User login
ERs are swamped with seriously ill patients, although many don’t have COVID
Inside the emergency department at Sparrow Hospital in Lansing, Mich., staff members are struggling to care for patients showing up much sicker than they’ve ever seen.
Tiffani Dusang, the ER’s nursing director, practically vibrates with pent-up anxiety, looking at patients lying on a long line of stretchers pushed up against the beige walls of the hospital hallways. “It’s hard to watch,” she said in a warm Texas twang.
But there’s nothing she can do. The ER’s 72 rooms are already filled.
“I always feel very, very bad when I walk down the hallway and see that people are in pain, or needing to sleep, or needing quiet. But they have to be in the hallway with, as you can see, 10 or 15 people walking by every minute,” Ms. Dusang said.
The scene is a stark contrast to where this emergency department — and thousands of others — were at the start of the pandemic. Except for initial hot spots like New York City, in spring 2020 many ERs across the country were often eerily empty. Terrified of contracting COVID-19, people who were sick with other things did their best to stay away from hospitals. Visits to emergency rooms dropped to half their typical levels, according to the Epic Health Research Network, and didn’t fully rebound until this summer.
But now, they’re too full.
Months of treatment delays have exacerbated chronic conditions and worsened symptoms. Doctors and nurses say the severity of illness ranges widely and includes abdominal pain, respiratory problems, blood clots, heart conditions and suicide attempts, among other conditions.
But they can hardly be accommodated. Emergency departments, ideally, are meant to be brief ports in a storm, with patients staying just long enough to be sent home with instructions to follow up with primary care physicians, or sufficiently stabilized to be transferred “upstairs” to inpatient or intensive care units.
Except now those long-term care floors are full too, with a mix of covid and non-covid patients. People coming to the ER get warehoused for hours, even days, forcing ER staffers to perform long-term care roles they weren’t trained to do.
At Sparrow, space is a valuable commodity in the ER: A separate section of the hospital was turned into an overflow unit. Stretchers stack up in halls. A row of brown reclining chairs lines a wall, intended for patients who aren’t sick enough for a stretcher but are too sick to stay in the main waiting room.
Forget privacy, Alejos Perrientoz learned when he arrived. He came to the ER because his arm had been tingling and painful for over a week. He couldn’t hold a cup of coffee. A nurse gave him a full physical exam in a brown recliner, which made him self-conscious about having his shirt lifted in front of strangers. “I felt a little uncomfortable,” he whispered. “But I have no choice, you know? I’m in the hallway. There’s no rooms.
“We could have done the physical in the parking lot,” he added, managing a laugh.
Even patients who arrive by ambulance are not guaranteed a room: One nurse runs triage, screening those who absolutely need a bed, and those who can be put in the waiting area.
“I hate that we even have to make that determination,” MS. Dusang said. Lately, staff members have been pulling out some patients already in the ER’s rooms when others arrive who are more critically ill. “No one likes to take someone out of the privacy of their room and say, ‘We’re going to put you in a hallway because we need to get care to someone else.’”
ER patients have grown sicker
“We are hearing from members in every part of the country,” said Dr. Lisa Moreno, president of the American Academy of Emergency Medicine. “The Midwest, the South, the Northeast, the West … they are seeing this exact same phenomenon.”
Although the number of ER visits returned to pre-COVID levels this summer, admission rates, from the ER to the hospital’s inpatient floors, are still almost 20% higher. That’s according to the most recent analysis by the Epic Health Research Network, which pulls data from more than 120 million patients across the country.
“It’s an early indicator that what’s happening in the ED is that we’re seeing more acute cases than we were pre-pandemic,” said Caleb Cox, a data scientist at Epic.
Less acute cases, such as people with health issues like rashes or conjunctivitis, still aren’t going to the ER as much as they used to. Instead, they may be opting for an urgent care center or their primary care doctor, Mr. Cox explained. Meanwhile, there has been an increase in people coming to the ER with more serious conditions, like strokes and heart attacks.
So, even though the total number of patients coming to ERs is about the same as before the pandemic, “that’s absolutely going to feel like [if I’m an ER doctor or nurse] I’m seeing more patients and I’m seeing more acute patients,” Mr. Cox said.
Dr. Moreno, the AAEM president, works at an emergency department in New Orleans. She said the level of illness, and the inability to admit patients quickly and move them to beds upstairs, has created a level of chaos she described as “not even humane.”
At the beginning of a recent shift, she heard a patient crying nearby and went to investigate. It was a paraplegic man who’d recently had surgery for colon cancer. His large post-operative wound was sealed with a device called a wound vac, which pulls fluid from the wound into a drainage tube attached to a portable vacuum pump.
But the wound vac had malfunctioned, which is why he had come to the ER. Staffers were so busy, however, that by the time Dr. Moreno came in, the fluid from his wound was leaking everywhere.
“When I went in, the bed was covered,” she recalled. “I mean, he was lying in a puddle of secretions from this wound. And he was crying, because he said to me, ‘I’m paralyzed. I can’t move to get away from all these secretions, and I know I’m going to end up getting an infection. I know I’m going to end up getting an ulcer. I’ve been laying in this for, like, eight or nine hours.’”
The nurse in charge of his care told Dr. Moreno she simply hadn’t had time to help this patient yet. “She said, ‘I’ve had so many patients to take care of, and so many critical patients. I started [an IV] drip on this person. This person is on a cardiac monitor. I just didn’t have time to get in there.’”
“This is not humane care,” Dr. Moreno said. “This is horrible care.”
But it’s what can happen when emergency department staffers don’t have the resources they need to deal with the onslaught of competing demands.
“All the nurses and doctors had the highest level of intent to do the right thing for the person,” Dr. Moreno said. “But because of the high acuity of … a large number of patients, the staffing ratio of nurse to patient, even the staffing ratio of doctor to patient, this guy did not get the care that he deserved to get, just as a human being.”
The instance of unintended neglect that Dr. Moreno saw is extreme, and not the experience of most patients who arrive at ERs these days. But the problem is not new: Even before the pandemic, ER overcrowding had been a “widespread problem and a source of patient harm, according to a recent commentary in NEJM Catalyst Innovations in Care Delivery.
“ED crowding is not an issue of inconvenience,” the authors wrote. “There is incontrovertible evidence that ED crowding leads to significant patient harm, including morbidity and mortality related to consequential delays of treatment for both high- and low-acuity patients.”
And already-overwhelmed staffers are burning out.
Burnout feeds staffing shortages, and vice versa
Every morning, Tiffani Dusang wakes up and checks her Sparrow email with one singular hope: that she will not see yet another nurse resignation letter in her inbox.
“I cannot tell you how many of them [the nurses] tell me they went home crying” after their shifts, she said.
Despite Ms. Dusang’s best efforts to support her staffers, they’re leaving too fast to be replaced, either to take higher-paying gigs as a travel nurse, to try a less-stressful type of nursing, or simply walking away from the profession entirely.
Kelly Spitz has been an emergency department nurse at Sparrow for 10 years. But, lately, she has also fantasized about leaving. “It has crossed my mind several times,” she said, and yet she continues to come back. “Because I have a team here. And I love what I do.” But then she started to cry. The issue is not the hard work, or even the stress. She struggles with not being able to give her patients the kind of care and attention she wants to give them, and that they need and deserve, she said.
She often thinks about a patient whose test results revealed terminal cancer, she said. Ms. Spitz spent all day working the phones, hustling case managers, trying to get hospice care set up in the man’s home. He was going to die, and she just didn’t want him to have to die in the hospital, where only one visitor was allowed. She wanted to get him home, and back with his family.
Finally, after many hours, they found an ambulance to take him home.
Three days later, the man’s family members called Ms. Spitz: He had died surrounded by family. They were calling to thank her.
“I felt like I did my job there, because I got him home,” she said. But that’s a rare feeling these days. “I just hope it gets better. I hope it gets better soon.”
Around 4 p.m. at Sparrow Hospital as one shift approached its end, Ms. Dusang faced a new crisis: The overnight shift was more short-staffed than usual.
“Can we get two inpatient nurses?” she asked, hoping to borrow two nurses from one of the hospital floors upstairs.
“Already tried,” replied nurse Troy Latunski.
Without more staff, it’s going to be hard to care for new patients who come in overnight — from car crashes to seizures or other emergencies.
But Mr. Latunski had a plan: He would go home, snatch a few hours of sleep and return at 11 p.m. to work the overnight shift in the ER’s overflow unit. That meant he would be largely caring for eight patients, alone. On just a few short hours of sleep. But lately that seemed to be their only, and best, option.
Ms. Dusang considered for a moment, took a deep breath and nodded. “OK,” she said.
“Go home. Get some sleep. Thank you,” she added, shooting Mr. Latunski a grateful smile. And then she pivoted, because another nurse was approaching with an urgent question. On to the next crisis.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation. This story is part of a partnership that includes Michigan Radio, NPR and KHN.
Inside the emergency department at Sparrow Hospital in Lansing, Mich., staff members are struggling to care for patients showing up much sicker than they’ve ever seen.
Tiffani Dusang, the ER’s nursing director, practically vibrates with pent-up anxiety, looking at patients lying on a long line of stretchers pushed up against the beige walls of the hospital hallways. “It’s hard to watch,” she said in a warm Texas twang.
But there’s nothing she can do. The ER’s 72 rooms are already filled.
“I always feel very, very bad when I walk down the hallway and see that people are in pain, or needing to sleep, or needing quiet. But they have to be in the hallway with, as you can see, 10 or 15 people walking by every minute,” Ms. Dusang said.
The scene is a stark contrast to where this emergency department — and thousands of others — were at the start of the pandemic. Except for initial hot spots like New York City, in spring 2020 many ERs across the country were often eerily empty. Terrified of contracting COVID-19, people who were sick with other things did their best to stay away from hospitals. Visits to emergency rooms dropped to half their typical levels, according to the Epic Health Research Network, and didn’t fully rebound until this summer.
But now, they’re too full.
Months of treatment delays have exacerbated chronic conditions and worsened symptoms. Doctors and nurses say the severity of illness ranges widely and includes abdominal pain, respiratory problems, blood clots, heart conditions and suicide attempts, among other conditions.
But they can hardly be accommodated. Emergency departments, ideally, are meant to be brief ports in a storm, with patients staying just long enough to be sent home with instructions to follow up with primary care physicians, or sufficiently stabilized to be transferred “upstairs” to inpatient or intensive care units.
Except now those long-term care floors are full too, with a mix of covid and non-covid patients. People coming to the ER get warehoused for hours, even days, forcing ER staffers to perform long-term care roles they weren’t trained to do.
At Sparrow, space is a valuable commodity in the ER: A separate section of the hospital was turned into an overflow unit. Stretchers stack up in halls. A row of brown reclining chairs lines a wall, intended for patients who aren’t sick enough for a stretcher but are too sick to stay in the main waiting room.
Forget privacy, Alejos Perrientoz learned when he arrived. He came to the ER because his arm had been tingling and painful for over a week. He couldn’t hold a cup of coffee. A nurse gave him a full physical exam in a brown recliner, which made him self-conscious about having his shirt lifted in front of strangers. “I felt a little uncomfortable,” he whispered. “But I have no choice, you know? I’m in the hallway. There’s no rooms.
“We could have done the physical in the parking lot,” he added, managing a laugh.
Even patients who arrive by ambulance are not guaranteed a room: One nurse runs triage, screening those who absolutely need a bed, and those who can be put in the waiting area.
“I hate that we even have to make that determination,” MS. Dusang said. Lately, staff members have been pulling out some patients already in the ER’s rooms when others arrive who are more critically ill. “No one likes to take someone out of the privacy of their room and say, ‘We’re going to put you in a hallway because we need to get care to someone else.’”
ER patients have grown sicker
“We are hearing from members in every part of the country,” said Dr. Lisa Moreno, president of the American Academy of Emergency Medicine. “The Midwest, the South, the Northeast, the West … they are seeing this exact same phenomenon.”
Although the number of ER visits returned to pre-COVID levels this summer, admission rates, from the ER to the hospital’s inpatient floors, are still almost 20% higher. That’s according to the most recent analysis by the Epic Health Research Network, which pulls data from more than 120 million patients across the country.
“It’s an early indicator that what’s happening in the ED is that we’re seeing more acute cases than we were pre-pandemic,” said Caleb Cox, a data scientist at Epic.
Less acute cases, such as people with health issues like rashes or conjunctivitis, still aren’t going to the ER as much as they used to. Instead, they may be opting for an urgent care center or their primary care doctor, Mr. Cox explained. Meanwhile, there has been an increase in people coming to the ER with more serious conditions, like strokes and heart attacks.
So, even though the total number of patients coming to ERs is about the same as before the pandemic, “that’s absolutely going to feel like [if I’m an ER doctor or nurse] I’m seeing more patients and I’m seeing more acute patients,” Mr. Cox said.
Dr. Moreno, the AAEM president, works at an emergency department in New Orleans. She said the level of illness, and the inability to admit patients quickly and move them to beds upstairs, has created a level of chaos she described as “not even humane.”
At the beginning of a recent shift, she heard a patient crying nearby and went to investigate. It was a paraplegic man who’d recently had surgery for colon cancer. His large post-operative wound was sealed with a device called a wound vac, which pulls fluid from the wound into a drainage tube attached to a portable vacuum pump.
But the wound vac had malfunctioned, which is why he had come to the ER. Staffers were so busy, however, that by the time Dr. Moreno came in, the fluid from his wound was leaking everywhere.
“When I went in, the bed was covered,” she recalled. “I mean, he was lying in a puddle of secretions from this wound. And he was crying, because he said to me, ‘I’m paralyzed. I can’t move to get away from all these secretions, and I know I’m going to end up getting an infection. I know I’m going to end up getting an ulcer. I’ve been laying in this for, like, eight or nine hours.’”
The nurse in charge of his care told Dr. Moreno she simply hadn’t had time to help this patient yet. “She said, ‘I’ve had so many patients to take care of, and so many critical patients. I started [an IV] drip on this person. This person is on a cardiac monitor. I just didn’t have time to get in there.’”
“This is not humane care,” Dr. Moreno said. “This is horrible care.”
But it’s what can happen when emergency department staffers don’t have the resources they need to deal with the onslaught of competing demands.
“All the nurses and doctors had the highest level of intent to do the right thing for the person,” Dr. Moreno said. “But because of the high acuity of … a large number of patients, the staffing ratio of nurse to patient, even the staffing ratio of doctor to patient, this guy did not get the care that he deserved to get, just as a human being.”
The instance of unintended neglect that Dr. Moreno saw is extreme, and not the experience of most patients who arrive at ERs these days. But the problem is not new: Even before the pandemic, ER overcrowding had been a “widespread problem and a source of patient harm, according to a recent commentary in NEJM Catalyst Innovations in Care Delivery.
“ED crowding is not an issue of inconvenience,” the authors wrote. “There is incontrovertible evidence that ED crowding leads to significant patient harm, including morbidity and mortality related to consequential delays of treatment for both high- and low-acuity patients.”
And already-overwhelmed staffers are burning out.
Burnout feeds staffing shortages, and vice versa
Every morning, Tiffani Dusang wakes up and checks her Sparrow email with one singular hope: that she will not see yet another nurse resignation letter in her inbox.
“I cannot tell you how many of them [the nurses] tell me they went home crying” after their shifts, she said.
Despite Ms. Dusang’s best efforts to support her staffers, they’re leaving too fast to be replaced, either to take higher-paying gigs as a travel nurse, to try a less-stressful type of nursing, or simply walking away from the profession entirely.
Kelly Spitz has been an emergency department nurse at Sparrow for 10 years. But, lately, she has also fantasized about leaving. “It has crossed my mind several times,” she said, and yet she continues to come back. “Because I have a team here. And I love what I do.” But then she started to cry. The issue is not the hard work, or even the stress. She struggles with not being able to give her patients the kind of care and attention she wants to give them, and that they need and deserve, she said.
She often thinks about a patient whose test results revealed terminal cancer, she said. Ms. Spitz spent all day working the phones, hustling case managers, trying to get hospice care set up in the man’s home. He was going to die, and she just didn’t want him to have to die in the hospital, where only one visitor was allowed. She wanted to get him home, and back with his family.
Finally, after many hours, they found an ambulance to take him home.
Three days later, the man’s family members called Ms. Spitz: He had died surrounded by family. They were calling to thank her.
“I felt like I did my job there, because I got him home,” she said. But that’s a rare feeling these days. “I just hope it gets better. I hope it gets better soon.”
Around 4 p.m. at Sparrow Hospital as one shift approached its end, Ms. Dusang faced a new crisis: The overnight shift was more short-staffed than usual.
“Can we get two inpatient nurses?” she asked, hoping to borrow two nurses from one of the hospital floors upstairs.
“Already tried,” replied nurse Troy Latunski.
Without more staff, it’s going to be hard to care for new patients who come in overnight — from car crashes to seizures or other emergencies.
But Mr. Latunski had a plan: He would go home, snatch a few hours of sleep and return at 11 p.m. to work the overnight shift in the ER’s overflow unit. That meant he would be largely caring for eight patients, alone. On just a few short hours of sleep. But lately that seemed to be their only, and best, option.
Ms. Dusang considered for a moment, took a deep breath and nodded. “OK,” she said.
“Go home. Get some sleep. Thank you,” she added, shooting Mr. Latunski a grateful smile. And then she pivoted, because another nurse was approaching with an urgent question. On to the next crisis.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation. This story is part of a partnership that includes Michigan Radio, NPR and KHN.
Inside the emergency department at Sparrow Hospital in Lansing, Mich., staff members are struggling to care for patients showing up much sicker than they’ve ever seen.
Tiffani Dusang, the ER’s nursing director, practically vibrates with pent-up anxiety, looking at patients lying on a long line of stretchers pushed up against the beige walls of the hospital hallways. “It’s hard to watch,” she said in a warm Texas twang.
But there’s nothing she can do. The ER’s 72 rooms are already filled.
“I always feel very, very bad when I walk down the hallway and see that people are in pain, or needing to sleep, or needing quiet. But they have to be in the hallway with, as you can see, 10 or 15 people walking by every minute,” Ms. Dusang said.
The scene is a stark contrast to where this emergency department — and thousands of others — were at the start of the pandemic. Except for initial hot spots like New York City, in spring 2020 many ERs across the country were often eerily empty. Terrified of contracting COVID-19, people who were sick with other things did their best to stay away from hospitals. Visits to emergency rooms dropped to half their typical levels, according to the Epic Health Research Network, and didn’t fully rebound until this summer.
But now, they’re too full.
Months of treatment delays have exacerbated chronic conditions and worsened symptoms. Doctors and nurses say the severity of illness ranges widely and includes abdominal pain, respiratory problems, blood clots, heart conditions and suicide attempts, among other conditions.
But they can hardly be accommodated. Emergency departments, ideally, are meant to be brief ports in a storm, with patients staying just long enough to be sent home with instructions to follow up with primary care physicians, or sufficiently stabilized to be transferred “upstairs” to inpatient or intensive care units.
Except now those long-term care floors are full too, with a mix of covid and non-covid patients. People coming to the ER get warehoused for hours, even days, forcing ER staffers to perform long-term care roles they weren’t trained to do.
At Sparrow, space is a valuable commodity in the ER: A separate section of the hospital was turned into an overflow unit. Stretchers stack up in halls. A row of brown reclining chairs lines a wall, intended for patients who aren’t sick enough for a stretcher but are too sick to stay in the main waiting room.
Forget privacy, Alejos Perrientoz learned when he arrived. He came to the ER because his arm had been tingling and painful for over a week. He couldn’t hold a cup of coffee. A nurse gave him a full physical exam in a brown recliner, which made him self-conscious about having his shirt lifted in front of strangers. “I felt a little uncomfortable,” he whispered. “But I have no choice, you know? I’m in the hallway. There’s no rooms.
“We could have done the physical in the parking lot,” he added, managing a laugh.
Even patients who arrive by ambulance are not guaranteed a room: One nurse runs triage, screening those who absolutely need a bed, and those who can be put in the waiting area.
“I hate that we even have to make that determination,” MS. Dusang said. Lately, staff members have been pulling out some patients already in the ER’s rooms when others arrive who are more critically ill. “No one likes to take someone out of the privacy of their room and say, ‘We’re going to put you in a hallway because we need to get care to someone else.’”
ER patients have grown sicker
“We are hearing from members in every part of the country,” said Dr. Lisa Moreno, president of the American Academy of Emergency Medicine. “The Midwest, the South, the Northeast, the West … they are seeing this exact same phenomenon.”
Although the number of ER visits returned to pre-COVID levels this summer, admission rates, from the ER to the hospital’s inpatient floors, are still almost 20% higher. That’s according to the most recent analysis by the Epic Health Research Network, which pulls data from more than 120 million patients across the country.
“It’s an early indicator that what’s happening in the ED is that we’re seeing more acute cases than we were pre-pandemic,” said Caleb Cox, a data scientist at Epic.
Less acute cases, such as people with health issues like rashes or conjunctivitis, still aren’t going to the ER as much as they used to. Instead, they may be opting for an urgent care center or their primary care doctor, Mr. Cox explained. Meanwhile, there has been an increase in people coming to the ER with more serious conditions, like strokes and heart attacks.
So, even though the total number of patients coming to ERs is about the same as before the pandemic, “that’s absolutely going to feel like [if I’m an ER doctor or nurse] I’m seeing more patients and I’m seeing more acute patients,” Mr. Cox said.
Dr. Moreno, the AAEM president, works at an emergency department in New Orleans. She said the level of illness, and the inability to admit patients quickly and move them to beds upstairs, has created a level of chaos she described as “not even humane.”
At the beginning of a recent shift, she heard a patient crying nearby and went to investigate. It was a paraplegic man who’d recently had surgery for colon cancer. His large post-operative wound was sealed with a device called a wound vac, which pulls fluid from the wound into a drainage tube attached to a portable vacuum pump.
But the wound vac had malfunctioned, which is why he had come to the ER. Staffers were so busy, however, that by the time Dr. Moreno came in, the fluid from his wound was leaking everywhere.
