User login
VIDEO: 5 years of additional AI no better than 2 in HR+ breast cancer
SAN ANTONIO – Clinical trials have shown a clear benefit for preventing breast cancer recurrence with aromatase inhibitor (AI) therapy following 5 years of tamoxifen. Yet the optimal duration for additional AI therapy following 5 years of endocrine therapy with tamoxifen, an AI, or sequential therapies is not known, according to Michael Gnant, MD, from the Medical University of Vienna.
In the ABCSG-16 trial, Dr. Gnant and his colleagues reported that 5 years of additional therapy with anastrozole (Arimidex) was no more effective than 2 additional years following the standard 5 years of initial endocrine therapy in postmenopausal women with hormone receptor–positive (HR+) breast cancer.
In this video interview at the San Antonio Breast Cancer Symposium, Dr. Gnant notes that, although some patients may still benefit from 5 years of additional therapy, the trial results suggest that most patients can be spared from such adverse events as risk for fractures associated with three additional and evidently unnecessary years of therapy.
The ABCSG-16 study was supported by AstraZeneca. Dr. Gnant disclosed research funding, honoraria, and travel funding from the company and others.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN ANTONIO – Clinical trials have shown a clear benefit for preventing breast cancer recurrence with aromatase inhibitor (AI) therapy following 5 years of tamoxifen. Yet the optimal duration for additional AI therapy following 5 years of endocrine therapy with tamoxifen, an AI, or sequential therapies is not known, according to Michael Gnant, MD, from the Medical University of Vienna.
In the ABCSG-16 trial, Dr. Gnant and his colleagues reported that 5 years of additional therapy with anastrozole (Arimidex) was no more effective than 2 additional years following the standard 5 years of initial endocrine therapy in postmenopausal women with hormone receptor–positive (HR+) breast cancer.
In this video interview at the San Antonio Breast Cancer Symposium, Dr. Gnant notes that, although some patients may still benefit from 5 years of additional therapy, the trial results suggest that most patients can be spared from such adverse events as risk for fractures associated with three additional and evidently unnecessary years of therapy.
The ABCSG-16 study was supported by AstraZeneca. Dr. Gnant disclosed research funding, honoraria, and travel funding from the company and others.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN ANTONIO – Clinical trials have shown a clear benefit for preventing breast cancer recurrence with aromatase inhibitor (AI) therapy following 5 years of tamoxifen. Yet the optimal duration for additional AI therapy following 5 years of endocrine therapy with tamoxifen, an AI, or sequential therapies is not known, according to Michael Gnant, MD, from the Medical University of Vienna.
In the ABCSG-16 trial, Dr. Gnant and his colleagues reported that 5 years of additional therapy with anastrozole (Arimidex) was no more effective than 2 additional years following the standard 5 years of initial endocrine therapy in postmenopausal women with hormone receptor–positive (HR+) breast cancer.
In this video interview at the San Antonio Breast Cancer Symposium, Dr. Gnant notes that, although some patients may still benefit from 5 years of additional therapy, the trial results suggest that most patients can be spared from such adverse events as risk for fractures associated with three additional and evidently unnecessary years of therapy.
The ABCSG-16 study was supported by AstraZeneca. Dr. Gnant disclosed research funding, honoraria, and travel funding from the company and others.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
REPORTING FROM SABCS 2017
Expanded hospital testing improves respiratory pathogen detection
SAN DIEGO – Systematic testing of acute respiratory illness patients can increase the likelihood of finding relevant pathogens, according to a study presented at an annual scientific meeting on infectious diseases.
Currently, hospitals conduct either nonroutine assessments or rely heavily on clinical laboratory testing among severe acute respiratory illness patients, which can lead to missing clinically key viruses.
Systematic testing expands on tests ordered and carried out at hospitals, expanding on them by testing for influenza, respiratory syncytial virus (RSV), human metapneumovirus, rhinovirus and enterovirus, adenovirus, coronavirus, and parainfluenza viruses 1-4. To test the efficacy of systematic testing, investigators studied 2,216 severe acute respiratory illness patients hospitalized in one of three hospitals in Minnesota during September 2015-August 2016. Patients were predominantly younger than 5 years old (57%) and had one or more chronic medical condition (63%).
Detection of at least one virus increased from 1,062 patients (48%) to 1,600 patients (72%) when comparing clinically ordered tests against expanded, systematic RT-PCR testing conducted through the Minnesota Health Department (MDH).
By patient age, viral detection increased by 27%, 24%, 18%, and 21% for patients aged younger than 5 years, 5-17 years, 18-64 years, and 65 years and older, respectively. Except for influenza viruses and RSV, the proportions of viruses identified, regardless of age, were all lower in hospital testing, compared with MDH testing.
“RSV targeting was almost systematic among children less than 5 years, but [accounted for] only 28% of RSV detection,” said Dr. Steffen in her presentation. “A smaller proportion of other respiratory viruses, including the human metapneumovirus, were detected at the hospital, and this was especially true for adults.”
Patients with rhinovirus and enterovirus saw a difference between hospital and expanded testing, increasing from a little over 300 patients detected, to nearly 800 patients.
“Patients admitted to the ICU were less likely to have a pathogen detection than those not admitted to the ICU, and those with one or more chronic medical condition had lower viral detection than those without,” Dr. Steffens said. “While testing at MDH did increase the percent of patients in each category, trends remained consistent and significant.”
Since testing information was only collected for patients with positive test results at the hospital, investigators were not able to compare testing practices between patients with and without viruses. This study may also have underrepresented pathogens detected through means other than the hospital laboratory, like rapid tests in emergency departments. The study was also limited by the short time frame of only 1 year.
The presenters reported no relevant financial disclosures.
SOURCE: Steffens A et al. Abstract 885.
SAN DIEGO – Systematic testing of acute respiratory illness patients can increase the likelihood of finding relevant pathogens, according to a study presented at an annual scientific meeting on infectious diseases.
Currently, hospitals conduct either nonroutine assessments or rely heavily on clinical laboratory testing among severe acute respiratory illness patients, which can lead to missing clinically key viruses.
Systematic testing expands on tests ordered and carried out at hospitals, expanding on them by testing for influenza, respiratory syncytial virus (RSV), human metapneumovirus, rhinovirus and enterovirus, adenovirus, coronavirus, and parainfluenza viruses 1-4. To test the efficacy of systematic testing, investigators studied 2,216 severe acute respiratory illness patients hospitalized in one of three hospitals in Minnesota during September 2015-August 2016. Patients were predominantly younger than 5 years old (57%) and had one or more chronic medical condition (63%).
Detection of at least one virus increased from 1,062 patients (48%) to 1,600 patients (72%) when comparing clinically ordered tests against expanded, systematic RT-PCR testing conducted through the Minnesota Health Department (MDH).
By patient age, viral detection increased by 27%, 24%, 18%, and 21% for patients aged younger than 5 years, 5-17 years, 18-64 years, and 65 years and older, respectively. Except for influenza viruses and RSV, the proportions of viruses identified, regardless of age, were all lower in hospital testing, compared with MDH testing.
“RSV targeting was almost systematic among children less than 5 years, but [accounted for] only 28% of RSV detection,” said Dr. Steffen in her presentation. “A smaller proportion of other respiratory viruses, including the human metapneumovirus, were detected at the hospital, and this was especially true for adults.”
Patients with rhinovirus and enterovirus saw a difference between hospital and expanded testing, increasing from a little over 300 patients detected, to nearly 800 patients.
“Patients admitted to the ICU were less likely to have a pathogen detection than those not admitted to the ICU, and those with one or more chronic medical condition had lower viral detection than those without,” Dr. Steffens said. “While testing at MDH did increase the percent of patients in each category, trends remained consistent and significant.”
Since testing information was only collected for patients with positive test results at the hospital, investigators were not able to compare testing practices between patients with and without viruses. This study may also have underrepresented pathogens detected through means other than the hospital laboratory, like rapid tests in emergency departments. The study was also limited by the short time frame of only 1 year.
The presenters reported no relevant financial disclosures.
SOURCE: Steffens A et al. Abstract 885.
SAN DIEGO – Systematic testing of acute respiratory illness patients can increase the likelihood of finding relevant pathogens, according to a study presented at an annual scientific meeting on infectious diseases.
Currently, hospitals conduct either nonroutine assessments or rely heavily on clinical laboratory testing among severe acute respiratory illness patients, which can lead to missing clinically key viruses.
Systematic testing expands on tests ordered and carried out at hospitals, expanding on them by testing for influenza, respiratory syncytial virus (RSV), human metapneumovirus, rhinovirus and enterovirus, adenovirus, coronavirus, and parainfluenza viruses 1-4. To test the efficacy of systematic testing, investigators studied 2,216 severe acute respiratory illness patients hospitalized in one of three hospitals in Minnesota during September 2015-August 2016. Patients were predominantly younger than 5 years old (57%) and had one or more chronic medical condition (63%).
Detection of at least one virus increased from 1,062 patients (48%) to 1,600 patients (72%) when comparing clinically ordered tests against expanded, systematic RT-PCR testing conducted through the Minnesota Health Department (MDH).
By patient age, viral detection increased by 27%, 24%, 18%, and 21% for patients aged younger than 5 years, 5-17 years, 18-64 years, and 65 years and older, respectively. Except for influenza viruses and RSV, the proportions of viruses identified, regardless of age, were all lower in hospital testing, compared with MDH testing.
“RSV targeting was almost systematic among children less than 5 years, but [accounted for] only 28% of RSV detection,” said Dr. Steffen in her presentation. “A smaller proportion of other respiratory viruses, including the human metapneumovirus, were detected at the hospital, and this was especially true for adults.”
Patients with rhinovirus and enterovirus saw a difference between hospital and expanded testing, increasing from a little over 300 patients detected, to nearly 800 patients.
“Patients admitted to the ICU were less likely to have a pathogen detection than those not admitted to the ICU, and those with one or more chronic medical condition had lower viral detection than those without,” Dr. Steffens said. “While testing at MDH did increase the percent of patients in each category, trends remained consistent and significant.”
Since testing information was only collected for patients with positive test results at the hospital, investigators were not able to compare testing practices between patients with and without viruses. This study may also have underrepresented pathogens detected through means other than the hospital laboratory, like rapid tests in emergency departments. The study was also limited by the short time frame of only 1 year.
The presenters reported no relevant financial disclosures.
SOURCE: Steffens A et al. Abstract 885.
REPORTING FROM ID WEEK 2017
Key clinical point:
Major finding: Among 2,216 patients studied, 1,600 (72%) were found to have at least one respiratory virus through expanded testing, compared with 1,062 (48%) patients tested through clincian-directed testing.
Study details: 2,351 severe acute respiratory illness patients hospitalized in one of three hospitals in Minnesota.
Disclosures: The presenter reported no relevant financial disclosures.
Source: Steffens A et al. Abstract 885.
Does Treating Sleep Apnea Improve Seizure Outcomes?
WASHINGTON, DC—Treatment of obstructive sleep apnea (OSA) with positive airway pressure (PAP) is associated with better one-year seizure outcomes in patients with epilepsy, according to a study presented at the 71st Annual Meeting of the American Epilepsy Society.
Prior research has suggested that sleep disturbances are more common in people with epilepsy than in age-matched controls and that more than 40% of people with epilepsy have OSA. PAP therapy has been associated with seizure reduction in small case series.
To compare long-term seizure control between patients with PAP-treated OSA, patients with untreated OSA, and patients without OSA, Thapanee Somboon, MD, a research fellow at the Sleep Disorders Center at Cleveland Clinic, and colleagues conducted a retrospective study of adults with epilepsy who underwent polysomnography at Cleveland Clinic between 1997 and 2015. Researchers compared patients’ seizure outcomes at one, three, and five years after polysomnography.
The study included 197 people with epilepsy, 122 of whom had OSA (ie, an apnea–hypopnea index of 5 or greater). Of the patients with OSA, 73 received PAP therapy. Mean age was about 44, 58% were female, and 70% had focal epilepsy. Patients with OSA were more likely to be older, have a higher BMI, and be male than those without OSA.
At one year, 63% of patients treated with PAP had a 50% or greater reduction in seizures from baseline, compared with 14% of patients with OSA who were not treated and 44% of patients who did not have OSA. Researchers also assessed successful seizure outcomes, which were defined as not having seizures at baseline and remaining seizure-free for a year, or having seizures at baseline but reporting a 50% or greater reduction in seizures over one year. Successful outcomes occurred in 85% of patients who were treated with PAP, 55% of patients with OSA who were untreated, and 65% of patients who did not have OSA.
After adjusting for baseline seizure freedom and antiepileptic drug standardized dose, patients with treated OSA remained more likely to have successful outcomes at one year. Comparisons at three and five years included fewer patients, and the differences at those time points were not statistically significant.
—Jake Remaly
WASHINGTON, DC—Treatment of obstructive sleep apnea (OSA) with positive airway pressure (PAP) is associated with better one-year seizure outcomes in patients with epilepsy, according to a study presented at the 71st Annual Meeting of the American Epilepsy Society.
Prior research has suggested that sleep disturbances are more common in people with epilepsy than in age-matched controls and that more than 40% of people with epilepsy have OSA. PAP therapy has been associated with seizure reduction in small case series.
To compare long-term seizure control between patients with PAP-treated OSA, patients with untreated OSA, and patients without OSA, Thapanee Somboon, MD, a research fellow at the Sleep Disorders Center at Cleveland Clinic, and colleagues conducted a retrospective study of adults with epilepsy who underwent polysomnography at Cleveland Clinic between 1997 and 2015. Researchers compared patients’ seizure outcomes at one, three, and five years after polysomnography.
The study included 197 people with epilepsy, 122 of whom had OSA (ie, an apnea–hypopnea index of 5 or greater). Of the patients with OSA, 73 received PAP therapy. Mean age was about 44, 58% were female, and 70% had focal epilepsy. Patients with OSA were more likely to be older, have a higher BMI, and be male than those without OSA.
At one year, 63% of patients treated with PAP had a 50% or greater reduction in seizures from baseline, compared with 14% of patients with OSA who were not treated and 44% of patients who did not have OSA. Researchers also assessed successful seizure outcomes, which were defined as not having seizures at baseline and remaining seizure-free for a year, or having seizures at baseline but reporting a 50% or greater reduction in seizures over one year. Successful outcomes occurred in 85% of patients who were treated with PAP, 55% of patients with OSA who were untreated, and 65% of patients who did not have OSA.
After adjusting for baseline seizure freedom and antiepileptic drug standardized dose, patients with treated OSA remained more likely to have successful outcomes at one year. Comparisons at three and five years included fewer patients, and the differences at those time points were not statistically significant.
—Jake Remaly
WASHINGTON, DC—Treatment of obstructive sleep apnea (OSA) with positive airway pressure (PAP) is associated with better one-year seizure outcomes in patients with epilepsy, according to a study presented at the 71st Annual Meeting of the American Epilepsy Society.
Prior research has suggested that sleep disturbances are more common in people with epilepsy than in age-matched controls and that more than 40% of people with epilepsy have OSA. PAP therapy has been associated with seizure reduction in small case series.
To compare long-term seizure control between patients with PAP-treated OSA, patients with untreated OSA, and patients without OSA, Thapanee Somboon, MD, a research fellow at the Sleep Disorders Center at Cleveland Clinic, and colleagues conducted a retrospective study of adults with epilepsy who underwent polysomnography at Cleveland Clinic between 1997 and 2015. Researchers compared patients’ seizure outcomes at one, three, and five years after polysomnography.
The study included 197 people with epilepsy, 122 of whom had OSA (ie, an apnea–hypopnea index of 5 or greater). Of the patients with OSA, 73 received PAP therapy. Mean age was about 44, 58% were female, and 70% had focal epilepsy. Patients with OSA were more likely to be older, have a higher BMI, and be male than those without OSA.
At one year, 63% of patients treated with PAP had a 50% or greater reduction in seizures from baseline, compared with 14% of patients with OSA who were not treated and 44% of patients who did not have OSA. Researchers also assessed successful seizure outcomes, which were defined as not having seizures at baseline and remaining seizure-free for a year, or having seizures at baseline but reporting a 50% or greater reduction in seizures over one year. Successful outcomes occurred in 85% of patients who were treated with PAP, 55% of patients with OSA who were untreated, and 65% of patients who did not have OSA.
After adjusting for baseline seizure freedom and antiepileptic drug standardized dose, patients with treated OSA remained more likely to have successful outcomes at one year. Comparisons at three and five years included fewer patients, and the differences at those time points were not statistically significant.
—Jake Remaly
New curriculum teaches value-based health care
While value has become an imperative in both training and health care delivery, few tools exist to teach hospitalists and other providers the basic concepts of value.
“Hospitalists are on the front lines of health care value delivery, and it is critical that we understand and embrace the concepts of value; however, we also need to be able to deliver upon these ideals,” said Christopher Moriates, MD, assistant dean for health care value at the University of Texas at Austin.
“As a hospitalist, I ensured that the content would be specifically applicable to our day-to-day world and experience,” Dr. Moriates said. “Using the modules, hospitalists can better understand how emerging tools, such as the University of Utah’s Value-Drive Outcome tool, can be used by hospitalists to improve value. The modules also dig into thorny subjects like understanding health care costs – for example, what really is the difference between costs and charges?”
The course is adaptive and interactive, using the latest in instructional technology, he said. Hospitalists can take the course independently and earn free CME credits; those who complete all three modules in this first collection will receive a certificate of completion and CME credit.
The goal is to release 10 modules over the course of this academic year, Dr. Moriates said. Future collections will cover “value-based health care delivery,” “how to deliver high-value care at the bedside,” and “how to deliver high-value care in systems.”
“As value-based health care is increasingly taught in medical schools and residency training, it is important for hospitalists – especially any of us that work with trainees – to be able to speak the same language and understand what our trainees now will know,” he said.
While value has become an imperative in both training and health care delivery, few tools exist to teach hospitalists and other providers the basic concepts of value.
“Hospitalists are on the front lines of health care value delivery, and it is critical that we understand and embrace the concepts of value; however, we also need to be able to deliver upon these ideals,” said Christopher Moriates, MD, assistant dean for health care value at the University of Texas at Austin.
“As a hospitalist, I ensured that the content would be specifically applicable to our day-to-day world and experience,” Dr. Moriates said. “Using the modules, hospitalists can better understand how emerging tools, such as the University of Utah’s Value-Drive Outcome tool, can be used by hospitalists to improve value. The modules also dig into thorny subjects like understanding health care costs – for example, what really is the difference between costs and charges?”
The course is adaptive and interactive, using the latest in instructional technology, he said. Hospitalists can take the course independently and earn free CME credits; those who complete all three modules in this first collection will receive a certificate of completion and CME credit.
The goal is to release 10 modules over the course of this academic year, Dr. Moriates said. Future collections will cover “value-based health care delivery,” “how to deliver high-value care at the bedside,” and “how to deliver high-value care in systems.”
“As value-based health care is increasingly taught in medical schools and residency training, it is important for hospitalists – especially any of us that work with trainees – to be able to speak the same language and understand what our trainees now will know,” he said.
While value has become an imperative in both training and health care delivery, few tools exist to teach hospitalists and other providers the basic concepts of value.
“Hospitalists are on the front lines of health care value delivery, and it is critical that we understand and embrace the concepts of value; however, we also need to be able to deliver upon these ideals,” said Christopher Moriates, MD, assistant dean for health care value at the University of Texas at Austin.
“As a hospitalist, I ensured that the content would be specifically applicable to our day-to-day world and experience,” Dr. Moriates said. “Using the modules, hospitalists can better understand how emerging tools, such as the University of Utah’s Value-Drive Outcome tool, can be used by hospitalists to improve value. The modules also dig into thorny subjects like understanding health care costs – for example, what really is the difference between costs and charges?”
The course is adaptive and interactive, using the latest in instructional technology, he said. Hospitalists can take the course independently and earn free CME credits; those who complete all three modules in this first collection will receive a certificate of completion and CME credit.
The goal is to release 10 modules over the course of this academic year, Dr. Moriates said. Future collections will cover “value-based health care delivery,” “how to deliver high-value care at the bedside,” and “how to deliver high-value care in systems.”
“As value-based health care is increasingly taught in medical schools and residency training, it is important for hospitalists – especially any of us that work with trainees – to be able to speak the same language and understand what our trainees now will know,” he said.
Clinical trial: Study underway of robot-assisted surgery for pelvic prolapse
Robotic Assisted Sacral Colpopexy: A Prospective Study Assessing Outcomes With Learning Curves is an open-label study that is being conducted on a new pelvic floor program for women with pelvic organ prolapse.
A prospective cohort of 100 patients will be recruited and the study will assess surgical time (total and specific essential portions), simulator training, and observed surgeon skills. Secondary endpoints include subjective outcomes for issues of sexual function and incontinence and adverse events such as genitourinary injury, blood loss, wound infection, and mesh erosion.
Kaiser Permanente is the trial sponsor, and patients aged 18-80 years who are undergoing robotic-assisted laparoscopic sacrocolpopexy with or without other procedures for pelvic organ prolapse are being recruited. For more details about the trial, visit https://goo.gl/pWq7qe.
SOURCE: ClinicalTrials.gov: NCT01535833.
Robotic Assisted Sacral Colpopexy: A Prospective Study Assessing Outcomes With Learning Curves is an open-label study that is being conducted on a new pelvic floor program for women with pelvic organ prolapse.
A prospective cohort of 100 patients will be recruited and the study will assess surgical time (total and specific essential portions), simulator training, and observed surgeon skills. Secondary endpoints include subjective outcomes for issues of sexual function and incontinence and adverse events such as genitourinary injury, blood loss, wound infection, and mesh erosion.
Kaiser Permanente is the trial sponsor, and patients aged 18-80 years who are undergoing robotic-assisted laparoscopic sacrocolpopexy with or without other procedures for pelvic organ prolapse are being recruited. For more details about the trial, visit https://goo.gl/pWq7qe.
SOURCE: ClinicalTrials.gov: NCT01535833.
Robotic Assisted Sacral Colpopexy: A Prospective Study Assessing Outcomes With Learning Curves is an open-label study that is being conducted on a new pelvic floor program for women with pelvic organ prolapse.
A prospective cohort of 100 patients will be recruited and the study will assess surgical time (total and specific essential portions), simulator training, and observed surgeon skills. Secondary endpoints include subjective outcomes for issues of sexual function and incontinence and adverse events such as genitourinary injury, blood loss, wound infection, and mesh erosion.
Kaiser Permanente is the trial sponsor, and patients aged 18-80 years who are undergoing robotic-assisted laparoscopic sacrocolpopexy with or without other procedures for pelvic organ prolapse are being recruited. For more details about the trial, visit https://goo.gl/pWq7qe.
SOURCE: ClinicalTrials.gov: NCT01535833.
SUMMARY FROM CLINICALTRIALS.GOV
Timing of Surgical Reduction and Stabilization of Talus Fracture-Dislocations
Take-Home Points
- There is a 41% rate of AVN or PTOA after operatively managed talus fracture.
- Surgical timing does not affect development of AVN or PTOA.
- Open fractures are associated with development of AVN and PTOA.
- Quality of reduction is likely more important than timing of reduction.
- Urgent surgical treatment is necessary for threatened soft tissue or neurovascular compromise.
Talus fractures are rare injuries that present a significant treatment dilemma.1-12 These fractures represent <1% of all fractures4 and are second only to calcaneus fractures in fractures of the hindfoot. Talus fractures with associated dislocations are even rarer and may provide treating surgeons with a significant surgical quandary.6,13-16
Talus fractures historically have been characterized by their anatomical location: head, neck, or body. Two systems are commonly used to classify talus fractures: Hawkins and AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association). The first, developed by Hawkins7 and modified by Canale and Kelly2 and Vallier and colleagues,1 identifies 4 basic fracture types with associated dislocations. The other system, published in 199617 and republished in 2007,18 uses the combined methods of AO and OTA to systematically describe talus fractures. Although these classification systems accurately describe talus fractures with associated dislocation, both have difficulty predicting clinical outcomes.1,19,20
Talus fractures commonly result in avascular necrosis (AVN) of the talus and posttraumatic osteoarthritis (PTOA) of the tibiotalar and subtalar joints.3,8,9,12,14-16 Hawkins7 initially described subchondral lucency as indicating revascularization of the talus after injury. AVN and PTOA rates traditionally have been thought to be related to a blood supply disruption, given the prognostic value of the Hawkins sign.1,7,12,21 New methods, including a dual-incision approach and expedited transfer to foot and ankle surgeons or orthopedic traumatologists, have improved reduction quality21-24 but not patient outcomes.3,5,8,9,12,14
Recently, time from injury to surgical intervention has been a topic of much discussion, and there have been studies on the specific effects of timing with respect to outcome.1,15,16 Vallier and colleagues,1 who wanted to identify injury characteristics predictive of osteonecrosis, found that delaying reduction and surgical fixation did not increase the risk of AVN. Another study found that urgent reduction of fracture-dislocation with delayed open reduction and internal fixation (ORIF) using a dual approach may improve clinical outcomes.21
In this vein, we conducted a study to evaluate the effect of time to surgical reduction of talus fractures and talus fracture-dislocations on the development of AVN and PTOA. We hypothesized that time to surgical reduction of talus fracture-dislocation as classified with the AO/OTA system would have no effect of the development of AVN/PTOA.
Methods
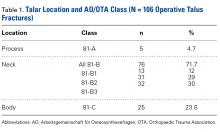
After this study received Institutional Review Board approval, we retrospectively reviewed the records on talus fractures surgically managed at a level I trauma center during the 10-year period 2003 to 2013. Of the 119 potential cases identified using Current Procedural Terminology code 28445 (ORIF of talus), 13 were excluded (12 for inaccurate coding or missing documentation, 1 for being a pediatric case), leaving 106 for analysis. Using the Hawkins and AO/OTA systems, 3 independent reviewers classified the injuries on plain radiographs.
Injury dates and times were obtained from the medical records. Operating room start times were also obtained. Surgical timing was defined as time from injury to operating room start. For cases without an injury time, time of presentation to emergency department was used.
Open fracture-dislocations were managed with intravenous antibiotics, urgent surgical irrigation, débridement, and immediate fixation or temporizing external fixation after reduction. All fractures were definitively managed with standard ORIF with an anteromedial, anterolateral, or dual approach and mini-fragment implants. After fixation, weight-bearing typically was restricted for 6 to 12 weeks.
Follow-up radiographs were evaluated. Presence or absence of Hawkins sign7 was noted on radiographs at 6 or 8 weeks, and all follow-up radiographs were evaluated for AVN as defined by increased radiographic density within the talar dome or collapse of the articular surface. All radiographs were evaluated for PTOA as defined by loss of joint space within the tibiotalar, subtalar, or talonavicular joint on follow-up radiographs.
Clinical outcomes were analyzed for development of AVN, PTOA, or secondary corrective surgery or arthrodesis. Continuous variables were evaluated with the t test, and the χ2 test was used to compare distributions of categorical variables. The Wilcoxon rank sum test was used to compare non-normally distributed variables. Significance was set at P < .05.
Results
Classification Analysis (Table 1)
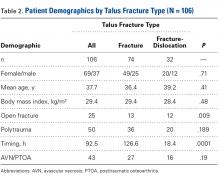
Subject Analysis (Table 2)
The mechanisms of injury were motor vehicle accident (70/106; 66%), fall from height (25; 24%), misstep (4), sports related (2), object falling on ankle (2), and not reported (3).
Of the 106 patients, 45 (42%) had isolated talus injuries, 35 had concomitant ipsilateral lower extremity injuries, 25 had concomitant contralateral lower extremity injuries, and 1 had a concomitant upper extremity injury.
Smoking status was everyday (14 patients), past (10), never (34), and unreported (48). Five patients reported a history of alcohol abuse, and 4 patients reported illicit drug use. Two had a history of atrial fibrillation, 9 had hypertension, 3 had hyperlipidemia, 3 had renal disease, 3 had heart disease, 4 had diabetes, 3 had lung disease, and 1 had a history of lung cancer.
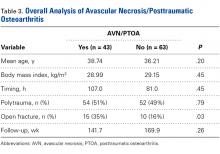
Overall Analysis of AVN/PTOA (Table 3)
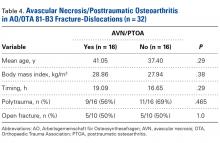
Analysis of AVN/PTOA in 81-B3 Fracture-Dislocations (Table 4)
Analysis of AVN/PTOA in All Other Talus Fractures (Table 5)
Discussion
Our results showed that time from talus fracture-dislocation to surgical reduction had no effect on development of AVN/PTOA. The findings in this largest series to date agree with earlier findings1,8,15,16,24 and add to the volume of literature suggesting that time to surgical reduction of talus fractures and talus fracture-dislocations does not markedly affect outcome.
Talus fractures continue to present a significant treatment dilemma. Despite recent improvements in surgical techniques and overall management of these injuries, rates of AVN and PTOA have not significantly decreased.1,16,23 At most treating facilities, talus fracture-dislocations are considered surgical emergencies/urgencies, and every effort is made to reduce and surgically address these injuries as soon as possible.1,13
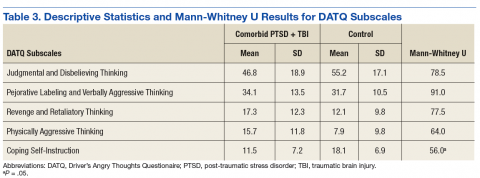
In this study, rates of AVN/PTOA were 41% (all talus fractures) and 50% (displaced talar neck fractures), and the difference was not significant (Table 3). These rates are higher but consistent with previously reported rates (range, 14%-49%).1,2,7-9,12,14,24 There was no difference in surgical timing for development of AVN/PTOA. We analyzed the cases of all patients who had talus fractures and developed AVN/PTOA (43/106). Within this group, there were no significant differences in surgical timing, age, sex, polytrauma, or BMI between patients who developed AVN/PTOA and those who did not. Compared with patients who did not develop AVN/PTOA, those who developed AVN/PTOA were significantly more likely to have open injuries. This finding, consistent with those in other reports9,12,13 (Table 3), indicates outcome is more likely related to injury severity and not necessarily injury class.
We retrospectively analyzed talus fractures and talus fracture-dislocations to determine if urgent surgical management affects outcomes. Current practice at our institution is to routinely reduce and surgically address these fractures urgently, often during the middle of the night, when orthopedic resources are reduced. Our study found a significant difference in surgical timing for patients with talus fracture-dislocations and patients with talus fractures without dislocations (Table 2). Given our findings, urgent surgical reduction and fixation are not indicated to preserve the talus blood supply and prevent AVN/PTOA, though we still recommend urgent surgical management in the setting of an open wound, skin necrosis, or soft-tissue/neurovascular compromise.
This study had several limitations, primarily related to its retrospective nature. Surgical timing was defined as time from injury, as noted in the medical record, to operating room start. In some instances, time of injury was not noted in the medical record, and time of presentation to emergency room was used instead. Thus, surgical timing for these patients may have been longer than identified. In addition, given the rare injury pattern and the retrospective design, this study was susceptible to type II error and may have been underpowered to detect whether time to surgical reduction predicted complications. Also, the study did not address functional outcome as measured by validated outcome scores. Outcome measures were obtained in many but not all cases, making functional outcome measurement difficult. Similarly, the quality of the anatomical reductions was not assessed, potentially affecting complication rates. Postoperative reduction assessment, possibly performed with computed tomography, is an avenue of further study.
