User login
Hyaluronidase for Skin Necrosis Induced by Amiodarone
To the Editor:
Amiodarone is an oral or intravenous (IV) drug commonly used to treat supraventricular and ventricular arrhythmia as well as atrial fibrillation.1 Adverse drug reactions associated with the use of amiodarone include pulmonary, gastrointestinal, thyroid, ocular, neurologic, and cutaneous reactions.1 Long-term use of amiodarone—typically more than 4 months—can lead to slate-gray skin discoloration and photosensitivity, both of which can be reversed with drug withdrawal.2,3 Phlebitis also has been described in less than 3% of patients who receive peripheral IV administration of amiodarone.4
Amiodarone-induced skin necrosis due to extravasation is a rare complication of this antiarrhythmic medication, with only 3 reported cases in the literature according to a PubMed search of articles indexed for MEDLINE using the search terms amiodarone and skin and (necrosis or ischemia or extravasation or reaction).5–7 Although hyaluronidase is a known therapy for extravasation of fluids, including parenteral nutrition and chemotherapy, its use for the treatment of extravasation from amiodarone is not well documented.6 We report a case of skin necrosis of the left dorsal forearm and the left dorsal and ventral hand following infusion of amiodarone through a peripheral IV line, which was treated with injections of hyaluronidase.
A 77-year-old man was admitted to the emergency department for sepsis secondary to cholangitis in the setting of an obstructive gallbladder stone. His medical history was notable for multivessel coronary artery disease and atrial flutter treated with ablation. One day after admission, endoscopic retrograde cholangiopancreatography was attempted and aborted due to atrial fibrillation with rapid ventricular response. A second endoscopic retrograde cholangiopancreatography attempt was made 4 days later, during which the patient underwent cardiac arrest. During this event, amiodarone was administered in a 200-mL solution (1.8 mg/mL) in 5% dextrose through a peripheral IV line in the left forearm. The patient was stabilized and transferred to the intensive care unit.
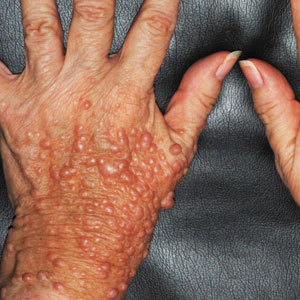
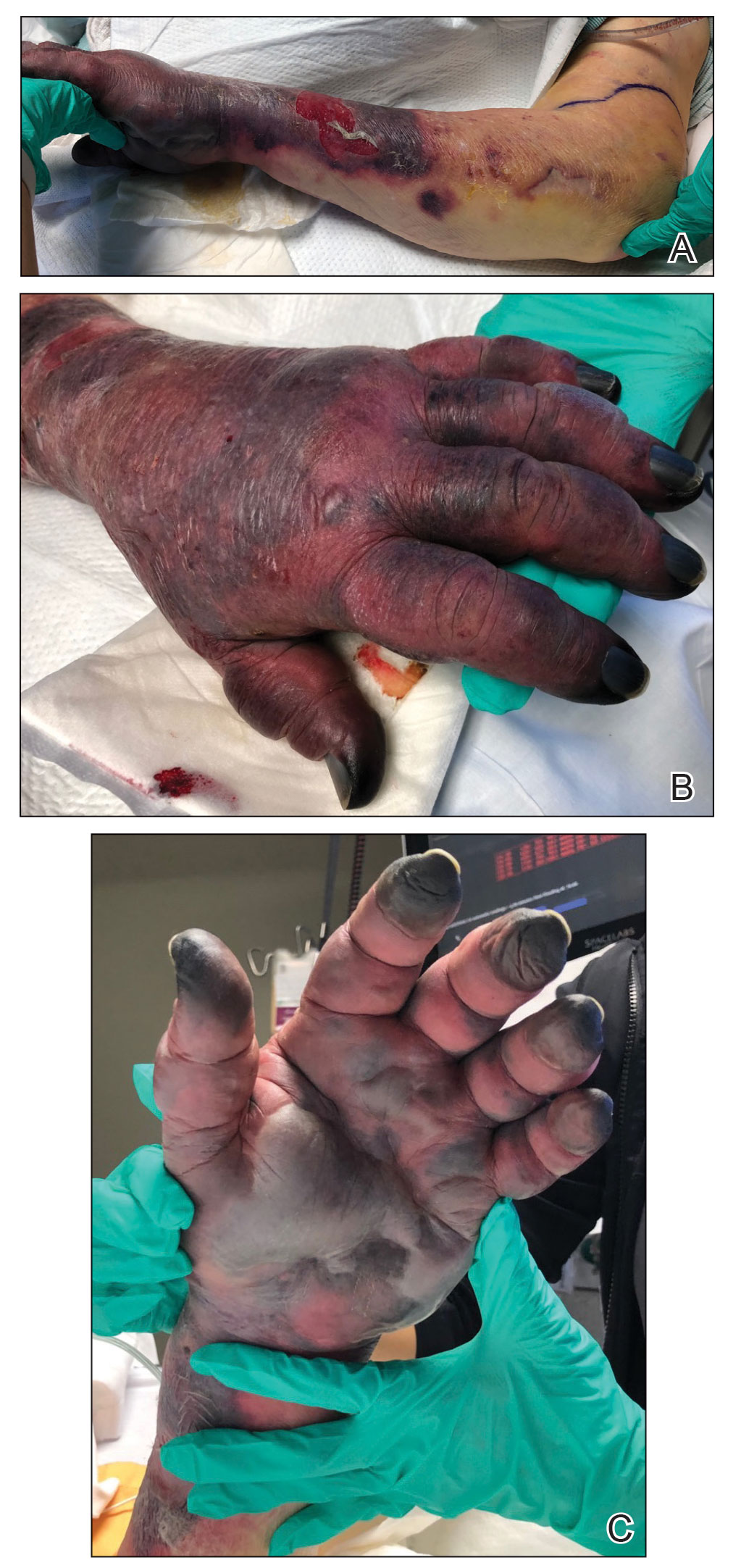
Twenty-four hours after amiodarone administration, erythema was noted on the left dorsal forearm. Within hours, the digits of the hand became a dark, dusky color, which spread to involve the forearm. Surgical debridement was not deemed necessary; the left arm was elevated, and warm compresses were applied regularly. Within the next week, the skin of the left hand and dorsal forearm had progressively worsened and took on a well-demarcated, dusky blue hue surrounded by an erythematous border involving the proximal forearm and upper arm (Figure 1A). The skin was fragile and had overlying bullae (Figure 1B).
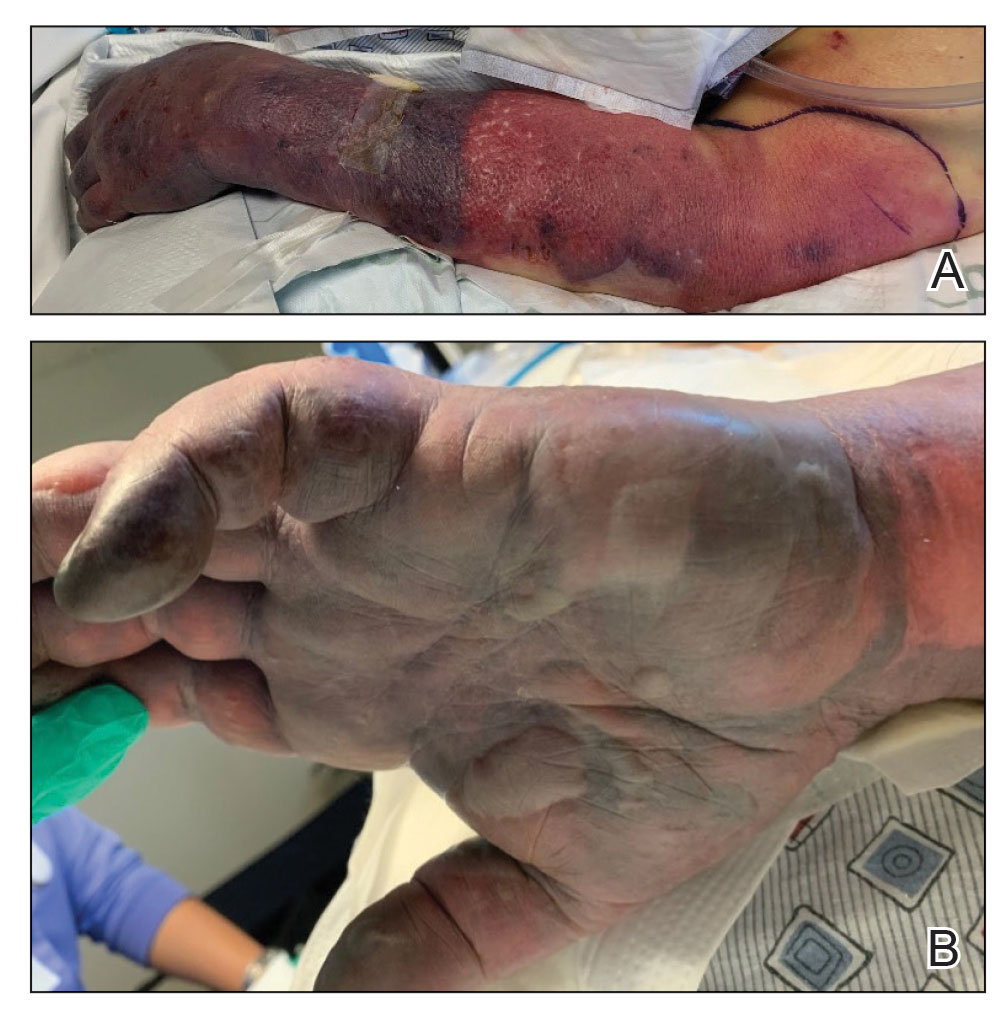
Hyaluronidase (1000 U) was injected into the surrounding areas of erythema, which resolved from the left proximal forearm to the elbow within 2 days after injection (Figure 2). The dusky violaceous patches were persistent, and the necrotic bullae were unchanged. Hyaluronidase (1000 U) was injected into necrotic skin of the left dorsal forearm and dorsal and ventral hand. No improvement was noted on subsequent evaluations of this area. While still an inpatient, he received wound care and twice-daily Doppler ultrasounds in the areas of necrosis. The patient lost sensation in the left hand with increased soft tissue necrosis and developed an eschar on the left dorsal forearm. Due to the progressive loss of function and necrosis, a partial forearm amputation was performed that healed well, and the patient experienced improvement in range of motion of the left upper extremity.
Well-known adverse reactions of amiodarone treatment include pulmonary fibrosis, hepatic dysfunction, hypothyroidism and hyperthyroidism, peripheral neuropathy, and corneal deposits.1 Cutaneous adverse reactions include photosensitivity (phototoxic and photoallergic reactions), hyperpigmentation, pseudoporphyria, and linear IgA bullous dermatosis. Less commonly, it also can cause urticaria, pruritus, erythema nodosum, purpura, and toxic epidermal necrolysis.3 Amiodarone-induced skin necrosis is rare, first described by Russell and Saltissi5 in 2006 in a 60-year-old man who developed dark discoloration and edema of the forearm 24 hours after initiation of an amiodarone peripheral IV. The patient was treated with hot or cold packs and steroid cream per the pharmaceutical company’s recommendations; however, patient outcomes were not discussed.5 A 77-year-old man who received subcutaneous amiodarone due to misplaced vascular access developed edema and bullae of the forearm followed by tissue necrosis, resulting in notably reduced mobility.6 Fox et al7 described a 60-year-old man who developed atrial fibrillation after emergent spinal fusion and laminectomy. He received intradermal hyaluronidase administration within 24 hours of developing severe pain from extravasation induced by amiodarone with no adverse outcomes and full recovery.7
There are numerous properties of amiodarone that may have resulted in the skin necrosis seen in these cases. The acidic pH (3.5–4.5) of amiodarone can contribute to coagulative necrosis, cellular desiccation, eschar formation, and edema.8 It also can contain additives such as polysorbate and benzyl alcohol, which may contribute to the drug’s vesicant properties.9
Current recommendations for IV administration of amiodarone include delivery through a central vein with high concentrations (>2 mg/mL) because peripheral infusion is slower and may cause phlebitis.4 In-line filters also may be a potential method of preventing phlebitis with peripheral IV administration of amiodarone.10 Extravasation of amiodarone can be treated nonpharmacologically with limb elevation and warm compresses, as these methods may promote vasodilation and enhance drug removal.5-7 However, when extravasation leads to progressive erythema and skin necrosis or is refractory to these therapies, intradermal injection of hyaluronidase should be considered. Hyaluronidase mediates the degradation of hyaluronic acid in the extracellular matrix, allowing for increased permeability of injected fluids into tissues and diluting the concentration of toxins at the site of exposure.9,11 It has been used to treat extravasation of fluids such as parenteral nutrition, electrolyte infusion, antibiotics, aminophylline, mannitol, and chemotherapy.11 Although hyaluronidase has been recognized as therapeutic for extravasation, there is no established consistent dosing or proper technique. In the setting of infiltration of chemotherapy, doses of hyaluronidase ranging from 150 to 1500 U/mL can be subcutaneously or intradermally injected into the site within 1 hour of extravasation. Side effects of using hyaluronidase are rare, including local pruritus, allergic reactions, urticaria, and angioedema.12
The patient described by Fox et al7 who fully recovered from amiodarone extravasation after hyaluronidase injections likely benefited from quick intervention, as he received amiodarone within 24 hours of the care team identifying initial erythema. Although our patient did have improvement of the areas of erythema on the forearm, evidence of skin and subcutaneous tissue necrosis on the left hand and proximal forearm was already apparent and not reversible, most likely caused by late intervention of intradermal hyaluronidase almost a week after the extravasation event. It is important to identify amiodarone as the source of extravasation and administer intradermal hyaluronidase in a timely fashion for extravasation refractory to conventional measurements to prevent progression to severe tissue damage.
Our case draws attention to the risk for skin necrosis with peripheral IV administration of amiodarone. Interventions include limb elevation, warm compresses, and consideration of intradermal hyaluronidase within 24 hours of extravasation, as this may reduce the severity of subsequent tissue damage with minimal side effects.
- Epstein AE, Olshansky B, Naccarelli GV, et al. Practical management guide for clinicians who treat patients with amiodarone. Am J Med. 2016;129:468-475. doi:10.1016/j.amjmed.2015.08.039
- Harris L, McKenna WJ, Rowland E, et al. Side effects of long-term amiodarone therapy. Circulation. 1983;67:45-51. doi:10.1161/01.cir.67.1.45
- Jaworski K, Walecka I, Rudnicka L, et al. Cutaneous adverse reactions of amiodarone. Med Sci Monit. 2014;20:2369-2372. doi:10.12659/MSM.890881
- Kowey Peter R, Marinchak Roger A, Rials Seth J, et al. Intravenous amiodarone. J Am Coll Cardiol. 1997;29:1190-1198. doi:10.1016/S0735-1097(97)00069-7
- Russell SJ, Saltissi S. Amiodarone induced skin necrosis. Heart. 2006;92:1395. doi:10.1136/hrt.2005.086157
- Grove EL. Skin necrosis and consequences of accidental subcutaneous administration of amiodarone. Ugeskr Laeger. 2015;177:V66928.
- Fox AN, Villanueva R, Miller JL. Management of amiodarone extravasation with intradermal hyaluronidase. Am J Health Syst Pharm. 2017;74:1545-1548. doi:10.2146/ajhp160737
- Reynolds PM, MacLaren R, Mueller SW, et al. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617-632. doi:https://doi.org/10.1002/phar.1396
- Le A, Patel S. Extravasation of noncytotoxic drugs: a review of the literature. Ann Pharmacother. 2014;48:870-886. doi:10.1177/1060028014527820
- Slim AM, Roth JE, Duffy B, et al. The incidence of phlebitis with intravenous amiodarone at guideline dose recommendations. Mil Med. 2007;172:1279-1283.
- Girish KS, Kemparaju K. The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci. 2007;80:1921-1943. doi:10.1016/j.lfs.2007.02.037
- Jung H. Hyaluronidase: an overview of its properties, applications, and side effects. Arch Plast Surg. 2020;47:297-300. doi:10.5999/aps.2020.00752
To the Editor:
Amiodarone is an oral or intravenous (IV) drug commonly used to treat supraventricular and ventricular arrhythmia as well as atrial fibrillation.1 Adverse drug reactions associated with the use of amiodarone include pulmonary, gastrointestinal, thyroid, ocular, neurologic, and cutaneous reactions.1 Long-term use of amiodarone—typically more than 4 months—can lead to slate-gray skin discoloration and photosensitivity, both of which can be reversed with drug withdrawal.2,3 Phlebitis also has been described in less than 3% of patients who receive peripheral IV administration of amiodarone.4
Amiodarone-induced skin necrosis due to extravasation is a rare complication of this antiarrhythmic medication, with only 3 reported cases in the literature according to a PubMed search of articles indexed for MEDLINE using the search terms amiodarone and skin and (necrosis or ischemia or extravasation or reaction).5–7 Although hyaluronidase is a known therapy for extravasation of fluids, including parenteral nutrition and chemotherapy, its use for the treatment of extravasation from amiodarone is not well documented.6 We report a case of skin necrosis of the left dorsal forearm and the left dorsal and ventral hand following infusion of amiodarone through a peripheral IV line, which was treated with injections of hyaluronidase.
A 77-year-old man was admitted to the emergency department for sepsis secondary to cholangitis in the setting of an obstructive gallbladder stone. His medical history was notable for multivessel coronary artery disease and atrial flutter treated with ablation. One day after admission, endoscopic retrograde cholangiopancreatography was attempted and aborted due to atrial fibrillation with rapid ventricular response. A second endoscopic retrograde cholangiopancreatography attempt was made 4 days later, during which the patient underwent cardiac arrest. During this event, amiodarone was administered in a 200-mL solution (1.8 mg/mL) in 5% dextrose through a peripheral IV line in the left forearm. The patient was stabilized and transferred to the intensive care unit.
Twenty-four hours after amiodarone administration, erythema was noted on the left dorsal forearm. Within hours, the digits of the hand became a dark, dusky color, which spread to involve the forearm. Surgical debridement was not deemed necessary; the left arm was elevated, and warm compresses were applied regularly. Within the next week, the skin of the left hand and dorsal forearm had progressively worsened and took on a well-demarcated, dusky blue hue surrounded by an erythematous border involving the proximal forearm and upper arm (Figure 1A). The skin was fragile and had overlying bullae (Figure 1B).

Hyaluronidase (1000 U) was injected into the surrounding areas of erythema, which resolved from the left proximal forearm to the elbow within 2 days after injection (Figure 2). The dusky violaceous patches were persistent, and the necrotic bullae were unchanged. Hyaluronidase (1000 U) was injected into necrotic skin of the left dorsal forearm and dorsal and ventral hand. No improvement was noted on subsequent evaluations of this area. While still an inpatient, he received wound care and twice-daily Doppler ultrasounds in the areas of necrosis. The patient lost sensation in the left hand with increased soft tissue necrosis and developed an eschar on the left dorsal forearm. Due to the progressive loss of function and necrosis, a partial forearm amputation was performed that healed well, and the patient experienced improvement in range of motion of the left upper extremity.

Well-known adverse reactions of amiodarone treatment include pulmonary fibrosis, hepatic dysfunction, hypothyroidism and hyperthyroidism, peripheral neuropathy, and corneal deposits.1 Cutaneous adverse reactions include photosensitivity (phototoxic and photoallergic reactions), hyperpigmentation, pseudoporphyria, and linear IgA bullous dermatosis. Less commonly, it also can cause urticaria, pruritus, erythema nodosum, purpura, and toxic epidermal necrolysis.3 Amiodarone-induced skin necrosis is rare, first described by Russell and Saltissi5 in 2006 in a 60-year-old man who developed dark discoloration and edema of the forearm 24 hours after initiation of an amiodarone peripheral IV. The patient was treated with hot or cold packs and steroid cream per the pharmaceutical company’s recommendations; however, patient outcomes were not discussed.5 A 77-year-old man who received subcutaneous amiodarone due to misplaced vascular access developed edema and bullae of the forearm followed by tissue necrosis, resulting in notably reduced mobility.6 Fox et al7 described a 60-year-old man who developed atrial fibrillation after emergent spinal fusion and laminectomy. He received intradermal hyaluronidase administration within 24 hours of developing severe pain from extravasation induced by amiodarone with no adverse outcomes and full recovery.7
There are numerous properties of amiodarone that may have resulted in the skin necrosis seen in these cases. The acidic pH (3.5–4.5) of amiodarone can contribute to coagulative necrosis, cellular desiccation, eschar formation, and edema.8 It also can contain additives such as polysorbate and benzyl alcohol, which may contribute to the drug’s vesicant properties.9
Current recommendations for IV administration of amiodarone include delivery through a central vein with high concentrations (>2 mg/mL) because peripheral infusion is slower and may cause phlebitis.4 In-line filters also may be a potential method of preventing phlebitis with peripheral IV administration of amiodarone.10 Extravasation of amiodarone can be treated nonpharmacologically with limb elevation and warm compresses, as these methods may promote vasodilation and enhance drug removal.5-7 However, when extravasation leads to progressive erythema and skin necrosis or is refractory to these therapies, intradermal injection of hyaluronidase should be considered. Hyaluronidase mediates the degradation of hyaluronic acid in the extracellular matrix, allowing for increased permeability of injected fluids into tissues and diluting the concentration of toxins at the site of exposure.9,11 It has been used to treat extravasation of fluids such as parenteral nutrition, electrolyte infusion, antibiotics, aminophylline, mannitol, and chemotherapy.11 Although hyaluronidase has been recognized as therapeutic for extravasation, there is no established consistent dosing or proper technique. In the setting of infiltration of chemotherapy, doses of hyaluronidase ranging from 150 to 1500 U/mL can be subcutaneously or intradermally injected into the site within 1 hour of extravasation. Side effects of using hyaluronidase are rare, including local pruritus, allergic reactions, urticaria, and angioedema.12
The patient described by Fox et al7 who fully recovered from amiodarone extravasation after hyaluronidase injections likely benefited from quick intervention, as he received amiodarone within 24 hours of the care team identifying initial erythema. Although our patient did have improvement of the areas of erythema on the forearm, evidence of skin and subcutaneous tissue necrosis on the left hand and proximal forearm was already apparent and not reversible, most likely caused by late intervention of intradermal hyaluronidase almost a week after the extravasation event. It is important to identify amiodarone as the source of extravasation and administer intradermal hyaluronidase in a timely fashion for extravasation refractory to conventional measurements to prevent progression to severe tissue damage.
Our case draws attention to the risk for skin necrosis with peripheral IV administration of amiodarone. Interventions include limb elevation, warm compresses, and consideration of intradermal hyaluronidase within 24 hours of extravasation, as this may reduce the severity of subsequent tissue damage with minimal side effects.
To the Editor:
Amiodarone is an oral or intravenous (IV) drug commonly used to treat supraventricular and ventricular arrhythmia as well as atrial fibrillation.1 Adverse drug reactions associated with the use of amiodarone include pulmonary, gastrointestinal, thyroid, ocular, neurologic, and cutaneous reactions.1 Long-term use of amiodarone—typically more than 4 months—can lead to slate-gray skin discoloration and photosensitivity, both of which can be reversed with drug withdrawal.2,3 Phlebitis also has been described in less than 3% of patients who receive peripheral IV administration of amiodarone.4
Amiodarone-induced skin necrosis due to extravasation is a rare complication of this antiarrhythmic medication, with only 3 reported cases in the literature according to a PubMed search of articles indexed for MEDLINE using the search terms amiodarone and skin and (necrosis or ischemia or extravasation or reaction).5–7 Although hyaluronidase is a known therapy for extravasation of fluids, including parenteral nutrition and chemotherapy, its use for the treatment of extravasation from amiodarone is not well documented.6 We report a case of skin necrosis of the left dorsal forearm and the left dorsal and ventral hand following infusion of amiodarone through a peripheral IV line, which was treated with injections of hyaluronidase.
A 77-year-old man was admitted to the emergency department for sepsis secondary to cholangitis in the setting of an obstructive gallbladder stone. His medical history was notable for multivessel coronary artery disease and atrial flutter treated with ablation. One day after admission, endoscopic retrograde cholangiopancreatography was attempted and aborted due to atrial fibrillation with rapid ventricular response. A second endoscopic retrograde cholangiopancreatography attempt was made 4 days later, during which the patient underwent cardiac arrest. During this event, amiodarone was administered in a 200-mL solution (1.8 mg/mL) in 5% dextrose through a peripheral IV line in the left forearm. The patient was stabilized and transferred to the intensive care unit.
Twenty-four hours after amiodarone administration, erythema was noted on the left dorsal forearm. Within hours, the digits of the hand became a dark, dusky color, which spread to involve the forearm. Surgical debridement was not deemed necessary; the left arm was elevated, and warm compresses were applied regularly. Within the next week, the skin of the left hand and dorsal forearm had progressively worsened and took on a well-demarcated, dusky blue hue surrounded by an erythematous border involving the proximal forearm and upper arm (Figure 1A). The skin was fragile and had overlying bullae (Figure 1B).

Hyaluronidase (1000 U) was injected into the surrounding areas of erythema, which resolved from the left proximal forearm to the elbow within 2 days after injection (Figure 2). The dusky violaceous patches were persistent, and the necrotic bullae were unchanged. Hyaluronidase (1000 U) was injected into necrotic skin of the left dorsal forearm and dorsal and ventral hand. No improvement was noted on subsequent evaluations of this area. While still an inpatient, he received wound care and twice-daily Doppler ultrasounds in the areas of necrosis. The patient lost sensation in the left hand with increased soft tissue necrosis and developed an eschar on the left dorsal forearm. Due to the progressive loss of function and necrosis, a partial forearm amputation was performed that healed well, and the patient experienced improvement in range of motion of the left upper extremity.

