User login
85-year-old woman • insomnia • abdominal discomfort • urge to move at night • Dx?
THE CASE
An 85-year-old woman with hypertension presented to our hospital with a 10-month history of insomnia along with abdominal discomfort. Several months prior, the patient had undergone an esophagogastroduodenoscopy, the results of which were normal, and had received diagnoses of psychogenic insomnia and abdominal pain from her previous physician. At that time, she was prescribed eszopiclone, but her insomnia did not improve. She did not complain of any other gastrointestinal symptoms.
On examination at our hospital, the patient’s abdomen was soft and nontender. Laboratory results were unremarkable. Abdominal computed tomography was performed to exclude obvious malignancy and showed no remarkable findings.
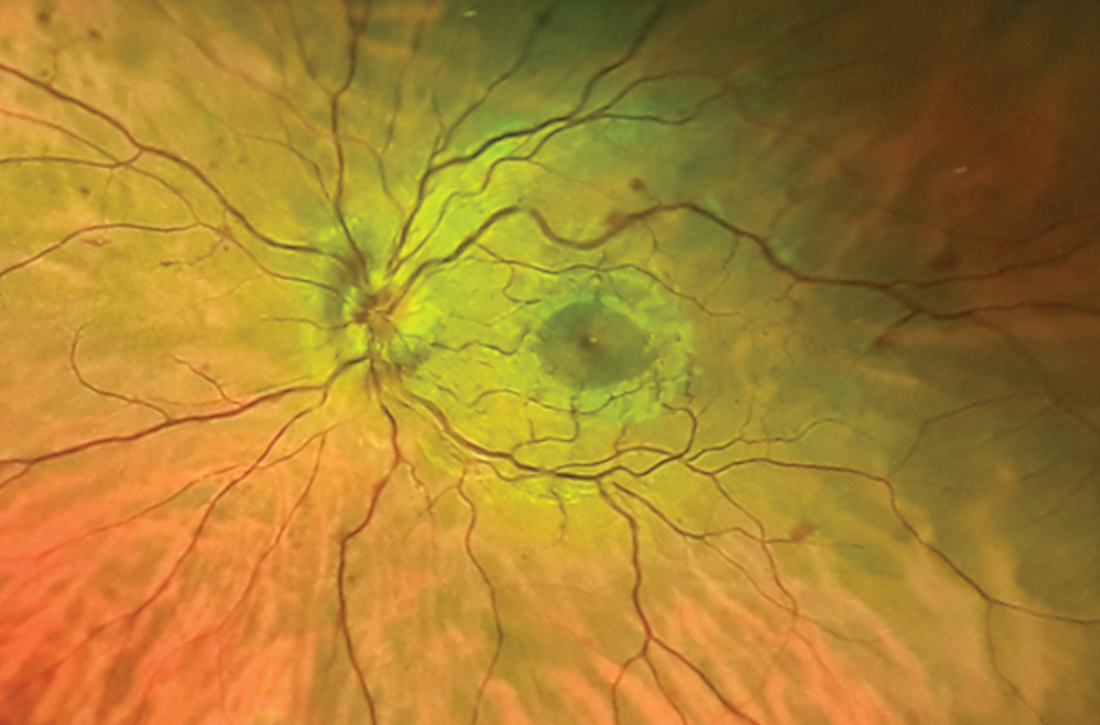
Additional history taking and physical examination were performed. The patient reported that she could sleep for only about 2 hours per night due to persistent severe discomfort around the umbilicus, which she described as “itching.” The discomfort occurred along with an urge to move while she laid in a state of relaxed wakefulness. This discomfort occurred no matter what position she laid in and improved if she walked or tapped around the umbilicus for a while. She denied any unusual or uncomfortable sensations in her lower extremities.
Her symptoms were absent during the daytime and not related to diet. Furthermore, she did not have any symptoms of anxiety and/or depression; a detailed neurologic examination, including cognitive assessment and extrapyramidal system, yielded unremarkable findings. Additional laboratory tests showed a mild iron deficiency (ferritin, 52.6 µ g/L; iron, 10.7 µ mol/L) without anemia.
THE DIAGNOSIS
Given the patient’s presentation and clinical history, the differential diagnosis included restless abdomen (which is a spectrum or a phenotypic variant of restless legs syndrome [RLS]) and its mimics, which include fibromyalgia and gastrointestinal tract diseases. We considered the characteristic symptoms of this case (ie, irresistible symptoms, lengthy duration of symptoms, and sleep problems) to better support the diagnosis of restless abdomen than its mimics.1 In particular, abdominal discomfort that led to insomnia was characteristic of restless abdomen, helping to pinpoint the diagnosis.
DISCUSSION
RLS is a common sensorimotor disorder that is characterized by an unpleasant urge to move the legs.2 RLS may manifest as an idiopathic condition, or it can be secondary to medical conditions such as iron deficiency and Parkinson disease.3,4 Because the unpleasant symptom is exacerbated in the evenings, patients with RLS frequently complain of sleep disturbance.
Cases of RLS-like sensory disorders, with symptoms involving sites other than the lower extremities (eg, arms, mouth, trunk, and genitals) recently have been reported.5-7 Among them is restless abdomen, a rare disorder that manifests with a restless abdominal sensation and worsens the quality of sleep and life.6
Continue to: Restless abdomen meets all...
Restless abdomen meets all other diagnostic criteria for RLS except for the affected anatomy.6,8 In most cases of restless abdomen, the uncomfortable sensation involves the abdomen, as well as other parts of the body (eg, legs and arms). Cases in which the symptoms are confined to the abdomen are rare, with only 7 reported to date. 6,8-10 All of these cases have involved patients older than 40 years. 6,8-10
Treatment is straightforward, but consider iron supplementation, as well
Because RLS or its variants degrade the quality of life and sleep in patients,3,4 appropriate therapy must be initiated early. Although the optimal treatment strategy for restless abdomen is yet to be established, an oral dopamine agonist—specifically, pramipexole—has been used successfully in almost all cases.6,8-10
Previous clinical research has shown that patients with RLS have low levels of iron in the brain and may benefit from iron supplementation, even if they are not anemic.3,4 Iron replacement is suggested for patients with RLS whose fasting serum ferritin level is ≤ 75 µg/L.4 It is not known to what extent iron deficiency is involved in the pathophysiology of restless abdomen, and further research is required to determine the optimal therapy for it.
Our patient was started on oral supplementation with sodium ferrous citrate (50 mg/d) based on an initial suspicion that iron deficiency was the cause of her restless abdomen. We also suggested that the patient undergo a fecal occult blood test or colonoscopy, but she declined because of her advanced age.
After 2 months of iron supplementation, the patient’s serum ferritin levels improved (100 µg/L) and her insomnia and abdominal discomfort improved a bit. However, 3 months after starting on the iron supplementation, her symptoms flared again.
Continue to: We then prescribed...
We then prescribed pramipexole 0.25 mg/d. The patient’s symptoms subsequently resolved, and she no longer experienced insomnia. This favorable response to dopamine agonist therapy supported the diagnosis of restless abdomen. The patient continues to take the pramipexole to prevent a relapse.
THE TAKEAWAY
Insomnia is a common presenting complaint in primary care and sleeping pills may be prescribed without adequate investigation of the cause. However, some patients may have serious underlying diseases.11
Although restless abdomen is a disorder that causes severe sleep disturbance and impairs the patient’s quality of sleep and life, it is not widely recognized by clinicians and may be misdiagnosed. When recognized, insomnia due to restless abdomen can be relieved by a simple therapy: oral dopamine agonists. Therefore, primary care physicians should consider restless abdomen as a potential cause of insomnia with abdominal symptoms.
CORRESPONDENCE
Hirohisa Fujikawa, MD, Department of Medical Education Studies, International Research Center for Medical Education, Graduate School of Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan; [email protected]
1. Hening WA, Allen RP, Washburn M, et al. The four diagnostic criteria for restless legs syndrome are unable to exclude confounding conditions (“mimics”). Sleep Med. 2009;10:976-981. doi: 10.1016/j.sleep.2008.09.015
2. Innes KE, Selfe TK, Agarwal P. Prevalence of restless legs syndrome in North American and Western European populations: a systematic review. Sleep Med. 2011;12:623-634. doi: 10.1016/j.sleep.2010.12.018
3. Bogan RK, Cheray JA. Restless legs syndrome: a review of diagnosis and management in primary care. Postgrad Med. 2013;125:99-111. doi: 10.3810/pgm.2013.05.2636
4. Silber MH, Buchfuhrer MJ, Earley CJ, et al. The management of restless legs syndrome: an updated algorithm. Mayo Clin Proc. 2021;96:1921-1937. doi: 10.1016/j.mayocp.2020.12.026
5. Aquino CC, Mestre T, Lang AE. Restless genital syndrome in Parkinson disease. JAMA Neurol. 2014;71:1559-1561. doi: 10.1001/jamaneurol.2014.1326
6. Pérez-Díaz H, Iranzo A, Rye DB, et al. Restless abdomen: a phenotypic variant of restless legs syndrome. Neurology. 2011;77:1283-1286. doi: 10.1212/WNL.0b013e318230207a
7. Sforza E, Hupin D, Roche F. Restless genital syndrome: differential diagnosis and treatment with pramipexole. J Clin Sleep Med. 2017;13:1109-1110. doi: 10.5664/jcsm.6736
8. Wang XX, Zhu XY, Wang Z, et al. Restless abdomen: a spectrum or a phenotype variant of restless legs syndrome? BMC Neurol. 2020;20:298. doi: 10.1186/s12883-020-01875-1
9. Esaki Y, Kitajima T, Tsuchiya A, et al. Periodic abdominal movements. Psychiatry Clin Neurosci. 2014;68:167. doi: 10.1111/pcn.12095
10. Baiardi S, La Morgia C, Mondini S, et al. A restless abdomen and propriospinal myoclonus like at sleep onset: an unusual overlap syndrome. BMJ Case Rep. 2015;2015:bcr2014206679. doi: 10.1136/bcr-2014-206679
11. Pavlova MK, Latreille V. Sleep disorders. Am J Med. 2019;132:292-299. doi: 10.1016/j.amjmed.2018.09.021
THE CASE
An 85-year-old woman with hypertension presented to our hospital with a 10-month history of insomnia along with abdominal discomfort. Several months prior, the patient had undergone an esophagogastroduodenoscopy, the results of which were normal, and had received diagnoses of psychogenic insomnia and abdominal pain from her previous physician. At that time, she was prescribed eszopiclone, but her insomnia did not improve. She did not complain of any other gastrointestinal symptoms.
On examination at our hospital, the patient’s abdomen was soft and nontender. Laboratory results were unremarkable. Abdominal computed tomography was performed to exclude obvious malignancy and showed no remarkable findings.
Additional history taking and physical examination were performed. The patient reported that she could sleep for only about 2 hours per night due to persistent severe discomfort around the umbilicus, which she described as “itching.” The discomfort occurred along with an urge to move while she laid in a state of relaxed wakefulness. This discomfort occurred no matter what position she laid in and improved if she walked or tapped around the umbilicus for a while. She denied any unusual or uncomfortable sensations in her lower extremities.
Her symptoms were absent during the daytime and not related to diet. Furthermore, she did not have any symptoms of anxiety and/or depression; a detailed neurologic examination, including cognitive assessment and extrapyramidal system, yielded unremarkable findings. Additional laboratory tests showed a mild iron deficiency (ferritin, 52.6 µ g/L; iron, 10.7 µ mol/L) without anemia.
THE DIAGNOSIS
Given the patient’s presentation and clinical history, the differential diagnosis included restless abdomen (which is a spectrum or a phenotypic variant of restless legs syndrome [RLS]) and its mimics, which include fibromyalgia and gastrointestinal tract diseases. We considered the characteristic symptoms of this case (ie, irresistible symptoms, lengthy duration of symptoms, and sleep problems) to better support the diagnosis of restless abdomen than its mimics.1 In particular, abdominal discomfort that led to insomnia was characteristic of restless abdomen, helping to pinpoint the diagnosis.
DISCUSSION
RLS is a common sensorimotor disorder that is characterized by an unpleasant urge to move the legs.2 RLS may manifest as an idiopathic condition, or it can be secondary to medical conditions such as iron deficiency and Parkinson disease.3,4 Because the unpleasant symptom is exacerbated in the evenings, patients with RLS frequently complain of sleep disturbance.
Cases of RLS-like sensory disorders, with symptoms involving sites other than the lower extremities (eg, arms, mouth, trunk, and genitals) recently have been reported.5-7 Among them is restless abdomen, a rare disorder that manifests with a restless abdominal sensation and worsens the quality of sleep and life.6
Continue to: Restless abdomen meets all...
Restless abdomen meets all other diagnostic criteria for RLS except for the affected anatomy.6,8 In most cases of restless abdomen, the uncomfortable sensation involves the abdomen, as well as other parts of the body (eg, legs and arms). Cases in which the symptoms are confined to the abdomen are rare, with only 7 reported to date. 6,8-10 All of these cases have involved patients older than 40 years. 6,8-10
Treatment is straightforward, but consider iron supplementation, as well
Because RLS or its variants degrade the quality of life and sleep in patients,3,4 appropriate therapy must be initiated early. Although the optimal treatment strategy for restless abdomen is yet to be established, an oral dopamine agonist—specifically, pramipexole—has been used successfully in almost all cases.6,8-10
Previous clinical research has shown that patients with RLS have low levels of iron in the brain and may benefit from iron supplementation, even if they are not anemic.3,4 Iron replacement is suggested for patients with RLS whose fasting serum ferritin level is ≤ 75 µg/L.4 It is not known to what extent iron deficiency is involved in the pathophysiology of restless abdomen, and further research is required to determine the optimal therapy for it.
Our patient was started on oral supplementation with sodium ferrous citrate (50 mg/d) based on an initial suspicion that iron deficiency was the cause of her restless abdomen. We also suggested that the patient undergo a fecal occult blood test or colonoscopy, but she declined because of her advanced age.
After 2 months of iron supplementation, the patient’s serum ferritin levels improved (100 µg/L) and her insomnia and abdominal discomfort improved a bit. However, 3 months after starting on the iron supplementation, her symptoms flared again.
Continue to: We then prescribed...
We then prescribed pramipexole 0.25 mg/d. The patient’s symptoms subsequently resolved, and she no longer experienced insomnia. This favorable response to dopamine agonist therapy supported the diagnosis of restless abdomen. The patient continues to take the pramipexole to prevent a relapse.
THE TAKEAWAY
Insomnia is a common presenting complaint in primary care and sleeping pills may be prescribed without adequate investigation of the cause. However, some patients may have serious underlying diseases.11
Although restless abdomen is a disorder that causes severe sleep disturbance and impairs the patient’s quality of sleep and life, it is not widely recognized by clinicians and may be misdiagnosed. When recognized, insomnia due to restless abdomen can be relieved by a simple therapy: oral dopamine agonists. Therefore, primary care physicians should consider restless abdomen as a potential cause of insomnia with abdominal symptoms.
CORRESPONDENCE
Hirohisa Fujikawa, MD, Department of Medical Education Studies, International Research Center for Medical Education, Graduate School of Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan; [email protected]
THE CASE
An 85-year-old woman with hypertension presented to our hospital with a 10-month history of insomnia along with abdominal discomfort. Several months prior, the patient had undergone an esophagogastroduodenoscopy, the results of which were normal, and had received diagnoses of psychogenic insomnia and abdominal pain from her previous physician. At that time, she was prescribed eszopiclone, but her insomnia did not improve. She did not complain of any other gastrointestinal symptoms.
On examination at our hospital, the patient’s abdomen was soft and nontender. Laboratory results were unremarkable. Abdominal computed tomography was performed to exclude obvious malignancy and showed no remarkable findings.
Additional history taking and physical examination were performed. The patient reported that she could sleep for only about 2 hours per night due to persistent severe discomfort around the umbilicus, which she described as “itching.” The discomfort occurred along with an urge to move while she laid in a state of relaxed wakefulness. This discomfort occurred no matter what position she laid in and improved if she walked or tapped around the umbilicus for a while. She denied any unusual or uncomfortable sensations in her lower extremities.
Her symptoms were absent during the daytime and not related to diet. Furthermore, she did not have any symptoms of anxiety and/or depression; a detailed neurologic examination, including cognitive assessment and extrapyramidal system, yielded unremarkable findings. Additional laboratory tests showed a mild iron deficiency (ferritin, 52.6 µ g/L; iron, 10.7 µ mol/L) without anemia.
THE DIAGNOSIS
Given the patient’s presentation and clinical history, the differential diagnosis included restless abdomen (which is a spectrum or a phenotypic variant of restless legs syndrome [RLS]) and its mimics, which include fibromyalgia and gastrointestinal tract diseases. We considered the characteristic symptoms of this case (ie, irresistible symptoms, lengthy duration of symptoms, and sleep problems) to better support the diagnosis of restless abdomen than its mimics.1 In particular, abdominal discomfort that led to insomnia was characteristic of restless abdomen, helping to pinpoint the diagnosis.
DISCUSSION
RLS is a common sensorimotor disorder that is characterized by an unpleasant urge to move the legs.2 RLS may manifest as an idiopathic condition, or it can be secondary to medical conditions such as iron deficiency and Parkinson disease.3,4 Because the unpleasant symptom is exacerbated in the evenings, patients with RLS frequently complain of sleep disturbance.
Cases of RLS-like sensory disorders, with symptoms involving sites other than the lower extremities (eg, arms, mouth, trunk, and genitals) recently have been reported.5-7 Among them is restless abdomen, a rare disorder that manifests with a restless abdominal sensation and worsens the quality of sleep and life.6
Continue to: Restless abdomen meets all...
Restless abdomen meets all other diagnostic criteria for RLS except for the affected anatomy.6,8 In most cases of restless abdomen, the uncomfortable sensation involves the abdomen, as well as other parts of the body (eg, legs and arms). Cases in which the symptoms are confined to the abdomen are rare, with only 7 reported to date. 6,8-10 All of these cases have involved patients older than 40 years. 6,8-10
Treatment is straightforward, but consider iron supplementation, as well
Because RLS or its variants degrade the quality of life and sleep in patients,3,4 appropriate therapy must be initiated early. Although the optimal treatment strategy for restless abdomen is yet to be established, an oral dopamine agonist—specifically, pramipexole—has been used successfully in almost all cases.6,8-10
Previous clinical research has shown that patients with RLS have low levels of iron in the brain and may benefit from iron supplementation, even if they are not anemic.3,4 Iron replacement is suggested for patients with RLS whose fasting serum ferritin level is ≤ 75 µg/L.4 It is not known to what extent iron deficiency is involved in the pathophysiology of restless abdomen, and further research is required to determine the optimal therapy for it.
Our patient was started on oral supplementation with sodium ferrous citrate (50 mg/d) based on an initial suspicion that iron deficiency was the cause of her restless abdomen. We also suggested that the patient undergo a fecal occult blood test or colonoscopy, but she declined because of her advanced age.
After 2 months of iron supplementation, the patient’s serum ferritin levels improved (100 µg/L) and her insomnia and abdominal discomfort improved a bit. However, 3 months after starting on the iron supplementation, her symptoms flared again.
Continue to: We then prescribed...
We then prescribed pramipexole 0.25 mg/d. The patient’s symptoms subsequently resolved, and she no longer experienced insomnia. This favorable response to dopamine agonist therapy supported the diagnosis of restless abdomen. The patient continues to take the pramipexole to prevent a relapse.
THE TAKEAWAY
Insomnia is a common presenting complaint in primary care and sleeping pills may be prescribed without adequate investigation of the cause. However, some patients may have serious underlying diseases.11
Although restless abdomen is a disorder that causes severe sleep disturbance and impairs the patient’s quality of sleep and life, it is not widely recognized by clinicians and may be misdiagnosed. When recognized, insomnia due to restless abdomen can be relieved by a simple therapy: oral dopamine agonists. Therefore, primary care physicians should consider restless abdomen as a potential cause of insomnia with abdominal symptoms.
CORRESPONDENCE
Hirohisa Fujikawa, MD, Department of Medical Education Studies, International Research Center for Medical Education, Graduate School of Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan; [email protected]
1. Hening WA, Allen RP, Washburn M, et al. The four diagnostic criteria for restless legs syndrome are unable to exclude confounding conditions (“mimics”). Sleep Med. 2009;10:976-981. doi: 10.1016/j.sleep.2008.09.015
2. Innes KE, Selfe TK, Agarwal P. Prevalence of restless legs syndrome in North American and Western European populations: a systematic review. Sleep Med. 2011;12:623-634. doi: 10.1016/j.sleep.2010.12.018
3. Bogan RK, Cheray JA. Restless legs syndrome: a review of diagnosis and management in primary care. Postgrad Med. 2013;125:99-111. doi: 10.3810/pgm.2013.05.2636
4. Silber MH, Buchfuhrer MJ, Earley CJ, et al. The management of restless legs syndrome: an updated algorithm. Mayo Clin Proc. 2021;96:1921-1937. doi: 10.1016/j.mayocp.2020.12.026
5. Aquino CC, Mestre T, Lang AE. Restless genital syndrome in Parkinson disease. JAMA Neurol. 2014;71:1559-1561. doi: 10.1001/jamaneurol.2014.1326
6. Pérez-Díaz H, Iranzo A, Rye DB, et al. Restless abdomen: a phenotypic variant of restless legs syndrome. Neurology. 2011;77:1283-1286. doi: 10.1212/WNL.0b013e318230207a
7. Sforza E, Hupin D, Roche F. Restless genital syndrome: differential diagnosis and treatment with pramipexole. J Clin Sleep Med. 2017;13:1109-1110. doi: 10.5664/jcsm.6736
8. Wang XX, Zhu XY, Wang Z, et al. Restless abdomen: a spectrum or a phenotype variant of restless legs syndrome? BMC Neurol. 2020;20:298. doi: 10.1186/s12883-020-01875-1
9. Esaki Y, Kitajima T, Tsuchiya A, et al. Periodic abdominal movements. Psychiatry Clin Neurosci. 2014;68:167. doi: 10.1111/pcn.12095
10. Baiardi S, La Morgia C, Mondini S, et al. A restless abdomen and propriospinal myoclonus like at sleep onset: an unusual overlap syndrome. BMJ Case Rep. 2015;2015:bcr2014206679. doi: 10.1136/bcr-2014-206679
11. Pavlova MK, Latreille V. Sleep disorders. Am J Med. 2019;132:292-299. doi: 10.1016/j.amjmed.2018.09.021
1. Hening WA, Allen RP, Washburn M, et al. The four diagnostic criteria for restless legs syndrome are unable to exclude confounding conditions (“mimics”). Sleep Med. 2009;10:976-981. doi: 10.1016/j.sleep.2008.09.015
2. Innes KE, Selfe TK, Agarwal P. Prevalence of restless legs syndrome in North American and Western European populations: a systematic review. Sleep Med. 2011;12:623-634. doi: 10.1016/j.sleep.2010.12.018
3. Bogan RK, Cheray JA. Restless legs syndrome: a review of diagnosis and management in primary care. Postgrad Med. 2013;125:99-111. doi: 10.3810/pgm.2013.05.2636
4. Silber MH, Buchfuhrer MJ, Earley CJ, et al. The management of restless legs syndrome: an updated algorithm. Mayo Clin Proc. 2021;96:1921-1937. doi: 10.1016/j.mayocp.2020.12.026
5. Aquino CC, Mestre T, Lang AE. Restless genital syndrome in Parkinson disease. JAMA Neurol. 2014;71:1559-1561. doi: 10.1001/jamaneurol.2014.1326
6. Pérez-Díaz H, Iranzo A, Rye DB, et al. Restless abdomen: a phenotypic variant of restless legs syndrome. Neurology. 2011;77:1283-1286. doi: 10.1212/WNL.0b013e318230207a
7. Sforza E, Hupin D, Roche F. Restless genital syndrome: differential diagnosis and treatment with pramipexole. J Clin Sleep Med. 2017;13:1109-1110. doi: 10.5664/jcsm.6736
8. Wang XX, Zhu XY, Wang Z, et al. Restless abdomen: a spectrum or a phenotype variant of restless legs syndrome? BMC Neurol. 2020;20:298. doi: 10.1186/s12883-020-01875-1
9. Esaki Y, Kitajima T, Tsuchiya A, et al. Periodic abdominal movements. Psychiatry Clin Neurosci. 2014;68:167. doi: 10.1111/pcn.12095
10. Baiardi S, La Morgia C, Mondini S, et al. A restless abdomen and propriospinal myoclonus like at sleep onset: an unusual overlap syndrome. BMJ Case Rep. 2015;2015:bcr2014206679. doi: 10.1136/bcr-2014-206679
11. Pavlova MK, Latreille V. Sleep disorders. Am J Med. 2019;132:292-299. doi: 10.1016/j.amjmed.2018.09.021
Healthy lifestyle mitigates effect of childhood cancer
Although people who survive a childhood cancer are at an increased risk of developing and dying from subsequent cancers, as well as heart disease and stroke, they can reduce this risk by following a healthy lifestyle, say U.S. investigators.
This message comes from a retrospective analysis of more than 34,000 childhood cancer survivors, which found that 40 years after the initial cancer diagnosis, the cumulative all-cause mortality rate was 23.3%, compared with less than 5% in the general population.
However, following a healthy lifestyle was associated with a 20% reduction in health-related mortality, independent of other factors, the analysis showed. This rose even further, up to a 30% reduction, among individuals who did not have hypertension or diabetes.
The study was published online in The Lancet.
“We identified that long-term survivors of childhood cancer are experiencing a large number of deaths in excess of what would be expected for the general, aging population,” first author Stephanie Dixon, MD, MPH, oncology department, St. Jude Children’s Research Hospital, Memphis, Tenn., said in a press release.
“These excess deaths are predominantly due to the same leading causes of death as in the general population,” including subsequent cancers, heart disease, cerebrovascular disease/stroke, chronic liver and kidney disease, and infectious diseases, she noted. However, in these childhood cancer survivors they are occurring “at a younger age and higher rate.”
