User login
Failed Alzheimer’s Trial May Offer Hopeful Signals
SAN DIEGO—Solanezumab may not have slowed the clinical progression of Alzheimer’s disease, but it provided valuable evidence for the amyloid hypothesis, experts said during a wide-ranging discussion of Eli Lilly and Company’s recent EXPEDITION3 trial.
Lilly representatives and EXPEDITION investigators presented the study’s results at the Ninth Annual Clinical Trials for Alzheimer’s Disease meeting. While solanezumab failed to meet its primary end point, it did achieve significance on several secondary end points—findings that should be read as encouraging, rather than as a defeat, according to Paul Aisen, MD, Director of the Alzheimer’s Therapeutic Research Institute at the University of Southern California, Los Angeles.
“We have here a negative study that confirms a beneficial treatment,” said Dr. Aisen, an EXPEDITION3 investigator. “We have a treatment that engages its target, binds to soluble amyloid, and, by virtue of that mechanism, is slowing cognitive and functional decline,” not only in EXPEDITION3, but in its predecessors, EXPEDITION and EXPEDITION2.
“This is not a refutation of the amyloid hypothesis, but a confirmation of it.”
Nevertheless, the drug failed its trial, he said. There was no statistically significant separation between solanezumab and placebo on the 14-item Alzheimer’s Disease Assessment Scale-Cognitive subscale (ADAS-Cog14), an assessment of cognitive function that was the study’s primary end point. The active treatment group experienced 11% less decline than did the placebo group, but the result was not significant.
If the result had been significant, “it would still be a small effect size,” which would have thrown into question the drug’s clinical utility, Dr. Aisen said. “We thought we might see a 30% slowing of decline on the ADAS-Cog, and it was disappointing to only get 11%. But that is also what we saw on the key [secondary end points]. Overall, the effect size looks to be about 12% to 13%, and that’s just too small.”
Three Phase III Trials
EXPEDITION3 was the last of a triad of solanezumab studies, all of which posted signals of cognitive and functional benefit in patients with mild Alzheimer’s disease. It was based on subgroup analyses of EXPEDITION and EXPEDITION2, both of which failed to meet their primary end points and included patients with mild and moderate disease. When researchers pooled the patients with mild disease from the first two studies, they found that solanezumab was associated with a 34% slowing of cognitive decline on the ADAS-Cog14. Lilly conducted EXPEDITION3 in an attempt to confirm those findings.
Lawrence S. Honig, MD, PhD, Professor of Neurology at Columbia University Medical Center in New York and principal investigator of the EXPEDITION3 study, detailed the study’s results, including biomarker data.
The study included about 2,000 patients with imaging-confirmed amyloid brain plaques and mild dementia due to Alzheimer’s disease. They were randomized to receive placebo or monthly injections of 400 mg of solanezumab for 80 weeks. The study was conducted at 210 sites in 11 countries.
While solanezumab’s effect on the ADAS-Cog was not significant, its effect on the Mini-Mental State Examination score was significant, with a 13% slowing of decline, compared with placebo. There was also a significant 5% difference in the Clinical Dementia Rating scale-Sum of Boxes score.
Outcomes were mixed in measures of function. On the Alzheimer’s Disease Cooperative Study Activities of Daily Living (ADCS-ADL) and its related measure, the ADCS-ADL inventory instrumental items, patients who received solanezumab had significant 15% and 14% differences, respectively, relative to placebo.
But differences on the Functional Activities Questionnaire, an informant measure of more complex activities, were not significant.
Biomarkers trended the right way, Dr. Honig noted. Solanezumab resulted in a 500- to 800-fold increase in amyloid beta in plasma, relative to placebo. There were no changes in amyloid brain plaques, as measured by PET imaging. This finding was not surprising because the antibody does not recognize fibrillar amyloid, Dr. Aisen said.
“What we expect to see with biomarkers differs based on the epitope targeted,” he said. “Solanezumab ignores plaques. It targets the middle of the peptide, binding to soluble amyloid beta. Now, how that helps [treat Alzheimer’s disease] is something of a debate, but it is important to recognize that it does not attack plaques. Instead, by tying up monomeric amyloid beta, it may change the dynamic exchange of various species of amyloid around plaques; the toxicity of amyloid is thought to reside as much in oligomeric species as in the fibrillar deposits. I see this [plasma amyloid beta increase] as confirming that it’s tying up monomeric amyloid species and that the result is a slowing of disease progression. I believe it is supportive of the amyloid hypothesis.”
Solanezumab had no significant effect on tau in CSF or on imaging, nor did it change the progression of ventricular enlargement, a marker of whole brain atrophy.
The antibody was safe, with 17% of patients who received solanezumab reporting an adverse event, compared with 19% of patients who received placebo. There were nine deaths in the solanezumab arm and 16 in the placebo arm; about 4% of each group discontinued treatment because of an adverse event.
In late November 2016, Eric Siemers, MD, Senior Medical Director of the Alzheimer’s Disease Global Development Team at Lilly, said the company would not seek regulatory approval for solanezumab based on the trial results. “We didn’t expect this to be a cure for this disease, but we did hope it would be the first drug to slow its progress.... We are very disappointed,” Dr. Siemers said during the panel discussion.
Further Trials of Solanezumab
He and Dr. Aisen confirmed, however, that two other trials using solanezumab in different populations would go forward. The Anti-Amyloid Treatment in Asymptomatic Alzheimer’s study (A4 study) is investigating its effect in cognitively healthy elderly people with Alzheimer’s disease risk factors, and the Dominantly Inherited Alzheimer’s Network (DIAN) study is investigating its effects in patients with autosomal dominant mutations in Alzheimer’s disease genes.
Dr. Aisen is excited about solanezumab’s potential to target the disease before cognitive symptoms develop. “I expect all antiamyloid treatments would work better when neurodegeneration is not extensive,” he said. “Any of the antiamyloid antibodies would theoretically be more effective at a preclinical stage of Alzheimer’s disease than even in the mild dementia stage.”
Maria Carrillo, PhD, Chief Science Officer of the Alzheimer’s Association, said that EXPEDITION3 was far from a path to nowhere and urged the research community, patients, and families to double down on their commitment to tackling the disease.
“These results stress the urgency for pushing forward harder,” Dr. Carrillo said. “This is not a time to slow down. It’s a time to ramp up our efforts. This is not the time to sit back and say, ‘The amyloid hypothesis has been the wrong pathway, and we need to drop it.’ But we also need to pursue other pathways, to broaden our approach, and to broaden the armamentarium our clinicians will need to combat this disease.
“This is not a win, true. But it gets us a little closer to one.”
—Michele G. Sullivan
SAN DIEGO—Solanezumab may not have slowed the clinical progression of Alzheimer’s disease, but it provided valuable evidence for the amyloid hypothesis, experts said during a wide-ranging discussion of Eli Lilly and Company’s recent EXPEDITION3 trial.
Lilly representatives and EXPEDITION investigators presented the study’s results at the Ninth Annual Clinical Trials for Alzheimer’s Disease meeting. While solanezumab failed to meet its primary end point, it did achieve significance on several secondary end points—findings that should be read as encouraging, rather than as a defeat, according to Paul Aisen, MD, Director of the Alzheimer’s Therapeutic Research Institute at the University of Southern California, Los Angeles.
“We have here a negative study that confirms a beneficial treatment,” said Dr. Aisen, an EXPEDITION3 investigator. “We have a treatment that engages its target, binds to soluble amyloid, and, by virtue of that mechanism, is slowing cognitive and functional decline,” not only in EXPEDITION3, but in its predecessors, EXPEDITION and EXPEDITION2.
“This is not a refutation of the amyloid hypothesis, but a confirmation of it.”
Nevertheless, the drug failed its trial, he said. There was no statistically significant separation between solanezumab and placebo on the 14-item Alzheimer’s Disease Assessment Scale-Cognitive subscale (ADAS-Cog14), an assessment of cognitive function that was the study’s primary end point. The active treatment group experienced 11% less decline than did the placebo group, but the result was not significant.
If the result had been significant, “it would still be a small effect size,” which would have thrown into question the drug’s clinical utility, Dr. Aisen said. “We thought we might see a 30% slowing of decline on the ADAS-Cog, and it was disappointing to only get 11%. But that is also what we saw on the key [secondary end points]. Overall, the effect size looks to be about 12% to 13%, and that’s just too small.”
Three Phase III Trials
EXPEDITION3 was the last of a triad of solanezumab studies, all of which posted signals of cognitive and functional benefit in patients with mild Alzheimer’s disease. It was based on subgroup analyses of EXPEDITION and EXPEDITION2, both of which failed to meet their primary end points and included patients with mild and moderate disease. When researchers pooled the patients with mild disease from the first two studies, they found that solanezumab was associated with a 34% slowing of cognitive decline on the ADAS-Cog14. Lilly conducted EXPEDITION3 in an attempt to confirm those findings.
Lawrence S. Honig, MD, PhD, Professor of Neurology at Columbia University Medical Center in New York and principal investigator of the EXPEDITION3 study, detailed the study’s results, including biomarker data.
The study included about 2,000 patients with imaging-confirmed amyloid brain plaques and mild dementia due to Alzheimer’s disease. They were randomized to receive placebo or monthly injections of 400 mg of solanezumab for 80 weeks. The study was conducted at 210 sites in 11 countries.
While solanezumab’s effect on the ADAS-Cog was not significant, its effect on the Mini-Mental State Examination score was significant, with a 13% slowing of decline, compared with placebo. There was also a significant 5% difference in the Clinical Dementia Rating scale-Sum of Boxes score.
Outcomes were mixed in measures of function. On the Alzheimer’s Disease Cooperative Study Activities of Daily Living (ADCS-ADL) and its related measure, the ADCS-ADL inventory instrumental items, patients who received solanezumab had significant 15% and 14% differences, respectively, relative to placebo.
But differences on the Functional Activities Questionnaire, an informant measure of more complex activities, were not significant.
Biomarkers trended the right way, Dr. Honig noted. Solanezumab resulted in a 500- to 800-fold increase in amyloid beta in plasma, relative to placebo. There were no changes in amyloid brain plaques, as measured by PET imaging. This finding was not surprising because the antibody does not recognize fibrillar amyloid, Dr. Aisen said.
“What we expect to see with biomarkers differs based on the epitope targeted,” he said. “Solanezumab ignores plaques. It targets the middle of the peptide, binding to soluble amyloid beta. Now, how that helps [treat Alzheimer’s disease] is something of a debate, but it is important to recognize that it does not attack plaques. Instead, by tying up monomeric amyloid beta, it may change the dynamic exchange of various species of amyloid around plaques; the toxicity of amyloid is thought to reside as much in oligomeric species as in the fibrillar deposits. I see this [plasma amyloid beta increase] as confirming that it’s tying up monomeric amyloid species and that the result is a slowing of disease progression. I believe it is supportive of the amyloid hypothesis.”
Solanezumab had no significant effect on tau in CSF or on imaging, nor did it change the progression of ventricular enlargement, a marker of whole brain atrophy.
The antibody was safe, with 17% of patients who received solanezumab reporting an adverse event, compared with 19% of patients who received placebo. There were nine deaths in the solanezumab arm and 16 in the placebo arm; about 4% of each group discontinued treatment because of an adverse event.
In late November 2016, Eric Siemers, MD, Senior Medical Director of the Alzheimer’s Disease Global Development Team at Lilly, said the company would not seek regulatory approval for solanezumab based on the trial results. “We didn’t expect this to be a cure for this disease, but we did hope it would be the first drug to slow its progress.... We are very disappointed,” Dr. Siemers said during the panel discussion.
Further Trials of Solanezumab
He and Dr. Aisen confirmed, however, that two other trials using solanezumab in different populations would go forward. The Anti-Amyloid Treatment in Asymptomatic Alzheimer’s study (A4 study) is investigating its effect in cognitively healthy elderly people with Alzheimer’s disease risk factors, and the Dominantly Inherited Alzheimer’s Network (DIAN) study is investigating its effects in patients with autosomal dominant mutations in Alzheimer’s disease genes.
Dr. Aisen is excited about solanezumab’s potential to target the disease before cognitive symptoms develop. “I expect all antiamyloid treatments would work better when neurodegeneration is not extensive,” he said. “Any of the antiamyloid antibodies would theoretically be more effective at a preclinical stage of Alzheimer’s disease than even in the mild dementia stage.”
Maria Carrillo, PhD, Chief Science Officer of the Alzheimer’s Association, said that EXPEDITION3 was far from a path to nowhere and urged the research community, patients, and families to double down on their commitment to tackling the disease.
“These results stress the urgency for pushing forward harder,” Dr. Carrillo said. “This is not a time to slow down. It’s a time to ramp up our efforts. This is not the time to sit back and say, ‘The amyloid hypothesis has been the wrong pathway, and we need to drop it.’ But we also need to pursue other pathways, to broaden our approach, and to broaden the armamentarium our clinicians will need to combat this disease.
“This is not a win, true. But it gets us a little closer to one.”
—Michele G. Sullivan
SAN DIEGO—Solanezumab may not have slowed the clinical progression of Alzheimer’s disease, but it provided valuable evidence for the amyloid hypothesis, experts said during a wide-ranging discussion of Eli Lilly and Company’s recent EXPEDITION3 trial.
Lilly representatives and EXPEDITION investigators presented the study’s results at the Ninth Annual Clinical Trials for Alzheimer’s Disease meeting. While solanezumab failed to meet its primary end point, it did achieve significance on several secondary end points—findings that should be read as encouraging, rather than as a defeat, according to Paul Aisen, MD, Director of the Alzheimer’s Therapeutic Research Institute at the University of Southern California, Los Angeles.
“We have here a negative study that confirms a beneficial treatment,” said Dr. Aisen, an EXPEDITION3 investigator. “We have a treatment that engages its target, binds to soluble amyloid, and, by virtue of that mechanism, is slowing cognitive and functional decline,” not only in EXPEDITION3, but in its predecessors, EXPEDITION and EXPEDITION2.
“This is not a refutation of the amyloid hypothesis, but a confirmation of it.”
Nevertheless, the drug failed its trial, he said. There was no statistically significant separation between solanezumab and placebo on the 14-item Alzheimer’s Disease Assessment Scale-Cognitive subscale (ADAS-Cog14), an assessment of cognitive function that was the study’s primary end point. The active treatment group experienced 11% less decline than did the placebo group, but the result was not significant.
If the result had been significant, “it would still be a small effect size,” which would have thrown into question the drug’s clinical utility, Dr. Aisen said. “We thought we might see a 30% slowing of decline on the ADAS-Cog, and it was disappointing to only get 11%. But that is also what we saw on the key [secondary end points]. Overall, the effect size looks to be about 12% to 13%, and that’s just too small.”
Three Phase III Trials
EXPEDITION3 was the last of a triad of solanezumab studies, all of which posted signals of cognitive and functional benefit in patients with mild Alzheimer’s disease. It was based on subgroup analyses of EXPEDITION and EXPEDITION2, both of which failed to meet their primary end points and included patients with mild and moderate disease. When researchers pooled the patients with mild disease from the first two studies, they found that solanezumab was associated with a 34% slowing of cognitive decline on the ADAS-Cog14. Lilly conducted EXPEDITION3 in an attempt to confirm those findings.
Lawrence S. Honig, MD, PhD, Professor of Neurology at Columbia University Medical Center in New York and principal investigator of the EXPEDITION3 study, detailed the study’s results, including biomarker data.
The study included about 2,000 patients with imaging-confirmed amyloid brain plaques and mild dementia due to Alzheimer’s disease. They were randomized to receive placebo or monthly injections of 400 mg of solanezumab for 80 weeks. The study was conducted at 210 sites in 11 countries.
While solanezumab’s effect on the ADAS-Cog was not significant, its effect on the Mini-Mental State Examination score was significant, with a 13% slowing of decline, compared with placebo. There was also a significant 5% difference in the Clinical Dementia Rating scale-Sum of Boxes score.
Outcomes were mixed in measures of function. On the Alzheimer’s Disease Cooperative Study Activities of Daily Living (ADCS-ADL) and its related measure, the ADCS-ADL inventory instrumental items, patients who received solanezumab had significant 15% and 14% differences, respectively, relative to placebo.
But differences on the Functional Activities Questionnaire, an informant measure of more complex activities, were not significant.
Biomarkers trended the right way, Dr. Honig noted. Solanezumab resulted in a 500- to 800-fold increase in amyloid beta in plasma, relative to placebo. There were no changes in amyloid brain plaques, as measured by PET imaging. This finding was not surprising because the antibody does not recognize fibrillar amyloid, Dr. Aisen said.
“What we expect to see with biomarkers differs based on the epitope targeted,” he said. “Solanezumab ignores plaques. It targets the middle of the peptide, binding to soluble amyloid beta. Now, how that helps [treat Alzheimer’s disease] is something of a debate, but it is important to recognize that it does not attack plaques. Instead, by tying up monomeric amyloid beta, it may change the dynamic exchange of various species of amyloid around plaques; the toxicity of amyloid is thought to reside as much in oligomeric species as in the fibrillar deposits. I see this [plasma amyloid beta increase] as confirming that it’s tying up monomeric amyloid species and that the result is a slowing of disease progression. I believe it is supportive of the amyloid hypothesis.”
Solanezumab had no significant effect on tau in CSF or on imaging, nor did it change the progression of ventricular enlargement, a marker of whole brain atrophy.
The antibody was safe, with 17% of patients who received solanezumab reporting an adverse event, compared with 19% of patients who received placebo. There were nine deaths in the solanezumab arm and 16 in the placebo arm; about 4% of each group discontinued treatment because of an adverse event.
In late November 2016, Eric Siemers, MD, Senior Medical Director of the Alzheimer’s Disease Global Development Team at Lilly, said the company would not seek regulatory approval for solanezumab based on the trial results. “We didn’t expect this to be a cure for this disease, but we did hope it would be the first drug to slow its progress.... We are very disappointed,” Dr. Siemers said during the panel discussion.
Further Trials of Solanezumab
He and Dr. Aisen confirmed, however, that two other trials using solanezumab in different populations would go forward. The Anti-Amyloid Treatment in Asymptomatic Alzheimer’s study (A4 study) is investigating its effect in cognitively healthy elderly people with Alzheimer’s disease risk factors, and the Dominantly Inherited Alzheimer’s Network (DIAN) study is investigating its effects in patients with autosomal dominant mutations in Alzheimer’s disease genes.
Dr. Aisen is excited about solanezumab’s potential to target the disease before cognitive symptoms develop. “I expect all antiamyloid treatments would work better when neurodegeneration is not extensive,” he said. “Any of the antiamyloid antibodies would theoretically be more effective at a preclinical stage of Alzheimer’s disease than even in the mild dementia stage.”
Maria Carrillo, PhD, Chief Science Officer of the Alzheimer’s Association, said that EXPEDITION3 was far from a path to nowhere and urged the research community, patients, and families to double down on their commitment to tackling the disease.
“These results stress the urgency for pushing forward harder,” Dr. Carrillo said. “This is not a time to slow down. It’s a time to ramp up our efforts. This is not the time to sit back and say, ‘The amyloid hypothesis has been the wrong pathway, and we need to drop it.’ But we also need to pursue other pathways, to broaden our approach, and to broaden the armamentarium our clinicians will need to combat this disease.
“This is not a win, true. But it gets us a little closer to one.”
—Michele G. Sullivan
Red-Blue Nodule on the Scalp
Metastatic Clear Cell Renal Cell Carcinoma
The differential diagnosis of cutaneous neoplasms with clear cells is broad. Clear cell features can be seen in primary tumors arising from the epidermis and cutaneous adnexa as well as in mesenchymal and melanocytic neoplasms. Furthermore, metastatic disease should be considered in the histologic differential diagnosis, as many visceral malignancies have clear cell features. This patient was subsequently found to have a large renal mass with metastasis to the lungs, spleen, and bone. The histologic findings support the diagnosis of metastatic clear cell renal cell carcinoma (RCC) to the skin.
Approximately 30% of patients with clear cell RCC present with metastatic disease with approximately 8% of those involving the skin.1,2 Cutaneous RCC metastases show a predilection for the head, especially the scalp. The clinical presentation is variable, but there often is a history of a rapidly growing brown, black, or purple nodule or plaque. A thorough review of the patient's history should be conducted if metastatic RCC is in the differential diagnosis, as it has been reported to occur up to 20 years after initial diagnosis.3
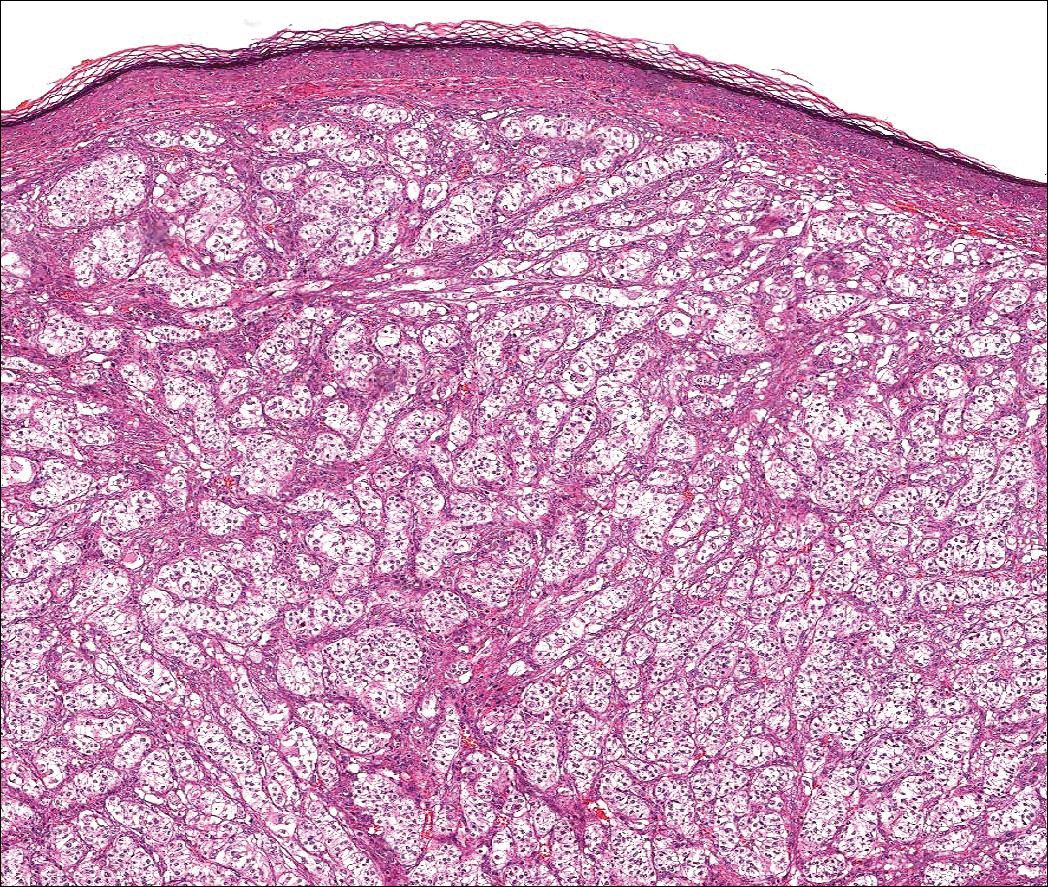
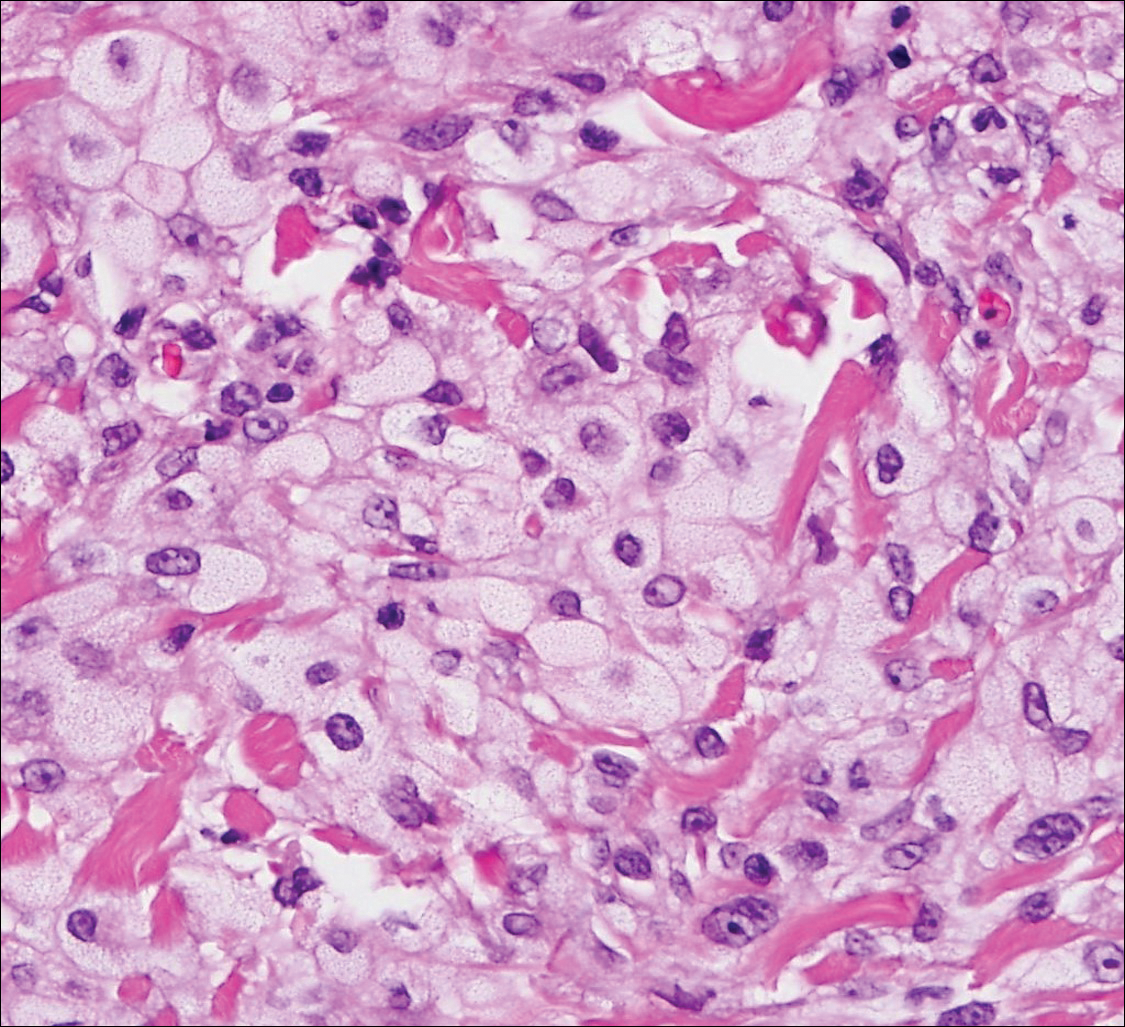
Histologically, clear cell RCC (quiz image) is composed of nests of tumor cells with clear cytoplasm and centrally located nuclei with prominent nucleoli. The clear cell features result from abundant cytoplasmic glycogen and lipid but may not be present in every case. One of the most important histologic features is the presence of delicate branching blood vessels (Figure 1). Numerous extravasated red blood cells also may be present. Positive immunohistochemical staining for PAX8, CD10, and RCC antigens support the diagnosis.4
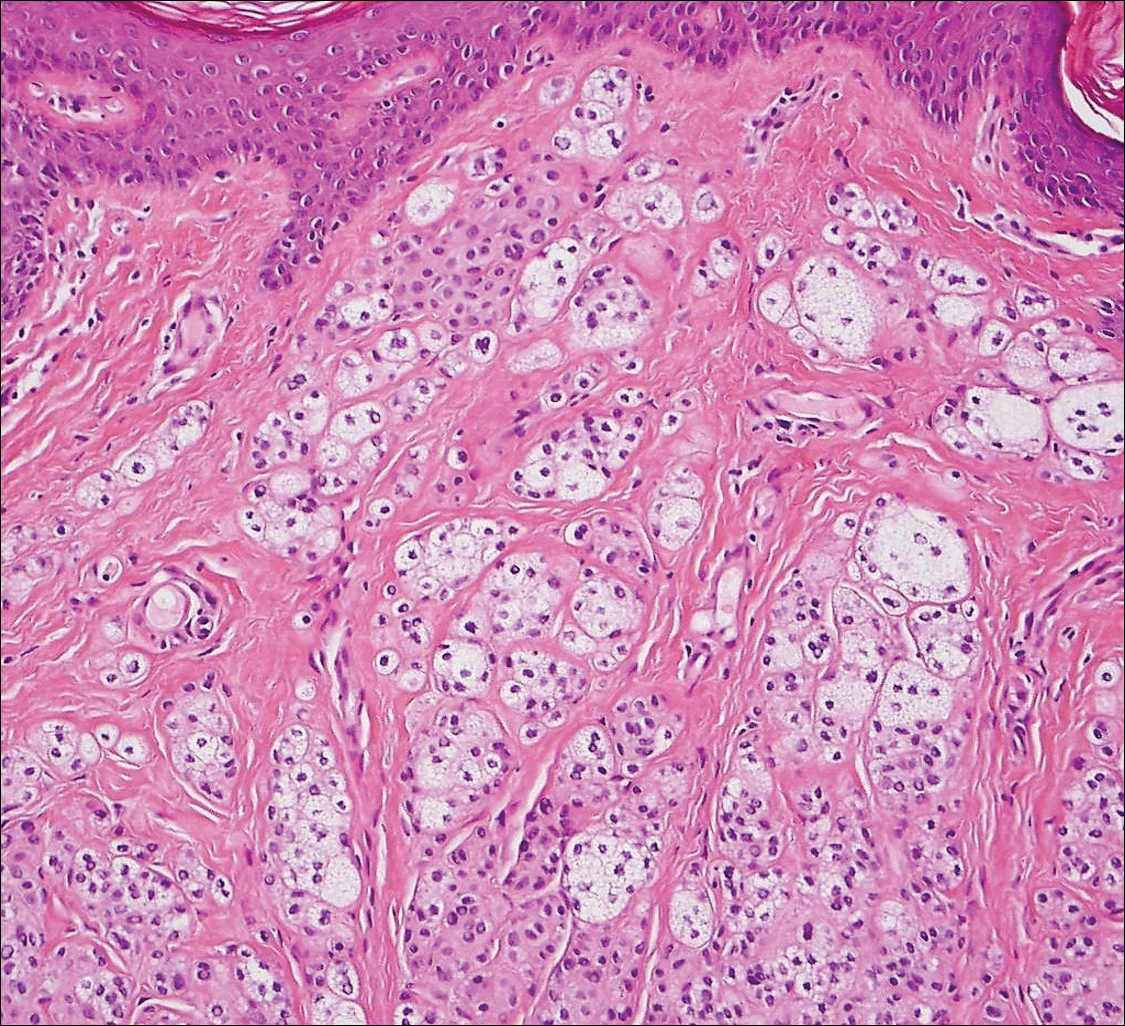
Balloon cell nevi (Figure 2) most commonly occur on the head and neck in adolescents and young adults but clinically are indistinguishable from other banal nevi. The nevus cells are large with foamy to finely vacuolated cytoplasm and lack atypia. The clear cell change is the result of melanosome degeneration and may be extensive. The presence of melanin pigment, nests of typical nevus cells, and positive staining with MART-1 can help distinguish the tumor from xanthomas and RCC.5
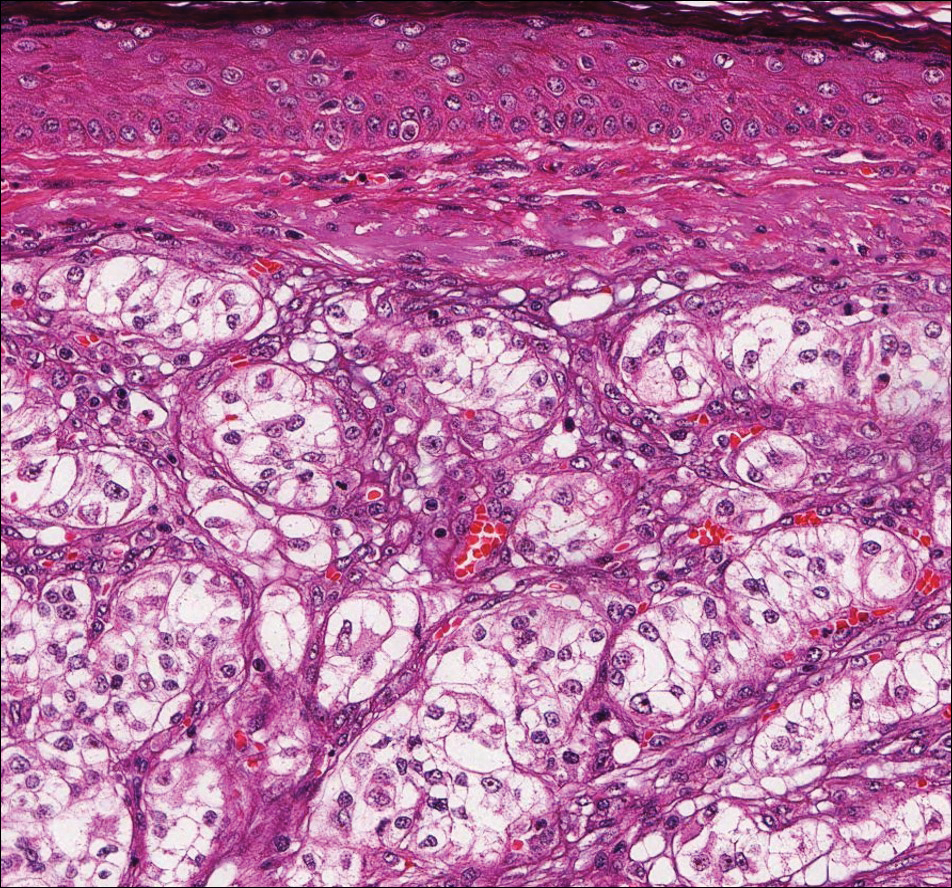
Clear cell hidradenoma (Figure 3) is a well-circumscribed tumor of sweat gland origin that arises in the dermis. The architecture usually is solid, cystic, or a combination of both. The cytology is classically bland with poroid, squamoid, or clear cell morphology. Clear cells that are positive on periodic acid-Schiff staining predominate in up to one-third of cases. Carcinoembryonic antigen and epithelial membrane antigen can be used to highlight the eosinophilic cuticles of ducts within solid areas.6

