase the risk of cardiovascular events in adult smokers.
Major finding: There were no significant differences among groups in rates of major adverse cardiovascular events, rates of other pertinent cardiovascular events, time to cardiovascular events, blood pressure, or heart rate.
Study details: Double-blind, randomized, multicenter, triple-dummy trial of 8,058 adult smokers receiving nicotine replacement therapy, bupropion, varenicline, or placebo (EAGLES).
Disclosures: GlaxoSmithKline and Pfizer sponsored the study and make the drugs. Dr. Benowitz disclosed a consulting relationship with Pfizer and other pharmaceutical companies. He also has been a paid expert witness in litigation against tobacco companies. Eight coinvestigators disclosed ties to Pfizer, GlaxoSmithKline, and other companies.
Source: Benowitz NL et al. JAMA Intern Med. 2018 Apr 9. doi: 10.1001/jamainternmed.2018.0397.
Epilepsy upped risk of unnatural death
People with epilepsy were about three times more likely to die from unnatural causes and five times more likely to die from unintentional medication poisoning than controls in a large study.
Opioid and psychotropic drugs were the main sources of poisoning deaths, said Hayley C. Gorton, PhD, of the University of Manchester, England, and her associates. Epilepsy also was associated with a twofold increase in risk of suicide. Providers should counsel patients with epilepsy about unintentional injuries, exercise caution when prescribing opioids, and monitor patients closely for suicidal thoughts, ideation, and behavior, the researchers wrote online April 9 in JAMA Neurology.
The study included 58,729 individuals with epilepsy and nearly 1.2 million controls matched by age, sex, and location. Data sources included the Clinical Practice Research Datalink in England and the Secure Anonymised Information Linkage Databank in Wales. The researchers identified unnatural deaths by querying relevant codes from the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision. The study spanned 1998-2014, with typically 4-8 years of follow-up.
Epilepsy was associated with a significantly increased risk of death from any unnatural cause (hazard ratio, 2.8; 95% confidence interval, 2.4-3.3), from accidental medication poisoning (HR, 5.0; 95% CI, 3.2-7.7), and from suicide (HR, 2.2; 95% CI, 1.5-3.1). Opioids were the most common cause of death from medication poisoning (56%), followed by psychotropic drugs (32%). Antiepileptic drugs were responsible for only about 10% of iatrogenic deaths.
As in prior studies, epilepsy was tied to numerous psychiatric comorbidities, including substance abuse disorders, anxiety, mood and eating disorders, personality disorders, and schizophrenia. Mental illness increases the risk of unintentional injury, poisoning, and suicide, the investigators noted. Mental illness and associated stigma also explain why epilepsy patients were three times more likely to die from homicide (HR, 3.5; 95% CI, 1.2-10.6), they wrote.
Funders included the National Institute for Health Research and Health and Care Research Wales. The researchers reported having no conflicts of interest.
SOURCE: Gorton HC et al. JAMA Neurol. 2019 Apr 9. doi: 10.1001/jamaneurol.2018.0333.
The study elucidates “an enormous problem hiding in plain sight” – persistently high rates of unnatural and medication-induced death among people with epilepsy, wrote Orrin Devinsky, MD, Anuradha Singh, MD, and Daniel Friedman, MD, in an accompanying editorial in JAMA Neurology.
“We need a new paradigm to ‘see’ patients and ‘understand’ their disorders and experiences,” the editorialists explained. Mood disorders, poor judgment, impulsive behavior, and cognitive impairment “are part of the disease biology as much as brain stem cardiopulmonary dysfunction is thought to contribute to sudden unexpected death in epilepsy.”
They called on the National Institute of Mental Health and the National Institute of Neurological Disorders and Stroke to encourage studies of “the tangled thicket where neurology and psychiatry meet.”
All three physicians are at NYU Langone Medical Center, New York. Dr. Devinsky disclosed ties to GW Pharmaceuticals and several other companies. No other disclosures were reported (JAMA Neurol. 2018 Apr 9. doi: 10.1001/jamaneurol.2018.0002).
The study elucidates “an enormous problem hiding in plain sight” – persistently high rates of unnatural and medication-induced death among people with epilepsy, wrote Orrin Devinsky, MD, Anuradha Singh, MD, and Daniel Friedman, MD, in an accompanying editorial in JAMA Neurology.
“We need a new paradigm to ‘see’ patients and ‘understand’ their disorders and experiences,” the editorialists explained. Mood disorders, poor judgment, impulsive behavior, and cognitive impairment “are part of the disease biology as much as brain stem cardiopulmonary dysfunction is thought to contribute to sudden unexpected death in epilepsy.”
They called on the National Institute of Mental Health and the National Institute of Neurological Disorders and Stroke to encourage studies of “the tangled thicket where neurology and psychiatry meet.”
All three physicians are at NYU Langone Medical Center, New York. Dr. Devinsky disclosed ties to GW Pharmaceuticals and several other companies. No other disclosures were reported (JAMA Neurol. 2018 Apr 9. doi: 10.1001/jamaneurol.2018.0002).
The study elucidates “an enormous problem hiding in plain sight” – persistently high rates of unnatural and medication-induced death among people with epilepsy, wrote Orrin Devinsky, MD, Anuradha Singh, MD, and Daniel Friedman, MD, in an accompanying editorial in JAMA Neurology.
“We need a new paradigm to ‘see’ patients and ‘understand’ their disorders and experiences,” the editorialists explained. Mood disorders, poor judgment, impulsive behavior, and cognitive impairment “are part of the disease biology as much as brain stem cardiopulmonary dysfunction is thought to contribute to sudden unexpected death in epilepsy.”
They called on the National Institute of Mental Health and the National Institute of Neurological Disorders and Stroke to encourage studies of “the tangled thicket where neurology and psychiatry meet.”
All three physicians are at NYU Langone Medical Center, New York. Dr. Devinsky disclosed ties to GW Pharmaceuticals and several other companies. No other disclosures were reported (JAMA Neurol. 2018 Apr 9. doi: 10.1001/jamaneurol.2018.0002).
People with epilepsy were about three times more likely to die from unnatural causes and five times more likely to die from unintentional medication poisoning than controls in a large study.
Opioid and psychotropic drugs were the main sources of poisoning deaths, said Hayley C. Gorton, PhD, of the University of Manchester, England, and her associates. Epilepsy also was associated with a twofold increase in risk of suicide. Providers should counsel patients with epilepsy about unintentional injuries, exercise caution when prescribing opioids, and monitor patients closely for suicidal thoughts, ideation, and behavior, the researchers wrote online April 9 in JAMA Neurology.
The study included 58,729 individuals with epilepsy and nearly 1.2 million controls matched by age, sex, and location. Data sources included the Clinical Practice Research Datalink in England and the Secure Anonymised Information Linkage Databank in Wales. The researchers identified unnatural deaths by querying relevant codes from the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision. The study spanned 1998-2014, with typically 4-8 years of follow-up.
Epilepsy was associated with a significantly increased risk of death from any unnatural cause (hazard ratio, 2.8; 95% confidence interval, 2.4-3.3), from accidental medication poisoning (HR, 5.0; 95% CI, 3.2-7.7), and from suicide (HR, 2.2; 95% CI, 1.5-3.1). Opioids were the most common cause of death from medication poisoning (56%), followed by psychotropic drugs (32%). Antiepileptic drugs were responsible for only about 10% of iatrogenic deaths.
As in prior studies, epilepsy was tied to numerous psychiatric comorbidities, including substance abuse disorders, anxiety, mood and eating disorders, personality disorders, and schizophrenia. Mental illness increases the risk of unintentional injury, poisoning, and suicide, the investigators noted. Mental illness and associated stigma also explain why epilepsy patients were three times more likely to die from homicide (HR, 3.5; 95% CI, 1.2-10.6), they wrote.
Funders included the National Institute for Health Research and Health and Care Research Wales. The researchers reported having no conflicts of interest.
SOURCE: Gorton HC et al. JAMA Neurol. 2019 Apr 9. doi: 10.1001/jamaneurol.2018.0333.
People with epilepsy were about three times more likely to die from unnatural causes and five times more likely to die from unintentional medication poisoning than controls in a large study.
Opioid and psychotropic drugs were the main sources of poisoning deaths, said Hayley C. Gorton, PhD, of the University of Manchester, England, and her associates. Epilepsy also was associated with a twofold increase in risk of suicide. Providers should counsel patients with epilepsy about unintentional injuries, exercise caution when prescribing opioids, and monitor patients closely for suicidal thoughts, ideation, and behavior, the researchers wrote online April 9 in JAMA Neurology.
The study included 58,729 individuals with epilepsy and nearly 1.2 million controls matched by age, sex, and location. Data sources included the Clinical Practice Research Datalink in England and the Secure Anonymised Information Linkage Databank in Wales. The researchers identified unnatural deaths by querying relevant codes from the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision. The study spanned 1998-2014, with typically 4-8 years of follow-up.
Epilepsy was associated with a significantly increased risk of death from any unnatural cause (hazard ratio, 2.8; 95% confidence interval, 2.4-3.3), from accidental medication poisoning (HR, 5.0; 95% CI, 3.2-7.7), and from suicide (HR, 2.2; 95% CI, 1.5-3.1). Opioids were the most common cause of death from medication poisoning (56%), followed by psychotropic drugs (32%). Antiepileptic drugs were responsible for only about 10% of iatrogenic deaths.
As in prior studies, epilepsy was tied to numerous psychiatric comorbidities, including substance abuse disorders, anxiety, mood and eating disorders, personality disorders, and schizophrenia. Mental illness increases the risk of unintentional injury, poisoning, and suicide, the investigators noted. Mental illness and associated stigma also explain why epilepsy patients were three times more likely to die from homicide (HR, 3.5; 95% CI, 1.2-10.6), they wrote.
Funders included the National Institute for Health Research and Health and Care Research Wales. The researchers reported having no conflicts of interest.
SOURCE: Gorton HC et al. JAMA Neurol. 2019 Apr 9. doi: 10.1001/jamaneurol.2018.0333.
FROM JAMA NEUROLOGY
Key clinical point: Epilepsy increases the risk of mortality from unnatural causes.
Major finding: Epilepsy was associated with a significantly increased risk of death from any unnatural cause (HR, 2.8), from accidental medication poisoning (HR, 5.0), and from suicide (HR, 2.2).
Study details: A population-based cohort study of more than 1 million people.
Disclosures: Funders included the National Institute for Health Research and Health and Care Research Wales. The researchers reported having no conflicts of interest.
Source: Gorton HC et al. JAMA Neurol. 2019 Apr 9. doi: 10.1001/jamaneurol.2018.0333.
Social support after Katrina may ease depressive, PTSD symptoms
The availability of social support after a traumatic event such as a natural disaster could help buffer the development of depressive and PTSD symptoms in individuals exposed to the event, according to research published April 5.
In the Journal of Traumatic Stress, researchers reported the results of a survey of 810 adults who were exposed to the third-deadliest hurricane in U.S. history – Hurricane Katrina – in August 2005. Of those adults, 259 were displaced by the hurricane and 546 were not displaced. All of the adults were residents of Mississippi before the hurricane. More than half of the participants were women (52.2%), most were white (73.5%), and their ages ranged from 18 to 91 years.
Interviewers who were supervised by doctoral level clinicians administered numerous self-report questionnaires 18-24 months after the hurricane. Among other measures, the interviews included the Composite International Interview for DSM-IV.
The researchers found a significant negative interaction between perceived social support received in the 2 months after the hurricane and depressive symptoms, both in displaced and nondisplaced individuals.
The study also showed that the number of Katrina-related traumatic events, and whether an individual had been displaced or not, were associated with depressive symptoms – even after accounting for potential confounders, such as the number of previous traumatic events, age, and minority status.
In addition, the study explored the interaction between the number of hurricane-related traumatic events, perceived social support received, and displacement status as predictors of each cluster of PTSD symptoms. Individuals who experienced greater numbers of hurricane-related traumatic events and were displaced by the event showed more reexperiencing, avoidance, and arousal symptoms. However, social support was associated with lower likelihood of all PTSD symptom clusters.
Nondisplaced individuals who experienced a greater number of hurricane-related traumatic events showed higher arousal and avoidance symptoms, but this was only significant in individuals who reported lower levels of social support.
“Unlike previous studies, by controlling for highly correlated variables: number of previous traumatic events experienced and disaster-related stressors,” wrote Adam P. McGuire, PhD, formerly of the University of Mississippi, Jackson, and now at the Veterans Integrated Service Network, and his coauthors.
The authors also commented on the “unexpected” finding that the significant buffering effect of social support was seen both in displaced and nondisplaced residents, “which suggests that perceived social support is linked to important cognitive and behavioral processes that reduce the likelihood of developing depressive symptoms [e.g., challenging negative beliefs about self], and those effects are not limited to nondisplaced disaster survivors.”
The study was supported by the National Institutes of Health and the Midwest Regional Postdoctoral Program in Eating Disorder Research. The authors had no conflicts of interest.
SOURCE: McGuire AP et al. J Trauma Stress. 2018 Apr 5. doi: 10.1002/jts.22270.
The availability of social support after a traumatic event such as a natural disaster could help buffer the development of depressive and PTSD symptoms in individuals exposed to the event, according to research published April 5.
In the Journal of Traumatic Stress, researchers reported the results of a survey of 810 adults who were exposed to the third-deadliest hurricane in U.S. history – Hurricane Katrina – in August 2005. Of those adults, 259 were displaced by the hurricane and 546 were not displaced. All of the adults were residents of Mississippi before the hurricane. More than half of the participants were women (52.2%), most were white (73.5%), and their ages ranged from 18 to 91 years.
Interviewers who were supervised by doctoral level clinicians administered numerous self-report questionnaires 18-24 months after the hurricane. Among other measures, the interviews included the Composite International Interview for DSM-IV.
The researchers found a significant negative interaction between perceived social support received in the 2 months after the hurricane and depressive symptoms, both in displaced and nondisplaced individuals.
The study also showed that the number of Katrina-related traumatic events, and whether an individual had been displaced or not, were associated with depressive symptoms – even after accounting for potential confounders, such as the number of previous traumatic events, age, and minority status.
In addition, the study explored the interaction between the number of hurricane-related traumatic events, perceived social support received, and displacement status as predictors of each cluster of PTSD symptoms. Individuals who experienced greater numbers of hurricane-related traumatic events and were displaced by the event showed more reexperiencing, avoidance, and arousal symptoms. However, social support was associated with lower likelihood of all PTSD symptom clusters.
Nondisplaced individuals who experienced a greater number of hurricane-related traumatic events showed higher arousal and avoidance symptoms, but this was only significant in individuals who reported lower levels of social support.
“Unlike previous studies, by controlling for highly correlated variables: number of previous traumatic events experienced and disaster-related stressors,” wrote Adam P. McGuire, PhD, formerly of the University of Mississippi, Jackson, and now at the Veterans Integrated Service Network, and his coauthors.
The authors also commented on the “unexpected” finding that the significant buffering effect of social support was seen both in displaced and nondisplaced residents, “which suggests that perceived social support is linked to important cognitive and behavioral processes that reduce the likelihood of developing depressive symptoms [e.g., challenging negative beliefs about self], and those effects are not limited to nondisplaced disaster survivors.”
The study was supported by the National Institutes of Health and the Midwest Regional Postdoctoral Program in Eating Disorder Research. The authors had no conflicts of interest.
SOURCE: McGuire AP et al. J Trauma Stress. 2018 Apr 5. doi: 10.1002/jts.22270.
The availability of social support after a traumatic event such as a natural disaster could help buffer the development of depressive and PTSD symptoms in individuals exposed to the event, according to research published April 5.
