User login
New ASE guideline on interventional echocardiography training
The American Society of Echocardiography (ASE) has issued guidance on all critical aspects of training for cardiology and anesthesiology trainees and postgraduate echocardiographers who plan to specialize in interventional echocardiography (IE).
The guideline outlines requirements of the training institution, the duration and core competencies of training, minimal procedural volume for competency in IE, and knowledge of specific structural health disease (SHD) procedures.
The 16-page guideline was published online in the Journal of the American Society of Echocardiography.
Specific skill set
IE is the primary imaging modality used to support and guide SHD interventions, such as heart valve replacements and other cardiac catheterization procedures, the writing group notes.
They say the “emerging specialty” of IE requires a specific set of skills to support an array of transcatheter therapies, with successful outcomes highly dependent on the skill of the echocardiography team.
“IE techniques are unique since imaging is performed in real-time, it is highly dependent on 3D and non-standard views, and it has immediate and profound implications for patient management,” Stephen H. Little, MD, ASE president and co-chair of the guideline writing group, says in a news release.
“Additionally, IE requires candid, accurate, and timely communication with other members of the multidisciplinary SHD team,” Dr. Little adds.
The new ASE guideline expands on the 2019 statement on echocardiography training put forward by the American College of Cardiology, American Heart Association, and ASE, by focusing specifically on interventional echocardiographers.
It outlines core competencies common to all transcatheter therapies, as well as specific transcatheter procedures. It provides consensus recommendations for specific knowledge, experience, and skills to be learned and demonstrated within an IE training program or during postgraduate training.
A “core principle” in the guideline states that the length of IE training or achieved number of procedures performed are less important than the demonstration of procedure-specific competencies within the milestone domains of knowledge, skill, and communication.
“Transcatheter therapies for SHD continue to grow at a rapid pace, which means that the demand for skilled interventional echocardiographers has steadily increased,” Vera H. Rigolin, MD, co-chair of the guideline writing, says in the release.
“Training standards are needed to ensure that interventional echocardiographers have the necessary expertise to provide fast, accurate, and high-quality image acquisition and interpretation in real-time,” Dr. Rigolin adds.
In addition, the guidelines states that use of simulation training has a role in IE training.
Virtual and simulation training could shorten the learning curve for trainees and, when combined with remote learning, could permit societies to standardize a teaching curriculum and allow the trainee to complete training in a reasonable timeframe. Simulator training may also improve access to training and thus promote diversity and inclusivity, the writing group says.
The guideline has been endorsed by 21 ASE international partners.
Writing group co-chairs Little and Rigolin have declared no conflicts of interest. A complete list of disclosures for the writing group is available with the original article.
A version of this article first appeared on Medscape.com.
The American Society of Echocardiography (ASE) has issued guidance on all critical aspects of training for cardiology and anesthesiology trainees and postgraduate echocardiographers who plan to specialize in interventional echocardiography (IE).
The guideline outlines requirements of the training institution, the duration and core competencies of training, minimal procedural volume for competency in IE, and knowledge of specific structural health disease (SHD) procedures.
The 16-page guideline was published online in the Journal of the American Society of Echocardiography.
Specific skill set
IE is the primary imaging modality used to support and guide SHD interventions, such as heart valve replacements and other cardiac catheterization procedures, the writing group notes.
They say the “emerging specialty” of IE requires a specific set of skills to support an array of transcatheter therapies, with successful outcomes highly dependent on the skill of the echocardiography team.
“IE techniques are unique since imaging is performed in real-time, it is highly dependent on 3D and non-standard views, and it has immediate and profound implications for patient management,” Stephen H. Little, MD, ASE president and co-chair of the guideline writing group, says in a news release.
“Additionally, IE requires candid, accurate, and timely communication with other members of the multidisciplinary SHD team,” Dr. Little adds.
The new ASE guideline expands on the 2019 statement on echocardiography training put forward by the American College of Cardiology, American Heart Association, and ASE, by focusing specifically on interventional echocardiographers.
It outlines core competencies common to all transcatheter therapies, as well as specific transcatheter procedures. It provides consensus recommendations for specific knowledge, experience, and skills to be learned and demonstrated within an IE training program or during postgraduate training.
A “core principle” in the guideline states that the length of IE training or achieved number of procedures performed are less important than the demonstration of procedure-specific competencies within the milestone domains of knowledge, skill, and communication.
“Transcatheter therapies for SHD continue to grow at a rapid pace, which means that the demand for skilled interventional echocardiographers has steadily increased,” Vera H. Rigolin, MD, co-chair of the guideline writing, says in the release.
“Training standards are needed to ensure that interventional echocardiographers have the necessary expertise to provide fast, accurate, and high-quality image acquisition and interpretation in real-time,” Dr. Rigolin adds.
In addition, the guidelines states that use of simulation training has a role in IE training.
Virtual and simulation training could shorten the learning curve for trainees and, when combined with remote learning, could permit societies to standardize a teaching curriculum and allow the trainee to complete training in a reasonable timeframe. Simulator training may also improve access to training and thus promote diversity and inclusivity, the writing group says.
The guideline has been endorsed by 21 ASE international partners.
Writing group co-chairs Little and Rigolin have declared no conflicts of interest. A complete list of disclosures for the writing group is available with the original article.
A version of this article first appeared on Medscape.com.
The American Society of Echocardiography (ASE) has issued guidance on all critical aspects of training for cardiology and anesthesiology trainees and postgraduate echocardiographers who plan to specialize in interventional echocardiography (IE).
The guideline outlines requirements of the training institution, the duration and core competencies of training, minimal procedural volume for competency in IE, and knowledge of specific structural health disease (SHD) procedures.
The 16-page guideline was published online in the Journal of the American Society of Echocardiography.
Specific skill set
IE is the primary imaging modality used to support and guide SHD interventions, such as heart valve replacements and other cardiac catheterization procedures, the writing group notes.
They say the “emerging specialty” of IE requires a specific set of skills to support an array of transcatheter therapies, with successful outcomes highly dependent on the skill of the echocardiography team.
“IE techniques are unique since imaging is performed in real-time, it is highly dependent on 3D and non-standard views, and it has immediate and profound implications for patient management,” Stephen H. Little, MD, ASE president and co-chair of the guideline writing group, says in a news release.
“Additionally, IE requires candid, accurate, and timely communication with other members of the multidisciplinary SHD team,” Dr. Little adds.
The new ASE guideline expands on the 2019 statement on echocardiography training put forward by the American College of Cardiology, American Heart Association, and ASE, by focusing specifically on interventional echocardiographers.
It outlines core competencies common to all transcatheter therapies, as well as specific transcatheter procedures. It provides consensus recommendations for specific knowledge, experience, and skills to be learned and demonstrated within an IE training program or during postgraduate training.
A “core principle” in the guideline states that the length of IE training or achieved number of procedures performed are less important than the demonstration of procedure-specific competencies within the milestone domains of knowledge, skill, and communication.
“Transcatheter therapies for SHD continue to grow at a rapid pace, which means that the demand for skilled interventional echocardiographers has steadily increased,” Vera H. Rigolin, MD, co-chair of the guideline writing, says in the release.
“Training standards are needed to ensure that interventional echocardiographers have the necessary expertise to provide fast, accurate, and high-quality image acquisition and interpretation in real-time,” Dr. Rigolin adds.
In addition, the guidelines states that use of simulation training has a role in IE training.
Virtual and simulation training could shorten the learning curve for trainees and, when combined with remote learning, could permit societies to standardize a teaching curriculum and allow the trainee to complete training in a reasonable timeframe. Simulator training may also improve access to training and thus promote diversity and inclusivity, the writing group says.
The guideline has been endorsed by 21 ASE international partners.
Writing group co-chairs Little and Rigolin have declared no conflicts of interest. A complete list of disclosures for the writing group is available with the original article.
A version of this article first appeared on Medscape.com.
Urban green and blue spaces linked to less psychological distress
The findings of the study, which was released ahead of its scheduled presentation at the annual meeting of the American Academy of Neurology, build on a growing understanding of the relationship between types and qualities of urban environments and dementia risk.
Adithya Vegaraju, a student at Washington State University, Spokane, led the study, which looked at data from the Washington State Behavioral Risk Factor Surveillance System to assess prevalence of serious psychological distress among 42,980 Washington state residents aged 65 and over.
The data, collected between 2011 and 2019, used a self-reported questionnaire to determine serious psychological distress, which is defined as a level of mental distress considered debilitating enough to warrant treatment.
Mr. Vegaraju and his coauthor Solmaz Amiri, DDes, also of Washington State University, used ZIP codes, along with U.S. census data, to approximate the urban adults’ proximity to green and blue spaces.
After controlling for potential confounders of age, sex, ethnicity, education, and marital status, the investigators found that people living within half a mile of green or blue spaces had a 17% lower risk of experiencing serious psychological distress, compared with people living farther from these spaces, the investigators said in a news release.
Implications for cognitive decline and dementia?
Psychological distress in adults has been linked in population-based longitudinal studies to later cognitive decline and dementia. One study in older adults found the risk of dementia to be more than 50% higher among adults aged 50-70 with persistent depression. Blue and green spaces have also been investigated in relation to neurodegenerative disease among older adults; a 2022 study looking at data from some 62 million Medicare beneficiaries found those living in areas with more vegetation saw lower risk of hospitalizations for Alzheimer’s disease and related dementias.
“Since we lack effective prevention methods or treatments for mild cognitive impairment and dementia, we need to get creative in how we look at these issues,” Dr. Amiri commented in a press statement about her and Mr. Vegaraju’s findings. “Our hope is that this study showing better mental health among people living close to parks and water will trigger other studies about how these benefits work and whether this proximity can help prevent or delay mild cognitive impairment and dementia.”
The investigators acknowledged that their findings were limited by reliance on a self-reported measure of psychological distress.
A bidirectional connection with depression and dementia
In a comment, Anjum Hajat, PhD, an epidemiologist at University of Washington School of Public Health in Seattle who has also studied the relationship between green space and dementia risk in older adults, noted some further apparent limitations of the new study, for which only an abstract was available at publication.
“It has been shown that people with depression are at higher risk for dementia, but the opposite is also true,” Dr. Hajat commented. “Those with dementia are more likely to develop depression. This bidirectionality makes this study abstract difficult to interpret since the study is based on cross-sectional data: Individuals are not followed over time to see which develops first, dementia or depression.”
Additionally, Dr. Hajat noted, the data used to determine proximity to green and blue spaces did not allow for the calculation of precise distances between subjects’ homes and these spaces.
Mr. Vegaraju and Dr. Amiri’s study had no outside support, and the investigators declared no conflicts of interest. Dr. Hajat declared no conflicts of interest.
The findings of the study, which was released ahead of its scheduled presentation at the annual meeting of the American Academy of Neurology, build on a growing understanding of the relationship between types and qualities of urban environments and dementia risk.
Adithya Vegaraju, a student at Washington State University, Spokane, led the study, which looked at data from the Washington State Behavioral Risk Factor Surveillance System to assess prevalence of serious psychological distress among 42,980 Washington state residents aged 65 and over.
The data, collected between 2011 and 2019, used a self-reported questionnaire to determine serious psychological distress, which is defined as a level of mental distress considered debilitating enough to warrant treatment.
Mr. Vegaraju and his coauthor Solmaz Amiri, DDes, also of Washington State University, used ZIP codes, along with U.S. census data, to approximate the urban adults’ proximity to green and blue spaces.
After controlling for potential confounders of age, sex, ethnicity, education, and marital status, the investigators found that people living within half a mile of green or blue spaces had a 17% lower risk of experiencing serious psychological distress, compared with people living farther from these spaces, the investigators said in a news release.
Implications for cognitive decline and dementia?
Psychological distress in adults has been linked in population-based longitudinal studies to later cognitive decline and dementia. One study in older adults found the risk of dementia to be more than 50% higher among adults aged 50-70 with persistent depression. Blue and green spaces have also been investigated in relation to neurodegenerative disease among older adults; a 2022 study looking at data from some 62 million Medicare beneficiaries found those living in areas with more vegetation saw lower risk of hospitalizations for Alzheimer’s disease and related dementias.
“Since we lack effective prevention methods or treatments for mild cognitive impairment and dementia, we need to get creative in how we look at these issues,” Dr. Amiri commented in a press statement about her and Mr. Vegaraju’s findings. “Our hope is that this study showing better mental health among people living close to parks and water will trigger other studies about how these benefits work and whether this proximity can help prevent or delay mild cognitive impairment and dementia.”
The investigators acknowledged that their findings were limited by reliance on a self-reported measure of psychological distress.
A bidirectional connection with depression and dementia
In a comment, Anjum Hajat, PhD, an epidemiologist at University of Washington School of Public Health in Seattle who has also studied the relationship between green space and dementia risk in older adults, noted some further apparent limitations of the new study, for which only an abstract was available at publication.
“It has been shown that people with depression are at higher risk for dementia, but the opposite is also true,” Dr. Hajat commented. “Those with dementia are more likely to develop depression. This bidirectionality makes this study abstract difficult to interpret since the study is based on cross-sectional data: Individuals are not followed over time to see which develops first, dementia or depression.”
Additionally, Dr. Hajat noted, the data used to determine proximity to green and blue spaces did not allow for the calculation of precise distances between subjects’ homes and these spaces.
Mr. Vegaraju and Dr. Amiri’s study had no outside support, and the investigators declared no conflicts of interest. Dr. Hajat declared no conflicts of interest.
The findings of the study, which was released ahead of its scheduled presentation at the annual meeting of the American Academy of Neurology, build on a growing understanding of the relationship between types and qualities of urban environments and dementia risk.
Adithya Vegaraju, a student at Washington State University, Spokane, led the study, which looked at data from the Washington State Behavioral Risk Factor Surveillance System to assess prevalence of serious psychological distress among 42,980 Washington state residents aged 65 and over.
The data, collected between 2011 and 2019, used a self-reported questionnaire to determine serious psychological distress, which is defined as a level of mental distress considered debilitating enough to warrant treatment.
Mr. Vegaraju and his coauthor Solmaz Amiri, DDes, also of Washington State University, used ZIP codes, along with U.S. census data, to approximate the urban adults’ proximity to green and blue spaces.
After controlling for potential confounders of age, sex, ethnicity, education, and marital status, the investigators found that people living within half a mile of green or blue spaces had a 17% lower risk of experiencing serious psychological distress, compared with people living farther from these spaces, the investigators said in a news release.
Implications for cognitive decline and dementia?
Psychological distress in adults has been linked in population-based longitudinal studies to later cognitive decline and dementia. One study in older adults found the risk of dementia to be more than 50% higher among adults aged 50-70 with persistent depression. Blue and green spaces have also been investigated in relation to neurodegenerative disease among older adults; a 2022 study looking at data from some 62 million Medicare beneficiaries found those living in areas with more vegetation saw lower risk of hospitalizations for Alzheimer’s disease and related dementias.
“Since we lack effective prevention methods or treatments for mild cognitive impairment and dementia, we need to get creative in how we look at these issues,” Dr. Amiri commented in a press statement about her and Mr. Vegaraju’s findings. “Our hope is that this study showing better mental health among people living close to parks and water will trigger other studies about how these benefits work and whether this proximity can help prevent or delay mild cognitive impairment and dementia.”
The investigators acknowledged that their findings were limited by reliance on a self-reported measure of psychological distress.
A bidirectional connection with depression and dementia
In a comment, Anjum Hajat, PhD, an epidemiologist at University of Washington School of Public Health in Seattle who has also studied the relationship between green space and dementia risk in older adults, noted some further apparent limitations of the new study, for which only an abstract was available at publication.
“It has been shown that people with depression are at higher risk for dementia, but the opposite is also true,” Dr. Hajat commented. “Those with dementia are more likely to develop depression. This bidirectionality makes this study abstract difficult to interpret since the study is based on cross-sectional data: Individuals are not followed over time to see which develops first, dementia or depression.”
Additionally, Dr. Hajat noted, the data used to determine proximity to green and blue spaces did not allow for the calculation of precise distances between subjects’ homes and these spaces.
Mr. Vegaraju and Dr. Amiri’s study had no outside support, and the investigators declared no conflicts of interest. Dr. Hajat declared no conflicts of interest.
FROM AAN 2023
Bad sleep cuts years off life, but exercise can save us
Experts recommend that most adults get 7-9 hours of sleep a night.
Plenty of research points to sleep and physical activity as crucial factors affecting life expectancy. Regular exercise can lengthen life, while too little or too much sleep may cut it short.
But evidence is growing that exercise may counteract the negative effects of poor sleep. A 2022 study found that being physically active for at least 25 minutes a day can erase the risk of early death associated with too much sleep or trouble falling asleep. And a 2021 study found that lower levels of physical activity may exacerbate the impact of poor sleep on early death, heart disease, and cancer.
The latest such study, published in the European Journal of Preventive Cardiology, suggests that higher volumes of exercise can virtually eliminate the risk of early death associated with sleeping too little or too long.
This study is unique, the researchers say, because it used accelerometers (motion-tracking sensors) to quantify sleep and physical activity. Other studies asked participants to report their own data, opening the door to false reports and mistakes.
Some 92,000 participants in the United Kingdom (mean age, 62 years; 56% women) wore the activity trackers for a week to measure how much they moved and slept. In the following 7 years, 3,080 participants died, mostly from cardiovascular disease or cancer.
As one might expect, the participants who were least likely to die also exercised the most and slept the “normal” amount (6-8 hours a night, as defined by the study).
Compared with that group, those who exercised the least and slept less than 6 hours were 2.5 times more likely to die during those 7 years (P < .001). Less active persons who got the recommended sleep were 79% more likely to die (P < .001). The risk was slightly higher than that for those who logged more than 8 hours a night.
But those risks disappeared for short- or long-sleeping participants who logged at least 150 minutes a week of moderate to vigorous activity.
“Exercise fights inflammatory and metabolic dysregulations and abnormal sympathetic nervous system activity,” said study author Jihui Zhang, PhD, of the Affiliated Brain Hospital of Guangzhou (China). Those problems are associated with cardiovascular diseases and other potentially fatal conditions.
More objective data – with tech
A study’s findings are only as good as the data it relies on. That’s why obtaining objective data not influenced by individual perception is key.
“Self-report questionnaires are prone to misperception, or recall or response bias,” Dr. Zhang explains.
Take sleep, for example. Research reveals that several factors can affect how we judge our sleep. When people have to sleep at irregular times, they often underestimate how many hours they sleep but overestimate how long they nap, found a study in the Journal of Clinical Sleep Medicine.
Another study showed that when people are under a lot of stress, they’ll report more sleep problems than they actually have, as revealed by an Actiheart monitor.
With exercise, participants often report doing more exercise, and doing it at a higher intensity, than objective measurements show they did. At the same time, self-reports typically don’t account for much of the unplanned, low-effort movement people do throughout the day.
Staying active when you’re tired
The study raises a practical question: If you don’t get the proper amount of sleep, how are you supposed to find the time, energy, and motivation to exercise?
The solution is to use one to fix the other.
Exercise and sleep have “a robust directional relationship,” Dr. Zhang said. Exercise improves sleep, while better sleep makes it easier to stick with an exercise program.
Ideally, that program will include a mix of cardio and resistance exercise, said Mitch Duncan, PhD, a professor of public health at the University of Newcastle, Australia.
As Dr. Duncan and his co-authors showed in a recent study, “the largest benefits to health occur when people do a combination of both aerobic and muscle-strengthening activity,” Dr. Duncan said.
“In terms of benefits to sleep, there doesn’t seem to be consistent evidence that favors either as being most effective.”
The timing or intensity of exercise doesn’t seem to matter much, either.
“But there is evidence that a greater duration contributes to larger improvements in sleep,” Dr. Duncan said.
In other words, longer workouts are generally better, but they don’t necessarily have to be super-intense.
The strongest evidence of all, however, shows that recent and regular exercise offer the biggest benefits at bedtime.
Today’s workout will improve tonight’s sleep. And the better you sleep tonight, the more likely you are to stick with the program.
A version of this article first appeared on WebMD.com.
Experts recommend that most adults get 7-9 hours of sleep a night.
Plenty of research points to sleep and physical activity as crucial factors affecting life expectancy. Regular exercise can lengthen life, while too little or too much sleep may cut it short.
But evidence is growing that exercise may counteract the negative effects of poor sleep. A 2022 study found that being physically active for at least 25 minutes a day can erase the risk of early death associated with too much sleep or trouble falling asleep. And a 2021 study found that lower levels of physical activity may exacerbate the impact of poor sleep on early death, heart disease, and cancer.
The latest such study, published in the European Journal of Preventive Cardiology, suggests that higher volumes of exercise can virtually eliminate the risk of early death associated with sleeping too little or too long.
This study is unique, the researchers say, because it used accelerometers (motion-tracking sensors) to quantify sleep and physical activity. Other studies asked participants to report their own data, opening the door to false reports and mistakes.
Some 92,000 participants in the United Kingdom (mean age, 62 years; 56% women) wore the activity trackers for a week to measure how much they moved and slept. In the following 7 years, 3,080 participants died, mostly from cardiovascular disease or cancer.
As one might expect, the participants who were least likely to die also exercised the most and slept the “normal” amount (6-8 hours a night, as defined by the study).
Compared with that group, those who exercised the least and slept less than 6 hours were 2.5 times more likely to die during those 7 years (P < .001). Less active persons who got the recommended sleep were 79% more likely to die (P < .001). The risk was slightly higher than that for those who logged more than 8 hours a night.
But those risks disappeared for short- or long-sleeping participants who logged at least 150 minutes a week of moderate to vigorous activity.
“Exercise fights inflammatory and metabolic dysregulations and abnormal sympathetic nervous system activity,” said study author Jihui Zhang, PhD, of the Affiliated Brain Hospital of Guangzhou (China). Those problems are associated with cardiovascular diseases and other potentially fatal conditions.
More objective data – with tech
A study’s findings are only as good as the data it relies on. That’s why obtaining objective data not influenced by individual perception is key.
“Self-report questionnaires are prone to misperception, or recall or response bias,” Dr. Zhang explains.
Take sleep, for example. Research reveals that several factors can affect how we judge our sleep. When people have to sleep at irregular times, they often underestimate how many hours they sleep but overestimate how long they nap, found a study in the Journal of Clinical Sleep Medicine.
Another study showed that when people are under a lot of stress, they’ll report more sleep problems than they actually have, as revealed by an Actiheart monitor.
With exercise, participants often report doing more exercise, and doing it at a higher intensity, than objective measurements show they did. At the same time, self-reports typically don’t account for much of the unplanned, low-effort movement people do throughout the day.
Staying active when you’re tired
The study raises a practical question: If you don’t get the proper amount of sleep, how are you supposed to find the time, energy, and motivation to exercise?
The solution is to use one to fix the other.
Exercise and sleep have “a robust directional relationship,” Dr. Zhang said. Exercise improves sleep, while better sleep makes it easier to stick with an exercise program.
Ideally, that program will include a mix of cardio and resistance exercise, said Mitch Duncan, PhD, a professor of public health at the University of Newcastle, Australia.
As Dr. Duncan and his co-authors showed in a recent study, “the largest benefits to health occur when people do a combination of both aerobic and muscle-strengthening activity,” Dr. Duncan said.
“In terms of benefits to sleep, there doesn’t seem to be consistent evidence that favors either as being most effective.”
The timing or intensity of exercise doesn’t seem to matter much, either.
“But there is evidence that a greater duration contributes to larger improvements in sleep,” Dr. Duncan said.
In other words, longer workouts are generally better, but they don’t necessarily have to be super-intense.
The strongest evidence of all, however, shows that recent and regular exercise offer the biggest benefits at bedtime.
Today’s workout will improve tonight’s sleep. And the better you sleep tonight, the more likely you are to stick with the program.
A version of this article first appeared on WebMD.com.
Experts recommend that most adults get 7-9 hours of sleep a night.
Plenty of research points to sleep and physical activity as crucial factors affecting life expectancy. Regular exercise can lengthen life, while too little or too much sleep may cut it short.
But evidence is growing that exercise may counteract the negative effects of poor sleep. A 2022 study found that being physically active for at least 25 minutes a day can erase the risk of early death associated with too much sleep or trouble falling asleep. And a 2021 study found that lower levels of physical activity may exacerbate the impact of poor sleep on early death, heart disease, and cancer.
The latest such study, published in the European Journal of Preventive Cardiology, suggests that higher volumes of exercise can virtually eliminate the risk of early death associated with sleeping too little or too long.
This study is unique, the researchers say, because it used accelerometers (motion-tracking sensors) to quantify sleep and physical activity. Other studies asked participants to report their own data, opening the door to false reports and mistakes.
Some 92,000 participants in the United Kingdom (mean age, 62 years; 56% women) wore the activity trackers for a week to measure how much they moved and slept. In the following 7 years, 3,080 participants died, mostly from cardiovascular disease or cancer.
As one might expect, the participants who were least likely to die also exercised the most and slept the “normal” amount (6-8 hours a night, as defined by the study).
Compared with that group, those who exercised the least and slept less than 6 hours were 2.5 times more likely to die during those 7 years (P < .001). Less active persons who got the recommended sleep were 79% more likely to die (P < .001). The risk was slightly higher than that for those who logged more than 8 hours a night.
But those risks disappeared for short- or long-sleeping participants who logged at least 150 minutes a week of moderate to vigorous activity.
“Exercise fights inflammatory and metabolic dysregulations and abnormal sympathetic nervous system activity,” said study author Jihui Zhang, PhD, of the Affiliated Brain Hospital of Guangzhou (China). Those problems are associated with cardiovascular diseases and other potentially fatal conditions.
More objective data – with tech
A study’s findings are only as good as the data it relies on. That’s why obtaining objective data not influenced by individual perception is key.
“Self-report questionnaires are prone to misperception, or recall or response bias,” Dr. Zhang explains.
Take sleep, for example. Research reveals that several factors can affect how we judge our sleep. When people have to sleep at irregular times, they often underestimate how many hours they sleep but overestimate how long they nap, found a study in the Journal of Clinical Sleep Medicine.
Another study showed that when people are under a lot of stress, they’ll report more sleep problems than they actually have, as revealed by an Actiheart monitor.
With exercise, participants often report doing more exercise, and doing it at a higher intensity, than objective measurements show they did. At the same time, self-reports typically don’t account for much of the unplanned, low-effort movement people do throughout the day.
Staying active when you’re tired
The study raises a practical question: If you don’t get the proper amount of sleep, how are you supposed to find the time, energy, and motivation to exercise?
The solution is to use one to fix the other.
Exercise and sleep have “a robust directional relationship,” Dr. Zhang said. Exercise improves sleep, while better sleep makes it easier to stick with an exercise program.
Ideally, that program will include a mix of cardio and resistance exercise, said Mitch Duncan, PhD, a professor of public health at the University of Newcastle, Australia.
As Dr. Duncan and his co-authors showed in a recent study, “the largest benefits to health occur when people do a combination of both aerobic and muscle-strengthening activity,” Dr. Duncan said.
“In terms of benefits to sleep, there doesn’t seem to be consistent evidence that favors either as being most effective.”
The timing or intensity of exercise doesn’t seem to matter much, either.
“But there is evidence that a greater duration contributes to larger improvements in sleep,” Dr. Duncan said.
In other words, longer workouts are generally better, but they don’t necessarily have to be super-intense.
The strongest evidence of all, however, shows that recent and regular exercise offer the biggest benefits at bedtime.
Today’s workout will improve tonight’s sleep. And the better you sleep tonight, the more likely you are to stick with the program.
A version of this article first appeared on WebMD.com.
FROM EUROPEAN JOURNAL OF PREVENTIVE CARDIOLOGY
NSAID use in diabetes may worsen risk for first HF hospitalization
suggests a prospective, controlled study.
Certain subgroups may account for much of the excess risk, the results suggest, including the very elderly, patients with uncontrolled diabetes, those prescribed an NSAID for the first time, and patients already taking both a renin-angiotensin system inhibitor (RASi) and a diuretic.
Such patients with a firm indication for NSAIDs potentially could “be the ones benefiting most from closer follow-up, reduced dosage, or other mitigation strategies,” Anders Holt, MD, said in an interview.
Dr. Holt, of Copenhagen University Hospital and Herlev-Gentofte Hospital in Hellerup, Denmark, is lead author on the analysis of Danish registry data published in the Journal of the American College of Cardiology. He presented essentially the same results in preliminary form at the 2022 annual congress of the European Society of Cardiology.
HF hospitalizations linked to NSAIDs, the published report notes, are often attributed to symptoms from temporary fluid overload, often without worsening cardiac function, that stem from the drugs’ renal effects.
“One could speculate,” Dr. Holt said, that such HF events might be less severe and even associated with better outcomes, compared with other forms of heart failure.