Strengths of this study include its large sample size (this was one of the largest studies of talus fractures), long follow-up (mean, 150 weeks), and novel use of AO/OTA classification.
We postulate that development of AVN/PTOA is not necessarily related to the urgency or timing of surgical reduction and fixation and is more likely related to injury severity. This idea is supported by the finding that development of AVN/PTOA was significantly correlated to open injuries in all talus fractures, including talus fracture-dislocations and isolated talus fractures.
Conclusion
Talus fracture-dislocations are devastating injuries with high rates of complications. In this study, open talus fractures, and fractures with associated tibiotalar or subtalar dislocations, had higher complication rates. Given the evidence presented, we recommend basing surgical timing on injury severity, not necessarily for AVN/PTOA prevention. Specifically, in the absence of an open wound, skin necrosis, or soft-tissue/neurovascular compromise, talus fracture-dislocations can be surgically reduced and stabilized when optimal resources are available.
1. Vallier HA, Reichard SG, Boyd AJ, Moore TA. A new look at the Hawkins classification for talar neck fractures: which features of injury and treatment are predictive of osteonecrosis? J Bone Joint Surg Am. 2014;96(3):192-197.
2. Canale ST, Kelly FB Jr. Fractures of the neck of the talus. Long-term evaluation of seventy-one cases. J Bone Joint Surg Am. 1978;60(2):143-156.
3. Ebraheim NA, Patil V, Owens C, Kandimalla Y. Clinical outcome of fractures of the talar body. Int Orthop. 2008;32(6):773-777.
4. Fortin PT, Balazsy JE. Talus fractures: evaluation and treatment. J Am Acad Orthop Surg. 2001;9(2):114-127.
5. Fournier A, Barba N, Steiger V, et al. Total talar fracture—long-term results of internal fixation of talar fractures. A multicentric study of 114 cases. Orthop Traumatol Surg Res. 2012;98(4 suppl):S48-S55.
6. Grob D, Simpson LA, Weber BG, Bray T. Operative treatment of displaced talus fractures. Clin Orthop Relat Res. 1985;(199):88-96.
7. Hawkins LG. Fractures of the neck of the talus. J Bone Joint Surg Am. 1970;52(5):991-1002.
8. Lindvall E, Haidukewych G, DiPasquale T, Herscovici D Jr, Sanders R. Open reduction and stable fixation of isolated, displaced talar neck and body fractures. J Bone Joint Surg Am. 2004;86(10):2229-2234.
9. Ohl X, Harisboure A, Hemery X, Dehoux E. Long-term follow-up after surgical treatment of talar fractures: twenty cases with an average follow-up of 7.5 years. Int Orthop. 2011;35(1):93-99.
10. Rammelt S, Zwipp H. Talar neck and body fractures. Injury. 2009;40(2):120-135.
11. Schulze W, Richter J, Russe O, Ingelfinger P, Muhr G. Surgical treatment of talus fractures: a retrospective study of 80 cases followed for 1-15 years. Acta Orthop Scand. 2002;73(3):344-351.
12. Vallier HA, Nork SE, Barei DP, Benirschke SK, Sangeorzan BJ. Talar neck fractures: results and outcomes. J Bone Joint Surg Am. 2004;86(8):1616-1624.
13. Patel R, Van Bergeyk A, Pinney S. Are displaced talar neck fractures surgical emergencies? A survey of orthopaedic trauma experts. Foot Ankle Int. 2005;26(5):378-381.
14. Sanders DW, Busam M, Hattwick E, Edwards JR, McAndrew MP, Johnson KD. Functional outcomes following displaced talar neck fractures. J Orthop Trauma. 2004;18(5):265-270.
15. Elgafy H, Ebraheim NA, Tile M, Stephen D, Kase J. Fractures of the talus: experience of two level 1 trauma centers. Foot Ankle Int. 2000;21(12):1023-1029.
16 Frawley PA, Hart JA, Young DA. Treatment outcome of major fractures of the talus. Foot Ankle Int. 1995;16(6):339-345.
17. Fracture and dislocation compendium. Orthopaedic Trauma Association committee for coding and classification. J Orthop Trauma. 1996;10(suppl 1):v-ix, 1-154.
18. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
19. Williams T, Barba N, Noailles T, et al. Total talar fracture—inter- and intra-observer reproducibility of two classification systems (Hawkins and AO) for central talar fractures. Orthop Traumatol Surg Res. 2012;98(4 suppl):S56-S65.
20. Zwipp H, Baumgart F, Cronier P, et al. Integral classification of injuries (ICI) to the bones, joints, and ligaments—application to injuries of the foot. Injury. 2004;35(suppl 2):SB3-SB9.
21. Xue Y, Zhang H, Pei F, et al. Treatment of displaced talar neck fractures using delayed procedures of plate fixation through dual approaches. Int Orthop. 2014;38(1):149-154.
22. Vallier HA, Nork SE, Benirschke SK, Sangeorzan BJ. Surgical treatment of talar body fractures. J Bone Joint Surg Am. 2003;85(9):1716-1724.
23. Fleuriau Chateau PB, Brokaw DS, Jelen BA, Scheid DK, Weber TG. Plate fixation of talar neck fractures: preliminary review of a new technique in twenty-three patients. J Orthop Trauma. 2002;16(4):213-219.
24. Vallier HA, Nork SE, Benirschke SK, Sangeorzan BJ. Surgical treatment of talar body fractures. J Bone Joint Surg Am. 2004;86(suppl 1, pt 2):180-192.
Take-Home Points
- There is a 41% rate of AVN or PTOA after operatively managed talus fracture.
- Surgical timing does not affect development of AVN or PTOA.
- Open fractures are associated with development of AVN and PTOA.
- Quality of reduction is likely more important than timing of reduction.
- Urgent surgical treatment is necessary for threatened soft tissue or neurovascular compromise.
Talus fractures are rare injuries that present a significant treatment dilemma.1-12 These fractures represent <1% of all fractures4 and are second only to calcaneus fractures in fractures of the hindfoot. Talus fractures with associated dislocations are even rarer and may provide treating surgeons with a significant surgical quandary.6,13-16
Talus fractures historically have been characterized by their anatomical location: head, neck, or body. Two systems are commonly used to classify talus fractures: Hawkins and AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association). The first, developed by Hawkins7 and modified by Canale and Kelly2 and Vallier and colleagues,1 identifies 4 basic fracture types with associated dislocations. The other system, published in 199617 and republished in 2007,18 uses the combined methods of AO and OTA to systematically describe talus fractures. Although these classification systems accurately describe talus fractures with associated dislocation, both have difficulty predicting clinical outcomes.1,19,20
Talus fractures commonly result in avascular necrosis (AVN) of the talus and posttraumatic osteoarthritis (PTOA) of the tibiotalar and subtalar joints.3,8,9,12,14-16 Hawkins7 initially described subchondral lucency as indicating revascularization of the talus after injury. AVN and PTOA rates traditionally have been thought to be related to a blood supply disruption, given the prognostic value of the Hawkins sign.1,7,12,21 New methods, including a dual-incision approach and expedited transfer to foot and ankle surgeons or orthopedic traumatologists, have improved reduction quality21-24 but not patient outcomes.3,5,8,9,12,14
Recently, time from injury to surgical intervention has been a topic of much discussion, and there have been studies on the specific effects of timing with respect to outcome.1,15,16 Vallier and colleagues,1 who wanted to identify injury characteristics predictive of osteonecrosis, found that delaying reduction and surgical fixation did not increase the risk of AVN. Another study found that urgent reduction of fracture-dislocation with delayed open reduction and internal fixation (ORIF) using a dual approach may improve clinical outcomes.21
In this vein, we conducted a study to evaluate the effect of time to surgical reduction of talus fractures and talus fracture-dislocations on the development of AVN and PTOA. We hypothesized that time to surgical reduction of talus fracture-dislocation as classified with the AO/OTA system would have no effect of the development of AVN/PTOA.
Methods
After this study received Institutional Review Board approval, we retrospectively reviewed the records on talus fractures surgically managed at a level I trauma center during the 10-year period 2003 to 2013. Of the 119 potential cases identified using Current Procedural Terminology code 28445 (ORIF of talus), 13 were excluded (12 for inaccurate coding or missing documentation, 1 for being a pediatric case), leaving 106 for analysis. Using the Hawkins and AO/OTA systems, 3 independent reviewers classified the injuries on plain radiographs.
Injury dates and times were obtained from the medical records. Operating room start times were also obtained. Surgical timing was defined as time from injury to operating room start. For cases without an injury time, time of presentation to emergency department was used.
Open fracture-dislocations were managed with intravenous antibiotics, urgent surgical irrigation, débridement, and immediate fixation or temporizing external fixation after reduction. All fractures were definitively managed with standard ORIF with an anteromedial, anterolateral, or dual approach and mini-fragment implants. After fixation, weight-bearing typically was restricted for 6 to 12 weeks.
Follow-up radiographs were evaluated. Presence or absence of Hawkins sign7 was noted on radiographs at 6 or 8 weeks, and all follow-up radiographs were evaluated for AVN as defined by increased radiographic density within the talar dome or collapse of the articular surface. All radiographs were evaluated for PTOA as defined by loss of joint space within the tibiotalar, subtalar, or talonavicular joint on follow-up radiographs.
Clinical outcomes were analyzed for development of AVN, PTOA, or secondary corrective surgery or arthrodesis. Continuous variables were evaluated with the t test, and the χ2 test was used to compare distributions of categorical variables. The Wilcoxon rank sum test was used to compare non-normally distributed variables. Significance was set at P < .05.
Results
Classification Analysis (Table 1)
Subject Analysis (Table 2)
The mechanisms of injury were motor vehicle accident (70/106; 66%), fall from height (25; 24%), misstep (4), sports related (2), object falling on ankle (2), and not reported (3).
Of the 106 patients, 45 (42%) had isolated talus injuries, 35 had concomitant ipsilateral lower extremity injuries, 25 had concomitant contralateral lower extremity injuries, and 1 had a concomitant upper extremity injury.
Smoking status was everyday (14 patients), past (10), never (34), and unreported (48). Five patients reported a history of alcohol abuse, and 4 patients reported illicit drug use. Two had a history of atrial fibrillation, 9 had hypertension, 3 had hyperlipidemia, 3 had renal disease, 3 had heart disease, 4 had diabetes, 3 had lung disease, and 1 had a history of lung cancer.
Overall Analysis of AVN/PTOA (Table 3)
Analysis of AVN/PTOA in 81-B3 Fracture-Dislocations (Table 4)
Analysis of AVN/PTOA in All Other Talus Fractures (Table 5)
Discussion
Our results showed that time from talus fracture-dislocation to surgical reduction had no effect on development of AVN/PTOA. The findings in this largest series to date agree with earlier findings1,8,15,16,24 and add to the volume of literature suggesting that time to surgical reduction of talus fractures and talus fracture-dislocations does not markedly affect outcome.
Talus fractures continue to present a significant treatment dilemma. Despite recent improvements in surgical techniques and overall management of these injuries, rates of AVN and PTOA have not significantly decreased.1,16,23 At most treating facilities, talus fracture-dislocations are considered surgical emergencies/urgencies, and every effort is made to reduce and surgically address these injuries as soon as possible.1,13
In this study, rates of AVN/PTOA were 41% (all talus fractures) and 50% (displaced talar neck fractures), and the difference was not significant (Table 3). These rates are higher but consistent with previously reported rates (range, 14%-49%).1,2,7-9,12,14,24 There was no difference in surgical timing for development of AVN/PTOA. We analyzed the cases of all patients who had talus fractures and developed AVN/PTOA (43/106). Within this group, there were no significant differences in surgical timing, age, sex, polytrauma, or BMI between patients who developed AVN/PTOA and those who did not. Compared with patients who did not develop AVN/PTOA, those who developed AVN/PTOA were significantly more likely to have open injuries. This finding, consistent with those in other reports9,12,13 (Table 3), indicates outcome is more likely related to injury severity and not necessarily injury class.
We retrospectively analyzed talus fractures and talus fracture-dislocations to determine if urgent surgical management affects outcomes. Current practice at our institution is to routinely reduce and surgically address these fractures urgently, often during the middle of the night, when orthopedic resources are reduced. Our study found a significant difference in surgical timing for patients with talus fracture-dislocations and patients with talus fractures without dislocations (Table 2). Given our findings, urgent surgical reduction and fixation are not indicated to preserve the talus blood supply and prevent AVN/PTOA, though we still recommend urgent surgical management in the setting of an open wound, skin necrosis, or soft-tissue/neurovascular compromise.
This study had several limitations, primarily related to its retrospective nature. Surgical timing was defined as time from injury, as noted in the medical record, to operating room start. In some instances, time of injury was not noted in the medical record, and time of presentation to emergency room was used instead. Thus, surgical timing for these patients may have been longer than identified. In addition, given the rare injury pattern and the retrospective design, this study was susceptible to type II error and may have been underpowered to detect whether time to surgical reduction predicted complications. Also, the study did not address functional outcome as measured by validated outcome scores. Outcome measures were obtained in many but not all cases, making functional outcome measurement difficult. Similarly, the quality of the anatomical reductions was not assessed, potentially affecting complication rates. Postoperative reduction assessment, possibly performed with computed tomography, is an avenue of further study.
Strengths of this study include its large sample size (this was one of the largest studies of talus fractures), long follow-up (mean, 150 weeks), and novel use of AO/OTA classification.
We postulate that development of AVN/PTOA is not necessarily related to the urgency or timing of surgical reduction and fixation and is more likely related to injury severity. This idea is supported by the finding that development of AVN/PTOA was significantly correlated to open injuries in all talus fractures, including talus fracture-dislocations and isolated talus fractures.
Conclusion
Talus fracture-dislocations are devastating injuries with high rates of complications. In this study, open talus fractures, and fractures with associated tibiotalar or subtalar dislocations, had higher complication rates. Given the evidence presented, we recommend basing surgical timing on injury severity, not necessarily for AVN/PTOA prevention. Specifically, in the absence of an open wound, skin necrosis, or soft-tissue/neurovascular compromise, talus fracture-dislocations can be surgically reduced and stabilized when optimal resources are available.
Take-Home Points
- There is a 41% rate of AVN or PTOA after operatively managed talus fracture.
- Surgical timing does not affect development of AVN or PTOA.
- Open fractures are associated with development of AVN and PTOA.
- Quality of reduction is likely more important than timing of reduction.
- Urgent surgical treatment is necessary for threatened soft tissue or neurovascular compromise.
Talus fractures are rare injuries that present a significant treatment dilemma.1-12 These fractures represent <1% of all fractures4 and are second only to calcaneus fractures in fractures of the hindfoot. Talus fractures with associated dislocations are even rarer and may provide treating surgeons with a significant surgical quandary.6,13-16
Talus fractures historically have been characterized by their anatomical location: head, neck, or body. Two systems are commonly used to classify talus fractures: Hawkins and AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association). The first, developed by Hawkins7 and modified by Canale and Kelly2 and Vallier and colleagues,1 identifies 4 basic fracture types with associated dislocations. The other system, published in 199617 and republished in 2007,18 uses the combined methods of AO and OTA to systematically describe talus fractures. Although these classification systems accurately describe talus fractures with associated dislocation, both have difficulty predicting clinical outcomes.1,19,20
Talus fractures commonly result in avascular necrosis (AVN) of the talus and posttraumatic osteoarthritis (PTOA) of the tibiotalar and subtalar joints.3,8,9,12,14-16 Hawkins7 initially described subchondral lucency as indicating revascularization of the talus after injury. AVN and PTOA rates traditionally have been thought to be related to a blood supply disruption, given the prognostic value of the Hawkins sign.1,7,12,21 New methods, including a dual-incision approach and expedited transfer to foot and ankle surgeons or orthopedic traumatologists, have improved reduction quality21-24 but not patient outcomes.3,5,8,9,12,14
Recently, time from injury to surgical intervention has been a topic of much discussion, and there have been studies on the specific effects of timing with respect to outcome.1,15,16 Vallier and colleagues,1 who wanted to identify injury characteristics predictive of osteonecrosis, found that delaying reduction and surgical fixation did not increase the risk of AVN. Another study found that urgent reduction of fracture-dislocation with delayed open reduction and internal fixation (ORIF) using a dual approach may improve clinical outcomes.21
In this vein, we conducted a study to evaluate the effect of time to surgical reduction of talus fractures and talus fracture-dislocations on the development of AVN and PTOA. We hypothesized that time to surgical reduction of talus fracture-dislocation as classified with the AO/OTA system would have no effect of the development of AVN/PTOA.
Methods
After this study received Institutional Review Board approval, we retrospectively reviewed the records on talus fractures surgically managed at a level I trauma center during the 10-year period 2003 to 2013. Of the 119 potential cases identified using Current Procedural Terminology code 28445 (ORIF of talus), 13 were excluded (12 for inaccurate coding or missing documentation, 1 for being a pediatric case), leaving 106 for analysis. Using the Hawkins and AO/OTA systems, 3 independent reviewers classified the injuries on plain radiographs.
Injury dates and times were obtained from the medical records. Operating room start times were also obtained. Surgical timing was defined as time from injury to operating room start. For cases without an injury time, time of presentation to emergency department was used.
Open fracture-dislocations were managed with intravenous antibiotics, urgent surgical irrigation, débridement, and immediate fixation or temporizing external fixation after reduction. All fractures were definitively managed with standard ORIF with an anteromedial, anterolateral, or dual approach and mini-fragment implants. After fixation, weight-bearing typically was restricted for 6 to 12 weeks.
Follow-up radiographs were evaluated. Presence or absence of Hawkins sign7 was noted on radiographs at 6 or 8 weeks, and all follow-up radiographs were evaluated for AVN as defined by increased radiographic density within the talar dome or collapse of the articular surface. All radiographs were evaluated for PTOA as defined by loss of joint space within the tibiotalar, subtalar, or talonavicular joint on follow-up radiographs.
Clinical outcomes were analyzed for development of AVN, PTOA, or secondary corrective surgery or arthrodesis. Continuous variables were evaluated with the t test, and the χ2 test was used to compare distributions of categorical variables. The Wilcoxon rank sum test was used to compare non-normally distributed variables. Significance was set at P < .05.
Results
Classification Analysis (Table 1)
Subject Analysis (Table 2)
The mechanisms of injury were motor vehicle accident (70/106; 66%), fall from height (25; 24%), misstep (4), sports related (2), object falling on ankle (2), and not reported (3).
Of the 106 patients, 45 (42%) had isolated talus injuries, 35 had concomitant ipsilateral lower extremity injuries, 25 had concomitant contralateral lower extremity injuries, and 1 had a concomitant upper extremity injury.
Smoking status was everyday (14 patients), past (10), never (34), and unreported (48). Five patients reported a history of alcohol abuse, and 4 patients reported illicit drug use. Two had a history of atrial fibrillation, 9 had hypertension, 3 had hyperlipidemia, 3 had renal disease, 3 had heart disease, 4 had diabetes, 3 had lung disease, and 1 had a history of lung cancer.
Overall Analysis of AVN/PTOA (Table 3)
Analysis of AVN/PTOA in 81-B3 Fracture-Dislocations (Table 4)
Analysis of AVN/PTOA in All Other Talus Fractures (Table 5)
Discussion
Our results showed that time from talus fracture-dislocation to surgical reduction had no effect on development of AVN/PTOA. The findings in this largest series to date agree with earlier findings1,8,15,16,24 and add to the volume of literature suggesting that time to surgical reduction of talus fractures and talus fracture-dislocations does not markedly affect outcome.
Talus fractures continue to present a significant treatment dilemma. Despite recent improvements in surgical techniques and overall management of these injuries, rates of AVN and PTOA have not significantly decreased.1,16,23 At most treating facilities, talus fracture-dislocations are considered surgical emergencies/urgencies, and every effort is made to reduce and surgically address these injuries as soon as possible.1,13
In this study, rates of AVN/PTOA were 41% (all talus fractures) and 50% (displaced talar neck fractures), and the difference was not significant (Table 3). These rates are higher but consistent with previously reported rates (range, 14%-49%).1,2,7-9,12,14,24 There was no difference in surgical timing for development of AVN/PTOA. We analyzed the cases of all patients who had talus fractures and developed AVN/PTOA (43/106). Within this group, there were no significant differences in surgical timing, age, sex, polytrauma, or BMI between patients who developed AVN/PTOA and those who did not. Compared with patients who did not develop AVN/PTOA, those who developed AVN/PTOA were significantly more likely to have open injuries. This finding, consistent with those in other reports9,12,13 (Table 3), indicates outcome is more likely related to injury severity and not necessarily injury class.
We retrospectively analyzed talus fractures and talus fracture-dislocations to determine if urgent surgical management affects outcomes. Current practice at our institution is to routinely reduce and surgically address these fractures urgently, often during the middle of the night, when orthopedic resources are reduced. Our study found a significant difference in surgical timing for patients with talus fracture-dislocations and patients with talus fractures without dislocations (Table 2). Given our findings, urgent surgical reduction and fixation are not indicated to preserve the talus blood supply and prevent AVN/PTOA, though we still recommend urgent surgical management in the setting of an open wound, skin necrosis, or soft-tissue/neurovascular compromise.
This study had several limitations, primarily related to its retrospective nature. Surgical timing was defined as time from injury, as noted in the medical record, to operating room start. In some instances, time of injury was not noted in the medical record, and time of presentation to emergency room was used instead. Thus, surgical timing for these patients may have been longer than identified. In addition, given the rare injury pattern and the retrospective design, this study was susceptible to type II error and may have been underpowered to detect whether time to surgical reduction predicted complications. Also, the study did not address functional outcome as measured by validated outcome scores. Outcome measures were obtained in many but not all cases, making functional outcome measurement difficult. Similarly, the quality of the anatomical reductions was not assessed, potentially affecting complication rates. Postoperative reduction assessment, possibly performed with computed tomography, is an avenue of further study.
Strengths of this study include its large sample size (this was one of the largest studies of talus fractures), long follow-up (mean, 150 weeks), and novel use of AO/OTA classification.
We postulate that development of AVN/PTOA is not necessarily related to the urgency or timing of surgical reduction and fixation and is more likely related to injury severity. This idea is supported by the finding that development of AVN/PTOA was significantly correlated to open injuries in all talus fractures, including talus fracture-dislocations and isolated talus fractures.
Conclusion
Talus fracture-dislocations are devastating injuries with high rates of complications. In this study, open talus fractures, and fractures with associated tibiotalar or subtalar dislocations, had higher complication rates. Given the evidence presented, we recommend basing surgical timing on injury severity, not necessarily for AVN/PTOA prevention. Specifically, in the absence of an open wound, skin necrosis, or soft-tissue/neurovascular compromise, talus fracture-dislocations can be surgically reduced and stabilized when optimal resources are available.
1. Vallier HA, Reichard SG, Boyd AJ, Moore TA. A new look at the Hawkins classification for talar neck fractures: which features of injury and treatment are predictive of osteonecrosis? J Bone Joint Surg Am. 2014;96(3):192-197.
2. Canale ST, Kelly FB Jr. Fractures of the neck of the talus. Long-term evaluation of seventy-one cases. J Bone Joint Surg Am. 1978;60(2):143-156.
3. Ebraheim NA, Patil V, Owens C, Kandimalla Y. Clinical outcome of fractures of the talar body. Int Orthop. 2008;32(6):773-777.
4. Fortin PT, Balazsy JE. Talus fractures: evaluation and treatment. J Am Acad Orthop Surg. 2001;9(2):114-127.
5. Fournier A, Barba N, Steiger V, et al. Total talar fracture—long-term results of internal fixation of talar fractures. A multicentric study of 114 cases. Orthop Traumatol Surg Res. 2012;98(4 suppl):S48-S55.
6. Grob D, Simpson LA, Weber BG, Bray T. Operative treatment of displaced talus fractures. Clin Orthop Relat Res. 1985;(199):88-96.
7. Hawkins LG. Fractures of the neck of the talus. J Bone Joint Surg Am. 1970;52(5):991-1002.
8. Lindvall E, Haidukewych G, DiPasquale T, Herscovici D Jr, Sanders R. Open reduction and stable fixation of isolated, displaced talar neck and body fractures. J Bone Joint Surg Am. 2004;86(10):2229-2234.
9. Ohl X, Harisboure A, Hemery X, Dehoux E. Long-term follow-up after surgical treatment of talar fractures: twenty cases with an average follow-up of 7.5 years. Int Orthop. 2011;35(1):93-99.
10. Rammelt S, Zwipp H. Talar neck and body fractures. Injury. 2009;40(2):120-135.
11. Schulze W, Richter J, Russe O, Ingelfinger P, Muhr G. Surgical treatment of talus fractures: a retrospective study of 80 cases followed for 1-15 years. Acta Orthop Scand. 2002;73(3):344-351.
12. Vallier HA, Nork SE, Barei DP, Benirschke SK, Sangeorzan BJ. Talar neck fractures: results and outcomes. J Bone Joint Surg Am. 2004;86(8):1616-1624.
13. Patel R, Van Bergeyk A, Pinney S. Are displaced talar neck fractures surgical emergencies? A survey of orthopaedic trauma experts. Foot Ankle Int. 2005;26(5):378-381.
14. Sanders DW, Busam M, Hattwick E, Edwards JR, McAndrew MP, Johnson KD. Functional outcomes following displaced talar neck fractures. J Orthop Trauma. 2004;18(5):265-270.
15. Elgafy H, Ebraheim NA, Tile M, Stephen D, Kase J. Fractures of the talus: experience of two level 1 trauma centers. Foot Ankle Int. 2000;21(12):1023-1029.
16 Frawley PA, Hart JA, Young DA. Treatment outcome of major fractures of the talus. Foot Ankle Int. 1995;16(6):339-345.
17. Fracture and dislocation compendium. Orthopaedic Trauma Association committee for coding and classification. J Orthop Trauma. 1996;10(suppl 1):v-ix, 1-154.
18. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
19. Williams T, Barba N, Noailles T, et al. Total talar fracture—inter- and intra-observer reproducibility of two classification systems (Hawkins and AO) for central talar fractures. Orthop Traumatol Surg Res. 2012;98(4 suppl):S56-S65.
20. Zwipp H, Baumgart F, Cronier P, et al. Integral classification of injuries (ICI) to the bones, joints, and ligaments—application to injuries of the foot. Injury. 2004;35(suppl 2):SB3-SB9.
21. Xue Y, Zhang H, Pei F, et al. Treatment of displaced talar neck fractures using delayed procedures of plate fixation through dual approaches. Int Orthop. 2014;38(1):149-154.
22. Vallier HA, Nork SE, Benirschke SK, Sangeorzan BJ. Surgical treatment of talar body fractures. J Bone Joint Surg Am. 2003;85(9):1716-1724.
23. Fleuriau Chateau PB, Brokaw DS, Jelen BA, Scheid DK, Weber TG. Plate fixation of talar neck fractures: preliminary review of a new technique in twenty-three patients. J Orthop Trauma. 2002;16(4):213-219.
24. Vallier HA, Nork SE, Benirschke SK, Sangeorzan BJ. Surgical treatment of talar body fractures. J Bone Joint Surg Am. 2004;86(suppl 1, pt 2):180-192.
1. Vallier HA, Reichard SG, Boyd AJ, Moore TA. A new look at the Hawkins classification for talar neck fractures: which features of injury and treatment are predictive of osteonecrosis? J Bone Joint Surg Am. 2014;96(3):192-197.
2. Canale ST, Kelly FB Jr. Fractures of the neck of the talus. Long-term evaluation of seventy-one cases. J Bone Joint Surg Am. 1978;60(2):143-156.
3. Ebraheim NA, Patil V, Owens C, Kandimalla Y. Clinical outcome of fractures of the talar body. Int Orthop. 2008;32(6):773-777.
4. Fortin PT, Balazsy JE. Talus fractures: evaluation and treatment. J Am Acad Orthop Surg. 2001;9(2):114-127.
5. Fournier A, Barba N, Steiger V, et al. Total talar fracture—long-term results of internal fixation of talar fractures. A multicentric study of 114 cases. Orthop Traumatol Surg Res. 2012;98(4 suppl):S48-S55.
6. Grob D, Simpson LA, Weber BG, Bray T. Operative treatment of displaced talus fractures. Clin Orthop Relat Res. 1985;(199):88-96.
7. Hawkins LG. Fractures of the neck of the talus. J Bone Joint Surg Am. 1970;52(5):991-1002.
8. Lindvall E, Haidukewych G, DiPasquale T, Herscovici D Jr, Sanders R. Open reduction and stable fixation of isolated, displaced talar neck and body fractures. J Bone Joint Surg Am. 2004;86(10):2229-2234.
9. Ohl X, Harisboure A, Hemery X, Dehoux E. Long-term follow-up after surgical treatment of talar fractures: twenty cases with an average follow-up of 7.5 years. Int Orthop. 2011;35(1):93-99.
10. Rammelt S, Zwipp H. Talar neck and body fractures. Injury. 2009;40(2):120-135.
11. Schulze W, Richter J, Russe O, Ingelfinger P, Muhr G. Surgical treatment of talus fractures: a retrospective study of 80 cases followed for 1-15 years. Acta Orthop Scand. 2002;73(3):344-351.
12. Vallier HA, Nork SE, Barei DP, Benirschke SK, Sangeorzan BJ. Talar neck fractures: results and outcomes. J Bone Joint Surg Am. 2004;86(8):1616-1624.
13. Patel R, Van Bergeyk A, Pinney S. Are displaced talar neck fractures surgical emergencies? A survey of orthopaedic trauma experts. Foot Ankle Int. 2005;26(5):378-381.
14. Sanders DW, Busam M, Hattwick E, Edwards JR, McAndrew MP, Johnson KD. Functional outcomes following displaced talar neck fractures. J Orthop Trauma. 2004;18(5):265-270.
15. Elgafy H, Ebraheim NA, Tile M, Stephen D, Kase J. Fractures of the talus: experience of two level 1 trauma centers. Foot Ankle Int. 2000;21(12):1023-1029.
16 Frawley PA, Hart JA, Young DA. Treatment outcome of major fractures of the talus. Foot Ankle Int. 1995;16(6):339-345.
17. Fracture and dislocation compendium. Orthopaedic Trauma Association committee for coding and classification. J Orthop Trauma. 1996;10(suppl 1):v-ix, 1-154.
18. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
19. Williams T, Barba N, Noailles T, et al. Total talar fracture—inter- and intra-observer reproducibility of two classification systems (Hawkins and AO) for central talar fractures. Orthop Traumatol Surg Res. 2012;98(4 suppl):S56-S65.
20. Zwipp H, Baumgart F, Cronier P, et al. Integral classification of injuries (ICI) to the bones, joints, and ligaments—application to injuries of the foot. Injury. 2004;35(suppl 2):SB3-SB9.