Well-known adverse reactions of amiodarone treatment include pulmonary fibrosis, hepatic dysfunction, hypothyroidism and hyperthyroidism, peripheral neuropathy, and corneal deposits.1 Cutaneous adverse reactions include photosensitivity (phototoxic and photoallergic reactions), hyperpigmentation, pseudoporphyria, and linear IgA bullous dermatosis. Less commonly, it also can cause urticaria, pruritus, erythema nodosum, purpura, and toxic epidermal necrolysis.3 Amiodarone-induced skin necrosis is rare, first described by Russell and Saltissi5 in 2006 in a 60-year-old man who developed dark discoloration and edema of the forearm 24 hours after initiation of an amiodarone peripheral IV. The patient was treated with hot or cold packs and steroid cream per the pharmaceutical company’s recommendations; however, patient outcomes were not discussed.5 A 77-year-old man who received subcutaneous amiodarone due to misplaced vascular access developed edema and bullae of the forearm followed by tissue necrosis, resulting in notably reduced mobility.6 Fox et al7 described a 60-year-old man who developed atrial fibrillation after emergent spinal fusion and laminectomy. He received intradermal hyaluronidase administration within 24 hours of developing severe pain from extravasation induced by amiodarone with no adverse outcomes and full recovery.7
There are numerous properties of amiodarone that may have resulted in the skin necrosis seen in these cases. The acidic pH (3.5–4.5) of amiodarone can contribute to coagulative necrosis, cellular desiccation, eschar formation, and edema.8 It also can contain additives such as polysorbate and benzyl alcohol, which may contribute to the drug’s vesicant properties.9
Current recommendations for IV administration of amiodarone include delivery through a central vein with high concentrations (>2 mg/mL) because peripheral infusion is slower and may cause phlebitis.4 In-line filters also may be a potential method of preventing phlebitis with peripheral IV administration of amiodarone.10 Extravasation of amiodarone can be treated nonpharmacologically with limb elevation and warm compresses, as these methods may promote vasodilation and enhance drug removal.5-7 However, when extravasation leads to progressive erythema and skin necrosis or is refractory to these therapies, intradermal injection of hyaluronidase should be considered. Hyaluronidase mediates the degradation of hyaluronic acid in the extracellular matrix, allowing for increased permeability of injected fluids into tissues and diluting the concentration of toxins at the site of exposure.9,11 It has been used to treat extravasation of fluids such as parenteral nutrition, electrolyte infusion, antibiotics, aminophylline, mannitol, and chemotherapy.11 Although hyaluronidase has been recognized as therapeutic for extravasation, there is no established consistent dosing or proper technique. In the setting of infiltration of chemotherapy, doses of hyaluronidase ranging from 150 to 1500 U/mL can be subcutaneously or intradermally injected into the site within 1 hour of extravasation. Side effects of using hyaluronidase are rare, including local pruritus, allergic reactions, urticaria, and angioedema.12
The patient described by Fox et al7 who fully recovered from amiodarone extravasation after hyaluronidase injections likely benefited from quick intervention, as he received amiodarone within 24 hours of the care team identifying initial erythema. Although our patient did have improvement of the areas of erythema on the forearm, evidence of skin and subcutaneous tissue necrosis on the left hand and proximal forearm was already apparent and not reversible, most likely caused by late intervention of intradermal hyaluronidase almost a week after the extravasation event. It is important to identify amiodarone as the source of extravasation and administer intradermal hyaluronidase in a timely fashion for extravasation refractory to conventional measurements to prevent progression to severe tissue damage.
Our case draws attention to the risk for skin necrosis with peripheral IV administration of amiodarone. Interventions include limb elevation, warm compresses, and consideration of intradermal hyaluronidase within 24 hours of extravasation, as this may reduce the severity of subsequent tissue damage with minimal side effects.
- Epstein AE, Olshansky B, Naccarelli GV, et al. Practical management guide for clinicians who treat patients with amiodarone. Am J Med. 2016;129:468-475. doi:10.1016/j.amjmed.2015.08.039
- Harris L, McKenna WJ, Rowland E, et al. Side effects of long-term amiodarone therapy. Circulation. 1983;67:45-51. doi:10.1161/01.cir.67.1.45
- Jaworski K, Walecka I, Rudnicka L, et al. Cutaneous adverse reactions of amiodarone. Med Sci Monit. 2014;20:2369-2372. doi:10.12659/MSM.890881
- Kowey Peter R, Marinchak Roger A, Rials Seth J, et al. Intravenous amiodarone. J Am Coll Cardiol. 1997;29:1190-1198. doi:10.1016/S0735-1097(97)00069-7
- Russell SJ, Saltissi S. Amiodarone induced skin necrosis. Heart. 2006;92:1395. doi:10.1136/hrt.2005.086157
- Grove EL. Skin necrosis and consequences of accidental subcutaneous administration of amiodarone. Ugeskr Laeger. 2015;177:V66928.
- Fox AN, Villanueva R, Miller JL. Management of amiodarone extravasation with intradermal hyaluronidase. Am J Health Syst Pharm. 2017;74:1545-1548. doi:10.2146/ajhp160737
- Reynolds PM, MacLaren R, Mueller SW, et al. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617-632. doi:https://doi.org/10.1002/phar.1396
- Le A, Patel S. Extravasation of noncytotoxic drugs: a review of the literature. Ann Pharmacother. 2014;48:870-886. doi:10.1177/1060028014527820
- Slim AM, Roth JE, Duffy B, et al. The incidence of phlebitis with intravenous amiodarone at guideline dose recommendations. Mil Med. 2007;172:1279-1283.
- Girish KS, Kemparaju K. The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci. 2007;80:1921-1943. doi:10.1016/j.lfs.2007.02.037
- Jung H. Hyaluronidase: an overview of its properties, applications, and side effects. Arch Plast Surg. 2020;47:297-300. doi:10.5999/aps.2020.00752
- Epstein AE, Olshansky B, Naccarelli GV, et al. Practical management guide for clinicians who treat patients with amiodarone. Am J Med. 2016;129:468-475. doi:10.1016/j.amjmed.2015.08.039
- Harris L, McKenna WJ, Rowland E, et al. Side effects of long-term amiodarone therapy. Circulation. 1983;67:45-51. doi:10.1161/01.cir.67.1.45
- Jaworski K, Walecka I, Rudnicka L, et al. Cutaneous adverse reactions of amiodarone. Med Sci Monit. 2014;20:2369-2372. doi:10.12659/MSM.890881
- Kowey Peter R, Marinchak Roger A, Rials Seth J, et al. Intravenous amiodarone. J Am Coll Cardiol. 1997;29:1190-1198. doi:10.1016/S0735-1097(97)00069-7
- Russell SJ, Saltissi S. Amiodarone induced skin necrosis. Heart. 2006;92:1395. doi:10.1136/hrt.2005.086157
- Grove EL. Skin necrosis and consequences of accidental subcutaneous administration of amiodarone. Ugeskr Laeger. 2015;177:V66928.
- Fox AN, Villanueva R, Miller JL. Management of amiodarone extravasation with intradermal hyaluronidase. Am J Health Syst Pharm. 2017;74:1545-1548. doi:10.2146/ajhp160737
- Reynolds PM, MacLaren R, Mueller SW, et al. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617-632. doi:https://doi.org/10.1002/phar.1396
- Le A, Patel S. Extravasation of noncytotoxic drugs: a review of the literature. Ann Pharmacother. 2014;48:870-886. doi:10.1177/1060028014527820
- Slim AM, Roth JE, Duffy B, et al. The incidence of phlebitis with intravenous amiodarone at guideline dose recommendations. Mil Med. 2007;172:1279-1283.
- Girish KS, Kemparaju K. The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci. 2007;80:1921-1943. doi:10.1016/j.lfs.2007.02.037
- Jung H. Hyaluronidase: an overview of its properties, applications, and side effects. Arch Plast Surg. 2020;47:297-300. doi:10.5999/aps.2020.00752
Practice Points
- Intravenous amiodarone administered peripherally can induce skin extravasation, leading to necrosis.
- Dermatologists should be aware that early intervention with intradermal hyaluronidase may reduce the severity of tissue damage caused by amiodarone-induced skin necrosis.
Genital Lentiginosis: A Benign Pigmentary Abnormality Often Raising Concern for Melanoma
To the Editor:
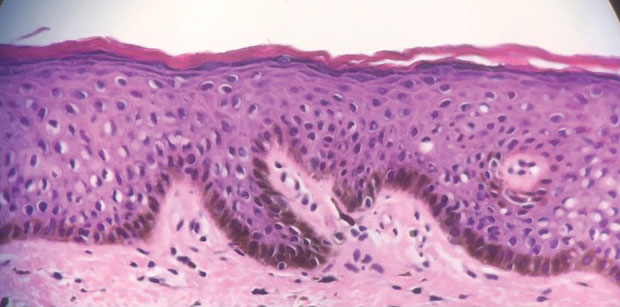
Genital lentiginosis (also known as mucosal melanotic macules, vulvar melanosis, penile melanosis, and penile lentigines) occurs in men and women.1 Lesions present in adult life as multifocal, asymmetrical, pigmented patches that can have a mottled appearance or exhibit skip areas. The irregular appearance of the pigmented areas often raises concern for melanoma. Biopsy reveals increased pigmentation along the basal layer of the epidermis; the irregular distribution of single melanocytes and pagetoid spread typical of melanoma in situ is not identified.
Genital lentiginosis usually occurs as an isolated finding; however, the condition can be a manifestation of Laugier-Hunziker syndrome, Carney complex, and Bannayan-Riley-Ruvalcaba syndrome.1-3 When it occurs as an isolated finding, the patient can be reassured and treatment is unnecessary. Because genital lentiginosis may mimic the appearance of melanoma, it is important for physicians to differentiate the two and make a correct diagnosis. We present a case of genital lentiginosis that mimicked vulvar melanoma.
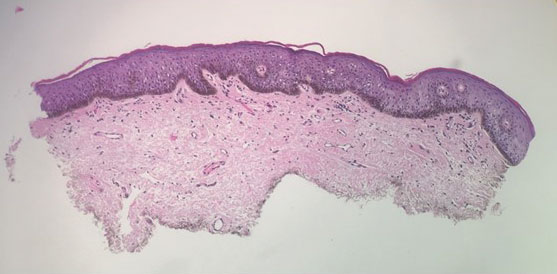
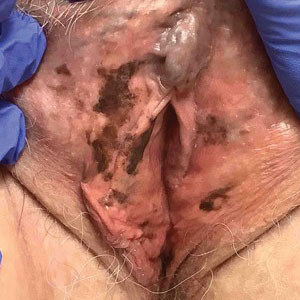
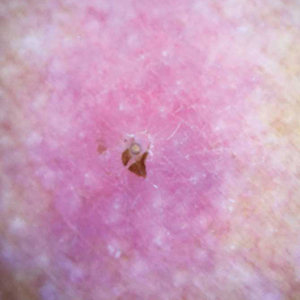
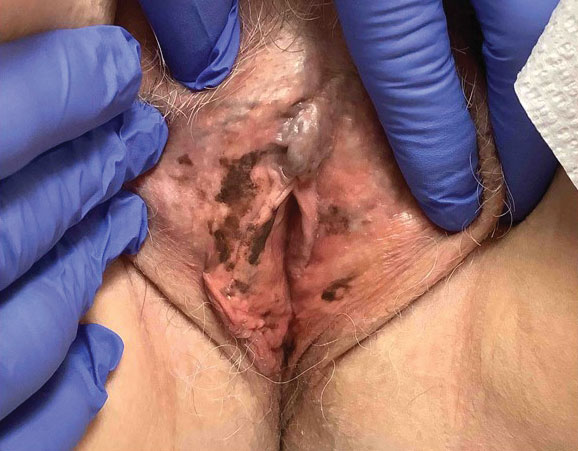
A 64-year-old woman was referred by her gynecologist to dermatology to rule out vulvar melanoma. The patient had a history of hypothyroidism and hypercholesterolemia but was otherwise in good health. Genital examination revealed asymptomatic pigmented macules and patches of unknown duration (Figure 1). Specimens were taken from 3 areas by punch biopsy to clarify the diagnosis. All 3 specimens showed identical features including basilar pigmentation, occasional melanophages in the papillary dermis, and no evidence of nests or pagetoid spread of atypical melanocytes (Figures 2 and 3). Histologic findings were diagnostic for genital lentiginosis. The patient was reassured, and no treatment was provided. At 6-month follow-up there was no change in clinical appearance.
Genital lentiginosis is characterized by brown lesions that can have a mottled appearance and often are associated with skip areas.1 Lesions can be strikingly irregular and darkly pigmented.
Although the lesions of genital lentiginosis most often are isolated findings, they can be a clue to several uncommon syndromes such as autosomal-dominant Bannayan-Riley-Ruvalcaba syndrome, which is associated with genital lentiginosis, intestinal polyposis, and macrocephaly.3 Vascular malformations, lipomatosis, verrucal keratoses, and acrochordons can occur. Bannayan-Riley-Ruvalcaba syndrome and Cowden syndrome may share genetic linkage; mutations in the tumor suppressor PTEN (phosphatase and tensin homolog deleted on chromosome ten) has been implicated in both syndromes.4 Underlying Carney complex should be excluded when genital lentiginosis is encountered.
Genital lentiginosis is idiopathic in most instances, but reports of lesions occurring after annular lichen planus suggest a possible mechanism.5 The disappearance of lentigines after imatinib therapy suggests a role for c-kit, a receptor tyrosine kinase that is involved in intracellular signaling, in some cases.6 At times, lesions can simulate trichrome vitiligo or have a reticulate pattern.7
Men and women present at different points in the course of disease. Men often present with penile lesions 14 years after onset, on average; they notice a gradual increase in the size of lesions. Because women can have greater difficulty self-examining the genital region, they tend to present much later in the course but often within a few months after initial inspection.1,8
Genital lentiginosis can mimic melanoma with nonhomogeneous pigmentation, asymmetry, and unilateral distribution, which makes dermoscopic assessment of colors helpful in narrowing the differential diagnosis. Melanoma is associated with combinations of gray, red, blue, and white, which are not found in genital lentiginosis.9
Biopsy of a genital lentigo is diagnostic, distinguishing the lesion from melanoma—failing to reveal the atypical melanocytes and pagetoid spread characteristic of melanoma in situ. Histologic findings can cause diagnostic difficulties when concurrent lichen sclerosus is associated with genital lentigines or nevi.10
Lentigines on sun-damaged skin or in the setting of xeroderma pigmentosum have been associated with melanoma,11-13 but genital lentigines are not considered a form of precancerous melanosis. In women, early diagnosis is important when there is concern for melanoma because the prognosis for vulvar melanoma is improved in thin lesions.14
Other entities in the differential include secondary syphilis, which commonly presents as macules and scaly papules and can be found on mucosal surfaces such as the oral cavity,15 as well as Kaposi sarcoma, which is characterized by purplish, brown, or black macules, plaques, and nodules, more commonly in immunosuppressed patients.16
To avoid unwarranted concern and unnecessary surgery, dermatologists should be aware of genital lentigines and their characteristic presentation in adults.
- Hwang L, Wilson H, Orengo I. Off-center fold: irregular, pigmented genital macules. Arch Dermatol. 2000;136:1559-1564. doi:10.1001/archderm.136.12.1559-b
- Rhodes AR, Silverman RA, Harrist TJ, et al. Mucocutaneous lentigines, cardiomucocutaneous myxomas, and multiple blue nevi: the “LAMB” syndrome. J Am Acad Dermatol. 1984;10:72-82. doi:10.1016/s0190-9622(84)80047-x
- Erkek E, Hizel S, Sanl C, et al. Clinical and histopathological findings in Bannayan-Riley-Ruvalcaba syndrome. J Am Acad Dermatol. 2005;53:639-643. doi:10.1016/j.jaad.2005.06.022
- Blum RR, Rahimizadeh A, Kardon N, et al. Genital lentigines in a 6-year-old boy with a family history of Cowden’s disease: clinical and genetic evidence of the linkage between Bannayan-Riley-Ruvalcaba syndrome and Cowden’s disease. J Cutan Med Surg. 2001;5:228-230. doi:10.1177/120347540100500307
- Isbary G, Dyall-Smith D, Coras-Stepanek B, et al. Penile lentigo (genital mucosal macule) following annular lichen planus: a possible association? Australas J Dermatol. 2014;55:159-161. doi:10.1111/ajd.12169
- Campbell T, Felsten L, Moore J. Disappearance of lentigines in a patient receiving imatinib treatment for familial gastrointestinal stromal tumor syndrome. Arch Dermatol. 2009;145:1313-1316. doi:10.1001/archdermatol.2009.263
- Romero- A, R, , et al. Reticulate genital pigmentation associated with localized vitiligo. Arch Dermatol. 2010; 146:574-575. doi:10.1001/archdermatol.2010.69
- Barnhill RL, Albert LS, Shama SK, et al. Genital lentiginosis: a clinical and histopathologic study. J Am Acad Dermatol. 1990;22:453-460. doi:10.1016/0190-9622(90)70064-o
- De Giorgi V, Gori A, Salvati L, et al. Clinical and dermoscopic features of vulvar melanosis over the last 20 years. JAMA Dermatol. 2020;156:1185–1191. doi:10.1001/jamadermatol.2020.2528
- El Shabrawi-Caelen L, Soyer HP, Schaeppi H, et al. Genital lentigines and melanocytic nevi with superimposed lichen sclerosus: a diagnostic challenge. J Am Acad Dermatol. 2004;50:690-694. doi:10.1016/j.jaad.2003.09.034
- Shatkin M, Helm MF, Muhlbauer A, et al. Solar lentigo evolving into fatal metastatic melanoma in a patient who initially refused surgery. N A J Med Sci. 2020;1:28-31. doi:10.7156/najms.2020.1301028
- Stern JB, Peck GL, Haupt HM, et al. Malignant melanoma in xeroderma pigmentosum: search for a precursor lesion. J Am Acad Dermatol. 1993;28:591-594. doi:10.1016/0190-9622(93)70079-9
- Byrom L, Barksdale S, Weedon D, et al. Unstable solar lentigo: a defined separate entity. Australas J Dermatol. 2016;57:229-234. doi:10.1111/ajd.12447
- Panizzon RG. Vulvar melanoma. Semin Dermatol. 1996;15:67-70. doi:10.1016/s1085-5629(96)80021-6
- Chapel TA. The signs and symptoms of secondary syphilis. Sex Transm Dis. 1980;7:161-164. doi:10.1097/00007435-198010000-00002
- Schwartz RA. Kaposi’s sarcoma: an update. J Surg Oncol. 2004;87:146-151. doi:10.1002/jso.20090
To the Editor:
Genital lentiginosis (also known as mucosal melanotic macules, vulvar melanosis, penile melanosis, and penile lentigines) occurs in men and women.1 Lesions present in adult life as multifocal, asymmetrical, pigmented patches that can have a mottled appearance or exhibit skip areas. The irregular appearance of the pigmented areas often raises concern for melanoma. Biopsy reveals increased pigmentation along the basal layer of the epidermis; the irregular distribution of single melanocytes and pagetoid spread typical of melanoma in situ is not identified.

Genital lentiginosis usually occurs as an isolated finding; however, the condition can be a manifestation of Laugier-Hunziker syndrome, Carney complex, and Bannayan-Riley-Ruvalcaba syndrome.1-3 When it occurs as an isolated finding, the patient can be reassured and treatment is unnecessary. Because genital lentiginosis may mimic the appearance of melanoma, it is important for physicians to differentiate the two and make a correct diagnosis. We present a case of genital lentiginosis that mimicked vulvar melanoma.

A 64-year-old woman was referred by her gynecologist to dermatology to rule out vulvar melanoma. The patient had a history of hypothyroidism and hypercholesterolemia but was otherwise in good health. Genital examination revealed asymptomatic pigmented macules and patches of unknown duration (Figure 1). Specimens were taken from 3 areas by punch biopsy to clarify the diagnosis. All 3 specimens showed identical features including basilar pigmentation, occasional melanophages in the papillary dermis, and no evidence of nests or pagetoid spread of atypical melanocytes (Figures 2 and 3). Histologic findings were diagnostic for genital lentiginosis. The patient was reassured, and no treatment was provided. At 6-month follow-up there was no change in clinical appearance.

Genital lentiginosis is characterized by brown lesions that can have a mottled appearance and often are associated with skip areas.1 Lesions can be strikingly irregular and darkly pigmented.
Although the lesions of genital lentiginosis most often are isolated findings, they can be a clue to several uncommon syndromes such as autosomal-dominant Bannayan-Riley-Ruvalcaba syndrome, which is associated with genital lentiginosis, intestinal polyposis, and macrocephaly.3 Vascular malformations, lipomatosis, verrucal keratoses, and acrochordons can occur. Bannayan-Riley-Ruvalcaba syndrome and Cowden syndrome may share genetic linkage; mutations in the tumor suppressor PTEN (phosphatase and tensin homolog deleted on chromosome ten) has been implicated in both syndromes.4 Underlying Carney complex should be excluded when genital lentiginosis is encountered.
Genital lentiginosis is idiopathic in most instances, but reports of lesions occurring after annular lichen planus suggest a possible mechanism.5 The disappearance of lentigines after imatinib therapy suggests a role for c-kit, a receptor tyrosine kinase that is involved in intracellular signaling, in some cases.6 At times, lesions can simulate trichrome vitiligo or have a reticulate pattern.7
Men and women present at different points in the course of disease. Men often present with penile lesions 14 years after onset, on average; they notice a gradual increase in the size of lesions. Because women can have greater difficulty self-examining the genital region, they tend to present much later in the course but often within a few months after initial inspection.1,8
Genital lentiginosis can mimic melanoma with nonhomogeneous pigmentation, asymmetry, and unilateral distribution, which makes dermoscopic assessment of colors helpful in narrowing the differential diagnosis. Melanoma is associated with combinations of gray, red, blue, and white, which are not found in genital lentiginosis.9
Biopsy of a genital lentigo is diagnostic, distinguishing the lesion from melanoma—failing to reveal the atypical melanocytes and pagetoid spread characteristic of melanoma in situ. Histologic findings can cause diagnostic difficulties when concurrent lichen sclerosus is associated with genital lentigines or nevi.10
Lentigines on sun-damaged skin or in the setting of xeroderma pigmentosum have been associated with melanoma,11-13 but genital lentigines are not considered a form of precancerous melanosis. In women, early diagnosis is important when there is concern for melanoma because the prognosis for vulvar melanoma is improved in thin lesions.14
Other entities in the differential include secondary syphilis, which commonly presents as macules and scaly papules and can be found on mucosal surfaces such as the oral cavity,15 as well as Kaposi sarcoma, which is characterized by purplish, brown, or black macules, plaques, and nodules, more commonly in immunosuppressed patients.16
To avoid unwarranted concern and unnecessary surgery, dermatologists should be aware of genital lentigines and their characteristic presentation in adults.
To the Editor:
Genital lentiginosis (also known as mucosal melanotic macules, vulvar melanosis, penile melanosis, and penile lentigines) occurs in men and women.1 Lesions present in adult life as multifocal, asymmetrical, pigmented patches that can have a mottled appearance or exhibit skip areas. The irregular appearance of the pigmented areas often raises concern for melanoma. Biopsy reveals increased pigmentation along the basal layer of the epidermis; the irregular distribution of single melanocytes and pagetoid spread typical of melanoma in situ is not identified.

Genital lentiginosis usually occurs as an isolated finding; however, the condition can be a manifestation of Laugier-Hunziker syndrome, Carney complex, and Bannayan-Riley-Ruvalcaba syndrome.1-3 When it occurs as an isolated finding, the patient can be reassured and treatment is unnecessary. Because genital lentiginosis may mimic the appearance of melanoma, it is important for physicians to differentiate the two and make a correct diagnosis. We present a case of genital lentiginosis that mimicked vulvar melanoma.

A 64-year-old woman was referred by her gynecologist to dermatology to rule out vulvar melanoma. The patient had a history of hypothyroidism and hypercholesterolemia but was otherwise in good health. Genital examination revealed asymptomatic pigmented macules and patches of unknown duration (Figure 1). Specimens were taken from 3 areas by punch biopsy to clarify the diagnosis. All 3 specimens showed identical features including basilar pigmentation, occasional melanophages in the papillary dermis, and no evidence of nests or pagetoid spread of atypical melanocytes (Figures 2 and 3). Histologic findings were diagnostic for genital lentiginosis. The patient was reassured, and no treatment was provided. At 6-month follow-up there was no change in clinical appearance.

