User login
Targeting depression: Primary care tips and tools
THE CASE
As you get ready to see your next patient, 52-year-old Jim M, you see in his chart that during an annual routine nurse screening (per office protocol), he scored positive for depressed mood/anhedonia on the Patient Health Questionnaire-2 (PHQ-2) and scored a 21 out of 27 on the full version (PHQ-9), suggesting that he has severe major depressive disorder and that antidepressants should be considered.
When you enter the exam room, you notice his sad expression, poor eye contact, and stooped posture. Mr. M says his wife “made him” come to see you. He reports low energy and not wanting to leave his house, which started about a year earlier after he lost his job. When you discuss his job loss and the impact it has had on him, he sheepishly admits to sometimes thinking that things would be better if he were dead. Upon further questioning, you learn that he does not have suicidal intentions or plans.
HOW WOULD YOU PROCEED WITH THIS PATIENT?
Depression is the most common mental health complaint in primary care settings; in 2015, an estimated 16.1 million (6.7%) adults in the United States ages 18 or older had at least one depressive episode in the past year.1 Depression results in significant health, work, and social life impairments,2 and comorbid anxiety is highly prevalent in patients with depression.
Primary care physicians see almost twice as many mental health patients as psychiatrists3 due to barriers in behavioral health treatment (such as wait times, cost, and stigma) and the fact that primary care physicians often provide first-line access to behavioral health resources. Depression is caused by biological, psychological, and social factors, and primary care physicians are ideally positioned to develop therapeutic, healing relationships with patients that coincide with the biopsychosocial model of the disease.4
This review will provide some useful tips and tools to ensure that these patients get the care they need.
Depression? Or are other factors at play?
Major depressive disorder (MDD) is defined as a clinically significant change in mood that lasts at least 2 weeks.5 The main symptoms of MDD include depressed mood and markedly diminished interest or pleasure; additional symptoms may include reduced self-esteem, weight/appetite changes, fatigue or reduced energy, guilt/worthlessness, decreased activity, poor concentration, and suicidal thinking.5 To meet the criteria for a diagnosis of MDD, patients must experience symptoms for most of the day, nearly every day. (Dysthymia or persistent depressive disorder is a type of depression that is milder and more chronic than MDD, but does not have as many symptoms as MDD.) The focus of this article will be on MDD.
Shared symptoms with other disorders
Depression often displays some of the same symptoms as bereavement disorder and adjustment disorder, as well as other conditions.
Grief over loss and depressive symptoms circumscribed to a stressor are considered bereavement disorder and adjustment disorder, respectively. These disorders are usually limited to weeks or months as the patient adapts to his/her particular situation.
Organic problems such as nutritional deficiencies and sleep apnea can cause, exacerbate, or mimic depression (TABLE 16). Pain and depression are often associated, in that chronic pain can precipitate or perpetuate depression.7
Bipolar disorder consists of both depressive and manic episodes; patients may be misdiagnosed and treated for depression alone.
Substance intoxication or withdrawal can precipitate or perpetuate depression. A period of abstinence of at least one month may be necessary to see if depressive symptoms persist or resolve.
Premenstrual dysphoric disorder is defined as a period of depressed mood that is limited to the final week before the onset of menses and resolves in the week post-menses.
How to make the diagnosis
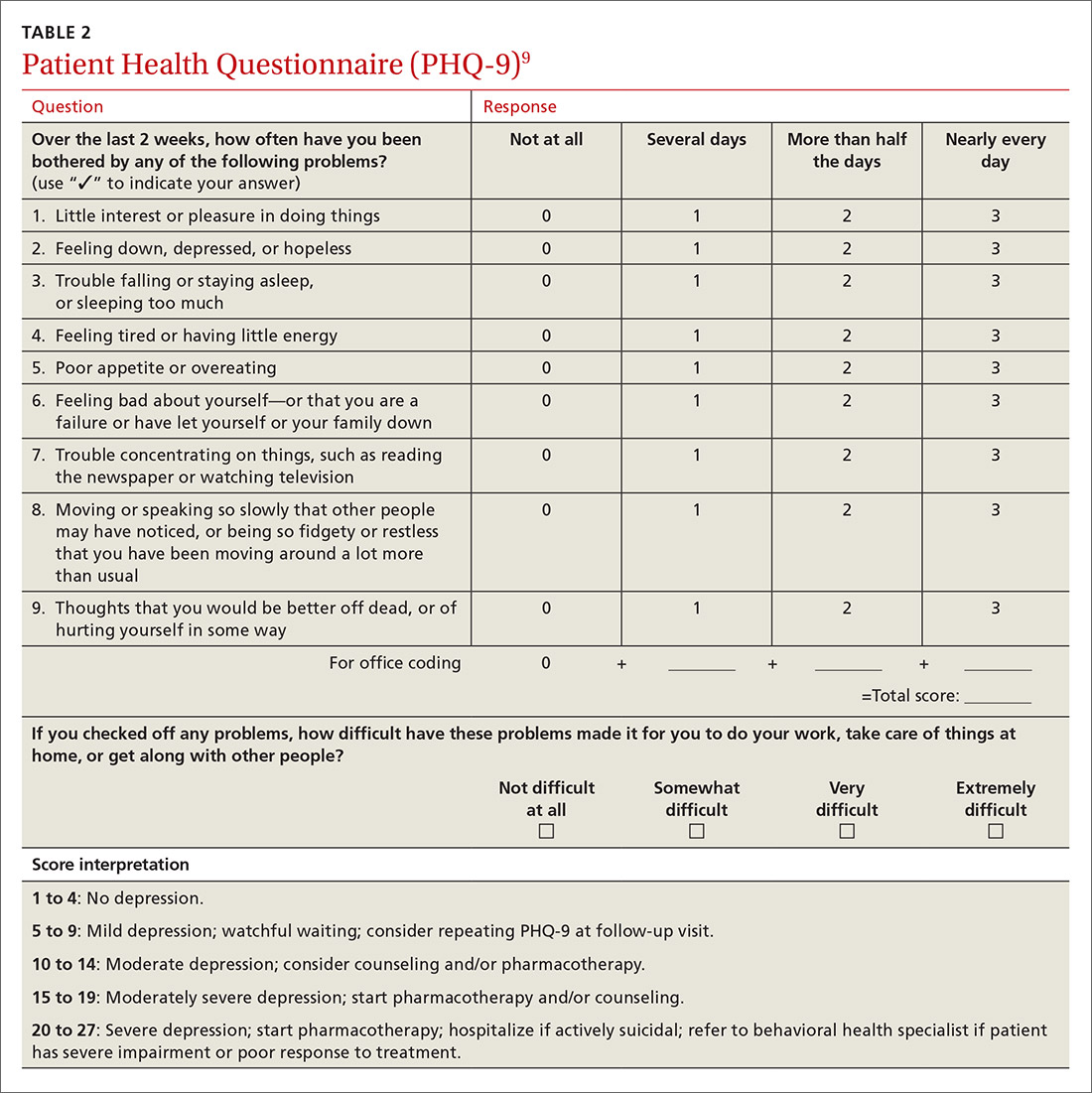
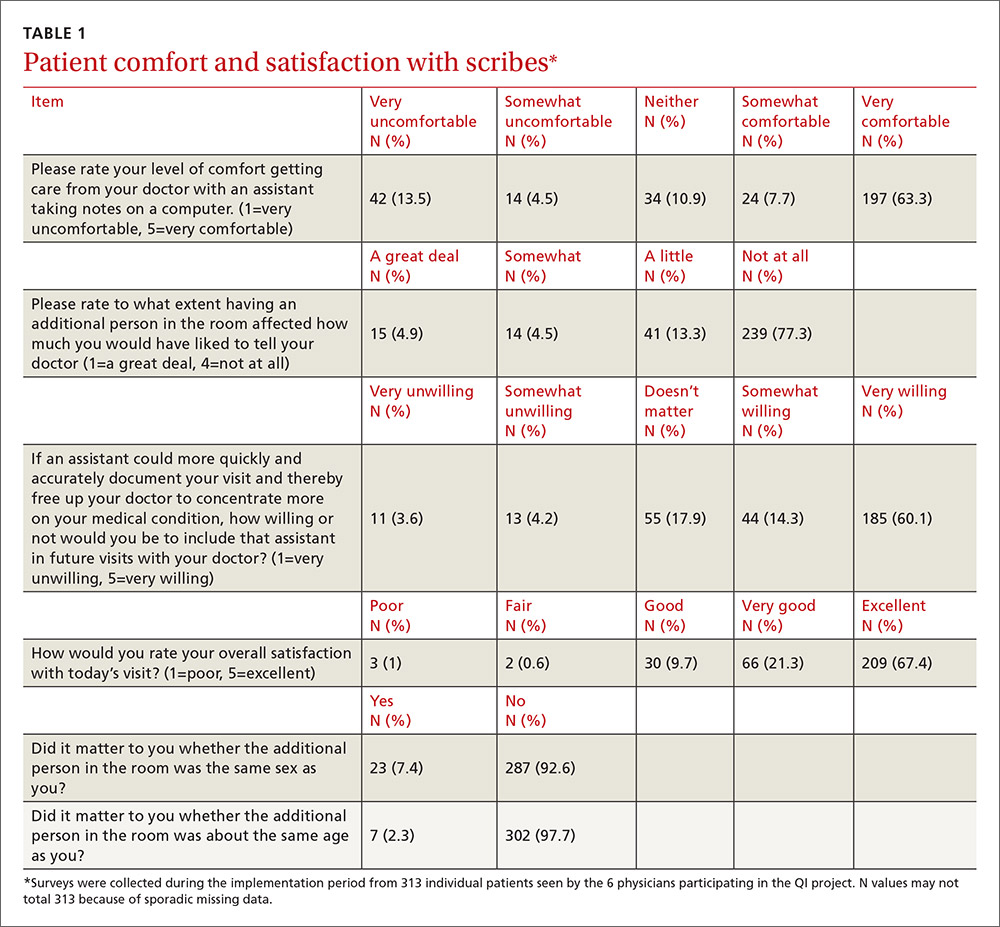
Inquiring about prolonged feelings of sadness and/or lack of enjoyment in activities is an effective way to begin the screening process for depression.8 Screening tools such as the PHQ-9 (TABLE 29), Beck Depression Inventory, Hamilton Rating Scale for Depression, and Geriatric Depression Scale are useful when combined with a clinical interview. Another useful tool is the Mood Disorder Questionnaire, which can help one determine if a patient is suffering from depression or bipolar disorder. It’s available at: http://www.dbsalliance.org/pdfs/MDQ.pdf. (Asking about a history of consecutive days of elevated, expansive, or irritable mood accompanied by increased activity or energy can also provide valuable insight.)
For its part, the US Preventive Services Task Force recommends screening adults for depression when adequate systems are in place (eg, referrals to settings that can provide necessary care) so as “to assure accurate diagnosis, effective treatment, and follow-up.”10-12
Assessing severity. Asking about functional impairments at work and at home and with academics and relationships will help determine severity, as will inquiring about a patient’s past or current suicidal thoughts. About two-thirds of all patients with depression contemplate suicide and 10% to 15% will attempt suicide.13
There is no evidence that inquiring about thoughts of death or suicide exacerbates suicidal risk.14,15 Confirming a diagnosis of MDD may require multiple visits, but should not delay treatment.
Making the most of the tools at your disposal
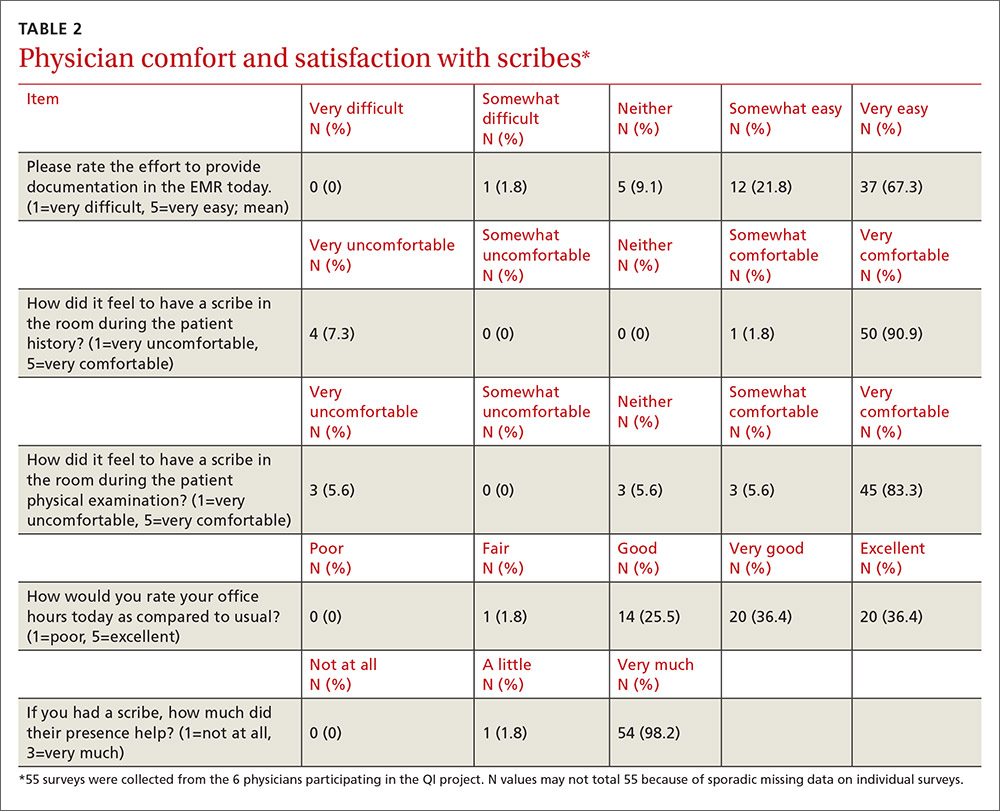
As a family physician (FP), you are especially well positioned to help patients suffering from MDD by offering education, counseling, and support; prescribing antidepressants; and coordinating care. Collaboration with behavioral health teams may be beneficial, especially in complex and treatment-resistant cases.
Counseling, alone or combined with pharmacotherapy, may improve patient outcomes.16,17 A first step may be recommending behavior modifications (such as adequate sleep, exercise, and a healthy diet). FPs can learn to utilize several counseling techniques, such as motivational interviewing, solution-focused therapy, and supportive therapy, for a variety of clinical situations in which behavioral change would be helpful.18 Establishing a therapeutic alliance through empathy and creating treatment expectations are key to helping patients overcome depression.19,20 Referral to a therapist can help identify and manage psychosocial factors that are often inherent in depression. Explaining to the patient that depression is best improved with a combination of medication and therapy is often helpful in motivating the patient to see a therapist.
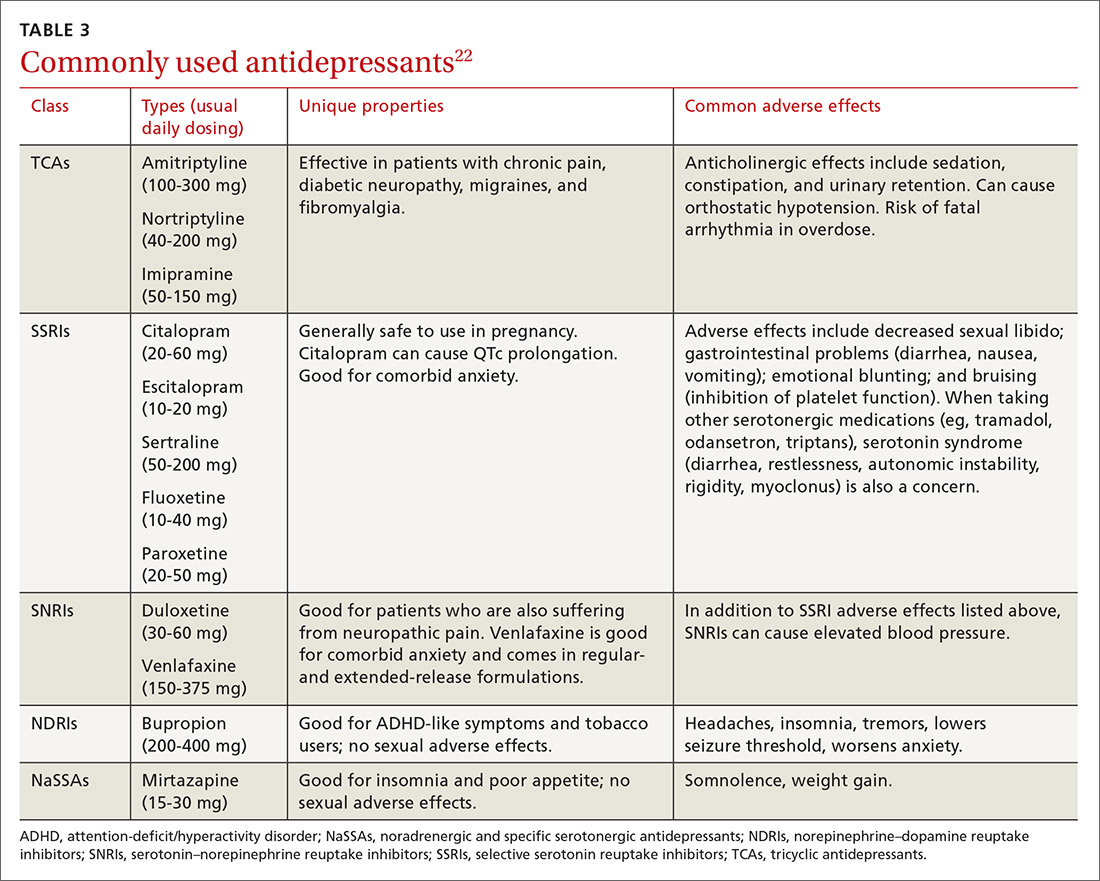
Selecting an antidepressant. There is insufficient evidence to show differences in remission rates or times to remission among antidepressants,21 so medication choice involves balancing factors such as cost, previous treatments, adverse effects, and comorbid conditions (TABLE 322). A recent systematic review and meta-analysis involving 66 studies and more than 15,000 patients found tricyclic/tetracyclic antidepressants and selective serotonin reuptake inhibitors (SSRIs) to have the best evidence for treatment of depression in the primary care setting.23 Ask the patient about previous antidepressant prescriptions they were given, if any, and weigh the benefits and adverse effects with the patient.
Patients may notice a partial response as early as one to 2 weeks after starting treatment with antidepressants, but it’s important to tell them that a full response can take up to 4 to 6 weeks. The goal of treatment is remission of depressive symptoms, which is defined as scoring below the cutoff point on a validated depression scale, such as less than 5 on the PHQ-9.24 It’s advisable to increase the antidepressant dose if the patient has a partial response and switch to a new class if the patient has no response or severe adverse effects.
Antidepressants should be maintained for at least 6 months or the length of a previous episode, whichever is greater.24 Prophylactic treatment should be considered for patients who have had severe episodes in the past (eg, a history of suicidal ideations and/or past hospitalizations). If an antidepressant is discontinued, it should be tapered over one to 2 weeks to minimize the risk of discontinuation syndrome (flu-like symptoms, nausea, insomnia, and hyperarousal). There is a lack of consistent evidence for the use of St. John’s wort, and as such, it is not recommended.24
Adjunct medications can also be used when remission does not occur after 8 to 12 weeks of maximum antidepressant doses. Insomnia, which is a common complaint in patients with MDD, can be treated with trazodone (an off-label indication), diphenhydramine, or melatonin. (See “Insomnia: Getting to the cause, facilitating relief.”) Benzodiazepines and other hypnotics (eg, zolpidem) can be used initially until antidepressants have had time to become effective. Antipsychotics such as aripiprazole, risperidone, quetiapine, and ziprasidone can be used to treat psychotic symptoms of depression or boost antidepressant effectiveness.25 Lithium and thyroxine are effective for treatment-resistant depression.26 Nutraceuticals such as S-Adenosyl-L-methionine, methylfolate, omega-3, and vitamin D can reduce depressive symptoms when combined with an antidepressant.27
There is some evidence to support combining 2 antidepressants from different classes (eg, an SSRI plus a serotonin–norepinephrine reuptake inhibitor [SNRI] or norepinephrine–dopamine reuptake inhibitor, or an SNRI plus a noradrenergic and specific serotonergic antidepressant) when adjunct therapy has proven ineffective.28
Inpatient psychiatric admission is warranted in severe cases, such as when a patient has active suicidal intentions/plans or poor self-care.
Your critical role, even when depression is co-managed
Collaborative care for depression (patient contact with both primary and behavioral health care providers in the same clinic) significantly improves clinical outcomes at 6 months compared to primary care treatment alone.29 Patients who have failed 2 therapeutic trials (at least 6-8 weeks of separate antidepressant treatments without response) are considered treatment-resistant.30 Referral to a psychiatrist is appropriate in this setting to determine alternative treatment options.
› CASE
Based on further conversation with Mr. M, you learn that he actually began exhibiting symptoms of depression (anhedonia, poor concentration, insomnia) years before he lost his job, but that he had considered the symptoms “normal” for his age. He reports that he didn’t want to socialize with others anymore and harbors feelings of worthlessness. You tell him that you believe he is suffering from MDD and talk to him about some options for treatment. You decide together to begin a trial of escitalopram 10 mg/d, as it was covered by his insurance, has minimal adverse effects, and was a good match for his symptoms. You also educate and instruct Mr. M on self-management goals such as limiting alcohol intake, eating at least 2 meals a day, walking with his wife each evening, and following a regular sleep schedule. You discuss a safety plan with Mr. M, should his depressive symptoms worsen. Specifically, you tell him that if he begins to have suicidal intentions or plans, he should call 911 or go to the nearest emergency department.
Mr. M returns 4 weeks later and reports that his mood has slightly improved, as evidenced by a brighter affect and increased energy, so you increase the dose of escitalopram to 20 mg/d. At his third visit 4 weeks later, Mr. M discloses a remote history of trauma and current intermittent heavy drinking. After offering support and education and discussing his options, you refer Mr. M to a counselor in your clinic through a “warm handoff” (the counselor is brought briefly into the current session with the patient to meet and set up an appointment). During this time, he is given information about an outpatient substance abuse treatment group.
Mr. M’s PHQ-9 improves by 8 points by his fourth visit 4 weeks later. He reports that he is still taking the escitalopram and you recommend he continue to take it. Mr. M tells you he’s been seeing the counselor at your clinic every other week and that he has begun attending meetings with the substance abuse group. He also says that he and his wife go out for walks now and then. Mr. M says he feels as though he is a failure, prompting you to recommend that he explore the cognitive distortions (ie, inaccurate thoughts that reinforce negative feelings) with his therapist.
You schedule another appointment with Mr. M in 3 months to keep track of his progress. Fortunately, Mr. M’s therapist works in the same clinic as you, so you can contact her to discuss his progress with therapy.
CORRESPONDENCE
Michael Raddock, MD, 2500 MetroHealth Drive, Cleveland, OH 44109; [email protected].
1. National Institute of Mental Health. Major depression among adults. National Institute of Mental Health Web site. Available at: http://www.nimh.nih.gov/health/statistics/prevalence/major-depression-among-adults.shtml. 2014. Accessed June 22, 2016.
2. Cameron C, Habert J, Anand L, et al. Optimizing the management of depression: primary care experience. Psychiatry Res. 2014;220:S45-S57.
3. Wang PS, Lane M, Olfson M, et al. Twelve-month use of mental health services in the United States: results from the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:629-640.
4. Schotte CK, Van Den Bossche B, De Doncker D, et al. A biopsychosocial model as a guide for psychoeducation and treatment of depression. Depress Anxiety. 2006;23:312-324.
5. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association, 2013:160-161.
6. Sadock BJ, Sadock VA. Kaplan and Sadock’s Synopsis of Psychiatry. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2003:830-834.
7. Fishbain DA, Cutler R, Rosomoff HL, et al. Chronic pain-associated depression: antecedent or consequence of chronic pain? A review. Clin J Pain. 1997;13:116-137.
8. Arroll B, Khin N, Kerse N. Screening for depression in primary care with two verbally asked questions: cross sectional study. BMJ. 2003;327:1144-1146.
9. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
10. US Preventive Services Task Force. Depression in adults: Screening. US Preventive Services Task Force Web site. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/depression-in-adults-screening. Accessed March 13, 2017.
11. Thombs BD, Ziegelstein RC. Does depression screening improve depression outcomes in primary care? BMJ. 2014;348:g1253.
12. Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for depression in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:380-387.
13. Sadock BJ, Sadock VA. Kaplan and Sadock’s Synopsis of Psychiatry. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2003:543.
14. Gould MS, Marrocco FA, Kleinman M, et al. Evaluating iatrogenic risk of youth suicide screening programs: a randomized controlled trial. JAMA. 2005;293:1635-1643.
15. Eynan R, Bergmans Y, Antony J, et al. The effects of suicide ideation assessments on urges to self-harm and suicide. Crisis. 2014;35:123-131.
16. Pampallona S, Bollini P, Tibaldi G, et al. Combined pharmacotherapy and psychological treatment for depression: a systematic review. Arch Gen Psychiatry. 2004;61:714-719.
17. Ishak WW, Ha K, Kapitanski N, et al. The impact of psychotherapy, pharmacotherapy, and their combination on quality of life in depression. Harv Rev Psychiatry. 2011;19:277-289.
18. Raddock M, Martukovich R, Berko E, et al. 7 tools to help patients adopt healthier behaviors. J Fam Pract. 2015;64:97-103.
19. Castonguay LG, Constantino MJ, Holtforth MG. The working alliance: Where are we and where should we go? Psychotherapy (Chic). 2006;43:271-279.
20. Greenberg RP, Constantino MJ, Bruce N. Are patient expectations still relevant for psychotherapy process and outcome? Clin Psychol Rev. 2006;26:657-678.
21. Warden D, Rush AJ, Trivedi MH, et al. The STAR*D Project results: a comprehensive review of findings. Curr Psychiatry Rep. 2007;9:449-459.
22. Sadock BJ, Sadock VA. Kaplan and Sadock’s Synopsis of Psychiatry. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2003:558.
23. Linde K, Kriston L, Rücker G, et al. Efficacy and acceptability of pharmacological treatments for depressive disorders in primary care: systematic review and network meta-analysis. Ann Fam Med. 2015;13:69-79.
24. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. 3rd ed. 2010. Available at: http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Accessed December 23, 2016.
25. Zhou X, Keitner GI, Qin B, et al. Atypical antipsychotic treatment for treatment-resistant depression: A systematic review and network meta-analysis. Int J Neuropsychopharmacol. 2015;18:pyv060.
26. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163:1519-1530; quiz 1665.
27. Sarris J, Murphy J, Mischoulon D, et al. Adjunctive nutraceuticals for depression: A systematic review and meta-analyses. Am J Psychiatry. 2016;173:575-587.
28. Dodd S, Horgan D, Malhi GS, et al. To combine or not to combine? A literature review of antidepressant combination therapy. J Affect Disord. 2005;89:1-11.
29. Gilbody S, Bower P, Fletcher J, et al. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med. 2006;166:2314-2321.
30. Papakostas GI, Fava M. Pharmacotherapy for Depression and Treatment-Resistant Depression. Hackensack, NJ: World Scientific. 2010:4.
THE CASE
As you get ready to see your next patient, 52-year-old Jim M, you see in his chart that during an annual routine nurse screening (per office protocol), he scored positive for depressed mood/anhedonia on the Patient Health Questionnaire-2 (PHQ-2) and scored a 21 out of 27 on the full version (PHQ-9), suggesting that he has severe major depressive disorder and that antidepressants should be considered.
When you enter the exam room, you notice his sad expression, poor eye contact, and stooped posture. Mr. M says his wife “made him” come to see you. He reports low energy and not wanting to leave his house, which started about a year earlier after he lost his job. When you discuss his job loss and the impact it has had on him, he sheepishly admits to sometimes thinking that things would be better if he were dead. Upon further questioning, you learn that he does not have suicidal intentions or plans.
HOW WOULD YOU PROCEED WITH THIS PATIENT?
Depression is the most common mental health complaint in primary care settings; in 2015, an estimated 16.1 million (6.7%) adults in the United States ages 18 or older had at least one depressive episode in the past year.1 Depression results in significant health, work, and social life impairments,2 and comorbid anxiety is highly prevalent in patients with depression.
Primary care physicians see almost twice as many mental health patients as psychiatrists3 due to barriers in behavioral health treatment (such as wait times, cost, and stigma) and the fact that primary care physicians often provide first-line access to behavioral health resources. Depression is caused by biological, psychological, and social factors, and primary care physicians are ideally positioned to develop therapeutic, healing relationships with patients that coincide with the biopsychosocial model of the disease.4
This review will provide some useful tips and tools to ensure that these patients get the care they need.