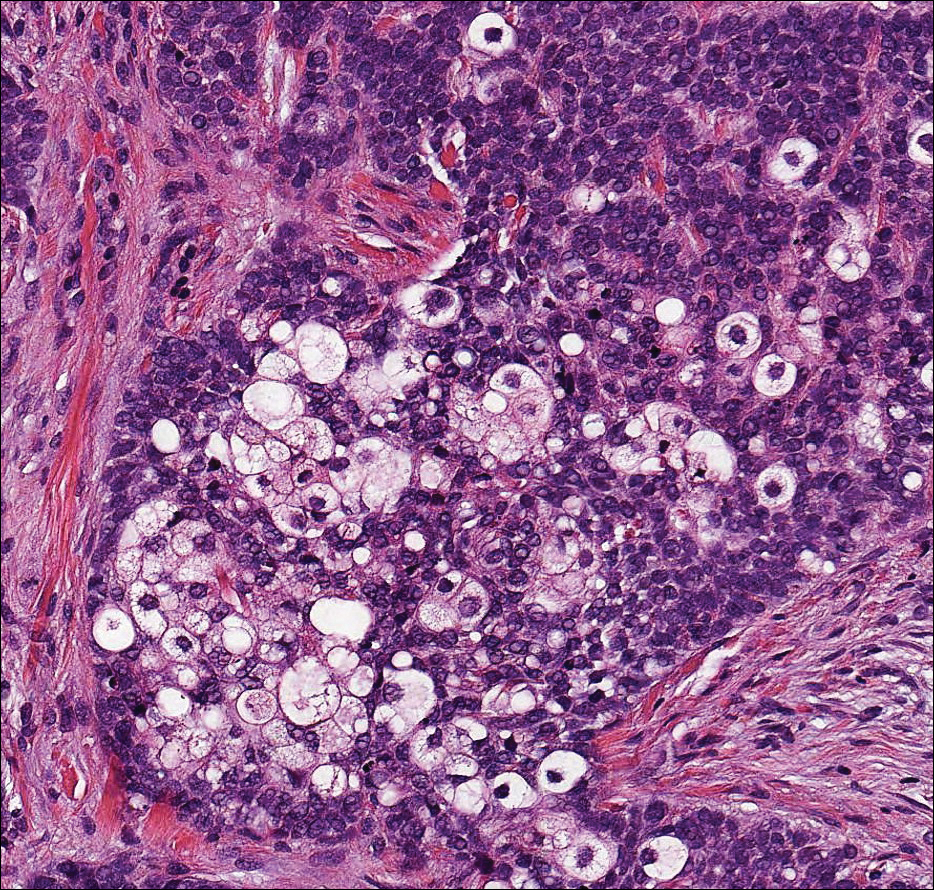
Sebaceous carcinoma (Figure 4) most frequently arises in a periorbital distribution, although extraocular lesions are known to occur. Histologically, there is a proliferation of both mature sebocytes and basaloid cells in the dermis, occasionally involving the epidermis. The mature sebocytes demonstrate clear cell features with foamy to vacuolated cytoplasm and large nuclei with scalloped borders. The clear cells may vary greatly in number and often are sparse in poorly differentiated tumors in which pleomorphic basaloid cells may predominate. The basaloid cells may resemble those of squamous or basal cell carcinoma, leading to a diagnostic dilemma in some cases. Special staining with Sudan black B and oil red O highlights the cytoplasmic lipid but must be performed on frozen section specimens. Although not entirely specific, immunohistochemical expression of epithelial membrane antigen, androgen receptor, and membranous vesicular adipophilin staining in sebaceous carcinoma can assist in the diagnosis.7
Cutaneous xanthomas (Figure 5) may arise in patients of any age and represent deposition of lipid-laden macrophages. Classification often is dependent on the clinical presentation; however, some subtypes demonstrate unique morphologic features (eg, verruciform xanthomas). Xanthomas classically arise in association with elevated serum lipids, but they also may occur in normolipemic patients. Individuals with Erdheim-Chester disease have an increased propensity to develop xanthelasma. Similarly, plane xanthomas have been associated with monoclonal gammopathy. Histologically, xanthomas are characterized by sheets of foamy macrophages within the dermis and subcutis. Positive immunohistochemical staining for CD68 highlighting the histiocytic nature of the cells and the absence of a delicate vascular network aid in the differentiation from RCC.
- Patterson JW, Hosler GA. Weedon's Skin Pathology. 4th ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2016.
- Alcaraz I, Cerroni L, Rutten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Calonje E, McKee PH. McKee's Pathology of the Skin. 4th ed. Edinburgh, Scotland: Elsevier/Saunders; 2012.
- Lin F, Prichard J. Handbook of Practical Immunohistochemistry: Frequently Asked Questions. 2nd ed. New York, NY: Springer; 2015.
- McKee PH, Calonje E. Diagnostic Atlas of Melanocytic Pathology. Edinburgh, Scotland: Mosby/Elsevier; 2009.
- Elston DM, Ferringer T, Ko CJ. Dermatopathology. 2nd ed. Philadelphia, PA: Saunders Elsevier; 2014.
- Ansai S, Takeichi H, Arase S, et al. Sebaceous carcinoma: an immunohistochemical reappraisal. Am J Dermatopathol. 2011;33:579-587.
Metastatic Clear Cell Renal Cell Carcinoma
The differential diagnosis of cutaneous neoplasms with clear cells is broad. Clear cell features can be seen in primary tumors arising from the epidermis and cutaneous adnexa as well as in mesenchymal and melanocytic neoplasms. Furthermore, metastatic disease should be considered in the histologic differential diagnosis, as many visceral malignancies have clear cell features. This patient was subsequently found to have a large renal mass with metastasis to the lungs, spleen, and bone. The histologic findings support the diagnosis of metastatic clear cell renal cell carcinoma (RCC) to the skin.
Approximately 30% of patients with clear cell RCC present with metastatic disease with approximately 8% of those involving the skin.1,2 Cutaneous RCC metastases show a predilection for the head, especially the scalp. The clinical presentation is variable, but there often is a history of a rapidly growing brown, black, or purple nodule or plaque. A thorough review of the patient's history should be conducted if metastatic RCC is in the differential diagnosis, as it has been reported to occur up to 20 years after initial diagnosis.3
Histologically, clear cell RCC (quiz image) is composed of nests of tumor cells with clear cytoplasm and centrally located nuclei with prominent nucleoli. The clear cell features result from abundant cytoplasmic glycogen and lipid but may not be present in every case. One of the most important histologic features is the presence of delicate branching blood vessels (Figure 1). Numerous extravasated red blood cells also may be present. Positive immunohistochemical staining for PAX8, CD10, and RCC antigens support the diagnosis.4

Balloon cell nevi (Figure 2) most commonly occur on the head and neck in adolescents and young adults but clinically are indistinguishable from other banal nevi. The nevus cells are large with foamy to finely vacuolated cytoplasm and lack atypia. The clear cell change is the result of melanosome degeneration and may be extensive. The presence of melanin pigment, nests of typical nevus cells, and positive staining with MART-1 can help distinguish the tumor from xanthomas and RCC.5

Clear cell hidradenoma (Figure 3) is a well-circumscribed tumor of sweat gland origin that arises in the dermis. The architecture usually is solid, cystic, or a combination of both. The cytology is classically bland with poroid, squamoid, or clear cell morphology. Clear cells that are positive on periodic acid-Schiff staining predominate in up to one-third of cases. Carcinoembryonic antigen and epithelial membrane antigen can be used to highlight the eosinophilic cuticles of ducts within solid areas.6

Sebaceous carcinoma (Figure 4) most frequently arises in a periorbital distribution, although extraocular lesions are known to occur. Histologically, there is a proliferation of both mature sebocytes and basaloid cells in the dermis, occasionally involving the epidermis. The mature sebocytes demonstrate clear cell features with foamy to vacuolated cytoplasm and large nuclei with scalloped borders. The clear cells may vary greatly in number and often are sparse in poorly differentiated tumors in which pleomorphic basaloid cells may predominate. The basaloid cells may resemble those of squamous or basal cell carcinoma, leading to a diagnostic dilemma in some cases. Special staining with Sudan black B and oil red O highlights the cytoplasmic lipid but must be performed on frozen section specimens. Although not entirely specific, immunohistochemical expression of epithelial membrane antigen, androgen receptor, and membranous vesicular adipophilin staining in sebaceous carcinoma can assist in the diagnosis.7

Cutaneous xanthomas (Figure 5) may arise in patients of any age and represent deposition of lipid-laden macrophages. Classification often is dependent on the clinical presentation; however, some subtypes demonstrate unique morphologic features (eg, verruciform xanthomas). Xanthomas classically arise in association with elevated serum lipids, but they also may occur in normolipemic patients. Individuals with Erdheim-Chester disease have an increased propensity to develop xanthelasma. Similarly, plane xanthomas have been associated with monoclonal gammopathy. Histologically, xanthomas are characterized by sheets of foamy macrophages within the dermis and subcutis. Positive immunohistochemical staining for CD68 highlighting the histiocytic nature of the cells and the absence of a delicate vascular network aid in the differentiation from RCC.

Metastatic Clear Cell Renal Cell Carcinoma
The differential diagnosis of cutaneous neoplasms with clear cells is broad. Clear cell features can be seen in primary tumors arising from the epidermis and cutaneous adnexa as well as in mesenchymal and melanocytic neoplasms. Furthermore, metastatic disease should be considered in the histologic differential diagnosis, as many visceral malignancies have clear cell features. This patient was subsequently found to have a large renal mass with metastasis to the lungs, spleen, and bone. The histologic findings support the diagnosis of metastatic clear cell renal cell carcinoma (RCC) to the skin.
Approximately 30% of patients with clear cell RCC present with metastatic disease with approximately 8% of those involving the skin.1,2 Cutaneous RCC metastases show a predilection for the head, especially the scalp. The clinical presentation is variable, but there often is a history of a rapidly growing brown, black, or purple nodule or plaque. A thorough review of the patient's history should be conducted if metastatic RCC is in the differential diagnosis, as it has been reported to occur up to 20 years after initial diagnosis.3
Histologically, clear cell RCC (quiz image) is composed of nests of tumor cells with clear cytoplasm and centrally located nuclei with prominent nucleoli. The clear cell features result from abundant cytoplasmic glycogen and lipid but may not be present in every case. One of the most important histologic features is the presence of delicate branching blood vessels (Figure 1). Numerous extravasated red blood cells also may be present. Positive immunohistochemical staining for PAX8, CD10, and RCC antigens support the diagnosis.4

Balloon cell nevi (Figure 2) most commonly occur on the head and neck in adolescents and young adults but clinically are indistinguishable from other banal nevi. The nevus cells are large with foamy to finely vacuolated cytoplasm and lack atypia. The clear cell change is the result of melanosome degeneration and may be extensive. The presence of melanin pigment, nests of typical nevus cells, and positive staining with MART-1 can help distinguish the tumor from xanthomas and RCC.5

Clear cell hidradenoma (Figure 3) is a well-circumscribed tumor of sweat gland origin that arises in the dermis. The architecture usually is solid, cystic, or a combination of both. The cytology is classically bland with poroid, squamoid, or clear cell morphology. Clear cells that are positive on periodic acid-Schiff staining predominate in up to one-third of cases. Carcinoembryonic antigen and epithelial membrane antigen can be used to highlight the eosinophilic cuticles of ducts within solid areas.6

Sebaceous carcinoma (Figure 4) most frequently arises in a periorbital distribution, although extraocular lesions are known to occur. Histologically, there is a proliferation of both mature sebocytes and basaloid cells in the dermis, occasionally involving the epidermis. The mature sebocytes demonstrate clear cell features with foamy to vacuolated cytoplasm and large nuclei with scalloped borders. The clear cells may vary greatly in number and often are sparse in poorly differentiated tumors in which pleomorphic basaloid cells may predominate. The basaloid cells may resemble those of squamous or basal cell carcinoma, leading to a diagnostic dilemma in some cases. Special staining with Sudan black B and oil red O highlights the cytoplasmic lipid but must be performed on frozen section specimens. Although not entirely specific, immunohistochemical expression of epithelial membrane antigen, androgen receptor, and membranous vesicular adipophilin staining in sebaceous carcinoma can assist in the diagnosis.7

Cutaneous xanthomas (Figure 5) may arise in patients of any age and represent deposition of lipid-laden macrophages. Classification often is dependent on the clinical presentation; however, some subtypes demonstrate unique morphologic features (eg, verruciform xanthomas). Xanthomas classically arise in association with elevated serum lipids, but they also may occur in normolipemic patients. Individuals with Erdheim-Chester disease have an increased propensity to develop xanthelasma. Similarly, plane xanthomas have been associated with monoclonal gammopathy. Histologically, xanthomas are characterized by sheets of foamy macrophages within the dermis and subcutis. Positive immunohistochemical staining for CD68 highlighting the histiocytic nature of the cells and the absence of a delicate vascular network aid in the differentiation from RCC.

