User login
Assessment of IV Edaravone Use in the Management of Amyotrophic Lateral Sclerosis
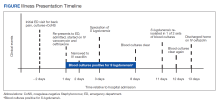
Amyotrophic lateral sclerosis (ALS) is an incurable neurodegenerative disorder that results in progressive deterioration of motor neurons in the ventral horn of the spinal cord, which results in loss of voluntary muscle movements.1 Eventually, typical daily tasks become difficult to perform, and as the disease progresses, the ability to eat and breathe is impaired.2 Reports from 2015 show the annual incidence of ALS is 5 cases per 100,000 people, with the total number of cases reported at more than 16,000 in the United States.3 In clinical practice, disease progression is routinely assessed by the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R). Typical decline is 1 point per month.4
Unfortunately, at this time, ALS care focuses on symptom management, including prevention of weight loss; implementation of communication strategies; and management of pain, constipation, excess secretions, cramping, and breathing. Despite copious research into treatment options, few exist. Riluzole is an oral medication administered twice daily and has been on the market since 1995.5-7 Efficacy was demonstrated in a study showing statistically significant survival at 12 months compared with controls (74% vs 58%, respectively; P = .014).6 Since its approval, riluzole has become part of standard-of-care ALS management.
In 2017, the US Food and Drug Administration (FDA) approved edaravone, an IV medication that was found to slow the progression of ALS in some patients.8-12 Oxidative stress caused by free radicals is hypothesized to increase the progression of ALS by motor neuron degradation.13 Edaravone works as a free radical and peroxynitrite scavenger and has been shown to eliminate lipid peroxides and hydroxyl radicals known to damage endothelial and neuronal cells.12
Given the mechanism of action of edaravone, it seemed to be a promising option to slow the progression of ALS. A 2019 systematic review analyzed 3 randomized studies with 367 patients and found a statistically significant difference in change in ALSFRS-R scores between patients treated with edaravone for 24 weeks compared with patients treated with the placebo (mean difference, 1.63; 95% CI, 0.26-3.00; P = .02).12 Secondary endpoints evaluated included percent forced vital capacity (%FVC), grip strength, and pinch strength: All showing no significant difference when comparing IV edaravone with placebo.
A 2022 postmarketing study of 324 patients with ALS evaluated the safety and efficacy of long-term edaravone treatment. IV edaravone therapy for > 24 weeks was well tolerated, although it was not associated with any disease-modifying benefit when comparing ALSFRS-R scores with patients not receiving edaravone over a median 13.9 months (ALSFRS-R points/month, -0.91 vs -0.85; P = .37).13 A third ALS treatment medication, sodium phenylbutyrate/taurursodiol was approved in 2022 but not available during our study period and not included here.14,15
Studies have shown an increased incidence of ALS in the veteran population. Veterans serving in the Gulf War were nearly twice as likely to develop ALS as those not serving in the Gulf.16 However, existing literature regarding the effectiveness of edaravone does not specifically examine the effect on this unique population. The objective of this study was to assess the effect of IV edaravone on ALS progression in veterans compared with veterans who received standard of care.
Methods
This study was conducted at a large, academic US Department of Veterans Affairs (VA) medical center. Patients with ALS are followed by a multidisciplinary clinic composed of a neurologist, pulmonologist, clinical pharmacist, social worker, speech therapist, physical therapist, occupational therapist, dietician, clinical psychologist, wheelchair clinic representative, and benefits representative. Patients are typically seen for a half-day appointment about every 3 months. During these visits, a comprehensive review of disease progression is performed. This review entails completion of the ALSFRS-R, physical examination, and pulmonary function testing. Speech intelligibility stage (SIS) is assessed by a speech therapist as well. SIS is scored from 1 (no detectable speech disorder) to 5 (no functional speech). All patients followed in this multidisciplinary ALS clinic receive standard-of-care treatment. This includes the discussion of treatment options that if appropriate are provided to help manage a wide range of complications associated with this disease (eg, pain, cramping, constipation, excessive secretions, weight loss, dysphagia). As a part of these personal discussions, treatment with riluzole is also offered as a standard-of-care pharmacologic option.
Study Design
This retrospective case-control study was conducted using electronic health record data to compare ALS progression in patients on IV edaravone therapy with standard of care. The Indiana University/Purdue University, Indianapolis Institutional Review Board and the VA Research and Development Committee approved the study. The control cohort received the standard of care. Patients in the case cohort received standard of care and edaravone 60 mg infusions daily for an initial cycle of 14 days on treatment, followed by 14 days off. All subsequent cycles were 10 of 14 days on treatment followed by 14 days off. The initial 2 doses were administered in the outpatient infusion clinic to monitor for a hypersensitivity reaction. Patients then had a peripherally inserted central catheter line placed and received doses on days 3 through 14 at home. A port was placed for subsequent cycles, which were also completed at home. Appropriateness of edaravone therapy was assessed by the neurologist at each follow-up appointment. Therapy was then discontinued if warranted based on disease progression or patient preference.
Study Population
Patients included were aged 18 to 75 years with diagnosed ALS. Patients with complications that might influence evaluation of medication efficacy (eg, Parkinson disease, schizophrenia, significant dementia, other major medical morbidity) were excluded. Patients were also excluded if they were on continuous bilevel positive airway pressure and/or had a total score of ≤ 3 points on ALSFRS-R items for dyspnea, orthopnea, or respiratory insufficiency. Due to our small sample size, patients were excluded if treatment was < 6 months, which is the gold standard of therapy duration established by clinical trials.9,11,12
The standard-of-care cohort included patients enrolled in the multidisciplinary clinic September 1, 2014 to August 31, 2017. These patients were compared in a 2:1 ratio with patients who received IV edaravone. The edaravone cohort included patients who initiated treatment with IV edaravone between September 1, 2017, and August 31, 2020. This date range prior to the approval of edaravone was chosen to compare patients at similar stages of disease progression and to have the largest sample size possible.
Data Collection
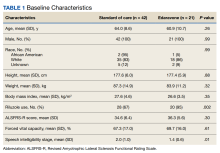
Data were obtained for eligible patients using the VA Computerized Patient Record System. Demographic data gathered for each patient included age, sex, weight, height, body mass index (BMI), race, and riluzole use.
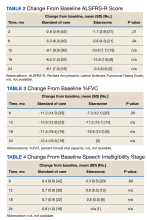
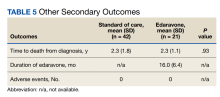
The primary endpoint was the change in ALSFRS-R score after 6 months of IV edaravone compared with standard-of-care ALS management. Secondary outcomes included change in ALSFRS-R scores 3, 12, 18, and 24 months after therapy initiation, change in %FVC and SIS 3, 6, 12, 18, and 24 months after therapy initiation, duration of edaravone completed (months), time to death (months), and adverse events.
Statistical Analysis
Comparisons between the edaravone and control groups for differences in patient characteristics were made using χ2 and 2-sample t tests for categorical and continuous variables, respectively. Comparisons between the 2 groups for differences in study outcomes (ALSFRS-R scores, %FVC, SIS) at each time point were evaluated using 2-sample t tests. Adverse events and adverse drug reactions were compared between groups using χ2 tests. Statistical significance was set at 0.05.
We estimated that a sample size of 21 subjects in the edaravone (case) group and 42 in the standard-of-care (control) group would be needed to achieve 80% power to detect a difference of 6.5 between the 2 groups for the change in ALSFRS-R scores. This 80% power was calculated based on a 2-sample t test, and assuming a 2-sided 5% significance level and a within-group SD of 8.5.9 Statistical analysis was conducted using Microsoft Excel.
Results
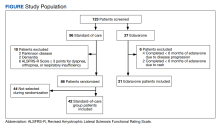
Of the 96 patients, 10 met exclusion criteria. From the remaining 86, 42 were randomly selected for the standard-of-care group. A total of 27 patients seen in multidisciplinary ALS clinic between September 1, 2017, and August 31, 2020, received at least 1 dose of IV edaravone. Of the 27 edaravone patients, 6 were excluded for not completing a total of 6 months of edaravone. Two of the 6 excluded developed a rash, which resolved within 1 week after discontinuing edaravone. The other 4 discontinued edaravone before 6 months because of disease progression.
Baseline Characteristics
Efficacy
Discussion
This 24-month, case-control retrospective study assessed efficacy and safety of IV edaravone for the management of ALS. Although the landmark edaravone study showed slowed progression of ALS at 6 and 12 months, the effectiveness of edaravone outside the clinical trial setting has been less compelling.9-11,13 A later study showed no difference in change in ALSFRS-R score at 6 months compared with that of the placebo group.7 In our study, no statistically significant difference was found for change in ALSFRS-R scores at 6 months.
Our study was unique given we evaluated a veteran population. The link between the military and ALS is largely unknown, although studies have shown increased incidence of ALS in people with a military history compared with that of the general population.16-18 Our study was also unique because it was single-centered in design and allowed for outcome assessments, including ALSFRS-R scores, SIS, and %FVC measurements, to all be conducted by the same practitioner to limit variability. Unfortunately, our sample size resulted in a cohort that was underpowered at 12, 18, and 24 months. In addition, there was a lack of data on chart review for SIS and %FVC measurements at 24 months. As ALS progresses toward end stage, SIS and %FVC measurements can become difficult and burdensome on the patient to obtain, and the ALS multidisciplinary team may decide not to gather these data points as ALS progresses. As a result, change in SIS and %FVC measurements were unable to be reported due to lack of gathering this information at the 24-month mark in the edaravone group. Due to the cost and administration burden associated with edaravone, it is important that assessment of disease progression is performed regularly to assess benefit and appropriateness of continued therapy. The oral formulation of edaravone was approved in 2022, shortly after the completion of data collection for this study.19,20 Although our study did not analyze oral edaravone, the administration burden of treatment would be reduced with the oral formulation, and we hypothesize there will be increased patient interest in ALS management with oral vs IV edaravone. Evaluation of long-term treatment for efficacy and safety beyond 24 months has not been evaluated. Future studies should continue to evaluate edaravone use in a larger veteran population.
Limitations
One limitation for our study alluded to earlier in the discussion was sample size. Although this study met power at the 6-month mark, it was limited by the number of patients who received more than 6 months of edaravone (n = 21). As a result, statistical analyses between treatment groups were underpowered at 12, 18, and 24 months. Our study had 80% power to detect a difference of 6.5 between the groups for the change in ALSFRS-R scores. Previous studies detected a statistically significant difference in ALSFRS-R scores, with a difference in ALSFRS-R scores of 2.49 between groups.8 Future studies should evaluate a larger sample size of patients who are prescribed edaravone.
Another limitation was that the edaravone and standard-of-care group data were gathered from different time periods. Two different time frames were selected to increase sample size by gathering data over a longer period and to account for patients who may have qualified for IV edaravone but could not receive it as it was not yet available on the market. There were no known changes to the standard of care between the time periods that would affect results. As noted previously, the standard-of-care group had fewer patients taking riluzole compared with the edaravone group, which may have confounded our results. We concluded patients opting for edaravone were more likely to trial riluzole, taken by mouth twice daily, before starting edaravone, a once-daily IV infusion.
Conclusions
No difference in the rate of ALS progression was noted between patients who received IV edaravone vs standard of care at 6 months. In addition, no difference was noted in other objective measures of disease progression, including %FVC, SIS, and time to death. As a result, the decision to initiate and continue edaravone therapy should be made on an individualized basis according to a prescriber’s clinical judgment and a patient’s goals. Edaravone therapy should be discontinued when disease progression occurs or when medication administration becomes a burden.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at Veteran Health Indiana.
1. Kiernan MC, Vucic S, Cheah BC, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377(9769):942-955. doi:10.1016/S0140-6736(10)61156-7
2. Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344(22):1688-1700. doi:0.1056/NEJM200105313442207
3. Mehta P, Kaye W, Raymond J, et al. Prevalence of amyotrophic lateral sclerosis–United States, 2015. MMWR Morb Mortal Wkly Rep. 2018;67(46):1285-1289. doi:10.15585/mmwr.mm6746a1
4. Castrillo-Viguera C, Grasso DL, Simpson E, Shefner J, Cudkowicz ME. Clinical significance in the change of decline in ALSFRS-R. Amyotroph Lateral Scler. 2010;11(1-2):178-180. doi:10.3109/17482960903093710
5. Rilutek. Package insert. Covis Pharmaceuticals; 1995.
6. Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330(9):585-591. doi:10.1056/NEJM199403033300901
7. Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet. 1996;347(9013):1425-1431. doi:10.1016/s0140-6736(96)91680-3
8. Radicava. Package insert. MT Pharma America Inc; 2017.
9. Abe K, Itoyama Y, Sobue G, et al. Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(7-8):610-617. doi:10.3109/21678421.2014.959024
10. Writing Group; Edaravone (MCI-186) ALS 19 Study Group. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16(7):505-512. doi:10.1016/S1474-4422(17)30115-1
11. Writing Group; Edaravone (MCI-186) ALS 19 Study Group. Exploratory double-blind, parallel-group, placebo-controlled study of edaravone (MCI-186) in amyotrophic lateral sclerosis (Japan ALS severity classification: Grade 3, requiring assistance for eating, excretion or ambulation). Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(suppl 1):40-48. doi:10.1080/21678421.2017.1361441
12. Luo L, Song Z, Li X, et al. Efficacy and safety of edaravone in treatment of amyotrophic lateral sclerosis–a systematic review and meta-analysis. Neurol Sci. 2019;40(2):235-241. doi:10.1007/s10072-018-3653-2
13. Witzel S, Maier A, Steinbach R, et al; German Motor Neuron Disease Network (MND-NET). Safety and effectiveness of long-term intravenous administration of edaravone for treatment of patients with amyotrophic lateral sclerosis. JAMA Neurol. 2022;79(2):121-130. doi:10.1001/jamaneurol.2021.4893
14. Paganoni S, Macklin EA, Hendrix S, et al. Trial of sodium phenylbutyrate-taurursodiol for amyotrophic lateral sclerosis. N Engl J Med. 2020;383(10):919-930. doi:10.1056/NEJMoa1916945
15. Relyvrio. Package insert. Amylyx Pharmaceuticals Inc; 2022.
16. McKay KA, Smith KA, Smertinaite L, Fang F, Ingre C, Taube F. Military service and related risk factors for amyotrophic lateral sclerosis. Acta Neurol Scand. 2021;143(1):39-50. doi:10.1111/ane.13345
17. Watanabe K, Tanaka M, Yuki S, Hirai M, Yamamoto Y. How is edaravone effective against acute ischemic stroke and amyotrophic lateral sclerosis? J Clin Biochem Nutr. 2018;62(1):20-38. doi:10.3164/jcbn.17-62
18. Horner RD, Kamins KG, Feussner JR, et al. Occurrence of amyotrophic lateral sclerosis among Gulf War veterans. Neurology. 2003;61(6):742-749. doi:10.1212/01.wnl.0000069922.32557.ca
19. Radicava ORS. Package insert. Mitsubishi Tanabe Pharma America Inc; 2022.
20. Shimizu H, Nishimura Y, Shiide Y, et al. Bioequivalence study of oral suspension and intravenous formulation of edaravone in healthy adult subjects. Clin Pharmacol Drug Dev. 2021;10(10):1188-1197. doi:10.1002/cpdd.952
Amyotrophic lateral sclerosis (ALS) is an incurable neurodegenerative disorder that results in progressive deterioration of motor neurons in the ventral horn of the spinal cord, which results in loss of voluntary muscle movements.1 Eventually, typical daily tasks become difficult to perform, and as the disease progresses, the ability to eat and breathe is impaired.2 Reports from 2015 show the annual incidence of ALS is 5 cases per 100,000 people, with the total number of cases reported at more than 16,000 in the United States.3 In clinical practice, disease progression is routinely assessed by the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R). Typical decline is 1 point per month.4
Unfortunately, at this time, ALS care focuses on symptom management, including prevention of weight loss; implementation of communication strategies; and management of pain, constipation, excess secretions, cramping, and breathing. Despite copious research into treatment options, few exist. Riluzole is an oral medication administered twice daily and has been on the market since 1995.5-7 Efficacy was demonstrated in a study showing statistically significant survival at 12 months compared with controls (74% vs 58%, respectively; P = .014).6 Since its approval, riluzole has become part of standard-of-care ALS management.
In 2017, the US Food and Drug Administration (FDA) approved edaravone, an IV medication that was found to slow the progression of ALS in some patients.8-12 Oxidative stress caused by free radicals is hypothesized to increase the progression of ALS by motor neuron degradation.13 Edaravone works as a free radical and peroxynitrite scavenger and has been shown to eliminate lipid peroxides and hydroxyl radicals known to damage endothelial and neuronal cells.12
Given the mechanism of action of edaravone, it seemed to be a promising option to slow the progression of ALS. A 2019 systematic review analyzed 3 randomized studies with 367 patients and found a statistically significant difference in change in ALSFRS-R scores between patients treated with edaravone for 24 weeks compared with patients treated with the placebo (mean difference, 1.63; 95% CI, 0.26-3.00; P = .02).12 Secondary endpoints evaluated included percent forced vital capacity (%FVC), grip strength, and pinch strength: All showing no significant difference when comparing IV edaravone with placebo.
A 2022 postmarketing study of 324 patients with ALS evaluated the safety and efficacy of long-term edaravone treatment. IV edaravone therapy for > 24 weeks was well tolerated, although it was not associated with any disease-modifying benefit when comparing ALSFRS-R scores with patients not receiving edaravone over a median 13.9 months (ALSFRS-R points/month, -0.91 vs -0.85; P = .37).13 A third ALS treatment medication, sodium phenylbutyrate/taurursodiol was approved in 2022 but not available during our study period and not included here.14,15
Studies have shown an increased incidence of ALS in the veteran population. Veterans serving in the Gulf War were nearly twice as likely to develop ALS as those not serving in the Gulf.16 However, existing literature regarding the effectiveness of edaravone does not specifically examine the effect on this unique population. The objective of this study was to assess the effect of IV edaravone on ALS progression in veterans compared with veterans who received standard of care.
Methods
This study was conducted at a large, academic US Department of Veterans Affairs (VA) medical center. Patients with ALS are followed by a multidisciplinary clinic composed of a neurologist, pulmonologist, clinical pharmacist, social worker, speech therapist, physical therapist, occupational therapist, dietician, clinical psychologist, wheelchair clinic representative, and benefits representative. Patients are typically seen for a half-day appointment about every 3 months. During these visits, a comprehensive review of disease progression is performed. This review entails completion of the ALSFRS-R, physical examination, and pulmonary function testing. Speech intelligibility stage (SIS) is assessed by a speech therapist as well. SIS is scored from 1 (no detectable speech disorder) to 5 (no functional speech). All patients followed in this multidisciplinary ALS clinic receive standard-of-care treatment. This includes the discussion of treatment options that if appropriate are provided to help manage a wide range of complications associated with this disease (eg, pain, cramping, constipation, excessive secretions, weight loss, dysphagia). As a part of these personal discussions, treatment with riluzole is also offered as a standard-of-care pharmacologic option.
Study Design
This retrospective case-control study was conducted using electronic health record data to compare ALS progression in patients on IV edaravone therapy with standard of care. The Indiana University/Purdue University, Indianapolis Institutional Review Board and the VA Research and Development Committee approved the study. The control cohort received the standard of care. Patients in the case cohort received standard of care and edaravone 60 mg infusions daily for an initial cycle of 14 days on treatment, followed by 14 days off. All subsequent cycles were 10 of 14 days on treatment followed by 14 days off. The initial 2 doses were administered in the outpatient infusion clinic to monitor for a hypersensitivity reaction. Patients then had a peripherally inserted central catheter line placed and received doses on days 3 through 14 at home. A port was placed for subsequent cycles, which were also completed at home. Appropriateness of edaravone therapy was assessed by the neurologist at each follow-up appointment. Therapy was then discontinued if warranted based on disease progression or patient preference.
Study Population
Patients included were aged 18 to 75 years with diagnosed ALS. Patients with complications that might influence evaluation of medication efficacy (eg, Parkinson disease, schizophrenia, significant dementia, other major medical morbidity) were excluded. Patients were also excluded if they were on continuous bilevel positive airway pressure and/or had a total score of ≤ 3 points on ALSFRS-R items for dyspnea, orthopnea, or respiratory insufficiency. Due to our small sample size, patients were excluded if treatment was < 6 months, which is the gold standard of therapy duration established by clinical trials.9,11,12
The standard-of-care cohort included patients enrolled in the multidisciplinary clinic September 1, 2014 to August 31, 2017. These patients were compared in a 2:1 ratio with patients who received IV edaravone. The edaravone cohort included patients who initiated treatment with IV edaravone between September 1, 2017, and August 31, 2020. This date range prior to the approval of edaravone was chosen to compare patients at similar stages of disease progression and to have the largest sample size possible.
Data Collection
Data were obtained for eligible patients using the VA Computerized Patient Record System. Demographic data gathered for each patient included age, sex, weight, height, body mass index (BMI), race, and riluzole use.
The primary endpoint was the change in ALSFRS-R score after 6 months of IV edaravone compared with standard-of-care ALS management. Secondary outcomes included change in ALSFRS-R scores 3, 12, 18, and 24 months after therapy initiation, change in %FVC and SIS 3, 6, 12, 18, and 24 months after therapy initiation, duration of edaravone completed (months), time to death (months), and adverse events.
Statistical Analysis
Comparisons between the edaravone and control groups for differences in patient characteristics were made using χ2 and 2-sample t tests for categorical and continuous variables, respectively. Comparisons between the 2 groups for differences in study outcomes (ALSFRS-R scores, %FVC, SIS) at each time point were evaluated using 2-sample t tests. Adverse events and adverse drug reactions were compared between groups using χ2 tests. Statistical significance was set at 0.05.
We estimated that a sample size of 21 subjects in the edaravone (case) group and 42 in the standard-of-care (control) group would be needed to achieve 80% power to detect a difference of 6.5 between the 2 groups for the change in ALSFRS-R scores. This 80% power was calculated based on a 2-sample t test, and assuming a 2-sided 5% significance level and a within-group SD of 8.5.9 Statistical analysis was conducted using Microsoft Excel.
Results
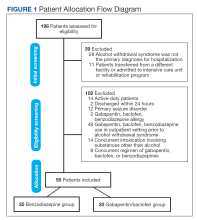
Of the 96 patients, 10 met exclusion criteria. From the remaining 86, 42 were randomly selected for the standard-of-care group. A total of 27 patients seen in multidisciplinary ALS clinic between September 1, 2017, and August 31, 2020, received at least 1 dose of IV edaravone. Of the 27 edaravone patients, 6 were excluded for not completing a total of 6 months of edaravone. Two of the 6 excluded developed a rash, which resolved within 1 week after discontinuing edaravone. The other 4 discontinued edaravone before 6 months because of disease progression.
Baseline Characteristics
Efficacy
Discussion
This 24-month, case-control retrospective study assessed efficacy and safety of IV edaravone for the management of ALS. Although the landmark edaravone study showed slowed progression of ALS at 6 and 12 months, the effectiveness of edaravone outside the clinical trial setting has been less compelling.9-11,13 A later study showed no difference in change in ALSFRS-R score at 6 months compared with that of the placebo group.7 In our study, no statistically significant difference was found for change in ALSFRS-R scores at 6 months.
Our study was unique given we evaluated a veteran population. The link between the military and ALS is largely unknown, although studies have shown increased incidence of ALS in people with a military history compared with that of the general population.16-18 Our study was also unique because it was single-centered in design and allowed for outcome assessments, including ALSFRS-R scores, SIS, and %FVC measurements, to all be conducted by the same practitioner to limit variability. Unfortunately, our sample size resulted in a cohort that was underpowered at 12, 18, and 24 months. In addition, there was a lack of data on chart review for SIS and %FVC measurements at 24 months. As ALS progresses toward end stage, SIS and %FVC measurements can become difficult and burdensome on the patient to obtain, and the ALS multidisciplinary team may decide not to gather these data points as ALS progresses. As a result, change in SIS and %FVC measurements were unable to be reported due to lack of gathering this information at the 24-month mark in the edaravone group. Due to the cost and administration burden associated with edaravone, it is important that assessment of disease progression is performed regularly to assess benefit and appropriateness of continued therapy. The oral formulation of edaravone was approved in 2022, shortly after the completion of data collection for this study.19,20 Although our study did not analyze oral edaravone, the administration burden of treatment would be reduced with the oral formulation, and we hypothesize there will be increased patient interest in ALS management with oral vs IV edaravone. Evaluation of long-term treatment for efficacy and safety beyond 24 months has not been evaluated. Future studies should continue to evaluate edaravone use in a larger veteran population.
Limitations
One limitation for our study alluded to earlier in the discussion was sample size. Although this study met power at the 6-month mark, it was limited by the number of patients who received more than 6 months of edaravone (n = 21). As a result, statistical analyses between treatment groups were underpowered at 12, 18, and 24 months. Our study had 80% power to detect a difference of 6.5 between the groups for the change in ALSFRS-R scores. Previous studies detected a statistically significant difference in ALSFRS-R scores, with a difference in ALSFRS-R scores of 2.49 between groups.8 Future studies should evaluate a larger sample size of patients who are prescribed edaravone.
Another limitation was that the edaravone and standard-of-care group data were gathered from different time periods. Two different time frames were selected to increase sample size by gathering data over a longer period and to account for patients who may have qualified for IV edaravone but could not receive it as it was not yet available on the market. There were no known changes to the standard of care between the time periods that would affect results. As noted previously, the standard-of-care group had fewer patients taking riluzole compared with the edaravone group, which may have confounded our results. We concluded patients opting for edaravone were more likely to trial riluzole, taken by mouth twice daily, before starting edaravone, a once-daily IV infusion.
Conclusions
No difference in the rate of ALS progression was noted between patients who received IV edaravone vs standard of care at 6 months. In addition, no difference was noted in other objective measures of disease progression, including %FVC, SIS, and time to death. As a result, the decision to initiate and continue edaravone therapy should be made on an individualized basis according to a prescriber’s clinical judgment and a patient’s goals. Edaravone therapy should be discontinued when disease progression occurs or when medication administration becomes a burden.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at Veteran Health Indiana.
Amyotrophic lateral sclerosis (ALS) is an incurable neurodegenerative disorder that results in progressive deterioration of motor neurons in the ventral horn of the spinal cord, which results in loss of voluntary muscle movements.1 Eventually, typical daily tasks become difficult to perform, and as the disease progresses, the ability to eat and breathe is impaired.2 Reports from 2015 show the annual incidence of ALS is 5 cases per 100,000 people, with the total number of cases reported at more than 16,000 in the United States.3 In clinical practice, disease progression is routinely assessed by the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R). Typical decline is 1 point per month.4
Unfortunately, at this time, ALS care focuses on symptom management, including prevention of weight loss; implementation of communication strategies; and management of pain, constipation, excess secretions, cramping, and breathing. Despite copious research into treatment options, few exist. Riluzole is an oral medication administered twice daily and has been on the market since 1995.5-7 Efficacy was demonstrated in a study showing statistically significant survival at 12 months compared with controls (74% vs 58%, respectively; P = .014).6 Since its approval, riluzole has become part of standard-of-care ALS management.
In 2017, the US Food and Drug Administration (FDA) approved edaravone, an IV medication that was found to slow the progression of ALS in some patients.8-12 Oxidative stress caused by free radicals is hypothesized to increase the progression of ALS by motor neuron degradation.13 Edaravone works as a free radical and peroxynitrite scavenger and has been shown to eliminate lipid peroxides and hydroxyl radicals known to damage endothelial and neuronal cells.12
Given the mechanism of action of edaravone, it seemed to be a promising option to slow the progression of ALS. A 2019 systematic review analyzed 3 randomized studies with 367 patients and found a statistically significant difference in change in ALSFRS-R scores between patients treated with edaravone for 24 weeks compared with patients treated with the placebo (mean difference, 1.63; 95% CI, 0.26-3.00; P = .02).12 Secondary endpoints evaluated included percent forced vital capacity (%FVC), grip strength, and pinch strength: All showing no significant difference when comparing IV edaravone with placebo.
A 2022 postmarketing study of 324 patients with ALS evaluated the safety and efficacy of long-term edaravone treatment. IV edaravone therapy for > 24 weeks was well tolerated, although it was not associated with any disease-modifying benefit when comparing ALSFRS-R scores with patients not receiving edaravone over a median 13.9 months (ALSFRS-R points/month, -0.91 vs -0.85; P = .37).13 A third ALS treatment medication, sodium phenylbutyrate/taurursodiol was approved in 2022 but not available during our study period and not included here.14,15
Studies have shown an increased incidence of ALS in the veteran population. Veterans serving in the Gulf War were nearly twice as likely to develop ALS as those not serving in the Gulf.16 However, existing literature regarding the effectiveness of edaravone does not specifically examine the effect on this unique population. The objective of this study was to assess the effect of IV edaravone on ALS progression in veterans compared with veterans who received standard of care.
Methods
This study was conducted at a large, academic US Department of Veterans Affairs (VA) medical center. Patients with ALS are followed by a multidisciplinary clinic composed of a neurologist, pulmonologist, clinical pharmacist, social worker, speech therapist, physical therapist, occupational therapist, dietician, clinical psychologist, wheelchair clinic representative, and benefits representative. Patients are typically seen for a half-day appointment about every 3 months. During these visits, a comprehensive review of disease progression is performed. This review entails completion of the ALSFRS-R, physical examination, and pulmonary function testing. Speech intelligibility stage (SIS) is assessed by a speech therapist as well. SIS is scored from 1 (no detectable speech disorder) to 5 (no functional speech). All patients followed in this multidisciplinary ALS clinic receive standard-of-care treatment. This includes the discussion of treatment options that if appropriate are provided to help manage a wide range of complications associated with this disease (eg, pain, cramping, constipation, excessive secretions, weight loss, dysphagia). As a part of these personal discussions, treatment with riluzole is also offered as a standard-of-care pharmacologic option.
Study Design
This retrospective case-control study was conducted using electronic health record data to compare ALS progression in patients on IV edaravone therapy with standard of care. The Indiana University/Purdue University, Indianapolis Institutional Review Board and the VA Research and Development Committee approved the study. The control cohort received the standard of care. Patients in the case cohort received standard of care and edaravone 60 mg infusions daily for an initial cycle of 14 days on treatment, followed by 14 days off. All subsequent cycles were 10 of 14 days on treatment followed by 14 days off. The initial 2 doses were administered in the outpatient infusion clinic to monitor for a hypersensitivity reaction. Patients then had a peripherally inserted central catheter line placed and received doses on days 3 through 14 at home. A port was placed for subsequent cycles, which were also completed at home. Appropriateness of edaravone therapy was assessed by the neurologist at each follow-up appointment. Therapy was then discontinued if warranted based on disease progression or patient preference.
Study Population
Patients included were aged 18 to 75 years with diagnosed ALS. Patients with complications that might influence evaluation of medication efficacy (eg, Parkinson disease, schizophrenia, significant dementia, other major medical morbidity) were excluded. Patients were also excluded if they were on continuous bilevel positive airway pressure and/or had a total score of ≤ 3 points on ALSFRS-R items for dyspnea, orthopnea, or respiratory insufficiency. Due to our small sample size, patients were excluded if treatment was < 6 months, which is the gold standard of therapy duration established by clinical trials.9,11,12
The standard-of-care cohort included patients enrolled in the multidisciplinary clinic September 1, 2014 to August 31, 2017. These patients were compared in a 2:1 ratio with patients who received IV edaravone. The edaravone cohort included patients who initiated treatment with IV edaravone between September 1, 2017, and August 31, 2020. This date range prior to the approval of edaravone was chosen to compare patients at similar stages of disease progression and to have the largest sample size possible.
Data Collection
Data were obtained for eligible patients using the VA Computerized Patient Record System. Demographic data gathered for each patient included age, sex, weight, height, body mass index (BMI), race, and riluzole use.
The primary endpoint was the change in ALSFRS-R score after 6 months of IV edaravone compared with standard-of-care ALS management. Secondary outcomes included change in ALSFRS-R scores 3, 12, 18, and 24 months after therapy initiation, change in %FVC and SIS 3, 6, 12, 18, and 24 months after therapy initiation, duration of edaravone completed (months), time to death (months), and adverse events.
Statistical Analysis
Comparisons between the edaravone and control groups for differences in patient characteristics were made using χ2 and 2-sample t tests for categorical and continuous variables, respectively. Comparisons between the 2 groups for differences in study outcomes (ALSFRS-R scores, %FVC, SIS) at each time point were evaluated using 2-sample t tests. Adverse events and adverse drug reactions were compared between groups using χ2 tests. Statistical significance was set at 0.05.
We estimated that a sample size of 21 subjects in the edaravone (case) group and 42 in the standard-of-care (control) group would be needed to achieve 80% power to detect a difference of 6.5 between the 2 groups for the change in ALSFRS-R scores. This 80% power was calculated based on a 2-sample t test, and assuming a 2-sided 5% significance level and a within-group SD of 8.5.9 Statistical analysis was conducted using Microsoft Excel.
Results
Of the 96 patients, 10 met exclusion criteria. From the remaining 86, 42 were randomly selected for the standard-of-care group. A total of 27 patients seen in multidisciplinary ALS clinic between September 1, 2017, and August 31, 2020, received at least 1 dose of IV edaravone. Of the 27 edaravone patients, 6 were excluded for not completing a total of 6 months of edaravone. Two of the 6 excluded developed a rash, which resolved within 1 week after discontinuing edaravone. The other 4 discontinued edaravone before 6 months because of disease progression.
Baseline Characteristics
Efficacy
Discussion
This 24-month, case-control retrospective study assessed efficacy and safety of IV edaravone for the management of ALS. Although the landmark edaravone study showed slowed progression of ALS at 6 and 12 months, the effectiveness of edaravone outside the clinical trial setting has been less compelling.9-11,13 A later study showed no difference in change in ALSFRS-R score at 6 months compared with that of the placebo group.7 In our study, no statistically significant difference was found for change in ALSFRS-R scores at 6 months.
Our study was unique given we evaluated a veteran population. The link between the military and ALS is largely unknown, although studies have shown increased incidence of ALS in people with a military history compared with that of the general population.16-18 Our study was also unique because it was single-centered in design and allowed for outcome assessments, including ALSFRS-R scores, SIS, and %FVC measurements, to all be conducted by the same practitioner to limit variability. Unfortunately, our sample size resulted in a cohort that was underpowered at 12, 18, and 24 months. In addition, there was a lack of data on chart review for SIS and %FVC measurements at 24 months. As ALS progresses toward end stage, SIS and %FVC measurements can become difficult and burdensome on the patient to obtain, and the ALS multidisciplinary team may decide not to gather these data points as ALS progresses. As a result, change in SIS and %FVC measurements were unable to be reported due to lack of gathering this information at the 24-month mark in the edaravone group. Due to the cost and administration burden associated with edaravone, it is important that assessment of disease progression is performed regularly to assess benefit and appropriateness of continued therapy. The oral formulation of edaravone was approved in 2022, shortly after the completion of data collection for this study.19,20 Although our study did not analyze oral edaravone, the administration burden of treatment would be reduced with the oral formulation, and we hypothesize there will be increased patient interest in ALS management with oral vs IV edaravone. Evaluation of long-term treatment for efficacy and safety beyond 24 months has not been evaluated. Future studies should continue to evaluate edaravone use in a larger veteran population.
Limitations
One limitation for our study alluded to earlier in the discussion was sample size. Although this study met power at the 6-month mark, it was limited by the number of patients who received more than 6 months of edaravone (n = 21). As a result, statistical analyses between treatment groups were underpowered at 12, 18, and 24 months. Our study had 80% power to detect a difference of 6.5 between the groups for the change in ALSFRS-R scores. Previous studies detected a statistically significant difference in ALSFRS-R scores, with a difference in ALSFRS-R scores of 2.49 between groups.8 Future studies should evaluate a larger sample size of patients who are prescribed edaravone.
Another limitation was that the edaravone and standard-of-care group data were gathered from different time periods. Two different time frames were selected to increase sample size by gathering data over a longer period and to account for patients who may have qualified for IV edaravone but could not receive it as it was not yet available on the market. There were no known changes to the standard of care between the time periods that would affect results. As noted previously, the standard-of-care group had fewer patients taking riluzole compared with the edaravone group, which may have confounded our results. We concluded patients opting for edaravone were more likely to trial riluzole, taken by mouth twice daily, before starting edaravone, a once-daily IV infusion.
Conclusions
No difference in the rate of ALS progression was noted between patients who received IV edaravone vs standard of care at 6 months. In addition, no difference was noted in other objective measures of disease progression, including %FVC, SIS, and time to death. As a result, the decision to initiate and continue edaravone therapy should be made on an individualized basis according to a prescriber’s clinical judgment and a patient’s goals. Edaravone therapy should be discontinued when disease progression occurs or when medication administration becomes a burden.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at Veteran Health Indiana.
1. Kiernan MC, Vucic S, Cheah BC, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377(9769):942-955. doi:10.1016/S0140-6736(10)61156-7
2. Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344(22):1688-1700. doi:0.1056/NEJM200105313442207
3. Mehta P, Kaye W, Raymond J, et al. Prevalence of amyotrophic lateral sclerosis–United States, 2015. MMWR Morb Mortal Wkly Rep. 2018;67(46):1285-1289. doi:10.15585/mmwr.mm6746a1
4. Castrillo-Viguera C, Grasso DL, Simpson E, Shefner J, Cudkowicz ME. Clinical significance in the change of decline in ALSFRS-R. Amyotroph Lateral Scler. 2010;11(1-2):178-180. doi:10.3109/17482960903093710
5. Rilutek. Package insert. Covis Pharmaceuticals; 1995.
6. Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330(9):585-591. doi:10.1056/NEJM199403033300901
7. Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet. 1996;347(9013):1425-1431. doi:10.1016/s0140-6736(96)91680-3
8. Radicava. Package insert. MT Pharma America Inc; 2017.
9. Abe K, Itoyama Y, Sobue G, et al. Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(7-8):610-617. doi:10.3109/21678421.2014.959024
10. Writing Group; Edaravone (MCI-186) ALS 19 Study Group. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16(7):505-512. doi:10.1016/S1474-4422(17)30115-1
11. Writing Group; Edaravone (MCI-186) ALS 19 Study Group. Exploratory double-blind, parallel-group, placebo-controlled study of edaravone (MCI-186) in amyotrophic lateral sclerosis (Japan ALS severity classification: Grade 3, requiring assistance for eating, excretion or ambulation). Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(suppl 1):40-48. doi:10.1080/21678421.2017.1361441
12. Luo L, Song Z, Li X, et al. Efficacy and safety of edaravone in treatment of amyotrophic lateral sclerosis–a systematic review and meta-analysis. Neurol Sci. 2019;40(2):235-241. doi:10.1007/s10072-018-3653-2
13. Witzel S, Maier A, Steinbach R, et al; German Motor Neuron Disease Network (MND-NET). Safety and effectiveness of long-term intravenous administration of edaravone for treatment of patients with amyotrophic lateral sclerosis. JAMA Neurol. 2022;79(2):121-130. doi:10.1001/jamaneurol.2021.4893
14. Paganoni S, Macklin EA, Hendrix S, et al. Trial of sodium phenylbutyrate-taurursodiol for amyotrophic lateral sclerosis. N Engl J Med. 2020;383(10):919-930. doi:10.1056/NEJMoa1916945
15. Relyvrio. Package insert. Amylyx Pharmaceuticals Inc; 2022.
16. McKay KA, Smith KA, Smertinaite L, Fang F, Ingre C, Taube F. Military service and related risk factors for amyotrophic lateral sclerosis. Acta Neurol Scand. 2021;143(1):39-50. doi:10.1111/ane.13345
17. Watanabe K, Tanaka M, Yuki S, Hirai M, Yamamoto Y. How is edaravone effective against acute ischemic stroke and amyotrophic lateral sclerosis? J Clin Biochem Nutr. 2018;62(1):20-38. doi:10.3164/jcbn.17-62
18. Horner RD, Kamins KG, Feussner JR, et al. Occurrence of amyotrophic lateral sclerosis among Gulf War veterans. Neurology. 2003;61(6):742-749. doi:10.1212/01.wnl.0000069922.32557.ca
19. Radicava ORS. Package insert. Mitsubishi Tanabe Pharma America Inc; 2022.
20. Shimizu H, Nishimura Y, Shiide Y, et al. Bioequivalence study of oral suspension and intravenous formulation of edaravone in healthy adult subjects. Clin Pharmacol Drug Dev. 2021;10(10):1188-1197. doi:10.1002/cpdd.952
1. Kiernan MC, Vucic S, Cheah BC, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377(9769):942-955. doi:10.1016/S0140-6736(10)61156-7
2. Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344(22):1688-1700. doi:0.1056/NEJM200105313442207
3. Mehta P, Kaye W, Raymond J, et al. Prevalence of amyotrophic lateral sclerosis–United States, 2015. MMWR Morb Mortal Wkly Rep. 2018;67(46):1285-1289. doi:10.15585/mmwr.mm6746a1
4. Castrillo-Viguera C, Grasso DL, Simpson E, Shefner J, Cudkowicz ME. Clinical significance in the change of decline in ALSFRS-R. Amyotroph Lateral Scler. 2010;11(1-2):178-180. doi:10.3109/17482960903093710
5. Rilutek. Package insert. Covis Pharmaceuticals; 1995.
6. Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330(9):585-591. doi:10.1056/NEJM199403033300901
7. Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet. 1996;347(9013):1425-1431. doi:10.1016/s0140-6736(96)91680-3
8. Radicava. Package insert. MT Pharma America Inc; 2017.
9. Abe K, Itoyama Y, Sobue G, et al. Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(7-8):610-617. doi:10.3109/21678421.2014.959024
10. Writing Group; Edaravone (MCI-186) ALS 19 Study Group. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16(7):505-512. doi:10.1016/S1474-4422(17)30115-1
11. Writing Group; Edaravone (MCI-186) ALS 19 Study Group. Exploratory double-blind, parallel-group, placebo-controlled study of edaravone (MCI-186) in amyotrophic lateral sclerosis (Japan ALS severity classification: Grade 3, requiring assistance for eating, excretion or ambulation). Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(suppl 1):40-48. doi:10.1080/21678421.2017.1361441
12. Luo L, Song Z, Li X, et al. Efficacy and safety of edaravone in treatment of amyotrophic lateral sclerosis–a systematic review and meta-analysis. Neurol Sci. 2019;40(2):235-241. doi:10.1007/s10072-018-3653-2
13. Witzel S, Maier A, Steinbach R, et al; German Motor Neuron Disease Network (MND-NET). Safety and effectiveness of long-term intravenous administration of edaravone for treatment of patients with amyotrophic lateral sclerosis. JAMA Neurol. 2022;79(2):121-130. doi:10.1001/jamaneurol.2021.4893
14. Paganoni S, Macklin EA, Hendrix S, et al. Trial of sodium phenylbutyrate-taurursodiol for amyotrophic lateral sclerosis. N Engl J Med. 2020;383(10):919-930. doi:10.1056/NEJMoa1916945
15. Relyvrio. Package insert. Amylyx Pharmaceuticals Inc; 2022.
16. McKay KA, Smith KA, Smertinaite L, Fang F, Ingre C, Taube F. Military service and related risk factors for amyotrophic lateral sclerosis. Acta Neurol Scand. 2021;143(1):39-50. doi:10.1111/ane.13345
17. Watanabe K, Tanaka M, Yuki S, Hirai M, Yamamoto Y. How is edaravone effective against acute ischemic stroke and amyotrophic lateral sclerosis? J Clin Biochem Nutr. 2018;62(1):20-38. doi:10.3164/jcbn.17-62
18. Horner RD, Kamins KG, Feussner JR, et al. Occurrence of amyotrophic lateral sclerosis among Gulf War veterans. Neurology. 2003;61(6):742-749. doi:10.1212/01.wnl.0000069922.32557.ca
19. Radicava ORS. Package insert. Mitsubishi Tanabe Pharma America Inc; 2022.
20. Shimizu H, Nishimura Y, Shiide Y, et al. Bioequivalence study of oral suspension and intravenous formulation of edaravone in healthy adult subjects. Clin Pharmacol Drug Dev. 2021;10(10):1188-1197. doi:10.1002/cpdd.952
Acute Painful Horner Syndrome as the First Presenting Sign of Carotid Artery Dissection
Horner syndrome is a rare condition that has no sex or race predilection and is characterized by the clinical triad of a miosis, anhidrosis, and small, unilateral ptosis. The prompt diagnosis and determination of the etiology of Horner syndrome are of utmost importance, as the condition can result from many life-threatening systemic complications. Horner syndrome is often asymptomatic but can have distinct, easily identified characteristics seen with an ophthalmic examination. This report describes a patient who presented with Horner syndrome resulting from an internal carotid artery dissection.
