User login
Children with infantile spasms or nonsyndromic epilepsy achieve similar outcomes
HOUSTON – Infants and children who had epilepsy that was not identified as being part of a syndrome fared slightly worse in developmental outcomes and pharmacoresistance than did those with West syndrome/infantile spasms, Dravet syndrome, or another type of syndromic epilepsy, according to a prospective multisite study.
But in the study of 775 patients from 17 American pediatric epilepsy centers, early age at diagnosis was associated with greater mortality, greater risk of developmental decline, and greater pharmacoresistance, regardless of seizure type.
Lead study author Anne Berg, PhD, and her colleagues wrote that they were surprised to see that children in their study population with nonsyndromic epilepsy (NSE) were slightly more likely to have pharmacoresistant seizures (PR).
Further, although logistic regression analysis showed that seizure etiology, younger age at onset, and PR all had independent contributions to developmental decline, “West/IS was not convincingly associated with developmental decline,” they said.
In a poster session at the annual meeting of the American Epilepsy Society, Dr. Berg, an epidemiologist and research professor of pediatric neurology at Northwestern University, Chicago, presented the findings of a study that examined outcomes for infants and children diagnosed with epilepsy.
Patients were prospectively identified during a 3-year period from 2012 to 2015. Patients were eligible if their epilepsy began before their third birthday, and if the epilepsy was initially diagnosed at one of the participating centers. Patient data were evaluated for seizure and developmental outcomes if the patient was followed for at least 6 months after diagnosis.
Of the 775 patients initially recruited, 367 (47.3%) were girls. The mean age of epilepsy onset (which usually meant age at first unprovoked seizure) was 11.1 months (standard deviation, 9.4). Most patients (n = 509; 65.7%) were diagnosed with epilepsy before the age of 1 year. Just 115 patients (14.8%) received their epilepsy diagnosis when they were older than 2 years.
A key outcome investigated by Dr. Berg and her colleagues was pharmacoresistance, identified as lack of seizure control (i.e., at least a 3-month seizure-free period) after trying two appropriate medications. Other outcome measures included tracking whether patients developed West/IS, and whether West/IS evolved into other seizure types. The investigators also tracked developmental delay after epilepsy diagnosis and collected data about deaths among participating patients.
About a quarter (27%) of patients had persistent PR; these were more likely to occur in children who were younger at the onset of epilepsy. PR were more common when seizures began before the age of 1 year, occurring in 30% of this patient population, whereas 20% of patients with seizure onset happening after 1 year of age had PR (P = .0008).
Other findings from the study revealed that infants whose NSE had an etiology of focal cortical dysplasia or of an acquired insult such as trauma were more than twice as likely to have their seizures evolve into WS/IS.
Of 214 children whose initial presentation was WS/IS, 49 (23%) developed new seizure types. Most of these (47 of 49) were infants. Patients with WS/IS due to tuberous sclerosis complex, infectious causes, hypoxic-ischemic encephalopathy, and cephalic brain disorders were more likely to develop new seizure types.
“At initial presentation of epilepsy, children with West/IS were more likely already to have developmental delay than children with other syndromes or NSE,” they said.
Of the 22 patient deaths that occurred during the study, all but 1 occurred in infants younger than 1 year. None of the deaths occurred in typically-developing children with unknown epilepsy etiology.
“West/IS is the only early life epilepsy with consensus guidelines for treatment,” noted Dr. Berg and her coauthors, speculating that the guidelines might contribute to the slightly better outcomes observed for this population in their study.
However, although some groups of infants and children in the study fared slightly better than others, “[F]or the most part, there are no clearly ‘low’ risk groups,” Dr. Berg and her colleagues said. “Our findings highlight that most, if not all, early life epilepsies pose serious risk for poor outcomes and are equally deserving of concerted efforts.”
Dr. Berg reported no relevant financial disclosures. The study was funded by the Pediatric Epilepsy Research Foundation, and conducted through the Pediatric Epilepsy Research Consortium.
[email protected]
On Twitter @karioakes
HOUSTON – Infants and children who had epilepsy that was not identified as being part of a syndrome fared slightly worse in developmental outcomes and pharmacoresistance than did those with West syndrome/infantile spasms, Dravet syndrome, or another type of syndromic epilepsy, according to a prospective multisite study.
But in the study of 775 patients from 17 American pediatric epilepsy centers, early age at diagnosis was associated with greater mortality, greater risk of developmental decline, and greater pharmacoresistance, regardless of seizure type.
Lead study author Anne Berg, PhD, and her colleagues wrote that they were surprised to see that children in their study population with nonsyndromic epilepsy (NSE) were slightly more likely to have pharmacoresistant seizures (PR).
Further, although logistic regression analysis showed that seizure etiology, younger age at onset, and PR all had independent contributions to developmental decline, “West/IS was not convincingly associated with developmental decline,” they said.
In a poster session at the annual meeting of the American Epilepsy Society, Dr. Berg, an epidemiologist and research professor of pediatric neurology at Northwestern University, Chicago, presented the findings of a study that examined outcomes for infants and children diagnosed with epilepsy.
Patients were prospectively identified during a 3-year period from 2012 to 2015. Patients were eligible if their epilepsy began before their third birthday, and if the epilepsy was initially diagnosed at one of the participating centers. Patient data were evaluated for seizure and developmental outcomes if the patient was followed for at least 6 months after diagnosis.
Of the 775 patients initially recruited, 367 (47.3%) were girls. The mean age of epilepsy onset (which usually meant age at first unprovoked seizure) was 11.1 months (standard deviation, 9.4). Most patients (n = 509; 65.7%) were diagnosed with epilepsy before the age of 1 year. Just 115 patients (14.8%) received their epilepsy diagnosis when they were older than 2 years.
A key outcome investigated by Dr. Berg and her colleagues was pharmacoresistance, identified as lack of seizure control (i.e., at least a 3-month seizure-free period) after trying two appropriate medications. Other outcome measures included tracking whether patients developed West/IS, and whether West/IS evolved into other seizure types. The investigators also tracked developmental delay after epilepsy diagnosis and collected data about deaths among participating patients.
About a quarter (27%) of patients had persistent PR; these were more likely to occur in children who were younger at the onset of epilepsy. PR were more common when seizures began before the age of 1 year, occurring in 30% of this patient population, whereas 20% of patients with seizure onset happening after 1 year of age had PR (P = .0008).
Other findings from the study revealed that infants whose NSE had an etiology of focal cortical dysplasia or of an acquired insult such as trauma were more than twice as likely to have their seizures evolve into WS/IS.
Of 214 children whose initial presentation was WS/IS, 49 (23%) developed new seizure types. Most of these (47 of 49) were infants. Patients with WS/IS due to tuberous sclerosis complex, infectious causes, hypoxic-ischemic encephalopathy, and cephalic brain disorders were more likely to develop new seizure types.
“At initial presentation of epilepsy, children with West/IS were more likely already to have developmental delay than children with other syndromes or NSE,” they said.
Of the 22 patient deaths that occurred during the study, all but 1 occurred in infants younger than 1 year. None of the deaths occurred in typically-developing children with unknown epilepsy etiology.
“West/IS is the only early life epilepsy with consensus guidelines for treatment,” noted Dr. Berg and her coauthors, speculating that the guidelines might contribute to the slightly better outcomes observed for this population in their study.
However, although some groups of infants and children in the study fared slightly better than others, “[F]or the most part, there are no clearly ‘low’ risk groups,” Dr. Berg and her colleagues said. “Our findings highlight that most, if not all, early life epilepsies pose serious risk for poor outcomes and are equally deserving of concerted efforts.”
Dr. Berg reported no relevant financial disclosures. The study was funded by the Pediatric Epilepsy Research Foundation, and conducted through the Pediatric Epilepsy Research Consortium.
[email protected]
On Twitter @karioakes
HOUSTON – Infants and children who had epilepsy that was not identified as being part of a syndrome fared slightly worse in developmental outcomes and pharmacoresistance than did those with West syndrome/infantile spasms, Dravet syndrome, or another type of syndromic epilepsy, according to a prospective multisite study.
But in the study of 775 patients from 17 American pediatric epilepsy centers, early age at diagnosis was associated with greater mortality, greater risk of developmental decline, and greater pharmacoresistance, regardless of seizure type.
Lead study author Anne Berg, PhD, and her colleagues wrote that they were surprised to see that children in their study population with nonsyndromic epilepsy (NSE) were slightly more likely to have pharmacoresistant seizures (PR).
Further, although logistic regression analysis showed that seizure etiology, younger age at onset, and PR all had independent contributions to developmental decline, “West/IS was not convincingly associated with developmental decline,” they said.
In a poster session at the annual meeting of the American Epilepsy Society, Dr. Berg, an epidemiologist and research professor of pediatric neurology at Northwestern University, Chicago, presented the findings of a study that examined outcomes for infants and children diagnosed with epilepsy.
Patients were prospectively identified during a 3-year period from 2012 to 2015. Patients were eligible if their epilepsy began before their third birthday, and if the epilepsy was initially diagnosed at one of the participating centers. Patient data were evaluated for seizure and developmental outcomes if the patient was followed for at least 6 months after diagnosis.
Of the 775 patients initially recruited, 367 (47.3%) were girls. The mean age of epilepsy onset (which usually meant age at first unprovoked seizure) was 11.1 months (standard deviation, 9.4). Most patients (n = 509; 65.7%) were diagnosed with epilepsy before the age of 1 year. Just 115 patients (14.8%) received their epilepsy diagnosis when they were older than 2 years.