Genital lentiginosis is characterized by brown lesions that can have a mottled appearance and often are associated with skip areas.1 Lesions can be strikingly irregular and darkly pigmented.
Although the lesions of genital lentiginosis most often are isolated findings, they can be a clue to several uncommon syndromes such as autosomal-dominant Bannayan-Riley-Ruvalcaba syndrome, which is associated with genital lentiginosis, intestinal polyposis, and macrocephaly.3 Vascular malformations, lipomatosis, verrucal keratoses, and acrochordons can occur. Bannayan-Riley-Ruvalcaba syndrome and Cowden syndrome may share genetic linkage; mutations in the tumor suppressor PTEN (phosphatase and tensin homolog deleted on chromosome ten) has been implicated in both syndromes.4 Underlying Carney complex should be excluded when genital lentiginosis is encountered.
Genital lentiginosis is idiopathic in most instances, but reports of lesions occurring after annular lichen planus suggest a possible mechanism.5 The disappearance of lentigines after imatinib therapy suggests a role for c-kit, a receptor tyrosine kinase that is involved in intracellular signaling, in some cases.6 At times, lesions can simulate trichrome vitiligo or have a reticulate pattern.7
Men and women present at different points in the course of disease. Men often present with penile lesions 14 years after onset, on average; they notice a gradual increase in the size of lesions. Because women can have greater difficulty self-examining the genital region, they tend to present much later in the course but often within a few months after initial inspection.1,8
Genital lentiginosis can mimic melanoma with nonhomogeneous pigmentation, asymmetry, and unilateral distribution, which makes dermoscopic assessment of colors helpful in narrowing the differential diagnosis. Melanoma is associated with combinations of gray, red, blue, and white, which are not found in genital lentiginosis.9
Biopsy of a genital lentigo is diagnostic, distinguishing the lesion from melanoma—failing to reveal the atypical melanocytes and pagetoid spread characteristic of melanoma in situ. Histologic findings can cause diagnostic difficulties when concurrent lichen sclerosus is associated with genital lentigines or nevi.10
Lentigines on sun-damaged skin or in the setting of xeroderma pigmentosum have been associated with melanoma,11-13 but genital lentigines are not considered a form of precancerous melanosis. In women, early diagnosis is important when there is concern for melanoma because the prognosis for vulvar melanoma is improved in thin lesions.14
Other entities in the differential include secondary syphilis, which commonly presents as macules and scaly papules and can be found on mucosal surfaces such as the oral cavity,15 as well as Kaposi sarcoma, which is characterized by purplish, brown, or black macules, plaques, and nodules, more commonly in immunosuppressed patients.16
To avoid unwarranted concern and unnecessary surgery, dermatologists should be aware of genital lentigines and their characteristic presentation in adults.
- Hwang L, Wilson H, Orengo I. Off-center fold: irregular, pigmented genital macules. Arch Dermatol. 2000;136:1559-1564. doi:10.1001/archderm.136.12.1559-b
- Rhodes AR, Silverman RA, Harrist TJ, et al. Mucocutaneous lentigines, cardiomucocutaneous myxomas, and multiple blue nevi: the “LAMB” syndrome. J Am Acad Dermatol. 1984;10:72-82. doi:10.1016/s0190-9622(84)80047-x
- Erkek E, Hizel S, Sanl C, et al. Clinical and histopathological findings in Bannayan-Riley-Ruvalcaba syndrome. J Am Acad Dermatol. 2005;53:639-643. doi:10.1016/j.jaad.2005.06.022
- Blum RR, Rahimizadeh A, Kardon N, et al. Genital lentigines in a 6-year-old boy with a family history of Cowden’s disease: clinical and genetic evidence of the linkage between Bannayan-Riley-Ruvalcaba syndrome and Cowden’s disease. J Cutan Med Surg. 2001;5:228-230. doi:10.1177/120347540100500307
- Isbary G, Dyall-Smith D, Coras-Stepanek B, et al. Penile lentigo (genital mucosal macule) following annular lichen planus: a possible association? Australas J Dermatol. 2014;55:159-161. doi:10.1111/ajd.12169
- Campbell T, Felsten L, Moore J. Disappearance of lentigines in a patient receiving imatinib treatment for familial gastrointestinal stromal tumor syndrome. Arch Dermatol. 2009;145:1313-1316. doi:10.1001/archdermatol.2009.263
- Romero- A, R, , et al. Reticulate genital pigmentation associated with localized vitiligo. Arch Dermatol. 2010; 146:574-575. doi:10.1001/archdermatol.2010.69
- Barnhill RL, Albert LS, Shama SK, et al. Genital lentiginosis: a clinical and histopathologic study. J Am Acad Dermatol. 1990;22:453-460. doi:10.1016/0190-9622(90)70064-o
- De Giorgi V, Gori A, Salvati L, et al. Clinical and dermoscopic features of vulvar melanosis over the last 20 years. JAMA Dermatol. 2020;156:1185–1191. doi:10.1001/jamadermatol.2020.2528
- El Shabrawi-Caelen L, Soyer HP, Schaeppi H, et al. Genital lentigines and melanocytic nevi with superimposed lichen sclerosus: a diagnostic challenge. J Am Acad Dermatol. 2004;50:690-694. doi:10.1016/j.jaad.2003.09.034
- Shatkin M, Helm MF, Muhlbauer A, et al. Solar lentigo evolving into fatal metastatic melanoma in a patient who initially refused surgery. N A J Med Sci. 2020;1:28-31. doi:10.7156/najms.2020.1301028
- Stern JB, Peck GL, Haupt HM, et al. Malignant melanoma in xeroderma pigmentosum: search for a precursor lesion. J Am Acad Dermatol. 1993;28:591-594. doi:10.1016/0190-9622(93)70079-9
- Byrom L, Barksdale S, Weedon D, et al. Unstable solar lentigo: a defined separate entity. Australas J Dermatol. 2016;57:229-234. doi:10.1111/ajd.12447
- Panizzon RG. Vulvar melanoma. Semin Dermatol. 1996;15:67-70. doi:10.1016/s1085-5629(96)80021-6
- Chapel TA. The signs and symptoms of secondary syphilis. Sex Transm Dis. 1980;7:161-164. doi:10.1097/00007435-198010000-00002
- Schwartz RA. Kaposi’s sarcoma: an update. J Surg Oncol. 2004;87:146-151. doi:10.1002/jso.20090
- Hwang L, Wilson H, Orengo I. Off-center fold: irregular, pigmented genital macules. Arch Dermatol. 2000;136:1559-1564. doi:10.1001/archderm.136.12.1559-b
- Rhodes AR, Silverman RA, Harrist TJ, et al. Mucocutaneous lentigines, cardiomucocutaneous myxomas, and multiple blue nevi: the “LAMB” syndrome. J Am Acad Dermatol. 1984;10:72-82. doi:10.1016/s0190-9622(84)80047-x
- Erkek E, Hizel S, Sanl C, et al. Clinical and histopathological findings in Bannayan-Riley-Ruvalcaba syndrome. J Am Acad Dermatol. 2005;53:639-643. doi:10.1016/j.jaad.2005.06.022
- Blum RR, Rahimizadeh A, Kardon N, et al. Genital lentigines in a 6-year-old boy with a family history of Cowden’s disease: clinical and genetic evidence of the linkage between Bannayan-Riley-Ruvalcaba syndrome and Cowden’s disease. J Cutan Med Surg. 2001;5:228-230. doi:10.1177/120347540100500307
- Isbary G, Dyall-Smith D, Coras-Stepanek B, et al. Penile lentigo (genital mucosal macule) following annular lichen planus: a possible association? Australas J Dermatol. 2014;55:159-161. doi:10.1111/ajd.12169
- Campbell T, Felsten L, Moore J. Disappearance of lentigines in a patient receiving imatinib treatment for familial gastrointestinal stromal tumor syndrome. Arch Dermatol. 2009;145:1313-1316. doi:10.1001/archdermatol.2009.263
- Romero- A, R, , et al. Reticulate genital pigmentation associated with localized vitiligo. Arch Dermatol. 2010; 146:574-575. doi:10.1001/archdermatol.2010.69
- Barnhill RL, Albert LS, Shama SK, et al. Genital lentiginosis: a clinical and histopathologic study. J Am Acad Dermatol. 1990;22:453-460. doi:10.1016/0190-9622(90)70064-o
- De Giorgi V, Gori A, Salvati L, et al. Clinical and dermoscopic features of vulvar melanosis over the last 20 years. JAMA Dermatol. 2020;156:1185–1191. doi:10.1001/jamadermatol.2020.2528
- El Shabrawi-Caelen L, Soyer HP, Schaeppi H, et al. Genital lentigines and melanocytic nevi with superimposed lichen sclerosus: a diagnostic challenge. J Am Acad Dermatol. 2004;50:690-694. doi:10.1016/j.jaad.2003.09.034
- Shatkin M, Helm MF, Muhlbauer A, et al. Solar lentigo evolving into fatal metastatic melanoma in a patient who initially refused surgery. N A J Med Sci. 2020;1:28-31. doi:10.7156/najms.2020.1301028
- Stern JB, Peck GL, Haupt HM, et al. Malignant melanoma in xeroderma pigmentosum: search for a precursor lesion. J Am Acad Dermatol. 1993;28:591-594. doi:10.1016/0190-9622(93)70079-9
- Byrom L, Barksdale S, Weedon D, et al. Unstable solar lentigo: a defined separate entity. Australas J Dermatol. 2016;57:229-234. doi:10.1111/ajd.12447
- Panizzon RG. Vulvar melanoma. Semin Dermatol. 1996;15:67-70. doi:10.1016/s1085-5629(96)80021-6
- Chapel TA. The signs and symptoms of secondary syphilis. Sex Transm Dis. 1980;7:161-164. doi:10.1097/00007435-198010000-00002
- Schwartz RA. Kaposi’s sarcoma: an update. J Surg Oncol. 2004;87:146-151. doi:10.1002/jso.20090
Practice Points
- The irregular appearance of genital lentiginosis—multifocal, asymmetric, irregular, and darkly pigmented patches—often raises concern for melanoma but is benign.
- Certain genetic conditions can present with genital lentiginosis.
- Dermoscopic assessment of the lesion color is highly helpful in narrowing the differential diagnosis; seeing no gray, red, blue, or white makes melanoma less likely.
- Be aware of genital lentigines and their characteristic presentation in adulthood to avoid unwarranted concern and unneeded surgery.
Atypical Localized Scleroderma Development During Nivolumab Therapy for Metastatic Lung Adenocarcinoma
To the Editor:
Immune checkpoint inhibitors such as anti–programmed cell death protein 1 (anti–PD-1) and anticytotoxic T lymphocyte–associated protein 4 therapies are a promising class of cancer therapeutics. However, they are associated with a variety of immune-related adverse events (irAEs), including cutaneous toxicity.1 The PD-1/programmed death ligand 1 (PD-L1) pathway is important for the maintenance of immune tolerance, and a blockade has been shown to lead to development of various autoimmune diseases.2 We present the case of a patient who developed new-onset localized scleroderma during treatment with the PD-1 inhibitor nivolumab.
A 65-year-old woman presented with a rash on the left thigh that was associated with pruritus, pain, and a pulling sensation. She had a history of stage IV lung adenocarcinoma, with a mass in the right upper lobe with metastatic foci to the left femur, right humerus, right hilar, and pretracheal lymph nodes. She received palliative radiation to the left femur and was started on carboplatin and pemetrexed. Metastasis to the liver was noted after completion of 6 cycles of therapy, and the patient’s treatment was changed to nivolumab. After 17 months on nivolumab therapy (2 years after initial diagnosis and 20 months after radiation therapy), she presented to our dermatology clinic with a cutaneous eruption on the buttocks that spread to the left thigh. The rash failed to improve after 1 month of treatment with emollients and triamcinolone cream 0.1%.
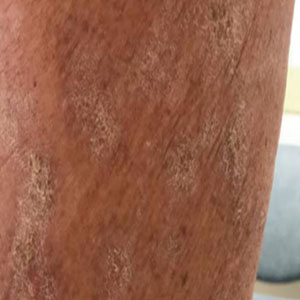
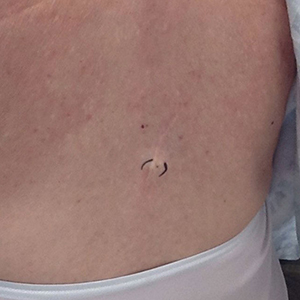
At the current presentation, which was 2 months after she initially presented to our clinic, dermatologic examination revealed erythematous and sclerotic plaques on the left lateral thigh (Figure 1A). Betamethasone cream 0.05% was prescribed, and nivolumab was discontinued due to progression of cutaneous symptoms. A punch biopsy from the left thigh demonstrated superficial dermal sclerosis that was suggestive of chronic radiation dermatitis; direct immunofluorescence testing was negative. The patient was started on prednisone 50 mg daily, which resulted in mild improvement in symptoms.
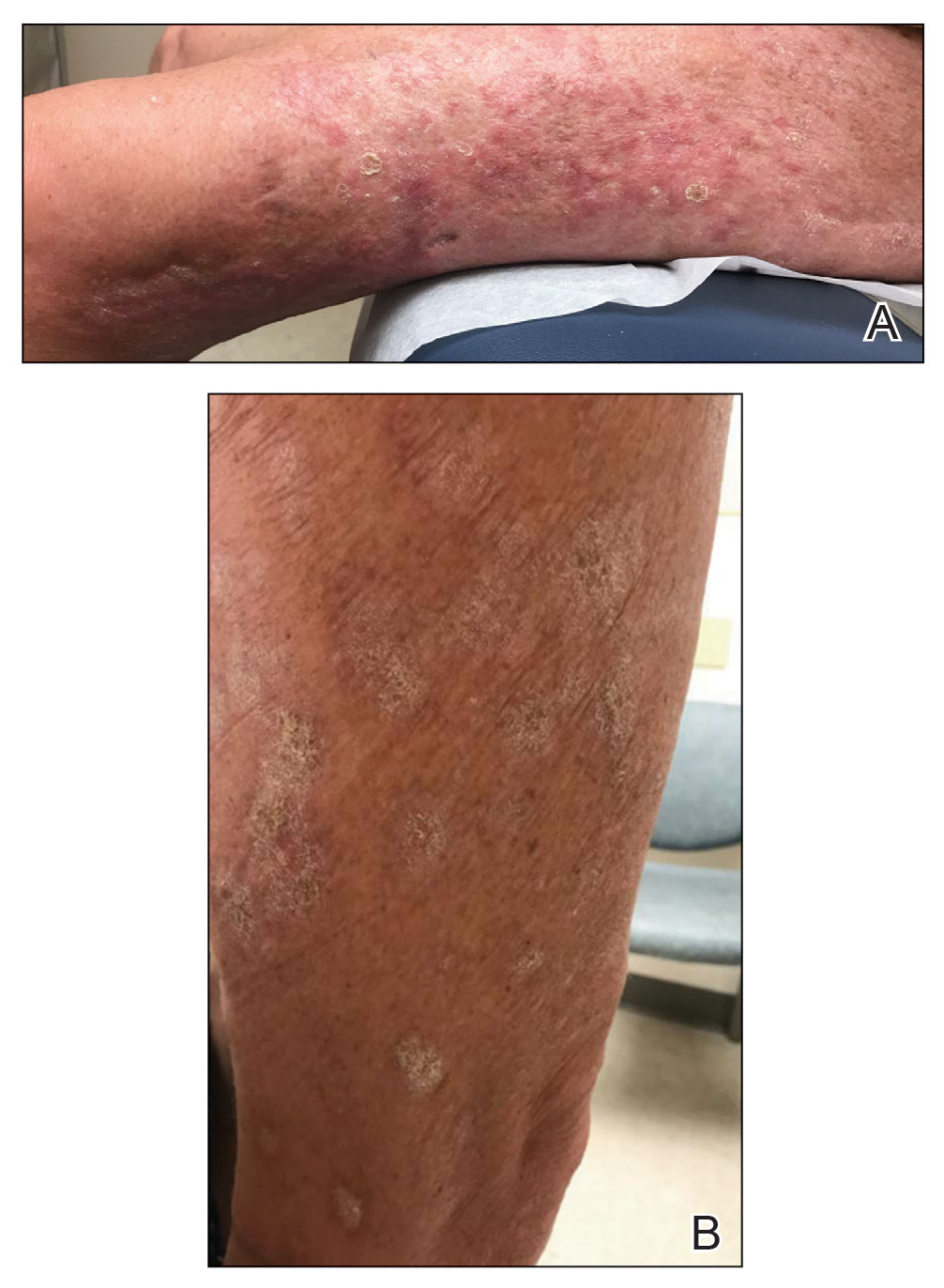
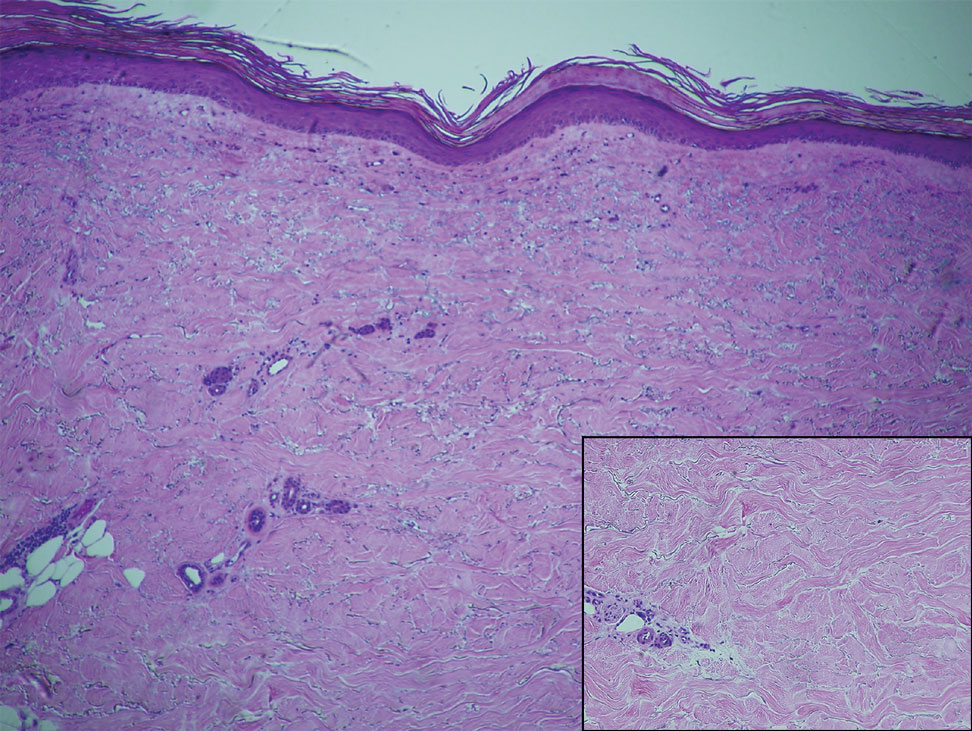
Within 6 months, new sclerotic plaques developed on the patient’s back and right thigh (Figure 1B). Because the lesions were located outside the radiation field of the left femur, a second biopsy was obtained from the right thigh. Histopathology revealed extensive dermal sclerosis and a perivascular lymphoplasmacytic infiltrate (Figure 2). An antinuclear antibody test was weakly positive (1:40, nucleolar pattern) with a negative extractable nuclear antigen panel result. Anti–double-stranded DNA, anti–topoisomerase 1, anti-Smith, antiribonucleoprotein, anti–Sjögren syndrome type A, anti–Sjögren syndrome type B, and anticentromere serology test results were negative. The patient denied decreased oral aperture, difficulty swallowing, or Raynaud phenomenon. Due to the atypical clinical presentation in the setting of PD-1 inhibitor therapy, the etiology of the eruption was potentially attributable to nivolumab. She was started on treatment with methotrexate 20 mg weekly and clobetasol cream 0.05% twice daily; she continued taking prednisone 5 mg daily. The cutaneous manifestations on the patient’s back completely resolved, and the legs continued to gradually improve on this regimen. Immunotherapy continued to be held due to skin toxicity.
Localized scleroderma is an autoimmune disorder characterized by inflammation and skin thickening. Overactive fibroblasts produce excess collagen, leading to the clinical symptoms of skin thickening, hardening, and discoloration.3 Lesions frequently develop on the arms, face, or legs and can present as patches or linear bands. Unlike systemic sclerosis, the internal organs typically are uninvolved; however, sclerotic lesions can be disfiguring and cause notable disability if they impede joint movement.
The PD-1/PD-L1 pathway is a negative regulator of the immune response that inactivates T cells and helps maintain self-tolerance. Modulation of the PD-1/PD-L1 pathway and overexpression of PD-L1 are seen in various cancers as a mechanism to help malignant cells avoid immune destruction.4 Conversely, inhibition of this pathway can be used to stimulate an antitumor immune response. This checkpoint inhibition strategy has been highly successful for the treatment of various cancers including melanoma and non–small cell lung carcinoma. There are several checkpoint inhibitors approved in the United States that are used for cancer therapy and target the PD-1/PD-L1 pathway, such as nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.4 A downside of checkpoint inhibitor treatment is that uncontrolled T-cell activation can lead to irAEs, including cutaneous eruptions, pruritus, diarrhea, colitis, hepatitis, endocrinopathies, pneumonitis, and renal insufficiency.5 These toxicities are reversible if treated appropriately but can cause notable morbidity and mortality if left unrecognized. Cutaneous eruption is one of the most common irAEs associated with anti–PD-1 and anti–PD-L1 therapies and can limit therapeutic efficacy, as the drug may need to be held or discontinued due to the severity of the eruption.6 Mid-potency to high-potency topical corticosteroids and systemic antihistamines are first-line treatments of grades 1 and 2 skin toxicities associated with PD-1 inhibitor therapy. For eruptions classified as grades 3 or 4 or refractory grade 2, discontinuation of the drug and systemic corticosteroids is recommended.7
The cutaneous eruption in immunotherapy-mediated dermatitis is thought to be largely mediated by activated T cells infiltrating the dermis.8 In localized scleroderma, increased tumor necrosis factor α, IFN-γ, IFN-γ–induced protein 10, and granulocyte macrophage colony stimulating factor activity have been shown to correlate with disease activity.9,10 Interestingly, increased tumor necrosis factor α and IFN-γ correlate with better response and increased overall survival in PD-1 inhibition therapy, suggesting a correlation between PD-1 inhibition and T helper activation as noted by the etiology of sclerosis in our patient.11 Additionally, history of radiation was a confounding factor in the diagnosis of our patient, as both sclerodermoid reactions and chronic radiation dermatitis can present with dermal sclerosis. However, the progression of disease outside of the radiation field excluded this etiology. Although new-onset sclerodermoid reactions have been reported with PD-1 inhibitors, they have been described secondary to sclerodermoid reactions from treatment with pembrolizumab.12,13 One case series reported a case of diffuse sclerodermoid reaction and a limited reaction in response to pembrolizumab treatment, while another case report described a relapse of generalized morphea in response to pembrolizumab treatment.12,13 One case of relapsing morphea in response to nivolumab treatment for stage IV lung adenocarcinoma also has been reported.14
Cutaneous toxicities are one of the most common irAEs associated with checkpoint inhibitors and are seen in more than one-third of treated patients. Most frequently, these irAEs manifest as spongiotic dermatitis on histopathology, but a broad spectrum of cutaneous reactions have been observed.15 Although sclerodermoid reactions have been reported with PD-1 inhibitors, most are described secondary to sclerodermoid reactions with pembrolizumab and involve relapse of previously diagnosed morphea rather than new-onset disease.12-14
Our case highlights new-onset localized scleroderma in the setting of nivolumab therapy that showed clinical improvement with methotrexate and topical and systemic steroids. This reaction pattern should be considered in all patients who develop cutaneous eruptions when treated with a PD-1 inhibitor. There should be a high index of suspicion for the potential occurrence of irAEs to ensure early recognition and treatment to minimize morbidity and maximize adherence to therapy for the underlying malignancy.
- Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ. 2018;360:k793.
- Dai S, Jia R, Zhang X, et al. The PD-1/PD-Ls pathway and autoimmune diseases. Cell Immunol. 2014;290:72-79.
- Badea I, Taylor M, Rosenberg A, et al. Pathogenesis and therapeutic approaches for improved topical treatment in localized scleroderma and systemic sclerosis. Rheumatology (Oxford). 2009;48:213-221.
- Constantinidou A, Alifieris C, Trafalis DT. Targeting programmed cell death-1 (PD-1) and ligand (PD-L1): a new era in cancer active immunotherapy. Pharmacol Ther. 2019;194:84-106.
- Villadolid J, Asim A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4:560-575.
- Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2016;27:1362.
- O’Kane GM, Labbé C, Doherty MK, et al. Monitoring and management of immune-related adverse events associated with programmed cell death protein-1 axis inhibitors in lung cancer. Oncologist. 2017;22:70-80.
- Shi VJ, Rodic N, Gettinger S, et al. Clinical and histologic features of lichenoid mucocutaneous eruptions due to anti-programmed celldeath 1 and anti-programmed cell death ligand 1 immunotherapy. JAMA Dermatol. 2016;152:1128-1136.
- Torok KS, Kurzinski K, Kelsey C, et al. Peripheral blood cytokine and chemokine profiles in juvenile localized scleroderma: T-helper cell-associated cytokine profiles. Semin Arthritis Rheum. 2015;45:284-293.
- Guo X, Higgs BW, Bay-Jensen AC, et al. Suppression of T cell activation and collagen accumulation by an anti-IFNAR1 mAb, anifrolumab, in adult patients with systemic sclerosis. J Invest Dermatol. 2015;135:2402-2409.
- Boutsikou E, Domvri K, Hardavella G, et al. Tumor necrosis factor, interferon-gamma and interleukins as predictive markers of antiprogrammed cell-death protein-1 treatment in advanced non-small cell lung cancer: a pragmatic approach in clinical practice. Ther Adv Med Oncol. 2018;10:1758835918768238.
- Barbosa NS, Wetter DA, Wieland CN, et al. Scleroderma induced by pembrolizumab: a case series. Mayo Clin Proc. 2017;92:1158-1163.
- Cheng MW, Hisaw LD, Bernet L. Generalized morphea in the setting of pembrolizumab. Int J Dermatol. 2019;58:736-738.
- Alegre-Sánchez A, Fonda-Pascual P, Saceda-Corralo D, et al. Relapse of morphea during nivolumab therapy for lung adenocarcinoma. Actas Dermosifiliogr. 2017;108:69-70.
- Sibaud V. Dermatologic reactions to immune checkpoint inhibitors: skin toxicities and immunotherapy. Am J Clin Dermatol. 2018;19:345-361.
To the Editor:
Immune checkpoint inhibitors such as anti–programmed cell death protein 1 (anti–PD-1) and anticytotoxic T lymphocyte–associated protein 4 therapies are a promising class of cancer therapeutics. However, they are associated with a variety of immune-related adverse events (irAEs), including cutaneous toxicity.1 The PD-1/programmed death ligand 1 (PD-L1) pathway is important for the maintenance of immune tolerance, and a blockade has been shown to lead to development of various autoimmune diseases.2 We present the case of a patient who developed new-onset localized scleroderma during treatment with the PD-1 inhibitor nivolumab.
A 65-year-old woman presented with a rash on the left thigh that was associated with pruritus, pain, and a pulling sensation. She had a history of stage IV lung adenocarcinoma, with a mass in the right upper lobe with metastatic foci to the left femur, right humerus, right hilar, and pretracheal lymph nodes. She received palliative radiation to the left femur and was started on carboplatin and pemetrexed. Metastasis to the liver was noted after completion of 6 cycles of therapy, and the patient’s treatment was changed to nivolumab. After 17 months on nivolumab therapy (2 years after initial diagnosis and 20 months after radiation therapy), she presented to our dermatology clinic with a cutaneous eruption on the buttocks that spread to the left thigh. The rash failed to improve after 1 month of treatment with emollients and triamcinolone cream 0.1%.
At the current presentation, which was 2 months after she initially presented to our clinic, dermatologic examination revealed erythematous and sclerotic plaques on the left lateral thigh (Figure 1A). Betamethasone cream 0.05% was prescribed, and nivolumab was discontinued due to progression of cutaneous symptoms. A punch biopsy from the left thigh demonstrated superficial dermal sclerosis that was suggestive of chronic radiation dermatitis; direct immunofluorescence testing was negative. The patient was started on prednisone 50 mg daily, which resulted in mild improvement in symptoms.

Within 6 months, new sclerotic plaques developed on the patient’s back and right thigh (Figure 1B). Because the lesions were located outside the radiation field of the left femur, a second biopsy was obtained from the right thigh. Histopathology revealed extensive dermal sclerosis and a perivascular lymphoplasmacytic infiltrate (Figure 2). An antinuclear antibody test was weakly positive (1:40, nucleolar pattern) with a negative extractable nuclear antigen panel result. Anti–double-stranded DNA, anti–topoisomerase 1, anti-Smith, antiribonucleoprotein, anti–Sjögren syndrome type A, anti–Sjögren syndrome type B, and anticentromere serology test results were negative. The patient denied decreased oral aperture, difficulty swallowing, or Raynaud phenomenon. Due to the atypical clinical presentation in the setting of PD-1 inhibitor therapy, the etiology of the eruption was potentially attributable to nivolumab. She was started on treatment with methotrexate 20 mg weekly and clobetasol cream 0.05% twice daily; she continued taking prednisone 5 mg daily. The cutaneous manifestations on the patient’s back completely resolved, and the legs continued to gradually improve on this regimen. Immunotherapy continued to be held due to skin toxicity.

Localized scleroderma is an autoimmune disorder characterized by inflammation and skin thickening. Overactive fibroblasts produce excess collagen, leading to the clinical symptoms of skin thickening, hardening, and discoloration.3 Lesions frequently develop on the arms, face, or legs and can present as patches or linear bands. Unlike systemic sclerosis, the internal organs typically are uninvolved; however, sclerotic lesions can be disfiguring and cause notable disability if they impede joint movement.
The PD-1/PD-L1 pathway is a negative regulator of the immune response that inactivates T cells and helps maintain self-tolerance. Modulation of the PD-1/PD-L1 pathway and overexpression of PD-L1 are seen in various cancers as a mechanism to help malignant cells avoid immune destruction.4 Conversely, inhibition of this pathway can be used to stimulate an antitumor immune response. This checkpoint inhibition strategy has been highly successful for the treatment of various cancers including melanoma and non–small cell lung carcinoma. There are several checkpoint inhibitors approved in the United States that are used for cancer therapy and target the PD-1/PD-L1 pathway, such as nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.4 A downside of checkpoint inhibitor treatment is that uncontrolled T-cell activation can lead to irAEs, including cutaneous eruptions, pruritus, diarrhea, colitis, hepatitis, endocrinopathies, pneumonitis, and renal insufficiency.5 These toxicities are reversible if treated appropriately but can cause notable morbidity and mortality if left unrecognized. Cutaneous eruption is one of the most common irAEs associated with anti–PD-1 and anti–PD-L1 therapies and can limit therapeutic efficacy, as the drug may need to be held or discontinued due to the severity of the eruption.6 Mid-potency to high-potency topical corticosteroids and systemic antihistamines are first-line treatments of grades 1 and 2 skin toxicities associated with PD-1 inhibitor therapy. For eruptions classified as grades 3 or 4 or refractory grade 2, discontinuation of the drug and systemic corticosteroids is recommended.7
The cutaneous eruption in immunotherapy-mediated dermatitis is thought to be largely mediated by activated T cells infiltrating the dermis.8 In localized scleroderma, increased tumor necrosis factor α, IFN-γ, IFN-γ–induced protein 10, and granulocyte macrophage colony stimulating factor activity have been shown to correlate with disease activity.9,10 Interestingly, increased tumor necrosis factor α and IFN-γ correlate with better response and increased overall survival in PD-1 inhibition therapy, suggesting a correlation between PD-1 inhibition and T helper activation as noted by the etiology of sclerosis in our patient.11 Additionally, history of radiation was a confounding factor in the diagnosis of our patient, as both sclerodermoid reactions and chronic radiation dermatitis can present with dermal sclerosis. However, the progression of disease outside of the radiation field excluded this etiology. Although new-onset sclerodermoid reactions have been reported with PD-1 inhibitors, they have been described secondary to sclerodermoid reactions from treatment with pembrolizumab.12,13 One case series reported a case of diffuse sclerodermoid reaction and a limited reaction in response to pembrolizumab treatment, while another case report described a relapse of generalized morphea in response to pembrolizumab treatment.12,13 One case of relapsing morphea in response to nivolumab treatment for stage IV lung adenocarcinoma also has been reported.14
Cutaneous toxicities are one of the most common irAEs associated with checkpoint inhibitors and are seen in more than one-third of treated patients. Most frequently, these irAEs manifest as spongiotic dermatitis on histopathology, but a broad spectrum of cutaneous reactions have been observed.15 Although sclerodermoid reactions have been reported with PD-1 inhibitors, most are described secondary to sclerodermoid reactions with pembrolizumab and involve relapse of previously diagnosed morphea rather than new-onset disease.12-14
Our case highlights new-onset localized scleroderma in the setting of nivolumab therapy that showed clinical improvement with methotrexate and topical and systemic steroids. This reaction pattern should be considered in all patients who develop cutaneous eruptions when treated with a PD-1 inhibitor. There should be a high index of suspicion for the potential occurrence of irAEs to ensure early recognition and treatment to minimize morbidity and maximize adherence to therapy for the underlying malignancy.
To the Editor:
Immune checkpoint inhibitors such as anti–programmed cell death protein 1 (anti–PD-1) and anticytotoxic T lymphocyte–associated protein 4 therapies are a promising class of cancer therapeutics. However, they are associated with a variety of immune-related adverse events (irAEs), including cutaneous toxicity.1 The PD-1/programmed death ligand 1 (PD-L1) pathway is important for the maintenance of immune tolerance, and a blockade has been shown to lead to development of various autoimmune diseases.2 We present the case of a patient who developed new-onset localized scleroderma during treatment with the PD-1 inhibitor nivolumab.
A 65-year-old woman presented with a rash on the left thigh that was associated with pruritus, pain, and a pulling sensation. She had a history of stage IV lung adenocarcinoma, with a mass in the right upper lobe with metastatic foci to the left femur, right humerus, right hilar, and pretracheal lymph nodes. She received palliative radiation to the left femur and was started on carboplatin and pemetrexed. Metastasis to the liver was noted after completion of 6 cycles of therapy, and the patient’s treatment was changed to nivolumab. After 17 months on nivolumab therapy (2 years after initial diagnosis and 20 months after radiation therapy), she presented to our dermatology clinic with a cutaneous eruption on the buttocks that spread to the left thigh. The rash failed to improve after 1 month of treatment with emollients and triamcinolone cream 0.1%.
At the current presentation, which was 2 months after she initially presented to our clinic, dermatologic examination revealed erythematous and sclerotic plaques on the left lateral thigh (Figure 1A). Betamethasone cream 0.05% was prescribed, and nivolumab was discontinued due to progression of cutaneous symptoms. A punch biopsy from the left thigh demonstrated superficial dermal sclerosis that was suggestive of chronic radiation dermatitis; direct immunofluorescence testing was negative. The patient was started on prednisone 50 mg daily, which resulted in mild improvement in symptoms.

Within 6 months, new sclerotic plaques developed on the patient’s back and right thigh (Figure 1B). Because the lesions were located outside the radiation field of the left femur, a second biopsy was obtained from the right thigh. Histopathology revealed extensive dermal sclerosis and a perivascular lymphoplasmacytic infiltrate (Figure 2). An antinuclear antibody test was weakly positive (1:40, nucleolar pattern) with a negative extractable nuclear antigen panel result. Anti–double-stranded DNA, anti–topoisomerase 1, anti-Smith, antiribonucleoprotein, anti–Sjögren syndrome type A, anti–Sjögren syndrome type B, and anticentromere serology test results were negative. The patient denied decreased oral aperture, difficulty swallowing, or Raynaud phenomenon. Due to the atypical clinical presentation in the setting of PD-1 inhibitor therapy, the etiology of the eruption was potentially attributable to nivolumab. She was started on treatment with methotrexate 20 mg weekly and clobetasol cream 0.05% twice daily; she continued taking prednisone 5 mg daily. The cutaneous manifestations on the patient’s back completely resolved, and the legs continued to gradually improve on this regimen. Immunotherapy continued to be held due to skin toxicity.