In the Journal of Traumatic Stress, researchers reported the results of a survey of 810 adults who were exposed to the third-deadliest hurricane in U.S. history – Hurricane Katrina – in August 2005. Of those adults, 259 were displaced by the hurricane and 546 were not displaced. All of the adults were residents of Mississippi before the hurricane. More than half of the participants were women (52.2%), most were white (73.5%), and their ages ranged from 18 to 91 years.
Interviewers who were supervised by doctoral level clinicians administered numerous self-report questionnaires 18-24 months after the hurricane. Among other measures, the interviews included the Composite International Interview for DSM-IV.
The researchers found a significant negative interaction between perceived social support received in the 2 months after the hurricane and depressive symptoms, both in displaced and nondisplaced individuals.
The study also showed that the number of Katrina-related traumatic events, and whether an individual had been displaced or not, were associated with depressive symptoms – even after accounting for potential confounders, such as the number of previous traumatic events, age, and minority status.
In addition, the study explored the interaction between the number of hurricane-related traumatic events, perceived social support received, and displacement status as predictors of each cluster of PTSD symptoms. Individuals who experienced greater numbers of hurricane-related traumatic events and were displaced by the event showed more reexperiencing, avoidance, and arousal symptoms. However, social support was associated with lower likelihood of all PTSD symptom clusters.
Nondisplaced individuals who experienced a greater number of hurricane-related traumatic events showed higher arousal and avoidance symptoms, but this was only significant in individuals who reported lower levels of social support.
“Unlike previous studies, by controlling for highly correlated variables: number of previous traumatic events experienced and disaster-related stressors,” wrote Adam P. McGuire, PhD, formerly of the University of Mississippi, Jackson, and now at the Veterans Integrated Service Network, and his coauthors.
The authors also commented on the “unexpected” finding that the significant buffering effect of social support was seen both in displaced and nondisplaced residents, “which suggests that perceived social support is linked to important cognitive and behavioral processes that reduce the likelihood of developing depressive symptoms [e.g., challenging negative beliefs about self], and those effects are not limited to nondisplaced disaster survivors.”
The study was supported by the National Institutes of Health and the Midwest Regional Postdoctoral Program in Eating Disorder Research. The authors had no conflicts of interest.
SOURCE: McGuire AP et al. J Trauma Stress. 2018 Apr 5. doi: 10.1002/jts.22270.
FROM THE JOURNAL OF TRAUMATIC STRESS
Key clinical point: Social support can reduce the impact of traumatic events such as hurricanes.
Major finding: Perceived social support was associated with reduced depressive symptoms.
Study details: A survey of 810 adults who were exposed to Hurricane Katrina while living in Mississippi.
Disclosures: The study was supported by the National Institutes of Health and the Midwest Regional Postdoctoral Program in Eating Disorder Research. The presenters had no conflicts of interest.
Source: McGuire AP et al. J Trauma Stress. 2018 Apr 5. doi: 10.1002/jts.22270.
Simvastatin, atorvastatin cut mortality risk for sepsis patients
a large health care database review has determined.
Among almost 53,000 sepsis patients, those who had been taking simvastatin were 28% less likely to die within 30 days of a sepsis admission than were patients not taking a statin. Atorvastatin conferred a similar significant survival benefit, reducing the risk of death by 22%, Chien-Chang Lee, MD and his colleagues wrote in the April issue of the journal CHEST®.
The drugs also exert a direct antimicrobial effect, he asserted.
“Of note, simvastatin was shown by several reports to have the most potent antibacterial activity,” targeting both methicillin-resistant and -sensitive Staphylococcus aureus, as well as gram negative and positive bacteria.
Dr. Lee and his colleagues extracted mortality and statin prescription data from the Taiwan National Health Insurance Database from 2000-2011. They looked at 30- and 90-day mortality in 52,737 patients who developed sepsis; the statins of interest were atorvastatin, simvastatin, and rosuvastatin. Patients had to have been taking the medication for at least 30 days before sepsis onset to be included, and patients taking more than one statin were excluded from the analysis.
Patients were a mean of 69 years old. About half had a lower respiratory infection. The remainder had infections within the abdomen, the biliary or urinary tract, skin, or orthopedic infections. There were no significant differences in comorbidities or in other medications taken among the three statin groups or the nonusers.
Of the entire cohort, 17% died by 30 days and nearly 23% by 90 days. Compared with those who had never received a statin, the statin users were 12% less likely to die by 30 days (hazard ratio, 0.88). Mortality at 90 days was also decreased, when compared with nonusers (HR, 0.93).
Simvastatin demonstrated the greatest benefit, with a 28% decreased risk of 30-day mortality (HR, 0.72). Atorvastatin followed, with a 22% risk reduction (HR, 0.78). Rosuvastatin exerted a nonsignificant 13% benefit.
The authors then examined 90-day mortality risks for the patients with a propensity matching score using a subgroup comprising 536 simvastatin users, 536 atorvastatin users, and 536 rosuvastatin users. Simvastatin was associated with a 23% reduction in 30-day mortality risk (HR, 0.77) and atorvastatin with a 21% reduction (HR, 0.79), when compared with rosuvastatin.
Statins’ antimicrobial properties are probably partially caused by their inactivation of the 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase pathway, Dr. Lee and his colleagues noted. In addition to being vital for cholesterol synthesis, this pathway “also contributes to the production of isoprenoids and lipid compounds that are essential for cell signaling and structure in the pathogen. Secondly, the chemical property of different types of statins may affect their targeting to bacteria. The lipophilic properties of simvastatin or atorvastatin may allow better binding to bacteria cell walls than the hydrophilic properties of rosuvastatin.”
The study was funded by the Taiwan National Science Foundation and Taiwan National Ministry of Science and Technology. Dr. Lee had no financial conflicts.
The statin-sepsis mortality link will probably never be definitively proven, but the study by Lee and colleagues gives us the best data so far on this intriguing connection, Steven Q. Simpson, MD and Joel D. Mermis, MD wrote in an accompanying editorial.
“It is unlikely that prospective randomized trials of statins for prevention of sepsis mortality will ever be undertaken, owing to the sheer number of patients that would require randomization in order to have adequate numbers who actually develop sepsis,” the colleagues wrote. “We believe that the next best thing to randomization and a prospective trial is exactly what the authors have done – identify a cohort, track them through time, even if nonconcurrently, and match cases to controls by propensity matching on important clinical characteristics.”
Nevertheless, the two said, “This brings us to one aspect of the study that leaves open a window for some doubt.”
Lee et al. extracted their data from a large national insurance claims database. These systems “are commonly believed to overestimate sepsis incidence,” Dr. Simpson and Dr. Mermis wrote. A 2009 U.S. study bore this out, they said. “That study showed that in the U.S in 2014, there were approximately 1.7 million cases of sepsis in a population of 330 million, for an annual incidence rate of five sepsis cases per 1,000 patient-years.”
However, a “quick calculation” of the Taiwan data suggests that the annual sepsis caseload is about 5,200 per year in a population of 23 million at risk – an annual incidence of only 0.2 cases per 1,000 patient-years.
“This represents an order of magnitude difference in sepsis incidence between the U.S. and Taiwan, providing some issues to ponder. Does Taiwan indeed have a lower incidence of sepsis by that much? If so, is the lower incidence related to genetics, environment, health care access, or other factors?
“Although Lee et al. have provided us with data of the highest quality that we can likely hope for, the book may not be quite closed, yet.”
Dr. Mermis and Dr. Simpson are pulmonologists at the University of Kansas, Kansas City. They made their comments in an editorial published in the April issue of CHEST® (Mermis JD and Simpson SQ. CHEST. 2018 April. doi: 10.1016/j.chest.2017.12.004.)
The statin-sepsis mortality link will probably never be definitively proven, but the study by Lee and colleagues gives us the best data so far on this intriguing connection, Steven Q. Simpson, MD and Joel D. Mermis, MD wrote in an accompanying editorial.
“It is unlikely that prospective randomized trials of statins for prevention of sepsis mortality will ever be undertaken, owing to the sheer number of patients that would require randomization in order to have adequate numbers who actually develop sepsis,” the colleagues wrote. “We believe that the next best thing to randomization and a prospective trial is exactly what the authors have done – identify a cohort, track them through time, even if nonconcurrently, and match cases to controls by propensity matching on important clinical characteristics.”
Nevertheless, the two said, “This brings us to one aspect of the study that leaves open a window for some doubt.”
Lee et al. extracted their data from a large national insurance claims database. These systems “are commonly believed to overestimate sepsis incidence,” Dr. Simpson and Dr. Mermis wrote. A 2009 U.S. study bore this out, they said. “That study showed that in the U.S in 2014, there were approximately 1.7 million cases of sepsis in a population of 330 million, for an annual incidence rate of five sepsis cases per 1,000 patient-years.”
However, a “quick calculation” of the Taiwan data suggests that the annual sepsis caseload is about 5,200 per year in a population of 23 million at risk – an annual incidence of only 0.2 cases per 1,000 patient-years.
“This represents an order of magnitude difference in sepsis incidence between the U.S. and Taiwan, providing some issues to ponder. Does Taiwan indeed have a lower incidence of sepsis by that much? If so, is the lower incidence related to genetics, environment, health care access, or other factors?
“Although Lee et al. have provided us with data of the highest quality that we can likely hope for, the book may not be quite closed, yet.”
Dr. Mermis and Dr. Simpson are pulmonologists at the University of Kansas, Kansas City. They made their comments in an editorial published in the April issue of CHEST® (Mermis JD and Simpson SQ. CHEST. 2018 April. doi: 10.1016/j.chest.2017.12.004.)
The statin-sepsis mortality link will probably never be definitively proven, but the study by Lee and colleagues gives us the best data so far on this intriguing connection, Steven Q. Simpson, MD and Joel D. Mermis, MD wrote in an accompanying editorial.
“It is unlikely that prospective randomized trials of statins for prevention of sepsis mortality will ever be undertaken, owing to the sheer number of patients that would require randomization in order to have adequate numbers who actually develop sepsis,” the colleagues wrote. “We believe that the next best thing to randomization and a prospective trial is exactly what the authors have done – identify a cohort, track them through time, even if nonconcurrently, and match cases to controls by propensity matching on important clinical characteristics.”
Nevertheless, the two said, “This brings us to one aspect of the study that leaves open a window for some doubt.”
Lee et al. extracted their data from a large national insurance claims database. These systems “are commonly believed to overestimate sepsis incidence,” Dr. Simpson and Dr. Mermis wrote. A 2009 U.S. study bore this out, they said. “That study showed that in the U.S in 2014, there were approximately 1.7 million cases of sepsis in a population of 330 million, for an annual incidence rate of five sepsis cases per 1,000 patient-years.”
However, a “quick calculation” of the Taiwan data suggests that the annual sepsis caseload is about 5,200 per year in a population of 23 million at risk – an annual incidence of only 0.2 cases per 1,000 patient-years.
“This represents an order of magnitude difference in sepsis incidence between the U.S. and Taiwan, providing some issues to ponder. Does Taiwan indeed have a lower incidence of sepsis by that much? If so, is the lower incidence related to genetics, environment, health care access, or other factors?
“Although Lee et al. have provided us with data of the highest quality that we can likely hope for, the book may not be quite closed, yet.”
Dr. Mermis and Dr. Simpson are pulmonologists at the University of Kansas, Kansas City. They made their comments in an editorial published in the April issue of CHEST® (Mermis JD and Simpson SQ. CHEST. 2018 April. doi: 10.1016/j.chest.2017.12.004.)
a large health care database review has determined.
Among almost 53,000 sepsis patients, those who had been taking simvastatin were 28% less likely to die within 30 days of a sepsis admission than were patients not taking a statin. Atorvastatin conferred a similar significant survival benefit, reducing the risk of death by 22%, Chien-Chang Lee, MD and his colleagues wrote in the April issue of the journal CHEST®.
The drugs also exert a direct antimicrobial effect, he asserted.
“Of note, simvastatin was shown by several reports to have the most potent antibacterial activity,” targeting both methicillin-resistant and -sensitive Staphylococcus aureus, as well as gram negative and positive bacteria.
Dr. Lee and his colleagues extracted mortality and statin prescription data from the Taiwan National Health Insurance Database from 2000-2011. They looked at 30- and 90-day mortality in 52,737 patients who developed sepsis; the statins of interest were atorvastatin, simvastatin, and rosuvastatin. Patients had to have been taking the medication for at least 30 days before sepsis onset to be included, and patients taking more than one statin were excluded from the analysis.
Patients were a mean of 69 years old. About half had a lower respiratory infection. The remainder had infections within the abdomen, the biliary or urinary tract, skin, or orthopedic infections. There were no significant differences in comorbidities or in other medications taken among the three statin groups or the nonusers.
Of the entire cohort, 17% died by 30 days and nearly 23% by 90 days. Compared with those who had never received a statin, the statin users were 12% less likely to die by 30 days (hazard ratio, 0.88). Mortality at 90 days was also decreased, when compared with nonusers (HR, 0.93).
Simvastatin demonstrated the greatest benefit, with a 28% decreased risk of 30-day mortality (HR, 0.72). Atorvastatin followed, with a 22% risk reduction (HR, 0.78). Rosuvastatin exerted a nonsignificant 13% benefit.
The authors then examined 90-day mortality risks for the patients with a propensity matching score using a subgroup comprising 536 simvastatin users, 536 atorvastatin users, and 536 rosuvastatin users. Simvastatin was associated with a 23% reduction in 30-day mortality risk (HR, 0.77) and atorvastatin with a 21% reduction (HR, 0.79), when compared with rosuvastatin.
Statins’ antimicrobial properties are probably partially caused by their inactivation of the 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase pathway, Dr. Lee and his colleagues noted. In addition to being vital for cholesterol synthesis, this pathway “also contributes to the production of isoprenoids and lipid compounds that are essential for cell signaling and structure in the pathogen. Secondly, the chemical property of different types of statins may affect their targeting to bacteria. The lipophilic properties of simvastatin or atorvastatin may allow better binding to bacteria cell walls than the hydrophilic properties of rosuvastatin.”
The study was funded by the Taiwan National Science Foundation and Taiwan National Ministry of Science and Technology. Dr. Lee had no financial conflicts.
a large health care database review has determined.
Among almost 53,000 sepsis patients, those who had been taking simvastatin were 28% less likely to die within 30 days of a sepsis admission than were patients not taking a statin. Atorvastatin conferred a similar significant survival benefit, reducing the risk of death by 22%, Chien-Chang Lee, MD and his colleagues wrote in the April issue of the journal CHEST®.
The drugs also exert a direct antimicrobial effect, he asserted.
“Of note, simvastatin was shown by several reports to have the most potent antibacterial activity,” targeting both methicillin-resistant and -sensitive Staphylococcus aureus, as well as gram negative and positive bacteria.
Dr. Lee and his colleagues extracted mortality and statin prescription data from the Taiwan National Health Insurance Database from 2000-2011. They looked at 30- and 90-day mortality in 52,737 patients who developed sepsis; the statins of interest were atorvastatin, simvastatin, and rosuvastatin. Patients had to have been taking the medication for at least 30 days before sepsis onset to be included, and patients taking more than one statin were excluded from the analysis.
Patients were a mean of 69 years old. About half had a lower respiratory infection. The remainder had infections within the abdomen, the biliary or urinary tract, skin, or orthopedic infections. There were no significant differences in comorbidities or in other medications taken among the three statin groups or the nonusers.
Of the entire cohort, 17% died by 30 days and nearly 23% by 90 days. Compared with those who had never received a statin, the statin users were 12% less likely to die by 30 days (hazard ratio, 0.88). Mortality at 90 days was also decreased, when compared with nonusers (HR, 0.93).
Simvastatin demonstrated the greatest benefit, with a 28% decreased risk of 30-day mortality (HR, 0.72). Atorvastatin followed, with a 22% risk reduction (HR, 0.78). Rosuvastatin exerted a nonsignificant 13% benefit.