A key outcome investigated by Dr. Berg and her colleagues was pharmacoresistance, identified as lack of seizure control (i.e., at least a 3-month seizure-free period) after trying two appropriate medications. Other outcome measures included tracking whether patients developed West/IS, and whether West/IS evolved into other seizure types. The investigators also tracked developmental delay after epilepsy diagnosis and collected data about deaths among participating patients.
About a quarter (27%) of patients had persistent PR; these were more likely to occur in children who were younger at the onset of epilepsy. PR were more common when seizures began before the age of 1 year, occurring in 30% of this patient population, whereas 20% of patients with seizure onset happening after 1 year of age had PR (P = .0008).
Other findings from the study revealed that infants whose NSE had an etiology of focal cortical dysplasia or of an acquired insult such as trauma were more than twice as likely to have their seizures evolve into WS/IS.
Of 214 children whose initial presentation was WS/IS, 49 (23%) developed new seizure types. Most of these (47 of 49) were infants. Patients with WS/IS due to tuberous sclerosis complex, infectious causes, hypoxic-ischemic encephalopathy, and cephalic brain disorders were more likely to develop new seizure types.
“At initial presentation of epilepsy, children with West/IS were more likely already to have developmental delay than children with other syndromes or NSE,” they said.
Of the 22 patient deaths that occurred during the study, all but 1 occurred in infants younger than 1 year. None of the deaths occurred in typically-developing children with unknown epilepsy etiology.
“West/IS is the only early life epilepsy with consensus guidelines for treatment,” noted Dr. Berg and her coauthors, speculating that the guidelines might contribute to the slightly better outcomes observed for this population in their study.
However, although some groups of infants and children in the study fared slightly better than others, “[F]or the most part, there are no clearly ‘low’ risk groups,” Dr. Berg and her colleagues said. “Our findings highlight that most, if not all, early life epilepsies pose serious risk for poor outcomes and are equally deserving of concerted efforts.”
Dr. Berg reported no relevant financial disclosures. The study was funded by the Pediatric Epilepsy Research Foundation, and conducted through the Pediatric Epilepsy Research Consortium.
[email protected]
On Twitter @karioakes
AT AES 2016
Key clinical point:
Major finding: Infantile spasms was not independently associated with worse developmental decline compared with nonsyndromic epilepsy.
Data source: Prospective study of 775 infants and children with epilepsy.
Disclosures: Dr. Berg reported no relevant financial disclosures. The study was funded by the Pediatric Epilepsy Research Foundation, and conducted through the Pediatric Epilepsy Research Consortium.
Shulkin Nominated to Replace McDonald at VA
In a surprise move, David Shulkin, MD, the current under secretary for health at the VA, has been nominated to take over as the VA Secretary to replace Robert McDonald. If confirmed, Dr. Shulkin would be the first nonveteran to head the agency.
Amid conversation about privatizing the VA, Dr. Shulkin had been a vocal proponent on Capital Hill and in medical journals for why the VA had a special responsibility to care for veterans. He has argued that the VA is especially qualified to handle the unique medical needs of veterans. Dr. Shulkin has also offered a number of strategies for reducing wait times, including expanding the scope of practice for advanced practice nurses, streamlining the adoption of innovative programs, and improving ageing information technology systems.
“As someone who spent more than 25 years managing private sector health care organizations and recently joined VA as its under secretary for health, I’ve had the unique opportunity to compare the health care systems,” Dr. Shulkin wrote in a Federal Practitioner editorial. “Over the past several months, I’ve met with veterans and their families, veterans service organizations, VA clinicians, facility staff, and veteran employees at all levels. Through these meetings and travel to dozens of facilities, I’ve come to realize that many of the essential services provided by the VA cannot be found in or even replicated in the private sector.”
Related: Shulkin Addresses APRN Rule, Health Care Vacancies, and Access
With just days to go before the inauguration of Donald Trump, only 2 cabinet-level positions had remained open. The delay caused worry on a number of fronts. “We cannot afford any lapse in leadership at the VA, especially at the Secretary level,” said AMVETS National Executive Director Joe Chenelly in a statement. “The transition between administrations naturally brings uncertainty, but that must be minimized with a timely decision by the incoming president regarding the VA Secretary.”
Members of Congress share similar concerns. “I am very concerned that the President-elect has yet to nominate a VA Secretary,” Sen. Jon Tester (D-MT) said in a press release. “If he needs more counsel before making this important decision, he should start by personally sitting down with our nation's veterans service organizations. Every day he continues to delay his decision, he jeopardizes the seamless transition that is needed to ensure this nation fulfills its commitment to the brave men and women who served.”
Related: Shulkin: VA "Not a Political Issue”
Who Else Was Under Consideration for the Job?
According to multiple reports, a number of people have been offered the position but have turned it down or were deemed unqualified for the position.
- Leo MacKay Jr.: A deputy VA secretary under President George W. Bush and currently a senior vice president at Lockheed Martin.
- Toby Cosgrove: Cleveland Clinic CEO; he also turned down a previous offer from President Obama.
- ADM Michelle Howard, USN
- Luis Quinonez, a businessman from Florida
- Jeff Miller, former U.S. House Veterans Affairs Committee Chairman (R-Fla.): critics raised concerns that Miller was not a veteran.
- Pete Hegseth: Iraq and Afghanistan veteran who leads Concerned Veterans for America, a conservative VSO, has been criticized for his advocacy for full privatization of VA health care. However, he is still considered to be a potential candidate.
- Scott Brown: veteran and former republican Senator from Massachusetts was criticized for not having any management experience.
- Sarah Palin: Former republican vice presidential candidate and Alasksa governor was criticized for not being a veteran or having experience running a large institution.
- Coast Guard Adm. Thad Allen: while his name has been mentioned in connection with the position, there is little information on his interest or criticism of him.
Veteran service organizations had been pushing the Trump transition team to consider retaining current VA secretary Robert McDonald, who is a republican.
In a surprise move, David Shulkin, MD, the current under secretary for health at the VA, has been nominated to take over as the VA Secretary to replace Robert McDonald. If confirmed, Dr. Shulkin would be the first nonveteran to head the agency.
Amid conversation about privatizing the VA, Dr. Shulkin had been a vocal proponent on Capital Hill and in medical journals for why the VA had a special responsibility to care for veterans. He has argued that the VA is especially qualified to handle the unique medical needs of veterans. Dr. Shulkin has also offered a number of strategies for reducing wait times, including expanding the scope of practice for advanced practice nurses, streamlining the adoption of innovative programs, and improving ageing information technology systems.
“As someone who spent more than 25 years managing private sector health care organizations and recently joined VA as its under secretary for health, I’ve had the unique opportunity to compare the health care systems,” Dr. Shulkin wrote in a Federal Practitioner editorial. “Over the past several months, I’ve met with veterans and their families, veterans service organizations, VA clinicians, facility staff, and veteran employees at all levels. Through these meetings and travel to dozens of facilities, I’ve come to realize that many of the essential services provided by the VA cannot be found in or even replicated in the private sector.”
Related: Shulkin Addresses APRN Rule, Health Care Vacancies, and Access
With just days to go before the inauguration of Donald Trump, only 2 cabinet-level positions had remained open. The delay caused worry on a number of fronts. “We cannot afford any lapse in leadership at the VA, especially at the Secretary level,” said AMVETS National Executive Director Joe Chenelly in a statement. “The transition between administrations naturally brings uncertainty, but that must be minimized with a timely decision by the incoming president regarding the VA Secretary.”
Members of Congress share similar concerns. “I am very concerned that the President-elect has yet to nominate a VA Secretary,” Sen. Jon Tester (D-MT) said in a press release. “If he needs more counsel before making this important decision, he should start by personally sitting down with our nation's veterans service organizations. Every day he continues to delay his decision, he jeopardizes the seamless transition that is needed to ensure this nation fulfills its commitment to the brave men and women who served.”
Related: Shulkin: VA "Not a Political Issue”
Who Else Was Under Consideration for the Job?
According to multiple reports, a number of people have been offered the position but have turned it down or were deemed unqualified for the position.
- Leo MacKay Jr.: A deputy VA secretary under President George W. Bush and currently a senior vice president at Lockheed Martin.
- Toby Cosgrove: Cleveland Clinic CEO; he also turned down a previous offer from President Obama.
- ADM Michelle Howard, USN
- Luis Quinonez, a businessman from Florida
- Jeff Miller, former U.S. House Veterans Affairs Committee Chairman (R-Fla.): critics raised concerns that Miller was not a veteran.
- Pete Hegseth: Iraq and Afghanistan veteran who leads Concerned Veterans for America, a conservative VSO, has been criticized for his advocacy for full privatization of VA health care. However, he is still considered to be a potential candidate.
- Scott Brown: veteran and former republican Senator from Massachusetts was criticized for not having any management experience.
- Sarah Palin: Former republican vice presidential candidate and Alasksa governor was criticized for not being a veteran or having experience running a large institution.
- Coast Guard Adm. Thad Allen: while his name has been mentioned in connection with the position, there is little information on his interest or criticism of him.
Veteran service organizations had been pushing the Trump transition team to consider retaining current VA secretary Robert McDonald, who is a republican.
In a surprise move, David Shulkin, MD, the current under secretary for health at the VA, has been nominated to take over as the VA Secretary to replace Robert McDonald. If confirmed, Dr. Shulkin would be the first nonveteran to head the agency.
Amid conversation about privatizing the VA, Dr. Shulkin had been a vocal proponent on Capital Hill and in medical journals for why the VA had a special responsibility to care for veterans. He has argued that the VA is especially qualified to handle the unique medical needs of veterans. Dr. Shulkin has also offered a number of strategies for reducing wait times, including expanding the scope of practice for advanced practice nurses, streamlining the adoption of innovative programs, and improving ageing information technology systems.