- Patterson JW, Hosler GA. Weedon's Skin Pathology. 4th ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2016.
- Alcaraz I, Cerroni L, Rutten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Calonje E, McKee PH. McKee's Pathology of the Skin. 4th ed. Edinburgh, Scotland: Elsevier/Saunders; 2012.
- Lin F, Prichard J. Handbook of Practical Immunohistochemistry: Frequently Asked Questions. 2nd ed. New York, NY: Springer; 2015.
- McKee PH, Calonje E. Diagnostic Atlas of Melanocytic Pathology. Edinburgh, Scotland: Mosby/Elsevier; 2009.
- Elston DM, Ferringer T, Ko CJ. Dermatopathology. 2nd ed. Philadelphia, PA: Saunders Elsevier; 2014.
- Ansai S, Takeichi H, Arase S, et al. Sebaceous carcinoma: an immunohistochemical reappraisal. Am J Dermatopathol. 2011;33:579-587.
- Patterson JW, Hosler GA. Weedon's Skin Pathology. 4th ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2016.
- Alcaraz I, Cerroni L, Rutten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Calonje E, McKee PH. McKee's Pathology of the Skin. 4th ed. Edinburgh, Scotland: Elsevier/Saunders; 2012.
- Lin F, Prichard J. Handbook of Practical Immunohistochemistry: Frequently Asked Questions. 2nd ed. New York, NY: Springer; 2015.
- McKee PH, Calonje E. Diagnostic Atlas of Melanocytic Pathology. Edinburgh, Scotland: Mosby/Elsevier; 2009.
- Elston DM, Ferringer T, Ko CJ. Dermatopathology. 2nd ed. Philadelphia, PA: Saunders Elsevier; 2014.
- Ansai S, Takeichi H, Arase S, et al. Sebaceous carcinoma: an immunohistochemical reappraisal. Am J Dermatopathol. 2011;33:579-587.
A 59-year-old man presented with a 1.5×1.0-cm asymptomatic, smooth, red-blue nodule on the left parietal scalp. The nodule had been rapidly enlarging over the last 3 weeks. After resection, the cut surface was golden yellow and focally hemorrhagic.
Focus on treating genital atrophy symptoms
As estrogen levels decline, postmenopausal women commonly experience uncomfortable and distressing symptoms of genital atrophy, or genitourinary syndrome of menopause (GSM). Moreover, aromatase inhibitors (AIs), increasingly used as adjuvant therapy by menopausal breast cancer survivors, contribute to vaginal dryness and sexual pain. This discussion focuses on studies of several local vaginal treatments (including a recently approved agent) that ameliorate GSM symptoms but do not appreciably raise serum sex steroid levels—reassuring data for certain patient populations.
EXPERT COMMENTARY
Andrew M. Kaunitz, MD, is University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology, University of Florida College of Medicine–Jacksonville. He is the Medical Director and Director of Menopause and Gynecologic Ultrasound Services, UF Women’s Health Specialists–Emerson. Dr. Kaunitz serves on the OBG Management Board of Editors.
Dr. Kaunitz reports that in 2015 he served on a contraception advisory board for Pfizer, which markets the low-dose estradiol vaginal ring.
Read expert commentary from Dr. Kaunitz
For women with early-stage breast cancer receiving an AI, is a vaginal estradiol ring or testosterone cream safe for genital atrophy?
Yes, according to results of a randomized, noncomparative short-term trial that found both agents improved vaginal dryness and sexual dysfunction and had little tendency to persistently elevate serum estradiol levels
Melisko ME, Goldman ME, Hwang J, et al. Vaginal testosterone cream vs estradiol vaginal ring for vaginal dryness or decreased libido in women receiving aromatase inhibitors for early-stage breast cancer: a randomized clinical trial [published online ahead of print November 10, 2016]. JAMA Oncol. doi: 10.1001/jamaoncol.2016.3904.
Long-term adjuvant AI therapy, which often causes vaginal dryness and sexual dysfunction, is recommended for postmenopausal women with hormone receptor-positive breast cancer. Although use of a vaginally administered low-dose 3-month estradiol ring as well as compounded testosterone cream is known to improve menopausal genital atrophy and sexual symptoms, little data address these agents' impact on serum estradiol levels in women using AIs.
In a safety evaluation study of these treatments performed at an academic US cancer center, Melisko and colleagues randomly assigned postmenopausal women with hormone receptor-positive breast cancer who reported vaginal dryness, sexual pain, or reduced sexual desire to 12 weeks of off-label treatment with an estradiol vaginal ring or intravaginal testosterone cream.
Related article:
Does extending aromatase-inhibitor use from 5 to 10 years benefit menopausal women with hormone-positive breast cancer?
Details of the study
Among 68 evaluable women (mean age, 56 years), mean baseline estradiol levels were 20 pg/mL (range, <2 to 127 pg/mL); estradiol levels were above the postmenopausal range (>10 pg/mL) in 37% of participants. During the 12-week trial, transient and persistent estradiol levels above this threshold were noted, respectively, in 4 and 0 women treated with the vaginal ring and in 4 and 4 women treated with testosterone cream. Estradiol levels assessed using commercially available (liquid chromatography and mass spectrometry) and research laboratory (radioimmune assay) methodology yielded similar results. In the testosterone cream group, persistent elevations above the normal postmenopausal range were common.
Atrophic vaginal changes, sexual desire, and sexual dysfunction improved in both treatment groups based on gynecologic examinations and sexual quality-of-life questionnaires completed at baseline and week 12.
The authors indicated that their current practice is to continue the estradiol vaginal ring or testosterone cream in AI users who experience symptomatic improvement with these formulations. They check serum estradiol levels every few months. A future large, long-term trial assessing the impact of off-label use of the estradiol vaginal ring on the incidence of recurrent disease in breast cancer survivors would provide definitive evidence of this treatment's safety.
--Andrew M. Kaunitz, MD
Read on for Dr. Kaunitz’s comments on a new dyspareunia treatment
What's new for the treatment of dyspareunia associated with GSM?
Intrarosa, a once-daily vaginal insert containing prasterone as the active ingredient, was recently approved for the treatment of moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy due to menopause
FDA approves Intrarosa for postmenopausal women experiencing pain during sex [news release]. Silver Spring, MD: US Food and Drug Administration; November 17, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm529641.htm. Accessed December 19, 2017.
Intrarosa [package insert]. Quebec City, Canada: Endoceutics Inc; 2016.
On November 17, 2016, the US Food and Drug Administration (FDA) approved Intrarosa, vaginal dehydroepiandrosterone (DHEA)--also known as prasterone--for women experiencing moderate to severe pain during sexual intercourse due to menopause-related genital atrophy, or GSM. In clinical trials, daily treatment with a 6.5-mg vaginal ovule of DHEA was found effective in reducing symptoms of atrophy. Vaginal discharge was the most common adverse effect.
After menopause, DHEA, which is produced largely by the adrenal glands, represents the dominant source of all sex steroids. DHEA is enzymatically transformed at the intracellular level into estrogens. Because estradiol is inactivated at the site of its synthesis, use of vaginal DHEA causes little if any rise in serum estradiol levels.1,2
Related article:
2014 Update on Fertility
Details of 2 studies
A pivotal randomized, double-blind, placebo-controlled phase 3 trial of intravaginal DHEA (6.5 mg daily) for treating postmenopausal dyspareunia in women with vulvovaginal atrophy was conducted over 12 weeks.1 The trial included 325 women treated with DHEA and 157 who received placebo.
All 4 coprimary objectives measured improved with treatment compared with mean baseline levels: percentage of parabasal cells in treated participants decreased by 27.7% over placebo (P<.0001); percentage of superficial cells increased by 8.44% over placebo (P<.0001); vaginal pH decreased by 0.66 pH unit over placebo (P<.0001); and pain with sexual activity decreased by 1.42 severity score unit from baseline or 0.36 unit over placebo (P = .0002). In addition, participant-reported moderate to severe vaginal dryness (present in 84% of women at baseline) improved considerably at 12 weeks, and gynecologic evaluation revealed improvements in vaginal secretions, epithelial integrity and surface thickness, and color.1
About 6% of participants reported vaginal discharge as an adverse effect. Levels of serum steroids remained within the normal range for postmenopausal women.1
Another study, in which authors integrated data from four phase 3 clinical trials of postmenopausal women with vulvovaginal atrophy treated with vaginal DHEA (n = 723) or placebo (n = 266) for 12 weeks, analyzed serum steroid levels measured at Day 1 and Week 12 by liquid chromatography-tandem mass spectrometry.2
At 12 weeks' treatment, mean levels of the most relevant sex steroid, serum estradiol, was noted to be 3.36 pg/mL, 19% below the normal postmenopausal value of 4.17 pg/mL.The mean level of estrone sulfate was noted to be 209 pg/mL, lower than the normal 220 pg/mL level in postmenopausal women. Further, androsterone glucuronide, the primary metabolite of androgens, also remained well within normal postmenopausal values.2
The authors concluded that the study data demonstrate that a daily 6.5-mg dose of intravaginal DHEA in postmenopausal women achieves the desired local efficacy (ie, amelioration of vulvovaginal atrophy symptoms) without systemic sex steroid exposure.2
The new information detailed in this article indicates that the recently FDA-approved vaginal DHEA (prasterone) ovules, as well as the 3-month low-dose estradiol vaginal ring, improve symptoms of genital atrophy without causing appreciable elevations in serum estradiol levels. This will be welcome news for all women with symptomatic genital atrophy, including those who have been treated for estrogen-sensitive cancers. Clinicians should be aware that, although package labeling for vaginal prasterone does not list a history of breast cancer as a contraindication, a history of breast cancer is listed in the Warning and Precautions section of package labeling, noting that this medication has not been studied in women with a history of breast cancer.
-- Andrew M. Kaunitz, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Labrie F, Archer DF, Koltun W, et al; VVA Prasterone Research Group. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause. 2016;23(3):243–256.
- Martel C, Labrie F, Archer DF, et al; Prasterone Clinical Research Group. Serum steroid concentrations remain within normal postmenopausal values in women receiving daily 6.5 mg intravaginal prasterone for 12 weeks. J Steroid Biochem Mol Biol. 2016;159:142–153.
As estrogen levels decline, postmenopausal women commonly experience uncomfortable and distressing symptoms of genital atrophy, or genitourinary syndrome of menopause (GSM). Moreover, aromatase inhibitors (AIs), increasingly used as adjuvant therapy by menopausal breast cancer survivors, contribute to vaginal dryness and sexual pain. This discussion focuses on studies of several local vaginal treatments (including a recently approved agent) that ameliorate GSM symptoms but do not appreciably raise serum sex steroid levels—reassuring data for certain patient populations.
EXPERT COMMENTARY
Andrew M. Kaunitz, MD, is University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology, University of Florida College of Medicine–Jacksonville. He is the Medical Director and Director of Menopause and Gynecologic Ultrasound Services, UF Women’s Health Specialists–Emerson. Dr. Kaunitz serves on the OBG Management Board of Editors.
Dr. Kaunitz reports that in 2015 he served on a contraception advisory board for Pfizer, which markets the low-dose estradiol vaginal ring.
Read expert commentary from Dr. Kaunitz
For women with early-stage breast cancer receiving an AI, is a vaginal estradiol ring or testosterone cream safe for genital atrophy?
Yes, according to results of a randomized, noncomparative short-term trial that found both agents improved vaginal dryness and sexual dysfunction and had little tendency to persistently elevate serum estradiol levels
Melisko ME, Goldman ME, Hwang J, et al. Vaginal testosterone cream vs estradiol vaginal ring for vaginal dryness or decreased libido in women receiving aromatase inhibitors for early-stage breast cancer: a randomized clinical trial [published online ahead of print November 10, 2016]. JAMA Oncol. doi: 10.1001/jamaoncol.2016.3904.
Long-term adjuvant AI therapy, which often causes vaginal dryness and sexual dysfunction, is recommended for postmenopausal women with hormone receptor-positive breast cancer. Although use of a vaginally administered low-dose 3-month estradiol ring as well as compounded testosterone cream is known to improve menopausal genital atrophy and sexual symptoms, little data address these agents' impact on serum estradiol levels in women using AIs.
In a safety evaluation study of these treatments performed at an academic US cancer center, Melisko and colleagues randomly assigned postmenopausal women with hormone receptor-positive breast cancer who reported vaginal dryness, sexual pain, or reduced sexual desire to 12 weeks of off-label treatment with an estradiol vaginal ring or intravaginal testosterone cream.
Related article:
Does extending aromatase-inhibitor use from 5 to 10 years benefit menopausal women with hormone-positive breast cancer?
Details of the study
Among 68 evaluable women (mean age, 56 years), mean baseline estradiol levels were 20 pg/mL (range, <2 to 127 pg/mL); estradiol levels were above the postmenopausal range (>10 pg/mL) in 37% of participants. During the 12-week trial, transient and persistent estradiol levels above this threshold were noted, respectively, in 4 and 0 women treated with the vaginal ring and in 4 and 4 women treated with testosterone cream. Estradiol levels assessed using commercially available (liquid chromatography and mass spectrometry) and research laboratory (radioimmune assay) methodology yielded similar results. In the testosterone cream group, persistent elevations above the normal postmenopausal range were common.
Atrophic vaginal changes, sexual desire, and sexual dysfunction improved in both treatment groups based on gynecologic examinations and sexual quality-of-life questionnaires completed at baseline and week 12.
The authors indicated that their current practice is to continue the estradiol vaginal ring or testosterone cream in AI users who experience symptomatic improvement with these formulations. They check serum estradiol levels every few months. A future large, long-term trial assessing the impact of off-label use of the estradiol vaginal ring on the incidence of recurrent disease in breast cancer survivors would provide definitive evidence of this treatment's safety.
--Andrew M. Kaunitz, MD
Read on for Dr. Kaunitz’s comments on a new dyspareunia treatment
What's new for the treatment of dyspareunia associated with GSM?
Intrarosa, a once-daily vaginal insert containing prasterone as the active ingredient, was recently approved for the treatment of moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy due to menopause
FDA approves Intrarosa for postmenopausal women experiencing pain during sex [news release]. Silver Spring, MD: US Food and Drug Administration; November 17, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm529641.htm. Accessed December 19, 2017.
Intrarosa [package insert]. Quebec City, Canada: Endoceutics Inc; 2016.
On November 17, 2016, the US Food and Drug Administration (FDA) approved Intrarosa, vaginal dehydroepiandrosterone (DHEA)--also known as prasterone--for women experiencing moderate to severe pain during sexual intercourse due to menopause-related genital atrophy, or GSM. In clinical trials, daily treatment with a 6.5-mg vaginal ovule of DHEA was found effective in reducing symptoms of atrophy. Vaginal discharge was the most common adverse effect.
After menopause, DHEA, which is produced largely by the adrenal glands, represents the dominant source of all sex steroids. DHEA is enzymatically transformed at the intracellular level into estrogens. Because estradiol is inactivated at the site of its synthesis, use of vaginal DHEA causes little if any rise in serum estradiol levels.1,2
Related article:
2014 Update on Fertility
Details of 2 studies
A pivotal randomized, double-blind, placebo-controlled phase 3 trial of intravaginal DHEA (6.5 mg daily) for treating postmenopausal dyspareunia in women with vulvovaginal atrophy was conducted over 12 weeks.1 The trial included 325 women treated with DHEA and 157 who received placebo.
All 4 coprimary objectives measured improved with treatment compared with mean baseline levels: percentage of parabasal cells in treated participants decreased by 27.7% over placebo (P<.0001); percentage of superficial cells increased by 8.44% over placebo (P<.0001); vaginal pH decreased by 0.66 pH unit over placebo (P<.0001); and pain with sexual activity decreased by 1.42 severity score unit from baseline or 0.36 unit over placebo (P = .0002). In addition, participant-reported moderate to severe vaginal dryness (present in 84% of women at baseline) improved considerably at 12 weeks, and gynecologic evaluation revealed improvements in vaginal secretions, epithelial integrity and surface thickness, and color.1
About 6% of participants reported vaginal discharge as an adverse effect. Levels of serum steroids remained within the normal range for postmenopausal women.1
Another study, in which authors integrated data from four phase 3 clinical trials of postmenopausal women with vulvovaginal atrophy treated with vaginal DHEA (n = 723) or placebo (n = 266) for 12 weeks, analyzed serum steroid levels measured at Day 1 and Week 12 by liquid chromatography-tandem mass spectrometry.2
At 12 weeks' treatment, mean levels of the most relevant sex steroid, serum estradiol, was noted to be 3.36 pg/mL, 19% below the normal postmenopausal value of 4.17 pg/mL.The mean level of estrone sulfate was noted to be 209 pg/mL, lower than the normal 220 pg/mL level in postmenopausal women. Further, androsterone glucuronide, the primary metabolite of androgens, also remained well within normal postmenopausal values.2
The authors concluded that the study data demonstrate that a daily 6.5-mg dose of intravaginal DHEA in postmenopausal women achieves the desired local efficacy (ie, amelioration of vulvovaginal atrophy symptoms) without systemic sex steroid exposure.2
The new information detailed in this article indicates that the recently FDA-approved vaginal DHEA (prasterone) ovules, as well as the 3-month low-dose estradiol vaginal ring, improve symptoms of genital atrophy without causing appreciable elevations in serum estradiol levels. This will be welcome news for all women with symptomatic genital atrophy, including those who have been treated for estrogen-sensitive cancers. Clinicians should be aware that, although package labeling for vaginal prasterone does not list a history of breast cancer as a contraindication, a history of breast cancer is listed in the Warning and Precautions section of package labeling, noting that this medication has not been studied in women with a history of breast cancer.
-- Andrew M. Kaunitz, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
As estrogen levels decline, postmenopausal women commonly experience uncomfortable and distressing symptoms of genital atrophy, or genitourinary syndrome of menopause (GSM). Moreover, aromatase inhibitors (AIs), increasingly used as adjuvant therapy by menopausal breast cancer survivors, contribute to vaginal dryness and sexual pain. This discussion focuses on studies of several local vaginal treatments (including a recently approved agent) that ameliorate GSM symptoms but do not appreciably raise serum sex steroid levels—reassuring data for certain patient populations.
EXPERT COMMENTARY
Andrew M. Kaunitz, MD, is University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology, University of Florida College of Medicine–Jacksonville. He is the Medical Director and Director of Menopause and Gynecologic Ultrasound Services, UF Women’s Health Specialists–Emerson. Dr. Kaunitz serves on the OBG Management Board of Editors.
Dr. Kaunitz reports that in 2015 he served on a contraception advisory board for Pfizer, which markets the low-dose estradiol vaginal ring.
Read expert commentary from Dr. Kaunitz
For women with early-stage breast cancer receiving an AI, is a vaginal estradiol ring or testosterone cream safe for genital atrophy?
Yes, according to results of a randomized, noncomparative short-term trial that found both agents improved vaginal dryness and sexual dysfunction and had little tendency to persistently elevate serum estradiol levels
Melisko ME, Goldman ME, Hwang J, et al. Vaginal testosterone cream vs estradiol vaginal ring for vaginal dryness or decreased libido in women receiving aromatase inhibitors for early-stage breast cancer: a randomized clinical trial [published online ahead of print November 10, 2016]. JAMA Oncol. doi: 10.1001/jamaoncol.2016.3904.
Long-term adjuvant AI therapy, which often causes vaginal dryness and sexual dysfunction, is recommended for postmenopausal women with hormone receptor-positive breast cancer. Although use of a vaginally administered low-dose 3-month estradiol ring as well as compounded testosterone cream is known to improve menopausal genital atrophy and sexual symptoms, little data address these agents' impact on serum estradiol levels in women using AIs.
In a safety evaluation study of these treatments performed at an academic US cancer center, Melisko and colleagues randomly assigned postmenopausal women with hormone receptor-positive breast cancer who reported vaginal dryness, sexual pain, or reduced sexual desire to 12 weeks of off-label treatment with an estradiol vaginal ring or intravaginal testosterone cream.
Related article:
Does extending aromatase-inhibitor use from 5 to 10 years benefit menopausal women with hormone-positive breast cancer?
Details of the study
Among 68 evaluable women (mean age, 56 years), mean baseline estradiol levels were 20 pg/mL (range, <2 to 127 pg/mL); estradiol levels were above the postmenopausal range (>10 pg/mL) in 37% of participants. During the 12-week trial, transient and persistent estradiol levels above this threshold were noted, respectively, in 4 and 0 women treated with the vaginal ring and in 4 and 4 women treated with testosterone cream. Estradiol levels assessed using commercially available (liquid chromatography and mass spectrometry) and research laboratory (radioimmune assay) methodology yielded similar results. In the testosterone cream group, persistent elevations above the normal postmenopausal range were common.
Atrophic vaginal changes, sexual desire, and sexual dysfunction improved in both treatment groups based on gynecologic examinations and sexual quality-of-life questionnaires completed at baseline and week 12.
The authors indicated that their current practice is to continue the estradiol vaginal ring or testosterone cream in AI users who experience symptomatic improvement with these formulations. They check serum estradiol levels every few months. A future large, long-term trial assessing the impact of off-label use of the estradiol vaginal ring on the incidence of recurrent disease in breast cancer survivors would provide definitive evidence of this treatment's safety.
--Andrew M. Kaunitz, MD
Read on for Dr. Kaunitz’s comments on a new dyspareunia treatment
What's new for the treatment of dyspareunia associated with GSM?
Intrarosa, a once-daily vaginal insert containing prasterone as the active ingredient, was recently approved for the treatment of moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy due to menopause
FDA approves Intrarosa for postmenopausal women experiencing pain during sex [news release]. Silver Spring, MD: US Food and Drug Administration; November 17, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm529641.htm. Accessed December 19, 2017.
Intrarosa [package insert]. Quebec City, Canada: Endoceutics Inc; 2016.
On November 17, 2016, the US Food and Drug Administration (FDA) approved Intrarosa, vaginal dehydroepiandrosterone (DHEA)--also known as prasterone--for women experiencing moderate to severe pain during sexual intercourse due to menopause-related genital atrophy, or GSM. In clinical trials, daily treatment with a 6.5-mg vaginal ovule of DHEA was found effective in reducing symptoms of atrophy. Vaginal discharge was the most common adverse effect.
After menopause, DHEA, which is produced largely by the adrenal glands, represents the dominant source of all sex steroids. DHEA is enzymatically transformed at the intracellular level into estrogens. Because estradiol is inactivated at the site of its synthesis, use of vaginal DHEA causes little if any rise in serum estradiol levels.1,2
Related article:
2014 Update on Fertility
Details of 2 studies
A pivotal randomized, double-blind, placebo-controlled phase 3 trial of intravaginal DHEA (6.5 mg daily) for treating postmenopausal dyspareunia in women with vulvovaginal atrophy was conducted over 12 weeks.1 The trial included 325 women treated with DHEA and 157 who received placebo.
All 4 coprimary objectives measured improved with treatment compared with mean baseline levels: percentage of parabasal cells in treated participants decreased by 27.7% over placebo (P<.0001); percentage of superficial cells increased by 8.44% over placebo (P<.0001); vaginal pH decreased by 0.66 pH unit over placebo (P<.0001); and pain with sexual activity decreased by 1.42 severity score unit from baseline or 0.36 unit over placebo (P = .0002). In addition, participant-reported moderate to severe vaginal dryness (present in 84% of women at baseline) improved considerably at 12 weeks, and gynecologic evaluation revealed improvements in vaginal secretions, epithelial integrity and surface thickness, and color.1
About 6% of participants reported vaginal discharge as an adverse effect. Levels of serum steroids remained within the normal range for postmenopausal women.1
Another study, in which authors integrated data from four phase 3 clinical trials of postmenopausal women with vulvovaginal atrophy treated with vaginal DHEA (n = 723) or placebo (n = 266) for 12 weeks, analyzed serum steroid levels measured at Day 1 and Week 12 by liquid chromatography-tandem mass spectrometry.2
At 12 weeks' treatment, mean levels of the most relevant sex steroid, serum estradiol, was noted to be 3.36 pg/mL, 19% below the normal postmenopausal value of 4.17 pg/mL.The mean level of estrone sulfate was noted to be 209 pg/mL, lower than the normal 220 pg/mL level in postmenopausal women. Further, androsterone glucuronide, the primary metabolite of androgens, also remained well within normal postmenopausal values.2
The authors concluded that the study data demonstrate that a daily 6.5-mg dose of intravaginal DHEA in postmenopausal women achieves the desired local efficacy (ie, amelioration of vulvovaginal atrophy symptoms) without systemic sex steroid exposure.2
The new information detailed in this article indicates that the recently FDA-approved vaginal DHEA (prasterone) ovules, as well as the 3-month low-dose estradiol vaginal ring, improve symptoms of genital atrophy without causing appreciable elevations in serum estradiol levels. This will be welcome news for all women with symptomatic genital atrophy, including those who have been treated for estrogen-sensitive cancers. Clinicians should be aware that, although package labeling for vaginal prasterone does not list a history of breast cancer as a contraindication, a history of breast cancer is listed in the Warning and Precautions section of package labeling, noting that this medication has not been studied in women with a history of breast cancer.
-- Andrew M. Kaunitz, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Labrie F, Archer DF, Koltun W, et al; VVA Prasterone Research Group. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause. 2016;23(3):243–256.
- Martel C, Labrie F, Archer DF, et al; Prasterone Clinical Research Group. Serum steroid concentrations remain within normal postmenopausal values in women receiving daily 6.5 mg intravaginal prasterone for 12 weeks. J Steroid Biochem Mol Biol. 2016;159:142–153.
- Labrie F, Archer DF, Koltun W, et al; VVA Prasterone Research Group. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause. 2016;23(3):243–256.
- Martel C, Labrie F, Archer DF, et al; Prasterone Clinical Research Group. Serum steroid concentrations remain within normal postmenopausal values in women receiving daily 6.5 mg intravaginal prasterone for 12 weeks. J Steroid Biochem Mol Biol. 2016;159:142–153.
Superficial Ulceration on the Vulva
Foscarnet-Induced Ulceration
Viral swabs were negative for herpes simplex virus. The diagnosis of foscarnet-induced ulceration was reached and the drug was discontinued. Symptomatic treatment with soap substitutes and lidocaine ointment was used.
Foscarnet is an antiviral agent used when resistance develops to first-line therapies.1 It is a pyrophosphate analogue that inhibits viral DNA polymerase, thereby preventing viral replication. It is used in treating cytomegalovirus and herpes simplex virus, which are resistant to first-line therapies, or patients who develop hematologic toxicity from antivirals. The main side effects of foscarnet include nephrotoxicity, alteration of calcium homeostasis, and malaise. Genital ulceration is a known side effect of therapy, though it is rare and more commonly seen in uncircumcised males. Approximately 94% of the drug is excreted unchanged in the urine, which causes an irritant dermatitis that is more pronounced in males as the urine stays in the subpreputial area.1
Vulval ulceration2 and penile ulceration3 has been reported in AIDS patients treated with foscarnet. In these patients, the onset of ulceration is temporally related to foscarnet therapy, occurring at approximately day 7 to 24 of treatment and resolving after discontinuation of therapy.
- Wagstaff AJ, Bryson HM. Foscarnet. a reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic use in immunocompromised patients with viral infections. Drugs. 1994;48:199-226.
- Lacey HB, Ness A, Mandal BK. Vulval ulceration associated with foscarnet. Genitourin Med. 1992;68:182.
- Moyle G, Barton S, Gazzard BG. Penile ulceration with foscarnet therapy. AIDS. 1993;7:140-141.
Foscarnet-Induced Ulceration
Viral swabs were negative for herpes simplex virus. The diagnosis of foscarnet-induced ulceration was reached and the drug was discontinued. Symptomatic treatment with soap substitutes and lidocaine ointment was used.
Foscarnet is an antiviral agent used when resistance develops to first-line therapies.1 It is a pyrophosphate analogue that inhibits viral DNA polymerase, thereby preventing viral replication. It is used in treating cytomegalovirus and herpes simplex virus, which are resistant to first-line therapies, or patients who develop hematologic toxicity from antivirals. The main side effects of foscarnet include nephrotoxicity, alteration of calcium homeostasis, and malaise. Genital ulceration is a known side effect of therapy, though it is rare and more commonly seen in uncircumcised males. Approximately 94% of the drug is excreted unchanged in the urine, which causes an irritant dermatitis that is more pronounced in males as the urine stays in the subpreputial area.1
Vulval ulceration2 and penile ulceration3 has been reported in AIDS patients treated with foscarnet. In these patients, the onset of ulceration is temporally related to foscarnet therapy, occurring at approximately day 7 to 24 of treatment and resolving after discontinuation of therapy.
Foscarnet-Induced Ulceration
Viral swabs were negative for herpes simplex virus. The diagnosis of foscarnet-induced ulceration was reached and the drug was discontinued. Symptomatic treatment with soap substitutes and lidocaine ointment was used.
Foscarnet is an antiviral agent used when resistance develops to first-line therapies.1 It is a pyrophosphate analogue that inhibits viral DNA polymerase, thereby preventing viral replication. It is used in treating cytomegalovirus and herpes simplex virus, which are resistant to first-line therapies, or patients who develop hematologic toxicity from antivirals. The main side effects of foscarnet include nephrotoxicity, alteration of calcium homeostasis, and malaise. Genital ulceration is a known side effect of therapy, though it is rare and more commonly seen in uncircumcised males. Approximately 94% of the drug is excreted unchanged in the urine, which causes an irritant dermatitis that is more pronounced in males as the urine stays in the subpreputial area.1
Vulval ulceration2 and penile ulceration3 has been reported in AIDS patients treated with foscarnet. In these patients, the onset of ulceration is temporally related to foscarnet therapy, occurring at approximately day 7 to 24 of treatment and resolving after discontinuation of therapy.
- Wagstaff AJ, Bryson HM. Foscarnet. a reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic use in immunocompromised patients with viral infections. Drugs. 1994;48:199-226.
- Lacey HB, Ness A, Mandal BK. Vulval ulceration associated with foscarnet. Genitourin Med. 1992;68:182.
- Moyle G, Barton S, Gazzard BG. Penile ulceration with foscarnet therapy. AIDS. 1993;7:140-141.
- Wagstaff AJ, Bryson HM. Foscarnet. a reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic use in immunocompromised patients with viral infections. Drugs. 1994;48:199-226.
- Lacey HB, Ness A, Mandal BK. Vulval ulceration associated with foscarnet. Genitourin Med. 1992;68:182.
- Moyle G, Barton S, Gazzard BG. Penile ulceration with foscarnet therapy. AIDS. 1993;7:140-141.
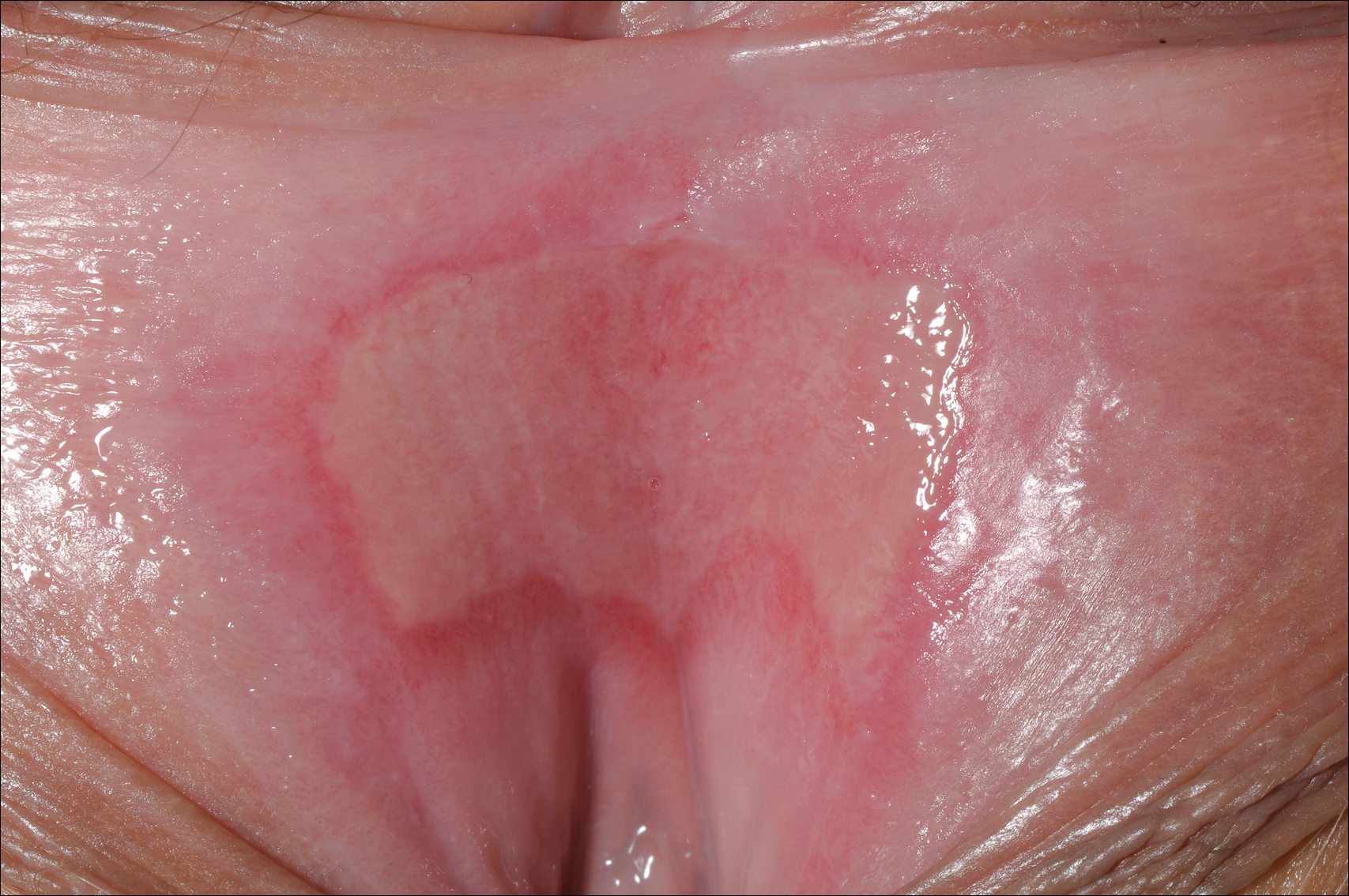
A 23-year-old woman who was immunosuppressed secondary to cyclophosphamide and prednisolone treatment of autoimmune panniculitis was admitted to intensive care with dyspnea. Cytomegalovirus and Pneumocystis jiroveci pneumonia were diagnosed on bronchoscopy and bronchial washings. Management with valganciclovir was started but worsened the patient's pancytopenia. She was started on intravenous foscarnet. After a week of therapy, the patient reported vulval soreness and painful micturition. On examination there was superficial ulceration of the labia minora. The affected area was symmetrical, and there was some extension into the vestibule. There were no vesicles or lesions on the cutaneous skin.
Overcoming LARC complications: 7 case challenges
The use of long-acting reversible contraceptive (LARC) methods has shown a steady increase in the United States. The major factors for increasing acceptance include high efficacy, ease of use, and an acceptable adverse effect profile. Since these methods require placement under the skin (implantable device) or into the uterus (intrauterine devices [IUDs]), unique management issues arise during their usage. Recently, the American College of Obstetricians and Gynecologists (ACOG) released a committee opinion addressing several of these clinical challenges—among them: pain with insertion, what to do when the IUD strings are not visualized, and the plan of action for a nonpalpable IUD or contraceptive implant.1 In this article we present 7 cases, and successful management approaches, that reflect ACOG’s recent recommendations and our extensive clinical experience.
Read the first CHALLENGE: Pain with IUD insertion
CHALLENGE 1: Pain with IUD insertion
CASE First-time, nulliparous IUD user apprehensive about insertion pain
A 21-year-old woman (G0) presents for placement of a 52-mg levonorgestrel IUD for contraception and treatment of dysmenorrhea. Her medical and surgical histories are unremarkable. She has heard that IUD insertion “is more painful if you haven’t had a baby yet” and she asks what treatments are available to aid in pain relief.
What can you offer her?
A number of approaches have been used to reduce IUD insertion pain, including:
- placing lidocaine gel into or on the cervix
- lidocaine paracervical block
- preinsertion use of misoprostol or nonsteroidal anti-inflammatory drugs.
Authors of a recent Cochrane review2 indicated that none of these approaches were particularly effective at reducing insertion pain for nulliparous women. Naproxen sodium 550 mg or tramadol 50 mg taken 1 hour prior to IUD insertion have been found to decrease IUD insertion pain in multiparous patients.3 Misoprostol, apart from being ineffective in reducing insertion pain, also requires use for a number of hours before insertion and can cause painful uterine cramping, upset stomach, and diarrhea.2 Some studies do suggest that use of a paracervical block does reduce the pain associated with tenaculum placement but not the IUD insertion itself.
Related article:
Benefit of self-administered vaginal lidocaine gel in IUD placement
A reasonable pain management strategy for nulliparous patients. Given these data, there is not an evidence-based IUD insertion pain management strategy that can be used for the nulliparous case patient. A practical approach for nulliparous patients is to offer naproxen sodium or tramadol, which have been found to be beneficial in multiparous patients, to a nulliparous patient. Additionally, lidocaine gel applied to the cervix or tenaculum-site injection can be considered for tenaculum-associated pain, although it does not appear to help significantly with IUD insertion pain. Misoprostol should be avoided as it does not alleviate the pain of insertion and it can cause bothersome adverse effects.
Read CHALLENGE 2: IUD strings not visualized
CHALLENGE 2: IUD strings not visualized
CASE No strings palpated 6 weeks after postpartum IUD placement
A 26-year-old woman (G2P2) presents to your office for a postpartum visit 6 weeks after an uncomplicated cesarean delivery at term. She had requested that a 52-mg levonorgestrel IUD be placed at the time of delivery, and the delivery report describes an uneventful placement. The patient has not been able to feel the IUD strings using her fingers and you do not find them on examination. She does not remember the IUD falling out.
What are the next steps in her management?
Failure to palpate the IUD strings by the user or failure to visualize the strings is a fairly common occurrence. This is especially true when an IUD is placed immediatelypostpartum, as in this patient’s case.
When the strings cannot be palpated, it is important to exclude pregnancy and recommend a form of backup contraception, such as condoms and emergency contraception if appropriate, until evaluation can be completed.
Steps to locate a device. In the office setting, the strings often can be located by inserting a cytobrush into the endocervical canal to extract them. If that maneuver fails to locate them, an ultrasound should be completed to determine if the device is in the uterus. If the ultrasound does not detect the device in the uterus, obtain an anteroposterior (AP) x-ray encompassing the entire abdomen and pelvis. All IUDs used in the United States are radiopaque and will be observed on x-ray if present. If the IUD is identified, operative removal is indicated.
Related article:
How to identify and localize IUDs on ultrasound
Intraperitoneal location. If an IUD is found in this location, it is usually the result of a perforation that occurred at the time of insertion. In general, the device can be removed via laparoscopy. Occasionally, laparotomy is needed if there is significant pelvic infection, possible bowel perforation, or if there is an inability to locate the device at laparoscopy.4 The copper IUD is more inflammatory than the levonorgestrel IUDs.
Abdominal location. No matter the IUD type, operative removal of intra-abdominal IUDs should take place expeditiously after they are discovered.
In the case of expulsion. If the IUD is not seen on x-ray, expulsion is the likely cause. Expulsion tends to be more common among5:
- parous users
- those younger than age 20
- placements that immediately follow a delivery or second-trimester abortion.
Nulliparity and type of device are not associated with increased risk of expulsion.
Read CHALLENGE 3: Difficult IUD removal
CHALLENGE 3: Difficult IUD removal
CASE Strings not palpated in a patient with history of LEEP
A 37-year-old woman (G3P2) presents to your office for IUD removal. She underwent a loop electrosurgical excision procedure 2 years ago for cervical intraepithelial neoplasia (CIN) 2 and since then has not been able to feel the IUD strings. On pelvic examination, you do not palpate or visualize the IUD strings after speculum placement.
How can you achieve IUD removal for your patient?
When a patient requests that her IUD be removed, but the strings are not visible and the woman is not pregnant, employ ultrasonography to confirm the IUD remains intrauterine and to rule out expulsion or perforation.
Employ alligator forceps or an IUD hook. Once intrauterine position is confirmed, use an alligator forceps of suitable length and with a small diameter to extract the device (FIGURE 1). It is useful to utilize ultrasonography for guidance during the removal procedure. The alligator forceps will grasp both the IUD device itself and IUD strings well, so either can be targeted during removal.
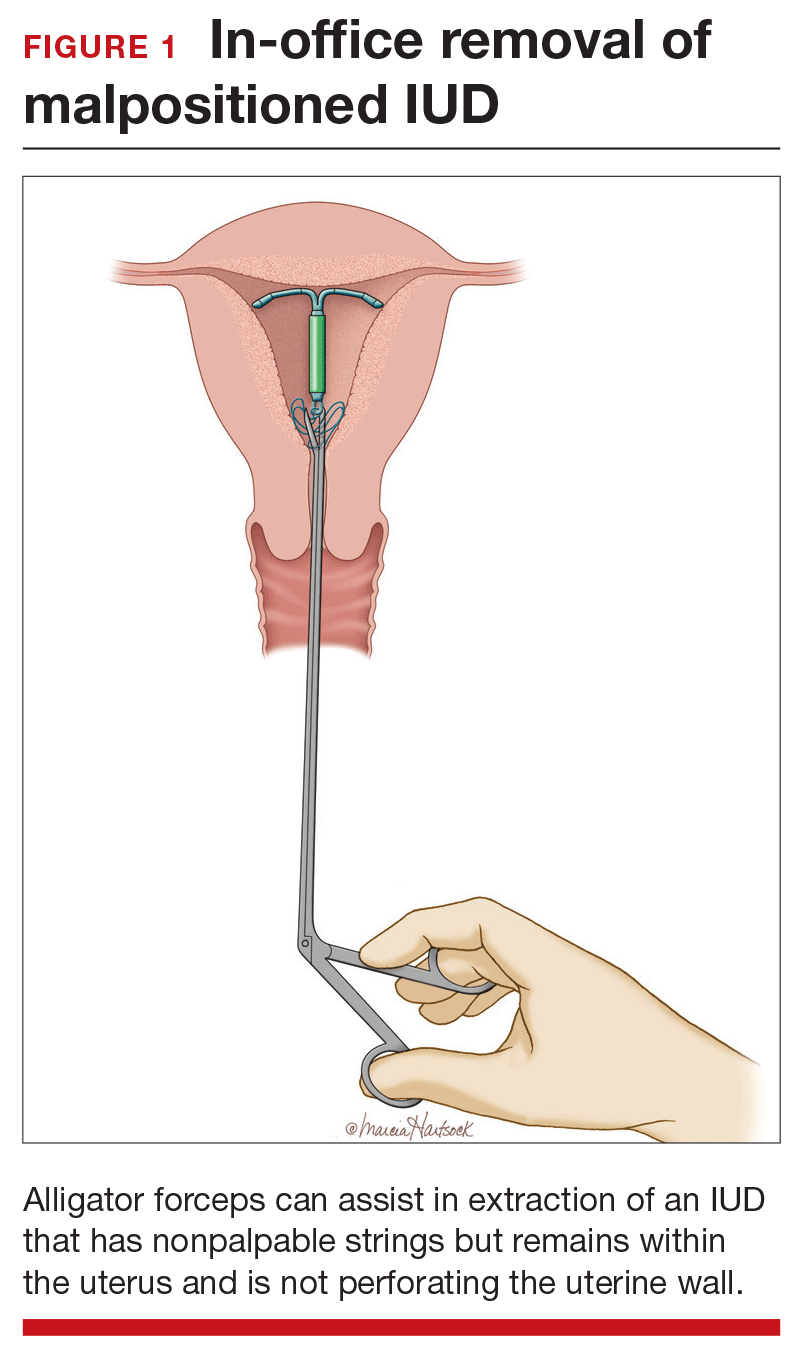
A second useful tool for IUD removal is an IUD hook (FIGURE 2). In a similar way that a curette is used for endometrial sampling, IUD hooks can be used to drag the IUD from the uterus.