Depression? Or are other factors at play?
Major depressive disorder (MDD) is defined as a clinically significant change in mood that lasts at least 2 weeks.5 The main symptoms of MDD include depressed mood and markedly diminished interest or pleasure; additional symptoms may include reduced self-esteem, weight/appetite changes, fatigue or reduced energy, guilt/worthlessness, decreased activity, poor concentration, and suicidal thinking.5 To meet the criteria for a diagnosis of MDD, patients must experience symptoms for most of the day, nearly every day. (Dysthymia or persistent depressive disorder is a type of depression that is milder and more chronic than MDD, but does not have as many symptoms as MDD.) The focus of this article will be on MDD.
Shared symptoms with other disorders
Depression often displays some of the same symptoms as bereavement disorder and adjustment disorder, as well as other conditions.
Grief over loss and depressive symptoms circumscribed to a stressor are considered bereavement disorder and adjustment disorder, respectively. These disorders are usually limited to weeks or months as the patient adapts to his/her particular situation.
Organic problems such as nutritional deficiencies and sleep apnea can cause, exacerbate, or mimic depression (TABLE 16). Pain and depression are often associated, in that chronic pain can precipitate or perpetuate depression.7
Bipolar disorder consists of both depressive and manic episodes; patients may be misdiagnosed and treated for depression alone.
Substance intoxication or withdrawal can precipitate or perpetuate depression. A period of abstinence of at least one month may be necessary to see if depressive symptoms persist or resolve.
Premenstrual dysphoric disorder is defined as a period of depressed mood that is limited to the final week before the onset of menses and resolves in the week post-menses.
How to make the diagnosis
Inquiring about prolonged feelings of sadness and/or lack of enjoyment in activities is an effective way to begin the screening process for depression.8 Screening tools such as the PHQ-9 (TABLE 29), Beck Depression Inventory, Hamilton Rating Scale for Depression, and Geriatric Depression Scale are useful when combined with a clinical interview. Another useful tool is the Mood Disorder Questionnaire, which can help one determine if a patient is suffering from depression or bipolar disorder. It’s available at: http://www.dbsalliance.org/pdfs/MDQ.pdf. (Asking about a history of consecutive days of elevated, expansive, or irritable mood accompanied by increased activity or energy can also provide valuable insight.)
For its part, the US Preventive Services Task Force recommends screening adults for depression when adequate systems are in place (eg, referrals to settings that can provide necessary care) so as “to assure accurate diagnosis, effective treatment, and follow-up.”10-12
Assessing severity. Asking about functional impairments at work and at home and with academics and relationships will help determine severity, as will inquiring about a patient’s past or current suicidal thoughts. About two-thirds of all patients with depression contemplate suicide and 10% to 15% will attempt suicide.13
There is no evidence that inquiring about thoughts of death or suicide exacerbates suicidal risk.14,15 Confirming a diagnosis of MDD may require multiple visits, but should not delay treatment.
Making the most of the tools at your disposal
As a family physician (FP), you are especially well positioned to help patients suffering from MDD by offering education, counseling, and support; prescribing antidepressants; and coordinating care. Collaboration with behavioral health teams may be beneficial, especially in complex and treatment-resistant cases.
Counseling, alone or combined with pharmacotherapy, may improve patient outcomes.16,17 A first step may be recommending behavior modifications (such as adequate sleep, exercise, and a healthy diet). FPs can learn to utilize several counseling techniques, such as motivational interviewing, solution-focused therapy, and supportive therapy, for a variety of clinical situations in which behavioral change would be helpful.18 Establishing a therapeutic alliance through empathy and creating treatment expectations are key to helping patients overcome depression.19,20 Referral to a therapist can help identify and manage psychosocial factors that are often inherent in depression. Explaining to the patient that depression is best improved with a combination of medication and therapy is often helpful in motivating the patient to see a therapist.
Selecting an antidepressant. There is insufficient evidence to show differences in remission rates or times to remission among antidepressants,21 so medication choice involves balancing factors such as cost, previous treatments, adverse effects, and comorbid conditions (TABLE 322). A recent systematic review and meta-analysis involving 66 studies and more than 15,000 patients found tricyclic/tetracyclic antidepressants and selective serotonin reuptake inhibitors (SSRIs) to have the best evidence for treatment of depression in the primary care setting.23 Ask the patient about previous antidepressant prescriptions they were given, if any, and weigh the benefits and adverse effects with the patient.
Patients may notice a partial response as early as one to 2 weeks after starting treatment with antidepressants, but it’s important to tell them that a full response can take up to 4 to 6 weeks. The goal of treatment is remission of depressive symptoms, which is defined as scoring below the cutoff point on a validated depression scale, such as less than 5 on the PHQ-9.24 It’s advisable to increase the antidepressant dose if the patient has a partial response and switch to a new class if the patient has no response or severe adverse effects.
Antidepressants should be maintained for at least 6 months or the length of a previous episode, whichever is greater.24 Prophylactic treatment should be considered for patients who have had severe episodes in the past (eg, a history of suicidal ideations and/or past hospitalizations). If an antidepressant is discontinued, it should be tapered over one to 2 weeks to minimize the risk of discontinuation syndrome (flu-like symptoms, nausea, insomnia, and hyperarousal). There is a lack of consistent evidence for the use of St. John’s wort, and as such, it is not recommended.24
Adjunct medications can also be used when remission does not occur after 8 to 12 weeks of maximum antidepressant doses. Insomnia, which is a common complaint in patients with MDD, can be treated with trazodone (an off-label indication), diphenhydramine, or melatonin. (See “Insomnia: Getting to the cause, facilitating relief.”) Benzodiazepines and other hypnotics (eg, zolpidem) can be used initially until antidepressants have had time to become effective. Antipsychotics such as aripiprazole, risperidone, quetiapine, and ziprasidone can be used to treat psychotic symptoms of depression or boost antidepressant effectiveness.25 Lithium and thyroxine are effective for treatment-resistant depression.26 Nutraceuticals such as S-Adenosyl-L-methionine, methylfolate, omega-3, and vitamin D can reduce depressive symptoms when combined with an antidepressant.27
There is some evidence to support combining 2 antidepressants from different classes (eg, an SSRI plus a serotonin–norepinephrine reuptake inhibitor [SNRI] or norepinephrine–dopamine reuptake inhibitor, or an SNRI plus a noradrenergic and specific serotonergic antidepressant) when adjunct therapy has proven ineffective.28
Inpatient psychiatric admission is warranted in severe cases, such as when a patient has active suicidal intentions/plans or poor self-care.
Your critical role, even when depression is co-managed
Collaborative care for depression (patient contact with both primary and behavioral health care providers in the same clinic) significantly improves clinical outcomes at 6 months compared to primary care treatment alone.29 Patients who have failed 2 therapeutic trials (at least 6-8 weeks of separate antidepressant treatments without response) are considered treatment-resistant.30 Referral to a psychiatrist is appropriate in this setting to determine alternative treatment options.
› CASE
Based on further conversation with Mr. M, you learn that he actually began exhibiting symptoms of depression (anhedonia, poor concentration, insomnia) years before he lost his job, but that he had considered the symptoms “normal” for his age. He reports that he didn’t want to socialize with others anymore and harbors feelings of worthlessness. You tell him that you believe he is suffering from MDD and talk to him about some options for treatment. You decide together to begin a trial of escitalopram 10 mg/d, as it was covered by his insurance, has minimal adverse effects, and was a good match for his symptoms. You also educate and instruct Mr. M on self-management goals such as limiting alcohol intake, eating at least 2 meals a day, walking with his wife each evening, and following a regular sleep schedule. You discuss a safety plan with Mr. M, should his depressive symptoms worsen. Specifically, you tell him that if he begins to have suicidal intentions or plans, he should call 911 or go to the nearest emergency department.
Mr. M returns 4 weeks later and reports that his mood has slightly improved, as evidenced by a brighter affect and increased energy, so you increase the dose of escitalopram to 20 mg/d. At his third visit 4 weeks later, Mr. M discloses a remote history of trauma and current intermittent heavy drinking. After offering support and education and discussing his options, you refer Mr. M to a counselor in your clinic through a “warm handoff” (the counselor is brought briefly into the current session with the patient to meet and set up an appointment). During this time, he is given information about an outpatient substance abuse treatment group.
Mr. M’s PHQ-9 improves by 8 points by his fourth visit 4 weeks later. He reports that he is still taking the escitalopram and you recommend he continue to take it. Mr. M tells you he’s been seeing the counselor at your clinic every other week and that he has begun attending meetings with the substance abuse group. He also says that he and his wife go out for walks now and then. Mr. M says he feels as though he is a failure, prompting you to recommend that he explore the cognitive distortions (ie, inaccurate thoughts that reinforce negative feelings) with his therapist.
You schedule another appointment with Mr. M in 3 months to keep track of his progress. Fortunately, Mr. M’s therapist works in the same clinic as you, so you can contact her to discuss his progress with therapy.
CORRESPONDENCE
Michael Raddock, MD, 2500 MetroHealth Drive, Cleveland, OH 44109; [email protected].
THE CASE
As you get ready to see your next patient, 52-year-old Jim M, you see in his chart that during an annual routine nurse screening (per office protocol), he scored positive for depressed mood/anhedonia on the Patient Health Questionnaire-2 (PHQ-2) and scored a 21 out of 27 on the full version (PHQ-9), suggesting that he has severe major depressive disorder and that antidepressants should be considered.
When you enter the exam room, you notice his sad expression, poor eye contact, and stooped posture. Mr. M says his wife “made him” come to see you. He reports low energy and not wanting to leave his house, which started about a year earlier after he lost his job. When you discuss his job loss and the impact it has had on him, he sheepishly admits to sometimes thinking that things would be better if he were dead. Upon further questioning, you learn that he does not have suicidal intentions or plans.
HOW WOULD YOU PROCEED WITH THIS PATIENT?
Depression is the most common mental health complaint in primary care settings; in 2015, an estimated 16.1 million (6.7%) adults in the United States ages 18 or older had at least one depressive episode in the past year.1 Depression results in significant health, work, and social life impairments,2 and comorbid anxiety is highly prevalent in patients with depression.
Primary care physicians see almost twice as many mental health patients as psychiatrists3 due to barriers in behavioral health treatment (such as wait times, cost, and stigma) and the fact that primary care physicians often provide first-line access to behavioral health resources. Depression is caused by biological, psychological, and social factors, and primary care physicians are ideally positioned to develop therapeutic, healing relationships with patients that coincide with the biopsychosocial model of the disease.4
This review will provide some useful tips and tools to ensure that these patients get the care they need.
Depression? Or are other factors at play?
Major depressive disorder (MDD) is defined as a clinically significant change in mood that lasts at least 2 weeks.5 The main symptoms of MDD include depressed mood and markedly diminished interest or pleasure; additional symptoms may include reduced self-esteem, weight/appetite changes, fatigue or reduced energy, guilt/worthlessness, decreased activity, poor concentration, and suicidal thinking.5 To meet the criteria for a diagnosis of MDD, patients must experience symptoms for most of the day, nearly every day. (Dysthymia or persistent depressive disorder is a type of depression that is milder and more chronic than MDD, but does not have as many symptoms as MDD.) The focus of this article will be on MDD.
Shared symptoms with other disorders
Depression often displays some of the same symptoms as bereavement disorder and adjustment disorder, as well as other conditions.
Grief over loss and depressive symptoms circumscribed to a stressor are considered bereavement disorder and adjustment disorder, respectively. These disorders are usually limited to weeks or months as the patient adapts to his/her particular situation.
Organic problems such as nutritional deficiencies and sleep apnea can cause, exacerbate, or mimic depression (TABLE 16). Pain and depression are often associated, in that chronic pain can precipitate or perpetuate depression.7
Bipolar disorder consists of both depressive and manic episodes; patients may be misdiagnosed and treated for depression alone.
Substance intoxication or withdrawal can precipitate or perpetuate depression. A period of abstinence of at least one month may be necessary to see if depressive symptoms persist or resolve.
Premenstrual dysphoric disorder is defined as a period of depressed mood that is limited to the final week before the onset of menses and resolves in the week post-menses.
How to make the diagnosis
Inquiring about prolonged feelings of sadness and/or lack of enjoyment in activities is an effective way to begin the screening process for depression.8 Screening tools such as the PHQ-9 (TABLE 29), Beck Depression Inventory, Hamilton Rating Scale for Depression, and Geriatric Depression Scale are useful when combined with a clinical interview. Another useful tool is the Mood Disorder Questionnaire, which can help one determine if a patient is suffering from depression or bipolar disorder. It’s available at: http://www.dbsalliance.org/pdfs/MDQ.pdf. (Asking about a history of consecutive days of elevated, expansive, or irritable mood accompanied by increased activity or energy can also provide valuable insight.)
For its part, the US Preventive Services Task Force recommends screening adults for depression when adequate systems are in place (eg, referrals to settings that can provide necessary care) so as “to assure accurate diagnosis, effective treatment, and follow-up.”10-12
Assessing severity. Asking about functional impairments at work and at home and with academics and relationships will help determine severity, as will inquiring about a patient’s past or current suicidal thoughts. About two-thirds of all patients with depression contemplate suicide and 10% to 15% will attempt suicide.13
There is no evidence that inquiring about thoughts of death or suicide exacerbates suicidal risk.14,15 Confirming a diagnosis of MDD may require multiple visits, but should not delay treatment.
Making the most of the tools at your disposal
As a family physician (FP), you are especially well positioned to help patients suffering from MDD by offering education, counseling, and support; prescribing antidepressants; and coordinating care. Collaboration with behavioral health teams may be beneficial, especially in complex and treatment-resistant cases.
Counseling, alone or combined with pharmacotherapy, may improve patient outcomes.16,17 A first step may be recommending behavior modifications (such as adequate sleep, exercise, and a healthy diet). FPs can learn to utilize several counseling techniques, such as motivational interviewing, solution-focused therapy, and supportive therapy, for a variety of clinical situations in which behavioral change would be helpful.18 Establishing a therapeutic alliance through empathy and creating treatment expectations are key to helping patients overcome depression.19,20 Referral to a therapist can help identify and manage psychosocial factors that are often inherent in depression. Explaining to the patient that depression is best improved with a combination of medication and therapy is often helpful in motivating the patient to see a therapist.
Selecting an antidepressant. There is insufficient evidence to show differences in remission rates or times to remission among antidepressants,21 so medication choice involves balancing factors such as cost, previous treatments, adverse effects, and comorbid conditions (TABLE 322). A recent systematic review and meta-analysis involving 66 studies and more than 15,000 patients found tricyclic/tetracyclic antidepressants and selective serotonin reuptake inhibitors (SSRIs) to have the best evidence for treatment of depression in the primary care setting.23 Ask the patient about previous antidepressant prescriptions they were given, if any, and weigh the benefits and adverse effects with the patient.
Patients may notice a partial response as early as one to 2 weeks after starting treatment with antidepressants, but it’s important to tell them that a full response can take up to 4 to 6 weeks. The goal of treatment is remission of depressive symptoms, which is defined as scoring below the cutoff point on a validated depression scale, such as less than 5 on the PHQ-9.24 It’s advisable to increase the antidepressant dose if the patient has a partial response and switch to a new class if the patient has no response or severe adverse effects.
Antidepressants should be maintained for at least 6 months or the length of a previous episode, whichever is greater.24 Prophylactic treatment should be considered for patients who have had severe episodes in the past (eg, a history of suicidal ideations and/or past hospitalizations). If an antidepressant is discontinued, it should be tapered over one to 2 weeks to minimize the risk of discontinuation syndrome (flu-like symptoms, nausea, insomnia, and hyperarousal). There is a lack of consistent evidence for the use of St. John’s wort, and as such, it is not recommended.24
Adjunct medications can also be used when remission does not occur after 8 to 12 weeks of maximum antidepressant doses. Insomnia, which is a common complaint in patients with MDD, can be treated with trazodone (an off-label indication), diphenhydramine, or melatonin. (See “Insomnia: Getting to the cause, facilitating relief.”) Benzodiazepines and other hypnotics (eg, zolpidem) can be used initially until antidepressants have had time to become effective. Antipsychotics such as aripiprazole, risperidone, quetiapine, and ziprasidone can be used to treat psychotic symptoms of depression or boost antidepressant effectiveness.25 Lithium and thyroxine are effective for treatment-resistant depression.26 Nutraceuticals such as S-Adenosyl-L-methionine, methylfolate, omega-3, and vitamin D can reduce depressive symptoms when combined with an antidepressant.27
There is some evidence to support combining 2 antidepressants from different classes (eg, an SSRI plus a serotonin–norepinephrine reuptake inhibitor [SNRI] or norepinephrine–dopamine reuptake inhibitor, or an SNRI plus a noradrenergic and specific serotonergic antidepressant) when adjunct therapy has proven ineffective.28
Inpatient psychiatric admission is warranted in severe cases, such as when a patient has active suicidal intentions/plans or poor self-care.
Your critical role, even when depression is co-managed
Collaborative care for depression (patient contact with both primary and behavioral health care providers in the same clinic) significantly improves clinical outcomes at 6 months compared to primary care treatment alone.29 Patients who have failed 2 therapeutic trials (at least 6-8 weeks of separate antidepressant treatments without response) are considered treatment-resistant.30 Referral to a psychiatrist is appropriate in this setting to determine alternative treatment options.
› CASE
Based on further conversation with Mr. M, you learn that he actually began exhibiting symptoms of depression (anhedonia, poor concentration, insomnia) years before he lost his job, but that he had considered the symptoms “normal” for his age. He reports that he didn’t want to socialize with others anymore and harbors feelings of worthlessness. You tell him that you believe he is suffering from MDD and talk to him about some options for treatment. You decide together to begin a trial of escitalopram 10 mg/d, as it was covered by his insurance, has minimal adverse effects, and was a good match for his symptoms. You also educate and instruct Mr. M on self-management goals such as limiting alcohol intake, eating at least 2 meals a day, walking with his wife each evening, and following a regular sleep schedule. You discuss a safety plan with Mr. M, should his depressive symptoms worsen. Specifically, you tell him that if he begins to have suicidal intentions or plans, he should call 911 or go to the nearest emergency department.
Mr. M returns 4 weeks later and reports that his mood has slightly improved, as evidenced by a brighter affect and increased energy, so you increase the dose of escitalopram to 20 mg/d. At his third visit 4 weeks later, Mr. M discloses a remote history of trauma and current intermittent heavy drinking. After offering support and education and discussing his options, you refer Mr. M to a counselor in your clinic through a “warm handoff” (the counselor is brought briefly into the current session with the patient to meet and set up an appointment). During this time, he is given information about an outpatient substance abuse treatment group.
Mr. M’s PHQ-9 improves by 8 points by his fourth visit 4 weeks later. He reports that he is still taking the escitalopram and you recommend he continue to take it. Mr. M tells you he’s been seeing the counselor at your clinic every other week and that he has begun attending meetings with the substance abuse group. He also says that he and his wife go out for walks now and then. Mr. M says he feels as though he is a failure, prompting you to recommend that he explore the cognitive distortions (ie, inaccurate thoughts that reinforce negative feelings) with his therapist.
You schedule another appointment with Mr. M in 3 months to keep track of his progress. Fortunately, Mr. M’s therapist works in the same clinic as you, so you can contact her to discuss his progress with therapy.
CORRESPONDENCE
Michael Raddock, MD, 2500 MetroHealth Drive, Cleveland, OH 44109; [email protected].
1. National Institute of Mental Health. Major depression among adults. National Institute of Mental Health Web site. Available at: http://www.nimh.nih.gov/health/statistics/prevalence/major-depression-among-adults.shtml. 2014. Accessed June 22, 2016.
2. Cameron C, Habert J, Anand L, et al. Optimizing the management of depression: primary care experience. Psychiatry Res. 2014;220:S45-S57.
3. Wang PS, Lane M, Olfson M, et al. Twelve-month use of mental health services in the United States: results from the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:629-640.
4. Schotte CK, Van Den Bossche B, De Doncker D, et al. A biopsychosocial model as a guide for psychoeducation and treatment of depression. Depress Anxiety. 2006;23:312-324.
5. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association, 2013:160-161.
6. Sadock BJ, Sadock VA. Kaplan and Sadock’s Synopsis of Psychiatry. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2003:830-834.
7. Fishbain DA, Cutler R, Rosomoff HL, et al. Chronic pain-associated depression: antecedent or consequence of chronic pain? A review. Clin J Pain. 1997;13:116-137.
8. Arroll B, Khin N, Kerse N. Screening for depression in primary care with two verbally asked questions: cross sectional study. BMJ. 2003;327:1144-1146.
9. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
10. US Preventive Services Task Force. Depression in adults: Screening. US Preventive Services Task Force Web site. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/depression-in-adults-screening. Accessed March 13, 2017.
11. Thombs BD, Ziegelstein RC. Does depression screening improve depression outcomes in primary care? BMJ. 2014;348:g1253.
12. Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for depression in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:380-387.
13. Sadock BJ, Sadock VA. Kaplan and Sadock’s Synopsis of Psychiatry. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2003:543.
14. Gould MS, Marrocco FA, Kleinman M, et al. Evaluating iatrogenic risk of youth suicide screening programs: a randomized controlled trial. JAMA. 2005;293:1635-1643.
15. Eynan R, Bergmans Y, Antony J, et al. The effects of suicide ideation assessments on urges to self-harm and suicide. Crisis. 2014;35:123-131.
16. Pampallona S, Bollini P, Tibaldi G, et al. Combined pharmacotherapy and psychological treatment for depression: a systematic review. Arch Gen Psychiatry. 2004;61:714-719.
17. Ishak WW, Ha K, Kapitanski N, et al. The impact of psychotherapy, pharmacotherapy, and their combination on quality of life in depression. Harv Rev Psychiatry. 2011;19:277-289.
18. Raddock M, Martukovich R, Berko E, et al. 7 tools to help patients adopt healthier behaviors. J Fam Pract. 2015;64:97-103.
19. Castonguay LG, Constantino MJ, Holtforth MG. The working alliance: Where are we and where should we go? Psychotherapy (Chic). 2006;43:271-279.
20. Greenberg RP, Constantino MJ, Bruce N. Are patient expectations still relevant for psychotherapy process and outcome? Clin Psychol Rev. 2006;26:657-678.
21. Warden D, Rush AJ, Trivedi MH, et al. The STAR*D Project results: a comprehensive review of findings. Curr Psychiatry Rep. 2007;9:449-459.
22. Sadock BJ, Sadock VA. Kaplan and Sadock’s Synopsis of Psychiatry. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2003:558.
23. Linde K, Kriston L, Rücker G, et al. Efficacy and acceptability of pharmacological treatments for depressive disorders in primary care: systematic review and network meta-analysis. Ann Fam Med. 2015;13:69-79.
24. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. 3rd ed. 2010. Available at: http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Accessed December 23, 2016.
25. Zhou X, Keitner GI, Qin B, et al. Atypical antipsychotic treatment for treatment-resistant depression: A systematic review and network meta-analysis. Int J Neuropsychopharmacol. 2015;18:pyv060.
26. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163:1519-1530; quiz 1665.
27. Sarris J, Murphy J, Mischoulon D, et al. Adjunctive nutraceuticals for depression: A systematic review and meta-analyses. Am J Psychiatry. 2016;173:575-587.
28. Dodd S, Horgan D, Malhi GS, et al. To combine or not to combine? A literature review of antidepressant combination therapy. J Affect Disord. 2005;89:1-11.
29. Gilbody S, Bower P, Fletcher J, et al. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med. 2006;166:2314-2321.
30. Papakostas GI, Fava M. Pharmacotherapy for Depression and Treatment-Resistant Depression. Hackensack, NJ: World Scientific. 2010:4.
1. National Institute of Mental Health. Major depression among adults. National Institute of Mental Health Web site. Available at: http://www.nimh.nih.gov/health/statistics/prevalence/major-depression-among-adults.shtml. 2014. Accessed June 22, 2016.
2. Cameron C, Habert J, Anand L, et al. Optimizing the management of depression: primary care experience. Psychiatry Res. 2014;220:S45-S57.
3. Wang PS, Lane M, Olfson M, et al. Twelve-month use of mental health services in the United States: results from the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:629-640.
4. Schotte CK, Van Den Bossche B, De Doncker D, et al. A biopsychosocial model as a guide for psychoeducation and treatment of depression. Depress Anxiety. 2006;23:312-324.
5. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association, 2013:160-161.
6. Sadock BJ, Sadock VA. Kaplan and Sadock’s Synopsis of Psychiatry. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2003:830-834.
7. Fishbain DA, Cutler R, Rosomoff HL, et al. Chronic pain-associated depression: antecedent or consequence of chronic pain? A review. Clin J Pain. 1997;13:116-137.
8. Arroll B, Khin N, Kerse N. Screening for depression in primary care with two verbally asked questions: cross sectional study. BMJ. 2003;327:1144-1146.
9. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
10. US Preventive Services Task Force. Depression in adults: Screening. US Preventive Services Task Force Web site. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/depression-in-adults-screening. Accessed March 13, 2017.
11. Thombs BD, Ziegelstein RC. Does depression screening improve depression outcomes in primary care? BMJ. 2014;348:g1253.
12. Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for depression in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:380-387.
13. Sadock BJ, Sadock VA. Kaplan and Sadock’s Synopsis of Psychiatry. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2003:543.
14. Gould MS, Marrocco FA, Kleinman M, et al. Evaluating iatrogenic risk of youth suicide screening programs: a randomized controlled trial. JAMA. 2005;293:1635-1643.
15. Eynan R, Bergmans Y, Antony J, et al. The effects of suicide ideation assessments on urges to self-harm and suicide. Crisis. 2014;35:123-131.
16. Pampallona S, Bollini P, Tibaldi G, et al. Combined pharmacotherapy and psychological treatment for depression: a systematic review. Arch Gen Psychiatry. 2004;61:714-719.
17. Ishak WW, Ha K, Kapitanski N, et al. The impact of psychotherapy, pharmacotherapy, and their combination on quality of life in depression. Harv Rev Psychiatry. 2011;19:277-289.
18. Raddock M, Martukovich R, Berko E, et al. 7 tools to help patients adopt healthier behaviors. J Fam Pract. 2015;64:97-103.
19. Castonguay LG, Constantino MJ, Holtforth MG. The working alliance: Where are we and where should we go? Psychotherapy (Chic). 2006;43:271-279.
20. Greenberg RP, Constantino MJ, Bruce N. Are patient expectations still relevant for psychotherapy process and outcome? Clin Psychol Rev. 2006;26:657-678.
21. Warden D, Rush AJ, Trivedi MH, et al. The STAR*D Project results: a comprehensive review of findings. Curr Psychiatry Rep. 2007;9:449-459.
22. Sadock BJ, Sadock VA. Kaplan and Sadock’s Synopsis of Psychiatry. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2003:558.
23. Linde K, Kriston L, Rücker G, et al. Efficacy and acceptability of pharmacological treatments for depressive disorders in primary care: systematic review and network meta-analysis. Ann Fam Med. 2015;13:69-79.
24. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. 3rd ed. 2010. Available at: http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Accessed December 23, 2016.
25. Zhou X, Keitner GI, Qin B, et al. Atypical antipsychotic treatment for treatment-resistant depression: A systematic review and network meta-analysis. Int J Neuropsychopharmacol. 2015;18:pyv060.
26. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163:1519-1530; quiz 1665.
27. Sarris J, Murphy J, Mischoulon D, et al. Adjunctive nutraceuticals for depression: A systematic review and meta-analyses. Am J Psychiatry. 2016;173:575-587.
28. Dodd S, Horgan D, Malhi GS, et al. To combine or not to combine? A literature review of antidepressant combination therapy. J Affect Disord. 2005;89:1-11.
29. Gilbody S, Bower P, Fletcher J, et al. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med. 2006;166:2314-2321.
30. Papakostas GI, Fava M. Pharmacotherapy for Depression and Treatment-Resistant Depression. Hackensack, NJ: World Scientific. 2010:4.