21. Xue Y, Zhang H, Pei F, et al. Treatment of displaced talar neck fractures using delayed procedures of plate fixation through dual approaches. Int Orthop. 2014;38(1):149-154.
22. Vallier HA, Nork SE, Benirschke SK, Sangeorzan BJ. Surgical treatment of talar body fractures. J Bone Joint Surg Am. 2003;85(9):1716-1724.
23. Fleuriau Chateau PB, Brokaw DS, Jelen BA, Scheid DK, Weber TG. Plate fixation of talar neck fractures: preliminary review of a new technique in twenty-three patients. J Orthop Trauma. 2002;16(4):213-219.
24. Vallier HA, Nork SE, Benirschke SK, Sangeorzan BJ. Surgical treatment of talar body fractures. J Bone Joint Surg Am. 2004;86(suppl 1, pt 2):180-192.
Biceps Tenodesis: A Comparison of Tendon-to-Bone and Tendon-to-Tendon Healing in a Rat Model
Take-Home Points
- Cellular healing response differs between bony and soft tissue biceps tenodesis.
- Bony tenodesis incites an inflammatory healing response.
- Bony tenodesis healing occurs at the tendon-bone interface.
- Intrasseous bony fixation leads to tendon degeneration within the bone.
- Tendon-to-tendon tenodesis may result in regenerative tendon healing.
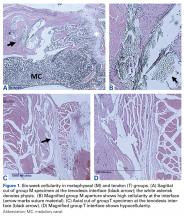
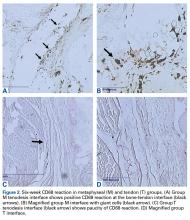
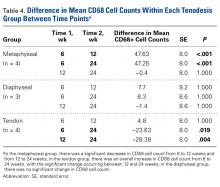
The long head of the biceps tendon (LHBT) is a well-established pain generator of the anterior shoulder1,2 and may be surgically addressed in refractory cases.3 According to a recent study of 44,932 cases, biceps tenodesis rates increased 80% over just 3 years (2008-2011).4 Nevertheless, optimal tenodesis location and technique remain controversial. Proximal and distal tenodesis, including numerous soft-tissue and bony techniques, have been described.5-7 Several studies have focused on the biomechanical strength of various fixation modalities.8-14 These data highlight the ongoing evolution of our understanding of biceps-labrum complex (BLC) disease.
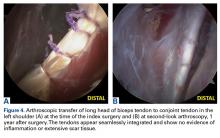
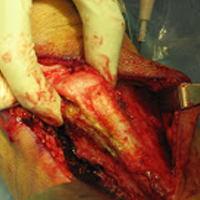
Over the years, tenodesis location has proved to be an important factor in outcomes.3,15-20 Several recent studies have elucidated the role of the extra-articular LHBT and the limited capabilities of diagnostic arthroscopy.15-17,20,21 Taylor and colleagues17 defined the bicipital tunnel as the extra-articular segment of LHBT and its fibro-osseous enclosure. The tunnel extends from the articular margin through the subpectoral region and can be divided into 3 zones: Zone 1 goes from the articular margin to the inferior margin of the subscapularis, zone 2 goes from the inferior margin of the subscapularis to the proximal margin of the pectoralis major tendon, and zone 3 is the subpectoral region. Zone 2 is often referred to as “no man’s land” for its relative invisibility from arthroscopy above and open exposure below.17,21 Notably, a recent study reported a 47% prevalence of hidden tunnel lesions in patients with chronic BLC disease symptoms.18 Other studies have shown that standard proximal tenodesis methods often fail to address LHBT pathology in this area, leading to residual symptoms.9,22 It is evident that tenodesis location and technique play important roles in patient outcomes. Sanders and colleagues16 found that the revision rate was significantly higher among patients who underwent biceps tenodesis without release of the bicipital tunnel sheath than among patients who underwent tenodesis with the release. Dr. O’Brien developed an alternative option: soft-tissue tenodesis with transfer of the LHBT to the conjoint tendon within the subdeltoid space.23,24 This technique addresses intra-articular and extra-articular tunnel disease while mitigating the complications associated with bony tenodesis. Early and midterm studies have shown this to be an effective intervention for chronically symptomatic BLC disease.25,26
Despite the abundance of literature on tenodesis techniques, no one has histologically evaluated the location-dependent healing and inflammatory responses. We conducted a study to determine the impact of tenodesis location on healing and inflammation in a rat model. We hypothesized that, compared with tendon-to-bone techniques, soft-tissue tenodesis would minimize inflammatory response and optimize healing.
Methods
The study was approved by the Institutional Animal Care and Use Committee at the Hospital for Special Surgery.
Animals
Biceps tenodesis was performed at 1 of 3 locations in 36 thirteen-week-old Sprague-Dawley rats (Charles River Laboratories). All rats were prepared for surgery by an experienced veterinary technician. Sedation was induced with isoflurane gas through a nose cone.
Surgical Procedure
Animals were randomly assigned to 3 different tenodesis groups: tendon-to-bone in the bicipital groove (metaphyseal, M); tendon-to-bone in the subpectoral region (diaphyseal, D); and soft tissue-to-soft tissue transfer to the conjoint tendon (T). A standard deltopectoral approach was used to expose the biceps tendon. The tendon was tagged with a 5-0 polypropylene suture and tenotomized at the level of the bicipital groove (zone 1). All wounds were irrigated and closed with 4-0 nylon suture.
For animals undergoing tendon-to-bone metaphyseal tenodesis, a 0.045-mm Kirschner wire was used to drill bicortically into the intertubercular sulcus. Wire positioning distal to the physeal plate was confirmed with fluoroscopy. A locking stitch of 5-0 polypropylene suture was run along the free edge of the tendon. The tendon was then passed through the bone tunnel in an anterior-to-posterior direction, and the limbs of the suture were tied around the lateral cortex.
The process was repeated for animals undergoing diaphyseal tenodesis; only the tenodesis location was different. The inferior border of the pectoralis major was identified, and a bicortical tunnel was made in the center of the diaphyseal bone. The tendon was then prepared and tenodesed to bone using the method already described.
In soft-tissue tenodesis, the conjoint tendon was identified and carefully dissected from surrounding tissues. The LHBT was then tenodesed to the attached conjoint tendon with interrupted simple stitches of 5-0 polypropylene suture.
The animals were allowed to bear weight on the operative limb immediately after surgery and without immobilization.
Specimen Harvest and Preparation
Four animals from each group were sacrificed at 6, 12, and 24 weeks. Harvested specimens were fixed in 10% neutral-buffered formalin solution. Bony specimens consisted of the upper half of the humerus and the tenodesed biceps tendon, and soft-tissue specimens consisted of the tenodesed LHBT-conjoint tendon complex. Bony specimens were decalcified in 10% ethylenediaminetetraacetic acid. All specimens were paraffin-embedded and sectioned at 7 microns.
Analysis of Cellularity
Sections were stained with hematoxylin-eosin. Overall cellularity at the tenodesis interface was quantified by averaging the nuclei count within 3 separate standardized ×20 magnification high power fields. Only nucleated cells were included in the cell count. Immunohistochemical staining with tenomodulin (Santa Cruz Laboratories, sc-49324) was performed to characterize the cell population at the interface. Deparaffinized sections underwent antigen retrieval with pronase for 30 minutes at 37°C and were incubated overnight with the anti-tenomodulin goat monoclonal antibody diluted to 1:200 in 1% phosphate-buffered saline. The prepared slides were then counterstained with methyl green. Specimens treated with tenomodulin were evaluated for presence or absence of a positive reaction at the tenodesis interface.
Analysis of Inflammation
Inflammation at the interface was evaluated with the CD68 macrophage marker (ABcam, ab31630). Deparaffinized sections underwent antigen retrieval with pronase for 30 minutes at 37°C and were incubated overnight with anti-CD68 mouse monoclonal antibodies diluted to 1:200 in 1% phosphate-buffered saline. The prepared slides were then counterstained with neutral red. Inflammation was quantified by averaging the number of reactive cells within 3 separate standardized ×20 magnification high power fields.
Statistical Analysis
Descriptive statistics were calculated for cell and macrophage counts for each group at every time point. Two-way analysis of variance was used to compare the cell and macrophage counts between groups at each time point as well as the count differences within each group between time points. P values were Bonferroni-corrected to account for the multiple comparisons between groups. P < .05 was used to signify statistical significance.
Results
All 36 animals survived to their designated harvest time without complications. Twelve specimens were successfully harvested at 6 weeks and another 12 at 24 weeks. At 12 weeks, tenodesis failure occurred in 1 animal in group D, leaving 11 specimens for analysis.
Cellularity
Within-group analysis revealed a trend of increasing cellularity at 12 weeks followed by a decrease at 24 weeks in all 3 groups (Table 2).
Inflammatory Response
During specimen processing, 1 group D specimen was severely degraded after pronase treatment, leaving 3 specimens for evaluation. Descriptive statistics for each group are listed in Table 3A.
At 6 weeks, mean CD68 cell count was significantly higher in group M than in group D (P = .011) and group T (P < .001) (Table 3B). Likewise, CD68 count was significantly higher in group D than in group T (P < .001). There were no differences in CD68 counts between the 2 bony tenodesis groups at 12 weeks (P = .486) or 24 weeks (P = .315). Both bony tenodesis groups, however, had persistently higher CD68 counts at 12 weeks when compared with group T (group M, P = .002; group D, P < .001). In these specimens, an inflammatory milieu characterized by a large accumulation of lymphocytes and giant cells was noted at the bone-tendon interface.
Tissue-Specific Staining
At 6 weeks, antigen retrieval resulted in severe degradation of 2 group M specimens, 2 group D specimens, and 1 group T specimen. The most notable tenomodulin reaction occurred in group T at the 6- and 12-week harvests, with the 6-week group having the most robust reaction. There was scant reaction in this group at 24 weeks.
Discussion
In this study, the healing response differed between bony and soft-tissue tenodesis techniques in a rat model. Tendon-to-bone tenodesis, both diaphyseal and metaphyseal, appeared to incite an inflammatory degenerative response, whereas tendon-to-tendon healing occurred in a more quiescent and perhaps even regenerative manner.
The early inflammatory response that occurred in the bony tenodesis groups is not unlike what occurs in fracture healing.27 The reaction was even more robust at 12 weeks, signifying an ongoing inflammatory process. In this context, tendon degeneration may plausibly explain the consistent absence of mature tendon within the tunnels at all 3 time points. Some tendon degeneration may be explained by the vascular damage that occurred during surgery, but this damage was a constant factor in all 3 study groups. Interestingly, group M showed the highest early CD68 counts, consistent with this being the more biologically active region of bone.28
Group T had significantly lower cell and macrophage counts throughout the study period, possibly indicating improved healing—an observation supported by a study in which the impact of macrophage depletion on bone-tendon interface healing was evaluated.29 The authors found that, in suppressing macrophage activity, the morphologic and biomechanical properties at the healing interface were significantly improved.29 These findings are consistent with Dr. O’Brien’s anecdotal experience with patients who previously underwent the biceps transfer; on second-look arthroscopy, there was complete seamless integration of tendon and conjoint tendon (Figure 4).
Studies have found that the inflammatory process is closely associated with pain, and pain syndromes such as fibromyalgia.30,31 Persistent inflammation, as seen in our bony tenodesis group, could explain the recalcitrant anterior shoulder pain that often occurs in patients after bony tenodesis of the LHBT.2,6,19,32
Studies have also suggested that osteoclasts at the bone-tendon interface—osteoclasts share a cell lineage with macrophages—may contribute to bone loss and tunnel widening.33,34 Osteoclasts are expected at the bone tunnel, as fracture healing occurs at the bone-tendon interface. These osteoclasts could have contributed to the strong CD68 reaction in our bony tenodesis groups. However, CD68 historically has been described as the classic macrophage marker.35 We specifically selected CD68 for this reason: Macrophages are the primary inflammatory cells involved in early healing and are key to the inflammatory process.36
Results of the tenomodulin analysis suggested 2 different healing processes are occurring in the bony and tendon groups. Tenomodulin is a known tenocyte marker for developing and mature tendon in both rats and humans.37,38 In our study, only group T had a positive tenomodulin reaction. Notably, the reaction occurred only at 6 and 12 weeks. This finding may indicate that a regenerative healing pattern becomes quiescent by 24 weeks. Indeed, it has been suggested that tenomodulin is a key regulator of tenocyte proliferation and tendon maturation.39
The complete absence of tenomodulin reaction in our bony tenodesis groups in the setting of significant inflammation further supports our theory of tendon degeneration within the tunnel. One potential explanation for this finding may be that as the tendon heals to the surface of the bone, the intra-osseous tendon is no longer load-bearing and is resorbed by the body through an inflammatory response. This finding differs from those in previous studies, which have described viable tendon within the bone tunnel at all time points up to 26 weeks.40 More recently, it has been suggested that callus formation at the external cortical tendon-bone interface is critical for healing and mechanical strength.41,42 In addition, recent studies have found a predominantly fibroblastic healing process at the midtunnel, potentially leading to the formation of loose fibrovascular tissue at the tendon-bone interface.43 These data, in concert with ours, call into question the rationale for performing intra-osseous tenodesis through bone tunnels.
Our study results, if confirmed in humans, will have significant clinical implications. If a similar effect can be confirmed in the human shoulder, one could argue that soft-tissue tenodesis may result in decreased postoperative shoulder pain. In addition, if tendon degeneration does occur within the intramedullary tunnel, surface fixation may be the better, safer alternative. Although older studies reported suboptimal strength with this type of fixation,8,44 more recent studies have found surface fixation strength equivalent to screw fixation strength.45,46 Such a shift in the treatment paradigm would obviate the need for violation of the humeral cortex, eliminating potential stress risers associated with screw fixation,47 and effectively eliminating the risk of iatrogenic fracture.48,49 It would be interesting to investigate what occurs histologically at the bone-tendon interface in surface fixation (ie, suture anchors). Would the inflammatory response at the surface be similar to the inflammatory intramedullary healing, or would it be similar to the quieter tendon-tendon healing? Answers to such questions have the potential to streamline the treatment algorithm for patients who require tenodesis.
Study Limitations
Our study had several limitations. First, as this was a basic science study using a rat model, its conclusions can only be extrapolated to humans. Second, given the nonspecific nature of the cellular analysis, we cannot draw any definitive conclusions about the cell population at the bone-tendon interface. For example, although tenomodulin is expressed by tenocytes, it is not an established specific marker for tenocytes and may be expressed by other fibroblastic cells. Still, our results provide insight into the local microenvironment and identify important differences between the tenodesis methods. Similarly, the complete absence of tendon within the bone tunnels suggests that an analysis of osteoclastic activity at the tenodesis interface may have been a valuable addition to the study. This finding, however, was unexpected, and we did not have the foresight to include it in our methods. A third limitation is that our fixation method essentially uses the suspension tenodesis method. This fixation method differs from the common fixation techniques used in the clinical setting. Testing of other fixation constructs would require a larger animal model. Furthermore, in suspension- type constructs, micromotion within the bone tunnel may independently elicit an inflammatory response. Inert suture was used in our fixation in order to reduce the risk of an iatrogenic inflammatory response. Last, it would have been valuable to perform a biomechanical analysis of the strength of each tenodesis construct. This was explored with our institution’s biomechanics team, but specimen size precluded successful analysis.
Conclusion
Our results indicated that, compared with tendon-to-tendon fixation, tendon-to-bone tenodesis produces a significantly greater inflammatory response at the tenodesis interface. An inflammatory milieu in the absence of tendon within the bony tunnel suggests intraosseous tendon degeneration. Tendon-to-tendon tenodesis, on the other hand, seems to limit the inflammatory response. In addition, a robust tenomodulin reaction in the early phases of tendon-to-tendon healing suggests regenerative healing. Our results showed a fundamental difference in the healing response between the 2 tenodesis methods. Further study is needed to evaluate the validity and applicability of our findings to the human patient population. Most important, our results underscore the need for more study to elucidate optimal tenodesis location and encourage orthopedic surgeons to reexamine current clinical practice patterns.
1. Alpantaki K, McLaughlin D, Karagogeos D, Hadjipavlou A, Kontakis G. Sympathetic and sensory neural elements in the tendon of the long head of the biceps. J Bone Joint Surg Am. 2005;87(7):1580-1583.
2. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
3. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
4. Werner BC, Brockmeier SF, Gwathmey FW. Trends in long head biceps tenodesis. Am J Sports Med. 2015;43(3):570-578.
5. Boileau P, Baque F, Valerio L, Ahrens P, Chuinard C, Trojani C. Isolated arthroscopic biceps tenotomy or tenodesis improves symptoms in patients with massive irreparable rotator cuff tears. J Bone Joint Surg Am. 2007;89(4):747-757.
6. Becker DA, Cofield RH. Tenodesis of the long head of the biceps brachii for chronic bicipital tendinitis. Long-term results. J Bone Joint Surg Am. 1989;71(3):376-381.
7. Richards DP, Burkhart SS. Arthroscopic-assisted biceps tenodesis for ruptures of the long head of biceps brachii: the cobra procedure. Arthroscopy. 2004;20(suppl 2):201-207.
8. Ozalay M, Akpinar S, Karaeminogullari O, et al. Mechanical strength of four different biceps tenodesis techniques. Arthroscopy. 2005;21(8):992-998.
9. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
10. Kilicoglu O, Koyuncu O, Demirhan M, et al. Time-dependent changes in failure loads of 3 biceps tenodesis techniques: in vivo study in a sheep model. Am J Sports Med. 2005;33(10):1536-1544.
11. Golish SR, Caldwell PE 3rd, Miller MD, et al. Interference screw versus suture anchor fixation for subpectoral tenodesis of the proximal biceps tendon: a cadaveric study. Arthroscopy. 2008;24(10):1103-1108.
12. Kusma M, Dienst M, Eckert J, Steimer O, Kohn D. Tenodesis of the long head of biceps brachii: cyclic testing of five methods of fixation in a porcine model. J Shoulder Elbow Surg. 2008;17(6):967-973.
13. Buchholz A, Martetschlager F, Siebenlist S, et al. Biomechanical comparison of intramedullary cortical button fixation and interference screw technique for subpectoral biceps tenodesis. Arthroscopy. 2013;29(5):845-853.
14. Werner BC, Lyons ML, Evans CL, et al. Arthroscopic suprapectoral and open subpectoral biceps tenodesis: a comparison of restoration of length-tension and mechanical strength between techniques. Arthroscopy. 2015;31(4):620-627.
15. Gilmer BB, DeMers AM, Guerrero D, Reid JB 3rd, Lubowitz JH, Guttmann D. Arthroscopic versus open comparison of long head of biceps tendon visualization and pathology in patients requiring tenodesis. Arthroscopy. 2015;31(1):29-34.
16. Sanders B, Lavery KP, Pennington S, Warner JJ. Clinical success of biceps tenodesis with and without release of the transverse humeral ligament. J Shoulder Elbow Surg. 2012;21(1):66-71.
17. Taylor SA, Fabricant PD, Bansal M, et al. The anatomy and histology of the bicipital tunnel of the shoulder. J Shoulder Elbow Surg. 2015;24(4):511-519.
18. Taylor SA, Khair MM, Gulotta LV, et al. Diagnostic glenohumeral arthroscopy fails to fully evaluate the biceps-labral complex. Arthroscopy. 2015;31(2):215-224.
19. Lutton DM, Gruson KI, Harrison AK, Gladstone JN, Flatow EL. Where to tenodese the biceps: proximal or distal? Clin Orthop Relat Res. 2011;469(4):1050-1055.
20. Moon SC, Cho NS, Rhee YG. Analysis of “hidden lesions” of the extra-articular biceps after subpectoral biceps tenodesis: the subpectoral portion as the optimal tenodesis site. Am J Sports Med. 2015;43(1):63-68.
21. Festa A, Allert J, Issa K, Tasto JP, Myer JJ. Visualization of the extra-articular portion of the long head of the biceps tendon during intra-articular shoulder arthroscopy. Arthroscopy. 2014;30(11):1413-1417.
22. Friedman DJ, Dunn JC, Higgins LD, Warner JJ. Proximal biceps tendon: injuries and management. Sports Med Arthrosc. 2008;16(3):162-169.
23. Verma NN, Drakos M, O’Brien SJ. Arthroscopic transfer of the long head biceps to the conjoint tendon. Arthroscopy. 2005;21(6):764.
24. O’Brien SJ, Taylor SA, DiPietro JR, Newman AM, Drakos MC, Voos JE. The arthroscopic “subdeltoid approach” to the anterior shoulder. J Shoulder Elbow Surg. 2013;22(4):e6-e10.
25. Drakos MC, Verma NN, Gulotta LV, et al. Arthroscopic transfer of the long head of the biceps tendon: functional outcome and clinical results. Arthroscopy. 2008;24(2):217-223.
26. Taylor SA, Fabricant PD, Baret NJ, et al. Midterm clinical outcomes for arthroscopic subdeltoid transfer of the long head of the biceps tendon to the conjoint tendon. Arthroscopy. 2014;30(12):1574-1581.
27. Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42(6):551-555.
28. Khan SN, Cammisa FP Jr, Sandhu HS, Diwan AD, Girardi FP, Lane JM. The biology of bone healing. J Am Acad Orthop Surg. 2005;13(1):77-86.
29. Hays PL, Kawamura S, Deng XH, et al. The role of macrophages in early healing of a tendon graft in a bone tunnel. J Bone Joint Surg Am. 2008;90(3):565-579.
30. Uhl RL, Roberts TT, Papaliodis DN, Mulligan MT, Dubin AH. Management of chronic musculoskeletal pain. J Am Acad Orthop Surg. 2014;22(2):101-110.
31. Kosek E, Altawil R, Kadetoff D, et al. Evidence of different mediators of central inflammation in dysfunctional and inflammatory pain—interleukin-8 in fibromyalgia and interleukin-1 β in rheumatoid arthritis. J Neuroimmunol. 2015;280:49-55.
32. Slenker NR, Lawson K, Ciccotti MG, Dodson CC, Cohen SB. Biceps tenotomy versus tenodesis: clinical outcomes. Arthroscopy. 2012;28(4):576-582.
33. Rodeo SA, Kawamura S, Kim HJ, Dynybil C, Ying L. Tendon healing in a bone tunnel differs at the tunnel entrance versus the tunnel exit: an effect of graft-tunnel motion? Am J Sports Med. 2006;34(11):1790-1800.
34. Hjorthaug GA, Madsen JE, Nordsletten L, Reinholt FP, Steen H, Dimmen S. Tendon to bone tunnel healing—a study on the time-dependent changes in biomechanics, bone remodeling, and histology in a rat model. J Orthop Res. 2015;33(2):216-223.
35. Pulford KA, Sipos A, Cordell JL, Stross WP, Mason DY. Distribution of the CD68 macrophage/myeloid associated antigen. Int Immunol. 1990;2(10):973-980.
36. Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4(3):281-286.
37. Qi J, Dmochowski JM, Banes AN, et al. Differential expression and cellular localization of novel isoforms of the tendon biomarker tenomodulin. J Appl Physiol (1985). 2012;113(6):861-871.
38. Jelinsky SA, Archambault J, Li L, Seeherman H. Tendon-selective genes identified from rat and human musculoskeletal tissues. J Orthop Res. 2010;28(3):289-297.
39. Docheva D, Hunziker EB, Fassler R, Brandau O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol Cell Biol. 2005;25(2):699-705.
40. Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75(12):1795-1803.
41. Silva MJ, Thomopoulos S, Kusano N, et al. Early healing of flexor tendon insertion site injuries: tunnel repair is mechanically and histologically inferior to surface repair in a canine model. J Orthop Res. 2006;24(5):990-1000.
42. Hibino N, Hamada Y, Sairyo K, Yukata K, Sano T, Yasui N. Callus formation during healing of the repaired tendon–bone junction. A rat experimental model. J Bone Joint Surg Br. 2007;89(11):1539-1544.
43. Bedi A, Kawamura S, Ying L, Rodeo SA. Differences in tendon graft healing between the intra-articular and extra-articular ends of a bone tunnel. HSS J. 2009;5(1):51-57.
44. Richards DP, Burkhart SS. A biomechanical analysis of two biceps tenodesis fixation techniques. Arthroscopy. 2005;21(7):861-866.
45. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
46. Baleani M, Francesconi D, Zani L, Giannini S, Snyder SJ. Suprapectoral biceps tenodesis: a biomechanical comparison of a new “soft anchor” tenodesis technique versus interference screw biceps tendon fixation. Clin Biomech. 2015;30(2):188-194.
47. Euler SA, Smith SD, Williams BT, Dornan GJ, Millett PJ, Wijdicks CA. Biomechanical analysis of subpectoral biceps tenodesis: effect of screw malpositioning on proximal humeral strength. Am J Sports Med. 2015;43(1):69-74.
48. Sears BW, Spencer EE, Getz CL. Humeral fracture following subpectoral biceps tenodesis in 2 active, healthy patients. J Shoulder Elbow Surg. 2011;20(6):e7-e11.
49. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis. Am J Sports Med. 2014;42(4):877-879.
Take-Home Points
- Cellular healing response differs between bony and soft tissue biceps tenodesis.
- Bony tenodesis incites an inflammatory healing response.
- Bony tenodesis healing occurs at the tendon-bone interface.
- Intrasseous bony fixation leads to tendon degeneration within the bone.
- Tendon-to-tendon tenodesis may result in regenerative tendon healing.
The long head of the biceps tendon (LHBT) is a well-established pain generator of the anterior shoulder1,2 and may be surgically addressed in refractory cases.3 According to a recent study of 44,932 cases, biceps tenodesis rates increased 80% over just 3 years (2008-2011).4 Nevertheless, optimal tenodesis location and technique remain controversial. Proximal and distal tenodesis, including numerous soft-tissue and bony techniques, have been described.5-7 Several studies have focused on the biomechanical strength of various fixation modalities.8-14 These data highlight the ongoing evolution of our understanding of biceps-labrum complex (BLC) disease.
Over the years, tenodesis location has proved to be an important factor in outcomes.3,15-20 Several recent studies have elucidated the role of the extra-articular LHBT and the limited capabilities of diagnostic arthroscopy.15-17,20,21 Taylor and colleagues17 defined the bicipital tunnel as the extra-articular segment of LHBT and its fibro-osseous enclosure. The tunnel extends from the articular margin through the subpectoral region and can be divided into 3 zones: Zone 1 goes from the articular margin to the inferior margin of the subscapularis, zone 2 goes from the inferior margin of the subscapularis to the proximal margin of the pectoralis major tendon, and zone 3 is the subpectoral region. Zone 2 is often referred to as “no man’s land” for its relative invisibility from arthroscopy above and open exposure below.17,21 Notably, a recent study reported a 47% prevalence of hidden tunnel lesions in patients with chronic BLC disease symptoms.18 Other studies have shown that standard proximal tenodesis methods often fail to address LHBT pathology in this area, leading to residual symptoms.9,22 It is evident that tenodesis location and technique play important roles in patient outcomes. Sanders and colleagues16 found that the revision rate was significantly higher among patients who underwent biceps tenodesis without release of the bicipital tunnel sheath than among patients who underwent tenodesis with the release. Dr. O’Brien developed an alternative option: soft-tissue tenodesis with transfer of the LHBT to the conjoint tendon within the subdeltoid space.23,24 This technique addresses intra-articular and extra-articular tunnel disease while mitigating the complications associated with bony tenodesis. Early and midterm studies have shown this to be an effective intervention for chronically symptomatic BLC disease.25,26
Despite the abundance of literature on tenodesis techniques, no one has histologically evaluated the location-dependent healing and inflammatory responses. We conducted a study to determine the impact of tenodesis location on healing and inflammation in a rat model. We hypothesized that, compared with tendon-to-bone techniques, soft-tissue tenodesis would minimize inflammatory response and optimize healing.
Methods
The study was approved by the Institutional Animal Care and Use Committee at the Hospital for Special Surgery.
Animals
Biceps tenodesis was performed at 1 of 3 locations in 36 thirteen-week-old Sprague-Dawley rats (Charles River Laboratories). All rats were prepared for surgery by an experienced veterinary technician. Sedation was induced with isoflurane gas through a nose cone.
Surgical Procedure
Animals were randomly assigned to 3 different tenodesis groups: tendon-to-bone in the bicipital groove (metaphyseal, M); tendon-to-bone in the subpectoral region (diaphyseal, D); and soft tissue-to-soft tissue transfer to the conjoint tendon (T). A standard deltopectoral approach was used to expose the biceps tendon. The tendon was tagged with a 5-0 polypropylene suture and tenotomized at the level of the bicipital groove (zone 1). All wounds were irrigated and closed with 4-0 nylon suture.
For animals undergoing tendon-to-bone metaphyseal tenodesis, a 0.045-mm Kirschner wire was used to drill bicortically into the intertubercular sulcus. Wire positioning distal to the physeal plate was confirmed with fluoroscopy. A locking stitch of 5-0 polypropylene suture was run along the free edge of the tendon. The tendon was then passed through the bone tunnel in an anterior-to-posterior direction, and the limbs of the suture were tied around the lateral cortex.
The process was repeated for animals undergoing diaphyseal tenodesis; only the tenodesis location was different. The inferior border of the pectoralis major was identified, and a bicortical tunnel was made in the center of the diaphyseal bone. The tendon was then prepared and tenodesed to bone using the method already described.
In soft-tissue tenodesis, the conjoint tendon was identified and carefully dissected from surrounding tissues. The LHBT was then tenodesed to the attached conjoint tendon with interrupted simple stitches of 5-0 polypropylene suture.
The animals were allowed to bear weight on the operative limb immediately after surgery and without immobilization.
Specimen Harvest and Preparation
Four animals from each group were sacrificed at 6, 12, and 24 weeks. Harvested specimens were fixed in 10% neutral-buffered formalin solution. Bony specimens consisted of the upper half of the humerus and the tenodesed biceps tendon, and soft-tissue specimens consisted of the tenodesed LHBT-conjoint tendon complex. Bony specimens were decalcified in 10% ethylenediaminetetraacetic acid. All specimens were paraffin-embedded and sectioned at 7 microns.
Analysis of Cellularity
Sections were stained with hematoxylin-eosin. Overall cellularity at the tenodesis interface was quantified by averaging the nuclei count within 3 separate standardized ×20 magnification high power fields. Only nucleated cells were included in the cell count. Immunohistochemical staining with tenomodulin (Santa Cruz Laboratories, sc-49324) was performed to characterize the cell population at the interface. Deparaffinized sections underwent antigen retrieval with pronase for 30 minutes at 37°C and were incubated overnight with the anti-tenomodulin goat monoclonal antibody diluted to 1:200 in 1% phosphate-buffered saline. The prepared slides were then counterstained with methyl green. Specimens treated with tenomodulin were evaluated for presence or absence of a positive reaction at the tenodesis interface.
Analysis of Inflammation
Inflammation at the interface was evaluated with the CD68 macrophage marker (ABcam, ab31630). Deparaffinized sections underwent antigen retrieval with pronase for 30 minutes at 37°C and were incubated overnight with anti-CD68 mouse monoclonal antibodies diluted to 1:200 in 1% phosphate-buffered saline. The prepared slides were then counterstained with neutral red. Inflammation was quantified by averaging the number of reactive cells within 3 separate standardized ×20 magnification high power fields.
