User login
Tool that lets patients report AEs proves reliable

receiving chemotherapy
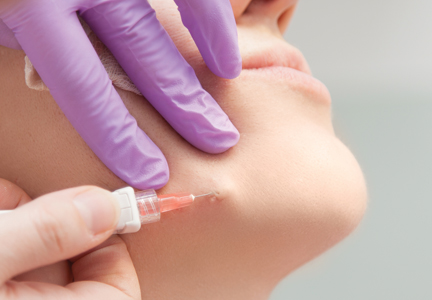
Photo by Rhoda Baer
Results of a multicenter study indicate that a tool cancer patients can use to report adverse events (AEs) is as accurate as other, established patient-reported and clinical measures.
The tool is the National Cancer Institute’s Patient Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE).
Study investigators were able to validate 119 of 124 PRO-CTCAE questions against 2 established measurement tools.
The 5 questions that were not validated could not be evaluated due to underrepresentation in the study population.
This research was published in JAMA Oncology.
“In most cancer clinical trials, information on side effects is collected by providers who have limited time with their patients, and current patient questionnaires are limited in scope and depth,” said study author Amylou Dueck, PhD, of the Mayo Clinic in Scottsdale, Arizona.
“PRO-CTCAE is a library of items for patients to directly report on the level of each of their symptoms, to enhance the reporting of side effects in cancer clinical trials, which is normally based on information from providers. The study itself is unprecedented, as more than 100 distinct questions about symptomatic adverse events were validated simultaneously.”
To assess the PRO-CTCAE, Dr Dueck and her colleagues recruited 975 cancer patients from 9 clinical practices across the US, including 7 cancer centers.
The patients had a range of cancers and were undergoing outpatient chemotherapy and/or radiation therapy. The investigators said these participants reflected the geographic, ethnic, racial, and economic diversity in cancer clinical trials.
The patients were asked to fill out the PRO-CTCAE questionnaire before appointments. The investigators then compared patient reports to clinician-reported Eastern Cooperative Oncology Group (ECOG) performance status and the European Organization for Research and Treatment of Cancer Core Quality of Life Questionnaire (QLQ-C30).
A majority of patients completed items on the PRO-CTCAE questionnaire at their first visit (96.4%, 940/975) and second visit (90.6%, 852/940).
Most patients (99.8%, 938/940) reported having at least 1 symptomatic AE, with 81.7% (768/940) reporting at least 1 AE as frequent, severe, and/or interfering “quite a bit” with daily activities.
To gauge the accuracy of the PRO-CTCAE, the investigators assessed construct validity, test-retest reliability, and responsiveness of PRO-CTCAE items.
Construct validity
The investigators explained that construct validity reflects the association between a new measurement tool and an established measure.
Construct validity is often investigated through convergent validity, which determines whether the new tool moves in the same direction as an established instrument, and known-groups validity, which determines whether the tool can distinguish between groups of patients who are thought to be distinct.
When the investigators considered all QLQ-C30 functioning/global scales, they found that all 124 items on the PRO-CTCAE questionnaire were associated in the expected direction with 1 or more scales. One hundred and fourteen of the PRO-CTCAE items demonstrated a meaningful correlation (Pearson r≥0.1), and 111 of them were statistically significant (P<0.05 for all).
Scores for 94 of 124 PRO-CTCAE items were higher among patients with an ECOG performance status of 2 to 4 (17.1% of patients) than among patients with a score of 0 to 1. The difference was significant for 58 of the items (P<0.05 for all).
Test-retest reliability and responsiveness
The investigators said they estimated test-retest reliability using the intraclass correlation coefficient (ICC), based on a 1-way analysis of variance model with an ICC of 0.7 or greater interpreted as high.
Test-retest reliability was 0.7 or greater for 36 of 49 prespecified PRO-CTCAE items. The median ICC was 0.76 [range, 0.53-0.96).
The investigators assessed the responsiveness of PRO-CTCAE items by comparing any change from the first visit to the second visit in 27 items that were selected a priori.
Correlations between PRO-CTCAE item changes and corresponding QLQ-C30 scale changes were significant for all 27 items (P≤0.006 for all).
“This is a landmark study demonstrating that meaningful information about adverse events can be elicited from patients themselves, which is a major step for advancing the patient-centeredness of clinical trials,” said study author Ethan Basch, MD, of the Lineberger Cancer Center of the University of North Carolina in Chapel Hill. ![]()

receiving chemotherapy
Photo by Rhoda Baer
Results of a multicenter study indicate that a tool cancer patients can use to report adverse events (AEs) is as accurate as other, established patient-reported and clinical measures.
The tool is the National Cancer Institute’s Patient Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE).
Study investigators were able to validate 119 of 124 PRO-CTCAE questions against 2 established measurement tools.
The 5 questions that were not validated could not be evaluated due to underrepresentation in the study population.
This research was published in JAMA Oncology.
“In most cancer clinical trials, information on side effects is collected by providers who have limited time with their patients, and current patient questionnaires are limited in scope and depth,” said study author Amylou Dueck, PhD, of the Mayo Clinic in Scottsdale, Arizona.
“PRO-CTCAE is a library of items for patients to directly report on the level of each of their symptoms, to enhance the reporting of side effects in cancer clinical trials, which is normally based on information from providers. The study itself is unprecedented, as more than 100 distinct questions about symptomatic adverse events were validated simultaneously.”
To assess the PRO-CTCAE, Dr Dueck and her colleagues recruited 975 cancer patients from 9 clinical practices across the US, including 7 cancer centers.
The patients had a range of cancers and were undergoing outpatient chemotherapy and/or radiation therapy. The investigators said these participants reflected the geographic, ethnic, racial, and economic diversity in cancer clinical trials.
The patients were asked to fill out the PRO-CTCAE questionnaire before appointments. The investigators then compared patient reports to clinician-reported Eastern Cooperative Oncology Group (ECOG) performance status and the European Organization for Research and Treatment of Cancer Core Quality of Life Questionnaire (QLQ-C30).
A majority of patients completed items on the PRO-CTCAE questionnaire at their first visit (96.4%, 940/975) and second visit (90.6%, 852/940).
Most patients (99.8%, 938/940) reported having at least 1 symptomatic AE, with 81.7% (768/940) reporting at least 1 AE as frequent, severe, and/or interfering “quite a bit” with daily activities.
To gauge the accuracy of the PRO-CTCAE, the investigators assessed construct validity, test-retest reliability, and responsiveness of PRO-CTCAE items.
Construct validity
The investigators explained that construct validity reflects the association between a new measurement tool and an established measure.
Construct validity is often investigated through convergent validity, which determines whether the new tool moves in the same direction as an established instrument, and known-groups validity, which determines whether the tool can distinguish between groups of patients who are thought to be distinct.
When the investigators considered all QLQ-C30 functioning/global scales, they found that all 124 items on the PRO-CTCAE questionnaire were associated in the expected direction with 1 or more scales. One hundred and fourteen of the PRO-CTCAE items demonstrated a meaningful correlation (Pearson r≥0.1), and 111 of them were statistically significant (P<0.05 for all).
Scores for 94 of 124 PRO-CTCAE items were higher among patients with an ECOG performance status of 2 to 4 (17.1% of patients) than among patients with a score of 0 to 1. The difference was significant for 58 of the items (P<0.05 for all).
Test-retest reliability and responsiveness
The investigators said they estimated test-retest reliability using the intraclass correlation coefficient (ICC), based on a 1-way analysis of variance model with an ICC of 0.7 or greater interpreted as high.
Test-retest reliability was 0.7 or greater for 36 of 49 prespecified PRO-CTCAE items. The median ICC was 0.76 [range, 0.53-0.96).
The investigators assessed the responsiveness of PRO-CTCAE items by comparing any change from the first visit to the second visit in 27 items that were selected a priori.
Correlations between PRO-CTCAE item changes and corresponding QLQ-C30 scale changes were significant for all 27 items (P≤0.006 for all).
“This is a landmark study demonstrating that meaningful information about adverse events can be elicited from patients themselves, which is a major step for advancing the patient-centeredness of clinical trials,” said study author Ethan Basch, MD, of the Lineberger Cancer Center of the University of North Carolina in Chapel Hill. ![]()

receiving chemotherapy
Photo by Rhoda Baer
Results of a multicenter study indicate that a tool cancer patients can use to report adverse events (AEs) is as accurate as other, established patient-reported and clinical measures.
The tool is the National Cancer Institute’s Patient Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE).
Study investigators were able to validate 119 of 124 PRO-CTCAE questions against 2 established measurement tools.
The 5 questions that were not validated could not be evaluated due to underrepresentation in the study population.
This research was published in JAMA Oncology.
“In most cancer clinical trials, information on side effects is collected by providers who have limited time with their patients, and current patient questionnaires are limited in scope and depth,” said study author Amylou Dueck, PhD, of the Mayo Clinic in Scottsdale, Arizona.
“PRO-CTCAE is a library of items for patients to directly report on the level of each of their symptoms, to enhance the reporting of side effects in cancer clinical trials, which is normally based on information from providers. The study itself is unprecedented, as more than 100 distinct questions about symptomatic adverse events were validated simultaneously.”
To assess the PRO-CTCAE, Dr Dueck and her colleagues recruited 975 cancer patients from 9 clinical practices across the US, including 7 cancer centers.
The patients had a range of cancers and were undergoing outpatient chemotherapy and/or radiation therapy. The investigators said these participants reflected the geographic, ethnic, racial, and economic diversity in cancer clinical trials.
The patients were asked to fill out the PRO-CTCAE questionnaire before appointments. The investigators then compared patient reports to clinician-reported Eastern Cooperative Oncology Group (ECOG) performance status and the European Organization for Research and Treatment of Cancer Core Quality of Life Questionnaire (QLQ-C30).
A majority of patients completed items on the PRO-CTCAE questionnaire at their first visit (96.4%, 940/975) and second visit (90.6%, 852/940).
Most patients (99.8%, 938/940) reported having at least 1 symptomatic AE, with 81.7% (768/940) reporting at least 1 AE as frequent, severe, and/or interfering “quite a bit” with daily activities.
To gauge the accuracy of the PRO-CTCAE, the investigators assessed construct validity, test-retest reliability, and responsiveness of PRO-CTCAE items.
Construct validity
The investigators explained that construct validity reflects the association between a new measurement tool and an established measure.
Construct validity is often investigated through convergent validity, which determines whether the new tool moves in the same direction as an established instrument, and known-groups validity, which determines whether the tool can distinguish between groups of patients who are thought to be distinct.
When the investigators considered all QLQ-C30 functioning/global scales, they found that all 124 items on the PRO-CTCAE questionnaire were associated in the expected direction with 1 or more scales. One hundred and fourteen of the PRO-CTCAE items demonstrated a meaningful correlation (Pearson r≥0.1), and 111 of them were statistically significant (P<0.05 for all).
Scores for 94 of 124 PRO-CTCAE items were higher among patients with an ECOG performance status of 2 to 4 (17.1% of patients) than among patients with a score of 0 to 1. The difference was significant for 58 of the items (P<0.05 for all).
Test-retest reliability and responsiveness
The investigators said they estimated test-retest reliability using the intraclass correlation coefficient (ICC), based on a 1-way analysis of variance model with an ICC of 0.7 or greater interpreted as high.
Test-retest reliability was 0.7 or greater for 36 of 49 prespecified PRO-CTCAE items. The median ICC was 0.76 [range, 0.53-0.96).
The investigators assessed the responsiveness of PRO-CTCAE items by comparing any change from the first visit to the second visit in 27 items that were selected a priori.
Correlations between PRO-CTCAE item changes and corresponding QLQ-C30 scale changes were significant for all 27 items (P≤0.006 for all).
“This is a landmark study demonstrating that meaningful information about adverse events can be elicited from patients themselves, which is a major step for advancing the patient-centeredness of clinical trials,” said study author Ethan Basch, MD, of the Lineberger Cancer Center of the University of North Carolina in Chapel Hill. ![]()
National Acute Medicine Programme
In 2009, Irish hospitals were experiencing ongoing and increasing overcrowding of emergency departments (EDs). This overcrowding and subsequent assessment delays are both associated with increased morbidity and mortality rates.[1, 2, 3, 4] The prevailing culture in many larger hospitals was to prioritize subspecialty care at the expense of the assessment and management of patients with undifferentiated acute medical presentations with nonspecific symptoms. The National Acute Medicine Programme (NAMP) was set up in 2010 by the Royal College of Physicians in Ireland (RCPI) and the Health Service Executive (HSE) to address this unsatisfactory management of acutely ill medical patients.
The objectives of the NAMP are categorized under 3 quality improvement principles: (1) Quality: to improve quality of care and patient safety by ensuring patients are seen by a nurse within 20 minutes and a senior doctor within 1 hour of arrival. (2) Access: to improve access by ensuring that the patient journey from presentation to decision to admit or discharge does not exceed 6 hours and to eliminate extended waiting periods on gurneys for medical patients. (3) Cost: to reduce cost and increase value by achieving bed savings through reduced overnight admissions and shortened lengths of stay.
The program was implemented by a small national team, which included hospital and public health physicians, nurses, a health and social care professional (HSCP), a general practitioner (GP), and a program manager. RCPI also set up a National Advisory Group of Consultant Physicians, comprised of representative medical consultants from all over the country, and key links were established with each acute hospital. The team aimed to develop a standardized model of care for all acutely ill medical patients and ensure its full implementation nationally.
METHODS
A literature review was undertaken to develop the standardized model of care in agreement with stakeholders and in consultation with patient groups.[5] The model of care required the establishment of acute medical assessment units (AMAUs), whose main function was to assess to discharge rather than admit to assess patients.[6, 7] At that time, only 8 of the 33 acute Irish hospitals that admitted medical patients had an AMAU. However, their function and operation varied greatly. In the remaining hospitals, all medical patients went to the ED, and from there were either admitted or discharged. Delays in access to senior clinicians, diagnostics, and allied health professionals such as, Occupational Therapists, Physiotherapists and Speech and Language Therapists often resulted in delays in assessment and treatment that could lead to overnight admissions.
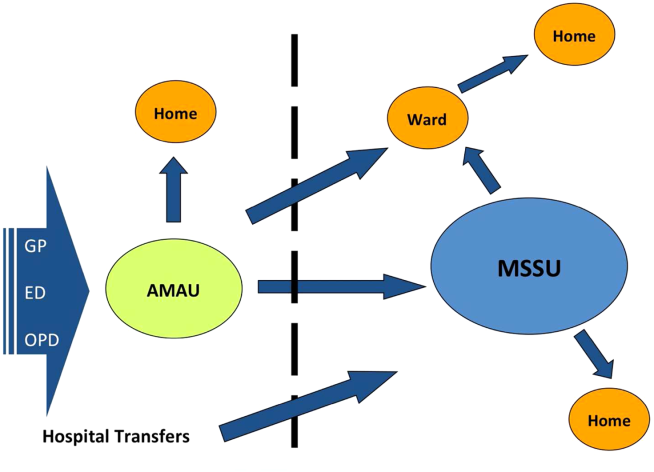
In the new model, all acute medical patients, except those requiring invasive monitoring, critical care, or special services such as oncology and dialysis, are referred to the AMAU by another doctor (ie. a GP, outpatient department, or ED physician), as shown in Figure 1. A senior physician in the AMAU then reviews the patient and decides to admit or discharge. This doctor can either be a dedicated physician with an interest in acute general medicine, or a specialist consultant rostered to work in the unit on a regular basis. Some patients are discharged the same day thanks to prompt review and treatment. Of those requiring overnight admission, some are streamed directly to specialist pathways (eg. coronary care unit). The remaining patients are admitted to the medical short‐stay unit (MSSU) under the care of an acute physician. Patients in the MSSU are then either discharged within 48 hours or go on to be transferred to a specialist ward.
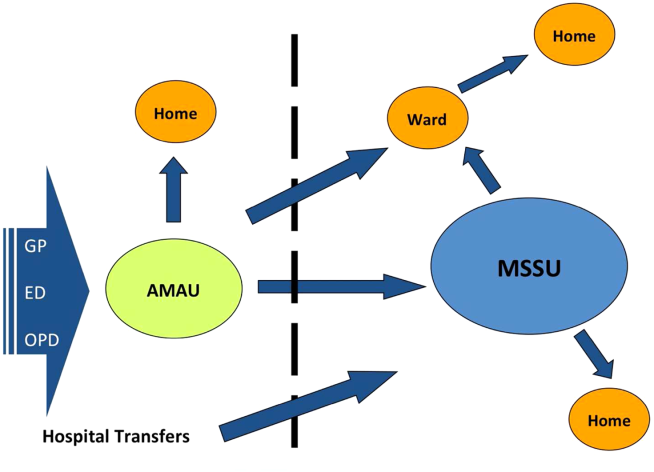
The model of care was therefore divided into 4 care pathways. National Health Service (NHS) admission data for 2008 to 2009 were used to calculate the proportion of patients who flowed through each pathway. The NHS has a wealth of experience in the development and use of AMAUs, having started implementing these units in the early 2000s. Therefore, the NHS estimates calculated above were used to set the national benchmarks for the NAMP. The four pathways are:
1. Ambulatory Care Pathway
Patients receive safe and effective treatment and are discharged on the same day. The NAMP benchmark was that at least 25% of AMAU admissions should follow this pathway of care.
2. Medical Short‐Stay Care Pathway
This pathway was developed for those patients who require inpatient care but are not expected to stay longer than 1 or 2 nights. The program benchmark was that 31% of patients should be discharged within 48 hours.
3. Routine Specialist Inpatient Care Pathway
Approximately 33% of medical admissions are expected to stay more than 2 days and less than 14 days in the hospital and have a straightforward discharge after their acute episode of care. These patients are admitted either directly to specialist medical wards from AMAU or via the MSSU within 2 days of arrival. Care is formally handed over from the AMAU team to the appropriate consultant physician upon transfer.
4. Appropriate Care and Discharge of Complex Patients Care Pathway
Frail older patients have complex care needs that continue following discharge, and their discharge requirements must be identified early during the acute care episode. The NAMP benchmark was that no more than 11% of medical admissions would fall into this pathway and require a length of stay (LoS) exceeding 14 days.
The flow model was used to build system capacity by modeling and predicting the expected demand on each AMAU to assist in forward planning The number of assessment spaces and ward beds required for each hospital were calculated by analyzing respective admission data for 2009 and applying target lengths of stay for medical patients to the flow model. The program team carried out this analysis for each of the 32 hospitals. The model of care also identified a number of practice changes under each pathway that would be required to achieve process changes and the resulting efficiency gains. Table 1 summarizes these.
|
| Ambulatory care pathway |
| Establishment of adequate assessment area |
| National early warning score within 20 minutes |
| Access to senior decision maker within 1 hour |
| Access to rapid diagnostics and HSCP assessment |
| Development of clinical criteria for transfer between ED and AMAU |
| Liaison with discharge planner |
| Clear pathways to specialist wards and community support |
| Close liaison with GP to ensure integrated care |
| Patient experience time in AMAU to be 6 hours or less |
| Medical short‐stay care pathway |
| Establishment of adequate short‐stay unit |
| Access to senior decision maker within 12 hours of transfer from AMAU |
| Twice daily consultant ward rounds |
| Access to prioritized diagnostics and HSCP assessment |
| Integrated discharge planning |
| Routine specialist inpatient care pathway |
| Daily consultant ward rounds |
| Weekend nurse/HSCP‐facilitated discharges |
| Active discharge planning with planned dates of discharge for every patient |
| Liaison with caregivers and community supports |
| Development of clinical criteria to support bidirectional flow to community hospitals within hospital groups |
| Appropriate care and discharge of complex patients care pathway |
| Early assessment and identification of complex patients |
| Streaming to care of the elderly services where appropriate |
| Proactive multidisciplinary discharge planning and liaison with funding agencies for referral to community placements and supports |
Hospitals were also categorized into 4 divisions or models as determined by the complexity of patients they admit. Model 1 hospitals are community units with subacute inpatient beds that can care for patients with rehabilitation, respite, or palliative care needs. Model 2 hospitals are small hospitals that provide inpatient and outpatient care for low‐risk, differentiated medical patients or refer on to associated higher complexity facilities. The majority of hospitals in the country are model 3 general hospitals, admitting 50% of all medical patients. Last, model 4 hospitals are the 8 regional tertiary referral centers in Ireland. A considerable volume of their patient workload remains inpatient admissions for routine specialist inpatient care.
Measuring success in the program's quality and access objectives required the development of a bespoke information technology (IT) system that is not yet operational, and therefore these objectives could not be audited.
A number of outcome measures or key performance indicators (KPIs) were developed to assess performance under each care pathway relative to the cost objectives of the NAMP as shown in Table 2. The available hospital inpatient enquiry (HIPE) data were analyzed by the program team to establish baseline performance metrics for each hospital. Initially, these data were only available to the NAMP 1 year in arrears. However, the NAMP worked with the hospitals and the HIPE system to improve the completeness and timeliness of the HIPE reporting, so that by the third quarter of 2011 monthly data were available. Audit cycles occurred on a continuous monthly basis, with feedback provided to each hospital and follow‐up of results conducted at a local level. This allowed for analysis of performance at a national, hospital group, and individual hospital level. Of note, it was only possible to analyze readmission rates to the same facility in the absence of a national unique patient identifier, and therefore readmission rates observed were of limited use as a quality measure.
| Care Pathway | Metric | National Target | 2010 | 2011 | 2012 | 2013* |
|---|---|---|---|---|---|---|
| ||||||
| Ambulatory care pathway | % of patients with LoS=0 | 25% | 11.5% | 12.9% | 18.8% | 23.2% |
| Medical short‐stay pathway | % of patients with LoS 12 days | 31% | 25.4% | 25.9% | 25.6% | 23.8% |
| Routine specialist inpatient pathway | % of patients with LoS>2 days | 44% | 63.1% | 61.2% | 55.6% | 53.1% |
| Complex care pathway | % of patients with LoS>14 days | 11% | 13.1% | 12.4% | 11.0% | 10.8% |
| % BDU of patients with LoS>30 days | 33% | 36.9% | 36.0% | 35.1% | 34.4% | |
| Routine and complex care pathway | Average LoS for those staying >2 days | 610 days | 12.9 | 12.7 | 12.4 | 12.4 |
| Summary metric | Overall average LoS | 5.8 days | 8.5 | 8.1 | 7.2 | 6.9 |
| No. of medical discharges | 202,567 | 206,250 | 235,167 | 253,083 | ||
RESULTS
The NAMP model of care was officially launched in December 2010.[6] Thirty‐two out of the 33 Irish hospitals that admit acute medical patients had adopted the model of care by the end of 2013. The program team performed an initial diagnostic meeting at each hospital to explain the program, discuss their individual baseline metrics, and collaboratively develop a hospital‐specific implementation plan. A local implementation and unscheduled care governance team, composed of senior management members and local GPs, was established in each hospital to identify ward spaces to be developed as AMAUs, reassign nursing staff to the AMAU from the wards, and organize the recruitment of new consultants with an interest in acute general medicine. The program team performed 2 to 3 visits per year to each hospital to obtain feedback on performance and support local improvement plans using appreciative enquiry. They also organized workshops and training for physicians, nurses, managers, and data managers to improve understanding of and engagement with the program. An acute medicine nurse interest group was convened to support nurses in the transition to clinical practice with a greater focus on ambulatory care. Annual conferences were held to present and discuss annual and cumulative audit results.
Table 2 presents the national KPI results for the cost and value objectives over the 3 years of implementation. The number of medical discharges increased from 202,567 in 2010 to 253,083 in 2013. The proportion of discharges that passed through the AMAU was 29% in 2013, considerably reducing the amount of patients seen through the ED and alleviating some of the overcrowding experienced there.
The proportion of medical patients who avoided admission increased from 11.5% to 23.2% in 2013. When examining the proportion of patients discharged within 48 hours, we combined results for the ambulatory care pathway (LoS=0) and the medical short‐stay pathway (LoS=12) and found a 10% increase nationally from 36.9% to 47% in 2013. In addition, the proportion of total medical bed‐days used (BDU) for patients with LoS over 30 days also improved by 2.5%. The program achieved an overall reduction of 0.5 days in those staying over 2 days nationally, and an overall reduction in average LoS (AvLoS) for all medical inpatients of 1.6 days (from 8.5 days 6.9 days) across the 3 years.
Table 3 shows the average change in KPIs from 2010 to 2013 by hospital model group. Looking at data by hospital group allowed results to be interpreted in a national context and identify any bottlenecks in the health system.
| Care pathway | Metric | National | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|---|
| |||||
| Ambulatory care pathway | % of patients with LoS=0 | 11.7% | 11.5% | 12% | 11.5% |
| Medical short‐stay pathway | % of patients with LoS 12 days | 1.6% | 5% | 2.3% | 0.3% |
| Routine specialist inpatient pathway | % of patients with LoS>2 days | 10% | 6.4% | 9.8% | 11.2% |
| Complex care pathway | % of patients with LoS>14 days | 2.3% | 0.4% | 1.7% | 4.1% |
| % BDU of patients with LoS>30 days | 2.5% | 1.9% | 0.2% | 4.9% | |
| Routine and complex care pathway | Average LoS for those staying >2 days | 0.5 | 0.7 | 0 | 1.4 |
| Summary metric | Overall average LoS | 1.6 | 0.4 | 1.0 | 2.6 |
During the 3‐year period, the role of model 2 hospitals changed from admitting all medical patients to only admitting differentiated medical patients referred from GPs. This is reflected in their KPI results, with an increasing proportion of patients with LoS greater than 14 days and the proportion of BDU occupied by those with LoS greater than 30 days. Data from the model 2 and 3 hospitals showed a considerable increase in same‐day discharges, with a concurrent decrease in percentage of patients staying in the hospital longer than 2 days. This translated to a national reduction in AvLoS of 1 day in this hospital group. Model 2 hospitals experienced small increases in both the AvLoS for those patients staying over 2 days (0.7%) and the proportion of BDU occupied by patients staying longer than 30 days (1.9%), whereas model 3 had experienced no real change in either of these metrics (0% and 0.2%, respectively). This reflected the limited availability of long‐term care facilities and protracted funding approval process nationally during the implementation period.
Model 4 hospitals experienced improvement across all KPIs. There was an 11.2% increase in the proportion of patients discharged within 48 hours and a 1.4‐day reduction in AvLoS for patients with LoS>2 days. A notable success within this hospital category was the 4.9% reduction in percentage of BDU by patients with LoS>30 days. The AvLoS for all medical admissions in this group remained above the national target at 8.6 days but did decrease considerably by 2.6 days from its baseline.
Data on 28‐day readmission to the same facility were used as a balancing measure but were only available for the latter 2 years. We found rates of 11% and 10% for 2012 and 2013, respectively. Patient experience of these new units should be assessed, but it was not possible to measure this during the implementation period.
DISCUSSION
The implementation of the NAMP has demonstrably streamlined the care of acute medical patients in Ireland. We report the results of this national transformational change brought about by the implementation of an evidence‐based model of care. The development of a flow model for each hospital improved the patient flow from assessment to discharge. Process improvement lies at the core of all the successes achieved by the program. The practice changes highlighted in Table 1 were pivotal in streamlining and improving the care of acutely ill medical patients. The focus on early access to senior decision making, early diagnostics, and a continuous, coordinated, multidisciplinary approach to care and discharge were central to the effective functioning of the AMAU and the resulting increase in avoided admissions.
Shortened lengths of stay are associated with better clinical outcomes and reduced exposure of patients to risk, and result in significant cost efficiencies accrued to the Irish health services.[2, 8] The adoption of ambulatory care and medical short‐stay pathways facilitated the 11.7% increase in avoided admissions and the reduction of 1.6 days in overall AvLoS nationally. This translates to significant cost savings for the Irish health system and likely improves clinical outcomes and reduced morbidity. We estimated these cost savings to be approximately 88.2 million by multiplying the number of bed days saved by the marginal cost of a bed day, which was quoted at 246 in 2012 by our Healthcare Pricing Office.