Partnering to optimize care of childhood cancer survivors
The number of childhood cancer survivors (CCSs) entering the adult health care system is increasing, a not-so-surprising trend when you consider that more than 80% of children and adolescents given a cancer diagnosis become long-term survivors.1 This patient population has a heightened risk for developing at least one chronic health problem, resulting from therapy. By the fourth decade of life, 88% of all CCSs will have a chronic condition,2 and about one-third develop a late effect that is either severe or life-threatening.3 In contrast to patients with many other pediatric chronic diseases that manifest at an early age and are progressive, CCSs are often physically well for many years, or decades, prior to their manifestation of late effects.4
Cancer survivorship has varying definitions; however, we define cancer survivorship as the phase of cancer care for individuals who have been diagnosed with cancer and have completed primary treatment for their disease.5 Cancer survivorship, which is becoming more widely acknowledged as a distinct and critically important phase of cancer care, includes:6
- “surveillance for recurrence,
- evaluation … and treatment of medical and psychosocial consequences of treatment,
- recommendations for screening for new primary cancers,
- health promotion recommendations, and
- provision of a written treatment summary and care plan to the patient and other health professionals.”
Although models of survivorship care vary, their common goal is to promote optimal health and well-being in cancer survivors, and to prevent and detect any health concerns that may be related to prior cancer diagnosis or treatment.
Some pediatric cancer survivors have not received recommended survivorship care because of a lack of insurance or limitations from pre-existing conditions.4,7 The Affordable Care Act may remove these barriers for many.8 Others, however, fail to receive such recommendations because national models of transition are lacking. Unique considerations for this population include their need to establish age appropriate, lifelong follow-up care (and education) from a primary care provider (PCP). Unfortunately, many CCSs become lost to follow-up and fail to receive recommended survivorship care when they discontinue the relationship with their pediatrician or family practitioner and their pediatric oncologist. Fewer than 25% of CCSs who have been successfully treated for cancer during childhood continue to be followed by a cancer center and are at risk for missing survivorship-focused care or recommended screening.4,9
PCPs are an invaluable link in helping CCSs to continue to receive recommended care and surveillance. However, PCPs experience barriers in providing cancer care because of a lack of timely and specific communication from oncologists and limited knowledge of guidelines and resources available to them.10 The purpose of this article is to share information with you, the family physician, about childhood cancer survivorship needs, available resources, and how partnering with pediatric oncologists may improve treatment and health outcomes for CCSs.
Providing for the future health of childhood cancer survivors
Numerous studies have outlined the myriad of potential late effects that CCSs may experience from disease and treatment.11,12 These effects can manifest at any time and can appear in virtually every body system from the central nervous system, to the lungs, heart, bones, and endocrine systems. CCSs' particular risk for late effects may result from many factors including cancer diagnosis, types of treatments (eg, surgery, chemotherapy, radiation, and stem-cell transplant), and dosages of medications, gender, and age at diagnosis.
Determining individual risk for late effects
The Children’s Oncology Group (COG) is the world’s largest organization devoted exclusively to childhood and adolescent cancer research, including the long-term health of cancer survivors. To help provide more individualized recommendations, COG has set forth risk-based, evidence-based, exposure-related clinical practice guidelines to offer recommendations for screening and management of late effects in survivors of childhood and adolescent cancers.13 (These guidelines, Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers, are available at http://www.survivorshipguidelines.org.) The purpose of the guidelines is to standardize and enhance follow-up care for CCSs throughout their lifespan.13 To remain current, a multidisciplinary task force reviews and incorporates findings from the medical literature—including evaluations of the cost-effectiveness of recommended testing—into guideline revisions at least every 5 years.
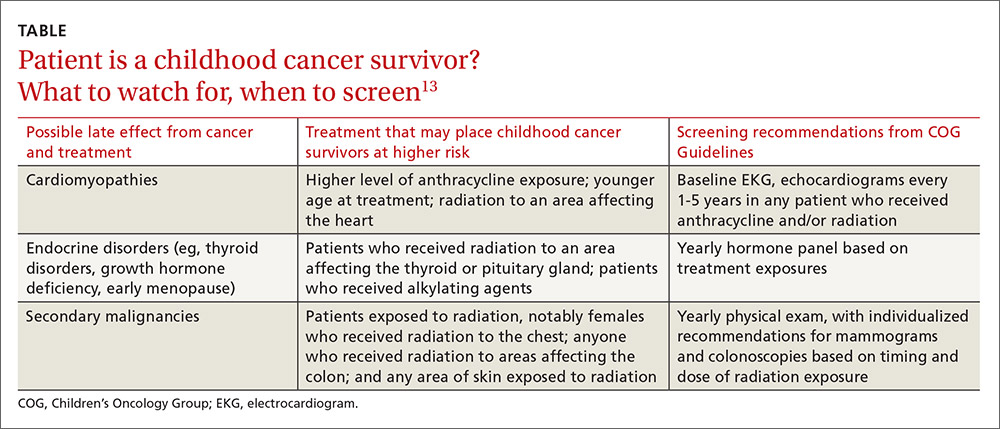
Some of the most severe or life-threatening late effects include cardiomyopathies, endocrine disorders, and secondary malignancies (TABLE).13 Ongoing follow-up care is based on a survivor’s individual risk level and the frequency of lifelong recommended screening. The majority of patients will require yearly follow-up with additional testing, such as echocardiograms occurring as infrequently as every 2 to 5 years. Patients who received more intense therapy, such as hematopoietic stem-cell transplants, will require follow-up (often including annual echocardiograms, blood work, and a thorough physical exam) every 6 months to one year. Common testing and surveillance include blood pressure checks, urinalyses, thyroid function tests, lipid panels, echocardiograms, and electrocardiograms.
After treatment, patients should receive survivorship care plans
For health care providers to use COG Guidelines effectively across medical disciplines, it is important to know critical pieces of the patient’s cancer diagnosis and treatment history. In 2006, the Institute of Medicine released a report14 recommending that all cancer survivors be given a comprehensive care summary and follow-up plan when they complete their primary cancer care. More recently, the Commission on Cancer of the American College of Surgeons has mandated that, in order to be a cancer program accredited by the Commission, all cancer patients must be given a survivorship care plan after completing treatment.15 Generated by the treating cancer center, these care plans are meant to concisely communicate a patient’s cancer diagnosis, treatment, and long-term risks to other health care providers (across disciplines and institutions).
What’s included in a survivorship care plan?
The survivorship care plan is a paper or electronic document created by the treating institution that contains 2 components: a treatment summary and a long-term care plan based on medical/treatment history. The treatment summary includes, at a minimum, general background information (eg, demographics, pertinent medical history, diagnostic details, and significant treatment complications) and a therapeutic summary (such as dates of treatment, protocol, and details of chemotherapy, radiation, hematopoietic stem-cell transplant, and/or surgery).
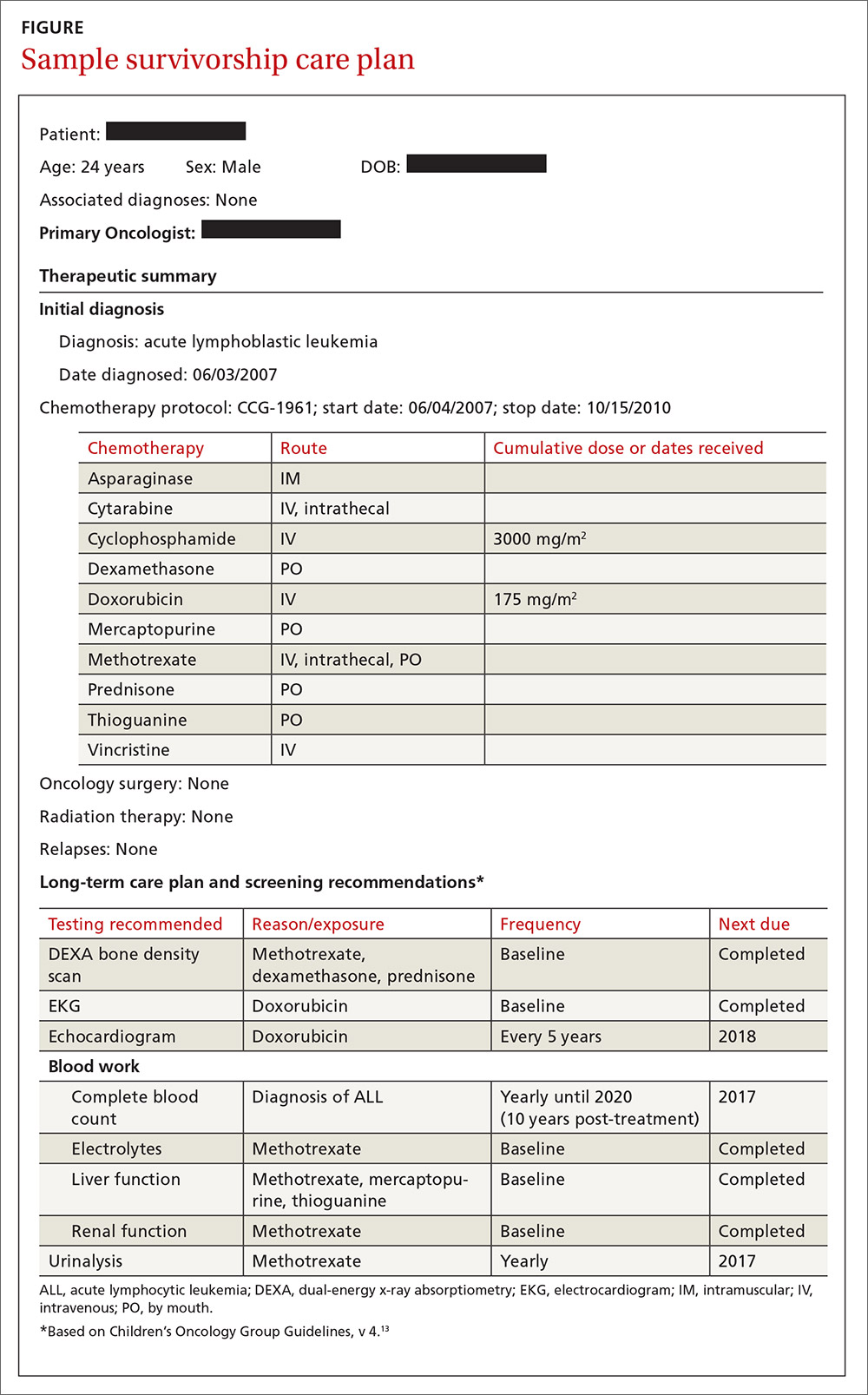
The second component, the long-term care plan, details potential long-term effects specific to the treatment received, and recommendations for ongoing follow-up related to long-term risk (FIGURE). The post-treatment plan is primarily based on COG Guideline recommendations. Many institutions are introducing an electronic-based survivorship care plan, either in addition to or in replacement of a paper-based care plan. Electronic-based care plans have several benefits for patients and providers, including increased accessibility, and some offer the ability to easily update follow-up recommendations, as guidelines change, without the need for manual entry.
Shared care for cancer survivors: Oncology and primary care
Numerous models of cancer survivorship care have been described, including care by the treating oncologist, a dedicated cancer survivorship program, or follow-up completed by PCPs. There is no consensus on the best model, although many have noted that shared care is a critically important component of successful cancer survivorship care,6,16–18 and appears to be the preferred model of PCPs.19
Shared care, as the name implies, involves care that is coordinated between 2 or more health providers across specialties or locations.20 This model has shown improved outcomes in other chronic disease-management models, such as those for diabetes21 and chronic renal disease.22 One study23 found that colorectal cancer survivors who were seen by both an oncologist and a PCP were significantly more likely to receive recommended testing and follow-up to promote overall health than when they were followed by either physician alone. Information sharing between oncology and PCPs is critical to maintaining and promoting optimal health and well-being in cancer survivors, and requires ongoing communication and a concerted effort to facilitate and maintain collaboration between oncology specialists and other health care providers.6,17
Role of the cancer center in survivorship care
Although every cancer center has a slightly different timeline and structure in terms of survivorship care, there are common themes across programs regarding the type of care provided. Immediately following treatment, care is focused on surveillance for recurrence, with appointments ranging from monthly to a few times a year. This care is most often provided by the primary oncologist.
The next phase of care is reached 2 to 5 years after treatment, when recurrence is no longer a significant risk, and care is focused on monitoring and treating late effects. Depending on the center, this care may be coordinated by a dedicated survivorship clinic, the primary oncologist, or the PCP. In some models,6 the survivorship team is integrated into the patient’s care from the beginning of treatment, while others do not become active in care until the patient is considered cured of disease. In all models, a survivorship care plan should be completed after treatment has ended and before transitioning care to a PCP.
In our institution’s model, we have a survivorship program that serves patients who are more than 5 years from the completion of their treatment. Our survivorship team is comprised of a pediatric oncologist, advanced practice practitioner (APP) coordinator, a project coordinator, a clinical social worker, and a research staff member. Patients are seen every one to 2 years, depending on their overall risk for late effects. For those who are seen every other year, we are available to the PCP for questions or concerns, and the survivorship team connects with the CCS by phone to screen for any change in health status that would alter recommendations for an earlier follow-up at the oncology center.
A typical visit to our survivorship clinic includes completion of an annual health questionnaire, which addresses current health issues, as well as screening for anxiety, depression, nicotine, alcohol, and drug use. This questionnaire is reviewed by the pediatric oncologist and is used to tailor screening, referrals, and patient education based on current complaints. The oncologist also performs a thorough physical exam with special attention to areas in which late effects may occur (eg, skin exam in areas of previous radiation). In addition, each patient receives an individualized treatment summary based on COG guidelines, which is updated before each visit by the APP coordinator. The APP coordinator reviews the document at each visit and offers patient education and health maintenance counseling.
Ensuring patients aren’t lost to follow-up. In our experience, numerous patients become lost to follow-up as they age, enter college or the workforce, or move away. So, rather than attempting to follow these patients for life, we work to transition patient care to a PCP of their choice, particularly if they are at least 21 years old and more than 10 years post-diagnosis. However, we will work to transition at any time at the request of the CCS. Even when a patient’s ongoing care is transitioned to a PCP, we will remain as a continuing resource to PCPs and CCSs on an as-needed basis.
Role of primary care providers in survivorship care
Every health care provider caring for a CCS should have a copy of the patient’s survivorship care plan. This document should be provided by the treating institution, but research has shown that as many as 86% of PCPs fail to receive this critical information.24 Any PCP who treats a patient with a history of cancer and has not received a survivorship care plan should contact the treating cancer center to request a copy. A properly prepared survivorship care plan summarizes the patient’s disease and treatment history, and provides a road map of the patient’s risk for long-term effects from disease and treatment.
The most important sections of the survivorship care plan for use in primary care will be the list of potential late effects and ongoing recommended testing. This list will help to guide the PCP’s differential and work-up for specific complaints. For example, knowing that a patient is at risk for a second malignancy because of radiation therapy may result in earlier diagnostic imaging, leading to a timelier diagnosis.
The COG screening recommendations that are generally included in a survivorship care plan are appropriate for survivors who are asymptomatic and presenting for routine, exposure-based medical follow-up. More extensive work-ups are presumed to be completed as clinically indicated. Consultation with a pediatric long-term follow-up clinic is also encouraged, particularly if a concern arises.
A complementary set of patient education materials, known as “Health Links,” accompany the COG guidelines to broaden their application and enhance patient follow-up visits. A survivorship care plan and the COG Guidelines help ensure that CCSs receive appropriate ongoing follow-up based on their history. A collaborative approach between Oncology and PCPs is essential to improve the quality of care for CCSs and to maintain the long-term health of this vulnerable population.
CORRESPONDENCE
Jean M. Tersak, Children’s Hospital of Pittsburgh of UPMC, 4401 Penn Avenue, 5th Floor Plaza Building, Pittsburgh, PA 15224; [email protected].
1. Ries LAG, Eisner MP, Kosary CL, et al, eds. SEER Cancer Statistics Review, 1975-2002. National Cancer Institute. Bethesda, MD. Available at: http://seer.cancer.gov/csr/1975_2002/. Accessed May 26, 2016.
2. Phillips SM, Padgett LS, Leisenring WM, et al. Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev. 2015;24:653-663.
3. Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572-1582.
4. Nathan PC, Greenberg ML, Ness KK, et al. Medical care in long-term survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2008;26:4401-4409.
5. Feuerstein M. Defining cancer survivorship. J Cancer Surviv. 2007;1:5-7.
6. McCabe MS, Jacobs LA. Clinical update: survivorship care—models and programs. Semin Oncol Nurs. 2012;28:e1-e8.
7. Oeffinger K, Mertens A, Hudson M, et al. Health care of young adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Ann Fam Med. 2004;2:61-70.
8. Mueller EL, Park ER, Davis MM. What the affordable care act means for survivors of pediatric cancer. J Clin Oncol. 2014;32:615-617.
9. Oeffinger KC. Longitudinal risk-based health care for adult survivors of childhood cancer. Curr Probl Cancer. 2003;27:143-167.
10. Lawrence RA, McLoone JK, Wakefield CE, et al. Primary care physicians’ perspectives of their role in cancer care: a systematic review. J Gen Intern Med. 2016:1-15.
11. Schwartz CL. Long-term survivors of childhood cancer: the late effects of therapy. Oncologist. 1999;4:45-54.
12. Late Effects of Treatment for Childhood Cancer (PDQ(R)): Health Professional Version [Internet]. Bethesda, MD: National Cancer Institute. Updated March 31, 2016. Available at: www.cancer.gov/types/childhood-cancers/late-effects-hp-pdq. Accessed June 2, 2016.
13. Children’s Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancer, Version 4.0. Monrovia CA: Children’s Oncology Group. 2013. Available at: www.survivorshipguidelines.org. Accessed June 2, 2016.
14. Hewitt M, Greenfield S, Stovall E, Committee on Cancer Survivorship: Improving Care and Quality of Life. National Cancer Policy Board, Institute of Medicine, National Research Council, eds. From cancer patient to cancer survivor: Lost in transition. Washington, DC: The National Academies Press; 2005.
15. Commission on Cancer [Internet]. Cancer Program Standards: Ensuring Patient-Centered Care. Chicago, IL: American College of Surgeons; 2015. Available at: https://www.facs.org/quality%20programs/cancer/coc/standards. Accessed June 2, 2016.
16. Askins MA, Moore BD. Preventing neurocognitive late effects in childhood cancer survivors. J Child Neurol. 2008;23:1160-1171.
17. McCabe MS, Jacobs L. Survivorship care: models and programs. Semin Oncol Nurs. 2008;24:202-207.
18. Oeffinger KC, McCabe MS. Models for delivering survivorship care. J Clin Oncol. 2006;24:5117-5124.
19. Potosky AL, Han PKJ, Rowland J, et al. Differences between primary care physicians’ and oncologists’ knowledge, attitudes and practices regarding the care of cancer survivors. J Gen Intern Med. 2011;26:1403-1410.
20. Gilbert SM, Miller DC, Hollenbeck BK, et al. Cancer survivorship: challenges and changing paradigms. J Urol. 2008;179:431-438.
21. Renders CM, Valk GD, de Sonnaville JJ, et al. Quality of care for patients with Type 2 diabetes mellitus—a long-term comparison of two quality improvement programmes in the Netherlands. Diabet Med. 2003;20:846-852.
22. Jones C, Roderick P, Harris S, et al. An evaluation of a shared primary and secondary care nephrology service for managing patients with moderate to advanced CKD. Am J Kidney Dis. 2006;47:103-114.
23. Earle CC, Neville BA. Under use of necessary care among cancer survivors. Cancer. 2004;101:1712-1719.
24. Sima JL, Perkins SM, Haggstrom DA. Primary care physician perceptions of adult survivors of childhood cancer. J Pediatr Hematol Oncol. 2014;36:118-124.
The number of childhood cancer survivors (CCSs) entering the adult health care system is increasing, a not-so-surprising trend when you consider that more than 80% of children and adolescents given a cancer diagnosis become long-term survivors.1 This patient population has a heightened risk for developing at least one chronic health problem, resulting from therapy. By the fourth decade of life, 88% of all CCSs will have a chronic condition,2 and about one-third develop a late effect that is either severe or life-threatening.3 In contrast to patients with many other pediatric chronic diseases that manifest at an early age and are progressive, CCSs are often physically well for many years, or decades, prior to their manifestation of late effects.4
Cancer survivorship has varying definitions; however, we define cancer survivorship as the phase of cancer care for individuals who have been diagnosed with cancer and have completed primary treatment for their disease.5 Cancer survivorship, which is becoming more widely acknowledged as a distinct and critically important phase of cancer care, includes:6
- “surveillance for recurrence,
- evaluation … and treatment of medical and psychosocial consequences of treatment,
- recommendations for screening for new primary cancers,
- health promotion recommendations, and
- provision of a written treatment summary and care plan to the patient and other health professionals.”
Although models of survivorship care vary, their common goal is to promote optimal health and well-being in cancer survivors, and to prevent and detect any health concerns that may be related to prior cancer diagnosis or treatment.
Some pediatric cancer survivors have not received recommended survivorship care because of a lack of insurance or limitations from pre-existing conditions.4,7 The Affordable Care Act may remove these barriers for many.8 Others, however, fail to receive such recommendations because national models of transition are lacking. Unique considerations for this population include their need to establish age appropriate, lifelong follow-up care (and education) from a primary care provider (PCP). Unfortunately, many CCSs become lost to follow-up and fail to receive recommended survivorship care when they discontinue the relationship with their pediatrician or family practitioner and their pediatric oncologist. Fewer than 25% of CCSs who have been successfully treated for cancer during childhood continue to be followed by a cancer center and are at risk for missing survivorship-focused care or recommended screening.4,9
PCPs are an invaluable link in helping CCSs to continue to receive recommended care and surveillance. However, PCPs experience barriers in providing cancer care because of a lack of timely and specific communication from oncologists and limited knowledge of guidelines and resources available to them.10 The purpose of this article is to share information with you, the family physician, about childhood cancer survivorship needs, available resources, and how partnering with pediatric oncologists may improve treatment and health outcomes for CCSs.
Providing for the future health of childhood cancer survivors
Numerous studies have outlined the myriad of potential late effects that CCSs may experience from disease and treatment.11,12 These effects can manifest at any time and can appear in virtually every body system from the central nervous system, to the lungs, heart, bones, and endocrine systems. CCSs' particular risk for late effects may result from many factors including cancer diagnosis, types of treatments (eg, surgery, chemotherapy, radiation, and stem-cell transplant), and dosages of medications, gender, and age at diagnosis.
Determining individual risk for late effects
The Children’s Oncology Group (COG) is the world’s largest organization devoted exclusively to childhood and adolescent cancer research, including the long-term health of cancer survivors. To help provide more individualized recommendations, COG has set forth risk-based, evidence-based, exposure-related clinical practice guidelines to offer recommendations for screening and management of late effects in survivors of childhood and adolescent cancers.13 (These guidelines, Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers, are available at http://www.survivorshipguidelines.org.) The purpose of the guidelines is to standardize and enhance follow-up care for CCSs throughout their lifespan.13 To remain current, a multidisciplinary task force reviews and incorporates findings from the medical literature—including evaluations of the cost-effectiveness of recommended testing—into guideline revisions at least every 5 years.
Some of the most severe or life-threatening late effects include cardiomyopathies, endocrine disorders, and secondary malignancies (TABLE).13 Ongoing follow-up care is based on a survivor’s individual risk level and the frequency of lifelong recommended screening. The majority of patients will require yearly follow-up with additional testing, such as echocardiograms occurring as infrequently as every 2 to 5 years. Patients who received more intense therapy, such as hematopoietic stem-cell transplants, will require follow-up (often including annual echocardiograms, blood work, and a thorough physical exam) every 6 months to one year. Common testing and surveillance include blood pressure checks, urinalyses, thyroid function tests, lipid panels, echocardiograms, and electrocardiograms.
After treatment, patients should receive survivorship care plans
For health care providers to use COG Guidelines effectively across medical disciplines, it is important to know critical pieces of the patient’s cancer diagnosis and treatment history. In 2006, the Institute of Medicine released a report14 recommending that all cancer survivors be given a comprehensive care summary and follow-up plan when they complete their primary cancer care. More recently, the Commission on Cancer of the American College of Surgeons has mandated that, in order to be a cancer program accredited by the Commission, all cancer patients must be given a survivorship care plan after completing treatment.15 Generated by the treating cancer center, these care plans are meant to concisely communicate a patient’s cancer diagnosis, treatment, and long-term risks to other health care providers (across disciplines and institutions).
What’s included in a survivorship care plan?
The survivorship care plan is a paper or electronic document created by the treating institution that contains 2 components: a treatment summary and a long-term care plan based on medical/treatment history. The treatment summary includes, at a minimum, general background information (eg, demographics, pertinent medical history, diagnostic details, and significant treatment complications) and a therapeutic summary (such as dates of treatment, protocol, and details of chemotherapy, radiation, hematopoietic stem-cell transplant, and/or surgery).
The second component, the long-term care plan, details potential long-term effects specific to the treatment received, and recommendations for ongoing follow-up related to long-term risk (FIGURE). The post-treatment plan is primarily based on COG Guideline recommendations. Many institutions are introducing an electronic-based survivorship care plan, either in addition to or in replacement of a paper-based care plan. Electronic-based care plans have several benefits for patients and providers, including increased accessibility, and some offer the ability to easily update follow-up recommendations, as guidelines change, without the need for manual entry.
Shared care for cancer survivors: Oncology and primary care
Numerous models of cancer survivorship care have been described, including care by the treating oncologist, a dedicated cancer survivorship program, or follow-up completed by PCPs. There is no consensus on the best model, although many have noted that shared care is a critically important component of successful cancer survivorship care,6,16–18 and appears to be the preferred model of PCPs.19
Shared care, as the name implies, involves care that is coordinated between 2 or more health providers across specialties or locations.20 This model has shown improved outcomes in other chronic disease-management models, such as those for diabetes21 and chronic renal disease.22 One study23 found that colorectal cancer survivors who were seen by both an oncologist and a PCP were significantly more likely to receive recommended testing and follow-up to promote overall health than when they were followed by either physician alone. Information sharing between oncology and PCPs is critical to maintaining and promoting optimal health and well-being in cancer survivors, and requires ongoing communication and a concerted effort to facilitate and maintain collaboration between oncology specialists and other health care providers.6,17
Role of the cancer center in survivorship care
Although every cancer center has a slightly different timeline and structure in terms of survivorship care, there are common themes across programs regarding the type of care provided. Immediately following treatment, care is focused on surveillance for recurrence, with appointments ranging from monthly to a few times a year. This care is most often provided by the primary oncologist.
The next phase of care is reached 2 to 5 years after treatment, when recurrence is no longer a significant risk, and care is focused on monitoring and treating late effects. Depending on the center, this care may be coordinated by a dedicated survivorship clinic, the primary oncologist, or the PCP. In some models,6 the survivorship team is integrated into the patient’s care from the beginning of treatment, while others do not become active in care until the patient is considered cured of disease. In all models, a survivorship care plan should be completed after treatment has ended and before transitioning care to a PCP.
In our institution’s model, we have a survivorship program that serves patients who are more than 5 years from the completion of their treatment. Our survivorship team is comprised of a pediatric oncologist, advanced practice practitioner (APP) coordinator, a project coordinator, a clinical social worker, and a research staff member. Patients are seen every one to 2 years, depending on their overall risk for late effects. For those who are seen every other year, we are available to the PCP for questions or concerns, and the survivorship team connects with the CCS by phone to screen for any change in health status that would alter recommendations for an earlier follow-up at the oncology center.
A typical visit to our survivorship clinic includes completion of an annual health questionnaire, which addresses current health issues, as well as screening for anxiety, depression, nicotine, alcohol, and drug use. This questionnaire is reviewed by the pediatric oncologist and is used to tailor screening, referrals, and patient education based on current complaints. The oncologist also performs a thorough physical exam with special attention to areas in which late effects may occur (eg, skin exam in areas of previous radiation). In addition, each patient receives an individualized treatment summary based on COG guidelines, which is updated before each visit by the APP coordinator. The APP coordinator reviews the document at each visit and offers patient education and health maintenance counseling.
Ensuring patients aren’t lost to follow-up. In our experience, numerous patients become lost to follow-up as they age, enter college or the workforce, or move away. So, rather than attempting to follow these patients for life, we work to transition patient care to a PCP of their choice, particularly if they are at least 21 years old and more than 10 years post-diagnosis. However, we will work to transition at any time at the request of the CCS. Even when a patient’s ongoing care is transitioned to a PCP, we will remain as a continuing resource to PCPs and CCSs on an as-needed basis.
Role of primary care providers in survivorship care
Every health care provider caring for a CCS should have a copy of the patient’s survivorship care plan. This document should be provided by the treating institution, but research has shown that as many as 86% of PCPs fail to receive this critical information.24 Any PCP who treats a patient with a history of cancer and has not received a survivorship care plan should contact the treating cancer center to request a copy. A properly prepared survivorship care plan summarizes the patient’s disease and treatment history, and provides a road map of the patient’s risk for long-term effects from disease and treatment.