“What was most exciting to see,” Dr. Dixon added, “was that, independent of prior treatment exposures and sociodemographic factors, a healthy lifestyle and absence of hypertension or diabetes were each associated with a reduced risk of health-related mortality.”
“This is important because our goal is to extend the life span of survivors and to improve their ‘health span’ as well,” said senior author Greg Armstrong, MD, MSCE, chair of the department of epidemiology and cancer control at St. Jude.
As such, “the study highlights the importance of encouraging survivors to practice healthy behaviors and maintain good control of cardiovascular disease risk factors,” emphasized coauthor Melissa M. Hudson, MD, director of the cancer survivorship division at St. Jude.
Future research should focus on interventions for modifiable lifestyle and cardiovascular risk factors that “may need to be specifically tailored to survivors, with the goal of reducing chronic disease development” and extending their lifespan, the researchers said.
Late effects of treatment
Childhood cancer has a tremendous success rate: In the United States, the 5-year survival rate is now more than 85%.
However, long-term survivors experience excess morbidity and late mortality compared with the general population, both of which are “attributable to late effects of treatment,” the team pointed out.
Their study focused on individuals who had been diagnosed with cancer before they were 21 years old and who had survived at least 5 years after the cancer diagnosis.
The median age at diagnosis was 6 years, and the most common diagnoses were acute lymphoblastic leukemia (36%), Hodgkin lymphoma (11%), astrocytoma (10%), and kidney tumors (8%).
The team identified 34,230 survivors who had been treated between Jan. 1, 1970, and Dec. 31, 1999, at 31 institutions in the United States and Canada.
They represented approximately 20% of all childhood cancer survivors in the United States over the study period. The team noted that 56% of the survivors were male, and the majority (64%) were non-Hispanic White.
The date and causes of death through December 2017 were obtained via linkage to the National Death Index, and cancer treatment information was collated for 21,418 survivors who provided consent. Lifestyle factors – including smoking, alcohol use, physical activity, and unhealthy weight – were graded on a score of 0-4.
Over a median follow-up of 29.1 years, there were 5,916 deaths, with 34% attributable to the recurrence or progression of the primary cancer, and 51.2% attributable to other causes, such as subsequent neoplasms, and cardiac, pulmonary, and other health-related causes.
Overall, survivors were at an elevated risk of death compared with the general population, at a standardized mortality ratio of 5.6. This ratio peaked at 5-9 years after diagnosis at an 18.1-fold increased risk of death compared with the general population.
Forty years or more from the initial diagnosis, two-thirds of the 131 per 10,000 person-years excess deaths from health-related causes were due to the top three causes of health-related death in the general population, the team reported.
This included an absolute excess risk of death from cancer of 54 per 10,000 person-years, an excess risk of heart disease mortality of 27 per 10,000 person-years, and an excess risk of cerebrovascular disease mortality of 10 per 10,000 person-years.
The individual cases of death contributing the greatest excess risk were gastrointestinal cancers (11 per 10,000 person-years), cerebrovascular disease (10 per 10,000 person-years), ischemic heart disease (10 per 10,000 person-years), and valvular heart disease (9 per 10,000 person-years).
The good news is that following a healthy lifestyle was associated with a 20% reduction in health-related mortality versus an unhealthy lifestyle (P = .0020).
Moreover, following even a moderately healthy lifestyle was associated with a 10% reduction in health-related mortality, the researchers noted.
The study was supported by grants from the National Cancer Institute, St. Jude Children’s Research Hospital Cancer Center Support, and the American Lebanese-Syrian Associated Charities. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although people who survive a childhood cancer are at an increased risk of developing and dying from subsequent cancers, as well as heart disease and stroke, they can reduce this risk by following a healthy lifestyle, say U.S. investigators.
This message comes from a retrospective analysis of more than 34,000 childhood cancer survivors, which found that 40 years after the initial cancer diagnosis, the cumulative all-cause mortality rate was 23.3%, compared with less than 5% in the general population.
However, following a healthy lifestyle was associated with a 20% reduction in health-related mortality, independent of other factors, the analysis showed. This rose even further, up to a 30% reduction, among individuals who did not have hypertension or diabetes.
The study was published online in The Lancet.
“We identified that long-term survivors of childhood cancer are experiencing a large number of deaths in excess of what would be expected for the general, aging population,” first author Stephanie Dixon, MD, MPH, oncology department, St. Jude Children’s Research Hospital, Memphis, Tenn., said in a press release.
“These excess deaths are predominantly due to the same leading causes of death as in the general population,” including subsequent cancers, heart disease, cerebrovascular disease/stroke, chronic liver and kidney disease, and infectious diseases, she noted. However, in these childhood cancer survivors they are occurring “at a younger age and higher rate.”
“What was most exciting to see,” Dr. Dixon added, “was that, independent of prior treatment exposures and sociodemographic factors, a healthy lifestyle and absence of hypertension or diabetes were each associated with a reduced risk of health-related mortality.”
“This is important because our goal is to extend the life span of survivors and to improve their ‘health span’ as well,” said senior author Greg Armstrong, MD, MSCE, chair of the department of epidemiology and cancer control at St. Jude.
As such, “the study highlights the importance of encouraging survivors to practice healthy behaviors and maintain good control of cardiovascular disease risk factors,” emphasized coauthor Melissa M. Hudson, MD, director of the cancer survivorship division at St. Jude.
Future research should focus on interventions for modifiable lifestyle and cardiovascular risk factors that “may need to be specifically tailored to survivors, with the goal of reducing chronic disease development” and extending their lifespan, the researchers said.
Late effects of treatment
Childhood cancer has a tremendous success rate: In the United States, the 5-year survival rate is now more than 85%.
However, long-term survivors experience excess morbidity and late mortality compared with the general population, both of which are “attributable to late effects of treatment,” the team pointed out.
Their study focused on individuals who had been diagnosed with cancer before they were 21 years old and who had survived at least 5 years after the cancer diagnosis.
The median age at diagnosis was 6 years, and the most common diagnoses were acute lymphoblastic leukemia (36%), Hodgkin lymphoma (11%), astrocytoma (10%), and kidney tumors (8%).
The team identified 34,230 survivors who had been treated between Jan. 1, 1970, and Dec. 31, 1999, at 31 institutions in the United States and Canada.
They represented approximately 20% of all childhood cancer survivors in the United States over the study period. The team noted that 56% of the survivors were male, and the majority (64%) were non-Hispanic White.
The date and causes of death through December 2017 were obtained via linkage to the National Death Index, and cancer treatment information was collated for 21,418 survivors who provided consent. Lifestyle factors – including smoking, alcohol use, physical activity, and unhealthy weight – were graded on a score of 0-4.
Over a median follow-up of 29.1 years, there were 5,916 deaths, with 34% attributable to the recurrence or progression of the primary cancer, and 51.2% attributable to other causes, such as subsequent neoplasms, and cardiac, pulmonary, and other health-related causes.
Overall, survivors were at an elevated risk of death compared with the general population, at a standardized mortality ratio of 5.6. This ratio peaked at 5-9 years after diagnosis at an 18.1-fold increased risk of death compared with the general population.
Forty years or more from the initial diagnosis, two-thirds of the 131 per 10,000 person-years excess deaths from health-related causes were due to the top three causes of health-related death in the general population, the team reported.
This included an absolute excess risk of death from cancer of 54 per 10,000 person-years, an excess risk of heart disease mortality of 27 per 10,000 person-years, and an excess risk of cerebrovascular disease mortality of 10 per 10,000 person-years.
The individual cases of death contributing the greatest excess risk were gastrointestinal cancers (11 per 10,000 person-years), cerebrovascular disease (10 per 10,000 person-years), ischemic heart disease (10 per 10,000 person-years), and valvular heart disease (9 per 10,000 person-years).
The good news is that following a healthy lifestyle was associated with a 20% reduction in health-related mortality versus an unhealthy lifestyle (P = .0020).
Moreover, following even a moderately healthy lifestyle was associated with a 10% reduction in health-related mortality, the researchers noted.
The study was supported by grants from the National Cancer Institute, St. Jude Children’s Research Hospital Cancer Center Support, and the American Lebanese-Syrian Associated Charities. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although people who survive a childhood cancer are at an increased risk of developing and dying from subsequent cancers, as well as heart disease and stroke, they can reduce this risk by following a healthy lifestyle, say U.S. investigators.
This message comes from a retrospective analysis of more than 34,000 childhood cancer survivors, which found that 40 years after the initial cancer diagnosis, the cumulative all-cause mortality rate was 23.3%, compared with less than 5% in the general population.
However, following a healthy lifestyle was associated with a 20% reduction in health-related mortality, independent of other factors, the analysis showed. This rose even further, up to a 30% reduction, among individuals who did not have hypertension or diabetes.
The study was published online in The Lancet.
“We identified that long-term survivors of childhood cancer are experiencing a large number of deaths in excess of what would be expected for the general, aging population,” first author Stephanie Dixon, MD, MPH, oncology department, St. Jude Children’s Research Hospital, Memphis, Tenn., said in a press release.
“These excess deaths are predominantly due to the same leading causes of death as in the general population,” including subsequent cancers, heart disease, cerebrovascular disease/stroke, chronic liver and kidney disease, and infectious diseases, she noted. However, in these childhood cancer survivors they are occurring “at a younger age and higher rate.”
“What was most exciting to see,” Dr. Dixon added, “was that, independent of prior treatment exposures and sociodemographic factors, a healthy lifestyle and absence of hypertension or diabetes were each associated with a reduced risk of health-related mortality.”
“This is important because our goal is to extend the life span of survivors and to improve their ‘health span’ as well,” said senior author Greg Armstrong, MD, MSCE, chair of the department of epidemiology and cancer control at St. Jude.
As such, “the study highlights the importance of encouraging survivors to practice healthy behaviors and maintain good control of cardiovascular disease risk factors,” emphasized coauthor Melissa M. Hudson, MD, director of the cancer survivorship division at St. Jude.
Future research should focus on interventions for modifiable lifestyle and cardiovascular risk factors that “may need to be specifically tailored to survivors, with the goal of reducing chronic disease development” and extending their lifespan, the researchers said.
Late effects of treatment
Childhood cancer has a tremendous success rate: In the United States, the 5-year survival rate is now more than 85%.
However, long-term survivors experience excess morbidity and late mortality compared with the general population, both of which are “attributable to late effects of treatment,” the team pointed out.
Their study focused on individuals who had been diagnosed with cancer before they were 21 years old and who had survived at least 5 years after the cancer diagnosis.
The median age at diagnosis was 6 years, and the most common diagnoses were acute lymphoblastic leukemia (36%), Hodgkin lymphoma (11%), astrocytoma (10%), and kidney tumors (8%).
The team identified 34,230 survivors who had been treated between Jan. 1, 1970, and Dec. 31, 1999, at 31 institutions in the United States and Canada.
They represented approximately 20% of all childhood cancer survivors in the United States over the study period. The team noted that 56% of the survivors were male, and the majority (64%) were non-Hispanic White.
The date and causes of death through December 2017 were obtained via linkage to the National Death Index, and cancer treatment information was collated for 21,418 survivors who provided consent. Lifestyle factors – including smoking, alcohol use, physical activity, and unhealthy weight – were graded on a score of 0-4.
Over a median follow-up of 29.1 years, there were 5,916 deaths, with 34% attributable to the recurrence or progression of the primary cancer, and 51.2% attributable to other causes, such as subsequent neoplasms, and cardiac, pulmonary, and other health-related causes.
Overall, survivors were at an elevated risk of death compared with the general population, at a standardized mortality ratio of 5.6. This ratio peaked at 5-9 years after diagnosis at an 18.1-fold increased risk of death compared with the general population.
Forty years or more from the initial diagnosis, two-thirds of the 131 per 10,000 person-years excess deaths from health-related causes were due to the top three causes of health-related death in the general population, the team reported.
This included an absolute excess risk of death from cancer of 54 per 10,000 person-years, an excess risk of heart disease mortality of 27 per 10,000 person-years, and an excess risk of cerebrovascular disease mortality of 10 per 10,000 person-years.
The individual cases of death contributing the greatest excess risk were gastrointestinal cancers (11 per 10,000 person-years), cerebrovascular disease (10 per 10,000 person-years), ischemic heart disease (10 per 10,000 person-years), and valvular heart disease (9 per 10,000 person-years).
The good news is that following a healthy lifestyle was associated with a 20% reduction in health-related mortality versus an unhealthy lifestyle (P = .0020).
Moreover, following even a moderately healthy lifestyle was associated with a 10% reduction in health-related mortality, the researchers noted.
The study was supported by grants from the National Cancer Institute, St. Jude Children’s Research Hospital Cancer Center Support, and the American Lebanese-Syrian Associated Charities. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Antimicrobial resistance requires a manifold response
BUENOS AIRES – Antimicrobial resistance (AMR) has become a global concern. And while one issue to be addressed is the deficit in research and development for new antibiotics, efforts to tackle this public health threat also should be directed toward promoting more rational prescription practices and strengthening the ability to identify the microorganisms responsible for infections, according to the World Health Organization. This was the conclusion reached at the fourth meeting of the WHO AMR Surveillance and Quality Assessment Collaborating Centres Network, which was held in Buenos Aires.
“We have to provide assistance to countries to ensure that the drugs are being used responsibly. We can come up with new antibiotics, but the issue at hand is not simply one of innovation: If nothing is done to correct inappropriate prescription practices and to overcome the lack of diagnostic laboratories at the country level, we’re going to miss out on those drugs as soon as they become available,” Kitty van Weezenbeek, MD, PhD, MPH, director of the AMR Surveillance, Prevention, and Control (AMR/SPC) Department at the WHO’s headquarters in Geneva, told this news organization.
Dr. van Weezenbeek pointed out that although there are currently no shortages of antimicrobials, the development and launch of new drugs that fight multidrug-resistant infections – infections for which there are few therapeutic options – has proceeded slowly. “It takes 10 to 15 years to develop a new antibiotic,” she said, adding that “the majority of pharmaceutical companies that had been engaged in the development of antimicrobials have filed for bankruptcy.”
In 2019, more people died – 1.2 million – from AMR than from malaria, tuberculosis, and HIV combined. Why are there so few market incentives when there is such a great need for those drugs? “One reason is that the pharmaceutical industry makes more money with long-term treatments, such as those for cancer and respiratory diseases. The other problem is that people everywhere are told not to use antibiotics,” said Dr. van Weezenbeek.
“A course of antibiotics lasts a few days, especially because we’re promoting rational use. Therefore, the trend is for the total amount of antimicrobials being used to be lower. So, it’s not as profitable,” added Carmem Lucia Pessoa-Silva, MD, PhD, head of the Surveillance, Evidence, and Laboratory Strengthening Unit of the WHO’s AMR/SPC Department.
On that note, Dr. van Weezenbeek mentioned that member countries are working with pharmaceutical companies and universities to address this problem. The WHO, for its part, has responded by implementing a global mechanism with a public health approach to create a “healthy” and equitable market for these medicines.
AMR is one of the top 10 global threats to human health. But it also has an impact on animal production, agricultural production, and the environment. Strategies to tackle AMR based on the One Health approach should involve all actors, social sectors, and citizens, according to Eva Jané Llopis, PhD, the representative of the Pan American Health Organization/WHO in Argentina.
At the root of the AMR problem is the widespread use of these drugs as growth promoters in animal production – for which several countries have enacted regulations – as well as “misunderstandings” between patients and physicians when there is not sufficient, timely access to laboratory diagnostics, especially in low- and middle-income countries.
“People think that if they’re given broad-spectrum antibiotics, they’re being prescribed the best antibiotics; and doctors, because there are no laboratory services, prescribe broad-spectrum antibiotics because they want to help patients. But that ends up causing more resistance to drugs, and thus, those antibiotics aren’t good for the patients,” said Dr. van Weezenbeek.
The WHO Global AMR and Use Surveillance System (GLASS) was launched in 2015. Its 2022 report, which marked the end of the system’s early implementation period, noted that the reported AMR rates are often lower in countries, territories, and areas with better testing coverage for most pathogen-drug-infection site combinations. However, as Dr. Pessoa-Silva acknowledged, monitoring “has not yet generated representative data,” because in many cases, countries either do not have surveillance systems or have only recently started implementing them.
Even so, the indicators that are available paint an increasingly worrisome picture. “For example, in many countries, resistance rates to first-line antibiotics were around 10%-20% with respect to Escherichia coli urinary tract infections and bloodstream bacteriologically confirmed infections. So, the risk of treatment failure is very high,” explained Dr. Pessoa-Silva.
The latest estimates indicate that every 2 or 3 minutes, somewhere in the world, a child dies from AMR. And the situation is particularly “dramatic” in neonatal intensive care units, where outbreaks of multidrug-resistant infections have a mortality rate of 50%, said Pilar Ramón-Pardo, MD, PhD, lead of the Special Program on AMR at the Pan American Health Organization, the WHO Regional Office for the Americas.
AMR rates also got worse during the pandemic because of the inappropriate prescription of massive amounts of antibiotics to hospitalized patients – something that was not in compliance with guidelines or protocols. Silvia Bertagnolio, MD, is an infectious disease specialist and the head of the Control and Response Strategies Unit in the WHO’s AMR Division. She spoke about the global clinical platform data pertaining to more than 1,500,000 patients who were hospitalized for COVID-19. Since 2020, 85% received antimicrobial treatment, despite the fact that only 5% had a concomitant infection at admission. “It’s easier to give antibiotics than to make a proper diagnosis,” said Dr. Bertagnolio.
This article was translated from Medscape’s Spanish edition and a version appeared on Medscape.com.
BUENOS AIRES – Antimicrobial resistance (AMR) has become a global concern. And while one issue to be addressed is the deficit in research and development for new antibiotics, efforts to tackle this public health threat also should be directed toward promoting more rational prescription practices and strengthening the ability to identify the microorganisms responsible for infections, according to the World Health Organization. This was the conclusion reached at the fourth meeting of the WHO AMR Surveillance and Quality Assessment Collaborating Centres Network, which was held in Buenos Aires.
“We have to provide assistance to countries to ensure that the drugs are being used responsibly. We can come up with new antibiotics, but the issue at hand is not simply one of innovation: If nothing is done to correct inappropriate prescription practices and to overcome the lack of diagnostic laboratories at the country level, we’re going to miss out on those drugs as soon as they become available,” Kitty van Weezenbeek, MD, PhD, MPH, director of the AMR Surveillance, Prevention, and Control (AMR/SPC) Department at the WHO’s headquarters in Geneva, told this news organization.
Dr. van Weezenbeek pointed out that although there are currently no shortages of antimicrobials, the development and launch of new drugs that fight multidrug-resistant infections – infections for which there are few therapeutic options – has proceeded slowly. “It takes 10 to 15 years to develop a new antibiotic,” she said, adding that “the majority of pharmaceutical companies that had been engaged in the development of antimicrobials have filed for bankruptcy.”
In 2019, more people died – 1.2 million – from AMR than from malaria, tuberculosis, and HIV combined. Why are there so few market incentives when there is such a great need for those drugs? “One reason is that the pharmaceutical industry makes more money with long-term treatments, such as those for cancer and respiratory diseases. The other problem is that people everywhere are told not to use antibiotics,” said Dr. van Weezenbeek.
“A course of antibiotics lasts a few days, especially because we’re promoting rational use. Therefore, the trend is for the total amount of antimicrobials being used to be lower. So, it’s not as profitable,” added Carmem Lucia Pessoa-Silva, MD, PhD, head of the Surveillance, Evidence, and Laboratory Strengthening Unit of the WHO’s AMR/SPC Department.
On that note, Dr. van Weezenbeek mentioned that member countries are working with pharmaceutical companies and universities to address this problem. The WHO, for its part, has responded by implementing a global mechanism with a public health approach to create a “healthy” and equitable market for these medicines.
AMR is one of the top 10 global threats to human health. But it also has an impact on animal production, agricultural production, and the environment. Strategies to tackle AMR based on the One Health approach should involve all actors, social sectors, and citizens, according to Eva Jané Llopis, PhD, the representative of the Pan American Health Organization/WHO in Argentina.
At the root of the AMR problem is the widespread use of these drugs as growth promoters in animal production – for which several countries have enacted regulations – as well as “misunderstandings” between patients and physicians when there is not sufficient, timely access to laboratory diagnostics, especially in low- and middle-income countries.
“People think that if they’re given broad-spectrum antibiotics, they’re being prescribed the best antibiotics; and doctors, because there are no laboratory services, prescribe broad-spectrum antibiotics because they want to help patients. But that ends up causing more resistance to drugs, and thus, those antibiotics aren’t good for the patients,” said Dr. van Weezenbeek.
The WHO Global AMR and Use Surveillance System (GLASS) was launched in 2015. Its 2022 report, which marked the end of the system’s early implementation period, noted that the reported AMR rates are often lower in countries, territories, and areas with better testing coverage for most pathogen-drug-infection site combinations. However, as Dr. Pessoa-Silva acknowledged, monitoring “has not yet generated representative data,” because in many cases, countries either do not have surveillance systems or have only recently started implementing them.
Even so, the indicators that are available paint an increasingly worrisome picture. “For example, in many countries, resistance rates to first-line antibiotics were around 10%-20% with respect to Escherichia coli urinary tract infections and bloodstream bacteriologically confirmed infections. So, the risk of treatment failure is very high,” explained Dr. Pessoa-Silva.
The latest estimates indicate that every 2 or 3 minutes, somewhere in the world, a child dies from AMR. And the situation is particularly “dramatic” in neonatal intensive care units, where outbreaks of multidrug-resistant infections have a mortality rate of 50%, said Pilar Ramón-Pardo, MD, PhD, lead of the Special Program on AMR at the Pan American Health Organization, the WHO Regional Office for the Americas.
AMR rates also got worse during the pandemic because of the inappropriate prescription of massive amounts of antibiotics to hospitalized patients – something that was not in compliance with guidelines or protocols. Silvia Bertagnolio, MD, is an infectious disease specialist and the head of the Control and Response Strategies Unit in the WHO’s AMR Division. She spoke about the global clinical platform data pertaining to more than 1,500,000 patients who were hospitalized for COVID-19. Since 2020, 85% received antimicrobial treatment, despite the fact that only 5% had a concomitant infection at admission. “It’s easier to give antibiotics than to make a proper diagnosis,” said Dr. Bertagnolio.
This article was translated from Medscape’s Spanish edition and a version appeared on Medscape.com.
BUENOS AIRES – Antimicrobial resistance (AMR) has become a global concern. And while one issue to be addressed is the deficit in research and development for new antibiotics, efforts to tackle this public health threat also should be directed toward promoting more rational prescription practices and strengthening the ability to identify the microorganisms responsible for infections, according to the World Health Organization. This was the conclusion reached at the fourth meeting of the WHO AMR Surveillance and Quality Assessment Collaborating Centres Network, which was held in Buenos Aires.
“We have to provide assistance to countries to ensure that the drugs are being used responsibly. We can come up with new antibiotics, but the issue at hand is not simply one of innovation: If nothing is done to correct inappropriate prescription practices and to overcome the lack of diagnostic laboratories at the country level, we’re going to miss out on those drugs as soon as they become available,” Kitty van Weezenbeek, MD, PhD, MPH, director of the AMR Surveillance, Prevention, and Control (AMR/SPC) Department at the WHO’s headquarters in Geneva, told this news organization.
Dr. van Weezenbeek pointed out that although there are currently no shortages of antimicrobials, the development and launch of new drugs that fight multidrug-resistant infections – infections for which there are few therapeutic options – has proceeded slowly. “It takes 10 to 15 years to develop a new antibiotic,” she said, adding that “the majority of pharmaceutical companies that had been engaged in the development of antimicrobials have filed for bankruptcy.”
In 2019, more people died – 1.2 million – from AMR than from malaria, tuberculosis, and HIV combined. Why are there so few market incentives when there is such a great need for those drugs? “One reason is that the pharmaceutical industry makes more money with long-term treatments, such as those for cancer and respiratory diseases. The other problem is that people everywhere are told not to use antibiotics,” said Dr. van Weezenbeek.
“A course of antibiotics lasts a few days, especially because we’re promoting rational use. Therefore, the trend is for the total amount of antimicrobials being used to be lower. So, it’s not as profitable,” added Carmem Lucia Pessoa-Silva, MD, PhD, head of the Surveillance, Evidence, and Laboratory Strengthening Unit of the WHO’s AMR/SPC Department.
On that note, Dr. van Weezenbeek mentioned that member countries are working with pharmaceutical companies and universities to address this problem. The WHO, for its part, has responded by implementing a global mechanism with a public health approach to create a “healthy” and equitable market for these medicines.
AMR is one of the top 10 global threats to human health. But it also has an impact on animal production, agricultural production, and the environment. Strategies to tackle AMR based on the One Health approach should involve all actors, social sectors, and citizens, according to Eva Jané Llopis, PhD, the representative of the Pan American Health Organization/WHO in Argentina.
At the root of the AMR problem is the widespread use of these drugs as growth promoters in animal production – for which several countries have enacted regulations – as well as “misunderstandings” between patients and physicians when there is not sufficient, timely access to laboratory diagnostics, especially in low- and middle-income countries.
“People think that if they’re given broad-spectrum antibiotics, they’re being prescribed the best antibiotics; and doctors, because there are no laboratory services, prescribe broad-spectrum antibiotics because they want to help patients. But that ends up causing more resistance to drugs, and thus, those antibiotics aren’t good for the patients,” said Dr. van Weezenbeek.
The WHO Global AMR and Use Surveillance System (GLASS) was launched in 2015. Its 2022 report, which marked the end of the system’s early implementation period, noted that the reported AMR rates are often lower in countries, territories, and areas with better testing coverage for most pathogen-drug-infection site combinations. However, as Dr. Pessoa-Silva acknowledged, monitoring “has not yet generated representative data,” because in many cases, countries either do not have surveillance systems or have only recently started implementing them.