“As someone who spent more than 25 years managing private sector health care organizations and recently joined VA as its under secretary for health, I’ve had the unique opportunity to compare the health care systems,” Dr. Shulkin wrote in a Federal Practitioner editorial. “Over the past several months, I’ve met with veterans and their families, veterans service organizations, VA clinicians, facility staff, and veteran employees at all levels. Through these meetings and travel to dozens of facilities, I’ve come to realize that many of the essential services provided by the VA cannot be found in or even replicated in the private sector.”
Related: Shulkin Addresses APRN Rule, Health Care Vacancies, and Access
With just days to go before the inauguration of Donald Trump, only 2 cabinet-level positions had remained open. The delay caused worry on a number of fronts. “We cannot afford any lapse in leadership at the VA, especially at the Secretary level,” said AMVETS National Executive Director Joe Chenelly in a statement. “The transition between administrations naturally brings uncertainty, but that must be minimized with a timely decision by the incoming president regarding the VA Secretary.”
Members of Congress share similar concerns. “I am very concerned that the President-elect has yet to nominate a VA Secretary,” Sen. Jon Tester (D-MT) said in a press release. “If he needs more counsel before making this important decision, he should start by personally sitting down with our nation's veterans service organizations. Every day he continues to delay his decision, he jeopardizes the seamless transition that is needed to ensure this nation fulfills its commitment to the brave men and women who served.”
Related: Shulkin: VA "Not a Political Issue”
Who Else Was Under Consideration for the Job?
According to multiple reports, a number of people have been offered the position but have turned it down or were deemed unqualified for the position.
- Leo MacKay Jr.: A deputy VA secretary under President George W. Bush and currently a senior vice president at Lockheed Martin.
- Toby Cosgrove: Cleveland Clinic CEO; he also turned down a previous offer from President Obama.
- ADM Michelle Howard, USN
- Luis Quinonez, a businessman from Florida
- Jeff Miller, former U.S. House Veterans Affairs Committee Chairman (R-Fla.): critics raised concerns that Miller was not a veteran.
- Pete Hegseth: Iraq and Afghanistan veteran who leads Concerned Veterans for America, a conservative VSO, has been criticized for his advocacy for full privatization of VA health care. However, he is still considered to be a potential candidate.
- Scott Brown: veteran and former republican Senator from Massachusetts was criticized for not having any management experience.
- Sarah Palin: Former republican vice presidential candidate and Alasksa governor was criticized for not being a veteran or having experience running a large institution.
- Coast Guard Adm. Thad Allen: while his name has been mentioned in connection with the position, there is little information on his interest or criticism of him.
Veteran service organizations had been pushing the Trump transition team to consider retaining current VA secretary Robert McDonald, who is a republican.
A new approach to treat MLL-rearranged leukemia?

Investigators may have discovered a new way to treat mixed-lineage leukemia (MLL)-rearranged leukemia, according to research published in Cell.
The team found they could disrupt the balance between wild-type MLL proteins and MLL chimeras.
This impeded MLL leukemia cell proliferation in vitro, delayed disease progression in a mouse model of MLL-AF9 leukemia, and prolonged survival in the mice.
The investigators are now attempting to translate these findings to the clinic.
“We’ve spent the last 20 years in my laboratory trying to molecularly understand how MLL translocations cause this rare and devastating form of leukemia in children so that we can use this information to develop an effective therapy for this cancer,” said lead investigator Ali Shilatifard, PhD, of Northwestern University Feinberg School of Medicine in Chicago, Illinois.
“Now, we’ve made a fundamentally important breakthrough.”
The investigators found that wild-type MLL protein is less stable than the MLL chimeras in MLL leukemia cells. They therefore theorized that stabilizing the wild-type copy of the protein would displace the mutated version that drives MLL-rearranged leukemia.
The team set out to identify factors regulating MLL protein degradation and found the ubiquitin-conjugating enzyme E2O (UBE2O).
The investigators said UBE2O regulates the stability of wild-type MLL in response to interleukin-1 signaling. And inhibiting interleukin-1 receptor-associated kinases (IRAKs) increases the stability and chromatin occupancy of wild-type MLL.
The team also found that IRAK inhibition displaces the MLL chimera and subunits of the super elongation complex at a subset of target genes (LGALS1, LMO2, and GNA15).
To determine the implications of these findings for treatment, the investigators tested an IRAK4 inhibitor in patient-derived cell lines, including MLL leukemia and non-MLL leukemia/lymphoma cells. The inhibitor preferentially impeded the growth of MLL-rearranged leukemia cells.
The team also tested IRAK inhibitors in a murine MLL-AF9 leukemia transplantation model. They injected the animals with IRAK inhibitors on day 19 after transplant, which is just before the mice succumb to leukemia.
The mice received injections with an IRAK1/4 inhibitor (8 mg/kg), an IRAK4 inhibitor (75 mg/kg), or vehicle control every other day for 10 days.
The investigators said both IRAK inhibitors significantly extended survival beyond the 27-day mark, when all of the vehicle-treated mice had succumbed to the disease. Two mice treated with an IRAK inhibitor (1 mouse for each drug) were still alive at day 55.
The team also treated mice with the IRAK inhibitors or vehicle control at 10 days after transplant.
Eight of the 10 mice that received the IRAK1/4 inhibitor had not developed MLL-AF9 leukemia as of day 55. And the same was true for 4 of the 9 mice that received the IRAK4 inhibitor.
However, all of the vehicle-treated mice had succumbed to the disease by the 31-day mark.
The investigators said they are now synthesizing better compounds and hope to eventually launch a phase 1 trial to test these compounds in Chicago. ![]()

Investigators may have discovered a new way to treat mixed-lineage leukemia (MLL)-rearranged leukemia, according to research published in Cell.
The team found they could disrupt the balance between wild-type MLL proteins and MLL chimeras.
This impeded MLL leukemia cell proliferation in vitro, delayed disease progression in a mouse model of MLL-AF9 leukemia, and prolonged survival in the mice.
The investigators are now attempting to translate these findings to the clinic.
“We’ve spent the last 20 years in my laboratory trying to molecularly understand how MLL translocations cause this rare and devastating form of leukemia in children so that we can use this information to develop an effective therapy for this cancer,” said lead investigator Ali Shilatifard, PhD, of Northwestern University Feinberg School of Medicine in Chicago, Illinois.
“Now, we’ve made a fundamentally important breakthrough.”
The investigators found that wild-type MLL protein is less stable than the MLL chimeras in MLL leukemia cells. They therefore theorized that stabilizing the wild-type copy of the protein would displace the mutated version that drives MLL-rearranged leukemia.
The team set out to identify factors regulating MLL protein degradation and found the ubiquitin-conjugating enzyme E2O (UBE2O).
The investigators said UBE2O regulates the stability of wild-type MLL in response to interleukin-1 signaling. And inhibiting interleukin-1 receptor-associated kinases (IRAKs) increases the stability and chromatin occupancy of wild-type MLL.
The team also found that IRAK inhibition displaces the MLL chimera and subunits of the super elongation complex at a subset of target genes (LGALS1, LMO2, and GNA15).
To determine the implications of these findings for treatment, the investigators tested an IRAK4 inhibitor in patient-derived cell lines, including MLL leukemia and non-MLL leukemia/lymphoma cells. The inhibitor preferentially impeded the growth of MLL-rearranged leukemia cells.
The team also tested IRAK inhibitors in a murine MLL-AF9 leukemia transplantation model. They injected the animals with IRAK inhibitors on day 19 after transplant, which is just before the mice succumb to leukemia.
The mice received injections with an IRAK1/4 inhibitor (8 mg/kg), an IRAK4 inhibitor (75 mg/kg), or vehicle control every other day for 10 days.
The investigators said both IRAK inhibitors significantly extended survival beyond the 27-day mark, when all of the vehicle-treated mice had succumbed to the disease. Two mice treated with an IRAK inhibitor (1 mouse for each drug) were still alive at day 55.
The team also treated mice with the IRAK inhibitors or vehicle control at 10 days after transplant.
Eight of the 10 mice that received the IRAK1/4 inhibitor had not developed MLL-AF9 leukemia as of day 55. And the same was true for 4 of the 9 mice that received the IRAK4 inhibitor.
However, all of the vehicle-treated mice had succumbed to the disease by the 31-day mark.
The investigators said they are now synthesizing better compounds and hope to eventually launch a phase 1 trial to test these compounds in Chicago. ![]()

Investigators may have discovered a new way to treat mixed-lineage leukemia (MLL)-rearranged leukemia, according to research published in Cell.
The team found they could disrupt the balance between wild-type MLL proteins and MLL chimeras.
This impeded MLL leukemia cell proliferation in vitro, delayed disease progression in a mouse model of MLL-AF9 leukemia, and prolonged survival in the mice.
The investigators are now attempting to translate these findings to the clinic.
“We’ve spent the last 20 years in my laboratory trying to molecularly understand how MLL translocations cause this rare and devastating form of leukemia in children so that we can use this information to develop an effective therapy for this cancer,” said lead investigator Ali Shilatifard, PhD, of Northwestern University Feinberg School of Medicine in Chicago, Illinois.
“Now, we’ve made a fundamentally important breakthrough.”
The investigators found that wild-type MLL protein is less stable than the MLL chimeras in MLL leukemia cells. They therefore theorized that stabilizing the wild-type copy of the protein would displace the mutated version that drives MLL-rearranged leukemia.
The team set out to identify factors regulating MLL protein degradation and found the ubiquitin-conjugating enzyme E2O (UBE2O).