The most important sections of the survivorship care plan for use in primary care will be the list of potential late effects and ongoing recommended testing. This list will help to guide the PCP’s differential and work-up for specific complaints. For example, knowing that a patient is at risk for a second malignancy because of radiation therapy may result in earlier diagnostic imaging, leading to a timelier diagnosis.
The COG screening recommendations that are generally included in a survivorship care plan are appropriate for survivors who are asymptomatic and presenting for routine, exposure-based medical follow-up. More extensive work-ups are presumed to be completed as clinically indicated. Consultation with a pediatric long-term follow-up clinic is also encouraged, particularly if a concern arises.
A complementary set of patient education materials, known as “Health Links,” accompany the COG guidelines to broaden their application and enhance patient follow-up visits. A survivorship care plan and the COG Guidelines help ensure that CCSs receive appropriate ongoing follow-up based on their history. A collaborative approach between Oncology and PCPs is essential to improve the quality of care for CCSs and to maintain the long-term health of this vulnerable population.
CORRESPONDENCE
Jean M. Tersak, Children’s Hospital of Pittsburgh of UPMC, 4401 Penn Avenue, 5th Floor Plaza Building, Pittsburgh, PA 15224; [email protected].
The number of childhood cancer survivors (CCSs) entering the adult health care system is increasing, a not-so-surprising trend when you consider that more than 80% of children and adolescents given a cancer diagnosis become long-term survivors.1 This patient population has a heightened risk for developing at least one chronic health problem, resulting from therapy. By the fourth decade of life, 88% of all CCSs will have a chronic condition,2 and about one-third develop a late effect that is either severe or life-threatening.3 In contrast to patients with many other pediatric chronic diseases that manifest at an early age and are progressive, CCSs are often physically well for many years, or decades, prior to their manifestation of late effects.4
Cancer survivorship has varying definitions; however, we define cancer survivorship as the phase of cancer care for individuals who have been diagnosed with cancer and have completed primary treatment for their disease.5 Cancer survivorship, which is becoming more widely acknowledged as a distinct and critically important phase of cancer care, includes:6
- “surveillance for recurrence,
- evaluation … and treatment of medical and psychosocial consequences of treatment,
- recommendations for screening for new primary cancers,
- health promotion recommendations, and
- provision of a written treatment summary and care plan to the patient and other health professionals.”
Although models of survivorship care vary, their common goal is to promote optimal health and well-being in cancer survivors, and to prevent and detect any health concerns that may be related to prior cancer diagnosis or treatment.
Some pediatric cancer survivors have not received recommended survivorship care because of a lack of insurance or limitations from pre-existing conditions.4,7 The Affordable Care Act may remove these barriers for many.8 Others, however, fail to receive such recommendations because national models of transition are lacking. Unique considerations for this population include their need to establish age appropriate, lifelong follow-up care (and education) from a primary care provider (PCP). Unfortunately, many CCSs become lost to follow-up and fail to receive recommended survivorship care when they discontinue the relationship with their pediatrician or family practitioner and their pediatric oncologist. Fewer than 25% of CCSs who have been successfully treated for cancer during childhood continue to be followed by a cancer center and are at risk for missing survivorship-focused care or recommended screening.4,9
PCPs are an invaluable link in helping CCSs to continue to receive recommended care and surveillance. However, PCPs experience barriers in providing cancer care because of a lack of timely and specific communication from oncologists and limited knowledge of guidelines and resources available to them.10 The purpose of this article is to share information with you, the family physician, about childhood cancer survivorship needs, available resources, and how partnering with pediatric oncologists may improve treatment and health outcomes for CCSs.
Providing for the future health of childhood cancer survivors
Numerous studies have outlined the myriad of potential late effects that CCSs may experience from disease and treatment.11,12 These effects can manifest at any time and can appear in virtually every body system from the central nervous system, to the lungs, heart, bones, and endocrine systems. CCSs' particular risk for late effects may result from many factors including cancer diagnosis, types of treatments (eg, surgery, chemotherapy, radiation, and stem-cell transplant), and dosages of medications, gender, and age at diagnosis.
Determining individual risk for late effects
The Children’s Oncology Group (COG) is the world’s largest organization devoted exclusively to childhood and adolescent cancer research, including the long-term health of cancer survivors. To help provide more individualized recommendations, COG has set forth risk-based, evidence-based, exposure-related clinical practice guidelines to offer recommendations for screening and management of late effects in survivors of childhood and adolescent cancers.13 (These guidelines, Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers, are available at http://www.survivorshipguidelines.org.) The purpose of the guidelines is to standardize and enhance follow-up care for CCSs throughout their lifespan.13 To remain current, a multidisciplinary task force reviews and incorporates findings from the medical literature—including evaluations of the cost-effectiveness of recommended testing—into guideline revisions at least every 5 years.
Some of the most severe or life-threatening late effects include cardiomyopathies, endocrine disorders, and secondary malignancies (TABLE).13 Ongoing follow-up care is based on a survivor’s individual risk level and the frequency of lifelong recommended screening. The majority of patients will require yearly follow-up with additional testing, such as echocardiograms occurring as infrequently as every 2 to 5 years. Patients who received more intense therapy, such as hematopoietic stem-cell transplants, will require follow-up (often including annual echocardiograms, blood work, and a thorough physical exam) every 6 months to one year. Common testing and surveillance include blood pressure checks, urinalyses, thyroid function tests, lipid panels, echocardiograms, and electrocardiograms.
After treatment, patients should receive survivorship care plans
For health care providers to use COG Guidelines effectively across medical disciplines, it is important to know critical pieces of the patient’s cancer diagnosis and treatment history. In 2006, the Institute of Medicine released a report14 recommending that all cancer survivors be given a comprehensive care summary and follow-up plan when they complete their primary cancer care. More recently, the Commission on Cancer of the American College of Surgeons has mandated that, in order to be a cancer program accredited by the Commission, all cancer patients must be given a survivorship care plan after completing treatment.15 Generated by the treating cancer center, these care plans are meant to concisely communicate a patient’s cancer diagnosis, treatment, and long-term risks to other health care providers (across disciplines and institutions).
What’s included in a survivorship care plan?
The survivorship care plan is a paper or electronic document created by the treating institution that contains 2 components: a treatment summary and a long-term care plan based on medical/treatment history. The treatment summary includes, at a minimum, general background information (eg, demographics, pertinent medical history, diagnostic details, and significant treatment complications) and a therapeutic summary (such as dates of treatment, protocol, and details of chemotherapy, radiation, hematopoietic stem-cell transplant, and/or surgery).
The second component, the long-term care plan, details potential long-term effects specific to the treatment received, and recommendations for ongoing follow-up related to long-term risk (FIGURE). The post-treatment plan is primarily based on COG Guideline recommendations. Many institutions are introducing an electronic-based survivorship care plan, either in addition to or in replacement of a paper-based care plan. Electronic-based care plans have several benefits for patients and providers, including increased accessibility, and some offer the ability to easily update follow-up recommendations, as guidelines change, without the need for manual entry.
Shared care for cancer survivors: Oncology and primary care
Numerous models of cancer survivorship care have been described, including care by the treating oncologist, a dedicated cancer survivorship program, or follow-up completed by PCPs. There is no consensus on the best model, although many have noted that shared care is a critically important component of successful cancer survivorship care,6,16–18 and appears to be the preferred model of PCPs.19
Shared care, as the name implies, involves care that is coordinated between 2 or more health providers across specialties or locations.20 This model has shown improved outcomes in other chronic disease-management models, such as those for diabetes21 and chronic renal disease.22 One study23 found that colorectal cancer survivors who were seen by both an oncologist and a PCP were significantly more likely to receive recommended testing and follow-up to promote overall health than when they were followed by either physician alone. Information sharing between oncology and PCPs is critical to maintaining and promoting optimal health and well-being in cancer survivors, and requires ongoing communication and a concerted effort to facilitate and maintain collaboration between oncology specialists and other health care providers.6,17
Role of the cancer center in survivorship care
Although every cancer center has a slightly different timeline and structure in terms of survivorship care, there are common themes across programs regarding the type of care provided. Immediately following treatment, care is focused on surveillance for recurrence, with appointments ranging from monthly to a few times a year. This care is most often provided by the primary oncologist.
The next phase of care is reached 2 to 5 years after treatment, when recurrence is no longer a significant risk, and care is focused on monitoring and treating late effects. Depending on the center, this care may be coordinated by a dedicated survivorship clinic, the primary oncologist, or the PCP. In some models,6 the survivorship team is integrated into the patient’s care from the beginning of treatment, while others do not become active in care until the patient is considered cured of disease. In all models, a survivorship care plan should be completed after treatment has ended and before transitioning care to a PCP.
In our institution’s model, we have a survivorship program that serves patients who are more than 5 years from the completion of their treatment. Our survivorship team is comprised of a pediatric oncologist, advanced practice practitioner (APP) coordinator, a project coordinator, a clinical social worker, and a research staff member. Patients are seen every one to 2 years, depending on their overall risk for late effects. For those who are seen every other year, we are available to the PCP for questions or concerns, and the survivorship team connects with the CCS by phone to screen for any change in health status that would alter recommendations for an earlier follow-up at the oncology center.
A typical visit to our survivorship clinic includes completion of an annual health questionnaire, which addresses current health issues, as well as screening for anxiety, depression, nicotine, alcohol, and drug use. This questionnaire is reviewed by the pediatric oncologist and is used to tailor screening, referrals, and patient education based on current complaints. The oncologist also performs a thorough physical exam with special attention to areas in which late effects may occur (eg, skin exam in areas of previous radiation). In addition, each patient receives an individualized treatment summary based on COG guidelines, which is updated before each visit by the APP coordinator. The APP coordinator reviews the document at each visit and offers patient education and health maintenance counseling.
Ensuring patients aren’t lost to follow-up. In our experience, numerous patients become lost to follow-up as they age, enter college or the workforce, or move away. So, rather than attempting to follow these patients for life, we work to transition patient care to a PCP of their choice, particularly if they are at least 21 years old and more than 10 years post-diagnosis. However, we will work to transition at any time at the request of the CCS. Even when a patient’s ongoing care is transitioned to a PCP, we will remain as a continuing resource to PCPs and CCSs on an as-needed basis.
Role of primary care providers in survivorship care
Every health care provider caring for a CCS should have a copy of the patient’s survivorship care plan. This document should be provided by the treating institution, but research has shown that as many as 86% of PCPs fail to receive this critical information.24 Any PCP who treats a patient with a history of cancer and has not received a survivorship care plan should contact the treating cancer center to request a copy. A properly prepared survivorship care plan summarizes the patient’s disease and treatment history, and provides a road map of the patient’s risk for long-term effects from disease and treatment.
The most important sections of the survivorship care plan for use in primary care will be the list of potential late effects and ongoing recommended testing. This list will help to guide the PCP’s differential and work-up for specific complaints. For example, knowing that a patient is at risk for a second malignancy because of radiation therapy may result in earlier diagnostic imaging, leading to a timelier diagnosis.
The COG screening recommendations that are generally included in a survivorship care plan are appropriate for survivors who are asymptomatic and presenting for routine, exposure-based medical follow-up. More extensive work-ups are presumed to be completed as clinically indicated. Consultation with a pediatric long-term follow-up clinic is also encouraged, particularly if a concern arises.
A complementary set of patient education materials, known as “Health Links,” accompany the COG guidelines to broaden their application and enhance patient follow-up visits. A survivorship care plan and the COG Guidelines help ensure that CCSs receive appropriate ongoing follow-up based on their history. A collaborative approach between Oncology and PCPs is essential to improve the quality of care for CCSs and to maintain the long-term health of this vulnerable population.
CORRESPONDENCE
Jean M. Tersak, Children’s Hospital of Pittsburgh of UPMC, 4401 Penn Avenue, 5th Floor Plaza Building, Pittsburgh, PA 15224; [email protected].
1. Ries LAG, Eisner MP, Kosary CL, et al, eds. SEER Cancer Statistics Review, 1975-2002. National Cancer Institute. Bethesda, MD. Available at: http://seer.cancer.gov/csr/1975_2002/. Accessed May 26, 2016.
2. Phillips SM, Padgett LS, Leisenring WM, et al. Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev. 2015;24:653-663.
3. Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572-1582.
4. Nathan PC, Greenberg ML, Ness KK, et al. Medical care in long-term survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2008;26:4401-4409.
5. Feuerstein M. Defining cancer survivorship. J Cancer Surviv. 2007;1:5-7.
6. McCabe MS, Jacobs LA. Clinical update: survivorship care—models and programs. Semin Oncol Nurs. 2012;28:e1-e8.
7. Oeffinger K, Mertens A, Hudson M, et al. Health care of young adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Ann Fam Med. 2004;2:61-70.
8. Mueller EL, Park ER, Davis MM. What the affordable care act means for survivors of pediatric cancer. J Clin Oncol. 2014;32:615-617.
9. Oeffinger KC. Longitudinal risk-based health care for adult survivors of childhood cancer. Curr Probl Cancer. 2003;27:143-167.
10. Lawrence RA, McLoone JK, Wakefield CE, et al. Primary care physicians’ perspectives of their role in cancer care: a systematic review. J Gen Intern Med. 2016:1-15.
11. Schwartz CL. Long-term survivors of childhood cancer: the late effects of therapy. Oncologist. 1999;4:45-54.
12. Late Effects of Treatment for Childhood Cancer (PDQ(R)): Health Professional Version [Internet]. Bethesda, MD: National Cancer Institute. Updated March 31, 2016. Available at: www.cancer.gov/types/childhood-cancers/late-effects-hp-pdq. Accessed June 2, 2016.
13. Children’s Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancer, Version 4.0. Monrovia CA: Children’s Oncology Group. 2013. Available at: www.survivorshipguidelines.org. Accessed June 2, 2016.
14. Hewitt M, Greenfield S, Stovall E, Committee on Cancer Survivorship: Improving Care and Quality of Life. National Cancer Policy Board, Institute of Medicine, National Research Council, eds. From cancer patient to cancer survivor: Lost in transition. Washington, DC: The National Academies Press; 2005.
15. Commission on Cancer [Internet]. Cancer Program Standards: Ensuring Patient-Centered Care. Chicago, IL: American College of Surgeons; 2015. Available at: https://www.facs.org/quality%20programs/cancer/coc/standards. Accessed June 2, 2016.
16. Askins MA, Moore BD. Preventing neurocognitive late effects in childhood cancer survivors. J Child Neurol. 2008;23:1160-1171.
17. McCabe MS, Jacobs L. Survivorship care: models and programs. Semin Oncol Nurs. 2008;24:202-207.
18. Oeffinger KC, McCabe MS. Models for delivering survivorship care. J Clin Oncol. 2006;24:5117-5124.
19. Potosky AL, Han PKJ, Rowland J, et al. Differences between primary care physicians’ and oncologists’ knowledge, attitudes and practices regarding the care of cancer survivors. J Gen Intern Med. 2011;26:1403-1410.
20. Gilbert SM, Miller DC, Hollenbeck BK, et al. Cancer survivorship: challenges and changing paradigms. J Urol. 2008;179:431-438.
21. Renders CM, Valk GD, de Sonnaville JJ, et al. Quality of care for patients with Type 2 diabetes mellitus—a long-term comparison of two quality improvement programmes in the Netherlands. Diabet Med. 2003;20:846-852.
22. Jones C, Roderick P, Harris S, et al. An evaluation of a shared primary and secondary care nephrology service for managing patients with moderate to advanced CKD. Am J Kidney Dis. 2006;47:103-114.
23. Earle CC, Neville BA. Under use of necessary care among cancer survivors. Cancer. 2004;101:1712-1719.
24. Sima JL, Perkins SM, Haggstrom DA. Primary care physician perceptions of adult survivors of childhood cancer. J Pediatr Hematol Oncol. 2014;36:118-124.
1. Ries LAG, Eisner MP, Kosary CL, et al, eds. SEER Cancer Statistics Review, 1975-2002. National Cancer Institute. Bethesda, MD. Available at: http://seer.cancer.gov/csr/1975_2002/. Accessed May 26, 2016.
2. Phillips SM, Padgett LS, Leisenring WM, et al. Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev. 2015;24:653-663.
3. Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572-1582.
4. Nathan PC, Greenberg ML, Ness KK, et al. Medical care in long-term survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2008;26:4401-4409.
5. Feuerstein M. Defining cancer survivorship. J Cancer Surviv. 2007;1:5-7.
6. McCabe MS, Jacobs LA. Clinical update: survivorship care—models and programs. Semin Oncol Nurs. 2012;28:e1-e8.
7. Oeffinger K, Mertens A, Hudson M, et al. Health care of young adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Ann Fam Med. 2004;2:61-70.
8. Mueller EL, Park ER, Davis MM. What the affordable care act means for survivors of pediatric cancer. J Clin Oncol. 2014;32:615-617.
9. Oeffinger KC. Longitudinal risk-based health care for adult survivors of childhood cancer. Curr Probl Cancer. 2003;27:143-167.
10. Lawrence RA, McLoone JK, Wakefield CE, et al. Primary care physicians’ perspectives of their role in cancer care: a systematic review. J Gen Intern Med. 2016:1-15.
11. Schwartz CL. Long-term survivors of childhood cancer: the late effects of therapy. Oncologist. 1999;4:45-54.
12. Late Effects of Treatment for Childhood Cancer (PDQ(R)): Health Professional Version [Internet]. Bethesda, MD: National Cancer Institute. Updated March 31, 2016. Available at: www.cancer.gov/types/childhood-cancers/late-effects-hp-pdq. Accessed June 2, 2016.
13. Children’s Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancer, Version 4.0. Monrovia CA: Children’s Oncology Group. 2013. Available at: www.survivorshipguidelines.org. Accessed June 2, 2016.
14. Hewitt M, Greenfield S, Stovall E, Committee on Cancer Survivorship: Improving Care and Quality of Life. National Cancer Policy Board, Institute of Medicine, National Research Council, eds. From cancer patient to cancer survivor: Lost in transition. Washington, DC: The National Academies Press; 2005.
15. Commission on Cancer [Internet]. Cancer Program Standards: Ensuring Patient-Centered Care. Chicago, IL: American College of Surgeons; 2015. Available at: https://www.facs.org/quality%20programs/cancer/coc/standards. Accessed June 2, 2016.
16. Askins MA, Moore BD. Preventing neurocognitive late effects in childhood cancer survivors. J Child Neurol. 2008;23:1160-1171.
17. McCabe MS, Jacobs L. Survivorship care: models and programs. Semin Oncol Nurs. 2008;24:202-207.
18. Oeffinger KC, McCabe MS. Models for delivering survivorship care. J Clin Oncol. 2006;24:5117-5124.
19. Potosky AL, Han PKJ, Rowland J, et al. Differences between primary care physicians’ and oncologists’ knowledge, attitudes and practices regarding the care of cancer survivors. J Gen Intern Med. 2011;26:1403-1410.
20. Gilbert SM, Miller DC, Hollenbeck BK, et al. Cancer survivorship: challenges and changing paradigms. J Urol. 2008;179:431-438.
21. Renders CM, Valk GD, de Sonnaville JJ, et al. Quality of care for patients with Type 2 diabetes mellitus—a long-term comparison of two quality improvement programmes in the Netherlands. Diabet Med. 2003;20:846-852.
22. Jones C, Roderick P, Harris S, et al. An evaluation of a shared primary and secondary care nephrology service for managing patients with moderate to advanced CKD. Am J Kidney Dis. 2006;47:103-114.
23. Earle CC, Neville BA. Under use of necessary care among cancer survivors. Cancer. 2004;101:1712-1719.
24. Sima JL, Perkins SM, Haggstrom DA. Primary care physician perceptions of adult survivors of childhood cancer. J Pediatr Hematol Oncol. 2014;36:118-124.
PRACTICE RECOMMENDATIONS
› Use the survivorship care plan from the patient’s primary oncologist to guide your screening and management of late effects. C
› Apply the Children’s Oncology Group Guidelines, which are risk-based, exposure-related, clinical practice guidelines, to direct screening and management of late effects in survivors of pediatric malignancies. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Can scribes boost FPs’ efficiency and job satisfaction?
ABSTRACT
Purpose Research in other medical specialties has shown that the addition of medical scribes to the clinical team enhances physicians’ practice experience and increases productivity. To date, literature on the implementation of scribes in primary care is limited. To determine the feasibility and benefits of implementing scribes in family medicine, we undertook a pilot mixed-method quality improvement (QI) study.
Methods In 2014, we incorporated 4 part-time scribes into an academic family medicine practice consisting of 7 physicians. We then measured, via survey and time-tracking data, the impact the scribes had on physician office hours and productivity, time spent on documentation, perceptions of work-life balance, and physician and patient satisfaction.
Results Six of the 7 faculty physicians participated. This study demonstrated that the use of scribes in a busy academic primary care practice substantially reduced the amount of time that family physicians spent on charting, improved work-life balance, and had good patient acceptance. Specifically, the physicians spent an average of 5.1 fewer hours/week (hrs/wk) on documentation, while various measures of productivity revealed increases ranging from 9.2% to 28.8%. Perhaps most important of all, when the results of the pilot study were annualized, they were projected to generate $168,600 per year—more than twice the $79,500 annual cost of 2 full-time equivalent scribes.
Surveys assessing work-life balance demonstrated improvement in the physicians’ perception of the administrative burden/paperwork related to practice and a decrease in their perception of the extent to which work encroached on their personal lives. In addition, survey data from 313 patients at the time of their ambulatory visit with a scribe present revealed a high level of comfort. Likewise, surveys completed by physicians after 55 clinical sessions (ie, blocks of consecutive, uninterrupted patient appointments; there are usually 2 sessions per day) revealed good to excellent ratings more than 90% of the time.
Conclusion In an outpatient family medicine clinic, the use of scribes substantially improved physicians’ efficiency, job satisfaction, and productivity without negatively impacting the patient experience.
While electronic medical records (EMRs) are important tools for improving patient care and communication, they bring with them an additional administrative burden for health care providers. In the emergency medicine literature, scribes have been reported to reduce that burden and improve clinicians’ productivity and satisfaction.1-4 Additionally, studies have reported increases in patient volume, generated billings, and provider morale, as well as decreases in emergency department (ED) lengths of stay.5 A recent review of the emergency medicine literature concluded that scribes have “the ability to allay the burden of documentation, improve throughput in the ED, and potentially enhance doctors’ satisfaction.”6
Similar benefits following scribe implementation have been reported in the literature of other specialties. A maternal-fetal medicine practice reported significant increases in generated billings and reimbursement.7 Increases in physician productivity and improvements in physician-patient interactions were reported in a cardiology clinic,8 and a urology practice reported high satisfaction and acceptance rates among both patients and physicians.9
Practice management literature and an article in The New York Times have anecdotally described the benefits of scribes in clinical practice10-12 with the latter noting that, “Physicians who use [scribes] say they feel liberated from the constant note-taking ...” and that “scribes have helped restore joy in the practice of medicine.”10
A small retrospective review that appeared in The Journal of Family Practice last year looked at the quality of scribes’ notes and found that they were rated slightly higher than physicians’ notes—at least for diabetes visits. However, it did not address the issues of physician productivity or satisfaction. (See "Medical scribes: How do their notes stack up?" 2016;65:155-159.)
The only family medicine study that we did find that addressed these 2 issues was one done in Oregon. The study noted that scribes enabled physicians to see 24 patients per day—up from 18, with accompanying improvements in physician “quality of life.”13 Absent from the literature are quantitative data on the feasibility and benefits of implementing scribes in family medicine.
Could a study at our facility offer some insights? In light of the paucity of published data on scribes in family medicine, and the fact that a survey conducted at our health center revealed that our faculty physicians felt overburdened by the administrative demands of clinical practice,14 we decided to study whether scribes might improve the work climate for clinicians at our family medicine residency training site. Our goal was to assess the impact of scribes on physician and patient satisfaction and on hours physicians spent on administrative tasks generated by clinical care.
METHODS
The study took place at the Barre Family Health Center (BFHC), a rural, freestanding family health center/residency site owned and operated by UMassMemorial Health Care (UMMHC), the major teaching/clinical affiliate of the University of Massachusetts Medical School. The health care providers of BFHC conduct 40,000 patient visits annually. Without scribes, the physicians typically dictated their notes at the end of the day, and they became available for review/sign off usually within 24 hours.
Six of the 7 faculty physicians working at BFHC in 2014 (including the lead author) participated in the pilot study (the seventh declined to participate). Three male and 3 female physicians between the ages of 34 and 65 years participated; they had been in practice between 5 and 40 years. All of the physicians had used an EMR for 5 years or more, and all but 2 had previously used a paper record. Residents and advanced practitioners did not participate because limited funding allowed for the hiring of only 2 full-time equivalent (FTE; 4 part-time) scribes.
Contracting for services. We contracted with an outside vendor for scribe services. Prior to their arrival at our health care center, the scribes received online training on medical vocabulary, note structure, billing and coding, and patient confidentiality (HIPAA). Once they arrived, on-site training detailed workflow, precharting, use of templates, the EMR and chart organization, and billing. In addition to typing notes into the EMR during patient visits, the scribes helped develop processes for scheduling, alerting patients to the scribe’s role, and defining when scribes should and should not be present in the exam room. The chief scribe created a monthly schedule, which enabled staff to determine which physician schedules should have extra appointment slots added. This was imperative because our parent institution mandated that new initiatives yield a 25% return on investment (ROI).
Using standard scripting and consent methods, nursing staff informed patients during rooming that the provider was working with a scribe, explained the scribe’s role, and asked about any objections to the scribe’s presence. Patients could decline scribe involvement, and all scribes were routinely excused during genital and rectal examinations.
Data collection
Data were collected during the 6-month trial period from May through October of 2014. The number of hours physicians spent at BFHC and at home working on clinical documentation was collected using a smartphone time-tracking application for two 3-week periods: the first period was in April 2014, before the scribes came on board; the second period was at the end of the 6-month scribe implementation period. In order to assess effects on productivity and whether the project was meeting the required ROI for continuation, we included a retrospective review of the EMR for both of the 3-week periods to document total clinical hours, number of clinic sessions (blocks of consecutive, uninterrupted appointments), average hours per session, the number of patient appointments scheduled per session, and the number of patient visits actually conducted per session (accounting for no-shows and unused appointments).
Physician work-life balance. We utilized 19 questions most relevant to this project’s focus from the 36-item Physician Work-Life Survey.15 Items were scored on a 5-point Likert scale ranging from ‘strongly disagree’ (1) to ‘strongly agree’ (5). The BFHC ambulatory manager distributed surveys to physicians immediately prior to the trial with scribes and 2 weeks after the conclusion of the 6-month trial.
Patient and provider satisfaction. During the 6-month intervention period, satisfaction surveys9 were distributed to patients by scribes at the end of the office visit and to physicians at the end of each scribed session, after notes were completed and reviewed. Patient surveys consisted of 6 closed-end questions regarding comfort level with the scribe in the exam room, willingness to have a scribe present for subsequent visits, importance of the scribe being the same gender/age as the patient, and overall satisfaction with the scribe’s presence (TABLE 1).
Physician surveys included 5 closed-end questions9 regarding comfort level with the scribe’s presence, ease of EMR documentation, change in office hours with having a scribe for that day’s session(s), and overall helpfulness of the scribe (TABLE 2). Open-ended questions on both surveys asked for additional comments or concerns regarding scribes and the scribe’s impact on patient encounters.
Our goal was to collect a minimum of 100 completed patient surveys and 50 completed physician surveys representing as many different patient demographics, visit types, days of the week, and times of day as possible. Surveys were anonymous and distributed during the second and third months of the trial, giving the scribes a one-month training and adjustment period.
Impact assessment, professional development needs. At the end of the 6-month study period, we held 2 focus groups—one with nurses and one with scribes. From the nurses, we solicited insights regarding the impact of scribes on patient volume, patient satisfaction, visit flow, and EMR documentation.
Scribes were asked about job skills needed, amount of training received, comfort in the exam room (both for themselves and patients), frequency of feedback received, balancing physician style with EMR documentation needs, and lessons learned.
