User login
Do new Alzheimer’s drugs get us closer to solving the Alzheimer’s disease riddle?
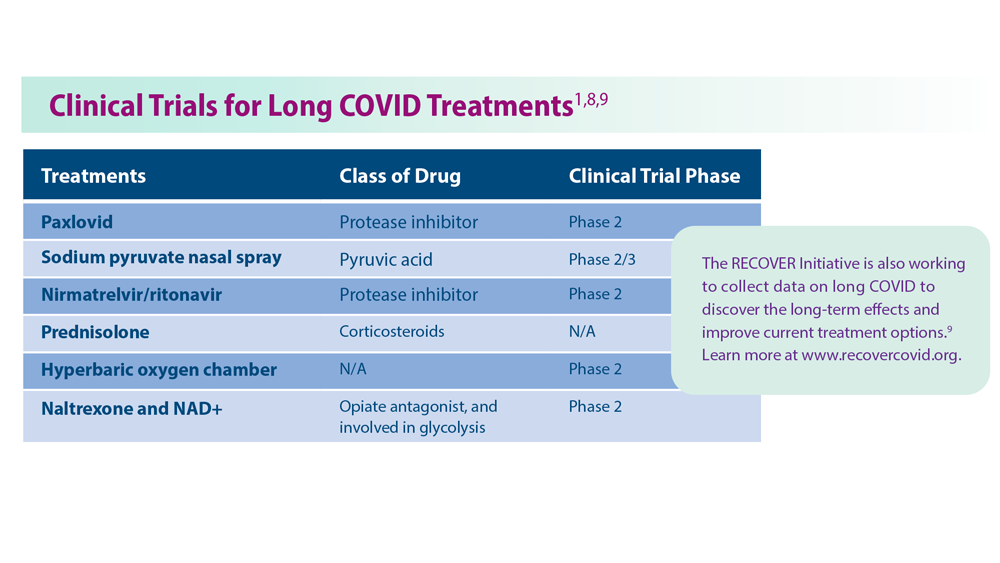
Two antiamyloid drugs were recently approved by the Food and Drug Administration for treating early-stage Alzheimer’s disease (AD). In trials of both lecanemab (Leqembi) and donanemab, a long-held neuropharmacologic dream was realized: Most amyloid plaques – the primary pathologic marker for AD – were eliminated from the brains of patients with late pre-AD or early AD.
Implications for the amyloid hypothesis
The reduction of amyloid plaques has been argued by many scientists and clinical authorities to be the likely pharmacologic solution for AD. These trials are appropriately viewed as a test of the hypothesis that amyloid bodies are a primary cause of the neurobehavioral symptoms we call AD.
In parallel with that striking reduction in amyloid bodies, drug-treated patients had an initially slower progression of neurobehavioral decline than did placebo-treated control patients. That slowing in symptom progression was accompanied by a modest but statistically significant difference in neurobehavioral ability. After several months in treatment, the rate of decline again paralleled that recorded in the control group. The sustained difference of about a half point on cognitive assessment scores separating treatment and control participants was well short of the 1.5-point difference typically considered clinically significant.
A small number of unexpected and unexplained deaths occurred in the treatment groups. Brain swelling and/or micro-hemorrhages were seen in 20%-30% of treated individuals. Significant brain shrinkage was recorded. These adverse findings are indicative of drug-induced trauma in the target organ for these drugs (i.e., the brain) and were the basis for a boxed warning label for drug usage. Antiamyloid drug treatment was not effective in patients who had higher initial numbers of amyloid plaques, indicating that these drugs would not measurably help the majority of AD patients, who are at more advanced disease stages.
These drugs do not appear to be an “answer” for AD. A modest delay in progression does not mean that we’re on a path to a “cure.” Treatment cost estimates are high – more than $80,000 per year. With requisite PET exams and high copays, patient accessibility issues will be daunting.
Of note, To the contrary, they add strong support for the counterargument that the emergence of amyloid plaques is an effect and not a fundamental cause of that progressive loss of neurologic function that we ultimately define as “Alzheimer’s disease.”
Time to switch gears
The more obvious path to winning the battle against this human scourge is prevention. A recent analysis published in The Lancet argued that about 40% of AD and other dementias are potentially preventable. I disagree. I believe that 80%-90% of prospective cases can be substantially delayed or prevented. Studies have shown that progression to AD or other dementias is driven primarily by the progressive deterioration of organic brain health, expressed by the loss of what psychologists have termed “cognitive reserve.” Cognitive reserve is resilience arising from active brain usage, akin to physical resilience attributable to a physically active life. Scientific studies have shown us that an individual’s cognitive resilience (reserve) is a greater predictor of risk for dementia than are amyloid plaques – indeed, greater than any combination of pathologic markers in dementia patients.
Building up cognitive reserve
It’s increasingly clear to this observer that cognitive reserve is synonymous with organic brain health. The primary factors that underlie cognitive reserve are processing speed in the brain, executive control, response withholding, memory acquisition, reasoning, and attention abilities. Faster, more accurate brains are necessarily more physically optimized. They necessarily sustain brain system connectivity. They are necessarily healthier. Such brains bear a relatively low risk of developing AD or other dementias, just as physically healthier bodies bear a lower risk of being prematurely banished to semi-permanent residence in an easy chair or a bed.
Brain health can be sustained by deploying inexpensive, self-administered, app-based assessments of neurologic performance limits, which inform patients and their medical teams about general brain health status. These assessments can help doctors guide their patients to adopt more intelligent brain-healthy lifestyles, or direct them to the “brain gym” to progressively exercise their brains in ways that contribute to rapid, potentially large-scale, rejuvenating improvements in physical and functional brain health.
Randomized controlled trials incorporating different combinations of physical exercise, diet, and cognitive training have recorded significant improvements in physical and functional neurologic status, indicating substantially advanced brain health. Consistent moderate-to-intense physical exercise, brain- and heart-healthy eating habits, and, particularly, computerized brain training have repeatedly been shown to improve cognitive function and physically rejuvenate the brain. With cognitive training in the right forms, improvements in processing speed and other measures manifest improving brain health and greater safety.
In the National Institutes of Health–funded ACTIVE study with more than 2,800 older adults, just 10-18 hours of a specific speed of processing training (now part of BrainHQ, a program that I was involved in developing) reduced the probability of a progression to dementia over the following 10 years by 29%, and by 48% in those who did the most training.
This approach is several orders of magnitude less expensive than the pricey new AD drugs. It presents less serious issues of accessibility and has no side effects. It delivers far more powerful therapeutic benefits in older normal and at-risk populations.
Sustained wellness supporting prevention is the far more sensible medical way forward to save people from AD and other dementias – at a far lower medical and societal cost.
Dr. Merzenich is professor emeritus, department of neuroscience, University of California, San Francisco. He reported conflicts of interest with Posit Science, Stronger Brains, and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Two antiamyloid drugs were recently approved by the Food and Drug Administration for treating early-stage Alzheimer’s disease (AD). In trials of both lecanemab (Leqembi) and donanemab, a long-held neuropharmacologic dream was realized: Most amyloid plaques – the primary pathologic marker for AD – were eliminated from the brains of patients with late pre-AD or early AD.
Implications for the amyloid hypothesis
The reduction of amyloid plaques has been argued by many scientists and clinical authorities to be the likely pharmacologic solution for AD. These trials are appropriately viewed as a test of the hypothesis that amyloid bodies are a primary cause of the neurobehavioral symptoms we call AD.
In parallel with that striking reduction in amyloid bodies, drug-treated patients had an initially slower progression of neurobehavioral decline than did placebo-treated control patients. That slowing in symptom progression was accompanied by a modest but statistically significant difference in neurobehavioral ability. After several months in treatment, the rate of decline again paralleled that recorded in the control group. The sustained difference of about a half point on cognitive assessment scores separating treatment and control participants was well short of the 1.5-point difference typically considered clinically significant.
A small number of unexpected and unexplained deaths occurred in the treatment groups. Brain swelling and/or micro-hemorrhages were seen in 20%-30% of treated individuals. Significant brain shrinkage was recorded. These adverse findings are indicative of drug-induced trauma in the target organ for these drugs (i.e., the brain) and were the basis for a boxed warning label for drug usage. Antiamyloid drug treatment was not effective in patients who had higher initial numbers of amyloid plaques, indicating that these drugs would not measurably help the majority of AD patients, who are at more advanced disease stages.
These drugs do not appear to be an “answer” for AD. A modest delay in progression does not mean that we’re on a path to a “cure.” Treatment cost estimates are high – more than $80,000 per year. With requisite PET exams and high copays, patient accessibility issues will be daunting.
Of note, To the contrary, they add strong support for the counterargument that the emergence of amyloid plaques is an effect and not a fundamental cause of that progressive loss of neurologic function that we ultimately define as “Alzheimer’s disease.”
Time to switch gears
The more obvious path to winning the battle against this human scourge is prevention. A recent analysis published in The Lancet argued that about 40% of AD and other dementias are potentially preventable. I disagree. I believe that 80%-90% of prospective cases can be substantially delayed or prevented. Studies have shown that progression to AD or other dementias is driven primarily by the progressive deterioration of organic brain health, expressed by the loss of what psychologists have termed “cognitive reserve.” Cognitive reserve is resilience arising from active brain usage, akin to physical resilience attributable to a physically active life. Scientific studies have shown us that an individual’s cognitive resilience (reserve) is a greater predictor of risk for dementia than are amyloid plaques – indeed, greater than any combination of pathologic markers in dementia patients.
Building up cognitive reserve
It’s increasingly clear to this observer that cognitive reserve is synonymous with organic brain health. The primary factors that underlie cognitive reserve are processing speed in the brain, executive control, response withholding, memory acquisition, reasoning, and attention abilities. Faster, more accurate brains are necessarily more physically optimized. They necessarily sustain brain system connectivity. They are necessarily healthier. Such brains bear a relatively low risk of developing AD or other dementias, just as physically healthier bodies bear a lower risk of being prematurely banished to semi-permanent residence in an easy chair or a bed.
Brain health can be sustained by deploying inexpensive, self-administered, app-based assessments of neurologic performance limits, which inform patients and their medical teams about general brain health status. These assessments can help doctors guide their patients to adopt more intelligent brain-healthy lifestyles, or direct them to the “brain gym” to progressively exercise their brains in ways that contribute to rapid, potentially large-scale, rejuvenating improvements in physical and functional brain health.
Randomized controlled trials incorporating different combinations of physical exercise, diet, and cognitive training have recorded significant improvements in physical and functional neurologic status, indicating substantially advanced brain health. Consistent moderate-to-intense physical exercise, brain- and heart-healthy eating habits, and, particularly, computerized brain training have repeatedly been shown to improve cognitive function and physically rejuvenate the brain. With cognitive training in the right forms, improvements in processing speed and other measures manifest improving brain health and greater safety.
In the National Institutes of Health–funded ACTIVE study with more than 2,800 older adults, just 10-18 hours of a specific speed of processing training (now part of BrainHQ, a program that I was involved in developing) reduced the probability of a progression to dementia over the following 10 years by 29%, and by 48% in those who did the most training.
This approach is several orders of magnitude less expensive than the pricey new AD drugs. It presents less serious issues of accessibility and has no side effects. It delivers far more powerful therapeutic benefits in older normal and at-risk populations.
Sustained wellness supporting prevention is the far more sensible medical way forward to save people from AD and other dementias – at a far lower medical and societal cost.
Dr. Merzenich is professor emeritus, department of neuroscience, University of California, San Francisco. He reported conflicts of interest with Posit Science, Stronger Brains, and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Two antiamyloid drugs were recently approved by the Food and Drug Administration for treating early-stage Alzheimer’s disease (AD). In trials of both lecanemab (Leqembi) and donanemab, a long-held neuropharmacologic dream was realized: Most amyloid plaques – the primary pathologic marker for AD – were eliminated from the brains of patients with late pre-AD or early AD.
Implications for the amyloid hypothesis
The reduction of amyloid plaques has been argued by many scientists and clinical authorities to be the likely pharmacologic solution for AD. These trials are appropriately viewed as a test of the hypothesis that amyloid bodies are a primary cause of the neurobehavioral symptoms we call AD.
In parallel with that striking reduction in amyloid bodies, drug-treated patients had an initially slower progression of neurobehavioral decline than did placebo-treated control patients. That slowing in symptom progression was accompanied by a modest but statistically significant difference in neurobehavioral ability. After several months in treatment, the rate of decline again paralleled that recorded in the control group. The sustained difference of about a half point on cognitive assessment scores separating treatment and control participants was well short of the 1.5-point difference typically considered clinically significant.
A small number of unexpected and unexplained deaths occurred in the treatment groups. Brain swelling and/or micro-hemorrhages were seen in 20%-30% of treated individuals. Significant brain shrinkage was recorded. These adverse findings are indicative of drug-induced trauma in the target organ for these drugs (i.e., the brain) and were the basis for a boxed warning label for drug usage. Antiamyloid drug treatment was not effective in patients who had higher initial numbers of amyloid plaques, indicating that these drugs would not measurably help the majority of AD patients, who are at more advanced disease stages.
These drugs do not appear to be an “answer” for AD. A modest delay in progression does not mean that we’re on a path to a “cure.” Treatment cost estimates are high – more than $80,000 per year. With requisite PET exams and high copays, patient accessibility issues will be daunting.