Anesthesia is not usually necessary for IUD removal with alligator forceps or an IUD hook, although it may be appropriate in select patients. Data are limited with regard to the utility of paracervical blocks in this situation.
Related article:
Surgical removal of malpositioned IUDs
Hysteroscopy is an option. If removal with an alligator forceps or IUD hook is unsuccessful, or if preferred by the clinician, hysteroscopic-guided removal is a management option. Hysteroscopic removal may be required if the IUD has become embedded in the uterine wall.
Read CHALLENGE 4: Nonfundal IUD location
CHALLENGE 4: Nonfundal IUD location
CASE Copper IUD found in lower uterine segment
A 31-year-old woman (G1P1) calls your office to report that she thinks her copper IUD strings are longer than before. Office examination confirms that the strings are noticeably longer than is typical. Pelvic ultrasonography shows the copper IUD in the lower uterine segment.
What is the appropriate course of action?
Occasionally, IUDs are noted to be located in the lower uterine segment (FIGURE 3) or cervix. With malposition, users may be experiencing cramping or abnormal bleeding.
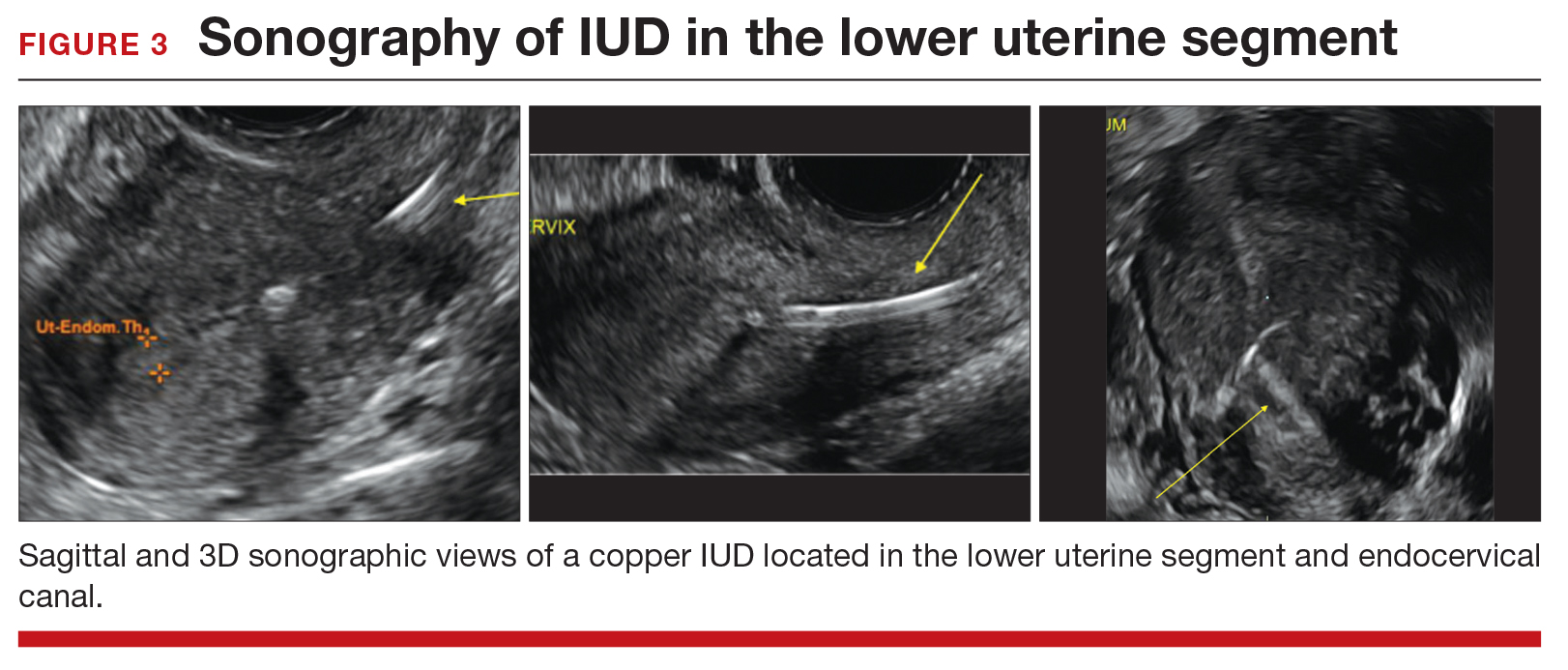
Cervical malposition calls for removal. ACOG advises that, regardless of a patient’s presenting symptoms, clinicians should remove IUDs located in the cervix (ie, the stem below the internal os) due to an increased risk of pregnancy and address the woman’s contraceptive needs.
Related article:
STOP relying on 2D ultrasound for IUD localization
Lower-uterine-segment malposition man‑agement less clear. If the patient is symptomatic, remove the device and initiate some form of contraception. If the woman is asymptomatic, the woman should be given the option of having the device removed or left in place. The mechanisms of action of both the copper and levonorgestrel-releasing IUDs suggest that this lower location is unlikely to be associated with a significant decrease in efficacy.
Unfortunately, it is difficult to estimate the risk of pregnancy for a patient whose device is located in the lower uterine segment. Braaten and Goldberg discussed case-controlled data in their 2012 article that suggest malposition may be more important to the efficacy of copper IUDs than of levonorgestrel IUDs.6,7 As unintended pregnancy is an important risk to avoid, ultimately, it is the woman’s decision as to whether she wants removal or continued IUD use.
Read CHALLENGE 5: Pregnancy in an IUD user
CHALLENGE 5: Pregnancy in an IUD user
CASE 3-year copper IUD user with positive pregnancy test
A 25-year-old woman (G3P2) presents to your office because of missed menses and a positive home pregnancy test. Her last menstrual period was 6 weeks ago. She has had a copper IUD in place for 3 years and can feel the strings herself. She has experienced light cramping but no bleeding. Office examination is notable for the IUD stem present at the external cervical os. While the pregnancy is unplanned, the patient desires that it continue.
Should you remove the IUD?
The pregnancy rate among IUD users is less than 1%—a rate that is equivalent to that experienced by women undergoing tubal sterilization. Although there is an overall low risk of pregnancy, a higher proportion of pregnancies among IUD users compared with nonusers are ectopic. Therefore, subsequent management of pregnancy in an IUD user needs to be determined by, using ultrasound, both the location of the pregnancy and whether the IUD is in place.
If an ectopic pregnancy is found, it may be managed medically or surgically with the IUD left in place if desired. If you find an intrauterine pregnancy that is undesired, the IUD can be removed at the time of a surgical abortion or before the initiation of a medical abortion.
If you fail to locate the IUD either before or after the abortion procedure, use an AP x-ray of the entire abdomen and pelvis to determine whether the IUD is in the peritoneal cavity or whether it was likely expelled prior to the pregnancy.
Related article:
In which clinical situations can the use of the 52-mg levonorgestrel-releasing IUD (Mirena) and the TCu380A copper-IUD (ParaGard) be extended?
With a desired pregnancy, if the strings are visible, remove the IUD with gentle traction. If the IUD is left in place, the risk of spontaneous abortion is significantly increased. If the strings are not seen, but the device was noted to be in the cervix by ultrasound, remove the device if the stem is below the internal cervical os. For IUDs that are located above the cervix, removal should not be attempted; counsel the patient about the increased risk of spontaneous abortion, infection, and preterm delivery.
Read CHALLENGE 6: Pregnancy in an implant user
CHALLENGE 6: Pregnancy in an implant user
CASE 3-week implant user with positive pregnancy test
Your 21-year-old patient who received a contraceptive implant 3 weeks earlier now pre‑sents with nausea and abdominal cramping. Her last menstrual period was 6 weeks ago. She has regular cycles that are 28 days in length. Results of urine pregnancy testing are positive. Prior to using the implant, the patient inconsistently used condoms.
How should you counsel your patient?
The rate of pregnancy among implant users is very low; it is estimated at 5 pregnancies per 10,000 implant users per year.8 As in this case, apparent “failures” of the contraceptive implant actually may represent placements that occurred before a very early pregnancy was recognized. Similar to IUDs, the proportion of pregnancies that are ectopic among implant users compared to nonusers may be higher.
With a pregnancy that is ectopic or that is intrauterine and undesired, the device may be left in and use continued after the pregnancy has been terminated. Although the effectiveness of medication abortion with pre-existing contraceptive implant in situ is not well known, researchers have demonstrated that medication abortion initiated at the same time as contraceptive implant insertion does not influence success of the medication abortion.9
Related article:
2016 Update on contraception
For women with desired intrauterine pregnancies, remove the device as soon as feasible and counsel the woman that there is no known teratogenic risk associated with the contraceptive implant.
Read CHALLENGE 7: Nonpalpable contraceptive implant
CHALLENGE 7: Nonpalpable contraceptive implant
CASE Patient requests device removal to attempt conception
A 30-year-old woman (G2P2) presents for contraceptive implant removal because she would like to have another child. The device was placed 30 months ago in the patient’s left arm. The insertion note in the patient’s medical record is unremarkable, and standard insertion technique was used. On physical examination, you cannot palpate the device.
What is your next course of action?
Nonpalpable implants, particularly if removal is desired, present a significant clinical challenge. Do not attempt removing a nonpalpable implant before trying to locate the device through past medical records or radiography. Records that describe the original insertion, particularly the location and type of device, are helpful.
Related article:
2015 Update on contraception
Appropriate imaging assistance. Ultrasonography with a high frequency linear array transducer (10 MHz or greater) may allow an experienced radiologist to identify the implant—including earlier versions without barium (Implanon) and later ones with barium (Nexplanon). Magnetic resonance imaging (MRI), computed tomography scan, or plain x-ray also can be used to detect a barium-containing device; MRI can be used to locate a non−barium-containing implant.
Carry out removal using ultrasonographic guidance. If a deep insertion is felt to be close to a neurovascular bundle, device removal should be carried out in an operating room by a surgeon familiar with the anatomy of the upper arm.
When an implant cannot be located despite radiography. This is an infrequent occurrence. Merck, the manufacturer of the etonorgestrel implant, provides advice and support in this circumstance. (Visit https://www.merckconnect.com/nexplanon/over view.html.)
Recently, published case reports detail episodes of implants inserted into the venous system with migration to the heart or lungs.10 While this phenomenon is considered rare, the manufacturer has recommended that insertion of the contraceptive implant avoid the sulcus between the biceps and triceps muscles.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 672: clinical challenges of long-acting reversible contraceptive methods. Obstet Gynecol. 2016;128(3):e69−e77.
- Lopez LM, Bernholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2015;(7):CD007373.
- Karabayirli S, Ayrim AA, Muslu B. Comparison of the analgesic effects of oral tramadol and naproxen sodium on pain relief during IUD insertion. J Minim Invasive Gynecol. 2012;19(5):581−584.
- Kho KA, Chamsy DJ. Perforated intraperitoneal intrauterine contraceptive devices: diagnosis, management, and clinical outcomes. J Minim Invasive Gynecol. 2014;21(4):596−601.
- Madden T, McNicholas C, Zhao Q, Secura GM, Eisenberg DL, Peipert JF. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014;124(4):718−726.
- Patil E, Bednarek PH. Immediate intrauterine device insertion following surgical abortion. Obstet Gynecol Clin North Am. 2015;42(4):583−546.
- Braaten and Goldberg. OBG Manag. Malpositioned IUDs: When you should intervene (and when you should not). OBG Manag. 2012;24(8):38−46.
- Trussell J. Contraceptive failure in the United States. Contraception. 2011;83(5):397−404.
- Raymond EG, Weaver MA, Tan YL, et al. Effect of immediate compared with delayed insertion of etonogestrel implants on medical abortion efficacy and repeat pregnancy: a randomized controlled trial. Obstet Gynecol. 2017;127(2):306−312.
- Rowlands S, Mansour D, Walling M. Intravascular migration of contraceptive implants: two more cases. Contraception. 2016. In press.
The use of long-acting reversible contraceptive (LARC) methods has shown a steady increase in the United States. The major factors for increasing acceptance include high efficacy, ease of use, and an acceptable adverse effect profile. Since these methods require placement under the skin (implantable device) or into the uterus (intrauterine devices [IUDs]), unique management issues arise during their usage. Recently, the American College of Obstetricians and Gynecologists (ACOG) released a committee opinion addressing several of these clinical challenges—among them: pain with insertion, what to do when the IUD strings are not visualized, and the plan of action for a nonpalpable IUD or contraceptive implant.1 In this article we present 7 cases, and successful management approaches, that reflect ACOG’s recent recommendations and our extensive clinical experience.
Read the first CHALLENGE: Pain with IUD insertion
CHALLENGE 1: Pain with IUD insertion
CASE First-time, nulliparous IUD user apprehensive about insertion pain
A 21-year-old woman (G0) presents for placement of a 52-mg levonorgestrel IUD for contraception and treatment of dysmenorrhea. Her medical and surgical histories are unremarkable. She has heard that IUD insertion “is more painful if you haven’t had a baby yet” and she asks what treatments are available to aid in pain relief.
What can you offer her?
A number of approaches have been used to reduce IUD insertion pain, including:
- placing lidocaine gel into or on the cervix
- lidocaine paracervical block
- preinsertion use of misoprostol or nonsteroidal anti-inflammatory drugs.
Authors of a recent Cochrane review2 indicated that none of these approaches were particularly effective at reducing insertion pain for nulliparous women. Naproxen sodium 550 mg or tramadol 50 mg taken 1 hour prior to IUD insertion have been found to decrease IUD insertion pain in multiparous patients.3 Misoprostol, apart from being ineffective in reducing insertion pain, also requires use for a number of hours before insertion and can cause painful uterine cramping, upset stomach, and diarrhea.2 Some studies do suggest that use of a paracervical block does reduce the pain associated with tenaculum placement but not the IUD insertion itself.
Related article:
Benefit of self-administered vaginal lidocaine gel in IUD placement
A reasonable pain management strategy for nulliparous patients. Given these data, there is not an evidence-based IUD insertion pain management strategy that can be used for the nulliparous case patient. A practical approach for nulliparous patients is to offer naproxen sodium or tramadol, which have been found to be beneficial in multiparous patients, to a nulliparous patient. Additionally, lidocaine gel applied to the cervix or tenaculum-site injection can be considered for tenaculum-associated pain, although it does not appear to help significantly with IUD insertion pain. Misoprostol should be avoided as it does not alleviate the pain of insertion and it can cause bothersome adverse effects.
Read CHALLENGE 2: IUD strings not visualized
CHALLENGE 2: IUD strings not visualized
CASE No strings palpated 6 weeks after postpartum IUD placement
A 26-year-old woman (G2P2) presents to your office for a postpartum visit 6 weeks after an uncomplicated cesarean delivery at term. She had requested that a 52-mg levonorgestrel IUD be placed at the time of delivery, and the delivery report describes an uneventful placement. The patient has not been able to feel the IUD strings using her fingers and you do not find them on examination. She does not remember the IUD falling out.
What are the next steps in her management?
Failure to palpate the IUD strings by the user or failure to visualize the strings is a fairly common occurrence. This is especially true when an IUD is placed immediatelypostpartum, as in this patient’s case.
When the strings cannot be palpated, it is important to exclude pregnancy and recommend a form of backup contraception, such as condoms and emergency contraception if appropriate, until evaluation can be completed.
Steps to locate a device. In the office setting, the strings often can be located by inserting a cytobrush into the endocervical canal to extract them. If that maneuver fails to locate them, an ultrasound should be completed to determine if the device is in the uterus. If the ultrasound does not detect the device in the uterus, obtain an anteroposterior (AP) x-ray encompassing the entire abdomen and pelvis. All IUDs used in the United States are radiopaque and will be observed on x-ray if present. If the IUD is identified, operative removal is indicated.
Related article:
How to identify and localize IUDs on ultrasound
Intraperitoneal location. If an IUD is found in this location, it is usually the result of a perforation that occurred at the time of insertion. In general, the device can be removed via laparoscopy. Occasionally, laparotomy is needed if there is significant pelvic infection, possible bowel perforation, or if there is an inability to locate the device at laparoscopy.4 The copper IUD is more inflammatory than the levonorgestrel IUDs.
Abdominal location. No matter the IUD type, operative removal of intra-abdominal IUDs should take place expeditiously after they are discovered.
In the case of expulsion. If the IUD is not seen on x-ray, expulsion is the likely cause. Expulsion tends to be more common among5:
- parous users
- those younger than age 20
- placements that immediately follow a delivery or second-trimester abortion.
Nulliparity and type of device are not associated with increased risk of expulsion.
Read CHALLENGE 3: Difficult IUD removal
CHALLENGE 3: Difficult IUD removal
CASE Strings not palpated in a patient with history of LEEP
A 37-year-old woman (G3P2) presents to your office for IUD removal. She underwent a loop electrosurgical excision procedure 2 years ago for cervical intraepithelial neoplasia (CIN) 2 and since then has not been able to feel the IUD strings. On pelvic examination, you do not palpate or visualize the IUD strings after speculum placement.
How can you achieve IUD removal for your patient?
When a patient requests that her IUD be removed, but the strings are not visible and the woman is not pregnant, employ ultrasonography to confirm the IUD remains intrauterine and to rule out expulsion or perforation.
Employ alligator forceps or an IUD hook. Once intrauterine position is confirmed, use an alligator forceps of suitable length and with a small diameter to extract the device (FIGURE 1). It is useful to utilize ultrasonography for guidance during the removal procedure. The alligator forceps will grasp both the IUD device itself and IUD strings well, so either can be targeted during removal.

A second useful tool for IUD removal is an IUD hook (FIGURE 2). In a similar way that a curette is used for endometrial sampling, IUD hooks can be used to drag the IUD from the uterus.

Anesthesia is not usually necessary for IUD removal with alligator forceps or an IUD hook, although it may be appropriate in select patients. Data are limited with regard to the utility of paracervical blocks in this situation.
Related article:
Surgical removal of malpositioned IUDs
Hysteroscopy is an option. If removal with an alligator forceps or IUD hook is unsuccessful, or if preferred by the clinician, hysteroscopic-guided removal is a management option. Hysteroscopic removal may be required if the IUD has become embedded in the uterine wall.
Read CHALLENGE 4: Nonfundal IUD location
CHALLENGE 4: Nonfundal IUD location
CASE Copper IUD found in lower uterine segment
A 31-year-old woman (G1P1) calls your office to report that she thinks her copper IUD strings are longer than before. Office examination confirms that the strings are noticeably longer than is typical. Pelvic ultrasonography shows the copper IUD in the lower uterine segment.
What is the appropriate course of action?
Occasionally, IUDs are noted to be located in the lower uterine segment (FIGURE 3) or cervix. With malposition, users may be experiencing cramping or abnormal bleeding.

