User login
Onchocerciasis
The larvae of Onchocerca volvulus, a nematode that is most commonly found in tropical Africa, Yemen, Central America, and South America, are transmitted by flies of the genus Simulium that breed near fast-flowing rivers.1 The flies bite the host and transmit the larvae, and the larvae then mature into adults within the skin and subcutis, forming nodules that typically are not painful. The worms may reside within the skin for years and produce microfilariae, which can migrate and cause visual impairment, blindness, or a pruritic papular rash.1
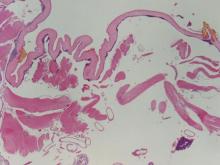
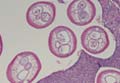
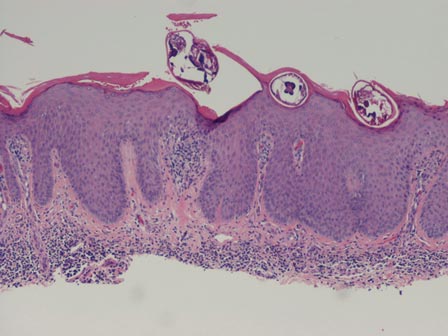
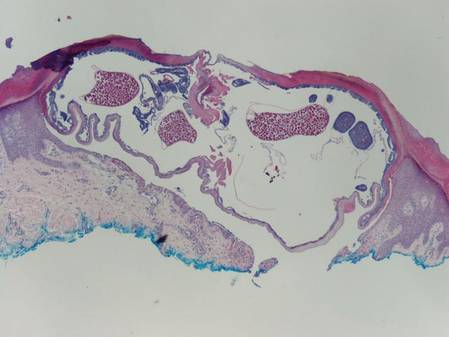
The nematode produces a nodule within the dermis or subcutis with surrounding fibrous tissue and a mixed inflammatory infiltrate with eosinophils (Figure 1). In some cases, microfilariae can be seen within the lymphatics or within the uteri of the worms.1 Male and female worms typically are present and have a corrugated cuticle with a thin underlying layer of striated muscle. The females have paired uteri, which usually contain microfilariae2 (Figure 2).
| 
|
Dirofilaria repens also is a nematode that produces a subcutaneous nodule with an inflammatory reaction. This worm typically has a thick cuticle with longitudinal ridges, long thick muscle, and lateral cords.3 Additionally, because humans are not the usual host, Dirofilaria species do not complete their lifecycle and typically are not gravid, unlike Onchocerca species.
Myiasis is the presence of fly larvae within the skin. The larvae demonstrate a thick hyaline cuticle with pigmented brown-yellow spikes (Figure 3). There is a thick muscular layer under the cuticle and a tubular tracheal system containing vertical striations. The digestive system has an epithelial lining with prominent vessels. Adipose tissue with granulated cytoplasm, prominent nuclei, and coarse chromatin also are present.4
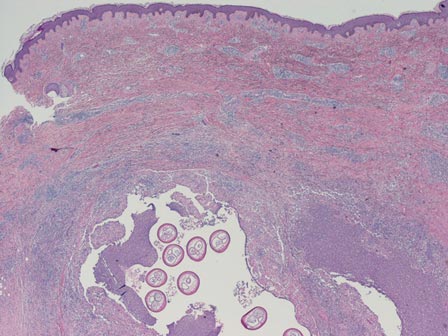
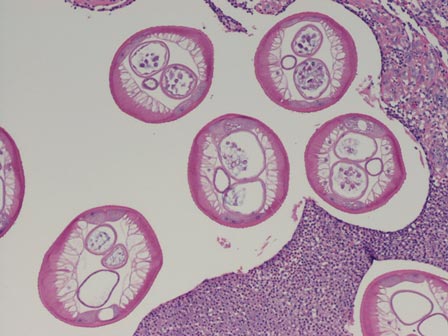
Scabies mites (Figure 4), ova, and scybala are present within the stratum corneum. A mixed inflammatory infiltrate also can be present.1 Tungiasis is caused by burrowing fleas and typically occurs on acral skin; therefore, it is more frequently found in the superficial portion of the skin. Erythrocytes usually are present in the gastrointestinal tract, and the females usually are gravid.2 A surrounding mixed inflammatory infiltrate is present, and necrosis also can occur (Figure 5).1
| 
|
1. Weedon D. Weedon’s Skin Pathology. 3rd ed. Edinburgh, Scotland: Churchill Livingstone Elsevier; 2010.
2. Elston DM, Ferringer T. Dermatopathology: Requisites in Dermatology. Edinburgh, Scotland: Saunders Elsevier; 2008.
3. Tzanetou K, Gasteratos S, Pantazopoulou A, et al. Subcutaneous dirofilariasis caused by Dirofilaria repens in Greece: a case report. J Cutan Pathol. 2009;36:892-895.
4. Fernandez-Flores A, Saeb-Lima M. Pulse granuloma of the lip: morphologic clues in its differential diagnosis. J Cutan Pathol. 2014;41:394-399.
The larvae of Onchocerca volvulus, a nematode that is most commonly found in tropical Africa, Yemen, Central America, and South America, are transmitted by flies of the genus Simulium that breed near fast-flowing rivers.1 The flies bite the host and transmit the larvae, and the larvae then mature into adults within the skin and subcutis, forming nodules that typically are not painful. The worms may reside within the skin for years and produce microfilariae, which can migrate and cause visual impairment, blindness, or a pruritic papular rash.1
The nematode produces a nodule within the dermis or subcutis with surrounding fibrous tissue and a mixed inflammatory infiltrate with eosinophils (Figure 1). In some cases, microfilariae can be seen within the lymphatics or within the uteri of the worms.1 Male and female worms typically are present and have a corrugated cuticle with a thin underlying layer of striated muscle. The females have paired uteri, which usually contain microfilariae2 (Figure 2).

| 
|
Dirofilaria repens also is a nematode that produces a subcutaneous nodule with an inflammatory reaction. This worm typically has a thick cuticle with longitudinal ridges, long thick muscle, and lateral cords.3 Additionally, because humans are not the usual host, Dirofilaria species do not complete their lifecycle and typically are not gravid, unlike Onchocerca species.
Myiasis is the presence of fly larvae within the skin. The larvae demonstrate a thick hyaline cuticle with pigmented brown-yellow spikes (Figure 3). There is a thick muscular layer under the cuticle and a tubular tracheal system containing vertical striations. The digestive system has an epithelial lining with prominent vessels. Adipose tissue with granulated cytoplasm, prominent nuclei, and coarse chromatin also are present.4
Scabies mites (Figure 4), ova, and scybala are present within the stratum corneum. A mixed inflammatory infiltrate also can be present.1 Tungiasis is caused by burrowing fleas and typically occurs on acral skin; therefore, it is more frequently found in the superficial portion of the skin. Erythrocytes usually are present in the gastrointestinal tract, and the females usually are gravid.2 A surrounding mixed inflammatory infiltrate is present, and necrosis also can occur (Figure 5).1

| 
|
The larvae of Onchocerca volvulus, a nematode that is most commonly found in tropical Africa, Yemen, Central America, and South America, are transmitted by flies of the genus Simulium that breed near fast-flowing rivers.1 The flies bite the host and transmit the larvae, and the larvae then mature into adults within the skin and subcutis, forming nodules that typically are not painful. The worms may reside within the skin for years and produce microfilariae, which can migrate and cause visual impairment, blindness, or a pruritic papular rash.1
The nematode produces a nodule within the dermis or subcutis with surrounding fibrous tissue and a mixed inflammatory infiltrate with eosinophils (Figure 1). In some cases, microfilariae can be seen within the lymphatics or within the uteri of the worms.1 Male and female worms typically are present and have a corrugated cuticle with a thin underlying layer of striated muscle. The females have paired uteri, which usually contain microfilariae2 (Figure 2).

| 
|
Dirofilaria repens also is a nematode that produces a subcutaneous nodule with an inflammatory reaction. This worm typically has a thick cuticle with longitudinal ridges, long thick muscle, and lateral cords.3 Additionally, because humans are not the usual host, Dirofilaria species do not complete their lifecycle and typically are not gravid, unlike Onchocerca species.
Myiasis is the presence of fly larvae within the skin. The larvae demonstrate a thick hyaline cuticle with pigmented brown-yellow spikes (Figure 3). There is a thick muscular layer under the cuticle and a tubular tracheal system containing vertical striations. The digestive system has an epithelial lining with prominent vessels. Adipose tissue with granulated cytoplasm, prominent nuclei, and coarse chromatin also are present.4
Scabies mites (Figure 4), ova, and scybala are present within the stratum corneum. A mixed inflammatory infiltrate also can be present.1 Tungiasis is caused by burrowing fleas and typically occurs on acral skin; therefore, it is more frequently found in the superficial portion of the skin. Erythrocytes usually are present in the gastrointestinal tract, and the females usually are gravid.2 A surrounding mixed inflammatory infiltrate is present, and necrosis also can occur (Figure 5).1

| 
|
1. Weedon D. Weedon’s Skin Pathology. 3rd ed. Edinburgh, Scotland: Churchill Livingstone Elsevier; 2010.
2. Elston DM, Ferringer T. Dermatopathology: Requisites in Dermatology. Edinburgh, Scotland: Saunders Elsevier; 2008.
3. Tzanetou K, Gasteratos S, Pantazopoulou A, et al. Subcutaneous dirofilariasis caused by Dirofilaria repens in Greece: a case report. J Cutan Pathol. 2009;36:892-895.
4. Fernandez-Flores A, Saeb-Lima M. Pulse granuloma of the lip: morphologic clues in its differential diagnosis. J Cutan Pathol. 2014;41:394-399.