Localized scleroderma is an autoimmune disorder characterized by inflammation and skin thickening. Overactive fibroblasts produce excess collagen, leading to the clinical symptoms of skin thickening, hardening, and discoloration.3 Lesions frequently develop on the arms, face, or legs and can present as patches or linear bands. Unlike systemic sclerosis, the internal organs typically are uninvolved; however, sclerotic lesions can be disfiguring and cause notable disability if they impede joint movement.
The PD-1/PD-L1 pathway is a negative regulator of the immune response that inactivates T cells and helps maintain self-tolerance. Modulation of the PD-1/PD-L1 pathway and overexpression of PD-L1 are seen in various cancers as a mechanism to help malignant cells avoid immune destruction.4 Conversely, inhibition of this pathway can be used to stimulate an antitumor immune response. This checkpoint inhibition strategy has been highly successful for the treatment of various cancers including melanoma and non–small cell lung carcinoma. There are several checkpoint inhibitors approved in the United States that are used for cancer therapy and target the PD-1/PD-L1 pathway, such as nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.4 A downside of checkpoint inhibitor treatment is that uncontrolled T-cell activation can lead to irAEs, including cutaneous eruptions, pruritus, diarrhea, colitis, hepatitis, endocrinopathies, pneumonitis, and renal insufficiency.5 These toxicities are reversible if treated appropriately but can cause notable morbidity and mortality if left unrecognized. Cutaneous eruption is one of the most common irAEs associated with anti–PD-1 and anti–PD-L1 therapies and can limit therapeutic efficacy, as the drug may need to be held or discontinued due to the severity of the eruption.6 Mid-potency to high-potency topical corticosteroids and systemic antihistamines are first-line treatments of grades 1 and 2 skin toxicities associated with PD-1 inhibitor therapy. For eruptions classified as grades 3 or 4 or refractory grade 2, discontinuation of the drug and systemic corticosteroids is recommended.7
The cutaneous eruption in immunotherapy-mediated dermatitis is thought to be largely mediated by activated T cells infiltrating the dermis.8 In localized scleroderma, increased tumor necrosis factor α, IFN-γ, IFN-γ–induced protein 10, and granulocyte macrophage colony stimulating factor activity have been shown to correlate with disease activity.9,10 Interestingly, increased tumor necrosis factor α and IFN-γ correlate with better response and increased overall survival in PD-1 inhibition therapy, suggesting a correlation between PD-1 inhibition and T helper activation as noted by the etiology of sclerosis in our patient.11 Additionally, history of radiation was a confounding factor in the diagnosis of our patient, as both sclerodermoid reactions and chronic radiation dermatitis can present with dermal sclerosis. However, the progression of disease outside of the radiation field excluded this etiology. Although new-onset sclerodermoid reactions have been reported with PD-1 inhibitors, they have been described secondary to sclerodermoid reactions from treatment with pembrolizumab.12,13 One case series reported a case of diffuse sclerodermoid reaction and a limited reaction in response to pembrolizumab treatment, while another case report described a relapse of generalized morphea in response to pembrolizumab treatment.12,13 One case of relapsing morphea in response to nivolumab treatment for stage IV lung adenocarcinoma also has been reported.14
Cutaneous toxicities are one of the most common irAEs associated with checkpoint inhibitors and are seen in more than one-third of treated patients. Most frequently, these irAEs manifest as spongiotic dermatitis on histopathology, but a broad spectrum of cutaneous reactions have been observed.15 Although sclerodermoid reactions have been reported with PD-1 inhibitors, most are described secondary to sclerodermoid reactions with pembrolizumab and involve relapse of previously diagnosed morphea rather than new-onset disease.12-14
Our case highlights new-onset localized scleroderma in the setting of nivolumab therapy that showed clinical improvement with methotrexate and topical and systemic steroids. This reaction pattern should be considered in all patients who develop cutaneous eruptions when treated with a PD-1 inhibitor. There should be a high index of suspicion for the potential occurrence of irAEs to ensure early recognition and treatment to minimize morbidity and maximize adherence to therapy for the underlying malignancy.
- Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ. 2018;360:k793.
- Dai S, Jia R, Zhang X, et al. The PD-1/PD-Ls pathway and autoimmune diseases. Cell Immunol. 2014;290:72-79.
- Badea I, Taylor M, Rosenberg A, et al. Pathogenesis and therapeutic approaches for improved topical treatment in localized scleroderma and systemic sclerosis. Rheumatology (Oxford). 2009;48:213-221.
- Constantinidou A, Alifieris C, Trafalis DT. Targeting programmed cell death-1 (PD-1) and ligand (PD-L1): a new era in cancer active immunotherapy. Pharmacol Ther. 2019;194:84-106.
- Villadolid J, Asim A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4:560-575.
- Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2016;27:1362.
- O’Kane GM, Labbé C, Doherty MK, et al. Monitoring and management of immune-related adverse events associated with programmed cell death protein-1 axis inhibitors in lung cancer. Oncologist. 2017;22:70-80.
- Shi VJ, Rodic N, Gettinger S, et al. Clinical and histologic features of lichenoid mucocutaneous eruptions due to anti-programmed celldeath 1 and anti-programmed cell death ligand 1 immunotherapy. JAMA Dermatol. 2016;152:1128-1136.
- Torok KS, Kurzinski K, Kelsey C, et al. Peripheral blood cytokine and chemokine profiles in juvenile localized scleroderma: T-helper cell-associated cytokine profiles. Semin Arthritis Rheum. 2015;45:284-293.
- Guo X, Higgs BW, Bay-Jensen AC, et al. Suppression of T cell activation and collagen accumulation by an anti-IFNAR1 mAb, anifrolumab, in adult patients with systemic sclerosis. J Invest Dermatol. 2015;135:2402-2409.
- Boutsikou E, Domvri K, Hardavella G, et al. Tumor necrosis factor, interferon-gamma and interleukins as predictive markers of antiprogrammed cell-death protein-1 treatment in advanced non-small cell lung cancer: a pragmatic approach in clinical practice. Ther Adv Med Oncol. 2018;10:1758835918768238.
- Barbosa NS, Wetter DA, Wieland CN, et al. Scleroderma induced by pembrolizumab: a case series. Mayo Clin Proc. 2017;92:1158-1163.
- Cheng MW, Hisaw LD, Bernet L. Generalized morphea in the setting of pembrolizumab. Int J Dermatol. 2019;58:736-738.
- Alegre-Sánchez A, Fonda-Pascual P, Saceda-Corralo D, et al. Relapse of morphea during nivolumab therapy for lung adenocarcinoma. Actas Dermosifiliogr. 2017;108:69-70.
- Sibaud V. Dermatologic reactions to immune checkpoint inhibitors: skin toxicities and immunotherapy. Am J Clin Dermatol. 2018;19:345-361.
- Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ. 2018;360:k793.
- Dai S, Jia R, Zhang X, et al. The PD-1/PD-Ls pathway and autoimmune diseases. Cell Immunol. 2014;290:72-79.
- Badea I, Taylor M, Rosenberg A, et al. Pathogenesis and therapeutic approaches for improved topical treatment in localized scleroderma and systemic sclerosis. Rheumatology (Oxford). 2009;48:213-221.
- Constantinidou A, Alifieris C, Trafalis DT. Targeting programmed cell death-1 (PD-1) and ligand (PD-L1): a new era in cancer active immunotherapy. Pharmacol Ther. 2019;194:84-106.
- Villadolid J, Asim A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4:560-575.
- Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2016;27:1362.
- O’Kane GM, Labbé C, Doherty MK, et al. Monitoring and management of immune-related adverse events associated with programmed cell death protein-1 axis inhibitors in lung cancer. Oncologist. 2017;22:70-80.
- Shi VJ, Rodic N, Gettinger S, et al. Clinical and histologic features of lichenoid mucocutaneous eruptions due to anti-programmed celldeath 1 and anti-programmed cell death ligand 1 immunotherapy. JAMA Dermatol. 2016;152:1128-1136.
- Torok KS, Kurzinski K, Kelsey C, et al. Peripheral blood cytokine and chemokine profiles in juvenile localized scleroderma: T-helper cell-associated cytokine profiles. Semin Arthritis Rheum. 2015;45:284-293.
- Guo X, Higgs BW, Bay-Jensen AC, et al. Suppression of T cell activation and collagen accumulation by an anti-IFNAR1 mAb, anifrolumab, in adult patients with systemic sclerosis. J Invest Dermatol. 2015;135:2402-2409.
- Boutsikou E, Domvri K, Hardavella G, et al. Tumor necrosis factor, interferon-gamma and interleukins as predictive markers of antiprogrammed cell-death protein-1 treatment in advanced non-small cell lung cancer: a pragmatic approach in clinical practice. Ther Adv Med Oncol. 2018;10:1758835918768238.
- Barbosa NS, Wetter DA, Wieland CN, et al. Scleroderma induced by pembrolizumab: a case series. Mayo Clin Proc. 2017;92:1158-1163.
- Cheng MW, Hisaw LD, Bernet L. Generalized morphea in the setting of pembrolizumab. Int J Dermatol. 2019;58:736-738.
- Alegre-Sánchez A, Fonda-Pascual P, Saceda-Corralo D, et al. Relapse of morphea during nivolumab therapy for lung adenocarcinoma. Actas Dermosifiliogr. 2017;108:69-70.
- Sibaud V. Dermatologic reactions to immune checkpoint inhibitors: skin toxicities and immunotherapy. Am J Clin Dermatol. 2018;19:345-361.
Practice Points
- Immune checkpoint inhibitors such as nivolumab, a programmed cell death protein 1 (PD-1) inhibitor, are associated with immune-related adverse events (irAEs) such as skin toxicity.
- Scleroderma should be considered in the differential diagnosis of patients who develop cutaneous eruptions during treatment with PD-1 inhibitors.
- To ensure prompt recognition and treatment, health care providers should maintain a high index of suspicion for development of cutaneous irAEs in patients using checkpoint inhibitors.
Ossification and Migration of a Nodule Following Calcium Hydroxylapatite Injection
To the Editor:
Calcium hydroxylapatite is an injectable filler approved by the US Food and Drug Administration for moderate to severe rhytides of the face and the treatment of facial lipodystrophy in patients with HIV.1 This long-lasting filler generally is well tolerated with minimal side effects; however, there have been reports of nodules or granulomatous formation following injection.2 We present a case of a migrating nodule following injection of a calcium hydroxylapatite filler that appeared ossified on radiographic imaging. We highlight this rarely reported phenomenon to increase awareness of this complication.
A 72-year-old woman presented to our clinic with a mass on the left cheek. The patient had a history of treatment with facial fillers but no notable medical conditions. She initially received hyaluronic acid injectable gel dermal filler twice—3 years apart—before switching to calcium hydroxylapatite injections twice—4 months apart—from an outside provider. One month after the second treatment, she noticed a mass on the left cheek and promptly returned to the provider who performed the calcium hydroxylapatite injections. The provider, who had originally injected in the infraorbital area, stated it was unlikely that the filler would have migrated to the mid cheek and referred the patient to a general dentist who suspected salivary gland pathology. The patient was referred to an oral and maxillofacial surgeon who suspected the mass was related to the parotid gland. Maxillofacial computed tomography (CT) revealed heterotopic ossification vs myositis ossificans, possibly related to the recent injection. The patient was eventually referred to the Division of Plastic Surgery, Department of Surgery, at the University of Texas Medical Branch (Galveston, Texas) for further evaluation. Physical examination revealed a 2×1-cm firm, mobile, nontender mass in the left cheek in the area of the buccinator muscles. The mass did not express any fluid and was most easily palpable from the oral cavity. Radiography findings showed that the calcium hydroxylapatite filler had migrated to this location and formed a nodule (Figure). Because calcium hydroxylapatite fillers generally last 12 to 18 months, we opted to observe the lesion for spontaneous resolution. Four months later, the patient presented to our clinic for follow-up and the mass had reduced in size and appeared to be spontaneously resolving.
We present a unique case of a migrating nodule that occurred after injection with calcium hydroxylapatite, which led to concern for neoplastic tumor formation. This complication is rare, and it is important for practitioners who inject calcium hydroxylapatite as well as those who these patients may be referred to for evaluation to be aware that migrating nodules can occur. This awareness can help reduce unnecessary referrals, medical procedures, and anxiety.
Calcium hydroxylapatite filler is composed of 30% calcium hydroxylapatite microspheres suspended in a 70% sodium carboxymethylcellulose gel. The water-soluble gel rapidly becomes absorbed upon injection; however, the microspheres form a scaffold for the production of newly synthesized collagen. The filling effect generally lasts 12 to 18 months.1
Calcium hydroxylapatite, similar to most fillers, generally is well tolerated with a low complication rate of 3%.1 Although nodule formation with calcium hydroxylapatite is rare, it is the most common adverse event and encompasses 96% of complications. The remaining 4% of complications include persistent inflammation, swelling, erythema, and technical mistakes leading to overcorrection.1 Migrating nodules are rare; however, Beer3 reported a similar case.
Treatment of calcium hydroxylapatite nodules depends on differentiating a cause based on the time of onset. Early nodules that occur within 1 to 2 weeks of the injection usually represent incorrect positioning of the filler and can be treated by massaging the nodule. Other more invasive techniques involve aspiration or injection of sterile water. Late-onset nodules have shown response to corticosteroid injections. For inflammatory nodules of infectious origin, antibiotics can be useful. Surgical excision of the nodule rarely is required, as most nodules will resolve spontaneously, even without intervention.1,2
Radiologic findings of calcium hydroxylapatite appear as high-attenuation linear streaks or masses on CT (280–700 HU) and as low to intermediate signal intensity on T1- or T2-weighted sequences on magnetic resonance imaging. Oftentimes, calcium hydroxylapatite has a similar radiographic appearance to bone and can persist for 2 years or more on radiographic imaging, longer than they are clinically visible.4 The nodule formation from injection with calcium hydroxylapatite can mimic pathologic conditions such as miliary osteomas, myositis ossificans, heterotrophic/dystrophic calcifications, and foreign bodies on CT. Our patient’s CT findings of high attenuation linear streaks and nodules of similar signal intensity to bone were consistent with those previously described in the radiographic literature.
Calcium hydroxylapatite fillers have a good safety profile, but it is important to recognize that nodule formation is a common adverse event and that migration of nodules can occur. Practitioners should recognize this possibility in patients presenting with new masses after filler injection before advocating for potentially invasive and costly procedures and diagnostic modalities.
- Kadouch JA. Calcium hydroxylapatite: a review on safety and complications. J Cosmet Dermatol. 2017;16:152-161.
- Moulinets I, Arnaud E, Bui P, et al. Foreign body reaction to Radiesse: 2 cases. Am J Dermatopathol. 2013;35:e37-40.
- Beer KR. Radiesse nodule of the lips from a distant injection site: report of a case and consideration of etiology and management. J Drugs Dermatol. 2007;6:846-847.
- Ginat DT, Schatz CJ. Imaging features of midface injectable fillers and associated complications. AJNR Am J Neuroradiol. 2013;34:1488-1495.
To the Editor:
Calcium hydroxylapatite is an injectable filler approved by the US Food and Drug Administration for moderate to severe rhytides of the face and the treatment of facial lipodystrophy in patients with HIV.1 This long-lasting filler generally is well tolerated with minimal side effects; however, there have been reports of nodules or granulomatous formation following injection.2 We present a case of a migrating nodule following injection of a calcium hydroxylapatite filler that appeared ossified on radiographic imaging. We highlight this rarely reported phenomenon to increase awareness of this complication.
A 72-year-old woman presented to our clinic with a mass on the left cheek. The patient had a history of treatment with facial fillers but no notable medical conditions. She initially received hyaluronic acid injectable gel dermal filler twice—3 years apart—before switching to calcium hydroxylapatite injections twice—4 months apart—from an outside provider. One month after the second treatment, she noticed a mass on the left cheek and promptly returned to the provider who performed the calcium hydroxylapatite injections. The provider, who had originally injected in the infraorbital area, stated it was unlikely that the filler would have migrated to the mid cheek and referred the patient to a general dentist who suspected salivary gland pathology. The patient was referred to an oral and maxillofacial surgeon who suspected the mass was related to the parotid gland. Maxillofacial computed tomography (CT) revealed heterotopic ossification vs myositis ossificans, possibly related to the recent injection. The patient was eventually referred to the Division of Plastic Surgery, Department of Surgery, at the University of Texas Medical Branch (Galveston, Texas) for further evaluation. Physical examination revealed a 2×1-cm firm, mobile, nontender mass in the left cheek in the area of the buccinator muscles. The mass did not express any fluid and was most easily palpable from the oral cavity. Radiography findings showed that the calcium hydroxylapatite filler had migrated to this location and formed a nodule (Figure). Because calcium hydroxylapatite fillers generally last 12 to 18 months, we opted to observe the lesion for spontaneous resolution. Four months later, the patient presented to our clinic for follow-up and the mass had reduced in size and appeared to be spontaneously resolving.

We present a unique case of a migrating nodule that occurred after injection with calcium hydroxylapatite, which led to concern for neoplastic tumor formation. This complication is rare, and it is important for practitioners who inject calcium hydroxylapatite as well as those who these patients may be referred to for evaluation to be aware that migrating nodules can occur. This awareness can help reduce unnecessary referrals, medical procedures, and anxiety.
Calcium hydroxylapatite filler is composed of 30% calcium hydroxylapatite microspheres suspended in a 70% sodium carboxymethylcellulose gel. The water-soluble gel rapidly becomes absorbed upon injection; however, the microspheres form a scaffold for the production of newly synthesized collagen. The filling effect generally lasts 12 to 18 months.1
Calcium hydroxylapatite, similar to most fillers, generally is well tolerated with a low complication rate of 3%.1 Although nodule formation with calcium hydroxylapatite is rare, it is the most common adverse event and encompasses 96% of complications. The remaining 4% of complications include persistent inflammation, swelling, erythema, and technical mistakes leading to overcorrection.1 Migrating nodules are rare; however, Beer3 reported a similar case.
Treatment of calcium hydroxylapatite nodules depends on differentiating a cause based on the time of onset. Early nodules that occur within 1 to 2 weeks of the injection usually represent incorrect positioning of the filler and can be treated by massaging the nodule. Other more invasive techniques involve aspiration or injection of sterile water. Late-onset nodules have shown response to corticosteroid injections. For inflammatory nodules of infectious origin, antibiotics can be useful. Surgical excision of the nodule rarely is required, as most nodules will resolve spontaneously, even without intervention.1,2
Radiologic findings of calcium hydroxylapatite appear as high-attenuation linear streaks or masses on CT (280–700 HU) and as low to intermediate signal intensity on T1- or T2-weighted sequences on magnetic resonance imaging. Oftentimes, calcium hydroxylapatite has a similar radiographic appearance to bone and can persist for 2 years or more on radiographic imaging, longer than they are clinically visible.4 The nodule formation from injection with calcium hydroxylapatite can mimic pathologic conditions such as miliary osteomas, myositis ossificans, heterotrophic/dystrophic calcifications, and foreign bodies on CT. Our patient’s CT findings of high attenuation linear streaks and nodules of similar signal intensity to bone were consistent with those previously described in the radiographic literature.
Calcium hydroxylapatite fillers have a good safety profile, but it is important to recognize that nodule formation is a common adverse event and that migration of nodules can occur. Practitioners should recognize this possibility in patients presenting with new masses after filler injection before advocating for potentially invasive and costly procedures and diagnostic modalities.
To the Editor:
Calcium hydroxylapatite is an injectable filler approved by the US Food and Drug Administration for moderate to severe rhytides of the face and the treatment of facial lipodystrophy in patients with HIV.1 This long-lasting filler generally is well tolerated with minimal side effects; however, there have been reports of nodules or granulomatous formation following injection.2 We present a case of a migrating nodule following injection of a calcium hydroxylapatite filler that appeared ossified on radiographic imaging. We highlight this rarely reported phenomenon to increase awareness of this complication.
A 72-year-old woman presented to our clinic with a mass on the left cheek. The patient had a history of treatment with facial fillers but no notable medical conditions. She initially received hyaluronic acid injectable gel dermal filler twice—3 years apart—before switching to calcium hydroxylapatite injections twice—4 months apart—from an outside provider. One month after the second treatment, she noticed a mass on the left cheek and promptly returned to the provider who performed the calcium hydroxylapatite injections. The provider, who had originally injected in the infraorbital area, stated it was unlikely that the filler would have migrated to the mid cheek and referred the patient to a general dentist who suspected salivary gland pathology. The patient was referred to an oral and maxillofacial surgeon who suspected the mass was related to the parotid gland. Maxillofacial computed tomography (CT) revealed heterotopic ossification vs myositis ossificans, possibly related to the recent injection. The patient was eventually referred to the Division of Plastic Surgery, Department of Surgery, at the University of Texas Medical Branch (Galveston, Texas) for further evaluation. Physical examination revealed a 2×1-cm firm, mobile, nontender mass in the left cheek in the area of the buccinator muscles. The mass did not express any fluid and was most easily palpable from the oral cavity. Radiography findings showed that the calcium hydroxylapatite filler had migrated to this location and formed a nodule (Figure). Because calcium hydroxylapatite fillers generally last 12 to 18 months, we opted to observe the lesion for spontaneous resolution. Four months later, the patient presented to our clinic for follow-up and the mass had reduced in size and appeared to be spontaneously resolving.

We present a unique case of a migrating nodule that occurred after injection with calcium hydroxylapatite, which led to concern for neoplastic tumor formation. This complication is rare, and it is important for practitioners who inject calcium hydroxylapatite as well as those who these patients may be referred to for evaluation to be aware that migrating nodules can occur. This awareness can help reduce unnecessary referrals, medical procedures, and anxiety.
Calcium hydroxylapatite filler is composed of 30% calcium hydroxylapatite microspheres suspended in a 70% sodium carboxymethylcellulose gel. The water-soluble gel rapidly becomes absorbed upon injection; however, the microspheres form a scaffold for the production of newly synthesized collagen. The filling effect generally lasts 12 to 18 months.1
Calcium hydroxylapatite, similar to most fillers, generally is well tolerated with a low complication rate of 3%.1 Although nodule formation with calcium hydroxylapatite is rare, it is the most common adverse event and encompasses 96% of complications. The remaining 4% of complications include persistent inflammation, swelling, erythema, and technical mistakes leading to overcorrection.1 Migrating nodules are rare; however, Beer3 reported a similar case.
Treatment of calcium hydroxylapatite nodules depends on differentiating a cause based on the time of onset. Early nodules that occur within 1 to 2 weeks of the injection usually represent incorrect positioning of the filler and can be treated by massaging the nodule. Other more invasive techniques involve aspiration or injection of sterile water. Late-onset nodules have shown response to corticosteroid injections. For inflammatory nodules of infectious origin, antibiotics can be useful. Surgical excision of the nodule rarely is required, as most nodules will resolve spontaneously, even without intervention.1,2
Radiologic findings of calcium hydroxylapatite appear as high-attenuation linear streaks or masses on CT (280–700 HU) and as low to intermediate signal intensity on T1- or T2-weighted sequences on magnetic resonance imaging. Oftentimes, calcium hydroxylapatite has a similar radiographic appearance to bone and can persist for 2 years or more on radiographic imaging, longer than they are clinically visible.4 The nodule formation from injection with calcium hydroxylapatite can mimic pathologic conditions such as miliary osteomas, myositis ossificans, heterotrophic/dystrophic calcifications, and foreign bodies on CT. Our patient’s CT findings of high attenuation linear streaks and nodules of similar signal intensity to bone were consistent with those previously described in the radiographic literature.
Calcium hydroxylapatite fillers have a good safety profile, but it is important to recognize that nodule formation is a common adverse event and that migration of nodules can occur. Practitioners should recognize this possibility in patients presenting with new masses after filler injection before advocating for potentially invasive and costly procedures and diagnostic modalities.
- Kadouch JA. Calcium hydroxylapatite: a review on safety and complications. J Cosmet Dermatol. 2017;16:152-161.
- Moulinets I, Arnaud E, Bui P, et al. Foreign body reaction to Radiesse: 2 cases. Am J Dermatopathol. 2013;35:e37-40.
- Beer KR. Radiesse nodule of the lips from a distant injection site: report of a case and consideration of etiology and management. J Drugs Dermatol. 2007;6:846-847.
- Ginat DT, Schatz CJ. Imaging features of midface injectable fillers and associated complications. AJNR Am J Neuroradiol. 2013;34:1488-1495.
- Kadouch JA. Calcium hydroxylapatite: a review on safety and complications. J Cosmet Dermatol. 2017;16:152-161.
- Moulinets I, Arnaud E, Bui P, et al. Foreign body reaction to Radiesse: 2 cases. Am J Dermatopathol. 2013;35:e37-40.
- Beer KR. Radiesse nodule of the lips from a distant injection site: report of a case and consideration of etiology and management. J Drugs Dermatol. 2007;6:846-847.
- Ginat DT, Schatz CJ. Imaging features of midface injectable fillers and associated complications. AJNR Am J Neuroradiol. 2013;34:1488-1495.
Practice Points
- Calcium hydroxylapatite filler can migrate and form nodules in distant locations from the original injection site.
- Practitioners of calcium hydroxylapatite fillers should be aware of the potential for nodule migration to avoid costly, time-consuming, and invasive referrals and procedures.
Unusual Bilateral Distribution of Neurofibromatosis Type 5 on the Distal Upper Extremities
To the Editor:
Segmental neurofibromatosis, or neurofibromatosis type 5 (NF5), is a rare subtype of neurofibromatosis type 1 (NF1)(also known as von Recklinghausen disease). Phenotypic manifestations of NF5 include café-au-lait macules, neurofibromas, or both in 1 or more adjacent dermatomes. In contrast to the systemic features of NF1, the dermatomal distribution of NF5 demonstrates mosaicism due to a spontaneous postzygotic mutation in the neurofibromin 1 gene, NF1. We describe an atypical presentation of NF5 with bilateral features on the upper extremities.
A 74-year-old woman presented with soft pink- to flesh-colored growths on the left dorsal forearm and hand that were observed incidentally during a Mohs procedure for treatment of a basal cell carcinoma on the upper cutaneous lip. The patient reported that the lesions initially appeared on the left dorsal hand at approximately 16 years of age and had since spread proximally up to the mid dorsal forearm over the course of her lifetime. She denied any pain but claimed the affected area could be itchy. The lesions did not interfere with her daily activities, but they negatively impacted her social life due to their cosmetic appearance as well as her fear that they could be contagious. She denied any family history of NF1.
Physical examination revealed innumerable soft, pink- to flesh-colored cutaneous nodules ranging from 3 to 9 mm in diameter clustered uniformly on the left dorsal hand and lower forearm within the C6, C7, and C8 dermatomal regions (Figure, A). A singular brown patch measuring 20 mm in diameter also was observed on the right dorsal hand within the C6 dermatome, which the patient reported had been present since birth (Figure, B). The nodules and pigmented patch were clinically diagnosed as cutaneous neurofibromas on the left arm and a café-au-lait macule on the right arm, each manifesting within the C6 dermatome on separate upper extremities. Lisch nodules, axillary freckling, and acoustic schwannomas were not observed. Because of the dermatomal distribution of the lesions and lack of family history of NF1, a diagnosis of bilateral NF5 was made. The patient stated she had declined treatment of the neurofibromas from her referring general dermatologist due to possible risk for recurrence.
Segmental neurofibromatosis was first described in 1931 by Gammel,1 and in 1982, segmental neurofibromatosis was classified as NF5 by Riccardi.2 After Tinschert et al3 later demonstrated NF5 to be a somatic mutation of NF1,3 Ruggieri and Huson4 proposed the term mosaic neurofibromatosis 1 in 2001.
While the prevalence of NF1 is 1 in 3000 individuals,5 NF5 is rare with an occurrence of 1 in 40,000.6 In NF5, a spontaneous NF1 gene mutation occurs on chromosome 17 in a dividing cell after conception.7 Individuals with NF5 are born mosaic with 2 genotypes—one normal and one abnormal—for the NF1 gene.8 This contrasts with the autosomal-dominant and systemic characteristics of NF1, which has the NF1 gene mutation in all cells. Patients with NF5 generally are not expected to have affected offspring because the spontaneous mutation usually arises in somatic cells; however, a postzygotic mutation in the gonadal region could potentially affect germline cells, resulting in vertical transmission, with documented cases of offspring with systemic NF1.4 Because of the risk for malignancy with systemic neurofibromatosis, early diagnosis with genetic counseling is imperative in patients with both NF1 and NF5.
Neurofibromatosis type 5 is a clinical diagnosis based on the presence of neurofibromas and/or café-au-lait macules in a dermatomal distribution. The clinical presentation depends on when and where the NF1 gene mutation occurs in utero as cells multiply, differentiate, and migrate.8 Earlier mutations result in a broader manifestation of NF5 in comparison to late mutations, which have more localized features. An NF1 gene mutation causes a loss of function of neurofibromin, a tumor suppressor protein, in Schwann cells and fibroblasts.8 This produces neurofibromas and café-au-lait macules, respectively.8
A large literature review on segmental neurofibromatosis by Garcia-Romero et al6 identified 320 individuals who did not meet full inclusion criteria for NF1 between 1977 and 2012. Overall, 76% of cases were unilaterally distributed. The investigators identified 157 individual case reports in which the most to least common presentation was pigmentary changes only, neurofibromas only, mixed pigmentary changes with neurofibromas, and plexiform neurofibromas only; however, many of these cases were children who may have later developed both neurofibromas and pigmentary changes during puberty.6 Additional features of NF5 may include freckling, Lisch nodules, optic gliomas, malignant peripheral nerve sheath tumors, skeletal abnormalities, precocious puberty, vascular malformations, hypertension, seizures, and/or learning difficulties based on the affected anatomy.
Segmental neurofibromatosis, or NF5, is a rare subtype of NF1. Our case demonstrates an unusual bilateral distribution of NF5 with cutaneous neurofibromas and a café-au-lait macule on the upper extremities. Awareness of variations of neurofibromatosis and their genetic implications is essential in establishing earlier clinical diagnoses in cases with subtle manifestations.
- Gammel JA. Localized neurofibromatosis. Arch Dermatol. 1931;24:712-713.
- Riccardi VM. Neurofibromatosis: clinical heterogeneity. Curr Probl Cancer. 1982;7:1-34.
- Tinschert S, Naumann I, Stegmann E, et al. Segmental neurofibromatosis is caused by somatic mutation of the neurofibromatosis type 1 (NF1) gene. Eur J Hum Genet. 2000;8:455-459.
- Ruggieri M, Huson SM. The clinical and diagnostic implications of mosaicism in the neurofibromatoses. Neurology. 2001;56:1433-1443.
- Crowe FW, Schull WJ, Neel JV. A Clinical, Pathological and Genetic Study of Multiple Neurofibromatosis. Charles C Thomas; 1956.
- García-Romero MT, Parkin P, Lara-Corrales I. Mosaic neurofibromatosis type 1: a systematic review. Pediatr Dermatol. 2016;33:9-17.
- Ledbetter DH, Rich DC, O’Connell P, et al. Precise localization of NF1 to 17q11.2 by balanced translocation. Am J Hum Genet. 1989;44:20-24.
- Redlick FP, Shaw JC. Segmental neurofibromatosis follows Blaschko’s lines or dermatomes depending on the cell line affected: case report and literature review. J Cutan Med Surg. 2004;8:353-356.
To the Editor:
Segmental neurofibromatosis, or neurofibromatosis type 5 (NF5), is a rare subtype of neurofibromatosis type 1 (NF1)(also known as von Recklinghausen disease). Phenotypic manifestations of NF5 include café-au-lait macules, neurofibromas, or both in 1 or more adjacent dermatomes. In contrast to the systemic features of NF1, the dermatomal distribution of NF5 demonstrates mosaicism due to a spontaneous postzygotic mutation in the neurofibromin 1 gene, NF1. We describe an atypical presentation of NF5 with bilateral features on the upper extremities.
A 74-year-old woman presented with soft pink- to flesh-colored growths on the left dorsal forearm and hand that were observed incidentally during a Mohs procedure for treatment of a basal cell carcinoma on the upper cutaneous lip. The patient reported that the lesions initially appeared on the left dorsal hand at approximately 16 years of age and had since spread proximally up to the mid dorsal forearm over the course of her lifetime. She denied any pain but claimed the affected area could be itchy. The lesions did not interfere with her daily activities, but they negatively impacted her social life due to their cosmetic appearance as well as her fear that they could be contagious. She denied any family history of NF1.
Physical examination revealed innumerable soft, pink- to flesh-colored cutaneous nodules ranging from 3 to 9 mm in diameter clustered uniformly on the left dorsal hand and lower forearm within the C6, C7, and C8 dermatomal regions (Figure, A). A singular brown patch measuring 20 mm in diameter also was observed on the right dorsal hand within the C6 dermatome, which the patient reported had been present since birth (Figure, B). The nodules and pigmented patch were clinically diagnosed as cutaneous neurofibromas on the left arm and a café-au-lait macule on the right arm, each manifesting within the C6 dermatome on separate upper extremities. Lisch nodules, axillary freckling, and acoustic schwannomas were not observed. Because of the dermatomal distribution of the lesions and lack of family history of NF1, a diagnosis of bilateral NF5 was made. The patient stated she had declined treatment of the neurofibromas from her referring general dermatologist due to possible risk for recurrence.

Segmental neurofibromatosis was first described in 1931 by Gammel,1 and in 1982, segmental neurofibromatosis was classified as NF5 by Riccardi.2 After Tinschert et al3 later demonstrated NF5 to be a somatic mutation of NF1,3 Ruggieri and Huson4 proposed the term mosaic neurofibromatosis 1 in 2001.
While the prevalence of NF1 is 1 in 3000 individuals,5 NF5 is rare with an occurrence of 1 in 40,000.6 In NF5, a spontaneous NF1 gene mutation occurs on chromosome 17 in a dividing cell after conception.7 Individuals with NF5 are born mosaic with 2 genotypes—one normal and one abnormal—for the NF1 gene.8 This contrasts with the autosomal-dominant and systemic characteristics of NF1, which has the NF1 gene mutation in all cells. Patients with NF5 generally are not expected to have affected offspring because the spontaneous mutation usually arises in somatic cells; however, a postzygotic mutation in the gonadal region could potentially affect germline cells, resulting in vertical transmission, with documented cases of offspring with systemic NF1.4 Because of the risk for malignancy with systemic neurofibromatosis, early diagnosis with genetic counseling is imperative in patients with both NF1 and NF5.
Neurofibromatosis type 5 is a clinical diagnosis based on the presence of neurofibromas and/or café-au-lait macules in a dermatomal distribution. The clinical presentation depends on when and where the NF1 gene mutation occurs in utero as cells multiply, differentiate, and migrate.8 Earlier mutations result in a broader manifestation of NF5 in comparison to late mutations, which have more localized features. An NF1 gene mutation causes a loss of function of neurofibromin, a tumor suppressor protein, in Schwann cells and fibroblasts.8 This produces neurofibromas and café-au-lait macules, respectively.8
A large literature review on segmental neurofibromatosis by Garcia-Romero et al6 identified 320 individuals who did not meet full inclusion criteria for NF1 between 1977 and 2012. Overall, 76% of cases were unilaterally distributed. The investigators identified 157 individual case reports in which the most to least common presentation was pigmentary changes only, neurofibromas only, mixed pigmentary changes with neurofibromas, and plexiform neurofibromas only; however, many of these cases were children who may have later developed both neurofibromas and pigmentary changes during puberty.6 Additional features of NF5 may include freckling, Lisch nodules, optic gliomas, malignant peripheral nerve sheath tumors, skeletal abnormalities, precocious puberty, vascular malformations, hypertension, seizures, and/or learning difficulties based on the affected anatomy.
Segmental neurofibromatosis, or NF5, is a rare subtype of NF1. Our case demonstrates an unusual bilateral distribution of NF5 with cutaneous neurofibromas and a café-au-lait macule on the upper extremities. Awareness of variations of neurofibromatosis and their genetic implications is essential in establishing earlier clinical diagnoses in cases with subtle manifestations.
To the Editor:
Segmental neurofibromatosis, or neurofibromatosis type 5 (NF5), is a rare subtype of neurofibromatosis type 1 (NF1)(also known as von Recklinghausen disease). Phenotypic manifestations of NF5 include café-au-lait macules, neurofibromas, or both in 1 or more adjacent dermatomes. In contrast to the systemic features of NF1, the dermatomal distribution of NF5 demonstrates mosaicism due to a spontaneous postzygotic mutation in the neurofibromin 1 gene, NF1. We describe an atypical presentation of NF5 with bilateral features on the upper extremities.
A 74-year-old woman presented with soft pink- to flesh-colored growths on the left dorsal forearm and hand that were observed incidentally during a Mohs procedure for treatment of a basal cell carcinoma on the upper cutaneous lip. The patient reported that the lesions initially appeared on the left dorsal hand at approximately 16 years of age and had since spread proximally up to the mid dorsal forearm over the course of her lifetime. She denied any pain but claimed the affected area could be itchy. The lesions did not interfere with her daily activities, but they negatively impacted her social life due to their cosmetic appearance as well as her fear that they could be contagious. She denied any family history of NF1.
Physical examination revealed innumerable soft, pink- to flesh-colored cutaneous nodules ranging from 3 to 9 mm in diameter clustered uniformly on the left dorsal hand and lower forearm within the C6, C7, and C8 dermatomal regions (Figure, A). A singular brown patch measuring 20 mm in diameter also was observed on the right dorsal hand within the C6 dermatome, which the patient reported had been present since birth (Figure, B). The nodules and pigmented patch were clinically diagnosed as cutaneous neurofibromas on the left arm and a café-au-lait macule on the right arm, each manifesting within the C6 dermatome on separate upper extremities. Lisch nodules, axillary freckling, and acoustic schwannomas were not observed. Because of the dermatomal distribution of the lesions and lack of family history of NF1, a diagnosis of bilateral NF5 was made. The patient stated she had declined treatment of the neurofibromas from her referring general dermatologist due to possible risk for recurrence.