But the current analysis provides a hint to the contrary, he observed. The 5-year mortality was similar for patients with HF linked to NSAIDs and those with other forms of HF, “which could suggest that NSAID-associated heart failure is more than transient fluid overload.”
The drugs may promote HF through direct effects on the heart by any of several proposed mechanisms, including “induction of arrhythmias and heart fibrosis, vasoconstriction, subclinical inflammation, and blood pressure elevation,” Dr. Holt said.
The current study doesn’t determine whether NSAID-associated HF stems from transient fluid overload or direct cardiac effects, but it’s “most likely both.”
In other limitations, the analysis is unable to “reliably explore” whether promotion of HF is an NSAID class effect, a “clinically relevant” point given the drugs’ varying effects on cardiovascular risk, states an accompanying editorial. Nor was it able to determine whether the drugs exert a dose-response effect on HF risk, noted Hassan Khan, MD, PhD, Norton Healthcare, Louisville, Ky., and Setor K. Kunutsor, MD, PhD, University of Leicester (England).
Still, “given the well-established relationship between the use of NSAIDs and increased HF, these findings are not unexpected because type 2 diabetes is also a major risk factor for HF.”
But it may be “premature to issue guideline recommendations based on a single observational study,” the editorialists wrote. “Further robust clinical trial evidence is needed to replicate these results and investigate the relationship of the type and dose of NSAIDs with HF risk. However, it should be realized that short-term or long-term use of NSAIDs may be detrimental to cardiovascular health.”
The analysis covered 23,308 patients from throughout Denmark with a type 2 diabetes diagnosis and no HF history who experienced a first HF hospitalization; their age averaged 76 years and 39% were women.
They served as their own controls; their NSAID exposures at two 28-day periods preceding the HF event, the one immediately before and the other preceding it by 56 days, were compared as the index and control periods, respectively.
Exposure to NSAIDs was defined as obtaining a prescription for celecoxib, diclofenac, ibuprofen, or naproxen, “as these are NSAIDs used primarily in Denmark,” the report states.
The odds ratios for HF hospitalization associated with NSAID exposure within 28 days preceding the event were 1.43 (95% confidence interval, 1.27-1.63) overall, 1.41 (95% CI, 1.16-1.71) for an NSAID given on top of both RASi and diuretics, 1.68 (95% CI, 1.00-2.88) for patients with elevated hemoglobin A1c, 1.78 (95% CI, 1.39-2.28) for those 80 or older, and 2.71 (95% CI, 1.78-4.23) for those with prior NSAID use.
That NSAID use and diabetes are each associated with increased risk for HF is well established, Dr. Holt observed. Yet the drugs had been prescribed to 16% of patients in the study.
“One of the more surprising findings, to me, was the quite substantial use of prescribed NSAIDs in a population of patients with diabetes, a patient group with a well-established cardiovascular risk,” he said.
“This patient group is only growing, so emphasis on the possible associations between even short-term NSAID use and incident heart failure is probably timely and perhaps needed.”
Dr. Holt and the study were supported by grants from Ib Mogens Kristiansens Almene Fond, Helsefonden, Snedkermester Sophus Jacobsen og hustru Astrid Jacobsen Fond, Marie og M.B. Richters Fond, and the Dagmar Marshalls Fond. Dr. Khan and Dr. Kunutsor reported no relevant relationships.
A version of this article first appeared on Medscape.com.
suggests a prospective, controlled study.
Certain subgroups may account for much of the excess risk, the results suggest, including the very elderly, patients with uncontrolled diabetes, those prescribed an NSAID for the first time, and patients already taking both a renin-angiotensin system inhibitor (RASi) and a diuretic.
Such patients with a firm indication for NSAIDs potentially could “be the ones benefiting most from closer follow-up, reduced dosage, or other mitigation strategies,” Anders Holt, MD, said in an interview.
Dr. Holt, of Copenhagen University Hospital and Herlev-Gentofte Hospital in Hellerup, Denmark, is lead author on the analysis of Danish registry data published in the Journal of the American College of Cardiology. He presented essentially the same results in preliminary form at the 2022 annual congress of the European Society of Cardiology.
HF hospitalizations linked to NSAIDs, the published report notes, are often attributed to symptoms from temporary fluid overload, often without worsening cardiac function, that stem from the drugs’ renal effects.
“One could speculate,” Dr. Holt said, that such HF events might be less severe and even associated with better outcomes, compared with other forms of heart failure.
But the current analysis provides a hint to the contrary, he observed. The 5-year mortality was similar for patients with HF linked to NSAIDs and those with other forms of HF, “which could suggest that NSAID-associated heart failure is more than transient fluid overload.”
The drugs may promote HF through direct effects on the heart by any of several proposed mechanisms, including “induction of arrhythmias and heart fibrosis, vasoconstriction, subclinical inflammation, and blood pressure elevation,” Dr. Holt said.
The current study doesn’t determine whether NSAID-associated HF stems from transient fluid overload or direct cardiac effects, but it’s “most likely both.”
In other limitations, the analysis is unable to “reliably explore” whether promotion of HF is an NSAID class effect, a “clinically relevant” point given the drugs’ varying effects on cardiovascular risk, states an accompanying editorial. Nor was it able to determine whether the drugs exert a dose-response effect on HF risk, noted Hassan Khan, MD, PhD, Norton Healthcare, Louisville, Ky., and Setor K. Kunutsor, MD, PhD, University of Leicester (England).
Still, “given the well-established relationship between the use of NSAIDs and increased HF, these findings are not unexpected because type 2 diabetes is also a major risk factor for HF.”
But it may be “premature to issue guideline recommendations based on a single observational study,” the editorialists wrote. “Further robust clinical trial evidence is needed to replicate these results and investigate the relationship of the type and dose of NSAIDs with HF risk. However, it should be realized that short-term or long-term use of NSAIDs may be detrimental to cardiovascular health.”
The analysis covered 23,308 patients from throughout Denmark with a type 2 diabetes diagnosis and no HF history who experienced a first HF hospitalization; their age averaged 76 years and 39% were women.
They served as their own controls; their NSAID exposures at two 28-day periods preceding the HF event, the one immediately before and the other preceding it by 56 days, were compared as the index and control periods, respectively.
Exposure to NSAIDs was defined as obtaining a prescription for celecoxib, diclofenac, ibuprofen, or naproxen, “as these are NSAIDs used primarily in Denmark,” the report states.
The odds ratios for HF hospitalization associated with NSAID exposure within 28 days preceding the event were 1.43 (95% confidence interval, 1.27-1.63) overall, 1.41 (95% CI, 1.16-1.71) for an NSAID given on top of both RASi and diuretics, 1.68 (95% CI, 1.00-2.88) for patients with elevated hemoglobin A1c, 1.78 (95% CI, 1.39-2.28) for those 80 or older, and 2.71 (95% CI, 1.78-4.23) for those with prior NSAID use.
That NSAID use and diabetes are each associated with increased risk for HF is well established, Dr. Holt observed. Yet the drugs had been prescribed to 16% of patients in the study.
“One of the more surprising findings, to me, was the quite substantial use of prescribed NSAIDs in a population of patients with diabetes, a patient group with a well-established cardiovascular risk,” he said.
“This patient group is only growing, so emphasis on the possible associations between even short-term NSAID use and incident heart failure is probably timely and perhaps needed.”
Dr. Holt and the study were supported by grants from Ib Mogens Kristiansens Almene Fond, Helsefonden, Snedkermester Sophus Jacobsen og hustru Astrid Jacobsen Fond, Marie og M.B. Richters Fond, and the Dagmar Marshalls Fond. Dr. Khan and Dr. Kunutsor reported no relevant relationships.
A version of this article first appeared on Medscape.com.
suggests a prospective, controlled study.
Certain subgroups may account for much of the excess risk, the results suggest, including the very elderly, patients with uncontrolled diabetes, those prescribed an NSAID for the first time, and patients already taking both a renin-angiotensin system inhibitor (RASi) and a diuretic.
Such patients with a firm indication for NSAIDs potentially could “be the ones benefiting most from closer follow-up, reduced dosage, or other mitigation strategies,” Anders Holt, MD, said in an interview.
Dr. Holt, of Copenhagen University Hospital and Herlev-Gentofte Hospital in Hellerup, Denmark, is lead author on the analysis of Danish registry data published in the Journal of the American College of Cardiology. He presented essentially the same results in preliminary form at the 2022 annual congress of the European Society of Cardiology.
HF hospitalizations linked to NSAIDs, the published report notes, are often attributed to symptoms from temporary fluid overload, often without worsening cardiac function, that stem from the drugs’ renal effects.
“One could speculate,” Dr. Holt said, that such HF events might be less severe and even associated with better outcomes, compared with other forms of heart failure.
But the current analysis provides a hint to the contrary, he observed. The 5-year mortality was similar for patients with HF linked to NSAIDs and those with other forms of HF, “which could suggest that NSAID-associated heart failure is more than transient fluid overload.”
The drugs may promote HF through direct effects on the heart by any of several proposed mechanisms, including “induction of arrhythmias and heart fibrosis, vasoconstriction, subclinical inflammation, and blood pressure elevation,” Dr. Holt said.
The current study doesn’t determine whether NSAID-associated HF stems from transient fluid overload or direct cardiac effects, but it’s “most likely both.”
In other limitations, the analysis is unable to “reliably explore” whether promotion of HF is an NSAID class effect, a “clinically relevant” point given the drugs’ varying effects on cardiovascular risk, states an accompanying editorial. Nor was it able to determine whether the drugs exert a dose-response effect on HF risk, noted Hassan Khan, MD, PhD, Norton Healthcare, Louisville, Ky., and Setor K. Kunutsor, MD, PhD, University of Leicester (England).
Still, “given the well-established relationship between the use of NSAIDs and increased HF, these findings are not unexpected because type 2 diabetes is also a major risk factor for HF.”
But it may be “premature to issue guideline recommendations based on a single observational study,” the editorialists wrote. “Further robust clinical trial evidence is needed to replicate these results and investigate the relationship of the type and dose of NSAIDs with HF risk. However, it should be realized that short-term or long-term use of NSAIDs may be detrimental to cardiovascular health.”
The analysis covered 23,308 patients from throughout Denmark with a type 2 diabetes diagnosis and no HF history who experienced a first HF hospitalization; their age averaged 76 years and 39% were women.
They served as their own controls; their NSAID exposures at two 28-day periods preceding the HF event, the one immediately before and the other preceding it by 56 days, were compared as the index and control periods, respectively.
Exposure to NSAIDs was defined as obtaining a prescription for celecoxib, diclofenac, ibuprofen, or naproxen, “as these are NSAIDs used primarily in Denmark,” the report states.
The odds ratios for HF hospitalization associated with NSAID exposure within 28 days preceding the event were 1.43 (95% confidence interval, 1.27-1.63) overall, 1.41 (95% CI, 1.16-1.71) for an NSAID given on top of both RASi and diuretics, 1.68 (95% CI, 1.00-2.88) for patients with elevated hemoglobin A1c, 1.78 (95% CI, 1.39-2.28) for those 80 or older, and 2.71 (95% CI, 1.78-4.23) for those with prior NSAID use.
That NSAID use and diabetes are each associated with increased risk for HF is well established, Dr. Holt observed. Yet the drugs had been prescribed to 16% of patients in the study.
“One of the more surprising findings, to me, was the quite substantial use of prescribed NSAIDs in a population of patients with diabetes, a patient group with a well-established cardiovascular risk,” he said.
“This patient group is only growing, so emphasis on the possible associations between even short-term NSAID use and incident heart failure is probably timely and perhaps needed.”
Dr. Holt and the study were supported by grants from Ib Mogens Kristiansens Almene Fond, Helsefonden, Snedkermester Sophus Jacobsen og hustru Astrid Jacobsen Fond, Marie og M.B. Richters Fond, and the Dagmar Marshalls Fond. Dr. Khan and Dr. Kunutsor reported no relevant relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
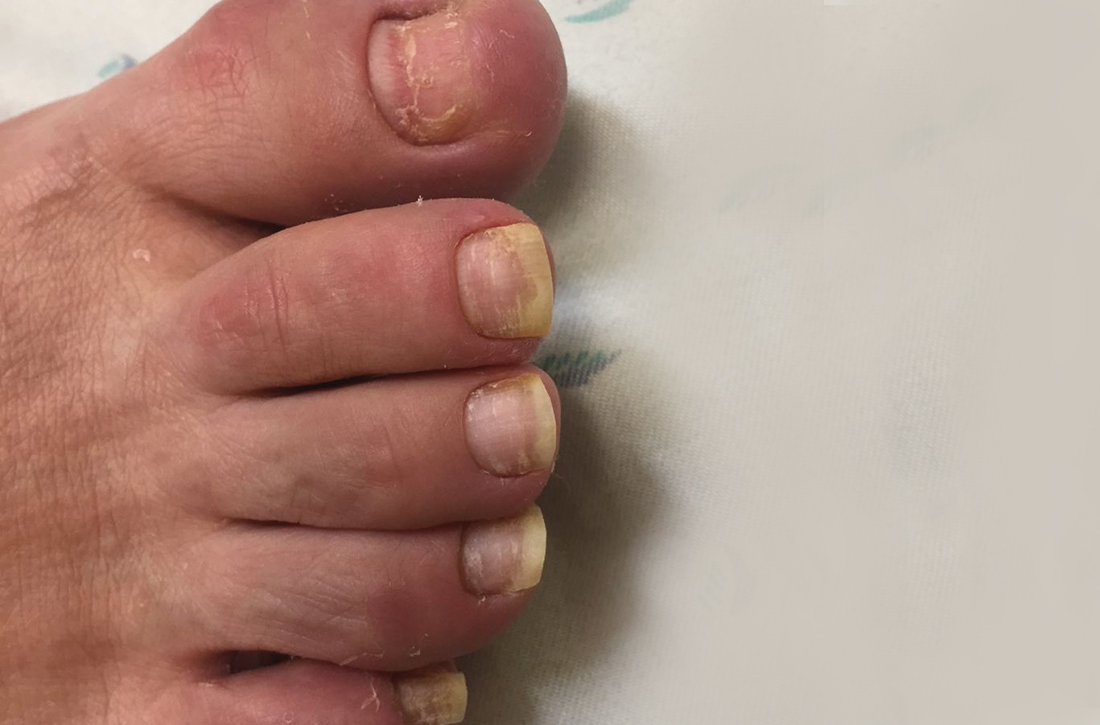
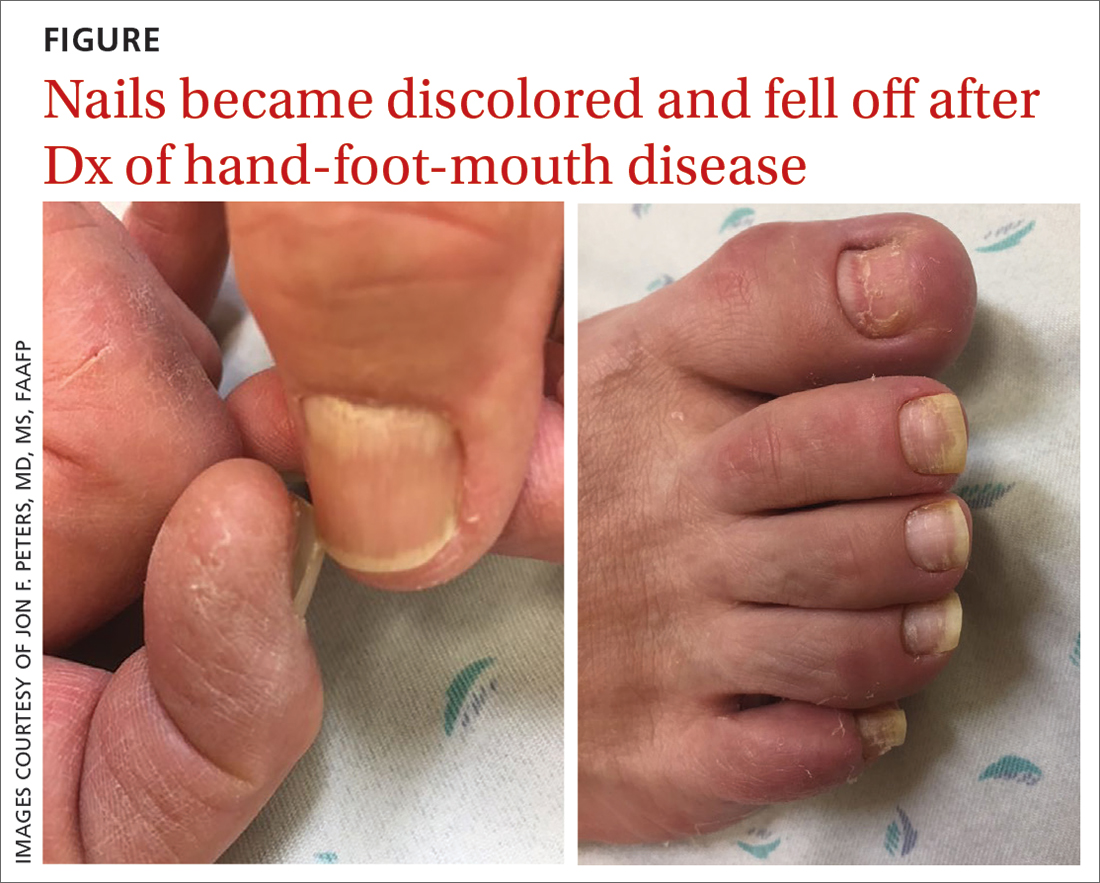
75-year-old man • recent history of hand-foot-mouth disease • discolored fingernails and toenails lifting from the proximal end • Dx?
THE CASE
A 75-year-old man sought care from his primary care physician because his “fingernails and toenails [were] all falling off.” He did not feel ill and had no other complaints. His vital signs were unremarkable. He had no history of malignancies, chronic skin conditions, or systemic diseases. His fingernails and toenails were discolored and lifting from the proximal end of his nail beds (FIGURE). One of his great toenails had already fallen off, 1 thumb nail was minimally attached with the cuticle, and the rest of his nails were loose and in the process of separating from their nail beds. There was no nail pitting, rash, or joint swelling and tenderness.
The patient reported that while on vacation in Hawaii 3 weeks earlier, he had sought care at an urgent care clinic for a painless rash on his hands and the soles of his feet. At that time, he did not feel ill or have mouth ulcers, penile discharge, or arthralgia. There had been no recent changes to his prescription medications, which included finasteride, terazosin, omeprazole, and an albuterol inhaler. He denied taking over-the-counter medications or supplements.
The physical exam at the urgent care had revealed multiple blotchy, dark, 0.5- to 1-cm nonpruritic lesions that were desquamating. No oral lesions were seen. He had been given a diagnosis of hand-foot-mouth disease (HFMD) and reassured that it would resolve on its own in about 10 days.
THE DIAGNOSIS
Several possible diagnoses for nail disorders came to mind with this patient, including onychomycosis, onychoschizia, onycholysis, and onychomadesis.
Onychomycosis is a chronic fungal infection of the nail that affects toenails more often than fingernails.1 The most common form is distal subungual onychomycosis, which begins distally and slowly migrates proximally through the nail matrix.1 Often onychomycosis affects only a few nails unless the patient is elderly or has comorbid conditions, and the nails rarely separate from the nail bed.
Onychoschizia involves lamellar splitting and peeling of the dorsal surface of the nail plate.2 Usually white discolorations appear on the distal edges of the nail.3 It is more common in women than in men and is often caused by nail dehydration from repeated excessive immersion in water with detergents or recurrent application of nail polish.2 However, the nails do not separate from the nail bed, and usually only the fingernails are involved.
Onycholysis is a nail attachment disorder in which the nail plate distally separates from the nail bed. Areas of separation will appear white or yellow. There are many etiologies for onycholysis, including trauma, psoriasis, fungal infection, and contact irritant reactions.3 It also can be caused by medications and thyroid disease.3,4
Continue to: Onychomadesis
Onychomadesis, sometimes considered a severe form of Beau’s line,5,6 is defined by the spontaneous separation of the nail plate from the nail matrix. Although the nail will initially remain attached, proximal shedding will eventually occur.7 When several nails are involved, a systemic source—such as an acute infection, autoimmune disease, medication, malignancy (eg, cutaneous T-cell lymphoma), Kawasaki disease, skin disorders (eg, pemphigus vulgaris or keratosis punctata et planters), or chemotherapy—may be the cause.6-8 If only a few nails are involved, it may be associated with trauma, and in rare cases, onychomadesis can be idiopathic.5,7
In this case, all signs pointed to onychomadesis. All of the patient’s nails were affected (discolored and lifting), his nail loss involved spontaneous proximal separation of the nail plate from the nail matrix, and he had a recent previous infection: HFMD.
DISCUSSION
Onychomadesis is a rare nail-shedding disorder thought to be caused by the temporary arrest of the nail matrix.8 It is a potential late complication of infection, such as HFMD,9 and was first reported in children in Chicago in 2000.10 Since then, onychomadesis has been noted in children in many countries.8 Reports of onychomadesis following HFMD in adults are rare, but it may be underreported because HFMD is more common in children and symptoms are usually minor in adults.11
Molecular studies have associated onychomadesis with coxsackievirus (CV)A6 and CVA10.4 Other serotypes associated with onychomadesis include CVB1, CVB2, CVA5, CVA16, and enteroviruses 71 and 9.4 Most known outbreaks seem to be caused by CVA6.4
No treatment is needed for onychomadesis; physicians can reassure patients that normal nail growth will begin within 1 to 4 months. Because onychomadesis is rare, it does not have its own billing code, so one can use code L60.8 for “Other nail disorders.”12
Our patient was seen in the primary care clinic 3 months after his initial visit. At that time, his nails were no longer discolored and no other abnormalities were present. All of the nails on his fingers and toes were firmly attached and growing normally.
THE TAKEAWAY
The sudden asymptomatic loss of multiple fingernails and toenails—especially with proximal nail shedding—is a rare disorder known as onychomadesis. It can be caused by various etiologies and can be a late complication of HFMD or other viral infections. Onychomadesis should be considered when evaluating older patients, particularly when all of their nails are involved after a viral infection.
CORRESPONDENCE
Jon F. Peters, MD, MS, FAAFP, 14486 SE Lyon Court, Happy Valley, OR 97086; [email protected]
1. Rodgers P, Bassler M. Treating onychomycosis. Am Fam Physician. 2001;63:663-672, 677-678.
2. Sparavigna A, Tenconi B, La Penna L. Efficacy and tolerability of a biomineral formulation for treatment of onychoschizia: a randomized trial. Clin Cosmet Investig Dermatol. 2019:12:355-362. doi: 10.2147/CCID.S187305
3. Singal A, Arora R. Nail as a window of systemic diseases. Indian Dermatol Online J. 2015;6:67-74. doi: 10.4103/2229-5178.153002
4. Cleveland Clinic. Onycholysis. Accessed March 1, 2023. https://my.clevelandclinic.org/health/diseases/22903-onycholysis
5. Chiu H-H, Liu M-T, Chung W-H, et al. The mechanism of onychomadesis (nail shedding) and Beau’s lines following hand-foot-mouth disease. Viruses. 2019;11:522. doi: 10.3390/v11060522
6. Suchonwanit P, Nitayavardhana S. Idiopathic sporadic onychomadesis of toenails. Case Rep Dermatol Med. 2016;2016:6451327. doi: 10.1155/2016/6451327
7. Hardin J, Haber RM. Onychomadesis: literature review. Br J Dermatol. 2015;172:592-596. doi: 10.1111/bjd.13339
8. Li D, Yang W, Xing X, et al. Onychomadesis and potential association with HFMD outbreak in a kindergarten in Hubei providence, China, 2017. BMC Infect Dis. 2019:19:995. doi: 10.1186/s12879-019-4560-8
9. Chiu HH, Wu CS, Lan CE. Onychomadesis: a late complication of hand, foot, and mouth disease. J Emerg Med. 2017;52:243-245. doi: 10.1016/j.jemermed.2016.01.034
10. Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11. doi: 10.1046/j.1525-1470.2000.01702.x
11. Scarfi F, Arunachalam M, Galeone M, et al. An uncommon onychomadesis in adults. Int J Derm. 2014;53:1392-1394. doi: 10.1111/j.1365-4632.2012.05774.x
12. ICD10Data.com. 2023 ICD-10-CM codes. Accessed February 15, 2023. www.icd10data.com/ICD10CM/codes
THE CASE
A 75-year-old man sought care from his primary care physician because his “fingernails and toenails [were] all falling off.” He did not feel ill and had no other complaints. His vital signs were unremarkable. He had no history of malignancies, chronic skin conditions, or systemic diseases. His fingernails and toenails were discolored and lifting from the proximal end of his nail beds (FIGURE). One of his great toenails had already fallen off, 1 thumb nail was minimally attached with the cuticle, and the rest of his nails were loose and in the process of separating from their nail beds. There was no nail pitting, rash, or joint swelling and tenderness.

The patient reported that while on vacation in Hawaii 3 weeks earlier, he had sought care at an urgent care clinic for a painless rash on his hands and the soles of his feet. At that time, he did not feel ill or have mouth ulcers, penile discharge, or arthralgia. There had been no recent changes to his prescription medications, which included finasteride, terazosin, omeprazole, and an albuterol inhaler. He denied taking over-the-counter medications or supplements.
The physical exam at the urgent care had revealed multiple blotchy, dark, 0.5- to 1-cm nonpruritic lesions that were desquamating. No oral lesions were seen. He had been given a diagnosis of hand-foot-mouth disease (HFMD) and reassured that it would resolve on its own in about 10 days.
THE DIAGNOSIS
Several possible diagnoses for nail disorders came to mind with this patient, including onychomycosis, onychoschizia, onycholysis, and onychomadesis.
Onychomycosis is a chronic fungal infection of the nail that affects toenails more often than fingernails.1 The most common form is distal subungual onychomycosis, which begins distally and slowly migrates proximally through the nail matrix.1 Often onychomycosis affects only a few nails unless the patient is elderly or has comorbid conditions, and the nails rarely separate from the nail bed.
Onychoschizia involves lamellar splitting and peeling of the dorsal surface of the nail plate.2 Usually white discolorations appear on the distal edges of the nail.3 It is more common in women than in men and is often caused by nail dehydration from repeated excessive immersion in water with detergents or recurrent application of nail polish.2 However, the nails do not separate from the nail bed, and usually only the fingernails are involved.
Onycholysis is a nail attachment disorder in which the nail plate distally separates from the nail bed. Areas of separation will appear white or yellow. There are many etiologies for onycholysis, including trauma, psoriasis, fungal infection, and contact irritant reactions.3 It also can be caused by medications and thyroid disease.3,4
Continue to: Onychomadesis
Onychomadesis, sometimes considered a severe form of Beau’s line,5,6 is defined by the spontaneous separation of the nail plate from the nail matrix. Although the nail will initially remain attached, proximal shedding will eventually occur.7 When several nails are involved, a systemic source—such as an acute infection, autoimmune disease, medication, malignancy (eg, cutaneous T-cell lymphoma), Kawasaki disease, skin disorders (eg, pemphigus vulgaris or keratosis punctata et planters), or chemotherapy—may be the cause.6-8 If only a few nails are involved, it may be associated with trauma, and in rare cases, onychomadesis can be idiopathic.5,7
In this case, all signs pointed to onychomadesis. All of the patient’s nails were affected (discolored and lifting), his nail loss involved spontaneous proximal separation of the nail plate from the nail matrix, and he had a recent previous infection: HFMD.
DISCUSSION
Onychomadesis is a rare nail-shedding disorder thought to be caused by the temporary arrest of the nail matrix.8 It is a potential late complication of infection, such as HFMD,9 and was first reported in children in Chicago in 2000.10 Since then, onychomadesis has been noted in children in many countries.8 Reports of onychomadesis following HFMD in adults are rare, but it may be underreported because HFMD is more common in children and symptoms are usually minor in adults.11
Molecular studies have associated onychomadesis with coxsackievirus (CV)A6 and CVA10.4 Other serotypes associated with onychomadesis include CVB1, CVB2, CVA5, CVA16, and enteroviruses 71 and 9.4 Most known outbreaks seem to be caused by CVA6.4
No treatment is needed for onychomadesis; physicians can reassure patients that normal nail growth will begin within 1 to 4 months. Because onychomadesis is rare, it does not have its own billing code, so one can use code L60.8 for “Other nail disorders.”12
Our patient was seen in the primary care clinic 3 months after his initial visit. At that time, his nails were no longer discolored and no other abnormalities were present. All of the nails on his fingers and toes were firmly attached and growing normally.