Case Presentation
A 61-year-old woman presented with periorbital pain with onset 3 days prior. The patient described the pain as 7 of 10 that had been worsening and was localized around and behind the right eye. She reported new-onset headaches on the right side over the past week with associated intermittent vision blurriness in the right eye. She had a history of mobility issues and had fallen backward about 1 week before, hitting the back of her head on the floor without direct trauma to the eye. She was symptomatic for light sensitivity, syncope, and dizziness, with reports of a recent history of transient ischemic attacks (TIAs) of unknown etiology, which had occurred in the months preceding her examination. She reported no jaw claudication, scalp tenderness, and neck or shoulder pain. She was unaware of any changes in her perspiration pattern on the right side of her face but mentioned that she had noticed her right upper eyelid drooping while looking in the mirror.
This patient had a routine eye examination 2 months before, which was remarkable for stable, nonfoveal involving adult-onset vitelliform dystrophy in the left eye and nuclear sclerotic cataracts and mild refractive error in both eyes. No iris heterochromia was noted, and her pupils were equal, round, and reactive to light. Her history was remarkable for chest pain, obesity, bipolar disorder, vertigo, transient cerebral ischemia, hypertension, hypercholesterolemia, alcohol use disorder, cocaine use disorder, and asthma. A carotid ultrasound had been performed 1 month before the onset of symptoms due to her history of TIAs, which showed no hemodynamically significant stenosis (> 50% stenosis) of either carotid artery. Her medications included oxybutynin chloride, amlodipine, acetaminophen, sertraline hydrochloride, lidocaine, albuterol, risperidone, hydroxyzine hydrochloride, lisinopril, omeprazole, once-daily baby aspirin, atorvastatin, and calcium.
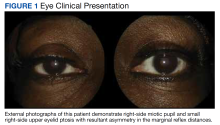
At the time of presentation, an ophthalmic examination revealed no decrease in visual acuity with a best-corrected visual acuity of 20/20 in the right and left eyes. The patient’s pupil sizes were unequal, with a smaller, more miotic right pupil with a greater difference between the pupil sizes in dim illumination (Figure 1).
As the patient had pathologic miosis, conditions causing pathologic mydriasis, such as Adie tonic pupil and cranial nerve III palsy, were ruled out. The presence of an acute, slight ptosis with pathologic miosis and pain in the ipsilateral eye with no reports of exposure to miotic pharmaceutical agents and no history of trauma to the globe or orbit eliminated other differentials, leading to a diagnosis of right-sided Horner syndrome. Due to concerns of acute onset periorbital and retrobulbar pain, she was referred to the emergency department with recommendations for computed tomography angiography (CTA), magnetic resonance imaging (MRI), and magnetic resonance angiogram (MRA) of the head and neck to rule out a carotid artery dissection.
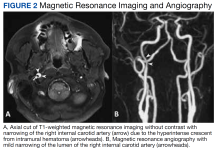
CTA revealed a focal linear filling defect in the right midinternal carotid artery, likely related to an internal carotid artery vascular flap. There was no evidence of proximal intracranial occlusive disease. MRI revealed a linear area of high-intensity signal projecting over the mid and distal right internal carotid artery lumen (Figure 2A).
Imaging suggested an internal carotid artery dissection, and the patient was admitted to the hospital for observation for 4 days. During this time, the patient was instructed to continue taking 81mg aspirin daily and to begin taking 75 mg clopidogrel bisulfate daily to prevent a cerebrovascular accident. Once stability was established, the patient was discharged with instructions to follow up with neurology and neuro-ophthalmology.
Discussion
Anisocoria is defined as a difference in pupil sizes between the eyes.1 This difference can be physiologic with no underlying pathology as an etiology of the condition. If underlying pathology causes anisocoria, it can result in dysfunction with mydriasis, leading to a more miotic pupil, or it can result from issues with miosis, leading to a more mydriatic pupil.1
To determine whether anisocoria is physiologic or pathologic, one must assess the patient’s pupil sizes in dim and bright illumination. If the difference in the pupil size is the same in both room illuminations (ie, the anisocoria is 2 mm in both bright and dim illumination, pupillary constriction and dilation are functioning normally), then the patient has physiologic anisocoria.1 If anisocoria is different in bright and dim illumination (ie, the anisocoria is 1 mm in bright and 3 mm in dim settings or 3 mm in bright and 1 mm in dim settings), the condition is related to pathology. To determine the underlying pathology of anisocoria in cases that are not physiologic, it is important to first determine whether the anisocoria is related to miotic or mydriatic dysfunction.1
If the anisocoria is greater in dim illumination, this suggests mydriatic dysfunction and could be a result of damage to the sympathetic pupillary pathway.1 The smaller or more miotic pupil in this instance is the pathologic pupil. If the anisocoria is greater in bright illumination, this suggests miotic dysfunction and could be a result of damage to the parasympathetic pathway.1 The larger or more mydriatic pupil in this instance is the pathologic pupil. Congenital abnormalities, such as iris colobomas, aniridia, and ectopic pupils, can result in a wide range of pupil sizes and shapes, including miotic or mydriatic pupils.1
Pathologic Mydriasis
Pathologic mydriatic pupils can result from dysfunction in the parasympathetic nervous system, which results in a pupil that is not sufficiently able to dilate with the removal of a light stimulus. Mydriatic pupils can be related to Adie tonic pupil, Argyll-Robertson pupil, third nerve palsy, trauma, surgeries, or pharmacologic mydriasis.2 The conditions that cause mydriasis can be readily differentiated from one another based on clinical examination.
Adie tonic pupil results from damage to the ciliary ganglion.2 While pupillary constriction in response to light will be absent or sluggish in an Adie pupil, the patient will have an intact but sluggish accommodative pupillary response; therefore, the pupil will still constrict with accommodation and convergence to focus on near objects, although slowly. This is known as light-near dissociation.2
Argyll-Robertson pupils are caused by damage to the Edinger-Westphal nucleus in the rostral midbrain.3 Lesions to this area of the brain are typically associated with neurosyphilis but also can be a result of Lyme disease, multiple sclerosis, encephalitis, neurosarcoidosis, herpes zoster, diabetes mellitus, and chronic alcohol misuse.3 Argyll Robertson pupils can appear very similar to a tonic pupil in that this condition will also have a dilated pupil and light-near dissociation.3 These pupils will differ in that they also tend to have an irregular shape (dyscoria), and the pupils will constrict briskly when focusing on near objects and dilate briskly when focusing on distant objects, not sluggishly, as in Adie tonic pupil.3
Mydriasis due to a third nerve palsy will present with ptosis and extraocular muscle dysfunction (including deficits to the superior rectus, medial rectus, inferior oblique, and inferior rectus), with the classic presentation of a completed palsy with the eye positioned “down and out” or the patient’s inability to look medially and superiorly with the affected eye.2
As in cases of pathologic mydriasis, a thorough and in-depth history can help determine traumatic, surgical and pharmacologic etiologies of a mydriatic pupil. It should be determined whether the patient has had any previous trauma or surgeries to the eye or has been in contact with any of the following: acetylcholine receptor antagonists (atropine, scopolamine, homatropine, cyclopentolate, and tropicamide), motion sickness patches (scopolamine), nasal vasoconstrictors, glycopyrrolate deodorants, and/or various plants (Jimson weed or plants belonging to the digitalis family, such as foxglove).2
Pathologic Miosis
Pathologic miotic pupils can result from dysfunction in the sympathetic nervous system and can be related to blunt or penetrating trauma to the orbit, Horner syndrome, and pharmacologic miosis.2 Horner syndrome will be accompanied by a slight ptosis and sometimes anhidrosis on the ipsilateral side of the face. To differentiate between traumatic and pharmacologic miosis, a detailed history should be obtained, paying close attention to injuries to the eyes or head and/or possible exposure to chemical or pharmaceutical agents, including prostaglandins, pilocarpine, organophosphates, and opiates.2
Horner Syndrome
Horner syndrome is a neurologic condition that results from damage to the oculosympathetic pathway.4 The oculosympathetic pathway is a 3-neuron pathway that begins in the hypothalamus and follows a circuitous route to ultimately innervate the facial sweat glands, the smooth muscles of the blood vessels in the orbit and face, the iris dilator muscle, and the Müller muscles of the superior and inferior eyelids.1,5 Therefore, this pathway’s functions include vasoconstriction of facial blood vessels, facial diaphoresis (sweating), pupillary dilation, and maintaining an open position of the eyelids.1
Oculosympathetic pathway anatomy. To understand the findings associated with Horner syndrome, it is necessary to understand the anatomy of this 3-neuron pathway.5 First-order neurons, or central neurons, arise in the posterolateral aspect of the hypothalamus, where they then descend through the midbrain, pons, medulla, and cervical spinal cord via the intermediolateral gray column.6 The fibers then synapse in the ciliospinal center of Budge at the level of cervical vertebra C8 to thoracic vertebra T2, which give rise to the preganglionic, or second-order neurons.6
Second-order neurons begin at the ciliospinal center of Budge and exit the spinal cord via the central roots, most at the level of thoracic vertebra T1, with the remainder leaving at the levels of cervical vertebra C8 and thoracic vertebra T2.7 After exiting the spinal cord, the second-order neurons loop around the subclavian artery, where they then ascend close to the apex of the lung to synapse with the cell bodies of the third-order neurons at the superior cervical ganglion near cervical vertebrae C2 and C3.7
After arising at the superior cervical ganglion, third-order neurons diverge to follow 2 different courses.7 A portion of the neurons travels along the external carotid artery to ultimately innervate the facial sweat glands, while the other portion of the neurons combines with the carotid plexus and travels within the walls of the internal carotid artery and through the cavernous sinus.7 The fibers then briefly join the abducens nerve before anastomosing with the ophthalmic division of the trigeminal nerve.7 After coursing through the superior orbital fissure, the fibers innervate the iris dilator and Müller muscles via the long ciliary nerves.7
Symptoms and signs. Patients with Horner syndrome can present with a variety of symptoms and signs. Patients may be largely asymptomatic or they may complain of a droopy eyelid and blurry vision. The full Horner syndrome triad consists of ipsilateral miosis, anhidrosis of the face, and mild ptosis of the upper eyelid with reverse ptosis of the lower eyelid.8 The difference in pupil size is greatest 4 to 5 seconds after switching from bright to dim room illumination due to dilation lag in the miotic pupil from poor innervation.1
Although the classical triad of ptosis, miosis, and anhidrosis is emphasized in the literature, the full triad may not always be present.4 This variation is due to the anatomy of the oculosympathetic pathway with branches of the nerve system separating at the superior cervical ganglion and following different pathways along the internal and external carotid arteries, resulting in anhidrosis only in Horner syndrome caused by lesions to the first- or second-order neurons.4,5 Because of this deviation of the nerve fibers in the pathway, the presence of miosis and a slight ptosis in the absence of anhidrosis should still strongly suggest Horner syndrome.
In addition to the classic triad, Horner syndrome can present with other ophthalmic findings, including conjunctival injection, changes in accommodation, and a small decrease in intraocular pressure usually by no more than 1 to 2 mm Hg.4 Congenital Horner syndrome is unique in that it can result in iris heterochromia, with the lighter eye being the affected eye.4
Due to the long and circuitous nature of the oculosympathetic pathway, damage can occur due to a wide variety of conditions (Table) and can present with many neurologic findings.7
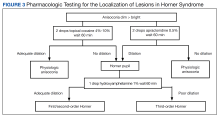
Localization of lesions. In Horner syndrome, 13% of lesions were present at first-order neurons, 44% at second-order neurons, and 43% at third-order neurons.7 While all these lesions have similar clinical presentations that can be difficult to differentiate, localization of the lesion within the oculosympathetic pathway is important to determine the underlying cause. This determination can be readily achieved in office with pharmacologic pupil testing (Figure 3).
Management. All acute Horner syndrome presentations should be referred for same-day evaluation to rule out potentially life-threatening conditions, such as a cerebrovascular accident, carotid artery dissection or aneurysm, and giant cell arteritis.10 The urgent evaluation should include CTA and MRI/MRA of the head and neck.5 If giant cell arteritis is suspected, it is also recommended to obtain urgent bloodwork, which should include complete blood count with differential, erythrocyte sedimentation rate, and C-reactive protein.5 Carotid angiography and CT of the chest also are indicated if the aforementioned tests are noncontributory, but these are less urgent and can be deferred for evaluation within 1 to 2 days after the initial diagnosis.10
In this patient’s case, an immediate neurologic evaluation was appropriate due to the acute and painful nature of her presentation. Ultimately, her Horner syndrome was determined to result from an internal carotid artery dissection. As indicated by Schievink, all acute Horner syndrome cases should be considered a result of a carotid artery dissection until proven otherwise, despite the presence or absence of any other signs or symptoms.11 This consideration is not only because of the potentially life-threatening sequelae associated with carotid dissections, but also because dissections have been shown to be the most common cause of ischemic strokes in young and middle-aged patients, accounting for 10% to 25% of all ischemic strokes.4,11
Carotid Artery Dissection
An artery dissection is typically the result of a tear of the
There are many causes of carotid artery dissections, such as structural defects of the arterial wall, fibromuscular dysplasia, cystic medial necrosis, and connective tissue disorders, including Ehlers-Danlos syndrome type IV, Marfan syndrome, autosomal dominant polycystic kidney disease, and osteogenesis imperfecta type I.13 Many environmental factors also can induce a carotid artery dissection, such as a history of anesthesia use, resuscitation with classic cardiopulmonary resuscitation techniques, head or neck trauma, chiropractic manipulation of the neck, and hyperextension or rotation of the neck, which can occur in activities such as yoga, painting a ceiling, coughing, vomiting, or sneezing.11
Patients with an internal carotid artery dissection typically present with pain on one side of the neck, face, or head, which can be accompanied by a partial Horner syndrome that results from damage to the oculosympathetic neurons traveling with the carotid plexus in the internal carotid artery wall.9,10 Unilateral facial or orbital pain has been noted to be present in half of patients and is typically accompanied by an ipsilateral headache.9 These symptoms are typically followed by cerebral or retinal ischemia within hours or days of onset and other ophthalmic conditions that can cause blindness, such as ischemic optic neuropathy or retinal artery occlusions, although these are rare.9
Due to the potential complications that can arise, carotid artery dissections require prompt treatment with antithrombotic therapy for 3 to 6 months to prevent carotid artery occlusion, which can result in a hemispheric cerebrovascular accident or TIAs.15 The options for antithrombotic therapy include anticoagulants, such as warfarin, and antiplatelets, such as aspirin. Studies have found similar rates of recurrent ischemic strokes in treatment with anticoagulants compared with antiplatelets, so both are reasonable therapeutic options.15,16 Following a carotid artery dissection diagnosis, patients should be evaluated by neurology to minimize other cardiovascular risk factors and prevent other complications.
Conclusions
Due to the potential life-threatening complications that can arise from conditions resulting in Horner syndrome, it is imperative that clinicians have a thorough understanding of the condition and its appropriate treatment and management modalities. Understanding the need for immediate testing to determine the underlying etiology of Horner syndrome can help prevent a decrease in a patient’s vision or quality of life, and in some cases, prevent death.
Acknowledgments
The author recognizes and thanks Kyle Stuard for his invaluable assistance in the editing of this manuscript
1. Yanoff M, Duker J. Ophthalmology. 5th ed. Elsevier; 2019.
2. Payne WN, Blair K, Barrett MJ. Anisocoria. StatPearls Publishing; 2022. Accessed February 1, 2023. https://www.ncbi.nlm.nih.gov/books/NBK470384
3. Lee A, Bindiganavile SH, Fan J, Al-Zubidi N, Bhatti MT. Argyll Robertson pupils. Accessed February 1, 2023. https://eyewiki.aao.org/Argyll_Robertson_Pupils
4. Kedar S, Prakalapakorn G, Yen M, et al. Horner syndrome. American Academy of Optometry. 2021. Accessed February 1, 2023. https://eyewiki.aao.org/Horner_Syndrome
5. Daroff R, Bradley W, Jankovic J. Bradley and Daroff’s Neurology in Clinical Practice. 8th ed. Elsevier; 2022.
6. Kanagalingam S, Miller NR. Horner syndrome: clinical perspectives. Eye Brain. 2015;7:35-46. doi:10.2147/EB.S63633
7. Lykstad J, Reddy V, Hanna A. Neuroanatomy, Pupillary Dilation Pathway. StatPearls Publishing; 2022. Updated August 11, 2021. Accessed February 1, 2023. https://www.ncbi.nlm.nih.gov/books/NBK535421
8. Friedman N, Kaiser P, Pineda R. The Massachusetts Eye and Ear Infirmary Illustrated Manual of Ophthalmology. 5th ed. Elsevier; 2020.
9. Silbert PL, Mokri B, Schievink WI. Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology. 1995;45(8):1517-1522. doi:10.1212/wnl.45.8.1517
10. Gervasio K, Peck T. The Will’s Eye Manual. 8th ed. Walters Kluwer; 2022.
11. Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906. doi:10.1056/NEJM200103223441206
12. Hart RG, Easton JD. Dissections of cervical and cerebral arteries. Neurol Clin. 1983;1(1):155-182.
13. Goodfriend SD, Tadi P, Koury R. Carotid Artery Dissection. StatPearls Publishing; 2022. Updated December 24, 2021. Accessed February 1, 2023. https://www.ncbi.nlm.nih.gov/books/NBK430835
14. Blum CA, Yaghi S. Cervical artery dissection: a review of the epidemiology, pathophysiology, treatment, and outcome. Arch Neurosci. 2015;2(4):e26670. doi:10.5812/archneurosci.26670
15. Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(1):227-276. doi:10.1161/STR.0b013e3181f7d043
16. Mohr JP, Thompson JL, Lazar RM, et al; Warfarin-Aspirin Recurrent Stroke Study Group. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001;345(20):1444-1451. doi:10.1056/NEJMoa011258
17. Davagnanam I, Fraser CL, Miszkiel K, Daniel CS, Plant GT. Adult Horner’s syndrome: a combined clinical, pharmacological, and imaging algorithm. Eye (Lond). 2013;27(3):291-298. doi:10.1038/eye.2012.281
Horner syndrome is a rare condition that has no sex or race predilection and is characterized by the clinical triad of a miosis, anhidrosis, and small, unilateral ptosis. The prompt diagnosis and determination of the etiology of Horner syndrome are of utmost importance, as the condition can result from many life-threatening systemic complications. Horner syndrome is often asymptomatic but can have distinct, easily identified characteristics seen with an ophthalmic examination. This report describes a patient who presented with Horner syndrome resulting from an internal carotid artery dissection.
Case Presentation
A 61-year-old woman presented with periorbital pain with onset 3 days prior. The patient described the pain as 7 of 10 that had been worsening and was localized around and behind the right eye. She reported new-onset headaches on the right side over the past week with associated intermittent vision blurriness in the right eye. She had a history of mobility issues and had fallen backward about 1 week before, hitting the back of her head on the floor without direct trauma to the eye. She was symptomatic for light sensitivity, syncope, and dizziness, with reports of a recent history of transient ischemic attacks (TIAs) of unknown etiology, which had occurred in the months preceding her examination. She reported no jaw claudication, scalp tenderness, and neck or shoulder pain. She was unaware of any changes in her perspiration pattern on the right side of her face but mentioned that she had noticed her right upper eyelid drooping while looking in the mirror.
This patient had a routine eye examination 2 months before, which was remarkable for stable, nonfoveal involving adult-onset vitelliform dystrophy in the left eye and nuclear sclerotic cataracts and mild refractive error in both eyes. No iris heterochromia was noted, and her pupils were equal, round, and reactive to light. Her history was remarkable for chest pain, obesity, bipolar disorder, vertigo, transient cerebral ischemia, hypertension, hypercholesterolemia, alcohol use disorder, cocaine use disorder, and asthma. A carotid ultrasound had been performed 1 month before the onset of symptoms due to her history of TIAs, which showed no hemodynamically significant stenosis (> 50% stenosis) of either carotid artery. Her medications included oxybutynin chloride, amlodipine, acetaminophen, sertraline hydrochloride, lidocaine, albuterol, risperidone, hydroxyzine hydrochloride, lisinopril, omeprazole, once-daily baby aspirin, atorvastatin, and calcium.
At the time of presentation, an ophthalmic examination revealed no decrease in visual acuity with a best-corrected visual acuity of 20/20 in the right and left eyes. The patient’s pupil sizes were unequal, with a smaller, more miotic right pupil with a greater difference between the pupil sizes in dim illumination (Figure 1).
As the patient had pathologic miosis, conditions causing pathologic mydriasis, such as Adie tonic pupil and cranial nerve III palsy, were ruled out. The presence of an acute, slight ptosis with pathologic miosis and pain in the ipsilateral eye with no reports of exposure to miotic pharmaceutical agents and no history of trauma to the globe or orbit eliminated other differentials, leading to a diagnosis of right-sided Horner syndrome. Due to concerns of acute onset periorbital and retrobulbar pain, she was referred to the emergency department with recommendations for computed tomography angiography (CTA), magnetic resonance imaging (MRI), and magnetic resonance angiogram (MRA) of the head and neck to rule out a carotid artery dissection.
CTA revealed a focal linear filling defect in the right midinternal carotid artery, likely related to an internal carotid artery vascular flap. There was no evidence of proximal intracranial occlusive disease. MRI revealed a linear area of high-intensity signal projecting over the mid and distal right internal carotid artery lumen (Figure 2A).
Imaging suggested an internal carotid artery dissection, and the patient was admitted to the hospital for observation for 4 days. During this time, the patient was instructed to continue taking 81mg aspirin daily and to begin taking 75 mg clopidogrel bisulfate daily to prevent a cerebrovascular accident. Once stability was established, the patient was discharged with instructions to follow up with neurology and neuro-ophthalmology.
Discussion
Anisocoria is defined as a difference in pupil sizes between the eyes.1 This difference can be physiologic with no underlying pathology as an etiology of the condition. If underlying pathology causes anisocoria, it can result in dysfunction with mydriasis, leading to a more miotic pupil, or it can result from issues with miosis, leading to a more mydriatic pupil.1
To determine whether anisocoria is physiologic or pathologic, one must assess the patient’s pupil sizes in dim and bright illumination. If the difference in the pupil size is the same in both room illuminations (ie, the anisocoria is 2 mm in both bright and dim illumination, pupillary constriction and dilation are functioning normally), then the patient has physiologic anisocoria.1 If anisocoria is different in bright and dim illumination (ie, the anisocoria is 1 mm in bright and 3 mm in dim settings or 3 mm in bright and 1 mm in dim settings), the condition is related to pathology. To determine the underlying pathology of anisocoria in cases that are not physiologic, it is important to first determine whether the anisocoria is related to miotic or mydriatic dysfunction.1
If the anisocoria is greater in dim illumination, this suggests mydriatic dysfunction and could be a result of damage to the sympathetic pupillary pathway.1 The smaller or more miotic pupil in this instance is the pathologic pupil. If the anisocoria is greater in bright illumination, this suggests miotic dysfunction and could be a result of damage to the parasympathetic pathway.1 The larger or more mydriatic pupil in this instance is the pathologic pupil. Congenital abnormalities, such as iris colobomas, aniridia, and ectopic pupils, can result in a wide range of pupil sizes and shapes, including miotic or mydriatic pupils.1
Pathologic Mydriasis
Pathologic mydriatic pupils can result from dysfunction in the parasympathetic nervous system, which results in a pupil that is not sufficiently able to dilate with the removal of a light stimulus. Mydriatic pupils can be related to Adie tonic pupil, Argyll-Robertson pupil, third nerve palsy, trauma, surgeries, or pharmacologic mydriasis.2 The conditions that cause mydriasis can be readily differentiated from one another based on clinical examination.
Adie tonic pupil results from damage to the ciliary ganglion.2 While pupillary constriction in response to light will be absent or sluggish in an Adie pupil, the patient will have an intact but sluggish accommodative pupillary response; therefore, the pupil will still constrict with accommodation and convergence to focus on near objects, although slowly. This is known as light-near dissociation.2
Argyll-Robertson pupils are caused by damage to the Edinger-Westphal nucleus in the rostral midbrain.3 Lesions to this area of the brain are typically associated with neurosyphilis but also can be a result of Lyme disease, multiple sclerosis, encephalitis, neurosarcoidosis, herpes zoster, diabetes mellitus, and chronic alcohol misuse.3 Argyll Robertson pupils can appear very similar to a tonic pupil in that this condition will also have a dilated pupil and light-near dissociation.3 These pupils will differ in that they also tend to have an irregular shape (dyscoria), and the pupils will constrict briskly when focusing on near objects and dilate briskly when focusing on distant objects, not sluggishly, as in Adie tonic pupil.3
Mydriasis due to a third nerve palsy will present with ptosis and extraocular muscle dysfunction (including deficits to the superior rectus, medial rectus, inferior oblique, and inferior rectus), with the classic presentation of a completed palsy with the eye positioned “down and out” or the patient’s inability to look medially and superiorly with the affected eye.2
As in cases of pathologic mydriasis, a thorough and in-depth history can help determine traumatic, surgical and pharmacologic etiologies of a mydriatic pupil. It should be determined whether the patient has had any previous trauma or surgeries to the eye or has been in contact with any of the following: acetylcholine receptor antagonists (atropine, scopolamine, homatropine, cyclopentolate, and tropicamide), motion sickness patches (scopolamine), nasal vasoconstrictors, glycopyrrolate deodorants, and/or various plants (Jimson weed or plants belonging to the digitalis family, such as foxglove).2
Pathologic Miosis
Pathologic miotic pupils can result from dysfunction in the sympathetic nervous system and can be related to blunt or penetrating trauma to the orbit, Horner syndrome, and pharmacologic miosis.2 Horner syndrome will be accompanied by a slight ptosis and sometimes anhidrosis on the ipsilateral side of the face. To differentiate between traumatic and pharmacologic miosis, a detailed history should be obtained, paying close attention to injuries to the eyes or head and/or possible exposure to chemical or pharmaceutical agents, including prostaglandins, pilocarpine, organophosphates, and opiates.2
Horner Syndrome
Horner syndrome is a neurologic condition that results from damage to the oculosympathetic pathway.4 The oculosympathetic pathway is a 3-neuron pathway that begins in the hypothalamus and follows a circuitous route to ultimately innervate the facial sweat glands, the smooth muscles of the blood vessels in the orbit and face, the iris dilator muscle, and the Müller muscles of the superior and inferior eyelids.1,5 Therefore, this pathway’s functions include vasoconstriction of facial blood vessels, facial diaphoresis (sweating), pupillary dilation, and maintaining an open position of the eyelids.1
Oculosympathetic pathway anatomy. To understand the findings associated with Horner syndrome, it is necessary to understand the anatomy of this 3-neuron pathway.5 First-order neurons, or central neurons, arise in the posterolateral aspect of the hypothalamus, where they then descend through the midbrain, pons, medulla, and cervical spinal cord via the intermediolateral gray column.6 The fibers then synapse in the ciliospinal center of Budge at the level of cervical vertebra C8 to thoracic vertebra T2, which give rise to the preganglionic, or second-order neurons.6
Second-order neurons begin at the ciliospinal center of Budge and exit the spinal cord via the central roots, most at the level of thoracic vertebra T1, with the remainder leaving at the levels of cervical vertebra C8 and thoracic vertebra T2.7 After exiting the spinal cord, the second-order neurons loop around the subclavian artery, where they then ascend close to the apex of the lung to synapse with the cell bodies of the third-order neurons at the superior cervical ganglion near cervical vertebrae C2 and C3.7
After arising at the superior cervical ganglion, third-order neurons diverge to follow 2 different courses.7 A portion of the neurons travels along the external carotid artery to ultimately innervate the facial sweat glands, while the other portion of the neurons combines with the carotid plexus and travels within the walls of the internal carotid artery and through the cavernous sinus.7 The fibers then briefly join the abducens nerve before anastomosing with the ophthalmic division of the trigeminal nerve.7 After coursing through the superior orbital fissure, the fibers innervate the iris dilator and Müller muscles via the long ciliary nerves.7
Symptoms and signs. Patients with Horner syndrome can present with a variety of symptoms and signs. Patients may be largely asymptomatic or they may complain of a droopy eyelid and blurry vision. The full Horner syndrome triad consists of ipsilateral miosis, anhidrosis of the face, and mild ptosis of the upper eyelid with reverse ptosis of the lower eyelid.8 The difference in pupil size is greatest 4 to 5 seconds after switching from bright to dim room illumination due to dilation lag in the miotic pupil from poor innervation.1
Although the classical triad of ptosis, miosis, and anhidrosis is emphasized in the literature, the full triad may not always be present.4 This variation is due to the anatomy of the oculosympathetic pathway with branches of the nerve system separating at the superior cervical ganglion and following different pathways along the internal and external carotid arteries, resulting in anhidrosis only in Horner syndrome caused by lesions to the first- or second-order neurons.4,5 Because of this deviation of the nerve fibers in the pathway, the presence of miosis and a slight ptosis in the absence of anhidrosis should still strongly suggest Horner syndrome.
In addition to the classic triad, Horner syndrome can present with other ophthalmic findings, including conjunctival injection, changes in accommodation, and a small decrease in intraocular pressure usually by no more than 1 to 2 mm Hg.4 Congenital Horner syndrome is unique in that it can result in iris heterochromia, with the lighter eye being the affected eye.4
Due to the long and circuitous nature of the oculosympathetic pathway, damage can occur due to a wide variety of conditions (Table) and can present with many neurologic findings.7
Localization of lesions. In Horner syndrome, 13% of lesions were present at first-order neurons, 44% at second-order neurons, and 43% at third-order neurons.7 While all these lesions have similar clinical presentations that can be difficult to differentiate, localization of the lesion within the oculosympathetic pathway is important to determine the underlying cause. This determination can be readily achieved in office with pharmacologic pupil testing (Figure 3).
Management. All acute Horner syndrome presentations should be referred for same-day evaluation to rule out potentially life-threatening conditions, such as a cerebrovascular accident, carotid artery dissection or aneurysm, and giant cell arteritis.10 The urgent evaluation should include CTA and MRI/MRA of the head and neck.5 If giant cell arteritis is suspected, it is also recommended to obtain urgent bloodwork, which should include complete blood count with differential, erythrocyte sedimentation rate, and C-reactive protein.5 Carotid angiography and CT of the chest also are indicated if the aforementioned tests are noncontributory, but these are less urgent and can be deferred for evaluation within 1 to 2 days after the initial diagnosis.10
In this patient’s case, an immediate neurologic evaluation was appropriate due to the acute and painful nature of her presentation. Ultimately, her Horner syndrome was determined to result from an internal carotid artery dissection. As indicated by Schievink, all acute Horner syndrome cases should be considered a result of a carotid artery dissection until proven otherwise, despite the presence or absence of any other signs or symptoms.11 This consideration is not only because of the potentially life-threatening sequelae associated with carotid dissections, but also because dissections have been shown to be the most common cause of ischemic strokes in young and middle-aged patients, accounting for 10% to 25% of all ischemic strokes.4,11
Carotid Artery Dissection
An artery dissection is typically the result of a tear of the
There are many causes of carotid artery dissections, such as structural defects of the arterial wall, fibromuscular dysplasia, cystic medial necrosis, and connective tissue disorders, including Ehlers-Danlos syndrome type IV, Marfan syndrome, autosomal dominant polycystic kidney disease, and osteogenesis imperfecta type I.13 Many environmental factors also can induce a carotid artery dissection, such as a history of anesthesia use, resuscitation with classic cardiopulmonary resuscitation techniques, head or neck trauma, chiropractic manipulation of the neck, and hyperextension or rotation of the neck, which can occur in activities such as yoga, painting a ceiling, coughing, vomiting, or sneezing.11
Patients with an internal carotid artery dissection typically present with pain on one side of the neck, face, or head, which can be accompanied by a partial Horner syndrome that results from damage to the oculosympathetic neurons traveling with the carotid plexus in the internal carotid artery wall.9,10 Unilateral facial or orbital pain has been noted to be present in half of patients and is typically accompanied by an ipsilateral headache.9 These symptoms are typically followed by cerebral or retinal ischemia within hours or days of onset and other ophthalmic conditions that can cause blindness, such as ischemic optic neuropathy or retinal artery occlusions, although these are rare.9
Due to the potential complications that can arise, carotid artery dissections require prompt treatment with antithrombotic therapy for 3 to 6 months to prevent carotid artery occlusion, which can result in a hemispheric cerebrovascular accident or TIAs.15 The options for antithrombotic therapy include anticoagulants, such as warfarin, and antiplatelets, such as aspirin. Studies have found similar rates of recurrent ischemic strokes in treatment with anticoagulants compared with antiplatelets, so both are reasonable therapeutic options.15,16 Following a carotid artery dissection diagnosis, patients should be evaluated by neurology to minimize other cardiovascular risk factors and prevent other complications.
Conclusions
Due to the potential life-threatening complications that can arise from conditions resulting in Horner syndrome, it is imperative that clinicians have a thorough understanding of the condition and its appropriate treatment and management modalities. Understanding the need for immediate testing to determine the underlying etiology of Horner syndrome can help prevent a decrease in a patient’s vision or quality of life, and in some cases, prevent death.
Acknowledgments
The author recognizes and thanks Kyle Stuard for his invaluable assistance in the editing of this manuscript
Horner syndrome is a rare condition that has no sex or race predilection and is characterized by the clinical triad of a miosis, anhidrosis, and small, unilateral ptosis. The prompt diagnosis and determination of the etiology of Horner syndrome are of utmost importance, as the condition can result from many life-threatening systemic complications. Horner syndrome is often asymptomatic but can have distinct, easily identified characteristics seen with an ophthalmic examination. This report describes a patient who presented with Horner syndrome resulting from an internal carotid artery dissection.
Case Presentation
A 61-year-old woman presented with periorbital pain with onset 3 days prior. The patient described the pain as 7 of 10 that had been worsening and was localized around and behind the right eye. She reported new-onset headaches on the right side over the past week with associated intermittent vision blurriness in the right eye. She had a history of mobility issues and had fallen backward about 1 week before, hitting the back of her head on the floor without direct trauma to the eye. She was symptomatic for light sensitivity, syncope, and dizziness, with reports of a recent history of transient ischemic attacks (TIAs) of unknown etiology, which had occurred in the months preceding her examination. She reported no jaw claudication, scalp tenderness, and neck or shoulder pain. She was unaware of any changes in her perspiration pattern on the right side of her face but mentioned that she had noticed her right upper eyelid drooping while looking in the mirror.
This patient had a routine eye examination 2 months before, which was remarkable for stable, nonfoveal involving adult-onset vitelliform dystrophy in the left eye and nuclear sclerotic cataracts and mild refractive error in both eyes. No iris heterochromia was noted, and her pupils were equal, round, and reactive to light. Her history was remarkable for chest pain, obesity, bipolar disorder, vertigo, transient cerebral ischemia, hypertension, hypercholesterolemia, alcohol use disorder, cocaine use disorder, and asthma. A carotid ultrasound had been performed 1 month before the onset of symptoms due to her history of TIAs, which showed no hemodynamically significant stenosis (> 50% stenosis) of either carotid artery. Her medications included oxybutynin chloride, amlodipine, acetaminophen, sertraline hydrochloride, lidocaine, albuterol, risperidone, hydroxyzine hydrochloride, lisinopril, omeprazole, once-daily baby aspirin, atorvastatin, and calcium.
At the time of presentation, an ophthalmic examination revealed no decrease in visual acuity with a best-corrected visual acuity of 20/20 in the right and left eyes. The patient’s pupil sizes were unequal, with a smaller, more miotic right pupil with a greater difference between the pupil sizes in dim illumination (Figure 1).
As the patient had pathologic miosis, conditions causing pathologic mydriasis, such as Adie tonic pupil and cranial nerve III palsy, were ruled out. The presence of an acute, slight ptosis with pathologic miosis and pain in the ipsilateral eye with no reports of exposure to miotic pharmaceutical agents and no history of trauma to the globe or orbit eliminated other differentials, leading to a diagnosis of right-sided Horner syndrome. Due to concerns of acute onset periorbital and retrobulbar pain, she was referred to the emergency department with recommendations for computed tomography angiography (CTA), magnetic resonance imaging (MRI), and magnetic resonance angiogram (MRA) of the head and neck to rule out a carotid artery dissection.
CTA revealed a focal linear filling defect in the right midinternal carotid artery, likely related to an internal carotid artery vascular flap. There was no evidence of proximal intracranial occlusive disease. MRI revealed a linear area of high-intensity signal projecting over the mid and distal right internal carotid artery lumen (Figure 2A).
Imaging suggested an internal carotid artery dissection, and the patient was admitted to the hospital for observation for 4 days. During this time, the patient was instructed to continue taking 81mg aspirin daily and to begin taking 75 mg clopidogrel bisulfate daily to prevent a cerebrovascular accident. Once stability was established, the patient was discharged with instructions to follow up with neurology and neuro-ophthalmology.
Discussion
Anisocoria is defined as a difference in pupil sizes between the eyes.1 This difference can be physiologic with no underlying pathology as an etiology of the condition. If underlying pathology causes anisocoria, it can result in dysfunction with mydriasis, leading to a more miotic pupil, or it can result from issues with miosis, leading to a more mydriatic pupil.1
To determine whether anisocoria is physiologic or pathologic, one must assess the patient’s pupil sizes in dim and bright illumination. If the difference in the pupil size is the same in both room illuminations (ie, the anisocoria is 2 mm in both bright and dim illumination, pupillary constriction and dilation are functioning normally), then the patient has physiologic anisocoria.1 If anisocoria is different in bright and dim illumination (ie, the anisocoria is 1 mm in bright and 3 mm in dim settings or 3 mm in bright and 1 mm in dim settings), the condition is related to pathology. To determine the underlying pathology of anisocoria in cases that are not physiologic, it is important to first determine whether the anisocoria is related to miotic or mydriatic dysfunction.1
If the anisocoria is greater in dim illumination, this suggests mydriatic dysfunction and could be a result of damage to the sympathetic pupillary pathway.1 The smaller or more miotic pupil in this instance is the pathologic pupil. If the anisocoria is greater in bright illumination, this suggests miotic dysfunction and could be a result of damage to the parasympathetic pathway.1 The larger or more mydriatic pupil in this instance is the pathologic pupil. Congenital abnormalities, such as iris colobomas, aniridia, and ectopic pupils, can result in a wide range of pupil sizes and shapes, including miotic or mydriatic pupils.1
Pathologic Mydriasis
Pathologic mydriatic pupils can result from dysfunction in the parasympathetic nervous system, which results in a pupil that is not sufficiently able to dilate with the removal of a light stimulus. Mydriatic pupils can be related to Adie tonic pupil, Argyll-Robertson pupil, third nerve palsy, trauma, surgeries, or pharmacologic mydriasis.2 The conditions that cause mydriasis can be readily differentiated from one another based on clinical examination.