Statistical Analysis
Descriptive statistics were calculated for cell and macrophage counts for each group at every time point. Two-way analysis of variance was used to compare the cell and macrophage counts between groups at each time point as well as the count differences within each group between time points. P values were Bonferroni-corrected to account for the multiple comparisons between groups. P < .05 was used to signify statistical significance.
Results
All 36 animals survived to their designated harvest time without complications. Twelve specimens were successfully harvested at 6 weeks and another 12 at 24 weeks. At 12 weeks, tenodesis failure occurred in 1 animal in group D, leaving 11 specimens for analysis.
Cellularity
Within-group analysis revealed a trend of increasing cellularity at 12 weeks followed by a decrease at 24 weeks in all 3 groups (Table 2).
Inflammatory Response
During specimen processing, 1 group D specimen was severely degraded after pronase treatment, leaving 3 specimens for evaluation. Descriptive statistics for each group are listed in Table 3A.
At 6 weeks, mean CD68 cell count was significantly higher in group M than in group D (P = .011) and group T (P < .001) (Table 3B). Likewise, CD68 count was significantly higher in group D than in group T (P < .001). There were no differences in CD68 counts between the 2 bony tenodesis groups at 12 weeks (P = .486) or 24 weeks (P = .315). Both bony tenodesis groups, however, had persistently higher CD68 counts at 12 weeks when compared with group T (group M, P = .002; group D, P < .001). In these specimens, an inflammatory milieu characterized by a large accumulation of lymphocytes and giant cells was noted at the bone-tendon interface.
Tissue-Specific Staining
At 6 weeks, antigen retrieval resulted in severe degradation of 2 group M specimens, 2 group D specimens, and 1 group T specimen. The most notable tenomodulin reaction occurred in group T at the 6- and 12-week harvests, with the 6-week group having the most robust reaction. There was scant reaction in this group at 24 weeks.
Discussion
In this study, the healing response differed between bony and soft-tissue tenodesis techniques in a rat model. Tendon-to-bone tenodesis, both diaphyseal and metaphyseal, appeared to incite an inflammatory degenerative response, whereas tendon-to-tendon healing occurred in a more quiescent and perhaps even regenerative manner.
The early inflammatory response that occurred in the bony tenodesis groups is not unlike what occurs in fracture healing.27 The reaction was even more robust at 12 weeks, signifying an ongoing inflammatory process. In this context, tendon degeneration may plausibly explain the consistent absence of mature tendon within the tunnels at all 3 time points. Some tendon degeneration may be explained by the vascular damage that occurred during surgery, but this damage was a constant factor in all 3 study groups. Interestingly, group M showed the highest early CD68 counts, consistent with this being the more biologically active region of bone.28
Group T had significantly lower cell and macrophage counts throughout the study period, possibly indicating improved healing—an observation supported by a study in which the impact of macrophage depletion on bone-tendon interface healing was evaluated.29 The authors found that, in suppressing macrophage activity, the morphologic and biomechanical properties at the healing interface were significantly improved.29 These findings are consistent with Dr. O’Brien’s anecdotal experience with patients who previously underwent the biceps transfer; on second-look arthroscopy, there was complete seamless integration of tendon and conjoint tendon (Figure 4).
Studies have found that the inflammatory process is closely associated with pain, and pain syndromes such as fibromyalgia.30,31 Persistent inflammation, as seen in our bony tenodesis group, could explain the recalcitrant anterior shoulder pain that often occurs in patients after bony tenodesis of the LHBT.2,6,19,32
Studies have also suggested that osteoclasts at the bone-tendon interface—osteoclasts share a cell lineage with macrophages—may contribute to bone loss and tunnel widening.33,34 Osteoclasts are expected at the bone tunnel, as fracture healing occurs at the bone-tendon interface. These osteoclasts could have contributed to the strong CD68 reaction in our bony tenodesis groups. However, CD68 historically has been described as the classic macrophage marker.35 We specifically selected CD68 for this reason: Macrophages are the primary inflammatory cells involved in early healing and are key to the inflammatory process.36
Results of the tenomodulin analysis suggested 2 different healing processes are occurring in the bony and tendon groups. Tenomodulin is a known tenocyte marker for developing and mature tendon in both rats and humans.37,38 In our study, only group T had a positive tenomodulin reaction. Notably, the reaction occurred only at 6 and 12 weeks. This finding may indicate that a regenerative healing pattern becomes quiescent by 24 weeks. Indeed, it has been suggested that tenomodulin is a key regulator of tenocyte proliferation and tendon maturation.39
The complete absence of tenomodulin reaction in our bony tenodesis groups in the setting of significant inflammation further supports our theory of tendon degeneration within the tunnel. One potential explanation for this finding may be that as the tendon heals to the surface of the bone, the intra-osseous tendon is no longer load-bearing and is resorbed by the body through an inflammatory response. This finding differs from those in previous studies, which have described viable tendon within the bone tunnel at all time points up to 26 weeks.40 More recently, it has been suggested that callus formation at the external cortical tendon-bone interface is critical for healing and mechanical strength.41,42 In addition, recent studies have found a predominantly fibroblastic healing process at the midtunnel, potentially leading to the formation of loose fibrovascular tissue at the tendon-bone interface.43 These data, in concert with ours, call into question the rationale for performing intra-osseous tenodesis through bone tunnels.
Our study results, if confirmed in humans, will have significant clinical implications. If a similar effect can be confirmed in the human shoulder, one could argue that soft-tissue tenodesis may result in decreased postoperative shoulder pain. In addition, if tendon degeneration does occur within the intramedullary tunnel, surface fixation may be the better, safer alternative. Although older studies reported suboptimal strength with this type of fixation,8,44 more recent studies have found surface fixation strength equivalent to screw fixation strength.45,46 Such a shift in the treatment paradigm would obviate the need for violation of the humeral cortex, eliminating potential stress risers associated with screw fixation,47 and effectively eliminating the risk of iatrogenic fracture.48,49 It would be interesting to investigate what occurs histologically at the bone-tendon interface in surface fixation (ie, suture anchors). Would the inflammatory response at the surface be similar to the inflammatory intramedullary healing, or would it be similar to the quieter tendon-tendon healing? Answers to such questions have the potential to streamline the treatment algorithm for patients who require tenodesis.
Study Limitations
Our study had several limitations. First, as this was a basic science study using a rat model, its conclusions can only be extrapolated to humans. Second, given the nonspecific nature of the cellular analysis, we cannot draw any definitive conclusions about the cell population at the bone-tendon interface. For example, although tenomodulin is expressed by tenocytes, it is not an established specific marker for tenocytes and may be expressed by other fibroblastic cells. Still, our results provide insight into the local microenvironment and identify important differences between the tenodesis methods. Similarly, the complete absence of tendon within the bone tunnels suggests that an analysis of osteoclastic activity at the tenodesis interface may have been a valuable addition to the study. This finding, however, was unexpected, and we did not have the foresight to include it in our methods. A third limitation is that our fixation method essentially uses the suspension tenodesis method. This fixation method differs from the common fixation techniques used in the clinical setting. Testing of other fixation constructs would require a larger animal model. Furthermore, in suspension- type constructs, micromotion within the bone tunnel may independently elicit an inflammatory response. Inert suture was used in our fixation in order to reduce the risk of an iatrogenic inflammatory response. Last, it would have been valuable to perform a biomechanical analysis of the strength of each tenodesis construct. This was explored with our institution’s biomechanics team, but specimen size precluded successful analysis.
Conclusion
Our results indicated that, compared with tendon-to-tendon fixation, tendon-to-bone tenodesis produces a significantly greater inflammatory response at the tenodesis interface. An inflammatory milieu in the absence of tendon within the bony tunnel suggests intraosseous tendon degeneration. Tendon-to-tendon tenodesis, on the other hand, seems to limit the inflammatory response. In addition, a robust tenomodulin reaction in the early phases of tendon-to-tendon healing suggests regenerative healing. Our results showed a fundamental difference in the healing response between the 2 tenodesis methods. Further study is needed to evaluate the validity and applicability of our findings to the human patient population. Most important, our results underscore the need for more study to elucidate optimal tenodesis location and encourage orthopedic surgeons to reexamine current clinical practice patterns.
Take-Home Points
- Cellular healing response differs between bony and soft tissue biceps tenodesis.
- Bony tenodesis incites an inflammatory healing response.
- Bony tenodesis healing occurs at the tendon-bone interface.
- Intrasseous bony fixation leads to tendon degeneration within the bone.
- Tendon-to-tendon tenodesis may result in regenerative tendon healing.
The long head of the biceps tendon (LHBT) is a well-established pain generator of the anterior shoulder1,2 and may be surgically addressed in refractory cases.3 According to a recent study of 44,932 cases, biceps tenodesis rates increased 80% over just 3 years (2008-2011).4 Nevertheless, optimal tenodesis location and technique remain controversial. Proximal and distal tenodesis, including numerous soft-tissue and bony techniques, have been described.5-7 Several studies have focused on the biomechanical strength of various fixation modalities.8-14 These data highlight the ongoing evolution of our understanding of biceps-labrum complex (BLC) disease.
Over the years, tenodesis location has proved to be an important factor in outcomes.3,15-20 Several recent studies have elucidated the role of the extra-articular LHBT and the limited capabilities of diagnostic arthroscopy.15-17,20,21 Taylor and colleagues17 defined the bicipital tunnel as the extra-articular segment of LHBT and its fibro-osseous enclosure. The tunnel extends from the articular margin through the subpectoral region and can be divided into 3 zones: Zone 1 goes from the articular margin to the inferior margin of the subscapularis, zone 2 goes from the inferior margin of the subscapularis to the proximal margin of the pectoralis major tendon, and zone 3 is the subpectoral region. Zone 2 is often referred to as “no man’s land” for its relative invisibility from arthroscopy above and open exposure below.17,21 Notably, a recent study reported a 47% prevalence of hidden tunnel lesions in patients with chronic BLC disease symptoms.18 Other studies have shown that standard proximal tenodesis methods often fail to address LHBT pathology in this area, leading to residual symptoms.9,22 It is evident that tenodesis location and technique play important roles in patient outcomes. Sanders and colleagues16 found that the revision rate was significantly higher among patients who underwent biceps tenodesis without release of the bicipital tunnel sheath than among patients who underwent tenodesis with the release. Dr. O’Brien developed an alternative option: soft-tissue tenodesis with transfer of the LHBT to the conjoint tendon within the subdeltoid space.23,24 This technique addresses intra-articular and extra-articular tunnel disease while mitigating the complications associated with bony tenodesis. Early and midterm studies have shown this to be an effective intervention for chronically symptomatic BLC disease.25,26
Despite the abundance of literature on tenodesis techniques, no one has histologically evaluated the location-dependent healing and inflammatory responses. We conducted a study to determine the impact of tenodesis location on healing and inflammation in a rat model. We hypothesized that, compared with tendon-to-bone techniques, soft-tissue tenodesis would minimize inflammatory response and optimize healing.
Methods
The study was approved by the Institutional Animal Care and Use Committee at the Hospital for Special Surgery.
Animals
Biceps tenodesis was performed at 1 of 3 locations in 36 thirteen-week-old Sprague-Dawley rats (Charles River Laboratories). All rats were prepared for surgery by an experienced veterinary technician. Sedation was induced with isoflurane gas through a nose cone.
Surgical Procedure
Animals were randomly assigned to 3 different tenodesis groups: tendon-to-bone in the bicipital groove (metaphyseal, M); tendon-to-bone in the subpectoral region (diaphyseal, D); and soft tissue-to-soft tissue transfer to the conjoint tendon (T). A standard deltopectoral approach was used to expose the biceps tendon. The tendon was tagged with a 5-0 polypropylene suture and tenotomized at the level of the bicipital groove (zone 1). All wounds were irrigated and closed with 4-0 nylon suture.
For animals undergoing tendon-to-bone metaphyseal tenodesis, a 0.045-mm Kirschner wire was used to drill bicortically into the intertubercular sulcus. Wire positioning distal to the physeal plate was confirmed with fluoroscopy. A locking stitch of 5-0 polypropylene suture was run along the free edge of the tendon. The tendon was then passed through the bone tunnel in an anterior-to-posterior direction, and the limbs of the suture were tied around the lateral cortex.
The process was repeated for animals undergoing diaphyseal tenodesis; only the tenodesis location was different. The inferior border of the pectoralis major was identified, and a bicortical tunnel was made in the center of the diaphyseal bone. The tendon was then prepared and tenodesed to bone using the method already described.
In soft-tissue tenodesis, the conjoint tendon was identified and carefully dissected from surrounding tissues. The LHBT was then tenodesed to the attached conjoint tendon with interrupted simple stitches of 5-0 polypropylene suture.
The animals were allowed to bear weight on the operative limb immediately after surgery and without immobilization.
Specimen Harvest and Preparation
Four animals from each group were sacrificed at 6, 12, and 24 weeks. Harvested specimens were fixed in 10% neutral-buffered formalin solution. Bony specimens consisted of the upper half of the humerus and the tenodesed biceps tendon, and soft-tissue specimens consisted of the tenodesed LHBT-conjoint tendon complex. Bony specimens were decalcified in 10% ethylenediaminetetraacetic acid. All specimens were paraffin-embedded and sectioned at 7 microns.
Analysis of Cellularity
Sections were stained with hematoxylin-eosin. Overall cellularity at the tenodesis interface was quantified by averaging the nuclei count within 3 separate standardized ×20 magnification high power fields. Only nucleated cells were included in the cell count. Immunohistochemical staining with tenomodulin (Santa Cruz Laboratories, sc-49324) was performed to characterize the cell population at the interface. Deparaffinized sections underwent antigen retrieval with pronase for 30 minutes at 37°C and were incubated overnight with the anti-tenomodulin goat monoclonal antibody diluted to 1:200 in 1% phosphate-buffered saline. The prepared slides were then counterstained with methyl green. Specimens treated with tenomodulin were evaluated for presence or absence of a positive reaction at the tenodesis interface.
Analysis of Inflammation
Inflammation at the interface was evaluated with the CD68 macrophage marker (ABcam, ab31630). Deparaffinized sections underwent antigen retrieval with pronase for 30 minutes at 37°C and were incubated overnight with anti-CD68 mouse monoclonal antibodies diluted to 1:200 in 1% phosphate-buffered saline. The prepared slides were then counterstained with neutral red. Inflammation was quantified by averaging the number of reactive cells within 3 separate standardized ×20 magnification high power fields.
Statistical Analysis
Descriptive statistics were calculated for cell and macrophage counts for each group at every time point. Two-way analysis of variance was used to compare the cell and macrophage counts between groups at each time point as well as the count differences within each group between time points. P values were Bonferroni-corrected to account for the multiple comparisons between groups. P < .05 was used to signify statistical significance.
Results
All 36 animals survived to their designated harvest time without complications. Twelve specimens were successfully harvested at 6 weeks and another 12 at 24 weeks. At 12 weeks, tenodesis failure occurred in 1 animal in group D, leaving 11 specimens for analysis.
Cellularity
Within-group analysis revealed a trend of increasing cellularity at 12 weeks followed by a decrease at 24 weeks in all 3 groups (Table 2).
Inflammatory Response
During specimen processing, 1 group D specimen was severely degraded after pronase treatment, leaving 3 specimens for evaluation. Descriptive statistics for each group are listed in Table 3A.
At 6 weeks, mean CD68 cell count was significantly higher in group M than in group D (P = .011) and group T (P < .001) (Table 3B). Likewise, CD68 count was significantly higher in group D than in group T (P < .001). There were no differences in CD68 counts between the 2 bony tenodesis groups at 12 weeks (P = .486) or 24 weeks (P = .315). Both bony tenodesis groups, however, had persistently higher CD68 counts at 12 weeks when compared with group T (group M, P = .002; group D, P < .001). In these specimens, an inflammatory milieu characterized by a large accumulation of lymphocytes and giant cells was noted at the bone-tendon interface.
Tissue-Specific Staining
At 6 weeks, antigen retrieval resulted in severe degradation of 2 group M specimens, 2 group D specimens, and 1 group T specimen. The most notable tenomodulin reaction occurred in group T at the 6- and 12-week harvests, with the 6-week group having the most robust reaction. There was scant reaction in this group at 24 weeks.
Discussion
In this study, the healing response differed between bony and soft-tissue tenodesis techniques in a rat model. Tendon-to-bone tenodesis, both diaphyseal and metaphyseal, appeared to incite an inflammatory degenerative response, whereas tendon-to-tendon healing occurred in a more quiescent and perhaps even regenerative manner.
The early inflammatory response that occurred in the bony tenodesis groups is not unlike what occurs in fracture healing.27 The reaction was even more robust at 12 weeks, signifying an ongoing inflammatory process. In this context, tendon degeneration may plausibly explain the consistent absence of mature tendon within the tunnels at all 3 time points. Some tendon degeneration may be explained by the vascular damage that occurred during surgery, but this damage was a constant factor in all 3 study groups. Interestingly, group M showed the highest early CD68 counts, consistent with this being the more biologically active region of bone.28
Group T had significantly lower cell and macrophage counts throughout the study period, possibly indicating improved healing—an observation supported by a study in which the impact of macrophage depletion on bone-tendon interface healing was evaluated.29 The authors found that, in suppressing macrophage activity, the morphologic and biomechanical properties at the healing interface were significantly improved.29 These findings are consistent with Dr. O’Brien’s anecdotal experience with patients who previously underwent the biceps transfer; on second-look arthroscopy, there was complete seamless integration of tendon and conjoint tendon (Figure 4).
Studies have found that the inflammatory process is closely associated with pain, and pain syndromes such as fibromyalgia.30,31 Persistent inflammation, as seen in our bony tenodesis group, could explain the recalcitrant anterior shoulder pain that often occurs in patients after bony tenodesis of the LHBT.2,6,19,32
Studies have also suggested that osteoclasts at the bone-tendon interface—osteoclasts share a cell lineage with macrophages—may contribute to bone loss and tunnel widening.33,34 Osteoclasts are expected at the bone tunnel, as fracture healing occurs at the bone-tendon interface. These osteoclasts could have contributed to the strong CD68 reaction in our bony tenodesis groups. However, CD68 historically has been described as the classic macrophage marker.35 We specifically selected CD68 for this reason: Macrophages are the primary inflammatory cells involved in early healing and are key to the inflammatory process.36
Results of the tenomodulin analysis suggested 2 different healing processes are occurring in the bony and tendon groups. Tenomodulin is a known tenocyte marker for developing and mature tendon in both rats and humans.37,38 In our study, only group T had a positive tenomodulin reaction. Notably, the reaction occurred only at 6 and 12 weeks. This finding may indicate that a regenerative healing pattern becomes quiescent by 24 weeks. Indeed, it has been suggested that tenomodulin is a key regulator of tenocyte proliferation and tendon maturation.39
The complete absence of tenomodulin reaction in our bony tenodesis groups in the setting of significant inflammation further supports our theory of tendon degeneration within the tunnel. One potential explanation for this finding may be that as the tendon heals to the surface of the bone, the intra-osseous tendon is no longer load-bearing and is resorbed by the body through an inflammatory response. This finding differs from those in previous studies, which have described viable tendon within the bone tunnel at all time points up to 26 weeks.40 More recently, it has been suggested that callus formation at the external cortical tendon-bone interface is critical for healing and mechanical strength.41,42 In addition, recent studies have found a predominantly fibroblastic healing process at the midtunnel, potentially leading to the formation of loose fibrovascular tissue at the tendon-bone interface.43 These data, in concert with ours, call into question the rationale for performing intra-osseous tenodesis through bone tunnels.
Our study results, if confirmed in humans, will have significant clinical implications. If a similar effect can be confirmed in the human shoulder, one could argue that soft-tissue tenodesis may result in decreased postoperative shoulder pain. In addition, if tendon degeneration does occur within the intramedullary tunnel, surface fixation may be the better, safer alternative. Although older studies reported suboptimal strength with this type of fixation,8,44 more recent studies have found surface fixation strength equivalent to screw fixation strength.45,46 Such a shift in the treatment paradigm would obviate the need for violation of the humeral cortex, eliminating potential stress risers associated with screw fixation,47 and effectively eliminating the risk of iatrogenic fracture.48,49 It would be interesting to investigate what occurs histologically at the bone-tendon interface in surface fixation (ie, suture anchors). Would the inflammatory response at the surface be similar to the inflammatory intramedullary healing, or would it be similar to the quieter tendon-tendon healing? Answers to such questions have the potential to streamline the treatment algorithm for patients who require tenodesis.
Study Limitations
Our study had several limitations. First, as this was a basic science study using a rat model, its conclusions can only be extrapolated to humans. Second, given the nonspecific nature of the cellular analysis, we cannot draw any definitive conclusions about the cell population at the bone-tendon interface. For example, although tenomodulin is expressed by tenocytes, it is not an established specific marker for tenocytes and may be expressed by other fibroblastic cells. Still, our results provide insight into the local microenvironment and identify important differences between the tenodesis methods. Similarly, the complete absence of tendon within the bone tunnels suggests that an analysis of osteoclastic activity at the tenodesis interface may have been a valuable addition to the study. This finding, however, was unexpected, and we did not have the foresight to include it in our methods. A third limitation is that our fixation method essentially uses the suspension tenodesis method. This fixation method differs from the common fixation techniques used in the clinical setting. Testing of other fixation constructs would require a larger animal model. Furthermore, in suspension- type constructs, micromotion within the bone tunnel may independently elicit an inflammatory response. Inert suture was used in our fixation in order to reduce the risk of an iatrogenic inflammatory response. Last, it would have been valuable to perform a biomechanical analysis of the strength of each tenodesis construct. This was explored with our institution’s biomechanics team, but specimen size precluded successful analysis.
Conclusion
Our results indicated that, compared with tendon-to-tendon fixation, tendon-to-bone tenodesis produces a significantly greater inflammatory response at the tenodesis interface. An inflammatory milieu in the absence of tendon within the bony tunnel suggests intraosseous tendon degeneration. Tendon-to-tendon tenodesis, on the other hand, seems to limit the inflammatory response. In addition, a robust tenomodulin reaction in the early phases of tendon-to-tendon healing suggests regenerative healing. Our results showed a fundamental difference in the healing response between the 2 tenodesis methods. Further study is needed to evaluate the validity and applicability of our findings to the human patient population. Most important, our results underscore the need for more study to elucidate optimal tenodesis location and encourage orthopedic surgeons to reexamine current clinical practice patterns.
1. Alpantaki K, McLaughlin D, Karagogeos D, Hadjipavlou A, Kontakis G. Sympathetic and sensory neural elements in the tendon of the long head of the biceps. J Bone Joint Surg Am. 2005;87(7):1580-1583.
2. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
3. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
4. Werner BC, Brockmeier SF, Gwathmey FW. Trends in long head biceps tenodesis. Am J Sports Med. 2015;43(3):570-578.
5. Boileau P, Baque F, Valerio L, Ahrens P, Chuinard C, Trojani C. Isolated arthroscopic biceps tenotomy or tenodesis improves symptoms in patients with massive irreparable rotator cuff tears. J Bone Joint Surg Am. 2007;89(4):747-757.
6. Becker DA, Cofield RH. Tenodesis of the long head of the biceps brachii for chronic bicipital tendinitis. Long-term results. J Bone Joint Surg Am. 1989;71(3):376-381.
7. Richards DP, Burkhart SS. Arthroscopic-assisted biceps tenodesis for ruptures of the long head of biceps brachii: the cobra procedure. Arthroscopy. 2004;20(suppl 2):201-207.
8. Ozalay M, Akpinar S, Karaeminogullari O, et al. Mechanical strength of four different biceps tenodesis techniques. Arthroscopy. 2005;21(8):992-998.
9. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
10. Kilicoglu O, Koyuncu O, Demirhan M, et al. Time-dependent changes in failure loads of 3 biceps tenodesis techniques: in vivo study in a sheep model. Am J Sports Med. 2005;33(10):1536-1544.
11. Golish SR, Caldwell PE 3rd, Miller MD, et al. Interference screw versus suture anchor fixation for subpectoral tenodesis of the proximal biceps tendon: a cadaveric study. Arthroscopy. 2008;24(10):1103-1108.
12. Kusma M, Dienst M, Eckert J, Steimer O, Kohn D. Tenodesis of the long head of biceps brachii: cyclic testing of five methods of fixation in a porcine model. J Shoulder Elbow Surg. 2008;17(6):967-973.
13. Buchholz A, Martetschlager F, Siebenlist S, et al. Biomechanical comparison of intramedullary cortical button fixation and interference screw technique for subpectoral biceps tenodesis. Arthroscopy. 2013;29(5):845-853.
14. Werner BC, Lyons ML, Evans CL, et al. Arthroscopic suprapectoral and open subpectoral biceps tenodesis: a comparison of restoration of length-tension and mechanical strength between techniques. Arthroscopy. 2015;31(4):620-627.
15. Gilmer BB, DeMers AM, Guerrero D, Reid JB 3rd, Lubowitz JH, Guttmann D. Arthroscopic versus open comparison of long head of biceps tendon visualization and pathology in patients requiring tenodesis. Arthroscopy. 2015;31(1):29-34.
16. Sanders B, Lavery KP, Pennington S, Warner JJ. Clinical success of biceps tenodesis with and without release of the transverse humeral ligament. J Shoulder Elbow Surg. 2012;21(1):66-71.
17. Taylor SA, Fabricant PD, Bansal M, et al. The anatomy and histology of the bicipital tunnel of the shoulder. J Shoulder Elbow Surg. 2015;24(4):511-519.
18. Taylor SA, Khair MM, Gulotta LV, et al. Diagnostic glenohumeral arthroscopy fails to fully evaluate the biceps-labral complex. Arthroscopy. 2015;31(2):215-224.
19. Lutton DM, Gruson KI, Harrison AK, Gladstone JN, Flatow EL. Where to tenodese the biceps: proximal or distal? Clin Orthop Relat Res. 2011;469(4):1050-1055.
20. Moon SC, Cho NS, Rhee YG. Analysis of “hidden lesions” of the extra-articular biceps after subpectoral biceps tenodesis: the subpectoral portion as the optimal tenodesis site. Am J Sports Med. 2015;43(1):63-68.
21. Festa A, Allert J, Issa K, Tasto JP, Myer JJ. Visualization of the extra-articular portion of the long head of the biceps tendon during intra-articular shoulder arthroscopy. Arthroscopy. 2014;30(11):1413-1417.
22. Friedman DJ, Dunn JC, Higgins LD, Warner JJ. Proximal biceps tendon: injuries and management. Sports Med Arthrosc. 2008;16(3):162-169.
23. Verma NN, Drakos M, O’Brien SJ. Arthroscopic transfer of the long head biceps to the conjoint tendon. Arthroscopy. 2005;21(6):764.
24. O’Brien SJ, Taylor SA, DiPietro JR, Newman AM, Drakos MC, Voos JE. The arthroscopic “subdeltoid approach” to the anterior shoulder. J Shoulder Elbow Surg. 2013;22(4):e6-e10.
25. Drakos MC, Verma NN, Gulotta LV, et al. Arthroscopic transfer of the long head of the biceps tendon: functional outcome and clinical results. Arthroscopy. 2008;24(2):217-223.
26. Taylor SA, Fabricant PD, Baret NJ, et al. Midterm clinical outcomes for arthroscopic subdeltoid transfer of the long head of the biceps tendon to the conjoint tendon. Arthroscopy. 2014;30(12):1574-1581.
27. Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42(6):551-555.
28. Khan SN, Cammisa FP Jr, Sandhu HS, Diwan AD, Girardi FP, Lane JM. The biology of bone healing. J Am Acad Orthop Surg. 2005;13(1):77-86.
29. Hays PL, Kawamura S, Deng XH, et al. The role of macrophages in early healing of a tendon graft in a bone tunnel. J Bone Joint Surg Am. 2008;90(3):565-579.
30. Uhl RL, Roberts TT, Papaliodis DN, Mulligan MT, Dubin AH. Management of chronic musculoskeletal pain. J Am Acad Orthop Surg. 2014;22(2):101-110.
31. Kosek E, Altawil R, Kadetoff D, et al. Evidence of different mediators of central inflammation in dysfunctional and inflammatory pain—interleukin-8 in fibromyalgia and interleukin-1 β in rheumatoid arthritis. J Neuroimmunol. 2015;280:49-55.
32. Slenker NR, Lawson K, Ciccotti MG, Dodson CC, Cohen SB. Biceps tenotomy versus tenodesis: clinical outcomes. Arthroscopy. 2012;28(4):576-582.
33. Rodeo SA, Kawamura S, Kim HJ, Dynybil C, Ying L. Tendon healing in a bone tunnel differs at the tunnel entrance versus the tunnel exit: an effect of graft-tunnel motion? Am J Sports Med. 2006;34(11):1790-1800.
34. Hjorthaug GA, Madsen JE, Nordsletten L, Reinholt FP, Steen H, Dimmen S. Tendon to bone tunnel healing—a study on the time-dependent changes in biomechanics, bone remodeling, and histology in a rat model. J Orthop Res. 2015;33(2):216-223.
35. Pulford KA, Sipos A, Cordell JL, Stross WP, Mason DY. Distribution of the CD68 macrophage/myeloid associated antigen. Int Immunol. 1990;2(10):973-980.
36. Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4(3):281-286.
37. Qi J, Dmochowski JM, Banes AN, et al. Differential expression and cellular localization of novel isoforms of the tendon biomarker tenomodulin. J Appl Physiol (1985). 2012;113(6):861-871.
38. Jelinsky SA, Archambault J, Li L, Seeherman H. Tendon-selective genes identified from rat and human musculoskeletal tissues. J Orthop Res. 2010;28(3):289-297.
39. Docheva D, Hunziker EB, Fassler R, Brandau O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol Cell Biol. 2005;25(2):699-705.
40. Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75(12):1795-1803.
41. Silva MJ, Thomopoulos S, Kusano N, et al. Early healing of flexor tendon insertion site injuries: tunnel repair is mechanically and histologically inferior to surface repair in a canine model. J Orthop Res. 2006;24(5):990-1000.
42. Hibino N, Hamada Y, Sairyo K, Yukata K, Sano T, Yasui N. Callus formation during healing of the repaired tendon–bone junction. A rat experimental model. J Bone Joint Surg Br. 2007;89(11):1539-1544.
43. Bedi A, Kawamura S, Ying L, Rodeo SA. Differences in tendon graft healing between the intra-articular and extra-articular ends of a bone tunnel. HSS J. 2009;5(1):51-57.
44. Richards DP, Burkhart SS. A biomechanical analysis of two biceps tenodesis fixation techniques. Arthroscopy. 2005;21(7):861-866.
45. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
46. Baleani M, Francesconi D, Zani L, Giannini S, Snyder SJ. Suprapectoral biceps tenodesis: a biomechanical comparison of a new “soft anchor” tenodesis technique versus interference screw biceps tendon fixation. Clin Biomech. 2015;30(2):188-194.