THE TAKEAWAY
The sudden asymptomatic loss of multiple fingernails and toenails—especially with proximal nail shedding—is a rare disorder known as onychomadesis. It can be caused by various etiologies and can be a late complication of HFMD or other viral infections. Onychomadesis should be considered when evaluating older patients, particularly when all of their nails are involved after a viral infection.
CORRESPONDENCE
Jon F. Peters, MD, MS, FAAFP, 14486 SE Lyon Court, Happy Valley, OR 97086; [email protected]
THE CASE
A 75-year-old man sought care from his primary care physician because his “fingernails and toenails [were] all falling off.” He did not feel ill and had no other complaints. His vital signs were unremarkable. He had no history of malignancies, chronic skin conditions, or systemic diseases. His fingernails and toenails were discolored and lifting from the proximal end of his nail beds (FIGURE). One of his great toenails had already fallen off, 1 thumb nail was minimally attached with the cuticle, and the rest of his nails were loose and in the process of separating from their nail beds. There was no nail pitting, rash, or joint swelling and tenderness.

The patient reported that while on vacation in Hawaii 3 weeks earlier, he had sought care at an urgent care clinic for a painless rash on his hands and the soles of his feet. At that time, he did not feel ill or have mouth ulcers, penile discharge, or arthralgia. There had been no recent changes to his prescription medications, which included finasteride, terazosin, omeprazole, and an albuterol inhaler. He denied taking over-the-counter medications or supplements.
The physical exam at the urgent care had revealed multiple blotchy, dark, 0.5- to 1-cm nonpruritic lesions that were desquamating. No oral lesions were seen. He had been given a diagnosis of hand-foot-mouth disease (HFMD) and reassured that it would resolve on its own in about 10 days.
THE DIAGNOSIS
Several possible diagnoses for nail disorders came to mind with this patient, including onychomycosis, onychoschizia, onycholysis, and onychomadesis.
Onychomycosis is a chronic fungal infection of the nail that affects toenails more often than fingernails.1 The most common form is distal subungual onychomycosis, which begins distally and slowly migrates proximally through the nail matrix.1 Often onychomycosis affects only a few nails unless the patient is elderly or has comorbid conditions, and the nails rarely separate from the nail bed.
Onychoschizia involves lamellar splitting and peeling of the dorsal surface of the nail plate.2 Usually white discolorations appear on the distal edges of the nail.3 It is more common in women than in men and is often caused by nail dehydration from repeated excessive immersion in water with detergents or recurrent application of nail polish.2 However, the nails do not separate from the nail bed, and usually only the fingernails are involved.
Onycholysis is a nail attachment disorder in which the nail plate distally separates from the nail bed. Areas of separation will appear white or yellow. There are many etiologies for onycholysis, including trauma, psoriasis, fungal infection, and contact irritant reactions.3 It also can be caused by medications and thyroid disease.3,4
Continue to: Onychomadesis
Onychomadesis, sometimes considered a severe form of Beau’s line,5,6 is defined by the spontaneous separation of the nail plate from the nail matrix. Although the nail will initially remain attached, proximal shedding will eventually occur.7 When several nails are involved, a systemic source—such as an acute infection, autoimmune disease, medication, malignancy (eg, cutaneous T-cell lymphoma), Kawasaki disease, skin disorders (eg, pemphigus vulgaris or keratosis punctata et planters), or chemotherapy—may be the cause.6-8 If only a few nails are involved, it may be associated with trauma, and in rare cases, onychomadesis can be idiopathic.5,7
In this case, all signs pointed to onychomadesis. All of the patient’s nails were affected (discolored and lifting), his nail loss involved spontaneous proximal separation of the nail plate from the nail matrix, and he had a recent previous infection: HFMD.
DISCUSSION
Onychomadesis is a rare nail-shedding disorder thought to be caused by the temporary arrest of the nail matrix.8 It is a potential late complication of infection, such as HFMD,9 and was first reported in children in Chicago in 2000.10 Since then, onychomadesis has been noted in children in many countries.8 Reports of onychomadesis following HFMD in adults are rare, but it may be underreported because HFMD is more common in children and symptoms are usually minor in adults.11
Molecular studies have associated onychomadesis with coxsackievirus (CV)A6 and CVA10.4 Other serotypes associated with onychomadesis include CVB1, CVB2, CVA5, CVA16, and enteroviruses 71 and 9.4 Most known outbreaks seem to be caused by CVA6.4
No treatment is needed for onychomadesis; physicians can reassure patients that normal nail growth will begin within 1 to 4 months. Because onychomadesis is rare, it does not have its own billing code, so one can use code L60.8 for “Other nail disorders.”12
Our patient was seen in the primary care clinic 3 months after his initial visit. At that time, his nails were no longer discolored and no other abnormalities were present. All of the nails on his fingers and toes were firmly attached and growing normally.
THE TAKEAWAY
The sudden asymptomatic loss of multiple fingernails and toenails—especially with proximal nail shedding—is a rare disorder known as onychomadesis. It can be caused by various etiologies and can be a late complication of HFMD or other viral infections. Onychomadesis should be considered when evaluating older patients, particularly when all of their nails are involved after a viral infection.
CORRESPONDENCE
Jon F. Peters, MD, MS, FAAFP, 14486 SE Lyon Court, Happy Valley, OR 97086; [email protected]
1. Rodgers P, Bassler M. Treating onychomycosis. Am Fam Physician. 2001;63:663-672, 677-678.
2. Sparavigna A, Tenconi B, La Penna L. Efficacy and tolerability of a biomineral formulation for treatment of onychoschizia: a randomized trial. Clin Cosmet Investig Dermatol. 2019:12:355-362. doi: 10.2147/CCID.S187305
3. Singal A, Arora R. Nail as a window of systemic diseases. Indian Dermatol Online J. 2015;6:67-74. doi: 10.4103/2229-5178.153002
4. Cleveland Clinic. Onycholysis. Accessed March 1, 2023. https://my.clevelandclinic.org/health/diseases/22903-onycholysis
5. Chiu H-H, Liu M-T, Chung W-H, et al. The mechanism of onychomadesis (nail shedding) and Beau’s lines following hand-foot-mouth disease. Viruses. 2019;11:522. doi: 10.3390/v11060522
6. Suchonwanit P, Nitayavardhana S. Idiopathic sporadic onychomadesis of toenails. Case Rep Dermatol Med. 2016;2016:6451327. doi: 10.1155/2016/6451327
7. Hardin J, Haber RM. Onychomadesis: literature review. Br J Dermatol. 2015;172:592-596. doi: 10.1111/bjd.13339
8. Li D, Yang W, Xing X, et al. Onychomadesis and potential association with HFMD outbreak in a kindergarten in Hubei providence, China, 2017. BMC Infect Dis. 2019:19:995. doi: 10.1186/s12879-019-4560-8
9. Chiu HH, Wu CS, Lan CE. Onychomadesis: a late complication of hand, foot, and mouth disease. J Emerg Med. 2017;52:243-245. doi: 10.1016/j.jemermed.2016.01.034
10. Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11. doi: 10.1046/j.1525-1470.2000.01702.x
11. Scarfi F, Arunachalam M, Galeone M, et al. An uncommon onychomadesis in adults. Int J Derm. 2014;53:1392-1394. doi: 10.1111/j.1365-4632.2012.05774.x
12. ICD10Data.com. 2023 ICD-10-CM codes. Accessed February 15, 2023. www.icd10data.com/ICD10CM/codes
1. Rodgers P, Bassler M. Treating onychomycosis. Am Fam Physician. 2001;63:663-672, 677-678.
2. Sparavigna A, Tenconi B, La Penna L. Efficacy and tolerability of a biomineral formulation for treatment of onychoschizia: a randomized trial. Clin Cosmet Investig Dermatol. 2019:12:355-362. doi: 10.2147/CCID.S187305
3. Singal A, Arora R. Nail as a window of systemic diseases. Indian Dermatol Online J. 2015;6:67-74. doi: 10.4103/2229-5178.153002
4. Cleveland Clinic. Onycholysis. Accessed March 1, 2023. https://my.clevelandclinic.org/health/diseases/22903-onycholysis
5. Chiu H-H, Liu M-T, Chung W-H, et al. The mechanism of onychomadesis (nail shedding) and Beau’s lines following hand-foot-mouth disease. Viruses. 2019;11:522. doi: 10.3390/v11060522
6. Suchonwanit P, Nitayavardhana S. Idiopathic sporadic onychomadesis of toenails. Case Rep Dermatol Med. 2016;2016:6451327. doi: 10.1155/2016/6451327
7. Hardin J, Haber RM. Onychomadesis: literature review. Br J Dermatol. 2015;172:592-596. doi: 10.1111/bjd.13339
8. Li D, Yang W, Xing X, et al. Onychomadesis and potential association with HFMD outbreak in a kindergarten in Hubei providence, China, 2017. BMC Infect Dis. 2019:19:995. doi: 10.1186/s12879-019-4560-8
9. Chiu HH, Wu CS, Lan CE. Onychomadesis: a late complication of hand, foot, and mouth disease. J Emerg Med. 2017;52:243-245. doi: 10.1016/j.jemermed.2016.01.034
10. Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11. doi: 10.1046/j.1525-1470.2000.01702.x
11. Scarfi F, Arunachalam M, Galeone M, et al. An uncommon onychomadesis in adults. Int J Derm. 2014;53:1392-1394. doi: 10.1111/j.1365-4632.2012.05774.x
12. ICD10Data.com. 2023 ICD-10-CM codes. Accessed February 15, 2023. www.icd10data.com/ICD10CM/codes
► Recent history of hand-foot-mouth disease
► Discolored fingernails and toenails lifting from the proximal end
Should RAAS blockade therapy be continued in patients with advanced renal disease?
Evidence summary
Mixed results, Yes, but no evidence of harm in continuing RAAS therapy
A 2014 cohort study assessed the effect of treatment with angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEIs/ARBs) on all-cause mortality in US veterans (N = 141,413) with non-dialysis chronic kidney disease (CKD)—defined as either a stable estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 or a stable eGFR ≥ 60 mL/min/1.73 m2 and an elevated urine microalbumin measurement.1 In an intention-to-treat analysis, ACEI/ARB treatment was associated with a significantly decreased risk for all-cause mortality (hazard ratio [HR] = 0.81; 95% CI, 0.78-0.84).
A 2018 meta-analysis analyzed data from 9 RCTs comparing RAAS blockade therapy to placebo or alternative antihypertensive agents in patients with non-dialysis CKD stages 3 to 5.2 Although the meta-analysis authors focused on patients with comorbid diabetes and non-dialysis CKD (N = 9797), some included studies had a mixed population (ie, only a subset of patients had diabetes). This, among other variances in characteristics, participants, interventions, and endpoints, resulted in different numbers of participants included in the data extraction and analysis of outcomes. Overall, there was no difference between the RAAS group and the control group in terms of all-cause mortality (N = 5309; risk ratio [RR] = 0.97; 95% CI, 0.85-1.10), cardiovascular mortality (N = 3748; RR = 1.03; 95% CI, 0.75-1.41), or adverse events (N = 1822; RR = 1.05; 95% CI, 0.89-1.25). Compared to the control group, the RAAS group was less likely to experience a nonfatal cardiovascular event (N = 6138; RR = 0.90; 95% CI, 0.81-1.00). For the composite endpoint of need for renal replacement therapy/doubling of serum creatinine, RAAS therapy was associated with reduced risk in both the overall population (N = 5202; RR = 0.81; 95% CI, 0.70-0.92) and in patients with comorbid diabetes (N = 3314; RR = 0.78; 95% CI, 0.67-0.90).
A 2022 open-label trial (STOP ACEi) randomly assigned 411 patients with stage 4 or 5 CKD to either continue (N = 205) or discontinue (N = 206) RAAS inhibitor therapy.3 The primary outcome measure was eGFR at 3 years. The difference in the rate of decline in eGFR between groups was –0.7% (95% CI, –2.5 to 1.0; P = .42), favoring the group that continued therapy.
Recommendations from others
After reviewing data from multiple clinical trials, the authors of the 2018 report from the National Kidney Foundation–Kidney Disease Outcomes Quality Initiative (NKF–KDOQI) concluded that the decision to continue or stop RAAS therapy in patients with advanced CKD should be individualized.4 Criteria that should be considered in the decision-making process include the presence or absence of large acute declines in eGFR (> 20% in the absence of a significant decrease in proteinuria), hypotension, or acute kidney injury with significant risk for worsening.
In 2021, the Renal Association and the Association of British Clinical Diabetologists published updated clinical practice guidelines for the management of hypertension and RAAS blockade in adults with diabetic kidney disease.5 Collective data indicated that, although outcomes varied based on type of diabetes (1 vs 2) and degree of proteinuria, blockade therapy overall led to improved outcomes; this was hypothesized to be due to the effects of reduced blood pressure. However, discontinuation of RAAS blockade therapy may be warranted when the patient (1) has a potassium level > 5 mmol/L pretreatment or ≥ 6 mmol/L with treatment, (2) demonstrates a decrease in eGFR > 25% or an increase in serum creatinine > 30% upon initiation of blockade, without another cause of renal deterioration, (3) is pregnant, or (4) has an acute illness with fluid depletion (in which case, RAAS therapy can be restarted 24 to 48 hours after recovery).
Editor’s takeaway
Evidence supports continuation of RAAS blockade, particularly in patients with significant comorbidities (diabetes and cardiovascular disease). Study data indicate continuation is either beneficial or neutral to further morbidity. The only caveat is that these patients should have their renal function and potassium level continuously monitored. The evidence should provide reassurance to patients and physicians that continuation is the correct course of action.
1. Molnar MZ, Kalantar-Zadeh K, Lott EH, et al. Angiotensin-converting enzyme inhibitor, angiotensin receptor blocker use, and mortality in patients with chronic kidney disease. J Am Coll Cardiol. 2014;63:650-658. doi: 10.1016/j.jacc.2013.10.050
2. Nistor I, De Sutter J, Drechsler C, et al. Effect of renin-angiotensin-aldosterone system blockade in adults with diabetes mellitus and advanced chronic kidney disease not on dialysis: a systematic review and meta-analysis. Nephrol Dial Transplant. 2018;33:12-22. doi: 10.1093/ndt/gfx072
3. Bhandari S, Mehta S, Khwaja A, et al. Renin-angiotensin system inhibition in advanced chronic kidney disease. N Engl J Med. 2022;387:2021-2032. doi: 10.1056/NEJMoa2210639
4. Weir MR, Lakkis JI, Jaar B, et al. Use of renin-angiotensin system blockade in advanced CKD: an NKF–KDOQI controversies report. Am J Kidney Dis. 2018;72:873-884. doi: 10.1053/j.ajkd.2018.06.010
5. Banerjee D, Winocour P, Chowdhury TA, et al. Management of hypertension and renin-angiotensin-aldosterone system blockade in adults with diabetic kidney disease: Association of British Clinical Diabetologists and the Renal Association UK guideline update 2021. BMC Nephrol. 2022;23:9. doi: 10.1186/s12882-021-02587-5
Evidence summary
Mixed results, Yes, but no evidence of harm in continuing RAAS therapy
A 2014 cohort study assessed the effect of treatment with angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEIs/ARBs) on all-cause mortality in US veterans (N = 141,413) with non-dialysis chronic kidney disease (CKD)—defined as either a stable estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 or a stable eGFR ≥ 60 mL/min/1.73 m2 and an elevated urine microalbumin measurement.1 In an intention-to-treat analysis, ACEI/ARB treatment was associated with a significantly decreased risk for all-cause mortality (hazard ratio [HR] = 0.81; 95% CI, 0.78-0.84).
A 2018 meta-analysis analyzed data from 9 RCTs comparing RAAS blockade therapy to placebo or alternative antihypertensive agents in patients with non-dialysis CKD stages 3 to 5.2 Although the meta-analysis authors focused on patients with comorbid diabetes and non-dialysis CKD (N = 9797), some included studies had a mixed population (ie, only a subset of patients had diabetes). This, among other variances in characteristics, participants, interventions, and endpoints, resulted in different numbers of participants included in the data extraction and analysis of outcomes. Overall, there was no difference between the RAAS group and the control group in terms of all-cause mortality (N = 5309; risk ratio [RR] = 0.97; 95% CI, 0.85-1.10), cardiovascular mortality (N = 3748; RR = 1.03; 95% CI, 0.75-1.41), or adverse events (N = 1822; RR = 1.05; 95% CI, 0.89-1.25). Compared to the control group, the RAAS group was less likely to experience a nonfatal cardiovascular event (N = 6138; RR = 0.90; 95% CI, 0.81-1.00). For the composite endpoint of need for renal replacement therapy/doubling of serum creatinine, RAAS therapy was associated with reduced risk in both the overall population (N = 5202; RR = 0.81; 95% CI, 0.70-0.92) and in patients with comorbid diabetes (N = 3314; RR = 0.78; 95% CI, 0.67-0.90).
A 2022 open-label trial (STOP ACEi) randomly assigned 411 patients with stage 4 or 5 CKD to either continue (N = 205) or discontinue (N = 206) RAAS inhibitor therapy.3 The primary outcome measure was eGFR at 3 years. The difference in the rate of decline in eGFR between groups was –0.7% (95% CI, –2.5 to 1.0; P = .42), favoring the group that continued therapy.
Recommendations from others
After reviewing data from multiple clinical trials, the authors of the 2018 report from the National Kidney Foundation–Kidney Disease Outcomes Quality Initiative (NKF–KDOQI) concluded that the decision to continue or stop RAAS therapy in patients with advanced CKD should be individualized.4 Criteria that should be considered in the decision-making process include the presence or absence of large acute declines in eGFR (> 20% in the absence of a significant decrease in proteinuria), hypotension, or acute kidney injury with significant risk for worsening.
In 2021, the Renal Association and the Association of British Clinical Diabetologists published updated clinical practice guidelines for the management of hypertension and RAAS blockade in adults with diabetic kidney disease.5 Collective data indicated that, although outcomes varied based on type of diabetes (1 vs 2) and degree of proteinuria, blockade therapy overall led to improved outcomes; this was hypothesized to be due to the effects of reduced blood pressure. However, discontinuation of RAAS blockade therapy may be warranted when the patient (1) has a potassium level > 5 mmol/L pretreatment or ≥ 6 mmol/L with treatment, (2) demonstrates a decrease in eGFR > 25% or an increase in serum creatinine > 30% upon initiation of blockade, without another cause of renal deterioration, (3) is pregnant, or (4) has an acute illness with fluid depletion (in which case, RAAS therapy can be restarted 24 to 48 hours after recovery).
Editor’s takeaway
Evidence supports continuation of RAAS blockade, particularly in patients with significant comorbidities (diabetes and cardiovascular disease). Study data indicate continuation is either beneficial or neutral to further morbidity. The only caveat is that these patients should have their renal function and potassium level continuously monitored. The evidence should provide reassurance to patients and physicians that continuation is the correct course of action.
Evidence summary
Mixed results, Yes, but no evidence of harm in continuing RAAS therapy
A 2014 cohort study assessed the effect of treatment with angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEIs/ARBs) on all-cause mortality in US veterans (N = 141,413) with non-dialysis chronic kidney disease (CKD)—defined as either a stable estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 or a stable eGFR ≥ 60 mL/min/1.73 m2 and an elevated urine microalbumin measurement.1 In an intention-to-treat analysis, ACEI/ARB treatment was associated with a significantly decreased risk for all-cause mortality (hazard ratio [HR] = 0.81; 95% CI, 0.78-0.84).
A 2018 meta-analysis analyzed data from 9 RCTs comparing RAAS blockade therapy to placebo or alternative antihypertensive agents in patients with non-dialysis CKD stages 3 to 5.2 Although the meta-analysis authors focused on patients with comorbid diabetes and non-dialysis CKD (N = 9797), some included studies had a mixed population (ie, only a subset of patients had diabetes). This, among other variances in characteristics, participants, interventions, and endpoints, resulted in different numbers of participants included in the data extraction and analysis of outcomes. Overall, there was no difference between the RAAS group and the control group in terms of all-cause mortality (N = 5309; risk ratio [RR] = 0.97; 95% CI, 0.85-1.10), cardiovascular mortality (N = 3748; RR = 1.03; 95% CI, 0.75-1.41), or adverse events (N = 1822; RR = 1.05; 95% CI, 0.89-1.25). Compared to the control group, the RAAS group was less likely to experience a nonfatal cardiovascular event (N = 6138; RR = 0.90; 95% CI, 0.81-1.00). For the composite endpoint of need for renal replacement therapy/doubling of serum creatinine, RAAS therapy was associated with reduced risk in both the overall population (N = 5202; RR = 0.81; 95% CI, 0.70-0.92) and in patients with comorbid diabetes (N = 3314; RR = 0.78; 95% CI, 0.67-0.90).
A 2022 open-label trial (STOP ACEi) randomly assigned 411 patients with stage 4 or 5 CKD to either continue (N = 205) or discontinue (N = 206) RAAS inhibitor therapy.3 The primary outcome measure was eGFR at 3 years. The difference in the rate of decline in eGFR between groups was –0.7% (95% CI, –2.5 to 1.0; P = .42), favoring the group that continued therapy.
Recommendations from others
After reviewing data from multiple clinical trials, the authors of the 2018 report from the National Kidney Foundation–Kidney Disease Outcomes Quality Initiative (NKF–KDOQI) concluded that the decision to continue or stop RAAS therapy in patients with advanced CKD should be individualized.4 Criteria that should be considered in the decision-making process include the presence or absence of large acute declines in eGFR (> 20% in the absence of a significant decrease in proteinuria), hypotension, or acute kidney injury with significant risk for worsening.
In 2021, the Renal Association and the Association of British Clinical Diabetologists published updated clinical practice guidelines for the management of hypertension and RAAS blockade in adults with diabetic kidney disease.5 Collective data indicated that, although outcomes varied based on type of diabetes (1 vs 2) and degree of proteinuria, blockade therapy overall led to improved outcomes; this was hypothesized to be due to the effects of reduced blood pressure. However, discontinuation of RAAS blockade therapy may be warranted when the patient (1) has a potassium level > 5 mmol/L pretreatment or ≥ 6 mmol/L with treatment, (2) demonstrates a decrease in eGFR > 25% or an increase in serum creatinine > 30% upon initiation of blockade, without another cause of renal deterioration, (3) is pregnant, or (4) has an acute illness with fluid depletion (in which case, RAAS therapy can be restarted 24 to 48 hours after recovery).
Editor’s takeaway
Evidence supports continuation of RAAS blockade, particularly in patients with significant comorbidities (diabetes and cardiovascular disease). Study data indicate continuation is either beneficial or neutral to further morbidity. The only caveat is that these patients should have their renal function and potassium level continuously monitored. The evidence should provide reassurance to patients and physicians that continuation is the correct course of action.
1. Molnar MZ, Kalantar-Zadeh K, Lott EH, et al. Angiotensin-converting enzyme inhibitor, angiotensin receptor blocker use, and mortality in patients with chronic kidney disease. J Am Coll Cardiol. 2014;63:650-658. doi: 10.1016/j.jacc.2013.10.050
2. Nistor I, De Sutter J, Drechsler C, et al. Effect of renin-angiotensin-aldosterone system blockade in adults with diabetes mellitus and advanced chronic kidney disease not on dialysis: a systematic review and meta-analysis. Nephrol Dial Transplant. 2018;33:12-22. doi: 10.1093/ndt/gfx072
3. Bhandari S, Mehta S, Khwaja A, et al. Renin-angiotensin system inhibition in advanced chronic kidney disease. N Engl J Med. 2022;387:2021-2032. doi: 10.1056/NEJMoa2210639
4. Weir MR, Lakkis JI, Jaar B, et al. Use of renin-angiotensin system blockade in advanced CKD: an NKF–KDOQI controversies report. Am J Kidney Dis. 2018;72:873-884. doi: 10.1053/j.ajkd.2018.06.010
5. Banerjee D, Winocour P, Chowdhury TA, et al. Management of hypertension and renin-angiotensin-aldosterone system blockade in adults with diabetic kidney disease: Association of British Clinical Diabetologists and the Renal Association UK guideline update 2021. BMC Nephrol. 2022;23:9. doi: 10.1186/s12882-021-02587-5
1. Molnar MZ, Kalantar-Zadeh K, Lott EH, et al. Angiotensin-converting enzyme inhibitor, angiotensin receptor blocker use, and mortality in patients with chronic kidney disease. J Am Coll Cardiol. 2014;63:650-658. doi: 10.1016/j.jacc.2013.10.050
2. Nistor I, De Sutter J, Drechsler C, et al. Effect of renin-angiotensin-aldosterone system blockade in adults with diabetes mellitus and advanced chronic kidney disease not on dialysis: a systematic review and meta-analysis. Nephrol Dial Transplant. 2018;33:12-22. doi: 10.1093/ndt/gfx072
3. Bhandari S, Mehta S, Khwaja A, et al. Renin-angiotensin system inhibition in advanced chronic kidney disease. N Engl J Med. 2022;387:2021-2032. doi: 10.1056/NEJMoa2210639
4. Weir MR, Lakkis JI, Jaar B, et al. Use of renin-angiotensin system blockade in advanced CKD: an NKF–KDOQI controversies report. Am J Kidney Dis. 2018;72:873-884. doi: 10.1053/j.ajkd.2018.06.010
5. Banerjee D, Winocour P, Chowdhury TA, et al. Management of hypertension and renin-angiotensin-aldosterone system blockade in adults with diabetic kidney disease: Association of British Clinical Diabetologists and the Renal Association UK guideline update 2021. BMC Nephrol. 2022;23:9. doi: 10.1186/s12882-021-02587-5
EVIDENCE-BASED REVIEW:
PROBABLY. Renin-angiotensin- aldosterone system (RAAS) blockade therapy should be continued in most patients with advanced renal disease and comorbid conditions; however, individualized treatment is warranted as data on the benefits and harms in all-cause mortality, cardiovascular mortality, and risk for renal replacement therapy are inconclusive (strength of recommendation [SOR]: B, based on observational studies, systematic reviews, and meta-analyses of randomized controlled trials [RCTs]). Certain patient populations, such as patients with diabetes or those with cardiovascular risk or history, may benefit most from continued RAAS blockade therapy (SOR: A, based on systematic reviews and meta-analyses of RCTs).
Sports: An underutilized tool for patients with disabilities
Approximately 6.5 million people in the United States have an intellectual disability, the most common type of developmental disability.1 People with disabilities are 3 times more likely to have heart disease, stroke, or diabetes than adults without disabilities.2
Sports as a treatment modality are not used to full advantage to combat these conditions in people with intellectual/developmental disabilities (IDDs). Participation in sport activities can lead to weight loss, reduce risk for cardiovascular disease, and optimize physical health. Sports also can help enhance social and communication skills and improve quality of life for this patient population (TABLE).3-6
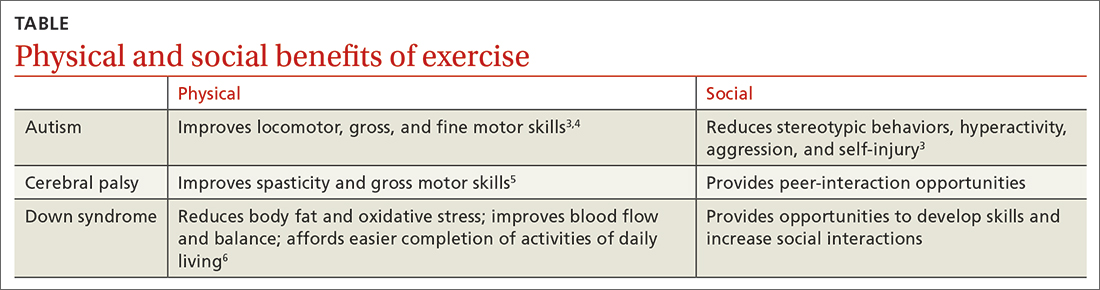
However, a 2014 report found that while inactive adults with disabilities (hearing, vision, cognition, mobility) were 50% more likely to report 1 or more chronic diseases than those who were physically active, only 44% of adults with disabilities who visited a health professional in the previous 12 months received a physical activity recommendation.7 In addition, more than 50% of adults with disabilities are not meeting US recommended exercise guidelines.7-9
Family physicians may not feel they have adequate training to counsel patients with IDDs. Additional limiting factors include dependence on caregivers for exercise participation, expense, transportation difficulties, a lack of choice in sporting activities, and the patient’s level of motivation.10The guidance reviewed here details how to modify the pre-participation sports physical exam specifically for patients with IDDs. It also provides sport and exercise recommendations for patients with 3 disabilities: Down syndrome, cerebral palsy, and autism spectrum disorder.