The authors then examined 90-day mortality risks for the patients with a propensity matching score using a subgroup comprising 536 simvastatin users, 536 atorvastatin users, and 536 rosuvastatin users. Simvastatin was associated with a 23% reduction in 30-day mortality risk (HR, 0.77) and atorvastatin with a 21% reduction (HR, 0.79), when compared with rosuvastatin.
Statins’ antimicrobial properties are probably partially caused by their inactivation of the 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase pathway, Dr. Lee and his colleagues noted. In addition to being vital for cholesterol synthesis, this pathway “also contributes to the production of isoprenoids and lipid compounds that are essential for cell signaling and structure in the pathogen. Secondly, the chemical property of different types of statins may affect their targeting to bacteria. The lipophilic properties of simvastatin or atorvastatin may allow better binding to bacteria cell walls than the hydrophilic properties of rosuvastatin.”
The study was funded by the Taiwan National Science Foundation and Taiwan National Ministry of Science and Technology. Dr. Lee had no financial conflicts.
FROM CHEST
Key clinical point: Simvastatin and atorvastatin were associated with decreased mortality risk among sepsis patients.
Major finding: Compared with those not taking the drugs, those taking simvastatin were 28% less likely to die by 30 days, and those taking atorvastatin were 22% less likely.
Study details: The database study comprised almost 54,000 sepsis cases over 11 years.
Disclosures: The study was funded by the Taiwan National Science Foundation and Taiwan National Ministry of Science and Technology. Dr. Lee had no financial conflicts.
Source: Lee C-C et al. CHEST. 2018 April;153(4):769-70.
Cardiovascular risk in type 2 diabetes: Patients are often clueless
ORLANDO – What most people with type 2 diabetes don’t know about their cardiovascular risk could get them killed.
A national online survey revealed a surprising lack of awareness among patients with type 2 diabetes and their loved ones regarding the well-established significantly increased risk of cardiovascular mortality associated with the disease.
The same low rate of awareness was present in the general population, as represented by the 1,004 controls who didn’t have type 2 diabetes or know anyone who did, Jonathan Pak, PharmD, reported at the annual meeting of the American College of Cardiology.
“We thought patient awareness would be higher, but some who I think understand this space better are surprised it’s even this high,” Dr. Pak said in an interview.
Putting aside the widespread lack of awareness of cardiovascular disease as the leading cause of death in patients with type 2 diabetes, 52% of the patients with type 2 diabetes and a similar proportion of their loved ones were unaware that type 2 diabetes is associated with any increased risk of cardiovascular disease and other macrovascular events, noted Dr. Pak, director of metabolism at Boehringer Ingelheim in Ridgefield, Conn.
There was a strong whiff of denial in the patients’ attitudes. For example, only 24% of the group with type 2 diabetes rated themselves as “likely” to have an amputation in the future, yet they rated 42% of others with type 2 disease as likely to undergo amputation. The same attitude pertained to the prospect of having an acute MI: It’s the others who are at increased risk, not me.
Encouragingly though, more than 80% of the type 2 diabetes patients who hadn’t realized they were at increased cardiovascular risk indicated that if they truly are at increased risk, they would take preventive measures to reduce that risk, including dietary modification and a conversation with their healthcare provider. In addition, 80% said their motivation in doing so would be to improve their quality of life, and 73% cited a desire to live longer and spend more time with their family, Dr. Pak observed.
“The findings from this survey highlight a huge opportunity to educate and inform, and for patients and their loved ones to act on,” he said.
The For Your Sweetheart survey was supported by Boehringer Ingelheim, Eli Lilly, and the Company Diabetes Alliance.
SOURCE: Pak J. ACC 18.
ORLANDO – What most people with type 2 diabetes don’t know about their cardiovascular risk could get them killed.
A national online survey revealed a surprising lack of awareness among patients with type 2 diabetes and their loved ones regarding the well-established significantly increased risk of cardiovascular mortality associated with the disease.
The same low rate of awareness was present in the general population, as represented by the 1,004 controls who didn’t have type 2 diabetes or know anyone who did, Jonathan Pak, PharmD, reported at the annual meeting of the American College of Cardiology.
“We thought patient awareness would be higher, but some who I think understand this space better are surprised it’s even this high,” Dr. Pak said in an interview.
Putting aside the widespread lack of awareness of cardiovascular disease as the leading cause of death in patients with type 2 diabetes, 52% of the patients with type 2 diabetes and a similar proportion of their loved ones were unaware that type 2 diabetes is associated with any increased risk of cardiovascular disease and other macrovascular events, noted Dr. Pak, director of metabolism at Boehringer Ingelheim in Ridgefield, Conn.
There was a strong whiff of denial in the patients’ attitudes. For example, only 24% of the group with type 2 diabetes rated themselves as “likely” to have an amputation in the future, yet they rated 42% of others with type 2 disease as likely to undergo amputation. The same attitude pertained to the prospect of having an acute MI: It’s the others who are at increased risk, not me.
Encouragingly though, more than 80% of the type 2 diabetes patients who hadn’t realized they were at increased cardiovascular risk indicated that if they truly are at increased risk, they would take preventive measures to reduce that risk, including dietary modification and a conversation with their healthcare provider. In addition, 80% said their motivation in doing so would be to improve their quality of life, and 73% cited a desire to live longer and spend more time with their family, Dr. Pak observed.
“The findings from this survey highlight a huge opportunity to educate and inform, and for patients and their loved ones to act on,” he said.
The For Your Sweetheart survey was supported by Boehringer Ingelheim, Eli Lilly, and the Company Diabetes Alliance.
SOURCE: Pak J. ACC 18.
ORLANDO – What most people with type 2 diabetes don’t know about their cardiovascular risk could get them killed.
A national online survey revealed a surprising lack of awareness among patients with type 2 diabetes and their loved ones regarding the well-established significantly increased risk of cardiovascular mortality associated with the disease.
The same low rate of awareness was present in the general population, as represented by the 1,004 controls who didn’t have type 2 diabetes or know anyone who did, Jonathan Pak, PharmD, reported at the annual meeting of the American College of Cardiology.
“We thought patient awareness would be higher, but some who I think understand this space better are surprised it’s even this high,” Dr. Pak said in an interview.
Putting aside the widespread lack of awareness of cardiovascular disease as the leading cause of death in patients with type 2 diabetes, 52% of the patients with type 2 diabetes and a similar proportion of their loved ones were unaware that type 2 diabetes is associated with any increased risk of cardiovascular disease and other macrovascular events, noted Dr. Pak, director of metabolism at Boehringer Ingelheim in Ridgefield, Conn.
There was a strong whiff of denial in the patients’ attitudes. For example, only 24% of the group with type 2 diabetes rated themselves as “likely” to have an amputation in the future, yet they rated 42% of others with type 2 disease as likely to undergo amputation. The same attitude pertained to the prospect of having an acute MI: It’s the others who are at increased risk, not me.
Encouragingly though, more than 80% of the type 2 diabetes patients who hadn’t realized they were at increased cardiovascular risk indicated that if they truly are at increased risk, they would take preventive measures to reduce that risk, including dietary modification and a conversation with their healthcare provider. In addition, 80% said their motivation in doing so would be to improve their quality of life, and 73% cited a desire to live longer and spend more time with their family, Dr. Pak observed.
“The findings from this survey highlight a huge opportunity to educate and inform, and for patients and their loved ones to act on,” he said.
The For Your Sweetheart survey was supported by Boehringer Ingelheim, Eli Lilly, and the Company Diabetes Alliance.
SOURCE: Pak J. ACC 18.
REPORTING FROM ACC 2018
Key clinical point: What most people with type 2 diabetes don’t know about their cardiovascular risk could get them killed.
Major finding: More than half of patients with type 2 diabetes who participated in an online survey were unaware that their disease places them at increased cardiovascular risk.
Study details: This online, cross-sectional survey included 1,869 respondents.
Disclosures: The For Your Sweetheart survey was supported by Boehringer Ingelheim and Eli Lilly. The presenter is a Boehringer Ingelheim employee.
Source: Pak J. ACC 18.
What’s Eating You? Ixodes Tick and Related Diseases, Part 2: Diagnosis and Treatment of Regional Tick-borne Diseases
The Ixodes tick is prevalent in temperate climates worldwide. During a blood meal, pathogens may be transmitted from the tick to its host. Borrelia burgdorferi, a spirochete responsible for Lyme disease, is the most prevalent pathogen transmitted by Ixodes ticks.1 Borrelia mayonii recently was identified as an additional cause of Lyme disease in the United States.2
The Ixodes tick also is associated with several less common pathogens, including Babesia microti and the tick-borne encephalitis virus, which have been recognized as Ixodes-associated pathogens for many years.3,4 Other pathogens have been identified, including Anaplasma phagocytophilum, recognized in the 1990s as the cause of human granulocytic anaplasmosis, as well as the Powassan virus and Borrelia miyamotoi.5-7 Additionally, tick paralysis has been associated with toxins in the saliva of various species of several genera of ticks, including some Ixodes species.8 Due to an overlap in geographic distribution (Figure) and disease presentations (eTable), it is important that physicians be familiar with these regional pathogens transmitted by Ixodes ticks.
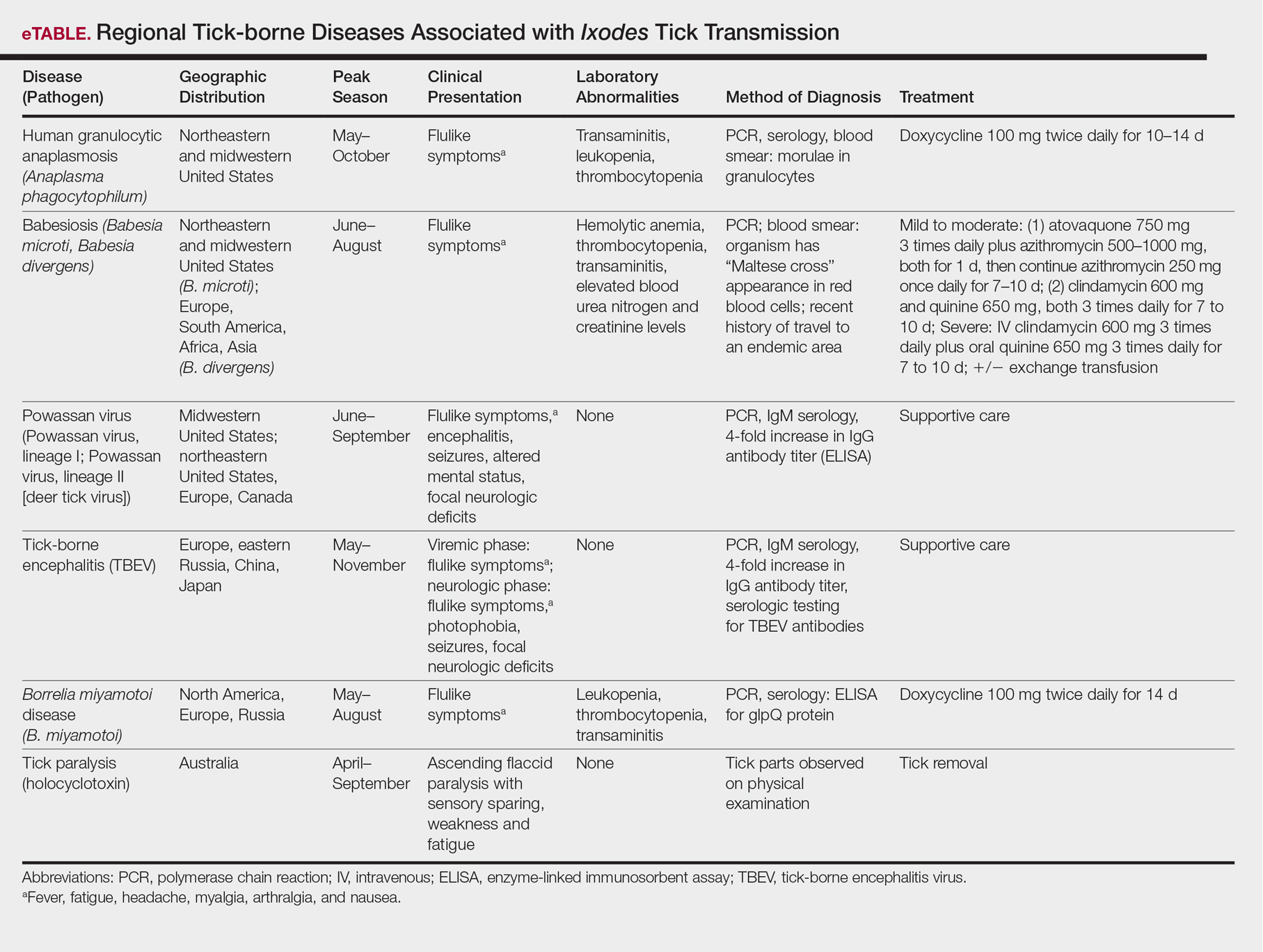
Human Granulocytic Anaplasmosis
Formerly known as human granulocytic ehrlichiosis, human granulocytic anaplasmosis is caused by A phagocytophilum and is transmitted by Ixodes scapularis, Ixodes pacificus, and Ixodes persulcatus. The incidence of human granulocytic anaplasmosis in the United States increased 12-fold from 2001 to 2011.9
Presenting symptoms generally are nonspecific, including fever, night sweats, headache, myalgias, and arthralgias, often resulting in misdiagnosis as a viral infection. Laboratory abnormalities include mild transaminitis, leukopenia, and thrombocytopenia.9,10 Although most infections resolve spontaneously, 3% of patients develop serious complications. The mortality rate is 0.6%.11
A diagnosis of human granulocytic anaplasmosis should be suspected in patients with a viral-like illness and exposure to ticks in an endemic area. The diagnosis can be confirmed by polymerase chain reaction (PCR), acute- and convalescent-phase serologic testing, or direct fluorescent antibody screening. Characteristic morulae may be present in granulocytes.12 Treatment typically includes doxycycline, which also covers B burgdorferi coinfection. When a diagnosis of human granulocytic anaplasmosis is suspected, treatment should never be delayed to await laboratory confirmation. If no clinical improvement is seen within 48 hours, alternate diagnoses or coinfection with B microti should be considered.10
Babesiosis
The protozoan B microti causes babesiosis in the United States, with Babesia divergens being more common in Europe.13 Reported cases of babesiosis in New York increased as much as 20-fold from 2001 to 2008.14 Transmission primarily is from the Ixodes tick but rarely can occur from blood transfusion.15 Tick attachment for at least 36 hours is required for transmission.13
The clinical presentation of babesiosis ranges from asymptomatic to fatal. Symptoms generally are nonspecific, resembling a viral infection and including headache, nausea, diarrhea, arthralgia, and myalgia. Laboratory evaluation may reveal hemolytic anemia, thrombocytopenia, transaminitis, and elevated blood urea nitrogen and creatinine levels.16 Rash is not typical. Resolution of symptoms generally occurs within 2 weeks of presentation, although anemia may persist for months.13 Severe disease is more common among elderly and immunocompromised patients. Complications include respiratory failure, renal failure, congestive heart failure, and disseminated intravascular coagulation. The mortality rate in the United States is approximately 10%.10,16
A diagnosis of babesiosis is made based on the presence of flulike symptoms, laboratory results, and history of recent travel to an endemic area. A thin blood smear allows identification of the organism in erythrocytes as ring forms or tetrads (a “Maltese cross” appearance).17 Polymerase chain reaction is more sensitive than a blood smear, especially in early disease.18 Indirect fluorescent antibody testing is species-specific but cannot verify active infection.10
Treatment of babesiosis is indicated for symptomatic patients with active infection. Positive serology alone is not an indication for treatment. Asymptomatic patients with positive serology should have diagnostic testing repeated in 3 months with subsequent treatment if parasitemia persists. Mild disease is treated with atovaquone plus azithromycin or clindamycin plus quinine. Severe babesiosis is treated with quinine and intravenous clindamycin and may require exchange transfusion.10 Coinfection with B burgdorferi should be considered in patients with flulike symptoms and erythema migrans or treatment failure. Coinfection is diagnosed by Lyme serology plus PCR for B microti. This is an important consideration because treatment of babesiosis does not eradicate B burgdorferi infection.19
Powassan Virus
Powassan virus is a flavivirus that causes encephalitis. It is transmitted by Ixodes cookei (Powassan virus, lineage I) in the Great Lakes region and by I scapularis (Powassan virus, lineage II, or deer tick virus) in the northeastern United States. Transmission can occur within 15 minutes of tick attachment.6,20,21
Patients typically present with fever, headache, altered mental status, seizures, and focal neurologic deficits. Gastrointestinal symptoms and rash also have been reported.21 The diagnosis is made based on clinical presentation and laboratory testing with PCR or enzyme-linked immunosorbent assay (ELISA). Cross-reactivity on ELISA exists, necessitating confirmation with a neutralizing antibody or PCR. Treatment is supportive. Corticosteroids and intravenous immunoglobulin have been proposed as treatment modalities, but evidence of their efficacy is limited.22
Tick-borne Encephalitis
Tick-borne encephalitis is caused by the flavivirus tick-borne encephalitis virus in Europe and Asia. The tick-borne encephalitis virus is transmitted by Ixodes ricinus in Europe and by Ixodes persulcatus in eastern Russia, China, and Japan. It also has been associated with consumption of unpasteurized milk.23,24
Tick-borne encephalitis presents in a biphasic pattern. The initial viremic phase can persist for as long as 8 days with headache, nausea, myalgia, and fever. One-third of patients then enter an asymptomatic phase, followed by virus penetration into the central nervous system. The neurologic phase produces continued headache and fever with photophobia, focal neurologic deficits, seizures, respiratory depression, or coma. Neurologic sequelae persist in 10% to 20% of patients.25,26
In the viremic stage, diagnosis is made with PCR or culture. During the latent phase or neurologic phase, serologic testing for tick-borne encephalitis virus antibodies is indicated. Neutralizing antibody evaluation may be necessary due to cross-reactivity among flaviviruses.27 Treatment is supportive. An inactivated vaccine is available for high-risk populations.28
Borrelia miyamotoi Disease
Borrelia miyamotoi is a symbiont of the Ixodes tick formerly believed to have no pathogenic significance; however, B miyamotoi was isolated in febrile patients in Russia in 20117 and was identified as a pathogen in both North America29 and Europe in 2013.30 Disease presentation includes nonspecific symptoms of fever, fatigue, headache, arthralgia, myalgia, and nausea. Rash is uncommon. Laboratory abnormalities include leukopenia, thrombocytopenia, and transaminitis.31,32 Meningoencephalitis may occur in immunocompromised patients.29,30
The diagnosis of B miyamotoi disease is confirmed by PCR or serology. An ELISA that is positive for B burgdorferi IgM but negative with confirmatory immunoblot suggests B miyamotoi disease. Seroconversion using a glpQ protein ELISA also can be assessed.31 If ELISA is positive, Lyme disease can be excluded because B burgdorferi does not possess g1pQ. Treatment is with doxycycline.32
Tick Paralysis
Tick paralysis is an intoxication with holocyclotoxin from the saliva of gravid hard ticks. In the United States, intoxication is associated with ticks of various species of Amblyomma, Dermacentor, and Ixodes in the Northwest, Southeast, and Northeast. In Australia, intoxication is associated with Ixodes.33 Patients present with weakness and fatigue, progressing to ascending flaccid paralysis with sensory sparing. The treatment is tick removal.8,33
Conclusion
Arthropods carry many regional pathogens. Physicians outside of those regions should seek a travel history and be alert for imported disease.
