User login
Hidden disparities: How language barriers reduce cancer care access
TOPLINE:
, a new study suggests.
METHODOLOGY:
- Language barriers between patients and physicians negatively affect the quality of care patients receive; however, less is known about how language barriers may affect patients’ access to cancer care.
- Researchers examined the impact of patients’ spoken language on their access to care for three types of cancer that disproportionately affect Hispanic and Asian populations (colon, lung, and thyroid cancer).
- Trained investigators who speak English, Spanish, or Mandarin called the general information line of 144 US hospitals in 12 states seeking an appointment.
- The primary outcome was whether the simulated patient caller was provided with next steps to access cancer care, defined as being given a clinic number or clinic transfer.
TAKEAWAY:
- Of the 1,296 calls made (432 in each language), 53% resulted in the caller receiving next steps to access cancer care.
- Spanish- and Mandarin-speaking callers were significantly less likely to receive information on next steps (37.7% and 27.5%, respectively), compared with English-speaking callers (93.5%).
- In multivariable logistic regression, non–English-speaking callers had lower odds of being given next steps to access cancer care (odds ratio, 0.04 for Spanish speakers; OR, 0.02 for Mandarin speakers).
- Compared with calls to teaching hospitals, calls to nonteaching hospitals were associated with lower odds of simulated callers receiving this next-step information (OR, 0.43).
IN PRACTICE:
“Our study provides actionable insights into existing linguistic disparities in cancer care access due to systems-level barriers present prior to evaluation by a physician,” the authors concluded. It is essential to “engage in efforts to mitigate these communication barriers that disproportionately impact the health of vulnerable patient populations with cancer.”
SOURCE:
The study, led by Debbie Chen, MD, University of Michigan, Ann Arbor, was published online Sept. 5 in the Journal of the National Comprehensive Cancer Network.
LIMITATIONS:
The researchers only assessed responses from the hospital general information line, and the findings do not reflect the type or quality of cancer care a patient may have received once seen and treated. The study did not capture the complexities of hospital call center workflows, which limited the authors’ ability to discern the reasons behind the observed outcomes.
DISCLOSURES:
The study was supported by the University of Michigan’s Rogel Cancer Center and the National Institute of Diabetes and Digestive and Kidney Diseases . The authors have disclosed no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
, a new study suggests.
METHODOLOGY:
- Language barriers between patients and physicians negatively affect the quality of care patients receive; however, less is known about how language barriers may affect patients’ access to cancer care.
- Researchers examined the impact of patients’ spoken language on their access to care for three types of cancer that disproportionately affect Hispanic and Asian populations (colon, lung, and thyroid cancer).
- Trained investigators who speak English, Spanish, or Mandarin called the general information line of 144 US hospitals in 12 states seeking an appointment.
- The primary outcome was whether the simulated patient caller was provided with next steps to access cancer care, defined as being given a clinic number or clinic transfer.
TAKEAWAY:
- Of the 1,296 calls made (432 in each language), 53% resulted in the caller receiving next steps to access cancer care.
- Spanish- and Mandarin-speaking callers were significantly less likely to receive information on next steps (37.7% and 27.5%, respectively), compared with English-speaking callers (93.5%).
- In multivariable logistic regression, non–English-speaking callers had lower odds of being given next steps to access cancer care (odds ratio, 0.04 for Spanish speakers; OR, 0.02 for Mandarin speakers).
- Compared with calls to teaching hospitals, calls to nonteaching hospitals were associated with lower odds of simulated callers receiving this next-step information (OR, 0.43).
IN PRACTICE:
“Our study provides actionable insights into existing linguistic disparities in cancer care access due to systems-level barriers present prior to evaluation by a physician,” the authors concluded. It is essential to “engage in efforts to mitigate these communication barriers that disproportionately impact the health of vulnerable patient populations with cancer.”
SOURCE:
The study, led by Debbie Chen, MD, University of Michigan, Ann Arbor, was published online Sept. 5 in the Journal of the National Comprehensive Cancer Network.
LIMITATIONS:
The researchers only assessed responses from the hospital general information line, and the findings do not reflect the type or quality of cancer care a patient may have received once seen and treated. The study did not capture the complexities of hospital call center workflows, which limited the authors’ ability to discern the reasons behind the observed outcomes.
DISCLOSURES:
The study was supported by the University of Michigan’s Rogel Cancer Center and the National Institute of Diabetes and Digestive and Kidney Diseases . The authors have disclosed no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
, a new study suggests.
METHODOLOGY:
- Language barriers between patients and physicians negatively affect the quality of care patients receive; however, less is known about how language barriers may affect patients’ access to cancer care.
- Researchers examined the impact of patients’ spoken language on their access to care for three types of cancer that disproportionately affect Hispanic and Asian populations (colon, lung, and thyroid cancer).
- Trained investigators who speak English, Spanish, or Mandarin called the general information line of 144 US hospitals in 12 states seeking an appointment.
- The primary outcome was whether the simulated patient caller was provided with next steps to access cancer care, defined as being given a clinic number or clinic transfer.
TAKEAWAY:
- Of the 1,296 calls made (432 in each language), 53% resulted in the caller receiving next steps to access cancer care.
- Spanish- and Mandarin-speaking callers were significantly less likely to receive information on next steps (37.7% and 27.5%, respectively), compared with English-speaking callers (93.5%).
- In multivariable logistic regression, non–English-speaking callers had lower odds of being given next steps to access cancer care (odds ratio, 0.04 for Spanish speakers; OR, 0.02 for Mandarin speakers).
- Compared with calls to teaching hospitals, calls to nonteaching hospitals were associated with lower odds of simulated callers receiving this next-step information (OR, 0.43).
IN PRACTICE:
“Our study provides actionable insights into existing linguistic disparities in cancer care access due to systems-level barriers present prior to evaluation by a physician,” the authors concluded. It is essential to “engage in efforts to mitigate these communication barriers that disproportionately impact the health of vulnerable patient populations with cancer.”
SOURCE:
The study, led by Debbie Chen, MD, University of Michigan, Ann Arbor, was published online Sept. 5 in the Journal of the National Comprehensive Cancer Network.
LIMITATIONS:
The researchers only assessed responses from the hospital general information line, and the findings do not reflect the type or quality of cancer care a patient may have received once seen and treated. The study did not capture the complexities of hospital call center workflows, which limited the authors’ ability to discern the reasons behind the observed outcomes.
DISCLOSURES:
The study was supported by the University of Michigan’s Rogel Cancer Center and the National Institute of Diabetes and Digestive and Kidney Diseases . The authors have disclosed no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
FROM JNCCN
3D-printed meds customize the exact dose for sick children
Convincing kids to take their medicine could become much easier. Researchers at Texas A&M University are developing a new method of pharmaceutical 3D printing with pediatric patients in mind.
They hope to print precisely dosed tablets in child-friendly shapes and flavors. While the effort is focused on two drugs for pediatric AIDS, the process could be used to print other medicines, including for adults.
Researchers from Britain, Australia, and the University of Texas at Austin are also in the early stages of 3D-printed medication projects. It’s a promising venture in the broader pursuit of “personalized medicine,” tailoring treatments to each patient’s unique needs.
Drug mass production fails to address pediatric patients, who often need different dosages and combinations of medicines as they grow. As a result, adult tablets are often crushed and dissolved in liquid – known as compounding – and given to children. But this can harm drug quality and make doses less precise.
“Suppose the child needs 3.4 milligrams and only a 10-milligram tablet is available. Once you manipulate the dosage from solid to liquid, how do you ensure that it has the same amount of drug in it?” said co-principal investigator Mansoor Khan, PhD, a professor of pharmaceutical sciences at Texas A&M.
Most pharmacies lack the equipment to test compounded drug quality, he said. And liquified drugs taste bad because the pill coating has been ground away.
“Flavor is a big issue,” said Olive Eckstein, MD, an assistant professor of pediatric hematology-oncology at Texas Children’s Hospital and Baylor College of Medicine, who is not involved in the research. “Hospitals will sometimes delay discharging pediatric patients because they can’t take their meds orally and have to get an IV formulation.”
Updating pharmaceutical 3D printing
The FDA approved a 3D-printed drug in 2015, but since then, progress has stalled, largely because the method relied on solvents to bind drug particles together. Over time, solvents can compromise shelf life, according to co-principal investigator Mathew Kuttolamadom, PhD, an associate professor of engineering at Texas A&M.
The Texas A&M team is using a different method, without solvents. First, they create a powder mixture of the drug, a biocompatible polymer (such as lactose), and a sheen, a pigment that colors the tablet and allows heat to be absorbed. Flavoring can also be added. Next, the mixture is heated in the printer chamber.
“The polymer should melt just enough. That gives the tablet structural strength. But it should not melt too much, whereby the drug can start dissolving into the polymer,” Dr. Kuttolamadom said.
The tablets are finished with precise applications of laser heat. Using computer-aided design software, the researchers can create tablets in almost any shape, such as “stars or teddy bears,” he said.
After much trial and error, the researchers have printed tablets that won’t break apart or become soggy.
Now they are testing how different laser scan speeds affect the structure of the tablet, which in turn affects the rate at which drugs dissolve. Slowing down the laser imparts more energy, strengthening the tablet structure and making drugs dissolve slower, for a longer release inside the body.
The researchers hope to develop machine learning models to test different laser speed combinations. Eventually, they could create tablets that combine drugs with different dissolve rates.
“The outside could be a rapid release, and the inside could be an extended release or a sustained release, or even a completely different drug,” Dr. Kuttolamadom said.
Older patients who take many daily medications could benefit from the technology. “Personalized tablets could be printed at your local pharmacy,” he said, “even before you leave your doctor’s office.”
A version of this article first appeared on WebMD.com.
Convincing kids to take their medicine could become much easier. Researchers at Texas A&M University are developing a new method of pharmaceutical 3D printing with pediatric patients in mind.
They hope to print precisely dosed tablets in child-friendly shapes and flavors. While the effort is focused on two drugs for pediatric AIDS, the process could be used to print other medicines, including for adults.
Researchers from Britain, Australia, and the University of Texas at Austin are also in the early stages of 3D-printed medication projects. It’s a promising venture in the broader pursuit of “personalized medicine,” tailoring treatments to each patient’s unique needs.
Drug mass production fails to address pediatric patients, who often need different dosages and combinations of medicines as they grow. As a result, adult tablets are often crushed and dissolved in liquid – known as compounding – and given to children. But this can harm drug quality and make doses less precise.
“Suppose the child needs 3.4 milligrams and only a 10-milligram tablet is available. Once you manipulate the dosage from solid to liquid, how do you ensure that it has the same amount of drug in it?” said co-principal investigator Mansoor Khan, PhD, a professor of pharmaceutical sciences at Texas A&M.
Most pharmacies lack the equipment to test compounded drug quality, he said. And liquified drugs taste bad because the pill coating has been ground away.
“Flavor is a big issue,” said Olive Eckstein, MD, an assistant professor of pediatric hematology-oncology at Texas Children’s Hospital and Baylor College of Medicine, who is not involved in the research. “Hospitals will sometimes delay discharging pediatric patients because they can’t take their meds orally and have to get an IV formulation.”
Updating pharmaceutical 3D printing
The FDA approved a 3D-printed drug in 2015, but since then, progress has stalled, largely because the method relied on solvents to bind drug particles together. Over time, solvents can compromise shelf life, according to co-principal investigator Mathew Kuttolamadom, PhD, an associate professor of engineering at Texas A&M.
The Texas A&M team is using a different method, without solvents. First, they create a powder mixture of the drug, a biocompatible polymer (such as lactose), and a sheen, a pigment that colors the tablet and allows heat to be absorbed. Flavoring can also be added. Next, the mixture is heated in the printer chamber.
“The polymer should melt just enough. That gives the tablet structural strength. But it should not melt too much, whereby the drug can start dissolving into the polymer,” Dr. Kuttolamadom said.
The tablets are finished with precise applications of laser heat. Using computer-aided design software, the researchers can create tablets in almost any shape, such as “stars or teddy bears,” he said.
After much trial and error, the researchers have printed tablets that won’t break apart or become soggy.
Now they are testing how different laser scan speeds affect the structure of the tablet, which in turn affects the rate at which drugs dissolve. Slowing down the laser imparts more energy, strengthening the tablet structure and making drugs dissolve slower, for a longer release inside the body.
The researchers hope to develop machine learning models to test different laser speed combinations. Eventually, they could create tablets that combine drugs with different dissolve rates.
“The outside could be a rapid release, and the inside could be an extended release or a sustained release, or even a completely different drug,” Dr. Kuttolamadom said.
Older patients who take many daily medications could benefit from the technology. “Personalized tablets could be printed at your local pharmacy,” he said, “even before you leave your doctor’s office.”
A version of this article first appeared on WebMD.com.
Convincing kids to take their medicine could become much easier. Researchers at Texas A&M University are developing a new method of pharmaceutical 3D printing with pediatric patients in mind.
They hope to print precisely dosed tablets in child-friendly shapes and flavors. While the effort is focused on two drugs for pediatric AIDS, the process could be used to print other medicines, including for adults.
Researchers from Britain, Australia, and the University of Texas at Austin are also in the early stages of 3D-printed medication projects. It’s a promising venture in the broader pursuit of “personalized medicine,” tailoring treatments to each patient’s unique needs.
Drug mass production fails to address pediatric patients, who often need different dosages and combinations of medicines as they grow. As a result, adult tablets are often crushed and dissolved in liquid – known as compounding – and given to children. But this can harm drug quality and make doses less precise.
“Suppose the child needs 3.4 milligrams and only a 10-milligram tablet is available. Once you manipulate the dosage from solid to liquid, how do you ensure that it has the same amount of drug in it?” said co-principal investigator Mansoor Khan, PhD, a professor of pharmaceutical sciences at Texas A&M.
Most pharmacies lack the equipment to test compounded drug quality, he said. And liquified drugs taste bad because the pill coating has been ground away.
“Flavor is a big issue,” said Olive Eckstein, MD, an assistant professor of pediatric hematology-oncology at Texas Children’s Hospital and Baylor College of Medicine, who is not involved in the research. “Hospitals will sometimes delay discharging pediatric patients because they can’t take their meds orally and have to get an IV formulation.”
Updating pharmaceutical 3D printing
The FDA approved a 3D-printed drug in 2015, but since then, progress has stalled, largely because the method relied on solvents to bind drug particles together. Over time, solvents can compromise shelf life, according to co-principal investigator Mathew Kuttolamadom, PhD, an associate professor of engineering at Texas A&M.
The Texas A&M team is using a different method, without solvents. First, they create a powder mixture of the drug, a biocompatible polymer (such as lactose), and a sheen, a pigment that colors the tablet and allows heat to be absorbed. Flavoring can also be added. Next, the mixture is heated in the printer chamber.
“The polymer should melt just enough. That gives the tablet structural strength. But it should not melt too much, whereby the drug can start dissolving into the polymer,” Dr. Kuttolamadom said.
The tablets are finished with precise applications of laser heat. Using computer-aided design software, the researchers can create tablets in almost any shape, such as “stars or teddy bears,” he said.
After much trial and error, the researchers have printed tablets that won’t break apart or become soggy.
Now they are testing how different laser scan speeds affect the structure of the tablet, which in turn affects the rate at which drugs dissolve. Slowing down the laser imparts more energy, strengthening the tablet structure and making drugs dissolve slower, for a longer release inside the body.
The researchers hope to develop machine learning models to test different laser speed combinations. Eventually, they could create tablets that combine drugs with different dissolve rates.
“The outside could be a rapid release, and the inside could be an extended release or a sustained release, or even a completely different drug,” Dr. Kuttolamadom said.
Older patients who take many daily medications could benefit from the technology. “Personalized tablets could be printed at your local pharmacy,” he said, “even before you leave your doctor’s office.”
A version of this article first appeared on WebMD.com.
Fracture risk factors described in patients with ankylosing spondylitis
TOPLINE:
Opioid use, older age, and fracture history increase the risk for fractures in older adults with ankylosing spondylitis (AS) based on a review of registry and Medicare claims data.
METHODOLOGY:
- Rheumatology Informatics System for Effectiveness (RISE) registry data were linked to Medicare claims from 2016 to 2018; each patient had two AS International Classification of Diseases–9 and –10 codes at least 30 days apart.
- The study population included 1426 adults with AS (mean age, 69.4 years) who had continuous Medicare enrollment (Parts A and B) for the entire follow-up period but did not have Medicare Advantage Plan (Part C).
- The researchers used a logistic regression analysis to identify factors associated with fractures including age, sex, and body mass index.
TAKEAWAYS:
- The overall incidence of fractures was 76.7 per 1,000 person-years.
- Older age, history of fracture, and opioid use at a morphine-equivalent dose > 30 mg (at least one prescription 30 or more days prior to the index date) were significantly associated with increased risk for fracture (odds ratios, 2.8, 5.24, and 1.86, respectively).
- Fracture risk was equally likely for men and women.
IN PRACTICE:
The study supports fracture risk-reduction strategies for men and women with AS and a fracture history, with added attention to opioid users.
SOURCE:
The first author of the study was Rachael Stovall, MD, of the University of California, San Francisco. The study was published Aug. 22, 2023, in Arthritis Care & Research.
LIMITATIONS:
The study does not include individuals younger than 65 years and references only first fractures. Some EHR data on variables including race, body mass index, national area deprivation index, and smoking status are incomplete.
DISCLOSURES:
The study was supported by various grants from the National Center for Advancing Translational Sciences, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Rheumatology Research Foundation.
A version of this article first appeared on Medscape.com.
TOPLINE:
Opioid use, older age, and fracture history increase the risk for fractures in older adults with ankylosing spondylitis (AS) based on a review of registry and Medicare claims data.
METHODOLOGY:
- Rheumatology Informatics System for Effectiveness (RISE) registry data were linked to Medicare claims from 2016 to 2018; each patient had two AS International Classification of Diseases–9 and –10 codes at least 30 days apart.
- The study population included 1426 adults with AS (mean age, 69.4 years) who had continuous Medicare enrollment (Parts A and B) for the entire follow-up period but did not have Medicare Advantage Plan (Part C).
- The researchers used a logistic regression analysis to identify factors associated with fractures including age, sex, and body mass index.
TAKEAWAYS:
- The overall incidence of fractures was 76.7 per 1,000 person-years.
- Older age, history of fracture, and opioid use at a morphine-equivalent dose > 30 mg (at least one prescription 30 or more days prior to the index date) were significantly associated with increased risk for fracture (odds ratios, 2.8, 5.24, and 1.86, respectively).
- Fracture risk was equally likely for men and women.
IN PRACTICE:
The study supports fracture risk-reduction strategies for men and women with AS and a fracture history, with added attention to opioid users.
SOURCE:
The first author of the study was Rachael Stovall, MD, of the University of California, San Francisco. The study was published Aug. 22, 2023, in Arthritis Care & Research.
LIMITATIONS:
The study does not include individuals younger than 65 years and references only first fractures. Some EHR data on variables including race, body mass index, national area deprivation index, and smoking status are incomplete.
DISCLOSURES:
The study was supported by various grants from the National Center for Advancing Translational Sciences, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Rheumatology Research Foundation.
A version of this article first appeared on Medscape.com.
TOPLINE:
Opioid use, older age, and fracture history increase the risk for fractures in older adults with ankylosing spondylitis (AS) based on a review of registry and Medicare claims data.
METHODOLOGY:
- Rheumatology Informatics System for Effectiveness (RISE) registry data were linked to Medicare claims from 2016 to 2018; each patient had two AS International Classification of Diseases–9 and –10 codes at least 30 days apart.
- The study population included 1426 adults with AS (mean age, 69.4 years) who had continuous Medicare enrollment (Parts A and B) for the entire follow-up period but did not have Medicare Advantage Plan (Part C).
- The researchers used a logistic regression analysis to identify factors associated with fractures including age, sex, and body mass index.
TAKEAWAYS:
- The overall incidence of fractures was 76.7 per 1,000 person-years.
- Older age, history of fracture, and opioid use at a morphine-equivalent dose > 30 mg (at least one prescription 30 or more days prior to the index date) were significantly associated with increased risk for fracture (odds ratios, 2.8, 5.24, and 1.86, respectively).
- Fracture risk was equally likely for men and women.
IN PRACTICE:
The study supports fracture risk-reduction strategies for men and women with AS and a fracture history, with added attention to opioid users.
SOURCE:
The first author of the study was Rachael Stovall, MD, of the University of California, San Francisco. The study was published Aug. 22, 2023, in Arthritis Care & Research.
LIMITATIONS:
The study does not include individuals younger than 65 years and references only first fractures. Some EHR data on variables including race, body mass index, national area deprivation index, and smoking status are incomplete.
DISCLOSURES:
The study was supported by various grants from the National Center for Advancing Translational Sciences, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Rheumatology Research Foundation.
A version of this article first appeared on Medscape.com.
From Breakouts to Bargains: Strategies for Patient-Centered, Cost-effective Acne Care
In the United States, acne affects 85% of adolescents and can persist into adulthood at a prevalence of 30% to 50% in adult women. 1,2 The pathogenesis of acne is multifactorial and involves hyperkeratinization of the follicle, bacterial colonization with Cutibacterium acnes , and increased androgen-induced sebum production, which together lead to inflammation. 3,4 A wide range of treatment guideline–recommended options are available, including benzoyl peroxide (BPO), topical retinoids, topical and oral antibiotics, antiandrogens, and isotretinoin. 5 However, these options vary widely in their clinical uses, effectiveness, and costs.
Why Cost-effective Acne Care Matters
Out-of-pocket spending by patients on acne treatments can be substantial, with surveys finding that acne patients often spend hundreds to thousands of dollars per year.6,7 In a poll conducted in 2019 by the Kaiser Family Foundation, 3 in 10 patients said they had not taken their medicine as prescribed because of costs.8 A mixed methods study by Ryskina et al9 found that 65% (17/26) of participants who reported primary nonadherence—intended to fill prescriptions but were unable to do so—cited cost or coverage-related barriers as the reason. With the continued rise of dermatologic drug prices and increased prevalence of high-deductible health plans, cost-effective treatment continues to grow in importance. Failure to consider cost-effective, patient-centered care may lead to increased financial toxicity, reduced adherence, and ultimately worse outcomes and patient satisfaction. We aim to review the cost-effectiveness of current prescription therapies for acne management and highlight the most cost-effective approaches to patients with mild to moderate acne as well as moderate to severe acne.
In this review, we will take a value-oriented framework.10 Value can be defined as the cost per outcome of interest. Therefore, a treatment does not necessarily need to be inexpensive to provide high value if it delivers outstanding clinical outcomes. In addition, we will focus on incremental cost-effectiveness relative to common alternatives (eg, a retinoid could deliver high value relative to a vehicle but still provide limited value compared to other available retinoids if it is more expensive but not more efficacious). When possible, we present data from cost-effectiveness studies.11,12 We also use recent available price data obtained from GoodRx on August 11, 2023, to guide this discussion.13 However, as comparative-effectiveness and cost-effectiveness studies rarely are performed for acne medications, much of this discussion will be based on expert opinion.