Of note, To the contrary, they add strong support for the counterargument that the emergence of amyloid plaques is an effect and not a fundamental cause of that progressive loss of neurologic function that we ultimately define as “Alzheimer’s disease.”
Time to switch gears
The more obvious path to winning the battle against this human scourge is prevention. A recent analysis published in The Lancet argued that about 40% of AD and other dementias are potentially preventable. I disagree. I believe that 80%-90% of prospective cases can be substantially delayed or prevented. Studies have shown that progression to AD or other dementias is driven primarily by the progressive deterioration of organic brain health, expressed by the loss of what psychologists have termed “cognitive reserve.” Cognitive reserve is resilience arising from active brain usage, akin to physical resilience attributable to a physically active life. Scientific studies have shown us that an individual’s cognitive resilience (reserve) is a greater predictor of risk for dementia than are amyloid plaques – indeed, greater than any combination of pathologic markers in dementia patients.
Building up cognitive reserve
It’s increasingly clear to this observer that cognitive reserve is synonymous with organic brain health. The primary factors that underlie cognitive reserve are processing speed in the brain, executive control, response withholding, memory acquisition, reasoning, and attention abilities. Faster, more accurate brains are necessarily more physically optimized. They necessarily sustain brain system connectivity. They are necessarily healthier. Such brains bear a relatively low risk of developing AD or other dementias, just as physically healthier bodies bear a lower risk of being prematurely banished to semi-permanent residence in an easy chair or a bed.
Brain health can be sustained by deploying inexpensive, self-administered, app-based assessments of neurologic performance limits, which inform patients and their medical teams about general brain health status. These assessments can help doctors guide their patients to adopt more intelligent brain-healthy lifestyles, or direct them to the “brain gym” to progressively exercise their brains in ways that contribute to rapid, potentially large-scale, rejuvenating improvements in physical and functional brain health.
Randomized controlled trials incorporating different combinations of physical exercise, diet, and cognitive training have recorded significant improvements in physical and functional neurologic status, indicating substantially advanced brain health. Consistent moderate-to-intense physical exercise, brain- and heart-healthy eating habits, and, particularly, computerized brain training have repeatedly been shown to improve cognitive function and physically rejuvenate the brain. With cognitive training in the right forms, improvements in processing speed and other measures manifest improving brain health and greater safety.
In the National Institutes of Health–funded ACTIVE study with more than 2,800 older adults, just 10-18 hours of a specific speed of processing training (now part of BrainHQ, a program that I was involved in developing) reduced the probability of a progression to dementia over the following 10 years by 29%, and by 48% in those who did the most training.
This approach is several orders of magnitude less expensive than the pricey new AD drugs. It presents less serious issues of accessibility and has no side effects. It delivers far more powerful therapeutic benefits in older normal and at-risk populations.
Sustained wellness supporting prevention is the far more sensible medical way forward to save people from AD and other dementias – at a far lower medical and societal cost.
Dr. Merzenich is professor emeritus, department of neuroscience, University of California, San Francisco. He reported conflicts of interest with Posit Science, Stronger Brains, and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Social media makes kids with type 1 diabetes feel less alone
After being diagnosed with type 1 diabetes in 2021, British teenager Johnny Bailey felt isolated. That’s when he turned to social media, where he found others living with type 1 diabetes. He began to share his experience and now has more than 329,000 followers on his TikTok account, where he regularly posts videos.
These include short clips of him demonstrating how he changes his FreeStyle Libre sensor for his flash glucose monitor. In the videos, Johnny appropriately places his sensor on the back of his arm with background music, makes facial expressions, and transforms a dreaded diabetes-related task into an experience that appears fun and entertaining. In the limited videos I was able to review, he follows all the appropriate steps for sensor placement.
Many youths living with type 1 diabetes struggle with living with a chronic medical condition. Because type 1 diabetes is a rare condition, affecting about 1 in 500 children in the United States, many youth may not meet anyone else their age with type 1 diabetes through school, social events, or extracurricular activities.
For adolescents with intensively managed conditions like type 1 diabetes, this can present numerous psychosocial challenges – specifically, many youth experience shame or stigma associated with managing type 1 diabetes.
Diabetes-specific tasks may include wearing an insulin pump, monitoring blood glucose with finger pricks or a continuous glucose monitor (CGM), giving injections of insulin before meals and snacks, adjusting times for meals and snacks based on metabolic needs, waking up in the middle of the night to treat high or low blood glucose – the list goes on and on.
One study estimated that the average time it takes a child with type 1 diabetes to perform diabetes-specific tasks is over 5 hours per day.
Although much of this diabetes management time is spent by parents, as children get older and become teenagers, they are gradually transitioning to taking on more of this responsibility themselves. Wearing diabetes technology (insulin pumps and CGMs) can draw unwanted attention, leading to diabetes-specific body image concerns. Kids may also have to excuse themselves from an activity to treat a low or high blood glucose, creating uncomfortable situations when others inquire about why the activity was interrupted. As a result, many youths will avoid managing their diabetes properly to avoid drawing unwanted attention, consequently put their health at risk.
Those who are afraid of placing their glucose sensor owing to fear of pain may be reassured by seeing Johnny placing his sensor with a smile on his face. Some of his content also highlights other stigmatizing situations that teens may face, for example someone with a judgmental look questioning why he needed to give an insulin injection here.
This highlights an important concept – that people with type 1 diabetes may face criticism when dosing insulin in public, but it doesn’t mean they should feel forced to manage diabetes in private unless they choose to. Johnny is an inspirational individual who has bravely taken his type 1 diabetes experiences and used his creative skills to make these seemingly boring health-related tasks fun, interesting, and accessible.
Social media has become an outlet for people with type 1 diabetes to connect with others who can relate to their experiences.
However, there’s another side to consider. Although social media may provide a great source of support for youth, it may also adversely affect mental health. Just as quickly as social media outlets have grown, so has concern over excessive social media use and its impact on adolescents’ mental health. There’s a growing body of literature that describes the negative mental health aspects related to social media use.
Some adolescents struggling to manage type 1 diabetes may feel worse when seeing others thrive on social media, which has the potential to worsen stigma and shame. Youth may wonder how someone else is able to manage their type 1 diabetes so well when they are facing so many challenges.
Short videos on social media provide an incomplete picture of living with type 1 diabetes – just a glimpse into others’ lives, and only the parts that they want others to see. Managing a chronic condition can’t be fully represented in 10-second videos. And if youths choose to post their type 1 diabetes experiences on social media, they also risk receiving backlash or criticism, which can negatively their impact mental health in return.
Furthermore, the content being posted may not always be accurate or educational, leading to the potential for some youth to misunderstand type 1 diabetes.
Although I wouldn’t discourage youth with type 1 diabetes from engaging on social media and viewing diabetes-related content, they need to know that social media is flooded with misinformation. Creating an open space for youth to ask their clinicians questions about type 1 diabetes–related topics they view on social media is vital to ensuring they are viewing accurate information, so they are able to continue to manage their diabetes safely.
As a pediatric endocrinologist, I sometimes share resources on social media with patients if I believe it will help them cope with their type 1 diabetes diagnosis and management. I have had numerous patients – many of whom have struggled to accept their diagnosis – mention with joy and excitement that they were following an organization addressing type 1 diabetes on social media.
When making suggestions, I may refer them to The Diabetes Link, an organization with resources for young adults with type 1 diabetes that creates a space to connect with other young adults with type 1 diabetes. diaTribe is another organization created and led by people with diabetes that has a plethora of resources and provides evidence-based education for patients. I have also shared Project 50-in-50, which highlights two individuals with type 1 diabetes hiking the highest peak in each state in less than 50 days. Being able to see type 1 diabetes in a positive light is a huge step toward a more positive outlook on diabetes management.
Dr. Nally is an assistant professor, department of pediatrics, and a pediatric endocrinologist, division of pediatric endocrinology, at Yale University, New Haven, Conn. She reported conflicts of interest with Medtronic and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
After being diagnosed with type 1 diabetes in 2021, British teenager Johnny Bailey felt isolated. That’s when he turned to social media, where he found others living with type 1 diabetes. He began to share his experience and now has more than 329,000 followers on his TikTok account, where he regularly posts videos.
These include short clips of him demonstrating how he changes his FreeStyle Libre sensor for his flash glucose monitor. In the videos, Johnny appropriately places his sensor on the back of his arm with background music, makes facial expressions, and transforms a dreaded diabetes-related task into an experience that appears fun and entertaining. In the limited videos I was able to review, he follows all the appropriate steps for sensor placement.
Many youths living with type 1 diabetes struggle with living with a chronic medical condition. Because type 1 diabetes is a rare condition, affecting about 1 in 500 children in the United States, many youth may not meet anyone else their age with type 1 diabetes through school, social events, or extracurricular activities.
For adolescents with intensively managed conditions like type 1 diabetes, this can present numerous psychosocial challenges – specifically, many youth experience shame or stigma associated with managing type 1 diabetes.
Diabetes-specific tasks may include wearing an insulin pump, monitoring blood glucose with finger pricks or a continuous glucose monitor (CGM), giving injections of insulin before meals and snacks, adjusting times for meals and snacks based on metabolic needs, waking up in the middle of the night to treat high or low blood glucose – the list goes on and on.
One study estimated that the average time it takes a child with type 1 diabetes to perform diabetes-specific tasks is over 5 hours per day.
Although much of this diabetes management time is spent by parents, as children get older and become teenagers, they are gradually transitioning to taking on more of this responsibility themselves. Wearing diabetes technology (insulin pumps and CGMs) can draw unwanted attention, leading to diabetes-specific body image concerns. Kids may also have to excuse themselves from an activity to treat a low or high blood glucose, creating uncomfortable situations when others inquire about why the activity was interrupted. As a result, many youths will avoid managing their diabetes properly to avoid drawing unwanted attention, consequently put their health at risk.
Those who are afraid of placing their glucose sensor owing to fear of pain may be reassured by seeing Johnny placing his sensor with a smile on his face. Some of his content also highlights other stigmatizing situations that teens may face, for example someone with a judgmental look questioning why he needed to give an insulin injection here.
This highlights an important concept – that people with type 1 diabetes may face criticism when dosing insulin in public, but it doesn’t mean they should feel forced to manage diabetes in private unless they choose to. Johnny is an inspirational individual who has bravely taken his type 1 diabetes experiences and used his creative skills to make these seemingly boring health-related tasks fun, interesting, and accessible.
Social media has become an outlet for people with type 1 diabetes to connect with others who can relate to their experiences.
However, there’s another side to consider. Although social media may provide a great source of support for youth, it may also adversely affect mental health. Just as quickly as social media outlets have grown, so has concern over excessive social media use and its impact on adolescents’ mental health. There’s a growing body of literature that describes the negative mental health aspects related to social media use.
Some adolescents struggling to manage type 1 diabetes may feel worse when seeing others thrive on social media, which has the potential to worsen stigma and shame. Youth may wonder how someone else is able to manage their type 1 diabetes so well when they are facing so many challenges.
Short videos on social media provide an incomplete picture of living with type 1 diabetes – just a glimpse into others’ lives, and only the parts that they want others to see. Managing a chronic condition can’t be fully represented in 10-second videos. And if youths choose to post their type 1 diabetes experiences on social media, they also risk receiving backlash or criticism, which can negatively their impact mental health in return.
Furthermore, the content being posted may not always be accurate or educational, leading to the potential for some youth to misunderstand type 1 diabetes.
Although I wouldn’t discourage youth with type 1 diabetes from engaging on social media and viewing diabetes-related content, they need to know that social media is flooded with misinformation. Creating an open space for youth to ask their clinicians questions about type 1 diabetes–related topics they view on social media is vital to ensuring they are viewing accurate information, so they are able to continue to manage their diabetes safely.
As a pediatric endocrinologist, I sometimes share resources on social media with patients if I believe it will help them cope with their type 1 diabetes diagnosis and management. I have had numerous patients – many of whom have struggled to accept their diagnosis – mention with joy and excitement that they were following an organization addressing type 1 diabetes on social media.
When making suggestions, I may refer them to The Diabetes Link, an organization with resources for young adults with type 1 diabetes that creates a space to connect with other young adults with type 1 diabetes. diaTribe is another organization created and led by people with diabetes that has a plethora of resources and provides evidence-based education for patients. I have also shared Project 50-in-50, which highlights two individuals with type 1 diabetes hiking the highest peak in each state in less than 50 days. Being able to see type 1 diabetes in a positive light is a huge step toward a more positive outlook on diabetes management.
Dr. Nally is an assistant professor, department of pediatrics, and a pediatric endocrinologist, division of pediatric endocrinology, at Yale University, New Haven, Conn. She reported conflicts of interest with Medtronic and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
After being diagnosed with type 1 diabetes in 2021, British teenager Johnny Bailey felt isolated. That’s when he turned to social media, where he found others living with type 1 diabetes. He began to share his experience and now has more than 329,000 followers on his TikTok account, where he regularly posts videos.
These include short clips of him demonstrating how he changes his FreeStyle Libre sensor for his flash glucose monitor. In the videos, Johnny appropriately places his sensor on the back of his arm with background music, makes facial expressions, and transforms a dreaded diabetes-related task into an experience that appears fun and entertaining. In the limited videos I was able to review, he follows all the appropriate steps for sensor placement.