- Steere AC, Grodzicki RL, Kornblatt AN, et al. The spirochetal etiology of Lyme disease. N Engl J Med. 1983;308:733-740.
- Dolan MC, Hojgaard A, Hoxmeier JC, et al. Vector competence of the blacklegged tick, Ixodes scapularis, for the recently recognized Lyme borreliosis spirochete Candidatus Borrelia mayonii. Ticks Tick Borne Dis. 2016;7:665-669.
- Rudzinska MA, Spielman A, Riek RF, et al. Intraerythrocytic ‘gametocytes’ of Babesia microti and their maturation in ticks. Can J Zool. 1979;57:424-434.
- Casals J, Olitsky PK. Enduring immunity following vaccination of mice with formalin-inactivated virus of Russian spring-summer (Far Eastern, tick-borne) encephalitis; correlation with serum-neutralizing and complement-fixing antibodies. J Exp Med. 1945;82:431-443.
- Magnarelli LA, Stafford KC III, Mather TN, et al. Hemocytic rickettsia-like organisms in ticks: serologic reactivity with antisera to Ehrlichiae and detection of DNA of agent of human granulocytic ehrlichiosis by PCR. J Clin Microbiol. 1995;33:2710-2714.
- McLean DM, Donohue WL. Powassan virus: isolation of virus from a fatal case of encephalitis. Can Med Assoc J. 1959;80:708-711.
- Platonov AE, Karan LS, Kolyasnikova NM, et al. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg Infect Dis. 2011;17:1816-1823.
- Diaz JH. A 60-year meta-analysis of tick paralysis in the United States: a predictable, preventable, and often misdiagnosed poisoning. J Med Toxicol. 2010;6:15-21.
- Bakken J, Dumler JS. Human granulocytic anaplasmosis. Infect Dis Clin North Am. 2015;29:341-355.
- Chapman AS, Bakken JS, Folk SM, et al; Tickborne Rickettsial Diseases Working Group; CDC. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis—United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep. 2006;55(RR-4):1-27.
- Dahlgren FS, Mandel EJ, Krebs JW, et al. Increasing incidence of Ehrlichia chaffeensis and Anaplasma phagocytophilum in the United States, 2000-2007. Am J Trop Med Hyg. 2011;85:124-130.
- Aguero-Rosenfeld ME. Diagnosis of human granulocytic ehrlichiosis: state of the art. Vector Borne Zoonotic Dis. 2002;2:233-239.
- Vannier EG, Diuk-Wasser MA, Ben Mamoun C, et al. Babesiosis. Infect Dis Clin North Am. 2015;29:357-370.
- Joseph JT, Roy SS, Shams N, et al. Babesiosis in Lower Hudson Valley, New York, USA. Emerg Infect Dis. 2011;17:843-847.
- McQuiston JH, Childs JE, Chamberland ME, et al. Transmission of tickborne agents by blood transfusions: a review of known and potential risks in the United States. Transfusion. 2000;40:274-284.
- Hatcher JC, Greenberg PD, Antique J, et al. Severe babesiosis in Long Island: review of 34 cases and their complications. Clin Infect Dis. 2001;32:1117-1125.
- Healy GR, Ruebush TK. Morphology of Babesia microti in human blood smears. Am J Clin Pathol. 1980;73:107-109.
- Kowalski TJ, Jobe DA, Dolan EC, et al. The emergence of clinically relevant babesiosis in southwestern Wisconsin. WMJ. 2015;114:152-157.
- Krause PJ, Telford SR III, Spielman A, et al. Concurrent Lyme disease and babesiosis. evidence for increased severity and duration of illness. JAMA. 1996;275:1657-1660.
- Centers for Disease Control and Prevention. Statistics & maps. http://www.cdc.gov/powassan/statistics.html. Updated February 14, 2017. Accessed December 11, 2017.
- Piantadosi A, Rubin DB, McQuillen DP, et al. Emerging cases of Powassan virus encephalitis in New England: clinical presentation, imaging, and review of the literature. Clin Infect Dis. 2016;62:707-713.
- El Khoury MY, Camargo JF, White JL, et al. Potential role of deer tick virus in Powassan encephalitis cases in Lyme disease-endemic areas of New York, U.S.A. Emerg Infect Dis. 2013;19:1926-1933.
- World Health Organization (WHO). Vaccines against tick-borne encephalitis: WHO position paper. Wkly Epidemiol Rec. 2011;86:241-256.
- Centers for Disease Control and Prevention (CDC). Tick-borne encephalitis among U.S. travelers to Europe and Asia—2000-2009. JAMA. 2010;303:2132-2135.
- Valarcher JF, Hägglund S, Juremalm M, et al. Tick-borne encephalitits. Rev Sci Tech. 2015;34:453-466.
- Schultze D, Dollenmaier G, Rohner A, et al. Benefit of detecting tick-borne encephalitis viremia in the first phase of illness. J Clin Virol. 2007;38:172-175.
- Holzmann H. Diagnosis of tick-borne encephalitis. Vaccine. 2003;21(suppl 1):S36-S40.
- Zavadska D, Anca I, André F, et al. Recommendations for tick-borne encephalitis vaccination from the Central European Vaccination Awareness Group. Hum Vaccin Immunother. 2013;9:362-374.
- Gugliotta JL, Goethert HK, Berardi VP, et al. Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. N Engl J Med. 2013;368:240-245.
- Hovius JW, de Wever B, Sohne M, et al. A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet. 2013;382:658.
- Molloy PJ, Telford SR III, Chowdri HR, et al. Borrelia miyamotoi disease in the northeastern United States: a case series. Ann Intern Med. 2015;163:91-98.
- Telford SR 3rd, Goethert HK, Molloy PJ, et al. Borrelia miyamotoi disease: neither Lyme disease nor relapsing fever. Clin Lab Med. 2015;35:867-882.
- Diaz JH. A comparative meta-analysis of tick paralysis in the United States and Australia. Clin Toxicol (Phila). 2015;53:874-883.
The Ixodes tick is prevalent in temperate climates worldwide. During a blood meal, pathogens may be transmitted from the tick to its host. Borrelia burgdorferi, a spirochete responsible for Lyme disease, is the most prevalent pathogen transmitted by Ixodes ticks.1 Borrelia mayonii recently was identified as an additional cause of Lyme disease in the United States.2
The Ixodes tick also is associated with several less common pathogens, including Babesia microti and the tick-borne encephalitis virus, which have been recognized as Ixodes-associated pathogens for many years.3,4 Other pathogens have been identified, including Anaplasma phagocytophilum, recognized in the 1990s as the cause of human granulocytic anaplasmosis, as well as the Powassan virus and Borrelia miyamotoi.5-7 Additionally, tick paralysis has been associated with toxins in the saliva of various species of several genera of ticks, including some Ixodes species.8 Due to an overlap in geographic distribution (Figure) and disease presentations (eTable), it is important that physicians be familiar with these regional pathogens transmitted by Ixodes ticks.


Human Granulocytic Anaplasmosis
Formerly known as human granulocytic ehrlichiosis, human granulocytic anaplasmosis is caused by A phagocytophilum and is transmitted by Ixodes scapularis, Ixodes pacificus, and Ixodes persulcatus. The incidence of human granulocytic anaplasmosis in the United States increased 12-fold from 2001 to 2011.9
Presenting symptoms generally are nonspecific, including fever, night sweats, headache, myalgias, and arthralgias, often resulting in misdiagnosis as a viral infection. Laboratory abnormalities include mild transaminitis, leukopenia, and thrombocytopenia.9,10 Although most infections resolve spontaneously, 3% of patients develop serious complications. The mortality rate is 0.6%.11
A diagnosis of human granulocytic anaplasmosis should be suspected in patients with a viral-like illness and exposure to ticks in an endemic area. The diagnosis can be confirmed by polymerase chain reaction (PCR), acute- and convalescent-phase serologic testing, or direct fluorescent antibody screening. Characteristic morulae may be present in granulocytes.12 Treatment typically includes doxycycline, which also covers B burgdorferi coinfection. When a diagnosis of human granulocytic anaplasmosis is suspected, treatment should never be delayed to await laboratory confirmation. If no clinical improvement is seen within 48 hours, alternate diagnoses or coinfection with B microti should be considered.10
Babesiosis
The protozoan B microti causes babesiosis in the United States, with Babesia divergens being more common in Europe.13 Reported cases of babesiosis in New York increased as much as 20-fold from 2001 to 2008.14 Transmission primarily is from the Ixodes tick but rarely can occur from blood transfusion.15 Tick attachment for at least 36 hours is required for transmission.13
The clinical presentation of babesiosis ranges from asymptomatic to fatal. Symptoms generally are nonspecific, resembling a viral infection and including headache, nausea, diarrhea, arthralgia, and myalgia. Laboratory evaluation may reveal hemolytic anemia, thrombocytopenia, transaminitis, and elevated blood urea nitrogen and creatinine levels.16 Rash is not typical. Resolution of symptoms generally occurs within 2 weeks of presentation, although anemia may persist for months.13 Severe disease is more common among elderly and immunocompromised patients. Complications include respiratory failure, renal failure, congestive heart failure, and disseminated intravascular coagulation. The mortality rate in the United States is approximately 10%.10,16
A diagnosis of babesiosis is made based on the presence of flulike symptoms, laboratory results, and history of recent travel to an endemic area. A thin blood smear allows identification of the organism in erythrocytes as ring forms or tetrads (a “Maltese cross” appearance).17 Polymerase chain reaction is more sensitive than a blood smear, especially in early disease.18 Indirect fluorescent antibody testing is species-specific but cannot verify active infection.10
Treatment of babesiosis is indicated for symptomatic patients with active infection. Positive serology alone is not an indication for treatment. Asymptomatic patients with positive serology should have diagnostic testing repeated in 3 months with subsequent treatment if parasitemia persists. Mild disease is treated with atovaquone plus azithromycin or clindamycin plus quinine. Severe babesiosis is treated with quinine and intravenous clindamycin and may require exchange transfusion.10 Coinfection with B burgdorferi should be considered in patients with flulike symptoms and erythema migrans or treatment failure. Coinfection is diagnosed by Lyme serology plus PCR for B microti. This is an important consideration because treatment of babesiosis does not eradicate B burgdorferi infection.19
Powassan Virus
Powassan virus is a flavivirus that causes encephalitis. It is transmitted by Ixodes cookei (Powassan virus, lineage I) in the Great Lakes region and by I scapularis (Powassan virus, lineage II, or deer tick virus) in the northeastern United States. Transmission can occur within 15 minutes of tick attachment.6,20,21
Patients typically present with fever, headache, altered mental status, seizures, and focal neurologic deficits. Gastrointestinal symptoms and rash also have been reported.21 The diagnosis is made based on clinical presentation and laboratory testing with PCR or enzyme-linked immunosorbent assay (ELISA). Cross-reactivity on ELISA exists, necessitating confirmation with a neutralizing antibody or PCR. Treatment is supportive. Corticosteroids and intravenous immunoglobulin have been proposed as treatment modalities, but evidence of their efficacy is limited.22
Tick-borne Encephalitis
Tick-borne encephalitis is caused by the flavivirus tick-borne encephalitis virus in Europe and Asia. The tick-borne encephalitis virus is transmitted by Ixodes ricinus in Europe and by Ixodes persulcatus in eastern Russia, China, and Japan. It also has been associated with consumption of unpasteurized milk.23,24
Tick-borne encephalitis presents in a biphasic pattern. The initial viremic phase can persist for as long as 8 days with headache, nausea, myalgia, and fever. One-third of patients then enter an asymptomatic phase, followed by virus penetration into the central nervous system. The neurologic phase produces continued headache and fever with photophobia, focal neurologic deficits, seizures, respiratory depression, or coma. Neurologic sequelae persist in 10% to 20% of patients.25,26
In the viremic stage, diagnosis is made with PCR or culture. During the latent phase or neurologic phase, serologic testing for tick-borne encephalitis virus antibodies is indicated. Neutralizing antibody evaluation may be necessary due to cross-reactivity among flaviviruses.27 Treatment is supportive. An inactivated vaccine is available for high-risk populations.28
Borrelia miyamotoi Disease
Borrelia miyamotoi is a symbiont of the Ixodes tick formerly believed to have no pathogenic significance; however, B miyamotoi was isolated in febrile patients in Russia in 20117 and was identified as a pathogen in both North America29 and Europe in 2013.30 Disease presentation includes nonspecific symptoms of fever, fatigue, headache, arthralgia, myalgia, and nausea. Rash is uncommon. Laboratory abnormalities include leukopenia, thrombocytopenia, and transaminitis.31,32 Meningoencephalitis may occur in immunocompromised patients.29,30
The diagnosis of B miyamotoi disease is confirmed by PCR or serology. An ELISA that is positive for B burgdorferi IgM but negative with confirmatory immunoblot suggests B miyamotoi disease. Seroconversion using a glpQ protein ELISA also can be assessed.31 If ELISA is positive, Lyme disease can be excluded because B burgdorferi does not possess g1pQ. Treatment is with doxycycline.32
Tick Paralysis
Tick paralysis is an intoxication with holocyclotoxin from the saliva of gravid hard ticks. In the United States, intoxication is associated with ticks of various species of Amblyomma, Dermacentor, and Ixodes in the Northwest, Southeast, and Northeast. In Australia, intoxication is associated with Ixodes.33 Patients present with weakness and fatigue, progressing to ascending flaccid paralysis with sensory sparing. The treatment is tick removal.8,33
Conclusion
Arthropods carry many regional pathogens. Physicians outside of those regions should seek a travel history and be alert for imported disease.