Treatment Categories
Topical Retinoids—There currently are 4 topical retinoids that are approved by the US Food and Drug Administration (FDA) for the treatment of acne: tretinoin, tazarotene, trifarotene, and adapalene. These drugs are vitamin A derivatives that bind retinoic acid receptors and function as comedolytic and anti-inflammatory agents.5 In general, generic tretinoin and adapalene products have the lowest cost (Table).

In network meta-analyses, tretinoin and adapalene often are highly ranked topical treatment options with respect to efficacy.14 Combined with their low cost, generic tretinoin and adapalene likely are excellent initial options for topical therapy from the standpoint of cost-effectiveness.15 Adapalene may be preferred in many situations because of its better photostability and compatibility with BPO.
Due to the importance of the vehicle in determining retinoid tolerability, efforts have been made to use encapsulation and polymeric emulsion technology to improve tolerability. Recently, polymeric lotion formulations of tretinoin and tazarotene have become available. In a phase 2 study, tazarotene lotion 0.045% was found to have equivalent efficacy and superior tolerability to tazarotene cream 0.1%.16 Although head-to-head data are not available, it is likely that tretinoin lotion may offer similar tolerability improvements.17 Although these formulations currently are more costly, this improved tolerability may be critical for some patients to be able to use topical retinoids, and the additional cost may be worthwhile. In addition, as these products lose market exclusivity, they may become more affordable and similarly priced to other topical retinoids. It is important to keep in mind that in clinical trials of tretinoin and adapalene, rates of dropout due to adverse events typically were 1% to 2%; therefore, because many patients can tolerate generic tretinoin and adapalene, at current prices the lotion formulations of retinoids may not be cost-effective relative to these generics.14
Trifarotene cream 0.005%, a fourth-generation topical retinoid that is highly sensitive for retinoic acid receptor γ, recently was FDA approved for the treatment of acne. Although trifarotene is efficacious for both facial and truncal acne, there is a lack of active comparator data compared to other topical retinoids.18 In a 2023 network meta-analysis, trifarotene was found to be both less efficacious and less tolerable compared to other topical retinoids.19 Thus, it is unclear if trifarotene offers any improved efficacy compared to other options, and it comes at a much higher cost (Table). In a tolerability study, trifarotene was found to be significantly more irritating than tazarotene lotion 0.045% and adapalene gel 0.3% (P<.05).20 Therefore, trifarotene cream 0.005% is unlikely to be a cost-effective option; in fact, it may be overall inferior to other topical retinoids, given its potentially lower tolerability.
Topical Antibiotics—There are 4 commonly prescribed topical antibiotics that are approved by the FDA for the treatment of acne: clindamycin, erythromycin, dapsone, and minocycline. The American Academy of Dermatology guidelines for the treatment of acne recommend concomitant use of BPO to prevent antibiotic resistance.5 Clindamycin is favored over erythromycin because of increasing antibiotic resistance to erythromycin.21 Inexpensive generic options in multiple vehicles (eg, solution, foam, gel) make clindamycin a highly cost-effective option when antibiotic therapy is desired as part of a topical regimen (Table).
The cost-effectiveness of dapsone gel and minocycline foam relative to clindamycin are less certain. Rates of resistance to minocycline are lower than clindamycin, and minocycline foam may be a reasonable alternative in patients who have not had success with other topical antibiotics, such as clindamycin.22 However, given the absence of comparative effectiveness data to suggest minocycline is more effective than clindamycin, it is difficult to justify the substantially higher cost for the typical patient. Although dapsone gel has been suggested as an option for adult women with acne, there are no data to support that it is any more effective than other topical antibiotics in this patient population.23 As generic dapsone prices decrease, it may become a reasonable alternative to clindamycin. In addition, the antineutrophil properties of dapsone may be useful in other acneform and inflammatory eruptions, such as scalp folliculitis and folliculitis decalvans.24
Combination Topicals—Current combination topical products include antibiotic and BPO, antibiotic and retinoid, and retinoid and BPO. Use of combination agents is recommended to reduce the risk for resistance and to enhance effectiveness. Combination products offer improved convenience, which is associated with better adherence and outcomes.25 Generic fixed-dose adapalene-BPO can be a highly cost-effective option that can sometimes be less expensive than the individual component products (Table). Similarly, fixed-dose clindamycin-BPO also is likely to be highly cost-effective. A network meta-analysis found fixed-dose adapalene-BPO to be the most efficacious topical treatment, though it also was found to be the most irritating—more so than fixed-dose clindamycin-BPO, which may have similar efficacy.14,26,27 Generic fixed-dose tretinoin-clindamycin offers improved convenience and adherence compared to the individual components, but it is more expensive, and its cost-effectiveness may be influenced by the importance of convenience for the patient.25 An encapsulated, fixed-dose tretinoin 0.1%–BPO 3% cream is FDA approved for acne, but the cost is high and there is a lack of comparative effectiveness data demonstrating advantages over generic fixed-dose adapalene-BPO products.
Topical Antiandrogen—Clascoterone was introduced in 2020 as the first FDA-approved topical medication to target the hormonal pathogenesis of acne, inhibiting the androgen receptors in the sebaceous gland.28 Because it is rapidly metabolized to cortexolone and does not have systemic antiandrogen effects, clascoterone can be used in both men and women with acne. In clinical trials, it had minimal side effects, including no evidence of irritability, which is an advantage over topical retinoids and BPO.29 In addition, a phase 2 study found that clascoterone may have similar to superior efficacy to tretinoin cream 0.05%.30 Although clascoterone has several strengths, including its efficacy, tolerability, and unique mechanism of action, its cost-effectiveness is limited due to its high cost (Table) and the need for twice-daily application, which reduces convenience. Clascoterone likely is best reserved for patients with a strong hormonal pathogenesis of their acne or difficulty tolerating other topicals, or as an additional therapy to complement other topicals.
Oral Antibiotics—Oral antibiotics are the most commonly prescribed systemic treatments for acne, particularly tetracyclines such as doxycycline, minocycline, and sarecycline.31-34 Doxycycline and minocycline are considered first-line oral antibiotic therapy in the United States and are inexpensive and easily accessible.5 Doxycycline generally is recommended over minocycline given lack of evidence of superior efficacy of minocycline and concerns about severe adverse cutaneous reactions and drug-induced lupus with minocycline.35
In recent years, there has been growing concern of the development of antibiotic resistance.5 Sarecycline is a narrow-spectrum tetracycline that was FDA approved for acne in 2018. In vitro studies demonstrate sarecycline maintains high efficacy against C acnes with less activity against other bacteria, particularly gram-negative enterobes.36 The selectivity of sarecycline may lessen alterations of the gut microbiome seen with other oral antibiotics and reduce gastrointestinal tract side effects. Although comparative effectiveness studies are lacking, sarecycline was efficacious in phase 3 trials with few side effects compared with placebo.37 However, at this time, given the absence of comparative effectiveness data and its high cost (Table), sarecycline likely is best reserved for patients with comorbidities (eg, gastrointestinal disease), those requiring long-term antibiotic therapy, or those with acne that has failed to respond to other oral antibiotics.
Hormonal Treatments—Hormonal treatments such as combined oral contraceptives (COCs) and spironolactone often are considered second-line options, though they may represent cost-effective and safe alternatives to oral antibiotics for women with moderate to severe acne.38-41 There currently are 4 COCs approved by the FDA for the treatment of moderate acne in postmenarcheal females: drospirenone-ethinyl estradiol (Yaz [Bayer HealthCare Pharmaceuticals, Inc]), ethinyl estradiol-norgestimate (Ortho Tri-Cyclen [Ortho-McNeil Pharmaceuticals, Inc]), drospirenone-ethinyl estradiol-levomefolate (Beyaz [Bayer HealthCare Pharmaceuticals, Inc]), and ethinyl estradiol-norethindrone acetate-ferrous fumarate (Estrostep Fe [Allergan USA, Inc]).5 Treatment with COCs has been shown to cause substantial reductions in lesion counts across all lesion types compared to placebo, and a meta-analysis of 24 randomized trials conducted by Arowojolu et al42 demonstrated no consistent differences in acne reduction among different COCs.43,44 Although oral antibiotics are associated with faster improvement than COCs, there is some evidence that they have similar efficacy at 6 months of therapy.45 Combined oral contraceptives are inexpensive and likely reflect a highly cost-effective option (Table).
Spironolactone is an aldosterone inhibitor and androgen receptor blocker that is used off label to treat acne. It is one of the least expensive systemic medications for acne (Table). Although randomized controlled trials are lacking, several large case series support the effectiveness of spironolactone for women with acne.38,46 In addition, observational data suggest spironolactone may have similar effectiveness to oral antibiotics.41 Spironolactone generally is well tolerated, with the most common adverse effects being menstrual irregularities, breast tenderness, and diuresis.47,48 Many of these adverse effects are dose dependent and less likely with the dosing used in acne care. Additionally, menstrual irregularities can be reduced by concomitant use of a COC.48
Although frequent potassium monitoring remains common among patients being treated with spironolactone, there is growing evidence to suggest that potassium monitoring is of low value in young healthy women with acne.49-51 Reducing this laboratory monitoring likely represents an opportunity to provide higher-value care to patients being treated with spironolactone. However, laboratory monitoring should be considered if risk factors for hyperkalemia are present (eg, older age, comorbidities, medications).51
Isotretinoin—Isotretinoin is the most efficacious treatment available for acne and has the unique property of being able to induce a remission of acne activity for many patients.5 Although it remains modestly expensive (Table), it may be less costly overall relative to other treatments that may need continued use over many years because it can induce a remission of acne activity. As with spironolactone, frequent laboratory monitoring remains common among patients being treated with isotretinoin. There is no evidence to support checking complete blood cell counts.52 Several observational studies and a Delphi consensus support reduced monitoring, such as checking lipids and alanine aminotransferase at baseline and peak dose in otherwise young healthy patients.53,54 A recent critically appraised topic published in the British Journal of Dermatology has proposed eliminating laboratory monitoring entirely.55 Reducing laboratory monitoring for patients being treated with isotretinoin has been estimated to potentially save $100 million to $200 million per year in the United States.52-54
Other Strategies to Reduce Patient Costs
Although choosing a cost-effective treatment approach is critical to preventing financial toxicity given poor coverage for acne care and the growth of high-deductible insurance plans, some patients may still experience high treatment costs.56 Because pharmacy costs often are inflated, potentially related to practices of pharmacy benefit managers, it often is possible to find better prices than the presented list price, either by using platforms such as GoodRx or through direct-to-patient mail-order pharmacies such as Cost Plus Drug.57 For branded medications, some patients may be eligible for patient-assistance programs, though they typically are not available for those with public insurance such as Medicare or Medicaid. Compounding pharmacies offer another approach to reduce cost and improve convenience for patients, but because the vehicle can influence the efficacy and tolerability of some topical medications, it is possible that these compounded formulations may not perform similarly to the original FDA-approved products.
Conclusion
For mild to moderate acne, multimodal topical therapy often is required. Fixed-dose combination adapalene-BPO and clindamycin-BPO are highly cost-effective options for most patients. Lotion formulations of topical retinoids may be useful in patients with difficulty tolerating other formulations. Clascoterone is a novel topical antiandrogen that is more expensive than other topical therapies but can complement other topical therapies and is well tolerated.
For moderate to severe acne, doxycycline or hormonal therapy (ie, COCs, spironolactone) are highly cost-effective options. Isotretinoin is recommended for severe or scarring acne. Reduced laboratory monitoring for spironolactone and isotretinoin is an opportunity to provide higher-value care.
- Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485. doi:10.1111/bjd.12149
- Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59. doi:10.1016/j.jaad.2007.06.045
- Webster GF. The pathophysiology of acne. Cutis. 2005;76(2 suppl):4-7.
- Degitz K, Placzek M, Borelli C, et al. Pathophysiology of acne. J Dtsch Dermatol Ges. 2007;5:316-323. doi:10.1111/j.1610-0387.2007.06274.x
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi:10.1016/j.jaad.2015.12.037
- Felmingham C, Kerr A, Veysey E. Costs incurred by patients with acne prior to dermatological consultation and their relation to patient income. Australas J Dermatol. 2020;61:384-386. doi:10.1111/ajd.13324
- Perche P, Singh R, Feldman S. Patient preferences for acne vulgaris treatment and barriers to care: a survey study. J Drugs Dermatol. 2022;21:1191-1195. doi:10.36849/JDD.6940
- KFF Health Tracking Poll—February 2019. Accessed August 9, 2023. https://files.kff.org/attachment/Topline-KFF-Health-Tracking-Poll-February-2019
- Ryskina KL, Goldberg E, Lott B, et al. The role of the physician in patient perceptions of barriers to primary adherence with acne medications. JAMA Dermatol. 2018;154:456-459. doi:10.1001/jamadermatol.2017.6144
- Porter ME. What is value in health care? N Engl J Med. 2010;363:2477-2481. doi:10.1056/NEJMp1011024
- Barbieri JS, Tan JKL, Adamson AS. Active comparator trial designs used to promote development of innovative new medications. Cutis. 2020;106:E4-E6. doi:10.12788/cutis.0067
- Miller J, Ly S, Mostaghimi A, et al. Use of active comparator trials for topical medications in dermatology. JAMA Dermatol. 2021;157:597-599. doi:10.1001/jamadermatol.2021.0356
- GoodRx. Accessed August 11, 2023. https://www.goodrx.com
- Stuart B, Maund E, Wilcox C, et al. Topical preparations for the treatment of mild‐to‐moderate acne vulgaris: systematic review and network meta‐analysis. Br J Dermatol. 2021;185:512-525. doi:10.1111/bjd.20080
- Mavranezouli I, Welton NJ, Daly CH, et al. Cost-effectiveness of topical pharmacological, oral pharmacological, physical and combined treatments for acne vulgaris. Clin Exp Dermatol. 2022;47:2176-2187. doi:10.1111/ced.15356
- Tanghetti E, Werschler W, Lain T, et al. Tazarotene 0.045% lotion for once-daily treatment of moderate-to-severe acne vulgaris: results from two phase 3 trials. J Drugs Dermatol. 2020;19:70-77. doi:10.36849/JDD.2020.3977
- Tyring SK, Kircik LH, Pariser DM, et al. Novel tretinoin 0.05% lotion for the once-daily treatment of moderate-to-severe acne vulgaris: assessment of efficacy and safety in patients aged 9 years and older. J Drugs Dermatol. 2018;17:1084-1091.
- Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 μg/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80:1691-1699. doi:10.1016/j.jaad.2019.02.044
- Huang CY, Chang IJ, Bolick N, et al. Comparative efficacy of pharmacological treatments for acne vulgaris: a network meta-analysis of 221 randomized controlled trials. Ann Fam Med. 2023;21:358-369. doi:10.1370/afm.2995
- Draelos ZD. Low irritation potential of tazarotene 0.045% lotion: head-to-head comparison to adapalene 0.3% gel and trifarotene 0.005% cream in two studies. J Dermatolog Treat. 2023;34:2166346. doi:10.1080/09546634.2023.2166346
- Dessinioti C, Katsambas A. Antibiotics and antimicrobial resistance in acne: epidemiological trends and clinical practice considerations. Yale J Biol Med. 2022;95:429-443.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;80:168-177. doi:10.1016/j.jaad.2018.08.020
- Wang X, Wang Z, Sun L, et al. Efficacy and safety of dapsone gel for acne: a systematic review and meta-analysis. Ann Palliat Med. 2022;11:611-620. doi:10.21037/apm-21-3935
- Melián-Olivera A, Burgos-Blasco P, Selda-Enríquez G, et al. Topical dapsone for folliculitis decalvans: a retrospective cohort study. J Am Acad Dermatol. 2022;87:150-151. doi:10.1016/j.jaad.2021.07.004
- Yentzer BA, Ade RA, Fountain JM, et al. Simplifying regimens promotes greater adherence and outcomes with topical acne medications: a randomized controlled trial. Cutis. 2010;86:103-108.
- Ting W. Randomized, observer-blind, split-face study to compare the irritation potential of 2 topical acne formulations over a 14-day treatment period. Cutis. 2012;90:91-96.
- Aschoff R, Möller S, Haase R, et al. Tolerability and efficacy ofclindamycin/tretinoin versus adapalene/benzoyl peroxide in the treatment of acne vulgaris. J Drugs Dermatol. 2021;20:295-301. doi:10.36849/JDD.2021.5641
- Rosette C, Agan FJ, Mazzetti A, et al. Cortexolone 17α-propionate (clascoterone) is a novel androgen receptor antagonist that inhibits production of lipids and inflammatory cytokines from sebocytes in vitro. J Drugs Dermatol. 2019;18:412-418.
- Hebert A, Thiboutot D, Stein Gold L, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630. doi:10.1001/jamadermatol.2020.0465
- Trifu V, Tiplica GS, Naumescu E, et al. Cortexolone 17α-propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. a pilot randomized, double-blind comparative study vs. placebo and tretinoin 0·05% cream. Br J Dermatol. 2011;165:177-183. doi:10.1111/j.1365-2133.2011.10332.x
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Guzman AK, Barbieri JS. Comparative analysis of prescribing patterns of tetracycline class antibiotics and spironolactone between advanced practice providers and physicians in the treatment of acne vulgaris. J Am Acad Dermatol. 2021;84:1119-1121. doi:10.1016/j.jaad.2020.06.044
- Barbieri JS, James WD, Margolis DJ. Trends in prescribing behavior of systemic agents used in the treatment of acne among dermatologists and nondermatologists: a retrospective analysis, 2004-2013. J Am Acad Dermatol. 2017;77:456-463.e4. doi:10.1016/j.jaad.2017.04.016
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297. doi:10.1001/jamadermatol.2018.4944
- Garner SE, Eady A, Bennett C, et al. Minocycline for acne vulgaris: efficacy and safety. Cochrane Database Syst Rev. 2012;2012:CD002086. doi:10.1002/14651858.CD002086.pub2
- Zhanel G, Critchley I, Lin LY, et al. Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob Agents Chemother. 2018;63:e01297-18. doi:10.1128/AAC.01297-18
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris: results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17:987-996.
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Barbieri JS, Choi JK, James WD, et al. Real-world drug usage survival of spironolactone versus oral antibiotics for the management of female patients with acne. J Am Acad Dermatol. 2019;81:848-851. doi:10.1016/j.jaad.2019.03.036
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments. J Am Acad Dermatol. 2019;80:538-549. doi:10.1016/j.jaad.2018.09.055
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;7:CD004425. doi:10.1002/14651858.CD004425.pub6
- Maloney JM, Dietze P, Watson D, et al. Treatment of acne using a 3-milligram drospirenone/20-microgram ethinyl estradiol oral contraceptive administered in a 24/4 regimen. Obstet Gynecol. 2008;112:773-781. doi:10.1097/AOG.0b013e318187e1c5
- Lucky AW, Koltun W, Thiboutot D, et al. A combined oral contraceptive containing 3-mg drospirenone/20-microg ethinyl estradiol in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled study evaluating lesion counts and participant self-assessment. Cutis. 2008;82:143-150.
- Koo EB, Petersen TD, Kimball AB. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J Am Acad Dermatol. 2014;71:450-459. doi:10.1016/j.jaad.2014.03.051
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502. doi:10.1067/mjd.2000.105557
- Layton AM, Eady EA, Whitehouse H, et al. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18:169-191. doi:10.1007/s40257-016-0245-x
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300. doi:10.1001/jamadermatol.2020.5468
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944. doi:10.1001/jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Barbieri JS, Shin DB, Wang S, et al. The clinical utility of laboratory monitoring during isotretinoin therapy for acne and changes to monitoring practices over time. J Am Acad Dermatol. 2020;82:72-79. doi:10.1016/j.jaad.2019.06.025
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory monitoring during isotretinoin therapy for acne: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:35-44. doi:10.1001/jamadermatol.2015.3091
- Xia E, Han J, Faletsky A, et al. Isotretinoin laboratory monitoring in acne treatment: a Delphi consensus study. JAMA Dermatol. 2022;158:942-948. doi:10.1001/jamadermatol.2022.2044
- Affleck A, Jackson D, Williams HC, et al. Is routine laboratory testing in healthy young patients taking isotretinoin necessary: a critically appraised topic. Br J Dermatol. 2022;187:857-865. doi:10.1111/bjd.21840
- Barbieri JS, LaChance A, Albrecht J. Double standards and inconsistencies in access to care-what constitutes a cosmetic treatment? JAMA Dermatol. 2023;159:245-246. doi:10.1001/jamadermatol.2022.6322
- Trish E, Van Nuys K, Popovian R. US consumers overpay for generic drugs. Schaeffer Center White Paper Series. May 31, 2022. doi:10.25549/m589-2268
In the United States, acne affects 85% of adolescents and can persist into adulthood at a prevalence of 30% to 50% in adult women. 1,2 The pathogenesis of acne is multifactorial and involves hyperkeratinization of the follicle, bacterial colonization with Cutibacterium acnes , and increased androgen-induced sebum production, which together lead to inflammation. 3,4 A wide range of treatment guideline–recommended options are available, including benzoyl peroxide (BPO), topical retinoids, topical and oral antibiotics, antiandrogens, and isotretinoin. 5 However, these options vary widely in their clinical uses, effectiveness, and costs.
Why Cost-effective Acne Care Matters
Out-of-pocket spending by patients on acne treatments can be substantial, with surveys finding that acne patients often spend hundreds to thousands of dollars per year.6,7 In a poll conducted in 2019 by the Kaiser Family Foundation, 3 in 10 patients said they had not taken their medicine as prescribed because of costs.8 A mixed methods study by Ryskina et al9 found that 65% (17/26) of participants who reported primary nonadherence—intended to fill prescriptions but were unable to do so—cited cost or coverage-related barriers as the reason. With the continued rise of dermatologic drug prices and increased prevalence of high-deductible health plans, cost-effective treatment continues to grow in importance. Failure to consider cost-effective, patient-centered care may lead to increased financial toxicity, reduced adherence, and ultimately worse outcomes and patient satisfaction. We aim to review the cost-effectiveness of current prescription therapies for acne management and highlight the most cost-effective approaches to patients with mild to moderate acne as well as moderate to severe acne.
In this review, we will take a value-oriented framework.10 Value can be defined as the cost per outcome of interest. Therefore, a treatment does not necessarily need to be inexpensive to provide high value if it delivers outstanding clinical outcomes. In addition, we will focus on incremental cost-effectiveness relative to common alternatives (eg, a retinoid could deliver high value relative to a vehicle but still provide limited value compared to other available retinoids if it is more expensive but not more efficacious). When possible, we present data from cost-effectiveness studies.11,12 We also use recent available price data obtained from GoodRx on August 11, 2023, to guide this discussion.13 However, as comparative-effectiveness and cost-effectiveness studies rarely are performed for acne medications, much of this discussion will be based on expert opinion.