Many youths living with type 1 diabetes struggle with living with a chronic medical condition. Because type 1 diabetes is a rare condition, affecting about 1 in 500 children in the United States, many youth may not meet anyone else their age with type 1 diabetes through school, social events, or extracurricular activities.
For adolescents with intensively managed conditions like type 1 diabetes, this can present numerous psychosocial challenges – specifically, many youth experience shame or stigma associated with managing type 1 diabetes.
Diabetes-specific tasks may include wearing an insulin pump, monitoring blood glucose with finger pricks or a continuous glucose monitor (CGM), giving injections of insulin before meals and snacks, adjusting times for meals and snacks based on metabolic needs, waking up in the middle of the night to treat high or low blood glucose – the list goes on and on.
One study estimated that the average time it takes a child with type 1 diabetes to perform diabetes-specific tasks is over 5 hours per day.
Although much of this diabetes management time is spent by parents, as children get older and become teenagers, they are gradually transitioning to taking on more of this responsibility themselves. Wearing diabetes technology (insulin pumps and CGMs) can draw unwanted attention, leading to diabetes-specific body image concerns. Kids may also have to excuse themselves from an activity to treat a low or high blood glucose, creating uncomfortable situations when others inquire about why the activity was interrupted. As a result, many youths will avoid managing their diabetes properly to avoid drawing unwanted attention, consequently put their health at risk.
Those who are afraid of placing their glucose sensor owing to fear of pain may be reassured by seeing Johnny placing his sensor with a smile on his face. Some of his content also highlights other stigmatizing situations that teens may face, for example someone with a judgmental look questioning why he needed to give an insulin injection here.
This highlights an important concept – that people with type 1 diabetes may face criticism when dosing insulin in public, but it doesn’t mean they should feel forced to manage diabetes in private unless they choose to. Johnny is an inspirational individual who has bravely taken his type 1 diabetes experiences and used his creative skills to make these seemingly boring health-related tasks fun, interesting, and accessible.
Social media has become an outlet for people with type 1 diabetes to connect with others who can relate to their experiences.
However, there’s another side to consider. Although social media may provide a great source of support for youth, it may also adversely affect mental health. Just as quickly as social media outlets have grown, so has concern over excessive social media use and its impact on adolescents’ mental health. There’s a growing body of literature that describes the negative mental health aspects related to social media use.
Some adolescents struggling to manage type 1 diabetes may feel worse when seeing others thrive on social media, which has the potential to worsen stigma and shame. Youth may wonder how someone else is able to manage their type 1 diabetes so well when they are facing so many challenges.
Short videos on social media provide an incomplete picture of living with type 1 diabetes – just a glimpse into others’ lives, and only the parts that they want others to see. Managing a chronic condition can’t be fully represented in 10-second videos. And if youths choose to post their type 1 diabetes experiences on social media, they also risk receiving backlash or criticism, which can negatively their impact mental health in return.
Furthermore, the content being posted may not always be accurate or educational, leading to the potential for some youth to misunderstand type 1 diabetes.
Although I wouldn’t discourage youth with type 1 diabetes from engaging on social media and viewing diabetes-related content, they need to know that social media is flooded with misinformation. Creating an open space for youth to ask their clinicians questions about type 1 diabetes–related topics they view on social media is vital to ensuring they are viewing accurate information, so they are able to continue to manage their diabetes safely.
As a pediatric endocrinologist, I sometimes share resources on social media with patients if I believe it will help them cope with their type 1 diabetes diagnosis and management. I have had numerous patients – many of whom have struggled to accept their diagnosis – mention with joy and excitement that they were following an organization addressing type 1 diabetes on social media.
When making suggestions, I may refer them to The Diabetes Link, an organization with resources for young adults with type 1 diabetes that creates a space to connect with other young adults with type 1 diabetes. diaTribe is another organization created and led by people with diabetes that has a plethora of resources and provides evidence-based education for patients. I have also shared Project 50-in-50, which highlights two individuals with type 1 diabetes hiking the highest peak in each state in less than 50 days. Being able to see type 1 diabetes in a positive light is a huge step toward a more positive outlook on diabetes management.
Dr. Nally is an assistant professor, department of pediatrics, and a pediatric endocrinologist, division of pediatric endocrinology, at Yale University, New Haven, Conn. She reported conflicts of interest with Medtronic and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Adolescents’ acute care use for eating disorders has risen
In a repeated cross-sectional study that examined population-based data from January 2017 through August 2022, ED visits increased by 121% above expected levels, and hospital admissions increased by 54% above expected among patients aged 10-17 years during the pandemic.
“We are hoping this study continues to heighten awareness of the importance of eating disorders, and also to bolster support for eating disorder programs so that we can adequately care for patients and address the increasing demand for treatment and services,” lead author Alene Toulany, MD, adolescent medicine specialist and researcher at the Hospital for Sick Children in Toronto, told this news organization.
The study was published in the Canadian Medical Association Journal.
‘A pressing concern’
The researchers used linked health administrative databases that included all patients in Ontario who were eligible for the Ontario Health Insurance Plan, which is publicly funded. They compared observed and expected rates of ED visits and hospitalizations for eating disorders between a prepandemic period (Jan. 1, 2017, to Feb. 29, 2020) and a pandemic period (Mar. 1, 2020, to Aug. 31, 2022). The researchers examined the following four age categories: adolescents (aged 10-17 years), young adults (aged 18-26 years), adults (aged 27-40 years), and older adults (aged 41-105 years).
Among adolescents, the observed rate of ED visits during the 30 pandemic months studied was 7.38 per 100,000 population, compared with 3.33 per 100,000 before the pandemic (incidence rate ratio [IRR], 2.21).
The rate of ED visits among young adults increased by 13% above the expected rate. It reached 2.79 per 100,000, compared with 2.46 per 100,000 in the prepandemic period (IRR, 1.13).
Among older adults, ED visits increased from 0.11 per 100,000 in the prepandemic period to 0.14 per 100,000 in the pandemic period (IRR, 1.15). The rate of ED visits among adults remained approximately the same.
The rate of hospital admissions among adolescents increased by 54% above the expected rate during the pandemic. The observed rate of hospital admissions before the pandemic was 5.74 per 100,000, vs. 8.82 per 100,000 during the pandemic (IRR, 1.54). Hospital admissions remained stable or decreased for the other age groups.
“Eating disorders have increased globally in children and adolescents during COVID,” said Dr. Toulany. “There are a number of risk factors contributing to this pandemic rise, including isolation, more time on social media, decreased access to care (as many in-person services were not available due to the pandemic), as well as fear of getting infected. All of these could contribute to an increased risk of developing an eating disorder or of making an existing one worse.”
Regardless of the cause, more investment in eating disorders research and eating disorder programs for adolescents and adults is needed, she said.
“The pandemic served as a catalyst, because it started to shed light on the prevalence of eating disorders, especially in young people. But it’s very important that we recognize that this has been a long-standing issue and a pressing concern that has been consistently overlooked and underfunded,” said Dr. Toulany.
Surging eating disorders
Commenting on the findings, Victor Fornari, MD, director of child and adolescent psychiatry at Zucker Hillside Hospital/Northwell Health in Glen Oaks, N.Y., said, “Our experience in the United States parallels what is described in this Canadian paper. This was a surge of eating disorders the likes of which I had not experienced in my career.” Dr. Fornari did not participate in the current study.
“I’ve been here for over 40 years, and the average number of our inpatients in our eating disorder program has been three to five and about a dozen patients in our day clinic at any one time. But in the spring of 2020, we surged to 20 inpatients and over 20 day patients,” Dr. Fornari said.
“We can speculate as to the reasons for this,” he continued. “Kids were isolated. School was closed. They spent more time on social media and the Internet. Their sports activities were curtailed. There was anxiety because the guidance that we were all offered to prevent contagion was increasing people’s anxiety about safety and danger. So, I think we saw dramatic rises in eating disorders in the same way we saw dramatic rises in anxiety and depression in adolescents, as well.”
Dr. Fornari cited social media as an important contributing factor to eating disorders, especially among vulnerable teenagers. “Many of these vulnerable kids are looking at pictures of people who are very thin and comparing themselves, feeling inadequate, feeling sad. Social media is one of the reasons why the rates of psychopathology amongst teens has skyrocketed in the last decade. The surgeon general recently said we should delay access to social media until age 16 because the younger kids are impressionable and vulnerable. I think there is wisdom there, but it is very hard to actually put into practice.”
Worsening mental health
“I thought this was very relevant research and an important contribution to our understanding of eating disorders during pandemic times,” said Simon Sherry, PhD, professor of psychology and neuroscience at Dalhousie University in Halifax, Nova Scotia. “It also dovetails with my own experience as a practitioner.” Dr. Sherry was not involved in the research.
The pandemic has been difficult for people with disordered eating for many reasons, Dr. Sherry said. “There was a massive disruption or ‘loss of normal’ around food. Restaurants closed, grocery shopping was disrupted, scarcity of food occurred, hoarding of food occurred. That meant that eating was difficult for all of us, but especially for individuals who were rigid and controlling around the consumption of food. In this COVID era, you would need flexibility and acceptance around eating, but if you had a narrow range of preferred foods and preferred shopping locations, no doubt the pandemic made this a lot worse.”
Certain forms of disordered eating would be much more likely during the pandemic, Dr. Sherry noted. “For example, binge eating is often triggered by psychological, social, and environmental events,” and those triggers were abundant at the beginning of the pandemic. Boredom, anxiety, depression, stress, loneliness, confinement, and isolation are among the triggers. “COVID-19-related stress was and is very fertile ground for the growth of emotional eating, binge eating, or turning to food to cope. Eating disorders tend to fester amid silence and isolation and inactivity, and that was very much our experience during the lockdown phase of the pandemic,” he said.
Dr. Sherry agrees with the need for more funding for eating disorders research. “We know in Canada that eating disorders are a very important and deadly issue that is chronically underfunded. We are not funding disordered eating in proportion to its prevalence or in proportion to the amount of harm and destruction it creates for individuals, their family members, and our society at large. The authors are absolutely correct to advocate for care in proportion to the prevalence and the damage associated with eating disorders,” he said.
The study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health, the Ministry of Long-Term Care, and the Canadian Institutes of Health Research (CIHR). Dr. Toulany, Dr. Fornari, and Dr. Sherry reported no relevant financial relationships. One study author reported receiving personal fees from the BMJ Group’s Archives of Diseases in Childhood and grants from CIHR, the Ontario Ministry of Health, the Centre for Addiction and Mental Health, and the Hospital for Sick Children. A second author reported funding from CIHR.
A version of this article first appeared on Medscape.com.
In a repeated cross-sectional study that examined population-based data from January 2017 through August 2022, ED visits increased by 121% above expected levels, and hospital admissions increased by 54% above expected among patients aged 10-17 years during the pandemic.
“We are hoping this study continues to heighten awareness of the importance of eating disorders, and also to bolster support for eating disorder programs so that we can adequately care for patients and address the increasing demand for treatment and services,” lead author Alene Toulany, MD, adolescent medicine specialist and researcher at the Hospital for Sick Children in Toronto, told this news organization.
The study was published in the Canadian Medical Association Journal.
‘A pressing concern’
The researchers used linked health administrative databases that included all patients in Ontario who were eligible for the Ontario Health Insurance Plan, which is publicly funded. They compared observed and expected rates of ED visits and hospitalizations for eating disorders between a prepandemic period (Jan. 1, 2017, to Feb. 29, 2020) and a pandemic period (Mar. 1, 2020, to Aug. 31, 2022). The researchers examined the following four age categories: adolescents (aged 10-17 years), young adults (aged 18-26 years), adults (aged 27-40 years), and older adults (aged 41-105 years).
Among adolescents, the observed rate of ED visits during the 30 pandemic months studied was 7.38 per 100,000 population, compared with 3.33 per 100,000 before the pandemic (incidence rate ratio [IRR], 2.21).
The rate of ED visits among young adults increased by 13% above the expected rate. It reached 2.79 per 100,000, compared with 2.46 per 100,000 in the prepandemic period (IRR, 1.13).
Among older adults, ED visits increased from 0.11 per 100,000 in the prepandemic period to 0.14 per 100,000 in the pandemic period (IRR, 1.15). The rate of ED visits among adults remained approximately the same.
The rate of hospital admissions among adolescents increased by 54% above the expected rate during the pandemic. The observed rate of hospital admissions before the pandemic was 5.74 per 100,000, vs. 8.82 per 100,000 during the pandemic (IRR, 1.54). Hospital admissions remained stable or decreased for the other age groups.
“Eating disorders have increased globally in children and adolescents during COVID,” said Dr. Toulany. “There are a number of risk factors contributing to this pandemic rise, including isolation, more time on social media, decreased access to care (as many in-person services were not available due to the pandemic), as well as fear of getting infected. All of these could contribute to an increased risk of developing an eating disorder or of making an existing one worse.”
Regardless of the cause, more investment in eating disorders research and eating disorder programs for adolescents and adults is needed, she said.
“The pandemic served as a catalyst, because it started to shed light on the prevalence of eating disorders, especially in young people. But it’s very important that we recognize that this has been a long-standing issue and a pressing concern that has been consistently overlooked and underfunded,” said Dr. Toulany.
Surging eating disorders
Commenting on the findings, Victor Fornari, MD, director of child and adolescent psychiatry at Zucker Hillside Hospital/Northwell Health in Glen Oaks, N.Y., said, “Our experience in the United States parallels what is described in this Canadian paper. This was a surge of eating disorders the likes of which I had not experienced in my career.” Dr. Fornari did not participate in the current study.