“When I went in, the bed was covered,” she recalled. “I mean, he was lying in a puddle of secretions from this wound. And he was crying, because he said to me, ‘I’m paralyzed. I can’t move to get away from all these secretions, and I know I’m going to end up getting an infection. I know I’m going to end up getting an ulcer. I’ve been laying in this for, like, eight or nine hours.’”
The nurse in charge of his care told Dr. Moreno she simply hadn’t had time to help this patient yet. “She said, ‘I’ve had so many patients to take care of, and so many critical patients. I started [an IV] drip on this person. This person is on a cardiac monitor. I just didn’t have time to get in there.’”
“This is not humane care,” Dr. Moreno said. “This is horrible care.”
But it’s what can happen when emergency department staffers don’t have the resources they need to deal with the onslaught of competing demands.
“All the nurses and doctors had the highest level of intent to do the right thing for the person,” Dr. Moreno said. “But because of the high acuity of … a large number of patients, the staffing ratio of nurse to patient, even the staffing ratio of doctor to patient, this guy did not get the care that he deserved to get, just as a human being.”
The instance of unintended neglect that Dr. Moreno saw is extreme, and not the experience of most patients who arrive at ERs these days. But the problem is not new: Even before the pandemic, ER overcrowding had been a “widespread problem and a source of patient harm, according to a recent commentary in NEJM Catalyst Innovations in Care Delivery.
“ED crowding is not an issue of inconvenience,” the authors wrote. “There is incontrovertible evidence that ED crowding leads to significant patient harm, including morbidity and mortality related to consequential delays of treatment for both high- and low-acuity patients.”
And already-overwhelmed staffers are burning out.
Burnout feeds staffing shortages, and vice versa
Every morning, Tiffani Dusang wakes up and checks her Sparrow email with one singular hope: that she will not see yet another nurse resignation letter in her inbox.
“I cannot tell you how many of them [the nurses] tell me they went home crying” after their shifts, she said.
Despite Ms. Dusang’s best efforts to support her staffers, they’re leaving too fast to be replaced, either to take higher-paying gigs as a travel nurse, to try a less-stressful type of nursing, or simply walking away from the profession entirely.
Kelly Spitz has been an emergency department nurse at Sparrow for 10 years. But, lately, she has also fantasized about leaving. “It has crossed my mind several times,” she said, and yet she continues to come back. “Because I have a team here. And I love what I do.” But then she started to cry. The issue is not the hard work, or even the stress. She struggles with not being able to give her patients the kind of care and attention she wants to give them, and that they need and deserve, she said.
She often thinks about a patient whose test results revealed terminal cancer, she said. Ms. Spitz spent all day working the phones, hustling case managers, trying to get hospice care set up in the man’s home. He was going to die, and she just didn’t want him to have to die in the hospital, where only one visitor was allowed. She wanted to get him home, and back with his family.
Finally, after many hours, they found an ambulance to take him home.
Three days later, the man’s family members called Ms. Spitz: He had died surrounded by family. They were calling to thank her.
“I felt like I did my job there, because I got him home,” she said. But that’s a rare feeling these days. “I just hope it gets better. I hope it gets better soon.”
Around 4 p.m. at Sparrow Hospital as one shift approached its end, Ms. Dusang faced a new crisis: The overnight shift was more short-staffed than usual.
“Can we get two inpatient nurses?” she asked, hoping to borrow two nurses from one of the hospital floors upstairs.
“Already tried,” replied nurse Troy Latunski.
Without more staff, it’s going to be hard to care for new patients who come in overnight — from car crashes to seizures or other emergencies.
But Mr. Latunski had a plan: He would go home, snatch a few hours of sleep and return at 11 p.m. to work the overnight shift in the ER’s overflow unit. That meant he would be largely caring for eight patients, alone. On just a few short hours of sleep. But lately that seemed to be their only, and best, option.
Ms. Dusang considered for a moment, took a deep breath and nodded. “OK,” she said.
“Go home. Get some sleep. Thank you,” she added, shooting Mr. Latunski a grateful smile. And then she pivoted, because another nurse was approaching with an urgent question. On to the next crisis.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation. This story is part of a partnership that includes Michigan Radio, NPR and KHN.
FDA authorizes Pfizer’s COVID-19 vaccine for kids
The move brings families with young children a step closer to resuming their normal activities, and it should help further slow transmission of the coronavirus virus in the United States.
States have already placed their orders for initial doses of the vaccines. The Oct. 29 FDA authorization triggers the shipment of millions of doses to pediatricians, family practice doctors, children’s hospitals, community health centers, and pharmacies.
Next, a panel of experts known as the Advisory Committee on Immunization Practices, or ACIP, will meet Nov. 2 to vote on recommendations for use of the vaccine.
As soon as the Centers for Disease Control and Prevention’s director signs off on those recommendations, children can get the shots, perhaps as early as Nov. 3.
Pfizer’s vaccine for children is 10 micrograms, or one-third of the dose given to teens and adults. Kids get two doses of the vaccine 3 weeks apart. In clinical trials, the most common side effects were pain at the injection site, fatigue, and headache. These side effects were mild and disappeared quickly. There were no serious adverse events detected in the studies, which included about 3,100 children. In one study, the vaccine was 90% effective at preventing COVID-19 infections with symptoms in younger children.
There are about 28 million children in the United States between the ages of 5 and 12.
“As a mother and a physician, I know that parents, caregivers, school staff, and children have been waiting for today’s authorization. Vaccinating younger children against COVID-19 will bring us closer to returning to a sense of normalcy,” Acting FDA Commissioner Janet Woodcock, MD, said in an FDA news release.
“Our comprehensive and rigorous evaluation of the data pertaining to the vaccine’s safety and effectiveness should help assure parents and guardians that this vaccine meets our high standards,” she said.
A version of this article first appeared on WebMD.com.
The move brings families with young children a step closer to resuming their normal activities, and it should help further slow transmission of the coronavirus virus in the United States.
States have already placed their orders for initial doses of the vaccines. The Oct. 29 FDA authorization triggers the shipment of millions of doses to pediatricians, family practice doctors, children’s hospitals, community health centers, and pharmacies.
Next, a panel of experts known as the Advisory Committee on Immunization Practices, or ACIP, will meet Nov. 2 to vote on recommendations for use of the vaccine.
As soon as the Centers for Disease Control and Prevention’s director signs off on those recommendations, children can get the shots, perhaps as early as Nov. 3.
Pfizer’s vaccine for children is 10 micrograms, or one-third of the dose given to teens and adults. Kids get two doses of the vaccine 3 weeks apart. In clinical trials, the most common side effects were pain at the injection site, fatigue, and headache. These side effects were mild and disappeared quickly. There were no serious adverse events detected in the studies, which included about 3,100 children. In one study, the vaccine was 90% effective at preventing COVID-19 infections with symptoms in younger children.
There are about 28 million children in the United States between the ages of 5 and 12.
“As a mother and a physician, I know that parents, caregivers, school staff, and children have been waiting for today’s authorization. Vaccinating younger children against COVID-19 will bring us closer to returning to a sense of normalcy,” Acting FDA Commissioner Janet Woodcock, MD, said in an FDA news release.
“Our comprehensive and rigorous evaluation of the data pertaining to the vaccine’s safety and effectiveness should help assure parents and guardians that this vaccine meets our high standards,” she said.
A version of this article first appeared on WebMD.com.
The move brings families with young children a step closer to resuming their normal activities, and it should help further slow transmission of the coronavirus virus in the United States.
States have already placed their orders for initial doses of the vaccines. The Oct. 29 FDA authorization triggers the shipment of millions of doses to pediatricians, family practice doctors, children’s hospitals, community health centers, and pharmacies.
Next, a panel of experts known as the Advisory Committee on Immunization Practices, or ACIP, will meet Nov. 2 to vote on recommendations for use of the vaccine.
As soon as the Centers for Disease Control and Prevention’s director signs off on those recommendations, children can get the shots, perhaps as early as Nov. 3.
Pfizer’s vaccine for children is 10 micrograms, or one-third of the dose given to teens and adults. Kids get two doses of the vaccine 3 weeks apart. In clinical trials, the most common side effects were pain at the injection site, fatigue, and headache. These side effects were mild and disappeared quickly. There were no serious adverse events detected in the studies, which included about 3,100 children. In one study, the vaccine was 90% effective at preventing COVID-19 infections with symptoms in younger children.
There are about 28 million children in the United States between the ages of 5 and 12.
“As a mother and a physician, I know that parents, caregivers, school staff, and children have been waiting for today’s authorization. Vaccinating younger children against COVID-19 will bring us closer to returning to a sense of normalcy,” Acting FDA Commissioner Janet Woodcock, MD, said in an FDA news release.
“Our comprehensive and rigorous evaluation of the data pertaining to the vaccine’s safety and effectiveness should help assure parents and guardians that this vaccine meets our high standards,” she said.
A version of this article first appeared on WebMD.com.
Antidepressant may cut COVID-19–related hospitalization, mortality: TOGETHER
The antidepressant fluvoxamine (Luvox) may prevent hospitalization and death in outpatients with COVID-19, new research suggests.
Results from the placebo-controlled, multisite, phase 3 TOGETHER trial showed that in COVID-19 outpatients at high risk for complications, hospitalizations were cut by 66% and deaths were reduced by 91% in those who tolerated fluvoxamine.
“Our trial has found that fluvoxamine, an inexpensive existing drug, reduces the need for advanced disease care in this high-risk population,” wrote the investigators, led by Gilmar Reis, MD, PhD, research division, Cardresearch, Belo Horizonte, Brazil.
The findings were published online Oct. 27 in The Lancet Global Health.
Alternative mechanisms
Fluvoxamine, a selective serotonin reuptake inhibitor (SSRI), is an antidepressant commonly prescribed for obsessive-compulsive disorder.
Besides its known effects on serotonin, the drug acts in other molecular pathways to dampen the production of inflammatory cytokines. Those alternative mechanisms are the ones believed to help patients with COVID-19, said coinvestigator Angela Reiersen, MD, child psychiatrist at Washington University, St. Louis.
Based on cell culture and mouse studies showing effects of the molecule’s binding to the sigma-1 receptor in the endoplasmic reticulum, Dr. Reiersen came up with the idea of testing if fluvoxamine could keep COVID-19 from progressing in newly infected patients.
Dr. Reiersen and psychiatrist Eric Lenze, MD, also from Washington University, led the phase 2 trial that initially suggested fluvoxamine’s promise as an outpatient medication. They are coinvestigators on the new phase 3 adaptive platform trial called TOGETHER, which was conducted by an international team of investigators in Brazil, Canada, and the United States.
For this latest study, researchers at McMaster University, Hamilton, Ont., partnered with the research clinic Cardresearch in Brazil to recruit unvaccinated, high-risk adults within 7 days of developing flu-like symptoms from COVID-19. They analyzed 1,497 newly symptomatic COVID-19 patients at 11 clinical sites in Brazil.
Patients entered the trial between January and August 2021 and were assigned to receive 100 mg fluvoxamine or placebo pills twice a day for 10 days. Investigators monitored participants through 28 days post treatment, noting whether complications developed requiring hospitalization or more than 6 hours of emergency care.
In the placebo group, 119 of 756 patients (15.7%) worsened to this extent. In comparison, 79 of 741 (10.7%) fluvoxamine-treated patients met these primary criteria. This represented a 32% reduction in hospitalizations and emergency visits.
Additional analysis requested
As Lancet Global Health reviewed these findings from the submitted manuscript, journal reviewers requested an additional “pre-protocol analysis” that was not specified in the trial’s original protocol. The request was to examine the subgroup of patients with good adherence (74% of treated group, 82% of placebo group).
Among these three quarters of patients who took at least 80% of their doses, benefits were better.
Fluvoxamine cut serious complications in this group by 66% and reduced mortality by 91%. In the placebo group, 12 people died compared with one who received the study drug.
from complications of the infection.
However, clinicians should note that the drug can cause side effects such as nausea, dizziness, and insomnia, she added. In addition, because it prevents the body from metabolizing caffeine, patients should limit their daily intake to half of a small cup of coffee or one can of soda or one tea while taking the drug.
Previous research has shown that fluvoxamine affects the metabolism of some drugs, such as theophylline, clozapine, olanzapine, and tizanidine.
Despite huge challenges with studying generic drugs as early COVID-19 treatment, the TOGETHER trial shows it is possible to produce quality evidence during a pandemic on a shoestring budget, noted co-principal investigator Edward Mills, PhD, professor in the department of health research methods, evidence, and impact at McMaster University.
To screen more than 12,000 patients and enroll 4,000 to test nine interventions, “our total budget was less than $8 million,” Dr. Mills said. The trial was funded by Fast Grants and the Rainwater Charitable Foundation.
‘A $10 medicine’
Commenting on the findings, David Boulware, MD, MPH, an infectious disease physician-researcher at the University of Minnesota in Minneapolis, noted fluvoxamine is “a $10 medicine that’s available and has a very good safety record.”
By comparison, a 5-day course of Merck’s antiviral molnupiravir, another oral drug that the company says can cut hospitalizations in COVID-19 outpatients, costs $700. However, the data have not been peer reviewed – and molnupiravir is not currently available and has unknown long-term safety implications, Dr. Boulware said.
Pharmaceutical companies typically spend tens of thousands of dollars on a trial evaluating a single drug, he noted.
In addition, the National Institutes of Health’s ACTIV-6 study, a nationwide trial on the effect of fluvoxamine and other repurposed generic drugs on thousands of COVID-19 outpatients, is a $110 million effort, according to Dr. Boulware, who cochairs its steering committee.
ACTIV-6 is currently enrolling outpatients with COVID-19 to test a lower dose of fluvoxamine, at 50 mg twice daily instead of the 100-mg dose used in the TOGETHER trial, as well as ivermectin and inhaled fluticasone. The COVID-OUT trial is also recruiting newly diagnosed COVID-19 patients to test various combinations of fluvoxamine, ivermectin, and the diabetes drug metformin.
Unanswered safety, efficacy questions
In an accompanying editorial in The Lancet Global Health, Otavio Berwanger, MD, cardiologist and clinical trialist, Academic Research Organization, Hospital Israelita Albert Einstein, São Paulo, Brazil, commends the investigators for rapidly generating evidence during the COVID-19 pandemic.
However, despite the important findings, “some questions related to efficacy and safety of fluvoxamine for patients with COVID-19 remain open,” Dr. Berwanger wrote.
The effects of the drug on reducing both mortality and hospitalizations also “still need addressing,” he noted.
“In addition, it remains to be established whether fluvoxamine has an additive effect to other therapies such as monoclonal antibodies and budesonide, and what is the optimal fluvoxamine therapeutic scheme,” wrote Dr. Berwanger.
In an interview, he noted that 74% of the Brazil population have currently received at least one dose of a COVID-19 vaccine and 52% have received two doses. In addition, deaths have gone down from 4,000 per day during the March-April second wave to about 400 per day. “That is still unfortunate and far from ideal,” he said. In total, they have had about 600,000 deaths because of COVID-19.
Asked whether public health authorities are now recommending fluvoxamine as an early treatment for COVID-19 based on the TOGETHER trial data, Dr. Berwanger answered, “Not yet.
“I believe medical and scientific societies will need to critically appraise the manuscript in order to inform their decisions and recommendations. This interesting trial adds another important piece of information in this regard,” he said.
Dr. Reiersen and Dr. Lenze are inventors on a patent application related to methods for treating COVID-19, which was filed by Washington University. Dr. Mills reports no relevant financial relationships, as does Dr. Boulware – except that the TOGETHER trial funders are also funding the University of Minnesota COVID-OUT trial. Dr. Berwanger reports having received research grants outside of the submitted work that were paid to his institution by AstraZeneca, Bayer, Amgen, Servier, Novartis, Pfizer, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
The antidepressant fluvoxamine (Luvox) may prevent hospitalization and death in outpatients with COVID-19, new research suggests.
Results from the placebo-controlled, multisite, phase 3 TOGETHER trial showed that in COVID-19 outpatients at high risk for complications, hospitalizations were cut by 66% and deaths were reduced by 91% in those who tolerated fluvoxamine.
“Our trial has found that fluvoxamine, an inexpensive existing drug, reduces the need for advanced disease care in this high-risk population,” wrote the investigators, led by Gilmar Reis, MD, PhD, research division, Cardresearch, Belo Horizonte, Brazil.
The findings were published online Oct. 27 in The Lancet Global Health.
Alternative mechanisms
Fluvoxamine, a selective serotonin reuptake inhibitor (SSRI), is an antidepressant commonly prescribed for obsessive-compulsive disorder.
Besides its known effects on serotonin, the drug acts in other molecular pathways to dampen the production of inflammatory cytokines. Those alternative mechanisms are the ones believed to help patients with COVID-19, said coinvestigator Angela Reiersen, MD, child psychiatrist at Washington University, St. Louis.
Based on cell culture and mouse studies showing effects of the molecule’s binding to the sigma-1 receptor in the endoplasmic reticulum, Dr. Reiersen came up with the idea of testing if fluvoxamine could keep COVID-19 from progressing in newly infected patients.
Dr. Reiersen and psychiatrist Eric Lenze, MD, also from Washington University, led the phase 2 trial that initially suggested fluvoxamine’s promise as an outpatient medication. They are coinvestigators on the new phase 3 adaptive platform trial called TOGETHER, which was conducted by an international team of investigators in Brazil, Canada, and the United States.
For this latest study, researchers at McMaster University, Hamilton, Ont., partnered with the research clinic Cardresearch in Brazil to recruit unvaccinated, high-risk adults within 7 days of developing flu-like symptoms from COVID-19. They analyzed 1,497 newly symptomatic COVID-19 patients at 11 clinical sites in Brazil.
Patients entered the trial between January and August 2021 and were assigned to receive 100 mg fluvoxamine or placebo pills twice a day for 10 days. Investigators monitored participants through 28 days post treatment, noting whether complications developed requiring hospitalization or more than 6 hours of emergency care.
In the placebo group, 119 of 756 patients (15.7%) worsened to this extent. In comparison, 79 of 741 (10.7%) fluvoxamine-treated patients met these primary criteria. This represented a 32% reduction in hospitalizations and emergency visits.
Additional analysis requested
As Lancet Global Health reviewed these findings from the submitted manuscript, journal reviewers requested an additional “pre-protocol analysis” that was not specified in the trial’s original protocol. The request was to examine the subgroup of patients with good adherence (74% of treated group, 82% of placebo group).
Among these three quarters of patients who took at least 80% of their doses, benefits were better.
Fluvoxamine cut serious complications in this group by 66% and reduced mortality by 91%. In the placebo group, 12 people died compared with one who received the study drug.
from complications of the infection.
However, clinicians should note that the drug can cause side effects such as nausea, dizziness, and insomnia, she added. In addition, because it prevents the body from metabolizing caffeine, patients should limit their daily intake to half of a small cup of coffee or one can of soda or one tea while taking the drug.
Previous research has shown that fluvoxamine affects the metabolism of some drugs, such as theophylline, clozapine, olanzapine, and tizanidine.
Despite huge challenges with studying generic drugs as early COVID-19 treatment, the TOGETHER trial shows it is possible to produce quality evidence during a pandemic on a shoestring budget, noted co-principal investigator Edward Mills, PhD, professor in the department of health research methods, evidence, and impact at McMaster University.
To screen more than 12,000 patients and enroll 4,000 to test nine interventions, “our total budget was less than $8 million,” Dr. Mills said. The trial was funded by Fast Grants and the Rainwater Charitable Foundation.
‘A $10 medicine’
Commenting on the findings, David Boulware, MD, MPH, an infectious disease physician-researcher at the University of Minnesota in Minneapolis, noted fluvoxamine is “a $10 medicine that’s available and has a very good safety record.”
By comparison, a 5-day course of Merck’s antiviral molnupiravir, another oral drug that the company says can cut hospitalizations in COVID-19 outpatients, costs $700. However, the data have not been peer reviewed – and molnupiravir is not currently available and has unknown long-term safety implications, Dr. Boulware said.
Pharmaceutical companies typically spend tens of thousands of dollars on a trial evaluating a single drug, he noted.
In addition, the National Institutes of Health’s ACTIV-6 study, a nationwide trial on the effect of fluvoxamine and other repurposed generic drugs on thousands of COVID-19 outpatients, is a $110 million effort, according to Dr. Boulware, who cochairs its steering committee.
ACTIV-6 is currently enrolling outpatients with COVID-19 to test a lower dose of fluvoxamine, at 50 mg twice daily instead of the 100-mg dose used in the TOGETHER trial, as well as ivermectin and inhaled fluticasone. The COVID-OUT trial is also recruiting newly diagnosed COVID-19 patients to test various combinations of fluvoxamine, ivermectin, and the diabetes drug metformin.
Unanswered safety, efficacy questions
In an accompanying editorial in The Lancet Global Health, Otavio Berwanger, MD, cardiologist and clinical trialist, Academic Research Organization, Hospital Israelita Albert Einstein, São Paulo, Brazil, commends the investigators for rapidly generating evidence during the COVID-19 pandemic.
However, despite the important findings, “some questions related to efficacy and safety of fluvoxamine for patients with COVID-19 remain open,” Dr. Berwanger wrote.
The effects of the drug on reducing both mortality and hospitalizations also “still need addressing,” he noted.
“In addition, it remains to be established whether fluvoxamine has an additive effect to other therapies such as monoclonal antibodies and budesonide, and what is the optimal fluvoxamine therapeutic scheme,” wrote Dr. Berwanger.
In an interview, he noted that 74% of the Brazil population have currently received at least one dose of a COVID-19 vaccine and 52% have received two doses. In addition, deaths have gone down from 4,000 per day during the March-April second wave to about 400 per day. “That is still unfortunate and far from ideal,” he said. In total, they have had about 600,000 deaths because of COVID-19.
Asked whether public health authorities are now recommending fluvoxamine as an early treatment for COVID-19 based on the TOGETHER trial data, Dr. Berwanger answered, “Not yet.
“I believe medical and scientific societies will need to critically appraise the manuscript in order to inform their decisions and recommendations. This interesting trial adds another important piece of information in this regard,” he said.