The investigators said UBE2O regulates the stability of wild-type MLL in response to interleukin-1 signaling. And inhibiting interleukin-1 receptor-associated kinases (IRAKs) increases the stability and chromatin occupancy of wild-type MLL.
The team also found that IRAK inhibition displaces the MLL chimera and subunits of the super elongation complex at a subset of target genes (LGALS1, LMO2, and GNA15).
To determine the implications of these findings for treatment, the investigators tested an IRAK4 inhibitor in patient-derived cell lines, including MLL leukemia and non-MLL leukemia/lymphoma cells. The inhibitor preferentially impeded the growth of MLL-rearranged leukemia cells.
The team also tested IRAK inhibitors in a murine MLL-AF9 leukemia transplantation model. They injected the animals with IRAK inhibitors on day 19 after transplant, which is just before the mice succumb to leukemia.
The mice received injections with an IRAK1/4 inhibitor (8 mg/kg), an IRAK4 inhibitor (75 mg/kg), or vehicle control every other day for 10 days.
The investigators said both IRAK inhibitors significantly extended survival beyond the 27-day mark, when all of the vehicle-treated mice had succumbed to the disease. Two mice treated with an IRAK inhibitor (1 mouse for each drug) were still alive at day 55.
The team also treated mice with the IRAK inhibitors or vehicle control at 10 days after transplant.
Eight of the 10 mice that received the IRAK1/4 inhibitor had not developed MLL-AF9 leukemia as of day 55. And the same was true for 4 of the 9 mice that received the IRAK4 inhibitor.
However, all of the vehicle-treated mice had succumbed to the disease by the 31-day mark.
The investigators said they are now synthesizing better compounds and hope to eventually launch a phase 1 trial to test these compounds in Chicago. ![]()
ACC releases guidance on anticoagulant use in NVAF

The American College of Cardiology (ACC) has published a guidance document for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation (NVAF).
The document includes recommendations on how and when to stop anticoagulants in NVAF patients undergoing surgery, deciding if a substitute medication should be used, and determining when it is safe for patients to resume anticoagulants after surgery.
The document was published in the Journal of the American College of Cardiology.
“With this new decision pathway [document], physicians will be able to make better-informed decisions, and this will contribute to improved patient outcomes,” said John U. Doherty, MD, chair of the document writing committee.
“In North America alone, more than 250,000 nonvalvular atrial fibrillation patients undergo surgery annually, so this document will impact many people.”
The document provides guidance on:
- The overall decision to keep a patient chronically on an anticoagulant by examining whether anticoagulation is warranted based on overall thrombotic risk.
- The decision to take the patient off an anticoagulant temporarily.
- How to temporarily stop the use of vitamin K antagonists and direct-acting oral anticoagulants.
- Deciding if bridging a patient—temporarily discontinuing an oral anticoagulant and replacing it with a subcutaneous or intravenous anticoagulant—before, during, and after surgery is the best choice.
- Deciding how to bridge before, during, and after surgery.
- Deciding how and when to restart the patient’s regular anticoagulant after surgery.


The American College of Cardiology (ACC) has published a guidance document for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation (NVAF).
The document includes recommendations on how and when to stop anticoagulants in NVAF patients undergoing surgery, deciding if a substitute medication should be used, and determining when it is safe for patients to resume anticoagulants after surgery.
The document was published in the Journal of the American College of Cardiology.
“With this new decision pathway [document], physicians will be able to make better-informed decisions, and this will contribute to improved patient outcomes,” said John U. Doherty, MD, chair of the document writing committee.
“In North America alone, more than 250,000 nonvalvular atrial fibrillation patients undergo surgery annually, so this document will impact many people.”
The document provides guidance on:
- The overall decision to keep a patient chronically on an anticoagulant by examining whether anticoagulation is warranted based on overall thrombotic risk.
- The decision to take the patient off an anticoagulant temporarily.
- How to temporarily stop the use of vitamin K antagonists and direct-acting oral anticoagulants.
- Deciding if bridging a patient—temporarily discontinuing an oral anticoagulant and replacing it with a subcutaneous or intravenous anticoagulant—before, during, and after surgery is the best choice.
- Deciding how to bridge before, during, and after surgery.
- Deciding how and when to restart the patient’s regular anticoagulant after surgery.


The American College of Cardiology (ACC) has published a guidance document for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation (NVAF).
The document includes recommendations on how and when to stop anticoagulants in NVAF patients undergoing surgery, deciding if a substitute medication should be used, and determining when it is safe for patients to resume anticoagulants after surgery.
The document was published in the Journal of the American College of Cardiology.
“With this new decision pathway [document], physicians will be able to make better-informed decisions, and this will contribute to improved patient outcomes,” said John U. Doherty, MD, chair of the document writing committee.
“In North America alone, more than 250,000 nonvalvular atrial fibrillation patients undergo surgery annually, so this document will impact many people.”
The document provides guidance on:
- The overall decision to keep a patient chronically on an anticoagulant by examining whether anticoagulation is warranted based on overall thrombotic risk.
- The decision to take the patient off an anticoagulant temporarily.
- How to temporarily stop the use of vitamin K antagonists and direct-acting oral anticoagulants.
- Deciding if bridging a patient—temporarily discontinuing an oral anticoagulant and replacing it with a subcutaneous or intravenous anticoagulant—before, during, and after surgery is the best choice.
- Deciding how to bridge before, during, and after surgery.
- Deciding how and when to restart the patient’s regular anticoagulant after surgery.

Team uses light to launch drugs from RBCs

Researchers say they’ve developed a technique that uses light to activate a drug stored in circulating red blood cells (RBCs) so the drug is released exactly when and where it’s needed.
The group believes the work could have profound implications for the field of drug delivery.
They say the technique could drastically reduce the amount of drug needed to treat diseases and therefore decrease the risk of side effects.
“Using light to treat a disease site has a lot of benefits beyond the ‘isn’t-that-cool’ factor,” said study author David Lawrence, PhD, of the University of North Carolina at Chapel Hill.
“Those benefits could include avoiding surgery and the risk of infection, making anesthesia unnecessary, and allowing people to treat themselves by shining a light on a problem area, such as an arthritic knee.”
Dr Lawrence and his colleagues described their technique in Angewandte Chemie.
The researchers attached various drug molecules (methotrexate, colchicine, and paclitaxel) to vitamin B12 and loaded the compounds into RBCs, which can circulate for up to 4 months, potentially providing a lasting reservoir of treatment that could be tapped as needed.
The team then demonstrated their ability to overcome a long-time technical hurdle: using long-wavelength light to penetrate deep enough into the body to break molecular bonds; in this case, the drug linked to vitamin B12.
Long-wavelength light can penetrate much more deeply into the body, but it doesn’t carry as much energy as short-wavelength light and cannot typically break molecular bonds.
To activate the drugs with long-wavelength light, the researchers had to determine how to do it in a way that required less energy.
“That’s the trick, and that’s where we’ve been successful,” Dr Lawrence said.
The team solved the energy problem by introducing a weak energy bond between vitamin B12 and the drug and then attaching a fluorescent molecule to the bond.
The fluorescent molecule acts as an antenna, capturing long-wavelength light and using it to cut the bond between the drug and the vitamin carrier.
Dr Lawrence noted that this technique could prove useful in treating cancers for which patients may need to receive a wide array of anticancer agents.
“The problem is, when you start using 4 or 5 very toxic drugs, you’re going to have intolerable side effects,” he said. “However, by focusing powerful drugs at a specific site, it may be possible to significantly reduce or eliminate the side effects that commonly accompany cancer chemotherapy.”
Dr Lawrence has created a company in partnership with the University of North Carolina, Iris BioMed, to further develop the technology to be used in humans. ![]()

Researchers say they’ve developed a technique that uses light to activate a drug stored in circulating red blood cells (RBCs) so the drug is released exactly when and where it’s needed.
The group believes the work could have profound implications for the field of drug delivery.
They say the technique could drastically reduce the amount of drug needed to treat diseases and therefore decrease the risk of side effects.
“Using light to treat a disease site has a lot of benefits beyond the ‘isn’t-that-cool’ factor,” said study author David Lawrence, PhD, of the University of North Carolina at Chapel Hill.
“Those benefits could include avoiding surgery and the risk of infection, making anesthesia unnecessary, and allowing people to treat themselves by shining a light on a problem area, such as an arthritic knee.”
Dr Lawrence and his colleagues described their technique in Angewandte Chemie.
The researchers attached various drug molecules (methotrexate, colchicine, and paclitaxel) to vitamin B12 and loaded the compounds into RBCs, which can circulate for up to 4 months, potentially providing a lasting reservoir of treatment that could be tapped as needed.
The team then demonstrated their ability to overcome a long-time technical hurdle: using long-wavelength light to penetrate deep enough into the body to break molecular bonds; in this case, the drug linked to vitamin B12.
Long-wavelength light can penetrate much more deeply into the body, but it doesn’t carry as much energy as short-wavelength light and cannot typically break molecular bonds.
To activate the drugs with long-wavelength light, the researchers had to determine how to do it in a way that required less energy.
“That’s the trick, and that’s where we’ve been successful,” Dr Lawrence said.
The team solved the energy problem by introducing a weak energy bond between vitamin B12 and the drug and then attaching a fluorescent molecule to the bond.
The fluorescent molecule acts as an antenna, capturing long-wavelength light and using it to cut the bond between the drug and the vitamin carrier.
Dr Lawrence noted that this technique could prove useful in treating cancers for which patients may need to receive a wide array of anticancer agents.