Data analysis
Data were analyzed using the software SPSS V22.0. Univariate statistics were used to analyze patient and physician satisfaction, as well as clinic volume, time tracking, and EMR documentation. Initially, bivariate statistics were used to examine pre- and post-trial physician and patient data, but then non-parametric comparisons were used because of small sample sizes (and the resulting data being distributed abnormally). Detailed focus group notes were reviewed by all study investigators and summarized for dominant themes to support the quantitative evaluation. Lastly, the study was evaluated by the University of Massachusetts Institutional Review Board and was waived from review/oversight because of its QI intent.
RESULTS
Physician findings. Fifty-five physician surveys were completed during the 6-month period (TABLE 2). All of the physicians who were asked to complete this short survey at the end of the day (after reviewing notes with their scribe) did so. Physicians reported a high degree of satisfaction with collaboration with scribes. Their comments reflected positive experiences, including an improved ability to remain on schedule, having assistance finding important information in the record, and having notes completed at the end of the session.
TABLE 3 shows high satisfaction with clinical roles and colleagues with no substantive changes over time regarding these questions. However, the incorporation of scribes had a positive impact on issues related to physician morale, due to changes in paperwork, administrative duties, and work schedules.
Review of patient scheduling and documentation (TABLE 4) revealed visits per clinical session increased 28.8% from 6.6 to 8.5, and for sessions with 10 or more appointment slots available, billable visits increased 9.2% from 8.7 to 9.5. This increase was a result of adding an additional appointment slot to the schedule when a scribe was assigned and a greater physician willingness to overbook when scribe assistance was available.
A comparison of time tracking pre- and post-intervention showed a 13% decrease in time spent in the clinic, from a 3-week average of 30.1 hrs/wk to 26.1 hrs/wk (TABLE 4). Time spent working at home decreased 38%, from a 3-week average of 2.9 hrs/wk to 1.8 hrs/wk. These reductions occurred despite average scheduled clinic hours being 18% higher (35.5 vs 30.1) during the post- vs pre-intervention measurement periods.
Patient findings. TABLE 1 summarizes the 313 patient responses. Less than 10% of patients declined to have a scribe during the visit. Patients reported a high level of comfort with the scribe and indicated that having a scribe in the room had little impact on what they would have liked to tell their doctor. Nearly all open-ended comments were positive and reflected feelings that the scribe’s presence enabled their provider to focus more on them and less on the computer.
Focus group findings
The scribe focus group identified a number of skills thought to be necessary to be successful in the job, including typing quickly; having technology/computer-searching strategy skills; and being detail-oriented, organized, and able to multitask. Scribes estimated that it took 2 to 6 weeks to feel comfortable doing the job. Physician feedback was preferred at the end of every session.
Lastly, the 4 scribes identified several challenges that should be addressed in future training, such as how to: 1. document a visit when the patient has a complicated medical history and the communication between the doctor and the patient is implicit; 2. incorporate the particulars of a visit into a patient’s full medical history; and 3. sift through the volume of previous notes when a physician has been seeing a patient for a long period of time.
The nurses’ focus group identified many positive effects on patient care. They reported no significant challenges with introducing scribes to patients. Improvements in timely availability of documentation enhanced their ability to respond quickly and more completely to patient queries. The nurses noted that the use of scribes improved patient care and made them “a better practice.”
DISCUSSION
This study demonstrated that the use of scribes in a busy academic primary care practice substantially reduced the amount of time that family practitioners spent on charting, improved work-life balance, and had good patient acceptance. Our time-tracking studies demonstrated that physicians spent 5.1 fewer hrs/wk working—4 fewer hrs/wk in the clinic, and 1.1 fewer hrs/wk outside of the clinic—while clinical hours and productivity per session increased. Patients reported high satisfaction with scribed visits and a willingness to have scribes in the future. Creating notes in real time and having immediate availability after the session was a plus for nursing staff in providing follow-up patient care.
Concerns by physicians that having another person in the room would alter the physician-patient relationship were not substantiated, perhaps because the staff routinely obtained consent and explained the scribe’s role. Consistent with previous work, we found no suggestion that a scribe’s presence affected patients’ willingness to discuss sensitive issues.9 Patients reacted positively to scribes who enabled physicians to focus more on the patient and less on charting.
Despite increased patient volume, physician morale improved. Physicians left work more than an hour earlier per day, on average, and spent over 1 hour less per week working on clinical documentation outside the office. Physician surveys showed an improvement in perceptions of how much work encroached on their personal life, consistent with the time-tracking data. These results have significant implications for clinician retention, productivity, and satisfaction.
Since our site is an academic training site, one might wonder how residents and advanced practitioners viewed this implementation, as they were not initially included. From the perspective of the administrators, this was a feasibility study. Clinicians who were not included understood that if this pilot was successful, the use of scribes would be expanded in the future. In fact, because of these positive results, our institution has expanded the scribe program, so that it now covers all clinical sessions for faculty in our center and is rolling out a similar program in 3 other departmental academic practices.
Financial implications. At the beginning of this initiative, our institution required that we cover the cost of the program plus generate a 25% ROI. Using a conservative 9.2% increase in billable visits, we extrapolated that utilizing 2 FTE scribes would result in an additional 860 visits annually. Per our hospital’s finance department, estimated revenue generated by our facility-based practice per visit is $196, including ancillaries. That means that additional visits would generate an estimated $168,600 annually—more than twice the $79,500 annual cost of 2 FTE scribes, yielding a 112% ROI. Furthermore, patient access improved by making more visits available. Beyond the positive direct ROI, the improvements in physician morale and work-life balance have positive implications for retention, likely substantially increasing the long-term, overall ROI.
Challenges. Implementing a new program in a large organization proved to be challenging. The biggest hurdle was convincing our institution’s administration and finance department that this new expense would pay for itself in both tangible (increased visits per session) and intangible (increased physician satisfaction and retention) ways. A cost-sharing arrangement proposed by our department’s administrator convinced hospital administration to move forward. Additional challenges included delays in getting the scribe program started because of vendor selection, purchasing new laptops for scribes, hiring and training scribes, developing new EMR templates, validating provider productivity, and legal/compliance approval of the scribe’s EMR documentation processes to meet third-party and accuracy/quality requirements—all taking longer than anticipated. However, we believe that our results indicate significant potential for other primary care practices.
Limitations. The number of physicians in the study was small, and they all worked in the same location. Social desirability could have biased patient and provider feedback, but our quantitative results were consistent with subjective assessments, suggesting that information bias potential was low. Patient and provider survey findings were also supported by qualitative assessments from both scribes and nursing staff. The size of the project did not lend itself to an analysis controlling for clustering by physician and/or scribe. The focus group discussions were not subject to rigorous qualitative analysis, potentially increasing the risk of biased interpretation. Lastly, we did not have the ability to directly compare sessions with and without scribes during the pilot.
Similarity to other findings. Despite these limitations, our findings are remarkably similar to those of Howard, et al,16 on the pilot implementation of scribes in a community health center, including good patient and clinician acceptance and increased productivity that more than offset the cost of the scribes. We expect that others implementing scribe services in primary care settings will experience similar results.
CORRESPONDENCE
Stephen T. Earls, MD, 151 Worcester Road, Barre, MA 01005; [email protected].
ACKNOWLEDGEMENT
The authors gratefully acknowledge the assistance of Barbara Fisher, MBA, vice president for ambulatory services; Nicholas Comeau, BS; and Brenda Rivard, administrative lead, Barre Family Health Center, UMassMemorial Health Care, in the preparation and execution of this study.
1. Walker K, Ben-Meir M, O’Mullane P, et al. Scribes in an Australian private emergency department: a description of physician productivity. Emerg Med Australas. 2014;26:543-548.
2. Arya R, Salovich DM, Ohman-Strickland P, et al. Impact of scribes on performance indicators in the emergency department. Acad Emerg Med. 2010;17:490-494.
3. Expanded scribe role boosts staff morale. ED Manag. 2009;21:75-77.
4. Scribes, EMR please docs, save $600,000. ED Manag. 2009;21:117-118.
5. Bastani A, Shaqiri B, Palomba K, et al. An ED scribe program is able to improve throughput time and patient satisfaction. Am J Emerg Med. 2014;32:399-402.
6. Cabilan CJ, Eley RM. Review article: potential of medical scribes to allay the burden of documentation and enhance efficiency in Australian emergency departments. Emerg Med Australas. 2015 Aug 13. [Epub ahead of print]
7. Hegstrom L, Leslie J, Hutchinson E, et al. Medical scribes: are scribe programs cost effective in an outpatient MFM setting? Am J Obstet Gynecol. 2013;208:S240.
8. Campbell LL, Case D, Crocker JE, et al. Using medical scribes in a physician practice. J AHIMA. 2012;83:64-69.
9. Koshy S, Feustel PJ, Hong M, et al. Scribes in an ambulatory urology practice: patient and physician satisfaction. J Urol. 2010;184:258-262.
10. Hafner K. A busy doctor’s right hand, ever ready to type. The New York Times. January 12, 2014. Available at: https://www.nytimes.com/2014/01/14/health/a-busy-doctors-right-hand-ever-ready-to-type.html?_r=0. Accessed February 6, 2017.
11. Brady K, Shariff A. Virtual medical scribes: making electronic medical records work for you. J Med Pract Manage. 2013;29:133-136.
12. Baugh R, Jones JE, Troff K, et al. Medical scribes. J Med Pract Manage. 2012;28:195-197.
13. Grimshaw H. Physician scribes improve productivity. Oak Street Medical allows doctors to spend more face time with patients, improve job satisfaction. MGMA Connex. 2012;12:27-28.
14. Morehead Associates, Inc. UMassMemorial Health Care: Physician Satisfaction Survey. 2013.
15. Konrad TR, Williams ES, Linzer M, et al. Measuring physician job satisfaction in a changing workplace and challenging environment. SGIM Career Satisfaction Study Group. Society of General Internal Medicine. Med Care. 1999;37:1174-1182.
16. Howard KA, Helé K, Salibi N, et al. BTW Informing change. Blue Shield of California Foundation. Adapting the EHR scribe model to community health centers: the experience of Shasta Community Health Center’s pilot. Available at: http://informingchange.com/cat-publications/adapting-the-ehr-scribe-model-to-community-health-centers-the-experience-of-shasta-community-health-centers-pilot. Accessed November 6, 2015.
ABSTRACT
Purpose Research in other medical specialties has shown that the addition of medical scribes to the clinical team enhances physicians’ practice experience and increases productivity. To date, literature on the implementation of scribes in primary care is limited. To determine the feasibility and benefits of implementing scribes in family medicine, we undertook a pilot mixed-method quality improvement (QI) study.
Methods In 2014, we incorporated 4 part-time scribes into an academic family medicine practice consisting of 7 physicians. We then measured, via survey and time-tracking data, the impact the scribes had on physician office hours and productivity, time spent on documentation, perceptions of work-life balance, and physician and patient satisfaction.
Results Six of the 7 faculty physicians participated. This study demonstrated that the use of scribes in a busy academic primary care practice substantially reduced the amount of time that family physicians spent on charting, improved work-life balance, and had good patient acceptance. Specifically, the physicians spent an average of 5.1 fewer hours/week (hrs/wk) on documentation, while various measures of productivity revealed increases ranging from 9.2% to 28.8%. Perhaps most important of all, when the results of the pilot study were annualized, they were projected to generate $168,600 per year—more than twice the $79,500 annual cost of 2 full-time equivalent scribes.
Surveys assessing work-life balance demonstrated improvement in the physicians’ perception of the administrative burden/paperwork related to practice and a decrease in their perception of the extent to which work encroached on their personal lives. In addition, survey data from 313 patients at the time of their ambulatory visit with a scribe present revealed a high level of comfort. Likewise, surveys completed by physicians after 55 clinical sessions (ie, blocks of consecutive, uninterrupted patient appointments; there are usually 2 sessions per day) revealed good to excellent ratings more than 90% of the time.
Conclusion In an outpatient family medicine clinic, the use of scribes substantially improved physicians’ efficiency, job satisfaction, and productivity without negatively impacting the patient experience.
While electronic medical records (EMRs) are important tools for improving patient care and communication, they bring with them an additional administrative burden for health care providers. In the emergency medicine literature, scribes have been reported to reduce that burden and improve clinicians’ productivity and satisfaction.1-4 Additionally, studies have reported increases in patient volume, generated billings, and provider morale, as well as decreases in emergency department (ED) lengths of stay.5 A recent review of the emergency medicine literature concluded that scribes have “the ability to allay the burden of documentation, improve throughput in the ED, and potentially enhance doctors’ satisfaction.”6
Similar benefits following scribe implementation have been reported in the literature of other specialties. A maternal-fetal medicine practice reported significant increases in generated billings and reimbursement.7 Increases in physician productivity and improvements in physician-patient interactions were reported in a cardiology clinic,8 and a urology practice reported high satisfaction and acceptance rates among both patients and physicians.9
Practice management literature and an article in The New York Times have anecdotally described the benefits of scribes in clinical practice10-12 with the latter noting that, “Physicians who use [scribes] say they feel liberated from the constant note-taking ...” and that “scribes have helped restore joy in the practice of medicine.”10
A small retrospective review that appeared in The Journal of Family Practice last year looked at the quality of scribes’ notes and found that they were rated slightly higher than physicians’ notes—at least for diabetes visits. However, it did not address the issues of physician productivity or satisfaction. (See "Medical scribes: How do their notes stack up?" 2016;65:155-159.)
The only family medicine study that we did find that addressed these 2 issues was one done in Oregon. The study noted that scribes enabled physicians to see 24 patients per day—up from 18, with accompanying improvements in physician “quality of life.”13 Absent from the literature are quantitative data on the feasibility and benefits of implementing scribes in family medicine.
Could a study at our facility offer some insights? In light of the paucity of published data on scribes in family medicine, and the fact that a survey conducted at our health center revealed that our faculty physicians felt overburdened by the administrative demands of clinical practice,14 we decided to study whether scribes might improve the work climate for clinicians at our family medicine residency training site. Our goal was to assess the impact of scribes on physician and patient satisfaction and on hours physicians spent on administrative tasks generated by clinical care.
METHODS
The study took place at the Barre Family Health Center (BFHC), a rural, freestanding family health center/residency site owned and operated by UMassMemorial Health Care (UMMHC), the major teaching/clinical affiliate of the University of Massachusetts Medical School. The health care providers of BFHC conduct 40,000 patient visits annually. Without scribes, the physicians typically dictated their notes at the end of the day, and they became available for review/sign off usually within 24 hours.
Six of the 7 faculty physicians working at BFHC in 2014 (including the lead author) participated in the pilot study (the seventh declined to participate). Three male and 3 female physicians between the ages of 34 and 65 years participated; they had been in practice between 5 and 40 years. All of the physicians had used an EMR for 5 years or more, and all but 2 had previously used a paper record. Residents and advanced practitioners did not participate because limited funding allowed for the hiring of only 2 full-time equivalent (FTE; 4 part-time) scribes.
Contracting for services. We contracted with an outside vendor for scribe services. Prior to their arrival at our health care center, the scribes received online training on medical vocabulary, note structure, billing and coding, and patient confidentiality (HIPAA). Once they arrived, on-site training detailed workflow, precharting, use of templates, the EMR and chart organization, and billing. In addition to typing notes into the EMR during patient visits, the scribes helped develop processes for scheduling, alerting patients to the scribe’s role, and defining when scribes should and should not be present in the exam room. The chief scribe created a monthly schedule, which enabled staff to determine which physician schedules should have extra appointment slots added. This was imperative because our parent institution mandated that new initiatives yield a 25% return on investment (ROI).
Using standard scripting and consent methods, nursing staff informed patients during rooming that the provider was working with a scribe, explained the scribe’s role, and asked about any objections to the scribe’s presence. Patients could decline scribe involvement, and all scribes were routinely excused during genital and rectal examinations.
Data collection
Data were collected during the 6-month trial period from May through October of 2014. The number of hours physicians spent at BFHC and at home working on clinical documentation was collected using a smartphone time-tracking application for two 3-week periods: the first period was in April 2014, before the scribes came on board; the second period was at the end of the 6-month scribe implementation period. In order to assess effects on productivity and whether the project was meeting the required ROI for continuation, we included a retrospective review of the EMR for both of the 3-week periods to document total clinical hours, number of clinic sessions (blocks of consecutive, uninterrupted appointments), average hours per session, the number of patient appointments scheduled per session, and the number of patient visits actually conducted per session (accounting for no-shows and unused appointments).
Physician work-life balance. We utilized 19 questions most relevant to this project’s focus from the 36-item Physician Work-Life Survey.15 Items were scored on a 5-point Likert scale ranging from ‘strongly disagree’ (1) to ‘strongly agree’ (5). The BFHC ambulatory manager distributed surveys to physicians immediately prior to the trial with scribes and 2 weeks after the conclusion of the 6-month trial.
Patient and provider satisfaction. During the 6-month intervention period, satisfaction surveys9 were distributed to patients by scribes at the end of the office visit and to physicians at the end of each scribed session, after notes were completed and reviewed. Patient surveys consisted of 6 closed-end questions regarding comfort level with the scribe in the exam room, willingness to have a scribe present for subsequent visits, importance of the scribe being the same gender/age as the patient, and overall satisfaction with the scribe’s presence (TABLE 1).
Physician surveys included 5 closed-end questions9 regarding comfort level with the scribe’s presence, ease of EMR documentation, change in office hours with having a scribe for that day’s session(s), and overall helpfulness of the scribe (TABLE 2). Open-ended questions on both surveys asked for additional comments or concerns regarding scribes and the scribe’s impact on patient encounters.
Our goal was to collect a minimum of 100 completed patient surveys and 50 completed physician surveys representing as many different patient demographics, visit types, days of the week, and times of day as possible. Surveys were anonymous and distributed during the second and third months of the trial, giving the scribes a one-month training and adjustment period.
Impact assessment, professional development needs. At the end of the 6-month study period, we held 2 focus groups—one with nurses and one with scribes. From the nurses, we solicited insights regarding the impact of scribes on patient volume, patient satisfaction, visit flow, and EMR documentation.
Scribes were asked about job skills needed, amount of training received, comfort in the exam room (both for themselves and patients), frequency of feedback received, balancing physician style with EMR documentation needs, and lessons learned.
Data analysis
Data were analyzed using the software SPSS V22.0. Univariate statistics were used to analyze patient and physician satisfaction, as well as clinic volume, time tracking, and EMR documentation. Initially, bivariate statistics were used to examine pre- and post-trial physician and patient data, but then non-parametric comparisons were used because of small sample sizes (and the resulting data being distributed abnormally). Detailed focus group notes were reviewed by all study investigators and summarized for dominant themes to support the quantitative evaluation. Lastly, the study was evaluated by the University of Massachusetts Institutional Review Board and was waived from review/oversight because of its QI intent.
RESULTS
Physician findings. Fifty-five physician surveys were completed during the 6-month period (TABLE 2). All of the physicians who were asked to complete this short survey at the end of the day (after reviewing notes with their scribe) did so. Physicians reported a high degree of satisfaction with collaboration with scribes. Their comments reflected positive experiences, including an improved ability to remain on schedule, having assistance finding important information in the record, and having notes completed at the end of the session.
TABLE 3 shows high satisfaction with clinical roles and colleagues with no substantive changes over time regarding these questions. However, the incorporation of scribes had a positive impact on issues related to physician morale, due to changes in paperwork, administrative duties, and work schedules.
Review of patient scheduling and documentation (TABLE 4) revealed visits per clinical session increased 28.8% from 6.6 to 8.5, and for sessions with 10 or more appointment slots available, billable visits increased 9.2% from 8.7 to 9.5. This increase was a result of adding an additional appointment slot to the schedule when a scribe was assigned and a greater physician willingness to overbook when scribe assistance was available.
A comparison of time tracking pre- and post-intervention showed a 13% decrease in time spent in the clinic, from a 3-week average of 30.1 hrs/wk to 26.1 hrs/wk (TABLE 4). Time spent working at home decreased 38%, from a 3-week average of 2.9 hrs/wk to 1.8 hrs/wk. These reductions occurred despite average scheduled clinic hours being 18% higher (35.5 vs 30.1) during the post- vs pre-intervention measurement periods.
Patient findings. TABLE 1 summarizes the 313 patient responses. Less than 10% of patients declined to have a scribe during the visit. Patients reported a high level of comfort with the scribe and indicated that having a scribe in the room had little impact on what they would have liked to tell their doctor. Nearly all open-ended comments were positive and reflected feelings that the scribe’s presence enabled their provider to focus more on them and less on the computer.
Focus group findings
The scribe focus group identified a number of skills thought to be necessary to be successful in the job, including typing quickly; having technology/computer-searching strategy skills; and being detail-oriented, organized, and able to multitask. Scribes estimated that it took 2 to 6 weeks to feel comfortable doing the job. Physician feedback was preferred at the end of every session.
Lastly, the 4 scribes identified several challenges that should be addressed in future training, such as how to: 1. document a visit when the patient has a complicated medical history and the communication between the doctor and the patient is implicit; 2. incorporate the particulars of a visit into a patient’s full medical history; and 3. sift through the volume of previous notes when a physician has been seeing a patient for a long period of time.
The nurses’ focus group identified many positive effects on patient care. They reported no significant challenges with introducing scribes to patients. Improvements in timely availability of documentation enhanced their ability to respond quickly and more completely to patient queries. The nurses noted that the use of scribes improved patient care and made them “a better practice.”
DISCUSSION
This study demonstrated that the use of scribes in a busy academic primary care practice substantially reduced the amount of time that family practitioners spent on charting, improved work-life balance, and had good patient acceptance. Our time-tracking studies demonstrated that physicians spent 5.1 fewer hrs/wk working—4 fewer hrs/wk in the clinic, and 1.1 fewer hrs/wk outside of the clinic—while clinical hours and productivity per session increased. Patients reported high satisfaction with scribed visits and a willingness to have scribes in the future. Creating notes in real time and having immediate availability after the session was a plus for nursing staff in providing follow-up patient care.
Concerns by physicians that having another person in the room would alter the physician-patient relationship were not substantiated, perhaps because the staff routinely obtained consent and explained the scribe’s role. Consistent with previous work, we found no suggestion that a scribe’s presence affected patients’ willingness to discuss sensitive issues.9 Patients reacted positively to scribes who enabled physicians to focus more on the patient and less on charting.
Despite increased patient volume, physician morale improved. Physicians left work more than an hour earlier per day, on average, and spent over 1 hour less per week working on clinical documentation outside the office. Physician surveys showed an improvement in perceptions of how much work encroached on their personal life, consistent with the time-tracking data. These results have significant implications for clinician retention, productivity, and satisfaction.
Since our site is an academic training site, one might wonder how residents and advanced practitioners viewed this implementation, as they were not initially included. From the perspective of the administrators, this was a feasibility study. Clinicians who were not included understood that if this pilot was successful, the use of scribes would be expanded in the future. In fact, because of these positive results, our institution has expanded the scribe program, so that it now covers all clinical sessions for faculty in our center and is rolling out a similar program in 3 other departmental academic practices.
Financial implications. At the beginning of this initiative, our institution required that we cover the cost of the program plus generate a 25% ROI. Using a conservative 9.2% increase in billable visits, we extrapolated that utilizing 2 FTE scribes would result in an additional 860 visits annually. Per our hospital’s finance department, estimated revenue generated by our facility-based practice per visit is $196, including ancillaries. That means that additional visits would generate an estimated $168,600 annually—more than twice the $79,500 annual cost of 2 FTE scribes, yielding a 112% ROI. Furthermore, patient access improved by making more visits available. Beyond the positive direct ROI, the improvements in physician morale and work-life balance have positive implications for retention, likely substantially increasing the long-term, overall ROI.
Challenges. Implementing a new program in a large organization proved to be challenging. The biggest hurdle was convincing our institution’s administration and finance department that this new expense would pay for itself in both tangible (increased visits per session) and intangible (increased physician satisfaction and retention) ways. A cost-sharing arrangement proposed by our department’s administrator convinced hospital administration to move forward. Additional challenges included delays in getting the scribe program started because of vendor selection, purchasing new laptops for scribes, hiring and training scribes, developing new EMR templates, validating provider productivity, and legal/compliance approval of the scribe’s EMR documentation processes to meet third-party and accuracy/quality requirements—all taking longer than anticipated. However, we believe that our results indicate significant potential for other primary care practices.
Limitations. The number of physicians in the study was small, and they all worked in the same location. Social desirability could have biased patient and provider feedback, but our quantitative results were consistent with subjective assessments, suggesting that information bias potential was low. Patient and provider survey findings were also supported by qualitative assessments from both scribes and nursing staff. The size of the project did not lend itself to an analysis controlling for clustering by physician and/or scribe. The focus group discussions were not subject to rigorous qualitative analysis, potentially increasing the risk of biased interpretation. Lastly, we did not have the ability to directly compare sessions with and without scribes during the pilot.
Similarity to other findings. Despite these limitations, our findings are remarkably similar to those of Howard, et al,16 on the pilot implementation of scribes in a community health center, including good patient and clinician acceptance and increased productivity that more than offset the cost of the scribes. We expect that others implementing scribe services in primary care settings will experience similar results.
CORRESPONDENCE
Stephen T. Earls, MD, 151 Worcester Road, Barre, MA 01005; [email protected].
ACKNOWLEDGEMENT
The authors gratefully acknowledge the assistance of Barbara Fisher, MBA, vice president for ambulatory services; Nicholas Comeau, BS; and Brenda Rivard, administrative lead, Barre Family Health Center, UMassMemorial Health Care, in the preparation and execution of this study.
ABSTRACT
Purpose Research in other medical specialties has shown that the addition of medical scribes to the clinical team enhances physicians’ practice experience and increases productivity. To date, literature on the implementation of scribes in primary care is limited. To determine the feasibility and benefits of implementing scribes in family medicine, we undertook a pilot mixed-method quality improvement (QI) study.
Methods In 2014, we incorporated 4 part-time scribes into an academic family medicine practice consisting of 7 physicians. We then measured, via survey and time-tracking data, the impact the scribes had on physician office hours and productivity, time spent on documentation, perceptions of work-life balance, and physician and patient satisfaction.
Results Six of the 7 faculty physicians participated. This study demonstrated that the use of scribes in a busy academic primary care practice substantially reduced the amount of time that family physicians spent on charting, improved work-life balance, and had good patient acceptance. Specifically, the physicians spent an average of 5.1 fewer hours/week (hrs/wk) on documentation, while various measures of productivity revealed increases ranging from 9.2% to 28.8%. Perhaps most important of all, when the results of the pilot study were annualized, they were projected to generate $168,600 per year—more than twice the $79,500 annual cost of 2 full-time equivalent scribes.
Surveys assessing work-life balance demonstrated improvement in the physicians’ perception of the administrative burden/paperwork related to practice and a decrease in their perception of the extent to which work encroached on their personal lives. In addition, survey data from 313 patients at the time of their ambulatory visit with a scribe present revealed a high level of comfort. Likewise, surveys completed by physicians after 55 clinical sessions (ie, blocks of consecutive, uninterrupted patient appointments; there are usually 2 sessions per day) revealed good to excellent ratings more than 90% of the time.
Conclusion In an outpatient family medicine clinic, the use of scribes substantially improved physicians’ efficiency, job satisfaction, and productivity without negatively impacting the patient experience.
While electronic medical records (EMRs) are important tools for improving patient care and communication, they bring with them an additional administrative burden for health care providers. In the emergency medicine literature, scribes have been reported to reduce that burden and improve clinicians’ productivity and satisfaction.1-4 Additionally, studies have reported increases in patient volume, generated billings, and provider morale, as well as decreases in emergency department (ED) lengths of stay.5 A recent review of the emergency medicine literature concluded that scribes have “the ability to allay the burden of documentation, improve throughput in the ED, and potentially enhance doctors’ satisfaction.”6
Similar benefits following scribe implementation have been reported in the literature of other specialties. A maternal-fetal medicine practice reported significant increases in generated billings and reimbursement.7 Increases in physician productivity and improvements in physician-patient interactions were reported in a cardiology clinic,8 and a urology practice reported high satisfaction and acceptance rates among both patients and physicians.9
Practice management literature and an article in The New York Times have anecdotally described the benefits of scribes in clinical practice10-12 with the latter noting that, “Physicians who use [scribes] say they feel liberated from the constant note-taking ...” and that “scribes have helped restore joy in the practice of medicine.”10
A small retrospective review that appeared in The Journal of Family Practice last year looked at the quality of scribes’ notes and found that they were rated slightly higher than physicians’ notes—at least for diabetes visits. However, it did not address the issues of physician productivity or satisfaction. (See "Medical scribes: How do their notes stack up?" 2016;65:155-159.)