Dr. Reiersen and Dr. Lenze are inventors on a patent application related to methods for treating COVID-19, which was filed by Washington University. Dr. Mills reports no relevant financial relationships, as does Dr. Boulware – except that the TOGETHER trial funders are also funding the University of Minnesota COVID-OUT trial. Dr. Berwanger reports having received research grants outside of the submitted work that were paid to his institution by AstraZeneca, Bayer, Amgen, Servier, Novartis, Pfizer, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
The antidepressant fluvoxamine (Luvox) may prevent hospitalization and death in outpatients with COVID-19, new research suggests.
Results from the placebo-controlled, multisite, phase 3 TOGETHER trial showed that in COVID-19 outpatients at high risk for complications, hospitalizations were cut by 66% and deaths were reduced by 91% in those who tolerated fluvoxamine.
“Our trial has found that fluvoxamine, an inexpensive existing drug, reduces the need for advanced disease care in this high-risk population,” wrote the investigators, led by Gilmar Reis, MD, PhD, research division, Cardresearch, Belo Horizonte, Brazil.
The findings were published online Oct. 27 in The Lancet Global Health.
Alternative mechanisms
Fluvoxamine, a selective serotonin reuptake inhibitor (SSRI), is an antidepressant commonly prescribed for obsessive-compulsive disorder.
Besides its known effects on serotonin, the drug acts in other molecular pathways to dampen the production of inflammatory cytokines. Those alternative mechanisms are the ones believed to help patients with COVID-19, said coinvestigator Angela Reiersen, MD, child psychiatrist at Washington University, St. Louis.
Based on cell culture and mouse studies showing effects of the molecule’s binding to the sigma-1 receptor in the endoplasmic reticulum, Dr. Reiersen came up with the idea of testing if fluvoxamine could keep COVID-19 from progressing in newly infected patients.
Dr. Reiersen and psychiatrist Eric Lenze, MD, also from Washington University, led the phase 2 trial that initially suggested fluvoxamine’s promise as an outpatient medication. They are coinvestigators on the new phase 3 adaptive platform trial called TOGETHER, which was conducted by an international team of investigators in Brazil, Canada, and the United States.
For this latest study, researchers at McMaster University, Hamilton, Ont., partnered with the research clinic Cardresearch in Brazil to recruit unvaccinated, high-risk adults within 7 days of developing flu-like symptoms from COVID-19. They analyzed 1,497 newly symptomatic COVID-19 patients at 11 clinical sites in Brazil.
Patients entered the trial between January and August 2021 and were assigned to receive 100 mg fluvoxamine or placebo pills twice a day for 10 days. Investigators monitored participants through 28 days post treatment, noting whether complications developed requiring hospitalization or more than 6 hours of emergency care.
In the placebo group, 119 of 756 patients (15.7%) worsened to this extent. In comparison, 79 of 741 (10.7%) fluvoxamine-treated patients met these primary criteria. This represented a 32% reduction in hospitalizations and emergency visits.
Additional analysis requested
As Lancet Global Health reviewed these findings from the submitted manuscript, journal reviewers requested an additional “pre-protocol analysis” that was not specified in the trial’s original protocol. The request was to examine the subgroup of patients with good adherence (74% of treated group, 82% of placebo group).
Among these three quarters of patients who took at least 80% of their doses, benefits were better.
Fluvoxamine cut serious complications in this group by 66% and reduced mortality by 91%. In the placebo group, 12 people died compared with one who received the study drug.
from complications of the infection.
However, clinicians should note that the drug can cause side effects such as nausea, dizziness, and insomnia, she added. In addition, because it prevents the body from metabolizing caffeine, patients should limit their daily intake to half of a small cup of coffee or one can of soda or one tea while taking the drug.
Previous research has shown that fluvoxamine affects the metabolism of some drugs, such as theophylline, clozapine, olanzapine, and tizanidine.
Despite huge challenges with studying generic drugs as early COVID-19 treatment, the TOGETHER trial shows it is possible to produce quality evidence during a pandemic on a shoestring budget, noted co-principal investigator Edward Mills, PhD, professor in the department of health research methods, evidence, and impact at McMaster University.
To screen more than 12,000 patients and enroll 4,000 to test nine interventions, “our total budget was less than $8 million,” Dr. Mills said. The trial was funded by Fast Grants and the Rainwater Charitable Foundation.
‘A $10 medicine’
Commenting on the findings, David Boulware, MD, MPH, an infectious disease physician-researcher at the University of Minnesota in Minneapolis, noted fluvoxamine is “a $10 medicine that’s available and has a very good safety record.”
By comparison, a 5-day course of Merck’s antiviral molnupiravir, another oral drug that the company says can cut hospitalizations in COVID-19 outpatients, costs $700. However, the data have not been peer reviewed – and molnupiravir is not currently available and has unknown long-term safety implications, Dr. Boulware said.
Pharmaceutical companies typically spend tens of thousands of dollars on a trial evaluating a single drug, he noted.
In addition, the National Institutes of Health’s ACTIV-6 study, a nationwide trial on the effect of fluvoxamine and other repurposed generic drugs on thousands of COVID-19 outpatients, is a $110 million effort, according to Dr. Boulware, who cochairs its steering committee.
ACTIV-6 is currently enrolling outpatients with COVID-19 to test a lower dose of fluvoxamine, at 50 mg twice daily instead of the 100-mg dose used in the TOGETHER trial, as well as ivermectin and inhaled fluticasone. The COVID-OUT trial is also recruiting newly diagnosed COVID-19 patients to test various combinations of fluvoxamine, ivermectin, and the diabetes drug metformin.
Unanswered safety, efficacy questions
In an accompanying editorial in The Lancet Global Health, Otavio Berwanger, MD, cardiologist and clinical trialist, Academic Research Organization, Hospital Israelita Albert Einstein, São Paulo, Brazil, commends the investigators for rapidly generating evidence during the COVID-19 pandemic.
However, despite the important findings, “some questions related to efficacy and safety of fluvoxamine for patients with COVID-19 remain open,” Dr. Berwanger wrote.
The effects of the drug on reducing both mortality and hospitalizations also “still need addressing,” he noted.
“In addition, it remains to be established whether fluvoxamine has an additive effect to other therapies such as monoclonal antibodies and budesonide, and what is the optimal fluvoxamine therapeutic scheme,” wrote Dr. Berwanger.
In an interview, he noted that 74% of the Brazil population have currently received at least one dose of a COVID-19 vaccine and 52% have received two doses. In addition, deaths have gone down from 4,000 per day during the March-April second wave to about 400 per day. “That is still unfortunate and far from ideal,” he said. In total, they have had about 600,000 deaths because of COVID-19.
Asked whether public health authorities are now recommending fluvoxamine as an early treatment for COVID-19 based on the TOGETHER trial data, Dr. Berwanger answered, “Not yet.
“I believe medical and scientific societies will need to critically appraise the manuscript in order to inform their decisions and recommendations. This interesting trial adds another important piece of information in this regard,” he said.
Dr. Reiersen and Dr. Lenze are inventors on a patent application related to methods for treating COVID-19, which was filed by Washington University. Dr. Mills reports no relevant financial relationships, as does Dr. Boulware – except that the TOGETHER trial funders are also funding the University of Minnesota COVID-OUT trial. Dr. Berwanger reports having received research grants outside of the submitted work that were paid to his institution by AstraZeneca, Bayer, Amgen, Servier, Novartis, Pfizer, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
In and out surgeries become the norm during pandemic
Urologist Ronney Abaza, MD, a robotic surgery specialist in Dublin, Ohio, and colleagues, reviewed robotic surgeries at their hospital during COVID-19 restrictions on surgery in Ohio between March 17 and June 5, 2020, and compared them with robotic procedures before COVID-19 and after restrictions were lifted. They published their results in Urology.
Since 2016, the hospital has offered the option of same-day discharge (SDD) to all robotic urologic surgery patients, regardless of procedure or patient-specific factors.
Among patients who had surgery during COVID-19 restrictions, 98% (87/89 patients) opted for SDD versus 52% in the group having surgery before the restrictions (P < .00001). After the COVID-19 surgery restrictions were lifted, the higher rate of SDD remained at 98%.
“There were no differences in 30-day complications or readmissions between SDD and overnight patients,” the authors write.
The right patient, the right motivation for successful surgery
Brian Lane, MD, PhD, a urologic oncologist with Spectrum Health in Grand Rapids, Michigan, told this news organization that, for nephrectomies, uptake of same-day discharge will continue to be slow.
“You have to have the right patient, the right patient motivation, and the surgery has to go smoothly,” he said. “If you start sending everyone home the same day, you will certainly see readmissions,” he said.
Dr. Lane is part of the Michigan Urologic Surgery Improvement Collaborative and he said the group recently looked at same-day discharge outcomes after robotic prostatectomies with SDD as compared with 1-2 nights in the hospital.
The work has not yet been published but, “There was a slight signal that there were increased readmissions with same-day discharge vs. 0-1 day,” he said.
A paper on outcomes of same-day discharge in total knee arthroplasty in the Journal of Bone & Joint Surgery found a higher risk of perioperative complications “including component failure, surgical site infection, knee stiffness, and deep vein thrombosis.” Researchers compared outcomes between 4,391 patients who underwent outpatient TKA and 128,951 patients who underwent inpatient TKA.
But for other many surgeries, same-day discharge numbers are increasing without worsening outcomes.
A paper in the Journal of Robotic Surgery found that same-day discharge following robotic-assisted endometrial cancer staging is “safe and feasible.”
Stephen Bradley, MD, MPH, with the Minneapolis Heart Institute in Minneapolis, and colleagues write in the Journal of the American College of Cardiology: Cardiovascular Interventions that they found a large increase in the use of same-day discharge after elective percutaneous coronary intervention (PCI) was not associated with worse 30-day mortality rates or readmission.
In that study, 114,461 patients were discharged the same day they underwent PCI. The proportion of patients who had a same-day discharge increased from 4.5% in 2009 to 28.6% in the fourth quarter of 2017.
Risk-adjusted 30-day mortality did not change in that time, while risk-adjusted rehospitalization decreased over time and more quickly when patients had same-day discharge.
Deepak L. Bhatt, MD, MPH, and Jonathan G. Sung, MBCHB, both of Brigham and Women’s Hospital Heart & Vascular Center, Harvard Medical School, Boston, wrote in an accompanying article that, “Advances in the devices and techniques of PCI have improved the safety and efficacy of the procedure. In selected patients, same-day discharge has become possible, and overnight in-hospital observation can be avoided. By reducing unnecessary hospital stays, both patients and hospitals could benefit.”
Evan Garden, a medical student at Icahn School of Medicine at Mount Sinai in New York, presented findings at the American Urological Association 2021 annual meeting that show patients selected for same-day discharge after partial or radical nephrectomy did not have increased rates of postoperative complications or readmissions in the immediate postoperative period, compared with standard discharge of 1-3 days.
Case studies in nephrectomy
While several case studies have looked at the feasibility and safety of performing partial and radical nephrectomy with same-day discharge in select cases, “this topic has not been addressed on a national level,” Mr. Garden said.
Few patients who have partial or radical nephrectomies have same-day discharges. The researchers found that fewer than 1% of patients who have either procedure in the sample studied were discharged the same day.
Researchers used the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database, a nationally representative deidentified database that prospectively tracks patient characteristics and 30-day perioperative outcomes for major inpatient and outpatient surgical procedures at more than 700 hospitals.
They extracted all minimally invasive partial and radical nephrectomies from 2012 to 2019 and refined the cohort to 28,140 patients who were theoretically eligible for same-day discharge: Of those, 237 (0.8%) had SSD, and 27,903 (99.2%) had a standard-length discharge (SLD).
The team found that there were no differences in 30-day complications or readmissions between same-day discharge (Clavien-Dindo [CD] I/II, 4.22%; CD III, 0%; CD IV, 1.27%; readmission, 4.64%); and SLD (CD I/II, 4.11%; CD III, 0.95%; CD IV, 0.79%; readmission, 3.90%; all P > .05).
Controlling for demographic and clinical variables, SDD was not associated with greater risk of 30-day complications or readmissions (CD I/II: odds ratio, 1.08; 95% confidence interval, 0.57-2.048; P = .813; CD IV: OR 1.699; 95% CI, 0.537-5.375; P = .367; readmission: OR, 1.254; 95% CI, 0.681-2.31; P = .467).
Mr. Garden and coauthors report no relevant financial relationships.
Dr. Lane reports no relevant financial relationships.
Urologist Ronney Abaza, MD, a robotic surgery specialist in Dublin, Ohio, and colleagues, reviewed robotic surgeries at their hospital during COVID-19 restrictions on surgery in Ohio between March 17 and June 5, 2020, and compared them with robotic procedures before COVID-19 and after restrictions were lifted. They published their results in Urology.
Since 2016, the hospital has offered the option of same-day discharge (SDD) to all robotic urologic surgery patients, regardless of procedure or patient-specific factors.
Among patients who had surgery during COVID-19 restrictions, 98% (87/89 patients) opted for SDD versus 52% in the group having surgery before the restrictions (P < .00001). After the COVID-19 surgery restrictions were lifted, the higher rate of SDD remained at 98%.
“There were no differences in 30-day complications or readmissions between SDD and overnight patients,” the authors write.
The right patient, the right motivation for successful surgery
Brian Lane, MD, PhD, a urologic oncologist with Spectrum Health in Grand Rapids, Michigan, told this news organization that, for nephrectomies, uptake of same-day discharge will continue to be slow.
“You have to have the right patient, the right patient motivation, and the surgery has to go smoothly,” he said. “If you start sending everyone home the same day, you will certainly see readmissions,” he said.
Dr. Lane is part of the Michigan Urologic Surgery Improvement Collaborative and he said the group recently looked at same-day discharge outcomes after robotic prostatectomies with SDD as compared with 1-2 nights in the hospital.
The work has not yet been published but, “There was a slight signal that there were increased readmissions with same-day discharge vs. 0-1 day,” he said.
A paper on outcomes of same-day discharge in total knee arthroplasty in the Journal of Bone & Joint Surgery found a higher risk of perioperative complications “including component failure, surgical site infection, knee stiffness, and deep vein thrombosis.” Researchers compared outcomes between 4,391 patients who underwent outpatient TKA and 128,951 patients who underwent inpatient TKA.
But for other many surgeries, same-day discharge numbers are increasing without worsening outcomes.
A paper in the Journal of Robotic Surgery found that same-day discharge following robotic-assisted endometrial cancer staging is “safe and feasible.”
Stephen Bradley, MD, MPH, with the Minneapolis Heart Institute in Minneapolis, and colleagues write in the Journal of the American College of Cardiology: Cardiovascular Interventions that they found a large increase in the use of same-day discharge after elective percutaneous coronary intervention (PCI) was not associated with worse 30-day mortality rates or readmission.
In that study, 114,461 patients were discharged the same day they underwent PCI. The proportion of patients who had a same-day discharge increased from 4.5% in 2009 to 28.6% in the fourth quarter of 2017.
Risk-adjusted 30-day mortality did not change in that time, while risk-adjusted rehospitalization decreased over time and more quickly when patients had same-day discharge.
Deepak L. Bhatt, MD, MPH, and Jonathan G. Sung, MBCHB, both of Brigham and Women’s Hospital Heart & Vascular Center, Harvard Medical School, Boston, wrote in an accompanying article that, “Advances in the devices and techniques of PCI have improved the safety and efficacy of the procedure. In selected patients, same-day discharge has become possible, and overnight in-hospital observation can be avoided. By reducing unnecessary hospital stays, both patients and hospitals could benefit.”
Evan Garden, a medical student at Icahn School of Medicine at Mount Sinai in New York, presented findings at the American Urological Association 2021 annual meeting that show patients selected for same-day discharge after partial or radical nephrectomy did not have increased rates of postoperative complications or readmissions in the immediate postoperative period, compared with standard discharge of 1-3 days.
Case studies in nephrectomy
While several case studies have looked at the feasibility and safety of performing partial and radical nephrectomy with same-day discharge in select cases, “this topic has not been addressed on a national level,” Mr. Garden said.
Few patients who have partial or radical nephrectomies have same-day discharges. The researchers found that fewer than 1% of patients who have either procedure in the sample studied were discharged the same day.
Researchers used the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database, a nationally representative deidentified database that prospectively tracks patient characteristics and 30-day perioperative outcomes for major inpatient and outpatient surgical procedures at more than 700 hospitals.
They extracted all minimally invasive partial and radical nephrectomies from 2012 to 2019 and refined the cohort to 28,140 patients who were theoretically eligible for same-day discharge: Of those, 237 (0.8%) had SSD, and 27,903 (99.2%) had a standard-length discharge (SLD).
The team found that there were no differences in 30-day complications or readmissions between same-day discharge (Clavien-Dindo [CD] I/II, 4.22%; CD III, 0%; CD IV, 1.27%; readmission, 4.64%); and SLD (CD I/II, 4.11%; CD III, 0.95%; CD IV, 0.79%; readmission, 3.90%; all P > .05).
Controlling for demographic and clinical variables, SDD was not associated with greater risk of 30-day complications or readmissions (CD I/II: odds ratio, 1.08; 95% confidence interval, 0.57-2.048; P = .813; CD IV: OR 1.699; 95% CI, 0.537-5.375; P = .367; readmission: OR, 1.254; 95% CI, 0.681-2.31; P = .467).
Mr. Garden and coauthors report no relevant financial relationships.
Dr. Lane reports no relevant financial relationships.
Urologist Ronney Abaza, MD, a robotic surgery specialist in Dublin, Ohio, and colleagues, reviewed robotic surgeries at their hospital during COVID-19 restrictions on surgery in Ohio between March 17 and June 5, 2020, and compared them with robotic procedures before COVID-19 and after restrictions were lifted. They published their results in Urology.
Since 2016, the hospital has offered the option of same-day discharge (SDD) to all robotic urologic surgery patients, regardless of procedure or patient-specific factors.
Among patients who had surgery during COVID-19 restrictions, 98% (87/89 patients) opted for SDD versus 52% in the group having surgery before the restrictions (P < .00001). After the COVID-19 surgery restrictions were lifted, the higher rate of SDD remained at 98%.
“There were no differences in 30-day complications or readmissions between SDD and overnight patients,” the authors write.
The right patient, the right motivation for successful surgery
Brian Lane, MD, PhD, a urologic oncologist with Spectrum Health in Grand Rapids, Michigan, told this news organization that, for nephrectomies, uptake of same-day discharge will continue to be slow.
“You have to have the right patient, the right patient motivation, and the surgery has to go smoothly,” he said. “If you start sending everyone home the same day, you will certainly see readmissions,” he said.
Dr. Lane is part of the Michigan Urologic Surgery Improvement Collaborative and he said the group recently looked at same-day discharge outcomes after robotic prostatectomies with SDD as compared with 1-2 nights in the hospital.
The work has not yet been published but, “There was a slight signal that there were increased readmissions with same-day discharge vs. 0-1 day,” he said.
A paper on outcomes of same-day discharge in total knee arthroplasty in the Journal of Bone & Joint Surgery found a higher risk of perioperative complications “including component failure, surgical site infection, knee stiffness, and deep vein thrombosis.” Researchers compared outcomes between 4,391 patients who underwent outpatient TKA and 128,951 patients who underwent inpatient TKA.
But for other many surgeries, same-day discharge numbers are increasing without worsening outcomes.
A paper in the Journal of Robotic Surgery found that same-day discharge following robotic-assisted endometrial cancer staging is “safe and feasible.”
Stephen Bradley, MD, MPH, with the Minneapolis Heart Institute in Minneapolis, and colleagues write in the Journal of the American College of Cardiology: Cardiovascular Interventions that they found a large increase in the use of same-day discharge after elective percutaneous coronary intervention (PCI) was not associated with worse 30-day mortality rates or readmission.
In that study, 114,461 patients were discharged the same day they underwent PCI. The proportion of patients who had a same-day discharge increased from 4.5% in 2009 to 28.6% in the fourth quarter of 2017.
Risk-adjusted 30-day mortality did not change in that time, while risk-adjusted rehospitalization decreased over time and more quickly when patients had same-day discharge.
Deepak L. Bhatt, MD, MPH, and Jonathan G. Sung, MBCHB, both of Brigham and Women’s Hospital Heart & Vascular Center, Harvard Medical School, Boston, wrote in an accompanying article that, “Advances in the devices and techniques of PCI have improved the safety and efficacy of the procedure. In selected patients, same-day discharge has become possible, and overnight in-hospital observation can be avoided. By reducing unnecessary hospital stays, both patients and hospitals could benefit.”
Evan Garden, a medical student at Icahn School of Medicine at Mount Sinai in New York, presented findings at the American Urological Association 2021 annual meeting that show patients selected for same-day discharge after partial or radical nephrectomy did not have increased rates of postoperative complications or readmissions in the immediate postoperative period, compared with standard discharge of 1-3 days.
Case studies in nephrectomy
While several case studies have looked at the feasibility and safety of performing partial and radical nephrectomy with same-day discharge in select cases, “this topic has not been addressed on a national level,” Mr. Garden said.
Few patients who have partial or radical nephrectomies have same-day discharges. The researchers found that fewer than 1% of patients who have either procedure in the sample studied were discharged the same day.
Researchers used the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database, a nationally representative deidentified database that prospectively tracks patient characteristics and 30-day perioperative outcomes for major inpatient and outpatient surgical procedures at more than 700 hospitals.
They extracted all minimally invasive partial and radical nephrectomies from 2012 to 2019 and refined the cohort to 28,140 patients who were theoretically eligible for same-day discharge: Of those, 237 (0.8%) had SSD, and 27,903 (99.2%) had a standard-length discharge (SLD).
The team found that there were no differences in 30-day complications or readmissions between same-day discharge (Clavien-Dindo [CD] I/II, 4.22%; CD III, 0%; CD IV, 1.27%; readmission, 4.64%); and SLD (CD I/II, 4.11%; CD III, 0.95%; CD IV, 0.79%; readmission, 3.90%; all P > .05).
Controlling for demographic and clinical variables, SDD was not associated with greater risk of 30-day complications or readmissions (CD I/II: odds ratio, 1.08; 95% confidence interval, 0.57-2.048; P = .813; CD IV: OR 1.699; 95% CI, 0.537-5.375; P = .367; readmission: OR, 1.254; 95% CI, 0.681-2.31; P = .467).
Mr. Garden and coauthors report no relevant financial relationships.