“I’ve been here for over 40 years, and the average number of our inpatients in our eating disorder program has been three to five and about a dozen patients in our day clinic at any one time. But in the spring of 2020, we surged to 20 inpatients and over 20 day patients,” Dr. Fornari said.
“We can speculate as to the reasons for this,” he continued. “Kids were isolated. School was closed. They spent more time on social media and the Internet. Their sports activities were curtailed. There was anxiety because the guidance that we were all offered to prevent contagion was increasing people’s anxiety about safety and danger. So, I think we saw dramatic rises in eating disorders in the same way we saw dramatic rises in anxiety and depression in adolescents, as well.”
Dr. Fornari cited social media as an important contributing factor to eating disorders, especially among vulnerable teenagers. “Many of these vulnerable kids are looking at pictures of people who are very thin and comparing themselves, feeling inadequate, feeling sad. Social media is one of the reasons why the rates of psychopathology amongst teens has skyrocketed in the last decade. The surgeon general recently said we should delay access to social media until age 16 because the younger kids are impressionable and vulnerable. I think there is wisdom there, but it is very hard to actually put into practice.”
Worsening mental health
“I thought this was very relevant research and an important contribution to our understanding of eating disorders during pandemic times,” said Simon Sherry, PhD, professor of psychology and neuroscience at Dalhousie University in Halifax, Nova Scotia. “It also dovetails with my own experience as a practitioner.” Dr. Sherry was not involved in the research.
The pandemic has been difficult for people with disordered eating for many reasons, Dr. Sherry said. “There was a massive disruption or ‘loss of normal’ around food. Restaurants closed, grocery shopping was disrupted, scarcity of food occurred, hoarding of food occurred. That meant that eating was difficult for all of us, but especially for individuals who were rigid and controlling around the consumption of food. In this COVID era, you would need flexibility and acceptance around eating, but if you had a narrow range of preferred foods and preferred shopping locations, no doubt the pandemic made this a lot worse.”
Certain forms of disordered eating would be much more likely during the pandemic, Dr. Sherry noted. “For example, binge eating is often triggered by psychological, social, and environmental events,” and those triggers were abundant at the beginning of the pandemic. Boredom, anxiety, depression, stress, loneliness, confinement, and isolation are among the triggers. “COVID-19-related stress was and is very fertile ground for the growth of emotional eating, binge eating, or turning to food to cope. Eating disorders tend to fester amid silence and isolation and inactivity, and that was very much our experience during the lockdown phase of the pandemic,” he said.
Dr. Sherry agrees with the need for more funding for eating disorders research. “We know in Canada that eating disorders are a very important and deadly issue that is chronically underfunded. We are not funding disordered eating in proportion to its prevalence or in proportion to the amount of harm and destruction it creates for individuals, their family members, and our society at large. The authors are absolutely correct to advocate for care in proportion to the prevalence and the damage associated with eating disorders,” he said.
The study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health, the Ministry of Long-Term Care, and the Canadian Institutes of Health Research (CIHR). Dr. Toulany, Dr. Fornari, and Dr. Sherry reported no relevant financial relationships. One study author reported receiving personal fees from the BMJ Group’s Archives of Diseases in Childhood and grants from CIHR, the Ontario Ministry of Health, the Centre for Addiction and Mental Health, and the Hospital for Sick Children. A second author reported funding from CIHR.
A version of this article first appeared on Medscape.com.
In a repeated cross-sectional study that examined population-based data from January 2017 through August 2022, ED visits increased by 121% above expected levels, and hospital admissions increased by 54% above expected among patients aged 10-17 years during the pandemic.
“We are hoping this study continues to heighten awareness of the importance of eating disorders, and also to bolster support for eating disorder programs so that we can adequately care for patients and address the increasing demand for treatment and services,” lead author Alene Toulany, MD, adolescent medicine specialist and researcher at the Hospital for Sick Children in Toronto, told this news organization.
The study was published in the Canadian Medical Association Journal.
‘A pressing concern’
The researchers used linked health administrative databases that included all patients in Ontario who were eligible for the Ontario Health Insurance Plan, which is publicly funded. They compared observed and expected rates of ED visits and hospitalizations for eating disorders between a prepandemic period (Jan. 1, 2017, to Feb. 29, 2020) and a pandemic period (Mar. 1, 2020, to Aug. 31, 2022). The researchers examined the following four age categories: adolescents (aged 10-17 years), young adults (aged 18-26 years), adults (aged 27-40 years), and older adults (aged 41-105 years).
Among adolescents, the observed rate of ED visits during the 30 pandemic months studied was 7.38 per 100,000 population, compared with 3.33 per 100,000 before the pandemic (incidence rate ratio [IRR], 2.21).
The rate of ED visits among young adults increased by 13% above the expected rate. It reached 2.79 per 100,000, compared with 2.46 per 100,000 in the prepandemic period (IRR, 1.13).
Among older adults, ED visits increased from 0.11 per 100,000 in the prepandemic period to 0.14 per 100,000 in the pandemic period (IRR, 1.15). The rate of ED visits among adults remained approximately the same.
The rate of hospital admissions among adolescents increased by 54% above the expected rate during the pandemic. The observed rate of hospital admissions before the pandemic was 5.74 per 100,000, vs. 8.82 per 100,000 during the pandemic (IRR, 1.54). Hospital admissions remained stable or decreased for the other age groups.
“Eating disorders have increased globally in children and adolescents during COVID,” said Dr. Toulany. “There are a number of risk factors contributing to this pandemic rise, including isolation, more time on social media, decreased access to care (as many in-person services were not available due to the pandemic), as well as fear of getting infected. All of these could contribute to an increased risk of developing an eating disorder or of making an existing one worse.”
Regardless of the cause, more investment in eating disorders research and eating disorder programs for adolescents and adults is needed, she said.
“The pandemic served as a catalyst, because it started to shed light on the prevalence of eating disorders, especially in young people. But it’s very important that we recognize that this has been a long-standing issue and a pressing concern that has been consistently overlooked and underfunded,” said Dr. Toulany.
Surging eating disorders
Commenting on the findings, Victor Fornari, MD, director of child and adolescent psychiatry at Zucker Hillside Hospital/Northwell Health in Glen Oaks, N.Y., said, “Our experience in the United States parallels what is described in this Canadian paper. This was a surge of eating disorders the likes of which I had not experienced in my career.” Dr. Fornari did not participate in the current study.
“I’ve been here for over 40 years, and the average number of our inpatients in our eating disorder program has been three to five and about a dozen patients in our day clinic at any one time. But in the spring of 2020, we surged to 20 inpatients and over 20 day patients,” Dr. Fornari said.
“We can speculate as to the reasons for this,” he continued. “Kids were isolated. School was closed. They spent more time on social media and the Internet. Their sports activities were curtailed. There was anxiety because the guidance that we were all offered to prevent contagion was increasing people’s anxiety about safety and danger. So, I think we saw dramatic rises in eating disorders in the same way we saw dramatic rises in anxiety and depression in adolescents, as well.”
Dr. Fornari cited social media as an important contributing factor to eating disorders, especially among vulnerable teenagers. “Many of these vulnerable kids are looking at pictures of people who are very thin and comparing themselves, feeling inadequate, feeling sad. Social media is one of the reasons why the rates of psychopathology amongst teens has skyrocketed in the last decade. The surgeon general recently said we should delay access to social media until age 16 because the younger kids are impressionable and vulnerable. I think there is wisdom there, but it is very hard to actually put into practice.”
Worsening mental health
“I thought this was very relevant research and an important contribution to our understanding of eating disorders during pandemic times,” said Simon Sherry, PhD, professor of psychology and neuroscience at Dalhousie University in Halifax, Nova Scotia. “It also dovetails with my own experience as a practitioner.” Dr. Sherry was not involved in the research.
The pandemic has been difficult for people with disordered eating for many reasons, Dr. Sherry said. “There was a massive disruption or ‘loss of normal’ around food. Restaurants closed, grocery shopping was disrupted, scarcity of food occurred, hoarding of food occurred. That meant that eating was difficult for all of us, but especially for individuals who were rigid and controlling around the consumption of food. In this COVID era, you would need flexibility and acceptance around eating, but if you had a narrow range of preferred foods and preferred shopping locations, no doubt the pandemic made this a lot worse.”
Certain forms of disordered eating would be much more likely during the pandemic, Dr. Sherry noted. “For example, binge eating is often triggered by psychological, social, and environmental events,” and those triggers were abundant at the beginning of the pandemic. Boredom, anxiety, depression, stress, loneliness, confinement, and isolation are among the triggers. “COVID-19-related stress was and is very fertile ground for the growth of emotional eating, binge eating, or turning to food to cope. Eating disorders tend to fester amid silence and isolation and inactivity, and that was very much our experience during the lockdown phase of the pandemic,” he said.
Dr. Sherry agrees with the need for more funding for eating disorders research. “We know in Canada that eating disorders are a very important and deadly issue that is chronically underfunded. We are not funding disordered eating in proportion to its prevalence or in proportion to the amount of harm and destruction it creates for individuals, their family members, and our society at large. The authors are absolutely correct to advocate for care in proportion to the prevalence and the damage associated with eating disorders,” he said.
The study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health, the Ministry of Long-Term Care, and the Canadian Institutes of Health Research (CIHR). Dr. Toulany, Dr. Fornari, and Dr. Sherry reported no relevant financial relationships. One study author reported receiving personal fees from the BMJ Group’s Archives of Diseases in Childhood and grants from CIHR, the Ontario Ministry of Health, the Centre for Addiction and Mental Health, and the Hospital for Sick Children. A second author reported funding from CIHR.
A version of this article first appeared on Medscape.com.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL
Hyperbaric oxygen therapy for traumatic brain injury: Promising or wishful thinking?
A recent review by Hadanny and colleagues recommends hyperbaric oxygen therapy (HBOT) for acute moderate to severe traumatic brain injury (TBI) and selected patients with prolonged postconcussive syndrome.
This article piqued my curiosity because I trained in HBOT more than 20 years ago. As a passionate scuba diver, my motivation was to master treatment for air embolism and decompression illness. Thankfully, these diving accidents are rare. However, I used HBOT for nonhealing wounds, and its efficacy was sometimes remarkable.
Paradoxical results with oxygen therapy
Although it may seem self-evident that “more oxygen is better” for medical illness, this is not necessarily true. I recently interviewed Ola Didrik Saugstad, MD, who demonstrated that the traditional practice of resuscitating newborns with 100% oxygen was more toxic than resuscitation with air (which contains 21% oxygen). His counterintuitive discovery led to a lifesaving change in the international newborn resuscitation guidelines.
The Food and Drug Administration has approved HBOT for a wide variety of conditions, but some practitioners enthusiastically promote it for off-label indications. These include antiaging, autism, multiple sclerosis, and the aforementioned TBI.
More than 50 years ago, HBOT was proposed for stroke, another disorder where the brain has been deprived of oxygen. Despite obvious logic, clinical trials have been unconvincing. The FDA has not approved HBOT for stroke.
HBOT in practice
During HBOT, the patient breathes 100% oxygen while the whole body is pressurized within a hyperbaric chamber. The chamber’s construction allows pressures above normal sea level of 1.0 atmosphere absolute (ATA). For example, The U.S. Navy Treatment Table for decompression sickness recommends 100% oxygen at 2.8 ATA. Chambers may hold one or more patients at a time.
The frequency of therapy varies but often consists of 20-60 sessions lasting 90-120 minutes. For off-label use like TBI, patients usually pay out of pocket. Given the multiple treatments, costs can add up.
Inconsistent evidence and sham controls
The unwieldy 33-page evidence review by Hadanny and colleagues cites multiple studies supporting HBOT for TBI. However, many, if not all, suffer from methodological flaws. These include vague inclusion criteria, lack of a control group, small patient numbers, treatment at different times since injury, poorly defined or varying HBOT protocols, varying outcome measures, and superficial results analysis.
A sham or control arm is essential for HBOT research trials, given the potential placebo effect of placing a human being inside a large, high-tech, sealed tube for an hour or more. In some sham-controlled studies, which consisted of low-pressure oxygen (that is, 1.3 ATA as sham vs. 2.4 ATA as treatment), all groups experienced symptom improvement. The review authors argue that the low-dose HBOT sham arms were biologically active and that the improvements seen mean that both high- and low-dose HBOT is therapeutic. The alternative explanation is that the placebo effect accounted for improvement in both groups.
The late Michael Bennett, a world authority on hyperbaric and underwater medicine, doubted that conventional HBOT sham controls could genuinely have a therapeutic effect, and I agree. The upcoming HOT-POCS trial (discussed below) should answer the question more definitively.
Mechanisms of action and safety
Mechanisms of benefit for HBOT include increased oxygen availability and angiogenesis. Animal research suggests that it may reduce secondary cell death from TBI, through stabilization of the blood-brain barrier and inflammation reduction.
HBOT is generally safe and well tolerated. A retrospective analysis of 1.5 million outpatient hyperbaric treatments revealed that less than 1% were associated with adverse events. The most common were ear and sinus barotrauma. Because HBOT uses increased air pressure, patients must equalize their ears and sinuses. Those who cannot because of altered consciousness, anatomical defects, or congestion must undergo myringotomy or terminate therapy. Claustrophobia was the second most common adverse effect. Convulsions and tension pneumocephalus were rare.