Thirty‐two of the 33 Irish hospitals that admit acute medical patients are now operating the program and achieving improvements in performance, as evidenced by ongoing audits. The priority given to the program by the RCPI and HSE has enabled the assignment of local implementation teams sustaining the focus on quality improvement at a local level. It also allowed for modest seed funding to be allocated for the appointment of 36 new consultants with an interest in acute general medicine. The cost of these additional consultants is offset by the considerable savings achieved through efficiency gains. An important challenge to implementation was the change in mindset required from local healthcare staff to divert patients away from the ED to the AMAU, and reassign staff and resources from other inpatient wards to the new unit. Visible clinical leadership from clinical directors, acute medicine hospital leads, senior nursing, and HSCP, together with management and local GPs, was essential in effecting this change. The program team also offered considerable support in this regard through advocacy and promotion of the program nationally. The implementation of the 4 care pathways represents a generational change in how medicine is practiced in Ireland. The development of acute medicine as a new specialty was strongly fostered by the program.
A number of disease‐specific clinical programs began operation during the implementation period and achieved reductions in AvLoS for some conditions such as chronic obstructive pulmonary disease and heart failure, contributing to varying degrees (2%6%) to the bed‐days savings achieved by the NAMP. During the 3‐year period, there was a 25% increase in medical discharges. This is partly due to the changing demographics and epidemiology of chronic diseases in the Irish population. This increased demand was absorbed by the system with no increase in acute bed usage. We estimated that approximately 1000 additional acute beds would have been required if the NAMP efficiencies had not been achieved. Concurrent financial constraints compounded the stress on the public health system by limiting the available staff and resources for the new AMAUs and by reducing the number of community and nursing home beds available. This obstructed the flow of older and frailer patients out of the acute setting and impacted negatively on the performance of some hospitals.
An important limitation in auditing success in the quality and access aims of the program was the absence of IT systems within the AMAUs. These have since been specified by the NAMP but have not yet been delivered to the service areas. In addition, a bespoke user interface, which allows hospitals to manipulate and benchmark their own performance, is being developed. This will facilitate more in‐depth auditing within hospitals at the ward and consultant team level. The lack of a unique patient identifier hindered our ability to measure true 28‐day readmission rates, which is a useful quality indicator.
Despite these contextual, cultural, and structural challenges, the NAMP successfully implemented an evidence‐based model of care across the country. Through its implementation, tangible improvements to the Irish health system were observed with expected benefits to the patient. The program successfully instituted an ongoing audit cycle to promote continuous improvement and identified areas for future work to build on the successes achieved.
Disclosure
Nothing to report.
- ,. The effect of emergency department crowding on patient outcomes: a literature review. Adv Emerg Nurs J. 2011;33(1):39–54.
- , , , et al. Effect of emergency department crowding on outcomes of admitted patients. Ann Emerg Med. 2013;61(6):605–611.e6.
- , , . The relationship between emergency department crowding and patient outcomes: a systematic review. J Nurs Scholarsh. 2014;46(2):106–115.
- , , , et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16(1):1–10.
- Acute medical assessment units: a literature review. 2012. [Unpublished Manuscript]
- National Acute Medicine Programme Working Group. Report of the National Acute Medicine Programme 2010. Retrieved on Sep 24, 2014 from, http://www.hse.ie/eng/about/Who/clinical/natclinprog/acutemedicineprogramme/report.pdf. [Retrieved]
- Royal College of Physicians. Acute medical care. The right person, in the right setting—first time. October 2007. Retrieved on Sep 24, 2014, from, https://www.rcplondon.ac.uk/sites/default/files/documents/acute_medical_care_final_for_web.pdf.
- . Increase in patient mortality at 10 days associated with emergency department overcrowding. Med J Aust. 2006;184(5):213–216.
In 2009, Irish hospitals were experiencing ongoing and increasing overcrowding of emergency departments (EDs). This overcrowding and subsequent assessment delays are both associated with increased morbidity and mortality rates.[1, 2, 3, 4] The prevailing culture in many larger hospitals was to prioritize subspecialty care at the expense of the assessment and management of patients with undifferentiated acute medical presentations with nonspecific symptoms. The National Acute Medicine Programme (NAMP) was set up in 2010 by the Royal College of Physicians in Ireland (RCPI) and the Health Service Executive (HSE) to address this unsatisfactory management of acutely ill medical patients.
The objectives of the NAMP are categorized under 3 quality improvement principles: (1) Quality: to improve quality of care and patient safety by ensuring patients are seen by a nurse within 20 minutes and a senior doctor within 1 hour of arrival. (2) Access: to improve access by ensuring that the patient journey from presentation to decision to admit or discharge does not exceed 6 hours and to eliminate extended waiting periods on gurneys for medical patients. (3) Cost: to reduce cost and increase value by achieving bed savings through reduced overnight admissions and shortened lengths of stay.
The program was implemented by a small national team, which included hospital and public health physicians, nurses, a health and social care professional (HSCP), a general practitioner (GP), and a program manager. RCPI also set up a National Advisory Group of Consultant Physicians, comprised of representative medical consultants from all over the country, and key links were established with each acute hospital. The team aimed to develop a standardized model of care for all acutely ill medical patients and ensure its full implementation nationally.
METHODS
A literature review was undertaken to develop the standardized model of care in agreement with stakeholders and in consultation with patient groups.[5] The model of care required the establishment of acute medical assessment units (AMAUs), whose main function was to assess to discharge rather than admit to assess patients.[6, 7] At that time, only 8 of the 33 acute Irish hospitals that admitted medical patients had an AMAU. However, their function and operation varied greatly. In the remaining hospitals, all medical patients went to the ED, and from there were either admitted or discharged. Delays in access to senior clinicians, diagnostics, and allied health professionals such as, Occupational Therapists, Physiotherapists and Speech and Language Therapists often resulted in delays in assessment and treatment that could lead to overnight admissions.
In the new model, all acute medical patients, except those requiring invasive monitoring, critical care, or special services such as oncology and dialysis, are referred to the AMAU by another doctor (ie. a GP, outpatient department, or ED physician), as shown in Figure 1. A senior physician in the AMAU then reviews the patient and decides to admit or discharge. This doctor can either be a dedicated physician with an interest in acute general medicine, or a specialist consultant rostered to work in the unit on a regular basis. Some patients are discharged the same day thanks to prompt review and treatment. Of those requiring overnight admission, some are streamed directly to specialist pathways (eg. coronary care unit). The remaining patients are admitted to the medical short‐stay unit (MSSU) under the care of an acute physician. Patients in the MSSU are then either discharged within 48 hours or go on to be transferred to a specialist ward.

The model of care was therefore divided into 4 care pathways. National Health Service (NHS) admission data for 2008 to 2009 were used to calculate the proportion of patients who flowed through each pathway. The NHS has a wealth of experience in the development and use of AMAUs, having started implementing these units in the early 2000s. Therefore, the NHS estimates calculated above were used to set the national benchmarks for the NAMP. The four pathways are:
1. Ambulatory Care Pathway
Patients receive safe and effective treatment and are discharged on the same day. The NAMP benchmark was that at least 25% of AMAU admissions should follow this pathway of care.
2. Medical Short‐Stay Care Pathway
This pathway was developed for those patients who require inpatient care but are not expected to stay longer than 1 or 2 nights. The program benchmark was that 31% of patients should be discharged within 48 hours.
3. Routine Specialist Inpatient Care Pathway
Approximately 33% of medical admissions are expected to stay more than 2 days and less than 14 days in the hospital and have a straightforward discharge after their acute episode of care. These patients are admitted either directly to specialist medical wards from AMAU or via the MSSU within 2 days of arrival. Care is formally handed over from the AMAU team to the appropriate consultant physician upon transfer.
4. Appropriate Care and Discharge of Complex Patients Care Pathway
Frail older patients have complex care needs that continue following discharge, and their discharge requirements must be identified early during the acute care episode. The NAMP benchmark was that no more than 11% of medical admissions would fall into this pathway and require a length of stay (LoS) exceeding 14 days.
The flow model was used to build system capacity by modeling and predicting the expected demand on each AMAU to assist in forward planning The number of assessment spaces and ward beds required for each hospital were calculated by analyzing respective admission data for 2009 and applying target lengths of stay for medical patients to the flow model. The program team carried out this analysis for each of the 32 hospitals. The model of care also identified a number of practice changes under each pathway that would be required to achieve process changes and the resulting efficiency gains. Table 1 summarizes these.
|
| Ambulatory care pathway |
| Establishment of adequate assessment area |
| National early warning score within 20 minutes |
| Access to senior decision maker within 1 hour |
| Access to rapid diagnostics and HSCP assessment |
| Development of clinical criteria for transfer between ED and AMAU |
| Liaison with discharge planner |
| Clear pathways to specialist wards and community support |
| Close liaison with GP to ensure integrated care |
| Patient experience time in AMAU to be 6 hours or less |
| Medical short‐stay care pathway |
| Establishment of adequate short‐stay unit |
| Access to senior decision maker within 12 hours of transfer from AMAU |
| Twice daily consultant ward rounds |
| Access to prioritized diagnostics and HSCP assessment |
| Integrated discharge planning |
| Routine specialist inpatient care pathway |
| Daily consultant ward rounds |
| Weekend nurse/HSCP‐facilitated discharges |
| Active discharge planning with planned dates of discharge for every patient |
| Liaison with caregivers and community supports |
| Development of clinical criteria to support bidirectional flow to community hospitals within hospital groups |
| Appropriate care and discharge of complex patients care pathway |
| Early assessment and identification of complex patients |
| Streaming to care of the elderly services where appropriate |
| Proactive multidisciplinary discharge planning and liaison with funding agencies for referral to community placements and supports |
Hospitals were also categorized into 4 divisions or models as determined by the complexity of patients they admit. Model 1 hospitals are community units with subacute inpatient beds that can care for patients with rehabilitation, respite, or palliative care needs. Model 2 hospitals are small hospitals that provide inpatient and outpatient care for low‐risk, differentiated medical patients or refer on to associated higher complexity facilities. The majority of hospitals in the country are model 3 general hospitals, admitting 50% of all medical patients. Last, model 4 hospitals are the 8 regional tertiary referral centers in Ireland. A considerable volume of their patient workload remains inpatient admissions for routine specialist inpatient care.
Measuring success in the program's quality and access objectives required the development of a bespoke information technology (IT) system that is not yet operational, and therefore these objectives could not be audited.
A number of outcome measures or key performance indicators (KPIs) were developed to assess performance under each care pathway relative to the cost objectives of the NAMP as shown in Table 2. The available hospital inpatient enquiry (HIPE) data were analyzed by the program team to establish baseline performance metrics for each hospital. Initially, these data were only available to the NAMP 1 year in arrears. However, the NAMP worked with the hospitals and the HIPE system to improve the completeness and timeliness of the HIPE reporting, so that by the third quarter of 2011 monthly data were available. Audit cycles occurred on a continuous monthly basis, with feedback provided to each hospital and follow‐up of results conducted at a local level. This allowed for analysis of performance at a national, hospital group, and individual hospital level. Of note, it was only possible to analyze readmission rates to the same facility in the absence of a national unique patient identifier, and therefore readmission rates observed were of limited use as a quality measure.
| Care Pathway | Metric | National Target | 2010 | 2011 | 2012 | 2013* |
|---|---|---|---|---|---|---|
| ||||||
| Ambulatory care pathway | % of patients with LoS=0 | 25% | 11.5% | 12.9% | 18.8% | 23.2% |
| Medical short‐stay pathway | % of patients with LoS 12 days | 31% | 25.4% | 25.9% | 25.6% | 23.8% |
| Routine specialist inpatient pathway | % of patients with LoS>2 days | 44% | 63.1% | 61.2% | 55.6% | 53.1% |
| Complex care pathway | % of patients with LoS>14 days | 11% | 13.1% | 12.4% | 11.0% | 10.8% |
| % BDU of patients with LoS>30 days | 33% | 36.9% | 36.0% | 35.1% | 34.4% | |
| Routine and complex care pathway | Average LoS for those staying >2 days | 610 days | 12.9 | 12.7 | 12.4 | 12.4 |
| Summary metric | Overall average LoS | 5.8 days | 8.5 | 8.1 | 7.2 | 6.9 |
| No. of medical discharges | 202,567 | 206,250 | 235,167 | 253,083 | ||
RESULTS
The NAMP model of care was officially launched in December 2010.[6] Thirty‐two out of the 33 Irish hospitals that admit acute medical patients had adopted the model of care by the end of 2013. The program team performed an initial diagnostic meeting at each hospital to explain the program, discuss their individual baseline metrics, and collaboratively develop a hospital‐specific implementation plan. A local implementation and unscheduled care governance team, composed of senior management members and local GPs, was established in each hospital to identify ward spaces to be developed as AMAUs, reassign nursing staff to the AMAU from the wards, and organize the recruitment of new consultants with an interest in acute general medicine. The program team performed 2 to 3 visits per year to each hospital to obtain feedback on performance and support local improvement plans using appreciative enquiry. They also organized workshops and training for physicians, nurses, managers, and data managers to improve understanding of and engagement with the program. An acute medicine nurse interest group was convened to support nurses in the transition to clinical practice with a greater focus on ambulatory care. Annual conferences were held to present and discuss annual and cumulative audit results.
Table 2 presents the national KPI results for the cost and value objectives over the 3 years of implementation. The number of medical discharges increased from 202,567 in 2010 to 253,083 in 2013. The proportion of discharges that passed through the AMAU was 29% in 2013, considerably reducing the amount of patients seen through the ED and alleviating some of the overcrowding experienced there.
The proportion of medical patients who avoided admission increased from 11.5% to 23.2% in 2013. When examining the proportion of patients discharged within 48 hours, we combined results for the ambulatory care pathway (LoS=0) and the medical short‐stay pathway (LoS=12) and found a 10% increase nationally from 36.9% to 47% in 2013. In addition, the proportion of total medical bed‐days used (BDU) for patients with LoS over 30 days also improved by 2.5%. The program achieved an overall reduction of 0.5 days in those staying over 2 days nationally, and an overall reduction in average LoS (AvLoS) for all medical inpatients of 1.6 days (from 8.5 days 6.9 days) across the 3 years.
Table 3 shows the average change in KPIs from 2010 to 2013 by hospital model group. Looking at data by hospital group allowed results to be interpreted in a national context and identify any bottlenecks in the health system.
| Care pathway | Metric | National | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|---|
| |||||
| Ambulatory care pathway | % of patients with LoS=0 | 11.7% | 11.5% | 12% | 11.5% |
| Medical short‐stay pathway | % of patients with LoS 12 days | 1.6% | 5% | 2.3% | 0.3% |
| Routine specialist inpatient pathway | % of patients with LoS>2 days | 10% | 6.4% | 9.8% | 11.2% |
| Complex care pathway | % of patients with LoS>14 days | 2.3% | 0.4% | 1.7% | 4.1% |
| % BDU of patients with LoS>30 days | 2.5% | 1.9% | 0.2% | 4.9% | |
| Routine and complex care pathway | Average LoS for those staying >2 days | 0.5 | 0.7 | 0 | 1.4 |
| Summary metric | Overall average LoS | 1.6 | 0.4 | 1.0 | 2.6 |
During the 3‐year period, the role of model 2 hospitals changed from admitting all medical patients to only admitting differentiated medical patients referred from GPs. This is reflected in their KPI results, with an increasing proportion of patients with LoS greater than 14 days and the proportion of BDU occupied by those with LoS greater than 30 days. Data from the model 2 and 3 hospitals showed a considerable increase in same‐day discharges, with a concurrent decrease in percentage of patients staying in the hospital longer than 2 days. This translated to a national reduction in AvLoS of 1 day in this hospital group. Model 2 hospitals experienced small increases in both the AvLoS for those patients staying over 2 days (0.7%) and the proportion of BDU occupied by patients staying longer than 30 days (1.9%), whereas model 3 had experienced no real change in either of these metrics (0% and 0.2%, respectively). This reflected the limited availability of long‐term care facilities and protracted funding approval process nationally during the implementation period.
Model 4 hospitals experienced improvement across all KPIs. There was an 11.2% increase in the proportion of patients discharged within 48 hours and a 1.4‐day reduction in AvLoS for patients with LoS>2 days. A notable success within this hospital category was the 4.9% reduction in percentage of BDU by patients with LoS>30 days. The AvLoS for all medical admissions in this group remained above the national target at 8.6 days but did decrease considerably by 2.6 days from its baseline.
Data on 28‐day readmission to the same facility were used as a balancing measure but were only available for the latter 2 years. We found rates of 11% and 10% for 2012 and 2013, respectively. Patient experience of these new units should be assessed, but it was not possible to measure this during the implementation period.
DISCUSSION
The implementation of the NAMP has demonstrably streamlined the care of acute medical patients in Ireland. We report the results of this national transformational change brought about by the implementation of an evidence‐based model of care. The development of a flow model for each hospital improved the patient flow from assessment to discharge. Process improvement lies at the core of all the successes achieved by the program. The practice changes highlighted in Table 1 were pivotal in streamlining and improving the care of acutely ill medical patients. The focus on early access to senior decision making, early diagnostics, and a continuous, coordinated, multidisciplinary approach to care and discharge were central to the effective functioning of the AMAU and the resulting increase in avoided admissions.
Shortened lengths of stay are associated with better clinical outcomes and reduced exposure of patients to risk, and result in significant cost efficiencies accrued to the Irish health services.[2, 8] The adoption of ambulatory care and medical short‐stay pathways facilitated the 11.7% increase in avoided admissions and the reduction of 1.6 days in overall AvLoS nationally. This translates to significant cost savings for the Irish health system and likely improves clinical outcomes and reduced morbidity. We estimated these cost savings to be approximately 88.2 million by multiplying the number of bed days saved by the marginal cost of a bed day, which was quoted at 246 in 2012 by our Healthcare Pricing Office.
Thirty‐two of the 33 Irish hospitals that admit acute medical patients are now operating the program and achieving improvements in performance, as evidenced by ongoing audits. The priority given to the program by the RCPI and HSE has enabled the assignment of local implementation teams sustaining the focus on quality improvement at a local level. It also allowed for modest seed funding to be allocated for the appointment of 36 new consultants with an interest in acute general medicine. The cost of these additional consultants is offset by the considerable savings achieved through efficiency gains. An important challenge to implementation was the change in mindset required from local healthcare staff to divert patients away from the ED to the AMAU, and reassign staff and resources from other inpatient wards to the new unit. Visible clinical leadership from clinical directors, acute medicine hospital leads, senior nursing, and HSCP, together with management and local GPs, was essential in effecting this change. The program team also offered considerable support in this regard through advocacy and promotion of the program nationally. The implementation of the 4 care pathways represents a generational change in how medicine is practiced in Ireland. The development of acute medicine as a new specialty was strongly fostered by the program.
A number of disease‐specific clinical programs began operation during the implementation period and achieved reductions in AvLoS for some conditions such as chronic obstructive pulmonary disease and heart failure, contributing to varying degrees (2%6%) to the bed‐days savings achieved by the NAMP. During the 3‐year period, there was a 25% increase in medical discharges. This is partly due to the changing demographics and epidemiology of chronic diseases in the Irish population. This increased demand was absorbed by the system with no increase in acute bed usage. We estimated that approximately 1000 additional acute beds would have been required if the NAMP efficiencies had not been achieved. Concurrent financial constraints compounded the stress on the public health system by limiting the available staff and resources for the new AMAUs and by reducing the number of community and nursing home beds available. This obstructed the flow of older and frailer patients out of the acute setting and impacted negatively on the performance of some hospitals.
An important limitation in auditing success in the quality and access aims of the program was the absence of IT systems within the AMAUs. These have since been specified by the NAMP but have not yet been delivered to the service areas. In addition, a bespoke user interface, which allows hospitals to manipulate and benchmark their own performance, is being developed. This will facilitate more in‐depth auditing within hospitals at the ward and consultant team level. The lack of a unique patient identifier hindered our ability to measure true 28‐day readmission rates, which is a useful quality indicator.
Despite these contextual, cultural, and structural challenges, the NAMP successfully implemented an evidence‐based model of care across the country. Through its implementation, tangible improvements to the Irish health system were observed with expected benefits to the patient. The program successfully instituted an ongoing audit cycle to promote continuous improvement and identified areas for future work to build on the successes achieved.
Disclosure
Nothing to report.
In 2009, Irish hospitals were experiencing ongoing and increasing overcrowding of emergency departments (EDs). This overcrowding and subsequent assessment delays are both associated with increased morbidity and mortality rates.[1, 2, 3, 4] The prevailing culture in many larger hospitals was to prioritize subspecialty care at the expense of the assessment and management of patients with undifferentiated acute medical presentations with nonspecific symptoms. The National Acute Medicine Programme (NAMP) was set up in 2010 by the Royal College of Physicians in Ireland (RCPI) and the Health Service Executive (HSE) to address this unsatisfactory management of acutely ill medical patients.
The objectives of the NAMP are categorized under 3 quality improvement principles: (1) Quality: to improve quality of care and patient safety by ensuring patients are seen by a nurse within 20 minutes and a senior doctor within 1 hour of arrival. (2) Access: to improve access by ensuring that the patient journey from presentation to decision to admit or discharge does not exceed 6 hours and to eliminate extended waiting periods on gurneys for medical patients. (3) Cost: to reduce cost and increase value by achieving bed savings through reduced overnight admissions and shortened lengths of stay.
The program was implemented by a small national team, which included hospital and public health physicians, nurses, a health and social care professional (HSCP), a general practitioner (GP), and a program manager. RCPI also set up a National Advisory Group of Consultant Physicians, comprised of representative medical consultants from all over the country, and key links were established with each acute hospital. The team aimed to develop a standardized model of care for all acutely ill medical patients and ensure its full implementation nationally.
METHODS
A literature review was undertaken to develop the standardized model of care in agreement with stakeholders and in consultation with patient groups.[5] The model of care required the establishment of acute medical assessment units (AMAUs), whose main function was to assess to discharge rather than admit to assess patients.[6, 7] At that time, only 8 of the 33 acute Irish hospitals that admitted medical patients had an AMAU. However, their function and operation varied greatly. In the remaining hospitals, all medical patients went to the ED, and from there were either admitted or discharged. Delays in access to senior clinicians, diagnostics, and allied health professionals such as, Occupational Therapists, Physiotherapists and Speech and Language Therapists often resulted in delays in assessment and treatment that could lead to overnight admissions.
In the new model, all acute medical patients, except those requiring invasive monitoring, critical care, or special services such as oncology and dialysis, are referred to the AMAU by another doctor (ie. a GP, outpatient department, or ED physician), as shown in Figure 1. A senior physician in the AMAU then reviews the patient and decides to admit or discharge. This doctor can either be a dedicated physician with an interest in acute general medicine, or a specialist consultant rostered to work in the unit on a regular basis. Some patients are discharged the same day thanks to prompt review and treatment. Of those requiring overnight admission, some are streamed directly to specialist pathways (eg. coronary care unit). The remaining patients are admitted to the medical short‐stay unit (MSSU) under the care of an acute physician. Patients in the MSSU are then either discharged within 48 hours or go on to be transferred to a specialist ward.

The model of care was therefore divided into 4 care pathways. National Health Service (NHS) admission data for 2008 to 2009 were used to calculate the proportion of patients who flowed through each pathway. The NHS has a wealth of experience in the development and use of AMAUs, having started implementing these units in the early 2000s. Therefore, the NHS estimates calculated above were used to set the national benchmarks for the NAMP. The four pathways are:
1. Ambulatory Care Pathway
Patients receive safe and effective treatment and are discharged on the same day. The NAMP benchmark was that at least 25% of AMAU admissions should follow this pathway of care.
2. Medical Short‐Stay Care Pathway
This pathway was developed for those patients who require inpatient care but are not expected to stay longer than 1 or 2 nights. The program benchmark was that 31% of patients should be discharged within 48 hours.
3. Routine Specialist Inpatient Care Pathway
Approximately 33% of medical admissions are expected to stay more than 2 days and less than 14 days in the hospital and have a straightforward discharge after their acute episode of care. These patients are admitted either directly to specialist medical wards from AMAU or via the MSSU within 2 days of arrival. Care is formally handed over from the AMAU team to the appropriate consultant physician upon transfer.
4. Appropriate Care and Discharge of Complex Patients Care Pathway
Frail older patients have complex care needs that continue following discharge, and their discharge requirements must be identified early during the acute care episode. The NAMP benchmark was that no more than 11% of medical admissions would fall into this pathway and require a length of stay (LoS) exceeding 14 days.
The flow model was used to build system capacity by modeling and predicting the expected demand on each AMAU to assist in forward planning The number of assessment spaces and ward beds required for each hospital were calculated by analyzing respective admission data for 2009 and applying target lengths of stay for medical patients to the flow model. The program team carried out this analysis for each of the 32 hospitals. The model of care also identified a number of practice changes under each pathway that would be required to achieve process changes and the resulting efficiency gains. Table 1 summarizes these.
|
| Ambulatory care pathway |
| Establishment of adequate assessment area |
| National early warning score within 20 minutes |
| Access to senior decision maker within 1 hour |
| Access to rapid diagnostics and HSCP assessment |
| Development of clinical criteria for transfer between ED and AMAU |
| Liaison with discharge planner |
| Clear pathways to specialist wards and community support |
| Close liaison with GP to ensure integrated care |
| Patient experience time in AMAU to be 6 hours or less |
| Medical short‐stay care pathway |
| Establishment of adequate short‐stay unit |
| Access to senior decision maker within 12 hours of transfer from AMAU |
| Twice daily consultant ward rounds |
| Access to prioritized diagnostics and HSCP assessment |
| Integrated discharge planning |
| Routine specialist inpatient care pathway |
| Daily consultant ward rounds |
| Weekend nurse/HSCP‐facilitated discharges |
| Active discharge planning with planned dates of discharge for every patient |
| Liaison with caregivers and community supports |
| Development of clinical criteria to support bidirectional flow to community hospitals within hospital groups |
| Appropriate care and discharge of complex patients care pathway |
| Early assessment and identification of complex patients |
| Streaming to care of the elderly services where appropriate |
| Proactive multidisciplinary discharge planning and liaison with funding agencies for referral to community placements and supports |
Hospitals were also categorized into 4 divisions or models as determined by the complexity of patients they admit. Model 1 hospitals are community units with subacute inpatient beds that can care for patients with rehabilitation, respite, or palliative care needs. Model 2 hospitals are small hospitals that provide inpatient and outpatient care for low‐risk, differentiated medical patients or refer on to associated higher complexity facilities. The majority of hospitals in the country are model 3 general hospitals, admitting 50% of all medical patients. Last, model 4 hospitals are the 8 regional tertiary referral centers in Ireland. A considerable volume of their patient workload remains inpatient admissions for routine specialist inpatient care.
Measuring success in the program's quality and access objectives required the development of a bespoke information technology (IT) system that is not yet operational, and therefore these objectives could not be audited.
A number of outcome measures or key performance indicators (KPIs) were developed to assess performance under each care pathway relative to the cost objectives of the NAMP as shown in Table 2. The available hospital inpatient enquiry (HIPE) data were analyzed by the program team to establish baseline performance metrics for each hospital. Initially, these data were only available to the NAMP 1 year in arrears. However, the NAMP worked with the hospitals and the HIPE system to improve the completeness and timeliness of the HIPE reporting, so that by the third quarter of 2011 monthly data were available. Audit cycles occurred on a continuous monthly basis, with feedback provided to each hospital and follow‐up of results conducted at a local level. This allowed for analysis of performance at a national, hospital group, and individual hospital level. Of note, it was only possible to analyze readmission rates to the same facility in the absence of a national unique patient identifier, and therefore readmission rates observed were of limited use as a quality measure.
| Care Pathway | Metric | National Target | 2010 | 2011 | 2012 | 2013* |
|---|---|---|---|---|---|---|
| ||||||
| Ambulatory care pathway | % of patients with LoS=0 | 25% | 11.5% | 12.9% | 18.8% | 23.2% |
| Medical short‐stay pathway | % of patients with LoS 12 days | 31% | 25.4% | 25.9% | 25.6% | 23.8% |
| Routine specialist inpatient pathway | % of patients with LoS>2 days | 44% | 63.1% | 61.2% | 55.6% | 53.1% |
| Complex care pathway | % of patients with LoS>14 days | 11% | 13.1% | 12.4% | 11.0% | 10.8% |
| % BDU of patients with LoS>30 days | 33% | 36.9% | 36.0% | 35.1% | 34.4% | |
| Routine and complex care pathway | Average LoS for those staying >2 days | 610 days | 12.9 | 12.7 | 12.4 | 12.4 |
| Summary metric | Overall average LoS | 5.8 days | 8.5 | 8.1 | 7.2 | 6.9 |
| No. of medical discharges | 202,567 | 206,250 | 235,167 | 253,083 | ||
RESULTS
The NAMP model of care was officially launched in December 2010.[6] Thirty‐two out of the 33 Irish hospitals that admit acute medical patients had adopted the model of care by the end of 2013. The program team performed an initial diagnostic meeting at each hospital to explain the program, discuss their individual baseline metrics, and collaboratively develop a hospital‐specific implementation plan. A local implementation and unscheduled care governance team, composed of senior management members and local GPs, was established in each hospital to identify ward spaces to be developed as AMAUs, reassign nursing staff to the AMAU from the wards, and organize the recruitment of new consultants with an interest in acute general medicine. The program team performed 2 to 3 visits per year to each hospital to obtain feedback on performance and support local improvement plans using appreciative enquiry. They also organized workshops and training for physicians, nurses, managers, and data managers to improve understanding of and engagement with the program. An acute medicine nurse interest group was convened to support nurses in the transition to clinical practice with a greater focus on ambulatory care. Annual conferences were held to present and discuss annual and cumulative audit results.