Treatment Categories
Topical Retinoids—There currently are 4 topical retinoids that are approved by the US Food and Drug Administration (FDA) for the treatment of acne: tretinoin, tazarotene, trifarotene, and adapalene. These drugs are vitamin A derivatives that bind retinoic acid receptors and function as comedolytic and anti-inflammatory agents.5 In general, generic tretinoin and adapalene products have the lowest cost (Table).

In network meta-analyses, tretinoin and adapalene often are highly ranked topical treatment options with respect to efficacy.14 Combined with their low cost, generic tretinoin and adapalene likely are excellent initial options for topical therapy from the standpoint of cost-effectiveness.15 Adapalene may be preferred in many situations because of its better photostability and compatibility with BPO.
Due to the importance of the vehicle in determining retinoid tolerability, efforts have been made to use encapsulation and polymeric emulsion technology to improve tolerability. Recently, polymeric lotion formulations of tretinoin and tazarotene have become available. In a phase 2 study, tazarotene lotion 0.045% was found to have equivalent efficacy and superior tolerability to tazarotene cream 0.1%.16 Although head-to-head data are not available, it is likely that tretinoin lotion may offer similar tolerability improvements.17 Although these formulations currently are more costly, this improved tolerability may be critical for some patients to be able to use topical retinoids, and the additional cost may be worthwhile. In addition, as these products lose market exclusivity, they may become more affordable and similarly priced to other topical retinoids. It is important to keep in mind that in clinical trials of tretinoin and adapalene, rates of dropout due to adverse events typically were 1% to 2%; therefore, because many patients can tolerate generic tretinoin and adapalene, at current prices the lotion formulations of retinoids may not be cost-effective relative to these generics.14
Trifarotene cream 0.005%, a fourth-generation topical retinoid that is highly sensitive for retinoic acid receptor γ, recently was FDA approved for the treatment of acne. Although trifarotene is efficacious for both facial and truncal acne, there is a lack of active comparator data compared to other topical retinoids.18 In a 2023 network meta-analysis, trifarotene was found to be both less efficacious and less tolerable compared to other topical retinoids.19 Thus, it is unclear if trifarotene offers any improved efficacy compared to other options, and it comes at a much higher cost (Table). In a tolerability study, trifarotene was found to be significantly more irritating than tazarotene lotion 0.045% and adapalene gel 0.3% (P<.05).20 Therefore, trifarotene cream 0.005% is unlikely to be a cost-effective option; in fact, it may be overall inferior to other topical retinoids, given its potentially lower tolerability.
Topical Antibiotics—There are 4 commonly prescribed topical antibiotics that are approved by the FDA for the treatment of acne: clindamycin, erythromycin, dapsone, and minocycline. The American Academy of Dermatology guidelines for the treatment of acne recommend concomitant use of BPO to prevent antibiotic resistance.5 Clindamycin is favored over erythromycin because of increasing antibiotic resistance to erythromycin.21 Inexpensive generic options in multiple vehicles (eg, solution, foam, gel) make clindamycin a highly cost-effective option when antibiotic therapy is desired as part of a topical regimen (Table).
The cost-effectiveness of dapsone gel and minocycline foam relative to clindamycin are less certain. Rates of resistance to minocycline are lower than clindamycin, and minocycline foam may be a reasonable alternative in patients who have not had success with other topical antibiotics, such as clindamycin.22 However, given the absence of comparative effectiveness data to suggest minocycline is more effective than clindamycin, it is difficult to justify the substantially higher cost for the typical patient. Although dapsone gel has been suggested as an option for adult women with acne, there are no data to support that it is any more effective than other topical antibiotics in this patient population.23 As generic dapsone prices decrease, it may become a reasonable alternative to clindamycin. In addition, the antineutrophil properties of dapsone may be useful in other acneform and inflammatory eruptions, such as scalp folliculitis and folliculitis decalvans.24
Combination Topicals—Current combination topical products include antibiotic and BPO, antibiotic and retinoid, and retinoid and BPO. Use of combination agents is recommended to reduce the risk for resistance and to enhance effectiveness. Combination products offer improved convenience, which is associated with better adherence and outcomes.25 Generic fixed-dose adapalene-BPO can be a highly cost-effective option that can sometimes be less expensive than the individual component products (Table). Similarly, fixed-dose clindamycin-BPO also is likely to be highly cost-effective. A network meta-analysis found fixed-dose adapalene-BPO to be the most efficacious topical treatment, though it also was found to be the most irritating—more so than fixed-dose clindamycin-BPO, which may have similar efficacy.14,26,27 Generic fixed-dose tretinoin-clindamycin offers improved convenience and adherence compared to the individual components, but it is more expensive, and its cost-effectiveness may be influenced by the importance of convenience for the patient.25 An encapsulated, fixed-dose tretinoin 0.1%–BPO 3% cream is FDA approved for acne, but the cost is high and there is a lack of comparative effectiveness data demonstrating advantages over generic fixed-dose adapalene-BPO products.
Topical Antiandrogen—Clascoterone was introduced in 2020 as the first FDA-approved topical medication to target the hormonal pathogenesis of acne, inhibiting the androgen receptors in the sebaceous gland.28 Because it is rapidly metabolized to cortexolone and does not have systemic antiandrogen effects, clascoterone can be used in both men and women with acne. In clinical trials, it had minimal side effects, including no evidence of irritability, which is an advantage over topical retinoids and BPO.29 In addition, a phase 2 study found that clascoterone may have similar to superior efficacy to tretinoin cream 0.05%.30 Although clascoterone has several strengths, including its efficacy, tolerability, and unique mechanism of action, its cost-effectiveness is limited due to its high cost (Table) and the need for twice-daily application, which reduces convenience. Clascoterone likely is best reserved for patients with a strong hormonal pathogenesis of their acne or difficulty tolerating other topicals, or as an additional therapy to complement other topicals.
Oral Antibiotics—Oral antibiotics are the most commonly prescribed systemic treatments for acne, particularly tetracyclines such as doxycycline, minocycline, and sarecycline.31-34 Doxycycline and minocycline are considered first-line oral antibiotic therapy in the United States and are inexpensive and easily accessible.5 Doxycycline generally is recommended over minocycline given lack of evidence of superior efficacy of minocycline and concerns about severe adverse cutaneous reactions and drug-induced lupus with minocycline.35
In recent years, there has been growing concern of the development of antibiotic resistance.5 Sarecycline is a narrow-spectrum tetracycline that was FDA approved for acne in 2018. In vitro studies demonstrate sarecycline maintains high efficacy against C acnes with less activity against other bacteria, particularly gram-negative enterobes.36 The selectivity of sarecycline may lessen alterations of the gut microbiome seen with other oral antibiotics and reduce gastrointestinal tract side effects. Although comparative effectiveness studies are lacking, sarecycline was efficacious in phase 3 trials with few side effects compared with placebo.37 However, at this time, given the absence of comparative effectiveness data and its high cost (Table), sarecycline likely is best reserved for patients with comorbidities (eg, gastrointestinal disease), those requiring long-term antibiotic therapy, or those with acne that has failed to respond to other oral antibiotics.
Hormonal Treatments—Hormonal treatments such as combined oral contraceptives (COCs) and spironolactone often are considered second-line options, though they may represent cost-effective and safe alternatives to oral antibiotics for women with moderate to severe acne.38-41 There currently are 4 COCs approved by the FDA for the treatment of moderate acne in postmenarcheal females: drospirenone-ethinyl estradiol (Yaz [Bayer HealthCare Pharmaceuticals, Inc]), ethinyl estradiol-norgestimate (Ortho Tri-Cyclen [Ortho-McNeil Pharmaceuticals, Inc]), drospirenone-ethinyl estradiol-levomefolate (Beyaz [Bayer HealthCare Pharmaceuticals, Inc]), and ethinyl estradiol-norethindrone acetate-ferrous fumarate (Estrostep Fe [Allergan USA, Inc]).5 Treatment with COCs has been shown to cause substantial reductions in lesion counts across all lesion types compared to placebo, and a meta-analysis of 24 randomized trials conducted by Arowojolu et al42 demonstrated no consistent differences in acne reduction among different COCs.43,44 Although oral antibiotics are associated with faster improvement than COCs, there is some evidence that they have similar efficacy at 6 months of therapy.45 Combined oral contraceptives are inexpensive and likely reflect a highly cost-effective option (Table).
Spironolactone is an aldosterone inhibitor and androgen receptor blocker that is used off label to treat acne. It is one of the least expensive systemic medications for acne (Table). Although randomized controlled trials are lacking, several large case series support the effectiveness of spironolactone for women with acne.38,46 In addition, observational data suggest spironolactone may have similar effectiveness to oral antibiotics.41 Spironolactone generally is well tolerated, with the most common adverse effects being menstrual irregularities, breast tenderness, and diuresis.47,48 Many of these adverse effects are dose dependent and less likely with the dosing used in acne care. Additionally, menstrual irregularities can be reduced by concomitant use of a COC.48
Although frequent potassium monitoring remains common among patients being treated with spironolactone, there is growing evidence to suggest that potassium monitoring is of low value in young healthy women with acne.49-51 Reducing this laboratory monitoring likely represents an opportunity to provide higher-value care to patients being treated with spironolactone. However, laboratory monitoring should be considered if risk factors for hyperkalemia are present (eg, older age, comorbidities, medications).51
Isotretinoin—Isotretinoin is the most efficacious treatment available for acne and has the unique property of being able to induce a remission of acne activity for many patients.5 Although it remains modestly expensive (Table), it may be less costly overall relative to other treatments that may need continued use over many years because it can induce a remission of acne activity. As with spironolactone, frequent laboratory monitoring remains common among patients being treated with isotretinoin. There is no evidence to support checking complete blood cell counts.52 Several observational studies and a Delphi consensus support reduced monitoring, such as checking lipids and alanine aminotransferase at baseline and peak dose in otherwise young healthy patients.53,54 A recent critically appraised topic published in the British Journal of Dermatology has proposed eliminating laboratory monitoring entirely.55 Reducing laboratory monitoring for patients being treated with isotretinoin has been estimated to potentially save $100 million to $200 million per year in the United States.52-54
Other Strategies to Reduce Patient Costs
Although choosing a cost-effective treatment approach is critical to preventing financial toxicity given poor coverage for acne care and the growth of high-deductible insurance plans, some patients may still experience high treatment costs.56 Because pharmacy costs often are inflated, potentially related to practices of pharmacy benefit managers, it often is possible to find better prices than the presented list price, either by using platforms such as GoodRx or through direct-to-patient mail-order pharmacies such as Cost Plus Drug.57 For branded medications, some patients may be eligible for patient-assistance programs, though they typically are not available for those with public insurance such as Medicare or Medicaid. Compounding pharmacies offer another approach to reduce cost and improve convenience for patients, but because the vehicle can influence the efficacy and tolerability of some topical medications, it is possible that these compounded formulations may not perform similarly to the original FDA-approved products.
Conclusion
For mild to moderate acne, multimodal topical therapy often is required. Fixed-dose combination adapalene-BPO and clindamycin-BPO are highly cost-effective options for most patients. Lotion formulations of topical retinoids may be useful in patients with difficulty tolerating other formulations. Clascoterone is a novel topical antiandrogen that is more expensive than other topical therapies but can complement other topical therapies and is well tolerated.
For moderate to severe acne, doxycycline or hormonal therapy (ie, COCs, spironolactone) are highly cost-effective options. Isotretinoin is recommended for severe or scarring acne. Reduced laboratory monitoring for spironolactone and isotretinoin is an opportunity to provide higher-value care.
In the United States, acne affects 85% of adolescents and can persist into adulthood at a prevalence of 30% to 50% in adult women. 1,2 The pathogenesis of acne is multifactorial and involves hyperkeratinization of the follicle, bacterial colonization with Cutibacterium acnes , and increased androgen-induced sebum production, which together lead to inflammation. 3,4 A wide range of treatment guideline–recommended options are available, including benzoyl peroxide (BPO), topical retinoids, topical and oral antibiotics, antiandrogens, and isotretinoin. 5 However, these options vary widely in their clinical uses, effectiveness, and costs.
Why Cost-effective Acne Care Matters
Out-of-pocket spending by patients on acne treatments can be substantial, with surveys finding that acne patients often spend hundreds to thousands of dollars per year.6,7 In a poll conducted in 2019 by the Kaiser Family Foundation, 3 in 10 patients said they had not taken their medicine as prescribed because of costs.8 A mixed methods study by Ryskina et al9 found that 65% (17/26) of participants who reported primary nonadherence—intended to fill prescriptions but were unable to do so—cited cost or coverage-related barriers as the reason. With the continued rise of dermatologic drug prices and increased prevalence of high-deductible health plans, cost-effective treatment continues to grow in importance. Failure to consider cost-effective, patient-centered care may lead to increased financial toxicity, reduced adherence, and ultimately worse outcomes and patient satisfaction. We aim to review the cost-effectiveness of current prescription therapies for acne management and highlight the most cost-effective approaches to patients with mild to moderate acne as well as moderate to severe acne.
In this review, we will take a value-oriented framework.10 Value can be defined as the cost per outcome of interest. Therefore, a treatment does not necessarily need to be inexpensive to provide high value if it delivers outstanding clinical outcomes. In addition, we will focus on incremental cost-effectiveness relative to common alternatives (eg, a retinoid could deliver high value relative to a vehicle but still provide limited value compared to other available retinoids if it is more expensive but not more efficacious). When possible, we present data from cost-effectiveness studies.11,12 We also use recent available price data obtained from GoodRx on August 11, 2023, to guide this discussion.13 However, as comparative-effectiveness and cost-effectiveness studies rarely are performed for acne medications, much of this discussion will be based on expert opinion.
Treatment Categories
Topical Retinoids—There currently are 4 topical retinoids that are approved by the US Food and Drug Administration (FDA) for the treatment of acne: tretinoin, tazarotene, trifarotene, and adapalene. These drugs are vitamin A derivatives that bind retinoic acid receptors and function as comedolytic and anti-inflammatory agents.5 In general, generic tretinoin and adapalene products have the lowest cost (Table).

In network meta-analyses, tretinoin and adapalene often are highly ranked topical treatment options with respect to efficacy.14 Combined with their low cost, generic tretinoin and adapalene likely are excellent initial options for topical therapy from the standpoint of cost-effectiveness.15 Adapalene may be preferred in many situations because of its better photostability and compatibility with BPO.
Due to the importance of the vehicle in determining retinoid tolerability, efforts have been made to use encapsulation and polymeric emulsion technology to improve tolerability. Recently, polymeric lotion formulations of tretinoin and tazarotene have become available. In a phase 2 study, tazarotene lotion 0.045% was found to have equivalent efficacy and superior tolerability to tazarotene cream 0.1%.16 Although head-to-head data are not available, it is likely that tretinoin lotion may offer similar tolerability improvements.17 Although these formulations currently are more costly, this improved tolerability may be critical for some patients to be able to use topical retinoids, and the additional cost may be worthwhile. In addition, as these products lose market exclusivity, they may become more affordable and similarly priced to other topical retinoids. It is important to keep in mind that in clinical trials of tretinoin and adapalene, rates of dropout due to adverse events typically were 1% to 2%; therefore, because many patients can tolerate generic tretinoin and adapalene, at current prices the lotion formulations of retinoids may not be cost-effective relative to these generics.14
Trifarotene cream 0.005%, a fourth-generation topical retinoid that is highly sensitive for retinoic acid receptor γ, recently was FDA approved for the treatment of acne. Although trifarotene is efficacious for both facial and truncal acne, there is a lack of active comparator data compared to other topical retinoids.18 In a 2023 network meta-analysis, trifarotene was found to be both less efficacious and less tolerable compared to other topical retinoids.19 Thus, it is unclear if trifarotene offers any improved efficacy compared to other options, and it comes at a much higher cost (Table). In a tolerability study, trifarotene was found to be significantly more irritating than tazarotene lotion 0.045% and adapalene gel 0.3% (P<.05).20 Therefore, trifarotene cream 0.005% is unlikely to be a cost-effective option; in fact, it may be overall inferior to other topical retinoids, given its potentially lower tolerability.
Topical Antibiotics—There are 4 commonly prescribed topical antibiotics that are approved by the FDA for the treatment of acne: clindamycin, erythromycin, dapsone, and minocycline. The American Academy of Dermatology guidelines for the treatment of acne recommend concomitant use of BPO to prevent antibiotic resistance.5 Clindamycin is favored over erythromycin because of increasing antibiotic resistance to erythromycin.21 Inexpensive generic options in multiple vehicles (eg, solution, foam, gel) make clindamycin a highly cost-effective option when antibiotic therapy is desired as part of a topical regimen (Table).
The cost-effectiveness of dapsone gel and minocycline foam relative to clindamycin are less certain. Rates of resistance to minocycline are lower than clindamycin, and minocycline foam may be a reasonable alternative in patients who have not had success with other topical antibiotics, such as clindamycin.22 However, given the absence of comparative effectiveness data to suggest minocycline is more effective than clindamycin, it is difficult to justify the substantially higher cost for the typical patient. Although dapsone gel has been suggested as an option for adult women with acne, there are no data to support that it is any more effective than other topical antibiotics in this patient population.23 As generic dapsone prices decrease, it may become a reasonable alternative to clindamycin. In addition, the antineutrophil properties of dapsone may be useful in other acneform and inflammatory eruptions, such as scalp folliculitis and folliculitis decalvans.24
Combination Topicals—Current combination topical products include antibiotic and BPO, antibiotic and retinoid, and retinoid and BPO. Use of combination agents is recommended to reduce the risk for resistance and to enhance effectiveness. Combination products offer improved convenience, which is associated with better adherence and outcomes.25 Generic fixed-dose adapalene-BPO can be a highly cost-effective option that can sometimes be less expensive than the individual component products (Table). Similarly, fixed-dose clindamycin-BPO also is likely to be highly cost-effective. A network meta-analysis found fixed-dose adapalene-BPO to be the most efficacious topical treatment, though it also was found to be the most irritating—more so than fixed-dose clindamycin-BPO, which may have similar efficacy.14,26,27 Generic fixed-dose tretinoin-clindamycin offers improved convenience and adherence compared to the individual components, but it is more expensive, and its cost-effectiveness may be influenced by the importance of convenience for the patient.25 An encapsulated, fixed-dose tretinoin 0.1%–BPO 3% cream is FDA approved for acne, but the cost is high and there is a lack of comparative effectiveness data demonstrating advantages over generic fixed-dose adapalene-BPO products.
Topical Antiandrogen—Clascoterone was introduced in 2020 as the first FDA-approved topical medication to target the hormonal pathogenesis of acne, inhibiting the androgen receptors in the sebaceous gland.28 Because it is rapidly metabolized to cortexolone and does not have systemic antiandrogen effects, clascoterone can be used in both men and women with acne. In clinical trials, it had minimal side effects, including no evidence of irritability, which is an advantage over topical retinoids and BPO.29 In addition, a phase 2 study found that clascoterone may have similar to superior efficacy to tretinoin cream 0.05%.30 Although clascoterone has several strengths, including its efficacy, tolerability, and unique mechanism of action, its cost-effectiveness is limited due to its high cost (Table) and the need for twice-daily application, which reduces convenience. Clascoterone likely is best reserved for patients with a strong hormonal pathogenesis of their acne or difficulty tolerating other topicals, or as an additional therapy to complement other topicals.
Oral Antibiotics—Oral antibiotics are the most commonly prescribed systemic treatments for acne, particularly tetracyclines such as doxycycline, minocycline, and sarecycline.31-34 Doxycycline and minocycline are considered first-line oral antibiotic therapy in the United States and are inexpensive and easily accessible.5 Doxycycline generally is recommended over minocycline given lack of evidence of superior efficacy of minocycline and concerns about severe adverse cutaneous reactions and drug-induced lupus with minocycline.35
In recent years, there has been growing concern of the development of antibiotic resistance.5 Sarecycline is a narrow-spectrum tetracycline that was FDA approved for acne in 2018. In vitro studies demonstrate sarecycline maintains high efficacy against C acnes with less activity against other bacteria, particularly gram-negative enterobes.36 The selectivity of sarecycline may lessen alterations of the gut microbiome seen with other oral antibiotics and reduce gastrointestinal tract side effects. Although comparative effectiveness studies are lacking, sarecycline was efficacious in phase 3 trials with few side effects compared with placebo.37 However, at this time, given the absence of comparative effectiveness data and its high cost (Table), sarecycline likely is best reserved for patients with comorbidities (eg, gastrointestinal disease), those requiring long-term antibiotic therapy, or those with acne that has failed to respond to other oral antibiotics.
Hormonal Treatments—Hormonal treatments such as combined oral contraceptives (COCs) and spironolactone often are considered second-line options, though they may represent cost-effective and safe alternatives to oral antibiotics for women with moderate to severe acne.38-41 There currently are 4 COCs approved by the FDA for the treatment of moderate acne in postmenarcheal females: drospirenone-ethinyl estradiol (Yaz [Bayer HealthCare Pharmaceuticals, Inc]), ethinyl estradiol-norgestimate (Ortho Tri-Cyclen [Ortho-McNeil Pharmaceuticals, Inc]), drospirenone-ethinyl estradiol-levomefolate (Beyaz [Bayer HealthCare Pharmaceuticals, Inc]), and ethinyl estradiol-norethindrone acetate-ferrous fumarate (Estrostep Fe [Allergan USA, Inc]).5 Treatment with COCs has been shown to cause substantial reductions in lesion counts across all lesion types compared to placebo, and a meta-analysis of 24 randomized trials conducted by Arowojolu et al42 demonstrated no consistent differences in acne reduction among different COCs.43,44 Although oral antibiotics are associated with faster improvement than COCs, there is some evidence that they have similar efficacy at 6 months of therapy.45 Combined oral contraceptives are inexpensive and likely reflect a highly cost-effective option (Table).
Spironolactone is an aldosterone inhibitor and androgen receptor blocker that is used off label to treat acne. It is one of the least expensive systemic medications for acne (Table). Although randomized controlled trials are lacking, several large case series support the effectiveness of spironolactone for women with acne.38,46 In addition, observational data suggest spironolactone may have similar effectiveness to oral antibiotics.41 Spironolactone generally is well tolerated, with the most common adverse effects being menstrual irregularities, breast tenderness, and diuresis.47,48 Many of these adverse effects are dose dependent and less likely with the dosing used in acne care. Additionally, menstrual irregularities can be reduced by concomitant use of a COC.48
Although frequent potassium monitoring remains common among patients being treated with spironolactone, there is growing evidence to suggest that potassium monitoring is of low value in young healthy women with acne.49-51 Reducing this laboratory monitoring likely represents an opportunity to provide higher-value care to patients being treated with spironolactone. However, laboratory monitoring should be considered if risk factors for hyperkalemia are present (eg, older age, comorbidities, medications).51
Isotretinoin—Isotretinoin is the most efficacious treatment available for acne and has the unique property of being able to induce a remission of acne activity for many patients.5 Although it remains modestly expensive (Table), it may be less costly overall relative to other treatments that may need continued use over many years because it can induce a remission of acne activity. As with spironolactone, frequent laboratory monitoring remains common among patients being treated with isotretinoin. There is no evidence to support checking complete blood cell counts.52 Several observational studies and a Delphi consensus support reduced monitoring, such as checking lipids and alanine aminotransferase at baseline and peak dose in otherwise young healthy patients.53,54 A recent critically appraised topic published in the British Journal of Dermatology has proposed eliminating laboratory monitoring entirely.55 Reducing laboratory monitoring for patients being treated with isotretinoin has been estimated to potentially save $100 million to $200 million per year in the United States.52-54
Other Strategies to Reduce Patient Costs
Although choosing a cost-effective treatment approach is critical to preventing financial toxicity given poor coverage for acne care and the growth of high-deductible insurance plans, some patients may still experience high treatment costs.56 Because pharmacy costs often are inflated, potentially related to practices of pharmacy benefit managers, it often is possible to find better prices than the presented list price, either by using platforms such as GoodRx or through direct-to-patient mail-order pharmacies such as Cost Plus Drug.57 For branded medications, some patients may be eligible for patient-assistance programs, though they typically are not available for those with public insurance such as Medicare or Medicaid. Compounding pharmacies offer another approach to reduce cost and improve convenience for patients, but because the vehicle can influence the efficacy and tolerability of some topical medications, it is possible that these compounded formulations may not perform similarly to the original FDA-approved products.
