User login
Tuberculosis: In and out of the airways
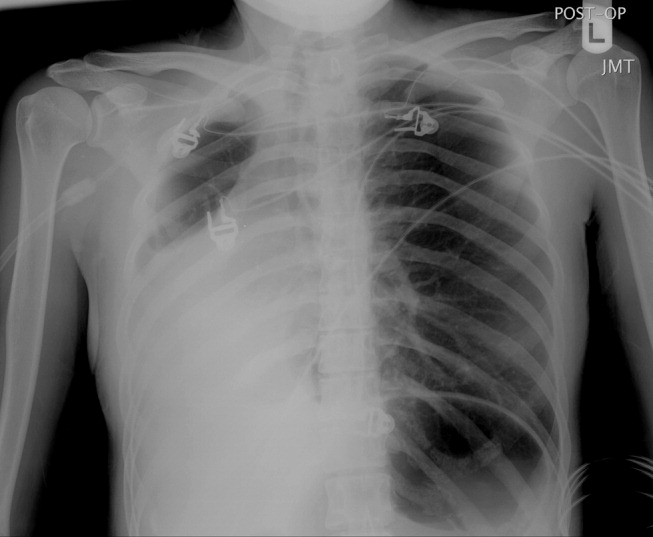
A 23‐year‐old Chinese woman presented with worsening exertional dyspnea. Her medical history was notable for pulmonary tuberculosis treated at the age of 16. Over the past 3 years, she reported progressive respiratory symptoms resulting in marked exercise intolerance. She denied any fevers, cough, or weight loss. On examination, she had right‐sided tracheal deviation but spoke comfortably. Her heart sounds were displaced and right‐sided breath sounds nearly absent. Chest x‐ray (Fig. 1) and subsequent CT revealed complete collapse of the right lung with associated hyperexpansion of the left lung and left‐to‐right mediastinal shift (Fig. 2, with an asterisk denoting the aortic arch; an arrow, the right main‐stem bronchus, which would soon terminate; and arrowheads, the collapsed right lung). No lung masses or effusions were noted; active TB had been ruled out with AFB sputums. Bronchoscopy revealed a fibrotic and stenotic right main‐stem bronchus (Fig. 3, with an asterisk denoting a patent left main‐stem bronchus and an arrow denoting a stenotic right main‐stem bronchus). Pulmonary manifestations of TB include parenchymal and endobronchial disease. Patients more likely to develop endobronchial disease include those with extensive pulmonary involvement, particularly cavitary lesions. Between 10% and 20% of patients with endobronchial disease will have normal CXRs, though CT scans may reveal endobronchial lesions or narrowing. Complications of endobronchial disease include obstruction, bronchiectasis, and tracheal or bronchial stenosis. Some airway obstructions may be associated with enlarging peribronchial nodes, which may erode into the airways as broncholiths. Steroids have been used to prevent long‐term complications, but their efficacy is still unclear. Repeated dilation, stenting, and resection all serve as management options for advanced endobronchial disease. In our patient, the extensive bronchial scarring and stenosis were most likely complications from past endobronchial infection. Unfortunately, attempts at balloon dilatation of her right main‐stem bronchus were unsuccessful, and she continues to have considerable exercise limitation. More prompt recognition of the disease may have allowed for an earlier and more successful intervention.
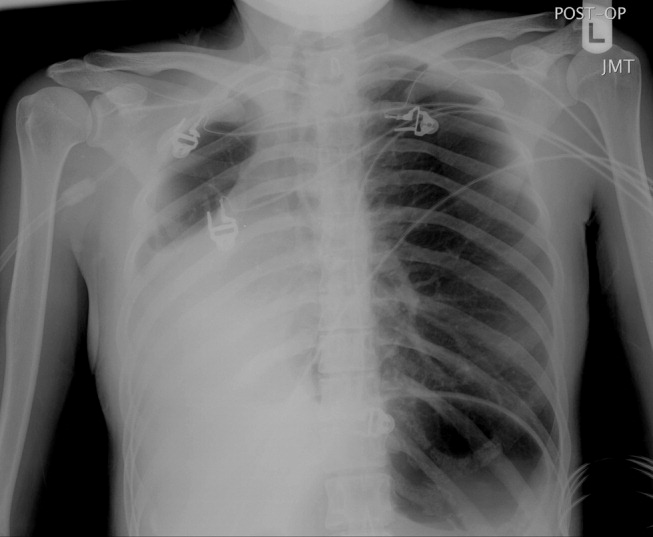
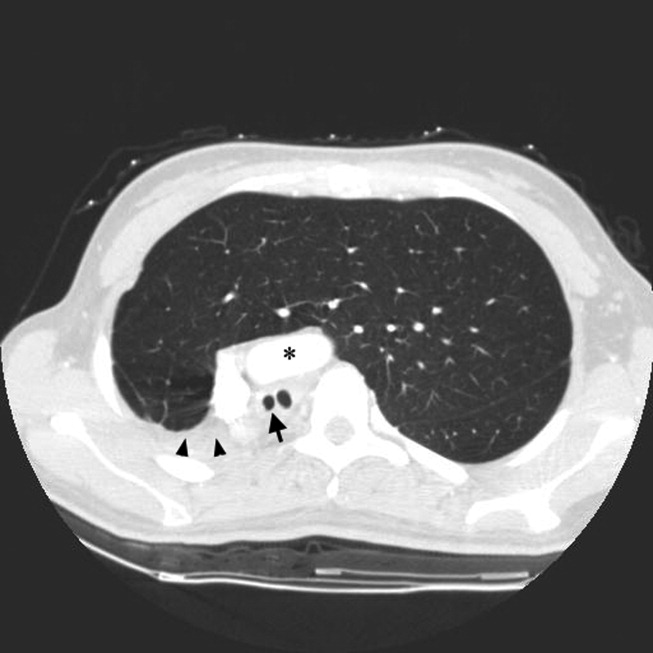
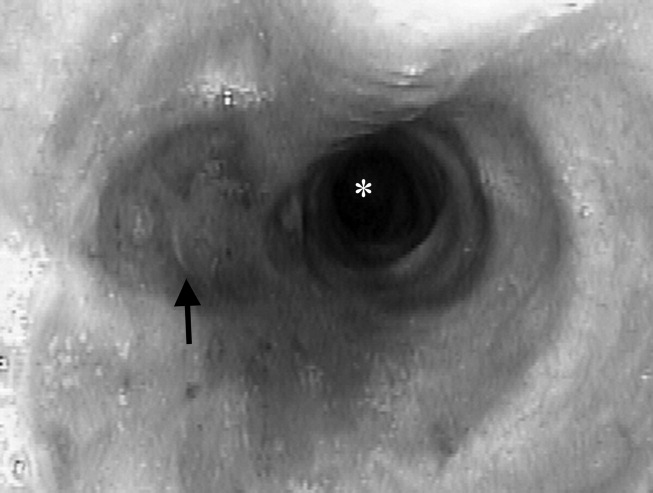
A 23‐year‐old Chinese woman presented with worsening exertional dyspnea. Her medical history was notable for pulmonary tuberculosis treated at the age of 16. Over the past 3 years, she reported progressive respiratory symptoms resulting in marked exercise intolerance. She denied any fevers, cough, or weight loss. On examination, she had right‐sided tracheal deviation but spoke comfortably. Her heart sounds were displaced and right‐sided breath sounds nearly absent. Chest x‐ray (Fig. 1) and subsequent CT revealed complete collapse of the right lung with associated hyperexpansion of the left lung and left‐to‐right mediastinal shift (Fig. 2, with an asterisk denoting the aortic arch; an arrow, the right main‐stem bronchus, which would soon terminate; and arrowheads, the collapsed right lung). No lung masses or effusions were noted; active TB had been ruled out with AFB sputums. Bronchoscopy revealed a fibrotic and stenotic right main‐stem bronchus (Fig. 3, with an asterisk denoting a patent left main‐stem bronchus and an arrow denoting a stenotic right main‐stem bronchus). Pulmonary manifestations of TB include parenchymal and endobronchial disease. Patients more likely to develop endobronchial disease include those with extensive pulmonary involvement, particularly cavitary lesions. Between 10% and 20% of patients with endobronchial disease will have normal CXRs, though CT scans may reveal endobronchial lesions or narrowing. Complications of endobronchial disease include obstruction, bronchiectasis, and tracheal or bronchial stenosis. Some airway obstructions may be associated with enlarging peribronchial nodes, which may erode into the airways as broncholiths. Steroids have been used to prevent long‐term complications, but their efficacy is still unclear. Repeated dilation, stenting, and resection all serve as management options for advanced endobronchial disease. In our patient, the extensive bronchial scarring and stenosis were most likely complications from past endobronchial infection. Unfortunately, attempts at balloon dilatation of her right main‐stem bronchus were unsuccessful, and she continues to have considerable exercise limitation. More prompt recognition of the disease may have allowed for an earlier and more successful intervention.



A 23‐year‐old Chinese woman presented with worsening exertional dyspnea. Her medical history was notable for pulmonary tuberculosis treated at the age of 16. Over the past 3 years, she reported progressive respiratory symptoms resulting in marked exercise intolerance. She denied any fevers, cough, or weight loss. On examination, she had right‐sided tracheal deviation but spoke comfortably. Her heart sounds were displaced and right‐sided breath sounds nearly absent. Chest x‐ray (Fig. 1) and subsequent CT revealed complete collapse of the right lung with associated hyperexpansion of the left lung and left‐to‐right mediastinal shift (Fig. 2, with an asterisk denoting the aortic arch; an arrow, the right main‐stem bronchus, which would soon terminate; and arrowheads, the collapsed right lung). No lung masses or effusions were noted; active TB had been ruled out with AFB sputums. Bronchoscopy revealed a fibrotic and stenotic right main‐stem bronchus (Fig. 3, with an asterisk denoting a patent left main‐stem bronchus and an arrow denoting a stenotic right main‐stem bronchus). Pulmonary manifestations of TB include parenchymal and endobronchial disease. Patients more likely to develop endobronchial disease include those with extensive pulmonary involvement, particularly cavitary lesions. Between 10% and 20% of patients with endobronchial disease will have normal CXRs, though CT scans may reveal endobronchial lesions or narrowing. Complications of endobronchial disease include obstruction, bronchiectasis, and tracheal or bronchial stenosis. Some airway obstructions may be associated with enlarging peribronchial nodes, which may erode into the airways as broncholiths. Steroids have been used to prevent long‐term complications, but their efficacy is still unclear. Repeated dilation, stenting, and resection all serve as management options for advanced endobronchial disease. In our patient, the extensive bronchial scarring and stenosis were most likely complications from past endobronchial infection. Unfortunately, attempts at balloon dilatation of her right main‐stem bronchus were unsuccessful, and she continues to have considerable exercise limitation. More prompt recognition of the disease may have allowed for an earlier and more successful intervention.



Rules of Engagement
Peripheral arterial disease (PAD) is defined by the presence of stenosis or occlusion in peripheral arterial beds.1, 2 Based on large population‐based screening surveys, the prevalence of this disease ranges between 5.5% and 26.7% and is dependent on age, atherothrombotic risk factors, and the coexistence of other atherothrombotic diseases.35 Symptoms of PAD include mild to intermittent claudication, ischemic rest pain, and tissue loss.2 Disease severity is classified according to either Fontaine's stages or Rutherford categories. These categorization schema have value in improving communication between physicians, which is important in ensuring continuity of care between the inpatient and outpatient settings (Table 1).2
| Stage | Fontaine | Rutherford | ||
|---|---|---|---|---|
| Clinical | Grade | Category | Clinical | |
| ||||
| I | Asymptomatic | 0 | 0 | Asymptomatic |
| IIa | Mild claudication | I | 1 | Mild claudication |
| IIb | Moderate‐severe claudication | I | 2 | Moderate claudication |
| III | Ischemic rest pain | I | 3 | Severe claudication |
| IV | Ulceration or gangrene | II | 4 | Ischemic rest pain |
| III | 5 | Minor tissue loss | ||
| IV | 6 | Ulceration or gangrene | ||
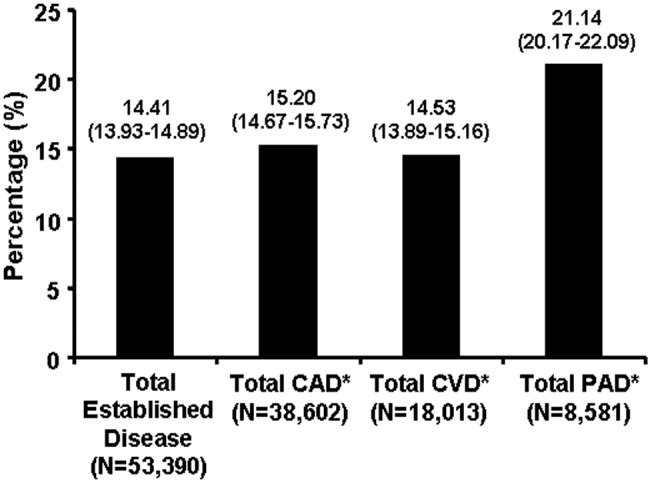
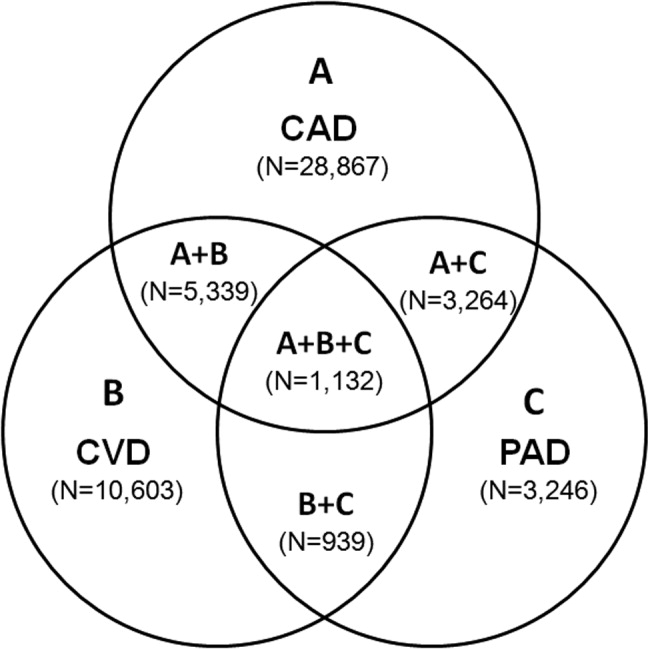
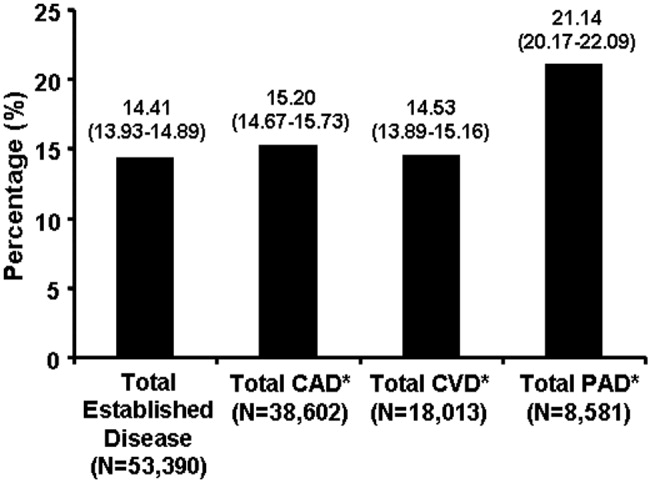
Patients with PAD are at increased risk of dying from or experiencing a cardiovascular event.68 Among patients diagnosed with PAD, coronary artery disease (CAD), or cerebrovascular disease (CVD), those with PAD have the highest 1‐year rate of cardiovascular death, MI, stroke, or vascular‐related hospitalization (Fig. 1).8 This risk is attributable in part to the high rate of association of PAD with other atherothrombotic diseases. The Reduction of Atherothrombosis for Continued Health (REACH) Registry found that approximately 60% of participants with documented PAD have polyvascular disease, defined by the coexistence of CAD and/or CVD. In comparison, 25% of participants with CAD and 40% of participants with CVD have polyvascular disease.8 Thus, PAD can be considered a powerful indicator of systemic atherothrombotic disease and a predictor of cardiovascular and cerebrovascular morbidity and mortality.1
Unfortunately, asymptomatic PAD is more common than its symptomatic counterpart.3 In addition, symptomatic patients often fail to notify their physicians about PAD‐associated symptoms because they attribute them to aging.3 As a result, this disease is underdiagnosed and undertreated.1 Accordingly, several medical associations and physician task forces have called for an increase in screening for PAD in at‐risk populations that include: patients older than 70, patients older than 50 who have concomitant atherothrombotic risk factors, and patients with atherothrombotic disease of single or multiple vascular beds.1, 9 In many cases hospitalists encounter patients at high‐risk for PAD whose DRG for admission might be unrelated to this disease. Nonetheless, hospitalists have the opportunity to improve patient outcomes by being capable of screening for undiagnosed PAD and initiating appropriate interventions to reduce the risk of life‐threatening cardiovascular events.
DIAGNOSIS
Peripheral arterial disease can be diagnosed by either noninvasive or invasive methods. The ankle‐to‐brachial index (ABI) is an accurate, practical, inexpensive, and noninvasive method for detecting PAD.1 The sensitivity of ABI in detecting PAD is 95% with 99% specificity,10 which makes the method superior to other indicators (eg, absence of a pedal pulse, presence of a femoral arterial bruit, slow venous filling, or cold/abnormally colored skin) assessed during a physical examination.11 Under normal conditions, the systolic pressure at the ankle should be equal to or greater than that recorded from the upper arm. As PAD narrows arteries, the systolic pressure decreases at sites distal to the area of arterial narrowing. A resting ABI is quantified by taking 2 readings each of ankle and brachial blood pressures with a handheld Doppler device while the patient is supine and dividing the highest ankle systolic pressure by the highest brachial pressure.12
An ABI between 0.9 and 1.30 is considered normal. Ratios between 0.7 and 0.89 indicate mild PAD, 0.4 and 0.69 moderate PAD, and an ABI 0.4 severe PAD when patients are more likely to have ischemic pain when at rest. An ABI > 1.3 usually indicates the presence of noncompressible vessels, which can be common in the elderly and patients with diabetes mellitus who have calcification of the distal arteries.1, 2 The ABI is also inversely related to the number of atherosclerotic risk factors and the risk of adverse cardiovascular events and death.6, 1316 To identify individuals with suspected or asymptomatic lower‐extremity PAD, ABI has a class I recommendation from the American College of Cardiology and American Heart Association (ACC/AHA) for patients who present with leg symptoms, who are 70 years and older, or who are 50 years and older with a history of smoking or diabetes.2 This enables physicians to make therapeutic interventions to reduce the risk of adverse vascular events in these patient cohorts.
Additional detection methods for PAD include measuring the ABI before and after exercise on a treadmill, if the patient is ambulatory, or exercise by performing 50 repetitions of raising the heels maximally off the floor, if the patient is not ambulatory. These tests determine the extent of claudication.2 Duplex ultrasound is used to establish the location and severity of stenosis and to follow PAD progression.2
Invasive evaluations for PAD are used primarily to confirm an initial diagnosis of PAD and assess its severity. These methods include a conventional angiogram, which is the most readily available and widely used technique for defining arterial stenosis. Magnetic resonance (MR) angiography with gadolinium and computed tomographic (CT) angiography are used to determine the location and degree of stenosis. Both MR and CT angiography have advantages and disadvantages but are considered interchangeable with one another in patients with contraindications to either method (Table 2).2
| Diagostic method | Benefits | Limitations |
|---|---|---|
| ||
| Magnetic resonance angiography (MRA) | Useful to assess PAD anatomy and presence of significant stenosis | Tends to overestimate degree of stenosis |
| Useful to select patients who are candidates for endovascular of surgical revascularization | May be inaccurate in arteries treated with metal stents | |
| Cannot be used in patients with contraindication to magnetic resonance technique | ||
| Computed tomographic angiography (CTA) | Useful to assess PAD anatomy and presence of significant stenosis | Single‐detector CT lacks accuracy to detect stenoses |
| Useful to select patients who are candidates for endovascular or surgical revascularization | Spatial Resolution lower than digital subtraction angiography | |
| Helpful to provide associated soft‐tissue diagnostic information that may be associated with PAD | Venous opacification can obscure arterial filling | |
| Patients with contraindications to MRA | Asymmetric opacification of legs may obscure arterial phase in some vessels | |
| Metal clips, stents, and prostheses do not cause significant CTA artifacts | Accuracy and effectiveness not as well determined as MRA | |
| Scan times are significantly faster | Treatment plans based on CTA have not been compared to those of catheter angiography | |
| Requires contrast and radiation | ||
| Use may be limited in individuals with renal dysfunction | ||
ANTIPLATELET THERAPY FOR REDUCTION OF VASCULAR EVENTS
Hospitalists utilize a wide array of therapies to treat and manage PAD. Acute complications of PAD may require interventions to prevent tissue loss or infection, revascularization procedures, or surgical amputation. Treatment of mild to moderate PAD focuses on atherothrombotic risk factor management, exercise therapy to improve limb function, and interventions to reduce the risk of adverse vascular events.2, 9 The remainder of this report focuses on the role of antiplatelet therapy (eg, aspirin and thienopyridines) in reducing the risk of vascular events in patients with PAD.
The Antiplatelet Trialists' Collaboration performed an overview analysis of randomized trials conducted prior to 1990 in order to determine the association of prolonged antiplatelet therapy with the occurrence of major vascular events. As a whole, therapies thought to act through inhibition of platelet aggregation, adhesion, or both reduced the incidence of vascular events by 33% in patients with PAD and those at high risk, and by 25% in all patient groups. Antiplatelet agents were also well tolerated; the absolute risk of fatal or nonmajor hemorrhage was low.17
A similar meta‐analysis was conducted of antiplatelet therapies in high‐risk patients with atherothrombosis by the Antithrombotic Trialists' Collaboration. Antiplatelet therapies taken together reduced the odds of patients experiencing vascular events by 22% (SE = 2%) across all trials and 23% (SE = 8%) in patients with PAD.18 Similar to the Antiplatelet Trialists' Collaboration study, the absolute risk of major and minor bleeding was low compared to the benefits of antiplatelet therapy.18 The results of these studies provide supporting evidence for the ACC/AHA class I recommendation for the use of antiplatelet therapy to reduce the risk of MI, stroke, or vascular death in patients with PAD.
The Antithrombotic Trialists' Collaboration also examined the risk reduction associated with a specific antiplatelet agent, aspirin. All doses of aspirin (75‐150, 160‐325, and 500‐1500 mg/day) reduced the odds by 23% (SE = 2%); high doses were no more effective than medium or low doses.18 Although the effects of aspirin was not analyzed in a subgroup analysis of patients with PAD, this study and others support the ACC/AHA class I recommendations for the use of aspirin to reduce the risk of MI, stroke, or vascular death in patients with PAD.2, 1921
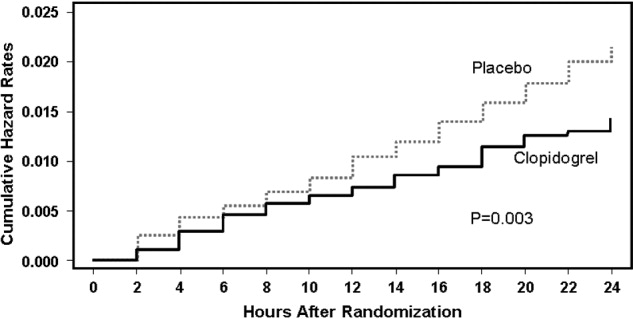
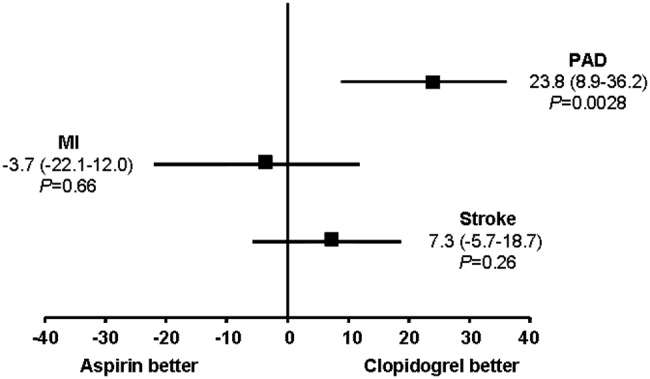
The CAPRIE trial compared the efficacy of another antiplatelet agent, clopidogrel, against aspirin in patients with PAD.22 Patients with a history of recent ischemic stroke, MI, or symptomatic PAD were randomized to receive either clopidogrel (75 mg/day) or aspirin (325 mg/day) for 1‐3 years (mean follow‐up time, 1.91 years). Study outcomes were the incidence of nonfatal MI, ischemic stroke, hemorrhagic stroke, leg amputation, and vascular deaths. The absolute risk reduction for all patients was 8.7% (95% confidence interval [CI], 0.3%‐16.5%) in favor of clopidogrel over aspirin. Moreover, subgroup analysis in patients with PAD revealed that clopidogrel reduced the risk of a vascular event by 23.8% (95% CI, 8.9%‐36.2%; P = 0.0028) compared with aspirin (Fig. 2). Clopidogrel and aspirin had similar safety profiles, but other studies have revealed bleeding incidence is numerically greater in patients treated with clopidogrel.2224 Although the CAPRIE trial is the only study to date to compare the efficacy of clopidogrel over aspirin in reducing vascular event in patients with PAD, its outcomes underlie the class I ACC/AHA recommendation for clopidogrel (75 mg/day) as an effective alternative to aspirin to reduce the risk of MI, stroke, or death in patients with PAD.2
CONCLUSIONS
Despite the availability of accurate, practical, and inexpensive diagnostic testing, PAD remains underdiagnosed and undertreated. Early detection of PAD and subsequent intervention by hospitalists are important because peripheral arterial disease is strongly associated with an increased risk of mortality and morbidity from adverse vascular events. The ACC/AHA recommends screening for asymptomatic patients at risk for this disease so that therapies that reduce the risk of an MI, stroke, or vascular death can be administered immediately. Antiplatelet agents reduce the risk of adverse vascular events in patients with PAD. The use of aspirin or clopidogrel is recommended in this cohort of patients. However, further study is necessary to determine the efficacy and safety of combination therapy with aspirin and clopidogrel in patients with PAD. It is also important to note that coordination of care between hospitalists and cardiologists is critical in the management of patients with this disease. However, the appropriate handoff of patients between these 2 groups of physicians depends on the local expertise and support structure of these health care professionals. Thus, an interdisciplinary approach utilizing guideline‐based patient care will allow hospitalists to refer patients accordingly, ensuring optimal outcomes in patients with PAD.
- ,,, et al.Prevention of Atherothrombotic Disease Network. Critical issues in peripheral arterial disease detection and management: a call to action.Arch Intern Med.2003;163:884–892.
- ,,, et al.ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic).Circulation.2006;113:e463–e654.
- ,,,,,.Peripheral arterial disease in the elderly: the Rotterdam Study.Arterioscler Thromb Vasc Biol.1998;18:185–192.
- ,,, et al.Peripheral arterial disease detection, awareness, and treatment in primary care.JAMA.2001;286:1317–1324.
- ,.Prevalence of and risk factors for peripheral arterial disease in the United States: Results from the National Health and Nutrition Examination Survey, 1999‐2000.Circulation.2004;110:738–743.
- ,,, et al.Mortality over a period of 10 years in patients with peripheral arterial disease.N Engl J Med.1992;326:381–386.
- ,.Vascular event rates in patients with atherosclerotic cerebrovascular disease.Arch Neurol.1992;49:857–863.
- ,,, et al.;REACH Registry Investigators. One‐year cardiovascular event rates in outpatients with atherothrombosis.JAMA.2007;297:1197–1206.
- ,,, et al.Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: A critical review.Circulation.1996;94:3026–3049.
- ,.Management of peripheral arterial disease (PAD): TASC Working Group. TransAtlantic Inter‐Society Consensus (TASC).J Vasc Surg.2000:31(1Pt 2):S1–S296.
- ,.Physical examination and chronic lower‐extremity ischemia.Arch Intern Med.1998;158:1357–1364.
- .Medical treatment of peripheral artery disease and claudication.N Engl J Med.2001;344:1608–1621.
- ,,, et al.Ankle‐arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group.Circulation.1993;88:837–845.
- ,,,.Morbidity and mortality in hypertensive adults with a low ankle/arm blood pressure index.JAMA.1993;270:487–489.
- ,,, et al.Ankle‐arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group.Arterioscler Thromb Vasc Biol.1999;19:538–545.
- ,,,,,;Framingham Study. The ankle‐brachial index in the elderly and risk of stroke, coronary disease, and death: the Framingham Study.Arch Intern Med.2003;163:1939–1942.
- Antiplatelet Trialists' Collaboration.Collaborative overview of randomized trials of antiplatelet therapy—1: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients.BMJ.1994;308:81–106.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.BMJ.2002;324:71–86.
- The Medical Research Council's General Practice Research Framework.Thrombosis prevention trial: randomised trial of low‐intensity oral anticoagulation with warfarin and low‐dose aspirin in the primary prevention of ischemic heart disease in men at increased risk.Lancet.1998;351:233–241.
- ,,, for theHOT Study Group.Effects of intensive blood pressure lowering and low‐dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial.Lancet1998;280:1930–1935.
- Collaborative Group of the Primary Prevention Project (PPP).Low‐dose aspirin and vitamin E in people at cardiovascular risk: a randomized trial in general practice.Lancet.2001;357:89–95.
- CAPRIE Steering Committee.A randomized, blinded, trial of clopidogrel versus aspirin in patients at risk of ischemic events (CAPRIE).Lancet.1996;348:1329–1339.
- ,,; for theCHARISMA Investigators.Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events.N Engl J Med.2006;354:1706–1717.
- ,,; on behalf of theMATCH investigators.Aspirin and clopidogrel compared with clopidogrel alone after ischaemic stroke or transient ischaemic attack in high‐risk patients (MATCH): randomised, double‐blind, placebo‐controlled trial.Lancet.2004;364:331–337.
Peripheral arterial disease (PAD) is defined by the presence of stenosis or occlusion in peripheral arterial beds.1, 2 Based on large population‐based screening surveys, the prevalence of this disease ranges between 5.5% and 26.7% and is dependent on age, atherothrombotic risk factors, and the coexistence of other atherothrombotic diseases.35 Symptoms of PAD include mild to intermittent claudication, ischemic rest pain, and tissue loss.2 Disease severity is classified according to either Fontaine's stages or Rutherford categories. These categorization schema have value in improving communication between physicians, which is important in ensuring continuity of care between the inpatient and outpatient settings (Table 1).2
| Stage | Fontaine | Rutherford | ||
|---|---|---|---|---|
| Clinical | Grade | Category | Clinical | |
| ||||
| I | Asymptomatic | 0 | 0 | Asymptomatic |
| IIa | Mild claudication | I | 1 | Mild claudication |
| IIb | Moderate‐severe claudication | I | 2 | Moderate claudication |
| III | Ischemic rest pain | I | 3 | Severe claudication |
| IV | Ulceration or gangrene | II | 4 | Ischemic rest pain |
| III | 5 | Minor tissue loss | ||
| IV | 6 | Ulceration or gangrene | ||
Patients with PAD are at increased risk of dying from or experiencing a cardiovascular event.68 Among patients diagnosed with PAD, coronary artery disease (CAD), or cerebrovascular disease (CVD), those with PAD have the highest 1‐year rate of cardiovascular death, MI, stroke, or vascular‐related hospitalization (Fig. 1).8 This risk is attributable in part to the high rate of association of PAD with other atherothrombotic diseases. The Reduction of Atherothrombosis for Continued Health (REACH) Registry found that approximately 60% of participants with documented PAD have polyvascular disease, defined by the coexistence of CAD and/or CVD. In comparison, 25% of participants with CAD and 40% of participants with CVD have polyvascular disease.8 Thus, PAD can be considered a powerful indicator of systemic atherothrombotic disease and a predictor of cardiovascular and cerebrovascular morbidity and mortality.1