1. Weedon D. Weedon’s Skin Pathology. 3rd ed. Edinburgh, Scotland: Churchill Livingstone Elsevier; 2010.
2. Elston DM, Ferringer T. Dermatopathology: Requisites in Dermatology. Edinburgh, Scotland: Saunders Elsevier; 2008.
3. Tzanetou K, Gasteratos S, Pantazopoulou A, et al. Subcutaneous dirofilariasis caused by Dirofilaria repens in Greece: a case report. J Cutan Pathol. 2009;36:892-895.
4. Fernandez-Flores A, Saeb-Lima M. Pulse granuloma of the lip: morphologic clues in its differential diagnosis. J Cutan Pathol. 2014;41:394-399.
Latest valvular disease guidelines bring big changes
SNOWMASS, COLO. – The 2014 American Heart Association/American College of Cardiology guidelines for the management of valvular heart disease break new ground in numerous ways, Dr. Rick A. Nishimura said at the Annual Cardiovascular Conference at Snowmass.
“We needed to do things differently. These guidelines were created in a different format from prior valvular heart disease guidelines. We wanted these guidelines to promote access to concise, relevant bytes of information at the point of care,” explained Dr. Nishimura, professor of medicine at the Mayo Clinic in Rochester, Minn., and cochair of the guidelines writing committee.
These guidelines – the first major revision in 8 years – introduce a new taxonomy and the first staging system for valvular heart disease. The guidelines also lower the threshold for intervention in asymptomatic patients, recommending surgical or catheter-based treatment at an earlier point in the disease process than ever before. And the guidelines introduce the concept of heart valve centers of excellence, offering a strong recommendation that patients be referred to those centers for procedures to be performed in the asymptomatic phase of disease (J. Am. Coll. Cardiol. 2014;63:2438-88).
These valvular heart disease guidelines place greater emphasis than before on the quality of the scientific evidence underlying recommendations. Since valvular heart disease is a field with a paucity of randomized trials, that meant cutting back.
“Our goal was, if there’s little evidence, don’t write a recommendation. So the number of recommendations went down, but at least the ones that were made were based on evidence,” the cardiologist noted.
Indeed, in the 2006 guidelines, more than 70% of the recommendations were Level of Evidence C and based solely upon expert opinion; in the new guidelines, that’s true for less than 50%. And the proportion of recommendations that are Level of Evidence B increased from 30% to 45%.
The 2014 update was prompted by huge changes in the field of valvular heart disease since 2006. For example, better data became available on the natural history of valvular heart disease. The old concept was not to operate on the asymptomatic patient with severe aortic stenosis and normal left ventricular function, but more recent natural history studies have shown that, left untreated, 72% of such patients will die or develop symptoms within 5 years.
So there has been a push to intervene earlier. Fortunately, that became doable, as recent years also brought improved noninvasive imaging, new catheter-based interventions, and refined surgical methods, enabling operators to safely lower the threshold for intervention in asymptomatic patients while at the same time extending procedural therapies to older, sicker populations.
Dr. Nishimura predicted that cardiologists and surgeons will find the new staging system clinically useful. The four stages, A-D, define the categories “at risk,” “progressive,” “asymptomatic severe,” and “symptomatic severe,” respectively. These categories are particularly helpful in determining how often to schedule patient follow-up and when to time intervention.
The guidelines recommend observation for patients who are Stage A or B and intervention when reasonable in patients who are Stage C2 or D. What bumps a patient with hemodynamically severe yet asymptomatic mitral regurgitation from Stage C1 to C2 is an left ventricular ejection fraction below 60% or a left ventricular end systolic dimension of 40 mm or more. In the setting of asymptomatic aortic stenosis, it’s a peak aortic valve velocity of 4.0 m/sec on Doppler echocardiography plus an LVEF of less than 50%.
The latest guidelines introduced the concept of heart valve centers of excellence in response to evidence of large variability across the country in terms of experience with valve operations. For example, the majority of centers perform fewer than 40 mitral valve repairs per year, and surgeons who perform mitral operations do a median of just five per year. The guideline committee, which included general and interventional cardiologists, surgeons, anesthesiologists, and imaging experts, was persuaded that those numbers are not sufficient to achieve optimal results in complex valve operations for asymptomatic patients.
The criteria for qualifying as a heart valve center of excellence, as defined in the guidelines, include having a multidisciplinary heart valve team, high patient volume, high-level surgical experience and expertise in complex valve procedures, and active participation in multicenter data registries and continuous quality improvement processes.
“The most important thing is you have to be very transparent with your data,” according to the cardiologist.
Ultimately, the most far-reaching change introduced in the current valvular heart disease guidelines is the switch from textbook format to what Dr. Nishimura calls structured data knowledge management.
“The AHA/ACC clinical practice guidelines are generally recognized as the flagship of U.S. cardiovascular medicine, but they’re like a library of old books. Clinically valuable knowledge is buried within documents that can be 200 pages long. What we need at the point of care is the gist: concise, relevant bytes of information that answer a specific clinical question, synthesized by experts,” Dr. Nishimura said.
The new approach is designed to counter the information overload that plagues contemporary medical practice. Each recommendation in the current valvular heart disease guidelines addresses a specific clinical question via a brief summary statement followed by a short explanatory paragraph, with accompanying references for those who seek additional details. This new format is designed to lead AHA/ACC clinical practice guidelines into the electronic information management future.
“In the future, you’ll go to your iPad or iPhone or whatever, type in search terms such as ‘anticoagulation for mechanical valves during pregnancy,’ and it will take you straight to the relevant knowledge byte. You can then click on ‘more’ and find out more and get to the supporting evidence tables. The knowledge chunks will be stored in a centralized knowledge management system. The nice thing about this is that it will be a living document that can easily be updated, instead of having to wait 8 years for a new version,” Dr. Nishimura explained.
He reported having no financial conflicts of interest.
SNOWMASS, COLO. – The 2014 American Heart Association/American College of Cardiology guidelines for the management of valvular heart disease break new ground in numerous ways, Dr. Rick A. Nishimura said at the Annual Cardiovascular Conference at Snowmass.
“We needed to do things differently. These guidelines were created in a different format from prior valvular heart disease guidelines. We wanted these guidelines to promote access to concise, relevant bytes of information at the point of care,” explained Dr. Nishimura, professor of medicine at the Mayo Clinic in Rochester, Minn., and cochair of the guidelines writing committee.
These guidelines – the first major revision in 8 years – introduce a new taxonomy and the first staging system for valvular heart disease. The guidelines also lower the threshold for intervention in asymptomatic patients, recommending surgical or catheter-based treatment at an earlier point in the disease process than ever before. And the guidelines introduce the concept of heart valve centers of excellence, offering a strong recommendation that patients be referred to those centers for procedures to be performed in the asymptomatic phase of disease (J. Am. Coll. Cardiol. 2014;63:2438-88).
These valvular heart disease guidelines place greater emphasis than before on the quality of the scientific evidence underlying recommendations. Since valvular heart disease is a field with a paucity of randomized trials, that meant cutting back.
“Our goal was, if there’s little evidence, don’t write a recommendation. So the number of recommendations went down, but at least the ones that were made were based on evidence,” the cardiologist noted.
Indeed, in the 2006 guidelines, more than 70% of the recommendations were Level of Evidence C and based solely upon expert opinion; in the new guidelines, that’s true for less than 50%. And the proportion of recommendations that are Level of Evidence B increased from 30% to 45%.
The 2014 update was prompted by huge changes in the field of valvular heart disease since 2006. For example, better data became available on the natural history of valvular heart disease. The old concept was not to operate on the asymptomatic patient with severe aortic stenosis and normal left ventricular function, but more recent natural history studies have shown that, left untreated, 72% of such patients will die or develop symptoms within 5 years.
So there has been a push to intervene earlier. Fortunately, that became doable, as recent years also brought improved noninvasive imaging, new catheter-based interventions, and refined surgical methods, enabling operators to safely lower the threshold for intervention in asymptomatic patients while at the same time extending procedural therapies to older, sicker populations.
Dr. Nishimura predicted that cardiologists and surgeons will find the new staging system clinically useful. The four stages, A-D, define the categories “at risk,” “progressive,” “asymptomatic severe,” and “symptomatic severe,” respectively. These categories are particularly helpful in determining how often to schedule patient follow-up and when to time intervention.
The guidelines recommend observation for patients who are Stage A or B and intervention when reasonable in patients who are Stage C2 or D. What bumps a patient with hemodynamically severe yet asymptomatic mitral regurgitation from Stage C1 to C2 is an left ventricular ejection fraction below 60% or a left ventricular end systolic dimension of 40 mm or more. In the setting of asymptomatic aortic stenosis, it’s a peak aortic valve velocity of 4.0 m/sec on Doppler echocardiography plus an LVEF of less than 50%.
The latest guidelines introduced the concept of heart valve centers of excellence in response to evidence of large variability across the country in terms of experience with valve operations. For example, the majority of centers perform fewer than 40 mitral valve repairs per year, and surgeons who perform mitral operations do a median of just five per year. The guideline committee, which included general and interventional cardiologists, surgeons, anesthesiologists, and imaging experts, was persuaded that those numbers are not sufficient to achieve optimal results in complex valve operations for asymptomatic patients.
The criteria for qualifying as a heart valve center of excellence, as defined in the guidelines, include having a multidisciplinary heart valve team, high patient volume, high-level surgical experience and expertise in complex valve procedures, and active participation in multicenter data registries and continuous quality improvement processes.
“The most important thing is you have to be very transparent with your data,” according to the cardiologist.
Ultimately, the most far-reaching change introduced in the current valvular heart disease guidelines is the switch from textbook format to what Dr. Nishimura calls structured data knowledge management.