Cervical malposition calls for removal. ACOG advises that, regardless of a patient’s presenting symptoms, clinicians should remove IUDs located in the cervix (ie, the stem below the internal os) due to an increased risk of pregnancy and address the woman’s contraceptive needs.
Related article:
STOP relying on 2D ultrasound for IUD localization
Lower-uterine-segment malposition man‑agement less clear. If the patient is symptomatic, remove the device and initiate some form of contraception. If the woman is asymptomatic, the woman should be given the option of having the device removed or left in place. The mechanisms of action of both the copper and levonorgestrel-releasing IUDs suggest that this lower location is unlikely to be associated with a significant decrease in efficacy.
Unfortunately, it is difficult to estimate the risk of pregnancy for a patient whose device is located in the lower uterine segment. Braaten and Goldberg discussed case-controlled data in their 2012 article that suggest malposition may be more important to the efficacy of copper IUDs than of levonorgestrel IUDs.6,7 As unintended pregnancy is an important risk to avoid, ultimately, it is the woman’s decision as to whether she wants removal or continued IUD use.
Read CHALLENGE 5: Pregnancy in an IUD user
CHALLENGE 5: Pregnancy in an IUD user
CASE 3-year copper IUD user with positive pregnancy test
A 25-year-old woman (G3P2) presents to your office because of missed menses and a positive home pregnancy test. Her last menstrual period was 6 weeks ago. She has had a copper IUD in place for 3 years and can feel the strings herself. She has experienced light cramping but no bleeding. Office examination is notable for the IUD stem present at the external cervical os. While the pregnancy is unplanned, the patient desires that it continue.
Should you remove the IUD?
The pregnancy rate among IUD users is less than 1%—a rate that is equivalent to that experienced by women undergoing tubal sterilization. Although there is an overall low risk of pregnancy, a higher proportion of pregnancies among IUD users compared with nonusers are ectopic. Therefore, subsequent management of pregnancy in an IUD user needs to be determined by, using ultrasound, both the location of the pregnancy and whether the IUD is in place.
If an ectopic pregnancy is found, it may be managed medically or surgically with the IUD left in place if desired. If you find an intrauterine pregnancy that is undesired, the IUD can be removed at the time of a surgical abortion or before the initiation of a medical abortion.
If you fail to locate the IUD either before or after the abortion procedure, use an AP x-ray of the entire abdomen and pelvis to determine whether the IUD is in the peritoneal cavity or whether it was likely expelled prior to the pregnancy.
Related article:
In which clinical situations can the use of the 52-mg levonorgestrel-releasing IUD (Mirena) and the TCu380A copper-IUD (ParaGard) be extended?
With a desired pregnancy, if the strings are visible, remove the IUD with gentle traction. If the IUD is left in place, the risk of spontaneous abortion is significantly increased. If the strings are not seen, but the device was noted to be in the cervix by ultrasound, remove the device if the stem is below the internal cervical os. For IUDs that are located above the cervix, removal should not be attempted; counsel the patient about the increased risk of spontaneous abortion, infection, and preterm delivery.
Read CHALLENGE 6: Pregnancy in an implant user
CHALLENGE 6: Pregnancy in an implant user
CASE 3-week implant user with positive pregnancy test
Your 21-year-old patient who received a contraceptive implant 3 weeks earlier now pre‑sents with nausea and abdominal cramping. Her last menstrual period was 6 weeks ago. She has regular cycles that are 28 days in length. Results of urine pregnancy testing are positive. Prior to using the implant, the patient inconsistently used condoms.
How should you counsel your patient?
The rate of pregnancy among implant users is very low; it is estimated at 5 pregnancies per 10,000 implant users per year.8 As in this case, apparent “failures” of the contraceptive implant actually may represent placements that occurred before a very early pregnancy was recognized. Similar to IUDs, the proportion of pregnancies that are ectopic among implant users compared to nonusers may be higher.
With a pregnancy that is ectopic or that is intrauterine and undesired, the device may be left in and use continued after the pregnancy has been terminated. Although the effectiveness of medication abortion with pre-existing contraceptive implant in situ is not well known, researchers have demonstrated that medication abortion initiated at the same time as contraceptive implant insertion does not influence success of the medication abortion.9
Related article:
2016 Update on contraception
For women with desired intrauterine pregnancies, remove the device as soon as feasible and counsel the woman that there is no known teratogenic risk associated with the contraceptive implant.
Read CHALLENGE 7: Nonpalpable contraceptive implant
CHALLENGE 7: Nonpalpable contraceptive implant
CASE Patient requests device removal to attempt conception
A 30-year-old woman (G2P2) presents for contraceptive implant removal because she would like to have another child. The device was placed 30 months ago in the patient’s left arm. The insertion note in the patient’s medical record is unremarkable, and standard insertion technique was used. On physical examination, you cannot palpate the device.
What is your next course of action?
Nonpalpable implants, particularly if removal is desired, present a significant clinical challenge. Do not attempt removing a nonpalpable implant before trying to locate the device through past medical records or radiography. Records that describe the original insertion, particularly the location and type of device, are helpful.
Related article:
2015 Update on contraception
Appropriate imaging assistance. Ultrasonography with a high frequency linear array transducer (10 MHz or greater) may allow an experienced radiologist to identify the implant—including earlier versions without barium (Implanon) and later ones with barium (Nexplanon). Magnetic resonance imaging (MRI), computed tomography scan, or plain x-ray also can be used to detect a barium-containing device; MRI can be used to locate a non−barium-containing implant.
Carry out removal using ultrasonographic guidance. If a deep insertion is felt to be close to a neurovascular bundle, device removal should be carried out in an operating room by a surgeon familiar with the anatomy of the upper arm.
When an implant cannot be located despite radiography. This is an infrequent occurrence. Merck, the manufacturer of the etonorgestrel implant, provides advice and support in this circumstance. (Visit https://www.merckconnect.com/nexplanon/over view.html.)
Recently, published case reports detail episodes of implants inserted into the venous system with migration to the heart or lungs.10 While this phenomenon is considered rare, the manufacturer has recommended that insertion of the contraceptive implant avoid the sulcus between the biceps and triceps muscles.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The use of long-acting reversible contraceptive (LARC) methods has shown a steady increase in the United States. The major factors for increasing acceptance include high efficacy, ease of use, and an acceptable adverse effect profile. Since these methods require placement under the skin (implantable device) or into the uterus (intrauterine devices [IUDs]), unique management issues arise during their usage. Recently, the American College of Obstetricians and Gynecologists (ACOG) released a committee opinion addressing several of these clinical challenges—among them: pain with insertion, what to do when the IUD strings are not visualized, and the plan of action for a nonpalpable IUD or contraceptive implant.1 In this article we present 7 cases, and successful management approaches, that reflect ACOG’s recent recommendations and our extensive clinical experience.
Read the first CHALLENGE: Pain with IUD insertion
CHALLENGE 1: Pain with IUD insertion
CASE First-time, nulliparous IUD user apprehensive about insertion pain
A 21-year-old woman (G0) presents for placement of a 52-mg levonorgestrel IUD for contraception and treatment of dysmenorrhea. Her medical and surgical histories are unremarkable. She has heard that IUD insertion “is more painful if you haven’t had a baby yet” and she asks what treatments are available to aid in pain relief.
What can you offer her?
A number of approaches have been used to reduce IUD insertion pain, including:
- placing lidocaine gel into or on the cervix
- lidocaine paracervical block
- preinsertion use of misoprostol or nonsteroidal anti-inflammatory drugs.
Authors of a recent Cochrane review2 indicated that none of these approaches were particularly effective at reducing insertion pain for nulliparous women. Naproxen sodium 550 mg or tramadol 50 mg taken 1 hour prior to IUD insertion have been found to decrease IUD insertion pain in multiparous patients.3 Misoprostol, apart from being ineffective in reducing insertion pain, also requires use for a number of hours before insertion and can cause painful uterine cramping, upset stomach, and diarrhea.2 Some studies do suggest that use of a paracervical block does reduce the pain associated with tenaculum placement but not the IUD insertion itself.
Related article:
Benefit of self-administered vaginal lidocaine gel in IUD placement
A reasonable pain management strategy for nulliparous patients. Given these data, there is not an evidence-based IUD insertion pain management strategy that can be used for the nulliparous case patient. A practical approach for nulliparous patients is to offer naproxen sodium or tramadol, which have been found to be beneficial in multiparous patients, to a nulliparous patient. Additionally, lidocaine gel applied to the cervix or tenaculum-site injection can be considered for tenaculum-associated pain, although it does not appear to help significantly with IUD insertion pain. Misoprostol should be avoided as it does not alleviate the pain of insertion and it can cause bothersome adverse effects.
Read CHALLENGE 2: IUD strings not visualized
CHALLENGE 2: IUD strings not visualized
CASE No strings palpated 6 weeks after postpartum IUD placement
A 26-year-old woman (G2P2) presents to your office for a postpartum visit 6 weeks after an uncomplicated cesarean delivery at term. She had requested that a 52-mg levonorgestrel IUD be placed at the time of delivery, and the delivery report describes an uneventful placement. The patient has not been able to feel the IUD strings using her fingers and you do not find them on examination. She does not remember the IUD falling out.
What are the next steps in her management?
Failure to palpate the IUD strings by the user or failure to visualize the strings is a fairly common occurrence. This is especially true when an IUD is placed immediatelypostpartum, as in this patient’s case.
When the strings cannot be palpated, it is important to exclude pregnancy and recommend a form of backup contraception, such as condoms and emergency contraception if appropriate, until evaluation can be completed.
Steps to locate a device. In the office setting, the strings often can be located by inserting a cytobrush into the endocervical canal to extract them. If that maneuver fails to locate them, an ultrasound should be completed to determine if the device is in the uterus. If the ultrasound does not detect the device in the uterus, obtain an anteroposterior (AP) x-ray encompassing the entire abdomen and pelvis. All IUDs used in the United States are radiopaque and will be observed on x-ray if present. If the IUD is identified, operative removal is indicated.
Related article:
How to identify and localize IUDs on ultrasound
Intraperitoneal location. If an IUD is found in this location, it is usually the result of a perforation that occurred at the time of insertion. In general, the device can be removed via laparoscopy. Occasionally, laparotomy is needed if there is significant pelvic infection, possible bowel perforation, or if there is an inability to locate the device at laparoscopy.4 The copper IUD is more inflammatory than the levonorgestrel IUDs.
Abdominal location. No matter the IUD type, operative removal of intra-abdominal IUDs should take place expeditiously after they are discovered.
In the case of expulsion. If the IUD is not seen on x-ray, expulsion is the likely cause. Expulsion tends to be more common among5:
- parous users
- those younger than age 20
- placements that immediately follow a delivery or second-trimester abortion.
Nulliparity and type of device are not associated with increased risk of expulsion.
Read CHALLENGE 3: Difficult IUD removal
CHALLENGE 3: Difficult IUD removal
CASE Strings not palpated in a patient with history of LEEP
A 37-year-old woman (G3P2) presents to your office for IUD removal. She underwent a loop electrosurgical excision procedure 2 years ago for cervical intraepithelial neoplasia (CIN) 2 and since then has not been able to feel the IUD strings. On pelvic examination, you do not palpate or visualize the IUD strings after speculum placement.
How can you achieve IUD removal for your patient?
When a patient requests that her IUD be removed, but the strings are not visible and the woman is not pregnant, employ ultrasonography to confirm the IUD remains intrauterine and to rule out expulsion or perforation.
Employ alligator forceps or an IUD hook. Once intrauterine position is confirmed, use an alligator forceps of suitable length and with a small diameter to extract the device (FIGURE 1). It is useful to utilize ultrasonography for guidance during the removal procedure. The alligator forceps will grasp both the IUD device itself and IUD strings well, so either can be targeted during removal.

A second useful tool for IUD removal is an IUD hook (FIGURE 2). In a similar way that a curette is used for endometrial sampling, IUD hooks can be used to drag the IUD from the uterus.

Anesthesia is not usually necessary for IUD removal with alligator forceps or an IUD hook, although it may be appropriate in select patients. Data are limited with regard to the utility of paracervical blocks in this situation.
Related article:
Surgical removal of malpositioned IUDs
Hysteroscopy is an option. If removal with an alligator forceps or IUD hook is unsuccessful, or if preferred by the clinician, hysteroscopic-guided removal is a management option. Hysteroscopic removal may be required if the IUD has become embedded in the uterine wall.
Read CHALLENGE 4: Nonfundal IUD location
CHALLENGE 4: Nonfundal IUD location
CASE Copper IUD found in lower uterine segment
A 31-year-old woman (G1P1) calls your office to report that she thinks her copper IUD strings are longer than before. Office examination confirms that the strings are noticeably longer than is typical. Pelvic ultrasonography shows the copper IUD in the lower uterine segment.
What is the appropriate course of action?
Occasionally, IUDs are noted to be located in the lower uterine segment (FIGURE 3) or cervix. With malposition, users may be experiencing cramping or abnormal bleeding.

Cervical malposition calls for removal. ACOG advises that, regardless of a patient’s presenting symptoms, clinicians should remove IUDs located in the cervix (ie, the stem below the internal os) due to an increased risk of pregnancy and address the woman’s contraceptive needs.
Related article:
STOP relying on 2D ultrasound for IUD localization
Lower-uterine-segment malposition man‑agement less clear. If the patient is symptomatic, remove the device and initiate some form of contraception. If the woman is asymptomatic, the woman should be given the option of having the device removed or left in place. The mechanisms of action of both the copper and levonorgestrel-releasing IUDs suggest that this lower location is unlikely to be associated with a significant decrease in efficacy.
Unfortunately, it is difficult to estimate the risk of pregnancy for a patient whose device is located in the lower uterine segment. Braaten and Goldberg discussed case-controlled data in their 2012 article that suggest malposition may be more important to the efficacy of copper IUDs than of levonorgestrel IUDs.6,7 As unintended pregnancy is an important risk to avoid, ultimately, it is the woman’s decision as to whether she wants removal or continued IUD use.
Read CHALLENGE 5: Pregnancy in an IUD user
CHALLENGE 5: Pregnancy in an IUD user
CASE 3-year copper IUD user with positive pregnancy test
A 25-year-old woman (G3P2) presents to your office because of missed menses and a positive home pregnancy test. Her last menstrual period was 6 weeks ago. She has had a copper IUD in place for 3 years and can feel the strings herself. She has experienced light cramping but no bleeding. Office examination is notable for the IUD stem present at the external cervical os. While the pregnancy is unplanned, the patient desires that it continue.
Should you remove the IUD?
The pregnancy rate among IUD users is less than 1%—a rate that is equivalent to that experienced by women undergoing tubal sterilization. Although there is an overall low risk of pregnancy, a higher proportion of pregnancies among IUD users compared with nonusers are ectopic. Therefore, subsequent management of pregnancy in an IUD user needs to be determined by, using ultrasound, both the location of the pregnancy and whether the IUD is in place.
If an ectopic pregnancy is found, it may be managed medically or surgically with the IUD left in place if desired. If you find an intrauterine pregnancy that is undesired, the IUD can be removed at the time of a surgical abortion or before the initiation of a medical abortion.
If you fail to locate the IUD either before or after the abortion procedure, use an AP x-ray of the entire abdomen and pelvis to determine whether the IUD is in the peritoneal cavity or whether it was likely expelled prior to the pregnancy.
Related article:
In which clinical situations can the use of the 52-mg levonorgestrel-releasing IUD (Mirena) and the TCu380A copper-IUD (ParaGard) be extended?
With a desired pregnancy, if the strings are visible, remove the IUD with gentle traction. If the IUD is left in place, the risk of spontaneous abortion is significantly increased. If the strings are not seen, but the device was noted to be in the cervix by ultrasound, remove the device if the stem is below the internal cervical os. For IUDs that are located above the cervix, removal should not be attempted; counsel the patient about the increased risk of spontaneous abortion, infection, and preterm delivery.
Read CHALLENGE 6: Pregnancy in an implant user
CHALLENGE 6: Pregnancy in an implant user
CASE 3-week implant user with positive pregnancy test
Your 21-year-old patient who received a contraceptive implant 3 weeks earlier now pre‑sents with nausea and abdominal cramping. Her last menstrual period was 6 weeks ago. She has regular cycles that are 28 days in length. Results of urine pregnancy testing are positive. Prior to using the implant, the patient inconsistently used condoms.
How should you counsel your patient?
The rate of pregnancy among implant users is very low; it is estimated at 5 pregnancies per 10,000 implant users per year.8 As in this case, apparent “failures” of the contraceptive implant actually may represent placements that occurred before a very early pregnancy was recognized. Similar to IUDs, the proportion of pregnancies that are ectopic among implant users compared to nonusers may be higher.
With a pregnancy that is ectopic or that is intrauterine and undesired, the device may be left in and use continued after the pregnancy has been terminated. Although the effectiveness of medication abortion with pre-existing contraceptive implant in situ is not well known, researchers have demonstrated that medication abortion initiated at the same time as contraceptive implant insertion does not influence success of the medication abortion.9
Related article:
2016 Update on contraception
For women with desired intrauterine pregnancies, remove the device as soon as feasible and counsel the woman that there is no known teratogenic risk associated with the contraceptive implant.
Read CHALLENGE 7: Nonpalpable contraceptive implant
CHALLENGE 7: Nonpalpable contraceptive implant
CASE Patient requests device removal to attempt conception
A 30-year-old woman (G2P2) presents for contraceptive implant removal because she would like to have another child. The device was placed 30 months ago in the patient’s left arm. The insertion note in the patient’s medical record is unremarkable, and standard insertion technique was used. On physical examination, you cannot palpate the device.
What is your next course of action?
Nonpalpable implants, particularly if removal is desired, present a significant clinical challenge. Do not attempt removing a nonpalpable implant before trying to locate the device through past medical records or radiography. Records that describe the original insertion, particularly the location and type of device, are helpful.
Related article:
2015 Update on contraception
Appropriate imaging assistance. Ultrasonography with a high frequency linear array transducer (10 MHz or greater) may allow an experienced radiologist to identify the implant—including earlier versions without barium (Implanon) and later ones with barium (Nexplanon). Magnetic resonance imaging (MRI), computed tomography scan, or plain x-ray also can be used to detect a barium-containing device; MRI can be used to locate a non−barium-containing implant.
Carry out removal using ultrasonographic guidance. If a deep insertion is felt to be close to a neurovascular bundle, device removal should be carried out in an operating room by a surgeon familiar with the anatomy of the upper arm.
When an implant cannot be located despite radiography. This is an infrequent occurrence. Merck, the manufacturer of the etonorgestrel implant, provides advice and support in this circumstance. (Visit https://www.merckconnect.com/nexplanon/over view.html.)
Recently, published case reports detail episodes of implants inserted into the venous system with migration to the heart or lungs.10 While this phenomenon is considered rare, the manufacturer has recommended that insertion of the contraceptive implant avoid the sulcus between the biceps and triceps muscles.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 672: clinical challenges of long-acting reversible contraceptive methods. Obstet Gynecol. 2016;128(3):e69−e77.
- Lopez LM, Bernholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2015;(7):CD007373.
- Karabayirli S, Ayrim AA, Muslu B. Comparison of the analgesic effects of oral tramadol and naproxen sodium on pain relief during IUD insertion. J Minim Invasive Gynecol. 2012;19(5):581−584.
- Kho KA, Chamsy DJ. Perforated intraperitoneal intrauterine contraceptive devices: diagnosis, management, and clinical outcomes. J Minim Invasive Gynecol. 2014;21(4):596−601.
- Madden T, McNicholas C, Zhao Q, Secura GM, Eisenberg DL, Peipert JF. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014;124(4):718−726.
- Patil E, Bednarek PH. Immediate intrauterine device insertion following surgical abortion. Obstet Gynecol Clin North Am. 2015;42(4):583−546.
- Braaten and Goldberg. OBG Manag. Malpositioned IUDs: When you should intervene (and when you should not). OBG Manag. 2012;24(8):38−46.
- Trussell J. Contraceptive failure in the United States. Contraception. 2011;83(5):397−404.
- Raymond EG, Weaver MA, Tan YL, et al. Effect of immediate compared with delayed insertion of etonogestrel implants on medical abortion efficacy and repeat pregnancy: a randomized controlled trial. Obstet Gynecol. 2017;127(2):306−312.
- Rowlands S, Mansour D, Walling M. Intravascular migration of contraceptive implants: two more cases. Contraception. 2016. In press.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 672: clinical challenges of long-acting reversible contraceptive methods. Obstet Gynecol. 2016;128(3):e69−e77.
- Lopez LM, Bernholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2015;(7):CD007373.
- Karabayirli S, Ayrim AA, Muslu B. Comparison of the analgesic effects of oral tramadol and naproxen sodium on pain relief during IUD insertion. J Minim Invasive Gynecol. 2012;19(5):581−584.
- Kho KA, Chamsy DJ. Perforated intraperitoneal intrauterine contraceptive devices: diagnosis, management, and clinical outcomes. J Minim Invasive Gynecol. 2014;21(4):596−601.
- Madden T, McNicholas C, Zhao Q, Secura GM, Eisenberg DL, Peipert JF. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014;124(4):718−726.
- Patil E, Bednarek PH. Immediate intrauterine device insertion following surgical abortion. Obstet Gynecol Clin North Am. 2015;42(4):583−546.
- Braaten and Goldberg. OBG Manag. Malpositioned IUDs: When you should intervene (and when you should not). OBG Manag. 2012;24(8):38−46.
- Trussell J. Contraceptive failure in the United States. Contraception. 2011;83(5):397−404.
- Raymond EG, Weaver MA, Tan YL, et al. Effect of immediate compared with delayed insertion of etonogestrel implants on medical abortion efficacy and repeat pregnancy: a randomized controlled trial. Obstet Gynecol. 2017;127(2):306−312.
- Rowlands S, Mansour D, Walling M. Intravascular migration of contraceptive implants: two more cases. Contraception. 2016. In press.
Preventing surgical site infections in hysterectomy
Surgical site infections are a major source of patient morbidity. They are also an important quality metric for surgeons and hospital systems, and are increasingly being linked to reimbursement.
They occur in approximately 2% of the 600,000 women undergoing hysterectomy in the United States each year. The U.S. Centers for Disease Control and Prevention defines surgical site infection (SSI) as an infection that occurs within 30 days of a procedure in the part of the body where the surgery took place. Most SSIs are superficial incisional, but they also include deep incisional or organ or space infections.
Classification
The incidence of SSI varies according to the classification of the wound, as defined by the National Academy of Sciences.1 Most hysterectomies are classified as clean-contaminated wounds because they involve entry into the mucosa of the genitourinary tract. However, hysterectomy with contamination of bowel flora, or in the setting of acute infection (such as suppurative pelvic inflammatory disease) are considered a contaminated wound class, and are associated with even higher rates of SSI.
Risk factors
The risk factors associated with SSI are both modifiable and unmodifiable. Broadly speaking, they include increased risk to endogenous flora (e.g., wound classification), increased exposure to exogenous flora (e.g., inadequate protection of a wound from external pathogens), and impairment of the body’s immune mechanisms to prevent and overcome infection (e.g., hypothermia and hypoglycemia).
Unmodifiable risk factors include increasing age, a history of radiation exposure, vascular disease, and a history of prior SSIs. Modifiable risk factors include obesity, tobacco use, immunosuppressive medications, hypoalbuminemia, route of hysterectomy, hair removal, preoperative infections (such as bacterial vaginosis), surgical scrub, skin and vaginal preparation, antimicrobial prophylaxis (inappropriate choice or timing, inadequate dosing or redosing), operative time, blood transfusion, surgical skill, and operating room characteristics (ventilation, increased OR traffic, and sterilization of surgical equipment).
Antimicrobial prophylaxis
The CDC and the American College of Obstetricians and Gynecologists (ACOG) have provided clear guidelines regarding methods to reduce SSI in hysterectomy.3,4 There is strong evidence for using antimicrobial prophylaxis for hysterectomy.
It is important that physicians confirm the validity of beta-lactam allergies with patients because there are higher rates of SSI with the use of non–beta-lactam regimens, even those endorsed by the CDC and ACOG.5
Antibiotics should be administered within 1 hour of skin incision, and ideally within 30 minutes. They should be discontinued within 24 hours. Dosing should be adjusted to weight, and antimicrobials should be redosed for long procedures (at intervals of two half-lives), and for increased blood loss.
Skin preparation
Hair removal should be avoided unless necessary for technical reasons. If it is required, it should be performed outside of the operative space using clippers, not razors. For patients colonized with methicillin-resistant S. aureus, there is supporting evidence for pretreatment with mupirocin ointment to the nares, and chlorhexidine showers for 5-10 days. Patients who have bacterial vaginosis should be treated before surgery to decrease the rate of vaginal cuff SSI.

If there is a planned or potential gastrointestinal procedure as part of the hysterectomy, the surgeon should consider using an impervious plastic wound protector in place of, or in addition to, other retractors. Preoperative oral antimicrobials with mechanical bowel preparation have been associated with decreased SSIs; however, this benefit is not observed with mechanical bowel preparation alone.
Wound closure
Surgical technique and wound closure techniques also impact SSI. Minimally invasive and vaginal hysterectomy routes are preferred, as these are associated with the lowest rates of SSI. Antimicrobial-impregnated suture materials appear to be unnecessary. Surgeons should ensure that there is delicate handling of tissues and closure of dead spaces. If the subcutaneous fat space depth measures more than 2.5 cm, it should be reapproximated with a rapidly-absorbing suture material.
Use of electrosurgery versus a scalpel when creating the incision does not appear to influence infection rates, nor does use of staples versus subcuticular suture during closure.7
Using a dilute iodine lavage in the subcutaneous space, opening a sterile closing tray, and having surgeons change gloves prior to skin closure should be considered. The CDC recommends keeping the skin dressing in place for 24 hours postoperatively.
Other strategies
Hyperglycemia is associated with impaired neutrophil response, and therefore blood glucose should be controlled before surgery (hemoglobin A1c levels of less than 7% preoperatively) and immediately postoperatively (less than 180 mg/dL within 18-24 hours after the end of anesthesia).
It is also important to minimize perioperative hypothermia (less than 35.5° F), as this also impairs the body’s immune response. Keeping operative room ambient temperatures higher, minimizing incision size, warming CO2 gas in minimally invasive procedures, warming fluids, and using extrinsic body warmers can help achieve this.
Excessive blood loss should be minimized because blood transfusion is associated with impaired macrophage function and increased risk for SSI.
In addition to teamwide (including nonsurgeon) strict adherence to hand hygiene, OR personnel should avoid unnecessary operating room traffic. Hospital officials should ensure that the facility’s ventilator systems are well maintained and that there is care and maintenance of air handlers.
Many strategies can be employed perioperatively to decrease SSI rates for hysterectomy. We advocate for a protocol-based approach (known as “bundling” strategies) to achieve consistency of practice and to maximize surgeon and institutional improvements in SSI rates. This is similar to the approach outlined in a recent consensus statement from the Council on Patient Safety in Women’s Health Care.8
A comprehensive multidisciplinary approach throughout the perioperative period is necessary. It is imperative that good communication exist with patients regarding SSIs after hysterectomy and how patients, surgeons, and hospitals can together minimize the risks of SSIs.
References
1. Altemeier WA. “Manual on Control of Infection in Surgical Patients” (Philadelphia: Lippincott Williams & Wilkins, 1984).
2. Rev Infect Dis. 1991 Sep-Oct;13(Suppl 10):S821-41.
3. Infect Control Hosp Epidemiol. 2014 Jun;35(6):605-27.
4. Obstet Gynecol. 2009 May;113(5):1180-9.
5. Obstet Gynecol. 2016 Feb;127(2):321-9.
6. Am J Obstet Gynecol. 2005 Feb;192(2):422-5.
7. J Gastrointest Surg. 2016 Dec;20(12):2083-92.
8. Obstet Gynecol. 2016 Dec 7. doi: 10.1097/AOG.0000000000001751.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Jackson-Moore is an associate professor in gynecologic oncology at UNC. They reported having no relevant financial disclosures.
Surgical site infections are a major source of patient morbidity. They are also an important quality metric for surgeons and hospital systems, and are increasingly being linked to reimbursement.
They occur in approximately 2% of the 600,000 women undergoing hysterectomy in the United States each year. The U.S. Centers for Disease Control and Prevention defines surgical site infection (SSI) as an infection that occurs within 30 days of a procedure in the part of the body where the surgery took place. Most SSIs are superficial incisional, but they also include deep incisional or organ or space infections.
Classification
The incidence of SSI varies according to the classification of the wound, as defined by the National Academy of Sciences.1 Most hysterectomies are classified as clean-contaminated wounds because they involve entry into the mucosa of the genitourinary tract. However, hysterectomy with contamination of bowel flora, or in the setting of acute infection (such as suppurative pelvic inflammatory disease) are considered a contaminated wound class, and are associated with even higher rates of SSI.
Risk factors
The risk factors associated with SSI are both modifiable and unmodifiable. Broadly speaking, they include increased risk to endogenous flora (e.g., wound classification), increased exposure to exogenous flora (e.g., inadequate protection of a wound from external pathogens), and impairment of the body’s immune mechanisms to prevent and overcome infection (e.g., hypothermia and hypoglycemia).
Unmodifiable risk factors include increasing age, a history of radiation exposure, vascular disease, and a history of prior SSIs. Modifiable risk factors include obesity, tobacco use, immunosuppressive medications, hypoalbuminemia, route of hysterectomy, hair removal, preoperative infections (such as bacterial vaginosis), surgical scrub, skin and vaginal preparation, antimicrobial prophylaxis (inappropriate choice or timing, inadequate dosing or redosing), operative time, blood transfusion, surgical skill, and operating room characteristics (ventilation, increased OR traffic, and sterilization of surgical equipment).
Antimicrobial prophylaxis
The CDC and the American College of Obstetricians and Gynecologists (ACOG) have provided clear guidelines regarding methods to reduce SSI in hysterectomy.3,4 There is strong evidence for using antimicrobial prophylaxis for hysterectomy.
It is important that physicians confirm the validity of beta-lactam allergies with patients because there are higher rates of SSI with the use of non–beta-lactam regimens, even those endorsed by the CDC and ACOG.5
Antibiotics should be administered within 1 hour of skin incision, and ideally within 30 minutes. They should be discontinued within 24 hours. Dosing should be adjusted to weight, and antimicrobials should be redosed for long procedures (at intervals of two half-lives), and for increased blood loss.
Skin preparation
Hair removal should be avoided unless necessary for technical reasons. If it is required, it should be performed outside of the operative space using clippers, not razors. For patients colonized with methicillin-resistant S. aureus, there is supporting evidence for pretreatment with mupirocin ointment to the nares, and chlorhexidine showers for 5-10 days. Patients who have bacterial vaginosis should be treated before surgery to decrease the rate of vaginal cuff SSI.