Dr. Lane reports no relevant financial relationships.
AHA/ACC issues first comprehensive guidance on chest pain
Clinicians should use standardized risk assessments, clinical pathways, and tools to evaluate and communicate with patients who present with chest pain (angina), advises a joint clinical practice guideline released by American Heart Association and American College of Cardiology.
While evaluation of chest pain has been covered in previous guidelines, this is the first comprehensive guideline from the AHA and ACC focused exclusively on the evaluation and diagnosis of chest pain.
“As our imaging technologies have evolved, we needed a contemporary approach to which patients need further testing, and which do not, in addition to what testing is effective,” Martha Gulati, MD, University of Arizona, Phoenix, and chair of the guideline writing group, said in an interview.
“Our hope is that we have provided an evidence-based approach to evaluating patients that will assist all of us who manage, diagnose, and treat patients who experience chest pain,” said Dr. Gulati, who is also president-elect of the American Society for Preventive Cardiology.
The guideline was simultaneously published online Oct. 28, 2021, in Circulation and the Journal of the American College of Cardiology.
‘Atypical’ is out, ‘noncardiac’ is in
Each year, chest pain sends more than 6.5 million adults to the ED and more than 4 million to outpatient clinics in the United States.
Yet, among all patients who come to the ED, only 5% will have acute coronary syndrome (ACS). More than half will ultimately have a noncardiac reason for their chest pain, including respiratory, musculoskeletal, gastrointestinal, psychological, or other causes.
The guideline says evaluating the severity and the cause of chest pain is essential and advises using standard risk assessments to determine if a patient is at low, intermediate, or high risk for having a cardiac event.
“I hope clinicians take from our guidelines the understanding that low-risk patients often do not need additional testing. And if we communicate this effectively with our patients – incorporating shared decision-making into our practice – we can reduce ‘overtesting’ in low-risk patients,” Dr. Gulati said in an interview.
The guideline notes that women are unique when presenting with ACS symptoms. While chest pain is the dominant and most common symptom for both men and women, women may be more likely to also have symptoms such as nausea and shortness of breath.
The guideline also encourages using the term “noncardiac” if heart disease is not suspected in a patient with angina and says the term “atypical” is a “misleading” descriptor of chest pain and should not be used.
“Words matter, and we need to move away from describing chest pain as ‘atypical’ because it has resulted in confusion when these words are used,” Dr. Gulati stressed.
“Rather than meaning a different way of presenting, it has taken on a meaning to imply it is not cardiac. It is more useful to talk about the probability of the pain being cardiac vs noncardiac,” Dr. Gulati explained.
No one best test for everyone
There is also a focus on evaluation of patients with chest pain who present to the ED. The initial goals of ED physicians should be to identify if there are life-threatening causes and to determine if there is a need for hospital admission or testing, the guideline states.
Thorough screening in the ED may help determine who is at high risk versus intermediate or low risk for a cardiac event. An individual deemed to be at low risk may be referred for additional evaluation in an outpatient setting rather than being admitted to the hospital, the authors wrote.
High-sensitivity cardiac troponins are the “preferred standard” for establishing a biomarker diagnosis of acute myocardial infarction, allowing for more accurate detection and exclusion of myocardial injury, they added.
“While there is no one ‘best test’ for every patient, the guideline emphasizes the tests that may be most appropriate, depending on the individual situation, and which ones won’t provide additional information; therefore, these tests should not be done just for the sake of doing them,” Dr. Gulati said in a news release.
“Appropriate testing is also dependent upon the technology and screening devices that are available at the hospital or healthcare center where the patient is receiving care. All imaging modalities highlighted in the guideline have an important role in the assessment of chest pain to help determine the underlying cause, with the goal of preventing a serious cardiac event,” Dr. Gulati added.
The guideline was prepared on behalf of and approved by the AHA and ACC Joint Committee on Clinical Practice Guidelines.
Five other partnering organizations participated in and approved the guideline: the American Society of Echocardiography, the American College of Chest Physicians, the Society for Academic Emergency Medicine, the Society of Cardiovascular Computed Tomography, and the Society for Cardiovascular Magnetic Resonance.
The writing group included representatives from each of the partnering organizations and experts in the field (cardiac intensivists, cardiac interventionalists, cardiac surgeons, cardiologists, emergency physicians, and epidemiologists), as well as a lay/patient representative.
The research had no commercial funding.
A version of this article first appeared on Medscape.com.
Clinicians should use standardized risk assessments, clinical pathways, and tools to evaluate and communicate with patients who present with chest pain (angina), advises a joint clinical practice guideline released by American Heart Association and American College of Cardiology.
While evaluation of chest pain has been covered in previous guidelines, this is the first comprehensive guideline from the AHA and ACC focused exclusively on the evaluation and diagnosis of chest pain.
“As our imaging technologies have evolved, we needed a contemporary approach to which patients need further testing, and which do not, in addition to what testing is effective,” Martha Gulati, MD, University of Arizona, Phoenix, and chair of the guideline writing group, said in an interview.
“Our hope is that we have provided an evidence-based approach to evaluating patients that will assist all of us who manage, diagnose, and treat patients who experience chest pain,” said Dr. Gulati, who is also president-elect of the American Society for Preventive Cardiology.
The guideline was simultaneously published online Oct. 28, 2021, in Circulation and the Journal of the American College of Cardiology.
‘Atypical’ is out, ‘noncardiac’ is in
Each year, chest pain sends more than 6.5 million adults to the ED and more than 4 million to outpatient clinics in the United States.
Yet, among all patients who come to the ED, only 5% will have acute coronary syndrome (ACS). More than half will ultimately have a noncardiac reason for their chest pain, including respiratory, musculoskeletal, gastrointestinal, psychological, or other causes.
The guideline says evaluating the severity and the cause of chest pain is essential and advises using standard risk assessments to determine if a patient is at low, intermediate, or high risk for having a cardiac event.
“I hope clinicians take from our guidelines the understanding that low-risk patients often do not need additional testing. And if we communicate this effectively with our patients – incorporating shared decision-making into our practice – we can reduce ‘overtesting’ in low-risk patients,” Dr. Gulati said in an interview.
The guideline notes that women are unique when presenting with ACS symptoms. While chest pain is the dominant and most common symptom for both men and women, women may be more likely to also have symptoms such as nausea and shortness of breath.
The guideline also encourages using the term “noncardiac” if heart disease is not suspected in a patient with angina and says the term “atypical” is a “misleading” descriptor of chest pain and should not be used.
“Words matter, and we need to move away from describing chest pain as ‘atypical’ because it has resulted in confusion when these words are used,” Dr. Gulati stressed.
“Rather than meaning a different way of presenting, it has taken on a meaning to imply it is not cardiac. It is more useful to talk about the probability of the pain being cardiac vs noncardiac,” Dr. Gulati explained.
No one best test for everyone
There is also a focus on evaluation of patients with chest pain who present to the ED. The initial goals of ED physicians should be to identify if there are life-threatening causes and to determine if there is a need for hospital admission or testing, the guideline states.
Thorough screening in the ED may help determine who is at high risk versus intermediate or low risk for a cardiac event. An individual deemed to be at low risk may be referred for additional evaluation in an outpatient setting rather than being admitted to the hospital, the authors wrote.
High-sensitivity cardiac troponins are the “preferred standard” for establishing a biomarker diagnosis of acute myocardial infarction, allowing for more accurate detection and exclusion of myocardial injury, they added.
“While there is no one ‘best test’ for every patient, the guideline emphasizes the tests that may be most appropriate, depending on the individual situation, and which ones won’t provide additional information; therefore, these tests should not be done just for the sake of doing them,” Dr. Gulati said in a news release.
“Appropriate testing is also dependent upon the technology and screening devices that are available at the hospital or healthcare center where the patient is receiving care. All imaging modalities highlighted in the guideline have an important role in the assessment of chest pain to help determine the underlying cause, with the goal of preventing a serious cardiac event,” Dr. Gulati added.
The guideline was prepared on behalf of and approved by the AHA and ACC Joint Committee on Clinical Practice Guidelines.
Five other partnering organizations participated in and approved the guideline: the American Society of Echocardiography, the American College of Chest Physicians, the Society for Academic Emergency Medicine, the Society of Cardiovascular Computed Tomography, and the Society for Cardiovascular Magnetic Resonance.
The writing group included representatives from each of the partnering organizations and experts in the field (cardiac intensivists, cardiac interventionalists, cardiac surgeons, cardiologists, emergency physicians, and epidemiologists), as well as a lay/patient representative.
The research had no commercial funding.
A version of this article first appeared on Medscape.com.
Clinicians should use standardized risk assessments, clinical pathways, and tools to evaluate and communicate with patients who present with chest pain (angina), advises a joint clinical practice guideline released by American Heart Association and American College of Cardiology.
While evaluation of chest pain has been covered in previous guidelines, this is the first comprehensive guideline from the AHA and ACC focused exclusively on the evaluation and diagnosis of chest pain.
“As our imaging technologies have evolved, we needed a contemporary approach to which patients need further testing, and which do not, in addition to what testing is effective,” Martha Gulati, MD, University of Arizona, Phoenix, and chair of the guideline writing group, said in an interview.
“Our hope is that we have provided an evidence-based approach to evaluating patients that will assist all of us who manage, diagnose, and treat patients who experience chest pain,” said Dr. Gulati, who is also president-elect of the American Society for Preventive Cardiology.
The guideline was simultaneously published online Oct. 28, 2021, in Circulation and the Journal of the American College of Cardiology.
‘Atypical’ is out, ‘noncardiac’ is in
Each year, chest pain sends more than 6.5 million adults to the ED and more than 4 million to outpatient clinics in the United States.
Yet, among all patients who come to the ED, only 5% will have acute coronary syndrome (ACS). More than half will ultimately have a noncardiac reason for their chest pain, including respiratory, musculoskeletal, gastrointestinal, psychological, or other causes.
The guideline says evaluating the severity and the cause of chest pain is essential and advises using standard risk assessments to determine if a patient is at low, intermediate, or high risk for having a cardiac event.
“I hope clinicians take from our guidelines the understanding that low-risk patients often do not need additional testing. And if we communicate this effectively with our patients – incorporating shared decision-making into our practice – we can reduce ‘overtesting’ in low-risk patients,” Dr. Gulati said in an interview.
The guideline notes that women are unique when presenting with ACS symptoms. While chest pain is the dominant and most common symptom for both men and women, women may be more likely to also have symptoms such as nausea and shortness of breath.
The guideline also encourages using the term “noncardiac” if heart disease is not suspected in a patient with angina and says the term “atypical” is a “misleading” descriptor of chest pain and should not be used.
“Words matter, and we need to move away from describing chest pain as ‘atypical’ because it has resulted in confusion when these words are used,” Dr. Gulati stressed.
“Rather than meaning a different way of presenting, it has taken on a meaning to imply it is not cardiac. It is more useful to talk about the probability of the pain being cardiac vs noncardiac,” Dr. Gulati explained.
No one best test for everyone
There is also a focus on evaluation of patients with chest pain who present to the ED. The initial goals of ED physicians should be to identify if there are life-threatening causes and to determine if there is a need for hospital admission or testing, the guideline states.
Thorough screening in the ED may help determine who is at high risk versus intermediate or low risk for a cardiac event. An individual deemed to be at low risk may be referred for additional evaluation in an outpatient setting rather than being admitted to the hospital, the authors wrote.
High-sensitivity cardiac troponins are the “preferred standard” for establishing a biomarker diagnosis of acute myocardial infarction, allowing for more accurate detection and exclusion of myocardial injury, they added.
“While there is no one ‘best test’ for every patient, the guideline emphasizes the tests that may be most appropriate, depending on the individual situation, and which ones won’t provide additional information; therefore, these tests should not be done just for the sake of doing them,” Dr. Gulati said in a news release.
“Appropriate testing is also dependent upon the technology and screening devices that are available at the hospital or healthcare center where the patient is receiving care. All imaging modalities highlighted in the guideline have an important role in the assessment of chest pain to help determine the underlying cause, with the goal of preventing a serious cardiac event,” Dr. Gulati added.
The guideline was prepared on behalf of and approved by the AHA and ACC Joint Committee on Clinical Practice Guidelines.
Five other partnering organizations participated in and approved the guideline: the American Society of Echocardiography, the American College of Chest Physicians, the Society for Academic Emergency Medicine, the Society of Cardiovascular Computed Tomography, and the Society for Cardiovascular Magnetic Resonance.
The writing group included representatives from each of the partnering organizations and experts in the field (cardiac intensivists, cardiac interventionalists, cardiac surgeons, cardiologists, emergency physicians, and epidemiologists), as well as a lay/patient representative.
The research had no commercial funding.
A version of this article first appeared on Medscape.com.
FDA panel votes to approve Pfizer’s vaccine for children
Seventeen of the 18 members of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) on Oct. 26 voted to recommend the 10-microgram shot for kids, which is one-third the dose given to adults.
One member, Michael Kurilla, MD, director of the division of clinical innovation at the National Institutes of Health, Bethesda, Md., abstained from voting.
If the FDA follows the recommendation, as it typically does, and issues an Emergency Use Authorization for the vaccine, the shots could be available within days.
After the FDA’s final decision, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will meet to make specific recommendations for its use. The CDC committee must stick closely to the conditions for use spelled out in the EUA, so their recommendations are likely to be similar to those made by the FDA. Their next meeting is scheduled for Nov. 2 and 3.
In the end, some on the panel felt uneasy with their decision.
“I voted yes primarily because I wanted to make sure that children who really need this vaccine, the Black and brown children of our country, get the vaccine,” said James Hildreth, MD, PhD, president and CEO of Meharry Medical College in Nashville.
“But to be honest, the best way to protect the health of some children will be to do nothing because they will be just fine,” he said.
Others said they were surprised by how difficult the decision had been.
“This is a much tougher one than we had expected going into it,” said committee member Eric Rubin, MD, editor and chief of the New England Journal of Medicine, during the FDA advisory committee’s meeting.
Ahead of the vote, the committee heard presentations outlining the expected benefits of vaccinating children along with potential risks.
“Children have been greatly impacted by the pandemic,” said Fiona Havers, MD, a medical officer with the CDC in Atlanta who reviewed the epidemiology of COVID-19 in kids.
In the second year of the pandemic, as more seniors have been vaccinated against the virus, COVID cases have largely shifted from older to younger age groups.
So far, there have been more than 1.9 million COVID-19 cases in children ages 5 through 11 in the United States.. Cases in kids saw a big jump in July and August with summer travel, schools reopening, and the dominance of the Delta variant.
And those are just the cases reported to the CDC. Regular testing of anonymous blood samples collected at sites across the United States indicates that 6 times as many kids have had COVID than what is reflected in official counts.
Last winter, blood sample testing showed about 13% of children had antibodies against the virus, suggesting they’d been infected. By this summer, that number had risen to 42%.
That figure clearly made an impression on many members of the committee who asked the FDA’s vaccine reviewers if they had tried to account for immunity from past infections in their modeling. They had not.
Some felt that even with a highly effective vaccine — new data presented by Pfizer showed the children’s dose was 90% effective at preventing symptomatic infections in kids — caution was warranted as much is still unknown about myocarditis, a rare side effect of the mRNA vaccines.
Myocarditis has been more common in younger age groups. It usually goes away over time but requires hospital care. It’s not known if myocarditis could have lingering effects for those who experience it.
There were no cases of myocarditis seen in Pfizer’s studies of the vaccine in children, and no other serious events were seen. Vaccine side effects reported in the Pfizer studies were mostly mild and included fatigue, headache, and pain at the injection site.
“We think we have optimized the immune response and minimized our reactions,” said William Gruber, MD, senior vice president vaccine research and clinical development at Pfizer.
But the studies didn’t include enough participants to pick up rare, but serious adverse events like myocarditis.
“We’re worried about a side effect that we can’t measure yet, but it’s probably real, and we see a benefit that isn’t the same as it is in older age groups,” said Dr. Rubin.
Benefits vs. risks
FDA modeled the benefits and risks for children under a variety of scenarios. The benefits of the vaccines to children very much depend on the amount of transmission in the community.
When transmission is high, the benefits to children — in terms of infections, hospitalizations, ICU admissions — clearly outweigh its risks.
But when COVID-19 rates are low in the community, as they were in June, FDA analysts predicted the vaccines might send more children to the hospital for myocarditis than the virus would.
The FDA noted that kids who are hospitalized for myocarditis tend not to be as ill as children with COVID-19, however.
“If the trends continue the way they are going, the emergency for children is not what we might think it would be. That was my concern,” Dr. Hildreth said.
But others warned against complacency.
“Thinking that this is going to be the end of the wave permanently may be a little overly optimistic,” said committee chairman Arnold Monto, MD, a professor of public health and epidemiology at the University of Michigan, Ann Arbor.
The majority of COVID-19 cases in children are mild. Only about 1% of kids are hospitalized for their infections, according to CDC data. But the rates of hospitalizations in kids are about 3 times higher for people of color — including Blacks, Hispanics, and Native Americans, as compared to Whites and Asian Americans.
Since the start of the pandemic, 94 children ages 5 to 11 have died, making it the eighth leading cause of death for kids this age last year.
More than 5,200 children have developed a delayed complication from their infections called Multi-System Inflammatory Syndrome (MIS-C).
MIS-C can be severe and require hospital care and can lead to myocarditis. Children ages 5 to 11 are the age group at greatest risk for this complication.
Kids can also get long COVID. There’s not a lot of data on how often this happens, though it appears to be less frequent in children than in adults.
But a survey in the United Kingdom found that 7%-8% of kids have symptoms from their infections that last longer than 12 weeks, Dr. Havers said. Symptoms that can linger for kids include fatigue, cough, muscle and joint pain, headaches, and insomnia.
More than 1 million children have been impacted by school closures so far this year, and quarantines have had lasting impacts on learning, social development, and mental health.
Even though kids aren’t usually COVID superspreaders, they can still pass the infection on to others.
“What is clear is that secondary transmission from children, both to other children and to adults, does occur,” Dr. Havers said.
For that reason, they can continue the spread of the virus and give it opportunities to mutate and become more dangerous.
Safety monitoring to continue
Some committee members referenced thousands of letters they had received within the past few days urging them to vote against the vaccine.
Jay Portnoy, MD, a professor of pediatrics at Children’s Mercy Hospital in Kansas City, Mo., said he had personally received about 4,000 emails.
“But I feel like I need to also represent the consumers, the parents that I see every day in the clinic who are terrified of sending their children to school because they’re not protected against COVID,” he said, explaining his vote to recommend authorization.
“Our kids are going to be dealing with this virus for many years to come. It’s going to come repeatedly. Getting this vaccine is just the first step that they can take to protect themselves from having bad outcomes,” Dr. Portnoy said.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, reminded members of the committee that there were several government surveillance systems in place to catch any potential safety issues in near real time.
“I really appreciate very much the concern here. The safety monitoring of this vaccine will continue,” Dr. Marks said. “I do view this as one of our greatest responsibilities.”
“I really am so grateful that we had this discussion and voted to approve,” said Capt. Amanda Cohn, MD, chief medical officer at the National Center for Immunization and Respiratory Diseases.
“I think the benefits in this age group really are super important even if they are lower than for other age groups.”
This article was updated 10/27/21.
A version of this article first appeared on WebMD.com.
Seventeen of the 18 members of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) on Oct. 26 voted to recommend the 10-microgram shot for kids, which is one-third the dose given to adults.
One member, Michael Kurilla, MD, director of the division of clinical innovation at the National Institutes of Health, Bethesda, Md., abstained from voting.
If the FDA follows the recommendation, as it typically does, and issues an Emergency Use Authorization for the vaccine, the shots could be available within days.
After the FDA’s final decision, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will meet to make specific recommendations for its use. The CDC committee must stick closely to the conditions for use spelled out in the EUA, so their recommendations are likely to be similar to those made by the FDA. Their next meeting is scheduled for Nov. 2 and 3.
In the end, some on the panel felt uneasy with their decision.
“I voted yes primarily because I wanted to make sure that children who really need this vaccine, the Black and brown children of our country, get the vaccine,” said James Hildreth, MD, PhD, president and CEO of Meharry Medical College in Nashville.
“But to be honest, the best way to protect the health of some children will be to do nothing because they will be just fine,” he said.
Others said they were surprised by how difficult the decision had been.
“This is a much tougher one than we had expected going into it,” said committee member Eric Rubin, MD, editor and chief of the New England Journal of Medicine, during the FDA advisory committee’s meeting.
Ahead of the vote, the committee heard presentations outlining the expected benefits of vaccinating children along with potential risks.
“Children have been greatly impacted by the pandemic,” said Fiona Havers, MD, a medical officer with the CDC in Atlanta who reviewed the epidemiology of COVID-19 in kids.
In the second year of the pandemic, as more seniors have been vaccinated against the virus, COVID cases have largely shifted from older to younger age groups.
So far, there have been more than 1.9 million COVID-19 cases in children ages 5 through 11 in the United States.. Cases in kids saw a big jump in July and August with summer travel, schools reopening, and the dominance of the Delta variant.
And those are just the cases reported to the CDC. Regular testing of anonymous blood samples collected at sites across the United States indicates that 6 times as many kids have had COVID than what is reflected in official counts.
Last winter, blood sample testing showed about 13% of children had antibodies against the virus, suggesting they’d been infected. By this summer, that number had risen to 42%.
That figure clearly made an impression on many members of the committee who asked the FDA’s vaccine reviewers if they had tried to account for immunity from past infections in their modeling. They had not.
Some felt that even with a highly effective vaccine — new data presented by Pfizer showed the children’s dose was 90% effective at preventing symptomatic infections in kids — caution was warranted as much is still unknown about myocarditis, a rare side effect of the mRNA vaccines.
Myocarditis has been more common in younger age groups. It usually goes away over time but requires hospital care. It’s not known if myocarditis could have lingering effects for those who experience it.
There were no cases of myocarditis seen in Pfizer’s studies of the vaccine in children, and no other serious events were seen. Vaccine side effects reported in the Pfizer studies were mostly mild and included fatigue, headache, and pain at the injection site.
“We think we have optimized the immune response and minimized our reactions,” said William Gruber, MD, senior vice president vaccine research and clinical development at Pfizer.