Worth noting: As is true for adults without disabilities, those with IDDs should participate in at least 150 minutes of moderate-intensity, or 75 minutes of vigorous intensity, aerobic physical activity each week.9 Recommend muscle-strengthening activities be performed at least 2 days each week.9
Exercise recommendations for patients with Down syndrome
One in every 700 babies receives a diagnosis of Down syndrome.11 Among its many possible manifestations—which include intellectual disability, heart disease, and diabetes—Down syndrome is associated with an increased risk for obesity, which makes exercise an extremely important lifestyle modification for these patients. Obesity can lead to obstructive sleep apnea causing cor pulmonale and even premature death. Continuous positive airway pressure intervention can be difficult in terms of patient compliance. However, weight loss through exercise and sports is an effective intervention to mitigate these obesity-related health comorbidities.
Pre-participation exam. A focused history and physical exam are often conducted before a patient engages in organized competitive or recreational sports. The pre-participation sports physical exam typically focuses on cardiac, neurologic, hereditary, and musculoskeletal disorders. While we recommend including these baseline elements as part of the exam for patients with disabilities, we also recommend modifying the exam to include disability-specific screening for associated comorbidities.
Continue to: For patients with Down syndrome...
For patients with Down syndrome, a complete pre-participation sports physical exam is warranted. Inquire specifically about neck pain or dislocations, heart murmur, cardiac surgery, seizures, sleep issues, history of congenital abdominal defect, hematologic malignancy, and bone pain as part of the focused physical exam.
Look for evidence of patellofemoral instability, pes planus, scoliosis, hallux deformities, decreased muscle tone, and muscular weakness. Check for cataracts and perform a thorough cardiovascular exam to assess for murmur or signs of chronic hypoxia, such as cyanosis. If a heart murmur is detected, refer the patient to a cardiologist.
Patients with Down syndrome are also at increased risk for atlantoaxial instability. A thorough neurologic evaluation to screen for this condition is indicated; however, routine radiologic screening is not needed.12
An annual complete blood cell count and thyroid-stimulating hormone test are recommended for all children with Down syndrome.13 For patients with Down syndrome who are 13 to 21 years of age, an echocardiogram also is recommended for concerning symptoms.13 Ferritin levels also should be assessed annually for patients who are younger than 13 years of age to check for iron-deficiency anemia.13 Consider high-risk screening strategies for patients with diabetes and metabolic syndrome.
Special considerations. Patients with Down syndrome were found to be injured more frequently than individuals with other disabilities during the Special Olympics.14 These patients may be hypersensitive to pain with prolonged pain responses, or unable to verbally communicate their pain or injury.15
Continue to: The complexity of pain assessment...
The complexity of pain assessment in patients with Down syndrome may increase the difficulty of accurately diagnosing an injury, leading to underdiagnosis or overdiagnosis. To increase accuracy of pain assessment in this setting, we recommend using the Wong-Baker FACES Pain Rating Scale or a numeric pain rating scale in verbal patients.15 In nonverbal patients, facial expressions are reliable indicators of pain.
Which exercise? Healthy patients with Down syndrome can participate in any sport. Aerobic exercise can help lower body fat, reduce oxidative stress, and improve blood flow.6 Muscle-strengthening exercises can lead to improved daily functioning and balance. Strength training and aerobic exercise benefit aging patients with Down syndrome who are struggling with obesity. Such exercise also helps increase bone mineral density and improve cardiovascular fitness, especially when initiated at a young age. Consistent exercise promotes positive health outcomes throughout the lifespan.16
Exercise recommendations for patients with cerebral palsy
Cerebral palsy, the most common motor disability in children, is associated with intellectual disability, seizures, respiratory insufficiency, scoliosis, osteoporosis, mood disorders, dysphagia, and speech and hearing impairment.17 The increasing survival of premature babies born with cerebral palsy and the growing prevalence of adults with the condition point to the importance of expanding one’s knowledge of how best to care for this population.18
Pre-participation exam. In addition to a complete sport physical exam, it’s important to further evaluate patients with cerebral palsy for epilepsy, joint contractures, muscle weakness, spinal deformities, and respiratory insufficiency. The Gross Motor Function Classification system, commonly used for patients with cerebral palsy, scores functional ability in 5 levels.18 Patients at Level I are the most mobile; patients at Level V need wheelchair transport in all settings.
Further evaluation of spinal deformities can be initiated with x-ray screening. Consider ordering dual x-ray absorptiometry scans to evaluate bone mass.17
Continue to: Special considerations
Special considerations. Patients with cerebral palsy have a heightened risk for depression and anxiety.19 Mental health can be assessed via the General Anxiety Disorder-7, the Patient Health Questionnaire-9, and the Ask Suicide-Screening Questions tools, among others. Mental health screening may need to be adjusted depending on the patient’s level of cognition and ability to communicate. The patient’s caregiver also can provide supplemental information.
Consider screening vitamin D levels in patients with cerebral palsy. Approximately 50% of adults with cerebral palsy are vitamin D–deficient secondary to sedentary behavior and lack of sun exposure.20-22
Optimal medical management has been shown to decrease muscle spasticity and may be beneficial before initiating an exercise program. For patients with moderate-to-severe symptoms, referral for physical therapy to further improve gross motor function and spasticity may be required before initiating an exercise program.
Which exercise? Individuals with cerebral palsy spend 76% to 99% of their waking hours being sedentary.5 Consequently, they typically have decreased cardiorespiratory endurance and decreased muscle strength. Strength training may improve muscle spasticity, gross motor function, joint health, and respiratory insufficiency.5 Even in those who function at Level IV-V of the Gross Motor Function Classification system, exercise reduces vertebral fractures and improves time spent standing.23 By improving endurance, spasticity, and strength with exercise, deconditioning can be mitigated.
Involvement in sports promotes peer interactions, personal interests, and positive self-identity. It can give a newfound passion for life. Additionally, families of children with disabilities who engage in leisure activities together have less caregiver burden.24,25 Sporting activities offer a way to optimize psychosocial well-being for the patient and the entire family.
Continue to: Dance promotes functionality...
Dance promotes functionality and psychosocial adjustment.26 Hippotherapy, defined as therapy and rehabilitation during which the patient interacts with horses, can diminish muscle spasticity.27 Aquatic therapy also may increase muscle strength.28
Sports for patients with autism spectrum disorder
Autism spectrum disorder is defined as persistent deficits in social communication and social interaction that are usually evident in the first 3 years of life.29 Autism can manifest with or without intellectual or language impairment. Patients with autism commonly have difficulty processing sensory stimuli and can experience “sensory overload.” More than half have a coexisting mental health disorder, such as attention-deficit/hyperactivity disorder, anxiety, depression, schizophrenia, or bipolar disorder.30
Aversions to foods and food selectivity, as well as adverse effects from medical treatment of autism-related agitation, result in a higher incidence of obesity in patients with autism.31,32
Pre-participation exam. In addition to a comprehensive pre-participation exam, the Autism Spectrum Syndrome Questionnaire (ASSQ) and Modified Checklist for Autism in Toddlers are tools to screen school-age children with normal cognition to mild intellectual disability.33 These questionnaires have limitations, however. For example, ASSQ has limited ability to identify the female autistic phenotype.34 As such, these are solely screening tools. Final diagnosis is based on clinical judgment.
Special considerations. Include screening for constipation or diarrhea, fiber intake, food aversions, and common mental health comorbidities using Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition criteria.29 Psychiatric referral may be necessary if certain previously undiagnosed condition(s) become apparent. The patient’s caregiver can provide supplemental information
Continue to: During the physical exam...
During the physical exam, limit sensory stimuli as much as possible, including lights and sounds. Verbalize components of the exam before touching a patient with autism who is sensitive to physical touch.
Which exercise? Participation in sports is an effective therapy for autism and can help patients develop communication skills and promote socialization. Vigorous exercise is associated with a reduction in stereotypic behaviors, hyperactivity, aggression, and self-injury.3 Sports also can offer an alternative channel for social interaction. Children with autism may have impaired or delayed motor skills, and exercise can improve motor skill proficiency.4
The prevalence of feeding problems in children with autism spectrum disorder is estimated to be as high as 90%, and close to 70% are selective eaters.31,35,36 For those with gastrointestinal disorders, exercise can exert positive effects on the microbiome-gut-brain axis.37 Additionally, patients with autism are much more likely to be overweight or obese.32 Physical activity offers those with autism health benefits similar to those for the general population.32
Children with autism spectrum disorder have similar odds of injury, including serious injury, relative to population controls.38 Karate and swimming are among the most researched sports therapy options for patients with autism.38-40 Both are shown to improve motor ability and reduce communication deficits.
Summing up
The literature, although limited, demonstrates that exercise and sports improve the health and well-being of people with IDDs throughout the lifespan, especially if childhood exercise/sports involvement is maintained.
Encourage your patients to participate in sports, but be aware of factors that can limit (or facilitate) participation.41 Exercise participation increases based on, among other things, the individual’s desire to be fit and active, skills practice, peer involvement, family support, accessible facilities, and skilled staff.10
Additional resources that can help people with IDDs access sports and recreational activities include the Special Olympics; Paralympics; YMCA; after-school programs; The American College of Sports Medicine; The National Center on Health, Physical Activity, and Disability; and disability-certified inclusive fitness trainers.
CORRESPONDENCE
Kristina Jones, BS, 12901 Bruce B. Downs Boulevard, Tampa, FL 33612; [email protected]
1. CDC. Addressing gaps in healthcare for individuals with intellectual disabilities. Updated October 15, 2019. Accessed January 21, 2023. www.cdc.gov/grand-rounds/pp/2019/20191015-intellectual-disabilities.html
2. CDC. Vital signs: adults with disabilities. Physical activity is for everybody. Updated November 16, 2018. Accessed January 21, 2023. www.cdc.gov/vitalsigns/disabilities/index.html
3. Di Palma D, Molisso V. Sport for autism. J Humanities Soc Pol. 2017;3:42-49.
4. Pan CY, Chu CH, Tsai CL, et al. The impacts of physical activity intervention on physical and cognitive outcomes in children with autism spectrum disorder. Autism. 2017;21:190-202. doi: 10.1177/1362361316633562
5. Verschuren O, Peterson MD, Balemans AC, et al. Exercise and physical activity recommendations for people with cerebral palsy. Dev Med Child Neurol. 2016;58:798-808. doi: 10.1111/dmcn.13053
6. Paul Y, Ellapen TJ, Barnard M, et al. The health benefits of exercise therapy for patients with Down syndrome: a systematic review. Afr J Disabil. 2019;8:576. doi: 10.4102/ajod.v8i0.576
7. Carroll DD, Courtney-Long EA, Stevens AC, et al. Vital signs: disability and physical activity—United States, 2009-2012. MMWR Morb Mortal Wkly Rep. 2014;63:407-413.
8. Rimmer JH. Physical activity for people with disabilities: how do we reach those with the greatest need? NAM Perspectives. Published April 6, 2015. Accessed March 23, 2023. https://nam.edu/perspectives-2015-physical-activity-for-people-with-disabilities-how-do-we-reach-those-with-the-greatest-need/
9. Department of Health and Human Services. Physical Activity Guidelines For Americans. 2nd edition. Published 2018. Accessed March 23, 2023. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
10. Darcy S, Dowse L. In search of a level playing field—the constraints and benefits of sport participation for people with intellectual disability. Disabil Soc. 2013;28:393-407. doi: 10.1080/ 09687599.2012.714258
11. Mai CT, Isenburg JL, Canfield MA, et al. National population‐based estimates for major birth defects, 2010-2014. Birth Defects Res. 2019;111:1420-1435. doi: 10.1002/bdr2.1589
12. MyŚliwiec A, Posłuszny A, Saulicz E, et al. Atlanto-axial instability in people with Down’s syndrome and its impact on the ability to perform sports activities—a review. J Hum Kinet. 2015;48:17-24. doi: 10.1515/hukin-2015-0087
13. Bunt CW, Bunt SK. Role of the family physician in the care of children with Down syndrome. Am Fam Physician. 2014;90:851-858.
14. McCormick DP, Niebuhr VN, Risser WL. Injury and illness surveillance at local Special Olympic Games. Br J Sports Med. 1990; 24:221-224. doi: 10.1136/bjsm.24.4.221
15. McGuire BE, Defrin R. Pain perception in people with Down syndrome: a synthesis of clinical and experimental research. Front Behav Neurosci. 2015;9. doi: 10.3389/fnbeh.2015.00194
16. Barnhart RC, Connolly B. Aging and Down syndrome: implications for physical therapy. Phys Ther. 2007;87:1399-1406. doi: 10.2522/ptj.20060334
17. Vitrikas K, Dalton H, Breish D. Cerebral palsy: an overview. Am Fam Physician. 2020;101:213-220.
18. Maenner MJ, Blumberg SJ, Kogan MD, et al. Prevalence of cerebral palsy and intellectual disability among children identified in two US national surveys, 2011-2013. Ann Epidemiol. 2016;26:222-226. doi: 10.1016/j.annepidem.2016.01.001
19. Smith KJ, Peterson MD, O’Connell NE, et al. Risk of depression and anxiety in adults with cerebral palsy. JAMA Neurol. 2019;76;294-300. doi: 10.1001/jamaneurol.2018.4147
20. Peterson MD, Haapala HJ, Chaddha A, et al. Abdominal obesity is an independent predictor of serum 25-hydroxyvitamin D deficiency in adults with cerebral palsy. Nutr Metab (Lond). 2014;11:22. doi: 10.1186/1743-7075-11-22
21. Yi YG, Jung SH, Bang MS. Emerging issues in cerebral palsy associated with aging: a physiatrist perspective. Ann Rehabil Med. 2019;43:241-249. doi: 10.5535/arm.2019.43.3.241
22. Sarathy K, Doshi C, Aroojis A. Clinical examination of children with cerebral palsy. Indian J Orthop. 2019;53:35-44. doi: 10.4103/ortho.IJOrtho_409_17
23. Caulton JM, Ward KA, Alsop CW, et al. A randomised controlled trial of standing programme on bone mineral density in non-ambulant children with cerebral palsy. Arch Dis Child. 2004;89:131-135. doi: 10.1136/adc.2002.009316
24. Clutterbuck G, Auld M, Johnston L. Active exercise interventions improve gross motor function of ambulant/semi-ambulant children with cerebral palsy: a systematic review. Disabil Rehabil. 2019;41:1131-1151. doi: 10.1080/09638288.2017.1422035
25. Shikako-Thomas K, Majnemer A, Law M, et al. Determinants of participation in leisure activities in children and youth with cerebral palsy: systematic review. Phys Occup Ther Pedi. 2008;28:155-169. doi: 10.1080/01942630802031834
26. Teixeira-Machado L, Azevedo-Santos I, DeSantana JM. Dance improves functionality and psychosocial adjustment in cerebral palsy: a randomized controlled clinical trial. Am J Phys Med Rehabil. 2017;96:424-429. doi: 10.1097/PHM.0000000000000646
27. Lucena-Antón D, Rosety-Rodríguez I, Moral-Munoz JA. Effects of a hippotherapy intervention on muscle spasticity in children with cerebral palsy: a randomized controlled trial. Complement Ther Clin Pract. 2018;31:188-192. doi: 10.1016/j.ctcp.2018.02.013
28. Roostaei M, Baharlouei H, Azadi H, et al. Effects of aquatic intervention on gross motor skills in children with cerebral palsy: a systematic review. Phys Occup Ther Pediatr. 2017;37:496-515. doi: 10.1080/01942638.2016.1247938
29. American Psychiatric Association. Autism spectrum disorder, section II. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. 2013:50-56.
30. Romero M, Aguilar JM, Del-Rey-Mejías Á, et al. Psychiatric comorbidities in autism spectrum disorder: a comparative study between DSM-IV-TR and DSM-5 diagnosis. Int J Clin Health Psychol. 2016;16:266-275. doi: 10.1016/j.ijchp.2016.03.001
31. Volkert VM, Vaz PC. Recent studies on feeding problems in children with autism. J Appl Behav Anal. 2015;43:155-159. doi: 10.1901/jaba.2010.43-155
32. Broder-Fingert S, Brazauskas K, Lindgren K, et al. Prevalence of overweight and obesity in a large clinical sample of children with autism. Acad Pediatr. 2014;14:408-414. doi: 10.1016/j.acap.2014.04.004. PMID: 24976353
33. Adachi M, Takahashi M, Takayanagi N, et al. Adaptation of the Autism Spectrum Screening Questionnaire (ASSQ) to preschool children. PLoS One. 2018;10;13:e0199590. doi: 10.1371/journal.pone.0199590
34. Kopp S. Gillberg C. The Autism Spectrum Screening Questionnaire (ASSQ)-Revised Extended Version (ASSQ-REV): an instrument for better capturing the autism phenotype in girls? A preliminary study involving 191 clinical cases and community controls. Res Develop Disabil. 2011:32: 2875-2888.
35. Kotak T. Piazza CC. Assessment and behavioral treatment of feeding and sleeping disorders in children with autism spectrum disorders. Child Adol Psych Clin North Am. 2008;17:887-905. doi: 10.1016/j.chc.2008.06.005
36. Twachtman-Reilly J, Amaral SC, Zebrowski PP. Addressing feeding behaviors in children on the autism spectrum in school-based settings: physiological and behavioral issues. Lang Speech Hear Serv Sch. 2008:39:261-272. doi: 10.1044/0161-1461(2008/025)
37. Dalton A, Mermier C, Zuhl M. Exercise influence on the microbiome-gut-brain axis. Gut Microbes. 2019;10:555-568. doi: 10.1080/19490976.2018.1562268
38. Iliadis I, Apteslis N. The role of physical education and exercise for children with autism spectrum disorder and the effects on socialization, communication, behavior, fitness, and quality of life. Dial Clin Neurosc Mental Health. 2020;3:71-78. doi: 10.26386/obrela.v3i1.178
39. Phung JN, Goldberg WA. Promoting executive functioning in children with autism spectrum disorder through mixed martial arts training. J Autism Dev Dis. 2019;49:3660-3684. doi: 10.1007/s10803-019-04072-3
40. Bahrami F, Movahedi A, Marandi SM, et al. The effect of karate techniques training on communication deficit of children with autism spectrum disorder. J Autism Dev Disord. 2016;46: 978-986. doi: 10.1007/s10803-015-2643-y
41. Shields N, Synnot A. Perceived barriers and facilitators to participation in physical activity for children with disability: a qualitative study. BMC Pediatr. 2016;16:9. doi: 10.1186/s12887-016-0544-7
Approximately 6.5 million people in the United States have an intellectual disability, the most common type of developmental disability.1 People with disabilities are 3 times more likely to have heart disease, stroke, or diabetes than adults without disabilities.2
Sports as a treatment modality are not used to full advantage to combat these conditions in people with intellectual/developmental disabilities (IDDs). Participation in sport activities can lead to weight loss, reduce risk for cardiovascular disease, and optimize physical health. Sports also can help enhance social and communication skills and improve quality of life for this patient population (TABLE).3-6

However, a 2014 report found that while inactive adults with disabilities (hearing, vision, cognition, mobility) were 50% more likely to report 1 or more chronic diseases than those who were physically active, only 44% of adults with disabilities who visited a health professional in the previous 12 months received a physical activity recommendation.7 In addition, more than 50% of adults with disabilities are not meeting US recommended exercise guidelines.7-9
Family physicians may not feel they have adequate training to counsel patients with IDDs. Additional limiting factors include dependence on caregivers for exercise participation, expense, transportation difficulties, a lack of choice in sporting activities, and the patient’s level of motivation.10The guidance reviewed here details how to modify the pre-participation sports physical exam specifically for patients with IDDs. It also provides sport and exercise recommendations for patients with 3 disabilities: Down syndrome, cerebral palsy, and autism spectrum disorder.
Worth noting: As is true for adults without disabilities, those with IDDs should participate in at least 150 minutes of moderate-intensity, or 75 minutes of vigorous intensity, aerobic physical activity each week.9 Recommend muscle-strengthening activities be performed at least 2 days each week.9
Exercise recommendations for patients with Down syndrome
One in every 700 babies receives a diagnosis of Down syndrome.11 Among its many possible manifestations—which include intellectual disability, heart disease, and diabetes—Down syndrome is associated with an increased risk for obesity, which makes exercise an extremely important lifestyle modification for these patients. Obesity can lead to obstructive sleep apnea causing cor pulmonale and even premature death. Continuous positive airway pressure intervention can be difficult in terms of patient compliance. However, weight loss through exercise and sports is an effective intervention to mitigate these obesity-related health comorbidities.
Pre-participation exam. A focused history and physical exam are often conducted before a patient engages in organized competitive or recreational sports. The pre-participation sports physical exam typically focuses on cardiac, neurologic, hereditary, and musculoskeletal disorders. While we recommend including these baseline elements as part of the exam for patients with disabilities, we also recommend modifying the exam to include disability-specific screening for associated comorbidities.
Continue to: For patients with Down syndrome...
For patients with Down syndrome, a complete pre-participation sports physical exam is warranted. Inquire specifically about neck pain or dislocations, heart murmur, cardiac surgery, seizures, sleep issues, history of congenital abdominal defect, hematologic malignancy, and bone pain as part of the focused physical exam.
Look for evidence of patellofemoral instability, pes planus, scoliosis, hallux deformities, decreased muscle tone, and muscular weakness. Check for cataracts and perform a thorough cardiovascular exam to assess for murmur or signs of chronic hypoxia, such as cyanosis. If a heart murmur is detected, refer the patient to a cardiologist.
Patients with Down syndrome are also at increased risk for atlantoaxial instability. A thorough neurologic evaluation to screen for this condition is indicated; however, routine radiologic screening is not needed.12
An annual complete blood cell count and thyroid-stimulating hormone test are recommended for all children with Down syndrome.13 For patients with Down syndrome who are 13 to 21 years of age, an echocardiogram also is recommended for concerning symptoms.13 Ferritin levels also should be assessed annually for patients who are younger than 13 years of age to check for iron-deficiency anemia.13 Consider high-risk screening strategies for patients with diabetes and metabolic syndrome.
Special considerations. Patients with Down syndrome were found to be injured more frequently than individuals with other disabilities during the Special Olympics.14 These patients may be hypersensitive to pain with prolonged pain responses, or unable to verbally communicate their pain or injury.15
Continue to: The complexity of pain assessment...
The complexity of pain assessment in patients with Down syndrome may increase the difficulty of accurately diagnosing an injury, leading to underdiagnosis or overdiagnosis. To increase accuracy of pain assessment in this setting, we recommend using the Wong-Baker FACES Pain Rating Scale or a numeric pain rating scale in verbal patients.15 In nonverbal patients, facial expressions are reliable indicators of pain.
Which exercise? Healthy patients with Down syndrome can participate in any sport. Aerobic exercise can help lower body fat, reduce oxidative stress, and improve blood flow.6 Muscle-strengthening exercises can lead to improved daily functioning and balance. Strength training and aerobic exercise benefit aging patients with Down syndrome who are struggling with obesity. Such exercise also helps increase bone mineral density and improve cardiovascular fitness, especially when initiated at a young age. Consistent exercise promotes positive health outcomes throughout the lifespan.16
Exercise recommendations for patients with cerebral palsy
Cerebral palsy, the most common motor disability in children, is associated with intellectual disability, seizures, respiratory insufficiency, scoliosis, osteoporosis, mood disorders, dysphagia, and speech and hearing impairment.17 The increasing survival of premature babies born with cerebral palsy and the growing prevalence of adults with the condition point to the importance of expanding one’s knowledge of how best to care for this population.18
Pre-participation exam. In addition to a complete sport physical exam, it’s important to further evaluate patients with cerebral palsy for epilepsy, joint contractures, muscle weakness, spinal deformities, and respiratory insufficiency. The Gross Motor Function Classification system, commonly used for patients with cerebral palsy, scores functional ability in 5 levels.18 Patients at Level I are the most mobile; patients at Level V need wheelchair transport in all settings.
Further evaluation of spinal deformities can be initiated with x-ray screening. Consider ordering dual x-ray absorptiometry scans to evaluate bone mass.17
Continue to: Special considerations
Special considerations. Patients with cerebral palsy have a heightened risk for depression and anxiety.19 Mental health can be assessed via the General Anxiety Disorder-7, the Patient Health Questionnaire-9, and the Ask Suicide-Screening Questions tools, among others. Mental health screening may need to be adjusted depending on the patient’s level of cognition and ability to communicate. The patient’s caregiver also can provide supplemental information.
Consider screening vitamin D levels in patients with cerebral palsy. Approximately 50% of adults with cerebral palsy are vitamin D–deficient secondary to sedentary behavior and lack of sun exposure.20-22
Optimal medical management has been shown to decrease muscle spasticity and may be beneficial before initiating an exercise program. For patients with moderate-to-severe symptoms, referral for physical therapy to further improve gross motor function and spasticity may be required before initiating an exercise program.
Which exercise? Individuals with cerebral palsy spend 76% to 99% of their waking hours being sedentary.5 Consequently, they typically have decreased cardiorespiratory endurance and decreased muscle strength. Strength training may improve muscle spasticity, gross motor function, joint health, and respiratory insufficiency.5 Even in those who function at Level IV-V of the Gross Motor Function Classification system, exercise reduces vertebral fractures and improves time spent standing.23 By improving endurance, spasticity, and strength with exercise, deconditioning can be mitigated.
Involvement in sports promotes peer interactions, personal interests, and positive self-identity. It can give a newfound passion for life. Additionally, families of children with disabilities who engage in leisure activities together have less caregiver burden.24,25 Sporting activities offer a way to optimize psychosocial well-being for the patient and the entire family.
Continue to: Dance promotes functionality...
Dance promotes functionality and psychosocial adjustment.26 Hippotherapy, defined as therapy and rehabilitation during which the patient interacts with horses, can diminish muscle spasticity.27 Aquatic therapy also may increase muscle strength.28
Sports for patients with autism spectrum disorder
Autism spectrum disorder is defined as persistent deficits in social communication and social interaction that are usually evident in the first 3 years of life.29 Autism can manifest with or without intellectual or language impairment. Patients with autism commonly have difficulty processing sensory stimuli and can experience “sensory overload.” More than half have a coexisting mental health disorder, such as attention-deficit/hyperactivity disorder, anxiety, depression, schizophrenia, or bipolar disorder.30
Aversions to foods and food selectivity, as well as adverse effects from medical treatment of autism-related agitation, result in a higher incidence of obesity in patients with autism.31,32
Pre-participation exam. In addition to a comprehensive pre-participation exam, the Autism Spectrum Syndrome Questionnaire (ASSQ) and Modified Checklist for Autism in Toddlers are tools to screen school-age children with normal cognition to mild intellectual disability.33 These questionnaires have limitations, however. For example, ASSQ has limited ability to identify the female autistic phenotype.34 As such, these are solely screening tools. Final diagnosis is based on clinical judgment.
Special considerations. Include screening for constipation or diarrhea, fiber intake, food aversions, and common mental health comorbidities using Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition criteria.29 Psychiatric referral may be necessary if certain previously undiagnosed condition(s) become apparent. The patient’s caregiver can provide supplemental information
Continue to: During the physical exam...
During the physical exam, limit sensory stimuli as much as possible, including lights and sounds. Verbalize components of the exam before touching a patient with autism who is sensitive to physical touch.
Which exercise? Participation in sports is an effective therapy for autism and can help patients develop communication skills and promote socialization. Vigorous exercise is associated with a reduction in stereotypic behaviors, hyperactivity, aggression, and self-injury.3 Sports also can offer an alternative channel for social interaction. Children with autism may have impaired or delayed motor skills, and exercise can improve motor skill proficiency.4
The prevalence of feeding problems in children with autism spectrum disorder is estimated to be as high as 90%, and close to 70% are selective eaters.31,35,36 For those with gastrointestinal disorders, exercise can exert positive effects on the microbiome-gut-brain axis.37 Additionally, patients with autism are much more likely to be overweight or obese.32 Physical activity offers those with autism health benefits similar to those for the general population.32
Children with autism spectrum disorder have similar odds of injury, including serious injury, relative to population controls.38 Karate and swimming are among the most researched sports therapy options for patients with autism.38-40 Both are shown to improve motor ability and reduce communication deficits.
Summing up
The literature, although limited, demonstrates that exercise and sports improve the health and well-being of people with IDDs throughout the lifespan, especially if childhood exercise/sports involvement is maintained.
Encourage your patients to participate in sports, but be aware of factors that can limit (or facilitate) participation.41 Exercise participation increases based on, among other things, the individual’s desire to be fit and active, skills practice, peer involvement, family support, accessible facilities, and skilled staff.10
Additional resources that can help people with IDDs access sports and recreational activities include the Special Olympics; Paralympics; YMCA; after-school programs; The American College of Sports Medicine; The National Center on Health, Physical Activity, and Disability; and disability-certified inclusive fitness trainers.