Conclusion
For mild to moderate acne, multimodal topical therapy often is required. Fixed-dose combination adapalene-BPO and clindamycin-BPO are highly cost-effective options for most patients. Lotion formulations of topical retinoids may be useful in patients with difficulty tolerating other formulations. Clascoterone is a novel topical antiandrogen that is more expensive than other topical therapies but can complement other topical therapies and is well tolerated.
For moderate to severe acne, doxycycline or hormonal therapy (ie, COCs, spironolactone) are highly cost-effective options. Isotretinoin is recommended for severe or scarring acne. Reduced laboratory monitoring for spironolactone and isotretinoin is an opportunity to provide higher-value care.
- Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485. doi:10.1111/bjd.12149
- Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59. doi:10.1016/j.jaad.2007.06.045
- Webster GF. The pathophysiology of acne. Cutis. 2005;76(2 suppl):4-7.
- Degitz K, Placzek M, Borelli C, et al. Pathophysiology of acne. J Dtsch Dermatol Ges. 2007;5:316-323. doi:10.1111/j.1610-0387.2007.06274.x
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi:10.1016/j.jaad.2015.12.037
- Felmingham C, Kerr A, Veysey E. Costs incurred by patients with acne prior to dermatological consultation and their relation to patient income. Australas J Dermatol. 2020;61:384-386. doi:10.1111/ajd.13324
- Perche P, Singh R, Feldman S. Patient preferences for acne vulgaris treatment and barriers to care: a survey study. J Drugs Dermatol. 2022;21:1191-1195. doi:10.36849/JDD.6940
- KFF Health Tracking Poll—February 2019. Accessed August 9, 2023. https://files.kff.org/attachment/Topline-KFF-Health-Tracking-Poll-February-2019
- Ryskina KL, Goldberg E, Lott B, et al. The role of the physician in patient perceptions of barriers to primary adherence with acne medications. JAMA Dermatol. 2018;154:456-459. doi:10.1001/jamadermatol.2017.6144
- Porter ME. What is value in health care? N Engl J Med. 2010;363:2477-2481. doi:10.1056/NEJMp1011024
- Barbieri JS, Tan JKL, Adamson AS. Active comparator trial designs used to promote development of innovative new medications. Cutis. 2020;106:E4-E6. doi:10.12788/cutis.0067
- Miller J, Ly S, Mostaghimi A, et al. Use of active comparator trials for topical medications in dermatology. JAMA Dermatol. 2021;157:597-599. doi:10.1001/jamadermatol.2021.0356
- GoodRx. Accessed August 11, 2023. https://www.goodrx.com
- Stuart B, Maund E, Wilcox C, et al. Topical preparations for the treatment of mild‐to‐moderate acne vulgaris: systematic review and network meta‐analysis. Br J Dermatol. 2021;185:512-525. doi:10.1111/bjd.20080
- Mavranezouli I, Welton NJ, Daly CH, et al. Cost-effectiveness of topical pharmacological, oral pharmacological, physical and combined treatments for acne vulgaris. Clin Exp Dermatol. 2022;47:2176-2187. doi:10.1111/ced.15356
- Tanghetti E, Werschler W, Lain T, et al. Tazarotene 0.045% lotion for once-daily treatment of moderate-to-severe acne vulgaris: results from two phase 3 trials. J Drugs Dermatol. 2020;19:70-77. doi:10.36849/JDD.2020.3977
- Tyring SK, Kircik LH, Pariser DM, et al. Novel tretinoin 0.05% lotion for the once-daily treatment of moderate-to-severe acne vulgaris: assessment of efficacy and safety in patients aged 9 years and older. J Drugs Dermatol. 2018;17:1084-1091.
- Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 μg/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80:1691-1699. doi:10.1016/j.jaad.2019.02.044
- Huang CY, Chang IJ, Bolick N, et al. Comparative efficacy of pharmacological treatments for acne vulgaris: a network meta-analysis of 221 randomized controlled trials. Ann Fam Med. 2023;21:358-369. doi:10.1370/afm.2995
- Draelos ZD. Low irritation potential of tazarotene 0.045% lotion: head-to-head comparison to adapalene 0.3% gel and trifarotene 0.005% cream in two studies. J Dermatolog Treat. 2023;34:2166346. doi:10.1080/09546634.2023.2166346
- Dessinioti C, Katsambas A. Antibiotics and antimicrobial resistance in acne: epidemiological trends and clinical practice considerations. Yale J Biol Med. 2022;95:429-443.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;80:168-177. doi:10.1016/j.jaad.2018.08.020
- Wang X, Wang Z, Sun L, et al. Efficacy and safety of dapsone gel for acne: a systematic review and meta-analysis. Ann Palliat Med. 2022;11:611-620. doi:10.21037/apm-21-3935
- Melián-Olivera A, Burgos-Blasco P, Selda-Enríquez G, et al. Topical dapsone for folliculitis decalvans: a retrospective cohort study. J Am Acad Dermatol. 2022;87:150-151. doi:10.1016/j.jaad.2021.07.004
- Yentzer BA, Ade RA, Fountain JM, et al. Simplifying regimens promotes greater adherence and outcomes with topical acne medications: a randomized controlled trial. Cutis. 2010;86:103-108.
- Ting W. Randomized, observer-blind, split-face study to compare the irritation potential of 2 topical acne formulations over a 14-day treatment period. Cutis. 2012;90:91-96.
- Aschoff R, Möller S, Haase R, et al. Tolerability and efficacy ofclindamycin/tretinoin versus adapalene/benzoyl peroxide in the treatment of acne vulgaris. J Drugs Dermatol. 2021;20:295-301. doi:10.36849/JDD.2021.5641
- Rosette C, Agan FJ, Mazzetti A, et al. Cortexolone 17α-propionate (clascoterone) is a novel androgen receptor antagonist that inhibits production of lipids and inflammatory cytokines from sebocytes in vitro. J Drugs Dermatol. 2019;18:412-418.
- Hebert A, Thiboutot D, Stein Gold L, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630. doi:10.1001/jamadermatol.2020.0465
- Trifu V, Tiplica GS, Naumescu E, et al. Cortexolone 17α-propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. a pilot randomized, double-blind comparative study vs. placebo and tretinoin 0·05% cream. Br J Dermatol. 2011;165:177-183. doi:10.1111/j.1365-2133.2011.10332.x
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Guzman AK, Barbieri JS. Comparative analysis of prescribing patterns of tetracycline class antibiotics and spironolactone between advanced practice providers and physicians in the treatment of acne vulgaris. J Am Acad Dermatol. 2021;84:1119-1121. doi:10.1016/j.jaad.2020.06.044
- Barbieri JS, James WD, Margolis DJ. Trends in prescribing behavior of systemic agents used in the treatment of acne among dermatologists and nondermatologists: a retrospective analysis, 2004-2013. J Am Acad Dermatol. 2017;77:456-463.e4. doi:10.1016/j.jaad.2017.04.016
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297. doi:10.1001/jamadermatol.2018.4944
- Garner SE, Eady A, Bennett C, et al. Minocycline for acne vulgaris: efficacy and safety. Cochrane Database Syst Rev. 2012;2012:CD002086. doi:10.1002/14651858.CD002086.pub2
- Zhanel G, Critchley I, Lin LY, et al. Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob Agents Chemother. 2018;63:e01297-18. doi:10.1128/AAC.01297-18
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris: results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17:987-996.
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Barbieri JS, Choi JK, James WD, et al. Real-world drug usage survival of spironolactone versus oral antibiotics for the management of female patients with acne. J Am Acad Dermatol. 2019;81:848-851. doi:10.1016/j.jaad.2019.03.036
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments. J Am Acad Dermatol. 2019;80:538-549. doi:10.1016/j.jaad.2018.09.055
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;7:CD004425. doi:10.1002/14651858.CD004425.pub6
- Maloney JM, Dietze P, Watson D, et al. Treatment of acne using a 3-milligram drospirenone/20-microgram ethinyl estradiol oral contraceptive administered in a 24/4 regimen. Obstet Gynecol. 2008;112:773-781. doi:10.1097/AOG.0b013e318187e1c5
- Lucky AW, Koltun W, Thiboutot D, et al. A combined oral contraceptive containing 3-mg drospirenone/20-microg ethinyl estradiol in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled study evaluating lesion counts and participant self-assessment. Cutis. 2008;82:143-150.
- Koo EB, Petersen TD, Kimball AB. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J Am Acad Dermatol. 2014;71:450-459. doi:10.1016/j.jaad.2014.03.051
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502. doi:10.1067/mjd.2000.105557
- Layton AM, Eady EA, Whitehouse H, et al. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18:169-191. doi:10.1007/s40257-016-0245-x
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300. doi:10.1001/jamadermatol.2020.5468
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944. doi:10.1001/jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Barbieri JS, Shin DB, Wang S, et al. The clinical utility of laboratory monitoring during isotretinoin therapy for acne and changes to monitoring practices over time. J Am Acad Dermatol. 2020;82:72-79. doi:10.1016/j.jaad.2019.06.025
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory monitoring during isotretinoin therapy for acne: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:35-44. doi:10.1001/jamadermatol.2015.3091
- Xia E, Han J, Faletsky A, et al. Isotretinoin laboratory monitoring in acne treatment: a Delphi consensus study. JAMA Dermatol. 2022;158:942-948. doi:10.1001/jamadermatol.2022.2044
- Affleck A, Jackson D, Williams HC, et al. Is routine laboratory testing in healthy young patients taking isotretinoin necessary: a critically appraised topic. Br J Dermatol. 2022;187:857-865. doi:10.1111/bjd.21840
- Barbieri JS, LaChance A, Albrecht J. Double standards and inconsistencies in access to care-what constitutes a cosmetic treatment? JAMA Dermatol. 2023;159:245-246. doi:10.1001/jamadermatol.2022.6322
- Trish E, Van Nuys K, Popovian R. US consumers overpay for generic drugs. Schaeffer Center White Paper Series. May 31, 2022. doi:10.25549/m589-2268
- Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485. doi:10.1111/bjd.12149
- Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59. doi:10.1016/j.jaad.2007.06.045
- Webster GF. The pathophysiology of acne. Cutis. 2005;76(2 suppl):4-7.
- Degitz K, Placzek M, Borelli C, et al. Pathophysiology of acne. J Dtsch Dermatol Ges. 2007;5:316-323. doi:10.1111/j.1610-0387.2007.06274.x
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi:10.1016/j.jaad.2015.12.037
- Felmingham C, Kerr A, Veysey E. Costs incurred by patients with acne prior to dermatological consultation and their relation to patient income. Australas J Dermatol. 2020;61:384-386. doi:10.1111/ajd.13324
- Perche P, Singh R, Feldman S. Patient preferences for acne vulgaris treatment and barriers to care: a survey study. J Drugs Dermatol. 2022;21:1191-1195. doi:10.36849/JDD.6940
- KFF Health Tracking Poll—February 2019. Accessed August 9, 2023. https://files.kff.org/attachment/Topline-KFF-Health-Tracking-Poll-February-2019
- Ryskina KL, Goldberg E, Lott B, et al. The role of the physician in patient perceptions of barriers to primary adherence with acne medications. JAMA Dermatol. 2018;154:456-459. doi:10.1001/jamadermatol.2017.6144
- Porter ME. What is value in health care? N Engl J Med. 2010;363:2477-2481. doi:10.1056/NEJMp1011024
- Barbieri JS, Tan JKL, Adamson AS. Active comparator trial designs used to promote development of innovative new medications. Cutis. 2020;106:E4-E6. doi:10.12788/cutis.0067
- Miller J, Ly S, Mostaghimi A, et al. Use of active comparator trials for topical medications in dermatology. JAMA Dermatol. 2021;157:597-599. doi:10.1001/jamadermatol.2021.0356
- GoodRx. Accessed August 11, 2023. https://www.goodrx.com
- Stuart B, Maund E, Wilcox C, et al. Topical preparations for the treatment of mild‐to‐moderate acne vulgaris: systematic review and network meta‐analysis. Br J Dermatol. 2021;185:512-525. doi:10.1111/bjd.20080
- Mavranezouli I, Welton NJ, Daly CH, et al. Cost-effectiveness of topical pharmacological, oral pharmacological, physical and combined treatments for acne vulgaris. Clin Exp Dermatol. 2022;47:2176-2187. doi:10.1111/ced.15356
- Tanghetti E, Werschler W, Lain T, et al. Tazarotene 0.045% lotion for once-daily treatment of moderate-to-severe acne vulgaris: results from two phase 3 trials. J Drugs Dermatol. 2020;19:70-77. doi:10.36849/JDD.2020.3977
- Tyring SK, Kircik LH, Pariser DM, et al. Novel tretinoin 0.05% lotion for the once-daily treatment of moderate-to-severe acne vulgaris: assessment of efficacy and safety in patients aged 9 years and older. J Drugs Dermatol. 2018;17:1084-1091.
- Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 μg/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80:1691-1699. doi:10.1016/j.jaad.2019.02.044
- Huang CY, Chang IJ, Bolick N, et al. Comparative efficacy of pharmacological treatments for acne vulgaris: a network meta-analysis of 221 randomized controlled trials. Ann Fam Med. 2023;21:358-369. doi:10.1370/afm.2995
- Draelos ZD. Low irritation potential of tazarotene 0.045% lotion: head-to-head comparison to adapalene 0.3% gel and trifarotene 0.005% cream in two studies. J Dermatolog Treat. 2023;34:2166346. doi:10.1080/09546634.2023.2166346
- Dessinioti C, Katsambas A. Antibiotics and antimicrobial resistance in acne: epidemiological trends and clinical practice considerations. Yale J Biol Med. 2022;95:429-443.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;80:168-177. doi:10.1016/j.jaad.2018.08.020
- Wang X, Wang Z, Sun L, et al. Efficacy and safety of dapsone gel for acne: a systematic review and meta-analysis. Ann Palliat Med. 2022;11:611-620. doi:10.21037/apm-21-3935
- Melián-Olivera A, Burgos-Blasco P, Selda-Enríquez G, et al. Topical dapsone for folliculitis decalvans: a retrospective cohort study. J Am Acad Dermatol. 2022;87:150-151. doi:10.1016/j.jaad.2021.07.004
- Yentzer BA, Ade RA, Fountain JM, et al. Simplifying regimens promotes greater adherence and outcomes with topical acne medications: a randomized controlled trial. Cutis. 2010;86:103-108.
- Ting W. Randomized, observer-blind, split-face study to compare the irritation potential of 2 topical acne formulations over a 14-day treatment period. Cutis. 2012;90:91-96.
- Aschoff R, Möller S, Haase R, et al. Tolerability and efficacy ofclindamycin/tretinoin versus adapalene/benzoyl peroxide in the treatment of acne vulgaris. J Drugs Dermatol. 2021;20:295-301. doi:10.36849/JDD.2021.5641
- Rosette C, Agan FJ, Mazzetti A, et al. Cortexolone 17α-propionate (clascoterone) is a novel androgen receptor antagonist that inhibits production of lipids and inflammatory cytokines from sebocytes in vitro. J Drugs Dermatol. 2019;18:412-418.
- Hebert A, Thiboutot D, Stein Gold L, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630. doi:10.1001/jamadermatol.2020.0465
- Trifu V, Tiplica GS, Naumescu E, et al. Cortexolone 17α-propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. a pilot randomized, double-blind comparative study vs. placebo and tretinoin 0·05% cream. Br J Dermatol. 2011;165:177-183. doi:10.1111/j.1365-2133.2011.10332.x
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Guzman AK, Barbieri JS. Comparative analysis of prescribing patterns of tetracycline class antibiotics and spironolactone between advanced practice providers and physicians in the treatment of acne vulgaris. J Am Acad Dermatol. 2021;84:1119-1121. doi:10.1016/j.jaad.2020.06.044
- Barbieri JS, James WD, Margolis DJ. Trends in prescribing behavior of systemic agents used in the treatment of acne among dermatologists and nondermatologists: a retrospective analysis, 2004-2013. J Am Acad Dermatol. 2017;77:456-463.e4. doi:10.1016/j.jaad.2017.04.016
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297. doi:10.1001/jamadermatol.2018.4944
- Garner SE, Eady A, Bennett C, et al. Minocycline for acne vulgaris: efficacy and safety. Cochrane Database Syst Rev. 2012;2012:CD002086. doi:10.1002/14651858.CD002086.pub2
- Zhanel G, Critchley I, Lin LY, et al. Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob Agents Chemother. 2018;63:e01297-18. doi:10.1128/AAC.01297-18
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris: results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17:987-996.
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Barbieri JS, Choi JK, James WD, et al. Real-world drug usage survival of spironolactone versus oral antibiotics for the management of female patients with acne. J Am Acad Dermatol. 2019;81:848-851. doi:10.1016/j.jaad.2019.03.036
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments. J Am Acad Dermatol. 2019;80:538-549. doi:10.1016/j.jaad.2018.09.055
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;7:CD004425. doi:10.1002/14651858.CD004425.pub6
- Maloney JM, Dietze P, Watson D, et al. Treatment of acne using a 3-milligram drospirenone/20-microgram ethinyl estradiol oral contraceptive administered in a 24/4 regimen. Obstet Gynecol. 2008;112:773-781. doi:10.1097/AOG.0b013e318187e1c5
- Lucky AW, Koltun W, Thiboutot D, et al. A combined oral contraceptive containing 3-mg drospirenone/20-microg ethinyl estradiol in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled study evaluating lesion counts and participant self-assessment. Cutis. 2008;82:143-150.
- Koo EB, Petersen TD, Kimball AB. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J Am Acad Dermatol. 2014;71:450-459. doi:10.1016/j.jaad.2014.03.051
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502. doi:10.1067/mjd.2000.105557
- Layton AM, Eady EA, Whitehouse H, et al. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18:169-191. doi:10.1007/s40257-016-0245-x
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300. doi:10.1001/jamadermatol.2020.5468
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944. doi:10.1001/jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Barbieri JS, Shin DB, Wang S, et al. The clinical utility of laboratory monitoring during isotretinoin therapy for acne and changes to monitoring practices over time. J Am Acad Dermatol. 2020;82:72-79. doi:10.1016/j.jaad.2019.06.025
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory monitoring during isotretinoin therapy for acne: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:35-44. doi:10.1001/jamadermatol.2015.3091
- Xia E, Han J, Faletsky A, et al. Isotretinoin laboratory monitoring in acne treatment: a Delphi consensus study. JAMA Dermatol. 2022;158:942-948. doi:10.1001/jamadermatol.2022.2044
- Affleck A, Jackson D, Williams HC, et al. Is routine laboratory testing in healthy young patients taking isotretinoin necessary: a critically appraised topic. Br J Dermatol. 2022;187:857-865. doi:10.1111/bjd.21840
- Barbieri JS, LaChance A, Albrecht J. Double standards and inconsistencies in access to care-what constitutes a cosmetic treatment? JAMA Dermatol. 2023;159:245-246. doi:10.1001/jamadermatol.2022.6322
- Trish E, Van Nuys K, Popovian R. US consumers overpay for generic drugs. Schaeffer Center White Paper Series. May 31, 2022. doi:10.25549/m589-2268
Practice Points
- For mild to moderate acne, fixed-dose combination adapalene–benzoyl peroxide and clindamycin–benzoyl peroxide are highly cost-effective options for most patients.
- For moderate to severe acne, doxycycline or hormonal therapy (ie, combined oral contraceptives, spironolactone) are highly cost-effective options.
- Reduction of laboratory monitoring for spironolactone and isotretinoin is an opportunity to provide higher-value care.
Precision blood test for rheumatoid arthritis receives Medicare coverage
Medicare will now cover a molecular diagnostic test to predict treatment response for certain patients with rheumatoid arthritis.
The blood test, PrismRA, is the first and only commercially available test that can help predict which patients with RA are unlikely to respond to tumor necrosis factor inhibitor therapy, according to the test manufacturer, Scipher Medicine.
“Precision medicine will now be accessible to many patients suffering from RA, a potentially debilitating disease if not treated with the right therapy,” Alif Saleh, the company’s chief executive officer, said in a press release on Sept. 7. “This coverage decision not only represents a significant benefit for patients today but also ushers in a new era of precision medicine in autoimmune diseases.”
PrismRA was first made available in December 2021 for commercial billing. The test costs about $5,000, but most patients with insurance coverage pay less than $75 out-of-pocket after insurance, according to Scipher.
On Sept. 1, 2022, the Medicare administrative contractor Palmetto GBA published a draft recommendation that the test should not be covered by the national health insurance program, stating that biomarker tests “have not yet demonstrated definitive value above the combination of available clinical, laboratory, and demographic data.”
During the comment period, clinicians urged the contractor to reconsider.
“I do not have a test or clinical assessment to inform me of the right biologic for my patients. I utilize the PrismRA test to inform me which biologic is the best start. Without this valuable tool, I am left with prescribing based on what is dictated by the patient’s insurance,” wrote one commenter.
These responses and additional data published during the comment period resulted in Palmetto GBA revising their decision.
“We agree that despite the many limitations of predictive biomarker tests, a review of the evidence supports their limited use given their demonstrated validity and utility,” the company wrote in response. “Specifically, when a nonresponse signature is obtained by the molecular signature response classifier, nearly 90% of those patients will prove to not clinically respond to TNFi therapies using multiple validated disease response criteria including the ACR50 and CDAI. For these patients, a change in management would ultimately serve to avoid time on an unnecessary therapy and shorten the time to an appropriate therapy.”
The local coverage determination provides Medicare coverage nationally for patients who have a confirmed diagnosis of moderate to severely active RA, have failed first-line therapy for RA treatment, and have not started biologic or targeted synthetic therapy for RA or who are being considered for an alternative class of targeted therapy due to failure of an initially targeted therapy despite adequate dosing.