The Ixodes tick is prevalent in temperate climates worldwide. During a blood meal, pathogens may be transmitted from the tick to its host. Borrelia burgdorferi, a spirochete responsible for Lyme disease, is the most prevalent pathogen transmitted by Ixodes ticks.1 Borrelia mayonii recently was identified as an additional cause of Lyme disease in the United States.2
The Ixodes tick also is associated with several less common pathogens, including Babesia microti and the tick-borne encephalitis virus, which have been recognized as Ixodes-associated pathogens for many years.3,4 Other pathogens have been identified, including Anaplasma phagocytophilum, recognized in the 1990s as the cause of human granulocytic anaplasmosis, as well as the Powassan virus and Borrelia miyamotoi.5-7 Additionally, tick paralysis has been associated with toxins in the saliva of various species of several genera of ticks, including some Ixodes species.8 Due to an overlap in geographic distribution (Figure) and disease presentations (eTable), it is important that physicians be familiar with these regional pathogens transmitted by Ixodes ticks.


Human Granulocytic Anaplasmosis
Formerly known as human granulocytic ehrlichiosis, human granulocytic anaplasmosis is caused by A phagocytophilum and is transmitted by Ixodes scapularis, Ixodes pacificus, and Ixodes persulcatus. The incidence of human granulocytic anaplasmosis in the United States increased 12-fold from 2001 to 2011.9
Presenting symptoms generally are nonspecific, including fever, night sweats, headache, myalgias, and arthralgias, often resulting in misdiagnosis as a viral infection. Laboratory abnormalities include mild transaminitis, leukopenia, and thrombocytopenia.9,10 Although most infections resolve spontaneously, 3% of patients develop serious complications. The mortality rate is 0.6%.11
A diagnosis of human granulocytic anaplasmosis should be suspected in patients with a viral-like illness and exposure to ticks in an endemic area. The diagnosis can be confirmed by polymerase chain reaction (PCR), acute- and convalescent-phase serologic testing, or direct fluorescent antibody screening. Characteristic morulae may be present in granulocytes.12 Treatment typically includes doxycycline, which also covers B burgdorferi coinfection. When a diagnosis of human granulocytic anaplasmosis is suspected, treatment should never be delayed to await laboratory confirmation. If no clinical improvement is seen within 48 hours, alternate diagnoses or coinfection with B microti should be considered.10
Babesiosis
The protozoan B microti causes babesiosis in the United States, with Babesia divergens being more common in Europe.13 Reported cases of babesiosis in New York increased as much as 20-fold from 2001 to 2008.14 Transmission primarily is from the Ixodes tick but rarely can occur from blood transfusion.15 Tick attachment for at least 36 hours is required for transmission.13
The clinical presentation of babesiosis ranges from asymptomatic to fatal. Symptoms generally are nonspecific, resembling a viral infection and including headache, nausea, diarrhea, arthralgia, and myalgia. Laboratory evaluation may reveal hemolytic anemia, thrombocytopenia, transaminitis, and elevated blood urea nitrogen and creatinine levels.16 Rash is not typical. Resolution of symptoms generally occurs within 2 weeks of presentation, although anemia may persist for months.13 Severe disease is more common among elderly and immunocompromised patients. Complications include respiratory failure, renal failure, congestive heart failure, and disseminated intravascular coagulation. The mortality rate in the United States is approximately 10%.10,16
A diagnosis of babesiosis is made based on the presence of flulike symptoms, laboratory results, and history of recent travel to an endemic area. A thin blood smear allows identification of the organism in erythrocytes as ring forms or tetrads (a “Maltese cross” appearance).17 Polymerase chain reaction is more sensitive than a blood smear, especially in early disease.18 Indirect fluorescent antibody testing is species-specific but cannot verify active infection.10
Treatment of babesiosis is indicated for symptomatic patients with active infection. Positive serology alone is not an indication for treatment. Asymptomatic patients with positive serology should have diagnostic testing repeated in 3 months with subsequent treatment if parasitemia persists. Mild disease is treated with atovaquone plus azithromycin or clindamycin plus quinine. Severe babesiosis is treated with quinine and intravenous clindamycin and may require exchange transfusion.10 Coinfection with B burgdorferi should be considered in patients with flulike symptoms and erythema migrans or treatment failure. Coinfection is diagnosed by Lyme serology plus PCR for B microti. This is an important consideration because treatment of babesiosis does not eradicate B burgdorferi infection.19
Powassan Virus
Powassan virus is a flavivirus that causes encephalitis. It is transmitted by Ixodes cookei (Powassan virus, lineage I) in the Great Lakes region and by I scapularis (Powassan virus, lineage II, or deer tick virus) in the northeastern United States. Transmission can occur within 15 minutes of tick attachment.6,20,21
Patients typically present with fever, headache, altered mental status, seizures, and focal neurologic deficits. Gastrointestinal symptoms and rash also have been reported.21 The diagnosis is made based on clinical presentation and laboratory testing with PCR or enzyme-linked immunosorbent assay (ELISA). Cross-reactivity on ELISA exists, necessitating confirmation with a neutralizing antibody or PCR. Treatment is supportive. Corticosteroids and intravenous immunoglobulin have been proposed as treatment modalities, but evidence of their efficacy is limited.22
Tick-borne Encephalitis
Tick-borne encephalitis is caused by the flavivirus tick-borne encephalitis virus in Europe and Asia. The tick-borne encephalitis virus is transmitted by Ixodes ricinus in Europe and by Ixodes persulcatus in eastern Russia, China, and Japan. It also has been associated with consumption of unpasteurized milk.23,24
Tick-borne encephalitis presents in a biphasic pattern. The initial viremic phase can persist for as long as 8 days with headache, nausea, myalgia, and fever. One-third of patients then enter an asymptomatic phase, followed by virus penetration into the central nervous system. The neurologic phase produces continued headache and fever with photophobia, focal neurologic deficits, seizures, respiratory depression, or coma. Neurologic sequelae persist in 10% to 20% of patients.25,26
In the viremic stage, diagnosis is made with PCR or culture. During the latent phase or neurologic phase, serologic testing for tick-borne encephalitis virus antibodies is indicated. Neutralizing antibody evaluation may be necessary due to cross-reactivity among flaviviruses.27 Treatment is supportive. An inactivated vaccine is available for high-risk populations.28
Borrelia miyamotoi Disease
Borrelia miyamotoi is a symbiont of the Ixodes tick formerly believed to have no pathogenic significance; however, B miyamotoi was isolated in febrile patients in Russia in 20117 and was identified as a pathogen in both North America29 and Europe in 2013.30 Disease presentation includes nonspecific symptoms of fever, fatigue, headache, arthralgia, myalgia, and nausea. Rash is uncommon. Laboratory abnormalities include leukopenia, thrombocytopenia, and transaminitis.31,32 Meningoencephalitis may occur in immunocompromised patients.29,30
The diagnosis of B miyamotoi disease is confirmed by PCR or serology. An ELISA that is positive for B burgdorferi IgM but negative with confirmatory immunoblot suggests B miyamotoi disease. Seroconversion using a glpQ protein ELISA also can be assessed.31 If ELISA is positive, Lyme disease can be excluded because B burgdorferi does not possess g1pQ. Treatment is with doxycycline.32
Tick Paralysis
Tick paralysis is an intoxication with holocyclotoxin from the saliva of gravid hard ticks. In the United States, intoxication is associated with ticks of various species of Amblyomma, Dermacentor, and Ixodes in the Northwest, Southeast, and Northeast. In Australia, intoxication is associated with Ixodes.33 Patients present with weakness and fatigue, progressing to ascending flaccid paralysis with sensory sparing. The treatment is tick removal.8,33
Conclusion
Arthropods carry many regional pathogens. Physicians outside of those regions should seek a travel history and be alert for imported disease.
- Steere AC, Grodzicki RL, Kornblatt AN, et al. The spirochetal etiology of Lyme disease. N Engl J Med. 1983;308:733-740.
- Dolan MC, Hojgaard A, Hoxmeier JC, et al. Vector competence of the blacklegged tick, Ixodes scapularis, for the recently recognized Lyme borreliosis spirochete Candidatus Borrelia mayonii. Ticks Tick Borne Dis. 2016;7:665-669.
- Rudzinska MA, Spielman A, Riek RF, et al. Intraerythrocytic ‘gametocytes’ of Babesia microti and their maturation in ticks. Can J Zool. 1979;57:424-434.
- Casals J, Olitsky PK. Enduring immunity following vaccination of mice with formalin-inactivated virus of Russian spring-summer (Far Eastern, tick-borne) encephalitis; correlation with serum-neutralizing and complement-fixing antibodies. J Exp Med. 1945;82:431-443.
- Magnarelli LA, Stafford KC III, Mather TN, et al. Hemocytic rickettsia-like organisms in ticks: serologic reactivity with antisera to Ehrlichiae and detection of DNA of agent of human granulocytic ehrlichiosis by PCR. J Clin Microbiol. 1995;33:2710-2714.
- McLean DM, Donohue WL. Powassan virus: isolation of virus from a fatal case of encephalitis. Can Med Assoc J. 1959;80:708-711.
- Platonov AE, Karan LS, Kolyasnikova NM, et al. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg Infect Dis. 2011;17:1816-1823.
- Diaz JH. A 60-year meta-analysis of tick paralysis in the United States: a predictable, preventable, and often misdiagnosed poisoning. J Med Toxicol. 2010;6:15-21.
- Bakken J, Dumler JS. Human granulocytic anaplasmosis. Infect Dis Clin North Am. 2015;29:341-355.
- Chapman AS, Bakken JS, Folk SM, et al; Tickborne Rickettsial Diseases Working Group; CDC. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis—United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep. 2006;55(RR-4):1-27.
- Dahlgren FS, Mandel EJ, Krebs JW, et al. Increasing incidence of Ehrlichia chaffeensis and Anaplasma phagocytophilum in the United States, 2000-2007. Am J Trop Med Hyg. 2011;85:124-130.
- Aguero-Rosenfeld ME. Diagnosis of human granulocytic ehrlichiosis: state of the art. Vector Borne Zoonotic Dis. 2002;2:233-239.
- Vannier EG, Diuk-Wasser MA, Ben Mamoun C, et al. Babesiosis. Infect Dis Clin North Am. 2015;29:357-370.
- Joseph JT, Roy SS, Shams N, et al. Babesiosis in Lower Hudson Valley, New York, USA. Emerg Infect Dis. 2011;17:843-847.
- McQuiston JH, Childs JE, Chamberland ME, et al. Transmission of tickborne agents by blood transfusions: a review of known and potential risks in the United States. Transfusion. 2000;40:274-284.
- Hatcher JC, Greenberg PD, Antique J, et al. Severe babesiosis in Long Island: review of 34 cases and their complications. Clin Infect Dis. 2001;32:1117-1125.
- Healy GR, Ruebush TK. Morphology of Babesia microti in human blood smears. Am J Clin Pathol. 1980;73:107-109.
- Kowalski TJ, Jobe DA, Dolan EC, et al. The emergence of clinically relevant babesiosis in southwestern Wisconsin. WMJ. 2015;114:152-157.
- Krause PJ, Telford SR III, Spielman A, et al. Concurrent Lyme disease and babesiosis. evidence for increased severity and duration of illness. JAMA. 1996;275:1657-1660.
- Centers for Disease Control and Prevention. Statistics & maps. http://www.cdc.gov/powassan/statistics.html. Updated February 14, 2017. Accessed December 11, 2017.
- Piantadosi A, Rubin DB, McQuillen DP, et al. Emerging cases of Powassan virus encephalitis in New England: clinical presentation, imaging, and review of the literature. Clin Infect Dis. 2016;62:707-713.
- El Khoury MY, Camargo JF, White JL, et al. Potential role of deer tick virus in Powassan encephalitis cases in Lyme disease-endemic areas of New York, U.S.A. Emerg Infect Dis. 2013;19:1926-1933.
- World Health Organization (WHO). Vaccines against tick-borne encephalitis: WHO position paper. Wkly Epidemiol Rec. 2011;86:241-256.
- Centers for Disease Control and Prevention (CDC). Tick-borne encephalitis among U.S. travelers to Europe and Asia—2000-2009. JAMA. 2010;303:2132-2135.
- Valarcher JF, Hägglund S, Juremalm M, et al. Tick-borne encephalitits. Rev Sci Tech. 2015;34:453-466.
- Schultze D, Dollenmaier G, Rohner A, et al. Benefit of detecting tick-borne encephalitis viremia in the first phase of illness. J Clin Virol. 2007;38:172-175.
- Holzmann H. Diagnosis of tick-borne encephalitis. Vaccine. 2003;21(suppl 1):S36-S40.
- Zavadska D, Anca I, André F, et al. Recommendations for tick-borne encephalitis vaccination from the Central European Vaccination Awareness Group. Hum Vaccin Immunother. 2013;9:362-374.
- Gugliotta JL, Goethert HK, Berardi VP, et al. Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. N Engl J Med. 2013;368:240-245.
- Hovius JW, de Wever B, Sohne M, et al. A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet. 2013;382:658.
- Molloy PJ, Telford SR III, Chowdri HR, et al. Borrelia miyamotoi disease in the northeastern United States: a case series. Ann Intern Med. 2015;163:91-98.
- Telford SR 3rd, Goethert HK, Molloy PJ, et al. Borrelia miyamotoi disease: neither Lyme disease nor relapsing fever. Clin Lab Med. 2015;35:867-882.
- Diaz JH. A comparative meta-analysis of tick paralysis in the United States and Australia. Clin Toxicol (Phila). 2015;53:874-883.
- Steere AC, Grodzicki RL, Kornblatt AN, et al. The spirochetal etiology of Lyme disease. N Engl J Med. 1983;308:733-740.
- Dolan MC, Hojgaard A, Hoxmeier JC, et al. Vector competence of the blacklegged tick, Ixodes scapularis, for the recently recognized Lyme borreliosis spirochete Candidatus Borrelia mayonii. Ticks Tick Borne Dis. 2016;7:665-669.
- Rudzinska MA, Spielman A, Riek RF, et al. Intraerythrocytic ‘gametocytes’ of Babesia microti and their maturation in ticks. Can J Zool. 1979;57:424-434.
- Casals J, Olitsky PK. Enduring immunity following vaccination of mice with formalin-inactivated virus of Russian spring-summer (Far Eastern, tick-borne) encephalitis; correlation with serum-neutralizing and complement-fixing antibodies. J Exp Med. 1945;82:431-443.