Segmental neurofibromatosis was first described in 1931 by Gammel,1 and in 1982, segmental neurofibromatosis was classified as NF5 by Riccardi.2 After Tinschert et al3 later demonstrated NF5 to be a somatic mutation of NF1,3 Ruggieri and Huson4 proposed the term mosaic neurofibromatosis 1 in 2001.
While the prevalence of NF1 is 1 in 3000 individuals,5 NF5 is rare with an occurrence of 1 in 40,000.6 In NF5, a spontaneous NF1 gene mutation occurs on chromosome 17 in a dividing cell after conception.7 Individuals with NF5 are born mosaic with 2 genotypes—one normal and one abnormal—for the NF1 gene.8 This contrasts with the autosomal-dominant and systemic characteristics of NF1, which has the NF1 gene mutation in all cells. Patients with NF5 generally are not expected to have affected offspring because the spontaneous mutation usually arises in somatic cells; however, a postzygotic mutation in the gonadal region could potentially affect germline cells, resulting in vertical transmission, with documented cases of offspring with systemic NF1.4 Because of the risk for malignancy with systemic neurofibromatosis, early diagnosis with genetic counseling is imperative in patients with both NF1 and NF5.
Neurofibromatosis type 5 is a clinical diagnosis based on the presence of neurofibromas and/or café-au-lait macules in a dermatomal distribution. The clinical presentation depends on when and where the NF1 gene mutation occurs in utero as cells multiply, differentiate, and migrate.8 Earlier mutations result in a broader manifestation of NF5 in comparison to late mutations, which have more localized features. An NF1 gene mutation causes a loss of function of neurofibromin, a tumor suppressor protein, in Schwann cells and fibroblasts.8 This produces neurofibromas and café-au-lait macules, respectively.8
A large literature review on segmental neurofibromatosis by Garcia-Romero et al6 identified 320 individuals who did not meet full inclusion criteria for NF1 between 1977 and 2012. Overall, 76% of cases were unilaterally distributed. The investigators identified 157 individual case reports in which the most to least common presentation was pigmentary changes only, neurofibromas only, mixed pigmentary changes with neurofibromas, and plexiform neurofibromas only; however, many of these cases were children who may have later developed both neurofibromas and pigmentary changes during puberty.6 Additional features of NF5 may include freckling, Lisch nodules, optic gliomas, malignant peripheral nerve sheath tumors, skeletal abnormalities, precocious puberty, vascular malformations, hypertension, seizures, and/or learning difficulties based on the affected anatomy.
Segmental neurofibromatosis, or NF5, is a rare subtype of NF1. Our case demonstrates an unusual bilateral distribution of NF5 with cutaneous neurofibromas and a café-au-lait macule on the upper extremities. Awareness of variations of neurofibromatosis and their genetic implications is essential in establishing earlier clinical diagnoses in cases with subtle manifestations.
- Gammel JA. Localized neurofibromatosis. Arch Dermatol. 1931;24:712-713.
- Riccardi VM. Neurofibromatosis: clinical heterogeneity. Curr Probl Cancer. 1982;7:1-34.
- Tinschert S, Naumann I, Stegmann E, et al. Segmental neurofibromatosis is caused by somatic mutation of the neurofibromatosis type 1 (NF1) gene. Eur J Hum Genet. 2000;8:455-459.
- Ruggieri M, Huson SM. The clinical and diagnostic implications of mosaicism in the neurofibromatoses. Neurology. 2001;56:1433-1443.
- Crowe FW, Schull WJ, Neel JV. A Clinical, Pathological and Genetic Study of Multiple Neurofibromatosis. Charles C Thomas; 1956.
- García-Romero MT, Parkin P, Lara-Corrales I. Mosaic neurofibromatosis type 1: a systematic review. Pediatr Dermatol. 2016;33:9-17.
- Ledbetter DH, Rich DC, O’Connell P, et al. Precise localization of NF1 to 17q11.2 by balanced translocation. Am J Hum Genet. 1989;44:20-24.
- Redlick FP, Shaw JC. Segmental neurofibromatosis follows Blaschko’s lines or dermatomes depending on the cell line affected: case report and literature review. J Cutan Med Surg. 2004;8:353-356.
- Gammel JA. Localized neurofibromatosis. Arch Dermatol. 1931;24:712-713.
- Riccardi VM. Neurofibromatosis: clinical heterogeneity. Curr Probl Cancer. 1982;7:1-34.
- Tinschert S, Naumann I, Stegmann E, et al. Segmental neurofibromatosis is caused by somatic mutation of the neurofibromatosis type 1 (NF1) gene. Eur J Hum Genet. 2000;8:455-459.
- Ruggieri M, Huson SM. The clinical and diagnostic implications of mosaicism in the neurofibromatoses. Neurology. 2001;56:1433-1443.
- Crowe FW, Schull WJ, Neel JV. A Clinical, Pathological and Genetic Study of Multiple Neurofibromatosis. Charles C Thomas; 1956.
- García-Romero MT, Parkin P, Lara-Corrales I. Mosaic neurofibromatosis type 1: a systematic review. Pediatr Dermatol. 2016;33:9-17.
- Ledbetter DH, Rich DC, O’Connell P, et al. Precise localization of NF1 to 17q11.2 by balanced translocation. Am J Hum Genet. 1989;44:20-24.
- Redlick FP, Shaw JC. Segmental neurofibromatosis follows Blaschko’s lines or dermatomes depending on the cell line affected: case report and literature review. J Cutan Med Surg. 2004;8:353-356.
Practice Points
- Segmental neurofibromatosis, or neurofibromatosis type 5 (NF5), is a rare subtype of neurofibromatosistype 1 (NF1)(also known as von Recklinghausen disease).
- Individuals with NF5 are born mosaic with 2 genotypes—one normal and one abnormal—for the neurofibromin 1 gene, NF1. This is in contrast to the autosomal-dominant and systemic characteristics of NF1, which has the NF1 gene mutation in all cells.
Vedolizumab-Induced Acne Fulminans: An Uncommon and Severe Adverse Effect
To the Editor:
Vedolizumab is an innovative monoclonal antibody targeted against the α4β7 integrin that is approved for treatment of moderate to severe ulcerative colitis and Crohn disease refractory to standard treatment.1 Vedolizumab is thought to be gut specific, blocking integrins specific to T lymphocytes destined for the gastrointestinal tract and their interaction with endothelial cells, thereby modulating the adaptive immune system in the gut without systemic immunosuppression.2 It generally is well tolerated, and acne rarely has been reported as an adverse event.3,4 We present a case of acne fulminans without systemic symptoms (AF-WOSS) as a severe side effect of vedolizumab that responded very well to systemic steroids and oral isotretinoin in addition to the discontinuation of treatment.
A 46-year-old obese man presented to our dermatology clinic with a chief complaint of rapidly progressive tender skin lesions. The patient had a long-standing history of severe fistulating and stricturing Crohn disease status post–bowel resection with ileostomy and had recently started treatment with vedolizumab after failing treatment with infliximab, adalimumab, certolizumab pegol, ustekinumab, and methotrexate. Several weeks after beginning infusions of vedolizumab, the patient began to develop many erythematous papules and pustules on the face, chest (Figure 1), and buttocks that rapidly progressed into painful and coalescing nodules and cysts over the next several months. He was prescribed benzoyl peroxide wash 10% as well as several weeks of oral doxycycline 100 mg twice daily with no improvement. The patient denied any other new medications or triggers, fever, chills, bone pain, headache, fatigue, or myalgia. The skin involvement continued to worsen with successive vedolizumab infusions over a period of 8 weeks, which ultimately resulted in cessation of vedolizumab.
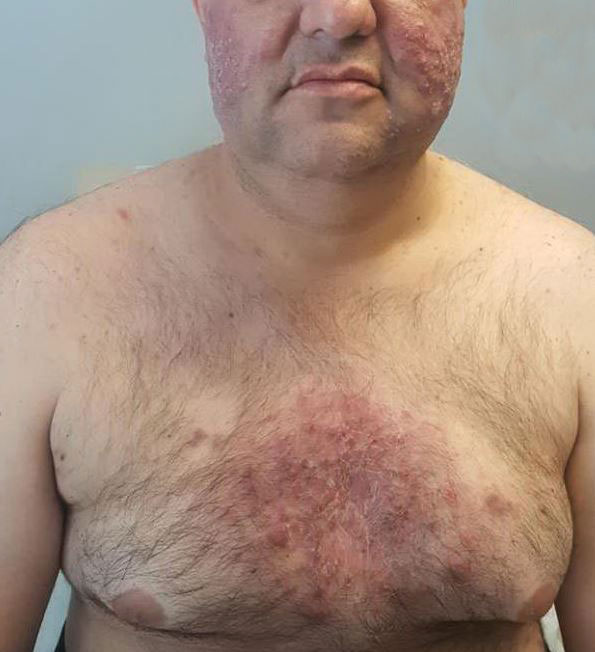
Physical examination revealed large, tender, pink, erythematous, and indurated plaques that were heavily studded with pink papules, pustules, and nodules on the cheeks (Figure 2), central chest, and buttocks. A punch biopsy of a pustule on the cheek showed ruptured suppurative folliculitis. The patient subsequently was diagnosed with AF-WOSS.
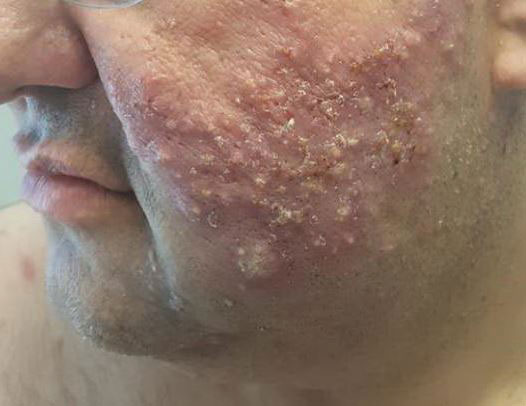
The patient then completed a 7-day course of sulfamethoxazole-trimethoprim followed by a 10-day course of amoxicillin-clavulanic acid, neither of which led to improvement of the lesions. He then was started on an oral prednisone taper (1 mg/kg starting dose) that ultimately totaled 14 weeks in length due to his frequent flares any time prednisone was decreased below 40 mg daily. After 3 weeks on the oral prednisone, the patient was started on 0.3 mg/kg of concomitant oral isotretinoin every other day, which slowly was increased as tolerated until he reached a goal dose of roughly 150 mg/kg, which resolved the acneform papules and pustules and allowed for successful tapering off the prednisone.
Many studies have been published regarding the safety and side-effect profile of vedolizumab, but most do not report acne as an adverse event.3-5 A German cohort study by Baumgart et al3 reported acne as a side effect in 15 of 212 (7.1%) patients but did not classify the severity. Another case report noted nodulocystic acne in a patient receiving vedolizumab for treatment of inflammatory bowel disease; however, this patient responded well to the use of a tetracycline antibiotic and was able to continue therapy with vedolizumab.5 Our patient demonstrated a severe and uncommon case of acne classified as AF-WOSS following initiation of therapy with vedolizumab, which required treatment with systemic steroids plus oral isotretinoin and resulted in cessation of vedolizumab.
As new therapies emerge, it is important to document new or severe adverse effects so providers can choose an appropriate therapy and adequately counsel patients regarding the side effects. Although vedolizumab was thought to have gut-specific action, there is new evidence to suggest that the principal ligand of the α4β7 integrin, mucosal addressin cell adhesion molecule-1, is not only expressed on gut endothelial cells but also on fibroblasts and melanomas, which may provide insight into the observed extraintestinal side effects of vedolizumab.6
- Smith MA, Mohammad RA. Vedolizumab: an α4β7 integrin inhibitor for inflammatory bowel diseases. Ann Pharmacother. 2014;48:1629-1635.
- Singh H, Grewal N, Arora E, et al. Vedolizumab: a novel anti-integrin drug for treatment of inflammatory bowel disease. J Nat Sci Bio Med. 2016;7:4-9.
- Baumgart DC, Bokemeyer B, Drabik A, et al. Vedolizumab induction therapy for inflammatory bowel disease in clinical practice: a nationwide consecutive German cohort study. Aliment Pharmacol Ther. 2016;43:1090-1102.
- Bye WA, Jairath V, Travis SPL. Systematic review: the safety of vedolizumab for the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:3-15.
- Gilhooley E, Doherty G, Lally A. Vedolizumab-induced acne in inflammatory bowel disease. Int J Dermatol. 2018;57:752-753.
- Leung E, Kanwar RK, Kanwar JR, et al. Mucosal vascular addressin cell adhesion molecule-1 is expressed outside the endothelial lineage on fibroblasts and melanoma cells. Immunol Cell Biol. 2003;81:320-327.
To the Editor:
Vedolizumab is an innovative monoclonal antibody targeted against the α4β7 integrin that is approved for treatment of moderate to severe ulcerative colitis and Crohn disease refractory to standard treatment.1 Vedolizumab is thought to be gut specific, blocking integrins specific to T lymphocytes destined for the gastrointestinal tract and their interaction with endothelial cells, thereby modulating the adaptive immune system in the gut without systemic immunosuppression.2 It generally is well tolerated, and acne rarely has been reported as an adverse event.3,4 We present a case of acne fulminans without systemic symptoms (AF-WOSS) as a severe side effect of vedolizumab that responded very well to systemic steroids and oral isotretinoin in addition to the discontinuation of treatment.
A 46-year-old obese man presented to our dermatology clinic with a chief complaint of rapidly progressive tender skin lesions. The patient had a long-standing history of severe fistulating and stricturing Crohn disease status post–bowel resection with ileostomy and had recently started treatment with vedolizumab after failing treatment with infliximab, adalimumab, certolizumab pegol, ustekinumab, and methotrexate. Several weeks after beginning infusions of vedolizumab, the patient began to develop many erythematous papules and pustules on the face, chest (Figure 1), and buttocks that rapidly progressed into painful and coalescing nodules and cysts over the next several months. He was prescribed benzoyl peroxide wash 10% as well as several weeks of oral doxycycline 100 mg twice daily with no improvement. The patient denied any other new medications or triggers, fever, chills, bone pain, headache, fatigue, or myalgia. The skin involvement continued to worsen with successive vedolizumab infusions over a period of 8 weeks, which ultimately resulted in cessation of vedolizumab.

Physical examination revealed large, tender, pink, erythematous, and indurated plaques that were heavily studded with pink papules, pustules, and nodules on the cheeks (Figure 2), central chest, and buttocks. A punch biopsy of a pustule on the cheek showed ruptured suppurative folliculitis. The patient subsequently was diagnosed with AF-WOSS.

The patient then completed a 7-day course of sulfamethoxazole-trimethoprim followed by a 10-day course of amoxicillin-clavulanic acid, neither of which led to improvement of the lesions. He then was started on an oral prednisone taper (1 mg/kg starting dose) that ultimately totaled 14 weeks in length due to his frequent flares any time prednisone was decreased below 40 mg daily. After 3 weeks on the oral prednisone, the patient was started on 0.3 mg/kg of concomitant oral isotretinoin every other day, which slowly was increased as tolerated until he reached a goal dose of roughly 150 mg/kg, which resolved the acneform papules and pustules and allowed for successful tapering off the prednisone.
Many studies have been published regarding the safety and side-effect profile of vedolizumab, but most do not report acne as an adverse event.3-5 A German cohort study by Baumgart et al3 reported acne as a side effect in 15 of 212 (7.1%) patients but did not classify the severity. Another case report noted nodulocystic acne in a patient receiving vedolizumab for treatment of inflammatory bowel disease; however, this patient responded well to the use of a tetracycline antibiotic and was able to continue therapy with vedolizumab.5 Our patient demonstrated a severe and uncommon case of acne classified as AF-WOSS following initiation of therapy with vedolizumab, which required treatment with systemic steroids plus oral isotretinoin and resulted in cessation of vedolizumab.
As new therapies emerge, it is important to document new or severe adverse effects so providers can choose an appropriate therapy and adequately counsel patients regarding the side effects. Although vedolizumab was thought to have gut-specific action, there is new evidence to suggest that the principal ligand of the α4β7 integrin, mucosal addressin cell adhesion molecule-1, is not only expressed on gut endothelial cells but also on fibroblasts and melanomas, which may provide insight into the observed extraintestinal side effects of vedolizumab.6
To the Editor:
Vedolizumab is an innovative monoclonal antibody targeted against the α4β7 integrin that is approved for treatment of moderate to severe ulcerative colitis and Crohn disease refractory to standard treatment.1 Vedolizumab is thought to be gut specific, blocking integrins specific to T lymphocytes destined for the gastrointestinal tract and their interaction with endothelial cells, thereby modulating the adaptive immune system in the gut without systemic immunosuppression.2 It generally is well tolerated, and acne rarely has been reported as an adverse event.3,4 We present a case of acne fulminans without systemic symptoms (AF-WOSS) as a severe side effect of vedolizumab that responded very well to systemic steroids and oral isotretinoin in addition to the discontinuation of treatment.
A 46-year-old obese man presented to our dermatology clinic with a chief complaint of rapidly progressive tender skin lesions. The patient had a long-standing history of severe fistulating and stricturing Crohn disease status post–bowel resection with ileostomy and had recently started treatment with vedolizumab after failing treatment with infliximab, adalimumab, certolizumab pegol, ustekinumab, and methotrexate. Several weeks after beginning infusions of vedolizumab, the patient began to develop many erythematous papules and pustules on the face, chest (Figure 1), and buttocks that rapidly progressed into painful and coalescing nodules and cysts over the next several months. He was prescribed benzoyl peroxide wash 10% as well as several weeks of oral doxycycline 100 mg twice daily with no improvement. The patient denied any other new medications or triggers, fever, chills, bone pain, headache, fatigue, or myalgia. The skin involvement continued to worsen with successive vedolizumab infusions over a period of 8 weeks, which ultimately resulted in cessation of vedolizumab.

Physical examination revealed large, tender, pink, erythematous, and indurated plaques that were heavily studded with pink papules, pustules, and nodules on the cheeks (Figure 2), central chest, and buttocks. A punch biopsy of a pustule on the cheek showed ruptured suppurative folliculitis. The patient subsequently was diagnosed with AF-WOSS.

The patient then completed a 7-day course of sulfamethoxazole-trimethoprim followed by a 10-day course of amoxicillin-clavulanic acid, neither of which led to improvement of the lesions. He then was started on an oral prednisone taper (1 mg/kg starting dose) that ultimately totaled 14 weeks in length due to his frequent flares any time prednisone was decreased below 40 mg daily. After 3 weeks on the oral prednisone, the patient was started on 0.3 mg/kg of concomitant oral isotretinoin every other day, which slowly was increased as tolerated until he reached a goal dose of roughly 150 mg/kg, which resolved the acneform papules and pustules and allowed for successful tapering off the prednisone.
Many studies have been published regarding the safety and side-effect profile of vedolizumab, but most do not report acne as an adverse event.3-5 A German cohort study by Baumgart et al3 reported acne as a side effect in 15 of 212 (7.1%) patients but did not classify the severity. Another case report noted nodulocystic acne in a patient receiving vedolizumab for treatment of inflammatory bowel disease; however, this patient responded well to the use of a tetracycline antibiotic and was able to continue therapy with vedolizumab.5 Our patient demonstrated a severe and uncommon case of acne classified as AF-WOSS following initiation of therapy with vedolizumab, which required treatment with systemic steroids plus oral isotretinoin and resulted in cessation of vedolizumab.
As new therapies emerge, it is important to document new or severe adverse effects so providers can choose an appropriate therapy and adequately counsel patients regarding the side effects. Although vedolizumab was thought to have gut-specific action, there is new evidence to suggest that the principal ligand of the α4β7 integrin, mucosal addressin cell adhesion molecule-1, is not only expressed on gut endothelial cells but also on fibroblasts and melanomas, which may provide insight into the observed extraintestinal side effects of vedolizumab.6
- Smith MA, Mohammad RA. Vedolizumab: an α4β7 integrin inhibitor for inflammatory bowel diseases. Ann Pharmacother. 2014;48:1629-1635.
- Singh H, Grewal N, Arora E, et al. Vedolizumab: a novel anti-integrin drug for treatment of inflammatory bowel disease. J Nat Sci Bio Med. 2016;7:4-9.
- Baumgart DC, Bokemeyer B, Drabik A, et al. Vedolizumab induction therapy for inflammatory bowel disease in clinical practice: a nationwide consecutive German cohort study. Aliment Pharmacol Ther. 2016;43:1090-1102.
- Bye WA, Jairath V, Travis SPL. Systematic review: the safety of vedolizumab for the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:3-15.
- Gilhooley E, Doherty G, Lally A. Vedolizumab-induced acne in inflammatory bowel disease. Int J Dermatol. 2018;57:752-753.
- Leung E, Kanwar RK, Kanwar JR, et al. Mucosal vascular addressin cell adhesion molecule-1 is expressed outside the endothelial lineage on fibroblasts and melanoma cells. Immunol Cell Biol. 2003;81:320-327.
- Smith MA, Mohammad RA. Vedolizumab: an α4β7 integrin inhibitor for inflammatory bowel diseases. Ann Pharmacother. 2014;48:1629-1635.
- Singh H, Grewal N, Arora E, et al. Vedolizumab: a novel anti-integrin drug for treatment of inflammatory bowel disease. J Nat Sci Bio Med. 2016;7:4-9.
- Baumgart DC, Bokemeyer B, Drabik A, et al. Vedolizumab induction therapy for inflammatory bowel disease in clinical practice: a nationwide consecutive German cohort study. Aliment Pharmacol Ther. 2016;43:1090-1102.
- Bye WA, Jairath V, Travis SPL. Systematic review: the safety of vedolizumab for the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:3-15.
- Gilhooley E, Doherty G, Lally A. Vedolizumab-induced acne in inflammatory bowel disease. Int J Dermatol. 2018;57:752-753.
- Leung E, Kanwar RK, Kanwar JR, et al. Mucosal vascular addressin cell adhesion molecule-1 is expressed outside the endothelial lineage on fibroblasts and melanoma cells. Immunol Cell Biol. 2003;81:320-327.
Practice Points
- Vedolizumab, a monoclonal antibody for the treatment of refractory inflammatory bowel disease, was found to cause acne fulminans without systemic symptoms.
- Vedolizumab previously was believed to be a gut-limited immune modulator.
- Off-target cutaneous effects may indicate wider expression of the target integrin of vedolizumab and should be recognized as the drug becomes more widely used.
Punked By the Punctum: Domestically Acquired Cutaneous Myiasis
To the Editor:
Cutaneous myiasis is a skin infestation with dipterous larvae that feed on the host’s tissue and cause a wide range of manifestations depending on the location of infestation. Cutaneous myiasis, which includes furuncular, wound, and migratory types, is the most common clinical form of this condition.1 It is endemic to tropical and subtropical areas and is not common in the United States, thus it can pose a diagnostic challenge when presenting in nonendemic areas. We present the case of a woman from Michigan who acquired furuncular myiasis without travel history to a tropical or subtropical locale.
A 72-year-old woman presented to our clinic with a chief concern of a burning, pruritic, migratory skin lesion on the left arm of approximately 1 week’s duration. She had a medical history of squamous cell carcinoma, keratoacanthoma, and multiple tick bites. She reported that the lesion started on the distal aspect of the left arm as an eraser-sized, perfectly round, raised bruise with a dark pepperlike bump in the center. The lesion then spread proximally over the course of 1 week, creating 3 more identical lesions. As one lesion resolved, a new lesion appeared approximately 2 to 4 cm proximal to the preceding lesion. The patient had traveled to England, Scotland, and Ireland 2 months prior but otherwise denied leaving the state of Michigan. She reported frequent exposure to gardens, meadows, and wetlands in search of milkweed and monarch butterfly larvae that she raises in northeast Michigan. She denied any recent illness or associated systemic symptoms. Initial evaluation by a primary care physician resulted in a diagnosis of a furuncle or tick bite; she completed a 10-day course of amoxicillin and a methylprednisolone dose pack without improvement.
Physical examination revealed a 1-cm, firm, violaceous nodule with a small distinct central punctum and surrounding erythema on the proximal aspect of the left arm. Dermoscopy revealed a pulsating motion and expulsion of serosanguineous fluid from the central punctum (Figure 1). Further inspection of the patient’s left arm exposed several noninflammatory puncta distal to the primary lesion spaced at 2- to 4-cm intervals.
Gross examination of a 6-mm punch biopsy from the primary inflammatory nodule uncovered a small, motile, gray-white larval organism in the inferior portion of the specimen (Figure 2). Histopathology revealed superficial and deep eosinophil-rich inflammation, fibrosis, and hemorrhage. There was a complex wedge-shaped organism with extensive internal muscle bounded by a thin cuticle bearing rows of chitinous hooklets located at one side within the deep dermis (Figure 3). The findings were consistent with a diagnosis of cutaneous myiasis. No further treatment was required, as the organism was completely excised with the biopsy.
The most common causative agents of furuncular myiasis obtained from travelers returning from Mexico and Central and South America are Dermatobia hominis and Cordylobia anthropophaga. Cases of furuncular myiasis acquired in the United States without recent foreign travel are rare. Most of these cases are caused by larvae of the Cuterebra species (also known as the rabbit botfly or rodent botfly).2 In a 2003 literature review by Safdar et al3 on 56 cases of furuncular myiasis in the United States, the median age of patients was 14 years, 87% of cases occurred in August and September, and most involved exposure in rural or suburban settings; 53% of cases presented in the northeastern United States.
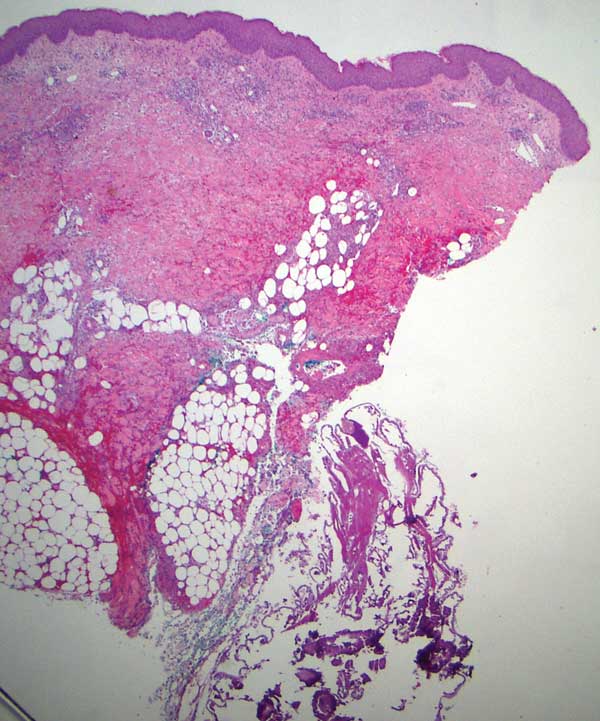
Furuncular myiasis occurs when the organism’s ova are deposited on the skin of a human host by the parent organism or a mosquito vector. The heat of the skin causes the eggs to hatch and the dipteran larvae must penetrate the skin within 20 days.1 Signs of infection typically are seen 6 to 10 days after infestation.3 The larvae then feed on human tissue and burrow deep in the dermis, forming an erythematous furunculoid nodule containing one or multiple maggots. After 5 to 10 weeks, the adult larvae drop to the ground, where they mature into adult organisms in the soil.1
The most reported symptoms of furuncular myiasis include pruritus, pain, and movement sensation, typically occurring suddenly at night.4 The most common presentation is a furunclelike lesion that exudes serosanguineous or purulent fluid,1 but there have been reports of vesicular, bullous, pustular, erosive, ecchymotic, and ulcerative lesions.5Dermatobia hominis usually presents on an exposed site, such as the scalp, face, and extremities. It may present with paroxysmal episodes of lancinating pain. Over time, the lesion usually heals without a scar, though hyperpigmentation and scarring can occur. The most reported complication is secondary bacterial infection.4 Local lymphadenopathy or systemic symptoms should raise concern for infection. Staphylococcus aureus and group B Streptococcus have been cultured from lesions.6,7
The differential diagnosis for myiasis should include furuncle, insect bite, insect prurigo, pyoderma, inflamed cyst, and tungiasis. Myiasis also can present similarly to severe soft tissue infections or cellulitis. If located on the breasts, it can be mistaken for periductal mastitis, a benign mass with microcalcification, or inflammatory carcinoma. Lastly, due to pain, erythema, pruritus, small vesicles, and crusting, it may be confused for herpes simplex virus.1
Furuncular myiasis typically is diagnosed based on clinical presentation, especially in endemic regions. In nonendemic areas, the patient’s history may reveal recent travel or predisposition to myiasis. In cases where there is uncertainty, dermoscopy may be used to identify the maggot in the lesion, or ultrasonography can be used to confirm myiasis through the detection of larval movement.8 Dermoscopy will reveal a furuncular lesion with a central opening surrounded by dilated blood vessels and a yellowish structure with black barblike spines.9 Within the dermis is a fibrous cystic sinus tract containing the dipteran larva. Laboratory studies typically are unremarkable. In chronic cases, a complete blood cell count and other laboratory tests may show systemic inflammation, peripheral eosinophilia, and elevated IgE.10 Biopsies of furuncular myiasis are not necessary for diagnosis. Histopathology reveals an ulcerated epidermis with or without hyperkeratosis and an inflammatory infiltrate composed of lymphocytes and neutrophils with eosinophils, fibroblasts, histiocytes, basophils, mast cells, plasma cells, and Langerhans cells within the dermis and subcutis.11
There are various approaches to treating furuncular myiasis, with the goal of complete removal of the larva and prevention of secondary infection. One treatment option is to apply a toxic substance to the larva, effectively killing it. Another approach is to force the larva to emerge via localized hypoxia, which can be done by occluding the punctum of the lesion for at least 24 hours. A complication of this method is suffocation of the larva without migration, leading to incomplete extraction and secondary infection.1 A third method is to surgically remove the larva, which allows for debridement of necrotic tissue surrounding the lesion if present.12 Ultrasonography also can be used therapeutically to aid in the removal of the larvae. The last method is to inject lidocaine into the base of the lesion, forcing the larva out of the punctum via fluid pressure.13 Oral treatments such as ivermectin are not recommended because they can result in the death of larvae within the lesion, leading to an inflammatory response.8
Furuncular myiasis is a form of cutaneous larvae infestation not commonly seen in individuals who do not live or travel in endemic, tropical, and subtropical regions. Diagnosis is based on clinical presentation, with imaging and laboratory studies available to supplement in unclear or atypical manifestations. Treatment involves complete removal of the larva, typically through forced evacuation via hypoxia or through surgical removal. Most cases resolve without notable scarring or other sequelae; however, in those who do have complications, the most common is secondary bacterial infection. Our patient’s absence of notable travel history and frequent environmental exposure in Michigan led us to believe the organism was from a domestic source. Our case underlines the importance of a thorough history and clinical examination of furuncular lesions including the use of dermoscopy to yield an appropriate diagnosis and treatment plan.
- Francesconi F, Lupi O. Myiasis. Clin Microbiol Rev. 2012;25:79-105. doi:10.1128/CMR.00010-11
- Schiff TA. Furuncular cutaneous myiasis caused by Cuterebra larva. J Am Acad Dermatol 1993;28:261-263.
- Safdar N, Young DK, Andes D. Autochthonous furuncular myiasis in the United States: case report and literature review. Clin Infect Dis. 2003;26:73-80.
- Mahal JJ, Sperling JD. Furuncular myiasis from Dermatobia hominus: a case of human botfly infestation. J Emerg Med. 2012;43:618-621.
- Francesconi F, Lupi O. Myiasis. In: Tyring SK, Lupi O, Hengge UR, eds. Tropical Dermatology. Elsevier; 2006:232-239.
- Gordon PM, Hepburn NC, Williams AE, et al. Cutaneous myiasis due to Dermatobia hominis: a report of six cases. Br J Dermatol. 1995;132:811-814.
- Hubler WR Jr, Rudolph AH, Dougherty EF. Dermal myiasis. Arch Dermatol. 1974;110:109-110.
- Quintanilla-Cedillo MR, León-Ureña H, Contreras-Ruiz J, et al. The value of Doppler ultrasound in diagnosis in 25 cases of furunculoid myiasis. Int J Dermatol. 2005;44:34-37.
- Bakos RM, Bakos L. Dermoscopic diagnosis of furuncular myiasis. Arch Dermatol. 2007;143:123-124.
- Varani S, Tassinari D, Elleri D, et al. A case of furuncular myiasis associated with systemic inflammation. Parasitol Int. 2007;56:330-333.
- Grogan TM, Payne CM, Spier C, et al. Cutaneous myiasis. immunohistologic and ultrastructural morphometric features of a human botfly lesion. Am J Dermatopathol. 1987;9:232-239.
- Krajewski A, Allen B, Hoss D, et al. Cutaneous myiasis. J Plast Reconstr Aesthet Surg. 2009;62:383-386.
- Lebwohl MG, Heymann WR, Berth-Jones J, et al. Myiasis: Treatment of Skin Diseases. Comprehensive Therapeutic Strategies. 2nd ed. Elsevier-Mosby; 2006.
To the Editor:
Cutaneous myiasis is a skin infestation with dipterous larvae that feed on the host’s tissue and cause a wide range of manifestations depending on the location of infestation. Cutaneous myiasis, which includes furuncular, wound, and migratory types, is the most common clinical form of this condition.1 It is endemic to tropical and subtropical areas and is not common in the United States, thus it can pose a diagnostic challenge when presenting in nonendemic areas. We present the case of a woman from Michigan who acquired furuncular myiasis without travel history to a tropical or subtropical locale.
A 72-year-old woman presented to our clinic with a chief concern of a burning, pruritic, migratory skin lesion on the left arm of approximately 1 week’s duration. She had a medical history of squamous cell carcinoma, keratoacanthoma, and multiple tick bites. She reported that the lesion started on the distal aspect of the left arm as an eraser-sized, perfectly round, raised bruise with a dark pepperlike bump in the center. The lesion then spread proximally over the course of 1 week, creating 3 more identical lesions. As one lesion resolved, a new lesion appeared approximately 2 to 4 cm proximal to the preceding lesion. The patient had traveled to England, Scotland, and Ireland 2 months prior but otherwise denied leaving the state of Michigan. She reported frequent exposure to gardens, meadows, and wetlands in search of milkweed and monarch butterfly larvae that she raises in northeast Michigan. She denied any recent illness or associated systemic symptoms. Initial evaluation by a primary care physician resulted in a diagnosis of a furuncle or tick bite; she completed a 10-day course of amoxicillin and a methylprednisolone dose pack without improvement.
Physical examination revealed a 1-cm, firm, violaceous nodule with a small distinct central punctum and surrounding erythema on the proximal aspect of the left arm. Dermoscopy revealed a pulsating motion and expulsion of serosanguineous fluid from the central punctum (Figure 1). Further inspection of the patient’s left arm exposed several noninflammatory puncta distal to the primary lesion spaced at 2- to 4-cm intervals.