Even so, the indicators that are available paint an increasingly worrisome picture. “For example, in many countries, resistance rates to first-line antibiotics were around 10%-20% with respect to Escherichia coli urinary tract infections and bloodstream bacteriologically confirmed infections. So, the risk of treatment failure is very high,” explained Dr. Pessoa-Silva.
The latest estimates indicate that every 2 or 3 minutes, somewhere in the world, a child dies from AMR. And the situation is particularly “dramatic” in neonatal intensive care units, where outbreaks of multidrug-resistant infections have a mortality rate of 50%, said Pilar Ramón-Pardo, MD, PhD, lead of the Special Program on AMR at the Pan American Health Organization, the WHO Regional Office for the Americas.
AMR rates also got worse during the pandemic because of the inappropriate prescription of massive amounts of antibiotics to hospitalized patients – something that was not in compliance with guidelines or protocols. Silvia Bertagnolio, MD, is an infectious disease specialist and the head of the Control and Response Strategies Unit in the WHO’s AMR Division. She spoke about the global clinical platform data pertaining to more than 1,500,000 patients who were hospitalized for COVID-19. Since 2020, 85% received antimicrobial treatment, despite the fact that only 5% had a concomitant infection at admission. “It’s easier to give antibiotics than to make a proper diagnosis,” said Dr. Bertagnolio.
This article was translated from Medscape’s Spanish edition and a version appeared on Medscape.com.
New Medicare rule streamlines prior authorization in Medicare Advantage plans
A new federal rule seeks to reduce Medicare Advantage insurance plans’ prior authorization burdens on physicians while also ensuring that enrollees have the same access to necessary care that they would receive under traditional fee-for-service Medicare.
The prior authorization changes, announced this week, are part of the Centers for Medicare & Medicaid Services’ 2024 update of policy changes for Medicare Advantage and Part D pharmacy plans
Medicare Advantage plans’ business practices have raised significant concerns in recent years. More than 28 million Americans were enrolled in a Medicare Advantage plan in 2022, which is nearly half of all Medicare enrollees, according to the Kaiser Family Foundation.
Medicare pays a fixed amount per enrollee per year to these privately run managed care plans, in contrast to traditional fee-for-service Medicare. Medicare Advantage plans have been criticized for aggressive marketing, for overbilling the federal government for care, and for using prior authorization to inappropriately deny needed care to patients.
About 13% of prior authorization requests that are denied by Medicare Advantage plans actually met Medicare coverage rules and should have been approved, the Office of the Inspector General at the U.S. Department of Health & Human Services reported in 2022.
The newly finalized rule now requires Medicare Advantage plans to do the following.
- Ensure that a prior authorization approval, once granted, remains valid for as long as medically necessary to avoid disruptions in care.
- Conduct an annual review of utilization management policies.
- Ensure that coverage denials based on medical necessity be reviewed by health care professionals with relevant expertise before a denial can be issued.
Physician groups welcomed the changes. In a statement, the American Medical Association said that an initial reading of the rule suggested CMS had “taken important steps toward right-sizing the prior authorization process.”
The Medical Group Management Association praised CMS in a statement for having limited “dangerous disruptions and delays to necessary patient care” resulting from the cumbersome processes of prior approval. With the new rules, CMS will provide greater consistency across Advantage plans as well as traditional Medicare, said Anders Gilberg, MGMA’s senior vice president of government affairs, in a statement.
Peer consideration
The final rule did disappoint physician groups in one key way. CMS rebuffed requests to have CMS require Advantage plans to use reviewers of the same specialty as treating physicians in handling disputes about prior authorization. CMS said it expects plans to exercise judgment in finding reviewers with “sufficient expertise to make an informed and supportable decision.”
“In some instances, we expect that plans will use a physician or other health care professional of the same specialty or subspecialty as the treating physician,” CMS said. “In other instances, we expect that plans will utilize a reviewer with specialized training, certification, or clinical experience in the applicable field of medicine.”
Medicare Advantage marketing ‘sowing confusion’
With this final rule, CMS also sought to protect consumers from “potentially misleading marketing practices” used in promoting Medicare Advantage and Part D prescription drug plans.
The agency said it had received complaints about people who have received official-looking promotional materials for Medicare that directed them not to government sources of information but to Medicare Advantage and Part D plans or their agents and brokers.
Ads now must mention a specific plan name, and they cannot use the Medicare name, CMS logo, Medicare card, or other government information in a misleading way, CMS said.
“CMS can see no value or purpose in a non-governmental entity’s use of the Medicare logo or HHS logo except for the express purpose of sowing confusion and misrepresenting itself as the government,” the agency said.
A version of this article first appeared on Medscape.com.
A new federal rule seeks to reduce Medicare Advantage insurance plans’ prior authorization burdens on physicians while also ensuring that enrollees have the same access to necessary care that they would receive under traditional fee-for-service Medicare.
The prior authorization changes, announced this week, are part of the Centers for Medicare & Medicaid Services’ 2024 update of policy changes for Medicare Advantage and Part D pharmacy plans
Medicare Advantage plans’ business practices have raised significant concerns in recent years. More than 28 million Americans were enrolled in a Medicare Advantage plan in 2022, which is nearly half of all Medicare enrollees, according to the Kaiser Family Foundation.
Medicare pays a fixed amount per enrollee per year to these privately run managed care plans, in contrast to traditional fee-for-service Medicare. Medicare Advantage plans have been criticized for aggressive marketing, for overbilling the federal government for care, and for using prior authorization to inappropriately deny needed care to patients.
About 13% of prior authorization requests that are denied by Medicare Advantage plans actually met Medicare coverage rules and should have been approved, the Office of the Inspector General at the U.S. Department of Health & Human Services reported in 2022.
The newly finalized rule now requires Medicare Advantage plans to do the following.
- Ensure that a prior authorization approval, once granted, remains valid for as long as medically necessary to avoid disruptions in care.
- Conduct an annual review of utilization management policies.
- Ensure that coverage denials based on medical necessity be reviewed by health care professionals with relevant expertise before a denial can be issued.
Physician groups welcomed the changes. In a statement, the American Medical Association said that an initial reading of the rule suggested CMS had “taken important steps toward right-sizing the prior authorization process.”
The Medical Group Management Association praised CMS in a statement for having limited “dangerous disruptions and delays to necessary patient care” resulting from the cumbersome processes of prior approval. With the new rules, CMS will provide greater consistency across Advantage plans as well as traditional Medicare, said Anders Gilberg, MGMA’s senior vice president of government affairs, in a statement.
Peer consideration
The final rule did disappoint physician groups in one key way. CMS rebuffed requests to have CMS require Advantage plans to use reviewers of the same specialty as treating physicians in handling disputes about prior authorization. CMS said it expects plans to exercise judgment in finding reviewers with “sufficient expertise to make an informed and supportable decision.”
“In some instances, we expect that plans will use a physician or other health care professional of the same specialty or subspecialty as the treating physician,” CMS said. “In other instances, we expect that plans will utilize a reviewer with specialized training, certification, or clinical experience in the applicable field of medicine.”
Medicare Advantage marketing ‘sowing confusion’
With this final rule, CMS also sought to protect consumers from “potentially misleading marketing practices” used in promoting Medicare Advantage and Part D prescription drug plans.
The agency said it had received complaints about people who have received official-looking promotional materials for Medicare that directed them not to government sources of information but to Medicare Advantage and Part D plans or their agents and brokers.
Ads now must mention a specific plan name, and they cannot use the Medicare name, CMS logo, Medicare card, or other government information in a misleading way, CMS said.
“CMS can see no value or purpose in a non-governmental entity’s use of the Medicare logo or HHS logo except for the express purpose of sowing confusion and misrepresenting itself as the government,” the agency said.
A version of this article first appeared on Medscape.com.
A new federal rule seeks to reduce Medicare Advantage insurance plans’ prior authorization burdens on physicians while also ensuring that enrollees have the same access to necessary care that they would receive under traditional fee-for-service Medicare.
The prior authorization changes, announced this week, are part of the Centers for Medicare & Medicaid Services’ 2024 update of policy changes for Medicare Advantage and Part D pharmacy plans
Medicare Advantage plans’ business practices have raised significant concerns in recent years. More than 28 million Americans were enrolled in a Medicare Advantage plan in 2022, which is nearly half of all Medicare enrollees, according to the Kaiser Family Foundation.
Medicare pays a fixed amount per enrollee per year to these privately run managed care plans, in contrast to traditional fee-for-service Medicare. Medicare Advantage plans have been criticized for aggressive marketing, for overbilling the federal government for care, and for using prior authorization to inappropriately deny needed care to patients.
About 13% of prior authorization requests that are denied by Medicare Advantage plans actually met Medicare coverage rules and should have been approved, the Office of the Inspector General at the U.S. Department of Health & Human Services reported in 2022.
The newly finalized rule now requires Medicare Advantage plans to do the following.
- Ensure that a prior authorization approval, once granted, remains valid for as long as medically necessary to avoid disruptions in care.
- Conduct an annual review of utilization management policies.
- Ensure that coverage denials based on medical necessity be reviewed by health care professionals with relevant expertise before a denial can be issued.
Physician groups welcomed the changes. In a statement, the American Medical Association said that an initial reading of the rule suggested CMS had “taken important steps toward right-sizing the prior authorization process.”
The Medical Group Management Association praised CMS in a statement for having limited “dangerous disruptions and delays to necessary patient care” resulting from the cumbersome processes of prior approval. With the new rules, CMS will provide greater consistency across Advantage plans as well as traditional Medicare, said Anders Gilberg, MGMA’s senior vice president of government affairs, in a statement.
Peer consideration
The final rule did disappoint physician groups in one key way. CMS rebuffed requests to have CMS require Advantage plans to use reviewers of the same specialty as treating physicians in handling disputes about prior authorization. CMS said it expects plans to exercise judgment in finding reviewers with “sufficient expertise to make an informed and supportable decision.”
“In some instances, we expect that plans will use a physician or other health care professional of the same specialty or subspecialty as the treating physician,” CMS said. “In other instances, we expect that plans will utilize a reviewer with specialized training, certification, or clinical experience in the applicable field of medicine.”
Medicare Advantage marketing ‘sowing confusion’
With this final rule, CMS also sought to protect consumers from “potentially misleading marketing practices” used in promoting Medicare Advantage and Part D prescription drug plans.
The agency said it had received complaints about people who have received official-looking promotional materials for Medicare that directed them not to government sources of information but to Medicare Advantage and Part D plans or their agents and brokers.
Ads now must mention a specific plan name, and they cannot use the Medicare name, CMS logo, Medicare card, or other government information in a misleading way, CMS said.
“CMS can see no value or purpose in a non-governmental entity’s use of the Medicare logo or HHS logo except for the express purpose of sowing confusion and misrepresenting itself as the government,” the agency said.
A version of this article first appeared on Medscape.com.
Why my independent GI practice started a GI fellowship program
In this video Naresh Gunaratnam, MD, discusses the gastroenterology fellowship program that Huron Gastroenterology developed with Trinity Health in Ann Arbor, Mich. Dr. Gunaratnam helped create the program because he and his colleagues felt that traditional fellowship programs don’t always provide information or guidance about non-academic career pathways in gastroenterology. Hear from Dr. Gunaratnam how the fellowship program at Huron Gastroenterology is training fellows to become excellent clinicians who care for patients in the community setting. He has no financial conflicts relative to the topics in this video.

In this video Naresh Gunaratnam, MD, discusses the gastroenterology fellowship program that Huron Gastroenterology developed with Trinity Health in Ann Arbor, Mich. Dr. Gunaratnam helped create the program because he and his colleagues felt that traditional fellowship programs don’t always provide information or guidance about non-academic career pathways in gastroenterology. Hear from Dr. Gunaratnam how the fellowship program at Huron Gastroenterology is training fellows to become excellent clinicians who care for patients in the community setting. He has no financial conflicts relative to the topics in this video.

In this video Naresh Gunaratnam, MD, discusses the gastroenterology fellowship program that Huron Gastroenterology developed with Trinity Health in Ann Arbor, Mich. Dr. Gunaratnam helped create the program because he and his colleagues felt that traditional fellowship programs don’t always provide information or guidance about non-academic career pathways in gastroenterology. Hear from Dr. Gunaratnam how the fellowship program at Huron Gastroenterology is training fellows to become excellent clinicians who care for patients in the community setting. He has no financial conflicts relative to the topics in this video.

TikTok offers to ‘balance your hormones’ are pure hokum
With more than 306 million views, #hormonebalance and #hormonebalancing are among the latest hacks to take over the social media platform TikTok, on which users post short videos. Influencers offer advice such as eating raw carrots for “happy hormones,” eating protein followed by fat for breakfast to regulate blood glucose, or taking vitamin B2 supplements for thyroid health.
Have you ever wondered if you were asleep during the lecture on “hormone balancing” in medical school? No, you weren’t. It was never a class for good reason, and you didn’t fail to read any such breakthrough studies in The New England Journal of Medicine either.
There are over 50 different hormones produced by humans and animals, regulating sleep, growth, metabolism and reproduction, among many other biological processes, so there is certainly no one-size-fits-all solution to ensure these are all working in perfect harmony.
When someone mentions “hormone balancing,” my mind wanders to the last time I took my car to have my tires rotated and balanced. If only it were as simple to balance hormones in real life. The best we can hope for is to get a specific hormone within the ideal physiologic range for that person’s age.
The term “hormone” can mean many things to different people. When a woman comes in with a hormone question, for example, it is often related to estrogen, followed by thyroid hormones. A wealth of misinformation exists in popular literature regarding these hormones alone.
Estrogen can be replaced, but not everyone needs it replaced. It depends on variables including age, underlying medical conditions, the time of day a test was drawn, and concomitant medications. Having low levels of a given hormone does not necessarily call for replacement either.
Insulin is another example of a hormone that can never completely be replaced in people with diabetes in a way that exactly mimics the normal physiologic release.
There are many lesser-known hormones that are measurable and replaceable but are also more difficult to reset to original manufacturer specifications.
A Google search for “hormone balancing” often sends you to “naturopaths” or “integrative medicine” practitioners, who often propose similar solutions to the TikTok influencers. Users are told that their hormones are out of whack and that restoring this “balance” can be achieved by purchasing whatever “natural products” or concoction they are selling.
These TikTok videos and online “experts” are the home-brewed versions of the strip-mall hormone specialists. TikTok videos claiming to help “balance hormones” typically don’t name a specific hormone either, or the end organs that each would have an impact on. Rather, they lump all hormones into a monolithic entity, implying that there is a single solution for all health problems. And personal testimonials extolling the benefits of a TikTok intervention don’t constitute proof of efficacy no matter how many “likes” they get. These influencers assume that viewers can “sense” their hormones are out of tune and no lab tests can convince them otherwise.
In these inflationary times, the cost of seeking medical care from conventional channels is increasingly prohibitive. It’s easy to understand the appeal of getting free advice from TikTok or some other Internet site. At best, following the advice will not have much impact; at worst, it could be harmful.
Don’t try this at home
There are some things that should never be tried at home, and do-it-yourself hormone replacement or remediation both fall under this umbrella.
Generally, the body does a good job of balancing its own hormones. Most patients don’t need to be worried if they’re in good health. If they’re in doubt, they should seek advice from a doctor, ideally an endocrinologist, but an ob.gyn. or general practitioner are also good options.
One of the first questions to ask a patient is “Which hormone are you worried about?” or “What health issue is it specifically that is bothering you?” Narrowing the focus to a single thing, if possible, will lead to a more efficient evaluation.
Often, patients arrive with multiple concerns written on little pieces of paper. These ubiquitous pieces of paper are the red flag for the flood of questions to follow.
Ordering the appropriate tests for the conditions they are concerned about can help put their minds at ease. If there are any specific deficiencies, or excesses in any hormones, then appropriate solutions can be discussed.
TikTok hormone-balancing solutions are simply the 21st-century version of the snake oil sold on late-night cable TV in the 1990s.
Needless to say, you should gently encourage your patients to stay away from these non–FDA-approved products, without making them feel stupid. Off-label use of hormones when these are not indicated is also to be avoided, unless a medical practitioner feels it is warranted.
Dr. de la Rosa is an endocrinologist in Englewood, Fla. He disclosed no conflicts of interest.
A version of this article first appeared on Medscape.com.
With more than 306 million views, #hormonebalance and #hormonebalancing are among the latest hacks to take over the social media platform TikTok, on which users post short videos. Influencers offer advice such as eating raw carrots for “happy hormones,” eating protein followed by fat for breakfast to regulate blood glucose, or taking vitamin B2 supplements for thyroid health.
Have you ever wondered if you were asleep during the lecture on “hormone balancing” in medical school? No, you weren’t. It was never a class for good reason, and you didn’t fail to read any such breakthrough studies in The New England Journal of Medicine either.
There are over 50 different hormones produced by humans and animals, regulating sleep, growth, metabolism and reproduction, among many other biological processes, so there is certainly no one-size-fits-all solution to ensure these are all working in perfect harmony.
When someone mentions “hormone balancing,” my mind wanders to the last time I took my car to have my tires rotated and balanced. If only it were as simple to balance hormones in real life. The best we can hope for is to get a specific hormone within the ideal physiologic range for that person’s age.
The term “hormone” can mean many things to different people. When a woman comes in with a hormone question, for example, it is often related to estrogen, followed by thyroid hormones. A wealth of misinformation exists in popular literature regarding these hormones alone.
Estrogen can be replaced, but not everyone needs it replaced. It depends on variables including age, underlying medical conditions, the time of day a test was drawn, and concomitant medications. Having low levels of a given hormone does not necessarily call for replacement either.
Insulin is another example of a hormone that can never completely be replaced in people with diabetes in a way that exactly mimics the normal physiologic release.
There are many lesser-known hormones that are measurable and replaceable but are also more difficult to reset to original manufacturer specifications.
A Google search for “hormone balancing” often sends you to “naturopaths” or “integrative medicine” practitioners, who often propose similar solutions to the TikTok influencers. Users are told that their hormones are out of whack and that restoring this “balance” can be achieved by purchasing whatever “natural products” or concoction they are selling.
These TikTok videos and online “experts” are the home-brewed versions of the strip-mall hormone specialists. TikTok videos claiming to help “balance hormones” typically don’t name a specific hormone either, or the end organs that each would have an impact on. Rather, they lump all hormones into a monolithic entity, implying that there is a single solution for all health problems. And personal testimonials extolling the benefits of a TikTok intervention don’t constitute proof of efficacy no matter how many “likes” they get. These influencers assume that viewers can “sense” their hormones are out of tune and no lab tests can convince them otherwise.
In these inflationary times, the cost of seeking medical care from conventional channels is increasingly prohibitive. It’s easy to understand the appeal of getting free advice from TikTok or some other Internet site. At best, following the advice will not have much impact; at worst, it could be harmful.
Don’t try this at home
There are some things that should never be tried at home, and do-it-yourself hormone replacement or remediation both fall under this umbrella.
Generally, the body does a good job of balancing its own hormones. Most patients don’t need to be worried if they’re in good health. If they’re in doubt, they should seek advice from a doctor, ideally an endocrinologist, but an ob.gyn. or general practitioner are also good options.
One of the first questions to ask a patient is “Which hormone are you worried about?” or “What health issue is it specifically that is bothering you?” Narrowing the focus to a single thing, if possible, will lead to a more efficient evaluation.
Often, patients arrive with multiple concerns written on little pieces of paper. These ubiquitous pieces of paper are the red flag for the flood of questions to follow.
Ordering the appropriate tests for the conditions they are concerned about can help put their minds at ease. If there are any specific deficiencies, or excesses in any hormones, then appropriate solutions can be discussed.
TikTok hormone-balancing solutions are simply the 21st-century version of the snake oil sold on late-night cable TV in the 1990s.
Needless to say, you should gently encourage your patients to stay away from these non–FDA-approved products, without making them feel stupid. Off-label use of hormones when these are not indicated is also to be avoided, unless a medical practitioner feels it is warranted.
Dr. de la Rosa is an endocrinologist in Englewood, Fla. He disclosed no conflicts of interest.
A version of this article first appeared on Medscape.com.
With more than 306 million views, #hormonebalance and #hormonebalancing are among the latest hacks to take over the social media platform TikTok, on which users post short videos. Influencers offer advice such as eating raw carrots for “happy hormones,” eating protein followed by fat for breakfast to regulate blood glucose, or taking vitamin B2 supplements for thyroid health.
Have you ever wondered if you were asleep during the lecture on “hormone balancing” in medical school? No, you weren’t. It was never a class for good reason, and you didn’t fail to read any such breakthrough studies in The New England Journal of Medicine either.
There are over 50 different hormones produced by humans and animals, regulating sleep, growth, metabolism and reproduction, among many other biological processes, so there is certainly no one-size-fits-all solution to ensure these are all working in perfect harmony.
When someone mentions “hormone balancing,” my mind wanders to the last time I took my car to have my tires rotated and balanced. If only it were as simple to balance hormones in real life. The best we can hope for is to get a specific hormone within the ideal physiologic range for that person’s age.
The term “hormone” can mean many things to different people. When a woman comes in with a hormone question, for example, it is often related to estrogen, followed by thyroid hormones. A wealth of misinformation exists in popular literature regarding these hormones alone.
Estrogen can be replaced, but not everyone needs it replaced. It depends on variables including age, underlying medical conditions, the time of day a test was drawn, and concomitant medications. Having low levels of a given hormone does not necessarily call for replacement either.
Insulin is another example of a hormone that can never completely be replaced in people with diabetes in a way that exactly mimics the normal physiologic release.
There are many lesser-known hormones that are measurable and replaceable but are also more difficult to reset to original manufacturer specifications.
A Google search for “hormone balancing” often sends you to “naturopaths” or “integrative medicine” practitioners, who often propose similar solutions to the TikTok influencers. Users are told that their hormones are out of whack and that restoring this “balance” can be achieved by purchasing whatever “natural products” or concoction they are selling.
These TikTok videos and online “experts” are the home-brewed versions of the strip-mall hormone specialists. TikTok videos claiming to help “balance hormones” typically don’t name a specific hormone either, or the end organs that each would have an impact on. Rather, they lump all hormones into a monolithic entity, implying that there is a single solution for all health problems. And personal testimonials extolling the benefits of a TikTok intervention don’t constitute proof of efficacy no matter how many “likes” they get. These influencers assume that viewers can “sense” their hormones are out of tune and no lab tests can convince them otherwise.
In these inflationary times, the cost of seeking medical care from conventional channels is increasingly prohibitive. It’s easy to understand the appeal of getting free advice from TikTok or some other Internet site. At best, following the advice will not have much impact; at worst, it could be harmful.
Don’t try this at home
There are some things that should never be tried at home, and do-it-yourself hormone replacement or remediation both fall under this umbrella.
Generally, the body does a good job of balancing its own hormones. Most patients don’t need to be worried if they’re in good health. If they’re in doubt, they should seek advice from a doctor, ideally an endocrinologist, but an ob.gyn. or general practitioner are also good options.
One of the first questions to ask a patient is “Which hormone are you worried about?” or “What health issue is it specifically that is bothering you?” Narrowing the focus to a single thing, if possible, will lead to a more efficient evaluation.
Often, patients arrive with multiple concerns written on little pieces of paper. These ubiquitous pieces of paper are the red flag for the flood of questions to follow.
Ordering the appropriate tests for the conditions they are concerned about can help put their minds at ease. If there are any specific deficiencies, or excesses in any hormones, then appropriate solutions can be discussed.
TikTok hormone-balancing solutions are simply the 21st-century version of the snake oil sold on late-night cable TV in the 1990s.
Needless to say, you should gently encourage your patients to stay away from these non–FDA-approved products, without making them feel stupid. Off-label use of hormones when these are not indicated is also to be avoided, unless a medical practitioner feels it is warranted.
Dr. de la Rosa is an endocrinologist in Englewood, Fla. He disclosed no conflicts of interest.
A version of this article first appeared on Medscape.com.
Does exercise help or hinder GERD?
PARIS – Frank Zerbib, MD, head of the department of gastroenterology at Bordeaux (France) University Hospital, broke them down during a session dedicated to exercise, which was the common theme of the JFHOD 2023, a French-speaking hepato-gastroenterology and digestive oncology conference held this year in Paris.
A contributing factor
Several factors can affect how exercise causes gastroesophageal reflux.
“Vigorous,” or mainly sports-related, exercise has a detrimental effect on GERD. Approximately 60% of athletes are said to report GERD symptoms connected to an increase in abdominal pressure. This is not because of obesity, but because of the abdominal contraction that occurs during exercise.
Other pathophysiological factors at the root of exercise-induced GERD can be involved in this phenomenon, namely a decrease in lower esophageal sphincter (LES) pressure and esophageal motility, in addition to phases of dissociation between the LES and the diaphragm, which is when most GERD episodes occur.
In such contexts, “it would appear that sports-related exercise has a relatively detrimental effect on the gastroesophageal junction and anti-GERD mechanisms,” said Dr. Zerbib. Meta-analyses provide answers to some questions, but not all; the situation is much less clear when it comes to non–sports-related exercise.”
Not so simple
“Taking into account only patients whose GERD has been confirmed through esophageal pH monitoring, exercise does not appear to significantly impact GERD symptoms or the characteristics seen on pH monitoring,” said Dr. Zerbib.
These results come from a study of 100 patients whose exercise level was assessed using the International Physical Activity Questionnaire and expressed using the standard metric of metabolic rate by minutes of performance during a week (METs-minute/week).
This questionnaire is used for most studies that assess exercise and separates patients into three groups (low, moderate, or high) based on their level of exercise. In essence, it considers the duration of exercise but not the type (that is, professional, recreational, and so on) or intensity, resulting in a key methodological issue to consider during the analysis, for example, of the results of a large meta-analysis on the topic.
The meta-analysis in question included 78,000 patients, of whom 10,000 had GERD symptoms.