- Magnarelli LA, Stafford KC III, Mather TN, et al. Hemocytic rickettsia-like organisms in ticks: serologic reactivity with antisera to Ehrlichiae and detection of DNA of agent of human granulocytic ehrlichiosis by PCR. J Clin Microbiol. 1995;33:2710-2714.
- McLean DM, Donohue WL. Powassan virus: isolation of virus from a fatal case of encephalitis. Can Med Assoc J. 1959;80:708-711.
- Platonov AE, Karan LS, Kolyasnikova NM, et al. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg Infect Dis. 2011;17:1816-1823.
- Diaz JH. A 60-year meta-analysis of tick paralysis in the United States: a predictable, preventable, and often misdiagnosed poisoning. J Med Toxicol. 2010;6:15-21.
- Bakken J, Dumler JS. Human granulocytic anaplasmosis. Infect Dis Clin North Am. 2015;29:341-355.
- Chapman AS, Bakken JS, Folk SM, et al; Tickborne Rickettsial Diseases Working Group; CDC. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis—United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep. 2006;55(RR-4):1-27.
- Dahlgren FS, Mandel EJ, Krebs JW, et al. Increasing incidence of Ehrlichia chaffeensis and Anaplasma phagocytophilum in the United States, 2000-2007. Am J Trop Med Hyg. 2011;85:124-130.
- Aguero-Rosenfeld ME. Diagnosis of human granulocytic ehrlichiosis: state of the art. Vector Borne Zoonotic Dis. 2002;2:233-239.
- Vannier EG, Diuk-Wasser MA, Ben Mamoun C, et al. Babesiosis. Infect Dis Clin North Am. 2015;29:357-370.
- Joseph JT, Roy SS, Shams N, et al. Babesiosis in Lower Hudson Valley, New York, USA. Emerg Infect Dis. 2011;17:843-847.
- McQuiston JH, Childs JE, Chamberland ME, et al. Transmission of tickborne agents by blood transfusions: a review of known and potential risks in the United States. Transfusion. 2000;40:274-284.
- Hatcher JC, Greenberg PD, Antique J, et al. Severe babesiosis in Long Island: review of 34 cases and their complications. Clin Infect Dis. 2001;32:1117-1125.
- Healy GR, Ruebush TK. Morphology of Babesia microti in human blood smears. Am J Clin Pathol. 1980;73:107-109.
- Kowalski TJ, Jobe DA, Dolan EC, et al. The emergence of clinically relevant babesiosis in southwestern Wisconsin. WMJ. 2015;114:152-157.
- Krause PJ, Telford SR III, Spielman A, et al. Concurrent Lyme disease and babesiosis. evidence for increased severity and duration of illness. JAMA. 1996;275:1657-1660.
- Centers for Disease Control and Prevention. Statistics & maps. http://www.cdc.gov/powassan/statistics.html. Updated February 14, 2017. Accessed December 11, 2017.
- Piantadosi A, Rubin DB, McQuillen DP, et al. Emerging cases of Powassan virus encephalitis in New England: clinical presentation, imaging, and review of the literature. Clin Infect Dis. 2016;62:707-713.
- El Khoury MY, Camargo JF, White JL, et al. Potential role of deer tick virus in Powassan encephalitis cases in Lyme disease-endemic areas of New York, U.S.A. Emerg Infect Dis. 2013;19:1926-1933.
- World Health Organization (WHO). Vaccines against tick-borne encephalitis: WHO position paper. Wkly Epidemiol Rec. 2011;86:241-256.
- Centers for Disease Control and Prevention (CDC). Tick-borne encephalitis among U.S. travelers to Europe and Asia—2000-2009. JAMA. 2010;303:2132-2135.
- Valarcher JF, Hägglund S, Juremalm M, et al. Tick-borne encephalitits. Rev Sci Tech. 2015;34:453-466.
- Schultze D, Dollenmaier G, Rohner A, et al. Benefit of detecting tick-borne encephalitis viremia in the first phase of illness. J Clin Virol. 2007;38:172-175.
- Holzmann H. Diagnosis of tick-borne encephalitis. Vaccine. 2003;21(suppl 1):S36-S40.
- Zavadska D, Anca I, André F, et al. Recommendations for tick-borne encephalitis vaccination from the Central European Vaccination Awareness Group. Hum Vaccin Immunother. 2013;9:362-374.
- Gugliotta JL, Goethert HK, Berardi VP, et al. Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. N Engl J Med. 2013;368:240-245.
- Hovius JW, de Wever B, Sohne M, et al. A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet. 2013;382:658.
- Molloy PJ, Telford SR III, Chowdri HR, et al. Borrelia miyamotoi disease in the northeastern United States: a case series. Ann Intern Med. 2015;163:91-98.
- Telford SR 3rd, Goethert HK, Molloy PJ, et al. Borrelia miyamotoi disease: neither Lyme disease nor relapsing fever. Clin Lab Med. 2015;35:867-882.
- Diaz JH. A comparative meta-analysis of tick paralysis in the United States and Australia. Clin Toxicol (Phila). 2015;53:874-883.
Practice Points
- Apart from the more familiar Borrelia burgdorferi, several less common pathogens associated with diseases transmitted by Ixodes ticks include Anaplasma phagocytophilum, Babesia microti, Borrelia miyamotoi, the Powassan virus, and the tick-borne encephalitis virus.
- Overlap in both the geographic distribution and the clinical presentations of these uncommon pathogens underscores the importance of being familiar with their capacity for causing illness and effective treatment.
- Intoxication with the saliva of some Ixodes species can cause an ascending flaccid tick paralysis.
Do industry payments increase prescribing for some targeted therapies?
Physicians receiving general payments from the company marketing a targeted cancer therapy were more likely to prescribe it in three out of six drugs evaluated, researchers reported.
Prescribing of sunitinib, dasatinib, and nilotinib was increased for physicians receiving such payments versus not receiving them, while prescribing of imatinib, sorafenib, and pazopanib were not, according to the analysis by Aaron P. Mitchell, MD, of the Lineberger Comprehensive Cancer Center, UNC School of Medicine, University of North Carolina at Chapel Hill, and his coauthors.
In previous studies, pharmaceutical industry payments to physicians have been associated with “higher-cost, brand-name pharmaceutical prescribing,” Dr. Mitchell and his colleagues wrote. The report was published in JAMA Internal Medicine.
“Whether industry payments are associated with physician treatment choice in oncology is uncertain,” they said.
To evaluate the association between payments to oncologists and drug selection, Dr. Mitchell and his colleagues linked Open Payments data from the Centers for Medicare & Medicaid Services to data from Medicare Part D Prescriber Public Use File for the years 2013-2014.
The primary variable in the study was payments received during 2013, according to investigators, and the primary outcome of the analysis was prescriptions filled during 2014.
Open Payments reported in 2013 had a total dollar value of $4.08 billion, including $1.20 billion paid to physicians, according to CMS data.
The researchers focused on targeted therapies for two therapeutic areas: metastatic renal cell carcinoma (RCC), including sorafenib, sunitinib, and pazopanib; and chronic myeloid leukemia (CML), including imatinib, dasatinib, and nilotinib.
They limited their analysis to physicians listed as oncologists who filled at least 20 prescriptions for each of the three drugs in metastatic RCC (n = 354) or in CML (n = 2,225).
Receiving payments categorized as “general,” such as gifts, speaker fees, meals, and travel, increased the odds of prescribing drugs for both metastatic RCC (odds ratio, 2.05; 95% confidence interval, 1.34-3.14; P = .001) and for CML (odds ratio, 1.29; 95% CI, 1.13-1.47; P less than .001).
By contrast, research payments did not increase the odds of prescribing those drugs, the investigators reported.
Looking at specific drugs, they found that receipt of general payments from a drug’s manufacturer was associated with increased prescribing of sunitinib (50.5% versus 34.4%, P = .01), dasatinib (13.8% versus 11.4%, P = .02), and nilotinib (15.4% vs 12.5%, P = .01).
However, no such association was found for sorafenib or pazopanib.
For imatinib, by contrast, investigators said industry payments were associated with a prescribing decrease.
“This may reflect a strategy by the manufacturer of imatinib, which also produces nilotinib, to promote switching to nilotinib before the patent expiration of imatinib in 2015,” the researchers wrote.
Dr. Mitchell and his coauthors reported no conflict of interest disclosures related to the study.
SOURCE: Mitchell AP, et al. JAMA Intern Med. 2018 Apr 9. doi: 0.1001/jamainternmed.2018.0776.
Physicians receiving general payments from the company marketing a targeted cancer therapy were more likely to prescribe it in three out of six drugs evaluated, researchers reported.
Prescribing of sunitinib, dasatinib, and nilotinib was increased for physicians receiving such payments versus not receiving them, while prescribing of imatinib, sorafenib, and pazopanib were not, according to the analysis by Aaron P. Mitchell, MD, of the Lineberger Comprehensive Cancer Center, UNC School of Medicine, University of North Carolina at Chapel Hill, and his coauthors.
In previous studies, pharmaceutical industry payments to physicians have been associated with “higher-cost, brand-name pharmaceutical prescribing,” Dr. Mitchell and his colleagues wrote. The report was published in JAMA Internal Medicine.
“Whether industry payments are associated with physician treatment choice in oncology is uncertain,” they said.
To evaluate the association between payments to oncologists and drug selection, Dr. Mitchell and his colleagues linked Open Payments data from the Centers for Medicare & Medicaid Services to data from Medicare Part D Prescriber Public Use File for the years 2013-2014.
The primary variable in the study was payments received during 2013, according to investigators, and the primary outcome of the analysis was prescriptions filled during 2014.
Open Payments reported in 2013 had a total dollar value of $4.08 billion, including $1.20 billion paid to physicians, according to CMS data.
The researchers focused on targeted therapies for two therapeutic areas: metastatic renal cell carcinoma (RCC), including sorafenib, sunitinib, and pazopanib; and chronic myeloid leukemia (CML), including imatinib, dasatinib, and nilotinib.
They limited their analysis to physicians listed as oncologists who filled at least 20 prescriptions for each of the three drugs in metastatic RCC (n = 354) or in CML (n = 2,225).
Receiving payments categorized as “general,” such as gifts, speaker fees, meals, and travel, increased the odds of prescribing drugs for both metastatic RCC (odds ratio, 2.05; 95% confidence interval, 1.34-3.14; P = .001) and for CML (odds ratio, 1.29; 95% CI, 1.13-1.47; P less than .001).
By contrast, research payments did not increase the odds of prescribing those drugs, the investigators reported.
Looking at specific drugs, they found that receipt of general payments from a drug’s manufacturer was associated with increased prescribing of sunitinib (50.5% versus 34.4%, P = .01), dasatinib (13.8% versus 11.4%, P = .02), and nilotinib (15.4% vs 12.5%, P = .01).
However, no such association was found for sorafenib or pazopanib.
For imatinib, by contrast, investigators said industry payments were associated with a prescribing decrease.
“This may reflect a strategy by the manufacturer of imatinib, which also produces nilotinib, to promote switching to nilotinib before the patent expiration of imatinib in 2015,” the researchers wrote.
Dr. Mitchell and his coauthors reported no conflict of interest disclosures related to the study.
SOURCE: Mitchell AP, et al. JAMA Intern Med. 2018 Apr 9. doi: 0.1001/jamainternmed.2018.0776.
Physicians receiving general payments from the company marketing a targeted cancer therapy were more likely to prescribe it in three out of six drugs evaluated, researchers reported.
Prescribing of sunitinib, dasatinib, and nilotinib was increased for physicians receiving such payments versus not receiving them, while prescribing of imatinib, sorafenib, and pazopanib were not, according to the analysis by Aaron P. Mitchell, MD, of the Lineberger Comprehensive Cancer Center, UNC School of Medicine, University of North Carolina at Chapel Hill, and his coauthors.
In previous studies, pharmaceutical industry payments to physicians have been associated with “higher-cost, brand-name pharmaceutical prescribing,” Dr. Mitchell and his colleagues wrote. The report was published in JAMA Internal Medicine.
“Whether industry payments are associated with physician treatment choice in oncology is uncertain,” they said.
To evaluate the association between payments to oncologists and drug selection, Dr. Mitchell and his colleagues linked Open Payments data from the Centers for Medicare & Medicaid Services to data from Medicare Part D Prescriber Public Use File for the years 2013-2014.
The primary variable in the study was payments received during 2013, according to investigators, and the primary outcome of the analysis was prescriptions filled during 2014.
Open Payments reported in 2013 had a total dollar value of $4.08 billion, including $1.20 billion paid to physicians, according to CMS data.
The researchers focused on targeted therapies for two therapeutic areas: metastatic renal cell carcinoma (RCC), including sorafenib, sunitinib, and pazopanib; and chronic myeloid leukemia (CML), including imatinib, dasatinib, and nilotinib.
They limited their analysis to physicians listed as oncologists who filled at least 20 prescriptions for each of the three drugs in metastatic RCC (n = 354) or in CML (n = 2,225).
Receiving payments categorized as “general,” such as gifts, speaker fees, meals, and travel, increased the odds of prescribing drugs for both metastatic RCC (odds ratio, 2.05; 95% confidence interval, 1.34-3.14; P = .001) and for CML (odds ratio, 1.29; 95% CI, 1.13-1.47; P less than .001).
By contrast, research payments did not increase the odds of prescribing those drugs, the investigators reported.
Looking at specific drugs, they found that receipt of general payments from a drug’s manufacturer was associated with increased prescribing of sunitinib (50.5% versus 34.4%, P = .01), dasatinib (13.8% versus 11.4%, P = .02), and nilotinib (15.4% vs 12.5%, P = .01).
However, no such association was found for sorafenib or pazopanib.
For imatinib, by contrast, investigators said industry payments were associated with a prescribing decrease.
“This may reflect a strategy by the manufacturer of imatinib, which also produces nilotinib, to promote switching to nilotinib before the patent expiration of imatinib in 2015,” the researchers wrote.
Dr. Mitchell and his coauthors reported no conflict of interest disclosures related to the study.
SOURCE: Mitchell AP, et al. JAMA Intern Med. 2018 Apr 9. doi: 0.1001/jamainternmed.2018.0776.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Oncologists receiving general payments from the company marketing a cancer drug were more likely to prescribe it in three out of six drugs evaluated.
Major finding: Prescribing was significantly increased for sunitinib (50.5% versus 34.4%, P = .01), dasatinib (13.8% versus 11.4%, P = .02), and nilotinib (15.4% vs. 12.5%, P = .01), but not for imatinib, sorafenib, or pazopanib.
Study details: An analysis of Centers for Medicare & Medicaid Services Open Payments data and Medicare Part D Prescriber Public Use File for the years 2013 to 2014.
Disclosures: The authors reported no conflict of interest disclosures related to the study.
Source: Mitchell AP et al. JAMA Intern Med. 2018 Apr 9. doi: 0.1001/jamainternmed.2018.0776.
SSRI exposure in utero may change brain structure and connectivity
Prenatal exposure to selective serotonin reuptake inhibitors (SSRIs) was associated with fetal brain development in brain regions important in emotional processing, results of an imaging study show.
Compared to controls, SSRI-exposed infants had significant gray matter volume expansion and increased white matter structural connectivity in the amygdala and insula, according to results published in JAMA Pediatrics.
“Our findings suggest a potential association between prenatal SSRI exposure, likely via aberrant serotonin signaling, and the development of the amygdala-insula circuit in the fetal brain,” wrote Claudia Lugo-Candelas, PhD, of Columbia University Medical Center, New York, and her coauthors.
An increasing number of pregnant women are taking SSRIs, in part due to increased awareness of the negative effects of untreated prenatal maternal depression (PMD), the investigators said in their report.
“Because untreated PMD poses risks to both the infant and mother, the decision to initiate, continue, or suspend SSRI treatment remains a clinical dilemma,” they wrote.