The LCD becomes effective for tests performed on or after Oct. 15, 2023.
A version of this article first appeared on Medscape.com.
Medicare will now cover a molecular diagnostic test to predict treatment response for certain patients with rheumatoid arthritis.
The blood test, PrismRA, is the first and only commercially available test that can help predict which patients with RA are unlikely to respond to tumor necrosis factor inhibitor therapy, according to the test manufacturer, Scipher Medicine.
“Precision medicine will now be accessible to many patients suffering from RA, a potentially debilitating disease if not treated with the right therapy,” Alif Saleh, the company’s chief executive officer, said in a press release on Sept. 7. “This coverage decision not only represents a significant benefit for patients today but also ushers in a new era of precision medicine in autoimmune diseases.”
PrismRA was first made available in December 2021 for commercial billing. The test costs about $5,000, but most patients with insurance coverage pay less than $75 out-of-pocket after insurance, according to Scipher.
On Sept. 1, 2022, the Medicare administrative contractor Palmetto GBA published a draft recommendation that the test should not be covered by the national health insurance program, stating that biomarker tests “have not yet demonstrated definitive value above the combination of available clinical, laboratory, and demographic data.”
During the comment period, clinicians urged the contractor to reconsider.
“I do not have a test or clinical assessment to inform me of the right biologic for my patients. I utilize the PrismRA test to inform me which biologic is the best start. Without this valuable tool, I am left with prescribing based on what is dictated by the patient’s insurance,” wrote one commenter.
These responses and additional data published during the comment period resulted in Palmetto GBA revising their decision.
“We agree that despite the many limitations of predictive biomarker tests, a review of the evidence supports their limited use given their demonstrated validity and utility,” the company wrote in response. “Specifically, when a nonresponse signature is obtained by the molecular signature response classifier, nearly 90% of those patients will prove to not clinically respond to TNFi therapies using multiple validated disease response criteria including the ACR50 and CDAI. For these patients, a change in management would ultimately serve to avoid time on an unnecessary therapy and shorten the time to an appropriate therapy.”
The local coverage determination provides Medicare coverage nationally for patients who have a confirmed diagnosis of moderate to severely active RA, have failed first-line therapy for RA treatment, and have not started biologic or targeted synthetic therapy for RA or who are being considered for an alternative class of targeted therapy due to failure of an initially targeted therapy despite adequate dosing.
The LCD becomes effective for tests performed on or after Oct. 15, 2023.
A version of this article first appeared on Medscape.com.
Medicare will now cover a molecular diagnostic test to predict treatment response for certain patients with rheumatoid arthritis.
The blood test, PrismRA, is the first and only commercially available test that can help predict which patients with RA are unlikely to respond to tumor necrosis factor inhibitor therapy, according to the test manufacturer, Scipher Medicine.
“Precision medicine will now be accessible to many patients suffering from RA, a potentially debilitating disease if not treated with the right therapy,” Alif Saleh, the company’s chief executive officer, said in a press release on Sept. 7. “This coverage decision not only represents a significant benefit for patients today but also ushers in a new era of precision medicine in autoimmune diseases.”
PrismRA was first made available in December 2021 for commercial billing. The test costs about $5,000, but most patients with insurance coverage pay less than $75 out-of-pocket after insurance, according to Scipher.
On Sept. 1, 2022, the Medicare administrative contractor Palmetto GBA published a draft recommendation that the test should not be covered by the national health insurance program, stating that biomarker tests “have not yet demonstrated definitive value above the combination of available clinical, laboratory, and demographic data.”
During the comment period, clinicians urged the contractor to reconsider.
“I do not have a test or clinical assessment to inform me of the right biologic for my patients. I utilize the PrismRA test to inform me which biologic is the best start. Without this valuable tool, I am left with prescribing based on what is dictated by the patient’s insurance,” wrote one commenter.
These responses and additional data published during the comment period resulted in Palmetto GBA revising their decision.
“We agree that despite the many limitations of predictive biomarker tests, a review of the evidence supports their limited use given their demonstrated validity and utility,” the company wrote in response. “Specifically, when a nonresponse signature is obtained by the molecular signature response classifier, nearly 90% of those patients will prove to not clinically respond to TNFi therapies using multiple validated disease response criteria including the ACR50 and CDAI. For these patients, a change in management would ultimately serve to avoid time on an unnecessary therapy and shorten the time to an appropriate therapy.”
The local coverage determination provides Medicare coverage nationally for patients who have a confirmed diagnosis of moderate to severely active RA, have failed first-line therapy for RA treatment, and have not started biologic or targeted synthetic therapy for RA or who are being considered for an alternative class of targeted therapy due to failure of an initially targeted therapy despite adequate dosing.
The LCD becomes effective for tests performed on or after Oct. 15, 2023.
A version of this article first appeared on Medscape.com.
BCR is unreliable surrogate for overall survival in prostate cancer
TOPLINE
METHODOLOGY
- In trials of localized prostate cancer, BCR remains a controversial surrogate endpoint for overall survival.
- The meta-analysis included 10,741 patients from 11 randomized clinical trials; the median follow-up was 9.2 years.
- Interventions included radiotherapy dose escalation, in which high-dose radiotherapy was compared with conventional radiotherapy (n = 3,639); short-term androgen deprivation therapy (ADT), in which radiotherapy plus short-term ADT was compared with radiotherapy alone (n = 3,930); and ADT prolongation, in which radiotherapy plus long-term ADT was compared with radiotherapy plus short-term ADT (n = 3,772).
- Prentice criteria and the two-stage meta-analytic approach were used to assess BCR as a surrogate endpoint for overall survival.
- The researchers assessed the treatment effect on BCR and on overall survival.
TAKEAWAY
- With regard to treatment effect on BCR, the three interventions significantly reduced BCR risk – dose escalation by 29%, short-term ADT by 47%, and ADT prolongation by 46%. With regard to survival, only short- and long-term ADT significantly improved overall survival, by 9% and 14%, respectively.
- At 48 months, BCR was associated with significantly increased mortality risk: 2.46-fold increased risk for dose escalation, 1.51-fold greater risk for short-term ADT, and 2.31-fold higher risk for ADT prolongation.
- However, after adjusting for BCR at 48 months, there was no significant treatment effect on overall survival (hazard ratio, 1.10; [95% confidence interval, 0.96-1.27]; HR, 0.96 [95% CI, 0.87-1.06]; HR, 1.00 [95% CI, 0.90-1.12], respectively).
- Patient-level correlation between time to BCR and overall survival was low after censoring for noncancer-related deaths. The correlation between BCR-free survival and overall survival ranged from low to moderate.
IN PRACTICE
Overall, “these results strongly suggest that BCR-based endpoints should not be the primary endpoint in randomized trials conducted for localized [prostate cancer],” the authors concluded. They added that metastasis-free survival represents a more appropriate measure.
SOURCE
The study was led by senior author Amar Kishan, MD, of the University of California, Los Angeles, and was published online in the Journal of Clinical Oncology.
LIMITATIONS
- The trials used different definitions of BCR – the older American Society of Therapeutic Radiation and Oncology definition, and the more current Phoenix criteria.
- Some trials were conducted more than 20 years ago, and a variety of factors, including patient selection, staging, diagnostic criteria, and therapeutic approaches, have evolved in that time.
- Quality of life was not captured.
DISCLOSURES
The study received support from Cancer Research UK, the UK National Health Service, the Prostate Cancer National Institutes of Health Specialized Programs of Research Excellence, the UK Department of Defense, the Prostate Cancer Foundation, and the American Society for Radiation Oncology. Authors’ relevant financial relationships are detailed in the published study.
A version of this article appeared on Medscape.com.
TOPLINE
METHODOLOGY
- In trials of localized prostate cancer, BCR remains a controversial surrogate endpoint for overall survival.
- The meta-analysis included 10,741 patients from 11 randomized clinical trials; the median follow-up was 9.2 years.
- Interventions included radiotherapy dose escalation, in which high-dose radiotherapy was compared with conventional radiotherapy (n = 3,639); short-term androgen deprivation therapy (ADT), in which radiotherapy plus short-term ADT was compared with radiotherapy alone (n = 3,930); and ADT prolongation, in which radiotherapy plus long-term ADT was compared with radiotherapy plus short-term ADT (n = 3,772).
- Prentice criteria and the two-stage meta-analytic approach were used to assess BCR as a surrogate endpoint for overall survival.
- The researchers assessed the treatment effect on BCR and on overall survival.
TAKEAWAY
- With regard to treatment effect on BCR, the three interventions significantly reduced BCR risk – dose escalation by 29%, short-term ADT by 47%, and ADT prolongation by 46%. With regard to survival, only short- and long-term ADT significantly improved overall survival, by 9% and 14%, respectively.
- At 48 months, BCR was associated with significantly increased mortality risk: 2.46-fold increased risk for dose escalation, 1.51-fold greater risk for short-term ADT, and 2.31-fold higher risk for ADT prolongation.
- However, after adjusting for BCR at 48 months, there was no significant treatment effect on overall survival (hazard ratio, 1.10; [95% confidence interval, 0.96-1.27]; HR, 0.96 [95% CI, 0.87-1.06]; HR, 1.00 [95% CI, 0.90-1.12], respectively).
- Patient-level correlation between time to BCR and overall survival was low after censoring for noncancer-related deaths. The correlation between BCR-free survival and overall survival ranged from low to moderate.
IN PRACTICE
Overall, “these results strongly suggest that BCR-based endpoints should not be the primary endpoint in randomized trials conducted for localized [prostate cancer],” the authors concluded. They added that metastasis-free survival represents a more appropriate measure.
SOURCE
The study was led by senior author Amar Kishan, MD, of the University of California, Los Angeles, and was published online in the Journal of Clinical Oncology.
LIMITATIONS
- The trials used different definitions of BCR – the older American Society of Therapeutic Radiation and Oncology definition, and the more current Phoenix criteria.
- Some trials were conducted more than 20 years ago, and a variety of factors, including patient selection, staging, diagnostic criteria, and therapeutic approaches, have evolved in that time.
- Quality of life was not captured.
DISCLOSURES
The study received support from Cancer Research UK, the UK National Health Service, the Prostate Cancer National Institutes of Health Specialized Programs of Research Excellence, the UK Department of Defense, the Prostate Cancer Foundation, and the American Society for Radiation Oncology. Authors’ relevant financial relationships are detailed in the published study.
A version of this article appeared on Medscape.com.
TOPLINE
METHODOLOGY
- In trials of localized prostate cancer, BCR remains a controversial surrogate endpoint for overall survival.
- The meta-analysis included 10,741 patients from 11 randomized clinical trials; the median follow-up was 9.2 years.
- Interventions included radiotherapy dose escalation, in which high-dose radiotherapy was compared with conventional radiotherapy (n = 3,639); short-term androgen deprivation therapy (ADT), in which radiotherapy plus short-term ADT was compared with radiotherapy alone (n = 3,930); and ADT prolongation, in which radiotherapy plus long-term ADT was compared with radiotherapy plus short-term ADT (n = 3,772).
- Prentice criteria and the two-stage meta-analytic approach were used to assess BCR as a surrogate endpoint for overall survival.
- The researchers assessed the treatment effect on BCR and on overall survival.
TAKEAWAY
- With regard to treatment effect on BCR, the three interventions significantly reduced BCR risk – dose escalation by 29%, short-term ADT by 47%, and ADT prolongation by 46%. With regard to survival, only short- and long-term ADT significantly improved overall survival, by 9% and 14%, respectively.
- At 48 months, BCR was associated with significantly increased mortality risk: 2.46-fold increased risk for dose escalation, 1.51-fold greater risk for short-term ADT, and 2.31-fold higher risk for ADT prolongation.
- However, after adjusting for BCR at 48 months, there was no significant treatment effect on overall survival (hazard ratio, 1.10; [95% confidence interval, 0.96-1.27]; HR, 0.96 [95% CI, 0.87-1.06]; HR, 1.00 [95% CI, 0.90-1.12], respectively).
- Patient-level correlation between time to BCR and overall survival was low after censoring for noncancer-related deaths. The correlation between BCR-free survival and overall survival ranged from low to moderate.
IN PRACTICE
Overall, “these results strongly suggest that BCR-based endpoints should not be the primary endpoint in randomized trials conducted for localized [prostate cancer],” the authors concluded. They added that metastasis-free survival represents a more appropriate measure.
SOURCE
The study was led by senior author Amar Kishan, MD, of the University of California, Los Angeles, and was published online in the Journal of Clinical Oncology.
LIMITATIONS
- The trials used different definitions of BCR – the older American Society of Therapeutic Radiation and Oncology definition, and the more current Phoenix criteria.
- Some trials were conducted more than 20 years ago, and a variety of factors, including patient selection, staging, diagnostic criteria, and therapeutic approaches, have evolved in that time.
- Quality of life was not captured.
DISCLOSURES
The study received support from Cancer Research UK, the UK National Health Service, the Prostate Cancer National Institutes of Health Specialized Programs of Research Excellence, the UK Department of Defense, the Prostate Cancer Foundation, and the American Society for Radiation Oncology. Authors’ relevant financial relationships are detailed in the published study.
A version of this article appeared on Medscape.com.
Ketogenic diet short-term may benefit women with PCOS
Ketogenic diets may improve reproductive hormone levels in women with polycystic ovary syndrome (PCOS), new research suggests.
In the first-ever systematic review and meta-analysis of clinical trials on the association, ketogenic diets followed for 45 days to 24 weeks showed improvements in the luteinizing hormone (LH)/follicle-stimulating hormone (FSH) ratio, serum free testosterone, and serum sex hormone binding globulin (SHBG).
Previous evidence supporting ketogenic diets in PCOS has been “relatively patchy,” and although there have been reviews on the topic, this is the first meta-analysis, write Karniza Khalid, MD, of the National Institutes of Health, Ministry of Health Malaysia, and colleagues.
Study co-author Syed A.A. Rizvi, MD, PhD, told this news organization: “Our paper supports the positive effects of short-term ketogenic diets on hormonal imbalances commonly associated with PCOS, a complex disease state associated with a multitude of presenting symptoms among individuals. Based on the presentation and individual patient circumstances, besides pharmacologic treatment, lifestyle changes and a ketogenic diet can lead to even faster improvements.”
However, Dr. Rizvi, a professor at the College of Biomedical Sciences, Larkin University, Miami, cautioned: “I would highly recommend a keto diet to women suffering from PCOS, but we all know every person has a different situation. Some may not want to change their diet, some may not be able to afford it, and for some it is just too much work. ... This is why any lifestyle change has to be discussed and planned carefully between patients and their health care providers.”
The findings were published online in the Journal of the Endocrine Society.
The literature search yielded seven qualifying studies of ketogenic diets, generally defined as a daily carbohydrate intake below 50 g while allowing variable amounts of fat and protein. A total of 170 participants were enrolled in the studies from Italy, China, and the United States.
Pooled data showed a significant association between ketogenic diet and reduced LH/FSH ratio (P < .001) and free testosterone (P < .001). There was also a significant increase in circulating SHBG (P = .002).
On the other hand, serum progesterone levels did not change significantly (P = .353).
Weight loss, a secondary outcome, was significantly greater with the ketogenic diet (P < .001).
“Since low-carbohydrate diets have shown to be effective in addressing obesity and type 2 diabetes, it makes sense that they would also be helpful to the patients with PCOS, and in fact, it has been the case,” Dr. Rizvi noted.
The exact mechanisms for the hormonal effects aren’t clear, but one theory is that the reduction in hyperinsulinemia from the ketogenic diet decreases stimulation of ovarian androgen production and increases SHBG levels. Another is that the physiologic ketosis induced by low carbohydrate intake reduces both circulating insulin and insulin-like growth factor-1, thereby suppressing the stimulus on the production of both ovarian and adrenal androgens.
The analysis didn’t include pregnancy rates. However, Dr. Rizvi noted, “there have been published studies showing that [patients with] PCOS on keto diets have significantly improved pregnancy rates, also including via [in vitro fertilization].”
The study received no outside funding. The authors have reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Ketogenic diets may improve reproductive hormone levels in women with polycystic ovary syndrome (PCOS), new research suggests.
In the first-ever systematic review and meta-analysis of clinical trials on the association, ketogenic diets followed for 45 days to 24 weeks showed improvements in the luteinizing hormone (LH)/follicle-stimulating hormone (FSH) ratio, serum free testosterone, and serum sex hormone binding globulin (SHBG).
Previous evidence supporting ketogenic diets in PCOS has been “relatively patchy,” and although there have been reviews on the topic, this is the first meta-analysis, write Karniza Khalid, MD, of the National Institutes of Health, Ministry of Health Malaysia, and colleagues.
Study co-author Syed A.A. Rizvi, MD, PhD, told this news organization: “Our paper supports the positive effects of short-term ketogenic diets on hormonal imbalances commonly associated with PCOS, a complex disease state associated with a multitude of presenting symptoms among individuals. Based on the presentation and individual patient circumstances, besides pharmacologic treatment, lifestyle changes and a ketogenic diet can lead to even faster improvements.”
However, Dr. Rizvi, a professor at the College of Biomedical Sciences, Larkin University, Miami, cautioned: “I would highly recommend a keto diet to women suffering from PCOS, but we all know every person has a different situation. Some may not want to change their diet, some may not be able to afford it, and for some it is just too much work. ... This is why any lifestyle change has to be discussed and planned carefully between patients and their health care providers.”
The findings were published online in the Journal of the Endocrine Society.
The literature search yielded seven qualifying studies of ketogenic diets, generally defined as a daily carbohydrate intake below 50 g while allowing variable amounts of fat and protein. A total of 170 participants were enrolled in the studies from Italy, China, and the United States.
Pooled data showed a significant association between ketogenic diet and reduced LH/FSH ratio (P < .001) and free testosterone (P < .001). There was also a significant increase in circulating SHBG (P = .002).
On the other hand, serum progesterone levels did not change significantly (P = .353).
Weight loss, a secondary outcome, was significantly greater with the ketogenic diet (P < .001).
“Since low-carbohydrate diets have shown to be effective in addressing obesity and type 2 diabetes, it makes sense that they would also be helpful to the patients with PCOS, and in fact, it has been the case,” Dr. Rizvi noted.
The exact mechanisms for the hormonal effects aren’t clear, but one theory is that the reduction in hyperinsulinemia from the ketogenic diet decreases stimulation of ovarian androgen production and increases SHBG levels. Another is that the physiologic ketosis induced by low carbohydrate intake reduces both circulating insulin and insulin-like growth factor-1, thereby suppressing the stimulus on the production of both ovarian and adrenal androgens.
The analysis didn’t include pregnancy rates. However, Dr. Rizvi noted, “there have been published studies showing that [patients with] PCOS on keto diets have significantly improved pregnancy rates, also including via [in vitro fertilization].”
The study received no outside funding. The authors have reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Ketogenic diets may improve reproductive hormone levels in women with polycystic ovary syndrome (PCOS), new research suggests.
In the first-ever systematic review and meta-analysis of clinical trials on the association, ketogenic diets followed for 45 days to 24 weeks showed improvements in the luteinizing hormone (LH)/follicle-stimulating hormone (FSH) ratio, serum free testosterone, and serum sex hormone binding globulin (SHBG).
Previous evidence supporting ketogenic diets in PCOS has been “relatively patchy,” and although there have been reviews on the topic, this is the first meta-analysis, write Karniza Khalid, MD, of the National Institutes of Health, Ministry of Health Malaysia, and colleagues.
Study co-author Syed A.A. Rizvi, MD, PhD, told this news organization: “Our paper supports the positive effects of short-term ketogenic diets on hormonal imbalances commonly associated with PCOS, a complex disease state associated with a multitude of presenting symptoms among individuals. Based on the presentation and individual patient circumstances, besides pharmacologic treatment, lifestyle changes and a ketogenic diet can lead to even faster improvements.”
However, Dr. Rizvi, a professor at the College of Biomedical Sciences, Larkin University, Miami, cautioned: “I would highly recommend a keto diet to women suffering from PCOS, but we all know every person has a different situation. Some may not want to change their diet, some may not be able to afford it, and for some it is just too much work. ... This is why any lifestyle change has to be discussed and planned carefully between patients and their health care providers.”
The findings were published online in the Journal of the Endocrine Society.
The literature search yielded seven qualifying studies of ketogenic diets, generally defined as a daily carbohydrate intake below 50 g while allowing variable amounts of fat and protein. A total of 170 participants were enrolled in the studies from Italy, China, and the United States.
Pooled data showed a significant association between ketogenic diet and reduced LH/FSH ratio (P < .001) and free testosterone (P < .001). There was also a significant increase in circulating SHBG (P = .002).
On the other hand, serum progesterone levels did not change significantly (P = .353).
Weight loss, a secondary outcome, was significantly greater with the ketogenic diet (P < .001).
“Since low-carbohydrate diets have shown to be effective in addressing obesity and type 2 diabetes, it makes sense that they would also be helpful to the patients with PCOS, and in fact, it has been the case,” Dr. Rizvi noted.
The exact mechanisms for the hormonal effects aren’t clear, but one theory is that the reduction in hyperinsulinemia from the ketogenic diet decreases stimulation of ovarian androgen production and increases SHBG levels. Another is that the physiologic ketosis induced by low carbohydrate intake reduces both circulating insulin and insulin-like growth factor-1, thereby suppressing the stimulus on the production of both ovarian and adrenal androgens.
The analysis didn’t include pregnancy rates. However, Dr. Rizvi noted, “there have been published studies showing that [patients with] PCOS on keto diets have significantly improved pregnancy rates, also including via [in vitro fertilization].”
The study received no outside funding. The authors have reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
No benefit of anti-inflammatory strategy in acute myocarditis
AMSTERDAM – A short course of the interleukin-1 receptor antagonist, anakinra, appeared safe but did not reduce complications of acute myocarditis in the ARAMIS trial.
The trial was presented at the annual congress of the European Society of Cardiology.
Lead investigator, Mathieu Kerneis, MD, Pitie Salpetriere APHP University Hospital, Paris, said this was the largest randomized controlled trial of patients with acute myocarditis and probably the first ever study in the acute setting of myocarditis patients diagnosed with cardiac magnetic resonance (CMR) imaging, not on biopsy, who are mostly at low risk for events.
He suggested that one of the reasons for the neutral result could have been the low-risk population involved and the low complication rate. “We enrolled an all-comer acute myocarditis population diagnosed with CMR, who were mostly at a low risk of complications,” he noted.
“I don’t think the story of anti-inflammatory drugs in acute myocarditis is over. This is just the beginning. This was the first trial, and it was just a phase 2 trial. We need further randomized trials to explore the potential benefit of an anti-inflammatory strategy in acute myocarditis patients at higher risk of complications. In addition, larger studies are needed to evaluate prolonged anti-inflammatory strategies in acute myocarditis patients at low-to-moderate risk of complications,” Dr. Kerneis concluded.
“It is very challenging to do a trial in high-risk patients with myocarditis as these patients are quite rare,” he added.
