User login
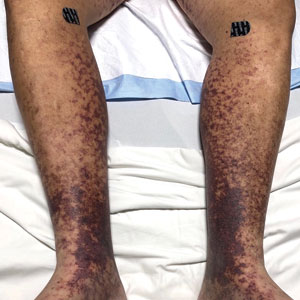
Retiform Purpura on the Lower Legs
The Diagnosis: Type I Cryoglobulinemia
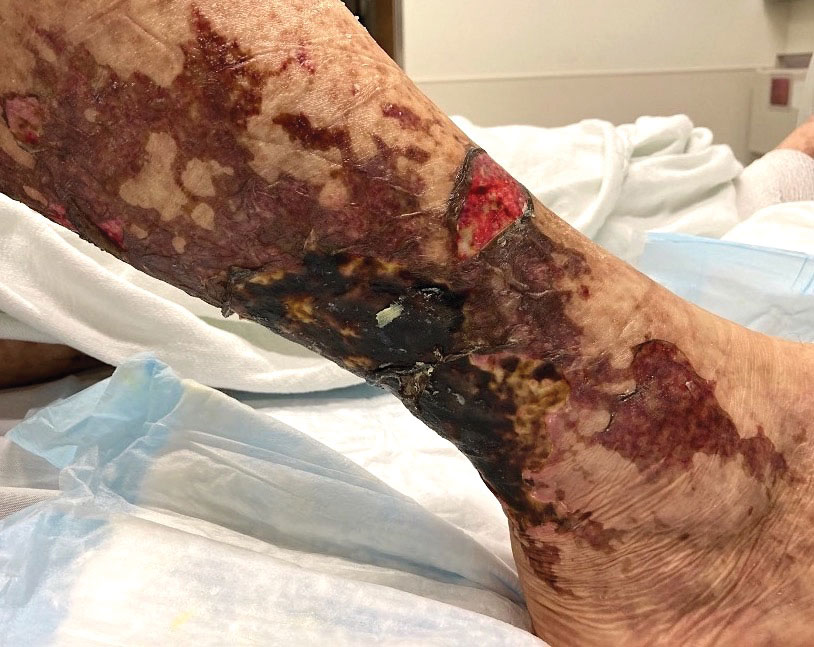
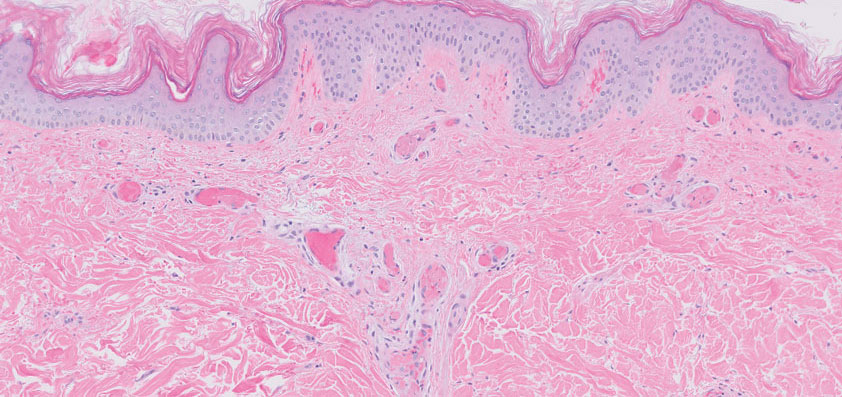
Retiform purpura with overlying necrosis subsequently developed over the course of a week following presentation (Figure 1). A skin biopsy showed fibrin thrombi and congestion of small- and medium-sized blood vessels, consistent with vasculopathy (Figure 2). Urinalysis revealed hematuria and proteinuria. A renal biopsy performed due to a continually elevated serum creatinine level revealed glomerulonephritis with numerous IgG1 lambda–restricted glomerular capillary hyaline thrombi, compatible with a lymphoproliferative disorder–associated type I cryoglobulinemia. A serum cryoglobulin immunofixation test confirmed type I cryoglobulinemia involving monoclonal IgG lambda. The combination of cutaneous, renal, and hematologic findings was consistent with type I cryoglobulinemia. A subsequent bone marrow biopsy demonstrated a CD20+ lambda–restricted plasma cell neoplasm. Initial treatment with high-dose corticosteroids followed by targeted treatment of the underlying hematologic condition with bortezomib, rituximab, and dexamethasone improved the skin disease.
Cryoglobulins are abnormal immunoglobulins that precipitate at temperatures below 37 °C. The persistent presence of cryoglobulins in the serum is termed cryoglobulinemia.1 Type I cryoglobulinemia is distinguished from mixed cryoglobulinemia—types II and III—by the presence of a single monoclonal immunoglobulin, typically IgM or IgG. It is associated with lymphoproliferative disorders, most commonly monoclonal gammopathy of undetermined significance and B-cell malignancies such as Waldenström macroglobulinemia, multiple myeloma, or chronic lymphocytic leukemia. Histopathology shows occlusion of small vessel lumina with homogenous eosinophilic material containing the monoclonal cryoprecipitate.2 Disease manifestations are caused by small vessel occlusion, which leads to ischemia and tissue damage.
Retiform purpura, livedo reticularis/racemosa, and necrosis leading to ulcers are the most common cutaneous clinical findings. Extracutaneous signs include peripheral neuropathy, arthralgia, Raynaud phenomenon, and acrocyanosis. Renal involvement, most commonly glomerulonephritis with associated proteinuria, is noted in 14% to 20% of cases.3,4 An elevated cryocrit can lead to symptoms of hyperviscosity syndrome.2
Treatment is difficult and primarily is focused on addressing the underlying hematologic condition, which is responsible for synthesis of the cryoglobulin. Decreasing cryoglobulin production leads to decreased occlusion of blood vessels, thus alleviating the ischemia and skin damage. Monoclonal gammopathy of undetermined significance–related type I cryoglobulinemia initially is treated with corticosteroids followed by rituximab if a CD20+ B-cell clone is identified.2 Bortezomib is recommended for cases associated with Waldenström macroglobulinemia and cases associated with multiple myeloma with concurrent renal failure. In patients with neuropathy, a lenalidomide-based treatment can be employed. Patients should be instructed to keep extremities warm.2 Diabetic foot care guidelines should be followed to prevent wound complications. The differential diagnosis for type I cryoglobulinemia includes other causes of retiform purpura–like angioinvasive fungal infection, antiphospholipid antibody syndrome, calciphylaxis, and livedoid vasculopathy.5 Angioinvasive fungal infections are caused by Candida, Aspergillus, and Mucorales species, as well as other hyaline molds. They typically occur in immunocompromised patients and invade the blood vessels via direct inoculation or dissemination.6 Patients present with retiform purpura but typically will be acutely ill with fevers and vital sign abnormalities. Histopathology with special stains often will identify the fungal organisms in the dermis or inside blood vessel walls with vessel wall destruction and hemorrhage.7 Accurate diagnosis is essential to selecting appropriate antifungal agents. If angioinvasive fungal infection is clinically suspected, treatment should begin before culture and histopathologic data are available.7
Antiphospholipid antibody syndrome is an autoimmune thrombophilia that can occur as primary disease or in association with other autoimmune conditions, most commonly systemic lupus erythematosus. Diagnosis requires the presence of antiphospholipid antibodies, such as lupus anticoagulant, anticardiolipin antibody, anti–β2-glycoprotein-1 antibody, with arterial or venous thrombosis and/or recurrent pregnancy loss. Paraproteinemia is not seen. The most common cutaneous finding is livedo reticularis, with livedo racemosa being a more distinctive finding.8 Small vessel thrombosis is seen histopathologically. Treatment includes antiplatelet and anticoagulant medications. Patients with refractory disease may benefit from additional therapy with hydroxychloroquine or intravenous immunoglobulins.8
Calciphylaxis is a rare depositional vasculopathy that often occurs in patients with end-stage renal disease on dialysis. Patients present with painful and poor-healing skin lesions including indurated nodules, violaceous plaques, and retiform purpura that typically affect areas of high adiposity such as the thighs, abdomen, and buttocks.9 Ulceration and superimposed infections are common complications. Histopathologically, small dermal and subcutaneous vessels demonstrate calcification, microthrombosis, and fibrointimal hyperplasia.9 Wound management is critically important in patients with calciphylaxis. Treatment with intravenous sodium thiosulfate is typical, but prognosis remains poor. Although livedoid vasculopathy may present with retiform purpura in the ankles, paraproteinemia is not seen and patients frequently present with punched-out ulcerations that tend to heal into atrophie blanche.10 Livedoid vasculopathy has been associated with underlying hypercoagulable states, connective tissue diseases, and chronic venous hypertension. Hypercoagulability and endothelial cell damage contribute to the formation of fibrin thrombi in the superficial dermal blood vessels. Histopathology demonstrates thickening of vessel walls and intraluminal hyaline thrombi. Successful treatment in most cases is achieved with anticoagulation therapy, typically rivaroxaban, especially in patients with underlying hypercoagulability. Antiplatelet therapy also may be considered, while anabolic agents have been shown to be helpful in patients with connective tissue disease.10
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j .jbspin.2019.01.016
- Muchtar E, Magen H, Gertz MA. How I treat cryoglobulinemia. Blood. 2017;129:289-298. doi:10.1182/blood-2016-09-719773
- Sidana S, Rajkumar SV, Dispenzieri A, et al. Clinical presentation and outcomes of patients with type 1 monoclonal cryoglobulinemia. Am J Hematol. 2017;92:668-673. doi:10.1002/ajh.24745
- Harel S, Mohr M, Jahn I, et al. Clinico-biological characteristics and treatment of type I monoclonal cryoglobulinaemia: a study of 64 cases. Br J Haematol. 2015;168:671-678. doi:10.1111/bjh.13196
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796. doi:10.1016/j.jaad.2019.07.112
- Shields BE, Rosenbach M, Brown-Joel Z, et al. Angioinvasive fungal infections impacting the skin: background, epidemiology, and clinical presentation. J Am Acad Dermatol. 2019;80:869-880.e5. doi:10.1016/j.jaad.2018.04.059
- Berger AP, Ford BA, Brown-Joel Z, et al. Angioinvasive fungal infections impacting the skin: diagnosis, management, and complications. J Am Acad Dermatol. 2019;80:883-898.e2. doi:10.1016/j.jaad.2018.04.058
- Negrini S, Pappalardo F, Murdaca G, et al. The antiphospholipid syndrome: from pathophysiology to treatment. Clin Exp Med. 2017;17:257-267. doi:10.1007/s10238-016-0430-5
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Georgesen C, Fox LP, Harp J. Retiform purpura: workup and therapeutic considerations in select conditions. J Am Acad Dermatol. 2020;82:799-816. doi:10.1016/j.jaad.2019.07.113
The Diagnosis: Type I Cryoglobulinemia
Retiform purpura with overlying necrosis subsequently developed over the course of a week following presentation (Figure 1). A skin biopsy showed fibrin thrombi and congestion of small- and medium-sized blood vessels, consistent with vasculopathy (Figure 2). Urinalysis revealed hematuria and proteinuria. A renal biopsy performed due to a continually elevated serum creatinine level revealed glomerulonephritis with numerous IgG1 lambda–restricted glomerular capillary hyaline thrombi, compatible with a lymphoproliferative disorder–associated type I cryoglobulinemia. A serum cryoglobulin immunofixation test confirmed type I cryoglobulinemia involving monoclonal IgG lambda. The combination of cutaneous, renal, and hematologic findings was consistent with type I cryoglobulinemia. A subsequent bone marrow biopsy demonstrated a CD20+ lambda–restricted plasma cell neoplasm. Initial treatment with high-dose corticosteroids followed by targeted treatment of the underlying hematologic condition with bortezomib, rituximab, and dexamethasone improved the skin disease.

Cryoglobulins are abnormal immunoglobulins that precipitate at temperatures below 37 °C. The persistent presence of cryoglobulins in the serum is termed cryoglobulinemia.1 Type I cryoglobulinemia is distinguished from mixed cryoglobulinemia—types II and III—by the presence of a single monoclonal immunoglobulin, typically IgM or IgG. It is associated with lymphoproliferative disorders, most commonly monoclonal gammopathy of undetermined significance and B-cell malignancies such as Waldenström macroglobulinemia, multiple myeloma, or chronic lymphocytic leukemia. Histopathology shows occlusion of small vessel lumina with homogenous eosinophilic material containing the monoclonal cryoprecipitate.2 Disease manifestations are caused by small vessel occlusion, which leads to ischemia and tissue damage.

Retiform purpura, livedo reticularis/racemosa, and necrosis leading to ulcers are the most common cutaneous clinical findings. Extracutaneous signs include peripheral neuropathy, arthralgia, Raynaud phenomenon, and acrocyanosis. Renal involvement, most commonly glomerulonephritis with associated proteinuria, is noted in 14% to 20% of cases.3,4 An elevated cryocrit can lead to symptoms of hyperviscosity syndrome.2
Treatment is difficult and primarily is focused on addressing the underlying hematologic condition, which is responsible for synthesis of the cryoglobulin. Decreasing cryoglobulin production leads to decreased occlusion of blood vessels, thus alleviating the ischemia and skin damage. Monoclonal gammopathy of undetermined significance–related type I cryoglobulinemia initially is treated with corticosteroids followed by rituximab if a CD20+ B-cell clone is identified.2 Bortezomib is recommended for cases associated with Waldenström macroglobulinemia and cases associated with multiple myeloma with concurrent renal failure. In patients with neuropathy, a lenalidomide-based treatment can be employed. Patients should be instructed to keep extremities warm.2 Diabetic foot care guidelines should be followed to prevent wound complications. The differential diagnosis for type I cryoglobulinemia includes other causes of retiform purpura–like angioinvasive fungal infection, antiphospholipid antibody syndrome, calciphylaxis, and livedoid vasculopathy.5 Angioinvasive fungal infections are caused by Candida, Aspergillus, and Mucorales species, as well as other hyaline molds. They typically occur in immunocompromised patients and invade the blood vessels via direct inoculation or dissemination.6 Patients present with retiform purpura but typically will be acutely ill with fevers and vital sign abnormalities. Histopathology with special stains often will identify the fungal organisms in the dermis or inside blood vessel walls with vessel wall destruction and hemorrhage.7 Accurate diagnosis is essential to selecting appropriate antifungal agents. If angioinvasive fungal infection is clinically suspected, treatment should begin before culture and histopathologic data are available.7
Antiphospholipid antibody syndrome is an autoimmune thrombophilia that can occur as primary disease or in association with other autoimmune conditions, most commonly systemic lupus erythematosus. Diagnosis requires the presence of antiphospholipid antibodies, such as lupus anticoagulant, anticardiolipin antibody, anti–β2-glycoprotein-1 antibody, with arterial or venous thrombosis and/or recurrent pregnancy loss. Paraproteinemia is not seen. The most common cutaneous finding is livedo reticularis, with livedo racemosa being a more distinctive finding.8 Small vessel thrombosis is seen histopathologically. Treatment includes antiplatelet and anticoagulant medications. Patients with refractory disease may benefit from additional therapy with hydroxychloroquine or intravenous immunoglobulins.8
Calciphylaxis is a rare depositional vasculopathy that often occurs in patients with end-stage renal disease on dialysis. Patients present with painful and poor-healing skin lesions including indurated nodules, violaceous plaques, and retiform purpura that typically affect areas of high adiposity such as the thighs, abdomen, and buttocks.9 Ulceration and superimposed infections are common complications. Histopathologically, small dermal and subcutaneous vessels demonstrate calcification, microthrombosis, and fibrointimal hyperplasia.9 Wound management is critically important in patients with calciphylaxis. Treatment with intravenous sodium thiosulfate is typical, but prognosis remains poor. Although livedoid vasculopathy may present with retiform purpura in the ankles, paraproteinemia is not seen and patients frequently present with punched-out ulcerations that tend to heal into atrophie blanche.10 Livedoid vasculopathy has been associated with underlying hypercoagulable states, connective tissue diseases, and chronic venous hypertension. Hypercoagulability and endothelial cell damage contribute to the formation of fibrin thrombi in the superficial dermal blood vessels. Histopathology demonstrates thickening of vessel walls and intraluminal hyaline thrombi. Successful treatment in most cases is achieved with anticoagulation therapy, typically rivaroxaban, especially in patients with underlying hypercoagulability. Antiplatelet therapy also may be considered, while anabolic agents have been shown to be helpful in patients with connective tissue disease.10
The Diagnosis: Type I Cryoglobulinemia
Retiform purpura with overlying necrosis subsequently developed over the course of a week following presentation (Figure 1). A skin biopsy showed fibrin thrombi and congestion of small- and medium-sized blood vessels, consistent with vasculopathy (Figure 2). Urinalysis revealed hematuria and proteinuria. A renal biopsy performed due to a continually elevated serum creatinine level revealed glomerulonephritis with numerous IgG1 lambda–restricted glomerular capillary hyaline thrombi, compatible with a lymphoproliferative disorder–associated type I cryoglobulinemia. A serum cryoglobulin immunofixation test confirmed type I cryoglobulinemia involving monoclonal IgG lambda. The combination of cutaneous, renal, and hematologic findings was consistent with type I cryoglobulinemia. A subsequent bone marrow biopsy demonstrated a CD20+ lambda–restricted plasma cell neoplasm. Initial treatment with high-dose corticosteroids followed by targeted treatment of the underlying hematologic condition with bortezomib, rituximab, and dexamethasone improved the skin disease.

Cryoglobulins are abnormal immunoglobulins that precipitate at temperatures below 37 °C. The persistent presence of cryoglobulins in the serum is termed cryoglobulinemia.1 Type I cryoglobulinemia is distinguished from mixed cryoglobulinemia—types II and III—by the presence of a single monoclonal immunoglobulin, typically IgM or IgG. It is associated with lymphoproliferative disorders, most commonly monoclonal gammopathy of undetermined significance and B-cell malignancies such as Waldenström macroglobulinemia, multiple myeloma, or chronic lymphocytic leukemia. Histopathology shows occlusion of small vessel lumina with homogenous eosinophilic material containing the monoclonal cryoprecipitate.2 Disease manifestations are caused by small vessel occlusion, which leads to ischemia and tissue damage.