Unfortunately, asymptomatic PAD is more common than its symptomatic counterpart.3 In addition, symptomatic patients often fail to notify their physicians about PAD‐associated symptoms because they attribute them to aging.3 As a result, this disease is underdiagnosed and undertreated.1 Accordingly, several medical associations and physician task forces have called for an increase in screening for PAD in at‐risk populations that include: patients older than 70, patients older than 50 who have concomitant atherothrombotic risk factors, and patients with atherothrombotic disease of single or multiple vascular beds.1, 9 In many cases hospitalists encounter patients at high‐risk for PAD whose DRG for admission might be unrelated to this disease. Nonetheless, hospitalists have the opportunity to improve patient outcomes by being capable of screening for undiagnosed PAD and initiating appropriate interventions to reduce the risk of life‐threatening cardiovascular events.
DIAGNOSIS
Peripheral arterial disease can be diagnosed by either noninvasive or invasive methods. The ankle‐to‐brachial index (ABI) is an accurate, practical, inexpensive, and noninvasive method for detecting PAD.1 The sensitivity of ABI in detecting PAD is 95% with 99% specificity,10 which makes the method superior to other indicators (eg, absence of a pedal pulse, presence of a femoral arterial bruit, slow venous filling, or cold/abnormally colored skin) assessed during a physical examination.11 Under normal conditions, the systolic pressure at the ankle should be equal to or greater than that recorded from the upper arm. As PAD narrows arteries, the systolic pressure decreases at sites distal to the area of arterial narrowing. A resting ABI is quantified by taking 2 readings each of ankle and brachial blood pressures with a handheld Doppler device while the patient is supine and dividing the highest ankle systolic pressure by the highest brachial pressure.12
An ABI between 0.9 and 1.30 is considered normal. Ratios between 0.7 and 0.89 indicate mild PAD, 0.4 and 0.69 moderate PAD, and an ABI 0.4 severe PAD when patients are more likely to have ischemic pain when at rest. An ABI > 1.3 usually indicates the presence of noncompressible vessels, which can be common in the elderly and patients with diabetes mellitus who have calcification of the distal arteries.1, 2 The ABI is also inversely related to the number of atherosclerotic risk factors and the risk of adverse cardiovascular events and death.6, 1316 To identify individuals with suspected or asymptomatic lower‐extremity PAD, ABI has a class I recommendation from the American College of Cardiology and American Heart Association (ACC/AHA) for patients who present with leg symptoms, who are 70 years and older, or who are 50 years and older with a history of smoking or diabetes.2 This enables physicians to make therapeutic interventions to reduce the risk of adverse vascular events in these patient cohorts.
Additional detection methods for PAD include measuring the ABI before and after exercise on a treadmill, if the patient is ambulatory, or exercise by performing 50 repetitions of raising the heels maximally off the floor, if the patient is not ambulatory. These tests determine the extent of claudication.2 Duplex ultrasound is used to establish the location and severity of stenosis and to follow PAD progression.2
Invasive evaluations for PAD are used primarily to confirm an initial diagnosis of PAD and assess its severity. These methods include a conventional angiogram, which is the most readily available and widely used technique for defining arterial stenosis. Magnetic resonance (MR) angiography with gadolinium and computed tomographic (CT) angiography are used to determine the location and degree of stenosis. Both MR and CT angiography have advantages and disadvantages but are considered interchangeable with one another in patients with contraindications to either method (Table 2).2
| Diagostic method | Benefits | Limitations |
|---|---|---|
| ||
| Magnetic resonance angiography (MRA) | Useful to assess PAD anatomy and presence of significant stenosis | Tends to overestimate degree of stenosis |
| Useful to select patients who are candidates for endovascular of surgical revascularization | May be inaccurate in arteries treated with metal stents | |
| Cannot be used in patients with contraindication to magnetic resonance technique | ||
| Computed tomographic angiography (CTA) | Useful to assess PAD anatomy and presence of significant stenosis | Single‐detector CT lacks accuracy to detect stenoses |
| Useful to select patients who are candidates for endovascular or surgical revascularization | Spatial Resolution lower than digital subtraction angiography | |
| Helpful to provide associated soft‐tissue diagnostic information that may be associated with PAD | Venous opacification can obscure arterial filling | |
| Patients with contraindications to MRA | Asymmetric opacification of legs may obscure arterial phase in some vessels | |
| Metal clips, stents, and prostheses do not cause significant CTA artifacts | Accuracy and effectiveness not as well determined as MRA | |
| Scan times are significantly faster | Treatment plans based on CTA have not been compared to those of catheter angiography | |
| Requires contrast and radiation | ||
| Use may be limited in individuals with renal dysfunction | ||
ANTIPLATELET THERAPY FOR REDUCTION OF VASCULAR EVENTS
Hospitalists utilize a wide array of therapies to treat and manage PAD. Acute complications of PAD may require interventions to prevent tissue loss or infection, revascularization procedures, or surgical amputation. Treatment of mild to moderate PAD focuses on atherothrombotic risk factor management, exercise therapy to improve limb function, and interventions to reduce the risk of adverse vascular events.2, 9 The remainder of this report focuses on the role of antiplatelet therapy (eg, aspirin and thienopyridines) in reducing the risk of vascular events in patients with PAD.
The Antiplatelet Trialists' Collaboration performed an overview analysis of randomized trials conducted prior to 1990 in order to determine the association of prolonged antiplatelet therapy with the occurrence of major vascular events. As a whole, therapies thought to act through inhibition of platelet aggregation, adhesion, or both reduced the incidence of vascular events by 33% in patients with PAD and those at high risk, and by 25% in all patient groups. Antiplatelet agents were also well tolerated; the absolute risk of fatal or nonmajor hemorrhage was low.17
A similar meta‐analysis was conducted of antiplatelet therapies in high‐risk patients with atherothrombosis by the Antithrombotic Trialists' Collaboration. Antiplatelet therapies taken together reduced the odds of patients experiencing vascular events by 22% (SE = 2%) across all trials and 23% (SE = 8%) in patients with PAD.18 Similar to the Antiplatelet Trialists' Collaboration study, the absolute risk of major and minor bleeding was low compared to the benefits of antiplatelet therapy.18 The results of these studies provide supporting evidence for the ACC/AHA class I recommendation for the use of antiplatelet therapy to reduce the risk of MI, stroke, or vascular death in patients with PAD.
The Antithrombotic Trialists' Collaboration also examined the risk reduction associated with a specific antiplatelet agent, aspirin. All doses of aspirin (75‐150, 160‐325, and 500‐1500 mg/day) reduced the odds by 23% (SE = 2%); high doses were no more effective than medium or low doses.18 Although the effects of aspirin was not analyzed in a subgroup analysis of patients with PAD, this study and others support the ACC/AHA class I recommendations for the use of aspirin to reduce the risk of MI, stroke, or vascular death in patients with PAD.2, 1921
The CAPRIE trial compared the efficacy of another antiplatelet agent, clopidogrel, against aspirin in patients with PAD.22 Patients with a history of recent ischemic stroke, MI, or symptomatic PAD were randomized to receive either clopidogrel (75 mg/day) or aspirin (325 mg/day) for 1‐3 years (mean follow‐up time, 1.91 years). Study outcomes were the incidence of nonfatal MI, ischemic stroke, hemorrhagic stroke, leg amputation, and vascular deaths. The absolute risk reduction for all patients was 8.7% (95% confidence interval [CI], 0.3%‐16.5%) in favor of clopidogrel over aspirin. Moreover, subgroup analysis in patients with PAD revealed that clopidogrel reduced the risk of a vascular event by 23.8% (95% CI, 8.9%‐36.2%; P = 0.0028) compared with aspirin (Fig. 2). Clopidogrel and aspirin had similar safety profiles, but other studies have revealed bleeding incidence is numerically greater in patients treated with clopidogrel.2224 Although the CAPRIE trial is the only study to date to compare the efficacy of clopidogrel over aspirin in reducing vascular event in patients with PAD, its outcomes underlie the class I ACC/AHA recommendation for clopidogrel (75 mg/day) as an effective alternative to aspirin to reduce the risk of MI, stroke, or death in patients with PAD.2

CONCLUSIONS
Despite the availability of accurate, practical, and inexpensive diagnostic testing, PAD remains underdiagnosed and undertreated. Early detection of PAD and subsequent intervention by hospitalists are important because peripheral arterial disease is strongly associated with an increased risk of mortality and morbidity from adverse vascular events. The ACC/AHA recommends screening for asymptomatic patients at risk for this disease so that therapies that reduce the risk of an MI, stroke, or vascular death can be administered immediately. Antiplatelet agents reduce the risk of adverse vascular events in patients with PAD. The use of aspirin or clopidogrel is recommended in this cohort of patients. However, further study is necessary to determine the efficacy and safety of combination therapy with aspirin and clopidogrel in patients with PAD. It is also important to note that coordination of care between hospitalists and cardiologists is critical in the management of patients with this disease. However, the appropriate handoff of patients between these 2 groups of physicians depends on the local expertise and support structure of these health care professionals. Thus, an interdisciplinary approach utilizing guideline‐based patient care will allow hospitalists to refer patients accordingly, ensuring optimal outcomes in patients with PAD.
Peripheral arterial disease (PAD) is defined by the presence of stenosis or occlusion in peripheral arterial beds.1, 2 Based on large population‐based screening surveys, the prevalence of this disease ranges between 5.5% and 26.7% and is dependent on age, atherothrombotic risk factors, and the coexistence of other atherothrombotic diseases.35 Symptoms of PAD include mild to intermittent claudication, ischemic rest pain, and tissue loss.2 Disease severity is classified according to either Fontaine's stages or Rutherford categories. These categorization schema have value in improving communication between physicians, which is important in ensuring continuity of care between the inpatient and outpatient settings (Table 1).2
| Stage | Fontaine | Rutherford | ||
|---|---|---|---|---|
| Clinical | Grade | Category | Clinical | |
| ||||
| I | Asymptomatic | 0 | 0 | Asymptomatic |
| IIa | Mild claudication | I | 1 | Mild claudication |
| IIb | Moderate‐severe claudication | I | 2 | Moderate claudication |
| III | Ischemic rest pain | I | 3 | Severe claudication |
| IV | Ulceration or gangrene | II | 4 | Ischemic rest pain |
| III | 5 | Minor tissue loss | ||
| IV | 6 | Ulceration or gangrene | ||
Patients with PAD are at increased risk of dying from or experiencing a cardiovascular event.68 Among patients diagnosed with PAD, coronary artery disease (CAD), or cerebrovascular disease (CVD), those with PAD have the highest 1‐year rate of cardiovascular death, MI, stroke, or vascular‐related hospitalization (Fig. 1).8 This risk is attributable in part to the high rate of association of PAD with other atherothrombotic diseases. The Reduction of Atherothrombosis for Continued Health (REACH) Registry found that approximately 60% of participants with documented PAD have polyvascular disease, defined by the coexistence of CAD and/or CVD. In comparison, 25% of participants with CAD and 40% of participants with CVD have polyvascular disease.8 Thus, PAD can be considered a powerful indicator of systemic atherothrombotic disease and a predictor of cardiovascular and cerebrovascular morbidity and mortality.1

Unfortunately, asymptomatic PAD is more common than its symptomatic counterpart.3 In addition, symptomatic patients often fail to notify their physicians about PAD‐associated symptoms because they attribute them to aging.3 As a result, this disease is underdiagnosed and undertreated.1 Accordingly, several medical associations and physician task forces have called for an increase in screening for PAD in at‐risk populations that include: patients older than 70, patients older than 50 who have concomitant atherothrombotic risk factors, and patients with atherothrombotic disease of single or multiple vascular beds.1, 9 In many cases hospitalists encounter patients at high‐risk for PAD whose DRG for admission might be unrelated to this disease. Nonetheless, hospitalists have the opportunity to improve patient outcomes by being capable of screening for undiagnosed PAD and initiating appropriate interventions to reduce the risk of life‐threatening cardiovascular events.
DIAGNOSIS
Peripheral arterial disease can be diagnosed by either noninvasive or invasive methods. The ankle‐to‐brachial index (ABI) is an accurate, practical, inexpensive, and noninvasive method for detecting PAD.1 The sensitivity of ABI in detecting PAD is 95% with 99% specificity,10 which makes the method superior to other indicators (eg, absence of a pedal pulse, presence of a femoral arterial bruit, slow venous filling, or cold/abnormally colored skin) assessed during a physical examination.11 Under normal conditions, the systolic pressure at the ankle should be equal to or greater than that recorded from the upper arm. As PAD narrows arteries, the systolic pressure decreases at sites distal to the area of arterial narrowing. A resting ABI is quantified by taking 2 readings each of ankle and brachial blood pressures with a handheld Doppler device while the patient is supine and dividing the highest ankle systolic pressure by the highest brachial pressure.12
An ABI between 0.9 and 1.30 is considered normal. Ratios between 0.7 and 0.89 indicate mild PAD, 0.4 and 0.69 moderate PAD, and an ABI 0.4 severe PAD when patients are more likely to have ischemic pain when at rest. An ABI > 1.3 usually indicates the presence of noncompressible vessels, which can be common in the elderly and patients with diabetes mellitus who have calcification of the distal arteries.1, 2 The ABI is also inversely related to the number of atherosclerotic risk factors and the risk of adverse cardiovascular events and death.6, 1316 To identify individuals with suspected or asymptomatic lower‐extremity PAD, ABI has a class I recommendation from the American College of Cardiology and American Heart Association (ACC/AHA) for patients who present with leg symptoms, who are 70 years and older, or who are 50 years and older with a history of smoking or diabetes.2 This enables physicians to make therapeutic interventions to reduce the risk of adverse vascular events in these patient cohorts.
Additional detection methods for PAD include measuring the ABI before and after exercise on a treadmill, if the patient is ambulatory, or exercise by performing 50 repetitions of raising the heels maximally off the floor, if the patient is not ambulatory. These tests determine the extent of claudication.2 Duplex ultrasound is used to establish the location and severity of stenosis and to follow PAD progression.2
Invasive evaluations for PAD are used primarily to confirm an initial diagnosis of PAD and assess its severity. These methods include a conventional angiogram, which is the most readily available and widely used technique for defining arterial stenosis. Magnetic resonance (MR) angiography with gadolinium and computed tomographic (CT) angiography are used to determine the location and degree of stenosis. Both MR and CT angiography have advantages and disadvantages but are considered interchangeable with one another in patients with contraindications to either method (Table 2).2
| Diagostic method | Benefits | Limitations |
|---|---|---|
| ||
| Magnetic resonance angiography (MRA) | Useful to assess PAD anatomy and presence of significant stenosis | Tends to overestimate degree of stenosis |
| Useful to select patients who are candidates for endovascular of surgical revascularization | May be inaccurate in arteries treated with metal stents | |
| Cannot be used in patients with contraindication to magnetic resonance technique | ||
| Computed tomographic angiography (CTA) | Useful to assess PAD anatomy and presence of significant stenosis | Single‐detector CT lacks accuracy to detect stenoses |
| Useful to select patients who are candidates for endovascular or surgical revascularization | Spatial Resolution lower than digital subtraction angiography | |
| Helpful to provide associated soft‐tissue diagnostic information that may be associated with PAD | Venous opacification can obscure arterial filling | |
| Patients with contraindications to MRA | Asymmetric opacification of legs may obscure arterial phase in some vessels | |
| Metal clips, stents, and prostheses do not cause significant CTA artifacts | Accuracy and effectiveness not as well determined as MRA | |
| Scan times are significantly faster | Treatment plans based on CTA have not been compared to those of catheter angiography | |
| Requires contrast and radiation | ||
| Use may be limited in individuals with renal dysfunction | ||
ANTIPLATELET THERAPY FOR REDUCTION OF VASCULAR EVENTS
Hospitalists utilize a wide array of therapies to treat and manage PAD. Acute complications of PAD may require interventions to prevent tissue loss or infection, revascularization procedures, or surgical amputation. Treatment of mild to moderate PAD focuses on atherothrombotic risk factor management, exercise therapy to improve limb function, and interventions to reduce the risk of adverse vascular events.2, 9 The remainder of this report focuses on the role of antiplatelet therapy (eg, aspirin and thienopyridines) in reducing the risk of vascular events in patients with PAD.
The Antiplatelet Trialists' Collaboration performed an overview analysis of randomized trials conducted prior to 1990 in order to determine the association of prolonged antiplatelet therapy with the occurrence of major vascular events. As a whole, therapies thought to act through inhibition of platelet aggregation, adhesion, or both reduced the incidence of vascular events by 33% in patients with PAD and those at high risk, and by 25% in all patient groups. Antiplatelet agents were also well tolerated; the absolute risk of fatal or nonmajor hemorrhage was low.17
A similar meta‐analysis was conducted of antiplatelet therapies in high‐risk patients with atherothrombosis by the Antithrombotic Trialists' Collaboration. Antiplatelet therapies taken together reduced the odds of patients experiencing vascular events by 22% (SE = 2%) across all trials and 23% (SE = 8%) in patients with PAD.18 Similar to the Antiplatelet Trialists' Collaboration study, the absolute risk of major and minor bleeding was low compared to the benefits of antiplatelet therapy.18 The results of these studies provide supporting evidence for the ACC/AHA class I recommendation for the use of antiplatelet therapy to reduce the risk of MI, stroke, or vascular death in patients with PAD.
The Antithrombotic Trialists' Collaboration also examined the risk reduction associated with a specific antiplatelet agent, aspirin. All doses of aspirin (75‐150, 160‐325, and 500‐1500 mg/day) reduced the odds by 23% (SE = 2%); high doses were no more effective than medium or low doses.18 Although the effects of aspirin was not analyzed in a subgroup analysis of patients with PAD, this study and others support the ACC/AHA class I recommendations for the use of aspirin to reduce the risk of MI, stroke, or vascular death in patients with PAD.2, 1921
The CAPRIE trial compared the efficacy of another antiplatelet agent, clopidogrel, against aspirin in patients with PAD.22 Patients with a history of recent ischemic stroke, MI, or symptomatic PAD were randomized to receive either clopidogrel (75 mg/day) or aspirin (325 mg/day) for 1‐3 years (mean follow‐up time, 1.91 years). Study outcomes were the incidence of nonfatal MI, ischemic stroke, hemorrhagic stroke, leg amputation, and vascular deaths. The absolute risk reduction for all patients was 8.7% (95% confidence interval [CI], 0.3%‐16.5%) in favor of clopidogrel over aspirin. Moreover, subgroup analysis in patients with PAD revealed that clopidogrel reduced the risk of a vascular event by 23.8% (95% CI, 8.9%‐36.2%; P = 0.0028) compared with aspirin (Fig. 2). Clopidogrel and aspirin had similar safety profiles, but other studies have revealed bleeding incidence is numerically greater in patients treated with clopidogrel.2224 Although the CAPRIE trial is the only study to date to compare the efficacy of clopidogrel over aspirin in reducing vascular event in patients with PAD, its outcomes underlie the class I ACC/AHA recommendation for clopidogrel (75 mg/day) as an effective alternative to aspirin to reduce the risk of MI, stroke, or death in patients with PAD.2