“The problem is, when you start using 4 or 5 very toxic drugs, you’re going to have intolerable side effects,” he said. “However, by focusing powerful drugs at a specific site, it may be possible to significantly reduce or eliminate the side effects that commonly accompany cancer chemotherapy.”
Dr Lawrence has created a company in partnership with the University of North Carolina, Iris BioMed, to further develop the technology to be used in humans. ![]()

Researchers say they’ve developed a technique that uses light to activate a drug stored in circulating red blood cells (RBCs) so the drug is released exactly when and where it’s needed.
The group believes the work could have profound implications for the field of drug delivery.
They say the technique could drastically reduce the amount of drug needed to treat diseases and therefore decrease the risk of side effects.
“Using light to treat a disease site has a lot of benefits beyond the ‘isn’t-that-cool’ factor,” said study author David Lawrence, PhD, of the University of North Carolina at Chapel Hill.
“Those benefits could include avoiding surgery and the risk of infection, making anesthesia unnecessary, and allowing people to treat themselves by shining a light on a problem area, such as an arthritic knee.”
Dr Lawrence and his colleagues described their technique in Angewandte Chemie.
The researchers attached various drug molecules (methotrexate, colchicine, and paclitaxel) to vitamin B12 and loaded the compounds into RBCs, which can circulate for up to 4 months, potentially providing a lasting reservoir of treatment that could be tapped as needed.
The team then demonstrated their ability to overcome a long-time technical hurdle: using long-wavelength light to penetrate deep enough into the body to break molecular bonds; in this case, the drug linked to vitamin B12.
Long-wavelength light can penetrate much more deeply into the body, but it doesn’t carry as much energy as short-wavelength light and cannot typically break molecular bonds.
To activate the drugs with long-wavelength light, the researchers had to determine how to do it in a way that required less energy.
“That’s the trick, and that’s where we’ve been successful,” Dr Lawrence said.
The team solved the energy problem by introducing a weak energy bond between vitamin B12 and the drug and then attaching a fluorescent molecule to the bond.
The fluorescent molecule acts as an antenna, capturing long-wavelength light and using it to cut the bond between the drug and the vitamin carrier.
Dr Lawrence noted that this technique could prove useful in treating cancers for which patients may need to receive a wide array of anticancer agents.
“The problem is, when you start using 4 or 5 very toxic drugs, you’re going to have intolerable side effects,” he said. “However, by focusing powerful drugs at a specific site, it may be possible to significantly reduce or eliminate the side effects that commonly accompany cancer chemotherapy.”
Dr Lawrence has created a company in partnership with the University of North Carolina, Iris BioMed, to further develop the technology to be used in humans. ![]()
Transfusing oldest blood may harm patients
Transfusions of blood that has been stored for 6 weeks can release large and potentially harmful amounts of iron into patients’ bloodstreams, a new study suggests.
Based on these findings, researchers are recommending the US Food and Drug Administration (FDA) reduce the maximum storage limit of red blood cells (RBCs) from 6 weeks to 5 weeks, as long as there is a sufficient supply of blood.
“Our recommendation will be controversial, but we think we have real data to support it,” said study author Steven Spitalnik, MD, of Columbia University College of Physicians and Surgeons — New York Presbyterian Hospital in New York, New York.
“Recent studies have concluded that transfusing old blood has no impact on patient outcomes, but those studies didn’t exclusively examine the oldest blood available for transfusions. Our new study found a real problem when transfusing blood that’s older than 5 weeks.”
Dr Spitalnik and his colleagues described their study in The Journal of Clinical Investigation.
The researchers randomly assigned a group of 60 healthy volunteers to receive a unit of RBCs that had been stored for 1, 2, 3, 4, 5, or 6 weeks. The volunteers were then monitored for 20 hours after transfusion.
The researchers found that a longer duration of RBC storage was associated with a progressive increase in extravascular hemolysis, decreasing post-transfusion RBC recovery, decreasing elevations in hematocrit, and increasing serum ferritin.
None of the volunteers were harmed by the transfusions, but subjects who received 6-week-old blood had outcomes associated with an increased risk of harm.
In 7 of the 9 subjects who received the 6-week-old blood (78%), the amount of iron entering the circulation exceeded the iron-uptake capacity of transferrin, producing circulating nontransferrin-bound iron. The same effect occurred in 1 of the 10 subjects who received 5-week-old blood (10%, P=0.003).
The area under the curve of the change in nontransferrin-bound iron was significantly higher for subjects who received the 6-week-old blood than for all other subjects (P<0.001). The same was true for serum iron (P<0.01) and transferrin saturation (P<0.001).
“Based on the amount of iron circulating in the blood of the volunteers who received 6-week-old blood, we’d predict that certain existing infections could be exacerbated,” said study author Eldad Hod, MD, also of Columbia University College of Physicians and Surgeons — New York Presbyterian Hospital.
“Thus, for ill, hospitalized patients, this excess iron could lead to serious complications,” Dr Spitalnik added.
The researchers said the true impact of 6-week-old blood on the rate of complications is likely to be small. However, since millions of Americans receive transfusions each year, even a 1% difference in complications could affect a large number of patients.
“It’s estimated that up to 10% to 20% of blood units used for transfusions have been stored for more than 5 weeks, so the number of patients who are likely to receive a unit of very old blood is substantial,” Dr Hod noted.
“Based on our findings of potential harm, we think the prudent thing to do, at this time, is for the FDA to reduce the maximum storage period,” Dr Spitalnik added.
“The UK, Ireland, the Netherlands, and the National Institutes of Health have limited storage to 35 days, and we think that can be achieved throughout the US without seriously affecting the blood supply.”
This study was supported by grants from the National Institutes of Health (HL115557 and UL1TR000040). ![]()
Transfusions of blood that has been stored for 6 weeks can release large and potentially harmful amounts of iron into patients’ bloodstreams, a new study suggests.
Based on these findings, researchers are recommending the US Food and Drug Administration (FDA) reduce the maximum storage limit of red blood cells (RBCs) from 6 weeks to 5 weeks, as long as there is a sufficient supply of blood.
“Our recommendation will be controversial, but we think we have real data to support it,” said study author Steven Spitalnik, MD, of Columbia University College of Physicians and Surgeons — New York Presbyterian Hospital in New York, New York.
“Recent studies have concluded that transfusing old blood has no impact on patient outcomes, but those studies didn’t exclusively examine the oldest blood available for transfusions. Our new study found a real problem when transfusing blood that’s older than 5 weeks.”
Dr Spitalnik and his colleagues described their study in The Journal of Clinical Investigation.
The researchers randomly assigned a group of 60 healthy volunteers to receive a unit of RBCs that had been stored for 1, 2, 3, 4, 5, or 6 weeks. The volunteers were then monitored for 20 hours after transfusion.
The researchers found that a longer duration of RBC storage was associated with a progressive increase in extravascular hemolysis, decreasing post-transfusion RBC recovery, decreasing elevations in hematocrit, and increasing serum ferritin.
None of the volunteers were harmed by the transfusions, but subjects who received 6-week-old blood had outcomes associated with an increased risk of harm.
In 7 of the 9 subjects who received the 6-week-old blood (78%), the amount of iron entering the circulation exceeded the iron-uptake capacity of transferrin, producing circulating nontransferrin-bound iron. The same effect occurred in 1 of the 10 subjects who received 5-week-old blood (10%, P=0.003).
The area under the curve of the change in nontransferrin-bound iron was significantly higher for subjects who received the 6-week-old blood than for all other subjects (P<0.001). The same was true for serum iron (P<0.01) and transferrin saturation (P<0.001).
“Based on the amount of iron circulating in the blood of the volunteers who received 6-week-old blood, we’d predict that certain existing infections could be exacerbated,” said study author Eldad Hod, MD, also of Columbia University College of Physicians and Surgeons — New York Presbyterian Hospital.
“Thus, for ill, hospitalized patients, this excess iron could lead to serious complications,” Dr Spitalnik added.
The researchers said the true impact of 6-week-old blood on the rate of complications is likely to be small. However, since millions of Americans receive transfusions each year, even a 1% difference in complications could affect a large number of patients.
“It’s estimated that up to 10% to 20% of blood units used for transfusions have been stored for more than 5 weeks, so the number of patients who are likely to receive a unit of very old blood is substantial,” Dr Hod noted.
“Based on our findings of potential harm, we think the prudent thing to do, at this time, is for the FDA to reduce the maximum storage period,” Dr Spitalnik added.
“The UK, Ireland, the Netherlands, and the National Institutes of Health have limited storage to 35 days, and we think that can be achieved throughout the US without seriously affecting the blood supply.”
This study was supported by grants from the National Institutes of Health (HL115557 and UL1TR000040). ![]()
Transfusions of blood that has been stored for 6 weeks can release large and potentially harmful amounts of iron into patients’ bloodstreams, a new study suggests.
Based on these findings, researchers are recommending the US Food and Drug Administration (FDA) reduce the maximum storage limit of red blood cells (RBCs) from 6 weeks to 5 weeks, as long as there is a sufficient supply of blood.
“Our recommendation will be controversial, but we think we have real data to support it,” said study author Steven Spitalnik, MD, of Columbia University College of Physicians and Surgeons — New York Presbyterian Hospital in New York, New York.
“Recent studies have concluded that transfusing old blood has no impact on patient outcomes, but those studies didn’t exclusively examine the oldest blood available for transfusions. Our new study found a real problem when transfusing blood that’s older than 5 weeks.”
Dr Spitalnik and his colleagues described their study in The Journal of Clinical Investigation.
The researchers randomly assigned a group of 60 healthy volunteers to receive a unit of RBCs that had been stored for 1, 2, 3, 4, 5, or 6 weeks. The volunteers were then monitored for 20 hours after transfusion.