CORRESPONDENCE
Kristina Jones, BS, 12901 Bruce B. Downs Boulevard, Tampa, FL 33612; [email protected]
Approximately 6.5 million people in the United States have an intellectual disability, the most common type of developmental disability.1 People with disabilities are 3 times more likely to have heart disease, stroke, or diabetes than adults without disabilities.2
Sports as a treatment modality are not used to full advantage to combat these conditions in people with intellectual/developmental disabilities (IDDs). Participation in sport activities can lead to weight loss, reduce risk for cardiovascular disease, and optimize physical health. Sports also can help enhance social and communication skills and improve quality of life for this patient population (TABLE).3-6

However, a 2014 report found that while inactive adults with disabilities (hearing, vision, cognition, mobility) were 50% more likely to report 1 or more chronic diseases than those who were physically active, only 44% of adults with disabilities who visited a health professional in the previous 12 months received a physical activity recommendation.7 In addition, more than 50% of adults with disabilities are not meeting US recommended exercise guidelines.7-9
Family physicians may not feel they have adequate training to counsel patients with IDDs. Additional limiting factors include dependence on caregivers for exercise participation, expense, transportation difficulties, a lack of choice in sporting activities, and the patient’s level of motivation.10The guidance reviewed here details how to modify the pre-participation sports physical exam specifically for patients with IDDs. It also provides sport and exercise recommendations for patients with 3 disabilities: Down syndrome, cerebral palsy, and autism spectrum disorder.
Worth noting: As is true for adults without disabilities, those with IDDs should participate in at least 150 minutes of moderate-intensity, or 75 minutes of vigorous intensity, aerobic physical activity each week.9 Recommend muscle-strengthening activities be performed at least 2 days each week.9
Exercise recommendations for patients with Down syndrome
One in every 700 babies receives a diagnosis of Down syndrome.11 Among its many possible manifestations—which include intellectual disability, heart disease, and diabetes—Down syndrome is associated with an increased risk for obesity, which makes exercise an extremely important lifestyle modification for these patients. Obesity can lead to obstructive sleep apnea causing cor pulmonale and even premature death. Continuous positive airway pressure intervention can be difficult in terms of patient compliance. However, weight loss through exercise and sports is an effective intervention to mitigate these obesity-related health comorbidities.
Pre-participation exam. A focused history and physical exam are often conducted before a patient engages in organized competitive or recreational sports. The pre-participation sports physical exam typically focuses on cardiac, neurologic, hereditary, and musculoskeletal disorders. While we recommend including these baseline elements as part of the exam for patients with disabilities, we also recommend modifying the exam to include disability-specific screening for associated comorbidities.
Continue to: For patients with Down syndrome...
For patients with Down syndrome, a complete pre-participation sports physical exam is warranted. Inquire specifically about neck pain or dislocations, heart murmur, cardiac surgery, seizures, sleep issues, history of congenital abdominal defect, hematologic malignancy, and bone pain as part of the focused physical exam.
Look for evidence of patellofemoral instability, pes planus, scoliosis, hallux deformities, decreased muscle tone, and muscular weakness. Check for cataracts and perform a thorough cardiovascular exam to assess for murmur or signs of chronic hypoxia, such as cyanosis. If a heart murmur is detected, refer the patient to a cardiologist.
Patients with Down syndrome are also at increased risk for atlantoaxial instability. A thorough neurologic evaluation to screen for this condition is indicated; however, routine radiologic screening is not needed.12
An annual complete blood cell count and thyroid-stimulating hormone test are recommended for all children with Down syndrome.13 For patients with Down syndrome who are 13 to 21 years of age, an echocardiogram also is recommended for concerning symptoms.13 Ferritin levels also should be assessed annually for patients who are younger than 13 years of age to check for iron-deficiency anemia.13 Consider high-risk screening strategies for patients with diabetes and metabolic syndrome.
Special considerations. Patients with Down syndrome were found to be injured more frequently than individuals with other disabilities during the Special Olympics.14 These patients may be hypersensitive to pain with prolonged pain responses, or unable to verbally communicate their pain or injury.15
Continue to: The complexity of pain assessment...
The complexity of pain assessment in patients with Down syndrome may increase the difficulty of accurately diagnosing an injury, leading to underdiagnosis or overdiagnosis. To increase accuracy of pain assessment in this setting, we recommend using the Wong-Baker FACES Pain Rating Scale or a numeric pain rating scale in verbal patients.15 In nonverbal patients, facial expressions are reliable indicators of pain.
Which exercise? Healthy patients with Down syndrome can participate in any sport. Aerobic exercise can help lower body fat, reduce oxidative stress, and improve blood flow.6 Muscle-strengthening exercises can lead to improved daily functioning and balance. Strength training and aerobic exercise benefit aging patients with Down syndrome who are struggling with obesity. Such exercise also helps increase bone mineral density and improve cardiovascular fitness, especially when initiated at a young age. Consistent exercise promotes positive health outcomes throughout the lifespan.16
Exercise recommendations for patients with cerebral palsy
Cerebral palsy, the most common motor disability in children, is associated with intellectual disability, seizures, respiratory insufficiency, scoliosis, osteoporosis, mood disorders, dysphagia, and speech and hearing impairment.17 The increasing survival of premature babies born with cerebral palsy and the growing prevalence of adults with the condition point to the importance of expanding one’s knowledge of how best to care for this population.18
Pre-participation exam. In addition to a complete sport physical exam, it’s important to further evaluate patients with cerebral palsy for epilepsy, joint contractures, muscle weakness, spinal deformities, and respiratory insufficiency. The Gross Motor Function Classification system, commonly used for patients with cerebral palsy, scores functional ability in 5 levels.18 Patients at Level I are the most mobile; patients at Level V need wheelchair transport in all settings.
Further evaluation of spinal deformities can be initiated with x-ray screening. Consider ordering dual x-ray absorptiometry scans to evaluate bone mass.17
Continue to: Special considerations
Special considerations. Patients with cerebral palsy have a heightened risk for depression and anxiety.19 Mental health can be assessed via the General Anxiety Disorder-7, the Patient Health Questionnaire-9, and the Ask Suicide-Screening Questions tools, among others. Mental health screening may need to be adjusted depending on the patient’s level of cognition and ability to communicate. The patient’s caregiver also can provide supplemental information.
Consider screening vitamin D levels in patients with cerebral palsy. Approximately 50% of adults with cerebral palsy are vitamin D–deficient secondary to sedentary behavior and lack of sun exposure.20-22
Optimal medical management has been shown to decrease muscle spasticity and may be beneficial before initiating an exercise program. For patients with moderate-to-severe symptoms, referral for physical therapy to further improve gross motor function and spasticity may be required before initiating an exercise program.
Which exercise? Individuals with cerebral palsy spend 76% to 99% of their waking hours being sedentary.5 Consequently, they typically have decreased cardiorespiratory endurance and decreased muscle strength. Strength training may improve muscle spasticity, gross motor function, joint health, and respiratory insufficiency.5 Even in those who function at Level IV-V of the Gross Motor Function Classification system, exercise reduces vertebral fractures and improves time spent standing.23 By improving endurance, spasticity, and strength with exercise, deconditioning can be mitigated.
Involvement in sports promotes peer interactions, personal interests, and positive self-identity. It can give a newfound passion for life. Additionally, families of children with disabilities who engage in leisure activities together have less caregiver burden.24,25 Sporting activities offer a way to optimize psychosocial well-being for the patient and the entire family.
Continue to: Dance promotes functionality...
Dance promotes functionality and psychosocial adjustment.26 Hippotherapy, defined as therapy and rehabilitation during which the patient interacts with horses, can diminish muscle spasticity.27 Aquatic therapy also may increase muscle strength.28
Sports for patients with autism spectrum disorder
Autism spectrum disorder is defined as persistent deficits in social communication and social interaction that are usually evident in the first 3 years of life.29 Autism can manifest with or without intellectual or language impairment. Patients with autism commonly have difficulty processing sensory stimuli and can experience “sensory overload.” More than half have a coexisting mental health disorder, such as attention-deficit/hyperactivity disorder, anxiety, depression, schizophrenia, or bipolar disorder.30
Aversions to foods and food selectivity, as well as adverse effects from medical treatment of autism-related agitation, result in a higher incidence of obesity in patients with autism.31,32
Pre-participation exam. In addition to a comprehensive pre-participation exam, the Autism Spectrum Syndrome Questionnaire (ASSQ) and Modified Checklist for Autism in Toddlers are tools to screen school-age children with normal cognition to mild intellectual disability.33 These questionnaires have limitations, however. For example, ASSQ has limited ability to identify the female autistic phenotype.34 As such, these are solely screening tools. Final diagnosis is based on clinical judgment.
Special considerations. Include screening for constipation or diarrhea, fiber intake, food aversions, and common mental health comorbidities using Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition criteria.29 Psychiatric referral may be necessary if certain previously undiagnosed condition(s) become apparent. The patient’s caregiver can provide supplemental information
Continue to: During the physical exam...
During the physical exam, limit sensory stimuli as much as possible, including lights and sounds. Verbalize components of the exam before touching a patient with autism who is sensitive to physical touch.
Which exercise? Participation in sports is an effective therapy for autism and can help patients develop communication skills and promote socialization. Vigorous exercise is associated with a reduction in stereotypic behaviors, hyperactivity, aggression, and self-injury.3 Sports also can offer an alternative channel for social interaction. Children with autism may have impaired or delayed motor skills, and exercise can improve motor skill proficiency.4
The prevalence of feeding problems in children with autism spectrum disorder is estimated to be as high as 90%, and close to 70% are selective eaters.31,35,36 For those with gastrointestinal disorders, exercise can exert positive effects on the microbiome-gut-brain axis.37 Additionally, patients with autism are much more likely to be overweight or obese.32 Physical activity offers those with autism health benefits similar to those for the general population.32
Children with autism spectrum disorder have similar odds of injury, including serious injury, relative to population controls.38 Karate and swimming are among the most researched sports therapy options for patients with autism.38-40 Both are shown to improve motor ability and reduce communication deficits.
Summing up
The literature, although limited, demonstrates that exercise and sports improve the health and well-being of people with IDDs throughout the lifespan, especially if childhood exercise/sports involvement is maintained.
Encourage your patients to participate in sports, but be aware of factors that can limit (or facilitate) participation.41 Exercise participation increases based on, among other things, the individual’s desire to be fit and active, skills practice, peer involvement, family support, accessible facilities, and skilled staff.10
Additional resources that can help people with IDDs access sports and recreational activities include the Special Olympics; Paralympics; YMCA; after-school programs; The American College of Sports Medicine; The National Center on Health, Physical Activity, and Disability; and disability-certified inclusive fitness trainers.
CORRESPONDENCE
Kristina Jones, BS, 12901 Bruce B. Downs Boulevard, Tampa, FL 33612; [email protected]
1. CDC. Addressing gaps in healthcare for individuals with intellectual disabilities. Updated October 15, 2019. Accessed January 21, 2023. www.cdc.gov/grand-rounds/pp/2019/20191015-intellectual-disabilities.html
2. CDC. Vital signs: adults with disabilities. Physical activity is for everybody. Updated November 16, 2018. Accessed January 21, 2023. www.cdc.gov/vitalsigns/disabilities/index.html
3. Di Palma D, Molisso V. Sport for autism. J Humanities Soc Pol. 2017;3:42-49.
4. Pan CY, Chu CH, Tsai CL, et al. The impacts of physical activity intervention on physical and cognitive outcomes in children with autism spectrum disorder. Autism. 2017;21:190-202. doi: 10.1177/1362361316633562
5. Verschuren O, Peterson MD, Balemans AC, et al. Exercise and physical activity recommendations for people with cerebral palsy. Dev Med Child Neurol. 2016;58:798-808. doi: 10.1111/dmcn.13053
6. Paul Y, Ellapen TJ, Barnard M, et al. The health benefits of exercise therapy for patients with Down syndrome: a systematic review. Afr J Disabil. 2019;8:576. doi: 10.4102/ajod.v8i0.576
7. Carroll DD, Courtney-Long EA, Stevens AC, et al. Vital signs: disability and physical activity—United States, 2009-2012. MMWR Morb Mortal Wkly Rep. 2014;63:407-413.
8. Rimmer JH. Physical activity for people with disabilities: how do we reach those with the greatest need? NAM Perspectives. Published April 6, 2015. Accessed March 23, 2023. https://nam.edu/perspectives-2015-physical-activity-for-people-with-disabilities-how-do-we-reach-those-with-the-greatest-need/
9. Department of Health and Human Services. Physical Activity Guidelines For Americans. 2nd edition. Published 2018. Accessed March 23, 2023. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
10. Darcy S, Dowse L. In search of a level playing field—the constraints and benefits of sport participation for people with intellectual disability. Disabil Soc. 2013;28:393-407. doi: 10.1080/ 09687599.2012.714258
11. Mai CT, Isenburg JL, Canfield MA, et al. National population‐based estimates for major birth defects, 2010-2014. Birth Defects Res. 2019;111:1420-1435. doi: 10.1002/bdr2.1589
12. MyŚliwiec A, Posłuszny A, Saulicz E, et al. Atlanto-axial instability in people with Down’s syndrome and its impact on the ability to perform sports activities—a review. J Hum Kinet. 2015;48:17-24. doi: 10.1515/hukin-2015-0087
13. Bunt CW, Bunt SK. Role of the family physician in the care of children with Down syndrome. Am Fam Physician. 2014;90:851-858.
14. McCormick DP, Niebuhr VN, Risser WL. Injury and illness surveillance at local Special Olympic Games. Br J Sports Med. 1990; 24:221-224. doi: 10.1136/bjsm.24.4.221
15. McGuire BE, Defrin R. Pain perception in people with Down syndrome: a synthesis of clinical and experimental research. Front Behav Neurosci. 2015;9. doi: 10.3389/fnbeh.2015.00194
16. Barnhart RC, Connolly B. Aging and Down syndrome: implications for physical therapy. Phys Ther. 2007;87:1399-1406. doi: 10.2522/ptj.20060334
17. Vitrikas K, Dalton H, Breish D. Cerebral palsy: an overview. Am Fam Physician. 2020;101:213-220.
18. Maenner MJ, Blumberg SJ, Kogan MD, et al. Prevalence of cerebral palsy and intellectual disability among children identified in two US national surveys, 2011-2013. Ann Epidemiol. 2016;26:222-226. doi: 10.1016/j.annepidem.2016.01.001
19. Smith KJ, Peterson MD, O’Connell NE, et al. Risk of depression and anxiety in adults with cerebral palsy. JAMA Neurol. 2019;76;294-300. doi: 10.1001/jamaneurol.2018.4147
20. Peterson MD, Haapala HJ, Chaddha A, et al. Abdominal obesity is an independent predictor of serum 25-hydroxyvitamin D deficiency in adults with cerebral palsy. Nutr Metab (Lond). 2014;11:22. doi: 10.1186/1743-7075-11-22
21. Yi YG, Jung SH, Bang MS. Emerging issues in cerebral palsy associated with aging: a physiatrist perspective. Ann Rehabil Med. 2019;43:241-249. doi: 10.5535/arm.2019.43.3.241
22. Sarathy K, Doshi C, Aroojis A. Clinical examination of children with cerebral palsy. Indian J Orthop. 2019;53:35-44. doi: 10.4103/ortho.IJOrtho_409_17
23. Caulton JM, Ward KA, Alsop CW, et al. A randomised controlled trial of standing programme on bone mineral density in non-ambulant children with cerebral palsy. Arch Dis Child. 2004;89:131-135. doi: 10.1136/adc.2002.009316
24. Clutterbuck G, Auld M, Johnston L. Active exercise interventions improve gross motor function of ambulant/semi-ambulant children with cerebral palsy: a systematic review. Disabil Rehabil. 2019;41:1131-1151. doi: 10.1080/09638288.2017.1422035
25. Shikako-Thomas K, Majnemer A, Law M, et al. Determinants of participation in leisure activities in children and youth with cerebral palsy: systematic review. Phys Occup Ther Pedi. 2008;28:155-169. doi: 10.1080/01942630802031834
26. Teixeira-Machado L, Azevedo-Santos I, DeSantana JM. Dance improves functionality and psychosocial adjustment in cerebral palsy: a randomized controlled clinical trial. Am J Phys Med Rehabil. 2017;96:424-429. doi: 10.1097/PHM.0000000000000646
27. Lucena-Antón D, Rosety-Rodríguez I, Moral-Munoz JA. Effects of a hippotherapy intervention on muscle spasticity in children with cerebral palsy: a randomized controlled trial. Complement Ther Clin Pract. 2018;31:188-192. doi: 10.1016/j.ctcp.2018.02.013
28. Roostaei M, Baharlouei H, Azadi H, et al. Effects of aquatic intervention on gross motor skills in children with cerebral palsy: a systematic review. Phys Occup Ther Pediatr. 2017;37:496-515. doi: 10.1080/01942638.2016.1247938
29. American Psychiatric Association. Autism spectrum disorder, section II. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. 2013:50-56.
30. Romero M, Aguilar JM, Del-Rey-Mejías Á, et al. Psychiatric comorbidities in autism spectrum disorder: a comparative study between DSM-IV-TR and DSM-5 diagnosis. Int J Clin Health Psychol. 2016;16:266-275. doi: 10.1016/j.ijchp.2016.03.001
31. Volkert VM, Vaz PC. Recent studies on feeding problems in children with autism. J Appl Behav Anal. 2015;43:155-159. doi: 10.1901/jaba.2010.43-155
32. Broder-Fingert S, Brazauskas K, Lindgren K, et al. Prevalence of overweight and obesity in a large clinical sample of children with autism. Acad Pediatr. 2014;14:408-414. doi: 10.1016/j.acap.2014.04.004. PMID: 24976353
33. Adachi M, Takahashi M, Takayanagi N, et al. Adaptation of the Autism Spectrum Screening Questionnaire (ASSQ) to preschool children. PLoS One. 2018;10;13:e0199590. doi: 10.1371/journal.pone.0199590
34. Kopp S. Gillberg C. The Autism Spectrum Screening Questionnaire (ASSQ)-Revised Extended Version (ASSQ-REV): an instrument for better capturing the autism phenotype in girls? A preliminary study involving 191 clinical cases and community controls. Res Develop Disabil. 2011:32: 2875-2888.
35. Kotak T. Piazza CC. Assessment and behavioral treatment of feeding and sleeping disorders in children with autism spectrum disorders. Child Adol Psych Clin North Am. 2008;17:887-905. doi: 10.1016/j.chc.2008.06.005
36. Twachtman-Reilly J, Amaral SC, Zebrowski PP. Addressing feeding behaviors in children on the autism spectrum in school-based settings: physiological and behavioral issues. Lang Speech Hear Serv Sch. 2008:39:261-272. doi: 10.1044/0161-1461(2008/025)
37. Dalton A, Mermier C, Zuhl M. Exercise influence on the microbiome-gut-brain axis. Gut Microbes. 2019;10:555-568. doi: 10.1080/19490976.2018.1562268
38. Iliadis I, Apteslis N. The role of physical education and exercise for children with autism spectrum disorder and the effects on socialization, communication, behavior, fitness, and quality of life. Dial Clin Neurosc Mental Health. 2020;3:71-78. doi: 10.26386/obrela.v3i1.178
39. Phung JN, Goldberg WA. Promoting executive functioning in children with autism spectrum disorder through mixed martial arts training. J Autism Dev Dis. 2019;49:3660-3684. doi: 10.1007/s10803-019-04072-3
40. Bahrami F, Movahedi A, Marandi SM, et al. The effect of karate techniques training on communication deficit of children with autism spectrum disorder. J Autism Dev Disord. 2016;46: 978-986. doi: 10.1007/s10803-015-2643-y
41. Shields N, Synnot A. Perceived barriers and facilitators to participation in physical activity for children with disability: a qualitative study. BMC Pediatr. 2016;16:9. doi: 10.1186/s12887-016-0544-7
1. CDC. Addressing gaps in healthcare for individuals with intellectual disabilities. Updated October 15, 2019. Accessed January 21, 2023. www.cdc.gov/grand-rounds/pp/2019/20191015-intellectual-disabilities.html
2. CDC. Vital signs: adults with disabilities. Physical activity is for everybody. Updated November 16, 2018. Accessed January 21, 2023. www.cdc.gov/vitalsigns/disabilities/index.html
3. Di Palma D, Molisso V. Sport for autism. J Humanities Soc Pol. 2017;3:42-49.
4. Pan CY, Chu CH, Tsai CL, et al. The impacts of physical activity intervention on physical and cognitive outcomes in children with autism spectrum disorder. Autism. 2017;21:190-202. doi: 10.1177/1362361316633562
5. Verschuren O, Peterson MD, Balemans AC, et al. Exercise and physical activity recommendations for people with cerebral palsy. Dev Med Child Neurol. 2016;58:798-808. doi: 10.1111/dmcn.13053
6. Paul Y, Ellapen TJ, Barnard M, et al. The health benefits of exercise therapy for patients with Down syndrome: a systematic review. Afr J Disabil. 2019;8:576. doi: 10.4102/ajod.v8i0.576
7. Carroll DD, Courtney-Long EA, Stevens AC, et al. Vital signs: disability and physical activity—United States, 2009-2012. MMWR Morb Mortal Wkly Rep. 2014;63:407-413.
8. Rimmer JH. Physical activity for people with disabilities: how do we reach those with the greatest need? NAM Perspectives. Published April 6, 2015. Accessed March 23, 2023. https://nam.edu/perspectives-2015-physical-activity-for-people-with-disabilities-how-do-we-reach-those-with-the-greatest-need/
9. Department of Health and Human Services. Physical Activity Guidelines For Americans. 2nd edition. Published 2018. Accessed March 23, 2023. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
10. Darcy S, Dowse L. In search of a level playing field—the constraints and benefits of sport participation for people with intellectual disability. Disabil Soc. 2013;28:393-407. doi: 10.1080/ 09687599.2012.714258
11. Mai CT, Isenburg JL, Canfield MA, et al. National population‐based estimates for major birth defects, 2010-2014. Birth Defects Res. 2019;111:1420-1435. doi: 10.1002/bdr2.1589
12. MyŚliwiec A, Posłuszny A, Saulicz E, et al. Atlanto-axial instability in people with Down’s syndrome and its impact on the ability to perform sports activities—a review. J Hum Kinet. 2015;48:17-24. doi: 10.1515/hukin-2015-0087
13. Bunt CW, Bunt SK. Role of the family physician in the care of children with Down syndrome. Am Fam Physician. 2014;90:851-858.
14. McCormick DP, Niebuhr VN, Risser WL. Injury and illness surveillance at local Special Olympic Games. Br J Sports Med. 1990; 24:221-224. doi: 10.1136/bjsm.24.4.221
15. McGuire BE, Defrin R. Pain perception in people with Down syndrome: a synthesis of clinical and experimental research. Front Behav Neurosci. 2015;9. doi: 10.3389/fnbeh.2015.00194
16. Barnhart RC, Connolly B. Aging and Down syndrome: implications for physical therapy. Phys Ther. 2007;87:1399-1406. doi: 10.2522/ptj.20060334
17. Vitrikas K, Dalton H, Breish D. Cerebral palsy: an overview. Am Fam Physician. 2020;101:213-220.
18. Maenner MJ, Blumberg SJ, Kogan MD, et al. Prevalence of cerebral palsy and intellectual disability among children identified in two US national surveys, 2011-2013. Ann Epidemiol. 2016;26:222-226. doi: 10.1016/j.annepidem.2016.01.001
19. Smith KJ, Peterson MD, O’Connell NE, et al. Risk of depression and anxiety in adults with cerebral palsy. JAMA Neurol. 2019;76;294-300. doi: 10.1001/jamaneurol.2018.4147
20. Peterson MD, Haapala HJ, Chaddha A, et al. Abdominal obesity is an independent predictor of serum 25-hydroxyvitamin D deficiency in adults with cerebral palsy. Nutr Metab (Lond). 2014;11:22. doi: 10.1186/1743-7075-11-22
21. Yi YG, Jung SH, Bang MS. Emerging issues in cerebral palsy associated with aging: a physiatrist perspective. Ann Rehabil Med. 2019;43:241-249. doi: 10.5535/arm.2019.43.3.241
22. Sarathy K, Doshi C, Aroojis A. Clinical examination of children with cerebral palsy. Indian J Orthop. 2019;53:35-44. doi: 10.4103/ortho.IJOrtho_409_17
23. Caulton JM, Ward KA, Alsop CW, et al. A randomised controlled trial of standing programme on bone mineral density in non-ambulant children with cerebral palsy. Arch Dis Child. 2004;89:131-135. doi: 10.1136/adc.2002.009316
24. Clutterbuck G, Auld M, Johnston L. Active exercise interventions improve gross motor function of ambulant/semi-ambulant children with cerebral palsy: a systematic review. Disabil Rehabil. 2019;41:1131-1151. doi: 10.1080/09638288.2017.1422035
25. Shikako-Thomas K, Majnemer A, Law M, et al. Determinants of participation in leisure activities in children and youth with cerebral palsy: systematic review. Phys Occup Ther Pedi. 2008;28:155-169. doi: 10.1080/01942630802031834
26. Teixeira-Machado L, Azevedo-Santos I, DeSantana JM. Dance improves functionality and psychosocial adjustment in cerebral palsy: a randomized controlled clinical trial. Am J Phys Med Rehabil. 2017;96:424-429. doi: 10.1097/PHM.0000000000000646
27. Lucena-Antón D, Rosety-Rodríguez I, Moral-Munoz JA. Effects of a hippotherapy intervention on muscle spasticity in children with cerebral palsy: a randomized controlled trial. Complement Ther Clin Pract. 2018;31:188-192. doi: 10.1016/j.ctcp.2018.02.013
28. Roostaei M, Baharlouei H, Azadi H, et al. Effects of aquatic intervention on gross motor skills in children with cerebral palsy: a systematic review. Phys Occup Ther Pediatr. 2017;37:496-515. doi: 10.1080/01942638.2016.1247938
29. American Psychiatric Association. Autism spectrum disorder, section II. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. 2013:50-56.
30. Romero M, Aguilar JM, Del-Rey-Mejías Á, et al. Psychiatric comorbidities in autism spectrum disorder: a comparative study between DSM-IV-TR and DSM-5 diagnosis. Int J Clin Health Psychol. 2016;16:266-275. doi: 10.1016/j.ijchp.2016.03.001
31. Volkert VM, Vaz PC. Recent studies on feeding problems in children with autism. J Appl Behav Anal. 2015;43:155-159. doi: 10.1901/jaba.2010.43-155
32. Broder-Fingert S, Brazauskas K, Lindgren K, et al. Prevalence of overweight and obesity in a large clinical sample of children with autism. Acad Pediatr. 2014;14:408-414. doi: 10.1016/j.acap.2014.04.004. PMID: 24976353
33. Adachi M, Takahashi M, Takayanagi N, et al. Adaptation of the Autism Spectrum Screening Questionnaire (ASSQ) to preschool children. PLoS One. 2018;10;13:e0199590. doi: 10.1371/journal.pone.0199590
34. Kopp S. Gillberg C. The Autism Spectrum Screening Questionnaire (ASSQ)-Revised Extended Version (ASSQ-REV): an instrument for better capturing the autism phenotype in girls? A preliminary study involving 191 clinical cases and community controls. Res Develop Disabil. 2011:32: 2875-2888.
35. Kotak T. Piazza CC. Assessment and behavioral treatment of feeding and sleeping disorders in children with autism spectrum disorders. Child Adol Psych Clin North Am. 2008;17:887-905. doi: 10.1016/j.chc.2008.06.005
36. Twachtman-Reilly J, Amaral SC, Zebrowski PP. Addressing feeding behaviors in children on the autism spectrum in school-based settings: physiological and behavioral issues. Lang Speech Hear Serv Sch. 2008:39:261-272. doi: 10.1044/0161-1461(2008/025)
37. Dalton A, Mermier C, Zuhl M. Exercise influence on the microbiome-gut-brain axis. Gut Microbes. 2019;10:555-568. doi: 10.1080/19490976.2018.1562268
38. Iliadis I, Apteslis N. The role of physical education and exercise for children with autism spectrum disorder and the effects on socialization, communication, behavior, fitness, and quality of life. Dial Clin Neurosc Mental Health. 2020;3:71-78. doi: 10.26386/obrela.v3i1.178
39. Phung JN, Goldberg WA. Promoting executive functioning in children with autism spectrum disorder through mixed martial arts training. J Autism Dev Dis. 2019;49:3660-3684. doi: 10.1007/s10803-019-04072-3
40. Bahrami F, Movahedi A, Marandi SM, et al. The effect of karate techniques training on communication deficit of children with autism spectrum disorder. J Autism Dev Disord. 2016;46: 978-986. doi: 10.1007/s10803-015-2643-y
41. Shields N, Synnot A. Perceived barriers and facilitators to participation in physical activity for children with disability: a qualitative study. BMC Pediatr. 2016;16:9. doi: 10.1186/s12887-016-0544-7
PRACTICE RECOMMENDATIONS
› Recommend physical activity as an adjunct to traditional medical management to maximize physical and psychosocial benefits in patients with intellectual/developmental disabilities. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Conversion disorder: An integrated care approach
THE CASE
Janice M* presented to the emergency department (ED) with worsening slurred speech. The 55-year-old patient’s history was significant for diabetes; hypertension; depression; sleep apnea; multiple transient ischemic attacks (TIAs) thought to be stress related; and left lower-extremity weakness secondary to prior infarct. Ms. M had been to the hospital multiple times in the previous 2 to 3 years for similar symptoms. Her most recent visit to the ED had been 2 months earlier.