47. Euler SA, Smith SD, Williams BT, Dornan GJ, Millett PJ, Wijdicks CA. Biomechanical analysis of subpectoral biceps tenodesis: effect of screw malpositioning on proximal humeral strength. Am J Sports Med. 2015;43(1):69-74.
48. Sears BW, Spencer EE, Getz CL. Humeral fracture following subpectoral biceps tenodesis in 2 active, healthy patients. J Shoulder Elbow Surg. 2011;20(6):e7-e11.
49. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis. Am J Sports Med. 2014;42(4):877-879.
1. Alpantaki K, McLaughlin D, Karagogeos D, Hadjipavlou A, Kontakis G. Sympathetic and sensory neural elements in the tendon of the long head of the biceps. J Bone Joint Surg Am. 2005;87(7):1580-1583.
2. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
3. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
4. Werner BC, Brockmeier SF, Gwathmey FW. Trends in long head biceps tenodesis. Am J Sports Med. 2015;43(3):570-578.
5. Boileau P, Baque F, Valerio L, Ahrens P, Chuinard C, Trojani C. Isolated arthroscopic biceps tenotomy or tenodesis improves symptoms in patients with massive irreparable rotator cuff tears. J Bone Joint Surg Am. 2007;89(4):747-757.
6. Becker DA, Cofield RH. Tenodesis of the long head of the biceps brachii for chronic bicipital tendinitis. Long-term results. J Bone Joint Surg Am. 1989;71(3):376-381.
7. Richards DP, Burkhart SS. Arthroscopic-assisted biceps tenodesis for ruptures of the long head of biceps brachii: the cobra procedure. Arthroscopy. 2004;20(suppl 2):201-207.
8. Ozalay M, Akpinar S, Karaeminogullari O, et al. Mechanical strength of four different biceps tenodesis techniques. Arthroscopy. 2005;21(8):992-998.
9. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
10. Kilicoglu O, Koyuncu O, Demirhan M, et al. Time-dependent changes in failure loads of 3 biceps tenodesis techniques: in vivo study in a sheep model. Am J Sports Med. 2005;33(10):1536-1544.
11. Golish SR, Caldwell PE 3rd, Miller MD, et al. Interference screw versus suture anchor fixation for subpectoral tenodesis of the proximal biceps tendon: a cadaveric study. Arthroscopy. 2008;24(10):1103-1108.
12. Kusma M, Dienst M, Eckert J, Steimer O, Kohn D. Tenodesis of the long head of biceps brachii: cyclic testing of five methods of fixation in a porcine model. J Shoulder Elbow Surg. 2008;17(6):967-973.
13. Buchholz A, Martetschlager F, Siebenlist S, et al. Biomechanical comparison of intramedullary cortical button fixation and interference screw technique for subpectoral biceps tenodesis. Arthroscopy. 2013;29(5):845-853.
14. Werner BC, Lyons ML, Evans CL, et al. Arthroscopic suprapectoral and open subpectoral biceps tenodesis: a comparison of restoration of length-tension and mechanical strength between techniques. Arthroscopy. 2015;31(4):620-627.
15. Gilmer BB, DeMers AM, Guerrero D, Reid JB 3rd, Lubowitz JH, Guttmann D. Arthroscopic versus open comparison of long head of biceps tendon visualization and pathology in patients requiring tenodesis. Arthroscopy. 2015;31(1):29-34.
16. Sanders B, Lavery KP, Pennington S, Warner JJ. Clinical success of biceps tenodesis with and without release of the transverse humeral ligament. J Shoulder Elbow Surg. 2012;21(1):66-71.
17. Taylor SA, Fabricant PD, Bansal M, et al. The anatomy and histology of the bicipital tunnel of the shoulder. J Shoulder Elbow Surg. 2015;24(4):511-519.
18. Taylor SA, Khair MM, Gulotta LV, et al. Diagnostic glenohumeral arthroscopy fails to fully evaluate the biceps-labral complex. Arthroscopy. 2015;31(2):215-224.
19. Lutton DM, Gruson KI, Harrison AK, Gladstone JN, Flatow EL. Where to tenodese the biceps: proximal or distal? Clin Orthop Relat Res. 2011;469(4):1050-1055.
20. Moon SC, Cho NS, Rhee YG. Analysis of “hidden lesions” of the extra-articular biceps after subpectoral biceps tenodesis: the subpectoral portion as the optimal tenodesis site. Am J Sports Med. 2015;43(1):63-68.
21. Festa A, Allert J, Issa K, Tasto JP, Myer JJ. Visualization of the extra-articular portion of the long head of the biceps tendon during intra-articular shoulder arthroscopy. Arthroscopy. 2014;30(11):1413-1417.
22. Friedman DJ, Dunn JC, Higgins LD, Warner JJ. Proximal biceps tendon: injuries and management. Sports Med Arthrosc. 2008;16(3):162-169.
23. Verma NN, Drakos M, O’Brien SJ. Arthroscopic transfer of the long head biceps to the conjoint tendon. Arthroscopy. 2005;21(6):764.
24. O’Brien SJ, Taylor SA, DiPietro JR, Newman AM, Drakos MC, Voos JE. The arthroscopic “subdeltoid approach” to the anterior shoulder. J Shoulder Elbow Surg. 2013;22(4):e6-e10.
25. Drakos MC, Verma NN, Gulotta LV, et al. Arthroscopic transfer of the long head of the biceps tendon: functional outcome and clinical results. Arthroscopy. 2008;24(2):217-223.
26. Taylor SA, Fabricant PD, Baret NJ, et al. Midterm clinical outcomes for arthroscopic subdeltoid transfer of the long head of the biceps tendon to the conjoint tendon. Arthroscopy. 2014;30(12):1574-1581.
27. Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42(6):551-555.
28. Khan SN, Cammisa FP Jr, Sandhu HS, Diwan AD, Girardi FP, Lane JM. The biology of bone healing. J Am Acad Orthop Surg. 2005;13(1):77-86.
29. Hays PL, Kawamura S, Deng XH, et al. The role of macrophages in early healing of a tendon graft in a bone tunnel. J Bone Joint Surg Am. 2008;90(3):565-579.
30. Uhl RL, Roberts TT, Papaliodis DN, Mulligan MT, Dubin AH. Management of chronic musculoskeletal pain. J Am Acad Orthop Surg. 2014;22(2):101-110.
31. Kosek E, Altawil R, Kadetoff D, et al. Evidence of different mediators of central inflammation in dysfunctional and inflammatory pain—interleukin-8 in fibromyalgia and interleukin-1 β in rheumatoid arthritis. J Neuroimmunol. 2015;280:49-55.
32. Slenker NR, Lawson K, Ciccotti MG, Dodson CC, Cohen SB. Biceps tenotomy versus tenodesis: clinical outcomes. Arthroscopy. 2012;28(4):576-582.
33. Rodeo SA, Kawamura S, Kim HJ, Dynybil C, Ying L. Tendon healing in a bone tunnel differs at the tunnel entrance versus the tunnel exit: an effect of graft-tunnel motion? Am J Sports Med. 2006;34(11):1790-1800.
34. Hjorthaug GA, Madsen JE, Nordsletten L, Reinholt FP, Steen H, Dimmen S. Tendon to bone tunnel healing—a study on the time-dependent changes in biomechanics, bone remodeling, and histology in a rat model. J Orthop Res. 2015;33(2):216-223.
35. Pulford KA, Sipos A, Cordell JL, Stross WP, Mason DY. Distribution of the CD68 macrophage/myeloid associated antigen. Int Immunol. 1990;2(10):973-980.
36. Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4(3):281-286.
37. Qi J, Dmochowski JM, Banes AN, et al. Differential expression and cellular localization of novel isoforms of the tendon biomarker tenomodulin. J Appl Physiol (1985). 2012;113(6):861-871.
38. Jelinsky SA, Archambault J, Li L, Seeherman H. Tendon-selective genes identified from rat and human musculoskeletal tissues. J Orthop Res. 2010;28(3):289-297.
39. Docheva D, Hunziker EB, Fassler R, Brandau O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol Cell Biol. 2005;25(2):699-705.
40. Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75(12):1795-1803.
41. Silva MJ, Thomopoulos S, Kusano N, et al. Early healing of flexor tendon insertion site injuries: tunnel repair is mechanically and histologically inferior to surface repair in a canine model. J Orthop Res. 2006;24(5):990-1000.
42. Hibino N, Hamada Y, Sairyo K, Yukata K, Sano T, Yasui N. Callus formation during healing of the repaired tendon–bone junction. A rat experimental model. J Bone Joint Surg Br. 2007;89(11):1539-1544.
43. Bedi A, Kawamura S, Ying L, Rodeo SA. Differences in tendon graft healing between the intra-articular and extra-articular ends of a bone tunnel. HSS J. 2009;5(1):51-57.
44. Richards DP, Burkhart SS. A biomechanical analysis of two biceps tenodesis fixation techniques. Arthroscopy. 2005;21(7):861-866.
45. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
46. Baleani M, Francesconi D, Zani L, Giannini S, Snyder SJ. Suprapectoral biceps tenodesis: a biomechanical comparison of a new “soft anchor” tenodesis technique versus interference screw biceps tendon fixation. Clin Biomech. 2015;30(2):188-194.
47. Euler SA, Smith SD, Williams BT, Dornan GJ, Millett PJ, Wijdicks CA. Biomechanical analysis of subpectoral biceps tenodesis: effect of screw malpositioning on proximal humeral strength. Am J Sports Med. 2015;43(1):69-74.
48. Sears BW, Spencer EE, Getz CL. Humeral fracture following subpectoral biceps tenodesis in 2 active, healthy patients. J Shoulder Elbow Surg. 2011;20(6):e7-e11.
49. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis. Am J Sports Med. 2014;42(4):877-879.
A Systematic Review of 21 Tibial Tubercle Osteotomy Studies and More Than 1000 Knees: Indications, Clinical Outcomes, Complications, and Reoperations
Take-Home Points
- TTO specifics depend on anatomy, radiographic alignment characteristics, and presence of chondral defects.
- Osteotomy and movement of the tibial tubercle can include anteriorization, anteromedialization, proximalization, medialization, or distalization.
- TTO was most commonly performed for isolated patellar instability in the presence of knee pain.
- Young women with prior surgery on the affected knee made up the primary patient population for this procedure.
- While TTO significantly improves knee pain and clinical outcome scores, >1 in 5 patients required reoperation for hardware removal.
Patellofemoral pain and patellofemoral instability are common orthopedic problems. Studies have found that 30% of patients 13 to 19 years old have patellofemoral pain and that 29 in 100,000 patients 10 to 17 years old have patellofemoral instability.1-3 The reported rate of recurrence after nonoperative management of patellofemoral instability is 33%.4 Tibial tubercle osteotomy (TTO), first described by Hauser5 in 1938, is an effective treatment option for many patellofemoral disorders.
TTO indications include patellofemoral maltracking or malalignment, patellar instability, patellofemoral arthritis, and focal patellofemoral chondral defects.6 With TTO, the goal is to move the tibial tubercle in a direction that will either improve patellar tracking or offload the medial or lateral patellar facet to improve pain and function.7,8 This action typically involves anterior, medial, lateral, or distal translation of the tibial tubercle, as posteriorization can lead to increased contact forces across the patellofemoral joint, resulting in accelerated patellofemoral wear and increased pain.9
We systematically reviewed the TTO literature to identify indications, clinical outcomes, complications, and reoperations. We hypothesized that the overall complication rate and the overall reoperation rate would both be <10%.
Clinical Evaluation of Patellofemoral Pathology
Patients with patellofemoral pain often report anterior knee pain, which typically begins gradually and is often activity related. Several symptoms may be present: pain with prolonged sitting with knees bent; pain on rising from a seated position; pain or crepitus with climbing stairs; and pain during repetitive activity such as running, squatting, or jumping. Location, duration, and onset of symptoms should be elicited. Patellofemoral instability can be described as dislocation events or subluxation events; number of events, mechanisms of injury, and resulting need for reduction should be documented. As age, sex, body mass index, and physical fitness are relevant to risk of recurrence, the physician should ask about general ligamentous laxity, other joint dislocations, and prior surgical intervention. Swelling or mechanical symptoms may indicate patellofemoral joint pathology.6,10
Physical examination of patients with patellofemoral pathology begins with assessment for overall limb alignment (including resting position of patella and corresponding quadriceps angle [Q-angle]), generalized ligamentous laxity (including hypermobile joints, evaluated with Brighton criteria), overall peri-knee muscle tone and strength, effusion, and gait pattern.
Common TTO Procedures
TTO specifics depend on anatomy, radiographic alignment characteristics, and presence of chondral defects. Essentially, the patella is translated to offload the affected areas. Osteotomy and movement of the tibial tubercle can include anteriorization, anteromedialization, proximalization, medialization, or distalization.
Methods
Search Strategy and Data Collection
We searched the PubMed (Medline) database for all English-language TTO studies published between database inception and April 9, 2015. After PROSPERO registration, and following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, we used the algorithm (“tibial” AND “tubercle” AND “osteotomy”) NOT (“total” AND “knee” AND “arthroplasty”) to search the literature. Inclusion criteria included level I-IV studies on TTO indications, operative findings, and outcomes. Exclusion criteria were non-English studies, unpublished studies, level V evidence, letters to the editor, editorials, review articles, basic science articles, technique articles, revision procedures, articles without clinical outcomes, and conference proceeding abstracts. Studies that reported on duplicate populations were included only with the most recent available clinical outcomes. All abstracts were reviewed in duplicate by Dr. Levy and Dr. Rao and assessed with respect to the criteria outlined. Then the same authors performed full-text reviews of eligible studies before including these studies in the systematic review.
Assessment of Study Quality
The quality of each TTO study in the review was assessed with a modified Coleman methodology score (MCMS), which ranges from 0 to 100. A study with an MCMS of <55 points is considered a poor-quality study.11
Data Synthesis and Statistical Analysis
Given that most of the included studies were level IV, a formal meta-analysis was not indicated. In this article, we report categorical data as frequencies with percentages and preoperative and postoperative continuous data as means (SDs), with weighted means based on number of patients in each study, where applicable. We used 2-tailed t tests for comparisons made with the free Meta-Analysis Calculator and Grapher (http://www.healthstrategy.com/meta/meta.pl ). Statistical significance was set at P < .05.
Results
Search Results and Included Studies
Only 1 study provided preoperative body mass index (27 kg/m2). There were 55.35% of patients who had prior surgery on the affected knee (6 studies reporting).
Preoperative Data
Preoperative pathologic, radiographic, and clinical scoring data were scarcely reported and nonuniform (Table 2). The most common pathology treated with TTO was isolated patellofemoral instability (746/1055 patients, 70.7%). The other pathologies addressed were isolated patellofemoral osteoarthritis/chondromalacia patellae (143, 13.6%), patellofemoral instability with patella alta (61, 5.8%), patellofemoral instability with patellofemoral osteoarthritis (45, 4.3%), isolated patella baja (41, 3.9%), isolated patella alta (19, 1.8%), and patellofemoral osteoarthritis with patella baja (2, 0.2%). Five hundred fifty-five patients (53%) had a preoperative complaint that included knee pain, and 809 (77%) reported preoperative patellar laxity or instability events. The imaging data reported were Q-angle, Insall-Salvati ratio, Caton-Deschamps index, Blackburne-Peel ratio, Outerbridge osteoarthritis grade, and TT-TG distance. Preoperative clinical scoring data most prominently included a visual analog scale (VAS) score of 70.50 (4 studies reporting), a Lysholm score of 59.19 (5 studies), and a Kujala score of 41.16 (4 studies). Shelbourne-Trumper and Cox-Insall scores were reported in 1 and 2 studies, respectively.
Operative Characteristics
Of the 21 studies, 12 reported only on patients who had TTO performed in isolation; in the other 9 studies, cohorts included patients who underwent concurrent procedures. In the 17 studies (856 patients) that listed numbers of patients who underwent specific concomitant procedures, 715 patients (83.5%) underwent an isolated TTO procedure, and the other 141 (16.5%) underwent either concomitant lateral femoral trochleoplasty, arthroscopic drilling of chondral lesions, patellar shaving chondroplasty, partial meniscectomy or concomitant meniscal repair, intra-articular loose body removal, and/or lateral release with or without medial plication.
Postoperative Data
There was a cumulative total of 79 complications (8% of cohort): 17 recurrent patellar dislocations (1.9%), 4 recurrent patellar subluxations (0.4%), 10 wound complications (1.0%), 2 intraoperative complications (0.2%), 14 tibial tubercle fractures (1.3%), 19 proximal tibia fractures (1.8%), 4 cases of anterior knee pain (0.4%), 4 cases of neuropraxia (0.4%), and 5 infections (0.5%). Of note, 219 knees (21%) required reoperation, but 170 (16.3%) of these were for painful hardware removal. Sixteen knees (1.5%) required revision TTO, 1 (0.1%) required subsequent high tibial osteotomy, 2 (0.2%) underwent patellofemoral arthroplasty for advanced arthritic changes, and 5 (0.5%) underwent total knee arthroplasty for advanced arthritic changes.
Studies With TTO Performed in Isolation
Twelve studies reported outcomes of isolated TTO procedures. In the 638 patients who underwent isolated TTO, the pathologies addressed were instability/laxity (429 patients, 67%), patellofemoral osteoarthritis (74, 12%), patella alta with instability (61, 10%), patellofemoral osteoarthritis with instability (31, 5%), patella baja (24, 4%), and patella alta (19, 3%). Pain was a preoperative issue in 289 (45%) of these patients and instability in 472 (74%).
Only 2.8% of patients experienced postoperative patellar dislocation events. Of the 12 studies, 2 reported VAS scores (34-point weighted mean improvement, 65 points before surgery to 31 after surgery), 3 reported Lysholm scores (30-point improvement, from 60 to 90), and 2 reported Kujala scores (21-point improvement, from 46 to 67).
Complication rates for this isolated-TTO pooled cohort of patients were 1.2% for revision TTOs, 0.5% for wound complications, 0.8% for tibial tubercle fractures, and 1.9% for proximal tibia fractures. In total, 16% of patients required hardware removal after surgery.
Discussion
This study found that TTO improved patient pain and clinical outcome scores despite having a high (16%) rate of reoperation for painful hardware in patients with preoperative pain or instability, or with patellofemoral osteoarthritis or aberrant patellar anatomy. This reoperation rate and the overall complication rate both exceeded our hypothesized 10% cumulative rate. However, <1% of patients required conversion to a definitive end-stage surgery (patellofemoral arthroplasty or total knee arthroplasty) by final follow-up, and the rates of comorbidities (anterior knee pain, wound infection, recurrent patellar subluxation/dislocation, tibial fracture) were relatively low.
Patellofemoral disorders are common in the general population and a frequent primary complaint on presentation to orthopedic offices. Having a thorough understanding of knee joint biomechanics is imperative when trying to determine whether surgery is appropriate for these complaints and how to proceed. Extensor mechanism abnormalities, including high lateral force vectors (or larger TT-TG distances) and excessive patellar tilt, can affect alignment and increase the risk for patellofemoral dislocations, patellofemoral anterior- based knee pains, and chondral lesions. Patella alta, an elevated patella, risks increased contact stresses between the patella and the trochlear groove33 and decreases the osseous constraints that inhibit dislocation of the patella with physiologic flexion of the joint.34 With TTO, the change in tuberosity position can alter angles in the extensor mechanism and thereby decrease joint reaction forces and patellofemoral contact area forces.35,36
Although its use began as an option for combating patellar instability events in patients with predisposed patellofemoral kinematics,5 TTO has evolved in its therapeutic uses to include offloading patellar and trochlear focal chondral lesions and slowing progression of patellofemoral arthritis. Multiple iterations and modifications of the procedure have involved distal and medial transfer of the tibial tuberosity, medialization alone, concurrent anterior and medial elevation of the tuberosity, and proximal or distal transfers, depending on the pathology being corrected. Although TTO is highly versatile in treating multiple patellofemoral joint pathologies, this study found that its primary indication continues to be patellar instability, with anteromedialization as the most common direction of tubercle transfer in support of the medial structures providing the medial force vector that keeps the patella in place. These medial structures include the medial patellofemoral ligament, the vastus medialis obliquus, the medial patellotibial ligament, and the medial retinaculum.
Also notable was the relatively high rate of reoperation after TTO. However, >75% of reoperations were performed to remove painful hardware, and the need for reoperation seemed to have no effect on the statistically significant overall preoperative-to-postoperative improvement in VAS, Lysholm, and Kujala scores. Rates of definitive surgery for end-stage patellofemoral changes, including patellofemoral arthroplasty and total knee arthroplasty, were quite low at the weighted mean follow-up of several years after surgery, suggesting a role for TTO in avoiding arthroplasty. Although the infection rate was <1%, the rate of tibial tubercle or proximal tibia fractures was a cumulative 3.1%. Patients should be counseled on this complication risk, as treatment can require cast immobilization and weight-bearing limitations.24
The 69% proportion of women in the overall cohort and the mean (SD) age of 27.68 (10.45) years highlight the primary patient population that undergoes TTO. Compared with men, young women are more likely to have aberrant patellofemoral biomechanics, owing to their native anatomy, including their relatively larger Q-angle and TT-TG distance and thus increased lateral translational force vectors on the patella.37 In addition, more than half of patients who are having TTO underwent previous surgery on the affected knee—an indication that TTO is still not universally considered first-line in addressing patellofemoral pathology.
Limitations of the Analysis
The limitations of this analysis derive from the limitations of the included studies, which were mostly retrospective case series with relatively short follow-up. The low MCMS (<55) of all 21 studies highlights their low quality as well. These studies showed considerable heterogeneity in their reporting of specific preoperative, intraoperative, and postoperative radiographic, physical examination, and clinical outcome scores, which may be indicative of the relatively low rate of use of TTO, a procedure originally described decades ago. These studies also showed ample heterogeneity in the specific radiographic parameters or outcome scales they used to present their data. We were therefore limited in our ability to cohesively summarize and provide cumulative data points from the patients as a unified cohort. There was substantial variety in the procedures performed, surgical techniques used, concomitant pathologies addressed at time of surgery, and diagnoses treated—indicating a performance bias. This additionally precluded any significant meta-analysis within the patient cohort. A higher quality study, a randomized controlled trial, is needed to answer more definitively and completely the questions we left unanswered, including the effect on radiographic parameters, additional clinical outcomes, and patient satisfaction.
Conclusion
TTO is most commonly performed for isolated patellar instability in the presence of knee pain. Other pathologies addressed are patellofemoral osteoarthritis, and patella alta and patella baja with and without associated knee pain. TTO significantly improves knee pain and clinical outcome scores, though 21% of patients (>1 in 5) require reoperation for hardware removal. Young women with prior surgery on the affected knee are the primary patient population.
1. Blond L, Hansen L. Patellofemoral pain syndrome in athletes: a 5.7- year retrospective follow-up study of 250 athletes. Acta Orthop Belg. 1998;64(4):393-400.
2. Fairbank JC, Pynsent PB, van Poortvliet JA, Phillips H. Mechanical factors in the incidence of knee pain in adolescents and young adults. J Bone Joint Surg Br. 1984;66(5):685-693.
3. Mehta VM, Inoue M, Nomura E, Fithian DC. An algorithm guiding the evaluation and treatment of acute primary patellar dislocations. Sports Med Arthrosc. 2007;15(2):78-81.
4. Erickson BJ, Mascarenhas R, Sayegh ET, et al. Does operative treatment of first-time patellar dislocations lead to increased patellofemoral stability? A systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(6):1207-1215.
5. Hauser E. Total tendon transplant for slipping patella. Surg Gynecol Obstet. 1938;66:199-214.
6. Sherman SL, Erickson BJ, Cvetanovich GL, et al. Tibial tuberosity osteotomy: indications, techniques, and outcomes. Am J Sports Med. 2014;42(8):2006-2017.
7. Hall MJ, Mandalia VI. Tibial tubercle osteotomy for patello-femoral joint disorders. Knee Surg Sports Traumatol Arthrosc. 2016;24(3):855-861.
8. Grawe B, Stein BS. Tibial tubercle osteotomy: indication and techniques. J Knee Surg. 2015;28(4):279-284.
9. Fulkerson JP. Disorders of the Patellofemoral Joint. 4th ed. Baltimore, MD: Williams & Wilkins; 1997.
10. Koh JL, Stewart C. Patellar instability. Clin Sports Med. 2014;33(3):461-476.
11. Coleman BD, Khan KM, Maffulli N, Cook JL, Wark JD. Studies of surgical outcome after patellar tendinopathy: clinical significance of methodological deficiencies and guidelines for future studies. Victorian Institute of Sport Tendon Study Group. Scand J Med Sci Sports. 2000;10(1):2-11.
12. Al-Sayyad MJ, Cameron JC. Functional outcome after tibial tubercle transfer for the painful patella alta. Clin Orthop Rel Res. 2002;(396):152-162.
13. Atkinson HD, Bailey CA, Anand S, Johal P, Oakeshott RD. Tibial tubercle advancement osteotomy with bone allograft for patellofemoral arthritis: a retrospective cohort study of 50 knees. Arch Orthop Trauma Surg. 2012;132(4):437-445.
14. Caton JH, Dejour D. Tibial tubercle osteotomy in patello-femoral instability and in patellar height abnormality. Int Orthop. 2010;34(2):305-309.
15. Dantas P, Nunes C, Moreira J, Amaral LB. Antero-medialisation of the tibial tubercle for patellar instability. Int Orthop. 2005;29(6):390-391.
16. Drexler M, Dwyer T, Marmor M, Sternheim A, Cameron HU, Cameron JC. The treatment of acquired patella baja with proximalize the tibial tuberosity. Knee Surg Sports Traumatol Arthrosc. 2013;21(11):2578-2583.
17. Eager MR, Bader DA, Kelly JD 4th, Moyer RA. Delayed fracture of the tibia following anteromedialization osteotomy of the tibial tubercle: a report of 5 cases. Am J Sports Med. 2004;32(4):1041-1048.
18. Ebinger TP, Boezaart A, Albright JP. Modifications of the Fulkerson osteotomy: a pilot study assessment of a novel technique of dynamic intraoperative determination of the adequacy of tubercle transfer. Iowa Orthop J. 2007;27:61-64.
19. Fulkerson JP, Becker GJ, Meaney JA, Miranda M, Folcik MA. Anteromedial tibial tubercle transfer without bone graft. Am J Sports Med. 1990;18(5):490-498.
20. Heatley FW, Allen PR, Patrick JH. Tibial tubercle advancement for anterior knee pain: a temporary or permanent solution. Clin Orthop Relat Res. 1986;(208):216-225.
21. Hirsh DM, Reddy DK. Experience with Maquet anterior tibial tubercle advancement for patellofemoral arthralgia. Clin Orthop Relat Res. 1980;(148):136-139.
22. Jack CM, Rajaratnam SS, Khan HO, Keast-Butler O, Butler-Manuel PA, Heatley FW. The modified tibial tubercle osteotomy for anterior knee pain due to chondromalacia patellae in adults: a five-year prospective study. Bone Joint Res. 2012;1(8):167-173.
23. Koëter S, Diks MJ, Anderson PG, Wymenga AB. A modified tibial tubercle osteotomy for patellar maltracking: results at two years. J Bone Joint Surg Br. 2007;89(2):180-185.
24. Luhmann SJ, Fuhrhop S, O’Donnell JC, Gordon JE. Tibial fractures after tibial tubercle osteotomies for patellar instability: a comparison of three osteotomy configurations. J Child Orthop. 2011;5(1):19-26.
25. Naranja RJ Jr, Reilly PJ, Kuhlman JR, Haut E, Torg JS. Long-term evaluation of the Elmslie-Trillat-Maquet procedure for patellofemoral dysfunction. Am J Sports Med. 1996;24(6):779-784.
26. Naveed MA, Ackroyd CE, Porteous AJ. Long-term (ten- to 15-year) outcome of arthroscopically assisted Elmslie-Trillat tibial tubercle osteotomy. Bone Joint J. 2013;95(4):478-485.
27. Paulos L, Swanson SC, Stoddard GJ, Barber-Westin S. Surgical correction of limb malalignment for instability of the patella: a comparison of 2 techniques. Am J Sports Med. 2009;37(7):1288-1300.
28. Pidoriano AJ, Weinstein RN, Buuck DA, Fulkerson JP. Correlation of patellar articular lesions with results from anteromedial tibial tubercle transfer. Am J Sports Med. 1997;25(4):533-537.
29. Shen HC, Chao KH, Huang GS, Pan RY, Lee CH. Combined proximal and distal realignment procedures to treat the habitual dislocation of the patella in adults. Am J Sports Med. 2007;35(12):2101-2108.
30. Stetson WB, Friedman MJ, Fulkerson JP, Cheng M, Buuck D. Fracture of the proximal tibia with immediate weightbearing after a Fulkerson osteotomy. Am J Sports Med. 1997;25(4):570-574.
31. Valenzuela L, Nemtala F, Orrego M, et al. Treatment of patellofemoral chondropathy with the Bandi tibial tubercle osteotomy: more than 10 years follow-up. Knee. 2011;18(2):94-97.
32. Wang CJ, Wong T, Ko JY, Siu KK. Triple positioning of tibial tubercle osteotomy for patellofemoral disorders. Knee. 2014;21(1):133-137.
33. Luyckx T, Didden K, Vandenneucker H, Labey L, Innocenti B, Bellemans J. Is there a biomechanical explanation for anterior knee pain in patients with patella alta? Influence of patellar height on patellofemoral contact force, contact area and contact pressure. J Bone Joint Surg Br. 2009;91(3):344-350.
34. Mayer C, Magnussen RA, Servien E, et al. Patellar tendon tenodesis in association with tibial tubercle distalization for the treatment of episodic patellar dislocation with patella alta. Am J Sports Med. 2012;40(2):346-351.
35. Maquet P. Advancement of the tibial tuberosity. Clin Orthop Relat Res. 1976;(115):225-230.
36. Lewallen DG, Riegger CL, Myers ER, Hayes WC. Effects of retinacular release and tibial tubercle elevation in patellofemoral degenerative joint disease. J Orthop Res. 1990;8(6):856-862.
37. Aglietti P, Insall JN, Cerulli G. Patellar pain and incongruence, I: measurements of incongruence. Clin Orthop Relat Res. 1983;(176):217-224.
Take-Home Points
- TTO specifics depend on anatomy, radiographic alignment characteristics, and presence of chondral defects.
- Osteotomy and movement of the tibial tubercle can include anteriorization, anteromedialization, proximalization, medialization, or distalization.
- TTO was most commonly performed for isolated patellar instability in the presence of knee pain.
- Young women with prior surgery on the affected knee made up the primary patient population for this procedure.
- While TTO significantly improves knee pain and clinical outcome scores, >1 in 5 patients required reoperation for hardware removal.
Patellofemoral pain and patellofemoral instability are common orthopedic problems. Studies have found that 30% of patients 13 to 19 years old have patellofemoral pain and that 29 in 100,000 patients 10 to 17 years old have patellofemoral instability.1-3 The reported rate of recurrence after nonoperative management of patellofemoral instability is 33%.4 Tibial tubercle osteotomy (TTO), first described by Hauser5 in 1938, is an effective treatment option for many patellofemoral disorders.