Inflammation of the myocardium
Dr. Kerneis explained that acute myocarditis is an inflammation of the myocardium that can cause permanent damage to the heart muscle and lead to myocardial infarction, stroke, heart failure, arrhythmias, and death. The condition can occur in individuals of all ages but is most frequent in young people. There is no specific treatment, but patients are generally treated with beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, and sometimes steroids.
Anakinra is an interleukin-1 receptor antagonist that works by targeting the interleukin-1β innate immune pathway. Anakinra is used for the treatment of rheumatoid arthritis and has shown efficacy in pericarditis. Dr. Kerneis noted that there have been several case reports of successful treatment with anakinra in acute myocarditis.
The ARAMIS trial – conducted at six academic centers in France – was the first randomized study to evaluate inhibition of the interleukin-1β innate immune pathway in myocarditis patients. The trial enrolled 120 hospitalized, symptomatic patients with chest pain, increased cardiac troponin, and acute myocarditis diagnosed using CMR. More than half had had a recent bacterial or viral infection.
Patients were randomized within 72 hours of hospital admission to a daily subcutaneous dose of anakinra 100 mg or placebo until hospital discharge. Patients in both groups received standard-of-care treatments, including an ACE inhibitor, for at least 1 month. Consistent with prior data, the median age of participants was 28 years and 90% were men.
The primary endpoint was the number of days free of myocarditis complications (heart failure requiring hospitalization, chest pain requiring medication, left ventricular ejection fraction less than 50%, and ventricular arrhythmias) within 28 days postdischarge.
There was no significant difference in this endpoint between the two arms, with a median of 30 days for anakinra versus 31 days for placebo.
Overall, the rate of the composite endpoint of myocarditis complications occurred in 13.7% of patients, and there was a numerical reduction in the number of patients with these myocarditis complications with anakinra – 6 patients (10.5%) in the anakinra group versus 10 patients (16.5%) in the placebo group (odds ratio, 0.59; 95% confidence interval, 0.19-1.78). This was driven by fewer patients with chest pain requiring new medication (two patients versus six patients).
The safety endpoint was the number of serious adverse events within 28 days postdischarge. This endpoint occurred in seven patients (12.1%) in the anakinra arm and six patients (10.2%) in the placebo arm, with no significant difference between groups. Cases of severe infection within 28 days postdischarge were reported in both arms.
Low-risk population
Designated discussant of the study at the ESC Hotline session, Enrico Ammirati, MD, PhD, University of Milano-Bicocca, Monza, Italy, said that patients involved in ARAMIS fit the profile of acute myocarditis and that the CMR diagnosis was positive in all the patients enrolled.
Dr. Ammirati agreed with Dr. Kerneis that the neutral results of the study were probably caused by the low-risk population. “If we look at retrospective registries, at 30 days there are zero cardiac deaths or heart transplants at 30 days in patients with a low-risk presentation.
“The ARAMIS trial has shown the feasibility of conducting studies in the setting of acute myocarditis, and even if the primary endpoint was neutral, some important data are still missing, such as change in ejection fraction and troponin levels,” he noted.
“In terms of future perspective, we are moving to assessing efficacy of anakinra or other immunosuppressive drugs from acute low risk patients to higher risk patients with heart failure and severe dysfunction,” he said.
Dr. Ammirati is the lead investigator of another ongoing study in such a higher-risk population; the MYTHS trial is investigating the use of intravenous steroids in patients with suspected acute myocarditis complicated by acute heart failure or cardiogenic shock, and an ejection fraction below 41%.
“So, we will have more results on the best treatment in this higher risk group of patients,” he concluded.
The ARAMIS trial was an academic study funded by the French Health Ministry and coordinated by the ACTION Group. Dr. Kerneis reports having received consulting fees from Kiniksa, Sanofi, and Bayer, and holds a patent for use of abatacept in immune checkpoint inhibitor (ICI)–induced myocarditis.
A version of this article first appeared on Medscape.com.
AMSTERDAM – A short course of the interleukin-1 receptor antagonist, anakinra, appeared safe but did not reduce complications of acute myocarditis in the ARAMIS trial.
The trial was presented at the annual congress of the European Society of Cardiology.
Lead investigator, Mathieu Kerneis, MD, Pitie Salpetriere APHP University Hospital, Paris, said this was the largest randomized controlled trial of patients with acute myocarditis and probably the first ever study in the acute setting of myocarditis patients diagnosed with cardiac magnetic resonance (CMR) imaging, not on biopsy, who are mostly at low risk for events.
He suggested that one of the reasons for the neutral result could have been the low-risk population involved and the low complication rate. “We enrolled an all-comer acute myocarditis population diagnosed with CMR, who were mostly at a low risk of complications,” he noted.
“I don’t think the story of anti-inflammatory drugs in acute myocarditis is over. This is just the beginning. This was the first trial, and it was just a phase 2 trial. We need further randomized trials to explore the potential benefit of an anti-inflammatory strategy in acute myocarditis patients at higher risk of complications. In addition, larger studies are needed to evaluate prolonged anti-inflammatory strategies in acute myocarditis patients at low-to-moderate risk of complications,” Dr. Kerneis concluded.
“It is very challenging to do a trial in high-risk patients with myocarditis as these patients are quite rare,” he added.
Inflammation of the myocardium
Dr. Kerneis explained that acute myocarditis is an inflammation of the myocardium that can cause permanent damage to the heart muscle and lead to myocardial infarction, stroke, heart failure, arrhythmias, and death. The condition can occur in individuals of all ages but is most frequent in young people. There is no specific treatment, but patients are generally treated with beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, and sometimes steroids.
Anakinra is an interleukin-1 receptor antagonist that works by targeting the interleukin-1β innate immune pathway. Anakinra is used for the treatment of rheumatoid arthritis and has shown efficacy in pericarditis. Dr. Kerneis noted that there have been several case reports of successful treatment with anakinra in acute myocarditis.
The ARAMIS trial – conducted at six academic centers in France – was the first randomized study to evaluate inhibition of the interleukin-1β innate immune pathway in myocarditis patients. The trial enrolled 120 hospitalized, symptomatic patients with chest pain, increased cardiac troponin, and acute myocarditis diagnosed using CMR. More than half had had a recent bacterial or viral infection.
Patients were randomized within 72 hours of hospital admission to a daily subcutaneous dose of anakinra 100 mg or placebo until hospital discharge. Patients in both groups received standard-of-care treatments, including an ACE inhibitor, for at least 1 month. Consistent with prior data, the median age of participants was 28 years and 90% were men.
The primary endpoint was the number of days free of myocarditis complications (heart failure requiring hospitalization, chest pain requiring medication, left ventricular ejection fraction less than 50%, and ventricular arrhythmias) within 28 days postdischarge.
There was no significant difference in this endpoint between the two arms, with a median of 30 days for anakinra versus 31 days for placebo.
Overall, the rate of the composite endpoint of myocarditis complications occurred in 13.7% of patients, and there was a numerical reduction in the number of patients with these myocarditis complications with anakinra – 6 patients (10.5%) in the anakinra group versus 10 patients (16.5%) in the placebo group (odds ratio, 0.59; 95% confidence interval, 0.19-1.78). This was driven by fewer patients with chest pain requiring new medication (two patients versus six patients).
The safety endpoint was the number of serious adverse events within 28 days postdischarge. This endpoint occurred in seven patients (12.1%) in the anakinra arm and six patients (10.2%) in the placebo arm, with no significant difference between groups. Cases of severe infection within 28 days postdischarge were reported in both arms.
Low-risk population
Designated discussant of the study at the ESC Hotline session, Enrico Ammirati, MD, PhD, University of Milano-Bicocca, Monza, Italy, said that patients involved in ARAMIS fit the profile of acute myocarditis and that the CMR diagnosis was positive in all the patients enrolled.
Dr. Ammirati agreed with Dr. Kerneis that the neutral results of the study were probably caused by the low-risk population. “If we look at retrospective registries, at 30 days there are zero cardiac deaths or heart transplants at 30 days in patients with a low-risk presentation.
“The ARAMIS trial has shown the feasibility of conducting studies in the setting of acute myocarditis, and even if the primary endpoint was neutral, some important data are still missing, such as change in ejection fraction and troponin levels,” he noted.
“In terms of future perspective, we are moving to assessing efficacy of anakinra or other immunosuppressive drugs from acute low risk patients to higher risk patients with heart failure and severe dysfunction,” he said.
Dr. Ammirati is the lead investigator of another ongoing study in such a higher-risk population; the MYTHS trial is investigating the use of intravenous steroids in patients with suspected acute myocarditis complicated by acute heart failure or cardiogenic shock, and an ejection fraction below 41%.
“So, we will have more results on the best treatment in this higher risk group of patients,” he concluded.
The ARAMIS trial was an academic study funded by the French Health Ministry and coordinated by the ACTION Group. Dr. Kerneis reports having received consulting fees from Kiniksa, Sanofi, and Bayer, and holds a patent for use of abatacept in immune checkpoint inhibitor (ICI)–induced myocarditis.
A version of this article first appeared on Medscape.com.
AMSTERDAM – A short course of the interleukin-1 receptor antagonist, anakinra, appeared safe but did not reduce complications of acute myocarditis in the ARAMIS trial.
The trial was presented at the annual congress of the European Society of Cardiology.
Lead investigator, Mathieu Kerneis, MD, Pitie Salpetriere APHP University Hospital, Paris, said this was the largest randomized controlled trial of patients with acute myocarditis and probably the first ever study in the acute setting of myocarditis patients diagnosed with cardiac magnetic resonance (CMR) imaging, not on biopsy, who are mostly at low risk for events.
He suggested that one of the reasons for the neutral result could have been the low-risk population involved and the low complication rate. “We enrolled an all-comer acute myocarditis population diagnosed with CMR, who were mostly at a low risk of complications,” he noted.
“I don’t think the story of anti-inflammatory drugs in acute myocarditis is over. This is just the beginning. This was the first trial, and it was just a phase 2 trial. We need further randomized trials to explore the potential benefit of an anti-inflammatory strategy in acute myocarditis patients at higher risk of complications. In addition, larger studies are needed to evaluate prolonged anti-inflammatory strategies in acute myocarditis patients at low-to-moderate risk of complications,” Dr. Kerneis concluded.
“It is very challenging to do a trial in high-risk patients with myocarditis as these patients are quite rare,” he added.
Inflammation of the myocardium
Dr. Kerneis explained that acute myocarditis is an inflammation of the myocardium that can cause permanent damage to the heart muscle and lead to myocardial infarction, stroke, heart failure, arrhythmias, and death. The condition can occur in individuals of all ages but is most frequent in young people. There is no specific treatment, but patients are generally treated with beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, and sometimes steroids.
Anakinra is an interleukin-1 receptor antagonist that works by targeting the interleukin-1β innate immune pathway. Anakinra is used for the treatment of rheumatoid arthritis and has shown efficacy in pericarditis. Dr. Kerneis noted that there have been several case reports of successful treatment with anakinra in acute myocarditis.
The ARAMIS trial – conducted at six academic centers in France – was the first randomized study to evaluate inhibition of the interleukin-1β innate immune pathway in myocarditis patients. The trial enrolled 120 hospitalized, symptomatic patients with chest pain, increased cardiac troponin, and acute myocarditis diagnosed using CMR. More than half had had a recent bacterial or viral infection.
Patients were randomized within 72 hours of hospital admission to a daily subcutaneous dose of anakinra 100 mg or placebo until hospital discharge. Patients in both groups received standard-of-care treatments, including an ACE inhibitor, for at least 1 month. Consistent with prior data, the median age of participants was 28 years and 90% were men.
The primary endpoint was the number of days free of myocarditis complications (heart failure requiring hospitalization, chest pain requiring medication, left ventricular ejection fraction less than 50%, and ventricular arrhythmias) within 28 days postdischarge.
There was no significant difference in this endpoint between the two arms, with a median of 30 days for anakinra versus 31 days for placebo.
Overall, the rate of the composite endpoint of myocarditis complications occurred in 13.7% of patients, and there was a numerical reduction in the number of patients with these myocarditis complications with anakinra – 6 patients (10.5%) in the anakinra group versus 10 patients (16.5%) in the placebo group (odds ratio, 0.59; 95% confidence interval, 0.19-1.78). This was driven by fewer patients with chest pain requiring new medication (two patients versus six patients).
The safety endpoint was the number of serious adverse events within 28 days postdischarge. This endpoint occurred in seven patients (12.1%) in the anakinra arm and six patients (10.2%) in the placebo arm, with no significant difference between groups. Cases of severe infection within 28 days postdischarge were reported in both arms.
Low-risk population
Designated discussant of the study at the ESC Hotline session, Enrico Ammirati, MD, PhD, University of Milano-Bicocca, Monza, Italy, said that patients involved in ARAMIS fit the profile of acute myocarditis and that the CMR diagnosis was positive in all the patients enrolled.
Dr. Ammirati agreed with Dr. Kerneis that the neutral results of the study were probably caused by the low-risk population. “If we look at retrospective registries, at 30 days there are zero cardiac deaths or heart transplants at 30 days in patients with a low-risk presentation.
“The ARAMIS trial has shown the feasibility of conducting studies in the setting of acute myocarditis, and even if the primary endpoint was neutral, some important data are still missing, such as change in ejection fraction and troponin levels,” he noted.
“In terms of future perspective, we are moving to assessing efficacy of anakinra or other immunosuppressive drugs from acute low risk patients to higher risk patients with heart failure and severe dysfunction,” he said.
Dr. Ammirati is the lead investigator of another ongoing study in such a higher-risk population; the MYTHS trial is investigating the use of intravenous steroids in patients with suspected acute myocarditis complicated by acute heart failure or cardiogenic shock, and an ejection fraction below 41%.
“So, we will have more results on the best treatment in this higher risk group of patients,” he concluded.
The ARAMIS trial was an academic study funded by the French Health Ministry and coordinated by the ACTION Group. Dr. Kerneis reports having received consulting fees from Kiniksa, Sanofi, and Bayer, and holds a patent for use of abatacept in immune checkpoint inhibitor (ICI)–induced myocarditis.
A version of this article first appeared on Medscape.com.
AT ESC CONGRESS 2023
The new normal in body temperature
This transcript has been edited for clarity.
Every branch of science has its constants. Physics has the speed of light, the gravitational constant, the Planck constant. Chemistry gives us Avogadro’s number, Faraday’s constant, the charge of an electron. Medicine isn’t quite as reliable as physics when it comes to these things, but insofar as there are any constants in medicine, might I suggest normal body temperature: 37° Celsius, 98.6° Fahrenheit.
Sure, serum sodium may be less variable and lactate concentration more clinically relevant, but even my 7-year-old knows that normal body temperature is 98.6°.
Except, as it turns out, 98.6° isn’t normal at all.
How did we arrive at 37.0° C for normal body temperature? We got it from this guy – German physician Carl Reinhold August Wunderlich, who, in addition to looking eerily like Luciano Pavarotti, was the first to realize that fever was not itself a disease but a symptom of one.
In 1851, Dr. Wunderlich released his measurements of more than 1 million body temperatures taken from 25,000 Germans – a painstaking process at the time, which employed a foot-long thermometer and took 20 minutes to obtain a measurement.
The average temperature measured, of course, was 37° C.
We’re more than 150 years post-Wunderlich right now, and the average person in the United States might be quite a bit different from the average German in 1850. Moreover, we can do a lot better than just measuring a ton of people and taking the average, because we have statistics. The problem with measuring a bunch of people and taking the average temperature as normal is that you can’t be sure that the people you are measuring are normal. There are obvious causes of elevated temperature that you could exclude. Let’s not take people with a respiratory infection or who are taking Tylenol, for example. But as highlighted in this paper in JAMA Internal Medicine, we can do a lot better than that.
The study leverages the fact that body temperature is typically measured during all medical office visits and recorded in the ever-present electronic medical record.
Researchers from Stanford identified 724,199 patient encounters with outpatient temperature data. They excluded extreme temperatures – less than 34° C or greater than 40° C – excluded patients under 20 or above 80 years, and excluded those with extremes of height, weight, or body mass index.
You end up with a distribution like this. Note that the peak is clearly lower than 37° C.
But we’re still not at “normal.” Some people would be seeing their doctor for conditions that affect body temperature, such as infection. You could use diagnosis codes to flag these individuals and drop them, but that feels a bit arbitrary.
I really love how the researchers used data to fix this problem. They used a technique called LIMIT (Laboratory Information Mining for Individualized Thresholds). It works like this:
Take all the temperature measurements and then identify the outliers – the very tails of the distribution.
Look at all the diagnosis codes in those distributions. Determine which diagnosis codes are overrepresented in those distributions. Now you have a data-driven way to say that yes, these diagnoses are associated with weird temperatures. Next, eliminate everyone with those diagnoses from the dataset. What you are left with is a normal population, or at least a population that doesn’t have a condition that seems to meaningfully affect temperature.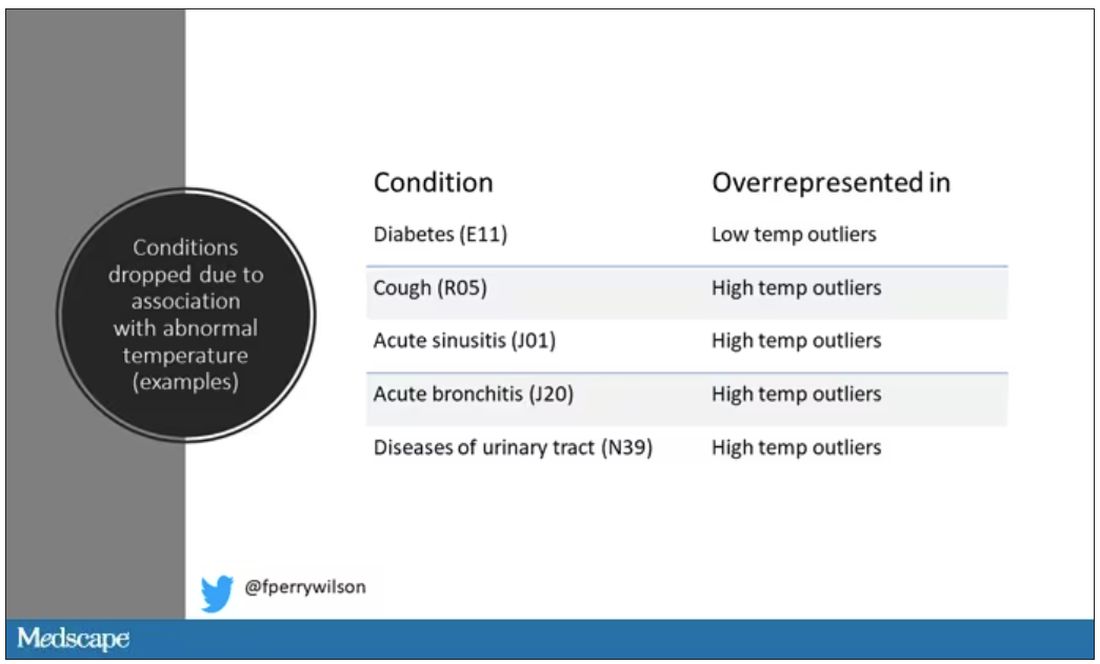
So, who was dropped? Well, a lot of people, actually. It turned out that diabetes was way overrepresented in the outlier group. Although 9.2% of the population had diabetes, 26% of people with very low temperatures did, so everyone with diabetes is removed from the dataset. While 5% of the population had a cough at their encounter, 7% of the people with very high temperature and 7% of the people with very low temperature had a cough, so everyone with cough gets thrown out.
The algorithm excluded people on antibiotics or who had sinusitis, urinary tract infections, pneumonia, and, yes, a diagnosis of “fever.” The list makes sense, which is always nice when you have a purely algorithmic classification system.
What do we have left? What is the real normal temperature? Ready?
It’s 36.64° C, or about 98.0° F.