The only family medicine study that we did find that addressed these 2 issues was one done in Oregon. The study noted that scribes enabled physicians to see 24 patients per day—up from 18, with accompanying improvements in physician “quality of life.”13 Absent from the literature are quantitative data on the feasibility and benefits of implementing scribes in family medicine.
Could a study at our facility offer some insights? In light of the paucity of published data on scribes in family medicine, and the fact that a survey conducted at our health center revealed that our faculty physicians felt overburdened by the administrative demands of clinical practice,14 we decided to study whether scribes might improve the work climate for clinicians at our family medicine residency training site. Our goal was to assess the impact of scribes on physician and patient satisfaction and on hours physicians spent on administrative tasks generated by clinical care.
METHODS
The study took place at the Barre Family Health Center (BFHC), a rural, freestanding family health center/residency site owned and operated by UMassMemorial Health Care (UMMHC), the major teaching/clinical affiliate of the University of Massachusetts Medical School. The health care providers of BFHC conduct 40,000 patient visits annually. Without scribes, the physicians typically dictated their notes at the end of the day, and they became available for review/sign off usually within 24 hours.
Six of the 7 faculty physicians working at BFHC in 2014 (including the lead author) participated in the pilot study (the seventh declined to participate). Three male and 3 female physicians between the ages of 34 and 65 years participated; they had been in practice between 5 and 40 years. All of the physicians had used an EMR for 5 years or more, and all but 2 had previously used a paper record. Residents and advanced practitioners did not participate because limited funding allowed for the hiring of only 2 full-time equivalent (FTE; 4 part-time) scribes.
Contracting for services. We contracted with an outside vendor for scribe services. Prior to their arrival at our health care center, the scribes received online training on medical vocabulary, note structure, billing and coding, and patient confidentiality (HIPAA). Once they arrived, on-site training detailed workflow, precharting, use of templates, the EMR and chart organization, and billing. In addition to typing notes into the EMR during patient visits, the scribes helped develop processes for scheduling, alerting patients to the scribe’s role, and defining when scribes should and should not be present in the exam room. The chief scribe created a monthly schedule, which enabled staff to determine which physician schedules should have extra appointment slots added. This was imperative because our parent institution mandated that new initiatives yield a 25% return on investment (ROI).
Using standard scripting and consent methods, nursing staff informed patients during rooming that the provider was working with a scribe, explained the scribe’s role, and asked about any objections to the scribe’s presence. Patients could decline scribe involvement, and all scribes were routinely excused during genital and rectal examinations.
Data collection
Data were collected during the 6-month trial period from May through October of 2014. The number of hours physicians spent at BFHC and at home working on clinical documentation was collected using a smartphone time-tracking application for two 3-week periods: the first period was in April 2014, before the scribes came on board; the second period was at the end of the 6-month scribe implementation period. In order to assess effects on productivity and whether the project was meeting the required ROI for continuation, we included a retrospective review of the EMR for both of the 3-week periods to document total clinical hours, number of clinic sessions (blocks of consecutive, uninterrupted appointments), average hours per session, the number of patient appointments scheduled per session, and the number of patient visits actually conducted per session (accounting for no-shows and unused appointments).
Physician work-life balance. We utilized 19 questions most relevant to this project’s focus from the 36-item Physician Work-Life Survey.15 Items were scored on a 5-point Likert scale ranging from ‘strongly disagree’ (1) to ‘strongly agree’ (5). The BFHC ambulatory manager distributed surveys to physicians immediately prior to the trial with scribes and 2 weeks after the conclusion of the 6-month trial.
Patient and provider satisfaction. During the 6-month intervention period, satisfaction surveys9 were distributed to patients by scribes at the end of the office visit and to physicians at the end of each scribed session, after notes were completed and reviewed. Patient surveys consisted of 6 closed-end questions regarding comfort level with the scribe in the exam room, willingness to have a scribe present for subsequent visits, importance of the scribe being the same gender/age as the patient, and overall satisfaction with the scribe’s presence (TABLE 1).
Physician surveys included 5 closed-end questions9 regarding comfort level with the scribe’s presence, ease of EMR documentation, change in office hours with having a scribe for that day’s session(s), and overall helpfulness of the scribe (TABLE 2). Open-ended questions on both surveys asked for additional comments or concerns regarding scribes and the scribe’s impact on patient encounters.
Our goal was to collect a minimum of 100 completed patient surveys and 50 completed physician surveys representing as many different patient demographics, visit types, days of the week, and times of day as possible. Surveys were anonymous and distributed during the second and third months of the trial, giving the scribes a one-month training and adjustment period.
Impact assessment, professional development needs. At the end of the 6-month study period, we held 2 focus groups—one with nurses and one with scribes. From the nurses, we solicited insights regarding the impact of scribes on patient volume, patient satisfaction, visit flow, and EMR documentation.
Scribes were asked about job skills needed, amount of training received, comfort in the exam room (both for themselves and patients), frequency of feedback received, balancing physician style with EMR documentation needs, and lessons learned.
Data analysis
Data were analyzed using the software SPSS V22.0. Univariate statistics were used to analyze patient and physician satisfaction, as well as clinic volume, time tracking, and EMR documentation. Initially, bivariate statistics were used to examine pre- and post-trial physician and patient data, but then non-parametric comparisons were used because of small sample sizes (and the resulting data being distributed abnormally). Detailed focus group notes were reviewed by all study investigators and summarized for dominant themes to support the quantitative evaluation. Lastly, the study was evaluated by the University of Massachusetts Institutional Review Board and was waived from review/oversight because of its QI intent.
RESULTS
Physician findings. Fifty-five physician surveys were completed during the 6-month period (TABLE 2). All of the physicians who were asked to complete this short survey at the end of the day (after reviewing notes with their scribe) did so. Physicians reported a high degree of satisfaction with collaboration with scribes. Their comments reflected positive experiences, including an improved ability to remain on schedule, having assistance finding important information in the record, and having notes completed at the end of the session.
TABLE 3 shows high satisfaction with clinical roles and colleagues with no substantive changes over time regarding these questions. However, the incorporation of scribes had a positive impact on issues related to physician morale, due to changes in paperwork, administrative duties, and work schedules.
Review of patient scheduling and documentation (TABLE 4) revealed visits per clinical session increased 28.8% from 6.6 to 8.5, and for sessions with 10 or more appointment slots available, billable visits increased 9.2% from 8.7 to 9.5. This increase was a result of adding an additional appointment slot to the schedule when a scribe was assigned and a greater physician willingness to overbook when scribe assistance was available.
A comparison of time tracking pre- and post-intervention showed a 13% decrease in time spent in the clinic, from a 3-week average of 30.1 hrs/wk to 26.1 hrs/wk (TABLE 4). Time spent working at home decreased 38%, from a 3-week average of 2.9 hrs/wk to 1.8 hrs/wk. These reductions occurred despite average scheduled clinic hours being 18% higher (35.5 vs 30.1) during the post- vs pre-intervention measurement periods.
Patient findings. TABLE 1 summarizes the 313 patient responses. Less than 10% of patients declined to have a scribe during the visit. Patients reported a high level of comfort with the scribe and indicated that having a scribe in the room had little impact on what they would have liked to tell their doctor. Nearly all open-ended comments were positive and reflected feelings that the scribe’s presence enabled their provider to focus more on them and less on the computer.
Focus group findings
The scribe focus group identified a number of skills thought to be necessary to be successful in the job, including typing quickly; having technology/computer-searching strategy skills; and being detail-oriented, organized, and able to multitask. Scribes estimated that it took 2 to 6 weeks to feel comfortable doing the job. Physician feedback was preferred at the end of every session.
Lastly, the 4 scribes identified several challenges that should be addressed in future training, such as how to: 1. document a visit when the patient has a complicated medical history and the communication between the doctor and the patient is implicit; 2. incorporate the particulars of a visit into a patient’s full medical history; and 3. sift through the volume of previous notes when a physician has been seeing a patient for a long period of time.
The nurses’ focus group identified many positive effects on patient care. They reported no significant challenges with introducing scribes to patients. Improvements in timely availability of documentation enhanced their ability to respond quickly and more completely to patient queries. The nurses noted that the use of scribes improved patient care and made them “a better practice.”
DISCUSSION
This study demonstrated that the use of scribes in a busy academic primary care practice substantially reduced the amount of time that family practitioners spent on charting, improved work-life balance, and had good patient acceptance. Our time-tracking studies demonstrated that physicians spent 5.1 fewer hrs/wk working—4 fewer hrs/wk in the clinic, and 1.1 fewer hrs/wk outside of the clinic—while clinical hours and productivity per session increased. Patients reported high satisfaction with scribed visits and a willingness to have scribes in the future. Creating notes in real time and having immediate availability after the session was a plus for nursing staff in providing follow-up patient care.
Concerns by physicians that having another person in the room would alter the physician-patient relationship were not substantiated, perhaps because the staff routinely obtained consent and explained the scribe’s role. Consistent with previous work, we found no suggestion that a scribe’s presence affected patients’ willingness to discuss sensitive issues.9 Patients reacted positively to scribes who enabled physicians to focus more on the patient and less on charting.
Despite increased patient volume, physician morale improved. Physicians left work more than an hour earlier per day, on average, and spent over 1 hour less per week working on clinical documentation outside the office. Physician surveys showed an improvement in perceptions of how much work encroached on their personal life, consistent with the time-tracking data. These results have significant implications for clinician retention, productivity, and satisfaction.
Since our site is an academic training site, one might wonder how residents and advanced practitioners viewed this implementation, as they were not initially included. From the perspective of the administrators, this was a feasibility study. Clinicians who were not included understood that if this pilot was successful, the use of scribes would be expanded in the future. In fact, because of these positive results, our institution has expanded the scribe program, so that it now covers all clinical sessions for faculty in our center and is rolling out a similar program in 3 other departmental academic practices.
Financial implications. At the beginning of this initiative, our institution required that we cover the cost of the program plus generate a 25% ROI. Using a conservative 9.2% increase in billable visits, we extrapolated that utilizing 2 FTE scribes would result in an additional 860 visits annually. Per our hospital’s finance department, estimated revenue generated by our facility-based practice per visit is $196, including ancillaries. That means that additional visits would generate an estimated $168,600 annually—more than twice the $79,500 annual cost of 2 FTE scribes, yielding a 112% ROI. Furthermore, patient access improved by making more visits available. Beyond the positive direct ROI, the improvements in physician morale and work-life balance have positive implications for retention, likely substantially increasing the long-term, overall ROI.
Challenges. Implementing a new program in a large organization proved to be challenging. The biggest hurdle was convincing our institution’s administration and finance department that this new expense would pay for itself in both tangible (increased visits per session) and intangible (increased physician satisfaction and retention) ways. A cost-sharing arrangement proposed by our department’s administrator convinced hospital administration to move forward. Additional challenges included delays in getting the scribe program started because of vendor selection, purchasing new laptops for scribes, hiring and training scribes, developing new EMR templates, validating provider productivity, and legal/compliance approval of the scribe’s EMR documentation processes to meet third-party and accuracy/quality requirements—all taking longer than anticipated. However, we believe that our results indicate significant potential for other primary care practices.
Limitations. The number of physicians in the study was small, and they all worked in the same location. Social desirability could have biased patient and provider feedback, but our quantitative results were consistent with subjective assessments, suggesting that information bias potential was low. Patient and provider survey findings were also supported by qualitative assessments from both scribes and nursing staff. The size of the project did not lend itself to an analysis controlling for clustering by physician and/or scribe. The focus group discussions were not subject to rigorous qualitative analysis, potentially increasing the risk of biased interpretation. Lastly, we did not have the ability to directly compare sessions with and without scribes during the pilot.
Similarity to other findings. Despite these limitations, our findings are remarkably similar to those of Howard, et al,16 on the pilot implementation of scribes in a community health center, including good patient and clinician acceptance and increased productivity that more than offset the cost of the scribes. We expect that others implementing scribe services in primary care settings will experience similar results.
CORRESPONDENCE
Stephen T. Earls, MD, 151 Worcester Road, Barre, MA 01005; [email protected].
ACKNOWLEDGEMENT
The authors gratefully acknowledge the assistance of Barbara Fisher, MBA, vice president for ambulatory services; Nicholas Comeau, BS; and Brenda Rivard, administrative lead, Barre Family Health Center, UMassMemorial Health Care, in the preparation and execution of this study.
1. Walker K, Ben-Meir M, O’Mullane P, et al. Scribes in an Australian private emergency department: a description of physician productivity. Emerg Med Australas. 2014;26:543-548.
2. Arya R, Salovich DM, Ohman-Strickland P, et al. Impact of scribes on performance indicators in the emergency department. Acad Emerg Med. 2010;17:490-494.
3. Expanded scribe role boosts staff morale. ED Manag. 2009;21:75-77.
4. Scribes, EMR please docs, save $600,000. ED Manag. 2009;21:117-118.
5. Bastani A, Shaqiri B, Palomba K, et al. An ED scribe program is able to improve throughput time and patient satisfaction. Am J Emerg Med. 2014;32:399-402.
6. Cabilan CJ, Eley RM. Review article: potential of medical scribes to allay the burden of documentation and enhance efficiency in Australian emergency departments. Emerg Med Australas. 2015 Aug 13. [Epub ahead of print]
7. Hegstrom L, Leslie J, Hutchinson E, et al. Medical scribes: are scribe programs cost effective in an outpatient MFM setting? Am J Obstet Gynecol. 2013;208:S240.
8. Campbell LL, Case D, Crocker JE, et al. Using medical scribes in a physician practice. J AHIMA. 2012;83:64-69.
9. Koshy S, Feustel PJ, Hong M, et al. Scribes in an ambulatory urology practice: patient and physician satisfaction. J Urol. 2010;184:258-262.
10. Hafner K. A busy doctor’s right hand, ever ready to type. The New York Times. January 12, 2014. Available at: https://www.nytimes.com/2014/01/14/health/a-busy-doctors-right-hand-ever-ready-to-type.html?_r=0. Accessed February 6, 2017.
11. Brady K, Shariff A. Virtual medical scribes: making electronic medical records work for you. J Med Pract Manage. 2013;29:133-136.
12. Baugh R, Jones JE, Troff K, et al. Medical scribes. J Med Pract Manage. 2012;28:195-197.
13. Grimshaw H. Physician scribes improve productivity. Oak Street Medical allows doctors to spend more face time with patients, improve job satisfaction. MGMA Connex. 2012;12:27-28.
14. Morehead Associates, Inc. UMassMemorial Health Care: Physician Satisfaction Survey. 2013.
15. Konrad TR, Williams ES, Linzer M, et al. Measuring physician job satisfaction in a changing workplace and challenging environment. SGIM Career Satisfaction Study Group. Society of General Internal Medicine. Med Care. 1999;37:1174-1182.
16. Howard KA, Helé K, Salibi N, et al. BTW Informing change. Blue Shield of California Foundation. Adapting the EHR scribe model to community health centers: the experience of Shasta Community Health Center’s pilot. Available at: http://informingchange.com/cat-publications/adapting-the-ehr-scribe-model-to-community-health-centers-the-experience-of-shasta-community-health-centers-pilot. Accessed November 6, 2015.
1. Walker K, Ben-Meir M, O’Mullane P, et al. Scribes in an Australian private emergency department: a description of physician productivity. Emerg Med Australas. 2014;26:543-548.
2. Arya R, Salovich DM, Ohman-Strickland P, et al. Impact of scribes on performance indicators in the emergency department. Acad Emerg Med. 2010;17:490-494.
3. Expanded scribe role boosts staff morale. ED Manag. 2009;21:75-77.
4. Scribes, EMR please docs, save $600,000. ED Manag. 2009;21:117-118.
5. Bastani A, Shaqiri B, Palomba K, et al. An ED scribe program is able to improve throughput time and patient satisfaction. Am J Emerg Med. 2014;32:399-402.
6. Cabilan CJ, Eley RM. Review article: potential of medical scribes to allay the burden of documentation and enhance efficiency in Australian emergency departments. Emerg Med Australas. 2015 Aug 13. [Epub ahead of print]
7. Hegstrom L, Leslie J, Hutchinson E, et al. Medical scribes: are scribe programs cost effective in an outpatient MFM setting? Am J Obstet Gynecol. 2013;208:S240.
8. Campbell LL, Case D, Crocker JE, et al. Using medical scribes in a physician practice. J AHIMA. 2012;83:64-69.
9. Koshy S, Feustel PJ, Hong M, et al. Scribes in an ambulatory urology practice: patient and physician satisfaction. J Urol. 2010;184:258-262.
10. Hafner K. A busy doctor’s right hand, ever ready to type. The New York Times. January 12, 2014. Available at: https://www.nytimes.com/2014/01/14/health/a-busy-doctors-right-hand-ever-ready-to-type.html?_r=0. Accessed February 6, 2017.
11. Brady K, Shariff A. Virtual medical scribes: making electronic medical records work for you. J Med Pract Manage. 2013;29:133-136.
12. Baugh R, Jones JE, Troff K, et al. Medical scribes. J Med Pract Manage. 2012;28:195-197.
13. Grimshaw H. Physician scribes improve productivity. Oak Street Medical allows doctors to spend more face time with patients, improve job satisfaction. MGMA Connex. 2012;12:27-28.
14. Morehead Associates, Inc. UMassMemorial Health Care: Physician Satisfaction Survey. 2013.
15. Konrad TR, Williams ES, Linzer M, et al. Measuring physician job satisfaction in a changing workplace and challenging environment. SGIM Career Satisfaction Study Group. Society of General Internal Medicine. Med Care. 1999;37:1174-1182.
16. Howard KA, Helé K, Salibi N, et al. BTW Informing change. Blue Shield of California Foundation. Adapting the EHR scribe model to community health centers: the experience of Shasta Community Health Center’s pilot. Available at: http://informingchange.com/cat-publications/adapting-the-ehr-scribe-model-to-community-health-centers-the-experience-of-shasta-community-health-centers-pilot. Accessed November 6, 2015.
Breast cancer screening: New study fans flames of old timing controversy
Muscle spasms, twitches in arm upon throwing • Dx?
THE CASE
A 31-year-old right-handed college baseball coach presented to his family physician (FP) with concerns about the “yips” in his right arm. His ability to throw a baseball had been gradually deteriorating. Involuntary upper right arm muscle contractions and spasms, which began intermittently when he was a teenager, were now a real problem for him as an adult. (See the video below.) The patient was having difficulty rolling a baseball underhand to players as part of infield practice and he was experiencing muscle spasms when lifting his right arm over his head. “Twitches” in the patient’s upper arm were making drinking difficult, but he had no problems feeding himself, writing, or performing other basic activities of daily living.
The patient experienced the same symptoms whether it was baseball season or not. He hadn’t noticed a change in symptoms with caffeine and denied use of any other stimulants in the last 4 years. His symptoms didn’t improve or worsen with greater or lesser quantity or quality of sleep or when he concentrated on stifling the involuntary movements. He had attempted to learn to throw left-handed to overcome the impairment, but was concerned that the same problem would occur in his left arm.
The patient had previously worked with a sports psychologist and hypnotherapist to overcome any potential subconscious performance anxiety, but this hadn’t helped. Stretching and strengthening with a physical therapist and numerous sessions with an acupuncturist hadn’t helped either. Despite this, he believed the problem to be primarily psychological.
The patient’s history included mild attention deficit disorder and exercise-induced asthma; his family history was negative for any movement or psychiatric disorders. He had 2 dislocation repairs on his left, non-throwing shoulder in his early twenties. His medications included fluticasone-salmeterol twice daily and albuterol, as needed.
The patient denied myalgia or arthralgia, decreased passive range of motion, shoulder or arm weakness, swelling, or muscle atrophy. He also didn’t have paresthesias in his right arm or hand, a resting tremor, difficulty moving (other than drinking from a cup), difficulty moving other extremities, dizziness, imbalance, or seizures.
The patient’s vital signs were normal. He had full range of motion and 5 out of 5 strength without pain during right shoulder abduction, external and internal rotation, an empty can test, a lower back lift off (Gerber’s) test, and a test of bicep and tricep strength, along with negative Neer and Hawkins tests.
There was no evidence of muscle wasting or asymmetry in the bilateral upper extremities. The patient’s deep tendon reflex grade was 2+ out of 4 in both of his arms. He didn’t have a sensory deficit to light touch in areas of C5 to T1 and he had normal cranial nerves II to XII. He had normal rapid alternating movements, heel-to-shin testing, and finger-to-nose testing, as well as a normal gait and Romberg test.
The patient provided a video showing the abnormal involuntary flexion of his shoulder when attempting to throw a baseball.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
THE DIAGNOSIS
The patient’s FP was aware of the “yips,” a condition that is commonly viewed as psychological or related to performance anxiety. (The “yips” are colloquially known as “Steve Blass Disease”—named after a Pittsburgh Pirates pitcher who suddenly lost the ability to control his pitches.1) But based on the patient’s clinical presentation and history of seeing a number of mental health care providers—in addition to his worsening symptoms—the FP ordered magnetic resonance imaging (MRI) of the brain. The MRI turned out to be unremarkable, so the patient was referred to Neurology.
In the general neurology clinic, a diagnosis of Wilson’s disease (a condition that leads to excess copper deposition in multiple organ systems, including the nervous system) was considered, as it can cause symptoms similar to those our patient was experiencing. However, a complete blood count, complete metabolic panel, antinuclear antibody test, ceruloplasmin test, and copper level were all normal, effectively ruling it out. An MRI of the cervical spine showed mild to moderate right foraminal stenosis at C3-4 and C5-6, but this did not explain the patient’s symptoms.
A diagnosis of paroxysmal exercise-induced dystonia was also considered at the time of the initial work-up, as our patient’s symptoms were most pronounced during physical activity. But this condition usually responds to antiepileptics, and carbamazepine and phenytoin were each tried for multiple months early in his evaluation without benefit.
3 factors led to a diagnosis of focal limb dystonia: Only our patient’s right arm was affected, his laboratory and imaging work-ups were negative, and he didn’t respond to antiepileptic treatment. Characterization of a movement disorder is based upon phenomenology. In this case, the patient had sustained abnormal posturing at the shoulder during right upper limb activation, which was only triggered with specific voluntary actions. This was consistent with dystonia, a movement disorder characterized by sustained or intermittent muscle contractions causing abnormal movements and/or postures—often initiated or worsened by voluntary action.2
DISCUSSION
The “yips,” or intermittent, transient tremors, jerks, or spasms3 that are seen in athletes, are well-documented in the lay press, but haven’t been significantly addressed in the medical literature.4 Stigma surrounding the condition among athletes likely leads to under-reporting. Athletes typically experience yips with fine motor movements, such as short putts in golf and pitching in baseball. In fact, while the majority of the medical literature on yips revolves around golfers, many talented baseball players have had their careers altered by the condition. The yips may also affect movements in sports like darts, cricket, table tennis, and billiards.
In 1984, dystonia was defined as a disorder of sensorimotor integration that results in co-contraction of agonist/antagonist muscles, and may be characterized by state dependence (exacerbation with specific activities) or sensory tricks (amelioration with specific types of sensory input).5 In 2013, the definition was revised to remove “co-contraction” from the definition because phenomenology alone is sufficient to make the diagnosis.1
Many athletes and sports fans believe the yips are caused by performance anxiety or related phobias, but evidence suggests that many athletes with the movement disorder may actually have focal limb dystonia.6,7 The yips can, however, lead to performance anxiety,3 but there has been no difference noted between the anxiety level of golfers with or without the yips.7 Psychological treatment approaches are commonly employed, but surface electromyograms have shown abnormal co-contraction of wrist flexor and extensor muscles in 5 out of 10 golfers with the yips (but 0 of those without) while putting—which is consistent with focal limb dystonia.8
Botulinum toxin injections are Tx of choice, but can cause weakness
Muscle relaxers, such as baclofen and benzodiazepines, as well as dopamine antagonists, can ameliorate dystonia.9 Focal limb dystonia may also respond to the antispasmodic trihexyphenidyl, but the dose must often be limited due to adverse effects such as nausea, dizziness, and anxiety.10
Botulinum toxin injections have proven effective for focal limb dystonia11 and are considered the treatment of choice. However, there are few reports on their use in athletes, where the adverse effect of weakness could affect performance. One case report also showed improvement of yips with acupuncture, although this has not been extensively studied.12
Our patient didn’t respond to low-dose (2 mg twice a day) trihexyphenidyl. Tetrabenazine, a dopamine depletor frequently used for hyperkinetic disorders, was not effective at 25 mg taken prior to coaching sessions. Higher doses of an anticholinergic could have been effective, but the patient declined our recommendation to pursue this (or botulinum toxin injections). He decided instead to train himself to use his left arm while coaching.
THE TAKEAWAY
Athletes who play sports that require precision movements commonly develop the yips. While the prevailing theory among athletes is that this is a psychological phenomenon, evidence shows that this may in fact be a neurologic focal dystonia caused by repetitive use. Greater awareness of yips as a possible organic, treatable neurologic condition is needed in order to stimulate more research on this topic.
1. Baseball’s head cases often prove baffling. USA Today Baseball Weekly. 2001. Available at: http://usatoday30.usatoday.com/sports/bbw/2001-02-07/2001-02-07-head.htm. Accessed March 15, 2017.
2. Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 2013;28:863-873.
3. Dhungana S, Jankovic J. Yips and other movement disorders in golfers. Mov Disord. 2013;28:576-581.
4. Stacy MA, ed. Handbook of dystonia. New York, NY: Informa Healthcare USA, Inc; 2007.
5. Fahn S, Marsden CD, Calne DB. Classification and investigation of dystonia. In: Marsden CD, Fahn S, eds. Movement disorders 2. London: Butterworths; 1987:332-358.
6. Smith AM, Adler CH, Crews D, et al. The ‘yips’ in golf: a continuum between a focal dystonia and choking. Sports Med. 2003;33:13-31.
7. Sachdev P. Golfers’ cramp: clinical characteristics and evidence against it being an anxiety disorder. Mov Disord. 1992;7:326-332.
8. Adler CH, Crews D, Hentz JG, et al. Abnormal co-contraction in yips-affected but not unaffected golfers: evidence for focal dystonia. Neurology. 2005;64:1813-1814.
9. Jankovic J. Treatment of hyperkinetic movement disorders. Lancet Neurol. 2009;8:844-856.
10. Jankovic J. Treatment of dystonia. Lancet Neurol. 2006;5:864-872.
11. Lungu C, Karp BI, Alter K, et al. Long-term follow-up of botulinum toxin therapy for focal hand dystonia: outcome at 10 years or more. Mov Disord. 2011;26:750-753.
12. Rosted P. Acupuncture for treatment of the yips?—a case report. Acupunct Med. 2005;23:188-189.
THE CASE
A 31-year-old right-handed college baseball coach presented to his family physician (FP) with concerns about the “yips” in his right arm. His ability to throw a baseball had been gradually deteriorating. Involuntary upper right arm muscle contractions and spasms, which began intermittently when he was a teenager, were now a real problem for him as an adult. (See the video below.) The patient was having difficulty rolling a baseball underhand to players as part of infield practice and he was experiencing muscle spasms when lifting his right arm over his head. “Twitches” in the patient’s upper arm were making drinking difficult, but he had no problems feeding himself, writing, or performing other basic activities of daily living.
The patient experienced the same symptoms whether it was baseball season or not. He hadn’t noticed a change in symptoms with caffeine and denied use of any other stimulants in the last 4 years. His symptoms didn’t improve or worsen with greater or lesser quantity or quality of sleep or when he concentrated on stifling the involuntary movements. He had attempted to learn to throw left-handed to overcome the impairment, but was concerned that the same problem would occur in his left arm.
The patient had previously worked with a sports psychologist and hypnotherapist to overcome any potential subconscious performance anxiety, but this hadn’t helped. Stretching and strengthening with a physical therapist and numerous sessions with an acupuncturist hadn’t helped either. Despite this, he believed the problem to be primarily psychological.
The patient’s history included mild attention deficit disorder and exercise-induced asthma; his family history was negative for any movement or psychiatric disorders. He had 2 dislocation repairs on his left, non-throwing shoulder in his early twenties. His medications included fluticasone-salmeterol twice daily and albuterol, as needed.
The patient denied myalgia or arthralgia, decreased passive range of motion, shoulder or arm weakness, swelling, or muscle atrophy. He also didn’t have paresthesias in his right arm or hand, a resting tremor, difficulty moving (other than drinking from a cup), difficulty moving other extremities, dizziness, imbalance, or seizures.
The patient’s vital signs were normal. He had full range of motion and 5 out of 5 strength without pain during right shoulder abduction, external and internal rotation, an empty can test, a lower back lift off (Gerber’s) test, and a test of bicep and tricep strength, along with negative Neer and Hawkins tests.