“The AHA/ACC clinical practice guidelines are generally recognized as the flagship of U.S. cardiovascular medicine, but they’re like a library of old books. Clinically valuable knowledge is buried within documents that can be 200 pages long. What we need at the point of care is the gist: concise, relevant bytes of information that answer a specific clinical question, synthesized by experts,” Dr. Nishimura said.
The new approach is designed to counter the information overload that plagues contemporary medical practice. Each recommendation in the current valvular heart disease guidelines addresses a specific clinical question via a brief summary statement followed by a short explanatory paragraph, with accompanying references for those who seek additional details. This new format is designed to lead AHA/ACC clinical practice guidelines into the electronic information management future.
“In the future, you’ll go to your iPad or iPhone or whatever, type in search terms such as ‘anticoagulation for mechanical valves during pregnancy,’ and it will take you straight to the relevant knowledge byte. You can then click on ‘more’ and find out more and get to the supporting evidence tables. The knowledge chunks will be stored in a centralized knowledge management system. The nice thing about this is that it will be a living document that can easily be updated, instead of having to wait 8 years for a new version,” Dr. Nishimura explained.
He reported having no financial conflicts of interest.
SNOWMASS, COLO. – The 2014 American Heart Association/American College of Cardiology guidelines for the management of valvular heart disease break new ground in numerous ways, Dr. Rick A. Nishimura said at the Annual Cardiovascular Conference at Snowmass.
“We needed to do things differently. These guidelines were created in a different format from prior valvular heart disease guidelines. We wanted these guidelines to promote access to concise, relevant bytes of information at the point of care,” explained Dr. Nishimura, professor of medicine at the Mayo Clinic in Rochester, Minn., and cochair of the guidelines writing committee.
These guidelines – the first major revision in 8 years – introduce a new taxonomy and the first staging system for valvular heart disease. The guidelines also lower the threshold for intervention in asymptomatic patients, recommending surgical or catheter-based treatment at an earlier point in the disease process than ever before. And the guidelines introduce the concept of heart valve centers of excellence, offering a strong recommendation that patients be referred to those centers for procedures to be performed in the asymptomatic phase of disease (J. Am. Coll. Cardiol. 2014;63:2438-88).
These valvular heart disease guidelines place greater emphasis than before on the quality of the scientific evidence underlying recommendations. Since valvular heart disease is a field with a paucity of randomized trials, that meant cutting back.
“Our goal was, if there’s little evidence, don’t write a recommendation. So the number of recommendations went down, but at least the ones that were made were based on evidence,” the cardiologist noted.
Indeed, in the 2006 guidelines, more than 70% of the recommendations were Level of Evidence C and based solely upon expert opinion; in the new guidelines, that’s true for less than 50%. And the proportion of recommendations that are Level of Evidence B increased from 30% to 45%.
The 2014 update was prompted by huge changes in the field of valvular heart disease since 2006. For example, better data became available on the natural history of valvular heart disease. The old concept was not to operate on the asymptomatic patient with severe aortic stenosis and normal left ventricular function, but more recent natural history studies have shown that, left untreated, 72% of such patients will die or develop symptoms within 5 years.
So there has been a push to intervene earlier. Fortunately, that became doable, as recent years also brought improved noninvasive imaging, new catheter-based interventions, and refined surgical methods, enabling operators to safely lower the threshold for intervention in asymptomatic patients while at the same time extending procedural therapies to older, sicker populations.
Dr. Nishimura predicted that cardiologists and surgeons will find the new staging system clinically useful. The four stages, A-D, define the categories “at risk,” “progressive,” “asymptomatic severe,” and “symptomatic severe,” respectively. These categories are particularly helpful in determining how often to schedule patient follow-up and when to time intervention.
The guidelines recommend observation for patients who are Stage A or B and intervention when reasonable in patients who are Stage C2 or D. What bumps a patient with hemodynamically severe yet asymptomatic mitral regurgitation from Stage C1 to C2 is an left ventricular ejection fraction below 60% or a left ventricular end systolic dimension of 40 mm or more. In the setting of asymptomatic aortic stenosis, it’s a peak aortic valve velocity of 4.0 m/sec on Doppler echocardiography plus an LVEF of less than 50%.
The latest guidelines introduced the concept of heart valve centers of excellence in response to evidence of large variability across the country in terms of experience with valve operations. For example, the majority of centers perform fewer than 40 mitral valve repairs per year, and surgeons who perform mitral operations do a median of just five per year. The guideline committee, which included general and interventional cardiologists, surgeons, anesthesiologists, and imaging experts, was persuaded that those numbers are not sufficient to achieve optimal results in complex valve operations for asymptomatic patients.
The criteria for qualifying as a heart valve center of excellence, as defined in the guidelines, include having a multidisciplinary heart valve team, high patient volume, high-level surgical experience and expertise in complex valve procedures, and active participation in multicenter data registries and continuous quality improvement processes.
“The most important thing is you have to be very transparent with your data,” according to the cardiologist.
Ultimately, the most far-reaching change introduced in the current valvular heart disease guidelines is the switch from textbook format to what Dr. Nishimura calls structured data knowledge management.
“The AHA/ACC clinical practice guidelines are generally recognized as the flagship of U.S. cardiovascular medicine, but they’re like a library of old books. Clinically valuable knowledge is buried within documents that can be 200 pages long. What we need at the point of care is the gist: concise, relevant bytes of information that answer a specific clinical question, synthesized by experts,” Dr. Nishimura said.
The new approach is designed to counter the information overload that plagues contemporary medical practice. Each recommendation in the current valvular heart disease guidelines addresses a specific clinical question via a brief summary statement followed by a short explanatory paragraph, with accompanying references for those who seek additional details. This new format is designed to lead AHA/ACC clinical practice guidelines into the electronic information management future.
“In the future, you’ll go to your iPad or iPhone or whatever, type in search terms such as ‘anticoagulation for mechanical valves during pregnancy,’ and it will take you straight to the relevant knowledge byte. You can then click on ‘more’ and find out more and get to the supporting evidence tables. The knowledge chunks will be stored in a centralized knowledge management system. The nice thing about this is that it will be a living document that can easily be updated, instead of having to wait 8 years for a new version,” Dr. Nishimura explained.
He reported having no financial conflicts of interest.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
26% 1-year death, stroke rate after TAVR
One year after transcatheter aortic valve replacement in the United States, the overall mortality was 23.7%, the stroke rate was 4.1%, and the composite outcome of death and stroke was 26.0%, according to a report published online March 10 in JAMA.
Long-term outcomes for TAVR haven’t been well studied until now, yet the procedure is being performed with increasing frequency for aortic stenosis in patients who are too high risk to undergo conventional surgical aortic valve replacement, said Dr. David R. Holmes Jr. of the Mayo Clinic, Rochester, Minn., and his associates.
They assessed 1-year outcomes by analyzing administrative data from the Centers for Medicare & Medicaid Services and clinical data from the Transcatheter Valve Therapies Registry, an initiative of the Society of Thoracic Surgeons and the American College of Cardiology. The study involved 12,182 patients who underwent TAVR at 299 medical centers across the country during a 19-month period. The patients’ median age was 84 years; 95% were white and 52% were women. The transfemoral approach was used in most patients, but alternative approaches were used in roughly 44%. As expected for an elderly, high-risk study population, baseline functional status was poor and comorbidities were common. They included reduced left ventricular ejection fraction (26% of patients), prior stroke (12%), moderate or severe lung disease (28%), renal failure (16%), peripheral vascular disease (32%), and atrial fibrillation (42%), Dr. Holmes and his associates reported (JAMA 2015 March 10 [doi:10.1001/jama.2015.1474]).
In addition to the mortality and stroke rates listed above, the 1-year rate of one rehospitalization was 24.4%, that of two rehospitalizations was 12.5%, and that of three or more rehospitalizations was 11.6%. The 1-year readmission rate specifically for stroke, heart failure, or repeat aortic valve intervention was 18.6%. These are important considerations for elderly, fragile patients because rehospitalizations indicate “an unacceptable quality-of-life outcome” and are very costly, the investigators noted.
Several baseline characteristics, including male sex, severe chronic obstructive pulmonary disease, dialysis-dependent end-stage renal disease, older age, higher STS Predicted Risk of Operative Mortality (PROM) score, a history of atrial fibrillation/flutter, and use of an access route (other than transfemoral), were found to be independently associated with higher 1-year mortality. Thus, “It may be possible to identify patients who may not benefit from this procedure and who should be counseled accordingly.” For example, in this study there was a small (77 patients) very high-risk subset of patients – aged 85-94 years, dependent on dialysis, and having an STS PROM score greater than 15% – whose 1-year mortality was 53.5%.
The STS and the ACC supported this study, and support the Transcatheter Valve Therapies Registry. Dr. Holmes reported having no relevant financial disclosures; his associates reported ties to Boston Scientific, Edwards Lifesciences, Janssen, Eli Lilly, Boehringer Ingelheim, Bayer, and AstraZeneca.
One year after transcatheter aortic valve replacement in the United States, the overall mortality was 23.7%, the stroke rate was 4.1%, and the composite outcome of death and stroke was 26.0%, according to a report published online March 10 in JAMA.
Long-term outcomes for TAVR haven’t been well studied until now, yet the procedure is being performed with increasing frequency for aortic stenosis in patients who are too high risk to undergo conventional surgical aortic valve replacement, said Dr. David R. Holmes Jr. of the Mayo Clinic, Rochester, Minn., and his associates.