Of course, normal temperature varied depending on the time of day it was measured – higher in the afternoon.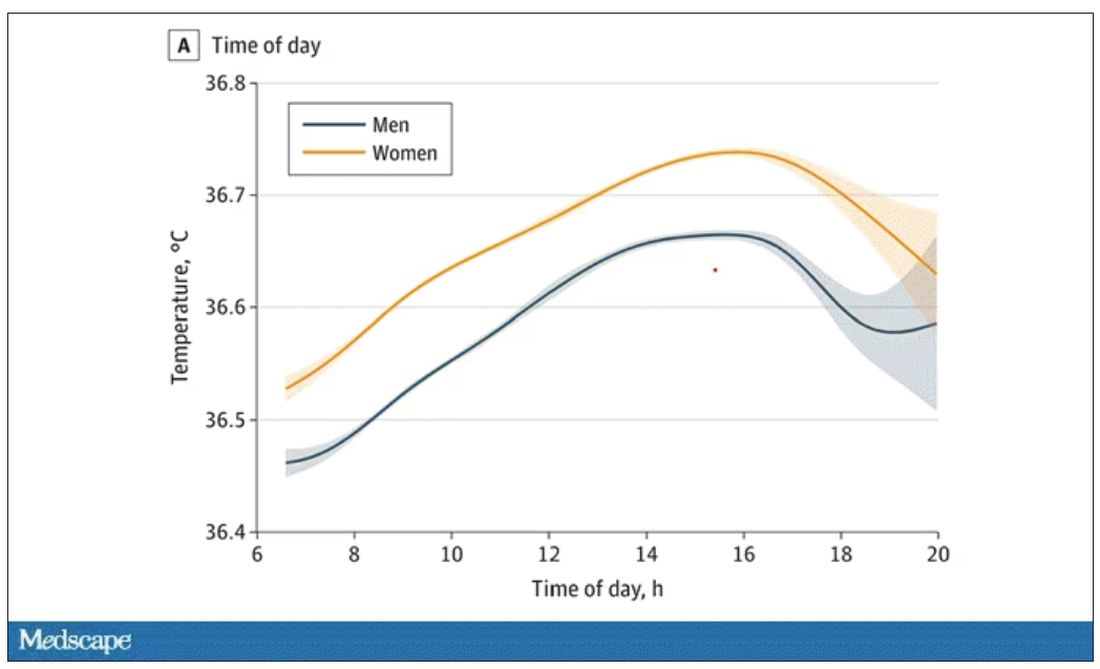
The normal temperature in women tended to be higher than in men. The normal temperature declined with age as well.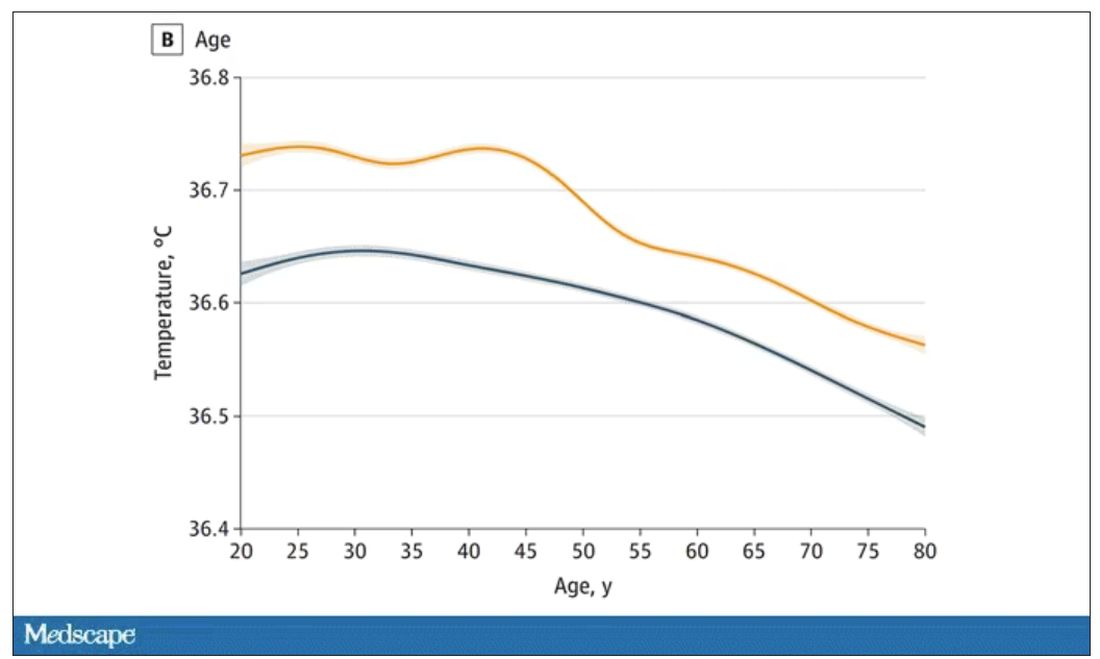
In fact, the researchers built a nice online calculator where you can enter your own, or your patient’s, parameters and calculate a normal body temperature for them. Here’s mine. My normal temperature at around 2 p.m. should be 36.7° C.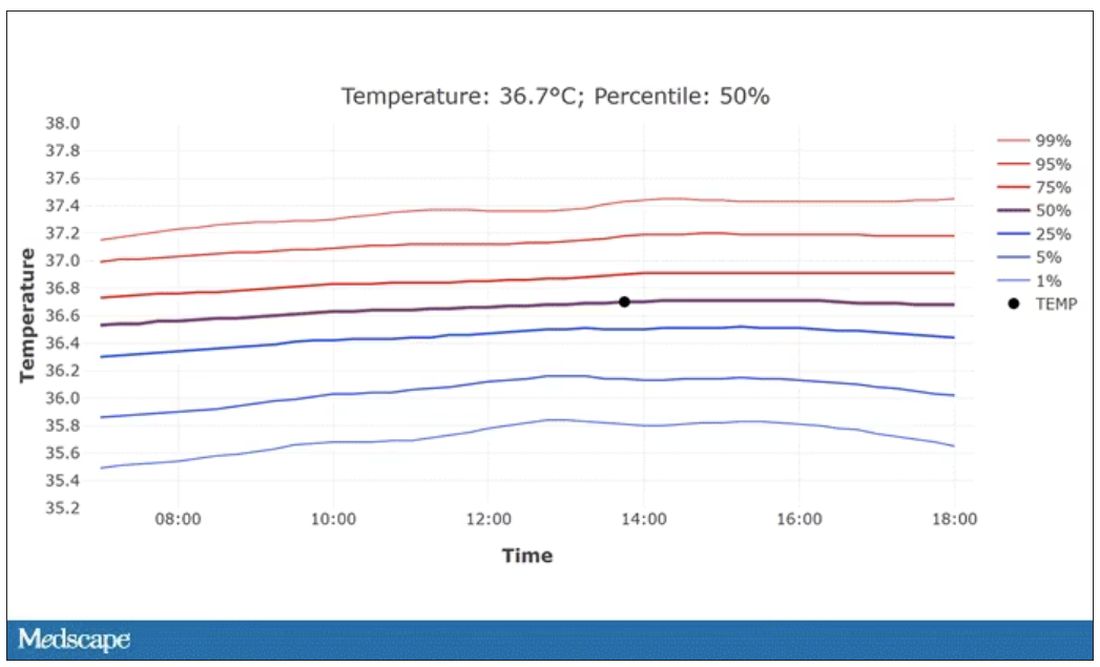
So, we’re all more cold-blooded than we thought. Is this just because of better methods? Maybe. But studies have actually shown that body temperature may be decreasing over time in humans, possibly because of the lower levels of inflammation we face in modern life (thanks to improvements in hygiene and antibiotics).
Of course, I’m sure some of you are asking yourselves whether any of this really matters. Is 37° C close enough?
Sure, this may be sort of puttering around the edges of physical diagnosis, but I think the methodology is really interesting and can obviously be applied to other broadly collected data points. But these data show us that thin, older individuals really do run cooler, and that we may need to pay more attention to a low-grade fever in that population than we otherwise would.
In any case, it’s time for a little re-education. If someone asks you what normal body temperature is, just say 36.6° C, 98.0° F. For his work in this area, I suggest we call it Wunderlich’s constant.
Dr. Wilson is associate professor of medicine and public health at Yale University, New Haven, Conn., and director of Yale’s Clinical and Translational Research Accelerator. He has no disclosures.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Every branch of science has its constants. Physics has the speed of light, the gravitational constant, the Planck constant. Chemistry gives us Avogadro’s number, Faraday’s constant, the charge of an electron. Medicine isn’t quite as reliable as physics when it comes to these things, but insofar as there are any constants in medicine, might I suggest normal body temperature: 37° Celsius, 98.6° Fahrenheit.
Sure, serum sodium may be less variable and lactate concentration more clinically relevant, but even my 7-year-old knows that normal body temperature is 98.6°.
Except, as it turns out, 98.6° isn’t normal at all.
How did we arrive at 37.0° C for normal body temperature? We got it from this guy – German physician Carl Reinhold August Wunderlich, who, in addition to looking eerily like Luciano Pavarotti, was the first to realize that fever was not itself a disease but a symptom of one.
In 1851, Dr. Wunderlich released his measurements of more than 1 million body temperatures taken from 25,000 Germans – a painstaking process at the time, which employed a foot-long thermometer and took 20 minutes to obtain a measurement.
The average temperature measured, of course, was 37° C.
We’re more than 150 years post-Wunderlich right now, and the average person in the United States might be quite a bit different from the average German in 1850. Moreover, we can do a lot better than just measuring a ton of people and taking the average, because we have statistics. The problem with measuring a bunch of people and taking the average temperature as normal is that you can’t be sure that the people you are measuring are normal. There are obvious causes of elevated temperature that you could exclude. Let’s not take people with a respiratory infection or who are taking Tylenol, for example. But as highlighted in this paper in JAMA Internal Medicine, we can do a lot better than that.
The study leverages the fact that body temperature is typically measured during all medical office visits and recorded in the ever-present electronic medical record.
Researchers from Stanford identified 724,199 patient encounters with outpatient temperature data. They excluded extreme temperatures – less than 34° C or greater than 40° C – excluded patients under 20 or above 80 years, and excluded those with extremes of height, weight, or body mass index.
You end up with a distribution like this. Note that the peak is clearly lower than 37° C.
But we’re still not at “normal.” Some people would be seeing their doctor for conditions that affect body temperature, such as infection. You could use diagnosis codes to flag these individuals and drop them, but that feels a bit arbitrary.
I really love how the researchers used data to fix this problem. They used a technique called LIMIT (Laboratory Information Mining for Individualized Thresholds). It works like this:
Take all the temperature measurements and then identify the outliers – the very tails of the distribution.
Look at all the diagnosis codes in those distributions. Determine which diagnosis codes are overrepresented in those distributions. Now you have a data-driven way to say that yes, these diagnoses are associated with weird temperatures. Next, eliminate everyone with those diagnoses from the dataset. What you are left with is a normal population, or at least a population that doesn’t have a condition that seems to meaningfully affect temperature.
So, who was dropped? Well, a lot of people, actually. It turned out that diabetes was way overrepresented in the outlier group. Although 9.2% of the population had diabetes, 26% of people with very low temperatures did, so everyone with diabetes is removed from the dataset. While 5% of the population had a cough at their encounter, 7% of the people with very high temperature and 7% of the people with very low temperature had a cough, so everyone with cough gets thrown out.
The algorithm excluded people on antibiotics or who had sinusitis, urinary tract infections, pneumonia, and, yes, a diagnosis of “fever.” The list makes sense, which is always nice when you have a purely algorithmic classification system.
What do we have left? What is the real normal temperature? Ready?
It’s 36.64° C, or about 98.0° F.
Of course, normal temperature varied depending on the time of day it was measured – higher in the afternoon.
The normal temperature in women tended to be higher than in men. The normal temperature declined with age as well.
In fact, the researchers built a nice online calculator where you can enter your own, or your patient’s, parameters and calculate a normal body temperature for them. Here’s mine. My normal temperature at around 2 p.m. should be 36.7° C.
So, we’re all more cold-blooded than we thought. Is this just because of better methods? Maybe. But studies have actually shown that body temperature may be decreasing over time in humans, possibly because of the lower levels of inflammation we face in modern life (thanks to improvements in hygiene and antibiotics).
Of course, I’m sure some of you are asking yourselves whether any of this really matters. Is 37° C close enough?
Sure, this may be sort of puttering around the edges of physical diagnosis, but I think the methodology is really interesting and can obviously be applied to other broadly collected data points. But these data show us that thin, older individuals really do run cooler, and that we may need to pay more attention to a low-grade fever in that population than we otherwise would.
In any case, it’s time for a little re-education. If someone asks you what normal body temperature is, just say 36.6° C, 98.0° F. For his work in this area, I suggest we call it Wunderlich’s constant.
Dr. Wilson is associate professor of medicine and public health at Yale University, New Haven, Conn., and director of Yale’s Clinical and Translational Research Accelerator. He has no disclosures.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Every branch of science has its constants. Physics has the speed of light, the gravitational constant, the Planck constant. Chemistry gives us Avogadro’s number, Faraday’s constant, the charge of an electron. Medicine isn’t quite as reliable as physics when it comes to these things, but insofar as there are any constants in medicine, might I suggest normal body temperature: 37° Celsius, 98.6° Fahrenheit.
Sure, serum sodium may be less variable and lactate concentration more clinically relevant, but even my 7-year-old knows that normal body temperature is 98.6°.
Except, as it turns out, 98.6° isn’t normal at all.
How did we arrive at 37.0° C for normal body temperature? We got it from this guy – German physician Carl Reinhold August Wunderlich, who, in addition to looking eerily like Luciano Pavarotti, was the first to realize that fever was not itself a disease but a symptom of one.
In 1851, Dr. Wunderlich released his measurements of more than 1 million body temperatures taken from 25,000 Germans – a painstaking process at the time, which employed a foot-long thermometer and took 20 minutes to obtain a measurement.
The average temperature measured, of course, was 37° C.
We’re more than 150 years post-Wunderlich right now, and the average person in the United States might be quite a bit different from the average German in 1850. Moreover, we can do a lot better than just measuring a ton of people and taking the average, because we have statistics. The problem with measuring a bunch of people and taking the average temperature as normal is that you can’t be sure that the people you are measuring are normal. There are obvious causes of elevated temperature that you could exclude. Let’s not take people with a respiratory infection or who are taking Tylenol, for example. But as highlighted in this paper in JAMA Internal Medicine, we can do a lot better than that.
The study leverages the fact that body temperature is typically measured during all medical office visits and recorded in the ever-present electronic medical record.
Researchers from Stanford identified 724,199 patient encounters with outpatient temperature data. They excluded extreme temperatures – less than 34° C or greater than 40° C – excluded patients under 20 or above 80 years, and excluded those with extremes of height, weight, or body mass index.
You end up with a distribution like this. Note that the peak is clearly lower than 37° C.
But we’re still not at “normal.” Some people would be seeing their doctor for conditions that affect body temperature, such as infection. You could use diagnosis codes to flag these individuals and drop them, but that feels a bit arbitrary.
I really love how the researchers used data to fix this problem. They used a technique called LIMIT (Laboratory Information Mining for Individualized Thresholds). It works like this:
Take all the temperature measurements and then identify the outliers – the very tails of the distribution.
Look at all the diagnosis codes in those distributions. Determine which diagnosis codes are overrepresented in those distributions. Now you have a data-driven way to say that yes, these diagnoses are associated with weird temperatures. Next, eliminate everyone with those diagnoses from the dataset. What you are left with is a normal population, or at least a population that doesn’t have a condition that seems to meaningfully affect temperature.
So, who was dropped? Well, a lot of people, actually. It turned out that diabetes was way overrepresented in the outlier group. Although 9.2% of the population had diabetes, 26% of people with very low temperatures did, so everyone with diabetes is removed from the dataset. While 5% of the population had a cough at their encounter, 7% of the people with very high temperature and 7% of the people with very low temperature had a cough, so everyone with cough gets thrown out.
The algorithm excluded people on antibiotics or who had sinusitis, urinary tract infections, pneumonia, and, yes, a diagnosis of “fever.” The list makes sense, which is always nice when you have a purely algorithmic classification system.
What do we have left? What is the real normal temperature? Ready?
It’s 36.64° C, or about 98.0° F.
Of course, normal temperature varied depending on the time of day it was measured – higher in the afternoon.
The normal temperature in women tended to be higher than in men. The normal temperature declined with age as well.
In fact, the researchers built a nice online calculator where you can enter your own, or your patient’s, parameters and calculate a normal body temperature for them. Here’s mine. My normal temperature at around 2 p.m. should be 36.7° C.
So, we’re all more cold-blooded than we thought. Is this just because of better methods? Maybe. But studies have actually shown that body temperature may be decreasing over time in humans, possibly because of the lower levels of inflammation we face in modern life (thanks to improvements in hygiene and antibiotics).
Of course, I’m sure some of you are asking yourselves whether any of this really matters. Is 37° C close enough?
Sure, this may be sort of puttering around the edges of physical diagnosis, but I think the methodology is really interesting and can obviously be applied to other broadly collected data points. But these data show us that thin, older individuals really do run cooler, and that we may need to pay more attention to a low-grade fever in that population than we otherwise would.
In any case, it’s time for a little re-education. If someone asks you what normal body temperature is, just say 36.6° C, 98.0° F. For his work in this area, I suggest we call it Wunderlich’s constant.
Dr. Wilson is associate professor of medicine and public health at Yale University, New Haven, Conn., and director of Yale’s Clinical and Translational Research Accelerator. He has no disclosures.
A version of this article appeared on Medscape.com.
The cult of the suicide risk assessment
Suicide is not a trivial matter – it upends families, robs partners of a loved one, prevents children from having a parent, and can destroy a parent’s most cherished being. It is not surprising that societies have repeatedly made it a goal to study and reduce suicide within their populations.
The suicide rate in the United States is trending upward, from about 10 per 100,000 in 2000 to about 15 per 100,000 in more recent reports. The increasing suicide rates have been accompanied by increasing distress among many strata of society. From a public health level, analysts are not just witnessing increasing suicide rates, but a shocking rise in all “deaths of despair,”1 among which suicide can be considered the ultimate example.
On an individual level, many know someone who has died of suicide or suffered from a serious suicide attempt. From the public health level to the individual level, advocacy has called for various interventions in the field of psychiatry to remedy this tragic problem.
Psychiatrists have been firsthand witnesses to this increasing demand for suicide interventions. When in residency, the norm was to perform a suicide risk assessment at the time of admission to the hospital and again at the time of discharge. As the years passed, the new normal within psychiatric hospitals has shifted to asking about suicidality on a daily basis.
In what seems to us like an escalating arms race, the emerging standard of care at many facilities is now not only for daily suicide risk assessments by each psychiatrist, but also to require nurses to ask about suicidality during every 8-hour shift – in addition to documented inquiries about suicidality by other allied staff on the psychiatric unit. As a result, it is not uncommon for a patient hospitalized at an academic center to receive more than half a dozen suicide risk assessments in a day (first by the medical student, at least once – often more than once – by the resident, again by the attending psychiatrist, then the social worker and three nurses in 24 hours).
One of the concerns about such an approach is the lack of logic inherent to many risk assessment tools and symptom scales. Many of us are familiar with the Patient Health Questionnaire (PHQ-9) to assess depression.2 The PHQ-9 asks to consider “over the last 2 weeks, how often have you ...” in relation to nine symptoms associated with depression. It has always defied reason to perform a PHQ-9 every day and expect the answers to change from “nearly every day” to “not at all,” considering only 1 day has passed since the last time the patient has answered the questions. Yet daily, or near daily, PHQ-9 scores are a frequently used tool of tracking symptom improvement in response to treatments, such as electroconvulsive therapy, performed multiple times a week.
One can argue that the patient’s perspective on how symptomatic he or she has been over the past 2 weeks may change rapidly with alleviation of a depressed mood. However, the PHQ-9 is both reported to be, and often regarded as, an objective score. If one wishes to utilize it as such, the defense of its use should not be that it is a subjective report with just as much utility as “Rate your depression on a scale of 0-27.”
Similarly, many suicide scales were intended to assess thoughts of suicide in the past month3 or have been re-tooled to address this particular concern by asking “since the last contact.”4 It is baffling to see a chart with many dozens of suicide risk assessments with at times widely differing answers, yet all measuring thoughts of suicide in the past month. Is one to expect the answer to “How many times have you had these thoughts [of suicide ideation]? (1) Less than once a week (2) Once a week ...” to change between 8 a.m. and noon? Furthermore, for the purpose of assessing acute risk of suicidality in the immediate future, to only consider symptoms since the last contact – or past 2 weeks, past month, etc. – is of unclear significance.
Provider liability
Another concern is the liability placed on providers. A common problem encountered in the inpatient setting is insurance companies refusing to reimburse a hospital stay for depressed patients denying suicidality.
Any provider in the position of caring for such a patient must ask: What is the likelihood of someone providing a false negative – a false denial of suicidality? Is the likelihood of a suicidal person denying suicidality different if asked 5 or 10 or more times in a day? There are innumerable instances where a patient at a very high risk of self-harm has denied suicidality, been discharged from the hospital, and suffered terrible consequences. Ethically, the psychiatrist aware of this risk is no more at ease discharging these patients, whether it is one suicide risk scale or a dozen that suggests a patient is at low risk.
Alternatively, it may feel untenable from a medicolegal perspective for a psychiatrist to discharge a patient denying suicidality when the chart includes over a dozen previously documented elevated suicide risk assessments in the past 72 hours. By placing the job of suicide risk assessment in the hands of providers of varying levels of training and responsibility, a situation is created in which the seasoned psychiatrist who would otherwise be comfortable discharging a patient feels unable to do so because every other note-writer in the record – from the triage nurse to the medical assistant to the sitter in the emergency department – has recorded the patient as high risk for suicide. When put in such a position, the thought often occurs that systems of care, rather than individual providers, are protected most by ever escalating requirements for suicide risk documentation. To make a clinical decision contrary to the body of suicide risk documentation puts the provider at risk of being scapegoated by the system of care, which can point to its illogical and ineffective, though profusely documented, suicide prevention protocols.
Limitations of risk assessments
Considering the ongoing rise in the use of suicide risk assessments, one would expect that the evidence for their efficacy was robust and well established. Yet a thorough review of suicide risk assessments funded by the MacArthur Foundation, which examined decades of research, came to disheartening conclusions: “predictive ability has not improved over the past 50 years”; “no risk factor category or subcategory is substantially stronger than any other”; and “predicting solely according to base rates may be comparable to prediction with current risk factors.”5
Those findings were consistent with the conclusions of many other studies, which have summarized the utility of suicide risk assessments as follows: “occurrence of suicide is too low to identify those individuals who are likely to die by suicide”;6 “suicide prediction models produce accurate overall classification models, but their accuracy of predicting a future event is near zero”;7 “risk stratification is too inaccurate to be clinically useful and might even be harmful”;8 “suicide risk prediction [lacks] any items or information that to a useful degree permit the identification of persons who will complete suicide”;9 “existing suicide prediction tools have little current clinical value”;10 “our current preoccupation with risk assessment has ... created a mythology with no evidence to support it.”11 And that’s to cite just a few.
Sadly, we have known about the limitations of suicide risk assessments for many decades. In 1983 a large VA prospective study, which aimed to identify veterans who will die by suicide, examined 4,800 patients with a wide range of instruments and measures.12 This study concluded that “discriminant analysis was clearly inadequate in correctly classifying the subjects. For an event as rare as suicide, our predictive tools and guides are simply not equal to the task.” The authors described the feelings of many in stating “courts and public opinion expect physicians to be able to pick out the particular persons who will later commit suicide. Although we may reconstruct causal chains and motives, we do not possess the tools to predict suicides.”
Yet, even several decades prior, in 1954, Dr. Albert Rosen performed an elegant statistical analysis and predicted that, considering the low base rate of suicide, suicide risk assessments are “of no practical value, for it would be impossible to treat the prodigious number of false positives.”13 It seems that we continue to be unable to accept Dr. Rosen’s premonition despite decades of confirmatory evidence.
“Quantity over quality”
Regardless of those sobering reports,
One can reasonably argue that the periodic performance of a suicide risk assessment may have clinical utility in reminding us of modifiable risk factors such as intoxication, social isolation, and access to lethal means. One can also reasonably argue that these risk assessments may provide useful education to patients and their families on epidemiological risk factors such as gender, age, and marital status. But our pursuit of serial suicide risk assessments throughout the day is encouraging providers to focus on a particular risk factor that changes from moment to moment and has particularly low validity, that being self-reported suicidality.
Reported suicidality is one of the few risk factors that can change from shift to shift. But 80% of people who die by suicide had not previously expressed suicidality, and 98.3% of people who have endorsed suicidality do not die by suicide.14 While the former statistic may improve with increased assessment, the later will likely worsen.
Suicide is not a trivial matter. We admire those that study it and advocate for better interventions. We have compassion for those who have suffered the loss of a loved one to suicide. Our patients have died as a result of the human limitations surrounding suicide prevention. Recognizing the weight of suicide and making an effort to avoid minimizing its immense consequences drive our desire to be honest with ourselves, our patients and their families, and society. That includes the unfortunate truth regarding the current state of the evidence and our ability to enact change.
It is our concern that the rising fascination with repeated suicide risk assessment is misguided in its current form and serves the purpose of appeasing administrators more than reflecting a scientific understanding of the literature. More sadly, we are concerned that this “quantity-over-quality” approach is yet another barrier to practicing what may be one of the few interventions with any hope of meaningfully impacting a patient’s risk of suicide in the clinical setting – spending time connecting with our patients.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Compton is a member of the psychiatry faculty at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research. Dr. Badre and Dr. Compton have no conflicts of interest.
References
1. Joint Economic Committee. (2019). Long Term Trends in Deaths of Despair. SCP Report 4-19.
2. Kroenke K and Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2013;32(9):509-15. doi: 10.3928/0048-5713-20020901-06.
3. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Lifetime/Recent.
4. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Since Last Contact.