CONCLUSIONS
Despite the availability of accurate, practical, and inexpensive diagnostic testing, PAD remains underdiagnosed and undertreated. Early detection of PAD and subsequent intervention by hospitalists are important because peripheral arterial disease is strongly associated with an increased risk of mortality and morbidity from adverse vascular events. The ACC/AHA recommends screening for asymptomatic patients at risk for this disease so that therapies that reduce the risk of an MI, stroke, or vascular death can be administered immediately. Antiplatelet agents reduce the risk of adverse vascular events in patients with PAD. The use of aspirin or clopidogrel is recommended in this cohort of patients. However, further study is necessary to determine the efficacy and safety of combination therapy with aspirin and clopidogrel in patients with PAD. It is also important to note that coordination of care between hospitalists and cardiologists is critical in the management of patients with this disease. However, the appropriate handoff of patients between these 2 groups of physicians depends on the local expertise and support structure of these health care professionals. Thus, an interdisciplinary approach utilizing guideline‐based patient care will allow hospitalists to refer patients accordingly, ensuring optimal outcomes in patients with PAD.
- ,,, et al.Prevention of Atherothrombotic Disease Network. Critical issues in peripheral arterial disease detection and management: a call to action.Arch Intern Med.2003;163:884–892.
- ,,, et al.ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic).Circulation.2006;113:e463–e654.
- ,,,,,.Peripheral arterial disease in the elderly: the Rotterdam Study.Arterioscler Thromb Vasc Biol.1998;18:185–192.
- ,,, et al.Peripheral arterial disease detection, awareness, and treatment in primary care.JAMA.2001;286:1317–1324.
- ,.Prevalence of and risk factors for peripheral arterial disease in the United States: Results from the National Health and Nutrition Examination Survey, 1999‐2000.Circulation.2004;110:738–743.
- ,,, et al.Mortality over a period of 10 years in patients with peripheral arterial disease.N Engl J Med.1992;326:381–386.
- ,.Vascular event rates in patients with atherosclerotic cerebrovascular disease.Arch Neurol.1992;49:857–863.
- ,,, et al.;REACH Registry Investigators. One‐year cardiovascular event rates in outpatients with atherothrombosis.JAMA.2007;297:1197–1206.
- ,,, et al.Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: A critical review.Circulation.1996;94:3026–3049.
- ,.Management of peripheral arterial disease (PAD): TASC Working Group. TransAtlantic Inter‐Society Consensus (TASC).J Vasc Surg.2000:31(1Pt 2):S1–S296.
- ,.Physical examination and chronic lower‐extremity ischemia.Arch Intern Med.1998;158:1357–1364.
- .Medical treatment of peripheral artery disease and claudication.N Engl J Med.2001;344:1608–1621.
- ,,, et al.Ankle‐arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group.Circulation.1993;88:837–845.
- ,,,.Morbidity and mortality in hypertensive adults with a low ankle/arm blood pressure index.JAMA.1993;270:487–489.
- ,,, et al.Ankle‐arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group.Arterioscler Thromb Vasc Biol.1999;19:538–545.
- ,,,,,;Framingham Study. The ankle‐brachial index in the elderly and risk of stroke, coronary disease, and death: the Framingham Study.Arch Intern Med.2003;163:1939–1942.
- Antiplatelet Trialists' Collaboration.Collaborative overview of randomized trials of antiplatelet therapy—1: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients.BMJ.1994;308:81–106.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.BMJ.2002;324:71–86.
- The Medical Research Council's General Practice Research Framework.Thrombosis prevention trial: randomised trial of low‐intensity oral anticoagulation with warfarin and low‐dose aspirin in the primary prevention of ischemic heart disease in men at increased risk.Lancet.1998;351:233–241.
- ,,, for theHOT Study Group.Effects of intensive blood pressure lowering and low‐dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial.Lancet1998;280:1930–1935.
- Collaborative Group of the Primary Prevention Project (PPP).Low‐dose aspirin and vitamin E in people at cardiovascular risk: a randomized trial in general practice.Lancet.2001;357:89–95.
- CAPRIE Steering Committee.A randomized, blinded, trial of clopidogrel versus aspirin in patients at risk of ischemic events (CAPRIE).Lancet.1996;348:1329–1339.
- ,,; for theCHARISMA Investigators.Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events.N Engl J Med.2006;354:1706–1717.
- ,,; on behalf of theMATCH investigators.Aspirin and clopidogrel compared with clopidogrel alone after ischaemic stroke or transient ischaemic attack in high‐risk patients (MATCH): randomised, double‐blind, placebo‐controlled trial.Lancet.2004;364:331–337.
- ,,, et al.Prevention of Atherothrombotic Disease Network. Critical issues in peripheral arterial disease detection and management: a call to action.Arch Intern Med.2003;163:884–892.
- ,,, et al.ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic).Circulation.2006;113:e463–e654.
- ,,,,,.Peripheral arterial disease in the elderly: the Rotterdam Study.Arterioscler Thromb Vasc Biol.1998;18:185–192.
- ,,, et al.Peripheral arterial disease detection, awareness, and treatment in primary care.JAMA.2001;286:1317–1324.
- ,.Prevalence of and risk factors for peripheral arterial disease in the United States: Results from the National Health and Nutrition Examination Survey, 1999‐2000.Circulation.2004;110:738–743.
- ,,, et al.Mortality over a period of 10 years in patients with peripheral arterial disease.N Engl J Med.1992;326:381–386.
- ,.Vascular event rates in patients with atherosclerotic cerebrovascular disease.Arch Neurol.1992;49:857–863.
- ,,, et al.;REACH Registry Investigators. One‐year cardiovascular event rates in outpatients with atherothrombosis.JAMA.2007;297:1197–1206.
- ,,, et al.Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: A critical review.Circulation.1996;94:3026–3049.
- ,.Management of peripheral arterial disease (PAD): TASC Working Group. TransAtlantic Inter‐Society Consensus (TASC).J Vasc Surg.2000:31(1Pt 2):S1–S296.
- ,.Physical examination and chronic lower‐extremity ischemia.Arch Intern Med.1998;158:1357–1364.
- .Medical treatment of peripheral artery disease and claudication.N Engl J Med.2001;344:1608–1621.
- ,,, et al.Ankle‐arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group.Circulation.1993;88:837–845.
- ,,,.Morbidity and mortality in hypertensive adults with a low ankle/arm blood pressure index.JAMA.1993;270:487–489.
- ,,, et al.Ankle‐arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group.Arterioscler Thromb Vasc Biol.1999;19:538–545.
- ,,,,,;Framingham Study. The ankle‐brachial index in the elderly and risk of stroke, coronary disease, and death: the Framingham Study.Arch Intern Med.2003;163:1939–1942.
- Antiplatelet Trialists' Collaboration.Collaborative overview of randomized trials of antiplatelet therapy—1: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients.BMJ.1994;308:81–106.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.BMJ.2002;324:71–86.
- The Medical Research Council's General Practice Research Framework.Thrombosis prevention trial: randomised trial of low‐intensity oral anticoagulation with warfarin and low‐dose aspirin in the primary prevention of ischemic heart disease in men at increased risk.Lancet.1998;351:233–241.
- ,,, for theHOT Study Group.Effects of intensive blood pressure lowering and low‐dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial.Lancet1998;280:1930–1935.
- Collaborative Group of the Primary Prevention Project (PPP).Low‐dose aspirin and vitamin E in people at cardiovascular risk: a randomized trial in general practice.Lancet.2001;357:89–95.
- CAPRIE Steering Committee.A randomized, blinded, trial of clopidogrel versus aspirin in patients at risk of ischemic events (CAPRIE).Lancet.1996;348:1329–1339.
- ,,; for theCHARISMA Investigators.Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events.N Engl J Med.2006;354:1706–1717.
- ,,; on behalf of theMATCH investigators.Aspirin and clopidogrel compared with clopidogrel alone after ischaemic stroke or transient ischaemic attack in high‐risk patients (MATCH): randomised, double‐blind, placebo‐controlled trial.Lancet.2004;364:331–337.
Rules of Engagement: Stroke
Stroke is the leading cause of disability and the third leading cause of death in the United States.1, 2 Each year approximately 700,000 strokes occur, 88% of which are considered ischemic; they predominately arise from atherothrombotic events in large or small cerebral vessels. Moreover, approximately 200,000 of these events are classified as recurrent.1 Patients who have had a stroke frequently also have coronary artery disease (CAD) and/or peripheral artery disease (PAD), putting them at high risk of adverse vascular events such as myocardial infarction (MI) or sudden vascular death.35 Hospitalists initiate and coordinate aggressive and rapid interventions in the acute care setting in order to minimize stroke progression and thus optimize outcomes. They also initiate long‐term treatments to prevent recurrence and secondary vascular events in the outpatient setting. Thus, the treatment plan developed by the hospitalist on admission is as important as the one created on discharge.
The hospitalist plays a central role in managing stroke. Prior to having an event, patients are at risk. The goal of clinical management is prevention. This is mainly focused on risk factor reduction and aspirin therapy. Outpatient medical providers direct this care. Once a stroke occurs and the victim is admitted to the hospital, the hospitalist becomes this patient's medical care coordinator. In the very acute phase, the goal of management is optimizing outcomes by restoring perfusion to ischemic tissue and minimizing injury progression. There are a number of interventions available to the hospitalist. If patients present within 3 hours of ictus, they may qualify for IV thrombolytic therapy and if within 6 hours for intra‐arterial therapy. If later, aspirin can have beneficial effects on outcomes. Also during this time, it is important to maintain adequate systemic perfusion, oxygenation/ventilation, cardiovascular function, and, importantly, close clinical monitoring.
STROKE MORTALITY
Stroke is a deadly diseaseas deadly as many malignancies. Most patients die of complications of vascular disease (eg, cerebrovascular, cardiovascular, and peripheral vascular diseases). The Oxfordshire Community Stroke Project and Perth Community Stroke Study has indicated that at least 50% of patients die within 5 years of a first‐time acute ischemic or hemorrhagic stroke. The highest risk of death occurs during the first year, with a mortality rate ranging between 31% and 36.5% (95% confidence interval [CI], 27%34% and 31.5%41.4%, respectively).6, 7 Moreover, the risk of death within 30 days after stroke was approximately 20%. The annual risk of death for patients who survived 1 year was 7% and 10% according to the Oxfordshire and Perth studies, respectively, which was approximately 2‐fold higher than that for stroke‐free patients of the same age and sex.6, 7
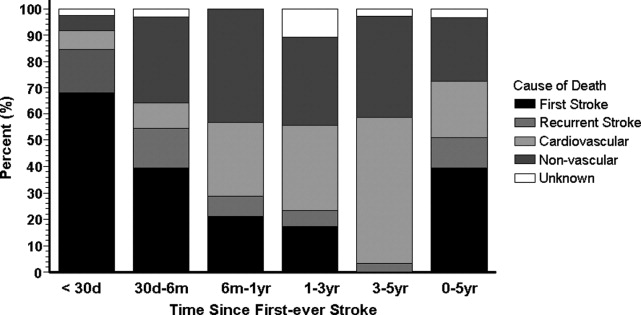
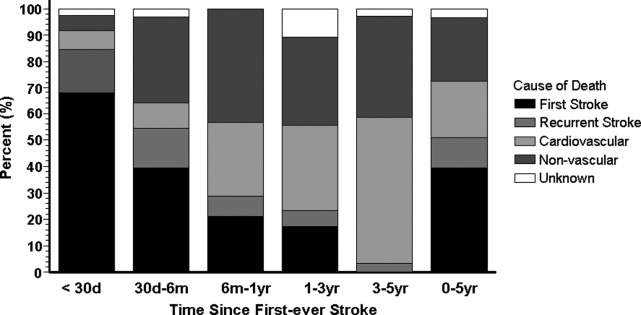
The proportion of death caused by stroke, recurrent stroke, cardiovascular events, or nonvascular events changes over time (Fig. 1). The Perth study showed that the predominant causes of death within the first 30 days were complications from the incident stroke and, to a lesser degree, recurrent stroke. Over time, cardiovascular events (eg, myocardial infarction, ruptured aortic aneurysms, PAD) become the most common cause of mortality in patients who have had a stroke. However, the risk of death from a recurrent stroke only diminishes slightly with time.7 This trend is consistent with the findings of the Oxfordshire study and the Northern Manhattan Stroke Study, which focused on long‐term survival after first‐ever ischemic stroke.6, 8 Thus, the short‐term goals of treatment implemented by hospitalists are to ensure survival and recovery from the index stroke, and the long‐term goals are to protect against recurrent stroke or secondary vascular events.
MANAGEMENT OF ACUTE ISCHEMIC STROKE
Stroke is no longer an untreatable disease. The introduction of thrombolytic therapy has provided an opportunity for medical providers to significantly improve short‐ and long‐term survival rates and functional outcomes of patients. Most ischemic strokes are caused by thrombotic arterial occlusions. Hence, thrombolytic therapy has been tested and approved for use in patients with acute ischemic stroke.9 The efficacy and safety of the thrombolytic agent, recombinant tissue plasminogen activator (rtPA), were demonstrated in the landmark National Institute of Neurological Disorders and Stroke (NINDS) rtPA Stroke Study.
When compared with patients who received placebo, the odds of a favorable treatment outcome increased by at least 30% in those who received rtPA within 3 hours of the onset of symptoms of an acute ischemic stroke. This benefit was sustained for 612 months.10, 11 Patients who received rtPA were at an increased risk for intracerebral hemorrhage, but this did not translate to an increased risk of death.10 Currently, this thrombolytic agent has a class I recommendation from the American Heart Association and American Stroke Association (AHA/ASA) for its administration within 3 hours of onset of ischemic stroke symptoms in patients who have no sign or history of subarachnoid hemorrhage and who meet the other 21 criteria based on those used in the NINDS study.9
Patients who arrive at the hospital 36 hours after symptom onset or those who have contraindications for IV rtPA may benefit from intra‐arterial administration of thrombolytic agents.12 However, there is no consensus on the optimal dose that should be delivered by intra‐arterial administration.13 In addition, this course of treatment requires rapid access to cerebral angiography and a qualified interventionalist, both of which may not be available to all hospitalists.9
If a patient presents beyond 6 hours, the hospitalist may initiate aspirin therapy, which has been shown to improve outcomes following acute stroke if therapy is begun within 48 hours. A planned meta‐analysis of approximately 40,000 patients with suspected ischemic stroke demonstrated that aspirin therapy proportionally reduces the risk of recurrent stroke and mortality from recurrent stroke or any other cause by 11% 3%. This benefit was apparent as early as 06 hours and as late as 2548 hours following stroke onset (Fig. 2), with only a slight increase in the risk of hemorrhagic stroke.14 The studies analyzed in the meta‐analysis underlie the AHA/ASA recommendations that aspirin (325 mg) be administered within 2448 hours of stroke onset or within 24 hours after thrombolytic therapy for the early management of ischemic stroke in adults.9 By contrast, heparin therapy is not a recommended treatment for acute ischemic stroke; its clinical benefits do not outweigh the risk of bleeding complications.9 In addition, clinical trial data do not support the use of heparin for cardioembolic stroke.13
The AHA/ASA has made several recommendations to enhance outcomes and to prevent complications after an acute ischemic stroke. These include the stabilization and management of blood pressure (BP) and blood glucose levels and protection against deep vein thrombosis.9 Hypertension in the peristroke period is expected and is generally not treated. The rationale is that cerebral blood flow (CBF) is autoregulated in healthy brain tissue. As such, CBF remains constant at 50 cc/100 g of tissue per minute over a wide range of mean arterial pressures: 60150 mm Hg. However, in ischemic brain regions, autoregulation is lost, resulting in a pressure passive perfusion state (ie, local CBF is dependent on systemic blood pressure). As an injured brain is hypermetabolic, CBF adequate to meet its needs is dependent on a higher than normal blood pressure. Thus, reduction of high BP might worsen ischemia.
From a clinical practice standpoint, patients' outpatient antihypertensive medications are frequently held, with no additional treatment given for blood pressure elevation. The exception is, should the patient become encephalopathic, blood pressure may need to be reduced, as this may represent a state of hypertensive encephalopathy or luxury perfusion. There are no data indicating the use of a specific hypertensive agent in reducing blood pressure in such a setting. The AHA/ASA guidelines for early management of ischemic stroke recommend the use of antihypertensive agents on a case‐by‐case basis; although as recommended by consensus, there may be IV administration of labetalol or nicardipine if there is evidence of hypertensive encephalopathy, the diastolic BP is >120 mm Hg or the systolic BP is >220 mm Hg.9
Blood glucose should be kept stable, between 80 and 120 mg/dL. This can be achieved with either an oral hypoglycemic agent or sliding‐scale insulin regimen. Venous thrombus formation after stroke is a very serious concern as it can result in pulmonary embolism. As soon as possible, sequential compression devices and agents such as unfractionated heparin, low‐molecular‐weight heparin (ie, enoxaparin, dalteparin), fondaparinux, warfarin, or aspirin should be initiated.9
Hyperthermia has been shown to worsen functional outcome following stroke.15 Thus, maintenance of normal body temperature is recommended. This can be achieved with acetaminophen. Causes other than acute brain injury such as infection need to be investigated and treated as appropriate. Induced hypothermia has long been considered a potential therapy for improving outcome from acute stroke. Although preclinical studies in animals support induced hypothermia as a beneficial approach, there has not yet been a successful human clinical trial demonstrating efficacy. In addition, hypotonic intravenous solutions have the potential to worsen cerebral edema. Thus, normal saline without dextrose may be preferable. However, conclusive evidence supporting the use of hypertonic and colloid solutions remains insufficient.
Other important issues are gastrointestinal prophylaxis, early mobilization, and nutrition. The nutritional needs of acute brain‐injured patients cannot be overemphasized. Caloric intake should be maintained at 140% to compensate for the hypermetabolic state of the brain and to avoid weight loss. Patients should not be fed or treated with oral medications until a speech and swallow study is conducted to determine the extent of dysphagia and dysarthria or aphasia.9 However, in general, patients who are alert can usually be administered their oral medications, but only after a swallow evaluation has been passed.
ANTIPLATELET THERAPY FOR STROKE PREVENTION
Primary Stroke Prevention
Aspirin has been shown to be efficacious in preventing first stroke in women. The evidence supporting aspirin use in women for primary prevention of stroke is from the Women's Health Study, which showed that the occurrence of first stroke could be reduced in women older than 45 years old by taking 100 mg of aspirin every other day as compared with placebo.16 The AHA/ASA recommends aspirin therapy for primary ischemic stroke prevention in women whose risk of stroke outweighs the risk of aspirin‐related bleeding. Unfortunately, there are not enough supporting data to recommend its use in men for primary stroke prevention.17
Secondary Stroke Prevention
Aspirin, clopidogrel, and the extended‐release dipyridamole‐aspirin combination are the most commonly used antiplatelet agents for secondary stroke prevention. Ticlopidine is indicated for prevention of recurrent stroke18 but has fallen out of use because of safety concerns, and dipyridamole confers little cardiovascular protection compared with the other antiplatelet agents. Aspirin is widely regarded as the first‐line agent for preventing recurrent stroke. The optimal dose of aspirin for reducing the risk of secondary stroke is uncertain. However, most practitioners use doses between 75 and 325 mg. The numerous studies supporting this have been summarized by Hennekens et al.19 The Antiplatelet Trialists Collaboration demonstrated that lower‐dose aspirin (75150 mg) is effective and can reduce secondary stroke by 25%.20 The European Stroke Prevention Study 2 (ESPS‐2) showed an 18% reduction in the risk of a recurrent stroke with only 50 mg of aspirin.21 The AHA/ASA recommends 50350 mg/day aspirin to reduce the risk of recurrent stroke and or vascular events in patients with ischemic stroke.5
In the CAPRIE study, clopidogrel was shown to be effective, but not superior to aspirin, in the reduction of recurrent stroke.22 Taking their similar safety and efficacy profiles into account and aspirin's low cost, the AHA/ASA concluded that clopidogrel is an acceptable but not preferable alternative to aspirin therapy for the reduction of recurrent strokes.5 The combination of clopidogrel and aspirin reduces secondary vascular events in high‐risk cardiovascular patients and can be considered in high‐risk stroke patients. The CHARISMA study revealed that a combination of clopidogrel and aspirin has benefit over aspirin alone in secondary prevention of a combined end point of stroke, MI, and CV death.23 However, this same study also showed that aspirin alone is superior to the combination in primary prevention of this same end point. Subgroup analysis demonstrated that the combination of clopidogrel and aspirin provided a significant benefit in further reducing nonfatal strokes over aspirin alone (P .05) and a trend toward reducing all ischemic strokes (P .10).24 The MATCH study showed no evidence that a combination of clopidogrel and aspirin was superior to aspirin alone in patients with recent TIA or stroke.25, 26 However, the impact of aspirin resistance in the MATCH study population was not quantified but may have affected the study results, as 80% of the patients were already taking aspirin on enrollment.24 Of significance is the finding in both CHARISMA and MATCH that the addition of aspirin to clopidogrel therapy conveys a higher risk for bleeding.26 Combining clopidogrel with aspirin therapy is not routinely recommended by the AHA/ASA to reduce the risk of recurrent stroke.5
The ESPS‐2 trial demonstrated that the combination of extended‐release (ER) dipyridamole and aspirin was superior to aspirin alone for reducing the risk of recurrent stroke in patients with ischemic stroke.21 However, the combination of ER dipyridamole and aspirin was not different from placebo in preventing myocardial infarction or CV death. Thus, the AHA/ASA recommends that the combination of ER‐dipyridamole/aspirin can be considered for secondary stroke prevention.5
LONG‐TERM MANAGEMENT FOR SECONDARY PREVENTION OF NONSTROKE VASCULAR EVENTS
In the subacute period, the hospitalist transitions the patient from acute to chronic care. Here, the goals are optimizing functional outcome and preventing recurrence. Still, during the first few days after ictus, the patient remains at risk for recurrent stroke, cerebral edema, and hemorrhagic transformation, so continued hospitalization is required. By 57 days later, the most significant risk period has elapsed. Physical and occupational therapy are initiated while patients are still hospitalized. Patient and family education about stroke and related diseases is done. A rational and comprehensive plan to reduce risk of secondary stroke is critical. This plan must include diet, tobacco, diabetes, blood pressure and excessive weight interventions. These may require care from a specialized team with members such as dieticians, exercise therapists, and tobacco interventionalists. Especially critical is instituting a discharge plan that highlights continued control of all modifiable risk factors and antiplatelet therapy. Finally, coordination with the patient's outpatient provider is paramount.
There is a developing awareness of the importance of the overlapping syndrome of combined stroke and cardiovascular and peripheral vascular risk. In leading clinical trials, the coexistence of coronary artery disease and cerebral artery disease is as high as 40%; thus, patients who have had a stroke are at high risk for other vascular events such as MI, critical limb ischemia, or vascular death. The AHA/ASA scientific statement on coronary risk evaluation recommends testing for CAD after ischemic stroke, as it has been suggested that asymptomatic CAD is highly prevalent among these patients.4 Diagnostic testing for CAD should be conducted outside the acute stroke setting and optimized based on stroke subtype and the health status of individual patients.4 Testing for PAD should also be done in patients with ischemic stroke when not otherwise contraindicated.27 Thus, the hospitalist should determine the stroke patient's risk of having coexisting CAD and/or PAD. If significant, then appropriate follow‐up testing either during the hospitalization or after discharge should be arranged.
To prevent secondary vascular events including stroke, effective management of common risk factors shared by stroke, CAD, and PAD is recommended. Long‐term treatment goals include control of hypertension, lipid and glucose management, smoking cessation, weight control, and integration of physical activity.4, 5, 27 Except for blood pressure control, many of these should be initiated while still in the hospital. Acute hospitalization is also an opportunity for patient and family education regarding risk factor reduction.
Antiplatelet therapies are also recommended and are associated with an absolute risk reduction of serious vascular events of 36 6 per 1000 persons with previous stroke or transient ischemic attack.20 Aspirin use in patients at high risk for atherothrombotic events has been shown to be effective in reducing the risk of myocardial infarction and other vascular events.20 The AHA‐recommended dose of aspirin for preventing sudden coronary syndrome is 81 mg/day or higher. Clopidogrel has been shown to be effective in reducing the risk of recurrent sudden coronary artery syndrome and progression of peripheral vascular disease.22 When combined with aspirin, clopidogrel has been shown to reduce recurrent sudden coronary syndrome.28, 29
CONCLUSIONS
The hospitalist is involved in the spectrum of stroke care, from management of stroke in the acute care setting to establishing long‐term treatments for prevention of secondary vascular events. As such, hospitalists can significantly affect the lives of patients with ischemic stroke. Current treatment guidelines for stroke recommend aggressive and rapid response in the acute setting. Long‐term treatments focus on risk reduction for recurrent stroke or for other vascular events such as MI or critical limb ischemia. Antiplatelet therapies are a component of long‐term treatments. Current research suggests that antiplatelet agents differ in reducing recurrent strokes versus nonstroke events. Thus, treatments should be based on a patient's individual risk factors for recurrent stroke and/or CAD or PAD. Although hospitalists will transfer care back to outpatient providers, the interventions initiated in the hospital will optimize the patient's future. In many ways, the patient's first step to a better health began when crossing the entrance of the hospital.
- ,,, et al.Heart disease and stroke statistics—2006 update: a report from the American Heart Association statistics committee and stroke statistics subcommittee.Circulation.2006;113:85–151.
- ,,,.Trends in the leading causes of death in the United States, 1970–2002.JAMA.2005;294:1255–1259.
- ,,, et al.One‐year cardiovascular event rates in outpatients with atherothrombosis.JAMA.2007;297:1197–1206.
- ,,, et al.Coronary risk evaluation in patients with transient ischemic attack and ischemic stroke: a scientific statement for healthcare professionals from the Stroke Council and the Council on Clinical Cardiology of the American Heart Association/American Stroke Association.Circulation.2003;108:1278–1290.
- ,,, et al.Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: A statement for healthcare professionals from the American Heart Association/American Stroke Association.Stroke.2006;37:577–617.
- ,,,,,.Long‐term survival after first‐ever stroke: the Oxfordshire Community Stroke Project.Stroke.1993;24:796–800.
- ,,, et al.Five‐year survival after first‐ever stroke and related prognostic factors in the Perth Community Stroke Study.Stroke.2000;31:2080–2086.
- ,,, et al.Mortality and causes of death after first ischemic stroke: the Northern Manhattan Stroke Study.Neurology.2001;57:2000–2005.
- ,,, et al.Guidelines for the early management of adults with ischemic stroke: A guideline from the American Heart Association/American Stroke Association.Stroke.2007;38:1655–1711.
- NINDS study group.Tissue plasminogen activator for acute ischemic stroke.N Engl J Med.1995;333:1581–1587.
- ,,, et al.Effects of tissue plasminogen activator for acute ischemic stroke at one year.N Engl J Med.1999;340:1781–1787.
- ,,, et al.Intraarterial recombinant tissue plasminogen activator for ischemic stroke: an accelerating dosing regimen.Neurosurgery.2000;47:473–476.
- ,,,,.Antithrombotic and thrombolytic therapy for ischemic stroke: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:483s–512s.
- ,,, et al.Indications for early aspirin use in acute ischemic stroke: a combined analysis of 40000 randomized patients from the Chinese acute stroke trial and the international stroke trial.Stroke.2000;31:1240–1249.
- ,,, et al.Body temperature in acute stroke: relation to stroke severity, infarct size, mortality, and outcome.Lancet.1996;347:422–425.
- ,,, et al.A randomized trial of low‐dose aspirin in the primary prevention of cardiovascular disease in women.N Engl J Med.2005;352:1293–1304.
- ,,, et al.Primary prevention of ischemic stroke: A guideline from the American Heart Association/American Stroke Association.Circulation.2006;113:873–823.
- ,,, et al.A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high‐risk patients.N Engl J Med.1989;321:501–517.
- ,,.Aspirin as a therapeutic agent in cardiovascular disease: a statement for healthcare professionals from the American Heart Association.Circulation.1997;96:2751–2753.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.BMJ.2002;324:71–86.
- ,,,,,.European stroke prevention study:2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke.J Neurol Sci.1996;143:1–13.
- CAPRIE steering committee.A randomized, blinded, trial of clopidogrel versus aspirin in patients at risk of ischemic events (CAPRIE).Lancet.1996;348:1329–1339.
- ,,, et al.Clopidogrel and aspiring versus aspirin alone for the prevention of atherothrombotic events.N Engl J Med.2006;354:1706–1717.
- ,,, et al.Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial.J Am Coll Cardiol.2007;49:1982–1988.
- ,,, et al.Aspirin and clopidogrel compared with clopidogrel alone after recent ischemic stroke or transient ischemic attack in high‐risk patients (MATCH): Randomized, double‐blind placebo‐controlled trial.Lancet.2004;364:331–337.
- .Role of aspirin in MATCH.Lancet.2004;364:1661.
- ,,, et al.ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic).Circulation.2006;113:463–654.
- CURE Trial Investigators.Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST‐segment elevation.N Engl J Med.2001;345:494–502.
- ,,, et al.Effects of pretreatment with clopidogrel and aspirin followed by long‐term therapy in patients undergoing percutaneous coronary intervention: PCI‐CURE study.Lancet.2001;358:527–533.
Stroke is the leading cause of disability and the third leading cause of death in the United States.1, 2 Each year approximately 700,000 strokes occur, 88% of which are considered ischemic; they predominately arise from atherothrombotic events in large or small cerebral vessels. Moreover, approximately 200,000 of these events are classified as recurrent.1 Patients who have had a stroke frequently also have coronary artery disease (CAD) and/or peripheral artery disease (PAD), putting them at high risk of adverse vascular events such as myocardial infarction (MI) or sudden vascular death.35 Hospitalists initiate and coordinate aggressive and rapid interventions in the acute care setting in order to minimize stroke progression and thus optimize outcomes. They also initiate long‐term treatments to prevent recurrence and secondary vascular events in the outpatient setting. Thus, the treatment plan developed by the hospitalist on admission is as important as the one created on discharge.
The hospitalist plays a central role in managing stroke. Prior to having an event, patients are at risk. The goal of clinical management is prevention. This is mainly focused on risk factor reduction and aspirin therapy. Outpatient medical providers direct this care. Once a stroke occurs and the victim is admitted to the hospital, the hospitalist becomes this patient's medical care coordinator. In the very acute phase, the goal of management is optimizing outcomes by restoring perfusion to ischemic tissue and minimizing injury progression. There are a number of interventions available to the hospitalist. If patients present within 3 hours of ictus, they may qualify for IV thrombolytic therapy and if within 6 hours for intra‐arterial therapy. If later, aspirin can have beneficial effects on outcomes. Also during this time, it is important to maintain adequate systemic perfusion, oxygenation/ventilation, cardiovascular function, and, importantly, close clinical monitoring.
STROKE MORTALITY
Stroke is a deadly diseaseas deadly as many malignancies. Most patients die of complications of vascular disease (eg, cerebrovascular, cardiovascular, and peripheral vascular diseases). The Oxfordshire Community Stroke Project and Perth Community Stroke Study has indicated that at least 50% of patients die within 5 years of a first‐time acute ischemic or hemorrhagic stroke. The highest risk of death occurs during the first year, with a mortality rate ranging between 31% and 36.5% (95% confidence interval [CI], 27%34% and 31.5%41.4%, respectively).6, 7 Moreover, the risk of death within 30 days after stroke was approximately 20%. The annual risk of death for patients who survived 1 year was 7% and 10% according to the Oxfordshire and Perth studies, respectively, which was approximately 2‐fold higher than that for stroke‐free patients of the same age and sex.6, 7
The proportion of death caused by stroke, recurrent stroke, cardiovascular events, or nonvascular events changes over time (Fig. 1). The Perth study showed that the predominant causes of death within the first 30 days were complications from the incident stroke and, to a lesser degree, recurrent stroke. Over time, cardiovascular events (eg, myocardial infarction, ruptured aortic aneurysms, PAD) become the most common cause of mortality in patients who have had a stroke. However, the risk of death from a recurrent stroke only diminishes slightly with time.7 This trend is consistent with the findings of the Oxfordshire study and the Northern Manhattan Stroke Study, which focused on long‐term survival after first‐ever ischemic stroke.6, 8 Thus, the short‐term goals of treatment implemented by hospitalists are to ensure survival and recovery from the index stroke, and the long‐term goals are to protect against recurrent stroke or secondary vascular events.

MANAGEMENT OF ACUTE ISCHEMIC STROKE
Stroke is no longer an untreatable disease. The introduction of thrombolytic therapy has provided an opportunity for medical providers to significantly improve short‐ and long‐term survival rates and functional outcomes of patients. Most ischemic strokes are caused by thrombotic arterial occlusions. Hence, thrombolytic therapy has been tested and approved for use in patients with acute ischemic stroke.9 The efficacy and safety of the thrombolytic agent, recombinant tissue plasminogen activator (rtPA), were demonstrated in the landmark National Institute of Neurological Disorders and Stroke (NINDS) rtPA Stroke Study.
When compared with patients who received placebo, the odds of a favorable treatment outcome increased by at least 30% in those who received rtPA within 3 hours of the onset of symptoms of an acute ischemic stroke. This benefit was sustained for 612 months.10, 11 Patients who received rtPA were at an increased risk for intracerebral hemorrhage, but this did not translate to an increased risk of death.10 Currently, this thrombolytic agent has a class I recommendation from the American Heart Association and American Stroke Association (AHA/ASA) for its administration within 3 hours of onset of ischemic stroke symptoms in patients who have no sign or history of subarachnoid hemorrhage and who meet the other 21 criteria based on those used in the NINDS study.9
Patients who arrive at the hospital 36 hours after symptom onset or those who have contraindications for IV rtPA may benefit from intra‐arterial administration of thrombolytic agents.12 However, there is no consensus on the optimal dose that should be delivered by intra‐arterial administration.13 In addition, this course of treatment requires rapid access to cerebral angiography and a qualified interventionalist, both of which may not be available to all hospitalists.9
If a patient presents beyond 6 hours, the hospitalist may initiate aspirin therapy, which has been shown to improve outcomes following acute stroke if therapy is begun within 48 hours. A planned meta‐analysis of approximately 40,000 patients with suspected ischemic stroke demonstrated that aspirin therapy proportionally reduces the risk of recurrent stroke and mortality from recurrent stroke or any other cause by 11% 3%. This benefit was apparent as early as 06 hours and as late as 2548 hours following stroke onset (Fig. 2), with only a slight increase in the risk of hemorrhagic stroke.14 The studies analyzed in the meta‐analysis underlie the AHA/ASA recommendations that aspirin (325 mg) be administered within 2448 hours of stroke onset or within 24 hours after thrombolytic therapy for the early management of ischemic stroke in adults.9 By contrast, heparin therapy is not a recommended treatment for acute ischemic stroke; its clinical benefits do not outweigh the risk of bleeding complications.9 In addition, clinical trial data do not support the use of heparin for cardioembolic stroke.13

The AHA/ASA has made several recommendations to enhance outcomes and to prevent complications after an acute ischemic stroke. These include the stabilization and management of blood pressure (BP) and blood glucose levels and protection against deep vein thrombosis.9 Hypertension in the peristroke period is expected and is generally not treated. The rationale is that cerebral blood flow (CBF) is autoregulated in healthy brain tissue. As such, CBF remains constant at 50 cc/100 g of tissue per minute over a wide range of mean arterial pressures: 60150 mm Hg. However, in ischemic brain regions, autoregulation is lost, resulting in a pressure passive perfusion state (ie, local CBF is dependent on systemic blood pressure). As an injured brain is hypermetabolic, CBF adequate to meet its needs is dependent on a higher than normal blood pressure. Thus, reduction of high BP might worsen ischemia.
From a clinical practice standpoint, patients' outpatient antihypertensive medications are frequently held, with no additional treatment given for blood pressure elevation. The exception is, should the patient become encephalopathic, blood pressure may need to be reduced, as this may represent a state of hypertensive encephalopathy or luxury perfusion. There are no data indicating the use of a specific hypertensive agent in reducing blood pressure in such a setting. The AHA/ASA guidelines for early management of ischemic stroke recommend the use of antihypertensive agents on a case‐by‐case basis; although as recommended by consensus, there may be IV administration of labetalol or nicardipine if there is evidence of hypertensive encephalopathy, the diastolic BP is >120 mm Hg or the systolic BP is >220 mm Hg.9
Blood glucose should be kept stable, between 80 and 120 mg/dL. This can be achieved with either an oral hypoglycemic agent or sliding‐scale insulin regimen. Venous thrombus formation after stroke is a very serious concern as it can result in pulmonary embolism. As soon as possible, sequential compression devices and agents such as unfractionated heparin, low‐molecular‐weight heparin (ie, enoxaparin, dalteparin), fondaparinux, warfarin, or aspirin should be initiated.9
Hyperthermia has been shown to worsen functional outcome following stroke.15 Thus, maintenance of normal body temperature is recommended. This can be achieved with acetaminophen. Causes other than acute brain injury such as infection need to be investigated and treated as appropriate. Induced hypothermia has long been considered a potential therapy for improving outcome from acute stroke. Although preclinical studies in animals support induced hypothermia as a beneficial approach, there has not yet been a successful human clinical trial demonstrating efficacy. In addition, hypotonic intravenous solutions have the potential to worsen cerebral edema. Thus, normal saline without dextrose may be preferable. However, conclusive evidence supporting the use of hypertonic and colloid solutions remains insufficient.
Other important issues are gastrointestinal prophylaxis, early mobilization, and nutrition. The nutritional needs of acute brain‐injured patients cannot be overemphasized. Caloric intake should be maintained at 140% to compensate for the hypermetabolic state of the brain and to avoid weight loss. Patients should not be fed or treated with oral medications until a speech and swallow study is conducted to determine the extent of dysphagia and dysarthria or aphasia.9 However, in general, patients who are alert can usually be administered their oral medications, but only after a swallow evaluation has been passed.
ANTIPLATELET THERAPY FOR STROKE PREVENTION
Primary Stroke Prevention
Aspirin has been shown to be efficacious in preventing first stroke in women. The evidence supporting aspirin use in women for primary prevention of stroke is from the Women's Health Study, which showed that the occurrence of first stroke could be reduced in women older than 45 years old by taking 100 mg of aspirin every other day as compared with placebo.16 The AHA/ASA recommends aspirin therapy for primary ischemic stroke prevention in women whose risk of stroke outweighs the risk of aspirin‐related bleeding. Unfortunately, there are not enough supporting data to recommend its use in men for primary stroke prevention.17
Secondary Stroke Prevention
Aspirin, clopidogrel, and the extended‐release dipyridamole‐aspirin combination are the most commonly used antiplatelet agents for secondary stroke prevention. Ticlopidine is indicated for prevention of recurrent stroke18 but has fallen out of use because of safety concerns, and dipyridamole confers little cardiovascular protection compared with the other antiplatelet agents. Aspirin is widely regarded as the first‐line agent for preventing recurrent stroke. The optimal dose of aspirin for reducing the risk of secondary stroke is uncertain. However, most practitioners use doses between 75 and 325 mg. The numerous studies supporting this have been summarized by Hennekens et al.19 The Antiplatelet Trialists Collaboration demonstrated that lower‐dose aspirin (75150 mg) is effective and can reduce secondary stroke by 25%.20 The European Stroke Prevention Study 2 (ESPS‐2) showed an 18% reduction in the risk of a recurrent stroke with only 50 mg of aspirin.21 The AHA/ASA recommends 50350 mg/day aspirin to reduce the risk of recurrent stroke and or vascular events in patients with ischemic stroke.5
In the CAPRIE study, clopidogrel was shown to be effective, but not superior to aspirin, in the reduction of recurrent stroke.22 Taking their similar safety and efficacy profiles into account and aspirin's low cost, the AHA/ASA concluded that clopidogrel is an acceptable but not preferable alternative to aspirin therapy for the reduction of recurrent strokes.5 The combination of clopidogrel and aspirin reduces secondary vascular events in high‐risk cardiovascular patients and can be considered in high‐risk stroke patients. The CHARISMA study revealed that a combination of clopidogrel and aspirin has benefit over aspirin alone in secondary prevention of a combined end point of stroke, MI, and CV death.23 However, this same study also showed that aspirin alone is superior to the combination in primary prevention of this same end point. Subgroup analysis demonstrated that the combination of clopidogrel and aspirin provided a significant benefit in further reducing nonfatal strokes over aspirin alone (P .05) and a trend toward reducing all ischemic strokes (P .10).24 The MATCH study showed no evidence that a combination of clopidogrel and aspirin was superior to aspirin alone in patients with recent TIA or stroke.25, 26 However, the impact of aspirin resistance in the MATCH study population was not quantified but may have affected the study results, as 80% of the patients were already taking aspirin on enrollment.24 Of significance is the finding in both CHARISMA and MATCH that the addition of aspirin to clopidogrel therapy conveys a higher risk for bleeding.26 Combining clopidogrel with aspirin therapy is not routinely recommended by the AHA/ASA to reduce the risk of recurrent stroke.5
The ESPS‐2 trial demonstrated that the combination of extended‐release (ER) dipyridamole and aspirin was superior to aspirin alone for reducing the risk of recurrent stroke in patients with ischemic stroke.21 However, the combination of ER dipyridamole and aspirin was not different from placebo in preventing myocardial infarction or CV death. Thus, the AHA/ASA recommends that the combination of ER‐dipyridamole/aspirin can be considered for secondary stroke prevention.5
LONG‐TERM MANAGEMENT FOR SECONDARY PREVENTION OF NONSTROKE VASCULAR EVENTS
In the subacute period, the hospitalist transitions the patient from acute to chronic care. Here, the goals are optimizing functional outcome and preventing recurrence. Still, during the first few days after ictus, the patient remains at risk for recurrent stroke, cerebral edema, and hemorrhagic transformation, so continued hospitalization is required. By 57 days later, the most significant risk period has elapsed. Physical and occupational therapy are initiated while patients are still hospitalized. Patient and family education about stroke and related diseases is done. A rational and comprehensive plan to reduce risk of secondary stroke is critical. This plan must include diet, tobacco, diabetes, blood pressure and excessive weight interventions. These may require care from a specialized team with members such as dieticians, exercise therapists, and tobacco interventionalists. Especially critical is instituting a discharge plan that highlights continued control of all modifiable risk factors and antiplatelet therapy. Finally, coordination with the patient's outpatient provider is paramount.
There is a developing awareness of the importance of the overlapping syndrome of combined stroke and cardiovascular and peripheral vascular risk. In leading clinical trials, the coexistence of coronary artery disease and cerebral artery disease is as high as 40%; thus, patients who have had a stroke are at high risk for other vascular events such as MI, critical limb ischemia, or vascular death. The AHA/ASA scientific statement on coronary risk evaluation recommends testing for CAD after ischemic stroke, as it has been suggested that asymptomatic CAD is highly prevalent among these patients.4 Diagnostic testing for CAD should be conducted outside the acute stroke setting and optimized based on stroke subtype and the health status of individual patients.4 Testing for PAD should also be done in patients with ischemic stroke when not otherwise contraindicated.27 Thus, the hospitalist should determine the stroke patient's risk of having coexisting CAD and/or PAD. If significant, then appropriate follow‐up testing either during the hospitalization or after discharge should be arranged.
To prevent secondary vascular events including stroke, effective management of common risk factors shared by stroke, CAD, and PAD is recommended. Long‐term treatment goals include control of hypertension, lipid and glucose management, smoking cessation, weight control, and integration of physical activity.4, 5, 27 Except for blood pressure control, many of these should be initiated while still in the hospital. Acute hospitalization is also an opportunity for patient and family education regarding risk factor reduction.
Antiplatelet therapies are also recommended and are associated with an absolute risk reduction of serious vascular events of 36 6 per 1000 persons with previous stroke or transient ischemic attack.20 Aspirin use in patients at high risk for atherothrombotic events has been shown to be effective in reducing the risk of myocardial infarction and other vascular events.20 The AHA‐recommended dose of aspirin for preventing sudden coronary syndrome is 81 mg/day or higher. Clopidogrel has been shown to be effective in reducing the risk of recurrent sudden coronary artery syndrome and progression of peripheral vascular disease.22 When combined with aspirin, clopidogrel has been shown to reduce recurrent sudden coronary syndrome.28, 29
CONCLUSIONS
The hospitalist is involved in the spectrum of stroke care, from management of stroke in the acute care setting to establishing long‐term treatments for prevention of secondary vascular events. As such, hospitalists can significantly affect the lives of patients with ischemic stroke. Current treatment guidelines for stroke recommend aggressive and rapid response in the acute setting. Long‐term treatments focus on risk reduction for recurrent stroke or for other vascular events such as MI or critical limb ischemia. Antiplatelet therapies are a component of long‐term treatments. Current research suggests that antiplatelet agents differ in reducing recurrent strokes versus nonstroke events. Thus, treatments should be based on a patient's individual risk factors for recurrent stroke and/or CAD or PAD. Although hospitalists will transfer care back to outpatient providers, the interventions initiated in the hospital will optimize the patient's future. In many ways, the patient's first step to a better health began when crossing the entrance of the hospital.
Stroke is the leading cause of disability and the third leading cause of death in the United States.1, 2 Each year approximately 700,000 strokes occur, 88% of which are considered ischemic; they predominately arise from atherothrombotic events in large or small cerebral vessels. Moreover, approximately 200,000 of these events are classified as recurrent.1 Patients who have had a stroke frequently also have coronary artery disease (CAD) and/or peripheral artery disease (PAD), putting them at high risk of adverse vascular events such as myocardial infarction (MI) or sudden vascular death.35 Hospitalists initiate and coordinate aggressive and rapid interventions in the acute care setting in order to minimize stroke progression and thus optimize outcomes. They also initiate long‐term treatments to prevent recurrence and secondary vascular events in the outpatient setting. Thus, the treatment plan developed by the hospitalist on admission is as important as the one created on discharge.
The hospitalist plays a central role in managing stroke. Prior to having an event, patients are at risk. The goal of clinical management is prevention. This is mainly focused on risk factor reduction and aspirin therapy. Outpatient medical providers direct this care. Once a stroke occurs and the victim is admitted to the hospital, the hospitalist becomes this patient's medical care coordinator. In the very acute phase, the goal of management is optimizing outcomes by restoring perfusion to ischemic tissue and minimizing injury progression. There are a number of interventions available to the hospitalist. If patients present within 3 hours of ictus, they may qualify for IV thrombolytic therapy and if within 6 hours for intra‐arterial therapy. If later, aspirin can have beneficial effects on outcomes. Also during this time, it is important to maintain adequate systemic perfusion, oxygenation/ventilation, cardiovascular function, and, importantly, close clinical monitoring.
STROKE MORTALITY
Stroke is a deadly diseaseas deadly as many malignancies. Most patients die of complications of vascular disease (eg, cerebrovascular, cardiovascular, and peripheral vascular diseases). The Oxfordshire Community Stroke Project and Perth Community Stroke Study has indicated that at least 50% of patients die within 5 years of a first‐time acute ischemic or hemorrhagic stroke. The highest risk of death occurs during the first year, with a mortality rate ranging between 31% and 36.5% (95% confidence interval [CI], 27%34% and 31.5%41.4%, respectively).6, 7 Moreover, the risk of death within 30 days after stroke was approximately 20%. The annual risk of death for patients who survived 1 year was 7% and 10% according to the Oxfordshire and Perth studies, respectively, which was approximately 2‐fold higher than that for stroke‐free patients of the same age and sex.6, 7
The proportion of death caused by stroke, recurrent stroke, cardiovascular events, or nonvascular events changes over time (Fig. 1). The Perth study showed that the predominant causes of death within the first 30 days were complications from the incident stroke and, to a lesser degree, recurrent stroke. Over time, cardiovascular events (eg, myocardial infarction, ruptured aortic aneurysms, PAD) become the most common cause of mortality in patients who have had a stroke. However, the risk of death from a recurrent stroke only diminishes slightly with time.7 This trend is consistent with the findings of the Oxfordshire study and the Northern Manhattan Stroke Study, which focused on long‐term survival after first‐ever ischemic stroke.6, 8 Thus, the short‐term goals of treatment implemented by hospitalists are to ensure survival and recovery from the index stroke, and the long‐term goals are to protect against recurrent stroke or secondary vascular events.