Adie tonic pupil results from damage to the ciliary ganglion.2 While pupillary constriction in response to light will be absent or sluggish in an Adie pupil, the patient will have an intact but sluggish accommodative pupillary response; therefore, the pupil will still constrict with accommodation and convergence to focus on near objects, although slowly. This is known as light-near dissociation.2
Argyll-Robertson pupils are caused by damage to the Edinger-Westphal nucleus in the rostral midbrain.3 Lesions to this area of the brain are typically associated with neurosyphilis but also can be a result of Lyme disease, multiple sclerosis, encephalitis, neurosarcoidosis, herpes zoster, diabetes mellitus, and chronic alcohol misuse.3 Argyll Robertson pupils can appear very similar to a tonic pupil in that this condition will also have a dilated pupil and light-near dissociation.3 These pupils will differ in that they also tend to have an irregular shape (dyscoria), and the pupils will constrict briskly when focusing on near objects and dilate briskly when focusing on distant objects, not sluggishly, as in Adie tonic pupil.3
Mydriasis due to a third nerve palsy will present with ptosis and extraocular muscle dysfunction (including deficits to the superior rectus, medial rectus, inferior oblique, and inferior rectus), with the classic presentation of a completed palsy with the eye positioned “down and out” or the patient’s inability to look medially and superiorly with the affected eye.2
As in cases of pathologic mydriasis, a thorough and in-depth history can help determine traumatic, surgical and pharmacologic etiologies of a mydriatic pupil. It should be determined whether the patient has had any previous trauma or surgeries to the eye or has been in contact with any of the following: acetylcholine receptor antagonists (atropine, scopolamine, homatropine, cyclopentolate, and tropicamide), motion sickness patches (scopolamine), nasal vasoconstrictors, glycopyrrolate deodorants, and/or various plants (Jimson weed or plants belonging to the digitalis family, such as foxglove).2
Pathologic Miosis
Pathologic miotic pupils can result from dysfunction in the sympathetic nervous system and can be related to blunt or penetrating trauma to the orbit, Horner syndrome, and pharmacologic miosis.2 Horner syndrome will be accompanied by a slight ptosis and sometimes anhidrosis on the ipsilateral side of the face. To differentiate between traumatic and pharmacologic miosis, a detailed history should be obtained, paying close attention to injuries to the eyes or head and/or possible exposure to chemical or pharmaceutical agents, including prostaglandins, pilocarpine, organophosphates, and opiates.2
Horner Syndrome
Horner syndrome is a neurologic condition that results from damage to the oculosympathetic pathway.4 The oculosympathetic pathway is a 3-neuron pathway that begins in the hypothalamus and follows a circuitous route to ultimately innervate the facial sweat glands, the smooth muscles of the blood vessels in the orbit and face, the iris dilator muscle, and the Müller muscles of the superior and inferior eyelids.1,5 Therefore, this pathway’s functions include vasoconstriction of facial blood vessels, facial diaphoresis (sweating), pupillary dilation, and maintaining an open position of the eyelids.1
Oculosympathetic pathway anatomy. To understand the findings associated with Horner syndrome, it is necessary to understand the anatomy of this 3-neuron pathway.5 First-order neurons, or central neurons, arise in the posterolateral aspect of the hypothalamus, where they then descend through the midbrain, pons, medulla, and cervical spinal cord via the intermediolateral gray column.6 The fibers then synapse in the ciliospinal center of Budge at the level of cervical vertebra C8 to thoracic vertebra T2, which give rise to the preganglionic, or second-order neurons.6
Second-order neurons begin at the ciliospinal center of Budge and exit the spinal cord via the central roots, most at the level of thoracic vertebra T1, with the remainder leaving at the levels of cervical vertebra C8 and thoracic vertebra T2.7 After exiting the spinal cord, the second-order neurons loop around the subclavian artery, where they then ascend close to the apex of the lung to synapse with the cell bodies of the third-order neurons at the superior cervical ganglion near cervical vertebrae C2 and C3.7
After arising at the superior cervical ganglion, third-order neurons diverge to follow 2 different courses.7 A portion of the neurons travels along the external carotid artery to ultimately innervate the facial sweat glands, while the other portion of the neurons combines with the carotid plexus and travels within the walls of the internal carotid artery and through the cavernous sinus.7 The fibers then briefly join the abducens nerve before anastomosing with the ophthalmic division of the trigeminal nerve.7 After coursing through the superior orbital fissure, the fibers innervate the iris dilator and Müller muscles via the long ciliary nerves.7
Symptoms and signs. Patients with Horner syndrome can present with a variety of symptoms and signs. Patients may be largely asymptomatic or they may complain of a droopy eyelid and blurry vision. The full Horner syndrome triad consists of ipsilateral miosis, anhidrosis of the face, and mild ptosis of the upper eyelid with reverse ptosis of the lower eyelid.8 The difference in pupil size is greatest 4 to 5 seconds after switching from bright to dim room illumination due to dilation lag in the miotic pupil from poor innervation.1
Although the classical triad of ptosis, miosis, and anhidrosis is emphasized in the literature, the full triad may not always be present.4 This variation is due to the anatomy of the oculosympathetic pathway with branches of the nerve system separating at the superior cervical ganglion and following different pathways along the internal and external carotid arteries, resulting in anhidrosis only in Horner syndrome caused by lesions to the first- or second-order neurons.4,5 Because of this deviation of the nerve fibers in the pathway, the presence of miosis and a slight ptosis in the absence of anhidrosis should still strongly suggest Horner syndrome.
In addition to the classic triad, Horner syndrome can present with other ophthalmic findings, including conjunctival injection, changes in accommodation, and a small decrease in intraocular pressure usually by no more than 1 to 2 mm Hg.4 Congenital Horner syndrome is unique in that it can result in iris heterochromia, with the lighter eye being the affected eye.4
Due to the long and circuitous nature of the oculosympathetic pathway, damage can occur due to a wide variety of conditions (Table) and can present with many neurologic findings.7
Localization of lesions. In Horner syndrome, 13% of lesions were present at first-order neurons, 44% at second-order neurons, and 43% at third-order neurons.7 While all these lesions have similar clinical presentations that can be difficult to differentiate, localization of the lesion within the oculosympathetic pathway is important to determine the underlying cause. This determination can be readily achieved in office with pharmacologic pupil testing (Figure 3).
Management. All acute Horner syndrome presentations should be referred for same-day evaluation to rule out potentially life-threatening conditions, such as a cerebrovascular accident, carotid artery dissection or aneurysm, and giant cell arteritis.10 The urgent evaluation should include CTA and MRI/MRA of the head and neck.5 If giant cell arteritis is suspected, it is also recommended to obtain urgent bloodwork, which should include complete blood count with differential, erythrocyte sedimentation rate, and C-reactive protein.5 Carotid angiography and CT of the chest also are indicated if the aforementioned tests are noncontributory, but these are less urgent and can be deferred for evaluation within 1 to 2 days after the initial diagnosis.10
In this patient’s case, an immediate neurologic evaluation was appropriate due to the acute and painful nature of her presentation. Ultimately, her Horner syndrome was determined to result from an internal carotid artery dissection. As indicated by Schievink, all acute Horner syndrome cases should be considered a result of a carotid artery dissection until proven otherwise, despite the presence or absence of any other signs or symptoms.11 This consideration is not only because of the potentially life-threatening sequelae associated with carotid dissections, but also because dissections have been shown to be the most common cause of ischemic strokes in young and middle-aged patients, accounting for 10% to 25% of all ischemic strokes.4,11
Carotid Artery Dissection
An artery dissection is typically the result of a tear of the
There are many causes of carotid artery dissections, such as structural defects of the arterial wall, fibromuscular dysplasia, cystic medial necrosis, and connective tissue disorders, including Ehlers-Danlos syndrome type IV, Marfan syndrome, autosomal dominant polycystic kidney disease, and osteogenesis imperfecta type I.13 Many environmental factors also can induce a carotid artery dissection, such as a history of anesthesia use, resuscitation with classic cardiopulmonary resuscitation techniques, head or neck trauma, chiropractic manipulation of the neck, and hyperextension or rotation of the neck, which can occur in activities such as yoga, painting a ceiling, coughing, vomiting, or sneezing.11
Patients with an internal carotid artery dissection typically present with pain on one side of the neck, face, or head, which can be accompanied by a partial Horner syndrome that results from damage to the oculosympathetic neurons traveling with the carotid plexus in the internal carotid artery wall.9,10 Unilateral facial or orbital pain has been noted to be present in half of patients and is typically accompanied by an ipsilateral headache.9 These symptoms are typically followed by cerebral or retinal ischemia within hours or days of onset and other ophthalmic conditions that can cause blindness, such as ischemic optic neuropathy or retinal artery occlusions, although these are rare.9
Due to the potential complications that can arise, carotid artery dissections require prompt treatment with antithrombotic therapy for 3 to 6 months to prevent carotid artery occlusion, which can result in a hemispheric cerebrovascular accident or TIAs.15 The options for antithrombotic therapy include anticoagulants, such as warfarin, and antiplatelets, such as aspirin. Studies have found similar rates of recurrent ischemic strokes in treatment with anticoagulants compared with antiplatelets, so both are reasonable therapeutic options.15,16 Following a carotid artery dissection diagnosis, patients should be evaluated by neurology to minimize other cardiovascular risk factors and prevent other complications.
Conclusions
Due to the potential life-threatening complications that can arise from conditions resulting in Horner syndrome, it is imperative that clinicians have a thorough understanding of the condition and its appropriate treatment and management modalities. Understanding the need for immediate testing to determine the underlying etiology of Horner syndrome can help prevent a decrease in a patient’s vision or quality of life, and in some cases, prevent death.
Acknowledgments
The author recognizes and thanks Kyle Stuard for his invaluable assistance in the editing of this manuscript
1. Yanoff M, Duker J. Ophthalmology. 5th ed. Elsevier; 2019.
2. Payne WN, Blair K, Barrett MJ. Anisocoria. StatPearls Publishing; 2022. Accessed February 1, 2023. https://www.ncbi.nlm.nih.gov/books/NBK470384
3. Lee A, Bindiganavile SH, Fan J, Al-Zubidi N, Bhatti MT. Argyll Robertson pupils. Accessed February 1, 2023. https://eyewiki.aao.org/Argyll_Robertson_Pupils
4. Kedar S, Prakalapakorn G, Yen M, et al. Horner syndrome. American Academy of Optometry. 2021. Accessed February 1, 2023. https://eyewiki.aao.org/Horner_Syndrome
5. Daroff R, Bradley W, Jankovic J. Bradley and Daroff’s Neurology in Clinical Practice. 8th ed. Elsevier; 2022.
6. Kanagalingam S, Miller NR. Horner syndrome: clinical perspectives. Eye Brain. 2015;7:35-46. doi:10.2147/EB.S63633
7. Lykstad J, Reddy V, Hanna A. Neuroanatomy, Pupillary Dilation Pathway. StatPearls Publishing; 2022. Updated August 11, 2021. Accessed February 1, 2023. https://www.ncbi.nlm.nih.gov/books/NBK535421
8. Friedman N, Kaiser P, Pineda R. The Massachusetts Eye and Ear Infirmary Illustrated Manual of Ophthalmology. 5th ed. Elsevier; 2020.
9. Silbert PL, Mokri B, Schievink WI. Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology. 1995;45(8):1517-1522. doi:10.1212/wnl.45.8.1517
10. Gervasio K, Peck T. The Will’s Eye Manual. 8th ed. Walters Kluwer; 2022.
11. Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906. doi:10.1056/NEJM200103223441206
12. Hart RG, Easton JD. Dissections of cervical and cerebral arteries. Neurol Clin. 1983;1(1):155-182.
13. Goodfriend SD, Tadi P, Koury R. Carotid Artery Dissection. StatPearls Publishing; 2022. Updated December 24, 2021. Accessed February 1, 2023. https://www.ncbi.nlm.nih.gov/books/NBK430835
14. Blum CA, Yaghi S. Cervical artery dissection: a review of the epidemiology, pathophysiology, treatment, and outcome. Arch Neurosci. 2015;2(4):e26670. doi:10.5812/archneurosci.26670
15. Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(1):227-276. doi:10.1161/STR.0b013e3181f7d043
16. Mohr JP, Thompson JL, Lazar RM, et al; Warfarin-Aspirin Recurrent Stroke Study Group. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001;345(20):1444-1451. doi:10.1056/NEJMoa011258
17. Davagnanam I, Fraser CL, Miszkiel K, Daniel CS, Plant GT. Adult Horner’s syndrome: a combined clinical, pharmacological, and imaging algorithm. Eye (Lond). 2013;27(3):291-298. doi:10.1038/eye.2012.281
1. Yanoff M, Duker J. Ophthalmology. 5th ed. Elsevier; 2019.
2. Payne WN, Blair K, Barrett MJ. Anisocoria. StatPearls Publishing; 2022. Accessed February 1, 2023. https://www.ncbi.nlm.nih.gov/books/NBK470384
3. Lee A, Bindiganavile SH, Fan J, Al-Zubidi N, Bhatti MT. Argyll Robertson pupils. Accessed February 1, 2023. https://eyewiki.aao.org/Argyll_Robertson_Pupils
4. Kedar S, Prakalapakorn G, Yen M, et al. Horner syndrome. American Academy of Optometry. 2021. Accessed February 1, 2023. https://eyewiki.aao.org/Horner_Syndrome
5. Daroff R, Bradley W, Jankovic J. Bradley and Daroff’s Neurology in Clinical Practice. 8th ed. Elsevier; 2022.
6. Kanagalingam S, Miller NR. Horner syndrome: clinical perspectives. Eye Brain. 2015;7:35-46. doi:10.2147/EB.S63633
7. Lykstad J, Reddy V, Hanna A. Neuroanatomy, Pupillary Dilation Pathway. StatPearls Publishing; 2022. Updated August 11, 2021. Accessed February 1, 2023. https://www.ncbi.nlm.nih.gov/books/NBK535421
8. Friedman N, Kaiser P, Pineda R. The Massachusetts Eye and Ear Infirmary Illustrated Manual of Ophthalmology. 5th ed. Elsevier; 2020.
9. Silbert PL, Mokri B, Schievink WI. Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology. 1995;45(8):1517-1522. doi:10.1212/wnl.45.8.1517
10. Gervasio K, Peck T. The Will’s Eye Manual. 8th ed. Walters Kluwer; 2022.
11. Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906. doi:10.1056/NEJM200103223441206
12. Hart RG, Easton JD. Dissections of cervical and cerebral arteries. Neurol Clin. 1983;1(1):155-182.
13. Goodfriend SD, Tadi P, Koury R. Carotid Artery Dissection. StatPearls Publishing; 2022. Updated December 24, 2021. Accessed February 1, 2023. https://www.ncbi.nlm.nih.gov/books/NBK430835
14. Blum CA, Yaghi S. Cervical artery dissection: a review of the epidemiology, pathophysiology, treatment, and outcome. Arch Neurosci. 2015;2(4):e26670. doi:10.5812/archneurosci.26670
15. Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(1):227-276. doi:10.1161/STR.0b013e3181f7d043
16. Mohr JP, Thompson JL, Lazar RM, et al; Warfarin-Aspirin Recurrent Stroke Study Group. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001;345(20):1444-1451. doi:10.1056/NEJMoa011258
17. Davagnanam I, Fraser CL, Miszkiel K, Daniel CS, Plant GT. Adult Horner’s syndrome: a combined clinical, pharmacological, and imaging algorithm. Eye (Lond). 2013;27(3):291-298. doi:10.1038/eye.2012.281
Evaluation of Gabapentin and Baclofen Combination for Inpatient Management of Alcohol Withdrawal Syndrome
Alcohol use disorder (AUD) is a chronic disease characterized by an impaired ability to control alcohol use that negatively impacts the social, occupational, and health aspects of patients’ lives.1 It is the third leading modifiable cause of death in the United States.2 About 50% of patients with AUD experience alcohol withdrawal syndrome (AWS) following abrupt cessation of alcohol use. AWS often presents with mild symptoms, such as headaches, nausea, vomiting, and anxiety. However, as many as 20% of patients experience severe and potentially life-threatening symptoms, such as tremors, delirium, hallucinations, and seizures within 48 hours of AWS onset.3
Benzodiazepines, such as lorazepam or chlordiazepoxide, are considered the gold standard for AWS.4 Benzodiazepines act by potentiation of γ-aminobutyric acid (GABA) receptors that produce inhibitory responses in the central nervous system (CNS). This mechanism is similar to the activity of ethanol, which acts primarily at the GABA-A receptors, resulting in facilitation of GABAergic transmission. The Clinical Institute Withdrawal Assessment (CIWA) of Alcohol scale is a commonly used tool to assess the severity of AWS and the appropriate dosing schedule of benzodiazepines.3 Multiple studies have demonstrated the superiority of using benzodiazepines, as they are beneficial for reducing withdrawal severity and incidence of delirium and seizures.5,6
Although benzodiazepines are effective, they are associated with serious adverse effects (AEs), such as respiratory depression, excessive sedation, and abuse potential.4 Older patients are at higher risk of these AEs, particularly oversedation. In addition, sudden discontinuation of a benzodiazepine treatment can result in anxiety, irritability, and insomnia, which might worsen AWS.
Given the safety concerns of benzodiazepines, alternative treatments for AWS management have been investigated, including gabapentin. Previous studies have demonstrated gabapentin might be effective for mild-to-moderate AWS management.7-9 Gabapentin exhibits its action by binding to the α2δ subunit of voltage-activated calcium channels with high affinity. Although the exact mechanism of action of gabapentin in AWS is unknown, it has been proposed that gabapentin normalizes GABA activation in the amygdala, which is associated with alcohol dependence.10 A systemic review conducted by Leung and colleagues found that gabapentin might be an option for the management of mild AWS.11 However, current evidence does not support the use of gabapentin monotherapy in patients with severe AWS, a history of seizures, or those at risk of delirium tremens (DTs) since there is a higher chance of complications.
Baclofen is another medication investigated by researchers for use in patients with AWS. Baclofen works by activating the GABA-B receptor, which results in the downregulation of GABA-A activity. This results in a negative feedback loop leading to a decrease in excitatory neurotransmitters that is similar to the effect produced by alcohol.12 However, there is limited evidence that baclofen is effective as monotherapy for the treatment of AWS. A Cochrane review previously evaluated baclofen use in AWS but found insufficient evidence of its efficacy and safety for this indication.
The Captain James A. Lovell Federal Health Care Center (CJALFHCC) in North Chicago, Illinois, currently uses a protocol in which the combination of gabapentin and baclofen is an option for AWS management in the inpatient setting. According to the current protocol, the combination of gabapentin and baclofen (g/b) is indicated for patients whose CIWA score is ≤ 8. If the CIWA score is 9 to 15, lorazepam or chlordiazepoxide should be used; if the CIWA score is 16 to 20, lorazepam should be used; and if the CIWA score is greater than 20, then lorazepam and dexmedetomidine are recommended. The protocol also lists certain patient characteristics, such as history of seizures, traumatic brain injury, or long duration of alcohol consumption, in which clinical judgment should be used to determine whether a described detoxification regimen is appropriate or whether the patient should be managed off-protocol.
Because to our knowledge, no current studies have investigated the use of g/b for inpatient AWS, the goal of this study was to evaluate its efficacy and safety. We hypothesized that AWS duration would be significantly different in patients who received g/b for AWS management compared with those treated with benzodiazepines.
Methods
We performed a retrospective cohort chart review at CJALFHCC. Data were collected from the facility’s electronic health record Computerized Patient Record System (CPRS). This study was approved by the Edward Hines Jr. Veterans Affairs Hospital Institutional Review Board.
Patient records were screened and included if they met the following criteria: (1) Patients aged ≥ 18 years who were hospitalized from January 1, 2014, to July 31, 2021, for the primary indication of AWS; (2) Patients who received a g/b or benzodiazepine protocol during AWS hospitalization. If a patient was admitted multiple times for AWS management, only the first admission was included for primary outcome analysis. Exclusion criteria were patients who were active-duty service members, discharged within 24 hours; patients with a primary seizure disorder; patients with known gabapentin, baclofen, or benzodiazepine allergy or intolerance. Patients who used gabapentin, baclofen, or benzodiazepines in an outpatient setting prior to AWS admission; had concurrent intoxication or overdose involving substances other than alcohol; had a concurrent regimen of gabapentin, baclofen, or benzodiazepines; or had initiation on adjuvant medications for AWS management (eg, divalproex, haloperidol, carbamazepine, or clonidine) also were excluded. Patients were categorized as those who received g/b as the initial therapy after admission or patients who received benzodiazepine therapy.
The primary outcome of this study was the length of stay (LOS), which was
CPRS was used to collect information including baseline demographics, blood alcohol content, CIWA scores throughout hospitalization, number of admissions for alcohol detoxification in the previous year, AWS readmission within 30 days after discharge, prior treatment with g/b, history of alcohol withdrawal seizures and DTs, hospital LOS, outpatient medications for AUD treatment, rates of conversions from g/b protocol to lorazepam, and rates of transition to a higher level of care.
Statistical Analysis
Study data were stored and analyzed using an Excel spreadsheet and IBM SPSS Statistics software. LOS was compared between the g/b and benzodiazepine groups using inferential statistics. An independent 2-sample t test was used to assess the primary outcome if data were normally distributed. If the collected data were not distributed normally, the Mann-Whitney U test was used. All other continuous variables were assessed by using independent t tests and categorical variables by using χ2 tests. A P value < .05 was considered statistically significant. Effect size of d = 0.42 was calculated based on a previous study with a similar research design as our study.9 We determined that if using an independent 2-sample t test for the primary outcome analysis, an estimated sample size of 178 subjects would provide the study with an 80% power to detect a difference at a 2-sided significance level with α = 0.05. If using the Mann-Whitney U test, 186 subjects would be required to provide identical power.
Results
We reviewed 196 patient health records, and 39 were initially excluded. The most common reason was that AWS was not the primary diagnosis for hospitalization (n = 28).
The Shapiro-Wilk tests showed a significant departure from normality in the benzodiazepine group W(35) = 0.805 (P < .001) and g/b group W(20) = 0.348 (P < .001) for the primary outcome.
Additionally, this study examined multiple secondary outcomes (Table 2).
Discussion
This retrospective chart review study found that LOS was shorter in patients with AWS treated with g/b compared with those treated with benzodiazepines, with no significant difference in safety outcomes such as seizures, DTs, or intensive care unit transfers. Although there was a statistically significant difference in the primary outcome between the 2 groups, it appears that patients on benzodiazepine therapy originally had more severe AWS presentation as their admission and maximum CIWA scores were statistically significantly higher compared with the g/b group. Thus, patients who were initially started on g/b had less serious AWS presentations. Based on this information we can conclude that the g/b combination may be an effective option for mild AWS management.
To our knowledge, this is the first study that has investigated the combination of g/b compared with benzodiazepines for AWS management in hospitalized patients. The research design of this project was adapted from the Bates and colleagues study that examined gabapentin monotherapy use for the treatment of patients hospitalized with AWS.9 We specifically used the primary outcome that they defined in their study since their LOS definition aimed to reflect clinically active withdrawal rather than simply hours of hospitalization, which would decrease the risk of confounding the primary outcome. The results of our research were similar to Bates and colleagues as they found that the gabapentin protocol appeared to be an effective and safe option compared with benzodiazepines for patients hospitalized with AWS.9
Limitations
This study has multiple limitations. As it was a retrospective chart review study, the data collection accuracy depends on accurate recordkeeping. Additionally, certain information was missing, such as CIWA scores for some patients. This study has limited external validity as most of the patients were older, White, and male, and the data collection was limited only to a single center. Therefore, it is uncertain whether the results of this study can be generalized to other populations. Also, this study had a small sample size, and we were not able to obtain the intended number of patients to achieve a power of 80%. Lastly, some background characteristics, such as admission and maximum CIWA scores, were not distributed equally between groups. Therefore, future studies are needed with a larger sample size that examine the LOS in the g/b group compared with the benzodiazepine group and in which CIWA scores are matched to reduce the effect of extraneous variables.
Conclusions
Gabapentin and baclofen combination seems to be an effective and safe alternative to benzodiazepines and may be considered for managing mild AWS in hospitalized patients, but additional research is needed to examine this regimen.
Acknowledgments
Research committee: Hong-Yen Vi, PharmD, BCPS; Shaiza Khan, PharmD, BCPS; Yinka Alaka, PharmD; Jennifer Kwon, PharmD, BCOP. Co-investigators: Zachary Rosenfeldt, PharmD, BCPS; Kaylee Caniff, PharmD, BCIDP.
1. National Institute on Alcohol Abuse and Alcoholism. Understanding alcohol use disorder. 2020. Updated April 2021. Accessed February 2, 2023. https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/understanding-alcohol-use-disorder
2. Moss HB. The impact of alcohol on society: a brief overview. Soc Work Public Health. 2013;28(3-4):175-177. doi:10.1080/19371918.2013.758987
3. Pace C. Alcohol withdrawal: epidemiology, clinical manifestations, course, assessment, and diagnosis. Accessed January 26, 2023. https://www.uptodate.com/contents/alcohol-withdrawal-epidemiology-clinical-manifestations-course-assessment-and-diagnosis
4. Sachdeva A, Choudhary M, Chandra M. Alcohol withdrawal syndrome: benzodiazepines and beyond. J Clin Diagn Res. 2015;9(9):VE01-VE07. doi:10.7860/JCDR/2015/13407.6538
5. Mayo-Smith MF. Pharmacological management of alcohol withdrawal. A meta-analysis and evidence-based practice guideline. American Society of Addiction Medicine Working Group on Pharmacological Management of Alcohol Withdrawal. JAMA. 1997;278(2):144-151. doi:10.1001/jama.278.2.144
6. Holbrook AM, Crowther R, Lotter A, Cheng C, King D. Meta-analysis of benzodiazepine use in the treatment of acute alcohol withdrawal. CMAJ. 1999;160(5):649-655.
7. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588. doi:10.1111/j.1530-0277.2009.00986.x
8. Leung JG, Rakocevic DB, Allen ND, et al. Use of a gabapentin protocol for the management of alcohol withdrawal: a preliminary experience expanding from the consultation-liaison psychiatry service. Psychosomatics. 2018;59(5):496-505. doi:10.1016/j.psym.2018.03.002
9. Bates RE, Leung JG, Morgan RJ 3rd, Fischer KM, Philbrick KL, Kung S. Retrospective analysis of gabapentin for alcohol withdrawal in the hospital setting: the Mayo Clinic experience. Mayo Clin Proc Innov Qual Outcomes. 2020;4(5):542-549. Published 2020 Aug 19. doi:10.1016/j.mayocpiqo.2020.06.002
10. Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77. doi:10.1001/jamainternmed.2013.11950
11. Leung JG, Hall-Flavin D, Nelson S, Schmidt KA, Schak KM. The role of gabapentin in the management of alcohol withdrawal and dependence. Ann Pharmacother. 2015;49(8):897-906. doi:10.1177/1060028015585849
12. Cooney G, Heydtmann M, Smith ID. Baclofen and the alcohol withdrawal syndrome-a short review. Front Psychiatry. 2019;9:773. doi:10.3389/fpsyt.2018.00773
13. Liu J, Wang LN. Baclofen for alcohol withdrawal. Cochrane Database Syst Rev. 2019;2019(11):CD008502. Published 2019 Nov 6. doi:10.1002/14651858.CD008502.pub6
Alcohol use disorder (AUD) is a chronic disease characterized by an impaired ability to control alcohol use that negatively impacts the social, occupational, and health aspects of patients’ lives.1 It is the third leading modifiable cause of death in the United States.2 About 50% of patients with AUD experience alcohol withdrawal syndrome (AWS) following abrupt cessation of alcohol use. AWS often presents with mild symptoms, such as headaches, nausea, vomiting, and anxiety. However, as many as 20% of patients experience severe and potentially life-threatening symptoms, such as tremors, delirium, hallucinations, and seizures within 48 hours of AWS onset.3
Benzodiazepines, such as lorazepam or chlordiazepoxide, are considered the gold standard for AWS.4 Benzodiazepines act by potentiation of γ-aminobutyric acid (GABA) receptors that produce inhibitory responses in the central nervous system (CNS). This mechanism is similar to the activity of ethanol, which acts primarily at the GABA-A receptors, resulting in facilitation of GABAergic transmission. The Clinical Institute Withdrawal Assessment (CIWA) of Alcohol scale is a commonly used tool to assess the severity of AWS and the appropriate dosing schedule of benzodiazepines.3 Multiple studies have demonstrated the superiority of using benzodiazepines, as they are beneficial for reducing withdrawal severity and incidence of delirium and seizures.5,6
Although benzodiazepines are effective, they are associated with serious adverse effects (AEs), such as respiratory depression, excessive sedation, and abuse potential.4 Older patients are at higher risk of these AEs, particularly oversedation. In addition, sudden discontinuation of a benzodiazepine treatment can result in anxiety, irritability, and insomnia, which might worsen AWS.
Given the safety concerns of benzodiazepines, alternative treatments for AWS management have been investigated, including gabapentin. Previous studies have demonstrated gabapentin might be effective for mild-to-moderate AWS management.7-9 Gabapentin exhibits its action by binding to the α2δ subunit of voltage-activated calcium channels with high affinity. Although the exact mechanism of action of gabapentin in AWS is unknown, it has been proposed that gabapentin normalizes GABA activation in the amygdala, which is associated with alcohol dependence.10 A systemic review conducted by Leung and colleagues found that gabapentin might be an option for the management of mild AWS.11 However, current evidence does not support the use of gabapentin monotherapy in patients with severe AWS, a history of seizures, or those at risk of delirium tremens (DTs) since there is a higher chance of complications.
Baclofen is another medication investigated by researchers for use in patients with AWS. Baclofen works by activating the GABA-B receptor, which results in the downregulation of GABA-A activity. This results in a negative feedback loop leading to a decrease in excitatory neurotransmitters that is similar to the effect produced by alcohol.12 However, there is limited evidence that baclofen is effective as monotherapy for the treatment of AWS. A Cochrane review previously evaluated baclofen use in AWS but found insufficient evidence of its efficacy and safety for this indication.
The Captain James A. Lovell Federal Health Care Center (CJALFHCC) in North Chicago, Illinois, currently uses a protocol in which the combination of gabapentin and baclofen is an option for AWS management in the inpatient setting. According to the current protocol, the combination of gabapentin and baclofen (g/b) is indicated for patients whose CIWA score is ≤ 8. If the CIWA score is 9 to 15, lorazepam or chlordiazepoxide should be used; if the CIWA score is 16 to 20, lorazepam should be used; and if the CIWA score is greater than 20, then lorazepam and dexmedetomidine are recommended. The protocol also lists certain patient characteristics, such as history of seizures, traumatic brain injury, or long duration of alcohol consumption, in which clinical judgment should be used to determine whether a described detoxification regimen is appropriate or whether the patient should be managed off-protocol.
Because to our knowledge, no current studies have investigated the use of g/b for inpatient AWS, the goal of this study was to evaluate its efficacy and safety. We hypothesized that AWS duration would be significantly different in patients who received g/b for AWS management compared with those treated with benzodiazepines.
Methods
We performed a retrospective cohort chart review at CJALFHCC. Data were collected from the facility’s electronic health record Computerized Patient Record System (CPRS). This study was approved by the Edward Hines Jr. Veterans Affairs Hospital Institutional Review Board.
Patient records were screened and included if they met the following criteria: (1) Patients aged ≥ 18 years who were hospitalized from January 1, 2014, to July 31, 2021, for the primary indication of AWS; (2) Patients who received a g/b or benzodiazepine protocol during AWS hospitalization. If a patient was admitted multiple times for AWS management, only the first admission was included for primary outcome analysis. Exclusion criteria were patients who were active-duty service members, discharged within 24 hours; patients with a primary seizure disorder; patients with known gabapentin, baclofen, or benzodiazepine allergy or intolerance. Patients who used gabapentin, baclofen, or benzodiazepines in an outpatient setting prior to AWS admission; had concurrent intoxication or overdose involving substances other than alcohol; had a concurrent regimen of gabapentin, baclofen, or benzodiazepines; or had initiation on adjuvant medications for AWS management (eg, divalproex, haloperidol, carbamazepine, or clonidine) also were excluded. Patients were categorized as those who received g/b as the initial therapy after admission or patients who received benzodiazepine therapy.
The primary outcome of this study was the length of stay (LOS), which was
CPRS was used to collect information including baseline demographics, blood alcohol content, CIWA scores throughout hospitalization, number of admissions for alcohol detoxification in the previous year, AWS readmission within 30 days after discharge, prior treatment with g/b, history of alcohol withdrawal seizures and DTs, hospital LOS, outpatient medications for AUD treatment, rates of conversions from g/b protocol to lorazepam, and rates of transition to a higher level of care.
Statistical Analysis
Study data were stored and analyzed using an Excel spreadsheet and IBM SPSS Statistics software. LOS was compared between the g/b and benzodiazepine groups using inferential statistics. An independent 2-sample t test was used to assess the primary outcome if data were normally distributed. If the collected data were not distributed normally, the Mann-Whitney U test was used. All other continuous variables were assessed by using independent t tests and categorical variables by using χ2 tests. A P value < .05 was considered statistically significant. Effect size of d = 0.42 was calculated based on a previous study with a similar research design as our study.9 We determined that if using an independent 2-sample t test for the primary outcome analysis, an estimated sample size of 178 subjects would provide the study with an 80% power to detect a difference at a 2-sided significance level with α = 0.05. If using the Mann-Whitney U test, 186 subjects would be required to provide identical power.
Results
We reviewed 196 patient health records, and 39 were initially excluded. The most common reason was that AWS was not the primary diagnosis for hospitalization (n = 28).
The Shapiro-Wilk tests showed a significant departure from normality in the benzodiazepine group W(35) = 0.805 (P < .001) and g/b group W(20) = 0.348 (P < .001) for the primary outcome.
Additionally, this study examined multiple secondary outcomes (Table 2).
Discussion
This retrospective chart review study found that LOS was shorter in patients with AWS treated with g/b compared with those treated with benzodiazepines, with no significant difference in safety outcomes such as seizures, DTs, or intensive care unit transfers. Although there was a statistically significant difference in the primary outcome between the 2 groups, it appears that patients on benzodiazepine therapy originally had more severe AWS presentation as their admission and maximum CIWA scores were statistically significantly higher compared with the g/b group. Thus, patients who were initially started on g/b had less serious AWS presentations. Based on this information we can conclude that the g/b combination may be an effective option for mild AWS management.
To our knowledge, this is the first study that has investigated the combination of g/b compared with benzodiazepines for AWS management in hospitalized patients. The research design of this project was adapted from the Bates and colleagues study that examined gabapentin monotherapy use for the treatment of patients hospitalized with AWS.9 We specifically used the primary outcome that they defined in their study since their LOS definition aimed to reflect clinically active withdrawal rather than simply hours of hospitalization, which would decrease the risk of confounding the primary outcome. The results of our research were similar to Bates and colleagues as they found that the gabapentin protocol appeared to be an effective and safe option compared with benzodiazepines for patients hospitalized with AWS.9
Limitations
This study has multiple limitations. As it was a retrospective chart review study, the data collection accuracy depends on accurate recordkeeping. Additionally, certain information was missing, such as CIWA scores for some patients. This study has limited external validity as most of the patients were older, White, and male, and the data collection was limited only to a single center. Therefore, it is uncertain whether the results of this study can be generalized to other populations. Also, this study had a small sample size, and we were not able to obtain the intended number of patients to achieve a power of 80%. Lastly, some background characteristics, such as admission and maximum CIWA scores, were not distributed equally between groups. Therefore, future studies are needed with a larger sample size that examine the LOS in the g/b group compared with the benzodiazepine group and in which CIWA scores are matched to reduce the effect of extraneous variables.
Conclusions
Gabapentin and baclofen combination seems to be an effective and safe alternative to benzodiazepines and may be considered for managing mild AWS in hospitalized patients, but additional research is needed to examine this regimen.
Acknowledgments
Research committee: Hong-Yen Vi, PharmD, BCPS; Shaiza Khan, PharmD, BCPS; Yinka Alaka, PharmD; Jennifer Kwon, PharmD, BCOP. Co-investigators: Zachary Rosenfeldt, PharmD, BCPS; Kaylee Caniff, PharmD, BCIDP.
Alcohol use disorder (AUD) is a chronic disease characterized by an impaired ability to control alcohol use that negatively impacts the social, occupational, and health aspects of patients’ lives.1 It is the third leading modifiable cause of death in the United States.2 About 50% of patients with AUD experience alcohol withdrawal syndrome (AWS) following abrupt cessation of alcohol use. AWS often presents with mild symptoms, such as headaches, nausea, vomiting, and anxiety. However, as many as 20% of patients experience severe and potentially life-threatening symptoms, such as tremors, delirium, hallucinations, and seizures within 48 hours of AWS onset.3
Benzodiazepines, such as lorazepam or chlordiazepoxide, are considered the gold standard for AWS.4 Benzodiazepines act by potentiation of γ-aminobutyric acid (GABA) receptors that produce inhibitory responses in the central nervous system (CNS). This mechanism is similar to the activity of ethanol, which acts primarily at the GABA-A receptors, resulting in facilitation of GABAergic transmission. The Clinical Institute Withdrawal Assessment (CIWA) of Alcohol scale is a commonly used tool to assess the severity of AWS and the appropriate dosing schedule of benzodiazepines.3 Multiple studies have demonstrated the superiority of using benzodiazepines, as they are beneficial for reducing withdrawal severity and incidence of delirium and seizures.5,6
Although benzodiazepines are effective, they are associated with serious adverse effects (AEs), such as respiratory depression, excessive sedation, and abuse potential.4 Older patients are at higher risk of these AEs, particularly oversedation. In addition, sudden discontinuation of a benzodiazepine treatment can result in anxiety, irritability, and insomnia, which might worsen AWS.
Given the safety concerns of benzodiazepines, alternative treatments for AWS management have been investigated, including gabapentin. Previous studies have demonstrated gabapentin might be effective for mild-to-moderate AWS management.7-9 Gabapentin exhibits its action by binding to the α2δ subunit of voltage-activated calcium channels with high affinity. Although the exact mechanism of action of gabapentin in AWS is unknown, it has been proposed that gabapentin normalizes GABA activation in the amygdala, which is associated with alcohol dependence.10 A systemic review conducted by Leung and colleagues found that gabapentin might be an option for the management of mild AWS.11 However, current evidence does not support the use of gabapentin monotherapy in patients with severe AWS, a history of seizures, or those at risk of delirium tremens (DTs) since there is a higher chance of complications.
Baclofen is another medication investigated by researchers for use in patients with AWS. Baclofen works by activating the GABA-B receptor, which results in the downregulation of GABA-A activity. This results in a negative feedback loop leading to a decrease in excitatory neurotransmitters that is similar to the effect produced by alcohol.12 However, there is limited evidence that baclofen is effective as monotherapy for the treatment of AWS. A Cochrane review previously evaluated baclofen use in AWS but found insufficient evidence of its efficacy and safety for this indication.
The Captain James A. Lovell Federal Health Care Center (CJALFHCC) in North Chicago, Illinois, currently uses a protocol in which the combination of gabapentin and baclofen is an option for AWS management in the inpatient setting. According to the current protocol, the combination of gabapentin and baclofen (g/b) is indicated for patients whose CIWA score is ≤ 8. If the CIWA score is 9 to 15, lorazepam or chlordiazepoxide should be used; if the CIWA score is 16 to 20, lorazepam should be used; and if the CIWA score is greater than 20, then lorazepam and dexmedetomidine are recommended. The protocol also lists certain patient characteristics, such as history of seizures, traumatic brain injury, or long duration of alcohol consumption, in which clinical judgment should be used to determine whether a described detoxification regimen is appropriate or whether the patient should be managed off-protocol.
Because to our knowledge, no current studies have investigated the use of g/b for inpatient AWS, the goal of this study was to evaluate its efficacy and safety. We hypothesized that AWS duration would be significantly different in patients who received g/b for AWS management compared with those treated with benzodiazepines.
Methods
We performed a retrospective cohort chart review at CJALFHCC. Data were collected from the facility’s electronic health record Computerized Patient Record System (CPRS). This study was approved by the Edward Hines Jr. Veterans Affairs Hospital Institutional Review Board.
Patient records were screened and included if they met the following criteria: (1) Patients aged ≥ 18 years who were hospitalized from January 1, 2014, to July 31, 2021, for the primary indication of AWS; (2) Patients who received a g/b or benzodiazepine protocol during AWS hospitalization. If a patient was admitted multiple times for AWS management, only the first admission was included for primary outcome analysis. Exclusion criteria were patients who were active-duty service members, discharged within 24 hours; patients with a primary seizure disorder; patients with known gabapentin, baclofen, or benzodiazepine allergy or intolerance. Patients who used gabapentin, baclofen, or benzodiazepines in an outpatient setting prior to AWS admission; had concurrent intoxication or overdose involving substances other than alcohol; had a concurrent regimen of gabapentin, baclofen, or benzodiazepines; or had initiation on adjuvant medications for AWS management (eg, divalproex, haloperidol, carbamazepine, or clonidine) also were excluded. Patients were categorized as those who received g/b as the initial therapy after admission or patients who received benzodiazepine therapy.
The primary outcome of this study was the length of stay (LOS), which was
CPRS was used to collect information including baseline demographics, blood alcohol content, CIWA scores throughout hospitalization, number of admissions for alcohol detoxification in the previous year, AWS readmission within 30 days after discharge, prior treatment with g/b, history of alcohol withdrawal seizures and DTs, hospital LOS, outpatient medications for AUD treatment, rates of conversions from g/b protocol to lorazepam, and rates of transition to a higher level of care.
Statistical Analysis
Study data were stored and analyzed using an Excel spreadsheet and IBM SPSS Statistics software. LOS was compared between the g/b and benzodiazepine groups using inferential statistics. An independent 2-sample t test was used to assess the primary outcome if data were normally distributed. If the collected data were not distributed normally, the Mann-Whitney U test was used. All other continuous variables were assessed by using independent t tests and categorical variables by using χ2 tests. A P value < .05 was considered statistically significant. Effect size of d = 0.42 was calculated based on a previous study with a similar research design as our study.9 We determined that if using an independent 2-sample t test for the primary outcome analysis, an estimated sample size of 178 subjects would provide the study with an 80% power to detect a difference at a 2-sided significance level with α = 0.05. If using the Mann-Whitney U test, 186 subjects would be required to provide identical power.
Results
We reviewed 196 patient health records, and 39 were initially excluded. The most common reason was that AWS was not the primary diagnosis for hospitalization (n = 28).
The Shapiro-Wilk tests showed a significant departure from normality in the benzodiazepine group W(35) = 0.805 (P < .001) and g/b group W(20) = 0.348 (P < .001) for the primary outcome.
Additionally, this study examined multiple secondary outcomes (Table 2).
Discussion
This retrospective chart review study found that LOS was shorter in patients with AWS treated with g/b compared with those treated with benzodiazepines, with no significant difference in safety outcomes such as seizures, DTs, or intensive care unit transfers. Although there was a statistically significant difference in the primary outcome between the 2 groups, it appears that patients on benzodiazepine therapy originally had more severe AWS presentation as their admission and maximum CIWA scores were statistically significantly higher compared with the g/b group. Thus, patients who were initially started on g/b had less serious AWS presentations. Based on this information we can conclude that the g/b combination may be an effective option for mild AWS management.