5. Franklin JC et al. Risk factors for suicidal thoughts and behaviors: A meta-analysis of 50 years of research. Psychol Bull. 2017 Feb;143(2):187-232. doi: 10.1037/bul0000084.
6. Beautrais AL. Further suicidal behavior among medically serious suicide attempters. Suicide Life Threat Behav. 2004 Spring;34(1):1-11. doi: 10.1521/suli.34.1.1.27772.
7. Belsher BE. Prediction models for suicide attempts and deaths: A systematic review and simulation. JAMA Psychiatry. 2019 Jun 1;76(6):642-651. doi: 10.1001/jamapsychiatry.2019.0174.
8. Carter G et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guideline for the management of deliberate self-harm. Aust N Z J Psychiatry. 2016 Oct;50(10):939-1000. doi: 10.1177/0004867416661039.
9. Fosse R et al. Predictors of suicide in the patient population admitted to a locked-door psychiatric acute ward. PLoS One. 2017 Mar 16;12(3):e0173958. doi: 10.1371/journal.pone.0173958.
10. Kessler RC et al. Suicide prediction models: A critical review of recent research with recommendations for the way forward. Mol Psychiatry. 2020 Jan;25(1):168-79. doi: 10.1038/s41380-019-0531-0.
11. Mulder R. Problems with suicide risk assessment. Aust N Z J Psychiatry. 2011 Aug;45(8):605-7. doi: 10.3109/00048674.2011.594786.
12. Pokorny AD. Prediction of suicide in psychiatric patients: Report of a prospective study. Arch Gen Psychiatry. 1983 Mar;40(3):249-57. doi: 10.1001/archpsyc.1983.01790030019002.
13. Rosen A. Detection of suicidal patients: An example of some limitations in the prediction of infrequent events. J Consult Psychol. 1954 Dec;18(6):397-403. doi: 10.1037/h0058579.
14. McHugh CM et al. (2019). Association between suicidal ideation and suicide: Meta-analyses of odds ratios, sensitivity, specificity and positive predictive value. BJPsych Open. 2019 Mar;5(2):e18. doi: 10.1192/bjo.2018.88.
Suicide is not a trivial matter – it upends families, robs partners of a loved one, prevents children from having a parent, and can destroy a parent’s most cherished being. It is not surprising that societies have repeatedly made it a goal to study and reduce suicide within their populations.
The suicide rate in the United States is trending upward, from about 10 per 100,000 in 2000 to about 15 per 100,000 in more recent reports. The increasing suicide rates have been accompanied by increasing distress among many strata of society. From a public health level, analysts are not just witnessing increasing suicide rates, but a shocking rise in all “deaths of despair,”1 among which suicide can be considered the ultimate example.
On an individual level, many know someone who has died of suicide or suffered from a serious suicide attempt. From the public health level to the individual level, advocacy has called for various interventions in the field of psychiatry to remedy this tragic problem.
Psychiatrists have been firsthand witnesses to this increasing demand for suicide interventions. When in residency, the norm was to perform a suicide risk assessment at the time of admission to the hospital and again at the time of discharge. As the years passed, the new normal within psychiatric hospitals has shifted to asking about suicidality on a daily basis.
In what seems to us like an escalating arms race, the emerging standard of care at many facilities is now not only for daily suicide risk assessments by each psychiatrist, but also to require nurses to ask about suicidality during every 8-hour shift – in addition to documented inquiries about suicidality by other allied staff on the psychiatric unit. As a result, it is not uncommon for a patient hospitalized at an academic center to receive more than half a dozen suicide risk assessments in a day (first by the medical student, at least once – often more than once – by the resident, again by the attending psychiatrist, then the social worker and three nurses in 24 hours).
One of the concerns about such an approach is the lack of logic inherent to many risk assessment tools and symptom scales. Many of us are familiar with the Patient Health Questionnaire (PHQ-9) to assess depression.2 The PHQ-9 asks to consider “over the last 2 weeks, how often have you ...” in relation to nine symptoms associated with depression. It has always defied reason to perform a PHQ-9 every day and expect the answers to change from “nearly every day” to “not at all,” considering only 1 day has passed since the last time the patient has answered the questions. Yet daily, or near daily, PHQ-9 scores are a frequently used tool of tracking symptom improvement in response to treatments, such as electroconvulsive therapy, performed multiple times a week.
One can argue that the patient’s perspective on how symptomatic he or she has been over the past 2 weeks may change rapidly with alleviation of a depressed mood. However, the PHQ-9 is both reported to be, and often regarded as, an objective score. If one wishes to utilize it as such, the defense of its use should not be that it is a subjective report with just as much utility as “Rate your depression on a scale of 0-27.”
Similarly, many suicide scales were intended to assess thoughts of suicide in the past month3 or have been re-tooled to address this particular concern by asking “since the last contact.”4 It is baffling to see a chart with many dozens of suicide risk assessments with at times widely differing answers, yet all measuring thoughts of suicide in the past month. Is one to expect the answer to “How many times have you had these thoughts [of suicide ideation]? (1) Less than once a week (2) Once a week ...” to change between 8 a.m. and noon? Furthermore, for the purpose of assessing acute risk of suicidality in the immediate future, to only consider symptoms since the last contact – or past 2 weeks, past month, etc. – is of unclear significance.
Provider liability
Another concern is the liability placed on providers. A common problem encountered in the inpatient setting is insurance companies refusing to reimburse a hospital stay for depressed patients denying suicidality.
Any provider in the position of caring for such a patient must ask: What is the likelihood of someone providing a false negative – a false denial of suicidality? Is the likelihood of a suicidal person denying suicidality different if asked 5 or 10 or more times in a day? There are innumerable instances where a patient at a very high risk of self-harm has denied suicidality, been discharged from the hospital, and suffered terrible consequences. Ethically, the psychiatrist aware of this risk is no more at ease discharging these patients, whether it is one suicide risk scale or a dozen that suggests a patient is at low risk.
Alternatively, it may feel untenable from a medicolegal perspective for a psychiatrist to discharge a patient denying suicidality when the chart includes over a dozen previously documented elevated suicide risk assessments in the past 72 hours. By placing the job of suicide risk assessment in the hands of providers of varying levels of training and responsibility, a situation is created in which the seasoned psychiatrist who would otherwise be comfortable discharging a patient feels unable to do so because every other note-writer in the record – from the triage nurse to the medical assistant to the sitter in the emergency department – has recorded the patient as high risk for suicide. When put in such a position, the thought often occurs that systems of care, rather than individual providers, are protected most by ever escalating requirements for suicide risk documentation. To make a clinical decision contrary to the body of suicide risk documentation puts the provider at risk of being scapegoated by the system of care, which can point to its illogical and ineffective, though profusely documented, suicide prevention protocols.
Limitations of risk assessments
Considering the ongoing rise in the use of suicide risk assessments, one would expect that the evidence for their efficacy was robust and well established. Yet a thorough review of suicide risk assessments funded by the MacArthur Foundation, which examined decades of research, came to disheartening conclusions: “predictive ability has not improved over the past 50 years”; “no risk factor category or subcategory is substantially stronger than any other”; and “predicting solely according to base rates may be comparable to prediction with current risk factors.”5
Those findings were consistent with the conclusions of many other studies, which have summarized the utility of suicide risk assessments as follows: “occurrence of suicide is too low to identify those individuals who are likely to die by suicide”;6 “suicide prediction models produce accurate overall classification models, but their accuracy of predicting a future event is near zero”;7 “risk stratification is too inaccurate to be clinically useful and might even be harmful”;8 “suicide risk prediction [lacks] any items or information that to a useful degree permit the identification of persons who will complete suicide”;9 “existing suicide prediction tools have little current clinical value”;10 “our current preoccupation with risk assessment has ... created a mythology with no evidence to support it.”11 And that’s to cite just a few.
Sadly, we have known about the limitations of suicide risk assessments for many decades. In 1983 a large VA prospective study, which aimed to identify veterans who will die by suicide, examined 4,800 patients with a wide range of instruments and measures.12 This study concluded that “discriminant analysis was clearly inadequate in correctly classifying the subjects. For an event as rare as suicide, our predictive tools and guides are simply not equal to the task.” The authors described the feelings of many in stating “courts and public opinion expect physicians to be able to pick out the particular persons who will later commit suicide. Although we may reconstruct causal chains and motives, we do not possess the tools to predict suicides.”
Yet, even several decades prior, in 1954, Dr. Albert Rosen performed an elegant statistical analysis and predicted that, considering the low base rate of suicide, suicide risk assessments are “of no practical value, for it would be impossible to treat the prodigious number of false positives.”13 It seems that we continue to be unable to accept Dr. Rosen’s premonition despite decades of confirmatory evidence.
“Quantity over quality”
Regardless of those sobering reports,
One can reasonably argue that the periodic performance of a suicide risk assessment may have clinical utility in reminding us of modifiable risk factors such as intoxication, social isolation, and access to lethal means. One can also reasonably argue that these risk assessments may provide useful education to patients and their families on epidemiological risk factors such as gender, age, and marital status. But our pursuit of serial suicide risk assessments throughout the day is encouraging providers to focus on a particular risk factor that changes from moment to moment and has particularly low validity, that being self-reported suicidality.
Reported suicidality is one of the few risk factors that can change from shift to shift. But 80% of people who die by suicide had not previously expressed suicidality, and 98.3% of people who have endorsed suicidality do not die by suicide.14 While the former statistic may improve with increased assessment, the later will likely worsen.
Suicide is not a trivial matter. We admire those that study it and advocate for better interventions. We have compassion for those who have suffered the loss of a loved one to suicide. Our patients have died as a result of the human limitations surrounding suicide prevention. Recognizing the weight of suicide and making an effort to avoid minimizing its immense consequences drive our desire to be honest with ourselves, our patients and their families, and society. That includes the unfortunate truth regarding the current state of the evidence and our ability to enact change.
It is our concern that the rising fascination with repeated suicide risk assessment is misguided in its current form and serves the purpose of appeasing administrators more than reflecting a scientific understanding of the literature. More sadly, we are concerned that this “quantity-over-quality” approach is yet another barrier to practicing what may be one of the few interventions with any hope of meaningfully impacting a patient’s risk of suicide in the clinical setting – spending time connecting with our patients.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Compton is a member of the psychiatry faculty at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research. Dr. Badre and Dr. Compton have no conflicts of interest.
References
1. Joint Economic Committee. (2019). Long Term Trends in Deaths of Despair. SCP Report 4-19.
2. Kroenke K and Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2013;32(9):509-15. doi: 10.3928/0048-5713-20020901-06.
3. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Lifetime/Recent.
4. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Since Last Contact.
5. Franklin JC et al. Risk factors for suicidal thoughts and behaviors: A meta-analysis of 50 years of research. Psychol Bull. 2017 Feb;143(2):187-232. doi: 10.1037/bul0000084.
6. Beautrais AL. Further suicidal behavior among medically serious suicide attempters. Suicide Life Threat Behav. 2004 Spring;34(1):1-11. doi: 10.1521/suli.34.1.1.27772.
7. Belsher BE. Prediction models for suicide attempts and deaths: A systematic review and simulation. JAMA Psychiatry. 2019 Jun 1;76(6):642-651. doi: 10.1001/jamapsychiatry.2019.0174.
8. Carter G et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guideline for the management of deliberate self-harm. Aust N Z J Psychiatry. 2016 Oct;50(10):939-1000. doi: 10.1177/0004867416661039.
9. Fosse R et al. Predictors of suicide in the patient population admitted to a locked-door psychiatric acute ward. PLoS One. 2017 Mar 16;12(3):e0173958. doi: 10.1371/journal.pone.0173958.
10. Kessler RC et al. Suicide prediction models: A critical review of recent research with recommendations for the way forward. Mol Psychiatry. 2020 Jan;25(1):168-79. doi: 10.1038/s41380-019-0531-0.
11. Mulder R. Problems with suicide risk assessment. Aust N Z J Psychiatry. 2011 Aug;45(8):605-7. doi: 10.3109/00048674.2011.594786.
12. Pokorny AD. Prediction of suicide in psychiatric patients: Report of a prospective study. Arch Gen Psychiatry. 1983 Mar;40(3):249-57. doi: 10.1001/archpsyc.1983.01790030019002.
13. Rosen A. Detection of suicidal patients: An example of some limitations in the prediction of infrequent events. J Consult Psychol. 1954 Dec;18(6):397-403. doi: 10.1037/h0058579.
14. McHugh CM et al. (2019). Association between suicidal ideation and suicide: Meta-analyses of odds ratios, sensitivity, specificity and positive predictive value. BJPsych Open. 2019 Mar;5(2):e18. doi: 10.1192/bjo.2018.88.
Suicide is not a trivial matter – it upends families, robs partners of a loved one, prevents children from having a parent, and can destroy a parent’s most cherished being. It is not surprising that societies have repeatedly made it a goal to study and reduce suicide within their populations.
The suicide rate in the United States is trending upward, from about 10 per 100,000 in 2000 to about 15 per 100,000 in more recent reports. The increasing suicide rates have been accompanied by increasing distress among many strata of society. From a public health level, analysts are not just witnessing increasing suicide rates, but a shocking rise in all “deaths of despair,”1 among which suicide can be considered the ultimate example.
On an individual level, many know someone who has died of suicide or suffered from a serious suicide attempt. From the public health level to the individual level, advocacy has called for various interventions in the field of psychiatry to remedy this tragic problem.
Psychiatrists have been firsthand witnesses to this increasing demand for suicide interventions. When in residency, the norm was to perform a suicide risk assessment at the time of admission to the hospital and again at the time of discharge. As the years passed, the new normal within psychiatric hospitals has shifted to asking about suicidality on a daily basis.
In what seems to us like an escalating arms race, the emerging standard of care at many facilities is now not only for daily suicide risk assessments by each psychiatrist, but also to require nurses to ask about suicidality during every 8-hour shift – in addition to documented inquiries about suicidality by other allied staff on the psychiatric unit. As a result, it is not uncommon for a patient hospitalized at an academic center to receive more than half a dozen suicide risk assessments in a day (first by the medical student, at least once – often more than once – by the resident, again by the attending psychiatrist, then the social worker and three nurses in 24 hours).
One of the concerns about such an approach is the lack of logic inherent to many risk assessment tools and symptom scales. Many of us are familiar with the Patient Health Questionnaire (PHQ-9) to assess depression.2 The PHQ-9 asks to consider “over the last 2 weeks, how often have you ...” in relation to nine symptoms associated with depression. It has always defied reason to perform a PHQ-9 every day and expect the answers to change from “nearly every day” to “not at all,” considering only 1 day has passed since the last time the patient has answered the questions. Yet daily, or near daily, PHQ-9 scores are a frequently used tool of tracking symptom improvement in response to treatments, such as electroconvulsive therapy, performed multiple times a week.
One can argue that the patient’s perspective on how symptomatic he or she has been over the past 2 weeks may change rapidly with alleviation of a depressed mood. However, the PHQ-9 is both reported to be, and often regarded as, an objective score. If one wishes to utilize it as such, the defense of its use should not be that it is a subjective report with just as much utility as “Rate your depression on a scale of 0-27.”
Similarly, many suicide scales were intended to assess thoughts of suicide in the past month3 or have been re-tooled to address this particular concern by asking “since the last contact.”4 It is baffling to see a chart with many dozens of suicide risk assessments with at times widely differing answers, yet all measuring thoughts of suicide in the past month. Is one to expect the answer to “How many times have you had these thoughts [of suicide ideation]? (1) Less than once a week (2) Once a week ...” to change between 8 a.m. and noon? Furthermore, for the purpose of assessing acute risk of suicidality in the immediate future, to only consider symptoms since the last contact – or past 2 weeks, past month, etc. – is of unclear significance.
Provider liability
Another concern is the liability placed on providers. A common problem encountered in the inpatient setting is insurance companies refusing to reimburse a hospital stay for depressed patients denying suicidality.
Any provider in the position of caring for such a patient must ask: What is the likelihood of someone providing a false negative – a false denial of suicidality? Is the likelihood of a suicidal person denying suicidality different if asked 5 or 10 or more times in a day? There are innumerable instances where a patient at a very high risk of self-harm has denied suicidality, been discharged from the hospital, and suffered terrible consequences. Ethically, the psychiatrist aware of this risk is no more at ease discharging these patients, whether it is one suicide risk scale or a dozen that suggests a patient is at low risk.
Alternatively, it may feel untenable from a medicolegal perspective for a psychiatrist to discharge a patient denying suicidality when the chart includes over a dozen previously documented elevated suicide risk assessments in the past 72 hours. By placing the job of suicide risk assessment in the hands of providers of varying levels of training and responsibility, a situation is created in which the seasoned psychiatrist who would otherwise be comfortable discharging a patient feels unable to do so because every other note-writer in the record – from the triage nurse to the medical assistant to the sitter in the emergency department – has recorded the patient as high risk for suicide. When put in such a position, the thought often occurs that systems of care, rather than individual providers, are protected most by ever escalating requirements for suicide risk documentation. To make a clinical decision contrary to the body of suicide risk documentation puts the provider at risk of being scapegoated by the system of care, which can point to its illogical and ineffective, though profusely documented, suicide prevention protocols.
Limitations of risk assessments
Considering the ongoing rise in the use of suicide risk assessments, one would expect that the evidence for their efficacy was robust and well established. Yet a thorough review of suicide risk assessments funded by the MacArthur Foundation, which examined decades of research, came to disheartening conclusions: “predictive ability has not improved over the past 50 years”; “no risk factor category or subcategory is substantially stronger than any other”; and “predicting solely according to base rates may be comparable to prediction with current risk factors.”5
Those findings were consistent with the conclusions of many other studies, which have summarized the utility of suicide risk assessments as follows: “occurrence of suicide is too low to identify those individuals who are likely to die by suicide”;6 “suicide prediction models produce accurate overall classification models, but their accuracy of predicting a future event is near zero”;7 “risk stratification is too inaccurate to be clinically useful and might even be harmful”;8 “suicide risk prediction [lacks] any items or information that to a useful degree permit the identification of persons who will complete suicide”;9 “existing suicide prediction tools have little current clinical value”;10 “our current preoccupation with risk assessment has ... created a mythology with no evidence to support it.”11 And that’s to cite just a few.
Sadly, we have known about the limitations of suicide risk assessments for many decades. In 1983 a large VA prospective study, which aimed to identify veterans who will die by suicide, examined 4,800 patients with a wide range of instruments and measures.12 This study concluded that “discriminant analysis was clearly inadequate in correctly classifying the subjects. For an event as rare as suicide, our predictive tools and guides are simply not equal to the task.” The authors described the feelings of many in stating “courts and public opinion expect physicians to be able to pick out the particular persons who will later commit suicide. Although we may reconstruct causal chains and motives, we do not possess the tools to predict suicides.”
Yet, even several decades prior, in 1954, Dr. Albert Rosen performed an elegant statistical analysis and predicted that, considering the low base rate of suicide, suicide risk assessments are “of no practical value, for it would be impossible to treat the prodigious number of false positives.”13 It seems that we continue to be unable to accept Dr. Rosen’s premonition despite decades of confirmatory evidence.
“Quantity over quality”
Regardless of those sobering reports,
One can reasonably argue that the periodic performance of a suicide risk assessment may have clinical utility in reminding us of modifiable risk factors such as intoxication, social isolation, and access to lethal means. One can also reasonably argue that these risk assessments may provide useful education to patients and their families on epidemiological risk factors such as gender, age, and marital status. But our pursuit of serial suicide risk assessments throughout the day is encouraging providers to focus on a particular risk factor that changes from moment to moment and has particularly low validity, that being self-reported suicidality.
Reported suicidality is one of the few risk factors that can change from shift to shift. But 80% of people who die by suicide had not previously expressed suicidality, and 98.3% of people who have endorsed suicidality do not die by suicide.14 While the former statistic may improve with increased assessment, the later will likely worsen.
Suicide is not a trivial matter. We admire those that study it and advocate for better interventions. We have compassion for those who have suffered the loss of a loved one to suicide. Our patients have died as a result of the human limitations surrounding suicide prevention. Recognizing the weight of suicide and making an effort to avoid minimizing its immense consequences drive our desire to be honest with ourselves, our patients and their families, and society. That includes the unfortunate truth regarding the current state of the evidence and our ability to enact change.
It is our concern that the rising fascination with repeated suicide risk assessment is misguided in its current form and serves the purpose of appeasing administrators more than reflecting a scientific understanding of the literature. More sadly, we are concerned that this “quantity-over-quality” approach is yet another barrier to practicing what may be one of the few interventions with any hope of meaningfully impacting a patient’s risk of suicide in the clinical setting – spending time connecting with our patients.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Compton is a member of the psychiatry faculty at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research. Dr. Badre and Dr. Compton have no conflicts of interest.
References
1. Joint Economic Committee. (2019). Long Term Trends in Deaths of Despair. SCP Report 4-19.
2. Kroenke K and Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2013;32(9):509-15. doi: 10.3928/0048-5713-20020901-06.
3. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Lifetime/Recent.
4. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Since Last Contact.
5. Franklin JC et al. Risk factors for suicidal thoughts and behaviors: A meta-analysis of 50 years of research. Psychol Bull. 2017 Feb;143(2):187-232. doi: 10.1037/bul0000084.
6. Beautrais AL. Further suicidal behavior among medically serious suicide attempters. Suicide Life Threat Behav. 2004 Spring;34(1):1-11. doi: 10.1521/suli.34.1.1.27772.
7. Belsher BE. Prediction models for suicide attempts and deaths: A systematic review and simulation. JAMA Psychiatry. 2019 Jun 1;76(6):642-651. doi: 10.1001/jamapsychiatry.2019.0174.
8. Carter G et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guideline for the management of deliberate self-harm. Aust N Z J Psychiatry. 2016 Oct;50(10):939-1000. doi: 10.1177/0004867416661039.
9. Fosse R et al. Predictors of suicide in the patient population admitted to a locked-door psychiatric acute ward. PLoS One. 2017 Mar 16;12(3):e0173958. doi: 10.1371/journal.pone.0173958.
10. Kessler RC et al. Suicide prediction models: A critical review of recent research with recommendations for the way forward. Mol Psychiatry. 2020 Jan;25(1):168-79. doi: 10.1038/s41380-019-0531-0.
11. Mulder R. Problems with suicide risk assessment. Aust N Z J Psychiatry. 2011 Aug;45(8):605-7. doi: 10.3109/00048674.2011.594786.
12. Pokorny AD. Prediction of suicide in psychiatric patients: Report of a prospective study. Arch Gen Psychiatry. 1983 Mar;40(3):249-57. doi: 10.1001/archpsyc.1983.01790030019002.
13. Rosen A. Detection of suicidal patients: An example of some limitations in the prediction of infrequent events. J Consult Psychol. 1954 Dec;18(6):397-403. doi: 10.1037/h0058579.
14. McHugh CM et al. (2019). Association between suicidal ideation and suicide: Meta-analyses of odds ratios, sensitivity, specificity and positive predictive value. BJPsych Open. 2019 Mar;5(2):e18. doi: 10.1192/bjo.2018.88.