Retiform purpura, livedo reticularis/racemosa, and necrosis leading to ulcers are the most common cutaneous clinical findings. Extracutaneous signs include peripheral neuropathy, arthralgia, Raynaud phenomenon, and acrocyanosis. Renal involvement, most commonly glomerulonephritis with associated proteinuria, is noted in 14% to 20% of cases.3,4 An elevated cryocrit can lead to symptoms of hyperviscosity syndrome.2
Treatment is difficult and primarily is focused on addressing the underlying hematologic condition, which is responsible for synthesis of the cryoglobulin. Decreasing cryoglobulin production leads to decreased occlusion of blood vessels, thus alleviating the ischemia and skin damage. Monoclonal gammopathy of undetermined significance–related type I cryoglobulinemia initially is treated with corticosteroids followed by rituximab if a CD20+ B-cell clone is identified.2 Bortezomib is recommended for cases associated with Waldenström macroglobulinemia and cases associated with multiple myeloma with concurrent renal failure. In patients with neuropathy, a lenalidomide-based treatment can be employed. Patients should be instructed to keep extremities warm.2 Diabetic foot care guidelines should be followed to prevent wound complications. The differential diagnosis for type I cryoglobulinemia includes other causes of retiform purpura–like angioinvasive fungal infection, antiphospholipid antibody syndrome, calciphylaxis, and livedoid vasculopathy.5 Angioinvasive fungal infections are caused by Candida, Aspergillus, and Mucorales species, as well as other hyaline molds. They typically occur in immunocompromised patients and invade the blood vessels via direct inoculation or dissemination.6 Patients present with retiform purpura but typically will be acutely ill with fevers and vital sign abnormalities. Histopathology with special stains often will identify the fungal organisms in the dermis or inside blood vessel walls with vessel wall destruction and hemorrhage.7 Accurate diagnosis is essential to selecting appropriate antifungal agents. If angioinvasive fungal infection is clinically suspected, treatment should begin before culture and histopathologic data are available.7
Antiphospholipid antibody syndrome is an autoimmune thrombophilia that can occur as primary disease or in association with other autoimmune conditions, most commonly systemic lupus erythematosus. Diagnosis requires the presence of antiphospholipid antibodies, such as lupus anticoagulant, anticardiolipin antibody, anti–β2-glycoprotein-1 antibody, with arterial or venous thrombosis and/or recurrent pregnancy loss. Paraproteinemia is not seen. The most common cutaneous finding is livedo reticularis, with livedo racemosa being a more distinctive finding.8 Small vessel thrombosis is seen histopathologically. Treatment includes antiplatelet and anticoagulant medications. Patients with refractory disease may benefit from additional therapy with hydroxychloroquine or intravenous immunoglobulins.8
Calciphylaxis is a rare depositional vasculopathy that often occurs in patients with end-stage renal disease on dialysis. Patients present with painful and poor-healing skin lesions including indurated nodules, violaceous plaques, and retiform purpura that typically affect areas of high adiposity such as the thighs, abdomen, and buttocks.9 Ulceration and superimposed infections are common complications. Histopathologically, small dermal and subcutaneous vessels demonstrate calcification, microthrombosis, and fibrointimal hyperplasia.9 Wound management is critically important in patients with calciphylaxis. Treatment with intravenous sodium thiosulfate is typical, but prognosis remains poor. Although livedoid vasculopathy may present with retiform purpura in the ankles, paraproteinemia is not seen and patients frequently present with punched-out ulcerations that tend to heal into atrophie blanche.10 Livedoid vasculopathy has been associated with underlying hypercoagulable states, connective tissue diseases, and chronic venous hypertension. Hypercoagulability and endothelial cell damage contribute to the formation of fibrin thrombi in the superficial dermal blood vessels. Histopathology demonstrates thickening of vessel walls and intraluminal hyaline thrombi. Successful treatment in most cases is achieved with anticoagulation therapy, typically rivaroxaban, especially in patients with underlying hypercoagulability. Antiplatelet therapy also may be considered, while anabolic agents have been shown to be helpful in patients with connective tissue disease.10
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j .jbspin.2019.01.016
- Muchtar E, Magen H, Gertz MA. How I treat cryoglobulinemia. Blood. 2017;129:289-298. doi:10.1182/blood-2016-09-719773
- Sidana S, Rajkumar SV, Dispenzieri A, et al. Clinical presentation and outcomes of patients with type 1 monoclonal cryoglobulinemia. Am J Hematol. 2017;92:668-673. doi:10.1002/ajh.24745
- Harel S, Mohr M, Jahn I, et al. Clinico-biological characteristics and treatment of type I monoclonal cryoglobulinaemia: a study of 64 cases. Br J Haematol. 2015;168:671-678. doi:10.1111/bjh.13196
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796. doi:10.1016/j.jaad.2019.07.112
- Shields BE, Rosenbach M, Brown-Joel Z, et al. Angioinvasive fungal infections impacting the skin: background, epidemiology, and clinical presentation. J Am Acad Dermatol. 2019;80:869-880.e5. doi:10.1016/j.jaad.2018.04.059
- Berger AP, Ford BA, Brown-Joel Z, et al. Angioinvasive fungal infections impacting the skin: diagnosis, management, and complications. J Am Acad Dermatol. 2019;80:883-898.e2. doi:10.1016/j.jaad.2018.04.058
- Negrini S, Pappalardo F, Murdaca G, et al. The antiphospholipid syndrome: from pathophysiology to treatment. Clin Exp Med. 2017;17:257-267. doi:10.1007/s10238-016-0430-5
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Georgesen C, Fox LP, Harp J. Retiform purpura: workup and therapeutic considerations in select conditions. J Am Acad Dermatol. 2020;82:799-816. doi:10.1016/j.jaad.2019.07.113
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j .jbspin.2019.01.016
- Muchtar E, Magen H, Gertz MA. How I treat cryoglobulinemia. Blood. 2017;129:289-298. doi:10.1182/blood-2016-09-719773
- Sidana S, Rajkumar SV, Dispenzieri A, et al. Clinical presentation and outcomes of patients with type 1 monoclonal cryoglobulinemia. Am J Hematol. 2017;92:668-673. doi:10.1002/ajh.24745
- Harel S, Mohr M, Jahn I, et al. Clinico-biological characteristics and treatment of type I monoclonal cryoglobulinaemia: a study of 64 cases. Br J Haematol. 2015;168:671-678. doi:10.1111/bjh.13196
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796. doi:10.1016/j.jaad.2019.07.112
- Shields BE, Rosenbach M, Brown-Joel Z, et al. Angioinvasive fungal infections impacting the skin: background, epidemiology, and clinical presentation. J Am Acad Dermatol. 2019;80:869-880.e5. doi:10.1016/j.jaad.2018.04.059
- Berger AP, Ford BA, Brown-Joel Z, et al. Angioinvasive fungal infections impacting the skin: diagnosis, management, and complications. J Am Acad Dermatol. 2019;80:883-898.e2. doi:10.1016/j.jaad.2018.04.058
- Negrini S, Pappalardo F, Murdaca G, et al. The antiphospholipid syndrome: from pathophysiology to treatment. Clin Exp Med. 2017;17:257-267. doi:10.1007/s10238-016-0430-5
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Georgesen C, Fox LP, Harp J. Retiform purpura: workup and therapeutic considerations in select conditions. J Am Acad Dermatol. 2020;82:799-816. doi:10.1016/j.jaad.2019.07.113
A 58-year-old man presented with a petechial and purpuric rash limited to the lower extremities. He reported that the rash had been present for months but worsened acutely over the last 3 days with new-onset dark urine, joint pain, and edema limiting his ability to walk. Physical examination showed areas of violaceous macules and papules on the legs and dorsal feet in a reticular distribution. Laboratory findings were remarkable for an elevated serum creatinine level of 2.75 mg/dL (reference range, 0.70–1.30 mg/dL), and serum immunofixation revealed the presence of markedly elevated IgG lambda monoclonal proteins. He was afebrile and his vital signs were stable. Dermatology, nephrology, and rheumatology services were consulted.
Single-dose psilocybin promising for resistant depression
PARIS –
Known as COMP360, the synthetic agent, a proprietary, purified form of psilocybin, improved symptoms related to mood and anhedonia while leaving aspects such as appetite and weight changes unaffected, reported investigators led by Guy M. Goodwin, PhD, emeritus professor of psychiatry, University of Oxford, England, and chief medical officer, COMPASS Pathways.
The study was presented at the European Psychiatric Association (EPA) 2023 Congress.
100 million affected
Affecting up to 100 million people globally, TRD is “not an official diagnosis,” although it is often defined as the failure to elicit a response with at least two antidepressant treatments, said Dr. Goodwin.
Compared to their counterparts with non-TRD, those with TRD experience higher relapse rates, higher rates of suicidal behavior, and more residual symptoms even when they do respond to treatment.
Previous results from the study known as P-TRD indicated that a single 25-mg dose of COMP360 significantly reduced depression scores for up to 12 weeks when given along with psychological support, although a later analysis suggested the effect subsequently dropped off.
The vast majority of the patients in the trial were naive to psychedelics, and so, Dr. Goodwin explained, they undergo a preparation phase during which they receive psychoeducation and have at least two visits with a therapist, who then stays with them during administration of the drug to offer support if they experience psychological distress.
Following the psilocybin session, participants go through a process known as integration, which involves two sessions with a therapist within 2 weeks.
“That, in our view, is essentially about safety, and about identifying problems that have arisen as a result of taking the drug,” said Dr. Goodwin.
The phase 2b trial examined changes in specific depression symptoms after psilocybin treatment in 233 patients with TRD. Participants were a mean age of 39.8 years and 59% were women. They were randomized to receive one of three doses of the drug: a 1-mg dose (n = 79), a 10-mg dose (n = 75), or a 25-mg dose (n = 79).
The primary outcome was changes in individual items on the Montgomery-Åsberg Depression Rating Scale (MADRS) and 16-item Quick Inventory of Depressive Symptomatology–Self Report (QIDS-SR-16) scale.
While the effect on overall depression scores is important, said Dr. Goodwin, many of the items included in the depression assessment scales are “uninformative.”
Reduction in ‘core’ symptoms
Participants were assessed by a blinded rater at baseline, day 1, day 2, and at 1, 2, 3, 6, 9, and 12 weeks after administration of COMP360. The primary endpoint was a reduction in individual items on MADRS and scores from baseline to 3 weeks. Individual items on the QIDS-SR-16 were rated by participants at the same time points.
Investigators found the largest mean changes from baseline were on reported and apparent sadness, lassitude, inability to feel, and concentration difficulties, with “very nice and clear dose-related differences,” Dr. Goodwin said.
The results indicate that the significant benefit with the largest dose at 3 weeks versus baseline was confined to items such as inability to feel and reported and apparent sadness on the MADRS and feeling sad and general interest on the QIDS-SR-16 (Table 1).
The results suggest the effect of COMP360 is “on the core symptoms of depression,” said Dr. Goodwin.
Results were similar for individual items on the QIDS-SR-16, with the greatest changes in items including feeling sad, general interest, energy level, falling asleep, view of myself, concentration/decision-making, and feeling down.
Other scale items, such as decreased appetite, feel restless, and weight changes, showed negligible changes in response to COMP360 therapy and were described by Dr. Goodwin as “inconsequential.”
“Essentially, these items are contributing nothing but noise to the signal,” he said.
He added the results of the study need to be replicated and that plans for phase 3 trials are underway. These studies, he said, are designed to convince the Food and Drug Administration that “this is not just a recreational drug, it’s a medicine.”
Enthusiasm running ahead of the data
Commenting on the findings, Bertha K. Madras, PhD, professor of psychobiology, department of psychiatry, Harvard Medical School, Boston, who was not involved in the study, said “hallucinogens are an intriguing class of drugs and I support ongoing high-quality research in this area.”
However, she told this news organization that the “breathtaking endorsement of this drug is far ahead of scientific data.”
She cited concerns such as the “narrow demographics” of participants, their previous experience with and expectations of hallucinogens, the “potential for symptom fluidity of enrollees,” such as depression evolving into psychosis, and the “undefined role” of the therapist during a hallucinogenic session.
“Finally, I am concerned that enthusiasm for therapeutic potential has been, and will continue to be, preempted and directed towards legalization and widespread access for vulnerable populations,” Dr. Madras said.
This, she said, “is occurring at breakneck speed in the U.S., with scant resistance or skepticism from the investigators engaged in therapeutic assessment.”
The study was funded by COMPASS Pathways. Dr. Goodwin has reported relationships with COMPASS Pathways, Buckley Psytech, Boehringer Ingelheim, Clerkenwell Health, EVA Pharma, Lundbeck, Janssen Global Services, Novartis, Ocean Neurosciences, P1vital, Sage Therapeutics, Servier, Takeda, and WebMD.
A version of this article first appeared on Medscape.com.
PARIS –
Known as COMP360, the synthetic agent, a proprietary, purified form of psilocybin, improved symptoms related to mood and anhedonia while leaving aspects such as appetite and weight changes unaffected, reported investigators led by Guy M. Goodwin, PhD, emeritus professor of psychiatry, University of Oxford, England, and chief medical officer, COMPASS Pathways.
The study was presented at the European Psychiatric Association (EPA) 2023 Congress.
100 million affected
Affecting up to 100 million people globally, TRD is “not an official diagnosis,” although it is often defined as the failure to elicit a response with at least two antidepressant treatments, said Dr. Goodwin.
Compared to their counterparts with non-TRD, those with TRD experience higher relapse rates, higher rates of suicidal behavior, and more residual symptoms even when they do respond to treatment.
Previous results from the study known as P-TRD indicated that a single 25-mg dose of COMP360 significantly reduced depression scores for up to 12 weeks when given along with psychological support, although a later analysis suggested the effect subsequently dropped off.
The vast majority of the patients in the trial were naive to psychedelics, and so, Dr. Goodwin explained, they undergo a preparation phase during which they receive psychoeducation and have at least two visits with a therapist, who then stays with them during administration of the drug to offer support if they experience psychological distress.
Following the psilocybin session, participants go through a process known as integration, which involves two sessions with a therapist within 2 weeks.
“That, in our view, is essentially about safety, and about identifying problems that have arisen as a result of taking the drug,” said Dr. Goodwin.
The phase 2b trial examined changes in specific depression symptoms after psilocybin treatment in 233 patients with TRD. Participants were a mean age of 39.8 years and 59% were women. They were randomized to receive one of three doses of the drug: a 1-mg dose (n = 79), a 10-mg dose (n = 75), or a 25-mg dose (n = 79).
The primary outcome was changes in individual items on the Montgomery-Åsberg Depression Rating Scale (MADRS) and 16-item Quick Inventory of Depressive Symptomatology–Self Report (QIDS-SR-16) scale.
While the effect on overall depression scores is important, said Dr. Goodwin, many of the items included in the depression assessment scales are “uninformative.”
Reduction in ‘core’ symptoms
Participants were assessed by a blinded rater at baseline, day 1, day 2, and at 1, 2, 3, 6, 9, and 12 weeks after administration of COMP360. The primary endpoint was a reduction in individual items on MADRS and scores from baseline to 3 weeks. Individual items on the QIDS-SR-16 were rated by participants at the same time points.
Investigators found the largest mean changes from baseline were on reported and apparent sadness, lassitude, inability to feel, and concentration difficulties, with “very nice and clear dose-related differences,” Dr. Goodwin said.
The results indicate that the significant benefit with the largest dose at 3 weeks versus baseline was confined to items such as inability to feel and reported and apparent sadness on the MADRS and feeling sad and general interest on the QIDS-SR-16 (Table 1).
The results suggest the effect of COMP360 is “on the core symptoms of depression,” said Dr. Goodwin.
Results were similar for individual items on the QIDS-SR-16, with the greatest changes in items including feeling sad, general interest, energy level, falling asleep, view of myself, concentration/decision-making, and feeling down.
Other scale items, such as decreased appetite, feel restless, and weight changes, showed negligible changes in response to COMP360 therapy and were described by Dr. Goodwin as “inconsequential.”
“Essentially, these items are contributing nothing but noise to the signal,” he said.
He added the results of the study need to be replicated and that plans for phase 3 trials are underway. These studies, he said, are designed to convince the Food and Drug Administration that “this is not just a recreational drug, it’s a medicine.”
Enthusiasm running ahead of the data
Commenting on the findings, Bertha K. Madras, PhD, professor of psychobiology, department of psychiatry, Harvard Medical School, Boston, who was not involved in the study, said “hallucinogens are an intriguing class of drugs and I support ongoing high-quality research in this area.”
However, she told this news organization that the “breathtaking endorsement of this drug is far ahead of scientific data.”
She cited concerns such as the “narrow demographics” of participants, their previous experience with and expectations of hallucinogens, the “potential for symptom fluidity of enrollees,” such as depression evolving into psychosis, and the “undefined role” of the therapist during a hallucinogenic session.
“Finally, I am concerned that enthusiasm for therapeutic potential has been, and will continue to be, preempted and directed towards legalization and widespread access for vulnerable populations,” Dr. Madras said.
This, she said, “is occurring at breakneck speed in the U.S., with scant resistance or skepticism from the investigators engaged in therapeutic assessment.”
The study was funded by COMPASS Pathways. Dr. Goodwin has reported relationships with COMPASS Pathways, Buckley Psytech, Boehringer Ingelheim, Clerkenwell Health, EVA Pharma, Lundbeck, Janssen Global Services, Novartis, Ocean Neurosciences, P1vital, Sage Therapeutics, Servier, Takeda, and WebMD.
A version of this article first appeared on Medscape.com.
PARIS –
Known as COMP360, the synthetic agent, a proprietary, purified form of psilocybin, improved symptoms related to mood and anhedonia while leaving aspects such as appetite and weight changes unaffected, reported investigators led by Guy M. Goodwin, PhD, emeritus professor of psychiatry, University of Oxford, England, and chief medical officer, COMPASS Pathways.
The study was presented at the European Psychiatric Association (EPA) 2023 Congress.
100 million affected
Affecting up to 100 million people globally, TRD is “not an official diagnosis,” although it is often defined as the failure to elicit a response with at least two antidepressant treatments, said Dr. Goodwin.
Compared to their counterparts with non-TRD, those with TRD experience higher relapse rates, higher rates of suicidal behavior, and more residual symptoms even when they do respond to treatment.
Previous results from the study known as P-TRD indicated that a single 25-mg dose of COMP360 significantly reduced depression scores for up to 12 weeks when given along with psychological support, although a later analysis suggested the effect subsequently dropped off.
The vast majority of the patients in the trial were naive to psychedelics, and so, Dr. Goodwin explained, they undergo a preparation phase during which they receive psychoeducation and have at least two visits with a therapist, who then stays with them during administration of the drug to offer support if they experience psychological distress.