MANAGEMENT OF ACUTE ISCHEMIC STROKE
Stroke is no longer an untreatable disease. The introduction of thrombolytic therapy has provided an opportunity for medical providers to significantly improve short‐ and long‐term survival rates and functional outcomes of patients. Most ischemic strokes are caused by thrombotic arterial occlusions. Hence, thrombolytic therapy has been tested and approved for use in patients with acute ischemic stroke.9 The efficacy and safety of the thrombolytic agent, recombinant tissue plasminogen activator (rtPA), were demonstrated in the landmark National Institute of Neurological Disorders and Stroke (NINDS) rtPA Stroke Study.
When compared with patients who received placebo, the odds of a favorable treatment outcome increased by at least 30% in those who received rtPA within 3 hours of the onset of symptoms of an acute ischemic stroke. This benefit was sustained for 612 months.10, 11 Patients who received rtPA were at an increased risk for intracerebral hemorrhage, but this did not translate to an increased risk of death.10 Currently, this thrombolytic agent has a class I recommendation from the American Heart Association and American Stroke Association (AHA/ASA) for its administration within 3 hours of onset of ischemic stroke symptoms in patients who have no sign or history of subarachnoid hemorrhage and who meet the other 21 criteria based on those used in the NINDS study.9
Patients who arrive at the hospital 36 hours after symptom onset or those who have contraindications for IV rtPA may benefit from intra‐arterial administration of thrombolytic agents.12 However, there is no consensus on the optimal dose that should be delivered by intra‐arterial administration.13 In addition, this course of treatment requires rapid access to cerebral angiography and a qualified interventionalist, both of which may not be available to all hospitalists.9
If a patient presents beyond 6 hours, the hospitalist may initiate aspirin therapy, which has been shown to improve outcomes following acute stroke if therapy is begun within 48 hours. A planned meta‐analysis of approximately 40,000 patients with suspected ischemic stroke demonstrated that aspirin therapy proportionally reduces the risk of recurrent stroke and mortality from recurrent stroke or any other cause by 11% 3%. This benefit was apparent as early as 06 hours and as late as 2548 hours following stroke onset (Fig. 2), with only a slight increase in the risk of hemorrhagic stroke.14 The studies analyzed in the meta‐analysis underlie the AHA/ASA recommendations that aspirin (325 mg) be administered within 2448 hours of stroke onset or within 24 hours after thrombolytic therapy for the early management of ischemic stroke in adults.9 By contrast, heparin therapy is not a recommended treatment for acute ischemic stroke; its clinical benefits do not outweigh the risk of bleeding complications.9 In addition, clinical trial data do not support the use of heparin for cardioembolic stroke.13

The AHA/ASA has made several recommendations to enhance outcomes and to prevent complications after an acute ischemic stroke. These include the stabilization and management of blood pressure (BP) and blood glucose levels and protection against deep vein thrombosis.9 Hypertension in the peristroke period is expected and is generally not treated. The rationale is that cerebral blood flow (CBF) is autoregulated in healthy brain tissue. As such, CBF remains constant at 50 cc/100 g of tissue per minute over a wide range of mean arterial pressures: 60150 mm Hg. However, in ischemic brain regions, autoregulation is lost, resulting in a pressure passive perfusion state (ie, local CBF is dependent on systemic blood pressure). As an injured brain is hypermetabolic, CBF adequate to meet its needs is dependent on a higher than normal blood pressure. Thus, reduction of high BP might worsen ischemia.
From a clinical practice standpoint, patients' outpatient antihypertensive medications are frequently held, with no additional treatment given for blood pressure elevation. The exception is, should the patient become encephalopathic, blood pressure may need to be reduced, as this may represent a state of hypertensive encephalopathy or luxury perfusion. There are no data indicating the use of a specific hypertensive agent in reducing blood pressure in such a setting. The AHA/ASA guidelines for early management of ischemic stroke recommend the use of antihypertensive agents on a case‐by‐case basis; although as recommended by consensus, there may be IV administration of labetalol or nicardipine if there is evidence of hypertensive encephalopathy, the diastolic BP is >120 mm Hg or the systolic BP is >220 mm Hg.9
Blood glucose should be kept stable, between 80 and 120 mg/dL. This can be achieved with either an oral hypoglycemic agent or sliding‐scale insulin regimen. Venous thrombus formation after stroke is a very serious concern as it can result in pulmonary embolism. As soon as possible, sequential compression devices and agents such as unfractionated heparin, low‐molecular‐weight heparin (ie, enoxaparin, dalteparin), fondaparinux, warfarin, or aspirin should be initiated.9
Hyperthermia has been shown to worsen functional outcome following stroke.15 Thus, maintenance of normal body temperature is recommended. This can be achieved with acetaminophen. Causes other than acute brain injury such as infection need to be investigated and treated as appropriate. Induced hypothermia has long been considered a potential therapy for improving outcome from acute stroke. Although preclinical studies in animals support induced hypothermia as a beneficial approach, there has not yet been a successful human clinical trial demonstrating efficacy. In addition, hypotonic intravenous solutions have the potential to worsen cerebral edema. Thus, normal saline without dextrose may be preferable. However, conclusive evidence supporting the use of hypertonic and colloid solutions remains insufficient.
Other important issues are gastrointestinal prophylaxis, early mobilization, and nutrition. The nutritional needs of acute brain‐injured patients cannot be overemphasized. Caloric intake should be maintained at 140% to compensate for the hypermetabolic state of the brain and to avoid weight loss. Patients should not be fed or treated with oral medications until a speech and swallow study is conducted to determine the extent of dysphagia and dysarthria or aphasia.9 However, in general, patients who are alert can usually be administered their oral medications, but only after a swallow evaluation has been passed.
ANTIPLATELET THERAPY FOR STROKE PREVENTION
Primary Stroke Prevention
Aspirin has been shown to be efficacious in preventing first stroke in women. The evidence supporting aspirin use in women for primary prevention of stroke is from the Women's Health Study, which showed that the occurrence of first stroke could be reduced in women older than 45 years old by taking 100 mg of aspirin every other day as compared with placebo.16 The AHA/ASA recommends aspirin therapy for primary ischemic stroke prevention in women whose risk of stroke outweighs the risk of aspirin‐related bleeding. Unfortunately, there are not enough supporting data to recommend its use in men for primary stroke prevention.17
Secondary Stroke Prevention
Aspirin, clopidogrel, and the extended‐release dipyridamole‐aspirin combination are the most commonly used antiplatelet agents for secondary stroke prevention. Ticlopidine is indicated for prevention of recurrent stroke18 but has fallen out of use because of safety concerns, and dipyridamole confers little cardiovascular protection compared with the other antiplatelet agents. Aspirin is widely regarded as the first‐line agent for preventing recurrent stroke. The optimal dose of aspirin for reducing the risk of secondary stroke is uncertain. However, most practitioners use doses between 75 and 325 mg. The numerous studies supporting this have been summarized by Hennekens et al.19 The Antiplatelet Trialists Collaboration demonstrated that lower‐dose aspirin (75150 mg) is effective and can reduce secondary stroke by 25%.20 The European Stroke Prevention Study 2 (ESPS‐2) showed an 18% reduction in the risk of a recurrent stroke with only 50 mg of aspirin.21 The AHA/ASA recommends 50350 mg/day aspirin to reduce the risk of recurrent stroke and or vascular events in patients with ischemic stroke.5
In the CAPRIE study, clopidogrel was shown to be effective, but not superior to aspirin, in the reduction of recurrent stroke.22 Taking their similar safety and efficacy profiles into account and aspirin's low cost, the AHA/ASA concluded that clopidogrel is an acceptable but not preferable alternative to aspirin therapy for the reduction of recurrent strokes.5 The combination of clopidogrel and aspirin reduces secondary vascular events in high‐risk cardiovascular patients and can be considered in high‐risk stroke patients. The CHARISMA study revealed that a combination of clopidogrel and aspirin has benefit over aspirin alone in secondary prevention of a combined end point of stroke, MI, and CV death.23 However, this same study also showed that aspirin alone is superior to the combination in primary prevention of this same end point. Subgroup analysis demonstrated that the combination of clopidogrel and aspirin provided a significant benefit in further reducing nonfatal strokes over aspirin alone (P .05) and a trend toward reducing all ischemic strokes (P .10).24 The MATCH study showed no evidence that a combination of clopidogrel and aspirin was superior to aspirin alone in patients with recent TIA or stroke.25, 26 However, the impact of aspirin resistance in the MATCH study population was not quantified but may have affected the study results, as 80% of the patients were already taking aspirin on enrollment.24 Of significance is the finding in both CHARISMA and MATCH that the addition of aspirin to clopidogrel therapy conveys a higher risk for bleeding.26 Combining clopidogrel with aspirin therapy is not routinely recommended by the AHA/ASA to reduce the risk of recurrent stroke.5
The ESPS‐2 trial demonstrated that the combination of extended‐release (ER) dipyridamole and aspirin was superior to aspirin alone for reducing the risk of recurrent stroke in patients with ischemic stroke.21 However, the combination of ER dipyridamole and aspirin was not different from placebo in preventing myocardial infarction or CV death. Thus, the AHA/ASA recommends that the combination of ER‐dipyridamole/aspirin can be considered for secondary stroke prevention.5
LONG‐TERM MANAGEMENT FOR SECONDARY PREVENTION OF NONSTROKE VASCULAR EVENTS
In the subacute period, the hospitalist transitions the patient from acute to chronic care. Here, the goals are optimizing functional outcome and preventing recurrence. Still, during the first few days after ictus, the patient remains at risk for recurrent stroke, cerebral edema, and hemorrhagic transformation, so continued hospitalization is required. By 57 days later, the most significant risk period has elapsed. Physical and occupational therapy are initiated while patients are still hospitalized. Patient and family education about stroke and related diseases is done. A rational and comprehensive plan to reduce risk of secondary stroke is critical. This plan must include diet, tobacco, diabetes, blood pressure and excessive weight interventions. These may require care from a specialized team with members such as dieticians, exercise therapists, and tobacco interventionalists. Especially critical is instituting a discharge plan that highlights continued control of all modifiable risk factors and antiplatelet therapy. Finally, coordination with the patient's outpatient provider is paramount.
There is a developing awareness of the importance of the overlapping syndrome of combined stroke and cardiovascular and peripheral vascular risk. In leading clinical trials, the coexistence of coronary artery disease and cerebral artery disease is as high as 40%; thus, patients who have had a stroke are at high risk for other vascular events such as MI, critical limb ischemia, or vascular death. The AHA/ASA scientific statement on coronary risk evaluation recommends testing for CAD after ischemic stroke, as it has been suggested that asymptomatic CAD is highly prevalent among these patients.4 Diagnostic testing for CAD should be conducted outside the acute stroke setting and optimized based on stroke subtype and the health status of individual patients.4 Testing for PAD should also be done in patients with ischemic stroke when not otherwise contraindicated.27 Thus, the hospitalist should determine the stroke patient's risk of having coexisting CAD and/or PAD. If significant, then appropriate follow‐up testing either during the hospitalization or after discharge should be arranged.
To prevent secondary vascular events including stroke, effective management of common risk factors shared by stroke, CAD, and PAD is recommended. Long‐term treatment goals include control of hypertension, lipid and glucose management, smoking cessation, weight control, and integration of physical activity.4, 5, 27 Except for blood pressure control, many of these should be initiated while still in the hospital. Acute hospitalization is also an opportunity for patient and family education regarding risk factor reduction.
Antiplatelet therapies are also recommended and are associated with an absolute risk reduction of serious vascular events of 36 6 per 1000 persons with previous stroke or transient ischemic attack.20 Aspirin use in patients at high risk for atherothrombotic events has been shown to be effective in reducing the risk of myocardial infarction and other vascular events.20 The AHA‐recommended dose of aspirin for preventing sudden coronary syndrome is 81 mg/day or higher. Clopidogrel has been shown to be effective in reducing the risk of recurrent sudden coronary artery syndrome and progression of peripheral vascular disease.22 When combined with aspirin, clopidogrel has been shown to reduce recurrent sudden coronary syndrome.28, 29
CONCLUSIONS
The hospitalist is involved in the spectrum of stroke care, from management of stroke in the acute care setting to establishing long‐term treatments for prevention of secondary vascular events. As such, hospitalists can significantly affect the lives of patients with ischemic stroke. Current treatment guidelines for stroke recommend aggressive and rapid response in the acute setting. Long‐term treatments focus on risk reduction for recurrent stroke or for other vascular events such as MI or critical limb ischemia. Antiplatelet therapies are a component of long‐term treatments. Current research suggests that antiplatelet agents differ in reducing recurrent strokes versus nonstroke events. Thus, treatments should be based on a patient's individual risk factors for recurrent stroke and/or CAD or PAD. Although hospitalists will transfer care back to outpatient providers, the interventions initiated in the hospital will optimize the patient's future. In many ways, the patient's first step to a better health began when crossing the entrance of the hospital.
- ,,, et al.Heart disease and stroke statistics—2006 update: a report from the American Heart Association statistics committee and stroke statistics subcommittee.Circulation.2006;113:85–151.
- ,,,.Trends in the leading causes of death in the United States, 1970–2002.JAMA.2005;294:1255–1259.
- ,,, et al.One‐year cardiovascular event rates in outpatients with atherothrombosis.JAMA.2007;297:1197–1206.
- ,,, et al.Coronary risk evaluation in patients with transient ischemic attack and ischemic stroke: a scientific statement for healthcare professionals from the Stroke Council and the Council on Clinical Cardiology of the American Heart Association/American Stroke Association.Circulation.2003;108:1278–1290.
- ,,, et al.Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: A statement for healthcare professionals from the American Heart Association/American Stroke Association.Stroke.2006;37:577–617.
- ,,,,,.Long‐term survival after first‐ever stroke: the Oxfordshire Community Stroke Project.Stroke.1993;24:796–800.
- ,,, et al.Five‐year survival after first‐ever stroke and related prognostic factors in the Perth Community Stroke Study.Stroke.2000;31:2080–2086.
- ,,, et al.Mortality and causes of death after first ischemic stroke: the Northern Manhattan Stroke Study.Neurology.2001;57:2000–2005.
- ,,, et al.Guidelines for the early management of adults with ischemic stroke: A guideline from the American Heart Association/American Stroke Association.Stroke.2007;38:1655–1711.
- NINDS study group.Tissue plasminogen activator for acute ischemic stroke.N Engl J Med.1995;333:1581–1587.
- ,,, et al.Effects of tissue plasminogen activator for acute ischemic stroke at one year.N Engl J Med.1999;340:1781–1787.
- ,,, et al.Intraarterial recombinant tissue plasminogen activator for ischemic stroke: an accelerating dosing regimen.Neurosurgery.2000;47:473–476.
- ,,,,.Antithrombotic and thrombolytic therapy for ischemic stroke: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:483s–512s.
- ,,, et al.Indications for early aspirin use in acute ischemic stroke: a combined analysis of 40000 randomized patients from the Chinese acute stroke trial and the international stroke trial.Stroke.2000;31:1240–1249.
- ,,, et al.Body temperature in acute stroke: relation to stroke severity, infarct size, mortality, and outcome.Lancet.1996;347:422–425.
- ,,, et al.A randomized trial of low‐dose aspirin in the primary prevention of cardiovascular disease in women.N Engl J Med.2005;352:1293–1304.
- ,,, et al.Primary prevention of ischemic stroke: A guideline from the American Heart Association/American Stroke Association.Circulation.2006;113:873–823.
- ,,, et al.A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high‐risk patients.N Engl J Med.1989;321:501–517.
- ,,.Aspirin as a therapeutic agent in cardiovascular disease: a statement for healthcare professionals from the American Heart Association.Circulation.1997;96:2751–2753.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.BMJ.2002;324:71–86.
- ,,,,,.European stroke prevention study:2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke.J Neurol Sci.1996;143:1–13.
- CAPRIE steering committee.A randomized, blinded, trial of clopidogrel versus aspirin in patients at risk of ischemic events (CAPRIE).Lancet.1996;348:1329–1339.
- ,,, et al.Clopidogrel and aspiring versus aspirin alone for the prevention of atherothrombotic events.N Engl J Med.2006;354:1706–1717.
- ,,, et al.Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial.J Am Coll Cardiol.2007;49:1982–1988.
- ,,, et al.Aspirin and clopidogrel compared with clopidogrel alone after recent ischemic stroke or transient ischemic attack in high‐risk patients (MATCH): Randomized, double‐blind placebo‐controlled trial.Lancet.2004;364:331–337.
- .Role of aspirin in MATCH.Lancet.2004;364:1661.
- ,,, et al.ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic).Circulation.2006;113:463–654.
- CURE Trial Investigators.Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST‐segment elevation.N Engl J Med.2001;345:494–502.
- ,,, et al.Effects of pretreatment with clopidogrel and aspirin followed by long‐term therapy in patients undergoing percutaneous coronary intervention: PCI‐CURE study.Lancet.2001;358:527–533.
- ,,, et al.Heart disease and stroke statistics—2006 update: a report from the American Heart Association statistics committee and stroke statistics subcommittee.Circulation.2006;113:85–151.
- ,,,.Trends in the leading causes of death in the United States, 1970–2002.JAMA.2005;294:1255–1259.
- ,,, et al.One‐year cardiovascular event rates in outpatients with atherothrombosis.JAMA.2007;297:1197–1206.
- ,,, et al.Coronary risk evaluation in patients with transient ischemic attack and ischemic stroke: a scientific statement for healthcare professionals from the Stroke Council and the Council on Clinical Cardiology of the American Heart Association/American Stroke Association.Circulation.2003;108:1278–1290.
- ,,, et al.Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: A statement for healthcare professionals from the American Heart Association/American Stroke Association.Stroke.2006;37:577–617.
- ,,,,,.Long‐term survival after first‐ever stroke: the Oxfordshire Community Stroke Project.Stroke.1993;24:796–800.
- ,,, et al.Five‐year survival after first‐ever stroke and related prognostic factors in the Perth Community Stroke Study.Stroke.2000;31:2080–2086.
- ,,, et al.Mortality and causes of death after first ischemic stroke: the Northern Manhattan Stroke Study.Neurology.2001;57:2000–2005.
- ,,, et al.Guidelines for the early management of adults with ischemic stroke: A guideline from the American Heart Association/American Stroke Association.Stroke.2007;38:1655–1711.
- NINDS study group.Tissue plasminogen activator for acute ischemic stroke.N Engl J Med.1995;333:1581–1587.
- ,,, et al.Effects of tissue plasminogen activator for acute ischemic stroke at one year.N Engl J Med.1999;340:1781–1787.
- ,,, et al.Intraarterial recombinant tissue plasminogen activator for ischemic stroke: an accelerating dosing regimen.Neurosurgery.2000;47:473–476.
- ,,,,.Antithrombotic and thrombolytic therapy for ischemic stroke: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:483s–512s.
- ,,, et al.Indications for early aspirin use in acute ischemic stroke: a combined analysis of 40000 randomized patients from the Chinese acute stroke trial and the international stroke trial.Stroke.2000;31:1240–1249.
- ,,, et al.Body temperature in acute stroke: relation to stroke severity, infarct size, mortality, and outcome.Lancet.1996;347:422–425.
- ,,, et al.A randomized trial of low‐dose aspirin in the primary prevention of cardiovascular disease in women.N Engl J Med.2005;352:1293–1304.
- ,,, et al.Primary prevention of ischemic stroke: A guideline from the American Heart Association/American Stroke Association.Circulation.2006;113:873–823.
- ,,, et al.A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high‐risk patients.N Engl J Med.1989;321:501–517.
- ,,.Aspirin as a therapeutic agent in cardiovascular disease: a statement for healthcare professionals from the American Heart Association.Circulation.1997;96:2751–2753.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.BMJ.2002;324:71–86.
- ,,,,,.European stroke prevention study:2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke.J Neurol Sci.1996;143:1–13.
- CAPRIE steering committee.A randomized, blinded, trial of clopidogrel versus aspirin in patients at risk of ischemic events (CAPRIE).Lancet.1996;348:1329–1339.
- ,,, et al.Clopidogrel and aspiring versus aspirin alone for the prevention of atherothrombotic events.N Engl J Med.2006;354:1706–1717.
- ,,, et al.Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial.J Am Coll Cardiol.2007;49:1982–1988.
- ,,, et al.Aspirin and clopidogrel compared with clopidogrel alone after recent ischemic stroke or transient ischemic attack in high‐risk patients (MATCH): Randomized, double‐blind placebo‐controlled trial.Lancet.2004;364:331–337.
- .Role of aspirin in MATCH.Lancet.2004;364:1661.
- ,,, et al.ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic).Circulation.2006;113:463–654.
- CURE Trial Investigators.Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST‐segment elevation.N Engl J Med.2001;345:494–502.
- ,,, et al.Effects of pretreatment with clopidogrel and aspirin followed by long‐term therapy in patients undergoing percutaneous coronary intervention: PCI‐CURE study.Lancet.2001;358:527–533.
Editorial: Rules of Engagement
Acute atherothrombotic events associated with ischemic heart disease and stroke are the first and third most common causes of death in the United States, respectively.1 Despite an overall decrease in age‐adjusted mortality since 1970 in the United States, the worldwide prevalence of these diseases is anticipated to sharply increase by 2020.1, 2 Caring for patients with atherothrombosis is now within the purview of hospitalists to a larger extent than ever before. In recognition of the expanding role of these health care professionals and to reduce the risk of adverse cardiovascular events in the outpatient setting, the Society of Hospital Medicine held a symposium during its 10th Annual Meeting.
Rules of Engagement: The Hospitalist and Atherothrombosis took place on May 24, 2007, in Dallas, Texas. This supplement summarizes the highlights from this symposium and reviews the causes and polyvascular nature of atherothrombosis. The role of the hospitalist in managing atherothrombotic disease and evidence‐based practices for the evaluation and treatment of patients with various manifestations of atherothrombotic disease are also discussed.
ARTERIAL THROMBOSIS AND ITS POLYVASCULAR NATURE
Atherothrombosis refers to the formation of large and occlusive mural thrombi that arise from the rupture of an atherosclerotic plaque. Myocardial infarction (MI), ischemic stroke, and acute limb ischemia are the most severe manifestations of this disease.3, 4 This process begins when denuded or inflamed endothelial cells develop properties that permit platelet adhesion. At the site of endothelial dysfunction, activation of adherent platelet results in the release of inflammatory and mitogenic factors. After a series of dynamic and repetitive processes including amplified platelet activation, monocyte chemotaxis, adhesion, transmigration, and lipoprotein retention, plaque formation occurs.5 Consequently, the rupture or erosion of an atherosclerotic plaque produces a higher degree of platelet adhesion, activation, and aggregation, causing the fibrotic organization of a mural thrombus.3
The number of persons with multiple, concomitant cardiovascular disease (CAD), cerebrovascular disease (CVD), and peripheral arterial disease (PAD) accentuates the polyvascular nature of atherothrombosis (Fig. 1). The international Reduction of Atherothrombosis for Continued Health (REACH) Registry demonstrated that 1‐year incidence rates of major cardiovascular events (eg, MI, stroke, death) were high in patients with an established atherothrombotic disease and increased with the number of concomitant vascular diseases.6 These data infer that the burden on the vascular system is considered extensive on diagnosis of a single atherothrombotic disease. Thus, aggressive therapies are needed to reduce the risk of recurrent or other cardiovascular events. The management of risk factors for atherothrombosis such as hypercholesterolemia, dyslipidemia, hypertension, and diabetes mellitus fall under specific disease‐specific guidelines for patients presenting with atherothrombotic diseases.712
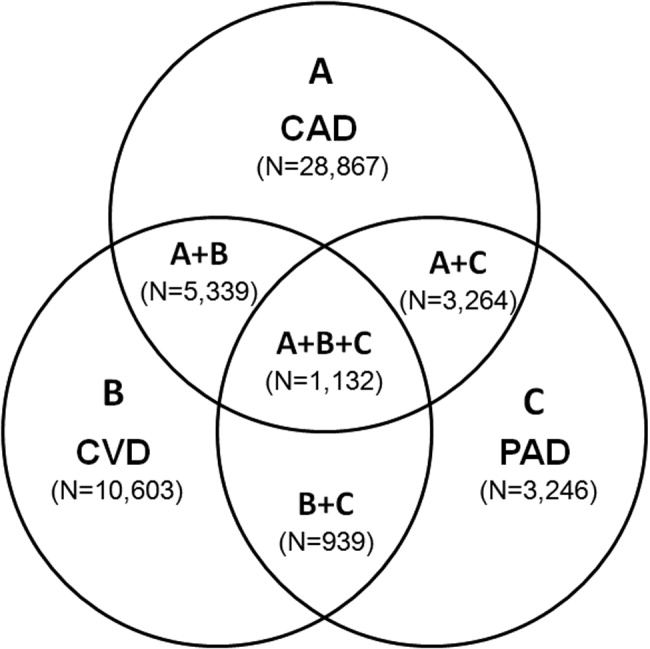
ANTIPLATELET THERAPIES
Antiplatelet therapies are used for the acute and long‐term treatment of patients after a thrombic event. Antiplatelet agents target the molecular mechanisms responsible for platelet activation and aggregation, such as the synthesis of thromboxane A2. On platelet activation, free arachidonic acid is converted to prostaglandin H2 (PGH2) by cyclooxygenase‐1 (COX‐1; Fig. 2). Further metabolism of PGH2 by thromboxane synthase produces thromboxane A2, which induces vasoconstriction (Fig. 2). Fortunately, the ability of platelets to produce COX‐1 is limited, and irreversible inhibition of this enzyme can impair thromboxane A2 synthesis for approximately 10 days.
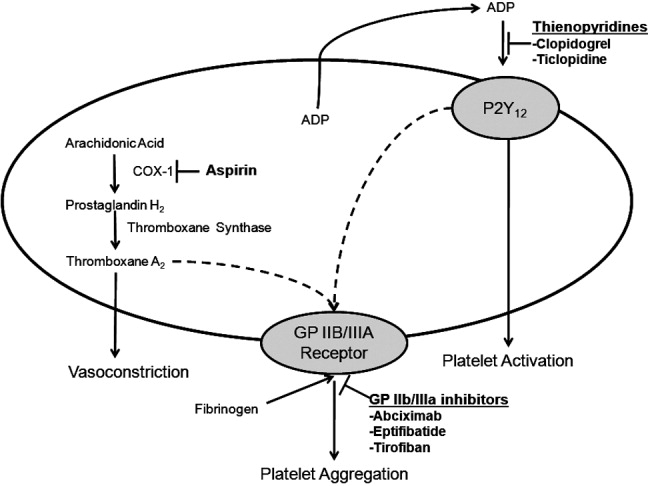
Aspirin is a potent COX‐1 inhibitor, whose effects are evident 1 hour after dosing (Fig. 2).4, 13 Aspirin effectively prevents fatal and nonfatal vascular events in healthy individuals and in patients who present with acute MI or ischemic stroke.13 Unfortunately, a proportion of patients are aspirin resistant. Recent studies have indicated that interactions with the nonsteroidal anti‐inflammatory drug (NSAID) ibuprofen may diminish the primary and secondary protective effects of aspirin and may contribute to aspirin resistance, although the origin of this remains unclear.
The results of a post hoc subgroup analysis of 22,071 apparently healthy male physicians randomized to take aspirin or placebo for 5 years indicated that individuals who used NSAIDs for at least 60 days/year increased their risk of MI by more than 2‐fold compared with those who did not use NSAIDs.14 A second study conducted in patients following a major adverse cardiovascular event showed that the combination of aspirin plus ibuprofen increased the adjusted relative risk of cardiovascular mortality over an 8‐year period compared with aspirin alone.15 However, the effects of NSAIDS on aspirin's ability to inhibit COX‐1 are reversible and only last for the dosing interval and body clearance time of the drug.16
Adeonsine diphosphate (ADP)dependent stimulation of the P2Y12 receptor is another target for antiplatelet therapy. On its release, ADP binds to the P2Y12 receptor on platelets, resulting in activation and aggregation (Fig. 2). Ticlopidine and clopidogrel are thienopyridines that may irreversibly modify the P2Y12 receptor (Fig. 2).13 Safety concerns associated with ticlopidine use, including severe neutropenia, have limited its administration. Conversely, clopidogrel is relatively well‐tolerated and can prevent cardiovascular events in patients with CAD, ischemic stroke, and PAD. This agent is an orally administered prodrug requiring activation by hepatic cytochrome P450 enzymes.13
Aspirin and thienopyridines do not inhibit platelet aggregation induced by the binding of fibrinogen to the platelet glycoprotein (GP) IIb/IIIa receptor (Fig. 2).4, 13 However, there are 3 commonly administered GP IIb/IIIa inhibitors: abciximab, eptifibatide, and tirofiban (Fig. 2).4 Abciximab is the fab fragment of the chimeric monoclonal antibody 7E3 and irreversibly inhibits the GP IIb/IIIa receptor. By contrast, eptifibatide is a cyclic heptapeptide, tirofiban is a nonpeptide, and both agents are reversible inhibitors. These agents are administered intravenously, and boluses are reserved for the short‐term treatment of atherothrombosis in patients undergoing percutaneous coronary intervention.13
CONCLUSIONS
Atherothrombosis is a systemic disease that often affects coronary, intracranial, and peripheral arterial beds concomitantly, which increases the probability of a thrombotic event. Aggressive treatments, including acute and long‐term antiplatelet therapies, are required to reduce the risks associated with atherothrombosis. This supplement reviews the evidence‐based approaches for managing atherothrombosis. It will provide hospitalists with the knowledge needed to treat patients with PAD, stroke, and acute coronary syndrome. First, the administration of antiplatelet therapies to patients with acute coronary syndrome will be described. Then, guidelines for the management of patients with acute ischemic stroke and the use of antiplatelet therapies to reduce mortality due to primary and secondary ischemic events will be reviewed. Finally, the role of the hospitalist in the diagnosis of PAD in asymptomatic patients and in those with confirmed atherothrombosis will be discussed.
- ,,,.Trends in the leading causes of death in the United States, 1970‐2002.JAMA.2005;294:1255–1259.
- ,.The global burden of disease, 1990‐2020.Nat Med.1998:4:1241–1243.
- ,,,.The pathogenesis of coronary artery disease and the acute coronary syndromes.N Engl J Med.1992;326:242–250.
- .Antiplatelet therapy.Am J Med.1996;101:199–209.
- ,,.Platelets in inflammation and atherogenesis.J Clin Invest.2005;115:3378–3384.
- ,,, et al.One‐year cardiovascular event rates in outpatients with atherothrombosis.JAMA.2007;297:1197–1206.
- ,,, et al.ACC/AHA 2000 guidelines for management of patients with unstable angina and non‐ST‐segment elevation myocardial infarction.J Am Coll Cardiol.2000;36:970–1062.
- ,,, et al.ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction.Circulation.2004;110:82–292.
- ,,, et al.Guidelines for the prevention of stroke in patients with ischemic stroke or transient ischemic attack. A statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke.Stroke.2006;37:557–617.
- ,,, et al.ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines.Circulation.2006;113:463–654.
- ,,.Inflammation and atherosclerosis.Circulation.2002;105:1135–1143.
- ,,, et al.AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update.J Am Coll Cardiol.2006;47:2130–2139.
- ,,, et al.Platelet‐active drugs: the relationships among dose, effectiveness, and side effects.Chest.2001;119:39–63.
- ,,, et al.Inhibition of clinical benefits of aspirin on first myocardial infarction by nonsteroidal anti‐inflammatory drugs.Circulation.2003;108:1191–1195.
- ,.The effect of ibuprofen on cardioprotective effects of aspirin.Lancet.2003;361:573–574.
- ,,, et al.Cyclooxygenase inhibitors and the antiplatelet effects of aspirin.N Engl J Med.2001;345:1809–1817.
Acute atherothrombotic events associated with ischemic heart disease and stroke are the first and third most common causes of death in the United States, respectively.1 Despite an overall decrease in age‐adjusted mortality since 1970 in the United States, the worldwide prevalence of these diseases is anticipated to sharply increase by 2020.1, 2 Caring for patients with atherothrombosis is now within the purview of hospitalists to a larger extent than ever before. In recognition of the expanding role of these health care professionals and to reduce the risk of adverse cardiovascular events in the outpatient setting, the Society of Hospital Medicine held a symposium during its 10th Annual Meeting.
Rules of Engagement: The Hospitalist and Atherothrombosis took place on May 24, 2007, in Dallas, Texas. This supplement summarizes the highlights from this symposium and reviews the causes and polyvascular nature of atherothrombosis. The role of the hospitalist in managing atherothrombotic disease and evidence‐based practices for the evaluation and treatment of patients with various manifestations of atherothrombotic disease are also discussed.
ARTERIAL THROMBOSIS AND ITS POLYVASCULAR NATURE
Atherothrombosis refers to the formation of large and occlusive mural thrombi that arise from the rupture of an atherosclerotic plaque. Myocardial infarction (MI), ischemic stroke, and acute limb ischemia are the most severe manifestations of this disease.3, 4 This process begins when denuded or inflamed endothelial cells develop properties that permit platelet adhesion. At the site of endothelial dysfunction, activation of adherent platelet results in the release of inflammatory and mitogenic factors. After a series of dynamic and repetitive processes including amplified platelet activation, monocyte chemotaxis, adhesion, transmigration, and lipoprotein retention, plaque formation occurs.5 Consequently, the rupture or erosion of an atherosclerotic plaque produces a higher degree of platelet adhesion, activation, and aggregation, causing the fibrotic organization of a mural thrombus.3
The number of persons with multiple, concomitant cardiovascular disease (CAD), cerebrovascular disease (CVD), and peripheral arterial disease (PAD) accentuates the polyvascular nature of atherothrombosis (Fig. 1). The international Reduction of Atherothrombosis for Continued Health (REACH) Registry demonstrated that 1‐year incidence rates of major cardiovascular events (eg, MI, stroke, death) were high in patients with an established atherothrombotic disease and increased with the number of concomitant vascular diseases.6 These data infer that the burden on the vascular system is considered extensive on diagnosis of a single atherothrombotic disease. Thus, aggressive therapies are needed to reduce the risk of recurrent or other cardiovascular events. The management of risk factors for atherothrombosis such as hypercholesterolemia, dyslipidemia, hypertension, and diabetes mellitus fall under specific disease‐specific guidelines for patients presenting with atherothrombotic diseases.712

ANTIPLATELET THERAPIES
Antiplatelet therapies are used for the acute and long‐term treatment of patients after a thrombic event. Antiplatelet agents target the molecular mechanisms responsible for platelet activation and aggregation, such as the synthesis of thromboxane A2. On platelet activation, free arachidonic acid is converted to prostaglandin H2 (PGH2) by cyclooxygenase‐1 (COX‐1; Fig. 2). Further metabolism of PGH2 by thromboxane synthase produces thromboxane A2, which induces vasoconstriction (Fig. 2). Fortunately, the ability of platelets to produce COX‐1 is limited, and irreversible inhibition of this enzyme can impair thromboxane A2 synthesis for approximately 10 days.