Based on the results, exercise decreases the risk of GERD by about one-third, after adjustment for body mass index (BMI). “This last point is important,” Dr. Zerbib noted, “since adjusting for BMI without providing the nonadjusted data fails to identify whether exercise decreases the risk of GERD because of the effect on the BMI.* What’s more, when it comes to complications of GERD, like Barrett’s esophagus or adenocarcinoma, the data are far fewer and less robust, with negative case-control studies for the most part.”
One of these two studies, which concerned non–sports-related exercise and the onset of Barrett’s esophagus, reported no association (odds ratio, 1.19; 95% confidence interval, 0.82-1.73).
“Exercise considered vigorous (sports-related) contributes to GERD by altering the antireflux barrier (LES/diaphragm dissociation) and increasing constraints on the esophageal junction (abdominal pressure). In the general population, regular exercise likely decreases the risk of pathological GERD. When it comes to complications of GERD, the data are not very robust, mostly because the studies omitted several exercise-related (healthy lifestyle) factors,” said Dr. Zerbib.
Several confounding factors
It’s difficult to issue an opinion under these conditions. There are several confounding factors that studies rarely address. Although the studies always included factors such as age, sex, or BMI, other parameters related to a healthy lifestyle, whether directly or indirectly connected to exercise, were never mentioned. Indeed, diet (such as high calorie or high fat) is known to lead to an increased incidence of GERD. The same goes for alcohol use. Occupation also likely plays a role, but the studies do not mention this.
“So, it’s easy to imagine that a patient who regularly exercises likely eats healthier than a sedentary patient, which comes with the likelihood of a lower risk of developing GERD symptoms,” said Dr. Zerbib. “Overall, evaluating the impact of exercise on GERD is no small feat. It can be said with relative certainty that exercise contributes to GERD through a proven pathophysiology. In the general population, however, exercise likely reduces the risk of GERD but not of its complications. Other than the impact on weight and abdominal obesity, the reality is that a lack of exercise is associated with a less healthy lifestyle and, therefore, behaviors that contribute to GERD.”
Dr. Zerbib reported no conflicts of interest connected to this presentation.
* From a pathophysiological standpoint, the evidence is clear that a high BMI increases the gastroesophageal pressure gradient and dissociation between the LES and the diaphragm, whether temporarily or permanently, as in the case of a hiatal hernia. Abdominal obesity increases constraints on the gastroesophageal junction and results in a two- to threefold increase in the risk of GERD and its complications.
This article was translated from the Medscape French Edition. A version appeared on Medscape.com.
PARIS – Frank Zerbib, MD, head of the department of gastroenterology at Bordeaux (France) University Hospital, broke them down during a session dedicated to exercise, which was the common theme of the JFHOD 2023, a French-speaking hepato-gastroenterology and digestive oncology conference held this year in Paris.
A contributing factor
Several factors can affect how exercise causes gastroesophageal reflux.
“Vigorous,” or mainly sports-related, exercise has a detrimental effect on GERD. Approximately 60% of athletes are said to report GERD symptoms connected to an increase in abdominal pressure. This is not because of obesity, but because of the abdominal contraction that occurs during exercise.
Other pathophysiological factors at the root of exercise-induced GERD can be involved in this phenomenon, namely a decrease in lower esophageal sphincter (LES) pressure and esophageal motility, in addition to phases of dissociation between the LES and the diaphragm, which is when most GERD episodes occur.
In such contexts, “it would appear that sports-related exercise has a relatively detrimental effect on the gastroesophageal junction and anti-GERD mechanisms,” said Dr. Zerbib. Meta-analyses provide answers to some questions, but not all; the situation is much less clear when it comes to non–sports-related exercise.”
Not so simple
“Taking into account only patients whose GERD has been confirmed through esophageal pH monitoring, exercise does not appear to significantly impact GERD symptoms or the characteristics seen on pH monitoring,” said Dr. Zerbib.
These results come from a study of 100 patients whose exercise level was assessed using the International Physical Activity Questionnaire and expressed using the standard metric of metabolic rate by minutes of performance during a week (METs-minute/week).
This questionnaire is used for most studies that assess exercise and separates patients into three groups (low, moderate, or high) based on their level of exercise. In essence, it considers the duration of exercise but not the type (that is, professional, recreational, and so on) or intensity, resulting in a key methodological issue to consider during the analysis, for example, of the results of a large meta-analysis on the topic.
The meta-analysis in question included 78,000 patients, of whom 10,000 had GERD symptoms.
Based on the results, exercise decreases the risk of GERD by about one-third, after adjustment for body mass index (BMI). “This last point is important,” Dr. Zerbib noted, “since adjusting for BMI without providing the nonadjusted data fails to identify whether exercise decreases the risk of GERD because of the effect on the BMI.* What’s more, when it comes to complications of GERD, like Barrett’s esophagus or adenocarcinoma, the data are far fewer and less robust, with negative case-control studies for the most part.”
One of these two studies, which concerned non–sports-related exercise and the onset of Barrett’s esophagus, reported no association (odds ratio, 1.19; 95% confidence interval, 0.82-1.73).
“Exercise considered vigorous (sports-related) contributes to GERD by altering the antireflux barrier (LES/diaphragm dissociation) and increasing constraints on the esophageal junction (abdominal pressure). In the general population, regular exercise likely decreases the risk of pathological GERD. When it comes to complications of GERD, the data are not very robust, mostly because the studies omitted several exercise-related (healthy lifestyle) factors,” said Dr. Zerbib.
Several confounding factors
It’s difficult to issue an opinion under these conditions. There are several confounding factors that studies rarely address. Although the studies always included factors such as age, sex, or BMI, other parameters related to a healthy lifestyle, whether directly or indirectly connected to exercise, were never mentioned. Indeed, diet (such as high calorie or high fat) is known to lead to an increased incidence of GERD. The same goes for alcohol use. Occupation also likely plays a role, but the studies do not mention this.
“So, it’s easy to imagine that a patient who regularly exercises likely eats healthier than a sedentary patient, which comes with the likelihood of a lower risk of developing GERD symptoms,” said Dr. Zerbib. “Overall, evaluating the impact of exercise on GERD is no small feat. It can be said with relative certainty that exercise contributes to GERD through a proven pathophysiology. In the general population, however, exercise likely reduces the risk of GERD but not of its complications. Other than the impact on weight and abdominal obesity, the reality is that a lack of exercise is associated with a less healthy lifestyle and, therefore, behaviors that contribute to GERD.”
Dr. Zerbib reported no conflicts of interest connected to this presentation.
* From a pathophysiological standpoint, the evidence is clear that a high BMI increases the gastroesophageal pressure gradient and dissociation between the LES and the diaphragm, whether temporarily or permanently, as in the case of a hiatal hernia. Abdominal obesity increases constraints on the gastroesophageal junction and results in a two- to threefold increase in the risk of GERD and its complications.
This article was translated from the Medscape French Edition. A version appeared on Medscape.com.
PARIS – Frank Zerbib, MD, head of the department of gastroenterology at Bordeaux (France) University Hospital, broke them down during a session dedicated to exercise, which was the common theme of the JFHOD 2023, a French-speaking hepato-gastroenterology and digestive oncology conference held this year in Paris.
A contributing factor
Several factors can affect how exercise causes gastroesophageal reflux.
“Vigorous,” or mainly sports-related, exercise has a detrimental effect on GERD. Approximately 60% of athletes are said to report GERD symptoms connected to an increase in abdominal pressure. This is not because of obesity, but because of the abdominal contraction that occurs during exercise.
Other pathophysiological factors at the root of exercise-induced GERD can be involved in this phenomenon, namely a decrease in lower esophageal sphincter (LES) pressure and esophageal motility, in addition to phases of dissociation between the LES and the diaphragm, which is when most GERD episodes occur.
In such contexts, “it would appear that sports-related exercise has a relatively detrimental effect on the gastroesophageal junction and anti-GERD mechanisms,” said Dr. Zerbib. Meta-analyses provide answers to some questions, but not all; the situation is much less clear when it comes to non–sports-related exercise.”
Not so simple
“Taking into account only patients whose GERD has been confirmed through esophageal pH monitoring, exercise does not appear to significantly impact GERD symptoms or the characteristics seen on pH monitoring,” said Dr. Zerbib.
These results come from a study of 100 patients whose exercise level was assessed using the International Physical Activity Questionnaire and expressed using the standard metric of metabolic rate by minutes of performance during a week (METs-minute/week).
This questionnaire is used for most studies that assess exercise and separates patients into three groups (low, moderate, or high) based on their level of exercise. In essence, it considers the duration of exercise but not the type (that is, professional, recreational, and so on) or intensity, resulting in a key methodological issue to consider during the analysis, for example, of the results of a large meta-analysis on the topic.
The meta-analysis in question included 78,000 patients, of whom 10,000 had GERD symptoms.
Based on the results, exercise decreases the risk of GERD by about one-third, after adjustment for body mass index (BMI). “This last point is important,” Dr. Zerbib noted, “since adjusting for BMI without providing the nonadjusted data fails to identify whether exercise decreases the risk of GERD because of the effect on the BMI.* What’s more, when it comes to complications of GERD, like Barrett’s esophagus or adenocarcinoma, the data are far fewer and less robust, with negative case-control studies for the most part.”
One of these two studies, which concerned non–sports-related exercise and the onset of Barrett’s esophagus, reported no association (odds ratio, 1.19; 95% confidence interval, 0.82-1.73).
“Exercise considered vigorous (sports-related) contributes to GERD by altering the antireflux barrier (LES/diaphragm dissociation) and increasing constraints on the esophageal junction (abdominal pressure). In the general population, regular exercise likely decreases the risk of pathological GERD. When it comes to complications of GERD, the data are not very robust, mostly because the studies omitted several exercise-related (healthy lifestyle) factors,” said Dr. Zerbib.
Several confounding factors
It’s difficult to issue an opinion under these conditions. There are several confounding factors that studies rarely address. Although the studies always included factors such as age, sex, or BMI, other parameters related to a healthy lifestyle, whether directly or indirectly connected to exercise, were never mentioned. Indeed, diet (such as high calorie or high fat) is known to lead to an increased incidence of GERD. The same goes for alcohol use. Occupation also likely plays a role, but the studies do not mention this.
“So, it’s easy to imagine that a patient who regularly exercises likely eats healthier than a sedentary patient, which comes with the likelihood of a lower risk of developing GERD symptoms,” said Dr. Zerbib. “Overall, evaluating the impact of exercise on GERD is no small feat. It can be said with relative certainty that exercise contributes to GERD through a proven pathophysiology. In the general population, however, exercise likely reduces the risk of GERD but not of its complications. Other than the impact on weight and abdominal obesity, the reality is that a lack of exercise is associated with a less healthy lifestyle and, therefore, behaviors that contribute to GERD.”
Dr. Zerbib reported no conflicts of interest connected to this presentation.
* From a pathophysiological standpoint, the evidence is clear that a high BMI increases the gastroesophageal pressure gradient and dissociation between the LES and the diaphragm, whether temporarily or permanently, as in the case of a hiatal hernia. Abdominal obesity increases constraints on the gastroesophageal junction and results in a two- to threefold increase in the risk of GERD and its complications.
This article was translated from the Medscape French Edition. A version appeared on Medscape.com.
Acute unilateral visual disturbance
A previously healthy 37-year-old runner presented to his primary care physician with acute-onset floaters and scotoma in his left eye, which he first noticed less than 24 hours earlier. He denied eye pain, diplopia, headache, fever, chills, slurred speech, weakness, or other focal neurologic deficits. His vital signs were normal.
Despite the acute visual disturbances, visual acuity was 20/20 in both eyes with corrective lenses; pupils were equal, round, and reactive to light and accommodation; and extraocular movements were intact. On a dilated funduscopic exam, the physician discovered edema of the optic cup, tortuous vasculature, and microhemorrhages in the left eye (FIGURE).
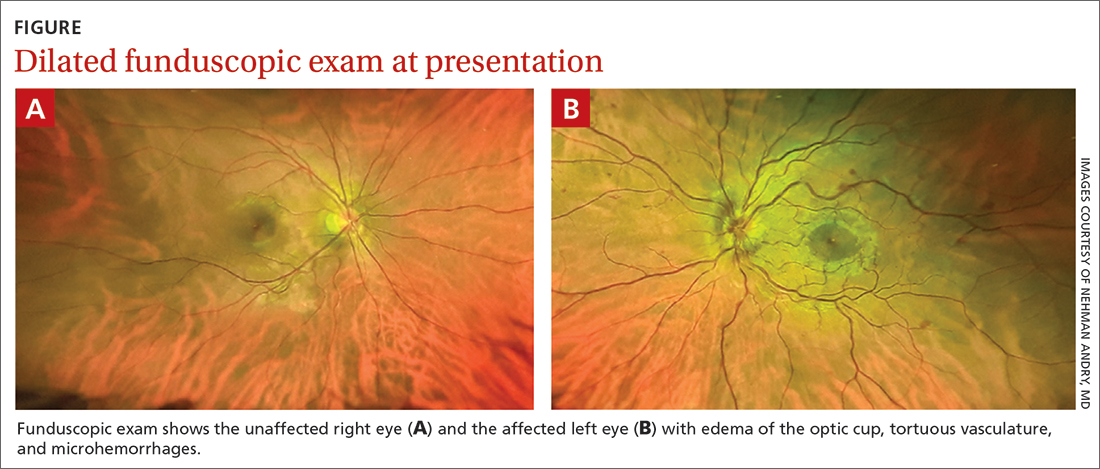
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Central retinal vein occlusion
The patient was given a diagnosis of central retinal vein occlusion (CRVO). In this condition, a blockage causes the central retinal vein to leak blood and excess fluid into the retina. This fluid can collect in the macula, leading to visual disturbance.
Retinal vein occlusion is the second most common retinal vascular disease in the United States and is one of the most common causes of vision loss in the elderly.1 Advancing age (≥ 70 years), increasing mean arterial blood pressure, and retinal atherosclerotic signs (focal narrowing, arteriovenous nicking, and opacification) are significant predictors of retinal vein occlusion.2 Other risk factors include diabetes, hyperlipidemia, cardiovascular disease, smoking, obesity, hypercoagulable state, and glaucoma.3-7 However, retinal vein occlusion may also occur in younger, healthier patients who lack the aforementioned risk factors. In such cases, thrombophilic risk factors should be considered.8
CRVO is classified as either ischemic or nonischemic (perfused) based on retinal angiography. More than 80% of CRVO cases are nonischemic,9 of which the majority has visual acuity better than 20/400, mild or no pupillary defect, and mild, unilateral visual changes.10 Nonischemic CRVO can progress to ischemic CRVO, which can result in permanent vision loss. Visual outcome is good in nonischemic CRVO and poor in ischemic CRVO.11 Early detection of poor prognostic features, such as macular edema and neovascularization, is essential for minimizing the risk for permanent damage.12
Dilated funduscopic exam of a patient with CRVO may reveal widespread retinal hemorrhages, markedly dilated and tortuous retinal vessels, cotton wool spots, optic disc or macular edema, and/or vitreous hemorrhages.10
Differential includes varied conditions that can affect vision
CRVO may manifest similarly to the following:
Proliferative diabetic retinopathy can manifest with retinal edema or vitreous and retinal hemorrhages, which also are seen in CRVO.13 Macular edema, retinal hemorrhage, and neovascularization on the optic disc or retinal surface also may be seen on funduscopy in proliferative diabetic retinopathy.14 However, proliferative diabetic retinopathy is often bilateral and gradual in onset in patients with longstanding, uncontrolled diabetes.
Continue to: Hyperviscosity retinopathy
Hyperviscosity retinopathy, which is commonly caused by plasma cell and erythrocyte disorders, also manifests similarly to CRVO. Two noticeable differences include its bilateral presentation and Roth spots, neither of which are commonly seen in CRVO. In addition to visual abnormalities, mucosal bleeding and neurologic abnormalities complete the classic triad of hyperviscosity.15
Ocular ischemic syndrome is often confused with diabetic retinopathies and CRVO on funduscopy. However, patients with this condition may have narrowed retinal arteries, perifoveal telangiectasias, and periorbital pain—findings rarely seen in CRVO.16 Because ocular ischemic syndrome is a manifestation of severe carotid artery atherosclerosis, constitutional symptoms also may be present.
The work-up
When CRVO is suspected, an extensive laboratory work-up is necessary to determine the underlying etiology, including: blood pressure, electrocardiogram, complete blood count, random glucose level, electrolytes, lipid panel, plasma protein electrophoresis, thyroid function tests, and inflammatory markers.1
Additional testing may be required for younger patients who lack vasculopathic risk factors, who have bilateral CRVO, or who have a personal or family history of thrombosis.1 These patients should be screened for thrombophilia, hypercoagulable disorders, and homocysteinuria.1
Cases of CRVO have been linked to dehydration as well, with acute vision changes occurring after strenuous exercise, excessive vomiting, or extended periods of fasting.17-19
Continue to: Treatment may include injections, surgery, or nothing at all
Treatment may include injections, surgery, or nothing at all
Currently, there are no proven treatments to reopen occluded retinal veins. Thus, management is directed at complications that contribute to vision loss, including macular edema and neovascularization.20-21 Intravitreal anti-vascular endothelial growth factor (VEGF) agents are recognized as first-line therapy for macular edema in numerous studies.22-26 Intravitreal corticosteroids are an alternative treatment for patients with macular edema who do not respond to anti-VEGF therapy; however, monitoring is required as these corticosteroids increase the risk for glaucoma and cataract formation.27 In patients with CRVO with neovascularization, panretinal laser photocoagulation may be used.28
Observation and monitoring for the development of complications, rather than initiation of treatment, is appropriate for patients with CRVO without macular edema or neovascularization, with follow-up intervals and duration dictated by the severity of visual loss and whether the CRVO was ischemic or nonischemic.
Our patient’s diagnosis was confirmed by retinal specialists with optic coherence tomography, gonioscopy, and fluorescein angiography. He underwent an extensive laboratory work-up and hypercoagulation studies to determine the etiology. All results returned within normal limits with the exception of a nonspecific pattern found on serum protein electrophoresis that suggested dehydration.
Given his negative hypercoagulation studies, normal laboratory values, and new exercise regimen, dehydration was concluded to be the likely etiology. Since his visual acuity was not affected, observation with bimonthly follow-up for 6 months was the management strategy. He was also encouraged to maintain adequate hydration during exercise. His vision returned to normal 2 weeks after the initial event, and he did not have recurrence during the monitoring period.
1. Woo SC, Lip GY, Lip PL. Associations of retinal artery occlusion and retinal vein occlusion to mortality, stroke, and myocardial infarction: a systematic review. Eye (Lond). 2016;30:1031-1038. doi: 10.1038/eye.2016.111
2. Cugati S, Wang JJ, Rochtchina E, et al. Ten-year incidence of retinal vein occlusion in an older population: the Blue Mountains Eye Study. Arch Ophthalmol. 2006;124:726. doi: 10.1001/archopht.124.5.726
3. O’Mahoney PR, Wong DT, Ray JG. Retinal vein occlusion and traditional risk factors for atherosclerosis. Arch Ophthalmol. 2008;126:692-699. doi: 10.1001/archopht.126.5.692
4. Hayreh SS, Zimmerman B, McCarthy MJ, et al. Systemic diseases associated with various types of retinal vein occlusion. Am J Ophthalmol. 2001;131:61-77. doi: 10.1016/s0002-9394(00)00709-1
5. Janssen MC, den Heijer M, Cruysberg JR, et al. Retinal vein occlusion: a form of venous thrombosis or a complication of atherosclerosis? A meta-analysis of thrombophilic factors. Thromb Haemost. 2005;93:1021-1026. doi: 10.1160/TH04-11-0768
6. Rehak M, Rehak J, Müller M, et al. The prevalence of activated protein C (APC) resistance and factor V Leiden is significantly higher in patients with retinal vein occlusion without general risk factors. Case-control study and meta-analysis. Thromb Haemost. 2008;99:925-929. doi: 10.1160/TH07-11-0658
7. Yin X, Li J, Zhang B, et al. Association of glaucoma with risk of retinal vein occlusion: a meta-analysis. Acta Ophthalmol. 2019;97:652-659. doi: 10.1111/aos.14141
8. Rehak M, Krcova V, Slavik L, et al. The role of thrombophilia in patients with retinal vein occlusion and no systemic risk factors. Can J Ophthalmol. 2010;45:171-175. doi: 10.3129/i09-273
9. Hayreh SS, Zimmerman MB, Podhajsky P. Incidence of various types of retinal vein occlusion and their recurrent and demographic characteristics. Am J Ophthalmol. 1994;117:429-441. doi: 10.1016/s0002-9394(14)70001-7
10. Hayreh SS, Klugman MR, Beri M, et al. Differentiation of ischemic from non-ischemic central retinal vein occlusion during the early acute phase. Graefes Arch Clin Exp Ophthalmol. 1990;228:201-217. doi: 10.1007/BF00920022
11. Hayreh SS, Podhajsky PA, Zimmerman MB. Natural history of visual outcome in central retinal vein occlusion. Ophthalmology. 2011;118:119-133. doi: 10.1016/j.ophtha.2010.04.019
12. Bakri SJ, Berrocal A, Capone A, et al. Retina health series: central retinal vein occlusion. American Society of Retina Specialists. January 2020. Accessed April 16, 2021. www.asrs.org/content/documents/fact-sheet-21-central-retinal-vein-occlusion-2020_1_asrs.pdf
13. Columbia University Department of Ophthalmology. Proliferative diabetic retinopathy (PDR). Accessed July 2, 2021. www.columbiaeye.org/education/digital-reference-of-ophthalmology/vitreous-retina/retinal-vascular-diseases/proliferative-diabetic-retinopathy-pdr
14. Mehta S. Diabetic retinopathy. Merck Manual Professional Version. Updated June 2021. Accessed July 11, 2021. www.merckmanuals.com/professional/eye-disorders/retinal-disorders/diabetic-retinopathy
15. Gertz MA. Acute hyperviscosity: syndromes and management. Blood 2018;132:1379-1385. doi: 10.1182/blood-2018-06-846816
16. Terelak-Borys B, Skonieczna K, Grabska-Liberek I. Ocular ischemic syndrome—a systematic review. Med Sci Monit. 2012;18: RA138-RA144. doi: 10.12659/msm.883260
17. Moisseiev E, Sagiv O, Lazar M. Intense exercise causing central retinal vein occlusion in a young patient: case report and review of the literature. Case Rep Ophthalmol. 2014;5:116-120. doi: 10.1159/000360904.
18. Weiss KD, Kuriyan AE, Flynn HW Jr. Central retinal vein occlusion after prolonged vomiting and repeated valsalva maneuvers associated with gastroenteritis and dehydration. Ophthalmic Surg Lasers Imaging Retina. 2014;45:e23-e25. doi: 10.3928/23258160-20140331-03
19. Jacobs DJ, Flynn HW, Pathengay A, et al. Central retinal vein occlusion after intense exercise: response to intravitreal bevacizumab. Ophthalmic Surg Lasers Imaging. 2011;42:e59-e62. doi: 10.3928/15428877-20110623-02
20. Mohamed Q, McIntosh RL, Saw SM, et al. Interventions for central retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2007;114:507-524. doi: 10.1016/j.ophtha. 2006.11.011
21. Berker N, Batman C. Surgical treatment of central retinal vein occlusion. Acta Ophthalmol. 2008;86:245-252. doi: 10.1111/j.1755-3768.2007.01144.x
22. Braithwaite T, Nanji AA, Greenberg PB. Anti-vascular endothelial growth factor for macular edema secondary to central retinal vein occlusion. Cochrane Database Syst Rev. 2010;10:CD007325. doi: 10.1002/14651858.CD007325.pub2
23. Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117:1124-1133. doi: 10.1016/j.ophtha.2010.02.022
24. Campochiaro PA, Brown DM, Awh CC, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology. 2011;118:2041-2049. doi: 10.1016/j.ophtha.2011. 02.038
25. Prasad AG, Schadlu R, Apte RS. Intravitreal pharmacotherapy: applications in retinal disease. Compr Ophthalmol Update. 2007; 8:259-269.
26. Brown DM, Heier JS, Clark WL, et al. Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. Am J Ophthalmol. 2013;155:429-437. doi: 10.1016/j.ajo.2012.09.026
27. Ip MS, Scott IU, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Arch Ophthalmol. 2009;127:1101-1114. doi: 10.1001/archophthalmol.2009.234
28. The Central Vein Occlusion Study Group. A randomized clinical trial of early panretinal photocoagulation for ischemic central vein occlusion. The Central Vein Occlusion Study Group N report. Ophthalmology. 1995;102:1434-1444.
A previously healthy 37-year-old runner presented to his primary care physician with acute-onset floaters and scotoma in his left eye, which he first noticed less than 24 hours earlier. He denied eye pain, diplopia, headache, fever, chills, slurred speech, weakness, or other focal neurologic deficits. His vital signs were normal.
Despite the acute visual disturbances, visual acuity was 20/20 in both eyes with corrective lenses; pupils were equal, round, and reactive to light and accommodation; and extraocular movements were intact. On a dilated funduscopic exam, the physician discovered edema of the optic cup, tortuous vasculature, and microhemorrhages in the left eye (FIGURE).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Central retinal vein occlusion
The patient was given a diagnosis of central retinal vein occlusion (CRVO). In this condition, a blockage causes the central retinal vein to leak blood and excess fluid into the retina. This fluid can collect in the macula, leading to visual disturbance.