They assessed 1-year outcomes by analyzing administrative data from the Centers for Medicare & Medicaid Services and clinical data from the Transcatheter Valve Therapies Registry, an initiative of the Society of Thoracic Surgeons and the American College of Cardiology. The study involved 12,182 patients who underwent TAVR at 299 medical centers across the country during a 19-month period. The patients’ median age was 84 years; 95% were white and 52% were women. The transfemoral approach was used in most patients, but alternative approaches were used in roughly 44%. As expected for an elderly, high-risk study population, baseline functional status was poor and comorbidities were common. They included reduced left ventricular ejection fraction (26% of patients), prior stroke (12%), moderate or severe lung disease (28%), renal failure (16%), peripheral vascular disease (32%), and atrial fibrillation (42%), Dr. Holmes and his associates reported (JAMA 2015 March 10 [doi:10.1001/jama.2015.1474]).
In addition to the mortality and stroke rates listed above, the 1-year rate of one rehospitalization was 24.4%, that of two rehospitalizations was 12.5%, and that of three or more rehospitalizations was 11.6%. The 1-year readmission rate specifically for stroke, heart failure, or repeat aortic valve intervention was 18.6%. These are important considerations for elderly, fragile patients because rehospitalizations indicate “an unacceptable quality-of-life outcome” and are very costly, the investigators noted.
Several baseline characteristics, including male sex, severe chronic obstructive pulmonary disease, dialysis-dependent end-stage renal disease, older age, higher STS Predicted Risk of Operative Mortality (PROM) score, a history of atrial fibrillation/flutter, and use of an access route (other than transfemoral), were found to be independently associated with higher 1-year mortality. Thus, “It may be possible to identify patients who may not benefit from this procedure and who should be counseled accordingly.” For example, in this study there was a small (77 patients) very high-risk subset of patients – aged 85-94 years, dependent on dialysis, and having an STS PROM score greater than 15% – whose 1-year mortality was 53.5%.
The STS and the ACC supported this study, and support the Transcatheter Valve Therapies Registry. Dr. Holmes reported having no relevant financial disclosures; his associates reported ties to Boston Scientific, Edwards Lifesciences, Janssen, Eli Lilly, Boehringer Ingelheim, Bayer, and AstraZeneca.
One year after transcatheter aortic valve replacement in the United States, the overall mortality was 23.7%, the stroke rate was 4.1%, and the composite outcome of death and stroke was 26.0%, according to a report published online March 10 in JAMA.
Long-term outcomes for TAVR haven’t been well studied until now, yet the procedure is being performed with increasing frequency for aortic stenosis in patients who are too high risk to undergo conventional surgical aortic valve replacement, said Dr. David R. Holmes Jr. of the Mayo Clinic, Rochester, Minn., and his associates.
They assessed 1-year outcomes by analyzing administrative data from the Centers for Medicare & Medicaid Services and clinical data from the Transcatheter Valve Therapies Registry, an initiative of the Society of Thoracic Surgeons and the American College of Cardiology. The study involved 12,182 patients who underwent TAVR at 299 medical centers across the country during a 19-month period. The patients’ median age was 84 years; 95% were white and 52% were women. The transfemoral approach was used in most patients, but alternative approaches were used in roughly 44%. As expected for an elderly, high-risk study population, baseline functional status was poor and comorbidities were common. They included reduced left ventricular ejection fraction (26% of patients), prior stroke (12%), moderate or severe lung disease (28%), renal failure (16%), peripheral vascular disease (32%), and atrial fibrillation (42%), Dr. Holmes and his associates reported (JAMA 2015 March 10 [doi:10.1001/jama.2015.1474]).
In addition to the mortality and stroke rates listed above, the 1-year rate of one rehospitalization was 24.4%, that of two rehospitalizations was 12.5%, and that of three or more rehospitalizations was 11.6%. The 1-year readmission rate specifically for stroke, heart failure, or repeat aortic valve intervention was 18.6%. These are important considerations for elderly, fragile patients because rehospitalizations indicate “an unacceptable quality-of-life outcome” and are very costly, the investigators noted.
Several baseline characteristics, including male sex, severe chronic obstructive pulmonary disease, dialysis-dependent end-stage renal disease, older age, higher STS Predicted Risk of Operative Mortality (PROM) score, a history of atrial fibrillation/flutter, and use of an access route (other than transfemoral), were found to be independently associated with higher 1-year mortality. Thus, “It may be possible to identify patients who may not benefit from this procedure and who should be counseled accordingly.” For example, in this study there was a small (77 patients) very high-risk subset of patients – aged 85-94 years, dependent on dialysis, and having an STS PROM score greater than 15% – whose 1-year mortality was 53.5%.
The STS and the ACC supported this study, and support the Transcatheter Valve Therapies Registry. Dr. Holmes reported having no relevant financial disclosures; his associates reported ties to Boston Scientific, Edwards Lifesciences, Janssen, Eli Lilly, Boehringer Ingelheim, Bayer, and AstraZeneca.
FROM JAMA
Key clinical point: Severe chronic obstructive pulmonary disease, end-stage renal disease, older age, higher STS PROM score, and use of an access route (other than transfemoral) are among the baseline characteristics linked with higher 1-year mortality after TAVR.
Major finding: The overall mortality was 23.7%, the stroke rate was 4.1%, and the composite rate of death and stroke was 26% 1 year after TAVR.
Data source: An analysis of data from the Transcatheter Valve Therapies Registry for 12,182 patients who underwent TAVR procedures at 299 U.S. hospitals during a 19-month period.
Disclosures: The Society of Thoracic Surgeons and the American College of Cardiology supported this study, and support the Transcatheter Valve Therapies Registry. Dr. Holmes reported having no relevant financial disclosures; his associates reported ties to Boston Scientific, Edwards Lifesciences, Janssen, Eli Lilly, Boehringer Ingelheim, Bayer, and AstraZeneca.
Leadership 101: Basic skills for early-career hospitalists
Early career hospitalists may think leadership roles automatically yield more money and free time, Dr. Gundersen says. Actually, being a leader requires stamina and the ability to weather the ups and downs that come with the leadership role. For example, will you be able to handle situations in which your decisions make others unhappy?
Deliver on Promises
Honest self-assessment is one of the most critical elements in becoming a leader, agrees Steven Deitelzweig, MD, MMM, FACP, FSVMB, RVT, VPMA, system chairman of hospital medicine and medical director of regional business development for Ochsner Health System in the greater New Orleans area. In addition to having good interpersonal skills, showing enthusiasm, and promoting your organization sincerely—what Dr. Deitelzweig labels “emotional intelligence”—prospective leaders need to be cognizant of delivering on promises.
“This is something I call a high ‘say/do’ ratio,” he explains, “and, simply put, it means that you accomplish what you say you will. At the end of the day, the only way anybody moves up is by being good at achieving results.”
If you start missing deadlines, you communicate that you are not reliable. Not all project implementation goes according to plan, of course, so when you encounter difficulties, early communication about obstacles is also key, he says.
Nuts and Bolts
Through SHM’s Leadership Academy, hospitalists can be trained in team management and other key leadership skills. An October 2015 session is scheduled in Austin.
Having trusted mentors is crucial, agreed both physicians, so that you can keep polishing your skill set and obtain honest feedback. These mentors should not be people to whom you directly report, and they need not be in the healthcare industry.
In fact, Dr. Gundersen says he’s known mentors for years “who have not been in the same specialty or even the same field, but who give me guidance and have helped make me into the leader I am.”
How do you assert your desire to be a leader? Dr. Deitelzweig suggests making your aspirations clear to your own group leaders. The annual review is an excellent juncture at which to discuss this, he says.
If you do not yet have project management or communications experience, ask your leaders whether they are familiar with training to help you develop those skills. In the current healthcare environment, the Affordable Care Act and reimbursement regulations mean that change will continue to be part of the leadership challenge.
“If you really want to be a leader,” Dr. Deitelzweig says, “you cannot be a naysayer. Make change work for you, look at it as an opportunity for you to innovate, and then show how valuable you can be.”
Gretchen Henkel is a freelance writer in California.
Listen Now
Listen to Dr. Gundersen dive further into the importance of mentors and assessing your personal qualities to be a good leader.
[audio mp3="http://www.the-hospitalist.org/wp-content/uploads/2015/03/Gundersen_CareerCenter1_FINAL_012515.mp3"][/audio]
Early career hospitalists may think leadership roles automatically yield more money and free time, Dr. Gundersen says. Actually, being a leader requires stamina and the ability to weather the ups and downs that come with the leadership role. For example, will you be able to handle situations in which your decisions make others unhappy?
Deliver on Promises
Honest self-assessment is one of the most critical elements in becoming a leader, agrees Steven Deitelzweig, MD, MMM, FACP, FSVMB, RVT, VPMA, system chairman of hospital medicine and medical director of regional business development for Ochsner Health System in the greater New Orleans area. In addition to having good interpersonal skills, showing enthusiasm, and promoting your organization sincerely—what Dr. Deitelzweig labels “emotional intelligence”—prospective leaders need to be cognizant of delivering on promises.
“This is something I call a high ‘say/do’ ratio,” he explains, “and, simply put, it means that you accomplish what you say you will. At the end of the day, the only way anybody moves up is by being good at achieving results.”
If you start missing deadlines, you communicate that you are not reliable. Not all project implementation goes according to plan, of course, so when you encounter difficulties, early communication about obstacles is also key, he says.
Nuts and Bolts
Through SHM’s Leadership Academy, hospitalists can be trained in team management and other key leadership skills. An October 2015 session is scheduled in Austin.
Having trusted mentors is crucial, agreed both physicians, so that you can keep polishing your skill set and obtain honest feedback. These mentors should not be people to whom you directly report, and they need not be in the healthcare industry.
In fact, Dr. Gundersen says he’s known mentors for years “who have not been in the same specialty or even the same field, but who give me guidance and have helped make me into the leader I am.”