Aspirin is a potent COX‐1 inhibitor, whose effects are evident 1 hour after dosing (Fig. 2).4, 13 Aspirin effectively prevents fatal and nonfatal vascular events in healthy individuals and in patients who present with acute MI or ischemic stroke.13 Unfortunately, a proportion of patients are aspirin resistant. Recent studies have indicated that interactions with the nonsteroidal anti‐inflammatory drug (NSAID) ibuprofen may diminish the primary and secondary protective effects of aspirin and may contribute to aspirin resistance, although the origin of this remains unclear.
The results of a post hoc subgroup analysis of 22,071 apparently healthy male physicians randomized to take aspirin or placebo for 5 years indicated that individuals who used NSAIDs for at least 60 days/year increased their risk of MI by more than 2‐fold compared with those who did not use NSAIDs.14 A second study conducted in patients following a major adverse cardiovascular event showed that the combination of aspirin plus ibuprofen increased the adjusted relative risk of cardiovascular mortality over an 8‐year period compared with aspirin alone.15 However, the effects of NSAIDS on aspirin's ability to inhibit COX‐1 are reversible and only last for the dosing interval and body clearance time of the drug.16
Adeonsine diphosphate (ADP)dependent stimulation of the P2Y12 receptor is another target for antiplatelet therapy. On its release, ADP binds to the P2Y12 receptor on platelets, resulting in activation and aggregation (Fig. 2). Ticlopidine and clopidogrel are thienopyridines that may irreversibly modify the P2Y12 receptor (Fig. 2).13 Safety concerns associated with ticlopidine use, including severe neutropenia, have limited its administration. Conversely, clopidogrel is relatively well‐tolerated and can prevent cardiovascular events in patients with CAD, ischemic stroke, and PAD. This agent is an orally administered prodrug requiring activation by hepatic cytochrome P450 enzymes.13
Aspirin and thienopyridines do not inhibit platelet aggregation induced by the binding of fibrinogen to the platelet glycoprotein (GP) IIb/IIIa receptor (Fig. 2).4, 13 However, there are 3 commonly administered GP IIb/IIIa inhibitors: abciximab, eptifibatide, and tirofiban (Fig. 2).4 Abciximab is the fab fragment of the chimeric monoclonal antibody 7E3 and irreversibly inhibits the GP IIb/IIIa receptor. By contrast, eptifibatide is a cyclic heptapeptide, tirofiban is a nonpeptide, and both agents are reversible inhibitors. These agents are administered intravenously, and boluses are reserved for the short‐term treatment of atherothrombosis in patients undergoing percutaneous coronary intervention.13
CONCLUSIONS
Atherothrombosis is a systemic disease that often affects coronary, intracranial, and peripheral arterial beds concomitantly, which increases the probability of a thrombotic event. Aggressive treatments, including acute and long‐term antiplatelet therapies, are required to reduce the risks associated with atherothrombosis. This supplement reviews the evidence‐based approaches for managing atherothrombosis. It will provide hospitalists with the knowledge needed to treat patients with PAD, stroke, and acute coronary syndrome. First, the administration of antiplatelet therapies to patients with acute coronary syndrome will be described. Then, guidelines for the management of patients with acute ischemic stroke and the use of antiplatelet therapies to reduce mortality due to primary and secondary ischemic events will be reviewed. Finally, the role of the hospitalist in the diagnosis of PAD in asymptomatic patients and in those with confirmed atherothrombosis will be discussed.
Acute atherothrombotic events associated with ischemic heart disease and stroke are the first and third most common causes of death in the United States, respectively.1 Despite an overall decrease in age‐adjusted mortality since 1970 in the United States, the worldwide prevalence of these diseases is anticipated to sharply increase by 2020.1, 2 Caring for patients with atherothrombosis is now within the purview of hospitalists to a larger extent than ever before. In recognition of the expanding role of these health care professionals and to reduce the risk of adverse cardiovascular events in the outpatient setting, the Society of Hospital Medicine held a symposium during its 10th Annual Meeting.
Rules of Engagement: The Hospitalist and Atherothrombosis took place on May 24, 2007, in Dallas, Texas. This supplement summarizes the highlights from this symposium and reviews the causes and polyvascular nature of atherothrombosis. The role of the hospitalist in managing atherothrombotic disease and evidence‐based practices for the evaluation and treatment of patients with various manifestations of atherothrombotic disease are also discussed.
ARTERIAL THROMBOSIS AND ITS POLYVASCULAR NATURE
Atherothrombosis refers to the formation of large and occlusive mural thrombi that arise from the rupture of an atherosclerotic plaque. Myocardial infarction (MI), ischemic stroke, and acute limb ischemia are the most severe manifestations of this disease.3, 4 This process begins when denuded or inflamed endothelial cells develop properties that permit platelet adhesion. At the site of endothelial dysfunction, activation of adherent platelet results in the release of inflammatory and mitogenic factors. After a series of dynamic and repetitive processes including amplified platelet activation, monocyte chemotaxis, adhesion, transmigration, and lipoprotein retention, plaque formation occurs.5 Consequently, the rupture or erosion of an atherosclerotic plaque produces a higher degree of platelet adhesion, activation, and aggregation, causing the fibrotic organization of a mural thrombus.3
The number of persons with multiple, concomitant cardiovascular disease (CAD), cerebrovascular disease (CVD), and peripheral arterial disease (PAD) accentuates the polyvascular nature of atherothrombosis (Fig. 1). The international Reduction of Atherothrombosis for Continued Health (REACH) Registry demonstrated that 1‐year incidence rates of major cardiovascular events (eg, MI, stroke, death) were high in patients with an established atherothrombotic disease and increased with the number of concomitant vascular diseases.6 These data infer that the burden on the vascular system is considered extensive on diagnosis of a single atherothrombotic disease. Thus, aggressive therapies are needed to reduce the risk of recurrent or other cardiovascular events. The management of risk factors for atherothrombosis such as hypercholesterolemia, dyslipidemia, hypertension, and diabetes mellitus fall under specific disease‐specific guidelines for patients presenting with atherothrombotic diseases.712

ANTIPLATELET THERAPIES
Antiplatelet therapies are used for the acute and long‐term treatment of patients after a thrombic event. Antiplatelet agents target the molecular mechanisms responsible for platelet activation and aggregation, such as the synthesis of thromboxane A2. On platelet activation, free arachidonic acid is converted to prostaglandin H2 (PGH2) by cyclooxygenase‐1 (COX‐1; Fig. 2). Further metabolism of PGH2 by thromboxane synthase produces thromboxane A2, which induces vasoconstriction (Fig. 2). Fortunately, the ability of platelets to produce COX‐1 is limited, and irreversible inhibition of this enzyme can impair thromboxane A2 synthesis for approximately 10 days.

Aspirin is a potent COX‐1 inhibitor, whose effects are evident 1 hour after dosing (Fig. 2).4, 13 Aspirin effectively prevents fatal and nonfatal vascular events in healthy individuals and in patients who present with acute MI or ischemic stroke.13 Unfortunately, a proportion of patients are aspirin resistant. Recent studies have indicated that interactions with the nonsteroidal anti‐inflammatory drug (NSAID) ibuprofen may diminish the primary and secondary protective effects of aspirin and may contribute to aspirin resistance, although the origin of this remains unclear.
The results of a post hoc subgroup analysis of 22,071 apparently healthy male physicians randomized to take aspirin or placebo for 5 years indicated that individuals who used NSAIDs for at least 60 days/year increased their risk of MI by more than 2‐fold compared with those who did not use NSAIDs.14 A second study conducted in patients following a major adverse cardiovascular event showed that the combination of aspirin plus ibuprofen increased the adjusted relative risk of cardiovascular mortality over an 8‐year period compared with aspirin alone.15 However, the effects of NSAIDS on aspirin's ability to inhibit COX‐1 are reversible and only last for the dosing interval and body clearance time of the drug.16
Adeonsine diphosphate (ADP)dependent stimulation of the P2Y12 receptor is another target for antiplatelet therapy. On its release, ADP binds to the P2Y12 receptor on platelets, resulting in activation and aggregation (Fig. 2). Ticlopidine and clopidogrel are thienopyridines that may irreversibly modify the P2Y12 receptor (Fig. 2).13 Safety concerns associated with ticlopidine use, including severe neutropenia, have limited its administration. Conversely, clopidogrel is relatively well‐tolerated and can prevent cardiovascular events in patients with CAD, ischemic stroke, and PAD. This agent is an orally administered prodrug requiring activation by hepatic cytochrome P450 enzymes.13
Aspirin and thienopyridines do not inhibit platelet aggregation induced by the binding of fibrinogen to the platelet glycoprotein (GP) IIb/IIIa receptor (Fig. 2).4, 13 However, there are 3 commonly administered GP IIb/IIIa inhibitors: abciximab, eptifibatide, and tirofiban (Fig. 2).4 Abciximab is the fab fragment of the chimeric monoclonal antibody 7E3 and irreversibly inhibits the GP IIb/IIIa receptor. By contrast, eptifibatide is a cyclic heptapeptide, tirofiban is a nonpeptide, and both agents are reversible inhibitors. These agents are administered intravenously, and boluses are reserved for the short‐term treatment of atherothrombosis in patients undergoing percutaneous coronary intervention.13
CONCLUSIONS
Atherothrombosis is a systemic disease that often affects coronary, intracranial, and peripheral arterial beds concomitantly, which increases the probability of a thrombotic event. Aggressive treatments, including acute and long‐term antiplatelet therapies, are required to reduce the risks associated with atherothrombosis. This supplement reviews the evidence‐based approaches for managing atherothrombosis. It will provide hospitalists with the knowledge needed to treat patients with PAD, stroke, and acute coronary syndrome. First, the administration of antiplatelet therapies to patients with acute coronary syndrome will be described. Then, guidelines for the management of patients with acute ischemic stroke and the use of antiplatelet therapies to reduce mortality due to primary and secondary ischemic events will be reviewed. Finally, the role of the hospitalist in the diagnosis of PAD in asymptomatic patients and in those with confirmed atherothrombosis will be discussed.
- ,,,.Trends in the leading causes of death in the United States, 1970‐2002.JAMA.2005;294:1255–1259.
- ,.The global burden of disease, 1990‐2020.Nat Med.1998:4:1241–1243.
- ,,,.The pathogenesis of coronary artery disease and the acute coronary syndromes.N Engl J Med.1992;326:242–250.
- .Antiplatelet therapy.Am J Med.1996;101:199–209.
- ,,.Platelets in inflammation and atherogenesis.J Clin Invest.2005;115:3378–3384.
- ,,, et al.One‐year cardiovascular event rates in outpatients with atherothrombosis.JAMA.2007;297:1197–1206.
- ,,, et al.ACC/AHA 2000 guidelines for management of patients with unstable angina and non‐ST‐segment elevation myocardial infarction.J Am Coll Cardiol.2000;36:970–1062.
- ,,, et al.ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction.Circulation.2004;110:82–292.
- ,,, et al.Guidelines for the prevention of stroke in patients with ischemic stroke or transient ischemic attack. A statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke.Stroke.2006;37:557–617.
- ,,, et al.ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines.Circulation.2006;113:463–654.
- ,,.Inflammation and atherosclerosis.Circulation.2002;105:1135–1143.
- ,,, et al.AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update.J Am Coll Cardiol.2006;47:2130–2139.
- ,,, et al.Platelet‐active drugs: the relationships among dose, effectiveness, and side effects.Chest.2001;119:39–63.
- ,,, et al.Inhibition of clinical benefits of aspirin on first myocardial infarction by nonsteroidal anti‐inflammatory drugs.Circulation.2003;108:1191–1195.
- ,.The effect of ibuprofen on cardioprotective effects of aspirin.Lancet.2003;361:573–574.
- ,,, et al.Cyclooxygenase inhibitors and the antiplatelet effects of aspirin.N Engl J Med.2001;345:1809–1817.
- ,,,.Trends in the leading causes of death in the United States, 1970‐2002.JAMA.2005;294:1255–1259.
- ,.The global burden of disease, 1990‐2020.Nat Med.1998:4:1241–1243.
- ,,,.The pathogenesis of coronary artery disease and the acute coronary syndromes.N Engl J Med.1992;326:242–250.
- .Antiplatelet therapy.Am J Med.1996;101:199–209.
- ,,.Platelets in inflammation and atherogenesis.J Clin Invest.2005;115:3378–3384.
- ,,, et al.One‐year cardiovascular event rates in outpatients with atherothrombosis.JAMA.2007;297:1197–1206.
- ,,, et al.ACC/AHA 2000 guidelines for management of patients with unstable angina and non‐ST‐segment elevation myocardial infarction.J Am Coll Cardiol.2000;36:970–1062.
- ,,, et al.ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction.Circulation.2004;110:82–292.
- ,,, et al.Guidelines for the prevention of stroke in patients with ischemic stroke or transient ischemic attack. A statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke.Stroke.2006;37:557–617.
- ,,, et al.ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines.Circulation.2006;113:463–654.
- ,,.Inflammation and atherosclerosis.Circulation.2002;105:1135–1143.
- ,,, et al.AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update.J Am Coll Cardiol.2006;47:2130–2139.
- ,,, et al.Platelet‐active drugs: the relationships among dose, effectiveness, and side effects.Chest.2001;119:39–63.
- ,,, et al.Inhibition of clinical benefits of aspirin on first myocardial infarction by nonsteroidal anti‐inflammatory drugs.Circulation.2003;108:1191–1195.
- ,.The effect of ibuprofen on cardioprotective effects of aspirin.Lancet.2003;361:573–574.
- ,,, et al.Cyclooxygenase inhibitors and the antiplatelet effects of aspirin.N Engl J Med.2001;345:1809–1817.
Rules of Engagement
Atherothrombosis triggered by plaque rupture reduces arterial blood flow, leading to myocardial ischemia and/or necrosis, in which the extent of occlusion relates to the clinical presentations of acute coronary syndrome (ACS): unstable angina (UA), non‐ST‐segment and ST‐segment elevated myocardial infarction (NSTEMI and STEMI, respectively). Although UA and NSTEMI may be indistinguishable at the time of presentation, NSTEMI is defined by myocardial necrosis and is differentiated by release of cardiac enzymes.1 In UA, the myocardial ischemia is reversible, without necrosis. Typically, STEMI results from the total occlusion of a large epicardial infarct‐related artery and is diagnosed by electrocardiography (ECG) and the release of cardiac enzymes.2 Strategies employed by emergency physicians and hospitalists to treat the spectrum of symptoms caused by ACS include pharmacotherapy and revascularization procedures. Coordination of care between these 2 groups of physicians, and appropriate handoff of patients from the ED to hospitalists, utilizing guideline‐based care pathways and treatment protocols, will ensure maximization of outcomes in patients with ACS.
TREATMENT STRATEGIES FOR ACUTE CORONARY SYNDROME
The American College of Cardiology and American Heart Association (ACC/AHA) guidelines indicate the need for rapid triage and aggressive treatment when managing patients with ACS.1, 2 Those patients with NSTEMI are quickly differentiated from those with STEMI, who are evaluated rapidly for pharmacological and/or revascularization therapy.2 Patients with UA/NSTEMI are further stratified according to their risk of death or nonfatal MI, and appropriate care pathways are instituted as indicated by their risk for adverse outcomes. These care pathways must be guideline driven to assure maximal effectiveness.
Percutaneous coronary intervention (PCI) and coronary artery bypass graft surgery (CABG) revascularization procedures have been highly successful with low complication rates in patients with ACS. Although revascularization in patients with UA/NSTEMI is determined in part by their risk stratification, those patients with STEMI are almost always candidates for PCI or CABG.
Unfortunately, vascular flow is not completely restored in many patients after undergoing revascularization, both STEMI and UA/NSTEMI. This may be attributed to microvascular damage caused by the formation of microemboli during the initial atherothrombotic event or revascularization.1, 2 Given that percutaneous catheters are incapable of restoring microvasculature patency, pharmacotherapy is the only alternative for this cohort of patients. Thus, acute antithrombotic therapies (antiplatelet agents and anticoagulants) are administered to maintain vascular flow in patients with ACS before and after revascularization. Maximization of the effectiveness of this antithrombotic therapy in the precatheterization period will decrease the occurrence of ischemia and the extent of infarction.
Patients with ACS often have multiple vulnerable plaques in addition to the culprit lesion responsible for the initial bout of ischemia. The presence of multiple vulnerable plaques increases the risk of secondary vascular events such as stroke, myocardial infarction (MI), or vascular death in patients with ACS.3, 4 Thus, ongoing anti‐inflammatory and antiplatelet therapies are needed to stabilize vulnerable plaques and prevent secondary vascular events.5 The ACC/AHA guidelines recommend sustained antiplatelet and antithrombin therapies to improve long‐term outcomes in patients with ACS.1
ASPIRIN THERAPY FOR ACUTE CORONARY SYNDROME
Aspirin effectively reduces the short‐term risk of myocardial ischemic events in patients with ACS. The RISC and ISIS‐2 studies showed a clinically relevant reduction in risk of MI or death after short durations of aspirin therapy in patients with UA (3 months) and suspected acute MI (5 weeks), respectively.6, 7 Although long‐term therapy with aspirin has not been tested among individual ACS subgroups, clinical studies have demonstrated that aspirin therapy effectively prevents MI, stroke, and vascular death in patients with prior MI or other vascular events. A meta‐analysis of 65 trials in patients with atherothrombosis showed that aspirin therapy reduces the odds of vascular events by 23% 2%.8 Moreover, aspirin has a class IA recommendation from the ACC/AHA for the management of patients with UA/NSTEMI or STEMI; in the absence of contraindications, it should be initiated as soon as possible and continued indefinitely.1, 2 On initial presentation of ACS, high‐dose aspirin is recommended (162‐325 mg), and lower doses (75‐162 mg), which minimize bleeding or gastrointestinal side effects, are indicated thereafter.1, 2
SUSTAINED ANTIPLATELET THERAPIES FORUA/NSTEMI
Clinical data have indicated that long‐term therapies may be more beneficial to patients with UA/NSTEMI than to those with STEMI. Antiplatelet therapy reduces the risk of vascular events in patients with UA/NSTEMI; benefits are noticeable soon after therapy is initiated. The sustained use of clopidogrel, an inhibitor of ADP‐dependent platelet activation, is at least as effective as aspirin in reducing long‐term vascular events in patients with atherothrombotic diseases, and the ACC/AHA recommends this agent be used when aspirin is contraindicated.1, 9
Recently, the Clopidogrel in Unstable Angina Recurrent Events (CURE) study demonstrated that the combination of clopidogrel and aspirin is superior to either agent alone in preventing vascular events in patients with UA/NSTEMI.10 Patients (N = 12,562) were randomized within 24 hours of UA/NSTEMI presentation to receive aspirin (75‐325 mg/day), an immediate loading dose of clopidogrel (300 mg) followed by once‐daily clopidogrel (75 mg), or a matching placebo for 3‐12 months.10 At the treating physicians' discretion, patients were treated with anticoagulants, revascularization procedures, or GP IIb/IIIa inhibitors after randomization. When used in combination with aspirin, clopidogrel reduced the risk of a major cardiovascular event by 20% (relative risk RR, 0.80; 95% CI, 0.72‐0.90; P .001) compared with aspirin alone.10
A retrospective subgroup analysis revealed that the therapeutic benefits of this drug combination increases as the risk of a vascular event increases in patients with UA/NSTEMI.11 In addition, the effects of clopidogrel and aspirin were apparent early after randomization (Fig. 1); a 34% reduction in the risk of major cardiovascular events (RR, 0.66; 95% CI, 0.51‐0.86; P .003) was observed within 24 hours postrandomization.12 Thirty days after randomization, the relative risk reduction in the composite end point of cardiovascular death and nonfatal MI, stroke, and refractory ischemia was 17% (RR, 0.83; 95% CI, 0.73‐0.93; P .002) for clopidogrel with aspirin.
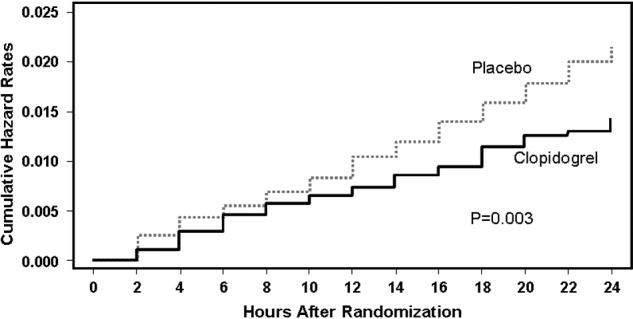
The combination of clopidogrel and aspirin was associated with a 38% (RR, 1.38; 95% CI, 1.13‐1.67; P = .001) increase in major bleeding episodes compared with patients taking aspirin alone. Conversely, the incidence of bleeding requiring surgical intervention, hemorrhagic stroke, and fatal hemorrhage between treatment groups did not differ significantly.10 The clinical benefits of clopidogrel and aspirin outweighed the risk of life‐threatening bleeding (RR, 0.84; 95% CI, 0.76‐0.93), even when the number of deaths and the number of life‐threatening bleeds were taken into account in the efficacy‐safety analysis.13 As a result, the ACC/AHA guidelines for the management of patients with UA/NSTEMI recommend that clopidogrel and aspirin should be administered to hospitalized patients as soon as possible and should be continued for 1 month and up to 12 months after the initial presentation of symptoms.1 The new ACC/AHA guidelines recommend upstream clopidogrel as a class IA treatment for UA/non‐ST‐elevation MI in patients who are managed either invasively (catheterization within 6‐24 hours) or conservatively (selectively invasive medical management, followed by catheterization if needed). This latter recommendation is much stronger than the prior guidelines and illustrates the importance of antiplatelet therapy as a part of medical management for intermediate‐ to high‐risk ACS.
ANTIPLATELET THERAPIES WITH REVASCULARIZATION PROCEDURES IN UA/NSTEMI
The efficacy of clopidogrel and aspirin has been examined in patients enrolled in the CURE study who underwent revascularization procedures (eg, PCI, CABG). These agents reduced the relative risk of cardiovascular death or nonfatal MI by 31% (RR, 0.69; 95% CI, 0.54‐0.87; P = .002) in patients who underwent PCI.14 Subgroup analysis of PCI timing relative to randomization showed that the risk of vascular events increased with time to revascularization. However, this relationship was not evident in patients treated with aspirin alone (Fig. 2).14 There was no significant difference in the incidence of major bleeding (RR, 1.2; 95% CI, 0.70‐1.78; P = .64) between the treatment groups from PCI to follow‐up.14, 15 Similar results have been reported elsewhere.16 As a result, ACC/AHA guidelines recommend the early administration of clopidogrel and aspirin to patients undergoing planned PCI, and should be continued up to 12 months following the procedure, unless it is contraindicated.1
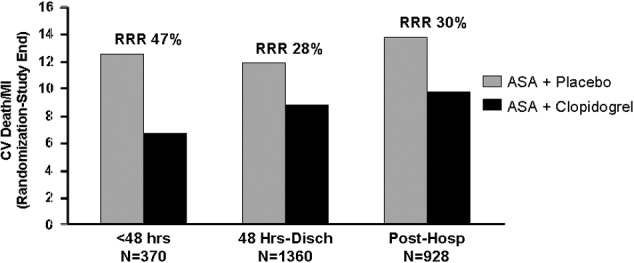
Clopidogrel and aspirin also reduced the risk of vascular death by 11% (RR, 0.89; 95% CI, 0.71‐1.11) in patients who underwent CABG.13 Study medications were stopped prior to the procedure. Major bleeding was not observed in patients who stopped medication at least 5 days prior to surgery and did not differ significantly from those who stopped therapy less than 5 days before CABG.13 Thus, the ACC/AHA recommends withholding clopidogrel therapy for 5‐7 days prior to elective CABG.1
Platelet glycoprotein (GP) IIb/IIIa inhibitors are another class of antiplatelet therapy that can be beneficial to patients with UA/NSTEMI undergoing PCI. Analysis of the CAPTURE, PURSUIT, and PRISM‐PLUS trials of the GP IIb/IIIa inhibitors abciximab, eptifibatide, and tirofiban, respectively, showed they effectively reduce the rates of death and/or MI (odds ratio [OR], 0.66; 95% CI, 0.54‐0.81) in patients with UA/STEMI prior to PCI, which was more pronounced (OR, 0.59; 95% CI, 0.44‐0.81) when outcomes were measured 48 hours after revascularization.17
The ISAR‐REACT 2 study examined the efficacy of abciximab with clopidogrel and aspirin in patients with NSTEMI prior to PCI.18 The primary end point was a composite of death, MI, and secondary urgent target revascularization 30 days after randomization. The addition of abciximab to 600 mg of the clopidogrel loading dose and aspirin reduced the risk of major adverse cardiovascular events by 25% (RR, 0.75; 95% CI, 0.58‐0.97; P = .03) 30 days after the initiation of therapy. However, subgroup analysis revealed that this therapeutic benefit was confined to patients with elevated troponin levels.18 The ACC/AHA also recommends the concomitant administration of GP IIb/IIIa inhibitors to patients receiving heparin, aspirin, and clopidogrel and undergoing planned PCI.1 The guideline recommendation is for either clopidogrel or a GP IIb/IIIa inhibitor in the invasive pathway (class IA), but both are recommended in patients with elevated troponin, recurrent ischemia, or delay to catheterization (class IIaB). This triple antiplatelet therapy is considered advantageous in the highest=risk NSTEMI patients. The short‐term and long‐term benefits of antiplatelet therapies are consistent across the UA/NSTEMI‐risk spectrum and galvanize the ACC/AHA recommendations for antithrombotic therapy in patients with UA/NSTEMI (Fig. 3).1
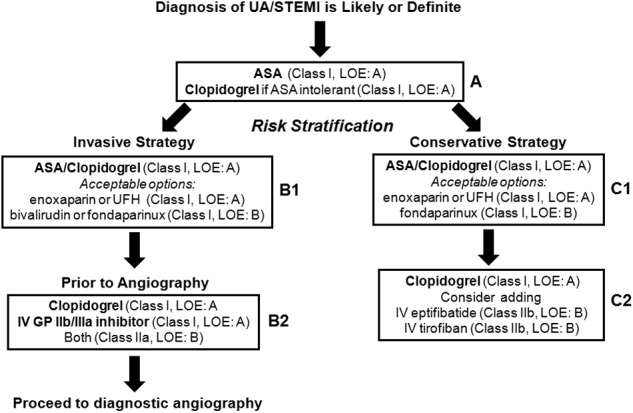
ANTIPLATELET THERAPIES FOR STEMI
The ACC/AHA has made several recommendations regarding the administration of clopidogrel in patients with STEMI. Clopidogrel should be administered to patients with contraindications to aspirin. After placement of a bare metal or drug‐eluting stent, this agent should be administered at least 1 month and less than 12 months after surgery, respectively. It should also be withheld at least 5‐7 days prior to CABG.2 These guidelines are largely based on clinical trials in patients with UA/STEMI or ACS.9, 15 Fortunately, several more recent studies have examined the use of antiplatelet therapy in patients with STEMI.
In the Clopidogrel as Adjunctive Reperfusion TherapyThrombolysis in Myocardial Infarction (CLARITY‐TIMI) 28 study, 3491 patients with STEMI were randomized to receive clopidogrel (300 mg loading dose, then 75 mg/day) or placebo. Patients were also treated with a fibrinolytic agent, aspirin, and unfractionated heparin and underwent angiography 48‐192 hours after randomization. Clopidogrel reduced the risk of detecting an occluded infarct‐related artery by angiography or recurrent MI/death prior to angiography by 36% (OR, 0.64; 95% CI, 0.53‐0.76; P .001) compared with placebo.19 It also reduced the risk of major adverse cardiovascular events 30 days after randomization by 20% (OR, 0.80; 95% CI, 0.65‐0.97; P = .03) compared with placebo, with no significant difference in the risk of bleeding between the treatment groups.
The PCI‐CLARITY study examined the efficacy of clopidogrel in patients undergoing PCI during the CLARITY‐TIMI 28 trial. Clopidogrel reduced the rate of major adverse cardiovascular events by 46% (OR, 0.54; 95% CI, 0.35‐0.85; P = .008) after PCI and 30 days after randomization, with no excess in major bleeding.20 Although the use of this agent along with contemporary reperfusion therapies in patients with STEMI is supported, further research into the sustained use of clopidogrel in STEMI is needed.
CONCLUSIONS
Patients with ACS require aggressive diagnosis and acute treatment. However, long‐term therapies are also needed to improve outcomes. Antiplatelet therapies are a key component of the treatment of ACS. The benefits of aspirin and clopidogrel combination therapy are evident early, and their sustained use improves the outcome of patients who receive medical therapy and/or revascularization procedures. Early initiation of antiplatelet therapy in patients with ACS is best accomplished with care pathways or ACS protocols that are guideline driven. Initiation of these protocols in the ED, with appropriate handoff to hospitalists, will ensure maximization of antiplatelet therapy for patients throughout the precatheterization medical management period. Although antiplatelet agents may be associated with an increased risk of bleeding in some patients, these risks can be minimized and are outweighed by the benefits of clopidogrel and aspirin.
- ,,, et al.ACC/AHA guidelines for the management of patients with unstable angina/non‐ST‐segment elevation myocardial infarction: executive summary.J Am Coll Cardiol.2007;50:e1–e157. Available at: http://www.acc.org.
- ,,, et al.ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction.Circulation.2004;110:82–292.
- Antiplatelet Trialists' Collaboration.Collaborative overview of randomized trials of antiplatelet therapy. Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients.BMJ.1994;308:81–106.
- ,,, et al.One‐year cardiovascular event rates in outpatients with atherothrombosis.JAMA.2007;297:1197–1206.
- ,,, et al.Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association.Circulation.2006;113:873–823.
- The RISC Group.Risk of myocardial infarction and death during treatment with low dose aspirin and intravenous heparin in men with unstable coronary artery disease.Lancet.1990;336:827–830.
- ISIS‐2 Collaborative Group.Randomized trial of intravenous streptokinase, oral aspirin, both or neither among 17,187 cases of suspected acute myocardial infarction: ISIS‐2.Lancet.1988;2:349–360.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.BMJ.2002;324:71–86.
- CAPRIE steering committee.A randomized, blinded, trial of clopidogrel versus aspirin in patients at risk of ischemic events (CAPRIE).Lancet.1996;348:1329–1339.
- CURE Trial Investigators.Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST‐segment elevation.N Engl J Med.2001;345:494–502.
- ,,, et al.Benefit of clopidogrel in patients with acute coronary syndromes without ST‐segment elevation in various risk groups.Circulation.2002;106:1622–1626.
- ,,, et al.Early and late effects of clopidogrel in patients with acute coronary syndromes.Circulation.2003;107:966–972.
- ,,, et al.Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non‐ST‐elevation acute coronary syndrome: The clopidogrel in unstable angina to prevent recurrent ischemic events (CURE) trial.Circulation.2004;110:1202–1208.
- ,,, et al.Benefit of clopidogrel according to timing of percutaneous coronary intervention in patients with acute coronary syndromes: Further results from the clopidogrel in unstable angina to prevent recurrent events (CURE) study.Am Heart J.2005;150:1177–1184.
- ,,, et al.Effects of pretreatment with clopidogrel and aspirin followed by long‐term therapy in patients undergoing percutaneous coronary intervention: the PCI‐CURE study.Lancet.2001;358:527–533.
- ,,, et al.Early and sustained dual oral antiplatelet therapy following percutaneous intervention. A randomized trial.JAMA.2002;288:2411–2420.
- ,,,,,.Platelet glycoprotein IIb/IIIa receptor inhibition in non‐ST‐elevation acute coronary syndromes: early benefit during medical treatment only, with additional protection during percutaneous coronary intervention.Circulation.1999;100:2045–2048.
- ,,, et al.Abciximab in patients with acute coronary syndromes undergoing percutaneous coronary intervention after clopidogrel pretreatment. The ISAR‐REACT2 randomized trial.JAMA.2006;295:1531–1538.
- ,,, et al.Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST‐segment elevation.N Engl J Med.2005;352:1179–1189.
- ,,, et al.Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST‐elevation myocardial infarction treated with fibrinolytics. The PCI‐CLARITY study.JAMA.2005;294:1224–1232.
Atherothrombosis triggered by plaque rupture reduces arterial blood flow, leading to myocardial ischemia and/or necrosis, in which the extent of occlusion relates to the clinical presentations of acute coronary syndrome (ACS): unstable angina (UA), non‐ST‐segment and ST‐segment elevated myocardial infarction (NSTEMI and STEMI, respectively). Although UA and NSTEMI may be indistinguishable at the time of presentation, NSTEMI is defined by myocardial necrosis and is differentiated by release of cardiac enzymes.1 In UA, the myocardial ischemia is reversible, without necrosis. Typically, STEMI results from the total occlusion of a large epicardial infarct‐related artery and is diagnosed by electrocardiography (ECG) and the release of cardiac enzymes.2 Strategies employed by emergency physicians and hospitalists to treat the spectrum of symptoms caused by ACS include pharmacotherapy and revascularization procedures. Coordination of care between these 2 groups of physicians, and appropriate handoff of patients from the ED to hospitalists, utilizing guideline‐based care pathways and treatment protocols, will ensure maximization of outcomes in patients with ACS.
TREATMENT STRATEGIES FOR ACUTE CORONARY SYNDROME
The American College of Cardiology and American Heart Association (ACC/AHA) guidelines indicate the need for rapid triage and aggressive treatment when managing patients with ACS.1, 2 Those patients with NSTEMI are quickly differentiated from those with STEMI, who are evaluated rapidly for pharmacological and/or revascularization therapy.2 Patients with UA/NSTEMI are further stratified according to their risk of death or nonfatal MI, and appropriate care pathways are instituted as indicated by their risk for adverse outcomes. These care pathways must be guideline driven to assure maximal effectiveness.
Percutaneous coronary intervention (PCI) and coronary artery bypass graft surgery (CABG) revascularization procedures have been highly successful with low complication rates in patients with ACS. Although revascularization in patients with UA/NSTEMI is determined in part by their risk stratification, those patients with STEMI are almost always candidates for PCI or CABG.
Unfortunately, vascular flow is not completely restored in many patients after undergoing revascularization, both STEMI and UA/NSTEMI. This may be attributed to microvascular damage caused by the formation of microemboli during the initial atherothrombotic event or revascularization.1, 2 Given that percutaneous catheters are incapable of restoring microvasculature patency, pharmacotherapy is the only alternative for this cohort of patients. Thus, acute antithrombotic therapies (antiplatelet agents and anticoagulants) are administered to maintain vascular flow in patients with ACS before and after revascularization. Maximization of the effectiveness of this antithrombotic therapy in the precatheterization period will decrease the occurrence of ischemia and the extent of infarction.
Patients with ACS often have multiple vulnerable plaques in addition to the culprit lesion responsible for the initial bout of ischemia. The presence of multiple vulnerable plaques increases the risk of secondary vascular events such as stroke, myocardial infarction (MI), or vascular death in patients with ACS.3, 4 Thus, ongoing anti‐inflammatory and antiplatelet therapies are needed to stabilize vulnerable plaques and prevent secondary vascular events.5 The ACC/AHA guidelines recommend sustained antiplatelet and antithrombin therapies to improve long‐term outcomes in patients with ACS.1
ASPIRIN THERAPY FOR ACUTE CORONARY SYNDROME
Aspirin effectively reduces the short‐term risk of myocardial ischemic events in patients with ACS. The RISC and ISIS‐2 studies showed a clinically relevant reduction in risk of MI or death after short durations of aspirin therapy in patients with UA (3 months) and suspected acute MI (5 weeks), respectively.6, 7 Although long‐term therapy with aspirin has not been tested among individual ACS subgroups, clinical studies have demonstrated that aspirin therapy effectively prevents MI, stroke, and vascular death in patients with prior MI or other vascular events. A meta‐analysis of 65 trials in patients with atherothrombosis showed that aspirin therapy reduces the odds of vascular events by 23% 2%.8 Moreover, aspirin has a class IA recommendation from the ACC/AHA for the management of patients with UA/NSTEMI or STEMI; in the absence of contraindications, it should be initiated as soon as possible and continued indefinitely.1, 2 On initial presentation of ACS, high‐dose aspirin is recommended (162‐325 mg), and lower doses (75‐162 mg), which minimize bleeding or gastrointestinal side effects, are indicated thereafter.1, 2
SUSTAINED ANTIPLATELET THERAPIES FORUA/NSTEMI
Clinical data have indicated that long‐term therapies may be more beneficial to patients with UA/NSTEMI than to those with STEMI. Antiplatelet therapy reduces the risk of vascular events in patients with UA/NSTEMI; benefits are noticeable soon after therapy is initiated. The sustained use of clopidogrel, an inhibitor of ADP‐dependent platelet activation, is at least as effective as aspirin in reducing long‐term vascular events in patients with atherothrombotic diseases, and the ACC/AHA recommends this agent be used when aspirin is contraindicated.1, 9
Recently, the Clopidogrel in Unstable Angina Recurrent Events (CURE) study demonstrated that the combination of clopidogrel and aspirin is superior to either agent alone in preventing vascular events in patients with UA/NSTEMI.10 Patients (N = 12,562) were randomized within 24 hours of UA/NSTEMI presentation to receive aspirin (75‐325 mg/day), an immediate loading dose of clopidogrel (300 mg) followed by once‐daily clopidogrel (75 mg), or a matching placebo for 3‐12 months.10 At the treating physicians' discretion, patients were treated with anticoagulants, revascularization procedures, or GP IIb/IIIa inhibitors after randomization. When used in combination with aspirin, clopidogrel reduced the risk of a major cardiovascular event by 20% (relative risk RR, 0.80; 95% CI, 0.72‐0.90; P .001) compared with aspirin alone.10
A retrospective subgroup analysis revealed that the therapeutic benefits of this drug combination increases as the risk of a vascular event increases in patients with UA/NSTEMI.11 In addition, the effects of clopidogrel and aspirin were apparent early after randomization (Fig. 1); a 34% reduction in the risk of major cardiovascular events (RR, 0.66; 95% CI, 0.51‐0.86; P .003) was observed within 24 hours postrandomization.12 Thirty days after randomization, the relative risk reduction in the composite end point of cardiovascular death and nonfatal MI, stroke, and refractory ischemia was 17% (RR, 0.83; 95% CI, 0.73‐0.93; P .002) for clopidogrel with aspirin.