The researchers found that a longer duration of RBC storage was associated with a progressive increase in extravascular hemolysis, decreasing post-transfusion RBC recovery, decreasing elevations in hematocrit, and increasing serum ferritin.
None of the volunteers were harmed by the transfusions, but subjects who received 6-week-old blood had outcomes associated with an increased risk of harm.
In 7 of the 9 subjects who received the 6-week-old blood (78%), the amount of iron entering the circulation exceeded the iron-uptake capacity of transferrin, producing circulating nontransferrin-bound iron. The same effect occurred in 1 of the 10 subjects who received 5-week-old blood (10%, P=0.003).
The area under the curve of the change in nontransferrin-bound iron was significantly higher for subjects who received the 6-week-old blood than for all other subjects (P<0.001). The same was true for serum iron (P<0.01) and transferrin saturation (P<0.001).
“Based on the amount of iron circulating in the blood of the volunteers who received 6-week-old blood, we’d predict that certain existing infections could be exacerbated,” said study author Eldad Hod, MD, also of Columbia University College of Physicians and Surgeons — New York Presbyterian Hospital.
“Thus, for ill, hospitalized patients, this excess iron could lead to serious complications,” Dr Spitalnik added.
The researchers said the true impact of 6-week-old blood on the rate of complications is likely to be small. However, since millions of Americans receive transfusions each year, even a 1% difference in complications could affect a large number of patients.
“It’s estimated that up to 10% to 20% of blood units used for transfusions have been stored for more than 5 weeks, so the number of patients who are likely to receive a unit of very old blood is substantial,” Dr Hod noted.
“Based on our findings of potential harm, we think the prudent thing to do, at this time, is for the FDA to reduce the maximum storage period,” Dr Spitalnik added.
“The UK, Ireland, the Netherlands, and the National Institutes of Health have limited storage to 35 days, and we think that can be achieved throughout the US without seriously affecting the blood supply.”
This study was supported by grants from the National Institutes of Health (HL115557 and UL1TR000040). ![]()
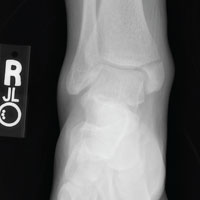
From Hydroplane to Ankle Pain
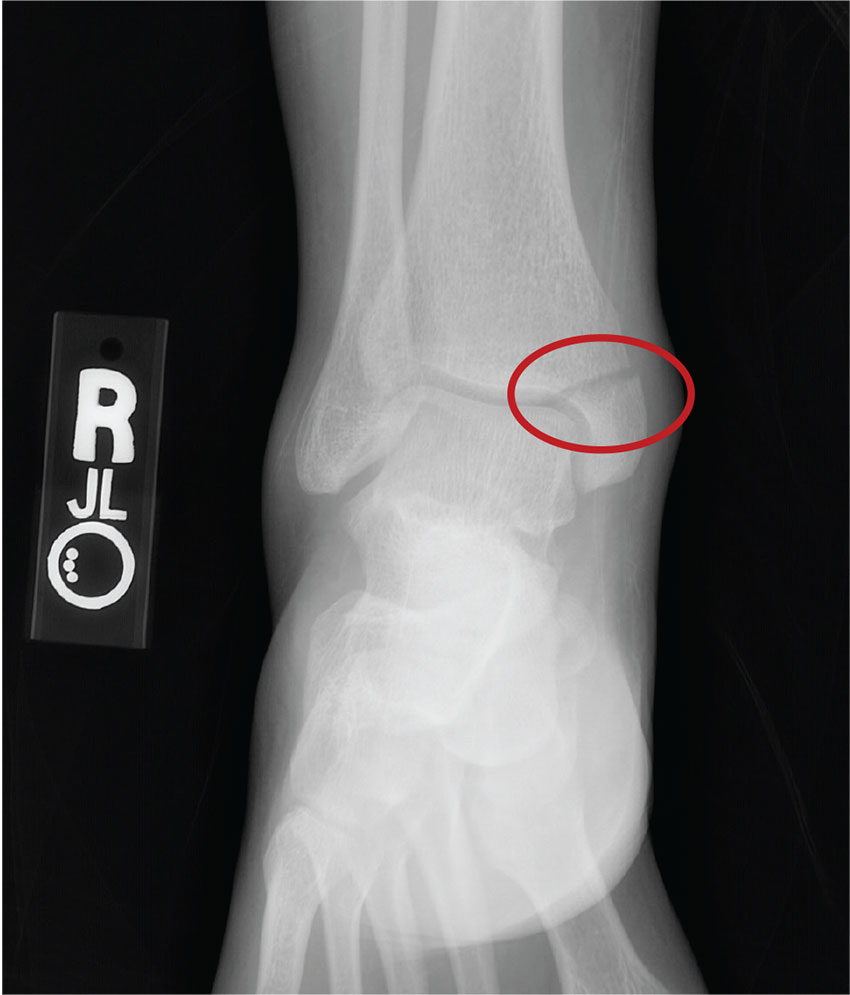
ANSWER
The radiograph shows an acute fracture of the medial malleolus. It is minimally displaced. The mortise joint appears intact. The patient was placed in a short leg splint for immobilization, and prompt orthopedic follow-up was arranged.

ANSWER
The radiograph shows an acute fracture of the medial malleolus. It is minimally displaced. The mortise joint appears intact. The patient was placed in a short leg splint for immobilization, and prompt orthopedic follow-up was arranged.

ANSWER
The radiograph shows an acute fracture of the medial malleolus. It is minimally displaced. The mortise joint appears intact. The patient was placed in a short leg splint for immobilization, and prompt orthopedic follow-up was arranged.

A 40-year-old woman presents to urgent care for evaluation of ankle pain following a car accident. She was a restrained driver who lost control of her vehicle while driving on wet roads. Her vehicle hit a telephone pole head on. There was no air bag deployment. Initially, she thought she was fine and declined EMS transport to a local hospital. But when she experienced severe pain bearing weight on her right foot, she opted to have it evaluated.
She denies any other complaints. Her medical history is otherwise unremarkable, and vital signs are normal. Physical examination of her right ankle demonstrates general soft-tissue swelling but no obvious deformity. She has moderate tenderness on both the medial and lateral aspects of her ankle. She has limited dorsiflexion and plantar flexion secondary to pain. Good distal pulses are palpable, and good capillary refill is noted in all of the toes.
A radiograph of the ankle is shown. What is your impression?
Nutrition expert to heart patients: ‘Eat some cheese’
NEW ORLEANS – While many Americans have been dithering over the relative health benefits of high- versus low-carbohydrate diets, various pop-culture weight loss programs, vegetarianism, gluten-free living, and other nutritional matters, a quiet revolution in mainstream scientific thinking has occurred regarding the role of full-fat dairy products.
Saturated fatty acid–rich dairy products, formerly viewed as the enemy of cardiovascular health, have gone from foe to friend, according to Arne Astrup, MD, professor and head of the department of nutrition, exercise and sports at the University of Copenhagen.
“From all I have seen, I think it’s quite safe to recommend that our diabetics and heart patients eat some cheese without being afraid of it. I don’t think there’s any harmful effect, and it could actually be very beneficial,” Dr. Astrup continued.
For example, a recent comprehensive meta-analysis of 31 prospective cohort studies found that a high dairy intake was associated with a 9% reduction in the risk of stroke, compared with low or no dairy consumption. Of note, high cheese intake was associated with an 18% lower risk of coronary heart disease (CHD) and a 13% reduction in risk of stroke (Br J Nutr. 2016;115[4]:737-50).
Dutch investigators reported based upon their meta-analysis of 18 prospective cohort studies with 8-26 years of follow-up that stroke risk fell by 7% for each 200 mL of milk consumed per day. Consumption of 25 g/day or more of cheese was associated with a 13% reduction in stroke risk and an 8% lower risk of CHD (J Am Heart Assoc. 2016 May 20;5[5]. doi: 10.1161/JAHA.115.002787).
“The totality of evidence – meta-analyses of both observational studies and randomized controlled trials – cannot find any harmful effects of cheese on body fat, metabolic syndrome, type 2 diabetes, or cardiovascular disease,” he said. “And cheese has beneficial effects on LDL cholesterol, blood pressure, and postprandial triglycerides as compared with butter containing the same amount of saturated fatty acids.”
The classic lipid hypothesis of cardiovascular disease holds that dietary saturated fat raises blood cholesterol, in turn accelerating atherosclerosis and resultant coronary heart disease. But the published literature of the past few years indicates it’s not that simple. All saturated fats are not equally harmful. They have very different biologic effects, and the food matrix in which they occur seems to be important. The saturated fatty acids found in red meat are clearly damaging. Ditto trans fats.
In contrast, the saturated fats present in milk, hard cheeses, and fermented dairy products such as yogurt have been shown in a variety of study formats to be cardioprotective. They also appear to protect against other chronic diseases as well, according to the researcher.
“If we look at all the different meta-analyses addressing the various cardiovascular risk factors, it really looks like cheese, despite its high content of sodium and saturated fat, seems to exert some beneficial effects. So I think we need to address the food matrix much more. We’ve done controlled feeding trials in humans and found that if we give subjects the same amount of saturated fat from either butter or cheese, you see following the cheese [that] the subjects do not increase their total or LDL-cholesterol as you would expect based upon their intake of saturated fat. So there’s something going on with cheese,” Dr. Astrup said.
What’s going on, he continued, is the saturated fats in cheese benefit from the company they keep. Fermented dairy products contain an arm-long list of potentially beneficial nutrients, including protein, calcium, short-chain fatty acids, bioactive peptides, and phospholipids.