But the studies didn’t include enough participants to pick up rare, but serious adverse events like myocarditis.
“We’re worried about a side effect that we can’t measure yet, but it’s probably real, and we see a benefit that isn’t the same as it is in older age groups,” said Dr. Rubin.
Benefits vs. risks
FDA modeled the benefits and risks for children under a variety of scenarios. The benefits of the vaccines to children very much depend on the amount of transmission in the community.
When transmission is high, the benefits to children — in terms of infections, hospitalizations, ICU admissions — clearly outweigh its risks.
But when COVID-19 rates are low in the community, as they were in June, FDA analysts predicted the vaccines might send more children to the hospital for myocarditis than the virus would.
The FDA noted that kids who are hospitalized for myocarditis tend not to be as ill as children with COVID-19, however.
“If the trends continue the way they are going, the emergency for children is not what we might think it would be. That was my concern,” Dr. Hildreth said.
But others warned against complacency.
“Thinking that this is going to be the end of the wave permanently may be a little overly optimistic,” said committee chairman Arnold Monto, MD, a professor of public health and epidemiology at the University of Michigan, Ann Arbor.
The majority of COVID-19 cases in children are mild. Only about 1% of kids are hospitalized for their infections, according to CDC data. But the rates of hospitalizations in kids are about 3 times higher for people of color — including Blacks, Hispanics, and Native Americans, as compared to Whites and Asian Americans.
Since the start of the pandemic, 94 children ages 5 to 11 have died, making it the eighth leading cause of death for kids this age last year.
More than 5,200 children have developed a delayed complication from their infections called Multi-System Inflammatory Syndrome (MIS-C).
MIS-C can be severe and require hospital care and can lead to myocarditis. Children ages 5 to 11 are the age group at greatest risk for this complication.
Kids can also get long COVID. There’s not a lot of data on how often this happens, though it appears to be less frequent in children than in adults.
But a survey in the United Kingdom found that 7%-8% of kids have symptoms from their infections that last longer than 12 weeks, Dr. Havers said. Symptoms that can linger for kids include fatigue, cough, muscle and joint pain, headaches, and insomnia.
More than 1 million children have been impacted by school closures so far this year, and quarantines have had lasting impacts on learning, social development, and mental health.
Even though kids aren’t usually COVID superspreaders, they can still pass the infection on to others.
“What is clear is that secondary transmission from children, both to other children and to adults, does occur,” Dr. Havers said.
For that reason, they can continue the spread of the virus and give it opportunities to mutate and become more dangerous.
Safety monitoring to continue
Some committee members referenced thousands of letters they had received within the past few days urging them to vote against the vaccine.
Jay Portnoy, MD, a professor of pediatrics at Children’s Mercy Hospital in Kansas City, Mo., said he had personally received about 4,000 emails.
“But I feel like I need to also represent the consumers, the parents that I see every day in the clinic who are terrified of sending their children to school because they’re not protected against COVID,” he said, explaining his vote to recommend authorization.
“Our kids are going to be dealing with this virus for many years to come. It’s going to come repeatedly. Getting this vaccine is just the first step that they can take to protect themselves from having bad outcomes,” Dr. Portnoy said.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, reminded members of the committee that there were several government surveillance systems in place to catch any potential safety issues in near real time.
“I really appreciate very much the concern here. The safety monitoring of this vaccine will continue,” Dr. Marks said. “I do view this as one of our greatest responsibilities.”
“I really am so grateful that we had this discussion and voted to approve,” said Capt. Amanda Cohn, MD, chief medical officer at the National Center for Immunization and Respiratory Diseases.
“I think the benefits in this age group really are super important even if they are lower than for other age groups.”
This article was updated 10/27/21.
A version of this article first appeared on WebMD.com.
Seventeen of the 18 members of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) on Oct. 26 voted to recommend the 10-microgram shot for kids, which is one-third the dose given to adults.
One member, Michael Kurilla, MD, director of the division of clinical innovation at the National Institutes of Health, Bethesda, Md., abstained from voting.
If the FDA follows the recommendation, as it typically does, and issues an Emergency Use Authorization for the vaccine, the shots could be available within days.
After the FDA’s final decision, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will meet to make specific recommendations for its use. The CDC committee must stick closely to the conditions for use spelled out in the EUA, so their recommendations are likely to be similar to those made by the FDA. Their next meeting is scheduled for Nov. 2 and 3.
In the end, some on the panel felt uneasy with their decision.
“I voted yes primarily because I wanted to make sure that children who really need this vaccine, the Black and brown children of our country, get the vaccine,” said James Hildreth, MD, PhD, president and CEO of Meharry Medical College in Nashville.
“But to be honest, the best way to protect the health of some children will be to do nothing because they will be just fine,” he said.
Others said they were surprised by how difficult the decision had been.
“This is a much tougher one than we had expected going into it,” said committee member Eric Rubin, MD, editor and chief of the New England Journal of Medicine, during the FDA advisory committee’s meeting.
Ahead of the vote, the committee heard presentations outlining the expected benefits of vaccinating children along with potential risks.
“Children have been greatly impacted by the pandemic,” said Fiona Havers, MD, a medical officer with the CDC in Atlanta who reviewed the epidemiology of COVID-19 in kids.
In the second year of the pandemic, as more seniors have been vaccinated against the virus, COVID cases have largely shifted from older to younger age groups.
So far, there have been more than 1.9 million COVID-19 cases in children ages 5 through 11 in the United States.. Cases in kids saw a big jump in July and August with summer travel, schools reopening, and the dominance of the Delta variant.
And those are just the cases reported to the CDC. Regular testing of anonymous blood samples collected at sites across the United States indicates that 6 times as many kids have had COVID than what is reflected in official counts.
Last winter, blood sample testing showed about 13% of children had antibodies against the virus, suggesting they’d been infected. By this summer, that number had risen to 42%.
That figure clearly made an impression on many members of the committee who asked the FDA’s vaccine reviewers if they had tried to account for immunity from past infections in their modeling. They had not.
Some felt that even with a highly effective vaccine — new data presented by Pfizer showed the children’s dose was 90% effective at preventing symptomatic infections in kids — caution was warranted as much is still unknown about myocarditis, a rare side effect of the mRNA vaccines.
Myocarditis has been more common in younger age groups. It usually goes away over time but requires hospital care. It’s not known if myocarditis could have lingering effects for those who experience it.
There were no cases of myocarditis seen in Pfizer’s studies of the vaccine in children, and no other serious events were seen. Vaccine side effects reported in the Pfizer studies were mostly mild and included fatigue, headache, and pain at the injection site.
“We think we have optimized the immune response and minimized our reactions,” said William Gruber, MD, senior vice president vaccine research and clinical development at Pfizer.
But the studies didn’t include enough participants to pick up rare, but serious adverse events like myocarditis.
“We’re worried about a side effect that we can’t measure yet, but it’s probably real, and we see a benefit that isn’t the same as it is in older age groups,” said Dr. Rubin.
Benefits vs. risks
FDA modeled the benefits and risks for children under a variety of scenarios. The benefits of the vaccines to children very much depend on the amount of transmission in the community.
When transmission is high, the benefits to children — in terms of infections, hospitalizations, ICU admissions — clearly outweigh its risks.
But when COVID-19 rates are low in the community, as they were in June, FDA analysts predicted the vaccines might send more children to the hospital for myocarditis than the virus would.
The FDA noted that kids who are hospitalized for myocarditis tend not to be as ill as children with COVID-19, however.
“If the trends continue the way they are going, the emergency for children is not what we might think it would be. That was my concern,” Dr. Hildreth said.
But others warned against complacency.
“Thinking that this is going to be the end of the wave permanently may be a little overly optimistic,” said committee chairman Arnold Monto, MD, a professor of public health and epidemiology at the University of Michigan, Ann Arbor.
The majority of COVID-19 cases in children are mild. Only about 1% of kids are hospitalized for their infections, according to CDC data. But the rates of hospitalizations in kids are about 3 times higher for people of color — including Blacks, Hispanics, and Native Americans, as compared to Whites and Asian Americans.
Since the start of the pandemic, 94 children ages 5 to 11 have died, making it the eighth leading cause of death for kids this age last year.
More than 5,200 children have developed a delayed complication from their infections called Multi-System Inflammatory Syndrome (MIS-C).
MIS-C can be severe and require hospital care and can lead to myocarditis. Children ages 5 to 11 are the age group at greatest risk for this complication.
Kids can also get long COVID. There’s not a lot of data on how often this happens, though it appears to be less frequent in children than in adults.
But a survey in the United Kingdom found that 7%-8% of kids have symptoms from their infections that last longer than 12 weeks, Dr. Havers said. Symptoms that can linger for kids include fatigue, cough, muscle and joint pain, headaches, and insomnia.
More than 1 million children have been impacted by school closures so far this year, and quarantines have had lasting impacts on learning, social development, and mental health.
Even though kids aren’t usually COVID superspreaders, they can still pass the infection on to others.
“What is clear is that secondary transmission from children, both to other children and to adults, does occur,” Dr. Havers said.
For that reason, they can continue the spread of the virus and give it opportunities to mutate and become more dangerous.
Safety monitoring to continue
Some committee members referenced thousands of letters they had received within the past few days urging them to vote against the vaccine.
Jay Portnoy, MD, a professor of pediatrics at Children’s Mercy Hospital in Kansas City, Mo., said he had personally received about 4,000 emails.
“But I feel like I need to also represent the consumers, the parents that I see every day in the clinic who are terrified of sending their children to school because they’re not protected against COVID,” he said, explaining his vote to recommend authorization.
“Our kids are going to be dealing with this virus for many years to come. It’s going to come repeatedly. Getting this vaccine is just the first step that they can take to protect themselves from having bad outcomes,” Dr. Portnoy said.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, reminded members of the committee that there were several government surveillance systems in place to catch any potential safety issues in near real time.
“I really appreciate very much the concern here. The safety monitoring of this vaccine will continue,” Dr. Marks said. “I do view this as one of our greatest responsibilities.”
“I really am so grateful that we had this discussion and voted to approve,” said Capt. Amanda Cohn, MD, chief medical officer at the National Center for Immunization and Respiratory Diseases.
“I think the benefits in this age group really are super important even if they are lower than for other age groups.”
This article was updated 10/27/21.
A version of this article first appeared on WebMD.com.
Opioid-induced adrenal insufficiency for the hospitalist
Consider OIAI, even among patients with common infections
Case
A 60-year-old woman with metastatic breast cancer using morphine extended release 30 mg twice daily and as-needed oxycodone for cancer-related pain presents with fever, dyspnea, and productive cough for 2 days. She also notes several weeks of fatigue, nausea, weight loss, and orthostatic lightheadedness. She is found to have pneumonia and is admitted for intravenous antibiotics. She remains borderline hypotensive after intravenous fluids and the hospitalist suspects opioid-induced adrenal insufficiency (OIAI).
How is OIAI diagnosed and managed?
Brief overview of issue
In the United States, 5.4% of the population is currently using long-term opioids.1 Patients using high doses of opioids for greater than 3 months are 40%-50% more likely to be hospitalized than those on a lower dose or no opioids.2 Hospitalists frequently encounter common opioid side effects such as constipation, nausea, and drowsiness, but may be less familiar with their effects on the endocrine system. Chronic, high-dose opioids can suppress the hypothalamic-pituitary-adrenal (HPA) axis and cause secondary, or central, adrenal insufficiency (AI).1
Recognition of OIAI is critical given the current opioid epidemic and life-threatening consequences of AI in systemically ill patients. While high-dose opioids may acutely suppress the HPA axis,3 OIAI is more commonly associated with long-term opioid use.4 The prevalence of OIAI among patients receiving long-term opioids ranges from 8.3% to 29%. This range reflects variations in opioid dose, duration of use, and different methods of assessing the HPA axis.1,4 When screening for HPA axis suppression in subjects taking chronic opioids, Lamprecht and colleagues found a prevalence of 22.5%.5 In comparison, Gibb and colleagues found the prevalence of secondary AI to be 8.3% in patients enrolled in a chronic pain clinic.6 Despite the high prevalence on biochemical screening, the clinical significance of OIAI is less clear. Clinical AI and adrenal crisis among patients on opioids are less frequent and mostly limited to case reports.7,8 In one retrospective cohort, one in 40 patients with OIAI presented with adrenal crisis during a hospitalization for viral gastroenteritis.9
With this prevalence, one would expect to diagnose OIAI more commonly in hospitalized patients. A concerning possibility is that this diagnosis is underrecognized because of either a lack of knowledge of the disease or the clinical overlap between the nonspecific symptoms of AI and other diagnoses. In patients reporting symptoms suggestive of OIAI, the diagnosis was delayed by a median of 12 months.9 The challenge for the hospitalist is to consider OIAI, even among patients with common infections such as pneumonia, viral gastroenteritis, or endocarditis who present with these nonspecific symptoms, while also avoiding unnecessary testing and treatment with glucocorticoids.
Overview of the data
Opiates and opioids exert their physiologic effect through activation of the mu, kappa, and delta receptors. These receptors are located throughout the body, including the hypothalamus and pituitary gland.4 Activation of these receptors results in tonic inhibition of the HPA axis and results in central AI.4 Central AI is characterized by a low a.m. cortisol, low adrenocorticotropic hormone (ACTH), and low dehydroepiandrosterone sulfate (DHEAS) levels.1,4 The low ACTH is indicative of central etiology. This effect of opioids is likely dose dependent with patients using more than 60 morphine-equivalent daily dose at greater risk.1,5
Unexplained or unresolved fatigue, musculoskeletal pain, nausea, vomiting, anorexia, abdominal pain, and orthostatic hypotension in a patient on chronic opioids should prompt consideration of OIAI.9 Once suspected, an 8 a.m. cortisol, ACTH level, and DHEAS level should be ordered. Because of the diurnal variation of cortisol levels, 8 a.m. values are best validated for diagnosis.10 While cutoffs differ, an 8 a.m. cortisol less than 5 mcg/dL combined with ACTH less than 10 pmol/L, and DHEAS less than 50 mcg/dL are highly suggestive of OIAI. Low or indeterminate baseline a.m. cortisol levels warrant confirmatory testing.4,10 While the insulin tolerance test is considered the gold standard, the high dose (250 mcg) cosyntropin stimulation test (CST) is the more commonly used test to diagnose and confirm AI. A CST peak response greater than 18-20 mcg/dL suggests an intact HPA axis (see Figure 1).10 This testing will diagnose central AI, but is not specific for OIAI. Other causes of central AI such as exogenous steroid use, pituitary pathology, and head trauma should be considered before attributing AI to opioids (see Table 1).4
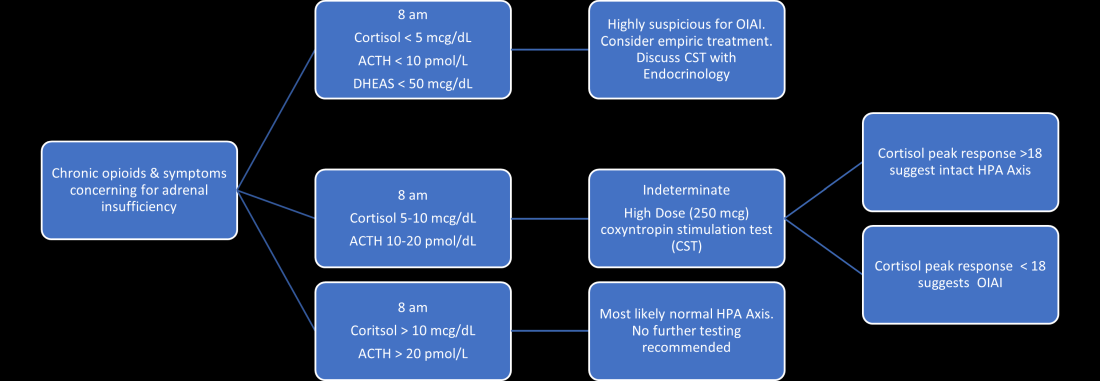
The abnormal CST in central AI is from chronic ACTH deficiency and lack of adrenal stimulation resulting in adrenal atrophy. Adrenal atrophy leaves the adrenal glands incapable of responding to exogenous ACTH. This process takes several weeks; therefore, those with ACTH suppression caused by recent high-dose opioid use or subacute pituitary injury may have an indeterminate or normal cortisol response to high-dose exogenous ACTH.4 Even in the setting of a normal CST, there may remain uncertainty in the diagnosis of OIAI. When evaluating for central AI, the sensitivity and negative likelihood ratio of the CST are only 0.64 and 0.39, respectively.4 In the same cohort of 40 patients with OIAI, 11 patients had a normal CST.9 The low-dose (1 mcg) CST may increase the sensitivity, but the use of this test is limited because of technical challenges.1 Endocrinology consultation can assist when the initial diagnostic and clinical presentation is unclear.
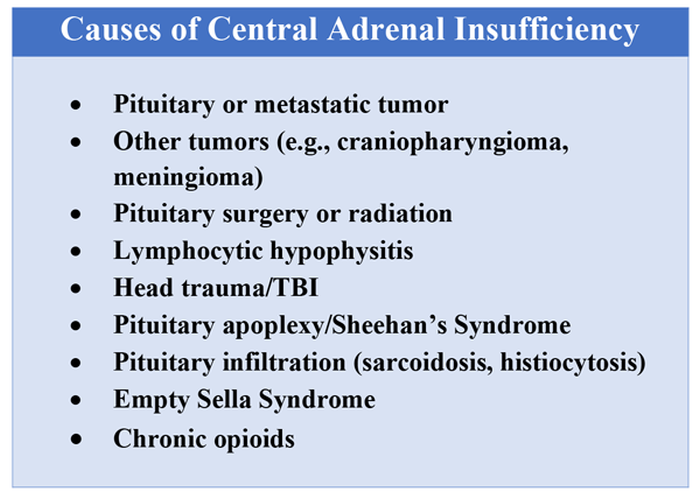
To manage a patient on opioid therapy who has laboratory data consistent with central AI, the clinician must weigh the severity of symptoms, probability of opioid weaning, and risks associated with glucocorticoid treatment. Patients presenting with acute adrenal crisis, hypotension, or critical illness should be managed with intravenous steroid replacement per existing guideline recommendations.10,11
Patients with mild symptoms of nausea, vomiting, or orthostatic symptoms that resolve with treatment of their admitting diagnosis but who have evidence of an abnormal HPA axis should be considered for weaning opioid therapy. Evidence suggests that OIAI is reversible with reduction and cessation of chronic opioid use.4,9 These patients may not need chronic steroid replacement; however, they should receive education on the symptoms of AI and potentially rescue steroids for home use in the setting of severe illness. Patients with OIAI admitted for surgical procedures should be managed in accordance with existing guidelines for perioperative stress dosing of glucocorticoids for AI.
Those with persisting symptoms of OIAI and an abnormal HPA axis require endocrinology consultation and glucocorticoid replacement. There is limited evidence that suggests low dose steroid replacement in patients with OIAI can improve subjective perception of bodily pain, activity level, and mood in chronic opioid users.9 Li and colleagues found that 16 of 23 patients experienced improvement of symptoms on glucocorticoids, and 15 were able to discontinue opioids completely.9 The authors speculated that the improvement in fatigue and musculoskeletal pain after steroid replacement is what allowed for successful opioid weaning. Seven of 10 of these patients with available follow-up had recovery of the HPA axis during the follow-up period.9 In central AI, doses as low as 10-20 mg/day of hydrocortisone have been used.10,11 Hospitalists should educate patients on recognizing symptoms of AI, as this low dose may not be sufficient to prevent adrenal crisis.
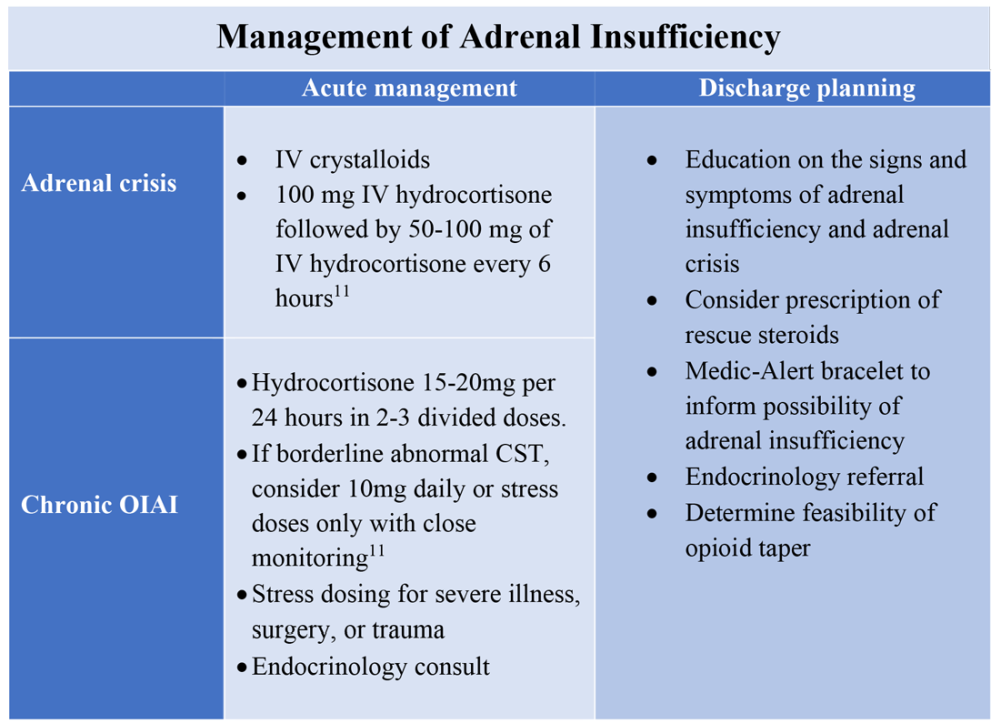
All patients with evidence of abnormalities in the HPA axis should receive a Medic-Alert bracelet to inform other providers of the possibility of adrenal crisis should a major trauma or critical illness render them unconscious.4,10 Since OIAI is a form of central AI, mineralocorticoid replacement is not generally necessary.11 Endocrinology follow-up can help wean steroids as the HPA axis recovers after weaning opioid therapy. Recognizing and diagnosing OIAI can identify patients with untreated symptoms who are at risk for adrenal crisis, improve communication with patients on benefits of weaning opioids, and provide valuable patient education and safe transition of care.