There was no evidence of muscle wasting or asymmetry in the bilateral upper extremities. The patient’s deep tendon reflex grade was 2+ out of 4 in both of his arms. He didn’t have a sensory deficit to light touch in areas of C5 to T1 and he had normal cranial nerves II to XII. He had normal rapid alternating movements, heel-to-shin testing, and finger-to-nose testing, as well as a normal gait and Romberg test.
The patient provided a video showing the abnormal involuntary flexion of his shoulder when attempting to throw a baseball.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
THE DIAGNOSIS
The patient’s FP was aware of the “yips,” a condition that is commonly viewed as psychological or related to performance anxiety. (The “yips” are colloquially known as “Steve Blass Disease”—named after a Pittsburgh Pirates pitcher who suddenly lost the ability to control his pitches.1) But based on the patient’s clinical presentation and history of seeing a number of mental health care providers—in addition to his worsening symptoms—the FP ordered magnetic resonance imaging (MRI) of the brain. The MRI turned out to be unremarkable, so the patient was referred to Neurology.
In the general neurology clinic, a diagnosis of Wilson’s disease (a condition that leads to excess copper deposition in multiple organ systems, including the nervous system) was considered, as it can cause symptoms similar to those our patient was experiencing. However, a complete blood count, complete metabolic panel, antinuclear antibody test, ceruloplasmin test, and copper level were all normal, effectively ruling it out. An MRI of the cervical spine showed mild to moderate right foraminal stenosis at C3-4 and C5-6, but this did not explain the patient’s symptoms.
A diagnosis of paroxysmal exercise-induced dystonia was also considered at the time of the initial work-up, as our patient’s symptoms were most pronounced during physical activity. But this condition usually responds to antiepileptics, and carbamazepine and phenytoin were each tried for multiple months early in his evaluation without benefit.
3 factors led to a diagnosis of focal limb dystonia: Only our patient’s right arm was affected, his laboratory and imaging work-ups were negative, and he didn’t respond to antiepileptic treatment. Characterization of a movement disorder is based upon phenomenology. In this case, the patient had sustained abnormal posturing at the shoulder during right upper limb activation, which was only triggered with specific voluntary actions. This was consistent with dystonia, a movement disorder characterized by sustained or intermittent muscle contractions causing abnormal movements and/or postures—often initiated or worsened by voluntary action.2
DISCUSSION
The “yips,” or intermittent, transient tremors, jerks, or spasms3 that are seen in athletes, are well-documented in the lay press, but haven’t been significantly addressed in the medical literature.4 Stigma surrounding the condition among athletes likely leads to under-reporting. Athletes typically experience yips with fine motor movements, such as short putts in golf and pitching in baseball. In fact, while the majority of the medical literature on yips revolves around golfers, many talented baseball players have had their careers altered by the condition. The yips may also affect movements in sports like darts, cricket, table tennis, and billiards.
In 1984, dystonia was defined as a disorder of sensorimotor integration that results in co-contraction of agonist/antagonist muscles, and may be characterized by state dependence (exacerbation with specific activities) or sensory tricks (amelioration with specific types of sensory input).5 In 2013, the definition was revised to remove “co-contraction” from the definition because phenomenology alone is sufficient to make the diagnosis.1
Many athletes and sports fans believe the yips are caused by performance anxiety or related phobias, but evidence suggests that many athletes with the movement disorder may actually have focal limb dystonia.6,7 The yips can, however, lead to performance anxiety,3 but there has been no difference noted between the anxiety level of golfers with or without the yips.7 Psychological treatment approaches are commonly employed, but surface electromyograms have shown abnormal co-contraction of wrist flexor and extensor muscles in 5 out of 10 golfers with the yips (but 0 of those without) while putting—which is consistent with focal limb dystonia.8
Botulinum toxin injections are Tx of choice, but can cause weakness
Muscle relaxers, such as baclofen and benzodiazepines, as well as dopamine antagonists, can ameliorate dystonia.9 Focal limb dystonia may also respond to the antispasmodic trihexyphenidyl, but the dose must often be limited due to adverse effects such as nausea, dizziness, and anxiety.10
Botulinum toxin injections have proven effective for focal limb dystonia11 and are considered the treatment of choice. However, there are few reports on their use in athletes, where the adverse effect of weakness could affect performance. One case report also showed improvement of yips with acupuncture, although this has not been extensively studied.12
Our patient didn’t respond to low-dose (2 mg twice a day) trihexyphenidyl. Tetrabenazine, a dopamine depletor frequently used for hyperkinetic disorders, was not effective at 25 mg taken prior to coaching sessions. Higher doses of an anticholinergic could have been effective, but the patient declined our recommendation to pursue this (or botulinum toxin injections). He decided instead to train himself to use his left arm while coaching.
THE TAKEAWAY
Athletes who play sports that require precision movements commonly develop the yips. While the prevailing theory among athletes is that this is a psychological phenomenon, evidence shows that this may in fact be a neurologic focal dystonia caused by repetitive use. Greater awareness of yips as a possible organic, treatable neurologic condition is needed in order to stimulate more research on this topic.
THE CASE
A 31-year-old right-handed college baseball coach presented to his family physician (FP) with concerns about the “yips” in his right arm. His ability to throw a baseball had been gradually deteriorating. Involuntary upper right arm muscle contractions and spasms, which began intermittently when he was a teenager, were now a real problem for him as an adult. (See the video below.) The patient was having difficulty rolling a baseball underhand to players as part of infield practice and he was experiencing muscle spasms when lifting his right arm over his head. “Twitches” in the patient’s upper arm were making drinking difficult, but he had no problems feeding himself, writing, or performing other basic activities of daily living.
The patient experienced the same symptoms whether it was baseball season or not. He hadn’t noticed a change in symptoms with caffeine and denied use of any other stimulants in the last 4 years. His symptoms didn’t improve or worsen with greater or lesser quantity or quality of sleep or when he concentrated on stifling the involuntary movements. He had attempted to learn to throw left-handed to overcome the impairment, but was concerned that the same problem would occur in his left arm.
The patient had previously worked with a sports psychologist and hypnotherapist to overcome any potential subconscious performance anxiety, but this hadn’t helped. Stretching and strengthening with a physical therapist and numerous sessions with an acupuncturist hadn’t helped either. Despite this, he believed the problem to be primarily psychological.
The patient’s history included mild attention deficit disorder and exercise-induced asthma; his family history was negative for any movement or psychiatric disorders. He had 2 dislocation repairs on his left, non-throwing shoulder in his early twenties. His medications included fluticasone-salmeterol twice daily and albuterol, as needed.
The patient denied myalgia or arthralgia, decreased passive range of motion, shoulder or arm weakness, swelling, or muscle atrophy. He also didn’t have paresthesias in his right arm or hand, a resting tremor, difficulty moving (other than drinking from a cup), difficulty moving other extremities, dizziness, imbalance, or seizures.
The patient’s vital signs were normal. He had full range of motion and 5 out of 5 strength without pain during right shoulder abduction, external and internal rotation, an empty can test, a lower back lift off (Gerber’s) test, and a test of bicep and tricep strength, along with negative Neer and Hawkins tests.
There was no evidence of muscle wasting or asymmetry in the bilateral upper extremities. The patient’s deep tendon reflex grade was 2+ out of 4 in both of his arms. He didn’t have a sensory deficit to light touch in areas of C5 to T1 and he had normal cranial nerves II to XII. He had normal rapid alternating movements, heel-to-shin testing, and finger-to-nose testing, as well as a normal gait and Romberg test.
The patient provided a video showing the abnormal involuntary flexion of his shoulder when attempting to throw a baseball.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
THE DIAGNOSIS
The patient’s FP was aware of the “yips,” a condition that is commonly viewed as psychological or related to performance anxiety. (The “yips” are colloquially known as “Steve Blass Disease”—named after a Pittsburgh Pirates pitcher who suddenly lost the ability to control his pitches.1) But based on the patient’s clinical presentation and history of seeing a number of mental health care providers—in addition to his worsening symptoms—the FP ordered magnetic resonance imaging (MRI) of the brain. The MRI turned out to be unremarkable, so the patient was referred to Neurology.
In the general neurology clinic, a diagnosis of Wilson’s disease (a condition that leads to excess copper deposition in multiple organ systems, including the nervous system) was considered, as it can cause symptoms similar to those our patient was experiencing. However, a complete blood count, complete metabolic panel, antinuclear antibody test, ceruloplasmin test, and copper level were all normal, effectively ruling it out. An MRI of the cervical spine showed mild to moderate right foraminal stenosis at C3-4 and C5-6, but this did not explain the patient’s symptoms.
A diagnosis of paroxysmal exercise-induced dystonia was also considered at the time of the initial work-up, as our patient’s symptoms were most pronounced during physical activity. But this condition usually responds to antiepileptics, and carbamazepine and phenytoin were each tried for multiple months early in his evaluation without benefit.
3 factors led to a diagnosis of focal limb dystonia: Only our patient’s right arm was affected, his laboratory and imaging work-ups were negative, and he didn’t respond to antiepileptic treatment. Characterization of a movement disorder is based upon phenomenology. In this case, the patient had sustained abnormal posturing at the shoulder during right upper limb activation, which was only triggered with specific voluntary actions. This was consistent with dystonia, a movement disorder characterized by sustained or intermittent muscle contractions causing abnormal movements and/or postures—often initiated or worsened by voluntary action.2
DISCUSSION
The “yips,” or intermittent, transient tremors, jerks, or spasms3 that are seen in athletes, are well-documented in the lay press, but haven’t been significantly addressed in the medical literature.4 Stigma surrounding the condition among athletes likely leads to under-reporting. Athletes typically experience yips with fine motor movements, such as short putts in golf and pitching in baseball. In fact, while the majority of the medical literature on yips revolves around golfers, many talented baseball players have had their careers altered by the condition. The yips may also affect movements in sports like darts, cricket, table tennis, and billiards.
In 1984, dystonia was defined as a disorder of sensorimotor integration that results in co-contraction of agonist/antagonist muscles, and may be characterized by state dependence (exacerbation with specific activities) or sensory tricks (amelioration with specific types of sensory input).5 In 2013, the definition was revised to remove “co-contraction” from the definition because phenomenology alone is sufficient to make the diagnosis.1
Many athletes and sports fans believe the yips are caused by performance anxiety or related phobias, but evidence suggests that many athletes with the movement disorder may actually have focal limb dystonia.6,7 The yips can, however, lead to performance anxiety,3 but there has been no difference noted between the anxiety level of golfers with or without the yips.7 Psychological treatment approaches are commonly employed, but surface electromyograms have shown abnormal co-contraction of wrist flexor and extensor muscles in 5 out of 10 golfers with the yips (but 0 of those without) while putting—which is consistent with focal limb dystonia.8
Botulinum toxin injections are Tx of choice, but can cause weakness
Muscle relaxers, such as baclofen and benzodiazepines, as well as dopamine antagonists, can ameliorate dystonia.9 Focal limb dystonia may also respond to the antispasmodic trihexyphenidyl, but the dose must often be limited due to adverse effects such as nausea, dizziness, and anxiety.10
Botulinum toxin injections have proven effective for focal limb dystonia11 and are considered the treatment of choice. However, there are few reports on their use in athletes, where the adverse effect of weakness could affect performance. One case report also showed improvement of yips with acupuncture, although this has not been extensively studied.12
Our patient didn’t respond to low-dose (2 mg twice a day) trihexyphenidyl. Tetrabenazine, a dopamine depletor frequently used for hyperkinetic disorders, was not effective at 25 mg taken prior to coaching sessions. Higher doses of an anticholinergic could have been effective, but the patient declined our recommendation to pursue this (or botulinum toxin injections). He decided instead to train himself to use his left arm while coaching.
THE TAKEAWAY
Athletes who play sports that require precision movements commonly develop the yips. While the prevailing theory among athletes is that this is a psychological phenomenon, evidence shows that this may in fact be a neurologic focal dystonia caused by repetitive use. Greater awareness of yips as a possible organic, treatable neurologic condition is needed in order to stimulate more research on this topic.
1. Baseball’s head cases often prove baffling. USA Today Baseball Weekly. 2001. Available at: http://usatoday30.usatoday.com/sports/bbw/2001-02-07/2001-02-07-head.htm. Accessed March 15, 2017.
2. Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 2013;28:863-873.
3. Dhungana S, Jankovic J. Yips and other movement disorders in golfers. Mov Disord. 2013;28:576-581.
4. Stacy MA, ed. Handbook of dystonia. New York, NY: Informa Healthcare USA, Inc; 2007.
5. Fahn S, Marsden CD, Calne DB. Classification and investigation of dystonia. In: Marsden CD, Fahn S, eds. Movement disorders 2. London: Butterworths; 1987:332-358.
6. Smith AM, Adler CH, Crews D, et al. The ‘yips’ in golf: a continuum between a focal dystonia and choking. Sports Med. 2003;33:13-31.
7. Sachdev P. Golfers’ cramp: clinical characteristics and evidence against it being an anxiety disorder. Mov Disord. 1992;7:326-332.
8. Adler CH, Crews D, Hentz JG, et al. Abnormal co-contraction in yips-affected but not unaffected golfers: evidence for focal dystonia. Neurology. 2005;64:1813-1814.
9. Jankovic J. Treatment of hyperkinetic movement disorders. Lancet Neurol. 2009;8:844-856.
10. Jankovic J. Treatment of dystonia. Lancet Neurol. 2006;5:864-872.
11. Lungu C, Karp BI, Alter K, et al. Long-term follow-up of botulinum toxin therapy for focal hand dystonia: outcome at 10 years or more. Mov Disord. 2011;26:750-753.
12. Rosted P. Acupuncture for treatment of the yips?—a case report. Acupunct Med. 2005;23:188-189.
1. Baseball’s head cases often prove baffling. USA Today Baseball Weekly. 2001. Available at: http://usatoday30.usatoday.com/sports/bbw/2001-02-07/2001-02-07-head.htm. Accessed March 15, 2017.
2. Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 2013;28:863-873.
3. Dhungana S, Jankovic J. Yips and other movement disorders in golfers. Mov Disord. 2013;28:576-581.
4. Stacy MA, ed. Handbook of dystonia. New York, NY: Informa Healthcare USA, Inc; 2007.
5. Fahn S, Marsden CD, Calne DB. Classification and investigation of dystonia. In: Marsden CD, Fahn S, eds. Movement disorders 2. London: Butterworths; 1987:332-358.
6. Smith AM, Adler CH, Crews D, et al. The ‘yips’ in golf: a continuum between a focal dystonia and choking. Sports Med. 2003;33:13-31.
7. Sachdev P. Golfers’ cramp: clinical characteristics and evidence against it being an anxiety disorder. Mov Disord. 1992;7:326-332.
8. Adler CH, Crews D, Hentz JG, et al. Abnormal co-contraction in yips-affected but not unaffected golfers: evidence for focal dystonia. Neurology. 2005;64:1813-1814.
9. Jankovic J. Treatment of hyperkinetic movement disorders. Lancet Neurol. 2009;8:844-856.
10. Jankovic J. Treatment of dystonia. Lancet Neurol. 2006;5:864-872.
11. Lungu C, Karp BI, Alter K, et al. Long-term follow-up of botulinum toxin therapy for focal hand dystonia: outcome at 10 years or more. Mov Disord. 2011;26:750-753.
12. Rosted P. Acupuncture for treatment of the yips?—a case report. Acupunct Med. 2005;23:188-189.
Choice of protease inhibitor may impact CVD risk in HIV+ patients
SEATTLE – A study of newer generation protease inhibitors (PIs) suggests that ritonavir-boosted atazanavir (ATV/r) has a better cardiovascular safety profile than ritonavir-boosted darunavir (DRV/r). After adjustment for age and other factors, patients taking DRV/r had a higher incidence rate ratio of cardiovascular disease during a 5-year follow-up compared to pre-exposure, while no such increase was seen in patients taking ATV/r.
Older protease inhibitors had been shown to be associated with increased CVD risk, but the newer generation of drugs had not been similarly examined. A previous analysis of the D:A:D (Data collection on Adverse events of Anti-HIV Drugs) study had shown no effect of ATV/r with cardiovascular risk, but the follow-up time was short.
The data suggest that ATV/r may be the best choice for patients at heightened risk of cardiovascular disease. “This is just the results from the first five years, and we need to reassess at some point, but according to what we know now, it does really look like a quite real effect,” Dr Ryom added.
The researchers examined longer-term results from 35,711 (47.8% white, 73.6% men, median age 44) participants in the D:A:D study, who were followed beginning January 1, 2009 through the earliest CVD diagnosis, last visit plus 6 months, or February 1, 2016.
After adjustment for baseline variables potentially on the causal pathway between PI use and cardiovascular disease, the researchers found that, compared to baseline pre-exposure levels, patients taking ATV/r had a 5-year incidence rate ratio (IRR) of CVD of 1.03 (95% confidence interval 0.90-1.18), while those taking DRV/r had an IRR 1.59 (95% CI, 1.33-1.91).
Time-updated adjustment analyses for factors potentially on the causal pathway made no meaningful difference in the calculation of incidence risk ratios. “This suggests that the association we say for boosted darunavir is not moderated by dyslipidemia, which is interesting and in contrast to what we saw in first-generation protease inhibitors,” Dr Ryom said during her talk.
Adjusting for bilirubin levels had no impact on the associations.
There was no data on drug dose, and due to the observational nature of the study, the researchers could not prove a causal association between CVD risk and use of either drug, but the results were convincing enough for Dr Ryom to consider a drug that appears friendlier to the cardiovascular system, particularly in high risk patients, though she also noted that there is evidence that ATV/r could lead to kidney stones and chronic kidney disease. “So it’s very difficult to balance the different risks. You really need to tailor your choice according to the patient that is in front of you,” Dr Ryom said.
The study was funded by The Oversight Committee for the Evaluation of Metabolic Complications of HAART, which included both academic and industry sources, and the Danish National Research Foundation. Dr Ryom reported having no financial disclosures.
SEATTLE – A study of newer generation protease inhibitors (PIs) suggests that ritonavir-boosted atazanavir (ATV/r) has a better cardiovascular safety profile than ritonavir-boosted darunavir (DRV/r). After adjustment for age and other factors, patients taking DRV/r had a higher incidence rate ratio of cardiovascular disease during a 5-year follow-up compared to pre-exposure, while no such increase was seen in patients taking ATV/r.
Older protease inhibitors had been shown to be associated with increased CVD risk, but the newer generation of drugs had not been similarly examined. A previous analysis of the D:A:D (Data collection on Adverse events of Anti-HIV Drugs) study had shown no effect of ATV/r with cardiovascular risk, but the follow-up time was short.
The data suggest that ATV/r may be the best choice for patients at heightened risk of cardiovascular disease. “This is just the results from the first five years, and we need to reassess at some point, but according to what we know now, it does really look like a quite real effect,” Dr Ryom added.
The researchers examined longer-term results from 35,711 (47.8% white, 73.6% men, median age 44) participants in the D:A:D study, who were followed beginning January 1, 2009 through the earliest CVD diagnosis, last visit plus 6 months, or February 1, 2016.
After adjustment for baseline variables potentially on the causal pathway between PI use and cardiovascular disease, the researchers found that, compared to baseline pre-exposure levels, patients taking ATV/r had a 5-year incidence rate ratio (IRR) of CVD of 1.03 (95% confidence interval 0.90-1.18), while those taking DRV/r had an IRR 1.59 (95% CI, 1.33-1.91).
Time-updated adjustment analyses for factors potentially on the causal pathway made no meaningful difference in the calculation of incidence risk ratios. “This suggests that the association we say for boosted darunavir is not moderated by dyslipidemia, which is interesting and in contrast to what we saw in first-generation protease inhibitors,” Dr Ryom said during her talk.
Adjusting for bilirubin levels had no impact on the associations.
There was no data on drug dose, and due to the observational nature of the study, the researchers could not prove a causal association between CVD risk and use of either drug, but the results were convincing enough for Dr Ryom to consider a drug that appears friendlier to the cardiovascular system, particularly in high risk patients, though she also noted that there is evidence that ATV/r could lead to kidney stones and chronic kidney disease. “So it’s very difficult to balance the different risks. You really need to tailor your choice according to the patient that is in front of you,” Dr Ryom said.
The study was funded by The Oversight Committee for the Evaluation of Metabolic Complications of HAART, which included both academic and industry sources, and the Danish National Research Foundation. Dr Ryom reported having no financial disclosures.
SEATTLE – A study of newer generation protease inhibitors (PIs) suggests that ritonavir-boosted atazanavir (ATV/r) has a better cardiovascular safety profile than ritonavir-boosted darunavir (DRV/r). After adjustment for age and other factors, patients taking DRV/r had a higher incidence rate ratio of cardiovascular disease during a 5-year follow-up compared to pre-exposure, while no such increase was seen in patients taking ATV/r.
Older protease inhibitors had been shown to be associated with increased CVD risk, but the newer generation of drugs had not been similarly examined. A previous analysis of the D:A:D (Data collection on Adverse events of Anti-HIV Drugs) study had shown no effect of ATV/r with cardiovascular risk, but the follow-up time was short.
The data suggest that ATV/r may be the best choice for patients at heightened risk of cardiovascular disease. “This is just the results from the first five years, and we need to reassess at some point, but according to what we know now, it does really look like a quite real effect,” Dr Ryom added.
The researchers examined longer-term results from 35,711 (47.8% white, 73.6% men, median age 44) participants in the D:A:D study, who were followed beginning January 1, 2009 through the earliest CVD diagnosis, last visit plus 6 months, or February 1, 2016.
After adjustment for baseline variables potentially on the causal pathway between PI use and cardiovascular disease, the researchers found that, compared to baseline pre-exposure levels, patients taking ATV/r had a 5-year incidence rate ratio (IRR) of CVD of 1.03 (95% confidence interval 0.90-1.18), while those taking DRV/r had an IRR 1.59 (95% CI, 1.33-1.91).
Time-updated adjustment analyses for factors potentially on the causal pathway made no meaningful difference in the calculation of incidence risk ratios. “This suggests that the association we say for boosted darunavir is not moderated by dyslipidemia, which is interesting and in contrast to what we saw in first-generation protease inhibitors,” Dr Ryom said during her talk.
Adjusting for bilirubin levels had no impact on the associations.
There was no data on drug dose, and due to the observational nature of the study, the researchers could not prove a causal association between CVD risk and use of either drug, but the results were convincing enough for Dr Ryom to consider a drug that appears friendlier to the cardiovascular system, particularly in high risk patients, though she also noted that there is evidence that ATV/r could lead to kidney stones and chronic kidney disease. “So it’s very difficult to balance the different risks. You really need to tailor your choice according to the patient that is in front of you,” Dr Ryom said.
The study was funded by The Oversight Committee for the Evaluation of Metabolic Complications of HAART, which included both academic and industry sources, and the Danish National Research Foundation. Dr Ryom reported having no financial disclosures.
AT CROI 2017
Key clinical point: Atazanavir may be safer for HIV-positive patients at high CVD risk.
Major finding: Patients taking darunavir had a 59% higher incidence of cardiovascular disease than those taking atazanavir.
Data source: Retrospective analysis of 35,711 patients.
Disclosures: The study was funded by The Oversight Committee for the Evaluation of Metabolic Complications of HAART, which included both academic and industry sources, and the Danish National Research Foundation. Dr Ryom reported having no financial disclosures.
In type 2 diabetes, chronotype may affect depressive symptoms
ORLANDO – For patients with type 2 diabetes, larks may have an edge on owls when it comes to mood, according to a new study. Significantly more depressive symptoms were seen in owls – individuals whose sleep time preference, or chronotype, was later-to-bed and later-to-rise – in a cohort of 476 patients with type 2 diabetes.
In work that accounted for regional differences in chronotype by drawing from two different geographic regions, a later chronotype was associated with a higher score on the Center for Epidemiologic Studies Depression scale (CES-D), after controlling for potentially confounding factors (P = .045 for each cohort).
The study’s senior author, Sirimon Reutrakul, MD, of the faculty of medicine at Mahidol University, Bangkok, Thailand, said that previous work had also shown that when depression in patients with diabetes is untreated, self-care and blood glucose control can suffer, and more complications of diabetes are seen.
Dr. Reutrakul’s facility teamed with Rush University School of Medicine in Chicago, administering questionnaires designed to assess where patients fell along the morningness-eveningness scale. For the Chicago cohort (n = 194, 70% female), the Morningness-Eveningness Questionnaire (MEQ) was administered, while the Thailand cohort (n = 282, 57% female) completed the Composite Score of Morningness (CSM). Both groups completed the CES-D to assess depressive symptoms and the Pittsburgh Sleep Quality Index (PSQI); hemoglobin A1c values for both groups were ascertained by medical record review.
The mean age for the two cohorts was similar (Chicago, 58.3 plus or minus 13.0 years; Thailand, 55.7 plus or minus 11.6 years). The Chicago patients who scored higher on the CES-D, indicating that they had more depressive symptoms, were more likely to have a later chronotype on unadjusted analysis (P = .001). They were also likely to be younger (P = .005) and to have poorer sleep quality (P less than .001). Patients with more depressive symptoms were significantly more likely to be female, non-white, to use insulin, and to have poor glycemic control.
For the patients in Thailand, more depressive symptoms were also associated with a later chronotype on unadjusted analysis (P less than .001); younger age (P = .019), and poor sleep quality (P less than .001) were also associated with more depressive symptoms. Correction for a number of potentially confounding variables yielded modest statistical significance (P = .045) of the depressive symptoms-later chronotype relationship in both cohorts.
The Chicago patients were at approximately latitude 42N, while the patients in Thailand were at about latitude 13N, much closer to the equator. Populations that live closer to the equator, where days and nights are of approximately equal length year-round, tend to have more larks than owls, wrote Dr. Reutrakul and her colleagues.
In these two cohorts, which differed both by ethnic composition and geographic distance from the equator, “later chronotype was found to be independently associated with depressive symptoms” for patients with type 2 diabetes, wrote Dr. Reutrakul and her coauthors in the abstract accompanying the presentation. “In addition, sleep disturbances, more prevalent in evening types, have been associated with depression,” which is common in type 2 diabetes, the investigators wrote.
Dr. Reutrakul acknowledged that the work found an “only modest,” though consistent, association between eveningness and depressive symptoms. “We need further research to explore a combination of interventions that help with circadian timing, such as light therapy and melatonin,” she said in a press statement preceding her presentation. “Learning more about the relationship between depression and circadian functioning might help us figure out strategies to improve physical and mental health for patients with diabetes…We need further research to explore a combination of interventions that help with circadian timing, such as light therapy and melatonin,” Dr. Reutrakul said.
Dr. Reutrakul reported financial relationships with Medtronic Minimed, Novo Nordisk, Merck, and Sanofi.
[email protected]
On Twitter @karioakes
ORLANDO – For patients with type 2 diabetes, larks may have an edge on owls when it comes to mood, according to a new study. Significantly more depressive symptoms were seen in owls – individuals whose sleep time preference, or chronotype, was later-to-bed and later-to-rise – in a cohort of 476 patients with type 2 diabetes.
In work that accounted for regional differences in chronotype by drawing from two different geographic regions, a later chronotype was associated with a higher score on the Center for Epidemiologic Studies Depression scale (CES-D), after controlling for potentially confounding factors (P = .045 for each cohort).
The study’s senior author, Sirimon Reutrakul, MD, of the faculty of medicine at Mahidol University, Bangkok, Thailand, said that previous work had also shown that when depression in patients with diabetes is untreated, self-care and blood glucose control can suffer, and more complications of diabetes are seen.
Dr. Reutrakul’s facility teamed with Rush University School of Medicine in Chicago, administering questionnaires designed to assess where patients fell along the morningness-eveningness scale. For the Chicago cohort (n = 194, 70% female), the Morningness-Eveningness Questionnaire (MEQ) was administered, while the Thailand cohort (n = 282, 57% female) completed the Composite Score of Morningness (CSM). Both groups completed the CES-D to assess depressive symptoms and the Pittsburgh Sleep Quality Index (PSQI); hemoglobin A1c values for both groups were ascertained by medical record review.
The mean age for the two cohorts was similar (Chicago, 58.3 plus or minus 13.0 years; Thailand, 55.7 plus or minus 11.6 years). The Chicago patients who scored higher on the CES-D, indicating that they had more depressive symptoms, were more likely to have a later chronotype on unadjusted analysis (P = .001). They were also likely to be younger (P = .005) and to have poorer sleep quality (P less than .001). Patients with more depressive symptoms were significantly more likely to be female, non-white, to use insulin, and to have poor glycemic control.