Following the psilocybin session, participants go through a process known as integration, which involves two sessions with a therapist within 2 weeks.
“That, in our view, is essentially about safety, and about identifying problems that have arisen as a result of taking the drug,” said Dr. Goodwin.
The phase 2b trial examined changes in specific depression symptoms after psilocybin treatment in 233 patients with TRD. Participants were a mean age of 39.8 years and 59% were women. They were randomized to receive one of three doses of the drug: a 1-mg dose (n = 79), a 10-mg dose (n = 75), or a 25-mg dose (n = 79).
The primary outcome was changes in individual items on the Montgomery-Åsberg Depression Rating Scale (MADRS) and 16-item Quick Inventory of Depressive Symptomatology–Self Report (QIDS-SR-16) scale.
While the effect on overall depression scores is important, said Dr. Goodwin, many of the items included in the depression assessment scales are “uninformative.”
Reduction in ‘core’ symptoms
Participants were assessed by a blinded rater at baseline, day 1, day 2, and at 1, 2, 3, 6, 9, and 12 weeks after administration of COMP360. The primary endpoint was a reduction in individual items on MADRS and scores from baseline to 3 weeks. Individual items on the QIDS-SR-16 were rated by participants at the same time points.
Investigators found the largest mean changes from baseline were on reported and apparent sadness, lassitude, inability to feel, and concentration difficulties, with “very nice and clear dose-related differences,” Dr. Goodwin said.
The results indicate that the significant benefit with the largest dose at 3 weeks versus baseline was confined to items such as inability to feel and reported and apparent sadness on the MADRS and feeling sad and general interest on the QIDS-SR-16 (Table 1).
The results suggest the effect of COMP360 is “on the core symptoms of depression,” said Dr. Goodwin.
Results were similar for individual items on the QIDS-SR-16, with the greatest changes in items including feeling sad, general interest, energy level, falling asleep, view of myself, concentration/decision-making, and feeling down.
Other scale items, such as decreased appetite, feel restless, and weight changes, showed negligible changes in response to COMP360 therapy and were described by Dr. Goodwin as “inconsequential.”
“Essentially, these items are contributing nothing but noise to the signal,” he said.
He added the results of the study need to be replicated and that plans for phase 3 trials are underway. These studies, he said, are designed to convince the Food and Drug Administration that “this is not just a recreational drug, it’s a medicine.”
Enthusiasm running ahead of the data
Commenting on the findings, Bertha K. Madras, PhD, professor of psychobiology, department of psychiatry, Harvard Medical School, Boston, who was not involved in the study, said “hallucinogens are an intriguing class of drugs and I support ongoing high-quality research in this area.”
However, she told this news organization that the “breathtaking endorsement of this drug is far ahead of scientific data.”
She cited concerns such as the “narrow demographics” of participants, their previous experience with and expectations of hallucinogens, the “potential for symptom fluidity of enrollees,” such as depression evolving into psychosis, and the “undefined role” of the therapist during a hallucinogenic session.
“Finally, I am concerned that enthusiasm for therapeutic potential has been, and will continue to be, preempted and directed towards legalization and widespread access for vulnerable populations,” Dr. Madras said.
This, she said, “is occurring at breakneck speed in the U.S., with scant resistance or skepticism from the investigators engaged in therapeutic assessment.”
The study was funded by COMPASS Pathways. Dr. Goodwin has reported relationships with COMPASS Pathways, Buckley Psytech, Boehringer Ingelheim, Clerkenwell Health, EVA Pharma, Lundbeck, Janssen Global Services, Novartis, Ocean Neurosciences, P1vital, Sage Therapeutics, Servier, Takeda, and WebMD.
A version of this article first appeared on Medscape.com.
AT EPA 2023
Parents of patients with rheumatic disease, MIS-C strongly hesitant of COVID vaccination
NEW ORLEANS – Parents’ concerns about vaccinating their children against COVID-19 remain a substantial barrier to immunizing children against the disease, whether those children have chronic rheumatologic conditions or a history of multisystem inflammatory syndrome in children (MIS-C), according to two studies presented at the Pediatric Rheumatology Symposium.
Parents of children who developed MIS-C after a SARS-CoV-2 infection were particularly hesitant to vaccinate, despite strong encouragement from health care professionals at Baylor College of Medicine, Houston, said the presenter of one of the studies.
“Unfortunately, it remains unclear who is susceptible and what the mechanisms are” when it comes to MIS-C, Mariana Sanchez Villa, MS, a research coordinator at Baylor, told attendees. “Because of this, there is much hesitancy to vaccinate children with a history of MIS-C against COVID-19 out of a fear that hyperinflammation may occur.”
Ms. Sanchez Villa reported findings on the vaccination rate among patients who had been hospitalized with MIS-C. The researchers included all 295 patients who presented at the hospital with MIS-C between May 2020 and October 2022. Overall, 5% of these patients had been vaccinated against COVID-19 before they were diagnosed with MIS-C. When all these patients and their families came to outpatient follow-up appointments after discharge, the subspecialist clinicians recommended the children receive the COVID-19 vaccine 3 months after discharge. The researchers then reviewed the patients’ charts to see who did and did not receive the vaccine, which they confirmed through the state’s immunization registry.
Among the 295 patients with MIS-C, 1 died, and 99 (34%) received at least one COVID-19 vaccine dose after their diagnosis, including 7 of the 15 who had also been vaccinated prior to their MIS-C diagnosis. Just over half of the vaccinated patients (58%) were male. They received their vaccine an average 8.8 months after their hospitalization, when they were an average 10 years old, and all but one of the vaccine doses they received were the Pfizer/BioNTech mRNA vaccine.
Only 9 of the 99 vaccinated patients are fully vaccinated, defined as receiving the primary series plus the recommended boosters. Of the other patients, 13 received only one dose of the vaccine, 60 received two doses, and 17 received at least three doses of the primary series doses but no bivalent boosters. Over a subsequent average 11 months of follow-up, none of the vaccinated patients returned to the hospital with a recurrence of MIS-C or any other hyperinflammatory condition. The seven patients who had been vaccinated both before and after their MIS-C diagnosis have also not had any recurrence of a hyperinflammatory condition.
“SARS-CoV-2 vaccination is well-tolerated by children with a history of MIS-C,” the researchers concluded. Ms. Sanchez Villa referenced two other studies, in The Pediatric Infectious Disease Journal and in JAMA Network Open, with similar findings on the safety of COVID-19 vaccination in patients who have had MIS-C. “This is reassuring as SARS-CoV-2 becomes endemic and annual vaccination against SARS-CoV-2 is considered.”
Dilan Dissanayake, MD, PhD, a rheumatologist at The Hospital for Sick Children in Toronto, who attended the presentation, told this news organization that data increasingly show a “synergistic protective effect” from COVID-19 infection and vaccination. That is, “having COVID or having MIS-C once doesn’t necessarily preclude you from having it again,” thereby supporting the importance of vaccination after an MIS-C diagnosis. In talking to parents about vaccinating, he has found it most helpful for them to hear about rheumatologists’ experience regarding COVID-19 vaccination.
“Particularly as the pandemic went on, being able to comfortably say that we have this large patient group, as well as collaborators across the world who have been monitoring for any safety issues, and that all the data has been reassuring” has been most useful for parents to hear, Dr. Dissanayake said.
The other study, led by Beth Rutstein, MD, MSCE, an attending rheumatologist at Children’s Hospital of Philadelphia, focused on the population of pediatric rheumatology patients by surveying pediatric rheumatologists who were members of the Childhood Arthritis and Rheumatology Research Alliance. The survey, conducted from March to May 2022, included questions about the rheumatologists’ COVID-19 vaccination practices as well as perceptions of the vaccine by the parents of their patients.
The 219 respondents included 74% pediatric rheumatologists and 21% fellows. Nearly all the respondents (98%) believed that any disease flares after COVID-19 vaccination would be mild and/or rare, and nearly all (98%) recommend their patients be vaccinated against COVID-19.
The primary finding from the study was that “we [rheumatologists] have different concerns from the families,” coauthor and presenter Vidya Sivaraman, MD, a pediatric rheumatologist at Nationwide Children’s Hospital and the Ohio State University in Columbus, told this news organization. “We’re more worried about the efficacy of the vaccine on immunosuppressive medications,” such as rituximab, which depletes B cells, Dr. Sivaraman said, but concerns about the vaccine’s immunogenicity or efficacy were very low among parents.
Just over half the clinicians surveyed (59%) were concerned about how effective the vaccine would be for their patients, especially those receiving immunosuppressive therapy. Health care professionals were most concerned about patients on rituximab – all clinicians reported concerns about the vaccine’s effectiveness in these patients – followed by patients taking systemic corticosteroids (86%), mycophenolate mofetil (59%), and Janus kinase inhibitors (46%).
Most clinicians (88%) reported that they had temporarily modified a patient’s immunosuppressive therapy to allow for vaccination, following guidelines by the American College of Rheumatology. Aside from a small proportion of health care professionals who checked patients’ post-vaccination serology primarily for research purposes, most clinicians (82%) did not collect this serology.
In regard to adverse events, the concern cited most often by respondents was myocarditis (76%), followed by development of new autoimmune conditions (29%) and thrombosis (22%), but the clinicians ranked these adverse events as low risk.
Meanwhile, the top three concerns about vaccination among parents, as reported to physicians, were worries about side effects, lack of long-term safety data on the vaccine, and misinformation they had heard, such as anxiety about changes to their child’s genetics or vaccination causing a COVID-19 infection. “They’re seeing things on social media from other parents [saying that COVID-19 vaccines are] going to affect their fertility, so they don’t want their daughters to get it,” Dr. Sivaraman said as another example of commonly cited misinformation.
Nearly half of the respondents (47%) said more than half of their families had concerns about side effects and the lack of data on long-term outcomes after vaccination. Only 8.5% of physicians said that fewer than 10% of their families were anxious about side effects. In addition, 39% of physicians said more than half of their families had concerns about misinformation they had heard, and only 16% of physicians had heard about misinformation concerns from fewer than 10% of their patients.
Other concerns cited by parents included their child’s disease flaring; lack of data on how well the vaccine would stimulate their child’s immune system; their child having already had COVID-19; and not believing COVID-19 was a major health risk to their child. Nearly every respondent (98%) said they had parents who turned down COVID-19 vaccination, and a majority (75%) reported that more than 10% of their patients had parents who were hesitant about COVID-19 vaccination.
No external funding was noted for either study. Ms. Sanchez Villa had no relevant financial relationships, but two abstract coauthors reported financial relationships with Pfizer and Moderna, and one reported a financial relationship with Novartis. Dr. Rutstein, Dr. Sivaraman, and Dr. Dissanayake had no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – Parents’ concerns about vaccinating their children against COVID-19 remain a substantial barrier to immunizing children against the disease, whether those children have chronic rheumatologic conditions or a history of multisystem inflammatory syndrome in children (MIS-C), according to two studies presented at the Pediatric Rheumatology Symposium.
Parents of children who developed MIS-C after a SARS-CoV-2 infection were particularly hesitant to vaccinate, despite strong encouragement from health care professionals at Baylor College of Medicine, Houston, said the presenter of one of the studies.
“Unfortunately, it remains unclear who is susceptible and what the mechanisms are” when it comes to MIS-C, Mariana Sanchez Villa, MS, a research coordinator at Baylor, told attendees. “Because of this, there is much hesitancy to vaccinate children with a history of MIS-C against COVID-19 out of a fear that hyperinflammation may occur.”
Ms. Sanchez Villa reported findings on the vaccination rate among patients who had been hospitalized with MIS-C. The researchers included all 295 patients who presented at the hospital with MIS-C between May 2020 and October 2022. Overall, 5% of these patients had been vaccinated against COVID-19 before they were diagnosed with MIS-C. When all these patients and their families came to outpatient follow-up appointments after discharge, the subspecialist clinicians recommended the children receive the COVID-19 vaccine 3 months after discharge. The researchers then reviewed the patients’ charts to see who did and did not receive the vaccine, which they confirmed through the state’s immunization registry.
Among the 295 patients with MIS-C, 1 died, and 99 (34%) received at least one COVID-19 vaccine dose after their diagnosis, including 7 of the 15 who had also been vaccinated prior to their MIS-C diagnosis. Just over half of the vaccinated patients (58%) were male. They received their vaccine an average 8.8 months after their hospitalization, when they were an average 10 years old, and all but one of the vaccine doses they received were the Pfizer/BioNTech mRNA vaccine.
Only 9 of the 99 vaccinated patients are fully vaccinated, defined as receiving the primary series plus the recommended boosters. Of the other patients, 13 received only one dose of the vaccine, 60 received two doses, and 17 received at least three doses of the primary series doses but no bivalent boosters. Over a subsequent average 11 months of follow-up, none of the vaccinated patients returned to the hospital with a recurrence of MIS-C or any other hyperinflammatory condition. The seven patients who had been vaccinated both before and after their MIS-C diagnosis have also not had any recurrence of a hyperinflammatory condition.
“SARS-CoV-2 vaccination is well-tolerated by children with a history of MIS-C,” the researchers concluded. Ms. Sanchez Villa referenced two other studies, in The Pediatric Infectious Disease Journal and in JAMA Network Open, with similar findings on the safety of COVID-19 vaccination in patients who have had MIS-C. “This is reassuring as SARS-CoV-2 becomes endemic and annual vaccination against SARS-CoV-2 is considered.”
Dilan Dissanayake, MD, PhD, a rheumatologist at The Hospital for Sick Children in Toronto, who attended the presentation, told this news organization that data increasingly show a “synergistic protective effect” from COVID-19 infection and vaccination. That is, “having COVID or having MIS-C once doesn’t necessarily preclude you from having it again,” thereby supporting the importance of vaccination after an MIS-C diagnosis. In talking to parents about vaccinating, he has found it most helpful for them to hear about rheumatologists’ experience regarding COVID-19 vaccination.
“Particularly as the pandemic went on, being able to comfortably say that we have this large patient group, as well as collaborators across the world who have been monitoring for any safety issues, and that all the data has been reassuring” has been most useful for parents to hear, Dr. Dissanayake said.
The other study, led by Beth Rutstein, MD, MSCE, an attending rheumatologist at Children’s Hospital of Philadelphia, focused on the population of pediatric rheumatology patients by surveying pediatric rheumatologists who were members of the Childhood Arthritis and Rheumatology Research Alliance. The survey, conducted from March to May 2022, included questions about the rheumatologists’ COVID-19 vaccination practices as well as perceptions of the vaccine by the parents of their patients.
The 219 respondents included 74% pediatric rheumatologists and 21% fellows. Nearly all the respondents (98%) believed that any disease flares after COVID-19 vaccination would be mild and/or rare, and nearly all (98%) recommend their patients be vaccinated against COVID-19.
The primary finding from the study was that “we [rheumatologists] have different concerns from the families,” coauthor and presenter Vidya Sivaraman, MD, a pediatric rheumatologist at Nationwide Children’s Hospital and the Ohio State University in Columbus, told this news organization. “We’re more worried about the efficacy of the vaccine on immunosuppressive medications,” such as rituximab, which depletes B cells, Dr. Sivaraman said, but concerns about the vaccine’s immunogenicity or efficacy were very low among parents.
Just over half the clinicians surveyed (59%) were concerned about how effective the vaccine would be for their patients, especially those receiving immunosuppressive therapy. Health care professionals were most concerned about patients on rituximab – all clinicians reported concerns about the vaccine’s effectiveness in these patients – followed by patients taking systemic corticosteroids (86%), mycophenolate mofetil (59%), and Janus kinase inhibitors (46%).
Most clinicians (88%) reported that they had temporarily modified a patient’s immunosuppressive therapy to allow for vaccination, following guidelines by the American College of Rheumatology. Aside from a small proportion of health care professionals who checked patients’ post-vaccination serology primarily for research purposes, most clinicians (82%) did not collect this serology.
In regard to adverse events, the concern cited most often by respondents was myocarditis (76%), followed by development of new autoimmune conditions (29%) and thrombosis (22%), but the clinicians ranked these adverse events as low risk.
Meanwhile, the top three concerns about vaccination among parents, as reported to physicians, were worries about side effects, lack of long-term safety data on the vaccine, and misinformation they had heard, such as anxiety about changes to their child’s genetics or vaccination causing a COVID-19 infection. “They’re seeing things on social media from other parents [saying that COVID-19 vaccines are] going to affect their fertility, so they don’t want their daughters to get it,” Dr. Sivaraman said as another example of commonly cited misinformation.
Nearly half of the respondents (47%) said more than half of their families had concerns about side effects and the lack of data on long-term outcomes after vaccination. Only 8.5% of physicians said that fewer than 10% of their families were anxious about side effects. In addition, 39% of physicians said more than half of their families had concerns about misinformation they had heard, and only 16% of physicians had heard about misinformation concerns from fewer than 10% of their patients.
Other concerns cited by parents included their child’s disease flaring; lack of data on how well the vaccine would stimulate their child’s immune system; their child having already had COVID-19; and not believing COVID-19 was a major health risk to their child. Nearly every respondent (98%) said they had parents who turned down COVID-19 vaccination, and a majority (75%) reported that more than 10% of their patients had parents who were hesitant about COVID-19 vaccination.
No external funding was noted for either study. Ms. Sanchez Villa had no relevant financial relationships, but two abstract coauthors reported financial relationships with Pfizer and Moderna, and one reported a financial relationship with Novartis. Dr. Rutstein, Dr. Sivaraman, and Dr. Dissanayake had no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – Parents’ concerns about vaccinating their children against COVID-19 remain a substantial barrier to immunizing children against the disease, whether those children have chronic rheumatologic conditions or a history of multisystem inflammatory syndrome in children (MIS-C), according to two studies presented at the Pediatric Rheumatology Symposium.
Parents of children who developed MIS-C after a SARS-CoV-2 infection were particularly hesitant to vaccinate, despite strong encouragement from health care professionals at Baylor College of Medicine, Houston, said the presenter of one of the studies.
“Unfortunately, it remains unclear who is susceptible and what the mechanisms are” when it comes to MIS-C, Mariana Sanchez Villa, MS, a research coordinator at Baylor, told attendees. “Because of this, there is much hesitancy to vaccinate children with a history of MIS-C against COVID-19 out of a fear that hyperinflammation may occur.”