To our knowledge, this is the first study that has investigated the combination of g/b compared with benzodiazepines for AWS management in hospitalized patients. The research design of this project was adapted from the Bates and colleagues study that examined gabapentin monotherapy use for the treatment of patients hospitalized with AWS.9 We specifically used the primary outcome that they defined in their study since their LOS definition aimed to reflect clinically active withdrawal rather than simply hours of hospitalization, which would decrease the risk of confounding the primary outcome. The results of our research were similar to Bates and colleagues as they found that the gabapentin protocol appeared to be an effective and safe option compared with benzodiazepines for patients hospitalized with AWS.9
Limitations
This study has multiple limitations. As it was a retrospective chart review study, the data collection accuracy depends on accurate recordkeeping. Additionally, certain information was missing, such as CIWA scores for some patients. This study has limited external validity as most of the patients were older, White, and male, and the data collection was limited only to a single center. Therefore, it is uncertain whether the results of this study can be generalized to other populations. Also, this study had a small sample size, and we were not able to obtain the intended number of patients to achieve a power of 80%. Lastly, some background characteristics, such as admission and maximum CIWA scores, were not distributed equally between groups. Therefore, future studies are needed with a larger sample size that examine the LOS in the g/b group compared with the benzodiazepine group and in which CIWA scores are matched to reduce the effect of extraneous variables.
Conclusions
Gabapentin and baclofen combination seems to be an effective and safe alternative to benzodiazepines and may be considered for managing mild AWS in hospitalized patients, but additional research is needed to examine this regimen.
Acknowledgments
Research committee: Hong-Yen Vi, PharmD, BCPS; Shaiza Khan, PharmD, BCPS; Yinka Alaka, PharmD; Jennifer Kwon, PharmD, BCOP. Co-investigators: Zachary Rosenfeldt, PharmD, BCPS; Kaylee Caniff, PharmD, BCIDP.
1. National Institute on Alcohol Abuse and Alcoholism. Understanding alcohol use disorder. 2020. Updated April 2021. Accessed February 2, 2023. https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/understanding-alcohol-use-disorder
2. Moss HB. The impact of alcohol on society: a brief overview. Soc Work Public Health. 2013;28(3-4):175-177. doi:10.1080/19371918.2013.758987
3. Pace C. Alcohol withdrawal: epidemiology, clinical manifestations, course, assessment, and diagnosis. Accessed January 26, 2023. https://www.uptodate.com/contents/alcohol-withdrawal-epidemiology-clinical-manifestations-course-assessment-and-diagnosis
4. Sachdeva A, Choudhary M, Chandra M. Alcohol withdrawal syndrome: benzodiazepines and beyond. J Clin Diagn Res. 2015;9(9):VE01-VE07. doi:10.7860/JCDR/2015/13407.6538
5. Mayo-Smith MF. Pharmacological management of alcohol withdrawal. A meta-analysis and evidence-based practice guideline. American Society of Addiction Medicine Working Group on Pharmacological Management of Alcohol Withdrawal. JAMA. 1997;278(2):144-151. doi:10.1001/jama.278.2.144
6. Holbrook AM, Crowther R, Lotter A, Cheng C, King D. Meta-analysis of benzodiazepine use in the treatment of acute alcohol withdrawal. CMAJ. 1999;160(5):649-655.
7. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588. doi:10.1111/j.1530-0277.2009.00986.x
8. Leung JG, Rakocevic DB, Allen ND, et al. Use of a gabapentin protocol for the management of alcohol withdrawal: a preliminary experience expanding from the consultation-liaison psychiatry service. Psychosomatics. 2018;59(5):496-505. doi:10.1016/j.psym.2018.03.002
9. Bates RE, Leung JG, Morgan RJ 3rd, Fischer KM, Philbrick KL, Kung S. Retrospective analysis of gabapentin for alcohol withdrawal in the hospital setting: the Mayo Clinic experience. Mayo Clin Proc Innov Qual Outcomes. 2020;4(5):542-549. Published 2020 Aug 19. doi:10.1016/j.mayocpiqo.2020.06.002
10. Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77. doi:10.1001/jamainternmed.2013.11950
11. Leung JG, Hall-Flavin D, Nelson S, Schmidt KA, Schak KM. The role of gabapentin in the management of alcohol withdrawal and dependence. Ann Pharmacother. 2015;49(8):897-906. doi:10.1177/1060028015585849
12. Cooney G, Heydtmann M, Smith ID. Baclofen and the alcohol withdrawal syndrome-a short review. Front Psychiatry. 2019;9:773. doi:10.3389/fpsyt.2018.00773
13. Liu J, Wang LN. Baclofen for alcohol withdrawal. Cochrane Database Syst Rev. 2019;2019(11):CD008502. Published 2019 Nov 6. doi:10.1002/14651858.CD008502.pub6
1. National Institute on Alcohol Abuse and Alcoholism. Understanding alcohol use disorder. 2020. Updated April 2021. Accessed February 2, 2023. https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/understanding-alcohol-use-disorder
2. Moss HB. The impact of alcohol on society: a brief overview. Soc Work Public Health. 2013;28(3-4):175-177. doi:10.1080/19371918.2013.758987
3. Pace C. Alcohol withdrawal: epidemiology, clinical manifestations, course, assessment, and diagnosis. Accessed January 26, 2023. https://www.uptodate.com/contents/alcohol-withdrawal-epidemiology-clinical-manifestations-course-assessment-and-diagnosis
4. Sachdeva A, Choudhary M, Chandra M. Alcohol withdrawal syndrome: benzodiazepines and beyond. J Clin Diagn Res. 2015;9(9):VE01-VE07. doi:10.7860/JCDR/2015/13407.6538
5. Mayo-Smith MF. Pharmacological management of alcohol withdrawal. A meta-analysis and evidence-based practice guideline. American Society of Addiction Medicine Working Group on Pharmacological Management of Alcohol Withdrawal. JAMA. 1997;278(2):144-151. doi:10.1001/jama.278.2.144
6. Holbrook AM, Crowther R, Lotter A, Cheng C, King D. Meta-analysis of benzodiazepine use in the treatment of acute alcohol withdrawal. CMAJ. 1999;160(5):649-655.
7. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588. doi:10.1111/j.1530-0277.2009.00986.x
8. Leung JG, Rakocevic DB, Allen ND, et al. Use of a gabapentin protocol for the management of alcohol withdrawal: a preliminary experience expanding from the consultation-liaison psychiatry service. Psychosomatics. 2018;59(5):496-505. doi:10.1016/j.psym.2018.03.002
9. Bates RE, Leung JG, Morgan RJ 3rd, Fischer KM, Philbrick KL, Kung S. Retrospective analysis of gabapentin for alcohol withdrawal in the hospital setting: the Mayo Clinic experience. Mayo Clin Proc Innov Qual Outcomes. 2020;4(5):542-549. Published 2020 Aug 19. doi:10.1016/j.mayocpiqo.2020.06.002
10. Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77. doi:10.1001/jamainternmed.2013.11950
11. Leung JG, Hall-Flavin D, Nelson S, Schmidt KA, Schak KM. The role of gabapentin in the management of alcohol withdrawal and dependence. Ann Pharmacother. 2015;49(8):897-906. doi:10.1177/1060028015585849
12. Cooney G, Heydtmann M, Smith ID. Baclofen and the alcohol withdrawal syndrome-a short review. Front Psychiatry. 2019;9:773. doi:10.3389/fpsyt.2018.00773
13. Liu J, Wang LN. Baclofen for alcohol withdrawal. Cochrane Database Syst Rev. 2019;2019(11):CD008502. Published 2019 Nov 6. doi:10.1002/14651858.CD008502.pub6
Battlefield Acupuncture vs Ketorolac for Treating Pain in the Emergency Department
Acute pain is a primary symptom for many patients who present to the emergency department (ED). The ED team is challenged with relieving pain while limiting harm from medications.1 A 2017 National Health Interview Survey showed that compared with nonveterans, more veterans reported pain in the previous 3 months, and the rate of severe pain was 40% higher in the veteran group especially among those who served during the era of wars in Afghanistan and Iraq.2
The American College of Emergency Physicians guidelines pain management guidelines recommend patient-centered shared decision making that includes patient education about treatment goals and expectations, and short- and long-term risks, as well as a preference toward pharmacologic treatment with nonopioid analgesics except for patients with severe pain or pain refractory to other drug and treatment modalities.3 There is a lack of evidence regarding superior efficacy of either opioid or nonopioid analgesics; therefore, the use of nonopioid analgesics, such as oral or topical nonsteroidal anti-inflammatory drugs (NSAIDs) or central analgesics, such as acetaminophen, is preferred for treating acute pain to mitigate adverse effects (AEs) and risks associated with opioid use.1,3,4 The US Department of Veterans Affairs (VA) and Department of Defense (DoD) guideline on managing opioid therapy for chronic pain, updated in 2017 and 2022, similarly recommends alternatives to opioids for mild-to-moderate acute pain and encourages multimodal pain care.5 However, use of other pharmacologic treatments, such as NSAIDs, is limited by AE profiles, patient contraindications, and severity of acute pain etiologies. There is a need for the expanded use of nonpharmacologic treatments for addressing pain in the veteran population.
The American College of Emergency Physicians guidelines recommend nonpharmacologic modalities, such as applying heat or cold, physical therapy, cognitive behavioral therapy, and acupuncture.3 A 2014 study reported that 37% to 46% of active duty and reserve military personnel use complementary and alternative medicine (CAM) for a variety of ailments, and there is increasing interest in the use of CAM as adjuncts to traditional therapies.6 According to one study, some CAM therapies are used significantly more by military personnel than used by civilians.7 However, the percentage of the veteran population using acupuncture in this study was small, and more information is needed to assess its use.
Auricular acupuncture originated in traditional Chinese medicine.8 Contemporary auricular acupuncture experts view this modality as a self-contained microsystem mapping portions of the ear to specific parts of the body and internal organs. The analgesic effects may be mediated through the central nervous system by local release of endorphins through nerve fiber activation and neurotransmitters—including serotonin, dopamine, and norepinephrine—leading to pre- and postsynaptic suppression of pain transmission.
Battlefield acupuncture (BFA) uses 5 set points anatomically located on each ear.9 Practitioners use small semipermanent, dartlike acupuncture needles. Patients could experience pain relief in a few minutes, which can last minutes, hours, days, weeks, or months depending on the pathology of the pain. This procedure developed in 2001 has been studied for different pain types and has shown benefit when used for postsurgical pain, chronic spinal cord injury−related neuropathic pain, and general chronic pain, as well as for other indications, such as insomnia, depression, and weight loss.8,10-13 In 2018, a randomized controlled trial compared postintervention numeric rating scale (NRS) pain scores in patients presenting to the ED with acute or acute-on-chronic lower back pain who received BFA as an adjunct to standard care vs standard care alone.14 Patients receiving BFA as an adjunct to standard care were found to have mean postintervention pain scores 1.7 points lower than those receiving standard care alone. This study demonstrated that BFA was feasible and well tolerated for lower back pain in the ED as an adjunct to standard care. The study was limited by the adjunct use of BFA rather than as monotherapy and by the practitioners’ discretion regarding standard care, which was not defined by the study’s authors.
The Jesse Brown Veterans Affairs Medical Center (JBVAMC) in Chicago, Illinois, offers several CAM modalities, such as exercise/movement therapy, chiropractic, art/music therapy, and relaxation workshops, which are widely used by veterans. Recent evidence suggests BFA could reduce pain scores as an adjunct or an alternative to pharmacologic therapy. We are interested in how CAM therapies, such as BFA, can help avoid AEs associated with opioid or NSAID therapy.
At the JBVAMC ED, ketorolac 15 mg is the preferred first-line treatment of acute, noncancer pain, based on the results of previous studies. In 2018 BFA was offered first to veterans presenting with acute or acute-on-chronic pain to the ED; however, its effectiveness for pain reduction vs ketorolac has not been evaluated in this patient population. Limited literature is available on BFA and its use in the ED. To our knowledge, this was the first observational study assessing the difference between a single session of BFA vs a single dose of ketorolac in treating noncancer acute or acute-on-chronic pain in the ED.
Methods
This study was a retrospective chart review of patients who presented to the JBVAMC ED with acute pain or acute-on-chronic pain, who received ketorolac or BFA. The study population was generated from a list of all IV and intramuscular (IM) ketorolac unit dose orders verified from June 1, 2018, through August 30, 2019, and a list of all BFA procedure notes signed from June 1, 2018, through August 30, 2019. Patients were included in the study if they had documented administration of IV or IM ketorolac or BFA between June 1, 2018, and August 30, 2019. Patients who received ketorolac doses other than 15 mg, the intervention was administered outside of the ED, received adjunct treatment in addition to the treatment intervention in the ED, had no baseline NRS pain score documented before the intervention, had an NRS pain score of < 4, had no postintervention NRS pain score documented within 6 hours, had a treatment indication other than pain, or had active cancer were excluded. As in previous JBVAMC studies, we used NRS pain score cutoffs (mild, moderate, severe, and very severe) based on Woo and colleagues’ meta-analysis and excluded scores < 4.15
Endpoints
The primary endpoint was the mean difference in NRS pain score before and after the intervention, determined by comparing the NRS pain score documented at triage to the ED with the first documented NRS pain score at least 30 minutes to 6 hours after treatment administration. The secondary endpoints included the number of patients prescribed pain medication at discharge, the number of patients who were discharged with no medications, and the number of patients admitted to the hospital. The safety endpoint included any AEs of the intervention. Subgroup analyses were performed comparing the mean difference in NRS pain score among subgroups classified by severity of baseline NRS pain score and pain location.
Statistical Analysis
Baseline characteristics and endpoints were analyzed using descriptive statistics. Categorical data were analyzed using Fisher exact test and z test for proportions, and continuous data were compared using t test and paired t test. An 80% power calculation determined that 84 patients per group were needed to detect a statistically significant difference in pain score reduction of 1.3 at a type-1 error rate of 0.05. The sample size was based on a calculation performed in a previously published study that compared IV ketorolac at 3 single-dose regimens for treating acute pain in the ED.16 The 1.3 pain score reduction is considered the minimum clinically significant difference in pain that could be detected with the NRS.17
Results
Sixty-one patients received BFA during the study period: 31 were excluded (26 received adjunct treatment in the ED, 2 had active cancer documented, 2 had an indication other than pain, and 1 received BFA outside of the ED), leaving 30 patients in the BFA cohort. During the study period, 1299 patients received ketorolac.
Baseline characteristics were similar between the 2 groups except for the average baseline NRS pain score, which was statistically significantly higher in the BFA vs ketorolac group (8.7 vs 7.7, respectively; P = .02). The mean age was 51 years in the BFA group and 48 years in the ketorolac group. Most patients in each cohort were male: 80% in the BFA group and 71% in the ketorolac group. The most common types of pain documented as the chief ED presentation included back, lower extremity, and head.
Endpoints
The mean difference in NRS pain score was 3.9 for the BFA group and 5.1 for the ketorolac group. Both were clinically and statistically significant reductions (P = .03 and P < .01), but the difference between the intervention groups in NRS score reduction was not statistically significant (P = .07).
For the secondary endpoint of outpatient prescriptions written at discharge, there was no significant difference between the groups except for oral NSAIDs, which were more likely to be prescribed to patients who received ketorolac (P = .01).
Subgroup Analysis
An analysis was performed for subgroups classified by baseline NRS pain score (mild: 4; moderate, 5 - 6; severe, 7 - 9; and very severe, 10). Data for mild pain was limited because a small number of patients received interventions. For moderate pain, the mean difference in NRS pain score for BFA and ketorolac was 3.5 and 3.8, respectively; for severe pain, 3.4 and 5.3; and for very severe pain, 4.6 and 6.4. There was a larger difference in the preintervention and postintervention NRS pain scores within severe pain and very severe pain groups.
Discussion
Both interventions resulted in a significant reduction in the mean NRS pain score of about 4 to 5 points within their group, and BFA resulted in a similar NRS pain score reduction compared with ketorolac 15 mg. Because the baseline NRS pain scores were significantly different between the BFA and ketorolac groups,
In this study, more patients in the BFA group presented to the ED with lower extremity pain, such as gout or neuropathy, compared with the ketorolac group; however, BFA did not result in a significantly different pain score reduction in this subgroup compared with ketorolac. Patients receiving BFA were more likely to receive topical analgesics or muscle relaxants at discharge; whereas those receiving ketorolac were significantly more likely to receive oral NSAIDs. Patients in this study also were more likely to be admitted to the hospital if they received ketorolac; however, for these patients, pain was secondary to their chief presentation, and the admitting physician’s familiarity with ketorolac might have been the reason for choosing this intervention. Reasons for the admissions were surgical observation, psychiatric stabilization, kidney/gallstones, rule out of acute coronary syndrome, pneumonia, and proctitis in the ketorolac group, and suicidal ideations in the BFA group.
Limitations
As a limited number of patients received BFA at JBVAMC, the study was not sufficiently powered to detect a difference in the primary outcome. Because BFA required a consultation to be entered in the electronic health record, in addition to time needed to perform the procedure, practitioners might have preferred IV/IM ketorolac during busy times in the ED, potentially leading to underrepresentation in the BFA group. Prescribing preferences might have differed among the rotating physicians, timing of the documentation of the NRS pain score could have differed based on the treatment intervention, and the investigators were unable to control or accurately assess whether patients had taken an analgesic medication before presenting to the ED.
Conclusions
NRS pain score reduction with BFA did not differ compared with ketorolac 15 mg for treating acute and acute-on-chronic pain in the ED. Although this study was underpowered, these results add to the limited existing literature, suggesting that both interventions could result in clinically significant pain score reductions for patients presenting to the ED with severe and very severe pain, making BFA a viable nonpharmacologic option. Future studies could include investigating the benefit of BFA in the veteran population by studying larger samples in the ED, surveying patients after their interventions to identify rates AEs, and exploring the use of BFA for chronic pain in the outpatient setting.
1. Cantrill SV, Brown MD, Carlisle RJ, et al. Clinical policy: critical issues in the prescribing of opioids for adult patients in the emergency department. Ann Emerg Med. 2012;60(4):499-525. doi:10.1016/j.annemergmed.2012.06.013
2. Nahin RL. Severe pain in veterans: the effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254. doi:10.1016/j.jpain.2016.10.021
3. Motov S, Strayer R, Hayes BD, et al. The treatment of acute pain in the emergency department: a white paper position statement prepared for the American Academy of Emergency Medicine. J Emerg Med. 2018;54(5):731-736. doi:10.1016/j.jemermed.2018.01.020
4. Samcam I, Papa L. Acute pain management in the emergency department. In: Prostran M, ed. Pain Management. IntechOpen; 2016. doi:10.5772/62861
5. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the use of opioids in the management of chronic pain. Accessed February 15, 2023. https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOpioidsCPG.pdf
6. Davis MT, Mulvaney-Day N, Larson MJ, Hoover R, Mauch D. Complementary and alternative medicine among veterans and military personnel: a synthesis of population surveys. Med Care. 2014;52(12 suppl 5):S83-590. doi:10.1097/MLR.0000000000000227
7. Goertz C, Marriott BP, Finch FD, et al. Military report more complementary and alternative medicine use than civilians. J Altern Complement Med. 2013;19(6):509-517. doi:10.1089/acm.2012.0108
8. King HC, Hickey AH, Connelly C. Auricular acupuncture: a brief introduction for military providers. Mil Med. 2013;178(8):867-874. doi:10.7205/MILMED-D-13-00075
9. Niemtzow RC. Battlefield acupuncture. Medical Acupunct. 2007;19(4):225-228. doi:10.1089/acu.2007.0603
10. Collinsworth KM, Goss DL. Battlefield acupuncture and physical therapy versus physical therapy alone after shoulder surgery. Med Acupunct. 2019;31(4):228-238. doi:10.1089/acu.2019.1372
11. Estores I, Chen K, Jackson B, Lao L, Gorman PH. Auricular acupuncture for spinal cord injury related neuropathic pain: a pilot controlled clinical trial. J Spinal Cord Med. 2017;40(4):432-438. doi:10.1080/10790268.2016.1141489
12. Federman DG, Radhakrishnan K, Gabriel L, Poulin LM, Kravetz JD. Group battlefield acupuncture in primary care for veterans with pain. South Med J. 2018;111(10):619-624. doi:10.14423/SMJ.0000000000000877
13. Garner BK, Hopkinson SG, Ketz AK, Landis CA, Trego LL. Auricular acupuncture for chronic pain and insomnia: a randomized clinical trial. Med Acupunct. 2018;30(5):262-272. doi:10.1089/acu.2018.1294
14. Fox LM, Murakami M, Danesh H, Manini AF. Battlefield acupuncture to treat low back pain in the emergency department. Am J Emerg Med. 2018; 36:1045-1048. doi:10.1016/j.ajem.2018.02.038
15. Woo A, Lechner B, Fu T, et al. Cut points for mild, moderate, and severe pain among cancer and non-cancer patients: a literature review. Ann Palliat Med. 2015;4(4):176-183. doi:10.3978/j.issn.2224-5820.2015.09.04
16. Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017;70(2):177-184. doi:10.1016/j.annemergmed.2016.10.014
17. Bijur PE, Latimer CT, Gallagher EJ. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med. 2003;10:390-392. doi:10.1111/j.1553-2712.2003.tb01355.
Acute pain is a primary symptom for many patients who present to the emergency department (ED). The ED team is challenged with relieving pain while limiting harm from medications.1 A 2017 National Health Interview Survey showed that compared with nonveterans, more veterans reported pain in the previous 3 months, and the rate of severe pain was 40% higher in the veteran group especially among those who served during the era of wars in Afghanistan and Iraq.2
The American College of Emergency Physicians guidelines pain management guidelines recommend patient-centered shared decision making that includes patient education about treatment goals and expectations, and short- and long-term risks, as well as a preference toward pharmacologic treatment with nonopioid analgesics except for patients with severe pain or pain refractory to other drug and treatment modalities.3 There is a lack of evidence regarding superior efficacy of either opioid or nonopioid analgesics; therefore, the use of nonopioid analgesics, such as oral or topical nonsteroidal anti-inflammatory drugs (NSAIDs) or central analgesics, such as acetaminophen, is preferred for treating acute pain to mitigate adverse effects (AEs) and risks associated with opioid use.1,3,4 The US Department of Veterans Affairs (VA) and Department of Defense (DoD) guideline on managing opioid therapy for chronic pain, updated in 2017 and 2022, similarly recommends alternatives to opioids for mild-to-moderate acute pain and encourages multimodal pain care.5 However, use of other pharmacologic treatments, such as NSAIDs, is limited by AE profiles, patient contraindications, and severity of acute pain etiologies. There is a need for the expanded use of nonpharmacologic treatments for addressing pain in the veteran population.
The American College of Emergency Physicians guidelines recommend nonpharmacologic modalities, such as applying heat or cold, physical therapy, cognitive behavioral therapy, and acupuncture.3 A 2014 study reported that 37% to 46% of active duty and reserve military personnel use complementary and alternative medicine (CAM) for a variety of ailments, and there is increasing interest in the use of CAM as adjuncts to traditional therapies.6 According to one study, some CAM therapies are used significantly more by military personnel than used by civilians.7 However, the percentage of the veteran population using acupuncture in this study was small, and more information is needed to assess its use.
Auricular acupuncture originated in traditional Chinese medicine.8 Contemporary auricular acupuncture experts view this modality as a self-contained microsystem mapping portions of the ear to specific parts of the body and internal organs. The analgesic effects may be mediated through the central nervous system by local release of endorphins through nerve fiber activation and neurotransmitters—including serotonin, dopamine, and norepinephrine—leading to pre- and postsynaptic suppression of pain transmission.
Battlefield acupuncture (BFA) uses 5 set points anatomically located on each ear.9 Practitioners use small semipermanent, dartlike acupuncture needles. Patients could experience pain relief in a few minutes, which can last minutes, hours, days, weeks, or months depending on the pathology of the pain. This procedure developed in 2001 has been studied for different pain types and has shown benefit when used for postsurgical pain, chronic spinal cord injury−related neuropathic pain, and general chronic pain, as well as for other indications, such as insomnia, depression, and weight loss.8,10-13 In 2018, a randomized controlled trial compared postintervention numeric rating scale (NRS) pain scores in patients presenting to the ED with acute or acute-on-chronic lower back pain who received BFA as an adjunct to standard care vs standard care alone.14 Patients receiving BFA as an adjunct to standard care were found to have mean postintervention pain scores 1.7 points lower than those receiving standard care alone. This study demonstrated that BFA was feasible and well tolerated for lower back pain in the ED as an adjunct to standard care. The study was limited by the adjunct use of BFA rather than as monotherapy and by the practitioners’ discretion regarding standard care, which was not defined by the study’s authors.
The Jesse Brown Veterans Affairs Medical Center (JBVAMC) in Chicago, Illinois, offers several CAM modalities, such as exercise/movement therapy, chiropractic, art/music therapy, and relaxation workshops, which are widely used by veterans. Recent evidence suggests BFA could reduce pain scores as an adjunct or an alternative to pharmacologic therapy. We are interested in how CAM therapies, such as BFA, can help avoid AEs associated with opioid or NSAID therapy.
At the JBVAMC ED, ketorolac 15 mg is the preferred first-line treatment of acute, noncancer pain, based on the results of previous studies. In 2018 BFA was offered first to veterans presenting with acute or acute-on-chronic pain to the ED; however, its effectiveness for pain reduction vs ketorolac has not been evaluated in this patient population. Limited literature is available on BFA and its use in the ED. To our knowledge, this was the first observational study assessing the difference between a single session of BFA vs a single dose of ketorolac in treating noncancer acute or acute-on-chronic pain in the ED.
Methods
This study was a retrospective chart review of patients who presented to the JBVAMC ED with acute pain or acute-on-chronic pain, who received ketorolac or BFA. The study population was generated from a list of all IV and intramuscular (IM) ketorolac unit dose orders verified from June 1, 2018, through August 30, 2019, and a list of all BFA procedure notes signed from June 1, 2018, through August 30, 2019. Patients were included in the study if they had documented administration of IV or IM ketorolac or BFA between June 1, 2018, and August 30, 2019. Patients who received ketorolac doses other than 15 mg, the intervention was administered outside of the ED, received adjunct treatment in addition to the treatment intervention in the ED, had no baseline NRS pain score documented before the intervention, had an NRS pain score of < 4, had no postintervention NRS pain score documented within 6 hours, had a treatment indication other than pain, or had active cancer were excluded. As in previous JBVAMC studies, we used NRS pain score cutoffs (mild, moderate, severe, and very severe) based on Woo and colleagues’ meta-analysis and excluded scores < 4.15
Endpoints
The primary endpoint was the mean difference in NRS pain score before and after the intervention, determined by comparing the NRS pain score documented at triage to the ED with the first documented NRS pain score at least 30 minutes to 6 hours after treatment administration. The secondary endpoints included the number of patients prescribed pain medication at discharge, the number of patients who were discharged with no medications, and the number of patients admitted to the hospital. The safety endpoint included any AEs of the intervention. Subgroup analyses were performed comparing the mean difference in NRS pain score among subgroups classified by severity of baseline NRS pain score and pain location.
Statistical Analysis
Baseline characteristics and endpoints were analyzed using descriptive statistics. Categorical data were analyzed using Fisher exact test and z test for proportions, and continuous data were compared using t test and paired t test. An 80% power calculation determined that 84 patients per group were needed to detect a statistically significant difference in pain score reduction of 1.3 at a type-1 error rate of 0.05. The sample size was based on a calculation performed in a previously published study that compared IV ketorolac at 3 single-dose regimens for treating acute pain in the ED.16 The 1.3 pain score reduction is considered the minimum clinically significant difference in pain that could be detected with the NRS.17
Results
Sixty-one patients received BFA during the study period: 31 were excluded (26 received adjunct treatment in the ED, 2 had active cancer documented, 2 had an indication other than pain, and 1 received BFA outside of the ED), leaving 30 patients in the BFA cohort. During the study period, 1299 patients received ketorolac.
Baseline characteristics were similar between the 2 groups except for the average baseline NRS pain score, which was statistically significantly higher in the BFA vs ketorolac group (8.7 vs 7.7, respectively; P = .02). The mean age was 51 years in the BFA group and 48 years in the ketorolac group. Most patients in each cohort were male: 80% in the BFA group and 71% in the ketorolac group. The most common types of pain documented as the chief ED presentation included back, lower extremity, and head.
Endpoints
The mean difference in NRS pain score was 3.9 for the BFA group and 5.1 for the ketorolac group. Both were clinically and statistically significant reductions (P = .03 and P < .01), but the difference between the intervention groups in NRS score reduction was not statistically significant (P = .07).
For the secondary endpoint of outpatient prescriptions written at discharge, there was no significant difference between the groups except for oral NSAIDs, which were more likely to be prescribed to patients who received ketorolac (P = .01).
Subgroup Analysis
An analysis was performed for subgroups classified by baseline NRS pain score (mild: 4; moderate, 5 - 6; severe, 7 - 9; and very severe, 10). Data for mild pain was limited because a small number of patients received interventions. For moderate pain, the mean difference in NRS pain score for BFA and ketorolac was 3.5 and 3.8, respectively; for severe pain, 3.4 and 5.3; and for very severe pain, 4.6 and 6.4. There was a larger difference in the preintervention and postintervention NRS pain scores within severe pain and very severe pain groups.
Discussion
Both interventions resulted in a significant reduction in the mean NRS pain score of about 4 to 5 points within their group, and BFA resulted in a similar NRS pain score reduction compared with ketorolac 15 mg. Because the baseline NRS pain scores were significantly different between the BFA and ketorolac groups,
In this study, more patients in the BFA group presented to the ED with lower extremity pain, such as gout or neuropathy, compared with the ketorolac group; however, BFA did not result in a significantly different pain score reduction in this subgroup compared with ketorolac. Patients receiving BFA were more likely to receive topical analgesics or muscle relaxants at discharge; whereas those receiving ketorolac were significantly more likely to receive oral NSAIDs. Patients in this study also were more likely to be admitted to the hospital if they received ketorolac; however, for these patients, pain was secondary to their chief presentation, and the admitting physician’s familiarity with ketorolac might have been the reason for choosing this intervention. Reasons for the admissions were surgical observation, psychiatric stabilization, kidney/gallstones, rule out of acute coronary syndrome, pneumonia, and proctitis in the ketorolac group, and suicidal ideations in the BFA group.
Limitations
As a limited number of patients received BFA at JBVAMC, the study was not sufficiently powered to detect a difference in the primary outcome. Because BFA required a consultation to be entered in the electronic health record, in addition to time needed to perform the procedure, practitioners might have preferred IV/IM ketorolac during busy times in the ED, potentially leading to underrepresentation in the BFA group. Prescribing preferences might have differed among the rotating physicians, timing of the documentation of the NRS pain score could have differed based on the treatment intervention, and the investigators were unable to control or accurately assess whether patients had taken an analgesic medication before presenting to the ED.
Conclusions
NRS pain score reduction with BFA did not differ compared with ketorolac 15 mg for treating acute and acute-on-chronic pain in the ED. Although this study was underpowered, these results add to the limited existing literature, suggesting that both interventions could result in clinically significant pain score reductions for patients presenting to the ED with severe and very severe pain, making BFA a viable nonpharmacologic option. Future studies could include investigating the benefit of BFA in the veteran population by studying larger samples in the ED, surveying patients after their interventions to identify rates AEs, and exploring the use of BFA for chronic pain in the outpatient setting.
Acute pain is a primary symptom for many patients who present to the emergency department (ED). The ED team is challenged with relieving pain while limiting harm from medications.1 A 2017 National Health Interview Survey showed that compared with nonveterans, more veterans reported pain in the previous 3 months, and the rate of severe pain was 40% higher in the veteran group especially among those who served during the era of wars in Afghanistan and Iraq.2
The American College of Emergency Physicians guidelines pain management guidelines recommend patient-centered shared decision making that includes patient education about treatment goals and expectations, and short- and long-term risks, as well as a preference toward pharmacologic treatment with nonopioid analgesics except for patients with severe pain or pain refractory to other drug and treatment modalities.3 There is a lack of evidence regarding superior efficacy of either opioid or nonopioid analgesics; therefore, the use of nonopioid analgesics, such as oral or topical nonsteroidal anti-inflammatory drugs (NSAIDs) or central analgesics, such as acetaminophen, is preferred for treating acute pain to mitigate adverse effects (AEs) and risks associated with opioid use.1,3,4 The US Department of Veterans Affairs (VA) and Department of Defense (DoD) guideline on managing opioid therapy for chronic pain, updated in 2017 and 2022, similarly recommends alternatives to opioids for mild-to-moderate acute pain and encourages multimodal pain care.5 However, use of other pharmacologic treatments, such as NSAIDs, is limited by AE profiles, patient contraindications, and severity of acute pain etiologies. There is a need for the expanded use of nonpharmacologic treatments for addressing pain in the veteran population.
The American College of Emergency Physicians guidelines recommend nonpharmacologic modalities, such as applying heat or cold, physical therapy, cognitive behavioral therapy, and acupuncture.3 A 2014 study reported that 37% to 46% of active duty and reserve military personnel use complementary and alternative medicine (CAM) for a variety of ailments, and there is increasing interest in the use of CAM as adjuncts to traditional therapies.6 According to one study, some CAM therapies are used significantly more by military personnel than used by civilians.7 However, the percentage of the veteran population using acupuncture in this study was small, and more information is needed to assess its use.
Auricular acupuncture originated in traditional Chinese medicine.8 Contemporary auricular acupuncture experts view this modality as a self-contained microsystem mapping portions of the ear to specific parts of the body and internal organs. The analgesic effects may be mediated through the central nervous system by local release of endorphins through nerve fiber activation and neurotransmitters—including serotonin, dopamine, and norepinephrine—leading to pre- and postsynaptic suppression of pain transmission.
Battlefield acupuncture (BFA) uses 5 set points anatomically located on each ear.9 Practitioners use small semipermanent, dartlike acupuncture needles. Patients could experience pain relief in a few minutes, which can last minutes, hours, days, weeks, or months depending on the pathology of the pain. This procedure developed in 2001 has been studied for different pain types and has shown benefit when used for postsurgical pain, chronic spinal cord injury−related neuropathic pain, and general chronic pain, as well as for other indications, such as insomnia, depression, and weight loss.8,10-13 In 2018, a randomized controlled trial compared postintervention numeric rating scale (NRS) pain scores in patients presenting to the ED with acute or acute-on-chronic lower back pain who received BFA as an adjunct to standard care vs standard care alone.14 Patients receiving BFA as an adjunct to standard care were found to have mean postintervention pain scores 1.7 points lower than those receiving standard care alone. This study demonstrated that BFA was feasible and well tolerated for lower back pain in the ED as an adjunct to standard care. The study was limited by the adjunct use of BFA rather than as monotherapy and by the practitioners’ discretion regarding standard care, which was not defined by the study’s authors.
The Jesse Brown Veterans Affairs Medical Center (JBVAMC) in Chicago, Illinois, offers several CAM modalities, such as exercise/movement therapy, chiropractic, art/music therapy, and relaxation workshops, which are widely used by veterans. Recent evidence suggests BFA could reduce pain scores as an adjunct or an alternative to pharmacologic therapy. We are interested in how CAM therapies, such as BFA, can help avoid AEs associated with opioid or NSAID therapy.
At the JBVAMC ED, ketorolac 15 mg is the preferred first-line treatment of acute, noncancer pain, based on the results of previous studies. In 2018 BFA was offered first to veterans presenting with acute or acute-on-chronic pain to the ED; however, its effectiveness for pain reduction vs ketorolac has not been evaluated in this patient population. Limited literature is available on BFA and its use in the ED. To our knowledge, this was the first observational study assessing the difference between a single session of BFA vs a single dose of ketorolac in treating noncancer acute or acute-on-chronic pain in the ED.
Methods
This study was a retrospective chart review of patients who presented to the JBVAMC ED with acute pain or acute-on-chronic pain, who received ketorolac or BFA. The study population was generated from a list of all IV and intramuscular (IM) ketorolac unit dose orders verified from June 1, 2018, through August 30, 2019, and a list of all BFA procedure notes signed from June 1, 2018, through August 30, 2019. Patients were included in the study if they had documented administration of IV or IM ketorolac or BFA between June 1, 2018, and August 30, 2019. Patients who received ketorolac doses other than 15 mg, the intervention was administered outside of the ED, received adjunct treatment in addition to the treatment intervention in the ED, had no baseline NRS pain score documented before the intervention, had an NRS pain score of < 4, had no postintervention NRS pain score documented within 6 hours, had a treatment indication other than pain, or had active cancer were excluded. As in previous JBVAMC studies, we used NRS pain score cutoffs (mild, moderate, severe, and very severe) based on Woo and colleagues’ meta-analysis and excluded scores < 4.15
Endpoints
The primary endpoint was the mean difference in NRS pain score before and after the intervention, determined by comparing the NRS pain score documented at triage to the ED with the first documented NRS pain score at least 30 minutes to 6 hours after treatment administration. The secondary endpoints included the number of patients prescribed pain medication at discharge, the number of patients who were discharged with no medications, and the number of patients admitted to the hospital. The safety endpoint included any AEs of the intervention. Subgroup analyses were performed comparing the mean difference in NRS pain score among subgroups classified by severity of baseline NRS pain score and pain location.
Statistical Analysis
Baseline characteristics and endpoints were analyzed using descriptive statistics. Categorical data were analyzed using Fisher exact test and z test for proportions, and continuous data were compared using t test and paired t test. An 80% power calculation determined that 84 patients per group were needed to detect a statistically significant difference in pain score reduction of 1.3 at a type-1 error rate of 0.05. The sample size was based on a calculation performed in a previously published study that compared IV ketorolac at 3 single-dose regimens for treating acute pain in the ED.16 The 1.3 pain score reduction is considered the minimum clinically significant difference in pain that could be detected with the NRS.17
Results
Sixty-one patients received BFA during the study period: 31 were excluded (26 received adjunct treatment in the ED, 2 had active cancer documented, 2 had an indication other than pain, and 1 received BFA outside of the ED), leaving 30 patients in the BFA cohort. During the study period, 1299 patients received ketorolac.
Baseline characteristics were similar between the 2 groups except for the average baseline NRS pain score, which was statistically significantly higher in the BFA vs ketorolac group (8.7 vs 7.7, respectively; P = .02). The mean age was 51 years in the BFA group and 48 years in the ketorolac group. Most patients in each cohort were male: 80% in the BFA group and 71% in the ketorolac group. The most common types of pain documented as the chief ED presentation included back, lower extremity, and head.
Endpoints
The mean difference in NRS pain score was 3.9 for the BFA group and 5.1 for the ketorolac group. Both were clinically and statistically significant reductions (P = .03 and P < .01), but the difference between the intervention groups in NRS score reduction was not statistically significant (P = .07).
For the secondary endpoint of outpatient prescriptions written at discharge, there was no significant difference between the groups except for oral NSAIDs, which were more likely to be prescribed to patients who received ketorolac (P = .01).
Subgroup Analysis
An analysis was performed for subgroups classified by baseline NRS pain score (mild: 4; moderate, 5 - 6; severe, 7 - 9; and very severe, 10). Data for mild pain was limited because a small number of patients received interventions. For moderate pain, the mean difference in NRS pain score for BFA and ketorolac was 3.5 and 3.8, respectively; for severe pain, 3.4 and 5.3; and for very severe pain, 4.6 and 6.4. There was a larger difference in the preintervention and postintervention NRS pain scores within severe pain and very severe pain groups.
Discussion
Both interventions resulted in a significant reduction in the mean NRS pain score of about 4 to 5 points within their group, and BFA resulted in a similar NRS pain score reduction compared with ketorolac 15 mg. Because the baseline NRS pain scores were significantly different between the BFA and ketorolac groups,
In this study, more patients in the BFA group presented to the ED with lower extremity pain, such as gout or neuropathy, compared with the ketorolac group; however, BFA did not result in a significantly different pain score reduction in this subgroup compared with ketorolac. Patients receiving BFA were more likely to receive topical analgesics or muscle relaxants at discharge; whereas those receiving ketorolac were significantly more likely to receive oral NSAIDs. Patients in this study also were more likely to be admitted to the hospital if they received ketorolac; however, for these patients, pain was secondary to their chief presentation, and the admitting physician’s familiarity with ketorolac might have been the reason for choosing this intervention. Reasons for the admissions were surgical observation, psychiatric stabilization, kidney/gallstones, rule out of acute coronary syndrome, pneumonia, and proctitis in the ketorolac group, and suicidal ideations in the BFA group.
Limitations
As a limited number of patients received BFA at JBVAMC, the study was not sufficiently powered to detect a difference in the primary outcome. Because BFA required a consultation to be entered in the electronic health record, in addition to time needed to perform the procedure, practitioners might have preferred IV/IM ketorolac during busy times in the ED, potentially leading to underrepresentation in the BFA group. Prescribing preferences might have differed among the rotating physicians, timing of the documentation of the NRS pain score could have differed based on the treatment intervention, and the investigators were unable to control or accurately assess whether patients had taken an analgesic medication before presenting to the ED.
Conclusions
NRS pain score reduction with BFA did not differ compared with ketorolac 15 mg for treating acute and acute-on-chronic pain in the ED. Although this study was underpowered, these results add to the limited existing literature, suggesting that both interventions could result in clinically significant pain score reductions for patients presenting to the ED with severe and very severe pain, making BFA a viable nonpharmacologic option. Future studies could include investigating the benefit of BFA in the veteran population by studying larger samples in the ED, surveying patients after their interventions to identify rates AEs, and exploring the use of BFA for chronic pain in the outpatient setting.