Table 2 presents the national KPI results for the cost and value objectives over the 3 years of implementation. The number of medical discharges increased from 202,567 in 2010 to 253,083 in 2013. The proportion of discharges that passed through the AMAU was 29% in 2013, considerably reducing the amount of patients seen through the ED and alleviating some of the overcrowding experienced there.
The proportion of medical patients who avoided admission increased from 11.5% to 23.2% in 2013. When examining the proportion of patients discharged within 48 hours, we combined results for the ambulatory care pathway (LoS=0) and the medical short‐stay pathway (LoS=12) and found a 10% increase nationally from 36.9% to 47% in 2013. In addition, the proportion of total medical bed‐days used (BDU) for patients with LoS over 30 days also improved by 2.5%. The program achieved an overall reduction of 0.5 days in those staying over 2 days nationally, and an overall reduction in average LoS (AvLoS) for all medical inpatients of 1.6 days (from 8.5 days 6.9 days) across the 3 years.
Table 3 shows the average change in KPIs from 2010 to 2013 by hospital model group. Looking at data by hospital group allowed results to be interpreted in a national context and identify any bottlenecks in the health system.
| Care pathway | Metric | National | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|---|
| |||||
| Ambulatory care pathway | % of patients with LoS=0 | 11.7% | 11.5% | 12% | 11.5% |
| Medical short‐stay pathway | % of patients with LoS 12 days | 1.6% | 5% | 2.3% | 0.3% |
| Routine specialist inpatient pathway | % of patients with LoS>2 days | 10% | 6.4% | 9.8% | 11.2% |
| Complex care pathway | % of patients with LoS>14 days | 2.3% | 0.4% | 1.7% | 4.1% |
| % BDU of patients with LoS>30 days | 2.5% | 1.9% | 0.2% | 4.9% | |
| Routine and complex care pathway | Average LoS for those staying >2 days | 0.5 | 0.7 | 0 | 1.4 |
| Summary metric | Overall average LoS | 1.6 | 0.4 | 1.0 | 2.6 |
During the 3‐year period, the role of model 2 hospitals changed from admitting all medical patients to only admitting differentiated medical patients referred from GPs. This is reflected in their KPI results, with an increasing proportion of patients with LoS greater than 14 days and the proportion of BDU occupied by those with LoS greater than 30 days. Data from the model 2 and 3 hospitals showed a considerable increase in same‐day discharges, with a concurrent decrease in percentage of patients staying in the hospital longer than 2 days. This translated to a national reduction in AvLoS of 1 day in this hospital group. Model 2 hospitals experienced small increases in both the AvLoS for those patients staying over 2 days (0.7%) and the proportion of BDU occupied by patients staying longer than 30 days (1.9%), whereas model 3 had experienced no real change in either of these metrics (0% and 0.2%, respectively). This reflected the limited availability of long‐term care facilities and protracted funding approval process nationally during the implementation period.
Model 4 hospitals experienced improvement across all KPIs. There was an 11.2% increase in the proportion of patients discharged within 48 hours and a 1.4‐day reduction in AvLoS for patients with LoS>2 days. A notable success within this hospital category was the 4.9% reduction in percentage of BDU by patients with LoS>30 days. The AvLoS for all medical admissions in this group remained above the national target at 8.6 days but did decrease considerably by 2.6 days from its baseline.
Data on 28‐day readmission to the same facility were used as a balancing measure but were only available for the latter 2 years. We found rates of 11% and 10% for 2012 and 2013, respectively. Patient experience of these new units should be assessed, but it was not possible to measure this during the implementation period.
DISCUSSION
The implementation of the NAMP has demonstrably streamlined the care of acute medical patients in Ireland. We report the results of this national transformational change brought about by the implementation of an evidence‐based model of care. The development of a flow model for each hospital improved the patient flow from assessment to discharge. Process improvement lies at the core of all the successes achieved by the program. The practice changes highlighted in Table 1 were pivotal in streamlining and improving the care of acutely ill medical patients. The focus on early access to senior decision making, early diagnostics, and a continuous, coordinated, multidisciplinary approach to care and discharge were central to the effective functioning of the AMAU and the resulting increase in avoided admissions.
Shortened lengths of stay are associated with better clinical outcomes and reduced exposure of patients to risk, and result in significant cost efficiencies accrued to the Irish health services.[2, 8] The adoption of ambulatory care and medical short‐stay pathways facilitated the 11.7% increase in avoided admissions and the reduction of 1.6 days in overall AvLoS nationally. This translates to significant cost savings for the Irish health system and likely improves clinical outcomes and reduced morbidity. We estimated these cost savings to be approximately 88.2 million by multiplying the number of bed days saved by the marginal cost of a bed day, which was quoted at 246 in 2012 by our Healthcare Pricing Office.
Thirty‐two of the 33 Irish hospitals that admit acute medical patients are now operating the program and achieving improvements in performance, as evidenced by ongoing audits. The priority given to the program by the RCPI and HSE has enabled the assignment of local implementation teams sustaining the focus on quality improvement at a local level. It also allowed for modest seed funding to be allocated for the appointment of 36 new consultants with an interest in acute general medicine. The cost of these additional consultants is offset by the considerable savings achieved through efficiency gains. An important challenge to implementation was the change in mindset required from local healthcare staff to divert patients away from the ED to the AMAU, and reassign staff and resources from other inpatient wards to the new unit. Visible clinical leadership from clinical directors, acute medicine hospital leads, senior nursing, and HSCP, together with management and local GPs, was essential in effecting this change. The program team also offered considerable support in this regard through advocacy and promotion of the program nationally. The implementation of the 4 care pathways represents a generational change in how medicine is practiced in Ireland. The development of acute medicine as a new specialty was strongly fostered by the program.
A number of disease‐specific clinical programs began operation during the implementation period and achieved reductions in AvLoS for some conditions such as chronic obstructive pulmonary disease and heart failure, contributing to varying degrees (2%6%) to the bed‐days savings achieved by the NAMP. During the 3‐year period, there was a 25% increase in medical discharges. This is partly due to the changing demographics and epidemiology of chronic diseases in the Irish population. This increased demand was absorbed by the system with no increase in acute bed usage. We estimated that approximately 1000 additional acute beds would have been required if the NAMP efficiencies had not been achieved. Concurrent financial constraints compounded the stress on the public health system by limiting the available staff and resources for the new AMAUs and by reducing the number of community and nursing home beds available. This obstructed the flow of older and frailer patients out of the acute setting and impacted negatively on the performance of some hospitals.
An important limitation in auditing success in the quality and access aims of the program was the absence of IT systems within the AMAUs. These have since been specified by the NAMP but have not yet been delivered to the service areas. In addition, a bespoke user interface, which allows hospitals to manipulate and benchmark their own performance, is being developed. This will facilitate more in‐depth auditing within hospitals at the ward and consultant team level. The lack of a unique patient identifier hindered our ability to measure true 28‐day readmission rates, which is a useful quality indicator.
Despite these contextual, cultural, and structural challenges, the NAMP successfully implemented an evidence‐based model of care across the country. Through its implementation, tangible improvements to the Irish health system were observed with expected benefits to the patient. The program successfully instituted an ongoing audit cycle to promote continuous improvement and identified areas for future work to build on the successes achieved.
Disclosure
Nothing to report.
- ,. The effect of emergency department crowding on patient outcomes: a literature review. Adv Emerg Nurs J. 2011;33(1):39–54.
- , , , et al. Effect of emergency department crowding on outcomes of admitted patients. Ann Emerg Med. 2013;61(6):605–611.e6.
- , , . The relationship between emergency department crowding and patient outcomes: a systematic review. J Nurs Scholarsh. 2014;46(2):106–115.
- , , , et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16(1):1–10.
- Acute medical assessment units: a literature review. 2012. [Unpublished Manuscript]
- National Acute Medicine Programme Working Group. Report of the National Acute Medicine Programme 2010. Retrieved on Sep 24, 2014 from, http://www.hse.ie/eng/about/Who/clinical/natclinprog/acutemedicineprogramme/report.pdf. [Retrieved]
- Royal College of Physicians. Acute medical care. The right person, in the right setting—first time. October 2007. Retrieved on Sep 24, 2014, from, https://www.rcplondon.ac.uk/sites/default/files/documents/acute_medical_care_final_for_web.pdf.
- . Increase in patient mortality at 10 days associated with emergency department overcrowding. Med J Aust. 2006;184(5):213–216.
- ,. The effect of emergency department crowding on patient outcomes: a literature review. Adv Emerg Nurs J. 2011;33(1):39–54.
- , , , et al. Effect of emergency department crowding on outcomes of admitted patients. Ann Emerg Med. 2013;61(6):605–611.e6.
- , , . The relationship between emergency department crowding and patient outcomes: a systematic review. J Nurs Scholarsh. 2014;46(2):106–115.
- , , , et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16(1):1–10.
- Acute medical assessment units: a literature review. 2012. [Unpublished Manuscript]
- National Acute Medicine Programme Working Group. Report of the National Acute Medicine Programme 2010. Retrieved on Sep 24, 2014 from, http://www.hse.ie/eng/about/Who/clinical/natclinprog/acutemedicineprogramme/report.pdf. [Retrieved]
- Royal College of Physicians. Acute medical care. The right person, in the right setting—first time. October 2007. Retrieved on Sep 24, 2014, from, https://www.rcplondon.ac.uk/sites/default/files/documents/acute_medical_care_final_for_web.pdf.
- . Increase in patient mortality at 10 days associated with emergency department overcrowding. Med J Aust. 2006;184(5):213–216.
© 2015 Society of Hospital Medicine
Bluish Pink, Nontender Lesion Worries Patient’s Mother
A 12-year-old girl is brought to dermatology by her mother for evaluation of a lesion on her arm. It’s been there for two years without causing symptoms—but lately it has grown, as has the mother’s concern.
The child is otherwise healthy. The mother reports that the child has neither a personal nor a family history of seizures.
EXAMINATION
A solitary, firm, subcutaneous nodule measuring 2 cm is located on the lateral aspect of the child’s left triceps. It is bluish pink, nontender, and firm on palpation. No other overlying skin changes are seen.
Lateral digital traction toward the center of the lesion produces no dimpling, while lateral traction toward its periphery accentuates the lesion’s central raised portion. The lesion is moderately mobile. No lymph nodes are felt on palpation of nodal sites in the area, and no other such lesions are found elsewhere on the child’s skin.
What is the diagnosis?
DISCUSSION
At this point, the differential included items such as pilomatricoma, dermatofibroma, calcinosis cutis, or epidermal cyst. The firm feel, bluish color, and shallow subcutaneous location of the lesion lent themselves to a provisional diagnosis of pilomatricoma, as did the patient’s age. But the fact that the lesion was changing was of sufficient concern to prompt removal.
Excision revealed a cystic lesion with cottage-cheese–like contents and a poorly defined wall, extending more than a centimeter into the subcutaneous tissue. It was removed in one piece and submitted to pathology. Primary closure completed the procedure.
The pathology report showed sheets of anucleate squamous cells (called ghost cells), benign viable nucleated squamous cells, and a center filled with multiple soft calcified granules. A positive von Kossa stain confirmed the expected diagnosis of pilomatricoma (PMC; also spelled pilomatrixoma).
PMCs, also known by their eponymous designation of calcifying epithelioma of Malherbe, are common, benign appendageal tumors derived from hair matrix. They usually manifest (as in this case) as a solitary subcutaneous firm mass, often with bluish discoloration, on the face, neck, or upper extremities. While they average around 2 cm, they can be as large as 15 cm in diameter. They are more common in children and occur slightly more often in girls.
There is some evidence that the tendency to develop PMCs is associated with increased levels of beta-catenin, which encourages cell growth by diminishing apoptosis. This mechanism is thought to promote malignant transformation of PMCs—a rare event.
As is often the case, the main concern about this patient’s lesion related to its unknown source and recent alteration. Aside from scarring, the patient was no worse off for its removal—and her mother was much relieved.
TAKE-HOME LEARNING POINTS
• PMCs are benign cystic lesions of appendageal origin, commonly found on the necks, faces, and upper extremities of children.
• Diagnostic clues for PMC include firm feel, subcutaneous location, bluish discoloration, and patient age.
• PMCs have poorly defined cyst walls and granular calcified contents.
• Except when occurring in multiples, PMCs have no pathologic implications.
• The term calcifying epithelioma of Malherbe is still in use, as is the alternate spelling of pilomatrixoma.
A 12-year-old girl is brought to dermatology by her mother for evaluation of a lesion on her arm. It’s been there for two years without causing symptoms—but lately it has grown, as has the mother’s concern.
The child is otherwise healthy. The mother reports that the child has neither a personal nor a family history of seizures.
EXAMINATION
A solitary, firm, subcutaneous nodule measuring 2 cm is located on the lateral aspect of the child’s left triceps. It is bluish pink, nontender, and firm on palpation. No other overlying skin changes are seen.
Lateral digital traction toward the center of the lesion produces no dimpling, while lateral traction toward its periphery accentuates the lesion’s central raised portion. The lesion is moderately mobile. No lymph nodes are felt on palpation of nodal sites in the area, and no other such lesions are found elsewhere on the child’s skin.
What is the diagnosis?
DISCUSSION
At this point, the differential included items such as pilomatricoma, dermatofibroma, calcinosis cutis, or epidermal cyst. The firm feel, bluish color, and shallow subcutaneous location of the lesion lent themselves to a provisional diagnosis of pilomatricoma, as did the patient’s age. But the fact that the lesion was changing was of sufficient concern to prompt removal.
Excision revealed a cystic lesion with cottage-cheese–like contents and a poorly defined wall, extending more than a centimeter into the subcutaneous tissue. It was removed in one piece and submitted to pathology. Primary closure completed the procedure.
The pathology report showed sheets of anucleate squamous cells (called ghost cells), benign viable nucleated squamous cells, and a center filled with multiple soft calcified granules. A positive von Kossa stain confirmed the expected diagnosis of pilomatricoma (PMC; also spelled pilomatrixoma).
PMCs, also known by their eponymous designation of calcifying epithelioma of Malherbe, are common, benign appendageal tumors derived from hair matrix. They usually manifest (as in this case) as a solitary subcutaneous firm mass, often with bluish discoloration, on the face, neck, or upper extremities. While they average around 2 cm, they can be as large as 15 cm in diameter. They are more common in children and occur slightly more often in girls.
There is some evidence that the tendency to develop PMCs is associated with increased levels of beta-catenin, which encourages cell growth by diminishing apoptosis. This mechanism is thought to promote malignant transformation of PMCs—a rare event.
As is often the case, the main concern about this patient’s lesion related to its unknown source and recent alteration. Aside from scarring, the patient was no worse off for its removal—and her mother was much relieved.
TAKE-HOME LEARNING POINTS
• PMCs are benign cystic lesions of appendageal origin, commonly found on the necks, faces, and upper extremities of children.
• Diagnostic clues for PMC include firm feel, subcutaneous location, bluish discoloration, and patient age.
• PMCs have poorly defined cyst walls and granular calcified contents.
• Except when occurring in multiples, PMCs have no pathologic implications.
• The term calcifying epithelioma of Malherbe is still in use, as is the alternate spelling of pilomatrixoma.
A 12-year-old girl is brought to dermatology by her mother for evaluation of a lesion on her arm. It’s been there for two years without causing symptoms—but lately it has grown, as has the mother’s concern.
The child is otherwise healthy. The mother reports that the child has neither a personal nor a family history of seizures.
EXAMINATION
A solitary, firm, subcutaneous nodule measuring 2 cm is located on the lateral aspect of the child’s left triceps. It is bluish pink, nontender, and firm on palpation. No other overlying skin changes are seen.
Lateral digital traction toward the center of the lesion produces no dimpling, while lateral traction toward its periphery accentuates the lesion’s central raised portion. The lesion is moderately mobile. No lymph nodes are felt on palpation of nodal sites in the area, and no other such lesions are found elsewhere on the child’s skin.
What is the diagnosis?
DISCUSSION
At this point, the differential included items such as pilomatricoma, dermatofibroma, calcinosis cutis, or epidermal cyst. The firm feel, bluish color, and shallow subcutaneous location of the lesion lent themselves to a provisional diagnosis of pilomatricoma, as did the patient’s age. But the fact that the lesion was changing was of sufficient concern to prompt removal.
Excision revealed a cystic lesion with cottage-cheese–like contents and a poorly defined wall, extending more than a centimeter into the subcutaneous tissue. It was removed in one piece and submitted to pathology. Primary closure completed the procedure.
The pathology report showed sheets of anucleate squamous cells (called ghost cells), benign viable nucleated squamous cells, and a center filled with multiple soft calcified granules. A positive von Kossa stain confirmed the expected diagnosis of pilomatricoma (PMC; also spelled pilomatrixoma).
PMCs, also known by their eponymous designation of calcifying epithelioma of Malherbe, are common, benign appendageal tumors derived from hair matrix. They usually manifest (as in this case) as a solitary subcutaneous firm mass, often with bluish discoloration, on the face, neck, or upper extremities. While they average around 2 cm, they can be as large as 15 cm in diameter. They are more common in children and occur slightly more often in girls.
There is some evidence that the tendency to develop PMCs is associated with increased levels of beta-catenin, which encourages cell growth by diminishing apoptosis. This mechanism is thought to promote malignant transformation of PMCs—a rare event.
As is often the case, the main concern about this patient’s lesion related to its unknown source and recent alteration. Aside from scarring, the patient was no worse off for its removal—and her mother was much relieved.
TAKE-HOME LEARNING POINTS
• PMCs are benign cystic lesions of appendageal origin, commonly found on the necks, faces, and upper extremities of children.
• Diagnostic clues for PMC include firm feel, subcutaneous location, bluish discoloration, and patient age.
• PMCs have poorly defined cyst walls and granular calcified contents.
• Except when occurring in multiples, PMCs have no pathologic implications.
• The term calcifying epithelioma of Malherbe is still in use, as is the alternate spelling of pilomatrixoma.
Stratum Corneum Absorption Kinetics of 2 Potent Topical Corticosteroid Formulations: A Pilot Study
The active ingredient of any pharmaceutical product is responsible for the agent’s efficacy and safety profile. This ingredient is extensively studied in clinical trials and evaluated by the US Food and Drug Administration before the product is commercially available. In dermatologic products, especially those for treating dermatoses, the vehicle in which the active ingredient is formulated also plays a role in drug delivery and indirectly impacts therapeutic outcomes, unlike excipients in oral medications. Topical vehicles must be stable, provide a suitable environment that will not degrade the active ingredient or affect its efficacy, and be cosmetically acceptable.1
Topical vehicles are formulated to maintain the stability of the active ingredient and allow it to readily penetrate the skin and reach its target area with minimal absorption into the bloodstream, thus avoiding systemic adverse events. A variety of vehicles can exist for a single active ingredient to accommodate different phases of disease and different anatomical sites where the disease may occur.2 For example, alcohol-based vehicles, sprays, and foams are preferred for the scalp where evaporation of the vehicle is beneficial to prevent greasiness of the hair, while ointments may be preferred due to their occlusive nature for areas with xerotic or thick skin from dermatoses.
Cosmetic acceptability of the vehicle may influence patient adherence to therapy. Housman et al3 assessed a variety of products formulated in different vehicles (ie, solutions, foams, emollients, gels, creams, ointments) for the treatment of psoriasis. Patients with psoriasis applied each test product to a quarter-sized area of normal skin on the forearm using a cotton swab and completed a preference questionnaire. By far, respondents significantly preferred solutions and foams over creams, gels, and ointments (P<.01). Side effects were rated to be the most important characteristics of topical therapy, followed by time needed for application, ease of application, and messiness.3 Presumably, if patients are frustrated with the topical product that they are using, adherence to the prescribed dosage and application instructions will diminish over time, leading to suboptimal steady-state levels of the product. If appropriate levels of the drug are not present at the target site, treatment will not be successful.
Steady-state levels of a topical drug at the site of action also are maintained via appropriate application frequency, most commonly once to 4 times daily for dermatologic products. Fluocinonide and halcinonide are class II (potent) corticosteroids indicated for the relief of inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses and usually are administered at least twice daily. In double-blind clinical studies comparing both products in the treatment of psoriasis, halcinonide resulted in more improved outcomes than fluocinonide.4-6 Sudilovsky and Clewe4 studied 140 patients with moderate to severe psoriasis. After 3 weeks of treatment, 44% showed superior results with halcinonide, 27% showed superior results with fluocinonide, 26% showed equal results with both products, and 3% showed no relief.4 Similarly, Close5 reported that 61% of patients showed superior results with halcinonide, 25% showed superior results with fluocinonide, 10% showed equal results with both products, and 4% showed no relief (N=50). Lynfield and Watsky6 reported that 56% of patients with severe psoriasis who were treated with halcinonide for 2 weeks showed improvement to normal or slight inflammation compared to 44% of patients treated with fluocinonide (N=59). All 3 studies used cream formulations of halcinonide and fluocinonide.
Recently, halcinonide cream was shown to have an immediate release into the stratum corneum that peaked within 1 hour of application and remained elevated for 6 hours before beginning to decline.7 These results support a biphasic release of halcinonide, which is in agreement with its formulation—that halcinonide exists in both a solution phase for immediate release into the skin and in a suspension phase that allows a sustained release after equilibrium is reached between the solution and suspension phases.8 Fluocinonide is not known to be formulated in a similar way. Its vehicle composition and penetration into the skin could explain the superior efficacy of halcinonide versus fluocinonide.
The current pilot study was conducted to compare the release pattern of fluocinonide cream versus halcinonide cream into the stratum corneum using an in vivo, noninvasive method. Results for halcin-onide have been previously published.7
Methods
Participants were sequestered in a controlled environment for the entire day to allow the skin to equilibrate prior to product application. The methodology for the application and quantification of halcinonide cream 0.1% into the stratum corneum of 5 participants using a tape-stripping protocol has been described elsewhere.7 Concordia Clinical Research institutional review board (Cedar Knolls, New Jersey) approved this study, which was conducted at Dermatology Consulting Services (High Point, North Carolina).
A 0.1-g dose of generic fluocinonide cream 0.05% was applied to four 2.5-cm circular sites on the forearm in 5 participants with normal skin until completely absorbed. Circular tape strips were subsequently placed on the application site at 1, 3, 6, and 9 hours posttreatment and were held for 10 seconds with a controlled pressure plunger to ensure adequate and consistent contact between the tape strip and the skin. The tape strip was removed with forceps, rolled with the skin scale inside, and placed in a glass vial. This procedure was repeated 6 times at 1 of 4 sites with a new tape strip at each time point to obtain samples from deeper skin layers. A total of 24 tape strips were collected from each participant.
All vials were frozen at -20°C and were shipped overnight to Robert Kellar, PhD, at the Center for Bioengineering Innovation at Northern Arizona University (Flagstaff, Arizona) for mass spectroscopy evaluation. Once received at the outside facility, the vials were stored at -20°C until analysis. Each sample was spiked with a known quantity of an appropriate reference standard and extracted with 1 mL acetonitrile at room temperature for 1 minute with agitation. New unused tape strips were spiked with a small amount of fluocinonide reference standard for extraction efficiency.
Extracts were evaporated to dryness under nitrogen gas, resuspended in 200 µL chromatography solvent, and quantified using liquid chromatography–mass spectrometry. To remove the skin scale from the tape strips, 10 mL of a solvent solution of 0.1 mg/mL fludrocortisone acetate in acetonitrile was dispensed into a 4 dram vial containing the tape strip. The vials were ultrasonicated and shaken for 10 to 15 minutes, and the samples were further diluted to 100-fold and were inverted several times to ensure complete dissolution of fluocinonide before liquid chromatography–mass spectrometry.
A standard curve ranging from the lower limit of quantification to the upper limit of quantification for the fluocinonide reference was used to determine the quantity of fluocinonide in each of the tape strips. Once the lower limit of quantification was reached in a given set of tape strip samples (1-, 3-, 6-, and 9-hour samples), the next 2 sequential tape strips in that set were analyzed to confirm fluocinonide was not detectable in deeper layers. Standard quality controls were analyzed to ensure run-to-run and sample-to-sample accuracy.
Each sample was analyzed in duplicate; 10 mg fluocinonide was used as a reference standard. The minimum detectable concentration of fluocinonide was 1 ng/mL.
Results
As expected, tape strip 1 from each participant contained the highest concentration of fluocinonide. This strip corresponded to the most superficial layer of skin. Concentrations decreased in deeper skin layers, as detected in strips 2 to 6.
In general, the average concentration of fluocin-onide in strip 1 for all 5 participants was highest at hour 1, with a subsequent decline at hours 3, 6, and 9; however, participant 1 showed a second peak in fluocinonide concentration at hour 6 (Figure 1). When the fluocinonide concentration in strips 1 to 6 was averaged for each participant at each time point, similar results were obtained: a general decline after hour 1, but a second prominent peak at hour 6 in participant 1 only. In participant 1, the average fluocinonide concentration for strips 1 to 6 was 393 ng/mL at hour 1 and declined to 208 ng/mL at hour 3; it increased to 451 ng/mL at hour 6 before declining again to 202 ng/mL at hour 9.
Because participant 1 was the only one to exhibit a second peak of fluocinonide concentration, it appears that measurements obtained from this participant may be outliers. When removing partici-pant 1 from the analysis of fluocinonide concentration in strip 1 at each time point, a clear decline is evident from hour 1 to hour 9 (Figure 2A, red line [partici-pants 2–5] vs blue line [participants 1–5]).
When the average concentration of fluocinonide was calculated in strips 1 to 6 from all participants, there was a general steady decline after hour 1 with a slight increase of 25 ng/mL at hour 6 (Figure 2B, blue line). This increase is due to the measurements obtained from participant 1; however, if partici-pant 1 is removed from the analysis, a constant decline is observed from hour 1 to hour 9 (Figure 2B, red line).
|
A prior study evaluated the penetration and absorption of halcinonide in the stratum corneum.7 In summary, halcinonide concentration peaked at hour 1 following application and remained elevated to hour 6, before beginning a slow decline. The average concentration of halcinonide from all participants in strips 1 to 6 reached 1350 ng/mL at hour 1, remained within 93% to 97% of this level (1253–1303 ng/mL) for the next 5 hours, and declined only 29% from the peak at hour 1 to hour 9 (958 ng/mL)(Figure 3, blue line).7 In contrast, the fluocinonide concentration in participants 2 to 5 from the current study reached 190 ng/mL at hour 1 and steadily declined 53% to 89 ng/mL by hour 9 (Figure 3, red line).
Two participants from the prior halcinonide study also were enrolled in the current fluocinonide study (referred to as participant A and B). In general, halcinonide levels in both participants remained elevated for 6 hours after application and declined 27.5% and 35.5%, respectively, by hour 9 (Figure 4). Participant A experienced a 20.5% dip in halcinonide concentration at hour 3 followed by an increase at hour 6; however, the halcinonide concentration at hour 9 was similar to hour 3.7 In contrast, fluocin-onide concentrations for these participants peaked at 1 hour and clearly declined approximately 60% over the next 8 hours.
Comment
The release of both fluocinonide and halcinonide into the skin was evaluated using dermal tape stripping on 4 sites on the forearms of healthy individuals. Cream formulations of each corticosteroid were evaluated in 5 participants, with 2 participants receiving both formulations during different study periods. In the prior study with halcinonide, the stratum corneum exhibited the highest concentration of the corticosteroid, with substantial declines beyond strip 6 (ie, strips 7–20).7 For this reason, only strips 1 to 6 were evaluated for corticosteroid penetration and absorption.