In the ED, the patient’s NIH stroke score was 1 for the presence of dysarthria, and a code for emergency stroke management was initiated. Ms. M was alert and oriented x 3, with no focal motor or sensory deficits noted. Computed tomography (CT) and CT angiography were negative for any acute abnormality. Throughout the course of the ED visit, her NIH score improved to 0. Ms. M exhibited staccato/stuttering speech, but it was believed that this would likely improve over the next few days.
According to the hospital neurologist, the ED work-up suggested either a TIA, stress-induced psychiatric speech disorder, or conversion disorder. The patient was discharged home in stable condition and was asked to follow up with the outpatient neurologist in 1 week.
Ms. M was seen approximately 2 weeks later in the outpatient neurology stroke clinic. Her symptoms had resolved, and she did not report any new or worsening symptoms. An outpatient stroke work-up was initiated, including magnetic resonance imaging (MRI) of the brain, echocardiography, and measurement of low-density lipoprotein and hemoglobin A1C; all results were unremarkable. Given the timeline for symptom improvement and results of the work-up, the patient was given a diagnosis of conversion disorder. Ms. M was encouraged to follow up with her primary care physician (PCP) for further medical management.
●
* The patient’s name has been changed to protect her identity.
What is conversion disorder, and how common is it?
According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, text revision, conversion disorder (also known as functional neurological symptom disorder) is characterized as a somatic symptom and related disorder.1 The prominent feature shared among disorders in this category is the presence of somatic symptoms that are associated with distress and impairment.
In conversion disorder, the focus is on symptoms that are neurologic in nature but are not due to underlying neurologic disease and are incongruent with typical patterns of presentation for any neurologic condition. Patients with conversion disorder may present with motor symptoms (eg, weakness, paralysis, tremor, dystonia), altered sensory or cognitive function, seizure-like symptoms, alterations in speech, or changes in swallowing.1,2
For a diagnosis of conversion disorder, the following criteria must be met1:
- The patient has 1 or more symptoms of altered voluntary motor or sensory function.
- Symptom presentation is incongruent with recognized neurologic or medical disease or conditions.
S ymptoms are not better explained by another medical or mental health condition.- There is significant distress or impairment in functioning due to symptoms or the deficit.
The etiology of conversion disorder has not been firmly established. While the literature suggests that psychological stressors play a role,3,4 an effort also has been made to better understand the underlying neural and biological basis. Specifically, studies have utilized brain imaging to explore brain pathways and mechanisms that could account for symptom presentation.5,6
Prevalence rates for conversion disorder vary depending on the population studied. While it is estimated that 5% of patients in a general hospital setting meet full criteria for conversion disorder,7 higher rates may exist in specialty settings; 1 study found that 30% of patients in a neurology specialty clinic exhibited symptoms that were medically unexplained.8
Continue to: In primary care...
In primary care, prevalence of conversion disorder can be difficult to pinpoint; however, 1 study indicated that physicians identified medically unexplained symptoms as the main presenting problem for nearly 20% of patients in a primary care setting.9 Therefore, it is important for family physicians (FPs) to be familiar with the assessment and treatment of conversion disorder (and other disorders in which medically unexplained symptoms may be at the core of the patient presentation).
The differential: Neurologic and psychiatric conditions
Patients with conversion disorder may present with a variety of neurologic symptoms that can mimic those of organic disease. This can pose a diagnostic challenge, increase the chance of misdiagnosis, and delay treatment.
Motor symptoms may include paralysis, gait disturbance, dysphagia, or aphasia. Patients also may have sensory symptoms, such as blindness, deafness, or anesthesia.10,11 As a result, it is important to rule out both urgent neurologic presentations, such as TIA, acute stroke, and brain tumor, and other chronic neurologic conditions, including multiple sclerosis, myasthenia gravis, and epilepsy.11,12
Multiple sclerosis will demonstrate characteristic lesions on MRI that differentiate it from conversion disorder.
Myasthenia gravis is distinguished by positive findings on autoantibodies testing and on electrophysiologic studies.
Continue to: Epilepsy
Epilepsy. Patients with conversion disorder may present with unresponsiveness and abnormal movements, such as generalized limb shaking and hip thrusting, that mimic an epileptic seizure. In contrast to epileptic seizures, psychogenic nonepileptic seizures may last longer, symptoms may wax and wane, and patients generally do not have bowel or bladder incontinence or sustain injury as they would during an actual seizure.12
There are several psychiatric/psychosocial conditions that also should be considered in the differential diagnosis of conversion disorder.
Somatic symptom disorder, like conversion disorder, produces somatic symptoms that can cause significant distress for patients. The difference in the 2 conditions is that symptoms of somatic symptom disorder may be compatible with a recognized neurologic or general medical condition, whereas in conversion disorder, the symptoms are not consistent with a recognized disease.1,12
Factitious disorder, similar to conversion disorder, can involve neurologic symptoms that are not attributed to disease. However, patients with factitious disorder deliberately simulate symptoms to receive medical care. A thorough clinical interview and physical exam can help to distinguish conversion disorder from factitious disorder.
Malingering is not a psychiatric condition but a behavior that involves intentionally feigning symptoms for the purpose of personal or financial gain. There is no evidence that patients with conversion disorder simulate their symptoms.12,13
Continue to: Negative results and positive signs point to the Dx
Negative results and positive signs point to the Dx
Conversion disorder is not a diagnosis of exclusion. Diagnosis requires detailed history taking and a thorough neurologic exam. Laboratory testing and neuroimaging are also important, and results will have to be negative to support the diagnosis.
Neurologic deficits with conversion disorder do not follow a known neurologic insult.14 There are many tests that can be used to distinguish functional symptoms vs organic symptoms. Two of the most well-known tests are the Hoover sign and the abductor sign, which will be positive in conversion disorder. Both can be performed easily in an outpatient setting.
The Hoover sign is considered positive when there is weakness of voluntary hip extension in the presence of normal involuntary hip extension during contralateral hip flexion against resistance. According to a meta-analysis of multiple studies of patients with conversion disorder, the overall estimated sensitivity of this test is 94% and the specificity, 99%.15
The abductor sign follows the same principle as the Hoover sign: When the patient abducts the nonparetic leg, both the nonparetic and “paretic” leg are strong. When the patient abducts just the “paretic” leg, both legs become weak.16
Other symptom evaluations. For patients who have functional seizures, video electroencephalography is helpful to distinguish functional seizures from “true” seizures.17,18 In conversion disorder, functional dysarthria normally resembles a stutter or speech that is extremely slow with long hesitations that are hard to interrupt.18 Dysphonia and functional dysphagia are also very common functional symptoms. Usually after extensive work-up, no organic cause of the patient’s symptoms is ever found.18
Continue to: Treatment requires an integrated team approach
Treatment requires an integrated team approach
Treatment for conversion disorder can be difficult due to the complex and not fully understood etiology of the condition. Due to its multifaceted nature, an integrated team approach can be beneficial at each stage, including assessment and intervention.
Explain the diagnosis clearly. An essential initial step in the treatment of conversion disorder is careful explanation of the diagnosis. Clear explanation of the terminology and presentation of conversion disorder may prevent the patient from misinterpreting their diagnosis as a suggestion that they are feigning or malingering symptoms or feeling that their symptoms or concerns are being dismissed.2 Understanding the condition can help improve the likelihood of the patient accepting the treatment plan and help decrease the likelihood of unnecessary testing, health care visits, and consultations. Developing a strong rapport with the patient is key when explaining the diagnosis.
Recommend cognitive behavioral therapy (CBT). In a meta-analysis of 15 randomized controlled trials, CBT significantly reduced somatic, anxious, and depressive symptoms and improved physical functioning in patients with somatoform disorders and medically unexplained symptoms.19 Another study, utilizing a case series, demonstrated significant improvement in social, emotional, and behavioral functioning in children and adolescents with functional neurologic symptoms (conversion disorder) post–CBT intervention.20
Given that research supports CBT’s effectiveness in the management of conversion disorder, it is beneficial to engage a behavioral health professional as a part of the treatment team to focus on factors such as stress management, development of coping skills, and treatment of underlying psychiatric conditions.
Consider these other options. The addition of medication management can be considered for patients with comorbid psychiatric disorders. Evidence suggests that physical therapy is helpful in the treatment of motor and gait dysfunction seen in conversion disorder.21,22 The role of hypnosis in the management of conversion disorder has also been studied, but more randomized clinical trials are needed to further explore this treatment.2,23,24
Continue to: The FP's role in coordination of care
The FP’s role in coordination of care
Conversion disorder can be challenging to diagnose and often involves a multidisciplinary approach. Patients with conversion disorder may see multiple clinicians as they undergo evaluation for their symptoms, but they usually are referred back to their PCP for management and coordination of care. Thus, the FP’s understanding of how the condition is diagnosed and appropriately managed is beneficial.
Open and effective communication among all members of the health care team can ensure consistency in treatment, a strong patient–provider relationship, favorable prognosis, and prevention of symptom relapse. FPs, by establishing a good rapport with patients, can help them understand the condition and the mind-body connection. Once other diagnoses have been ruled out, the FP can provide reassurance to patients and minimize further diagnostic testing.
The prognosis of conversion disorder is associated with symptom duration25; thus, consultation between FPs and mental health providers is essential. The FP also can be integral in the recognition of psychiatric comorbidities, such as anxiety and depression, helping to ensure that these conditions also are treated appropriately.25,26
THE CASE
Ms. M was referred to a neuropsychologist for further assessment, and the diagnosis of conversion disorder was confirmed. She was then referred to a family medicine behavioral health psychologist for CBT. The initial consult indicated that psychological stressors were contributing to symptoms, and Ms. M was diagnosed with depression and anxiety as well as conversion disorder.
Treatment started with patient education. The treatment framework was carefully explained to Ms. M, with a focus on identifying possible symptom triggers, helping her build a more effective stress response, increasing skills to more effectively manage stressors, and managing underlying psychiatric disorders (ie, depression, anxiety).
Ms. M continued regular visits with the family medicine behavioral health psychologist for CBT and followed up with her PCP as needed to manage chronic health conditions and stroke risk factors. The patient was able to implement skills discussed in treatment sessions, including identifying triggers and implementing coping skills (eg, managing negative thoughts that contribute to symptoms, setting boundaries) to manage stressors.
Her depressive and anxious symptoms improved, as indicated by symptom measurement tools and self-report. The frequency and severity of episodes of slurred speech and muscle weakness decreased, and the patient reported only 1 ED visit related to speech difficulties in the 2 years while following up with the behavioral health psychologist.
CORRESPONDENCE
Kristen J. Alston, PhD, University of Mississippi Medical Center, 2400 North State Street, Jackson, MS 39216; [email protected]
1. American Psychiatric Association. Somatic symptom and related disorders. In: Diagnostic and Statistical Manual of Mental Disorders. 5th edition, text revision. American Psychiatric Association Publishing; 2022. doi: 10.1176/appi.books.9780890425787.x09_Somatic_Symptom_and_Related_Disorders
2. O’Neal MA, Baslet G. Treatment for patients with a functional neurological disorder (conversion disorder): an integrated approach. Am J Psychiatry. 2018;175:307-314. doi: 10.1176/appi.ajp.2017.17040450
3. Roelofs K, Spinhoven P, Sandijck P, et al. The impact of early trauma and recent life-events on symptom severity in patients with conversion disorder. J Nerv Ment Dis. 2005;193:508-514. doi: 10.1097/01.nmd.0000172472.60197.4d
4. Nicholson TR, Aybek S, Craig T, et al. Life events and escape in conversion disorder. Psychol Med. 2016;46:2617-2626. doi: 10.1017/S0033291716000714
5. Ejareh Dar M, Kanaan RA. Uncovering the etiology of conversion disorder: insights from functional neuroimaging. Neuropsychiatr Dis Treat. 2016;12:143-153. doi: 10.2147/NDT.S65880
6. Aybek S, Vuilleumier P. Imaging studies of functional neurologic disorders. Handb Clin Neurol. 2016;139:73-84. doi: 10.1016/B978-0-12-801772-2.00007-2
7. Folks DG, Ford CV, Regan WM. Conversion symptoms in a general hospital. Psychosomatics. 1984;25:285-295. doi: 10.1016/S0033-3182(84)73046-5
8. Carson AJ, Best S, Postma K, et al. The outcome of neurology outpatients with medically unexplained symptoms: a prospective cohort study. J Neurol Neurosurg Psychiatry. 2003;74:897-900. doi: 10.1136/jnnp.74.7.897
9. Peveler R, Kilkenny L, Kinmonth AL. Medically unexplained physical symptoms in primary care: a comparison of self-report screening questionnaires and clinical opinion. J Psychosom Res. 1997;42:245-252. doi: 10.1016/s0022-3999(96)00292-9
10. Tobiano PS, Wang HE, McCausland JB, et al. A case of conversion disorder presenting as a severe acute stroke. J Emerg Med. 2006;30:283-286. doi: 10.1016/j.jemermed.2005.05.024
11. Chou HY, Weng MC, Huang MH, et al. Conversion disorder in stroke: a case report. Kaohsiung J Med Sci. 2006;22:586-589. doi: 10.1016/S1607-551X(09)70357-2
12. Peeling JL, Muzio MR. Conversion disorder. StatPearls [Internet]. Updated May 19, 2021. Accessed March 14, 2023. www.ncbi.nlm.nih.gov/books/NBK551567/
13. Ali S, Jabeen S, Pate RJ, et al. Conversion disorder—mind versus body: a review. Innov Clin Neurosci. 2015;12:27-33.
14. Hurwitz TA. Somatization and conversion disorder. Can J Psychiatry. 2004;49:172-178. doi: 10.1177/070674370404900304
15. Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85:180-190. doi: 10.1136/jnnp-2012-304607
16. Sonoo M. Abductor sign: a reliable new sign to detect unilateral non-organic paresis of the lower limb. J Neurol Neurosurg Psychiatry. 2004;75:121-125.
17. Tsui P, Deptula A, Yuan DY. Conversion disorder, functional neurological symptom disorder, and chronic pain: comorbidity, assessment, and treatment. Curr Pain Headache Rep. 2017;21:29. doi: 10.1007/s11916-017-0627-7
18. Stone J, Carson A, Sharpe M. Functional symptoms and signs in neurology: assessment and diagnosis. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i2-i12. doi: 10.1136/jnnp.2004.061655
19. Liu J, Gill NS, Teodorczuk A, et al. The efficacy of cognitive behavioural therapy in somatoform disorders and medically unexplained physical symptoms: a meta-analysis of randomized controlled trials. J Affect Disord. 2019;245:98-112. doi: 10.1016/j.jad.2018.10.114
20. McFarlane FA, Allcott-Watson H, Hadji-Michael M, et al. Cognitive-behavioural treatment of functional neurological symptoms (conversion disorder) in children and adolescents: a case series. Eur J Paediatr Neurol. 2019;23:317-328. doi: 10.1016/j.ejpn.2018.12.002
21. Ness D. Physical therapy management for conversion disorder: case series. J Neurol Phys Ther. 2007;31:30-39. doi: 10.1097/01.npt.0000260571.77487.14
22. Nielsen G, Ricciardi L, Demartini B, et al. Outcomes of a 5-day physiotherapy programme for functional (psychogenic) motor disorders. J Neurol. 2015;262:674-681. doi: 10.1007/s00415-014-7631-1
23. Sanyal R, Raseta M, Natarajan I, et al. The use of hypnotherapy as treatment for functional stroke: a case series from a single center in the UK. Int J Stroke. 2022;17:59-66. doi: 10.1177/1747493021995590
24. Moene FC, Spinhoven P, Hoogduin KA, et al. A randomized controlled clinical trial of a hypnosis-based treatment for patients with conversion disorder, motor type. Int J Clin Exp Hypn. 2003;51:29-50. doi: 10.1076/iceh.51.1.29.14067
25. Feinstein A. Conversion disorder: advances in our understanding. CMAJ. 2011;183:915-920. doi: 10.1503/cmaj.110490
26. Kurlansik SL, Maffei MS. Somatic symptom disorder. Am Fam Physician. 2016;93:49-54.
THE CASE
Janice M* presented to the emergency department (ED) with worsening slurred speech. The 55-year-old patient’s history was significant for diabetes; hypertension; depression; sleep apnea; multiple transient ischemic attacks (TIAs) thought to be stress related; and left lower-extremity weakness secondary to prior infarct. Ms. M had been to the hospital multiple times in the previous 2 to 3 years for similar symptoms. Her most recent visit to the ED had been 2 months earlier.
In the ED, the patient’s NIH stroke score was 1 for the presence of dysarthria, and a code for emergency stroke management was initiated. Ms. M was alert and oriented x 3, with no focal motor or sensory deficits noted. Computed tomography (CT) and CT angiography were negative for any acute abnormality. Throughout the course of the ED visit, her NIH score improved to 0. Ms. M exhibited staccato/stuttering speech, but it was believed that this would likely improve over the next few days.
According to the hospital neurologist, the ED work-up suggested either a TIA, stress-induced psychiatric speech disorder, or conversion disorder. The patient was discharged home in stable condition and was asked to follow up with the outpatient neurologist in 1 week.
Ms. M was seen approximately 2 weeks later in the outpatient neurology stroke clinic. Her symptoms had resolved, and she did not report any new or worsening symptoms. An outpatient stroke work-up was initiated, including magnetic resonance imaging (MRI) of the brain, echocardiography, and measurement of low-density lipoprotein and hemoglobin A1C; all results were unremarkable. Given the timeline for symptom improvement and results of the work-up, the patient was given a diagnosis of conversion disorder. Ms. M was encouraged to follow up with her primary care physician (PCP) for further medical management.
●
* The patient’s name has been changed to protect her identity.
What is conversion disorder, and how common is it?
According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, text revision, conversion disorder (also known as functional neurological symptom disorder) is characterized as a somatic symptom and related disorder.1 The prominent feature shared among disorders in this category is the presence of somatic symptoms that are associated with distress and impairment.
In conversion disorder, the focus is on symptoms that are neurologic in nature but are not due to underlying neurologic disease and are incongruent with typical patterns of presentation for any neurologic condition. Patients with conversion disorder may present with motor symptoms (eg, weakness, paralysis, tremor, dystonia), altered sensory or cognitive function, seizure-like symptoms, alterations in speech, or changes in swallowing.1,2
For a diagnosis of conversion disorder, the following criteria must be met1:
- The patient has 1 or more symptoms of altered voluntary motor or sensory function.
- Symptom presentation is incongruent with recognized neurologic or medical disease or conditions.
S ymptoms are not better explained by another medical or mental health condition.- There is significant distress or impairment in functioning due to symptoms or the deficit.
The etiology of conversion disorder has not been firmly established. While the literature suggests that psychological stressors play a role,3,4 an effort also has been made to better understand the underlying neural and biological basis. Specifically, studies have utilized brain imaging to explore brain pathways and mechanisms that could account for symptom presentation.5,6
Prevalence rates for conversion disorder vary depending on the population studied. While it is estimated that 5% of patients in a general hospital setting meet full criteria for conversion disorder,7 higher rates may exist in specialty settings; 1 study found that 30% of patients in a neurology specialty clinic exhibited symptoms that were medically unexplained.8
Continue to: In primary care...
In primary care, prevalence of conversion disorder can be difficult to pinpoint; however, 1 study indicated that physicians identified medically unexplained symptoms as the main presenting problem for nearly 20% of patients in a primary care setting.9 Therefore, it is important for family physicians (FPs) to be familiar with the assessment and treatment of conversion disorder (and other disorders in which medically unexplained symptoms may be at the core of the patient presentation).
The differential: Neurologic and psychiatric conditions
Patients with conversion disorder may present with a variety of neurologic symptoms that can mimic those of organic disease. This can pose a diagnostic challenge, increase the chance of misdiagnosis, and delay treatment.
Motor symptoms may include paralysis, gait disturbance, dysphagia, or aphasia. Patients also may have sensory symptoms, such as blindness, deafness, or anesthesia.10,11 As a result, it is important to rule out both urgent neurologic presentations, such as TIA, acute stroke, and brain tumor, and other chronic neurologic conditions, including multiple sclerosis, myasthenia gravis, and epilepsy.11,12
Multiple sclerosis will demonstrate characteristic lesions on MRI that differentiate it from conversion disorder.
Myasthenia gravis is distinguished by positive findings on autoantibodies testing and on electrophysiologic studies.
Continue to: Epilepsy
Epilepsy. Patients with conversion disorder may present with unresponsiveness and abnormal movements, such as generalized limb shaking and hip thrusting, that mimic an epileptic seizure. In contrast to epileptic seizures, psychogenic nonepileptic seizures may last longer, symptoms may wax and wane, and patients generally do not have bowel or bladder incontinence or sustain injury as they would during an actual seizure.12
There are several psychiatric/psychosocial conditions that also should be considered in the differential diagnosis of conversion disorder.
Somatic symptom disorder, like conversion disorder, produces somatic symptoms that can cause significant distress for patients. The difference in the 2 conditions is that symptoms of somatic symptom disorder may be compatible with a recognized neurologic or general medical condition, whereas in conversion disorder, the symptoms are not consistent with a recognized disease.1,12
Factitious disorder, similar to conversion disorder, can involve neurologic symptoms that are not attributed to disease. However, patients with factitious disorder deliberately simulate symptoms to receive medical care. A thorough clinical interview and physical exam can help to distinguish conversion disorder from factitious disorder.
Malingering is not a psychiatric condition but a behavior that involves intentionally feigning symptoms for the purpose of personal or financial gain. There is no evidence that patients with conversion disorder simulate their symptoms.12,13
Continue to: Negative results and positive signs point to the Dx
Negative results and positive signs point to the Dx
Conversion disorder is not a diagnosis of exclusion. Diagnosis requires detailed history taking and a thorough neurologic exam. Laboratory testing and neuroimaging are also important, and results will have to be negative to support the diagnosis.
Neurologic deficits with conversion disorder do not follow a known neurologic insult.14 There are many tests that can be used to distinguish functional symptoms vs organic symptoms. Two of the most well-known tests are the Hoover sign and the abductor sign, which will be positive in conversion disorder. Both can be performed easily in an outpatient setting.
The Hoover sign is considered positive when there is weakness of voluntary hip extension in the presence of normal involuntary hip extension during contralateral hip flexion against resistance. According to a meta-analysis of multiple studies of patients with conversion disorder, the overall estimated sensitivity of this test is 94% and the specificity, 99%.15
The abductor sign follows the same principle as the Hoover sign: When the patient abducts the nonparetic leg, both the nonparetic and “paretic” leg are strong. When the patient abducts just the “paretic” leg, both legs become weak.16
Other symptom evaluations. For patients who have functional seizures, video electroencephalography is helpful to distinguish functional seizures from “true” seizures.17,18 In conversion disorder, functional dysarthria normally resembles a stutter or speech that is extremely slow with long hesitations that are hard to interrupt.18 Dysphonia and functional dysphagia are also very common functional symptoms. Usually after extensive work-up, no organic cause of the patient’s symptoms is ever found.18
Continue to: Treatment requires an integrated team approach
Treatment requires an integrated team approach
Treatment for conversion disorder can be difficult due to the complex and not fully understood etiology of the condition. Due to its multifaceted nature, an integrated team approach can be beneficial at each stage, including assessment and intervention.
Explain the diagnosis clearly. An essential initial step in the treatment of conversion disorder is careful explanation of the diagnosis. Clear explanation of the terminology and presentation of conversion disorder may prevent the patient from misinterpreting their diagnosis as a suggestion that they are feigning or malingering symptoms or feeling that their symptoms or concerns are being dismissed.2 Understanding the condition can help improve the likelihood of the patient accepting the treatment plan and help decrease the likelihood of unnecessary testing, health care visits, and consultations. Developing a strong rapport with the patient is key when explaining the diagnosis.
Recommend cognitive behavioral therapy (CBT). In a meta-analysis of 15 randomized controlled trials, CBT significantly reduced somatic, anxious, and depressive symptoms and improved physical functioning in patients with somatoform disorders and medically unexplained symptoms.19 Another study, utilizing a case series, demonstrated significant improvement in social, emotional, and behavioral functioning in children and adolescents with functional neurologic symptoms (conversion disorder) post–CBT intervention.20
Given that research supports CBT’s effectiveness in the management of conversion disorder, it is beneficial to engage a behavioral health professional as a part of the treatment team to focus on factors such as stress management, development of coping skills, and treatment of underlying psychiatric conditions.
Consider these other options. The addition of medication management can be considered for patients with comorbid psychiatric disorders. Evidence suggests that physical therapy is helpful in the treatment of motor and gait dysfunction seen in conversion disorder.21,22 The role of hypnosis in the management of conversion disorder has also been studied, but more randomized clinical trials are needed to further explore this treatment.2,23,24
Continue to: The FP's role in coordination of care
The FP’s role in coordination of care
Conversion disorder can be challenging to diagnose and often involves a multidisciplinary approach. Patients with conversion disorder may see multiple clinicians as they undergo evaluation for their symptoms, but they usually are referred back to their PCP for management and coordination of care. Thus, the FP’s understanding of how the condition is diagnosed and appropriately managed is beneficial.
Open and effective communication among all members of the health care team can ensure consistency in treatment, a strong patient–provider relationship, favorable prognosis, and prevention of symptom relapse. FPs, by establishing a good rapport with patients, can help them understand the condition and the mind-body connection. Once other diagnoses have been ruled out, the FP can provide reassurance to patients and minimize further diagnostic testing.
The prognosis of conversion disorder is associated with symptom duration25; thus, consultation between FPs and mental health providers is essential. The FP also can be integral in the recognition of psychiatric comorbidities, such as anxiety and depression, helping to ensure that these conditions also are treated appropriately.25,26
THE CASE
Ms. M was referred to a neuropsychologist for further assessment, and the diagnosis of conversion disorder was confirmed. She was then referred to a family medicine behavioral health psychologist for CBT. The initial consult indicated that psychological stressors were contributing to symptoms, and Ms. M was diagnosed with depression and anxiety as well as conversion disorder.
Treatment started with patient education. The treatment framework was carefully explained to Ms. M, with a focus on identifying possible symptom triggers, helping her build a more effective stress response, increasing skills to more effectively manage stressors, and managing underlying psychiatric disorders (ie, depression, anxiety).
Ms. M continued regular visits with the family medicine behavioral health psychologist for CBT and followed up with her PCP as needed to manage chronic health conditions and stroke risk factors. The patient was able to implement skills discussed in treatment sessions, including identifying triggers and implementing coping skills (eg, managing negative thoughts that contribute to symptoms, setting boundaries) to manage stressors.
Her depressive and anxious symptoms improved, as indicated by symptom measurement tools and self-report. The frequency and severity of episodes of slurred speech and muscle weakness decreased, and the patient reported only 1 ED visit related to speech difficulties in the 2 years while following up with the behavioral health psychologist.
CORRESPONDENCE
Kristen J. Alston, PhD, University of Mississippi Medical Center, 2400 North State Street, Jackson, MS 39216; [email protected]
THE CASE
Janice M* presented to the emergency department (ED) with worsening slurred speech. The 55-year-old patient’s history was significant for diabetes; hypertension; depression; sleep apnea; multiple transient ischemic attacks (TIAs) thought to be stress related; and left lower-extremity weakness secondary to prior infarct. Ms. M had been to the hospital multiple times in the previous 2 to 3 years for similar symptoms. Her most recent visit to the ED had been 2 months earlier.
In the ED, the patient’s NIH stroke score was 1 for the presence of dysarthria, and a code for emergency stroke management was initiated. Ms. M was alert and oriented x 3, with no focal motor or sensory deficits noted. Computed tomography (CT) and CT angiography were negative for any acute abnormality. Throughout the course of the ED visit, her NIH score improved to 0. Ms. M exhibited staccato/stuttering speech, but it was believed that this would likely improve over the next few days.