If there is a planned or potential gastrointestinal procedure as part of the hysterectomy, the surgeon should consider using an impervious plastic wound protector in place of, or in addition to, other retractors. Preoperative oral antimicrobials with mechanical bowel preparation have been associated with decreased SSIs; however, this benefit is not observed with mechanical bowel preparation alone.
Wound closure
Surgical technique and wound closure techniques also impact SSI. Minimally invasive and vaginal hysterectomy routes are preferred, as these are associated with the lowest rates of SSI. Antimicrobial-impregnated suture materials appear to be unnecessary. Surgeons should ensure that there is delicate handling of tissues and closure of dead spaces. If the subcutaneous fat space depth measures more than 2.5 cm, it should be reapproximated with a rapidly-absorbing suture material.
Use of electrosurgery versus a scalpel when creating the incision does not appear to influence infection rates, nor does use of staples versus subcuticular suture during closure.7
Using a dilute iodine lavage in the subcutaneous space, opening a sterile closing tray, and having surgeons change gloves prior to skin closure should be considered. The CDC recommends keeping the skin dressing in place for 24 hours postoperatively.
Other strategies
Hyperglycemia is associated with impaired neutrophil response, and therefore blood glucose should be controlled before surgery (hemoglobin A1c levels of less than 7% preoperatively) and immediately postoperatively (less than 180 mg/dL within 18-24 hours after the end of anesthesia).
It is also important to minimize perioperative hypothermia (less than 35.5° F), as this also impairs the body’s immune response. Keeping operative room ambient temperatures higher, minimizing incision size, warming CO2 gas in minimally invasive procedures, warming fluids, and using extrinsic body warmers can help achieve this.
Excessive blood loss should be minimized because blood transfusion is associated with impaired macrophage function and increased risk for SSI.
In addition to teamwide (including nonsurgeon) strict adherence to hand hygiene, OR personnel should avoid unnecessary operating room traffic. Hospital officials should ensure that the facility’s ventilator systems are well maintained and that there is care and maintenance of air handlers.
Many strategies can be employed perioperatively to decrease SSI rates for hysterectomy. We advocate for a protocol-based approach (known as “bundling” strategies) to achieve consistency of practice and to maximize surgeon and institutional improvements in SSI rates. This is similar to the approach outlined in a recent consensus statement from the Council on Patient Safety in Women’s Health Care.8
A comprehensive multidisciplinary approach throughout the perioperative period is necessary. It is imperative that good communication exist with patients regarding SSIs after hysterectomy and how patients, surgeons, and hospitals can together minimize the risks of SSIs.
References
1. Altemeier WA. “Manual on Control of Infection in Surgical Patients” (Philadelphia: Lippincott Williams & Wilkins, 1984).
2. Rev Infect Dis. 1991 Sep-Oct;13(Suppl 10):S821-41.
3. Infect Control Hosp Epidemiol. 2014 Jun;35(6):605-27.
4. Obstet Gynecol. 2009 May;113(5):1180-9.
5. Obstet Gynecol. 2016 Feb;127(2):321-9.
6. Am J Obstet Gynecol. 2005 Feb;192(2):422-5.
7. J Gastrointest Surg. 2016 Dec;20(12):2083-92.
8. Obstet Gynecol. 2016 Dec 7. doi: 10.1097/AOG.0000000000001751.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Jackson-Moore is an associate professor in gynecologic oncology at UNC. They reported having no relevant financial disclosures.
Surgical site infections are a major source of patient morbidity. They are also an important quality metric for surgeons and hospital systems, and are increasingly being linked to reimbursement.
They occur in approximately 2% of the 600,000 women undergoing hysterectomy in the United States each year. The U.S. Centers for Disease Control and Prevention defines surgical site infection (SSI) as an infection that occurs within 30 days of a procedure in the part of the body where the surgery took place. Most SSIs are superficial incisional, but they also include deep incisional or organ or space infections.
Classification
The incidence of SSI varies according to the classification of the wound, as defined by the National Academy of Sciences.1 Most hysterectomies are classified as clean-contaminated wounds because they involve entry into the mucosa of the genitourinary tract. However, hysterectomy with contamination of bowel flora, or in the setting of acute infection (such as suppurative pelvic inflammatory disease) are considered a contaminated wound class, and are associated with even higher rates of SSI.
Risk factors
The risk factors associated with SSI are both modifiable and unmodifiable. Broadly speaking, they include increased risk to endogenous flora (e.g., wound classification), increased exposure to exogenous flora (e.g., inadequate protection of a wound from external pathogens), and impairment of the body’s immune mechanisms to prevent and overcome infection (e.g., hypothermia and hypoglycemia).
Unmodifiable risk factors include increasing age, a history of radiation exposure, vascular disease, and a history of prior SSIs. Modifiable risk factors include obesity, tobacco use, immunosuppressive medications, hypoalbuminemia, route of hysterectomy, hair removal, preoperative infections (such as bacterial vaginosis), surgical scrub, skin and vaginal preparation, antimicrobial prophylaxis (inappropriate choice or timing, inadequate dosing or redosing), operative time, blood transfusion, surgical skill, and operating room characteristics (ventilation, increased OR traffic, and sterilization of surgical equipment).
Antimicrobial prophylaxis
The CDC and the American College of Obstetricians and Gynecologists (ACOG) have provided clear guidelines regarding methods to reduce SSI in hysterectomy.3,4 There is strong evidence for using antimicrobial prophylaxis for hysterectomy.
It is important that physicians confirm the validity of beta-lactam allergies with patients because there are higher rates of SSI with the use of non–beta-lactam regimens, even those endorsed by the CDC and ACOG.5
Antibiotics should be administered within 1 hour of skin incision, and ideally within 30 minutes. They should be discontinued within 24 hours. Dosing should be adjusted to weight, and antimicrobials should be redosed for long procedures (at intervals of two half-lives), and for increased blood loss.
Skin preparation
Hair removal should be avoided unless necessary for technical reasons. If it is required, it should be performed outside of the operative space using clippers, not razors. For patients colonized with methicillin-resistant S. aureus, there is supporting evidence for pretreatment with mupirocin ointment to the nares, and chlorhexidine showers for 5-10 days. Patients who have bacterial vaginosis should be treated before surgery to decrease the rate of vaginal cuff SSI.

If there is a planned or potential gastrointestinal procedure as part of the hysterectomy, the surgeon should consider using an impervious plastic wound protector in place of, or in addition to, other retractors. Preoperative oral antimicrobials with mechanical bowel preparation have been associated with decreased SSIs; however, this benefit is not observed with mechanical bowel preparation alone.
Wound closure
Surgical technique and wound closure techniques also impact SSI. Minimally invasive and vaginal hysterectomy routes are preferred, as these are associated with the lowest rates of SSI. Antimicrobial-impregnated suture materials appear to be unnecessary. Surgeons should ensure that there is delicate handling of tissues and closure of dead spaces. If the subcutaneous fat space depth measures more than 2.5 cm, it should be reapproximated with a rapidly-absorbing suture material.
Use of electrosurgery versus a scalpel when creating the incision does not appear to influence infection rates, nor does use of staples versus subcuticular suture during closure.7
Using a dilute iodine lavage in the subcutaneous space, opening a sterile closing tray, and having surgeons change gloves prior to skin closure should be considered. The CDC recommends keeping the skin dressing in place for 24 hours postoperatively.
Other strategies
Hyperglycemia is associated with impaired neutrophil response, and therefore blood glucose should be controlled before surgery (hemoglobin A1c levels of less than 7% preoperatively) and immediately postoperatively (less than 180 mg/dL within 18-24 hours after the end of anesthesia).
It is also important to minimize perioperative hypothermia (less than 35.5° F), as this also impairs the body’s immune response. Keeping operative room ambient temperatures higher, minimizing incision size, warming CO2 gas in minimally invasive procedures, warming fluids, and using extrinsic body warmers can help achieve this.
Excessive blood loss should be minimized because blood transfusion is associated with impaired macrophage function and increased risk for SSI.
In addition to teamwide (including nonsurgeon) strict adherence to hand hygiene, OR personnel should avoid unnecessary operating room traffic. Hospital officials should ensure that the facility’s ventilator systems are well maintained and that there is care and maintenance of air handlers.
Many strategies can be employed perioperatively to decrease SSI rates for hysterectomy. We advocate for a protocol-based approach (known as “bundling” strategies) to achieve consistency of practice and to maximize surgeon and institutional improvements in SSI rates. This is similar to the approach outlined in a recent consensus statement from the Council on Patient Safety in Women’s Health Care.8
A comprehensive multidisciplinary approach throughout the perioperative period is necessary. It is imperative that good communication exist with patients regarding SSIs after hysterectomy and how patients, surgeons, and hospitals can together minimize the risks of SSIs.
References
1. Altemeier WA. “Manual on Control of Infection in Surgical Patients” (Philadelphia: Lippincott Williams & Wilkins, 1984).
2. Rev Infect Dis. 1991 Sep-Oct;13(Suppl 10):S821-41.
3. Infect Control Hosp Epidemiol. 2014 Jun;35(6):605-27.
4. Obstet Gynecol. 2009 May;113(5):1180-9.
5. Obstet Gynecol. 2016 Feb;127(2):321-9.
6. Am J Obstet Gynecol. 2005 Feb;192(2):422-5.
7. J Gastrointest Surg. 2016 Dec;20(12):2083-92.
8. Obstet Gynecol. 2016 Dec 7. doi: 10.1097/AOG.0000000000001751.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Jackson-Moore is an associate professor in gynecologic oncology at UNC. They reported having no relevant financial disclosures.
TRUST: How to build a support net for ObGyns affected by a medical error
An estimated 98,000 Americans die each year due to medical errors. This is an attention-grabbing statistic—from the year 2000.1 A recent study (published in 2016) reported that medical errors are the third leading cause of death in the United States, ranking just behind heart disease and cancer.2
As expected, much has been done to reduce medical errors and improve patient safety as a result of these publications. Quality, safety, and outcomes are paramount, as evidenced by the Institute of Health Care Improvement’s “triple aim”: reduce cost of care, improve quality of care, and improve patient outcomes.3
While these 3 aims are of paramount importance, this article seeks to portray the “quadruple aim,” with an additional focus on physician well-being. Patients and their families (first victims) are not the only ones affected by medical errors. Clinicians are, too, and these effects can be devastating. Here I offer concrete strategies to support providers involved in medical errors, including tips on developing a formal support program. First, however, I describe the devastating effects medical errors can have on providers and the signs of a second victim.
Related article:
Medical errors: Caring for the second victim (you)
The scope of the problem
In 2000, it was Dr. Albert Wu’s publication in The British Medical Journal titled “Medical Error: The Second Victim” (the doctor who makes mistakes needs help too), that first addressed this important topic.4 In his article he shared a case of another house officer who missed signs of a pericardial tamponade and was judged incompetent by peers due to his mistake.
As physicians, we do not intrinsically support colleagues who have experienced a medical error. We all have taken, with pride and commitment, our Hippocratic Oath of “do no harm,” yet we are often held to standards of perfection by society, peers, and, above all, ourselves. Have technologic wonders and precise laboratory tests supplanted the adage “doctors are only human”? Dr. Wu also points out in this landmark essay his observation and dismay at the lack of empathy, sympathy, and compassion shown by peers when medical errors occur. All of these elements are needed for the healing of those involved to take place. If they are not provided, dysfunctional coping mechanisms ensue.4
Incidence of medical errors
Despite the Institute of Medicine report from 20001 and the recent study from Johns Hopkins,2 determining the exact number of errors and incidents is not easy. Most data reporting is sparse. A prospective longitudinal study of perceived medical errors and resident distress estimated medical errors to be between 5% and 10% in hospitalized patients, but that it could be up to 50%.5 According to a 2005 study, approximately one-third of internal medicine residents report at least 1 major medical error during their 3 years of training, while 18% of multidisciplinary residents report an adverse event under their care in the previous week.6
Related article:
Medical errors: Meeting ethical obligations and reducing liability with proper communication
Who is at risk of becoming a second victim?
Any and all clinicians can become a second victim, and the state can be realized at varying points in the process of an experienced medical error. The circumstances of the initial error and the severity of the effect on the patient and/or the damaged physician−patient relationship can affect whether or not there is a second victim. A second victim also can emerge as a result of peers’ or colleagues’ comments and lack of empathy or support. Certainly a lawsuit can produce a second victim.7
How often do physicians become second victims?
The prevalence of second victims has a large variation in estimates. A 2006 study estimates a prevalence of 10.4%.8 In 2010, the estimate was 30%, and a prevalence of 43.3% was reported in 2000.9,10 Regarding emotional distress within a year of a major adverse event, 30% of almost 900 providers reported these feelings.11 Other studies note 50% of health care workers reported feelings consistent with those of a second victim.7
Next: What are the symptoms of a second victim?
The signs of, and long-lasting risks for, a second victim
Second victims are at risk for several well-documented symptoms, regardless of their stage of training, including6:
- depression (in fact, they have a 3-fold risk)
- decrease in overall quality of life
- increase in burnout
- increase in feelings of distress, guilt, and shame, which may be long lasting.
Health care providers as second victims also may experience shock and hopelessness, sleep disturbance, social avoidance, intrusive thoughts and nightmares, and poor memory and concentration. Interestingly, these emotions and reactions are indistinguishable from posttraumatic stress disorder. These continued symptoms can have short- and long-term implications for physicians, patients, and the health care organization.12
Next: How to support those affected by a medical error
How to support all of those affected by a medical error
Over the past decade or so, much attention has been paid to creating safer health systems, improving outcomes and patient satisfaction, and recognizing the needs of patients and families of first victims when medical errors occur. Much less has been done to acknowledge and address the needs of struggling clinicians.
Provide nurturing discussions and sympathy
Hospital systems do have embedded processes to review outcomes and medical errors, including, among others, peer review, quality improvement, morbidity and mortality review, and root cause analysis. Unfortunately, often a “name, blame, shame game” can result from the overall process, with certain individuals or groups of individuals singled out, and only worsen the incidence and effects of the second victim. Ideally, system processes for addressing medical errors should allow for an environment more focused on nurturing discussions to prevent error and recognize all the factors contributing to an error.
Of course in any outcome or error investigation, the goal is to identify what happened, what factors contributed to the incident, and what can be done to prevent future occurrences. The concern for the family as priority is understandable, as is the desire to prevent a lawsuit. The lack of attention and sympathy to the health care provider involved contributes to the second victim.7
It is all too easy to blame, even in a Just Culture. Deficiencies in sympathy and attention can occur without a system whose culture is focused on “name, blame, shame.” A Just Culture, as defined by the Institute for Healthcare Improvement, is one in which individuals come forward with a mistake without fear of punishment. Such a culture balances the need to learn from our mistakes and the need to have disciplinary action.13
David Marx, an outcomes engineer and author of “Whack a Mole: The Price We Pay for Expecting Perfection,” touts a Just Culture as one having the following sets of beliefs:
- recognition that professionals will make mistakes
- recognition that even professionals will develop unhealthy norms
- a fierce intolerance for reckless conduct.
He strongly asserts that human error be consoled while reckless behavior be punished.14 Punishing human error is a setup for the second victim.
Read on for tips to develop a coping program
Tips for developing a coping program
In 2009, Scott and colleagues described 6 stages of a second victim. These are:
- Stage 1: Chaos and event repair
- Stage 2: Intrusive thoughts, “what if”
- Stage 3: Restoring personal identity
- Stage 4: Enduring the inquisition
- Stage 5: Obtaining emotional first aid
- Stage 6: Moving on or dropping out; surviving and/or thriving
Throughout the stages, second victims look for support and share their experience of the medical error event, as well as their personal and professional impact of the error.15
A 2007 study that examined the emotional impact of medical errors on physicians revealed some startling data. A full 82% of physicians expressed interest in counseling to help cope with their distress. And 90% felt there was inadequate support at their hospitals or health care organizations for this distress.16
Use The Joint Commission’s toolkit
Unfortunately, there are only a few well-documented second-victim support programs in the United States, despite the growing evidence of the emotional distress that second victims experience. Many hospitals do not know how to develop or implement such a support system. Recognizing this challenge, The Joint Commission developed a toolkit to assist health care organizations in developing a second-victim program. The toolkit consists of 10 modules (TABLE) designed to assist organizations not only to implement a second-victim support process but also to customize it to their specific institutional culture. This toolkit can be downloaded for free or used online. Within the first year of its availability, over 6,000 people visited the website and there were more than 700 requests for a download.17

Follow forYOU’s example
An example and well-recognized second-victim support program is the “forYOU” team at the University of Missouri. The program is free to employees, confidential, and available 24-7. Its purpose is “providing care and support to our staff,” by helping members understand the phenomenon of the second victim and quickly returning members to a satisfying professional practice.18
The “forYOU” team was created in 2007 under the direction of the University of Missouri Health Care’s Office of Clinical Effectiveness with the goals of increasing institutional awareness, providing a second victim with a “safe zone,” and allowing for the expression of emotions and reactions in a confidential setting. Team members are multidisciplinary and include physicians, nurses, respiratory therapists, social workers, and chaplains. They strive to normalize the feelings and thoughts second victims experience after a stressful outcome or event. Team members are highly trained in second-victim responses and the stages of coping. The program has established institutional actions to each of the 6 stages (FIGURE).19

Read on to learn how peer mentors are crucial to a support program
Establish TRUST
At the Carilion Clinic in Roanoke, Virginia, we too have developed a second-victim support program for all of our employees: TRUST. In the beginning stages, we quickly reaffirmed the challenges in developing such a program.
Initial challenges you will face. First, education on what a second victim is needs to be recognized. The fact that not everyone experiences second-victim emotions needs to be validated. Administrators and staff must be convinced that needing support is not a sign of weakness. And the program must ensure confidentiality and recruit mentors. These are just a few of the obstacles we faced on our path to program realization. Our journey to develop our second-victim program was approximately 5 years and required participation, affirmation, and support from all levels of the organization.
Our program name embodies its inherent purpose and goals. TRUST stands for:
- Treatment that is just. Second victims deserve the right of a presumption that their intentions were good, and should be able to depend on organizational leaders for integrity, fairness, just treatment, and shared accountability for outcomes.
- Respect. Second victims deserve respect and common decency and should not be blamed and shamed for human fallibility.
- Understanding and compassion. Second victims need compassionate help to grieve and heal.
- Supportive care. Second victims are entitled to psychological and support services that are delivered in a professional and organized way.
- Transparency and opportunity to contribute. Second victims have a right to participate in the learning gathered from the event, to share important causal information with the organization, and to be provided with an opportunity to heal by contributing to the prevention of future events.
Employ peer mentors, who serve a vital role
We have identified the need to develop a more direct and active approach to the TRUST program’s recruitment and established a subcommittee to begin this process. We began by asking leaders to nominate potential peer mentors and spoke about the program and asked for volunteers at various hospital committees. Once we had most disciplines represented, leaders were asked to take an assessment for emotional intelligence.
Other than the initial training for the TRUST program, the time requirement for participation for peer mentors is likely less than an hour per month. The dedicated time certainly is dependent on how much support the second victim is requiring, however, and varies. We encourage the peer supporters to be aware of their time constraints and establish parameters for the relationship in a direct but supportive way.
Since the inception of the TRUST Team in September 2014, we have trained 12 peer mentors, 10 of whom currently still serve in that capacity. We have 3 additional peers awaiting training. To date, The TRUST team has supported 19 clinicians/staff, including 3 ACPs, 9 nurses, 6 physicians, and 1 other (pharmacist). Of those 10, 3 are still actively receiving support so closing data have yet to be collected. Of the 16 who have been closed, 6 were referred for ongoing support and 10 were able to return to baseline with TRUST Team Supports.
Related article:
Who is liable when a surgical error occurs?
Just surviving the medical error is not the goal
Medical errors are inevitable, and the effects on providers can be devastating. It is important that physicians and institutions are aware of the signs and symptoms of a second victim as well as provide support to them. Institutions must have a just culture in which all members of the health care team can come forward with medical errors without the fear of punishment. Ideally, these institutions also have a second-victim support system that identifies those who need assistance and assist all health care clinicians not only to survive the effects of medical errors but also to thrive after receiving the necessary support.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- To err is human: Building a safer health system. Kohn LT, Corrigan JM, Donaldson MS, eds. Washington, DC: National Academy Press; 2000. http://www.nap.edu/books/0309068371/html. Accessed December 18, 2016.
- Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139.
- Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Affairs (Millwood). 2008;27(3):759−769. http://www.ihi.org/resources/Pages/Publications/TripleAimCareHealthandCost.aspx. Accessed December 18, 2016.
- Wu AW. Medical error: The second victim. The doctor who makes the mistake needs help too. BMJ . 2000;320(7237):726−727.
- West CP, Huschka MM, Novotny PJ, et al. Association of perceived medical errors with resident distress and empathy: a prospective longitudinal study. JAMA. 2006;296(9):1071−1078.
- Jagsi R, Kitch BT, Weinstein DF, Campbell EG, Hetter M, Weissman JS. Residents report on adverse events and their causes. Arch Intern Med. 2005;165(22):2607−2613.
- Wu AW, Steckelberg RC. Medical error, incident investigation, and the second victim: doing better but feeling worse? BMJ Qual Saf. 2012;21(4):267−270.
- Lander LI, Connor JA, Shah RK, Kentala E, Healy, GB, Roberson DW. Otolaryngologists’ responses to errors and adverse events. Laryngoscope. 2006;116(7):1114−1120.
- Scott SD, Hirschinger LE, Cox KR. Sharing the load. Rescuing the healer after trauma. RN. 2008;71(12):38−40,42−43.
- Wolf ZR. Stress management in response to practice errors: critical events in professional practice. PA-PSRS Patient Safety Advisory. 2005;2:1−2.
- Scott SD, Hirschinger LE, Cox KR, et al. Caring for our own: deploying a systemwide second victim rapid response team. Jt Comm J Qual Patient Saf. 2010;36(5):233−240.
- Edrees HH, Paine LA, Feroli ER, Wu AW. Health care workers as second victims of medical errors. Pol Arch Med Wewn. 2011;121(4):101−108.
- Leonard M. Organizational fairness/Just Culture. Cambridge, MA: Institute for Healthcare Improvement; 2012. http://app.ihi.org/extranetng/content/58886256-47d8-4f9c-bf7b-0afc352f013a/0efbd6cd-d0a3-4353-ad84-c86d07f499e1/4_5_Just%20Culture_ML.pdf. Accessed December 18, 2016.
- Marx D. Whack-a-Mole: The Price We Pay for Expecting Perfection. Plano, TX: By Your Side Studios; 2009.
- Scott SD, Hirschinger LE, Cox KR, McCoig M, Brandt J, Hall LW. The natural history of recovery for the healthcare provider “second victim” after adverse patient events. Qual Saf Health Care. 2009;18(5):325−330.
- Waterman AD, Garbutt J, Hazel E, et al. The emotional impact of medical errors on practicing physicians in the United States and Canada. Jt Comm J Qual Patient Saf. 2007;33(8):467−476.
- Pratt S, Kenney L, Scott SD, Wu AW. How to develop a second victim support program: a toolkit for health care organizations. Jt Comm J Qual Patient Saf. 2012;38(5):235−240,193.
- forYOU Team. Caring for our own. University of Missouri Health System website. http://www.muhealth.org/about/quality-of-care/office-of-clinical-effectiveness/foryou-team/. Accessed December 18, 2016.
- Second victim trajectory. Columbia, MO: University of Missouri Health System; 2009. http://www.muhealth.org/app/files/public/1390/6StagesRecovery.pdf. Accessed December 19, 2016.
An estimated 98,000 Americans die each year due to medical errors. This is an attention-grabbing statistic—from the year 2000.1 A recent study (published in 2016) reported that medical errors are the third leading cause of death in the United States, ranking just behind heart disease and cancer.2
As expected, much has been done to reduce medical errors and improve patient safety as a result of these publications. Quality, safety, and outcomes are paramount, as evidenced by the Institute of Health Care Improvement’s “triple aim”: reduce cost of care, improve quality of care, and improve patient outcomes.3
While these 3 aims are of paramount importance, this article seeks to portray the “quadruple aim,” with an additional focus on physician well-being. Patients and their families (first victims) are not the only ones affected by medical errors. Clinicians are, too, and these effects can be devastating. Here I offer concrete strategies to support providers involved in medical errors, including tips on developing a formal support program. First, however, I describe the devastating effects medical errors can have on providers and the signs of a second victim.
Related article:
Medical errors: Caring for the second victim (you)
The scope of the problem
In 2000, it was Dr. Albert Wu’s publication in The British Medical Journal titled “Medical Error: The Second Victim” (the doctor who makes mistakes needs help too), that first addressed this important topic.4 In his article he shared a case of another house officer who missed signs of a pericardial tamponade and was judged incompetent by peers due to his mistake.
As physicians, we do not intrinsically support colleagues who have experienced a medical error. We all have taken, with pride and commitment, our Hippocratic Oath of “do no harm,” yet we are often held to standards of perfection by society, peers, and, above all, ourselves. Have technologic wonders and precise laboratory tests supplanted the adage “doctors are only human”? Dr. Wu also points out in this landmark essay his observation and dismay at the lack of empathy, sympathy, and compassion shown by peers when medical errors occur. All of these elements are needed for the healing of those involved to take place. If they are not provided, dysfunctional coping mechanisms ensue.4
Incidence of medical errors
Despite the Institute of Medicine report from 20001 and the recent study from Johns Hopkins,2 determining the exact number of errors and incidents is not easy. Most data reporting is sparse. A prospective longitudinal study of perceived medical errors and resident distress estimated medical errors to be between 5% and 10% in hospitalized patients, but that it could be up to 50%.5 According to a 2005 study, approximately one-third of internal medicine residents report at least 1 major medical error during their 3 years of training, while 18% of multidisciplinary residents report an adverse event under their care in the previous week.6
Related article:
Medical errors: Meeting ethical obligations and reducing liability with proper communication
Who is at risk of becoming a second victim?
Any and all clinicians can become a second victim, and the state can be realized at varying points in the process of an experienced medical error. The circumstances of the initial error and the severity of the effect on the patient and/or the damaged physician−patient relationship can affect whether or not there is a second victim. A second victim also can emerge as a result of peers’ or colleagues’ comments and lack of empathy or support. Certainly a lawsuit can produce a second victim.7
How often do physicians become second victims?
The prevalence of second victims has a large variation in estimates. A 2006 study estimates a prevalence of 10.4%.8 In 2010, the estimate was 30%, and a prevalence of 43.3% was reported in 2000.9,10 Regarding emotional distress within a year of a major adverse event, 30% of almost 900 providers reported these feelings.11 Other studies note 50% of health care workers reported feelings consistent with those of a second victim.7
Next: What are the symptoms of a second victim?
The signs of, and long-lasting risks for, a second victim
Second victims are at risk for several well-documented symptoms, regardless of their stage of training, including6:
- depression (in fact, they have a 3-fold risk)
- decrease in overall quality of life
- increase in burnout
- increase in feelings of distress, guilt, and shame, which may be long lasting.
Health care providers as second victims also may experience shock and hopelessness, sleep disturbance, social avoidance, intrusive thoughts and nightmares, and poor memory and concentration. Interestingly, these emotions and reactions are indistinguishable from posttraumatic stress disorder. These continued symptoms can have short- and long-term implications for physicians, patients, and the health care organization.12
Next: How to support those affected by a medical error
How to support all of those affected by a medical error
Over the past decade or so, much attention has been paid to creating safer health systems, improving outcomes and patient satisfaction, and recognizing the needs of patients and families of first victims when medical errors occur. Much less has been done to acknowledge and address the needs of struggling clinicians.
Provide nurturing discussions and sympathy
Hospital systems do have embedded processes to review outcomes and medical errors, including, among others, peer review, quality improvement, morbidity and mortality review, and root cause analysis. Unfortunately, often a “name, blame, shame game” can result from the overall process, with certain individuals or groups of individuals singled out, and only worsen the incidence and effects of the second victim. Ideally, system processes for addressing medical errors should allow for an environment more focused on nurturing discussions to prevent error and recognize all the factors contributing to an error.
Of course in any outcome or error investigation, the goal is to identify what happened, what factors contributed to the incident, and what can be done to prevent future occurrences. The concern for the family as priority is understandable, as is the desire to prevent a lawsuit. The lack of attention and sympathy to the health care provider involved contributes to the second victim.7
It is all too easy to blame, even in a Just Culture. Deficiencies in sympathy and attention can occur without a system whose culture is focused on “name, blame, shame.” A Just Culture, as defined by the Institute for Healthcare Improvement, is one in which individuals come forward with a mistake without fear of punishment. Such a culture balances the need to learn from our mistakes and the need to have disciplinary action.13
David Marx, an outcomes engineer and author of “Whack a Mole: The Price We Pay for Expecting Perfection,” touts a Just Culture as one having the following sets of beliefs:
- recognition that professionals will make mistakes
- recognition that even professionals will develop unhealthy norms
- a fierce intolerance for reckless conduct.
He strongly asserts that human error be consoled while reckless behavior be punished.14 Punishing human error is a setup for the second victim.
Read on for tips to develop a coping program
Tips for developing a coping program
In 2009, Scott and colleagues described 6 stages of a second victim. These are:
- Stage 1: Chaos and event repair
- Stage 2: Intrusive thoughts, “what if”
- Stage 3: Restoring personal identity
- Stage 4: Enduring the inquisition
- Stage 5: Obtaining emotional first aid
- Stage 6: Moving on or dropping out; surviving and/or thriving
Throughout the stages, second victims look for support and share their experience of the medical error event, as well as their personal and professional impact of the error.15
A 2007 study that examined the emotional impact of medical errors on physicians revealed some startling data. A full 82% of physicians expressed interest in counseling to help cope with their distress. And 90% felt there was inadequate support at their hospitals or health care organizations for this distress.16
Use The Joint Commission’s toolkit
Unfortunately, there are only a few well-documented second-victim support programs in the United States, despite the growing evidence of the emotional distress that second victims experience. Many hospitals do not know how to develop or implement such a support system. Recognizing this challenge, The Joint Commission developed a toolkit to assist health care organizations in developing a second-victim program. The toolkit consists of 10 modules (TABLE) designed to assist organizations not only to implement a second-victim support process but also to customize it to their specific institutional culture. This toolkit can be downloaded for free or used online. Within the first year of its availability, over 6,000 people visited the website and there were more than 700 requests for a download.17