Ms. Sanchez Villa reported findings on the vaccination rate among patients who had been hospitalized with MIS-C. The researchers included all 295 patients who presented at the hospital with MIS-C between May 2020 and October 2022. Overall, 5% of these patients had been vaccinated against COVID-19 before they were diagnosed with MIS-C. When all these patients and their families came to outpatient follow-up appointments after discharge, the subspecialist clinicians recommended the children receive the COVID-19 vaccine 3 months after discharge. The researchers then reviewed the patients’ charts to see who did and did not receive the vaccine, which they confirmed through the state’s immunization registry.
Among the 295 patients with MIS-C, 1 died, and 99 (34%) received at least one COVID-19 vaccine dose after their diagnosis, including 7 of the 15 who had also been vaccinated prior to their MIS-C diagnosis. Just over half of the vaccinated patients (58%) were male. They received their vaccine an average 8.8 months after their hospitalization, when they were an average 10 years old, and all but one of the vaccine doses they received were the Pfizer/BioNTech mRNA vaccine.
Only 9 of the 99 vaccinated patients are fully vaccinated, defined as receiving the primary series plus the recommended boosters. Of the other patients, 13 received only one dose of the vaccine, 60 received two doses, and 17 received at least three doses of the primary series doses but no bivalent boosters. Over a subsequent average 11 months of follow-up, none of the vaccinated patients returned to the hospital with a recurrence of MIS-C or any other hyperinflammatory condition. The seven patients who had been vaccinated both before and after their MIS-C diagnosis have also not had any recurrence of a hyperinflammatory condition.
“SARS-CoV-2 vaccination is well-tolerated by children with a history of MIS-C,” the researchers concluded. Ms. Sanchez Villa referenced two other studies, in The Pediatric Infectious Disease Journal and in JAMA Network Open, with similar findings on the safety of COVID-19 vaccination in patients who have had MIS-C. “This is reassuring as SARS-CoV-2 becomes endemic and annual vaccination against SARS-CoV-2 is considered.”
Dilan Dissanayake, MD, PhD, a rheumatologist at The Hospital for Sick Children in Toronto, who attended the presentation, told this news organization that data increasingly show a “synergistic protective effect” from COVID-19 infection and vaccination. That is, “having COVID or having MIS-C once doesn’t necessarily preclude you from having it again,” thereby supporting the importance of vaccination after an MIS-C diagnosis. In talking to parents about vaccinating, he has found it most helpful for them to hear about rheumatologists’ experience regarding COVID-19 vaccination.
“Particularly as the pandemic went on, being able to comfortably say that we have this large patient group, as well as collaborators across the world who have been monitoring for any safety issues, and that all the data has been reassuring” has been most useful for parents to hear, Dr. Dissanayake said.
The other study, led by Beth Rutstein, MD, MSCE, an attending rheumatologist at Children’s Hospital of Philadelphia, focused on the population of pediatric rheumatology patients by surveying pediatric rheumatologists who were members of the Childhood Arthritis and Rheumatology Research Alliance. The survey, conducted from March to May 2022, included questions about the rheumatologists’ COVID-19 vaccination practices as well as perceptions of the vaccine by the parents of their patients.
The 219 respondents included 74% pediatric rheumatologists and 21% fellows. Nearly all the respondents (98%) believed that any disease flares after COVID-19 vaccination would be mild and/or rare, and nearly all (98%) recommend their patients be vaccinated against COVID-19.
The primary finding from the study was that “we [rheumatologists] have different concerns from the families,” coauthor and presenter Vidya Sivaraman, MD, a pediatric rheumatologist at Nationwide Children’s Hospital and the Ohio State University in Columbus, told this news organization. “We’re more worried about the efficacy of the vaccine on immunosuppressive medications,” such as rituximab, which depletes B cells, Dr. Sivaraman said, but concerns about the vaccine’s immunogenicity or efficacy were very low among parents.
Just over half the clinicians surveyed (59%) were concerned about how effective the vaccine would be for their patients, especially those receiving immunosuppressive therapy. Health care professionals were most concerned about patients on rituximab – all clinicians reported concerns about the vaccine’s effectiveness in these patients – followed by patients taking systemic corticosteroids (86%), mycophenolate mofetil (59%), and Janus kinase inhibitors (46%).
Most clinicians (88%) reported that they had temporarily modified a patient’s immunosuppressive therapy to allow for vaccination, following guidelines by the American College of Rheumatology. Aside from a small proportion of health care professionals who checked patients’ post-vaccination serology primarily for research purposes, most clinicians (82%) did not collect this serology.
In regard to adverse events, the concern cited most often by respondents was myocarditis (76%), followed by development of new autoimmune conditions (29%) and thrombosis (22%), but the clinicians ranked these adverse events as low risk.
Meanwhile, the top three concerns about vaccination among parents, as reported to physicians, were worries about side effects, lack of long-term safety data on the vaccine, and misinformation they had heard, such as anxiety about changes to their child’s genetics or vaccination causing a COVID-19 infection. “They’re seeing things on social media from other parents [saying that COVID-19 vaccines are] going to affect their fertility, so they don’t want their daughters to get it,” Dr. Sivaraman said as another example of commonly cited misinformation.
Nearly half of the respondents (47%) said more than half of their families had concerns about side effects and the lack of data on long-term outcomes after vaccination. Only 8.5% of physicians said that fewer than 10% of their families were anxious about side effects. In addition, 39% of physicians said more than half of their families had concerns about misinformation they had heard, and only 16% of physicians had heard about misinformation concerns from fewer than 10% of their patients.
Other concerns cited by parents included their child’s disease flaring; lack of data on how well the vaccine would stimulate their child’s immune system; their child having already had COVID-19; and not believing COVID-19 was a major health risk to their child. Nearly every respondent (98%) said they had parents who turned down COVID-19 vaccination, and a majority (75%) reported that more than 10% of their patients had parents who were hesitant about COVID-19 vaccination.
No external funding was noted for either study. Ms. Sanchez Villa had no relevant financial relationships, but two abstract coauthors reported financial relationships with Pfizer and Moderna, and one reported a financial relationship with Novartis. Dr. Rutstein, Dr. Sivaraman, and Dr. Dissanayake had no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT PRSYM 2023
The earlier baricitinib for severe alopecia areata is started, the better
NEW ORLEANS – In the nearly 1 year .
“The journey to JAK inhibition in alopecia areata has been incredible,” Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the center for eczema and itch at Northwestern University, Chicago, said at the annual meeting of the American Academy of Dermatology. “JAK inhibitors are here to stay, and I think baricitinib offers an amazing opportunity for the right patients.”
The efficacy and safety of baricitinib (Olumiant) for AA was studied in two randomized, double-blind, placebo-controlled trials (BRAVE-AA1 and BRAVE-AA2) with patients who had at least 50% scalp hair loss as measured by the Severity of Alopecia Tool (SALT) for more than 6 months. Patients in these trials received either a placebo, 2 mg of baricitinib, or 4 mg of baricitinib every day. The primary measurement of efficacy for both trials was the proportion of patients who achieved a SALT score of 20 or less, or at least 80% scalp hair coverage at week 36. The researchers found that 36%-39% of individuals in the 4-mg arm achieved a SALT score of less than 20, compared with 19%-23% of individuals in the 2 mg arm. Similar outcomes were observed for eyebrow and eyelash hair loss.
Most adverse events observed in BRAVE-AA1 and BRAVE-AA2 were in the mild to moderate range, and the actual number of adverse events leading to permanent discontinuation was extremely low. The most common adverse events were upper respiratory tract infections, headache, nasopharyngitis, acne, urinary tract infections, and an increase in blood creatine kinase.
Baricitinib is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or other potent immunosuppressants, Dr. Chovatiya said. Required lab evaluations include baseline testing for tuberculosis and viral hepatitis; CBC, hepatic function, and renal function at baseline and then as clinically indicated; and lipids after 12 weeks of therapy, then as clinically indicated. The recommended starting dose of baricitinib is 2 mg per day, which can be increased to 4 mg per day if the response is not adequate. “However, for patients with nearly complete or complete scalp hair loss, with or without substantial eyelash or eyebrow hair loss, 4 mg once daily is recommended,” he said. “Once an adequate response is achieved, it’s recommended to reduce from 4 to 2 mg daily.”
52-week, 76-week data
According to pooled data from BRAVE-AA1 and BRAVE-AA2 published online March 1, 2023, efficacy continues to increase out to 52 weeks. Specifically, by week 52, 39% of individuals in the 4 mg arm achieved a SALT score of 20 or less, compared with 22.6% of individuals in the 2 mg arm. “You see similar linear growth in the eyebrow and eyelash response loss as well,” Dr. Chovatiya said.
In other findings, patients in the 4 mg treatment arm who achieved a SALT score of 20 or less at week 52 were eligible for randomized down titration, provided that they had stayed on the same dose of baricitinib from initial randomization. According to data from baricitinib manufacturer Eli Lilly, 77.5% of patients who stepped down to the 2 mg dose from the 4 mg dose at week 52 achieved a SALT score of 20 or less at week 76, Dr. Chovatiya said. “If I can keep someone on 4 mg that’s great, but it looks like you can go to a lower dose and do a pretty good job,” he said.
Patients in the baricitinib arms who achieved a SALT score of 20 or less at week 52 were eligible for randomized withdrawal, provided that they had stayed on the same dose of the drug from initial randomization. According to Dr. Chovatiya, 89.4% of individuals who remained on the 4 mg dose to week 76 maintained a SALT score of 20 or less, compared with 33.3% of those who switched from the 4 mg to placebo. “The takeaway here is that clinically, longitudinal treatment looks to be required in this time period” for continued efficacy, he said. “However, what this looks like in the real world remains to be seen.”
A recently published integrated analysis of safety data from BRAVE-AA1 and BRAVE-AA2 reported that no deaths occurred and of the few reported serious infections, nearly half were COVID-19. There was a single case of multidermatomal herpes zoster and no cases of tuberculosis. One patient with risk factors for MI had an MI during a placebo-controlled period, and one study participant with a history of COVID-19 infection developed a pulmonary embolism at day 638. There was one case each of chronic lymphocytic leukemia, B-cell lymphoma, breast cancer, and appendicitis.
Baseline severity and treatment response
“Does treatment response vary with baseline disease status?” Dr. Chovatiya asked. “Yes. People with very severe hair loss [defined as a SALT score of 95 or higher] tended to do worse, while the rest of the study population did even better – an almost twofold difference. This means that you want to treat as early as you possibly can. It’s interesting to note that you don’t see this difference as much in the case of eyebrows and eyelashes. This makes sense, though. Eyebrows and eyelashes probably behave differently in terms of growth than the scalp does.”
Certain baseline characteristics of patients in BRAVE-AA1 and BRAVE-AA2 portended better outcomes. Women tended to fare better than men, but individuals who had longer histories of AA did not respond well. “People who had a shorter duration of their current episode of AA also did better than people who had a longer current episode, so we want to think about treating as soon as we possibly can,” Dr. Chovatiya said.
Dr. Chovatiya disclosed that he is a consultant to, a speaker for, investigator, and/or a member of the advisory board for several pharmaceutical companies, including Eli Lilly.
NEW ORLEANS – In the nearly 1 year .
“The journey to JAK inhibition in alopecia areata has been incredible,” Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the center for eczema and itch at Northwestern University, Chicago, said at the annual meeting of the American Academy of Dermatology. “JAK inhibitors are here to stay, and I think baricitinib offers an amazing opportunity for the right patients.”
The efficacy and safety of baricitinib (Olumiant) for AA was studied in two randomized, double-blind, placebo-controlled trials (BRAVE-AA1 and BRAVE-AA2) with patients who had at least 50% scalp hair loss as measured by the Severity of Alopecia Tool (SALT) for more than 6 months. Patients in these trials received either a placebo, 2 mg of baricitinib, or 4 mg of baricitinib every day. The primary measurement of efficacy for both trials was the proportion of patients who achieved a SALT score of 20 or less, or at least 80% scalp hair coverage at week 36. The researchers found that 36%-39% of individuals in the 4-mg arm achieved a SALT score of less than 20, compared with 19%-23% of individuals in the 2 mg arm. Similar outcomes were observed for eyebrow and eyelash hair loss.
Most adverse events observed in BRAVE-AA1 and BRAVE-AA2 were in the mild to moderate range, and the actual number of adverse events leading to permanent discontinuation was extremely low. The most common adverse events were upper respiratory tract infections, headache, nasopharyngitis, acne, urinary tract infections, and an increase in blood creatine kinase.
Baricitinib is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or other potent immunosuppressants, Dr. Chovatiya said. Required lab evaluations include baseline testing for tuberculosis and viral hepatitis; CBC, hepatic function, and renal function at baseline and then as clinically indicated; and lipids after 12 weeks of therapy, then as clinically indicated. The recommended starting dose of baricitinib is 2 mg per day, which can be increased to 4 mg per day if the response is not adequate. “However, for patients with nearly complete or complete scalp hair loss, with or without substantial eyelash or eyebrow hair loss, 4 mg once daily is recommended,” he said. “Once an adequate response is achieved, it’s recommended to reduce from 4 to 2 mg daily.”
52-week, 76-week data
According to pooled data from BRAVE-AA1 and BRAVE-AA2 published online March 1, 2023, efficacy continues to increase out to 52 weeks. Specifically, by week 52, 39% of individuals in the 4 mg arm achieved a SALT score of 20 or less, compared with 22.6% of individuals in the 2 mg arm. “You see similar linear growth in the eyebrow and eyelash response loss as well,” Dr. Chovatiya said.
In other findings, patients in the 4 mg treatment arm who achieved a SALT score of 20 or less at week 52 were eligible for randomized down titration, provided that they had stayed on the same dose of baricitinib from initial randomization. According to data from baricitinib manufacturer Eli Lilly, 77.5% of patients who stepped down to the 2 mg dose from the 4 mg dose at week 52 achieved a SALT score of 20 or less at week 76, Dr. Chovatiya said. “If I can keep someone on 4 mg that’s great, but it looks like you can go to a lower dose and do a pretty good job,” he said.
Patients in the baricitinib arms who achieved a SALT score of 20 or less at week 52 were eligible for randomized withdrawal, provided that they had stayed on the same dose of the drug from initial randomization. According to Dr. Chovatiya, 89.4% of individuals who remained on the 4 mg dose to week 76 maintained a SALT score of 20 or less, compared with 33.3% of those who switched from the 4 mg to placebo. “The takeaway here is that clinically, longitudinal treatment looks to be required in this time period” for continued efficacy, he said. “However, what this looks like in the real world remains to be seen.”
A recently published integrated analysis of safety data from BRAVE-AA1 and BRAVE-AA2 reported that no deaths occurred and of the few reported serious infections, nearly half were COVID-19. There was a single case of multidermatomal herpes zoster and no cases of tuberculosis. One patient with risk factors for MI had an MI during a placebo-controlled period, and one study participant with a history of COVID-19 infection developed a pulmonary embolism at day 638. There was one case each of chronic lymphocytic leukemia, B-cell lymphoma, breast cancer, and appendicitis.
Baseline severity and treatment response
“Does treatment response vary with baseline disease status?” Dr. Chovatiya asked. “Yes. People with very severe hair loss [defined as a SALT score of 95 or higher] tended to do worse, while the rest of the study population did even better – an almost twofold difference. This means that you want to treat as early as you possibly can. It’s interesting to note that you don’t see this difference as much in the case of eyebrows and eyelashes. This makes sense, though. Eyebrows and eyelashes probably behave differently in terms of growth than the scalp does.”
Certain baseline characteristics of patients in BRAVE-AA1 and BRAVE-AA2 portended better outcomes. Women tended to fare better than men, but individuals who had longer histories of AA did not respond well. “People who had a shorter duration of their current episode of AA also did better than people who had a longer current episode, so we want to think about treating as soon as we possibly can,” Dr. Chovatiya said.
Dr. Chovatiya disclosed that he is a consultant to, a speaker for, investigator, and/or a member of the advisory board for several pharmaceutical companies, including Eli Lilly.
NEW ORLEANS – In the nearly 1 year .
“The journey to JAK inhibition in alopecia areata has been incredible,” Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the center for eczema and itch at Northwestern University, Chicago, said at the annual meeting of the American Academy of Dermatology. “JAK inhibitors are here to stay, and I think baricitinib offers an amazing opportunity for the right patients.”
The efficacy and safety of baricitinib (Olumiant) for AA was studied in two randomized, double-blind, placebo-controlled trials (BRAVE-AA1 and BRAVE-AA2) with patients who had at least 50% scalp hair loss as measured by the Severity of Alopecia Tool (SALT) for more than 6 months. Patients in these trials received either a placebo, 2 mg of baricitinib, or 4 mg of baricitinib every day. The primary measurement of efficacy for both trials was the proportion of patients who achieved a SALT score of 20 or less, or at least 80% scalp hair coverage at week 36. The researchers found that 36%-39% of individuals in the 4-mg arm achieved a SALT score of less than 20, compared with 19%-23% of individuals in the 2 mg arm. Similar outcomes were observed for eyebrow and eyelash hair loss.
Most adverse events observed in BRAVE-AA1 and BRAVE-AA2 were in the mild to moderate range, and the actual number of adverse events leading to permanent discontinuation was extremely low. The most common adverse events were upper respiratory tract infections, headache, nasopharyngitis, acne, urinary tract infections, and an increase in blood creatine kinase.
Baricitinib is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or other potent immunosuppressants, Dr. Chovatiya said. Required lab evaluations include baseline testing for tuberculosis and viral hepatitis; CBC, hepatic function, and renal function at baseline and then as clinically indicated; and lipids after 12 weeks of therapy, then as clinically indicated. The recommended starting dose of baricitinib is 2 mg per day, which can be increased to 4 mg per day if the response is not adequate. “However, for patients with nearly complete or complete scalp hair loss, with or without substantial eyelash or eyebrow hair loss, 4 mg once daily is recommended,” he said. “Once an adequate response is achieved, it’s recommended to reduce from 4 to 2 mg daily.”
52-week, 76-week data
According to pooled data from BRAVE-AA1 and BRAVE-AA2 published online March 1, 2023, efficacy continues to increase out to 52 weeks. Specifically, by week 52, 39% of individuals in the 4 mg arm achieved a SALT score of 20 or less, compared with 22.6% of individuals in the 2 mg arm. “You see similar linear growth in the eyebrow and eyelash response loss as well,” Dr. Chovatiya said.
In other findings, patients in the 4 mg treatment arm who achieved a SALT score of 20 or less at week 52 were eligible for randomized down titration, provided that they had stayed on the same dose of baricitinib from initial randomization. According to data from baricitinib manufacturer Eli Lilly, 77.5% of patients who stepped down to the 2 mg dose from the 4 mg dose at week 52 achieved a SALT score of 20 or less at week 76, Dr. Chovatiya said. “If I can keep someone on 4 mg that’s great, but it looks like you can go to a lower dose and do a pretty good job,” he said.