According to the hospital neurologist, the ED work-up suggested either a TIA, stress-induced psychiatric speech disorder, or conversion disorder. The patient was discharged home in stable condition and was asked to follow up with the outpatient neurologist in 1 week.
Ms. M was seen approximately 2 weeks later in the outpatient neurology stroke clinic. Her symptoms had resolved, and she did not report any new or worsening symptoms. An outpatient stroke work-up was initiated, including magnetic resonance imaging (MRI) of the brain, echocardiography, and measurement of low-density lipoprotein and hemoglobin A1C; all results were unremarkable. Given the timeline for symptom improvement and results of the work-up, the patient was given a diagnosis of conversion disorder. Ms. M was encouraged to follow up with her primary care physician (PCP) for further medical management.
●
* The patient’s name has been changed to protect her identity.
What is conversion disorder, and how common is it?
According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, text revision, conversion disorder (also known as functional neurological symptom disorder) is characterized as a somatic symptom and related disorder.1 The prominent feature shared among disorders in this category is the presence of somatic symptoms that are associated with distress and impairment.
In conversion disorder, the focus is on symptoms that are neurologic in nature but are not due to underlying neurologic disease and are incongruent with typical patterns of presentation for any neurologic condition. Patients with conversion disorder may present with motor symptoms (eg, weakness, paralysis, tremor, dystonia), altered sensory or cognitive function, seizure-like symptoms, alterations in speech, or changes in swallowing.1,2
For a diagnosis of conversion disorder, the following criteria must be met1:
- The patient has 1 or more symptoms of altered voluntary motor or sensory function.
- Symptom presentation is incongruent with recognized neurologic or medical disease or conditions.
S ymptoms are not better explained by another medical or mental health condition.- There is significant distress or impairment in functioning due to symptoms or the deficit.
The etiology of conversion disorder has not been firmly established. While the literature suggests that psychological stressors play a role,3,4 an effort also has been made to better understand the underlying neural and biological basis. Specifically, studies have utilized brain imaging to explore brain pathways and mechanisms that could account for symptom presentation.5,6
Prevalence rates for conversion disorder vary depending on the population studied. While it is estimated that 5% of patients in a general hospital setting meet full criteria for conversion disorder,7 higher rates may exist in specialty settings; 1 study found that 30% of patients in a neurology specialty clinic exhibited symptoms that were medically unexplained.8
Continue to: In primary care...
In primary care, prevalence of conversion disorder can be difficult to pinpoint; however, 1 study indicated that physicians identified medically unexplained symptoms as the main presenting problem for nearly 20% of patients in a primary care setting.9 Therefore, it is important for family physicians (FPs) to be familiar with the assessment and treatment of conversion disorder (and other disorders in which medically unexplained symptoms may be at the core of the patient presentation).
The differential: Neurologic and psychiatric conditions
Patients with conversion disorder may present with a variety of neurologic symptoms that can mimic those of organic disease. This can pose a diagnostic challenge, increase the chance of misdiagnosis, and delay treatment.
Motor symptoms may include paralysis, gait disturbance, dysphagia, or aphasia. Patients also may have sensory symptoms, such as blindness, deafness, or anesthesia.10,11 As a result, it is important to rule out both urgent neurologic presentations, such as TIA, acute stroke, and brain tumor, and other chronic neurologic conditions, including multiple sclerosis, myasthenia gravis, and epilepsy.11,12
Multiple sclerosis will demonstrate characteristic lesions on MRI that differentiate it from conversion disorder.
Myasthenia gravis is distinguished by positive findings on autoantibodies testing and on electrophysiologic studies.
Continue to: Epilepsy
Epilepsy. Patients with conversion disorder may present with unresponsiveness and abnormal movements, such as generalized limb shaking and hip thrusting, that mimic an epileptic seizure. In contrast to epileptic seizures, psychogenic nonepileptic seizures may last longer, symptoms may wax and wane, and patients generally do not have bowel or bladder incontinence or sustain injury as they would during an actual seizure.12
There are several psychiatric/psychosocial conditions that also should be considered in the differential diagnosis of conversion disorder.
Somatic symptom disorder, like conversion disorder, produces somatic symptoms that can cause significant distress for patients. The difference in the 2 conditions is that symptoms of somatic symptom disorder may be compatible with a recognized neurologic or general medical condition, whereas in conversion disorder, the symptoms are not consistent with a recognized disease.1,12
Factitious disorder, similar to conversion disorder, can involve neurologic symptoms that are not attributed to disease. However, patients with factitious disorder deliberately simulate symptoms to receive medical care. A thorough clinical interview and physical exam can help to distinguish conversion disorder from factitious disorder.
Malingering is not a psychiatric condition but a behavior that involves intentionally feigning symptoms for the purpose of personal or financial gain. There is no evidence that patients with conversion disorder simulate their symptoms.12,13
Continue to: Negative results and positive signs point to the Dx
Negative results and positive signs point to the Dx
Conversion disorder is not a diagnosis of exclusion. Diagnosis requires detailed history taking and a thorough neurologic exam. Laboratory testing and neuroimaging are also important, and results will have to be negative to support the diagnosis.
Neurologic deficits with conversion disorder do not follow a known neurologic insult.14 There are many tests that can be used to distinguish functional symptoms vs organic symptoms. Two of the most well-known tests are the Hoover sign and the abductor sign, which will be positive in conversion disorder. Both can be performed easily in an outpatient setting.
The Hoover sign is considered positive when there is weakness of voluntary hip extension in the presence of normal involuntary hip extension during contralateral hip flexion against resistance. According to a meta-analysis of multiple studies of patients with conversion disorder, the overall estimated sensitivity of this test is 94% and the specificity, 99%.15
The abductor sign follows the same principle as the Hoover sign: When the patient abducts the nonparetic leg, both the nonparetic and “paretic” leg are strong. When the patient abducts just the “paretic” leg, both legs become weak.16
Other symptom evaluations. For patients who have functional seizures, video electroencephalography is helpful to distinguish functional seizures from “true” seizures.17,18 In conversion disorder, functional dysarthria normally resembles a stutter or speech that is extremely slow with long hesitations that are hard to interrupt.18 Dysphonia and functional dysphagia are also very common functional symptoms. Usually after extensive work-up, no organic cause of the patient’s symptoms is ever found.18
Continue to: Treatment requires an integrated team approach
Treatment requires an integrated team approach
Treatment for conversion disorder can be difficult due to the complex and not fully understood etiology of the condition. Due to its multifaceted nature, an integrated team approach can be beneficial at each stage, including assessment and intervention.
Explain the diagnosis clearly. An essential initial step in the treatment of conversion disorder is careful explanation of the diagnosis. Clear explanation of the terminology and presentation of conversion disorder may prevent the patient from misinterpreting their diagnosis as a suggestion that they are feigning or malingering symptoms or feeling that their symptoms or concerns are being dismissed.2 Understanding the condition can help improve the likelihood of the patient accepting the treatment plan and help decrease the likelihood of unnecessary testing, health care visits, and consultations. Developing a strong rapport with the patient is key when explaining the diagnosis.
Recommend cognitive behavioral therapy (CBT). In a meta-analysis of 15 randomized controlled trials, CBT significantly reduced somatic, anxious, and depressive symptoms and improved physical functioning in patients with somatoform disorders and medically unexplained symptoms.19 Another study, utilizing a case series, demonstrated significant improvement in social, emotional, and behavioral functioning in children and adolescents with functional neurologic symptoms (conversion disorder) post–CBT intervention.20
Given that research supports CBT’s effectiveness in the management of conversion disorder, it is beneficial to engage a behavioral health professional as a part of the treatment team to focus on factors such as stress management, development of coping skills, and treatment of underlying psychiatric conditions.
Consider these other options. The addition of medication management can be considered for patients with comorbid psychiatric disorders. Evidence suggests that physical therapy is helpful in the treatment of motor and gait dysfunction seen in conversion disorder.21,22 The role of hypnosis in the management of conversion disorder has also been studied, but more randomized clinical trials are needed to further explore this treatment.2,23,24
Continue to: The FP's role in coordination of care
The FP’s role in coordination of care
Conversion disorder can be challenging to diagnose and often involves a multidisciplinary approach. Patients with conversion disorder may see multiple clinicians as they undergo evaluation for their symptoms, but they usually are referred back to their PCP for management and coordination of care. Thus, the FP’s understanding of how the condition is diagnosed and appropriately managed is beneficial.
Open and effective communication among all members of the health care team can ensure consistency in treatment, a strong patient–provider relationship, favorable prognosis, and prevention of symptom relapse. FPs, by establishing a good rapport with patients, can help them understand the condition and the mind-body connection. Once other diagnoses have been ruled out, the FP can provide reassurance to patients and minimize further diagnostic testing.
The prognosis of conversion disorder is associated with symptom duration25; thus, consultation between FPs and mental health providers is essential. The FP also can be integral in the recognition of psychiatric comorbidities, such as anxiety and depression, helping to ensure that these conditions also are treated appropriately.25,26
THE CASE
Ms. M was referred to a neuropsychologist for further assessment, and the diagnosis of conversion disorder was confirmed. She was then referred to a family medicine behavioral health psychologist for CBT. The initial consult indicated that psychological stressors were contributing to symptoms, and Ms. M was diagnosed with depression and anxiety as well as conversion disorder.
Treatment started with patient education. The treatment framework was carefully explained to Ms. M, with a focus on identifying possible symptom triggers, helping her build a more effective stress response, increasing skills to more effectively manage stressors, and managing underlying psychiatric disorders (ie, depression, anxiety).
Ms. M continued regular visits with the family medicine behavioral health psychologist for CBT and followed up with her PCP as needed to manage chronic health conditions and stroke risk factors. The patient was able to implement skills discussed in treatment sessions, including identifying triggers and implementing coping skills (eg, managing negative thoughts that contribute to symptoms, setting boundaries) to manage stressors.
Her depressive and anxious symptoms improved, as indicated by symptom measurement tools and self-report. The frequency and severity of episodes of slurred speech and muscle weakness decreased, and the patient reported only 1 ED visit related to speech difficulties in the 2 years while following up with the behavioral health psychologist.
CORRESPONDENCE
Kristen J. Alston, PhD, University of Mississippi Medical Center, 2400 North State Street, Jackson, MS 39216; [email protected]
1. American Psychiatric Association. Somatic symptom and related disorders. In: Diagnostic and Statistical Manual of Mental Disorders. 5th edition, text revision. American Psychiatric Association Publishing; 2022. doi: 10.1176/appi.books.9780890425787.x09_Somatic_Symptom_and_Related_Disorders
2. O’Neal MA, Baslet G. Treatment for patients with a functional neurological disorder (conversion disorder): an integrated approach. Am J Psychiatry. 2018;175:307-314. doi: 10.1176/appi.ajp.2017.17040450
3. Roelofs K, Spinhoven P, Sandijck P, et al. The impact of early trauma and recent life-events on symptom severity in patients with conversion disorder. J Nerv Ment Dis. 2005;193:508-514. doi: 10.1097/01.nmd.0000172472.60197.4d
4. Nicholson TR, Aybek S, Craig T, et al. Life events and escape in conversion disorder. Psychol Med. 2016;46:2617-2626. doi: 10.1017/S0033291716000714
5. Ejareh Dar M, Kanaan RA. Uncovering the etiology of conversion disorder: insights from functional neuroimaging. Neuropsychiatr Dis Treat. 2016;12:143-153. doi: 10.2147/NDT.S65880
6. Aybek S, Vuilleumier P. Imaging studies of functional neurologic disorders. Handb Clin Neurol. 2016;139:73-84. doi: 10.1016/B978-0-12-801772-2.00007-2
7. Folks DG, Ford CV, Regan WM. Conversion symptoms in a general hospital. Psychosomatics. 1984;25:285-295. doi: 10.1016/S0033-3182(84)73046-5
8. Carson AJ, Best S, Postma K, et al. The outcome of neurology outpatients with medically unexplained symptoms: a prospective cohort study. J Neurol Neurosurg Psychiatry. 2003;74:897-900. doi: 10.1136/jnnp.74.7.897
9. Peveler R, Kilkenny L, Kinmonth AL. Medically unexplained physical symptoms in primary care: a comparison of self-report screening questionnaires and clinical opinion. J Psychosom Res. 1997;42:245-252. doi: 10.1016/s0022-3999(96)00292-9
10. Tobiano PS, Wang HE, McCausland JB, et al. A case of conversion disorder presenting as a severe acute stroke. J Emerg Med. 2006;30:283-286. doi: 10.1016/j.jemermed.2005.05.024
11. Chou HY, Weng MC, Huang MH, et al. Conversion disorder in stroke: a case report. Kaohsiung J Med Sci. 2006;22:586-589. doi: 10.1016/S1607-551X(09)70357-2
12. Peeling JL, Muzio MR. Conversion disorder. StatPearls [Internet]. Updated May 19, 2021. Accessed March 14, 2023. www.ncbi.nlm.nih.gov/books/NBK551567/
13. Ali S, Jabeen S, Pate RJ, et al. Conversion disorder—mind versus body: a review. Innov Clin Neurosci. 2015;12:27-33.
14. Hurwitz TA. Somatization and conversion disorder. Can J Psychiatry. 2004;49:172-178. doi: 10.1177/070674370404900304
15. Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85:180-190. doi: 10.1136/jnnp-2012-304607
16. Sonoo M. Abductor sign: a reliable new sign to detect unilateral non-organic paresis of the lower limb. J Neurol Neurosurg Psychiatry. 2004;75:121-125.
17. Tsui P, Deptula A, Yuan DY. Conversion disorder, functional neurological symptom disorder, and chronic pain: comorbidity, assessment, and treatment. Curr Pain Headache Rep. 2017;21:29. doi: 10.1007/s11916-017-0627-7
18. Stone J, Carson A, Sharpe M. Functional symptoms and signs in neurology: assessment and diagnosis. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i2-i12. doi: 10.1136/jnnp.2004.061655
19. Liu J, Gill NS, Teodorczuk A, et al. The efficacy of cognitive behavioural therapy in somatoform disorders and medically unexplained physical symptoms: a meta-analysis of randomized controlled trials. J Affect Disord. 2019;245:98-112. doi: 10.1016/j.jad.2018.10.114
20. McFarlane FA, Allcott-Watson H, Hadji-Michael M, et al. Cognitive-behavioural treatment of functional neurological symptoms (conversion disorder) in children and adolescents: a case series. Eur J Paediatr Neurol. 2019;23:317-328. doi: 10.1016/j.ejpn.2018.12.002
21. Ness D. Physical therapy management for conversion disorder: case series. J Neurol Phys Ther. 2007;31:30-39. doi: 10.1097/01.npt.0000260571.77487.14
22. Nielsen G, Ricciardi L, Demartini B, et al. Outcomes of a 5-day physiotherapy programme for functional (psychogenic) motor disorders. J Neurol. 2015;262:674-681. doi: 10.1007/s00415-014-7631-1
23. Sanyal R, Raseta M, Natarajan I, et al. The use of hypnotherapy as treatment for functional stroke: a case series from a single center in the UK. Int J Stroke. 2022;17:59-66. doi: 10.1177/1747493021995590
24. Moene FC, Spinhoven P, Hoogduin KA, et al. A randomized controlled clinical trial of a hypnosis-based treatment for patients with conversion disorder, motor type. Int J Clin Exp Hypn. 2003;51:29-50. doi: 10.1076/iceh.51.1.29.14067
25. Feinstein A. Conversion disorder: advances in our understanding. CMAJ. 2011;183:915-920. doi: 10.1503/cmaj.110490
26. Kurlansik SL, Maffei MS. Somatic symptom disorder. Am Fam Physician. 2016;93:49-54.
1. American Psychiatric Association. Somatic symptom and related disorders. In: Diagnostic and Statistical Manual of Mental Disorders. 5th edition, text revision. American Psychiatric Association Publishing; 2022. doi: 10.1176/appi.books.9780890425787.x09_Somatic_Symptom_and_Related_Disorders
2. O’Neal MA, Baslet G. Treatment for patients with a functional neurological disorder (conversion disorder): an integrated approach. Am J Psychiatry. 2018;175:307-314. doi: 10.1176/appi.ajp.2017.17040450
3. Roelofs K, Spinhoven P, Sandijck P, et al. The impact of early trauma and recent life-events on symptom severity in patients with conversion disorder. J Nerv Ment Dis. 2005;193:508-514. doi: 10.1097/01.nmd.0000172472.60197.4d
4. Nicholson TR, Aybek S, Craig T, et al. Life events and escape in conversion disorder. Psychol Med. 2016;46:2617-2626. doi: 10.1017/S0033291716000714
5. Ejareh Dar M, Kanaan RA. Uncovering the etiology of conversion disorder: insights from functional neuroimaging. Neuropsychiatr Dis Treat. 2016;12:143-153. doi: 10.2147/NDT.S65880
6. Aybek S, Vuilleumier P. Imaging studies of functional neurologic disorders. Handb Clin Neurol. 2016;139:73-84. doi: 10.1016/B978-0-12-801772-2.00007-2
7. Folks DG, Ford CV, Regan WM. Conversion symptoms in a general hospital. Psychosomatics. 1984;25:285-295. doi: 10.1016/S0033-3182(84)73046-5
8. Carson AJ, Best S, Postma K, et al. The outcome of neurology outpatients with medically unexplained symptoms: a prospective cohort study. J Neurol Neurosurg Psychiatry. 2003;74:897-900. doi: 10.1136/jnnp.74.7.897
9. Peveler R, Kilkenny L, Kinmonth AL. Medically unexplained physical symptoms in primary care: a comparison of self-report screening questionnaires and clinical opinion. J Psychosom Res. 1997;42:245-252. doi: 10.1016/s0022-3999(96)00292-9
10. Tobiano PS, Wang HE, McCausland JB, et al. A case of conversion disorder presenting as a severe acute stroke. J Emerg Med. 2006;30:283-286. doi: 10.1016/j.jemermed.2005.05.024
11. Chou HY, Weng MC, Huang MH, et al. Conversion disorder in stroke: a case report. Kaohsiung J Med Sci. 2006;22:586-589. doi: 10.1016/S1607-551X(09)70357-2
12. Peeling JL, Muzio MR. Conversion disorder. StatPearls [Internet]. Updated May 19, 2021. Accessed March 14, 2023. www.ncbi.nlm.nih.gov/books/NBK551567/
13. Ali S, Jabeen S, Pate RJ, et al. Conversion disorder—mind versus body: a review. Innov Clin Neurosci. 2015;12:27-33.
14. Hurwitz TA. Somatization and conversion disorder. Can J Psychiatry. 2004;49:172-178. doi: 10.1177/070674370404900304
15. Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85:180-190. doi: 10.1136/jnnp-2012-304607
16. Sonoo M. Abductor sign: a reliable new sign to detect unilateral non-organic paresis of the lower limb. J Neurol Neurosurg Psychiatry. 2004;75:121-125.
17. Tsui P, Deptula A, Yuan DY. Conversion disorder, functional neurological symptom disorder, and chronic pain: comorbidity, assessment, and treatment. Curr Pain Headache Rep. 2017;21:29. doi: 10.1007/s11916-017-0627-7
18. Stone J, Carson A, Sharpe M. Functional symptoms and signs in neurology: assessment and diagnosis. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i2-i12. doi: 10.1136/jnnp.2004.061655
19. Liu J, Gill NS, Teodorczuk A, et al. The efficacy of cognitive behavioural therapy in somatoform disorders and medically unexplained physical symptoms: a meta-analysis of randomized controlled trials. J Affect Disord. 2019;245:98-112. doi: 10.1016/j.jad.2018.10.114
20. McFarlane FA, Allcott-Watson H, Hadji-Michael M, et al. Cognitive-behavioural treatment of functional neurological symptoms (conversion disorder) in children and adolescents: a case series. Eur J Paediatr Neurol. 2019;23:317-328. doi: 10.1016/j.ejpn.2018.12.002
21. Ness D. Physical therapy management for conversion disorder: case series. J Neurol Phys Ther. 2007;31:30-39. doi: 10.1097/01.npt.0000260571.77487.14
22. Nielsen G, Ricciardi L, Demartini B, et al. Outcomes of a 5-day physiotherapy programme for functional (psychogenic) motor disorders. J Neurol. 2015;262:674-681. doi: 10.1007/s00415-014-7631-1
23. Sanyal R, Raseta M, Natarajan I, et al. The use of hypnotherapy as treatment for functional stroke: a case series from a single center in the UK. Int J Stroke. 2022;17:59-66. doi: 10.1177/1747493021995590
24. Moene FC, Spinhoven P, Hoogduin KA, et al. A randomized controlled clinical trial of a hypnosis-based treatment for patients with conversion disorder, motor type. Int J Clin Exp Hypn. 2003;51:29-50. doi: 10.1076/iceh.51.1.29.14067
25. Feinstein A. Conversion disorder: advances in our understanding. CMAJ. 2011;183:915-920. doi: 10.1503/cmaj.110490
26. Kurlansik SL, Maffei MS. Somatic symptom disorder. Am Fam Physician. 2016;93:49-54.
Is combination pharmacotherapy effective for patients with acute depression?
ILLUSTRATIVE CASE
A healthy 33-year-old woman presents to your office with a 3-month history of depressed mood. She reports difficulty concentrating, insomnia, decreased appetite, and generalized fatigue. She denies suicidal or homicidal ideation, substance misuse, or history consistent with manic episodes. Her vital signs are normal and overall her physical examination is unremarkable, although the patient is tearful when discussing her mood. Using shared decision-making, you and the patient determine it is appropriate to initiate pharmacotherapy. Is there a role for combination pharmacotherapy to treat this patient’s acute depression?
Unipolar depression is a highly prevalent condition, estimated to affect 21% of US adults at some point in their lifetime.2 It is the second leading cause of disability in the United States, with an estimated economic impact of more than $200 billion annually.3
The diagnosis of unipolar depression is based on the criteria set forth in the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) and commonly includes depressed mood, anhedonia, sleep disturbance, appetite changes, fatigue, feelings of worthlessness or guilt, decreased ability to concentrate, and psychomotor symptoms occurring over at least a 2-week period.4 Symptoms represent a decrease in functioning from previous levels that are not attributable to another medical condition or substance, and must not include a history of past manic or hypomanic episodes. Thoughts of death and suicidal ideation are common.
Several systematic reviews and meta-analyses have shown that a combination of psychotherapy and pharmacotherapy is more efficacious for treatment of unipolar depression than either therapy alone.5-7 As for which medication is most effective and tolerable, multiple systematic reviews and meta-analyses have not demonstrated superiority of 1 second-generation antidepressant (eg, SSRIs, SNRIs) over another.7,8
General practice guidelines support titration of the dose or a switch in monotherapy medications until treatment response is achieved, prior to initiation of a second agent. When an adjunctive medication is considered, there are several options: a second-generation antipsychotic, a second antidepressant from a different class, thyroid hormone, and lithium. Special consideration is given to the adverse effect profile and potential tolerability; higher adverse effect profiles are observed with second-generation antipsychotics and lithium.9
It is not common practice to initiate 2 antidepressants for a new diagnosis of acute depression. The systematic review and meta-analysis conducted by Henssler et al1 attempted to provide evidence to support the efficacy and tolerability of specific antidepressants when used in combination for initial treatment of acute depression. Of note, a 2008 national survey showed that a majority of psychotropic medications in the United States are prescribed by primary care physicians (73.6%) rather than psychiatrists, making this analysis relevant to family physicians.10
STUDY SUMMARY
Combination pharmacotherapy yields superior efficacy in acute depression
This 2022 systematic review and meta-analysis (39 randomized clinical trials [RCTs]; N = 6751) compared the efficacy and tolerability of monotherapy to combination therapy in the treatment of patients with acute depression.1 The study also aimed to address which specific combination therapies were superior.
Continue to: Selected RCTs included...
Selected RCTs included an intervention group using a combination of 2 antidepressants, regardless of dosage, and a control group of patients taking antidepressant monotherapy. Studies evaluated both patients being treated for the first time and those with a previously inadequate response to medical treatment. All participants were ages 18 years or older (mean age not reported) and had received a diagnosis of depressive disorder according to standard operationalized criteria; patients with multiple psychiatric comorbidities were not excluded.
Studies used various standardized questionnaires—most frequently, the Hamilton Depression Rating Scale (HDRS) and the Montgomery-Åsberg Depression Rating Scale (MADRS)—to determine the severity of depression at baseline and following treatment. The HDRS is a 17-item depression scale and the MADRS is a 10-item depression scale; for both, higher scores indicate worsening depression. Follow-up time ranged from 2 to 12 weeks.
The primary outcome was treatment efficacy measured as the standardized mean difference (SMD). Secondary outcomes included remission (normal-range scores) and response to treatment (eg, ≥ 50% reduction in scores), as defined by the study authors.
Combination therapy was determined to have superior efficacy relative to monotherapy (SMD = 0.31; 95% CI, 0.19-0.44; P < .001). Combinations with a presynaptic α2-autoreceptor antagonist (eg, mirtazapine, trazodone, or mianserin [the last of which is not approved by the US Food and Drug Administration for use in the United States]) and a monoamine reuptake inhibitor (eg, an SSRI, SNRI, or TCA) were superior to other combinations (SMD = 0.37; 95% CI, 0.19-0.55). Combinations that included bupropion were not superior to monotherapy (SMD = 0.10; 95% CI, –0.07 to 0.27).
Secondary outcomes revealed combination therapy to be superior to monotherapy with respect to remission (odds ratio [OR] = 1.52; 95% CI, 1.20-1.92) and response (OR = 1.40; 95% CI, 1.15-1.69). Subgroup analyses showed that combinations with presynaptic α2-autoreceptor antagonists led to improved remission (OR = 1.42; 95% CI, 1.01-2.01) and response (OR = 1.49; 95% CI, 1.18-1.87) compared with monotherapy, whereas combinations that included bupropion were not superior to monotherapy. For patients who dropped out of treatment for any reason, including adverse drug events, results for combination pharmacotherapy and monotherapy were similar.
Continue to: WHAT'S NEW
WHAT’S NEW
One combination proved more effective than others
Current clinical guidelines indicate the suitability of trialing pharmacologic monotherapy during the acute phase of depression treatment prior to initiating an adjunctive medication.9 All classes of medication investigated in this meta-analysis are generally regarded as first-line therapies, although they are rarely started in combination. This study’s findings suggest that combination pharmacotherapy, especially with a presynaptic α2-autoreceptor antagonist (eg, mirtazapine, trazodone) and a monoamine reuptake inhibitor (eg, an SSRI, SNRI, or a TCA), is superior to monotherapy, both at the time of treatment initiation and in patients with previous inadequate pharmacologic response.
CAVEATS
Potential limitations due to publication bias
Concerns about publication bias and significant study heterogeneity may limit the generalizability of these findings. However, conclusions were robust in a subgroup analysis that was restricted to publications with low risk for bias.
CHALLENGES TO IMPLEMENTATION
None to report
There are no major challenges to implementing this combination treatment. Importantly, there were no differences in tolerability between monotherapy and combination treatment.
1. Henssler J, Alexander D, Schwarzer G, et al. Combining antidepressants vs antidepressant monotherapy for treatment of patients with acute depression: a systematic review and meta-analysis. JAMA Psychiatry. 2022;79:300-312. doi: 10.1001/jamapsychiatry.2021.4313
2. Hasin DS, Sarvet AL, Meyers JL, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry. 2018;75:336-346. doi: 10.1001/jamapsychiatry.2017.4602
3. Greenberg PE, Fournier AA, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76:155-162. doi: 10.4088/JCP.14m09298
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
5. Cuijpers P, Reynolds CF III, Donker T, et al. Personalized treatment of adult depression: medication, psychotherapy, or both? A systematic review. Depress Anxiety. 2012;29:855-864. doi: 10.1002/da.21985
6. Cuijpers P, van Straten A, Hollon SD, et al. The contribution of active medication to combined treatments of psychotherapy and pharmacotherapy for adult depression: a meta-analysis. Acta Psychiatr Scand. 2010;121:415-423. doi: 10.1111/j.1600-0447.2009.01513.x
7. Thase ME, Greenhouse JB, Frank E, et al. Treatment of major depression with psychotherapy or psychotherapy-pharmacotherapy combinations. Arch Gen Psychiatry. 1997;54: 1009-1015. doi: 10.1001/archpsyc.1997.01830230043006
8. Gartlehner G, Hansen RA, Morgan LC, et al. Comparative benefits and harms of second-generation antidepressants for treating major depressive disorder: an updated meta-analysis. Ann Intern Med. 2011;155:722-785. doi: 10.7326/0003-4819-155-11-201112060-00009
9. American Psychiatric Association. Practice Guideline for the Treatment of Patients With Major Depressive Disorder. 3rd ed. American Psychiatric Association; 2010. Accessed February 27, 2023. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf
10. Mojtabai R, Olfson M. National patterns in antidepressant treatment by psychiatrists and general medical providers: results from the national comorbidity survey replication. J Clin Psychiatry. 2008;69:1064-1074. doi: 10.4088/jcp.v69n0704
ILLUSTRATIVE CASE
A healthy 33-year-old woman presents to your office with a 3-month history of depressed mood. She reports difficulty concentrating, insomnia, decreased appetite, and generalized fatigue. She denies suicidal or homicidal ideation, substance misuse, or history consistent with manic episodes. Her vital signs are normal and overall her physical examination is unremarkable, although the patient is tearful when discussing her mood. Using shared decision-making, you and the patient determine it is appropriate to initiate pharmacotherapy. Is there a role for combination pharmacotherapy to treat this patient’s acute depression?