Results from strip 1 indicated immediate absorption of corticosteroid (fluocinonide and halcinonide) into the skin. Unlike the release of halcinonide, which demonstrated a clear sustained release over 6 hours before decreasing,7 fluocinonide concentrations began declining immediately after peaking at hour 1 and continued to decline up to hour 9. Only participant 1 exhibited a second peak of fluocinonide concentration at hour 6; the rest of the participants did not. This second peak is most likely an anomaly due to the small number of participants rather than a true elevation.
Given the rapid decline of fluocinonide concentration over the 9 hours compared with the more gradual decline of halcinonide concentration, there appears to be no evidence of a biphasic sustained release of fluocinonide from its vehicle. This difference in release pattern from each corticosteroid’s respective vehicle may explain in part the different clinical outcomes in comparative studies.4-6
It is known that vehicle composition affects corticosteroid diffusion from the vehicle to the skin surface and subsequent penetration into the skin.9 Either process can determine the overall effectiveness of the product. Ayres and Hooper10 evaluated the penetration of 4 topical preparations of cortisol. Product 1 delivered 16 times more cortisol to the skin than product 2, 8 times more than product 3, and 3 times more than product 4. Because all the preparations contained cortisol-free alcohol, these differences were attributed to the vehicle in which the cortisol was formulated. Products 1 and 4 both contained 10% urea, but the urea in product 1 was a powder in a cream base and the urea in product 4 was in a stabilizing emulsified base. Product 2 contained a propylene glycol/water base and product 3 was a water-miscible cream.10
Generic corticosteroid products have been observed in clinical practice and have been shown in vasoconstriction assays to be less and more potent than their brand-name equivalents.2,11 Vasoconstriction assays are the standard for assessing the potency of topical corticosteroids and predicting their clinical efficacy.2 One study reported significant differences in therapeutic effectiveness between generic formulations and their brand-name equivalents.12 Kenalog cream 0.1% (multiple manufacturers) was significantly more potent than any of the generic triamcinolone creams tested (P<.05); in fact, Kenalog cream 0.025% (multiple manufacturers) was statistically superior to all the generic triamcinolone creams 0.1%. Moreover, Artistocort A ointment 0.1% (Lederele Laboratories) and Valisone cream 0.1% (Schering Corporation) also were more potent than their generics at the same concentration in the same vehicle type.12 A second study also observed that 2 of 6 generic formulations had significantly less vasoconstriction than their respective brand-name formulations.11 A brand-name betamethasone valerate cream produced significantly greater vasoconstriction than its generic equivalent, and a brand-name betamethasone dipropionate cream produced greater vasoconstriction than one generic and equal vasoconstriction to another generic. Additionally, the vasoconstriction measured with Diprosone was greater than that measured with Diprolene, another brand-name product of betamethasone dipropionate.11 Diprosone and Diprolene differ in their vehicle content. The latter, a class I corticosteroid, contains a modified vehicle high in propylene glycol, whereas the former contains less propylene glycol and thus is classified as a class III corticosteroid. Propylene glycol allows hydrophobic molecules such as corticosteroids to dissolve more fully in the vehicle.12
Ostrenga et al1 studied the solubility of corticosteroids in different vehicles and, as expected, corticosteroids that fully solubilized in the vehicle exhibited better penetration into the skin on assessment with vasoconstriction assays. Corticosteroids in a suspension, on the other hand, showed slower penetration into the skin.1,13 A balance between the solution and suspension phase would allow a drug to rapidly penetrate the skin upon application, and when this pool of solubilized drug was depleted, additional drug could penetrate into the skin from the suspension phase. Based on the tape strip results from the current study it appears that halcinonide, which is manufactured in a biphasic formulation, follows this pattern of penetration and absorption into the stratum corneum. In contrast, fluocinonide appears to exist in a soluble state without much, if any, amount in a suspension phase because it had no sustained release during the 9 hours after application.
Common belief among dermatologists is that long-term use of corticosteroids leads to tachyphylaxis,14 which can be attributed to poor patient adherence. If patients skip doses, then the steady state of the product at the target site is not maintained. It is interesting to speculate that using agents with more sustained release beyond the time of application (such as halcinonide) may preserve steady-state levels even when patients are neglectful of the next medication application. Corticosteroids that work in 2 phases such as halcinonide may minimize tachyphylaxis experienced with prolonged use of corticosteroids.
Fluocinonide and halcinonide are both class II high-potency corticosteroids as shown on outcomes from vasoconstrictor assays, which assess the extent to which a corticosteroid causes cutaneous vasoconstriction or blanching in normal healthy individuals.15 The assay depends on the molecule diffusing from the vehicle, penetrating the skin, and causing a reaction (blanching) that is then evaluated. The assay cannot effectively evaluate the rate of continued diffusion and skin penetration beyond the appearance of blanching. In contrast, the tape-stripping method provides an inside look at the extent of penetration of the corticosteroid beyond the skin surface and the rate of its clearance from different skin layers. In the current study, the levels of fluocinonide declined after peaking at 1 hour after application, but the levels of halcinonide clearly remained elevated after peaking at the same time point. Most likely, vasoconstrictor studies would not be able to differentiate between the concentrations of the 2 products in the stratum corneum beyond the first hour after application.
Tape stripping, or dermatopharmacokinetics, has advantages over vasoconstriction assays in studying corticosteroid penetration and clearance from the stratum corneum. At one point, the US Food and Drug Administration had included tape stripping in its preliminary guidelines for generic topical bioequivalence studies until data from the same formulation generated from 2 different laboratories produced different results.16 Since that time, much work has been done with tape stripping to ensure its consistency. Weigmann et al17 demonstrated equivalent results with clobetasol using vasoconstriction and tape stripping, and Wiedersberg et al18 demonstrated the same with betamethasone. For the current study, the fluocinonide and halcinonide formulations were weighed prior to application so that the same dose was tested in all participants. A plunger was used to produce consistent pressure at all application sites to control for the amount of skin that was stripped off with the tape. Results for both corticosteroids were consistent between the participants. Variability in the data was detected; however, this observation is most likely due to the small number of participants in the studies.
Conclusion
In summary, this pilot study demonstrated that fluocinonide concentration in the stratum corneum peaks within the first hour of application before beginning a steady general decline. There was no evidence of sustained release. In contrast, halcin-onide demonstrated a sustained release for 6 hours after application. Halcinonide is formulated in a cream base in which the corticosteroid is present in a solution and suspension phase that allows for sustained delivery in skin over time. Fluocinonide does not appear to be formulated in the same way, and its concentrations in the stratum corneum begin to decline 1 hour after application.
Acknowledgement
Thank you to Robert Kellar, PhD, at the Center for Bioengineering Innovation at Northern Arizona University, Flagstaff, for conducting the liquid chromatography–mass spectrometry.
1. Ostrenga J, Haleblian J, Poulsen B, et al. Vehicle design for a new topical steroid, fluocinonide. J Invest Dermatol. 1971;56:392-399.
2. Rathi SK, D’Souza P. Rational and ethical use of topical corticosteroids based on safety and efficacy. Indian J Dermatol. 2012;57:251-259.
3. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
4. Sudilovsky A, Clewe TH. Comparative efficacy of halcin-onide and fluocinonide creams in psoriasis and eczematous dermatoses. J Clin Pharmacol. 1975;15:779-784.
5. Close JE. Double-blind comparison of topical halcinonide and fluocinonide in the treatment of psoriasis. Int J Dermatol. 1976;15:534-537.
6. Lynfield Y, Watsky M. Psoriasis: topical corticosteroid therapy. Cutis. 1976;18:133, 136-137.
7. Draelos ZD. Demonstration of the biphasic release of 0.1% halcinonide cream. J Drugs Dermatol. 2015;14:89-90.
8. Bagatell FK. Halcinonide: a new potent topical anti-inflammatory drug. Cutis. 1974;14:459-462.
9. Ostrenga J, Steinmetz C, Poulsen B. Significance of vehicle composition. I. relationship between topical vehicle composition, skin penetrability, and clinical efficacy. J Pharm Sci. 1971;60:1175-1179.
10. Ayres PJ, Hooper G. Assessment of the skin penetration properties of different carrier vehicles for topically applied cortisol. Br J Dermatol. 1978;99:307-317.
11. Olsen EA. Double-blind controlled comparison of generic and trade-name topical steroids using the vasoconstriction assay. Arch Dermatol. 1991;127:197-201.
12. Stoughton RB. Are generic formulations equivalent to trade name topical glucocorticoids? Arch Dermatol. 1987;123:1312-1314.
13. Poulsen BJ, Young E, Coquilla V, et al. Effect of topical vehicle composition on the in vitro release of fluocinolone acetonide and its acetate ester. J Pharm Sci. 1968;57:928-933.
14. Taheri A, Cantrell J, Feldman SR. Tachyphylaxis to topical glucocorticoids: what is the evidence? Dermatol Online J. 2013;19:18954.
15. Ference JD, Last AR. Choosing topical corticosteroids. Am Fam Physician. 2009;79:135-140.
16. Pershing LK, Nelson JL, Corlett JL, et al. Assessment of dermatopharmacokinetic approach in the bioequivalence determination of topical tretinoin gel products. J Am Acad Dermatol. 2003;48:740-751.
17. Weigmann H, Lademann J, v Pelchrzim R, et al. Bioavailability of clobetasol propionate-quantification of drug concentrations in the stratum corneum by dermatopharmacokinetics using tape stripping. Skin Pharmacol Appl Skin Physiol. 1999;12:46-53.
18. Wiedersberg S, Naik A, Leopold CS, et al. Pharmacodynamics and dermatopharmacokinetics of betamethasone 17-valerate: assessment of topical bioavailability. Br J Dermatol. 2009;160:676-686.
The active ingredient of any pharmaceutical product is responsible for the agent’s efficacy and safety profile. This ingredient is extensively studied in clinical trials and evaluated by the US Food and Drug Administration before the product is commercially available. In dermatologic products, especially those for treating dermatoses, the vehicle in which the active ingredient is formulated also plays a role in drug delivery and indirectly impacts therapeutic outcomes, unlike excipients in oral medications. Topical vehicles must be stable, provide a suitable environment that will not degrade the active ingredient or affect its efficacy, and be cosmetically acceptable.1
Topical vehicles are formulated to maintain the stability of the active ingredient and allow it to readily penetrate the skin and reach its target area with minimal absorption into the bloodstream, thus avoiding systemic adverse events. A variety of vehicles can exist for a single active ingredient to accommodate different phases of disease and different anatomical sites where the disease may occur.2 For example, alcohol-based vehicles, sprays, and foams are preferred for the scalp where evaporation of the vehicle is beneficial to prevent greasiness of the hair, while ointments may be preferred due to their occlusive nature for areas with xerotic or thick skin from dermatoses.
Cosmetic acceptability of the vehicle may influence patient adherence to therapy. Housman et al3 assessed a variety of products formulated in different vehicles (ie, solutions, foams, emollients, gels, creams, ointments) for the treatment of psoriasis. Patients with psoriasis applied each test product to a quarter-sized area of normal skin on the forearm using a cotton swab and completed a preference questionnaire. By far, respondents significantly preferred solutions and foams over creams, gels, and ointments (P<.01). Side effects were rated to be the most important characteristics of topical therapy, followed by time needed for application, ease of application, and messiness.3 Presumably, if patients are frustrated with the topical product that they are using, adherence to the prescribed dosage and application instructions will diminish over time, leading to suboptimal steady-state levels of the product. If appropriate levels of the drug are not present at the target site, treatment will not be successful.
Steady-state levels of a topical drug at the site of action also are maintained via appropriate application frequency, most commonly once to 4 times daily for dermatologic products. Fluocinonide and halcinonide are class II (potent) corticosteroids indicated for the relief of inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses and usually are administered at least twice daily. In double-blind clinical studies comparing both products in the treatment of psoriasis, halcinonide resulted in more improved outcomes than fluocinonide.4-6 Sudilovsky and Clewe4 studied 140 patients with moderate to severe psoriasis. After 3 weeks of treatment, 44% showed superior results with halcinonide, 27% showed superior results with fluocinonide, 26% showed equal results with both products, and 3% showed no relief.4 Similarly, Close5 reported that 61% of patients showed superior results with halcinonide, 25% showed superior results with fluocinonide, 10% showed equal results with both products, and 4% showed no relief (N=50). Lynfield and Watsky6 reported that 56% of patients with severe psoriasis who were treated with halcinonide for 2 weeks showed improvement to normal or slight inflammation compared to 44% of patients treated with fluocinonide (N=59). All 3 studies used cream formulations of halcinonide and fluocinonide.
Recently, halcinonide cream was shown to have an immediate release into the stratum corneum that peaked within 1 hour of application and remained elevated for 6 hours before beginning to decline.7 These results support a biphasic release of halcinonide, which is in agreement with its formulation—that halcinonide exists in both a solution phase for immediate release into the skin and in a suspension phase that allows a sustained release after equilibrium is reached between the solution and suspension phases.8 Fluocinonide is not known to be formulated in a similar way. Its vehicle composition and penetration into the skin could explain the superior efficacy of halcinonide versus fluocinonide.
The current pilot study was conducted to compare the release pattern of fluocinonide cream versus halcinonide cream into the stratum corneum using an in vivo, noninvasive method. Results for halcin-onide have been previously published.7
Methods
Participants were sequestered in a controlled environment for the entire day to allow the skin to equilibrate prior to product application. The methodology for the application and quantification of halcinonide cream 0.1% into the stratum corneum of 5 participants using a tape-stripping protocol has been described elsewhere.7 Concordia Clinical Research institutional review board (Cedar Knolls, New Jersey) approved this study, which was conducted at Dermatology Consulting Services (High Point, North Carolina).
A 0.1-g dose of generic fluocinonide cream 0.05% was applied to four 2.5-cm circular sites on the forearm in 5 participants with normal skin until completely absorbed. Circular tape strips were subsequently placed on the application site at 1, 3, 6, and 9 hours posttreatment and were held for 10 seconds with a controlled pressure plunger to ensure adequate and consistent contact between the tape strip and the skin. The tape strip was removed with forceps, rolled with the skin scale inside, and placed in a glass vial. This procedure was repeated 6 times at 1 of 4 sites with a new tape strip at each time point to obtain samples from deeper skin layers. A total of 24 tape strips were collected from each participant.
All vials were frozen at -20°C and were shipped overnight to Robert Kellar, PhD, at the Center for Bioengineering Innovation at Northern Arizona University (Flagstaff, Arizona) for mass spectroscopy evaluation. Once received at the outside facility, the vials were stored at -20°C until analysis. Each sample was spiked with a known quantity of an appropriate reference standard and extracted with 1 mL acetonitrile at room temperature for 1 minute with agitation. New unused tape strips were spiked with a small amount of fluocinonide reference standard for extraction efficiency.
Extracts were evaporated to dryness under nitrogen gas, resuspended in 200 µL chromatography solvent, and quantified using liquid chromatography–mass spectrometry. To remove the skin scale from the tape strips, 10 mL of a solvent solution of 0.1 mg/mL fludrocortisone acetate in acetonitrile was dispensed into a 4 dram vial containing the tape strip. The vials were ultrasonicated and shaken for 10 to 15 minutes, and the samples were further diluted to 100-fold and were inverted several times to ensure complete dissolution of fluocinonide before liquid chromatography–mass spectrometry.
A standard curve ranging from the lower limit of quantification to the upper limit of quantification for the fluocinonide reference was used to determine the quantity of fluocinonide in each of the tape strips. Once the lower limit of quantification was reached in a given set of tape strip samples (1-, 3-, 6-, and 9-hour samples), the next 2 sequential tape strips in that set were analyzed to confirm fluocinonide was not detectable in deeper layers. Standard quality controls were analyzed to ensure run-to-run and sample-to-sample accuracy.
Each sample was analyzed in duplicate; 10 mg fluocinonide was used as a reference standard. The minimum detectable concentration of fluocinonide was 1 ng/mL.
Results
As expected, tape strip 1 from each participant contained the highest concentration of fluocinonide. This strip corresponded to the most superficial layer of skin. Concentrations decreased in deeper skin layers, as detected in strips 2 to 6.
In general, the average concentration of fluocin-onide in strip 1 for all 5 participants was highest at hour 1, with a subsequent decline at hours 3, 6, and 9; however, participant 1 showed a second peak in fluocinonide concentration at hour 6 (Figure 1). When the fluocinonide concentration in strips 1 to 6 was averaged for each participant at each time point, similar results were obtained: a general decline after hour 1, but a second prominent peak at hour 6 in participant 1 only. In participant 1, the average fluocinonide concentration for strips 1 to 6 was 393 ng/mL at hour 1 and declined to 208 ng/mL at hour 3; it increased to 451 ng/mL at hour 6 before declining again to 202 ng/mL at hour 9.
Because participant 1 was the only one to exhibit a second peak of fluocinonide concentration, it appears that measurements obtained from this participant may be outliers. When removing partici-pant 1 from the analysis of fluocinonide concentration in strip 1 at each time point, a clear decline is evident from hour 1 to hour 9 (Figure 2A, red line [partici-pants 2–5] vs blue line [participants 1–5]).
When the average concentration of fluocinonide was calculated in strips 1 to 6 from all participants, there was a general steady decline after hour 1 with a slight increase of 25 ng/mL at hour 6 (Figure 2B, blue line). This increase is due to the measurements obtained from participant 1; however, if partici-pant 1 is removed from the analysis, a constant decline is observed from hour 1 to hour 9 (Figure 2B, red line).
|
A prior study evaluated the penetration and absorption of halcinonide in the stratum corneum.7 In summary, halcinonide concentration peaked at hour 1 following application and remained elevated to hour 6, before beginning a slow decline. The average concentration of halcinonide from all participants in strips 1 to 6 reached 1350 ng/mL at hour 1, remained within 93% to 97% of this level (1253–1303 ng/mL) for the next 5 hours, and declined only 29% from the peak at hour 1 to hour 9 (958 ng/mL)(Figure 3, blue line).7 In contrast, the fluocinonide concentration in participants 2 to 5 from the current study reached 190 ng/mL at hour 1 and steadily declined 53% to 89 ng/mL by hour 9 (Figure 3, red line).
Two participants from the prior halcinonide study also were enrolled in the current fluocinonide study (referred to as participant A and B). In general, halcinonide levels in both participants remained elevated for 6 hours after application and declined 27.5% and 35.5%, respectively, by hour 9 (Figure 4). Participant A experienced a 20.5% dip in halcinonide concentration at hour 3 followed by an increase at hour 6; however, the halcinonide concentration at hour 9 was similar to hour 3.7 In contrast, fluocin-onide concentrations for these participants peaked at 1 hour and clearly declined approximately 60% over the next 8 hours.
Comment
The release of both fluocinonide and halcinonide into the skin was evaluated using dermal tape stripping on 4 sites on the forearms of healthy individuals. Cream formulations of each corticosteroid were evaluated in 5 participants, with 2 participants receiving both formulations during different study periods. In the prior study with halcinonide, the stratum corneum exhibited the highest concentration of the corticosteroid, with substantial declines beyond strip 6 (ie, strips 7–20).7 For this reason, only strips 1 to 6 were evaluated for corticosteroid penetration and absorption.
Results from strip 1 indicated immediate absorption of corticosteroid (fluocinonide and halcinonide) into the skin. Unlike the release of halcinonide, which demonstrated a clear sustained release over 6 hours before decreasing,7 fluocinonide concentrations began declining immediately after peaking at hour 1 and continued to decline up to hour 9. Only participant 1 exhibited a second peak of fluocinonide concentration at hour 6; the rest of the participants did not. This second peak is most likely an anomaly due to the small number of participants rather than a true elevation.
Given the rapid decline of fluocinonide concentration over the 9 hours compared with the more gradual decline of halcinonide concentration, there appears to be no evidence of a biphasic sustained release of fluocinonide from its vehicle. This difference in release pattern from each corticosteroid’s respective vehicle may explain in part the different clinical outcomes in comparative studies.4-6
It is known that vehicle composition affects corticosteroid diffusion from the vehicle to the skin surface and subsequent penetration into the skin.9 Either process can determine the overall effectiveness of the product. Ayres and Hooper10 evaluated the penetration of 4 topical preparations of cortisol. Product 1 delivered 16 times more cortisol to the skin than product 2, 8 times more than product 3, and 3 times more than product 4. Because all the preparations contained cortisol-free alcohol, these differences were attributed to the vehicle in which the cortisol was formulated. Products 1 and 4 both contained 10% urea, but the urea in product 1 was a powder in a cream base and the urea in product 4 was in a stabilizing emulsified base. Product 2 contained a propylene glycol/water base and product 3 was a water-miscible cream.10
Generic corticosteroid products have been observed in clinical practice and have been shown in vasoconstriction assays to be less and more potent than their brand-name equivalents.2,11 Vasoconstriction assays are the standard for assessing the potency of topical corticosteroids and predicting their clinical efficacy.2 One study reported significant differences in therapeutic effectiveness between generic formulations and their brand-name equivalents.12 Kenalog cream 0.1% (multiple manufacturers) was significantly more potent than any of the generic triamcinolone creams tested (P<.05); in fact, Kenalog cream 0.025% (multiple manufacturers) was statistically superior to all the generic triamcinolone creams 0.1%. Moreover, Artistocort A ointment 0.1% (Lederele Laboratories) and Valisone cream 0.1% (Schering Corporation) also were more potent than their generics at the same concentration in the same vehicle type.12 A second study also observed that 2 of 6 generic formulations had significantly less vasoconstriction than their respective brand-name formulations.11 A brand-name betamethasone valerate cream produced significantly greater vasoconstriction than its generic equivalent, and a brand-name betamethasone dipropionate cream produced greater vasoconstriction than one generic and equal vasoconstriction to another generic. Additionally, the vasoconstriction measured with Diprosone was greater than that measured with Diprolene, another brand-name product of betamethasone dipropionate.11 Diprosone and Diprolene differ in their vehicle content. The latter, a class I corticosteroid, contains a modified vehicle high in propylene glycol, whereas the former contains less propylene glycol and thus is classified as a class III corticosteroid. Propylene glycol allows hydrophobic molecules such as corticosteroids to dissolve more fully in the vehicle.12
Ostrenga et al1 studied the solubility of corticosteroids in different vehicles and, as expected, corticosteroids that fully solubilized in the vehicle exhibited better penetration into the skin on assessment with vasoconstriction assays. Corticosteroids in a suspension, on the other hand, showed slower penetration into the skin.1,13 A balance between the solution and suspension phase would allow a drug to rapidly penetrate the skin upon application, and when this pool of solubilized drug was depleted, additional drug could penetrate into the skin from the suspension phase. Based on the tape strip results from the current study it appears that halcinonide, which is manufactured in a biphasic formulation, follows this pattern of penetration and absorption into the stratum corneum. In contrast, fluocinonide appears to exist in a soluble state without much, if any, amount in a suspension phase because it had no sustained release during the 9 hours after application.
Common belief among dermatologists is that long-term use of corticosteroids leads to tachyphylaxis,14 which can be attributed to poor patient adherence. If patients skip doses, then the steady state of the product at the target site is not maintained. It is interesting to speculate that using agents with more sustained release beyond the time of application (such as halcinonide) may preserve steady-state levels even when patients are neglectful of the next medication application. Corticosteroids that work in 2 phases such as halcinonide may minimize tachyphylaxis experienced with prolonged use of corticosteroids.
Fluocinonide and halcinonide are both class II high-potency corticosteroids as shown on outcomes from vasoconstrictor assays, which assess the extent to which a corticosteroid causes cutaneous vasoconstriction or blanching in normal healthy individuals.15 The assay depends on the molecule diffusing from the vehicle, penetrating the skin, and causing a reaction (blanching) that is then evaluated. The assay cannot effectively evaluate the rate of continued diffusion and skin penetration beyond the appearance of blanching. In contrast, the tape-stripping method provides an inside look at the extent of penetration of the corticosteroid beyond the skin surface and the rate of its clearance from different skin layers. In the current study, the levels of fluocinonide declined after peaking at 1 hour after application, but the levels of halcinonide clearly remained elevated after peaking at the same time point. Most likely, vasoconstrictor studies would not be able to differentiate between the concentrations of the 2 products in the stratum corneum beyond the first hour after application.
Tape stripping, or dermatopharmacokinetics, has advantages over vasoconstriction assays in studying corticosteroid penetration and clearance from the stratum corneum. At one point, the US Food and Drug Administration had included tape stripping in its preliminary guidelines for generic topical bioequivalence studies until data from the same formulation generated from 2 different laboratories produced different results.16 Since that time, much work has been done with tape stripping to ensure its consistency. Weigmann et al17 demonstrated equivalent results with clobetasol using vasoconstriction and tape stripping, and Wiedersberg et al18 demonstrated the same with betamethasone. For the current study, the fluocinonide and halcinonide formulations were weighed prior to application so that the same dose was tested in all participants. A plunger was used to produce consistent pressure at all application sites to control for the amount of skin that was stripped off with the tape. Results for both corticosteroids were consistent between the participants. Variability in the data was detected; however, this observation is most likely due to the small number of participants in the studies.
Conclusion
In summary, this pilot study demonstrated that fluocinonide concentration in the stratum corneum peaks within the first hour of application before beginning a steady general decline. There was no evidence of sustained release. In contrast, halcin-onide demonstrated a sustained release for 6 hours after application. Halcinonide is formulated in a cream base in which the corticosteroid is present in a solution and suspension phase that allows for sustained delivery in skin over time. Fluocinonide does not appear to be formulated in the same way, and its concentrations in the stratum corneum begin to decline 1 hour after application.
Acknowledgement
Thank you to Robert Kellar, PhD, at the Center for Bioengineering Innovation at Northern Arizona University, Flagstaff, for conducting the liquid chromatography–mass spectrometry.
The active ingredient of any pharmaceutical product is responsible for the agent’s efficacy and safety profile. This ingredient is extensively studied in clinical trials and evaluated by the US Food and Drug Administration before the product is commercially available. In dermatologic products, especially those for treating dermatoses, the vehicle in which the active ingredient is formulated also plays a role in drug delivery and indirectly impacts therapeutic outcomes, unlike excipients in oral medications. Topical vehicles must be stable, provide a suitable environment that will not degrade the active ingredient or affect its efficacy, and be cosmetically acceptable.1
Topical vehicles are formulated to maintain the stability of the active ingredient and allow it to readily penetrate the skin and reach its target area with minimal absorption into the bloodstream, thus avoiding systemic adverse events. A variety of vehicles can exist for a single active ingredient to accommodate different phases of disease and different anatomical sites where the disease may occur.2 For example, alcohol-based vehicles, sprays, and foams are preferred for the scalp where evaporation of the vehicle is beneficial to prevent greasiness of the hair, while ointments may be preferred due to their occlusive nature for areas with xerotic or thick skin from dermatoses.
Cosmetic acceptability of the vehicle may influence patient adherence to therapy. Housman et al3 assessed a variety of products formulated in different vehicles (ie, solutions, foams, emollients, gels, creams, ointments) for the treatment of psoriasis. Patients with psoriasis applied each test product to a quarter-sized area of normal skin on the forearm using a cotton swab and completed a preference questionnaire. By far, respondents significantly preferred solutions and foams over creams, gels, and ointments (P<.01). Side effects were rated to be the most important characteristics of topical therapy, followed by time needed for application, ease of application, and messiness.3 Presumably, if patients are frustrated with the topical product that they are using, adherence to the prescribed dosage and application instructions will diminish over time, leading to suboptimal steady-state levels of the product. If appropriate levels of the drug are not present at the target site, treatment will not be successful.
Steady-state levels of a topical drug at the site of action also are maintained via appropriate application frequency, most commonly once to 4 times daily for dermatologic products. Fluocinonide and halcinonide are class II (potent) corticosteroids indicated for the relief of inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses and usually are administered at least twice daily. In double-blind clinical studies comparing both products in the treatment of psoriasis, halcinonide resulted in more improved outcomes than fluocinonide.4-6 Sudilovsky and Clewe4 studied 140 patients with moderate to severe psoriasis. After 3 weeks of treatment, 44% showed superior results with halcinonide, 27% showed superior results with fluocinonide, 26% showed equal results with both products, and 3% showed no relief.4 Similarly, Close5 reported that 61% of patients showed superior results with halcinonide, 25% showed superior results with fluocinonide, 10% showed equal results with both products, and 4% showed no relief (N=50). Lynfield and Watsky6 reported that 56% of patients with severe psoriasis who were treated with halcinonide for 2 weeks showed improvement to normal or slight inflammation compared to 44% of patients treated with fluocinonide (N=59). All 3 studies used cream formulations of halcinonide and fluocinonide.