Gross examination of a 6-mm punch biopsy from the primary inflammatory nodule uncovered a small, motile, gray-white larval organism in the inferior portion of the specimen (Figure 2). Histopathology revealed superficial and deep eosinophil-rich inflammation, fibrosis, and hemorrhage. There was a complex wedge-shaped organism with extensive internal muscle bounded by a thin cuticle bearing rows of chitinous hooklets located at one side within the deep dermis (Figure 3). The findings were consistent with a diagnosis of cutaneous myiasis. No further treatment was required, as the organism was completely excised with the biopsy.

The most common causative agents of furuncular myiasis obtained from travelers returning from Mexico and Central and South America are Dermatobia hominis and Cordylobia anthropophaga. Cases of furuncular myiasis acquired in the United States without recent foreign travel are rare. Most of these cases are caused by larvae of the Cuterebra species (also known as the rabbit botfly or rodent botfly).2 In a 2003 literature review by Safdar et al3 on 56 cases of furuncular myiasis in the United States, the median age of patients was 14 years, 87% of cases occurred in August and September, and most involved exposure in rural or suburban settings; 53% of cases presented in the northeastern United States.

Furuncular myiasis occurs when the organism’s ova are deposited on the skin of a human host by the parent organism or a mosquito vector. The heat of the skin causes the eggs to hatch and the dipteran larvae must penetrate the skin within 20 days.1 Signs of infection typically are seen 6 to 10 days after infestation.3 The larvae then feed on human tissue and burrow deep in the dermis, forming an erythematous furunculoid nodule containing one or multiple maggots. After 5 to 10 weeks, the adult larvae drop to the ground, where they mature into adult organisms in the soil.1
The most reported symptoms of furuncular myiasis include pruritus, pain, and movement sensation, typically occurring suddenly at night.4 The most common presentation is a furunclelike lesion that exudes serosanguineous or purulent fluid,1 but there have been reports of vesicular, bullous, pustular, erosive, ecchymotic, and ulcerative lesions.5Dermatobia hominis usually presents on an exposed site, such as the scalp, face, and extremities. It may present with paroxysmal episodes of lancinating pain. Over time, the lesion usually heals without a scar, though hyperpigmentation and scarring can occur. The most reported complication is secondary bacterial infection.4 Local lymphadenopathy or systemic symptoms should raise concern for infection. Staphylococcus aureus and group B Streptococcus have been cultured from lesions.6,7
The differential diagnosis for myiasis should include furuncle, insect bite, insect prurigo, pyoderma, inflamed cyst, and tungiasis. Myiasis also can present similarly to severe soft tissue infections or cellulitis. If located on the breasts, it can be mistaken for periductal mastitis, a benign mass with microcalcification, or inflammatory carcinoma. Lastly, due to pain, erythema, pruritus, small vesicles, and crusting, it may be confused for herpes simplex virus.1
Furuncular myiasis typically is diagnosed based on clinical presentation, especially in endemic regions. In nonendemic areas, the patient’s history may reveal recent travel or predisposition to myiasis. In cases where there is uncertainty, dermoscopy may be used to identify the maggot in the lesion, or ultrasonography can be used to confirm myiasis through the detection of larval movement.8 Dermoscopy will reveal a furuncular lesion with a central opening surrounded by dilated blood vessels and a yellowish structure with black barblike spines.9 Within the dermis is a fibrous cystic sinus tract containing the dipteran larva. Laboratory studies typically are unremarkable. In chronic cases, a complete blood cell count and other laboratory tests may show systemic inflammation, peripheral eosinophilia, and elevated IgE.10 Biopsies of furuncular myiasis are not necessary for diagnosis. Histopathology reveals an ulcerated epidermis with or without hyperkeratosis and an inflammatory infiltrate composed of lymphocytes and neutrophils with eosinophils, fibroblasts, histiocytes, basophils, mast cells, plasma cells, and Langerhans cells within the dermis and subcutis.11
There are various approaches to treating furuncular myiasis, with the goal of complete removal of the larva and prevention of secondary infection. One treatment option is to apply a toxic substance to the larva, effectively killing it. Another approach is to force the larva to emerge via localized hypoxia, which can be done by occluding the punctum of the lesion for at least 24 hours. A complication of this method is suffocation of the larva without migration, leading to incomplete extraction and secondary infection.1 A third method is to surgically remove the larva, which allows for debridement of necrotic tissue surrounding the lesion if present.12 Ultrasonography also can be used therapeutically to aid in the removal of the larvae. The last method is to inject lidocaine into the base of the lesion, forcing the larva out of the punctum via fluid pressure.13 Oral treatments such as ivermectin are not recommended because they can result in the death of larvae within the lesion, leading to an inflammatory response.8
Furuncular myiasis is a form of cutaneous larvae infestation not commonly seen in individuals who do not live or travel in endemic, tropical, and subtropical regions. Diagnosis is based on clinical presentation, with imaging and laboratory studies available to supplement in unclear or atypical manifestations. Treatment involves complete removal of the larva, typically through forced evacuation via hypoxia or through surgical removal. Most cases resolve without notable scarring or other sequelae; however, in those who do have complications, the most common is secondary bacterial infection. Our patient’s absence of notable travel history and frequent environmental exposure in Michigan led us to believe the organism was from a domestic source. Our case underlines the importance of a thorough history and clinical examination of furuncular lesions including the use of dermoscopy to yield an appropriate diagnosis and treatment plan.
To the Editor:
Cutaneous myiasis is a skin infestation with dipterous larvae that feed on the host’s tissue and cause a wide range of manifestations depending on the location of infestation. Cutaneous myiasis, which includes furuncular, wound, and migratory types, is the most common clinical form of this condition.1 It is endemic to tropical and subtropical areas and is not common in the United States, thus it can pose a diagnostic challenge when presenting in nonendemic areas. We present the case of a woman from Michigan who acquired furuncular myiasis without travel history to a tropical or subtropical locale.
A 72-year-old woman presented to our clinic with a chief concern of a burning, pruritic, migratory skin lesion on the left arm of approximately 1 week’s duration. She had a medical history of squamous cell carcinoma, keratoacanthoma, and multiple tick bites. She reported that the lesion started on the distal aspect of the left arm as an eraser-sized, perfectly round, raised bruise with a dark pepperlike bump in the center. The lesion then spread proximally over the course of 1 week, creating 3 more identical lesions. As one lesion resolved, a new lesion appeared approximately 2 to 4 cm proximal to the preceding lesion. The patient had traveled to England, Scotland, and Ireland 2 months prior but otherwise denied leaving the state of Michigan. She reported frequent exposure to gardens, meadows, and wetlands in search of milkweed and monarch butterfly larvae that she raises in northeast Michigan. She denied any recent illness or associated systemic symptoms. Initial evaluation by a primary care physician resulted in a diagnosis of a furuncle or tick bite; she completed a 10-day course of amoxicillin and a methylprednisolone dose pack without improvement.
Physical examination revealed a 1-cm, firm, violaceous nodule with a small distinct central punctum and surrounding erythema on the proximal aspect of the left arm. Dermoscopy revealed a pulsating motion and expulsion of serosanguineous fluid from the central punctum (Figure 1). Further inspection of the patient’s left arm exposed several noninflammatory puncta distal to the primary lesion spaced at 2- to 4-cm intervals.

Gross examination of a 6-mm punch biopsy from the primary inflammatory nodule uncovered a small, motile, gray-white larval organism in the inferior portion of the specimen (Figure 2). Histopathology revealed superficial and deep eosinophil-rich inflammation, fibrosis, and hemorrhage. There was a complex wedge-shaped organism with extensive internal muscle bounded by a thin cuticle bearing rows of chitinous hooklets located at one side within the deep dermis (Figure 3). The findings were consistent with a diagnosis of cutaneous myiasis. No further treatment was required, as the organism was completely excised with the biopsy.

The most common causative agents of furuncular myiasis obtained from travelers returning from Mexico and Central and South America are Dermatobia hominis and Cordylobia anthropophaga. Cases of furuncular myiasis acquired in the United States without recent foreign travel are rare. Most of these cases are caused by larvae of the Cuterebra species (also known as the rabbit botfly or rodent botfly).2 In a 2003 literature review by Safdar et al3 on 56 cases of furuncular myiasis in the United States, the median age of patients was 14 years, 87% of cases occurred in August and September, and most involved exposure in rural or suburban settings; 53% of cases presented in the northeastern United States.

Furuncular myiasis occurs when the organism’s ova are deposited on the skin of a human host by the parent organism or a mosquito vector. The heat of the skin causes the eggs to hatch and the dipteran larvae must penetrate the skin within 20 days.1 Signs of infection typically are seen 6 to 10 days after infestation.3 The larvae then feed on human tissue and burrow deep in the dermis, forming an erythematous furunculoid nodule containing one or multiple maggots. After 5 to 10 weeks, the adult larvae drop to the ground, where they mature into adult organisms in the soil.1
The most reported symptoms of furuncular myiasis include pruritus, pain, and movement sensation, typically occurring suddenly at night.4 The most common presentation is a furunclelike lesion that exudes serosanguineous or purulent fluid,1 but there have been reports of vesicular, bullous, pustular, erosive, ecchymotic, and ulcerative lesions.5Dermatobia hominis usually presents on an exposed site, such as the scalp, face, and extremities. It may present with paroxysmal episodes of lancinating pain. Over time, the lesion usually heals without a scar, though hyperpigmentation and scarring can occur. The most reported complication is secondary bacterial infection.4 Local lymphadenopathy or systemic symptoms should raise concern for infection. Staphylococcus aureus and group B Streptococcus have been cultured from lesions.6,7
The differential diagnosis for myiasis should include furuncle, insect bite, insect prurigo, pyoderma, inflamed cyst, and tungiasis. Myiasis also can present similarly to severe soft tissue infections or cellulitis. If located on the breasts, it can be mistaken for periductal mastitis, a benign mass with microcalcification, or inflammatory carcinoma. Lastly, due to pain, erythema, pruritus, small vesicles, and crusting, it may be confused for herpes simplex virus.1
Furuncular myiasis typically is diagnosed based on clinical presentation, especially in endemic regions. In nonendemic areas, the patient’s history may reveal recent travel or predisposition to myiasis. In cases where there is uncertainty, dermoscopy may be used to identify the maggot in the lesion, or ultrasonography can be used to confirm myiasis through the detection of larval movement.8 Dermoscopy will reveal a furuncular lesion with a central opening surrounded by dilated blood vessels and a yellowish structure with black barblike spines.9 Within the dermis is a fibrous cystic sinus tract containing the dipteran larva. Laboratory studies typically are unremarkable. In chronic cases, a complete blood cell count and other laboratory tests may show systemic inflammation, peripheral eosinophilia, and elevated IgE.10 Biopsies of furuncular myiasis are not necessary for diagnosis. Histopathology reveals an ulcerated epidermis with or without hyperkeratosis and an inflammatory infiltrate composed of lymphocytes and neutrophils with eosinophils, fibroblasts, histiocytes, basophils, mast cells, plasma cells, and Langerhans cells within the dermis and subcutis.11
There are various approaches to treating furuncular myiasis, with the goal of complete removal of the larva and prevention of secondary infection. One treatment option is to apply a toxic substance to the larva, effectively killing it. Another approach is to force the larva to emerge via localized hypoxia, which can be done by occluding the punctum of the lesion for at least 24 hours. A complication of this method is suffocation of the larva without migration, leading to incomplete extraction and secondary infection.1 A third method is to surgically remove the larva, which allows for debridement of necrotic tissue surrounding the lesion if present.12 Ultrasonography also can be used therapeutically to aid in the removal of the larvae. The last method is to inject lidocaine into the base of the lesion, forcing the larva out of the punctum via fluid pressure.13 Oral treatments such as ivermectin are not recommended because they can result in the death of larvae within the lesion, leading to an inflammatory response.8
Furuncular myiasis is a form of cutaneous larvae infestation not commonly seen in individuals who do not live or travel in endemic, tropical, and subtropical regions. Diagnosis is based on clinical presentation, with imaging and laboratory studies available to supplement in unclear or atypical manifestations. Treatment involves complete removal of the larva, typically through forced evacuation via hypoxia or through surgical removal. Most cases resolve without notable scarring or other sequelae; however, in those who do have complications, the most common is secondary bacterial infection. Our patient’s absence of notable travel history and frequent environmental exposure in Michigan led us to believe the organism was from a domestic source. Our case underlines the importance of a thorough history and clinical examination of furuncular lesions including the use of dermoscopy to yield an appropriate diagnosis and treatment plan.
- Francesconi F, Lupi O. Myiasis. Clin Microbiol Rev. 2012;25:79-105. doi:10.1128/CMR.00010-11
- Schiff TA. Furuncular cutaneous myiasis caused by Cuterebra larva. J Am Acad Dermatol 1993;28:261-263.
- Safdar N, Young DK, Andes D. Autochthonous furuncular myiasis in the United States: case report and literature review. Clin Infect Dis. 2003;26:73-80.
- Mahal JJ, Sperling JD. Furuncular myiasis from Dermatobia hominus: a case of human botfly infestation. J Emerg Med. 2012;43:618-621.
- Francesconi F, Lupi O. Myiasis. In: Tyring SK, Lupi O, Hengge UR, eds. Tropical Dermatology. Elsevier; 2006:232-239.
- Gordon PM, Hepburn NC, Williams AE, et al. Cutaneous myiasis due to Dermatobia hominis: a report of six cases. Br J Dermatol. 1995;132:811-814.
- Hubler WR Jr, Rudolph AH, Dougherty EF. Dermal myiasis. Arch Dermatol. 1974;110:109-110.
- Quintanilla-Cedillo MR, León-Ureña H, Contreras-Ruiz J, et al. The value of Doppler ultrasound in diagnosis in 25 cases of furunculoid myiasis. Int J Dermatol. 2005;44:34-37.
- Bakos RM, Bakos L. Dermoscopic diagnosis of furuncular myiasis. Arch Dermatol. 2007;143:123-124.
- Varani S, Tassinari D, Elleri D, et al. A case of furuncular myiasis associated with systemic inflammation. Parasitol Int. 2007;56:330-333.
- Grogan TM, Payne CM, Spier C, et al. Cutaneous myiasis. immunohistologic and ultrastructural morphometric features of a human botfly lesion. Am J Dermatopathol. 1987;9:232-239.
- Krajewski A, Allen B, Hoss D, et al. Cutaneous myiasis. J Plast Reconstr Aesthet Surg. 2009;62:383-386.
- Lebwohl MG, Heymann WR, Berth-Jones J, et al. Myiasis: Treatment of Skin Diseases. Comprehensive Therapeutic Strategies. 2nd ed. Elsevier-Mosby; 2006.
- Francesconi F, Lupi O. Myiasis. Clin Microbiol Rev. 2012;25:79-105. doi:10.1128/CMR.00010-11
- Schiff TA. Furuncular cutaneous myiasis caused by Cuterebra larva. J Am Acad Dermatol 1993;28:261-263.
- Safdar N, Young DK, Andes D. Autochthonous furuncular myiasis in the United States: case report and literature review. Clin Infect Dis. 2003;26:73-80.
- Mahal JJ, Sperling JD. Furuncular myiasis from Dermatobia hominus: a case of human botfly infestation. J Emerg Med. 2012;43:618-621.
- Francesconi F, Lupi O. Myiasis. In: Tyring SK, Lupi O, Hengge UR, eds. Tropical Dermatology. Elsevier; 2006:232-239.
- Gordon PM, Hepburn NC, Williams AE, et al. Cutaneous myiasis due to Dermatobia hominis: a report of six cases. Br J Dermatol. 1995;132:811-814.
- Hubler WR Jr, Rudolph AH, Dougherty EF. Dermal myiasis. Arch Dermatol. 1974;110:109-110.
- Quintanilla-Cedillo MR, León-Ureña H, Contreras-Ruiz J, et al. The value of Doppler ultrasound in diagnosis in 25 cases of furunculoid myiasis. Int J Dermatol. 2005;44:34-37.
- Bakos RM, Bakos L. Dermoscopic diagnosis of furuncular myiasis. Arch Dermatol. 2007;143:123-124.
- Varani S, Tassinari D, Elleri D, et al. A case of furuncular myiasis associated with systemic inflammation. Parasitol Int. 2007;56:330-333.
- Grogan TM, Payne CM, Spier C, et al. Cutaneous myiasis. immunohistologic and ultrastructural morphometric features of a human botfly lesion. Am J Dermatopathol. 1987;9:232-239.
- Krajewski A, Allen B, Hoss D, et al. Cutaneous myiasis. J Plast Reconstr Aesthet Surg. 2009;62:383-386.
- Lebwohl MG, Heymann WR, Berth-Jones J, et al. Myiasis: Treatment of Skin Diseases. Comprehensive Therapeutic Strategies. 2nd ed. Elsevier-Mosby; 2006.
Practice Points
- Cutaneous myiasis is a skin infestation with dipterous larvae that feed on the host’s tissue and cause a wide range of manifestations depending on the location of infestation. It consists of 3 types: furuncular, wound, and migratory forms.
- It is uncommon in the United States and not typically seen in patients who have no history of recent travel to tropical or subtropical areas.
- The most common cause of African furuncular myiasis acquired in the United States is larvae of the Cuterebra species (also known as the rabbit botfly or rodent botfly).
Ultra-Late Cutaneous Melanoma Recurrence Following 49 Years of Quiescence
To the Editor:
Ultra-late melanoma recurrence represents a minority of cases in which the quiescent period lasts longer than 15 years, and epidemiologic studies have reported recurrence rates of 6% to 10% during the ultra-late period.1 Even more uncommon are cases that span many decades (eg, >30 years), but all are useful in understanding the cellular behavior leading to the reactivation of fully excised melanomas. Few cases have been reported in which recurrence occurs more than 35 years after the original diagnosis of melanoma. Unfortunately, mechanisms underlying this long stable quiescence and subsequent reactivation are poorly understood, which is why it is important to identify and document cases. We present a case of local recurrence of cutaneous melanoma on the patient’s lower back after a 49-year disease-free period.
A 78-year-old White woman presented to a private dermatology office for a full-body skin examination. She had a medical history of a cutaneous melanoma that had been removed on the lower back 49 years prior; Parkinson disease of 10 years’ duration; and an enlarged thyroid nodule with decreased thyrotropin and hyperthyroidism, atrial fibrillation, mitral valve prolapse, osteoarthritis in the knees, and actinic keratoses, all of which were chronic conditions lasting years to decades. She was taking several medications for these medical conditions. Her surgical history included a hysterectomy, hip replacement, hernia repair, cardioversion, and tonsillectomy in childhood. Her family medical history included breast cancer in her paternal grandmother and aunt; hypertension in her father; and sarcoma in her mother at 78 years of age, which initially was identified in the sacrum and metastasized to the lungs causing death. No family history of melanoma or other skin cancers was reported. Prior to the original diagnosis of melanoma at 29 years of age, she had no history of skin cancer or any other medical condition other than acne. The patient did report spending a great deal of time in the sun during high school.
The patient reported developing the original cutaneous melanoma during her second pregnancy at 29 years of age and recalled that it was excised with wide margins. There had been a mole on her back that was present for years but changed in size during pregnancy, prompting the original visit to the primary care physician for evaluation. Remarkably, the original pathology report was obtained from the patient and revealed a specimen consisting of a 3.7×1.7-cm skin
Physical examination at the current presentation 49 years later revealed an even-bordered 2-mm black macule that was located approximately 1 cm from the original melanoma excision scar line (Figure). A biopsy was performed and sent to a dermatopathologist. Microscopic evaluation revealed nests, islands, and sheets of atypical epithelioid melanocytes extending through the dermis between collagen bundles. The melanocytes varied in size and shape with moderate nuclear pleomorphism present. Scattered mitotic figures and necrotic melanocytes were present, which most likely represented cutaneous satellite metastases of melanoma. Subsequent chest radiography, full-body positron emission tomography, and standard laboratory blood tests were unremarkable except for an enlarged right thyroid gland and moderate cardiomegaly. The patient was sent to a surgical oncologist for excision with wide surgical margins, and she elected not to have a sentinel lymph node biopsy. At follow-up 3, 6, 12, and 24 months later, there were no signs of recurrence based on direct clinical examination. The patient subsequently was lost to follow-up.
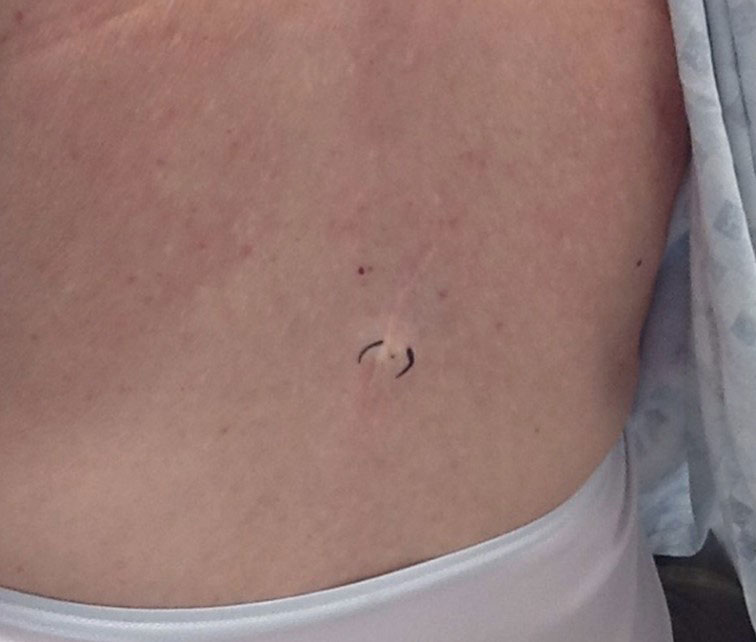
Recurrence rates of melanoma vary by stage and age at diagnosis, but prior studies have reported a recurrence rate of approximately 6% after 10 or more years following the initial diagnosis.2 Ultra-late recurrences of approximately 4 decades or more are extremely rare. A PubMed search of articles indexed for MEDLINE using the terms melanoma and ultra-late recurrence revealed 4 reported cases with a quiescent period of 38 or more years.3-6 All cases were metastatic melanomas in women; spanned 38, 40, 41, and 45 years from the initial melanoma diagnosis to recurrence; and all of the recurrences except one were regional or distal metastatic lesions (eg, lymph node, brain). In one case, both the original and recurrent lesions occurred on the left elbow.6 The original lesions occurred on the legs, elbow, and back of the neck, and there were no notable concomitant medical conditions. The patients were aged 72, 73, 73, and 84 years at recurrence.3-6 However, generalizations from these cases are limited given the potential for selection bias (eg, men may be less likely to visit a clinic for follow-up and nevi examination) and the likelihood that many cases of ultra-late melanoma recurrence are unrecognized or unreported.
More recently, genomic analyses on melanoma lesions occurring 30 years apart confirmed that the second lesion was indeed a recurrence, although with numerous additional mutations.7 The specific mechanisms underlying the dormancy and subsequent reemergence of metastatic lesions are unclear, but
It also is worth highlighting the concomitant diagnosis of Parkinson disease in our patient. In recent years, Parkinson disease has been linked to melanoma in both epidemiologic and genetic studies. For example, one large-scale study found a 50% increased risk for developing Parkinson disease in patients with melanoma (and vice versa), and this finding has been replicated in other studies.10 Moreover, patients with Parkinson disease have a 2-fold increase in their risk for developing melanoma, demonstrating that it is a bidirectional pathway. Not surprisingly, associations between melanin and neuromelanin pathways have been identified as a potential link between these diseases, and scientists are in the process of understanding the genetic components of both.10 It is unknown if specific genetic mutations contributed to both diseases in our case, but follow-up genetic testing on the recurrent melanoma specimen currently is being pursued.
The 49-year quiescent period in our case of recurrent cutaneous malignant melanoma potentially represents the longest ultra-late recurrence of melanoma in the literature to date based on a review of indexed publications. Moreover, it is relatively unique compared to other similar cases in that the recurrence was within a centimeter of the original excisional scar. Most metastases occur in locoregional lymph nodes or the lungs3; therefore, it is unusual to find one so close to the original lesion, especially one that occurred decades later. Factors associated with ultra-late recurrences are unknown, primarily because of the rarity of these cases as well as the biases and other factors that limit existing studies. However, genetic sequencing may provide information regarding these factors and related processes. Genetic sequencing specifically points to a small cell group remaining after excision of the primary tumor, which mutates while proliferating. Low antigenicity and tolerance to immunity during the quiescent period may explain the long duration of dormancy.6 More recently, there have been efforts to identify immunohistochemical signatures that may predict late recurrences, though the data are preliminary in nature.11
Given the latency period and location of the recurrence, our case demonstrates that even fully excised melanomas may recur locally many decades later, hence patients should be aware of the importance of a lifetime of vigilance after being diagnosed with melanoma.
- Tsao H, Cosimi AB, Sober AJ. Ultra-late recurrence (15 years or longer) of cutaneous melanoma. Cancer. 1997;79:2361-2370.
- Faries MB, Steen S, Ye X, et al. Late recurrence in melanoma: clinical implications of lost dormancy. J Am Coll Surg. 2013;217:27-34.
- Mansour D, Kejariwal D. It is never too late: ultra-late recurrence of melanoma with distant metastases [published online March 8, 2012]. BMJ Case Rep. 2012:bcr0120125474. doi:10.1136/bcr.01.2012.5474
- Saleh D, Peach AHS. Ultra-late recurrence of malignant melanoma after 40 years of quiescent disease. J Surg Oncol. 2011;103:290-291.
- Goodenough J, Cozon CL, Liew SH. An incidental finding of a nodal recurrence of cutaneous malignant melanoma after a 45-year disease-free period [published online June 4, 2014]. BMJ Case Rep. 2014:bcr2014204289. doi:10.1136/bcr-2014-204289
- Nakamura M, Obayashi M, Yoshimitsu M, et al. Comparative whole-exome sequencing of an ultra-late recurrent malignant melanoma. Br J Dermatol. 2021;184:762-763.
- Miller JJ, Lofgren KA, Hughes SR, et al. Genomic analysis of melanoma evolution following a 30-year disease-free interval. J Cutan Pathol. 2017;44:805-808.
- North JP, Kageshita T, Pinkel D, et al. Distribution and significance of occult intraepidermal tumor cells surrounding primary melanoma. J Invest Dermatol. 2008;128:2024-2030.
- Massi G, LeBoit PE. Recurrent and persistent melanoma. In: Massi G, LeBoit PE, eds. Histological Diagnosis of Nevi and Melanoma. 2nd ed. Springer-Verlag; 2014:689-698.
- Bose A, Petsko GA, Eliezer D. Parkinson’s disease and melanoma: co-occurrence and mechanisms. J Parkinsons Dis. 2018;8:385-398.
- Reschke R, Dumann K, Ziemer M. Risk stratification and clinical characteristics of patients with late recurrence of melanoma (>10 years).J Clin Med. 2022;11:2026.
To the Editor:
Ultra-late melanoma recurrence represents a minority of cases in which the quiescent period lasts longer than 15 years, and epidemiologic studies have reported recurrence rates of 6% to 10% during the ultra-late period.1 Even more uncommon are cases that span many decades (eg, >30 years), but all are useful in understanding the cellular behavior leading to the reactivation of fully excised melanomas. Few cases have been reported in which recurrence occurs more than 35 years after the original diagnosis of melanoma. Unfortunately, mechanisms underlying this long stable quiescence and subsequent reactivation are poorly understood, which is why it is important to identify and document cases. We present a case of local recurrence of cutaneous melanoma on the patient’s lower back after a 49-year disease-free period.
A 78-year-old White woman presented to a private dermatology office for a full-body skin examination. She had a medical history of a cutaneous melanoma that had been removed on the lower back 49 years prior; Parkinson disease of 10 years’ duration; and an enlarged thyroid nodule with decreased thyrotropin and hyperthyroidism, atrial fibrillation, mitral valve prolapse, osteoarthritis in the knees, and actinic keratoses, all of which were chronic conditions lasting years to decades. She was taking several medications for these medical conditions. Her surgical history included a hysterectomy, hip replacement, hernia repair, cardioversion, and tonsillectomy in childhood. Her family medical history included breast cancer in her paternal grandmother and aunt; hypertension in her father; and sarcoma in her mother at 78 years of age, which initially was identified in the sacrum and metastasized to the lungs causing death. No family history of melanoma or other skin cancers was reported. Prior to the original diagnosis of melanoma at 29 years of age, she had no history of skin cancer or any other medical condition other than acne. The patient did report spending a great deal of time in the sun during high school.
The patient reported developing the original cutaneous melanoma during her second pregnancy at 29 years of age and recalled that it was excised with wide margins. There had been a mole on her back that was present for years but changed in size during pregnancy, prompting the original visit to the primary care physician for evaluation. Remarkably, the original pathology report was obtained from the patient and revealed a specimen consisting of a 3.7×1.7-cm skin
Physical examination at the current presentation 49 years later revealed an even-bordered 2-mm black macule that was located approximately 1 cm from the original melanoma excision scar line (Figure). A biopsy was performed and sent to a dermatopathologist. Microscopic evaluation revealed nests, islands, and sheets of atypical epithelioid melanocytes extending through the dermis between collagen bundles. The melanocytes varied in size and shape with moderate nuclear pleomorphism present. Scattered mitotic figures and necrotic melanocytes were present, which most likely represented cutaneous satellite metastases of melanoma. Subsequent chest radiography, full-body positron emission tomography, and standard laboratory blood tests were unremarkable except for an enlarged right thyroid gland and moderate cardiomegaly. The patient was sent to a surgical oncologist for excision with wide surgical margins, and she elected not to have a sentinel lymph node biopsy. At follow-up 3, 6, 12, and 24 months later, there were no signs of recurrence based on direct clinical examination. The patient subsequently was lost to follow-up.