Retinal vein occlusion is the second most common retinal vascular disease in the United States and is one of the most common causes of vision loss in the elderly.1 Advancing age (≥ 70 years), increasing mean arterial blood pressure, and retinal atherosclerotic signs (focal narrowing, arteriovenous nicking, and opacification) are significant predictors of retinal vein occlusion.2 Other risk factors include diabetes, hyperlipidemia, cardiovascular disease, smoking, obesity, hypercoagulable state, and glaucoma.3-7 However, retinal vein occlusion may also occur in younger, healthier patients who lack the aforementioned risk factors. In such cases, thrombophilic risk factors should be considered.8
CRVO is classified as either ischemic or nonischemic (perfused) based on retinal angiography. More than 80% of CRVO cases are nonischemic,9 of which the majority has visual acuity better than 20/400, mild or no pupillary defect, and mild, unilateral visual changes.10 Nonischemic CRVO can progress to ischemic CRVO, which can result in permanent vision loss. Visual outcome is good in nonischemic CRVO and poor in ischemic CRVO.11 Early detection of poor prognostic features, such as macular edema and neovascularization, is essential for minimizing the risk for permanent damage.12
Dilated funduscopic exam of a patient with CRVO may reveal widespread retinal hemorrhages, markedly dilated and tortuous retinal vessels, cotton wool spots, optic disc or macular edema, and/or vitreous hemorrhages.10
Differential includes varied conditions that can affect vision
CRVO may manifest similarly to the following:
Proliferative diabetic retinopathy can manifest with retinal edema or vitreous and retinal hemorrhages, which also are seen in CRVO.13 Macular edema, retinal hemorrhage, and neovascularization on the optic disc or retinal surface also may be seen on funduscopy in proliferative diabetic retinopathy.14 However, proliferative diabetic retinopathy is often bilateral and gradual in onset in patients with longstanding, uncontrolled diabetes.
Continue to: Hyperviscosity retinopathy
Hyperviscosity retinopathy, which is commonly caused by plasma cell and erythrocyte disorders, also manifests similarly to CRVO. Two noticeable differences include its bilateral presentation and Roth spots, neither of which are commonly seen in CRVO. In addition to visual abnormalities, mucosal bleeding and neurologic abnormalities complete the classic triad of hyperviscosity.15
Ocular ischemic syndrome is often confused with diabetic retinopathies and CRVO on funduscopy. However, patients with this condition may have narrowed retinal arteries, perifoveal telangiectasias, and periorbital pain—findings rarely seen in CRVO.16 Because ocular ischemic syndrome is a manifestation of severe carotid artery atherosclerosis, constitutional symptoms also may be present.
The work-up
When CRVO is suspected, an extensive laboratory work-up is necessary to determine the underlying etiology, including: blood pressure, electrocardiogram, complete blood count, random glucose level, electrolytes, lipid panel, plasma protein electrophoresis, thyroid function tests, and inflammatory markers.1
Additional testing may be required for younger patients who lack vasculopathic risk factors, who have bilateral CRVO, or who have a personal or family history of thrombosis.1 These patients should be screened for thrombophilia, hypercoagulable disorders, and homocysteinuria.1
Cases of CRVO have been linked to dehydration as well, with acute vision changes occurring after strenuous exercise, excessive vomiting, or extended periods of fasting.17-19
Continue to: Treatment may include injections, surgery, or nothing at all
Treatment may include injections, surgery, or nothing at all
Currently, there are no proven treatments to reopen occluded retinal veins. Thus, management is directed at complications that contribute to vision loss, including macular edema and neovascularization.20-21 Intravitreal anti-vascular endothelial growth factor (VEGF) agents are recognized as first-line therapy for macular edema in numerous studies.22-26 Intravitreal corticosteroids are an alternative treatment for patients with macular edema who do not respond to anti-VEGF therapy; however, monitoring is required as these corticosteroids increase the risk for glaucoma and cataract formation.27 In patients with CRVO with neovascularization, panretinal laser photocoagulation may be used.28
Observation and monitoring for the development of complications, rather than initiation of treatment, is appropriate for patients with CRVO without macular edema or neovascularization, with follow-up intervals and duration dictated by the severity of visual loss and whether the CRVO was ischemic or nonischemic.
Our patient’s diagnosis was confirmed by retinal specialists with optic coherence tomography, gonioscopy, and fluorescein angiography. He underwent an extensive laboratory work-up and hypercoagulation studies to determine the etiology. All results returned within normal limits with the exception of a nonspecific pattern found on serum protein electrophoresis that suggested dehydration.
Given his negative hypercoagulation studies, normal laboratory values, and new exercise regimen, dehydration was concluded to be the likely etiology. Since his visual acuity was not affected, observation with bimonthly follow-up for 6 months was the management strategy. He was also encouraged to maintain adequate hydration during exercise. His vision returned to normal 2 weeks after the initial event, and he did not have recurrence during the monitoring period.
A previously healthy 37-year-old runner presented to his primary care physician with acute-onset floaters and scotoma in his left eye, which he first noticed less than 24 hours earlier. He denied eye pain, diplopia, headache, fever, chills, slurred speech, weakness, or other focal neurologic deficits. His vital signs were normal.
Despite the acute visual disturbances, visual acuity was 20/20 in both eyes with corrective lenses; pupils were equal, round, and reactive to light and accommodation; and extraocular movements were intact. On a dilated funduscopic exam, the physician discovered edema of the optic cup, tortuous vasculature, and microhemorrhages in the left eye (FIGURE).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Central retinal vein occlusion
The patient was given a diagnosis of central retinal vein occlusion (CRVO). In this condition, a blockage causes the central retinal vein to leak blood and excess fluid into the retina. This fluid can collect in the macula, leading to visual disturbance.
Retinal vein occlusion is the second most common retinal vascular disease in the United States and is one of the most common causes of vision loss in the elderly.1 Advancing age (≥ 70 years), increasing mean arterial blood pressure, and retinal atherosclerotic signs (focal narrowing, arteriovenous nicking, and opacification) are significant predictors of retinal vein occlusion.2 Other risk factors include diabetes, hyperlipidemia, cardiovascular disease, smoking, obesity, hypercoagulable state, and glaucoma.3-7 However, retinal vein occlusion may also occur in younger, healthier patients who lack the aforementioned risk factors. In such cases, thrombophilic risk factors should be considered.8
CRVO is classified as either ischemic or nonischemic (perfused) based on retinal angiography. More than 80% of CRVO cases are nonischemic,9 of which the majority has visual acuity better than 20/400, mild or no pupillary defect, and mild, unilateral visual changes.10 Nonischemic CRVO can progress to ischemic CRVO, which can result in permanent vision loss. Visual outcome is good in nonischemic CRVO and poor in ischemic CRVO.11 Early detection of poor prognostic features, such as macular edema and neovascularization, is essential for minimizing the risk for permanent damage.12
Dilated funduscopic exam of a patient with CRVO may reveal widespread retinal hemorrhages, markedly dilated and tortuous retinal vessels, cotton wool spots, optic disc or macular edema, and/or vitreous hemorrhages.10
Differential includes varied conditions that can affect vision
CRVO may manifest similarly to the following:
Proliferative diabetic retinopathy can manifest with retinal edema or vitreous and retinal hemorrhages, which also are seen in CRVO.13 Macular edema, retinal hemorrhage, and neovascularization on the optic disc or retinal surface also may be seen on funduscopy in proliferative diabetic retinopathy.14 However, proliferative diabetic retinopathy is often bilateral and gradual in onset in patients with longstanding, uncontrolled diabetes.
Continue to: Hyperviscosity retinopathy
Hyperviscosity retinopathy, which is commonly caused by plasma cell and erythrocyte disorders, also manifests similarly to CRVO. Two noticeable differences include its bilateral presentation and Roth spots, neither of which are commonly seen in CRVO. In addition to visual abnormalities, mucosal bleeding and neurologic abnormalities complete the classic triad of hyperviscosity.15
Ocular ischemic syndrome is often confused with diabetic retinopathies and CRVO on funduscopy. However, patients with this condition may have narrowed retinal arteries, perifoveal telangiectasias, and periorbital pain—findings rarely seen in CRVO.16 Because ocular ischemic syndrome is a manifestation of severe carotid artery atherosclerosis, constitutional symptoms also may be present.
The work-up
When CRVO is suspected, an extensive laboratory work-up is necessary to determine the underlying etiology, including: blood pressure, electrocardiogram, complete blood count, random glucose level, electrolytes, lipid panel, plasma protein electrophoresis, thyroid function tests, and inflammatory markers.1
Additional testing may be required for younger patients who lack vasculopathic risk factors, who have bilateral CRVO, or who have a personal or family history of thrombosis.1 These patients should be screened for thrombophilia, hypercoagulable disorders, and homocysteinuria.1
Cases of CRVO have been linked to dehydration as well, with acute vision changes occurring after strenuous exercise, excessive vomiting, or extended periods of fasting.17-19
Continue to: Treatment may include injections, surgery, or nothing at all
Treatment may include injections, surgery, or nothing at all
Currently, there are no proven treatments to reopen occluded retinal veins. Thus, management is directed at complications that contribute to vision loss, including macular edema and neovascularization.20-21 Intravitreal anti-vascular endothelial growth factor (VEGF) agents are recognized as first-line therapy for macular edema in numerous studies.22-26 Intravitreal corticosteroids are an alternative treatment for patients with macular edema who do not respond to anti-VEGF therapy; however, monitoring is required as these corticosteroids increase the risk for glaucoma and cataract formation.27 In patients with CRVO with neovascularization, panretinal laser photocoagulation may be used.28
Observation and monitoring for the development of complications, rather than initiation of treatment, is appropriate for patients with CRVO without macular edema or neovascularization, with follow-up intervals and duration dictated by the severity of visual loss and whether the CRVO was ischemic or nonischemic.
Our patient’s diagnosis was confirmed by retinal specialists with optic coherence tomography, gonioscopy, and fluorescein angiography. He underwent an extensive laboratory work-up and hypercoagulation studies to determine the etiology. All results returned within normal limits with the exception of a nonspecific pattern found on serum protein electrophoresis that suggested dehydration.
Given his negative hypercoagulation studies, normal laboratory values, and new exercise regimen, dehydration was concluded to be the likely etiology. Since his visual acuity was not affected, observation with bimonthly follow-up for 6 months was the management strategy. He was also encouraged to maintain adequate hydration during exercise. His vision returned to normal 2 weeks after the initial event, and he did not have recurrence during the monitoring period.
1. Woo SC, Lip GY, Lip PL. Associations of retinal artery occlusion and retinal vein occlusion to mortality, stroke, and myocardial infarction: a systematic review. Eye (Lond). 2016;30:1031-1038. doi: 10.1038/eye.2016.111
2. Cugati S, Wang JJ, Rochtchina E, et al. Ten-year incidence of retinal vein occlusion in an older population: the Blue Mountains Eye Study. Arch Ophthalmol. 2006;124:726. doi: 10.1001/archopht.124.5.726
3. O’Mahoney PR, Wong DT, Ray JG. Retinal vein occlusion and traditional risk factors for atherosclerosis. Arch Ophthalmol. 2008;126:692-699. doi: 10.1001/archopht.126.5.692
4. Hayreh SS, Zimmerman B, McCarthy MJ, et al. Systemic diseases associated with various types of retinal vein occlusion. Am J Ophthalmol. 2001;131:61-77. doi: 10.1016/s0002-9394(00)00709-1
5. Janssen MC, den Heijer M, Cruysberg JR, et al. Retinal vein occlusion: a form of venous thrombosis or a complication of atherosclerosis? A meta-analysis of thrombophilic factors. Thromb Haemost. 2005;93:1021-1026. doi: 10.1160/TH04-11-0768
6. Rehak M, Rehak J, Müller M, et al. The prevalence of activated protein C (APC) resistance and factor V Leiden is significantly higher in patients with retinal vein occlusion without general risk factors. Case-control study and meta-analysis. Thromb Haemost. 2008;99:925-929. doi: 10.1160/TH07-11-0658
7. Yin X, Li J, Zhang B, et al. Association of glaucoma with risk of retinal vein occlusion: a meta-analysis. Acta Ophthalmol. 2019;97:652-659. doi: 10.1111/aos.14141
8. Rehak M, Krcova V, Slavik L, et al. The role of thrombophilia in patients with retinal vein occlusion and no systemic risk factors. Can J Ophthalmol. 2010;45:171-175. doi: 10.3129/i09-273
9. Hayreh SS, Zimmerman MB, Podhajsky P. Incidence of various types of retinal vein occlusion and their recurrent and demographic characteristics. Am J Ophthalmol. 1994;117:429-441. doi: 10.1016/s0002-9394(14)70001-7
10. Hayreh SS, Klugman MR, Beri M, et al. Differentiation of ischemic from non-ischemic central retinal vein occlusion during the early acute phase. Graefes Arch Clin Exp Ophthalmol. 1990;228:201-217. doi: 10.1007/BF00920022
11. Hayreh SS, Podhajsky PA, Zimmerman MB. Natural history of visual outcome in central retinal vein occlusion. Ophthalmology. 2011;118:119-133. doi: 10.1016/j.ophtha.2010.04.019
12. Bakri SJ, Berrocal A, Capone A, et al. Retina health series: central retinal vein occlusion. American Society of Retina Specialists. January 2020. Accessed April 16, 2021. www.asrs.org/content/documents/fact-sheet-21-central-retinal-vein-occlusion-2020_1_asrs.pdf
13. Columbia University Department of Ophthalmology. Proliferative diabetic retinopathy (PDR). Accessed July 2, 2021. www.columbiaeye.org/education/digital-reference-of-ophthalmology/vitreous-retina/retinal-vascular-diseases/proliferative-diabetic-retinopathy-pdr
14. Mehta S. Diabetic retinopathy. Merck Manual Professional Version. Updated June 2021. Accessed July 11, 2021. www.merckmanuals.com/professional/eye-disorders/retinal-disorders/diabetic-retinopathy
15. Gertz MA. Acute hyperviscosity: syndromes and management. Blood 2018;132:1379-1385. doi: 10.1182/blood-2018-06-846816
16. Terelak-Borys B, Skonieczna K, Grabska-Liberek I. Ocular ischemic syndrome—a systematic review. Med Sci Monit. 2012;18: RA138-RA144. doi: 10.12659/msm.883260
17. Moisseiev E, Sagiv O, Lazar M. Intense exercise causing central retinal vein occlusion in a young patient: case report and review of the literature. Case Rep Ophthalmol. 2014;5:116-120. doi: 10.1159/000360904.
18. Weiss KD, Kuriyan AE, Flynn HW Jr. Central retinal vein occlusion after prolonged vomiting and repeated valsalva maneuvers associated with gastroenteritis and dehydration. Ophthalmic Surg Lasers Imaging Retina. 2014;45:e23-e25. doi: 10.3928/23258160-20140331-03
19. Jacobs DJ, Flynn HW, Pathengay A, et al. Central retinal vein occlusion after intense exercise: response to intravitreal bevacizumab. Ophthalmic Surg Lasers Imaging. 2011;42:e59-e62. doi: 10.3928/15428877-20110623-02
20. Mohamed Q, McIntosh RL, Saw SM, et al. Interventions for central retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2007;114:507-524. doi: 10.1016/j.ophtha. 2006.11.011
21. Berker N, Batman C. Surgical treatment of central retinal vein occlusion. Acta Ophthalmol. 2008;86:245-252. doi: 10.1111/j.1755-3768.2007.01144.x
22. Braithwaite T, Nanji AA, Greenberg PB. Anti-vascular endothelial growth factor for macular edema secondary to central retinal vein occlusion. Cochrane Database Syst Rev. 2010;10:CD007325. doi: 10.1002/14651858.CD007325.pub2
23. Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117:1124-1133. doi: 10.1016/j.ophtha.2010.02.022
24. Campochiaro PA, Brown DM, Awh CC, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology. 2011;118:2041-2049. doi: 10.1016/j.ophtha.2011. 02.038
25. Prasad AG, Schadlu R, Apte RS. Intravitreal pharmacotherapy: applications in retinal disease. Compr Ophthalmol Update. 2007; 8:259-269.
26. Brown DM, Heier JS, Clark WL, et al. Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. Am J Ophthalmol. 2013;155:429-437. doi: 10.1016/j.ajo.2012.09.026
27. Ip MS, Scott IU, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Arch Ophthalmol. 2009;127:1101-1114. doi: 10.1001/archophthalmol.2009.234
28. The Central Vein Occlusion Study Group. A randomized clinical trial of early panretinal photocoagulation for ischemic central vein occlusion. The Central Vein Occlusion Study Group N report. Ophthalmology. 1995;102:1434-1444.
1. Woo SC, Lip GY, Lip PL. Associations of retinal artery occlusion and retinal vein occlusion to mortality, stroke, and myocardial infarction: a systematic review. Eye (Lond). 2016;30:1031-1038. doi: 10.1038/eye.2016.111
2. Cugati S, Wang JJ, Rochtchina E, et al. Ten-year incidence of retinal vein occlusion in an older population: the Blue Mountains Eye Study. Arch Ophthalmol. 2006;124:726. doi: 10.1001/archopht.124.5.726
3. O’Mahoney PR, Wong DT, Ray JG. Retinal vein occlusion and traditional risk factors for atherosclerosis. Arch Ophthalmol. 2008;126:692-699. doi: 10.1001/archopht.126.5.692
4. Hayreh SS, Zimmerman B, McCarthy MJ, et al. Systemic diseases associated with various types of retinal vein occlusion. Am J Ophthalmol. 2001;131:61-77. doi: 10.1016/s0002-9394(00)00709-1
5. Janssen MC, den Heijer M, Cruysberg JR, et al. Retinal vein occlusion: a form of venous thrombosis or a complication of atherosclerosis? A meta-analysis of thrombophilic factors. Thromb Haemost. 2005;93:1021-1026. doi: 10.1160/TH04-11-0768
6. Rehak M, Rehak J, Müller M, et al. The prevalence of activated protein C (APC) resistance and factor V Leiden is significantly higher in patients with retinal vein occlusion without general risk factors. Case-control study and meta-analysis. Thromb Haemost. 2008;99:925-929. doi: 10.1160/TH07-11-0658
7. Yin X, Li J, Zhang B, et al. Association of glaucoma with risk of retinal vein occlusion: a meta-analysis. Acta Ophthalmol. 2019;97:652-659. doi: 10.1111/aos.14141
8. Rehak M, Krcova V, Slavik L, et al. The role of thrombophilia in patients with retinal vein occlusion and no systemic risk factors. Can J Ophthalmol. 2010;45:171-175. doi: 10.3129/i09-273
9. Hayreh SS, Zimmerman MB, Podhajsky P. Incidence of various types of retinal vein occlusion and their recurrent and demographic characteristics. Am J Ophthalmol. 1994;117:429-441. doi: 10.1016/s0002-9394(14)70001-7
10. Hayreh SS, Klugman MR, Beri M, et al. Differentiation of ischemic from non-ischemic central retinal vein occlusion during the early acute phase. Graefes Arch Clin Exp Ophthalmol. 1990;228:201-217. doi: 10.1007/BF00920022
11. Hayreh SS, Podhajsky PA, Zimmerman MB. Natural history of visual outcome in central retinal vein occlusion. Ophthalmology. 2011;118:119-133. doi: 10.1016/j.ophtha.2010.04.019
12. Bakri SJ, Berrocal A, Capone A, et al. Retina health series: central retinal vein occlusion. American Society of Retina Specialists. January 2020. Accessed April 16, 2021. www.asrs.org/content/documents/fact-sheet-21-central-retinal-vein-occlusion-2020_1_asrs.pdf
13. Columbia University Department of Ophthalmology. Proliferative diabetic retinopathy (PDR). Accessed July 2, 2021. www.columbiaeye.org/education/digital-reference-of-ophthalmology/vitreous-retina/retinal-vascular-diseases/proliferative-diabetic-retinopathy-pdr
14. Mehta S. Diabetic retinopathy. Merck Manual Professional Version. Updated June 2021. Accessed July 11, 2021. www.merckmanuals.com/professional/eye-disorders/retinal-disorders/diabetic-retinopathy
15. Gertz MA. Acute hyperviscosity: syndromes and management. Blood 2018;132:1379-1385. doi: 10.1182/blood-2018-06-846816
16. Terelak-Borys B, Skonieczna K, Grabska-Liberek I. Ocular ischemic syndrome—a systematic review. Med Sci Monit. 2012;18: RA138-RA144. doi: 10.12659/msm.883260
17. Moisseiev E, Sagiv O, Lazar M. Intense exercise causing central retinal vein occlusion in a young patient: case report and review of the literature. Case Rep Ophthalmol. 2014;5:116-120. doi: 10.1159/000360904.
18. Weiss KD, Kuriyan AE, Flynn HW Jr. Central retinal vein occlusion after prolonged vomiting and repeated valsalva maneuvers associated with gastroenteritis and dehydration. Ophthalmic Surg Lasers Imaging Retina. 2014;45:e23-e25. doi: 10.3928/23258160-20140331-03
19. Jacobs DJ, Flynn HW, Pathengay A, et al. Central retinal vein occlusion after intense exercise: response to intravitreal bevacizumab. Ophthalmic Surg Lasers Imaging. 2011;42:e59-e62. doi: 10.3928/15428877-20110623-02
20. Mohamed Q, McIntosh RL, Saw SM, et al. Interventions for central retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2007;114:507-524. doi: 10.1016/j.ophtha. 2006.11.011
21. Berker N, Batman C. Surgical treatment of central retinal vein occlusion. Acta Ophthalmol. 2008;86:245-252. doi: 10.1111/j.1755-3768.2007.01144.x
22. Braithwaite T, Nanji AA, Greenberg PB. Anti-vascular endothelial growth factor for macular edema secondary to central retinal vein occlusion. Cochrane Database Syst Rev. 2010;10:CD007325. doi: 10.1002/14651858.CD007325.pub2
23. Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117:1124-1133. doi: 10.1016/j.ophtha.2010.02.022
24. Campochiaro PA, Brown DM, Awh CC, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology. 2011;118:2041-2049. doi: 10.1016/j.ophtha.2011. 02.038
25. Prasad AG, Schadlu R, Apte RS. Intravitreal pharmacotherapy: applications in retinal disease. Compr Ophthalmol Update. 2007; 8:259-269.
26. Brown DM, Heier JS, Clark WL, et al. Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. Am J Ophthalmol. 2013;155:429-437. doi: 10.1016/j.ajo.2012.09.026
27. Ip MS, Scott IU, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Arch Ophthalmol. 2009;127:1101-1114. doi: 10.1001/archophthalmol.2009.234
28. The Central Vein Occlusion Study Group. A randomized clinical trial of early panretinal photocoagulation for ischemic central vein occlusion. The Central Vein Occlusion Study Group N report. Ophthalmology. 1995;102:1434-1444.
One emergency is ending, and we’re ready for the next
I’ve always thought it was interesting that the first cases of COVID-19 were reported to the World Health Organization on December 31, 2019.1 How close we came to having COVID-20! On January 31, 2020, the US Department of Health and Human Services declared a national public health emergency due to COVID-19, and it’s been in effect ever since.
A national public health emergency allows the Department of Health and Human Services to access and designate funds to diagnose, treat, and prevent disease in response to the emergency. The declaration also facilitates the Centers for Disease Control and Prevention response to an infectious disease emergency. There are provisions for modifications to Medicare, Medicaid, and the Children’s Health Insurance Program so clinicians can continue seeing patients and be reimbursed for doing so, even in a situation in which the emergency disrupts usual reporting and documentation requirements. The declaration is essentially a shortcut through the typical bureaucracy that too often gums up the practice of medicine2; it allows for the rapid deployment of funds and personnel to a community affected by an emergency.
Unprecedented change. In the early days, plastic partitions were erected between patients in the hospital, and the scarce supply of N-95 masks was stored in paper bags and baked at low temperatures in ovens overnight.
My hospital enacted its incident command response procedures, just as we did the day our community experienced a mass shooting—except incident command stayed open for months. We had to adapt quickly. My office never closed to in-person visits; we decided that we took care of too many people who did not have other access to care to make closing practical. My practice partners and I spent a Friday afternoon in March 2020 writing policies. A policy for our residency practice. A policy for how to see patients who might have COVID. A policy for how to cover the residents and faculty when we inevitably got sick. A policy for how to do telehealth visits. By the following Monday, when the office reopened, we had already trained the staff on the new policies, and we were ready to implement them with our patients.
As COVID and our knowledge about it changed, we rewrote those policies dozens of times, and each time the staff retrained in a hurry. We all learned so much so quickly. So as the official public health emergency comes to an end, there are things that I think I will take from it, and things that I wish all of medicine could take from it too.
We adapted as a team. I will never forget the stress of the early days of the emergency, when the patient volume was overwhelming and the death rate was staggering. But shining through those dark times were wonderful moments of connection with the teams with which I worked. I think about the residents whose training shifted suddenly to full-time COVID, the nurses who learned new things every weekend for so many months, and everyone who went out on a limb to do the right thing.
We provided care without bureaucracy. I wish medicine could leave the bureaucracy behind along with the emergency. It was so much easier to practice medicine when we knew that the testing and treatment were covered, without “we’ll see” or “it depends on your insurance.” Telehealth is probably here to stay, thanks to widespread uptake by patients and clinicians alike during the pandemic. My wish is that we can make it as easy as possible to use going forward, instead of choosing to return to a more restricted and difficult path.3,4
Family physicians have much to be proud of. We can look back on the COVID-19 public health emergency as a time when we absorbed a huge amount of rapidly changing information and showed our adaptability to a frightening and uncertain environment. We are not returning to the office, as so many Americans are these days, because we never left the many settings where family physicians practice. We remained at work during the emergency and we took care of our patients.
When the next emergency is declared—whether it be national or local—we will once again be there for our patients.
1. CDC. CDC museum COVID-19 timeline. Updated March 15, 2023. Accessed March 28, 2023. www.cdc.gov/museum/timeline/covid19.html
2. US Department of Health and Human Services Administration for Strategic Preparedness & Response. A public health emer-gency declaration. Accessed March 28, 2023. https://aspr.hhs.gov/legal/PHE/Pages/Public-Health-Emergency-Declaration.aspx
3. US Department of Health and Human Services. Telehealth policy changes after the COVID-19 public health emergency. Updated February 16, 2023. Accessed March 28, 2023. https://telehealth.hhs.gov/providers/policy-changes-during-the-covid-19-public-health-emergency/policy-changes-after-the-covid-19-public-health-emergency
4. Cox C, Kates J, Cubanski J, et al. The end of the COVID-19 public health emergency: details on health coverage and access. Kaiser Family Foundation. Published February 3, 2023. Accessed March 28, 2023. www.kff.org/policy-watch/the-end-of-the-covid-19-public-health-emergency-details-on-health-coverage-and-access/
I’ve always thought it was interesting that the first cases of COVID-19 were reported to the World Health Organization on December 31, 2019.1 How close we came to having COVID-20! On January 31, 2020, the US Department of Health and Human Services declared a national public health emergency due to COVID-19, and it’s been in effect ever since.