1. Cantrill SV, Brown MD, Carlisle RJ, et al. Clinical policy: critical issues in the prescribing of opioids for adult patients in the emergency department. Ann Emerg Med. 2012;60(4):499-525. doi:10.1016/j.annemergmed.2012.06.013
2. Nahin RL. Severe pain in veterans: the effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254. doi:10.1016/j.jpain.2016.10.021
3. Motov S, Strayer R, Hayes BD, et al. The treatment of acute pain in the emergency department: a white paper position statement prepared for the American Academy of Emergency Medicine. J Emerg Med. 2018;54(5):731-736. doi:10.1016/j.jemermed.2018.01.020
4. Samcam I, Papa L. Acute pain management in the emergency department. In: Prostran M, ed. Pain Management. IntechOpen; 2016. doi:10.5772/62861
5. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the use of opioids in the management of chronic pain. Accessed February 15, 2023. https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOpioidsCPG.pdf
6. Davis MT, Mulvaney-Day N, Larson MJ, Hoover R, Mauch D. Complementary and alternative medicine among veterans and military personnel: a synthesis of population surveys. Med Care. 2014;52(12 suppl 5):S83-590. doi:10.1097/MLR.0000000000000227
7. Goertz C, Marriott BP, Finch FD, et al. Military report more complementary and alternative medicine use than civilians. J Altern Complement Med. 2013;19(6):509-517. doi:10.1089/acm.2012.0108
8. King HC, Hickey AH, Connelly C. Auricular acupuncture: a brief introduction for military providers. Mil Med. 2013;178(8):867-874. doi:10.7205/MILMED-D-13-00075
9. Niemtzow RC. Battlefield acupuncture. Medical Acupunct. 2007;19(4):225-228. doi:10.1089/acu.2007.0603
10. Collinsworth KM, Goss DL. Battlefield acupuncture and physical therapy versus physical therapy alone after shoulder surgery. Med Acupunct. 2019;31(4):228-238. doi:10.1089/acu.2019.1372
11. Estores I, Chen K, Jackson B, Lao L, Gorman PH. Auricular acupuncture for spinal cord injury related neuropathic pain: a pilot controlled clinical trial. J Spinal Cord Med. 2017;40(4):432-438. doi:10.1080/10790268.2016.1141489
12. Federman DG, Radhakrishnan K, Gabriel L, Poulin LM, Kravetz JD. Group battlefield acupuncture in primary care for veterans with pain. South Med J. 2018;111(10):619-624. doi:10.14423/SMJ.0000000000000877
13. Garner BK, Hopkinson SG, Ketz AK, Landis CA, Trego LL. Auricular acupuncture for chronic pain and insomnia: a randomized clinical trial. Med Acupunct. 2018;30(5):262-272. doi:10.1089/acu.2018.1294
14. Fox LM, Murakami M, Danesh H, Manini AF. Battlefield acupuncture to treat low back pain in the emergency department. Am J Emerg Med. 2018; 36:1045-1048. doi:10.1016/j.ajem.2018.02.038
15. Woo A, Lechner B, Fu T, et al. Cut points for mild, moderate, and severe pain among cancer and non-cancer patients: a literature review. Ann Palliat Med. 2015;4(4):176-183. doi:10.3978/j.issn.2224-5820.2015.09.04
16. Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017;70(2):177-184. doi:10.1016/j.annemergmed.2016.10.014
17. Bijur PE, Latimer CT, Gallagher EJ. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med. 2003;10:390-392. doi:10.1111/j.1553-2712.2003.tb01355.
1. Cantrill SV, Brown MD, Carlisle RJ, et al. Clinical policy: critical issues in the prescribing of opioids for adult patients in the emergency department. Ann Emerg Med. 2012;60(4):499-525. doi:10.1016/j.annemergmed.2012.06.013
2. Nahin RL. Severe pain in veterans: the effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254. doi:10.1016/j.jpain.2016.10.021
3. Motov S, Strayer R, Hayes BD, et al. The treatment of acute pain in the emergency department: a white paper position statement prepared for the American Academy of Emergency Medicine. J Emerg Med. 2018;54(5):731-736. doi:10.1016/j.jemermed.2018.01.020
4. Samcam I, Papa L. Acute pain management in the emergency department. In: Prostran M, ed. Pain Management. IntechOpen; 2016. doi:10.5772/62861
5. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the use of opioids in the management of chronic pain. Accessed February 15, 2023. https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOpioidsCPG.pdf
6. Davis MT, Mulvaney-Day N, Larson MJ, Hoover R, Mauch D. Complementary and alternative medicine among veterans and military personnel: a synthesis of population surveys. Med Care. 2014;52(12 suppl 5):S83-590. doi:10.1097/MLR.0000000000000227
7. Goertz C, Marriott BP, Finch FD, et al. Military report more complementary and alternative medicine use than civilians. J Altern Complement Med. 2013;19(6):509-517. doi:10.1089/acm.2012.0108
8. King HC, Hickey AH, Connelly C. Auricular acupuncture: a brief introduction for military providers. Mil Med. 2013;178(8):867-874. doi:10.7205/MILMED-D-13-00075
9. Niemtzow RC. Battlefield acupuncture. Medical Acupunct. 2007;19(4):225-228. doi:10.1089/acu.2007.0603
10. Collinsworth KM, Goss DL. Battlefield acupuncture and physical therapy versus physical therapy alone after shoulder surgery. Med Acupunct. 2019;31(4):228-238. doi:10.1089/acu.2019.1372
11. Estores I, Chen K, Jackson B, Lao L, Gorman PH. Auricular acupuncture for spinal cord injury related neuropathic pain: a pilot controlled clinical trial. J Spinal Cord Med. 2017;40(4):432-438. doi:10.1080/10790268.2016.1141489
12. Federman DG, Radhakrishnan K, Gabriel L, Poulin LM, Kravetz JD. Group battlefield acupuncture in primary care for veterans with pain. South Med J. 2018;111(10):619-624. doi:10.14423/SMJ.0000000000000877
13. Garner BK, Hopkinson SG, Ketz AK, Landis CA, Trego LL. Auricular acupuncture for chronic pain and insomnia: a randomized clinical trial. Med Acupunct. 2018;30(5):262-272. doi:10.1089/acu.2018.1294
14. Fox LM, Murakami M, Danesh H, Manini AF. Battlefield acupuncture to treat low back pain in the emergency department. Am J Emerg Med. 2018; 36:1045-1048. doi:10.1016/j.ajem.2018.02.038
15. Woo A, Lechner B, Fu T, et al. Cut points for mild, moderate, and severe pain among cancer and non-cancer patients: a literature review. Ann Palliat Med. 2015;4(4):176-183. doi:10.3978/j.issn.2224-5820.2015.09.04
16. Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017;70(2):177-184. doi:10.1016/j.annemergmed.2016.10.014
17. Bijur PE, Latimer CT, Gallagher EJ. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med. 2003;10:390-392. doi:10.1111/j.1553-2712.2003.tb01355.
High-Grade Staphylococcus lugdunensis Bacteremia in a Patient on Home Hemodialysis
Staphylococcus lugdunensis (S lugdunensis) is a species of coagulase-negative Staphylococcus (CoNS) and a constituent of human skin flora. Unlike other strains of CoNS, however, S lugdunensis has gained notoriety for virulence that resembles Staphylococcus aureus (S aureus). S lugdunensis is now recognized as an important nosocomial pathogen and cause of prosthetic device infections, including vascular catheter infections. We present a case of persistent S lugdunensis bacteremia occurring in a patient on hemodialysis (HD) without any implanted prosthetic materials.
Case Presentation
A 60-year-old man with a history of uncontrolled type 2 diabetes mellitus (T2DM) and end-stage renal disease on home HD via arteriovenous fistula (AVF) presented to the emergency department (ED) for evaluation of subacute progressive low back pain. His symptoms began abruptly 2 weeks prior to presentation without any identifiable trigger or trauma. His pain localized to the lower thoracic spine, radiating anteriorly into his abdomen. He reported tactile fever for several days before presentation but no chills, night sweats, paresthesia, weakness, or bowel/bladder incontinence. He had no recent surgeries, implanted hardware, or invasive procedures involving the spine. HD was performed 5 times a week at home with a family member cannulating his AVF via buttonhole technique. He initially sought evaluation in a community hospital several days prior, where he underwent magnetic resonance imaging (MRI) of the thoracic spine. He was discharged from the community ED with oral opioids prior to the MRI results. He presented to West Los Angeles Veterans Affairs Medical Center (WLAVAMC) ED when MRI results came back indicating abnormalities and he reported recalcitrant pain.
On arrival at WLAVAMC, the patient was afebrile with a heart rate of 107 bpm and blood pressure of 152/97 mm Hg. The remainder of his vital signs were normal. The physical examination revealed midline tenderness on palpation of the distal thoracic and proximal lumbar spine. Muscle strength was 4 of 5 in the bilateral hip flexors, though this was limited by pain. The remainder of his neurologic examination was nonfocal. The cardiac examination was unremarkable with no murmurs auscultated. His left upper extremity AVF had an audible bruit and palpable thrill. The skin examination was notable for acanthosis nigricans but no areas of skin erythema or induration and no obvious stigmata of infective endocarditis.
The initial laboratory workup was remarkable for a white blood cell (WBC) count of 10.0 × 103/µL with left shift, blood urea nitrogen level of 59 mg/dL, and creatinine level of 9.3 mg/dL. The patient’s erythrocyte sedimentation rate (ESR) was 45 mm/h (reference range, ≤ 20 mm/h) and C-reactive protein level was > 8.0 mg/L (reference range, ≤ 0.74 mg/L). Two months prior the hemoglobin A1c had been recorded at 9.9%.
Given his intractable low back pain and elevated inflammatory markers, the patient underwent an MRI of the thoracic and lumbar spine with contrast while in the ED. This MRI revealed abnormal marrow edema in the T11-T12 vertebrae with abnormal fluid signal in the T11-T12 disc space. Subjacent paravertebral edema also was noted. There was no well-defined fluid collection or abnormal signal in the spinal cord. Taken together, these findings were concerning for T11-T12 discitis with osteomyelitis.
Two sets of blood cultures were obtained, and empiric IV vancomycin and ceftriaxone were started. Interventional radiology was consulted for consideration of vertebral biopsy but deferred while awaiting blood culture data. Neurosurgery also was consulted and recommended nonoperative management given his nonfocal neurologic examination and imaging without evidence of abscess. Both sets of blood cultures collected on admission later grew methicillin-sensitive S lugdunensis, a species of CoNS. A transthoracic and later transesophageal echocardiogram did not show any valvular vegetations. The patient’s antibiotic regimen was narrowed to IV oxacillin based on susceptibility data. It was later discovered that both blood cultures obtained during his outside ED encounter were also growing S lugdunensis.
The patient’s S lugdunensis bacteremia persisted for the first 8 days of his admission despite appropriate dosing of oxacillin. During this time, the patient remained afebrile with stable vital signs and a normal WBC count. Positron emission tomography was obtained to evaluate for potential sources of his persistent bacteremia. Aside from tracer uptake in the T11-T12 vertebral bodies and intervertebral disc space, no other areas showed suspicious uptake. Neurosurgery reevaluated the patient and again recommended nonoperative management. Blood cultures cleared and based on recommendations from an infectious disease specialist, the patient was transitioned to IV cefazolin dosed 3 times weekly after HD, which was transitioned to an outpatient dialysis center. The patient continued taking cefazolin for 6 weeks with subsequent improvement in back pain and normalization of inflammatory markers at outpatient follow-up.
Discussion
CoNS are a major contributor to human skin flora, a common contaminant of blood cultures, and an important cause of nosocomial bloodstream infections.1,2 These species have a predilection for forming biofilms, making CoNS a major cause of prosthetic device infections.3 S lugdunensis is a CoNS species that was first described in 1988.4 In addition to foreign body–related infections, S lugdunensis has been implicated in bone/joint infections, native valve endocarditis, toxic shock syndrome, and brain abscesses.5-8 Infections due to S lugdunensis are notorious for their aggressive and fulminant courses. With its increased virulence that is atypical of other CoNS, S lugdunensis has understandably been likened more to S aureus.
Prior cases have been reported of S lugdunensis bacteremia in patients using HD. However, the suspected source of bacteremia in these cases has generally been central venous catheters.9-12
Notably, our patient’s AVF was accessed using the buttonhole technique for his home HD sessions, which involves cannulating the same site along the fistula until an epithelialized track has formed from scar tissue. At later HD sessions, duller needles can then be used to cannulate this same track. In contrast, the rope-ladder technique involves cannulating a different site along the fistula until the entire length of the fistula has been used. Patients report higher levels of satisfaction with the buttonhole technique, citing decreased pain, decreased oozing, and the perception of easier cannulation by HD nurses.14 However, the buttonhole technique also appears to confer a higher risk of vascular access-related bloodstream infection when compared with the rope-ladder technique.13,15,16
The buttonhole technique is hypothesized to increase infection risk due to the repeated use of the same site for needle entry. Skin flora, including CoNS, may colonize the scab that forms after dialysis access. If proper sterilization techniques are not rigorously followed, the bacteria colonizing the scab and adjacent skin may be introduced into a patient’s bloodstream during needle puncture. Loss of skin integrity due to frequent cannulation of the same site may also contribute to this increased infection risk. It is relevant to recall that our patient received HD 5 times weekly using the buttonhole technique. The use of the buttonhole technique, frequency of his HD sessions, unclear sterilization methods, and immune dysfunction related to his uncontrolled T2DM and renal disease all likely contributed to our patient’s bacteremia.
Using topical mupirocin for prophylaxis at the intended buttonhole puncture site has shown promising results in decreasing rates of S aureus bacteremia.17 It is unclear whether this intervention also would be effective against S lugdunensis. Increasing rates of mupirocin resistance have been reported among S lugdunensis isolates in dialysis settings, but further research in this area is warranted.18
There are no established treatment guidelines for S lugdunensis infections. In vitro studies suggest that S lugdunensis is susceptible to a wide variety of antibiotics. The mecA gene is a major determinant of methicillin resistance that is commonly observed among CoNS but is uncommonly seen with S lugdunensis.5 In a study by Tan and colleagues of 106 S lugdunensis isolates, they found that only 5 (4.7%) were mecA positive.19
Vancomycin is generally reasonable for empiric antibiotic coverage of staphylococci while speciation is pending. However, if S lugdunensis is isolated, its favorable susceptibility pattern typically allows for de-escalation to an antistaphylococcal β-lactam, such as oxacillin or nafcillin. In cases of bloodstream infections caused by methicillin-sensitive S aureus, treatment with a β-lactam has demonstrated superiority over vancomycin due to the lower rates of treatment failure and mortality with β-lactams.20,21 It is unknown whether β-lactams is superior for treating bacteremia with methicillin-sensitive S lugdunensis.
Our patient’s isolate of S lugdunensis was pansensitive to all antibiotics tested, including penicillin. These susceptibility data were used to guide the de-escalation of his empiric vancomycin and ceftriaxone to oxacillin on hospital day 1.
Due to their virulence, bloodstream infections caused by S aureus and S lugdunensis often require more than timely antimicrobial treatment to ensure eradication. Consultation with an infectious disease specialist to manage patients with S aureus bacteremia has been proven to reduce mortality.25 A similar mortality benefit is seen when infectious disease specialists are consulted for S lugdunensis bacteremia.26 This mortality benefit is likely explained by S lugdunensis’ propensity to cause aggressive, metastatic infections. In such cases, infectious disease consultants may recommend additional imaging (eg, transthoracic echocardiogram) to evaluate for occult sources of infection, advocate for appropriate source control, and guide the selection of an appropriate antibiotic course to ensure resolution of the bacteremia.
Conclusions
S lugdunensis is an increasingly recognized cause of nosocomial bloodstream infections. Given the commonalities in virulence that S lugdunensis shares with S aureus, treatment of bacteremia caused by either species should follow similar management principles: prompt initiation of IV antistaphylococcal therapy, a thorough evaluation for the source(s) of bacteremia as well as metastatic complications, and consultation with an infectious disease specialist. This case report also highlights the importance of considering a patient’s AVF as a potential source for infection even in the absence of localized signs of infection. The buttonhole method of AVF cannulation was thought to be a major contributor to the development and persistence of our patient’s bacteremia. This risk should be discussed with patients using a shared decision-making approach when developing a dialysis treatment plan.
1. Huebner J, Goldmann DA. Coagulase-negative staphylococci: role as pathogens. Annu Rev Med. 1999;50(1):223-236. doi:10.1146/annurev.med.50.1.223
2. Beekmann SE, Diekema DJ, Doern GV. Determining the clinical significance of coagulase-negative staphylococci isolated from blood cultures. Infect Control Hosp Epidemiol. 2005;26(6):559-566. doi:10.1086/502584
3. Arrecubieta C, Toba FA, von Bayern M, et al. SdrF, a Staphylococcus epidermidis surface protein, contributes to the initiation of ventricular assist device driveline–related infections. PLoS Pathog. 2009;5(5):e1000411. doi.10.1371/journal.ppat.1000411
4. Freney J, Brun Y, Bes M, et al. Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., two species from human clinical specimens. Int J Syst Bacteriol. 1988;38(2):168-172. doi:10.1099/00207713-38-2-168
5. Frank KL, del Pozo JL, Patel R. From clinical microbiology to infection pathogenesis: how daring to be different works for Staphylococcus lugdunensis. Clin Microbiol Rev. 2008;21(1):111-133. doi:10.1128/CMR.00036-07
6. Anguera I, Del Río A, Miró JM; Hospital Clinic Endocarditis Study Group. Staphylococcus lugdunensis infective endocarditis: description of 10 cases and analysis of native valve, prosthetic valve, and pacemaker lead endocarditis clinical profiles. Heart. 2005;91(2):e10. doi:10.1136/hrt.2004.040659
7. Pareja J, Gupta K, Koziel H. The toxic shock syndrome and Staphylococcus lugdunensis bacteremia. Ann Intern Med. 1998;128(7):603-604. doi:10.7326/0003-4819-128-7-199804010-00029
8. Woznowski M, Quack I, Bölke E, et al. Fulminant Staphylococcus lugdunensis septicaemia following a pelvic varicella-zoster virus infection in an immune-deficient patient: a case report. Eur J Med Res. 201;15(9):410-414. doi:10.1186/2047-783x-15-9-410
9. Mallappallil M, Salifu M, Woredekal Y, et al. Staphylococcus lugdunensis bacteremia in hemodialysis patients. Int J Microbiol Res. 2012;4(2):178-181. doi:10.9735/0975-5276.4.2.178-181
10. Shuttleworth R, Colby W. Staphylococcus lugdunensis endocarditis. J Clin Microbiol. 1992;30(8):5. doi:10.1128/jcm.30.8.1948-1952.1992
11. Conner RC, Byrnes TJ, Clough LA, Myers JP. Staphylococcus lugdunensis tricuspid valve endocarditis associated with home hemodialysis therapy: report of a case and review of the literature. Infect Dis Clin Pract. 2012;20(3):182-183. doi:1097/IPC.0b013e318245d4f1
12. Kamaraju S, Nelson K, Williams D, Ayenew W, Modi K. Staphylococcus lugdunensis pulmonary valve endocarditis in a patient on chronic hemodialysis. Am J Nephrol. 1999;19(5):605-608. doi:1097/IPC.0b013e318245d4f1
13. Lok C, Sontrop J, Faratro R, Chan C, Zimmerman DL. Frequent hemodialysis fistula infectious complications. Nephron Extra. 2014;4(3):159-167. doi:10.1159/000366477
14. Hashmi A, Cheema MQ, Moss AH. Hemodialysis patients’ experience with and attitudes toward the buttonhole technique for arteriovenous fistula cannulation. Clin Nephrol. 2010;74(5):346-350. doi:10.5414/cnp74346
15. Lyman M, Nguyen DB, Shugart A, Gruhler H, Lines C, Patel PR. Risk of vascular access infection associated with buttonhole cannulation of fistulas: data from the National Healthcare Safety Network. Am J Kidney Dis. 2020;76(1):82-89. doi:10.1053/j.ajkd.2019.11.006
16. MacRae JM, Ahmed SB, Atkar R, Hemmelgarn BR. A randomized trial comparing buttonhole with rope ladder needling in conventional hemodialysis patients. Clin J Am Soc Nephrol. 2012;7(10):1632-1638. doi:10.2215/CJN.02730312
17. Nesrallah GE, Cuerden M, Wong JHS, Pierratos A. Staphylococcus aureus bacteremia and buttonhole cannulation: long-term safety and efficacy of mupirocin prophylaxis. Clin J Am Soc Nephrol. 2010;5(6):1047-1053. doi:10.2215/CJN.00280110
18. Ho PL, Liu MCJ, Chow KH, et al. Emergence of ileS2 -carrying, multidrug-resistant plasmids in Staphylococcus lugdunensis. Antimicrob Agents Chemother. 2016;60(10):6411-6414. doi:10.1128/AAC.00948-16
19. Tan TY, Ng SY, He J. Microbiological characteristics, presumptive identification, and antibiotic susceptibilities of Staphylococcus lugdunensis. J Clin Microbiol. 2008;46(7):2393-2395. doi:10.1128/JCM.00740-08
20. Chang FY, Peacock JE, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore). 2003;82(5):333-339. doi:10.1097/01.md.0000091184.93122.09
21. Shurland S, Zhan M, Bradham DD, Roghmann MC. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28(3):273-279. doi:10.1086/512627
22. Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med. 1991;115(9):674. doi:10.7326/0003-4819-115-9-674
23. Fowler VG, Karchmer AW, Tally FP, et al; S. aureus Endocarditis and Bacteremia Study Group. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355(7):653-665 . doi:10.1056/NEJMoa053783
24. Duhon B, Dallas S, Velasquez ST, Hand E. Staphylococcus lugdunensis bacteremia and endocarditis treated with cefazolin and rifampin. Am J Health Syst Pharm. 2015;72(13):1114-1118. doi:10.2146/ajhp140498
25. Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore). 2009;88(5):263-267. doi:10.1097/MD.0b013e3181b8fccb
26. Forsblom E, Högnäs E, Syrjänen J, Järvinen A. Infectious diseases specialist consultation in Staphylococcus lugdunensis bacteremia. PLoS ONE. 2021;16(10):e0258511. doi:10.1371/journal.pone.0258511
Staphylococcus lugdunensis (S lugdunensis) is a species of coagulase-negative Staphylococcus (CoNS) and a constituent of human skin flora. Unlike other strains of CoNS, however, S lugdunensis has gained notoriety for virulence that resembles Staphylococcus aureus (S aureus). S lugdunensis is now recognized as an important nosocomial pathogen and cause of prosthetic device infections, including vascular catheter infections. We present a case of persistent S lugdunensis bacteremia occurring in a patient on hemodialysis (HD) without any implanted prosthetic materials.
Case Presentation
A 60-year-old man with a history of uncontrolled type 2 diabetes mellitus (T2DM) and end-stage renal disease on home HD via arteriovenous fistula (AVF) presented to the emergency department (ED) for evaluation of subacute progressive low back pain. His symptoms began abruptly 2 weeks prior to presentation without any identifiable trigger or trauma. His pain localized to the lower thoracic spine, radiating anteriorly into his abdomen. He reported tactile fever for several days before presentation but no chills, night sweats, paresthesia, weakness, or bowel/bladder incontinence. He had no recent surgeries, implanted hardware, or invasive procedures involving the spine. HD was performed 5 times a week at home with a family member cannulating his AVF via buttonhole technique. He initially sought evaluation in a community hospital several days prior, where he underwent magnetic resonance imaging (MRI) of the thoracic spine. He was discharged from the community ED with oral opioids prior to the MRI results. He presented to West Los Angeles Veterans Affairs Medical Center (WLAVAMC) ED when MRI results came back indicating abnormalities and he reported recalcitrant pain.
On arrival at WLAVAMC, the patient was afebrile with a heart rate of 107 bpm and blood pressure of 152/97 mm Hg. The remainder of his vital signs were normal. The physical examination revealed midline tenderness on palpation of the distal thoracic and proximal lumbar spine. Muscle strength was 4 of 5 in the bilateral hip flexors, though this was limited by pain. The remainder of his neurologic examination was nonfocal. The cardiac examination was unremarkable with no murmurs auscultated. His left upper extremity AVF had an audible bruit and palpable thrill. The skin examination was notable for acanthosis nigricans but no areas of skin erythema or induration and no obvious stigmata of infective endocarditis.
The initial laboratory workup was remarkable for a white blood cell (WBC) count of 10.0 × 103/µL with left shift, blood urea nitrogen level of 59 mg/dL, and creatinine level of 9.3 mg/dL. The patient’s erythrocyte sedimentation rate (ESR) was 45 mm/h (reference range, ≤ 20 mm/h) and C-reactive protein level was > 8.0 mg/L (reference range, ≤ 0.74 mg/L). Two months prior the hemoglobin A1c had been recorded at 9.9%.
Given his intractable low back pain and elevated inflammatory markers, the patient underwent an MRI of the thoracic and lumbar spine with contrast while in the ED. This MRI revealed abnormal marrow edema in the T11-T12 vertebrae with abnormal fluid signal in the T11-T12 disc space. Subjacent paravertebral edema also was noted. There was no well-defined fluid collection or abnormal signal in the spinal cord. Taken together, these findings were concerning for T11-T12 discitis with osteomyelitis.
Two sets of blood cultures were obtained, and empiric IV vancomycin and ceftriaxone were started. Interventional radiology was consulted for consideration of vertebral biopsy but deferred while awaiting blood culture data. Neurosurgery also was consulted and recommended nonoperative management given his nonfocal neurologic examination and imaging without evidence of abscess. Both sets of blood cultures collected on admission later grew methicillin-sensitive S lugdunensis, a species of CoNS. A transthoracic and later transesophageal echocardiogram did not show any valvular vegetations. The patient’s antibiotic regimen was narrowed to IV oxacillin based on susceptibility data. It was later discovered that both blood cultures obtained during his outside ED encounter were also growing S lugdunensis.
The patient’s S lugdunensis bacteremia persisted for the first 8 days of his admission despite appropriate dosing of oxacillin. During this time, the patient remained afebrile with stable vital signs and a normal WBC count. Positron emission tomography was obtained to evaluate for potential sources of his persistent bacteremia. Aside from tracer uptake in the T11-T12 vertebral bodies and intervertebral disc space, no other areas showed suspicious uptake. Neurosurgery reevaluated the patient and again recommended nonoperative management. Blood cultures cleared and based on recommendations from an infectious disease specialist, the patient was transitioned to IV cefazolin dosed 3 times weekly after HD, which was transitioned to an outpatient dialysis center. The patient continued taking cefazolin for 6 weeks with subsequent improvement in back pain and normalization of inflammatory markers at outpatient follow-up.
Discussion
CoNS are a major contributor to human skin flora, a common contaminant of blood cultures, and an important cause of nosocomial bloodstream infections.1,2 These species have a predilection for forming biofilms, making CoNS a major cause of prosthetic device infections.3 S lugdunensis is a CoNS species that was first described in 1988.4 In addition to foreign body–related infections, S lugdunensis has been implicated in bone/joint infections, native valve endocarditis, toxic shock syndrome, and brain abscesses.5-8 Infections due to S lugdunensis are notorious for their aggressive and fulminant courses. With its increased virulence that is atypical of other CoNS, S lugdunensis has understandably been likened more to S aureus.
Prior cases have been reported of S lugdunensis bacteremia in patients using HD. However, the suspected source of bacteremia in these cases has generally been central venous catheters.9-12
Notably, our patient’s AVF was accessed using the buttonhole technique for his home HD sessions, which involves cannulating the same site along the fistula until an epithelialized track has formed from scar tissue. At later HD sessions, duller needles can then be used to cannulate this same track. In contrast, the rope-ladder technique involves cannulating a different site along the fistula until the entire length of the fistula has been used. Patients report higher levels of satisfaction with the buttonhole technique, citing decreased pain, decreased oozing, and the perception of easier cannulation by HD nurses.14 However, the buttonhole technique also appears to confer a higher risk of vascular access-related bloodstream infection when compared with the rope-ladder technique.13,15,16
The buttonhole technique is hypothesized to increase infection risk due to the repeated use of the same site for needle entry. Skin flora, including CoNS, may colonize the scab that forms after dialysis access. If proper sterilization techniques are not rigorously followed, the bacteria colonizing the scab and adjacent skin may be introduced into a patient’s bloodstream during needle puncture. Loss of skin integrity due to frequent cannulation of the same site may also contribute to this increased infection risk. It is relevant to recall that our patient received HD 5 times weekly using the buttonhole technique. The use of the buttonhole technique, frequency of his HD sessions, unclear sterilization methods, and immune dysfunction related to his uncontrolled T2DM and renal disease all likely contributed to our patient’s bacteremia.
Using topical mupirocin for prophylaxis at the intended buttonhole puncture site has shown promising results in decreasing rates of S aureus bacteremia.17 It is unclear whether this intervention also would be effective against S lugdunensis. Increasing rates of mupirocin resistance have been reported among S lugdunensis isolates in dialysis settings, but further research in this area is warranted.18
There are no established treatment guidelines for S lugdunensis infections. In vitro studies suggest that S lugdunensis is susceptible to a wide variety of antibiotics. The mecA gene is a major determinant of methicillin resistance that is commonly observed among CoNS but is uncommonly seen with S lugdunensis.5 In a study by Tan and colleagues of 106 S lugdunensis isolates, they found that only 5 (4.7%) were mecA positive.19
Vancomycin is generally reasonable for empiric antibiotic coverage of staphylococci while speciation is pending. However, if S lugdunensis is isolated, its favorable susceptibility pattern typically allows for de-escalation to an antistaphylococcal β-lactam, such as oxacillin or nafcillin. In cases of bloodstream infections caused by methicillin-sensitive S aureus, treatment with a β-lactam has demonstrated superiority over vancomycin due to the lower rates of treatment failure and mortality with β-lactams.20,21 It is unknown whether β-lactams is superior for treating bacteremia with methicillin-sensitive S lugdunensis.
Our patient’s isolate of S lugdunensis was pansensitive to all antibiotics tested, including penicillin. These susceptibility data were used to guide the de-escalation of his empiric vancomycin and ceftriaxone to oxacillin on hospital day 1.
Due to their virulence, bloodstream infections caused by S aureus and S lugdunensis often require more than timely antimicrobial treatment to ensure eradication. Consultation with an infectious disease specialist to manage patients with S aureus bacteremia has been proven to reduce mortality.25 A similar mortality benefit is seen when infectious disease specialists are consulted for S lugdunensis bacteremia.26 This mortality benefit is likely explained by S lugdunensis’ propensity to cause aggressive, metastatic infections. In such cases, infectious disease consultants may recommend additional imaging (eg, transthoracic echocardiogram) to evaluate for occult sources of infection, advocate for appropriate source control, and guide the selection of an appropriate antibiotic course to ensure resolution of the bacteremia.
Conclusions
S lugdunensis is an increasingly recognized cause of nosocomial bloodstream infections. Given the commonalities in virulence that S lugdunensis shares with S aureus, treatment of bacteremia caused by either species should follow similar management principles: prompt initiation of IV antistaphylococcal therapy, a thorough evaluation for the source(s) of bacteremia as well as metastatic complications, and consultation with an infectious disease specialist. This case report also highlights the importance of considering a patient’s AVF as a potential source for infection even in the absence of localized signs of infection. The buttonhole method of AVF cannulation was thought to be a major contributor to the development and persistence of our patient’s bacteremia. This risk should be discussed with patients using a shared decision-making approach when developing a dialysis treatment plan.
Staphylococcus lugdunensis (S lugdunensis) is a species of coagulase-negative Staphylococcus (CoNS) and a constituent of human skin flora. Unlike other strains of CoNS, however, S lugdunensis has gained notoriety for virulence that resembles Staphylococcus aureus (S aureus). S lugdunensis is now recognized as an important nosocomial pathogen and cause of prosthetic device infections, including vascular catheter infections. We present a case of persistent S lugdunensis bacteremia occurring in a patient on hemodialysis (HD) without any implanted prosthetic materials.
Case Presentation
A 60-year-old man with a history of uncontrolled type 2 diabetes mellitus (T2DM) and end-stage renal disease on home HD via arteriovenous fistula (AVF) presented to the emergency department (ED) for evaluation of subacute progressive low back pain. His symptoms began abruptly 2 weeks prior to presentation without any identifiable trigger or trauma. His pain localized to the lower thoracic spine, radiating anteriorly into his abdomen. He reported tactile fever for several days before presentation but no chills, night sweats, paresthesia, weakness, or bowel/bladder incontinence. He had no recent surgeries, implanted hardware, or invasive procedures involving the spine. HD was performed 5 times a week at home with a family member cannulating his AVF via buttonhole technique. He initially sought evaluation in a community hospital several days prior, where he underwent magnetic resonance imaging (MRI) of the thoracic spine. He was discharged from the community ED with oral opioids prior to the MRI results. He presented to West Los Angeles Veterans Affairs Medical Center (WLAVAMC) ED when MRI results came back indicating abnormalities and he reported recalcitrant pain.
On arrival at WLAVAMC, the patient was afebrile with a heart rate of 107 bpm and blood pressure of 152/97 mm Hg. The remainder of his vital signs were normal. The physical examination revealed midline tenderness on palpation of the distal thoracic and proximal lumbar spine. Muscle strength was 4 of 5 in the bilateral hip flexors, though this was limited by pain. The remainder of his neurologic examination was nonfocal. The cardiac examination was unremarkable with no murmurs auscultated. His left upper extremity AVF had an audible bruit and palpable thrill. The skin examination was notable for acanthosis nigricans but no areas of skin erythema or induration and no obvious stigmata of infective endocarditis.
The initial laboratory workup was remarkable for a white blood cell (WBC) count of 10.0 × 103/µL with left shift, blood urea nitrogen level of 59 mg/dL, and creatinine level of 9.3 mg/dL. The patient’s erythrocyte sedimentation rate (ESR) was 45 mm/h (reference range, ≤ 20 mm/h) and C-reactive protein level was > 8.0 mg/L (reference range, ≤ 0.74 mg/L). Two months prior the hemoglobin A1c had been recorded at 9.9%.
Given his intractable low back pain and elevated inflammatory markers, the patient underwent an MRI of the thoracic and lumbar spine with contrast while in the ED. This MRI revealed abnormal marrow edema in the T11-T12 vertebrae with abnormal fluid signal in the T11-T12 disc space. Subjacent paravertebral edema also was noted. There was no well-defined fluid collection or abnormal signal in the spinal cord. Taken together, these findings were concerning for T11-T12 discitis with osteomyelitis.
Two sets of blood cultures were obtained, and empiric IV vancomycin and ceftriaxone were started. Interventional radiology was consulted for consideration of vertebral biopsy but deferred while awaiting blood culture data. Neurosurgery also was consulted and recommended nonoperative management given his nonfocal neurologic examination and imaging without evidence of abscess. Both sets of blood cultures collected on admission later grew methicillin-sensitive S lugdunensis, a species of CoNS. A transthoracic and later transesophageal echocardiogram did not show any valvular vegetations. The patient’s antibiotic regimen was narrowed to IV oxacillin based on susceptibility data. It was later discovered that both blood cultures obtained during his outside ED encounter were also growing S lugdunensis.
The patient’s S lugdunensis bacteremia persisted for the first 8 days of his admission despite appropriate dosing of oxacillin. During this time, the patient remained afebrile with stable vital signs and a normal WBC count. Positron emission tomography was obtained to evaluate for potential sources of his persistent bacteremia. Aside from tracer uptake in the T11-T12 vertebral bodies and intervertebral disc space, no other areas showed suspicious uptake. Neurosurgery reevaluated the patient and again recommended nonoperative management. Blood cultures cleared and based on recommendations from an infectious disease specialist, the patient was transitioned to IV cefazolin dosed 3 times weekly after HD, which was transitioned to an outpatient dialysis center. The patient continued taking cefazolin for 6 weeks with subsequent improvement in back pain and normalization of inflammatory markers at outpatient follow-up.
Discussion
CoNS are a major contributor to human skin flora, a common contaminant of blood cultures, and an important cause of nosocomial bloodstream infections.1,2 These species have a predilection for forming biofilms, making CoNS a major cause of prosthetic device infections.3 S lugdunensis is a CoNS species that was first described in 1988.4 In addition to foreign body–related infections, S lugdunensis has been implicated in bone/joint infections, native valve endocarditis, toxic shock syndrome, and brain abscesses.5-8 Infections due to S lugdunensis are notorious for their aggressive and fulminant courses. With its increased virulence that is atypical of other CoNS, S lugdunensis has understandably been likened more to S aureus.
Prior cases have been reported of S lugdunensis bacteremia in patients using HD. However, the suspected source of bacteremia in these cases has generally been central venous catheters.9-12
Notably, our patient’s AVF was accessed using the buttonhole technique for his home HD sessions, which involves cannulating the same site along the fistula until an epithelialized track has formed from scar tissue. At later HD sessions, duller needles can then be used to cannulate this same track. In contrast, the rope-ladder technique involves cannulating a different site along the fistula until the entire length of the fistula has been used. Patients report higher levels of satisfaction with the buttonhole technique, citing decreased pain, decreased oozing, and the perception of easier cannulation by HD nurses.14 However, the buttonhole technique also appears to confer a higher risk of vascular access-related bloodstream infection when compared with the rope-ladder technique.13,15,16
The buttonhole technique is hypothesized to increase infection risk due to the repeated use of the same site for needle entry. Skin flora, including CoNS, may colonize the scab that forms after dialysis access. If proper sterilization techniques are not rigorously followed, the bacteria colonizing the scab and adjacent skin may be introduced into a patient’s bloodstream during needle puncture. Loss of skin integrity due to frequent cannulation of the same site may also contribute to this increased infection risk. It is relevant to recall that our patient received HD 5 times weekly using the buttonhole technique. The use of the buttonhole technique, frequency of his HD sessions, unclear sterilization methods, and immune dysfunction related to his uncontrolled T2DM and renal disease all likely contributed to our patient’s bacteremia.
Using topical mupirocin for prophylaxis at the intended buttonhole puncture site has shown promising results in decreasing rates of S aureus bacteremia.17 It is unclear whether this intervention also would be effective against S lugdunensis. Increasing rates of mupirocin resistance have been reported among S lugdunensis isolates in dialysis settings, but further research in this area is warranted.18
There are no established treatment guidelines for S lugdunensis infections. In vitro studies suggest that S lugdunensis is susceptible to a wide variety of antibiotics. The mecA gene is a major determinant of methicillin resistance that is commonly observed among CoNS but is uncommonly seen with S lugdunensis.5 In a study by Tan and colleagues of 106 S lugdunensis isolates, they found that only 5 (4.7%) were mecA positive.19
Vancomycin is generally reasonable for empiric antibiotic coverage of staphylococci while speciation is pending. However, if S lugdunensis is isolated, its favorable susceptibility pattern typically allows for de-escalation to an antistaphylococcal β-lactam, such as oxacillin or nafcillin. In cases of bloodstream infections caused by methicillin-sensitive S aureus, treatment with a β-lactam has demonstrated superiority over vancomycin due to the lower rates of treatment failure and mortality with β-lactams.20,21 It is unknown whether β-lactams is superior for treating bacteremia with methicillin-sensitive S lugdunensis.
Our patient’s isolate of S lugdunensis was pansensitive to all antibiotics tested, including penicillin. These susceptibility data were used to guide the de-escalation of his empiric vancomycin and ceftriaxone to oxacillin on hospital day 1.
Due to their virulence, bloodstream infections caused by S aureus and S lugdunensis often require more than timely antimicrobial treatment to ensure eradication. Consultation with an infectious disease specialist to manage patients with S aureus bacteremia has been proven to reduce mortality.25 A similar mortality benefit is seen when infectious disease specialists are consulted for S lugdunensis bacteremia.26 This mortality benefit is likely explained by S lugdunensis’ propensity to cause aggressive, metastatic infections. In such cases, infectious disease consultants may recommend additional imaging (eg, transthoracic echocardiogram) to evaluate for occult sources of infection, advocate for appropriate source control, and guide the selection of an appropriate antibiotic course to ensure resolution of the bacteremia.
Conclusions
S lugdunensis is an increasingly recognized cause of nosocomial bloodstream infections. Given the commonalities in virulence that S lugdunensis shares with S aureus, treatment of bacteremia caused by either species should follow similar management principles: prompt initiation of IV antistaphylococcal therapy, a thorough evaluation for the source(s) of bacteremia as well as metastatic complications, and consultation with an infectious disease specialist. This case report also highlights the importance of considering a patient’s AVF as a potential source for infection even in the absence of localized signs of infection. The buttonhole method of AVF cannulation was thought to be a major contributor to the development and persistence of our patient’s bacteremia. This risk should be discussed with patients using a shared decision-making approach when developing a dialysis treatment plan.