TTO indications include patellofemoral maltracking or malalignment, patellar instability, patellofemoral arthritis, and focal patellofemoral chondral defects.6 With TTO, the goal is to move the tibial tubercle in a direction that will either improve patellar tracking or offload the medial or lateral patellar facet to improve pain and function.7,8 This action typically involves anterior, medial, lateral, or distal translation of the tibial tubercle, as posteriorization can lead to increased contact forces across the patellofemoral joint, resulting in accelerated patellofemoral wear and increased pain.9
We systematically reviewed the TTO literature to identify indications, clinical outcomes, complications, and reoperations. We hypothesized that the overall complication rate and the overall reoperation rate would both be <10%.
Clinical Evaluation of Patellofemoral Pathology
Patients with patellofemoral pain often report anterior knee pain, which typically begins gradually and is often activity related. Several symptoms may be present: pain with prolonged sitting with knees bent; pain on rising from a seated position; pain or crepitus with climbing stairs; and pain during repetitive activity such as running, squatting, or jumping. Location, duration, and onset of symptoms should be elicited. Patellofemoral instability can be described as dislocation events or subluxation events; number of events, mechanisms of injury, and resulting need for reduction should be documented. As age, sex, body mass index, and physical fitness are relevant to risk of recurrence, the physician should ask about general ligamentous laxity, other joint dislocations, and prior surgical intervention. Swelling or mechanical symptoms may indicate patellofemoral joint pathology.6,10
Physical examination of patients with patellofemoral pathology begins with assessment for overall limb alignment (including resting position of patella and corresponding quadriceps angle [Q-angle]), generalized ligamentous laxity (including hypermobile joints, evaluated with Brighton criteria), overall peri-knee muscle tone and strength, effusion, and gait pattern.
Common TTO Procedures
TTO specifics depend on anatomy, radiographic alignment characteristics, and presence of chondral defects. Essentially, the patella is translated to offload the affected areas. Osteotomy and movement of the tibial tubercle can include anteriorization, anteromedialization, proximalization, medialization, or distalization.
Methods
Search Strategy and Data Collection
We searched the PubMed (Medline) database for all English-language TTO studies published between database inception and April 9, 2015. After PROSPERO registration, and following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, we used the algorithm (“tibial” AND “tubercle” AND “osteotomy”) NOT (“total” AND “knee” AND “arthroplasty”) to search the literature. Inclusion criteria included level I-IV studies on TTO indications, operative findings, and outcomes. Exclusion criteria were non-English studies, unpublished studies, level V evidence, letters to the editor, editorials, review articles, basic science articles, technique articles, revision procedures, articles without clinical outcomes, and conference proceeding abstracts. Studies that reported on duplicate populations were included only with the most recent available clinical outcomes. All abstracts were reviewed in duplicate by Dr. Levy and Dr. Rao and assessed with respect to the criteria outlined. Then the same authors performed full-text reviews of eligible studies before including these studies in the systematic review.
Assessment of Study Quality
The quality of each TTO study in the review was assessed with a modified Coleman methodology score (MCMS), which ranges from 0 to 100. A study with an MCMS of <55 points is considered a poor-quality study.11
Data Synthesis and Statistical Analysis
Given that most of the included studies were level IV, a formal meta-analysis was not indicated. In this article, we report categorical data as frequencies with percentages and preoperative and postoperative continuous data as means (SDs), with weighted means based on number of patients in each study, where applicable. We used 2-tailed t tests for comparisons made with the free Meta-Analysis Calculator and Grapher (http://www.healthstrategy.com/meta/meta.pl ). Statistical significance was set at P < .05.
Results
Search Results and Included Studies
Only 1 study provided preoperative body mass index (27 kg/m2). There were 55.35% of patients who had prior surgery on the affected knee (6 studies reporting).
Preoperative Data
Preoperative pathologic, radiographic, and clinical scoring data were scarcely reported and nonuniform (Table 2). The most common pathology treated with TTO was isolated patellofemoral instability (746/1055 patients, 70.7%). The other pathologies addressed were isolated patellofemoral osteoarthritis/chondromalacia patellae (143, 13.6%), patellofemoral instability with patella alta (61, 5.8%), patellofemoral instability with patellofemoral osteoarthritis (45, 4.3%), isolated patella baja (41, 3.9%), isolated patella alta (19, 1.8%), and patellofemoral osteoarthritis with patella baja (2, 0.2%). Five hundred fifty-five patients (53%) had a preoperative complaint that included knee pain, and 809 (77%) reported preoperative patellar laxity or instability events. The imaging data reported were Q-angle, Insall-Salvati ratio, Caton-Deschamps index, Blackburne-Peel ratio, Outerbridge osteoarthritis grade, and TT-TG distance. Preoperative clinical scoring data most prominently included a visual analog scale (VAS) score of 70.50 (4 studies reporting), a Lysholm score of 59.19 (5 studies), and a Kujala score of 41.16 (4 studies). Shelbourne-Trumper and Cox-Insall scores were reported in 1 and 2 studies, respectively.
Operative Characteristics
Of the 21 studies, 12 reported only on patients who had TTO performed in isolation; in the other 9 studies, cohorts included patients who underwent concurrent procedures. In the 17 studies (856 patients) that listed numbers of patients who underwent specific concomitant procedures, 715 patients (83.5%) underwent an isolated TTO procedure, and the other 141 (16.5%) underwent either concomitant lateral femoral trochleoplasty, arthroscopic drilling of chondral lesions, patellar shaving chondroplasty, partial meniscectomy or concomitant meniscal repair, intra-articular loose body removal, and/or lateral release with or without medial plication.
Postoperative Data
There was a cumulative total of 79 complications (8% of cohort): 17 recurrent patellar dislocations (1.9%), 4 recurrent patellar subluxations (0.4%), 10 wound complications (1.0%), 2 intraoperative complications (0.2%), 14 tibial tubercle fractures (1.3%), 19 proximal tibia fractures (1.8%), 4 cases of anterior knee pain (0.4%), 4 cases of neuropraxia (0.4%), and 5 infections (0.5%). Of note, 219 knees (21%) required reoperation, but 170 (16.3%) of these were for painful hardware removal. Sixteen knees (1.5%) required revision TTO, 1 (0.1%) required subsequent high tibial osteotomy, 2 (0.2%) underwent patellofemoral arthroplasty for advanced arthritic changes, and 5 (0.5%) underwent total knee arthroplasty for advanced arthritic changes.
Studies With TTO Performed in Isolation
Twelve studies reported outcomes of isolated TTO procedures. In the 638 patients who underwent isolated TTO, the pathologies addressed were instability/laxity (429 patients, 67%), patellofemoral osteoarthritis (74, 12%), patella alta with instability (61, 10%), patellofemoral osteoarthritis with instability (31, 5%), patella baja (24, 4%), and patella alta (19, 3%). Pain was a preoperative issue in 289 (45%) of these patients and instability in 472 (74%).
Only 2.8% of patients experienced postoperative patellar dislocation events. Of the 12 studies, 2 reported VAS scores (34-point weighted mean improvement, 65 points before surgery to 31 after surgery), 3 reported Lysholm scores (30-point improvement, from 60 to 90), and 2 reported Kujala scores (21-point improvement, from 46 to 67).
Complication rates for this isolated-TTO pooled cohort of patients were 1.2% for revision TTOs, 0.5% for wound complications, 0.8% for tibial tubercle fractures, and 1.9% for proximal tibia fractures. In total, 16% of patients required hardware removal after surgery.
Discussion
This study found that TTO improved patient pain and clinical outcome scores despite having a high (16%) rate of reoperation for painful hardware in patients with preoperative pain or instability, or with patellofemoral osteoarthritis or aberrant patellar anatomy. This reoperation rate and the overall complication rate both exceeded our hypothesized 10% cumulative rate. However, <1% of patients required conversion to a definitive end-stage surgery (patellofemoral arthroplasty or total knee arthroplasty) by final follow-up, and the rates of comorbidities (anterior knee pain, wound infection, recurrent patellar subluxation/dislocation, tibial fracture) were relatively low.
Patellofemoral disorders are common in the general population and a frequent primary complaint on presentation to orthopedic offices. Having a thorough understanding of knee joint biomechanics is imperative when trying to determine whether surgery is appropriate for these complaints and how to proceed. Extensor mechanism abnormalities, including high lateral force vectors (or larger TT-TG distances) and excessive patellar tilt, can affect alignment and increase the risk for patellofemoral dislocations, patellofemoral anterior- based knee pains, and chondral lesions. Patella alta, an elevated patella, risks increased contact stresses between the patella and the trochlear groove33 and decreases the osseous constraints that inhibit dislocation of the patella with physiologic flexion of the joint.34 With TTO, the change in tuberosity position can alter angles in the extensor mechanism and thereby decrease joint reaction forces and patellofemoral contact area forces.35,36
Although its use began as an option for combating patellar instability events in patients with predisposed patellofemoral kinematics,5 TTO has evolved in its therapeutic uses to include offloading patellar and trochlear focal chondral lesions and slowing progression of patellofemoral arthritis. Multiple iterations and modifications of the procedure have involved distal and medial transfer of the tibial tuberosity, medialization alone, concurrent anterior and medial elevation of the tuberosity, and proximal or distal transfers, depending on the pathology being corrected. Although TTO is highly versatile in treating multiple patellofemoral joint pathologies, this study found that its primary indication continues to be patellar instability, with anteromedialization as the most common direction of tubercle transfer in support of the medial structures providing the medial force vector that keeps the patella in place. These medial structures include the medial patellofemoral ligament, the vastus medialis obliquus, the medial patellotibial ligament, and the medial retinaculum.
Also notable was the relatively high rate of reoperation after TTO. However, >75% of reoperations were performed to remove painful hardware, and the need for reoperation seemed to have no effect on the statistically significant overall preoperative-to-postoperative improvement in VAS, Lysholm, and Kujala scores. Rates of definitive surgery for end-stage patellofemoral changes, including patellofemoral arthroplasty and total knee arthroplasty, were quite low at the weighted mean follow-up of several years after surgery, suggesting a role for TTO in avoiding arthroplasty. Although the infection rate was <1%, the rate of tibial tubercle or proximal tibia fractures was a cumulative 3.1%. Patients should be counseled on this complication risk, as treatment can require cast immobilization and weight-bearing limitations.24
The 69% proportion of women in the overall cohort and the mean (SD) age of 27.68 (10.45) years highlight the primary patient population that undergoes TTO. Compared with men, young women are more likely to have aberrant patellofemoral biomechanics, owing to their native anatomy, including their relatively larger Q-angle and TT-TG distance and thus increased lateral translational force vectors on the patella.37 In addition, more than half of patients who are having TTO underwent previous surgery on the affected knee—an indication that TTO is still not universally considered first-line in addressing patellofemoral pathology.
Limitations of the Analysis
The limitations of this analysis derive from the limitations of the included studies, which were mostly retrospective case series with relatively short follow-up. The low MCMS (<55) of all 21 studies highlights their low quality as well. These studies showed considerable heterogeneity in their reporting of specific preoperative, intraoperative, and postoperative radiographic, physical examination, and clinical outcome scores, which may be indicative of the relatively low rate of use of TTO, a procedure originally described decades ago. These studies also showed ample heterogeneity in the specific radiographic parameters or outcome scales they used to present their data. We were therefore limited in our ability to cohesively summarize and provide cumulative data points from the patients as a unified cohort. There was substantial variety in the procedures performed, surgical techniques used, concomitant pathologies addressed at time of surgery, and diagnoses treated—indicating a performance bias. This additionally precluded any significant meta-analysis within the patient cohort. A higher quality study, a randomized controlled trial, is needed to answer more definitively and completely the questions we left unanswered, including the effect on radiographic parameters, additional clinical outcomes, and patient satisfaction.
Conclusion
TTO is most commonly performed for isolated patellar instability in the presence of knee pain. Other pathologies addressed are patellofemoral osteoarthritis, and patella alta and patella baja with and without associated knee pain. TTO significantly improves knee pain and clinical outcome scores, though 21% of patients (>1 in 5) require reoperation for hardware removal. Young women with prior surgery on the affected knee are the primary patient population.
Take-Home Points
- TTO specifics depend on anatomy, radiographic alignment characteristics, and presence of chondral defects.
- Osteotomy and movement of the tibial tubercle can include anteriorization, anteromedialization, proximalization, medialization, or distalization.
- TTO was most commonly performed for isolated patellar instability in the presence of knee pain.
- Young women with prior surgery on the affected knee made up the primary patient population for this procedure.
- While TTO significantly improves knee pain and clinical outcome scores, >1 in 5 patients required reoperation for hardware removal.
Patellofemoral pain and patellofemoral instability are common orthopedic problems. Studies have found that 30% of patients 13 to 19 years old have patellofemoral pain and that 29 in 100,000 patients 10 to 17 years old have patellofemoral instability.1-3 The reported rate of recurrence after nonoperative management of patellofemoral instability is 33%.4 Tibial tubercle osteotomy (TTO), first described by Hauser5 in 1938, is an effective treatment option for many patellofemoral disorders.
TTO indications include patellofemoral maltracking or malalignment, patellar instability, patellofemoral arthritis, and focal patellofemoral chondral defects.6 With TTO, the goal is to move the tibial tubercle in a direction that will either improve patellar tracking or offload the medial or lateral patellar facet to improve pain and function.7,8 This action typically involves anterior, medial, lateral, or distal translation of the tibial tubercle, as posteriorization can lead to increased contact forces across the patellofemoral joint, resulting in accelerated patellofemoral wear and increased pain.9
We systematically reviewed the TTO literature to identify indications, clinical outcomes, complications, and reoperations. We hypothesized that the overall complication rate and the overall reoperation rate would both be <10%.
Clinical Evaluation of Patellofemoral Pathology
Patients with patellofemoral pain often report anterior knee pain, which typically begins gradually and is often activity related. Several symptoms may be present: pain with prolonged sitting with knees bent; pain on rising from a seated position; pain or crepitus with climbing stairs; and pain during repetitive activity such as running, squatting, or jumping. Location, duration, and onset of symptoms should be elicited. Patellofemoral instability can be described as dislocation events or subluxation events; number of events, mechanisms of injury, and resulting need for reduction should be documented. As age, sex, body mass index, and physical fitness are relevant to risk of recurrence, the physician should ask about general ligamentous laxity, other joint dislocations, and prior surgical intervention. Swelling or mechanical symptoms may indicate patellofemoral joint pathology.6,10
Physical examination of patients with patellofemoral pathology begins with assessment for overall limb alignment (including resting position of patella and corresponding quadriceps angle [Q-angle]), generalized ligamentous laxity (including hypermobile joints, evaluated with Brighton criteria), overall peri-knee muscle tone and strength, effusion, and gait pattern.
Common TTO Procedures
TTO specifics depend on anatomy, radiographic alignment characteristics, and presence of chondral defects. Essentially, the patella is translated to offload the affected areas. Osteotomy and movement of the tibial tubercle can include anteriorization, anteromedialization, proximalization, medialization, or distalization.
Methods
Search Strategy and Data Collection
We searched the PubMed (Medline) database for all English-language TTO studies published between database inception and April 9, 2015. After PROSPERO registration, and following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, we used the algorithm (“tibial” AND “tubercle” AND “osteotomy”) NOT (“total” AND “knee” AND “arthroplasty”) to search the literature. Inclusion criteria included level I-IV studies on TTO indications, operative findings, and outcomes. Exclusion criteria were non-English studies, unpublished studies, level V evidence, letters to the editor, editorials, review articles, basic science articles, technique articles, revision procedures, articles without clinical outcomes, and conference proceeding abstracts. Studies that reported on duplicate populations were included only with the most recent available clinical outcomes. All abstracts were reviewed in duplicate by Dr. Levy and Dr. Rao and assessed with respect to the criteria outlined. Then the same authors performed full-text reviews of eligible studies before including these studies in the systematic review.
Assessment of Study Quality
The quality of each TTO study in the review was assessed with a modified Coleman methodology score (MCMS), which ranges from 0 to 100. A study with an MCMS of <55 points is considered a poor-quality study.11
Data Synthesis and Statistical Analysis
Given that most of the included studies were level IV, a formal meta-analysis was not indicated. In this article, we report categorical data as frequencies with percentages and preoperative and postoperative continuous data as means (SDs), with weighted means based on number of patients in each study, where applicable. We used 2-tailed t tests for comparisons made with the free Meta-Analysis Calculator and Grapher (http://www.healthstrategy.com/meta/meta.pl ). Statistical significance was set at P < .05.
Results
Search Results and Included Studies
Only 1 study provided preoperative body mass index (27 kg/m2). There were 55.35% of patients who had prior surgery on the affected knee (6 studies reporting).
Preoperative Data
Preoperative pathologic, radiographic, and clinical scoring data were scarcely reported and nonuniform (Table 2). The most common pathology treated with TTO was isolated patellofemoral instability (746/1055 patients, 70.7%). The other pathologies addressed were isolated patellofemoral osteoarthritis/chondromalacia patellae (143, 13.6%), patellofemoral instability with patella alta (61, 5.8%), patellofemoral instability with patellofemoral osteoarthritis (45, 4.3%), isolated patella baja (41, 3.9%), isolated patella alta (19, 1.8%), and patellofemoral osteoarthritis with patella baja (2, 0.2%). Five hundred fifty-five patients (53%) had a preoperative complaint that included knee pain, and 809 (77%) reported preoperative patellar laxity or instability events. The imaging data reported were Q-angle, Insall-Salvati ratio, Caton-Deschamps index, Blackburne-Peel ratio, Outerbridge osteoarthritis grade, and TT-TG distance. Preoperative clinical scoring data most prominently included a visual analog scale (VAS) score of 70.50 (4 studies reporting), a Lysholm score of 59.19 (5 studies), and a Kujala score of 41.16 (4 studies). Shelbourne-Trumper and Cox-Insall scores were reported in 1 and 2 studies, respectively.
Operative Characteristics
Of the 21 studies, 12 reported only on patients who had TTO performed in isolation; in the other 9 studies, cohorts included patients who underwent concurrent procedures. In the 17 studies (856 patients) that listed numbers of patients who underwent specific concomitant procedures, 715 patients (83.5%) underwent an isolated TTO procedure, and the other 141 (16.5%) underwent either concomitant lateral femoral trochleoplasty, arthroscopic drilling of chondral lesions, patellar shaving chondroplasty, partial meniscectomy or concomitant meniscal repair, intra-articular loose body removal, and/or lateral release with or without medial plication.
Postoperative Data
There was a cumulative total of 79 complications (8% of cohort): 17 recurrent patellar dislocations (1.9%), 4 recurrent patellar subluxations (0.4%), 10 wound complications (1.0%), 2 intraoperative complications (0.2%), 14 tibial tubercle fractures (1.3%), 19 proximal tibia fractures (1.8%), 4 cases of anterior knee pain (0.4%), 4 cases of neuropraxia (0.4%), and 5 infections (0.5%). Of note, 219 knees (21%) required reoperation, but 170 (16.3%) of these were for painful hardware removal. Sixteen knees (1.5%) required revision TTO, 1 (0.1%) required subsequent high tibial osteotomy, 2 (0.2%) underwent patellofemoral arthroplasty for advanced arthritic changes, and 5 (0.5%) underwent total knee arthroplasty for advanced arthritic changes.
Studies With TTO Performed in Isolation
Twelve studies reported outcomes of isolated TTO procedures. In the 638 patients who underwent isolated TTO, the pathologies addressed were instability/laxity (429 patients, 67%), patellofemoral osteoarthritis (74, 12%), patella alta with instability (61, 10%), patellofemoral osteoarthritis with instability (31, 5%), patella baja (24, 4%), and patella alta (19, 3%). Pain was a preoperative issue in 289 (45%) of these patients and instability in 472 (74%).
Only 2.8% of patients experienced postoperative patellar dislocation events. Of the 12 studies, 2 reported VAS scores (34-point weighted mean improvement, 65 points before surgery to 31 after surgery), 3 reported Lysholm scores (30-point improvement, from 60 to 90), and 2 reported Kujala scores (21-point improvement, from 46 to 67).
Complication rates for this isolated-TTO pooled cohort of patients were 1.2% for revision TTOs, 0.5% for wound complications, 0.8% for tibial tubercle fractures, and 1.9% for proximal tibia fractures. In total, 16% of patients required hardware removal after surgery.
Discussion
This study found that TTO improved patient pain and clinical outcome scores despite having a high (16%) rate of reoperation for painful hardware in patients with preoperative pain or instability, or with patellofemoral osteoarthritis or aberrant patellar anatomy. This reoperation rate and the overall complication rate both exceeded our hypothesized 10% cumulative rate. However, <1% of patients required conversion to a definitive end-stage surgery (patellofemoral arthroplasty or total knee arthroplasty) by final follow-up, and the rates of comorbidities (anterior knee pain, wound infection, recurrent patellar subluxation/dislocation, tibial fracture) were relatively low.
Patellofemoral disorders are common in the general population and a frequent primary complaint on presentation to orthopedic offices. Having a thorough understanding of knee joint biomechanics is imperative when trying to determine whether surgery is appropriate for these complaints and how to proceed. Extensor mechanism abnormalities, including high lateral force vectors (or larger TT-TG distances) and excessive patellar tilt, can affect alignment and increase the risk for patellofemoral dislocations, patellofemoral anterior- based knee pains, and chondral lesions. Patella alta, an elevated patella, risks increased contact stresses between the patella and the trochlear groove33 and decreases the osseous constraints that inhibit dislocation of the patella with physiologic flexion of the joint.34 With TTO, the change in tuberosity position can alter angles in the extensor mechanism and thereby decrease joint reaction forces and patellofemoral contact area forces.35,36
Although its use began as an option for combating patellar instability events in patients with predisposed patellofemoral kinematics,5 TTO has evolved in its therapeutic uses to include offloading patellar and trochlear focal chondral lesions and slowing progression of patellofemoral arthritis. Multiple iterations and modifications of the procedure have involved distal and medial transfer of the tibial tuberosity, medialization alone, concurrent anterior and medial elevation of the tuberosity, and proximal or distal transfers, depending on the pathology being corrected. Although TTO is highly versatile in treating multiple patellofemoral joint pathologies, this study found that its primary indication continues to be patellar instability, with anteromedialization as the most common direction of tubercle transfer in support of the medial structures providing the medial force vector that keeps the patella in place. These medial structures include the medial patellofemoral ligament, the vastus medialis obliquus, the medial patellotibial ligament, and the medial retinaculum.
Also notable was the relatively high rate of reoperation after TTO. However, >75% of reoperations were performed to remove painful hardware, and the need for reoperation seemed to have no effect on the statistically significant overall preoperative-to-postoperative improvement in VAS, Lysholm, and Kujala scores. Rates of definitive surgery for end-stage patellofemoral changes, including patellofemoral arthroplasty and total knee arthroplasty, were quite low at the weighted mean follow-up of several years after surgery, suggesting a role for TTO in avoiding arthroplasty. Although the infection rate was <1%, the rate of tibial tubercle or proximal tibia fractures was a cumulative 3.1%. Patients should be counseled on this complication risk, as treatment can require cast immobilization and weight-bearing limitations.24
The 69% proportion of women in the overall cohort and the mean (SD) age of 27.68 (10.45) years highlight the primary patient population that undergoes TTO. Compared with men, young women are more likely to have aberrant patellofemoral biomechanics, owing to their native anatomy, including their relatively larger Q-angle and TT-TG distance and thus increased lateral translational force vectors on the patella.37 In addition, more than half of patients who are having TTO underwent previous surgery on the affected knee—an indication that TTO is still not universally considered first-line in addressing patellofemoral pathology.
Limitations of the Analysis
The limitations of this analysis derive from the limitations of the included studies, which were mostly retrospective case series with relatively short follow-up. The low MCMS (<55) of all 21 studies highlights their low quality as well. These studies showed considerable heterogeneity in their reporting of specific preoperative, intraoperative, and postoperative radiographic, physical examination, and clinical outcome scores, which may be indicative of the relatively low rate of use of TTO, a procedure originally described decades ago. These studies also showed ample heterogeneity in the specific radiographic parameters or outcome scales they used to present their data. We were therefore limited in our ability to cohesively summarize and provide cumulative data points from the patients as a unified cohort. There was substantial variety in the procedures performed, surgical techniques used, concomitant pathologies addressed at time of surgery, and diagnoses treated—indicating a performance bias. This additionally precluded any significant meta-analysis within the patient cohort. A higher quality study, a randomized controlled trial, is needed to answer more definitively and completely the questions we left unanswered, including the effect on radiographic parameters, additional clinical outcomes, and patient satisfaction.
Conclusion
TTO is most commonly performed for isolated patellar instability in the presence of knee pain. Other pathologies addressed are patellofemoral osteoarthritis, and patella alta and patella baja with and without associated knee pain. TTO significantly improves knee pain and clinical outcome scores, though 21% of patients (>1 in 5) require reoperation for hardware removal. Young women with prior surgery on the affected knee are the primary patient population.
1. Blond L, Hansen L. Patellofemoral pain syndrome in athletes: a 5.7- year retrospective follow-up study of 250 athletes. Acta Orthop Belg. 1998;64(4):393-400.
2. Fairbank JC, Pynsent PB, van Poortvliet JA, Phillips H. Mechanical factors in the incidence of knee pain in adolescents and young adults. J Bone Joint Surg Br. 1984;66(5):685-693.
3. Mehta VM, Inoue M, Nomura E, Fithian DC. An algorithm guiding the evaluation and treatment of acute primary patellar dislocations. Sports Med Arthrosc. 2007;15(2):78-81.
4. Erickson BJ, Mascarenhas R, Sayegh ET, et al. Does operative treatment of first-time patellar dislocations lead to increased patellofemoral stability? A systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(6):1207-1215.
5. Hauser E. Total tendon transplant for slipping patella. Surg Gynecol Obstet. 1938;66:199-214.
6. Sherman SL, Erickson BJ, Cvetanovich GL, et al. Tibial tuberosity osteotomy: indications, techniques, and outcomes. Am J Sports Med. 2014;42(8):2006-2017.
7. Hall MJ, Mandalia VI. Tibial tubercle osteotomy for patello-femoral joint disorders. Knee Surg Sports Traumatol Arthrosc. 2016;24(3):855-861.
8. Grawe B, Stein BS. Tibial tubercle osteotomy: indication and techniques. J Knee Surg. 2015;28(4):279-284.
9. Fulkerson JP. Disorders of the Patellofemoral Joint. 4th ed. Baltimore, MD: Williams & Wilkins; 1997.
10. Koh JL, Stewart C. Patellar instability. Clin Sports Med. 2014;33(3):461-476.
11. Coleman BD, Khan KM, Maffulli N, Cook JL, Wark JD. Studies of surgical outcome after patellar tendinopathy: clinical significance of methodological deficiencies and guidelines for future studies. Victorian Institute of Sport Tendon Study Group. Scand J Med Sci Sports. 2000;10(1):2-11.
12. Al-Sayyad MJ, Cameron JC. Functional outcome after tibial tubercle transfer for the painful patella alta. Clin Orthop Rel Res. 2002;(396):152-162.
13. Atkinson HD, Bailey CA, Anand S, Johal P, Oakeshott RD. Tibial tubercle advancement osteotomy with bone allograft for patellofemoral arthritis: a retrospective cohort study of 50 knees. Arch Orthop Trauma Surg. 2012;132(4):437-445.
14. Caton JH, Dejour D. Tibial tubercle osteotomy in patello-femoral instability and in patellar height abnormality. Int Orthop. 2010;34(2):305-309.
15. Dantas P, Nunes C, Moreira J, Amaral LB. Antero-medialisation of the tibial tubercle for patellar instability. Int Orthop. 2005;29(6):390-391.
16. Drexler M, Dwyer T, Marmor M, Sternheim A, Cameron HU, Cameron JC. The treatment of acquired patella baja with proximalize the tibial tuberosity. Knee Surg Sports Traumatol Arthrosc. 2013;21(11):2578-2583.
17. Eager MR, Bader DA, Kelly JD 4th, Moyer RA. Delayed fracture of the tibia following anteromedialization osteotomy of the tibial tubercle: a report of 5 cases. Am J Sports Med. 2004;32(4):1041-1048.
18. Ebinger TP, Boezaart A, Albright JP. Modifications of the Fulkerson osteotomy: a pilot study assessment of a novel technique of dynamic intraoperative determination of the adequacy of tubercle transfer. Iowa Orthop J. 2007;27:61-64.
19. Fulkerson JP, Becker GJ, Meaney JA, Miranda M, Folcik MA. Anteromedial tibial tubercle transfer without bone graft. Am J Sports Med. 1990;18(5):490-498.
20. Heatley FW, Allen PR, Patrick JH. Tibial tubercle advancement for anterior knee pain: a temporary or permanent solution. Clin Orthop Relat Res. 1986;(208):216-225.
21. Hirsh DM, Reddy DK. Experience with Maquet anterior tibial tubercle advancement for patellofemoral arthralgia. Clin Orthop Relat Res. 1980;(148):136-139.
22. Jack CM, Rajaratnam SS, Khan HO, Keast-Butler O, Butler-Manuel PA, Heatley FW. The modified tibial tubercle osteotomy for anterior knee pain due to chondromalacia patellae in adults: a five-year prospective study. Bone Joint Res. 2012;1(8):167-173.
23. Koëter S, Diks MJ, Anderson PG, Wymenga AB. A modified tibial tubercle osteotomy for patellar maltracking: results at two years. J Bone Joint Surg Br. 2007;89(2):180-185.
24. Luhmann SJ, Fuhrhop S, O’Donnell JC, Gordon JE. Tibial fractures after tibial tubercle osteotomies for patellar instability: a comparison of three osteotomy configurations. J Child Orthop. 2011;5(1):19-26.
25. Naranja RJ Jr, Reilly PJ, Kuhlman JR, Haut E, Torg JS. Long-term evaluation of the Elmslie-Trillat-Maquet procedure for patellofemoral dysfunction. Am J Sports Med. 1996;24(6):779-784.
26. Naveed MA, Ackroyd CE, Porteous AJ. Long-term (ten- to 15-year) outcome of arthroscopically assisted Elmslie-Trillat tibial tubercle osteotomy. Bone Joint J. 2013;95(4):478-485.
27. Paulos L, Swanson SC, Stoddard GJ, Barber-Westin S. Surgical correction of limb malalignment for instability of the patella: a comparison of 2 techniques. Am J Sports Med. 2009;37(7):1288-1300.
28. Pidoriano AJ, Weinstein RN, Buuck DA, Fulkerson JP. Correlation of patellar articular lesions with results from anteromedial tibial tubercle transfer. Am J Sports Med. 1997;25(4):533-537.
29. Shen HC, Chao KH, Huang GS, Pan RY, Lee CH. Combined proximal and distal realignment procedures to treat the habitual dislocation of the patella in adults. Am J Sports Med. 2007;35(12):2101-2108.