Patients in the baricitinib arms who achieved a SALT score of 20 or less at week 52 were eligible for randomized withdrawal, provided that they had stayed on the same dose of the drug from initial randomization. According to Dr. Chovatiya, 89.4% of individuals who remained on the 4 mg dose to week 76 maintained a SALT score of 20 or less, compared with 33.3% of those who switched from the 4 mg to placebo. “The takeaway here is that clinically, longitudinal treatment looks to be required in this time period” for continued efficacy, he said. “However, what this looks like in the real world remains to be seen.”
A recently published integrated analysis of safety data from BRAVE-AA1 and BRAVE-AA2 reported that no deaths occurred and of the few reported serious infections, nearly half were COVID-19. There was a single case of multidermatomal herpes zoster and no cases of tuberculosis. One patient with risk factors for MI had an MI during a placebo-controlled period, and one study participant with a history of COVID-19 infection developed a pulmonary embolism at day 638. There was one case each of chronic lymphocytic leukemia, B-cell lymphoma, breast cancer, and appendicitis.
Baseline severity and treatment response
“Does treatment response vary with baseline disease status?” Dr. Chovatiya asked. “Yes. People with very severe hair loss [defined as a SALT score of 95 or higher] tended to do worse, while the rest of the study population did even better – an almost twofold difference. This means that you want to treat as early as you possibly can. It’s interesting to note that you don’t see this difference as much in the case of eyebrows and eyelashes. This makes sense, though. Eyebrows and eyelashes probably behave differently in terms of growth than the scalp does.”
Certain baseline characteristics of patients in BRAVE-AA1 and BRAVE-AA2 portended better outcomes. Women tended to fare better than men, but individuals who had longer histories of AA did not respond well. “People who had a shorter duration of their current episode of AA also did better than people who had a longer current episode, so we want to think about treating as soon as we possibly can,” Dr. Chovatiya said.
Dr. Chovatiya disclosed that he is a consultant to, a speaker for, investigator, and/or a member of the advisory board for several pharmaceutical companies, including Eli Lilly.
AT AAD 2023
Study offers dozens of reasons to cut sugar
A new compilation of nearly all research to date on the health impacts of sugar offers dozens of reasons to cut back.
The studies accounted for decades of research on the topic, stretching back to the beginning of the largest electronic databases for scientific papers.
The result is a list that cites the world’s most common health problems like heart disease, diabetes, obesity, high blood pressure, heart attack, high cholesterol, cancer, and depression. The findings were published in the BMJ. Researchers looked at studies that evaluated the impacts of consuming free sugars, which means any food that contains processed or naturally occurring sugars like table sugar, honey, or maple syrup. Sugar found in whole fruits and vegetables and in milk is not free sugar.
U.S. dietary guidelines recommend getting no more than 10% of daily calories from added sugars. For a typical 2,000-calorie-per-day diet, that equals no more than 200 calories, or about 12 teaspoons. The CDC reports that the average person consumes 17 teaspoons per day, with the largest sources being sugar-sweetened beverages, desserts, and snacks. (For context: one 12-ounce can of soda contains the equivalent of 9 teaspoons of sugar, according to beverage maker Coca-Cola.)
The new analysis also found links between sugary beverage consumption and other diet and lifestyle characteristics that may contribute to health problems.
“People who consumed sugar-sweetened beverages more frequently were likely to ingest more total and saturated fat, carbohydrate, and sodium, and less fruit, fiber, dairy products, and whole grain foods,” the authors wrote. “This dietary pattern was also associated with more frequent smoking and drinking, lower physical activity levels, and more time spent watching television. Therefore, the role of these confounding factors should be taken into consideration when explaining the association between sugar consumption and burden of disease.”
Recommendations for limiting sugar consumption are in place worldwide, the authors noted. They concluded that more needs to be done given the known health dangers of sugar.
“To change sugar consumption patterns, especially for children and adolescents, a combination of widespread public health education and policies worldwide is urgently needed,” they said.
A version of this article first appeared on WebMD.com.
A new compilation of nearly all research to date on the health impacts of sugar offers dozens of reasons to cut back.
The studies accounted for decades of research on the topic, stretching back to the beginning of the largest electronic databases for scientific papers.
The result is a list that cites the world’s most common health problems like heart disease, diabetes, obesity, high blood pressure, heart attack, high cholesterol, cancer, and depression. The findings were published in the BMJ. Researchers looked at studies that evaluated the impacts of consuming free sugars, which means any food that contains processed or naturally occurring sugars like table sugar, honey, or maple syrup. Sugar found in whole fruits and vegetables and in milk is not free sugar.
U.S. dietary guidelines recommend getting no more than 10% of daily calories from added sugars. For a typical 2,000-calorie-per-day diet, that equals no more than 200 calories, or about 12 teaspoons. The CDC reports that the average person consumes 17 teaspoons per day, with the largest sources being sugar-sweetened beverages, desserts, and snacks. (For context: one 12-ounce can of soda contains the equivalent of 9 teaspoons of sugar, according to beverage maker Coca-Cola.)
The new analysis also found links between sugary beverage consumption and other diet and lifestyle characteristics that may contribute to health problems.
“People who consumed sugar-sweetened beverages more frequently were likely to ingest more total and saturated fat, carbohydrate, and sodium, and less fruit, fiber, dairy products, and whole grain foods,” the authors wrote. “This dietary pattern was also associated with more frequent smoking and drinking, lower physical activity levels, and more time spent watching television. Therefore, the role of these confounding factors should be taken into consideration when explaining the association between sugar consumption and burden of disease.”
Recommendations for limiting sugar consumption are in place worldwide, the authors noted. They concluded that more needs to be done given the known health dangers of sugar.
“To change sugar consumption patterns, especially for children and adolescents, a combination of widespread public health education and policies worldwide is urgently needed,” they said.
A version of this article first appeared on WebMD.com.
A new compilation of nearly all research to date on the health impacts of sugar offers dozens of reasons to cut back.
The studies accounted for decades of research on the topic, stretching back to the beginning of the largest electronic databases for scientific papers.
The result is a list that cites the world’s most common health problems like heart disease, diabetes, obesity, high blood pressure, heart attack, high cholesterol, cancer, and depression. The findings were published in the BMJ. Researchers looked at studies that evaluated the impacts of consuming free sugars, which means any food that contains processed or naturally occurring sugars like table sugar, honey, or maple syrup. Sugar found in whole fruits and vegetables and in milk is not free sugar.
U.S. dietary guidelines recommend getting no more than 10% of daily calories from added sugars. For a typical 2,000-calorie-per-day diet, that equals no more than 200 calories, or about 12 teaspoons. The CDC reports that the average person consumes 17 teaspoons per day, with the largest sources being sugar-sweetened beverages, desserts, and snacks. (For context: one 12-ounce can of soda contains the equivalent of 9 teaspoons of sugar, according to beverage maker Coca-Cola.)
The new analysis also found links between sugary beverage consumption and other diet and lifestyle characteristics that may contribute to health problems.
“People who consumed sugar-sweetened beverages more frequently were likely to ingest more total and saturated fat, carbohydrate, and sodium, and less fruit, fiber, dairy products, and whole grain foods,” the authors wrote. “This dietary pattern was also associated with more frequent smoking and drinking, lower physical activity levels, and more time spent watching television. Therefore, the role of these confounding factors should be taken into consideration when explaining the association between sugar consumption and burden of disease.”
Recommendations for limiting sugar consumption are in place worldwide, the authors noted. They concluded that more needs to be done given the known health dangers of sugar.
“To change sugar consumption patterns, especially for children and adolescents, a combination of widespread public health education and policies worldwide is urgently needed,” they said.
A version of this article first appeared on WebMD.com.
FROM THE BMJ
Antiphospholipid antibodies linked to future CV events
The presence of antiphospholipid antibodies is associated with an increased risk for future cardiovascular events, according to a new study.
The findings point to possible new approaches to risk stratification and the potential for new therapeutic targets in heart disease.
“In this study of the general population, we found that two antiphospholipid antibodies were associated with an increased risk of having a serious cardiovascular event over a follow-up of 8 years,” coauthor Jason Knight, MD, University of Michigan, Ann Arbor, said in an interview.
“If confirmed in further studies, these findings could be used to identify a subgroup of patients who need more careful monitoring and more aggressive risk-factor modification, and if the increased risk linked to these antibodies is high enough, it may also justify preemptive treatments such as the anticoagulants that are routinely used in antiphospholipid syndrome,” Dr. Knight said.
“The long-term vision is that we may identify some people in the general population who would benefit from treating the immune system for the prevention and treatment of cardiovascular disease instead of, or in addition to, using typical cardiovascular medications,” he added.
The study was published online in JAMA Network Open.
Individuals with autoimmune and inflammatory diseases have a greater risk for cardiovascular events than expected based on traditional cardiovascular risk factors, with mechanisms proposed to explain this risk including inflammation-mediated disruption of vascular integrity and activation of platelets and coagulation pathways, the authors explained. However, the role of autoantibodies remains unclear.
They noted that antiphospholipid antibodies can activate endothelial cells, platelets, and neutrophils, and some patients with persistently circulating antiphospholipid antibodies can develop antiphospholipid syndrome – an acquired thromboinflammatory disease characterized by arterial, venous, and microvascular thrombotic events and obstetric complications.
Cross-sectional studies have shown that antiphospholipid antibodies are acutely present in up to 17.4% of patients with stroke or transient ischemic attack, and small cohort studies have suggested that such antibodies may be present in 1%-12% of seemingly healthy individuals. However, the impact of sex, race, and ethnicity on the prevalence of antiphospholipid antibodies and their association with atherosclerotic cardiovascular disease is not known.
The researchers conducted the current study to look at the association between antiphospholipid antibodies and future risk for atherosclerotic cardiovascular events.
They analyzed data from 2,427 participants in the population-based Dallas Heart Study who had no history of atherosclerotic cardiovascular disease or autoimmune diseases requiring immunosuppressive medications at the time of blood sampling at study entry in 2007-2009.
Eight different types of antiphospholipid antibodies were measured, and data on cardiovascular events over the next 8 years was recorded.
Results showed that 14.5% of the cohort tested positive for one of these antiphospholipid antibodies at the start of the study, with approximately one-third of those detected at a moderate or high titer.
The researchers also found that the IgA isotypes of two antiphospholipid antibodies – anticardiolipin and anti-beta-2 glycoprotein – were associated with future atherosclerotic cardiovascular events.
After adjustment for other known risk factors, individuals testing positive for the IgA isotype of anticardiolipin had an almost five times increased risk (hazard ratio, 4.92) of the primary endpoint (myocardial infarction, stroke, coronary revascularization, or cardiovascular death); while those testing positive for anti–beta2-glycoprotein had an almost three times increased risk (HR, 2.91).
Furthermore, there was what appeared to be a dose effect. People with the highest levels of these antibodies also had the highest risk for cardiovascular events, with up to an almost 10-fold increased risk with the higher level of anticardiolipin.
Dr. Knight said that more research into the IgA isotypes of these antiphospholipid antibodies is needed.
“Most of the mechanistic work in the antiphospholipid syndrome field has focused on IgG antiphospholipid antibodies. While we commonly find these IgA antibodies in patients with APS, the extent to which they contribute to disease has not been firmly established,” he said. “The fact that IgA was the primary hit in our unbiased screen suggests that there is more to the story and we need to better understand the implications of having these antibodies in circulation, and what specific problems they may be causing.”
Noting that antiphospholipid antibodies can form transiently after certain situations, such as infections, Dr. Knight said that further studies were needed with repeat blood testing to detect the chronic presence of the antibodies.
He added that information of venous thromboses was not available in this study and “perhaps some of the other antibodies might have stood out if we were able to analyze for different outcomes.”
This study was supported by a Pfizer Aspire Award. Dr. Knight reported receiving research funding and consulting fees from Jazz Pharmaceuticals outside the submitted work.
A version of this article first appeared on Medscape.com.
The presence of antiphospholipid antibodies is associated with an increased risk for future cardiovascular events, according to a new study.
The findings point to possible new approaches to risk stratification and the potential for new therapeutic targets in heart disease.
“In this study of the general population, we found that two antiphospholipid antibodies were associated with an increased risk of having a serious cardiovascular event over a follow-up of 8 years,” coauthor Jason Knight, MD, University of Michigan, Ann Arbor, said in an interview.
“If confirmed in further studies, these findings could be used to identify a subgroup of patients who need more careful monitoring and more aggressive risk-factor modification, and if the increased risk linked to these antibodies is high enough, it may also justify preemptive treatments such as the anticoagulants that are routinely used in antiphospholipid syndrome,” Dr. Knight said.
“The long-term vision is that we may identify some people in the general population who would benefit from treating the immune system for the prevention and treatment of cardiovascular disease instead of, or in addition to, using typical cardiovascular medications,” he added.
The study was published online in JAMA Network Open.
Individuals with autoimmune and inflammatory diseases have a greater risk for cardiovascular events than expected based on traditional cardiovascular risk factors, with mechanisms proposed to explain this risk including inflammation-mediated disruption of vascular integrity and activation of platelets and coagulation pathways, the authors explained. However, the role of autoantibodies remains unclear.
They noted that antiphospholipid antibodies can activate endothelial cells, platelets, and neutrophils, and some patients with persistently circulating antiphospholipid antibodies can develop antiphospholipid syndrome – an acquired thromboinflammatory disease characterized by arterial, venous, and microvascular thrombotic events and obstetric complications.
Cross-sectional studies have shown that antiphospholipid antibodies are acutely present in up to 17.4% of patients with stroke or transient ischemic attack, and small cohort studies have suggested that such antibodies may be present in 1%-12% of seemingly healthy individuals. However, the impact of sex, race, and ethnicity on the prevalence of antiphospholipid antibodies and their association with atherosclerotic cardiovascular disease is not known.
The researchers conducted the current study to look at the association between antiphospholipid antibodies and future risk for atherosclerotic cardiovascular events.
They analyzed data from 2,427 participants in the population-based Dallas Heart Study who had no history of atherosclerotic cardiovascular disease or autoimmune diseases requiring immunosuppressive medications at the time of blood sampling at study entry in 2007-2009.
Eight different types of antiphospholipid antibodies were measured, and data on cardiovascular events over the next 8 years was recorded.
Results showed that 14.5% of the cohort tested positive for one of these antiphospholipid antibodies at the start of the study, with approximately one-third of those detected at a moderate or high titer.
The researchers also found that the IgA isotypes of two antiphospholipid antibodies – anticardiolipin and anti-beta-2 glycoprotein – were associated with future atherosclerotic cardiovascular events.
After adjustment for other known risk factors, individuals testing positive for the IgA isotype of anticardiolipin had an almost five times increased risk (hazard ratio, 4.92) of the primary endpoint (myocardial infarction, stroke, coronary revascularization, or cardiovascular death); while those testing positive for anti–beta2-glycoprotein had an almost three times increased risk (HR, 2.91).
Furthermore, there was what appeared to be a dose effect. People with the highest levels of these antibodies also had the highest risk for cardiovascular events, with up to an almost 10-fold increased risk with the higher level of anticardiolipin.
Dr. Knight said that more research into the IgA isotypes of these antiphospholipid antibodies is needed.
“Most of the mechanistic work in the antiphospholipid syndrome field has focused on IgG antiphospholipid antibodies. While we commonly find these IgA antibodies in patients with APS, the extent to which they contribute to disease has not been firmly established,” he said. “The fact that IgA was the primary hit in our unbiased screen suggests that there is more to the story and we need to better understand the implications of having these antibodies in circulation, and what specific problems they may be causing.”
Noting that antiphospholipid antibodies can form transiently after certain situations, such as infections, Dr. Knight said that further studies were needed with repeat blood testing to detect the chronic presence of the antibodies.
He added that information of venous thromboses was not available in this study and “perhaps some of the other antibodies might have stood out if we were able to analyze for different outcomes.”
This study was supported by a Pfizer Aspire Award. Dr. Knight reported receiving research funding and consulting fees from Jazz Pharmaceuticals outside the submitted work.
A version of this article first appeared on Medscape.com.
The presence of antiphospholipid antibodies is associated with an increased risk for future cardiovascular events, according to a new study.
The findings point to possible new approaches to risk stratification and the potential for new therapeutic targets in heart disease.
“In this study of the general population, we found that two antiphospholipid antibodies were associated with an increased risk of having a serious cardiovascular event over a follow-up of 8 years,” coauthor Jason Knight, MD, University of Michigan, Ann Arbor, said in an interview.
“If confirmed in further studies, these findings could be used to identify a subgroup of patients who need more careful monitoring and more aggressive risk-factor modification, and if the increased risk linked to these antibodies is high enough, it may also justify preemptive treatments such as the anticoagulants that are routinely used in antiphospholipid syndrome,” Dr. Knight said.
“The long-term vision is that we may identify some people in the general population who would benefit from treating the immune system for the prevention and treatment of cardiovascular disease instead of, or in addition to, using typical cardiovascular medications,” he added.
The study was published online in JAMA Network Open.
Individuals with autoimmune and inflammatory diseases have a greater risk for cardiovascular events than expected based on traditional cardiovascular risk factors, with mechanisms proposed to explain this risk including inflammation-mediated disruption of vascular integrity and activation of platelets and coagulation pathways, the authors explained. However, the role of autoantibodies remains unclear.
They noted that antiphospholipid antibodies can activate endothelial cells, platelets, and neutrophils, and some patients with persistently circulating antiphospholipid antibodies can develop antiphospholipid syndrome – an acquired thromboinflammatory disease characterized by arterial, venous, and microvascular thrombotic events and obstetric complications.
Cross-sectional studies have shown that antiphospholipid antibodies are acutely present in up to 17.4% of patients with stroke or transient ischemic attack, and small cohort studies have suggested that such antibodies may be present in 1%-12% of seemingly healthy individuals. However, the impact of sex, race, and ethnicity on the prevalence of antiphospholipid antibodies and their association with atherosclerotic cardiovascular disease is not known.
The researchers conducted the current study to look at the association between antiphospholipid antibodies and future risk for atherosclerotic cardiovascular events.
They analyzed data from 2,427 participants in the population-based Dallas Heart Study who had no history of atherosclerotic cardiovascular disease or autoimmune diseases requiring immunosuppressive medications at the time of blood sampling at study entry in 2007-2009.