Recently, halcinonide cream was shown to have an immediate release into the stratum corneum that peaked within 1 hour of application and remained elevated for 6 hours before beginning to decline.7 These results support a biphasic release of halcinonide, which is in agreement with its formulation—that halcinonide exists in both a solution phase for immediate release into the skin and in a suspension phase that allows a sustained release after equilibrium is reached between the solution and suspension phases.8 Fluocinonide is not known to be formulated in a similar way. Its vehicle composition and penetration into the skin could explain the superior efficacy of halcinonide versus fluocinonide.
The current pilot study was conducted to compare the release pattern of fluocinonide cream versus halcinonide cream into the stratum corneum using an in vivo, noninvasive method. Results for halcin-onide have been previously published.7
Methods
Participants were sequestered in a controlled environment for the entire day to allow the skin to equilibrate prior to product application. The methodology for the application and quantification of halcinonide cream 0.1% into the stratum corneum of 5 participants using a tape-stripping protocol has been described elsewhere.7 Concordia Clinical Research institutional review board (Cedar Knolls, New Jersey) approved this study, which was conducted at Dermatology Consulting Services (High Point, North Carolina).
A 0.1-g dose of generic fluocinonide cream 0.05% was applied to four 2.5-cm circular sites on the forearm in 5 participants with normal skin until completely absorbed. Circular tape strips were subsequently placed on the application site at 1, 3, 6, and 9 hours posttreatment and were held for 10 seconds with a controlled pressure plunger to ensure adequate and consistent contact between the tape strip and the skin. The tape strip was removed with forceps, rolled with the skin scale inside, and placed in a glass vial. This procedure was repeated 6 times at 1 of 4 sites with a new tape strip at each time point to obtain samples from deeper skin layers. A total of 24 tape strips were collected from each participant.
All vials were frozen at -20°C and were shipped overnight to Robert Kellar, PhD, at the Center for Bioengineering Innovation at Northern Arizona University (Flagstaff, Arizona) for mass spectroscopy evaluation. Once received at the outside facility, the vials were stored at -20°C until analysis. Each sample was spiked with a known quantity of an appropriate reference standard and extracted with 1 mL acetonitrile at room temperature for 1 minute with agitation. New unused tape strips were spiked with a small amount of fluocinonide reference standard for extraction efficiency.
Extracts were evaporated to dryness under nitrogen gas, resuspended in 200 µL chromatography solvent, and quantified using liquid chromatography–mass spectrometry. To remove the skin scale from the tape strips, 10 mL of a solvent solution of 0.1 mg/mL fludrocortisone acetate in acetonitrile was dispensed into a 4 dram vial containing the tape strip. The vials were ultrasonicated and shaken for 10 to 15 minutes, and the samples were further diluted to 100-fold and were inverted several times to ensure complete dissolution of fluocinonide before liquid chromatography–mass spectrometry.
A standard curve ranging from the lower limit of quantification to the upper limit of quantification for the fluocinonide reference was used to determine the quantity of fluocinonide in each of the tape strips. Once the lower limit of quantification was reached in a given set of tape strip samples (1-, 3-, 6-, and 9-hour samples), the next 2 sequential tape strips in that set were analyzed to confirm fluocinonide was not detectable in deeper layers. Standard quality controls were analyzed to ensure run-to-run and sample-to-sample accuracy.
Each sample was analyzed in duplicate; 10 mg fluocinonide was used as a reference standard. The minimum detectable concentration of fluocinonide was 1 ng/mL.
Results
As expected, tape strip 1 from each participant contained the highest concentration of fluocinonide. This strip corresponded to the most superficial layer of skin. Concentrations decreased in deeper skin layers, as detected in strips 2 to 6.
In general, the average concentration of fluocin-onide in strip 1 for all 5 participants was highest at hour 1, with a subsequent decline at hours 3, 6, and 9; however, participant 1 showed a second peak in fluocinonide concentration at hour 6 (Figure 1). When the fluocinonide concentration in strips 1 to 6 was averaged for each participant at each time point, similar results were obtained: a general decline after hour 1, but a second prominent peak at hour 6 in participant 1 only. In participant 1, the average fluocinonide concentration for strips 1 to 6 was 393 ng/mL at hour 1 and declined to 208 ng/mL at hour 3; it increased to 451 ng/mL at hour 6 before declining again to 202 ng/mL at hour 9.
Because participant 1 was the only one to exhibit a second peak of fluocinonide concentration, it appears that measurements obtained from this participant may be outliers. When removing partici-pant 1 from the analysis of fluocinonide concentration in strip 1 at each time point, a clear decline is evident from hour 1 to hour 9 (Figure 2A, red line [partici-pants 2–5] vs blue line [participants 1–5]).
When the average concentration of fluocinonide was calculated in strips 1 to 6 from all participants, there was a general steady decline after hour 1 with a slight increase of 25 ng/mL at hour 6 (Figure 2B, blue line). This increase is due to the measurements obtained from participant 1; however, if partici-pant 1 is removed from the analysis, a constant decline is observed from hour 1 to hour 9 (Figure 2B, red line).
|
A prior study evaluated the penetration and absorption of halcinonide in the stratum corneum.7 In summary, halcinonide concentration peaked at hour 1 following application and remained elevated to hour 6, before beginning a slow decline. The average concentration of halcinonide from all participants in strips 1 to 6 reached 1350 ng/mL at hour 1, remained within 93% to 97% of this level (1253–1303 ng/mL) for the next 5 hours, and declined only 29% from the peak at hour 1 to hour 9 (958 ng/mL)(Figure 3, blue line).7 In contrast, the fluocinonide concentration in participants 2 to 5 from the current study reached 190 ng/mL at hour 1 and steadily declined 53% to 89 ng/mL by hour 9 (Figure 3, red line).
Two participants from the prior halcinonide study also were enrolled in the current fluocinonide study (referred to as participant A and B). In general, halcinonide levels in both participants remained elevated for 6 hours after application and declined 27.5% and 35.5%, respectively, by hour 9 (Figure 4). Participant A experienced a 20.5% dip in halcinonide concentration at hour 3 followed by an increase at hour 6; however, the halcinonide concentration at hour 9 was similar to hour 3.7 In contrast, fluocin-onide concentrations for these participants peaked at 1 hour and clearly declined approximately 60% over the next 8 hours.
Comment
The release of both fluocinonide and halcinonide into the skin was evaluated using dermal tape stripping on 4 sites on the forearms of healthy individuals. Cream formulations of each corticosteroid were evaluated in 5 participants, with 2 participants receiving both formulations during different study periods. In the prior study with halcinonide, the stratum corneum exhibited the highest concentration of the corticosteroid, with substantial declines beyond strip 6 (ie, strips 7–20).7 For this reason, only strips 1 to 6 were evaluated for corticosteroid penetration and absorption.
Results from strip 1 indicated immediate absorption of corticosteroid (fluocinonide and halcinonide) into the skin. Unlike the release of halcinonide, which demonstrated a clear sustained release over 6 hours before decreasing,7 fluocinonide concentrations began declining immediately after peaking at hour 1 and continued to decline up to hour 9. Only participant 1 exhibited a second peak of fluocinonide concentration at hour 6; the rest of the participants did not. This second peak is most likely an anomaly due to the small number of participants rather than a true elevation.
Given the rapid decline of fluocinonide concentration over the 9 hours compared with the more gradual decline of halcinonide concentration, there appears to be no evidence of a biphasic sustained release of fluocinonide from its vehicle. This difference in release pattern from each corticosteroid’s respective vehicle may explain in part the different clinical outcomes in comparative studies.4-6
It is known that vehicle composition affects corticosteroid diffusion from the vehicle to the skin surface and subsequent penetration into the skin.9 Either process can determine the overall effectiveness of the product. Ayres and Hooper10 evaluated the penetration of 4 topical preparations of cortisol. Product 1 delivered 16 times more cortisol to the skin than product 2, 8 times more than product 3, and 3 times more than product 4. Because all the preparations contained cortisol-free alcohol, these differences were attributed to the vehicle in which the cortisol was formulated. Products 1 and 4 both contained 10% urea, but the urea in product 1 was a powder in a cream base and the urea in product 4 was in a stabilizing emulsified base. Product 2 contained a propylene glycol/water base and product 3 was a water-miscible cream.10
Generic corticosteroid products have been observed in clinical practice and have been shown in vasoconstriction assays to be less and more potent than their brand-name equivalents.2,11 Vasoconstriction assays are the standard for assessing the potency of topical corticosteroids and predicting their clinical efficacy.2 One study reported significant differences in therapeutic effectiveness between generic formulations and their brand-name equivalents.12 Kenalog cream 0.1% (multiple manufacturers) was significantly more potent than any of the generic triamcinolone creams tested (P<.05); in fact, Kenalog cream 0.025% (multiple manufacturers) was statistically superior to all the generic triamcinolone creams 0.1%. Moreover, Artistocort A ointment 0.1% (Lederele Laboratories) and Valisone cream 0.1% (Schering Corporation) also were more potent than their generics at the same concentration in the same vehicle type.12 A second study also observed that 2 of 6 generic formulations had significantly less vasoconstriction than their respective brand-name formulations.11 A brand-name betamethasone valerate cream produced significantly greater vasoconstriction than its generic equivalent, and a brand-name betamethasone dipropionate cream produced greater vasoconstriction than one generic and equal vasoconstriction to another generic. Additionally, the vasoconstriction measured with Diprosone was greater than that measured with Diprolene, another brand-name product of betamethasone dipropionate.11 Diprosone and Diprolene differ in their vehicle content. The latter, a class I corticosteroid, contains a modified vehicle high in propylene glycol, whereas the former contains less propylene glycol and thus is classified as a class III corticosteroid. Propylene glycol allows hydrophobic molecules such as corticosteroids to dissolve more fully in the vehicle.12
Ostrenga et al1 studied the solubility of corticosteroids in different vehicles and, as expected, corticosteroids that fully solubilized in the vehicle exhibited better penetration into the skin on assessment with vasoconstriction assays. Corticosteroids in a suspension, on the other hand, showed slower penetration into the skin.1,13 A balance between the solution and suspension phase would allow a drug to rapidly penetrate the skin upon application, and when this pool of solubilized drug was depleted, additional drug could penetrate into the skin from the suspension phase. Based on the tape strip results from the current study it appears that halcinonide, which is manufactured in a biphasic formulation, follows this pattern of penetration and absorption into the stratum corneum. In contrast, fluocinonide appears to exist in a soluble state without much, if any, amount in a suspension phase because it had no sustained release during the 9 hours after application.
Common belief among dermatologists is that long-term use of corticosteroids leads to tachyphylaxis,14 which can be attributed to poor patient adherence. If patients skip doses, then the steady state of the product at the target site is not maintained. It is interesting to speculate that using agents with more sustained release beyond the time of application (such as halcinonide) may preserve steady-state levels even when patients are neglectful of the next medication application. Corticosteroids that work in 2 phases such as halcinonide may minimize tachyphylaxis experienced with prolonged use of corticosteroids.
Fluocinonide and halcinonide are both class II high-potency corticosteroids as shown on outcomes from vasoconstrictor assays, which assess the extent to which a corticosteroid causes cutaneous vasoconstriction or blanching in normal healthy individuals.15 The assay depends on the molecule diffusing from the vehicle, penetrating the skin, and causing a reaction (blanching) that is then evaluated. The assay cannot effectively evaluate the rate of continued diffusion and skin penetration beyond the appearance of blanching. In contrast, the tape-stripping method provides an inside look at the extent of penetration of the corticosteroid beyond the skin surface and the rate of its clearance from different skin layers. In the current study, the levels of fluocinonide declined after peaking at 1 hour after application, but the levels of halcinonide clearly remained elevated after peaking at the same time point. Most likely, vasoconstrictor studies would not be able to differentiate between the concentrations of the 2 products in the stratum corneum beyond the first hour after application.
Tape stripping, or dermatopharmacokinetics, has advantages over vasoconstriction assays in studying corticosteroid penetration and clearance from the stratum corneum. At one point, the US Food and Drug Administration had included tape stripping in its preliminary guidelines for generic topical bioequivalence studies until data from the same formulation generated from 2 different laboratories produced different results.16 Since that time, much work has been done with tape stripping to ensure its consistency. Weigmann et al17 demonstrated equivalent results with clobetasol using vasoconstriction and tape stripping, and Wiedersberg et al18 demonstrated the same with betamethasone. For the current study, the fluocinonide and halcinonide formulations were weighed prior to application so that the same dose was tested in all participants. A plunger was used to produce consistent pressure at all application sites to control for the amount of skin that was stripped off with the tape. Results for both corticosteroids were consistent between the participants. Variability in the data was detected; however, this observation is most likely due to the small number of participants in the studies.
Conclusion
In summary, this pilot study demonstrated that fluocinonide concentration in the stratum corneum peaks within the first hour of application before beginning a steady general decline. There was no evidence of sustained release. In contrast, halcin-onide demonstrated a sustained release for 6 hours after application. Halcinonide is formulated in a cream base in which the corticosteroid is present in a solution and suspension phase that allows for sustained delivery in skin over time. Fluocinonide does not appear to be formulated in the same way, and its concentrations in the stratum corneum begin to decline 1 hour after application.
Acknowledgement
Thank you to Robert Kellar, PhD, at the Center for Bioengineering Innovation at Northern Arizona University, Flagstaff, for conducting the liquid chromatography–mass spectrometry.
1. Ostrenga J, Haleblian J, Poulsen B, et al. Vehicle design for a new topical steroid, fluocinonide. J Invest Dermatol. 1971;56:392-399.
2. Rathi SK, D’Souza P. Rational and ethical use of topical corticosteroids based on safety and efficacy. Indian J Dermatol. 2012;57:251-259.
3. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
4. Sudilovsky A, Clewe TH. Comparative efficacy of halcin-onide and fluocinonide creams in psoriasis and eczematous dermatoses. J Clin Pharmacol. 1975;15:779-784.
5. Close JE. Double-blind comparison of topical halcinonide and fluocinonide in the treatment of psoriasis. Int J Dermatol. 1976;15:534-537.
6. Lynfield Y, Watsky M. Psoriasis: topical corticosteroid therapy. Cutis. 1976;18:133, 136-137.
7. Draelos ZD. Demonstration of the biphasic release of 0.1% halcinonide cream. J Drugs Dermatol. 2015;14:89-90.
8. Bagatell FK. Halcinonide: a new potent topical anti-inflammatory drug. Cutis. 1974;14:459-462.
9. Ostrenga J, Steinmetz C, Poulsen B. Significance of vehicle composition. I. relationship between topical vehicle composition, skin penetrability, and clinical efficacy. J Pharm Sci. 1971;60:1175-1179.
10. Ayres PJ, Hooper G. Assessment of the skin penetration properties of different carrier vehicles for topically applied cortisol. Br J Dermatol. 1978;99:307-317.
11. Olsen EA. Double-blind controlled comparison of generic and trade-name topical steroids using the vasoconstriction assay. Arch Dermatol. 1991;127:197-201.
12. Stoughton RB. Are generic formulations equivalent to trade name topical glucocorticoids? Arch Dermatol. 1987;123:1312-1314.
13. Poulsen BJ, Young E, Coquilla V, et al. Effect of topical vehicle composition on the in vitro release of fluocinolone acetonide and its acetate ester. J Pharm Sci. 1968;57:928-933.
14. Taheri A, Cantrell J, Feldman SR. Tachyphylaxis to topical glucocorticoids: what is the evidence? Dermatol Online J. 2013;19:18954.
15. Ference JD, Last AR. Choosing topical corticosteroids. Am Fam Physician. 2009;79:135-140.
16. Pershing LK, Nelson JL, Corlett JL, et al. Assessment of dermatopharmacokinetic approach in the bioequivalence determination of topical tretinoin gel products. J Am Acad Dermatol. 2003;48:740-751.
17. Weigmann H, Lademann J, v Pelchrzim R, et al. Bioavailability of clobetasol propionate-quantification of drug concentrations in the stratum corneum by dermatopharmacokinetics using tape stripping. Skin Pharmacol Appl Skin Physiol. 1999;12:46-53.
18. Wiedersberg S, Naik A, Leopold CS, et al. Pharmacodynamics and dermatopharmacokinetics of betamethasone 17-valerate: assessment of topical bioavailability. Br J Dermatol. 2009;160:676-686.
1. Ostrenga J, Haleblian J, Poulsen B, et al. Vehicle design for a new topical steroid, fluocinonide. J Invest Dermatol. 1971;56:392-399.
2. Rathi SK, D’Souza P. Rational and ethical use of topical corticosteroids based on safety and efficacy. Indian J Dermatol. 2012;57:251-259.
3. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
4. Sudilovsky A, Clewe TH. Comparative efficacy of halcin-onide and fluocinonide creams in psoriasis and eczematous dermatoses. J Clin Pharmacol. 1975;15:779-784.
5. Close JE. Double-blind comparison of topical halcinonide and fluocinonide in the treatment of psoriasis. Int J Dermatol. 1976;15:534-537.
6. Lynfield Y, Watsky M. Psoriasis: topical corticosteroid therapy. Cutis. 1976;18:133, 136-137.
7. Draelos ZD. Demonstration of the biphasic release of 0.1% halcinonide cream. J Drugs Dermatol. 2015;14:89-90.
8. Bagatell FK. Halcinonide: a new potent topical anti-inflammatory drug. Cutis. 1974;14:459-462.
9. Ostrenga J, Steinmetz C, Poulsen B. Significance of vehicle composition. I. relationship between topical vehicle composition, skin penetrability, and clinical efficacy. J Pharm Sci. 1971;60:1175-1179.
10. Ayres PJ, Hooper G. Assessment of the skin penetration properties of different carrier vehicles for topically applied cortisol. Br J Dermatol. 1978;99:307-317.
11. Olsen EA. Double-blind controlled comparison of generic and trade-name topical steroids using the vasoconstriction assay. Arch Dermatol. 1991;127:197-201.
12. Stoughton RB. Are generic formulations equivalent to trade name topical glucocorticoids? Arch Dermatol. 1987;123:1312-1314.
13. Poulsen BJ, Young E, Coquilla V, et al. Effect of topical vehicle composition on the in vitro release of fluocinolone acetonide and its acetate ester. J Pharm Sci. 1968;57:928-933.
14. Taheri A, Cantrell J, Feldman SR. Tachyphylaxis to topical glucocorticoids: what is the evidence? Dermatol Online J. 2013;19:18954.
15. Ference JD, Last AR. Choosing topical corticosteroids. Am Fam Physician. 2009;79:135-140.
16. Pershing LK, Nelson JL, Corlett JL, et al. Assessment of dermatopharmacokinetic approach in the bioequivalence determination of topical tretinoin gel products. J Am Acad Dermatol. 2003;48:740-751.
17. Weigmann H, Lademann J, v Pelchrzim R, et al. Bioavailability of clobetasol propionate-quantification of drug concentrations in the stratum corneum by dermatopharmacokinetics using tape stripping. Skin Pharmacol Appl Skin Physiol. 1999;12:46-53.
18. Wiedersberg S, Naik A, Leopold CS, et al. Pharmacodynamics and dermatopharmacokinetics of betamethasone 17-valerate: assessment of topical bioavailability. Br J Dermatol. 2009;160:676-686.
Practice Points
- Fluocinonide concentration in the stratum corneum peaks within the first hour of application and then begins a steady decline.
- Halcinonide concentration also peaks within the first hour of application and remains elevated for 6 hours after application.
- Halcinonide, rather than fluocinonide, may provide clinical benefits in between doses because of its sustained release hours after application.
Product News: 08 2015
Epiduo Forte Gel
Galderma Laboratories, LP, announces US Food and Drug Administration approval of antibiotic-free Epiduo Forte (adapalene 0.3% and benzoyl peroxide 2.5%) Gel for the once-daily topical treatment of acne vulgaris. Epiduo Forte Gel contains a high concentration of adapalene, which works to unclog blocked pores and inhibits the release of proinflammatory mediators. Benzoyl peroxide offers antimicrobial properties. Epiduo Forte Gel can be considered for long-term use in patients with moderate to severe acne. The formulation is offered in a pump to provide convenient once-daily dosing. Epiduo Forte Gel will be available by prescription in early September 2015. For more information, visit www.galdermausa.com.
Odomzo
Novartis Pharmaceuticals Corporation obtains US Food and Drug Administration approval of Odomzo (sonidegib) 200-mg capsules for the treatment of adult patients with locally advanced basal cell carcinoma that has recurred following surgery or radiation therapy, or those who are not candidates for surgery or radiation therapy. Odomzo is an oral selective smoothened (SMO) inhibitor. SMO is a molecule that regulates the hedgehog signaling pathway, which plays a critical role in stem cell maintenance and tissue repair as well as in advanced basal cell carcinoma. For more information, visit www.novartis.com.
Physical Matte UV Defense SPF 50
SkinCeuticals introduces Physical Matte UV Defense SPF 50 sunscreen. This 100% mineral sunscreen is formulated with oil-absorbing powders to help minimize the appearance of sweat and oil to ensure a long-lasting matte finish while also providing broad-spectrum UVA/UVB protection. Physical Matte UV DefenseSPF 50 sunscreen is formulated for those with normal, combination, or oily skin with an uneven texture that is prone to shine. The mousse texture enhances application. It can be used alone or under makeup. For more information, visit www.skinceuticals.com.
Silagen Scar Refinement System
NewMedical Technology, Inc, introduces the Silagen Scar Refinement System, a line of 100% medical-grade silicone scar therapies. Silagen 100% Pure Silicone Gel is available in 15- or 30-g airless pumps. The Silagen line also includes a wide variety of silicone gel sheets, strips, and shapes with advanced adhesion technology and optimal silicone thickness. The gel has a silky feel and fast drying time. Silagen is physician dispensed. For more information, visit www.silagen.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected].
Epiduo Forte Gel
Galderma Laboratories, LP, announces US Food and Drug Administration approval of antibiotic-free Epiduo Forte (adapalene 0.3% and benzoyl peroxide 2.5%) Gel for the once-daily topical treatment of acne vulgaris. Epiduo Forte Gel contains a high concentration of adapalene, which works to unclog blocked pores and inhibits the release of proinflammatory mediators. Benzoyl peroxide offers antimicrobial properties. Epiduo Forte Gel can be considered for long-term use in patients with moderate to severe acne. The formulation is offered in a pump to provide convenient once-daily dosing. Epiduo Forte Gel will be available by prescription in early September 2015. For more information, visit www.galdermausa.com.
Odomzo
Novartis Pharmaceuticals Corporation obtains US Food and Drug Administration approval of Odomzo (sonidegib) 200-mg capsules for the treatment of adult patients with locally advanced basal cell carcinoma that has recurred following surgery or radiation therapy, or those who are not candidates for surgery or radiation therapy. Odomzo is an oral selective smoothened (SMO) inhibitor. SMO is a molecule that regulates the hedgehog signaling pathway, which plays a critical role in stem cell maintenance and tissue repair as well as in advanced basal cell carcinoma. For more information, visit www.novartis.com.
Physical Matte UV Defense SPF 50
SkinCeuticals introduces Physical Matte UV Defense SPF 50 sunscreen. This 100% mineral sunscreen is formulated with oil-absorbing powders to help minimize the appearance of sweat and oil to ensure a long-lasting matte finish while also providing broad-spectrum UVA/UVB protection. Physical Matte UV DefenseSPF 50 sunscreen is formulated for those with normal, combination, or oily skin with an uneven texture that is prone to shine. The mousse texture enhances application. It can be used alone or under makeup. For more information, visit www.skinceuticals.com.
Silagen Scar Refinement System
NewMedical Technology, Inc, introduces the Silagen Scar Refinement System, a line of 100% medical-grade silicone scar therapies. Silagen 100% Pure Silicone Gel is available in 15- or 30-g airless pumps. The Silagen line also includes a wide variety of silicone gel sheets, strips, and shapes with advanced adhesion technology and optimal silicone thickness. The gel has a silky feel and fast drying time. Silagen is physician dispensed. For more information, visit www.silagen.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected].
Epiduo Forte Gel
Galderma Laboratories, LP, announces US Food and Drug Administration approval of antibiotic-free Epiduo Forte (adapalene 0.3% and benzoyl peroxide 2.5%) Gel for the once-daily topical treatment of acne vulgaris. Epiduo Forte Gel contains a high concentration of adapalene, which works to unclog blocked pores and inhibits the release of proinflammatory mediators. Benzoyl peroxide offers antimicrobial properties. Epiduo Forte Gel can be considered for long-term use in patients with moderate to severe acne. The formulation is offered in a pump to provide convenient once-daily dosing. Epiduo Forte Gel will be available by prescription in early September 2015. For more information, visit www.galdermausa.com.
Odomzo
Novartis Pharmaceuticals Corporation obtains US Food and Drug Administration approval of Odomzo (sonidegib) 200-mg capsules for the treatment of adult patients with locally advanced basal cell carcinoma that has recurred following surgery or radiation therapy, or those who are not candidates for surgery or radiation therapy. Odomzo is an oral selective smoothened (SMO) inhibitor. SMO is a molecule that regulates the hedgehog signaling pathway, which plays a critical role in stem cell maintenance and tissue repair as well as in advanced basal cell carcinoma. For more information, visit www.novartis.com.
Physical Matte UV Defense SPF 50
SkinCeuticals introduces Physical Matte UV Defense SPF 50 sunscreen. This 100% mineral sunscreen is formulated with oil-absorbing powders to help minimize the appearance of sweat and oil to ensure a long-lasting matte finish while also providing broad-spectrum UVA/UVB protection. Physical Matte UV DefenseSPF 50 sunscreen is formulated for those with normal, combination, or oily skin with an uneven texture that is prone to shine. The mousse texture enhances application. It can be used alone or under makeup. For more information, visit www.skinceuticals.com.
Silagen Scar Refinement System
NewMedical Technology, Inc, introduces the Silagen Scar Refinement System, a line of 100% medical-grade silicone scar therapies. Silagen 100% Pure Silicone Gel is available in 15- or 30-g airless pumps. The Silagen line also includes a wide variety of silicone gel sheets, strips, and shapes with advanced adhesion technology and optimal silicone thickness. The gel has a silky feel and fast drying time. Silagen is physician dispensed. For more information, visit www.silagen.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected].
Update on Hyaluronic Acid Fillers for Facial Rejuvenation
Facial rejuvenation has become increasingly popular, with nonsurgical and noninvasive procedures comprising a large part of aesthetic practice. According to the American Society for Aesthetic Plastic Surgery, Americans spent $12 billion on cosmetic procedures in 2014, with more than 10 million surgical and nonsurgical procedures performed. The top 5 nonsurgical procedures for both men and women combined were botulinum toxin, hyaluronic acid (HA), hair removal, chemical peel, and microdermabrasion.1
The first dermal filler used was bovine collagen, which was approved by the US Food and Drug Administration (FDA) in 1981. Despite its efficacy in the correction of facial rhytides, bovine collagen required allergy testing prior to use and was discontinued in 2010. Dermal fillers have evolved over the years, and newer products that are superior to earlier fillers with regard to longevity, safety, and tolerability and that do not require allergy testing have become available; however, advances in the use of dermal fillers are not only related to the development of newer products but also to evolving injection techniques. Initially, the aim of treatment with dermal fillers was to correct lines and wrinkles, but an increased understanding of the complex changes that occur with aging have changed our approach to one of volume replacement, with an emphasis on volume restoration in the midface. This approach requires an in-depth understanding of facial anatomy as well as the interactions of the skin, soft tissue, muscle, and bone. Furthermore, placement of filler in specific fat compartments can provide a more natural appearance and an all-around youthful face.2 In this article, we discuss HA fillers that have gained FDA approval within the last 5 years (Table).