Perhaps the most concerning risk of HBOT for patients with TBI is the potential waste of human and financial resources.
Desperate physicians and patients
As a neurologist who regularly treats patients with TBI, I share the review authors’ frustration regarding the limited efficacy of available treatments. However, the suboptimal efficacy of currently available therapy is insufficient justification to recommend HBOT.
With respect to chronic TBI, it is difficult to imagine how HBOT could reverse brain injury that has been present for months or years. No other therapy exists that reliably encourages neuronal regeneration or prevents the development of posttraumatic epilepsy.
Frank Conidi, MD, a board-certified sports neurologist and headache specialist, shared his thoughts via email. He agrees that HBOT may have a role in TBI, but after reviewing Hadanny and colleagues’ paper, he concluded that there is insufficient evidence for the use of HBOT in all forms of TBI. He would like to see large multicenter, well-designed studies with standardized pressures and duration and a standard definition of the various types of head injury.
Ongoing research
There are at least five ongoing trials on HBOT for TBI or postconcussive syndrome, including the well-designed placebo-controlled HOT-POCS study. The latter has a novel placebo gas system that addresses Hadanny and colleagues’ contention that even low-dose HBOT might be effective.
The placebo arm in HOT-POCS mimics the HBO environment but provides only 0.21 ATA of oxygen, the same as room air. The active arm provides 100% oxygen at 2.0 ATA. If patients in both arms improve, the benefit will be caused by a placebo response, not HBOT.
Conflict of interest
Another concern with the review is that all three authors are affiliated with Aviv Scientific. This company has an exclusive partnership with the world’s largest hyperbaric medicine and research facility, the Sagol Center at Shamir Medical Center in Be’er Ya’akov, Israel.
This conflict of interest does not a priori invalidate their conclusions. However, official HBOT guidelines from a leading organization like the Undersea and Hyperbaric Medicine Society or the American Academy of Neurology would be preferable.
Conclusion
There is an urgent unmet need for more effective treatments for postconcussive syndrome and chronic TBI.
The review authors’ recommendations for HBOT seem premature. They are arguably a disservice to the many desperate patients and their families who will be tempted to expend valuable resources of time and money for an appealing but unproven therapy. Appropriately designed placebo-controlled studies such as HOT-POCS will help separate fact from wishful thinking.
Dr. Wilner is associate professor of neurology at University of Tennessee Health Science Center, Memphis. He reported a conflict of interest with Accordant Health Services.
A version of this article first appeared on Medscape.com.
A recent review by Hadanny and colleagues recommends hyperbaric oxygen therapy (HBOT) for acute moderate to severe traumatic brain injury (TBI) and selected patients with prolonged postconcussive syndrome.
This article piqued my curiosity because I trained in HBOT more than 20 years ago. As a passionate scuba diver, my motivation was to master treatment for air embolism and decompression illness. Thankfully, these diving accidents are rare. However, I used HBOT for nonhealing wounds, and its efficacy was sometimes remarkable.
Paradoxical results with oxygen therapy
Although it may seem self-evident that “more oxygen is better” for medical illness, this is not necessarily true. I recently interviewed Ola Didrik Saugstad, MD, who demonstrated that the traditional practice of resuscitating newborns with 100% oxygen was more toxic than resuscitation with air (which contains 21% oxygen). His counterintuitive discovery led to a lifesaving change in the international newborn resuscitation guidelines.
The Food and Drug Administration has approved HBOT for a wide variety of conditions, but some practitioners enthusiastically promote it for off-label indications. These include antiaging, autism, multiple sclerosis, and the aforementioned TBI.
More than 50 years ago, HBOT was proposed for stroke, another disorder where the brain has been deprived of oxygen. Despite obvious logic, clinical trials have been unconvincing. The FDA has not approved HBOT for stroke.
HBOT in practice
During HBOT, the patient breathes 100% oxygen while the whole body is pressurized within a hyperbaric chamber. The chamber’s construction allows pressures above normal sea level of 1.0 atmosphere absolute (ATA). For example, The U.S. Navy Treatment Table for decompression sickness recommends 100% oxygen at 2.8 ATA. Chambers may hold one or more patients at a time.
The frequency of therapy varies but often consists of 20-60 sessions lasting 90-120 minutes. For off-label use like TBI, patients usually pay out of pocket. Given the multiple treatments, costs can add up.
Inconsistent evidence and sham controls
The unwieldy 33-page evidence review by Hadanny and colleagues cites multiple studies supporting HBOT for TBI. However, many, if not all, suffer from methodological flaws. These include vague inclusion criteria, lack of a control group, small patient numbers, treatment at different times since injury, poorly defined or varying HBOT protocols, varying outcome measures, and superficial results analysis.
A sham or control arm is essential for HBOT research trials, given the potential placebo effect of placing a human being inside a large, high-tech, sealed tube for an hour or more. In some sham-controlled studies, which consisted of low-pressure oxygen (that is, 1.3 ATA as sham vs. 2.4 ATA as treatment), all groups experienced symptom improvement. The review authors argue that the low-dose HBOT sham arms were biologically active and that the improvements seen mean that both high- and low-dose HBOT is therapeutic. The alternative explanation is that the placebo effect accounted for improvement in both groups.
The late Michael Bennett, a world authority on hyperbaric and underwater medicine, doubted that conventional HBOT sham controls could genuinely have a therapeutic effect, and I agree. The upcoming HOT-POCS trial (discussed below) should answer the question more definitively.
Mechanisms of action and safety
Mechanisms of benefit for HBOT include increased oxygen availability and angiogenesis. Animal research suggests that it may reduce secondary cell death from TBI, through stabilization of the blood-brain barrier and inflammation reduction.
HBOT is generally safe and well tolerated. A retrospective analysis of 1.5 million outpatient hyperbaric treatments revealed that less than 1% were associated with adverse events. The most common were ear and sinus barotrauma. Because HBOT uses increased air pressure, patients must equalize their ears and sinuses. Those who cannot because of altered consciousness, anatomical defects, or congestion must undergo myringotomy or terminate therapy. Claustrophobia was the second most common adverse effect. Convulsions and tension pneumocephalus were rare.
Perhaps the most concerning risk of HBOT for patients with TBI is the potential waste of human and financial resources.
Desperate physicians and patients
As a neurologist who regularly treats patients with TBI, I share the review authors’ frustration regarding the limited efficacy of available treatments. However, the suboptimal efficacy of currently available therapy is insufficient justification to recommend HBOT.
With respect to chronic TBI, it is difficult to imagine how HBOT could reverse brain injury that has been present for months or years. No other therapy exists that reliably encourages neuronal regeneration or prevents the development of posttraumatic epilepsy.
Frank Conidi, MD, a board-certified sports neurologist and headache specialist, shared his thoughts via email. He agrees that HBOT may have a role in TBI, but after reviewing Hadanny and colleagues’ paper, he concluded that there is insufficient evidence for the use of HBOT in all forms of TBI. He would like to see large multicenter, well-designed studies with standardized pressures and duration and a standard definition of the various types of head injury.
Ongoing research
There are at least five ongoing trials on HBOT for TBI or postconcussive syndrome, including the well-designed placebo-controlled HOT-POCS study. The latter has a novel placebo gas system that addresses Hadanny and colleagues’ contention that even low-dose HBOT might be effective.
The placebo arm in HOT-POCS mimics the HBO environment but provides only 0.21 ATA of oxygen, the same as room air. The active arm provides 100% oxygen at 2.0 ATA. If patients in both arms improve, the benefit will be caused by a placebo response, not HBOT.
Conflict of interest
Another concern with the review is that all three authors are affiliated with Aviv Scientific. This company has an exclusive partnership with the world’s largest hyperbaric medicine and research facility, the Sagol Center at Shamir Medical Center in Be’er Ya’akov, Israel.
This conflict of interest does not a priori invalidate their conclusions. However, official HBOT guidelines from a leading organization like the Undersea and Hyperbaric Medicine Society or the American Academy of Neurology would be preferable.
Conclusion
There is an urgent unmet need for more effective treatments for postconcussive syndrome and chronic TBI.
The review authors’ recommendations for HBOT seem premature. They are arguably a disservice to the many desperate patients and their families who will be tempted to expend valuable resources of time and money for an appealing but unproven therapy. Appropriately designed placebo-controlled studies such as HOT-POCS will help separate fact from wishful thinking.
Dr. Wilner is associate professor of neurology at University of Tennessee Health Science Center, Memphis. He reported a conflict of interest with Accordant Health Services.
A version of this article first appeared on Medscape.com.
A recent review by Hadanny and colleagues recommends hyperbaric oxygen therapy (HBOT) for acute moderate to severe traumatic brain injury (TBI) and selected patients with prolonged postconcussive syndrome.
This article piqued my curiosity because I trained in HBOT more than 20 years ago. As a passionate scuba diver, my motivation was to master treatment for air embolism and decompression illness. Thankfully, these diving accidents are rare. However, I used HBOT for nonhealing wounds, and its efficacy was sometimes remarkable.
Paradoxical results with oxygen therapy
Although it may seem self-evident that “more oxygen is better” for medical illness, this is not necessarily true. I recently interviewed Ola Didrik Saugstad, MD, who demonstrated that the traditional practice of resuscitating newborns with 100% oxygen was more toxic than resuscitation with air (which contains 21% oxygen). His counterintuitive discovery led to a lifesaving change in the international newborn resuscitation guidelines.
The Food and Drug Administration has approved HBOT for a wide variety of conditions, but some practitioners enthusiastically promote it for off-label indications. These include antiaging, autism, multiple sclerosis, and the aforementioned TBI.
More than 50 years ago, HBOT was proposed for stroke, another disorder where the brain has been deprived of oxygen. Despite obvious logic, clinical trials have been unconvincing. The FDA has not approved HBOT for stroke.
HBOT in practice
During HBOT, the patient breathes 100% oxygen while the whole body is pressurized within a hyperbaric chamber. The chamber’s construction allows pressures above normal sea level of 1.0 atmosphere absolute (ATA). For example, The U.S. Navy Treatment Table for decompression sickness recommends 100% oxygen at 2.8 ATA. Chambers may hold one or more patients at a time.
The frequency of therapy varies but often consists of 20-60 sessions lasting 90-120 minutes. For off-label use like TBI, patients usually pay out of pocket. Given the multiple treatments, costs can add up.
Inconsistent evidence and sham controls
The unwieldy 33-page evidence review by Hadanny and colleagues cites multiple studies supporting HBOT for TBI. However, many, if not all, suffer from methodological flaws. These include vague inclusion criteria, lack of a control group, small patient numbers, treatment at different times since injury, poorly defined or varying HBOT protocols, varying outcome measures, and superficial results analysis.
A sham or control arm is essential for HBOT research trials, given the potential placebo effect of placing a human being inside a large, high-tech, sealed tube for an hour or more. In some sham-controlled studies, which consisted of low-pressure oxygen (that is, 1.3 ATA as sham vs. 2.4 ATA as treatment), all groups experienced symptom improvement. The review authors argue that the low-dose HBOT sham arms were biologically active and that the improvements seen mean that both high- and low-dose HBOT is therapeutic. The alternative explanation is that the placebo effect accounted for improvement in both groups.
The late Michael Bennett, a world authority on hyperbaric and underwater medicine, doubted that conventional HBOT sham controls could genuinely have a therapeutic effect, and I agree. The upcoming HOT-POCS trial (discussed below) should answer the question more definitively.
Mechanisms of action and safety
Mechanisms of benefit for HBOT include increased oxygen availability and angiogenesis. Animal research suggests that it may reduce secondary cell death from TBI, through stabilization of the blood-brain barrier and inflammation reduction.
HBOT is generally safe and well tolerated. A retrospective analysis of 1.5 million outpatient hyperbaric treatments revealed that less than 1% were associated with adverse events. The most common were ear and sinus barotrauma. Because HBOT uses increased air pressure, patients must equalize their ears and sinuses. Those who cannot because of altered consciousness, anatomical defects, or congestion must undergo myringotomy or terminate therapy. Claustrophobia was the second most common adverse effect. Convulsions and tension pneumocephalus were rare.
Perhaps the most concerning risk of HBOT for patients with TBI is the potential waste of human and financial resources.
Desperate physicians and patients
As a neurologist who regularly treats patients with TBI, I share the review authors’ frustration regarding the limited efficacy of available treatments. However, the suboptimal efficacy of currently available therapy is insufficient justification to recommend HBOT.
With respect to chronic TBI, it is difficult to imagine how HBOT could reverse brain injury that has been present for months or years. No other therapy exists that reliably encourages neuronal regeneration or prevents the development of posttraumatic epilepsy.
Frank Conidi, MD, a board-certified sports neurologist and headache specialist, shared his thoughts via email. He agrees that HBOT may have a role in TBI, but after reviewing Hadanny and colleagues’ paper, he concluded that there is insufficient evidence for the use of HBOT in all forms of TBI. He would like to see large multicenter, well-designed studies with standardized pressures and duration and a standard definition of the various types of head injury.
Ongoing research
There are at least five ongoing trials on HBOT for TBI or postconcussive syndrome, including the well-designed placebo-controlled HOT-POCS study. The latter has a novel placebo gas system that addresses Hadanny and colleagues’ contention that even low-dose HBOT might be effective.