Take, for example, calcium: “We’ve found the calcium content of cheese completely modifies the metabolism of the saturated fat. The calcium seems to bind the bile acids and fatty acids, resulting in increased fecal fat secretion,” according to Dr. Astrup.
Although at the AHA meeting he focused mainly on the effects of cheese and other dairy products on cardiovascular health, in a recent review article he expanded upon the scientific evidence regarding the impact of these foods on the risks of obesity, type 2 diabetes, cancer, and osteoporosis (Food Nutr Res. 2016 Nov 22;60:32527).
There is solid evidence that a diet high in dairy products reduces the risk of childhood obesity and enhances body composition in adults. It aids in weight loss by promoting satiety during periods of energy restriction. A recent meta-analysis of observational studies found an inverse relationship between consumption of fermented dairy products – yogurt and cheese – and risk of type 2 diabetes (Am J Clin Nutr. 2016 Apr;103[4]:1111-24).
Regarding cancer, the World Cancer Research Fund has issued a series of evidence reviews concluding that dairy products probably protect against colorectal, breast, gastric, and bladder cancer. The jury is still out regarding prostate cancer risk.
A wealth of evidence indicates dairy consumption has a beneficial effect on bone health in children and adolescents. However, meta-analyses haven’t shown a protective effect against osteoporosis and fractures in adults. This is consistent with the adage that osteoporosis is a pediatric disease with geriatric consequences, Dr. Astrup noted.
He reported receiving research grants from the Danish Dairy Research Foundation, the Global Dairy Platform, the Danish Agriculture and Food Council, and the European Milk Forum. He serves on advisory boards for the Dutch Beer Knowledge Institute, Suntory, Weight Watchers, and several food companies.
NEW ORLEANS – While many Americans have been dithering over the relative health benefits of high- versus low-carbohydrate diets, various pop-culture weight loss programs, vegetarianism, gluten-free living, and other nutritional matters, a quiet revolution in mainstream scientific thinking has occurred regarding the role of full-fat dairy products.
Saturated fatty acid–rich dairy products, formerly viewed as the enemy of cardiovascular health, have gone from foe to friend, according to Arne Astrup, MD, professor and head of the department of nutrition, exercise and sports at the University of Copenhagen.
“From all I have seen, I think it’s quite safe to recommend that our diabetics and heart patients eat some cheese without being afraid of it. I don’t think there’s any harmful effect, and it could actually be very beneficial,” Dr. Astrup continued.
For example, a recent comprehensive meta-analysis of 31 prospective cohort studies found that a high dairy intake was associated with a 9% reduction in the risk of stroke, compared with low or no dairy consumption. Of note, high cheese intake was associated with an 18% lower risk of coronary heart disease (CHD) and a 13% reduction in risk of stroke (Br J Nutr. 2016;115[4]:737-50).
Dutch investigators reported based upon their meta-analysis of 18 prospective cohort studies with 8-26 years of follow-up that stroke risk fell by 7% for each 200 mL of milk consumed per day. Consumption of 25 g/day or more of cheese was associated with a 13% reduction in stroke risk and an 8% lower risk of CHD (J Am Heart Assoc. 2016 May 20;5[5]. doi: 10.1161/JAHA.115.002787).
“The totality of evidence – meta-analyses of both observational studies and randomized controlled trials – cannot find any harmful effects of cheese on body fat, metabolic syndrome, type 2 diabetes, or cardiovascular disease,” he said. “And cheese has beneficial effects on LDL cholesterol, blood pressure, and postprandial triglycerides as compared with butter containing the same amount of saturated fatty acids.”
The classic lipid hypothesis of cardiovascular disease holds that dietary saturated fat raises blood cholesterol, in turn accelerating atherosclerosis and resultant coronary heart disease. But the published literature of the past few years indicates it’s not that simple. All saturated fats are not equally harmful. They have very different biologic effects, and the food matrix in which they occur seems to be important. The saturated fatty acids found in red meat are clearly damaging. Ditto trans fats.
In contrast, the saturated fats present in milk, hard cheeses, and fermented dairy products such as yogurt have been shown in a variety of study formats to be cardioprotective. They also appear to protect against other chronic diseases as well, according to the researcher.
“If we look at all the different meta-analyses addressing the various cardiovascular risk factors, it really looks like cheese, despite its high content of sodium and saturated fat, seems to exert some beneficial effects. So I think we need to address the food matrix much more. We’ve done controlled feeding trials in humans and found that if we give subjects the same amount of saturated fat from either butter or cheese, you see following the cheese [that] the subjects do not increase their total or LDL-cholesterol as you would expect based upon their intake of saturated fat. So there’s something going on with cheese,” Dr. Astrup said.
What’s going on, he continued, is the saturated fats in cheese benefit from the company they keep. Fermented dairy products contain an arm-long list of potentially beneficial nutrients, including protein, calcium, short-chain fatty acids, bioactive peptides, and phospholipids.
Take, for example, calcium: “We’ve found the calcium content of cheese completely modifies the metabolism of the saturated fat. The calcium seems to bind the bile acids and fatty acids, resulting in increased fecal fat secretion,” according to Dr. Astrup.
Although at the AHA meeting he focused mainly on the effects of cheese and other dairy products on cardiovascular health, in a recent review article he expanded upon the scientific evidence regarding the impact of these foods on the risks of obesity, type 2 diabetes, cancer, and osteoporosis (Food Nutr Res. 2016 Nov 22;60:32527).
There is solid evidence that a diet high in dairy products reduces the risk of childhood obesity and enhances body composition in adults. It aids in weight loss by promoting satiety during periods of energy restriction. A recent meta-analysis of observational studies found an inverse relationship between consumption of fermented dairy products – yogurt and cheese – and risk of type 2 diabetes (Am J Clin Nutr. 2016 Apr;103[4]:1111-24).
Regarding cancer, the World Cancer Research Fund has issued a series of evidence reviews concluding that dairy products probably protect against colorectal, breast, gastric, and bladder cancer. The jury is still out regarding prostate cancer risk.
A wealth of evidence indicates dairy consumption has a beneficial effect on bone health in children and adolescents. However, meta-analyses haven’t shown a protective effect against osteoporosis and fractures in adults. This is consistent with the adage that osteoporosis is a pediatric disease with geriatric consequences, Dr. Astrup noted.
He reported receiving research grants from the Danish Dairy Research Foundation, the Global Dairy Platform, the Danish Agriculture and Food Council, and the European Milk Forum. He serves on advisory boards for the Dutch Beer Knowledge Institute, Suntory, Weight Watchers, and several food companies.
NEW ORLEANS – While many Americans have been dithering over the relative health benefits of high- versus low-carbohydrate diets, various pop-culture weight loss programs, vegetarianism, gluten-free living, and other nutritional matters, a quiet revolution in mainstream scientific thinking has occurred regarding the role of full-fat dairy products.
Saturated fatty acid–rich dairy products, formerly viewed as the enemy of cardiovascular health, have gone from foe to friend, according to Arne Astrup, MD, professor and head of the department of nutrition, exercise and sports at the University of Copenhagen.
“From all I have seen, I think it’s quite safe to recommend that our diabetics and heart patients eat some cheese without being afraid of it. I don’t think there’s any harmful effect, and it could actually be very beneficial,” Dr. Astrup continued.
For example, a recent comprehensive meta-analysis of 31 prospective cohort studies found that a high dairy intake was associated with a 9% reduction in the risk of stroke, compared with low or no dairy consumption. Of note, high cheese intake was associated with an 18% lower risk of coronary heart disease (CHD) and a 13% reduction in risk of stroke (Br J Nutr. 2016;115[4]:737-50).
Dutch investigators reported based upon their meta-analysis of 18 prospective cohort studies with 8-26 years of follow-up that stroke risk fell by 7% for each 200 mL of milk consumed per day. Consumption of 25 g/day or more of cheese was associated with a 13% reduction in stroke risk and an 8% lower risk of CHD (J Am Heart Assoc. 2016 May 20;5[5]. doi: 10.1161/JAHA.115.002787).
“The totality of evidence – meta-analyses of both observational studies and randomized controlled trials – cannot find any harmful effects of cheese on body fat, metabolic syndrome, type 2 diabetes, or cardiovascular disease,” he said. “And cheese has beneficial effects on LDL cholesterol, blood pressure, and postprandial triglycerides as compared with butter containing the same amount of saturated fatty acids.”
The classic lipid hypothesis of cardiovascular disease holds that dietary saturated fat raises blood cholesterol, in turn accelerating atherosclerosis and resultant coronary heart disease. But the published literature of the past few years indicates it’s not that simple. All saturated fats are not equally harmful. They have very different biologic effects, and the food matrix in which they occur seems to be important. The saturated fatty acids found in red meat are clearly damaging. Ditto trans fats.
In contrast, the saturated fats present in milk, hard cheeses, and fermented dairy products such as yogurt have been shown in a variety of study formats to be cardioprotective. They also appear to protect against other chronic diseases as well, according to the researcher.
“If we look at all the different meta-analyses addressing the various cardiovascular risk factors, it really looks like cheese, despite its high content of sodium and saturated fat, seems to exert some beneficial effects. So I think we need to address the food matrix much more. We’ve done controlled feeding trials in humans and found that if we give subjects the same amount of saturated fat from either butter or cheese, you see following the cheese [that] the subjects do not increase their total or LDL-cholesterol as you would expect based upon their intake of saturated fat. So there’s something going on with cheese,” Dr. Astrup said.