Follow forYOU’s example
An example and well-recognized second-victim support program is the “forYOU” team at the University of Missouri. The program is free to employees, confidential, and available 24-7. Its purpose is “providing care and support to our staff,” by helping members understand the phenomenon of the second victim and quickly returning members to a satisfying professional practice.18
The “forYOU” team was created in 2007 under the direction of the University of Missouri Health Care’s Office of Clinical Effectiveness with the goals of increasing institutional awareness, providing a second victim with a “safe zone,” and allowing for the expression of emotions and reactions in a confidential setting. Team members are multidisciplinary and include physicians, nurses, respiratory therapists, social workers, and chaplains. They strive to normalize the feelings and thoughts second victims experience after a stressful outcome or event. Team members are highly trained in second-victim responses and the stages of coping. The program has established institutional actions to each of the 6 stages (FIGURE).19

Read on to learn how peer mentors are crucial to a support program
Establish TRUST
At the Carilion Clinic in Roanoke, Virginia, we too have developed a second-victim support program for all of our employees: TRUST. In the beginning stages, we quickly reaffirmed the challenges in developing such a program.
Initial challenges you will face. First, education on what a second victim is needs to be recognized. The fact that not everyone experiences second-victim emotions needs to be validated. Administrators and staff must be convinced that needing support is not a sign of weakness. And the program must ensure confidentiality and recruit mentors. These are just a few of the obstacles we faced on our path to program realization. Our journey to develop our second-victim program was approximately 5 years and required participation, affirmation, and support from all levels of the organization.
Our program name embodies its inherent purpose and goals. TRUST stands for:
- Treatment that is just. Second victims deserve the right of a presumption that their intentions were good, and should be able to depend on organizational leaders for integrity, fairness, just treatment, and shared accountability for outcomes.
- Respect. Second victims deserve respect and common decency and should not be blamed and shamed for human fallibility.
- Understanding and compassion. Second victims need compassionate help to grieve and heal.
- Supportive care. Second victims are entitled to psychological and support services that are delivered in a professional and organized way.
- Transparency and opportunity to contribute. Second victims have a right to participate in the learning gathered from the event, to share important causal information with the organization, and to be provided with an opportunity to heal by contributing to the prevention of future events.
Employ peer mentors, who serve a vital role
We have identified the need to develop a more direct and active approach to the TRUST program’s recruitment and established a subcommittee to begin this process. We began by asking leaders to nominate potential peer mentors and spoke about the program and asked for volunteers at various hospital committees. Once we had most disciplines represented, leaders were asked to take an assessment for emotional intelligence.
Other than the initial training for the TRUST program, the time requirement for participation for peer mentors is likely less than an hour per month. The dedicated time certainly is dependent on how much support the second victim is requiring, however, and varies. We encourage the peer supporters to be aware of their time constraints and establish parameters for the relationship in a direct but supportive way.
Since the inception of the TRUST Team in September 2014, we have trained 12 peer mentors, 10 of whom currently still serve in that capacity. We have 3 additional peers awaiting training. To date, The TRUST team has supported 19 clinicians/staff, including 3 ACPs, 9 nurses, 6 physicians, and 1 other (pharmacist). Of those 10, 3 are still actively receiving support so closing data have yet to be collected. Of the 16 who have been closed, 6 were referred for ongoing support and 10 were able to return to baseline with TRUST Team Supports.
Related article:
Who is liable when a surgical error occurs?
Just surviving the medical error is not the goal
Medical errors are inevitable, and the effects on providers can be devastating. It is important that physicians and institutions are aware of the signs and symptoms of a second victim as well as provide support to them. Institutions must have a just culture in which all members of the health care team can come forward with medical errors without the fear of punishment. Ideally, these institutions also have a second-victim support system that identifies those who need assistance and assist all health care clinicians not only to survive the effects of medical errors but also to thrive after receiving the necessary support.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
An estimated 98,000 Americans die each year due to medical errors. This is an attention-grabbing statistic—from the year 2000.1 A recent study (published in 2016) reported that medical errors are the third leading cause of death in the United States, ranking just behind heart disease and cancer.2
As expected, much has been done to reduce medical errors and improve patient safety as a result of these publications. Quality, safety, and outcomes are paramount, as evidenced by the Institute of Health Care Improvement’s “triple aim”: reduce cost of care, improve quality of care, and improve patient outcomes.3
While these 3 aims are of paramount importance, this article seeks to portray the “quadruple aim,” with an additional focus on physician well-being. Patients and their families (first victims) are not the only ones affected by medical errors. Clinicians are, too, and these effects can be devastating. Here I offer concrete strategies to support providers involved in medical errors, including tips on developing a formal support program. First, however, I describe the devastating effects medical errors can have on providers and the signs of a second victim.
Related article:
Medical errors: Caring for the second victim (you)
The scope of the problem
In 2000, it was Dr. Albert Wu’s publication in The British Medical Journal titled “Medical Error: The Second Victim” (the doctor who makes mistakes needs help too), that first addressed this important topic.4 In his article he shared a case of another house officer who missed signs of a pericardial tamponade and was judged incompetent by peers due to his mistake.
As physicians, we do not intrinsically support colleagues who have experienced a medical error. We all have taken, with pride and commitment, our Hippocratic Oath of “do no harm,” yet we are often held to standards of perfection by society, peers, and, above all, ourselves. Have technologic wonders and precise laboratory tests supplanted the adage “doctors are only human”? Dr. Wu also points out in this landmark essay his observation and dismay at the lack of empathy, sympathy, and compassion shown by peers when medical errors occur. All of these elements are needed for the healing of those involved to take place. If they are not provided, dysfunctional coping mechanisms ensue.4
Incidence of medical errors
Despite the Institute of Medicine report from 20001 and the recent study from Johns Hopkins,2 determining the exact number of errors and incidents is not easy. Most data reporting is sparse. A prospective longitudinal study of perceived medical errors and resident distress estimated medical errors to be between 5% and 10% in hospitalized patients, but that it could be up to 50%.5 According to a 2005 study, approximately one-third of internal medicine residents report at least 1 major medical error during their 3 years of training, while 18% of multidisciplinary residents report an adverse event under their care in the previous week.6
Related article:
Medical errors: Meeting ethical obligations and reducing liability with proper communication
Who is at risk of becoming a second victim?
Any and all clinicians can become a second victim, and the state can be realized at varying points in the process of an experienced medical error. The circumstances of the initial error and the severity of the effect on the patient and/or the damaged physician−patient relationship can affect whether or not there is a second victim. A second victim also can emerge as a result of peers’ or colleagues’ comments and lack of empathy or support. Certainly a lawsuit can produce a second victim.7
How often do physicians become second victims?
The prevalence of second victims has a large variation in estimates. A 2006 study estimates a prevalence of 10.4%.8 In 2010, the estimate was 30%, and a prevalence of 43.3% was reported in 2000.9,10 Regarding emotional distress within a year of a major adverse event, 30% of almost 900 providers reported these feelings.11 Other studies note 50% of health care workers reported feelings consistent with those of a second victim.7
Next: What are the symptoms of a second victim?
The signs of, and long-lasting risks for, a second victim
Second victims are at risk for several well-documented symptoms, regardless of their stage of training, including6:
- depression (in fact, they have a 3-fold risk)
- decrease in overall quality of life
- increase in burnout
- increase in feelings of distress, guilt, and shame, which may be long lasting.
Health care providers as second victims also may experience shock and hopelessness, sleep disturbance, social avoidance, intrusive thoughts and nightmares, and poor memory and concentration. Interestingly, these emotions and reactions are indistinguishable from posttraumatic stress disorder. These continued symptoms can have short- and long-term implications for physicians, patients, and the health care organization.12
Next: How to support those affected by a medical error
How to support all of those affected by a medical error
Over the past decade or so, much attention has been paid to creating safer health systems, improving outcomes and patient satisfaction, and recognizing the needs of patients and families of first victims when medical errors occur. Much less has been done to acknowledge and address the needs of struggling clinicians.
Provide nurturing discussions and sympathy
Hospital systems do have embedded processes to review outcomes and medical errors, including, among others, peer review, quality improvement, morbidity and mortality review, and root cause analysis. Unfortunately, often a “name, blame, shame game” can result from the overall process, with certain individuals or groups of individuals singled out, and only worsen the incidence and effects of the second victim. Ideally, system processes for addressing medical errors should allow for an environment more focused on nurturing discussions to prevent error and recognize all the factors contributing to an error.
Of course in any outcome or error investigation, the goal is to identify what happened, what factors contributed to the incident, and what can be done to prevent future occurrences. The concern for the family as priority is understandable, as is the desire to prevent a lawsuit. The lack of attention and sympathy to the health care provider involved contributes to the second victim.7
It is all too easy to blame, even in a Just Culture. Deficiencies in sympathy and attention can occur without a system whose culture is focused on “name, blame, shame.” A Just Culture, as defined by the Institute for Healthcare Improvement, is one in which individuals come forward with a mistake without fear of punishment. Such a culture balances the need to learn from our mistakes and the need to have disciplinary action.13
David Marx, an outcomes engineer and author of “Whack a Mole: The Price We Pay for Expecting Perfection,” touts a Just Culture as one having the following sets of beliefs:
- recognition that professionals will make mistakes
- recognition that even professionals will develop unhealthy norms
- a fierce intolerance for reckless conduct.
He strongly asserts that human error be consoled while reckless behavior be punished.14 Punishing human error is a setup for the second victim.
Read on for tips to develop a coping program
Tips for developing a coping program
In 2009, Scott and colleagues described 6 stages of a second victim. These are:
- Stage 1: Chaos and event repair
- Stage 2: Intrusive thoughts, “what if”
- Stage 3: Restoring personal identity
- Stage 4: Enduring the inquisition
- Stage 5: Obtaining emotional first aid
- Stage 6: Moving on or dropping out; surviving and/or thriving
Throughout the stages, second victims look for support and share their experience of the medical error event, as well as their personal and professional impact of the error.15
A 2007 study that examined the emotional impact of medical errors on physicians revealed some startling data. A full 82% of physicians expressed interest in counseling to help cope with their distress. And 90% felt there was inadequate support at their hospitals or health care organizations for this distress.16
Use The Joint Commission’s toolkit
Unfortunately, there are only a few well-documented second-victim support programs in the United States, despite the growing evidence of the emotional distress that second victims experience. Many hospitals do not know how to develop or implement such a support system. Recognizing this challenge, The Joint Commission developed a toolkit to assist health care organizations in developing a second-victim program. The toolkit consists of 10 modules (TABLE) designed to assist organizations not only to implement a second-victim support process but also to customize it to their specific institutional culture. This toolkit can be downloaded for free or used online. Within the first year of its availability, over 6,000 people visited the website and there were more than 700 requests for a download.17

Follow forYOU’s example
An example and well-recognized second-victim support program is the “forYOU” team at the University of Missouri. The program is free to employees, confidential, and available 24-7. Its purpose is “providing care and support to our staff,” by helping members understand the phenomenon of the second victim and quickly returning members to a satisfying professional practice.18
The “forYOU” team was created in 2007 under the direction of the University of Missouri Health Care’s Office of Clinical Effectiveness with the goals of increasing institutional awareness, providing a second victim with a “safe zone,” and allowing for the expression of emotions and reactions in a confidential setting. Team members are multidisciplinary and include physicians, nurses, respiratory therapists, social workers, and chaplains. They strive to normalize the feelings and thoughts second victims experience after a stressful outcome or event. Team members are highly trained in second-victim responses and the stages of coping. The program has established institutional actions to each of the 6 stages (FIGURE).19

Read on to learn how peer mentors are crucial to a support program
Establish TRUST
At the Carilion Clinic in Roanoke, Virginia, we too have developed a second-victim support program for all of our employees: TRUST. In the beginning stages, we quickly reaffirmed the challenges in developing such a program.
Initial challenges you will face. First, education on what a second victim is needs to be recognized. The fact that not everyone experiences second-victim emotions needs to be validated. Administrators and staff must be convinced that needing support is not a sign of weakness. And the program must ensure confidentiality and recruit mentors. These are just a few of the obstacles we faced on our path to program realization. Our journey to develop our second-victim program was approximately 5 years and required participation, affirmation, and support from all levels of the organization.
Our program name embodies its inherent purpose and goals. TRUST stands for:
- Treatment that is just. Second victims deserve the right of a presumption that their intentions were good, and should be able to depend on organizational leaders for integrity, fairness, just treatment, and shared accountability for outcomes.
- Respect. Second victims deserve respect and common decency and should not be blamed and shamed for human fallibility.
- Understanding and compassion. Second victims need compassionate help to grieve and heal.
- Supportive care. Second victims are entitled to psychological and support services that are delivered in a professional and organized way.
- Transparency and opportunity to contribute. Second victims have a right to participate in the learning gathered from the event, to share important causal information with the organization, and to be provided with an opportunity to heal by contributing to the prevention of future events.
Employ peer mentors, who serve a vital role
We have identified the need to develop a more direct and active approach to the TRUST program’s recruitment and established a subcommittee to begin this process. We began by asking leaders to nominate potential peer mentors and spoke about the program and asked for volunteers at various hospital committees. Once we had most disciplines represented, leaders were asked to take an assessment for emotional intelligence.
Other than the initial training for the TRUST program, the time requirement for participation for peer mentors is likely less than an hour per month. The dedicated time certainly is dependent on how much support the second victim is requiring, however, and varies. We encourage the peer supporters to be aware of their time constraints and establish parameters for the relationship in a direct but supportive way.
Since the inception of the TRUST Team in September 2014, we have trained 12 peer mentors, 10 of whom currently still serve in that capacity. We have 3 additional peers awaiting training. To date, The TRUST team has supported 19 clinicians/staff, including 3 ACPs, 9 nurses, 6 physicians, and 1 other (pharmacist). Of those 10, 3 are still actively receiving support so closing data have yet to be collected. Of the 16 who have been closed, 6 were referred for ongoing support and 10 were able to return to baseline with TRUST Team Supports.
Related article:
Who is liable when a surgical error occurs?
Just surviving the medical error is not the goal
Medical errors are inevitable, and the effects on providers can be devastating. It is important that physicians and institutions are aware of the signs and symptoms of a second victim as well as provide support to them. Institutions must have a just culture in which all members of the health care team can come forward with medical errors without the fear of punishment. Ideally, these institutions also have a second-victim support system that identifies those who need assistance and assist all health care clinicians not only to survive the effects of medical errors but also to thrive after receiving the necessary support.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- To err is human: Building a safer health system. Kohn LT, Corrigan JM, Donaldson MS, eds. Washington, DC: National Academy Press; 2000. http://www.nap.edu/books/0309068371/html. Accessed December 18, 2016.
- Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139.
- Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Affairs (Millwood). 2008;27(3):759−769. http://www.ihi.org/resources/Pages/Publications/TripleAimCareHealthandCost.aspx. Accessed December 18, 2016.
- Wu AW. Medical error: The second victim. The doctor who makes the mistake needs help too. BMJ . 2000;320(7237):726−727.
- West CP, Huschka MM, Novotny PJ, et al. Association of perceived medical errors with resident distress and empathy: a prospective longitudinal study. JAMA. 2006;296(9):1071−1078.
- Jagsi R, Kitch BT, Weinstein DF, Campbell EG, Hetter M, Weissman JS. Residents report on adverse events and their causes. Arch Intern Med. 2005;165(22):2607−2613.
- Wu AW, Steckelberg RC. Medical error, incident investigation, and the second victim: doing better but feeling worse? BMJ Qual Saf. 2012;21(4):267−270.
- Lander LI, Connor JA, Shah RK, Kentala E, Healy, GB, Roberson DW. Otolaryngologists’ responses to errors and adverse events. Laryngoscope. 2006;116(7):1114−1120.
- Scott SD, Hirschinger LE, Cox KR. Sharing the load. Rescuing the healer after trauma. RN. 2008;71(12):38−40,42−43.
- Wolf ZR. Stress management in response to practice errors: critical events in professional practice. PA-PSRS Patient Safety Advisory. 2005;2:1−2.
- Scott SD, Hirschinger LE, Cox KR, et al. Caring for our own: deploying a systemwide second victim rapid response team. Jt Comm J Qual Patient Saf. 2010;36(5):233−240.
- Edrees HH, Paine LA, Feroli ER, Wu AW. Health care workers as second victims of medical errors. Pol Arch Med Wewn. 2011;121(4):101−108.
- Leonard M. Organizational fairness/Just Culture. Cambridge, MA: Institute for Healthcare Improvement; 2012. http://app.ihi.org/extranetng/content/58886256-47d8-4f9c-bf7b-0afc352f013a/0efbd6cd-d0a3-4353-ad84-c86d07f499e1/4_5_Just%20Culture_ML.pdf. Accessed December 18, 2016.
- Marx D. Whack-a-Mole: The Price We Pay for Expecting Perfection. Plano, TX: By Your Side Studios; 2009.
- Scott SD, Hirschinger LE, Cox KR, McCoig M, Brandt J, Hall LW. The natural history of recovery for the healthcare provider “second victim” after adverse patient events. Qual Saf Health Care. 2009;18(5):325−330.
- Waterman AD, Garbutt J, Hazel E, et al. The emotional impact of medical errors on practicing physicians in the United States and Canada. Jt Comm J Qual Patient Saf. 2007;33(8):467−476.
- Pratt S, Kenney L, Scott SD, Wu AW. How to develop a second victim support program: a toolkit for health care organizations. Jt Comm J Qual Patient Saf. 2012;38(5):235−240,193.
- forYOU Team. Caring for our own. University of Missouri Health System website. http://www.muhealth.org/about/quality-of-care/office-of-clinical-effectiveness/foryou-team/. Accessed December 18, 2016.
- Second victim trajectory. Columbia, MO: University of Missouri Health System; 2009. http://www.muhealth.org/app/files/public/1390/6StagesRecovery.pdf. Accessed December 19, 2016.
- To err is human: Building a safer health system. Kohn LT, Corrigan JM, Donaldson MS, eds. Washington, DC: National Academy Press; 2000. http://www.nap.edu/books/0309068371/html. Accessed December 18, 2016.
- Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139.
- Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Affairs (Millwood). 2008;27(3):759−769. http://www.ihi.org/resources/Pages/Publications/TripleAimCareHealthandCost.aspx. Accessed December 18, 2016.
- Wu AW. Medical error: The second victim. The doctor who makes the mistake needs help too. BMJ . 2000;320(7237):726−727.
- West CP, Huschka MM, Novotny PJ, et al. Association of perceived medical errors with resident distress and empathy: a prospective longitudinal study. JAMA. 2006;296(9):1071−1078.
- Jagsi R, Kitch BT, Weinstein DF, Campbell EG, Hetter M, Weissman JS. Residents report on adverse events and their causes. Arch Intern Med. 2005;165(22):2607−2613.
- Wu AW, Steckelberg RC. Medical error, incident investigation, and the second victim: doing better but feeling worse? BMJ Qual Saf. 2012;21(4):267−270.
- Lander LI, Connor JA, Shah RK, Kentala E, Healy, GB, Roberson DW. Otolaryngologists’ responses to errors and adverse events. Laryngoscope. 2006;116(7):1114−1120.
- Scott SD, Hirschinger LE, Cox KR. Sharing the load. Rescuing the healer after trauma. RN. 2008;71(12):38−40,42−43.
- Wolf ZR. Stress management in response to practice errors: critical events in professional practice. PA-PSRS Patient Safety Advisory. 2005;2:1−2.
- Scott SD, Hirschinger LE, Cox KR, et al. Caring for our own: deploying a systemwide second victim rapid response team. Jt Comm J Qual Patient Saf. 2010;36(5):233−240.
- Edrees HH, Paine LA, Feroli ER, Wu AW. Health care workers as second victims of medical errors. Pol Arch Med Wewn. 2011;121(4):101−108.
- Leonard M. Organizational fairness/Just Culture. Cambridge, MA: Institute for Healthcare Improvement; 2012. http://app.ihi.org/extranetng/content/58886256-47d8-4f9c-bf7b-0afc352f013a/0efbd6cd-d0a3-4353-ad84-c86d07f499e1/4_5_Just%20Culture_ML.pdf. Accessed December 18, 2016.
- Marx D. Whack-a-Mole: The Price We Pay for Expecting Perfection. Plano, TX: By Your Side Studios; 2009.
- Scott SD, Hirschinger LE, Cox KR, McCoig M, Brandt J, Hall LW. The natural history of recovery for the healthcare provider “second victim” after adverse patient events. Qual Saf Health Care. 2009;18(5):325−330.
- Waterman AD, Garbutt J, Hazel E, et al. The emotional impact of medical errors on practicing physicians in the United States and Canada. Jt Comm J Qual Patient Saf. 2007;33(8):467−476.
- Pratt S, Kenney L, Scott SD, Wu AW. How to develop a second victim support program: a toolkit for health care organizations. Jt Comm J Qual Patient Saf. 2012;38(5):235−240,193.
- forYOU Team. Caring for our own. University of Missouri Health System website. http://www.muhealth.org/about/quality-of-care/office-of-clinical-effectiveness/foryou-team/. Accessed December 18, 2016.
- Second victim trajectory. Columbia, MO: University of Missouri Health System; 2009. http://www.muhealth.org/app/files/public/1390/6StagesRecovery.pdf. Accessed December 19, 2016.
Three good apps for calculating the date of delivery
Technology has changed--and continues to change--the practice of medicine. Health care providers access word processing programs, e-mail, and electronic medical records using desktop and laptop computers. Now, providers are accessing these same tools with handheld devices such as smartphones, tablets, and "phablets" (a class of mobile devices designed to combine the form of a smartphone and a tablet).
Critical to the popularity and functionality of handheld devices are mobile applications, also known as "apps." An app is a self-contained program or piece of software designed to run on handheld devices to perform a specific purpose. App overload and app inaccuracy, however, are major problems. Health care providers do not have the time to search through thousands of medical apps in app stores to find specialty-related apps that might be useful in their practice--or to check the accuracy of those apps.
Vetted apps for ObGyns
My team's research has focused on identifying apps for ObGyns to use in clinical practice.1 In the process, we have developed the APPLICATIONS scoring system, which contains objective and subjective components to help differentiate among the accurate apps.2 This new quarterly "App review" series in OBG Management will showcase recommended apps for the busy ObGyn in the hope of improving work efficiency and the provider-patient relationship.
First up: Apps for calculating the date of delivery. This first app review focuses on pregnancy wheels, or due date calculators. Calculator apps are preferred over other types of apps such as procedure/case documentation apps, as providers use smartphones at point of care to allow rapid decision making.3 Calculating the estimated date of delivery (EDD) and gestational age (GA) is an important, vital task for providers of obstetric care. In fact, new guidelines for calculating EDD were recently developed by the American College of Obstetricians and Gynecologists (ACOG), the American Institute of Ultrasound in Medicine (AIUM), and the Society for Maternal-Fetal Medicine (SMFM).4 Notably, pregnancy wheel apps are more accurate than paper wheels.5 My team checked the accuracy of the pregnancy wheel apps by applying strict criteria to ensure the correct EDD and GA and then scored them in a systematic, nonbiased, conflict-of-interest-free manner.2
Related article:
Elective induction of labor at 39 (vs 41) weeks: Caveats and considerations
The TABLE below lists the top 3 recommended pregnancy wheel or due date calculator apps vetted by our research. The apps are listed alphabetically, and details for each app are provided based on a shortened version of the APPLICATIONS scoring system, APPLI--app comprehensiveness, price, platform, literature use, and important special features.
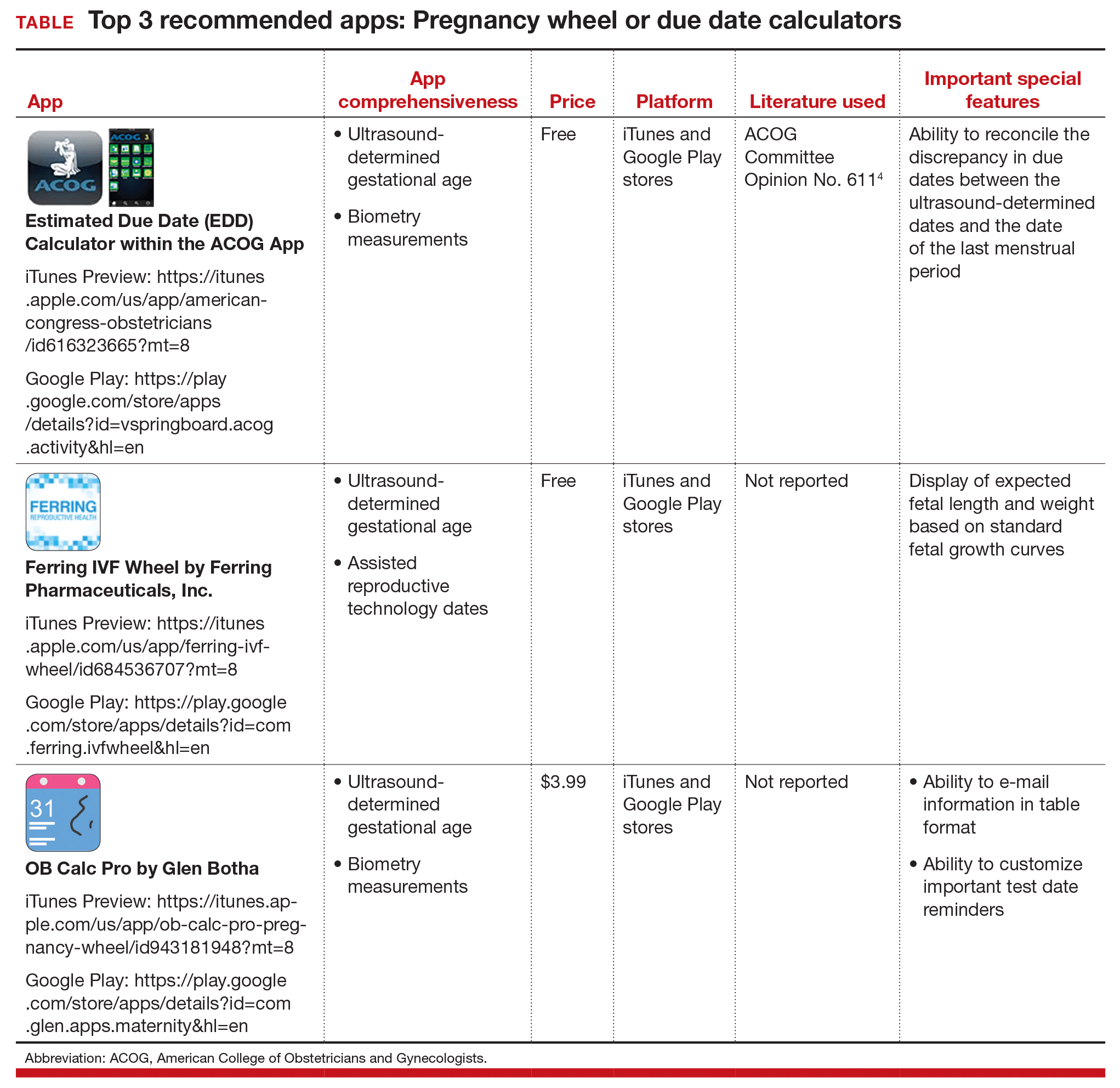
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Farag S, Chyjek K, Chen KT. Identification of iPhone and iPad applications for obstetrics and gynecology providers. Obstet Gynecol. 2014;124(5):941-945.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478-1483.
- Payne KB, Wharrad H, Watts K. Smartphone and medical related App use among medical students and junior doctors in the United Kingdom (UK): a regional survey. BMC Inform Decis Mak. 2012;12:121.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 611. Method for estimating due date. Obstet Gynecol. 2014;124(4):863-866.
- Chambliss LR, Clark SL. Paper gestational age wheels are generally inaccurate. Am J Obstet Gynecol. 2014;210(2):145.e1-e4.
Technology has changed--and continues to change--the practice of medicine. Health care providers access word processing programs, e-mail, and electronic medical records using desktop and laptop computers. Now, providers are accessing these same tools with handheld devices such as smartphones, tablets, and "phablets" (a class of mobile devices designed to combine the form of a smartphone and a tablet).
Critical to the popularity and functionality of handheld devices are mobile applications, also known as "apps." An app is a self-contained program or piece of software designed to run on handheld devices to perform a specific purpose. App overload and app inaccuracy, however, are major problems. Health care providers do not have the time to search through thousands of medical apps in app stores to find specialty-related apps that might be useful in their practice--or to check the accuracy of those apps.
Vetted apps for ObGyns
My team's research has focused on identifying apps for ObGyns to use in clinical practice.1 In the process, we have developed the APPLICATIONS scoring system, which contains objective and subjective components to help differentiate among the accurate apps.2 This new quarterly "App review" series in OBG Management will showcase recommended apps for the busy ObGyn in the hope of improving work efficiency and the provider-patient relationship.
First up: Apps for calculating the date of delivery. This first app review focuses on pregnancy wheels, or due date calculators. Calculator apps are preferred over other types of apps such as procedure/case documentation apps, as providers use smartphones at point of care to allow rapid decision making.3 Calculating the estimated date of delivery (EDD) and gestational age (GA) is an important, vital task for providers of obstetric care. In fact, new guidelines for calculating EDD were recently developed by the American College of Obstetricians and Gynecologists (ACOG), the American Institute of Ultrasound in Medicine (AIUM), and the Society for Maternal-Fetal Medicine (SMFM).4 Notably, pregnancy wheel apps are more accurate than paper wheels.5 My team checked the accuracy of the pregnancy wheel apps by applying strict criteria to ensure the correct EDD and GA and then scored them in a systematic, nonbiased, conflict-of-interest-free manner.2
Related article:
Elective induction of labor at 39 (vs 41) weeks: Caveats and considerations
The TABLE below lists the top 3 recommended pregnancy wheel or due date calculator apps vetted by our research. The apps are listed alphabetically, and details for each app are provided based on a shortened version of the APPLICATIONS scoring system, APPLI--app comprehensiveness, price, platform, literature use, and important special features.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Technology has changed--and continues to change--the practice of medicine. Health care providers access word processing programs, e-mail, and electronic medical records using desktop and laptop computers. Now, providers are accessing these same tools with handheld devices such as smartphones, tablets, and "phablets" (a class of mobile devices designed to combine the form of a smartphone and a tablet).
Critical to the popularity and functionality of handheld devices are mobile applications, also known as "apps." An app is a self-contained program or piece of software designed to run on handheld devices to perform a specific purpose. App overload and app inaccuracy, however, are major problems. Health care providers do not have the time to search through thousands of medical apps in app stores to find specialty-related apps that might be useful in their practice--or to check the accuracy of those apps.
Vetted apps for ObGyns
My team's research has focused on identifying apps for ObGyns to use in clinical practice.1 In the process, we have developed the APPLICATIONS scoring system, which contains objective and subjective components to help differentiate among the accurate apps.2 This new quarterly "App review" series in OBG Management will showcase recommended apps for the busy ObGyn in the hope of improving work efficiency and the provider-patient relationship.
First up: Apps for calculating the date of delivery. This first app review focuses on pregnancy wheels, or due date calculators. Calculator apps are preferred over other types of apps such as procedure/case documentation apps, as providers use smartphones at point of care to allow rapid decision making.3 Calculating the estimated date of delivery (EDD) and gestational age (GA) is an important, vital task for providers of obstetric care. In fact, new guidelines for calculating EDD were recently developed by the American College of Obstetricians and Gynecologists (ACOG), the American Institute of Ultrasound in Medicine (AIUM), and the Society for Maternal-Fetal Medicine (SMFM).4 Notably, pregnancy wheel apps are more accurate than paper wheels.5 My team checked the accuracy of the pregnancy wheel apps by applying strict criteria to ensure the correct EDD and GA and then scored them in a systematic, nonbiased, conflict-of-interest-free manner.2
Related article:
Elective induction of labor at 39 (vs 41) weeks: Caveats and considerations
The TABLE below lists the top 3 recommended pregnancy wheel or due date calculator apps vetted by our research. The apps are listed alphabetically, and details for each app are provided based on a shortened version of the APPLICATIONS scoring system, APPLI--app comprehensiveness, price, platform, literature use, and important special features.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Farag S, Chyjek K, Chen KT. Identification of iPhone and iPad applications for obstetrics and gynecology providers. Obstet Gynecol. 2014;124(5):941-945.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478-1483.
- Payne KB, Wharrad H, Watts K. Smartphone and medical related App use among medical students and junior doctors in the United Kingdom (UK): a regional survey. BMC Inform Decis Mak. 2012;12:121.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 611. Method for estimating due date. Obstet Gynecol. 2014;124(4):863-866.
- Chambliss LR, Clark SL. Paper gestational age wheels are generally inaccurate. Am J Obstet Gynecol. 2014;210(2):145.e1-e4.
- Farag S, Chyjek K, Chen KT. Identification of iPhone and iPad applications for obstetrics and gynecology providers. Obstet Gynecol. 2014;124(5):941-945.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478-1483.
- Payne KB, Wharrad H, Watts K. Smartphone and medical related App use among medical students and junior doctors in the United Kingdom (UK): a regional survey. BMC Inform Decis Mak. 2012;12:121.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 611. Method for estimating due date. Obstet Gynecol. 2014;124(4):863-866.
- Chambliss LR, Clark SL. Paper gestational age wheels are generally inaccurate. Am J Obstet Gynecol. 2014;210(2):145.e1-e4.
Intervention relieves distress in cancer patients