Application of the data to the original case
To make the diagnosis of OIAI, 8 a.m. cortisol, ACTH, and DHEAS should be obtained. Her cortisol was less than 5 mcg/dL, ACTH was 6 pmol/L and DHEAS was 30 mcg/dL. A high dose CST was performed with 30-minute and 60-minute cortisol values of 6 mcg/dL and 9 mcg/dL, respectively. The abnormal CST and low ACTH indicate central AI. She should undergo testing for other etiologies of central AI, such as a brain MRI and pituitary hormone testing, before confirming the diagnosis of OIAI.
The insufficient adrenal response to ACTH in the setting of infection and hypotension should prompt glucocorticoid replacement. Tapering opioids could result in recovery of the HPA axis, though may not be realistic in this patient with chronic cancer-related pain. If the patient is at high risk for adverse effects of glucocorticoids, repeat testing of the HPA axis in the outpatient setting can assess if the patient truly needs steroid replacement daily rather than only during physiologic stress. The patient should be given a Medic-Alert bracelet and instructions on symptoms of AI and stress dosing upon discharge.
Bottom line
OIAI is underrecognized because of central adrenal insufficiency. Knowing its clinical characteristics, diagnostic pathways, and treatment options aids in recognition and management.
Dr. Cunningham, Dr. Munoa, and Dr. Indovina are based in the division of hospital medicine at Denver Health and Hospital Authority.
References
1. Donegan D. Opioid induced adrenal insufficiency: What is new? Curr Opin Endocrinol Diabetes Obes. 2019 Jun;26(3):133-8. doi: 10.1097/MED.0000000000000474.
2. Liang Y and Turner BJ. Opioid risk measure for hospitalization. J Hosp Med. 2015 July;10(7):425-31. doi: 10.1002/jhm.2350.
3. Policola C et al. Adrenal insufficiency in acute oral opiate therapy. Endocrinol Diabetes Metab Case Rep. 2014;2014:130071. doi: 10.1530/EDM-13-0071.
4. Donegan D and Bancos I. Opioid-induced adrenal insufficiency. Mayo Clin Proc. 2018 July;93(7):937-44. doi: 10.1016/j.mayocp.2018.04.010.
5. Lamprecht A et al. Secondary adrenal insufficiency and pituitary dysfunction in oral/transdermal opioid users with non-cancer pain. Eur J Endocrinol. 2018 Dec 1;179(6):353-62. doi: 10.1530/EJE-18-0530.
6. Gibb FW et al. Adrenal insufficiency in patients on long-term opioid analgesia. Clin Endocrinol (Oxf). 2016 June;85(6):831-5. doi:10.1111/cen.13125.
7. Abs R et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000 June;85(6):2215-22. doi: 10.1210/jcem.85.6.6615.
8. Tabet EJ et al. Opioid-induced hypoadrenalism resulting in fasting hypoglycaemia. BMJ Case Rep. 2019 Dec 11;12(12):e230551. doi: 10.1136/bcr-2019-230551.
9. Li T et al. Clinical presentation and outcomes of opioid induced adrenal insufficiency. Endocr Pract. 2020 Nov;26(11):1291-1297. doi: 10.4158/EP-2020-0297.
10. Grossman AB. Clinical Review: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010 Nov;95(11):4855-63. doi: 10.1210/jc.2010-0982.
11. Charmandari E et al. Adrenal insufficiency. Lancet. 2014 June 21;383(9935):2152-67. doi: 10.1016/S0140-6736(13)61684-0.
Key points
- Opioids can cause central adrenal insufficiency because of tonic suppression of the HPA axis. This effect is likely dose dependent, and reversible upon tapering or withdrawal of opioids.
- The prevalence of biochemical OIAI in chronic opioid users of 8%-29% clinical AI is less frequent but may be underrecognized in hospitalized patients leading to delayed diagnosis.
- Diagnosis of central adrenal insufficiency is based upon low 8 a.m. cortisol and ACTH levels and/or an abnormal CST. OIAI is the likely etiology in patients on chronic opioids for whom other causes of central adrenal insufficiency have been ruled out.
- Management with glucocorticoid replacement is variable depending on clinical presentation, severity of HPA axis suppression, and ability to wean opioid therapy. Patient education regarding symptoms of AI and stress dosing is essential.
Additional reading
Grossman AB. Clinical Review: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010 Nov;95(11):4855-63. doi: 10.1210/jc.2010-0982.
Donegan D and Bancos I. Opioid-induced adrenal insufficiency. Mayo Clin Proc. 2018 July;93(7):937-44. doi: 10.1016/j.mayocp.2018.04.010.
Li T et al. Clinical presentation and outcomes of opioid induced adrenal insufficiency. Endocr Pract. 2020 Nov;26(11):1291-7. doi: 10.4158/EP-2020-0297.
Quiz
A 55-year-old man with chronic back pain, for which he takes a total of 90 mg of oral morphine daily, is admitted for pyelonephritis with fever, nausea, vomiting, dysuria, and abdominal pain. He is febrile and tachycardic on presentation, but his vitals quickly normalize after hydration and antibiotics. About 48 hours into his hospitalization his fevers, dysuria, and abdominal pain have resolved, but he has persistent nausea and headaches. On further questioning, he also reports weight loss and fatigue over the past 3 weeks. He is found to have a morning cortisol level less than 5 mcg/dL, as well as low levels of ACTH and DHEAS. OIAI is suspected.
Which of the following is true about management?
A. Glucocorticoid replacement therapy with oral hydrocortisone should be considered to improve his symptoms.
B. Tapering off opioids is unlikely to resolve his adrenal insufficiency.
C. Stress dose steroids should be started immediately with high-dose intravenous hydrocortisone.
D. Given high clinical suspicion for OIAI, further testing for other etiologies of central adrenal insufficiency is not recommended.
Explanation of correct answer
The correct answer is A. This patient’s ongoing nonspecific symptoms that have persisted despite treatment of his acute pyelonephritis are likely caused by adrenal insufficiency. In a symptomatic patient with OIAI, treatment with oral hydrocortisone should be considered to control symptoms and facilitate tapering opioids. Tapering and stopping opioids often leads to recovery of the HPA axis and resolution of the OIAI. Tapering opioids should be considered a mainstay of therapy for OIAI when clinically appropriate, as in this patient with chronic benign pain. Stress dose steroids are not indicated in the absence of critical illness, adrenal crisis, or major surgery. OIAI is a diagnosis of exclusion, and patients should undergo workup for other causes of secondary adrenal insufficiency.
Consider OIAI, even among patients with common infections
Consider OIAI, even among patients with common infections
Case
A 60-year-old woman with metastatic breast cancer using morphine extended release 30 mg twice daily and as-needed oxycodone for cancer-related pain presents with fever, dyspnea, and productive cough for 2 days. She also notes several weeks of fatigue, nausea, weight loss, and orthostatic lightheadedness. She is found to have pneumonia and is admitted for intravenous antibiotics. She remains borderline hypotensive after intravenous fluids and the hospitalist suspects opioid-induced adrenal insufficiency (OIAI).
How is OIAI diagnosed and managed?
Brief overview of issue
In the United States, 5.4% of the population is currently using long-term opioids.1 Patients using high doses of opioids for greater than 3 months are 40%-50% more likely to be hospitalized than those on a lower dose or no opioids.2 Hospitalists frequently encounter common opioid side effects such as constipation, nausea, and drowsiness, but may be less familiar with their effects on the endocrine system. Chronic, high-dose opioids can suppress the hypothalamic-pituitary-adrenal (HPA) axis and cause secondary, or central, adrenal insufficiency (AI).1
Recognition of OIAI is critical given the current opioid epidemic and life-threatening consequences of AI in systemically ill patients. While high-dose opioids may acutely suppress the HPA axis,3 OIAI is more commonly associated with long-term opioid use.4 The prevalence of OIAI among patients receiving long-term opioids ranges from 8.3% to 29%. This range reflects variations in opioid dose, duration of use, and different methods of assessing the HPA axis.1,4 When screening for HPA axis suppression in subjects taking chronic opioids, Lamprecht and colleagues found a prevalence of 22.5%.5 In comparison, Gibb and colleagues found the prevalence of secondary AI to be 8.3% in patients enrolled in a chronic pain clinic.6 Despite the high prevalence on biochemical screening, the clinical significance of OIAI is less clear. Clinical AI and adrenal crisis among patients on opioids are less frequent and mostly limited to case reports.7,8 In one retrospective cohort, one in 40 patients with OIAI presented with adrenal crisis during a hospitalization for viral gastroenteritis.9
With this prevalence, one would expect to diagnose OIAI more commonly in hospitalized patients. A concerning possibility is that this diagnosis is underrecognized because of either a lack of knowledge of the disease or the clinical overlap between the nonspecific symptoms of AI and other diagnoses. In patients reporting symptoms suggestive of OIAI, the diagnosis was delayed by a median of 12 months.9 The challenge for the hospitalist is to consider OIAI, even among patients with common infections such as pneumonia, viral gastroenteritis, or endocarditis who present with these nonspecific symptoms, while also avoiding unnecessary testing and treatment with glucocorticoids.
Overview of the data
Opiates and opioids exert their physiologic effect through activation of the mu, kappa, and delta receptors. These receptors are located throughout the body, including the hypothalamus and pituitary gland.4 Activation of these receptors results in tonic inhibition of the HPA axis and results in central AI.4 Central AI is characterized by a low a.m. cortisol, low adrenocorticotropic hormone (ACTH), and low dehydroepiandrosterone sulfate (DHEAS) levels.1,4 The low ACTH is indicative of central etiology. This effect of opioids is likely dose dependent with patients using more than 60 morphine-equivalent daily dose at greater risk.1,5
Unexplained or unresolved fatigue, musculoskeletal pain, nausea, vomiting, anorexia, abdominal pain, and orthostatic hypotension in a patient on chronic opioids should prompt consideration of OIAI.9 Once suspected, an 8 a.m. cortisol, ACTH level, and DHEAS level should be ordered. Because of the diurnal variation of cortisol levels, 8 a.m. values are best validated for diagnosis.10 While cutoffs differ, an 8 a.m. cortisol less than 5 mcg/dL combined with ACTH less than 10 pmol/L, and DHEAS less than 50 mcg/dL are highly suggestive of OIAI. Low or indeterminate baseline a.m. cortisol levels warrant confirmatory testing.4,10 While the insulin tolerance test is considered the gold standard, the high dose (250 mcg) cosyntropin stimulation test (CST) is the more commonly used test to diagnose and confirm AI. A CST peak response greater than 18-20 mcg/dL suggests an intact HPA axis (see Figure 1).10 This testing will diagnose central AI, but is not specific for OIAI. Other causes of central AI such as exogenous steroid use, pituitary pathology, and head trauma should be considered before attributing AI to opioids (see Table 1).4

The abnormal CST in central AI is from chronic ACTH deficiency and lack of adrenal stimulation resulting in adrenal atrophy. Adrenal atrophy leaves the adrenal glands incapable of responding to exogenous ACTH. This process takes several weeks; therefore, those with ACTH suppression caused by recent high-dose opioid use or subacute pituitary injury may have an indeterminate or normal cortisol response to high-dose exogenous ACTH.4 Even in the setting of a normal CST, there may remain uncertainty in the diagnosis of OIAI. When evaluating for central AI, the sensitivity and negative likelihood ratio of the CST are only 0.64 and 0.39, respectively.4 In the same cohort of 40 patients with OIAI, 11 patients had a normal CST.9 The low-dose (1 mcg) CST may increase the sensitivity, but the use of this test is limited because of technical challenges.1 Endocrinology consultation can assist when the initial diagnostic and clinical presentation is unclear.

To manage a patient on opioid therapy who has laboratory data consistent with central AI, the clinician must weigh the severity of symptoms, probability of opioid weaning, and risks associated with glucocorticoid treatment. Patients presenting with acute adrenal crisis, hypotension, or critical illness should be managed with intravenous steroid replacement per existing guideline recommendations.10,11
Patients with mild symptoms of nausea, vomiting, or orthostatic symptoms that resolve with treatment of their admitting diagnosis but who have evidence of an abnormal HPA axis should be considered for weaning opioid therapy. Evidence suggests that OIAI is reversible with reduction and cessation of chronic opioid use.4,9 These patients may not need chronic steroid replacement; however, they should receive education on the symptoms of AI and potentially rescue steroids for home use in the setting of severe illness. Patients with OIAI admitted for surgical procedures should be managed in accordance with existing guidelines for perioperative stress dosing of glucocorticoids for AI.
Those with persisting symptoms of OIAI and an abnormal HPA axis require endocrinology consultation and glucocorticoid replacement. There is limited evidence that suggests low dose steroid replacement in patients with OIAI can improve subjective perception of bodily pain, activity level, and mood in chronic opioid users.9 Li and colleagues found that 16 of 23 patients experienced improvement of symptoms on glucocorticoids, and 15 were able to discontinue opioids completely.9 The authors speculated that the improvement in fatigue and musculoskeletal pain after steroid replacement is what allowed for successful opioid weaning. Seven of 10 of these patients with available follow-up had recovery of the HPA axis during the follow-up period.9 In central AI, doses as low as 10-20 mg/day of hydrocortisone have been used.10,11 Hospitalists should educate patients on recognizing symptoms of AI, as this low dose may not be sufficient to prevent adrenal crisis.

All patients with evidence of abnormalities in the HPA axis should receive a Medic-Alert bracelet to inform other providers of the possibility of adrenal crisis should a major trauma or critical illness render them unconscious.4,10 Since OIAI is a form of central AI, mineralocorticoid replacement is not generally necessary.11 Endocrinology follow-up can help wean steroids as the HPA axis recovers after weaning opioid therapy. Recognizing and diagnosing OIAI can identify patients with untreated symptoms who are at risk for adrenal crisis, improve communication with patients on benefits of weaning opioids, and provide valuable patient education and safe transition of care.
Application of the data to the original case
To make the diagnosis of OIAI, 8 a.m. cortisol, ACTH, and DHEAS should be obtained. Her cortisol was less than 5 mcg/dL, ACTH was 6 pmol/L and DHEAS was 30 mcg/dL. A high dose CST was performed with 30-minute and 60-minute cortisol values of 6 mcg/dL and 9 mcg/dL, respectively. The abnormal CST and low ACTH indicate central AI. She should undergo testing for other etiologies of central AI, such as a brain MRI and pituitary hormone testing, before confirming the diagnosis of OIAI.
The insufficient adrenal response to ACTH in the setting of infection and hypotension should prompt glucocorticoid replacement. Tapering opioids could result in recovery of the HPA axis, though may not be realistic in this patient with chronic cancer-related pain. If the patient is at high risk for adverse effects of glucocorticoids, repeat testing of the HPA axis in the outpatient setting can assess if the patient truly needs steroid replacement daily rather than only during physiologic stress. The patient should be given a Medic-Alert bracelet and instructions on symptoms of AI and stress dosing upon discharge.
Bottom line
OIAI is underrecognized because of central adrenal insufficiency. Knowing its clinical characteristics, diagnostic pathways, and treatment options aids in recognition and management.
Dr. Cunningham, Dr. Munoa, and Dr. Indovina are based in the division of hospital medicine at Denver Health and Hospital Authority.
References
1. Donegan D. Opioid induced adrenal insufficiency: What is new? Curr Opin Endocrinol Diabetes Obes. 2019 Jun;26(3):133-8. doi: 10.1097/MED.0000000000000474.
2. Liang Y and Turner BJ. Opioid risk measure for hospitalization. J Hosp Med. 2015 July;10(7):425-31. doi: 10.1002/jhm.2350.
3. Policola C et al. Adrenal insufficiency in acute oral opiate therapy. Endocrinol Diabetes Metab Case Rep. 2014;2014:130071. doi: 10.1530/EDM-13-0071.
4. Donegan D and Bancos I. Opioid-induced adrenal insufficiency. Mayo Clin Proc. 2018 July;93(7):937-44. doi: 10.1016/j.mayocp.2018.04.010.
5. Lamprecht A et al. Secondary adrenal insufficiency and pituitary dysfunction in oral/transdermal opioid users with non-cancer pain. Eur J Endocrinol. 2018 Dec 1;179(6):353-62. doi: 10.1530/EJE-18-0530.
6. Gibb FW et al. Adrenal insufficiency in patients on long-term opioid analgesia. Clin Endocrinol (Oxf). 2016 June;85(6):831-5. doi:10.1111/cen.13125.
7. Abs R et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000 June;85(6):2215-22. doi: 10.1210/jcem.85.6.6615.
8. Tabet EJ et al. Opioid-induced hypoadrenalism resulting in fasting hypoglycaemia. BMJ Case Rep. 2019 Dec 11;12(12):e230551. doi: 10.1136/bcr-2019-230551.
9. Li T et al. Clinical presentation and outcomes of opioid induced adrenal insufficiency. Endocr Pract. 2020 Nov;26(11):1291-1297. doi: 10.4158/EP-2020-0297.
10. Grossman AB. Clinical Review: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010 Nov;95(11):4855-63. doi: 10.1210/jc.2010-0982.
11. Charmandari E et al. Adrenal insufficiency. Lancet. 2014 June 21;383(9935):2152-67. doi: 10.1016/S0140-6736(13)61684-0.
Key points
- Opioids can cause central adrenal insufficiency because of tonic suppression of the HPA axis. This effect is likely dose dependent, and reversible upon tapering or withdrawal of opioids.
- The prevalence of biochemical OIAI in chronic opioid users of 8%-29% clinical AI is less frequent but may be underrecognized in hospitalized patients leading to delayed diagnosis.
- Diagnosis of central adrenal insufficiency is based upon low 8 a.m. cortisol and ACTH levels and/or an abnormal CST. OIAI is the likely etiology in patients on chronic opioids for whom other causes of central adrenal insufficiency have been ruled out.
- Management with glucocorticoid replacement is variable depending on clinical presentation, severity of HPA axis suppression, and ability to wean opioid therapy. Patient education regarding symptoms of AI and stress dosing is essential.
Additional reading
Grossman AB. Clinical Review: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010 Nov;95(11):4855-63. doi: 10.1210/jc.2010-0982.
Donegan D and Bancos I. Opioid-induced adrenal insufficiency. Mayo Clin Proc. 2018 July;93(7):937-44. doi: 10.1016/j.mayocp.2018.04.010.
Li T et al. Clinical presentation and outcomes of opioid induced adrenal insufficiency. Endocr Pract. 2020 Nov;26(11):1291-7. doi: 10.4158/EP-2020-0297.
Quiz
A 55-year-old man with chronic back pain, for which he takes a total of 90 mg of oral morphine daily, is admitted for pyelonephritis with fever, nausea, vomiting, dysuria, and abdominal pain. He is febrile and tachycardic on presentation, but his vitals quickly normalize after hydration and antibiotics. About 48 hours into his hospitalization his fevers, dysuria, and abdominal pain have resolved, but he has persistent nausea and headaches. On further questioning, he also reports weight loss and fatigue over the past 3 weeks. He is found to have a morning cortisol level less than 5 mcg/dL, as well as low levels of ACTH and DHEAS. OIAI is suspected.
Which of the following is true about management?
A. Glucocorticoid replacement therapy with oral hydrocortisone should be considered to improve his symptoms.
B. Tapering off opioids is unlikely to resolve his adrenal insufficiency.
C. Stress dose steroids should be started immediately with high-dose intravenous hydrocortisone.
D. Given high clinical suspicion for OIAI, further testing for other etiologies of central adrenal insufficiency is not recommended.
Explanation of correct answer
The correct answer is A. This patient’s ongoing nonspecific symptoms that have persisted despite treatment of his acute pyelonephritis are likely caused by adrenal insufficiency. In a symptomatic patient with OIAI, treatment with oral hydrocortisone should be considered to control symptoms and facilitate tapering opioids. Tapering and stopping opioids often leads to recovery of the HPA axis and resolution of the OIAI. Tapering opioids should be considered a mainstay of therapy for OIAI when clinically appropriate, as in this patient with chronic benign pain. Stress dose steroids are not indicated in the absence of critical illness, adrenal crisis, or major surgery. OIAI is a diagnosis of exclusion, and patients should undergo workup for other causes of secondary adrenal insufficiency.
Case
A 60-year-old woman with metastatic breast cancer using morphine extended release 30 mg twice daily and as-needed oxycodone for cancer-related pain presents with fever, dyspnea, and productive cough for 2 days. She also notes several weeks of fatigue, nausea, weight loss, and orthostatic lightheadedness. She is found to have pneumonia and is admitted for intravenous antibiotics. She remains borderline hypotensive after intravenous fluids and the hospitalist suspects opioid-induced adrenal insufficiency (OIAI).
How is OIAI diagnosed and managed?
Brief overview of issue
In the United States, 5.4% of the population is currently using long-term opioids.1 Patients using high doses of opioids for greater than 3 months are 40%-50% more likely to be hospitalized than those on a lower dose or no opioids.2 Hospitalists frequently encounter common opioid side effects such as constipation, nausea, and drowsiness, but may be less familiar with their effects on the endocrine system. Chronic, high-dose opioids can suppress the hypothalamic-pituitary-adrenal (HPA) axis and cause secondary, or central, adrenal insufficiency (AI).1
Recognition of OIAI is critical given the current opioid epidemic and life-threatening consequences of AI in systemically ill patients. While high-dose opioids may acutely suppress the HPA axis,3 OIAI is more commonly associated with long-term opioid use.4 The prevalence of OIAI among patients receiving long-term opioids ranges from 8.3% to 29%. This range reflects variations in opioid dose, duration of use, and different methods of assessing the HPA axis.1,4 When screening for HPA axis suppression in subjects taking chronic opioids, Lamprecht and colleagues found a prevalence of 22.5%.5 In comparison, Gibb and colleagues found the prevalence of secondary AI to be 8.3% in patients enrolled in a chronic pain clinic.6 Despite the high prevalence on biochemical screening, the clinical significance of OIAI is less clear. Clinical AI and adrenal crisis among patients on opioids are less frequent and mostly limited to case reports.7,8 In one retrospective cohort, one in 40 patients with OIAI presented with adrenal crisis during a hospitalization for viral gastroenteritis.9
With this prevalence, one would expect to diagnose OIAI more commonly in hospitalized patients. A concerning possibility is that this diagnosis is underrecognized because of either a lack of knowledge of the disease or the clinical overlap between the nonspecific symptoms of AI and other diagnoses. In patients reporting symptoms suggestive of OIAI, the diagnosis was delayed by a median of 12 months.9 The challenge for the hospitalist is to consider OIAI, even among patients with common infections such as pneumonia, viral gastroenteritis, or endocarditis who present with these nonspecific symptoms, while also avoiding unnecessary testing and treatment with glucocorticoids.