What’s going on, he continued, is the saturated fats in cheese benefit from the company they keep. Fermented dairy products contain an arm-long list of potentially beneficial nutrients, including protein, calcium, short-chain fatty acids, bioactive peptides, and phospholipids.
Take, for example, calcium: “We’ve found the calcium content of cheese completely modifies the metabolism of the saturated fat. The calcium seems to bind the bile acids and fatty acids, resulting in increased fecal fat secretion,” according to Dr. Astrup.
Although at the AHA meeting he focused mainly on the effects of cheese and other dairy products on cardiovascular health, in a recent review article he expanded upon the scientific evidence regarding the impact of these foods on the risks of obesity, type 2 diabetes, cancer, and osteoporosis (Food Nutr Res. 2016 Nov 22;60:32527).
There is solid evidence that a diet high in dairy products reduces the risk of childhood obesity and enhances body composition in adults. It aids in weight loss by promoting satiety during periods of energy restriction. A recent meta-analysis of observational studies found an inverse relationship between consumption of fermented dairy products – yogurt and cheese – and risk of type 2 diabetes (Am J Clin Nutr. 2016 Apr;103[4]:1111-24).
Regarding cancer, the World Cancer Research Fund has issued a series of evidence reviews concluding that dairy products probably protect against colorectal, breast, gastric, and bladder cancer. The jury is still out regarding prostate cancer risk.
A wealth of evidence indicates dairy consumption has a beneficial effect on bone health in children and adolescents. However, meta-analyses haven’t shown a protective effect against osteoporosis and fractures in adults. This is consistent with the adage that osteoporosis is a pediatric disease with geriatric consequences, Dr. Astrup noted.
He reported receiving research grants from the Danish Dairy Research Foundation, the Global Dairy Platform, the Danish Agriculture and Food Council, and the European Milk Forum. He serves on advisory boards for the Dutch Beer Knowledge Institute, Suntory, Weight Watchers, and several food companies.
FROM THE AHA SCIENTIFIC SESSIONS
Chest-worn seizure detection device shows promise
HOUSTON – An investigative, chest-worn device shows promise for detecting a wide range of seizure types in children with epilepsy, results from a small single-center study showed.
“Our goal is for parents to have more peace of mind, not feeling like they have to watch their kids all night long,” one of the study authors, Kristin H. Gilchrist, PhD, said in an interview at the annual meeting of the American Epilepsy Society. “There are currently many wearable devices marketed for fitness [purposes], but if we can leverage some of these heart rate monitors and use them to detect seizures with specialized algorithms, that would be ideal.”
Approximately 60% of patients had a seizure during the monitoring period. Seizures without any clinical response or those lasting less than 10 seconds (such as single myoclonic jerks or clusters) were excluded from analysis, as were subjects with multiple seizures per hour because the autonomic signals often did not return to baseline, and this seizure frequency is outside of the intended use of the monitor. After exclusions, the algorithm was evaluated on 10 children with a mean age of 12 years. For additional validation, the algorithm was also evaluated with the Massachusetts Institute of Technology, Boston, PhysioNet database with ECG from five adults with partial seizures (Neurology. 1999;53:1590-2).The algorithm without motion parameters detected all seizures classified as tonic-clonic (3/3) or atonic/clonic/tonic (4/4), and 3/9 and 7/10 of focal seizures from the CNMC and MIT subjects, respectively. In the CNMC dataset, the motion algorithm detected all seizures classified as tonic-clonic (3/3), half of those categorized as atonic/clonic/tonic (2/4), and one of nine classified as focal seizures.
In the CNMC dataset, false positives averaged one per 14 hours, however, the majority of false positives occurred in a few patients with poor sensor data quality. More than half of the subjects (70%) had no false positives. One false positive occurred in the 16.8 hours of MIT data.
“In addition to an alert application, we have a technology that can be beneficial to clinic-based studies to quantify how many seizures people are having,” Dr. Gilchrist said. “Hopefully someday it will reach the commercial market.” She reported having no financial disclosures.
HOUSTON – An investigative, chest-worn device shows promise for detecting a wide range of seizure types in children with epilepsy, results from a small single-center study showed.
“Our goal is for parents to have more peace of mind, not feeling like they have to watch their kids all night long,” one of the study authors, Kristin H. Gilchrist, PhD, said in an interview at the annual meeting of the American Epilepsy Society. “There are currently many wearable devices marketed for fitness [purposes], but if we can leverage some of these heart rate monitors and use them to detect seizures with specialized algorithms, that would be ideal.”
Approximately 60% of patients had a seizure during the monitoring period. Seizures without any clinical response or those lasting less than 10 seconds (such as single myoclonic jerks or clusters) were excluded from analysis, as were subjects with multiple seizures per hour because the autonomic signals often did not return to baseline, and this seizure frequency is outside of the intended use of the monitor. After exclusions, the algorithm was evaluated on 10 children with a mean age of 12 years. For additional validation, the algorithm was also evaluated with the Massachusetts Institute of Technology, Boston, PhysioNet database with ECG from five adults with partial seizures (Neurology. 1999;53:1590-2).The algorithm without motion parameters detected all seizures classified as tonic-clonic (3/3) or atonic/clonic/tonic (4/4), and 3/9 and 7/10 of focal seizures from the CNMC and MIT subjects, respectively. In the CNMC dataset, the motion algorithm detected all seizures classified as tonic-clonic (3/3), half of those categorized as atonic/clonic/tonic (2/4), and one of nine classified as focal seizures.
In the CNMC dataset, false positives averaged one per 14 hours, however, the majority of false positives occurred in a few patients with poor sensor data quality. More than half of the subjects (70%) had no false positives. One false positive occurred in the 16.8 hours of MIT data.
“In addition to an alert application, we have a technology that can be beneficial to clinic-based studies to quantify how many seizures people are having,” Dr. Gilchrist said. “Hopefully someday it will reach the commercial market.” She reported having no financial disclosures.
HOUSTON – An investigative, chest-worn device shows promise for detecting a wide range of seizure types in children with epilepsy, results from a small single-center study showed.
“Our goal is for parents to have more peace of mind, not feeling like they have to watch their kids all night long,” one of the study authors, Kristin H. Gilchrist, PhD, said in an interview at the annual meeting of the American Epilepsy Society. “There are currently many wearable devices marketed for fitness [purposes], but if we can leverage some of these heart rate monitors and use them to detect seizures with specialized algorithms, that would be ideal.”
Approximately 60% of patients had a seizure during the monitoring period. Seizures without any clinical response or those lasting less than 10 seconds (such as single myoclonic jerks or clusters) were excluded from analysis, as were subjects with multiple seizures per hour because the autonomic signals often did not return to baseline, and this seizure frequency is outside of the intended use of the monitor. After exclusions, the algorithm was evaluated on 10 children with a mean age of 12 years. For additional validation, the algorithm was also evaluated with the Massachusetts Institute of Technology, Boston, PhysioNet database with ECG from five adults with partial seizures (Neurology. 1999;53:1590-2).The algorithm without motion parameters detected all seizures classified as tonic-clonic (3/3) or atonic/clonic/tonic (4/4), and 3/9 and 7/10 of focal seizures from the CNMC and MIT subjects, respectively. In the CNMC dataset, the motion algorithm detected all seizures classified as tonic-clonic (3/3), half of those categorized as atonic/clonic/tonic (2/4), and one of nine classified as focal seizures.
In the CNMC dataset, false positives averaged one per 14 hours, however, the majority of false positives occurred in a few patients with poor sensor data quality. More than half of the subjects (70%) had no false positives. One false positive occurred in the 16.8 hours of MIT data.
“In addition to an alert application, we have a technology that can be beneficial to clinic-based studies to quantify how many seizures people are having,” Dr. Gilchrist said. “Hopefully someday it will reach the commercial market.” She reported having no financial disclosures.
AT AES 2016
Key clinical point:
Major finding: The algorithm without motion parameters detected all seizures classified as tonic-clonic (3/3) or atonic/clonic/tonic (4/4), and 3 of 9 classified as focal seizures.
Data source: A clinic-based study of 10 epilepsy patients with a mean age of 12 years who wore an investigative device to detect seizures.
Disclosures: The study was funded by a grant from the National Institutes of Health. Dr. Gilchrist reported having no financial disclosures.
Survey: Docs see health care improvements as unlikely in 2017
Physicians appear to be quite pessimistic about the chances for improving health care quality, costs, and access in 2017, according to a survey conducted by InCrowd, a market insights technology firm.
Of the 150 physicians who responded to the 3-minute “mobile microsurvey” conducted on Dec. 4, 2016, 70% said it was unlikely that the cost of health care would improve under the incoming Trump administration, 69% said it was unlikely that access to care would improve, and 60% said it was unlikely that the quality of health care would improve, InCrowd reported.
Physicians appear to be quite pessimistic about the chances for improving health care quality, costs, and access in 2017, according to a survey conducted by InCrowd, a market insights technology firm.
Of the 150 physicians who responded to the 3-minute “mobile microsurvey” conducted on Dec. 4, 2016, 70% said it was unlikely that the cost of health care would improve under the incoming Trump administration, 69% said it was unlikely that access to care would improve, and 60% said it was unlikely that the quality of health care would improve, InCrowd reported.
Physicians appear to be quite pessimistic about the chances for improving health care quality, costs, and access in 2017, according to a survey conducted by InCrowd, a market insights technology firm.
Of the 150 physicians who responded to the 3-minute “mobile microsurvey” conducted on Dec. 4, 2016, 70% said it was unlikely that the cost of health care would improve under the incoming Trump administration, 69% said it was unlikely that access to care would improve, and 60% said it was unlikely that the quality of health care would improve, InCrowd reported.