How do you assert your desire to be a leader? Dr. Deitelzweig suggests making your aspirations clear to your own group leaders. The annual review is an excellent juncture at which to discuss this, he says.
If you do not yet have project management or communications experience, ask your leaders whether they are familiar with training to help you develop those skills. In the current healthcare environment, the Affordable Care Act and reimbursement regulations mean that change will continue to be part of the leadership challenge.
“If you really want to be a leader,” Dr. Deitelzweig says, “you cannot be a naysayer. Make change work for you, look at it as an opportunity for you to innovate, and then show how valuable you can be.”
Gretchen Henkel is a freelance writer in California.
Listen Now
Listen to Dr. Gundersen dive further into the importance of mentors and assessing your personal qualities to be a good leader.
[audio mp3="http://www.the-hospitalist.org/wp-content/uploads/2015/03/Gundersen_CareerCenter1_FINAL_012515.mp3"][/audio]
Early career hospitalists may think leadership roles automatically yield more money and free time, Dr. Gundersen says. Actually, being a leader requires stamina and the ability to weather the ups and downs that come with the leadership role. For example, will you be able to handle situations in which your decisions make others unhappy?
Deliver on Promises
Honest self-assessment is one of the most critical elements in becoming a leader, agrees Steven Deitelzweig, MD, MMM, FACP, FSVMB, RVT, VPMA, system chairman of hospital medicine and medical director of regional business development for Ochsner Health System in the greater New Orleans area. In addition to having good interpersonal skills, showing enthusiasm, and promoting your organization sincerely—what Dr. Deitelzweig labels “emotional intelligence”—prospective leaders need to be cognizant of delivering on promises.
“This is something I call a high ‘say/do’ ratio,” he explains, “and, simply put, it means that you accomplish what you say you will. At the end of the day, the only way anybody moves up is by being good at achieving results.”
If you start missing deadlines, you communicate that you are not reliable. Not all project implementation goes according to plan, of course, so when you encounter difficulties, early communication about obstacles is also key, he says.
Nuts and Bolts
Through SHM’s Leadership Academy, hospitalists can be trained in team management and other key leadership skills. An October 2015 session is scheduled in Austin.
Having trusted mentors is crucial, agreed both physicians, so that you can keep polishing your skill set and obtain honest feedback. These mentors should not be people to whom you directly report, and they need not be in the healthcare industry.
In fact, Dr. Gundersen says he’s known mentors for years “who have not been in the same specialty or even the same field, but who give me guidance and have helped make me into the leader I am.”
How do you assert your desire to be a leader? Dr. Deitelzweig suggests making your aspirations clear to your own group leaders. The annual review is an excellent juncture at which to discuss this, he says.
If you do not yet have project management or communications experience, ask your leaders whether they are familiar with training to help you develop those skills. In the current healthcare environment, the Affordable Care Act and reimbursement regulations mean that change will continue to be part of the leadership challenge.
“If you really want to be a leader,” Dr. Deitelzweig says, “you cannot be a naysayer. Make change work for you, look at it as an opportunity for you to innovate, and then show how valuable you can be.”
Gretchen Henkel is a freelance writer in California.
Listen Now
Listen to Dr. Gundersen dive further into the importance of mentors and assessing your personal qualities to be a good leader.
[audio mp3="http://www.the-hospitalist.org/wp-content/uploads/2015/03/Gundersen_CareerCenter1_FINAL_012515.mp3"][/audio]
Federal Program to Cut Hospital Readmissions Turns Out Modest Results
A new report has found that only a small number of groups included in a government-funded experiment to cut Medicare readmissions actually produced results. However, the less-than-hoped-for results don't necessarily indicate failure, a hospitalist and readmissions expert says.
The Community-based Care Transitions Program (CCTP) is one of several test care-delivery models created by the Affordable Care Act. Its main goal is to improve transitions of Medicare patients from the hospital to community-based settings, such as nursing homes, rehabilitation facilities, and government agencies that provide services to the elderly, and thereby reduce readmissions.
However, a new report [PDF] commissioned by the Centers for Medicare & Medicaid Services found that only four CCTP groups of the 48 studied significantly cut readmissions compared with those of a control group. The report was finished in May 2014 but wasn't made public until January.
"There are so few examples in healthcare where resource alignment makes sense with what we believe are the ideal ways of practicing," says Jeffrey L. Greenwald, MD, associate professor of medicine at Harvard Medical School and a member of the Inpatient Clinician Educator Service at Massachusetts General Hospital, both in Boston. "Things like CCTP, where you have an opportunity to partner a hospital with a community-based organization that can help to support patient transitions still looks promising despite its warts."
CCTP, funded with $300 million over five years, signed its first round of deals with community agencies in late 2011. The report covered partial 2012 results from groups participating in the early rounds.
Dr. Greenwald is one of the cofounders of SHM's Project BOOST, a yearlong QI program in which hospital teams are paired with mentors to help them improve care transitions. He explained that BOOST teams typically need at least 18-24 months to show positive results on length-of-stay or readmissions reductions.
"These are long processes that don't turn around overnight," Dr. Greenwald adds. "If they do, it's probably because [the hospital team] put in place something that is not sustainable, and the minute they stop measuring and keeping an eye on it, it will likely deteriorate.
"There's no magic bullet in care transitions."
Visit our website for more information on ways hospitals can reduce readmissions.
A new report has found that only a small number of groups included in a government-funded experiment to cut Medicare readmissions actually produced results. However, the less-than-hoped-for results don't necessarily indicate failure, a hospitalist and readmissions expert says.
The Community-based Care Transitions Program (CCTP) is one of several test care-delivery models created by the Affordable Care Act. Its main goal is to improve transitions of Medicare patients from the hospital to community-based settings, such as nursing homes, rehabilitation facilities, and government agencies that provide services to the elderly, and thereby reduce readmissions.
However, a new report [PDF] commissioned by the Centers for Medicare & Medicaid Services found that only four CCTP groups of the 48 studied significantly cut readmissions compared with those of a control group. The report was finished in May 2014 but wasn't made public until January.
"There are so few examples in healthcare where resource alignment makes sense with what we believe are the ideal ways of practicing," says Jeffrey L. Greenwald, MD, associate professor of medicine at Harvard Medical School and a member of the Inpatient Clinician Educator Service at Massachusetts General Hospital, both in Boston. "Things like CCTP, where you have an opportunity to partner a hospital with a community-based organization that can help to support patient transitions still looks promising despite its warts."
CCTP, funded with $300 million over five years, signed its first round of deals with community agencies in late 2011. The report covered partial 2012 results from groups participating in the early rounds.
Dr. Greenwald is one of the cofounders of SHM's Project BOOST, a yearlong QI program in which hospital teams are paired with mentors to help them improve care transitions. He explained that BOOST teams typically need at least 18-24 months to show positive results on length-of-stay or readmissions reductions.
"These are long processes that don't turn around overnight," Dr. Greenwald adds. "If they do, it's probably because [the hospital team] put in place something that is not sustainable, and the minute they stop measuring and keeping an eye on it, it will likely deteriorate.
"There's no magic bullet in care transitions."
Visit our website for more information on ways hospitals can reduce readmissions.
A new report has found that only a small number of groups included in a government-funded experiment to cut Medicare readmissions actually produced results. However, the less-than-hoped-for results don't necessarily indicate failure, a hospitalist and readmissions expert says.
The Community-based Care Transitions Program (CCTP) is one of several test care-delivery models created by the Affordable Care Act. Its main goal is to improve transitions of Medicare patients from the hospital to community-based settings, such as nursing homes, rehabilitation facilities, and government agencies that provide services to the elderly, and thereby reduce readmissions.
However, a new report [PDF] commissioned by the Centers for Medicare & Medicaid Services found that only four CCTP groups of the 48 studied significantly cut readmissions compared with those of a control group. The report was finished in May 2014 but wasn't made public until January.
"There are so few examples in healthcare where resource alignment makes sense with what we believe are the ideal ways of practicing," says Jeffrey L. Greenwald, MD, associate professor of medicine at Harvard Medical School and a member of the Inpatient Clinician Educator Service at Massachusetts General Hospital, both in Boston. "Things like CCTP, where you have an opportunity to partner a hospital with a community-based organization that can help to support patient transitions still looks promising despite its warts."
CCTP, funded with $300 million over five years, signed its first round of deals with community agencies in late 2011. The report covered partial 2012 results from groups participating in the early rounds.
Dr. Greenwald is one of the cofounders of SHM's Project BOOST, a yearlong QI program in which hospital teams are paired with mentors to help them improve care transitions. He explained that BOOST teams typically need at least 18-24 months to show positive results on length-of-stay or readmissions reductions.
"These are long processes that don't turn around overnight," Dr. Greenwald adds. "If they do, it's probably because [the hospital team] put in place something that is not sustainable, and the minute they stop measuring and keeping an eye on it, it will likely deteriorate.
"There's no magic bullet in care transitions."
Visit our website for more information on ways hospitals can reduce readmissions.
Hospitalists Gear Up for HM15
Hospital medicine will descend on Washington, D.C., again.
At least 2,500 attendees are expected at SHM's annual meeting, HM15, which kicks off March 29 at the Gaylord National Resort & Convention Center in National Harbor, Md. The four-day conference—SHM's third in the nation's capital in six years—ends April 1 and encompasses:
- Seven pre-courses on March 29 that can be applied toward continuing medical education credits;
- Dozens of educational sessions over three days, including the debut of its "Young Hospitalists" track on March 30;
- The largest Research, Innovations, and Clinical Vignettes poster competition ever; and
- Plenary sessions from patient-safety guru Peter Pronovost, MD, PhD, FCCM; HM pioneer Robert Wachter, MD, MHM; and Maureen Bisognano, president and CEO of the Institute for Healthcare Improvement.