The combination of clopidogrel and aspirin was associated with a 38% (RR, 1.38; 95% CI, 1.13‐1.67; P = .001) increase in major bleeding episodes compared with patients taking aspirin alone. Conversely, the incidence of bleeding requiring surgical intervention, hemorrhagic stroke, and fatal hemorrhage between treatment groups did not differ significantly.10 The clinical benefits of clopidogrel and aspirin outweighed the risk of life‐threatening bleeding (RR, 0.84; 95% CI, 0.76‐0.93), even when the number of deaths and the number of life‐threatening bleeds were taken into account in the efficacy‐safety analysis.13 As a result, the ACC/AHA guidelines for the management of patients with UA/NSTEMI recommend that clopidogrel and aspirin should be administered to hospitalized patients as soon as possible and should be continued for 1 month and up to 12 months after the initial presentation of symptoms.1 The new ACC/AHA guidelines recommend upstream clopidogrel as a class IA treatment for UA/non‐ST‐elevation MI in patients who are managed either invasively (catheterization within 6‐24 hours) or conservatively (selectively invasive medical management, followed by catheterization if needed). This latter recommendation is much stronger than the prior guidelines and illustrates the importance of antiplatelet therapy as a part of medical management for intermediate‐ to high‐risk ACS.
ANTIPLATELET THERAPIES WITH REVASCULARIZATION PROCEDURES IN UA/NSTEMI
The efficacy of clopidogrel and aspirin has been examined in patients enrolled in the CURE study who underwent revascularization procedures (eg, PCI, CABG). These agents reduced the relative risk of cardiovascular death or nonfatal MI by 31% (RR, 0.69; 95% CI, 0.54‐0.87; P = .002) in patients who underwent PCI.14 Subgroup analysis of PCI timing relative to randomization showed that the risk of vascular events increased with time to revascularization. However, this relationship was not evident in patients treated with aspirin alone (Fig. 2).14 There was no significant difference in the incidence of major bleeding (RR, 1.2; 95% CI, 0.70‐1.78; P = .64) between the treatment groups from PCI to follow‐up.14, 15 Similar results have been reported elsewhere.16 As a result, ACC/AHA guidelines recommend the early administration of clopidogrel and aspirin to patients undergoing planned PCI, and should be continued up to 12 months following the procedure, unless it is contraindicated.1

Clopidogrel and aspirin also reduced the risk of vascular death by 11% (RR, 0.89; 95% CI, 0.71‐1.11) in patients who underwent CABG.13 Study medications were stopped prior to the procedure. Major bleeding was not observed in patients who stopped medication at least 5 days prior to surgery and did not differ significantly from those who stopped therapy less than 5 days before CABG.13 Thus, the ACC/AHA recommends withholding clopidogrel therapy for 5‐7 days prior to elective CABG.1
Platelet glycoprotein (GP) IIb/IIIa inhibitors are another class of antiplatelet therapy that can be beneficial to patients with UA/NSTEMI undergoing PCI. Analysis of the CAPTURE, PURSUIT, and PRISM‐PLUS trials of the GP IIb/IIIa inhibitors abciximab, eptifibatide, and tirofiban, respectively, showed they effectively reduce the rates of death and/or MI (odds ratio [OR], 0.66; 95% CI, 0.54‐0.81) in patients with UA/STEMI prior to PCI, which was more pronounced (OR, 0.59; 95% CI, 0.44‐0.81) when outcomes were measured 48 hours after revascularization.17
The ISAR‐REACT 2 study examined the efficacy of abciximab with clopidogrel and aspirin in patients with NSTEMI prior to PCI.18 The primary end point was a composite of death, MI, and secondary urgent target revascularization 30 days after randomization. The addition of abciximab to 600 mg of the clopidogrel loading dose and aspirin reduced the risk of major adverse cardiovascular events by 25% (RR, 0.75; 95% CI, 0.58‐0.97; P = .03) 30 days after the initiation of therapy. However, subgroup analysis revealed that this therapeutic benefit was confined to patients with elevated troponin levels.18 The ACC/AHA also recommends the concomitant administration of GP IIb/IIIa inhibitors to patients receiving heparin, aspirin, and clopidogrel and undergoing planned PCI.1 The guideline recommendation is for either clopidogrel or a GP IIb/IIIa inhibitor in the invasive pathway (class IA), but both are recommended in patients with elevated troponin, recurrent ischemia, or delay to catheterization (class IIaB). This triple antiplatelet therapy is considered advantageous in the highest=risk NSTEMI patients. The short‐term and long‐term benefits of antiplatelet therapies are consistent across the UA/NSTEMI‐risk spectrum and galvanize the ACC/AHA recommendations for antithrombotic therapy in patients with UA/NSTEMI (Fig. 3).1

ANTIPLATELET THERAPIES FOR STEMI
The ACC/AHA has made several recommendations regarding the administration of clopidogrel in patients with STEMI. Clopidogrel should be administered to patients with contraindications to aspirin. After placement of a bare metal or drug‐eluting stent, this agent should be administered at least 1 month and less than 12 months after surgery, respectively. It should also be withheld at least 5‐7 days prior to CABG.2 These guidelines are largely based on clinical trials in patients with UA/STEMI or ACS.9, 15 Fortunately, several more recent studies have examined the use of antiplatelet therapy in patients with STEMI.
In the Clopidogrel as Adjunctive Reperfusion TherapyThrombolysis in Myocardial Infarction (CLARITY‐TIMI) 28 study, 3491 patients with STEMI were randomized to receive clopidogrel (300 mg loading dose, then 75 mg/day) or placebo. Patients were also treated with a fibrinolytic agent, aspirin, and unfractionated heparin and underwent angiography 48‐192 hours after randomization. Clopidogrel reduced the risk of detecting an occluded infarct‐related artery by angiography or recurrent MI/death prior to angiography by 36% (OR, 0.64; 95% CI, 0.53‐0.76; P .001) compared with placebo.19 It also reduced the risk of major adverse cardiovascular events 30 days after randomization by 20% (OR, 0.80; 95% CI, 0.65‐0.97; P = .03) compared with placebo, with no significant difference in the risk of bleeding between the treatment groups.
The PCI‐CLARITY study examined the efficacy of clopidogrel in patients undergoing PCI during the CLARITY‐TIMI 28 trial. Clopidogrel reduced the rate of major adverse cardiovascular events by 46% (OR, 0.54; 95% CI, 0.35‐0.85; P = .008) after PCI and 30 days after randomization, with no excess in major bleeding.20 Although the use of this agent along with contemporary reperfusion therapies in patients with STEMI is supported, further research into the sustained use of clopidogrel in STEMI is needed.
CONCLUSIONS
Patients with ACS require aggressive diagnosis and acute treatment. However, long‐term therapies are also needed to improve outcomes. Antiplatelet therapies are a key component of the treatment of ACS. The benefits of aspirin and clopidogrel combination therapy are evident early, and their sustained use improves the outcome of patients who receive medical therapy and/or revascularization procedures. Early initiation of antiplatelet therapy in patients with ACS is best accomplished with care pathways or ACS protocols that are guideline driven. Initiation of these protocols in the ED, with appropriate handoff to hospitalists, will ensure maximization of antiplatelet therapy for patients throughout the precatheterization medical management period. Although antiplatelet agents may be associated with an increased risk of bleeding in some patients, these risks can be minimized and are outweighed by the benefits of clopidogrel and aspirin.
Atherothrombosis triggered by plaque rupture reduces arterial blood flow, leading to myocardial ischemia and/or necrosis, in which the extent of occlusion relates to the clinical presentations of acute coronary syndrome (ACS): unstable angina (UA), non‐ST‐segment and ST‐segment elevated myocardial infarction (NSTEMI and STEMI, respectively). Although UA and NSTEMI may be indistinguishable at the time of presentation, NSTEMI is defined by myocardial necrosis and is differentiated by release of cardiac enzymes.1 In UA, the myocardial ischemia is reversible, without necrosis. Typically, STEMI results from the total occlusion of a large epicardial infarct‐related artery and is diagnosed by electrocardiography (ECG) and the release of cardiac enzymes.2 Strategies employed by emergency physicians and hospitalists to treat the spectrum of symptoms caused by ACS include pharmacotherapy and revascularization procedures. Coordination of care between these 2 groups of physicians, and appropriate handoff of patients from the ED to hospitalists, utilizing guideline‐based care pathways and treatment protocols, will ensure maximization of outcomes in patients with ACS.
TREATMENT STRATEGIES FOR ACUTE CORONARY SYNDROME
The American College of Cardiology and American Heart Association (ACC/AHA) guidelines indicate the need for rapid triage and aggressive treatment when managing patients with ACS.1, 2 Those patients with NSTEMI are quickly differentiated from those with STEMI, who are evaluated rapidly for pharmacological and/or revascularization therapy.2 Patients with UA/NSTEMI are further stratified according to their risk of death or nonfatal MI, and appropriate care pathways are instituted as indicated by their risk for adverse outcomes. These care pathways must be guideline driven to assure maximal effectiveness.
Percutaneous coronary intervention (PCI) and coronary artery bypass graft surgery (CABG) revascularization procedures have been highly successful with low complication rates in patients with ACS. Although revascularization in patients with UA/NSTEMI is determined in part by their risk stratification, those patients with STEMI are almost always candidates for PCI or CABG.
Unfortunately, vascular flow is not completely restored in many patients after undergoing revascularization, both STEMI and UA/NSTEMI. This may be attributed to microvascular damage caused by the formation of microemboli during the initial atherothrombotic event or revascularization.1, 2 Given that percutaneous catheters are incapable of restoring microvasculature patency, pharmacotherapy is the only alternative for this cohort of patients. Thus, acute antithrombotic therapies (antiplatelet agents and anticoagulants) are administered to maintain vascular flow in patients with ACS before and after revascularization. Maximization of the effectiveness of this antithrombotic therapy in the precatheterization period will decrease the occurrence of ischemia and the extent of infarction.
Patients with ACS often have multiple vulnerable plaques in addition to the culprit lesion responsible for the initial bout of ischemia. The presence of multiple vulnerable plaques increases the risk of secondary vascular events such as stroke, myocardial infarction (MI), or vascular death in patients with ACS.3, 4 Thus, ongoing anti‐inflammatory and antiplatelet therapies are needed to stabilize vulnerable plaques and prevent secondary vascular events.5 The ACC/AHA guidelines recommend sustained antiplatelet and antithrombin therapies to improve long‐term outcomes in patients with ACS.1
ASPIRIN THERAPY FOR ACUTE CORONARY SYNDROME
Aspirin effectively reduces the short‐term risk of myocardial ischemic events in patients with ACS. The RISC and ISIS‐2 studies showed a clinically relevant reduction in risk of MI or death after short durations of aspirin therapy in patients with UA (3 months) and suspected acute MI (5 weeks), respectively.6, 7 Although long‐term therapy with aspirin has not been tested among individual ACS subgroups, clinical studies have demonstrated that aspirin therapy effectively prevents MI, stroke, and vascular death in patients with prior MI or other vascular events. A meta‐analysis of 65 trials in patients with atherothrombosis showed that aspirin therapy reduces the odds of vascular events by 23% 2%.8 Moreover, aspirin has a class IA recommendation from the ACC/AHA for the management of patients with UA/NSTEMI or STEMI; in the absence of contraindications, it should be initiated as soon as possible and continued indefinitely.1, 2 On initial presentation of ACS, high‐dose aspirin is recommended (162‐325 mg), and lower doses (75‐162 mg), which minimize bleeding or gastrointestinal side effects, are indicated thereafter.1, 2
SUSTAINED ANTIPLATELET THERAPIES FORUA/NSTEMI
Clinical data have indicated that long‐term therapies may be more beneficial to patients with UA/NSTEMI than to those with STEMI. Antiplatelet therapy reduces the risk of vascular events in patients with UA/NSTEMI; benefits are noticeable soon after therapy is initiated. The sustained use of clopidogrel, an inhibitor of ADP‐dependent platelet activation, is at least as effective as aspirin in reducing long‐term vascular events in patients with atherothrombotic diseases, and the ACC/AHA recommends this agent be used when aspirin is contraindicated.1, 9
Recently, the Clopidogrel in Unstable Angina Recurrent Events (CURE) study demonstrated that the combination of clopidogrel and aspirin is superior to either agent alone in preventing vascular events in patients with UA/NSTEMI.10 Patients (N = 12,562) were randomized within 24 hours of UA/NSTEMI presentation to receive aspirin (75‐325 mg/day), an immediate loading dose of clopidogrel (300 mg) followed by once‐daily clopidogrel (75 mg), or a matching placebo for 3‐12 months.10 At the treating physicians' discretion, patients were treated with anticoagulants, revascularization procedures, or GP IIb/IIIa inhibitors after randomization. When used in combination with aspirin, clopidogrel reduced the risk of a major cardiovascular event by 20% (relative risk RR, 0.80; 95% CI, 0.72‐0.90; P .001) compared with aspirin alone.10
A retrospective subgroup analysis revealed that the therapeutic benefits of this drug combination increases as the risk of a vascular event increases in patients with UA/NSTEMI.11 In addition, the effects of clopidogrel and aspirin were apparent early after randomization (Fig. 1); a 34% reduction in the risk of major cardiovascular events (RR, 0.66; 95% CI, 0.51‐0.86; P .003) was observed within 24 hours postrandomization.12 Thirty days after randomization, the relative risk reduction in the composite end point of cardiovascular death and nonfatal MI, stroke, and refractory ischemia was 17% (RR, 0.83; 95% CI, 0.73‐0.93; P .002) for clopidogrel with aspirin.

The combination of clopidogrel and aspirin was associated with a 38% (RR, 1.38; 95% CI, 1.13‐1.67; P = .001) increase in major bleeding episodes compared with patients taking aspirin alone. Conversely, the incidence of bleeding requiring surgical intervention, hemorrhagic stroke, and fatal hemorrhage between treatment groups did not differ significantly.10 The clinical benefits of clopidogrel and aspirin outweighed the risk of life‐threatening bleeding (RR, 0.84; 95% CI, 0.76‐0.93), even when the number of deaths and the number of life‐threatening bleeds were taken into account in the efficacy‐safety analysis.13 As a result, the ACC/AHA guidelines for the management of patients with UA/NSTEMI recommend that clopidogrel and aspirin should be administered to hospitalized patients as soon as possible and should be continued for 1 month and up to 12 months after the initial presentation of symptoms.1 The new ACC/AHA guidelines recommend upstream clopidogrel as a class IA treatment for UA/non‐ST‐elevation MI in patients who are managed either invasively (catheterization within 6‐24 hours) or conservatively (selectively invasive medical management, followed by catheterization if needed). This latter recommendation is much stronger than the prior guidelines and illustrates the importance of antiplatelet therapy as a part of medical management for intermediate‐ to high‐risk ACS.
ANTIPLATELET THERAPIES WITH REVASCULARIZATION PROCEDURES IN UA/NSTEMI
The efficacy of clopidogrel and aspirin has been examined in patients enrolled in the CURE study who underwent revascularization procedures (eg, PCI, CABG). These agents reduced the relative risk of cardiovascular death or nonfatal MI by 31% (RR, 0.69; 95% CI, 0.54‐0.87; P = .002) in patients who underwent PCI.14 Subgroup analysis of PCI timing relative to randomization showed that the risk of vascular events increased with time to revascularization. However, this relationship was not evident in patients treated with aspirin alone (Fig. 2).14 There was no significant difference in the incidence of major bleeding (RR, 1.2; 95% CI, 0.70‐1.78; P = .64) between the treatment groups from PCI to follow‐up.14, 15 Similar results have been reported elsewhere.16 As a result, ACC/AHA guidelines recommend the early administration of clopidogrel and aspirin to patients undergoing planned PCI, and should be continued up to 12 months following the procedure, unless it is contraindicated.1

Clopidogrel and aspirin also reduced the risk of vascular death by 11% (RR, 0.89; 95% CI, 0.71‐1.11) in patients who underwent CABG.13 Study medications were stopped prior to the procedure. Major bleeding was not observed in patients who stopped medication at least 5 days prior to surgery and did not differ significantly from those who stopped therapy less than 5 days before CABG.13 Thus, the ACC/AHA recommends withholding clopidogrel therapy for 5‐7 days prior to elective CABG.1
Platelet glycoprotein (GP) IIb/IIIa inhibitors are another class of antiplatelet therapy that can be beneficial to patients with UA/NSTEMI undergoing PCI. Analysis of the CAPTURE, PURSUIT, and PRISM‐PLUS trials of the GP IIb/IIIa inhibitors abciximab, eptifibatide, and tirofiban, respectively, showed they effectively reduce the rates of death and/or MI (odds ratio [OR], 0.66; 95% CI, 0.54‐0.81) in patients with UA/STEMI prior to PCI, which was more pronounced (OR, 0.59; 95% CI, 0.44‐0.81) when outcomes were measured 48 hours after revascularization.17
The ISAR‐REACT 2 study examined the efficacy of abciximab with clopidogrel and aspirin in patients with NSTEMI prior to PCI.18 The primary end point was a composite of death, MI, and secondary urgent target revascularization 30 days after randomization. The addition of abciximab to 600 mg of the clopidogrel loading dose and aspirin reduced the risk of major adverse cardiovascular events by 25% (RR, 0.75; 95% CI, 0.58‐0.97; P = .03) 30 days after the initiation of therapy. However, subgroup analysis revealed that this therapeutic benefit was confined to patients with elevated troponin levels.18 The ACC/AHA also recommends the concomitant administration of GP IIb/IIIa inhibitors to patients receiving heparin, aspirin, and clopidogrel and undergoing planned PCI.1 The guideline recommendation is for either clopidogrel or a GP IIb/IIIa inhibitor in the invasive pathway (class IA), but both are recommended in patients with elevated troponin, recurrent ischemia, or delay to catheterization (class IIaB). This triple antiplatelet therapy is considered advantageous in the highest=risk NSTEMI patients. The short‐term and long‐term benefits of antiplatelet therapies are consistent across the UA/NSTEMI‐risk spectrum and galvanize the ACC/AHA recommendations for antithrombotic therapy in patients with UA/NSTEMI (Fig. 3).1