Overview of the data
Opiates and opioids exert their physiologic effect through activation of the mu, kappa, and delta receptors. These receptors are located throughout the body, including the hypothalamus and pituitary gland.4 Activation of these receptors results in tonic inhibition of the HPA axis and results in central AI.4 Central AI is characterized by a low a.m. cortisol, low adrenocorticotropic hormone (ACTH), and low dehydroepiandrosterone sulfate (DHEAS) levels.1,4 The low ACTH is indicative of central etiology. This effect of opioids is likely dose dependent with patients using more than 60 morphine-equivalent daily dose at greater risk.1,5
Unexplained or unresolved fatigue, musculoskeletal pain, nausea, vomiting, anorexia, abdominal pain, and orthostatic hypotension in a patient on chronic opioids should prompt consideration of OIAI.9 Once suspected, an 8 a.m. cortisol, ACTH level, and DHEAS level should be ordered. Because of the diurnal variation of cortisol levels, 8 a.m. values are best validated for diagnosis.10 While cutoffs differ, an 8 a.m. cortisol less than 5 mcg/dL combined with ACTH less than 10 pmol/L, and DHEAS less than 50 mcg/dL are highly suggestive of OIAI. Low or indeterminate baseline a.m. cortisol levels warrant confirmatory testing.4,10 While the insulin tolerance test is considered the gold standard, the high dose (250 mcg) cosyntropin stimulation test (CST) is the more commonly used test to diagnose and confirm AI. A CST peak response greater than 18-20 mcg/dL suggests an intact HPA axis (see Figure 1).10 This testing will diagnose central AI, but is not specific for OIAI. Other causes of central AI such as exogenous steroid use, pituitary pathology, and head trauma should be considered before attributing AI to opioids (see Table 1).4

The abnormal CST in central AI is from chronic ACTH deficiency and lack of adrenal stimulation resulting in adrenal atrophy. Adrenal atrophy leaves the adrenal glands incapable of responding to exogenous ACTH. This process takes several weeks; therefore, those with ACTH suppression caused by recent high-dose opioid use or subacute pituitary injury may have an indeterminate or normal cortisol response to high-dose exogenous ACTH.4 Even in the setting of a normal CST, there may remain uncertainty in the diagnosis of OIAI. When evaluating for central AI, the sensitivity and negative likelihood ratio of the CST are only 0.64 and 0.39, respectively.4 In the same cohort of 40 patients with OIAI, 11 patients had a normal CST.9 The low-dose (1 mcg) CST may increase the sensitivity, but the use of this test is limited because of technical challenges.1 Endocrinology consultation can assist when the initial diagnostic and clinical presentation is unclear.

To manage a patient on opioid therapy who has laboratory data consistent with central AI, the clinician must weigh the severity of symptoms, probability of opioid weaning, and risks associated with glucocorticoid treatment. Patients presenting with acute adrenal crisis, hypotension, or critical illness should be managed with intravenous steroid replacement per existing guideline recommendations.10,11
Patients with mild symptoms of nausea, vomiting, or orthostatic symptoms that resolve with treatment of their admitting diagnosis but who have evidence of an abnormal HPA axis should be considered for weaning opioid therapy. Evidence suggests that OIAI is reversible with reduction and cessation of chronic opioid use.4,9 These patients may not need chronic steroid replacement; however, they should receive education on the symptoms of AI and potentially rescue steroids for home use in the setting of severe illness. Patients with OIAI admitted for surgical procedures should be managed in accordance with existing guidelines for perioperative stress dosing of glucocorticoids for AI.
Those with persisting symptoms of OIAI and an abnormal HPA axis require endocrinology consultation and glucocorticoid replacement. There is limited evidence that suggests low dose steroid replacement in patients with OIAI can improve subjective perception of bodily pain, activity level, and mood in chronic opioid users.9 Li and colleagues found that 16 of 23 patients experienced improvement of symptoms on glucocorticoids, and 15 were able to discontinue opioids completely.9 The authors speculated that the improvement in fatigue and musculoskeletal pain after steroid replacement is what allowed for successful opioid weaning. Seven of 10 of these patients with available follow-up had recovery of the HPA axis during the follow-up period.9 In central AI, doses as low as 10-20 mg/day of hydrocortisone have been used.10,11 Hospitalists should educate patients on recognizing symptoms of AI, as this low dose may not be sufficient to prevent adrenal crisis.

All patients with evidence of abnormalities in the HPA axis should receive a Medic-Alert bracelet to inform other providers of the possibility of adrenal crisis should a major trauma or critical illness render them unconscious.4,10 Since OIAI is a form of central AI, mineralocorticoid replacement is not generally necessary.11 Endocrinology follow-up can help wean steroids as the HPA axis recovers after weaning opioid therapy. Recognizing and diagnosing OIAI can identify patients with untreated symptoms who are at risk for adrenal crisis, improve communication with patients on benefits of weaning opioids, and provide valuable patient education and safe transition of care.
Application of the data to the original case
To make the diagnosis of OIAI, 8 a.m. cortisol, ACTH, and DHEAS should be obtained. Her cortisol was less than 5 mcg/dL, ACTH was 6 pmol/L and DHEAS was 30 mcg/dL. A high dose CST was performed with 30-minute and 60-minute cortisol values of 6 mcg/dL and 9 mcg/dL, respectively. The abnormal CST and low ACTH indicate central AI. She should undergo testing for other etiologies of central AI, such as a brain MRI and pituitary hormone testing, before confirming the diagnosis of OIAI.
The insufficient adrenal response to ACTH in the setting of infection and hypotension should prompt glucocorticoid replacement. Tapering opioids could result in recovery of the HPA axis, though may not be realistic in this patient with chronic cancer-related pain. If the patient is at high risk for adverse effects of glucocorticoids, repeat testing of the HPA axis in the outpatient setting can assess if the patient truly needs steroid replacement daily rather than only during physiologic stress. The patient should be given a Medic-Alert bracelet and instructions on symptoms of AI and stress dosing upon discharge.
Bottom line
OIAI is underrecognized because of central adrenal insufficiency. Knowing its clinical characteristics, diagnostic pathways, and treatment options aids in recognition and management.
Dr. Cunningham, Dr. Munoa, and Dr. Indovina are based in the division of hospital medicine at Denver Health and Hospital Authority.
References
1. Donegan D. Opioid induced adrenal insufficiency: What is new? Curr Opin Endocrinol Diabetes Obes. 2019 Jun;26(3):133-8. doi: 10.1097/MED.0000000000000474.
2. Liang Y and Turner BJ. Opioid risk measure for hospitalization. J Hosp Med. 2015 July;10(7):425-31. doi: 10.1002/jhm.2350.
3. Policola C et al. Adrenal insufficiency in acute oral opiate therapy. Endocrinol Diabetes Metab Case Rep. 2014;2014:130071. doi: 10.1530/EDM-13-0071.
4. Donegan D and Bancos I. Opioid-induced adrenal insufficiency. Mayo Clin Proc. 2018 July;93(7):937-44. doi: 10.1016/j.mayocp.2018.04.010.
5. Lamprecht A et al. Secondary adrenal insufficiency and pituitary dysfunction in oral/transdermal opioid users with non-cancer pain. Eur J Endocrinol. 2018 Dec 1;179(6):353-62. doi: 10.1530/EJE-18-0530.
6. Gibb FW et al. Adrenal insufficiency in patients on long-term opioid analgesia. Clin Endocrinol (Oxf). 2016 June;85(6):831-5. doi:10.1111/cen.13125.
7. Abs R et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000 June;85(6):2215-22. doi: 10.1210/jcem.85.6.6615.
8. Tabet EJ et al. Opioid-induced hypoadrenalism resulting in fasting hypoglycaemia. BMJ Case Rep. 2019 Dec 11;12(12):e230551. doi: 10.1136/bcr-2019-230551.
9. Li T et al. Clinical presentation and outcomes of opioid induced adrenal insufficiency. Endocr Pract. 2020 Nov;26(11):1291-1297. doi: 10.4158/EP-2020-0297.
10. Grossman AB. Clinical Review: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010 Nov;95(11):4855-63. doi: 10.1210/jc.2010-0982.
11. Charmandari E et al. Adrenal insufficiency. Lancet. 2014 June 21;383(9935):2152-67. doi: 10.1016/S0140-6736(13)61684-0.
Key points
- Opioids can cause central adrenal insufficiency because of tonic suppression of the HPA axis. This effect is likely dose dependent, and reversible upon tapering or withdrawal of opioids.
- The prevalence of biochemical OIAI in chronic opioid users of 8%-29% clinical AI is less frequent but may be underrecognized in hospitalized patients leading to delayed diagnosis.
- Diagnosis of central adrenal insufficiency is based upon low 8 a.m. cortisol and ACTH levels and/or an abnormal CST. OIAI is the likely etiology in patients on chronic opioids for whom other causes of central adrenal insufficiency have been ruled out.
- Management with glucocorticoid replacement is variable depending on clinical presentation, severity of HPA axis suppression, and ability to wean opioid therapy. Patient education regarding symptoms of AI and stress dosing is essential.
Additional reading
Grossman AB. Clinical Review: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010 Nov;95(11):4855-63. doi: 10.1210/jc.2010-0982.
Donegan D and Bancos I. Opioid-induced adrenal insufficiency. Mayo Clin Proc. 2018 July;93(7):937-44. doi: 10.1016/j.mayocp.2018.04.010.
Li T et al. Clinical presentation and outcomes of opioid induced adrenal insufficiency. Endocr Pract. 2020 Nov;26(11):1291-7. doi: 10.4158/EP-2020-0297.
Quiz
A 55-year-old man with chronic back pain, for which he takes a total of 90 mg of oral morphine daily, is admitted for pyelonephritis with fever, nausea, vomiting, dysuria, and abdominal pain. He is febrile and tachycardic on presentation, but his vitals quickly normalize after hydration and antibiotics. About 48 hours into his hospitalization his fevers, dysuria, and abdominal pain have resolved, but he has persistent nausea and headaches. On further questioning, he also reports weight loss and fatigue over the past 3 weeks. He is found to have a morning cortisol level less than 5 mcg/dL, as well as low levels of ACTH and DHEAS. OIAI is suspected.
Which of the following is true about management?
A. Glucocorticoid replacement therapy with oral hydrocortisone should be considered to improve his symptoms.
B. Tapering off opioids is unlikely to resolve his adrenal insufficiency.
C. Stress dose steroids should be started immediately with high-dose intravenous hydrocortisone.
D. Given high clinical suspicion for OIAI, further testing for other etiologies of central adrenal insufficiency is not recommended.
Explanation of correct answer
The correct answer is A. This patient’s ongoing nonspecific symptoms that have persisted despite treatment of his acute pyelonephritis are likely caused by adrenal insufficiency. In a symptomatic patient with OIAI, treatment with oral hydrocortisone should be considered to control symptoms and facilitate tapering opioids. Tapering and stopping opioids often leads to recovery of the HPA axis and resolution of the OIAI. Tapering opioids should be considered a mainstay of therapy for OIAI when clinically appropriate, as in this patient with chronic benign pain. Stress dose steroids are not indicated in the absence of critical illness, adrenal crisis, or major surgery. OIAI is a diagnosis of exclusion, and patients should undergo workup for other causes of secondary adrenal insufficiency.
Unvaccinated people likely to catch COVID repeatedly
according to a recent study published in The Lancet Microbe.
Since COVID-19 hasn’t existed for long enough to perform a long-term study, researchers at Yale University and the University of North Carolina at Charlotte looked at reinfection data for six other human-infecting coronaviruses, including SARS and MERS.
“Reinfection can reasonably happen in three months or less,” Jeffrey Townsend, PhD, lead study author and a biostatistics professor at the Yale School of Public Health, said in a statement.
“Therefore, those who have been naturally infected should get vaccinated,” he said. “Previous infection alone can offer very little long-term protection against subsequent infections.”
The research team looked at post-infection data for six coronaviruses between 1984-2020 and found reinfection ranged from 128 days to 28 years. They calculated that reinfection with COVID-19 would likely occur between 3 months to 5 years after peak antibody response, with an average of 16 months. This is less than half the duration seen for other coronaviruses that circulate among humans.
The risk of COVID-19 reinfection is about 5% at three months, which jumps to 50% after 17 months, the research team found. Reinfection could become increasingly common as immunity wanes and new variants develop, they said.
“We tend to think about immunity as being immune or not immune. Our study cautions that we instead should be more focused on the risk of reinfection through time,” Alex Dornburg, PhD, senior study author and assistant professor of bioinformatics and genomics at UNC, said in the statement.
“As new variants arise, previous immune responses become less effective at combating the virus,” he said. “Those who were naturally infected early in the pandemic are increasingly likely to become reinfected in the near future.”
Study estimates are based on average times of declining immunity across different coronaviruses, the researchers told the Yale Daily News. At the individual level, people have different levels of immunity, which can provide shorter or longer duration of protection based on immune status, immunity within a community, age, underlying health conditions, environmental exposure, and other factors.
The research team said that preventive health measures and global distribution of vaccines will be “critical” in minimizing reinfection and COVID-19 deaths. In areas with low vaccination rates, for instance, unvaccinated people should continue safety practices such as social distancing, wearing masks, and proper indoor ventilation to avoid reinfection.
“We need to be very aware of the fact that this disease is likely to be circulating over the long term and that we don’t have this long-term immunity that many people seem to be hoping to rely on in order to protect them from disease,” Dr. Townsend told the newspaper.
A version of this article first appeared on WebMD.com.
according to a recent study published in The Lancet Microbe.
Since COVID-19 hasn’t existed for long enough to perform a long-term study, researchers at Yale University and the University of North Carolina at Charlotte looked at reinfection data for six other human-infecting coronaviruses, including SARS and MERS.
“Reinfection can reasonably happen in three months or less,” Jeffrey Townsend, PhD, lead study author and a biostatistics professor at the Yale School of Public Health, said in a statement.
“Therefore, those who have been naturally infected should get vaccinated,” he said. “Previous infection alone can offer very little long-term protection against subsequent infections.”
The research team looked at post-infection data for six coronaviruses between 1984-2020 and found reinfection ranged from 128 days to 28 years. They calculated that reinfection with COVID-19 would likely occur between 3 months to 5 years after peak antibody response, with an average of 16 months. This is less than half the duration seen for other coronaviruses that circulate among humans.
The risk of COVID-19 reinfection is about 5% at three months, which jumps to 50% after 17 months, the research team found. Reinfection could become increasingly common as immunity wanes and new variants develop, they said.
“We tend to think about immunity as being immune or not immune. Our study cautions that we instead should be more focused on the risk of reinfection through time,” Alex Dornburg, PhD, senior study author and assistant professor of bioinformatics and genomics at UNC, said in the statement.
“As new variants arise, previous immune responses become less effective at combating the virus,” he said. “Those who were naturally infected early in the pandemic are increasingly likely to become reinfected in the near future.”
Study estimates are based on average times of declining immunity across different coronaviruses, the researchers told the Yale Daily News. At the individual level, people have different levels of immunity, which can provide shorter or longer duration of protection based on immune status, immunity within a community, age, underlying health conditions, environmental exposure, and other factors.
The research team said that preventive health measures and global distribution of vaccines will be “critical” in minimizing reinfection and COVID-19 deaths. In areas with low vaccination rates, for instance, unvaccinated people should continue safety practices such as social distancing, wearing masks, and proper indoor ventilation to avoid reinfection.
“We need to be very aware of the fact that this disease is likely to be circulating over the long term and that we don’t have this long-term immunity that many people seem to be hoping to rely on in order to protect them from disease,” Dr. Townsend told the newspaper.
A version of this article first appeared on WebMD.com.
according to a recent study published in The Lancet Microbe.
Since COVID-19 hasn’t existed for long enough to perform a long-term study, researchers at Yale University and the University of North Carolina at Charlotte looked at reinfection data for six other human-infecting coronaviruses, including SARS and MERS.
“Reinfection can reasonably happen in three months or less,” Jeffrey Townsend, PhD, lead study author and a biostatistics professor at the Yale School of Public Health, said in a statement.
“Therefore, those who have been naturally infected should get vaccinated,” he said. “Previous infection alone can offer very little long-term protection against subsequent infections.”
The research team looked at post-infection data for six coronaviruses between 1984-2020 and found reinfection ranged from 128 days to 28 years. They calculated that reinfection with COVID-19 would likely occur between 3 months to 5 years after peak antibody response, with an average of 16 months. This is less than half the duration seen for other coronaviruses that circulate among humans.
The risk of COVID-19 reinfection is about 5% at three months, which jumps to 50% after 17 months, the research team found. Reinfection could become increasingly common as immunity wanes and new variants develop, they said.
“We tend to think about immunity as being immune or not immune. Our study cautions that we instead should be more focused on the risk of reinfection through time,” Alex Dornburg, PhD, senior study author and assistant professor of bioinformatics and genomics at UNC, said in the statement.
“As new variants arise, previous immune responses become less effective at combating the virus,” he said. “Those who were naturally infected early in the pandemic are increasingly likely to become reinfected in the near future.”
Study estimates are based on average times of declining immunity across different coronaviruses, the researchers told the Yale Daily News. At the individual level, people have different levels of immunity, which can provide shorter or longer duration of protection based on immune status, immunity within a community, age, underlying health conditions, environmental exposure, and other factors.
The research team said that preventive health measures and global distribution of vaccines will be “critical” in minimizing reinfection and COVID-19 deaths. In areas with low vaccination rates, for instance, unvaccinated people should continue safety practices such as social distancing, wearing masks, and proper indoor ventilation to avoid reinfection.
“We need to be very aware of the fact that this disease is likely to be circulating over the long term and that we don’t have this long-term immunity that many people seem to be hoping to rely on in order to protect them from disease,” Dr. Townsend told the newspaper.
A version of this article first appeared on WebMD.com.
Fluoroquinolones linked to sudden death risk for those on hemodialysis
, a large observational study suggests.
However, in many cases, the absolute risk is relatively small, and the antimicrobial benefits of a fluoroquinolone may outweigh the potential cardiac risks, the researchers say.
“Pathogen-directed treatment of respiratory infections is of the utmost importance. Respiratory fluoroquinolones should be prescribed whenever an amoxicillin-based antibiotic offers suboptimal antimicrobial coverage and clinicians should consider electrocardiographic monitoring,” first author Magdalene M. Assimon, PharmD, PhD, University of North Carolina, Chapel Hill, told this news organization.
The study was published online Oct. 20 in JAMA Cardiology (doi: 10.1001/jamacardio.2021.4234).
Nearly twofold increased risk
The QT interval-prolonging potential of fluoroquinolone antibiotics are well known. However, evidence linking respiratory fluoroquinolones to adverse cardiac outcomes in the hemodialysis population is limited.
These new observational findings are based on a total of 626,322 antibiotic treatment episodes among 264,968 adults (mean age, 61 years; 51% men) receiving in-center hemodialysis – with respiratory fluoroquinolone making up 40.2% of treatment episodes and amoxicillin-based antibiotic treatment episodes making up 59.8%.
The rate of SCD within 5 days of outpatient initiation of a study antibiotic was 105.7 per 100,000 people prescribed a respiratory fluoroquinolone (levofloxacin or moxifloxacin) versus with 40.0 per 100,000 prescribed amoxicillin or amoxicillin with clavulanic acid (weighted hazard ratio: 1.95; 95% confidence interval, 1.57-2.41).
The authors estimate that one additional SCD would occur during a 5-day follow-up period for every 2,273 respiratory fluoroquinolone treatment episodes. Consistent associations were seen when follow-up was extended to 7, 10, and 14 days.
“Our data suggest that curtailing respiratory fluoroquinolone prescribing may be one actionable strategy to mitigate SCD risk in the hemodialysis population. However, the associated absolute risk reduction would be relatively small,” wrote the authors.
They noted that the rate of SCD in the hemodialysis population exceeds that of the general population by more than 20-fold. Most patients undergoing hemodialysis have a least one risk factor for drug-induced QT interval prolongation.
In the current study, nearly 20% of hemodialysis patients prescribed a respiratory fluoroquinolone were taking other medications with known risk for torsades de pointes.
“Our results emphasize the importance of performing a thorough medication review and considering pharmacodynamic drug interactions before prescribing new drug therapies for any condition,” Dr. Assimon and colleagues advised.
They suggest that clinicians consider electrocardiographic monitoring before and during fluoroquinolone therapy in hemodialysis patients, especially in high-risk individuals.
Valuable study
Reached for comment, Ankur Shah, MD, of the division of kidney diseases and hypertension, Brown University, Providence, R.I., called the analysis “valuable” and said the results are “consistent with the known association of cardiac arrhythmias with respiratory fluoroquinolone use in the general population, postulated to be due to increased risk of torsades de pointes from QTc prolongation. This abnormal heart rhythm can lead to sudden cardiac death.
“Notably, the population receiving respiratory fluoroquinolones had a higher incidence of cardiac disease at baseline, but the risk persisted after adjustment for this increased burden of comorbidity,” Dr. Shah said in an interview. He was not involved in the current research.
Dr. Shah cautioned that observational data such as these should be considered more “hypothesis-generating than practice-changing, as there may be unrecognized confounders or differences in the population that received the respiratory fluoroquinolones.
“A prospective randomized trial would provide a definitive answer, but in the interim, caution should be taken in using respiratory fluoroquinolones when local bacterial resistance patterns or patient-specific data offer another option,” Dr. Shah concluded.