Animal studies suggest atypical serotonergic signaling from prenatal SSRI exposure could change fetal brain development and affect function later in life, they explained.
Studies in humans have produced mixed results, but in a recent national registry study including more than 15,000 individuals exposed to SSRIs prenatally, exposure was linked to increased rates of depression.
To evaluate the impact of prenatal SSRI exposure on brain development, Dr. Lugo-Candelas and colleagues used structural and diffusion magnetic resonance imaging (MRI) to evaluate the brains of 98 infants.
They included 16 infants with in utero exposure to SSRIs, 21 born to mothers with untreated maternal depression, and 61 healthy control subjects, all evaluated between 2011 and 2016.
Infants exposed to SSRIs in utero had significant (P less than .05) gray matter volume expansion versus controls in both the right amygdala (Cohen’s d, 0.65; 95 %CI, 0.06-1.23) and the right insula (Cohen’s d = 0.86; 95% CI, 0.26-1.14).
The SSRI-exposed infants also had a significant (P less than .05) increase in connectivity between the right amygdala and the right insula versus controls (Cohen’s d, 0.99; 95% CI, 0.40-1.57).
Whether these neurodevelopmental changes translate into long-term behavioral or psychological outcomes should be evaluated in subsequent studies, Dr. Lugo-Candelas and colleagues said.
Abnormalities in amygdala-insula circuitry may lead to anxiety or depression, they wrote.
“The structurally primed circuit in the infant brains could lead to maladaptive fear processing in their later life, such as generalization of conditioned fear or negative attention bias,” they added.
Dr. Lugo-Candelas reported no conflicts of interest related to the study. One study coauthor reported research support from Shire Pharmaceuticals and Aevi Genomics.
SOURCE: Lugo-Candelas C, et al. JAMA Pediatr. 2018 Apr 9. doi: 10.1001/jamapediatrics.2017.5227.
Prenatal exposure to selective serotonin reuptake inhibitors (SSRIs) was associated with fetal brain development in brain regions important in emotional processing, results of an imaging study show.
Compared to controls, SSRI-exposed infants had significant gray matter volume expansion and increased white matter structural connectivity in the amygdala and insula, according to results published in JAMA Pediatrics.
“Our findings suggest a potential association between prenatal SSRI exposure, likely via aberrant serotonin signaling, and the development of the amygdala-insula circuit in the fetal brain,” wrote Claudia Lugo-Candelas, PhD, of Columbia University Medical Center, New York, and her coauthors.
An increasing number of pregnant women are taking SSRIs, in part due to increased awareness of the negative effects of untreated prenatal maternal depression (PMD), the investigators said in their report.
“Because untreated PMD poses risks to both the infant and mother, the decision to initiate, continue, or suspend SSRI treatment remains a clinical dilemma,” they wrote.
Animal studies suggest atypical serotonergic signaling from prenatal SSRI exposure could change fetal brain development and affect function later in life, they explained.
Studies in humans have produced mixed results, but in a recent national registry study including more than 15,000 individuals exposed to SSRIs prenatally, exposure was linked to increased rates of depression.
To evaluate the impact of prenatal SSRI exposure on brain development, Dr. Lugo-Candelas and colleagues used structural and diffusion magnetic resonance imaging (MRI) to evaluate the brains of 98 infants.
They included 16 infants with in utero exposure to SSRIs, 21 born to mothers with untreated maternal depression, and 61 healthy control subjects, all evaluated between 2011 and 2016.
Infants exposed to SSRIs in utero had significant (P less than .05) gray matter volume expansion versus controls in both the right amygdala (Cohen’s d, 0.65; 95 %CI, 0.06-1.23) and the right insula (Cohen’s d = 0.86; 95% CI, 0.26-1.14).
The SSRI-exposed infants also had a significant (P less than .05) increase in connectivity between the right amygdala and the right insula versus controls (Cohen’s d, 0.99; 95% CI, 0.40-1.57).
Whether these neurodevelopmental changes translate into long-term behavioral or psychological outcomes should be evaluated in subsequent studies, Dr. Lugo-Candelas and colleagues said.
Abnormalities in amygdala-insula circuitry may lead to anxiety or depression, they wrote.
“The structurally primed circuit in the infant brains could lead to maladaptive fear processing in their later life, such as generalization of conditioned fear or negative attention bias,” they added.
Dr. Lugo-Candelas reported no conflicts of interest related to the study. One study coauthor reported research support from Shire Pharmaceuticals and Aevi Genomics.
SOURCE: Lugo-Candelas C, et al. JAMA Pediatr. 2018 Apr 9. doi: 10.1001/jamapediatrics.2017.5227.
Prenatal exposure to selective serotonin reuptake inhibitors (SSRIs) was associated with fetal brain development in brain regions important in emotional processing, results of an imaging study show.
Compared to controls, SSRI-exposed infants had significant gray matter volume expansion and increased white matter structural connectivity in the amygdala and insula, according to results published in JAMA Pediatrics.
“Our findings suggest a potential association between prenatal SSRI exposure, likely via aberrant serotonin signaling, and the development of the amygdala-insula circuit in the fetal brain,” wrote Claudia Lugo-Candelas, PhD, of Columbia University Medical Center, New York, and her coauthors.
An increasing number of pregnant women are taking SSRIs, in part due to increased awareness of the negative effects of untreated prenatal maternal depression (PMD), the investigators said in their report.
“Because untreated PMD poses risks to both the infant and mother, the decision to initiate, continue, or suspend SSRI treatment remains a clinical dilemma,” they wrote.
Animal studies suggest atypical serotonergic signaling from prenatal SSRI exposure could change fetal brain development and affect function later in life, they explained.
Studies in humans have produced mixed results, but in a recent national registry study including more than 15,000 individuals exposed to SSRIs prenatally, exposure was linked to increased rates of depression.
To evaluate the impact of prenatal SSRI exposure on brain development, Dr. Lugo-Candelas and colleagues used structural and diffusion magnetic resonance imaging (MRI) to evaluate the brains of 98 infants.
They included 16 infants with in utero exposure to SSRIs, 21 born to mothers with untreated maternal depression, and 61 healthy control subjects, all evaluated between 2011 and 2016.
Infants exposed to SSRIs in utero had significant (P less than .05) gray matter volume expansion versus controls in both the right amygdala (Cohen’s d, 0.65; 95 %CI, 0.06-1.23) and the right insula (Cohen’s d = 0.86; 95% CI, 0.26-1.14).
The SSRI-exposed infants also had a significant (P less than .05) increase in connectivity between the right amygdala and the right insula versus controls (Cohen’s d, 0.99; 95% CI, 0.40-1.57).
Whether these neurodevelopmental changes translate into long-term behavioral or psychological outcomes should be evaluated in subsequent studies, Dr. Lugo-Candelas and colleagues said.
Abnormalities in amygdala-insula circuitry may lead to anxiety or depression, they wrote.
“The structurally primed circuit in the infant brains could lead to maladaptive fear processing in their later life, such as generalization of conditioned fear or negative attention bias,” they added.
Dr. Lugo-Candelas reported no conflicts of interest related to the study. One study coauthor reported research support from Shire Pharmaceuticals and Aevi Genomics.
SOURCE: Lugo-Candelas C, et al. JAMA Pediatr. 2018 Apr 9. doi: 10.1001/jamapediatrics.2017.5227.
FROM JAMA PEDIATRICS
Key clinical point: Prenatal selective serotonin reuptake inhibitor (SSRI) exposure was associated with fetal brain development, especially in regions of the brain important to emotional processing.
Major finding: Compared to controls, SSRI-exposed infants had significant gray matter volume expansion and increased white matter structural connectivity in the amygdala and insula.
Study details: A two-center cohort study of data collected between 2011 and 2016 for 98 infants, including 16 with in utero exposure to SSRIs.
Disclosures: One study author reported research support from Shire Pharmaceuticals and Aevi Genomics.
Source: Lugo-Candelas C et al. JAMA Pediatr. 2018 Apr 9. doi: 10.1001/jamapediatrics.2017.5227.
The outcomes of “GOLD 2017”
After the Global Initiative for Chronic Obstructive Lung Disease released updated recommendations for grading COPD patients’ level of disease in November of 2016, Imran Iftikhar, MD, tried to incorporate them into his practice, but he encountered problems.
For one thing, the new classification system, which became known as GOLD 2017, uncoupled spirometry results from the ABCD treatment algorithm. “I found it wasn’t really helping me in terms of prognostication or COPD management,” said Dr. Iftikhar, section chief of pulmonary and critical care at Emory Saint Joseph’s Hospital, Atlanta. “Although the purpose of the GOLD classification was not really meant for prognostication, most practicing physicians are frequently asked about prognosis by patients, and I am not sure if the 2017 reclassification really helps with that.”
The GOLD 2017 classification simplified the chronic obstructive pulmonary disease staging that was available from 2011 to 2015 from three variables (spirometry thresholds, exacerbation risk, and dyspnea scale) to two variables (exacerbation risk and dyspnea scale). In the 2017 report, authors of the new guidelines characterized forced expiratory volume in 1 second (FEV1) as “a poor predictor of disease status” and proposed that clinicians derive ABCD groups exclusively from patient symptoms and their exacerbations. FEV1 is an “important parameter at the population level” in predicting hospitalization and mortality, the authors wrote, but keeping results separate “acknowledges the limitations of FEV1 in making treatment decisions for individualized patient care and highlights the importance of patient symptoms and exacerbation risks in guiding therapies in COPD.”
According to Meilan Han, MD, MS, a member of the GOLD Science Committee, since release of the 2017 guidelines, “clinicians have indicated that they like the flexibility the system provides in separating spirometry, symptoms, and exacerbation risk as this more accurately reflects the heterogeneity we see in the COPD patient population.” Nevertheless, how this approach influences long-term outcomes remains unclear.
Daniel Ouellette, MD, FCCP, a pulmonologist with the Henry Ford Health System in Detroit, described the GOLD 2017 criteria as “a good step forward” but said he wasn’t sure if the optimal or perfect tool exists for categorizing COPD patients’ level of disease.
“I think what we see is an effort to use all of these criteria to help us better treat our patients. I think ,” he said in an interview.
“All guidelines need to be modified as further research becomes available. I think that the frontiers of this area are going to be to incorporate new elements such as tobacco history, more emphasis on clinical signs and symptoms, and use of markers other than spirometry, such as eosinophil count, to categorize patients with COPD,” Dr. Ouellette added.
In an analysis of the GOLD 2017 criteria applied to 819 COPD patients in Spain and the United States, published online Nov. 3, 2017, in the American Journal of Respiratory and Critical Care Medicine, Carlos Cabrera López, MD, and his colleagues concluded that the mortality risk was better predicted by the 2015 GOLD classification system than by the 2017 iteration (Am J Respir Crit Care Med. 2018 Feb. doi: 10.101164/rccm.201707-1363OC).
The distribution of Charlson index scores also changed. Whereas group D was higher than B in 2015, they became similar in the 2017 system. For her part, Dr. Han emphasized that the primary goal of the GOLD ABCD classification system is to categorize patients with respect to treatment groups. “Current therapy targets symptoms and exacerbations, which are the key current elements of the classification schema,” she said in an interview. “The results of the Cabrera Lopez analysis are not necessarily unexpected, as FEV1 is associated with mortality.”
In a prospective, multicenter analysis, Portuguese researchers compared the performance of GOLD 2011 and 2017 in terms of how 200 COPD patients were reclassified, the level of agreement between the two iterations, and the performance of each to predict future exacerbations (COPD. 2018 Feb;15[1]; 21-6). They found that about half of patients classified as GOLD D under the 2011 guidelines became classified as GOLD B when the 2017 version was used, and the extent of agreement between the two iterations was moderate (P less than .001). They also found that the two versions of the guidelines were equivalently effective at predicting exacerbations (69.7% vs. 67.6% in the 2011 and 2017 iterations, respectively). In addition, patients who met the criteria for a GOLD B grouping in the 2017 iteration exacerbated 17% more often and had a lower percent predicted post bronchodilator FEV1 than did those who met the criteria for a GOLD B classification under the 2011 guidelines.
Dr. Han, who is also an associate professor of medicine at the University of Michigan Hospital, acknowledged that GOLD 2017 has resulted in the reclassification of some previously group D patients as group B patients. “Our primary goal is to aid clinicians with the diagnosis and management of patients with COPD,” she said. “We look forward to additional data coming in from ongoing clinical trials that will provide longer term data to further refine treatment algorithms.”
In a recent study of more than 33,000 Danish patients older than age 30 with COPD, researchers led by Anne Gedebjerg, MD, found that the GOLD 2017 ABCD classification did not predict all-cause and respiratory mortality more accurately than previous GOLD iterations from 2007 and 2011. Area under the curve for all-cause mortality was 0.61 for GOLD 2007, 0.61 for GOLD 2011, and 0.63 for GOLD 2017, while the area under the curve for respiratory mortality was 0.64 for GOLD 2007, 0.63 for GOLD 2011, and 0.65 for GOLD 2017 (Lancet Respir Med. 2018 Jan;6[3]:204-12).
However, when the spirometric stages 1-4 were combined with the A to D groupings based on symptoms and exacerbations, the 2017 classification predicted mortality with greater accuracy, compared with previous iterations (P less than .0001). “My practice is very much like this paper,” Dr. Iftikhar said. “I use both the spirometric grade and the ABCD grouping to specify which ‘group’ and ‘grade’ my patient belongs to. I think future investigators need to combine ABCD with spirometry classification to see how we can improve the classification system.”
In a commentary published in the same issue of the Lancet Respiratory Medicine as the large Danish study, Joan B. Soriano, MD, PhD, wrote that the 2011 GOLD guideline’s collapse of four spirometric thresholds (greater than 80%, 50%-80%, 30%-50%, and less than 30%) into just two (greater than 50% or 50% or less) “reduced the system’s ability to inform and predict mortality from the short term up to 10 years” (Lancet Respir Med. 2018 Jan;6[3]:165-6).
Lung function remains the best available biomarker for life expectancy in both patients with COPD and the general population,” wrote Dr. Soriano, a respiratory medicine researcher based in Madrid, Spain.
Additional important outcomes
Dr. Ouellette noted that while mortality is an important outcome for COPD patients, it’s not the only outcome of interest. “In addition to [trying to] help people live longer, which is certainly a desirable goal, we also want to make people be able to be more functional during their life, have fewer hospitalizations, and have less of a need of other types of supportive medical care for worsening of their disease,” he said. “The fact that the current guidelines don’t improve mortality more than the previous ones may not be a negative thing. It may tell us that the previous guidelines already did a pretty good job of helping us to improve mortality.”
Dr. Ouellette was quick to add that none of inhaled drugs currently available to treat COPD have been conclusively shown to improve mortality. “The only things we know that improve mortality for COPD patients are quitting smoking and using oxygen if a patient meets predefined goals for oxygen,” he said. “So the fact that GOLD criteria doesn’t improve mortality shouldn’t make us think that it’s not a useful tool. We already know that the medicines may not help people live longer.”
Dr. Han pointed out that spirometry “is still used to further clarify the choice of therapy recommended based on the nature and degree of airflow obstruction in light of severity of patient symptoms. The data are still designed to be used in conjunction to personalize therapy for patients.”
She added that the GOLD Science Committee “welcomes additional data analyses so that future recommendations can be further refined.”
Dr. Han disclosed that she has consulted for Boehringer Ingelheim, AstraZeneca, and GlaxoSmithKline. She has also received in-kind research support from Novartis and Sunovion.
Dr. Iftikhar reported having no financial disclosures. Dr. Ouellette is a member of CHEST® Physician’s editorial advisory board. He disclosed being part of a federally funded study being carried out by the Patient-Centered Outcomes Research Institute.
There was no industry involvement in the GOLD 2017 report, but many of its authors and board members had pharmaceutical company ties, and GOLD’s treatment advice relies on data from industry-sponsored studies.