chemotherapy
Photo by Rhoda Baer
Results of a small study suggest a single dose of the hallucinogenic drug psilocybin, when combined with counseling, can significantly lessen psychological distress in cancer patients for months at a time.
The study showed that psychological counseling and a single dose of psilocybin brought relief from distress that lasted for more than 6 months in a majority of the subjects monitored.
This was based on clinical evaluation scores for anxiety and depression.
“Our results represent the strongest evidence to date of a clinical benefit from psilocybin therapy, with the potential to transform care for patients with cancer-related psychological distress,” said study author Stephen Ross, MD, of New York University School of Medicine in New York, New York.
“If larger clinical trials prove successful, then we could ultimately have available a safe, effective, and inexpensive medication—dispensed under strict control—to alleviate the distress that increases suicide rates among cancer patients.”
Dr Ross and his colleagues reported the results of their study in the Journal of Psychopharmacology alongside a related study and 11 accompanying editorials.
Dr Ross’s study included 29 patients with cancer-related anxiety and depression. Their mean age was 56, and 62% were female. Ninety percent were Caucasian, and 10% were classified as “other” race.
Patients had breast cancer (31%), reproductive cancers (28%), digestive cancers (17%), leukemia/lymphoma (14%), and other cancers (10%).
All patients had been diagnosed as suffering from serious psychological distress related to their disease.
Treatment
Half of the patients were randomly assigned to receive a 0.3 mg/kg dose of psilocybin, and half received a vitamin placebo (250 mg of niacin) known to produce a “rush” that mimics a hallucinogenic drug experience.
Approximately half way through the study’s monitoring period (after 7 weeks), all patients switched treatments. Those who initially received psilocybin took a single dose of niacin, and vice-versa. Neither patients nor researchers knew who had first received psilocybin or placebo.
All patients were provided with tailored counseling from a psychiatrist, psychologist, nurse, or social worker. And the patients were monitored for side effects and improvements in their mental state.
Safety
The researchers said there were no serious adverse events (AEs), either medical or psychiatric, that were attributed to psilocybin.
The most common medical AEs that were attributable to psilocybin were non-clinically significant elevations in blood pressure and heart rate (76%), headaches/migraines (28%), and nausea (14%).
The most common psychiatric AEs attributable to psilocybin were transient anxiety (17%) and transient psychotic-like symptoms (7%; 1 case of transient paranoid ideation and 1 case of transient thought disorder).
Efficacy
The researchers said that, prior to the crossover, psilocybin produced immediate, substantial, and sustained improvements in anxiety and depression.
Specifically, patients who received psilocybin first had significant improvements in responses on the Hospital Anxiety and Depression Scale and the Beck Depression Inventory, when compared to patients who received niacin first.
The differences were significant 1 day after the patients’ first session and 7 weeks after the first session (P≤0.01 for all).
At the 6.5-month follow-up, 60% to 80% of participants continued with clinically significant reductions in depression or anxiety.
The researchers said a key finding of this study was that improvements in clinical evaluation scores for anxiety and depression lasted for the study’s extended monitoring period, which was 8 months for those who took psilocybin first.
Patients also reported post-psilocybin improvements in their quality of life, such as going out more, greater energy, getting along better with family members, and doing well at work. Some reported variations of spirituality, unusual peacefulness, and increased feelings of altruism.
“Our study showed that psilocybin facilitated experiences that drove reductions in psychological distress,” said study author Anthony Bossis, PhD, of New York University School of Medicine. “And if it’s true for cancer care, then it could apply to other stressful medical conditions.”
He cautioned that patients should not consume psilocybin on their own or without supervision from a physician and a trained counselor.
“Psilocybin therapy may not work for everyone,” he noted. “And some groups, such as people with schizophrenia, as well as adolescents, should not be treated with it.” ![]()

chemotherapy
Photo by Rhoda Baer
Results of a small study suggest a single dose of the hallucinogenic drug psilocybin, when combined with counseling, can significantly lessen psychological distress in cancer patients for months at a time.
The study showed that psychological counseling and a single dose of psilocybin brought relief from distress that lasted for more than 6 months in a majority of the subjects monitored.
This was based on clinical evaluation scores for anxiety and depression.
“Our results represent the strongest evidence to date of a clinical benefit from psilocybin therapy, with the potential to transform care for patients with cancer-related psychological distress,” said study author Stephen Ross, MD, of New York University School of Medicine in New York, New York.
“If larger clinical trials prove successful, then we could ultimately have available a safe, effective, and inexpensive medication—dispensed under strict control—to alleviate the distress that increases suicide rates among cancer patients.”
Dr Ross and his colleagues reported the results of their study in the Journal of Psychopharmacology alongside a related study and 11 accompanying editorials.
Dr Ross’s study included 29 patients with cancer-related anxiety and depression. Their mean age was 56, and 62% were female. Ninety percent were Caucasian, and 10% were classified as “other” race.
Patients had breast cancer (31%), reproductive cancers (28%), digestive cancers (17%), leukemia/lymphoma (14%), and other cancers (10%).
All patients had been diagnosed as suffering from serious psychological distress related to their disease.
Treatment
Half of the patients were randomly assigned to receive a 0.3 mg/kg dose of psilocybin, and half received a vitamin placebo (250 mg of niacin) known to produce a “rush” that mimics a hallucinogenic drug experience.
Approximately half way through the study’s monitoring period (after 7 weeks), all patients switched treatments. Those who initially received psilocybin took a single dose of niacin, and vice-versa. Neither patients nor researchers knew who had first received psilocybin or placebo.
All patients were provided with tailored counseling from a psychiatrist, psychologist, nurse, or social worker. And the patients were monitored for side effects and improvements in their mental state.
Safety
The researchers said there were no serious adverse events (AEs), either medical or psychiatric, that were attributed to psilocybin.
The most common medical AEs that were attributable to psilocybin were non-clinically significant elevations in blood pressure and heart rate (76%), headaches/migraines (28%), and nausea (14%).
The most common psychiatric AEs attributable to psilocybin were transient anxiety (17%) and transient psychotic-like symptoms (7%; 1 case of transient paranoid ideation and 1 case of transient thought disorder).
Efficacy
The researchers said that, prior to the crossover, psilocybin produced immediate, substantial, and sustained improvements in anxiety and depression.
Specifically, patients who received psilocybin first had significant improvements in responses on the Hospital Anxiety and Depression Scale and the Beck Depression Inventory, when compared to patients who received niacin first.
The differences were significant 1 day after the patients’ first session and 7 weeks after the first session (P≤0.01 for all).
At the 6.5-month follow-up, 60% to 80% of participants continued with clinically significant reductions in depression or anxiety.
The researchers said a key finding of this study was that improvements in clinical evaluation scores for anxiety and depression lasted for the study’s extended monitoring period, which was 8 months for those who took psilocybin first.
Patients also reported post-psilocybin improvements in their quality of life, such as going out more, greater energy, getting along better with family members, and doing well at work. Some reported variations of spirituality, unusual peacefulness, and increased feelings of altruism.
“Our study showed that psilocybin facilitated experiences that drove reductions in psychological distress,” said study author Anthony Bossis, PhD, of New York University School of Medicine. “And if it’s true for cancer care, then it could apply to other stressful medical conditions.”
He cautioned that patients should not consume psilocybin on their own or without supervision from a physician and a trained counselor.
“Psilocybin therapy may not work for everyone,” he noted. “And some groups, such as people with schizophrenia, as well as adolescents, should not be treated with it.” ![]()

chemotherapy
Photo by Rhoda Baer
Results of a small study suggest a single dose of the hallucinogenic drug psilocybin, when combined with counseling, can significantly lessen psychological distress in cancer patients for months at a time.
The study showed that psychological counseling and a single dose of psilocybin brought relief from distress that lasted for more than 6 months in a majority of the subjects monitored.
This was based on clinical evaluation scores for anxiety and depression.
“Our results represent the strongest evidence to date of a clinical benefit from psilocybin therapy, with the potential to transform care for patients with cancer-related psychological distress,” said study author Stephen Ross, MD, of New York University School of Medicine in New York, New York.
“If larger clinical trials prove successful, then we could ultimately have available a safe, effective, and inexpensive medication—dispensed under strict control—to alleviate the distress that increases suicide rates among cancer patients.”
Dr Ross and his colleagues reported the results of their study in the Journal of Psychopharmacology alongside a related study and 11 accompanying editorials.
Dr Ross’s study included 29 patients with cancer-related anxiety and depression. Their mean age was 56, and 62% were female. Ninety percent were Caucasian, and 10% were classified as “other” race.
Patients had breast cancer (31%), reproductive cancers (28%), digestive cancers (17%), leukemia/lymphoma (14%), and other cancers (10%).
All patients had been diagnosed as suffering from serious psychological distress related to their disease.
Treatment
Half of the patients were randomly assigned to receive a 0.3 mg/kg dose of psilocybin, and half received a vitamin placebo (250 mg of niacin) known to produce a “rush” that mimics a hallucinogenic drug experience.
Approximately half way through the study’s monitoring period (after 7 weeks), all patients switched treatments. Those who initially received psilocybin took a single dose of niacin, and vice-versa. Neither patients nor researchers knew who had first received psilocybin or placebo.
All patients were provided with tailored counseling from a psychiatrist, psychologist, nurse, or social worker. And the patients were monitored for side effects and improvements in their mental state.
Safety
The researchers said there were no serious adverse events (AEs), either medical or psychiatric, that were attributed to psilocybin.
The most common medical AEs that were attributable to psilocybin were non-clinically significant elevations in blood pressure and heart rate (76%), headaches/migraines (28%), and nausea (14%).
The most common psychiatric AEs attributable to psilocybin were transient anxiety (17%) and transient psychotic-like symptoms (7%; 1 case of transient paranoid ideation and 1 case of transient thought disorder).
Efficacy
The researchers said that, prior to the crossover, psilocybin produced immediate, substantial, and sustained improvements in anxiety and depression.
Specifically, patients who received psilocybin first had significant improvements in responses on the Hospital Anxiety and Depression Scale and the Beck Depression Inventory, when compared to patients who received niacin first.
The differences were significant 1 day after the patients’ first session and 7 weeks after the first session (P≤0.01 for all).
At the 6.5-month follow-up, 60% to 80% of participants continued with clinically significant reductions in depression or anxiety.
The researchers said a key finding of this study was that improvements in clinical evaluation scores for anxiety and depression lasted for the study’s extended monitoring period, which was 8 months for those who took psilocybin first.
Patients also reported post-psilocybin improvements in their quality of life, such as going out more, greater energy, getting along better with family members, and doing well at work. Some reported variations of spirituality, unusual peacefulness, and increased feelings of altruism.
“Our study showed that psilocybin facilitated experiences that drove reductions in psychological distress,” said study author Anthony Bossis, PhD, of New York University School of Medicine. “And if it’s true for cancer care, then it could apply to other stressful medical conditions.”
He cautioned that patients should not consume psilocybin on their own or without supervision from a physician and a trained counselor.
“Psilocybin therapy may not work for everyone,” he noted. “And some groups, such as people with schizophrenia, as well as adolescents, should not be treated with it.” ![]()
Infection in AML patient prompts discovery

Photo courtesy of
Janice Carr/CDC
The quest to understand a prolonged infection in an infant with acute myeloid leukemia (AML) has led to the discovery of a mutation that allows bacteria to tolerate antibiotic therapy.
Researchers described this discovery in the journal mBio.
“These findings detail a ‘perfect storm’ for development of antibiotic tolerance by bacteria that already pose a clinical challenge,” said study author Jason Rosch, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“The same conditions may be present in other patients with immune systems that have been compromised by chemotherapy or disease,” added co-author Joshua Wolf, MBBS, also of St. Jude.
The “perfect storm” involved a patient who was 6 weeks old when she was diagnosed with AML. The treatment wiped out her white blood cells, and, despite infection-control measures, she developed a bloodstream infection with vancomycin-resistant Enterococcus faecium (VRE).
The infection persisted for 26 days and only resolved after her immune system recovered. She then successfully completed AML treatment.
In-depth DNA sequencing of 22 VRE samples collected during the patient’s infection helped researchers link the prolonged infection to a point mutation in the relA gene of VRE.
The mutation inappropriately activated the stringent response pathway, which bacteria use to survive under stress and to tolerate antibiotics.
The mutation resulted in elevated levels of the signaling molecule alarmone, and this likely primed the bacteria to survive exposure to multiple antibiotics, the researchers said.
The team also noted that relA-mutant VRE was susceptible to the antibiotics linezolid and daptomycin in minimum inhibitory concentration testing and during planktonic growth.
However, when growing in biofilm, relA-mutant VRE could tolerate high doses of both antibiotics.
“This mutation has particular clinical significance because the antibiotics involved, linezolid and daptomycin, are the last line of defense against VRE infection,” Dr Wolf said.
Among the compounds in development for the treatment of bacterial biofilms is the experimental antibiotic ADEP-4. In this study, ADEP-4 killed relA-mutant and non-mutant VRE growing in biofilm in the lab.
“In the future, compounds like ADEP-4 may provide a new approach to resolving persistent infections,” Dr Wolf said.
Dr Rosch noted that evidence gleaned from tracking the evolution of VRE throughout the infection suggested the patient’s immune-compromised state was essential to survival of the mutant VRE.
Gene transcription was altered significantly in relA-mutant VRE and produced biofilms that were less robust and possibly unlikely to otherwise survive.
“The case expands our understanding of the role of the stringent response in susceptibility and tolerance to a wide range of antibiotics, especially in biofilms,” Dr Rosch said. “It also demonstrates that these mutations can develop and gain a foothold during a human infection.” ![]()

Photo courtesy of
Janice Carr/CDC
The quest to understand a prolonged infection in an infant with acute myeloid leukemia (AML) has led to the discovery of a mutation that allows bacteria to tolerate antibiotic therapy.
Researchers described this discovery in the journal mBio.
“These findings detail a ‘perfect storm’ for development of antibiotic tolerance by bacteria that already pose a clinical challenge,” said study author Jason Rosch, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“The same conditions may be present in other patients with immune systems that have been compromised by chemotherapy or disease,” added co-author Joshua Wolf, MBBS, also of St. Jude.
The “perfect storm” involved a patient who was 6 weeks old when she was diagnosed with AML. The treatment wiped out her white blood cells, and, despite infection-control measures, she developed a bloodstream infection with vancomycin-resistant Enterococcus faecium (VRE).
The infection persisted for 26 days and only resolved after her immune system recovered. She then successfully completed AML treatment.
In-depth DNA sequencing of 22 VRE samples collected during the patient’s infection helped researchers link the prolonged infection to a point mutation in the relA gene of VRE.
The mutation inappropriately activated the stringent response pathway, which bacteria use to survive under stress and to tolerate antibiotics.
The mutation resulted in elevated levels of the signaling molecule alarmone, and this likely primed the bacteria to survive exposure to multiple antibiotics, the researchers said.
The team also noted that relA-mutant VRE was susceptible to the antibiotics linezolid and daptomycin in minimum inhibitory concentration testing and during planktonic growth.
However, when growing in biofilm, relA-mutant VRE could tolerate high doses of both antibiotics.
“This mutation has particular clinical significance because the antibiotics involved, linezolid and daptomycin, are the last line of defense against VRE infection,” Dr Wolf said.
Among the compounds in development for the treatment of bacterial biofilms is the experimental antibiotic ADEP-4. In this study, ADEP-4 killed relA-mutant and non-mutant VRE growing in biofilm in the lab.
“In the future, compounds like ADEP-4 may provide a new approach to resolving persistent infections,” Dr Wolf said.
Dr Rosch noted that evidence gleaned from tracking the evolution of VRE throughout the infection suggested the patient’s immune-compromised state was essential to survival of the mutant VRE.
Gene transcription was altered significantly in relA-mutant VRE and produced biofilms that were less robust and possibly unlikely to otherwise survive.
“The case expands our understanding of the role of the stringent response in susceptibility and tolerance to a wide range of antibiotics, especially in biofilms,” Dr Rosch said. “It also demonstrates that these mutations can develop and gain a foothold during a human infection.” ![]()

Photo courtesy of
Janice Carr/CDC
The quest to understand a prolonged infection in an infant with acute myeloid leukemia (AML) has led to the discovery of a mutation that allows bacteria to tolerate antibiotic therapy.
Researchers described this discovery in the journal mBio.
“These findings detail a ‘perfect storm’ for development of antibiotic tolerance by bacteria that already pose a clinical challenge,” said study author Jason Rosch, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“The same conditions may be present in other patients with immune systems that have been compromised by chemotherapy or disease,” added co-author Joshua Wolf, MBBS, also of St. Jude.
The “perfect storm” involved a patient who was 6 weeks old when she was diagnosed with AML. The treatment wiped out her white blood cells, and, despite infection-control measures, she developed a bloodstream infection with vancomycin-resistant Enterococcus faecium (VRE).
The infection persisted for 26 days and only resolved after her immune system recovered. She then successfully completed AML treatment.
In-depth DNA sequencing of 22 VRE samples collected during the patient’s infection helped researchers link the prolonged infection to a point mutation in the relA gene of VRE.
The mutation inappropriately activated the stringent response pathway, which bacteria use to survive under stress and to tolerate antibiotics.
The mutation resulted in elevated levels of the signaling molecule alarmone, and this likely primed the bacteria to survive exposure to multiple antibiotics, the researchers said.
The team also noted that relA-mutant VRE was susceptible to the antibiotics linezolid and daptomycin in minimum inhibitory concentration testing and during planktonic growth.
However, when growing in biofilm, relA-mutant VRE could tolerate high doses of both antibiotics.
“This mutation has particular clinical significance because the antibiotics involved, linezolid and daptomycin, are the last line of defense against VRE infection,” Dr Wolf said.
Among the compounds in development for the treatment of bacterial biofilms is the experimental antibiotic ADEP-4. In this study, ADEP-4 killed relA-mutant and non-mutant VRE growing in biofilm in the lab.
“In the future, compounds like ADEP-4 may provide a new approach to resolving persistent infections,” Dr Wolf said.
Dr Rosch noted that evidence gleaned from tracking the evolution of VRE throughout the infection suggested the patient’s immune-compromised state was essential to survival of the mutant VRE.
Gene transcription was altered significantly in relA-mutant VRE and produced biofilms that were less robust and possibly unlikely to otherwise survive.
“The case expands our understanding of the role of the stringent response in susceptibility and tolerance to a wide range of antibiotics, especially in biofilms,” Dr Rosch said. “It also demonstrates that these mutations can develop and gain a foothold during a human infection.” ![]()