ANTIPLATELET THERAPIES FOR STEMI
The ACC/AHA has made several recommendations regarding the administration of clopidogrel in patients with STEMI. Clopidogrel should be administered to patients with contraindications to aspirin. After placement of a bare metal or drug‐eluting stent, this agent should be administered at least 1 month and less than 12 months after surgery, respectively. It should also be withheld at least 5‐7 days prior to CABG.2 These guidelines are largely based on clinical trials in patients with UA/STEMI or ACS.9, 15 Fortunately, several more recent studies have examined the use of antiplatelet therapy in patients with STEMI.
In the Clopidogrel as Adjunctive Reperfusion TherapyThrombolysis in Myocardial Infarction (CLARITY‐TIMI) 28 study, 3491 patients with STEMI were randomized to receive clopidogrel (300 mg loading dose, then 75 mg/day) or placebo. Patients were also treated with a fibrinolytic agent, aspirin, and unfractionated heparin and underwent angiography 48‐192 hours after randomization. Clopidogrel reduced the risk of detecting an occluded infarct‐related artery by angiography or recurrent MI/death prior to angiography by 36% (OR, 0.64; 95% CI, 0.53‐0.76; P .001) compared with placebo.19 It also reduced the risk of major adverse cardiovascular events 30 days after randomization by 20% (OR, 0.80; 95% CI, 0.65‐0.97; P = .03) compared with placebo, with no significant difference in the risk of bleeding between the treatment groups.
The PCI‐CLARITY study examined the efficacy of clopidogrel in patients undergoing PCI during the CLARITY‐TIMI 28 trial. Clopidogrel reduced the rate of major adverse cardiovascular events by 46% (OR, 0.54; 95% CI, 0.35‐0.85; P = .008) after PCI and 30 days after randomization, with no excess in major bleeding.20 Although the use of this agent along with contemporary reperfusion therapies in patients with STEMI is supported, further research into the sustained use of clopidogrel in STEMI is needed.
CONCLUSIONS
Patients with ACS require aggressive diagnosis and acute treatment. However, long‐term therapies are also needed to improve outcomes. Antiplatelet therapies are a key component of the treatment of ACS. The benefits of aspirin and clopidogrel combination therapy are evident early, and their sustained use improves the outcome of patients who receive medical therapy and/or revascularization procedures. Early initiation of antiplatelet therapy in patients with ACS is best accomplished with care pathways or ACS protocols that are guideline driven. Initiation of these protocols in the ED, with appropriate handoff to hospitalists, will ensure maximization of antiplatelet therapy for patients throughout the precatheterization medical management period. Although antiplatelet agents may be associated with an increased risk of bleeding in some patients, these risks can be minimized and are outweighed by the benefits of clopidogrel and aspirin.
- ,,, et al.ACC/AHA guidelines for the management of patients with unstable angina/non‐ST‐segment elevation myocardial infarction: executive summary.J Am Coll Cardiol.2007;50:e1–e157. Available at: http://www.acc.org.
- ,,, et al.ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction.Circulation.2004;110:82–292.
- Antiplatelet Trialists' Collaboration.Collaborative overview of randomized trials of antiplatelet therapy. Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients.BMJ.1994;308:81–106.
- ,,, et al.One‐year cardiovascular event rates in outpatients with atherothrombosis.JAMA.2007;297:1197–1206.
- ,,, et al.Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association.Circulation.2006;113:873–823.
- The RISC Group.Risk of myocardial infarction and death during treatment with low dose aspirin and intravenous heparin in men with unstable coronary artery disease.Lancet.1990;336:827–830.
- ISIS‐2 Collaborative Group.Randomized trial of intravenous streptokinase, oral aspirin, both or neither among 17,187 cases of suspected acute myocardial infarction: ISIS‐2.Lancet.1988;2:349–360.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.BMJ.2002;324:71–86.
- CAPRIE steering committee.A randomized, blinded, trial of clopidogrel versus aspirin in patients at risk of ischemic events (CAPRIE).Lancet.1996;348:1329–1339.
- CURE Trial Investigators.Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST‐segment elevation.N Engl J Med.2001;345:494–502.
- ,,, et al.Benefit of clopidogrel in patients with acute coronary syndromes without ST‐segment elevation in various risk groups.Circulation.2002;106:1622–1626.
- ,,, et al.Early and late effects of clopidogrel in patients with acute coronary syndromes.Circulation.2003;107:966–972.
- ,,, et al.Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non‐ST‐elevation acute coronary syndrome: The clopidogrel in unstable angina to prevent recurrent ischemic events (CURE) trial.Circulation.2004;110:1202–1208.
- ,,, et al.Benefit of clopidogrel according to timing of percutaneous coronary intervention in patients with acute coronary syndromes: Further results from the clopidogrel in unstable angina to prevent recurrent events (CURE) study.Am Heart J.2005;150:1177–1184.
- ,,, et al.Effects of pretreatment with clopidogrel and aspirin followed by long‐term therapy in patients undergoing percutaneous coronary intervention: the PCI‐CURE study.Lancet.2001;358:527–533.
- ,,, et al.Early and sustained dual oral antiplatelet therapy following percutaneous intervention. A randomized trial.JAMA.2002;288:2411–2420.
- ,,,,,.Platelet glycoprotein IIb/IIIa receptor inhibition in non‐ST‐elevation acute coronary syndromes: early benefit during medical treatment only, with additional protection during percutaneous coronary intervention.Circulation.1999;100:2045–2048.
- ,,, et al.Abciximab in patients with acute coronary syndromes undergoing percutaneous coronary intervention after clopidogrel pretreatment. The ISAR‐REACT2 randomized trial.JAMA.2006;295:1531–1538.
- ,,, et al.Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST‐segment elevation.N Engl J Med.2005;352:1179–1189.
- ,,, et al.Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST‐elevation myocardial infarction treated with fibrinolytics. The PCI‐CLARITY study.JAMA.2005;294:1224–1232.
- ,,, et al.ACC/AHA guidelines for the management of patients with unstable angina/non‐ST‐segment elevation myocardial infarction: executive summary.J Am Coll Cardiol.2007;50:e1–e157. Available at: http://www.acc.org.
- ,,, et al.ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction.Circulation.2004;110:82–292.
- Antiplatelet Trialists' Collaboration.Collaborative overview of randomized trials of antiplatelet therapy. Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients.BMJ.1994;308:81–106.
- ,,, et al.One‐year cardiovascular event rates in outpatients with atherothrombosis.JAMA.2007;297:1197–1206.
- ,,, et al.Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association.Circulation.2006;113:873–823.
- The RISC Group.Risk of myocardial infarction and death during treatment with low dose aspirin and intravenous heparin in men with unstable coronary artery disease.Lancet.1990;336:827–830.
- ISIS‐2 Collaborative Group.Randomized trial of intravenous streptokinase, oral aspirin, both or neither among 17,187 cases of suspected acute myocardial infarction: ISIS‐2.Lancet.1988;2:349–360.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.BMJ.2002;324:71–86.
- CAPRIE steering committee.A randomized, blinded, trial of clopidogrel versus aspirin in patients at risk of ischemic events (CAPRIE).Lancet.1996;348:1329–1339.
- CURE Trial Investigators.Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST‐segment elevation.N Engl J Med.2001;345:494–502.
- ,,, et al.Benefit of clopidogrel in patients with acute coronary syndromes without ST‐segment elevation in various risk groups.Circulation.2002;106:1622–1626.
- ,,, et al.Early and late effects of clopidogrel in patients with acute coronary syndromes.Circulation.2003;107:966–972.
- ,,, et al.Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non‐ST‐elevation acute coronary syndrome: The clopidogrel in unstable angina to prevent recurrent ischemic events (CURE) trial.Circulation.2004;110:1202–1208.
- ,,, et al.Benefit of clopidogrel according to timing of percutaneous coronary intervention in patients with acute coronary syndromes: Further results from the clopidogrel in unstable angina to prevent recurrent events (CURE) study.Am Heart J.2005;150:1177–1184.
- ,,, et al.Effects of pretreatment with clopidogrel and aspirin followed by long‐term therapy in patients undergoing percutaneous coronary intervention: the PCI‐CURE study.Lancet.2001;358:527–533.
- ,,, et al.Early and sustained dual oral antiplatelet therapy following percutaneous intervention. A randomized trial.JAMA.2002;288:2411–2420.
- ,,,,,.Platelet glycoprotein IIb/IIIa receptor inhibition in non‐ST‐elevation acute coronary syndromes: early benefit during medical treatment only, with additional protection during percutaneous coronary intervention.Circulation.1999;100:2045–2048.
- ,,, et al.Abciximab in patients with acute coronary syndromes undergoing percutaneous coronary intervention after clopidogrel pretreatment. The ISAR‐REACT2 randomized trial.JAMA.2006;295:1531–1538.
- ,,, et al.Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST‐segment elevation.N Engl J Med.2005;352:1179–1189.
- ,,, et al.Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST‐elevation myocardial infarction treated with fibrinolytics. The PCI‐CLARITY study.JAMA.2005;294:1224–1232.
FDA says heparin contamination was likely intentional
The contaminant found in heparin was most likely introduced intentionally, according to the US Food and Drug Administration.
In a Senate hearing on April 15, FDA Commissioner Andrew von Eschenbach said the FDA suspects the contaminant, oversulfated chondroitin sulfate, was introduced to increase profits.
However, it is unclear where in the chain of production the contaminant was added and, therefore, who would be responsible.
Baxter International Inc. and Scientific Protein Laboratories Inc. (SPL), suppliers of heparin, say the contaminant was not added in their factories.
Baxter said it has been seeking access to consolidators and workshops in China that handled the crude material before it went to SPL. According to spokeswoman Erin Gardiner, Baxter has not determined how or why the contaminant was introduced.
SPL has issued a press release claiming its lack of involvement in the contamination.
The company said, “Based upon testing and reports from around the world, it is clear that the contamination occurred on a widespread basis earlier in the Chinese heparin raw material supply chain, before those materials reached Changzhou SPL and SPL.”
Baxter began recalling heparin January 17 of this year, after receiving reports of allergic reactions and deaths resulting from use of the drug. Recalls of heparin have continued since that time.
In mid-March, the FDA released the news that a contaminant was found in crude lots of heparin at a Chinese processing plant. The substance was identified as over-sulfated chondroitin sulfate, which mimics heparin and is cheaper to produce than real heparin.
Earlier this month, the FDA said a total of 62 people have died since January as a result of heparin use. ![]()
The contaminant found in heparin was most likely introduced intentionally, according to the US Food and Drug Administration.
In a Senate hearing on April 15, FDA Commissioner Andrew von Eschenbach said the FDA suspects the contaminant, oversulfated chondroitin sulfate, was introduced to increase profits.
However, it is unclear where in the chain of production the contaminant was added and, therefore, who would be responsible.
Baxter International Inc. and Scientific Protein Laboratories Inc. (SPL), suppliers of heparin, say the contaminant was not added in their factories.
Baxter said it has been seeking access to consolidators and workshops in China that handled the crude material before it went to SPL. According to spokeswoman Erin Gardiner, Baxter has not determined how or why the contaminant was introduced.
SPL has issued a press release claiming its lack of involvement in the contamination.
The company said, “Based upon testing and reports from around the world, it is clear that the contamination occurred on a widespread basis earlier in the Chinese heparin raw material supply chain, before those materials reached Changzhou SPL and SPL.”
Baxter began recalling heparin January 17 of this year, after receiving reports of allergic reactions and deaths resulting from use of the drug. Recalls of heparin have continued since that time.
In mid-March, the FDA released the news that a contaminant was found in crude lots of heparin at a Chinese processing plant. The substance was identified as over-sulfated chondroitin sulfate, which mimics heparin and is cheaper to produce than real heparin.
Earlier this month, the FDA said a total of 62 people have died since January as a result of heparin use. ![]()
The contaminant found in heparin was most likely introduced intentionally, according to the US Food and Drug Administration.
In a Senate hearing on April 15, FDA Commissioner Andrew von Eschenbach said the FDA suspects the contaminant, oversulfated chondroitin sulfate, was introduced to increase profits.
However, it is unclear where in the chain of production the contaminant was added and, therefore, who would be responsible.
Baxter International Inc. and Scientific Protein Laboratories Inc. (SPL), suppliers of heparin, say the contaminant was not added in their factories.
Baxter said it has been seeking access to consolidators and workshops in China that handled the crude material before it went to SPL. According to spokeswoman Erin Gardiner, Baxter has not determined how or why the contaminant was introduced.
SPL has issued a press release claiming its lack of involvement in the contamination.
The company said, “Based upon testing and reports from around the world, it is clear that the contamination occurred on a widespread basis earlier in the Chinese heparin raw material supply chain, before those materials reached Changzhou SPL and SPL.”
Baxter began recalling heparin January 17 of this year, after receiving reports of allergic reactions and deaths resulting from use of the drug. Recalls of heparin have continued since that time.
In mid-March, the FDA released the news that a contaminant was found in crude lots of heparin at a Chinese processing plant. The substance was identified as over-sulfated chondroitin sulfate, which mimics heparin and is cheaper to produce than real heparin.
Earlier this month, the FDA said a total of 62 people have died since January as a result of heparin use. ![]()
When Crisis Comes
One hospitalist spent three weeks without a break treating victims of Hurricane Katrina in 2005. Another couldn’t get to work when the I-35W bridge collapsed in Minneapolis on Aug. 1, 2007, but there were enough physicians on hand for that tragedy and fewer victims to treat than feared.
Yet another shudders when he recalls treating victims of an 89-car pile-up caused by a dust storm in southern Idaho.
Not all hospitalists have been in the trenches treating victims of disasters. But two emerging trends likely will put hospitalists on the front lines of preparing for disasters and treating victims.
The first is the increasing recognition that there are many threats to the safety of the public, including terrorism, natural disasters, disease outbreaks, and criminal acts like the mass killings a year ago at Virginia Tech in Blacksburg.
The second is the rapidly expanding role hospitalists have in caring for critically ill and injured patients.
“Hospitalists will be a key,” says Timothy Close, senior safety officer for the University of Colorado Hospital in Denver and chairman of its emergency management committee. “Because of their understanding of all hospital services and treatments, they can handle a multitude of clinical roles. Facilities should deploy hospitalists’ understanding of the organization to facilitate patient care.”
Close, who has 15 years of experience in planning and preparedness, urges organizations to implement plans “that are realistic and doable based on local resources and conditions.” He also urges facilities to conduct emergency drills and have hospitalists participate.
He has dealt with crises wrought by fires, workplace violence, severe weather, and abductions, but adds it is important to remember that “you never know what’s going to happen.”
Close helped treat the victims of the dust storm pile-up. “It was caused by an unfortunate series of events,” he says. “A new land owner plowed during a dry time, and when the winds came it was catastrophic. The cars ran right into the dust cloud with zero visibility.”
Prepare for the Unseen
Lisa Kirkland, MD, a hospitalist at the Mayo Clinic in Rochester, Minn., agrees disaster planning should be local in the sense of preparing for specific events. Tornadoes are the most likely weather-related crisis to occur in Rochester, she says, and the area is not a prime terrorism target.
Yet disasters don’t have to happen suddenly or involve mass casualties. “A disaster is anything that overwhelms the usual system,” she says. “Putting a community under quarantine during an outbreak of influenza or bird flu, for example, could require the initiation of disaster plans since staff couldn’t get to hospitals.”
In this sort of scenario, like during the SARS outbreak in Toronto in 2003, patient care would be largely medical, rather than surgical, so hospitalists would be key providers of treatment, Dr. Kirkland says.
Hospitalists would also be key in maintaining effective communication, internally and with the outside world because of their thorough knowledge of hospital services, she adds.
Some 75 miles away in Minneapolis, many victims of the I-35W bridge collapse were taken to Hennepin County Medical Center (HCMC). Glen Varns, MD, hospitalist program leader at HCMC, was unable to get to work because he lives on the other side of the bridge. But he says hospitalists played a critical role in dealing with the crisis.
“Since our hospitalists are most familiar with the inner workings of the facility, they played a huge role in determining who needed to be hospitalized and where in the hospital they would best be treated,” he says. “This included reviewing the existing patient census when the collapse happened so we could discharge and transfer inpatients appropriately to ensure that the hospital was in the best position to deal with the collapse victims.”
Because the bridge collapsed during the early evening, there was plenty of staff on-hand to treat the victims, including residents who worked hand-in-hand with hospitalists in making admission and transfer decisions.
Challenge for Hospitalists
In smaller facilities where there are no residents, or in small emergency departments (ED) and intensive-care units, hospitalists will and should have even more critical roles in handling disasters and planning for them, Dr. Varns says.
He believes all hospitalists—but especially those in small, nonteaching facilities—should get triage training. “Hospitalists have a very broad skill set—especially with increasing responsibility for co-management of surgical cases—but they should develop triage skills,” says Dr. Varns, who suggests hospitalists take a two or three-day advanced trauma life support course.
Steven B. Deitelzweig, MD, FACP, system chairman, department of hospital medicine and vice president of medical affairs for the Ochsner Health System in the New Orleans area, agrees.
“I think the folks who are closest to guiding the care should be offering input into triage decisions,” he says. “Hospitalists can be invaluable in doing triage of inpatients. They provide objective detailed information.”
Dr. Deitelzweig, who experienced the three-week lock-down following Katrina, suggests hospitalist groups create a system of prioritizing evacuation of patients—including what kind of support they’ll need.
He believes hospitalists will be invaluable during crises because they are “front-line decision-makers, along with ED physicians and intensivists.” Hospitalists should be on disaster-preparedness committees and a key part of communication during an actual crisis, he urges.
“Communication is critical during a crisis—and hospitalists know their systems,” he continues, noting that Ochsner has out-of-state cell phones, satellite phones, ham radios, spectral light phones, radio frequency antennas in secure places, and more.
In addition to equipment and supplies, hospitalists need to be prepared to do whatever is needed in a crisis, Dr. Deitelzweig says. “In a disaster, you might have to do a procedure usually done by a specialist—with supervision—to extend that person,” he says. “You also may have to go past the physician role. That’s where leadership shows. Our CEO served food in the cafeteria during Katrina. During a disaster, you have to be a flat organization and just do what needs to be done. That gives emotional support to everyone.”
Still, the need to prepare before a disaster cannot be overemphasized, he says.
Ochsner now has two teams of pre-selected physicians, including hospitalists, dedicated to working through specific types of crises. Having the list of essential personnel online at all times is intended to prevent last-minute scurrying around to find the right people, he says.
In addition, providing balanced scheduling—especially in long-lasting crisis situations like Katrina—is important, says Dr. Deitelzweig. “Timing for release must be included, and having more staff on hand than necessary can help alleviate stress,” he advises.
Lessons of Katrina
Neal Axon, MD, an assistant professor at the Medical University of South Carolina, says he and his colleagues learned from those who went through Katrina as they prepared for the most likely disaster in Charleston: a severe hurricane.
Dr. Axon, a senior hospitalist in his group, says the facility has a system that generates e-mail, pages, text messages, and cell phone calls to keep hospital staff informed about potential crises. He also says the preparedness plan provides for relief of staff working for extended periods.
In addition, the hospital has trailers and inflatable tents to extend its facilities if there is a surge in patients. It also has a facility to provide decontamination for exposure to chemicals and radiation.
Brian Bossard, MD, director of Inpatient Physician Associates and medical staff quality designee at BryanLGH Medical Center in Lincoln Neb., says preparedness plans should be tested and updated regularly—especially the systems used to call in staff.
Dr. Bossard strongly believes hospitalists should be involved in disaster planning: “Every day hospitalists work hospital systems. We have a broad scope and perspective. That’s what you need in a disaster.” TH
Karla Feuer is a journalist based in New York.
One hospitalist spent three weeks without a break treating victims of Hurricane Katrina in 2005. Another couldn’t get to work when the I-35W bridge collapsed in Minneapolis on Aug. 1, 2007, but there were enough physicians on hand for that tragedy and fewer victims to treat than feared.
Yet another shudders when he recalls treating victims of an 89-car pile-up caused by a dust storm in southern Idaho.
Not all hospitalists have been in the trenches treating victims of disasters. But two emerging trends likely will put hospitalists on the front lines of preparing for disasters and treating victims.
The first is the increasing recognition that there are many threats to the safety of the public, including terrorism, natural disasters, disease outbreaks, and criminal acts like the mass killings a year ago at Virginia Tech in Blacksburg.
The second is the rapidly expanding role hospitalists have in caring for critically ill and injured patients.
“Hospitalists will be a key,” says Timothy Close, senior safety officer for the University of Colorado Hospital in Denver and chairman of its emergency management committee. “Because of their understanding of all hospital services and treatments, they can handle a multitude of clinical roles. Facilities should deploy hospitalists’ understanding of the organization to facilitate patient care.”
Close, who has 15 years of experience in planning and preparedness, urges organizations to implement plans “that are realistic and doable based on local resources and conditions.” He also urges facilities to conduct emergency drills and have hospitalists participate.
He has dealt with crises wrought by fires, workplace violence, severe weather, and abductions, but adds it is important to remember that “you never know what’s going to happen.”
Close helped treat the victims of the dust storm pile-up. “It was caused by an unfortunate series of events,” he says. “A new land owner plowed during a dry time, and when the winds came it was catastrophic. The cars ran right into the dust cloud with zero visibility.”
Prepare for the Unseen
Lisa Kirkland, MD, a hospitalist at the Mayo Clinic in Rochester, Minn., agrees disaster planning should be local in the sense of preparing for specific events. Tornadoes are the most likely weather-related crisis to occur in Rochester, she says, and the area is not a prime terrorism target.
Yet disasters don’t have to happen suddenly or involve mass casualties. “A disaster is anything that overwhelms the usual system,” she says. “Putting a community under quarantine during an outbreak of influenza or bird flu, for example, could require the initiation of disaster plans since staff couldn’t get to hospitals.”
In this sort of scenario, like during the SARS outbreak in Toronto in 2003, patient care would be largely medical, rather than surgical, so hospitalists would be key providers of treatment, Dr. Kirkland says.
Hospitalists would also be key in maintaining effective communication, internally and with the outside world because of their thorough knowledge of hospital services, she adds.
Some 75 miles away in Minneapolis, many victims of the I-35W bridge collapse were taken to Hennepin County Medical Center (HCMC). Glen Varns, MD, hospitalist program leader at HCMC, was unable to get to work because he lives on the other side of the bridge. But he says hospitalists played a critical role in dealing with the crisis.
“Since our hospitalists are most familiar with the inner workings of the facility, they played a huge role in determining who needed to be hospitalized and where in the hospital they would best be treated,” he says. “This included reviewing the existing patient census when the collapse happened so we could discharge and transfer inpatients appropriately to ensure that the hospital was in the best position to deal with the collapse victims.”
Because the bridge collapsed during the early evening, there was plenty of staff on-hand to treat the victims, including residents who worked hand-in-hand with hospitalists in making admission and transfer decisions.
Challenge for Hospitalists
In smaller facilities where there are no residents, or in small emergency departments (ED) and intensive-care units, hospitalists will and should have even more critical roles in handling disasters and planning for them, Dr. Varns says.
He believes all hospitalists—but especially those in small, nonteaching facilities—should get triage training. “Hospitalists have a very broad skill set—especially with increasing responsibility for co-management of surgical cases—but they should develop triage skills,” says Dr. Varns, who suggests hospitalists take a two or three-day advanced trauma life support course.
Steven B. Deitelzweig, MD, FACP, system chairman, department of hospital medicine and vice president of medical affairs for the Ochsner Health System in the New Orleans area, agrees.
“I think the folks who are closest to guiding the care should be offering input into triage decisions,” he says. “Hospitalists can be invaluable in doing triage of inpatients. They provide objective detailed information.”
Dr. Deitelzweig, who experienced the three-week lock-down following Katrina, suggests hospitalist groups create a system of prioritizing evacuation of patients—including what kind of support they’ll need.
He believes hospitalists will be invaluable during crises because they are “front-line decision-makers, along with ED physicians and intensivists.” Hospitalists should be on disaster-preparedness committees and a key part of communication during an actual crisis, he urges.
“Communication is critical during a crisis—and hospitalists know their systems,” he continues, noting that Ochsner has out-of-state cell phones, satellite phones, ham radios, spectral light phones, radio frequency antennas in secure places, and more.
In addition to equipment and supplies, hospitalists need to be prepared to do whatever is needed in a crisis, Dr. Deitelzweig says. “In a disaster, you might have to do a procedure usually done by a specialist—with supervision—to extend that person,” he says. “You also may have to go past the physician role. That’s where leadership shows. Our CEO served food in the cafeteria during Katrina. During a disaster, you have to be a flat organization and just do what needs to be done. That gives emotional support to everyone.”
Still, the need to prepare before a disaster cannot be overemphasized, he says.
Ochsner now has two teams of pre-selected physicians, including hospitalists, dedicated to working through specific types of crises. Having the list of essential personnel online at all times is intended to prevent last-minute scurrying around to find the right people, he says.
In addition, providing balanced scheduling—especially in long-lasting crisis situations like Katrina—is important, says Dr. Deitelzweig. “Timing for release must be included, and having more staff on hand than necessary can help alleviate stress,” he advises.
Lessons of Katrina
Neal Axon, MD, an assistant professor at the Medical University of South Carolina, says he and his colleagues learned from those who went through Katrina as they prepared for the most likely disaster in Charleston: a severe hurricane.
Dr. Axon, a senior hospitalist in his group, says the facility has a system that generates e-mail, pages, text messages, and cell phone calls to keep hospital staff informed about potential crises. He also says the preparedness plan provides for relief of staff working for extended periods.
In addition, the hospital has trailers and inflatable tents to extend its facilities if there is a surge in patients. It also has a facility to provide decontamination for exposure to chemicals and radiation.
Brian Bossard, MD, director of Inpatient Physician Associates and medical staff quality designee at BryanLGH Medical Center in Lincoln Neb., says preparedness plans should be tested and updated regularly—especially the systems used to call in staff.
Dr. Bossard strongly believes hospitalists should be involved in disaster planning: “Every day hospitalists work hospital systems. We have a broad scope and perspective. That’s what you need in a disaster.” TH
Karla Feuer is a journalist based in New York.
One hospitalist spent three weeks without a break treating victims of Hurricane Katrina in 2005. Another couldn’t get to work when the I-35W bridge collapsed in Minneapolis on Aug. 1, 2007, but there were enough physicians on hand for that tragedy and fewer victims to treat than feared.
Yet another shudders when he recalls treating victims of an 89-car pile-up caused by a dust storm in southern Idaho.
Not all hospitalists have been in the trenches treating victims of disasters. But two emerging trends likely will put hospitalists on the front lines of preparing for disasters and treating victims.
The first is the increasing recognition that there are many threats to the safety of the public, including terrorism, natural disasters, disease outbreaks, and criminal acts like the mass killings a year ago at Virginia Tech in Blacksburg.
The second is the rapidly expanding role hospitalists have in caring for critically ill and injured patients.
“Hospitalists will be a key,” says Timothy Close, senior safety officer for the University of Colorado Hospital in Denver and chairman of its emergency management committee. “Because of their understanding of all hospital services and treatments, they can handle a multitude of clinical roles. Facilities should deploy hospitalists’ understanding of the organization to facilitate patient care.”
Close, who has 15 years of experience in planning and preparedness, urges organizations to implement plans “that are realistic and doable based on local resources and conditions.” He also urges facilities to conduct emergency drills and have hospitalists participate.
He has dealt with crises wrought by fires, workplace violence, severe weather, and abductions, but adds it is important to remember that “you never know what’s going to happen.”
Close helped treat the victims of the dust storm pile-up. “It was caused by an unfortunate series of events,” he says. “A new land owner plowed during a dry time, and when the winds came it was catastrophic. The cars ran right into the dust cloud with zero visibility.”
Prepare for the Unseen
Lisa Kirkland, MD, a hospitalist at the Mayo Clinic in Rochester, Minn., agrees disaster planning should be local in the sense of preparing for specific events. Tornadoes are the most likely weather-related crisis to occur in Rochester, she says, and the area is not a prime terrorism target.
Yet disasters don’t have to happen suddenly or involve mass casualties. “A disaster is anything that overwhelms the usual system,” she says. “Putting a community under quarantine during an outbreak of influenza or bird flu, for example, could require the initiation of disaster plans since staff couldn’t get to hospitals.”
In this sort of scenario, like during the SARS outbreak in Toronto in 2003, patient care would be largely medical, rather than surgical, so hospitalists would be key providers of treatment, Dr. Kirkland says.
Hospitalists would also be key in maintaining effective communication, internally and with the outside world because of their thorough knowledge of hospital services, she adds.
Some 75 miles away in Minneapolis, many victims of the I-35W bridge collapse were taken to Hennepin County Medical Center (HCMC). Glen Varns, MD, hospitalist program leader at HCMC, was unable to get to work because he lives on the other side of the bridge. But he says hospitalists played a critical role in dealing with the crisis.
“Since our hospitalists are most familiar with the inner workings of the facility, they played a huge role in determining who needed to be hospitalized and where in the hospital they would best be treated,” he says. “This included reviewing the existing patient census when the collapse happened so we could discharge and transfer inpatients appropriately to ensure that the hospital was in the best position to deal with the collapse victims.”
Because the bridge collapsed during the early evening, there was plenty of staff on-hand to treat the victims, including residents who worked hand-in-hand with hospitalists in making admission and transfer decisions.
Challenge for Hospitalists
In smaller facilities where there are no residents, or in small emergency departments (ED) and intensive-care units, hospitalists will and should have even more critical roles in handling disasters and planning for them, Dr. Varns says.
He believes all hospitalists—but especially those in small, nonteaching facilities—should get triage training. “Hospitalists have a very broad skill set—especially with increasing responsibility for co-management of surgical cases—but they should develop triage skills,” says Dr. Varns, who suggests hospitalists take a two or three-day advanced trauma life support course.
Steven B. Deitelzweig, MD, FACP, system chairman, department of hospital medicine and vice president of medical affairs for the Ochsner Health System in the New Orleans area, agrees.
“I think the folks who are closest to guiding the care should be offering input into triage decisions,” he says. “Hospitalists can be invaluable in doing triage of inpatients. They provide objective detailed information.”
Dr. Deitelzweig, who experienced the three-week lock-down following Katrina, suggests hospitalist groups create a system of prioritizing evacuation of patients—including what kind of support they’ll need.
He believes hospitalists will be invaluable during crises because they are “front-line decision-makers, along with ED physicians and intensivists.” Hospitalists should be on disaster-preparedness committees and a key part of communication during an actual crisis, he urges.
“Communication is critical during a crisis—and hospitalists know their systems,” he continues, noting that Ochsner has out-of-state cell phones, satellite phones, ham radios, spectral light phones, radio frequency antennas in secure places, and more.
In addition to equipment and supplies, hospitalists need to be prepared to do whatever is needed in a crisis, Dr. Deitelzweig says. “In a disaster, you might have to do a procedure usually done by a specialist—with supervision—to extend that person,” he says. “You also may have to go past the physician role. That’s where leadership shows. Our CEO served food in the cafeteria during Katrina. During a disaster, you have to be a flat organization and just do what needs to be done. That gives emotional support to everyone.”
Still, the need to prepare before a disaster cannot be overemphasized, he says.
Ochsner now has two teams of pre-selected physicians, including hospitalists, dedicated to working through specific types of crises. Having the list of essential personnel online at all times is intended to prevent last-minute scurrying around to find the right people, he says.
In addition, providing balanced scheduling—especially in long-lasting crisis situations like Katrina—is important, says Dr. Deitelzweig. “Timing for release must be included, and having more staff on hand than necessary can help alleviate stress,” he advises.
Lessons of Katrina
Neal Axon, MD, an assistant professor at the Medical University of South Carolina, says he and his colleagues learned from those who went through Katrina as they prepared for the most likely disaster in Charleston: a severe hurricane.
Dr. Axon, a senior hospitalist in his group, says the facility has a system that generates e-mail, pages, text messages, and cell phone calls to keep hospital staff informed about potential crises. He also says the preparedness plan provides for relief of staff working for extended periods.
In addition, the hospital has trailers and inflatable tents to extend its facilities if there is a surge in patients. It also has a facility to provide decontamination for exposure to chemicals and radiation.
Brian Bossard, MD, director of Inpatient Physician Associates and medical staff quality designee at BryanLGH Medical Center in Lincoln Neb., says preparedness plans should be tested and updated regularly—especially the systems used to call in staff.
Dr. Bossard strongly believes hospitalists should be involved in disaster planning: “Every day hospitalists work hospital systems. We have a broad scope and perspective. That’s what you need in a disaster.” TH
Karla Feuer is a journalist based in New York.
Manage Your Work Flow
As a soon-to-be-attending hospitalist, you’ll shortly be on your own directing patient care. According to SHM data, you will see 12 to 18 patients per day, if not more. You understand the medicine, but how can you optimize your day to make it home in time? Here’s how you can direct your workday more efficiently.
1) Organize: This should come as no surprise. This crucial skill, reiterated during residency training, will prove invaluable as a practicing hospitalist. It certainly helps to maintain a structured and accurate daily census. Keeping a list of things to do handy and refreshing that list keeps you from having to rethink or reread your notes. On some occasions, doing things rather than writing them down to complete later can be faster. Whether you utilize handheld PCs or note cards, find a method that works for you.
2) Plan your day: If you know you are going to have a busy day, accept it. Start the day with a positive attitude and know you have to keep moving and can’t get stuck on trivial things. See your sickest patients first, or the ones you know will require a lot of time. Also, see your potential discharges as early in the day as possible to optimize the discharge process and pinpoint potential problems. If you are the attending on a teaching service, spend time with your resident to go over the structure of a typical day.
3) Consolidate: If you have patients in different areas in the hospital, start with the areas where you have the most patients—especially the sickest ones. See all the patients in proximity to each other. Avoid running between the computer, the chart, and the patient’s room for every patient. Lump some of these tasks together and avoid losing time.
4) Avoid hold music: Instead of paging people and waiting for a call back, send a text message to increase your efficiency. Try to contact (and wait for) someone to call you back while you are doing something productive—like writing a note. You can also make other work-related calls, such as to families and consultants, during your commute to or from work. This saves you some time when you are in the hospital.
5) Delegate: A lot of new hospitalists have difficulty relinquishing control—similar to when they made the transition from intern to resident. As an academic attending, don’t micromanage. Rather, attend to the global issues and problems that might need a greater degree of attending involvement, such as challenging family situations. This requires a certain degree of trust in your resident.
Assign specific responsibilities to members of your team (residents, interns, and medical students) and go over their roles. If you are not at an academic institution, you can still delegate tasks like procedures (central lines, spinal taps, thoracentesis, and paracentesis). There are other specialists in the hospital who perform these procedures more frequently and more efficiently than you.
6) Give yourself a time limit: To improve efficiency, some people find it helpful to give themselves a time limit to get their work done. Making this time limit practical may help get you home at reasonable hour. Also, learn to gracefully extract yourself from chatty patients, family, or colleagues if time is short.
7) Document efficiently: When rounding on patients, make sure the note is written when you see the patient—then move on. You can always come back for an addendum if needed. Group your note writing as much as possible on each floor. When admitting or discharging a patient, do all documentation at once, including notes and orders. This way you don’t waste time getting back to information you have in front of you. If there is a history and physical available on a new admission or consult, print it out and use it as a template during the patient interview. It helps to confirm details with the patient and fill in gaps. If time permits, prepare discharge papers and prescriptions in advance of anticipated discharges to save time on the day of discharge.
8) Define inpatient vs. outpatient management: Differentiate between important inpatient workup and evaluation that can be performed on an outpatient basis to save time and reduce length of stay. When patients can safely leave the hospital to continue work-ups and follow-ups with their primary care providers and specialists, you gain more time the following day—when you are no longer rounding on them.
9) Schedule a time to see family members: Conversations with family members are usually more productive if those times are scheduled. If possible, schedule them after you have seen a bulk of your patients to avoid feeling pressured to cut the meeting short. It is important to know who the family spokesperson is for large families so you can refer other family members to them and avoid multiple call-backs.
10) Develop and maintain good relationships: Your cordial interaction with various hospital department staff (nursing, case management, social work, radiology, and physical therapy to name a few) will help facilitate the inpatient care plan. It certainly helps not to have to wait two or more days to have a diagnostic test performed or assessment made. Sustaining a healthy working relationship promotes an understanding of your expectations for inpatient care.
11) Advocate for constructive change: Much inefficiency is systems based. Thinking about what interferes with your effectiveness in your system and suggesting changes can help a lot. For example, if your institution is going to switch to an electronic medical record, it certainly helps for you or a member of your hospitalist team to get involved in the implementation. Many hospitals are invested in quality improvement—and hospitalists are and should be at the forefront of this change.
As a hospitalist, you can initiate changes within your practice and your hospital’s system to keep things efficient. Making those adjustments sometimes takes time—but they’re well worth the effort.
Meanwhile, maximizing your efficiency can help promote patient throughput, enhance patient satisfaction, improve quality of care and increase job satisfaction. TH
Dr. Magnet is a hospitalist at the Singing River Hospital System on the Gulf Coast of Mississippi and a member of SHM’s Young Physician Committee.
As a soon-to-be-attending hospitalist, you’ll shortly be on your own directing patient care. According to SHM data, you will see 12 to 18 patients per day, if not more. You understand the medicine, but how can you optimize your day to make it home in time? Here’s how you can direct your workday more efficiently.
1) Organize: This should come as no surprise. This crucial skill, reiterated during residency training, will prove invaluable as a practicing hospitalist. It certainly helps to maintain a structured and accurate daily census. Keeping a list of things to do handy and refreshing that list keeps you from having to rethink or reread your notes. On some occasions, doing things rather than writing them down to complete later can be faster. Whether you utilize handheld PCs or note cards, find a method that works for you.
2) Plan your day: If you know you are going to have a busy day, accept it. Start the day with a positive attitude and know you have to keep moving and can’t get stuck on trivial things. See your sickest patients first, or the ones you know will require a lot of time. Also, see your potential discharges as early in the day as possible to optimize the discharge process and pinpoint potential problems. If you are the attending on a teaching service, spend time with your resident to go over the structure of a typical day.
3) Consolidate: If you have patients in different areas in the hospital, start with the areas where you have the most patients—especially the sickest ones. See all the patients in proximity to each other. Avoid running between the computer, the chart, and the patient’s room for every patient. Lump some of these tasks together and avoid losing time.
4) Avoid hold music: Instead of paging people and waiting for a call back, send a text message to increase your efficiency. Try to contact (and wait for) someone to call you back while you are doing something productive—like writing a note. You can also make other work-related calls, such as to families and consultants, during your commute to or from work. This saves you some time when you are in the hospital.