1. Huebner J, Goldmann DA. Coagulase-negative staphylococci: role as pathogens. Annu Rev Med. 1999;50(1):223-236. doi:10.1146/annurev.med.50.1.223
2. Beekmann SE, Diekema DJ, Doern GV. Determining the clinical significance of coagulase-negative staphylococci isolated from blood cultures. Infect Control Hosp Epidemiol. 2005;26(6):559-566. doi:10.1086/502584
3. Arrecubieta C, Toba FA, von Bayern M, et al. SdrF, a Staphylococcus epidermidis surface protein, contributes to the initiation of ventricular assist device driveline–related infections. PLoS Pathog. 2009;5(5):e1000411. doi.10.1371/journal.ppat.1000411
4. Freney J, Brun Y, Bes M, et al. Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., two species from human clinical specimens. Int J Syst Bacteriol. 1988;38(2):168-172. doi:10.1099/00207713-38-2-168
5. Frank KL, del Pozo JL, Patel R. From clinical microbiology to infection pathogenesis: how daring to be different works for Staphylococcus lugdunensis. Clin Microbiol Rev. 2008;21(1):111-133. doi:10.1128/CMR.00036-07
6. Anguera I, Del Río A, Miró JM; Hospital Clinic Endocarditis Study Group. Staphylococcus lugdunensis infective endocarditis: description of 10 cases and analysis of native valve, prosthetic valve, and pacemaker lead endocarditis clinical profiles. Heart. 2005;91(2):e10. doi:10.1136/hrt.2004.040659
7. Pareja J, Gupta K, Koziel H. The toxic shock syndrome and Staphylococcus lugdunensis bacteremia. Ann Intern Med. 1998;128(7):603-604. doi:10.7326/0003-4819-128-7-199804010-00029
8. Woznowski M, Quack I, Bölke E, et al. Fulminant Staphylococcus lugdunensis septicaemia following a pelvic varicella-zoster virus infection in an immune-deficient patient: a case report. Eur J Med Res. 201;15(9):410-414. doi:10.1186/2047-783x-15-9-410
9. Mallappallil M, Salifu M, Woredekal Y, et al. Staphylococcus lugdunensis bacteremia in hemodialysis patients. Int J Microbiol Res. 2012;4(2):178-181. doi:10.9735/0975-5276.4.2.178-181
10. Shuttleworth R, Colby W. Staphylococcus lugdunensis endocarditis. J Clin Microbiol. 1992;30(8):5. doi:10.1128/jcm.30.8.1948-1952.1992
11. Conner RC, Byrnes TJ, Clough LA, Myers JP. Staphylococcus lugdunensis tricuspid valve endocarditis associated with home hemodialysis therapy: report of a case and review of the literature. Infect Dis Clin Pract. 2012;20(3):182-183. doi:1097/IPC.0b013e318245d4f1
12. Kamaraju S, Nelson K, Williams D, Ayenew W, Modi K. Staphylococcus lugdunensis pulmonary valve endocarditis in a patient on chronic hemodialysis. Am J Nephrol. 1999;19(5):605-608. doi:1097/IPC.0b013e318245d4f1
13. Lok C, Sontrop J, Faratro R, Chan C, Zimmerman DL. Frequent hemodialysis fistula infectious complications. Nephron Extra. 2014;4(3):159-167. doi:10.1159/000366477
14. Hashmi A, Cheema MQ, Moss AH. Hemodialysis patients’ experience with and attitudes toward the buttonhole technique for arteriovenous fistula cannulation. Clin Nephrol. 2010;74(5):346-350. doi:10.5414/cnp74346
15. Lyman M, Nguyen DB, Shugart A, Gruhler H, Lines C, Patel PR. Risk of vascular access infection associated with buttonhole cannulation of fistulas: data from the National Healthcare Safety Network. Am J Kidney Dis. 2020;76(1):82-89. doi:10.1053/j.ajkd.2019.11.006
16. MacRae JM, Ahmed SB, Atkar R, Hemmelgarn BR. A randomized trial comparing buttonhole with rope ladder needling in conventional hemodialysis patients. Clin J Am Soc Nephrol. 2012;7(10):1632-1638. doi:10.2215/CJN.02730312
17. Nesrallah GE, Cuerden M, Wong JHS, Pierratos A. Staphylococcus aureus bacteremia and buttonhole cannulation: long-term safety and efficacy of mupirocin prophylaxis. Clin J Am Soc Nephrol. 2010;5(6):1047-1053. doi:10.2215/CJN.00280110
18. Ho PL, Liu MCJ, Chow KH, et al. Emergence of ileS2 -carrying, multidrug-resistant plasmids in Staphylococcus lugdunensis. Antimicrob Agents Chemother. 2016;60(10):6411-6414. doi:10.1128/AAC.00948-16
19. Tan TY, Ng SY, He J. Microbiological characteristics, presumptive identification, and antibiotic susceptibilities of Staphylococcus lugdunensis. J Clin Microbiol. 2008;46(7):2393-2395. doi:10.1128/JCM.00740-08
20. Chang FY, Peacock JE, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore). 2003;82(5):333-339. doi:10.1097/01.md.0000091184.93122.09
21. Shurland S, Zhan M, Bradham DD, Roghmann MC. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28(3):273-279. doi:10.1086/512627
22. Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med. 1991;115(9):674. doi:10.7326/0003-4819-115-9-674
23. Fowler VG, Karchmer AW, Tally FP, et al; S. aureus Endocarditis and Bacteremia Study Group. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355(7):653-665 . doi:10.1056/NEJMoa053783
24. Duhon B, Dallas S, Velasquez ST, Hand E. Staphylococcus lugdunensis bacteremia and endocarditis treated with cefazolin and rifampin. Am J Health Syst Pharm. 2015;72(13):1114-1118. doi:10.2146/ajhp140498
25. Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore). 2009;88(5):263-267. doi:10.1097/MD.0b013e3181b8fccb
26. Forsblom E, Högnäs E, Syrjänen J, Järvinen A. Infectious diseases specialist consultation in Staphylococcus lugdunensis bacteremia. PLoS ONE. 2021;16(10):e0258511. doi:10.1371/journal.pone.0258511
1. Huebner J, Goldmann DA. Coagulase-negative staphylococci: role as pathogens. Annu Rev Med. 1999;50(1):223-236. doi:10.1146/annurev.med.50.1.223
2. Beekmann SE, Diekema DJ, Doern GV. Determining the clinical significance of coagulase-negative staphylococci isolated from blood cultures. Infect Control Hosp Epidemiol. 2005;26(6):559-566. doi:10.1086/502584
3. Arrecubieta C, Toba FA, von Bayern M, et al. SdrF, a Staphylococcus epidermidis surface protein, contributes to the initiation of ventricular assist device driveline–related infections. PLoS Pathog. 2009;5(5):e1000411. doi.10.1371/journal.ppat.1000411
4. Freney J, Brun Y, Bes M, et al. Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., two species from human clinical specimens. Int J Syst Bacteriol. 1988;38(2):168-172. doi:10.1099/00207713-38-2-168
5. Frank KL, del Pozo JL, Patel R. From clinical microbiology to infection pathogenesis: how daring to be different works for Staphylococcus lugdunensis. Clin Microbiol Rev. 2008;21(1):111-133. doi:10.1128/CMR.00036-07
6. Anguera I, Del Río A, Miró JM; Hospital Clinic Endocarditis Study Group. Staphylococcus lugdunensis infective endocarditis: description of 10 cases and analysis of native valve, prosthetic valve, and pacemaker lead endocarditis clinical profiles. Heart. 2005;91(2):e10. doi:10.1136/hrt.2004.040659
7. Pareja J, Gupta K, Koziel H. The toxic shock syndrome and Staphylococcus lugdunensis bacteremia. Ann Intern Med. 1998;128(7):603-604. doi:10.7326/0003-4819-128-7-199804010-00029
8. Woznowski M, Quack I, Bölke E, et al. Fulminant Staphylococcus lugdunensis septicaemia following a pelvic varicella-zoster virus infection in an immune-deficient patient: a case report. Eur J Med Res. 201;15(9):410-414. doi:10.1186/2047-783x-15-9-410
9. Mallappallil M, Salifu M, Woredekal Y, et al. Staphylococcus lugdunensis bacteremia in hemodialysis patients. Int J Microbiol Res. 2012;4(2):178-181. doi:10.9735/0975-5276.4.2.178-181
10. Shuttleworth R, Colby W. Staphylococcus lugdunensis endocarditis. J Clin Microbiol. 1992;30(8):5. doi:10.1128/jcm.30.8.1948-1952.1992
11. Conner RC, Byrnes TJ, Clough LA, Myers JP. Staphylococcus lugdunensis tricuspid valve endocarditis associated with home hemodialysis therapy: report of a case and review of the literature. Infect Dis Clin Pract. 2012;20(3):182-183. doi:1097/IPC.0b013e318245d4f1
12. Kamaraju S, Nelson K, Williams D, Ayenew W, Modi K. Staphylococcus lugdunensis pulmonary valve endocarditis in a patient on chronic hemodialysis. Am J Nephrol. 1999;19(5):605-608. doi:1097/IPC.0b013e318245d4f1
13. Lok C, Sontrop J, Faratro R, Chan C, Zimmerman DL. Frequent hemodialysis fistula infectious complications. Nephron Extra. 2014;4(3):159-167. doi:10.1159/000366477
14. Hashmi A, Cheema MQ, Moss AH. Hemodialysis patients’ experience with and attitudes toward the buttonhole technique for arteriovenous fistula cannulation. Clin Nephrol. 2010;74(5):346-350. doi:10.5414/cnp74346
15. Lyman M, Nguyen DB, Shugart A, Gruhler H, Lines C, Patel PR. Risk of vascular access infection associated with buttonhole cannulation of fistulas: data from the National Healthcare Safety Network. Am J Kidney Dis. 2020;76(1):82-89. doi:10.1053/j.ajkd.2019.11.006
16. MacRae JM, Ahmed SB, Atkar R, Hemmelgarn BR. A randomized trial comparing buttonhole with rope ladder needling in conventional hemodialysis patients. Clin J Am Soc Nephrol. 2012;7(10):1632-1638. doi:10.2215/CJN.02730312
17. Nesrallah GE, Cuerden M, Wong JHS, Pierratos A. Staphylococcus aureus bacteremia and buttonhole cannulation: long-term safety and efficacy of mupirocin prophylaxis. Clin J Am Soc Nephrol. 2010;5(6):1047-1053. doi:10.2215/CJN.00280110
18. Ho PL, Liu MCJ, Chow KH, et al. Emergence of ileS2 -carrying, multidrug-resistant plasmids in Staphylococcus lugdunensis. Antimicrob Agents Chemother. 2016;60(10):6411-6414. doi:10.1128/AAC.00948-16
19. Tan TY, Ng SY, He J. Microbiological characteristics, presumptive identification, and antibiotic susceptibilities of Staphylococcus lugdunensis. J Clin Microbiol. 2008;46(7):2393-2395. doi:10.1128/JCM.00740-08
20. Chang FY, Peacock JE, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore). 2003;82(5):333-339. doi:10.1097/01.md.0000091184.93122.09
21. Shurland S, Zhan M, Bradham DD, Roghmann MC. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28(3):273-279. doi:10.1086/512627
22. Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med. 1991;115(9):674. doi:10.7326/0003-4819-115-9-674
23. Fowler VG, Karchmer AW, Tally FP, et al; S. aureus Endocarditis and Bacteremia Study Group. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355(7):653-665 . doi:10.1056/NEJMoa053783
24. Duhon B, Dallas S, Velasquez ST, Hand E. Staphylococcus lugdunensis bacteremia and endocarditis treated with cefazolin and rifampin. Am J Health Syst Pharm. 2015;72(13):1114-1118. doi:10.2146/ajhp140498
25. Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore). 2009;88(5):263-267. doi:10.1097/MD.0b013e3181b8fccb
26. Forsblom E, Högnäs E, Syrjänen J, Järvinen A. Infectious diseases specialist consultation in Staphylococcus lugdunensis bacteremia. PLoS ONE. 2021;16(10):e0258511. doi:10.1371/journal.pone.0258511
VA-Based Peritoneal Dialysis Program Feasibility Considerations and Process Outline
Compared with hemodialysis (HD), peritoneal dialysis (PD) offers comparable survival and superior patient-centered and health services outcomes.1,2 This has prompted repeated calls over the past 2 decades for policies to increase the use of home dialysis and, more specifically, for PD in the United States.3,4
Veterans comprise nearly 10% of the population with end-stage kidney disease (ESKD) burden; > 50,000 US veterans are currently on dialysis.5,6 A majority of these veterans receive their chronic kidney disease (CKD) care through their affiliated US Department of Veterans Affairs (VA) medical centers (VAMCs).
To address these needs, the VHA National Kidney Disease Program (NKDP) formed a 4-member PD workgroup in 2019. Considering the breadth of challenges involved, the PD workgroup broadly designed its approach based on the I CARE (Integrity, Commitment, Advocacy, Respect, and Excellence) VA Core Values.
This review focuses on the initial deliberations of the PD access subgroup and provides a guide to establishing a new local VA PD program.
Step 1: Prerequisites
A functional nephrology service is a bedrock prerequisite for establishing a new PD program. A clinician champion capable of leading the effort is equally necessary. Occasionally, the prevalent ESKD economic and health care burden prompts local VAMC leadership to consider a new PD program to improve the quality or availability of services. More commonly, though, the nephrology section and the clinician champion are the first to recognize the need. In either scenario, the champion will require support and advocacy at multiple levels of local leadership, ie, the section or department chief, facility chief of staff, VAMC director, and the Veterans Integrated Service Network (VISN) director. The foremost task for the champion is to assess local clinical and infrastructure needs.
Goal Alignment
Any new VA nephrology program needs to be evaluated for its overall congruence with the local and national VA missions to improve the accessibility, integration, quality, and innovation of care for veterans. The following considerations are likely to apply to many VA systems.
Accessibility. A VHA directive recommends that all veterans be provided with the opportunity to choose and use any form of dialysis, especially home dialysis.9 Transitioning a veteran seamlessly from advanced CKD to PD requires the execution of multiple sequential processes in the pre-ESKD period, beginning with early identification of advanced CKD, timely referral to nephrology, education for shared dialysis decision making, coordination of care, and PD training and therapy.10 Splitting this sequence between VA and community-based care creates obstacles, including multiple approvals through VA Community Care Services that may substantially increase wait time and effort. This onerous process may be a significant deterrent against pursuing PD and increases the odds of emergency or inpatient initiation. Furthermore, the lack of PD availability limits the knowledge and experience among staff designated to assist veterans, which may result in inappropriate advocacy for HD or delay the transition to PD. Together, these processes can increase morbidity and health care use, and significantly delay or eliminate PD. Finally, many veterans reside in rural or remote areas where the expertise and the availability of PD may be unreliable. Establishing PD services within the local VAMC can improve access to PD, reduce the lead time needed to coordinate the transition to ESKD, and assist individual veterans in making an informed choice about dialysis. The program champion will need to identify and highlight all accessibility barriers within their business plan.
Integration. Many veterans receiving dialysis care at community-based facilities continue to receive nonnephrology care in the VA. This creates a parallel health care system with concerns for duplication of efforts and processes, suboptimal quality of care, and increased risk of medical errors. Establishing VA PD services increases access and integration of nephrology with other VA care.
Excellence. Studies of many chronic diseases have shown superior patient satisfaction and equal or superior quality of care delivered by the VA compared with that of non-VA facilities.11-14 Similarly, mortality rates for veterans receiving CKD and ESKD care in VA are lower compared with those at non-VA facilities.15-17 While these outcomes have not been examined for PD, integration of PD with VA care may lead to an improved overall quality of care and greater loyalty to the VA.
Innovation. Due to its integrated health care infrastructure, the VA is uniquely positioned to implement patient-centered and evidence-based pre-ESKD interventions that may improve outcomes. Prior studies have shown that pre-ESKD kidney disease education (KDE) improves pre- and post-ESKD outcomes, reduces health care costs, and leads to higher selection and use of home dialysis therapies.18-20 The VA recommends that all veterans with advanced CKD be provided access to pre-ESKD care and KDE. Unfortunately, KDE is uncommon among non-VA clinicians. A recent USRDS analysis reported that < 1% of patients with ESKD received pre-ESKD KDE.21 The ongoing Evaluate and Assess the effects of Comprehensive Pre-ESKD kidney disease Education on home dialysis in Veterans Trial (NCT04064086) should provide further evidence.
Step 2: Feasibility
A business plan requires the realistic projections of the costs and accounting for gains of the new clinical program. While there is limited guidance on personnel requirements when planning a PD program, we provide estimated resources needed to successfully establish and run a PD program (eAppendix 1, available online at doi:10.12788/fp.0356).
Clinical Considerations
Secondary or tertiary care VAMCs with multiple medical and surgical specialties routinely provide complex inpatient care. For these facilities, the lack of inpatient PD poses an obstacle to the provision of specialized nonnephrology care to veterans with ESKD, who are frequent users of such complex care. These considerations argue for the need for at least inpatient PD services at VAMCs that provide complex medical care for many veterans receiving PD in the community.
Deliberations for outpatient PD programs should be based on the clinical demands of ESKD care, the number of veterans likely to use PD, and growth projections. While there is no established minimum number that guarantees cost-effectiveness, most existing VA outpatient PD programs provide services for about 5 to 25 veterans. A local census can provide estimations of future PD needs. Travel considerations (ie, distance, terrain, traffic) may affect eligibility for purchased care and the decision where to receive PD. Many veterans may prefer PD from the local VAMC if it is convenient and allows them to maintain centralized VA care. Potential patients can be surveyed to gauge interest in receiving VA-based PD. Facilities providing structured pre-ESKD KDE may hold greater potential for PD growth, and it is important to highlight KDE infrastructure in the business plan.
Infrastructure
Spatial needs including clinic space and storage space for consumables, supplies, and equipment should be part of infrastructure requirements. The program champion may need to examine the available space for suitability and adequacy of the PD program early in the process. Ventilation renovations in the PD rooms should be incorporated into budget calculations. Water access for handwashing and PD effluent drainage should be confirmed, and if the program intends to establish home HD, additional considerations for the storage and water supply may be required. The VHA Handbook outlines the infrastructure requirements for a dialysis program.22 The VA has established national vendor contracts for dialysis equipment and consumables. However, a new PD program may need further guidance regarding the local agencies that provide administrative support and assist patients.
Telehealth technology has enabled many VAMCs to overcome geographical barriers for rural veterans.23 Ongoing expansion of community-based outpatient clinics (CBOCs) to include more rural locations is improving access to specialty care, while the launch of VA Video Connect (VVC) has further improved outreach. Investigators from Minneapolis have demonstrated the feasibility of multidisciplinary home-based telehealth management of veterans with CKD.24 Several existing nephrology sections across the VHA use a combination of VVC and CBOC-facilitated clinic visits to provide some pre-ESKD and ESKD care, including KDE, PD home visits and training, and comprehensive ESKD care visits. Recent changes in the clinical care pattern during the COVID-19 pandemic have further eased ESKD telehealth protocols. Integrating the projected use of telehealth in collaboration with existing resources available through the VHA NKDP can allow the local champion to improve the financial feasibility and long-term success of a new PD program.
Clinicians
Experience and expertise in managing PD vary among nephrologists. A recent survey found that only 11% of second-year nephrology trainees felt fully prepared to manage PD patients and 27% felt that they were minimally prepared.25 Thus, it is important to ensure that adequately trained nephrologists are available locally before initiating a new program, and if needed, coverage across VHS or VISN can be explored. One potential method to enhance practitioner comfort in PD is the use of existing peer-to-peer education through the VA Kidney Specialty Care Access Network-Extension for Community Health care Outcomes program that links health care professionals in rural areas with specialists at a tertiary care center.23 Nurses are a primary pillar for the success of home dialysis programs and the lack of a trained nursing workforce can be a significant limitation. Similarly, while the placement and management of complications related to PD catheters are not technically challenging, the availability of interventionists (either a surgeon or trained interventional radiologist) should be part of the business plan.
Financial Considerations
The financial considerations involving a new PD program within the VHA are complex (eAppendix 2, available online at doi:10.12788/fp.0356). ESKD is one of the most complex and costly comorbidities. It is a major determinant of the expenditure and revenue generation for facilities. The Veterans Equitable Resource Allocation system classifies ESKD on repeated dialysis as price category 10, indicating high complexity and cost. The VAMC workload and facility budget allocation is assessed annually and increases as the population of price group 10 veterans increases. VHA also provides additional Veterans Equitable Resource Allocation funds to VAMCs, which can improve the bottom line for VA-based dialysis units. Providing PD facilitates outpatient and inpatient management of comorbidities, allowing for substantial cost savings while improving the quality of nonrenal care. Outsourcing dialysis care can reduce the administrative burden, although, it deprives the VAMC of all dialysis-associated revenues while bearing the cost of all nonrenal and some renal care. The net effect is reduced facility productivity. In aggregate, establishing a local dialysis program requires greater financial resources for the capital and personnel costs; however, if captured appropriately these funds can be a major source of revenue and savings for the local VAMC.
Indirect costs are important for financial projections. Most community dialysis units operate as outpatient units, whereas all but a handful of the VA dialysis units operate within or near a VAMC. As a result, the VA units providing maintenance dialysis are regularly classified as inpatient centers while providing largely outpatient services, which negatively impacts overhead cost calculations. The predominant use of in-center HD as the default modality further sets an erroneously high baseline for the indirect cost of the VA-based PD services, especially considering that the principal savings of the home dialysis are through the reduction in the labor and capital costs. A rudimentary make-buy model for the in-center HD is available through the NKDP, and establishing a similar model for PD programs may be useful.
Cost considerations also may vary based on the model of ESKD care used locally. Of the 71 hospital-based and free-standing VA HD facilities, only 33 provide PD services, with 5 units providing only inpatient PD. The financial burden of establishing a fully operational outpatient PD program will be based on whether it is targeting a new unit or is expanding. The costs for equipment rental, disposables, and supplies vary based on the VA contract negotiations but are standardized across the nation with approved cost-of-living geographic adjustments. Caution needs to be exercised in employing a phased-hiring approach, as newer programs may require proportionally larger nursing resources due to greater needs for KDE, transitioning services, and training for PD. A target census-based hiring schedule should be negotiated with leadership before launch. If existing labor mapping does not allow for cross-coverage, part-time positions for physicians may be considered. Travel nurses, especially for PD training, can be considered to meet labor needs when long-term projections prohibit permanent full-time hires.
Finally, the balance sheet of a new program needs to account for different scenarios. In addition to nephrology costs, outsourcing veterans for PD services incurs multiple costs (eg, administrative, social work). Facilities with inpatient PD services alone are likely already bearing a component of the medications (including antibiotics) and/or surgical costs for their outsourced patients. These hidden costs are infrequently counted in projections. Facilities without inpatient PD cannot provide complex nonrenal care to ESKD patients on PD, even when the center is well equipped to provide it. These facilities also bear the cost of outsourcing even for complications related to PD. While a full estimation of these services varies, the hidden cost savings of many procedures or inpatient admissions, such as cardiovascular or musculoskeletal surgeries, can exceed those of dialysis in this complex population.
Step 3: proposal
There are no standardized formats for presenting a VHA business proposal; however, this outline provides a template. The business proposal should be designed to effectively communicate the collective data that describe the needs and requirements of a PD program to the local, regional, and national leadership. Not every rationale presented here will apply to an individual proposal and the local champion will need to tailor their rationale for their locale. A sample business plan is shown in eAppendix 3 (available online at doi:10.12788/fp.0356). VHA Handbook of dialysis requires that a PD nurse has a minimum of 12 months of nursing experience with at least 3 months of PD experience.25 Nursing training, education, and support should be discussed with nursing leadership and included in the business plan. Similarly, arrangements for laboratory, pharmacy, and prosthetics services and/or logistics to facilitate procurement of the needed devices, disposables, and supplies are essential and should be highlighted in the business plan.
Approval Process
Postapproval Process
Once approved, the champion will need to work closely with various services and managers to oversee infrastructural renovations and execute the hiring plans, establish standard operating procedures (SOPs), standardize staff proficiencies and functional statements, and finalize quality assessment parameters. Home dialysis standards have been addressed by NKDP and The Joint Commission. While PD requires home visits to assess the appropriateness of the environment, the PD program is accredited under hospital-based therapy. Standards and performance metrics should be incorporated into all the VA PD programs for standardization and assessment. Based on guidance from the VHA Handbook, quality metrics, such as dialysis adequacy, and rates of infection should be monitored and reviewed. The dialysis director may need to consider more frequent program evaluations in the first year to ensure appropriate troubleshooting. The VA infrastructure has developed the resources for a central repository for the PD SOPs and quality metrics, which can be obtained and adapted for the local program. Similarly, veteran satisfaction can be assessed through existing resources. Finally, the dialysis director can join the National VHA Dialysis Director listserv for regular updates on the existing and new VHA policies and NKDP updates.
Conclusions
Establishing a new PD program within a local federal infrastructure can appear daunting, both in terms of planning as well as approvals. However, the provision of home-based dialysis therapies may be beneficial to those in rural settings with limited access to in-center dialysis modalities as well as to those who seek autonomy and lifestyle independence in their medical care. Collaborations with the VHA NKDP or PD workgroup can help overcome many of the procedural hurdles, provide guidance about infrastructure and resource allocation and utilization, and provide easy access to established SOPs and quality parameters.
Acknowledgments
We acknowledge the late Dr. Catherine Do for her significant contribution to this manuscript. We also extend our sincere thanks to Dr. Holly Mattix-Kramer (Edward Hines Jr. Veterans Affairs Hospital and Loyola University Medical Center) for her prompt and valuable feedback on this manuscript.
1. Jung HY, Jeon Y, Park Y, et al. Better quality of life of peritoneal dialysis compared to hemodialysis over a two-year period after dialysis initiation. Sci Rep. 2019;9(1):10266. Published 2019 Jul 16. doi:10.1038/s41598-019-46744-1
2. Wong B, Ravani P, Oliver MJ, et al. Comparison of patient survival between hemodialysis and peritoneal dialysis among patients eligible for both modalities. Am J Kidney Dis. 2018;71(3):344-351. doi:10.1053/j.ajkd.2017.08.028
3. Chan CT, Collins K, Ditschman EP, et al. Overcoming barriers for uptake and continued use of home dialysis: an NKF-KDOQI Conference report. Am J Kidney Dis. 2020;75(6):926-934. doi:10.1053/j.ajkd.2019.11.007
4. Executive Order 13879: Advancing American kidney health. Fed Regist. 2019; 84(135):33817-33819. https://www.govinfo.gov/content/pkg/FR-2019-07-15/pdf/2019-15159.pdf
5. Patel TG, Pogach LM, Barth RH. CKD screening and management in the Veterans Health Administration: the impact of system organization and an innovative electronic record. Am J Kidney Dis. 2009;53(suppl 3):S78-S85. doi:10.1053/j.ajkd.2008.07.051
6. Saran R, Pearson A, Tilea A, et al. Burden and cost of caring for US veterans with CKD: initial findings from the VA Renal Information System (VA-REINS). Am J Kidney Dis. 2021;77(3):397-405. doi:10.1053/j.ajkd.2020.07.013
7. Sloan CE, Coffman CJ, Sanders LL, et al. Trends in peritoneal dialysis use in the United States after Medicare payment reform. Clin J Am Soc Nephrol. 2019;14(12):1763-1772. doi:10.2215/CJN.05910519
8. VA Maintaining Internal Systems and Strengthening Integrated Outside Networks Act of 2018. HR 5674. 115th Congress; Report No. 115-671, Part 1. May 3, 2018. Accessed February 9, 2023. https://www.congress.gov/115/bills/hr5674/BILLS-115hr5674rh.pdf
9. US Department of Veterans Affairs, Veterans Health Administration. Chronic kidney disease prevention, early recognition, and management. VHA Directive 1053. March 17, 2020. Accessed February 9, 2023. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8737
10. Blake PG, Quinn RR, Oliver MJ. Peritoneal dialysis and the process of modality selection. Perit Dial Int. 2013;33(3):233-241. doi:10.3747/pdi.2012.00119
11. Stroupe KT, Hynes DM, Giobbie-Hurder A, et al. Patient satisfaction and use of Veterans Affairs versus non-Veterans Affairs healthcare services by veterans. Med Care. 2005;43(5):453-460. doi:10.1097/01.mlr.0000160377.82164.d3
12. Anhang Price R, Sloss EM, Cefalu M, Farmer CM, Hussey PS. Comparing quality of care in Veterans Affairs and non-Veterans Affairs settings. J Gen Intern Med. 2018;33(10):1631-1638. doi:10.1007/s11606-018-4433-7
13. Blay E Jr, DeLancey JO, Hewitt DB, Chung JW, Bilimoria KY. Initial public reporting of quality at Veterans Affairs vs non-Veterans Affairs hospitals. JAMA Intern Med. 2017;177(6):882-885. doi:10.1001/jamainternmed.2017.0605
14. Nuti SV, Qin L, Krumholz HM. Outcome after admission at Veterans Affairs vs non-Veterans Affairs hospitals--reply. JAMA. 2016;316(3):346. doi:10.1001/jama.2016.5394
15. Streja E, Kovesdy CP, Soohoo M, et al. Dialysis provider and outcomes among United States veterans who transition to dialysis. Clin J Am Soc Nephrol. 2018;13(7):1055-1062. doi:10.2215/CJN.12951117
16. Wang V, Coffman CJ, Stechuchak KM, et al. Survival among veterans obtaining dialysis in VA and non-VA settings. J Am Soc Nephrol. 2019;30(1):159-168. doi:10.1681/ASN.2018050521
17. Kurella Tamura M, Thomas IC, Montez-Rath ME, et al. Dialysis initiation and mortality among older veterans with kidney failure treated in Medicare vs the Department of Veterans Affairs. JAMA Intern Med. 2018;178(5):657-664. doi:10.1001/jamainternmed.2018.0411
18. Devins GM, Mendelssohn DC, Barré PE, Taub K, Binik YM. Predialysis psychoeducational intervention extends survival in CKD: a 20-year follow-up. Am J Kidney Dis. 2005;46(6):1088-1098. doi:10.1053/j.ajkd.2005.08.017
19. Devoe DJ, Wong B, James MT, et al. Patient education and peritoneal dialysis modality selection: a systematic review and meta-analysis. Am J Kidney Dis. 2016;68(3):422-433. doi:10.1053/j.ajkd.2016.02.053
20. Lin E, Chertow GM, Yan B, Malcolm E, Goldhaber-Fiebert JD. Cost-effectiveness of multidisciplinary care in mild to moderate chronic kidney disease in the United States: A modeling study. PLoS Med. 2018;15(3):e1002532. Published 2018 Mar 27. doi:10.1371/journal.pmed.1002532
21. Shukla AM, Bozorgmehri S, Ruchi R, et al. Utilization of CMS pre-ESRD Kidney Disease Education services and its associations with the home dialysis therapies. Perit Dial Int. 2021;41(5):453-462. doi:10.1177/0896860820975586
22. US Dept of Veterans Affairs, Veterans Health Administration. Criteria and standards for VA dialysis programs. VHA Directive 1601. 2016. May 23, 2016. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3205
23. Crowley ST, Belcher J, Choudhury D, et al. Targeting access to kidney care via telehealth: the VA experience. Adv Chronic Kidney Dis. 2017;24(1):22-30. doi:10.1053/j.ackd.2016.11.005
24. Ishani A, Christopher J, Palmer D, et al. Telehealth by an interprofessional team in patients with CKD: a randomized controlled trial. Am J Kidney Dis. 2016;68(1):41-49. doi:10.1053/j.ajkd.2016.01.018
25. Gupta N, Taber-Hight EB, Miller BW. Perceptions of home dialysis training and experience among US nephrology fellows. Am J Kidney Dis. 2021;77(5):713-718.e1. doi:10.1053/j.ajkd.2020.09.014
Compared with hemodialysis (HD), peritoneal dialysis (PD) offers comparable survival and superior patient-centered and health services outcomes.1,2 This has prompted repeated calls over the past 2 decades for policies to increase the use of home dialysis and, more specifically, for PD in the United States.3,4
Veterans comprise nearly 10% of the population with end-stage kidney disease (ESKD) burden; > 50,000 US veterans are currently on dialysis.5,6 A majority of these veterans receive their chronic kidney disease (CKD) care through their affiliated US Department of Veterans Affairs (VA) medical centers (VAMCs).
To address these needs, the VHA National Kidney Disease Program (NKDP) formed a 4-member PD workgroup in 2019. Considering the breadth of challenges involved, the PD workgroup broadly designed its approach based on the I CARE (Integrity, Commitment, Advocacy, Respect, and Excellence) VA Core Values.
This review focuses on the initial deliberations of the PD access subgroup and provides a guide to establishing a new local VA PD program.
Step 1: Prerequisites
A functional nephrology service is a bedrock prerequisite for establishing a new PD program. A clinician champion capable of leading the effort is equally necessary. Occasionally, the prevalent ESKD economic and health care burden prompts local VAMC leadership to consider a new PD program to improve the quality or availability of services. More commonly, though, the nephrology section and the clinician champion are the first to recognize the need. In either scenario, the champion will require support and advocacy at multiple levels of local leadership, ie, the section or department chief, facility chief of staff, VAMC director, and the Veterans Integrated Service Network (VISN) director. The foremost task for the champion is to assess local clinical and infrastructure needs.
Goal Alignment
Any new VA nephrology program needs to be evaluated for its overall congruence with the local and national VA missions to improve the accessibility, integration, quality, and innovation of care for veterans. The following considerations are likely to apply to many VA systems.
Accessibility. A VHA directive recommends that all veterans be provided with the opportunity to choose and use any form of dialysis, especially home dialysis.9 Transitioning a veteran seamlessly from advanced CKD to PD requires the execution of multiple sequential processes in the pre-ESKD period, beginning with early identification of advanced CKD, timely referral to nephrology, education for shared dialysis decision making, coordination of care, and PD training and therapy.10 Splitting this sequence between VA and community-based care creates obstacles, including multiple approvals through VA Community Care Services that may substantially increase wait time and effort. This onerous process may be a significant deterrent against pursuing PD and increases the odds of emergency or inpatient initiation. Furthermore, the lack of PD availability limits the knowledge and experience among staff designated to assist veterans, which may result in inappropriate advocacy for HD or delay the transition to PD. Together, these processes can increase morbidity and health care use, and significantly delay or eliminate PD. Finally, many veterans reside in rural or remote areas where the expertise and the availability of PD may be unreliable. Establishing PD services within the local VAMC can improve access to PD, reduce the lead time needed to coordinate the transition to ESKD, and assist individual veterans in making an informed choice about dialysis. The program champion will need to identify and highlight all accessibility barriers within their business plan.
Integration. Many veterans receiving dialysis care at community-based facilities continue to receive nonnephrology care in the VA. This creates a parallel health care system with concerns for duplication of efforts and processes, suboptimal quality of care, and increased risk of medical errors. Establishing VA PD services increases access and integration of nephrology with other VA care.
Excellence. Studies of many chronic diseases have shown superior patient satisfaction and equal or superior quality of care delivered by the VA compared with that of non-VA facilities.11-14 Similarly, mortality rates for veterans receiving CKD and ESKD care in VA are lower compared with those at non-VA facilities.15-17 While these outcomes have not been examined for PD, integration of PD with VA care may lead to an improved overall quality of care and greater loyalty to the VA.
Innovation. Due to its integrated health care infrastructure, the VA is uniquely positioned to implement patient-centered and evidence-based pre-ESKD interventions that may improve outcomes. Prior studies have shown that pre-ESKD kidney disease education (KDE) improves pre- and post-ESKD outcomes, reduces health care costs, and leads to higher selection and use of home dialysis therapies.18-20 The VA recommends that all veterans with advanced CKD be provided access to pre-ESKD care and KDE. Unfortunately, KDE is uncommon among non-VA clinicians. A recent USRDS analysis reported that < 1% of patients with ESKD received pre-ESKD KDE.21 The ongoing Evaluate and Assess the effects of Comprehensive Pre-ESKD kidney disease Education on home dialysis in Veterans Trial (NCT04064086) should provide further evidence.
Step 2: Feasibility
A business plan requires the realistic projections of the costs and accounting for gains of the new clinical program. While there is limited guidance on personnel requirements when planning a PD program, we provide estimated resources needed to successfully establish and run a PD program (eAppendix 1, available online at doi:10.12788/fp.0356).
Clinical Considerations
Secondary or tertiary care VAMCs with multiple medical and surgical specialties routinely provide complex inpatient care. For these facilities, the lack of inpatient PD poses an obstacle to the provision of specialized nonnephrology care to veterans with ESKD, who are frequent users of such complex care. These considerations argue for the need for at least inpatient PD services at VAMCs that provide complex medical care for many veterans receiving PD in the community.
Deliberations for outpatient PD programs should be based on the clinical demands of ESKD care, the number of veterans likely to use PD, and growth projections. While there is no established minimum number that guarantees cost-effectiveness, most existing VA outpatient PD programs provide services for about 5 to 25 veterans. A local census can provide estimations of future PD needs. Travel considerations (ie, distance, terrain, traffic) may affect eligibility for purchased care and the decision where to receive PD. Many veterans may prefer PD from the local VAMC if it is convenient and allows them to maintain centralized VA care. Potential patients can be surveyed to gauge interest in receiving VA-based PD. Facilities providing structured pre-ESKD KDE may hold greater potential for PD growth, and it is important to highlight KDE infrastructure in the business plan.
Infrastructure
Spatial needs including clinic space and storage space for consumables, supplies, and equipment should be part of infrastructure requirements. The program champion may need to examine the available space for suitability and adequacy of the PD program early in the process. Ventilation renovations in the PD rooms should be incorporated into budget calculations. Water access for handwashing and PD effluent drainage should be confirmed, and if the program intends to establish home HD, additional considerations for the storage and water supply may be required. The VHA Handbook outlines the infrastructure requirements for a dialysis program.22 The VA has established national vendor contracts for dialysis equipment and consumables. However, a new PD program may need further guidance regarding the local agencies that provide administrative support and assist patients.
Telehealth technology has enabled many VAMCs to overcome geographical barriers for rural veterans.23 Ongoing expansion of community-based outpatient clinics (CBOCs) to include more rural locations is improving access to specialty care, while the launch of VA Video Connect (VVC) has further improved outreach. Investigators from Minneapolis have demonstrated the feasibility of multidisciplinary home-based telehealth management of veterans with CKD.24 Several existing nephrology sections across the VHA use a combination of VVC and CBOC-facilitated clinic visits to provide some pre-ESKD and ESKD care, including KDE, PD home visits and training, and comprehensive ESKD care visits. Recent changes in the clinical care pattern during the COVID-19 pandemic have further eased ESKD telehealth protocols. Integrating the projected use of telehealth in collaboration with existing resources available through the VHA NKDP can allow the local champion to improve the financial feasibility and long-term success of a new PD program.
Clinicians
Experience and expertise in managing PD vary among nephrologists. A recent survey found that only 11% of second-year nephrology trainees felt fully prepared to manage PD patients and 27% felt that they were minimally prepared.25 Thus, it is important to ensure that adequately trained nephrologists are available locally before initiating a new program, and if needed, coverage across VHS or VISN can be explored. One potential method to enhance practitioner comfort in PD is the use of existing peer-to-peer education through the VA Kidney Specialty Care Access Network-Extension for Community Health care Outcomes program that links health care professionals in rural areas with specialists at a tertiary care center.23 Nurses are a primary pillar for the success of home dialysis programs and the lack of a trained nursing workforce can be a significant limitation. Similarly, while the placement and management of complications related to PD catheters are not technically challenging, the availability of interventionists (either a surgeon or trained interventional radiologist) should be part of the business plan.
Financial Considerations
The financial considerations involving a new PD program within the VHA are complex (eAppendix 2, available online at doi:10.12788/fp.0356). ESKD is one of the most complex and costly comorbidities. It is a major determinant of the expenditure and revenue generation for facilities. The Veterans Equitable Resource Allocation system classifies ESKD on repeated dialysis as price category 10, indicating high complexity and cost. The VAMC workload and facility budget allocation is assessed annually and increases as the population of price group 10 veterans increases. VHA also provides additional Veterans Equitable Resource Allocation funds to VAMCs, which can improve the bottom line for VA-based dialysis units. Providing PD facilitates outpatient and inpatient management of comorbidities, allowing for substantial cost savings while improving the quality of nonrenal care. Outsourcing dialysis care can reduce the administrative burden, although, it deprives the VAMC of all dialysis-associated revenues while bearing the cost of all nonrenal and some renal care. The net effect is reduced facility productivity. In aggregate, establishing a local dialysis program requires greater financial resources for the capital and personnel costs; however, if captured appropriately these funds can be a major source of revenue and savings for the local VAMC.
Indirect costs are important for financial projections. Most community dialysis units operate as outpatient units, whereas all but a handful of the VA dialysis units operate within or near a VAMC. As a result, the VA units providing maintenance dialysis are regularly classified as inpatient centers while providing largely outpatient services, which negatively impacts overhead cost calculations. The predominant use of in-center HD as the default modality further sets an erroneously high baseline for the indirect cost of the VA-based PD services, especially considering that the principal savings of the home dialysis are through the reduction in the labor and capital costs. A rudimentary make-buy model for the in-center HD is available through the NKDP, and establishing a similar model for PD programs may be useful.
Cost considerations also may vary based on the model of ESKD care used locally. Of the 71 hospital-based and free-standing VA HD facilities, only 33 provide PD services, with 5 units providing only inpatient PD. The financial burden of establishing a fully operational outpatient PD program will be based on whether it is targeting a new unit or is expanding. The costs for equipment rental, disposables, and supplies vary based on the VA contract negotiations but are standardized across the nation with approved cost-of-living geographic adjustments. Caution needs to be exercised in employing a phased-hiring approach, as newer programs may require proportionally larger nursing resources due to greater needs for KDE, transitioning services, and training for PD. A target census-based hiring schedule should be negotiated with leadership before launch. If existing labor mapping does not allow for cross-coverage, part-time positions for physicians may be considered. Travel nurses, especially for PD training, can be considered to meet labor needs when long-term projections prohibit permanent full-time hires.
Finally, the balance sheet of a new program needs to account for different scenarios. In addition to nephrology costs, outsourcing veterans for PD services incurs multiple costs (eg, administrative, social work). Facilities with inpatient PD services alone are likely already bearing a component of the medications (including antibiotics) and/or surgical costs for their outsourced patients. These hidden costs are infrequently counted in projections. Facilities without inpatient PD cannot provide complex nonrenal care to ESKD patients on PD, even when the center is well equipped to provide it. These facilities also bear the cost of outsourcing even for complications related to PD. While a full estimation of these services varies, the hidden cost savings of many procedures or inpatient admissions, such as cardiovascular or musculoskeletal surgeries, can exceed those of dialysis in this complex population.
Step 3: proposal
There are no standardized formats for presenting a VHA business proposal; however, this outline provides a template. The business proposal should be designed to effectively communicate the collective data that describe the needs and requirements of a PD program to the local, regional, and national leadership. Not every rationale presented here will apply to an individual proposal and the local champion will need to tailor their rationale for their locale. A sample business plan is shown in eAppendix 3 (available online at doi:10.12788/fp.0356). VHA Handbook of dialysis requires that a PD nurse has a minimum of 12 months of nursing experience with at least 3 months of PD experience.25 Nursing training, education, and support should be discussed with nursing leadership and included in the business plan. Similarly, arrangements for laboratory, pharmacy, and prosthetics services and/or logistics to facilitate procurement of the needed devices, disposables, and supplies are essential and should be highlighted in the business plan.
Approval Process
Postapproval Process
Once approved, the champion will need to work closely with various services and managers to oversee infrastructural renovations and execute the hiring plans, establish standard operating procedures (SOPs), standardize staff proficiencies and functional statements, and finalize quality assessment parameters. Home dialysis standards have been addressed by NKDP and The Joint Commission. While PD requires home visits to assess the appropriateness of the environment, the PD program is accredited under hospital-based therapy. Standards and performance metrics should be incorporated into all the VA PD programs for standardization and assessment. Based on guidance from the VHA Handbook, quality metrics, such as dialysis adequacy, and rates of infection should be monitored and reviewed. The dialysis director may need to consider more frequent program evaluations in the first year to ensure appropriate troubleshooting. The VA infrastructure has developed the resources for a central repository for the PD SOPs and quality metrics, which can be obtained and adapted for the local program. Similarly, veteran satisfaction can be assessed through existing resources. Finally, the dialysis director can join the National VHA Dialysis Director listserv for regular updates on the existing and new VHA policies and NKDP updates.
Conclusions
Establishing a new PD program within a local federal infrastructure can appear daunting, both in terms of planning as well as approvals. However, the provision of home-based dialysis therapies may be beneficial to those in rural settings with limited access to in-center dialysis modalities as well as to those who seek autonomy and lifestyle independence in their medical care. Collaborations with the VHA NKDP or PD workgroup can help overcome many of the procedural hurdles, provide guidance about infrastructure and resource allocation and utilization, and provide easy access to established SOPs and quality parameters.