Unipolar depression is a highly prevalent condition, estimated to affect 21% of US adults at some point in their lifetime.2 It is the second leading cause of disability in the United States, with an estimated economic impact of more than $200 billion annually.3
The diagnosis of unipolar depression is based on the criteria set forth in the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) and commonly includes depressed mood, anhedonia, sleep disturbance, appetite changes, fatigue, feelings of worthlessness or guilt, decreased ability to concentrate, and psychomotor symptoms occurring over at least a 2-week period.4 Symptoms represent a decrease in functioning from previous levels that are not attributable to another medical condition or substance, and must not include a history of past manic or hypomanic episodes. Thoughts of death and suicidal ideation are common.
Several systematic reviews and meta-analyses have shown that a combination of psychotherapy and pharmacotherapy is more efficacious for treatment of unipolar depression than either therapy alone.5-7 As for which medication is most effective and tolerable, multiple systematic reviews and meta-analyses have not demonstrated superiority of 1 second-generation antidepressant (eg, SSRIs, SNRIs) over another.7,8
General practice guidelines support titration of the dose or a switch in monotherapy medications until treatment response is achieved, prior to initiation of a second agent. When an adjunctive medication is considered, there are several options: a second-generation antipsychotic, a second antidepressant from a different class, thyroid hormone, and lithium. Special consideration is given to the adverse effect profile and potential tolerability; higher adverse effect profiles are observed with second-generation antipsychotics and lithium.9
It is not common practice to initiate 2 antidepressants for a new diagnosis of acute depression. The systematic review and meta-analysis conducted by Henssler et al1 attempted to provide evidence to support the efficacy and tolerability of specific antidepressants when used in combination for initial treatment of acute depression. Of note, a 2008 national survey showed that a majority of psychotropic medications in the United States are prescribed by primary care physicians (73.6%) rather than psychiatrists, making this analysis relevant to family physicians.10
STUDY SUMMARY
Combination pharmacotherapy yields superior efficacy in acute depression
This 2022 systematic review and meta-analysis (39 randomized clinical trials [RCTs]; N = 6751) compared the efficacy and tolerability of monotherapy to combination therapy in the treatment of patients with acute depression.1 The study also aimed to address which specific combination therapies were superior.
Continue to: Selected RCTs included...
Selected RCTs included an intervention group using a combination of 2 antidepressants, regardless of dosage, and a control group of patients taking antidepressant monotherapy. Studies evaluated both patients being treated for the first time and those with a previously inadequate response to medical treatment. All participants were ages 18 years or older (mean age not reported) and had received a diagnosis of depressive disorder according to standard operationalized criteria; patients with multiple psychiatric comorbidities were not excluded.
Studies used various standardized questionnaires—most frequently, the Hamilton Depression Rating Scale (HDRS) and the Montgomery-Åsberg Depression Rating Scale (MADRS)—to determine the severity of depression at baseline and following treatment. The HDRS is a 17-item depression scale and the MADRS is a 10-item depression scale; for both, higher scores indicate worsening depression. Follow-up time ranged from 2 to 12 weeks.
The primary outcome was treatment efficacy measured as the standardized mean difference (SMD). Secondary outcomes included remission (normal-range scores) and response to treatment (eg, ≥ 50% reduction in scores), as defined by the study authors.
Combination therapy was determined to have superior efficacy relative to monotherapy (SMD = 0.31; 95% CI, 0.19-0.44; P < .001). Combinations with a presynaptic α2-autoreceptor antagonist (eg, mirtazapine, trazodone, or mianserin [the last of which is not approved by the US Food and Drug Administration for use in the United States]) and a monoamine reuptake inhibitor (eg, an SSRI, SNRI, or TCA) were superior to other combinations (SMD = 0.37; 95% CI, 0.19-0.55). Combinations that included bupropion were not superior to monotherapy (SMD = 0.10; 95% CI, –0.07 to 0.27).
Secondary outcomes revealed combination therapy to be superior to monotherapy with respect to remission (odds ratio [OR] = 1.52; 95% CI, 1.20-1.92) and response (OR = 1.40; 95% CI, 1.15-1.69). Subgroup analyses showed that combinations with presynaptic α2-autoreceptor antagonists led to improved remission (OR = 1.42; 95% CI, 1.01-2.01) and response (OR = 1.49; 95% CI, 1.18-1.87) compared with monotherapy, whereas combinations that included bupropion were not superior to monotherapy. For patients who dropped out of treatment for any reason, including adverse drug events, results for combination pharmacotherapy and monotherapy were similar.
Continue to: WHAT'S NEW
WHAT’S NEW
One combination proved more effective than others
Current clinical guidelines indicate the suitability of trialing pharmacologic monotherapy during the acute phase of depression treatment prior to initiating an adjunctive medication.9 All classes of medication investigated in this meta-analysis are generally regarded as first-line therapies, although they are rarely started in combination. This study’s findings suggest that combination pharmacotherapy, especially with a presynaptic α2-autoreceptor antagonist (eg, mirtazapine, trazodone) and a monoamine reuptake inhibitor (eg, an SSRI, SNRI, or a TCA), is superior to monotherapy, both at the time of treatment initiation and in patients with previous inadequate pharmacologic response.
CAVEATS
Potential limitations due to publication bias
Concerns about publication bias and significant study heterogeneity may limit the generalizability of these findings. However, conclusions were robust in a subgroup analysis that was restricted to publications with low risk for bias.
CHALLENGES TO IMPLEMENTATION
None to report
There are no major challenges to implementing this combination treatment. Importantly, there were no differences in tolerability between monotherapy and combination treatment.
ILLUSTRATIVE CASE
A healthy 33-year-old woman presents to your office with a 3-month history of depressed mood. She reports difficulty concentrating, insomnia, decreased appetite, and generalized fatigue. She denies suicidal or homicidal ideation, substance misuse, or history consistent with manic episodes. Her vital signs are normal and overall her physical examination is unremarkable, although the patient is tearful when discussing her mood. Using shared decision-making, you and the patient determine it is appropriate to initiate pharmacotherapy. Is there a role for combination pharmacotherapy to treat this patient’s acute depression?
Unipolar depression is a highly prevalent condition, estimated to affect 21% of US adults at some point in their lifetime.2 It is the second leading cause of disability in the United States, with an estimated economic impact of more than $200 billion annually.3
The diagnosis of unipolar depression is based on the criteria set forth in the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) and commonly includes depressed mood, anhedonia, sleep disturbance, appetite changes, fatigue, feelings of worthlessness or guilt, decreased ability to concentrate, and psychomotor symptoms occurring over at least a 2-week period.4 Symptoms represent a decrease in functioning from previous levels that are not attributable to another medical condition or substance, and must not include a history of past manic or hypomanic episodes. Thoughts of death and suicidal ideation are common.
Several systematic reviews and meta-analyses have shown that a combination of psychotherapy and pharmacotherapy is more efficacious for treatment of unipolar depression than either therapy alone.5-7 As for which medication is most effective and tolerable, multiple systematic reviews and meta-analyses have not demonstrated superiority of 1 second-generation antidepressant (eg, SSRIs, SNRIs) over another.7,8
General practice guidelines support titration of the dose or a switch in monotherapy medications until treatment response is achieved, prior to initiation of a second agent. When an adjunctive medication is considered, there are several options: a second-generation antipsychotic, a second antidepressant from a different class, thyroid hormone, and lithium. Special consideration is given to the adverse effect profile and potential tolerability; higher adverse effect profiles are observed with second-generation antipsychotics and lithium.9
It is not common practice to initiate 2 antidepressants for a new diagnosis of acute depression. The systematic review and meta-analysis conducted by Henssler et al1 attempted to provide evidence to support the efficacy and tolerability of specific antidepressants when used in combination for initial treatment of acute depression. Of note, a 2008 national survey showed that a majority of psychotropic medications in the United States are prescribed by primary care physicians (73.6%) rather than psychiatrists, making this analysis relevant to family physicians.10
STUDY SUMMARY
Combination pharmacotherapy yields superior efficacy in acute depression
This 2022 systematic review and meta-analysis (39 randomized clinical trials [RCTs]; N = 6751) compared the efficacy and tolerability of monotherapy to combination therapy in the treatment of patients with acute depression.1 The study also aimed to address which specific combination therapies were superior.
Continue to: Selected RCTs included...
Selected RCTs included an intervention group using a combination of 2 antidepressants, regardless of dosage, and a control group of patients taking antidepressant monotherapy. Studies evaluated both patients being treated for the first time and those with a previously inadequate response to medical treatment. All participants were ages 18 years or older (mean age not reported) and had received a diagnosis of depressive disorder according to standard operationalized criteria; patients with multiple psychiatric comorbidities were not excluded.
Studies used various standardized questionnaires—most frequently, the Hamilton Depression Rating Scale (HDRS) and the Montgomery-Åsberg Depression Rating Scale (MADRS)—to determine the severity of depression at baseline and following treatment. The HDRS is a 17-item depression scale and the MADRS is a 10-item depression scale; for both, higher scores indicate worsening depression. Follow-up time ranged from 2 to 12 weeks.
The primary outcome was treatment efficacy measured as the standardized mean difference (SMD). Secondary outcomes included remission (normal-range scores) and response to treatment (eg, ≥ 50% reduction in scores), as defined by the study authors.
Combination therapy was determined to have superior efficacy relative to monotherapy (SMD = 0.31; 95% CI, 0.19-0.44; P < .001). Combinations with a presynaptic α2-autoreceptor antagonist (eg, mirtazapine, trazodone, or mianserin [the last of which is not approved by the US Food and Drug Administration for use in the United States]) and a monoamine reuptake inhibitor (eg, an SSRI, SNRI, or TCA) were superior to other combinations (SMD = 0.37; 95% CI, 0.19-0.55). Combinations that included bupropion were not superior to monotherapy (SMD = 0.10; 95% CI, –0.07 to 0.27).
Secondary outcomes revealed combination therapy to be superior to monotherapy with respect to remission (odds ratio [OR] = 1.52; 95% CI, 1.20-1.92) and response (OR = 1.40; 95% CI, 1.15-1.69). Subgroup analyses showed that combinations with presynaptic α2-autoreceptor antagonists led to improved remission (OR = 1.42; 95% CI, 1.01-2.01) and response (OR = 1.49; 95% CI, 1.18-1.87) compared with monotherapy, whereas combinations that included bupropion were not superior to monotherapy. For patients who dropped out of treatment for any reason, including adverse drug events, results for combination pharmacotherapy and monotherapy were similar.
Continue to: WHAT'S NEW
WHAT’S NEW
One combination proved more effective than others
Current clinical guidelines indicate the suitability of trialing pharmacologic monotherapy during the acute phase of depression treatment prior to initiating an adjunctive medication.9 All classes of medication investigated in this meta-analysis are generally regarded as first-line therapies, although they are rarely started in combination. This study’s findings suggest that combination pharmacotherapy, especially with a presynaptic α2-autoreceptor antagonist (eg, mirtazapine, trazodone) and a monoamine reuptake inhibitor (eg, an SSRI, SNRI, or a TCA), is superior to monotherapy, both at the time of treatment initiation and in patients with previous inadequate pharmacologic response.
CAVEATS
Potential limitations due to publication bias
Concerns about publication bias and significant study heterogeneity may limit the generalizability of these findings. However, conclusions were robust in a subgroup analysis that was restricted to publications with low risk for bias.
CHALLENGES TO IMPLEMENTATION
None to report
There are no major challenges to implementing this combination treatment. Importantly, there were no differences in tolerability between monotherapy and combination treatment.
1. Henssler J, Alexander D, Schwarzer G, et al. Combining antidepressants vs antidepressant monotherapy for treatment of patients with acute depression: a systematic review and meta-analysis. JAMA Psychiatry. 2022;79:300-312. doi: 10.1001/jamapsychiatry.2021.4313
2. Hasin DS, Sarvet AL, Meyers JL, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry. 2018;75:336-346. doi: 10.1001/jamapsychiatry.2017.4602
3. Greenberg PE, Fournier AA, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76:155-162. doi: 10.4088/JCP.14m09298
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
5. Cuijpers P, Reynolds CF III, Donker T, et al. Personalized treatment of adult depression: medication, psychotherapy, or both? A systematic review. Depress Anxiety. 2012;29:855-864. doi: 10.1002/da.21985
6. Cuijpers P, van Straten A, Hollon SD, et al. The contribution of active medication to combined treatments of psychotherapy and pharmacotherapy for adult depression: a meta-analysis. Acta Psychiatr Scand. 2010;121:415-423. doi: 10.1111/j.1600-0447.2009.01513.x
7. Thase ME, Greenhouse JB, Frank E, et al. Treatment of major depression with psychotherapy or psychotherapy-pharmacotherapy combinations. Arch Gen Psychiatry. 1997;54: 1009-1015. doi: 10.1001/archpsyc.1997.01830230043006
8. Gartlehner G, Hansen RA, Morgan LC, et al. Comparative benefits and harms of second-generation antidepressants for treating major depressive disorder: an updated meta-analysis. Ann Intern Med. 2011;155:722-785. doi: 10.7326/0003-4819-155-11-201112060-00009
9. American Psychiatric Association. Practice Guideline for the Treatment of Patients With Major Depressive Disorder. 3rd ed. American Psychiatric Association; 2010. Accessed February 27, 2023. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf
10. Mojtabai R, Olfson M. National patterns in antidepressant treatment by psychiatrists and general medical providers: results from the national comorbidity survey replication. J Clin Psychiatry. 2008;69:1064-1074. doi: 10.4088/jcp.v69n0704
1. Henssler J, Alexander D, Schwarzer G, et al. Combining antidepressants vs antidepressant monotherapy for treatment of patients with acute depression: a systematic review and meta-analysis. JAMA Psychiatry. 2022;79:300-312. doi: 10.1001/jamapsychiatry.2021.4313
2. Hasin DS, Sarvet AL, Meyers JL, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry. 2018;75:336-346. doi: 10.1001/jamapsychiatry.2017.4602
3. Greenberg PE, Fournier AA, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76:155-162. doi: 10.4088/JCP.14m09298
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
5. Cuijpers P, Reynolds CF III, Donker T, et al. Personalized treatment of adult depression: medication, psychotherapy, or both? A systematic review. Depress Anxiety. 2012;29:855-864. doi: 10.1002/da.21985
6. Cuijpers P, van Straten A, Hollon SD, et al. The contribution of active medication to combined treatments of psychotherapy and pharmacotherapy for adult depression: a meta-analysis. Acta Psychiatr Scand. 2010;121:415-423. doi: 10.1111/j.1600-0447.2009.01513.x
7. Thase ME, Greenhouse JB, Frank E, et al. Treatment of major depression with psychotherapy or psychotherapy-pharmacotherapy combinations. Arch Gen Psychiatry. 1997;54: 1009-1015. doi: 10.1001/archpsyc.1997.01830230043006
8. Gartlehner G, Hansen RA, Morgan LC, et al. Comparative benefits and harms of second-generation antidepressants for treating major depressive disorder: an updated meta-analysis. Ann Intern Med. 2011;155:722-785. doi: 10.7326/0003-4819-155-11-201112060-00009
9. American Psychiatric Association. Practice Guideline for the Treatment of Patients With Major Depressive Disorder. 3rd ed. American Psychiatric Association; 2010. Accessed February 27, 2023. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf
10. Mojtabai R, Olfson M. National patterns in antidepressant treatment by psychiatrists and general medical providers: results from the national comorbidity survey replication. J Clin Psychiatry. 2008;69:1064-1074. doi: 10.4088/jcp.v69n0704
PRACTICE CHANGER
Use a combination of a presynaptic α2-autoreceptor antagonist (eg, mirtazapine or trazodone) and a monoamine reuptake inhibitor (eg, selective serotonin reuptake inhibitor [SSRI], serotonin-norepinephrine reuptake inhibitor [SNRI], or tricyclic antidepressant [TCA]) to treat acute depression in adult patients.
STRENGTH OF RECOMMENDATION
A: Based on a single systematic review with meta-analysis.1
Henssler J, Alexander D, Schwarzer G, et al. Combining antidepressants vs antidepressant monotherapy for treatment of patients with acute depression: a systematic review and meta-analysis. JAMA Psychiatry. 2022;79:300-312. doi: 10.1001/jamapsychiatry.2021.4313
AFib risk with cancer drugs underestimated
Atrial fibrillation (AFib) is a known and serious side effect of some cancer treatments, but it is underreported in cancer drug trials, French investigators said in a new report.
As a result, oncologists likely underestimate the risk of atrial fibrillation when new cancer drugs come to market, they said.
The team came to these conclusions after conducting a meta-analysis of 191 phase 2 or 3 clinical trials that included 26,604 patients. The trials investigated 15 anticancer drugs used as monotherapy.
The meta-analysis showed that the annualized incidence rate of AFib ranged from 0.26 cases per 100 person-years – about the same as placebo – to 4.92 cases, a nearly 20 times’ higher risk.
Rates were the highest for ibrutinib, clofarabine, and ponatinib.
The study was published in JACC: CardioOncology, a journal of the American College of Cardiology.
Actual rates of AFib are probably higher than what they found in this meta-analysis, the authors suspect, because most oncology trials only identify and report severe cases of AFib that require immediate medical attention. Less severe cases can also lead to serious complications, including strokes, but they go unreported, said the investigators, led by Joachim Alexandre, MD, PhD, a member of the cardio-oncology program at the University of Caen Normandie Hospital Center, France.
“These findings suggest a global and systemic underreporting and/or underidentification of cardiotoxicity among cancer clinical trial participants,” and AFib reporting is “particularly affected,” they said.
Call for routine monitoring
The root of the problem is the lack of routine rhythm monitoring in cancer trials. This in turn “leads to a significant underestimation of AFib incidence” and rates “markedly lower than those observed among real-life” patients, the authors pointed out.
To address the issue, Dr. Alexandre and his team called for routine cardiac monitoring in trials to capture the true incidence of AFib and to “clearly define which anticancer drugs are significantly associated” with the condition.
Approached for comment, Michael G. Fradley, MD, medical director of cardio-oncology at the University of Pennsylvania, Philadelphia, agreed.
“It’s incredibly important” to “identify the drugs most likely to cause arrhythmias and determine the best prevention and treatment strategies. Unfortunately, systematic evaluation of arrhythmias in cancer clinical trials has often been lacking,” Dr. Fradley told this news organization.
The investigators said the issue is particularly pressing for drugs known to be associated with AFib. For Bruton’s tyrosine kinase inhibitors such as ibrutinib, for instance, they call for standardize AFib detection in trials “not only on 12-lead ECGs” for symptomatic AFib but also with “longer-term ambulatory monitoring or insertable cardiac monitors to detect subclinical AFib.”
Dr. Fradley said there might also be a role for newer wearable technologies that can detect arrhythmias through a skin patch or by other means.
Details of the meta-analysis
The investigators pulled the 191 studies they used in their meta-analysis from the ClinicalTrials.gov database.
The trials covered anticancer drugs used as monotherapy up to Sept. 18, 2020. Almost half were randomized trials, but only seven had placebo arms. Trials involving hematologic cancers outnumbered those involving solid tumors.
The 15 drugs examined were dacarbazine, abiraterone, clofarabine, azacitidine, ibrutinib, nilotinib, ponatinib, midostaurin, ipilimumab, aldesleukin, lenalidomide, pomalidomide, rituximab, bortezomib, and docetaxel.
The annualized incidence AFib rates per 100 person-years were 4.92 cases for ibrutinib, 2.38 cases for clofarabine, and 2.35 cases for ponatinib.
The lowest AFib rates were for ipilimumab (0.26 cases), rituximab (0.27), and nilotinib (0.29).
For placebo, the annualized rate was 0.25 cases per 100 person-years.
The team said caution is warranted regarding their estimations for clofarabine and midostaurin (0.65 cases) because no trials were registered after September 2009, when adverse event reporting became mandatory. As a result, estimates may be artificially low.
One of the limits of the study is that it focused on monotherapy in an age when combination treatment is generally the rule for cancer, the authors noted.
No external funding was reported for the study. Dr. Alexandre has received honoraria for presentations and consulting fees from Bayer, BMS, Pfizer, Amgen, and Bioserenity.
A version of this article first appeared on Medscape.com.
Atrial fibrillation (AFib) is a known and serious side effect of some cancer treatments, but it is underreported in cancer drug trials, French investigators said in a new report.
As a result, oncologists likely underestimate the risk of atrial fibrillation when new cancer drugs come to market, they said.
The team came to these conclusions after conducting a meta-analysis of 191 phase 2 or 3 clinical trials that included 26,604 patients. The trials investigated 15 anticancer drugs used as monotherapy.
The meta-analysis showed that the annualized incidence rate of AFib ranged from 0.26 cases per 100 person-years – about the same as placebo – to 4.92 cases, a nearly 20 times’ higher risk.
Rates were the highest for ibrutinib, clofarabine, and ponatinib.
The study was published in JACC: CardioOncology, a journal of the American College of Cardiology.
Actual rates of AFib are probably higher than what they found in this meta-analysis, the authors suspect, because most oncology trials only identify and report severe cases of AFib that require immediate medical attention. Less severe cases can also lead to serious complications, including strokes, but they go unreported, said the investigators, led by Joachim Alexandre, MD, PhD, a member of the cardio-oncology program at the University of Caen Normandie Hospital Center, France.
“These findings suggest a global and systemic underreporting and/or underidentification of cardiotoxicity among cancer clinical trial participants,” and AFib reporting is “particularly affected,” they said.
Call for routine monitoring
The root of the problem is the lack of routine rhythm monitoring in cancer trials. This in turn “leads to a significant underestimation of AFib incidence” and rates “markedly lower than those observed among real-life” patients, the authors pointed out.
To address the issue, Dr. Alexandre and his team called for routine cardiac monitoring in trials to capture the true incidence of AFib and to “clearly define which anticancer drugs are significantly associated” with the condition.
Approached for comment, Michael G. Fradley, MD, medical director of cardio-oncology at the University of Pennsylvania, Philadelphia, agreed.
“It’s incredibly important” to “identify the drugs most likely to cause arrhythmias and determine the best prevention and treatment strategies. Unfortunately, systematic evaluation of arrhythmias in cancer clinical trials has often been lacking,” Dr. Fradley told this news organization.
The investigators said the issue is particularly pressing for drugs known to be associated with AFib. For Bruton’s tyrosine kinase inhibitors such as ibrutinib, for instance, they call for standardize AFib detection in trials “not only on 12-lead ECGs” for symptomatic AFib but also with “longer-term ambulatory monitoring or insertable cardiac monitors to detect subclinical AFib.”
Dr. Fradley said there might also be a role for newer wearable technologies that can detect arrhythmias through a skin patch or by other means.
Details of the meta-analysis
The investigators pulled the 191 studies they used in their meta-analysis from the ClinicalTrials.gov database.
The trials covered anticancer drugs used as monotherapy up to Sept. 18, 2020. Almost half were randomized trials, but only seven had placebo arms. Trials involving hematologic cancers outnumbered those involving solid tumors.
The 15 drugs examined were dacarbazine, abiraterone, clofarabine, azacitidine, ibrutinib, nilotinib, ponatinib, midostaurin, ipilimumab, aldesleukin, lenalidomide, pomalidomide, rituximab, bortezomib, and docetaxel.
The annualized incidence AFib rates per 100 person-years were 4.92 cases for ibrutinib, 2.38 cases for clofarabine, and 2.35 cases for ponatinib.
The lowest AFib rates were for ipilimumab (0.26 cases), rituximab (0.27), and nilotinib (0.29).
For placebo, the annualized rate was 0.25 cases per 100 person-years.
The team said caution is warranted regarding their estimations for clofarabine and midostaurin (0.65 cases) because no trials were registered after September 2009, when adverse event reporting became mandatory. As a result, estimates may be artificially low.
One of the limits of the study is that it focused on monotherapy in an age when combination treatment is generally the rule for cancer, the authors noted.
No external funding was reported for the study. Dr. Alexandre has received honoraria for presentations and consulting fees from Bayer, BMS, Pfizer, Amgen, and Bioserenity.
A version of this article first appeared on Medscape.com.
Atrial fibrillation (AFib) is a known and serious side effect of some cancer treatments, but it is underreported in cancer drug trials, French investigators said in a new report.
As a result, oncologists likely underestimate the risk of atrial fibrillation when new cancer drugs come to market, they said.
The team came to these conclusions after conducting a meta-analysis of 191 phase 2 or 3 clinical trials that included 26,604 patients. The trials investigated 15 anticancer drugs used as monotherapy.
The meta-analysis showed that the annualized incidence rate of AFib ranged from 0.26 cases per 100 person-years – about the same as placebo – to 4.92 cases, a nearly 20 times’ higher risk.
Rates were the highest for ibrutinib, clofarabine, and ponatinib.
The study was published in JACC: CardioOncology, a journal of the American College of Cardiology.
Actual rates of AFib are probably higher than what they found in this meta-analysis, the authors suspect, because most oncology trials only identify and report severe cases of AFib that require immediate medical attention. Less severe cases can also lead to serious complications, including strokes, but they go unreported, said the investigators, led by Joachim Alexandre, MD, PhD, a member of the cardio-oncology program at the University of Caen Normandie Hospital Center, France.
“These findings suggest a global and systemic underreporting and/or underidentification of cardiotoxicity among cancer clinical trial participants,” and AFib reporting is “particularly affected,” they said.
Call for routine monitoring
The root of the problem is the lack of routine rhythm monitoring in cancer trials. This in turn “leads to a significant underestimation of AFib incidence” and rates “markedly lower than those observed among real-life” patients, the authors pointed out.
To address the issue, Dr. Alexandre and his team called for routine cardiac monitoring in trials to capture the true incidence of AFib and to “clearly define which anticancer drugs are significantly associated” with the condition.
Approached for comment, Michael G. Fradley, MD, medical director of cardio-oncology at the University of Pennsylvania, Philadelphia, agreed.
“It’s incredibly important” to “identify the drugs most likely to cause arrhythmias and determine the best prevention and treatment strategies. Unfortunately, systematic evaluation of arrhythmias in cancer clinical trials has often been lacking,” Dr. Fradley told this news organization.
The investigators said the issue is particularly pressing for drugs known to be associated with AFib. For Bruton’s tyrosine kinase inhibitors such as ibrutinib, for instance, they call for standardize AFib detection in trials “not only on 12-lead ECGs” for symptomatic AFib but also with “longer-term ambulatory monitoring or insertable cardiac monitors to detect subclinical AFib.”
Dr. Fradley said there might also be a role for newer wearable technologies that can detect arrhythmias through a skin patch or by other means.
Details of the meta-analysis
The investigators pulled the 191 studies they used in their meta-analysis from the ClinicalTrials.gov database.
The trials covered anticancer drugs used as monotherapy up to Sept. 18, 2020. Almost half were randomized trials, but only seven had placebo arms. Trials involving hematologic cancers outnumbered those involving solid tumors.
The 15 drugs examined were dacarbazine, abiraterone, clofarabine, azacitidine, ibrutinib, nilotinib, ponatinib, midostaurin, ipilimumab, aldesleukin, lenalidomide, pomalidomide, rituximab, bortezomib, and docetaxel.
The annualized incidence AFib rates per 100 person-years were 4.92 cases for ibrutinib, 2.38 cases for clofarabine, and 2.35 cases for ponatinib.
The lowest AFib rates were for ipilimumab (0.26 cases), rituximab (0.27), and nilotinib (0.29).
For placebo, the annualized rate was 0.25 cases per 100 person-years.
The team said caution is warranted regarding their estimations for clofarabine and midostaurin (0.65 cases) because no trials were registered after September 2009, when adverse event reporting became mandatory. As a result, estimates may be artificially low.
One of the limits of the study is that it focused on monotherapy in an age when combination treatment is generally the rule for cancer, the authors noted.
No external funding was reported for the study. Dr. Alexandre has received honoraria for presentations and consulting fees from Bayer, BMS, Pfizer, Amgen, and Bioserenity.
A version of this article first appeared on Medscape.com.