Recurrence rates of melanoma vary by stage and age at diagnosis, but prior studies have reported a recurrence rate of approximately 6% after 10 or more years following the initial diagnosis.2 Ultra-late recurrences of approximately 4 decades or more are extremely rare. A PubMed search of articles indexed for MEDLINE using the terms melanoma and ultra-late recurrence revealed 4 reported cases with a quiescent period of 38 or more years.3-6 All cases were metastatic melanomas in women; spanned 38, 40, 41, and 45 years from the initial melanoma diagnosis to recurrence; and all of the recurrences except one were regional or distal metastatic lesions (eg, lymph node, brain). In one case, both the original and recurrent lesions occurred on the left elbow.6 The original lesions occurred on the legs, elbow, and back of the neck, and there were no notable concomitant medical conditions. The patients were aged 72, 73, 73, and 84 years at recurrence.3-6 However, generalizations from these cases are limited given the potential for selection bias (eg, men may be less likely to visit a clinic for follow-up and nevi examination) and the likelihood that many cases of ultra-late melanoma recurrence are unrecognized or unreported.
More recently, genomic analyses on melanoma lesions occurring 30 years apart confirmed that the second lesion was indeed a recurrence, although with numerous additional mutations.7 The specific mechanisms underlying the dormancy and subsequent reemergence of metastatic lesions are unclear, but
It also is worth highlighting the concomitant diagnosis of Parkinson disease in our patient. In recent years, Parkinson disease has been linked to melanoma in both epidemiologic and genetic studies. For example, one large-scale study found a 50% increased risk for developing Parkinson disease in patients with melanoma (and vice versa), and this finding has been replicated in other studies.10 Moreover, patients with Parkinson disease have a 2-fold increase in their risk for developing melanoma, demonstrating that it is a bidirectional pathway. Not surprisingly, associations between melanin and neuromelanin pathways have been identified as a potential link between these diseases, and scientists are in the process of understanding the genetic components of both.10 It is unknown if specific genetic mutations contributed to both diseases in our case, but follow-up genetic testing on the recurrent melanoma specimen currently is being pursued.
The 49-year quiescent period in our case of recurrent cutaneous malignant melanoma potentially represents the longest ultra-late recurrence of melanoma in the literature to date based on a review of indexed publications. Moreover, it is relatively unique compared to other similar cases in that the recurrence was within a centimeter of the original excisional scar. Most metastases occur in locoregional lymph nodes or the lungs3; therefore, it is unusual to find one so close to the original lesion, especially one that occurred decades later. Factors associated with ultra-late recurrences are unknown, primarily because of the rarity of these cases as well as the biases and other factors that limit existing studies. However, genetic sequencing may provide information regarding these factors and related processes. Genetic sequencing specifically points to a small cell group remaining after excision of the primary tumor, which mutates while proliferating. Low antigenicity and tolerance to immunity during the quiescent period may explain the long duration of dormancy.6 More recently, there have been efforts to identify immunohistochemical signatures that may predict late recurrences, though the data are preliminary in nature.11
Given the latency period and location of the recurrence, our case demonstrates that even fully excised melanomas may recur locally many decades later, hence patients should be aware of the importance of a lifetime of vigilance after being diagnosed with melanoma.
To the Editor:
Ultra-late melanoma recurrence represents a minority of cases in which the quiescent period lasts longer than 15 years, and epidemiologic studies have reported recurrence rates of 6% to 10% during the ultra-late period.1 Even more uncommon are cases that span many decades (eg, >30 years), but all are useful in understanding the cellular behavior leading to the reactivation of fully excised melanomas. Few cases have been reported in which recurrence occurs more than 35 years after the original diagnosis of melanoma. Unfortunately, mechanisms underlying this long stable quiescence and subsequent reactivation are poorly understood, which is why it is important to identify and document cases. We present a case of local recurrence of cutaneous melanoma on the patient’s lower back after a 49-year disease-free period.
A 78-year-old White woman presented to a private dermatology office for a full-body skin examination. She had a medical history of a cutaneous melanoma that had been removed on the lower back 49 years prior; Parkinson disease of 10 years’ duration; and an enlarged thyroid nodule with decreased thyrotropin and hyperthyroidism, atrial fibrillation, mitral valve prolapse, osteoarthritis in the knees, and actinic keratoses, all of which were chronic conditions lasting years to decades. She was taking several medications for these medical conditions. Her surgical history included a hysterectomy, hip replacement, hernia repair, cardioversion, and tonsillectomy in childhood. Her family medical history included breast cancer in her paternal grandmother and aunt; hypertension in her father; and sarcoma in her mother at 78 years of age, which initially was identified in the sacrum and metastasized to the lungs causing death. No family history of melanoma or other skin cancers was reported. Prior to the original diagnosis of melanoma at 29 years of age, she had no history of skin cancer or any other medical condition other than acne. The patient did report spending a great deal of time in the sun during high school.
The patient reported developing the original cutaneous melanoma during her second pregnancy at 29 years of age and recalled that it was excised with wide margins. There had been a mole on her back that was present for years but changed in size during pregnancy, prompting the original visit to the primary care physician for evaluation. Remarkably, the original pathology report was obtained from the patient and revealed a specimen consisting of a 3.7×1.7-cm skin
Physical examination at the current presentation 49 years later revealed an even-bordered 2-mm black macule that was located approximately 1 cm from the original melanoma excision scar line (Figure). A biopsy was performed and sent to a dermatopathologist. Microscopic evaluation revealed nests, islands, and sheets of atypical epithelioid melanocytes extending through the dermis between collagen bundles. The melanocytes varied in size and shape with moderate nuclear pleomorphism present. Scattered mitotic figures and necrotic melanocytes were present, which most likely represented cutaneous satellite metastases of melanoma. Subsequent chest radiography, full-body positron emission tomography, and standard laboratory blood tests were unremarkable except for an enlarged right thyroid gland and moderate cardiomegaly. The patient was sent to a surgical oncologist for excision with wide surgical margins, and she elected not to have a sentinel lymph node biopsy. At follow-up 3, 6, 12, and 24 months later, there were no signs of recurrence based on direct clinical examination. The patient subsequently was lost to follow-up.

Recurrence rates of melanoma vary by stage and age at diagnosis, but prior studies have reported a recurrence rate of approximately 6% after 10 or more years following the initial diagnosis.2 Ultra-late recurrences of approximately 4 decades or more are extremely rare. A PubMed search of articles indexed for MEDLINE using the terms melanoma and ultra-late recurrence revealed 4 reported cases with a quiescent period of 38 or more years.3-6 All cases were metastatic melanomas in women; spanned 38, 40, 41, and 45 years from the initial melanoma diagnosis to recurrence; and all of the recurrences except one were regional or distal metastatic lesions (eg, lymph node, brain). In one case, both the original and recurrent lesions occurred on the left elbow.6 The original lesions occurred on the legs, elbow, and back of the neck, and there were no notable concomitant medical conditions. The patients were aged 72, 73, 73, and 84 years at recurrence.3-6 However, generalizations from these cases are limited given the potential for selection bias (eg, men may be less likely to visit a clinic for follow-up and nevi examination) and the likelihood that many cases of ultra-late melanoma recurrence are unrecognized or unreported.
More recently, genomic analyses on melanoma lesions occurring 30 years apart confirmed that the second lesion was indeed a recurrence, although with numerous additional mutations.7 The specific mechanisms underlying the dormancy and subsequent reemergence of metastatic lesions are unclear, but
It also is worth highlighting the concomitant diagnosis of Parkinson disease in our patient. In recent years, Parkinson disease has been linked to melanoma in both epidemiologic and genetic studies. For example, one large-scale study found a 50% increased risk for developing Parkinson disease in patients with melanoma (and vice versa), and this finding has been replicated in other studies.10 Moreover, patients with Parkinson disease have a 2-fold increase in their risk for developing melanoma, demonstrating that it is a bidirectional pathway. Not surprisingly, associations between melanin and neuromelanin pathways have been identified as a potential link between these diseases, and scientists are in the process of understanding the genetic components of both.10 It is unknown if specific genetic mutations contributed to both diseases in our case, but follow-up genetic testing on the recurrent melanoma specimen currently is being pursued.
The 49-year quiescent period in our case of recurrent cutaneous malignant melanoma potentially represents the longest ultra-late recurrence of melanoma in the literature to date based on a review of indexed publications. Moreover, it is relatively unique compared to other similar cases in that the recurrence was within a centimeter of the original excisional scar. Most metastases occur in locoregional lymph nodes or the lungs3; therefore, it is unusual to find one so close to the original lesion, especially one that occurred decades later. Factors associated with ultra-late recurrences are unknown, primarily because of the rarity of these cases as well as the biases and other factors that limit existing studies. However, genetic sequencing may provide information regarding these factors and related processes. Genetic sequencing specifically points to a small cell group remaining after excision of the primary tumor, which mutates while proliferating. Low antigenicity and tolerance to immunity during the quiescent period may explain the long duration of dormancy.6 More recently, there have been efforts to identify immunohistochemical signatures that may predict late recurrences, though the data are preliminary in nature.11
Given the latency period and location of the recurrence, our case demonstrates that even fully excised melanomas may recur locally many decades later, hence patients should be aware of the importance of a lifetime of vigilance after being diagnosed with melanoma.
- Tsao H, Cosimi AB, Sober AJ. Ultra-late recurrence (15 years or longer) of cutaneous melanoma. Cancer. 1997;79:2361-2370.
- Faries MB, Steen S, Ye X, et al. Late recurrence in melanoma: clinical implications of lost dormancy. J Am Coll Surg. 2013;217:27-34.
- Mansour D, Kejariwal D. It is never too late: ultra-late recurrence of melanoma with distant metastases [published online March 8, 2012]. BMJ Case Rep. 2012:bcr0120125474. doi:10.1136/bcr.01.2012.5474
- Saleh D, Peach AHS. Ultra-late recurrence of malignant melanoma after 40 years of quiescent disease. J Surg Oncol. 2011;103:290-291.
- Goodenough J, Cozon CL, Liew SH. An incidental finding of a nodal recurrence of cutaneous malignant melanoma after a 45-year disease-free period [published online June 4, 2014]. BMJ Case Rep. 2014:bcr2014204289. doi:10.1136/bcr-2014-204289
- Nakamura M, Obayashi M, Yoshimitsu M, et al. Comparative whole-exome sequencing of an ultra-late recurrent malignant melanoma. Br J Dermatol. 2021;184:762-763.
- Miller JJ, Lofgren KA, Hughes SR, et al. Genomic analysis of melanoma evolution following a 30-year disease-free interval. J Cutan Pathol. 2017;44:805-808.
- North JP, Kageshita T, Pinkel D, et al. Distribution and significance of occult intraepidermal tumor cells surrounding primary melanoma. J Invest Dermatol. 2008;128:2024-2030.
- Massi G, LeBoit PE. Recurrent and persistent melanoma. In: Massi G, LeBoit PE, eds. Histological Diagnosis of Nevi and Melanoma. 2nd ed. Springer-Verlag; 2014:689-698.
- Bose A, Petsko GA, Eliezer D. Parkinson’s disease and melanoma: co-occurrence and mechanisms. J Parkinsons Dis. 2018;8:385-398.
- Reschke R, Dumann K, Ziemer M. Risk stratification and clinical characteristics of patients with late recurrence of melanoma (>10 years).J Clin Med. 2022;11:2026.
- Tsao H, Cosimi AB, Sober AJ. Ultra-late recurrence (15 years or longer) of cutaneous melanoma. Cancer. 1997;79:2361-2370.
- Faries MB, Steen S, Ye X, et al. Late recurrence in melanoma: clinical implications of lost dormancy. J Am Coll Surg. 2013;217:27-34.
- Mansour D, Kejariwal D. It is never too late: ultra-late recurrence of melanoma with distant metastases [published online March 8, 2012]. BMJ Case Rep. 2012:bcr0120125474. doi:10.1136/bcr.01.2012.5474
- Saleh D, Peach AHS. Ultra-late recurrence of malignant melanoma after 40 years of quiescent disease. J Surg Oncol. 2011;103:290-291.
- Goodenough J, Cozon CL, Liew SH. An incidental finding of a nodal recurrence of cutaneous malignant melanoma after a 45-year disease-free period [published online June 4, 2014]. BMJ Case Rep. 2014:bcr2014204289. doi:10.1136/bcr-2014-204289
- Nakamura M, Obayashi M, Yoshimitsu M, et al. Comparative whole-exome sequencing of an ultra-late recurrent malignant melanoma. Br J Dermatol. 2021;184:762-763.
- Miller JJ, Lofgren KA, Hughes SR, et al. Genomic analysis of melanoma evolution following a 30-year disease-free interval. J Cutan Pathol. 2017;44:805-808.
- North JP, Kageshita T, Pinkel D, et al. Distribution and significance of occult intraepidermal tumor cells surrounding primary melanoma. J Invest Dermatol. 2008;128:2024-2030.
- Massi G, LeBoit PE. Recurrent and persistent melanoma. In: Massi G, LeBoit PE, eds. Histological Diagnosis of Nevi and Melanoma. 2nd ed. Springer-Verlag; 2014:689-698.
- Bose A, Petsko GA, Eliezer D. Parkinson’s disease and melanoma: co-occurrence and mechanisms. J Parkinsons Dis. 2018;8:385-398.
- Reschke R, Dumann K, Ziemer M. Risk stratification and clinical characteristics of patients with late recurrence of melanoma (>10 years).J Clin Med. 2022;11:2026.
Practice Points
- In some cases of ultra-late malignant melanoma recurrence, the quiescent period can last more than 30 years.
- There does not appear to be specificity with location since ultra-late melanoma recurrences can occur locally, regionally, and distally, and original lesions appear to be randomly distributed in these cases.
- Mechanisms for ultra-late melanoma recurrence are poorly understood; histologically, unrecognizable aberrations in the skin beyond the histopathologic margins may represent an early phase of disease that lies dormant for many years before reemerging in response to external or immunologic changes.
- Patients with malignant melanoma are at a higher risk for developing Parkinson disease (and vice versa) given the link between melanin and neuromelanin pathways.
Transverse Leukonychia and Beau Lines Following COVID-19 Vaccination
To the Editor:
Nail abnormalities associated with SARS-CoV-2 infection that have been reported in the medical literature include nail psoriasis,1 Beau lines,2 onychomadesis,3 heterogeneous red-white discoloration of the nail bed,4 transverse orange nail lesions,3 and the red half‐moon nail sign.3,5 It has been hypothesized that these nail findings may be an indication of microvascular injury to the distal subungual arcade of the digit or may be indicative of a procoagulant state.5,6 Currently, there is limited knowledge of the effect of COVID-19 vaccines on nail changes. We report a patient who presented with transverse leukonychia (Mees lines) and Beau lines shortly after each dose of the Pfizer-BioNTech COVID-19 messenger RNA vaccine was administered (with a total of 2 doses administered on presentation).
A 64-year-old woman with a history of rheumatoid arthritis presented with peeling of the fingernails and proximal white discoloration of several fingernails of 2 months’ duration. The patient first noticed whitening of the nails 3 weeks after she recevied the first dose of the COVID-19 vaccine. Five days after receiving the second, she presented to the dermatology clinic and exhibited transverse leukonychia in most fingernails (Figure 1).
Six weeks following the second dose of the COVID-19 vaccine, the patient returned to the dermatology clinic with Beau lines on the second and third fingernails on the right hand (Figure 2A). Subtle erythema of the proximal nail folds and distal fingers was observed in both hands. The patient also exhibited mild onychorrhexis of the left thumbnail and mottled red-brown discoloration of the third finger on the left hand (Figure 2B). Splinter hemorrhages and melanonychia of several fingernails also were observed. Our patient denied any known history of infection with SARS-CoV-2, which was confirmed by a negative COVID-19 polymerase chain reaction test result. She also denied fevers, chills, nausea, and vomiting, she and reported feeling generally well in the context of these postvaccination nail changes.
She reported no trauma or worsening of rheumatoid arthritis before or after COVID-19 vaccination. She was seronegative for rheumatoid arthritis and was being treated with hydroxychloroquine for the last year and methotrexate for the last 2 years. After each dose of the vaccine, methotrexate was withheld for 1 week and then resumed.
Subsequent follow-up examinations revealed the migration and resolution of transverse leukonychia and Beau lines. There also was interval improvement of the splinter hemorrhages. At 17 weeks following the second vaccine dose, all transverse leukonychia and Beau lines had resolved (Figure 3). The patient’s melanonychia remained unchanged.
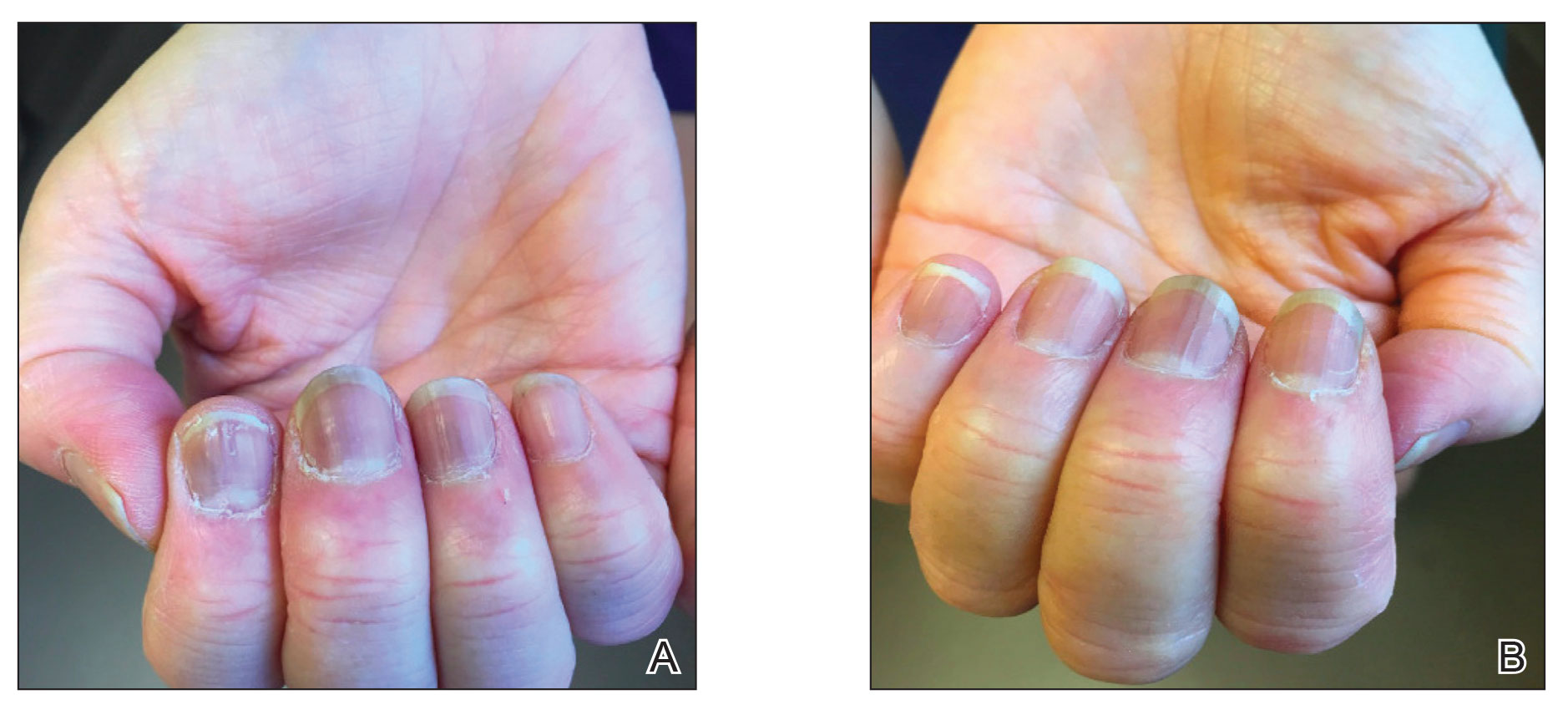
Laboratory evaluations drawn 1 month following the first dose of the COVID-19 vaccine, including comprehensive metabolic panel; erythrocyte sedimentation rate; C-reactive protein; and vitamin B12, ferritin, and iron levels were within reference range. The complete blood cell count only showed a mildly decreased white blood cell count (3.55×103/µL [reference range, 4.16–9.95×103/µL]) and mildly elevated mean corpuscular volume (101.9 fL [reference range, 79.3–98.6 fL), both near the patient’s baseline values prior to vaccination.
Documented cutaneous manifestations of SARS‐CoV‐2 infection have included perniolike lesions (known as COVID toes) and vesicular, urticarial, petechial, livedoid, or retiform purpura eruptions. Less frequently, nail findings in patients infected with COVID-19 have been reported, including Beau lines,2 onychomadesis,3 transverse leukonychia,3,7 and the red half‐moon nail sign.3,5 Single or multiple nails may be affected. Although the pathogenesis of nail manifestations related to COVID-19 remains unclear, complement-mediated microvascular injury and thrombosis as well as the procoagulant state, which have been associated with COVID-19, may offer possible explanations.5,6 The presence of microvascular abnormalities was observed in a nail fold video capillaroscopy study of the nails of 82 patients with COVID-19, revealing pericapillary edema, capillary ectasia, sludge flow, meandering capillaries and microvascular derangement, and low capillary density.8
Our patient exhibited transverse leukonychia of the fingernails, which is thought to result from abnormal keratinization of the nail plate due to systemic disorders that induce a temporary dysfunction of nail growth.9 Fernandez-Nieto et al7 reported transverse leukonychia in a patient with COVID-19 that was hypothesized to be due to a transitory nail matrix injury.
Beau lines and onychomadesis, which represent nail matrix arrest, commonly are seen with systemic drug treatments such as chemotherapy and in infectious diseases that precipitate systemic illness, such as hand, foot, and mouth disease. Although histologic examination was not performed in our patient due to cosmetic concerns, we believe that inflammation induced by the vaccine response also can trigger nail abnormalities such as transverse leukonychia and Beau lines. Both SARS-CoV-2 infections and the COVID-19 messenger RNA vaccines can induce systemic inflammation largely due a TH1-dominant response, and they also can trigger other inflammatory conditions. Reports of lichen planus and psoriasis triggered by vaccination—the hepatitis B vaccine,10 influenza vaccine,11 and even COVID-19 vaccines1,12—have been reported. Beau lines have been observed to spontaneously resolve in a self-limiting manner in asymptomatic patients with COVID-19.
Interestingly, our patient only showed 2 nails with Beau lines. We hypothesize that the immune response triggered by vaccination was more subdued than that caused by SARS-CoV-2 infection. Additionally, our patient was already being treated with immunosuppressants, which may have been associated with a reduced immune response despite being withheld right before vaccination. One may debate whether the nail abnormalities observed in our patient constituted an isolated finding from COVID-19 vaccination or were caused by reactivation of rheumatoid arthritis. We favor the former, as the rheumatoid arthritis remained stable before and after COVID-19 vaccination. Laboratory evaluations and physical examination revealed no evidence of flares, and our patient was otherwise healthy. Although the splinter hemorrhages also improved, it is difficult to comment as to whether they were caused by the vaccine or had existed prior to vaccination. However, we believe the melanonychia observed in the nails was unrelated to the vaccine and was likely a chronic manifestation due to long-term hydroxychloroquine and/or methotrexate use.
Given accelerated global vaccination efforts to control the COVID-19 pandemic, more cases of adverse nail manifestations associated with COVID-19 vaccines are expected. Dermatologists should be aware of and use the reported nail findings to educate patients and reassure them that ungual abnormalities are potential adverse effects of COVID-19 vaccines, but they should not discourage vaccination because they usually are temporary and self-resolving.
- Ricardo JW, Lipner SR. Case of de novo nail psoriasis triggered by the second dose of Pfizer-BioNTech BNT162b2 COVID-19 messenger RNA vaccine. JAAD Case Rep. 2021;17:18-20.
- Deng J, Ngo T, Zhu TH, et al. Telogen effluvium, Beau lines, and acral peeling associated with COVID-19 infection. JAAD Case Rep. 2021;13:138-140.
- Hadeler E, Morrison BW, Tosti A. A review of nail findings associated with COVID-19 infection. J Eur Acad Dermatol Venereol. 2021;35:E699-E709.
- Demir B, Yuksel EI, Cicek D, et al. Heterogeneous red-white discoloration of the nail bed and distal onycholysis in a patient with COVID-19. J Eur Acad Dermatol Venereol. 2021;35:E551-E553.
- Neri I, Guglielmo A, Virdi A, et al. The red half-moon nail sign: a novel manifestation of coronavirus infection. J Eur Acad Dermatol Venereol. 2020;34:E663-E665.
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13.
- Fernandez-Nieto D, Jimenez-Cauhe J, Ortega-Quijano D, et al. Transverse leukonychia (Mees’ lines) nail alterations in a COVID-19 patient. Dermatol Ther. 2020;33:E13863.
- Natalello G, De Luca G, Gigante L, et al. Nailfold capillaroscopy findings in patients with coronavirus disease 2019: broadening the spectrum of COVID-19 microvascular involvement [published online September 17, 2020]. Microvasc Res. doi:10.1016/j.mvr.2020.104071
- Piccolo V, Corneli P, Zalaudek I, et al. Mees’ lines because of chemotherapy for Hodgkin’s lymphoma. Int J Dermatol. 2020;59:E38.
- Miteva L. Bullous lichen planus with nail involvement induced by hepatitis B vaccine in a child. Int J Dermatol. 2005;44:142-144.
- Gunes AT, Fetil E, Akarsu S, et al. Possible triggering effect of influenza vaccination on psoriasis [published online August 25, 2015]. J Immunol Res. doi:10.1155/2015/258430
- Hiltun I, Sarriugarte J, Martínez-de-Espronceda I, et al. Lichen planus arising after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:e414-e415.
To the Editor:
Nail abnormalities associated with SARS-CoV-2 infection that have been reported in the medical literature include nail psoriasis,1 Beau lines,2 onychomadesis,3 heterogeneous red-white discoloration of the nail bed,4 transverse orange nail lesions,3 and the red half‐moon nail sign.3,5 It has been hypothesized that these nail findings may be an indication of microvascular injury to the distal subungual arcade of the digit or may be indicative of a procoagulant state.5,6 Currently, there is limited knowledge of the effect of COVID-19 vaccines on nail changes. We report a patient who presented with transverse leukonychia (Mees lines) and Beau lines shortly after each dose of the Pfizer-BioNTech COVID-19 messenger RNA vaccine was administered (with a total of 2 doses administered on presentation).
A 64-year-old woman with a history of rheumatoid arthritis presented with peeling of the fingernails and proximal white discoloration of several fingernails of 2 months’ duration. The patient first noticed whitening of the nails 3 weeks after she recevied the first dose of the COVID-19 vaccine. Five days after receiving the second, she presented to the dermatology clinic and exhibited transverse leukonychia in most fingernails (Figure 1).

Six weeks following the second dose of the COVID-19 vaccine, the patient returned to the dermatology clinic with Beau lines on the second and third fingernails on the right hand (Figure 2A). Subtle erythema of the proximal nail folds and distal fingers was observed in both hands. The patient also exhibited mild onychorrhexis of the left thumbnail and mottled red-brown discoloration of the third finger on the left hand (Figure 2B). Splinter hemorrhages and melanonychia of several fingernails also were observed. Our patient denied any known history of infection with SARS-CoV-2, which was confirmed by a negative COVID-19 polymerase chain reaction test result. She also denied fevers, chills, nausea, and vomiting, she and reported feeling generally well in the context of these postvaccination nail changes.

She reported no trauma or worsening of rheumatoid arthritis before or after COVID-19 vaccination. She was seronegative for rheumatoid arthritis and was being treated with hydroxychloroquine for the last year and methotrexate for the last 2 years. After each dose of the vaccine, methotrexate was withheld for 1 week and then resumed.
Subsequent follow-up examinations revealed the migration and resolution of transverse leukonychia and Beau lines. There also was interval improvement of the splinter hemorrhages. At 17 weeks following the second vaccine dose, all transverse leukonychia and Beau lines had resolved (Figure 3). The patient’s melanonychia remained unchanged.

Laboratory evaluations drawn 1 month following the first dose of the COVID-19 vaccine, including comprehensive metabolic panel; erythrocyte sedimentation rate; C-reactive protein; and vitamin B12, ferritin, and iron levels were within reference range. The complete blood cell count only showed a mildly decreased white blood cell count (3.55×103/µL [reference range, 4.16–9.95×103/µL]) and mildly elevated mean corpuscular volume (101.9 fL [reference range, 79.3–98.6 fL), both near the patient’s baseline values prior to vaccination.
Documented cutaneous manifestations of SARS‐CoV‐2 infection have included perniolike lesions (known as COVID toes) and vesicular, urticarial, petechial, livedoid, or retiform purpura eruptions. Less frequently, nail findings in patients infected with COVID-19 have been reported, including Beau lines,2 onychomadesis,3 transverse leukonychia,3,7 and the red half‐moon nail sign.3,5 Single or multiple nails may be affected. Although the pathogenesis of nail manifestations related to COVID-19 remains unclear, complement-mediated microvascular injury and thrombosis as well as the procoagulant state, which have been associated with COVID-19, may offer possible explanations.5,6 The presence of microvascular abnormalities was observed in a nail fold video capillaroscopy study of the nails of 82 patients with COVID-19, revealing pericapillary edema, capillary ectasia, sludge flow, meandering capillaries and microvascular derangement, and low capillary density.8
Our patient exhibited transverse leukonychia of the fingernails, which is thought to result from abnormal keratinization of the nail plate due to systemic disorders that induce a temporary dysfunction of nail growth.9 Fernandez-Nieto et al7 reported transverse leukonychia in a patient with COVID-19 that was hypothesized to be due to a transitory nail matrix injury.
Beau lines and onychomadesis, which represent nail matrix arrest, commonly are seen with systemic drug treatments such as chemotherapy and in infectious diseases that precipitate systemic illness, such as hand, foot, and mouth disease. Although histologic examination was not performed in our patient due to cosmetic concerns, we believe that inflammation induced by the vaccine response also can trigger nail abnormalities such as transverse leukonychia and Beau lines. Both SARS-CoV-2 infections and the COVID-19 messenger RNA vaccines can induce systemic inflammation largely due a TH1-dominant response, and they also can trigger other inflammatory conditions. Reports of lichen planus and psoriasis triggered by vaccination—the hepatitis B vaccine,10 influenza vaccine,11 and even COVID-19 vaccines1,12—have been reported. Beau lines have been observed to spontaneously resolve in a self-limiting manner in asymptomatic patients with COVID-19.
Interestingly, our patient only showed 2 nails with Beau lines. We hypothesize that the immune response triggered by vaccination was more subdued than that caused by SARS-CoV-2 infection. Additionally, our patient was already being treated with immunosuppressants, which may have been associated with a reduced immune response despite being withheld right before vaccination. One may debate whether the nail abnormalities observed in our patient constituted an isolated finding from COVID-19 vaccination or were caused by reactivation of rheumatoid arthritis. We favor the former, as the rheumatoid arthritis remained stable before and after COVID-19 vaccination. Laboratory evaluations and physical examination revealed no evidence of flares, and our patient was otherwise healthy. Although the splinter hemorrhages also improved, it is difficult to comment as to whether they were caused by the vaccine or had existed prior to vaccination. However, we believe the melanonychia observed in the nails was unrelated to the vaccine and was likely a chronic manifestation due to long-term hydroxychloroquine and/or methotrexate use.
Given accelerated global vaccination efforts to control the COVID-19 pandemic, more cases of adverse nail manifestations associated with COVID-19 vaccines are expected. Dermatologists should be aware of and use the reported nail findings to educate patients and reassure them that ungual abnormalities are potential adverse effects of COVID-19 vaccines, but they should not discourage vaccination because they usually are temporary and self-resolving.
To the Editor:
Nail abnormalities associated with SARS-CoV-2 infection that have been reported in the medical literature include nail psoriasis,1 Beau lines,2 onychomadesis,3 heterogeneous red-white discoloration of the nail bed,4 transverse orange nail lesions,3 and the red half‐moon nail sign.3,5 It has been hypothesized that these nail findings may be an indication of microvascular injury to the distal subungual arcade of the digit or may be indicative of a procoagulant state.5,6 Currently, there is limited knowledge of the effect of COVID-19 vaccines on nail changes. We report a patient who presented with transverse leukonychia (Mees lines) and Beau lines shortly after each dose of the Pfizer-BioNTech COVID-19 messenger RNA vaccine was administered (with a total of 2 doses administered on presentation).
A 64-year-old woman with a history of rheumatoid arthritis presented with peeling of the fingernails and proximal white discoloration of several fingernails of 2 months’ duration. The patient first noticed whitening of the nails 3 weeks after she recevied the first dose of the COVID-19 vaccine. Five days after receiving the second, she presented to the dermatology clinic and exhibited transverse leukonychia in most fingernails (Figure 1).