"The opportunity to learn about faculty development, the opportunity to learn about administrative concerns in running a hospital medicine program, the opportunity to address quality improvement…the opportunity to meet other folks who are doing very similar work and learn from them, all of those things exist,” says assistant course director Melissa Mattison, MD, SFHM.
In addition, SHM's advocacy event, Hospitalists on the Hill Day, is scheduled for April 1 and will see physicians holding hundreds of meetings with Capitol Hill legislators and staffers.
"Every congressman has physicians in their community, and they value the opinion of those physicians," SHM Public Policy Committee Chair Ron Greeno, MD, MHM, says. "Nothing is more effective than having one of our members meet with a representative from their home district about the issues that we care about."
Visit our website for more information on HM15.
Hospital medicine will descend on Washington, D.C., again.
At least 2,500 attendees are expected at SHM's annual meeting, HM15, which kicks off March 29 at the Gaylord National Resort & Convention Center in National Harbor, Md. The four-day conference—SHM's third in the nation's capital in six years—ends April 1 and encompasses:
- Seven pre-courses on March 29 that can be applied toward continuing medical education credits;
- Dozens of educational sessions over three days, including the debut of its "Young Hospitalists" track on March 30;
- The largest Research, Innovations, and Clinical Vignettes poster competition ever; and
- Plenary sessions from patient-safety guru Peter Pronovost, MD, PhD, FCCM; HM pioneer Robert Wachter, MD, MHM; and Maureen Bisognano, president and CEO of the Institute for Healthcare Improvement.
"The opportunity to learn about faculty development, the opportunity to learn about administrative concerns in running a hospital medicine program, the opportunity to address quality improvement…the opportunity to meet other folks who are doing very similar work and learn from them, all of those things exist,” says assistant course director Melissa Mattison, MD, SFHM.
In addition, SHM's advocacy event, Hospitalists on the Hill Day, is scheduled for April 1 and will see physicians holding hundreds of meetings with Capitol Hill legislators and staffers.
"Every congressman has physicians in their community, and they value the opinion of those physicians," SHM Public Policy Committee Chair Ron Greeno, MD, MHM, says. "Nothing is more effective than having one of our members meet with a representative from their home district about the issues that we care about."
Visit our website for more information on HM15.
Hospital medicine will descend on Washington, D.C., again.
At least 2,500 attendees are expected at SHM's annual meeting, HM15, which kicks off March 29 at the Gaylord National Resort & Convention Center in National Harbor, Md. The four-day conference—SHM's third in the nation's capital in six years—ends April 1 and encompasses:
- Seven pre-courses on March 29 that can be applied toward continuing medical education credits;
- Dozens of educational sessions over three days, including the debut of its "Young Hospitalists" track on March 30;
- The largest Research, Innovations, and Clinical Vignettes poster competition ever; and
- Plenary sessions from patient-safety guru Peter Pronovost, MD, PhD, FCCM; HM pioneer Robert Wachter, MD, MHM; and Maureen Bisognano, president and CEO of the Institute for Healthcare Improvement.
"The opportunity to learn about faculty development, the opportunity to learn about administrative concerns in running a hospital medicine program, the opportunity to address quality improvement…the opportunity to meet other folks who are doing very similar work and learn from them, all of those things exist,” says assistant course director Melissa Mattison, MD, SFHM.
In addition, SHM's advocacy event, Hospitalists on the Hill Day, is scheduled for April 1 and will see physicians holding hundreds of meetings with Capitol Hill legislators and staffers.
"Every congressman has physicians in their community, and they value the opinion of those physicians," SHM Public Policy Committee Chair Ron Greeno, MD, MHM, says. "Nothing is more effective than having one of our members meet with a representative from their home district about the issues that we care about."
Visit our website for more information on HM15.
Ranolazine plus beta-blockers might prevent postop AF
PHOENIX – Twice-daily ranolazine following adult cardiac surgery seemed to protect against atrial fibrillation, based on a retrospective cohort study at the University of Florida Jacksonville Medical Center.
Ranolazine (Ranexa) was dosed orally at 1,000 mg the morning of surgery, and then resumed after extubation, generally the night of surgery. The goal was 1,000 mg orally twice a day, for a maximum of 7 hospital days; patients usually went home before then, so they received an average of nine doses. The drug was discontinued at discharge.
Six (10.5%) of 57 patients in the ranolazine group developed postoperative atrial fibrillation (POAF) versus 26 (45.6%) of 57 matched controls (P < .0001). The first case came at postop day 3 in the ranolazine group, but within 24 hours in the control group. One person in the ranolazine group and one in the control group had a history of AF.
There was no statistical difference in ICU length of stay, 30-day readmission for cardiac causes, or 30-day cardiovascular mortality; the one cardiovascular death was in the control group.
Two-thirds of the patients had coronary artery bypass grafts, and the rest had either valve surgery or a combination of both surgeries. Patients were 60 years old on average, and two-thirds were men, Drayton Hammond, Pharm.D., said at the Critical Care Congress, sponsored by the Society for Critical Care Medicine.
Ranolazine is indicated for chronic angina, not POAF prevention, but some previous investigations have suggested a possible benefit. A randomized, controlled clinical trial is currently looking into the matter.
At least retrospectively, the drug was “beneficial, definitely. There is about a 35% absolute-risk reduction,” said Dr. Hammond, who conducted the study while at the Jacksonville hospital.
Doctors there continue to use ranolazine for postop AF prophylaxis, as they see fit, said Dr. Hammond, now an assistant professor of pharmacy practice at the University of Arkansas for Medical Sciences, in Little Rock.
Gilead, the maker of the Ranexa, is also working on a ranolazine-dronedarone combination for paroxysmal AF.
More than half of the ranolazine patients in the study developed symptomatic hypotension within 72 hours of surgery, versus about a third in the control group (P = .0004). The drug was discontinued in one ranolazine patient because of hypotension. The problem resolved after 72 hours.
“We don’t have a good explanation” for the side effect. Perhaps there were differences in myocardial stunning or vasopressor use between the groups, but “we had the same three surgeons” for all the cases, Dr. Hammond said.
Ranolazine labeling notes the risk of hypotension and orthostatic hypotension. Labeling also warns of QT interval prolongation and renal failure in susceptible patients. The investigators found no between-group differences in bradycardia, new renal failure, or neurological events.
Overall, 53 (93%) patients in the ranolazine group were on postoperative beta-blockers, and 54 (94.7%) on postop statins; 48 (84.2%) in the control group were on beta-blockers postop and 47 (82.5%) on statins. Beta-blockers are first-line treatment to prevent postop AF; patients on any other antiarrhythmic were excluded from the trial, as were those who died during surgery.
PHOENIX – Twice-daily ranolazine following adult cardiac surgery seemed to protect against atrial fibrillation, based on a retrospective cohort study at the University of Florida Jacksonville Medical Center.
Ranolazine (Ranexa) was dosed orally at 1,000 mg the morning of surgery, and then resumed after extubation, generally the night of surgery. The goal was 1,000 mg orally twice a day, for a maximum of 7 hospital days; patients usually went home before then, so they received an average of nine doses. The drug was discontinued at discharge.
Six (10.5%) of 57 patients in the ranolazine group developed postoperative atrial fibrillation (POAF) versus 26 (45.6%) of 57 matched controls (P < .0001). The first case came at postop day 3 in the ranolazine group, but within 24 hours in the control group. One person in the ranolazine group and one in the control group had a history of AF.
There was no statistical difference in ICU length of stay, 30-day readmission for cardiac causes, or 30-day cardiovascular mortality; the one cardiovascular death was in the control group.
Two-thirds of the patients had coronary artery bypass grafts, and the rest had either valve surgery or a combination of both surgeries. Patients were 60 years old on average, and two-thirds were men, Drayton Hammond, Pharm.D., said at the Critical Care Congress, sponsored by the Society for Critical Care Medicine.
Ranolazine is indicated for chronic angina, not POAF prevention, but some previous investigations have suggested a possible benefit. A randomized, controlled clinical trial is currently looking into the matter.
At least retrospectively, the drug was “beneficial, definitely. There is about a 35% absolute-risk reduction,” said Dr. Hammond, who conducted the study while at the Jacksonville hospital.
Doctors there continue to use ranolazine for postop AF prophylaxis, as they see fit, said Dr. Hammond, now an assistant professor of pharmacy practice at the University of Arkansas for Medical Sciences, in Little Rock.
Gilead, the maker of the Ranexa, is also working on a ranolazine-dronedarone combination for paroxysmal AF.
More than half of the ranolazine patients in the study developed symptomatic hypotension within 72 hours of surgery, versus about a third in the control group (P = .0004). The drug was discontinued in one ranolazine patient because of hypotension. The problem resolved after 72 hours.
“We don’t have a good explanation” for the side effect. Perhaps there were differences in myocardial stunning or vasopressor use between the groups, but “we had the same three surgeons” for all the cases, Dr. Hammond said.
Ranolazine labeling notes the risk of hypotension and orthostatic hypotension. Labeling also warns of QT interval prolongation and renal failure in susceptible patients. The investigators found no between-group differences in bradycardia, new renal failure, or neurological events.
Overall, 53 (93%) patients in the ranolazine group were on postoperative beta-blockers, and 54 (94.7%) on postop statins; 48 (84.2%) in the control group were on beta-blockers postop and 47 (82.5%) on statins. Beta-blockers are first-line treatment to prevent postop AF; patients on any other antiarrhythmic were excluded from the trial, as were those who died during surgery.
PHOENIX – Twice-daily ranolazine following adult cardiac surgery seemed to protect against atrial fibrillation, based on a retrospective cohort study at the University of Florida Jacksonville Medical Center.