The placebo arm in HOT-POCS mimics the HBO environment but provides only 0.21 ATA of oxygen, the same as room air. The active arm provides 100% oxygen at 2.0 ATA. If patients in both arms improve, the benefit will be caused by a placebo response, not HBOT.
Conflict of interest
Another concern with the review is that all three authors are affiliated with Aviv Scientific. This company has an exclusive partnership with the world’s largest hyperbaric medicine and research facility, the Sagol Center at Shamir Medical Center in Be’er Ya’akov, Israel.
This conflict of interest does not a priori invalidate their conclusions. However, official HBOT guidelines from a leading organization like the Undersea and Hyperbaric Medicine Society or the American Academy of Neurology would be preferable.
Conclusion
There is an urgent unmet need for more effective treatments for postconcussive syndrome and chronic TBI.
The review authors’ recommendations for HBOT seem premature. They are arguably a disservice to the many desperate patients and their families who will be tempted to expend valuable resources of time and money for an appealing but unproven therapy. Appropriately designed placebo-controlled studies such as HOT-POCS will help separate fact from wishful thinking.
Dr. Wilner is associate professor of neurology at University of Tennessee Health Science Center, Memphis. He reported a conflict of interest with Accordant Health Services.
A version of this article first appeared on Medscape.com.
The future for the primary care physician?
“The doctor won’t see you now.”
The editor of the alumni magazine had succeeded in getting my attention. The shock value of the headline hooked me and I was drawn in to chase down the research. A study by a team of researchers at Harvard Medical School has published a study in the British Medical Journal revealing that “from 2013 to 2019 the share of U.S. health care visits delivered by nonphysicians such as nurse practitioners or physician assistants increased from 14% to 26%.” In other words, at more than a quarter of the health care visits in this country the patient is not seen by a physician. The percent seen by advanced-practice providers varied by complaint and specialty. For example, 47% of patients with a respiratory complaint saw a nurse practitioner or physicians assistant, while for an eye condition only 13% were seen by an advanced-practice provider. However, overall the increase was dramatic.
It doesn’t require much deep thinking to come up with some explanations for this shift in provider involvement. It boils down to supply and demand. Compared with other similar nations, we have fewer physicians. Physicians are leaving the profession for a variety of reasons, including disappointment with their work-life balance and a sense that too much of their day is devoted to meaningless work with user unfriendly computer systems.
The number of nurse practitioners and physician assistants is growing faster than that of physicians. In fact it has been predicted that over the next 2 decades advanced-practice providers will increase by more than 50% while the physician pool will grow by less than 5%.
We can mull over the how-we-got-here ad infinitum, but this recent study suggests that we had better hustle and invest some time rethinking the role of a physician and how we should adapt our education system to better prepare for those choosing the path through medical school. This mirror gazing is particularly critical for those of us doing primary care.
While in years past I often heard a discontented grumble from patients that “I was ‘only’ seen by the nurse practitioner,” this complaint has become much less frequent as patients have gained more experience with advanced-practice providers and have begun to accept the new reality and see the change as inevitable.
When someone tells me that their daughter or nephew or second cousin is planning on becoming a doctor, I pause and listen patiently as they go on proudly about it before asking if the young person has considered becoming a physician assistant. I say, “Ya know, if I were 60 years younger I think I would bypass medical school and become a nurse practitioner because they get to do all the cool things that I enjoyed about seeing patients. Sure, my mother wouldn’t have been able to introduce me as her son the doctor. But, my parents and I would have spent less time and money on my training, and I would have had less administrative hassle heaped on me once I went into practice.”
The essence of good primary care is Availability, Continuity, and Expertise. The first two attributes aren’t taught in medical school and rely on commitment and having enough bodies to deliver the care. When it comes to expertise, how important is the broad and deep education of traditional medical school when the provider is seeing the relatively narrow spectrum of bread-and-butter everyday complaints that fill the day for most primary care providers? Particularly, when the population has already been preselected by age, geography, and socioeconomic factors.
The usual argument against my assertion is that a higher-priced and more arduous education pathway better provides the physician with the tools to deal with the outliers, the diagnostic enigmas. My reply is that any good provider regardless of his or her years spent in training is taught to first admit what they don’t know. When faced with an enigma, call a consultant or, in the near future, access a Chatbot.
If the natural market and economic forces continue to drive the growth of advanced-practice providers, what role(s) remains for the medical school–trained primary care provider? Does she or he remain the leader of a team of providers? Does she or he still see patients and somehow remain the first among equals?
While patients seem to be warming to the notion of seeing a nonphysician provider, I often still hear the complaint but “I see a different provider every time I go to the office.” Continuity is one of those three keystones of quality primary care. It is not incompatible with a team concept of care delivery, but it does require a commitment to the concept and creative scheduling that allows the patient to see the same provider at almost every visit. I’m not sure where having a first-among-equals provider fits into that scheme. Is it just one more “different” provider?
Maybe the medical school–trained provider becomes a consultant physician, much as the British and Canadian systems seem to work. She or he would see patients only after the advanced primary care provider has done an evaluation and is unsure of the next step. Would this be at the same site or electronically? Is there a time lag? In my old-school interpretation, if the visit is not the same day or maybe the next day then it doesn’t satisfy my Availability requirement of primary care.
Primarily an educator who generally doesn’t see patients but instead trains advanced primary care providers, organizes them into teams that function to provide care in a timely fashion that emphasizes Continuity, and then performs ongoing, real time assessments to assure that care provided is at the highest level of Expertise.
It sounds like an interesting and challenging job description requiring a deep and broad education. Just not one that appeals to me. I would rather be a nurse practitioner or physician’s assistant who is on the front line and hands on.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected]
“The doctor won’t see you now.”
The editor of the alumni magazine had succeeded in getting my attention. The shock value of the headline hooked me and I was drawn in to chase down the research. A study by a team of researchers at Harvard Medical School has published a study in the British Medical Journal revealing that “from 2013 to 2019 the share of U.S. health care visits delivered by nonphysicians such as nurse practitioners or physician assistants increased from 14% to 26%.” In other words, at more than a quarter of the health care visits in this country the patient is not seen by a physician. The percent seen by advanced-practice providers varied by complaint and specialty. For example, 47% of patients with a respiratory complaint saw a nurse practitioner or physicians assistant, while for an eye condition only 13% were seen by an advanced-practice provider. However, overall the increase was dramatic.
It doesn’t require much deep thinking to come up with some explanations for this shift in provider involvement. It boils down to supply and demand. Compared with other similar nations, we have fewer physicians. Physicians are leaving the profession for a variety of reasons, including disappointment with their work-life balance and a sense that too much of their day is devoted to meaningless work with user unfriendly computer systems.
The number of nurse practitioners and physician assistants is growing faster than that of physicians. In fact it has been predicted that over the next 2 decades advanced-practice providers will increase by more than 50% while the physician pool will grow by less than 5%.
We can mull over the how-we-got-here ad infinitum, but this recent study suggests that we had better hustle and invest some time rethinking the role of a physician and how we should adapt our education system to better prepare for those choosing the path through medical school. This mirror gazing is particularly critical for those of us doing primary care.
While in years past I often heard a discontented grumble from patients that “I was ‘only’ seen by the nurse practitioner,” this complaint has become much less frequent as patients have gained more experience with advanced-practice providers and have begun to accept the new reality and see the change as inevitable.
When someone tells me that their daughter or nephew or second cousin is planning on becoming a doctor, I pause and listen patiently as they go on proudly about it before asking if the young person has considered becoming a physician assistant. I say, “Ya know, if I were 60 years younger I think I would bypass medical school and become a nurse practitioner because they get to do all the cool things that I enjoyed about seeing patients. Sure, my mother wouldn’t have been able to introduce me as her son the doctor. But, my parents and I would have spent less time and money on my training, and I would have had less administrative hassle heaped on me once I went into practice.”
The essence of good primary care is Availability, Continuity, and Expertise. The first two attributes aren’t taught in medical school and rely on commitment and having enough bodies to deliver the care. When it comes to expertise, how important is the broad and deep education of traditional medical school when the provider is seeing the relatively narrow spectrum of bread-and-butter everyday complaints that fill the day for most primary care providers? Particularly, when the population has already been preselected by age, geography, and socioeconomic factors.
The usual argument against my assertion is that a higher-priced and more arduous education pathway better provides the physician with the tools to deal with the outliers, the diagnostic enigmas. My reply is that any good provider regardless of his or her years spent in training is taught to first admit what they don’t know. When faced with an enigma, call a consultant or, in the near future, access a Chatbot.
If the natural market and economic forces continue to drive the growth of advanced-practice providers, what role(s) remains for the medical school–trained primary care provider? Does she or he remain the leader of a team of providers? Does she or he still see patients and somehow remain the first among equals?
While patients seem to be warming to the notion of seeing a nonphysician provider, I often still hear the complaint but “I see a different provider every time I go to the office.” Continuity is one of those three keystones of quality primary care. It is not incompatible with a team concept of care delivery, but it does require a commitment to the concept and creative scheduling that allows the patient to see the same provider at almost every visit. I’m not sure where having a first-among-equals provider fits into that scheme. Is it just one more “different” provider?
Maybe the medical school–trained provider becomes a consultant physician, much as the British and Canadian systems seem to work. She or he would see patients only after the advanced primary care provider has done an evaluation and is unsure of the next step. Would this be at the same site or electronically? Is there a time lag? In my old-school interpretation, if the visit is not the same day or maybe the next day then it doesn’t satisfy my Availability requirement of primary care.
Primarily an educator who generally doesn’t see patients but instead trains advanced primary care providers, organizes them into teams that function to provide care in a timely fashion that emphasizes Continuity, and then performs ongoing, real time assessments to assure that care provided is at the highest level of Expertise.
It sounds like an interesting and challenging job description requiring a deep and broad education. Just not one that appeals to me. I would rather be a nurse practitioner or physician’s assistant who is on the front line and hands on.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected]
“The doctor won’t see you now.”
The editor of the alumni magazine had succeeded in getting my attention. The shock value of the headline hooked me and I was drawn in to chase down the research. A study by a team of researchers at Harvard Medical School has published a study in the British Medical Journal revealing that “from 2013 to 2019 the share of U.S. health care visits delivered by nonphysicians such as nurse practitioners or physician assistants increased from 14% to 26%.” In other words, at more than a quarter of the health care visits in this country the patient is not seen by a physician. The percent seen by advanced-practice providers varied by complaint and specialty. For example, 47% of patients with a respiratory complaint saw a nurse practitioner or physicians assistant, while for an eye condition only 13% were seen by an advanced-practice provider. However, overall the increase was dramatic.
It doesn’t require much deep thinking to come up with some explanations for this shift in provider involvement. It boils down to supply and demand. Compared with other similar nations, we have fewer physicians. Physicians are leaving the profession for a variety of reasons, including disappointment with their work-life balance and a sense that too much of their day is devoted to meaningless work with user unfriendly computer systems.
The number of nurse practitioners and physician assistants is growing faster than that of physicians. In fact it has been predicted that over the next 2 decades advanced-practice providers will increase by more than 50% while the physician pool will grow by less than 5%.
We can mull over the how-we-got-here ad infinitum, but this recent study suggests that we had better hustle and invest some time rethinking the role of a physician and how we should adapt our education system to better prepare for those choosing the path through medical school. This mirror gazing is particularly critical for those of us doing primary care.
While in years past I often heard a discontented grumble from patients that “I was ‘only’ seen by the nurse practitioner,” this complaint has become much less frequent as patients have gained more experience with advanced-practice providers and have begun to accept the new reality and see the change as inevitable.
When someone tells me that their daughter or nephew or second cousin is planning on becoming a doctor, I pause and listen patiently as they go on proudly about it before asking if the young person has considered becoming a physician assistant. I say, “Ya know, if I were 60 years younger I think I would bypass medical school and become a nurse practitioner because they get to do all the cool things that I enjoyed about seeing patients. Sure, my mother wouldn’t have been able to introduce me as her son the doctor. But, my parents and I would have spent less time and money on my training, and I would have had less administrative hassle heaped on me once I went into practice.”
The essence of good primary care is Availability, Continuity, and Expertise. The first two attributes aren’t taught in medical school and rely on commitment and having enough bodies to deliver the care. When it comes to expertise, how important is the broad and deep education of traditional medical school when the provider is seeing the relatively narrow spectrum of bread-and-butter everyday complaints that fill the day for most primary care providers? Particularly, when the population has already been preselected by age, geography, and socioeconomic factors.
The usual argument against my assertion is that a higher-priced and more arduous education pathway better provides the physician with the tools to deal with the outliers, the diagnostic enigmas. My reply is that any good provider regardless of his or her years spent in training is taught to first admit what they don’t know. When faced with an enigma, call a consultant or, in the near future, access a Chatbot.
If the natural market and economic forces continue to drive the growth of advanced-practice providers, what role(s) remains for the medical school–trained primary care provider? Does she or he remain the leader of a team of providers? Does she or he still see patients and somehow remain the first among equals?
While patients seem to be warming to the notion of seeing a nonphysician provider, I often still hear the complaint but “I see a different provider every time I go to the office.” Continuity is one of those three keystones of quality primary care. It is not incompatible with a team concept of care delivery, but it does require a commitment to the concept and creative scheduling that allows the patient to see the same provider at almost every visit. I’m not sure where having a first-among-equals provider fits into that scheme. Is it just one more “different” provider?