For the patients in Thailand, more depressive symptoms were also associated with a later chronotype on unadjusted analysis (P less than .001); younger age (P = .019), and poor sleep quality (P less than .001) were also associated with more depressive symptoms. Correction for a number of potentially confounding variables yielded modest statistical significance (P = .045) of the depressive symptoms-later chronotype relationship in both cohorts.
The Chicago patients were at approximately latitude 42N, while the patients in Thailand were at about latitude 13N, much closer to the equator. Populations that live closer to the equator, where days and nights are of approximately equal length year-round, tend to have more larks than owls, wrote Dr. Reutrakul and her colleagues.
In these two cohorts, which differed both by ethnic composition and geographic distance from the equator, “later chronotype was found to be independently associated with depressive symptoms” for patients with type 2 diabetes, wrote Dr. Reutrakul and her coauthors in the abstract accompanying the presentation. “In addition, sleep disturbances, more prevalent in evening types, have been associated with depression,” which is common in type 2 diabetes, the investigators wrote.
Dr. Reutrakul acknowledged that the work found an “only modest,” though consistent, association between eveningness and depressive symptoms. “We need further research to explore a combination of interventions that help with circadian timing, such as light therapy and melatonin,” she said in a press statement preceding her presentation. “Learning more about the relationship between depression and circadian functioning might help us figure out strategies to improve physical and mental health for patients with diabetes…We need further research to explore a combination of interventions that help with circadian timing, such as light therapy and melatonin,” Dr. Reutrakul said.
Dr. Reutrakul reported financial relationships with Medtronic Minimed, Novo Nordisk, Merck, and Sanofi.
[email protected]
On Twitter @karioakes
ORLANDO – For patients with type 2 diabetes, larks may have an edge on owls when it comes to mood, according to a new study. Significantly more depressive symptoms were seen in owls – individuals whose sleep time preference, or chronotype, was later-to-bed and later-to-rise – in a cohort of 476 patients with type 2 diabetes.
In work that accounted for regional differences in chronotype by drawing from two different geographic regions, a later chronotype was associated with a higher score on the Center for Epidemiologic Studies Depression scale (CES-D), after controlling for potentially confounding factors (P = .045 for each cohort).
The study’s senior author, Sirimon Reutrakul, MD, of the faculty of medicine at Mahidol University, Bangkok, Thailand, said that previous work had also shown that when depression in patients with diabetes is untreated, self-care and blood glucose control can suffer, and more complications of diabetes are seen.
Dr. Reutrakul’s facility teamed with Rush University School of Medicine in Chicago, administering questionnaires designed to assess where patients fell along the morningness-eveningness scale. For the Chicago cohort (n = 194, 70% female), the Morningness-Eveningness Questionnaire (MEQ) was administered, while the Thailand cohort (n = 282, 57% female) completed the Composite Score of Morningness (CSM). Both groups completed the CES-D to assess depressive symptoms and the Pittsburgh Sleep Quality Index (PSQI); hemoglobin A1c values for both groups were ascertained by medical record review.
The mean age for the two cohorts was similar (Chicago, 58.3 plus or minus 13.0 years; Thailand, 55.7 plus or minus 11.6 years). The Chicago patients who scored higher on the CES-D, indicating that they had more depressive symptoms, were more likely to have a later chronotype on unadjusted analysis (P = .001). They were also likely to be younger (P = .005) and to have poorer sleep quality (P less than .001). Patients with more depressive symptoms were significantly more likely to be female, non-white, to use insulin, and to have poor glycemic control.
For the patients in Thailand, more depressive symptoms were also associated with a later chronotype on unadjusted analysis (P less than .001); younger age (P = .019), and poor sleep quality (P less than .001) were also associated with more depressive symptoms. Correction for a number of potentially confounding variables yielded modest statistical significance (P = .045) of the depressive symptoms-later chronotype relationship in both cohorts.
The Chicago patients were at approximately latitude 42N, while the patients in Thailand were at about latitude 13N, much closer to the equator. Populations that live closer to the equator, where days and nights are of approximately equal length year-round, tend to have more larks than owls, wrote Dr. Reutrakul and her colleagues.
In these two cohorts, which differed both by ethnic composition and geographic distance from the equator, “later chronotype was found to be independently associated with depressive symptoms” for patients with type 2 diabetes, wrote Dr. Reutrakul and her coauthors in the abstract accompanying the presentation. “In addition, sleep disturbances, more prevalent in evening types, have been associated with depression,” which is common in type 2 diabetes, the investigators wrote.
Dr. Reutrakul acknowledged that the work found an “only modest,” though consistent, association between eveningness and depressive symptoms. “We need further research to explore a combination of interventions that help with circadian timing, such as light therapy and melatonin,” she said in a press statement preceding her presentation. “Learning more about the relationship between depression and circadian functioning might help us figure out strategies to improve physical and mental health for patients with diabetes…We need further research to explore a combination of interventions that help with circadian timing, such as light therapy and melatonin,” Dr. Reutrakul said.
Dr. Reutrakul reported financial relationships with Medtronic Minimed, Novo Nordisk, Merck, and Sanofi.
[email protected]
On Twitter @karioakes
AT ENDO 2017
Key clinical point:
Major finding: Later chronotype was associated with more depressive symptoms in 2 cohorts with type 2 diabetes (P = .045)
Data source: Cross-sectional study of 476 patients with type 2 diabetes in Chicago and Thailand..
Disclosures: Dr. Reutrakul reported financial relationships with Medtronic Minimed, Merck, Novo Nordisk, and Sanofi.
What do doctors want from health reform?
With the demise of Republican repeal and replace legislation, analysts say the landscape is ripe for repairs to the Affordable Care Act or for additional legislation that both political parties could support. So what do physicians want from health reform?
The first step should be stabilizing the health insurance marketplaces by strengthening and perhaps extending risk mitigation measures such as the risk adjustment, risk corridors, and reinsurance provisions of the law, said Patricia Salber, MD, an internist and health care consultant who blogs at TheDoctorWeighsIn.com. Those three ACA provisions were intended to promote insurer competition on the basis of quality and value and promote insurance market stability.
Keeping premiums at manageable levels for patients should also be addressed, said William J. Burke, DO, dean of Ohio University Heritage College of Osteopathic Medicine.
That was echoed in a poll taken by this news organization. Of 390 respondents, fully half (50%) said they would repair the ACA by stabilizing premiums and out-of-pocket costs for patients as of April 2. About 11% stated they would increase payment rates for care provided to Medicaid patients, and 10% said they would return the primary care incentive payment. About 9% of those surveyed would address workforce issues exacerbated by more patients in the system.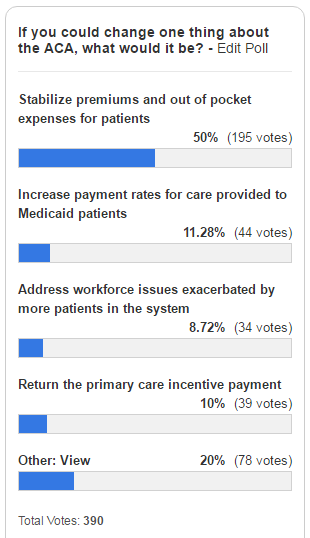
Improving reimbursement for Medicaid services is a necessary health reform change, agreed Diane J. Horvath-Cosper MD, an obstetrician-gynecologist and reproductive health advocacy fellow for Physicians for Reproductive Health, a reproductive rights advocacy organization.
“Reimbursement rates are so low that sometimes [physicians] have to limit the number of Medicaid patients to be able to pay staff,” Dr. Horvath said in an interview. “That’s a terrible position to put physicians in because we want to be able to see as many people who want to see us.”
Speaking of Medicaid, Dr. Salber adds that governors should be encouraged to continue expanding Medicaid to eliminate the coverage gap for the “near poor” that exists in states that did not participate in the expansion.
“Now that the [American Health Care Act] has failed, I think we will see some expansion take place organically even in states that were deeply opposed before,” she said.
“The volume of prior authorizations that all physicians face, but especially primary care physicians, is huge,” Dr. Munger said in an interview. “In many cases, we’re having to hire extra staff just to handle all of the prior authorizations. Every patient may not just have one prior authorization, but they may require two or three or four prior authorizations each month or quarterly. It really detracts from meaningful time you can spend with the patient.”
For starters, doctors should provide care to patients based on mutually agreed terms and without the interference of insurers, Dr. Orient said in an interview. In such a private medicine system, patients would pay doctors for services, and patients would then file claims with their insurer for reimbursement. Similarly, physicians should not be at the mercy of Medicare for payment, Dr. Orient said.
“Doctors can sign away their rights if they want in a Medicare participation agreement,” she said. “Doctors who do not sign the agreement to take assignment in all cases doctors should be freed of price controls and coding demands. Their patients should be allowed to file their own simple claims to Medicare with an itemized bill as they did before the 1990s law that requires physicians to submit the claims. Non-participating doctors should be exempted from MACRA [the Medicare Access and CHIP Reauthorization Act], and without the price controls, there is no need for [Recovery Audit Contractors] and other auditors.”
While contraceptive care was strengthened by the ACA, Dr. Horvath said further efforts should be made to improve coverage and level the playing field for reproductive medicine. In addition, she said that abortion should be treated a valid medical procedure, rather than parsed out, and both public and private insurers should be required to pay for the procedure, she said.
“I would love to see strengthened provisions for contraception coverage,” Dr. Horvath said. “[We need to] make sure that doesn’t get bargained away. The other thing is to expand coverage and make sure every method is covered, not just one method in each category.”
However, Robert Doherty, ACP senior vice president of governmental affairs and public policy, said the college is concerned that the current administration may fail to maintain the ACA now that its proposed repeal law has fallen through.
Without aggressively pushing ACA enrollment for younger patients and continued support for the individual mandate, more insurers may pull out of the marketplaces, and the ACA could implode, Mr. Doherty said.
“There are a number of ways that Republicans could either make things better or worse with action or inaction,” Mr. Doherty said during the press conference. “The insurance [companies] have gone to this administration with a wish list of things that will help keep them in the market. What remains to be seen is whether this administration is going to be receptive. If they don’t aggressively enforce the requirement that people buy coverage, more younger people will opt out and stay out until they get sick. That would make the problem of adverse selection even worse and could create the death cycle for insurance.”
Dr. Price consistently answered that Americans should be able to select the kinds of coverage they want. What “we believe is that individuals ought to be able to have access to the kind of coverage that they select for themselves and for their families and not what the government forces them to buy,” Dr. Price testified, echoing the message from his confirmation hearings.
He was also pressed on issues such as the individual mandate, and while noting that it is his duty to uphold the law of the land, he also remained noncommittal in answering questions about whether he would direct the agency to enforce the individual mandate. The first executive order from President Trump beginning his administration gave the agency discretion to not enforce mandates if they caused harm.
[email protected]
On Twitter @legal_med
Gregory Twachtman contributed to this report.
With the demise of Republican repeal and replace legislation, analysts say the landscape is ripe for repairs to the Affordable Care Act or for additional legislation that both political parties could support. So what do physicians want from health reform?
The first step should be stabilizing the health insurance marketplaces by strengthening and perhaps extending risk mitigation measures such as the risk adjustment, risk corridors, and reinsurance provisions of the law, said Patricia Salber, MD, an internist and health care consultant who blogs at TheDoctorWeighsIn.com. Those three ACA provisions were intended to promote insurer competition on the basis of quality and value and promote insurance market stability.
Keeping premiums at manageable levels for patients should also be addressed, said William J. Burke, DO, dean of Ohio University Heritage College of Osteopathic Medicine.
That was echoed in a poll taken by this news organization. Of 390 respondents, fully half (50%) said they would repair the ACA by stabilizing premiums and out-of-pocket costs for patients as of April 2. About 11% stated they would increase payment rates for care provided to Medicaid patients, and 10% said they would return the primary care incentive payment. About 9% of those surveyed would address workforce issues exacerbated by more patients in the system.
Improving reimbursement for Medicaid services is a necessary health reform change, agreed Diane J. Horvath-Cosper MD, an obstetrician-gynecologist and reproductive health advocacy fellow for Physicians for Reproductive Health, a reproductive rights advocacy organization.
“Reimbursement rates are so low that sometimes [physicians] have to limit the number of Medicaid patients to be able to pay staff,” Dr. Horvath said in an interview. “That’s a terrible position to put physicians in because we want to be able to see as many people who want to see us.”
Speaking of Medicaid, Dr. Salber adds that governors should be encouraged to continue expanding Medicaid to eliminate the coverage gap for the “near poor” that exists in states that did not participate in the expansion.
“Now that the [American Health Care Act] has failed, I think we will see some expansion take place organically even in states that were deeply opposed before,” she said.
“The volume of prior authorizations that all physicians face, but especially primary care physicians, is huge,” Dr. Munger said in an interview. “In many cases, we’re having to hire extra staff just to handle all of the prior authorizations. Every patient may not just have one prior authorization, but they may require two or three or four prior authorizations each month or quarterly. It really detracts from meaningful time you can spend with the patient.”
For starters, doctors should provide care to patients based on mutually agreed terms and without the interference of insurers, Dr. Orient said in an interview. In such a private medicine system, patients would pay doctors for services, and patients would then file claims with their insurer for reimbursement. Similarly, physicians should not be at the mercy of Medicare for payment, Dr. Orient said.
“Doctors can sign away their rights if they want in a Medicare participation agreement,” she said. “Doctors who do not sign the agreement to take assignment in all cases doctors should be freed of price controls and coding demands. Their patients should be allowed to file their own simple claims to Medicare with an itemized bill as they did before the 1990s law that requires physicians to submit the claims. Non-participating doctors should be exempted from MACRA [the Medicare Access and CHIP Reauthorization Act], and without the price controls, there is no need for [Recovery Audit Contractors] and other auditors.”
While contraceptive care was strengthened by the ACA, Dr. Horvath said further efforts should be made to improve coverage and level the playing field for reproductive medicine. In addition, she said that abortion should be treated a valid medical procedure, rather than parsed out, and both public and private insurers should be required to pay for the procedure, she said.
“I would love to see strengthened provisions for contraception coverage,” Dr. Horvath said. “[We need to] make sure that doesn’t get bargained away. The other thing is to expand coverage and make sure every method is covered, not just one method in each category.”
However, Robert Doherty, ACP senior vice president of governmental affairs and public policy, said the college is concerned that the current administration may fail to maintain the ACA now that its proposed repeal law has fallen through.
Without aggressively pushing ACA enrollment for younger patients and continued support for the individual mandate, more insurers may pull out of the marketplaces, and the ACA could implode, Mr. Doherty said.
“There are a number of ways that Republicans could either make things better or worse with action or inaction,” Mr. Doherty said during the press conference. “The insurance [companies] have gone to this administration with a wish list of things that will help keep them in the market. What remains to be seen is whether this administration is going to be receptive. If they don’t aggressively enforce the requirement that people buy coverage, more younger people will opt out and stay out until they get sick. That would make the problem of adverse selection even worse and could create the death cycle for insurance.”
Dr. Price consistently answered that Americans should be able to select the kinds of coverage they want. What “we believe is that individuals ought to be able to have access to the kind of coverage that they select for themselves and for their families and not what the government forces them to buy,” Dr. Price testified, echoing the message from his confirmation hearings.
He was also pressed on issues such as the individual mandate, and while noting that it is his duty to uphold the law of the land, he also remained noncommittal in answering questions about whether he would direct the agency to enforce the individual mandate. The first executive order from President Trump beginning his administration gave the agency discretion to not enforce mandates if they caused harm.
[email protected]
On Twitter @legal_med
Gregory Twachtman contributed to this report.
With the demise of Republican repeal and replace legislation, analysts say the landscape is ripe for repairs to the Affordable Care Act or for additional legislation that both political parties could support. So what do physicians want from health reform?
The first step should be stabilizing the health insurance marketplaces by strengthening and perhaps extending risk mitigation measures such as the risk adjustment, risk corridors, and reinsurance provisions of the law, said Patricia Salber, MD, an internist and health care consultant who blogs at TheDoctorWeighsIn.com. Those three ACA provisions were intended to promote insurer competition on the basis of quality and value and promote insurance market stability.
Keeping premiums at manageable levels for patients should also be addressed, said William J. Burke, DO, dean of Ohio University Heritage College of Osteopathic Medicine.
That was echoed in a poll taken by this news organization. Of 390 respondents, fully half (50%) said they would repair the ACA by stabilizing premiums and out-of-pocket costs for patients as of April 2. About 11% stated they would increase payment rates for care provided to Medicaid patients, and 10% said they would return the primary care incentive payment. About 9% of those surveyed would address workforce issues exacerbated by more patients in the system.
Improving reimbursement for Medicaid services is a necessary health reform change, agreed Diane J. Horvath-Cosper MD, an obstetrician-gynecologist and reproductive health advocacy fellow for Physicians for Reproductive Health, a reproductive rights advocacy organization.
“Reimbursement rates are so low that sometimes [physicians] have to limit the number of Medicaid patients to be able to pay staff,” Dr. Horvath said in an interview. “That’s a terrible position to put physicians in because we want to be able to see as many people who want to see us.”
Speaking of Medicaid, Dr. Salber adds that governors should be encouraged to continue expanding Medicaid to eliminate the coverage gap for the “near poor” that exists in states that did not participate in the expansion.
“Now that the [American Health Care Act] has failed, I think we will see some expansion take place organically even in states that were deeply opposed before,” she said.
“The volume of prior authorizations that all physicians face, but especially primary care physicians, is huge,” Dr. Munger said in an interview. “In many cases, we’re having to hire extra staff just to handle all of the prior authorizations. Every patient may not just have one prior authorization, but they may require two or three or four prior authorizations each month or quarterly. It really detracts from meaningful time you can spend with the patient.”
For starters, doctors should provide care to patients based on mutually agreed terms and without the interference of insurers, Dr. Orient said in an interview. In such a private medicine system, patients would pay doctors for services, and patients would then file claims with their insurer for reimbursement. Similarly, physicians should not be at the mercy of Medicare for payment, Dr. Orient said.
“Doctors can sign away their rights if they want in a Medicare participation agreement,” she said. “Doctors who do not sign the agreement to take assignment in all cases doctors should be freed of price controls and coding demands. Their patients should be allowed to file their own simple claims to Medicare with an itemized bill as they did before the 1990s law that requires physicians to submit the claims. Non-participating doctors should be exempted from MACRA [the Medicare Access and CHIP Reauthorization Act], and without the price controls, there is no need for [Recovery Audit Contractors] and other auditors.”
While contraceptive care was strengthened by the ACA, Dr. Horvath said further efforts should be made to improve coverage and level the playing field for reproductive medicine. In addition, she said that abortion should be treated a valid medical procedure, rather than parsed out, and both public and private insurers should be required to pay for the procedure, she said.
“I would love to see strengthened provisions for contraception coverage,” Dr. Horvath said. “[We need to] make sure that doesn’t get bargained away. The other thing is to expand coverage and make sure every method is covered, not just one method in each category.”
However, Robert Doherty, ACP senior vice president of governmental affairs and public policy, said the college is concerned that the current administration may fail to maintain the ACA now that its proposed repeal law has fallen through.
Without aggressively pushing ACA enrollment for younger patients and continued support for the individual mandate, more insurers may pull out of the marketplaces, and the ACA could implode, Mr. Doherty said.
“There are a number of ways that Republicans could either make things better or worse with action or inaction,” Mr. Doherty said during the press conference. “The insurance [companies] have gone to this administration with a wish list of things that will help keep them in the market. What remains to be seen is whether this administration is going to be receptive. If they don’t aggressively enforce the requirement that people buy coverage, more younger people will opt out and stay out until they get sick. That would make the problem of adverse selection even worse and could create the death cycle for insurance.”
Dr. Price consistently answered that Americans should be able to select the kinds of coverage they want. What “we believe is that individuals ought to be able to have access to the kind of coverage that they select for themselves and for their families and not what the government forces them to buy,” Dr. Price testified, echoing the message from his confirmation hearings.
He was also pressed on issues such as the individual mandate, and while noting that it is his duty to uphold the law of the land, he also remained noncommittal in answering questions about whether he would direct the agency to enforce the individual mandate. The first executive order from President Trump beginning his administration gave the agency discretion to not enforce mandates if they caused harm.
[email protected]
On Twitter @legal_med
Gregory Twachtman contributed to this report.
Statins may protect against HIV rebound
SEATTLE – Statins appeared to reduce the risk of viral rebound in HIV patients on antiretroviral therapy, suggesting modest antiviral activity, according to a review of 19,324 HIV-positive veterans who started combination ART from 1995-2011.
It’s been shown before that statins are active against HIV in vitro; the new study is likely the first to show an effect in actual patients, and it was only found in those who used statins consistently, perhaps because of the drugs’ short half-life. “In addition to their cardiovascular benefits, statins could increase the durability of successful antiretroviral therapy. Whether this effect is direct or mediated by [statins’] anti-inflammatory properties merits further evaluation,” concluded investigators led by Henning Drechsler, MD, an infectious disease specialist at the Dallas Veterans Affairs Medical Center.
After adjusting for a range of confounders, including demographics, substance use, and ART adherence assessed by pharmacy fill records, the team found that statins use within 7 days of testing was associated with almost a 20% drop in the risk of decreased risk of VF (adjusted HR 0.81, 95% 0.75-0.88), with a similar benefit for use within 3 months. There was no protective effect for blood pressure drugs, and a slight increase for patients on cardioprotective aspirin.
About a third of the patients – almost all men, around 48 years old – had at least tried statins, generally after their viral loads were suppressed and most often pravastatin or simvastatin. Statins’ protection against VF was present over the whole study period and among all types of ART and levels, but became less pronounced after 2005 in patients with optimal ART adherence, and in those taking newer options. The median observation time was 5.9 years.
“I am very confident that this is not a data fluke,” Dr. Drechsler said at the 2017 Conference on Retroviruses and Opportunistic Infections. He noted that there may even be role for statin use in HIV patients without dyslipidemia, but with the current study, “it’s hard to say. You really do need a prospect study.”
He plans to keep looking into the issue. Meanwhile, a large trial of statin use in HIV is underway.
Dr. Drechsler said he had no disclosures.
SEATTLE – Statins appeared to reduce the risk of viral rebound in HIV patients on antiretroviral therapy, suggesting modest antiviral activity, according to a review of 19,324 HIV-positive veterans who started combination ART from 1995-2011.
It’s been shown before that statins are active against HIV in vitro; the new study is likely the first to show an effect in actual patients, and it was only found in those who used statins consistently, perhaps because of the drugs’ short half-life. “In addition to their cardiovascular benefits, statins could increase the durability of successful antiretroviral therapy. Whether this effect is direct or mediated by [statins’] anti-inflammatory properties merits further evaluation,” concluded investigators led by Henning Drechsler, MD, an infectious disease specialist at the Dallas Veterans Affairs Medical Center.
After adjusting for a range of confounders, including demographics, substance use, and ART adherence assessed by pharmacy fill records, the team found that statins use within 7 days of testing was associated with almost a 20% drop in the risk of decreased risk of VF (adjusted HR 0.81, 95% 0.75-0.88), with a similar benefit for use within 3 months. There was no protective effect for blood pressure drugs, and a slight increase for patients on cardioprotective aspirin.
About a third of the patients – almost all men, around 48 years old – had at least tried statins, generally after their viral loads were suppressed and most often pravastatin or simvastatin. Statins’ protection against VF was present over the whole study period and among all types of ART and levels, but became less pronounced after 2005 in patients with optimal ART adherence, and in those taking newer options. The median observation time was 5.9 years.
“I am very confident that this is not a data fluke,” Dr. Drechsler said at the 2017 Conference on Retroviruses and Opportunistic Infections. He noted that there may even be role for statin use in HIV patients without dyslipidemia, but with the current study, “it’s hard to say. You really do need a prospect study.”
He plans to keep looking into the issue. Meanwhile, a large trial of statin use in HIV is underway.
Dr. Drechsler said he had no disclosures.
SEATTLE – Statins appeared to reduce the risk of viral rebound in HIV patients on antiretroviral therapy, suggesting modest antiviral activity, according to a review of 19,324 HIV-positive veterans who started combination ART from 1995-2011.
It’s been shown before that statins are active against HIV in vitro; the new study is likely the first to show an effect in actual patients, and it was only found in those who used statins consistently, perhaps because of the drugs’ short half-life. “In addition to their cardiovascular benefits, statins could increase the durability of successful antiretroviral therapy. Whether this effect is direct or mediated by [statins’] anti-inflammatory properties merits further evaluation,” concluded investigators led by Henning Drechsler, MD, an infectious disease specialist at the Dallas Veterans Affairs Medical Center.
After adjusting for a range of confounders, including demographics, substance use, and ART adherence assessed by pharmacy fill records, the team found that statins use within 7 days of testing was associated with almost a 20% drop in the risk of decreased risk of VF (adjusted HR 0.81, 95% 0.75-0.88), with a similar benefit for use within 3 months. There was no protective effect for blood pressure drugs, and a slight increase for patients on cardioprotective aspirin.
About a third of the patients – almost all men, around 48 years old – had at least tried statins, generally after their viral loads were suppressed and most often pravastatin or simvastatin. Statins’ protection against VF was present over the whole study period and among all types of ART and levels, but became less pronounced after 2005 in patients with optimal ART adherence, and in those taking newer options. The median observation time was 5.9 years.
“I am very confident that this is not a data fluke,” Dr. Drechsler said at the 2017 Conference on Retroviruses and Opportunistic Infections. He noted that there may even be role for statin use in HIV patients without dyslipidemia, but with the current study, “it’s hard to say. You really do need a prospect study.”
He plans to keep looking into the issue. Meanwhile, a large trial of statin use in HIV is underway.
Dr. Drechsler said he had no disclosures.
AT CROI 2017
Ebola vaccine maintains immune response after 1 year
People who received an Ebola virus vaccine maintained immune response one year after vaccination, according to a research letter from Rebecca L.Winslow, MRCGP, of the University of Oxford (UK) and her associates.
The immune response of 64 vaccine recipients of European descent 360 days after receiving either adenovirus type 26 vector vaccine encoding Ebolavirus glycoprotein (Ad26.ZEBOV) followed by modified vaccinia virus Ankara vector vaccine (MVA-BN-Filo), or MVA-BN-Filo followed by Ad26.ZEBOV. The 360 day follow-up occurred 120 days after the previous follow-up, during which time no significant adverse events were reported.
“Immune responses may differ in a sub-Saharan African population; these vaccine candidates are being assessed in this region. Additional research is also warranted to explore the persistence of immunity beyond 1 year following immunization and response to booster doses of vaccine,” the investigators concluded.
Find the full research letter in JAMA (doi: 10.1001/jama.2016.20644)
People who received an Ebola virus vaccine maintained immune response one year after vaccination, according to a research letter from Rebecca L.Winslow, MRCGP, of the University of Oxford (UK) and her associates.
The immune response of 64 vaccine recipients of European descent 360 days after receiving either adenovirus type 26 vector vaccine encoding Ebolavirus glycoprotein (Ad26.ZEBOV) followed by modified vaccinia virus Ankara vector vaccine (MVA-BN-Filo), or MVA-BN-Filo followed by Ad26.ZEBOV. The 360 day follow-up occurred 120 days after the previous follow-up, during which time no significant adverse events were reported.
“Immune responses may differ in a sub-Saharan African population; these vaccine candidates are being assessed in this region. Additional research is also warranted to explore the persistence of immunity beyond 1 year following immunization and response to booster doses of vaccine,” the investigators concluded.
Find the full research letter in JAMA (doi: 10.1001/jama.2016.20644)
People who received an Ebola virus vaccine maintained immune response one year after vaccination, according to a research letter from Rebecca L.Winslow, MRCGP, of the University of Oxford (UK) and her associates.
The immune response of 64 vaccine recipients of European descent 360 days after receiving either adenovirus type 26 vector vaccine encoding Ebolavirus glycoprotein (Ad26.ZEBOV) followed by modified vaccinia virus Ankara vector vaccine (MVA-BN-Filo), or MVA-BN-Filo followed by Ad26.ZEBOV. The 360 day follow-up occurred 120 days after the previous follow-up, during which time no significant adverse events were reported.
“Immune responses may differ in a sub-Saharan African population; these vaccine candidates are being assessed in this region. Additional research is also warranted to explore the persistence of immunity beyond 1 year following immunization and response to booster doses of vaccine,” the investigators concluded.
Find the full research letter in JAMA (doi: 10.1001/jama.2016.20644)
FROM JAMA