Eight different types of antiphospholipid antibodies were measured, and data on cardiovascular events over the next 8 years was recorded.
Results showed that 14.5% of the cohort tested positive for one of these antiphospholipid antibodies at the start of the study, with approximately one-third of those detected at a moderate or high titer.
The researchers also found that the IgA isotypes of two antiphospholipid antibodies – anticardiolipin and anti-beta-2 glycoprotein – were associated with future atherosclerotic cardiovascular events.
After adjustment for other known risk factors, individuals testing positive for the IgA isotype of anticardiolipin had an almost five times increased risk (hazard ratio, 4.92) of the primary endpoint (myocardial infarction, stroke, coronary revascularization, or cardiovascular death); while those testing positive for anti–beta2-glycoprotein had an almost three times increased risk (HR, 2.91).
Furthermore, there was what appeared to be a dose effect. People with the highest levels of these antibodies also had the highest risk for cardiovascular events, with up to an almost 10-fold increased risk with the higher level of anticardiolipin.
Dr. Knight said that more research into the IgA isotypes of these antiphospholipid antibodies is needed.
“Most of the mechanistic work in the antiphospholipid syndrome field has focused on IgG antiphospholipid antibodies. While we commonly find these IgA antibodies in patients with APS, the extent to which they contribute to disease has not been firmly established,” he said. “The fact that IgA was the primary hit in our unbiased screen suggests that there is more to the story and we need to better understand the implications of having these antibodies in circulation, and what specific problems they may be causing.”
Noting that antiphospholipid antibodies can form transiently after certain situations, such as infections, Dr. Knight said that further studies were needed with repeat blood testing to detect the chronic presence of the antibodies.
He added that information of venous thromboses was not available in this study and “perhaps some of the other antibodies might have stood out if we were able to analyze for different outcomes.”
This study was supported by a Pfizer Aspire Award. Dr. Knight reported receiving research funding and consulting fees from Jazz Pharmaceuticals outside the submitted work.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Why 9 is not too young for the HPV vaccine
For Sonja O’Leary, MD, higher rates of vaccination against human papillomavirus came with the flip of a switch.
Dr. O’Leary, the interim director of service for outpatient pediatric services at Denver Health and Hospital Authority, and her colleagues saw rates of HPV and other childhood immunizations drop during the COVID-19 pandemic and decided to act. Their health system, which includes 28 federally qualified health centers, offers vaccines at any inpatient or outpatient visit based on alerts from their electronic health record.
“It was actually really simple; it was really just changing our best-practice alert,” Dr. O’Leary said. Beginning in May 2021, and after notifying clinic staff of the impending change, DHHA dropped the alert for first dose of HPV from age 11 to 9.
The approach worked. Compared with the first 5 months of 2021, the percentage of children aged 9-13 years with an in-person visit who received at least one dose of HPV vaccine between June 2021 and August 2022 rose from 30.3% to 42.8% – a 41% increase. The share who received two doses by age 13 years more than doubled, from 19.3% to 42.7%, Dr. O’Leary said.
Frustrated efforts
Although those figures might seem to make an iron-clad case for earlier vaccinations against HPV – which is responsible for nearly 35,000 cases of cancer annually – factors beyond statistics have frustrated efforts to increase acceptance of the shots.
Data published in 2022 from the U.S. Centers for Disease Control and Prevention found that 89.6% of teens aged 13-17 years received at least one dose of tetanus, diphtheria, and acellular pertussis vaccine, and 89% got one or more doses of meningococcal conjugate vaccine. However, only 76.9% had received one or more doses of HPV vaccine. The rate of receiving both doses needed for full protection was much lower (61.7%).
Both the American Academy of Pediatrics and the American Cancer Society now endorse the strategy of offering HPV vaccine as early as age 9, which avoids the need for multiple shots at a single visit and results in more kids getting both doses. In a recent study that surveyed primary care professionals who see pediatric patients, 21% were already offering HPV vaccine at age 9, and another 48% were willing to try the approach.
What was the most common objection to the earlier age? Nearly three-quarters of clinicians said they felt that parents weren’t ready to talk about HPV vaccination yet.
Noel Brewer, PhD, one of the authors of the survey study, wondered why clinicians feel the need to bring up sex at all. “Providers should never be talking about sex when they are talking about vaccine, because that’s not the point,” said Dr. Brewer, the distinguished professor in public health at the University of North Carolina at Chapel Hill. He pointed out that providers don’t talk about the route of transmission for any other vaccine.
Dr. Brewer led a randomized controlled trial that trained pediatric clinicians in the “announcement” strategy, in which the clinician announces the vaccines that are due at that visit. If the parent hesitates, the clinician then probes further to identify and address their concerns and provides more information. If the parent is still not convinced, the clinician notes the discussion in the chart and tries again at the next visit.
The strategy was effective: Intervention clinics had a 5.4% higher rate of HPV vaccination coverage than control clinics after six months. Dr. Brewer and his colleagues have trained over 1,700 providers in the technique since 2020.
A cancer – not STI – vaccine
Although DHHA hasn’t participated in Dr. Brewer’s training, Dr. O’Leary and her colleagues take a similar approach of simply stating which vaccines the child should receive that day. And they talk about HPV as a cancer vaccine instead of one to prevent a sexually transmitted infection.
In her experience, this emphasis changes the conversation. Dr. O’Leary described a typical comment from parents as, “Oh, of course I would give my child a vaccine that could prevent cancer.”
Ana Rodriguez, MD, MPH, an obstetrician, became interested in raising rates of vaccination against HPV after watching too many women battle a preventable cancer. She worked for several years in the Rio Grande Valley along the U.S. border with Mexico, an impoverished rural area with poor access to health care and high rates of HPV infection.
“I would treat women very young – not even 30 years of age – already fighting advanced precancerous lesions secondary to HPV,” said Dr. Rodriguez, an associate professor of Obstetrics & Gynecology at the University of Texas Medical Branch at Galveston.
In 2016, when Texas ranked 47th in the nation for rates of up-to-date HPV vaccination, Dr. Rodriguez helped launch a community-based educational campaign in four rural counties in the Rio Grande Valley using social media, radio, and in-person meetings with school PTA members and members of school boards to educate staff and parents about the need for vaccination against the infection.
In 2019, the team began offering the vaccine to children ages 9-12 years at back-to-school events, progress report nights, and other school events, pivoting to outdoor events using a mobile vaccine van after COVID-19 struck. They recently published a study showing that 73.6% of students who received their first dose of vaccine at age 11 or younger completed the series, compared with only 45.1% of children who got their first dose at age 12 or older.
Dr. Rodriguez encountered parents who felt 9 or 10 years old was too young because their children were not going to be sexually active anytime soon. Her response was to describe HPV as a tool to prevent cancer, telling parents, “If you vaccinate your kids young enough, they will be protected for life.”
Lifetime protection is another point in favor of giving HPV vaccine prior to Tdap and MenACWY. The response to the two-dose series of HPV in preadolescents is robust and long-lasting, with no downside to giving it a few years earlier. In contrast, immunity to MenACWY wanes after a few years, so the immunization must be given before children enter high school, when their risk for meningitis increases.
The annual toll of deaths in the United States from meningococcus, tetanus, diphtheria, and pertussis typically totals less than 100, whereas cancer deaths attributable to HPV infection number in the thousands each year. And that may be the best reason for attempting new strategies to help HPV vaccination rates catch up to the rest of the preteen vaccines.
Dr. Brewer’s work was supported by the Gillings School of Global Public Health, the Lineberger Comprehensive Cancer Center at the University of North Carolina, and from training grants from the National Cancer Institute. Dr. Brewer has received research funding from Merck, Pfizer, and GSK and served as a paid advisor for Merck. Dr. O’Leary reports no relevant financial relationships. Dr. Rodriguez received a grant from the Cancer Prevention Research Institute of Texas, and the study was supported by the Institute for Translational Sciences at the University of Texas Medical Branch.
A version of this article first appeared on Medscape.com.
For Sonja O’Leary, MD, higher rates of vaccination against human papillomavirus came with the flip of a switch.
Dr. O’Leary, the interim director of service for outpatient pediatric services at Denver Health and Hospital Authority, and her colleagues saw rates of HPV and other childhood immunizations drop during the COVID-19 pandemic and decided to act. Their health system, which includes 28 federally qualified health centers, offers vaccines at any inpatient or outpatient visit based on alerts from their electronic health record.
“It was actually really simple; it was really just changing our best-practice alert,” Dr. O’Leary said. Beginning in May 2021, and after notifying clinic staff of the impending change, DHHA dropped the alert for first dose of HPV from age 11 to 9.
The approach worked. Compared with the first 5 months of 2021, the percentage of children aged 9-13 years with an in-person visit who received at least one dose of HPV vaccine between June 2021 and August 2022 rose from 30.3% to 42.8% – a 41% increase. The share who received two doses by age 13 years more than doubled, from 19.3% to 42.7%, Dr. O’Leary said.
Frustrated efforts
Although those figures might seem to make an iron-clad case for earlier vaccinations against HPV – which is responsible for nearly 35,000 cases of cancer annually – factors beyond statistics have frustrated efforts to increase acceptance of the shots.
Data published in 2022 from the U.S. Centers for Disease Control and Prevention found that 89.6% of teens aged 13-17 years received at least one dose of tetanus, diphtheria, and acellular pertussis vaccine, and 89% got one or more doses of meningococcal conjugate vaccine. However, only 76.9% had received one or more doses of HPV vaccine. The rate of receiving both doses needed for full protection was much lower (61.7%).
Both the American Academy of Pediatrics and the American Cancer Society now endorse the strategy of offering HPV vaccine as early as age 9, which avoids the need for multiple shots at a single visit and results in more kids getting both doses. In a recent study that surveyed primary care professionals who see pediatric patients, 21% were already offering HPV vaccine at age 9, and another 48% were willing to try the approach.
What was the most common objection to the earlier age? Nearly three-quarters of clinicians said they felt that parents weren’t ready to talk about HPV vaccination yet.
Noel Brewer, PhD, one of the authors of the survey study, wondered why clinicians feel the need to bring up sex at all. “Providers should never be talking about sex when they are talking about vaccine, because that’s not the point,” said Dr. Brewer, the distinguished professor in public health at the University of North Carolina at Chapel Hill. He pointed out that providers don’t talk about the route of transmission for any other vaccine.
Dr. Brewer led a randomized controlled trial that trained pediatric clinicians in the “announcement” strategy, in which the clinician announces the vaccines that are due at that visit. If the parent hesitates, the clinician then probes further to identify and address their concerns and provides more information. If the parent is still not convinced, the clinician notes the discussion in the chart and tries again at the next visit.
The strategy was effective: Intervention clinics had a 5.4% higher rate of HPV vaccination coverage than control clinics after six months. Dr. Brewer and his colleagues have trained over 1,700 providers in the technique since 2020.
A cancer – not STI – vaccine
Although DHHA hasn’t participated in Dr. Brewer’s training, Dr. O’Leary and her colleagues take a similar approach of simply stating which vaccines the child should receive that day. And they talk about HPV as a cancer vaccine instead of one to prevent a sexually transmitted infection.
In her experience, this emphasis changes the conversation. Dr. O’Leary described a typical comment from parents as, “Oh, of course I would give my child a vaccine that could prevent cancer.”
Ana Rodriguez, MD, MPH, an obstetrician, became interested in raising rates of vaccination against HPV after watching too many women battle a preventable cancer. She worked for several years in the Rio Grande Valley along the U.S. border with Mexico, an impoverished rural area with poor access to health care and high rates of HPV infection.
“I would treat women very young – not even 30 years of age – already fighting advanced precancerous lesions secondary to HPV,” said Dr. Rodriguez, an associate professor of Obstetrics & Gynecology at the University of Texas Medical Branch at Galveston.
In 2016, when Texas ranked 47th in the nation for rates of up-to-date HPV vaccination, Dr. Rodriguez helped launch a community-based educational campaign in four rural counties in the Rio Grande Valley using social media, radio, and in-person meetings with school PTA members and members of school boards to educate staff and parents about the need for vaccination against the infection.
In 2019, the team began offering the vaccine to children ages 9-12 years at back-to-school events, progress report nights, and other school events, pivoting to outdoor events using a mobile vaccine van after COVID-19 struck. They recently published a study showing that 73.6% of students who received their first dose of vaccine at age 11 or younger completed the series, compared with only 45.1% of children who got their first dose at age 12 or older.
Dr. Rodriguez encountered parents who felt 9 or 10 years old was too young because their children were not going to be sexually active anytime soon. Her response was to describe HPV as a tool to prevent cancer, telling parents, “If you vaccinate your kids young enough, they will be protected for life.”
Lifetime protection is another point in favor of giving HPV vaccine prior to Tdap and MenACWY. The response to the two-dose series of HPV in preadolescents is robust and long-lasting, with no downside to giving it a few years earlier. In contrast, immunity to MenACWY wanes after a few years, so the immunization must be given before children enter high school, when their risk for meningitis increases.
The annual toll of deaths in the United States from meningococcus, tetanus, diphtheria, and pertussis typically totals less than 100, whereas cancer deaths attributable to HPV infection number in the thousands each year. And that may be the best reason for attempting new strategies to help HPV vaccination rates catch up to the rest of the preteen vaccines.
Dr. Brewer’s work was supported by the Gillings School of Global Public Health, the Lineberger Comprehensive Cancer Center at the University of North Carolina, and from training grants from the National Cancer Institute. Dr. Brewer has received research funding from Merck, Pfizer, and GSK and served as a paid advisor for Merck. Dr. O’Leary reports no relevant financial relationships. Dr. Rodriguez received a grant from the Cancer Prevention Research Institute of Texas, and the study was supported by the Institute for Translational Sciences at the University of Texas Medical Branch.
A version of this article first appeared on Medscape.com.
For Sonja O’Leary, MD, higher rates of vaccination against human papillomavirus came with the flip of a switch.
Dr. O’Leary, the interim director of service for outpatient pediatric services at Denver Health and Hospital Authority, and her colleagues saw rates of HPV and other childhood immunizations drop during the COVID-19 pandemic and decided to act. Their health system, which includes 28 federally qualified health centers, offers vaccines at any inpatient or outpatient visit based on alerts from their electronic health record.
“It was actually really simple; it was really just changing our best-practice alert,” Dr. O’Leary said. Beginning in May 2021, and after notifying clinic staff of the impending change, DHHA dropped the alert for first dose of HPV from age 11 to 9.
The approach worked. Compared with the first 5 months of 2021, the percentage of children aged 9-13 years with an in-person visit who received at least one dose of HPV vaccine between June 2021 and August 2022 rose from 30.3% to 42.8% – a 41% increase. The share who received two doses by age 13 years more than doubled, from 19.3% to 42.7%, Dr. O’Leary said.
Frustrated efforts
Although those figures might seem to make an iron-clad case for earlier vaccinations against HPV – which is responsible for nearly 35,000 cases of cancer annually – factors beyond statistics have frustrated efforts to increase acceptance of the shots.
Data published in 2022 from the U.S. Centers for Disease Control and Prevention found that 89.6% of teens aged 13-17 years received at least one dose of tetanus, diphtheria, and acellular pertussis vaccine, and 89% got one or more doses of meningococcal conjugate vaccine. However, only 76.9% had received one or more doses of HPV vaccine. The rate of receiving both doses needed for full protection was much lower (61.7%).
Both the American Academy of Pediatrics and the American Cancer Society now endorse the strategy of offering HPV vaccine as early as age 9, which avoids the need for multiple shots at a single visit and results in more kids getting both doses. In a recent study that surveyed primary care professionals who see pediatric patients, 21% were already offering HPV vaccine at age 9, and another 48% were willing to try the approach.
What was the most common objection to the earlier age? Nearly three-quarters of clinicians said they felt that parents weren’t ready to talk about HPV vaccination yet.
Noel Brewer, PhD, one of the authors of the survey study, wondered why clinicians feel the need to bring up sex at all. “Providers should never be talking about sex when they are talking about vaccine, because that’s not the point,” said Dr. Brewer, the distinguished professor in public health at the University of North Carolina at Chapel Hill. He pointed out that providers don’t talk about the route of transmission for any other vaccine.
Dr. Brewer led a randomized controlled trial that trained pediatric clinicians in the “announcement” strategy, in which the clinician announces the vaccines that are due at that visit. If the parent hesitates, the clinician then probes further to identify and address their concerns and provides more information. If the parent is still not convinced, the clinician notes the discussion in the chart and tries again at the next visit.
The strategy was effective: Intervention clinics had a 5.4% higher rate of HPV vaccination coverage than control clinics after six months. Dr. Brewer and his colleagues have trained over 1,700 providers in the technique since 2020.
A cancer – not STI – vaccine
Although DHHA hasn’t participated in Dr. Brewer’s training, Dr. O’Leary and her colleagues take a similar approach of simply stating which vaccines the child should receive that day. And they talk about HPV as a cancer vaccine instead of one to prevent a sexually transmitted infection.
In her experience, this emphasis changes the conversation. Dr. O’Leary described a typical comment from parents as, “Oh, of course I would give my child a vaccine that could prevent cancer.”
Ana Rodriguez, MD, MPH, an obstetrician, became interested in raising rates of vaccination against HPV after watching too many women battle a preventable cancer. She worked for several years in the Rio Grande Valley along the U.S. border with Mexico, an impoverished rural area with poor access to health care and high rates of HPV infection.
“I would treat women very young – not even 30 years of age – already fighting advanced precancerous lesions secondary to HPV,” said Dr. Rodriguez, an associate professor of Obstetrics & Gynecology at the University of Texas Medical Branch at Galveston.
In 2016, when Texas ranked 47th in the nation for rates of up-to-date HPV vaccination, Dr. Rodriguez helped launch a community-based educational campaign in four rural counties in the Rio Grande Valley using social media, radio, and in-person meetings with school PTA members and members of school boards to educate staff and parents about the need for vaccination against the infection.
In 2019, the team began offering the vaccine to children ages 9-12 years at back-to-school events, progress report nights, and other school events, pivoting to outdoor events using a mobile vaccine van after COVID-19 struck. They recently published a study showing that 73.6% of students who received their first dose of vaccine at age 11 or younger completed the series, compared with only 45.1% of children who got their first dose at age 12 or older.
Dr. Rodriguez encountered parents who felt 9 or 10 years old was too young because their children were not going to be sexually active anytime soon. Her response was to describe HPV as a tool to prevent cancer, telling parents, “If you vaccinate your kids young enough, they will be protected for life.”