Maybe the medical school–trained provider becomes a consultant physician, much as the British and Canadian systems seem to work. She or he would see patients only after the advanced primary care provider has done an evaluation and is unsure of the next step. Would this be at the same site or electronically? Is there a time lag? In my old-school interpretation, if the visit is not the same day or maybe the next day then it doesn’t satisfy my Availability requirement of primary care.
Primarily an educator who generally doesn’t see patients but instead trains advanced primary care providers, organizes them into teams that function to provide care in a timely fashion that emphasizes Continuity, and then performs ongoing, real time assessments to assure that care provided is at the highest level of Expertise.
It sounds like an interesting and challenging job description requiring a deep and broad education. Just not one that appeals to me. I would rather be a nurse practitioner or physician’s assistant who is on the front line and hands on.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected]
Progressive Pulmonary Fibrosis: Understanding Its Many Forms
- Raghu G et al. Am J Respir Crit Care Med. 2022;205(9):e18-e47. doi:10.1164/rccm.202202-0399ST
- Cottin V et al. Front Med (Lausanne). 2022;9:799912. doi:10.3389/fmed.2022.799912
- Molina-Molina M et al. Expert Rev Respir Med. 2022;16(7):765-774. doi:10.1080/17476348.2022.2107508
- Cottin V. Am J Respir Crit Care Med. 2023;207(1):11-13. doi:10.1164/rccm.202208-1639ED
- Wijsenbeek M, Cottin V. N Engl J Med. 2020;383(10):958-968. doi:10.1056/NEJMra2005230
- Chiu YH et al. Front Med (Lausanne). 2023;10:1106560. doi:10.3389/fmed.2023.1106560
- Wong AW et al. BMC Pulm Med. 2022;22(1):148. doi:10.1186/s12890-022-01922-2
- Raghu G et al. Am J Respir Crit Care Med. 2022;205(9):e18-e47. doi:10.1164/rccm.202202-0399ST
- Cottin V et al. Front Med (Lausanne). 2022;9:799912. doi:10.3389/fmed.2022.799912
- Molina-Molina M et al. Expert Rev Respir Med. 2022;16(7):765-774. doi:10.1080/17476348.2022.2107508
- Cottin V. Am J Respir Crit Care Med. 2023;207(1):11-13. doi:10.1164/rccm.202208-1639ED
- Wijsenbeek M, Cottin V. N Engl J Med. 2020;383(10):958-968. doi:10.1056/NEJMra2005230
- Chiu YH et al. Front Med (Lausanne). 2023;10:1106560. doi:10.3389/fmed.2023.1106560
- Wong AW et al. BMC Pulm Med. 2022;22(1):148. doi:10.1186/s12890-022-01922-2
- Raghu G et al. Am J Respir Crit Care Med. 2022;205(9):e18-e47. doi:10.1164/rccm.202202-0399ST
- Cottin V et al. Front Med (Lausanne). 2022;9:799912. doi:10.3389/fmed.2022.799912
- Molina-Molina M et al. Expert Rev Respir Med. 2022;16(7):765-774. doi:10.1080/17476348.2022.2107508
- Cottin V. Am J Respir Crit Care Med. 2023;207(1):11-13. doi:10.1164/rccm.202208-1639ED
- Wijsenbeek M, Cottin V. N Engl J Med. 2020;383(10):958-968. doi:10.1056/NEJMra2005230
- Chiu YH et al. Front Med (Lausanne). 2023;10:1106560. doi:10.3389/fmed.2023.1106560
- Wong AW et al. BMC Pulm Med. 2022;22(1):148. doi:10.1186/s12890-022-01922-2
Long COVID: Advocating for Patients and Implementing Effective Techniques
1. Lutchmansingh DD et al. Semin Respir Crit Care Med. 2023;44(1):130-142. doi:10.1055/s-0042-1759568
2. Davis HE et al. Nat Rev Microbiol. 2023;21(3):133-146. doi:10.1038/s41579-022-00846-2
3. Ahmed H et al. J Rehabil Med. 2020;52(5):jrm00063. doi:10.2340/16501977-2694
4. Resources. Long COVID Physio. Accessed May 31, 2023. https://longcovid.physio/resources
5. Long COVID: What do the latest data show? KFF. Published January 26, 2023. Accessed May 31, 2023. https://www.kff.org/policy-watch/long-covid-what-do-latest-data-show/
6. Castanares-Zapatero D et al. Ann Med. 2022;54(1):1473-1487. doi:10.1080/07853890.2022.2076901
7. Mehandru S, Merad M. Nat Immunol. 2022;23(2):194-202. doi:10.1038/s41590-021-01104-y
8. Dhooria S et al. Eur Respir J. 2022;59(2):2102930. doi:10.1183/13993003.02930-2021
9. Researching COVID to enhance recovery. RECOVER. Accessed May 31, 2023. https://recovercovid.org/
1. Lutchmansingh DD et al. Semin Respir Crit Care Med. 2023;44(1):130-142. doi:10.1055/s-0042-1759568
2. Davis HE et al. Nat Rev Microbiol. 2023;21(3):133-146. doi:10.1038/s41579-022-00846-2
3. Ahmed H et al. J Rehabil Med. 2020;52(5):jrm00063. doi:10.2340/16501977-2694
4. Resources. Long COVID Physio. Accessed May 31, 2023. https://longcovid.physio/resources
5. Long COVID: What do the latest data show? KFF. Published January 26, 2023. Accessed May 31, 2023. https://www.kff.org/policy-watch/long-covid-what-do-latest-data-show/
6. Castanares-Zapatero D et al. Ann Med. 2022;54(1):1473-1487. doi:10.1080/07853890.2022.2076901
7. Mehandru S, Merad M. Nat Immunol. 2022;23(2):194-202. doi:10.1038/s41590-021-01104-y
8. Dhooria S et al. Eur Respir J. 2022;59(2):2102930. doi:10.1183/13993003.02930-2021
9. Researching COVID to enhance recovery. RECOVER. Accessed May 31, 2023. https://recovercovid.org/
1. Lutchmansingh DD et al. Semin Respir Crit Care Med. 2023;44(1):130-142. doi:10.1055/s-0042-1759568
2. Davis HE et al. Nat Rev Microbiol. 2023;21(3):133-146. doi:10.1038/s41579-022-00846-2
3. Ahmed H et al. J Rehabil Med. 2020;52(5):jrm00063. doi:10.2340/16501977-2694
4. Resources. Long COVID Physio. Accessed May 31, 2023. https://longcovid.physio/resources
5. Long COVID: What do the latest data show? KFF. Published January 26, 2023. Accessed May 31, 2023. https://www.kff.org/policy-watch/long-covid-what-do-latest-data-show/
6. Castanares-Zapatero D et al. Ann Med. 2022;54(1):1473-1487. doi:10.1080/07853890.2022.2076901
7. Mehandru S, Merad M. Nat Immunol. 2022;23(2):194-202. doi:10.1038/s41590-021-01104-y
8. Dhooria S et al. Eur Respir J. 2022;59(2):2102930. doi:10.1183/13993003.02930-2021
9. Researching COVID to enhance recovery. RECOVER. Accessed May 31, 2023. https://recovercovid.org/
Asthma Across a Woman’s Lifespan
1. Chowdhury NU et al. Eur Respir Rev. 2021;30(162):210067. doi:10.1183/16000617.0067-2021
2. Perikleous EP et al. J Pers Med. 2022;12(6):999. doi:10.3390/jpm12060999
3. Khaleva E et al. Clin Transl Allergy. 2020;10:40. doi:10.1186/s13601-020-00340-z
4. Robijn AL et al. Curr Opin Pulm Med. 2019;25(1):11-17. doi:10.1097/MCP.0000000000000538
5. Bravo-Solarte DC et al. Allergy Asthma Proc. 2023;44(1):24-34. doi:10.2500/aap.2023.44.220077
6. Wang G et al. J Matern Fetal Neonatal Med. 2014;27(9):934-942. doi:10.3109/14767058.2013.847080
7. Hough KP et al. Front Med (Lausanne). 2020;7:191. doi:10.3389/fmed.2020.00191
8. Triebner K et al. Am J Respir Crit Care Med. 2017;195(8):1058-1065. doi:10.1164/rccm.201605-0968OC
9. Bacharier LB, Jackson DJ. J Allergy Clin Immunol. 2023;151(3):581-589. doi:10.1016/j.jaci.2023.01.002
10. An amazing journey: how young lungs develop. American Lung Association. Published May 11, 2018. Accessed June 28, 2023. https://www.lung.org/blog/how-young-lungs-develop
11. Strunk RC et al. J Allergy Clin Immunol. 2006;118(5):1040-1047. doi:10.1016/j.jaci.2006.07.053
12. Kaplan A, Price D. J Asthma Allergy. 2020;13:39-49. doi:10.2147/JAA.S233268
1. Chowdhury NU et al. Eur Respir Rev. 2021;30(162):210067. doi:10.1183/16000617.0067-2021
2. Perikleous EP et al. J Pers Med. 2022;12(6):999. doi:10.3390/jpm12060999
3. Khaleva E et al. Clin Transl Allergy. 2020;10:40. doi:10.1186/s13601-020-00340-z
4. Robijn AL et al. Curr Opin Pulm Med. 2019;25(1):11-17. doi:10.1097/MCP.0000000000000538
5. Bravo-Solarte DC et al. Allergy Asthma Proc. 2023;44(1):24-34. doi:10.2500/aap.2023.44.220077
6. Wang G et al. J Matern Fetal Neonatal Med. 2014;27(9):934-942. doi:10.3109/14767058.2013.847080
7. Hough KP et al. Front Med (Lausanne). 2020;7:191. doi:10.3389/fmed.2020.00191
8. Triebner K et al. Am J Respir Crit Care Med. 2017;195(8):1058-1065. doi:10.1164/rccm.201605-0968OC
9. Bacharier LB, Jackson DJ. J Allergy Clin Immunol. 2023;151(3):581-589. doi:10.1016/j.jaci.2023.01.002
10. An amazing journey: how young lungs develop. American Lung Association. Published May 11, 2018. Accessed June 28, 2023. https://www.lung.org/blog/how-young-lungs-develop
11. Strunk RC et al. J Allergy Clin Immunol. 2006;118(5):1040-1047. doi:10.1016/j.jaci.2006.07.053
12. Kaplan A, Price D. J Asthma Allergy. 2020;13:39-49. doi:10.2147/JAA.S233268
1. Chowdhury NU et al. Eur Respir Rev. 2021;30(162):210067. doi:10.1183/16000617.0067-2021
2. Perikleous EP et al. J Pers Med. 2022;12(6):999. doi:10.3390/jpm12060999
3. Khaleva E et al. Clin Transl Allergy. 2020;10:40. doi:10.1186/s13601-020-00340-z
4. Robijn AL et al. Curr Opin Pulm Med. 2019;25(1):11-17. doi:10.1097/MCP.0000000000000538
5. Bravo-Solarte DC et al. Allergy Asthma Proc. 2023;44(1):24-34. doi:10.2500/aap.2023.44.220077
6. Wang G et al. J Matern Fetal Neonatal Med. 2014;27(9):934-942. doi:10.3109/14767058.2013.847080
7. Hough KP et al. Front Med (Lausanne). 2020;7:191. doi:10.3389/fmed.2020.00191
8. Triebner K et al. Am J Respir Crit Care Med. 2017;195(8):1058-1065. doi:10.1164/rccm.201605-0968OC
9. Bacharier LB, Jackson DJ. J Allergy Clin Immunol. 2023;151(3):581-589. doi:10.1016/j.jaci.2023.01.002
10. An amazing journey: how young lungs develop. American Lung Association. Published May 11, 2018. Accessed June 28, 2023. https://www.lung.org/blog/how-young-lungs-develop
11. Strunk RC et al. J Allergy Clin Immunol. 2006;118(5):1040-1047. doi:10.1016/j.jaci.2006.07.053
12. Kaplan A, Price D. J Asthma Allergy. 2020;13:39-49. doi:10.2147/JAA.S233268
Pulmonology Data Trends 2023 (Slideshow)
CHEST Physician presents the 2023 edition of Pulmonology Data Trends (click to read). This special issue provides updates on hot topics in pulmonology through original infographics and visual storytelling.
In this issue:
Long-Awaited RSV Vaccines Now Available for Older Adults and Pediatric Patients
Burton L. Lesnick, MD, FCCP
Decreasing Pulmonary Embolism-Related Mortality
Parth Rali, MD
Addressing Physician Burnout in Pulmonology and Critical Care
Kelly Vranas, MD, MCR
Updated Guidelines for COPD Management: 2023 GOLD Strategy Report
Muhammad Adrish, MD, MBA, FCCP, FCCM
Progressive Pulmonary Fibrosis: Understanding Its Many Forms
Tejaswini Kulkarni, MD, MPH, FCCP
Sleep Apnea: Comorbidities, Racial Disparities, Weight Guidelines, and Alternatives to CPAP
Lauren Tobias, MD, FCCP
Lung Cancer Screening: A Need for Adjunctive Testing
Eric S. Edell, MD, FCCP
Asthma Across a Woman’s Lifespan
Navitha Ramesh, MD, FCCP
Tuberculosis Management: Returning to Pre-Pandemic Priorities
Patricio Escalante, MD, MSc, FCCP, and Paige K. Marty, MD
Long COVID: Advocating for Patients and Implementing Effective Techniques
Kyle B. Enfield, MD, MS, FSHEA, FCCM
CHEST Physician presents the 2023 edition of Pulmonology Data Trends (click to read). This special issue provides updates on hot topics in pulmonology through original infographics and visual storytelling.