A national public health emergency allows the Department of Health and Human Services to access and designate funds to diagnose, treat, and prevent disease in response to the emergency. The declaration also facilitates the Centers for Disease Control and Prevention response to an infectious disease emergency. There are provisions for modifications to Medicare, Medicaid, and the Children’s Health Insurance Program so clinicians can continue seeing patients and be reimbursed for doing so, even in a situation in which the emergency disrupts usual reporting and documentation requirements. The declaration is essentially a shortcut through the typical bureaucracy that too often gums up the practice of medicine2; it allows for the rapid deployment of funds and personnel to a community affected by an emergency.
Unprecedented change. In the early days, plastic partitions were erected between patients in the hospital, and the scarce supply of N-95 masks was stored in paper bags and baked at low temperatures in ovens overnight.
My hospital enacted its incident command response procedures, just as we did the day our community experienced a mass shooting—except incident command stayed open for months. We had to adapt quickly. My office never closed to in-person visits; we decided that we took care of too many people who did not have other access to care to make closing practical. My practice partners and I spent a Friday afternoon in March 2020 writing policies. A policy for our residency practice. A policy for how to see patients who might have COVID. A policy for how to cover the residents and faculty when we inevitably got sick. A policy for how to do telehealth visits. By the following Monday, when the office reopened, we had already trained the staff on the new policies, and we were ready to implement them with our patients.
As COVID and our knowledge about it changed, we rewrote those policies dozens of times, and each time the staff retrained in a hurry. We all learned so much so quickly. So as the official public health emergency comes to an end, there are things that I think I will take from it, and things that I wish all of medicine could take from it too.
We adapted as a team. I will never forget the stress of the early days of the emergency, when the patient volume was overwhelming and the death rate was staggering. But shining through those dark times were wonderful moments of connection with the teams with which I worked. I think about the residents whose training shifted suddenly to full-time COVID, the nurses who learned new things every weekend for so many months, and everyone who went out on a limb to do the right thing.
We provided care without bureaucracy. I wish medicine could leave the bureaucracy behind along with the emergency. It was so much easier to practice medicine when we knew that the testing and treatment were covered, without “we’ll see” or “it depends on your insurance.” Telehealth is probably here to stay, thanks to widespread uptake by patients and clinicians alike during the pandemic. My wish is that we can make it as easy as possible to use going forward, instead of choosing to return to a more restricted and difficult path.3,4
Family physicians have much to be proud of. We can look back on the COVID-19 public health emergency as a time when we absorbed a huge amount of rapidly changing information and showed our adaptability to a frightening and uncertain environment. We are not returning to the office, as so many Americans are these days, because we never left the many settings where family physicians practice. We remained at work during the emergency and we took care of our patients.
When the next emergency is declared—whether it be national or local—we will once again be there for our patients.
I’ve always thought it was interesting that the first cases of COVID-19 were reported to the World Health Organization on December 31, 2019.1 How close we came to having COVID-20! On January 31, 2020, the US Department of Health and Human Services declared a national public health emergency due to COVID-19, and it’s been in effect ever since.
A national public health emergency allows the Department of Health and Human Services to access and designate funds to diagnose, treat, and prevent disease in response to the emergency. The declaration also facilitates the Centers for Disease Control and Prevention response to an infectious disease emergency. There are provisions for modifications to Medicare, Medicaid, and the Children’s Health Insurance Program so clinicians can continue seeing patients and be reimbursed for doing so, even in a situation in which the emergency disrupts usual reporting and documentation requirements. The declaration is essentially a shortcut through the typical bureaucracy that too often gums up the practice of medicine2; it allows for the rapid deployment of funds and personnel to a community affected by an emergency.
Unprecedented change. In the early days, plastic partitions were erected between patients in the hospital, and the scarce supply of N-95 masks was stored in paper bags and baked at low temperatures in ovens overnight.
My hospital enacted its incident command response procedures, just as we did the day our community experienced a mass shooting—except incident command stayed open for months. We had to adapt quickly. My office never closed to in-person visits; we decided that we took care of too many people who did not have other access to care to make closing practical. My practice partners and I spent a Friday afternoon in March 2020 writing policies. A policy for our residency practice. A policy for how to see patients who might have COVID. A policy for how to cover the residents and faculty when we inevitably got sick. A policy for how to do telehealth visits. By the following Monday, when the office reopened, we had already trained the staff on the new policies, and we were ready to implement them with our patients.
As COVID and our knowledge about it changed, we rewrote those policies dozens of times, and each time the staff retrained in a hurry. We all learned so much so quickly. So as the official public health emergency comes to an end, there are things that I think I will take from it, and things that I wish all of medicine could take from it too.
We adapted as a team. I will never forget the stress of the early days of the emergency, when the patient volume was overwhelming and the death rate was staggering. But shining through those dark times were wonderful moments of connection with the teams with which I worked. I think about the residents whose training shifted suddenly to full-time COVID, the nurses who learned new things every weekend for so many months, and everyone who went out on a limb to do the right thing.
We provided care without bureaucracy. I wish medicine could leave the bureaucracy behind along with the emergency. It was so much easier to practice medicine when we knew that the testing and treatment were covered, without “we’ll see” or “it depends on your insurance.” Telehealth is probably here to stay, thanks to widespread uptake by patients and clinicians alike during the pandemic. My wish is that we can make it as easy as possible to use going forward, instead of choosing to return to a more restricted and difficult path.3,4
Family physicians have much to be proud of. We can look back on the COVID-19 public health emergency as a time when we absorbed a huge amount of rapidly changing information and showed our adaptability to a frightening and uncertain environment. We are not returning to the office, as so many Americans are these days, because we never left the many settings where family physicians practice. We remained at work during the emergency and we took care of our patients.
When the next emergency is declared—whether it be national or local—we will once again be there for our patients.
1. CDC. CDC museum COVID-19 timeline. Updated March 15, 2023. Accessed March 28, 2023. www.cdc.gov/museum/timeline/covid19.html
2. US Department of Health and Human Services Administration for Strategic Preparedness & Response. A public health emer-gency declaration. Accessed March 28, 2023. https://aspr.hhs.gov/legal/PHE/Pages/Public-Health-Emergency-Declaration.aspx
3. US Department of Health and Human Services. Telehealth policy changes after the COVID-19 public health emergency. Updated February 16, 2023. Accessed March 28, 2023. https://telehealth.hhs.gov/providers/policy-changes-during-the-covid-19-public-health-emergency/policy-changes-after-the-covid-19-public-health-emergency
4. Cox C, Kates J, Cubanski J, et al. The end of the COVID-19 public health emergency: details on health coverage and access. Kaiser Family Foundation. Published February 3, 2023. Accessed March 28, 2023. www.kff.org/policy-watch/the-end-of-the-covid-19-public-health-emergency-details-on-health-coverage-and-access/
1. CDC. CDC museum COVID-19 timeline. Updated March 15, 2023. Accessed March 28, 2023. www.cdc.gov/museum/timeline/covid19.html
2. US Department of Health and Human Services Administration for Strategic Preparedness & Response. A public health emer-gency declaration. Accessed March 28, 2023. https://aspr.hhs.gov/legal/PHE/Pages/Public-Health-Emergency-Declaration.aspx
3. US Department of Health and Human Services. Telehealth policy changes after the COVID-19 public health emergency. Updated February 16, 2023. Accessed March 28, 2023. https://telehealth.hhs.gov/providers/policy-changes-during-the-covid-19-public-health-emergency/policy-changes-after-the-covid-19-public-health-emergency
4. Cox C, Kates J, Cubanski J, et al. The end of the COVID-19 public health emergency: details on health coverage and access. Kaiser Family Foundation. Published February 3, 2023. Accessed March 28, 2023. www.kff.org/policy-watch/the-end-of-the-covid-19-public-health-emergency-details-on-health-coverage-and-access/
Hyperlipidemia management: A calibrated approach
An elevated serum level of cholesterol has been recognized as a risk factor for atherosclerotic cardiovascular disease (ASCVD) since the publication of the Framingham Study in 1961.1 Although clinical outcomes related to ASCVD have improved in recent decades, ASCVD remains the leading cause of morbidity and mortality across the globe and remains, in the United States, the leading cause of death among most racial and ethnic groups. Much of this persistent disease burden can be attributed to inadequate control of ASCVD risk factors and suboptimal implementation of prevention strategies in the general population.2
The most recent (2019) iteration of the American College of Cardiology/American Heart Association (ACC/AHA) Guideline on the Primary Prevention of Cardiovascular Disease emphasizes a comprehensive, patient-centered, team-based approach to the management of ASCVD risk factors.2 In this article, I review how, first, medication to reduce ASCVD risk should be considered only when a patient’s risk is sufficiently high and, second, shared decision-making and social determinants of health should, in all cases, guide and inform optimal implementation of treatment.2
Estimating risk for ASCVDby ascertaining LDL-C
The Friedewald equation. Traditionally, low-density lipoprotein cholesterol (LDL-C) is estimated using the Friedewald equationa applied to a fasting lipid profile. In patients who have a low level of LDL-C (< 70 mg/dL), however, the Friedewald equation becomes less accurate; in patients with hypertriglyceridemia (TG ≥ 400 mg/dL), estimation of LDL-C is invalid.
The Martin–Hopkins equation offers a validated estimation of LDL-C when the LDL-C value is < 70 mg/dL.3 This equation—in which the fixed factor of 5 used in the Friedewald equation to estimate very-low-density lipoprotein cholesterol is replaced by an adjustable factor that is based on the patient’s non-HDL-C (ie, TC – HDL-C) and TG values—is preferred by the ACC/AHA Task Force on Clinical Practice Guidelines in this clinical circumstance.4
National Institutes of Health equation. This newer equation provides an accurate estimate of the LDL-C level in patients whose TG value is ≤ 800 mg/dL. The equation has not been fully validated for clinical use, however.5
Direct measurement obviates the need for an equation to estimate LDL-C, but the test is not available in all health care settings.
For adults ≥ 20 years of age who are not receiving lipid-lowering therapy, a nonfasting lipid profile can be used to estimate ASCVD risk and document the baseline LDL-C level. If the TG level is ≥ 400 mg/dL, the test should be administered in the fasting state.4
Continue to: Apolipoprotein B
Apolipoprotein B. Alternatively, apolipoprotein B (apoB) can be measured. Because each LDL-C particle contains 1 apoB molecule, the apoB level describes the LDL-C level more accurately than a calculation of LDL-C. Many patients with type 2 diabetes and metabolic syndrome have a relatively low calculated LDL-C (thereby falsely reassuring the testing clinician) but have an elevated apoB level. An apoB level ≥ 130 mg/dL corresponds to an LDL-C level >160 mg/dL.4
Calculation of non-HDL-C. Because the nonfasting state does not have a significant impact on a patient’s TC and HDL-C levels, the non-HDL-C level also can be calculated from the results of a nonfasting lipid profile.
Non-HDL-C and apoB are equivalent predictors of ASCVD risk. These 2 assessments might offer better risk estimation than other available tools in patients who have type 2 diabetes and metabolic syndrome.6
Applying the estimate of 10-year ASCVD risk
Your recommendation for preventive intervention, such as lipid-lowering therapy, should be based on the estimated 10-year risk for ASCVD. Although multiple validated risk assessment tools are available, ACC/AHA recommends the pooled cohort risk equations (PCE), introduced in the 2013 ACC/AHA cholesterol treatment guidelines. The Framingham Heart Study now recommends the ACC/AHA PCE for risk assessment as well.7
The PCE, developed from 5 large cohorts, is based on hard atherosclerotic events: nonfatal myocardial infarction, death from coronary artery disease, and stroke. The ACC/AHA PCE is the only risk assessment tool developed using a significant percentage of patients who self-identify as Black.8 Alternatives to the ACC/AHA PCE include:
- Multi-ethnic Study of Atherosclerosis (MESA) 10-year ASCVD risk calculator, which incorporates the coronary artery calcium (CAC) score.
- Reynolds Risk Score, which incorporates high-sensitivity C-reactive protein measurement and a family history of premature ASCVD.9
Continue to: How much does lifestyle modification actually matter?
How much does lifestyle modification actually matter?
The absolute impact of diet and exercise on lipid parameters is relatively modest. No studies have demonstrated a reduction in adverse cardiovascular outcomes with specific interventions regarding diet or activity.
Diet. Nevertheless, ACC/AHA recommends that at-risk patients follow a dietary pattern that (1) emphasizes vegetables, fruits, and whole grains and (2) limits sweets, sugar-sweetened beverages, and red meat.
Saturated fat should constitute no more than 5% or 6% of total calories. In controlled-feeding trials,10 for every 1% of calories from saturated fat that are replaced with carbohydrate or monounsaturated or polyunsaturated fat, the LDL-C level was found to decline by as much as 1.8 mg/dL. Evidence is insufficient to assert that lowering dietary cholesterol reduces LDL-C.11
Activity. Trials of aerobic physical activity, compared with a more sedentary activity pattern, have demonstrated a reduction in the LDL-C level of as much as 6 mg/dL. All adult patients should be counseled to engage in aerobic physical activity of moderate or vigorous intensity—averaging ≥ 40 minutes per session, 3 or 4 sessions per week.11
Primary prevention:Stratification by age
40 to 75 years. ACC/AHA recommends that you routinely assess traditional cardiovascular risk factors for these patients and calculate their 10-year risk for ASCVD using the PCE. Statin therapy as primary prevention is indicated for 3 major groups (TABLE 1).4 The US Preventive Services Task Force (USPSTF) recommends a 10-year ASCVD risk ≥ 10%, in conjunction with 1 or more additional CVD risk factors (dyslipidemia, diabetes, hypertension, smoking), as the threshold for initiating low- or moderate-intensity statin therapy in this age group.12
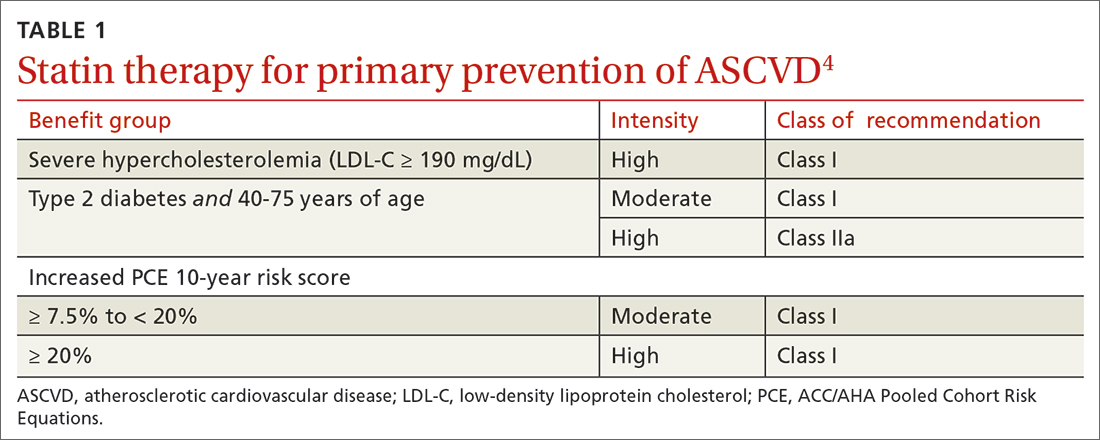
Continue to: In adults at borderline risk...
In adults at borderline risk (5% to < 7.5% 10-year ASCVD risk) or intermediate risk (≥ 7.5% to < 20% 10-year ASCVD risk), consider risk-enhancing factors to better inform your recommendation for preventive interventions. In these 2 groups, the presence of risk-enhancing factors might justify moderate-intensity statin therapy (TABLE 24).
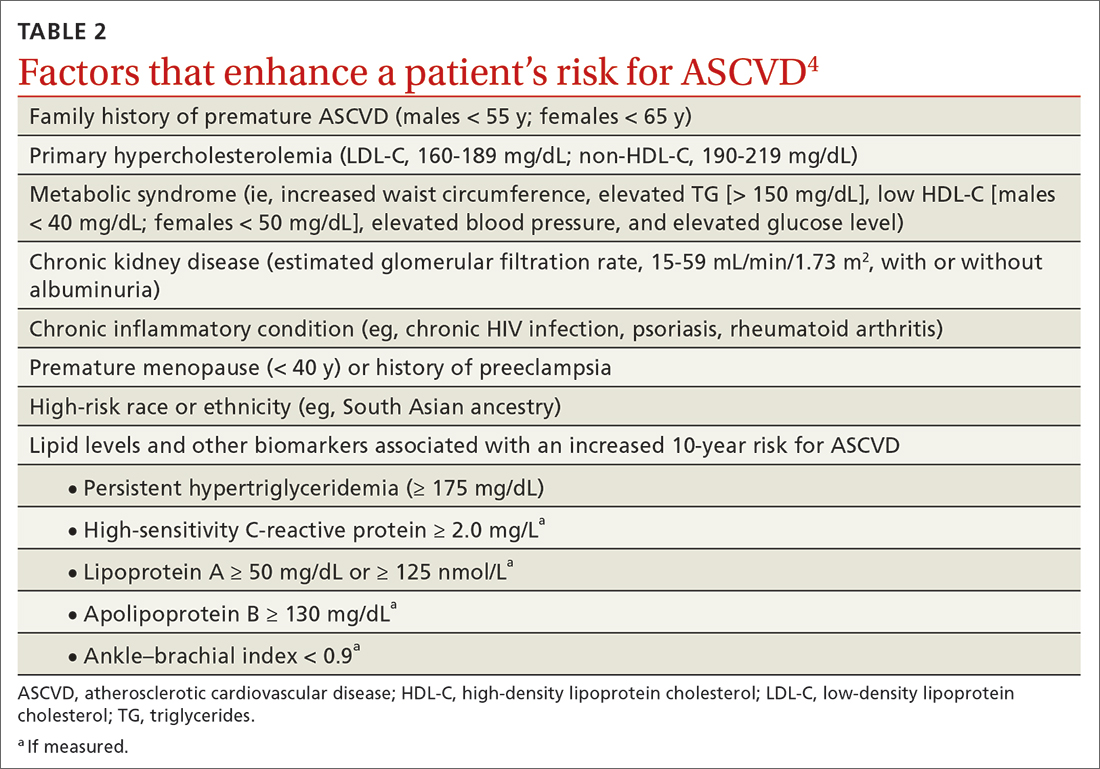
If your decision regarding preventive intervention remains uncertain, measuring CAC might further guide your discussion with the patient.4 When the CAC score is:
- 0 Agatston units and higher-risk conditions (eg, diabetes, family history of premature coronary artery disease, smoking) are absent, statin therapy can be withheld; reassess ASCVD risk in 5 to 10 years.
- 1-99 Agatston units, statin therapy can be started, especially for patients ≥ 55 years of age.
- ≥ 100 Agatston units or ≥ 75th percentile, statin therapy is indicated for all patients, regardless of additional risk factors.4
Because statins promote progression from unstable, inflammatory atherosclerotic plaque to more stable, calcified plaque, CAC scoring is not valid in patients already on statin therapy.13
In primary prevention, patients who have been classified as having low or intermediate risk, based on ASCVD risk scoring, with a CAC score of 0 Agatston units, have an annual all-cause mortality < 1%, regardless of age and gender. Patients classified as being at high risk, based on ASCVD risk scoring, with a CAC score of 0 Agatston units, have a significantly lower annual mortality than low- or intermediate-risk patients with a CAC score > 0 Agatston units.14
20 to 39 years. Focus on evaluation of lifetime ASCVD risk, rather than short-term (10-year) risk. Lifestyle modification is the primary intervention for younger patients; for those with moderate hypercholesterolemia (LDL-C, 160-189 mg/dL) and a family history of premature ASCVD, however, consider statin therapy. For patients with LDL-C ≥ 190 mg/dL, lifetime ASCVD risk is markedly increased, and high-intensity statin therapy is recommended, regardless of age. In this group, reassess ASCVD risk factors every 4 to 6 years.4
Continue to: > 75 years, without ASCVD
> 75 years, without ASCVD. In this group, the benefit of statin therapy is less clear and might be lessened by an increased potential for adverse effects. A meta-analysis of 28 trials demonstrated that people ages > 75 years had a 24% relative reduction in major coronary events for every 38.7 mg/dL (1.0 mmol/L) reduction in LDL-C, which is comparable to the risk reduction seen in people ages 40 to 75 years.15
With increasing age, however, the relative reduction in major coronary events with statin therapy decreased,15 although other trials have not demonstrated age heterogeneity.16 Because people > 75 years of age have a significantly higher ASCVD event rate, a comparable relative rate reduction with statin therapy results in a larger absolute rate reduction (ARR) and, therefore, a smaller number needed to treat (NNT) to prevent an event, compared to the NNT in younger people.
Secondary prevention
ACC/AHA guidelines define clinical ASCVD as a history of:
- acute coronary syndrome
- myocardial infarction
- coronary or other arterial revascularization
- cerebrovascular event
- symptomatic peripheral artery disease, including aortic aneurysm.