Six weeks following the second dose of the COVID-19 vaccine, the patient returned to the dermatology clinic with Beau lines on the second and third fingernails on the right hand (Figure 2A). Subtle erythema of the proximal nail folds and distal fingers was observed in both hands. The patient also exhibited mild onychorrhexis of the left thumbnail and mottled red-brown discoloration of the third finger on the left hand (Figure 2B). Splinter hemorrhages and melanonychia of several fingernails also were observed. Our patient denied any known history of infection with SARS-CoV-2, which was confirmed by a negative COVID-19 polymerase chain reaction test result. She also denied fevers, chills, nausea, and vomiting, she and reported feeling generally well in the context of these postvaccination nail changes.

She reported no trauma or worsening of rheumatoid arthritis before or after COVID-19 vaccination. She was seronegative for rheumatoid arthritis and was being treated with hydroxychloroquine for the last year and methotrexate for the last 2 years. After each dose of the vaccine, methotrexate was withheld for 1 week and then resumed.
Subsequent follow-up examinations revealed the migration and resolution of transverse leukonychia and Beau lines. There also was interval improvement of the splinter hemorrhages. At 17 weeks following the second vaccine dose, all transverse leukonychia and Beau lines had resolved (Figure 3). The patient’s melanonychia remained unchanged.

Laboratory evaluations drawn 1 month following the first dose of the COVID-19 vaccine, including comprehensive metabolic panel; erythrocyte sedimentation rate; C-reactive protein; and vitamin B12, ferritin, and iron levels were within reference range. The complete blood cell count only showed a mildly decreased white blood cell count (3.55×103/µL [reference range, 4.16–9.95×103/µL]) and mildly elevated mean corpuscular volume (101.9 fL [reference range, 79.3–98.6 fL), both near the patient’s baseline values prior to vaccination.
Documented cutaneous manifestations of SARS‐CoV‐2 infection have included perniolike lesions (known as COVID toes) and vesicular, urticarial, petechial, livedoid, or retiform purpura eruptions. Less frequently, nail findings in patients infected with COVID-19 have been reported, including Beau lines,2 onychomadesis,3 transverse leukonychia,3,7 and the red half‐moon nail sign.3,5 Single or multiple nails may be affected. Although the pathogenesis of nail manifestations related to COVID-19 remains unclear, complement-mediated microvascular injury and thrombosis as well as the procoagulant state, which have been associated with COVID-19, may offer possible explanations.5,6 The presence of microvascular abnormalities was observed in a nail fold video capillaroscopy study of the nails of 82 patients with COVID-19, revealing pericapillary edema, capillary ectasia, sludge flow, meandering capillaries and microvascular derangement, and low capillary density.8
Our patient exhibited transverse leukonychia of the fingernails, which is thought to result from abnormal keratinization of the nail plate due to systemic disorders that induce a temporary dysfunction of nail growth.9 Fernandez-Nieto et al7 reported transverse leukonychia in a patient with COVID-19 that was hypothesized to be due to a transitory nail matrix injury.
Beau lines and onychomadesis, which represent nail matrix arrest, commonly are seen with systemic drug treatments such as chemotherapy and in infectious diseases that precipitate systemic illness, such as hand, foot, and mouth disease. Although histologic examination was not performed in our patient due to cosmetic concerns, we believe that inflammation induced by the vaccine response also can trigger nail abnormalities such as transverse leukonychia and Beau lines. Both SARS-CoV-2 infections and the COVID-19 messenger RNA vaccines can induce systemic inflammation largely due a TH1-dominant response, and they also can trigger other inflammatory conditions. Reports of lichen planus and psoriasis triggered by vaccination—the hepatitis B vaccine,10 influenza vaccine,11 and even COVID-19 vaccines1,12—have been reported. Beau lines have been observed to spontaneously resolve in a self-limiting manner in asymptomatic patients with COVID-19.
Interestingly, our patient only showed 2 nails with Beau lines. We hypothesize that the immune response triggered by vaccination was more subdued than that caused by SARS-CoV-2 infection. Additionally, our patient was already being treated with immunosuppressants, which may have been associated with a reduced immune response despite being withheld right before vaccination. One may debate whether the nail abnormalities observed in our patient constituted an isolated finding from COVID-19 vaccination or were caused by reactivation of rheumatoid arthritis. We favor the former, as the rheumatoid arthritis remained stable before and after COVID-19 vaccination. Laboratory evaluations and physical examination revealed no evidence of flares, and our patient was otherwise healthy. Although the splinter hemorrhages also improved, it is difficult to comment as to whether they were caused by the vaccine or had existed prior to vaccination. However, we believe the melanonychia observed in the nails was unrelated to the vaccine and was likely a chronic manifestation due to long-term hydroxychloroquine and/or methotrexate use.
Given accelerated global vaccination efforts to control the COVID-19 pandemic, more cases of adverse nail manifestations associated with COVID-19 vaccines are expected. Dermatologists should be aware of and use the reported nail findings to educate patients and reassure them that ungual abnormalities are potential adverse effects of COVID-19 vaccines, but they should not discourage vaccination because they usually are temporary and self-resolving.
- Ricardo JW, Lipner SR. Case of de novo nail psoriasis triggered by the second dose of Pfizer-BioNTech BNT162b2 COVID-19 messenger RNA vaccine. JAAD Case Rep. 2021;17:18-20.
- Deng J, Ngo T, Zhu TH, et al. Telogen effluvium, Beau lines, and acral peeling associated with COVID-19 infection. JAAD Case Rep. 2021;13:138-140.
- Hadeler E, Morrison BW, Tosti A. A review of nail findings associated with COVID-19 infection. J Eur Acad Dermatol Venereol. 2021;35:E699-E709.
- Demir B, Yuksel EI, Cicek D, et al. Heterogeneous red-white discoloration of the nail bed and distal onycholysis in a patient with COVID-19. J Eur Acad Dermatol Venereol. 2021;35:E551-E553.
- Neri I, Guglielmo A, Virdi A, et al. The red half-moon nail sign: a novel manifestation of coronavirus infection. J Eur Acad Dermatol Venereol. 2020;34:E663-E665.
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13.
- Fernandez-Nieto D, Jimenez-Cauhe J, Ortega-Quijano D, et al. Transverse leukonychia (Mees’ lines) nail alterations in a COVID-19 patient. Dermatol Ther. 2020;33:E13863.
- Natalello G, De Luca G, Gigante L, et al. Nailfold capillaroscopy findings in patients with coronavirus disease 2019: broadening the spectrum of COVID-19 microvascular involvement [published online September 17, 2020]. Microvasc Res. doi:10.1016/j.mvr.2020.104071
- Piccolo V, Corneli P, Zalaudek I, et al. Mees’ lines because of chemotherapy for Hodgkin’s lymphoma. Int J Dermatol. 2020;59:E38.
- Miteva L. Bullous lichen planus with nail involvement induced by hepatitis B vaccine in a child. Int J Dermatol. 2005;44:142-144.
- Gunes AT, Fetil E, Akarsu S, et al. Possible triggering effect of influenza vaccination on psoriasis [published online August 25, 2015]. J Immunol Res. doi:10.1155/2015/258430
- Hiltun I, Sarriugarte J, Martínez-de-Espronceda I, et al. Lichen planus arising after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:e414-e415.
- Ricardo JW, Lipner SR. Case of de novo nail psoriasis triggered by the second dose of Pfizer-BioNTech BNT162b2 COVID-19 messenger RNA vaccine. JAAD Case Rep. 2021;17:18-20.
- Deng J, Ngo T, Zhu TH, et al. Telogen effluvium, Beau lines, and acral peeling associated with COVID-19 infection. JAAD Case Rep. 2021;13:138-140.
- Hadeler E, Morrison BW, Tosti A. A review of nail findings associated with COVID-19 infection. J Eur Acad Dermatol Venereol. 2021;35:E699-E709.
- Demir B, Yuksel EI, Cicek D, et al. Heterogeneous red-white discoloration of the nail bed and distal onycholysis in a patient with COVID-19. J Eur Acad Dermatol Venereol. 2021;35:E551-E553.
- Neri I, Guglielmo A, Virdi A, et al. The red half-moon nail sign: a novel manifestation of coronavirus infection. J Eur Acad Dermatol Venereol. 2020;34:E663-E665.
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13.
- Fernandez-Nieto D, Jimenez-Cauhe J, Ortega-Quijano D, et al. Transverse leukonychia (Mees’ lines) nail alterations in a COVID-19 patient. Dermatol Ther. 2020;33:E13863.
- Natalello G, De Luca G, Gigante L, et al. Nailfold capillaroscopy findings in patients with coronavirus disease 2019: broadening the spectrum of COVID-19 microvascular involvement [published online September 17, 2020]. Microvasc Res. doi:10.1016/j.mvr.2020.104071
- Piccolo V, Corneli P, Zalaudek I, et al. Mees’ lines because of chemotherapy for Hodgkin’s lymphoma. Int J Dermatol. 2020;59:E38.
- Miteva L. Bullous lichen planus with nail involvement induced by hepatitis B vaccine in a child. Int J Dermatol. 2005;44:142-144.
- Gunes AT, Fetil E, Akarsu S, et al. Possible triggering effect of influenza vaccination on psoriasis [published online August 25, 2015]. J Immunol Res. doi:10.1155/2015/258430
- Hiltun I, Sarriugarte J, Martínez-de-Espronceda I, et al. Lichen planus arising after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:e414-e415.
Practice Points
- Given accelerated global vaccination efforts to control the COVID-19 pandemic, cases of nail changes associated with COVID-19 vaccines are expected.
- Nail abnormalities are a potential general, temporary, and self-limiting adverse effect of COVID-19 vaccines that should not discourage patients from getting vaccinated.
Parameters of Scratch Pleasurability in the Management of Pruritic Conditions
To the Editor:
The itch-scratch cycle refers to the sequence created when a pruritic skin condition leads to scratching and skin barrier disruption, ultimately facilitating secondary skin changes and neural activation that prolongs pruritus. In patients with pruritic conditions, the itch-scratch cycle often can run unrestrained, with patients unaware of their scratching habits. Understanding what drives a patient to scratch, such as the pleasure gained from scratching, may be beneficial for dermatologists combating a patient’s scratching habits. The earliest documented attempts to understand the mechanism of an itch were made in Greece around the fifth century, but the pathophysiology of this sensation still is not fully understood. The Latin term pruritus refers to itching, irritation, or sexual excitement, while the Greek term knêsmos and related words also denote itch in an irritating or pleasurable sense.1 This paradoxical duality of irritation and pleasure is a phenomenon all too well understood by those affected with pruritic symptoms.
Although there are many measured characteristics of an itch, the pleasure granted from scratching an itch rarely is addressed. Understanding the factors influencing the pleasurability of scratching could help improve management and outcomes of patients’ pruritic conditions.
Pruritus is associated with a wide array of etiologies including dermatologic, infectious, metabolic, and autoimmune, but unanimously it evokes a strong desire to scratch. Scratching an itch often yields temporary relief from the irritation by dispensing a complex sensory concoction between pleasure and pain.2 The neurobiology behind this pleasure phenomenon is inconclusive. Some hypotheses point to how scratching-induced pleasure may be derived from the deactivation or inhibition of the unpleasant sensation of an itch in the central nervous system, the stimulation of the reward signals in the C-fiber system in the peripheral nervous system, the release of pruritis-inhibiting prostaglandin D2, or a combination of these pathways. Levels of sensation and pleasure induced from itch attenuation by scratching even vary based on anatomic location. One study demonstrated that, when compared to the forearms, the ankles and back perceived baseline induced itch most intensely, but no significant difference in perceived itch intensity was found between the ankles and back. Additionally, scratching an itchy back or ankle notably induced more pleasure when compared to the forearms, but there was no significant difference in scratching pleasurability between the ankle and back.3
Although there are adequate questionnaires and scales (eg, ItchyQoL,4 Skindex-16, Skindex-29) to quantify the severity of pruritus and its effects on a patient’s quality of life, these measurements do not assess the pleasure yielded from scratching, the impact of scratch pleasure on the patient experience, or the effect of scratch pleasure on the disease state.4 It appears that there are inadequate assessment tools to define factors associated with the pleasurability of scratching. A PubMed search of articles indexed for MEDLINE using the terms scratching pleasure scale and pruritus pleasure questionnaire yielded scarce results measuring patient perspectives on scratching-associated pleasure. A pertinent study performed by O’Neill et al5 compared the differences in itch characteristics between patients with psoriasis and those with atopic dermatitis using a web-based questionnaire featuring a numerical pleasure scale (ranging from −5 [highly unpleasurable] to +5 [highly pleasurable]) on an 11-point Likert scale. The questionnaire sought to measure the effects of scratching during a typical episode of itch within the past 2 weeks. Scratching was found pleasurable in both groups of patients.5 Another web-based questionnaire that characterized pleasurability in scratching a typical episode of itch in individuals with atopic dermatitis using a −5 to +5 Likert scale (−5 [highly unpleasurable] to +5 [highly pleasurable]) found that most participants perceived scratching as pleasurable and that there was a positive correlation between itch intensity and scratch pleasurability.6 Both of these studies quantified that scratching an itch is pleasurable, a correlation that may not come as a surprise. This direct correlation suggests that a more detailed analysis of this scratch pleasure could be beneficial in the management of pruritic conditions.
Treating the underlying cause of an itch is key to inhibiting the sensation; in some cases, anti-itch medications must be used. Current medications have limited effects on itch relief, but an expanding understanding of itch pathophysiology through clinical and laboratory research in the fields of dermatology, immunology, and neurology is paving the way for promising new therapeutic medications.7-11 In a review of the literature, Sanders and Akiyama12 elucidated the influence of stress and anxiety in scratching an itch and the way in which both pharmacologic and nonpharmacologic (ie, psychological and educational interventions) may be used to help break the itch-scratch cycle. Possible techniques include habit-reversal training, relaxation therapy, and cognitive behavioral therapy.13 Understanding patient perspectives on the pleasure yielded from scratching an itch and the disease factors that influence this pleasure seeking are paramount to reducing patient scratching. In understanding the pleasurability of scratching in pruritic conditions, the itch-scratch cycle and its accompanying deleterious effects (eg, stress, anxiety, pain, infection, secondary skin changes) can be broken.
The pleasure yielded from scratching an itch is a component of patient scratching habits that should be analyzed and quantified to reduce itch in pruritic conditions, mitigate damaging consequences of scratching, and improve the quality of life of patients with pruritic conditions. Furthermore, this understanding may help guide clinicians in management, such as counseling patients on the itch-scratch cycle and deciding which forthcoming medications could ameliorate a patient’s pruritic symptoms.
- Weisshaar E, Grüll V, König A, et al. The symptom of itch in medical history: highlights through the centuries. Int J Dermatol. 2009;48:1385-1394.
- Lavery MJ, Kinney MO, Mochizuki H, et al. Pruritus: an overview. what drives people to scratch an itch? Ulster Med J. 2016;85:164-173.
- Bin Saif GA, Papoiu ADP, Banari L, et al. The pleasurability of scratching an itch: a psychophysical and topographical assessment. Br J Dermatol. 2012;166:981-985.
- Desai NS, Poindexter GB, Monthrope YM, et al. A pilot quality-of-life instrument for pruritus. J Am Acad Dermatol. 2008;59:234-244.
- O’Neill JL, Chan YH, Rapp SR, et al. Differences in itch characteristics between psoriasis and atopic dermatitis patients: results of a web-based questionnaire. Acta Derm Venereol. 2011;91:537-540.
- Dawn A, Papoiu ADP, Chan YH, et al. Itch characteristics in atopic dermatitis: results of a web-based questionnaire. Br J Dermatol. 2009;160:642-644.
- Yosipovitch G, Rosen JD, Hashimoto T. Itch: from mechanism to (novel) therapeutic approaches. J Allergy Clin Immunol. 2018;142:1375-1390.
- Yosipovitch G, Misery L, Proksch E, et al. Skin barrier damage and itch: review of mechanisms, topical management and future directions. Acta Derm Venereol. 2019;99:1201-1209.
- Dong X, Dong X. Peripheral and central mechanisms of itch. Neuron. 2018;98:482-494.
- Lerner EA. Pathophysiology of itch. Dermatol Clin. 2018;36:175-177.
- Cevikbas F, Lerner EA. Physiology and pathophysiology of itch. Physiol Rev. 2020;100:945-982.
- Sanders KM, Akiyama T. The vicious cycle of itch and anxiety. Neurosci Biobehav Rev. 2018;87:17-26.
- Sanders KM, Nattkemper LA, Yosipovitch G. Advances in understanding itching and scratching: a new era of targeted treatments [published online August 22, 2016]. F1000Res. doi:10.12688/f1000research.8659.
To the Editor:
The itch-scratch cycle refers to the sequence created when a pruritic skin condition leads to scratching and skin barrier disruption, ultimately facilitating secondary skin changes and neural activation that prolongs pruritus. In patients with pruritic conditions, the itch-scratch cycle often can run unrestrained, with patients unaware of their scratching habits. Understanding what drives a patient to scratch, such as the pleasure gained from scratching, may be beneficial for dermatologists combating a patient’s scratching habits. The earliest documented attempts to understand the mechanism of an itch were made in Greece around the fifth century, but the pathophysiology of this sensation still is not fully understood. The Latin term pruritus refers to itching, irritation, or sexual excitement, while the Greek term knêsmos and related words also denote itch in an irritating or pleasurable sense.1 This paradoxical duality of irritation and pleasure is a phenomenon all too well understood by those affected with pruritic symptoms.
Although there are many measured characteristics of an itch, the pleasure granted from scratching an itch rarely is addressed. Understanding the factors influencing the pleasurability of scratching could help improve management and outcomes of patients’ pruritic conditions.
Pruritus is associated with a wide array of etiologies including dermatologic, infectious, metabolic, and autoimmune, but unanimously it evokes a strong desire to scratch. Scratching an itch often yields temporary relief from the irritation by dispensing a complex sensory concoction between pleasure and pain.2 The neurobiology behind this pleasure phenomenon is inconclusive. Some hypotheses point to how scratching-induced pleasure may be derived from the deactivation or inhibition of the unpleasant sensation of an itch in the central nervous system, the stimulation of the reward signals in the C-fiber system in the peripheral nervous system, the release of pruritis-inhibiting prostaglandin D2, or a combination of these pathways. Levels of sensation and pleasure induced from itch attenuation by scratching even vary based on anatomic location. One study demonstrated that, when compared to the forearms, the ankles and back perceived baseline induced itch most intensely, but no significant difference in perceived itch intensity was found between the ankles and back. Additionally, scratching an itchy back or ankle notably induced more pleasure when compared to the forearms, but there was no significant difference in scratching pleasurability between the ankle and back.3
Although there are adequate questionnaires and scales (eg, ItchyQoL,4 Skindex-16, Skindex-29) to quantify the severity of pruritus and its effects on a patient’s quality of life, these measurements do not assess the pleasure yielded from scratching, the impact of scratch pleasure on the patient experience, or the effect of scratch pleasure on the disease state.4 It appears that there are inadequate assessment tools to define factors associated with the pleasurability of scratching. A PubMed search of articles indexed for MEDLINE using the terms scratching pleasure scale and pruritus pleasure questionnaire yielded scarce results measuring patient perspectives on scratching-associated pleasure. A pertinent study performed by O’Neill et al5 compared the differences in itch characteristics between patients with psoriasis and those with atopic dermatitis using a web-based questionnaire featuring a numerical pleasure scale (ranging from −5 [highly unpleasurable] to +5 [highly pleasurable]) on an 11-point Likert scale. The questionnaire sought to measure the effects of scratching during a typical episode of itch within the past 2 weeks. Scratching was found pleasurable in both groups of patients.5 Another web-based questionnaire that characterized pleasurability in scratching a typical episode of itch in individuals with atopic dermatitis using a −5 to +5 Likert scale (−5 [highly unpleasurable] to +5 [highly pleasurable]) found that most participants perceived scratching as pleasurable and that there was a positive correlation between itch intensity and scratch pleasurability.6 Both of these studies quantified that scratching an itch is pleasurable, a correlation that may not come as a surprise. This direct correlation suggests that a more detailed analysis of this scratch pleasure could be beneficial in the management of pruritic conditions.
Treating the underlying cause of an itch is key to inhibiting the sensation; in some cases, anti-itch medications must be used. Current medications have limited effects on itch relief, but an expanding understanding of itch pathophysiology through clinical and laboratory research in the fields of dermatology, immunology, and neurology is paving the way for promising new therapeutic medications.7-11 In a review of the literature, Sanders and Akiyama12 elucidated the influence of stress and anxiety in scratching an itch and the way in which both pharmacologic and nonpharmacologic (ie, psychological and educational interventions) may be used to help break the itch-scratch cycle. Possible techniques include habit-reversal training, relaxation therapy, and cognitive behavioral therapy.13 Understanding patient perspectives on the pleasure yielded from scratching an itch and the disease factors that influence this pleasure seeking are paramount to reducing patient scratching. In understanding the pleasurability of scratching in pruritic conditions, the itch-scratch cycle and its accompanying deleterious effects (eg, stress, anxiety, pain, infection, secondary skin changes) can be broken.
The pleasure yielded from scratching an itch is a component of patient scratching habits that should be analyzed and quantified to reduce itch in pruritic conditions, mitigate damaging consequences of scratching, and improve the quality of life of patients with pruritic conditions. Furthermore, this understanding may help guide clinicians in management, such as counseling patients on the itch-scratch cycle and deciding which forthcoming medications could ameliorate a patient’s pruritic symptoms.
To the Editor:
The itch-scratch cycle refers to the sequence created when a pruritic skin condition leads to scratching and skin barrier disruption, ultimately facilitating secondary skin changes and neural activation that prolongs pruritus. In patients with pruritic conditions, the itch-scratch cycle often can run unrestrained, with patients unaware of their scratching habits. Understanding what drives a patient to scratch, such as the pleasure gained from scratching, may be beneficial for dermatologists combating a patient’s scratching habits. The earliest documented attempts to understand the mechanism of an itch were made in Greece around the fifth century, but the pathophysiology of this sensation still is not fully understood. The Latin term pruritus refers to itching, irritation, or sexual excitement, while the Greek term knêsmos and related words also denote itch in an irritating or pleasurable sense.1 This paradoxical duality of irritation and pleasure is a phenomenon all too well understood by those affected with pruritic symptoms.
Although there are many measured characteristics of an itch, the pleasure granted from scratching an itch rarely is addressed. Understanding the factors influencing the pleasurability of scratching could help improve management and outcomes of patients’ pruritic conditions.
Pruritus is associated with a wide array of etiologies including dermatologic, infectious, metabolic, and autoimmune, but unanimously it evokes a strong desire to scratch. Scratching an itch often yields temporary relief from the irritation by dispensing a complex sensory concoction between pleasure and pain.2 The neurobiology behind this pleasure phenomenon is inconclusive. Some hypotheses point to how scratching-induced pleasure may be derived from the deactivation or inhibition of the unpleasant sensation of an itch in the central nervous system, the stimulation of the reward signals in the C-fiber system in the peripheral nervous system, the release of pruritis-inhibiting prostaglandin D2, or a combination of these pathways. Levels of sensation and pleasure induced from itch attenuation by scratching even vary based on anatomic location. One study demonstrated that, when compared to the forearms, the ankles and back perceived baseline induced itch most intensely, but no significant difference in perceived itch intensity was found between the ankles and back. Additionally, scratching an itchy back or ankle notably induced more pleasure when compared to the forearms, but there was no significant difference in scratching pleasurability between the ankle and back.3
Although there are adequate questionnaires and scales (eg, ItchyQoL,4 Skindex-16, Skindex-29) to quantify the severity of pruritus and its effects on a patient’s quality of life, these measurements do not assess the pleasure yielded from scratching, the impact of scratch pleasure on the patient experience, or the effect of scratch pleasure on the disease state.4 It appears that there are inadequate assessment tools to define factors associated with the pleasurability of scratching. A PubMed search of articles indexed for MEDLINE using the terms scratching pleasure scale and pruritus pleasure questionnaire yielded scarce results measuring patient perspectives on scratching-associated pleasure. A pertinent study performed by O’Neill et al5 compared the differences in itch characteristics between patients with psoriasis and those with atopic dermatitis using a web-based questionnaire featuring a numerical pleasure scale (ranging from −5 [highly unpleasurable] to +5 [highly pleasurable]) on an 11-point Likert scale. The questionnaire sought to measure the effects of scratching during a typical episode of itch within the past 2 weeks. Scratching was found pleasurable in both groups of patients.5 Another web-based questionnaire that characterized pleasurability in scratching a typical episode of itch in individuals with atopic dermatitis using a −5 to +5 Likert scale (−5 [highly unpleasurable] to +5 [highly pleasurable]) found that most participants perceived scratching as pleasurable and that there was a positive correlation between itch intensity and scratch pleasurability.6 Both of these studies quantified that scratching an itch is pleasurable, a correlation that may not come as a surprise. This direct correlation suggests that a more detailed analysis of this scratch pleasure could be beneficial in the management of pruritic conditions.
Treating the underlying cause of an itch is key to inhibiting the sensation; in some cases, anti-itch medications must be used. Current medications have limited effects on itch relief, but an expanding understanding of itch pathophysiology through clinical and laboratory research in the fields of dermatology, immunology, and neurology is paving the way for promising new therapeutic medications.7-11 In a review of the literature, Sanders and Akiyama12 elucidated the influence of stress and anxiety in scratching an itch and the way in which both pharmacologic and nonpharmacologic (ie, psychological and educational interventions) may be used to help break the itch-scratch cycle. Possible techniques include habit-reversal training, relaxation therapy, and cognitive behavioral therapy.13 Understanding patient perspectives on the pleasure yielded from scratching an itch and the disease factors that influence this pleasure seeking are paramount to reducing patient scratching. In understanding the pleasurability of scratching in pruritic conditions, the itch-scratch cycle and its accompanying deleterious effects (eg, stress, anxiety, pain, infection, secondary skin changes) can be broken.
The pleasure yielded from scratching an itch is a component of patient scratching habits that should be analyzed and quantified to reduce itch in pruritic conditions, mitigate damaging consequences of scratching, and improve the quality of life of patients with pruritic conditions. Furthermore, this understanding may help guide clinicians in management, such as counseling patients on the itch-scratch cycle and deciding which forthcoming medications could ameliorate a patient’s pruritic symptoms.
- Weisshaar E, Grüll V, König A, et al. The symptom of itch in medical history: highlights through the centuries. Int J Dermatol. 2009;48:1385-1394.
- Lavery MJ, Kinney MO, Mochizuki H, et al. Pruritus: an overview. what drives people to scratch an itch? Ulster Med J. 2016;85:164-173.
- Bin Saif GA, Papoiu ADP, Banari L, et al. The pleasurability of scratching an itch: a psychophysical and topographical assessment. Br J Dermatol. 2012;166:981-985.
- Desai NS, Poindexter GB, Monthrope YM, et al. A pilot quality-of-life instrument for pruritus. J Am Acad Dermatol. 2008;59:234-244.
- O’Neill JL, Chan YH, Rapp SR, et al. Differences in itch characteristics between psoriasis and atopic dermatitis patients: results of a web-based questionnaire. Acta Derm Venereol. 2011;91:537-540.
- Dawn A, Papoiu ADP, Chan YH, et al. Itch characteristics in atopic dermatitis: results of a web-based questionnaire. Br J Dermatol. 2009;160:642-644.
- Yosipovitch G, Rosen JD, Hashimoto T. Itch: from mechanism to (novel) therapeutic approaches. J Allergy Clin Immunol. 2018;142:1375-1390.
- Yosipovitch G, Misery L, Proksch E, et al. Skin barrier damage and itch: review of mechanisms, topical management and future directions. Acta Derm Venereol. 2019;99:1201-1209.
- Dong X, Dong X. Peripheral and central mechanisms of itch. Neuron. 2018;98:482-494.
- Lerner EA. Pathophysiology of itch. Dermatol Clin. 2018;36:175-177.
- Cevikbas F, Lerner EA. Physiology and pathophysiology of itch. Physiol Rev. 2020;100:945-982.
- Sanders KM, Akiyama T. The vicious cycle of itch and anxiety. Neurosci Biobehav Rev. 2018;87:17-26.
- Sanders KM, Nattkemper LA, Yosipovitch G. Advances in understanding itching and scratching: a new era of targeted treatments [published online August 22, 2016]. F1000Res. doi:10.12688/f1000research.8659.
- Weisshaar E, Grüll V, König A, et al. The symptom of itch in medical history: highlights through the centuries. Int J Dermatol. 2009;48:1385-1394.
- Lavery MJ, Kinney MO, Mochizuki H, et al. Pruritus: an overview. what drives people to scratch an itch? Ulster Med J. 2016;85:164-173.
- Bin Saif GA, Papoiu ADP, Banari L, et al. The pleasurability of scratching an itch: a psychophysical and topographical assessment. Br J Dermatol. 2012;166:981-985.
- Desai NS, Poindexter GB, Monthrope YM, et al. A pilot quality-of-life instrument for pruritus. J Am Acad Dermatol. 2008;59:234-244.
- O’Neill JL, Chan YH, Rapp SR, et al. Differences in itch characteristics between psoriasis and atopic dermatitis patients: results of a web-based questionnaire. Acta Derm Venereol. 2011;91:537-540.
- Dawn A, Papoiu ADP, Chan YH, et al. Itch characteristics in atopic dermatitis: results of a web-based questionnaire. Br J Dermatol. 2009;160:642-644.
- Yosipovitch G, Rosen JD, Hashimoto T. Itch: from mechanism to (novel) therapeutic approaches. J Allergy Clin Immunol. 2018;142:1375-1390.
- Yosipovitch G, Misery L, Proksch E, et al. Skin barrier damage and itch: review of mechanisms, topical management and future directions. Acta Derm Venereol. 2019;99:1201-1209.
- Dong X, Dong X. Peripheral and central mechanisms of itch. Neuron. 2018;98:482-494.
- Lerner EA. Pathophysiology of itch. Dermatol Clin. 2018;36:175-177.
- Cevikbas F, Lerner EA. Physiology and pathophysiology of itch. Physiol Rev. 2020;100:945-982.
- Sanders KM, Akiyama T. The vicious cycle of itch and anxiety. Neurosci Biobehav Rev. 2018;87:17-26.
- Sanders KM, Nattkemper LA, Yosipovitch G. Advances in understanding itching and scratching: a new era of targeted treatments [published online August 22, 2016]. F1000Res. doi:10.12688/f1000research.8659.
Practice Points
- In individuals with pruritic skin conditions, the itch-scratch cycle can have damaging consequences such as anxiety, infection, and secondary skin changes.
- Understanding the pleasurability of scratching in pruritic skin conditions allows providers to help patients break the itch-scratch cycle and improve quality of life.