Acknowledgments
We acknowledge the late Dr. Catherine Do for her significant contribution to this manuscript. We also extend our sincere thanks to Dr. Holly Mattix-Kramer (Edward Hines Jr. Veterans Affairs Hospital and Loyola University Medical Center) for her prompt and valuable feedback on this manuscript.
Compared with hemodialysis (HD), peritoneal dialysis (PD) offers comparable survival and superior patient-centered and health services outcomes.1,2 This has prompted repeated calls over the past 2 decades for policies to increase the use of home dialysis and, more specifically, for PD in the United States.3,4
Veterans comprise nearly 10% of the population with end-stage kidney disease (ESKD) burden; > 50,000 US veterans are currently on dialysis.5,6 A majority of these veterans receive their chronic kidney disease (CKD) care through their affiliated US Department of Veterans Affairs (VA) medical centers (VAMCs).
To address these needs, the VHA National Kidney Disease Program (NKDP) formed a 4-member PD workgroup in 2019. Considering the breadth of challenges involved, the PD workgroup broadly designed its approach based on the I CARE (Integrity, Commitment, Advocacy, Respect, and Excellence) VA Core Values.
This review focuses on the initial deliberations of the PD access subgroup and provides a guide to establishing a new local VA PD program.
Step 1: Prerequisites
A functional nephrology service is a bedrock prerequisite for establishing a new PD program. A clinician champion capable of leading the effort is equally necessary. Occasionally, the prevalent ESKD economic and health care burden prompts local VAMC leadership to consider a new PD program to improve the quality or availability of services. More commonly, though, the nephrology section and the clinician champion are the first to recognize the need. In either scenario, the champion will require support and advocacy at multiple levels of local leadership, ie, the section or department chief, facility chief of staff, VAMC director, and the Veterans Integrated Service Network (VISN) director. The foremost task for the champion is to assess local clinical and infrastructure needs.
Goal Alignment
Any new VA nephrology program needs to be evaluated for its overall congruence with the local and national VA missions to improve the accessibility, integration, quality, and innovation of care for veterans. The following considerations are likely to apply to many VA systems.
Accessibility. A VHA directive recommends that all veterans be provided with the opportunity to choose and use any form of dialysis, especially home dialysis.9 Transitioning a veteran seamlessly from advanced CKD to PD requires the execution of multiple sequential processes in the pre-ESKD period, beginning with early identification of advanced CKD, timely referral to nephrology, education for shared dialysis decision making, coordination of care, and PD training and therapy.10 Splitting this sequence between VA and community-based care creates obstacles, including multiple approvals through VA Community Care Services that may substantially increase wait time and effort. This onerous process may be a significant deterrent against pursuing PD and increases the odds of emergency or inpatient initiation. Furthermore, the lack of PD availability limits the knowledge and experience among staff designated to assist veterans, which may result in inappropriate advocacy for HD or delay the transition to PD. Together, these processes can increase morbidity and health care use, and significantly delay or eliminate PD. Finally, many veterans reside in rural or remote areas where the expertise and the availability of PD may be unreliable. Establishing PD services within the local VAMC can improve access to PD, reduce the lead time needed to coordinate the transition to ESKD, and assist individual veterans in making an informed choice about dialysis. The program champion will need to identify and highlight all accessibility barriers within their business plan.
Integration. Many veterans receiving dialysis care at community-based facilities continue to receive nonnephrology care in the VA. This creates a parallel health care system with concerns for duplication of efforts and processes, suboptimal quality of care, and increased risk of medical errors. Establishing VA PD services increases access and integration of nephrology with other VA care.
Excellence. Studies of many chronic diseases have shown superior patient satisfaction and equal or superior quality of care delivered by the VA compared with that of non-VA facilities.11-14 Similarly, mortality rates for veterans receiving CKD and ESKD care in VA are lower compared with those at non-VA facilities.15-17 While these outcomes have not been examined for PD, integration of PD with VA care may lead to an improved overall quality of care and greater loyalty to the VA.
Innovation. Due to its integrated health care infrastructure, the VA is uniquely positioned to implement patient-centered and evidence-based pre-ESKD interventions that may improve outcomes. Prior studies have shown that pre-ESKD kidney disease education (KDE) improves pre- and post-ESKD outcomes, reduces health care costs, and leads to higher selection and use of home dialysis therapies.18-20 The VA recommends that all veterans with advanced CKD be provided access to pre-ESKD care and KDE. Unfortunately, KDE is uncommon among non-VA clinicians. A recent USRDS analysis reported that < 1% of patients with ESKD received pre-ESKD KDE.21 The ongoing Evaluate and Assess the effects of Comprehensive Pre-ESKD kidney disease Education on home dialysis in Veterans Trial (NCT04064086) should provide further evidence.
Step 2: Feasibility
A business plan requires the realistic projections of the costs and accounting for gains of the new clinical program. While there is limited guidance on personnel requirements when planning a PD program, we provide estimated resources needed to successfully establish and run a PD program (eAppendix 1, available online at doi:10.12788/fp.0356).
Clinical Considerations
Secondary or tertiary care VAMCs with multiple medical and surgical specialties routinely provide complex inpatient care. For these facilities, the lack of inpatient PD poses an obstacle to the provision of specialized nonnephrology care to veterans with ESKD, who are frequent users of such complex care. These considerations argue for the need for at least inpatient PD services at VAMCs that provide complex medical care for many veterans receiving PD in the community.
Deliberations for outpatient PD programs should be based on the clinical demands of ESKD care, the number of veterans likely to use PD, and growth projections. While there is no established minimum number that guarantees cost-effectiveness, most existing VA outpatient PD programs provide services for about 5 to 25 veterans. A local census can provide estimations of future PD needs. Travel considerations (ie, distance, terrain, traffic) may affect eligibility for purchased care and the decision where to receive PD. Many veterans may prefer PD from the local VAMC if it is convenient and allows them to maintain centralized VA care. Potential patients can be surveyed to gauge interest in receiving VA-based PD. Facilities providing structured pre-ESKD KDE may hold greater potential for PD growth, and it is important to highlight KDE infrastructure in the business plan.
Infrastructure
Spatial needs including clinic space and storage space for consumables, supplies, and equipment should be part of infrastructure requirements. The program champion may need to examine the available space for suitability and adequacy of the PD program early in the process. Ventilation renovations in the PD rooms should be incorporated into budget calculations. Water access for handwashing and PD effluent drainage should be confirmed, and if the program intends to establish home HD, additional considerations for the storage and water supply may be required. The VHA Handbook outlines the infrastructure requirements for a dialysis program.22 The VA has established national vendor contracts for dialysis equipment and consumables. However, a new PD program may need further guidance regarding the local agencies that provide administrative support and assist patients.
Telehealth technology has enabled many VAMCs to overcome geographical barriers for rural veterans.23 Ongoing expansion of community-based outpatient clinics (CBOCs) to include more rural locations is improving access to specialty care, while the launch of VA Video Connect (VVC) has further improved outreach. Investigators from Minneapolis have demonstrated the feasibility of multidisciplinary home-based telehealth management of veterans with CKD.24 Several existing nephrology sections across the VHA use a combination of VVC and CBOC-facilitated clinic visits to provide some pre-ESKD and ESKD care, including KDE, PD home visits and training, and comprehensive ESKD care visits. Recent changes in the clinical care pattern during the COVID-19 pandemic have further eased ESKD telehealth protocols. Integrating the projected use of telehealth in collaboration with existing resources available through the VHA NKDP can allow the local champion to improve the financial feasibility and long-term success of a new PD program.
Clinicians
Experience and expertise in managing PD vary among nephrologists. A recent survey found that only 11% of second-year nephrology trainees felt fully prepared to manage PD patients and 27% felt that they were minimally prepared.25 Thus, it is important to ensure that adequately trained nephrologists are available locally before initiating a new program, and if needed, coverage across VHS or VISN can be explored. One potential method to enhance practitioner comfort in PD is the use of existing peer-to-peer education through the VA Kidney Specialty Care Access Network-Extension for Community Health care Outcomes program that links health care professionals in rural areas with specialists at a tertiary care center.23 Nurses are a primary pillar for the success of home dialysis programs and the lack of a trained nursing workforce can be a significant limitation. Similarly, while the placement and management of complications related to PD catheters are not technically challenging, the availability of interventionists (either a surgeon or trained interventional radiologist) should be part of the business plan.
Financial Considerations
The financial considerations involving a new PD program within the VHA are complex (eAppendix 2, available online at doi:10.12788/fp.0356). ESKD is one of the most complex and costly comorbidities. It is a major determinant of the expenditure and revenue generation for facilities. The Veterans Equitable Resource Allocation system classifies ESKD on repeated dialysis as price category 10, indicating high complexity and cost. The VAMC workload and facility budget allocation is assessed annually and increases as the population of price group 10 veterans increases. VHA also provides additional Veterans Equitable Resource Allocation funds to VAMCs, which can improve the bottom line for VA-based dialysis units. Providing PD facilitates outpatient and inpatient management of comorbidities, allowing for substantial cost savings while improving the quality of nonrenal care. Outsourcing dialysis care can reduce the administrative burden, although, it deprives the VAMC of all dialysis-associated revenues while bearing the cost of all nonrenal and some renal care. The net effect is reduced facility productivity. In aggregate, establishing a local dialysis program requires greater financial resources for the capital and personnel costs; however, if captured appropriately these funds can be a major source of revenue and savings for the local VAMC.
Indirect costs are important for financial projections. Most community dialysis units operate as outpatient units, whereas all but a handful of the VA dialysis units operate within or near a VAMC. As a result, the VA units providing maintenance dialysis are regularly classified as inpatient centers while providing largely outpatient services, which negatively impacts overhead cost calculations. The predominant use of in-center HD as the default modality further sets an erroneously high baseline for the indirect cost of the VA-based PD services, especially considering that the principal savings of the home dialysis are through the reduction in the labor and capital costs. A rudimentary make-buy model for the in-center HD is available through the NKDP, and establishing a similar model for PD programs may be useful.
Cost considerations also may vary based on the model of ESKD care used locally. Of the 71 hospital-based and free-standing VA HD facilities, only 33 provide PD services, with 5 units providing only inpatient PD. The financial burden of establishing a fully operational outpatient PD program will be based on whether it is targeting a new unit or is expanding. The costs for equipment rental, disposables, and supplies vary based on the VA contract negotiations but are standardized across the nation with approved cost-of-living geographic adjustments. Caution needs to be exercised in employing a phased-hiring approach, as newer programs may require proportionally larger nursing resources due to greater needs for KDE, transitioning services, and training for PD. A target census-based hiring schedule should be negotiated with leadership before launch. If existing labor mapping does not allow for cross-coverage, part-time positions for physicians may be considered. Travel nurses, especially for PD training, can be considered to meet labor needs when long-term projections prohibit permanent full-time hires.
Finally, the balance sheet of a new program needs to account for different scenarios. In addition to nephrology costs, outsourcing veterans for PD services incurs multiple costs (eg, administrative, social work). Facilities with inpatient PD services alone are likely already bearing a component of the medications (including antibiotics) and/or surgical costs for their outsourced patients. These hidden costs are infrequently counted in projections. Facilities without inpatient PD cannot provide complex nonrenal care to ESKD patients on PD, even when the center is well equipped to provide it. These facilities also bear the cost of outsourcing even for complications related to PD. While a full estimation of these services varies, the hidden cost savings of many procedures or inpatient admissions, such as cardiovascular or musculoskeletal surgeries, can exceed those of dialysis in this complex population.
Step 3: proposal
There are no standardized formats for presenting a VHA business proposal; however, this outline provides a template. The business proposal should be designed to effectively communicate the collective data that describe the needs and requirements of a PD program to the local, regional, and national leadership. Not every rationale presented here will apply to an individual proposal and the local champion will need to tailor their rationale for their locale. A sample business plan is shown in eAppendix 3 (available online at doi:10.12788/fp.0356). VHA Handbook of dialysis requires that a PD nurse has a minimum of 12 months of nursing experience with at least 3 months of PD experience.25 Nursing training, education, and support should be discussed with nursing leadership and included in the business plan. Similarly, arrangements for laboratory, pharmacy, and prosthetics services and/or logistics to facilitate procurement of the needed devices, disposables, and supplies are essential and should be highlighted in the business plan.
Approval Process
Postapproval Process
Once approved, the champion will need to work closely with various services and managers to oversee infrastructural renovations and execute the hiring plans, establish standard operating procedures (SOPs), standardize staff proficiencies and functional statements, and finalize quality assessment parameters. Home dialysis standards have been addressed by NKDP and The Joint Commission. While PD requires home visits to assess the appropriateness of the environment, the PD program is accredited under hospital-based therapy. Standards and performance metrics should be incorporated into all the VA PD programs for standardization and assessment. Based on guidance from the VHA Handbook, quality metrics, such as dialysis adequacy, and rates of infection should be monitored and reviewed. The dialysis director may need to consider more frequent program evaluations in the first year to ensure appropriate troubleshooting. The VA infrastructure has developed the resources for a central repository for the PD SOPs and quality metrics, which can be obtained and adapted for the local program. Similarly, veteran satisfaction can be assessed through existing resources. Finally, the dialysis director can join the National VHA Dialysis Director listserv for regular updates on the existing and new VHA policies and NKDP updates.
Conclusions
Establishing a new PD program within a local federal infrastructure can appear daunting, both in terms of planning as well as approvals. However, the provision of home-based dialysis therapies may be beneficial to those in rural settings with limited access to in-center dialysis modalities as well as to those who seek autonomy and lifestyle independence in their medical care. Collaborations with the VHA NKDP or PD workgroup can help overcome many of the procedural hurdles, provide guidance about infrastructure and resource allocation and utilization, and provide easy access to established SOPs and quality parameters.
Acknowledgments
We acknowledge the late Dr. Catherine Do for her significant contribution to this manuscript. We also extend our sincere thanks to Dr. Holly Mattix-Kramer (Edward Hines Jr. Veterans Affairs Hospital and Loyola University Medical Center) for her prompt and valuable feedback on this manuscript.
1. Jung HY, Jeon Y, Park Y, et al. Better quality of life of peritoneal dialysis compared to hemodialysis over a two-year period after dialysis initiation. Sci Rep. 2019;9(1):10266. Published 2019 Jul 16. doi:10.1038/s41598-019-46744-1
2. Wong B, Ravani P, Oliver MJ, et al. Comparison of patient survival between hemodialysis and peritoneal dialysis among patients eligible for both modalities. Am J Kidney Dis. 2018;71(3):344-351. doi:10.1053/j.ajkd.2017.08.028
3. Chan CT, Collins K, Ditschman EP, et al. Overcoming barriers for uptake and continued use of home dialysis: an NKF-KDOQI Conference report. Am J Kidney Dis. 2020;75(6):926-934. doi:10.1053/j.ajkd.2019.11.007
4. Executive Order 13879: Advancing American kidney health. Fed Regist. 2019; 84(135):33817-33819. https://www.govinfo.gov/content/pkg/FR-2019-07-15/pdf/2019-15159.pdf
5. Patel TG, Pogach LM, Barth RH. CKD screening and management in the Veterans Health Administration: the impact of system organization and an innovative electronic record. Am J Kidney Dis. 2009;53(suppl 3):S78-S85. doi:10.1053/j.ajkd.2008.07.051
6. Saran R, Pearson A, Tilea A, et al. Burden and cost of caring for US veterans with CKD: initial findings from the VA Renal Information System (VA-REINS). Am J Kidney Dis. 2021;77(3):397-405. doi:10.1053/j.ajkd.2020.07.013
7. Sloan CE, Coffman CJ, Sanders LL, et al. Trends in peritoneal dialysis use in the United States after Medicare payment reform. Clin J Am Soc Nephrol. 2019;14(12):1763-1772. doi:10.2215/CJN.05910519
8. VA Maintaining Internal Systems and Strengthening Integrated Outside Networks Act of 2018. HR 5674. 115th Congress; Report No. 115-671, Part 1. May 3, 2018. Accessed February 9, 2023. https://www.congress.gov/115/bills/hr5674/BILLS-115hr5674rh.pdf
9. US Department of Veterans Affairs, Veterans Health Administration. Chronic kidney disease prevention, early recognition, and management. VHA Directive 1053. March 17, 2020. Accessed February 9, 2023. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8737
10. Blake PG, Quinn RR, Oliver MJ. Peritoneal dialysis and the process of modality selection. Perit Dial Int. 2013;33(3):233-241. doi:10.3747/pdi.2012.00119
11. Stroupe KT, Hynes DM, Giobbie-Hurder A, et al. Patient satisfaction and use of Veterans Affairs versus non-Veterans Affairs healthcare services by veterans. Med Care. 2005;43(5):453-460. doi:10.1097/01.mlr.0000160377.82164.d3
12. Anhang Price R, Sloss EM, Cefalu M, Farmer CM, Hussey PS. Comparing quality of care in Veterans Affairs and non-Veterans Affairs settings. J Gen Intern Med. 2018;33(10):1631-1638. doi:10.1007/s11606-018-4433-7
13. Blay E Jr, DeLancey JO, Hewitt DB, Chung JW, Bilimoria KY. Initial public reporting of quality at Veterans Affairs vs non-Veterans Affairs hospitals. JAMA Intern Med. 2017;177(6):882-885. doi:10.1001/jamainternmed.2017.0605
14. Nuti SV, Qin L, Krumholz HM. Outcome after admission at Veterans Affairs vs non-Veterans Affairs hospitals--reply. JAMA. 2016;316(3):346. doi:10.1001/jama.2016.5394
15. Streja E, Kovesdy CP, Soohoo M, et al. Dialysis provider and outcomes among United States veterans who transition to dialysis. Clin J Am Soc Nephrol. 2018;13(7):1055-1062. doi:10.2215/CJN.12951117
16. Wang V, Coffman CJ, Stechuchak KM, et al. Survival among veterans obtaining dialysis in VA and non-VA settings. J Am Soc Nephrol. 2019;30(1):159-168. doi:10.1681/ASN.2018050521
17. Kurella Tamura M, Thomas IC, Montez-Rath ME, et al. Dialysis initiation and mortality among older veterans with kidney failure treated in Medicare vs the Department of Veterans Affairs. JAMA Intern Med. 2018;178(5):657-664. doi:10.1001/jamainternmed.2018.0411
18. Devins GM, Mendelssohn DC, Barré PE, Taub K, Binik YM. Predialysis psychoeducational intervention extends survival in CKD: a 20-year follow-up. Am J Kidney Dis. 2005;46(6):1088-1098. doi:10.1053/j.ajkd.2005.08.017
19. Devoe DJ, Wong B, James MT, et al. Patient education and peritoneal dialysis modality selection: a systematic review and meta-analysis. Am J Kidney Dis. 2016;68(3):422-433. doi:10.1053/j.ajkd.2016.02.053
20. Lin E, Chertow GM, Yan B, Malcolm E, Goldhaber-Fiebert JD. Cost-effectiveness of multidisciplinary care in mild to moderate chronic kidney disease in the United States: A modeling study. PLoS Med. 2018;15(3):e1002532. Published 2018 Mar 27. doi:10.1371/journal.pmed.1002532
21. Shukla AM, Bozorgmehri S, Ruchi R, et al. Utilization of CMS pre-ESRD Kidney Disease Education services and its associations with the home dialysis therapies. Perit Dial Int. 2021;41(5):453-462. doi:10.1177/0896860820975586
22. US Dept of Veterans Affairs, Veterans Health Administration. Criteria and standards for VA dialysis programs. VHA Directive 1601. 2016. May 23, 2016. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3205
23. Crowley ST, Belcher J, Choudhury D, et al. Targeting access to kidney care via telehealth: the VA experience. Adv Chronic Kidney Dis. 2017;24(1):22-30. doi:10.1053/j.ackd.2016.11.005
24. Ishani A, Christopher J, Palmer D, et al. Telehealth by an interprofessional team in patients with CKD: a randomized controlled trial. Am J Kidney Dis. 2016;68(1):41-49. doi:10.1053/j.ajkd.2016.01.018
25. Gupta N, Taber-Hight EB, Miller BW. Perceptions of home dialysis training and experience among US nephrology fellows. Am J Kidney Dis. 2021;77(5):713-718.e1. doi:10.1053/j.ajkd.2020.09.014
1. Jung HY, Jeon Y, Park Y, et al. Better quality of life of peritoneal dialysis compared to hemodialysis over a two-year period after dialysis initiation. Sci Rep. 2019;9(1):10266. Published 2019 Jul 16. doi:10.1038/s41598-019-46744-1
2. Wong B, Ravani P, Oliver MJ, et al. Comparison of patient survival between hemodialysis and peritoneal dialysis among patients eligible for both modalities. Am J Kidney Dis. 2018;71(3):344-351. doi:10.1053/j.ajkd.2017.08.028
3. Chan CT, Collins K, Ditschman EP, et al. Overcoming barriers for uptake and continued use of home dialysis: an NKF-KDOQI Conference report. Am J Kidney Dis. 2020;75(6):926-934. doi:10.1053/j.ajkd.2019.11.007
4. Executive Order 13879: Advancing American kidney health. Fed Regist. 2019; 84(135):33817-33819. https://www.govinfo.gov/content/pkg/FR-2019-07-15/pdf/2019-15159.pdf
5. Patel TG, Pogach LM, Barth RH. CKD screening and management in the Veterans Health Administration: the impact of system organization and an innovative electronic record. Am J Kidney Dis. 2009;53(suppl 3):S78-S85. doi:10.1053/j.ajkd.2008.07.051
6. Saran R, Pearson A, Tilea A, et al. Burden and cost of caring for US veterans with CKD: initial findings from the VA Renal Information System (VA-REINS). Am J Kidney Dis. 2021;77(3):397-405. doi:10.1053/j.ajkd.2020.07.013
7. Sloan CE, Coffman CJ, Sanders LL, et al. Trends in peritoneal dialysis use in the United States after Medicare payment reform. Clin J Am Soc Nephrol. 2019;14(12):1763-1772. doi:10.2215/CJN.05910519
8. VA Maintaining Internal Systems and Strengthening Integrated Outside Networks Act of 2018. HR 5674. 115th Congress; Report No. 115-671, Part 1. May 3, 2018. Accessed February 9, 2023. https://www.congress.gov/115/bills/hr5674/BILLS-115hr5674rh.pdf
9. US Department of Veterans Affairs, Veterans Health Administration. Chronic kidney disease prevention, early recognition, and management. VHA Directive 1053. March 17, 2020. Accessed February 9, 2023. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8737
10. Blake PG, Quinn RR, Oliver MJ. Peritoneal dialysis and the process of modality selection. Perit Dial Int. 2013;33(3):233-241. doi:10.3747/pdi.2012.00119
11. Stroupe KT, Hynes DM, Giobbie-Hurder A, et al. Patient satisfaction and use of Veterans Affairs versus non-Veterans Affairs healthcare services by veterans. Med Care. 2005;43(5):453-460. doi:10.1097/01.mlr.0000160377.82164.d3
12. Anhang Price R, Sloss EM, Cefalu M, Farmer CM, Hussey PS. Comparing quality of care in Veterans Affairs and non-Veterans Affairs settings. J Gen Intern Med. 2018;33(10):1631-1638. doi:10.1007/s11606-018-4433-7
13. Blay E Jr, DeLancey JO, Hewitt DB, Chung JW, Bilimoria KY. Initial public reporting of quality at Veterans Affairs vs non-Veterans Affairs hospitals. JAMA Intern Med. 2017;177(6):882-885. doi:10.1001/jamainternmed.2017.0605
14. Nuti SV, Qin L, Krumholz HM. Outcome after admission at Veterans Affairs vs non-Veterans Affairs hospitals--reply. JAMA. 2016;316(3):346. doi:10.1001/jama.2016.5394
15. Streja E, Kovesdy CP, Soohoo M, et al. Dialysis provider and outcomes among United States veterans who transition to dialysis. Clin J Am Soc Nephrol. 2018;13(7):1055-1062. doi:10.2215/CJN.12951117
16. Wang V, Coffman CJ, Stechuchak KM, et al. Survival among veterans obtaining dialysis in VA and non-VA settings. J Am Soc Nephrol. 2019;30(1):159-168. doi:10.1681/ASN.2018050521
17. Kurella Tamura M, Thomas IC, Montez-Rath ME, et al. Dialysis initiation and mortality among older veterans with kidney failure treated in Medicare vs the Department of Veterans Affairs. JAMA Intern Med. 2018;178(5):657-664. doi:10.1001/jamainternmed.2018.0411
18. Devins GM, Mendelssohn DC, Barré PE, Taub K, Binik YM. Predialysis psychoeducational intervention extends survival in CKD: a 20-year follow-up. Am J Kidney Dis. 2005;46(6):1088-1098. doi:10.1053/j.ajkd.2005.08.017
19. Devoe DJ, Wong B, James MT, et al. Patient education and peritoneal dialysis modality selection: a systematic review and meta-analysis. Am J Kidney Dis. 2016;68(3):422-433. doi:10.1053/j.ajkd.2016.02.053
20. Lin E, Chertow GM, Yan B, Malcolm E, Goldhaber-Fiebert JD. Cost-effectiveness of multidisciplinary care in mild to moderate chronic kidney disease in the United States: A modeling study. PLoS Med. 2018;15(3):e1002532. Published 2018 Mar 27. doi:10.1371/journal.pmed.1002532
21. Shukla AM, Bozorgmehri S, Ruchi R, et al. Utilization of CMS pre-ESRD Kidney Disease Education services and its associations with the home dialysis therapies. Perit Dial Int. 2021;41(5):453-462. doi:10.1177/0896860820975586
22. US Dept of Veterans Affairs, Veterans Health Administration. Criteria and standards for VA dialysis programs. VHA Directive 1601. 2016. May 23, 2016. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3205
23. Crowley ST, Belcher J, Choudhury D, et al. Targeting access to kidney care via telehealth: the VA experience. Adv Chronic Kidney Dis. 2017;24(1):22-30. doi:10.1053/j.ackd.2016.11.005
24. Ishani A, Christopher J, Palmer D, et al. Telehealth by an interprofessional team in patients with CKD: a randomized controlled trial. Am J Kidney Dis. 2016;68(1):41-49. doi:10.1053/j.ajkd.2016.01.018
25. Gupta N, Taber-Hight EB, Miller BW. Perceptions of home dialysis training and experience among US nephrology fellows. Am J Kidney Dis. 2021;77(5):713-718.e1. doi:10.1053/j.ajkd.2020.09.014
Oophorectomies continue to dominate torsion treatment
Prompt surgical management is essential in cases of ovarian torsion in order to salvage ovarian function, and recent studies have shown that conservative management with detorsion does not increase postoperative complications, compared with oophorectomy, wrote Hannah Ryles, MD, of the University of Pennsylvania, Philadelphia, and colleagues.
The American College of Obstetricians and Gynecologists issued practice guidelines in November 2016 that recommended ovarian conservation rather than oophorectomy to manage adnexal torsion in women wishing to preserve fertility. However, the impact of this guideline on clinical practice and surgical patterns remains unclear, the researchers said.
In a study published in Obstetrics and Gynecology, the researchers reviewed data from 402 patients who underwent surgeries before the updated ACOG guidelines (2008-2016) and 1,389 who underwent surgeries after the guidelines (2017-2020). Surgery data came from the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database. The study population included women aged 18-50 years who underwent adnexal torsion surgery and were identified as having either oophorectomy or ovarian conservation surgery.
A total of 1,791 surgeries performed for adnexal torsion were included in the study; 542 (30.3%) involved ovarian conservation and 1,249 (69.7%) involved oophorectomy.
The proportion of oophorectomies was similar during the periods before and after the guidelines (71.9% vs. 69.1%; P = .16). However, the proportion of oophorectomies changed significantly across the entire study period, by approximately –1.6% each year.
Factors significantly associated with oophorectomy compared with ovarian conservation included older age (35 years vs. 28 years), higher body mass index (29.2 kg/m2 vs. 27.5 kg/m2), anemia (12.2% vs. 7.2%), hypertension (10.4% vs. 3.1%), and higher American Society of Anesthesiologists classification.
“There remains no defined acceptable rate of oophorectomy; this decision involves multiple factors, such as fertility and other patient desires after a risk and benefit discussion, menopausal status, concern for malignancy, and safety and feasibility of conservative procedures,” the researchers wrote in their discussion. However, in emergency situations, it may be difficult to determine a patient’s preferences, and a lack of desire for future fertility may be presumed, which may contribute to the relatively high oophorectomy rates over time, they said.
The findings were limited by several factors including the retrospective design and lack of data on surgical history, histopathology, and intraoperative appearance of the ovary, as well as lack of clinical data including the time from presentation to diagnosis or surgery, the researchers noted. “Although we were also unable to determine obstetric history and fertility desires, our median age of 32 years reflects a young cohort that was limited to women of reproductive age,” they added.
However, the results reflect studies suggesting that clinical practice often lags behind updated guidelines, and the findings were strengthened by the use of the NSQIP database and reflect a need for greater efforts to promote ovarian conservation in accordance with the current guidelines, the researchers concluded.
Consider unilateral oophorectomy
The current study highlights the discrepancy between the ACOG guidelines and clinical practice, with “disappointingly low” rates of ovarian preservation in the adult population, wrote Riley J. Young, MD, and Kimberly A. Kho, MD, both of the University of Texas Southwestern Medical Center, Dallas, in an accompanying editorial. The reasons for the discrepancy include clinical concerns for conserving a torsed ovary and the difficulty of assessing fertility desires in an emergency situation, they said.
However, consideration of unilateral oophorectomy as an option should be part of clinical decision-making, according to the editorialists. Previous studies suggest that retention of a single ovarian may still allow for a successful pregnancy, and the effects of unilateral oophorectomy have been studied in infertility and assisted reproductive technology settings.
Women with a single ovary have fewer eggs and require higher amounts of gonadotropins, but pregnancy is possible, the editorialists said. However, the long-term effects of unilateral oophorectomy are uncertain, and potential detrimental outcomes include increased mortality and cognitive impairment; therefore “we aim for premenopausal ovaries simply to be conserved, whether fertility is the stated goal or not,” they noted. This may include consideration of unilateral oophorectomy. “Each ovary conserved at midnight moves us closer to a more acceptable ovarian conservation rate,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Kho disclosed funding to her institution from Hologic for being on an investigator-initiated study, Dr. Young had no financial conflicts to disclose.
Prompt surgical management is essential in cases of ovarian torsion in order to salvage ovarian function, and recent studies have shown that conservative management with detorsion does not increase postoperative complications, compared with oophorectomy, wrote Hannah Ryles, MD, of the University of Pennsylvania, Philadelphia, and colleagues.
The American College of Obstetricians and Gynecologists issued practice guidelines in November 2016 that recommended ovarian conservation rather than oophorectomy to manage adnexal torsion in women wishing to preserve fertility. However, the impact of this guideline on clinical practice and surgical patterns remains unclear, the researchers said.
In a study published in Obstetrics and Gynecology, the researchers reviewed data from 402 patients who underwent surgeries before the updated ACOG guidelines (2008-2016) and 1,389 who underwent surgeries after the guidelines (2017-2020). Surgery data came from the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database. The study population included women aged 18-50 years who underwent adnexal torsion surgery and were identified as having either oophorectomy or ovarian conservation surgery.
A total of 1,791 surgeries performed for adnexal torsion were included in the study; 542 (30.3%) involved ovarian conservation and 1,249 (69.7%) involved oophorectomy.
The proportion of oophorectomies was similar during the periods before and after the guidelines (71.9% vs. 69.1%; P = .16). However, the proportion of oophorectomies changed significantly across the entire study period, by approximately –1.6% each year.
Factors significantly associated with oophorectomy compared with ovarian conservation included older age (35 years vs. 28 years), higher body mass index (29.2 kg/m2 vs. 27.5 kg/m2), anemia (12.2% vs. 7.2%), hypertension (10.4% vs. 3.1%), and higher American Society of Anesthesiologists classification.
“There remains no defined acceptable rate of oophorectomy; this decision involves multiple factors, such as fertility and other patient desires after a risk and benefit discussion, menopausal status, concern for malignancy, and safety and feasibility of conservative procedures,” the researchers wrote in their discussion. However, in emergency situations, it may be difficult to determine a patient’s preferences, and a lack of desire for future fertility may be presumed, which may contribute to the relatively high oophorectomy rates over time, they said.
The findings were limited by several factors including the retrospective design and lack of data on surgical history, histopathology, and intraoperative appearance of the ovary, as well as lack of clinical data including the time from presentation to diagnosis or surgery, the researchers noted. “Although we were also unable to determine obstetric history and fertility desires, our median age of 32 years reflects a young cohort that was limited to women of reproductive age,” they added.
However, the results reflect studies suggesting that clinical practice often lags behind updated guidelines, and the findings were strengthened by the use of the NSQIP database and reflect a need for greater efforts to promote ovarian conservation in accordance with the current guidelines, the researchers concluded.
Consider unilateral oophorectomy
The current study highlights the discrepancy between the ACOG guidelines and clinical practice, with “disappointingly low” rates of ovarian preservation in the adult population, wrote Riley J. Young, MD, and Kimberly A. Kho, MD, both of the University of Texas Southwestern Medical Center, Dallas, in an accompanying editorial. The reasons for the discrepancy include clinical concerns for conserving a torsed ovary and the difficulty of assessing fertility desires in an emergency situation, they said.
However, consideration of unilateral oophorectomy as an option should be part of clinical decision-making, according to the editorialists. Previous studies suggest that retention of a single ovarian may still allow for a successful pregnancy, and the effects of unilateral oophorectomy have been studied in infertility and assisted reproductive technology settings.
Women with a single ovary have fewer eggs and require higher amounts of gonadotropins, but pregnancy is possible, the editorialists said. However, the long-term effects of unilateral oophorectomy are uncertain, and potential detrimental outcomes include increased mortality and cognitive impairment; therefore “we aim for premenopausal ovaries simply to be conserved, whether fertility is the stated goal or not,” they noted. This may include consideration of unilateral oophorectomy. “Each ovary conserved at midnight moves us closer to a more acceptable ovarian conservation rate,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Kho disclosed funding to her institution from Hologic for being on an investigator-initiated study, Dr. Young had no financial conflicts to disclose.
Prompt surgical management is essential in cases of ovarian torsion in order to salvage ovarian function, and recent studies have shown that conservative management with detorsion does not increase postoperative complications, compared with oophorectomy, wrote Hannah Ryles, MD, of the University of Pennsylvania, Philadelphia, and colleagues.
The American College of Obstetricians and Gynecologists issued practice guidelines in November 2016 that recommended ovarian conservation rather than oophorectomy to manage adnexal torsion in women wishing to preserve fertility. However, the impact of this guideline on clinical practice and surgical patterns remains unclear, the researchers said.
In a study published in Obstetrics and Gynecology, the researchers reviewed data from 402 patients who underwent surgeries before the updated ACOG guidelines (2008-2016) and 1,389 who underwent surgeries after the guidelines (2017-2020). Surgery data came from the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database. The study population included women aged 18-50 years who underwent adnexal torsion surgery and were identified as having either oophorectomy or ovarian conservation surgery.
A total of 1,791 surgeries performed for adnexal torsion were included in the study; 542 (30.3%) involved ovarian conservation and 1,249 (69.7%) involved oophorectomy.
The proportion of oophorectomies was similar during the periods before and after the guidelines (71.9% vs. 69.1%; P = .16). However, the proportion of oophorectomies changed significantly across the entire study period, by approximately –1.6% each year.
Factors significantly associated with oophorectomy compared with ovarian conservation included older age (35 years vs. 28 years), higher body mass index (29.2 kg/m2 vs. 27.5 kg/m2), anemia (12.2% vs. 7.2%), hypertension (10.4% vs. 3.1%), and higher American Society of Anesthesiologists classification.
“There remains no defined acceptable rate of oophorectomy; this decision involves multiple factors, such as fertility and other patient desires after a risk and benefit discussion, menopausal status, concern for malignancy, and safety and feasibility of conservative procedures,” the researchers wrote in their discussion. However, in emergency situations, it may be difficult to determine a patient’s preferences, and a lack of desire for future fertility may be presumed, which may contribute to the relatively high oophorectomy rates over time, they said.
The findings were limited by several factors including the retrospective design and lack of data on surgical history, histopathology, and intraoperative appearance of the ovary, as well as lack of clinical data including the time from presentation to diagnosis or surgery, the researchers noted. “Although we were also unable to determine obstetric history and fertility desires, our median age of 32 years reflects a young cohort that was limited to women of reproductive age,” they added.
However, the results reflect studies suggesting that clinical practice often lags behind updated guidelines, and the findings were strengthened by the use of the NSQIP database and reflect a need for greater efforts to promote ovarian conservation in accordance with the current guidelines, the researchers concluded.
Consider unilateral oophorectomy
The current study highlights the discrepancy between the ACOG guidelines and clinical practice, with “disappointingly low” rates of ovarian preservation in the adult population, wrote Riley J. Young, MD, and Kimberly A. Kho, MD, both of the University of Texas Southwestern Medical Center, Dallas, in an accompanying editorial. The reasons for the discrepancy include clinical concerns for conserving a torsed ovary and the difficulty of assessing fertility desires in an emergency situation, they said.
However, consideration of unilateral oophorectomy as an option should be part of clinical decision-making, according to the editorialists. Previous studies suggest that retention of a single ovarian may still allow for a successful pregnancy, and the effects of unilateral oophorectomy have been studied in infertility and assisted reproductive technology settings.
Women with a single ovary have fewer eggs and require higher amounts of gonadotropins, but pregnancy is possible, the editorialists said. However, the long-term effects of unilateral oophorectomy are uncertain, and potential detrimental outcomes include increased mortality and cognitive impairment; therefore “we aim for premenopausal ovaries simply to be conserved, whether fertility is the stated goal or not,” they noted. This may include consideration of unilateral oophorectomy. “Each ovary conserved at midnight moves us closer to a more acceptable ovarian conservation rate,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Kho disclosed funding to her institution from Hologic for being on an investigator-initiated study, Dr. Young had no financial conflicts to disclose.
FROM OBSTETRICS & GYNECOLOGY
Melasma
THE COMPARISON
A Melasma on the face of a Hispanic woman, with hyperpigmentation on the cheeks, bridge of the nose, and upper lip.
B Melasma on the face of a Malaysian woman, with hyperpigmentation on the upper cheeks and bridge of the nose.
C Melasma on the face of an African woman, with hyperpigmentation on the upper cheeks and lateral to the eyes.
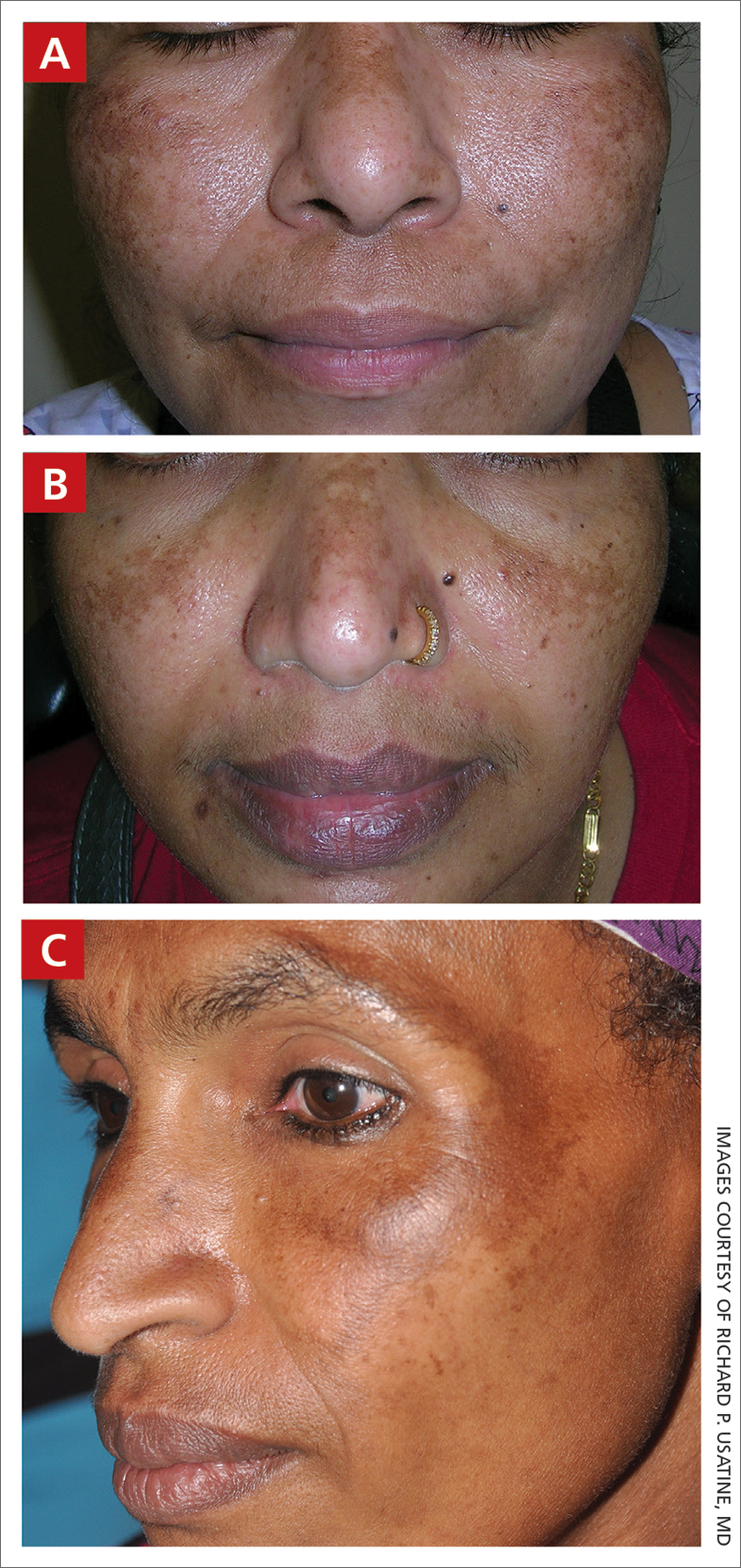
Melasma (also known as chloasma) is a pigmentary disorder that causes chronic symmetric hyperpigmentation on the face. In patients with darker skin tones, centrofacial areas are affected.1 Increased deposition of melanin distributed in the dermis leads to dermal melanosis. Newer research suggests that mast cell and keratinocyte interactions, altered gene regulation, neovascularization, and disruptions in the basement membrane cause melasma.2 Patients present with epidermal or dermal melasma or a combination of both (mixed melasma).3 Wood lamp examination is helpful to distinguish between epidermal and dermal melasma. Dermal and mixed melasma can be difficult to treat and require multimodal treatments.