30. Stetson WB, Friedman MJ, Fulkerson JP, Cheng M, Buuck D. Fracture of the proximal tibia with immediate weightbearing after a Fulkerson osteotomy. Am J Sports Med. 1997;25(4):570-574.
31. Valenzuela L, Nemtala F, Orrego M, et al. Treatment of patellofemoral chondropathy with the Bandi tibial tubercle osteotomy: more than 10 years follow-up. Knee. 2011;18(2):94-97.
32. Wang CJ, Wong T, Ko JY, Siu KK. Triple positioning of tibial tubercle osteotomy for patellofemoral disorders. Knee. 2014;21(1):133-137.
33. Luyckx T, Didden K, Vandenneucker H, Labey L, Innocenti B, Bellemans J. Is there a biomechanical explanation for anterior knee pain in patients with patella alta? Influence of patellar height on patellofemoral contact force, contact area and contact pressure. J Bone Joint Surg Br. 2009;91(3):344-350.
34. Mayer C, Magnussen RA, Servien E, et al. Patellar tendon tenodesis in association with tibial tubercle distalization for the treatment of episodic patellar dislocation with patella alta. Am J Sports Med. 2012;40(2):346-351.
35. Maquet P. Advancement of the tibial tuberosity. Clin Orthop Relat Res. 1976;(115):225-230.
36. Lewallen DG, Riegger CL, Myers ER, Hayes WC. Effects of retinacular release and tibial tubercle elevation in patellofemoral degenerative joint disease. J Orthop Res. 1990;8(6):856-862.
37. Aglietti P, Insall JN, Cerulli G. Patellar pain and incongruence, I: measurements of incongruence. Clin Orthop Relat Res. 1983;(176):217-224.
1. Blond L, Hansen L. Patellofemoral pain syndrome in athletes: a 5.7- year retrospective follow-up study of 250 athletes. Acta Orthop Belg. 1998;64(4):393-400.
2. Fairbank JC, Pynsent PB, van Poortvliet JA, Phillips H. Mechanical factors in the incidence of knee pain in adolescents and young adults. J Bone Joint Surg Br. 1984;66(5):685-693.
3. Mehta VM, Inoue M, Nomura E, Fithian DC. An algorithm guiding the evaluation and treatment of acute primary patellar dislocations. Sports Med Arthrosc. 2007;15(2):78-81.
4. Erickson BJ, Mascarenhas R, Sayegh ET, et al. Does operative treatment of first-time patellar dislocations lead to increased patellofemoral stability? A systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(6):1207-1215.
5. Hauser E. Total tendon transplant for slipping patella. Surg Gynecol Obstet. 1938;66:199-214.
6. Sherman SL, Erickson BJ, Cvetanovich GL, et al. Tibial tuberosity osteotomy: indications, techniques, and outcomes. Am J Sports Med. 2014;42(8):2006-2017.
7. Hall MJ, Mandalia VI. Tibial tubercle osteotomy for patello-femoral joint disorders. Knee Surg Sports Traumatol Arthrosc. 2016;24(3):855-861.
8. Grawe B, Stein BS. Tibial tubercle osteotomy: indication and techniques. J Knee Surg. 2015;28(4):279-284.
9. Fulkerson JP. Disorders of the Patellofemoral Joint. 4th ed. Baltimore, MD: Williams & Wilkins; 1997.
10. Koh JL, Stewart C. Patellar instability. Clin Sports Med. 2014;33(3):461-476.
11. Coleman BD, Khan KM, Maffulli N, Cook JL, Wark JD. Studies of surgical outcome after patellar tendinopathy: clinical significance of methodological deficiencies and guidelines for future studies. Victorian Institute of Sport Tendon Study Group. Scand J Med Sci Sports. 2000;10(1):2-11.
12. Al-Sayyad MJ, Cameron JC. Functional outcome after tibial tubercle transfer for the painful patella alta. Clin Orthop Rel Res. 2002;(396):152-162.
13. Atkinson HD, Bailey CA, Anand S, Johal P, Oakeshott RD. Tibial tubercle advancement osteotomy with bone allograft for patellofemoral arthritis: a retrospective cohort study of 50 knees. Arch Orthop Trauma Surg. 2012;132(4):437-445.
14. Caton JH, Dejour D. Tibial tubercle osteotomy in patello-femoral instability and in patellar height abnormality. Int Orthop. 2010;34(2):305-309.
15. Dantas P, Nunes C, Moreira J, Amaral LB. Antero-medialisation of the tibial tubercle for patellar instability. Int Orthop. 2005;29(6):390-391.
16. Drexler M, Dwyer T, Marmor M, Sternheim A, Cameron HU, Cameron JC. The treatment of acquired patella baja with proximalize the tibial tuberosity. Knee Surg Sports Traumatol Arthrosc. 2013;21(11):2578-2583.
17. Eager MR, Bader DA, Kelly JD 4th, Moyer RA. Delayed fracture of the tibia following anteromedialization osteotomy of the tibial tubercle: a report of 5 cases. Am J Sports Med. 2004;32(4):1041-1048.
18. Ebinger TP, Boezaart A, Albright JP. Modifications of the Fulkerson osteotomy: a pilot study assessment of a novel technique of dynamic intraoperative determination of the adequacy of tubercle transfer. Iowa Orthop J. 2007;27:61-64.
19. Fulkerson JP, Becker GJ, Meaney JA, Miranda M, Folcik MA. Anteromedial tibial tubercle transfer without bone graft. Am J Sports Med. 1990;18(5):490-498.
20. Heatley FW, Allen PR, Patrick JH. Tibial tubercle advancement for anterior knee pain: a temporary or permanent solution. Clin Orthop Relat Res. 1986;(208):216-225.
21. Hirsh DM, Reddy DK. Experience with Maquet anterior tibial tubercle advancement for patellofemoral arthralgia. Clin Orthop Relat Res. 1980;(148):136-139.
22. Jack CM, Rajaratnam SS, Khan HO, Keast-Butler O, Butler-Manuel PA, Heatley FW. The modified tibial tubercle osteotomy for anterior knee pain due to chondromalacia patellae in adults: a five-year prospective study. Bone Joint Res. 2012;1(8):167-173.
23. Koëter S, Diks MJ, Anderson PG, Wymenga AB. A modified tibial tubercle osteotomy for patellar maltracking: results at two years. J Bone Joint Surg Br. 2007;89(2):180-185.
24. Luhmann SJ, Fuhrhop S, O’Donnell JC, Gordon JE. Tibial fractures after tibial tubercle osteotomies for patellar instability: a comparison of three osteotomy configurations. J Child Orthop. 2011;5(1):19-26.
25. Naranja RJ Jr, Reilly PJ, Kuhlman JR, Haut E, Torg JS. Long-term evaluation of the Elmslie-Trillat-Maquet procedure for patellofemoral dysfunction. Am J Sports Med. 1996;24(6):779-784.
26. Naveed MA, Ackroyd CE, Porteous AJ. Long-term (ten- to 15-year) outcome of arthroscopically assisted Elmslie-Trillat tibial tubercle osteotomy. Bone Joint J. 2013;95(4):478-485.
27. Paulos L, Swanson SC, Stoddard GJ, Barber-Westin S. Surgical correction of limb malalignment for instability of the patella: a comparison of 2 techniques. Am J Sports Med. 2009;37(7):1288-1300.
28. Pidoriano AJ, Weinstein RN, Buuck DA, Fulkerson JP. Correlation of patellar articular lesions with results from anteromedial tibial tubercle transfer. Am J Sports Med. 1997;25(4):533-537.
29. Shen HC, Chao KH, Huang GS, Pan RY, Lee CH. Combined proximal and distal realignment procedures to treat the habitual dislocation of the patella in adults. Am J Sports Med. 2007;35(12):2101-2108.
30. Stetson WB, Friedman MJ, Fulkerson JP, Cheng M, Buuck D. Fracture of the proximal tibia with immediate weightbearing after a Fulkerson osteotomy. Am J Sports Med. 1997;25(4):570-574.
31. Valenzuela L, Nemtala F, Orrego M, et al. Treatment of patellofemoral chondropathy with the Bandi tibial tubercle osteotomy: more than 10 years follow-up. Knee. 2011;18(2):94-97.
32. Wang CJ, Wong T, Ko JY, Siu KK. Triple positioning of tibial tubercle osteotomy for patellofemoral disorders. Knee. 2014;21(1):133-137.
33. Luyckx T, Didden K, Vandenneucker H, Labey L, Innocenti B, Bellemans J. Is there a biomechanical explanation for anterior knee pain in patients with patella alta? Influence of patellar height on patellofemoral contact force, contact area and contact pressure. J Bone Joint Surg Br. 2009;91(3):344-350.
34. Mayer C, Magnussen RA, Servien E, et al. Patellar tendon tenodesis in association with tibial tubercle distalization for the treatment of episodic patellar dislocation with patella alta. Am J Sports Med. 2012;40(2):346-351.
35. Maquet P. Advancement of the tibial tuberosity. Clin Orthop Relat Res. 1976;(115):225-230.
36. Lewallen DG, Riegger CL, Myers ER, Hayes WC. Effects of retinacular release and tibial tubercle elevation in patellofemoral degenerative joint disease. J Orthop Res. 1990;8(6):856-862.
37. Aglietti P, Insall JN, Cerulli G. Patellar pain and incongruence, I: measurements of incongruence. Clin Orthop Relat Res. 1983;(176):217-224.
Driving-Related Coping Thoughts in Post-9/11 Combat Veterans With and Without Comorbid PTSD and TBI
Combat veterans who have served in Iraq and Afghanistan in the post-9/11 era face unique reintegration challenges, one being the transition from driving in combat zones to driving at home.1 Relative to previous conflicts, post-9/11 combat involves increased participation in road patrols and convoys along with more prevalent threats of improvised explosive devices (IEDs).1,2 Roadside ambushes designed to destroy or stop vehicles became a common warfare strategy, meaning that driving became an inherently dangerous combat maneuver.3
The modern combat driving framework includes cognitive tools (eg, targeted aggression and tactical awareness) combined with specific behaviors (eg, driving unpredictably fast, using rapid lane changes, and keeping other vehicles at a distance to avoid IEDs).4 This framework is adaptive and lifesaving in combat zones, but it can be maladaptive and dangerous in civilian environments. Service members face difficulty in updating this cognitive framework after leaving combat zones and may continue to experience specific cognitions (eg, “the world is dangerous”; “that car holds an IED”) while driving on civilian roads.3,5-8
The high prevalence of posttraumatic stress disorder (PTSD) and traumatic brain injury (TBI) in post-9/11 veterans may complicate reintegration. Both PTSD and TBI are considered signature wounds of these conflicts.8-11 Traumatic brain injury may be sustained as a result of blast injury or other mechanism, including a closed head injury or penetrating brain injury.10 Previous literature indicated that both PTSD and TBI across all severities are related to deficits in executive functioning, attention, and memory.12-16
In addition to cognitive deficits, veterans with PTSD also may experience cognitive misappraisal, in which they are more likely to perceive ambiguous stimuli as threatening because of an inability to suppress trauma-related schema and associations.5,17,18 Examples of roadside-specific trauma triggers include busy highways, traffic, loud or distracting noises, and vehicles of similar make and model as those commonly rigged with IEDs in Iraq or Afghanistan.2,7
Blast injury
Prior research suggests that veterans with PTSD and/or TBI experience significantly higher levels of anxiety in response to common roadside stimuli (ie, an overpass or stop sign) while driving than do veterans without either PTSD or TBI.3 Cognitive behavioral therapy (CBT) interventions have been developed and systematically evaluated for treating anxiety.21 The goal of CBT is to identify and change dysfunctional cognitions that result in biased information processing. Cognitive restructuring, the process by which problematic cognitions (negative automatic thoughts) are identified and examined for distortions, is one method of accomplishing this goal. Distortions then are disputed and rebutted with assistance from the clinician.22 A strategy for restructuring negative automatic thoughts is coping self-instruction, which centers on identifying when negative automatic thoughts are focused on others’ behavior, accepting that their behavior cannot be changed, and using positive coping behaviors to minimize negative automatic thoughts.23
The link between history of comorbid PTSD and TBI and combat driving, current driving anxiety, and coping strategies has not yet been extensively studied in veterans. Thus, the aim of the current study is to determine whether veterans with comorbid PTSD and TBI utilize coping self-instruction behind the wheel. Driving-specific coping self-instruction involves generating thoughts that are adaptive and accepting of others’ driving behaviors (eg, “Just turn up the radio and tune them out”). It was hypothesized that veterans with comorbid PTSD and TBI would endorse fewer coping self-instruction thoughts than would veterans without either PTSD or TBI.
Methods
The current project is part of a larger study that examines driving behaviors of post-9/11 combat veterans at the Michael J. Crescenz Veterans Affairs Medical Center in Philadelphia, Pennsylvania. Thirty-two male veterans aged between 22 and 48 years (M = 31.6, SD = 6.9) were included in the sample. Twenty-three were diagnosed with comorbid PTSD and TBI and 9 veterans with no major psychiatric or physical
Assessment
All participants completed a battery of questionnaires, including the Driver’s Angry Thoughts Questionnaire (DATQ).23 The DATQ was used to investigate the specific thoughts that veterans experienced while driving.23 Participants indicated on a Likert scale from 1 (not at all) to 5 (all the time) how often they experienced any of 65 thoughts while driving. Each item was categorized into 1 of 5 distinct subscales (Table 2). A frequency score was generated for each of the 5 subscales. Each subscale had good internal consistency and convergent, divergent, and predictive validity. The Coping Self-Instruction subscale, which is defined as engaging in relaxing thoughts to accept others’ driving behaviors, was of primary interest. It is a 9-item scale (frequency score can range from 9 to 45) with good reliability (α = .83).23
Given the small and unequal sample sizes, nonparametric independent samples Mann-Whitney U-tests were selected to compare frequency of driving-related thoughts across veterans with comorbid PTSD and TBI and those of veterans without either PTSD or TBI.
Results
Descriptive statistics and results for each DATQ subscale are reported in Table 3. Group comparisons revealed that veterans with comorbid PTSD and TBI endorsed statistically significantly fewer coping self-instruction thoughts while driving (M = 11.5, SD = 7.2) than did combat veterans without either PTSD or TBI (M = 18.1, SD = 6.9; U = 56.0, P = .05). Conversely, frequency of angry thoughts were statistically significant in their difference as a function of PTSD or TBI diagnostic status.
Discussion
While driving, veterans with PTSD or TBI endorsed statistically significantly fewer coping self-instruction thoughts than did veterans without either PTSD or TBI. Prior research suggests that veterans with PTSD or TBI experience greater anxiety than do veterans without either condition while driving.2,3 Taken together, this suggests that veterans with PTSD or TBI may lack efficient cognitive coping strategies related to the anxiety they experience while driving. Furthermore, the groups did not significantly differ in frequency of angry thoughts behind the wheel. This result was expected based on prior analyses that suggested that veterans with and without PTSD or TBI endorsed feelings of aggression, impatience, and frustration while driving at similar frequencies.3
Because all veterans in the current sample were exposed to combat, these results help to parse out the unique contribution of PTSD and TBI diagnoses on driving in civilian environments. Exposure to combat plus diagnoses of PTSD or TBI may be related to veterans’ ability to cope with typical driving situations at home. In the context of prior literature, results suggest that veterans with PTSD or TBI automatically may perceive neutral roadside stimuli as threatening, feel anxious in response to this perceived threat, and be ill-equipped to cope with this anxiety.3,5,17,18 According to CBT models, negative automatic thoughts play a critical role in maintaining anxiety.24 Particular cognitive distortions associated with PTSD symptomatology and combat driving experiences, such as misperceiving ambiguous stimuli as threatening because of an inability to suppress trauma-related schema and associations, may therefore maintain driving anxiety following military separation.
Research on CBT interventions suggests that cognitive restructuring, including coping self-instruction, are effective treatments to reduce anxiety.22,24 The current findings suggest that combat veterans with PTSD and TBI who experience driving anxiety endorse significantly fewer coping self-instruction thoughts than do controls in response to anxiety-provoking driving situations. In fact, prior research suggests that a majority of veterans experiencing driving-related anxiety do not seek help for their symptoms, and many of those who do prefer to reach out to friends rather than mental health professionals.2 However, due to their high levels of anxiety, these veterans likely would benefit from CBT interventions specifically targeted to coping strategies for civilian driving. These coping strategies should focus on recognizing that common roadside stimuli are not necessarily threatening in civilian environments. This type of cognitive restructuring may help veterans better manage anxiety while driving.
Limitations
The current study is limited by its small and unequal sample sizes and lack of a noncombat exposure comparison group. Additionally, while this study highlights a potential relationship between reduced cognitive coping strategies and behind-the-wheel anxiety in veterans with PTSD or TBI, causal inferences cannot be made. It is possible that individuals without coping strategies who are deployed to combat are more likely to develop PTSD or TBI. Being equipped with few coping strategies may then lead these veterans to experience greater anxiety while driving. Conversely, PTSD and TBI symptoms may prevent veterans from developing coping strategies over time.
Furthermore, the comorbid PTSD and TBI group was separated from the military for significantly longer than was the control group. Future studies using a longitudinal design could better examine the potential causal relationship between comorbid PTSD and TBI and coping and determine whether endorsement of coping self-instruction changes as a function of time since military separation.
Veterans in the current study report a variety of deployment experiences and locations. Methods of combat, type of vehicle, driving terrain, and prevalence of IEDs changed over the multiple post-9/11 military campaigns. Veterans who were deployed to Iraq in the mid-2000s were instructed to drive quickly and erratically to avoid IEDs and mortars, whereas veterans deployed in later years were taught to drive slowly and carefully to hunt for IEDs in heavily armored vehicles.3 Seventy-five percent of the veterans with PTSD or TBI in the current sample were deployed to Iraq in the early to mid-2000s, compared with 33% of the veterans without PTSD or TBI. Thus, the 2 groups in the current sample may have experienced different combat environments, which could impact how they perceived roadside stimuli. Future studies should recruit a larger and more balanced sample to better determine whether specific combat experiences impact coping strategies while driving.
Conclusion
To the best of the authors’ knowledge, the current study is the first to examine specific types of thoughts that veterans with and without PTSD or TBI experience while driving on civilian roads. Veterans with PTSD or TBI are not engaging in as many coping self-instruction thoughts behind the wheel, despite experiencing greater anxiety than that of veterans without either PTSD or TBI. Cognitive behavioral therapy interventions for anxiety include engaging in coping self-instruction during anxiety-provoking situations.22 Therefore, veterans with PTSD or TBI may benefit from learning targeted coping self-instruction thoughts that they can utilize when anxiety-provoking situations arise behind the wheel. Results suggest that clinicians should work with veterans with comorbid PTSD and TBI to develop specific coping self-instruction statements that they can utilize internally when faced with anxiety-provoking driving situations.
Acknowledgments
This study is the result of work supported by the Council on Brain Injury (grant #260472). The authors thank Dr. Rosette Biester for her guidance.
1. Belmont PJ, Schoenfeld AJ, Goodman G. Epidemiology of combat wounds in Operation Iraqi Freedom and Operation Enduring Freedom: orthopaedic burden of disease. J Surg Orthop Adv. 2010;19(1):2-7.
2. Zinzow HM, Brooks J, Stern EB. Driving-related anxiety in recently deployed service members: cues, mental health correlates, and help-seeking behavior. Mil Med. 2013;178(3):e357-e361.
3. Whipple EK, Schultheis MT, Robinson KM. Preliminary findings of a novel measure of driving behaviors in veterans with comorbid TBI and PTSD. J Rehabil Res Dev. 2016;53(6):827-838.
4. Adler AB, Bliese PD, McGurk D, Hoge CW, Castro CA. Battlemind debriefing and battlemind training as early interventions with soldiers returning from Iraq: randomization by platoon. J Consult Clin Psychol. 2009;77(5):928-940.
5. Amick MM, Kraft M, McGlinchey R. Driving simulator performance of veterans from the Iraq and Afghanistan wars. J Rehabil Res Dev. 2013;50(4):463-470.
6. Classen S, Cormack NL, Winter SM, et al. Efficacy of an occupational therapy driving intervention for returning combat veterans. OTJR (Thorofare NJ). 2014;34(4):177-182.
7. Hannold EM, Classen S, Winter S, Lanford DN, Levy CE. Exploratory pilot study of driving perceptions among OIF/OEF veterans with mTBI and PTSD. J Rehabil Res Dev. 2013;50(10):1315-1330.
8. Lew HL, Kraft M, Pogoda TK, Amick MM, Woods P, Cifu DX. Prevalence and characteristics of driving difficulties in Operation Iraqi Freedom/Operation Enduring Freedom combat returnees. J Rehabil Res Dev. 2011;48(8):913-925.
9. Arthur DC, MacDermid S, Kiley KC; Defense Health Board Task Force on Mental Health. An Achievable Vision: Report of the Department of Defense Task Force on Mental Health. Falls Church, VA: Defense Health Board; 2007.
10. Tanielian T, Jaycox LH, eds. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Santa Monica, CA: RAND Corporation; 2008.
11. Independent Review Group. Rebuilding the Trust: Independent Review Group Report on Rehabilitation Care and Administrative Processes at Walter Reed Army Medical Center and National Naval Medical Center. Arlington, VA: Independent Review Group; 2007
12. Bailie JM, Cole WR, Ivins B, et al. The experience, expression, and control of anger following traumatic brain injury in a military sample. J Head Trauma Rehabil. 2015;30(1):12-20.
13. Campbell TA, Nelson LA, Lumpkin R, Yoash-Gantz RE, Pickett TC, McCormick CL. Neuropsychological measures of processing speed and executive functioning in combat veterans with PTSD, TBI, and comorbid TBI/PTSD. Psychiatr Ann. 2009;39(8):796-803.
14. Classen S, Levy C, Meyer DL, Bewernitz M, Lanford DN, Mann WC. Simulated driving performance of combat veterans with mild tramatic brain injury and posttraumatic stress disorder: a pilot study. Am J Occup Ther. 2011;65(4):419-427.
15. Lew HL, Amick MM, Kraft M, Stein MB, Cifu DX. Potential driving issues in combat returnees. NeuroRehabilitation. 2010;26(3):271-278.
16. Vasterling JL, Brailey K, Allain AN, Duke LM, Constans JI, Sutker PB. Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology. 2002;16(1):5-14.
17. Kimble MO, Kaufman ML, Leonard LL, et al. Sentence completion test in veterans with and without PTSD: preliminary findings. Psychiatry Res. 2002;113(3):303-307.
18. Kuhn E, Drescher K, Ruzek J, Rosen C. Aggressive and unsafe driving in male veterans receiving residential treatment for PTSD. J Trauma Stress. 2010;23(3):399-402.
19. Stein MB, McAllister TW. Exploring the convergence of posttraumatic stress disorder and mild traumatic brain injury. Am J Psychiatry. 2009;166(7):768-776.
20. Hill JJ III, Mobo BH Jr, Cullen MR. Separating deployment-related traumatic brain injury and posttraumatic stress disorder in veterans: preliminary findings from the Veterans Affairs traumatic brain injury screening program. Am J Phys Med Rehabil. 2009;88(8):605-614.
21. Hofmann SG, Smits JA. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69(4):621-632.
22. Hope DA, Burns JA, Hayes SA, Herbert JD, Warner MD. Automatic thoughts and cognitive restructuring in cognitive behavioral group therapy for social anxiety disorder. Cognit Ther Res. 2010;34(1):1-12.
23. Deffenbacher JL, Petrilli RT, Lynch RS, Oetting ER, Swaim RC. The driver’s angry thoughts questionnaire: a measure of angry cognitions when driving. Cognit Ther Res. 2003;27(4):383-402.
24. Beck AT, Emery G, Greenberg RL. Anxiety Disorders and Phobias: A Cognitive Perspective. Rev. paperback ed. New York, NY: Basic Books; 2005.
Combat veterans who have served in Iraq and Afghanistan in the post-9/11 era face unique reintegration challenges, one being the transition from driving in combat zones to driving at home.1 Relative to previous conflicts, post-9/11 combat involves increased participation in road patrols and convoys along with more prevalent threats of improvised explosive devices (IEDs).1,2 Roadside ambushes designed to destroy or stop vehicles became a common warfare strategy, meaning that driving became an inherently dangerous combat maneuver.3
The modern combat driving framework includes cognitive tools (eg, targeted aggression and tactical awareness) combined with specific behaviors (eg, driving unpredictably fast, using rapid lane changes, and keeping other vehicles at a distance to avoid IEDs).4 This framework is adaptive and lifesaving in combat zones, but it can be maladaptive and dangerous in civilian environments. Service members face difficulty in updating this cognitive framework after leaving combat zones and may continue to experience specific cognitions (eg, “the world is dangerous”; “that car holds an IED”) while driving on civilian roads.3,5-8
The high prevalence of posttraumatic stress disorder (PTSD) and traumatic brain injury (TBI) in post-9/11 veterans may complicate reintegration. Both PTSD and TBI are considered signature wounds of these conflicts.8-11 Traumatic brain injury may be sustained as a result of blast injury or other mechanism, including a closed head injury or penetrating brain injury.10 Previous literature indicated that both PTSD and TBI across all severities are related to deficits in executive functioning, attention, and memory.12-16
In addition to cognitive deficits, veterans with PTSD also may experience cognitive misappraisal, in which they are more likely to perceive ambiguous stimuli as threatening because of an inability to suppress trauma-related schema and associations.5,17,18 Examples of roadside-specific trauma triggers include busy highways, traffic, loud or distracting noises, and vehicles of similar make and model as those commonly rigged with IEDs in Iraq or Afghanistan.2,7
Blast injury
Prior research suggests that veterans with PTSD and/or TBI experience significantly higher levels of anxiety in response to common roadside stimuli (ie, an overpass or stop sign) while driving than do veterans without either PTSD or TBI.3 Cognitive behavioral therapy (CBT) interventions have been developed and systematically evaluated for treating anxiety.21 The goal of CBT is to identify and change dysfunctional cognitions that result in biased information processing. Cognitive restructuring, the process by which problematic cognitions (negative automatic thoughts) are identified and examined for distortions, is one method of accomplishing this goal. Distortions then are disputed and rebutted with assistance from the clinician.22 A strategy for restructuring negative automatic thoughts is coping self-instruction, which centers on identifying when negative automatic thoughts are focused on others’ behavior, accepting that their behavior cannot be changed, and using positive coping behaviors to minimize negative automatic thoughts.23
The link between history of comorbid PTSD and TBI and combat driving, current driving anxiety, and coping strategies has not yet been extensively studied in veterans. Thus, the aim of the current study is to determine whether veterans with comorbid PTSD and TBI utilize coping self-instruction behind the wheel. Driving-specific coping self-instruction involves generating thoughts that are adaptive and accepting of others’ driving behaviors (eg, “Just turn up the radio and tune them out”). It was hypothesized that veterans with comorbid PTSD and TBI would endorse fewer coping self-instruction thoughts than would veterans without either PTSD or TBI.
Methods
The current project is part of a larger study that examines driving behaviors of post-9/11 combat veterans at the Michael J. Crescenz Veterans Affairs Medical Center in Philadelphia, Pennsylvania. Thirty-two male veterans aged between 22 and 48 years (M = 31.6, SD = 6.9) were included in the sample. Twenty-three were diagnosed with comorbid PTSD and TBI and 9 veterans with no major psychiatric or physical
Assessment
All participants completed a battery of questionnaires, including the Driver’s Angry Thoughts Questionnaire (DATQ).23 The DATQ was used to investigate the specific thoughts that veterans experienced while driving.23 Participants indicated on a Likert scale from 1 (not at all) to 5 (all the time) how often they experienced any of 65 thoughts while driving. Each item was categorized into 1 of 5 distinct subscales (Table 2). A frequency score was generated for each of the 5 subscales. Each subscale had good internal consistency and convergent, divergent, and predictive validity. The Coping Self-Instruction subscale, which is defined as engaging in relaxing thoughts to accept others’ driving behaviors, was of primary interest. It is a 9-item scale (frequency score can range from 9 to 45) with good reliability (α = .83).23
Given the small and unequal sample sizes, nonparametric independent samples Mann-Whitney U-tests were selected to compare frequency of driving-related thoughts across veterans with comorbid PTSD and TBI and those of veterans without either PTSD or TBI.
Results
Descriptive statistics and results for each DATQ subscale are reported in Table 3. Group comparisons revealed that veterans with comorbid PTSD and TBI endorsed statistically significantly fewer coping self-instruction thoughts while driving (M = 11.5, SD = 7.2) than did combat veterans without either PTSD or TBI (M = 18.1, SD = 6.9; U = 56.0, P = .05). Conversely, frequency of angry thoughts were statistically significant in their difference as a function of PTSD or TBI diagnostic status.
Discussion
While driving, veterans with PTSD or TBI endorsed statistically significantly fewer coping self-instruction thoughts than did veterans without either PTSD or TBI. Prior research suggests that veterans with PTSD or TBI experience greater anxiety than do veterans without either condition while driving.2,3 Taken together, this suggests that veterans with PTSD or TBI may lack efficient cognitive coping strategies related to the anxiety they experience while driving. Furthermore, the groups did not significantly differ in frequency of angry thoughts behind the wheel. This result was expected based on prior analyses that suggested that veterans with and without PTSD or TBI endorsed feelings of aggression, impatience, and frustration while driving at similar frequencies.3
Because all veterans in the current sample were exposed to combat, these results help to parse out the unique contribution of PTSD and TBI diagnoses on driving in civilian environments. Exposure to combat plus diagnoses of PTSD or TBI may be related to veterans’ ability to cope with typical driving situations at home. In the context of prior literature, results suggest that veterans with PTSD or TBI automatically may perceive neutral roadside stimuli as threatening, feel anxious in response to this perceived threat, and be ill-equipped to cope with this anxiety.3,5,17,18 According to CBT models, negative automatic thoughts play a critical role in maintaining anxiety.24 Particular cognitive distortions associated with PTSD symptomatology and combat driving experiences, such as misperceiving ambiguous stimuli as threatening because of an inability to suppress trauma-related schema and associations, may therefore maintain driving anxiety following military separation.
Research on CBT interventions suggests that cognitive restructuring, including coping self-instruction, are effective treatments to reduce anxiety.22,24 The current findings suggest that combat veterans with PTSD and TBI who experience driving anxiety endorse significantly fewer coping self-instruction thoughts than do controls in response to anxiety-provoking driving situations. In fact, prior research suggests that a majority of veterans experiencing driving-related anxiety do not seek help for their symptoms, and many of those who do prefer to reach out to friends rather than mental health professionals.2 However, due to their high levels of anxiety, these veterans likely would benefit from CBT interventions specifically targeted to coping strategies for civilian driving. These coping strategies should focus on recognizing that common roadside stimuli are not necessarily threatening in civilian environments. This type of cognitive restructuring may help veterans better manage anxiety while driving.
Limitations
The current study is limited by its small and unequal sample sizes and lack of a noncombat exposure comparison group. Additionally, while this study highlights a potential relationship between reduced cognitive coping strategies and behind-the-wheel anxiety in veterans with PTSD or TBI, causal inferences cannot be made. It is possible that individuals without coping strategies who are deployed to combat are more likely to develop PTSD or TBI. Being equipped with few coping strategies may then lead these veterans to experience greater anxiety while driving. Conversely, PTSD and TBI symptoms may prevent veterans from developing coping strategies over time.