Overview of HA Fillers
Hyaluronic acid is a naturally occurring linear glycosaminoglycan with a disaccharide unit, which repeats several thousand times.3 Hyaluronic acid is an essential part of the extracellular matrix of many tissues including the dermis and plays an important role in tissue growth, development, and wound healing. Hyaluronic acid is hygroscopic and absorbs water extensively, thus creating volume.4 Treatment with HA fillers is popular, as they are biocompatible and have a low potential for allergic reactions. They also are easy to use and reversible.4 The first HA filler to gain FDA approval was Restylane (Galderma Laboratories, LP). Currently, several HA fillers are approved in the United States, and each product differs from the others in polymer chain length, degree of HA concentration, particle size, gel consistency, gel hardness, gel viscosity, and degree of water solubility, as well as amount and degree of cross-linking. Cross-linking is essential to avoid enzymatic degradation by endogenous hyaluronidase when injected into the skin and thus to prolong the product’s half-life.5 Cross-linkers used to manufacture HA fillers include 1,4-butanediol diglycidyl ether and divinyl sulfone. More concentrated products with a greater degree of cross-linking provide increased longevity, but they are associated with a higher risk for inflammation and nodule formation. The elastic modulus (G′) is a measure of the firmness of dermal fillers, describing their resistance to deformation. Materials with a higher G′ are stiffer and are meant for deeper injections. Hyaluronic acid fillers can be further classified as biphasic or monophasic. Biphasic fillers (eg, Restylane, Perlane [Galderma Laboratories, LP]) contain a range of microsphere sizes, while monophasic fillers (eg, Juvéderm [Allergan, Inc], Belotero Balance [Merz North America, Inc]) contain homogeneous microspheres. Although randomized clinical trials have reported comparable efficacy and durability of biphasic and monophasic fillers when used to treat the nasolabial folds,6-8 monophasic HA fillers are more cohesive and may not migrate as much following injection.
Restylane Family
Restylane was the first FDA-approved HA filler, gaining its approval in 2003. Restylane is a nonanimal stabilized HA (NASHA) that is produced from the fermentation of equine streptococci. It is cross-linked with 1,4-butanediol diglycidyl ether with a 1% degree of cross-linking. Restylane has an HA concentration of 20 mg/mL. The particle size range of Restylane and Restylane-L is 330 to 430 mm. Restylane (and also Perlane) get passed through sizing screens via sieves and are quantified by their size. The longevity of HA fillers is approximately 6 months; however, various factors affect the product’s longevity, such as the degree of cross-linking, treatment area, and the patient’s metabolism. Restylane-L, which was FDA approved in 2012, is a newer product with 0.3% lidocaine incorporated into the syringe itself. It was the first product from the Restylane range to be approved for lip augmentation. The addition of lidocaine (designated by the L in the product name) does not affect the longevity of a filler.9
The newest FDA-approved HA filler was Restylane Silk (approved in 2014), which has been specifically designed for lip augmentation and correction of perioral rhytides. To avoid postprocedural swelling, it generally is recommended that Restylane Silk be injected slowly. If required, a short course of oral prednisone may be administered after the procedure to treat any edema. Restylane Silk is less viscous than Restylane and requires less pressure to inject. Therefore, it is more suited for treatment of fine perioral lines, as it flows more easily. Because it contains 0.3% lidocaine, discomfort usually is minimal, with treatment lasting approximately 30 to 60 minutes. In the author’s experience (G.G.), Restylane Silk provides a softer correction, though one has to be careful to inject slowly to avoid postinjection swelling. Restylane Silk also may be suitable for neck rejuvenation (off label), but several treatment sessions usually are required. Patients should be warned that they are likely to experience ecchymoses. In our experience, the effects of Restylane Silk injections last approximately 6 to 9 months.
Juvéderm Family
The first Juvéderm product was approved by the FDA in 2006. Juvéderm is a bacterium-derived NASHA. Injectable gel formulations of Juvéderm, including Juvéderm Ultra and Juvéderm Ultra Plus, are FDA approved for the correction of moderate to severe facial wrinkles and folds. The first products in the Juvéderm line were produced using a technology called Hylacross technology, with cohesive molecules of cross-linked HA. In contrast to the sizing technology used by Restylane and Perlane, the Hylacross technology does not break up the cross-linked HA by passing the product through sizing screens via sieves, but instead produces monophasic gels. These Juvéderm products have a high concentration of cross-linked HAs, which accounts for their longevity, and they are soft and easy to use.
Juvéderm Voluma XC was FDA approved in 2013. It is a 20-mg/mL, smooth, highly cohesive, viscous HA, gel that is manufactured using Vycross technology, a combination of low- and high-molecular-weight HA, and it is the only HA filler that is indicated for deep injection for cheek augmentation,10 as it creates a lift due to its higher G′ and a low swelling capacity, with results lasting up to 2 years. The mean volume administered over the initial 4-week period of one study was 5.1 mL.11
Belotero Balance
Belotero Balance was approved by the FDA in 2011 for the correction of moderate to severe facial wrinkles and folds (eg, nasolabial folds).12 Belotero Balance is an HA filler with a cohesive polydensified matrix technology and low elasticity and viscosity. It has the lowest G′ of the currently available dermal fillers12 and therefore is associated with increased injection precision. Belotero Balance is ideal for superficial injections,13 such as forehead lines, vermilion border, tear trough, atrophic scars, and neck lines (off label). Some clinicians reconstitute Belotero Balance with lidocaine (off label) to provide a more pain-free procedure. In our experience, results typically last at least 6 to 8 months.
Complications
Hyaluronic acid fillers share the same adverse events across the product lines. The most common reactions include erythema, swelling, and bruising, which often are unavoidable and may be considered expected effects. Less-frequent events include contour irregularities; product migration; bluish discoloration known as the Tyndall effect, which is more likely to occur with superficial injections; nodules; infection at the injection site; scarring; and vascular occlusion, potentially leading to blindness.14 These more severe complications often can be avoided. Appropriate skin preparation and a sterile technique are critical in preventing infections, while deep placement of filler material reduces the risk for Tyndall effect, nodules, and scarring. Skin necrosis occurs by external compression of the blood supply by the product or occlusion via direct injection into a vessel. Aspirating prior to injection, administering lower volumes, and tenting the skin to inject more superficially can reduce the risk for skin necrosis. Every clinician needs to be able to rapidly recognize the signs of necrosis and to administer urgent therapy, such as the application of warm gauze and nitroglycerin paste, tapping the area to facilitate vasodilatation, and injecting hyaluronidase when required.
On the Horizon
Other dermal fillers that may gain FDA approval in the next few years include Teosyal (Laboratories Teoxane Geneva),6 a new range of monophasic NASHA products that provide high viscosity and elasticity with results lasting 6 to 9 months, and potentially Juvéderm Volbella, a smooth, nonparticle, viscous HA gel developed specifically for the lip area with results lasting up to 1 year.15
Conclusion
Over the last decade, the popularity of dermal fillers has steadily increased, and fillers have become a cornerstone of aesthetic medicine. The increased number of available products necessitates thorough knowledge by the treating physician to ensure optimal outcomes. There is no universal filler that can achieve ideal outcomes in all anatomic sites or in all patients. Often a combination approach may be ideal, such as the use of a product with a higher G′ for re-volumization, followed by a filler with a lower G′ for superficial injection. Some patients may also benefit from a combination of both dermal fillers and neurotoxin injections, either on the same day or at separate visits, which may increase the longevity of the filler.
1. The American Society for Aesthetic Plastic Surgery reports Americans spent more than 12 billion in 2014; procedures for men up 43% over five year period [news release]. New York, NY: American Society for Aesthetic Plastic Surgery; March 11, 2015. http://www.surgery.org/media/news-releases/the-american-society-for-aesthetic-plastic-surgery-reports-americans-spent-more-than-12-billion-in-2014--pro. Accessed July 7, 2015.
2. Fitzgerald R, Rubin AG. Filler placement and the fat compartments. Dermatol Clin. 2014;32:37-50.
3. Cowman MK, Matsuoka S. Experimental approaches to hyaluronan structure. Carbohydr Res. 2005;340:791-809.
4. Lee A, Grummer SE, Kriegel D, et al. Hyaluronidase. Dermatol Surg. 2010;36:1071-1077.
5. Kablik J, Monheit GD, Yu L, et al. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol Surg. 2009;35(suppl 1):302-312.
6. Nast A, Reytan N, Hartmann V, et al. Efficacy and durability of two hyaluronic acid-based fillers in the correction of nasolabial folds: results of a prospective, randomized, double-blind, actively controlled clinical pilot study. Dermatol Surg. 2011;37:768-775.
7. Ascher B, Bayerl C, Brun P, et al. Efficacy and safety of a new hyaluronic acid dermal filler in the treatment of severe nasolabial lines: 6-month interim results of a randomized, evaluator-blinded, intra-individual comparison study. J Cosmet Dermatol. 2011;10:94-98.
8. Rzany B, Bayerl C, Bodokh I, et al. Efficacy and safety of a new hyaluronic acid dermal filler in the treatment of moderate nasolabial folds: 6-month interim results of a randomized, evaluator-blinded, intra-individual comparison study. J Cosmet Laser Ther. 2011;13:107-112.
9. Lupo MP, Swetman G, Waller W. The effect of lidocaine when mixed with large gel particle hyaluronic acid filler tolerability and longevity: a six-month trial. J Drugs Dermatol. 2010;9:1097-1100.
10. Ho D, Jagdeo J. Biological properties of a new volumizing hyaluronic acid filler: a systematic review. J Drugs Dermatol. 2015;14:50-54.
11. Callan P, Goodman GJ, Carlisle I, et al. Efficacy and safety of a hyaluronic acid filler in subjects treated for correction of midface volume deficiency: a 24 month study. Clin Cosmet Investig Dermatol. 2013;6:81-89.
12. Hevia O, Cohen BH, Howell DJ. Safety and efficacy of a cohesive polydensified matrix hyaluronic acid for the correction of infraorbital hollow: an observational study with results at 40 weeks. J Drugs Dermatol. 2014;13:1030-1036.
13. Lorenc ZP, Fagien S, Flynn TC, et al. Clinical application and assessment of Belotero: a roundtable discussion. Plast Reconstr Surg. 2013;132(4, suppl 2):69S-76S.
14. Carruthers JD, Fagien S, Rohrich RJ, et al. Blindness caused by cosmetic filler injection: a review of cause and therapy. Plast Reconstr Surg. 2014;134:1197-1201.
15. Eccleston D, Murphy DK. Juvéderm(®) Volbella™ in the perioral area: a 12-month prospective, multicenter, open-label study. Clin Cosmet Investig Dermatol. 2012;5:167-172.
Facial rejuvenation has become increasingly popular, with nonsurgical and noninvasive procedures comprising a large part of aesthetic practice. According to the American Society for Aesthetic Plastic Surgery, Americans spent $12 billion on cosmetic procedures in 2014, with more than 10 million surgical and nonsurgical procedures performed. The top 5 nonsurgical procedures for both men and women combined were botulinum toxin, hyaluronic acid (HA), hair removal, chemical peel, and microdermabrasion.1
The first dermal filler used was bovine collagen, which was approved by the US Food and Drug Administration (FDA) in 1981. Despite its efficacy in the correction of facial rhytides, bovine collagen required allergy testing prior to use and was discontinued in 2010. Dermal fillers have evolved over the years, and newer products that are superior to earlier fillers with regard to longevity, safety, and tolerability and that do not require allergy testing have become available; however, advances in the use of dermal fillers are not only related to the development of newer products but also to evolving injection techniques. Initially, the aim of treatment with dermal fillers was to correct lines and wrinkles, but an increased understanding of the complex changes that occur with aging have changed our approach to one of volume replacement, with an emphasis on volume restoration in the midface. This approach requires an in-depth understanding of facial anatomy as well as the interactions of the skin, soft tissue, muscle, and bone. Furthermore, placement of filler in specific fat compartments can provide a more natural appearance and an all-around youthful face.2 In this article, we discuss HA fillers that have gained FDA approval within the last 5 years (Table).
Overview of HA Fillers
Hyaluronic acid is a naturally occurring linear glycosaminoglycan with a disaccharide unit, which repeats several thousand times.3 Hyaluronic acid is an essential part of the extracellular matrix of many tissues including the dermis and plays an important role in tissue growth, development, and wound healing. Hyaluronic acid is hygroscopic and absorbs water extensively, thus creating volume.4 Treatment with HA fillers is popular, as they are biocompatible and have a low potential for allergic reactions. They also are easy to use and reversible.4 The first HA filler to gain FDA approval was Restylane (Galderma Laboratories, LP). Currently, several HA fillers are approved in the United States, and each product differs from the others in polymer chain length, degree of HA concentration, particle size, gel consistency, gel hardness, gel viscosity, and degree of water solubility, as well as amount and degree of cross-linking. Cross-linking is essential to avoid enzymatic degradation by endogenous hyaluronidase when injected into the skin and thus to prolong the product’s half-life.5 Cross-linkers used to manufacture HA fillers include 1,4-butanediol diglycidyl ether and divinyl sulfone. More concentrated products with a greater degree of cross-linking provide increased longevity, but they are associated with a higher risk for inflammation and nodule formation. The elastic modulus (G′) is a measure of the firmness of dermal fillers, describing their resistance to deformation. Materials with a higher G′ are stiffer and are meant for deeper injections. Hyaluronic acid fillers can be further classified as biphasic or monophasic. Biphasic fillers (eg, Restylane, Perlane [Galderma Laboratories, LP]) contain a range of microsphere sizes, while monophasic fillers (eg, Juvéderm [Allergan, Inc], Belotero Balance [Merz North America, Inc]) contain homogeneous microspheres. Although randomized clinical trials have reported comparable efficacy and durability of biphasic and monophasic fillers when used to treat the nasolabial folds,6-8 monophasic HA fillers are more cohesive and may not migrate as much following injection.
Restylane Family
Restylane was the first FDA-approved HA filler, gaining its approval in 2003. Restylane is a nonanimal stabilized HA (NASHA) that is produced from the fermentation of equine streptococci. It is cross-linked with 1,4-butanediol diglycidyl ether with a 1% degree of cross-linking. Restylane has an HA concentration of 20 mg/mL. The particle size range of Restylane and Restylane-L is 330 to 430 mm. Restylane (and also Perlane) get passed through sizing screens via sieves and are quantified by their size. The longevity of HA fillers is approximately 6 months; however, various factors affect the product’s longevity, such as the degree of cross-linking, treatment area, and the patient’s metabolism. Restylane-L, which was FDA approved in 2012, is a newer product with 0.3% lidocaine incorporated into the syringe itself. It was the first product from the Restylane range to be approved for lip augmentation. The addition of lidocaine (designated by the L in the product name) does not affect the longevity of a filler.9
The newest FDA-approved HA filler was Restylane Silk (approved in 2014), which has been specifically designed for lip augmentation and correction of perioral rhytides. To avoid postprocedural swelling, it generally is recommended that Restylane Silk be injected slowly. If required, a short course of oral prednisone may be administered after the procedure to treat any edema. Restylane Silk is less viscous than Restylane and requires less pressure to inject. Therefore, it is more suited for treatment of fine perioral lines, as it flows more easily. Because it contains 0.3% lidocaine, discomfort usually is minimal, with treatment lasting approximately 30 to 60 minutes. In the author’s experience (G.G.), Restylane Silk provides a softer correction, though one has to be careful to inject slowly to avoid postinjection swelling. Restylane Silk also may be suitable for neck rejuvenation (off label), but several treatment sessions usually are required. Patients should be warned that they are likely to experience ecchymoses. In our experience, the effects of Restylane Silk injections last approximately 6 to 9 months.
Juvéderm Family
The first Juvéderm product was approved by the FDA in 2006. Juvéderm is a bacterium-derived NASHA. Injectable gel formulations of Juvéderm, including Juvéderm Ultra and Juvéderm Ultra Plus, are FDA approved for the correction of moderate to severe facial wrinkles and folds. The first products in the Juvéderm line were produced using a technology called Hylacross technology, with cohesive molecules of cross-linked HA. In contrast to the sizing technology used by Restylane and Perlane, the Hylacross technology does not break up the cross-linked HA by passing the product through sizing screens via sieves, but instead produces monophasic gels. These Juvéderm products have a high concentration of cross-linked HAs, which accounts for their longevity, and they are soft and easy to use.
Juvéderm Voluma XC was FDA approved in 2013. It is a 20-mg/mL, smooth, highly cohesive, viscous HA, gel that is manufactured using Vycross technology, a combination of low- and high-molecular-weight HA, and it is the only HA filler that is indicated for deep injection for cheek augmentation,10 as it creates a lift due to its higher G′ and a low swelling capacity, with results lasting up to 2 years. The mean volume administered over the initial 4-week period of one study was 5.1 mL.11
Belotero Balance
Belotero Balance was approved by the FDA in 2011 for the correction of moderate to severe facial wrinkles and folds (eg, nasolabial folds).12 Belotero Balance is an HA filler with a cohesive polydensified matrix technology and low elasticity and viscosity. It has the lowest G′ of the currently available dermal fillers12 and therefore is associated with increased injection precision. Belotero Balance is ideal for superficial injections,13 such as forehead lines, vermilion border, tear trough, atrophic scars, and neck lines (off label). Some clinicians reconstitute Belotero Balance with lidocaine (off label) to provide a more pain-free procedure. In our experience, results typically last at least 6 to 8 months.
Complications
Hyaluronic acid fillers share the same adverse events across the product lines. The most common reactions include erythema, swelling, and bruising, which often are unavoidable and may be considered expected effects. Less-frequent events include contour irregularities; product migration; bluish discoloration known as the Tyndall effect, which is more likely to occur with superficial injections; nodules; infection at the injection site; scarring; and vascular occlusion, potentially leading to blindness.14 These more severe complications often can be avoided. Appropriate skin preparation and a sterile technique are critical in preventing infections, while deep placement of filler material reduces the risk for Tyndall effect, nodules, and scarring. Skin necrosis occurs by external compression of the blood supply by the product or occlusion via direct injection into a vessel. Aspirating prior to injection, administering lower volumes, and tenting the skin to inject more superficially can reduce the risk for skin necrosis. Every clinician needs to be able to rapidly recognize the signs of necrosis and to administer urgent therapy, such as the application of warm gauze and nitroglycerin paste, tapping the area to facilitate vasodilatation, and injecting hyaluronidase when required.
On the Horizon
Other dermal fillers that may gain FDA approval in the next few years include Teosyal (Laboratories Teoxane Geneva),6 a new range of monophasic NASHA products that provide high viscosity and elasticity with results lasting 6 to 9 months, and potentially Juvéderm Volbella, a smooth, nonparticle, viscous HA gel developed specifically for the lip area with results lasting up to 1 year.15
Conclusion
Over the last decade, the popularity of dermal fillers has steadily increased, and fillers have become a cornerstone of aesthetic medicine. The increased number of available products necessitates thorough knowledge by the treating physician to ensure optimal outcomes. There is no universal filler that can achieve ideal outcomes in all anatomic sites or in all patients. Often a combination approach may be ideal, such as the use of a product with a higher G′ for re-volumization, followed by a filler with a lower G′ for superficial injection. Some patients may also benefit from a combination of both dermal fillers and neurotoxin injections, either on the same day or at separate visits, which may increase the longevity of the filler.
Facial rejuvenation has become increasingly popular, with nonsurgical and noninvasive procedures comprising a large part of aesthetic practice. According to the American Society for Aesthetic Plastic Surgery, Americans spent $12 billion on cosmetic procedures in 2014, with more than 10 million surgical and nonsurgical procedures performed. The top 5 nonsurgical procedures for both men and women combined were botulinum toxin, hyaluronic acid (HA), hair removal, chemical peel, and microdermabrasion.1
The first dermal filler used was bovine collagen, which was approved by the US Food and Drug Administration (FDA) in 1981. Despite its efficacy in the correction of facial rhytides, bovine collagen required allergy testing prior to use and was discontinued in 2010. Dermal fillers have evolved over the years, and newer products that are superior to earlier fillers with regard to longevity, safety, and tolerability and that do not require allergy testing have become available; however, advances in the use of dermal fillers are not only related to the development of newer products but also to evolving injection techniques. Initially, the aim of treatment with dermal fillers was to correct lines and wrinkles, but an increased understanding of the complex changes that occur with aging have changed our approach to one of volume replacement, with an emphasis on volume restoration in the midface. This approach requires an in-depth understanding of facial anatomy as well as the interactions of the skin, soft tissue, muscle, and bone. Furthermore, placement of filler in specific fat compartments can provide a more natural appearance and an all-around youthful face.2 In this article, we discuss HA fillers that have gained FDA approval within the last 5 years (Table).
Overview of HA Fillers
Hyaluronic acid is a naturally occurring linear glycosaminoglycan with a disaccharide unit, which repeats several thousand times.3 Hyaluronic acid is an essential part of the extracellular matrix of many tissues including the dermis and plays an important role in tissue growth, development, and wound healing. Hyaluronic acid is hygroscopic and absorbs water extensively, thus creating volume.4 Treatment with HA fillers is popular, as they are biocompatible and have a low potential for allergic reactions. They also are easy to use and reversible.4 The first HA filler to gain FDA approval was Restylane (Galderma Laboratories, LP). Currently, several HA fillers are approved in the United States, and each product differs from the others in polymer chain length, degree of HA concentration, particle size, gel consistency, gel hardness, gel viscosity, and degree of water solubility, as well as amount and degree of cross-linking. Cross-linking is essential to avoid enzymatic degradation by endogenous hyaluronidase when injected into the skin and thus to prolong the product’s half-life.5 Cross-linkers used to manufacture HA fillers include 1,4-butanediol diglycidyl ether and divinyl sulfone. More concentrated products with a greater degree of cross-linking provide increased longevity, but they are associated with a higher risk for inflammation and nodule formation. The elastic modulus (G′) is a measure of the firmness of dermal fillers, describing their resistance to deformation. Materials with a higher G′ are stiffer and are meant for deeper injections. Hyaluronic acid fillers can be further classified as biphasic or monophasic. Biphasic fillers (eg, Restylane, Perlane [Galderma Laboratories, LP]) contain a range of microsphere sizes, while monophasic fillers (eg, Juvéderm [Allergan, Inc], Belotero Balance [Merz North America, Inc]) contain homogeneous microspheres. Although randomized clinical trials have reported comparable efficacy and durability of biphasic and monophasic fillers when used to treat the nasolabial folds,6-8 monophasic HA fillers are more cohesive and may not migrate as much following injection.
Restylane Family
Restylane was the first FDA-approved HA filler, gaining its approval in 2003. Restylane is a nonanimal stabilized HA (NASHA) that is produced from the fermentation of equine streptococci. It is cross-linked with 1,4-butanediol diglycidyl ether with a 1% degree of cross-linking. Restylane has an HA concentration of 20 mg/mL. The particle size range of Restylane and Restylane-L is 330 to 430 mm. Restylane (and also Perlane) get passed through sizing screens via sieves and are quantified by their size. The longevity of HA fillers is approximately 6 months; however, various factors affect the product’s longevity, such as the degree of cross-linking, treatment area, and the patient’s metabolism. Restylane-L, which was FDA approved in 2012, is a newer product with 0.3% lidocaine incorporated into the syringe itself. It was the first product from the Restylane range to be approved for lip augmentation. The addition of lidocaine (designated by the L in the product name) does not affect the longevity of a filler.9
The newest FDA-approved HA filler was Restylane Silk (approved in 2014), which has been specifically designed for lip augmentation and correction of perioral rhytides. To avoid postprocedural swelling, it generally is recommended that Restylane Silk be injected slowly. If required, a short course of oral prednisone may be administered after the procedure to treat any edema. Restylane Silk is less viscous than Restylane and requires less pressure to inject. Therefore, it is more suited for treatment of fine perioral lines, as it flows more easily. Because it contains 0.3% lidocaine, discomfort usually is minimal, with treatment lasting approximately 30 to 60 minutes. In the author’s experience (G.G.), Restylane Silk provides a softer correction, though one has to be careful to inject slowly to avoid postinjection swelling. Restylane Silk also may be suitable for neck rejuvenation (off label), but several treatment sessions usually are required. Patients should be warned that they are likely to experience ecchymoses. In our experience, the effects of Restylane Silk injections last approximately 6 to 9 months.
Juvéderm Family
The first Juvéderm product was approved by the FDA in 2006. Juvéderm is a bacterium-derived NASHA. Injectable gel formulations of Juvéderm, including Juvéderm Ultra and Juvéderm Ultra Plus, are FDA approved for the correction of moderate to severe facial wrinkles and folds. The first products in the Juvéderm line were produced using a technology called Hylacross technology, with cohesive molecules of cross-linked HA. In contrast to the sizing technology used by Restylane and Perlane, the Hylacross technology does not break up the cross-linked HA by passing the product through sizing screens via sieves, but instead produces monophasic gels. These Juvéderm products have a high concentration of cross-linked HAs, which accounts for their longevity, and they are soft and easy to use.
Juvéderm Voluma XC was FDA approved in 2013. It is a 20-mg/mL, smooth, highly cohesive, viscous HA, gel that is manufactured using Vycross technology, a combination of low- and high-molecular-weight HA, and it is the only HA filler that is indicated for deep injection for cheek augmentation,10 as it creates a lift due to its higher G′ and a low swelling capacity, with results lasting up to 2 years. The mean volume administered over the initial 4-week period of one study was 5.1 mL.11
Belotero Balance
Belotero Balance was approved by the FDA in 2011 for the correction of moderate to severe facial wrinkles and folds (eg, nasolabial folds).12 Belotero Balance is an HA filler with a cohesive polydensified matrix technology and low elasticity and viscosity. It has the lowest G′ of the currently available dermal fillers12 and therefore is associated with increased injection precision. Belotero Balance is ideal for superficial injections,13 such as forehead lines, vermilion border, tear trough, atrophic scars, and neck lines (off label). Some clinicians reconstitute Belotero Balance with lidocaine (off label) to provide a more pain-free procedure. In our experience, results typically last at least 6 to 8 months.
Complications
Hyaluronic acid fillers share the same adverse events across the product lines. The most common reactions include erythema, swelling, and bruising, which often are unavoidable and may be considered expected effects. Less-frequent events include contour irregularities; product migration; bluish discoloration known as the Tyndall effect, which is more likely to occur with superficial injections; nodules; infection at the injection site; scarring; and vascular occlusion, potentially leading to blindness.14 These more severe complications often can be avoided. Appropriate skin preparation and a sterile technique are critical in preventing infections, while deep placement of filler material reduces the risk for Tyndall effect, nodules, and scarring. Skin necrosis occurs by external compression of the blood supply by the product or occlusion via direct injection into a vessel. Aspirating prior to injection, administering lower volumes, and tenting the skin to inject more superficially can reduce the risk for skin necrosis. Every clinician needs to be able to rapidly recognize the signs of necrosis and to administer urgent therapy, such as the application of warm gauze and nitroglycerin paste, tapping the area to facilitate vasodilatation, and injecting hyaluronidase when required.
On the Horizon
Other dermal fillers that may gain FDA approval in the next few years include Teosyal (Laboratories Teoxane Geneva),6 a new range of monophasic NASHA products that provide high viscosity and elasticity with results lasting 6 to 9 months, and potentially Juvéderm Volbella, a smooth, nonparticle, viscous HA gel developed specifically for the lip area with results lasting up to 1 year.15
Conclusion
Over the last decade, the popularity of dermal fillers has steadily increased, and fillers have become a cornerstone of aesthetic medicine. The increased number of available products necessitates thorough knowledge by the treating physician to ensure optimal outcomes. There is no universal filler that can achieve ideal outcomes in all anatomic sites or in all patients. Often a combination approach may be ideal, such as the use of a product with a higher G′ for re-volumization, followed by a filler with a lower G′ for superficial injection. Some patients may also benefit from a combination of both dermal fillers and neurotoxin injections, either on the same day or at separate visits, which may increase the longevity of the filler.
1. The American Society for Aesthetic Plastic Surgery reports Americans spent more than 12 billion in 2014; procedures for men up 43% over five year period [news release]. New York, NY: American Society for Aesthetic Plastic Surgery; March 11, 2015. http://www.surgery.org/media/news-releases/the-american-society-for-aesthetic-plastic-surgery-reports-americans-spent-more-than-12-billion-in-2014--pro. Accessed July 7, 2015.