Lifetime protection is another point in favor of giving HPV vaccine prior to Tdap and MenACWY. The response to the two-dose series of HPV in preadolescents is robust and long-lasting, with no downside to giving it a few years earlier. In contrast, immunity to MenACWY wanes after a few years, so the immunization must be given before children enter high school, when their risk for meningitis increases.
The annual toll of deaths in the United States from meningococcus, tetanus, diphtheria, and pertussis typically totals less than 100, whereas cancer deaths attributable to HPV infection number in the thousands each year. And that may be the best reason for attempting new strategies to help HPV vaccination rates catch up to the rest of the preteen vaccines.
Dr. Brewer’s work was supported by the Gillings School of Global Public Health, the Lineberger Comprehensive Cancer Center at the University of North Carolina, and from training grants from the National Cancer Institute. Dr. Brewer has received research funding from Merck, Pfizer, and GSK and served as a paid advisor for Merck. Dr. O’Leary reports no relevant financial relationships. Dr. Rodriguez received a grant from the Cancer Prevention Research Institute of Texas, and the study was supported by the Institute for Translational Sciences at the University of Texas Medical Branch.
A version of this article first appeared on Medscape.com.
Racial disparities not found in chronic hepatitis B treatment initiation
Researchers studying differences in treatment initiation for chronic hepatitis B (CHB) among a large, multiracial cohort in North America did not find evidence of disparities by race or socioeconomic status.
.
That gap suggests that treatment guidelines need to be simplified and that efforts to increase hepatitis B virus (HBV) awareness and train more clinicians are needed to achieve the World Health Organization’s goal of eliminating HBV by 2030, the researchers write.
The Hepatitis B Research Network study was published online in JAMA Network Open.
The prevalence of CHB in the United States is estimated at 2.4 million. It disproportionately affects persons of Asian or African descent, the investigators note. Their study examined whether treatment initiation and outcomes differ between African American and Black, Asian, and White participants, as well as between African American and Black participants born in North America and East or West Africa.
The research involved 1,550 adult patients: 1,157 Asian American, 193 African American or Black (39 born in the United States, 90 in East Africa, 53 in West Africa, and 11 elsewhere), 157 White, and 43 who identified as being of “other races.” All had CHB but were not receiving antiviral treatment at enrollment.
Participants came from 20 centers in the United States and one in Canada. They underwent clinical and laboratory assessments and could receive anti-HBV treatment after they enrolled. Enrollment was from Jan. 14, 2011, to Jan. 28, 2018. Participants were followed at 12 and 24 weeks and every 24 weeks thereafter in the longitudinal cohort study by Mandana Khalili, MD, division of gastroenterology and hepatology, University of California, San Francisco, and colleagues.
Information on patients’ country of birth, duration of U.S. or Canadian residency, educational level, employment, insurance, prior antiviral treatment, family history of HBV or hepatocellular carcinoma (HCC), and mode of transmission were collected by research coordinators.
Treatment initiation
During the study period, slightly fewer than one-third (32.5%) of the participants initiated treatment. The incidences were 4.8 per 100 person-years in African American or Black participants, 9.9 per 100 person-years in Asian participants, 6.6 per 100 person-years in White participants, and 7.9 per 100 person-years in those of other races (P < .001).
A lower percentage of African American and Black participants (14%) met the American Association for the Study of Liver Diseases treatment criteria, compared with Asian (22%) and White (27%) participants (P = .01).
When the researchers compared cumulative probability of initiating treatment by race for those who met criteria for treatment, they found no significant differences by race.
At 72 weeks, initiation probability was 0.45 for African American and Black patients and 0.51 for Asian and White patients (P = .68). Similarly, among African American and Black participants who met treatment criteria, there were no significant differences in cumulative probability of treatment by region of birth.
The cumulative percentage of treatment initiation for those who met guideline-based criteria was 62%.
“Among participants with a treatment indication, treatment rates did not differ significantly by race, despite marked differences in educational level, income, and type of health care insurance across the racial groups,” the researchers write. “Moreover, race was not an independent estimator of treatment initiation when adjusting for known factors associated with a higher risk of adverse clinical outcomes, namely, HBV DNA, disease severity, sex, and age.”
Adverse liver outcomes (hepatic decompensation, HCC, liver transplant, and death) were rare and did not vary significantly by race, the researchers write.
One study limitation is that participants were linked to specialty liver clinics, so the findings may not be generalizable to patients who receive care in other settings, the authors note.
The results are “reassuring,” said senior author Anna S. Lok, MD, division of gastroenterology and hepatology at University of Michigan in Ann Arbor. However, she noted, study participants had already overcome barriers to receiving care at major academic centers.
“Once you get into the big academic liver centers, then maybe everything is equal, but in the real world, a lot of people don’t ever get to the big liver centers,” she said. The question becomes: “Are we serving only a portion of the patient population?”
Many factors drive the decision to undergo treatment, including the doctor’s opinion as to need and the patient’s desire to receive treatment, she said.
The study participants who were more likely to get treated were those with higher-level disease who had a stronger indication for treatment, Dr. Lok said.
Finding the disparities
Centers for Disease Control and Prevention statistics show that Black people are 3.9 times more likely to have CHB and 2.5 times more likely to die from it than White people, notes H. Nina Kim, MD, with the department of medicine, University of Washington, Seattle, in an accompanying invited commentary.
“The fact that we have not observed racial disparities in treatment initiation does not mean none exist; it means we have to look harder to find them,” she writes.
“We need to examine whether our guidelines for HBV treatment are so complex that it becomes the purview of specialists, thereby restricting access and deepening inequities,” Dr. Kim adds. “We should look closely at retention in care, the step that precedes treatment, and stratify this outcome by race and ethnicity.”
Primary care physicians in some regions might find it difficult to manage patients who have hepatitis B because they see so few of them, Dr. Lok noted.
Dr. Khalili has received grants and consulting fees from Gilead Sciences Inc and grants from Intercept Pharmaceuticals outside the submitted work. Dr. Lok has received grants from Target and consultant fees from Abbott, Ambys, Arbutus, Chroma, Clear B, Enanta, Enochian, GNI, GlaxoSmithKline, Eli Lilly, and Virion outside the submitted work. Coauthors have received grants, consulting fees, or personal fees from Bayer, Boston Scientific, Exact Sciences, Fujifilm Medical Sciences, Gilead Sciences, Glycotest, Redhill Biopharma, Target RWE, MedEd Design, Pontifax, Global Life, the Lynx Group, AstraZeneca, Eisai, Novartis Venture Fund, Grail, QED Therapeutics, Genentech, Hepion Pharmaceuticals, Roche, Abbott, AbbVie, and Pfizer. Dr. Kim has received grants from Gilead Sciences (paid to her institution) outside the submitted work.
A version of this article first appeared on Medscape.com.
Researchers studying differences in treatment initiation for chronic hepatitis B (CHB) among a large, multiracial cohort in North America did not find evidence of disparities by race or socioeconomic status.
.
That gap suggests that treatment guidelines need to be simplified and that efforts to increase hepatitis B virus (HBV) awareness and train more clinicians are needed to achieve the World Health Organization’s goal of eliminating HBV by 2030, the researchers write.
The Hepatitis B Research Network study was published online in JAMA Network Open.
The prevalence of CHB in the United States is estimated at 2.4 million. It disproportionately affects persons of Asian or African descent, the investigators note. Their study examined whether treatment initiation and outcomes differ between African American and Black, Asian, and White participants, as well as between African American and Black participants born in North America and East or West Africa.
The research involved 1,550 adult patients: 1,157 Asian American, 193 African American or Black (39 born in the United States, 90 in East Africa, 53 in West Africa, and 11 elsewhere), 157 White, and 43 who identified as being of “other races.” All had CHB but were not receiving antiviral treatment at enrollment.
Participants came from 20 centers in the United States and one in Canada. They underwent clinical and laboratory assessments and could receive anti-HBV treatment after they enrolled. Enrollment was from Jan. 14, 2011, to Jan. 28, 2018. Participants were followed at 12 and 24 weeks and every 24 weeks thereafter in the longitudinal cohort study by Mandana Khalili, MD, division of gastroenterology and hepatology, University of California, San Francisco, and colleagues.
Information on patients’ country of birth, duration of U.S. or Canadian residency, educational level, employment, insurance, prior antiviral treatment, family history of HBV or hepatocellular carcinoma (HCC), and mode of transmission were collected by research coordinators.
Treatment initiation
During the study period, slightly fewer than one-third (32.5%) of the participants initiated treatment. The incidences were 4.8 per 100 person-years in African American or Black participants, 9.9 per 100 person-years in Asian participants, 6.6 per 100 person-years in White participants, and 7.9 per 100 person-years in those of other races (P < .001).
A lower percentage of African American and Black participants (14%) met the American Association for the Study of Liver Diseases treatment criteria, compared with Asian (22%) and White (27%) participants (P = .01).
When the researchers compared cumulative probability of initiating treatment by race for those who met criteria for treatment, they found no significant differences by race.
At 72 weeks, initiation probability was 0.45 for African American and Black patients and 0.51 for Asian and White patients (P = .68). Similarly, among African American and Black participants who met treatment criteria, there were no significant differences in cumulative probability of treatment by region of birth.
The cumulative percentage of treatment initiation for those who met guideline-based criteria was 62%.
“Among participants with a treatment indication, treatment rates did not differ significantly by race, despite marked differences in educational level, income, and type of health care insurance across the racial groups,” the researchers write. “Moreover, race was not an independent estimator of treatment initiation when adjusting for known factors associated with a higher risk of adverse clinical outcomes, namely, HBV DNA, disease severity, sex, and age.”
Adverse liver outcomes (hepatic decompensation, HCC, liver transplant, and death) were rare and did not vary significantly by race, the researchers write.
One study limitation is that participants were linked to specialty liver clinics, so the findings may not be generalizable to patients who receive care in other settings, the authors note.
The results are “reassuring,” said senior author Anna S. Lok, MD, division of gastroenterology and hepatology at University of Michigan in Ann Arbor. However, she noted, study participants had already overcome barriers to receiving care at major academic centers.
“Once you get into the big academic liver centers, then maybe everything is equal, but in the real world, a lot of people don’t ever get to the big liver centers,” she said. The question becomes: “Are we serving only a portion of the patient population?”
Many factors drive the decision to undergo treatment, including the doctor’s opinion as to need and the patient’s desire to receive treatment, she said.
The study participants who were more likely to get treated were those with higher-level disease who had a stronger indication for treatment, Dr. Lok said.
Finding the disparities
Centers for Disease Control and Prevention statistics show that Black people are 3.9 times more likely to have CHB and 2.5 times more likely to die from it than White people, notes H. Nina Kim, MD, with the department of medicine, University of Washington, Seattle, in an accompanying invited commentary.
“The fact that we have not observed racial disparities in treatment initiation does not mean none exist; it means we have to look harder to find them,” she writes.
“We need to examine whether our guidelines for HBV treatment are so complex that it becomes the purview of specialists, thereby restricting access and deepening inequities,” Dr. Kim adds. “We should look closely at retention in care, the step that precedes treatment, and stratify this outcome by race and ethnicity.”
Primary care physicians in some regions might find it difficult to manage patients who have hepatitis B because they see so few of them, Dr. Lok noted.
Dr. Khalili has received grants and consulting fees from Gilead Sciences Inc and grants from Intercept Pharmaceuticals outside the submitted work. Dr. Lok has received grants from Target and consultant fees from Abbott, Ambys, Arbutus, Chroma, Clear B, Enanta, Enochian, GNI, GlaxoSmithKline, Eli Lilly, and Virion outside the submitted work. Coauthors have received grants, consulting fees, or personal fees from Bayer, Boston Scientific, Exact Sciences, Fujifilm Medical Sciences, Gilead Sciences, Glycotest, Redhill Biopharma, Target RWE, MedEd Design, Pontifax, Global Life, the Lynx Group, AstraZeneca, Eisai, Novartis Venture Fund, Grail, QED Therapeutics, Genentech, Hepion Pharmaceuticals, Roche, Abbott, AbbVie, and Pfizer. Dr. Kim has received grants from Gilead Sciences (paid to her institution) outside the submitted work.
A version of this article first appeared on Medscape.com.
Researchers studying differences in treatment initiation for chronic hepatitis B (CHB) among a large, multiracial cohort in North America did not find evidence of disparities by race or socioeconomic status.
.
That gap suggests that treatment guidelines need to be simplified and that efforts to increase hepatitis B virus (HBV) awareness and train more clinicians are needed to achieve the World Health Organization’s goal of eliminating HBV by 2030, the researchers write.
The Hepatitis B Research Network study was published online in JAMA Network Open.
The prevalence of CHB in the United States is estimated at 2.4 million. It disproportionately affects persons of Asian or African descent, the investigators note. Their study examined whether treatment initiation and outcomes differ between African American and Black, Asian, and White participants, as well as between African American and Black participants born in North America and East or West Africa.
The research involved 1,550 adult patients: 1,157 Asian American, 193 African American or Black (39 born in the United States, 90 in East Africa, 53 in West Africa, and 11 elsewhere), 157 White, and 43 who identified as being of “other races.” All had CHB but were not receiving antiviral treatment at enrollment.
Participants came from 20 centers in the United States and one in Canada. They underwent clinical and laboratory assessments and could receive anti-HBV treatment after they enrolled. Enrollment was from Jan. 14, 2011, to Jan. 28, 2018. Participants were followed at 12 and 24 weeks and every 24 weeks thereafter in the longitudinal cohort study by Mandana Khalili, MD, division of gastroenterology and hepatology, University of California, San Francisco, and colleagues.
Information on patients’ country of birth, duration of U.S. or Canadian residency, educational level, employment, insurance, prior antiviral treatment, family history of HBV or hepatocellular carcinoma (HCC), and mode of transmission were collected by research coordinators.
Treatment initiation
During the study period, slightly fewer than one-third (32.5%) of the participants initiated treatment. The incidences were 4.8 per 100 person-years in African American or Black participants, 9.9 per 100 person-years in Asian participants, 6.6 per 100 person-years in White participants, and 7.9 per 100 person-years in those of other races (P < .001).
A lower percentage of African American and Black participants (14%) met the American Association for the Study of Liver Diseases treatment criteria, compared with Asian (22%) and White (27%) participants (P = .01).
When the researchers compared cumulative probability of initiating treatment by race for those who met criteria for treatment, they found no significant differences by race.
At 72 weeks, initiation probability was 0.45 for African American and Black patients and 0.51 for Asian and White patients (P = .68). Similarly, among African American and Black participants who met treatment criteria, there were no significant differences in cumulative probability of treatment by region of birth.
The cumulative percentage of treatment initiation for those who met guideline-based criteria was 62%.
“Among participants with a treatment indication, treatment rates did not differ significantly by race, despite marked differences in educational level, income, and type of health care insurance across the racial groups,” the researchers write. “Moreover, race was not an independent estimator of treatment initiation when adjusting for known factors associated with a higher risk of adverse clinical outcomes, namely, HBV DNA, disease severity, sex, and age.”
Adverse liver outcomes (hepatic decompensation, HCC, liver transplant, and death) were rare and did not vary significantly by race, the researchers write.
One study limitation is that participants were linked to specialty liver clinics, so the findings may not be generalizable to patients who receive care in other settings, the authors note.
The results are “reassuring,” said senior author Anna S. Lok, MD, division of gastroenterology and hepatology at University of Michigan in Ann Arbor. However, she noted, study participants had already overcome barriers to receiving care at major academic centers.
“Once you get into the big academic liver centers, then maybe everything is equal, but in the real world, a lot of people don’t ever get to the big liver centers,” she said. The question becomes: “Are we serving only a portion of the patient population?”
Many factors drive the decision to undergo treatment, including the doctor’s opinion as to need and the patient’s desire to receive treatment, she said.
The study participants who were more likely to get treated were those with higher-level disease who had a stronger indication for treatment, Dr. Lok said.
Finding the disparities
Centers for Disease Control and Prevention statistics show that Black people are 3.9 times more likely to have CHB and 2.5 times more likely to die from it than White people, notes H. Nina Kim, MD, with the department of medicine, University of Washington, Seattle, in an accompanying invited commentary.
“The fact that we have not observed racial disparities in treatment initiation does not mean none exist; it means we have to look harder to find them,” she writes.
“We need to examine whether our guidelines for HBV treatment are so complex that it becomes the purview of specialists, thereby restricting access and deepening inequities,” Dr. Kim adds. “We should look closely at retention in care, the step that precedes treatment, and stratify this outcome by race and ethnicity.”
Primary care physicians in some regions might find it difficult to manage patients who have hepatitis B because they see so few of them, Dr. Lok noted.
Dr. Khalili has received grants and consulting fees from Gilead Sciences Inc and grants from Intercept Pharmaceuticals outside the submitted work. Dr. Lok has received grants from Target and consultant fees from Abbott, Ambys, Arbutus, Chroma, Clear B, Enanta, Enochian, GNI, GlaxoSmithKline, Eli Lilly, and Virion outside the submitted work. Coauthors have received grants, consulting fees, or personal fees from Bayer, Boston Scientific, Exact Sciences, Fujifilm Medical Sciences, Gilead Sciences, Glycotest, Redhill Biopharma, Target RWE, MedEd Design, Pontifax, Global Life, the Lynx Group, AstraZeneca, Eisai, Novartis Venture Fund, Grail, QED Therapeutics, Genentech, Hepion Pharmaceuticals, Roche, Abbott, AbbVie, and Pfizer. Dr. Kim has received grants from Gilead Sciences (paid to her institution) outside the submitted work.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Long COVID hitting some states, minorities, women harder
More than one in four adults sickened by the virus go on to have long COVID, according to a new report from the U.S. Census Bureau. Overall, nearly 15% of all American adults – more than 38 million people nationwide – have had long COVID at some point since the start of the pandemic, according to the report.
The report, based on survey data collected between March 1 and 13, defines long COVID as symptoms lasting at least 3 months that people didn’t have before getting infected with the virus.
It is the second recent look at who is most likely to face long COVID. A similar study, published in March, found that women, smokers, and those who had severe COVID-19 infections are most likely to have the disorder
The Census Bureau report found that while 27% of adults nationwide have had long COVID after getting infected with the virus, the condition has impacted some states more than others. The proportion of residents hit with long COVID ranged from a low of 18.8% in New Jersey to a high of 40.7% in West Virginia.