Epidemiology
Melasma commonly affects women ages 20 to 40 years,4 with a female to male ratio of 9:1.5 Potential triggers of melasma include hormones (eg, pregnancy, oral contraceptives, hormone replacement therapy) and exposure to UV light.2,5 Melasma occurs in patients of all racial and ethnic backgrounds; however, the prevalence is higher in patients with darker skin tones.2
Key clinical features in people with darker skin tones
Melasma commonly manifests as symmetrically distributed, reticulated (lacy), dark brown to grayish brown patches on the cheeks, nose, forehead, upper lip, and chin in patients with darker skin tones.5 The pigment can be tan brown in patients with lighter skin tones. Given that postinflammatory hyperpigmentation and other pigmentary disorders can cause a similar appearance, a biopsy sometimes is needed to confirm the diagnosis, but melasma is diagnosed via physical examination in most patients. Melasma can be misdiagnosed as postinflammatory hyperpigmentation, solar lentigines, exogenous ochronosis, and Hori nevus.5
Worth noting
Prevention
- Daily sunscreen use is critical to prevent worsening of melasma. Sunscreen may not appear cosmetically elegant on darker skin tones, which creates a barrier to its use.6 Protection from both sunlight and visible light is necessary. Visible light, including light from light bulbs and device-emitted blue light, can worsen melasma. Iron oxides in tinted sunscreen offer protection from visible light.
- Physicians can recommend sunscreens that are more transparent or tinted for a better cosmetic match.
- Severe flares of melasma can occur with sun exposure despite good control with medications and laser modalities.
Treatment
- First-line therapies include topical hydroquinone 2% to 4%, tretinoin, azelaic acid, kojic acid, or ascorbic acid (vitamin C). A popular topical compound is a steroid, tretinoin, and hydroquinone.1,5 Over-the-counter hydroquinone has been removed from the market due to safety concerns; however, it is still first line in the treatment of melasma. If hydroquinone is prescribed, treatment intervals of 6 to 8 weeks followed by a hydroquinone-free period is advised to reduce the risk for exogenous ochronosis (a paradoxical darkening of the skin).
- Chemical peels are second-line treatments that are effective for melasma. Improvement in epidermal melasma has been shown with chemical peels containing Jessner solution, salicylic acid, or a-hydroxy acid. Patients with dermal and mixed melasma have seen improvement with trichloroacetic acid 25% to 35% with or without Jessner solution.1
- Cysteamine is a topical treatment created from the degradation of coenzyme A. It disrupts the synthesis of melanin to create a more even skin tone. It may be recommended in combination with sunscreen as a first-line or secondline topical therapy.
- Oral tranexamic acid is a third-line treatment that is an analogue for lysine. It decreases prostaglandin production, which leads to a lower number of tyrosine precursors available for the creation of melanin. Tranexamic acid has been shown to lighten the appearance of melasma.7 The most common and dangerous adverse effect of tranexamic acid is blood clots, and this treatment should be avoided in those on combination (estrogen and progestin) contraceptives or those with a personal or family history of clotting disorders.8
- Fourth-line treatments such as lasers (performed by dermatologists) can destroy the deposition of pigment while avoiding destruction of epidermal keratinocytes.1,9,10 They also are commonly employed in refractive melasma. The most common lasers are nonablative fractionated lasers and low-fluence Q-switched lasers. The Q-switched Nd:YAG and picosecond lasers are safe for treating melasma in darker skin tones. Ablative fractionated lasers such as CO2 lasers and erbium:YAG lasers also have been used in the treatment of melasma; however, there is still an extremely high risk for postinflammatory dyspigmentation 1 to 2 months after the procedure.10
- Although there is still a risk for rebound hyperpigmentation after laser treatment, use of topical hydroquinone pretreatment may help decrease postoperative hyperpigmentation.1,5 Patients who are treated with the incorrect laser or overtreated may develop postinflammatory hyperpigmentation, rebound hyperpigmentation, or hypopigmentation.
Health disparity highlight
Melasma, most common in patients with skin of color, is a common chronic pigmentation disorder that is cosmetically and psychologically burdensome,11 leading to decreased quality of life, emotional functioning, and self-esteem.12 Clinicians should counsel patients and work closely on long-term management. The treatment options for melasma are considered cosmetic and may be cost prohibitive for many to cover out of pocket. Topical treatments have been found to be the most cost-effective.13 Some compounding pharmacies and drug discount programs provide more affordable treatment pricing; however, some patients are still unable to afford these options.
1. Cunha PR, Kroumpouzos G. Melasma and vitiligo: novel and experimental therapies. J Clin Exp Derm Res. 2016;7:2. doi:10.4172/2155-9554.1000e106
2. Rajanala S, Maymone MBC, Vashi NA. Melasma pathogenesis: a review of the latest research, pathological findings, and investigational therapies. Dermatol Online J. 2019;25:13030/qt47b7r28c.
3. Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
4. Achar A, Rathi SK. Melasma: a clinico-epidemiological study of 312 cases. Indian J Dermatol. 2011;56:380-382.
5. Ogbechie-Godec OA, Elbuluk N. Melasma: an up-to-date comprehensive review. Dermatol Ther. 2017;7:305-318.
6. Morquette AJ, Waples ER, Heath CR. The importance of cosmetically elegant sunscreen in skin of color populations. J Cosmet Dermatol. 2022;21:1337-1338.
7. Taraz M, Nikham S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatol Ther. 2017;30(3). doi:10.1111/dth.12465
8. Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
9. Castanedo-Cazares JP, Hernandez-Blanco D, Carlos-Ortega B, et al. Near-visible light and UV photoprotection in the treatment of melasma: a double-blind randomized trial. Photodermatol Photoimmunol Photomed. 2014;30:35-42.
10. Trivedi MK, Yang FC, Cho BK. A review of laser and light therapy in melasma. Int J Womens Dermatol. 2017;3:11-20.
11. Dodmani PN, Deshmukh AR. Assessment of quality of life of melasma patients as per melasma quality of life scale (MELASQoL). Pigment Int. 2020;7:75-79.
12. Balkrishnan R, McMichael A, Camacho FT, et al. Development and validation of a health‐related quality of life instrument for women with melasma. Br J Dermatol. 2003;149:572-577.
13. Alikhan A, Daly M, Wu J, et al. Cost-effectiveness of a hydroquinone/tretinoin/fluocinolone acetonide cream combination in treating melasma in the United States. J Dermatolog Treat. 2010;21:276-281.
THE COMPARISON
A Melasma on the face of a Hispanic woman, with hyperpigmentation on the cheeks, bridge of the nose, and upper lip.
B Melasma on the face of a Malaysian woman, with hyperpigmentation on the upper cheeks and bridge of the nose.
C Melasma on the face of an African woman, with hyperpigmentation on the upper cheeks and lateral to the eyes.

Melasma (also known as chloasma) is a pigmentary disorder that causes chronic symmetric hyperpigmentation on the face. In patients with darker skin tones, centrofacial areas are affected.1 Increased deposition of melanin distributed in the dermis leads to dermal melanosis. Newer research suggests that mast cell and keratinocyte interactions, altered gene regulation, neovascularization, and disruptions in the basement membrane cause melasma.2 Patients present with epidermal or dermal melasma or a combination of both (mixed melasma).3 Wood lamp examination is helpful to distinguish between epidermal and dermal melasma. Dermal and mixed melasma can be difficult to treat and require multimodal treatments.
Epidemiology
Melasma commonly affects women ages 20 to 40 years,4 with a female to male ratio of 9:1.5 Potential triggers of melasma include hormones (eg, pregnancy, oral contraceptives, hormone replacement therapy) and exposure to UV light.2,5 Melasma occurs in patients of all racial and ethnic backgrounds; however, the prevalence is higher in patients with darker skin tones.2
Key clinical features in people with darker skin tones
Melasma commonly manifests as symmetrically distributed, reticulated (lacy), dark brown to grayish brown patches on the cheeks, nose, forehead, upper lip, and chin in patients with darker skin tones.5 The pigment can be tan brown in patients with lighter skin tones. Given that postinflammatory hyperpigmentation and other pigmentary disorders can cause a similar appearance, a biopsy sometimes is needed to confirm the diagnosis, but melasma is diagnosed via physical examination in most patients. Melasma can be misdiagnosed as postinflammatory hyperpigmentation, solar lentigines, exogenous ochronosis, and Hori nevus.5
Worth noting
Prevention
- Daily sunscreen use is critical to prevent worsening of melasma. Sunscreen may not appear cosmetically elegant on darker skin tones, which creates a barrier to its use.6 Protection from both sunlight and visible light is necessary. Visible light, including light from light bulbs and device-emitted blue light, can worsen melasma. Iron oxides in tinted sunscreen offer protection from visible light.
- Physicians can recommend sunscreens that are more transparent or tinted for a better cosmetic match.
- Severe flares of melasma can occur with sun exposure despite good control with medications and laser modalities.
Treatment
- First-line therapies include topical hydroquinone 2% to 4%, tretinoin, azelaic acid, kojic acid, or ascorbic acid (vitamin C). A popular topical compound is a steroid, tretinoin, and hydroquinone.1,5 Over-the-counter hydroquinone has been removed from the market due to safety concerns; however, it is still first line in the treatment of melasma. If hydroquinone is prescribed, treatment intervals of 6 to 8 weeks followed by a hydroquinone-free period is advised to reduce the risk for exogenous ochronosis (a paradoxical darkening of the skin).
- Chemical peels are second-line treatments that are effective for melasma. Improvement in epidermal melasma has been shown with chemical peels containing Jessner solution, salicylic acid, or a-hydroxy acid. Patients with dermal and mixed melasma have seen improvement with trichloroacetic acid 25% to 35% with or without Jessner solution.1
- Cysteamine is a topical treatment created from the degradation of coenzyme A. It disrupts the synthesis of melanin to create a more even skin tone. It may be recommended in combination with sunscreen as a first-line or secondline topical therapy.
- Oral tranexamic acid is a third-line treatment that is an analogue for lysine. It decreases prostaglandin production, which leads to a lower number of tyrosine precursors available for the creation of melanin. Tranexamic acid has been shown to lighten the appearance of melasma.7 The most common and dangerous adverse effect of tranexamic acid is blood clots, and this treatment should be avoided in those on combination (estrogen and progestin) contraceptives or those with a personal or family history of clotting disorders.8
- Fourth-line treatments such as lasers (performed by dermatologists) can destroy the deposition of pigment while avoiding destruction of epidermal keratinocytes.1,9,10 They also are commonly employed in refractive melasma. The most common lasers are nonablative fractionated lasers and low-fluence Q-switched lasers. The Q-switched Nd:YAG and picosecond lasers are safe for treating melasma in darker skin tones. Ablative fractionated lasers such as CO2 lasers and erbium:YAG lasers also have been used in the treatment of melasma; however, there is still an extremely high risk for postinflammatory dyspigmentation 1 to 2 months after the procedure.10
- Although there is still a risk for rebound hyperpigmentation after laser treatment, use of topical hydroquinone pretreatment may help decrease postoperative hyperpigmentation.1,5 Patients who are treated with the incorrect laser or overtreated may develop postinflammatory hyperpigmentation, rebound hyperpigmentation, or hypopigmentation.
Health disparity highlight
Melasma, most common in patients with skin of color, is a common chronic pigmentation disorder that is cosmetically and psychologically burdensome,11 leading to decreased quality of life, emotional functioning, and self-esteem.12 Clinicians should counsel patients and work closely on long-term management. The treatment options for melasma are considered cosmetic and may be cost prohibitive for many to cover out of pocket. Topical treatments have been found to be the most cost-effective.13 Some compounding pharmacies and drug discount programs provide more affordable treatment pricing; however, some patients are still unable to afford these options.
THE COMPARISON
A Melasma on the face of a Hispanic woman, with hyperpigmentation on the cheeks, bridge of the nose, and upper lip.
B Melasma on the face of a Malaysian woman, with hyperpigmentation on the upper cheeks and bridge of the nose.
C Melasma on the face of an African woman, with hyperpigmentation on the upper cheeks and lateral to the eyes.

Melasma (also known as chloasma) is a pigmentary disorder that causes chronic symmetric hyperpigmentation on the face. In patients with darker skin tones, centrofacial areas are affected.1 Increased deposition of melanin distributed in the dermis leads to dermal melanosis. Newer research suggests that mast cell and keratinocyte interactions, altered gene regulation, neovascularization, and disruptions in the basement membrane cause melasma.2 Patients present with epidermal or dermal melasma or a combination of both (mixed melasma).3 Wood lamp examination is helpful to distinguish between epidermal and dermal melasma. Dermal and mixed melasma can be difficult to treat and require multimodal treatments.
Epidemiology
Melasma commonly affects women ages 20 to 40 years,4 with a female to male ratio of 9:1.5 Potential triggers of melasma include hormones (eg, pregnancy, oral contraceptives, hormone replacement therapy) and exposure to UV light.2,5 Melasma occurs in patients of all racial and ethnic backgrounds; however, the prevalence is higher in patients with darker skin tones.2
Key clinical features in people with darker skin tones
Melasma commonly manifests as symmetrically distributed, reticulated (lacy), dark brown to grayish brown patches on the cheeks, nose, forehead, upper lip, and chin in patients with darker skin tones.5 The pigment can be tan brown in patients with lighter skin tones. Given that postinflammatory hyperpigmentation and other pigmentary disorders can cause a similar appearance, a biopsy sometimes is needed to confirm the diagnosis, but melasma is diagnosed via physical examination in most patients. Melasma can be misdiagnosed as postinflammatory hyperpigmentation, solar lentigines, exogenous ochronosis, and Hori nevus.5
Worth noting
Prevention
- Daily sunscreen use is critical to prevent worsening of melasma. Sunscreen may not appear cosmetically elegant on darker skin tones, which creates a barrier to its use.6 Protection from both sunlight and visible light is necessary. Visible light, including light from light bulbs and device-emitted blue light, can worsen melasma. Iron oxides in tinted sunscreen offer protection from visible light.
- Physicians can recommend sunscreens that are more transparent or tinted for a better cosmetic match.
- Severe flares of melasma can occur with sun exposure despite good control with medications and laser modalities.
Treatment
- First-line therapies include topical hydroquinone 2% to 4%, tretinoin, azelaic acid, kojic acid, or ascorbic acid (vitamin C). A popular topical compound is a steroid, tretinoin, and hydroquinone.1,5 Over-the-counter hydroquinone has been removed from the market due to safety concerns; however, it is still first line in the treatment of melasma. If hydroquinone is prescribed, treatment intervals of 6 to 8 weeks followed by a hydroquinone-free period is advised to reduce the risk for exogenous ochronosis (a paradoxical darkening of the skin).
- Chemical peels are second-line treatments that are effective for melasma. Improvement in epidermal melasma has been shown with chemical peels containing Jessner solution, salicylic acid, or a-hydroxy acid. Patients with dermal and mixed melasma have seen improvement with trichloroacetic acid 25% to 35% with or without Jessner solution.1
- Cysteamine is a topical treatment created from the degradation of coenzyme A. It disrupts the synthesis of melanin to create a more even skin tone. It may be recommended in combination with sunscreen as a first-line or secondline topical therapy.
- Oral tranexamic acid is a third-line treatment that is an analogue for lysine. It decreases prostaglandin production, which leads to a lower number of tyrosine precursors available for the creation of melanin. Tranexamic acid has been shown to lighten the appearance of melasma.7 The most common and dangerous adverse effect of tranexamic acid is blood clots, and this treatment should be avoided in those on combination (estrogen and progestin) contraceptives or those with a personal or family history of clotting disorders.8
- Fourth-line treatments such as lasers (performed by dermatologists) can destroy the deposition of pigment while avoiding destruction of epidermal keratinocytes.1,9,10 They also are commonly employed in refractive melasma. The most common lasers are nonablative fractionated lasers and low-fluence Q-switched lasers. The Q-switched Nd:YAG and picosecond lasers are safe for treating melasma in darker skin tones. Ablative fractionated lasers such as CO2 lasers and erbium:YAG lasers also have been used in the treatment of melasma; however, there is still an extremely high risk for postinflammatory dyspigmentation 1 to 2 months after the procedure.10
- Although there is still a risk for rebound hyperpigmentation after laser treatment, use of topical hydroquinone pretreatment may help decrease postoperative hyperpigmentation.1,5 Patients who are treated with the incorrect laser or overtreated may develop postinflammatory hyperpigmentation, rebound hyperpigmentation, or hypopigmentation.
Health disparity highlight
Melasma, most common in patients with skin of color, is a common chronic pigmentation disorder that is cosmetically and psychologically burdensome,11 leading to decreased quality of life, emotional functioning, and self-esteem.12 Clinicians should counsel patients and work closely on long-term management. The treatment options for melasma are considered cosmetic and may be cost prohibitive for many to cover out of pocket. Topical treatments have been found to be the most cost-effective.13 Some compounding pharmacies and drug discount programs provide more affordable treatment pricing; however, some patients are still unable to afford these options.
1. Cunha PR, Kroumpouzos G. Melasma and vitiligo: novel and experimental therapies. J Clin Exp Derm Res. 2016;7:2. doi:10.4172/2155-9554.1000e106
2. Rajanala S, Maymone MBC, Vashi NA. Melasma pathogenesis: a review of the latest research, pathological findings, and investigational therapies. Dermatol Online J. 2019;25:13030/qt47b7r28c.
3. Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
4. Achar A, Rathi SK. Melasma: a clinico-epidemiological study of 312 cases. Indian J Dermatol. 2011;56:380-382.
5. Ogbechie-Godec OA, Elbuluk N. Melasma: an up-to-date comprehensive review. Dermatol Ther. 2017;7:305-318.
6. Morquette AJ, Waples ER, Heath CR. The importance of cosmetically elegant sunscreen in skin of color populations. J Cosmet Dermatol. 2022;21:1337-1338.
7. Taraz M, Nikham S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatol Ther. 2017;30(3). doi:10.1111/dth.12465
8. Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
9. Castanedo-Cazares JP, Hernandez-Blanco D, Carlos-Ortega B, et al. Near-visible light and UV photoprotection in the treatment of melasma: a double-blind randomized trial. Photodermatol Photoimmunol Photomed. 2014;30:35-42.
10. Trivedi MK, Yang FC, Cho BK. A review of laser and light therapy in melasma. Int J Womens Dermatol. 2017;3:11-20.
11. Dodmani PN, Deshmukh AR. Assessment of quality of life of melasma patients as per melasma quality of life scale (MELASQoL). Pigment Int. 2020;7:75-79.
12. Balkrishnan R, McMichael A, Camacho FT, et al. Development and validation of a health‐related quality of life instrument for women with melasma. Br J Dermatol. 2003;149:572-577.
13. Alikhan A, Daly M, Wu J, et al. Cost-effectiveness of a hydroquinone/tretinoin/fluocinolone acetonide cream combination in treating melasma in the United States. J Dermatolog Treat. 2010;21:276-281.
1. Cunha PR, Kroumpouzos G. Melasma and vitiligo: novel and experimental therapies. J Clin Exp Derm Res. 2016;7:2. doi:10.4172/2155-9554.1000e106
2. Rajanala S, Maymone MBC, Vashi NA. Melasma pathogenesis: a review of the latest research, pathological findings, and investigational therapies. Dermatol Online J. 2019;25:13030/qt47b7r28c.
3. Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
4. Achar A, Rathi SK. Melasma: a clinico-epidemiological study of 312 cases. Indian J Dermatol. 2011;56:380-382.
5. Ogbechie-Godec OA, Elbuluk N. Melasma: an up-to-date comprehensive review. Dermatol Ther. 2017;7:305-318.
6. Morquette AJ, Waples ER, Heath CR. The importance of cosmetically elegant sunscreen in skin of color populations. J Cosmet Dermatol. 2022;21:1337-1338.
7. Taraz M, Nikham S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatol Ther. 2017;30(3). doi:10.1111/dth.12465
8. Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
9. Castanedo-Cazares JP, Hernandez-Blanco D, Carlos-Ortega B, et al. Near-visible light and UV photoprotection in the treatment of melasma: a double-blind randomized trial. Photodermatol Photoimmunol Photomed. 2014;30:35-42.
10. Trivedi MK, Yang FC, Cho BK. A review of laser and light therapy in melasma. Int J Womens Dermatol. 2017;3:11-20.
11. Dodmani PN, Deshmukh AR. Assessment of quality of life of melasma patients as per melasma quality of life scale (MELASQoL). Pigment Int. 2020;7:75-79.
12. Balkrishnan R, McMichael A, Camacho FT, et al. Development and validation of a health‐related quality of life instrument for women with melasma. Br J Dermatol. 2003;149:572-577.
13. Alikhan A, Daly M, Wu J, et al. Cost-effectiveness of a hydroquinone/tretinoin/fluocinolone acetonide cream combination in treating melasma in the United States. J Dermatolog Treat. 2010;21:276-281.
Study gives new insight into timing of combo treatment in metastatic NSCLC
However, patients still fared poorly on average since overall survival remained low and didn’t change significantly.
While not conclusive, the new research – released at European Lung Cancer Congress 2023 – offers early insight into the best timing for the experimental combination treatment, study coauthor Yanyan Lou, MD, PhD, an oncologist at Mayo Clinic in Jacksonville, Fla., said in an interview.
The wide availability of radiation therapy could also allow the therapy to be administered even in regions with poor access to sophisticated medical care, she said. “Radiation is a very feasible approach that pretty much everybody in your community can get.”
Radiotherapy is typically not added to immunotherapy in patients with non–small cell lung cancer. But “there has been recent interest in the combination: Would tumor necrosis from radiation enhance the immunogenicity of the tumor and thus enhance the effect of immunotherapy?” oncologist Toby Campbell, MD, of University of Wisconsin–Madison, said in an interview.
Research has indeed suggested that the treatments may have a synergistic effect, he said, and it’s clear that “strategies to try and increase immunogenicity are an important area to investigate.”
But he cautioned that “we have a long way to go to understanding how immunogenicity works and how the gut microbiome, tumor, immunotherapy, and the immune system interact with one another.”
For the new study, researchers retrospectively analyzed cases of 225 patients with metastatic non–small cell lung cancer (male = 56%, median age = 68, 79% adenocarcinoma) who were treated with immunotherapy at Mayo Clinic–Jacksonville from 2011 to 2022. The study excluded those who received targeted therapy or prior concurrent chemoradiotherapy and durvalumab.
The most common metastases were bone and central nervous system types (41% and 25%, respectively). Fifty-six percent of patients received radiotherapy before or during immunotherapy. Another 27% never received radiotherapy, and 17% received it after immunotherapy was discontinued.
Common types of immunotherapy included pembrolizumab (78%), nivolumab (14%), and atezolizumab (12%).
Overall, the researchers found no statistically significant differences in various outcomes between patients who received radiotherapy before or during immunotherapy compared with those who didn’t get radiotherapy (progression-free survival: 5.9 vs. 5.5 months, P = .66; overall survival: 16.9 vs. 13.1 months, P = .84; immune-related adverse events: 26.2% vs. 34.4%, P = .24).
However, the researchers found that progression-free survival was significantly higher in one group: Those who received radiotherapy 1-12 months before immunotherapy vs. those who received it less than 1 month before (12.6 vs. 4.2 months, hazard ratio [HR], 0.46, 95% confidence interval [CI], 0.26-0.83, P = .005,) and those who never received radiotherapy (12.6 vs. 5.5 months, HR, 0.56, 95% CI, 0.36-0.89, P = .0197).
There wasn’t a statistically significant difference in overall survival.
The small number of subjects and the variation in treatment protocols may have prevented the study from revealing a survival benefit, Dr. Lou said.
As for adverse effects, she said a preliminary analysis didn’t turn up any.
It’s not clear why a 1- to 12-month gap between radiotherapy and immunotherapy may be most effective, she said. Moving forward, “we need validate this in a large cohort,” she noted.
In regard to cost, immunotherapy is notoriously expensive. Pembrolizumab, for example, has a list price of $10,897 per 200-mg dose given every 3 weeks, and patients may take the drug for a year or two.
Dr. Campbell, who didn’t take part in the new study, said it suggests that research into radiation-immunotherapy combination treatment may be worthwhile.
No funding was reported. The study authors and Dr. Campbell reported no disclosures.
However, patients still fared poorly on average since overall survival remained low and didn’t change significantly.
While not conclusive, the new research – released at European Lung Cancer Congress 2023 – offers early insight into the best timing for the experimental combination treatment, study coauthor Yanyan Lou, MD, PhD, an oncologist at Mayo Clinic in Jacksonville, Fla., said in an interview.
The wide availability of radiation therapy could also allow the therapy to be administered even in regions with poor access to sophisticated medical care, she said. “Radiation is a very feasible approach that pretty much everybody in your community can get.”
Radiotherapy is typically not added to immunotherapy in patients with non–small cell lung cancer. But “there has been recent interest in the combination: Would tumor necrosis from radiation enhance the immunogenicity of the tumor and thus enhance the effect of immunotherapy?” oncologist Toby Campbell, MD, of University of Wisconsin–Madison, said in an interview.
Research has indeed suggested that the treatments may have a synergistic effect, he said, and it’s clear that “strategies to try and increase immunogenicity are an important area to investigate.”
But he cautioned that “we have a long way to go to understanding how immunogenicity works and how the gut microbiome, tumor, immunotherapy, and the immune system interact with one another.”
For the new study, researchers retrospectively analyzed cases of 225 patients with metastatic non–small cell lung cancer (male = 56%, median age = 68, 79% adenocarcinoma) who were treated with immunotherapy at Mayo Clinic–Jacksonville from 2011 to 2022. The study excluded those who received targeted therapy or prior concurrent chemoradiotherapy and durvalumab.
The most common metastases were bone and central nervous system types (41% and 25%, respectively). Fifty-six percent of patients received radiotherapy before or during immunotherapy. Another 27% never received radiotherapy, and 17% received it after immunotherapy was discontinued.
Common types of immunotherapy included pembrolizumab (78%), nivolumab (14%), and atezolizumab (12%).
Overall, the researchers found no statistically significant differences in various outcomes between patients who received radiotherapy before or during immunotherapy compared with those who didn’t get radiotherapy (progression-free survival: 5.9 vs. 5.5 months, P = .66; overall survival: 16.9 vs. 13.1 months, P = .84; immune-related adverse events: 26.2% vs. 34.4%, P = .24).
However, the researchers found that progression-free survival was significantly higher in one group: Those who received radiotherapy 1-12 months before immunotherapy vs. those who received it less than 1 month before (12.6 vs. 4.2 months, hazard ratio [HR], 0.46, 95% confidence interval [CI], 0.26-0.83, P = .005,) and those who never received radiotherapy (12.6 vs. 5.5 months, HR, 0.56, 95% CI, 0.36-0.89, P = .0197).
There wasn’t a statistically significant difference in overall survival.
The small number of subjects and the variation in treatment protocols may have prevented the study from revealing a survival benefit, Dr. Lou said.
As for adverse effects, she said a preliminary analysis didn’t turn up any.
It’s not clear why a 1- to 12-month gap between radiotherapy and immunotherapy may be most effective, she said. Moving forward, “we need validate this in a large cohort,” she noted.
In regard to cost, immunotherapy is notoriously expensive. Pembrolizumab, for example, has a list price of $10,897 per 200-mg dose given every 3 weeks, and patients may take the drug for a year or two.
Dr. Campbell, who didn’t take part in the new study, said it suggests that research into radiation-immunotherapy combination treatment may be worthwhile.
No funding was reported. The study authors and Dr. Campbell reported no disclosures.
However, patients still fared poorly on average since overall survival remained low and didn’t change significantly.
While not conclusive, the new research – released at European Lung Cancer Congress 2023 – offers early insight into the best timing for the experimental combination treatment, study coauthor Yanyan Lou, MD, PhD, an oncologist at Mayo Clinic in Jacksonville, Fla., said in an interview.
The wide availability of radiation therapy could also allow the therapy to be administered even in regions with poor access to sophisticated medical care, she said. “Radiation is a very feasible approach that pretty much everybody in your community can get.”
Radiotherapy is typically not added to immunotherapy in patients with non–small cell lung cancer. But “there has been recent interest in the combination: Would tumor necrosis from radiation enhance the immunogenicity of the tumor and thus enhance the effect of immunotherapy?” oncologist Toby Campbell, MD, of University of Wisconsin–Madison, said in an interview.
Research has indeed suggested that the treatments may have a synergistic effect, he said, and it’s clear that “strategies to try and increase immunogenicity are an important area to investigate.”
But he cautioned that “we have a long way to go to understanding how immunogenicity works and how the gut microbiome, tumor, immunotherapy, and the immune system interact with one another.”
For the new study, researchers retrospectively analyzed cases of 225 patients with metastatic non–small cell lung cancer (male = 56%, median age = 68, 79% adenocarcinoma) who were treated with immunotherapy at Mayo Clinic–Jacksonville from 2011 to 2022. The study excluded those who received targeted therapy or prior concurrent chemoradiotherapy and durvalumab.
The most common metastases were bone and central nervous system types (41% and 25%, respectively). Fifty-six percent of patients received radiotherapy before or during immunotherapy. Another 27% never received radiotherapy, and 17% received it after immunotherapy was discontinued.
Common types of immunotherapy included pembrolizumab (78%), nivolumab (14%), and atezolizumab (12%).
Overall, the researchers found no statistically significant differences in various outcomes between patients who received radiotherapy before or during immunotherapy compared with those who didn’t get radiotherapy (progression-free survival: 5.9 vs. 5.5 months, P = .66; overall survival: 16.9 vs. 13.1 months, P = .84; immune-related adverse events: 26.2% vs. 34.4%, P = .24).
However, the researchers found that progression-free survival was significantly higher in one group: Those who received radiotherapy 1-12 months before immunotherapy vs. those who received it less than 1 month before (12.6 vs. 4.2 months, hazard ratio [HR], 0.46, 95% confidence interval [CI], 0.26-0.83, P = .005,) and those who never received radiotherapy (12.6 vs. 5.5 months, HR, 0.56, 95% CI, 0.36-0.89, P = .0197).
There wasn’t a statistically significant difference in overall survival.
The small number of subjects and the variation in treatment protocols may have prevented the study from revealing a survival benefit, Dr. Lou said.
As for adverse effects, she said a preliminary analysis didn’t turn up any.
It’s not clear why a 1- to 12-month gap between radiotherapy and immunotherapy may be most effective, she said. Moving forward, “we need validate this in a large cohort,” she noted.
In regard to cost, immunotherapy is notoriously expensive. Pembrolizumab, for example, has a list price of $10,897 per 200-mg dose given every 3 weeks, and patients may take the drug for a year or two.
Dr. Campbell, who didn’t take part in the new study, said it suggests that research into radiation-immunotherapy combination treatment may be worthwhile.
No funding was reported. The study authors and Dr. Campbell reported no disclosures.
FROM ELCC 2023
Long-term heavy smoking quadruples likelihood of lung cancer vs. less heavy smoking
People who have smoked an average of 1 pack a day for 20-39 years tripled their tumor risk versus less-heavy smokers and 30-fold versus those who never smoked. For those who smoked the equivalent of 1 pack for 40-60 years, or 2 packs for 20-30 years, the risk levels grew by fourfold and 40-fold, respectively. For those who’ve smoked even more, the likelihood of developing lung cancer is high, but the risk remains stable and doesn’t grow more over time, according to the analysis.
The report, released at the annual European Lung Cancer Congress 2023 meeting, and an earlier related study “underscore the importance of smoking abstinence and early smoking cessation,” said study lead author J. Anthony Nations, MD, MBA, in an interview.
The earlier study, published in JAMA Oncology, relied on a “pack-year” analysis to evaluate the risk of lung cancer in smokers. A pack-year refers to the cigarette use of a person who smoked a pack a day for 1 year. It’s the equivalent of smoking half a pack for 2 years or 2 packs for 6 months.
By this measure, a smoker with 20 pack-years of cigarette use smoked the equivalent of a pack a day for 20 years or 2 packs a day for 10 years. U.S. guidelines recommend annual low-dose CT lung cancer screening in adults who are aged 50-80, have more than 20 pack-years of tobacco exposure, and either currently smoke or quit within the last 15 years.
The JAMA Oncology report “showed that, compared with never-smokers, current heavy and nonheavy smokers had [a] 40 and 10 times higher risk of lung cancer, respectively,” said Dr. Nations, who is also a pulmonologist with Washington D.C. Veterans Affairs Medical Center. “A smoking history of greater than 20 pack-years was considered heavy, but current heavy smokers had a median pack-year smoking history of 50 pack-years. This observation prompted us to want to look more closely at pack-year smoking history.”
For the new analysis, researchers tracked 2,505 older adults (mean age, 73 ± 5.7 years; 69% women, 17% African American) in the Cardiovascular Health Study. Of those, 532 were current smokers (18% less than 20 pack-years, 30% 20-39 pack-years, 34% 40–59 pack-years, and 18% greater than 60 pack-years).
Lung cancer occurred in 0.5% of those who never smoked, 5% of those who smoked less than 20 pack-years, 14.6% of those who smoked 20-39 pack-years, 17.7% of those who smoked 40-59 pack-years, and 16.0% for those who smoked more than 60 pack-years. In an analysis adjusted for age, sex, race, and competing risk of death, researchers found that those who smoked less than 20 pack-years were 9.73 times more likely to develop lung cancer than those who never smoked (hazard ratio, 9.73). The HRs of lung cancer versus never-smokers for the other groups were 30.33 (20-39 pack-years), 42.97 (40-59 pack-years), and 46.02 (greater than 60 pack-years.).
“While it was not surprising that the risk of lung cancer in current heavy smokers would be proportionately greater in smokers with higher pack-year smoking history, we were surprised to see that the risk almost plateaued in the heaviest current smokers,” Dr. Nations said.
As for the clinical message from the findings, Dr. Nations said they reveal that quitting smoking makes a difference in lung cancer risk, even after many years of heavy smoking. “Smokers who quit after a 30–pack-year smoking history will not incur the higher risk of those with a 40– or 50–pack-year smoking history.”
The previous JAMA Oncology paper also showed that quitting pays dividends by reducing lung cancer risk. Subjects with at least 20 pack-years of smoking who quit less than 15 years ago nearly halved their excess risk of lung cancer, compared with similar current smokers who didn’t quit.
In an interview, cancer researcher Robert J. Volk, PhD, of the University of Texas MD Anderson Cancer Center, Houston, praised the new analysis but noted that it has limitations: “The sample is fairly small – 532 adults who currently smoke – and the subgroups based on pack-years are even smaller.”
No study funding is reported. The study authors and Dr. Volk reported no disclosures.
*This article was updated on 4/17/23.
People who have smoked an average of 1 pack a day for 20-39 years tripled their tumor risk versus less-heavy smokers and 30-fold versus those who never smoked. For those who smoked the equivalent of 1 pack for 40-60 years, or 2 packs for 20-30 years, the risk levels grew by fourfold and 40-fold, respectively. For those who’ve smoked even more, the likelihood of developing lung cancer is high, but the risk remains stable and doesn’t grow more over time, according to the analysis.
The report, released at the annual European Lung Cancer Congress 2023 meeting, and an earlier related study “underscore the importance of smoking abstinence and early smoking cessation,” said study lead author J. Anthony Nations, MD, MBA, in an interview.
The earlier study, published in JAMA Oncology, relied on a “pack-year” analysis to evaluate the risk of lung cancer in smokers. A pack-year refers to the cigarette use of a person who smoked a pack a day for 1 year. It’s the equivalent of smoking half a pack for 2 years or 2 packs for 6 months.
By this measure, a smoker with 20 pack-years of cigarette use smoked the equivalent of a pack a day for 20 years or 2 packs a day for 10 years. U.S. guidelines recommend annual low-dose CT lung cancer screening in adults who are aged 50-80, have more than 20 pack-years of tobacco exposure, and either currently smoke or quit within the last 15 years.
The JAMA Oncology report “showed that, compared with never-smokers, current heavy and nonheavy smokers had [a] 40 and 10 times higher risk of lung cancer, respectively,” said Dr. Nations, who is also a pulmonologist with Washington D.C. Veterans Affairs Medical Center. “A smoking history of greater than 20 pack-years was considered heavy, but current heavy smokers had a median pack-year smoking history of 50 pack-years. This observation prompted us to want to look more closely at pack-year smoking history.”
For the new analysis, researchers tracked 2,505 older adults (mean age, 73 ± 5.7 years; 69% women, 17% African American) in the Cardiovascular Health Study. Of those, 532 were current smokers (18% less than 20 pack-years, 30% 20-39 pack-years, 34% 40–59 pack-years, and 18% greater than 60 pack-years).
Lung cancer occurred in 0.5% of those who never smoked, 5% of those who smoked less than 20 pack-years, 14.6% of those who smoked 20-39 pack-years, 17.7% of those who smoked 40-59 pack-years, and 16.0% for those who smoked more than 60 pack-years. In an analysis adjusted for age, sex, race, and competing risk of death, researchers found that those who smoked less than 20 pack-years were 9.73 times more likely to develop lung cancer than those who never smoked (hazard ratio, 9.73). The HRs of lung cancer versus never-smokers for the other groups were 30.33 (20-39 pack-years), 42.97 (40-59 pack-years), and 46.02 (greater than 60 pack-years.).
“While it was not surprising that the risk of lung cancer in current heavy smokers would be proportionately greater in smokers with higher pack-year smoking history, we were surprised to see that the risk almost plateaued in the heaviest current smokers,” Dr. Nations said.
As for the clinical message from the findings, Dr. Nations said they reveal that quitting smoking makes a difference in lung cancer risk, even after many years of heavy smoking. “Smokers who quit after a 30–pack-year smoking history will not incur the higher risk of those with a 40– or 50–pack-year smoking history.”
The previous JAMA Oncology paper also showed that quitting pays dividends by reducing lung cancer risk. Subjects with at least 20 pack-years of smoking who quit less than 15 years ago nearly halved their excess risk of lung cancer, compared with similar current smokers who didn’t quit.
In an interview, cancer researcher Robert J. Volk, PhD, of the University of Texas MD Anderson Cancer Center, Houston, praised the new analysis but noted that it has limitations: “The sample is fairly small – 532 adults who currently smoke – and the subgroups based on pack-years are even smaller.”
No study funding is reported. The study authors and Dr. Volk reported no disclosures.
*This article was updated on 4/17/23.
People who have smoked an average of 1 pack a day for 20-39 years tripled their tumor risk versus less-heavy smokers and 30-fold versus those who never smoked. For those who smoked the equivalent of 1 pack for 40-60 years, or 2 packs for 20-30 years, the risk levels grew by fourfold and 40-fold, respectively. For those who’ve smoked even more, the likelihood of developing lung cancer is high, but the risk remains stable and doesn’t grow more over time, according to the analysis.
The report, released at the annual European Lung Cancer Congress 2023 meeting, and an earlier related study “underscore the importance of smoking abstinence and early smoking cessation,” said study lead author J. Anthony Nations, MD, MBA, in an interview.
The earlier study, published in JAMA Oncology, relied on a “pack-year” analysis to evaluate the risk of lung cancer in smokers. A pack-year refers to the cigarette use of a person who smoked a pack a day for 1 year. It’s the equivalent of smoking half a pack for 2 years or 2 packs for 6 months.
By this measure, a smoker with 20 pack-years of cigarette use smoked the equivalent of a pack a day for 20 years or 2 packs a day for 10 years. U.S. guidelines recommend annual low-dose CT lung cancer screening in adults who are aged 50-80, have more than 20 pack-years of tobacco exposure, and either currently smoke or quit within the last 15 years.
The JAMA Oncology report “showed that, compared with never-smokers, current heavy and nonheavy smokers had [a] 40 and 10 times higher risk of lung cancer, respectively,” said Dr. Nations, who is also a pulmonologist with Washington D.C. Veterans Affairs Medical Center. “A smoking history of greater than 20 pack-years was considered heavy, but current heavy smokers had a median pack-year smoking history of 50 pack-years. This observation prompted us to want to look more closely at pack-year smoking history.”
For the new analysis, researchers tracked 2,505 older adults (mean age, 73 ± 5.7 years; 69% women, 17% African American) in the Cardiovascular Health Study. Of those, 532 were current smokers (18% less than 20 pack-years, 30% 20-39 pack-years, 34% 40–59 pack-years, and 18% greater than 60 pack-years).
Lung cancer occurred in 0.5% of those who never smoked, 5% of those who smoked less than 20 pack-years, 14.6% of those who smoked 20-39 pack-years, 17.7% of those who smoked 40-59 pack-years, and 16.0% for those who smoked more than 60 pack-years. In an analysis adjusted for age, sex, race, and competing risk of death, researchers found that those who smoked less than 20 pack-years were 9.73 times more likely to develop lung cancer than those who never smoked (hazard ratio, 9.73). The HRs of lung cancer versus never-smokers for the other groups were 30.33 (20-39 pack-years), 42.97 (40-59 pack-years), and 46.02 (greater than 60 pack-years.).
“While it was not surprising that the risk of lung cancer in current heavy smokers would be proportionately greater in smokers with higher pack-year smoking history, we were surprised to see that the risk almost plateaued in the heaviest current smokers,” Dr. Nations said.
As for the clinical message from the findings, Dr. Nations said they reveal that quitting smoking makes a difference in lung cancer risk, even after many years of heavy smoking. “Smokers who quit after a 30–pack-year smoking history will not incur the higher risk of those with a 40– or 50–pack-year smoking history.”
The previous JAMA Oncology paper also showed that quitting pays dividends by reducing lung cancer risk. Subjects with at least 20 pack-years of smoking who quit less than 15 years ago nearly halved their excess risk of lung cancer, compared with similar current smokers who didn’t quit.
In an interview, cancer researcher Robert J. Volk, PhD, of the University of Texas MD Anderson Cancer Center, Houston, praised the new analysis but noted that it has limitations: “The sample is fairly small – 532 adults who currently smoke – and the subgroups based on pack-years are even smaller.”
No study funding is reported. The study authors and Dr. Volk reported no disclosures.
*This article was updated on 4/17/23.
FROM ELCC 2023