2. Fitzgerald R, Rubin AG. Filler placement and the fat compartments. Dermatol Clin. 2014;32:37-50.
3. Cowman MK, Matsuoka S. Experimental approaches to hyaluronan structure. Carbohydr Res. 2005;340:791-809.
4. Lee A, Grummer SE, Kriegel D, et al. Hyaluronidase. Dermatol Surg. 2010;36:1071-1077.
5. Kablik J, Monheit GD, Yu L, et al. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol Surg. 2009;35(suppl 1):302-312.
6. Nast A, Reytan N, Hartmann V, et al. Efficacy and durability of two hyaluronic acid-based fillers in the correction of nasolabial folds: results of a prospective, randomized, double-blind, actively controlled clinical pilot study. Dermatol Surg. 2011;37:768-775.
7. Ascher B, Bayerl C, Brun P, et al. Efficacy and safety of a new hyaluronic acid dermal filler in the treatment of severe nasolabial lines: 6-month interim results of a randomized, evaluator-blinded, intra-individual comparison study. J Cosmet Dermatol. 2011;10:94-98.
8. Rzany B, Bayerl C, Bodokh I, et al. Efficacy and safety of a new hyaluronic acid dermal filler in the treatment of moderate nasolabial folds: 6-month interim results of a randomized, evaluator-blinded, intra-individual comparison study. J Cosmet Laser Ther. 2011;13:107-112.
9. Lupo MP, Swetman G, Waller W. The effect of lidocaine when mixed with large gel particle hyaluronic acid filler tolerability and longevity: a six-month trial. J Drugs Dermatol. 2010;9:1097-1100.
10. Ho D, Jagdeo J. Biological properties of a new volumizing hyaluronic acid filler: a systematic review. J Drugs Dermatol. 2015;14:50-54.
11. Callan P, Goodman GJ, Carlisle I, et al. Efficacy and safety of a hyaluronic acid filler in subjects treated for correction of midface volume deficiency: a 24 month study. Clin Cosmet Investig Dermatol. 2013;6:81-89.
12. Hevia O, Cohen BH, Howell DJ. Safety and efficacy of a cohesive polydensified matrix hyaluronic acid for the correction of infraorbital hollow: an observational study with results at 40 weeks. J Drugs Dermatol. 2014;13:1030-1036.
13. Lorenc ZP, Fagien S, Flynn TC, et al. Clinical application and assessment of Belotero: a roundtable discussion. Plast Reconstr Surg. 2013;132(4, suppl 2):69S-76S.
14. Carruthers JD, Fagien S, Rohrich RJ, et al. Blindness caused by cosmetic filler injection: a review of cause and therapy. Plast Reconstr Surg. 2014;134:1197-1201.
15. Eccleston D, Murphy DK. Juvéderm(®) Volbella™ in the perioral area: a 12-month prospective, multicenter, open-label study. Clin Cosmet Investig Dermatol. 2012;5:167-172.
1. The American Society for Aesthetic Plastic Surgery reports Americans spent more than 12 billion in 2014; procedures for men up 43% over five year period [news release]. New York, NY: American Society for Aesthetic Plastic Surgery; March 11, 2015. http://www.surgery.org/media/news-releases/the-american-society-for-aesthetic-plastic-surgery-reports-americans-spent-more-than-12-billion-in-2014--pro. Accessed July 7, 2015.
2. Fitzgerald R, Rubin AG. Filler placement and the fat compartments. Dermatol Clin. 2014;32:37-50.
3. Cowman MK, Matsuoka S. Experimental approaches to hyaluronan structure. Carbohydr Res. 2005;340:791-809.
4. Lee A, Grummer SE, Kriegel D, et al. Hyaluronidase. Dermatol Surg. 2010;36:1071-1077.
5. Kablik J, Monheit GD, Yu L, et al. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol Surg. 2009;35(suppl 1):302-312.
6. Nast A, Reytan N, Hartmann V, et al. Efficacy and durability of two hyaluronic acid-based fillers in the correction of nasolabial folds: results of a prospective, randomized, double-blind, actively controlled clinical pilot study. Dermatol Surg. 2011;37:768-775.
7. Ascher B, Bayerl C, Brun P, et al. Efficacy and safety of a new hyaluronic acid dermal filler in the treatment of severe nasolabial lines: 6-month interim results of a randomized, evaluator-blinded, intra-individual comparison study. J Cosmet Dermatol. 2011;10:94-98.
8. Rzany B, Bayerl C, Bodokh I, et al. Efficacy and safety of a new hyaluronic acid dermal filler in the treatment of moderate nasolabial folds: 6-month interim results of a randomized, evaluator-blinded, intra-individual comparison study. J Cosmet Laser Ther. 2011;13:107-112.
9. Lupo MP, Swetman G, Waller W. The effect of lidocaine when mixed with large gel particle hyaluronic acid filler tolerability and longevity: a six-month trial. J Drugs Dermatol. 2010;9:1097-1100.
10. Ho D, Jagdeo J. Biological properties of a new volumizing hyaluronic acid filler: a systematic review. J Drugs Dermatol. 2015;14:50-54.
11. Callan P, Goodman GJ, Carlisle I, et al. Efficacy and safety of a hyaluronic acid filler in subjects treated for correction of midface volume deficiency: a 24 month study. Clin Cosmet Investig Dermatol. 2013;6:81-89.
12. Hevia O, Cohen BH, Howell DJ. Safety and efficacy of a cohesive polydensified matrix hyaluronic acid for the correction of infraorbital hollow: an observational study with results at 40 weeks. J Drugs Dermatol. 2014;13:1030-1036.
13. Lorenc ZP, Fagien S, Flynn TC, et al. Clinical application and assessment of Belotero: a roundtable discussion. Plast Reconstr Surg. 2013;132(4, suppl 2):69S-76S.
14. Carruthers JD, Fagien S, Rohrich RJ, et al. Blindness caused by cosmetic filler injection: a review of cause and therapy. Plast Reconstr Surg. 2014;134:1197-1201.
15. Eccleston D, Murphy DK. Juvéderm(®) Volbella™ in the perioral area: a 12-month prospective, multicenter, open-label study. Clin Cosmet Investig Dermatol. 2012;5:167-172.
Practice Points
- Restylane Silk is useful for the treatment of fine perioral lines.
- Juvéderm Voluma XC is a newer product in the Juvéderm range and is indicated for cheek augmentation.
- Belotero Balance has the lowest G′ of the currently available dermal fillers and allows greater precision.
Caps on malpractice damages
Question: Which of the following statements regarding statutory caps on malpractice damages is best?
A. All states have such a statutory provision.
B. The provision limits the recovery of both economic and noneconomic losses.
C. It’s constitutional.
D. It’s not constitutional.
E. Whether it’s constitutional depends on the jurisdiction.
Answer: E. In 1975, California enacted its historic Medical Injury Compensation Reform Act (MICRA),1 the state legislature declaring that there was “a major health care crisis in the State of California attributable to skyrocketing malpractice premium costs and resulting in a potential breakdown of the health delivery system.”
The rationale was to provide some predictability, because noneconomic damages are difficult to quantify, and jury sympathy may result in unrealistically high payments. It was believed that damages for pain and suffering, for example, often contributed to runaway jury verdicts, prompting one indignant observer to write: “In making arguments for pain and suffering awards, both sides attempt to win the jurors’ sympathies with highly emotional evidence. A blind plaintiff will receive careful instruction to come to court with his [guide] dog, and to dab at his eyes with a handkerchief.”2
One of the main provisions of MICRA is to limit noneconomic recovery for injuries arising out of medical negligence. It caps noneconomic damages – for example, pain and suffering, disfigurement, emotional distress, loss of consortium, and other nonpecuniary losses – at $250,000. The law does not restrict recovery of economic damages such as wage loss, medical expenses, and future lost income.
California is the pioneer state to institute this tort reform measure, and about a dozen other states have followed suit, such as Proposition 12 in Texas, which limits noneconomic damages to $750,000 – $250,000 from the defendant doctor and $500,000 from the hospital.
Many tort reformists hail MICRA as the prototype success story, crediting it for bringing California’s malpractice insurance premiums from one of the highest levels in the nation to one of its lowest. A 2004 study reported that states with caps have a loss ratio (losses plus costs over premiums) that is 12% lower than in those without damage caps.3 Lower premiums in turn are linked to greater physician entry into the locality, especially for high-risk specialists.
In addition, caps may have a salutary effect on the wasteful practice of defensive medicine. A 2007 report by the American Medical Association confirms and extends an earlier study that reached such conclusions.
However, recent medical malpractice rates are generally no longer rising or even falling – both in states that had enacted tort reform and in states that had not. This may mean that other interventions such as medical error recognition and reduction are also effective.
Unsurprisingly, caps on damages have been challenged on constitutional grounds, as a violation of the equal rights amendment and the patient’s right to a jury trial. Two recent cases with divergent results – one on California, and the other in Florida – illustrate the state of flux over this controversy.
In Chan v. Curran, the plaintiff sought to relitigate the constitutionality of the California damage cap, but the appellate court ruled for the doctor defendant.4 The case alleged a wrongful death when the patient died from hemorrhage related to warfarin (Coumadin) use during open heart surgery.
The plaintiff argued that MICRA’s rationale was irrelevant, because there was no longer a malpractice insurance crisis in California – thus, restrictions placed on the quantum of damages are not rationally related to any legitimate state interest.
Furthermore, by limiting the amount of noneconomic damages to $250,000, MICRA violated equal protection and discouraged or inhibited attorneys from taking up malpractice cases on a contingency fee basis. Finally, the plaintiff argued that under the statute, a litigant is deprived of the right to a jury trial.
The court rejected all of these arguments, and reaffirmed the constitutionality of MICRA in line with earlier decisions that began with California’s Supreme Court decision in the Fein v. Permanente Medical Group case.5
On the other hand, the recent case of Estate of Michelle Evette McCall v. U.S. found the Florida Supreme Court ruling for the plaintiff.6 There, the court deemed unconstitutional Florida’s statute limiting wrongful death damages in medical malpractice to $1 million.
The case involved a young mother who died of massive hemorrhage following a cesarean section. In a 5-2 decision, the court held that the statute was arbitrary, reasoning that “the statutory cap on wrongful death noneconomic damages fails because it imposes unfair and illogical burdens on injured parties.”
Unlike California, the Florida court found that the cap bears no rational relationship to any perceived malpractice insurance crisis. And, while saving a modest amount for many, the statute imposed devastating costs on those who are most grievously injured, as well as on cases affecting multiple claimants.
The court commented that “the finding by the Legislature and the Task Force that Florida was in the midst of a bona fide medical malpractice crisis, threatening the access of Floridians to health care, is dubious and questionable at the very best.” The court also noted that four malpractice carriers actually increased their net income by more than 4,300% between 2003 and 2010.
In 2010, the Illinois Supreme Court also held in Lebron v. Gottlieb Memorial Hospital that the state’s $500,000 cap for noneconomic damages was unconstitutional, being in violation of the separation of powers doctrine.7 Only judges are empowered to reduce excessive verdicts, termed a remittitur. Thus, a statutory damage cap amounted to a “legislative remittitur” that invaded the power of the judiciary and violated the constitutional requirement of separation of powers.
The battle over caps continues unabated, with the trend appearing to favor the plaintiff bar. Florida’s ruling was the eighth state supreme court decision that held damage caps unconstitutional, joining Alabama, Georgia, Illinois, Missouri, New Hampshire, Oregon, and Washington. Five other states – Arizona, Arkansas, Kentucky, Pennsylvania, and Wyoming – already have state constitutional prohibitions on damage caps.
References
1. Medical Injury Compensation Reform Act of 1975, Cal. Civ. Proc. Code § 3333.2 (West 1982).
2. O’Connell, J. Offers That Can’t Be Refused: Foreclosure of Personal Injury Claims by Defendants’ Prompt Tender of Claimants’ Net Economic Losses. 77 N.W.U.L. Rev. 589, 591 (1982).
3. Thorpe, K. The Medical Malpractice Crisis: Recent Trends and the Impact of State Tort Reforms, Health Affairs 2004, Jan 21 [doi:10.1377/hlthaff.w4.20].
4. Chan v. Curran, 237 Cal. App. 4th 601 (Cal.Ct.App. 2015).
5. Fein v. Permanente Medical Group, 695 P.2d 665 (Cal. 1985).
6. Estate of Michelle Evette McCall v. U.S., 2014 Fla. LEXIS 933 (Fla. Mar. 13, 2014).
7. Lebron v. Gottlieb Memorial Hospital, 930 N.E.2d 895 (Ill. 2010).
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, and currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, “Medical Malpractice: Understanding the Law, Managing the Risk,” and his 2012 Halsbury treatise, “Medical Negligence and Professional Misconduct.” For additional information, readers may contact the author at [email protected].
Question: Which of the following statements regarding statutory caps on malpractice damages is best?
A. All states have such a statutory provision.
B. The provision limits the recovery of both economic and noneconomic losses.
C. It’s constitutional.
D. It’s not constitutional.
E. Whether it’s constitutional depends on the jurisdiction.
Answer: E. In 1975, California enacted its historic Medical Injury Compensation Reform Act (MICRA),1 the state legislature declaring that there was “a major health care crisis in the State of California attributable to skyrocketing malpractice premium costs and resulting in a potential breakdown of the health delivery system.”
The rationale was to provide some predictability, because noneconomic damages are difficult to quantify, and jury sympathy may result in unrealistically high payments. It was believed that damages for pain and suffering, for example, often contributed to runaway jury verdicts, prompting one indignant observer to write: “In making arguments for pain and suffering awards, both sides attempt to win the jurors’ sympathies with highly emotional evidence. A blind plaintiff will receive careful instruction to come to court with his [guide] dog, and to dab at his eyes with a handkerchief.”2
One of the main provisions of MICRA is to limit noneconomic recovery for injuries arising out of medical negligence. It caps noneconomic damages – for example, pain and suffering, disfigurement, emotional distress, loss of consortium, and other nonpecuniary losses – at $250,000. The law does not restrict recovery of economic damages such as wage loss, medical expenses, and future lost income.
California is the pioneer state to institute this tort reform measure, and about a dozen other states have followed suit, such as Proposition 12 in Texas, which limits noneconomic damages to $750,000 – $250,000 from the defendant doctor and $500,000 from the hospital.
Many tort reformists hail MICRA as the prototype success story, crediting it for bringing California’s malpractice insurance premiums from one of the highest levels in the nation to one of its lowest. A 2004 study reported that states with caps have a loss ratio (losses plus costs over premiums) that is 12% lower than in those without damage caps.3 Lower premiums in turn are linked to greater physician entry into the locality, especially for high-risk specialists.
In addition, caps may have a salutary effect on the wasteful practice of defensive medicine. A 2007 report by the American Medical Association confirms and extends an earlier study that reached such conclusions.
However, recent medical malpractice rates are generally no longer rising or even falling – both in states that had enacted tort reform and in states that had not. This may mean that other interventions such as medical error recognition and reduction are also effective.
Unsurprisingly, caps on damages have been challenged on constitutional grounds, as a violation of the equal rights amendment and the patient’s right to a jury trial. Two recent cases with divergent results – one on California, and the other in Florida – illustrate the state of flux over this controversy.
In Chan v. Curran, the plaintiff sought to relitigate the constitutionality of the California damage cap, but the appellate court ruled for the doctor defendant.4 The case alleged a wrongful death when the patient died from hemorrhage related to warfarin (Coumadin) use during open heart surgery.
The plaintiff argued that MICRA’s rationale was irrelevant, because there was no longer a malpractice insurance crisis in California – thus, restrictions placed on the quantum of damages are not rationally related to any legitimate state interest.
Furthermore, by limiting the amount of noneconomic damages to $250,000, MICRA violated equal protection and discouraged or inhibited attorneys from taking up malpractice cases on a contingency fee basis. Finally, the plaintiff argued that under the statute, a litigant is deprived of the right to a jury trial.
The court rejected all of these arguments, and reaffirmed the constitutionality of MICRA in line with earlier decisions that began with California’s Supreme Court decision in the Fein v. Permanente Medical Group case.5
On the other hand, the recent case of Estate of Michelle Evette McCall v. U.S. found the Florida Supreme Court ruling for the plaintiff.6 There, the court deemed unconstitutional Florida’s statute limiting wrongful death damages in medical malpractice to $1 million.
The case involved a young mother who died of massive hemorrhage following a cesarean section. In a 5-2 decision, the court held that the statute was arbitrary, reasoning that “the statutory cap on wrongful death noneconomic damages fails because it imposes unfair and illogical burdens on injured parties.”
Unlike California, the Florida court found that the cap bears no rational relationship to any perceived malpractice insurance crisis. And, while saving a modest amount for many, the statute imposed devastating costs on those who are most grievously injured, as well as on cases affecting multiple claimants.
The court commented that “the finding by the Legislature and the Task Force that Florida was in the midst of a bona fide medical malpractice crisis, threatening the access of Floridians to health care, is dubious and questionable at the very best.” The court also noted that four malpractice carriers actually increased their net income by more than 4,300% between 2003 and 2010.
In 2010, the Illinois Supreme Court also held in Lebron v. Gottlieb Memorial Hospital that the state’s $500,000 cap for noneconomic damages was unconstitutional, being in violation of the separation of powers doctrine.7 Only judges are empowered to reduce excessive verdicts, termed a remittitur. Thus, a statutory damage cap amounted to a “legislative remittitur” that invaded the power of the judiciary and violated the constitutional requirement of separation of powers.
The battle over caps continues unabated, with the trend appearing to favor the plaintiff bar. Florida’s ruling was the eighth state supreme court decision that held damage caps unconstitutional, joining Alabama, Georgia, Illinois, Missouri, New Hampshire, Oregon, and Washington. Five other states – Arizona, Arkansas, Kentucky, Pennsylvania, and Wyoming – already have state constitutional prohibitions on damage caps.
References
1. Medical Injury Compensation Reform Act of 1975, Cal. Civ. Proc. Code § 3333.2 (West 1982).
2. O’Connell, J. Offers That Can’t Be Refused: Foreclosure of Personal Injury Claims by Defendants’ Prompt Tender of Claimants’ Net Economic Losses. 77 N.W.U.L. Rev. 589, 591 (1982).
3. Thorpe, K. The Medical Malpractice Crisis: Recent Trends and the Impact of State Tort Reforms, Health Affairs 2004, Jan 21 [doi:10.1377/hlthaff.w4.20].
4. Chan v. Curran, 237 Cal. App. 4th 601 (Cal.Ct.App. 2015).
5. Fein v. Permanente Medical Group, 695 P.2d 665 (Cal. 1985).
6. Estate of Michelle Evette McCall v. U.S., 2014 Fla. LEXIS 933 (Fla. Mar. 13, 2014).
7. Lebron v. Gottlieb Memorial Hospital, 930 N.E.2d 895 (Ill. 2010).
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, and currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, “Medical Malpractice: Understanding the Law, Managing the Risk,” and his 2012 Halsbury treatise, “Medical Negligence and Professional Misconduct.” For additional information, readers may contact the author at [email protected].
Question: Which of the following statements regarding statutory caps on malpractice damages is best?
A. All states have such a statutory provision.
B. The provision limits the recovery of both economic and noneconomic losses.
C. It’s constitutional.
D. It’s not constitutional.
E. Whether it’s constitutional depends on the jurisdiction.
Answer: E. In 1975, California enacted its historic Medical Injury Compensation Reform Act (MICRA),1 the state legislature declaring that there was “a major health care crisis in the State of California attributable to skyrocketing malpractice premium costs and resulting in a potential breakdown of the health delivery system.”
The rationale was to provide some predictability, because noneconomic damages are difficult to quantify, and jury sympathy may result in unrealistically high payments. It was believed that damages for pain and suffering, for example, often contributed to runaway jury verdicts, prompting one indignant observer to write: “In making arguments for pain and suffering awards, both sides attempt to win the jurors’ sympathies with highly emotional evidence. A blind plaintiff will receive careful instruction to come to court with his [guide] dog, and to dab at his eyes with a handkerchief.”2
One of the main provisions of MICRA is to limit noneconomic recovery for injuries arising out of medical negligence. It caps noneconomic damages – for example, pain and suffering, disfigurement, emotional distress, loss of consortium, and other nonpecuniary losses – at $250,000. The law does not restrict recovery of economic damages such as wage loss, medical expenses, and future lost income.
California is the pioneer state to institute this tort reform measure, and about a dozen other states have followed suit, such as Proposition 12 in Texas, which limits noneconomic damages to $750,000 – $250,000 from the defendant doctor and $500,000 from the hospital.
Many tort reformists hail MICRA as the prototype success story, crediting it for bringing California’s malpractice insurance premiums from one of the highest levels in the nation to one of its lowest. A 2004 study reported that states with caps have a loss ratio (losses plus costs over premiums) that is 12% lower than in those without damage caps.3 Lower premiums in turn are linked to greater physician entry into the locality, especially for high-risk specialists.
In addition, caps may have a salutary effect on the wasteful practice of defensive medicine. A 2007 report by the American Medical Association confirms and extends an earlier study that reached such conclusions.
However, recent medical malpractice rates are generally no longer rising or even falling – both in states that had enacted tort reform and in states that had not. This may mean that other interventions such as medical error recognition and reduction are also effective.
Unsurprisingly, caps on damages have been challenged on constitutional grounds, as a violation of the equal rights amendment and the patient’s right to a jury trial. Two recent cases with divergent results – one on California, and the other in Florida – illustrate the state of flux over this controversy.
In Chan v. Curran, the plaintiff sought to relitigate the constitutionality of the California damage cap, but the appellate court ruled for the doctor defendant.4 The case alleged a wrongful death when the patient died from hemorrhage related to warfarin (Coumadin) use during open heart surgery.
The plaintiff argued that MICRA’s rationale was irrelevant, because there was no longer a malpractice insurance crisis in California – thus, restrictions placed on the quantum of damages are not rationally related to any legitimate state interest.
Furthermore, by limiting the amount of noneconomic damages to $250,000, MICRA violated equal protection and discouraged or inhibited attorneys from taking up malpractice cases on a contingency fee basis. Finally, the plaintiff argued that under the statute, a litigant is deprived of the right to a jury trial.
The court rejected all of these arguments, and reaffirmed the constitutionality of MICRA in line with earlier decisions that began with California’s Supreme Court decision in the Fein v. Permanente Medical Group case.5
On the other hand, the recent case of Estate of Michelle Evette McCall v. U.S. found the Florida Supreme Court ruling for the plaintiff.6 There, the court deemed unconstitutional Florida’s statute limiting wrongful death damages in medical malpractice to $1 million.
The case involved a young mother who died of massive hemorrhage following a cesarean section. In a 5-2 decision, the court held that the statute was arbitrary, reasoning that “the statutory cap on wrongful death noneconomic damages fails because it imposes unfair and illogical burdens on injured parties.”
Unlike California, the Florida court found that the cap bears no rational relationship to any perceived malpractice insurance crisis. And, while saving a modest amount for many, the statute imposed devastating costs on those who are most grievously injured, as well as on cases affecting multiple claimants.
The court commented that “the finding by the Legislature and the Task Force that Florida was in the midst of a bona fide medical malpractice crisis, threatening the access of Floridians to health care, is dubious and questionable at the very best.” The court also noted that four malpractice carriers actually increased their net income by more than 4,300% between 2003 and 2010.
In 2010, the Illinois Supreme Court also held in Lebron v. Gottlieb Memorial Hospital that the state’s $500,000 cap for noneconomic damages was unconstitutional, being in violation of the separation of powers doctrine.7 Only judges are empowered to reduce excessive verdicts, termed a remittitur. Thus, a statutory damage cap amounted to a “legislative remittitur” that invaded the power of the judiciary and violated the constitutional requirement of separation of powers.
The battle over caps continues unabated, with the trend appearing to favor the plaintiff bar. Florida’s ruling was the eighth state supreme court decision that held damage caps unconstitutional, joining Alabama, Georgia, Illinois, Missouri, New Hampshire, Oregon, and Washington. Five other states – Arizona, Arkansas, Kentucky, Pennsylvania, and Wyoming – already have state constitutional prohibitions on damage caps.
References
1. Medical Injury Compensation Reform Act of 1975, Cal. Civ. Proc. Code § 3333.2 (West 1982).
2. O’Connell, J. Offers That Can’t Be Refused: Foreclosure of Personal Injury Claims by Defendants’ Prompt Tender of Claimants’ Net Economic Losses. 77 N.W.U.L. Rev. 589, 591 (1982).
3. Thorpe, K. The Medical Malpractice Crisis: Recent Trends and the Impact of State Tort Reforms, Health Affairs 2004, Jan 21 [doi:10.1377/hlthaff.w4.20].
4. Chan v. Curran, 237 Cal. App. 4th 601 (Cal.Ct.App. 2015).
5. Fein v. Permanente Medical Group, 695 P.2d 665 (Cal. 1985).
6. Estate of Michelle Evette McCall v. U.S., 2014 Fla. LEXIS 933 (Fla. Mar. 13, 2014).
7. Lebron v. Gottlieb Memorial Hospital, 930 N.E.2d 895 (Ill. 2010).
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, and currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, “Medical Malpractice: Understanding the Law, Managing the Risk,” and his 2012 Halsbury treatise, “Medical Negligence and Professional Misconduct.” For additional information, readers may contact the author at [email protected].
What Is Your Diagnosis? Verrucous Carcinoma
An 81-year-old woman presented for evaluation of a nodule on the right labia majora that had been present for 1 year. She had a history of intertriginous psoriasis, and several biopsies were performed at an outside facility over the last 5 years that revealed psoriasis but were otherwise noncontributory. Physical examination revealed erythema and scaling on the buttocks with maceration in the intertriginous area (top) and the perineum associated with a verrucous nodule (bottom).
The Diagnosis: Verrucous Carcinoma
Biopsies of early lesions often may be difficult to interpret without clinicopathological correlation. Our patient’s tumor was associated with intertriginous psoriasis, which was the only abnormality previously noted on superficial biopsies performed at an outside facility. The patient was scheduled for an excisional biopsy due to the large tumor size and clinical suspicion that the prior biopsies were inadequate and failed to demonstrate the primary underlying pathology. Excisional biopsy of the verrucous tumor revealed epithelium composed of keratinocytes with glassy cytoplasm. Papillomatosis was noted along with an endophytic component of well-differentiated epithelial cells extending into the dermis in a bulbous pattern consistent with the verrucous carcinoma variant of squamous cell carcinoma (SCC)(Figure). Verrucous carcinoma often requires correlation with both the clinical and histopathologic findings for definitive diagnosis, as keratinocytes often appear to be well differentiated.1
Verrucous carcinoma may begin as an innocuous papule that slowly grows into a large fungating tumor. Verrucous carcinomas typically are slow growing, exophytic, and low grade. The etiology of verrucous carcinoma is not clear, and the role of human papillomavirus (HPV) infection is controversial.2 Best classified as a well-differentiated SCC, verrucous carcinoma rarely metastasizes but may invade adjacent tissues.
Differential diagnoses include a giant inflamed seborrheic keratosis, condyloma acuminatum, rupioid psoriasis, and inflammatory linear verrucous epidermal nevus (ILVEN). Although large and inflamed seborrheic keratoses may have squamous eddies that mimic SCC, seborrheic keratoses do not invade the dermis and typically have a well-circumscribed stuck-on appearance. Abnormal mitotic figures are not identified. Condylomas are genital warts caused by HPV infection that often are clustered, well circumscribed, and exophytic. Large lesions can be difficult to distinguish from verrucous carcinomas, and biopsy generally reveals koilocytes identified by perinuclear clearing and raisinlike nuclei. Immunohistochemical staining and in situ hybridization studies can be of value in diagnosis and in identifying those lesions that are at high risk for malignant transformation. High-risk condylomas are associated with HPV-16, HPV-18, HPV-31, HPV-33, HPV-35, and HPV-39, as well as other types, whereas low-risk condylomas are associated with HPV-6, HPV-11, HPV-42, and others.2 Differentiating squamous cell hyperplasia from squamous cell carcinoma in situ also can be aided by immunohistochemistry. Squamous cell hyperplasia is usually negative for INK4 p16Ink4A and p53 and exhibits variable Ki-67 staining. Differentiated squamous cell carcinoma in situ exhibits a profile that is p16Ink4A negative, Ki-67 positive, and exhibits variable p53 staining.3 Basaloid and warty intraepithelial neoplasia is consistently p16Ink4A positive, Ki-67 positive, and variably positive for p53.3 Therefore, p16 staining of high-grade areas is a useful biomarker that can help establish diagnosis of associated squamous cell carcinoma.4 The role of papillomaviruses in the development of nonmelanoma skin cancer is an area of active study, and research suggests that papillomaviruses may have a much greater role than previously suspected.5
At times, psoriasis may be markedly hyperkeratotic, clinically mimicking a verrucous neoplasm. This hyperkeratotic type of psoriasis is known as rupioid psoriasis. However, these psoriatic lesions are exophytic, are associated with spongiform pustules, and lack the atypia and endophytic pattern typically seen with verrucous carcinoma. An ILVEN also lacks atypia and an endophytic pattern and usually presents in childhood as a persistent linear plaque, rather than the verrucous plaque noted in our patient. Squamous cell carcinoma has been reported to arise in the setting of verrucoid ILVEN but is exceptionally uncommon.6
Successful treatment of verrucous carcinoma is best achieved by complete excision. Oral retinoids and immunomodulators such as imiquimod also may be of value.7 Our patient’s tumor qualifies as T2N0M0 because it was greater than 2 cm in size.8 A Breslow thickness of 2 mm or greater and Clark level IV are high-risk features associated with a worse prognosis, but clinical evaluation of our patient’s lymph nodes was unremarkable and no distant metastases were identified. Our patient continues to do well with no evidence of recurrence.