5) Delegate: A lot of new hospitalists have difficulty relinquishing control—similar to when they made the transition from intern to resident. As an academic attending, don’t micromanage. Rather, attend to the global issues and problems that might need a greater degree of attending involvement, such as challenging family situations. This requires a certain degree of trust in your resident.
Assign specific responsibilities to members of your team (residents, interns, and medical students) and go over their roles. If you are not at an academic institution, you can still delegate tasks like procedures (central lines, spinal taps, thoracentesis, and paracentesis). There are other specialists in the hospital who perform these procedures more frequently and more efficiently than you.
6) Give yourself a time limit: To improve efficiency, some people find it helpful to give themselves a time limit to get their work done. Making this time limit practical may help get you home at reasonable hour. Also, learn to gracefully extract yourself from chatty patients, family, or colleagues if time is short.
7) Document efficiently: When rounding on patients, make sure the note is written when you see the patient—then move on. You can always come back for an addendum if needed. Group your note writing as much as possible on each floor. When admitting or discharging a patient, do all documentation at once, including notes and orders. This way you don’t waste time getting back to information you have in front of you. If there is a history and physical available on a new admission or consult, print it out and use it as a template during the patient interview. It helps to confirm details with the patient and fill in gaps. If time permits, prepare discharge papers and prescriptions in advance of anticipated discharges to save time on the day of discharge.
8) Define inpatient vs. outpatient management: Differentiate between important inpatient workup and evaluation that can be performed on an outpatient basis to save time and reduce length of stay. When patients can safely leave the hospital to continue work-ups and follow-ups with their primary care providers and specialists, you gain more time the following day—when you are no longer rounding on them.
9) Schedule a time to see family members: Conversations with family members are usually more productive if those times are scheduled. If possible, schedule them after you have seen a bulk of your patients to avoid feeling pressured to cut the meeting short. It is important to know who the family spokesperson is for large families so you can refer other family members to them and avoid multiple call-backs.
10) Develop and maintain good relationships: Your cordial interaction with various hospital department staff (nursing, case management, social work, radiology, and physical therapy to name a few) will help facilitate the inpatient care plan. It certainly helps not to have to wait two or more days to have a diagnostic test performed or assessment made. Sustaining a healthy working relationship promotes an understanding of your expectations for inpatient care.
11) Advocate for constructive change: Much inefficiency is systems based. Thinking about what interferes with your effectiveness in your system and suggesting changes can help a lot. For example, if your institution is going to switch to an electronic medical record, it certainly helps for you or a member of your hospitalist team to get involved in the implementation. Many hospitals are invested in quality improvement—and hospitalists are and should be at the forefront of this change.
As a hospitalist, you can initiate changes within your practice and your hospital’s system to keep things efficient. Making those adjustments sometimes takes time—but they’re well worth the effort.
Meanwhile, maximizing your efficiency can help promote patient throughput, enhance patient satisfaction, improve quality of care and increase job satisfaction. TH
Dr. Magnet is a hospitalist at the Singing River Hospital System on the Gulf Coast of Mississippi and a member of SHM’s Young Physician Committee.
As a soon-to-be-attending hospitalist, you’ll shortly be on your own directing patient care. According to SHM data, you will see 12 to 18 patients per day, if not more. You understand the medicine, but how can you optimize your day to make it home in time? Here’s how you can direct your workday more efficiently.
1) Organize: This should come as no surprise. This crucial skill, reiterated during residency training, will prove invaluable as a practicing hospitalist. It certainly helps to maintain a structured and accurate daily census. Keeping a list of things to do handy and refreshing that list keeps you from having to rethink or reread your notes. On some occasions, doing things rather than writing them down to complete later can be faster. Whether you utilize handheld PCs or note cards, find a method that works for you.
2) Plan your day: If you know you are going to have a busy day, accept it. Start the day with a positive attitude and know you have to keep moving and can’t get stuck on trivial things. See your sickest patients first, or the ones you know will require a lot of time. Also, see your potential discharges as early in the day as possible to optimize the discharge process and pinpoint potential problems. If you are the attending on a teaching service, spend time with your resident to go over the structure of a typical day.
3) Consolidate: If you have patients in different areas in the hospital, start with the areas where you have the most patients—especially the sickest ones. See all the patients in proximity to each other. Avoid running between the computer, the chart, and the patient’s room for every patient. Lump some of these tasks together and avoid losing time.
4) Avoid hold music: Instead of paging people and waiting for a call back, send a text message to increase your efficiency. Try to contact (and wait for) someone to call you back while you are doing something productive—like writing a note. You can also make other work-related calls, such as to families and consultants, during your commute to or from work. This saves you some time when you are in the hospital.
5) Delegate: A lot of new hospitalists have difficulty relinquishing control—similar to when they made the transition from intern to resident. As an academic attending, don’t micromanage. Rather, attend to the global issues and problems that might need a greater degree of attending involvement, such as challenging family situations. This requires a certain degree of trust in your resident.
Assign specific responsibilities to members of your team (residents, interns, and medical students) and go over their roles. If you are not at an academic institution, you can still delegate tasks like procedures (central lines, spinal taps, thoracentesis, and paracentesis). There are other specialists in the hospital who perform these procedures more frequently and more efficiently than you.
6) Give yourself a time limit: To improve efficiency, some people find it helpful to give themselves a time limit to get their work done. Making this time limit practical may help get you home at reasonable hour. Also, learn to gracefully extract yourself from chatty patients, family, or colleagues if time is short.
7) Document efficiently: When rounding on patients, make sure the note is written when you see the patient—then move on. You can always come back for an addendum if needed. Group your note writing as much as possible on each floor. When admitting or discharging a patient, do all documentation at once, including notes and orders. This way you don’t waste time getting back to information you have in front of you. If there is a history and physical available on a new admission or consult, print it out and use it as a template during the patient interview. It helps to confirm details with the patient and fill in gaps. If time permits, prepare discharge papers and prescriptions in advance of anticipated discharges to save time on the day of discharge.
8) Define inpatient vs. outpatient management: Differentiate between important inpatient workup and evaluation that can be performed on an outpatient basis to save time and reduce length of stay. When patients can safely leave the hospital to continue work-ups and follow-ups with their primary care providers and specialists, you gain more time the following day—when you are no longer rounding on them.
9) Schedule a time to see family members: Conversations with family members are usually more productive if those times are scheduled. If possible, schedule them after you have seen a bulk of your patients to avoid feeling pressured to cut the meeting short. It is important to know who the family spokesperson is for large families so you can refer other family members to them and avoid multiple call-backs.
10) Develop and maintain good relationships: Your cordial interaction with various hospital department staff (nursing, case management, social work, radiology, and physical therapy to name a few) will help facilitate the inpatient care plan. It certainly helps not to have to wait two or more days to have a diagnostic test performed or assessment made. Sustaining a healthy working relationship promotes an understanding of your expectations for inpatient care.
11) Advocate for constructive change: Much inefficiency is systems based. Thinking about what interferes with your effectiveness in your system and suggesting changes can help a lot. For example, if your institution is going to switch to an electronic medical record, it certainly helps for you or a member of your hospitalist team to get involved in the implementation. Many hospitals are invested in quality improvement—and hospitalists are and should be at the forefront of this change.
As a hospitalist, you can initiate changes within your practice and your hospital’s system to keep things efficient. Making those adjustments sometimes takes time—but they’re well worth the effort.
Meanwhile, maximizing your efficiency can help promote patient throughput, enhance patient satisfaction, improve quality of care and increase job satisfaction. TH
Dr. Magnet is a hospitalist at the Singing River Hospital System on the Gulf Coast of Mississippi and a member of SHM’s Young Physician Committee.
Is That Your Patient?
How many times have you been asked a medical question outside the hospital? Undoubtedly, it happens too many times to count.
An acquaintance asks about a strange pain; you look at a rash on your neighbor’s son; you guide a nurse when she can’t reach a patient’s physician; a colleague asks for a curbside consult; or you provide medical advice over the phone to another provider while on-call at the hospital. When do any of the people in these situations become your patient?
Unfortunately, there is no easy answer. Legally, the question of whether a physician-patient relationship is created is determined on a case-by-case basis. As a general rule, if a physician undertakes to treat or provide medical care, a physician-patient relationship exists and the physician contracts to exercise reasonable skill in providing the care.
Implied Relationships
Absent an express agreement to enter a physician-patient relationship, the law may imply a relationship based on conduct that demonstrates consent to a relationship. A patient demonstrates consent by seeking medical services. Consent may also be implied when, for example, a patient needs emergency care, services are provided at the request of a treating physician, or treatment is mandated by a court.
Physicians consent to a relationship by diagnosing, treating, or otherwise providing care. A physician can also consent simply because of a working arrangement with a hospital or other entity—such as an agreement to accept assignment of patients.
In determining whether a physician-patient relationship has been created, consider the absence or existence of affirmative acts by a physician. For example, when a physician receives a call from a patient’s treating physician and the two physicians discuss the patient, the conversation might not create a physician-patient relationship if the consulting physician does not expressly provide an opinion. This is because there is no affirmative action upon which a court can imply a duty. Likewise, an on-call doctor does not create a physician-patient relationship simply by being on-call when she does not see, treat, or participate in the care of a patient.
Conversely, acts sufficient to create a physician-patient relationship exist when an on-call or consulting physician offers advice, provides treatment, or discharges a patient. Notably, an implied duty can be inferred even if the physician has not had direct contact with a patient if the court determines the physician’s conduct has interfered with a patient’s interests—thereby entitling the patient to legal protection.
Duties of Physicians
Even absent a physician-patient relationship, the law can impose general duties on physicians. Physicians have a duty to use reasonable care in regard to affirmative conduct when it is foreseeable that another might be injured.
For example, the Colorado Supreme Court found that an anesthesiologist owed a general duty to hospital patients who were not his patients when the physician’s failure to properly dispose of medication exposed patients to a foreseeable risk of harm.
The court has also found that a physician retained by defendants in a personal injury lawsuit owed a duty of reasonable care when subjecting the plaintiff to medical examinations. Similarly, the court concluded that an independent medical examiner could be liable for any injury the examiner causes during an examination, even though the examiner does not owe a duty to accurately diagnose the patient.
Is That Your Patient?
Ultimately, a physician-patient relationship and its corresponding duties arise when reasonable people would recognize a duty and agree that it exists. You must analyze your conduct and interactions, including your:
- Communication with patients or nonpatients (including e-mail or conversations in passing);
- On-call status;
- Agreements with facilities, a service, or other providers to accept patients;
- Degree of responsibility for a given patient’s care;
- Charges or fee discussion;
- Affirmative acts of care or treatment as distinguished from examination solely for the benefit of a third party;
- Initiation of contact with the patient or patient’s family;
- Referral from another physician or non-physician; and
- Consultations with other physicians, either formal or informal and whether different areas of expertise are involved or specific care or advise is given.
Ultimate determination of whether a physician-patient relationship exists is fact-specific—and no single fact is definitive. The above factors may guide you in assessing the nature of your interactions with patients and your attendant responsibilities. TH
Patrick O’Rourke works in the Office of University Counsel, Department of Litigation, University of Colorado, Denver.
How many times have you been asked a medical question outside the hospital? Undoubtedly, it happens too many times to count.
An acquaintance asks about a strange pain; you look at a rash on your neighbor’s son; you guide a nurse when she can’t reach a patient’s physician; a colleague asks for a curbside consult; or you provide medical advice over the phone to another provider while on-call at the hospital. When do any of the people in these situations become your patient?
Unfortunately, there is no easy answer. Legally, the question of whether a physician-patient relationship is created is determined on a case-by-case basis. As a general rule, if a physician undertakes to treat or provide medical care, a physician-patient relationship exists and the physician contracts to exercise reasonable skill in providing the care.
Implied Relationships
Absent an express agreement to enter a physician-patient relationship, the law may imply a relationship based on conduct that demonstrates consent to a relationship. A patient demonstrates consent by seeking medical services. Consent may also be implied when, for example, a patient needs emergency care, services are provided at the request of a treating physician, or treatment is mandated by a court.
Physicians consent to a relationship by diagnosing, treating, or otherwise providing care. A physician can also consent simply because of a working arrangement with a hospital or other entity—such as an agreement to accept assignment of patients.
In determining whether a physician-patient relationship has been created, consider the absence or existence of affirmative acts by a physician. For example, when a physician receives a call from a patient’s treating physician and the two physicians discuss the patient, the conversation might not create a physician-patient relationship if the consulting physician does not expressly provide an opinion. This is because there is no affirmative action upon which a court can imply a duty. Likewise, an on-call doctor does not create a physician-patient relationship simply by being on-call when she does not see, treat, or participate in the care of a patient.
Conversely, acts sufficient to create a physician-patient relationship exist when an on-call or consulting physician offers advice, provides treatment, or discharges a patient. Notably, an implied duty can be inferred even if the physician has not had direct contact with a patient if the court determines the physician’s conduct has interfered with a patient’s interests—thereby entitling the patient to legal protection.
Duties of Physicians
Even absent a physician-patient relationship, the law can impose general duties on physicians. Physicians have a duty to use reasonable care in regard to affirmative conduct when it is foreseeable that another might be injured.
For example, the Colorado Supreme Court found that an anesthesiologist owed a general duty to hospital patients who were not his patients when the physician’s failure to properly dispose of medication exposed patients to a foreseeable risk of harm.
The court has also found that a physician retained by defendants in a personal injury lawsuit owed a duty of reasonable care when subjecting the plaintiff to medical examinations. Similarly, the court concluded that an independent medical examiner could be liable for any injury the examiner causes during an examination, even though the examiner does not owe a duty to accurately diagnose the patient.
Is That Your Patient?
Ultimately, a physician-patient relationship and its corresponding duties arise when reasonable people would recognize a duty and agree that it exists. You must analyze your conduct and interactions, including your:
- Communication with patients or nonpatients (including e-mail or conversations in passing);
- On-call status;
- Agreements with facilities, a service, or other providers to accept patients;
- Degree of responsibility for a given patient’s care;
- Charges or fee discussion;
- Affirmative acts of care or treatment as distinguished from examination solely for the benefit of a third party;
- Initiation of contact with the patient or patient’s family;
- Referral from another physician or non-physician; and
- Consultations with other physicians, either formal or informal and whether different areas of expertise are involved or specific care or advise is given.
Ultimate determination of whether a physician-patient relationship exists is fact-specific—and no single fact is definitive. The above factors may guide you in assessing the nature of your interactions with patients and your attendant responsibilities. TH
Patrick O’Rourke works in the Office of University Counsel, Department of Litigation, University of Colorado, Denver.
How many times have you been asked a medical question outside the hospital? Undoubtedly, it happens too many times to count.
An acquaintance asks about a strange pain; you look at a rash on your neighbor’s son; you guide a nurse when she can’t reach a patient’s physician; a colleague asks for a curbside consult; or you provide medical advice over the phone to another provider while on-call at the hospital. When do any of the people in these situations become your patient?
Unfortunately, there is no easy answer. Legally, the question of whether a physician-patient relationship is created is determined on a case-by-case basis. As a general rule, if a physician undertakes to treat or provide medical care, a physician-patient relationship exists and the physician contracts to exercise reasonable skill in providing the care.
Implied Relationships
Absent an express agreement to enter a physician-patient relationship, the law may imply a relationship based on conduct that demonstrates consent to a relationship. A patient demonstrates consent by seeking medical services. Consent may also be implied when, for example, a patient needs emergency care, services are provided at the request of a treating physician, or treatment is mandated by a court.
Physicians consent to a relationship by diagnosing, treating, or otherwise providing care. A physician can also consent simply because of a working arrangement with a hospital or other entity—such as an agreement to accept assignment of patients.
In determining whether a physician-patient relationship has been created, consider the absence or existence of affirmative acts by a physician. For example, when a physician receives a call from a patient’s treating physician and the two physicians discuss the patient, the conversation might not create a physician-patient relationship if the consulting physician does not expressly provide an opinion. This is because there is no affirmative action upon which a court can imply a duty. Likewise, an on-call doctor does not create a physician-patient relationship simply by being on-call when she does not see, treat, or participate in the care of a patient.
Conversely, acts sufficient to create a physician-patient relationship exist when an on-call or consulting physician offers advice, provides treatment, or discharges a patient. Notably, an implied duty can be inferred even if the physician has not had direct contact with a patient if the court determines the physician’s conduct has interfered with a patient’s interests—thereby entitling the patient to legal protection.
Duties of Physicians
Even absent a physician-patient relationship, the law can impose general duties on physicians. Physicians have a duty to use reasonable care in regard to affirmative conduct when it is foreseeable that another might be injured.
For example, the Colorado Supreme Court found that an anesthesiologist owed a general duty to hospital patients who were not his patients when the physician’s failure to properly dispose of medication exposed patients to a foreseeable risk of harm.
The court has also found that a physician retained by defendants in a personal injury lawsuit owed a duty of reasonable care when subjecting the plaintiff to medical examinations. Similarly, the court concluded that an independent medical examiner could be liable for any injury the examiner causes during an examination, even though the examiner does not owe a duty to accurately diagnose the patient.
Is That Your Patient?
Ultimately, a physician-patient relationship and its corresponding duties arise when reasonable people would recognize a duty and agree that it exists. You must analyze your conduct and interactions, including your:
- Communication with patients or nonpatients (including e-mail or conversations in passing);
- On-call status;
- Agreements with facilities, a service, or other providers to accept patients;
- Degree of responsibility for a given patient’s care;
- Charges or fee discussion;
- Affirmative acts of care or treatment as distinguished from examination solely for the benefit of a third party;
- Initiation of contact with the patient or patient’s family;
- Referral from another physician or non-physician; and
- Consultations with other physicians, either formal or informal and whether different areas of expertise are involved or specific care or advise is given.
Ultimate determination of whether a physician-patient relationship exists is fact-specific—and no single fact is definitive. The above factors may guide you in assessing the nature of your interactions with patients and your attendant responsibilities. TH
Patrick O’Rourke works in the Office of University Counsel, Department of Litigation, University of Colorado, Denver.
Document Patient History
Documentation in the medical record serves many purposes: communication among healthcare professionals, evidence of patient care, and justification for provider claims.
Although these three aspects of documentation are intertwined, the first two prevent physicians from paying settlements involving malpractice allegations, while the last one assists in obtaining appropriate reimbursement for services rendered. This is the first of a three-part series that will focus on claim reporting and outline the documentation guidelines set forth by the Centers for Medicare and Medicaid Services (CMS) in conjunction with the American Medical Association (AMA).
1995, 1997 Guidelines
Two sets of documentation guidelines are in place, referred to as the 1995 and 1997 guidelines. Increased criticism of the ambiguity in the 1995 guidelines from auditors and providers inspired development of the 1997 guidelines.
While the 1997 guidelines were intended to create a more objective and unified approach to documentation, the level of specificity required brought criticism and frustration. But while the physician community balked, most auditors praised these efforts.
To satisfy all parties and allow physicians to document as they prefer, both sets of guidelines remain. Physicians can document according to either style, and auditors are obligated to review provider records against both sets of guidelines, selecting the final visit level with the set that best supports provider documentation.
Elements of History
Chief complaint (CC): The CC is the reason for the visit as stated in the patient’s own words. This must be present for each encounter, and should reference a specific condition or complaint (e.g., patient complains of abdominal pain).
History of present illness (HPI): This is a description of the present illness as it developed. It is typically formatted and documented with reference to location, quality, severity, timing, context, modifying factors, and associated signs/symptoms as related to the chief complaint. The HPI may be classified as brief (a comment on fewer than HPI elements) or extended (a comment on more than four HPI elements). Sample documentation of an extended HPI is: “The patient has intermittent (duration), sharp (quality) pain in the right upper quadrant (location) without associated nausea, vomiting, or diarrhea (associated signs/symptoms).”
The 1997 guidelines offer an alternate format for documenting the HPI. In contrast to the standard method above, the physician may list and status the patient’s chronic or inactive conditions. An extended HPI consists of the status of at least three chronic or inactive conditions (e.g., “Diabetes controlled by oral medication; extrinsic asthma without acute exacerbation in past six months; hypertension stable with pressures ranging from 130-140/80-90”). Failing to document the status negates the opportunity for the physician to receive HPI credit. Instead, he will receive credit for a past medical history.
The HPI should never be documented by ancillary staff (e.g., registered nurse, medical assistant, students). HPI might be documented by residents (e.g., residents, fellows, interns) or nonphysician providers (nurse practitioners and physician assistants) when utilizing the Teaching Physician Rules or Split-Shared Billing Rules, respectively (teaching Physician Rules and Split-Shared Billing Rules will be addressed in an upcoming issue).
Review of systems (ROS): This is a series of questions used to elicit information about additional signs, symptoms, or problems currently or previously experienced by the patient:
- Constitutional;
- Eyes; ears, nose, mouth, throat;
- Cardiovascular;
- Respiratory;
- Gastrointestinal;
- Genitourinary;
- Musculoskeletal;
- Integumentary (including skin and/or breast);
- Neurological;
- Psychiatric;
- Endocrine;
- Hematologic/lymphatic; and
- Allergic/immunologic.
The ROS may be classified as brief (a comment on one system), expanded (a comment on two to nine systems), or complete (a comment on more than 10 systems).
Documentation of a complete ROS (more than 10 systems) can occur in two ways:
- The physician can individually document each system. For example: “No fever/chills (constitutional) or blurred vision (eyes); no chest pain (cardiovascular); shortness of breath (respiratory); or belly pain (gastrointestinal); etc.”; or
- The physician can document the positive findings and pertinent negative findings related to the chief complaint, along with a comment that “all other systems are negative.” This latter statement is not accepted by all local Medicare contractors.
Information involving the ROS can be documented by anyone, including the patient. If documented by someone else (e.g., a medical student) other than residents under the Teaching Physician Rules or nonphysician providers under the Split-Shared Billing Rules, the physician should reference the documented ROS in his progress note. Re-documentation of the ROS is not necessary unless a revision is required.
Past, family, and social history (PFSH): Documentation of PFSH involves data obtained about the patient’s previous illness or medical conditions/therapies, family occurrences with illness, and relevant patient activities. The PFSH can be classified as pertinent (a comment on one history) or complete (a comment in each of the three histories). Documentation that exemplifies a complete PFSH is: “Patient currently on Prilosec 20 mg daily; family history of Barrett’s esophagus; no tobacco or alcohol use.”
As with ROS, the PFSH can be documented by anyone, including the patient. If documented by someone else (e.g., a medical student) other than residents under the Teaching Physician Rules or nonphysician providers under the Split-Shared Billing Rules, the physician should reference the documented PFSH in his progress note. Re-documentation of the PFSH is not necessary unless a revision is required. It is important to note that while documentation of the PFSH is required when billing higher level consultations (99254-99255) or initial inpatient care (99221-99223), it is not required when reporting subsequent hospital care services (99231-99233).
Levels of History
There are four levels of history, determined by the number of elements documented in the progress note (see Table 1, p. 21). The physician must meet all the requirements in a specific level of history before assigning it.
If all of the required elements in a given history level are not documented, the level assigned is that of the least documented element. For example, physician documentation may include four HPI elements and a complete PFSH, yet only eight ROS. The physician can only receive credit for a detailed history. If the physician submitted a claim for 99222 (initial hospital care requiring a comprehensive history, a comprehensive exam, and moderate-complexity decision making), documentation would not support the reported service due to the underdocumented ROS. Deficiencies in the ROS and family history are the most common physician documentation errors involving the history component.
A specific level of history is associated with each type of physician encounter, and must be documented accordingly (see Table 2, right). The most common visit categories provided by hospitalists that include documentation requirements for history are initial inpatient consultations, initial hospital care, subsequent hospital care, and initial observation care. Other visit categories, such as critical care and discharge day management, have neither associated levels of history nor documentation requirements for historical elements. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is also on the faculty of SHM’s inpatient coding course.
Documentation in the medical record serves many purposes: communication among healthcare professionals, evidence of patient care, and justification for provider claims.
Although these three aspects of documentation are intertwined, the first two prevent physicians from paying settlements involving malpractice allegations, while the last one assists in obtaining appropriate reimbursement for services rendered. This is the first of a three-part series that will focus on claim reporting and outline the documentation guidelines set forth by the Centers for Medicare and Medicaid Services (CMS) in conjunction with the American Medical Association (AMA).
1995, 1997 Guidelines
Two sets of documentation guidelines are in place, referred to as the 1995 and 1997 guidelines. Increased criticism of the ambiguity in the 1995 guidelines from auditors and providers inspired development of the 1997 guidelines.
While the 1997 guidelines were intended to create a more objective and unified approach to documentation, the level of specificity required brought criticism and frustration. But while the physician community balked, most auditors praised these efforts.
To satisfy all parties and allow physicians to document as they prefer, both sets of guidelines remain. Physicians can document according to either style, and auditors are obligated to review provider records against both sets of guidelines, selecting the final visit level with the set that best supports provider documentation.
Elements of History
Chief complaint (CC): The CC is the reason for the visit as stated in the patient’s own words. This must be present for each encounter, and should reference a specific condition or complaint (e.g., patient complains of abdominal pain).
History of present illness (HPI): This is a description of the present illness as it developed. It is typically formatted and documented with reference to location, quality, severity, timing, context, modifying factors, and associated signs/symptoms as related to the chief complaint. The HPI may be classified as brief (a comment on fewer than HPI elements) or extended (a comment on more than four HPI elements). Sample documentation of an extended HPI is: “The patient has intermittent (duration), sharp (quality) pain in the right upper quadrant (location) without associated nausea, vomiting, or diarrhea (associated signs/symptoms).”
The 1997 guidelines offer an alternate format for documenting the HPI. In contrast to the standard method above, the physician may list and status the patient’s chronic or inactive conditions. An extended HPI consists of the status of at least three chronic or inactive conditions (e.g., “Diabetes controlled by oral medication; extrinsic asthma without acute exacerbation in past six months; hypertension stable with pressures ranging from 130-140/80-90”). Failing to document the status negates the opportunity for the physician to receive HPI credit. Instead, he will receive credit for a past medical history.
The HPI should never be documented by ancillary staff (e.g., registered nurse, medical assistant, students). HPI might be documented by residents (e.g., residents, fellows, interns) or nonphysician providers (nurse practitioners and physician assistants) when utilizing the Teaching Physician Rules or Split-Shared Billing Rules, respectively (teaching Physician Rules and Split-Shared Billing Rules will be addressed in an upcoming issue).
Review of systems (ROS): This is a series of questions used to elicit information about additional signs, symptoms, or problems currently or previously experienced by the patient:
- Constitutional;
- Eyes; ears, nose, mouth, throat;
- Cardiovascular;
- Respiratory;
- Gastrointestinal;
- Genitourinary;
- Musculoskeletal;
- Integumentary (including skin and/or breast);
- Neurological;
- Psychiatric;
- Endocrine;
- Hematologic/lymphatic; and
- Allergic/immunologic.
The ROS may be classified as brief (a comment on one system), expanded (a comment on two to nine systems), or complete (a comment on more than 10 systems).
Documentation of a complete ROS (more than 10 systems) can occur in two ways:
- The physician can individually document each system. For example: “No fever/chills (constitutional) or blurred vision (eyes); no chest pain (cardiovascular); shortness of breath (respiratory); or belly pain (gastrointestinal); etc.”; or
- The physician can document the positive findings and pertinent negative findings related to the chief complaint, along with a comment that “all other systems are negative.” This latter statement is not accepted by all local Medicare contractors.
Information involving the ROS can be documented by anyone, including the patient. If documented by someone else (e.g., a medical student) other than residents under the Teaching Physician Rules or nonphysician providers under the Split-Shared Billing Rules, the physician should reference the documented ROS in his progress note. Re-documentation of the ROS is not necessary unless a revision is required.
Past, family, and social history (PFSH): Documentation of PFSH involves data obtained about the patient’s previous illness or medical conditions/therapies, family occurrences with illness, and relevant patient activities. The PFSH can be classified as pertinent (a comment on one history) or complete (a comment in each of the three histories). Documentation that exemplifies a complete PFSH is: “Patient currently on Prilosec 20 mg daily; family history of Barrett’s esophagus; no tobacco or alcohol use.”
As with ROS, the PFSH can be documented by anyone, including the patient. If documented by someone else (e.g., a medical student) other than residents under the Teaching Physician Rules or nonphysician providers under the Split-Shared Billing Rules, the physician should reference the documented PFSH in his progress note. Re-documentation of the PFSH is not necessary unless a revision is required. It is important to note that while documentation of the PFSH is required when billing higher level consultations (99254-99255) or initial inpatient care (99221-99223), it is not required when reporting subsequent hospital care services (99231-99233).
Levels of History
There are four levels of history, determined by the number of elements documented in the progress note (see Table 1, p. 21). The physician must meet all the requirements in a specific level of history before assigning it.
If all of the required elements in a given history level are not documented, the level assigned is that of the least documented element. For example, physician documentation may include four HPI elements and a complete PFSH, yet only eight ROS. The physician can only receive credit for a detailed history. If the physician submitted a claim for 99222 (initial hospital care requiring a comprehensive history, a comprehensive exam, and moderate-complexity decision making), documentation would not support the reported service due to the underdocumented ROS. Deficiencies in the ROS and family history are the most common physician documentation errors involving the history component.
A specific level of history is associated with each type of physician encounter, and must be documented accordingly (see Table 2, right). The most common visit categories provided by hospitalists that include documentation requirements for history are initial inpatient consultations, initial hospital care, subsequent hospital care, and initial observation care. Other visit categories, such as critical care and discharge day management, have neither associated levels of history nor documentation requirements for historical elements. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is also on the faculty of SHM’s inpatient coding course.
Documentation in the medical record serves many purposes: communication among healthcare professionals, evidence of patient care, and justification for provider claims.
Although these three aspects of documentation are intertwined, the first two prevent physicians from paying settlements involving malpractice allegations, while the last one assists in obtaining appropriate reimbursement for services rendered. This is the first of a three-part series that will focus on claim reporting and outline the documentation guidelines set forth by the Centers for Medicare and Medicaid Services (CMS) in conjunction with the American Medical Association (AMA).
1995, 1997 Guidelines
Two sets of documentation guidelines are in place, referred to as the 1995 and 1997 guidelines. Increased criticism of the ambiguity in the 1995 guidelines from auditors and providers inspired development of the 1997 guidelines.
While the 1997 guidelines were intended to create a more objective and unified approach to documentation, the level of specificity required brought criticism and frustration. But while the physician community balked, most auditors praised these efforts.
To satisfy all parties and allow physicians to document as they prefer, both sets of guidelines remain. Physicians can document according to either style, and auditors are obligated to review provider records against both sets of guidelines, selecting the final visit level with the set that best supports provider documentation.
Elements of History
Chief complaint (CC): The CC is the reason for the visit as stated in the patient’s own words. This must be present for each encounter, and should reference a specific condition or complaint (e.g., patient complains of abdominal pain).
History of present illness (HPI): This is a description of the present illness as it developed. It is typically formatted and documented with reference to location, quality, severity, timing, context, modifying factors, and associated signs/symptoms as related to the chief complaint. The HPI may be classified as brief (a comment on fewer than HPI elements) or extended (a comment on more than four HPI elements). Sample documentation of an extended HPI is: “The patient has intermittent (duration), sharp (quality) pain in the right upper quadrant (location) without associated nausea, vomiting, or diarrhea (associated signs/symptoms).”
The 1997 guidelines offer an alternate format for documenting the HPI. In contrast to the standard method above, the physician may list and status the patient’s chronic or inactive conditions. An extended HPI consists of the status of at least three chronic or inactive conditions (e.g., “Diabetes controlled by oral medication; extrinsic asthma without acute exacerbation in past six months; hypertension stable with pressures ranging from 130-140/80-90”). Failing to document the status negates the opportunity for the physician to receive HPI credit. Instead, he will receive credit for a past medical history.
The HPI should never be documented by ancillary staff (e.g., registered nurse, medical assistant, students). HPI might be documented by residents (e.g., residents, fellows, interns) or nonphysician providers (nurse practitioners and physician assistants) when utilizing the Teaching Physician Rules or Split-Shared Billing Rules, respectively (teaching Physician Rules and Split-Shared Billing Rules will be addressed in an upcoming issue).
Review of systems (ROS): This is a series of questions used to elicit information about additional signs, symptoms, or problems currently or previously experienced by the patient:
- Constitutional;
- Eyes; ears, nose, mouth, throat;
- Cardiovascular;
- Respiratory;
- Gastrointestinal;
- Genitourinary;
- Musculoskeletal;
- Integumentary (including skin and/or breast);
- Neurological;
- Psychiatric;
- Endocrine;
- Hematologic/lymphatic; and
- Allergic/immunologic.
The ROS may be classified as brief (a comment on one system), expanded (a comment on two to nine systems), or complete (a comment on more than 10 systems).
Documentation of a complete ROS (more than 10 systems) can occur in two ways:
- The physician can individually document each system. For example: “No fever/chills (constitutional) or blurred vision (eyes); no chest pain (cardiovascular); shortness of breath (respiratory); or belly pain (gastrointestinal); etc.”; or
- The physician can document the positive findings and pertinent negative findings related to the chief complaint, along with a comment that “all other systems are negative.” This latter statement is not accepted by all local Medicare contractors.
Information involving the ROS can be documented by anyone, including the patient. If documented by someone else (e.g., a medical student) other than residents under the Teaching Physician Rules or nonphysician providers under the Split-Shared Billing Rules, the physician should reference the documented ROS in his progress note. Re-documentation of the ROS is not necessary unless a revision is required.
Past, family, and social history (PFSH): Documentation of PFSH involves data obtained about the patient’s previous illness or medical conditions/therapies, family occurrences with illness, and relevant patient activities. The PFSH can be classified as pertinent (a comment on one history) or complete (a comment in each of the three histories). Documentation that exemplifies a complete PFSH is: “Patient currently on Prilosec 20 mg daily; family history of Barrett’s esophagus; no tobacco or alcohol use.”
As with ROS, the PFSH can be documented by anyone, including the patient. If documented by someone else (e.g., a medical student) other than residents under the Teaching Physician Rules or nonphysician providers under the Split-Shared Billing Rules, the physician should reference the documented PFSH in his progress note. Re-documentation of the PFSH is not necessary unless a revision is required. It is important to note that while documentation of the PFSH is required when billing higher level consultations (99254-99255) or initial inpatient care (99221-99223), it is not required when reporting subsequent hospital care services (99231-99233).
Levels of History
There are four levels of history, determined by the number of elements documented in the progress note (see Table 1, p. 21). The physician must meet all the requirements in a specific level of history before assigning it.
If all of the required elements in a given history level are not documented, the level assigned is that of the least documented element. For example, physician documentation may include four HPI elements and a complete PFSH, yet only eight ROS. The physician can only receive credit for a detailed history. If the physician submitted a claim for 99222 (initial hospital care requiring a comprehensive history, a comprehensive exam, and moderate-complexity decision making), documentation would not support the reported service due to the underdocumented ROS. Deficiencies in the ROS and family history are the most common physician documentation errors involving the history component.
A specific level of history is associated with each type of physician encounter, and must be documented accordingly (see Table 2, right). The most common visit categories provided by hospitalists that include documentation requirements for history are initial inpatient consultations, initial hospital care, subsequent hospital care, and initial observation care. Other visit categories, such as critical care and discharge day management, have neither associated levels of history nor documentation requirements for historical elements. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is also on the faculty of SHM’s inpatient coding course.