In this issue:
Long-Awaited RSV Vaccines Now Available for Older Adults and Pediatric Patients
Burton L. Lesnick, MD, FCCP
Decreasing Pulmonary Embolism-Related Mortality
Parth Rali, MD
Addressing Physician Burnout in Pulmonology and Critical Care
Kelly Vranas, MD, MCR
Updated Guidelines for COPD Management: 2023 GOLD Strategy Report
Muhammad Adrish, MD, MBA, FCCP, FCCM
Progressive Pulmonary Fibrosis: Understanding Its Many Forms
Tejaswini Kulkarni, MD, MPH, FCCP
Sleep Apnea: Comorbidities, Racial Disparities, Weight Guidelines, and Alternatives to CPAP
Lauren Tobias, MD, FCCP
Lung Cancer Screening: A Need for Adjunctive Testing
Eric S. Edell, MD, FCCP
Asthma Across a Woman’s Lifespan
Navitha Ramesh, MD, FCCP
Tuberculosis Management: Returning to Pre-Pandemic Priorities
Patricio Escalante, MD, MSc, FCCP, and Paige K. Marty, MD
Long COVID: Advocating for Patients and Implementing Effective Techniques
Kyle B. Enfield, MD, MS, FSHEA, FCCM
CHEST Physician presents the 2023 edition of Pulmonology Data Trends (click to read). This special issue provides updates on hot topics in pulmonology through original infographics and visual storytelling.
In this issue:
Long-Awaited RSV Vaccines Now Available for Older Adults and Pediatric Patients
Burton L. Lesnick, MD, FCCP
Decreasing Pulmonary Embolism-Related Mortality
Parth Rali, MD
Addressing Physician Burnout in Pulmonology and Critical Care
Kelly Vranas, MD, MCR
Updated Guidelines for COPD Management: 2023 GOLD Strategy Report
Muhammad Adrish, MD, MBA, FCCP, FCCM
Progressive Pulmonary Fibrosis: Understanding Its Many Forms
Tejaswini Kulkarni, MD, MPH, FCCP
Sleep Apnea: Comorbidities, Racial Disparities, Weight Guidelines, and Alternatives to CPAP
Lauren Tobias, MD, FCCP
Lung Cancer Screening: A Need for Adjunctive Testing
Eric S. Edell, MD, FCCP
Asthma Across a Woman’s Lifespan
Navitha Ramesh, MD, FCCP
Tuberculosis Management: Returning to Pre-Pandemic Priorities
Patricio Escalante, MD, MSc, FCCP, and Paige K. Marty, MD
Long COVID: Advocating for Patients and Implementing Effective Techniques
Kyle B. Enfield, MD, MS, FSHEA, FCCM
Higher RT doses can boost lifespan, reduce risk of death in LS-SCLC patients
SAN DIEGO – , according to a new multicenter, open-label, randomized phase III trial.
Among 224 patients in China, aged 18-70, those randomly assigned to receive volumetric-modulated arc radiotherapy of high-dose, hypofractionated thoracic radiotherapy of 54 Gy in 30 fractions had a much higher median overall survival (62.4 months) than those who received the standard dose of 45 Gy in 30 fractions (43.1 months, P = .001), reported Jiayi Yu, PhD, of Beijing University Cancer Hospital and Institute and colleagues at the annual meeting of the American Society for Radiation Oncology.
Median progression-free survival was also higher in the 54 Gy group (30.5 months vs. 16.7 months in the 45 Gy group, P = .044).
Kristin Higgins, MD, of Winship Cancer Institute of Emory University, Atlanta, provided perspective at the ASTRO session following Dr. Yu’s presentation. She noted that the study population is quite different than that of LS-SCLC patients in the United States, where patients are often older and more likely to have a history of smoking.
“We need more technical details to understand how to deliver this regimen in clinical practice, and it may not be applicable for all patients,” she said. Still, she added that “a key takeaway here is that optimizing the radiotherapy component of treatment is very important.”
Both groups received chemotherapy. “Higher-dose thoracic radiation therapy concurrently with chemotherapy is an alternative therapeutic option,” Dr. Yu said at an ASTRO presentation.
As Dr. Yu noted, twice-daily thoracic radiotherapy of 45 Gy in 30 fractions and concurrent chemotherapy has been the standard treatment for LS-SCLC for the last 20 years. Trials failed to show benefits for once-daily 66-Gy (33 fractions) or 70-Gy treatment (35 fractions), but a phase 2 trial published in 2023 did indicate that twice-daily treatment of 60 Gy (40 fractions) improved survival without boosting side effects.
For the new study, researchers tracked 224 patients from 2017 to 2021 who were previously untreated or had received specific chemotherapy treatments and had ECOG performance status scores of 0 or 1; 108 patients were randomly assigned to the 54-Gy arm and 116 to the 45-Gy arm. All were recruited at 16 public hospitals in China.
The median age in the two groups were 60 in the 54-Gy arm and 62 in the 45-Gy arm; the percentages of women were similar (45.4% and 45.7%, respectively). Most were current or former smokers (62.0% and 61.2%, respectively).
The researchers closed the trial in April 2021 because of the survival benefit in the 54-Gy arm, and patients were tracked through January 2023 for a median 45 months.
Nearly three-quarters of patients in the 54-Gy arm survived to 2 years (77.7%) vs. 53.4% in the 45-Gy arm, a 41% reduction in risk of death. Adverse events were similar between the groups, with 1 reported treatment-related death (myocardial infarction), in the 54-Gy group.
In an interview, Kenneth Rosenzweig, MD, chairman of the department of radiation oncology at Icahn School of Medicine at Mount Sinai, New York, praised the study. It’s “no surprise” that higher radiation doses are well-tolerated since “our ability to shield normal tissue has improved” over the years, said Dr. Rosenzweig, who served as a moderator of the ASTRO session where the research was presented.
However, he cautioned that hypofractionation is still “intense” and may not be appropriate for certain patients. And he added that some clinics may not be set up to provide twice-daily treatments.
Information about study funding was not provided. The study authors have no disclosures. Dr. Higgins discloses relationships with AstraZeneca and Regeneron (advisory board), Jazz (funded research), and Janssen and Picture Health (consulting). Dr. Rosenzweig has no disclosures.
SAN DIEGO – , according to a new multicenter, open-label, randomized phase III trial.
Among 224 patients in China, aged 18-70, those randomly assigned to receive volumetric-modulated arc radiotherapy of high-dose, hypofractionated thoracic radiotherapy of 54 Gy in 30 fractions had a much higher median overall survival (62.4 months) than those who received the standard dose of 45 Gy in 30 fractions (43.1 months, P = .001), reported Jiayi Yu, PhD, of Beijing University Cancer Hospital and Institute and colleagues at the annual meeting of the American Society for Radiation Oncology.
Median progression-free survival was also higher in the 54 Gy group (30.5 months vs. 16.7 months in the 45 Gy group, P = .044).
Kristin Higgins, MD, of Winship Cancer Institute of Emory University, Atlanta, provided perspective at the ASTRO session following Dr. Yu’s presentation. She noted that the study population is quite different than that of LS-SCLC patients in the United States, where patients are often older and more likely to have a history of smoking.
“We need more technical details to understand how to deliver this regimen in clinical practice, and it may not be applicable for all patients,” she said. Still, she added that “a key takeaway here is that optimizing the radiotherapy component of treatment is very important.”
Both groups received chemotherapy. “Higher-dose thoracic radiation therapy concurrently with chemotherapy is an alternative therapeutic option,” Dr. Yu said at an ASTRO presentation.
As Dr. Yu noted, twice-daily thoracic radiotherapy of 45 Gy in 30 fractions and concurrent chemotherapy has been the standard treatment for LS-SCLC for the last 20 years. Trials failed to show benefits for once-daily 66-Gy (33 fractions) or 70-Gy treatment (35 fractions), but a phase 2 trial published in 2023 did indicate that twice-daily treatment of 60 Gy (40 fractions) improved survival without boosting side effects.
For the new study, researchers tracked 224 patients from 2017 to 2021 who were previously untreated or had received specific chemotherapy treatments and had ECOG performance status scores of 0 or 1; 108 patients were randomly assigned to the 54-Gy arm and 116 to the 45-Gy arm. All were recruited at 16 public hospitals in China.
The median age in the two groups were 60 in the 54-Gy arm and 62 in the 45-Gy arm; the percentages of women were similar (45.4% and 45.7%, respectively). Most were current or former smokers (62.0% and 61.2%, respectively).
The researchers closed the trial in April 2021 because of the survival benefit in the 54-Gy arm, and patients were tracked through January 2023 for a median 45 months.
Nearly three-quarters of patients in the 54-Gy arm survived to 2 years (77.7%) vs. 53.4% in the 45-Gy arm, a 41% reduction in risk of death. Adverse events were similar between the groups, with 1 reported treatment-related death (myocardial infarction), in the 54-Gy group.
In an interview, Kenneth Rosenzweig, MD, chairman of the department of radiation oncology at Icahn School of Medicine at Mount Sinai, New York, praised the study. It’s “no surprise” that higher radiation doses are well-tolerated since “our ability to shield normal tissue has improved” over the years, said Dr. Rosenzweig, who served as a moderator of the ASTRO session where the research was presented.
However, he cautioned that hypofractionation is still “intense” and may not be appropriate for certain patients. And he added that some clinics may not be set up to provide twice-daily treatments.
Information about study funding was not provided. The study authors have no disclosures. Dr. Higgins discloses relationships with AstraZeneca and Regeneron (advisory board), Jazz (funded research), and Janssen and Picture Health (consulting). Dr. Rosenzweig has no disclosures.
SAN DIEGO – , according to a new multicenter, open-label, randomized phase III trial.
Among 224 patients in China, aged 18-70, those randomly assigned to receive volumetric-modulated arc radiotherapy of high-dose, hypofractionated thoracic radiotherapy of 54 Gy in 30 fractions had a much higher median overall survival (62.4 months) than those who received the standard dose of 45 Gy in 30 fractions (43.1 months, P = .001), reported Jiayi Yu, PhD, of Beijing University Cancer Hospital and Institute and colleagues at the annual meeting of the American Society for Radiation Oncology.
Median progression-free survival was also higher in the 54 Gy group (30.5 months vs. 16.7 months in the 45 Gy group, P = .044).
Kristin Higgins, MD, of Winship Cancer Institute of Emory University, Atlanta, provided perspective at the ASTRO session following Dr. Yu’s presentation. She noted that the study population is quite different than that of LS-SCLC patients in the United States, where patients are often older and more likely to have a history of smoking.
“We need more technical details to understand how to deliver this regimen in clinical practice, and it may not be applicable for all patients,” she said. Still, she added that “a key takeaway here is that optimizing the radiotherapy component of treatment is very important.”
Both groups received chemotherapy. “Higher-dose thoracic radiation therapy concurrently with chemotherapy is an alternative therapeutic option,” Dr. Yu said at an ASTRO presentation.
As Dr. Yu noted, twice-daily thoracic radiotherapy of 45 Gy in 30 fractions and concurrent chemotherapy has been the standard treatment for LS-SCLC for the last 20 years. Trials failed to show benefits for once-daily 66-Gy (33 fractions) or 70-Gy treatment (35 fractions), but a phase 2 trial published in 2023 did indicate that twice-daily treatment of 60 Gy (40 fractions) improved survival without boosting side effects.
For the new study, researchers tracked 224 patients from 2017 to 2021 who were previously untreated or had received specific chemotherapy treatments and had ECOG performance status scores of 0 or 1; 108 patients were randomly assigned to the 54-Gy arm and 116 to the 45-Gy arm. All were recruited at 16 public hospitals in China.
The median age in the two groups were 60 in the 54-Gy arm and 62 in the 45-Gy arm; the percentages of women were similar (45.4% and 45.7%, respectively). Most were current or former smokers (62.0% and 61.2%, respectively).
The researchers closed the trial in April 2021 because of the survival benefit in the 54-Gy arm, and patients were tracked through January 2023 for a median 45 months.
Nearly three-quarters of patients in the 54-Gy arm survived to 2 years (77.7%) vs. 53.4% in the 45-Gy arm, a 41% reduction in risk of death. Adverse events were similar between the groups, with 1 reported treatment-related death (myocardial infarction), in the 54-Gy group.
In an interview, Kenneth Rosenzweig, MD, chairman of the department of radiation oncology at Icahn School of Medicine at Mount Sinai, New York, praised the study. It’s “no surprise” that higher radiation doses are well-tolerated since “our ability to shield normal tissue has improved” over the years, said Dr. Rosenzweig, who served as a moderator of the ASTRO session where the research was presented.
However, he cautioned that hypofractionation is still “intense” and may not be appropriate for certain patients. And he added that some clinics may not be set up to provide twice-daily treatments.
Information about study funding was not provided. The study authors have no disclosures. Dr. Higgins discloses relationships with AstraZeneca and Regeneron (advisory board), Jazz (funded research), and Janssen and Picture Health (consulting). Dr. Rosenzweig has no disclosures.
AT ASTRO 2023