High-intensity statin therapy is indicated for all patients ≤ 75 years who have clinical ASCVD. In patients > 75 years, consider a taper to moderate-intensity statin therapy. An upper age limit for seeing benefit from statin therapy in secondary prevention has not been identified.4
In high-risk patients, if LDL-C remains ≥ 70 mg/dL despite maximally tolerated statin therapy, ezetimibe (discussed in the next section) can be added. In very-high-risk patients, if LDL-C remains ≥ 70 mg/dL despite maximally tolerated statin therapy plus ezetimibe, a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor (also discussed next) can be added. Always precede initiation of a PCSK9 inhibitor with a discussion of the net benefit, safety, and cost with the patient.4
Continue to: Options for lipid-lowering pharmacotherapy
Options for lipid-lowering pharmacotherapy
Statins (formally, hydroxymethylglutaryl-coenzyme A reductase inhibitors) offer the most predictable reduction in ASCVD risk of any lipid-lowering therapy. The evidence report that accompanied the 2016 USPSTF guidelines on statins for the prevention of cardiovascular disease (CVD) stated that low- or moderate-dosage statin therapy is associated with approximately a 30% relative risk reduction (RRR) in CVD events and CVD deaths and a 10% to 15% RRR in all-cause mortality.17
High-intensity statin therapy reduces LDL-C by ≥ 50%. Moderate-intensity statin therapy reduces LDL-C by 30% to 49% (TABLE 3).4
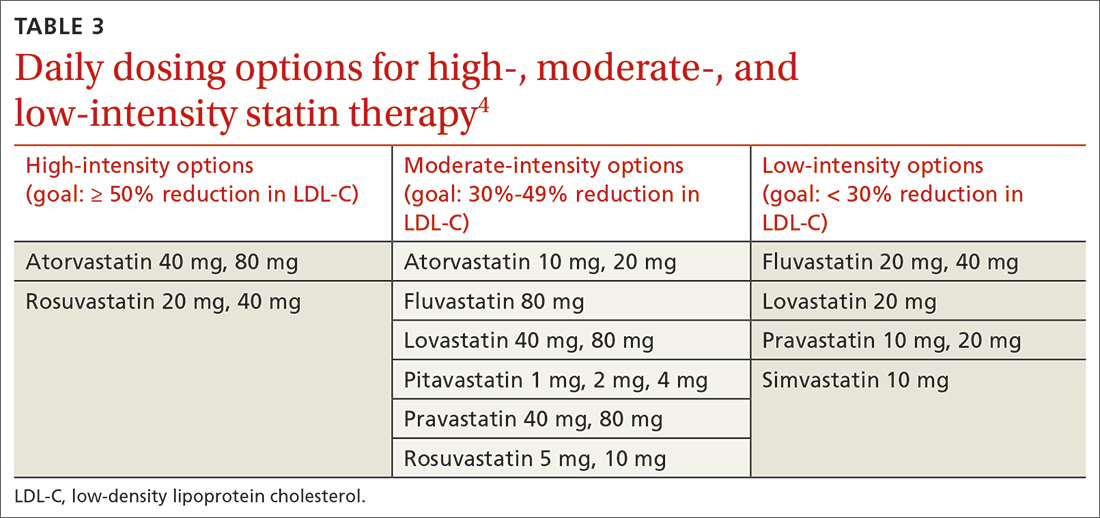
Statins are not without risk: A 2016 report18 estimated that treating 10,000 patients with a statin for 5 years would cause 1 case of rhabdomyolysis, 5 cases of myopathy, 75 new cases of diabetes, and 7 cases of hemorrhagic stroke. The same treatment would, however, avert approximately 1000 CVD events among patients with preexisting disease and approximately 500 CVD events among patients at elevated risk but without preexisting disease.18
Ezetimibe, a selective cholesterol-absorption inhibitor, lowers LDL-C by 13% to 20% and typically is well tolerated. The use of ezetimibe in ASCVD risk reduction is supported by a single randomized controlled trial of more than 18,000 patients with recent acute coronary syndrome. Adding ezetimibe to simvastatin 40 mg resulted in a 2% absolute reduction in major adverse cardiovascular events over a median follow-up of 6 years (NNT = 50), compared to simvastatin alone.19 ACC/AHA guidelines recommend adding ezetimibe to maximally tolerated statin therapy in patients with clinical ASCVD who do not reach their goal LDL reduction with a statin alone. Ezetimibe also can be considered a statin alternative in patients who are statin intolerant.4
PCSK9 inhibitors. When added to statin therapy, evolocumab and alirocumab—monoclonal antibodies that inhibit PCSK9—offer an incremental decrease in LDL-C of approximately 60%.20-22 In a meta-analysis of 35 trials evaluating the incremental benefit of PCSK9 inhibitor therapy, a significant reduction in cardiovascular events, including myocardial infarction (ARR = 1.3%; NNT = 77), stroke (ARR = 0.4%; NNT = 250), and coronary revascularization (ARR = 1.6%; NNT = 63) was reported. No significant difference was observed in all-cause or cardiovascular mortality.21,23
Continue to: Inclisiran
Inclisiran, an injectable small-interfering RNA that inhibits PCSK9 synthesis, provides an incremental decrease in LDL-C of > 50% in patients already receiving statin therapy. Meta-analysis of 3 small cardiovascular outcomes trials revealed no significant difference in the rate of myocardial infarction, stroke, or cardiovascular mortality with inclisiran compared to placebo. Larger outcomes trials are underway and might offer additional insight into this agent’s role in ASCVD risk management.24
Omega-3 fatty acids. Multiple trials have demonstrated that adding omega-3 fatty acids to usual lipid-lowering therapy does not offer a consistent reduction in adverse cardiovascular outcomes, despite providing a significant reduction in TG levels. In a high-risk population with persistently elevated TG despite statin therapy, icosapent ethyl, a purified eicosapentaenoic acid ethyl ester, reduced major ASCVD outcomes by 25% over a median 4.9 years (ARR = 4.8%; NNT = 21), and cardiovascular death by 20% (ARR = 0.9%; NNT = 111), compared with a mineral oil placebo.25 Subsequent trials, using a corn oil placebo, failed to duplicate these data26—raising concern that the mineral oil comparator might have altered results of the eicosapentaenoic acid ethyl ester study.27,28
Bempedoic acid is a small-molecule inhibitor of ATP citrate lyase that increases LDL uptake by the liver. Pooled data from studies of bempedoic acid show, on average, a 15% reduction in TC, a 23% reduction in LDL-C, and a 6% increase in HDL-C, without a significant change in TG.29 In statin-intolerant patients, bempedoic acid reduced major ASCVD outcomes by 13% over a median 40 months (ARR = 1.6%; NNT = 63), with no significant reduction in cardiovascular death.30
Niacin. Two large trials failed to demonstrate improvement in major cardiovascular events or other clinical benefit when niacin is added to moderate-intensity statin therapy, despite a significant increase in the HDL-C level (on average, 6 mg/dL) and a decrease in the LDL-C level (10-12 mg/dL) and TG (42 mg/dL).31,32
Fenofibrate lowers TG and increases HDL-C but does not consistently improve cardiovascular outcomes.33 In a trial of patients with type 2 diabetes and persistent dyslipidemia (serum TG > 204 mg/dL; HDL-C < 34 mg/dL) despite statin therapy, adding fenofibrate reduced CVD outcomes by 4.9%—although this absolute difference did not reach statistical significance.34
Neither niacin nor fenofibrate is considered useful for reducing ASCVD risk across broad populations.4
Follow-up to assess progress toward goals
Recheck the lipid profile 4 to 12 weeks after starting lipid-lowering therapy to verify adherence to medication and assess response. The primary goal is the percentage reduction in LDL-C based on ASCVD risk. An additional goal for very-high-risk patients is an LDL-C value ≤ 70 mg/dL. If the reduction in LDL-C is less than desired and adherence is assured, consider titrating the statin dosage or augmenting statin therapy with a nonstatin drug (eg, ezetimibe), or both.4
CORRESPONDENCE
Jonathon M. Firnhaber, MD, MAEd, MBA, East Carolina University, Family Medicine Center, 101 Heart Drive, Greenville, NC 27834; [email protected]
1. Kannel WB, Dawber TR, Kagan A, et al. Factors of risk in the development of coronary heart disease—six-year follow-up experience. The Framingham Study. Ann Intern Med. 1961;55:33. doi: 10.7326/0003-4819-55-1-33
2. Arnett DK, Blumenthal RS, Albert MA, et al; American Association of Cardiovascular and Pulmonary Rehabilitation, American Geriatrics Society, American Society of Preventive Cardiology, and Preventive Cardiovascular Nurses Association. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596-e646. doi: 10.1161/CIR.0000000000000678
3. Martin SS, Blaha MJ, Elshazly MB, et al. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA. 2013;310:2061-2068. doi: 10.1001/jama.2013.280532
4. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation. 2019;139:e1082-1143. doi: 10.1161/CIR.0000000000000625
5. Sampson M, Ling C, Sun Q, et al. A new equation for calculation of low-density lipoprotein cholesterol in patients with normolipidemia and/or hypertriglyceridemia. JAMA Cardiol. 2020;5:540-548. doi: 10.1001/jamacardio.2020.0013
6. Sniderman AD, Williams K, Contois JH, et al. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2011;4:337-345. doi: 10.1161/CIRCOUTCOMES.110.959247
7. Framingham Heart Study. Cardiovascular disease (10-year risk). Accessed February 14, 2023. www.framinghamheartstudy.org/fhs-risk-functions/cardiovascular-disease-10-year-risk/
8. Stone NJ, Robinson JG, Lichtenstein AH, et al; . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation. 2014;129(25 suppl 2):S1-S45. doi: 10.1161/01.cir.0000437738.63853.7a
9. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(suppl 2):1-87. doi: 10.4158/EP171764.APPGL
10. Mensink RP, Zock PL, Kester ADM, et al. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146
11. Eckel RH, Jakicic JM, Ard JD, et al; . 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S76-S99. doi: 10.1161/01.cir.0000437740.48606.d1
12. Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1997-2007. doi: 10.1001/jama.2016.15450
13. Lee S-E, Chang H-J, Sung JM, et al. Effects of statins on coronary atherosclerotic plaques: the PARADIGM study. JACC Cardiovasc Imaging. 2018;11:1475-1484. doi: 10.1016/j.jcmg.2018.04.015
14. Valenti V, Hartaigh B, Heo R, et al. A 15-year warranty period for asymptomatic individuals without coronary artery calcium: a prospective follow-up of 9,715 individuals. JACC Cardiovasc Imaging. 2015;8:900-909. doi: 10.1016/j.jcmg.2015.01.025
15. Armitage J, Baigent C, Barnes E, et al; . Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393:407-415. doi: 10.1016/S0140-6736(18)31942-1
16. Ridker PM, Lonn E, Paynter NP, et al. Primary prevention with statin therapy in the elderly: new meta-analyses from the contemporary JUPITER and HOPE-3 randomized trials. Circulation. 2017;135:1979-1981. doi: 10.1161/CIRCULATIONAHA.117.028271
17. Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008-2024. doi: 10.1001/jama.2015.15629
18. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532-2561. doi: 10.1016/S0140-6736(16)31357-5
19. Cannon CP, Blazing MA, Giugliano RP, et al; . Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397. doi: 10.1056/NEJMoa1410489
20. Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:2373-2384. doi: 10.1001/jama.2016.16951
21. Sabatine MS, Giugliano RP, Wiviott SD, et al; . Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509. doi: 10.1056/NEJMoa1500858
22. Robinson JG, Farnier M, Krempf M, et al; . Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499. doi: 10.1056/NEJMoa1501031
23. Karatasakis A, Danek BA, Karacsonyi J, et al. Effect of PCSK9 inhibitors on clinical outcomes in patients with hypercholesterolemia: a meta‐analysis of 35 randomized controlled trials. J Am Heart Assoc. 2017;6:e006910. doi: 10.1161/JAHA.117.006910
24. Khan SA, Naz A, Qamar Masood M, et al. Meta-analysis of inclisiran for the treatment of hypercholesterolemia. Am J Cardiol. 2020;134:69-73. doi: 10.1016/j.amjcard.2020.08.018
25. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22. doi: 10.1056/NEJMoa1812792
26. Nicholls SJ, Lincoff AM, Garcia M, et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 2020;324:2268-2280. doi: 10.1001/jama.2020.22258
27. Nissen SE, Lincoff AM, Wolski K, et al. Association between achieved ω-3 fatty acid levels and major adverse cardiovascular outcomes in patients with high cardiovascular risk. JAMA Cardiol. 2021;6:1-8. doi: 10.1001/jamacardio.2021.1157
28. US Food and Drug Administration. Briefing document: Endocrinologic and Metabolic Drugs Advisory Committee meeting, November 14, 2019. Accessed February 15, 2023. www.fda.gov/media/132477/download
29. Cicero AFG, Fogacci F, Hernandez AV, et al. Efficacy and safety of bempedoic acid for the treatment of hypercholesterolemia: a systematic review and meta-analysis. PLOS Med. 2020;17:e1003121. doi: 10.1371/journal.pmed.1003121
30. Nissen SE, Lincoff AM, Brennan D, et al; CLEAR Outcomes Investigators. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N Engl J Med. Published online March 4, 2023. doi: 10.1056/NEJMoa2215024
31. Landray MJ, Haynes R, Hopewell JC, et al; . Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203-212. doi: 10.1056/NEJMoa1300955
32. Boden WE, Probstfield JL, Anderson T, et al; AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267. doi: 10.1056/NEJMoa1107579
33. Elam MB, Ginsberg HN, Lovato LC, et al; ACCORDION Study Investigators. Association of fenofibrate therapy with long-term cardiovascular risk in statin-treated patients with type 2 diabetes. JAMA Cardiol. 2017;2:370-380. doi: 10.1001/jamacardio.2016.4828
34. Ginsberg HN, Elam MB, Lovato LC, et al; ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574. doi: 10.1056/NEJMoa1001282
An elevated serum level of cholesterol has been recognized as a risk factor for atherosclerotic cardiovascular disease (ASCVD) since the publication of the Framingham Study in 1961.1 Although clinical outcomes related to ASCVD have improved in recent decades, ASCVD remains the leading cause of morbidity and mortality across the globe and remains, in the United States, the leading cause of death among most racial and ethnic groups. Much of this persistent disease burden can be attributed to inadequate control of ASCVD risk factors and suboptimal implementation of prevention strategies in the general population.2

The most recent (2019) iteration of the American College of Cardiology/American Heart Association (ACC/AHA) Guideline on the Primary Prevention of Cardiovascular Disease emphasizes a comprehensive, patient-centered, team-based approach to the management of ASCVD risk factors.2 In this article, I review how, first, medication to reduce ASCVD risk should be considered only when a patient’s risk is sufficiently high and, second, shared decision-making and social determinants of health should, in all cases, guide and inform optimal implementation of treatment.2
Estimating risk for ASCVDby ascertaining LDL-C
The Friedewald equation. Traditionally, low-density lipoprotein cholesterol (LDL-C) is estimated using the Friedewald equationa applied to a fasting lipid profile. In patients who have a low level of LDL-C (< 70 mg/dL), however, the Friedewald equation becomes less accurate; in patients with hypertriglyceridemia (TG ≥ 400 mg/dL), estimation of LDL-C is invalid.
The Martin–Hopkins equation offers a validated estimation of LDL-C when the LDL-C value is < 70 mg/dL.3 This equation—in which the fixed factor of 5 used in the Friedewald equation to estimate very-low-density lipoprotein cholesterol is replaced by an adjustable factor that is based on the patient’s non-HDL-C (ie, TC – HDL-C) and TG values—is preferred by the ACC/AHA Task Force on Clinical Practice Guidelines in this clinical circumstance.4
National Institutes of Health equation. This newer equation provides an accurate estimate of the LDL-C level in patients whose TG value is ≤ 800 mg/dL. The equation has not been fully validated for clinical use, however.5
Direct measurement obviates the need for an equation to estimate LDL-C, but the test is not available in all health care settings.
For adults ≥ 20 years of age who are not receiving lipid-lowering therapy, a nonfasting lipid profile can be used to estimate ASCVD risk and document the baseline LDL-C level. If the TG level is ≥ 400 mg/dL, the test should be administered in the fasting state.4
Continue to: Apolipoprotein B
Apolipoprotein B. Alternatively, apolipoprotein B (apoB) can be measured. Because each LDL-C particle contains 1 apoB molecule, the apoB level describes the LDL-C level more accurately than a calculation of LDL-C. Many patients with type 2 diabetes and metabolic syndrome have a relatively low calculated LDL-C (thereby falsely reassuring the testing clinician) but have an elevated apoB level. An apoB level ≥ 130 mg/dL corresponds to an LDL-C level >160 mg/dL.4
Calculation of non-HDL-C. Because the nonfasting state does not have a significant impact on a patient’s TC and HDL-C levels, the non-HDL-C level also can be calculated from the results of a nonfasting lipid profile.
Non-HDL-C and apoB are equivalent predictors of ASCVD risk. These 2 assessments might offer better risk estimation than other available tools in patients who have type 2 diabetes and metabolic syndrome.6
Applying the estimate of 10-year ASCVD risk
Your recommendation for preventive intervention, such as lipid-lowering therapy, should be based on the estimated 10-year risk for ASCVD. Although multiple validated risk assessment tools are available, ACC/AHA recommends the pooled cohort risk equations (PCE), introduced in the 2013 ACC/AHA cholesterol treatment guidelines. The Framingham Heart Study now recommends the ACC/AHA PCE for risk assessment as well.7
The PCE, developed from 5 large cohorts, is based on hard atherosclerotic events: nonfatal myocardial infarction, death from coronary artery disease, and stroke. The ACC/AHA PCE is the only risk assessment tool developed using a significant percentage of patients who self-identify as Black.8 Alternatives to the ACC/AHA PCE include:
- Multi-ethnic Study of Atherosclerosis (MESA) 10-year ASCVD risk calculator, which incorporates the coronary artery calcium (CAC) score.
- Reynolds Risk Score, which incorporates high-sensitivity C-reactive protein measurement and a family history of premature ASCVD.9
Continue to: How much does lifestyle modification actually matter?
How much does lifestyle modification actually matter?
The absolute impact of diet and exercise on lipid parameters is relatively modest. No studies have demonstrated a reduction in adverse cardiovascular outcomes with specific interventions regarding diet or activity.
Diet. Nevertheless, ACC/AHA recommends that at-risk patients follow a dietary pattern that (1) emphasizes vegetables, fruits, and whole grains and (2) limits sweets, sugar-sweetened beverages, and red meat.
Saturated fat should constitute no more than 5% or 6% of total calories. In controlled-feeding trials,10 for every 1% of calories from saturated fat that are replaced with carbohydrate or monounsaturated or polyunsaturated fat, the LDL-C level was found to decline by as much as 1.8 mg/dL. Evidence is insufficient to assert that lowering dietary cholesterol reduces LDL-C.11
Activity. Trials of aerobic physical activity, compared with a more sedentary activity pattern, have demonstrated a reduction in the LDL-C level of as much as 6 mg/dL. All adult patients should be counseled to engage in aerobic physical activity of moderate or vigorous intensity—averaging ≥ 40 minutes per session, 3 or 4 sessions per week.11
Primary prevention:Stratification by age
40 to 75 years. ACC/AHA recommends that you routinely assess traditional cardiovascular risk factors for these patients and calculate their 10-year risk for ASCVD using the PCE. Statin therapy as primary prevention is indicated for 3 major groups (TABLE 1).4 The US Preventive Services Task Force (USPSTF) recommends a 10-year ASCVD risk ≥ 10%, in conjunction with 1 or more additional CVD risk factors (dyslipidemia, diabetes, hypertension, smoking), as the threshold for initiating low- or moderate-intensity statin therapy in this age group.12

Continue to: In adults at borderline risk...
In adults at borderline risk (5% to < 7.5% 10-year ASCVD risk) or intermediate risk (≥ 7.5% to < 20% 10-year ASCVD risk), consider risk-enhancing factors to better inform your recommendation for preventive interventions. In these 2 groups, the presence of risk-enhancing factors might justify moderate-intensity statin therapy (TABLE 24).

If your decision regarding preventive intervention remains uncertain, measuring CAC might further guide your discussion with the patient.4 When the CAC score is:
- 0 Agatston units and higher-risk conditions (eg, diabetes, family history of premature coronary artery disease, smoking) are absent, statin therapy can be withheld; reassess ASCVD risk in 5 to 10 years.
- 1-99 Agatston units, statin therapy can be started, especially for patients ≥ 55 years of age.
- ≥ 100 Agatston units or ≥ 75th percentile, statin therapy is indicated for all patients, regardless of additional risk factors.4
Because statins promote progression from unstable, inflammatory atherosclerotic plaque to more stable, calcified plaque, CAC scoring is not valid in patients already on statin therapy.13
In primary prevention, patients who have been classified as having low or intermediate risk, based on ASCVD risk scoring, with a CAC score of 0 Agatston units, have an annual all-cause mortality < 1%, regardless of age and gender. Patients classified as being at high risk, based on ASCVD risk scoring, with a CAC score of 0 Agatston units, have a significantly lower annual mortality than low- or intermediate-risk patients with a CAC score > 0 Agatston units.14
20 to 39 years. Focus on evaluation of lifetime ASCVD risk, rather than short-term (10-year) risk. Lifestyle modification is the primary intervention for younger patients; for those with moderate hypercholesterolemia (LDL-C, 160-189 mg/dL) and a family history of premature ASCVD, however, consider statin therapy. For patients with LDL-C ≥ 190 mg/dL, lifetime ASCVD risk is markedly increased, and high-intensity statin therapy is recommended, regardless of age. In this group, reassess ASCVD risk factors every 4 to 6 years.4
Continue to: > 75 years, without ASCVD
> 75 years, without ASCVD. In this group, the benefit of statin therapy is less clear and might be lessened by an increased potential for adverse effects. A meta-analysis of 28 trials demonstrated that people ages > 75 years had a 24% relative reduction in major coronary events for every 38.7 mg/dL (1.0 mmol/L) reduction in LDL-C, which is comparable to the risk reduction seen in people ages 40 to 75 years.15
With increasing age, however, the relative reduction in major coronary events with statin therapy decreased,15 although other trials have not demonstrated age heterogeneity.16 Because people > 75 years of age have a significantly higher ASCVD event rate, a comparable relative rate reduction with statin therapy results in a larger absolute rate reduction (ARR) and, therefore, a smaller number needed to treat (NNT) to prevent an event, compared to the NNT in younger people.
Secondary prevention
ACC/AHA guidelines define clinical ASCVD as a history of:
- acute coronary syndrome
- myocardial infarction
- coronary or other arterial revascularization
- cerebrovascular event
- symptomatic peripheral artery disease, including aortic aneurysm.
High-intensity statin therapy is indicated for all patients ≤ 75 years who have clinical ASCVD. In patients > 75 years, consider a taper to moderate-intensity statin therapy. An upper age limit for seeing benefit from statin therapy in secondary prevention has not been identified.4
In high-risk patients, if LDL-C remains ≥ 70 mg/dL despite maximally tolerated statin therapy, ezetimibe (discussed in the next section) can be added. In very-high-risk patients, if LDL-C remains ≥ 70 mg/dL despite maximally tolerated statin therapy plus ezetimibe, a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor (also discussed next) can be added. Always precede initiation of a PCSK9 inhibitor with a discussion of the net benefit, safety, and cost with the patient.4
Continue to: Options for lipid-lowering pharmacotherapy
Options for lipid-lowering pharmacotherapy
Statins (formally, hydroxymethylglutaryl-coenzyme A reductase inhibitors) offer the most predictable reduction in ASCVD risk of any lipid-lowering therapy. The evidence report that accompanied the 2016 USPSTF guidelines on statins for the prevention of cardiovascular disease (CVD) stated that low- or moderate-dosage statin therapy is associated with approximately a 30% relative risk reduction (RRR) in CVD events and CVD deaths and a 10% to 15% RRR in all-cause mortality.17
High-intensity statin therapy reduces LDL-C by ≥ 50%. Moderate-intensity statin therapy reduces LDL-C by 30% to 49% (TABLE 3).4

Statins are not without risk: A 2016 report18 estimated that treating 10,000 patients with a statin for 5 years would cause 1 case of rhabdomyolysis, 5 cases of myopathy, 75 new cases of diabetes, and 7 cases of hemorrhagic stroke. The same treatment would, however, avert approximately 1000 CVD events among patients with preexisting disease and approximately 500 CVD events among patients at elevated risk but without preexisting disease.18
Ezetimibe, a selective cholesterol-absorption inhibitor, lowers LDL-C by 13% to 20% and typically is well tolerated. The use of ezetimibe in ASCVD risk reduction is supported by a single randomized controlled trial of more than 18,000 patients with recent acute coronary syndrome. Adding ezetimibe to simvastatin 40 mg resulted in a 2% absolute reduction in major adverse cardiovascular events over a median follow-up of 6 years (NNT = 50), compared to simvastatin alone.19 ACC/AHA guidelines recommend adding ezetimibe to maximally tolerated statin therapy in patients with clinical ASCVD who do not reach their goal LDL reduction with a statin alone. Ezetimibe also can be considered a statin alternative in patients who are statin intolerant.4
PCSK9 inhibitors. When added to statin therapy, evolocumab and alirocumab—monoclonal antibodies that inhibit PCSK9—offer an incremental decrease in LDL-C of approximately 60%.20-22 In a meta-analysis of 35 trials evaluating the incremental benefit of PCSK9 inhibitor therapy, a significant reduction in cardiovascular events, including myocardial infarction (ARR = 1.3%; NNT = 77), stroke (ARR = 0.4%; NNT = 250), and coronary revascularization (ARR = 1.6%; NNT = 63) was reported. No significant difference was observed in all-cause or cardiovascular mortality.21,23
Continue to: Inclisiran
Inclisiran, an injectable small-interfering RNA that inhibits PCSK9 synthesis, provides an incremental decrease in LDL-C of > 50% in patients already receiving statin therapy. Meta-analysis of 3 small cardiovascular outcomes trials revealed no significant difference in the rate of myocardial infarction, stroke, or cardiovascular mortality with inclisiran compared to placebo. Larger outcomes trials are underway and might offer additional insight into this agent’s role in ASCVD risk management.24
Omega-3 fatty acids. Multiple trials have demonstrated that adding omega-3 fatty acids to usual lipid-lowering therapy does not offer a consistent reduction in adverse cardiovascular outcomes, despite providing a significant reduction in TG levels. In a high-risk population with persistently elevated TG despite statin therapy, icosapent ethyl, a purified eicosapentaenoic acid ethyl ester, reduced major ASCVD outcomes by 25% over a median 4.9 years (ARR = 4.8%; NNT = 21), and cardiovascular death by 20% (ARR = 0.9%; NNT = 111), compared with a mineral oil placebo.25 Subsequent trials, using a corn oil placebo, failed to duplicate these data26—raising concern that the mineral oil comparator might have altered results of the eicosapentaenoic acid ethyl ester study.27,28
Bempedoic acid is a small-molecule inhibitor of ATP citrate lyase that increases LDL uptake by the liver. Pooled data from studies of bempedoic acid show, on average, a 15% reduction in TC, a 23% reduction in LDL-C, and a 6% increase in HDL-C, without a significant change in TG.29 In statin-intolerant patients, bempedoic acid reduced major ASCVD outcomes by 13% over a median 40 months (ARR = 1.6%; NNT = 63), with no significant reduction in cardiovascular death.30
Niacin. Two large trials failed to demonstrate improvement in major cardiovascular events or other clinical benefit when niacin is added to moderate-intensity statin therapy, despite a significant increase in the HDL-C level (on average, 6 mg/dL) and a decrease in the LDL-C level (10-12 mg/dL) and TG (42 mg/dL).31,32
Fenofibrate lowers TG and increases HDL-C but does not consistently improve cardiovascular outcomes.33 In a trial of patients with type 2 diabetes and persistent dyslipidemia (serum TG > 204 mg/dL; HDL-C < 34 mg/dL) despite statin therapy, adding fenofibrate reduced CVD outcomes by 4.9%—although this absolute difference did not reach statistical significance.34
Neither niacin nor fenofibrate is considered useful for reducing ASCVD risk across broad populations.4
Follow-up to assess progress toward goals
Recheck the lipid profile 4 to 12 weeks after starting lipid-lowering therapy to verify adherence to medication and assess response. The primary goal is the percentage reduction in LDL-C based on ASCVD risk. An additional goal for very-high-risk patients is an LDL-C value ≤ 70 mg/dL. If the reduction in LDL-C is less than desired and adherence is assured, consider titrating the statin dosage or augmenting statin therapy with a nonstatin drug (eg, ezetimibe), or both.4
CORRESPONDENCE
Jonathon M. Firnhaber, MD, MAEd, MBA, East Carolina University, Family Medicine Center, 101 Heart Drive, Greenville, NC 27834; [email protected]
An elevated serum level of cholesterol has been recognized as a risk factor for atherosclerotic cardiovascular disease (ASCVD) since the publication of the Framingham Study in 1961.1 Although clinical outcomes related to ASCVD have improved in recent decades, ASCVD remains the leading cause of morbidity and mortality across the globe and remains, in the United States, the leading cause of death among most racial and ethnic groups. Much of this persistent disease burden can be attributed to inadequate control of ASCVD risk factors and suboptimal implementation of prevention strategies in the general population.2

The most recent (2019) iteration of the American College of Cardiology/American Heart Association (ACC/AHA) Guideline on the Primary Prevention of Cardiovascular Disease emphasizes a comprehensive, patient-centered, team-based approach to the management of ASCVD risk factors.2 In this article, I review how, first, medication to reduce ASCVD risk should be considered only when a patient’s risk is sufficiently high and, second, shared decision-making and social determinants of health should, in all cases, guide and inform optimal implementation of treatment.2
Estimating risk for ASCVDby ascertaining LDL-C
The Friedewald equation. Traditionally, low-density lipoprotein cholesterol (LDL-C) is estimated using the Friedewald equationa applied to a fasting lipid profile. In patients who have a low level of LDL-C (< 70 mg/dL), however, the Friedewald equation becomes less accurate; in patients with hypertriglyceridemia (TG ≥ 400 mg/dL), estimation of LDL-C is invalid.