After the Global Initiative for Chronic Obstructive Lung Disease released updated recommendations for grading COPD patients’ level of disease in November of 2016, Imran Iftikhar, MD, tried to incorporate them into his practice, but he encountered problems.
For one thing, the new classification system, which became known as GOLD 2017, uncoupled spirometry results from the ABCD treatment algorithm. “I found it wasn’t really helping me in terms of prognostication or COPD management,” said Dr. Iftikhar, section chief of pulmonary and critical care at Emory Saint Joseph’s Hospital, Atlanta. “Although the purpose of the GOLD classification was not really meant for prognostication, most practicing physicians are frequently asked about prognosis by patients, and I am not sure if the 2017 reclassification really helps with that.”
The GOLD 2017 classification simplified the chronic obstructive pulmonary disease staging that was available from 2011 to 2015 from three variables (spirometry thresholds, exacerbation risk, and dyspnea scale) to two variables (exacerbation risk and dyspnea scale). In the 2017 report, authors of the new guidelines characterized forced expiratory volume in 1 second (FEV1) as “a poor predictor of disease status” and proposed that clinicians derive ABCD groups exclusively from patient symptoms and their exacerbations. FEV1 is an “important parameter at the population level” in predicting hospitalization and mortality, the authors wrote, but keeping results separate “acknowledges the limitations of FEV1 in making treatment decisions for individualized patient care and highlights the importance of patient symptoms and exacerbation risks in guiding therapies in COPD.”
According to Meilan Han, MD, MS, a member of the GOLD Science Committee, since release of the 2017 guidelines, “clinicians have indicated that they like the flexibility the system provides in separating spirometry, symptoms, and exacerbation risk as this more accurately reflects the heterogeneity we see in the COPD patient population.” Nevertheless, how this approach influences long-term outcomes remains unclear.
Daniel Ouellette, MD, FCCP, a pulmonologist with the Henry Ford Health System in Detroit, described the GOLD 2017 criteria as “a good step forward” but said he wasn’t sure if the optimal or perfect tool exists for categorizing COPD patients’ level of disease.
“I think what we see is an effort to use all of these criteria to help us better treat our patients. I think ,” he said in an interview.
“All guidelines need to be modified as further research becomes available. I think that the frontiers of this area are going to be to incorporate new elements such as tobacco history, more emphasis on clinical signs and symptoms, and use of markers other than spirometry, such as eosinophil count, to categorize patients with COPD,” Dr. Ouellette added.
In an analysis of the GOLD 2017 criteria applied to 819 COPD patients in Spain and the United States, published online Nov. 3, 2017, in the American Journal of Respiratory and Critical Care Medicine, Carlos Cabrera López, MD, and his colleagues concluded that the mortality risk was better predicted by the 2015 GOLD classification system than by the 2017 iteration (Am J Respir Crit Care Med. 2018 Feb. doi: 10.101164/rccm.201707-1363OC).
The distribution of Charlson index scores also changed. Whereas group D was higher than B in 2015, they became similar in the 2017 system. For her part, Dr. Han emphasized that the primary goal of the GOLD ABCD classification system is to categorize patients with respect to treatment groups. “Current therapy targets symptoms and exacerbations, which are the key current elements of the classification schema,” she said in an interview. “The results of the Cabrera Lopez analysis are not necessarily unexpected, as FEV1 is associated with mortality.”
In a prospective, multicenter analysis, Portuguese researchers compared the performance of GOLD 2011 and 2017 in terms of how 200 COPD patients were reclassified, the level of agreement between the two iterations, and the performance of each to predict future exacerbations (COPD. 2018 Feb;15[1]; 21-6). They found that about half of patients classified as GOLD D under the 2011 guidelines became classified as GOLD B when the 2017 version was used, and the extent of agreement between the two iterations was moderate (P less than .001). They also found that the two versions of the guidelines were equivalently effective at predicting exacerbations (69.7% vs. 67.6% in the 2011 and 2017 iterations, respectively). In addition, patients who met the criteria for a GOLD B grouping in the 2017 iteration exacerbated 17% more often and had a lower percent predicted post bronchodilator FEV1 than did those who met the criteria for a GOLD B classification under the 2011 guidelines.
Dr. Han, who is also an associate professor of medicine at the University of Michigan Hospital, acknowledged that GOLD 2017 has resulted in the reclassification of some previously group D patients as group B patients. “Our primary goal is to aid clinicians with the diagnosis and management of patients with COPD,” she said. “We look forward to additional data coming in from ongoing clinical trials that will provide longer term data to further refine treatment algorithms.”
In a recent study of more than 33,000 Danish patients older than age 30 with COPD, researchers led by Anne Gedebjerg, MD, found that the GOLD 2017 ABCD classification did not predict all-cause and respiratory mortality more accurately than previous GOLD iterations from 2007 and 2011. Area under the curve for all-cause mortality was 0.61 for GOLD 2007, 0.61 for GOLD 2011, and 0.63 for GOLD 2017, while the area under the curve for respiratory mortality was 0.64 for GOLD 2007, 0.63 for GOLD 2011, and 0.65 for GOLD 2017 (Lancet Respir Med. 2018 Jan;6[3]:204-12).
However, when the spirometric stages 1-4 were combined with the A to D groupings based on symptoms and exacerbations, the 2017 classification predicted mortality with greater accuracy, compared with previous iterations (P less than .0001). “My practice is very much like this paper,” Dr. Iftikhar said. “I use both the spirometric grade and the ABCD grouping to specify which ‘group’ and ‘grade’ my patient belongs to. I think future investigators need to combine ABCD with spirometry classification to see how we can improve the classification system.”
In a commentary published in the same issue of the Lancet Respiratory Medicine as the large Danish study, Joan B. Soriano, MD, PhD, wrote that the 2011 GOLD guideline’s collapse of four spirometric thresholds (greater than 80%, 50%-80%, 30%-50%, and less than 30%) into just two (greater than 50% or 50% or less) “reduced the system’s ability to inform and predict mortality from the short term up to 10 years” (Lancet Respir Med. 2018 Jan;6[3]:165-6).
Lung function remains the best available biomarker for life expectancy in both patients with COPD and the general population,” wrote Dr. Soriano, a respiratory medicine researcher based in Madrid, Spain.
Additional important outcomes
Dr. Ouellette noted that while mortality is an important outcome for COPD patients, it’s not the only outcome of interest. “In addition to [trying to] help people live longer, which is certainly a desirable goal, we also want to make people be able to be more functional during their life, have fewer hospitalizations, and have less of a need of other types of supportive medical care for worsening of their disease,” he said. “The fact that the current guidelines don’t improve mortality more than the previous ones may not be a negative thing. It may tell us that the previous guidelines already did a pretty good job of helping us to improve mortality.”
Dr. Ouellette was quick to add that none of inhaled drugs currently available to treat COPD have been conclusively shown to improve mortality. “The only things we know that improve mortality for COPD patients are quitting smoking and using oxygen if a patient meets predefined goals for oxygen,” he said. “So the fact that GOLD criteria doesn’t improve mortality shouldn’t make us think that it’s not a useful tool. We already know that the medicines may not help people live longer.”
Dr. Han pointed out that spirometry “is still used to further clarify the choice of therapy recommended based on the nature and degree of airflow obstruction in light of severity of patient symptoms. The data are still designed to be used in conjunction to personalize therapy for patients.”
She added that the GOLD Science Committee “welcomes additional data analyses so that future recommendations can be further refined.”
Dr. Han disclosed that she has consulted for Boehringer Ingelheim, AstraZeneca, and GlaxoSmithKline. She has also received in-kind research support from Novartis and Sunovion.
Dr. Iftikhar reported having no financial disclosures. Dr. Ouellette is a member of CHEST® Physician’s editorial advisory board. He disclosed being part of a federally funded study being carried out by the Patient-Centered Outcomes Research Institute.
There was no industry involvement in the GOLD 2017 report, but many of its authors and board members had pharmaceutical company ties, and GOLD’s treatment advice relies on data from industry-sponsored studies.
After the Global Initiative for Chronic Obstructive Lung Disease released updated recommendations for grading COPD patients’ level of disease in November of 2016, Imran Iftikhar, MD, tried to incorporate them into his practice, but he encountered problems.
For one thing, the new classification system, which became known as GOLD 2017, uncoupled spirometry results from the ABCD treatment algorithm. “I found it wasn’t really helping me in terms of prognostication or COPD management,” said Dr. Iftikhar, section chief of pulmonary and critical care at Emory Saint Joseph’s Hospital, Atlanta. “Although the purpose of the GOLD classification was not really meant for prognostication, most practicing physicians are frequently asked about prognosis by patients, and I am not sure if the 2017 reclassification really helps with that.”
The GOLD 2017 classification simplified the chronic obstructive pulmonary disease staging that was available from 2011 to 2015 from three variables (spirometry thresholds, exacerbation risk, and dyspnea scale) to two variables (exacerbation risk and dyspnea scale). In the 2017 report, authors of the new guidelines characterized forced expiratory volume in 1 second (FEV1) as “a poor predictor of disease status” and proposed that clinicians derive ABCD groups exclusively from patient symptoms and their exacerbations. FEV1 is an “important parameter at the population level” in predicting hospitalization and mortality, the authors wrote, but keeping results separate “acknowledges the limitations of FEV1 in making treatment decisions for individualized patient care and highlights the importance of patient symptoms and exacerbation risks in guiding therapies in COPD.”
According to Meilan Han, MD, MS, a member of the GOLD Science Committee, since release of the 2017 guidelines, “clinicians have indicated that they like the flexibility the system provides in separating spirometry, symptoms, and exacerbation risk as this more accurately reflects the heterogeneity we see in the COPD patient population.” Nevertheless, how this approach influences long-term outcomes remains unclear.
Daniel Ouellette, MD, FCCP, a pulmonologist with the Henry Ford Health System in Detroit, described the GOLD 2017 criteria as “a good step forward” but said he wasn’t sure if the optimal or perfect tool exists for categorizing COPD patients’ level of disease.
“I think what we see is an effort to use all of these criteria to help us better treat our patients. I think ,” he said in an interview.
“All guidelines need to be modified as further research becomes available. I think that the frontiers of this area are going to be to incorporate new elements such as tobacco history, more emphasis on clinical signs and symptoms, and use of markers other than spirometry, such as eosinophil count, to categorize patients with COPD,” Dr. Ouellette added.
In an analysis of the GOLD 2017 criteria applied to 819 COPD patients in Spain and the United States, published online Nov. 3, 2017, in the American Journal of Respiratory and Critical Care Medicine, Carlos Cabrera López, MD, and his colleagues concluded that the mortality risk was better predicted by the 2015 GOLD classification system than by the 2017 iteration (Am J Respir Crit Care Med. 2018 Feb. doi: 10.101164/rccm.201707-1363OC).
The distribution of Charlson index scores also changed. Whereas group D was higher than B in 2015, they became similar in the 2017 system. For her part, Dr. Han emphasized that the primary goal of the GOLD ABCD classification system is to categorize patients with respect to treatment groups. “Current therapy targets symptoms and exacerbations, which are the key current elements of the classification schema,” she said in an interview. “The results of the Cabrera Lopez analysis are not necessarily unexpected, as FEV1 is associated with mortality.”
In a prospective, multicenter analysis, Portuguese researchers compared the performance of GOLD 2011 and 2017 in terms of how 200 COPD patients were reclassified, the level of agreement between the two iterations, and the performance of each to predict future exacerbations (COPD. 2018 Feb;15[1]; 21-6). They found that about half of patients classified as GOLD D under the 2011 guidelines became classified as GOLD B when the 2017 version was used, and the extent of agreement between the two iterations was moderate (P less than .001). They also found that the two versions of the guidelines were equivalently effective at predicting exacerbations (69.7% vs. 67.6% in the 2011 and 2017 iterations, respectively). In addition, patients who met the criteria for a GOLD B grouping in the 2017 iteration exacerbated 17% more often and had a lower percent predicted post bronchodilator FEV1 than did those who met the criteria for a GOLD B classification under the 2011 guidelines.
Dr. Han, who is also an associate professor of medicine at the University of Michigan Hospital, acknowledged that GOLD 2017 has resulted in the reclassification of some previously group D patients as group B patients. “Our primary goal is to aid clinicians with the diagnosis and management of patients with COPD,” she said. “We look forward to additional data coming in from ongoing clinical trials that will provide longer term data to further refine treatment algorithms.”
In a recent study of more than 33,000 Danish patients older than age 30 with COPD, researchers led by Anne Gedebjerg, MD, found that the GOLD 2017 ABCD classification did not predict all-cause and respiratory mortality more accurately than previous GOLD iterations from 2007 and 2011. Area under the curve for all-cause mortality was 0.61 for GOLD 2007, 0.61 for GOLD 2011, and 0.63 for GOLD 2017, while the area under the curve for respiratory mortality was 0.64 for GOLD 2007, 0.63 for GOLD 2011, and 0.65 for GOLD 2017 (Lancet Respir Med. 2018 Jan;6[3]:204-12).
However, when the spirometric stages 1-4 were combined with the A to D groupings based on symptoms and exacerbations, the 2017 classification predicted mortality with greater accuracy, compared with previous iterations (P less than .0001). “My practice is very much like this paper,” Dr. Iftikhar said. “I use both the spirometric grade and the ABCD grouping to specify which ‘group’ and ‘grade’ my patient belongs to. I think future investigators need to combine ABCD with spirometry classification to see how we can improve the classification system.”
In a commentary published in the same issue of the Lancet Respiratory Medicine as the large Danish study, Joan B. Soriano, MD, PhD, wrote that the 2011 GOLD guideline’s collapse of four spirometric thresholds (greater than 80%, 50%-80%, 30%-50%, and less than 30%) into just two (greater than 50% or 50% or less) “reduced the system’s ability to inform and predict mortality from the short term up to 10 years” (Lancet Respir Med. 2018 Jan;6[3]:165-6).
Lung function remains the best available biomarker for life expectancy in both patients with COPD and the general population,” wrote Dr. Soriano, a respiratory medicine researcher based in Madrid, Spain.
Additional important outcomes
Dr. Ouellette noted that while mortality is an important outcome for COPD patients, it’s not the only outcome of interest. “In addition to [trying to] help people live longer, which is certainly a desirable goal, we also want to make people be able to be more functional during their life, have fewer hospitalizations, and have less of a need of other types of supportive medical care for worsening of their disease,” he said. “The fact that the current guidelines don’t improve mortality more than the previous ones may not be a negative thing. It may tell us that the previous guidelines already did a pretty good job of helping us to improve mortality.”
Dr. Ouellette was quick to add that none of inhaled drugs currently available to treat COPD have been conclusively shown to improve mortality. “The only things we know that improve mortality for COPD patients are quitting smoking and using oxygen if a patient meets predefined goals for oxygen,” he said. “So the fact that GOLD criteria doesn’t improve mortality shouldn’t make us think that it’s not a useful tool. We already know that the medicines may not help people live longer.”
Dr. Han pointed out that spirometry “is still used to further clarify the choice of therapy recommended based on the nature and degree of airflow obstruction in light of severity of patient symptoms. The data are still designed to be used in conjunction to personalize therapy for patients.”
She added that the GOLD Science Committee “welcomes additional data analyses so that future recommendations can be further refined.”
Dr. Han disclosed that she has consulted for Boehringer Ingelheim, AstraZeneca, and GlaxoSmithKline. She has also received in-kind research support from Novartis and Sunovion.
Dr. Iftikhar reported having no financial disclosures. Dr. Ouellette is a member of CHEST® Physician’s editorial advisory board. He disclosed being part of a federally funded study being carried out by the Patient-Centered Outcomes Research Institute.
There was no industry involvement in the GOLD 2017 report, but many of its authors and board members had pharmaceutical company ties, and GOLD’s treatment advice relies on data from industry-sponsored studies.