Ranolazine (Ranexa) was dosed orally at 1,000 mg the morning of surgery, and then resumed after extubation, generally the night of surgery. The goal was 1,000 mg orally twice a day, for a maximum of 7 hospital days; patients usually went home before then, so they received an average of nine doses. The drug was discontinued at discharge.
Six (10.5%) of 57 patients in the ranolazine group developed postoperative atrial fibrillation (POAF) versus 26 (45.6%) of 57 matched controls (P < .0001). The first case came at postop day 3 in the ranolazine group, but within 24 hours in the control group. One person in the ranolazine group and one in the control group had a history of AF.
There was no statistical difference in ICU length of stay, 30-day readmission for cardiac causes, or 30-day cardiovascular mortality; the one cardiovascular death was in the control group.
Two-thirds of the patients had coronary artery bypass grafts, and the rest had either valve surgery or a combination of both surgeries. Patients were 60 years old on average, and two-thirds were men, Drayton Hammond, Pharm.D., said at the Critical Care Congress, sponsored by the Society for Critical Care Medicine.
Ranolazine is indicated for chronic angina, not POAF prevention, but some previous investigations have suggested a possible benefit. A randomized, controlled clinical trial is currently looking into the matter.
At least retrospectively, the drug was “beneficial, definitely. There is about a 35% absolute-risk reduction,” said Dr. Hammond, who conducted the study while at the Jacksonville hospital.
Doctors there continue to use ranolazine for postop AF prophylaxis, as they see fit, said Dr. Hammond, now an assistant professor of pharmacy practice at the University of Arkansas for Medical Sciences, in Little Rock.
Gilead, the maker of the Ranexa, is also working on a ranolazine-dronedarone combination for paroxysmal AF.
More than half of the ranolazine patients in the study developed symptomatic hypotension within 72 hours of surgery, versus about a third in the control group (P = .0004). The drug was discontinued in one ranolazine patient because of hypotension. The problem resolved after 72 hours.
“We don’t have a good explanation” for the side effect. Perhaps there were differences in myocardial stunning or vasopressor use between the groups, but “we had the same three surgeons” for all the cases, Dr. Hammond said.
Ranolazine labeling notes the risk of hypotension and orthostatic hypotension. Labeling also warns of QT interval prolongation and renal failure in susceptible patients. The investigators found no between-group differences in bradycardia, new renal failure, or neurological events.
Overall, 53 (93%) patients in the ranolazine group were on postoperative beta-blockers, and 54 (94.7%) on postop statins; 48 (84.2%) in the control group were on beta-blockers postop and 47 (82.5%) on statins. Beta-blockers are first-line treatment to prevent postop AF; patients on any other antiarrhythmic were excluded from the trial, as were those who died during surgery.
AT THE CRITICAL CARE CONGRESS
Key clinical point: Ranolazine’s protective effect seems to come at the cost of symptomatic hypotension in the first 3 days after surgery.
Major finding: Postop atrial fibrillation occurred in 6 (10.5%) of 57 patients in the ranolazine group and 26 (45.6%) of 57 matched controls (P < .0001).
Data source: Retrospective cohort study of postop follow-up in 114 adults who had cardiac surgery.
Disclosures: There was no outside funding for the work. The investigators said they have no financial relationship with Gilead, maker of ranolazine (Ranexa).
CHADS2 predicts postop atrial fibrillation
PHOENIX – For every unit increase in baseline CHADS2 score, the risk of postop atrial fibrillation increases by 17%, according to a retrospective chart review of 1,550 adults who had major vascular or thoracic surgery at the Mayo Clinic in Rochester, Minn.
On multivariate analysis, postop day 1 Sequential Organ Failure Assessment score (HR 1.08, 95% CI 1.03-1.12, per unit increase) and cumulative fluid balance (HR 1.03, 95% CI 1.01-1.06, per 1,000 mL) also correlated with the risk for new-onset atrial fibrillation (AF).
Baseline calcium channel blockers protected against new-onset AF (HR 0.52, 95% CI 0.37-0.73), but, paradoxically, the risk increased with baseline (HR 1.78, 95% CI 1.24-2.56) and postop (HR 1.44, 95% CI 1.05-1.99) beta-blocker use.
The relationship of CHADS2 to new-onset AF (HR 1.17, 95% CI 1.04-1.31) could prove handy in the surgical ICU because “everyone is familiar with it, and it’s easy to calculate.” CHADS2 (heart failure, hypertension, age, diabetes, prior stroke) has also recently been shown to predict AF after cardiac surgery, said lead investigator Kirstin Kooda, Pharm.D., a critical care pharmacist at Mayo.
The beta-blocker finding was a surprise, since beta-blockers are a standard AF treatment, Dr. Kooda said at the Critical Care Congress, sponsored by the Society for Critical Care Medicine. About 80% (175) of new-onset AF patients were on baseline beta-blockers, versus about 68% (892) who did not develop AF. Patients using beta-blockers received them the morning of surgery, and resumed them a median of 7 hours afterward. There were no significant differences in heart rates during surgery.
The team excluded patients with any history of AF and censored patients if they developed it, so the drugs’ use probably wasn’t related to a concern about the condition. Just under 70% of patients in both groups had baseline hypertension, another indication for the drugs.
Even so, the finding is probably real given the number of patients in the study. Most likely, the drugs were markers for additional risk factors not captured in the study, Dr. Kooda said.
Overall, 112 (20.7%) of the 540 thoracic patients and 107 (11%) of the 1,010 vascular patients developed new-onset AF a median of 55 hours after surgery. The incidence difference and timing are in line with previous reports.
The mean age in the AF group was 70 years, and in the non-AF group it was 66 years. In both, 65% were men, 5% had heart failure, 30% had diabetes, and 10% had prior strokes. Patients with pacemakers and recent myocardial infarctions – also possible settings for beta-blockers – were excluded from the trial.
The majority of the vascular cases were open aortic aneurysms, aortic bypasses, and thrombectomies or endarterectomies of central arteries. Most of the thoracic surgeries were lobectomies, pneumonectomies, and wedge or chest wall resections.
PHOENIX – For every unit increase in baseline CHADS2 score, the risk of postop atrial fibrillation increases by 17%, according to a retrospective chart review of 1,550 adults who had major vascular or thoracic surgery at the Mayo Clinic in Rochester, Minn.
On multivariate analysis, postop day 1 Sequential Organ Failure Assessment score (HR 1.08, 95% CI 1.03-1.12, per unit increase) and cumulative fluid balance (HR 1.03, 95% CI 1.01-1.06, per 1,000 mL) also correlated with the risk for new-onset atrial fibrillation (AF).
Baseline calcium channel blockers protected against new-onset AF (HR 0.52, 95% CI 0.37-0.73), but, paradoxically, the risk increased with baseline (HR 1.78, 95% CI 1.24-2.56) and postop (HR 1.44, 95% CI 1.05-1.99) beta-blocker use.
The relationship of CHADS2 to new-onset AF (HR 1.17, 95% CI 1.04-1.31) could prove handy in the surgical ICU because “everyone is familiar with it, and it’s easy to calculate.” CHADS2 (heart failure, hypertension, age, diabetes, prior stroke) has also recently been shown to predict AF after cardiac surgery, said lead investigator Kirstin Kooda, Pharm.D., a critical care pharmacist at Mayo.
The beta-blocker finding was a surprise, since beta-blockers are a standard AF treatment, Dr. Kooda said at the Critical Care Congress, sponsored by the Society for Critical Care Medicine. About 80% (175) of new-onset AF patients were on baseline beta-blockers, versus about 68% (892) who did not develop AF. Patients using beta-blockers received them the morning of surgery, and resumed them a median of 7 hours afterward. There were no significant differences in heart rates during surgery.
The team excluded patients with any history of AF and censored patients if they developed it, so the drugs’ use probably wasn’t related to a concern about the condition. Just under 70% of patients in both groups had baseline hypertension, another indication for the drugs.
Even so, the finding is probably real given the number of patients in the study. Most likely, the drugs were markers for additional risk factors not captured in the study, Dr. Kooda said.
Overall, 112 (20.7%) of the 540 thoracic patients and 107 (11%) of the 1,010 vascular patients developed new-onset AF a median of 55 hours after surgery. The incidence difference and timing are in line with previous reports.
The mean age in the AF group was 70 years, and in the non-AF group it was 66 years. In both, 65% were men, 5% had heart failure, 30% had diabetes, and 10% had prior strokes. Patients with pacemakers and recent myocardial infarctions – also possible settings for beta-blockers – were excluded from the trial.
The majority of the vascular cases were open aortic aneurysms, aortic bypasses, and thrombectomies or endarterectomies of central arteries. Most of the thoracic surgeries were lobectomies, pneumonectomies, and wedge or chest wall resections.
PHOENIX – For every unit increase in baseline CHADS2 score, the risk of postop atrial fibrillation increases by 17%, according to a retrospective chart review of 1,550 adults who had major vascular or thoracic surgery at the Mayo Clinic in Rochester, Minn.
On multivariate analysis, postop day 1 Sequential Organ Failure Assessment score (HR 1.08, 95% CI 1.03-1.12, per unit increase) and cumulative fluid balance (HR 1.03, 95% CI 1.01-1.06, per 1,000 mL) also correlated with the risk for new-onset atrial fibrillation (AF).
Baseline calcium channel blockers protected against new-onset AF (HR 0.52, 95% CI 0.37-0.73), but, paradoxically, the risk increased with baseline (HR 1.78, 95% CI 1.24-2.56) and postop (HR 1.44, 95% CI 1.05-1.99) beta-blocker use.