The Martin–Hopkins equation offers a validated estimation of LDL-C when the LDL-C value is < 70 mg/dL.3 This equation—in which the fixed factor of 5 used in the Friedewald equation to estimate very-low-density lipoprotein cholesterol is replaced by an adjustable factor that is based on the patient’s non-HDL-C (ie, TC – HDL-C) and TG values—is preferred by the ACC/AHA Task Force on Clinical Practice Guidelines in this clinical circumstance.4
National Institutes of Health equation. This newer equation provides an accurate estimate of the LDL-C level in patients whose TG value is ≤ 800 mg/dL. The equation has not been fully validated for clinical use, however.5
Direct measurement obviates the need for an equation to estimate LDL-C, but the test is not available in all health care settings.
For adults ≥ 20 years of age who are not receiving lipid-lowering therapy, a nonfasting lipid profile can be used to estimate ASCVD risk and document the baseline LDL-C level. If the TG level is ≥ 400 mg/dL, the test should be administered in the fasting state.4
Continue to: Apolipoprotein B
Apolipoprotein B. Alternatively, apolipoprotein B (apoB) can be measured. Because each LDL-C particle contains 1 apoB molecule, the apoB level describes the LDL-C level more accurately than a calculation of LDL-C. Many patients with type 2 diabetes and metabolic syndrome have a relatively low calculated LDL-C (thereby falsely reassuring the testing clinician) but have an elevated apoB level. An apoB level ≥ 130 mg/dL corresponds to an LDL-C level >160 mg/dL.4
Calculation of non-HDL-C. Because the nonfasting state does not have a significant impact on a patient’s TC and HDL-C levels, the non-HDL-C level also can be calculated from the results of a nonfasting lipid profile.
Non-HDL-C and apoB are equivalent predictors of ASCVD risk. These 2 assessments might offer better risk estimation than other available tools in patients who have type 2 diabetes and metabolic syndrome.6
Applying the estimate of 10-year ASCVD risk
Your recommendation for preventive intervention, such as lipid-lowering therapy, should be based on the estimated 10-year risk for ASCVD. Although multiple validated risk assessment tools are available, ACC/AHA recommends the pooled cohort risk equations (PCE), introduced in the 2013 ACC/AHA cholesterol treatment guidelines. The Framingham Heart Study now recommends the ACC/AHA PCE for risk assessment as well.7
The PCE, developed from 5 large cohorts, is based on hard atherosclerotic events: nonfatal myocardial infarction, death from coronary artery disease, and stroke. The ACC/AHA PCE is the only risk assessment tool developed using a significant percentage of patients who self-identify as Black.8 Alternatives to the ACC/AHA PCE include:
- Multi-ethnic Study of Atherosclerosis (MESA) 10-year ASCVD risk calculator, which incorporates the coronary artery calcium (CAC) score.
- Reynolds Risk Score, which incorporates high-sensitivity C-reactive protein measurement and a family history of premature ASCVD.9
Continue to: How much does lifestyle modification actually matter?
How much does lifestyle modification actually matter?
The absolute impact of diet and exercise on lipid parameters is relatively modest. No studies have demonstrated a reduction in adverse cardiovascular outcomes with specific interventions regarding diet or activity.
Diet. Nevertheless, ACC/AHA recommends that at-risk patients follow a dietary pattern that (1) emphasizes vegetables, fruits, and whole grains and (2) limits sweets, sugar-sweetened beverages, and red meat.
Saturated fat should constitute no more than 5% or 6% of total calories. In controlled-feeding trials,10 for every 1% of calories from saturated fat that are replaced with carbohydrate or monounsaturated or polyunsaturated fat, the LDL-C level was found to decline by as much as 1.8 mg/dL. Evidence is insufficient to assert that lowering dietary cholesterol reduces LDL-C.11
Activity. Trials of aerobic physical activity, compared with a more sedentary activity pattern, have demonstrated a reduction in the LDL-C level of as much as 6 mg/dL. All adult patients should be counseled to engage in aerobic physical activity of moderate or vigorous intensity—averaging ≥ 40 minutes per session, 3 or 4 sessions per week.11
Primary prevention:Stratification by age
40 to 75 years. ACC/AHA recommends that you routinely assess traditional cardiovascular risk factors for these patients and calculate their 10-year risk for ASCVD using the PCE. Statin therapy as primary prevention is indicated for 3 major groups (TABLE 1).4 The US Preventive Services Task Force (USPSTF) recommends a 10-year ASCVD risk ≥ 10%, in conjunction with 1 or more additional CVD risk factors (dyslipidemia, diabetes, hypertension, smoking), as the threshold for initiating low- or moderate-intensity statin therapy in this age group.12

Continue to: In adults at borderline risk...
In adults at borderline risk (5% to < 7.5% 10-year ASCVD risk) or intermediate risk (≥ 7.5% to < 20% 10-year ASCVD risk), consider risk-enhancing factors to better inform your recommendation for preventive interventions. In these 2 groups, the presence of risk-enhancing factors might justify moderate-intensity statin therapy (TABLE 24).

If your decision regarding preventive intervention remains uncertain, measuring CAC might further guide your discussion with the patient.4 When the CAC score is:
- 0 Agatston units and higher-risk conditions (eg, diabetes, family history of premature coronary artery disease, smoking) are absent, statin therapy can be withheld; reassess ASCVD risk in 5 to 10 years.
- 1-99 Agatston units, statin therapy can be started, especially for patients ≥ 55 years of age.
- ≥ 100 Agatston units or ≥ 75th percentile, statin therapy is indicated for all patients, regardless of additional risk factors.4
Because statins promote progression from unstable, inflammatory atherosclerotic plaque to more stable, calcified plaque, CAC scoring is not valid in patients already on statin therapy.13
In primary prevention, patients who have been classified as having low or intermediate risk, based on ASCVD risk scoring, with a CAC score of 0 Agatston units, have an annual all-cause mortality < 1%, regardless of age and gender. Patients classified as being at high risk, based on ASCVD risk scoring, with a CAC score of 0 Agatston units, have a significantly lower annual mortality than low- or intermediate-risk patients with a CAC score > 0 Agatston units.14
20 to 39 years. Focus on evaluation of lifetime ASCVD risk, rather than short-term (10-year) risk. Lifestyle modification is the primary intervention for younger patients; for those with moderate hypercholesterolemia (LDL-C, 160-189 mg/dL) and a family history of premature ASCVD, however, consider statin therapy. For patients with LDL-C ≥ 190 mg/dL, lifetime ASCVD risk is markedly increased, and high-intensity statin therapy is recommended, regardless of age. In this group, reassess ASCVD risk factors every 4 to 6 years.4
Continue to: > 75 years, without ASCVD
> 75 years, without ASCVD. In this group, the benefit of statin therapy is less clear and might be lessened by an increased potential for adverse effects. A meta-analysis of 28 trials demonstrated that people ages > 75 years had a 24% relative reduction in major coronary events for every 38.7 mg/dL (1.0 mmol/L) reduction in LDL-C, which is comparable to the risk reduction seen in people ages 40 to 75 years.15
With increasing age, however, the relative reduction in major coronary events with statin therapy decreased,15 although other trials have not demonstrated age heterogeneity.16 Because people > 75 years of age have a significantly higher ASCVD event rate, a comparable relative rate reduction with statin therapy results in a larger absolute rate reduction (ARR) and, therefore, a smaller number needed to treat (NNT) to prevent an event, compared to the NNT in younger people.
Secondary prevention
ACC/AHA guidelines define clinical ASCVD as a history of:
- acute coronary syndrome
- myocardial infarction
- coronary or other arterial revascularization
- cerebrovascular event
- symptomatic peripheral artery disease, including aortic aneurysm.
High-intensity statin therapy is indicated for all patients ≤ 75 years who have clinical ASCVD. In patients > 75 years, consider a taper to moderate-intensity statin therapy. An upper age limit for seeing benefit from statin therapy in secondary prevention has not been identified.4
In high-risk patients, if LDL-C remains ≥ 70 mg/dL despite maximally tolerated statin therapy, ezetimibe (discussed in the next section) can be added. In very-high-risk patients, if LDL-C remains ≥ 70 mg/dL despite maximally tolerated statin therapy plus ezetimibe, a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor (also discussed next) can be added. Always precede initiation of a PCSK9 inhibitor with a discussion of the net benefit, safety, and cost with the patient.4
Continue to: Options for lipid-lowering pharmacotherapy
Options for lipid-lowering pharmacotherapy
Statins (formally, hydroxymethylglutaryl-coenzyme A reductase inhibitors) offer the most predictable reduction in ASCVD risk of any lipid-lowering therapy. The evidence report that accompanied the 2016 USPSTF guidelines on statins for the prevention of cardiovascular disease (CVD) stated that low- or moderate-dosage statin therapy is associated with approximately a 30% relative risk reduction (RRR) in CVD events and CVD deaths and a 10% to 15% RRR in all-cause mortality.17
High-intensity statin therapy reduces LDL-C by ≥ 50%. Moderate-intensity statin therapy reduces LDL-C by 30% to 49% (TABLE 3).4

Statins are not without risk: A 2016 report18 estimated that treating 10,000 patients with a statin for 5 years would cause 1 case of rhabdomyolysis, 5 cases of myopathy, 75 new cases of diabetes, and 7 cases of hemorrhagic stroke. The same treatment would, however, avert approximately 1000 CVD events among patients with preexisting disease and approximately 500 CVD events among patients at elevated risk but without preexisting disease.18
Ezetimibe, a selective cholesterol-absorption inhibitor, lowers LDL-C by 13% to 20% and typically is well tolerated. The use of ezetimibe in ASCVD risk reduction is supported by a single randomized controlled trial of more than 18,000 patients with recent acute coronary syndrome. Adding ezetimibe to simvastatin 40 mg resulted in a 2% absolute reduction in major adverse cardiovascular events over a median follow-up of 6 years (NNT = 50), compared to simvastatin alone.19 ACC/AHA guidelines recommend adding ezetimibe to maximally tolerated statin therapy in patients with clinical ASCVD who do not reach their goal LDL reduction with a statin alone. Ezetimibe also can be considered a statin alternative in patients who are statin intolerant.4
PCSK9 inhibitors. When added to statin therapy, evolocumab and alirocumab—monoclonal antibodies that inhibit PCSK9—offer an incremental decrease in LDL-C of approximately 60%.20-22 In a meta-analysis of 35 trials evaluating the incremental benefit of PCSK9 inhibitor therapy, a significant reduction in cardiovascular events, including myocardial infarction (ARR = 1.3%; NNT = 77), stroke (ARR = 0.4%; NNT = 250), and coronary revascularization (ARR = 1.6%; NNT = 63) was reported. No significant difference was observed in all-cause or cardiovascular mortality.21,23
Continue to: Inclisiran
Inclisiran, an injectable small-interfering RNA that inhibits PCSK9 synthesis, provides an incremental decrease in LDL-C of > 50% in patients already receiving statin therapy. Meta-analysis of 3 small cardiovascular outcomes trials revealed no significant difference in the rate of myocardial infarction, stroke, or cardiovascular mortality with inclisiran compared to placebo. Larger outcomes trials are underway and might offer additional insight into this agent’s role in ASCVD risk management.24
Omega-3 fatty acids. Multiple trials have demonstrated that adding omega-3 fatty acids to usual lipid-lowering therapy does not offer a consistent reduction in adverse cardiovascular outcomes, despite providing a significant reduction in TG levels. In a high-risk population with persistently elevated TG despite statin therapy, icosapent ethyl, a purified eicosapentaenoic acid ethyl ester, reduced major ASCVD outcomes by 25% over a median 4.9 years (ARR = 4.8%; NNT = 21), and cardiovascular death by 20% (ARR = 0.9%; NNT = 111), compared with a mineral oil placebo.25 Subsequent trials, using a corn oil placebo, failed to duplicate these data26—raising concern that the mineral oil comparator might have altered results of the eicosapentaenoic acid ethyl ester study.27,28
Bempedoic acid is a small-molecule inhibitor of ATP citrate lyase that increases LDL uptake by the liver. Pooled data from studies of bempedoic acid show, on average, a 15% reduction in TC, a 23% reduction in LDL-C, and a 6% increase in HDL-C, without a significant change in TG.29 In statin-intolerant patients, bempedoic acid reduced major ASCVD outcomes by 13% over a median 40 months (ARR = 1.6%; NNT = 63), with no significant reduction in cardiovascular death.30
Niacin. Two large trials failed to demonstrate improvement in major cardiovascular events or other clinical benefit when niacin is added to moderate-intensity statin therapy, despite a significant increase in the HDL-C level (on average, 6 mg/dL) and a decrease in the LDL-C level (10-12 mg/dL) and TG (42 mg/dL).31,32
Fenofibrate lowers TG and increases HDL-C but does not consistently improve cardiovascular outcomes.33 In a trial of patients with type 2 diabetes and persistent dyslipidemia (serum TG > 204 mg/dL; HDL-C < 34 mg/dL) despite statin therapy, adding fenofibrate reduced CVD outcomes by 4.9%—although this absolute difference did not reach statistical significance.34
Neither niacin nor fenofibrate is considered useful for reducing ASCVD risk across broad populations.4
Follow-up to assess progress toward goals
Recheck the lipid profile 4 to 12 weeks after starting lipid-lowering therapy to verify adherence to medication and assess response. The primary goal is the percentage reduction in LDL-C based on ASCVD risk. An additional goal for very-high-risk patients is an LDL-C value ≤ 70 mg/dL. If the reduction in LDL-C is less than desired and adherence is assured, consider titrating the statin dosage or augmenting statin therapy with a nonstatin drug (eg, ezetimibe), or both.4
CORRESPONDENCE
Jonathon M. Firnhaber, MD, MAEd, MBA, East Carolina University, Family Medicine Center, 101 Heart Drive, Greenville, NC 27834; [email protected]
1. Kannel WB, Dawber TR, Kagan A, et al. Factors of risk in the development of coronary heart disease—six-year follow-up experience. The Framingham Study. Ann Intern Med. 1961;55:33. doi: 10.7326/0003-4819-55-1-33
2. Arnett DK, Blumenthal RS, Albert MA, et al; American Association of Cardiovascular and Pulmonary Rehabilitation, American Geriatrics Society, American Society of Preventive Cardiology, and Preventive Cardiovascular Nurses Association. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596-e646. doi: 10.1161/CIR.0000000000000678
3. Martin SS, Blaha MJ, Elshazly MB, et al. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA. 2013;310:2061-2068. doi: 10.1001/jama.2013.280532
4. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation. 2019;139:e1082-1143. doi: 10.1161/CIR.0000000000000625
5. Sampson M, Ling C, Sun Q, et al. A new equation for calculation of low-density lipoprotein cholesterol in patients with normolipidemia and/or hypertriglyceridemia. JAMA Cardiol. 2020;5:540-548. doi: 10.1001/jamacardio.2020.0013
6. Sniderman AD, Williams K, Contois JH, et al. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2011;4:337-345. doi: 10.1161/CIRCOUTCOMES.110.959247
7. Framingham Heart Study. Cardiovascular disease (10-year risk). Accessed February 14, 2023. www.framinghamheartstudy.org/fhs-risk-functions/cardiovascular-disease-10-year-risk/
8. Stone NJ, Robinson JG, Lichtenstein AH, et al; . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation. 2014;129(25 suppl 2):S1-S45. doi: 10.1161/01.cir.0000437738.63853.7a
9. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(suppl 2):1-87. doi: 10.4158/EP171764.APPGL
10. Mensink RP, Zock PL, Kester ADM, et al. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146
11. Eckel RH, Jakicic JM, Ard JD, et al; . 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S76-S99. doi: 10.1161/01.cir.0000437740.48606.d1
12. Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1997-2007. doi: 10.1001/jama.2016.15450
13. Lee S-E, Chang H-J, Sung JM, et al. Effects of statins on coronary atherosclerotic plaques: the PARADIGM study. JACC Cardiovasc Imaging. 2018;11:1475-1484. doi: 10.1016/j.jcmg.2018.04.015
14. Valenti V, Hartaigh B, Heo R, et al. A 15-year warranty period for asymptomatic individuals without coronary artery calcium: a prospective follow-up of 9,715 individuals. JACC Cardiovasc Imaging. 2015;8:900-909. doi: 10.1016/j.jcmg.2015.01.025
15. Armitage J, Baigent C, Barnes E, et al; . Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393:407-415. doi: 10.1016/S0140-6736(18)31942-1
16. Ridker PM, Lonn E, Paynter NP, et al. Primary prevention with statin therapy in the elderly: new meta-analyses from the contemporary JUPITER and HOPE-3 randomized trials. Circulation. 2017;135:1979-1981. doi: 10.1161/CIRCULATIONAHA.117.028271
17. Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008-2024. doi: 10.1001/jama.2015.15629
18. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532-2561. doi: 10.1016/S0140-6736(16)31357-5
19. Cannon CP, Blazing MA, Giugliano RP, et al; . Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397. doi: 10.1056/NEJMoa1410489
20. Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:2373-2384. doi: 10.1001/jama.2016.16951
21. Sabatine MS, Giugliano RP, Wiviott SD, et al; . Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509. doi: 10.1056/NEJMoa1500858
22. Robinson JG, Farnier M, Krempf M, et al; . Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499. doi: 10.1056/NEJMoa1501031
23. Karatasakis A, Danek BA, Karacsonyi J, et al. Effect of PCSK9 inhibitors on clinical outcomes in patients with hypercholesterolemia: a meta‐analysis of 35 randomized controlled trials. J Am Heart Assoc. 2017;6:e006910. doi: 10.1161/JAHA.117.006910
24. Khan SA, Naz A, Qamar Masood M, et al. Meta-analysis of inclisiran for the treatment of hypercholesterolemia. Am J Cardiol. 2020;134:69-73. doi: 10.1016/j.amjcard.2020.08.018
25. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22. doi: 10.1056/NEJMoa1812792
26. Nicholls SJ, Lincoff AM, Garcia M, et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 2020;324:2268-2280. doi: 10.1001/jama.2020.22258
27. Nissen SE, Lincoff AM, Wolski K, et al. Association between achieved ω-3 fatty acid levels and major adverse cardiovascular outcomes in patients with high cardiovascular risk. JAMA Cardiol. 2021;6:1-8. doi: 10.1001/jamacardio.2021.1157
28. US Food and Drug Administration. Briefing document: Endocrinologic and Metabolic Drugs Advisory Committee meeting, November 14, 2019. Accessed February 15, 2023. www.fda.gov/media/132477/download
29. Cicero AFG, Fogacci F, Hernandez AV, et al. Efficacy and safety of bempedoic acid for the treatment of hypercholesterolemia: a systematic review and meta-analysis. PLOS Med. 2020;17:e1003121. doi: 10.1371/journal.pmed.1003121
30. Nissen SE, Lincoff AM, Brennan D, et al; CLEAR Outcomes Investigators. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N Engl J Med. Published online March 4, 2023. doi: 10.1056/NEJMoa2215024
31. Landray MJ, Haynes R, Hopewell JC, et al; . Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203-212. doi: 10.1056/NEJMoa1300955
32. Boden WE, Probstfield JL, Anderson T, et al; AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267. doi: 10.1056/NEJMoa1107579
33. Elam MB, Ginsberg HN, Lovato LC, et al; ACCORDION Study Investigators. Association of fenofibrate therapy with long-term cardiovascular risk in statin-treated patients with type 2 diabetes. JAMA Cardiol. 2017;2:370-380. doi: 10.1001/jamacardio.2016.4828
34. Ginsberg HN, Elam MB, Lovato LC, et al; ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574. doi: 10.1056/NEJMoa1001282
1. Kannel WB, Dawber TR, Kagan A, et al. Factors of risk in the development of coronary heart disease—six-year follow-up experience. The Framingham Study. Ann Intern Med. 1961;55:33. doi: 10.7326/0003-4819-55-1-33
2. Arnett DK, Blumenthal RS, Albert MA, et al; American Association of Cardiovascular and Pulmonary Rehabilitation, American Geriatrics Society, American Society of Preventive Cardiology, and Preventive Cardiovascular Nurses Association. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596-e646. doi: 10.1161/CIR.0000000000000678
3. Martin SS, Blaha MJ, Elshazly MB, et al. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA. 2013;310:2061-2068. doi: 10.1001/jama.2013.280532
4. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation. 2019;139:e1082-1143. doi: 10.1161/CIR.0000000000000625
5. Sampson M, Ling C, Sun Q, et al. A new equation for calculation of low-density lipoprotein cholesterol in patients with normolipidemia and/or hypertriglyceridemia. JAMA Cardiol. 2020;5:540-548. doi: 10.1001/jamacardio.2020.0013
6. Sniderman AD, Williams K, Contois JH, et al. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2011;4:337-345. doi: 10.1161/CIRCOUTCOMES.110.959247
7. Framingham Heart Study. Cardiovascular disease (10-year risk). Accessed February 14, 2023. www.framinghamheartstudy.org/fhs-risk-functions/cardiovascular-disease-10-year-risk/
8. Stone NJ, Robinson JG, Lichtenstein AH, et al; . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation. 2014;129(25 suppl 2):S1-S45. doi: 10.1161/01.cir.0000437738.63853.7a
9. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(suppl 2):1-87. doi: 10.4158/EP171764.APPGL
10. Mensink RP, Zock PL, Kester ADM, et al. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146
11. Eckel RH, Jakicic JM, Ard JD, et al; . 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S76-S99. doi: 10.1161/01.cir.0000437740.48606.d1
12. Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1997-2007. doi: 10.1001/jama.2016.15450
13. Lee S-E, Chang H-J, Sung JM, et al. Effects of statins on coronary atherosclerotic plaques: the PARADIGM study. JACC Cardiovasc Imaging. 2018;11:1475-1484. doi: 10.1016/j.jcmg.2018.04.015
14. Valenti V, Hartaigh B, Heo R, et al. A 15-year warranty period for asymptomatic individuals without coronary artery calcium: a prospective follow-up of 9,715 individuals. JACC Cardiovasc Imaging. 2015;8:900-909. doi: 10.1016/j.jcmg.2015.01.025
15. Armitage J, Baigent C, Barnes E, et al; . Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393:407-415. doi: 10.1016/S0140-6736(18)31942-1
16. Ridker PM, Lonn E, Paynter NP, et al. Primary prevention with statin therapy in the elderly: new meta-analyses from the contemporary JUPITER and HOPE-3 randomized trials. Circulation. 2017;135:1979-1981. doi: 10.1161/CIRCULATIONAHA.117.028271
17. Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008-2024. doi: 10.1001/jama.2015.15629
18. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532-2561. doi: 10.1016/S0140-6736(16)31357-5
19. Cannon CP, Blazing MA, Giugliano RP, et al; . Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397. doi: 10.1056/NEJMoa1410489
20. Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:2373-2384. doi: 10.1001/jama.2016.16951
21. Sabatine MS, Giugliano RP, Wiviott SD, et al; . Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509. doi: 10.1056/NEJMoa1500858
22. Robinson JG, Farnier M, Krempf M, et al; . Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499. doi: 10.1056/NEJMoa1501031
23. Karatasakis A, Danek BA, Karacsonyi J, et al. Effect of PCSK9 inhibitors on clinical outcomes in patients with hypercholesterolemia: a meta‐analysis of 35 randomized controlled trials. J Am Heart Assoc. 2017;6:e006910. doi: 10.1161/JAHA.117.006910
24. Khan SA, Naz A, Qamar Masood M, et al. Meta-analysis of inclisiran for the treatment of hypercholesterolemia. Am J Cardiol. 2020;134:69-73. doi: 10.1016/j.amjcard.2020.08.018
25. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22. doi: 10.1056/NEJMoa1812792
26. Nicholls SJ, Lincoff AM, Garcia M, et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 2020;324:2268-2280. doi: 10.1001/jama.2020.22258
27. Nissen SE, Lincoff AM, Wolski K, et al. Association between achieved ω-3 fatty acid levels and major adverse cardiovascular outcomes in patients with high cardiovascular risk. JAMA Cardiol. 2021;6:1-8. doi: 10.1001/jamacardio.2021.1157
28. US Food and Drug Administration. Briefing document: Endocrinologic and Metabolic Drugs Advisory Committee meeting, November 14, 2019. Accessed February 15, 2023. www.fda.gov/media/132477/download
29. Cicero AFG, Fogacci F, Hernandez AV, et al. Efficacy and safety of bempedoic acid for the treatment of hypercholesterolemia: a systematic review and meta-analysis. PLOS Med. 2020;17:e1003121. doi: 10.1371/journal.pmed.1003121
30. Nissen SE, Lincoff AM, Brennan D, et al; CLEAR Outcomes Investigators. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N Engl J Med. Published online March 4, 2023. doi: 10.1056/NEJMoa2215024
31. Landray MJ, Haynes R, Hopewell JC, et al; . Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203-212. doi: 10.1056/NEJMoa1300955
32. Boden WE, Probstfield JL, Anderson T, et al; AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267. doi: 10.1056/NEJMoa1107579
33. Elam MB, Ginsberg HN, Lovato LC, et al; ACCORDION Study Investigators. Association of fenofibrate therapy with long-term cardiovascular risk in statin-treated patients with type 2 diabetes. JAMA Cardiol. 2017;2:370-380. doi: 10.1001/jamacardio.2016.4828
34. Ginsberg HN, Elam MB, Lovato LC, et al; ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574. doi: 10.1056/NEJMoa1001282
PRACTICE RECOMMENDATIONS
› Use an alternative to the Friedewald equation, such as the Martin–Hopkins equation, to estimate the low-density lipoprotein cholesterol (LDL-C) value; order direct measurement of LDL-C; or calculate non–high-density lipoprotein cholesterol to assess the risk for atherosclerotic cardiovascular disease (ASCVD) in patients who have a low LDL-C or a high triglycerides level. C
› Consider the impact of ASCVD risk-enhancing factors and coronary artery calcium scoring in making a recommendation to begin lipid-lowering therapy in intermediate-risk patients. C
› Add ezetimibe if a statin does not sufficiently lower LDL-C or if a patient cannot tolerate an adequate dosage of the statin. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series