1. Bambao C, Nofech-Mozes S, Shier M. Giant condyloma versus verrucous carcinoma: a case report. J Low Genit Tract Dis. 2010;14:230-233.
2. Asiaf A, Ahmad ST, Mohannad SO, et al. Review of the current knowledge on the epidemiology, pathogenesis, and prevention of human papillomavirus infection. Eur J Cancer Prev. 2014;23:206-224.
3. Chaux A, Pfannl R, Rodríguez IM, et al. Distinctive immunohistochemical profile of penile intraepithelial lesions: a study of 74 cases. Am J Surg Pathol. 2011;35:553-562.
4. Darragh TM, Colgan TJ, Cox JT, et al. The lower anogenital squamous terminology standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Arch Pathol Lab Med. 2012;136:1266-1297.
5. Aldabagh B, Angeles J, Cardones AR, et al. Cutaneous squamous cell carcinoma and human papillomavirus: is there an association? Dermatol Surg. 2013;39:1-23.
6. Turk BG, Ertam I, Urkmez A, et al. Development of squamous cell carcinoma on an inflammatory linear verrucous epidermal nevus in the genital area. Cutis. 2012;89:273-275.
7. Erkek E, Basar H, Bozdogan O, et al. Giant condyloma acuminata of Buschke-Löwenstein: successful treatment with a combination of surgical excision, oral acitretin and topical imiquimod. Clin Exp Dermatol. 2009;34:366-368.
8. Cutaneous squamous cell carcinoma and other cutaneous carcinomas. In: Edge SB, Byrd DR, Compton CC, et al, eds. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010:301-314.
An 81-year-old woman presented for evaluation of a nodule on the right labia majora that had been present for 1 year. She had a history of intertriginous psoriasis, and several biopsies were performed at an outside facility over the last 5 years that revealed psoriasis but were otherwise noncontributory. Physical examination revealed erythema and scaling on the buttocks with maceration in the intertriginous area (top) and the perineum associated with a verrucous nodule (bottom).
The Diagnosis: Verrucous Carcinoma
Biopsies of early lesions often may be difficult to interpret without clinicopathological correlation. Our patient’s tumor was associated with intertriginous psoriasis, which was the only abnormality previously noted on superficial biopsies performed at an outside facility. The patient was scheduled for an excisional biopsy due to the large tumor size and clinical suspicion that the prior biopsies were inadequate and failed to demonstrate the primary underlying pathology. Excisional biopsy of the verrucous tumor revealed epithelium composed of keratinocytes with glassy cytoplasm. Papillomatosis was noted along with an endophytic component of well-differentiated epithelial cells extending into the dermis in a bulbous pattern consistent with the verrucous carcinoma variant of squamous cell carcinoma (SCC)(Figure). Verrucous carcinoma often requires correlation with both the clinical and histopathologic findings for definitive diagnosis, as keratinocytes often appear to be well differentiated.1
Verrucous carcinoma may begin as an innocuous papule that slowly grows into a large fungating tumor. Verrucous carcinomas typically are slow growing, exophytic, and low grade. The etiology of verrucous carcinoma is not clear, and the role of human papillomavirus (HPV) infection is controversial.2 Best classified as a well-differentiated SCC, verrucous carcinoma rarely metastasizes but may invade adjacent tissues.
Differential diagnoses include a giant inflamed seborrheic keratosis, condyloma acuminatum, rupioid psoriasis, and inflammatory linear verrucous epidermal nevus (ILVEN). Although large and inflamed seborrheic keratoses may have squamous eddies that mimic SCC, seborrheic keratoses do not invade the dermis and typically have a well-circumscribed stuck-on appearance. Abnormal mitotic figures are not identified. Condylomas are genital warts caused by HPV infection that often are clustered, well circumscribed, and exophytic. Large lesions can be difficult to distinguish from verrucous carcinomas, and biopsy generally reveals koilocytes identified by perinuclear clearing and raisinlike nuclei. Immunohistochemical staining and in situ hybridization studies can be of value in diagnosis and in identifying those lesions that are at high risk for malignant transformation. High-risk condylomas are associated with HPV-16, HPV-18, HPV-31, HPV-33, HPV-35, and HPV-39, as well as other types, whereas low-risk condylomas are associated with HPV-6, HPV-11, HPV-42, and others.2 Differentiating squamous cell hyperplasia from squamous cell carcinoma in situ also can be aided by immunohistochemistry. Squamous cell hyperplasia is usually negative for INK4 p16Ink4A and p53 and exhibits variable Ki-67 staining. Differentiated squamous cell carcinoma in situ exhibits a profile that is p16Ink4A negative, Ki-67 positive, and exhibits variable p53 staining.3 Basaloid and warty intraepithelial neoplasia is consistently p16Ink4A positive, Ki-67 positive, and variably positive for p53.3 Therefore, p16 staining of high-grade areas is a useful biomarker that can help establish diagnosis of associated squamous cell carcinoma.4 The role of papillomaviruses in the development of nonmelanoma skin cancer is an area of active study, and research suggests that papillomaviruses may have a much greater role than previously suspected.5
At times, psoriasis may be markedly hyperkeratotic, clinically mimicking a verrucous neoplasm. This hyperkeratotic type of psoriasis is known as rupioid psoriasis. However, these psoriatic lesions are exophytic, are associated with spongiform pustules, and lack the atypia and endophytic pattern typically seen with verrucous carcinoma. An ILVEN also lacks atypia and an endophytic pattern and usually presents in childhood as a persistent linear plaque, rather than the verrucous plaque noted in our patient. Squamous cell carcinoma has been reported to arise in the setting of verrucoid ILVEN but is exceptionally uncommon.6
Successful treatment of verrucous carcinoma is best achieved by complete excision. Oral retinoids and immunomodulators such as imiquimod also may be of value.7 Our patient’s tumor qualifies as T2N0M0 because it was greater than 2 cm in size.8 A Breslow thickness of 2 mm or greater and Clark level IV are high-risk features associated with a worse prognosis, but clinical evaluation of our patient’s lymph nodes was unremarkable and no distant metastases were identified. Our patient continues to do well with no evidence of recurrence.
An 81-year-old woman presented for evaluation of a nodule on the right labia majora that had been present for 1 year. She had a history of intertriginous psoriasis, and several biopsies were performed at an outside facility over the last 5 years that revealed psoriasis but were otherwise noncontributory. Physical examination revealed erythema and scaling on the buttocks with maceration in the intertriginous area (top) and the perineum associated with a verrucous nodule (bottom).
The Diagnosis: Verrucous Carcinoma
Biopsies of early lesions often may be difficult to interpret without clinicopathological correlation. Our patient’s tumor was associated with intertriginous psoriasis, which was the only abnormality previously noted on superficial biopsies performed at an outside facility. The patient was scheduled for an excisional biopsy due to the large tumor size and clinical suspicion that the prior biopsies were inadequate and failed to demonstrate the primary underlying pathology. Excisional biopsy of the verrucous tumor revealed epithelium composed of keratinocytes with glassy cytoplasm. Papillomatosis was noted along with an endophytic component of well-differentiated epithelial cells extending into the dermis in a bulbous pattern consistent with the verrucous carcinoma variant of squamous cell carcinoma (SCC)(Figure). Verrucous carcinoma often requires correlation with both the clinical and histopathologic findings for definitive diagnosis, as keratinocytes often appear to be well differentiated.1
Verrucous carcinoma may begin as an innocuous papule that slowly grows into a large fungating tumor. Verrucous carcinomas typically are slow growing, exophytic, and low grade. The etiology of verrucous carcinoma is not clear, and the role of human papillomavirus (HPV) infection is controversial.2 Best classified as a well-differentiated SCC, verrucous carcinoma rarely metastasizes but may invade adjacent tissues.
Differential diagnoses include a giant inflamed seborrheic keratosis, condyloma acuminatum, rupioid psoriasis, and inflammatory linear verrucous epidermal nevus (ILVEN). Although large and inflamed seborrheic keratoses may have squamous eddies that mimic SCC, seborrheic keratoses do not invade the dermis and typically have a well-circumscribed stuck-on appearance. Abnormal mitotic figures are not identified. Condylomas are genital warts caused by HPV infection that often are clustered, well circumscribed, and exophytic. Large lesions can be difficult to distinguish from verrucous carcinomas, and biopsy generally reveals koilocytes identified by perinuclear clearing and raisinlike nuclei. Immunohistochemical staining and in situ hybridization studies can be of value in diagnosis and in identifying those lesions that are at high risk for malignant transformation. High-risk condylomas are associated with HPV-16, HPV-18, HPV-31, HPV-33, HPV-35, and HPV-39, as well as other types, whereas low-risk condylomas are associated with HPV-6, HPV-11, HPV-42, and others.2 Differentiating squamous cell hyperplasia from squamous cell carcinoma in situ also can be aided by immunohistochemistry. Squamous cell hyperplasia is usually negative for INK4 p16Ink4A and p53 and exhibits variable Ki-67 staining. Differentiated squamous cell carcinoma in situ exhibits a profile that is p16Ink4A negative, Ki-67 positive, and exhibits variable p53 staining.3 Basaloid and warty intraepithelial neoplasia is consistently p16Ink4A positive, Ki-67 positive, and variably positive for p53.3 Therefore, p16 staining of high-grade areas is a useful biomarker that can help establish diagnosis of associated squamous cell carcinoma.4 The role of papillomaviruses in the development of nonmelanoma skin cancer is an area of active study, and research suggests that papillomaviruses may have a much greater role than previously suspected.5
At times, psoriasis may be markedly hyperkeratotic, clinically mimicking a verrucous neoplasm. This hyperkeratotic type of psoriasis is known as rupioid psoriasis. However, these psoriatic lesions are exophytic, are associated with spongiform pustules, and lack the atypia and endophytic pattern typically seen with verrucous carcinoma. An ILVEN also lacks atypia and an endophytic pattern and usually presents in childhood as a persistent linear plaque, rather than the verrucous plaque noted in our patient. Squamous cell carcinoma has been reported to arise in the setting of verrucoid ILVEN but is exceptionally uncommon.6
Successful treatment of verrucous carcinoma is best achieved by complete excision. Oral retinoids and immunomodulators such as imiquimod also may be of value.7 Our patient’s tumor qualifies as T2N0M0 because it was greater than 2 cm in size.8 A Breslow thickness of 2 mm or greater and Clark level IV are high-risk features associated with a worse prognosis, but clinical evaluation of our patient’s lymph nodes was unremarkable and no distant metastases were identified. Our patient continues to do well with no evidence of recurrence.
1. Bambao C, Nofech-Mozes S, Shier M. Giant condyloma versus verrucous carcinoma: a case report. J Low Genit Tract Dis. 2010;14:230-233.
2. Asiaf A, Ahmad ST, Mohannad SO, et al. Review of the current knowledge on the epidemiology, pathogenesis, and prevention of human papillomavirus infection. Eur J Cancer Prev. 2014;23:206-224.
3. Chaux A, Pfannl R, Rodríguez IM, et al. Distinctive immunohistochemical profile of penile intraepithelial lesions: a study of 74 cases. Am J Surg Pathol. 2011;35:553-562.
4. Darragh TM, Colgan TJ, Cox JT, et al. The lower anogenital squamous terminology standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Arch Pathol Lab Med. 2012;136:1266-1297.
5. Aldabagh B, Angeles J, Cardones AR, et al. Cutaneous squamous cell carcinoma and human papillomavirus: is there an association? Dermatol Surg. 2013;39:1-23.
6. Turk BG, Ertam I, Urkmez A, et al. Development of squamous cell carcinoma on an inflammatory linear verrucous epidermal nevus in the genital area. Cutis. 2012;89:273-275.
7. Erkek E, Basar H, Bozdogan O, et al. Giant condyloma acuminata of Buschke-Löwenstein: successful treatment with a combination of surgical excision, oral acitretin and topical imiquimod. Clin Exp Dermatol. 2009;34:366-368.
8. Cutaneous squamous cell carcinoma and other cutaneous carcinomas. In: Edge SB, Byrd DR, Compton CC, et al, eds. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010:301-314.
1. Bambao C, Nofech-Mozes S, Shier M. Giant condyloma versus verrucous carcinoma: a case report. J Low Genit Tract Dis. 2010;14:230-233.
2. Asiaf A, Ahmad ST, Mohannad SO, et al. Review of the current knowledge on the epidemiology, pathogenesis, and prevention of human papillomavirus infection. Eur J Cancer Prev. 2014;23:206-224.
3. Chaux A, Pfannl R, Rodríguez IM, et al. Distinctive immunohistochemical profile of penile intraepithelial lesions: a study of 74 cases. Am J Surg Pathol. 2011;35:553-562.
4. Darragh TM, Colgan TJ, Cox JT, et al. The lower anogenital squamous terminology standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Arch Pathol Lab Med. 2012;136:1266-1297.
5. Aldabagh B, Angeles J, Cardones AR, et al. Cutaneous squamous cell carcinoma and human papillomavirus: is there an association? Dermatol Surg. 2013;39:1-23.
6. Turk BG, Ertam I, Urkmez A, et al. Development of squamous cell carcinoma on an inflammatory linear verrucous epidermal nevus in the genital area. Cutis. 2012;89:273-275.
7. Erkek E, Basar H, Bozdogan O, et al. Giant condyloma acuminata of Buschke-Löwenstein: successful treatment with a combination of surgical excision, oral acitretin and topical imiquimod. Clin Exp Dermatol. 2009;34:366-368.
8. Cutaneous squamous cell carcinoma and other cutaneous carcinomas. In: Edge SB, Byrd DR, Compton CC, et al, eds. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010:301-314.
ACIP releases 2015-2016 flu vaccine recommendations
Influenza vaccination is recommended for all patients aged 6 months and older, as long as they don’t have a contraindication, according to a report Aug. 7 in Morbidity and Mortality Weekly Report.
Trivalent influenza vaccines for the 2015-2016 season will contain hemagglutinin (HA) derived from an H1N1-like virus, an H3N2-like virus, and a B/Phuket/3073/2013-like (Yamagata lineage) virus. Quadrivalent vaccines will contain those components, as well as a B/Brisbane/60/2008-like (Victoria lineage) virus, the same virus recommended for quadrivalent formulations in the 2013-14 and 2014-15 seasons, ACIP said in a statement.
New FDA-approved vaccines include Afluria, the Fluzone Intradermal Quadrivalent vaccine (both approved in 2014 for adults aged 18-64 years), and an expanded age indication for Flublok, which is now indicated for adults aged 18 years and older.
The live attenuated influenza vaccine (LAIV) should not be used in certain populations, including those aged less than 2 years or greater than 49 years; children aged 2-17 years taking aspirin; pateints with severe allergic reactions to the vaccine; pregnant women; and those with egg allergies, among others. Either the LAIV or the inactivated influenza vaccine (IIV) is appropriate for administration in healthy children aged 2-8 years, ACIP said.
For a detailed explanation of the recommendations, see MMWR.
Influenza vaccination is recommended for all patients aged 6 months and older, as long as they don’t have a contraindication, according to a report Aug. 7 in Morbidity and Mortality Weekly Report.
Trivalent influenza vaccines for the 2015-2016 season will contain hemagglutinin (HA) derived from an H1N1-like virus, an H3N2-like virus, and a B/Phuket/3073/2013-like (Yamagata lineage) virus. Quadrivalent vaccines will contain those components, as well as a B/Brisbane/60/2008-like (Victoria lineage) virus, the same virus recommended for quadrivalent formulations in the 2013-14 and 2014-15 seasons, ACIP said in a statement.
New FDA-approved vaccines include Afluria, the Fluzone Intradermal Quadrivalent vaccine (both approved in 2014 for adults aged 18-64 years), and an expanded age indication for Flublok, which is now indicated for adults aged 18 years and older.
The live attenuated influenza vaccine (LAIV) should not be used in certain populations, including those aged less than 2 years or greater than 49 years; children aged 2-17 years taking aspirin; pateints with severe allergic reactions to the vaccine; pregnant women; and those with egg allergies, among others. Either the LAIV or the inactivated influenza vaccine (IIV) is appropriate for administration in healthy children aged 2-8 years, ACIP said.
For a detailed explanation of the recommendations, see MMWR.
Influenza vaccination is recommended for all patients aged 6 months and older, as long as they don’t have a contraindication, according to a report Aug. 7 in Morbidity and Mortality Weekly Report.
Trivalent influenza vaccines for the 2015-2016 season will contain hemagglutinin (HA) derived from an H1N1-like virus, an H3N2-like virus, and a B/Phuket/3073/2013-like (Yamagata lineage) virus. Quadrivalent vaccines will contain those components, as well as a B/Brisbane/60/2008-like (Victoria lineage) virus, the same virus recommended for quadrivalent formulations in the 2013-14 and 2014-15 seasons, ACIP said in a statement.
New FDA-approved vaccines include Afluria, the Fluzone Intradermal Quadrivalent vaccine (both approved in 2014 for adults aged 18-64 years), and an expanded age indication for Flublok, which is now indicated for adults aged 18 years and older.
The live attenuated influenza vaccine (LAIV) should not be used in certain populations, including those aged less than 2 years or greater than 49 years; children aged 2-17 years taking aspirin; pateints with severe allergic reactions to the vaccine; pregnant women; and those with egg allergies, among others. Either the LAIV or the inactivated influenza vaccine (IIV) is appropriate for administration in healthy children aged 2-8 years, ACIP said.
For a detailed explanation of the recommendations, see MMWR.
Modifiable risk factors foretell colonic anastomotic leak
CHICAGO – Several modifiable risk factors predicted the development of anastomotic leak following elective colon resection, a large national analysis found.
“Preoperative smoking cessation, preoperative administration of oral antibiotic bowel preparation, and laparoscopic approach are modifiable factors that could reduce the risk of anastomotic leak,” Dr. Cindy Wu said at the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) National Conference.
Anastomotic leakage results in increased morbidity and mortality, yet the current literature analyzing risk factors for this complication is generally limited to retrospective studies of single institutions, she said.
To examine data from a larger sample of colectomy patients from multiple centers, the investigators used the NSQIP Participant Use Data File specifically targeted to colectomy to identify 14,848 patients who underwent elective colon resection from 2012 to 2013. Chi-square, Wald chi-square, and logistic regression analyses were performed examining patient factors (sex, race, comorbidities, smoking status, American Society of Anesthesiologists class, functional status, steroid use, and preoperative albumin), oncologic factors (chemotherapy, tumor stage, and presence or absence of disseminated cancer), and operative factors (wound class, mechanical bowel preparation, oral antibiotic preparation, surgical approach, colectomy site, surgical indication, and operative time).
In all, 3.4%, or 498 patients, experienced an anastomotic leak, which is consistent with the literature, Dr. Wu of Temple University in Philadelphia said. Of these patients, 101 required no intervention, while 272 required surgery and 125 needed percutaneous drainage. The mean age of the patients was 60.7 years and 57% were male.
In a univariate analysis, male sex (chi-square = 17.4; P less than .01), diabetes controlled with either oral medication or insulin (X2 = 9.5; P less than .01), and smoking within the last year (X2 = 20.4; P less than .01) were associated with a greater incidence of anastomotic leak.
Other risk factors that were significant in additional univariate analysis were ASA class (X2 = 23.3; P = .0001), functional status (X2 = 9.15; P = .01), 10% weight loss over the last 6 months (X2 = 5.83; P = .02), wound class (X2 = 10.8; P = .01), mechanical bowel preparation (X2 = 5.89; P = .01), lack of oral antibiotic preparation (X2 = 17.5; P less than .0001), open vs. laparoscopic/minimally invasive surgery (X2 = 60.0; P less than .0001), chemotherapy in the last 90 days (X2 = 23.1; P less than .0001), and presence of disseminated cancer (X2 = 7.41; P = .01), Dr. Wu said.
With all of these factors taken into account in multivariate analysis, independent predictors of an increased risk of anastomotic leak were male sex (odds ratio, 1.74; P = .01), tobacco use (OR, 1.73; P = .03), and lack of a preoperative oral antibiotic bowel preparation (OR, 1.79; P less than .01).
Interestingly, use of a laparoscopic technique was protective against the development of anastomotic leakage (OR, 0.54; P less than .01), she said.
The authors reported having no relevant financial disclosures.
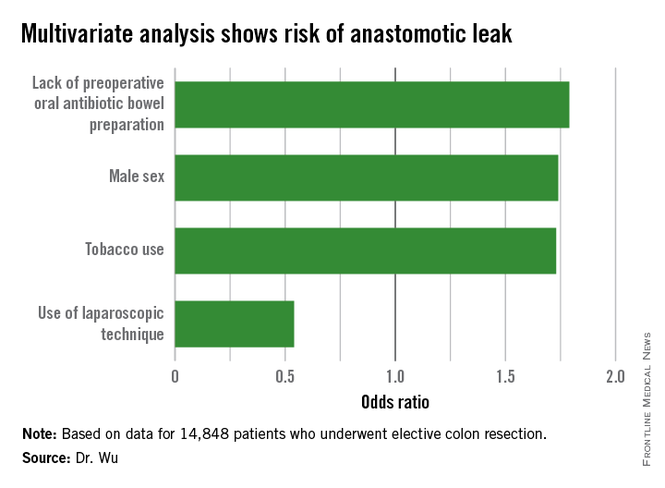
CHICAGO – Several modifiable risk factors predicted the development of anastomotic leak following elective colon resection, a large national analysis found.
“Preoperative smoking cessation, preoperative administration of oral antibiotic bowel preparation, and laparoscopic approach are modifiable factors that could reduce the risk of anastomotic leak,” Dr. Cindy Wu said at the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) National Conference.
Anastomotic leakage results in increased morbidity and mortality, yet the current literature analyzing risk factors for this complication is generally limited to retrospective studies of single institutions, she said.
To examine data from a larger sample of colectomy patients from multiple centers, the investigators used the NSQIP Participant Use Data File specifically targeted to colectomy to identify 14,848 patients who underwent elective colon resection from 2012 to 2013. Chi-square, Wald chi-square, and logistic regression analyses were performed examining patient factors (sex, race, comorbidities, smoking status, American Society of Anesthesiologists class, functional status, steroid use, and preoperative albumin), oncologic factors (chemotherapy, tumor stage, and presence or absence of disseminated cancer), and operative factors (wound class, mechanical bowel preparation, oral antibiotic preparation, surgical approach, colectomy site, surgical indication, and operative time).
In all, 3.4%, or 498 patients, experienced an anastomotic leak, which is consistent with the literature, Dr. Wu of Temple University in Philadelphia said. Of these patients, 101 required no intervention, while 272 required surgery and 125 needed percutaneous drainage. The mean age of the patients was 60.7 years and 57% were male.
In a univariate analysis, male sex (chi-square = 17.4; P less than .01), diabetes controlled with either oral medication or insulin (X2 = 9.5; P less than .01), and smoking within the last year (X2 = 20.4; P less than .01) were associated with a greater incidence of anastomotic leak.
Other risk factors that were significant in additional univariate analysis were ASA class (X2 = 23.3; P = .0001), functional status (X2 = 9.15; P = .01), 10% weight loss over the last 6 months (X2 = 5.83; P = .02), wound class (X2 = 10.8; P = .01), mechanical bowel preparation (X2 = 5.89; P = .01), lack of oral antibiotic preparation (X2 = 17.5; P less than .0001), open vs. laparoscopic/minimally invasive surgery (X2 = 60.0; P less than .0001), chemotherapy in the last 90 days (X2 = 23.1; P less than .0001), and presence of disseminated cancer (X2 = 7.41; P = .01), Dr. Wu said.
With all of these factors taken into account in multivariate analysis, independent predictors of an increased risk of anastomotic leak were male sex (odds ratio, 1.74; P = .01), tobacco use (OR, 1.73; P = .03), and lack of a preoperative oral antibiotic bowel preparation (OR, 1.79; P less than .01).
Interestingly, use of a laparoscopic technique was protective against the development of anastomotic leakage (OR, 0.54; P less than .01), she said.
The authors reported having no relevant financial disclosures.

CHICAGO – Several modifiable risk factors predicted the development of anastomotic leak following elective colon resection, a large national analysis found.
“Preoperative smoking cessation, preoperative administration of oral antibiotic bowel preparation, and laparoscopic approach are modifiable factors that could reduce the risk of anastomotic leak,” Dr. Cindy Wu said at the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) National Conference.
Anastomotic leakage results in increased morbidity and mortality, yet the current literature analyzing risk factors for this complication is generally limited to retrospective studies of single institutions, she said.
To examine data from a larger sample of colectomy patients from multiple centers, the investigators used the NSQIP Participant Use Data File specifically targeted to colectomy to identify 14,848 patients who underwent elective colon resection from 2012 to 2013. Chi-square, Wald chi-square, and logistic regression analyses were performed examining patient factors (sex, race, comorbidities, smoking status, American Society of Anesthesiologists class, functional status, steroid use, and preoperative albumin), oncologic factors (chemotherapy, tumor stage, and presence or absence of disseminated cancer), and operative factors (wound class, mechanical bowel preparation, oral antibiotic preparation, surgical approach, colectomy site, surgical indication, and operative time).
In all, 3.4%, or 498 patients, experienced an anastomotic leak, which is consistent with the literature, Dr. Wu of Temple University in Philadelphia said. Of these patients, 101 required no intervention, while 272 required surgery and 125 needed percutaneous drainage. The mean age of the patients was 60.7 years and 57% were male.
In a univariate analysis, male sex (chi-square = 17.4; P less than .01), diabetes controlled with either oral medication or insulin (X2 = 9.5; P less than .01), and smoking within the last year (X2 = 20.4; P less than .01) were associated with a greater incidence of anastomotic leak.
Other risk factors that were significant in additional univariate analysis were ASA class (X2 = 23.3; P = .0001), functional status (X2 = 9.15; P = .01), 10% weight loss over the last 6 months (X2 = 5.83; P = .02), wound class (X2 = 10.8; P = .01), mechanical bowel preparation (X2 = 5.89; P = .01), lack of oral antibiotic preparation (X2 = 17.5; P less than .0001), open vs. laparoscopic/minimally invasive surgery (X2 = 60.0; P less than .0001), chemotherapy in the last 90 days (X2 = 23.1; P less than .0001), and presence of disseminated cancer (X2 = 7.41; P = .01), Dr. Wu said.
With all of these factors taken into account in multivariate analysis, independent predictors of an increased risk of anastomotic leak were male sex (odds ratio, 1.74; P = .01), tobacco use (OR, 1.73; P = .03), and lack of a preoperative oral antibiotic bowel preparation (OR, 1.79; P less than .01).
Interestingly, use of a laparoscopic technique was protective against the development of anastomotic leakage (OR, 0.54; P less than .01), she said.
The authors reported having no relevant financial disclosures.

AT THE ACS NSQIP NATIONAL CONFERENCE
Key clinical point: Altering specific patient and operative factors can modify the risk of anastomotic leakage after colectomy.
Major finding: Male sex (OR, 1.74), tobacco use (OR, 1.73), and lack of an oral antibiotic bowel preparation (OR, 1.79) predicted anastomotic leak.
Data source: A retrospective study of 14,848 elective colectomies.
Disclosures: The authors reported having no relevant financial disclosures.