The relationship of CHADS2 to new-onset AF (HR 1.17, 95% CI 1.04-1.31) could prove handy in the surgical ICU because “everyone is familiar with it, and it’s easy to calculate.” CHADS2 (heart failure, hypertension, age, diabetes, prior stroke) has also recently been shown to predict AF after cardiac surgery, said lead investigator Kirstin Kooda, Pharm.D., a critical care pharmacist at Mayo.
The beta-blocker finding was a surprise, since beta-blockers are a standard AF treatment, Dr. Kooda said at the Critical Care Congress, sponsored by the Society for Critical Care Medicine. About 80% (175) of new-onset AF patients were on baseline beta-blockers, versus about 68% (892) who did not develop AF. Patients using beta-blockers received them the morning of surgery, and resumed them a median of 7 hours afterward. There were no significant differences in heart rates during surgery.
The team excluded patients with any history of AF and censored patients if they developed it, so the drugs’ use probably wasn’t related to a concern about the condition. Just under 70% of patients in both groups had baseline hypertension, another indication for the drugs.
Even so, the finding is probably real given the number of patients in the study. Most likely, the drugs were markers for additional risk factors not captured in the study, Dr. Kooda said.
Overall, 112 (20.7%) of the 540 thoracic patients and 107 (11%) of the 1,010 vascular patients developed new-onset AF a median of 55 hours after surgery. The incidence difference and timing are in line with previous reports.
The mean age in the AF group was 70 years, and in the non-AF group it was 66 years. In both, 65% were men, 5% had heart failure, 30% had diabetes, and 10% had prior strokes. Patients with pacemakers and recent myocardial infarctions – also possible settings for beta-blockers – were excluded from the trial.
The majority of the vascular cases were open aortic aneurysms, aortic bypasses, and thrombectomies or endarterectomies of central arteries. Most of the thoracic surgeries were lobectomies, pneumonectomies, and wedge or chest wall resections.
AT THE CRITICAL CARE CONGRESS
Key clinical point: Postop atrial fibrillation is more likely if patients go into surgery with an elevated CHADS 2 score.
Major finding: For every unit increase in baseline CHADS2 score, there is a 17% increase in the risk of new-onset AF following major vascular or thoracic surgery (HR 1.17, 95% CI 1.04-1.31).
Data source: Retrospective chart review of 1,550 adult patients.
Disclosures: The investigators said they had no disclosures. No outside funding was reported for the work.
Genetic blood profiles can estimate risk levels of VTE patients
Through genetic analysis, researchers used gene expression profiles to differentiate between several clinical phenotypes of VTE and distinguish high-risk patients from both low-risk patients and healthy controls, in a study published in Thrombosis Research.
Dr. Deborah A. Lewis of Duke University Medical Center and her associates used differential expression analysis to find several genes previously identified as potentially having a role in the development of thrombotic disorders, including SELP, KLKB1, ANXA5, andCD46. They then compared the genetic profiles of 107 patients, separated into low-, moderate-, or high-risk groups based on their clinical presentations of VTE, as well as 25 controls.
The most accurate comparisons were between the high-risk and low-risk groups, the high-risk group and the healthy controls, and the low-risk group and healthy controls, where the AUC levels were 0.81, 0.84 and 0.80 respectively.
“The profiles obtained … provide insights into approaches that might be useful in the identification of individuals with a single thrombotic event who are at highest risk for a recurrent VTE after completing a standard course of therapy,” the investigators wrote.
For the full article, click here (Thromb. Res. 2015 [doi:10.1016/j.thromres.2015.02.003]).
Through genetic analysis, researchers used gene expression profiles to differentiate between several clinical phenotypes of VTE and distinguish high-risk patients from both low-risk patients and healthy controls, in a study published in Thrombosis Research.
Dr. Deborah A. Lewis of Duke University Medical Center and her associates used differential expression analysis to find several genes previously identified as potentially having a role in the development of thrombotic disorders, including SELP, KLKB1, ANXA5, andCD46. They then compared the genetic profiles of 107 patients, separated into low-, moderate-, or high-risk groups based on their clinical presentations of VTE, as well as 25 controls.
The most accurate comparisons were between the high-risk and low-risk groups, the high-risk group and the healthy controls, and the low-risk group and healthy controls, where the AUC levels were 0.81, 0.84 and 0.80 respectively.
“The profiles obtained … provide insights into approaches that might be useful in the identification of individuals with a single thrombotic event who are at highest risk for a recurrent VTE after completing a standard course of therapy,” the investigators wrote.
For the full article, click here (Thromb. Res. 2015 [doi:10.1016/j.thromres.2015.02.003]).
Through genetic analysis, researchers used gene expression profiles to differentiate between several clinical phenotypes of VTE and distinguish high-risk patients from both low-risk patients and healthy controls, in a study published in Thrombosis Research.
Dr. Deborah A. Lewis of Duke University Medical Center and her associates used differential expression analysis to find several genes previously identified as potentially having a role in the development of thrombotic disorders, including SELP, KLKB1, ANXA5, andCD46. They then compared the genetic profiles of 107 patients, separated into low-, moderate-, or high-risk groups based on their clinical presentations of VTE, as well as 25 controls.
The most accurate comparisons were between the high-risk and low-risk groups, the high-risk group and the healthy controls, and the low-risk group and healthy controls, where the AUC levels were 0.81, 0.84 and 0.80 respectively.
“The profiles obtained … provide insights into approaches that might be useful in the identification of individuals with a single thrombotic event who are at highest risk for a recurrent VTE after completing a standard course of therapy,” the investigators wrote.
For the full article, click here (Thromb. Res. 2015 [doi:10.1016/j.thromres.2015.02.003]).
Esophagogastric cancer patients on chemotherapy more likely to develop VTE
Incidence rates for developing venous thromboembolism (VTE) among esophagogastric cancer patients undergoing neoadjuvant chemotherapy in combination with curative intended surgery were significantly higher among patients with initial stage III and IV cancers and gastric cancer, according to a new study published in Thrombosis Research.
In the clinical prospective study, 129 patients with lower esophageal, gastroesophageal, and gastric cancer were examined between 2008 and 2011. Baseline assessments were recorded via bilateral compression ultrasound (biCUS) for deep vein thrombosis and computer tomography pulmonary angiography for pulmonary embolism. The patients received a chemotherapy regimen of oxaliplatin, capecitabine, and epirubicin, with curative intended surgery, and were examined before undergoing preoperative chemotherapy, surgery, and postoperative chemotherapy. The researchers encountered 21 VTE cases, or 16% of the total number of patients examined, with VTE incidences twice as likely to be asymptomatic than symptomatic.
The authors noted that state-of-the-art technology helped boost VTE detection rates among asymptomatic patients, and older studies may have underreported incidences of the disease.
“Although our study only included 129 patients, the systematic use of biCUS strongly suggests that the frequency of VTE is much greater than that previously reported for these types of cancer,” wrote Dr. Anders Christian Larsen and his associates at Aalborg University Hospital, Denmark.
Read more here (Thrombosis Research 2015 [doi:10.1016/j.thromres.2015.01.021]).
Incidence rates for developing venous thromboembolism (VTE) among esophagogastric cancer patients undergoing neoadjuvant chemotherapy in combination with curative intended surgery were significantly higher among patients with initial stage III and IV cancers and gastric cancer, according to a new study published in Thrombosis Research.
In the clinical prospective study, 129 patients with lower esophageal, gastroesophageal, and gastric cancer were examined between 2008 and 2011. Baseline assessments were recorded via bilateral compression ultrasound (biCUS) for deep vein thrombosis and computer tomography pulmonary angiography for pulmonary embolism. The patients received a chemotherapy regimen of oxaliplatin, capecitabine, and epirubicin, with curative intended surgery, and were examined before undergoing preoperative chemotherapy, surgery, and postoperative chemotherapy. The researchers encountered 21 VTE cases, or 16% of the total number of patients examined, with VTE incidences twice as likely to be asymptomatic than symptomatic.
The authors noted that state-of-the-art technology helped boost VTE detection rates among asymptomatic patients, and older studies may have underreported incidences of the disease.
“Although our study only included 129 patients, the systematic use of biCUS strongly suggests that the frequency of VTE is much greater than that previously reported for these types of cancer,” wrote Dr. Anders Christian Larsen and his associates at Aalborg University Hospital, Denmark.
Read more here (Thrombosis Research 2015 [doi:10.1016/j.thromres.2015.01.021]).
Incidence rates for developing venous thromboembolism (VTE) among esophagogastric cancer patients undergoing neoadjuvant chemotherapy in combination with curative intended surgery were significantly higher among patients with initial stage III and IV cancers and gastric cancer, according to a new study published in Thrombosis Research.
In the clinical prospective study, 129 patients with lower esophageal, gastroesophageal, and gastric cancer were examined between 2008 and 2011. Baseline assessments were recorded via bilateral compression ultrasound (biCUS) for deep vein thrombosis and computer tomography pulmonary angiography for pulmonary embolism. The patients received a chemotherapy regimen of oxaliplatin, capecitabine, and epirubicin, with curative intended surgery, and were examined before undergoing preoperative chemotherapy, surgery, and postoperative chemotherapy. The researchers encountered 21 VTE cases, or 16% of the total number of patients examined, with VTE incidences twice as likely to be asymptomatic than symptomatic.
The authors noted that state-of-the-art technology helped boost VTE detection rates among asymptomatic patients, and older studies may have underreported incidences of the disease.
“Although our study only included 129 patients, the systematic use of biCUS strongly suggests that the frequency of VTE is much greater than that previously reported for these types of cancer,” wrote Dr. Anders Christian Larsen and his associates at Aalborg University Hospital, Denmark.
Read more here (Thrombosis Research 2015 [doi:10.1016/j.thromres.2015.01.021]).