Dr. Assimon reported receiving grants from the Renal Research Institute (a subsidiary of Fresenius Medical Care), honoraria from the International Society of Nephrology for serving as a statistical reviewer for Kidney International Reports, and honoraria from the American Society of Nephrology for serving as an editorial fellow for the Journal of the American Society of Nephrology. Dr. Shah has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a large observational study suggests.
However, in many cases, the absolute risk is relatively small, and the antimicrobial benefits of a fluoroquinolone may outweigh the potential cardiac risks, the researchers say.
“Pathogen-directed treatment of respiratory infections is of the utmost importance. Respiratory fluoroquinolones should be prescribed whenever an amoxicillin-based antibiotic offers suboptimal antimicrobial coverage and clinicians should consider electrocardiographic monitoring,” first author Magdalene M. Assimon, PharmD, PhD, University of North Carolina, Chapel Hill, told this news organization.
The study was published online Oct. 20 in JAMA Cardiology (doi: 10.1001/jamacardio.2021.4234).
Nearly twofold increased risk
The QT interval-prolonging potential of fluoroquinolone antibiotics are well known. However, evidence linking respiratory fluoroquinolones to adverse cardiac outcomes in the hemodialysis population is limited.
These new observational findings are based on a total of 626,322 antibiotic treatment episodes among 264,968 adults (mean age, 61 years; 51% men) receiving in-center hemodialysis – with respiratory fluoroquinolone making up 40.2% of treatment episodes and amoxicillin-based antibiotic treatment episodes making up 59.8%.
The rate of SCD within 5 days of outpatient initiation of a study antibiotic was 105.7 per 100,000 people prescribed a respiratory fluoroquinolone (levofloxacin or moxifloxacin) versus with 40.0 per 100,000 prescribed amoxicillin or amoxicillin with clavulanic acid (weighted hazard ratio: 1.95; 95% confidence interval, 1.57-2.41).
The authors estimate that one additional SCD would occur during a 5-day follow-up period for every 2,273 respiratory fluoroquinolone treatment episodes. Consistent associations were seen when follow-up was extended to 7, 10, and 14 days.
“Our data suggest that curtailing respiratory fluoroquinolone prescribing may be one actionable strategy to mitigate SCD risk in the hemodialysis population. However, the associated absolute risk reduction would be relatively small,” wrote the authors.
They noted that the rate of SCD in the hemodialysis population exceeds that of the general population by more than 20-fold. Most patients undergoing hemodialysis have a least one risk factor for drug-induced QT interval prolongation.
In the current study, nearly 20% of hemodialysis patients prescribed a respiratory fluoroquinolone were taking other medications with known risk for torsades de pointes.
“Our results emphasize the importance of performing a thorough medication review and considering pharmacodynamic drug interactions before prescribing new drug therapies for any condition,” Dr. Assimon and colleagues advised.
They suggest that clinicians consider electrocardiographic monitoring before and during fluoroquinolone therapy in hemodialysis patients, especially in high-risk individuals.
Valuable study
Reached for comment, Ankur Shah, MD, of the division of kidney diseases and hypertension, Brown University, Providence, R.I., called the analysis “valuable” and said the results are “consistent with the known association of cardiac arrhythmias with respiratory fluoroquinolone use in the general population, postulated to be due to increased risk of torsades de pointes from QTc prolongation. This abnormal heart rhythm can lead to sudden cardiac death.
“Notably, the population receiving respiratory fluoroquinolones had a higher incidence of cardiac disease at baseline, but the risk persisted after adjustment for this increased burden of comorbidity,” Dr. Shah said in an interview. He was not involved in the current research.
Dr. Shah cautioned that observational data such as these should be considered more “hypothesis-generating than practice-changing, as there may be unrecognized confounders or differences in the population that received the respiratory fluoroquinolones.
“A prospective randomized trial would provide a definitive answer, but in the interim, caution should be taken in using respiratory fluoroquinolones when local bacterial resistance patterns or patient-specific data offer another option,” Dr. Shah concluded.
Dr. Assimon reported receiving grants from the Renal Research Institute (a subsidiary of Fresenius Medical Care), honoraria from the International Society of Nephrology for serving as a statistical reviewer for Kidney International Reports, and honoraria from the American Society of Nephrology for serving as an editorial fellow for the Journal of the American Society of Nephrology. Dr. Shah has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a large observational study suggests.
However, in many cases, the absolute risk is relatively small, and the antimicrobial benefits of a fluoroquinolone may outweigh the potential cardiac risks, the researchers say.
“Pathogen-directed treatment of respiratory infections is of the utmost importance. Respiratory fluoroquinolones should be prescribed whenever an amoxicillin-based antibiotic offers suboptimal antimicrobial coverage and clinicians should consider electrocardiographic monitoring,” first author Magdalene M. Assimon, PharmD, PhD, University of North Carolina, Chapel Hill, told this news organization.
The study was published online Oct. 20 in JAMA Cardiology (doi: 10.1001/jamacardio.2021.4234).
Nearly twofold increased risk
The QT interval-prolonging potential of fluoroquinolone antibiotics are well known. However, evidence linking respiratory fluoroquinolones to adverse cardiac outcomes in the hemodialysis population is limited.
These new observational findings are based on a total of 626,322 antibiotic treatment episodes among 264,968 adults (mean age, 61 years; 51% men) receiving in-center hemodialysis – with respiratory fluoroquinolone making up 40.2% of treatment episodes and amoxicillin-based antibiotic treatment episodes making up 59.8%.
The rate of SCD within 5 days of outpatient initiation of a study antibiotic was 105.7 per 100,000 people prescribed a respiratory fluoroquinolone (levofloxacin or moxifloxacin) versus with 40.0 per 100,000 prescribed amoxicillin or amoxicillin with clavulanic acid (weighted hazard ratio: 1.95; 95% confidence interval, 1.57-2.41).
The authors estimate that one additional SCD would occur during a 5-day follow-up period for every 2,273 respiratory fluoroquinolone treatment episodes. Consistent associations were seen when follow-up was extended to 7, 10, and 14 days.
“Our data suggest that curtailing respiratory fluoroquinolone prescribing may be one actionable strategy to mitigate SCD risk in the hemodialysis population. However, the associated absolute risk reduction would be relatively small,” wrote the authors.
They noted that the rate of SCD in the hemodialysis population exceeds that of the general population by more than 20-fold. Most patients undergoing hemodialysis have a least one risk factor for drug-induced QT interval prolongation.
In the current study, nearly 20% of hemodialysis patients prescribed a respiratory fluoroquinolone were taking other medications with known risk for torsades de pointes.
“Our results emphasize the importance of performing a thorough medication review and considering pharmacodynamic drug interactions before prescribing new drug therapies for any condition,” Dr. Assimon and colleagues advised.
They suggest that clinicians consider electrocardiographic monitoring before and during fluoroquinolone therapy in hemodialysis patients, especially in high-risk individuals.
Valuable study
Reached for comment, Ankur Shah, MD, of the division of kidney diseases and hypertension, Brown University, Providence, R.I., called the analysis “valuable” and said the results are “consistent with the known association of cardiac arrhythmias with respiratory fluoroquinolone use in the general population, postulated to be due to increased risk of torsades de pointes from QTc prolongation. This abnormal heart rhythm can lead to sudden cardiac death.
“Notably, the population receiving respiratory fluoroquinolones had a higher incidence of cardiac disease at baseline, but the risk persisted after adjustment for this increased burden of comorbidity,” Dr. Shah said in an interview. He was not involved in the current research.
Dr. Shah cautioned that observational data such as these should be considered more “hypothesis-generating than practice-changing, as there may be unrecognized confounders or differences in the population that received the respiratory fluoroquinolones.
“A prospective randomized trial would provide a definitive answer, but in the interim, caution should be taken in using respiratory fluoroquinolones when local bacterial resistance patterns or patient-specific data offer another option,” Dr. Shah concluded.
Dr. Assimon reported receiving grants from the Renal Research Institute (a subsidiary of Fresenius Medical Care), honoraria from the International Society of Nephrology for serving as a statistical reviewer for Kidney International Reports, and honoraria from the American Society of Nephrology for serving as an editorial fellow for the Journal of the American Society of Nephrology. Dr. Shah has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A pill for C. difficile works by increasing microbiome diversity
CP101, under development by Finch Therapeutics, proved more effective than a placebo in preventing recurrent infections for up to 24 weeks.
The CP101 capsules contain a powder of freeze-dried human stools from screened donors. They restore natural diversity that has been disrupted by antibiotics, said Jessica Allegretti, MD, MPH a gastroenterologist at Brigham and Women’s Hospital in Boston.
The treatment offers an alternative to fecal microbiota transplant, which can effectively treat antibiotic-resistant C. difficile infections but is difficult to standardize and administer – and doesn’t have full approval from the U.S. Food and Drug Administration, she added.
“I think this marks a moment in this space where we’re going to have better, safer, and more available options for patients,” she said in an interview. “It’s exciting.”
Dr. Allegretti is an author on three presentations of results from PRISM3, a phase 2 trial of CP101. They will be presented this week at the annual meeting of the American College of Gastroenterology. These results extend out to 24 weeks, whereas the 8-week results of this trial were presented a year ago at the same meeting.
Study details
The study enrolled 198 people who received antibiotics for recurrent C. difficile infections. Some patients had two or more recurrences, while others had only one recurrence but were 65 years of age or older.
“That was a unique aspect of this study, to see the effect of bringing a therapy like CP101 earlier in the treatment paradigm,” said Dr. Allegretti. “You can imagine for an older, frail, or more fragile patient that you would want to get rid of this [infection] earlier.”
After waiting 2-6 days for the antibiotics to wash out, the researchers randomly assigned 102 of these patients to take the CP101 pills orally and 96 to take placebo pills, both without bowel preparation.
The two groups were not significantly different in age, gender, comorbidities, the number of C. difficile recurrences, or the type of test used to diagnose the infection (PCR-based vs. toxin EIA-based).
After 8 weeks, 74.5% of those given the CP101 pills had not had a recurrence, compared with 61.5% of those given the placebo. The difference was just barely statistically significant (P = .0488).
Sixteen weeks later, the effect endured, with 73.5% of the CP101 group and 59.4% of the placebo group still free of recurrence. The statistical significance of the difference improved slightly (P = .0347).
Drug-related emergent adverse events were similar between the two groups: 16.3% for the CP101 group vs. 19.2% for the placebo group. These were mostly gastrointestinal symptoms, and none were serious.
Some of the patients received vancomycin as a first-line treatment for C. difficile infections, and the researchers wondered if the washout period was not sufficient to purge that antibiotic, leaving enough to interfere with the effectiveness of CP101.
Therefore, they separately analyzed 40 patients treated with fidaxomicin, which they expected to wash out more quickly. Among these patients, 81% who received CP101 were free of recurrences, at 8 weeks and 24 weeks. This compared with 42.1% of those who received the placebo, at both time points. This difference was more statistically significant (P = .0211).
Understanding how it works
To understand better how CP101 achieves its effects, the researchers collected stool samples from the patients and counted the number of different kinds organisms in each sample.
At baseline, the patients had about the same number, but after a week the diversity was greater in the patients treated with CP101, and that difference had increased at week 8. The researchers also found much less diversity of organisms in the stools of those patients who had recurrences of C. difficile infection.
The diversity of microbes in the successfully treated patients appeared to have been introduced by CP101. Dr. Allegretti and colleagues measured the number of organisms in the stool samples that came from CP101. They found that 96% of patients colonized by the CP101 organisms had avoided recurrence of the C. difficile infections, compared with 54.2% of those patients not colonized by these microbes.
“We now have some microbiome-based markers that show us as early as week 1 that the patient is going to be cured or not,” Dr. Allegretti said.
Based on these results, Finch plans to launch a phase 3 trial soon, she said.
The data on colonization is interesting because it has not been found with fecal microbiota transplants, said Purna Kashyap, MBBS, codirector of the Microbiome Program at the Mayo Clinic College of Medicine in Rochester, Minn., who was not involved in the study.
But to better interpret the data, it would be helpful to know more about how the placebo and CP101 groups compared at baseline with regard to medications, immunosuppression, and antibiotics used to treat the C. difficile infections, Dr. Kashyap said. He was struck by the lower cure rate in the portion of the placebo group treated with fidaxomicin.
“Overall, I think these are exciting observations based on the data but require careful review of the entire data to make sense of [them], which will happen when it goes through peer review,” he told this news organization in an email.
Several other standardized microbiota restoration products are under development, including at least two other capsules. In contrast to CP101, which is made up of whole stool, VE303 (Vedanta Biosciences) is a “rationally defined bacterial consortium,” and SER-109 (Seres Therapeutics) is a “consortium of highly purified Firmicutes spores.” VE303 has completed a phase 2 trial, and SER-109 has completed a phase 3 trial.
Dr. Allegretti is a consultant for Finch Therapeutics, which funded the trial. Dr. Kashyap has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CP101, under development by Finch Therapeutics, proved more effective than a placebo in preventing recurrent infections for up to 24 weeks.
The CP101 capsules contain a powder of freeze-dried human stools from screened donors. They restore natural diversity that has been disrupted by antibiotics, said Jessica Allegretti, MD, MPH a gastroenterologist at Brigham and Women’s Hospital in Boston.
The treatment offers an alternative to fecal microbiota transplant, which can effectively treat antibiotic-resistant C. difficile infections but is difficult to standardize and administer – and doesn’t have full approval from the U.S. Food and Drug Administration, she added.
“I think this marks a moment in this space where we’re going to have better, safer, and more available options for patients,” she said in an interview. “It’s exciting.”
Dr. Allegretti is an author on three presentations of results from PRISM3, a phase 2 trial of CP101. They will be presented this week at the annual meeting of the American College of Gastroenterology. These results extend out to 24 weeks, whereas the 8-week results of this trial were presented a year ago at the same meeting.
Study details
The study enrolled 198 people who received antibiotics for recurrent C. difficile infections. Some patients had two or more recurrences, while others had only one recurrence but were 65 years of age or older.
“That was a unique aspect of this study, to see the effect of bringing a therapy like CP101 earlier in the treatment paradigm,” said Dr. Allegretti. “You can imagine for an older, frail, or more fragile patient that you would want to get rid of this [infection] earlier.”
After waiting 2-6 days for the antibiotics to wash out, the researchers randomly assigned 102 of these patients to take the CP101 pills orally and 96 to take placebo pills, both without bowel preparation.
The two groups were not significantly different in age, gender, comorbidities, the number of C. difficile recurrences, or the type of test used to diagnose the infection (PCR-based vs. toxin EIA-based).
After 8 weeks, 74.5% of those given the CP101 pills had not had a recurrence, compared with 61.5% of those given the placebo. The difference was just barely statistically significant (P = .0488).
Sixteen weeks later, the effect endured, with 73.5% of the CP101 group and 59.4% of the placebo group still free of recurrence. The statistical significance of the difference improved slightly (P = .0347).
Drug-related emergent adverse events were similar between the two groups: 16.3% for the CP101 group vs. 19.2% for the placebo group. These were mostly gastrointestinal symptoms, and none were serious.
Some of the patients received vancomycin as a first-line treatment for C. difficile infections, and the researchers wondered if the washout period was not sufficient to purge that antibiotic, leaving enough to interfere with the effectiveness of CP101.
Therefore, they separately analyzed 40 patients treated with fidaxomicin, which they expected to wash out more quickly. Among these patients, 81% who received CP101 were free of recurrences, at 8 weeks and 24 weeks. This compared with 42.1% of those who received the placebo, at both time points. This difference was more statistically significant (P = .0211).
Understanding how it works
To understand better how CP101 achieves its effects, the researchers collected stool samples from the patients and counted the number of different kinds organisms in each sample.
At baseline, the patients had about the same number, but after a week the diversity was greater in the patients treated with CP101, and that difference had increased at week 8. The researchers also found much less diversity of organisms in the stools of those patients who had recurrences of C. difficile infection.
The diversity of microbes in the successfully treated patients appeared to have been introduced by CP101. Dr. Allegretti and colleagues measured the number of organisms in the stool samples that came from CP101. They found that 96% of patients colonized by the CP101 organisms had avoided recurrence of the C. difficile infections, compared with 54.2% of those patients not colonized by these microbes.
“We now have some microbiome-based markers that show us as early as week 1 that the patient is going to be cured or not,” Dr. Allegretti said.
Based on these results, Finch plans to launch a phase 3 trial soon, she said.
The data on colonization is interesting because it has not been found with fecal microbiota transplants, said Purna Kashyap, MBBS, codirector of the Microbiome Program at the Mayo Clinic College of Medicine in Rochester, Minn., who was not involved in the study.
But to better interpret the data, it would be helpful to know more about how the placebo and CP101 groups compared at baseline with regard to medications, immunosuppression, and antibiotics used to treat the C. difficile infections, Dr. Kashyap said. He was struck by the lower cure rate in the portion of the placebo group treated with fidaxomicin.
“Overall, I think these are exciting observations based on the data but require careful review of the entire data to make sense of [them], which will happen when it goes through peer review,” he told this news organization in an email.
Several other standardized microbiota restoration products are under development, including at least two other capsules. In contrast to CP101, which is made up of whole stool, VE303 (Vedanta Biosciences) is a “rationally defined bacterial consortium,” and SER-109 (Seres Therapeutics) is a “consortium of highly purified Firmicutes spores.” VE303 has completed a phase 2 trial, and SER-109 has completed a phase 3 trial.
Dr. Allegretti is a consultant for Finch Therapeutics, which funded the trial. Dr. Kashyap has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CP101, under development by Finch Therapeutics, proved more effective than a placebo in preventing recurrent infections for up to 24 weeks.
The CP101 capsules contain a powder of freeze-dried human stools from screened donors. They restore natural diversity that has been disrupted by antibiotics, said Jessica Allegretti, MD, MPH a gastroenterologist at Brigham and Women’s Hospital in Boston.
The treatment offers an alternative to fecal microbiota transplant, which can effectively treat antibiotic-resistant C. difficile infections but is difficult to standardize and administer – and doesn’t have full approval from the U.S. Food and Drug Administration, she added.
“I think this marks a moment in this space where we’re going to have better, safer, and more available options for patients,” she said in an interview. “It’s exciting.”
Dr. Allegretti is an author on three presentations of results from PRISM3, a phase 2 trial of CP101. They will be presented this week at the annual meeting of the American College of Gastroenterology. These results extend out to 24 weeks, whereas the 8-week results of this trial were presented a year ago at the same meeting.
Study details
The study enrolled 198 people who received antibiotics for recurrent C. difficile infections. Some patients had two or more recurrences, while others had only one recurrence but were 65 years of age or older.
“That was a unique aspect of this study, to see the effect of bringing a therapy like CP101 earlier in the treatment paradigm,” said Dr. Allegretti. “You can imagine for an older, frail, or more fragile patient that you would want to get rid of this [infection] earlier.”
After waiting 2-6 days for the antibiotics to wash out, the researchers randomly assigned 102 of these patients to take the CP101 pills orally and 96 to take placebo pills, both without bowel preparation.
The two groups were not significantly different in age, gender, comorbidities, the number of C. difficile recurrences, or the type of test used to diagnose the infection (PCR-based vs. toxin EIA-based).
After 8 weeks, 74.5% of those given the CP101 pills had not had a recurrence, compared with 61.5% of those given the placebo. The difference was just barely statistically significant (P = .0488).
Sixteen weeks later, the effect endured, with 73.5% of the CP101 group and 59.4% of the placebo group still free of recurrence. The statistical significance of the difference improved slightly (P = .0347).
Drug-related emergent adverse events were similar between the two groups: 16.3% for the CP101 group vs. 19.2% for the placebo group. These were mostly gastrointestinal symptoms, and none were serious.
Some of the patients received vancomycin as a first-line treatment for C. difficile infections, and the researchers wondered if the washout period was not sufficient to purge that antibiotic, leaving enough to interfere with the effectiveness of CP101.
Therefore, they separately analyzed 40 patients treated with fidaxomicin, which they expected to wash out more quickly. Among these patients, 81% who received CP101 were free of recurrences, at 8 weeks and 24 weeks. This compared with 42.1% of those who received the placebo, at both time points. This difference was more statistically significant (P = .0211).
Understanding how it works
To understand better how CP101 achieves its effects, the researchers collected stool samples from the patients and counted the number of different kinds organisms in each sample.
At baseline, the patients had about the same number, but after a week the diversity was greater in the patients treated with CP101, and that difference had increased at week 8. The researchers also found much less diversity of organisms in the stools of those patients who had recurrences of C. difficile infection.
The diversity of microbes in the successfully treated patients appeared to have been introduced by CP101. Dr. Allegretti and colleagues measured the number of organisms in the stool samples that came from CP101. They found that 96% of patients colonized by the CP101 organisms had avoided recurrence of the C. difficile infections, compared with 54.2% of those patients not colonized by these microbes.
“We now have some microbiome-based markers that show us as early as week 1 that the patient is going to be cured or not,” Dr. Allegretti said.
Based on these results, Finch plans to launch a phase 3 trial soon, she said.
The data on colonization is interesting because it has not been found with fecal microbiota transplants, said Purna Kashyap, MBBS, codirector of the Microbiome Program at the Mayo Clinic College of Medicine in Rochester, Minn., who was not involved in the study.
But to better interpret the data, it would be helpful to know more about how the placebo and CP101 groups compared at baseline with regard to medications, immunosuppression, and antibiotics used to treat the C. difficile infections, Dr. Kashyap said. He was struck by the lower cure rate in the portion of the placebo group treated with fidaxomicin.
“Overall, I think these are exciting observations based on the data but require careful review of the entire data to make sense of [them], which will happen when it goes through peer review,” he told this news organization in an email.
Several other standardized microbiota restoration products are under development, including at least two other capsules. In contrast to CP101, which is made up of whole stool, VE303 (Vedanta Biosciences) is a “rationally defined bacterial consortium,” and SER-109 (Seres Therapeutics) is a “consortium of highly purified Firmicutes spores.” VE303 has completed a phase 2 trial, and SER-109 has completed a phase 3 trial.
Dr. Allegretti is a consultant for Finch Therapeutics, which funded the trial. Dr. Kashyap has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACG 2021