Furthermore, the comorbid PTSD and TBI group was separated from the military for significantly longer than was the control group. Future studies using a longitudinal design could better examine the potential causal relationship between comorbid PTSD and TBI and coping and determine whether endorsement of coping self-instruction changes as a function of time since military separation.
Veterans in the current study report a variety of deployment experiences and locations. Methods of combat, type of vehicle, driving terrain, and prevalence of IEDs changed over the multiple post-9/11 military campaigns. Veterans who were deployed to Iraq in the mid-2000s were instructed to drive quickly and erratically to avoid IEDs and mortars, whereas veterans deployed in later years were taught to drive slowly and carefully to hunt for IEDs in heavily armored vehicles.3 Seventy-five percent of the veterans with PTSD or TBI in the current sample were deployed to Iraq in the early to mid-2000s, compared with 33% of the veterans without PTSD or TBI. Thus, the 2 groups in the current sample may have experienced different combat environments, which could impact how they perceived roadside stimuli. Future studies should recruit a larger and more balanced sample to better determine whether specific combat experiences impact coping strategies while driving.
Conclusion
To the best of the authors’ knowledge, the current study is the first to examine specific types of thoughts that veterans with and without PTSD or TBI experience while driving on civilian roads. Veterans with PTSD or TBI are not engaging in as many coping self-instruction thoughts behind the wheel, despite experiencing greater anxiety than that of veterans without either PTSD or TBI. Cognitive behavioral therapy interventions for anxiety include engaging in coping self-instruction during anxiety-provoking situations.22 Therefore, veterans with PTSD or TBI may benefit from learning targeted coping self-instruction thoughts that they can utilize when anxiety-provoking situations arise behind the wheel. Results suggest that clinicians should work with veterans with comorbid PTSD and TBI to develop specific coping self-instruction statements that they can utilize internally when faced with anxiety-provoking driving situations.
Acknowledgments
This study is the result of work supported by the Council on Brain Injury (grant #260472). The authors thank Dr. Rosette Biester for her guidance.
Combat veterans who have served in Iraq and Afghanistan in the post-9/11 era face unique reintegration challenges, one being the transition from driving in combat zones to driving at home.1 Relative to previous conflicts, post-9/11 combat involves increased participation in road patrols and convoys along with more prevalent threats of improvised explosive devices (IEDs).1,2 Roadside ambushes designed to destroy or stop vehicles became a common warfare strategy, meaning that driving became an inherently dangerous combat maneuver.3
The modern combat driving framework includes cognitive tools (eg, targeted aggression and tactical awareness) combined with specific behaviors (eg, driving unpredictably fast, using rapid lane changes, and keeping other vehicles at a distance to avoid IEDs).4 This framework is adaptive and lifesaving in combat zones, but it can be maladaptive and dangerous in civilian environments. Service members face difficulty in updating this cognitive framework after leaving combat zones and may continue to experience specific cognitions (eg, “the world is dangerous”; “that car holds an IED”) while driving on civilian roads.3,5-8
The high prevalence of posttraumatic stress disorder (PTSD) and traumatic brain injury (TBI) in post-9/11 veterans may complicate reintegration. Both PTSD and TBI are considered signature wounds of these conflicts.8-11 Traumatic brain injury may be sustained as a result of blast injury or other mechanism, including a closed head injury or penetrating brain injury.10 Previous literature indicated that both PTSD and TBI across all severities are related to deficits in executive functioning, attention, and memory.12-16
In addition to cognitive deficits, veterans with PTSD also may experience cognitive misappraisal, in which they are more likely to perceive ambiguous stimuli as threatening because of an inability to suppress trauma-related schema and associations.5,17,18 Examples of roadside-specific trauma triggers include busy highways, traffic, loud or distracting noises, and vehicles of similar make and model as those commonly rigged with IEDs in Iraq or Afghanistan.2,7
Blast injury
Prior research suggests that veterans with PTSD and/or TBI experience significantly higher levels of anxiety in response to common roadside stimuli (ie, an overpass or stop sign) while driving than do veterans without either PTSD or TBI.3 Cognitive behavioral therapy (CBT) interventions have been developed and systematically evaluated for treating anxiety.21 The goal of CBT is to identify and change dysfunctional cognitions that result in biased information processing. Cognitive restructuring, the process by which problematic cognitions (negative automatic thoughts) are identified and examined for distortions, is one method of accomplishing this goal. Distortions then are disputed and rebutted with assistance from the clinician.22 A strategy for restructuring negative automatic thoughts is coping self-instruction, which centers on identifying when negative automatic thoughts are focused on others’ behavior, accepting that their behavior cannot be changed, and using positive coping behaviors to minimize negative automatic thoughts.23
The link between history of comorbid PTSD and TBI and combat driving, current driving anxiety, and coping strategies has not yet been extensively studied in veterans. Thus, the aim of the current study is to determine whether veterans with comorbid PTSD and TBI utilize coping self-instruction behind the wheel. Driving-specific coping self-instruction involves generating thoughts that are adaptive and accepting of others’ driving behaviors (eg, “Just turn up the radio and tune them out”). It was hypothesized that veterans with comorbid PTSD and TBI would endorse fewer coping self-instruction thoughts than would veterans without either PTSD or TBI.
Methods
The current project is part of a larger study that examines driving behaviors of post-9/11 combat veterans at the Michael J. Crescenz Veterans Affairs Medical Center in Philadelphia, Pennsylvania. Thirty-two male veterans aged between 22 and 48 years (M = 31.6, SD = 6.9) were included in the sample. Twenty-three were diagnosed with comorbid PTSD and TBI and 9 veterans with no major psychiatric or physical
Assessment
All participants completed a battery of questionnaires, including the Driver’s Angry Thoughts Questionnaire (DATQ).23 The DATQ was used to investigate the specific thoughts that veterans experienced while driving.23 Participants indicated on a Likert scale from 1 (not at all) to 5 (all the time) how often they experienced any of 65 thoughts while driving. Each item was categorized into 1 of 5 distinct subscales (Table 2). A frequency score was generated for each of the 5 subscales. Each subscale had good internal consistency and convergent, divergent, and predictive validity. The Coping Self-Instruction subscale, which is defined as engaging in relaxing thoughts to accept others’ driving behaviors, was of primary interest. It is a 9-item scale (frequency score can range from 9 to 45) with good reliability (α = .83).23
Given the small and unequal sample sizes, nonparametric independent samples Mann-Whitney U-tests were selected to compare frequency of driving-related thoughts across veterans with comorbid PTSD and TBI and those of veterans without either PTSD or TBI.
Results
Descriptive statistics and results for each DATQ subscale are reported in Table 3. Group comparisons revealed that veterans with comorbid PTSD and TBI endorsed statistically significantly fewer coping self-instruction thoughts while driving (M = 11.5, SD = 7.2) than did combat veterans without either PTSD or TBI (M = 18.1, SD = 6.9; U = 56.0, P = .05). Conversely, frequency of angry thoughts were statistically significant in their difference as a function of PTSD or TBI diagnostic status.
Discussion
While driving, veterans with PTSD or TBI endorsed statistically significantly fewer coping self-instruction thoughts than did veterans without either PTSD or TBI. Prior research suggests that veterans with PTSD or TBI experience greater anxiety than do veterans without either condition while driving.2,3 Taken together, this suggests that veterans with PTSD or TBI may lack efficient cognitive coping strategies related to the anxiety they experience while driving. Furthermore, the groups did not significantly differ in frequency of angry thoughts behind the wheel. This result was expected based on prior analyses that suggested that veterans with and without PTSD or TBI endorsed feelings of aggression, impatience, and frustration while driving at similar frequencies.3
Because all veterans in the current sample were exposed to combat, these results help to parse out the unique contribution of PTSD and TBI diagnoses on driving in civilian environments. Exposure to combat plus diagnoses of PTSD or TBI may be related to veterans’ ability to cope with typical driving situations at home. In the context of prior literature, results suggest that veterans with PTSD or TBI automatically may perceive neutral roadside stimuli as threatening, feel anxious in response to this perceived threat, and be ill-equipped to cope with this anxiety.3,5,17,18 According to CBT models, negative automatic thoughts play a critical role in maintaining anxiety.24 Particular cognitive distortions associated with PTSD symptomatology and combat driving experiences, such as misperceiving ambiguous stimuli as threatening because of an inability to suppress trauma-related schema and associations, may therefore maintain driving anxiety following military separation.
Research on CBT interventions suggests that cognitive restructuring, including coping self-instruction, are effective treatments to reduce anxiety.22,24 The current findings suggest that combat veterans with PTSD and TBI who experience driving anxiety endorse significantly fewer coping self-instruction thoughts than do controls in response to anxiety-provoking driving situations. In fact, prior research suggests that a majority of veterans experiencing driving-related anxiety do not seek help for their symptoms, and many of those who do prefer to reach out to friends rather than mental health professionals.2 However, due to their high levels of anxiety, these veterans likely would benefit from CBT interventions specifically targeted to coping strategies for civilian driving. These coping strategies should focus on recognizing that common roadside stimuli are not necessarily threatening in civilian environments. This type of cognitive restructuring may help veterans better manage anxiety while driving.
Limitations
The current study is limited by its small and unequal sample sizes and lack of a noncombat exposure comparison group. Additionally, while this study highlights a potential relationship between reduced cognitive coping strategies and behind-the-wheel anxiety in veterans with PTSD or TBI, causal inferences cannot be made. It is possible that individuals without coping strategies who are deployed to combat are more likely to develop PTSD or TBI. Being equipped with few coping strategies may then lead these veterans to experience greater anxiety while driving. Conversely, PTSD and TBI symptoms may prevent veterans from developing coping strategies over time.
Furthermore, the comorbid PTSD and TBI group was separated from the military for significantly longer than was the control group. Future studies using a longitudinal design could better examine the potential causal relationship between comorbid PTSD and TBI and coping and determine whether endorsement of coping self-instruction changes as a function of time since military separation.
Veterans in the current study report a variety of deployment experiences and locations. Methods of combat, type of vehicle, driving terrain, and prevalence of IEDs changed over the multiple post-9/11 military campaigns. Veterans who were deployed to Iraq in the mid-2000s were instructed to drive quickly and erratically to avoid IEDs and mortars, whereas veterans deployed in later years were taught to drive slowly and carefully to hunt for IEDs in heavily armored vehicles.3 Seventy-five percent of the veterans with PTSD or TBI in the current sample were deployed to Iraq in the early to mid-2000s, compared with 33% of the veterans without PTSD or TBI. Thus, the 2 groups in the current sample may have experienced different combat environments, which could impact how they perceived roadside stimuli. Future studies should recruit a larger and more balanced sample to better determine whether specific combat experiences impact coping strategies while driving.
Conclusion
To the best of the authors’ knowledge, the current study is the first to examine specific types of thoughts that veterans with and without PTSD or TBI experience while driving on civilian roads. Veterans with PTSD or TBI are not engaging in as many coping self-instruction thoughts behind the wheel, despite experiencing greater anxiety than that of veterans without either PTSD or TBI. Cognitive behavioral therapy interventions for anxiety include engaging in coping self-instruction during anxiety-provoking situations.22 Therefore, veterans with PTSD or TBI may benefit from learning targeted coping self-instruction thoughts that they can utilize when anxiety-provoking situations arise behind the wheel. Results suggest that clinicians should work with veterans with comorbid PTSD and TBI to develop specific coping self-instruction statements that they can utilize internally when faced with anxiety-provoking driving situations.
Acknowledgments
This study is the result of work supported by the Council on Brain Injury (grant #260472). The authors thank Dr. Rosette Biester for her guidance.
1. Belmont PJ, Schoenfeld AJ, Goodman G. Epidemiology of combat wounds in Operation Iraqi Freedom and Operation Enduring Freedom: orthopaedic burden of disease. J Surg Orthop Adv. 2010;19(1):2-7.
2. Zinzow HM, Brooks J, Stern EB. Driving-related anxiety in recently deployed service members: cues, mental health correlates, and help-seeking behavior. Mil Med. 2013;178(3):e357-e361.
3. Whipple EK, Schultheis MT, Robinson KM. Preliminary findings of a novel measure of driving behaviors in veterans with comorbid TBI and PTSD. J Rehabil Res Dev. 2016;53(6):827-838.
4. Adler AB, Bliese PD, McGurk D, Hoge CW, Castro CA. Battlemind debriefing and battlemind training as early interventions with soldiers returning from Iraq: randomization by platoon. J Consult Clin Psychol. 2009;77(5):928-940.
5. Amick MM, Kraft M, McGlinchey R. Driving simulator performance of veterans from the Iraq and Afghanistan wars. J Rehabil Res Dev. 2013;50(4):463-470.
6. Classen S, Cormack NL, Winter SM, et al. Efficacy of an occupational therapy driving intervention for returning combat veterans. OTJR (Thorofare NJ). 2014;34(4):177-182.
7. Hannold EM, Classen S, Winter S, Lanford DN, Levy CE. Exploratory pilot study of driving perceptions among OIF/OEF veterans with mTBI and PTSD. J Rehabil Res Dev. 2013;50(10):1315-1330.
8. Lew HL, Kraft M, Pogoda TK, Amick MM, Woods P, Cifu DX. Prevalence and characteristics of driving difficulties in Operation Iraqi Freedom/Operation Enduring Freedom combat returnees. J Rehabil Res Dev. 2011;48(8):913-925.
9. Arthur DC, MacDermid S, Kiley KC; Defense Health Board Task Force on Mental Health. An Achievable Vision: Report of the Department of Defense Task Force on Mental Health. Falls Church, VA: Defense Health Board; 2007.
10. Tanielian T, Jaycox LH, eds. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Santa Monica, CA: RAND Corporation; 2008.
11. Independent Review Group. Rebuilding the Trust: Independent Review Group Report on Rehabilitation Care and Administrative Processes at Walter Reed Army Medical Center and National Naval Medical Center. Arlington, VA: Independent Review Group; 2007
12. Bailie JM, Cole WR, Ivins B, et al. The experience, expression, and control of anger following traumatic brain injury in a military sample. J Head Trauma Rehabil. 2015;30(1):12-20.
13. Campbell TA, Nelson LA, Lumpkin R, Yoash-Gantz RE, Pickett TC, McCormick CL. Neuropsychological measures of processing speed and executive functioning in combat veterans with PTSD, TBI, and comorbid TBI/PTSD. Psychiatr Ann. 2009;39(8):796-803.
14. Classen S, Levy C, Meyer DL, Bewernitz M, Lanford DN, Mann WC. Simulated driving performance of combat veterans with mild tramatic brain injury and posttraumatic stress disorder: a pilot study. Am J Occup Ther. 2011;65(4):419-427.
15. Lew HL, Amick MM, Kraft M, Stein MB, Cifu DX. Potential driving issues in combat returnees. NeuroRehabilitation. 2010;26(3):271-278.
16. Vasterling JL, Brailey K, Allain AN, Duke LM, Constans JI, Sutker PB. Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology. 2002;16(1):5-14.
17. Kimble MO, Kaufman ML, Leonard LL, et al. Sentence completion test in veterans with and without PTSD: preliminary findings. Psychiatry Res. 2002;113(3):303-307.
18. Kuhn E, Drescher K, Ruzek J, Rosen C. Aggressive and unsafe driving in male veterans receiving residential treatment for PTSD. J Trauma Stress. 2010;23(3):399-402.
19. Stein MB, McAllister TW. Exploring the convergence of posttraumatic stress disorder and mild traumatic brain injury. Am J Psychiatry. 2009;166(7):768-776.
20. Hill JJ III, Mobo BH Jr, Cullen MR. Separating deployment-related traumatic brain injury and posttraumatic stress disorder in veterans: preliminary findings from the Veterans Affairs traumatic brain injury screening program. Am J Phys Med Rehabil. 2009;88(8):605-614.
21. Hofmann SG, Smits JA. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69(4):621-632.
22. Hope DA, Burns JA, Hayes SA, Herbert JD, Warner MD. Automatic thoughts and cognitive restructuring in cognitive behavioral group therapy for social anxiety disorder. Cognit Ther Res. 2010;34(1):1-12.
23. Deffenbacher JL, Petrilli RT, Lynch RS, Oetting ER, Swaim RC. The driver’s angry thoughts questionnaire: a measure of angry cognitions when driving. Cognit Ther Res. 2003;27(4):383-402.
24. Beck AT, Emery G, Greenberg RL. Anxiety Disorders and Phobias: A Cognitive Perspective. Rev. paperback ed. New York, NY: Basic Books; 2005.
1. Belmont PJ, Schoenfeld AJ, Goodman G. Epidemiology of combat wounds in Operation Iraqi Freedom and Operation Enduring Freedom: orthopaedic burden of disease. J Surg Orthop Adv. 2010;19(1):2-7.
2. Zinzow HM, Brooks J, Stern EB. Driving-related anxiety in recently deployed service members: cues, mental health correlates, and help-seeking behavior. Mil Med. 2013;178(3):e357-e361.
3. Whipple EK, Schultheis MT, Robinson KM. Preliminary findings of a novel measure of driving behaviors in veterans with comorbid TBI and PTSD. J Rehabil Res Dev. 2016;53(6):827-838.
4. Adler AB, Bliese PD, McGurk D, Hoge CW, Castro CA. Battlemind debriefing and battlemind training as early interventions with soldiers returning from Iraq: randomization by platoon. J Consult Clin Psychol. 2009;77(5):928-940.
5. Amick MM, Kraft M, McGlinchey R. Driving simulator performance of veterans from the Iraq and Afghanistan wars. J Rehabil Res Dev. 2013;50(4):463-470.
6. Classen S, Cormack NL, Winter SM, et al. Efficacy of an occupational therapy driving intervention for returning combat veterans. OTJR (Thorofare NJ). 2014;34(4):177-182.
7. Hannold EM, Classen S, Winter S, Lanford DN, Levy CE. Exploratory pilot study of driving perceptions among OIF/OEF veterans with mTBI and PTSD. J Rehabil Res Dev. 2013;50(10):1315-1330.
8. Lew HL, Kraft M, Pogoda TK, Amick MM, Woods P, Cifu DX. Prevalence and characteristics of driving difficulties in Operation Iraqi Freedom/Operation Enduring Freedom combat returnees. J Rehabil Res Dev. 2011;48(8):913-925.
9. Arthur DC, MacDermid S, Kiley KC; Defense Health Board Task Force on Mental Health. An Achievable Vision: Report of the Department of Defense Task Force on Mental Health. Falls Church, VA: Defense Health Board; 2007.
10. Tanielian T, Jaycox LH, eds. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Santa Monica, CA: RAND Corporation; 2008.
11. Independent Review Group. Rebuilding the Trust: Independent Review Group Report on Rehabilitation Care and Administrative Processes at Walter Reed Army Medical Center and National Naval Medical Center. Arlington, VA: Independent Review Group; 2007
12. Bailie JM, Cole WR, Ivins B, et al. The experience, expression, and control of anger following traumatic brain injury in a military sample. J Head Trauma Rehabil. 2015;30(1):12-20.
13. Campbell TA, Nelson LA, Lumpkin R, Yoash-Gantz RE, Pickett TC, McCormick CL. Neuropsychological measures of processing speed and executive functioning in combat veterans with PTSD, TBI, and comorbid TBI/PTSD. Psychiatr Ann. 2009;39(8):796-803.
14. Classen S, Levy C, Meyer DL, Bewernitz M, Lanford DN, Mann WC. Simulated driving performance of combat veterans with mild tramatic brain injury and posttraumatic stress disorder: a pilot study. Am J Occup Ther. 2011;65(4):419-427.
15. Lew HL, Amick MM, Kraft M, Stein MB, Cifu DX. Potential driving issues in combat returnees. NeuroRehabilitation. 2010;26(3):271-278.
16. Vasterling JL, Brailey K, Allain AN, Duke LM, Constans JI, Sutker PB. Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology. 2002;16(1):5-14.
17. Kimble MO, Kaufman ML, Leonard LL, et al. Sentence completion test in veterans with and without PTSD: preliminary findings. Psychiatry Res. 2002;113(3):303-307.
18. Kuhn E, Drescher K, Ruzek J, Rosen C. Aggressive and unsafe driving in male veterans receiving residential treatment for PTSD. J Trauma Stress. 2010;23(3):399-402.
19. Stein MB, McAllister TW. Exploring the convergence of posttraumatic stress disorder and mild traumatic brain injury. Am J Psychiatry. 2009;166(7):768-776.
20. Hill JJ III, Mobo BH Jr, Cullen MR. Separating deployment-related traumatic brain injury and posttraumatic stress disorder in veterans: preliminary findings from the Veterans Affairs traumatic brain injury screening program. Am J Phys Med Rehabil. 2009;88(8):605-614.
21. Hofmann SG, Smits JA. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69(4):621-632.
22. Hope DA, Burns JA, Hayes SA, Herbert JD, Warner MD. Automatic thoughts and cognitive restructuring in cognitive behavioral group therapy for social anxiety disorder. Cognit Ther Res. 2010;34(1):1-12.
23. Deffenbacher JL, Petrilli RT, Lynch RS, Oetting ER, Swaim RC. The driver’s angry thoughts questionnaire: a measure of angry cognitions when driving. Cognit Ther Res. 2003;27(4):383-402.
24. Beck AT, Emery G, Greenberg RL. Anxiety Disorders and Phobias: A Cognitive Perspective. Rev. paperback ed. New York, NY: Basic Books; 2005.
No evidence for perioperative AI benefit in early ER/PR+ breast cancer
SAN ANTONIO – Skip the perioperative aromatase inhibitor (AI) therapy in women with early stage hormone receptor-positive breast cancer, because it doesn’t make a difference in either time to recurrence or overall survival, British investigators advise.
Among nearly 4,500 women enrolled in the POETIC trial, time to recurrence (TTR) and overall survival (OS) were similar whether patients received AI therapy within 2 weeks of surgery or did not, reported John Robertson, MD, from the University of Nottingham, England, and his colleagues.
“There is no evidence of improved clinical outcome with perioperative aromatase inhibitor therapy,” he said at the San Antonio Breast Cancer Symposium.
The trial wasn’t all for naught, however, because it also provided further evidence that measuring Ki67 levels at baseline and at 2 weeks of therapy offer significant and independent prognostic information.
“If your baseline Ki67 is low, the prognosis is good, suggesting no need for 2 weeks of AI treatment and a second Ki67 measurement,” Dr. Robertson said.
However, if the Ki67 level is 10% or higher at baseline, a Ki67 measure after 2 weeks of AI therapy can be used to guide additional therapy.
“If the 2-week Ki67 is low, patients will do relatively well, with 8.4% recurrences, and may need no additional treatment beyond standard of care. If, however, your Ki67 at 2 weeks remains high, you have a poor prognosis with an almost 20% 5-year recurrence risk, and those patients should be considered for additional chemotherapy, and/or for trials looking at new agents,” he said.
POETIC was a randomized phase 3 trial in postmenopausal women with newly diagnosed estrogen– and progesterone receptor–positive invasive breast cancer. Patients were randomized on a 2:1 basis, respectively, to either perioperative therapy with an AI (letrozole or anastrozole) for 2 weeks, surgery, and an additional 2 weeks of AI treatment, or to surgery with no perioperative therapy within a 2-week window. Patients could receive further treatment in accordance with local practice.
Of 4,486 patients randomized, 4,480 were available for analysis, including 2,528 with both baseline and 2-week Ki67 in the perioperative therapy arm, and 678 with paired Ki67 readings in the no-therapy arm.
For the primary endpoint of TTR, the curves were superimposable, with 5-year TTR of 90.9% in the perioperative AI groups, and 90.3% in the no perioperative AI group, translating into an absolute difference of only 0.52%.
Similarly, there were no differences in OS at 5 years, at 89.0% vs. 89.5%, respectively (absolute difference, 0.51%), with survival curves virtually indistinguishable from one another.
The events contributing to TTR, including local or distant recurrence and breast cancer deaths were equally distributed between the trial arms.
For patients with high baseline Ki67 values (10% or greater) at both baseline and after 2 weeks of perioperative AI therapy, the 5-year absolute risk for recurrence was 19.6%, compared with 8.9% for those with high baseline but low 2-week Ki67 levels, and 4.5% for those with low levels at both time points. The hazard ratio for patients with high Ki67 at both time points was 2.22 (P less than .001).
The POETIC trial was supported by Cancer Research UK. Dr. Robertson did not report disclosure information.
SOURCE: Robertson J et al., Abstract GS1-03
SAN ANTONIO – Skip the perioperative aromatase inhibitor (AI) therapy in women with early stage hormone receptor-positive breast cancer, because it doesn’t make a difference in either time to recurrence or overall survival, British investigators advise.
Among nearly 4,500 women enrolled in the POETIC trial, time to recurrence (TTR) and overall survival (OS) were similar whether patients received AI therapy within 2 weeks of surgery or did not, reported John Robertson, MD, from the University of Nottingham, England, and his colleagues.
“There is no evidence of improved clinical outcome with perioperative aromatase inhibitor therapy,” he said at the San Antonio Breast Cancer Symposium.
The trial wasn’t all for naught, however, because it also provided further evidence that measuring Ki67 levels at baseline and at 2 weeks of therapy offer significant and independent prognostic information.
“If your baseline Ki67 is low, the prognosis is good, suggesting no need for 2 weeks of AI treatment and a second Ki67 measurement,” Dr. Robertson said.
However, if the Ki67 level is 10% or higher at baseline, a Ki67 measure after 2 weeks of AI therapy can be used to guide additional therapy.
“If the 2-week Ki67 is low, patients will do relatively well, with 8.4% recurrences, and may need no additional treatment beyond standard of care. If, however, your Ki67 at 2 weeks remains high, you have a poor prognosis with an almost 20% 5-year recurrence risk, and those patients should be considered for additional chemotherapy, and/or for trials looking at new agents,” he said.
POETIC was a randomized phase 3 trial in postmenopausal women with newly diagnosed estrogen– and progesterone receptor–positive invasive breast cancer. Patients were randomized on a 2:1 basis, respectively, to either perioperative therapy with an AI (letrozole or anastrozole) for 2 weeks, surgery, and an additional 2 weeks of AI treatment, or to surgery with no perioperative therapy within a 2-week window. Patients could receive further treatment in accordance with local practice.
Of 4,486 patients randomized, 4,480 were available for analysis, including 2,528 with both baseline and 2-week Ki67 in the perioperative therapy arm, and 678 with paired Ki67 readings in the no-therapy arm.
For the primary endpoint of TTR, the curves were superimposable, with 5-year TTR of 90.9% in the perioperative AI groups, and 90.3% in the no perioperative AI group, translating into an absolute difference of only 0.52%.
Similarly, there were no differences in OS at 5 years, at 89.0% vs. 89.5%, respectively (absolute difference, 0.51%), with survival curves virtually indistinguishable from one another.
The events contributing to TTR, including local or distant recurrence and breast cancer deaths were equally distributed between the trial arms.
For patients with high baseline Ki67 values (10% or greater) at both baseline and after 2 weeks of perioperative AI therapy, the 5-year absolute risk for recurrence was 19.6%, compared with 8.9% for those with high baseline but low 2-week Ki67 levels, and 4.5% for those with low levels at both time points. The hazard ratio for patients with high Ki67 at both time points was 2.22 (P less than .001).
The POETIC trial was supported by Cancer Research UK. Dr. Robertson did not report disclosure information.
SOURCE: Robertson J et al., Abstract GS1-03
SAN ANTONIO – Skip the perioperative aromatase inhibitor (AI) therapy in women with early stage hormone receptor-positive breast cancer, because it doesn’t make a difference in either time to recurrence or overall survival, British investigators advise.
Among nearly 4,500 women enrolled in the POETIC trial, time to recurrence (TTR) and overall survival (OS) were similar whether patients received AI therapy within 2 weeks of surgery or did not, reported John Robertson, MD, from the University of Nottingham, England, and his colleagues.
“There is no evidence of improved clinical outcome with perioperative aromatase inhibitor therapy,” he said at the San Antonio Breast Cancer Symposium.
The trial wasn’t all for naught, however, because it also provided further evidence that measuring Ki67 levels at baseline and at 2 weeks of therapy offer significant and independent prognostic information.
“If your baseline Ki67 is low, the prognosis is good, suggesting no need for 2 weeks of AI treatment and a second Ki67 measurement,” Dr. Robertson said.
However, if the Ki67 level is 10% or higher at baseline, a Ki67 measure after 2 weeks of AI therapy can be used to guide additional therapy.
“If the 2-week Ki67 is low, patients will do relatively well, with 8.4% recurrences, and may need no additional treatment beyond standard of care. If, however, your Ki67 at 2 weeks remains high, you have a poor prognosis with an almost 20% 5-year recurrence risk, and those patients should be considered for additional chemotherapy, and/or for trials looking at new agents,” he said.
POETIC was a randomized phase 3 trial in postmenopausal women with newly diagnosed estrogen– and progesterone receptor–positive invasive breast cancer. Patients were randomized on a 2:1 basis, respectively, to either perioperative therapy with an AI (letrozole or anastrozole) for 2 weeks, surgery, and an additional 2 weeks of AI treatment, or to surgery with no perioperative therapy within a 2-week window. Patients could receive further treatment in accordance with local practice.
Of 4,486 patients randomized, 4,480 were available for analysis, including 2,528 with both baseline and 2-week Ki67 in the perioperative therapy arm, and 678 with paired Ki67 readings in the no-therapy arm.
For the primary endpoint of TTR, the curves were superimposable, with 5-year TTR of 90.9% in the perioperative AI groups, and 90.3% in the no perioperative AI group, translating into an absolute difference of only 0.52%.
Similarly, there were no differences in OS at 5 years, at 89.0% vs. 89.5%, respectively (absolute difference, 0.51%), with survival curves virtually indistinguishable from one another.
The events contributing to TTR, including local or distant recurrence and breast cancer deaths were equally distributed between the trial arms.
For patients with high baseline Ki67 values (10% or greater) at both baseline and after 2 weeks of perioperative AI therapy, the 5-year absolute risk for recurrence was 19.6%, compared with 8.9% for those with high baseline but low 2-week Ki67 levels, and 4.5% for those with low levels at both time points. The hazard ratio for patients with high Ki67 at both time points was 2.22 (P less than .001).
The POETIC trial was supported by Cancer Research UK. Dr. Robertson did not report disclosure information.
SOURCE: Robertson J et al., Abstract GS1-03
REPORTING FROM SABCS 2017
Key clinical point: Perioperative aromatase inhibitor therapy did not offer a survival benefit in women with hormone receptor–positive early stage breast cancer.
Major finding: Both 5-year time to recurrence and overall survival rates were identical among women who received perioperative AI therapy and those who did not.
Data source: Randomized phase 3 trial with analysis of data on 4,480 women.
Disclosures: The POETIC trial was supported by Cancer Research UK. Dr. Robertson did not report disclosure information.
Source: Robertson J et al., Abstract GS1-03.