Other states with long COVID rates well below the national average include Alaska, Maryland, New York, and Wisconsin. At the other end of the spectrum, the states with rates well above the national average include Kentucky, Mississippi, New Mexico, Nevada, South Carolina, South Dakota, and Wyoming.
Long COVID rates also varied by age, gender, and race. People in their 50s were most at risk, with about 31% of those infected by the virus going on to have long COVID, followed by those in their 40s, at more than 29%.
Far more women (almost 33%) than men (21%) with COVID infections got long COVID. And when researchers looked at long COVID rates based on gender identity, they found that transgender adults were more than twice as likely to have long COVID than cisgender males. Bisexual adults also had much higher long COVID rates than straight, gay, or lesbian people.
Long COVID was also much more common among Hispanic adults, affecting almost 29% of those infected with the virus, than among White or Black people, who had long COVID rates similar to the national average of 27%. Asian adults had lower long COVID rates than the national average, at less than 20%.
People with disabilities were also at higher risk, with long COVID rates of almost 47%, compared with 24% among adults without disabilities.
A version of this article first appeared on WebMD.com.
More than one in four adults sickened by the virus go on to have long COVID, according to a new report from the U.S. Census Bureau. Overall, nearly 15% of all American adults – more than 38 million people nationwide – have had long COVID at some point since the start of the pandemic, according to the report.
The report, based on survey data collected between March 1 and 13, defines long COVID as symptoms lasting at least 3 months that people didn’t have before getting infected with the virus.
It is the second recent look at who is most likely to face long COVID. A similar study, published in March, found that women, smokers, and those who had severe COVID-19 infections are most likely to have the disorder
The Census Bureau report found that while 27% of adults nationwide have had long COVID after getting infected with the virus, the condition has impacted some states more than others. The proportion of residents hit with long COVID ranged from a low of 18.8% in New Jersey to a high of 40.7% in West Virginia.
Other states with long COVID rates well below the national average include Alaska, Maryland, New York, and Wisconsin. At the other end of the spectrum, the states with rates well above the national average include Kentucky, Mississippi, New Mexico, Nevada, South Carolina, South Dakota, and Wyoming.
Long COVID rates also varied by age, gender, and race. People in their 50s were most at risk, with about 31% of those infected by the virus going on to have long COVID, followed by those in their 40s, at more than 29%.
Far more women (almost 33%) than men (21%) with COVID infections got long COVID. And when researchers looked at long COVID rates based on gender identity, they found that transgender adults were more than twice as likely to have long COVID than cisgender males. Bisexual adults also had much higher long COVID rates than straight, gay, or lesbian people.
Long COVID was also much more common among Hispanic adults, affecting almost 29% of those infected with the virus, than among White or Black people, who had long COVID rates similar to the national average of 27%. Asian adults had lower long COVID rates than the national average, at less than 20%.
People with disabilities were also at higher risk, with long COVID rates of almost 47%, compared with 24% among adults without disabilities.
A version of this article first appeared on WebMD.com.
More than one in four adults sickened by the virus go on to have long COVID, according to a new report from the U.S. Census Bureau. Overall, nearly 15% of all American adults – more than 38 million people nationwide – have had long COVID at some point since the start of the pandemic, according to the report.
The report, based on survey data collected between March 1 and 13, defines long COVID as symptoms lasting at least 3 months that people didn’t have before getting infected with the virus.
It is the second recent look at who is most likely to face long COVID. A similar study, published in March, found that women, smokers, and those who had severe COVID-19 infections are most likely to have the disorder
The Census Bureau report found that while 27% of adults nationwide have had long COVID after getting infected with the virus, the condition has impacted some states more than others. The proportion of residents hit with long COVID ranged from a low of 18.8% in New Jersey to a high of 40.7% in West Virginia.
Other states with long COVID rates well below the national average include Alaska, Maryland, New York, and Wisconsin. At the other end of the spectrum, the states with rates well above the national average include Kentucky, Mississippi, New Mexico, Nevada, South Carolina, South Dakota, and Wyoming.
Long COVID rates also varied by age, gender, and race. People in their 50s were most at risk, with about 31% of those infected by the virus going on to have long COVID, followed by those in their 40s, at more than 29%.
Far more women (almost 33%) than men (21%) with COVID infections got long COVID. And when researchers looked at long COVID rates based on gender identity, they found that transgender adults were more than twice as likely to have long COVID than cisgender males. Bisexual adults also had much higher long COVID rates than straight, gay, or lesbian people.
Long COVID was also much more common among Hispanic adults, affecting almost 29% of those infected with the virus, than among White or Black people, who had long COVID rates similar to the national average of 27%. Asian adults had lower long COVID rates than the national average, at less than 20%.
People with disabilities were also at higher risk, with long COVID rates of almost 47%, compared with 24% among adults without disabilities.
A version of this article first appeared on WebMD.com.
Survival gains after surgery for small pancreatic NETs?
Overall, researchers found that surgical resection was associated with a 42% improvement in overall survival among patients with small tumors of 1.1-2.0 cm, but not tumors 1 cm or smaller. Among those with 1.1- to 2.0-cm tumors, the survival benefit following surgery was most notable among patients aged 64 years or younger and those with no comorbidities.
The findings were published in JAMA Network Open.
While surgical resection has been the first-line treatment for patients with functional or symptomatic localized, low-grade pancreatic NETs, surgery for asymptomatic low-grade nonfunctional pancreatic NETs of 2 cm or less “remains unclear even in consensus guidelines,” study author Richard D. Schulick, MD, MBA, of the University of Colorado at Denver, Aurora, and colleagues write.
Consensus guidelines from the European Neuroendocrine Tumor Society, for instance, indicate surveillance for these smaller tumors, while those from the Japan Neuroendocrine Tumor Society recommend surgery. The National Comprehensive Cancer Network (NCCN), which recently updated its guidelines, suggests observation as an option for patients with tumors as large as 2.0 cm but who are strongly considering resection.
To determine whether surgical resection of these smaller lesions influences overall survival, the team combed the U.S. National Cancer Database and identified 4,641 patients with nonfunctional pancreatic NETs up to 2.0 cm in size.
Researchers divided patients by tumor sizes of up to 1 cm (group 1a) and 1.1-2.0 cm (group 1b) and examined a range of variables, including age, comorbidities, tumor location and differentiation, and overall survival.
Overall, 1,278 patients had tumors measuring up to 1.0 cm (group 1a), and 3,363 had tumors measuring 1.1-2.0 cm (group 1b). The mean age across both groups was 60.5 years; about half were men, and most (77.4%) were White.
Over a median follow-up of 47.1 months, the surgical resection rate was significantly lower among patients in group 1a (82.0%) than in group 1b (87.0%). Patients who underwent resection, on average, were younger and were more likely to have tumors located in the pancreas tail and to have clinical lymph node metastasis.
Overall, the team found that surgical resection was associated with longer overall survival for patients with tumors of 1.1-2.0 cm (hazard ratio, 0.58) but not 1 cm or smaller (HR, 0.68; P = .12).
Among patients in group 1b (those with 1.1- to 2.0-cm tumors), the team also found that age 64 years or younger (adjusted HR, 0.34), treatment at academic institutions (aHR, 0.40), absence of comorbidities (aHR, 0.53), absence of clinical lymph node metastasis (aHR, 0.54), as well as tumors in the body (aHR, 0.36) and tail (aHR, 0.37) of the pancreas were significantly associated with increased survival after surgical resection.
Among patients with resected small nonmetastatic nonfunctional pancreatic NETs, pathologic lymph node metastasis (HR, 1.28; P = .43) and lymphovascular invasion (HR, 0.85; P = .75) were not associated with overall survival.
The results of the study “support an association between surgical resection and increased survival in select patients” among those with tumors 1.1-2.0 cm, Dr. Schulick and colleagues write.
James R. Howe, MD, who was not involved in the research, highlighted that the study tries to answer an important clinical problem: What should we do with small, nonfunctional pancreatic NETs?
However, he noted “significant selection bias” among patients included in the dataset.
More than 80% of patients with tumors under 1 cm underwent surgery, which “is not consistent with what most people would do in practice,” said Dr. Howe, of the division of surgical oncology and endocrine surgery, University of Iowa Hospitals and Clinics, Iowa City. “Most would be observed and might not make it into the National Cancer Database.”
Dr. Howe pointed to an even larger group of patients with pancreatic NETs who were not included in the database – those with CT evidence of a pancreatic NET but without biopsy confirmation.
With many patients potentially missing from the data, “it is very difficult to know that patients with tumors 1.1-2.0 cm in size are really benefiting from surgery, as suggested in the article,” he said.
Dr. Howe highlighted a recent interim analysis that indicated that active surveillance is the “preferred approach” for tumors no larger than 2 cm.
Dr. Schulick and the research team acknowledge limitations in their dataset, including the potential for coding errors and lack of information on the Ki-67 index, symptoms, incidental diagnosis, and recurrence.
Overall, though, the authors conclude that the findings “support the recommendations of the NCCN guidelines to resect small [nonfunctional pancreatic] NETs for selected patients” but need “to be further investigated to verify the results.”
The study was supported by a grant from the Japan Society for the Promotion of Science Overseas Challenge Program for Young Researchers and a grant from the Mochida Memorial Foundation for Medical and Pharmaceutical Research. Dr. Schulick is the inventor of a patent licensed to DynamiCure and has received laboratory equipment from Haemonetics outside the submitted work. Other authors also have relevant financial relationships.
A version of this article first appeared on Medscape.com.
Overall, researchers found that surgical resection was associated with a 42% improvement in overall survival among patients with small tumors of 1.1-2.0 cm, but not tumors 1 cm or smaller. Among those with 1.1- to 2.0-cm tumors, the survival benefit following surgery was most notable among patients aged 64 years or younger and those with no comorbidities.
The findings were published in JAMA Network Open.
While surgical resection has been the first-line treatment for patients with functional or symptomatic localized, low-grade pancreatic NETs, surgery for asymptomatic low-grade nonfunctional pancreatic NETs of 2 cm or less “remains unclear even in consensus guidelines,” study author Richard D. Schulick, MD, MBA, of the University of Colorado at Denver, Aurora, and colleagues write.
Consensus guidelines from the European Neuroendocrine Tumor Society, for instance, indicate surveillance for these smaller tumors, while those from the Japan Neuroendocrine Tumor Society recommend surgery. The National Comprehensive Cancer Network (NCCN), which recently updated its guidelines, suggests observation as an option for patients with tumors as large as 2.0 cm but who are strongly considering resection.
To determine whether surgical resection of these smaller lesions influences overall survival, the team combed the U.S. National Cancer Database and identified 4,641 patients with nonfunctional pancreatic NETs up to 2.0 cm in size.
Researchers divided patients by tumor sizes of up to 1 cm (group 1a) and 1.1-2.0 cm (group 1b) and examined a range of variables, including age, comorbidities, tumor location and differentiation, and overall survival.
Overall, 1,278 patients had tumors measuring up to 1.0 cm (group 1a), and 3,363 had tumors measuring 1.1-2.0 cm (group 1b). The mean age across both groups was 60.5 years; about half were men, and most (77.4%) were White.
Over a median follow-up of 47.1 months, the surgical resection rate was significantly lower among patients in group 1a (82.0%) than in group 1b (87.0%). Patients who underwent resection, on average, were younger and were more likely to have tumors located in the pancreas tail and to have clinical lymph node metastasis.
Overall, the team found that surgical resection was associated with longer overall survival for patients with tumors of 1.1-2.0 cm (hazard ratio, 0.58) but not 1 cm or smaller (HR, 0.68; P = .12).
Among patients in group 1b (those with 1.1- to 2.0-cm tumors), the team also found that age 64 years or younger (adjusted HR, 0.34), treatment at academic institutions (aHR, 0.40), absence of comorbidities (aHR, 0.53), absence of clinical lymph node metastasis (aHR, 0.54), as well as tumors in the body (aHR, 0.36) and tail (aHR, 0.37) of the pancreas were significantly associated with increased survival after surgical resection.
Among patients with resected small nonmetastatic nonfunctional pancreatic NETs, pathologic lymph node metastasis (HR, 1.28; P = .43) and lymphovascular invasion (HR, 0.85; P = .75) were not associated with overall survival.
The results of the study “support an association between surgical resection and increased survival in select patients” among those with tumors 1.1-2.0 cm, Dr. Schulick and colleagues write.
James R. Howe, MD, who was not involved in the research, highlighted that the study tries to answer an important clinical problem: What should we do with small, nonfunctional pancreatic NETs?
However, he noted “significant selection bias” among patients included in the dataset.
More than 80% of patients with tumors under 1 cm underwent surgery, which “is not consistent with what most people would do in practice,” said Dr. Howe, of the division of surgical oncology and endocrine surgery, University of Iowa Hospitals and Clinics, Iowa City. “Most would be observed and might not make it into the National Cancer Database.”
Dr. Howe pointed to an even larger group of patients with pancreatic NETs who were not included in the database – those with CT evidence of a pancreatic NET but without biopsy confirmation.
With many patients potentially missing from the data, “it is very difficult to know that patients with tumors 1.1-2.0 cm in size are really benefiting from surgery, as suggested in the article,” he said.
Dr. Howe highlighted a recent interim analysis that indicated that active surveillance is the “preferred approach” for tumors no larger than 2 cm.
Dr. Schulick and the research team acknowledge limitations in their dataset, including the potential for coding errors and lack of information on the Ki-67 index, symptoms, incidental diagnosis, and recurrence.
Overall, though, the authors conclude that the findings “support the recommendations of the NCCN guidelines to resect small [nonfunctional pancreatic] NETs for selected patients” but need “to be further investigated to verify the results.”
The study was supported by a grant from the Japan Society for the Promotion of Science Overseas Challenge Program for Young Researchers and a grant from the Mochida Memorial Foundation for Medical and Pharmaceutical Research. Dr. Schulick is the inventor of a patent licensed to DynamiCure and has received laboratory equipment from Haemonetics outside the submitted work. Other authors also have relevant financial relationships.
A version of this article first appeared on Medscape.com.
Overall, researchers found that surgical resection was associated with a 42% improvement in overall survival among patients with small tumors of 1.1-2.0 cm, but not tumors 1 cm or smaller. Among those with 1.1- to 2.0-cm tumors, the survival benefit following surgery was most notable among patients aged 64 years or younger and those with no comorbidities.
The findings were published in JAMA Network Open.
While surgical resection has been the first-line treatment for patients with functional or symptomatic localized, low-grade pancreatic NETs, surgery for asymptomatic low-grade nonfunctional pancreatic NETs of 2 cm or less “remains unclear even in consensus guidelines,” study author Richard D. Schulick, MD, MBA, of the University of Colorado at Denver, Aurora, and colleagues write.
Consensus guidelines from the European Neuroendocrine Tumor Society, for instance, indicate surveillance for these smaller tumors, while those from the Japan Neuroendocrine Tumor Society recommend surgery. The National Comprehensive Cancer Network (NCCN), which recently updated its guidelines, suggests observation as an option for patients with tumors as large as 2.0 cm but who are strongly considering resection.
To determine whether surgical resection of these smaller lesions influences overall survival, the team combed the U.S. National Cancer Database and identified 4,641 patients with nonfunctional pancreatic NETs up to 2.0 cm in size.
Researchers divided patients by tumor sizes of up to 1 cm (group 1a) and 1.1-2.0 cm (group 1b) and examined a range of variables, including age, comorbidities, tumor location and differentiation, and overall survival.
Overall, 1,278 patients had tumors measuring up to 1.0 cm (group 1a), and 3,363 had tumors measuring 1.1-2.0 cm (group 1b). The mean age across both groups was 60.5 years; about half were men, and most (77.4%) were White.
Over a median follow-up of 47.1 months, the surgical resection rate was significantly lower among patients in group 1a (82.0%) than in group 1b (87.0%). Patients who underwent resection, on average, were younger and were more likely to have tumors located in the pancreas tail and to have clinical lymph node metastasis.
Overall, the team found that surgical resection was associated with longer overall survival for patients with tumors of 1.1-2.0 cm (hazard ratio, 0.58) but not 1 cm or smaller (HR, 0.68; P = .12).
Among patients in group 1b (those with 1.1- to 2.0-cm tumors), the team also found that age 64 years or younger (adjusted HR, 0.34), treatment at academic institutions (aHR, 0.40), absence of comorbidities (aHR, 0.53), absence of clinical lymph node metastasis (aHR, 0.54), as well as tumors in the body (aHR, 0.36) and tail (aHR, 0.37) of the pancreas were significantly associated with increased survival after surgical resection.
Among patients with resected small nonmetastatic nonfunctional pancreatic NETs, pathologic lymph node metastasis (HR, 1.28; P = .43) and lymphovascular invasion (HR, 0.85; P = .75) were not associated with overall survival.
The results of the study “support an association between surgical resection and increased survival in select patients” among those with tumors 1.1-2.0 cm, Dr. Schulick and colleagues write.
James R. Howe, MD, who was not involved in the research, highlighted that the study tries to answer an important clinical problem: What should we do with small, nonfunctional pancreatic NETs?
However, he noted “significant selection bias” among patients included in the dataset.
More than 80% of patients with tumors under 1 cm underwent surgery, which “is not consistent with what most people would do in practice,” said Dr. Howe, of the division of surgical oncology and endocrine surgery, University of Iowa Hospitals and Clinics, Iowa City. “Most would be observed and might not make it into the National Cancer Database.”
Dr. Howe pointed to an even larger group of patients with pancreatic NETs who were not included in the database – those with CT evidence of a pancreatic NET but without biopsy confirmation.
With many patients potentially missing from the data, “it is very difficult to know that patients with tumors 1.1-2.0 cm in size are really benefiting from surgery, as suggested in the article,” he said.
Dr. Howe highlighted a recent interim analysis that indicated that active surveillance is the “preferred approach” for tumors no larger than 2 cm.
Dr. Schulick and the research team acknowledge limitations in their dataset, including the potential for coding errors and lack of information on the Ki-67 index, symptoms, incidental diagnosis, and recurrence.
Overall, though, the authors conclude that the findings “support the recommendations of the NCCN guidelines to resect small [nonfunctional pancreatic] NETs for selected patients” but need “to be further investigated to verify the results.”
The study was supported by a grant from the Japan Society for the Promotion of Science Overseas Challenge Program for Young Researchers and a grant from the Mochida Memorial Foundation for Medical and Pharmaceutical Research. Dr. Schulick is the inventor of a patent licensed to DynamiCure and has received laboratory equipment from Haemonetics outside the submitted work. Other authors also have relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN