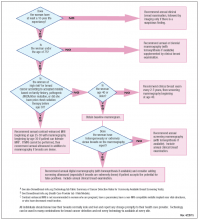
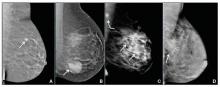
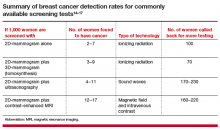
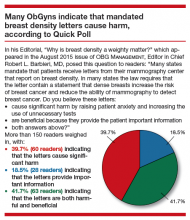
User login
Manual vacuum aspiration: A safe and effective treatment for early miscarriage
Case Miscarriage in a 29-year-old woman
A woman (G0P0) presents to her gynecologist with amenorrhea for 3 months and a positive home urine pregnancy test. She is 29 years of age. She denies any bleeding or pain and intends to continue the pregnancy, though it was unplanned. Results of office ultrasonography to assess fetal viability reveal an intrauterine gestation with an 8-mm fetal pole but no heartbeat. The diagnosis is miscarriage.
This case illustrates a typical miscarriage diagnosis; most women with miscarriage are asymptomatic and without serious bleeding requiring emergency intervention. The management options include surgical, medical, and expectant. Women should be offered all 3 of these, and clinicians should explain the risks and benefits of each approach. But while each strategy can be safe, effective, and acceptable, many women, as well as their health care providers, will benefit from office-based uterine aspiration. In this article, we present the data available on office-based manual vacuum aspiration (MVA) as well as procedure pointers and urge you to consider MVA in your practice for your patients.
Surgical management
Surgical management of miscarriage offers several clear advantages over medical and expectant management. Perhaps the most important advantage to patients is that surgery offers rapid resolution of miscarriage with the shortest duration of bleeding.1,2 When skilled providers perform electric vacuum aspiration (EVA) or MVA in outpatient or emergency department settings, successful uterine evacuation is completed in a single medical encounter 99% of the time.1 By comparison, several follow-up visits and additional ultrasounds may be required during medical or expectant management. Uterine aspiration rarely requires an operating room (OR). Such a setting should be limited to cases in which the clinical picture reflects:
- hemodynamic instability with active uterine bleeding
- serious uterine infection
- the presence of medical comorbidities in patients who may benefit from additional blood bank and anesthesia resources.
Office-based MVA
Office-based MVA is well tolerated when performed using a combination of verbal distraction and reassurance, oral nonsteroidal anti-inflammatory drugs (NSAIDs), and a paracervical block with or without intravenous sedation.
Evidence on managing pain at MVA. Multiple studies have assessed preprocedure and postprocedure pain using NSAIDs, oral anxiolytics, and local anesthesia at the time of EVA or MVA.3,4 Renner and colleagues found that women who received a paracervical block prior to MVA or EVA reported moderate levels of pain, according to a 100-point visual analogue scale (VAS), at the time of cervical dilation (mean, 42) and uterine aspiration (mean, 63).4 In this same study, patients’ willingness to treat a future pregnancy with EVA or MVA using local anesthesia and their overall satisfaction with the procedure was high (mean, 90 on 100-point VAS).
In-office advantages over the OR. Women and clinicians can avoid the extensive scheduling delays associated with ORs, as well as the complications associated with medical and expectant management, if office-based EVA and MVA services are readily available. Compared with surgical management of miscarriage in an OR, office-based EVA and MVA are faster to complete. For example, Dalton and colleagues compared patients undergoing first-trimester procedures in an office setting with those undergoing a procedure in an OR. The mean procedure time for women treated in an office was 10 minutes, compared with 19 minutes for women treated in the OR. In addition, women treated in an office setting spent a mean total of 97 minutes at the office; women treated in an OR spent a mean total of 290 minutes at the hospital.5
Patients’ satisfaction with care provided in the OR was comparable to patients’ satisfaction with care provided in a medical office. In fact, the median total satisfaction score was high among women who had a procedure in either setting (office score, 19 of 20; OR score, 20 of 20).
Cost and equipment for in-office MVA
Office-based surgical management of miscarriage is more cost-effective than OR-based management. In 2006, Dalton and colleagues conducted a cost analysis and found that average charges for office-based MVA were less than half the cost of charges for a dilation and curettage (D&C) in the OR ($968 vs $1,965, respectively).5
More recently, these researchers found that usual care (expectant or OR management) was more costly than a model that also included medical and office-based surgical options. They found that the expanded care model—with use of the OR only when needed—cost $1,033.29 per case. This was compared with $1,247.58 per case when management options did not include medical and office-based surgical treatments.6
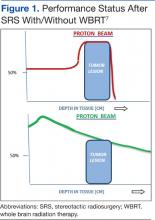
The cost of supplies needed to initiate MVA services within an established outpatient gynecologist’s office is modest. Equipment includes manual vacuum aspirators; disposable cannulae of various sizes; reusable plastic or metal dilators; supplies for disinfection, allowing reuse of MVA aspirators; and supplies for examination of products of conception (POC; FIGURE 1).
According to WomanCare Global, manufacturer of the IPAS MVA Plus, equipment should be sterilized after each use with soap and water, medical cleaning solution (such as Cidex, SPOROX II, etc.), or autoclaving.7 If 2 reusable aspirators are purchased along with dilators, disposable cannulae, and tools for tissue assessment, the price of supplies is estimated at US $500.8 WomanCare Global also offers prepackaged, single-use aspirator kits, which may be ideal for the emergency department setting.9
The procedure
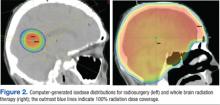
To view a video on the MVA device and procedure, including step-by-step technique (FIGURE 2), local anesthesia administration, choosing cannula size, and cervical dilation, visit the Managing Early Pregnancy Loss Web site (http://www.earlypregnancylossresources.org) and access “Videos.” The video “Uterine aspiration for EPL” is available under password protection and broken into chapters for viewing ease.
The risk of endometritis after surgical management of miscarriage is low. Antibiotic prophylaxis prior to MVA or EVA should be considered. Experts recommend giving a single dose of doxycycline 200 mg orally at least 1 hour prior to uterine aspiration.2,10
Use of EVA or MVA for outpatient management of miscarriage yields the opportunity to conduct immediate gross examination of the evacuated tissue and to verify the presence of complete POC. The process is simple: rinse the specimen through a sieve with water or saline, placed in a clear glass container under a small water bath and backlit on a light box. This allows clinicians to separate uterine decidua and pregnancy tissues. “Floating” tissue in this manner is especially useful in patients with pregnancy of unknown location, as immediate confirmation of a gestational sac rules out ectopic pregnancy.
Examine evacuated tissue for macroscopic evidence of pregnancy. Chorionic villi, which arise from syncytiotrophoblasts, can be seen with the naked eye. Immediate evaluation of POC is also useful for patients who desire diagnostic testing to ascertain a cause of their miscarriage because evacuated tissue stored in saline may be sent to a laboratory for cytogenetic analysis.
Medical management
Management of miscarriage with misoprostol is also safe and acceptable to women, though it has a lower success rate than surgical management.
Comparing efficacy: Medical vs surgical management. The Management of Early Pregnancy Failure Trial (MEPF) is the largest randomized controlled trial comparing medical management of miscarriage to surgical management. This multicenter study compared treatment with office-based EVA or MVA to vaginal misoprostol 800 µg. A repeat dose of vaginal misoprostol was offered 48 hours after the initial dose if a gestational sac was present on ultrasound.
Findings from the MEPF trial revealed a 71% complete uterine evacuation rate after 1 dose of misoprostol and an 84% rate after 2 doses.1 The average (SD) reported pain score documented within 48 hours of treatment with misoprostol or MVA/EVA was moderate (5.7 cm [2.4] on 10-cm VAS). The rate of infection or hospitalization was less than 1% in both treatment groups.
These data should provide patients who are clinically stable and who wish to avoid an invasive procedure reassurance that using medication for the management of miscarriage is a reasonable option.
Misoprostol. Use of misoprostol is associated with a longer median duration of bleeding compared with suction aspiration. After misoprostol, bleeding usually begins after several hours and may continue for weeks.11 Based on 2-week prospective bleeding diary entries from the MEPF trial, women who used misoprostol for management of miscarriage were more likely to have any bleeding during the 2 weeks after initiation of treatment, compared with women who had suction aspiration.12
Clinically significant changes in hemoglobin levels are more common in women treated with misoprostol than in those who choose EVA or MVA; however, these differences rarely require hospitalization or transfusion.1 Women who are considering use of misoprostol should be aware of common adverse effects, including nausea, vomiting, diarrhea, and low-grade temperature.
Medical management of miscarriage requires multiple office visits with repeat ultrasounds or serum beta–human chorionic gonadotropin (β-hCG) levels to confirm treatment success. In cases of medication failure (persistent gestational sac with or without bleeding) or suspected retained POC (endometrial stripe greater than 30 mm measured on ultrasound or persistent vaginal bleeding remote from treatment), women should be prepared for surgical resolution of pregnancy and clinicians should be able to perform an office-based procedure.
Expectant management
Women who choose the “watch and wait” approach should be advised that the process is unpredictable and occasionally requires urgent surgical intervention. Successful resolution of pregnancies that are expectantly managed depends on the type of miscarriage diagnosed at initial presentation. Luise and colleagues conducted a prospective study of 451 women with miscarriage who declined medical and surgical management. They found that the watch-and-wait approach was successful in 91% of women with an incomplete abortion, 76% of women with missed abortion, and 66% of women with anembryonic pregnancies.13 Success was defined by the absence of vaginal bleeding and an anterior-posterior endometrial stripe measuring less than 15 mm 4 weeks after initial diagnosis of miscarriage.
Like medical management for miscarriage, expectant management requires multiple office visits plus repeat ultrasounds or β-hCG measurement trends to confirm treatment success. Women who fail expectant management will require medical or surgical intervention to resolve the pregnancy. For those who are seeking pregnancy right away, the unpredictability and longer time to resolution of miscarriage may render expectant management anxiety provoking and unacceptable.
Etiology: Do true and perceived causes match?
Miscarriage during the first 13 weeks of gestation occurs in at least 10% of all clinically diagnosed pregnancies.10 A recent survey administered by Bardos and colleagues
assessed perceived prevalence and causes of miscarriage in more than 1,000 US men and women.14 The majority of respondents believed miscarriage is uncommon, occurring in less than 5% of pregnancies. Respondents also believed stressful events, lifting heavy objects, and prior use of intrauterine or hormonal contraception are often to blame for pregnancy loss.
Despite more than 3 decades of data confirming that more than 60% of early losses are associated with chromosomal abnormalities and that an additional 18% may be associated with fetal anomalies, women often blame themselves.15 Bardos and colleagues found that 47% of women felt guilty about the experience of miscarriage.
Diagnosis: Updated ultrasonography criteria issued
When miscarriage is suspected based on symptoms of pain and bleeding in preg-
nancy, obtain a thorough history and conduct a limited physical examination. If an intrauterine pregnancy (IUP) was previously identified, a repeat ultrasound can confirm the presence or absence of the gestational sac. If an IUP has not been documented, then additional studies, including serial serum β-hCG examinations and ultrasonography, are essential to rule out ectopic pregnancy. Rh status should be determined and a 50-µg dose of Rh(D)-immune globulin administered to Rh(D)-unsensitized women within 72 hours of documented bleeding.
Ultrasonography is often used to diagnose miscarriage. Many gynecologists use ultrasound criteria based on studies conducted in the early 1990s that define nonviability by an empty gestational sac with mean gestational sac diameter greater than 16 mm or a crown-rump length (CRL) without evidence of fetal cardiac activity greater than 5 mm.10 In 2012, members of the Society of Radiologists in Ultrasound Multispecialty Panel on Early First Trimester Diagnosis of Miscarriage and Exclusion of a Viable Intrauterine Pregnancy developed more conservative criteria for the diagnosis of miscarriage.16
Doubilet and colleagues suggested new cutoffs, based on their reanalysis of 2 large prospective studies conducted in the United Kingdom.17 Calculations for these new cut-offs are based on mathematical adjustments for interobserver variability. Strict adherence to these more conservative criteria is sensible when a pregnancy is desired. For women who do not want to continue the pregnancy there is no medical justification for using this diagnostic process. Indeed, delays can lead to stress and poor outcomes including emergent surgical management for spontaneous and heavy bleeding.
Culture change is needed
Patients’ beliefs and scientific evidence about miscarriage are incongruous. By making simple changes in practice and providing straightforward patient education, ObGyns
can demystify the causes of miscarriage and improve its management. In particular, providing office-based MVA when requested can streamline treatment for many women. For too long, patients have blamed themselves for miscarriage and physicians have relied on D&C in the OR. Changes in culture surrounding miscarriage are
long overdue.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Zhang J, Gilles JM, Barnhart K, Creinin MD, Westhoff C, Frederick MM. A comparison of medical management with misoprostol and surgical management for early pregnancy failure. N Eng J Med. 2005;353(8):761−769.
2. Paul M, Lichtenberg ES, Borgatta L, Grimes DA, Stubblefield PG, Creinin MD, eds. Management of Unintended and Abnormal Pregnancy: Comprehensive Abortion Care. Oxford, United Kingdom: Wiley-Blackwell; 2009.
3. Edelman A, Nichols MD, Jensen J. Comparison of pain and time of procedures with two first-trimester abortion techniques performed by residents and faculty. Am J Obstet Gynecol. 2001;184(7):1564−1567.
4. Renner RM, Nichols MD, Jensen JT, Li H, Edelman AB. Paracervical block for pain control in first-trimester surgical abortion: a randomized controlled trial. Obstet Gynecol. 2012;119(5):1030−1037.
5. Dalton VK, Harris L, Weisman CS, Guire K, Castleman L, Lebovic D. Patient p, satisfaction, and resource use in office evacuation of early pregnancy failure. Obstet Gynecol. 2006;108(1):103−110.
6. Dalton VK, Liang A, Hutton DW, Zochowski MK, Fendrick AM. Beyond usual care: the economic consequences of expanding treatment options in early pregnancy loss. Am J Obstet Gynecol. 2015;212(2):177.e171−177.e176.
7. Ipas. Ipas start-up kit for integrating manual vacuum aspiration (MVA) for early pregnancy loss into women’s reproductive healthcare services. Chapel Hill, NC: Ipas; 2009.
8. MVA Products page. HPSRx Web site. http://www.hpsrx.com/mva-products.html. Accessed October 13, 2015.
9. Kinariwala M, Quinley KE, Datner EM, Schreiber CA. Manual vacuum aspiration in the emergency department for management of early pregnancy failure. Am J Emerg Med. 2013;31(1):244−247.
10. The American College of Obstetricians and Gynecologists. Practice Bulletin No. 150: early pregnancy loss. Obstet Gynecol. 2015;125(5):1258−1267.
11. Meckstroth KR, Whitaker AK, Bertisch S, Goldberg AB, Darney PD. Misoprostol administered by epithelial routes: drug absorption and uterine response. Obstet Gynecol. 2006;108(3 Part 1):582−590.
12. Davis AR, Hendlish SK, Westhoff C, et al. Bleeding patterns after misoprostol vs surgical treatment of early pregnancy failure: results from a randomized trial. Am J Obstet Gynecol. 2007;196(1):31.e31−31.e37.
13. Luise C, Jermy K, May C, Costello G, Collins WP, Bourne TH. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. BMJ. 2002;324(7342):873−875.
14. Bardos J, Hercz D, Friedenthal J, Missmer SA, Williams Z. A national survey on public perceptions of miscarriage. Obstet Gynecol. 2015;125(6):1313−1320.
15. The Practice Committee of the American Society for Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98(5):1103−1111.
16. Doubilet PM, Benson CB, Bourne T, Blaivas M. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Eng JMed. 2013;369(15):1443−1451.
17. Abdallah Y, Daemen A, Kirk E, et al. Limitations of current definitions of miscarriage using mean gestational sac diameter and crown–rump length measurements: a multicenter observational study. Ultrasound Obstet Gynecol. 2011;38(5):497−502.
Case Miscarriage in a 29-year-old woman
A woman (G0P0) presents to her gynecologist with amenorrhea for 3 months and a positive home urine pregnancy test. She is 29 years of age. She denies any bleeding or pain and intends to continue the pregnancy, though it was unplanned. Results of office ultrasonography to assess fetal viability reveal an intrauterine gestation with an 8-mm fetal pole but no heartbeat. The diagnosis is miscarriage.
This case illustrates a typical miscarriage diagnosis; most women with miscarriage are asymptomatic and without serious bleeding requiring emergency intervention. The management options include surgical, medical, and expectant. Women should be offered all 3 of these, and clinicians should explain the risks and benefits of each approach. But while each strategy can be safe, effective, and acceptable, many women, as well as their health care providers, will benefit from office-based uterine aspiration. In this article, we present the data available on office-based manual vacuum aspiration (MVA) as well as procedure pointers and urge you to consider MVA in your practice for your patients.
Surgical management
Surgical management of miscarriage offers several clear advantages over medical and expectant management. Perhaps the most important advantage to patients is that surgery offers rapid resolution of miscarriage with the shortest duration of bleeding.1,2 When skilled providers perform electric vacuum aspiration (EVA) or MVA in outpatient or emergency department settings, successful uterine evacuation is completed in a single medical encounter 99% of the time.1 By comparison, several follow-up visits and additional ultrasounds may be required during medical or expectant management. Uterine aspiration rarely requires an operating room (OR). Such a setting should be limited to cases in which the clinical picture reflects:
- hemodynamic instability with active uterine bleeding
- serious uterine infection
- the presence of medical comorbidities in patients who may benefit from additional blood bank and anesthesia resources.
Office-based MVA
Office-based MVA is well tolerated when performed using a combination of verbal distraction and reassurance, oral nonsteroidal anti-inflammatory drugs (NSAIDs), and a paracervical block with or without intravenous sedation.
Evidence on managing pain at MVA. Multiple studies have assessed preprocedure and postprocedure pain using NSAIDs, oral anxiolytics, and local anesthesia at the time of EVA or MVA.3,4 Renner and colleagues found that women who received a paracervical block prior to MVA or EVA reported moderate levels of pain, according to a 100-point visual analogue scale (VAS), at the time of cervical dilation (mean, 42) and uterine aspiration (mean, 63).4 In this same study, patients’ willingness to treat a future pregnancy with EVA or MVA using local anesthesia and their overall satisfaction with the procedure was high (mean, 90 on 100-point VAS).
In-office advantages over the OR. Women and clinicians can avoid the extensive scheduling delays associated with ORs, as well as the complications associated with medical and expectant management, if office-based EVA and MVA services are readily available. Compared with surgical management of miscarriage in an OR, office-based EVA and MVA are faster to complete. For example, Dalton and colleagues compared patients undergoing first-trimester procedures in an office setting with those undergoing a procedure in an OR. The mean procedure time for women treated in an office was 10 minutes, compared with 19 minutes for women treated in the OR. In addition, women treated in an office setting spent a mean total of 97 minutes at the office; women treated in an OR spent a mean total of 290 minutes at the hospital.5
Patients’ satisfaction with care provided in the OR was comparable to patients’ satisfaction with care provided in a medical office. In fact, the median total satisfaction score was high among women who had a procedure in either setting (office score, 19 of 20; OR score, 20 of 20).
Cost and equipment for in-office MVA
Office-based surgical management of miscarriage is more cost-effective than OR-based management. In 2006, Dalton and colleagues conducted a cost analysis and found that average charges for office-based MVA were less than half the cost of charges for a dilation and curettage (D&C) in the OR ($968 vs $1,965, respectively).5
More recently, these researchers found that usual care (expectant or OR management) was more costly than a model that also included medical and office-based surgical options. They found that the expanded care model—with use of the OR only when needed—cost $1,033.29 per case. This was compared with $1,247.58 per case when management options did not include medical and office-based surgical treatments.6
The cost of supplies needed to initiate MVA services within an established outpatient gynecologist’s office is modest. Equipment includes manual vacuum aspirators; disposable cannulae of various sizes; reusable plastic or metal dilators; supplies for disinfection, allowing reuse of MVA aspirators; and supplies for examination of products of conception (POC; FIGURE 1).
According to WomanCare Global, manufacturer of the IPAS MVA Plus, equipment should be sterilized after each use with soap and water, medical cleaning solution (such as Cidex, SPOROX II, etc.), or autoclaving.7 If 2 reusable aspirators are purchased along with dilators, disposable cannulae, and tools for tissue assessment, the price of supplies is estimated at US $500.8 WomanCare Global also offers prepackaged, single-use aspirator kits, which may be ideal for the emergency department setting.9
The procedure
To view a video on the MVA device and procedure, including step-by-step technique (FIGURE 2), local anesthesia administration, choosing cannula size, and cervical dilation, visit the Managing Early Pregnancy Loss Web site (http://www.earlypregnancylossresources.org) and access “Videos.” The video “Uterine aspiration for EPL” is available under password protection and broken into chapters for viewing ease.
The risk of endometritis after surgical management of miscarriage is low. Antibiotic prophylaxis prior to MVA or EVA should be considered. Experts recommend giving a single dose of doxycycline 200 mg orally at least 1 hour prior to uterine aspiration.2,10
Use of EVA or MVA for outpatient management of miscarriage yields the opportunity to conduct immediate gross examination of the evacuated tissue and to verify the presence of complete POC. The process is simple: rinse the specimen through a sieve with water or saline, placed in a clear glass container under a small water bath and backlit on a light box. This allows clinicians to separate uterine decidua and pregnancy tissues. “Floating” tissue in this manner is especially useful in patients with pregnancy of unknown location, as immediate confirmation of a gestational sac rules out ectopic pregnancy.
Examine evacuated tissue for macroscopic evidence of pregnancy. Chorionic villi, which arise from syncytiotrophoblasts, can be seen with the naked eye. Immediate evaluation of POC is also useful for patients who desire diagnostic testing to ascertain a cause of their miscarriage because evacuated tissue stored in saline may be sent to a laboratory for cytogenetic analysis.
Medical management
Management of miscarriage with misoprostol is also safe and acceptable to women, though it has a lower success rate than surgical management.
Comparing efficacy: Medical vs surgical management. The Management of Early Pregnancy Failure Trial (MEPF) is the largest randomized controlled trial comparing medical management of miscarriage to surgical management. This multicenter study compared treatment with office-based EVA or MVA to vaginal misoprostol 800 µg. A repeat dose of vaginal misoprostol was offered 48 hours after the initial dose if a gestational sac was present on ultrasound.
Findings from the MEPF trial revealed a 71% complete uterine evacuation rate after 1 dose of misoprostol and an 84% rate after 2 doses.1 The average (SD) reported pain score documented within 48 hours of treatment with misoprostol or MVA/EVA was moderate (5.7 cm [2.4] on 10-cm VAS). The rate of infection or hospitalization was less than 1% in both treatment groups.
These data should provide patients who are clinically stable and who wish to avoid an invasive procedure reassurance that using medication for the management of miscarriage is a reasonable option.
Misoprostol. Use of misoprostol is associated with a longer median duration of bleeding compared with suction aspiration. After misoprostol, bleeding usually begins after several hours and may continue for weeks.11 Based on 2-week prospective bleeding diary entries from the MEPF trial, women who used misoprostol for management of miscarriage were more likely to have any bleeding during the 2 weeks after initiation of treatment, compared with women who had suction aspiration.12
Clinically significant changes in hemoglobin levels are more common in women treated with misoprostol than in those who choose EVA or MVA; however, these differences rarely require hospitalization or transfusion.1 Women who are considering use of misoprostol should be aware of common adverse effects, including nausea, vomiting, diarrhea, and low-grade temperature.
Medical management of miscarriage requires multiple office visits with repeat ultrasounds or serum beta–human chorionic gonadotropin (β-hCG) levels to confirm treatment success. In cases of medication failure (persistent gestational sac with or without bleeding) or suspected retained POC (endometrial stripe greater than 30 mm measured on ultrasound or persistent vaginal bleeding remote from treatment), women should be prepared for surgical resolution of pregnancy and clinicians should be able to perform an office-based procedure.
Expectant management
Women who choose the “watch and wait” approach should be advised that the process is unpredictable and occasionally requires urgent surgical intervention. Successful resolution of pregnancies that are expectantly managed depends on the type of miscarriage diagnosed at initial presentation. Luise and colleagues conducted a prospective study of 451 women with miscarriage who declined medical and surgical management. They found that the watch-and-wait approach was successful in 91% of women with an incomplete abortion, 76% of women with missed abortion, and 66% of women with anembryonic pregnancies.13 Success was defined by the absence of vaginal bleeding and an anterior-posterior endometrial stripe measuring less than 15 mm 4 weeks after initial diagnosis of miscarriage.
Like medical management for miscarriage, expectant management requires multiple office visits plus repeat ultrasounds or β-hCG measurement trends to confirm treatment success. Women who fail expectant management will require medical or surgical intervention to resolve the pregnancy. For those who are seeking pregnancy right away, the unpredictability and longer time to resolution of miscarriage may render expectant management anxiety provoking and unacceptable.
Etiology: Do true and perceived causes match?
Miscarriage during the first 13 weeks of gestation occurs in at least 10% of all clinically diagnosed pregnancies.10 A recent survey administered by Bardos and colleagues
assessed perceived prevalence and causes of miscarriage in more than 1,000 US men and women.14 The majority of respondents believed miscarriage is uncommon, occurring in less than 5% of pregnancies. Respondents also believed stressful events, lifting heavy objects, and prior use of intrauterine or hormonal contraception are often to blame for pregnancy loss.
Despite more than 3 decades of data confirming that more than 60% of early losses are associated with chromosomal abnormalities and that an additional 18% may be associated with fetal anomalies, women often blame themselves.15 Bardos and colleagues found that 47% of women felt guilty about the experience of miscarriage.
Diagnosis: Updated ultrasonography criteria issued
When miscarriage is suspected based on symptoms of pain and bleeding in preg-
nancy, obtain a thorough history and conduct a limited physical examination. If an intrauterine pregnancy (IUP) was previously identified, a repeat ultrasound can confirm the presence or absence of the gestational sac. If an IUP has not been documented, then additional studies, including serial serum β-hCG examinations and ultrasonography, are essential to rule out ectopic pregnancy. Rh status should be determined and a 50-µg dose of Rh(D)-immune globulin administered to Rh(D)-unsensitized women within 72 hours of documented bleeding.
Ultrasonography is often used to diagnose miscarriage. Many gynecologists use ultrasound criteria based on studies conducted in the early 1990s that define nonviability by an empty gestational sac with mean gestational sac diameter greater than 16 mm or a crown-rump length (CRL) without evidence of fetal cardiac activity greater than 5 mm.10 In 2012, members of the Society of Radiologists in Ultrasound Multispecialty Panel on Early First Trimester Diagnosis of Miscarriage and Exclusion of a Viable Intrauterine Pregnancy developed more conservative criteria for the diagnosis of miscarriage.16
Doubilet and colleagues suggested new cutoffs, based on their reanalysis of 2 large prospective studies conducted in the United Kingdom.17 Calculations for these new cut-offs are based on mathematical adjustments for interobserver variability. Strict adherence to these more conservative criteria is sensible when a pregnancy is desired. For women who do not want to continue the pregnancy there is no medical justification for using this diagnostic process. Indeed, delays can lead to stress and poor outcomes including emergent surgical management for spontaneous and heavy bleeding.
Culture change is needed
Patients’ beliefs and scientific evidence about miscarriage are incongruous. By making simple changes in practice and providing straightforward patient education, ObGyns
can demystify the causes of miscarriage and improve its management. In particular, providing office-based MVA when requested can streamline treatment for many women. For too long, patients have blamed themselves for miscarriage and physicians have relied on D&C in the OR. Changes in culture surrounding miscarriage are
long overdue.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Case Miscarriage in a 29-year-old woman
A woman (G0P0) presents to her gynecologist with amenorrhea for 3 months and a positive home urine pregnancy test. She is 29 years of age. She denies any bleeding or pain and intends to continue the pregnancy, though it was unplanned. Results of office ultrasonography to assess fetal viability reveal an intrauterine gestation with an 8-mm fetal pole but no heartbeat. The diagnosis is miscarriage.
This case illustrates a typical miscarriage diagnosis; most women with miscarriage are asymptomatic and without serious bleeding requiring emergency intervention. The management options include surgical, medical, and expectant. Women should be offered all 3 of these, and clinicians should explain the risks and benefits of each approach. But while each strategy can be safe, effective, and acceptable, many women, as well as their health care providers, will benefit from office-based uterine aspiration. In this article, we present the data available on office-based manual vacuum aspiration (MVA) as well as procedure pointers and urge you to consider MVA in your practice for your patients.
Surgical management
Surgical management of miscarriage offers several clear advantages over medical and expectant management. Perhaps the most important advantage to patients is that surgery offers rapid resolution of miscarriage with the shortest duration of bleeding.1,2 When skilled providers perform electric vacuum aspiration (EVA) or MVA in outpatient or emergency department settings, successful uterine evacuation is completed in a single medical encounter 99% of the time.1 By comparison, several follow-up visits and additional ultrasounds may be required during medical or expectant management. Uterine aspiration rarely requires an operating room (OR). Such a setting should be limited to cases in which the clinical picture reflects:
- hemodynamic instability with active uterine bleeding
- serious uterine infection
- the presence of medical comorbidities in patients who may benefit from additional blood bank and anesthesia resources.
Office-based MVA
Office-based MVA is well tolerated when performed using a combination of verbal distraction and reassurance, oral nonsteroidal anti-inflammatory drugs (NSAIDs), and a paracervical block with or without intravenous sedation.
Evidence on managing pain at MVA. Multiple studies have assessed preprocedure and postprocedure pain using NSAIDs, oral anxiolytics, and local anesthesia at the time of EVA or MVA.3,4 Renner and colleagues found that women who received a paracervical block prior to MVA or EVA reported moderate levels of pain, according to a 100-point visual analogue scale (VAS), at the time of cervical dilation (mean, 42) and uterine aspiration (mean, 63).4 In this same study, patients’ willingness to treat a future pregnancy with EVA or MVA using local anesthesia and their overall satisfaction with the procedure was high (mean, 90 on 100-point VAS).
In-office advantages over the OR. Women and clinicians can avoid the extensive scheduling delays associated with ORs, as well as the complications associated with medical and expectant management, if office-based EVA and MVA services are readily available. Compared with surgical management of miscarriage in an OR, office-based EVA and MVA are faster to complete. For example, Dalton and colleagues compared patients undergoing first-trimester procedures in an office setting with those undergoing a procedure in an OR. The mean procedure time for women treated in an office was 10 minutes, compared with 19 minutes for women treated in the OR. In addition, women treated in an office setting spent a mean total of 97 minutes at the office; women treated in an OR spent a mean total of 290 minutes at the hospital.5
Patients’ satisfaction with care provided in the OR was comparable to patients’ satisfaction with care provided in a medical office. In fact, the median total satisfaction score was high among women who had a procedure in either setting (office score, 19 of 20; OR score, 20 of 20).
Cost and equipment for in-office MVA
Office-based surgical management of miscarriage is more cost-effective than OR-based management. In 2006, Dalton and colleagues conducted a cost analysis and found that average charges for office-based MVA were less than half the cost of charges for a dilation and curettage (D&C) in the OR ($968 vs $1,965, respectively).5
More recently, these researchers found that usual care (expectant or OR management) was more costly than a model that also included medical and office-based surgical options. They found that the expanded care model—with use of the OR only when needed—cost $1,033.29 per case. This was compared with $1,247.58 per case when management options did not include medical and office-based surgical treatments.6
The cost of supplies needed to initiate MVA services within an established outpatient gynecologist’s office is modest. Equipment includes manual vacuum aspirators; disposable cannulae of various sizes; reusable plastic or metal dilators; supplies for disinfection, allowing reuse of MVA aspirators; and supplies for examination of products of conception (POC; FIGURE 1).
According to WomanCare Global, manufacturer of the IPAS MVA Plus, equipment should be sterilized after each use with soap and water, medical cleaning solution (such as Cidex, SPOROX II, etc.), or autoclaving.7 If 2 reusable aspirators are purchased along with dilators, disposable cannulae, and tools for tissue assessment, the price of supplies is estimated at US $500.8 WomanCare Global also offers prepackaged, single-use aspirator kits, which may be ideal for the emergency department setting.9
The procedure
To view a video on the MVA device and procedure, including step-by-step technique (FIGURE 2), local anesthesia administration, choosing cannula size, and cervical dilation, visit the Managing Early Pregnancy Loss Web site (http://www.earlypregnancylossresources.org) and access “Videos.” The video “Uterine aspiration for EPL” is available under password protection and broken into chapters for viewing ease.
The risk of endometritis after surgical management of miscarriage is low. Antibiotic prophylaxis prior to MVA or EVA should be considered. Experts recommend giving a single dose of doxycycline 200 mg orally at least 1 hour prior to uterine aspiration.2,10
Use of EVA or MVA for outpatient management of miscarriage yields the opportunity to conduct immediate gross examination of the evacuated tissue and to verify the presence of complete POC. The process is simple: rinse the specimen through a sieve with water or saline, placed in a clear glass container under a small water bath and backlit on a light box. This allows clinicians to separate uterine decidua and pregnancy tissues. “Floating” tissue in this manner is especially useful in patients with pregnancy of unknown location, as immediate confirmation of a gestational sac rules out ectopic pregnancy.
Examine evacuated tissue for macroscopic evidence of pregnancy. Chorionic villi, which arise from syncytiotrophoblasts, can be seen with the naked eye. Immediate evaluation of POC is also useful for patients who desire diagnostic testing to ascertain a cause of their miscarriage because evacuated tissue stored in saline may be sent to a laboratory for cytogenetic analysis.
Medical management
Management of miscarriage with misoprostol is also safe and acceptable to women, though it has a lower success rate than surgical management.
Comparing efficacy: Medical vs surgical management. The Management of Early Pregnancy Failure Trial (MEPF) is the largest randomized controlled trial comparing medical management of miscarriage to surgical management. This multicenter study compared treatment with office-based EVA or MVA to vaginal misoprostol 800 µg. A repeat dose of vaginal misoprostol was offered 48 hours after the initial dose if a gestational sac was present on ultrasound.
Findings from the MEPF trial revealed a 71% complete uterine evacuation rate after 1 dose of misoprostol and an 84% rate after 2 doses.1 The average (SD) reported pain score documented within 48 hours of treatment with misoprostol or MVA/EVA was moderate (5.7 cm [2.4] on 10-cm VAS). The rate of infection or hospitalization was less than 1% in both treatment groups.
These data should provide patients who are clinically stable and who wish to avoid an invasive procedure reassurance that using medication for the management of miscarriage is a reasonable option.
Misoprostol. Use of misoprostol is associated with a longer median duration of bleeding compared with suction aspiration. After misoprostol, bleeding usually begins after several hours and may continue for weeks.11 Based on 2-week prospective bleeding diary entries from the MEPF trial, women who used misoprostol for management of miscarriage were more likely to have any bleeding during the 2 weeks after initiation of treatment, compared with women who had suction aspiration.12
Clinically significant changes in hemoglobin levels are more common in women treated with misoprostol than in those who choose EVA or MVA; however, these differences rarely require hospitalization or transfusion.1 Women who are considering use of misoprostol should be aware of common adverse effects, including nausea, vomiting, diarrhea, and low-grade temperature.
Medical management of miscarriage requires multiple office visits with repeat ultrasounds or serum beta–human chorionic gonadotropin (β-hCG) levels to confirm treatment success. In cases of medication failure (persistent gestational sac with or without bleeding) or suspected retained POC (endometrial stripe greater than 30 mm measured on ultrasound or persistent vaginal bleeding remote from treatment), women should be prepared for surgical resolution of pregnancy and clinicians should be able to perform an office-based procedure.
Expectant management
Women who choose the “watch and wait” approach should be advised that the process is unpredictable and occasionally requires urgent surgical intervention. Successful resolution of pregnancies that are expectantly managed depends on the type of miscarriage diagnosed at initial presentation. Luise and colleagues conducted a prospective study of 451 women with miscarriage who declined medical and surgical management. They found that the watch-and-wait approach was successful in 91% of women with an incomplete abortion, 76% of women with missed abortion, and 66% of women with anembryonic pregnancies.13 Success was defined by the absence of vaginal bleeding and an anterior-posterior endometrial stripe measuring less than 15 mm 4 weeks after initial diagnosis of miscarriage.
Like medical management for miscarriage, expectant management requires multiple office visits plus repeat ultrasounds or β-hCG measurement trends to confirm treatment success. Women who fail expectant management will require medical or surgical intervention to resolve the pregnancy. For those who are seeking pregnancy right away, the unpredictability and longer time to resolution of miscarriage may render expectant management anxiety provoking and unacceptable.
Etiology: Do true and perceived causes match?
Miscarriage during the first 13 weeks of gestation occurs in at least 10% of all clinically diagnosed pregnancies.10 A recent survey administered by Bardos and colleagues
assessed perceived prevalence and causes of miscarriage in more than 1,000 US men and women.14 The majority of respondents believed miscarriage is uncommon, occurring in less than 5% of pregnancies. Respondents also believed stressful events, lifting heavy objects, and prior use of intrauterine or hormonal contraception are often to blame for pregnancy loss.
Despite more than 3 decades of data confirming that more than 60% of early losses are associated with chromosomal abnormalities and that an additional 18% may be associated with fetal anomalies, women often blame themselves.15 Bardos and colleagues found that 47% of women felt guilty about the experience of miscarriage.
Diagnosis: Updated ultrasonography criteria issued
When miscarriage is suspected based on symptoms of pain and bleeding in preg-
nancy, obtain a thorough history and conduct a limited physical examination. If an intrauterine pregnancy (IUP) was previously identified, a repeat ultrasound can confirm the presence or absence of the gestational sac. If an IUP has not been documented, then additional studies, including serial serum β-hCG examinations and ultrasonography, are essential to rule out ectopic pregnancy. Rh status should be determined and a 50-µg dose of Rh(D)-immune globulin administered to Rh(D)-unsensitized women within 72 hours of documented bleeding.
Ultrasonography is often used to diagnose miscarriage. Many gynecologists use ultrasound criteria based on studies conducted in the early 1990s that define nonviability by an empty gestational sac with mean gestational sac diameter greater than 16 mm or a crown-rump length (CRL) without evidence of fetal cardiac activity greater than 5 mm.10 In 2012, members of the Society of Radiologists in Ultrasound Multispecialty Panel on Early First Trimester Diagnosis of Miscarriage and Exclusion of a Viable Intrauterine Pregnancy developed more conservative criteria for the diagnosis of miscarriage.16
Doubilet and colleagues suggested new cutoffs, based on their reanalysis of 2 large prospective studies conducted in the United Kingdom.17 Calculations for these new cut-offs are based on mathematical adjustments for interobserver variability. Strict adherence to these more conservative criteria is sensible when a pregnancy is desired. For women who do not want to continue the pregnancy there is no medical justification for using this diagnostic process. Indeed, delays can lead to stress and poor outcomes including emergent surgical management for spontaneous and heavy bleeding.
Culture change is needed
Patients’ beliefs and scientific evidence about miscarriage are incongruous. By making simple changes in practice and providing straightforward patient education, ObGyns
can demystify the causes of miscarriage and improve its management. In particular, providing office-based MVA when requested can streamline treatment for many women. For too long, patients have blamed themselves for miscarriage and physicians have relied on D&C in the OR. Changes in culture surrounding miscarriage are
long overdue.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Zhang J, Gilles JM, Barnhart K, Creinin MD, Westhoff C, Frederick MM. A comparison of medical management with misoprostol and surgical management for early pregnancy failure. N Eng J Med. 2005;353(8):761−769.
2. Paul M, Lichtenberg ES, Borgatta L, Grimes DA, Stubblefield PG, Creinin MD, eds. Management of Unintended and Abnormal Pregnancy: Comprehensive Abortion Care. Oxford, United Kingdom: Wiley-Blackwell; 2009.
3. Edelman A, Nichols MD, Jensen J. Comparison of pain and time of procedures with two first-trimester abortion techniques performed by residents and faculty. Am J Obstet Gynecol. 2001;184(7):1564−1567.
4. Renner RM, Nichols MD, Jensen JT, Li H, Edelman AB. Paracervical block for pain control in first-trimester surgical abortion: a randomized controlled trial. Obstet Gynecol. 2012;119(5):1030−1037.
5. Dalton VK, Harris L, Weisman CS, Guire K, Castleman L, Lebovic D. Patient p, satisfaction, and resource use in office evacuation of early pregnancy failure. Obstet Gynecol. 2006;108(1):103−110.
6. Dalton VK, Liang A, Hutton DW, Zochowski MK, Fendrick AM. Beyond usual care: the economic consequences of expanding treatment options in early pregnancy loss. Am J Obstet Gynecol. 2015;212(2):177.e171−177.e176.
7. Ipas. Ipas start-up kit for integrating manual vacuum aspiration (MVA) for early pregnancy loss into women’s reproductive healthcare services. Chapel Hill, NC: Ipas; 2009.
8. MVA Products page. HPSRx Web site. http://www.hpsrx.com/mva-products.html. Accessed October 13, 2015.
9. Kinariwala M, Quinley KE, Datner EM, Schreiber CA. Manual vacuum aspiration in the emergency department for management of early pregnancy failure. Am J Emerg Med. 2013;31(1):244−247.
10. The American College of Obstetricians and Gynecologists. Practice Bulletin No. 150: early pregnancy loss. Obstet Gynecol. 2015;125(5):1258−1267.
11. Meckstroth KR, Whitaker AK, Bertisch S, Goldberg AB, Darney PD. Misoprostol administered by epithelial routes: drug absorption and uterine response. Obstet Gynecol. 2006;108(3 Part 1):582−590.
12. Davis AR, Hendlish SK, Westhoff C, et al. Bleeding patterns after misoprostol vs surgical treatment of early pregnancy failure: results from a randomized trial. Am J Obstet Gynecol. 2007;196(1):31.e31−31.e37.
13. Luise C, Jermy K, May C, Costello G, Collins WP, Bourne TH. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. BMJ. 2002;324(7342):873−875.
14. Bardos J, Hercz D, Friedenthal J, Missmer SA, Williams Z. A national survey on public perceptions of miscarriage. Obstet Gynecol. 2015;125(6):1313−1320.
15. The Practice Committee of the American Society for Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98(5):1103−1111.
16. Doubilet PM, Benson CB, Bourne T, Blaivas M. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Eng JMed. 2013;369(15):1443−1451.
17. Abdallah Y, Daemen A, Kirk E, et al. Limitations of current definitions of miscarriage using mean gestational sac diameter and crown–rump length measurements: a multicenter observational study. Ultrasound Obstet Gynecol. 2011;38(5):497−502.
1. Zhang J, Gilles JM, Barnhart K, Creinin MD, Westhoff C, Frederick MM. A comparison of medical management with misoprostol and surgical management for early pregnancy failure. N Eng J Med. 2005;353(8):761−769.
2. Paul M, Lichtenberg ES, Borgatta L, Grimes DA, Stubblefield PG, Creinin MD, eds. Management of Unintended and Abnormal Pregnancy: Comprehensive Abortion Care. Oxford, United Kingdom: Wiley-Blackwell; 2009.
3. Edelman A, Nichols MD, Jensen J. Comparison of pain and time of procedures with two first-trimester abortion techniques performed by residents and faculty. Am J Obstet Gynecol. 2001;184(7):1564−1567.
4. Renner RM, Nichols MD, Jensen JT, Li H, Edelman AB. Paracervical block for pain control in first-trimester surgical abortion: a randomized controlled trial. Obstet Gynecol. 2012;119(5):1030−1037.
5. Dalton VK, Harris L, Weisman CS, Guire K, Castleman L, Lebovic D. Patient p, satisfaction, and resource use in office evacuation of early pregnancy failure. Obstet Gynecol. 2006;108(1):103−110.
6. Dalton VK, Liang A, Hutton DW, Zochowski MK, Fendrick AM. Beyond usual care: the economic consequences of expanding treatment options in early pregnancy loss. Am J Obstet Gynecol. 2015;212(2):177.e171−177.e176.
7. Ipas. Ipas start-up kit for integrating manual vacuum aspiration (MVA) for early pregnancy loss into women’s reproductive healthcare services. Chapel Hill, NC: Ipas; 2009.
8. MVA Products page. HPSRx Web site. http://www.hpsrx.com/mva-products.html. Accessed October 13, 2015.
9. Kinariwala M, Quinley KE, Datner EM, Schreiber CA. Manual vacuum aspiration in the emergency department for management of early pregnancy failure. Am J Emerg Med. 2013;31(1):244−247.
10. The American College of Obstetricians and Gynecologists. Practice Bulletin No. 150: early pregnancy loss. Obstet Gynecol. 2015;125(5):1258−1267.
11. Meckstroth KR, Whitaker AK, Bertisch S, Goldberg AB, Darney PD. Misoprostol administered by epithelial routes: drug absorption and uterine response. Obstet Gynecol. 2006;108(3 Part 1):582−590.
12. Davis AR, Hendlish SK, Westhoff C, et al. Bleeding patterns after misoprostol vs surgical treatment of early pregnancy failure: results from a randomized trial. Am J Obstet Gynecol. 2007;196(1):31.e31−31.e37.
13. Luise C, Jermy K, May C, Costello G, Collins WP, Bourne TH. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. BMJ. 2002;324(7342):873−875.
14. Bardos J, Hercz D, Friedenthal J, Missmer SA, Williams Z. A national survey on public perceptions of miscarriage. Obstet Gynecol. 2015;125(6):1313−1320.
15. The Practice Committee of the American Society for Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98(5):1103−1111.
16. Doubilet PM, Benson CB, Bourne T, Blaivas M. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Eng JMed. 2013;369(15):1443−1451.
17. Abdallah Y, Daemen A, Kirk E, et al. Limitations of current definitions of miscarriage using mean gestational sac diameter and crown–rump length measurements: a multicenter observational study. Ultrasound Obstet Gynecol. 2011;38(5):497−502.
In this Article
- Advantages of office-based manual vacuum aspiration
- Medical management
- Current diagnostic criteria
Medial Patellar Subluxation: Diagnosis and Treatment
Medial patellar subluxation (MPS) is a disabling condition caused by an imbalance in the medial and lateral forces in the normal knee, allowing the patella to displace medially. Normally, the patella glides appropriately in the femoral trochlea, but alteration in this medial–lateral equilibrium can lead to pain and instability.1 MPS was first described in 1987 by Betz and colleagues2 as a complication of lateral retinacular release. Since then, multiple cases of iatrogenic, traumatic, and isolated medial subluxation have been reported.3–15 However, MPS after lateral release is the most common cause, accounting for the majority of published cases, whereas only 8 cases of isolated MPS have been reported to date.
Optimal treatment for MPS is not well understood. To better comprehend and manage MPS, we must fully appreciate the pathoanatomy, biomechanics, and current research. In this review, we focus on the anatomy of the lateral retinaculum, diagnosis and treatment of MPS, and outcomes of current treatment techniques.
Anatomy
In 1980, Fulkerson and Gossling16 delineated the anatomy of the knee joint lateral retinaculum. They described a 2-layered system with separate distinct anatomical structures. The lateral retinaculum is oriented longitudinally with the knee extended but exerts a posterolateral force on the lateral aspect of the patella as the knee is flexed. The superficial layer is composed of oblique fibers of the lateral retinaculum originating from the iliotibial band and the vastus lateralis fascia and inserting into the lateral margin of the patella and the patella tendon. The deep layer of the retinaculum consists of several structures, including the deep transverse retinaculum, lateral patellofemoral ligament (LPFL), and the patellotibial band.
Over the years, several studies have described the importance of the lateral retinaculum and, in particular, the LPFL. Examining the functional anatomy of the knee in 1962, Kaplan17 first described the lateral epicondylopatellar ligament as a palpable thickening of the joint capsule. Reider and colleagues18 later named this structure the lateral patellofemoral ligament in their anatomical study of 21 fresh cadaver knees. They described its width as ranging from 3 to 10 mm. In a comprehensive cadaveric study of the LPFL, Navarro and colleagues19,20 found it to be a distinct structure present in all 20 of their dissected specimens. They found its femoral insertion at the lateral epicondyle with a fanlike expansion of the fibers predominantly in the posterior region proximal to the lateral epicondyle. The patellar insertion was found in the posterior half and upper lateral aspect, also with expanded fibers. Mean length of the LPFL is 42.1 mm, and mean width is 16.1 mm.
Medial and lateral forces are balanced in a normal knee, and the patella glides appropriately in the femoral trochlea. Alteration in this medial–lateral equilibrium can lead to pain and instability.1 Normally, the patella lies laterally with the knee extended, but in early flexion the patella moves medially as it engages in the trochlea. As the knee continues to flex, the patella flexes and translates distally.21 By 45°, the patella is fully engaged in the trochlear groove throughout the remainder of the knee’s range of motion (ROM).
Lateral release procedures, as described in the literature, result in sectioning of both layers of the lateral retinaculum. In a biomechanical study, Merican and colleagues22 found that staged release of the lateral retinaculum reduced the medial stability of the patellofemoral joint progressively, making it easier to push the patella medially. At 30° of flexion, the transverse fibers of the midsection of the lateral retinaculum were found to be the main contributor to the lateral restraint of the patella. When the release extends too far proximally, the transverse fibers that anchor the lateral patella and the vastus lateralis oblique tendon to the iliotibial band are disrupted. Subsequent loss of a dynamic muscular pull in the orientation of the lateral stabilizing structures results in medial subluxation in a range from full knee extension to about 30° of flexion.
Furthermore, the attachments of the LPFL and the orientation of its fibers suggest that the LPFL may have a significant role in limiting medial excursion of the patella. Vieira and colleagues23 resected the LPFL in 10 fresh cadaver knees. They noticed that, after resection, the patella spontaneously traveled medially, demonstrating the importance of this ligament in patellar stability. In cases of isolated MPS, there have been no reports of associated pathology, such as muscular imbalance or coronal/rotational malalignment of the lower extremity. With an intact lateral retinaculum, medial subluxation is likely caused by pathology in the normal histologic structure of the LPFL and lateral retinaculum. However, the histologic structure of the LPFL and its contribution to the understanding of the pathoetiology of MPS have not been documented.
Diagnosis
MPS diagnosis can be challenging. Often, clinical examination findings are subtle, and radiographs may not show significant pathology. The most accurate diagnosis is obtained by combining patient history, physical examination findings, imaging studies, and diagnostic arthroscopy.
Patient History
Patients with MPS report chronic pain localized to the inferior medial patella and anterior-medial joint line. Occasionally, they complain of crepitus and intermittent swelling. Other symptoms include pain with knee flexion activity, such as squatting and climbing or descending stairs. Some patients describe episodes of giving way and feelings of instability. Often, they are aware the direction of instability is medial. The pain typically is not relieved by medication, physical therapy, or bracing.
Physical Examination
MPS must be identified by clinical examination. Peripatellar tenderness is typically noted. There is often no effusion or crepitus, but the patella is unstable in early flexion. Active and passive ROM is painful through the first 30° of knee flexion. The patient may have a positive medial apprehension test7 in which he or she experiences apprehension of the patella being subluxated with a medially directed force on the lateral border of the patella.
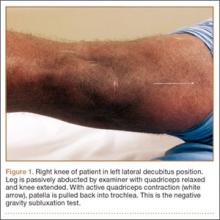
The gravity subluxation test described by Nonweiler and DeLee6 is useful in detecting MPS after lateral release and indicates that the vastus lateralis muscle has been detached from the patella and that the lateral retinaculum is lax. In this test, the patient is positioned in the lateral decubitus position with the involved knee farthest from the table. In this position, gravity causes the patella to subluxate out of the trochlea. The test is positive for MPS when a voluntary contraction of the quadriceps does not center the patella into the trochlear groove. Patients with MPS without previous lateral release can have the patella subluxate medially in the lateral decubitus position, but it is pulled back into the trochlea with active quadriceps contraction (Figure 1).
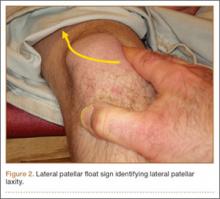
Patients with MPS often have lateral patellar laxity (LPL), which allows the patella to rotate upward on the lateral side and skid across the medial facet of the femoral trochlea. A physical examination sign combining lateral patellar glide and tilt was described by Shneider24 to identify LPL. This “lateral patellar float” sign is present when the patella translates laterally and rotates or tilts upward with medial pressure on the patella (Figure 2). Another maneuver to test for subtle MPS involves manually centering the patella in the trochlea during active knee flexion and extension. The involved knee is examined in the seated position. The examiner attempts to center the patella in the trochlea with a laterally directed force from the examiner’s thumb on the medial border of the patella. This will usually provide immediate relief as the patient actively ranges the knee.
Imaging Studies
Diagnostic imaging is a crucial component of the evaluation and treatment decision process. Plain radiographs often are not helpful in diagnosing MPS but may provide additional information.5 A variety of radiographic measurements have been described as indicators of structural disease, but there is a lack of comprehensive information recommending radiographic evaluation and interpretation of patients with patellofemoral dysfunction. It is crucial that orthopedic surgeons have common and consistent radiographic views for plain radiographic assessment that can serve as a basis for accurate diagnosis and surgical decision-making.
Standard knee radiographs should include a standing anteroposterior view of bilateral knees, a standing lateral view of the symptomatic knee in 30° of flexion, a patellar axial view, and a tunnel view. These views, occasionally combined with magnetic resonance imaging (MRI), can yield information vital to surgical decision-making. Image quality is highly technique-dependent, and variability in patient positioning can substantially affect the ability to properly diagnose structural abnormalities. For improved diagnostic accuracy and disease classification, radiographs must be obtained with use of the same standardized imaging protocol.
Kinetic MRI was shown by Shellock and colleagues25 to provide diagnostic information related to patellar malalignment. As kinetic MRI can image the patellofemoral joint within the initial 20° to 30° of flexion, it is useful in detecting some of the more subtle patellar tracking problems. In their study of 43 knees (40 patients) with symptoms after lateral release, Shellock and colleagues25 found that 27 knees (63%) had medial subluxation of the patella as the knee moved from extension to flexion. Furthermore, MPS was noted on the contralateral, unoperated knee in 17 (43%) of the 40 patients.
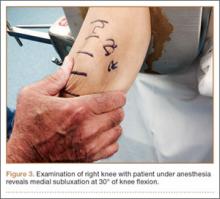
Diagnostic Arthroscopy
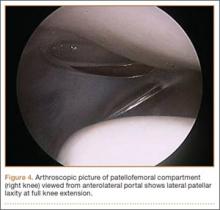
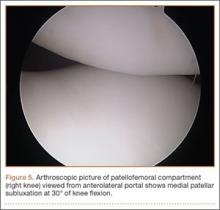
Once MPS is suspected after a thorough history and physical examination, examination under anesthesia accompanied by diagnostic arthroscopy confirms the diagnosis. Lateral forces are applied to the patella in full knee extension and 30° of flexion (Figure 3). During arthroscopy, the patellofemoral compartment is viewed from the anterolateral portal. With the knee at full extension, the lateral laxity and medial tilt of the patella can be identified (Figure 4). As the knee is flexed to 30°, the patella moves medially and can subluxate over the edge of the medial facet of the trochlea (Figure 5).
Treatment
Nonsurgical Management
Treatment of MPS depends entirely on making an accurate diagnosis and determining the degree of impairment. Patients with symptomatic MPS should initially undergo supervised rehabilitation focusing on balancing the medial and lateral forces that influence patellar tracking. Patients should be evaluated for specific muscle tightness, weakness, and biomechanical abnormalities. Each problem should be addressed with an individualized rehabilitation prescription. Emphasis is placed on balance, proprioception, and strengthening of the quadriceps, hip abductors/external rotators, and abdominal core muscle groups.
In some patients, symptomatic MPS may be reduced with a patella-stabilizing brace with a medial buttress.3,5,26 Although bracing should be regarded as an adjuvant to a structured physical therapy program, it can also be helpful in confirming the diagnosis of MPS. Shannon and Keene3 reported that all patients in their study experienced significant pain relief and decreased medial patellar subluxations when they wore a medial patella–stabilizing brace. Shellock and colleagues25 used kinematic MRI to investigate the effect of a patella-realignment brace and found that bracing counteracted patellar subluxation in the majority of knees studied.
Surgical Management
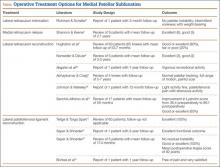
When conservative management fails and patients continue to experience pain and instability, surgical intervention is often required. Although various surgical techniques have been used (Table),3–6,8–10,14,15,27,28 the optimal surgical treatment for MPS has not been identified.
Lateral Retinaculum Imbrication. Lateral retinaculum imbrication has been used to centralize patella tracking and stabilize the patella. Richman and Scheller5 reported on a 17-year-old patient who had isolated medial subluxation of the patella without having undergone a previous lateral release. At 3-month follow-up, there was no recurrent instability; there was only intermittent medial knee soreness with weight-bearing activity.
Lateral Retinaculum Repair/Reconstruction. Hughston and colleagues8 treated 65 knees for MPS. Most had undergone lateral release. Of the 65 knees, 39 were treated with direct repair of the lateral retinaculum, and 26 with reconstruction of the lateral patellotibial ligament using locally available tissue, such as strips of iliotibial band or patellar tendon. Results were good to excellent in 80% of patients at a mean follow-up of 53.7 months. Nonweiler and DeLee6 reconstructed the lateral retinaculum in 5 patients with MPS that developed after isolated lateral retinacular release. Four (80%) of the 5 patients had no symptoms or physical signs of instability at a mean follow-up of 3.3 years. Results were excellent (3 knees) and good (2 knees) according to the Merchant and Mercer rating scale. Akşahin and colleagues28 reported on a single case of spontaneous medial patellar instability. At surgery, imbrication of the lateral structures failed to prevent the medial subluxation. Lateral patellotibial ligament augmentation was performed using an iliotibial band flap that effectively corrected the instability. At 1 year, the patient was characterized as engaging in vigorous recreational activity, according to the clinical score defined by Hughston and colleagues.8 He had mild pain with competitive sports but no pain with daily activity. Abhaykumar and Craig9 reported on 4 surgically treated knees with medial instability. They reconstructed the lateral retinaculum using a strip of fascia lata. By follow-up (5-7 years), each knee had its instability resolved and full ROM restored. Johnson and Wakeley26 reported on a case of iatrogenic MPS after lateral release. Treatment consisted of mobilization and direct repair of the lateral retinaculum. At 12-month follow-up, there was no instability. Although symptom-free with light activity, the patient had patellofemoral pain with strenuous activity. Sanchis-Alfonso and colleagues14 reported the results of isolated lateral retinacular reconstruction for iatrogenic MPS in 17 patients. At mean follow-up of 56 months, results were good or excellent in 65% of patients, and the Lysholm score improved from 36.4 preoperatively to 86.1 postoperatively.
Medial Retinaculum Release. Medial retinaculum release has been used as an alternative to open reconstruction. Shannon and Keene3 reported the results of medial retinacular release procedures on 9 knees. Four (44%) of the 9 patients had either spontaneous or traumatic onset of instability. All cases were treated with arthroscopic medial retinacular release, extending 2 cm medial to the superior pole of the patella down to the anteromedial portal. This avoided releasing the attachment of the vastus medialis oblique muscle to the patella and removing its dynamic medial stabilizing force. At a mean follow-up of 2.7 years, both medial subluxation and knee pain were relieved in all 9 knees without complications or further realignment surgery. Results were excellent in 6 knees (66.7%) and good in 3 knees (33.3%). Shannon and Keene3 emphasized that the procedure should not be used in patients with hypermobile patellae or in cases of failed lateral retinacular releases in which MPS is not clearly and carefully documented.
LPFL Reconstruction. Before coming to our practice, most patients have tried several months of formal physical rehabilitation, medications, and bracing. Many have already had surgical procedures, including arthroscopy, lateral release, and tibial tubercle transfer. When the diagnosis of MPS is suspected after a thorough history and physical examination, LPFL reconstruction is offered. Management of MPS with LPFL reconstruction has yielded excellent and reliable clinical results. Teitge and Torga Spak10 described an LPFL reconstruction technique that is used as a salvage procedure in managing medial iatrogenic patellar instability (the patient’s own quadriceps tendon is used). In their experience, direct repair or imbrication of the lateral retinaculum failed to provide long-term stability because medial excursion usually appeared after 1 year. The 60 patients’ outcomes were excellent with respect to patellar stability, and there were no cases of recurrent subluxation. Borbas and colleagues15 reported a case of LPFL reconstruction in a symptomatic medial subluxated patella resulting from TKA and extended lateral release. Using a free gracilis autograft through patellar bone tunnels to reconstruct the LPFL, the patient was free of pain and very satisfied with the result at 1 year postoperatively. Our current strategy is anatomical reconstruction of the LPFL using a quadriceps tendon graft and no bone tunnels, screws, or anchors in the patella.27 We previously reported a single case of isolated medial instability.4 At 2-year follow-up, there was no recurrent instability, and the functional outcome was excellent. This LPFL reconstruction method has been used in 10 patients with isolated MPS. There has been no residual medial subluxation on follow-up ranging from 3 months to 2 years. Outcome studies are in progress.
Rehabilitation. The initial goal of rehabilitation after surgical reconstruction of the lateral retinaculum or LPFL is to protect the healing soft tissues, restore normal knee ROM, and normalize gait. The knee is immobilized in a brace for weight-bearing activity for 4 to 6 weeks, until limb control is sufficient to prevent rotational stress on the knee. Gradual increase to full weight-bearing without bracing is permitted as quadriceps strength is restored. As motion is regained, strength, balance, and proprioception are emphasized for the entire lower extremity and core.
Functional limb training, including rotational activity, begins at 12 weeks. As strength and neuromuscular control progress, single-leg activity may be started with particular attention to proper alignment of the pelvis and the entire lower extremity. For competitive or recreational athletes, the final stages of rehabilitation focus on dynamic lower extremity control during sport-specific movements. Patients return to unrestricted activity by 6 months to 1 year after surgery.
Summary
MPS is a disabling condition that can limit daily functional activity because of apprehension and pain. Initially described as a complication of lateral retinacular release, isolated MPS can occur in the absence of a previous lateral release. Thorough physical examination and identification during arthroscopy are crucial for proper MPS diagnosis and management. When nonsurgical measures fail, LPFL reconstruction can provide patellofemoral stability and excellent functional outcomes.
1. Marumoto JM, Jordan C, Akins R. A biomechanical comparison of lateral retinacular releases. Am J Sports Med. 1995;23(2):151-155.
2. Betz RR, Magill JT, Lonergan RP. The percutaneous lateral retinacular release. Am J Sports Med. 1987;15(5):477-482.
3. Shannon BD, Keene JS. Results of arthroscopic medial retinacular release for treatment of medial subluxation of the patella. Am J Sports Med. 2007;35(7):1180-1187.
4. Saper MG, Shneider DA. Medial patellar subluxation without previous lateral release: a case report. J Pediatr Orthop B. 2014;23(4):350-353.
5. Richman NM, Scheller AD Jr. Medial subluxation of the patella without previous lateral retinacular release. Orthopedics. 1998;21(7):810-813.
6. Nonweiler DE, DeLee JC. The diagnosis and treatment of medial subluxation of the patella after lateral retinacular release. Am J Sports Med. 1994;22(5):680-686.
7. Hughston JC, Deese M. Medial subluxation of the patella as a complication of lateral retinacular release. Am J Sports Med. 1988;16(4):383-388.
8. Hughston JC, Flandry F, Brinker MR, Terry GC, Mills JC 3rd. Surgical correction of medial subluxation of the patella. Am J Sports Med. 1996;24(4):486-491.
9. Abhaykumar S, Craig DM. Fascia lata sling reconstruction for recurrent medial dislocation of the patella. The Knee. 1999;6(1):55-57.
10. Teitge RA, Torga Spak R. Lateral patellofemoral ligament reconstruction. Arthroscopy. 2004;20(9):998-1002.
11. Kusano M, Horibe S, Tanaka Y, et al. Simultaneous MPFL and LPFL reconstruction for recurrent lateral patellar dislocation with medial patellofemoral instability. Asia-Pac J Sports Med Arthrosc Rehabil Technol. 2014;1:42-46.
12. Saper MG, Shneider DA. Simultaneous medial and lateral patellofemoral ligament reconstruction for combined medial and lateral patellar subluxation. Arthrosc Tech. 2014,3(2):e227-e231.
13. Udagawa K, Niki Y, Matsumoto H, et al. Lateral patellar retinaculum reconstruction for medial patellar instability following lateral retinacular release: a case report. Knee. 2014;21(1):336-339.
14. Sanchis-Alfonso V, Montesinos-Berry E, Monllau JC, Merchant AC. Results of isolated lateral retinacular reconstruction for iatrogenic medial patellar instability. Arthroscopy. 2015;31(3):422-427.
15. Borbas P, Koch PP, Fucentese SF. Lateral patellofemoral ligament reconstruction using a free gracilis autograft. Orthopedics. 2014;37(7):e665-e668.
16. Fulkerson JP, Gossling H. Anatomy of the knee joint lateral retinaculum. Clin Orthop Relat Res. 1980;153:183-188.
17. Kaplan E. Some aspects of functional anatomy of the human knee joint. Clin Orthop Relat Res. 1962;23:18-29.
18. Reider B, Marshall J, Koslin B, Ring B, Girgis F. The anterior aspect of the knee joint. J Bone Joint Surg Am. 1981;63(3):351-356.
19. Navarro MS, Navarro RD, Akita Junior J, Cohen M. Anatomical study of the lateral patellofemoral ligament in cadaver knees. Rev Bras Ortop. 2008;43(7):300-307.
20. Navarro MS, Beltrani Filho CA, Akita Junior J, Navarro RD, Cohen M. Relationship between the lateral patellofemoral ligament and the width of the lateral patellar facet. Acta Ortop Bras. 2010;18(1):19-22.
21. Salsich GB, Ward SR, Terk MR, Powers CM. In vivo assessment of patellofemoral joint contact area in individuals who are pain free. Clin Orthop Relat Res. 2003;417:277-284.
22. Merican AM, Kondo E, Amis AA. The effect on patellofemoral joint stability of selective cutting of lateral retinacular and capsular structures. J Biomech. 2009;42(3):291-296.
23. Vieira EL, Vieira EÁ, da Silva RT, Berlfein PA, Abdalla RJ, Cohen M. An anatomic study of the iliotibial tract. Arthroscopy. 2007;23(3):269-274.
24. Shneider DA. Lateral patellar laxity—identification, significance, treatment. Poster session presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; February 25-28, 2009; Las Vegas, NV.
25. Shellock FG, Mink JH, Deutsch A, Fox JM, Ferkel RD. Evaluation of patients with persistent symptoms after lateral retinacular release by kinematic magnetic resonance imaging of the patellofemoral joint. Arthroscopy. 1990;6(3):226-234.
26. Johnson DP, Wakeley C. Reconstruction of the lateral patellar retinaculum following lateral release: a case report. Knee Surg Sports Traumatol Arthrosc. 2002;10(6):361-363.
27. Saper MG, Shneider DA. Lateral patellofemoral ligament reconstruction using a quadriceps tendon graft. Arthrosc Tech. 2014;3(4):e445-e448.
28. Akşahin E, Yumrukçal F, Yüksel HY, Doğruyol D, Celebi L. Role of pathophysiology of patellofemoral instability in the treatment of spontaneous medial patellofemoral subluxation: a case report. J Med Case Rep. 2010;4:148.
Medial patellar subluxation (MPS) is a disabling condition caused by an imbalance in the medial and lateral forces in the normal knee, allowing the patella to displace medially. Normally, the patella glides appropriately in the femoral trochlea, but alteration in this medial–lateral equilibrium can lead to pain and instability.1 MPS was first described in 1987 by Betz and colleagues2 as a complication of lateral retinacular release. Since then, multiple cases of iatrogenic, traumatic, and isolated medial subluxation have been reported.3–15 However, MPS after lateral release is the most common cause, accounting for the majority of published cases, whereas only 8 cases of isolated MPS have been reported to date.
Optimal treatment for MPS is not well understood. To better comprehend and manage MPS, we must fully appreciate the pathoanatomy, biomechanics, and current research. In this review, we focus on the anatomy of the lateral retinaculum, diagnosis and treatment of MPS, and outcomes of current treatment techniques.
Anatomy
In 1980, Fulkerson and Gossling16 delineated the anatomy of the knee joint lateral retinaculum. They described a 2-layered system with separate distinct anatomical structures. The lateral retinaculum is oriented longitudinally with the knee extended but exerts a posterolateral force on the lateral aspect of the patella as the knee is flexed. The superficial layer is composed of oblique fibers of the lateral retinaculum originating from the iliotibial band and the vastus lateralis fascia and inserting into the lateral margin of the patella and the patella tendon. The deep layer of the retinaculum consists of several structures, including the deep transverse retinaculum, lateral patellofemoral ligament (LPFL), and the patellotibial band.
Over the years, several studies have described the importance of the lateral retinaculum and, in particular, the LPFL. Examining the functional anatomy of the knee in 1962, Kaplan17 first described the lateral epicondylopatellar ligament as a palpable thickening of the joint capsule. Reider and colleagues18 later named this structure the lateral patellofemoral ligament in their anatomical study of 21 fresh cadaver knees. They described its width as ranging from 3 to 10 mm. In a comprehensive cadaveric study of the LPFL, Navarro and colleagues19,20 found it to be a distinct structure present in all 20 of their dissected specimens. They found its femoral insertion at the lateral epicondyle with a fanlike expansion of the fibers predominantly in the posterior region proximal to the lateral epicondyle. The patellar insertion was found in the posterior half and upper lateral aspect, also with expanded fibers. Mean length of the LPFL is 42.1 mm, and mean width is 16.1 mm.
Medial and lateral forces are balanced in a normal knee, and the patella glides appropriately in the femoral trochlea. Alteration in this medial–lateral equilibrium can lead to pain and instability.1 Normally, the patella lies laterally with the knee extended, but in early flexion the patella moves medially as it engages in the trochlea. As the knee continues to flex, the patella flexes and translates distally.21 By 45°, the patella is fully engaged in the trochlear groove throughout the remainder of the knee’s range of motion (ROM).
Lateral release procedures, as described in the literature, result in sectioning of both layers of the lateral retinaculum. In a biomechanical study, Merican and colleagues22 found that staged release of the lateral retinaculum reduced the medial stability of the patellofemoral joint progressively, making it easier to push the patella medially. At 30° of flexion, the transverse fibers of the midsection of the lateral retinaculum were found to be the main contributor to the lateral restraint of the patella. When the release extends too far proximally, the transverse fibers that anchor the lateral patella and the vastus lateralis oblique tendon to the iliotibial band are disrupted. Subsequent loss of a dynamic muscular pull in the orientation of the lateral stabilizing structures results in medial subluxation in a range from full knee extension to about 30° of flexion.
Furthermore, the attachments of the LPFL and the orientation of its fibers suggest that the LPFL may have a significant role in limiting medial excursion of the patella. Vieira and colleagues23 resected the LPFL in 10 fresh cadaver knees. They noticed that, after resection, the patella spontaneously traveled medially, demonstrating the importance of this ligament in patellar stability. In cases of isolated MPS, there have been no reports of associated pathology, such as muscular imbalance or coronal/rotational malalignment of the lower extremity. With an intact lateral retinaculum, medial subluxation is likely caused by pathology in the normal histologic structure of the LPFL and lateral retinaculum. However, the histologic structure of the LPFL and its contribution to the understanding of the pathoetiology of MPS have not been documented.
Diagnosis
MPS diagnosis can be challenging. Often, clinical examination findings are subtle, and radiographs may not show significant pathology. The most accurate diagnosis is obtained by combining patient history, physical examination findings, imaging studies, and diagnostic arthroscopy.
Patient History
Patients with MPS report chronic pain localized to the inferior medial patella and anterior-medial joint line. Occasionally, they complain of crepitus and intermittent swelling. Other symptoms include pain with knee flexion activity, such as squatting and climbing or descending stairs. Some patients describe episodes of giving way and feelings of instability. Often, they are aware the direction of instability is medial. The pain typically is not relieved by medication, physical therapy, or bracing.
Physical Examination
MPS must be identified by clinical examination. Peripatellar tenderness is typically noted. There is often no effusion or crepitus, but the patella is unstable in early flexion. Active and passive ROM is painful through the first 30° of knee flexion. The patient may have a positive medial apprehension test7 in which he or she experiences apprehension of the patella being subluxated with a medially directed force on the lateral border of the patella.
The gravity subluxation test described by Nonweiler and DeLee6 is useful in detecting MPS after lateral release and indicates that the vastus lateralis muscle has been detached from the patella and that the lateral retinaculum is lax. In this test, the patient is positioned in the lateral decubitus position with the involved knee farthest from the table. In this position, gravity causes the patella to subluxate out of the trochlea. The test is positive for MPS when a voluntary contraction of the quadriceps does not center the patella into the trochlear groove. Patients with MPS without previous lateral release can have the patella subluxate medially in the lateral decubitus position, but it is pulled back into the trochlea with active quadriceps contraction (Figure 1).
Patients with MPS often have lateral patellar laxity (LPL), which allows the patella to rotate upward on the lateral side and skid across the medial facet of the femoral trochlea. A physical examination sign combining lateral patellar glide and tilt was described by Shneider24 to identify LPL. This “lateral patellar float” sign is present when the patella translates laterally and rotates or tilts upward with medial pressure on the patella (Figure 2). Another maneuver to test for subtle MPS involves manually centering the patella in the trochlea during active knee flexion and extension. The involved knee is examined in the seated position. The examiner attempts to center the patella in the trochlea with a laterally directed force from the examiner’s thumb on the medial border of the patella. This will usually provide immediate relief as the patient actively ranges the knee.
Imaging Studies
Diagnostic imaging is a crucial component of the evaluation and treatment decision process. Plain radiographs often are not helpful in diagnosing MPS but may provide additional information.5 A variety of radiographic measurements have been described as indicators of structural disease, but there is a lack of comprehensive information recommending radiographic evaluation and interpretation of patients with patellofemoral dysfunction. It is crucial that orthopedic surgeons have common and consistent radiographic views for plain radiographic assessment that can serve as a basis for accurate diagnosis and surgical decision-making.
Standard knee radiographs should include a standing anteroposterior view of bilateral knees, a standing lateral view of the symptomatic knee in 30° of flexion, a patellar axial view, and a tunnel view. These views, occasionally combined with magnetic resonance imaging (MRI), can yield information vital to surgical decision-making. Image quality is highly technique-dependent, and variability in patient positioning can substantially affect the ability to properly diagnose structural abnormalities. For improved diagnostic accuracy and disease classification, radiographs must be obtained with use of the same standardized imaging protocol.
Kinetic MRI was shown by Shellock and colleagues25 to provide diagnostic information related to patellar malalignment. As kinetic MRI can image the patellofemoral joint within the initial 20° to 30° of flexion, it is useful in detecting some of the more subtle patellar tracking problems. In their study of 43 knees (40 patients) with symptoms after lateral release, Shellock and colleagues25 found that 27 knees (63%) had medial subluxation of the patella as the knee moved from extension to flexion. Furthermore, MPS was noted on the contralateral, unoperated knee in 17 (43%) of the 40 patients.
Diagnostic Arthroscopy
Once MPS is suspected after a thorough history and physical examination, examination under anesthesia accompanied by diagnostic arthroscopy confirms the diagnosis. Lateral forces are applied to the patella in full knee extension and 30° of flexion (Figure 3). During arthroscopy, the patellofemoral compartment is viewed from the anterolateral portal. With the knee at full extension, the lateral laxity and medial tilt of the patella can be identified (Figure 4). As the knee is flexed to 30°, the patella moves medially and can subluxate over the edge of the medial facet of the trochlea (Figure 5).
Treatment
Nonsurgical Management
Treatment of MPS depends entirely on making an accurate diagnosis and determining the degree of impairment. Patients with symptomatic MPS should initially undergo supervised rehabilitation focusing on balancing the medial and lateral forces that influence patellar tracking. Patients should be evaluated for specific muscle tightness, weakness, and biomechanical abnormalities. Each problem should be addressed with an individualized rehabilitation prescription. Emphasis is placed on balance, proprioception, and strengthening of the quadriceps, hip abductors/external rotators, and abdominal core muscle groups.
In some patients, symptomatic MPS may be reduced with a patella-stabilizing brace with a medial buttress.3,5,26 Although bracing should be regarded as an adjuvant to a structured physical therapy program, it can also be helpful in confirming the diagnosis of MPS. Shannon and Keene3 reported that all patients in their study experienced significant pain relief and decreased medial patellar subluxations when they wore a medial patella–stabilizing brace. Shellock and colleagues25 used kinematic MRI to investigate the effect of a patella-realignment brace and found that bracing counteracted patellar subluxation in the majority of knees studied.
Surgical Management
When conservative management fails and patients continue to experience pain and instability, surgical intervention is often required. Although various surgical techniques have been used (Table),3–6,8–10,14,15,27,28 the optimal surgical treatment for MPS has not been identified.
Lateral Retinaculum Imbrication. Lateral retinaculum imbrication has been used to centralize patella tracking and stabilize the patella. Richman and Scheller5 reported on a 17-year-old patient who had isolated medial subluxation of the patella without having undergone a previous lateral release. At 3-month follow-up, there was no recurrent instability; there was only intermittent medial knee soreness with weight-bearing activity.
Lateral Retinaculum Repair/Reconstruction. Hughston and colleagues8 treated 65 knees for MPS. Most had undergone lateral release. Of the 65 knees, 39 were treated with direct repair of the lateral retinaculum, and 26 with reconstruction of the lateral patellotibial ligament using locally available tissue, such as strips of iliotibial band or patellar tendon. Results were good to excellent in 80% of patients at a mean follow-up of 53.7 months. Nonweiler and DeLee6 reconstructed the lateral retinaculum in 5 patients with MPS that developed after isolated lateral retinacular release. Four (80%) of the 5 patients had no symptoms or physical signs of instability at a mean follow-up of 3.3 years. Results were excellent (3 knees) and good (2 knees) according to the Merchant and Mercer rating scale. Akşahin and colleagues28 reported on a single case of spontaneous medial patellar instability. At surgery, imbrication of the lateral structures failed to prevent the medial subluxation. Lateral patellotibial ligament augmentation was performed using an iliotibial band flap that effectively corrected the instability. At 1 year, the patient was characterized as engaging in vigorous recreational activity, according to the clinical score defined by Hughston and colleagues.8 He had mild pain with competitive sports but no pain with daily activity. Abhaykumar and Craig9 reported on 4 surgically treated knees with medial instability. They reconstructed the lateral retinaculum using a strip of fascia lata. By follow-up (5-7 years), each knee had its instability resolved and full ROM restored. Johnson and Wakeley26 reported on a case of iatrogenic MPS after lateral release. Treatment consisted of mobilization and direct repair of the lateral retinaculum. At 12-month follow-up, there was no instability. Although symptom-free with light activity, the patient had patellofemoral pain with strenuous activity. Sanchis-Alfonso and colleagues14 reported the results of isolated lateral retinacular reconstruction for iatrogenic MPS in 17 patients. At mean follow-up of 56 months, results were good or excellent in 65% of patients, and the Lysholm score improved from 36.4 preoperatively to 86.1 postoperatively.
Medial Retinaculum Release. Medial retinaculum release has been used as an alternative to open reconstruction. Shannon and Keene3 reported the results of medial retinacular release procedures on 9 knees. Four (44%) of the 9 patients had either spontaneous or traumatic onset of instability. All cases were treated with arthroscopic medial retinacular release, extending 2 cm medial to the superior pole of the patella down to the anteromedial portal. This avoided releasing the attachment of the vastus medialis oblique muscle to the patella and removing its dynamic medial stabilizing force. At a mean follow-up of 2.7 years, both medial subluxation and knee pain were relieved in all 9 knees without complications or further realignment surgery. Results were excellent in 6 knees (66.7%) and good in 3 knees (33.3%). Shannon and Keene3 emphasized that the procedure should not be used in patients with hypermobile patellae or in cases of failed lateral retinacular releases in which MPS is not clearly and carefully documented.
LPFL Reconstruction. Before coming to our practice, most patients have tried several months of formal physical rehabilitation, medications, and bracing. Many have already had surgical procedures, including arthroscopy, lateral release, and tibial tubercle transfer. When the diagnosis of MPS is suspected after a thorough history and physical examination, LPFL reconstruction is offered. Management of MPS with LPFL reconstruction has yielded excellent and reliable clinical results. Teitge and Torga Spak10 described an LPFL reconstruction technique that is used as a salvage procedure in managing medial iatrogenic patellar instability (the patient’s own quadriceps tendon is used). In their experience, direct repair or imbrication of the lateral retinaculum failed to provide long-term stability because medial excursion usually appeared after 1 year. The 60 patients’ outcomes were excellent with respect to patellar stability, and there were no cases of recurrent subluxation. Borbas and colleagues15 reported a case of LPFL reconstruction in a symptomatic medial subluxated patella resulting from TKA and extended lateral release. Using a free gracilis autograft through patellar bone tunnels to reconstruct the LPFL, the patient was free of pain and very satisfied with the result at 1 year postoperatively. Our current strategy is anatomical reconstruction of the LPFL using a quadriceps tendon graft and no bone tunnels, screws, or anchors in the patella.27 We previously reported a single case of isolated medial instability.4 At 2-year follow-up, there was no recurrent instability, and the functional outcome was excellent. This LPFL reconstruction method has been used in 10 patients with isolated MPS. There has been no residual medial subluxation on follow-up ranging from 3 months to 2 years. Outcome studies are in progress.
Rehabilitation. The initial goal of rehabilitation after surgical reconstruction of the lateral retinaculum or LPFL is to protect the healing soft tissues, restore normal knee ROM, and normalize gait. The knee is immobilized in a brace for weight-bearing activity for 4 to 6 weeks, until limb control is sufficient to prevent rotational stress on the knee. Gradual increase to full weight-bearing without bracing is permitted as quadriceps strength is restored. As motion is regained, strength, balance, and proprioception are emphasized for the entire lower extremity and core.
Functional limb training, including rotational activity, begins at 12 weeks. As strength and neuromuscular control progress, single-leg activity may be started with particular attention to proper alignment of the pelvis and the entire lower extremity. For competitive or recreational athletes, the final stages of rehabilitation focus on dynamic lower extremity control during sport-specific movements. Patients return to unrestricted activity by 6 months to 1 year after surgery.
Summary
MPS is a disabling condition that can limit daily functional activity because of apprehension and pain. Initially described as a complication of lateral retinacular release, isolated MPS can occur in the absence of a previous lateral release. Thorough physical examination and identification during arthroscopy are crucial for proper MPS diagnosis and management. When nonsurgical measures fail, LPFL reconstruction can provide patellofemoral stability and excellent functional outcomes.
Medial patellar subluxation (MPS) is a disabling condition caused by an imbalance in the medial and lateral forces in the normal knee, allowing the patella to displace medially. Normally, the patella glides appropriately in the femoral trochlea, but alteration in this medial–lateral equilibrium can lead to pain and instability.1 MPS was first described in 1987 by Betz and colleagues2 as a complication of lateral retinacular release. Since then, multiple cases of iatrogenic, traumatic, and isolated medial subluxation have been reported.3–15 However, MPS after lateral release is the most common cause, accounting for the majority of published cases, whereas only 8 cases of isolated MPS have been reported to date.
Optimal treatment for MPS is not well understood. To better comprehend and manage MPS, we must fully appreciate the pathoanatomy, biomechanics, and current research. In this review, we focus on the anatomy of the lateral retinaculum, diagnosis and treatment of MPS, and outcomes of current treatment techniques.
Anatomy
In 1980, Fulkerson and Gossling16 delineated the anatomy of the knee joint lateral retinaculum. They described a 2-layered system with separate distinct anatomical structures. The lateral retinaculum is oriented longitudinally with the knee extended but exerts a posterolateral force on the lateral aspect of the patella as the knee is flexed. The superficial layer is composed of oblique fibers of the lateral retinaculum originating from the iliotibial band and the vastus lateralis fascia and inserting into the lateral margin of the patella and the patella tendon. The deep layer of the retinaculum consists of several structures, including the deep transverse retinaculum, lateral patellofemoral ligament (LPFL), and the patellotibial band.
Over the years, several studies have described the importance of the lateral retinaculum and, in particular, the LPFL. Examining the functional anatomy of the knee in 1962, Kaplan17 first described the lateral epicondylopatellar ligament as a palpable thickening of the joint capsule. Reider and colleagues18 later named this structure the lateral patellofemoral ligament in their anatomical study of 21 fresh cadaver knees. They described its width as ranging from 3 to 10 mm. In a comprehensive cadaveric study of the LPFL, Navarro and colleagues19,20 found it to be a distinct structure present in all 20 of their dissected specimens. They found its femoral insertion at the lateral epicondyle with a fanlike expansion of the fibers predominantly in the posterior region proximal to the lateral epicondyle. The patellar insertion was found in the posterior half and upper lateral aspect, also with expanded fibers. Mean length of the LPFL is 42.1 mm, and mean width is 16.1 mm.
Medial and lateral forces are balanced in a normal knee, and the patella glides appropriately in the femoral trochlea. Alteration in this medial–lateral equilibrium can lead to pain and instability.1 Normally, the patella lies laterally with the knee extended, but in early flexion the patella moves medially as it engages in the trochlea. As the knee continues to flex, the patella flexes and translates distally.21 By 45°, the patella is fully engaged in the trochlear groove throughout the remainder of the knee’s range of motion (ROM).
Lateral release procedures, as described in the literature, result in sectioning of both layers of the lateral retinaculum. In a biomechanical study, Merican and colleagues22 found that staged release of the lateral retinaculum reduced the medial stability of the patellofemoral joint progressively, making it easier to push the patella medially. At 30° of flexion, the transverse fibers of the midsection of the lateral retinaculum were found to be the main contributor to the lateral restraint of the patella. When the release extends too far proximally, the transverse fibers that anchor the lateral patella and the vastus lateralis oblique tendon to the iliotibial band are disrupted. Subsequent loss of a dynamic muscular pull in the orientation of the lateral stabilizing structures results in medial subluxation in a range from full knee extension to about 30° of flexion.
Furthermore, the attachments of the LPFL and the orientation of its fibers suggest that the LPFL may have a significant role in limiting medial excursion of the patella. Vieira and colleagues23 resected the LPFL in 10 fresh cadaver knees. They noticed that, after resection, the patella spontaneously traveled medially, demonstrating the importance of this ligament in patellar stability. In cases of isolated MPS, there have been no reports of associated pathology, such as muscular imbalance or coronal/rotational malalignment of the lower extremity. With an intact lateral retinaculum, medial subluxation is likely caused by pathology in the normal histologic structure of the LPFL and lateral retinaculum. However, the histologic structure of the LPFL and its contribution to the understanding of the pathoetiology of MPS have not been documented.
Diagnosis
MPS diagnosis can be challenging. Often, clinical examination findings are subtle, and radiographs may not show significant pathology. The most accurate diagnosis is obtained by combining patient history, physical examination findings, imaging studies, and diagnostic arthroscopy.
Patient History
Patients with MPS report chronic pain localized to the inferior medial patella and anterior-medial joint line. Occasionally, they complain of crepitus and intermittent swelling. Other symptoms include pain with knee flexion activity, such as squatting and climbing or descending stairs. Some patients describe episodes of giving way and feelings of instability. Often, they are aware the direction of instability is medial. The pain typically is not relieved by medication, physical therapy, or bracing.
Physical Examination
MPS must be identified by clinical examination. Peripatellar tenderness is typically noted. There is often no effusion or crepitus, but the patella is unstable in early flexion. Active and passive ROM is painful through the first 30° of knee flexion. The patient may have a positive medial apprehension test7 in which he or she experiences apprehension of the patella being subluxated with a medially directed force on the lateral border of the patella.
The gravity subluxation test described by Nonweiler and DeLee6 is useful in detecting MPS after lateral release and indicates that the vastus lateralis muscle has been detached from the patella and that the lateral retinaculum is lax. In this test, the patient is positioned in the lateral decubitus position with the involved knee farthest from the table. In this position, gravity causes the patella to subluxate out of the trochlea. The test is positive for MPS when a voluntary contraction of the quadriceps does not center the patella into the trochlear groove. Patients with MPS without previous lateral release can have the patella subluxate medially in the lateral decubitus position, but it is pulled back into the trochlea with active quadriceps contraction (Figure 1).
Patients with MPS often have lateral patellar laxity (LPL), which allows the patella to rotate upward on the lateral side and skid across the medial facet of the femoral trochlea. A physical examination sign combining lateral patellar glide and tilt was described by Shneider24 to identify LPL. This “lateral patellar float” sign is present when the patella translates laterally and rotates or tilts upward with medial pressure on the patella (Figure 2). Another maneuver to test for subtle MPS involves manually centering the patella in the trochlea during active knee flexion and extension. The involved knee is examined in the seated position. The examiner attempts to center the patella in the trochlea with a laterally directed force from the examiner’s thumb on the medial border of the patella. This will usually provide immediate relief as the patient actively ranges the knee.
Imaging Studies
Diagnostic imaging is a crucial component of the evaluation and treatment decision process. Plain radiographs often are not helpful in diagnosing MPS but may provide additional information.5 A variety of radiographic measurements have been described as indicators of structural disease, but there is a lack of comprehensive information recommending radiographic evaluation and interpretation of patients with patellofemoral dysfunction. It is crucial that orthopedic surgeons have common and consistent radiographic views for plain radiographic assessment that can serve as a basis for accurate diagnosis and surgical decision-making.
Standard knee radiographs should include a standing anteroposterior view of bilateral knees, a standing lateral view of the symptomatic knee in 30° of flexion, a patellar axial view, and a tunnel view. These views, occasionally combined with magnetic resonance imaging (MRI), can yield information vital to surgical decision-making. Image quality is highly technique-dependent, and variability in patient positioning can substantially affect the ability to properly diagnose structural abnormalities. For improved diagnostic accuracy and disease classification, radiographs must be obtained with use of the same standardized imaging protocol.
Kinetic MRI was shown by Shellock and colleagues25 to provide diagnostic information related to patellar malalignment. As kinetic MRI can image the patellofemoral joint within the initial 20° to 30° of flexion, it is useful in detecting some of the more subtle patellar tracking problems. In their study of 43 knees (40 patients) with symptoms after lateral release, Shellock and colleagues25 found that 27 knees (63%) had medial subluxation of the patella as the knee moved from extension to flexion. Furthermore, MPS was noted on the contralateral, unoperated knee in 17 (43%) of the 40 patients.
Diagnostic Arthroscopy
Once MPS is suspected after a thorough history and physical examination, examination under anesthesia accompanied by diagnostic arthroscopy confirms the diagnosis. Lateral forces are applied to the patella in full knee extension and 30° of flexion (Figure 3). During arthroscopy, the patellofemoral compartment is viewed from the anterolateral portal. With the knee at full extension, the lateral laxity and medial tilt of the patella can be identified (Figure 4). As the knee is flexed to 30°, the patella moves medially and can subluxate over the edge of the medial facet of the trochlea (Figure 5).
Treatment
Nonsurgical Management
Treatment of MPS depends entirely on making an accurate diagnosis and determining the degree of impairment. Patients with symptomatic MPS should initially undergo supervised rehabilitation focusing on balancing the medial and lateral forces that influence patellar tracking. Patients should be evaluated for specific muscle tightness, weakness, and biomechanical abnormalities. Each problem should be addressed with an individualized rehabilitation prescription. Emphasis is placed on balance, proprioception, and strengthening of the quadriceps, hip abductors/external rotators, and abdominal core muscle groups.
In some patients, symptomatic MPS may be reduced with a patella-stabilizing brace with a medial buttress.3,5,26 Although bracing should be regarded as an adjuvant to a structured physical therapy program, it can also be helpful in confirming the diagnosis of MPS. Shannon and Keene3 reported that all patients in their study experienced significant pain relief and decreased medial patellar subluxations when they wore a medial patella–stabilizing brace. Shellock and colleagues25 used kinematic MRI to investigate the effect of a patella-realignment brace and found that bracing counteracted patellar subluxation in the majority of knees studied.
Surgical Management
When conservative management fails and patients continue to experience pain and instability, surgical intervention is often required. Although various surgical techniques have been used (Table),3–6,8–10,14,15,27,28 the optimal surgical treatment for MPS has not been identified.
Lateral Retinaculum Imbrication. Lateral retinaculum imbrication has been used to centralize patella tracking and stabilize the patella. Richman and Scheller5 reported on a 17-year-old patient who had isolated medial subluxation of the patella without having undergone a previous lateral release. At 3-month follow-up, there was no recurrent instability; there was only intermittent medial knee soreness with weight-bearing activity.
Lateral Retinaculum Repair/Reconstruction. Hughston and colleagues8 treated 65 knees for MPS. Most had undergone lateral release. Of the 65 knees, 39 were treated with direct repair of the lateral retinaculum, and 26 with reconstruction of the lateral patellotibial ligament using locally available tissue, such as strips of iliotibial band or patellar tendon. Results were good to excellent in 80% of patients at a mean follow-up of 53.7 months. Nonweiler and DeLee6 reconstructed the lateral retinaculum in 5 patients with MPS that developed after isolated lateral retinacular release. Four (80%) of the 5 patients had no symptoms or physical signs of instability at a mean follow-up of 3.3 years. Results were excellent (3 knees) and good (2 knees) according to the Merchant and Mercer rating scale. Akşahin and colleagues28 reported on a single case of spontaneous medial patellar instability. At surgery, imbrication of the lateral structures failed to prevent the medial subluxation. Lateral patellotibial ligament augmentation was performed using an iliotibial band flap that effectively corrected the instability. At 1 year, the patient was characterized as engaging in vigorous recreational activity, according to the clinical score defined by Hughston and colleagues.8 He had mild pain with competitive sports but no pain with daily activity. Abhaykumar and Craig9 reported on 4 surgically treated knees with medial instability. They reconstructed the lateral retinaculum using a strip of fascia lata. By follow-up (5-7 years), each knee had its instability resolved and full ROM restored. Johnson and Wakeley26 reported on a case of iatrogenic MPS after lateral release. Treatment consisted of mobilization and direct repair of the lateral retinaculum. At 12-month follow-up, there was no instability. Although symptom-free with light activity, the patient had patellofemoral pain with strenuous activity. Sanchis-Alfonso and colleagues14 reported the results of isolated lateral retinacular reconstruction for iatrogenic MPS in 17 patients. At mean follow-up of 56 months, results were good or excellent in 65% of patients, and the Lysholm score improved from 36.4 preoperatively to 86.1 postoperatively.
Medial Retinaculum Release. Medial retinaculum release has been used as an alternative to open reconstruction. Shannon and Keene3 reported the results of medial retinacular release procedures on 9 knees. Four (44%) of the 9 patients had either spontaneous or traumatic onset of instability. All cases were treated with arthroscopic medial retinacular release, extending 2 cm medial to the superior pole of the patella down to the anteromedial portal. This avoided releasing the attachment of the vastus medialis oblique muscle to the patella and removing its dynamic medial stabilizing force. At a mean follow-up of 2.7 years, both medial subluxation and knee pain were relieved in all 9 knees without complications or further realignment surgery. Results were excellent in 6 knees (66.7%) and good in 3 knees (33.3%). Shannon and Keene3 emphasized that the procedure should not be used in patients with hypermobile patellae or in cases of failed lateral retinacular releases in which MPS is not clearly and carefully documented.
LPFL Reconstruction. Before coming to our practice, most patients have tried several months of formal physical rehabilitation, medications, and bracing. Many have already had surgical procedures, including arthroscopy, lateral release, and tibial tubercle transfer. When the diagnosis of MPS is suspected after a thorough history and physical examination, LPFL reconstruction is offered. Management of MPS with LPFL reconstruction has yielded excellent and reliable clinical results. Teitge and Torga Spak10 described an LPFL reconstruction technique that is used as a salvage procedure in managing medial iatrogenic patellar instability (the patient’s own quadriceps tendon is used). In their experience, direct repair or imbrication of the lateral retinaculum failed to provide long-term stability because medial excursion usually appeared after 1 year. The 60 patients’ outcomes were excellent with respect to patellar stability, and there were no cases of recurrent subluxation. Borbas and colleagues15 reported a case of LPFL reconstruction in a symptomatic medial subluxated patella resulting from TKA and extended lateral release. Using a free gracilis autograft through patellar bone tunnels to reconstruct the LPFL, the patient was free of pain and very satisfied with the result at 1 year postoperatively. Our current strategy is anatomical reconstruction of the LPFL using a quadriceps tendon graft and no bone tunnels, screws, or anchors in the patella.27 We previously reported a single case of isolated medial instability.4 At 2-year follow-up, there was no recurrent instability, and the functional outcome was excellent. This LPFL reconstruction method has been used in 10 patients with isolated MPS. There has been no residual medial subluxation on follow-up ranging from 3 months to 2 years. Outcome studies are in progress.
Rehabilitation. The initial goal of rehabilitation after surgical reconstruction of the lateral retinaculum or LPFL is to protect the healing soft tissues, restore normal knee ROM, and normalize gait. The knee is immobilized in a brace for weight-bearing activity for 4 to 6 weeks, until limb control is sufficient to prevent rotational stress on the knee. Gradual increase to full weight-bearing without bracing is permitted as quadriceps strength is restored. As motion is regained, strength, balance, and proprioception are emphasized for the entire lower extremity and core.
Functional limb training, including rotational activity, begins at 12 weeks. As strength and neuromuscular control progress, single-leg activity may be started with particular attention to proper alignment of the pelvis and the entire lower extremity. For competitive or recreational athletes, the final stages of rehabilitation focus on dynamic lower extremity control during sport-specific movements. Patients return to unrestricted activity by 6 months to 1 year after surgery.
Summary
MPS is a disabling condition that can limit daily functional activity because of apprehension and pain. Initially described as a complication of lateral retinacular release, isolated MPS can occur in the absence of a previous lateral release. Thorough physical examination and identification during arthroscopy are crucial for proper MPS diagnosis and management. When nonsurgical measures fail, LPFL reconstruction can provide patellofemoral stability and excellent functional outcomes.
1. Marumoto JM, Jordan C, Akins R. A biomechanical comparison of lateral retinacular releases. Am J Sports Med. 1995;23(2):151-155.
2. Betz RR, Magill JT, Lonergan RP. The percutaneous lateral retinacular release. Am J Sports Med. 1987;15(5):477-482.
3. Shannon BD, Keene JS. Results of arthroscopic medial retinacular release for treatment of medial subluxation of the patella. Am J Sports Med. 2007;35(7):1180-1187.
4. Saper MG, Shneider DA. Medial patellar subluxation without previous lateral release: a case report. J Pediatr Orthop B. 2014;23(4):350-353.
5. Richman NM, Scheller AD Jr. Medial subluxation of the patella without previous lateral retinacular release. Orthopedics. 1998;21(7):810-813.
6. Nonweiler DE, DeLee JC. The diagnosis and treatment of medial subluxation of the patella after lateral retinacular release. Am J Sports Med. 1994;22(5):680-686.
7. Hughston JC, Deese M. Medial subluxation of the patella as a complication of lateral retinacular release. Am J Sports Med. 1988;16(4):383-388.
8. Hughston JC, Flandry F, Brinker MR, Terry GC, Mills JC 3rd. Surgical correction of medial subluxation of the patella. Am J Sports Med. 1996;24(4):486-491.
9. Abhaykumar S, Craig DM. Fascia lata sling reconstruction for recurrent medial dislocation of the patella. The Knee. 1999;6(1):55-57.
10. Teitge RA, Torga Spak R. Lateral patellofemoral ligament reconstruction. Arthroscopy. 2004;20(9):998-1002.
11. Kusano M, Horibe S, Tanaka Y, et al. Simultaneous MPFL and LPFL reconstruction for recurrent lateral patellar dislocation with medial patellofemoral instability. Asia-Pac J Sports Med Arthrosc Rehabil Technol. 2014;1:42-46.
12. Saper MG, Shneider DA. Simultaneous medial and lateral patellofemoral ligament reconstruction for combined medial and lateral patellar subluxation. Arthrosc Tech. 2014,3(2):e227-e231.
13. Udagawa K, Niki Y, Matsumoto H, et al. Lateral patellar retinaculum reconstruction for medial patellar instability following lateral retinacular release: a case report. Knee. 2014;21(1):336-339.
14. Sanchis-Alfonso V, Montesinos-Berry E, Monllau JC, Merchant AC. Results of isolated lateral retinacular reconstruction for iatrogenic medial patellar instability. Arthroscopy. 2015;31(3):422-427.
15. Borbas P, Koch PP, Fucentese SF. Lateral patellofemoral ligament reconstruction using a free gracilis autograft. Orthopedics. 2014;37(7):e665-e668.
16. Fulkerson JP, Gossling H. Anatomy of the knee joint lateral retinaculum. Clin Orthop Relat Res. 1980;153:183-188.
17. Kaplan E. Some aspects of functional anatomy of the human knee joint. Clin Orthop Relat Res. 1962;23:18-29.
18. Reider B, Marshall J, Koslin B, Ring B, Girgis F. The anterior aspect of the knee joint. J Bone Joint Surg Am. 1981;63(3):351-356.
19. Navarro MS, Navarro RD, Akita Junior J, Cohen M. Anatomical study of the lateral patellofemoral ligament in cadaver knees. Rev Bras Ortop. 2008;43(7):300-307.
20. Navarro MS, Beltrani Filho CA, Akita Junior J, Navarro RD, Cohen M. Relationship between the lateral patellofemoral ligament and the width of the lateral patellar facet. Acta Ortop Bras. 2010;18(1):19-22.
21. Salsich GB, Ward SR, Terk MR, Powers CM. In vivo assessment of patellofemoral joint contact area in individuals who are pain free. Clin Orthop Relat Res. 2003;417:277-284.
22. Merican AM, Kondo E, Amis AA. The effect on patellofemoral joint stability of selective cutting of lateral retinacular and capsular structures. J Biomech. 2009;42(3):291-296.
23. Vieira EL, Vieira EÁ, da Silva RT, Berlfein PA, Abdalla RJ, Cohen M. An anatomic study of the iliotibial tract. Arthroscopy. 2007;23(3):269-274.
24. Shneider DA. Lateral patellar laxity—identification, significance, treatment. Poster session presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; February 25-28, 2009; Las Vegas, NV.
25. Shellock FG, Mink JH, Deutsch A, Fox JM, Ferkel RD. Evaluation of patients with persistent symptoms after lateral retinacular release by kinematic magnetic resonance imaging of the patellofemoral joint. Arthroscopy. 1990;6(3):226-234.
26. Johnson DP, Wakeley C. Reconstruction of the lateral patellar retinaculum following lateral release: a case report. Knee Surg Sports Traumatol Arthrosc. 2002;10(6):361-363.
27. Saper MG, Shneider DA. Lateral patellofemoral ligament reconstruction using a quadriceps tendon graft. Arthrosc Tech. 2014;3(4):e445-e448.
28. Akşahin E, Yumrukçal F, Yüksel HY, Doğruyol D, Celebi L. Role of pathophysiology of patellofemoral instability in the treatment of spontaneous medial patellofemoral subluxation: a case report. J Med Case Rep. 2010;4:148.
1. Marumoto JM, Jordan C, Akins R. A biomechanical comparison of lateral retinacular releases. Am J Sports Med. 1995;23(2):151-155.
2. Betz RR, Magill JT, Lonergan RP. The percutaneous lateral retinacular release. Am J Sports Med. 1987;15(5):477-482.
3. Shannon BD, Keene JS. Results of arthroscopic medial retinacular release for treatment of medial subluxation of the patella. Am J Sports Med. 2007;35(7):1180-1187.
4. Saper MG, Shneider DA. Medial patellar subluxation without previous lateral release: a case report. J Pediatr Orthop B. 2014;23(4):350-353.
5. Richman NM, Scheller AD Jr. Medial subluxation of the patella without previous lateral retinacular release. Orthopedics. 1998;21(7):810-813.
6. Nonweiler DE, DeLee JC. The diagnosis and treatment of medial subluxation of the patella after lateral retinacular release. Am J Sports Med. 1994;22(5):680-686.
7. Hughston JC, Deese M. Medial subluxation of the patella as a complication of lateral retinacular release. Am J Sports Med. 1988;16(4):383-388.
8. Hughston JC, Flandry F, Brinker MR, Terry GC, Mills JC 3rd. Surgical correction of medial subluxation of the patella. Am J Sports Med. 1996;24(4):486-491.
9. Abhaykumar S, Craig DM. Fascia lata sling reconstruction for recurrent medial dislocation of the patella. The Knee. 1999;6(1):55-57.
10. Teitge RA, Torga Spak R. Lateral patellofemoral ligament reconstruction. Arthroscopy. 2004;20(9):998-1002.
11. Kusano M, Horibe S, Tanaka Y, et al. Simultaneous MPFL and LPFL reconstruction for recurrent lateral patellar dislocation with medial patellofemoral instability. Asia-Pac J Sports Med Arthrosc Rehabil Technol. 2014;1:42-46.
12. Saper MG, Shneider DA. Simultaneous medial and lateral patellofemoral ligament reconstruction for combined medial and lateral patellar subluxation. Arthrosc Tech. 2014,3(2):e227-e231.
13. Udagawa K, Niki Y, Matsumoto H, et al. Lateral patellar retinaculum reconstruction for medial patellar instability following lateral retinacular release: a case report. Knee. 2014;21(1):336-339.
14. Sanchis-Alfonso V, Montesinos-Berry E, Monllau JC, Merchant AC. Results of isolated lateral retinacular reconstruction for iatrogenic medial patellar instability. Arthroscopy. 2015;31(3):422-427.
15. Borbas P, Koch PP, Fucentese SF. Lateral patellofemoral ligament reconstruction using a free gracilis autograft. Orthopedics. 2014;37(7):e665-e668.
16. Fulkerson JP, Gossling H. Anatomy of the knee joint lateral retinaculum. Clin Orthop Relat Res. 1980;153:183-188.
17. Kaplan E. Some aspects of functional anatomy of the human knee joint. Clin Orthop Relat Res. 1962;23:18-29.
18. Reider B, Marshall J, Koslin B, Ring B, Girgis F. The anterior aspect of the knee joint. J Bone Joint Surg Am. 1981;63(3):351-356.
19. Navarro MS, Navarro RD, Akita Junior J, Cohen M. Anatomical study of the lateral patellofemoral ligament in cadaver knees. Rev Bras Ortop. 2008;43(7):300-307.
20. Navarro MS, Beltrani Filho CA, Akita Junior J, Navarro RD, Cohen M. Relationship between the lateral patellofemoral ligament and the width of the lateral patellar facet. Acta Ortop Bras. 2010;18(1):19-22.
21. Salsich GB, Ward SR, Terk MR, Powers CM. In vivo assessment of patellofemoral joint contact area in individuals who are pain free. Clin Orthop Relat Res. 2003;417:277-284.
22. Merican AM, Kondo E, Amis AA. The effect on patellofemoral joint stability of selective cutting of lateral retinacular and capsular structures. J Biomech. 2009;42(3):291-296.
23. Vieira EL, Vieira EÁ, da Silva RT, Berlfein PA, Abdalla RJ, Cohen M. An anatomic study of the iliotibial tract. Arthroscopy. 2007;23(3):269-274.
24. Shneider DA. Lateral patellar laxity—identification, significance, treatment. Poster session presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; February 25-28, 2009; Las Vegas, NV.
25. Shellock FG, Mink JH, Deutsch A, Fox JM, Ferkel RD. Evaluation of patients with persistent symptoms after lateral retinacular release by kinematic magnetic resonance imaging of the patellofemoral joint. Arthroscopy. 1990;6(3):226-234.
26. Johnson DP, Wakeley C. Reconstruction of the lateral patellar retinaculum following lateral release: a case report. Knee Surg Sports Traumatol Arthrosc. 2002;10(6):361-363.
27. Saper MG, Shneider DA. Lateral patellofemoral ligament reconstruction using a quadriceps tendon graft. Arthrosc Tech. 2014;3(4):e445-e448.
28. Akşahin E, Yumrukçal F, Yüksel HY, Doğruyol D, Celebi L. Role of pathophysiology of patellofemoral instability in the treatment of spontaneous medial patellofemoral subluxation: a case report. J Med Case Rep. 2010;4:148.
Biceps Tenodesis and Superior Labrum Anterior to Posterior (SLAP) Tears
Injuries of the superior labrum–biceps complex (SLBC) have been recognized as a cause of shoulder pain since they were first described by Andrews and colleagues1 in 1985. Superior labrum anterior to posterior (SLAP) tears are relatively uncommon injuries of the shoulder, and their true incidence is difficult to establish. However, recently there has been a significant increase in the reported incidence and operative treatment of SLAP tears.2 SLAP tears can occur in isolation, but they are commonly seen in association with other shoulder lesions, including rotator cuff tear, Bankart lesion, glenohumeral arthritis, acromioclavicular joint pathology, and subacromial impingement.
Although SLAP tears are well described and classified,3-6 our understanding of symptomatic SLAP tears and of their contribution to glenohumeral instability is limited. Diagnosing a SLAP tear on the basis of history and physical examination is a clinical challenge. Pain is the most common presentation of SLAP tears, though localization and characterization of pain are variable and nonspecific.7 The mechanism of injury is helpful in acute presentation (traction injury; fall on outstretched, abducted arm), but an overhead athlete may present with no distinct mechanism other than chronic, repetitive use of the shoulder.8-11 Numerous provocative physical examination tests have been used to assist in the diagnosis of SLAP tear, yet there is no consensus regarding the ideal physical examination test, with high sensitivity, specificity, and accuracy.12-14 Magnetic resonance arthrography, the gold standard imaging modality, is highly sensitive and specific (>95%) for diagnosing SLAP tears.
SLAP tear management is based on lesion type and severity, age, functional demands, and presence of coexisting intra-articular lesions. Management options include nonoperative treatment, débridement or repair of SLBC, biceps tenotomy, and biceps tenodesis.15-19
In this 5-point review, we present an evidence-based analysis of the role of the SLBC in glenohumeral stability and the role of biceps tenodesis in the management of SLAP tears.
1. Role of SLBC in stability of glenohumeral joint
The anatomy of the SLBC has been well described,20,21 and there is consensus that SLBC pathology can be a source of shoulder pain. The superior labrum is relatively more mobile than the rest of the glenoid labrum, and it provides attachment to the long head of the biceps tendon (LHBT) and the superior glenohumeral and middle glenohumeral ligaments.
The functional role of the SLBC in glenohumeral stability and its contribution to the pathogenesis of shoulder instability are not clearly defined. Our understanding of SLBC function is largely derived from simulated cadaveric experiments of SLAP tears. Controlled laboratory studies with simulated type II SLAP tears in cadavers have shown significantly increased glenohumeral translation in the anterior-posterior and superior-inferior directions, suggesting a role of the superior labrum in maintaining glenohumeral stability.22-26 Interestingly, there is conflicting evidence regarding restoration of normal glenohumeral translation in cadaveric shoulders after repair of simulated SLAP lesions in the presence or absence of simulated anterior capsular laxity.22,25-27 However, it is important to understand the limitations of cadaveric experiments in order to appreciate and truly comprehend the results of these experiments. There are inconsistencies in the size of simulated type II SLAP lesions in different studies, which can affect the degree of glenohumeral translation and the results of repair.23-25,28 The amount of glenohumeral translation noticed after simulated SLAP tears in cadavers, though statistically significant, is small in amplitude, and its relevance may not translate to a clinically significant level. The impact of dynamic components of stability (eg, rotator cuff muscles), capsular stretch, and other in vivo variables that affect glenohumeral stability are unaccounted for during cadaveric experiments.
LHBT is a recognized cause of shoulder pain, but its contribution to shoulder stability is a point of continued debate. According to one school of thought, LHBT is a vestigial structure that can be sacrificed without any loss of stability. Another school of thought holds that LHBT is an important active stabilizer of the glenohumeral joint. Cadaveric studies have demonstrated that loading the LHBT decreases glenohumeral translation and rotational range of motion, especially in lower and mid ranges of abduction.23,29,30 Furthermore, LHBT contributes to anterior glenohumeral stability by resisting torsional forces in the abducted and externally rotated shoulder and reducing stress on the inferior glenohumeral ligaments.31-33 Strauss and colleagues22 recently found that simulated anterior and posterior type II SLAP lesions in cadaveric shoulders increased glenohumeral translation in all planes, and biceps tenodesis did not further worsen this abnormal glenohumeral translation. Furthermore, repair of posterior SLAP lesions along with biceps tenodesis restored abnormal glenohumeral translation with no significant difference from the baseline in any plane of motion. Again, the limitations of cadaveric studies should be considered when interpreting these results and applying them clinically.
2. Biceps tenodesis as primary treatment for SLAP tears
A growing body of evidence suggests that primary tenodesis of LHBT may be an effective alternative treatment to SLAP repairs in select patients.34-36 However, the evidence is weak, and high-quality studies comparing SLAP repair and primary biceps tenodesis are required in order to make a strong recommendation for one technique over another. Gupta and colleagues35 retrospectively analyzed 28 cases of concomitant SLAP tear and biceps tendonitis treated with primary open subpectoral biceps tenodesis. There was significant improvement in patients’ functional outcome scores postoperatively [SANE (Single Assessment Numeric Evaluation), ASES (American Shoulder and Elbow Surgeons shoulder index), SST (Simple Shoulder Test), VAS (visual analog scale), and SF-12 (Short Form-12)]. In addition, 80% of patients were satisfied with their outcome. Mean age was 43.7 years. Forty-two percent of patients had a worker’s compensation claim. Interestingly, 15 patients in this cohort had a type I SLAP tear. Boileau and colleagues34 prospectively followed 25 cases of type II SLAP tear treated with either SLAP repair (10 patients; mean age, 37 years) or primary arthroscopic biceps tenodesis (15 patients; mean age, 52 years). Compared with the SLAP repair group, the biceps tenodesis group had significantly higher rates of satisfaction and return to previous level of sports participation. However, group assignments were nonrandomized, and the decision to treat a patient with SLAP repair versus biceps tenodesis was made by the senior surgeon purely on the basis of age (SLAP repair for patients under 30 years). Ek and colleagues36 retrospectively compared the cases of 10 patients who underwent SLAP repair (mean age, 32 years) and 15 who underwent biceps tenodesis (mean age, 47 years) for type II SLAP tear. There was no significant difference between the groups with respect to outcome scores, return to play or preinjury activity level, or complications.
There continues to be significant debate as to which patient will benefit from primary SLAP repair versus biceps tenodesis. Multiple factors are involved: age, presence of associated shoulder pathology, occupation, preinjury activity level, and worker’s compensation status. Age has convincingly been shown to affect the outcomes of treatment of type II SLAP tears.34,35,37-40 There is consensus that patients over age 40 years will benefit from primary biceps tenodesis for SLAP tears. However, the evidence for this recommendation is weak.
3. Biceps tenodesis and failed SLAP repair
The definition of a failed SLAP repair is not well documented in the literature, but dissatisfaction after SLAP repair can result from continued shoulder pain, poor shoulder function, or inability to return to preinjury functional level.15,41 The etiologic determination and treatment of a failed SLAP repair are challenging, and outcomes of revision SLAP repair are not very promising.42,43 Biceps tenodesis has been proposed as an alternative treatment to revision SLAP repair for failed SLAP repair. McCormick and colleagues41 prospectively evaluated 42 patients (mean age, 39.2 years; minimum follow-up, 2 years) with failed type II SLAP repairs that were treated with open subpectoral biceps tenodesis. There was significant improvement in ASES, SANE, and Western Ontario Shoulder Instability Index (WOSI) outcome scores and in postoperative shoulder range of motion at a mean follow-up of 3.6 years. One patient had transient musculocutaneous neurapraxia after surgery. In a retrospective cohort study, Gupta and colleagues44 found significant improvement in ASES, SANE, SST, SF-12, and VAS outcome scores in 11 patients who underwent open subpectoral biceps tenodesis for failed arthroscopic SLAP repair (mean age at surgery, 40 years; mean follow-up, 26 months). Three of the 11 patients had worker’s compensation claims, and there were no complications and no revision surgeries required after biceps tenodesis. Werner and colleagues16 retrospectively evaluated 17 patients who underwent biceps tenodesis for failed SLAP repair (mean age, 39 years; minimum follow-up, 2 years). Twenty-nine percent of patients had worker’s compensation claims. Compared with the contralateral shoulder, the treated shoulder had better postoperative ASES, SANE, SST, and Veteran RAND 36-item health survey outcome scores; range of motion was near normal.
There are no high-quality studies comparing revision SLAP repair and biceps tenodesis in the management of failed SLAP repair.16,41-44 Case series studies have found improved outcomes and pain relief after biceps tenodesis for failed SLAP repair, but the quality of evidence has been poor (level IV evidence).16,41-44 The senior author recommends treating failed SLAP repairs with biceps tenodesis.
4. Biceps tenodesis as treatment option for SLAP tear in overhead throwing athletes
Biceps tenodesis is a potential alternative treatment to SLAP repair in overhead throwing athletes. Although outcome scores and satisfaction rates after SLAP repair are high in overhead athletes, the rates of return to sport are relatively low, especially in baseball players.38,45-47 In a level III cohort study, Boileau and colleagues34 found that 13 (87%) of 15 patients with type II SLAP tears, including 8 overhead athletes, had returned to their previous level of activity by a mean of 30 months after biceps tenodesis. In contrast, only 2 of 10 patients returned to their previous level of activity after SLAP repair. Interestingly, 3 patients who underwent biceps tenodesis for failed SLAP repair returned to overhead sports. Schöffl and colleagues48 reported on the outcomes of biceps tenodesis for SLAP lesions in 6 high-level rock climbers. By a mean follow-up of 6 months, all 6 patients had returned to their previous level of climbing. Their satisfaction rate was 96.8%. Gupta and colleagues35 reported on a cohort of 28 patients who underwent biceps tenodesis for SLAP tears and concomitant biceps tendonitis. Of the 8 athletes in the group, 5 were able to return to their previous level of play, and 1 was able to return to a lower level of sporting activity. There was significant improvement from preoperative to postoperative scores on ASES, SST, SANE, VAS, SF-12 overall, and SF-12 components.
Chalmers and colleagues49 recently described motion analyses with simultaneous surface electromyographic measurements in 18 baseball pitchers. Of these 18 players, 7 were uninjured (controls), 6 were pitching after SLAP repair, and 5 were pitching after subpectoral biceps tenodesis. There were no significant differences between controls and postoperative patients with respect to pitching kinematics. Interestingly, compared with the controls and the patients who underwent open biceps tenodesis, the patients who underwent SLAP repair had altered patterns of thoracic rotation during pitching. However, the clinical significance of this finding and the impact of this finding on pitching efficacy are not currently known.
Biceps tenodesis as a primary procedure for type II SLAP lesion in an overhead athlete is a concept in evolution. Increasing evidence suggests a role for primary biceps tenodesis in an overhead athlete with type II SLAP lesion and concomitant biceps pathology. However, this evidence is of poor quality, and the strength of the recommendation is weak. Still to be determined is whether return to preinjury performance level is better with primary biceps tenodesis or with SLAP repair in overhead athletes with type II SLAP lesion. As per the senior author’s treatment algorithm, we prefer SLAP repair for overhead athletes with type II SLAP tears and reserve biceps tenodesis for cases involving significant biceps pathology and/or clinical symptoms involving the bicipital groove consistent with extra-articular biceps pain.
5. Biceps tenodesis for type II SLAP tear in contact athletes and occupations demanding heavy labor (blue-collar jobs)
SLAP tears are less common in contact athletes, and there is general agreement that SLAP repair outcomes are better in contact athletes than in overhead athletes. In a retrospective review of 18 rugby players with SLAP tears, Funk and Snow50 reported excellent results and quicker return to sport after SLAP repair. Patients with isolated SLAP tears had the earliest return to play. Enad and colleagues51 reported SLAP repair outcomes in an active military population. SLAP tears are more common in the military versus the general population because of the unique physical demands placed on military personnel. The authors retrospectively reviewed 27 cases of type II SLAP tears treated with SLAP repair and suture anchors. Outcomes were measured at a mean of 30.5 months after surgery. Twenty-four (89%) of the 27 patients had good to excellent results, and 94% had returned to active duty by a mean of 4.4 months after SLAP repair.
Given the poor-quality evidence in the literature, we believe that biceps tenodesis should be reserved for revision surgery in contact athletes. There is insufficient evidence to recommend biceps tenodesis as primary treatment for type II SLAP tears in contact athletes. SLAP repair should be performed for primary SLAP lesions in contact athletes and for patients in physically demanding professions (eg, military, laborer, weightlifter).
Conclusion
SLAP tears can result in persistent shoulder pain and dysfunction. SLAP tear management depends on lesion type and severity, age, and functional demands. SLAP repair is the treatment of choice for type II SLAP lesions in young, active patients. Biceps tenodesis is a preferred alternative to SLAP repair in failed SLAP repair and in type II SLAP patients who are older than 40 years and who are less active and have a worker’s compensation claim. These recommendations are based on poor-quality evidence. There is an unmet need for randomized clinical studies comparing SLAP repair with biceps tenodesis for type II SLAP tears in different patient populations so as to optimize the current decision-making algorithm for SLAP tears.
1. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
2. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopaedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
3. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
4. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
5. Powell SE, Nord KD, Ryu RKN. The diagnosis, classification, and treatment of SLAP lesions. Oper Tech Sports Med. 2012;20(1):46-56.
6. Maffet MW, Gartsman GM, Moseley B. Superior labrum-biceps tendon complex lesions of the shoulder. Am J Sports Med. 1995;23(1):93-98.
7. Kim TK, Queale WS, Cosgarea AJ, McFarland EG. Clinical features of the different types of SLAP lesions: an analysis of one hundred and thirty-nine cases. J Bone Joint Surg Am. 2003;85(1):66-71.
8. Abrams GD, Safran MR. Diagnosis and management of superior labrum anterior posterior lesions in overhead athletes. Br J Sports Med. 2010;44(5):311-318.
9. Keener JD, Brophy RH. Superior labral tears of the shoulder: pathogenesis, evaluation, and treatment. J Am Acad Orthop Surg. 2009;17(10):627-637.
10. Abrams GD, Hussey KE, Harris JD, Cole BJ. Clinical results of combined meniscus and femoral osteochondral allograft transplantation: minimum 2-year follow-up. Arthroscopy. 2014;30(8):964-970.e1.
11. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
12. Virk MS, Arciero RA. Superior labrum anterior to posterior tears and glenohumeral instability. Instr Course Lect. 2013;62:501-514.
13. Calvert E, Chambers GK, Regan W, Hawkins RH, Leith JM. Special physical examination tests for superior labrum anterior posterior shoulder tears are clinically limited and invalid: a diagnostic systematic review. J Clin Epidemiol. 2009;62(5):558-563.
14. Jones GL, Galluch DB. Clinical assessment of superior glenoid labral lesions: a systematic review. Clin Orthop Relat Res. 2007;455:45-51.
15. Werner BC, Brockmeier SF, Miller MD. Etiology, diagnosis, and management of failed SLAP repair. J Am Acad Orthop Surg. 2014;22(9):554-565.
16. Werner BC, Pehlivan HC, Hart JM, et al. Biceps tenodesis is a viable option for salvage of failed SLAP repair. J Shoulder Elbow Surg. 2014;23(8):e179-e184.
17. Erickson J, Lavery K, Monica J, Gatt C, Dhawan A. Surgical treatment of symptomatic superior labrum anterior-posterior tears in patients older than 40 years: a systematic review. Am J Sports Med. 2015;43(5):1274-1282.
18. Huri G, Hyun YS, Garbis NG, McFarland EG. Treatment of superior labrum anterior posterior lesions: a literature review. Acta Orthop Traumatol Turc. 2014;48(3):290-297.
19. Li X, Lin TJ, Jager M, et al. Management of type II superior labrum anterior posterior lesions: a review of the literature. Orthop Rev. 2010;2(1):e6.
20. Cooper DE, Arnoczky SP, O’Brien SJ, Warren RF, DiCarlo E, Allen AA. Anatomy, histology, and vascularity of the glenoid labrum. An anatomical study. J Bone Joint Surg Am. 1992;74(1):46-52.
21. Vangsness CT, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
22. Strauss EJ, Salata MJ, Sershon RA, et al. Role of the superior labrum after biceps tenodesis in glenohumeral stability. J Shoulder Elbow Surg. 2014;23(4):485-491.
23. Pagnani MJ, Deng XH, Warren RF, Torzilli PA, Altchek DW. Effect of lesions of the superior portion of the glenoid labrum on glenohumeral translation. J Bone Joint Surg Am. 1995;77(7):1003-1010.
24. McMahon PJ, Burkart A, Musahl V, Debski RE. Glenohumeral translations are increased after a type II superior labrum anterior-posterior lesion: a cadaveric study of severity of passive stabilizer injury. J Shoulder Elbow Surg. 2004;13(1):39-44.
25. Burkart A, Debski R, Musahl V, McMahon P, Woo SL. Biomechanical tests for type II SLAP lesions of the shoulder joint before and after arthroscopic repair [in German]. Orthopade. 2003;32(7):600-607.
26. Panossian VR, Mihata T, Tibone JE, Fitzpatrick MJ, McGarry MH, Lee TQ. Biomechanical analysis of isolated type II SLAP lesions and repair. J Shoulder Elbow Surg. 2005;14(5):529-534.
27. Mihata T, McGarry MH, Tibone JE, Fitzpatrick MJ, Kinoshita M, Lee TQ. Biomechanical assessment of type II superior labral anterior-posterior (SLAP) lesions associated with anterior shoulder capsular laxity as seen in throwers: a cadaveric study. Am J Sports Med. 2008;36(8):1604-1610.
28. Youm T, Tibone JE, ElAttrache NS, McGarry MH, Lee TQ. Simulated type II superior labral anterior posterior lesions do not alter the path of glenohumeral articulation: a cadaveric biomechanical study. Am J Sports Med. 2008;36(4):767-774.
29. Youm T, ElAttrache NS, Tibone JE, McGarry MH, Lee TQ. The effect of the long head of the biceps on glenohumeral kinematics. J Shoulder Elbow Surg. 2009;18(1):122-129.
30. McGarry MH, Nguyen ML, Quigley RJ, Hanypsiak B, Gupta R, Lee TQ. The effect of long and short head biceps loading on glenohumeral joint rotational range of motion and humeral head position [published online ahead of print September 26, 2014]. Knee Surg Sports Traumatol Arthrosc.
31. Glousman R, Jobe F, Tibone J, Moynes D, Antonelli D, Perry J. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am. 1988;70(2):220-226.
32. Gowan ID, Jobe FW, Tibone JE, Perry J, Moynes DR. A comparative electromyographic analysis of the shoulder during pitching. Professional versus amateur pitchers. Am J Sports Med. 1987;15(6):586-590.
33. Rodosky MW, Harner CD, Fu FH. The role of the long head of the biceps muscle and superior glenoid labrum in anterior stability of the shoulder. Am J Sports Med. 1994;22(1):121-130.
34. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
35. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
36. Ek ET, Shi LL, Tompson JD, Freehill MT, Warner JJ. Surgical treatment of isolated type II superior labrum anterior-posterior (SLAP) lesions: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2014;23(7):1059-1065.
37. Alpert JM, Wuerz TH, O’Donnell TF, Carroll KM, Brucker NN, Gill TJ. The effect of age on the outcomes of arthroscopic repair of type II superior labral anterior and posterior lesions. Am J Sports Med. 2010;38(11):2299-2303.
38. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
39. Denard PJ, Lädermann A, Burkhart SS. Long-term outcome after arthroscopic repair of type II SLAP lesions: results according to age and workers’ compensation status. Arthroscopy. 2012;28(4):451-457.
40. Burns JP, Bahk M, Snyder SJ. Superior labral tears: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2011;20(2 suppl):S2-S8.
41. McCormick F, Nwachukwu BU, Solomon D, et al. The efficacy of biceps tenodesis in the treatment of failed superior labral anterior posterior repairs. Am J Sports Med. 2014;42(4):820-825.
42. Katz LM, Hsu S, Miller SL, et al. Poor outcomes after SLAP repair: descriptive analysis and prognosis. Arthroscopy. 2009;25(8):849-855.
43. Park S, Glousman RE. Outcomes of revision arthroscopic type II superior labral anterior posterior repairs. Am J Sports Med. 2011;39(6):1290-1294.
44. Gupta AK, Bruce B, Klosterman EL, McCormick F, Harris J, Romeo AA. Subpectoral biceps tenodesis for failed type II SLAP repair. Orthopedics. 2013;36(6):e723-e728.
45. Neuman BJ, Boisvert CB, Reiter B, Lawson K, Ciccotti MG, Cohen SB. Results of arthroscopic repair of type II superior labral anterior posterior lesions in overhead athletes: assessment of return to preinjury playing level and satisfaction. Am J Sports Med. 2011;39(9):1883-1888.
46. Fedoriw WW, Ramkumar P, McCulloch PC, Lintner DM. Return to play after treatment of superior labral tears in professional baseball players. Am J Sports Med. 2014;42(5):1155-1160.
47. Park JY, Chung SW, Jeon SH, Lee JG, Oh KS. Clinical and radiological outcomes of type 2 superior labral anterior posterior repairs in elite overhead athletes. Am J Sports Med. 2013;41(6):1372-1379.
48. Schöffl V, Popp D, Dickschass J, Küpper T. Superior labral anterior-posterior lesions in rock climbers—primary double tenodesis? Clin J Sport Med. 2011;21(3):261-263.
49. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
50. Funk L, Snow M. SLAP tears of the glenoid labrum in contact athletes. Clin J Sport Med. 2007;17(1):1-4.
51. Enad JG, Gaines RJ, White SM, Kurtz CA. Arthroscopic superior labrum anterior-posterior repair in military patients. J Shoulder Elbow Surg. 2007;16(3):300-305.
Injuries of the superior labrum–biceps complex (SLBC) have been recognized as a cause of shoulder pain since they were first described by Andrews and colleagues1 in 1985. Superior labrum anterior to posterior (SLAP) tears are relatively uncommon injuries of the shoulder, and their true incidence is difficult to establish. However, recently there has been a significant increase in the reported incidence and operative treatment of SLAP tears.2 SLAP tears can occur in isolation, but they are commonly seen in association with other shoulder lesions, including rotator cuff tear, Bankart lesion, glenohumeral arthritis, acromioclavicular joint pathology, and subacromial impingement.
Although SLAP tears are well described and classified,3-6 our understanding of symptomatic SLAP tears and of their contribution to glenohumeral instability is limited. Diagnosing a SLAP tear on the basis of history and physical examination is a clinical challenge. Pain is the most common presentation of SLAP tears, though localization and characterization of pain are variable and nonspecific.7 The mechanism of injury is helpful in acute presentation (traction injury; fall on outstretched, abducted arm), but an overhead athlete may present with no distinct mechanism other than chronic, repetitive use of the shoulder.8-11 Numerous provocative physical examination tests have been used to assist in the diagnosis of SLAP tear, yet there is no consensus regarding the ideal physical examination test, with high sensitivity, specificity, and accuracy.12-14 Magnetic resonance arthrography, the gold standard imaging modality, is highly sensitive and specific (>95%) for diagnosing SLAP tears.
SLAP tear management is based on lesion type and severity, age, functional demands, and presence of coexisting intra-articular lesions. Management options include nonoperative treatment, débridement or repair of SLBC, biceps tenotomy, and biceps tenodesis.15-19
In this 5-point review, we present an evidence-based analysis of the role of the SLBC in glenohumeral stability and the role of biceps tenodesis in the management of SLAP tears.
1. Role of SLBC in stability of glenohumeral joint
The anatomy of the SLBC has been well described,20,21 and there is consensus that SLBC pathology can be a source of shoulder pain. The superior labrum is relatively more mobile than the rest of the glenoid labrum, and it provides attachment to the long head of the biceps tendon (LHBT) and the superior glenohumeral and middle glenohumeral ligaments.
The functional role of the SLBC in glenohumeral stability and its contribution to the pathogenesis of shoulder instability are not clearly defined. Our understanding of SLBC function is largely derived from simulated cadaveric experiments of SLAP tears. Controlled laboratory studies with simulated type II SLAP tears in cadavers have shown significantly increased glenohumeral translation in the anterior-posterior and superior-inferior directions, suggesting a role of the superior labrum in maintaining glenohumeral stability.22-26 Interestingly, there is conflicting evidence regarding restoration of normal glenohumeral translation in cadaveric shoulders after repair of simulated SLAP lesions in the presence or absence of simulated anterior capsular laxity.22,25-27 However, it is important to understand the limitations of cadaveric experiments in order to appreciate and truly comprehend the results of these experiments. There are inconsistencies in the size of simulated type II SLAP lesions in different studies, which can affect the degree of glenohumeral translation and the results of repair.23-25,28 The amount of glenohumeral translation noticed after simulated SLAP tears in cadavers, though statistically significant, is small in amplitude, and its relevance may not translate to a clinically significant level. The impact of dynamic components of stability (eg, rotator cuff muscles), capsular stretch, and other in vivo variables that affect glenohumeral stability are unaccounted for during cadaveric experiments.
LHBT is a recognized cause of shoulder pain, but its contribution to shoulder stability is a point of continued debate. According to one school of thought, LHBT is a vestigial structure that can be sacrificed without any loss of stability. Another school of thought holds that LHBT is an important active stabilizer of the glenohumeral joint. Cadaveric studies have demonstrated that loading the LHBT decreases glenohumeral translation and rotational range of motion, especially in lower and mid ranges of abduction.23,29,30 Furthermore, LHBT contributes to anterior glenohumeral stability by resisting torsional forces in the abducted and externally rotated shoulder and reducing stress on the inferior glenohumeral ligaments.31-33 Strauss and colleagues22 recently found that simulated anterior and posterior type II SLAP lesions in cadaveric shoulders increased glenohumeral translation in all planes, and biceps tenodesis did not further worsen this abnormal glenohumeral translation. Furthermore, repair of posterior SLAP lesions along with biceps tenodesis restored abnormal glenohumeral translation with no significant difference from the baseline in any plane of motion. Again, the limitations of cadaveric studies should be considered when interpreting these results and applying them clinically.
2. Biceps tenodesis as primary treatment for SLAP tears
A growing body of evidence suggests that primary tenodesis of LHBT may be an effective alternative treatment to SLAP repairs in select patients.34-36 However, the evidence is weak, and high-quality studies comparing SLAP repair and primary biceps tenodesis are required in order to make a strong recommendation for one technique over another. Gupta and colleagues35 retrospectively analyzed 28 cases of concomitant SLAP tear and biceps tendonitis treated with primary open subpectoral biceps tenodesis. There was significant improvement in patients’ functional outcome scores postoperatively [SANE (Single Assessment Numeric Evaluation), ASES (American Shoulder and Elbow Surgeons shoulder index), SST (Simple Shoulder Test), VAS (visual analog scale), and SF-12 (Short Form-12)]. In addition, 80% of patients were satisfied with their outcome. Mean age was 43.7 years. Forty-two percent of patients had a worker’s compensation claim. Interestingly, 15 patients in this cohort had a type I SLAP tear. Boileau and colleagues34 prospectively followed 25 cases of type II SLAP tear treated with either SLAP repair (10 patients; mean age, 37 years) or primary arthroscopic biceps tenodesis (15 patients; mean age, 52 years). Compared with the SLAP repair group, the biceps tenodesis group had significantly higher rates of satisfaction and return to previous level of sports participation. However, group assignments were nonrandomized, and the decision to treat a patient with SLAP repair versus biceps tenodesis was made by the senior surgeon purely on the basis of age (SLAP repair for patients under 30 years). Ek and colleagues36 retrospectively compared the cases of 10 patients who underwent SLAP repair (mean age, 32 years) and 15 who underwent biceps tenodesis (mean age, 47 years) for type II SLAP tear. There was no significant difference between the groups with respect to outcome scores, return to play or preinjury activity level, or complications.
There continues to be significant debate as to which patient will benefit from primary SLAP repair versus biceps tenodesis. Multiple factors are involved: age, presence of associated shoulder pathology, occupation, preinjury activity level, and worker’s compensation status. Age has convincingly been shown to affect the outcomes of treatment of type II SLAP tears.34,35,37-40 There is consensus that patients over age 40 years will benefit from primary biceps tenodesis for SLAP tears. However, the evidence for this recommendation is weak.
3. Biceps tenodesis and failed SLAP repair
The definition of a failed SLAP repair is not well documented in the literature, but dissatisfaction after SLAP repair can result from continued shoulder pain, poor shoulder function, or inability to return to preinjury functional level.15,41 The etiologic determination and treatment of a failed SLAP repair are challenging, and outcomes of revision SLAP repair are not very promising.42,43 Biceps tenodesis has been proposed as an alternative treatment to revision SLAP repair for failed SLAP repair. McCormick and colleagues41 prospectively evaluated 42 patients (mean age, 39.2 years; minimum follow-up, 2 years) with failed type II SLAP repairs that were treated with open subpectoral biceps tenodesis. There was significant improvement in ASES, SANE, and Western Ontario Shoulder Instability Index (WOSI) outcome scores and in postoperative shoulder range of motion at a mean follow-up of 3.6 years. One patient had transient musculocutaneous neurapraxia after surgery. In a retrospective cohort study, Gupta and colleagues44 found significant improvement in ASES, SANE, SST, SF-12, and VAS outcome scores in 11 patients who underwent open subpectoral biceps tenodesis for failed arthroscopic SLAP repair (mean age at surgery, 40 years; mean follow-up, 26 months). Three of the 11 patients had worker’s compensation claims, and there were no complications and no revision surgeries required after biceps tenodesis. Werner and colleagues16 retrospectively evaluated 17 patients who underwent biceps tenodesis for failed SLAP repair (mean age, 39 years; minimum follow-up, 2 years). Twenty-nine percent of patients had worker’s compensation claims. Compared with the contralateral shoulder, the treated shoulder had better postoperative ASES, SANE, SST, and Veteran RAND 36-item health survey outcome scores; range of motion was near normal.
There are no high-quality studies comparing revision SLAP repair and biceps tenodesis in the management of failed SLAP repair.16,41-44 Case series studies have found improved outcomes and pain relief after biceps tenodesis for failed SLAP repair, but the quality of evidence has been poor (level IV evidence).16,41-44 The senior author recommends treating failed SLAP repairs with biceps tenodesis.
4. Biceps tenodesis as treatment option for SLAP tear in overhead throwing athletes
Biceps tenodesis is a potential alternative treatment to SLAP repair in overhead throwing athletes. Although outcome scores and satisfaction rates after SLAP repair are high in overhead athletes, the rates of return to sport are relatively low, especially in baseball players.38,45-47 In a level III cohort study, Boileau and colleagues34 found that 13 (87%) of 15 patients with type II SLAP tears, including 8 overhead athletes, had returned to their previous level of activity by a mean of 30 months after biceps tenodesis. In contrast, only 2 of 10 patients returned to their previous level of activity after SLAP repair. Interestingly, 3 patients who underwent biceps tenodesis for failed SLAP repair returned to overhead sports. Schöffl and colleagues48 reported on the outcomes of biceps tenodesis for SLAP lesions in 6 high-level rock climbers. By a mean follow-up of 6 months, all 6 patients had returned to their previous level of climbing. Their satisfaction rate was 96.8%. Gupta and colleagues35 reported on a cohort of 28 patients who underwent biceps tenodesis for SLAP tears and concomitant biceps tendonitis. Of the 8 athletes in the group, 5 were able to return to their previous level of play, and 1 was able to return to a lower level of sporting activity. There was significant improvement from preoperative to postoperative scores on ASES, SST, SANE, VAS, SF-12 overall, and SF-12 components.
Chalmers and colleagues49 recently described motion analyses with simultaneous surface electromyographic measurements in 18 baseball pitchers. Of these 18 players, 7 were uninjured (controls), 6 were pitching after SLAP repair, and 5 were pitching after subpectoral biceps tenodesis. There were no significant differences between controls and postoperative patients with respect to pitching kinematics. Interestingly, compared with the controls and the patients who underwent open biceps tenodesis, the patients who underwent SLAP repair had altered patterns of thoracic rotation during pitching. However, the clinical significance of this finding and the impact of this finding on pitching efficacy are not currently known.
Biceps tenodesis as a primary procedure for type II SLAP lesion in an overhead athlete is a concept in evolution. Increasing evidence suggests a role for primary biceps tenodesis in an overhead athlete with type II SLAP lesion and concomitant biceps pathology. However, this evidence is of poor quality, and the strength of the recommendation is weak. Still to be determined is whether return to preinjury performance level is better with primary biceps tenodesis or with SLAP repair in overhead athletes with type II SLAP lesion. As per the senior author’s treatment algorithm, we prefer SLAP repair for overhead athletes with type II SLAP tears and reserve biceps tenodesis for cases involving significant biceps pathology and/or clinical symptoms involving the bicipital groove consistent with extra-articular biceps pain.
5. Biceps tenodesis for type II SLAP tear in contact athletes and occupations demanding heavy labor (blue-collar jobs)
SLAP tears are less common in contact athletes, and there is general agreement that SLAP repair outcomes are better in contact athletes than in overhead athletes. In a retrospective review of 18 rugby players with SLAP tears, Funk and Snow50 reported excellent results and quicker return to sport after SLAP repair. Patients with isolated SLAP tears had the earliest return to play. Enad and colleagues51 reported SLAP repair outcomes in an active military population. SLAP tears are more common in the military versus the general population because of the unique physical demands placed on military personnel. The authors retrospectively reviewed 27 cases of type II SLAP tears treated with SLAP repair and suture anchors. Outcomes were measured at a mean of 30.5 months after surgery. Twenty-four (89%) of the 27 patients had good to excellent results, and 94% had returned to active duty by a mean of 4.4 months after SLAP repair.
Given the poor-quality evidence in the literature, we believe that biceps tenodesis should be reserved for revision surgery in contact athletes. There is insufficient evidence to recommend biceps tenodesis as primary treatment for type II SLAP tears in contact athletes. SLAP repair should be performed for primary SLAP lesions in contact athletes and for patients in physically demanding professions (eg, military, laborer, weightlifter).
Conclusion
SLAP tears can result in persistent shoulder pain and dysfunction. SLAP tear management depends on lesion type and severity, age, and functional demands. SLAP repair is the treatment of choice for type II SLAP lesions in young, active patients. Biceps tenodesis is a preferred alternative to SLAP repair in failed SLAP repair and in type II SLAP patients who are older than 40 years and who are less active and have a worker’s compensation claim. These recommendations are based on poor-quality evidence. There is an unmet need for randomized clinical studies comparing SLAP repair with biceps tenodesis for type II SLAP tears in different patient populations so as to optimize the current decision-making algorithm for SLAP tears.
Injuries of the superior labrum–biceps complex (SLBC) have been recognized as a cause of shoulder pain since they were first described by Andrews and colleagues1 in 1985. Superior labrum anterior to posterior (SLAP) tears are relatively uncommon injuries of the shoulder, and their true incidence is difficult to establish. However, recently there has been a significant increase in the reported incidence and operative treatment of SLAP tears.2 SLAP tears can occur in isolation, but they are commonly seen in association with other shoulder lesions, including rotator cuff tear, Bankart lesion, glenohumeral arthritis, acromioclavicular joint pathology, and subacromial impingement.
Although SLAP tears are well described and classified,3-6 our understanding of symptomatic SLAP tears and of their contribution to glenohumeral instability is limited. Diagnosing a SLAP tear on the basis of history and physical examination is a clinical challenge. Pain is the most common presentation of SLAP tears, though localization and characterization of pain are variable and nonspecific.7 The mechanism of injury is helpful in acute presentation (traction injury; fall on outstretched, abducted arm), but an overhead athlete may present with no distinct mechanism other than chronic, repetitive use of the shoulder.8-11 Numerous provocative physical examination tests have been used to assist in the diagnosis of SLAP tear, yet there is no consensus regarding the ideal physical examination test, with high sensitivity, specificity, and accuracy.12-14 Magnetic resonance arthrography, the gold standard imaging modality, is highly sensitive and specific (>95%) for diagnosing SLAP tears.
SLAP tear management is based on lesion type and severity, age, functional demands, and presence of coexisting intra-articular lesions. Management options include nonoperative treatment, débridement or repair of SLBC, biceps tenotomy, and biceps tenodesis.15-19
In this 5-point review, we present an evidence-based analysis of the role of the SLBC in glenohumeral stability and the role of biceps tenodesis in the management of SLAP tears.
1. Role of SLBC in stability of glenohumeral joint
The anatomy of the SLBC has been well described,20,21 and there is consensus that SLBC pathology can be a source of shoulder pain. The superior labrum is relatively more mobile than the rest of the glenoid labrum, and it provides attachment to the long head of the biceps tendon (LHBT) and the superior glenohumeral and middle glenohumeral ligaments.
The functional role of the SLBC in glenohumeral stability and its contribution to the pathogenesis of shoulder instability are not clearly defined. Our understanding of SLBC function is largely derived from simulated cadaveric experiments of SLAP tears. Controlled laboratory studies with simulated type II SLAP tears in cadavers have shown significantly increased glenohumeral translation in the anterior-posterior and superior-inferior directions, suggesting a role of the superior labrum in maintaining glenohumeral stability.22-26 Interestingly, there is conflicting evidence regarding restoration of normal glenohumeral translation in cadaveric shoulders after repair of simulated SLAP lesions in the presence or absence of simulated anterior capsular laxity.22,25-27 However, it is important to understand the limitations of cadaveric experiments in order to appreciate and truly comprehend the results of these experiments. There are inconsistencies in the size of simulated type II SLAP lesions in different studies, which can affect the degree of glenohumeral translation and the results of repair.23-25,28 The amount of glenohumeral translation noticed after simulated SLAP tears in cadavers, though statistically significant, is small in amplitude, and its relevance may not translate to a clinically significant level. The impact of dynamic components of stability (eg, rotator cuff muscles), capsular stretch, and other in vivo variables that affect glenohumeral stability are unaccounted for during cadaveric experiments.
LHBT is a recognized cause of shoulder pain, but its contribution to shoulder stability is a point of continued debate. According to one school of thought, LHBT is a vestigial structure that can be sacrificed without any loss of stability. Another school of thought holds that LHBT is an important active stabilizer of the glenohumeral joint. Cadaveric studies have demonstrated that loading the LHBT decreases glenohumeral translation and rotational range of motion, especially in lower and mid ranges of abduction.23,29,30 Furthermore, LHBT contributes to anterior glenohumeral stability by resisting torsional forces in the abducted and externally rotated shoulder and reducing stress on the inferior glenohumeral ligaments.31-33 Strauss and colleagues22 recently found that simulated anterior and posterior type II SLAP lesions in cadaveric shoulders increased glenohumeral translation in all planes, and biceps tenodesis did not further worsen this abnormal glenohumeral translation. Furthermore, repair of posterior SLAP lesions along with biceps tenodesis restored abnormal glenohumeral translation with no significant difference from the baseline in any plane of motion. Again, the limitations of cadaveric studies should be considered when interpreting these results and applying them clinically.
2. Biceps tenodesis as primary treatment for SLAP tears
A growing body of evidence suggests that primary tenodesis of LHBT may be an effective alternative treatment to SLAP repairs in select patients.34-36 However, the evidence is weak, and high-quality studies comparing SLAP repair and primary biceps tenodesis are required in order to make a strong recommendation for one technique over another. Gupta and colleagues35 retrospectively analyzed 28 cases of concomitant SLAP tear and biceps tendonitis treated with primary open subpectoral biceps tenodesis. There was significant improvement in patients’ functional outcome scores postoperatively [SANE (Single Assessment Numeric Evaluation), ASES (American Shoulder and Elbow Surgeons shoulder index), SST (Simple Shoulder Test), VAS (visual analog scale), and SF-12 (Short Form-12)]. In addition, 80% of patients were satisfied with their outcome. Mean age was 43.7 years. Forty-two percent of patients had a worker’s compensation claim. Interestingly, 15 patients in this cohort had a type I SLAP tear. Boileau and colleagues34 prospectively followed 25 cases of type II SLAP tear treated with either SLAP repair (10 patients; mean age, 37 years) or primary arthroscopic biceps tenodesis (15 patients; mean age, 52 years). Compared with the SLAP repair group, the biceps tenodesis group had significantly higher rates of satisfaction and return to previous level of sports participation. However, group assignments were nonrandomized, and the decision to treat a patient with SLAP repair versus biceps tenodesis was made by the senior surgeon purely on the basis of age (SLAP repair for patients under 30 years). Ek and colleagues36 retrospectively compared the cases of 10 patients who underwent SLAP repair (mean age, 32 years) and 15 who underwent biceps tenodesis (mean age, 47 years) for type II SLAP tear. There was no significant difference between the groups with respect to outcome scores, return to play or preinjury activity level, or complications.
There continues to be significant debate as to which patient will benefit from primary SLAP repair versus biceps tenodesis. Multiple factors are involved: age, presence of associated shoulder pathology, occupation, preinjury activity level, and worker’s compensation status. Age has convincingly been shown to affect the outcomes of treatment of type II SLAP tears.34,35,37-40 There is consensus that patients over age 40 years will benefit from primary biceps tenodesis for SLAP tears. However, the evidence for this recommendation is weak.
3. Biceps tenodesis and failed SLAP repair
The definition of a failed SLAP repair is not well documented in the literature, but dissatisfaction after SLAP repair can result from continued shoulder pain, poor shoulder function, or inability to return to preinjury functional level.15,41 The etiologic determination and treatment of a failed SLAP repair are challenging, and outcomes of revision SLAP repair are not very promising.42,43 Biceps tenodesis has been proposed as an alternative treatment to revision SLAP repair for failed SLAP repair. McCormick and colleagues41 prospectively evaluated 42 patients (mean age, 39.2 years; minimum follow-up, 2 years) with failed type II SLAP repairs that were treated with open subpectoral biceps tenodesis. There was significant improvement in ASES, SANE, and Western Ontario Shoulder Instability Index (WOSI) outcome scores and in postoperative shoulder range of motion at a mean follow-up of 3.6 years. One patient had transient musculocutaneous neurapraxia after surgery. In a retrospective cohort study, Gupta and colleagues44 found significant improvement in ASES, SANE, SST, SF-12, and VAS outcome scores in 11 patients who underwent open subpectoral biceps tenodesis for failed arthroscopic SLAP repair (mean age at surgery, 40 years; mean follow-up, 26 months). Three of the 11 patients had worker’s compensation claims, and there were no complications and no revision surgeries required after biceps tenodesis. Werner and colleagues16 retrospectively evaluated 17 patients who underwent biceps tenodesis for failed SLAP repair (mean age, 39 years; minimum follow-up, 2 years). Twenty-nine percent of patients had worker’s compensation claims. Compared with the contralateral shoulder, the treated shoulder had better postoperative ASES, SANE, SST, and Veteran RAND 36-item health survey outcome scores; range of motion was near normal.
There are no high-quality studies comparing revision SLAP repair and biceps tenodesis in the management of failed SLAP repair.16,41-44 Case series studies have found improved outcomes and pain relief after biceps tenodesis for failed SLAP repair, but the quality of evidence has been poor (level IV evidence).16,41-44 The senior author recommends treating failed SLAP repairs with biceps tenodesis.
4. Biceps tenodesis as treatment option for SLAP tear in overhead throwing athletes
Biceps tenodesis is a potential alternative treatment to SLAP repair in overhead throwing athletes. Although outcome scores and satisfaction rates after SLAP repair are high in overhead athletes, the rates of return to sport are relatively low, especially in baseball players.38,45-47 In a level III cohort study, Boileau and colleagues34 found that 13 (87%) of 15 patients with type II SLAP tears, including 8 overhead athletes, had returned to their previous level of activity by a mean of 30 months after biceps tenodesis. In contrast, only 2 of 10 patients returned to their previous level of activity after SLAP repair. Interestingly, 3 patients who underwent biceps tenodesis for failed SLAP repair returned to overhead sports. Schöffl and colleagues48 reported on the outcomes of biceps tenodesis for SLAP lesions in 6 high-level rock climbers. By a mean follow-up of 6 months, all 6 patients had returned to their previous level of climbing. Their satisfaction rate was 96.8%. Gupta and colleagues35 reported on a cohort of 28 patients who underwent biceps tenodesis for SLAP tears and concomitant biceps tendonitis. Of the 8 athletes in the group, 5 were able to return to their previous level of play, and 1 was able to return to a lower level of sporting activity. There was significant improvement from preoperative to postoperative scores on ASES, SST, SANE, VAS, SF-12 overall, and SF-12 components.
Chalmers and colleagues49 recently described motion analyses with simultaneous surface electromyographic measurements in 18 baseball pitchers. Of these 18 players, 7 were uninjured (controls), 6 were pitching after SLAP repair, and 5 were pitching after subpectoral biceps tenodesis. There were no significant differences between controls and postoperative patients with respect to pitching kinematics. Interestingly, compared with the controls and the patients who underwent open biceps tenodesis, the patients who underwent SLAP repair had altered patterns of thoracic rotation during pitching. However, the clinical significance of this finding and the impact of this finding on pitching efficacy are not currently known.
Biceps tenodesis as a primary procedure for type II SLAP lesion in an overhead athlete is a concept in evolution. Increasing evidence suggests a role for primary biceps tenodesis in an overhead athlete with type II SLAP lesion and concomitant biceps pathology. However, this evidence is of poor quality, and the strength of the recommendation is weak. Still to be determined is whether return to preinjury performance level is better with primary biceps tenodesis or with SLAP repair in overhead athletes with type II SLAP lesion. As per the senior author’s treatment algorithm, we prefer SLAP repair for overhead athletes with type II SLAP tears and reserve biceps tenodesis for cases involving significant biceps pathology and/or clinical symptoms involving the bicipital groove consistent with extra-articular biceps pain.
5. Biceps tenodesis for type II SLAP tear in contact athletes and occupations demanding heavy labor (blue-collar jobs)
SLAP tears are less common in contact athletes, and there is general agreement that SLAP repair outcomes are better in contact athletes than in overhead athletes. In a retrospective review of 18 rugby players with SLAP tears, Funk and Snow50 reported excellent results and quicker return to sport after SLAP repair. Patients with isolated SLAP tears had the earliest return to play. Enad and colleagues51 reported SLAP repair outcomes in an active military population. SLAP tears are more common in the military versus the general population because of the unique physical demands placed on military personnel. The authors retrospectively reviewed 27 cases of type II SLAP tears treated with SLAP repair and suture anchors. Outcomes were measured at a mean of 30.5 months after surgery. Twenty-four (89%) of the 27 patients had good to excellent results, and 94% had returned to active duty by a mean of 4.4 months after SLAP repair.
Given the poor-quality evidence in the literature, we believe that biceps tenodesis should be reserved for revision surgery in contact athletes. There is insufficient evidence to recommend biceps tenodesis as primary treatment for type II SLAP tears in contact athletes. SLAP repair should be performed for primary SLAP lesions in contact athletes and for patients in physically demanding professions (eg, military, laborer, weightlifter).
Conclusion
SLAP tears can result in persistent shoulder pain and dysfunction. SLAP tear management depends on lesion type and severity, age, and functional demands. SLAP repair is the treatment of choice for type II SLAP lesions in young, active patients. Biceps tenodesis is a preferred alternative to SLAP repair in failed SLAP repair and in type II SLAP patients who are older than 40 years and who are less active and have a worker’s compensation claim. These recommendations are based on poor-quality evidence. There is an unmet need for randomized clinical studies comparing SLAP repair with biceps tenodesis for type II SLAP tears in different patient populations so as to optimize the current decision-making algorithm for SLAP tears.
1. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
2. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopaedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
3. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
4. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
5. Powell SE, Nord KD, Ryu RKN. The diagnosis, classification, and treatment of SLAP lesions. Oper Tech Sports Med. 2012;20(1):46-56.
6. Maffet MW, Gartsman GM, Moseley B. Superior labrum-biceps tendon complex lesions of the shoulder. Am J Sports Med. 1995;23(1):93-98.
7. Kim TK, Queale WS, Cosgarea AJ, McFarland EG. Clinical features of the different types of SLAP lesions: an analysis of one hundred and thirty-nine cases. J Bone Joint Surg Am. 2003;85(1):66-71.
8. Abrams GD, Safran MR. Diagnosis and management of superior labrum anterior posterior lesions in overhead athletes. Br J Sports Med. 2010;44(5):311-318.
9. Keener JD, Brophy RH. Superior labral tears of the shoulder: pathogenesis, evaluation, and treatment. J Am Acad Orthop Surg. 2009;17(10):627-637.
10. Abrams GD, Hussey KE, Harris JD, Cole BJ. Clinical results of combined meniscus and femoral osteochondral allograft transplantation: minimum 2-year follow-up. Arthroscopy. 2014;30(8):964-970.e1.
11. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
12. Virk MS, Arciero RA. Superior labrum anterior to posterior tears and glenohumeral instability. Instr Course Lect. 2013;62:501-514.
13. Calvert E, Chambers GK, Regan W, Hawkins RH, Leith JM. Special physical examination tests for superior labrum anterior posterior shoulder tears are clinically limited and invalid: a diagnostic systematic review. J Clin Epidemiol. 2009;62(5):558-563.
14. Jones GL, Galluch DB. Clinical assessment of superior glenoid labral lesions: a systematic review. Clin Orthop Relat Res. 2007;455:45-51.
15. Werner BC, Brockmeier SF, Miller MD. Etiology, diagnosis, and management of failed SLAP repair. J Am Acad Orthop Surg. 2014;22(9):554-565.
16. Werner BC, Pehlivan HC, Hart JM, et al. Biceps tenodesis is a viable option for salvage of failed SLAP repair. J Shoulder Elbow Surg. 2014;23(8):e179-e184.
17. Erickson J, Lavery K, Monica J, Gatt C, Dhawan A. Surgical treatment of symptomatic superior labrum anterior-posterior tears in patients older than 40 years: a systematic review. Am J Sports Med. 2015;43(5):1274-1282.
18. Huri G, Hyun YS, Garbis NG, McFarland EG. Treatment of superior labrum anterior posterior lesions: a literature review. Acta Orthop Traumatol Turc. 2014;48(3):290-297.
19. Li X, Lin TJ, Jager M, et al. Management of type II superior labrum anterior posterior lesions: a review of the literature. Orthop Rev. 2010;2(1):e6.
20. Cooper DE, Arnoczky SP, O’Brien SJ, Warren RF, DiCarlo E, Allen AA. Anatomy, histology, and vascularity of the glenoid labrum. An anatomical study. J Bone Joint Surg Am. 1992;74(1):46-52.
21. Vangsness CT, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
22. Strauss EJ, Salata MJ, Sershon RA, et al. Role of the superior labrum after biceps tenodesis in glenohumeral stability. J Shoulder Elbow Surg. 2014;23(4):485-491.
23. Pagnani MJ, Deng XH, Warren RF, Torzilli PA, Altchek DW. Effect of lesions of the superior portion of the glenoid labrum on glenohumeral translation. J Bone Joint Surg Am. 1995;77(7):1003-1010.
24. McMahon PJ, Burkart A, Musahl V, Debski RE. Glenohumeral translations are increased after a type II superior labrum anterior-posterior lesion: a cadaveric study of severity of passive stabilizer injury. J Shoulder Elbow Surg. 2004;13(1):39-44.
25. Burkart A, Debski R, Musahl V, McMahon P, Woo SL. Biomechanical tests for type II SLAP lesions of the shoulder joint before and after arthroscopic repair [in German]. Orthopade. 2003;32(7):600-607.
26. Panossian VR, Mihata T, Tibone JE, Fitzpatrick MJ, McGarry MH, Lee TQ. Biomechanical analysis of isolated type II SLAP lesions and repair. J Shoulder Elbow Surg. 2005;14(5):529-534.
27. Mihata T, McGarry MH, Tibone JE, Fitzpatrick MJ, Kinoshita M, Lee TQ. Biomechanical assessment of type II superior labral anterior-posterior (SLAP) lesions associated with anterior shoulder capsular laxity as seen in throwers: a cadaveric study. Am J Sports Med. 2008;36(8):1604-1610.
28. Youm T, Tibone JE, ElAttrache NS, McGarry MH, Lee TQ. Simulated type II superior labral anterior posterior lesions do not alter the path of glenohumeral articulation: a cadaveric biomechanical study. Am J Sports Med. 2008;36(4):767-774.
29. Youm T, ElAttrache NS, Tibone JE, McGarry MH, Lee TQ. The effect of the long head of the biceps on glenohumeral kinematics. J Shoulder Elbow Surg. 2009;18(1):122-129.
30. McGarry MH, Nguyen ML, Quigley RJ, Hanypsiak B, Gupta R, Lee TQ. The effect of long and short head biceps loading on glenohumeral joint rotational range of motion and humeral head position [published online ahead of print September 26, 2014]. Knee Surg Sports Traumatol Arthrosc.
31. Glousman R, Jobe F, Tibone J, Moynes D, Antonelli D, Perry J. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am. 1988;70(2):220-226.
32. Gowan ID, Jobe FW, Tibone JE, Perry J, Moynes DR. A comparative electromyographic analysis of the shoulder during pitching. Professional versus amateur pitchers. Am J Sports Med. 1987;15(6):586-590.
33. Rodosky MW, Harner CD, Fu FH. The role of the long head of the biceps muscle and superior glenoid labrum in anterior stability of the shoulder. Am J Sports Med. 1994;22(1):121-130.
34. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
35. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
36. Ek ET, Shi LL, Tompson JD, Freehill MT, Warner JJ. Surgical treatment of isolated type II superior labrum anterior-posterior (SLAP) lesions: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2014;23(7):1059-1065.
37. Alpert JM, Wuerz TH, O’Donnell TF, Carroll KM, Brucker NN, Gill TJ. The effect of age on the outcomes of arthroscopic repair of type II superior labral anterior and posterior lesions. Am J Sports Med. 2010;38(11):2299-2303.
38. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
39. Denard PJ, Lädermann A, Burkhart SS. Long-term outcome after arthroscopic repair of type II SLAP lesions: results according to age and workers’ compensation status. Arthroscopy. 2012;28(4):451-457.
40. Burns JP, Bahk M, Snyder SJ. Superior labral tears: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2011;20(2 suppl):S2-S8.
41. McCormick F, Nwachukwu BU, Solomon D, et al. The efficacy of biceps tenodesis in the treatment of failed superior labral anterior posterior repairs. Am J Sports Med. 2014;42(4):820-825.
42. Katz LM, Hsu S, Miller SL, et al. Poor outcomes after SLAP repair: descriptive analysis and prognosis. Arthroscopy. 2009;25(8):849-855.
43. Park S, Glousman RE. Outcomes of revision arthroscopic type II superior labral anterior posterior repairs. Am J Sports Med. 2011;39(6):1290-1294.
44. Gupta AK, Bruce B, Klosterman EL, McCormick F, Harris J, Romeo AA. Subpectoral biceps tenodesis for failed type II SLAP repair. Orthopedics. 2013;36(6):e723-e728.
45. Neuman BJ, Boisvert CB, Reiter B, Lawson K, Ciccotti MG, Cohen SB. Results of arthroscopic repair of type II superior labral anterior posterior lesions in overhead athletes: assessment of return to preinjury playing level and satisfaction. Am J Sports Med. 2011;39(9):1883-1888.
46. Fedoriw WW, Ramkumar P, McCulloch PC, Lintner DM. Return to play after treatment of superior labral tears in professional baseball players. Am J Sports Med. 2014;42(5):1155-1160.
47. Park JY, Chung SW, Jeon SH, Lee JG, Oh KS. Clinical and radiological outcomes of type 2 superior labral anterior posterior repairs in elite overhead athletes. Am J Sports Med. 2013;41(6):1372-1379.
48. Schöffl V, Popp D, Dickschass J, Küpper T. Superior labral anterior-posterior lesions in rock climbers—primary double tenodesis? Clin J Sport Med. 2011;21(3):261-263.
49. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
50. Funk L, Snow M. SLAP tears of the glenoid labrum in contact athletes. Clin J Sport Med. 2007;17(1):1-4.
51. Enad JG, Gaines RJ, White SM, Kurtz CA. Arthroscopic superior labrum anterior-posterior repair in military patients. J Shoulder Elbow Surg. 2007;16(3):300-305.
1. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
2. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopaedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
3. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
4. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
5. Powell SE, Nord KD, Ryu RKN. The diagnosis, classification, and treatment of SLAP lesions. Oper Tech Sports Med. 2012;20(1):46-56.
6. Maffet MW, Gartsman GM, Moseley B. Superior labrum-biceps tendon complex lesions of the shoulder. Am J Sports Med. 1995;23(1):93-98.
7. Kim TK, Queale WS, Cosgarea AJ, McFarland EG. Clinical features of the different types of SLAP lesions: an analysis of one hundred and thirty-nine cases. J Bone Joint Surg Am. 2003;85(1):66-71.
8. Abrams GD, Safran MR. Diagnosis and management of superior labrum anterior posterior lesions in overhead athletes. Br J Sports Med. 2010;44(5):311-318.
9. Keener JD, Brophy RH. Superior labral tears of the shoulder: pathogenesis, evaluation, and treatment. J Am Acad Orthop Surg. 2009;17(10):627-637.
10. Abrams GD, Hussey KE, Harris JD, Cole BJ. Clinical results of combined meniscus and femoral osteochondral allograft transplantation: minimum 2-year follow-up. Arthroscopy. 2014;30(8):964-970.e1.
11. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
12. Virk MS, Arciero RA. Superior labrum anterior to posterior tears and glenohumeral instability. Instr Course Lect. 2013;62:501-514.
13. Calvert E, Chambers GK, Regan W, Hawkins RH, Leith JM. Special physical examination tests for superior labrum anterior posterior shoulder tears are clinically limited and invalid: a diagnostic systematic review. J Clin Epidemiol. 2009;62(5):558-563.
14. Jones GL, Galluch DB. Clinical assessment of superior glenoid labral lesions: a systematic review. Clin Orthop Relat Res. 2007;455:45-51.
15. Werner BC, Brockmeier SF, Miller MD. Etiology, diagnosis, and management of failed SLAP repair. J Am Acad Orthop Surg. 2014;22(9):554-565.
16. Werner BC, Pehlivan HC, Hart JM, et al. Biceps tenodesis is a viable option for salvage of failed SLAP repair. J Shoulder Elbow Surg. 2014;23(8):e179-e184.
17. Erickson J, Lavery K, Monica J, Gatt C, Dhawan A. Surgical treatment of symptomatic superior labrum anterior-posterior tears in patients older than 40 years: a systematic review. Am J Sports Med. 2015;43(5):1274-1282.
18. Huri G, Hyun YS, Garbis NG, McFarland EG. Treatment of superior labrum anterior posterior lesions: a literature review. Acta Orthop Traumatol Turc. 2014;48(3):290-297.
19. Li X, Lin TJ, Jager M, et al. Management of type II superior labrum anterior posterior lesions: a review of the literature. Orthop Rev. 2010;2(1):e6.
20. Cooper DE, Arnoczky SP, O’Brien SJ, Warren RF, DiCarlo E, Allen AA. Anatomy, histology, and vascularity of the glenoid labrum. An anatomical study. J Bone Joint Surg Am. 1992;74(1):46-52.
21. Vangsness CT, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
22. Strauss EJ, Salata MJ, Sershon RA, et al. Role of the superior labrum after biceps tenodesis in glenohumeral stability. J Shoulder Elbow Surg. 2014;23(4):485-491.
23. Pagnani MJ, Deng XH, Warren RF, Torzilli PA, Altchek DW. Effect of lesions of the superior portion of the glenoid labrum on glenohumeral translation. J Bone Joint Surg Am. 1995;77(7):1003-1010.
24. McMahon PJ, Burkart A, Musahl V, Debski RE. Glenohumeral translations are increased after a type II superior labrum anterior-posterior lesion: a cadaveric study of severity of passive stabilizer injury. J Shoulder Elbow Surg. 2004;13(1):39-44.
25. Burkart A, Debski R, Musahl V, McMahon P, Woo SL. Biomechanical tests for type II SLAP lesions of the shoulder joint before and after arthroscopic repair [in German]. Orthopade. 2003;32(7):600-607.
26. Panossian VR, Mihata T, Tibone JE, Fitzpatrick MJ, McGarry MH, Lee TQ. Biomechanical analysis of isolated type II SLAP lesions and repair. J Shoulder Elbow Surg. 2005;14(5):529-534.
27. Mihata T, McGarry MH, Tibone JE, Fitzpatrick MJ, Kinoshita M, Lee TQ. Biomechanical assessment of type II superior labral anterior-posterior (SLAP) lesions associated with anterior shoulder capsular laxity as seen in throwers: a cadaveric study. Am J Sports Med. 2008;36(8):1604-1610.
28. Youm T, Tibone JE, ElAttrache NS, McGarry MH, Lee TQ. Simulated type II superior labral anterior posterior lesions do not alter the path of glenohumeral articulation: a cadaveric biomechanical study. Am J Sports Med. 2008;36(4):767-774.
29. Youm T, ElAttrache NS, Tibone JE, McGarry MH, Lee TQ. The effect of the long head of the biceps on glenohumeral kinematics. J Shoulder Elbow Surg. 2009;18(1):122-129.
30. McGarry MH, Nguyen ML, Quigley RJ, Hanypsiak B, Gupta R, Lee TQ. The effect of long and short head biceps loading on glenohumeral joint rotational range of motion and humeral head position [published online ahead of print September 26, 2014]. Knee Surg Sports Traumatol Arthrosc.
31. Glousman R, Jobe F, Tibone J, Moynes D, Antonelli D, Perry J. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am. 1988;70(2):220-226.
32. Gowan ID, Jobe FW, Tibone JE, Perry J, Moynes DR. A comparative electromyographic analysis of the shoulder during pitching. Professional versus amateur pitchers. Am J Sports Med. 1987;15(6):586-590.
33. Rodosky MW, Harner CD, Fu FH. The role of the long head of the biceps muscle and superior glenoid labrum in anterior stability of the shoulder. Am J Sports Med. 1994;22(1):121-130.
34. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
35. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
36. Ek ET, Shi LL, Tompson JD, Freehill MT, Warner JJ. Surgical treatment of isolated type II superior labrum anterior-posterior (SLAP) lesions: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2014;23(7):1059-1065.
37. Alpert JM, Wuerz TH, O’Donnell TF, Carroll KM, Brucker NN, Gill TJ. The effect of age on the outcomes of arthroscopic repair of type II superior labral anterior and posterior lesions. Am J Sports Med. 2010;38(11):2299-2303.
38. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
39. Denard PJ, Lädermann A, Burkhart SS. Long-term outcome after arthroscopic repair of type II SLAP lesions: results according to age and workers’ compensation status. Arthroscopy. 2012;28(4):451-457.
40. Burns JP, Bahk M, Snyder SJ. Superior labral tears: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2011;20(2 suppl):S2-S8.
41. McCormick F, Nwachukwu BU, Solomon D, et al. The efficacy of biceps tenodesis in the treatment of failed superior labral anterior posterior repairs. Am J Sports Med. 2014;42(4):820-825.
42. Katz LM, Hsu S, Miller SL, et al. Poor outcomes after SLAP repair: descriptive analysis and prognosis. Arthroscopy. 2009;25(8):849-855.
43. Park S, Glousman RE. Outcomes of revision arthroscopic type II superior labral anterior posterior repairs. Am J Sports Med. 2011;39(6):1290-1294.
44. Gupta AK, Bruce B, Klosterman EL, McCormick F, Harris J, Romeo AA. Subpectoral biceps tenodesis for failed type II SLAP repair. Orthopedics. 2013;36(6):e723-e728.
45. Neuman BJ, Boisvert CB, Reiter B, Lawson K, Ciccotti MG, Cohen SB. Results of arthroscopic repair of type II superior labral anterior posterior lesions in overhead athletes: assessment of return to preinjury playing level and satisfaction. Am J Sports Med. 2011;39(9):1883-1888.
46. Fedoriw WW, Ramkumar P, McCulloch PC, Lintner DM. Return to play after treatment of superior labral tears in professional baseball players. Am J Sports Med. 2014;42(5):1155-1160.
47. Park JY, Chung SW, Jeon SH, Lee JG, Oh KS. Clinical and radiological outcomes of type 2 superior labral anterior posterior repairs in elite overhead athletes. Am J Sports Med. 2013;41(6):1372-1379.
48. Schöffl V, Popp D, Dickschass J, Küpper T. Superior labral anterior-posterior lesions in rock climbers—primary double tenodesis? Clin J Sport Med. 2011;21(3):261-263.
49. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
50. Funk L, Snow M. SLAP tears of the glenoid labrum in contact athletes. Clin J Sport Med. 2007;17(1):1-4.
51. Enad JG, Gaines RJ, White SM, Kurtz CA. Arthroscopic superior labrum anterior-posterior repair in military patients. J Shoulder Elbow Surg. 2007;16(3):300-305.
Implications of the GOLD COPD Classification and Guidelines
After a busy day in the primary care clinic, having finished the day’s dictations and called a patient to discuss the results of his lipid panel, Dr. B reviews tomorrow’s schedule, and notices 2 patients with a primary diagnosis of chronic obstructive pulmonary disease (COPD). Dr. B recalls a recent publication on changes in the classification of COPD by the Global Initiative for Chronic Obstructive Lung Disease (GOLD).1 She remembers the main message being the degree of airway obstruction as measured by the forced expiratory volume in the first second (FEV1) is now considered insufficient to classify COPD severity and to make a therapeutic decision. This paradigm shift contradicts the familiar concept that FEV1 is the cornerstone piece of information in COPD, resulting in some degree of uncertainty about how to apply this in the practice. Dr. B. considers a multitude of practical questions, including: Is there a good reason to change the classification of COPD? How easy is it to use?
Will it make any therapeutic differences to my patients? In this article, the authors attempt to answer these and other questions prompted by the recent changes in the GOLD classification, with emphasis on its clinical use.
A Heterogeneous Condition
Spirometry is central to the diagnosis of obstructive lung diseases, including COPD and asthma. The diagnosis of COPD requires demonstration of an obstructive ventilatory defect in the spirometry, usually defined as a ratio of FEV1 to forced vital capacity (FVC) below 70% (FEV1/FVC < 0.7). FEV1 is still important, not only to confirm the diagnosis of airflow obstruction, but because it predicts mortality when severely reduced. However, during the last decade severity of airflow limitation has been challenged as a descriptor of both symptom burden and consequences of COPD by data from large studies.2 For example, it has been demonstrated that 2 patients with the same degree of obstruction, measured by the FEV1 percentage predicted, can provide the physician with very different experiences about the impact of their disease in daily life.3 These differences extend to the severity of their dyspnea; their exercise capacity, as seen in the sixminute walking distance test (6MWD); or their perceived quality of life (QOL), measured by the score on the Saint George’s Respiratory Questionnaire (SGRQ). These measures of disease impact show an extremely low correlation with FEV1: a correlation of 0.36 with the severity of dyspnea, 0.34 with 6MWD, and 0.38 with the SGRQ total score.2 These newer studies imply that while spirometry is important, it captures only a small portion of the symptomatic and functional impact of COPD.
Increasing interest in understanding the differences between COPD subjects has been the main motivation in identifying distinct COPD phenotypes, subgroups of patients with similar disease experience, probable similar underlying pathogenic mechanisms, similar outcomes, and perhaps specific treatment alternatives.4,5 The severity of airflow limitation, as measured by FEV1 percent predicted, is not always related with some of the emerging COPD phenotypes (eg, chronic bronchitis predominant phenotype, frequent exacerbation phenotype).6-8 Chronic bronchitis can be present across the whole spectrum of spirometry severity, and is always associated with poorer QOL and worse clinical outcomes. Similarly, there are patients with frequent exacerbation phenotype (defined as ≥ 2 exacerbations/year) at every level of airflow obstruction, and the phenotype tends to be stable, meaning that previous frequent exacerbations are a good predictor of future exacerbations.8
With all this information, participants in the development of the GOLD guidelines determined that although FEV1 is still a good descriptor of COPD severity and potential for poor outcomes (exacerbation frequency, mortality), a more comprehensive description of COPD needed the addition of data on the impact of symptoms (particularly dyspnea), and the future risk of poor COPD related events (exacerbations, death, disease progression).5 Hence, in response to Dr. B.’s question, it seems that a new approach to the way that we classify COPD was overdue, making it important to gather additional patient information, beyond FEV1.
GOLD Category Classification
An important difference from previous classifications is that the new GOLD categories use lettered groups, from A to D, not just grades of severity; however, the severity of the ventilatory defect measured by FEV1 is still graded from 1 to 4 and is still part of the classification.9
Placing a patient in the new groups is based on 2 questions: (1) How severe are the symptoms, particularly dyspnea; and (2) Is the patient at high or low risk of poor COPD-related outcomes? The first question (symptoms severity) can be systematically approached using 1 of 2 different instruments to grade COPD symptoms: the modified Medical Research Council dyspnea score (mMRC) or the COPD Assessment Test (CAT), a more recently developed instrument to quantify COPD impact.10,11
Use of CAT score, a more comprehensive descriptor of COPD impact, is the preferred method by guideline developers. If the practitioner is more familiar with the mMRC and wishes to use it instead, the result can be simplified as low (0-1 points) or high symptoms burden (≥ 2 points). The mMRC is based on the answer to the level of effort triggering dyspnea: a score of 1 means that the patient “get[s] shorter of breath when hurrying on a level surface or walking up a slight hill”; a score of 2 means that the patient “walk[s] slower than people of the same age while walking on a level surface because of breathlessness, or I have to stop for breath when walking on my own pace on the level.” Hence, the first step to classify a patient can be as simple as asking about dyspnea, surely part of the history taking process.
The second question (risk of poor COPD-related outcomes) can be answered by using the grade of obstruction by FEV1 or asking about the frequency of exacerbations in the previous year. If FEV1 is used, those with FEV1 percentage predicted ≥ 50% are considered as “low risk”; if the airflow obstruction is more severe (previously grades 3-4), the patient is at “high risk” of future events. If the exacerbation frequency is used, ≤ 1 outpatient-treated exacerbation in the previous year qualify as “low,” and ≥ 2 as “high risk.” There is an additional alternative way to identify high risk: all patients with any (≥ 1 per year) exacerbation requiring hospital admission are considered at high risk.
The next step is combining both symptoms and risk to create 4 mutually exclusive groups, which will be relevant to select the appropriate treatment (Table 1). The groups can also be represented graphically using a 2x2 figure, with the horizontal axis being symptoms severity, and the vertical (risk) either FEV1 or exacerbation history (Figure 1). If there is a discrepancy between the risk judged by lung function and history of exacerbations, it is recommended to use the answer corresponding to the worse category.12
GOLD Guideline-Based Treatment
The new classification should also help to identify the patient’s main needs: controlling symptoms, reducing future risks, or both. Based on the results of available randomized clinical trials, GOLD guideline developers suggest grouptailored strategies of management (Table 2, Figure 2).
Group A: Low Risk and Low Symptoms
The goal is to treat only as needed, using shortacting medications. No preference was given to the type of short-acting medication and the practitioner could select between short-acting beta agonists (SABAs) or short-acting anticholinergic (also known as short-acting antimuscarinic [SAMA]) medication as first-line therapy. Second-line therapy includes either the combination of both families of short-acting medications in 1 inhaler, or the use of 1 long-acting inhaler. As a rule of thumb, no patient in this group should be on more than 1 inhaler, and the combination of short and long-acting medications is not part of the recommendations. Patients in group A, and indeed everyone with COPD, benefit from respiratory immunizations and tobacco cessation.
Group B: Low Risk, High Symptoms
Again, the goal of treatment is symptom control. Based on the available evidence, this can be achieved using long-acting bronchodilators, without the need of inhaled corticosteroids (ICS). The first line of treatment should be just 1 bronchodilator, either a long-acting antimuscarinic (LAMA) or long-actingbeta agonist (LABA). These could be used together as second-line treatment (LAMA plus LABA), still without indication for ICS. It is important to remember that dyspnea, or other symptoms, could also be a manifestation of comorbid conditions, such as cardiovascular disease, obesity, deconditioning, and musculoskeletal diseases.13 When spirometry is not used to confirm the diagnosis of COPD, patients may receive incremental types of inhalers instead of being evaluated for other causes of dyspnea, which might have led to more appropriate specific therapy.14 As a result, judicious evaluation of the patient’s symptomsis recommended. The guidelines also recommend programs that increase physical activity for this group of patients, as well to those in groups C and D, as this can improve symptoms and decrease risk of exacerbations.15
Group C: High Risk, Low Symptoms
The combination of ICS/LABA is the first-line therapy for this group, based on data showing the superiority of the ICS/LABA combination over monotherapy to reduce exacerbations and symptoms, as well as to improve QOL.16,17 Monotherapy LABA is also a first-line GOLD recommendation. Selecting between ICS/LABA vs LABA should be individualized based on the reason that the patient was judged as high risk. In the authors’ practice, if
the risk is based only in spirometry values, using LABA as monotherapy is a good choice, while if the definition of high risk was based on the frequency of exacerbations, ICS/LABA is the first choice. The GOLD guidelines list the combination of LABA/LAMA as second-line therapy.
Group D: High Risk and High Symptoms
First-line therapy for this group is essentially the same that for group C, with similar considerations. The combination of LABA/LAMA is also recommended as second-line therapy, as well as the use of ICS/LABA and LAMA (all 3 major classes of controller medications together). It is worth noting that phosphodiesterase-4 inhibitors (PDE4-inh, roflumilast being the best known) can be considered as a third-line of therapy (in group C) or as part of secondline combinations (in group D).
Benefits and Limitations of GOLD
There is no doubt that the new classification system and treatment guidelines are a significant step forward, intended to foster the development of more personalized decisions for COPD patients. The guidelines are the first attempt to incorporate the concepts of phenotypes (frequent exacerbation phenotype), disease heterogeneity (the variation in outcomes for the same degree of airflow obstruction), and the differences between the burden of symptoms and the risk of outcomes. The guidelines incorporate the need to weigh the benefits and risks of medications at the individual level (eg, ICS without an accompanying long-acting agent are not recommended in any group, and ICS use is reserved for those with high risk, especially if the designation is based on exacerbation frequency). The guidelines also stress the importance of examining comorbidities, emphysizing that their management should in no way be altered just because the patient also has COPD. Relative to the previous staging based only on FEV1 values, this new classification system has been shown to have appropriate predictive ability and association with the risk of exacerbations, and better correlation with measures of quality of life and costs of care.18,19 The guidelines, initially released in 2011 and slightly updated recently, are in continuous development and have been subject to intense evaluation.
Some limitations have been found (eg, the classification is still not the best predictor of mortality, but has the same ability to predict hospital admission as the previous spirometry-based system).18,20,21 Hence, it should be no surprise that modifications will likely be released in the near future.
The treatment recommendations associated with the current classification are based on the best evidence available and expert opinion, as no published clinical trials have compared the group-based therapy system to standard therapies. Evaluations of their effectiveness in real-life practice are still to be released. Previous, less complex guidelines, based on spirometry stages, were followed < 60% of the time in actual practice, thus it will be surprising to find high adherence to the current recommendations, but evaluations are still in progress.22
Conclusion
The best way for primary care providers to incorporate the GOLD guidelines into daily practice is to remember that COPD is very heterogeneous. Although spirometry is important, it is also essential to inquire about exacerbation frequency and symptoms severity. It is encouraging that for each of the relevant questions needed to classify the patient, there is a clear, easy to remember cut point. First, look at symptoms (low or high burden, based on the presence of dyspnea), then to judge risk look at FEV1 percentage predicted (using 50% as a cutpoint) and at exacerbation frequency (using 2 per year as the cut point). With those simple questions, build the groups, based on the combination of answers, and select the appropriate therapy. The general assumptions are that short-acting medications are appropriate for infrequent symptoms, long-acting medications are used to control symptoms and prevent exacerbations in more severe disease, and that ICS (always in combination with LABA) are reserved for those in the high-risk groups, especially if high risk is defined by frequent exacerbations. This summary should be supplemented with the judicious use of the tables and figures provided in this review, and available with detailed description and discussion in the original sources.1
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner; Frontline Medical Communications Inc.; the Department of Defense, or its Components; and the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347-365.
2. Agusti A, Calverley PM, Celli B, et al; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) investigators. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:122.
3. Jones PW. Health status and the spiral of decline. COPD. 2009;6(1):59-63.
4. Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182(5):598-604.
5. Han MK, Kazerooni EA, Lynch DA, et al; COPDGene Investigators. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology. 2011;261(1):274-282.
6. Kim V, Han MK, Vance GB, et al; COPDGene Investigators. The chronic bronchitic phenotype of COPD: an analysis of the COPDGene Study. Chest. 2011;140(3):626-633.
7. Wedzicha JA, Brill SE, Allinson JP, Donaldson GC. Mechanisms and impact of the frequent exacerbator phenotype in chronic obstructive pulmonary disease. BMC Med. 2013;11:181.
8. Hurst JR, Vestbo J, Anzueto A, et al; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128-1138.
9. Qaseem A, Wilt TJ, Weinberger SE, et al; American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179-191.
10. Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121(5):1434-1440.
11. Lee SD, Huang MS, Kang J, et al; Investigators of the Predictive Ability of CAT in Acute Exacerbations of COPD (PACE) Study. The COPD assessment test (CAT) assists prediction of COPD exacerbations in high-risk patients. Respir Med. 2014;108(4):600-608.
12. Haughney J, Gruffydd-Jones K, Roberts J, Lee AJ, Hardwell A, McGarvey L. The distribution of COPD in UK general practice using the new GOLD classification. Eur Respir J. 2014;43(4):993-1002.
13. Martinez CH, Han MK. Contribution of the environment and comorbidities to chronic obstructive pulmonary disease phenotypes. Med Clin North Am. 2012;96(4):713-727.
14. Collins BF, Feemster LC, Rinne ST, Au DH. Factors predictive of airflow obstruction among veterans with presumed empiric diagnosis and treatment of COPD. Chest. 2015;147(2):369-376.
15. Puhan MA, Gimeno-Santos E, Scharplatz M, Troosters T, Walters EH, Steurer J. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;(10):CD005305.
16. Nannini LJ, Poole P, Milan SJ, Kesterton A. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus inhaled corticosteroids alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;8:CD006826.
17. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9:CD006829.
18. Goossens LM, Leimer I, Metzdorf N, Becker K, Rutten-van Mölken MP. Does the 2013 GOLD classification improve the ability to predict lung function decline, exacerbations and mortality: a post-hoc analysis of the 4-year UPLIFT trial. BMC Pulm Med. 2014;14:163.
19. Boland MR, Tsiachristas A, Kruis AL, Chavannes NH, Rutten-van Mölken MP. Are GOLD ABCD groups better associated with health status and costs than GOLD 1234 grades? A cross-sectional study. Prim Care Respir J. 2014;23(1):30-37.
20. Han MK, Muellerova H, Curran-Everett D, et al. GOLD 2011 disease severity classification in COPDGene: a prospective cohort study. Lancet Respir Med. 2013;(1):43-50.
21. Johannessen A, Nilsen RM, Storebø M, Gulsvik A, Eagan T, Bakke P. Comparison of 2011 and 2007 Global Initiative for Chronic Obstructive Lung Disease guidelines for predicting mortality and hospitalization. Am J Respir Crit Care Med. 2013;188(1):51-59.
22. Sharif R, Cuevas CR, Wang Y, Arora M, Sharma G. Guideline adherence in management of stable chronic obstructive pulmonary disease. Respir Med. 2013;107(7):1046-1052.
After a busy day in the primary care clinic, having finished the day’s dictations and called a patient to discuss the results of his lipid panel, Dr. B reviews tomorrow’s schedule, and notices 2 patients with a primary diagnosis of chronic obstructive pulmonary disease (COPD). Dr. B recalls a recent publication on changes in the classification of COPD by the Global Initiative for Chronic Obstructive Lung Disease (GOLD).1 She remembers the main message being the degree of airway obstruction as measured by the forced expiratory volume in the first second (FEV1) is now considered insufficient to classify COPD severity and to make a therapeutic decision. This paradigm shift contradicts the familiar concept that FEV1 is the cornerstone piece of information in COPD, resulting in some degree of uncertainty about how to apply this in the practice. Dr. B. considers a multitude of practical questions, including: Is there a good reason to change the classification of COPD? How easy is it to use?
Will it make any therapeutic differences to my patients? In this article, the authors attempt to answer these and other questions prompted by the recent changes in the GOLD classification, with emphasis on its clinical use.
A Heterogeneous Condition
Spirometry is central to the diagnosis of obstructive lung diseases, including COPD and asthma. The diagnosis of COPD requires demonstration of an obstructive ventilatory defect in the spirometry, usually defined as a ratio of FEV1 to forced vital capacity (FVC) below 70% (FEV1/FVC < 0.7). FEV1 is still important, not only to confirm the diagnosis of airflow obstruction, but because it predicts mortality when severely reduced. However, during the last decade severity of airflow limitation has been challenged as a descriptor of both symptom burden and consequences of COPD by data from large studies.2 For example, it has been demonstrated that 2 patients with the same degree of obstruction, measured by the FEV1 percentage predicted, can provide the physician with very different experiences about the impact of their disease in daily life.3 These differences extend to the severity of their dyspnea; their exercise capacity, as seen in the sixminute walking distance test (6MWD); or their perceived quality of life (QOL), measured by the score on the Saint George’s Respiratory Questionnaire (SGRQ). These measures of disease impact show an extremely low correlation with FEV1: a correlation of 0.36 with the severity of dyspnea, 0.34 with 6MWD, and 0.38 with the SGRQ total score.2 These newer studies imply that while spirometry is important, it captures only a small portion of the symptomatic and functional impact of COPD.
Increasing interest in understanding the differences between COPD subjects has been the main motivation in identifying distinct COPD phenotypes, subgroups of patients with similar disease experience, probable similar underlying pathogenic mechanisms, similar outcomes, and perhaps specific treatment alternatives.4,5 The severity of airflow limitation, as measured by FEV1 percent predicted, is not always related with some of the emerging COPD phenotypes (eg, chronic bronchitis predominant phenotype, frequent exacerbation phenotype).6-8 Chronic bronchitis can be present across the whole spectrum of spirometry severity, and is always associated with poorer QOL and worse clinical outcomes. Similarly, there are patients with frequent exacerbation phenotype (defined as ≥ 2 exacerbations/year) at every level of airflow obstruction, and the phenotype tends to be stable, meaning that previous frequent exacerbations are a good predictor of future exacerbations.8
With all this information, participants in the development of the GOLD guidelines determined that although FEV1 is still a good descriptor of COPD severity and potential for poor outcomes (exacerbation frequency, mortality), a more comprehensive description of COPD needed the addition of data on the impact of symptoms (particularly dyspnea), and the future risk of poor COPD related events (exacerbations, death, disease progression).5 Hence, in response to Dr. B.’s question, it seems that a new approach to the way that we classify COPD was overdue, making it important to gather additional patient information, beyond FEV1.
GOLD Category Classification
An important difference from previous classifications is that the new GOLD categories use lettered groups, from A to D, not just grades of severity; however, the severity of the ventilatory defect measured by FEV1 is still graded from 1 to 4 and is still part of the classification.9
Placing a patient in the new groups is based on 2 questions: (1) How severe are the symptoms, particularly dyspnea; and (2) Is the patient at high or low risk of poor COPD-related outcomes? The first question (symptoms severity) can be systematically approached using 1 of 2 different instruments to grade COPD symptoms: the modified Medical Research Council dyspnea score (mMRC) or the COPD Assessment Test (CAT), a more recently developed instrument to quantify COPD impact.10,11
Use of CAT score, a more comprehensive descriptor of COPD impact, is the preferred method by guideline developers. If the practitioner is more familiar with the mMRC and wishes to use it instead, the result can be simplified as low (0-1 points) or high symptoms burden (≥ 2 points). The mMRC is based on the answer to the level of effort triggering dyspnea: a score of 1 means that the patient “get[s] shorter of breath when hurrying on a level surface or walking up a slight hill”; a score of 2 means that the patient “walk[s] slower than people of the same age while walking on a level surface because of breathlessness, or I have to stop for breath when walking on my own pace on the level.” Hence, the first step to classify a patient can be as simple as asking about dyspnea, surely part of the history taking process.
The second question (risk of poor COPD-related outcomes) can be answered by using the grade of obstruction by FEV1 or asking about the frequency of exacerbations in the previous year. If FEV1 is used, those with FEV1 percentage predicted ≥ 50% are considered as “low risk”; if the airflow obstruction is more severe (previously grades 3-4), the patient is at “high risk” of future events. If the exacerbation frequency is used, ≤ 1 outpatient-treated exacerbation in the previous year qualify as “low,” and ≥ 2 as “high risk.” There is an additional alternative way to identify high risk: all patients with any (≥ 1 per year) exacerbation requiring hospital admission are considered at high risk.
The next step is combining both symptoms and risk to create 4 mutually exclusive groups, which will be relevant to select the appropriate treatment (Table 1). The groups can also be represented graphically using a 2x2 figure, with the horizontal axis being symptoms severity, and the vertical (risk) either FEV1 or exacerbation history (Figure 1). If there is a discrepancy between the risk judged by lung function and history of exacerbations, it is recommended to use the answer corresponding to the worse category.12
GOLD Guideline-Based Treatment
The new classification should also help to identify the patient’s main needs: controlling symptoms, reducing future risks, or both. Based on the results of available randomized clinical trials, GOLD guideline developers suggest grouptailored strategies of management (Table 2, Figure 2).
Group A: Low Risk and Low Symptoms
The goal is to treat only as needed, using shortacting medications. No preference was given to the type of short-acting medication and the practitioner could select between short-acting beta agonists (SABAs) or short-acting anticholinergic (also known as short-acting antimuscarinic [SAMA]) medication as first-line therapy. Second-line therapy includes either the combination of both families of short-acting medications in 1 inhaler, or the use of 1 long-acting inhaler. As a rule of thumb, no patient in this group should be on more than 1 inhaler, and the combination of short and long-acting medications is not part of the recommendations. Patients in group A, and indeed everyone with COPD, benefit from respiratory immunizations and tobacco cessation.
Group B: Low Risk, High Symptoms
Again, the goal of treatment is symptom control. Based on the available evidence, this can be achieved using long-acting bronchodilators, without the need of inhaled corticosteroids (ICS). The first line of treatment should be just 1 bronchodilator, either a long-acting antimuscarinic (LAMA) or long-actingbeta agonist (LABA). These could be used together as second-line treatment (LAMA plus LABA), still without indication for ICS. It is important to remember that dyspnea, or other symptoms, could also be a manifestation of comorbid conditions, such as cardiovascular disease, obesity, deconditioning, and musculoskeletal diseases.13 When spirometry is not used to confirm the diagnosis of COPD, patients may receive incremental types of inhalers instead of being evaluated for other causes of dyspnea, which might have led to more appropriate specific therapy.14 As a result, judicious evaluation of the patient’s symptomsis recommended. The guidelines also recommend programs that increase physical activity for this group of patients, as well to those in groups C and D, as this can improve symptoms and decrease risk of exacerbations.15
Group C: High Risk, Low Symptoms
The combination of ICS/LABA is the first-line therapy for this group, based on data showing the superiority of the ICS/LABA combination over monotherapy to reduce exacerbations and symptoms, as well as to improve QOL.16,17 Monotherapy LABA is also a first-line GOLD recommendation. Selecting between ICS/LABA vs LABA should be individualized based on the reason that the patient was judged as high risk. In the authors’ practice, if
the risk is based only in spirometry values, using LABA as monotherapy is a good choice, while if the definition of high risk was based on the frequency of exacerbations, ICS/LABA is the first choice. The GOLD guidelines list the combination of LABA/LAMA as second-line therapy.
Group D: High Risk and High Symptoms
First-line therapy for this group is essentially the same that for group C, with similar considerations. The combination of LABA/LAMA is also recommended as second-line therapy, as well as the use of ICS/LABA and LAMA (all 3 major classes of controller medications together). It is worth noting that phosphodiesterase-4 inhibitors (PDE4-inh, roflumilast being the best known) can be considered as a third-line of therapy (in group C) or as part of secondline combinations (in group D).
Benefits and Limitations of GOLD
There is no doubt that the new classification system and treatment guidelines are a significant step forward, intended to foster the development of more personalized decisions for COPD patients. The guidelines are the first attempt to incorporate the concepts of phenotypes (frequent exacerbation phenotype), disease heterogeneity (the variation in outcomes for the same degree of airflow obstruction), and the differences between the burden of symptoms and the risk of outcomes. The guidelines incorporate the need to weigh the benefits and risks of medications at the individual level (eg, ICS without an accompanying long-acting agent are not recommended in any group, and ICS use is reserved for those with high risk, especially if the designation is based on exacerbation frequency). The guidelines also stress the importance of examining comorbidities, emphysizing that their management should in no way be altered just because the patient also has COPD. Relative to the previous staging based only on FEV1 values, this new classification system has been shown to have appropriate predictive ability and association with the risk of exacerbations, and better correlation with measures of quality of life and costs of care.18,19 The guidelines, initially released in 2011 and slightly updated recently, are in continuous development and have been subject to intense evaluation.
Some limitations have been found (eg, the classification is still not the best predictor of mortality, but has the same ability to predict hospital admission as the previous spirometry-based system).18,20,21 Hence, it should be no surprise that modifications will likely be released in the near future.
The treatment recommendations associated with the current classification are based on the best evidence available and expert opinion, as no published clinical trials have compared the group-based therapy system to standard therapies. Evaluations of their effectiveness in real-life practice are still to be released. Previous, less complex guidelines, based on spirometry stages, were followed < 60% of the time in actual practice, thus it will be surprising to find high adherence to the current recommendations, but evaluations are still in progress.22
Conclusion
The best way for primary care providers to incorporate the GOLD guidelines into daily practice is to remember that COPD is very heterogeneous. Although spirometry is important, it is also essential to inquire about exacerbation frequency and symptoms severity. It is encouraging that for each of the relevant questions needed to classify the patient, there is a clear, easy to remember cut point. First, look at symptoms (low or high burden, based on the presence of dyspnea), then to judge risk look at FEV1 percentage predicted (using 50% as a cutpoint) and at exacerbation frequency (using 2 per year as the cut point). With those simple questions, build the groups, based on the combination of answers, and select the appropriate therapy. The general assumptions are that short-acting medications are appropriate for infrequent symptoms, long-acting medications are used to control symptoms and prevent exacerbations in more severe disease, and that ICS (always in combination with LABA) are reserved for those in the high-risk groups, especially if high risk is defined by frequent exacerbations. This summary should be supplemented with the judicious use of the tables and figures provided in this review, and available with detailed description and discussion in the original sources.1
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner; Frontline Medical Communications Inc.; the Department of Defense, or its Components; and the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
After a busy day in the primary care clinic, having finished the day’s dictations and called a patient to discuss the results of his lipid panel, Dr. B reviews tomorrow’s schedule, and notices 2 patients with a primary diagnosis of chronic obstructive pulmonary disease (COPD). Dr. B recalls a recent publication on changes in the classification of COPD by the Global Initiative for Chronic Obstructive Lung Disease (GOLD).1 She remembers the main message being the degree of airway obstruction as measured by the forced expiratory volume in the first second (FEV1) is now considered insufficient to classify COPD severity and to make a therapeutic decision. This paradigm shift contradicts the familiar concept that FEV1 is the cornerstone piece of information in COPD, resulting in some degree of uncertainty about how to apply this in the practice. Dr. B. considers a multitude of practical questions, including: Is there a good reason to change the classification of COPD? How easy is it to use?
Will it make any therapeutic differences to my patients? In this article, the authors attempt to answer these and other questions prompted by the recent changes in the GOLD classification, with emphasis on its clinical use.
A Heterogeneous Condition
Spirometry is central to the diagnosis of obstructive lung diseases, including COPD and asthma. The diagnosis of COPD requires demonstration of an obstructive ventilatory defect in the spirometry, usually defined as a ratio of FEV1 to forced vital capacity (FVC) below 70% (FEV1/FVC < 0.7). FEV1 is still important, not only to confirm the diagnosis of airflow obstruction, but because it predicts mortality when severely reduced. However, during the last decade severity of airflow limitation has been challenged as a descriptor of both symptom burden and consequences of COPD by data from large studies.2 For example, it has been demonstrated that 2 patients with the same degree of obstruction, measured by the FEV1 percentage predicted, can provide the physician with very different experiences about the impact of their disease in daily life.3 These differences extend to the severity of their dyspnea; their exercise capacity, as seen in the sixminute walking distance test (6MWD); or their perceived quality of life (QOL), measured by the score on the Saint George’s Respiratory Questionnaire (SGRQ). These measures of disease impact show an extremely low correlation with FEV1: a correlation of 0.36 with the severity of dyspnea, 0.34 with 6MWD, and 0.38 with the SGRQ total score.2 These newer studies imply that while spirometry is important, it captures only a small portion of the symptomatic and functional impact of COPD.
Increasing interest in understanding the differences between COPD subjects has been the main motivation in identifying distinct COPD phenotypes, subgroups of patients with similar disease experience, probable similar underlying pathogenic mechanisms, similar outcomes, and perhaps specific treatment alternatives.4,5 The severity of airflow limitation, as measured by FEV1 percent predicted, is not always related with some of the emerging COPD phenotypes (eg, chronic bronchitis predominant phenotype, frequent exacerbation phenotype).6-8 Chronic bronchitis can be present across the whole spectrum of spirometry severity, and is always associated with poorer QOL and worse clinical outcomes. Similarly, there are patients with frequent exacerbation phenotype (defined as ≥ 2 exacerbations/year) at every level of airflow obstruction, and the phenotype tends to be stable, meaning that previous frequent exacerbations are a good predictor of future exacerbations.8
With all this information, participants in the development of the GOLD guidelines determined that although FEV1 is still a good descriptor of COPD severity and potential for poor outcomes (exacerbation frequency, mortality), a more comprehensive description of COPD needed the addition of data on the impact of symptoms (particularly dyspnea), and the future risk of poor COPD related events (exacerbations, death, disease progression).5 Hence, in response to Dr. B.’s question, it seems that a new approach to the way that we classify COPD was overdue, making it important to gather additional patient information, beyond FEV1.
GOLD Category Classification
An important difference from previous classifications is that the new GOLD categories use lettered groups, from A to D, not just grades of severity; however, the severity of the ventilatory defect measured by FEV1 is still graded from 1 to 4 and is still part of the classification.9
Placing a patient in the new groups is based on 2 questions: (1) How severe are the symptoms, particularly dyspnea; and (2) Is the patient at high or low risk of poor COPD-related outcomes? The first question (symptoms severity) can be systematically approached using 1 of 2 different instruments to grade COPD symptoms: the modified Medical Research Council dyspnea score (mMRC) or the COPD Assessment Test (CAT), a more recently developed instrument to quantify COPD impact.10,11
Use of CAT score, a more comprehensive descriptor of COPD impact, is the preferred method by guideline developers. If the practitioner is more familiar with the mMRC and wishes to use it instead, the result can be simplified as low (0-1 points) or high symptoms burden (≥ 2 points). The mMRC is based on the answer to the level of effort triggering dyspnea: a score of 1 means that the patient “get[s] shorter of breath when hurrying on a level surface or walking up a slight hill”; a score of 2 means that the patient “walk[s] slower than people of the same age while walking on a level surface because of breathlessness, or I have to stop for breath when walking on my own pace on the level.” Hence, the first step to classify a patient can be as simple as asking about dyspnea, surely part of the history taking process.
The second question (risk of poor COPD-related outcomes) can be answered by using the grade of obstruction by FEV1 or asking about the frequency of exacerbations in the previous year. If FEV1 is used, those with FEV1 percentage predicted ≥ 50% are considered as “low risk”; if the airflow obstruction is more severe (previously grades 3-4), the patient is at “high risk” of future events. If the exacerbation frequency is used, ≤ 1 outpatient-treated exacerbation in the previous year qualify as “low,” and ≥ 2 as “high risk.” There is an additional alternative way to identify high risk: all patients with any (≥ 1 per year) exacerbation requiring hospital admission are considered at high risk.
The next step is combining both symptoms and risk to create 4 mutually exclusive groups, which will be relevant to select the appropriate treatment (Table 1). The groups can also be represented graphically using a 2x2 figure, with the horizontal axis being symptoms severity, and the vertical (risk) either FEV1 or exacerbation history (Figure 1). If there is a discrepancy between the risk judged by lung function and history of exacerbations, it is recommended to use the answer corresponding to the worse category.12
GOLD Guideline-Based Treatment
The new classification should also help to identify the patient’s main needs: controlling symptoms, reducing future risks, or both. Based on the results of available randomized clinical trials, GOLD guideline developers suggest grouptailored strategies of management (Table 2, Figure 2).
Group A: Low Risk and Low Symptoms
The goal is to treat only as needed, using shortacting medications. No preference was given to the type of short-acting medication and the practitioner could select between short-acting beta agonists (SABAs) or short-acting anticholinergic (also known as short-acting antimuscarinic [SAMA]) medication as first-line therapy. Second-line therapy includes either the combination of both families of short-acting medications in 1 inhaler, or the use of 1 long-acting inhaler. As a rule of thumb, no patient in this group should be on more than 1 inhaler, and the combination of short and long-acting medications is not part of the recommendations. Patients in group A, and indeed everyone with COPD, benefit from respiratory immunizations and tobacco cessation.
Group B: Low Risk, High Symptoms
Again, the goal of treatment is symptom control. Based on the available evidence, this can be achieved using long-acting bronchodilators, without the need of inhaled corticosteroids (ICS). The first line of treatment should be just 1 bronchodilator, either a long-acting antimuscarinic (LAMA) or long-actingbeta agonist (LABA). These could be used together as second-line treatment (LAMA plus LABA), still without indication for ICS. It is important to remember that dyspnea, or other symptoms, could also be a manifestation of comorbid conditions, such as cardiovascular disease, obesity, deconditioning, and musculoskeletal diseases.13 When spirometry is not used to confirm the diagnosis of COPD, patients may receive incremental types of inhalers instead of being evaluated for other causes of dyspnea, which might have led to more appropriate specific therapy.14 As a result, judicious evaluation of the patient’s symptomsis recommended. The guidelines also recommend programs that increase physical activity for this group of patients, as well to those in groups C and D, as this can improve symptoms and decrease risk of exacerbations.15
Group C: High Risk, Low Symptoms
The combination of ICS/LABA is the first-line therapy for this group, based on data showing the superiority of the ICS/LABA combination over monotherapy to reduce exacerbations and symptoms, as well as to improve QOL.16,17 Monotherapy LABA is also a first-line GOLD recommendation. Selecting between ICS/LABA vs LABA should be individualized based on the reason that the patient was judged as high risk. In the authors’ practice, if
the risk is based only in spirometry values, using LABA as monotherapy is a good choice, while if the definition of high risk was based on the frequency of exacerbations, ICS/LABA is the first choice. The GOLD guidelines list the combination of LABA/LAMA as second-line therapy.
Group D: High Risk and High Symptoms
First-line therapy for this group is essentially the same that for group C, with similar considerations. The combination of LABA/LAMA is also recommended as second-line therapy, as well as the use of ICS/LABA and LAMA (all 3 major classes of controller medications together). It is worth noting that phosphodiesterase-4 inhibitors (PDE4-inh, roflumilast being the best known) can be considered as a third-line of therapy (in group C) or as part of secondline combinations (in group D).
Benefits and Limitations of GOLD
There is no doubt that the new classification system and treatment guidelines are a significant step forward, intended to foster the development of more personalized decisions for COPD patients. The guidelines are the first attempt to incorporate the concepts of phenotypes (frequent exacerbation phenotype), disease heterogeneity (the variation in outcomes for the same degree of airflow obstruction), and the differences between the burden of symptoms and the risk of outcomes. The guidelines incorporate the need to weigh the benefits and risks of medications at the individual level (eg, ICS without an accompanying long-acting agent are not recommended in any group, and ICS use is reserved for those with high risk, especially if the designation is based on exacerbation frequency). The guidelines also stress the importance of examining comorbidities, emphysizing that their management should in no way be altered just because the patient also has COPD. Relative to the previous staging based only on FEV1 values, this new classification system has been shown to have appropriate predictive ability and association with the risk of exacerbations, and better correlation with measures of quality of life and costs of care.18,19 The guidelines, initially released in 2011 and slightly updated recently, are in continuous development and have been subject to intense evaluation.
Some limitations have been found (eg, the classification is still not the best predictor of mortality, but has the same ability to predict hospital admission as the previous spirometry-based system).18,20,21 Hence, it should be no surprise that modifications will likely be released in the near future.
The treatment recommendations associated with the current classification are based on the best evidence available and expert opinion, as no published clinical trials have compared the group-based therapy system to standard therapies. Evaluations of their effectiveness in real-life practice are still to be released. Previous, less complex guidelines, based on spirometry stages, were followed < 60% of the time in actual practice, thus it will be surprising to find high adherence to the current recommendations, but evaluations are still in progress.22
Conclusion
The best way for primary care providers to incorporate the GOLD guidelines into daily practice is to remember that COPD is very heterogeneous. Although spirometry is important, it is also essential to inquire about exacerbation frequency and symptoms severity. It is encouraging that for each of the relevant questions needed to classify the patient, there is a clear, easy to remember cut point. First, look at symptoms (low or high burden, based on the presence of dyspnea), then to judge risk look at FEV1 percentage predicted (using 50% as a cutpoint) and at exacerbation frequency (using 2 per year as the cut point). With those simple questions, build the groups, based on the combination of answers, and select the appropriate therapy. The general assumptions are that short-acting medications are appropriate for infrequent symptoms, long-acting medications are used to control symptoms and prevent exacerbations in more severe disease, and that ICS (always in combination with LABA) are reserved for those in the high-risk groups, especially if high risk is defined by frequent exacerbations. This summary should be supplemented with the judicious use of the tables and figures provided in this review, and available with detailed description and discussion in the original sources.1
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner; Frontline Medical Communications Inc.; the Department of Defense, or its Components; and the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347-365.
2. Agusti A, Calverley PM, Celli B, et al; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) investigators. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:122.
3. Jones PW. Health status and the spiral of decline. COPD. 2009;6(1):59-63.
4. Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182(5):598-604.
5. Han MK, Kazerooni EA, Lynch DA, et al; COPDGene Investigators. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology. 2011;261(1):274-282.
6. Kim V, Han MK, Vance GB, et al; COPDGene Investigators. The chronic bronchitic phenotype of COPD: an analysis of the COPDGene Study. Chest. 2011;140(3):626-633.
7. Wedzicha JA, Brill SE, Allinson JP, Donaldson GC. Mechanisms and impact of the frequent exacerbator phenotype in chronic obstructive pulmonary disease. BMC Med. 2013;11:181.
8. Hurst JR, Vestbo J, Anzueto A, et al; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128-1138.
9. Qaseem A, Wilt TJ, Weinberger SE, et al; American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179-191.
10. Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121(5):1434-1440.
11. Lee SD, Huang MS, Kang J, et al; Investigators of the Predictive Ability of CAT in Acute Exacerbations of COPD (PACE) Study. The COPD assessment test (CAT) assists prediction of COPD exacerbations in high-risk patients. Respir Med. 2014;108(4):600-608.
12. Haughney J, Gruffydd-Jones K, Roberts J, Lee AJ, Hardwell A, McGarvey L. The distribution of COPD in UK general practice using the new GOLD classification. Eur Respir J. 2014;43(4):993-1002.
13. Martinez CH, Han MK. Contribution of the environment and comorbidities to chronic obstructive pulmonary disease phenotypes. Med Clin North Am. 2012;96(4):713-727.
14. Collins BF, Feemster LC, Rinne ST, Au DH. Factors predictive of airflow obstruction among veterans with presumed empiric diagnosis and treatment of COPD. Chest. 2015;147(2):369-376.
15. Puhan MA, Gimeno-Santos E, Scharplatz M, Troosters T, Walters EH, Steurer J. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;(10):CD005305.
16. Nannini LJ, Poole P, Milan SJ, Kesterton A. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus inhaled corticosteroids alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;8:CD006826.
17. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9:CD006829.
18. Goossens LM, Leimer I, Metzdorf N, Becker K, Rutten-van Mölken MP. Does the 2013 GOLD classification improve the ability to predict lung function decline, exacerbations and mortality: a post-hoc analysis of the 4-year UPLIFT trial. BMC Pulm Med. 2014;14:163.
19. Boland MR, Tsiachristas A, Kruis AL, Chavannes NH, Rutten-van Mölken MP. Are GOLD ABCD groups better associated with health status and costs than GOLD 1234 grades? A cross-sectional study. Prim Care Respir J. 2014;23(1):30-37.
20. Han MK, Muellerova H, Curran-Everett D, et al. GOLD 2011 disease severity classification in COPDGene: a prospective cohort study. Lancet Respir Med. 2013;(1):43-50.
21. Johannessen A, Nilsen RM, Storebø M, Gulsvik A, Eagan T, Bakke P. Comparison of 2011 and 2007 Global Initiative for Chronic Obstructive Lung Disease guidelines for predicting mortality and hospitalization. Am J Respir Crit Care Med. 2013;188(1):51-59.
22. Sharif R, Cuevas CR, Wang Y, Arora M, Sharma G. Guideline adherence in management of stable chronic obstructive pulmonary disease. Respir Med. 2013;107(7):1046-1052.
1. Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347-365.
2. Agusti A, Calverley PM, Celli B, et al; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) investigators. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:122.
3. Jones PW. Health status and the spiral of decline. COPD. 2009;6(1):59-63.
4. Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182(5):598-604.
5. Han MK, Kazerooni EA, Lynch DA, et al; COPDGene Investigators. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology. 2011;261(1):274-282.
6. Kim V, Han MK, Vance GB, et al; COPDGene Investigators. The chronic bronchitic phenotype of COPD: an analysis of the COPDGene Study. Chest. 2011;140(3):626-633.
7. Wedzicha JA, Brill SE, Allinson JP, Donaldson GC. Mechanisms and impact of the frequent exacerbator phenotype in chronic obstructive pulmonary disease. BMC Med. 2013;11:181.
8. Hurst JR, Vestbo J, Anzueto A, et al; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128-1138.
9. Qaseem A, Wilt TJ, Weinberger SE, et al; American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179-191.
10. Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121(5):1434-1440.
11. Lee SD, Huang MS, Kang J, et al; Investigators of the Predictive Ability of CAT in Acute Exacerbations of COPD (PACE) Study. The COPD assessment test (CAT) assists prediction of COPD exacerbations in high-risk patients. Respir Med. 2014;108(4):600-608.
12. Haughney J, Gruffydd-Jones K, Roberts J, Lee AJ, Hardwell A, McGarvey L. The distribution of COPD in UK general practice using the new GOLD classification. Eur Respir J. 2014;43(4):993-1002.
13. Martinez CH, Han MK. Contribution of the environment and comorbidities to chronic obstructive pulmonary disease phenotypes. Med Clin North Am. 2012;96(4):713-727.
14. Collins BF, Feemster LC, Rinne ST, Au DH. Factors predictive of airflow obstruction among veterans with presumed empiric diagnosis and treatment of COPD. Chest. 2015;147(2):369-376.
15. Puhan MA, Gimeno-Santos E, Scharplatz M, Troosters T, Walters EH, Steurer J. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;(10):CD005305.
16. Nannini LJ, Poole P, Milan SJ, Kesterton A. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus inhaled corticosteroids alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;8:CD006826.
17. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9:CD006829.
18. Goossens LM, Leimer I, Metzdorf N, Becker K, Rutten-van Mölken MP. Does the 2013 GOLD classification improve the ability to predict lung function decline, exacerbations and mortality: a post-hoc analysis of the 4-year UPLIFT trial. BMC Pulm Med. 2014;14:163.
19. Boland MR, Tsiachristas A, Kruis AL, Chavannes NH, Rutten-van Mölken MP. Are GOLD ABCD groups better associated with health status and costs than GOLD 1234 grades? A cross-sectional study. Prim Care Respir J. 2014;23(1):30-37.
20. Han MK, Muellerova H, Curran-Everett D, et al. GOLD 2011 disease severity classification in COPDGene: a prospective cohort study. Lancet Respir Med. 2013;(1):43-50.
21. Johannessen A, Nilsen RM, Storebø M, Gulsvik A, Eagan T, Bakke P. Comparison of 2011 and 2007 Global Initiative for Chronic Obstructive Lung Disease guidelines for predicting mortality and hospitalization. Am J Respir Crit Care Med. 2013;188(1):51-59.
22. Sharif R, Cuevas CR, Wang Y, Arora M, Sharma G. Guideline adherence in management of stable chronic obstructive pulmonary disease. Respir Med. 2013;107(7):1046-1052.
The Asthma-COPD Overlap Syndrome
Asthma and chronic obstructive pulmonary disease (COPD) are common obstructive airway diseases frequently seen by clinicians in practice. Approximately 25 million Americans are reported to have asthma, and 15 million Americans have been diagnosed with COPD.1,2 An additional 24 million American adults have evidence of impaired lung function, suggestive of an under diagnosis of COPD.3 According to the National Heart, Lung and Blood Institute, the costs of COPD and asthma totaled $68.0 billion in 2008, of which $53.7 billion were direct costs.4 A subset of patients with asthma and COPD have characteristics of both disorders and are described clinically as having asthma-COPD overlap syndrome (ACOS).5 Patients with ACOS have a higher burden of disease and health care utilization and increasing recognition of this condition is critical. This article will review the identification, epidemiology, diagnostic evaluation, and basic treatment strategy for ACOS. This information should assist the primary care physician (PCP) in his or her approach to this condition.
The distinction between asthma and COPD is usually most evident to the clinician at the extremes of age. Asthma typically develops in childhood, manifests with classic symptoms of recurrent chest tightness, cough, wheeze, and dyspnea, and tends to be associated with atopic disorders. Chronic obstructive pulmonary disease typically manifests later in life, is insidious with productive cough and dyspnea being prominent symptoms, and tends to be associated with tobacco smoking. In addition, asthma is characterized by intermittent, reversible airflow obstruction, whereas COPD has persistent and irreversible airflow obstruction. As such, a nonsmoking atopic younger patient with a history of recurrent childhood wheezing with reversible airflow obstruction would favor a diagnosis of asthma. In contrast, an older patient with a history of tobacco smoking with chronic cough and dyspnea with evidence of fixed obstruction would favor a diagnosis of COPD.
Although asthma and COPD can present “classically,” many clinicians recognize that these disorders may present with overlapping features that make distinguishing between the two diagnostically challenging. Soriano and colleagues succinctly outlined the difficulties in distinguishing between asthma and COPD8:
- The conditions are viewed as part of a disease continuum;
- They have strong overlapping features
- There is no incentive to differentiate whether their treatment and prognosis are the same
- There are a lack of clear guidelines as to how the distinction can be made in clinical practice
- Uncertain criteria are used by physicians to classify patients as having asthma or COPD
The term ACOS is a clinical descriptive one and has not been clearly defined as evidenced by the multitude of descriptions in the literature. Soler-Cataluña and colleagues defined the clinical phenotype as “overlap phenotype COPD-asthma” based on the presence of major and minor criteria.9 Major criteria consisted of a postbronchodilator increase of forced expiratory volume in 1 second (FEV1) ≥ 12% and ≥ 400 mL, and eosinophilia in sputum in addition to a personal history of asthma. Minor criteria included high total immunoglobulinE (IgE), personal history of atopy, and a postbronchodilator increase of FEV1 ≥ 12% and ≥ 200 mL on ≥ 2 occasions.
Zeki and colleagues defined ACOS as: (1) asthma with partially reversible airflow obstruction, with or without emphysema or reduced carbon monoxide diffusion capacity (DLCO) to < 80% predicted; and (2) COPD with emphysema accompanied by reversible or partially reversible airflow obstruction, with or without environmental allergies or reduced DLCO.10 Louie and colleagues proposed the following major criteria for ACOS: a physician diagnosis of asthma and COPD in the same patient, history of evidence of atopy, elevated total IgE, aged ≥ 40 years, smoking > 10 pack-years, postbronchodilator FEV1< 80% predicted and FEV1/forced vital capacity (FVC) < 70%.11 Minor criteria consisted of a postbronchodilator increase of FEV1 by ≥ 15% or ≥ 12% and ≥ 200 mL following albuterol.
The Global Initiative for Asthma/Global Initiative for Chronic Obstructive Lung Disease published a joint consensus document on ACOS, which described a stepwise approach to diagnosis based on defining characteristics.5 To distinguish between the diagnosis of asthma, COPD, and ACOS in an adult patient, the guideline focuses on the features that are felt to be most helpful in distinguishing the syndromes in stepwise fashion. The physician should first assemble the features that favor a diagnosis of asthma or COPD, then compare the number of features in favor of a diagnosis of asthma or COPD, and finally consider the level of certainty around the diagnosis of asthma or COPD or whether there are features of both, suggesting ACOS.
Frequency
In 1995, the American Thoracic Society guidelines defined 11 distinct obstructive lung disease syndromes and identified overlap syndromes in 6 of them.12 Soriano and colleagues quantified the subpopulations of these patients by analyzing the U.S. National Health and Nutrition Examination III survey and the U.K. General Practice Research Database and reported an increased frequency of overlapping diagnosis of asthma and COPD with advancing age, with an estimated prevalence for < 10% in patients aged < 50 years and > 50% in patients aged ≥ 80 years.8 A study of patients aged > 50 years by Marsh and colleagues reported a combined syndrome of asthma and COPD to be the most common phenotype as confirmed by spirometry.13 In this study, 62% of subjects with the combined asthma and COPD phenotype were current or former smokers. In a study of 44 adults aged > 55 years with stable asthma or COPD, Gibson and colleague reported that 16% and 21%, respectively, could be categorized as having overlap syndrome.14 As in previous studies, those with overlap syndrome and COPD were predominantly ex-smokers.
Braman and colleagues characterized asthma in subjects aged > 70 years.15 Compared with those who developed asthma at an advanced age, those with early onset asthma had a significantly greater degree of airflow obstruction on pre- and postbronchodilator testing. This study suggested that long-standing asthma may lead to chronic persistent airflow obstruction and mimic COPD.
A longitudinal study by Vonk and colleagues reported that 16% of patients with asthma had developed incomplete airflow reversibility after 21 to 33 years of followup.16 De Marco and colleagues found the prevalence of asthma-COPD overlap to be 1.6%, 2.1%, and 4.5% in the 20 to 44, 45 to 64, and 65 to 84 years age groups, respectively, through a screening questionnaire of the general Italian population in concurrence with previous studies, noting an increased prevalence of ACOS in the elderly.17 Lee and colleagues described those with ACOS as older, male asthmatics, who have a higher lifetime smoking history and generally worse lung function.18
Quality of Life, Morbidity, and Moratality
In addition to being more prevalent in the elderly, ACOS is associated with more severe symptoms, impairment in quality of life (QOL), more frequent exacerbations, and high health care utilization. The ACOS phenotype is also at risk for accelerated decline in lung function secondary to its association with advancing age, tobacco smoking, presence of bronchial hyper-reactivity, and exacerbations.14
Burrows and colleagues described the characteristics and course of asthma in subjects aged > 65 years and concluded that asthma in this group may be associated with severe symptoms, higher death rates, and chronic airway obstruction.19 In this study, the subjects with suspected ACOS smoked at least 20 pack-years and had a significantly lower mean FEV1 (48.1% predicted ± 23.7) than any other group. Kauppi and colleagues reported on health-related QOL (HRQOL) and found that when compared to subjects with asthma or COPD only, the overlap group had the poorest HRQOL score.20 Chung and colleagues reported a similar reduction on self-rated health in the overlap group as well.21 Miravitles and colleagues reported that 17.4% of subjects previously diagnosed with COPD belonged to the COPD-asthma overlap phenotype.22 The overlap phenotype in this study had more dyspnea, wheezing, exacerbations, worse respiratory-specific QOL, and reduced levels of physical activity. Soriano and colleagues identified higher relative risks for pneumonia and respiratory infections in individuals aged > 65 years with asthma and COPD.23 In a study of hospital discharge registry data covering the Finnish population, Andersén and colleagues reported that the average numbers of treatment periods during 2000 to 2009 were 2.1 in asthma, 3.4 in COPD, and 6.0 in ACOS.24 Panizza and colleagues reported that long-standing asthma was associated with chronic airflow obstruction and increased risk of mortality.25
Although patients with both asthma and COPD are at risk for exacerbations, those with ACOS are at risk for more frequent and severe exacerbations.26 In the PLATINO study population, subjects with ACOS had higher risk for exacerbations, hospitalization, and worse general health status when compared with those with COPD.27 Frequent exacerbations of COPD leads to a greater loss of lung function compared with those who have infrequent exacerbations.14 A lower FEV1 is associated with increased disease severity in both asthma and COPD, and this is of particular concern to those with ACOS.
Of significance is the association of the ACOS phenotype with tobacco smoking. Although asthma is a risk factor for accelerated lung function decline, smoking status significantly accelerates the decline, and the loss may be even greater in those with asthma who smoke.28,29 This can ultimately predispose patients to the ACOS phenotype. Fortunately, quitting smoking can slow the decline in lung function as reported in the Lung Health Study.30 The annual decline in FEV1 in subjects who quit smoking at the beginning of the 11-year study was 30.2 mL /year for men and 21.5 mL /year for women. For those who continued smoking, the decline in FEV1 was 66.1 mL /year in men and 54.2 mL /year in women. For those with ACOS, treating tobacco use and dependence should be regarded as a primary and specific intervention.
Diagnosis
Spirometry is required for the appropriate diagnosis of obstructive lung disease and should be performed at least annually for assessment of control and disease progression.5,31,32 Postbronchodilator spirometry is necessary to determine whether obstruction (ie, FEV1/FVC < 0.7), if present, is reversible.32 In asthma, airway obstruction following bronchodilator administration is typically fully reversible.5 In COPD, patients will remain obstructed following postbronchodilator administration regardless of the FEV1 response.32 In ACOS, the postbronchodilator FEV1/FVC typically remains obstructed.5 A normal postbronchodilator FEV1/FVC is not compatible with the diagnosis of ACOS unless there is other evidence of chronic airflow limitation.5 Although spirometry confirms the presence of chronic airflow obstruction, it is of limited value in distinguishing between asthma with fixed airflow obstruction, COPD, and ACOS.5 At times, specialized investigations, such as carbon monoxide diffusion capacity on pulmonary function testing and chest imaging, may also be used to help distinguish between asthma and COPD.5,31,32
Treatment
Although much has been published on the recognition and identification of ACOS, there is a paucity of information on the effectiveness of therapeutics for this population. Patients with ACOS are frequently excluded from clinical studies involving asthma and COPD, which limits the generalization of findings from these trials to these patients. Although a comprehensive review of the available treatments for obstructive airway disease is beyond the scope of this article, some management tenets will be discussed.
In general, inhaled corticosteroids (ICS) are the cornerstone of the pharmacologic management of patients with persistent asthma, whereas inhaled bronchodilators (beta 2-agonists and anticholinergics) are the therapeutic mainstay for patients with COPD.31,32 In those with ACOS, the default position should be to start treatment with low or moderate dose ICS in recognition of the role of ICS in preventing morbidity and mortality in those with asthma.5 Depending on severity, a long-acting beta 2-agonist (LABA) could be added or continued if already prescribed for those with ACOS.5 Patients should not be treated with a LABA without ICS if there are features of asthma.5
Treatment of ACOS should also include advice about other therapeutic strategies such as smoking cessation, pulmonary rehabilitation, influenza and pneumococcal vaccinations, and treatment of other comorbid conditions.5 The treatment goals of ACOS are similar to those of asthma and COPD in that they are driven by controlling symptoms, optimizing health status and QOL, and preventing exacerbations. Although there are currently no disease-modifying medications that can alter the progression of airway obstruction in either asthma or COPD, smoking cessation is an essential component of the successful management of all obstructive airway disorders, because it is a modifiable risk factor.
The initial management of asthma and COPD can be carried out at the primary care level. All current guidelines for asthma, COPD, and ACOS provide
recommendations for specialty referral for further diagnostic and therapeutic consideations.5,31,32 As ACOS is associated with more severe disease and greater health care utilization, specialty referral for this subgroup should be considered.
Conclusion
Although there is no generally agreed term or defining features for ACOS, it is commonly recognized that a proportion of older patients who present with symptoms of chronic airway obstruction have features of both asthma and COPD. It is broadly recognized that distinguishing asthma from COPD can be problematic, particularly in smokers and the elderly. In addition, as these patients have frequent exacerbations, a poor QOL, a more rapid decline in lung function, and high mortality, identification of this subgroup is important. The lack of clinical trials to help guide therapeutic interventions in this syndrome is problematic as the extrapolation of data from asthma and/or COPD trials may not be applicable. Further studies in therapeutics for those with ACOS are warranted.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner; Frontline Medical Communications Inc.; the Department of Defense, or its Components; and the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Centers for Disease Control and Prevention. Asthma in the US: CDC Vital Signs. CDC Website. http://www.cdc.gov/vitalsigns/asthma/. Updated May 3, 2011. Accessed October 27, 2014.
2. Centers for Disease Control and Prevention. What is COPD? CDC Website. http://www.cdc.gov/copd/. Updated November 13, 2013. Accessed October 27, 2014.
3. American Lung Association. Chronic Obstructive Pulmonary Disease (COPD) Fact Sheet. American Lung Association Website. http://www.lung.org/lung-disease/copd/resources/facts-figures/COPD-Fact-Sheet.html. Published May 2014. Accessed October 27, 2014.
4. National Heart, Lung, and Blood Institute. Morbidity and Mortality: 2012 Chart Book on Cardiovascular, Lung and Blood Diseases. National Heart, Lung, and Blood Institute Website. https://www.nhlbi.nih.gov/files/docs/research/2012_ChartBook_508.pdf. Accessed January 6, 2015.
5. Global Initiative for Asthma/Global Initiative for Chronic Obstructive Lung Disease. Diagnosis of Diseases of Chronic Airflow Limitation: Asthma COPD and Asthma-COPD Overlap Syndrome (ACOS). Global Initiative for Asthma Website. http://www.ginasthma.org/documents/14. Accessed August 10, 2015.
6. Tam A, Sin DD. Pathobiologic mechanisms of chronic obstructive pulmonary disease. Med Clin North Am. 2012;96(4):681-698.
7. Silva GE, Sherrill DL, Guerra S, Barbee RA. Asthma as a risk factor for COPD in a longitudinal study. Chest. 2004;126(1):59-65.
8. Soriano JB, Davis KJ, Coleman B, Visick G, Mannino D, Pride NB. The proportional Venn diagram of obstructive lung disease: two approximations from the United States and the United Kingdom. Chest. 2003;124(2):474-481.
9. Soler-Cataluña JJ, Cosío B, Izquierdo JL, et al. Consensus document on the overlap phenotype COPD-asthma in COPD. Arch Bronconeumol. 2012;48(9):331-337.
10. Zeki AA, Schivo M, Chan A, Albertson TE, Louie S. The asthma-COPD overlap syndrome: a common clinical problem in the elderly. J Allergy (Cairo). 2011;2011:861926.
11. Louie S, Zeki AA, Schivo M, et al. The asthma-chronic obstructive pulmonary disease overlap syndrome: pharmacotherapeutic considerations. Expert Rev Clin Pharmacol. 2013;6(2):197-219.
12. American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1995;152 (5 pt 2):S77-S121.
13. Marsh SE, Travers J, Weatherall M, et al. Proportional classifications of COPD phenotypes [published correction appears in Thorax. 2014;69(7):672]. Thorax. 2008;63(9):761-767.
14. Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax. 2009;64(8):728-735.
15. Braman SS, Kaemmerlen JT, Davis SM. Asthma in the elderly: a comparison between patients with recently acquired and long-standing disease. Am Rev Respir Dis. 1991;143(2):336-340.
16. Vonk JM, Jongepier H, Panhuysen Cl, Schouten JP, Bleecker ER, Postma DS. Risk factors associated with the presence of irreversible airflow limitation and reduced transfer coefficient in patients with asthma after 26 years of follow up. Thorax. 2003;58(4):322-327.
17. de Marco R, Pesce G, Marcon A, et al. The coexistence of asthma and chronic obstructive pulmonary disease (COPD): prevalence and risk factors in young, middle-aged and elderly people from the general population. PLoS One. 2013;8(5):e62985.
18. Lee HY, Kang JY, Yoon HK, et al. Clinical characteristics of asthma combined with COPD feature. Yonsei Med J. 2014;55(4):980-986.
19. Burrows B, Barbee RA, Cline MG, Knudson RJ, Lebowitz MD. Characteristics of asthma among elderly adults in a sample of the general population. Chest. 1991;100(4):935-942.
20. Kauppi P, Kupiainen H, Lindqvust A, et al. Overlap syndrome of asthma and COPD predicts low quality of life. J Asthma. 2011;48(3):279-285.
21. Chung JW, Kong KA, Lee JH, Lee SJ, Ryu YJ, Chang JH. Characteristics and self-rated health of overlap syndrome. Int J Chron Obstruct Pulmon Dis. 2014;9:795-804.
22. Miravitles M, Soriano JB, Ancochea J, et al. Characterisation of the overlap COPDasthma phenotype. Focus on physical activity and health status. Respir Med. 2013;107(7):1053-1060.
23. Soriano JB, Visick GT, Mullerova H, Payvandi N, Hansell AL. Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest. 2005;128(4):2099-2107.
24. Andersén H, Lampela P, Nevanlinna A, SäynäJakangas O, Keistinen T. High hospital burden in overlap syndrome of asthma and COPD. Clin Respir J. 2013;7(4):342-346.
25. Panizza JA, James AL, Ryan G, de Klerk N, Finucane KE. Mortality and airflow obstruction in asthma: a 17-year follow-up study. Intern Med J. 2006;36(12):773-780.
26. Hardin M, Silverman EK, Barr RG, et al; COPDGene Investigators. The clinical features of the overlap between COPD and asthma. Respir Res. 2011;12:127.
27. Menezes AM, Montes de Oca M, Pérez-Padilla R, et al; PLATINO Team. Increased risk of exacerbation and hospitalization in subjects with an overlap phenotype: COPD-asthma. Chest. 2014;145(2):297-304.
28. Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med. 1998;339(17):1194-1200.
29. James AL, Palmer LJ, Kicic E, et al. Decline in lung function in the Busselton Health Study: the effects of asthma and cigarette smoking. Am J Respir Crit Care Med. 2005;171(2):109-114.
30. Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med. 2002;166(5):675-679.
31. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma Website. http://www.ginasthma.org/documents/4. Revised 2014. Accessed October 27, 2014.
32. Global Initiative for Chronic Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Global Initiative for Chronic Lung Disease Website. http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis management.html. Published January 2014. Accessed 27 October 2014.
Asthma and chronic obstructive pulmonary disease (COPD) are common obstructive airway diseases frequently seen by clinicians in practice. Approximately 25 million Americans are reported to have asthma, and 15 million Americans have been diagnosed with COPD.1,2 An additional 24 million American adults have evidence of impaired lung function, suggestive of an under diagnosis of COPD.3 According to the National Heart, Lung and Blood Institute, the costs of COPD and asthma totaled $68.0 billion in 2008, of which $53.7 billion were direct costs.4 A subset of patients with asthma and COPD have characteristics of both disorders and are described clinically as having asthma-COPD overlap syndrome (ACOS).5 Patients with ACOS have a higher burden of disease and health care utilization and increasing recognition of this condition is critical. This article will review the identification, epidemiology, diagnostic evaluation, and basic treatment strategy for ACOS. This information should assist the primary care physician (PCP) in his or her approach to this condition.
The distinction between asthma and COPD is usually most evident to the clinician at the extremes of age. Asthma typically develops in childhood, manifests with classic symptoms of recurrent chest tightness, cough, wheeze, and dyspnea, and tends to be associated with atopic disorders. Chronic obstructive pulmonary disease typically manifests later in life, is insidious with productive cough and dyspnea being prominent symptoms, and tends to be associated with tobacco smoking. In addition, asthma is characterized by intermittent, reversible airflow obstruction, whereas COPD has persistent and irreversible airflow obstruction. As such, a nonsmoking atopic younger patient with a history of recurrent childhood wheezing with reversible airflow obstruction would favor a diagnosis of asthma. In contrast, an older patient with a history of tobacco smoking with chronic cough and dyspnea with evidence of fixed obstruction would favor a diagnosis of COPD.
Although asthma and COPD can present “classically,” many clinicians recognize that these disorders may present with overlapping features that make distinguishing between the two diagnostically challenging. Soriano and colleagues succinctly outlined the difficulties in distinguishing between asthma and COPD8:
- The conditions are viewed as part of a disease continuum;
- They have strong overlapping features
- There is no incentive to differentiate whether their treatment and prognosis are the same
- There are a lack of clear guidelines as to how the distinction can be made in clinical practice
- Uncertain criteria are used by physicians to classify patients as having asthma or COPD
The term ACOS is a clinical descriptive one and has not been clearly defined as evidenced by the multitude of descriptions in the literature. Soler-Cataluña and colleagues defined the clinical phenotype as “overlap phenotype COPD-asthma” based on the presence of major and minor criteria.9 Major criteria consisted of a postbronchodilator increase of forced expiratory volume in 1 second (FEV1) ≥ 12% and ≥ 400 mL, and eosinophilia in sputum in addition to a personal history of asthma. Minor criteria included high total immunoglobulinE (IgE), personal history of atopy, and a postbronchodilator increase of FEV1 ≥ 12% and ≥ 200 mL on ≥ 2 occasions.
Zeki and colleagues defined ACOS as: (1) asthma with partially reversible airflow obstruction, with or without emphysema or reduced carbon monoxide diffusion capacity (DLCO) to < 80% predicted; and (2) COPD with emphysema accompanied by reversible or partially reversible airflow obstruction, with or without environmental allergies or reduced DLCO.10 Louie and colleagues proposed the following major criteria for ACOS: a physician diagnosis of asthma and COPD in the same patient, history of evidence of atopy, elevated total IgE, aged ≥ 40 years, smoking > 10 pack-years, postbronchodilator FEV1< 80% predicted and FEV1/forced vital capacity (FVC) < 70%.11 Minor criteria consisted of a postbronchodilator increase of FEV1 by ≥ 15% or ≥ 12% and ≥ 200 mL following albuterol.
The Global Initiative for Asthma/Global Initiative for Chronic Obstructive Lung Disease published a joint consensus document on ACOS, which described a stepwise approach to diagnosis based on defining characteristics.5 To distinguish between the diagnosis of asthma, COPD, and ACOS in an adult patient, the guideline focuses on the features that are felt to be most helpful in distinguishing the syndromes in stepwise fashion. The physician should first assemble the features that favor a diagnosis of asthma or COPD, then compare the number of features in favor of a diagnosis of asthma or COPD, and finally consider the level of certainty around the diagnosis of asthma or COPD or whether there are features of both, suggesting ACOS.
Frequency
In 1995, the American Thoracic Society guidelines defined 11 distinct obstructive lung disease syndromes and identified overlap syndromes in 6 of them.12 Soriano and colleagues quantified the subpopulations of these patients by analyzing the U.S. National Health and Nutrition Examination III survey and the U.K. General Practice Research Database and reported an increased frequency of overlapping diagnosis of asthma and COPD with advancing age, with an estimated prevalence for < 10% in patients aged < 50 years and > 50% in patients aged ≥ 80 years.8 A study of patients aged > 50 years by Marsh and colleagues reported a combined syndrome of asthma and COPD to be the most common phenotype as confirmed by spirometry.13 In this study, 62% of subjects with the combined asthma and COPD phenotype were current or former smokers. In a study of 44 adults aged > 55 years with stable asthma or COPD, Gibson and colleague reported that 16% and 21%, respectively, could be categorized as having overlap syndrome.14 As in previous studies, those with overlap syndrome and COPD were predominantly ex-smokers.
Braman and colleagues characterized asthma in subjects aged > 70 years.15 Compared with those who developed asthma at an advanced age, those with early onset asthma had a significantly greater degree of airflow obstruction on pre- and postbronchodilator testing. This study suggested that long-standing asthma may lead to chronic persistent airflow obstruction and mimic COPD.
A longitudinal study by Vonk and colleagues reported that 16% of patients with asthma had developed incomplete airflow reversibility after 21 to 33 years of followup.16 De Marco and colleagues found the prevalence of asthma-COPD overlap to be 1.6%, 2.1%, and 4.5% in the 20 to 44, 45 to 64, and 65 to 84 years age groups, respectively, through a screening questionnaire of the general Italian population in concurrence with previous studies, noting an increased prevalence of ACOS in the elderly.17 Lee and colleagues described those with ACOS as older, male asthmatics, who have a higher lifetime smoking history and generally worse lung function.18
Quality of Life, Morbidity, and Moratality
In addition to being more prevalent in the elderly, ACOS is associated with more severe symptoms, impairment in quality of life (QOL), more frequent exacerbations, and high health care utilization. The ACOS phenotype is also at risk for accelerated decline in lung function secondary to its association with advancing age, tobacco smoking, presence of bronchial hyper-reactivity, and exacerbations.14
Burrows and colleagues described the characteristics and course of asthma in subjects aged > 65 years and concluded that asthma in this group may be associated with severe symptoms, higher death rates, and chronic airway obstruction.19 In this study, the subjects with suspected ACOS smoked at least 20 pack-years and had a significantly lower mean FEV1 (48.1% predicted ± 23.7) than any other group. Kauppi and colleagues reported on health-related QOL (HRQOL) and found that when compared to subjects with asthma or COPD only, the overlap group had the poorest HRQOL score.20 Chung and colleagues reported a similar reduction on self-rated health in the overlap group as well.21 Miravitles and colleagues reported that 17.4% of subjects previously diagnosed with COPD belonged to the COPD-asthma overlap phenotype.22 The overlap phenotype in this study had more dyspnea, wheezing, exacerbations, worse respiratory-specific QOL, and reduced levels of physical activity. Soriano and colleagues identified higher relative risks for pneumonia and respiratory infections in individuals aged > 65 years with asthma and COPD.23 In a study of hospital discharge registry data covering the Finnish population, Andersén and colleagues reported that the average numbers of treatment periods during 2000 to 2009 were 2.1 in asthma, 3.4 in COPD, and 6.0 in ACOS.24 Panizza and colleagues reported that long-standing asthma was associated with chronic airflow obstruction and increased risk of mortality.25
Although patients with both asthma and COPD are at risk for exacerbations, those with ACOS are at risk for more frequent and severe exacerbations.26 In the PLATINO study population, subjects with ACOS had higher risk for exacerbations, hospitalization, and worse general health status when compared with those with COPD.27 Frequent exacerbations of COPD leads to a greater loss of lung function compared with those who have infrequent exacerbations.14 A lower FEV1 is associated with increased disease severity in both asthma and COPD, and this is of particular concern to those with ACOS.
Of significance is the association of the ACOS phenotype with tobacco smoking. Although asthma is a risk factor for accelerated lung function decline, smoking status significantly accelerates the decline, and the loss may be even greater in those with asthma who smoke.28,29 This can ultimately predispose patients to the ACOS phenotype. Fortunately, quitting smoking can slow the decline in lung function as reported in the Lung Health Study.30 The annual decline in FEV1 in subjects who quit smoking at the beginning of the 11-year study was 30.2 mL /year for men and 21.5 mL /year for women. For those who continued smoking, the decline in FEV1 was 66.1 mL /year in men and 54.2 mL /year in women. For those with ACOS, treating tobacco use and dependence should be regarded as a primary and specific intervention.
Diagnosis
Spirometry is required for the appropriate diagnosis of obstructive lung disease and should be performed at least annually for assessment of control and disease progression.5,31,32 Postbronchodilator spirometry is necessary to determine whether obstruction (ie, FEV1/FVC < 0.7), if present, is reversible.32 In asthma, airway obstruction following bronchodilator administration is typically fully reversible.5 In COPD, patients will remain obstructed following postbronchodilator administration regardless of the FEV1 response.32 In ACOS, the postbronchodilator FEV1/FVC typically remains obstructed.5 A normal postbronchodilator FEV1/FVC is not compatible with the diagnosis of ACOS unless there is other evidence of chronic airflow limitation.5 Although spirometry confirms the presence of chronic airflow obstruction, it is of limited value in distinguishing between asthma with fixed airflow obstruction, COPD, and ACOS.5 At times, specialized investigations, such as carbon monoxide diffusion capacity on pulmonary function testing and chest imaging, may also be used to help distinguish between asthma and COPD.5,31,32
Treatment
Although much has been published on the recognition and identification of ACOS, there is a paucity of information on the effectiveness of therapeutics for this population. Patients with ACOS are frequently excluded from clinical studies involving asthma and COPD, which limits the generalization of findings from these trials to these patients. Although a comprehensive review of the available treatments for obstructive airway disease is beyond the scope of this article, some management tenets will be discussed.
In general, inhaled corticosteroids (ICS) are the cornerstone of the pharmacologic management of patients with persistent asthma, whereas inhaled bronchodilators (beta 2-agonists and anticholinergics) are the therapeutic mainstay for patients with COPD.31,32 In those with ACOS, the default position should be to start treatment with low or moderate dose ICS in recognition of the role of ICS in preventing morbidity and mortality in those with asthma.5 Depending on severity, a long-acting beta 2-agonist (LABA) could be added or continued if already prescribed for those with ACOS.5 Patients should not be treated with a LABA without ICS if there are features of asthma.5
Treatment of ACOS should also include advice about other therapeutic strategies such as smoking cessation, pulmonary rehabilitation, influenza and pneumococcal vaccinations, and treatment of other comorbid conditions.5 The treatment goals of ACOS are similar to those of asthma and COPD in that they are driven by controlling symptoms, optimizing health status and QOL, and preventing exacerbations. Although there are currently no disease-modifying medications that can alter the progression of airway obstruction in either asthma or COPD, smoking cessation is an essential component of the successful management of all obstructive airway disorders, because it is a modifiable risk factor.
The initial management of asthma and COPD can be carried out at the primary care level. All current guidelines for asthma, COPD, and ACOS provide
recommendations for specialty referral for further diagnostic and therapeutic consideations.5,31,32 As ACOS is associated with more severe disease and greater health care utilization, specialty referral for this subgroup should be considered.
Conclusion
Although there is no generally agreed term or defining features for ACOS, it is commonly recognized that a proportion of older patients who present with symptoms of chronic airway obstruction have features of both asthma and COPD. It is broadly recognized that distinguishing asthma from COPD can be problematic, particularly in smokers and the elderly. In addition, as these patients have frequent exacerbations, a poor QOL, a more rapid decline in lung function, and high mortality, identification of this subgroup is important. The lack of clinical trials to help guide therapeutic interventions in this syndrome is problematic as the extrapolation of data from asthma and/or COPD trials may not be applicable. Further studies in therapeutics for those with ACOS are warranted.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner; Frontline Medical Communications Inc.; the Department of Defense, or its Components; and the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
Asthma and chronic obstructive pulmonary disease (COPD) are common obstructive airway diseases frequently seen by clinicians in practice. Approximately 25 million Americans are reported to have asthma, and 15 million Americans have been diagnosed with COPD.1,2 An additional 24 million American adults have evidence of impaired lung function, suggestive of an under diagnosis of COPD.3 According to the National Heart, Lung and Blood Institute, the costs of COPD and asthma totaled $68.0 billion in 2008, of which $53.7 billion were direct costs.4 A subset of patients with asthma and COPD have characteristics of both disorders and are described clinically as having asthma-COPD overlap syndrome (ACOS).5 Patients with ACOS have a higher burden of disease and health care utilization and increasing recognition of this condition is critical. This article will review the identification, epidemiology, diagnostic evaluation, and basic treatment strategy for ACOS. This information should assist the primary care physician (PCP) in his or her approach to this condition.
The distinction between asthma and COPD is usually most evident to the clinician at the extremes of age. Asthma typically develops in childhood, manifests with classic symptoms of recurrent chest tightness, cough, wheeze, and dyspnea, and tends to be associated with atopic disorders. Chronic obstructive pulmonary disease typically manifests later in life, is insidious with productive cough and dyspnea being prominent symptoms, and tends to be associated with tobacco smoking. In addition, asthma is characterized by intermittent, reversible airflow obstruction, whereas COPD has persistent and irreversible airflow obstruction. As such, a nonsmoking atopic younger patient with a history of recurrent childhood wheezing with reversible airflow obstruction would favor a diagnosis of asthma. In contrast, an older patient with a history of tobacco smoking with chronic cough and dyspnea with evidence of fixed obstruction would favor a diagnosis of COPD.
Although asthma and COPD can present “classically,” many clinicians recognize that these disorders may present with overlapping features that make distinguishing between the two diagnostically challenging. Soriano and colleagues succinctly outlined the difficulties in distinguishing between asthma and COPD8:
- The conditions are viewed as part of a disease continuum;
- They have strong overlapping features
- There is no incentive to differentiate whether their treatment and prognosis are the same
- There are a lack of clear guidelines as to how the distinction can be made in clinical practice
- Uncertain criteria are used by physicians to classify patients as having asthma or COPD
The term ACOS is a clinical descriptive one and has not been clearly defined as evidenced by the multitude of descriptions in the literature. Soler-Cataluña and colleagues defined the clinical phenotype as “overlap phenotype COPD-asthma” based on the presence of major and minor criteria.9 Major criteria consisted of a postbronchodilator increase of forced expiratory volume in 1 second (FEV1) ≥ 12% and ≥ 400 mL, and eosinophilia in sputum in addition to a personal history of asthma. Minor criteria included high total immunoglobulinE (IgE), personal history of atopy, and a postbronchodilator increase of FEV1 ≥ 12% and ≥ 200 mL on ≥ 2 occasions.
Zeki and colleagues defined ACOS as: (1) asthma with partially reversible airflow obstruction, with or without emphysema or reduced carbon monoxide diffusion capacity (DLCO) to < 80% predicted; and (2) COPD with emphysema accompanied by reversible or partially reversible airflow obstruction, with or without environmental allergies or reduced DLCO.10 Louie and colleagues proposed the following major criteria for ACOS: a physician diagnosis of asthma and COPD in the same patient, history of evidence of atopy, elevated total IgE, aged ≥ 40 years, smoking > 10 pack-years, postbronchodilator FEV1< 80% predicted and FEV1/forced vital capacity (FVC) < 70%.11 Minor criteria consisted of a postbronchodilator increase of FEV1 by ≥ 15% or ≥ 12% and ≥ 200 mL following albuterol.
The Global Initiative for Asthma/Global Initiative for Chronic Obstructive Lung Disease published a joint consensus document on ACOS, which described a stepwise approach to diagnosis based on defining characteristics.5 To distinguish between the diagnosis of asthma, COPD, and ACOS in an adult patient, the guideline focuses on the features that are felt to be most helpful in distinguishing the syndromes in stepwise fashion. The physician should first assemble the features that favor a diagnosis of asthma or COPD, then compare the number of features in favor of a diagnosis of asthma or COPD, and finally consider the level of certainty around the diagnosis of asthma or COPD or whether there are features of both, suggesting ACOS.
Frequency
In 1995, the American Thoracic Society guidelines defined 11 distinct obstructive lung disease syndromes and identified overlap syndromes in 6 of them.12 Soriano and colleagues quantified the subpopulations of these patients by analyzing the U.S. National Health and Nutrition Examination III survey and the U.K. General Practice Research Database and reported an increased frequency of overlapping diagnosis of asthma and COPD with advancing age, with an estimated prevalence for < 10% in patients aged < 50 years and > 50% in patients aged ≥ 80 years.8 A study of patients aged > 50 years by Marsh and colleagues reported a combined syndrome of asthma and COPD to be the most common phenotype as confirmed by spirometry.13 In this study, 62% of subjects with the combined asthma and COPD phenotype were current or former smokers. In a study of 44 adults aged > 55 years with stable asthma or COPD, Gibson and colleague reported that 16% and 21%, respectively, could be categorized as having overlap syndrome.14 As in previous studies, those with overlap syndrome and COPD were predominantly ex-smokers.
Braman and colleagues characterized asthma in subjects aged > 70 years.15 Compared with those who developed asthma at an advanced age, those with early onset asthma had a significantly greater degree of airflow obstruction on pre- and postbronchodilator testing. This study suggested that long-standing asthma may lead to chronic persistent airflow obstruction and mimic COPD.
A longitudinal study by Vonk and colleagues reported that 16% of patients with asthma had developed incomplete airflow reversibility after 21 to 33 years of followup.16 De Marco and colleagues found the prevalence of asthma-COPD overlap to be 1.6%, 2.1%, and 4.5% in the 20 to 44, 45 to 64, and 65 to 84 years age groups, respectively, through a screening questionnaire of the general Italian population in concurrence with previous studies, noting an increased prevalence of ACOS in the elderly.17 Lee and colleagues described those with ACOS as older, male asthmatics, who have a higher lifetime smoking history and generally worse lung function.18
Quality of Life, Morbidity, and Moratality
In addition to being more prevalent in the elderly, ACOS is associated with more severe symptoms, impairment in quality of life (QOL), more frequent exacerbations, and high health care utilization. The ACOS phenotype is also at risk for accelerated decline in lung function secondary to its association with advancing age, tobacco smoking, presence of bronchial hyper-reactivity, and exacerbations.14
Burrows and colleagues described the characteristics and course of asthma in subjects aged > 65 years and concluded that asthma in this group may be associated with severe symptoms, higher death rates, and chronic airway obstruction.19 In this study, the subjects with suspected ACOS smoked at least 20 pack-years and had a significantly lower mean FEV1 (48.1% predicted ± 23.7) than any other group. Kauppi and colleagues reported on health-related QOL (HRQOL) and found that when compared to subjects with asthma or COPD only, the overlap group had the poorest HRQOL score.20 Chung and colleagues reported a similar reduction on self-rated health in the overlap group as well.21 Miravitles and colleagues reported that 17.4% of subjects previously diagnosed with COPD belonged to the COPD-asthma overlap phenotype.22 The overlap phenotype in this study had more dyspnea, wheezing, exacerbations, worse respiratory-specific QOL, and reduced levels of physical activity. Soriano and colleagues identified higher relative risks for pneumonia and respiratory infections in individuals aged > 65 years with asthma and COPD.23 In a study of hospital discharge registry data covering the Finnish population, Andersén and colleagues reported that the average numbers of treatment periods during 2000 to 2009 were 2.1 in asthma, 3.4 in COPD, and 6.0 in ACOS.24 Panizza and colleagues reported that long-standing asthma was associated with chronic airflow obstruction and increased risk of mortality.25
Although patients with both asthma and COPD are at risk for exacerbations, those with ACOS are at risk for more frequent and severe exacerbations.26 In the PLATINO study population, subjects with ACOS had higher risk for exacerbations, hospitalization, and worse general health status when compared with those with COPD.27 Frequent exacerbations of COPD leads to a greater loss of lung function compared with those who have infrequent exacerbations.14 A lower FEV1 is associated with increased disease severity in both asthma and COPD, and this is of particular concern to those with ACOS.
Of significance is the association of the ACOS phenotype with tobacco smoking. Although asthma is a risk factor for accelerated lung function decline, smoking status significantly accelerates the decline, and the loss may be even greater in those with asthma who smoke.28,29 This can ultimately predispose patients to the ACOS phenotype. Fortunately, quitting smoking can slow the decline in lung function as reported in the Lung Health Study.30 The annual decline in FEV1 in subjects who quit smoking at the beginning of the 11-year study was 30.2 mL /year for men and 21.5 mL /year for women. For those who continued smoking, the decline in FEV1 was 66.1 mL /year in men and 54.2 mL /year in women. For those with ACOS, treating tobacco use and dependence should be regarded as a primary and specific intervention.
Diagnosis
Spirometry is required for the appropriate diagnosis of obstructive lung disease and should be performed at least annually for assessment of control and disease progression.5,31,32 Postbronchodilator spirometry is necessary to determine whether obstruction (ie, FEV1/FVC < 0.7), if present, is reversible.32 In asthma, airway obstruction following bronchodilator administration is typically fully reversible.5 In COPD, patients will remain obstructed following postbronchodilator administration regardless of the FEV1 response.32 In ACOS, the postbronchodilator FEV1/FVC typically remains obstructed.5 A normal postbronchodilator FEV1/FVC is not compatible with the diagnosis of ACOS unless there is other evidence of chronic airflow limitation.5 Although spirometry confirms the presence of chronic airflow obstruction, it is of limited value in distinguishing between asthma with fixed airflow obstruction, COPD, and ACOS.5 At times, specialized investigations, such as carbon monoxide diffusion capacity on pulmonary function testing and chest imaging, may also be used to help distinguish between asthma and COPD.5,31,32
Treatment
Although much has been published on the recognition and identification of ACOS, there is a paucity of information on the effectiveness of therapeutics for this population. Patients with ACOS are frequently excluded from clinical studies involving asthma and COPD, which limits the generalization of findings from these trials to these patients. Although a comprehensive review of the available treatments for obstructive airway disease is beyond the scope of this article, some management tenets will be discussed.
In general, inhaled corticosteroids (ICS) are the cornerstone of the pharmacologic management of patients with persistent asthma, whereas inhaled bronchodilators (beta 2-agonists and anticholinergics) are the therapeutic mainstay for patients with COPD.31,32 In those with ACOS, the default position should be to start treatment with low or moderate dose ICS in recognition of the role of ICS in preventing morbidity and mortality in those with asthma.5 Depending on severity, a long-acting beta 2-agonist (LABA) could be added or continued if already prescribed for those with ACOS.5 Patients should not be treated with a LABA without ICS if there are features of asthma.5
Treatment of ACOS should also include advice about other therapeutic strategies such as smoking cessation, pulmonary rehabilitation, influenza and pneumococcal vaccinations, and treatment of other comorbid conditions.5 The treatment goals of ACOS are similar to those of asthma and COPD in that they are driven by controlling symptoms, optimizing health status and QOL, and preventing exacerbations. Although there are currently no disease-modifying medications that can alter the progression of airway obstruction in either asthma or COPD, smoking cessation is an essential component of the successful management of all obstructive airway disorders, because it is a modifiable risk factor.
The initial management of asthma and COPD can be carried out at the primary care level. All current guidelines for asthma, COPD, and ACOS provide
recommendations for specialty referral for further diagnostic and therapeutic consideations.5,31,32 As ACOS is associated with more severe disease and greater health care utilization, specialty referral for this subgroup should be considered.
Conclusion
Although there is no generally agreed term or defining features for ACOS, it is commonly recognized that a proportion of older patients who present with symptoms of chronic airway obstruction have features of both asthma and COPD. It is broadly recognized that distinguishing asthma from COPD can be problematic, particularly in smokers and the elderly. In addition, as these patients have frequent exacerbations, a poor QOL, a more rapid decline in lung function, and high mortality, identification of this subgroup is important. The lack of clinical trials to help guide therapeutic interventions in this syndrome is problematic as the extrapolation of data from asthma and/or COPD trials may not be applicable. Further studies in therapeutics for those with ACOS are warranted.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner; Frontline Medical Communications Inc.; the Department of Defense, or its Components; and the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Centers for Disease Control and Prevention. Asthma in the US: CDC Vital Signs. CDC Website. http://www.cdc.gov/vitalsigns/asthma/. Updated May 3, 2011. Accessed October 27, 2014.
2. Centers for Disease Control and Prevention. What is COPD? CDC Website. http://www.cdc.gov/copd/. Updated November 13, 2013. Accessed October 27, 2014.
3. American Lung Association. Chronic Obstructive Pulmonary Disease (COPD) Fact Sheet. American Lung Association Website. http://www.lung.org/lung-disease/copd/resources/facts-figures/COPD-Fact-Sheet.html. Published May 2014. Accessed October 27, 2014.
4. National Heart, Lung, and Blood Institute. Morbidity and Mortality: 2012 Chart Book on Cardiovascular, Lung and Blood Diseases. National Heart, Lung, and Blood Institute Website. https://www.nhlbi.nih.gov/files/docs/research/2012_ChartBook_508.pdf. Accessed January 6, 2015.
5. Global Initiative for Asthma/Global Initiative for Chronic Obstructive Lung Disease. Diagnosis of Diseases of Chronic Airflow Limitation: Asthma COPD and Asthma-COPD Overlap Syndrome (ACOS). Global Initiative for Asthma Website. http://www.ginasthma.org/documents/14. Accessed August 10, 2015.
6. Tam A, Sin DD. Pathobiologic mechanisms of chronic obstructive pulmonary disease. Med Clin North Am. 2012;96(4):681-698.
7. Silva GE, Sherrill DL, Guerra S, Barbee RA. Asthma as a risk factor for COPD in a longitudinal study. Chest. 2004;126(1):59-65.
8. Soriano JB, Davis KJ, Coleman B, Visick G, Mannino D, Pride NB. The proportional Venn diagram of obstructive lung disease: two approximations from the United States and the United Kingdom. Chest. 2003;124(2):474-481.
9. Soler-Cataluña JJ, Cosío B, Izquierdo JL, et al. Consensus document on the overlap phenotype COPD-asthma in COPD. Arch Bronconeumol. 2012;48(9):331-337.
10. Zeki AA, Schivo M, Chan A, Albertson TE, Louie S. The asthma-COPD overlap syndrome: a common clinical problem in the elderly. J Allergy (Cairo). 2011;2011:861926.
11. Louie S, Zeki AA, Schivo M, et al. The asthma-chronic obstructive pulmonary disease overlap syndrome: pharmacotherapeutic considerations. Expert Rev Clin Pharmacol. 2013;6(2):197-219.
12. American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1995;152 (5 pt 2):S77-S121.
13. Marsh SE, Travers J, Weatherall M, et al. Proportional classifications of COPD phenotypes [published correction appears in Thorax. 2014;69(7):672]. Thorax. 2008;63(9):761-767.
14. Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax. 2009;64(8):728-735.
15. Braman SS, Kaemmerlen JT, Davis SM. Asthma in the elderly: a comparison between patients with recently acquired and long-standing disease. Am Rev Respir Dis. 1991;143(2):336-340.
16. Vonk JM, Jongepier H, Panhuysen Cl, Schouten JP, Bleecker ER, Postma DS. Risk factors associated with the presence of irreversible airflow limitation and reduced transfer coefficient in patients with asthma after 26 years of follow up. Thorax. 2003;58(4):322-327.
17. de Marco R, Pesce G, Marcon A, et al. The coexistence of asthma and chronic obstructive pulmonary disease (COPD): prevalence and risk factors in young, middle-aged and elderly people from the general population. PLoS One. 2013;8(5):e62985.
18. Lee HY, Kang JY, Yoon HK, et al. Clinical characteristics of asthma combined with COPD feature. Yonsei Med J. 2014;55(4):980-986.
19. Burrows B, Barbee RA, Cline MG, Knudson RJ, Lebowitz MD. Characteristics of asthma among elderly adults in a sample of the general population. Chest. 1991;100(4):935-942.
20. Kauppi P, Kupiainen H, Lindqvust A, et al. Overlap syndrome of asthma and COPD predicts low quality of life. J Asthma. 2011;48(3):279-285.
21. Chung JW, Kong KA, Lee JH, Lee SJ, Ryu YJ, Chang JH. Characteristics and self-rated health of overlap syndrome. Int J Chron Obstruct Pulmon Dis. 2014;9:795-804.
22. Miravitles M, Soriano JB, Ancochea J, et al. Characterisation of the overlap COPDasthma phenotype. Focus on physical activity and health status. Respir Med. 2013;107(7):1053-1060.
23. Soriano JB, Visick GT, Mullerova H, Payvandi N, Hansell AL. Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest. 2005;128(4):2099-2107.
24. Andersén H, Lampela P, Nevanlinna A, SäynäJakangas O, Keistinen T. High hospital burden in overlap syndrome of asthma and COPD. Clin Respir J. 2013;7(4):342-346.
25. Panizza JA, James AL, Ryan G, de Klerk N, Finucane KE. Mortality and airflow obstruction in asthma: a 17-year follow-up study. Intern Med J. 2006;36(12):773-780.
26. Hardin M, Silverman EK, Barr RG, et al; COPDGene Investigators. The clinical features of the overlap between COPD and asthma. Respir Res. 2011;12:127.
27. Menezes AM, Montes de Oca M, Pérez-Padilla R, et al; PLATINO Team. Increased risk of exacerbation and hospitalization in subjects with an overlap phenotype: COPD-asthma. Chest. 2014;145(2):297-304.
28. Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med. 1998;339(17):1194-1200.
29. James AL, Palmer LJ, Kicic E, et al. Decline in lung function in the Busselton Health Study: the effects of asthma and cigarette smoking. Am J Respir Crit Care Med. 2005;171(2):109-114.
30. Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med. 2002;166(5):675-679.
31. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma Website. http://www.ginasthma.org/documents/4. Revised 2014. Accessed October 27, 2014.
32. Global Initiative for Chronic Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Global Initiative for Chronic Lung Disease Website. http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis management.html. Published January 2014. Accessed 27 October 2014.
1. Centers for Disease Control and Prevention. Asthma in the US: CDC Vital Signs. CDC Website. http://www.cdc.gov/vitalsigns/asthma/. Updated May 3, 2011. Accessed October 27, 2014.
2. Centers for Disease Control and Prevention. What is COPD? CDC Website. http://www.cdc.gov/copd/. Updated November 13, 2013. Accessed October 27, 2014.
3. American Lung Association. Chronic Obstructive Pulmonary Disease (COPD) Fact Sheet. American Lung Association Website. http://www.lung.org/lung-disease/copd/resources/facts-figures/COPD-Fact-Sheet.html. Published May 2014. Accessed October 27, 2014.
4. National Heart, Lung, and Blood Institute. Morbidity and Mortality: 2012 Chart Book on Cardiovascular, Lung and Blood Diseases. National Heart, Lung, and Blood Institute Website. https://www.nhlbi.nih.gov/files/docs/research/2012_ChartBook_508.pdf. Accessed January 6, 2015.
5. Global Initiative for Asthma/Global Initiative for Chronic Obstructive Lung Disease. Diagnosis of Diseases of Chronic Airflow Limitation: Asthma COPD and Asthma-COPD Overlap Syndrome (ACOS). Global Initiative for Asthma Website. http://www.ginasthma.org/documents/14. Accessed August 10, 2015.
6. Tam A, Sin DD. Pathobiologic mechanisms of chronic obstructive pulmonary disease. Med Clin North Am. 2012;96(4):681-698.
7. Silva GE, Sherrill DL, Guerra S, Barbee RA. Asthma as a risk factor for COPD in a longitudinal study. Chest. 2004;126(1):59-65.
8. Soriano JB, Davis KJ, Coleman B, Visick G, Mannino D, Pride NB. The proportional Venn diagram of obstructive lung disease: two approximations from the United States and the United Kingdom. Chest. 2003;124(2):474-481.
9. Soler-Cataluña JJ, Cosío B, Izquierdo JL, et al. Consensus document on the overlap phenotype COPD-asthma in COPD. Arch Bronconeumol. 2012;48(9):331-337.
10. Zeki AA, Schivo M, Chan A, Albertson TE, Louie S. The asthma-COPD overlap syndrome: a common clinical problem in the elderly. J Allergy (Cairo). 2011;2011:861926.
11. Louie S, Zeki AA, Schivo M, et al. The asthma-chronic obstructive pulmonary disease overlap syndrome: pharmacotherapeutic considerations. Expert Rev Clin Pharmacol. 2013;6(2):197-219.
12. American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1995;152 (5 pt 2):S77-S121.
13. Marsh SE, Travers J, Weatherall M, et al. Proportional classifications of COPD phenotypes [published correction appears in Thorax. 2014;69(7):672]. Thorax. 2008;63(9):761-767.
14. Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax. 2009;64(8):728-735.
15. Braman SS, Kaemmerlen JT, Davis SM. Asthma in the elderly: a comparison between patients with recently acquired and long-standing disease. Am Rev Respir Dis. 1991;143(2):336-340.
16. Vonk JM, Jongepier H, Panhuysen Cl, Schouten JP, Bleecker ER, Postma DS. Risk factors associated with the presence of irreversible airflow limitation and reduced transfer coefficient in patients with asthma after 26 years of follow up. Thorax. 2003;58(4):322-327.
17. de Marco R, Pesce G, Marcon A, et al. The coexistence of asthma and chronic obstructive pulmonary disease (COPD): prevalence and risk factors in young, middle-aged and elderly people from the general population. PLoS One. 2013;8(5):e62985.
18. Lee HY, Kang JY, Yoon HK, et al. Clinical characteristics of asthma combined with COPD feature. Yonsei Med J. 2014;55(4):980-986.
19. Burrows B, Barbee RA, Cline MG, Knudson RJ, Lebowitz MD. Characteristics of asthma among elderly adults in a sample of the general population. Chest. 1991;100(4):935-942.
20. Kauppi P, Kupiainen H, Lindqvust A, et al. Overlap syndrome of asthma and COPD predicts low quality of life. J Asthma. 2011;48(3):279-285.
21. Chung JW, Kong KA, Lee JH, Lee SJ, Ryu YJ, Chang JH. Characteristics and self-rated health of overlap syndrome. Int J Chron Obstruct Pulmon Dis. 2014;9:795-804.
22. Miravitles M, Soriano JB, Ancochea J, et al. Characterisation of the overlap COPDasthma phenotype. Focus on physical activity and health status. Respir Med. 2013;107(7):1053-1060.
23. Soriano JB, Visick GT, Mullerova H, Payvandi N, Hansell AL. Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest. 2005;128(4):2099-2107.
24. Andersén H, Lampela P, Nevanlinna A, SäynäJakangas O, Keistinen T. High hospital burden in overlap syndrome of asthma and COPD. Clin Respir J. 2013;7(4):342-346.
25. Panizza JA, James AL, Ryan G, de Klerk N, Finucane KE. Mortality and airflow obstruction in asthma: a 17-year follow-up study. Intern Med J. 2006;36(12):773-780.
26. Hardin M, Silverman EK, Barr RG, et al; COPDGene Investigators. The clinical features of the overlap between COPD and asthma. Respir Res. 2011;12:127.
27. Menezes AM, Montes de Oca M, Pérez-Padilla R, et al; PLATINO Team. Increased risk of exacerbation and hospitalization in subjects with an overlap phenotype: COPD-asthma. Chest. 2014;145(2):297-304.
28. Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med. 1998;339(17):1194-1200.
29. James AL, Palmer LJ, Kicic E, et al. Decline in lung function in the Busselton Health Study: the effects of asthma and cigarette smoking. Am J Respir Crit Care Med. 2005;171(2):109-114.
30. Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med. 2002;166(5):675-679.
31. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma Website. http://www.ginasthma.org/documents/4. Revised 2014. Accessed October 27, 2014.
32. Global Initiative for Chronic Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Global Initiative for Chronic Lung Disease Website. http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis management.html. Published January 2014. Accessed 27 October 2014.
Role of Radiosurgery in the Treatment of Brain Metastasis
Since the 1980s, patients with a single intracranial metastatic lesion traditionally have been treated with surgery followed by whole brain radiation therapy (WBRT). However, there is growing concern about the debilitating cognitive effects associated with WBRT in long-term survivors.
Limbrick and colleagues studied the outcomes of surgery followed by stereotactic radiosurgery (SRS) instead of WBRT and found that the less invasive surgical resection (SR) followed by SRS was an equally effective therapeutic option for the treatment of patients with limited metastatic disease to the brain.1 Median overall survival (OS) was 20 months and was 22 and 13 months for Classes 1 and 2 recursive partitioning analysis (RPA) patients, respectively. Recursive partitioning analysis refers to 3 prognostic classes based on a database of 3 trial studies and 1,200 patients (Table 1).2 According to RPA, the best survival was observed in Class 1 patients, and the worst survival was seen in Class 3 patients. Limbrick and colleagues found that survival outcome was equivalent to or greater than that reported by other studies using surgery plus WBRT or SRS plus WBRT.1 The WBRT was not used and was reserved as salvage therapy in cases of initial failure such as disease progression of brain metastasis.
Radiation Therapies
Stereotactic radiosurgery is not a surgical procedure but a newly developed radiotherapy technique. It is a highly precise, intensive form of radiation therapy, focused on the tumor, with the goal of protecting the surrounding normal brain tissue as much as possible. Radiosurgery was initially introduced with the Gamma Knife by Lars Leksell several decades ago in order to deliver an intense radiation dose to a small, well-defined, single focal point using extreme precision. Stereotactic radiosurgery delivers efficient and focused radiation treatment to the tumor lesion.
There are 2 practical and commercially available radiation delivery systems for SRS: linear accelerator (LINAC)-based radiosurgery and Gamma Knife systems. Use of the Gamma Knife is limited largely to treatment of central nervous system (CNS) malignancies and certain head and neck cancers. Linear accelerator-based SRS is applicable to neoplasms in any organ system of the body (Table 2).
Proton therapy is yet another evolving and completely different mode of radiation therapy. There are currently 14 proton therapy centers in operation in the U.S., and 11 more centers are now under construction. Proton therapy uses charged heavy-particle therapy using proton beams, whereas conventional LINAC-based radiotherapy is X-ray radiotherapy, which uses high energy photon beams. Because of their relatively large mass, protons have little scatter of radiation to surrounding normal structures and can remain sharply focused on the tumor lesion. Accordingly, proton therapy delivers negligible radiation doses beyond tumor lesions, and much of the surrounding normal tissues can be saved from excessive and unnecessary radiation doses.
Related: Bone Metastasis: A Concise Overview
A single proton beam produces a narrow Bragg peak dose distribution at depth, and multiple consecutive stepwise series of different energies of proton beams are needed to administer complete coverage of the target tumor volume. The accumulation of these beam energies produces a uniform radiation dose distribution covering the entire tumor volume (Figure 1). In spite of the theoretical beneficial effects of proton beam therapy, more clinical experience is needed for it to be validated. Even then, the significantly higher costs of proton therapy represent another barrier to its wider implementation. Proton beam radiosurgery is still, in large part, an evolving technology, not widely and uniformly available.
Role of Radiosurgery
Photon (X-ray)-based radiosurgery can be an alternative to craniotomy. Patients can return to their activities immediately after treatment. The ideal candidate for radiosurgery should have a small tumor (1-3 cm is best) with a well-defined margin. Retrospective studies reported no significant difference in therapy outcomes between the 2 therapies.3,4 Additional benefits of radiosurgery include low morbidity and mortality. Furthermore, radiosurgery can be applied to tumors near critical structures, such as the thalamus, basal ganglia, and brainstem, that are otherwise surgically inaccessible.
Most brain metastases are well defined and spherical, so they are ideally treated using SRS (Figure 1). Additionally, the brain is encased in the bony skull, which prevents significant intrafraction motion and provides a reproducible fidulial for accurate setup. Radiosurgery can tailor the radiation dose in order to precisely concentrate radiation distribution to the tumor lesion with a rapid dose falloff beyond the margin of the tumor bed, so surrounding normal brain tissues are spared from high-dose radiation. In sharp contrast, WBRT indiscriminately irradiates the entire brain without sparing the adjacent normal brain tissue (Figure 2). However, because of its limited dose distribution, radiosurgery offers no protection elsewhere in the brain from future metastasis, which is a benefit of whole brain radiation.
Future Use of SBRT
Based on successful experience with intracranial lesions, stereotactic techniques have been expanded to additional anatomical body sites other than the brain. Stereotactic body radiation therapy (SBRT), also called stereotactic body ablative radiotherapy, is progressively gaining acceptance and is being applied to various extracranial tumors, especially lung cancers and hepatic malignancies. Dosimetric studies and early phase clinical trials have clearly established the feasibility, safety, and efficacy of SBRT for certain tumor sites, such as lung, liver, kidney, spine, and paraspinal tumors. Additionally, SBRT may reduce treatment time and therapy costs and thus provide increased convenience to patients.
Effectiveness of SRS
Stafinski and colleagues conducted a meta-analysis of randomized trials to study the effectiveness of SRS in improving the survival as well as the quality of life (QOL) and functional status following SRS of patients with brain metastasis.5 This study found that SRS plus WBRT increased OS for patients with single brain metastasis compared with WBRT alone. Although no significant difference in OS was found in patients with multiple brain metastases, the addition of SRS to WBRT improved the local control and functional independence of this group of patients.
Related: Palliative Radiotherapy for the Management of Metastatic Cancer
Kondziolka and colleagues reported a local failure rate at 1 year of merely 8% following SRS boost therapy after WBRT compared with 100% without SRS.6 There was also a remarkable difference in median time to local failure—36 months vs 6 months, respectively. A randomized study designed to assess the possible benefit of SRS for the treatment of brain metastasis found a survival gain for patients with a single brain metastasis with a median survival time of 6.5 months (SRS) vs 4.9 months (no SRS).7
There are sparse data and reporting related to QOL measurements after SRS for brain metastasis. Andrews and colleagues reported improved functional and independent abilities at 6 months after completion of SRS therapy.7 The criteria used in that study for performance assessments included the Karnofsky Performance Status (KPS) scale, the need for steroid use, and mental status. They found that KPS improvement was statistically significant, and patients were able to decrease the dosage of steroid medication at 6 months after therapy with SRS (Table 3). Despite these reports suggesting superior outcomes with SRS, more rigorous investigations that adequately control for other factors influencing QOL in patients with cancer are needed.
Two major limitations of SRS include large tumor size and multiple numbers of metastatic brain lesions. As the radiation dose to adjacent normal brain tissue quickly increases with larger tumor lesions (> 3-4 cm), the complication risks consequently rise proportionally, necessitating a decrease in the prescribed dose. Patients with poor performance status (< 70 KPS) and presence of active/progressive extracranial disease are also not ideal candidates for SRS.
Other unfavorable conditions for SRS include life expectancy of < 6 months, metastatic lesions in the posterior fossa, and severe acute CNS symptoms due to increased intracranial pressure, brain edema, or massive tumor effects. These factors do not necessarily contraindicate SRS but can increase the risks of such treatment. The authors recommend an experienced multispecialty approach to patients presenting with these findings.
Managing Brain Metastastis
To prevent symptoms related to brain edema (due to brain tumor itself and/or radiation-induced edema), steroid medication is generally administered to most patients, 1 to 3 days prior to initiation of radiation therapy. Corticosteroid use typically results in rapid improvement of existing CNS symptoms, such as headaches, and helps prevent the development of additional CNS symptoms due to radiation therapy-induced cerebral edema. A dexamethasone dose as low as 4 mg per day may be effective for prophylaxis if no symptoms or signs of increased intracranial pressure or altered consciousness exist. If the patient experiences symptomatic elevations in intracranial pressure, however, a 16-mg dose of dexamethasone per day orally, following a loading dose of 10-mg IV dexamethasone, should be considered. The latter scenario is not common.
Related: Pulmonary Vein Thrombosis Associated With Metastatic Carcinoma
The benefits of steroids, however, need to be carefully balanced against the possible adverse effects (AEs) associated with steroid use, including peripheral edema, gastrointestinal bleeding, risk of infections, hyperglycemia, insomnia, as well as mental status changes, such as anxiety, depression, and confusion. In long-term users, the additional AEs of oral candidiasis and osteoporosis should also be taken into account.
Craniotomy vs SRS
A retrospective study by Schöggl and colleagues compared single brain metastasis cases treated using either Gamma Knife or brain surgery followed by WBRT (30 Gy/10 fractions).3 Local control was significantly better after radiosurgery (95% vs 83%), and median survival was 12 months and 9 months after radiosurgery and brain surgery, respectively. There was no significant difference in OS.
Another comparative study of SR and SRS for solitary brain metastasis revealed no statistically significant difference in survival between the 2 therapeutic modalities (SR or SRS); the 1-year survival rate was 62% (SR) and 56% (SRS).4 A significant prognostic factor for survival was a good performance status of the patients. There was, however, a significant difference in local tumor control: None of the patients in the SRS group experienced local recurrence in contrast to 19 (58%) patients in the SR group.
Whereas selection criteria and treatment choice depend to a large extent on tumor location, tumor size, and availability of SRS, most studies demonstrated that both surgery and SRS result in comparable OS rates for patients with a single brain metastasis.
Multiple Brain Metastases
Jawahar and colleagues studied the role of SRS for multiple brain metastases.8 In their retrospective review of 50 patients with ≥ 3 brain metastases, they found an overall response rate (RR) of 82% and a median survival of 12 months after SRS. As a result of similar studies and their own data, Hasegawa and colleagues recommended radiosurgery alone as initial therapy for patients with a limited number of brain metastases.9
SRS vs SRS Plus WBRT
Studies on the role of SRS plus WBRT are somewhat conflicting. A Radiation Therapy Oncology Group study revealed statistically significant improvement in median survival when SRS boost therapy was added to WBRT in patients with a single brain metastasis compared with SRS alone.5 According to another study, the addition of SRS to WBRT provided better intracranial and local control of metastatic tumors.10
A randomized controlled study by Aoyama and colleagues reported no survival improvement using SRS and WBRT in patients with 1 to 4 brain metastases compared with SRS alone.11 In addition, a retrospective review found no difference in median survival outcomes between SRS alone and SRS plus WBRT (Table 4). In the absence of a clear survival benefit with the use of both modalities and in light of the added toxicity of WBRT, most clinicians have ceased offering both modalities upfront and instead reserve WBRT as a salvage option in cases of subsequent intracranial progression of disease.
SRS vs WBRT
In general, both SR (crainotomy) and SRS for the treatment of brain metastases seem to be effective therapeutic modalities. Comparisons of both treatments did not reveal significant differences and showed similar outcomes after treatment of smaller lesions. For example, Rades and colleagues reported that SRS alone is as effective as surgery and WBRT for limited metastatic lesions (< 2) in the brain.16 Either SRS or surgery can be used, depending on performance status and metastatic burden (size, location, number of lesions, etc).
There are some inconsistencies in the final results of various studies, such as survival, local tumor control, mortality rate, and pattern of failures. For large, symptomatic brain metastasis, initial surgical debulking remains the preferred approach as a way of achieving immediate decompression and relief of swelling/symptoms. Additionally, for patients who have > 10 brain lesions and/or a histology that corroborates diffuse subclinical involvement of the brain parenchyma (eg, small-cell lung cancer), WBRT is also typically preferred to upfront SRS. Alternatively, radiosurgery is the preferred method for fewer and smaller lesions as a way of minimizing the toxicity from whole brain irradiation. The optimal treatment of multiple small brain metastases remains controversial with some investigators recommending SRS for > 4 metastases only in the setting of controlled extracranial disease based on the more favorable expected survival of such patients.
Multidisciplinary Approach for Lung and Breast Cancers
Prognostic outcomes of patients with brain metastases can vary by primary cancer type. Therefore, clinicians should consider cancer-specific management and tailor their recommendation for specific types of radiation depending on the individual cancer diagnosis. Various investigators have attempted to develop disease-specific prognostic tools to aid clinicians in their decision making. For example, Sperduto and colleagues analyzed significant indexes and diagnosis-specific prognostic factors and published the diagnostic-specific graded prognostic assessment factors.17 They were able to identify several significant prognostic factors, specific to different primary cancer types.
Bimodality Therapies
For certain cancers such as lung and breast primary cancers, bimodality therapy using chemotherapy and radiation treatment should be considered based on promising responses reported in the literature.
Recent studies on the efficacy of chemotherapy for brain metastases from small-cell lung cancer (43%-82%) have also been reported.18-20 Postmus and colleagues reported superior RR of 57% with combination chemotherapy and radiation vs a 22% RR for chemotherapy alone.21 They also found favorable long-term survival trends in patients treated with combined radiochemotherapy.
The efficacy of chemotherapy in non-small cell carcinoma of the lung has been reported in multiple phase 2 studies using various chemotherapeutic agents. The reported RR ranged from 35% to 50%.22-24 Comparative studies of combined chemoradiotherapy demonstrated a 33% RR in contrast to a 27% RR for combined therapy or chemotherapy alone, respectively. However, no difference was noted in median survival rates.25
Care must be taken when interpreting these studies due to heterogeneity of the patient population studied and a lack of data on potential synergistic toxicities between radiation to the CNS and systemic therapy. The authors generally avoid concurrent chemotherapy during CNS irradiation in patients who may have significant survival times > 1 year.
The prognosis of breast cancer patients with brain metastasis largely depends on the number and size of metastatic brain lesions, performance status, extracranial or systemic involvement, and systemic treatment following brain irradiation. The median survival of patients with brain metastasis and radiation therapy is generally about 18 months. The median survival for patients with breast cancer who develop brain metastasis was 3 years from diagnosis of the primary breast cancer.26
Recent advances in systemic agents/options for patients with breast cancer can significantly affect the decision-making process in regard to the treatment of brain lesions in these patients. For example, a few retrospective studies have clearly demonstrated the beneficial effect of trastuzumab in patients with breast cancer with brain metastasis. The median OS in HER2-positive patients with brain metastasis was significantly extended to 41 months when treated with HER2-targeted trastuzumab vs only 13 months for patients who received no treatment.27,28 As a result of the expected prolonged survival, SRS for small and isolated brain lesions has recently become a much more attractive option as a way of mitigating the deleterious long-term effect of whole brain irradiation (memory decline, somnolence, etc).
Summary
Stereotactic radiosurgery is a newly developed radiation therapy technique of highly conformal and focused radiation. For the treatment of patients with favorable prognostic factors and limited brain metastases, especially single brain metastasis, crainiotomy and SRS seems similarly effective and appropriate choices of therapy. Some studies question the possible benefits of additional WBRT to local therapy, such as crainiotomy or radiosurgery.
Some authors recommend deferral of WBRT after local brain therapy and reserving it for salvage therapy in cases of recurrence or progression of brain disease because of possible long-term AEs of whole brain irradiation as well as deterioration of QOL in long-term survivors. Thus, the role of additional WBRT to other local therapy has not been fully settled; further randomized studies may be necessary. Due to the controversies and complexities surrounding the treatment choices for patients with brain disease, all treatment decisions should be individualized and should involve close multidisciplinary collaboration between neurosurgeons, medical oncologists, and radiation oncologists.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Limbrick DD Jr, Lusis EA, Chicoine MR, et al. Combined surgical resection and stereotactic radiosurgery for treatment of cerebral metastases. Surg Neurol. 2009;71(3):280-288.
2. Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745-751.
3. Schöggl A, Kitz K, Reddy M, et al. Defining the role of stereotactic radiosurgery versus microsurgery in the treatment of single brain metastases. Acta Neurochir (Wien). 2000;142(6):621-626.
4. O’Neill BP, Iturria NJ, Link MJ, Pollock BE, Ballman KV, O’Fallon JR. A comparison of surgical resection and stereotactic radiosurgery in the treatment of solitary brain metastases. Int J Radiat Oncol Biol Phys. 2003;55(5):1169-1176.
5. Stafinski T, Jhangri GS, Yan E, Manon D. Effectiveness of stereotactic radiosurgery alone or in combination with whole brain radiotherapy compared to conventional surgery and/or whole brain radiotherapy for the treatment of one or more brain metastases: a systematic review and meta-analysis. Cancer Treat Rev. 2006;32(3):203-213.
6. Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45(2):427-434.
7. Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665-1672.
8. Jawahar A, Shaya M, Campbell P, et al. Role of stereotactic radiosurgery as a primary treatment option in the management of newly diagnosed multiple (3-6) intracranial metastases. Surg Neurol. 2005;64(3):207-212.
9. Hasegawa T, Kondziolka D, Flickinger JC, Germanwala A, Lunsford LD. Brain metastases treated with radiosurgery alone: an alternative to whole brain radiotherapy? Neurosurgery. 2003;52(6):1318-1326.
10. Rades D, Kueter JD, Hornung D, et al. Comparison of stereotactic radiosurgery (SRS) alone and whole brain radiotherapy (WBRT) plus a stereotactic boost (WBRT+SRS) for one to three brain metastases. Strahlenther Onkol. 2008;184(12):655-662.
11. Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483-2491.
12. Chidel MA, Suh JH, Reddy CA, Chao ST, Lundbeck MF, Barnett GH. Application of recursive partitioning analysis and evaluation of the use of whole brain radiation among patients treated with stereotactic radiosurgery for newly diagnosed brain metastases. Int J Radiat Oncol Biol Phys. 2000;47(4):993-999.
13. Sneed PK, Lamborn KR, Forstner JM, et al. Radiosurgery for brain metastases: is whole brain radiotherapy necessary? Int J Radiat Oncol Biol Phys. 1999;43(3):549-558.
14. Noel G, Medioni J, Valery CA, et al. Three irradiation treatment options including radiosurgery for brain metastases from primary lung cancer. Lung Cancer. 2003;41(3):333-343.
15. Hoffman R, Sneed PK, McDermott MW, et al. Radiosurgery for brain metastases from primary lung carcinoma. Cancer J. 2001;7(2):121-131.
16. Rades D, Bohlen G, Pluemer A, et al. Stereotactic radiosurgery alone versus resection plus whole brain radiotherapy for 1 or 2 brain metastases in recursive partitioning analysis class 1 and 2 patients. Cancer. 2007;109(12):2515-2521.
17. Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77(3):655-661.
18. Twelves CJ, Souhami RL, Harper PG, et al. The response of cerebral metastases in small cell lung cancer to systemic chemotherapy. Br J Cancer. 1990;61(1):147-150.
19. Tanaka H, Takifuj N, Masuda N, et al. [Systemic chemotherapy for brain metastases from small-cell lung cancer]. Nihon Kyobu Shikkan Gakkai Zasshi. 1993;31(4):492-497. Japanese.
20. Lee JS, Murphy WK, Glisson BS, Dhingra HM, Holoye PY, Hong WK. Primary chemotherapy of brain metastasis in small-cell lung cancer. J Clin Oncol. 1989;7(7):216-222.
21. Postmus PE, Haaxma-Reiche H, Smit EF, et al. Treatment of brain metastases of small-cell lung cancer: comparing teniposide and teniposide with whole-brain radiotherapy—a phase III study of the European Organisation for the Research and Treatment of Cancer Lung Cancer Cooperative Group. J Clin Oncol. 2000;18(19):3400-3408.
22. Cortes J, Rodriguez J, Aramendia JM, et al. Frontline paclitaxel/cisplatin-based chemotherapy in brain metastases from non-small-cell lung cancer. Oncology. 2003;64(1):28-35.
23. Minotti V, Crinò L, Meacci ML, et al. Chemotherapy with cisplatin and teniposide for cerebral metastases in non-small cell lung cancer. Lung Cancer. 1998;20(2):23-28.
24. Fujita A, Fukuoka S, Takabatake H, Tagaki S, Sekine K. Combination chemotherapy of cisplatin, ifosfamide, and irinotecan with rhG-CSF support in patient with brain metastases from non-small cell lung cancer. Oncology. 2000;59(4):291-295.
25. Robinet G, Thomas R, Breton JL, et al. Results of a phase III study of early versus delayed whole brain radiotherapy with concurrent cisplatin and vinorelbine combination in inoperable brain metastasis of non-small-cell lung cancer: Groupe Français de Pneumo-Cancérologie (GFPC) Protocol 95-1. Ann Oncol. 2001;12(1):29-67.
26. Kiricuta IC, Kölbl O, Willner J, Bohndorf W. Central nervous system metastases in breast cancer. J Cancer Res Clin Oncol. 1992;118(7):542-546.
27. Berghoff AS, Bago-Horvath Z, Dubsky P, et al. Impact of HER-2-targeted therapy on overall survival in patients with HER-2 positive metastatic breast cancer. Breast J. 2013;19(2):149-155.
28. Park IH, Ro J, Lee KS, Nam BH, Kwon Y, Shin KH. Truastzumab treatment beyond brain progression in HER2-positive metastatic breast cancer. Ann Oncol. 2009;20(1):56-62.
Since the 1980s, patients with a single intracranial metastatic lesion traditionally have been treated with surgery followed by whole brain radiation therapy (WBRT). However, there is growing concern about the debilitating cognitive effects associated with WBRT in long-term survivors.
Limbrick and colleagues studied the outcomes of surgery followed by stereotactic radiosurgery (SRS) instead of WBRT and found that the less invasive surgical resection (SR) followed by SRS was an equally effective therapeutic option for the treatment of patients with limited metastatic disease to the brain.1 Median overall survival (OS) was 20 months and was 22 and 13 months for Classes 1 and 2 recursive partitioning analysis (RPA) patients, respectively. Recursive partitioning analysis refers to 3 prognostic classes based on a database of 3 trial studies and 1,200 patients (Table 1).2 According to RPA, the best survival was observed in Class 1 patients, and the worst survival was seen in Class 3 patients. Limbrick and colleagues found that survival outcome was equivalent to or greater than that reported by other studies using surgery plus WBRT or SRS plus WBRT.1 The WBRT was not used and was reserved as salvage therapy in cases of initial failure such as disease progression of brain metastasis.
Radiation Therapies
Stereotactic radiosurgery is not a surgical procedure but a newly developed radiotherapy technique. It is a highly precise, intensive form of radiation therapy, focused on the tumor, with the goal of protecting the surrounding normal brain tissue as much as possible. Radiosurgery was initially introduced with the Gamma Knife by Lars Leksell several decades ago in order to deliver an intense radiation dose to a small, well-defined, single focal point using extreme precision. Stereotactic radiosurgery delivers efficient and focused radiation treatment to the tumor lesion.
There are 2 practical and commercially available radiation delivery systems for SRS: linear accelerator (LINAC)-based radiosurgery and Gamma Knife systems. Use of the Gamma Knife is limited largely to treatment of central nervous system (CNS) malignancies and certain head and neck cancers. Linear accelerator-based SRS is applicable to neoplasms in any organ system of the body (Table 2).
Proton therapy is yet another evolving and completely different mode of radiation therapy. There are currently 14 proton therapy centers in operation in the U.S., and 11 more centers are now under construction. Proton therapy uses charged heavy-particle therapy using proton beams, whereas conventional LINAC-based radiotherapy is X-ray radiotherapy, which uses high energy photon beams. Because of their relatively large mass, protons have little scatter of radiation to surrounding normal structures and can remain sharply focused on the tumor lesion. Accordingly, proton therapy delivers negligible radiation doses beyond tumor lesions, and much of the surrounding normal tissues can be saved from excessive and unnecessary radiation doses.
Related: Bone Metastasis: A Concise Overview
A single proton beam produces a narrow Bragg peak dose distribution at depth, and multiple consecutive stepwise series of different energies of proton beams are needed to administer complete coverage of the target tumor volume. The accumulation of these beam energies produces a uniform radiation dose distribution covering the entire tumor volume (Figure 1). In spite of the theoretical beneficial effects of proton beam therapy, more clinical experience is needed for it to be validated. Even then, the significantly higher costs of proton therapy represent another barrier to its wider implementation. Proton beam radiosurgery is still, in large part, an evolving technology, not widely and uniformly available.
Role of Radiosurgery
Photon (X-ray)-based radiosurgery can be an alternative to craniotomy. Patients can return to their activities immediately after treatment. The ideal candidate for radiosurgery should have a small tumor (1-3 cm is best) with a well-defined margin. Retrospective studies reported no significant difference in therapy outcomes between the 2 therapies.3,4 Additional benefits of radiosurgery include low morbidity and mortality. Furthermore, radiosurgery can be applied to tumors near critical structures, such as the thalamus, basal ganglia, and brainstem, that are otherwise surgically inaccessible.
Most brain metastases are well defined and spherical, so they are ideally treated using SRS (Figure 1). Additionally, the brain is encased in the bony skull, which prevents significant intrafraction motion and provides a reproducible fidulial for accurate setup. Radiosurgery can tailor the radiation dose in order to precisely concentrate radiation distribution to the tumor lesion with a rapid dose falloff beyond the margin of the tumor bed, so surrounding normal brain tissues are spared from high-dose radiation. In sharp contrast, WBRT indiscriminately irradiates the entire brain without sparing the adjacent normal brain tissue (Figure 2). However, because of its limited dose distribution, radiosurgery offers no protection elsewhere in the brain from future metastasis, which is a benefit of whole brain radiation.
Future Use of SBRT
Based on successful experience with intracranial lesions, stereotactic techniques have been expanded to additional anatomical body sites other than the brain. Stereotactic body radiation therapy (SBRT), also called stereotactic body ablative radiotherapy, is progressively gaining acceptance and is being applied to various extracranial tumors, especially lung cancers and hepatic malignancies. Dosimetric studies and early phase clinical trials have clearly established the feasibility, safety, and efficacy of SBRT for certain tumor sites, such as lung, liver, kidney, spine, and paraspinal tumors. Additionally, SBRT may reduce treatment time and therapy costs and thus provide increased convenience to patients.
Effectiveness of SRS
Stafinski and colleagues conducted a meta-analysis of randomized trials to study the effectiveness of SRS in improving the survival as well as the quality of life (QOL) and functional status following SRS of patients with brain metastasis.5 This study found that SRS plus WBRT increased OS for patients with single brain metastasis compared with WBRT alone. Although no significant difference in OS was found in patients with multiple brain metastases, the addition of SRS to WBRT improved the local control and functional independence of this group of patients.
Related: Palliative Radiotherapy for the Management of Metastatic Cancer
Kondziolka and colleagues reported a local failure rate at 1 year of merely 8% following SRS boost therapy after WBRT compared with 100% without SRS.6 There was also a remarkable difference in median time to local failure—36 months vs 6 months, respectively. A randomized study designed to assess the possible benefit of SRS for the treatment of brain metastasis found a survival gain for patients with a single brain metastasis with a median survival time of 6.5 months (SRS) vs 4.9 months (no SRS).7
There are sparse data and reporting related to QOL measurements after SRS for brain metastasis. Andrews and colleagues reported improved functional and independent abilities at 6 months after completion of SRS therapy.7 The criteria used in that study for performance assessments included the Karnofsky Performance Status (KPS) scale, the need for steroid use, and mental status. They found that KPS improvement was statistically significant, and patients were able to decrease the dosage of steroid medication at 6 months after therapy with SRS (Table 3). Despite these reports suggesting superior outcomes with SRS, more rigorous investigations that adequately control for other factors influencing QOL in patients with cancer are needed.
Two major limitations of SRS include large tumor size and multiple numbers of metastatic brain lesions. As the radiation dose to adjacent normal brain tissue quickly increases with larger tumor lesions (> 3-4 cm), the complication risks consequently rise proportionally, necessitating a decrease in the prescribed dose. Patients with poor performance status (< 70 KPS) and presence of active/progressive extracranial disease are also not ideal candidates for SRS.
Other unfavorable conditions for SRS include life expectancy of < 6 months, metastatic lesions in the posterior fossa, and severe acute CNS symptoms due to increased intracranial pressure, brain edema, or massive tumor effects. These factors do not necessarily contraindicate SRS but can increase the risks of such treatment. The authors recommend an experienced multispecialty approach to patients presenting with these findings.
Managing Brain Metastastis
To prevent symptoms related to brain edema (due to brain tumor itself and/or radiation-induced edema), steroid medication is generally administered to most patients, 1 to 3 days prior to initiation of radiation therapy. Corticosteroid use typically results in rapid improvement of existing CNS symptoms, such as headaches, and helps prevent the development of additional CNS symptoms due to radiation therapy-induced cerebral edema. A dexamethasone dose as low as 4 mg per day may be effective for prophylaxis if no symptoms or signs of increased intracranial pressure or altered consciousness exist. If the patient experiences symptomatic elevations in intracranial pressure, however, a 16-mg dose of dexamethasone per day orally, following a loading dose of 10-mg IV dexamethasone, should be considered. The latter scenario is not common.
Related: Pulmonary Vein Thrombosis Associated With Metastatic Carcinoma
The benefits of steroids, however, need to be carefully balanced against the possible adverse effects (AEs) associated with steroid use, including peripheral edema, gastrointestinal bleeding, risk of infections, hyperglycemia, insomnia, as well as mental status changes, such as anxiety, depression, and confusion. In long-term users, the additional AEs of oral candidiasis and osteoporosis should also be taken into account.
Craniotomy vs SRS
A retrospective study by Schöggl and colleagues compared single brain metastasis cases treated using either Gamma Knife or brain surgery followed by WBRT (30 Gy/10 fractions).3 Local control was significantly better after radiosurgery (95% vs 83%), and median survival was 12 months and 9 months after radiosurgery and brain surgery, respectively. There was no significant difference in OS.
Another comparative study of SR and SRS for solitary brain metastasis revealed no statistically significant difference in survival between the 2 therapeutic modalities (SR or SRS); the 1-year survival rate was 62% (SR) and 56% (SRS).4 A significant prognostic factor for survival was a good performance status of the patients. There was, however, a significant difference in local tumor control: None of the patients in the SRS group experienced local recurrence in contrast to 19 (58%) patients in the SR group.
Whereas selection criteria and treatment choice depend to a large extent on tumor location, tumor size, and availability of SRS, most studies demonstrated that both surgery and SRS result in comparable OS rates for patients with a single brain metastasis.
Multiple Brain Metastases
Jawahar and colleagues studied the role of SRS for multiple brain metastases.8 In their retrospective review of 50 patients with ≥ 3 brain metastases, they found an overall response rate (RR) of 82% and a median survival of 12 months after SRS. As a result of similar studies and their own data, Hasegawa and colleagues recommended radiosurgery alone as initial therapy for patients with a limited number of brain metastases.9
SRS vs SRS Plus WBRT
Studies on the role of SRS plus WBRT are somewhat conflicting. A Radiation Therapy Oncology Group study revealed statistically significant improvement in median survival when SRS boost therapy was added to WBRT in patients with a single brain metastasis compared with SRS alone.5 According to another study, the addition of SRS to WBRT provided better intracranial and local control of metastatic tumors.10
A randomized controlled study by Aoyama and colleagues reported no survival improvement using SRS and WBRT in patients with 1 to 4 brain metastases compared with SRS alone.11 In addition, a retrospective review found no difference in median survival outcomes between SRS alone and SRS plus WBRT (Table 4). In the absence of a clear survival benefit with the use of both modalities and in light of the added toxicity of WBRT, most clinicians have ceased offering both modalities upfront and instead reserve WBRT as a salvage option in cases of subsequent intracranial progression of disease.
SRS vs WBRT
In general, both SR (crainotomy) and SRS for the treatment of brain metastases seem to be effective therapeutic modalities. Comparisons of both treatments did not reveal significant differences and showed similar outcomes after treatment of smaller lesions. For example, Rades and colleagues reported that SRS alone is as effective as surgery and WBRT for limited metastatic lesions (< 2) in the brain.16 Either SRS or surgery can be used, depending on performance status and metastatic burden (size, location, number of lesions, etc).
There are some inconsistencies in the final results of various studies, such as survival, local tumor control, mortality rate, and pattern of failures. For large, symptomatic brain metastasis, initial surgical debulking remains the preferred approach as a way of achieving immediate decompression and relief of swelling/symptoms. Additionally, for patients who have > 10 brain lesions and/or a histology that corroborates diffuse subclinical involvement of the brain parenchyma (eg, small-cell lung cancer), WBRT is also typically preferred to upfront SRS. Alternatively, radiosurgery is the preferred method for fewer and smaller lesions as a way of minimizing the toxicity from whole brain irradiation. The optimal treatment of multiple small brain metastases remains controversial with some investigators recommending SRS for > 4 metastases only in the setting of controlled extracranial disease based on the more favorable expected survival of such patients.
Multidisciplinary Approach for Lung and Breast Cancers
Prognostic outcomes of patients with brain metastases can vary by primary cancer type. Therefore, clinicians should consider cancer-specific management and tailor their recommendation for specific types of radiation depending on the individual cancer diagnosis. Various investigators have attempted to develop disease-specific prognostic tools to aid clinicians in their decision making. For example, Sperduto and colleagues analyzed significant indexes and diagnosis-specific prognostic factors and published the diagnostic-specific graded prognostic assessment factors.17 They were able to identify several significant prognostic factors, specific to different primary cancer types.
Bimodality Therapies
For certain cancers such as lung and breast primary cancers, bimodality therapy using chemotherapy and radiation treatment should be considered based on promising responses reported in the literature.
Recent studies on the efficacy of chemotherapy for brain metastases from small-cell lung cancer (43%-82%) have also been reported.18-20 Postmus and colleagues reported superior RR of 57% with combination chemotherapy and radiation vs a 22% RR for chemotherapy alone.21 They also found favorable long-term survival trends in patients treated with combined radiochemotherapy.
The efficacy of chemotherapy in non-small cell carcinoma of the lung has been reported in multiple phase 2 studies using various chemotherapeutic agents. The reported RR ranged from 35% to 50%.22-24 Comparative studies of combined chemoradiotherapy demonstrated a 33% RR in contrast to a 27% RR for combined therapy or chemotherapy alone, respectively. However, no difference was noted in median survival rates.25
Care must be taken when interpreting these studies due to heterogeneity of the patient population studied and a lack of data on potential synergistic toxicities between radiation to the CNS and systemic therapy. The authors generally avoid concurrent chemotherapy during CNS irradiation in patients who may have significant survival times > 1 year.
The prognosis of breast cancer patients with brain metastasis largely depends on the number and size of metastatic brain lesions, performance status, extracranial or systemic involvement, and systemic treatment following brain irradiation. The median survival of patients with brain metastasis and radiation therapy is generally about 18 months. The median survival for patients with breast cancer who develop brain metastasis was 3 years from diagnosis of the primary breast cancer.26
Recent advances in systemic agents/options for patients with breast cancer can significantly affect the decision-making process in regard to the treatment of brain lesions in these patients. For example, a few retrospective studies have clearly demonstrated the beneficial effect of trastuzumab in patients with breast cancer with brain metastasis. The median OS in HER2-positive patients with brain metastasis was significantly extended to 41 months when treated with HER2-targeted trastuzumab vs only 13 months for patients who received no treatment.27,28 As a result of the expected prolonged survival, SRS for small and isolated brain lesions has recently become a much more attractive option as a way of mitigating the deleterious long-term effect of whole brain irradiation (memory decline, somnolence, etc).
Summary
Stereotactic radiosurgery is a newly developed radiation therapy technique of highly conformal and focused radiation. For the treatment of patients with favorable prognostic factors and limited brain metastases, especially single brain metastasis, crainiotomy and SRS seems similarly effective and appropriate choices of therapy. Some studies question the possible benefits of additional WBRT to local therapy, such as crainiotomy or radiosurgery.
Some authors recommend deferral of WBRT after local brain therapy and reserving it for salvage therapy in cases of recurrence or progression of brain disease because of possible long-term AEs of whole brain irradiation as well as deterioration of QOL in long-term survivors. Thus, the role of additional WBRT to other local therapy has not been fully settled; further randomized studies may be necessary. Due to the controversies and complexities surrounding the treatment choices for patients with brain disease, all treatment decisions should be individualized and should involve close multidisciplinary collaboration between neurosurgeons, medical oncologists, and radiation oncologists.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Since the 1980s, patients with a single intracranial metastatic lesion traditionally have been treated with surgery followed by whole brain radiation therapy (WBRT). However, there is growing concern about the debilitating cognitive effects associated with WBRT in long-term survivors.
Limbrick and colleagues studied the outcomes of surgery followed by stereotactic radiosurgery (SRS) instead of WBRT and found that the less invasive surgical resection (SR) followed by SRS was an equally effective therapeutic option for the treatment of patients with limited metastatic disease to the brain.1 Median overall survival (OS) was 20 months and was 22 and 13 months for Classes 1 and 2 recursive partitioning analysis (RPA) patients, respectively. Recursive partitioning analysis refers to 3 prognostic classes based on a database of 3 trial studies and 1,200 patients (Table 1).2 According to RPA, the best survival was observed in Class 1 patients, and the worst survival was seen in Class 3 patients. Limbrick and colleagues found that survival outcome was equivalent to or greater than that reported by other studies using surgery plus WBRT or SRS plus WBRT.1 The WBRT was not used and was reserved as salvage therapy in cases of initial failure such as disease progression of brain metastasis.
Radiation Therapies
Stereotactic radiosurgery is not a surgical procedure but a newly developed radiotherapy technique. It is a highly precise, intensive form of radiation therapy, focused on the tumor, with the goal of protecting the surrounding normal brain tissue as much as possible. Radiosurgery was initially introduced with the Gamma Knife by Lars Leksell several decades ago in order to deliver an intense radiation dose to a small, well-defined, single focal point using extreme precision. Stereotactic radiosurgery delivers efficient and focused radiation treatment to the tumor lesion.
There are 2 practical and commercially available radiation delivery systems for SRS: linear accelerator (LINAC)-based radiosurgery and Gamma Knife systems. Use of the Gamma Knife is limited largely to treatment of central nervous system (CNS) malignancies and certain head and neck cancers. Linear accelerator-based SRS is applicable to neoplasms in any organ system of the body (Table 2).
Proton therapy is yet another evolving and completely different mode of radiation therapy. There are currently 14 proton therapy centers in operation in the U.S., and 11 more centers are now under construction. Proton therapy uses charged heavy-particle therapy using proton beams, whereas conventional LINAC-based radiotherapy is X-ray radiotherapy, which uses high energy photon beams. Because of their relatively large mass, protons have little scatter of radiation to surrounding normal structures and can remain sharply focused on the tumor lesion. Accordingly, proton therapy delivers negligible radiation doses beyond tumor lesions, and much of the surrounding normal tissues can be saved from excessive and unnecessary radiation doses.
Related: Bone Metastasis: A Concise Overview
A single proton beam produces a narrow Bragg peak dose distribution at depth, and multiple consecutive stepwise series of different energies of proton beams are needed to administer complete coverage of the target tumor volume. The accumulation of these beam energies produces a uniform radiation dose distribution covering the entire tumor volume (Figure 1). In spite of the theoretical beneficial effects of proton beam therapy, more clinical experience is needed for it to be validated. Even then, the significantly higher costs of proton therapy represent another barrier to its wider implementation. Proton beam radiosurgery is still, in large part, an evolving technology, not widely and uniformly available.
Role of Radiosurgery
Photon (X-ray)-based radiosurgery can be an alternative to craniotomy. Patients can return to their activities immediately after treatment. The ideal candidate for radiosurgery should have a small tumor (1-3 cm is best) with a well-defined margin. Retrospective studies reported no significant difference in therapy outcomes between the 2 therapies.3,4 Additional benefits of radiosurgery include low morbidity and mortality. Furthermore, radiosurgery can be applied to tumors near critical structures, such as the thalamus, basal ganglia, and brainstem, that are otherwise surgically inaccessible.
Most brain metastases are well defined and spherical, so they are ideally treated using SRS (Figure 1). Additionally, the brain is encased in the bony skull, which prevents significant intrafraction motion and provides a reproducible fidulial for accurate setup. Radiosurgery can tailor the radiation dose in order to precisely concentrate radiation distribution to the tumor lesion with a rapid dose falloff beyond the margin of the tumor bed, so surrounding normal brain tissues are spared from high-dose radiation. In sharp contrast, WBRT indiscriminately irradiates the entire brain without sparing the adjacent normal brain tissue (Figure 2). However, because of its limited dose distribution, radiosurgery offers no protection elsewhere in the brain from future metastasis, which is a benefit of whole brain radiation.
Future Use of SBRT
Based on successful experience with intracranial lesions, stereotactic techniques have been expanded to additional anatomical body sites other than the brain. Stereotactic body radiation therapy (SBRT), also called stereotactic body ablative radiotherapy, is progressively gaining acceptance and is being applied to various extracranial tumors, especially lung cancers and hepatic malignancies. Dosimetric studies and early phase clinical trials have clearly established the feasibility, safety, and efficacy of SBRT for certain tumor sites, such as lung, liver, kidney, spine, and paraspinal tumors. Additionally, SBRT may reduce treatment time and therapy costs and thus provide increased convenience to patients.
Effectiveness of SRS
Stafinski and colleagues conducted a meta-analysis of randomized trials to study the effectiveness of SRS in improving the survival as well as the quality of life (QOL) and functional status following SRS of patients with brain metastasis.5 This study found that SRS plus WBRT increased OS for patients with single brain metastasis compared with WBRT alone. Although no significant difference in OS was found in patients with multiple brain metastases, the addition of SRS to WBRT improved the local control and functional independence of this group of patients.
Related: Palliative Radiotherapy for the Management of Metastatic Cancer
Kondziolka and colleagues reported a local failure rate at 1 year of merely 8% following SRS boost therapy after WBRT compared with 100% without SRS.6 There was also a remarkable difference in median time to local failure—36 months vs 6 months, respectively. A randomized study designed to assess the possible benefit of SRS for the treatment of brain metastasis found a survival gain for patients with a single brain metastasis with a median survival time of 6.5 months (SRS) vs 4.9 months (no SRS).7
There are sparse data and reporting related to QOL measurements after SRS for brain metastasis. Andrews and colleagues reported improved functional and independent abilities at 6 months after completion of SRS therapy.7 The criteria used in that study for performance assessments included the Karnofsky Performance Status (KPS) scale, the need for steroid use, and mental status. They found that KPS improvement was statistically significant, and patients were able to decrease the dosage of steroid medication at 6 months after therapy with SRS (Table 3). Despite these reports suggesting superior outcomes with SRS, more rigorous investigations that adequately control for other factors influencing QOL in patients with cancer are needed.
Two major limitations of SRS include large tumor size and multiple numbers of metastatic brain lesions. As the radiation dose to adjacent normal brain tissue quickly increases with larger tumor lesions (> 3-4 cm), the complication risks consequently rise proportionally, necessitating a decrease in the prescribed dose. Patients with poor performance status (< 70 KPS) and presence of active/progressive extracranial disease are also not ideal candidates for SRS.
Other unfavorable conditions for SRS include life expectancy of < 6 months, metastatic lesions in the posterior fossa, and severe acute CNS symptoms due to increased intracranial pressure, brain edema, or massive tumor effects. These factors do not necessarily contraindicate SRS but can increase the risks of such treatment. The authors recommend an experienced multispecialty approach to patients presenting with these findings.
Managing Brain Metastastis
To prevent symptoms related to brain edema (due to brain tumor itself and/or radiation-induced edema), steroid medication is generally administered to most patients, 1 to 3 days prior to initiation of radiation therapy. Corticosteroid use typically results in rapid improvement of existing CNS symptoms, such as headaches, and helps prevent the development of additional CNS symptoms due to radiation therapy-induced cerebral edema. A dexamethasone dose as low as 4 mg per day may be effective for prophylaxis if no symptoms or signs of increased intracranial pressure or altered consciousness exist. If the patient experiences symptomatic elevations in intracranial pressure, however, a 16-mg dose of dexamethasone per day orally, following a loading dose of 10-mg IV dexamethasone, should be considered. The latter scenario is not common.
Related: Pulmonary Vein Thrombosis Associated With Metastatic Carcinoma
The benefits of steroids, however, need to be carefully balanced against the possible adverse effects (AEs) associated with steroid use, including peripheral edema, gastrointestinal bleeding, risk of infections, hyperglycemia, insomnia, as well as mental status changes, such as anxiety, depression, and confusion. In long-term users, the additional AEs of oral candidiasis and osteoporosis should also be taken into account.
Craniotomy vs SRS
A retrospective study by Schöggl and colleagues compared single brain metastasis cases treated using either Gamma Knife or brain surgery followed by WBRT (30 Gy/10 fractions).3 Local control was significantly better after radiosurgery (95% vs 83%), and median survival was 12 months and 9 months after radiosurgery and brain surgery, respectively. There was no significant difference in OS.
Another comparative study of SR and SRS for solitary brain metastasis revealed no statistically significant difference in survival between the 2 therapeutic modalities (SR or SRS); the 1-year survival rate was 62% (SR) and 56% (SRS).4 A significant prognostic factor for survival was a good performance status of the patients. There was, however, a significant difference in local tumor control: None of the patients in the SRS group experienced local recurrence in contrast to 19 (58%) patients in the SR group.
Whereas selection criteria and treatment choice depend to a large extent on tumor location, tumor size, and availability of SRS, most studies demonstrated that both surgery and SRS result in comparable OS rates for patients with a single brain metastasis.
Multiple Brain Metastases
Jawahar and colleagues studied the role of SRS for multiple brain metastases.8 In their retrospective review of 50 patients with ≥ 3 brain metastases, they found an overall response rate (RR) of 82% and a median survival of 12 months after SRS. As a result of similar studies and their own data, Hasegawa and colleagues recommended radiosurgery alone as initial therapy for patients with a limited number of brain metastases.9
SRS vs SRS Plus WBRT
Studies on the role of SRS plus WBRT are somewhat conflicting. A Radiation Therapy Oncology Group study revealed statistically significant improvement in median survival when SRS boost therapy was added to WBRT in patients with a single brain metastasis compared with SRS alone.5 According to another study, the addition of SRS to WBRT provided better intracranial and local control of metastatic tumors.10
A randomized controlled study by Aoyama and colleagues reported no survival improvement using SRS and WBRT in patients with 1 to 4 brain metastases compared with SRS alone.11 In addition, a retrospective review found no difference in median survival outcomes between SRS alone and SRS plus WBRT (Table 4). In the absence of a clear survival benefit with the use of both modalities and in light of the added toxicity of WBRT, most clinicians have ceased offering both modalities upfront and instead reserve WBRT as a salvage option in cases of subsequent intracranial progression of disease.
SRS vs WBRT
In general, both SR (crainotomy) and SRS for the treatment of brain metastases seem to be effective therapeutic modalities. Comparisons of both treatments did not reveal significant differences and showed similar outcomes after treatment of smaller lesions. For example, Rades and colleagues reported that SRS alone is as effective as surgery and WBRT for limited metastatic lesions (< 2) in the brain.16 Either SRS or surgery can be used, depending on performance status and metastatic burden (size, location, number of lesions, etc).
There are some inconsistencies in the final results of various studies, such as survival, local tumor control, mortality rate, and pattern of failures. For large, symptomatic brain metastasis, initial surgical debulking remains the preferred approach as a way of achieving immediate decompression and relief of swelling/symptoms. Additionally, for patients who have > 10 brain lesions and/or a histology that corroborates diffuse subclinical involvement of the brain parenchyma (eg, small-cell lung cancer), WBRT is also typically preferred to upfront SRS. Alternatively, radiosurgery is the preferred method for fewer and smaller lesions as a way of minimizing the toxicity from whole brain irradiation. The optimal treatment of multiple small brain metastases remains controversial with some investigators recommending SRS for > 4 metastases only in the setting of controlled extracranial disease based on the more favorable expected survival of such patients.
Multidisciplinary Approach for Lung and Breast Cancers
Prognostic outcomes of patients with brain metastases can vary by primary cancer type. Therefore, clinicians should consider cancer-specific management and tailor their recommendation for specific types of radiation depending on the individual cancer diagnosis. Various investigators have attempted to develop disease-specific prognostic tools to aid clinicians in their decision making. For example, Sperduto and colleagues analyzed significant indexes and diagnosis-specific prognostic factors and published the diagnostic-specific graded prognostic assessment factors.17 They were able to identify several significant prognostic factors, specific to different primary cancer types.
Bimodality Therapies
For certain cancers such as lung and breast primary cancers, bimodality therapy using chemotherapy and radiation treatment should be considered based on promising responses reported in the literature.
Recent studies on the efficacy of chemotherapy for brain metastases from small-cell lung cancer (43%-82%) have also been reported.18-20 Postmus and colleagues reported superior RR of 57% with combination chemotherapy and radiation vs a 22% RR for chemotherapy alone.21 They also found favorable long-term survival trends in patients treated with combined radiochemotherapy.
The efficacy of chemotherapy in non-small cell carcinoma of the lung has been reported in multiple phase 2 studies using various chemotherapeutic agents. The reported RR ranged from 35% to 50%.22-24 Comparative studies of combined chemoradiotherapy demonstrated a 33% RR in contrast to a 27% RR for combined therapy or chemotherapy alone, respectively. However, no difference was noted in median survival rates.25
Care must be taken when interpreting these studies due to heterogeneity of the patient population studied and a lack of data on potential synergistic toxicities between radiation to the CNS and systemic therapy. The authors generally avoid concurrent chemotherapy during CNS irradiation in patients who may have significant survival times > 1 year.
The prognosis of breast cancer patients with brain metastasis largely depends on the number and size of metastatic brain lesions, performance status, extracranial or systemic involvement, and systemic treatment following brain irradiation. The median survival of patients with brain metastasis and radiation therapy is generally about 18 months. The median survival for patients with breast cancer who develop brain metastasis was 3 years from diagnosis of the primary breast cancer.26
Recent advances in systemic agents/options for patients with breast cancer can significantly affect the decision-making process in regard to the treatment of brain lesions in these patients. For example, a few retrospective studies have clearly demonstrated the beneficial effect of trastuzumab in patients with breast cancer with brain metastasis. The median OS in HER2-positive patients with brain metastasis was significantly extended to 41 months when treated with HER2-targeted trastuzumab vs only 13 months for patients who received no treatment.27,28 As a result of the expected prolonged survival, SRS for small and isolated brain lesions has recently become a much more attractive option as a way of mitigating the deleterious long-term effect of whole brain irradiation (memory decline, somnolence, etc).
Summary
Stereotactic radiosurgery is a newly developed radiation therapy technique of highly conformal and focused radiation. For the treatment of patients with favorable prognostic factors and limited brain metastases, especially single brain metastasis, crainiotomy and SRS seems similarly effective and appropriate choices of therapy. Some studies question the possible benefits of additional WBRT to local therapy, such as crainiotomy or radiosurgery.
Some authors recommend deferral of WBRT after local brain therapy and reserving it for salvage therapy in cases of recurrence or progression of brain disease because of possible long-term AEs of whole brain irradiation as well as deterioration of QOL in long-term survivors. Thus, the role of additional WBRT to other local therapy has not been fully settled; further randomized studies may be necessary. Due to the controversies and complexities surrounding the treatment choices for patients with brain disease, all treatment decisions should be individualized and should involve close multidisciplinary collaboration between neurosurgeons, medical oncologists, and radiation oncologists.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Limbrick DD Jr, Lusis EA, Chicoine MR, et al. Combined surgical resection and stereotactic radiosurgery for treatment of cerebral metastases. Surg Neurol. 2009;71(3):280-288.
2. Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745-751.
3. Schöggl A, Kitz K, Reddy M, et al. Defining the role of stereotactic radiosurgery versus microsurgery in the treatment of single brain metastases. Acta Neurochir (Wien). 2000;142(6):621-626.
4. O’Neill BP, Iturria NJ, Link MJ, Pollock BE, Ballman KV, O’Fallon JR. A comparison of surgical resection and stereotactic radiosurgery in the treatment of solitary brain metastases. Int J Radiat Oncol Biol Phys. 2003;55(5):1169-1176.
5. Stafinski T, Jhangri GS, Yan E, Manon D. Effectiveness of stereotactic radiosurgery alone or in combination with whole brain radiotherapy compared to conventional surgery and/or whole brain radiotherapy for the treatment of one or more brain metastases: a systematic review and meta-analysis. Cancer Treat Rev. 2006;32(3):203-213.
6. Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45(2):427-434.
7. Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665-1672.
8. Jawahar A, Shaya M, Campbell P, et al. Role of stereotactic radiosurgery as a primary treatment option in the management of newly diagnosed multiple (3-6) intracranial metastases. Surg Neurol. 2005;64(3):207-212.
9. Hasegawa T, Kondziolka D, Flickinger JC, Germanwala A, Lunsford LD. Brain metastases treated with radiosurgery alone: an alternative to whole brain radiotherapy? Neurosurgery. 2003;52(6):1318-1326.
10. Rades D, Kueter JD, Hornung D, et al. Comparison of stereotactic radiosurgery (SRS) alone and whole brain radiotherapy (WBRT) plus a stereotactic boost (WBRT+SRS) for one to three brain metastases. Strahlenther Onkol. 2008;184(12):655-662.
11. Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483-2491.
12. Chidel MA, Suh JH, Reddy CA, Chao ST, Lundbeck MF, Barnett GH. Application of recursive partitioning analysis and evaluation of the use of whole brain radiation among patients treated with stereotactic radiosurgery for newly diagnosed brain metastases. Int J Radiat Oncol Biol Phys. 2000;47(4):993-999.
13. Sneed PK, Lamborn KR, Forstner JM, et al. Radiosurgery for brain metastases: is whole brain radiotherapy necessary? Int J Radiat Oncol Biol Phys. 1999;43(3):549-558.
14. Noel G, Medioni J, Valery CA, et al. Three irradiation treatment options including radiosurgery for brain metastases from primary lung cancer. Lung Cancer. 2003;41(3):333-343.
15. Hoffman R, Sneed PK, McDermott MW, et al. Radiosurgery for brain metastases from primary lung carcinoma. Cancer J. 2001;7(2):121-131.
16. Rades D, Bohlen G, Pluemer A, et al. Stereotactic radiosurgery alone versus resection plus whole brain radiotherapy for 1 or 2 brain metastases in recursive partitioning analysis class 1 and 2 patients. Cancer. 2007;109(12):2515-2521.
17. Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77(3):655-661.
18. Twelves CJ, Souhami RL, Harper PG, et al. The response of cerebral metastases in small cell lung cancer to systemic chemotherapy. Br J Cancer. 1990;61(1):147-150.
19. Tanaka H, Takifuj N, Masuda N, et al. [Systemic chemotherapy for brain metastases from small-cell lung cancer]. Nihon Kyobu Shikkan Gakkai Zasshi. 1993;31(4):492-497. Japanese.
20. Lee JS, Murphy WK, Glisson BS, Dhingra HM, Holoye PY, Hong WK. Primary chemotherapy of brain metastasis in small-cell lung cancer. J Clin Oncol. 1989;7(7):216-222.
21. Postmus PE, Haaxma-Reiche H, Smit EF, et al. Treatment of brain metastases of small-cell lung cancer: comparing teniposide and teniposide with whole-brain radiotherapy—a phase III study of the European Organisation for the Research and Treatment of Cancer Lung Cancer Cooperative Group. J Clin Oncol. 2000;18(19):3400-3408.
22. Cortes J, Rodriguez J, Aramendia JM, et al. Frontline paclitaxel/cisplatin-based chemotherapy in brain metastases from non-small-cell lung cancer. Oncology. 2003;64(1):28-35.
23. Minotti V, Crinò L, Meacci ML, et al. Chemotherapy with cisplatin and teniposide for cerebral metastases in non-small cell lung cancer. Lung Cancer. 1998;20(2):23-28.
24. Fujita A, Fukuoka S, Takabatake H, Tagaki S, Sekine K. Combination chemotherapy of cisplatin, ifosfamide, and irinotecan with rhG-CSF support in patient with brain metastases from non-small cell lung cancer. Oncology. 2000;59(4):291-295.
25. Robinet G, Thomas R, Breton JL, et al. Results of a phase III study of early versus delayed whole brain radiotherapy with concurrent cisplatin and vinorelbine combination in inoperable brain metastasis of non-small-cell lung cancer: Groupe Français de Pneumo-Cancérologie (GFPC) Protocol 95-1. Ann Oncol. 2001;12(1):29-67.
26. Kiricuta IC, Kölbl O, Willner J, Bohndorf W. Central nervous system metastases in breast cancer. J Cancer Res Clin Oncol. 1992;118(7):542-546.
27. Berghoff AS, Bago-Horvath Z, Dubsky P, et al. Impact of HER-2-targeted therapy on overall survival in patients with HER-2 positive metastatic breast cancer. Breast J. 2013;19(2):149-155.
28. Park IH, Ro J, Lee KS, Nam BH, Kwon Y, Shin KH. Truastzumab treatment beyond brain progression in HER2-positive metastatic breast cancer. Ann Oncol. 2009;20(1):56-62.
1. Limbrick DD Jr, Lusis EA, Chicoine MR, et al. Combined surgical resection and stereotactic radiosurgery for treatment of cerebral metastases. Surg Neurol. 2009;71(3):280-288.
2. Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745-751.
3. Schöggl A, Kitz K, Reddy M, et al. Defining the role of stereotactic radiosurgery versus microsurgery in the treatment of single brain metastases. Acta Neurochir (Wien). 2000;142(6):621-626.
4. O’Neill BP, Iturria NJ, Link MJ, Pollock BE, Ballman KV, O’Fallon JR. A comparison of surgical resection and stereotactic radiosurgery in the treatment of solitary brain metastases. Int J Radiat Oncol Biol Phys. 2003;55(5):1169-1176.
5. Stafinski T, Jhangri GS, Yan E, Manon D. Effectiveness of stereotactic radiosurgery alone or in combination with whole brain radiotherapy compared to conventional surgery and/or whole brain radiotherapy for the treatment of one or more brain metastases: a systematic review and meta-analysis. Cancer Treat Rev. 2006;32(3):203-213.
6. Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45(2):427-434.
7. Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665-1672.
8. Jawahar A, Shaya M, Campbell P, et al. Role of stereotactic radiosurgery as a primary treatment option in the management of newly diagnosed multiple (3-6) intracranial metastases. Surg Neurol. 2005;64(3):207-212.
9. Hasegawa T, Kondziolka D, Flickinger JC, Germanwala A, Lunsford LD. Brain metastases treated with radiosurgery alone: an alternative to whole brain radiotherapy? Neurosurgery. 2003;52(6):1318-1326.
10. Rades D, Kueter JD, Hornung D, et al. Comparison of stereotactic radiosurgery (SRS) alone and whole brain radiotherapy (WBRT) plus a stereotactic boost (WBRT+SRS) for one to three brain metastases. Strahlenther Onkol. 2008;184(12):655-662.
11. Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483-2491.
12. Chidel MA, Suh JH, Reddy CA, Chao ST, Lundbeck MF, Barnett GH. Application of recursive partitioning analysis and evaluation of the use of whole brain radiation among patients treated with stereotactic radiosurgery for newly diagnosed brain metastases. Int J Radiat Oncol Biol Phys. 2000;47(4):993-999.
13. Sneed PK, Lamborn KR, Forstner JM, et al. Radiosurgery for brain metastases: is whole brain radiotherapy necessary? Int J Radiat Oncol Biol Phys. 1999;43(3):549-558.
14. Noel G, Medioni J, Valery CA, et al. Three irradiation treatment options including radiosurgery for brain metastases from primary lung cancer. Lung Cancer. 2003;41(3):333-343.
15. Hoffman R, Sneed PK, McDermott MW, et al. Radiosurgery for brain metastases from primary lung carcinoma. Cancer J. 2001;7(2):121-131.
16. Rades D, Bohlen G, Pluemer A, et al. Stereotactic radiosurgery alone versus resection plus whole brain radiotherapy for 1 or 2 brain metastases in recursive partitioning analysis class 1 and 2 patients. Cancer. 2007;109(12):2515-2521.
17. Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77(3):655-661.
18. Twelves CJ, Souhami RL, Harper PG, et al. The response of cerebral metastases in small cell lung cancer to systemic chemotherapy. Br J Cancer. 1990;61(1):147-150.
19. Tanaka H, Takifuj N, Masuda N, et al. [Systemic chemotherapy for brain metastases from small-cell lung cancer]. Nihon Kyobu Shikkan Gakkai Zasshi. 1993;31(4):492-497. Japanese.
20. Lee JS, Murphy WK, Glisson BS, Dhingra HM, Holoye PY, Hong WK. Primary chemotherapy of brain metastasis in small-cell lung cancer. J Clin Oncol. 1989;7(7):216-222.
21. Postmus PE, Haaxma-Reiche H, Smit EF, et al. Treatment of brain metastases of small-cell lung cancer: comparing teniposide and teniposide with whole-brain radiotherapy—a phase III study of the European Organisation for the Research and Treatment of Cancer Lung Cancer Cooperative Group. J Clin Oncol. 2000;18(19):3400-3408.
22. Cortes J, Rodriguez J, Aramendia JM, et al. Frontline paclitaxel/cisplatin-based chemotherapy in brain metastases from non-small-cell lung cancer. Oncology. 2003;64(1):28-35.
23. Minotti V, Crinò L, Meacci ML, et al. Chemotherapy with cisplatin and teniposide for cerebral metastases in non-small cell lung cancer. Lung Cancer. 1998;20(2):23-28.
24. Fujita A, Fukuoka S, Takabatake H, Tagaki S, Sekine K. Combination chemotherapy of cisplatin, ifosfamide, and irinotecan with rhG-CSF support in patient with brain metastases from non-small cell lung cancer. Oncology. 2000;59(4):291-295.
25. Robinet G, Thomas R, Breton JL, et al. Results of a phase III study of early versus delayed whole brain radiotherapy with concurrent cisplatin and vinorelbine combination in inoperable brain metastasis of non-small-cell lung cancer: Groupe Français de Pneumo-Cancérologie (GFPC) Protocol 95-1. Ann Oncol. 2001;12(1):29-67.
26. Kiricuta IC, Kölbl O, Willner J, Bohndorf W. Central nervous system metastases in breast cancer. J Cancer Res Clin Oncol. 1992;118(7):542-546.
27. Berghoff AS, Bago-Horvath Z, Dubsky P, et al. Impact of HER-2-targeted therapy on overall survival in patients with HER-2 positive metastatic breast cancer. Breast J. 2013;19(2):149-155.
28. Park IH, Ro J, Lee KS, Nam BH, Kwon Y, Shin KH. Truastzumab treatment beyond brain progression in HER2-positive metastatic breast cancer. Ann Oncol. 2009;20(1):56-62.
Type 1 Neurofibromatosis (von Recklinghausen Disease)
Type 1 neurofibromatosis (NF1), or von Recklinghausen disease, is a multisystem disorder affecting approximately 1 in 3500 people in South East Wales.1 Type 1 neurofibromatosis has been described in the literature since the 13th century but was not recognized as a distinct disorder until 1882 in Friedrich Daniel von Recklinghausen’s landmark publication “On Multiple Fibromas of the Skin and Their Relationship to Multiple Neuromas.”2
Genetics
Type 1 neurofibromatosis is an autosomal-dominant disorder with a nearly even split between spontaneous and inherited mutations. It is characterized by neurofibromas, which are complex tumors composed of axonal processes, Schwann cells, fibroblasts, perineural cells, and mast cells. The NF1 gene (neurofibromin 1), discovered in 1990,3 is located on chromosome 17q11.2 and encodes for the protein neurofibromin. This large gene (60 exons and >300 kilobases of genomic DNA) has one of the highest rates of spontaneous mutations in the entire human genome.4,5 Mutations exhibited by the gene are complete deletions, insertions, and nonsense and splicing mutations. Ultimately, these mutations may result in a loss of heterozygosity of the NF1 gene (a somatic loss of the second NF1 allele). Segmental, generalized, or gonadal forms of NF1 demonstrate mosaicism.6
Pathogenesis
Neurofibromin, the NF1 gene product, is a tumor suppressor expressed in many cells, primarily in neurons, glial cells, and Schwann cells, and is seen early in melanocyte development.7 The MAPK/ERK signaling pathway is a complex series of signals and interactions involved in cell growth and proliferation.5 Under normal conditions, neurofibromin, an RAS GTPase–activating protein promotes the conversion of the active RAS-GTP bound form to an inactive RAS-GDP bound form, thereby suppressing cell growth8,9; however, other possible effects are being investigated.10 Mast cells have been implicated in contributing to inflammation in the plexiform neurofibroma microenvironment of NF1.11,12 In addition, haploinsufficiency of NF1 (NF1+/−) and c-kit signaling in the hematopoietic system have been implicated in tumor progression. Accumulation of additional mutations of multiple genes, including INK4A/ARF and the protein p53, may be responsible for malignant transformation. These revelations of molecular and cellular mechanisms involved with NF1 tumorigenesis have led to trials of targeted therapies including the mammalian target of rapamycin and tyrosine kinase inhibitor imatinib mesylate, which is demonstrating promising preclinical results for treatment of peripheral nerve sheath tumors.13,14
Diagnosis
Seven cardinal diagnostic criteria have been delineated for NF1, at least 2 of which must be met to diagnose an individual with the condition.15 These criteria include (1) six or more café au lait macules (5 mm in diameter in prepubertal patients, 15 mm in postpubertal patients); (2) axillary or inguinal freckles (>2 freckles); (3) two or more typical neurofibromas or 1 plexiform neurofibroma, (4) optic nerve glioma, (5) two or more iris hamartomas (Lisch nodules), often only identified through slit-lamp examination by an ophthalmologist; (6) sphenoid dysplasia or typical long bone abnormalities such as pseudoarthritis; and (7) first-degree relative with NF1. Diagnosis may be difficult in patients who exhibit some dermatologic features of interest but who do not fully meet the diagnostic criteria.
Skin manifestations of NF1 may present in restricted segments of the body. It has been reported that half of those with NF1 are the first in their family to have the disease.16 Children with 6 or more café au lait macules alone and no family history of neurofibromatosis should be followed up, as their chances of developing NF1 are high.17 Occasionally, Lisch nodules may be the only clinical feature. Type 1 neurofibromatosis mutation analysis may be used to confirm the diagnosis in uncertain cases as well as prenatal diagnosis. However, genetic testing is not routinely advocated, and expert consultation is advised before it is undertaken. Furthermore, biopsy of asymptomatic cutaneous neurofibromas should not be undertaken for diagnostic purposes in individuals with confirmed NF1.18
Hyperintense lesions on T2-weighted magnetic resonance imaging (MRI) of the brain (formerly known as unidentified bright objects) probably are caused by aberrant myelination or gliosis and are pathognomonic of NF1.19The presence of these lesions can assist in the diagnosis of NF1, but MRI under anesthesia is not warranted for this purpose in children, who may not be able to stay still during the test.20
Physicians should not only be able to identify the cardinal skin features of NF1 but also the less common cutaneous and extracutaneous findings, which may indicate the need for referral to a dermatologist and/or neurologist.1 Café au lait macules (CALMs) are among the salient features of NF1. Classically, these lesions are well demarcated with smooth, regular, “coast of California” borders (unlike irregular “coast of Maine” borders) and a homogeneous appearance. Although the resemblance to the color of coffee in milk has earned these lesions their name, their color can range from tan to dark brown. The presence of multiple CALMs is highly suggestive of NF1.21 The prevalence of CALMs in the general population has varied from 3% to 36% depending on the study groups selected, but the presence of multiple CALMs in the general population typically is less than 1%.22 Frequently, CALMs are the first sign of NF1, occurring in 99% of NF1 patients within the first year of life. Patients continue to develop lesions throughout childhood, but they often fade in adulthood.23
Freckling of the skin folds is the most common of the cardinal criteria for NF1. Other sites include under the neck and breasts, around the lips, and trunk. Their size ranges from 1 to 3 mm, distinguishing them from CALMs. Considered nearly pathognomonic, NF1 generally occurs in children aged 3 to 5 years in the axillae or groin.15,24
Cutaneous neurofibromas generally are cutaneous/dermal tumors that are dome shaped, soft, fleshy, and flesh colored to slightly hyperpigmented. Subcutaneous tumors are firm and nodular. Neurofibromas usually do not become apparent until puberty and may continue to increase in size and number throughout adulthood. Pregnancy also is associated with increased tumor growth.25 The tumors are comprised of Schwann cells, fibroblasts, mast cells, and perineural cells. There also is an admixture of collagen and extracellular matrix.26 The cutaneous and extracutaneous manifestations of NF1 are outlined in Table 1 and Table 2.27-31
Management
Type 1 neurofibromatosis needs to be differentiated from other conditions based on careful clinical examination. Additionally, histopathologic examination of the lesions, imaging studies (eg, MRI), echocardiography, regular skeletal roentgenogram, and detailed ophthalmologic examination are important to look for any visceral involvement. Painful and bleeding tumors and cosmetic enhancement warrant surgical intervention, including various surgical techniques and lasers.32,33 Application of sunscreen may make pigmentary alterations less noticeable over time. Although not often located on the face, CALMs also may be amenable to various makeup products. Various studies have demonstrated improvement of freckling and CALMs with topical vitamin D3 analogues and lovastatin (β-hydroxy-β-methylglutaryl-CoA reductase inhibitors)34-36; however, this needs further exploration. Rapamycin has demonstrated efficacy in reducing tumor volume in animal studies by inhibiting the mammalian target of rapamycin cellular pathway.37 Imatinib mesylate has demonstrated efficacy both in vivo and in vitro in mouse models by targeting the platelet-derived growth-factor receptors α and β.38
1. Boyd KP, Korf BR, Theos A. Neurofibromatosis type 1. J Am Acad Dermatol. 2009;61:1-14.
2. von Recklinghausen FV. Ueber die multiplen Fibrome der Haut und ihre Beziehung zu den Multiplen Neuromen. Berlin, Germany: August Hirschwald; 1882.
3. Viskochil D, Buchberg AM, Xu G, et al. Deletions and a translocation interrupt a cloned gene at the neurofibromatosis type 1 locus. Cell. 1990;62:187-192.
4. Theos A, Korf BR; American College of Physicians; American Physiological Society. Pathophysiology of neurofibromatosis type 1. Ann lntern Med. 2006;144:842-849.
5. Messiaen LM, Callens T, Mortier G, et al. Exhaustive mutation analysis of the NF1 gene allows identification of 95% of mutations and reveals a high frequency of unusual splicing defects. Hum Mutat. 2000;15:541-555.
6. Ruggieri M, Huson SM. The clinical and diagnostic implications of mosaicism in the neurofibromatoses. Neurology. 2001;56:1433-1443.
7. Stocker KM, Baizer L, Coston T, et al. Regulated expression of neurofibromin in migrating neural crest cells of avian embryos. J Neurobiol. 1995;27:535-552.
8. Maertens O, De Schepper S, Vandesompele J, et al. Molecular dissection of isolated disease features in mosaic neurofibromatosis type 1. Am J Hum Genet. 2007;81:243-251.
9. De Schepper S, Maertens O, Callens T, et al. Somatic mutation analysis in NF1 café au lait spots reveals two NF1 hits in the melanocytes. J Invest Dermatol. 2008;128:1050-1053.
10. Patrakitkomjorn S, Kobayahi D, Morikawa T, et al. Neurofibromatosis type 1 (NF1) tumor suppressor, neurofibromin, regulates the neuronal differentiation of PC12 cells via its associating protein, CRMP-2 [published online ahead of print. J Biol Chem. 2008;283:9399-9413.
11. Yang FC, Ingram DA, Chen S, et al. Nf1-dependent tumors require a microenvironment containing Nf1+/− and c-kit-dependent bone marrow. Cell. 2008;135:437-448.
12. Yang FC, Chen S, Clegg T, et al. Nf1+/- mast cells induce neurofibroma like phenotypes through secreted TGF-β signaling. Hum Mol Genet. 2006;15:2421-2437.
13. Gottfried ON, Viskochil DH, Couldwell WT. Neurofibromatosis type 1 and tumorigenesis: molecular mechanisms and therapeutic implications. Neurosurg Focus. 2010;28:E8.
14. Le LQ, Parada LF. Tumor microenvironment and neurofibromatosis type I: connecting the GAPs. Oncogene. 2007;26:4609-4616.
15. Gutmann DH, Aylsworth A, Carey JC, et al. The diagnostic evaluation and multidisciplinary management of neurofibromatosis 1 and neurofibromatosis 2. JAMA. 1997;278:51-57.
16. Huson SM, Harper PS, Compston DA. Von Recklinghausen neurofibromatosis. a clinical and population study in south-east Wales. Brain. 1988;111(pt 6):1355-1381.
17. Korf BR. Diagnostic outcome in children with multiple café au lait spots. Pediatrics. 1992;90:924-927.
18. Ferner RE, Huson SM, Thomas N, et al. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J Med Genet. 2007;44:81-88.
19. DiPaolo DP, Zimmerman RA, Rorke LB, et al. Neurofibromatosis type 1: pathologic substrate of high-signal-intensity foci in the brain. Radiology. 1995;195:721-724.
20. DeBella K, Poskitt K, Szudek J, et al. Use of “unidentified bright objects” on MRI for diagnosis of neurofibromatosis 1 in children. Neurology. 2000;54:1646-1651.
21. Whitehouse D. Diagnostic value of the café-au-lait spot in children. Arch Dis Child. 1966;41:316-319.
22. Landau M, Krafchik BR. The diagnostic value of café-au-lait macules. J Am Acad Dermatol. 1999;40(6, pt 1):877-890.
23. DeBella K, Szudek J, Friedman JM. Use of the National Institutes of Health criteria for diagnosis of neurofibromatosis 1 in children. Pediatrics. 2000;105(3, pt 1):608-614.
24. Obringer AC, Meadows AT, Zackai EH. The diagnosis of neurofibromatosis-l in the child under the age of 6 years. Am J Dis Child. 1989;143:717-719.
25. Page PZ, Page GP, Ecosse E, et al. Impact of neurofibromatosis 1 on quality of life: a cross-sectional study of 176 American cases. Am J Med Genet A. 2006;140:1893-1898.
26. Maertens O, Brems H. Vandesompele J, et al. Comprehensive NF1 screening on cultured Schwann cells from neurofibromas. Hum Mutat. 2006;27:1030-1040.
27. Huson S, Jones D, Beck L. Ophthalmic manifestations of neurofibromatosis. Br J Ophthalmol. 1987;71:235-238.
28. Listernick R, Ferner RE, Liu GT, et al. Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. Ann Neurol. 2007;61:189-198.
29. Levine TM, Materek A, Abel J, et al. Cognitive profile of neurofibromatosis type 1. Semin Pediatr Neurol. 2006;13:8-20.
30. Lammert M, Kappler M, Mautner VF, et al. Decreased bone mineral density in patients with neurofibromatosis 1. Osteoporos Int. 2005;16:1161-1166.
31. Schindeler A, Little DG. Recent insights into bone development, homeostasis, and repair in type 1 neurofibromatosis (NFl). Bone. 2008;42:616-622.
32. Yoo KH, Kim BJ, Rho YK, et al. A case of diffuse neurofibroma of the scalp. Ann Dermatol. 2009;21:46-48.
33. Onesti MG, Carella S, Spinelli G, et al. A study of 17 patients affected with plexiform neurofibromas in upper and lower extremities: comparison between different surgical techniques. Acta Chir Plast. 2009;51:35-40.
34. Yoshida Y, Sato N, Furumura M, et al. Treatment of pigmented lesions of neurofibromatosis 1 with intense pulsed-radio frequency in combination with topical application of vitamin D3 ointment. J Dermatol. 2007;34:227-230.
35. Nakayama J, Kiryu H, Urabe K, et al. Vitamin D3 analogues improve café au lait spots in patients with von Recklinghausen’s disease: experimental and clinical studies. Eur J Dermatol. 1999;9:202-206.
36. Lammert M, Friedman JM, Roth HJ, et al. Vitamin D deficiency associated with number of neurofibromas in neurofibromatosis 1. J Med Genet. 2006;43:810-813.
37. Hegedus B, Banerjee D, Yeh TH, et al. Preclinical cancer therapy in a mouse model of neurofibromatosis-1 optic glioma. Cancer Res. 2008;68:1520-1528.
38. Demestre M, Herzberg J, Holtkamp N, et al. Imatinib mesylate (Glivec) inhibits Schwann cell viability and reduces the size of human plexiform neurofibroma in a xenograft model. J Neurooncol. 2010;98:11-19.
Type 1 neurofibromatosis (NF1), or von Recklinghausen disease, is a multisystem disorder affecting approximately 1 in 3500 people in South East Wales.1 Type 1 neurofibromatosis has been described in the literature since the 13th century but was not recognized as a distinct disorder until 1882 in Friedrich Daniel von Recklinghausen’s landmark publication “On Multiple Fibromas of the Skin and Their Relationship to Multiple Neuromas.”2
Genetics
Type 1 neurofibromatosis is an autosomal-dominant disorder with a nearly even split between spontaneous and inherited mutations. It is characterized by neurofibromas, which are complex tumors composed of axonal processes, Schwann cells, fibroblasts, perineural cells, and mast cells. The NF1 gene (neurofibromin 1), discovered in 1990,3 is located on chromosome 17q11.2 and encodes for the protein neurofibromin. This large gene (60 exons and >300 kilobases of genomic DNA) has one of the highest rates of spontaneous mutations in the entire human genome.4,5 Mutations exhibited by the gene are complete deletions, insertions, and nonsense and splicing mutations. Ultimately, these mutations may result in a loss of heterozygosity of the NF1 gene (a somatic loss of the second NF1 allele). Segmental, generalized, or gonadal forms of NF1 demonstrate mosaicism.6
Pathogenesis
Neurofibromin, the NF1 gene product, is a tumor suppressor expressed in many cells, primarily in neurons, glial cells, and Schwann cells, and is seen early in melanocyte development.7 The MAPK/ERK signaling pathway is a complex series of signals and interactions involved in cell growth and proliferation.5 Under normal conditions, neurofibromin, an RAS GTPase–activating protein promotes the conversion of the active RAS-GTP bound form to an inactive RAS-GDP bound form, thereby suppressing cell growth8,9; however, other possible effects are being investigated.10 Mast cells have been implicated in contributing to inflammation in the plexiform neurofibroma microenvironment of NF1.11,12 In addition, haploinsufficiency of NF1 (NF1+/−) and c-kit signaling in the hematopoietic system have been implicated in tumor progression. Accumulation of additional mutations of multiple genes, including INK4A/ARF and the protein p53, may be responsible for malignant transformation. These revelations of molecular and cellular mechanisms involved with NF1 tumorigenesis have led to trials of targeted therapies including the mammalian target of rapamycin and tyrosine kinase inhibitor imatinib mesylate, which is demonstrating promising preclinical results for treatment of peripheral nerve sheath tumors.13,14
Diagnosis
Seven cardinal diagnostic criteria have been delineated for NF1, at least 2 of which must be met to diagnose an individual with the condition.15 These criteria include (1) six or more café au lait macules (5 mm in diameter in prepubertal patients, 15 mm in postpubertal patients); (2) axillary or inguinal freckles (>2 freckles); (3) two or more typical neurofibromas or 1 plexiform neurofibroma, (4) optic nerve glioma, (5) two or more iris hamartomas (Lisch nodules), often only identified through slit-lamp examination by an ophthalmologist; (6) sphenoid dysplasia or typical long bone abnormalities such as pseudoarthritis; and (7) first-degree relative with NF1. Diagnosis may be difficult in patients who exhibit some dermatologic features of interest but who do not fully meet the diagnostic criteria.
Skin manifestations of NF1 may present in restricted segments of the body. It has been reported that half of those with NF1 are the first in their family to have the disease.16 Children with 6 or more café au lait macules alone and no family history of neurofibromatosis should be followed up, as their chances of developing NF1 are high.17 Occasionally, Lisch nodules may be the only clinical feature. Type 1 neurofibromatosis mutation analysis may be used to confirm the diagnosis in uncertain cases as well as prenatal diagnosis. However, genetic testing is not routinely advocated, and expert consultation is advised before it is undertaken. Furthermore, biopsy of asymptomatic cutaneous neurofibromas should not be undertaken for diagnostic purposes in individuals with confirmed NF1.18
Hyperintense lesions on T2-weighted magnetic resonance imaging (MRI) of the brain (formerly known as unidentified bright objects) probably are caused by aberrant myelination or gliosis and are pathognomonic of NF1.19The presence of these lesions can assist in the diagnosis of NF1, but MRI under anesthesia is not warranted for this purpose in children, who may not be able to stay still during the test.20
Physicians should not only be able to identify the cardinal skin features of NF1 but also the less common cutaneous and extracutaneous findings, which may indicate the need for referral to a dermatologist and/or neurologist.1 Café au lait macules (CALMs) are among the salient features of NF1. Classically, these lesions are well demarcated with smooth, regular, “coast of California” borders (unlike irregular “coast of Maine” borders) and a homogeneous appearance. Although the resemblance to the color of coffee in milk has earned these lesions their name, their color can range from tan to dark brown. The presence of multiple CALMs is highly suggestive of NF1.21 The prevalence of CALMs in the general population has varied from 3% to 36% depending on the study groups selected, but the presence of multiple CALMs in the general population typically is less than 1%.22 Frequently, CALMs are the first sign of NF1, occurring in 99% of NF1 patients within the first year of life. Patients continue to develop lesions throughout childhood, but they often fade in adulthood.23
Freckling of the skin folds is the most common of the cardinal criteria for NF1. Other sites include under the neck and breasts, around the lips, and trunk. Their size ranges from 1 to 3 mm, distinguishing them from CALMs. Considered nearly pathognomonic, NF1 generally occurs in children aged 3 to 5 years in the axillae or groin.15,24
Cutaneous neurofibromas generally are cutaneous/dermal tumors that are dome shaped, soft, fleshy, and flesh colored to slightly hyperpigmented. Subcutaneous tumors are firm and nodular. Neurofibromas usually do not become apparent until puberty and may continue to increase in size and number throughout adulthood. Pregnancy also is associated with increased tumor growth.25 The tumors are comprised of Schwann cells, fibroblasts, mast cells, and perineural cells. There also is an admixture of collagen and extracellular matrix.26 The cutaneous and extracutaneous manifestations of NF1 are outlined in Table 1 and Table 2.27-31
Management
Type 1 neurofibromatosis needs to be differentiated from other conditions based on careful clinical examination. Additionally, histopathologic examination of the lesions, imaging studies (eg, MRI), echocardiography, regular skeletal roentgenogram, and detailed ophthalmologic examination are important to look for any visceral involvement. Painful and bleeding tumors and cosmetic enhancement warrant surgical intervention, including various surgical techniques and lasers.32,33 Application of sunscreen may make pigmentary alterations less noticeable over time. Although not often located on the face, CALMs also may be amenable to various makeup products. Various studies have demonstrated improvement of freckling and CALMs with topical vitamin D3 analogues and lovastatin (β-hydroxy-β-methylglutaryl-CoA reductase inhibitors)34-36; however, this needs further exploration. Rapamycin has demonstrated efficacy in reducing tumor volume in animal studies by inhibiting the mammalian target of rapamycin cellular pathway.37 Imatinib mesylate has demonstrated efficacy both in vivo and in vitro in mouse models by targeting the platelet-derived growth-factor receptors α and β.38
Type 1 neurofibromatosis (NF1), or von Recklinghausen disease, is a multisystem disorder affecting approximately 1 in 3500 people in South East Wales.1 Type 1 neurofibromatosis has been described in the literature since the 13th century but was not recognized as a distinct disorder until 1882 in Friedrich Daniel von Recklinghausen’s landmark publication “On Multiple Fibromas of the Skin and Their Relationship to Multiple Neuromas.”2
Genetics
Type 1 neurofibromatosis is an autosomal-dominant disorder with a nearly even split between spontaneous and inherited mutations. It is characterized by neurofibromas, which are complex tumors composed of axonal processes, Schwann cells, fibroblasts, perineural cells, and mast cells. The NF1 gene (neurofibromin 1), discovered in 1990,3 is located on chromosome 17q11.2 and encodes for the protein neurofibromin. This large gene (60 exons and >300 kilobases of genomic DNA) has one of the highest rates of spontaneous mutations in the entire human genome.4,5 Mutations exhibited by the gene are complete deletions, insertions, and nonsense and splicing mutations. Ultimately, these mutations may result in a loss of heterozygosity of the NF1 gene (a somatic loss of the second NF1 allele). Segmental, generalized, or gonadal forms of NF1 demonstrate mosaicism.6
Pathogenesis
Neurofibromin, the NF1 gene product, is a tumor suppressor expressed in many cells, primarily in neurons, glial cells, and Schwann cells, and is seen early in melanocyte development.7 The MAPK/ERK signaling pathway is a complex series of signals and interactions involved in cell growth and proliferation.5 Under normal conditions, neurofibromin, an RAS GTPase–activating protein promotes the conversion of the active RAS-GTP bound form to an inactive RAS-GDP bound form, thereby suppressing cell growth8,9; however, other possible effects are being investigated.10 Mast cells have been implicated in contributing to inflammation in the plexiform neurofibroma microenvironment of NF1.11,12 In addition, haploinsufficiency of NF1 (NF1+/−) and c-kit signaling in the hematopoietic system have been implicated in tumor progression. Accumulation of additional mutations of multiple genes, including INK4A/ARF and the protein p53, may be responsible for malignant transformation. These revelations of molecular and cellular mechanisms involved with NF1 tumorigenesis have led to trials of targeted therapies including the mammalian target of rapamycin and tyrosine kinase inhibitor imatinib mesylate, which is demonstrating promising preclinical results for treatment of peripheral nerve sheath tumors.13,14
Diagnosis
Seven cardinal diagnostic criteria have been delineated for NF1, at least 2 of which must be met to diagnose an individual with the condition.15 These criteria include (1) six or more café au lait macules (5 mm in diameter in prepubertal patients, 15 mm in postpubertal patients); (2) axillary or inguinal freckles (>2 freckles); (3) two or more typical neurofibromas or 1 plexiform neurofibroma, (4) optic nerve glioma, (5) two or more iris hamartomas (Lisch nodules), often only identified through slit-lamp examination by an ophthalmologist; (6) sphenoid dysplasia or typical long bone abnormalities such as pseudoarthritis; and (7) first-degree relative with NF1. Diagnosis may be difficult in patients who exhibit some dermatologic features of interest but who do not fully meet the diagnostic criteria.
Skin manifestations of NF1 may present in restricted segments of the body. It has been reported that half of those with NF1 are the first in their family to have the disease.16 Children with 6 or more café au lait macules alone and no family history of neurofibromatosis should be followed up, as their chances of developing NF1 are high.17 Occasionally, Lisch nodules may be the only clinical feature. Type 1 neurofibromatosis mutation analysis may be used to confirm the diagnosis in uncertain cases as well as prenatal diagnosis. However, genetic testing is not routinely advocated, and expert consultation is advised before it is undertaken. Furthermore, biopsy of asymptomatic cutaneous neurofibromas should not be undertaken for diagnostic purposes in individuals with confirmed NF1.18
Hyperintense lesions on T2-weighted magnetic resonance imaging (MRI) of the brain (formerly known as unidentified bright objects) probably are caused by aberrant myelination or gliosis and are pathognomonic of NF1.19The presence of these lesions can assist in the diagnosis of NF1, but MRI under anesthesia is not warranted for this purpose in children, who may not be able to stay still during the test.20
Physicians should not only be able to identify the cardinal skin features of NF1 but also the less common cutaneous and extracutaneous findings, which may indicate the need for referral to a dermatologist and/or neurologist.1 Café au lait macules (CALMs) are among the salient features of NF1. Classically, these lesions are well demarcated with smooth, regular, “coast of California” borders (unlike irregular “coast of Maine” borders) and a homogeneous appearance. Although the resemblance to the color of coffee in milk has earned these lesions their name, their color can range from tan to dark brown. The presence of multiple CALMs is highly suggestive of NF1.21 The prevalence of CALMs in the general population has varied from 3% to 36% depending on the study groups selected, but the presence of multiple CALMs in the general population typically is less than 1%.22 Frequently, CALMs are the first sign of NF1, occurring in 99% of NF1 patients within the first year of life. Patients continue to develop lesions throughout childhood, but they often fade in adulthood.23
Freckling of the skin folds is the most common of the cardinal criteria for NF1. Other sites include under the neck and breasts, around the lips, and trunk. Their size ranges from 1 to 3 mm, distinguishing them from CALMs. Considered nearly pathognomonic, NF1 generally occurs in children aged 3 to 5 years in the axillae or groin.15,24
Cutaneous neurofibromas generally are cutaneous/dermal tumors that are dome shaped, soft, fleshy, and flesh colored to slightly hyperpigmented. Subcutaneous tumors are firm and nodular. Neurofibromas usually do not become apparent until puberty and may continue to increase in size and number throughout adulthood. Pregnancy also is associated with increased tumor growth.25 The tumors are comprised of Schwann cells, fibroblasts, mast cells, and perineural cells. There also is an admixture of collagen and extracellular matrix.26 The cutaneous and extracutaneous manifestations of NF1 are outlined in Table 1 and Table 2.27-31
Management
Type 1 neurofibromatosis needs to be differentiated from other conditions based on careful clinical examination. Additionally, histopathologic examination of the lesions, imaging studies (eg, MRI), echocardiography, regular skeletal roentgenogram, and detailed ophthalmologic examination are important to look for any visceral involvement. Painful and bleeding tumors and cosmetic enhancement warrant surgical intervention, including various surgical techniques and lasers.32,33 Application of sunscreen may make pigmentary alterations less noticeable over time. Although not often located on the face, CALMs also may be amenable to various makeup products. Various studies have demonstrated improvement of freckling and CALMs with topical vitamin D3 analogues and lovastatin (β-hydroxy-β-methylglutaryl-CoA reductase inhibitors)34-36; however, this needs further exploration. Rapamycin has demonstrated efficacy in reducing tumor volume in animal studies by inhibiting the mammalian target of rapamycin cellular pathway.37 Imatinib mesylate has demonstrated efficacy both in vivo and in vitro in mouse models by targeting the platelet-derived growth-factor receptors α and β.38
1. Boyd KP, Korf BR, Theos A. Neurofibromatosis type 1. J Am Acad Dermatol. 2009;61:1-14.
2. von Recklinghausen FV. Ueber die multiplen Fibrome der Haut und ihre Beziehung zu den Multiplen Neuromen. Berlin, Germany: August Hirschwald; 1882.
3. Viskochil D, Buchberg AM, Xu G, et al. Deletions and a translocation interrupt a cloned gene at the neurofibromatosis type 1 locus. Cell. 1990;62:187-192.
4. Theos A, Korf BR; American College of Physicians; American Physiological Society. Pathophysiology of neurofibromatosis type 1. Ann lntern Med. 2006;144:842-849.
5. Messiaen LM, Callens T, Mortier G, et al. Exhaustive mutation analysis of the NF1 gene allows identification of 95% of mutations and reveals a high frequency of unusual splicing defects. Hum Mutat. 2000;15:541-555.
6. Ruggieri M, Huson SM. The clinical and diagnostic implications of mosaicism in the neurofibromatoses. Neurology. 2001;56:1433-1443.
7. Stocker KM, Baizer L, Coston T, et al. Regulated expression of neurofibromin in migrating neural crest cells of avian embryos. J Neurobiol. 1995;27:535-552.
8. Maertens O, De Schepper S, Vandesompele J, et al. Molecular dissection of isolated disease features in mosaic neurofibromatosis type 1. Am J Hum Genet. 2007;81:243-251.
9. De Schepper S, Maertens O, Callens T, et al. Somatic mutation analysis in NF1 café au lait spots reveals two NF1 hits in the melanocytes. J Invest Dermatol. 2008;128:1050-1053.
10. Patrakitkomjorn S, Kobayahi D, Morikawa T, et al. Neurofibromatosis type 1 (NF1) tumor suppressor, neurofibromin, regulates the neuronal differentiation of PC12 cells via its associating protein, CRMP-2 [published online ahead of print. J Biol Chem. 2008;283:9399-9413.
11. Yang FC, Ingram DA, Chen S, et al. Nf1-dependent tumors require a microenvironment containing Nf1+/− and c-kit-dependent bone marrow. Cell. 2008;135:437-448.
12. Yang FC, Chen S, Clegg T, et al. Nf1+/- mast cells induce neurofibroma like phenotypes through secreted TGF-β signaling. Hum Mol Genet. 2006;15:2421-2437.
13. Gottfried ON, Viskochil DH, Couldwell WT. Neurofibromatosis type 1 and tumorigenesis: molecular mechanisms and therapeutic implications. Neurosurg Focus. 2010;28:E8.
14. Le LQ, Parada LF. Tumor microenvironment and neurofibromatosis type I: connecting the GAPs. Oncogene. 2007;26:4609-4616.
15. Gutmann DH, Aylsworth A, Carey JC, et al. The diagnostic evaluation and multidisciplinary management of neurofibromatosis 1 and neurofibromatosis 2. JAMA. 1997;278:51-57.
16. Huson SM, Harper PS, Compston DA. Von Recklinghausen neurofibromatosis. a clinical and population study in south-east Wales. Brain. 1988;111(pt 6):1355-1381.
17. Korf BR. Diagnostic outcome in children with multiple café au lait spots. Pediatrics. 1992;90:924-927.
18. Ferner RE, Huson SM, Thomas N, et al. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J Med Genet. 2007;44:81-88.
19. DiPaolo DP, Zimmerman RA, Rorke LB, et al. Neurofibromatosis type 1: pathologic substrate of high-signal-intensity foci in the brain. Radiology. 1995;195:721-724.
20. DeBella K, Poskitt K, Szudek J, et al. Use of “unidentified bright objects” on MRI for diagnosis of neurofibromatosis 1 in children. Neurology. 2000;54:1646-1651.
21. Whitehouse D. Diagnostic value of the café-au-lait spot in children. Arch Dis Child. 1966;41:316-319.
22. Landau M, Krafchik BR. The diagnostic value of café-au-lait macules. J Am Acad Dermatol. 1999;40(6, pt 1):877-890.
23. DeBella K, Szudek J, Friedman JM. Use of the National Institutes of Health criteria for diagnosis of neurofibromatosis 1 in children. Pediatrics. 2000;105(3, pt 1):608-614.
24. Obringer AC, Meadows AT, Zackai EH. The diagnosis of neurofibromatosis-l in the child under the age of 6 years. Am J Dis Child. 1989;143:717-719.
25. Page PZ, Page GP, Ecosse E, et al. Impact of neurofibromatosis 1 on quality of life: a cross-sectional study of 176 American cases. Am J Med Genet A. 2006;140:1893-1898.
26. Maertens O, Brems H. Vandesompele J, et al. Comprehensive NF1 screening on cultured Schwann cells from neurofibromas. Hum Mutat. 2006;27:1030-1040.
27. Huson S, Jones D, Beck L. Ophthalmic manifestations of neurofibromatosis. Br J Ophthalmol. 1987;71:235-238.
28. Listernick R, Ferner RE, Liu GT, et al. Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. Ann Neurol. 2007;61:189-198.
29. Levine TM, Materek A, Abel J, et al. Cognitive profile of neurofibromatosis type 1. Semin Pediatr Neurol. 2006;13:8-20.
30. Lammert M, Kappler M, Mautner VF, et al. Decreased bone mineral density in patients with neurofibromatosis 1. Osteoporos Int. 2005;16:1161-1166.
31. Schindeler A, Little DG. Recent insights into bone development, homeostasis, and repair in type 1 neurofibromatosis (NFl). Bone. 2008;42:616-622.
32. Yoo KH, Kim BJ, Rho YK, et al. A case of diffuse neurofibroma of the scalp. Ann Dermatol. 2009;21:46-48.
33. Onesti MG, Carella S, Spinelli G, et al. A study of 17 patients affected with plexiform neurofibromas in upper and lower extremities: comparison between different surgical techniques. Acta Chir Plast. 2009;51:35-40.
34. Yoshida Y, Sato N, Furumura M, et al. Treatment of pigmented lesions of neurofibromatosis 1 with intense pulsed-radio frequency in combination with topical application of vitamin D3 ointment. J Dermatol. 2007;34:227-230.
35. Nakayama J, Kiryu H, Urabe K, et al. Vitamin D3 analogues improve café au lait spots in patients with von Recklinghausen’s disease: experimental and clinical studies. Eur J Dermatol. 1999;9:202-206.
36. Lammert M, Friedman JM, Roth HJ, et al. Vitamin D deficiency associated with number of neurofibromas in neurofibromatosis 1. J Med Genet. 2006;43:810-813.
37. Hegedus B, Banerjee D, Yeh TH, et al. Preclinical cancer therapy in a mouse model of neurofibromatosis-1 optic glioma. Cancer Res. 2008;68:1520-1528.
38. Demestre M, Herzberg J, Holtkamp N, et al. Imatinib mesylate (Glivec) inhibits Schwann cell viability and reduces the size of human plexiform neurofibroma in a xenograft model. J Neurooncol. 2010;98:11-19.
1. Boyd KP, Korf BR, Theos A. Neurofibromatosis type 1. J Am Acad Dermatol. 2009;61:1-14.
2. von Recklinghausen FV. Ueber die multiplen Fibrome der Haut und ihre Beziehung zu den Multiplen Neuromen. Berlin, Germany: August Hirschwald; 1882.
3. Viskochil D, Buchberg AM, Xu G, et al. Deletions and a translocation interrupt a cloned gene at the neurofibromatosis type 1 locus. Cell. 1990;62:187-192.
4. Theos A, Korf BR; American College of Physicians; American Physiological Society. Pathophysiology of neurofibromatosis type 1. Ann lntern Med. 2006;144:842-849.
5. Messiaen LM, Callens T, Mortier G, et al. Exhaustive mutation analysis of the NF1 gene allows identification of 95% of mutations and reveals a high frequency of unusual splicing defects. Hum Mutat. 2000;15:541-555.
6. Ruggieri M, Huson SM. The clinical and diagnostic implications of mosaicism in the neurofibromatoses. Neurology. 2001;56:1433-1443.
7. Stocker KM, Baizer L, Coston T, et al. Regulated expression of neurofibromin in migrating neural crest cells of avian embryos. J Neurobiol. 1995;27:535-552.
8. Maertens O, De Schepper S, Vandesompele J, et al. Molecular dissection of isolated disease features in mosaic neurofibromatosis type 1. Am J Hum Genet. 2007;81:243-251.
9. De Schepper S, Maertens O, Callens T, et al. Somatic mutation analysis in NF1 café au lait spots reveals two NF1 hits in the melanocytes. J Invest Dermatol. 2008;128:1050-1053.
10. Patrakitkomjorn S, Kobayahi D, Morikawa T, et al. Neurofibromatosis type 1 (NF1) tumor suppressor, neurofibromin, regulates the neuronal differentiation of PC12 cells via its associating protein, CRMP-2 [published online ahead of print. J Biol Chem. 2008;283:9399-9413.
11. Yang FC, Ingram DA, Chen S, et al. Nf1-dependent tumors require a microenvironment containing Nf1+/− and c-kit-dependent bone marrow. Cell. 2008;135:437-448.
12. Yang FC, Chen S, Clegg T, et al. Nf1+/- mast cells induce neurofibroma like phenotypes through secreted TGF-β signaling. Hum Mol Genet. 2006;15:2421-2437.
13. Gottfried ON, Viskochil DH, Couldwell WT. Neurofibromatosis type 1 and tumorigenesis: molecular mechanisms and therapeutic implications. Neurosurg Focus. 2010;28:E8.
14. Le LQ, Parada LF. Tumor microenvironment and neurofibromatosis type I: connecting the GAPs. Oncogene. 2007;26:4609-4616.
15. Gutmann DH, Aylsworth A, Carey JC, et al. The diagnostic evaluation and multidisciplinary management of neurofibromatosis 1 and neurofibromatosis 2. JAMA. 1997;278:51-57.
16. Huson SM, Harper PS, Compston DA. Von Recklinghausen neurofibromatosis. a clinical and population study in south-east Wales. Brain. 1988;111(pt 6):1355-1381.
17. Korf BR. Diagnostic outcome in children with multiple café au lait spots. Pediatrics. 1992;90:924-927.
18. Ferner RE, Huson SM, Thomas N, et al. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J Med Genet. 2007;44:81-88.
19. DiPaolo DP, Zimmerman RA, Rorke LB, et al. Neurofibromatosis type 1: pathologic substrate of high-signal-intensity foci in the brain. Radiology. 1995;195:721-724.
20. DeBella K, Poskitt K, Szudek J, et al. Use of “unidentified bright objects” on MRI for diagnosis of neurofibromatosis 1 in children. Neurology. 2000;54:1646-1651.
21. Whitehouse D. Diagnostic value of the café-au-lait spot in children. Arch Dis Child. 1966;41:316-319.
22. Landau M, Krafchik BR. The diagnostic value of café-au-lait macules. J Am Acad Dermatol. 1999;40(6, pt 1):877-890.
23. DeBella K, Szudek J, Friedman JM. Use of the National Institutes of Health criteria for diagnosis of neurofibromatosis 1 in children. Pediatrics. 2000;105(3, pt 1):608-614.
24. Obringer AC, Meadows AT, Zackai EH. The diagnosis of neurofibromatosis-l in the child under the age of 6 years. Am J Dis Child. 1989;143:717-719.
25. Page PZ, Page GP, Ecosse E, et al. Impact of neurofibromatosis 1 on quality of life: a cross-sectional study of 176 American cases. Am J Med Genet A. 2006;140:1893-1898.
26. Maertens O, Brems H. Vandesompele J, et al. Comprehensive NF1 screening on cultured Schwann cells from neurofibromas. Hum Mutat. 2006;27:1030-1040.
27. Huson S, Jones D, Beck L. Ophthalmic manifestations of neurofibromatosis. Br J Ophthalmol. 1987;71:235-238.
28. Listernick R, Ferner RE, Liu GT, et al. Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. Ann Neurol. 2007;61:189-198.
29. Levine TM, Materek A, Abel J, et al. Cognitive profile of neurofibromatosis type 1. Semin Pediatr Neurol. 2006;13:8-20.
30. Lammert M, Kappler M, Mautner VF, et al. Decreased bone mineral density in patients with neurofibromatosis 1. Osteoporos Int. 2005;16:1161-1166.
31. Schindeler A, Little DG. Recent insights into bone development, homeostasis, and repair in type 1 neurofibromatosis (NFl). Bone. 2008;42:616-622.
32. Yoo KH, Kim BJ, Rho YK, et al. A case of diffuse neurofibroma of the scalp. Ann Dermatol. 2009;21:46-48.
33. Onesti MG, Carella S, Spinelli G, et al. A study of 17 patients affected with plexiform neurofibromas in upper and lower extremities: comparison between different surgical techniques. Acta Chir Plast. 2009;51:35-40.
34. Yoshida Y, Sato N, Furumura M, et al. Treatment of pigmented lesions of neurofibromatosis 1 with intense pulsed-radio frequency in combination with topical application of vitamin D3 ointment. J Dermatol. 2007;34:227-230.
35. Nakayama J, Kiryu H, Urabe K, et al. Vitamin D3 analogues improve café au lait spots in patients with von Recklinghausen’s disease: experimental and clinical studies. Eur J Dermatol. 1999;9:202-206.
36. Lammert M, Friedman JM, Roth HJ, et al. Vitamin D deficiency associated with number of neurofibromas in neurofibromatosis 1. J Med Genet. 2006;43:810-813.
37. Hegedus B, Banerjee D, Yeh TH, et al. Preclinical cancer therapy in a mouse model of neurofibromatosis-1 optic glioma. Cancer Res. 2008;68:1520-1528.
38. Demestre M, Herzberg J, Holtkamp N, et al. Imatinib mesylate (Glivec) inhibits Schwann cell viability and reduces the size of human plexiform neurofibroma in a xenograft model. J Neurooncol. 2010;98:11-19.
Practice Points
- Histopathology and magnetic resonance imaging are useful in diagnosing type 1 neurofibromatosis (NF1).
- Newer treatments like statins and tyrosine kinase inhibitors are worth exploring in NF1.
Get smart about dense breasts
It’s a movement that shows no signs of abating. Women in 24 states, encompassing 67% of American women, now receive some level of notification after their mammogram about breast density. Individual patient advocates continue to push for notification, and states are likely to continue to draft bills. On the national level, a federal standard is being pursued through both federal legislation and federal regulation. Clinicians practicing in states with an “inform” law, either already in effect or imminent, will be tasked with engaging in new patient conversations arising from density notification. Are you ready to answer your patients’ questions?
Navigating inconsistent data and expert comments about density and discerning which patients may benefit from additional screening can create challenges in addressing a patient’s questions about the implications of her dense tissue. If you feel less than equipped to address these issues, you are not alone. A recent survey of clinicians, con- ducted after California’s breast density notification law went into effect, showed that only 6% were comfortable answering patients’ questions relating to breast density. Seventy-five percent of respondents indicated they wanted more education on the topic.1
For women having mammography, dense breast tissue is most important because it can mask detection of cancers, and women may want to have additional screening beyond mammography. Women with dense breasts are also at increased risk for developing breast cancer. For clinicians who are on the front lines of care for women undergoing screening, the most important action items are:
- identifying who needs more screening
- weighing the risks and benefits of such additional screening.
To assist you in informing patient discussions, in this article we answer some of the most frequently asked questions of ObGyns.
Which breasts are considered dense?
The American College of Radiology recommends that density be reported in 1 of 4 categories depending on the relative amounts of fat and fibroglandular tissue2:
- almost entirely fatty—on mammography most of the tissue appears dark gray while small amounts of dense (or fibroglandular) tissue display as light gray or white.
- scattered fibroglandular density—scattered areas of dense tissue mixed with fat. Even in breasts with scattered areas of breast tissue, cancers sometimes can be missed when they resemble areas of normal tissue or are within an area of denser tissue.
- heterogeneously dense—there are large portions of the breast where dense tissue could hide masses.
- extremely dense—most of the breast appears to consist of dense tissue, creating a “white out” situation and making it extremely difficult to see through.
Breasts that are either heterogeneously dense or extremely dense are considered “dense.” About 40% of women older than age 40 have dense breasts.3
Case study: Imaging of a cancerous breast mass in a 48-year-old woman with dense breasts
This patient has heterogeneously dense breast tissue. Standard 2D mediolateral oblique (MLO) digital mammogram (A) and MLO tomosynthesis 1-mm slice (B) reveal subtle possible distortion (arrow) in the upper right breast. On tomosynthesis, the distortion is better seen, as is the underlying irregular mass (red circle).
Ultrasound (US) directed to the mass (C) shows an irregular hypoechoic (dark gray) mass (marked by calipers), compatible with cancer. US-guided core needle biopsy revealed grade 2 invasive ductal cancer with associated ductal carcinoma in situ.
Axial magnetic resonance imaging of the right breast obtained after contrast injection, and after computer subtraction of nonenhanced image (D), shows irregular spiculated enhancing (white) mass (arrow) due to the known carcinoma.
Images: Courtesy Wendie Berg, MD, PhD
Who needs more screening?
The FIGURE is a screening decision support tool representing the consensus opinion of several medical experts based on the best available scientific evidence to optimize breast cancer detection.
Do dense breasts affect the risk of developing breast cancer?
Yes. Dense breasts are a risk factor for breast cancer. According to the American Cancer Society’s Breast Cancer Facts & Figures 2013−2014, “The risk of breast cancer in-creases with increasing breast density; women with very high breast density have a 4- to 6-fold increased risk of breast cancer compared to women with the least dense breasts.”4,5
There are several reasons that dense tissue increases risk. First, the glands tend to be made up of relatively actively dividing cells that can mutate and become cancerous (the more glandular tissue present, the greater the risk). Second, the local environment around the glands may produce certain growth hormones that stimulate cells to divide, and this seems to occur more in fibrous tissue than in fatty tissue.
Most women have breast density somewhere in the middle range, with their risk for breast cancer falling in between those with extremely dense breasts and those with fatty breasts.6 The risk for developing breast cancer is influenced by a combination of many different factors, including age, family history of cancer (particularly breast or ovarian cancer), and prior atypical breast biopsies. There currently is no reliable way to fully account for the interplay of breast density, family history, prior biopsy results, and other factors in determining overall risk. Importantly, more than half of all women who develop breast cancer have no known risk factors other than being female and aging.
Is your medical support staff “density ready?”
We’re all familiar with the adage that a picture is worth a thousand words. While the medical support personnel in your office are likely quite familiar with imaging reports and the terminology used in describing dense breasts, they may be quite unfamiliar with what a fatty versus dense breast actually looks like on a mammogram, and how cancer may display in each. Illustrated examples, as seen here, are useful for reference.
In the fatty breast (A), a small cancer (arrow) is seen easily. In a breast categorized as scattered fibroglandular density (B), a large cancer is easily seen (arrow) in the relatively fatty portion of the breast, though a small cancer could have been hidden in areas with normal glandular tissue.
In a breast categorized as heterogeneously dense (C), a 4-cm (about 1.5-inch) cancer (arrows) is hidden by the dense breast tissue. This cancer also has spread to a lymph node under the arm (curved arrow).
In an extremely dense breast (D), a cancer is seen because part of it is located in the back of the breast where there is a small amount of dark fat making it easier to see (arrow and triangle marker indicating lump). If this cancer had been located near the nipple and completely surrounded by white (dense) tissue, it probably would not have been seen on mammography.
Image: Courtesy of Dr. Regina Hooley and DenseBreast-info.org
Are screening mammography outcomes different for women with dense versus fatty breasts?
Yes. Cancer is more likely to be clinically detected in the interval between mammography screens (defined as interval cancer) in women with dense breasts. Such interval cancers tend to be more aggressive and have worse outcomes. Compared with those in fatty breasts, cancers found in dense breasts more often7:
- are locally advanced (stage IIb and III)
- are multifocal or multicentric
- require a mastectomy (rather than a lumpectomy).
Does supplemental screening beyond mammography save lives?
Mammography is the only imaging screening modality that has been studied by multiple randomized controlled trials with mortality as an endpoint. Across those trials, mammography has been shown to reduce deaths due to breast cancer. The randomized trials that show a benefit from mammography are those in which mammography increased detection of invasive breast cancers before they spread to lymph nodes.8
No randomized controlled trial has yet been reported on any other imaging screening modality, but it is expected that other screening tests that increase detection of node-negative invasive breast cancers beyond mammography should further reduce breast cancer mortality.
Proving the mortality benefit of any supplemental screening modality would require a very large, very expensive randomized controlled trial with 15 to 20 years of follow-up. Given the speed of technologic developments, any results likely would be obsolete by the trial’s conclusion. What we do know is that women at high risk for breast cancer who undergo annual magnetic resonance imaging (MRI) screening are less likely to have advanced breast cancer than their counterparts who were not screened with MRI.9
We also know that average-risk women who are screened with ultrasonography in addition to mammography are unlikely to have palpable cancer in the interval between screens,10,11 with the rates of such interval cancers similar to women with fatty breasts screened only with mammography. The cancers found only on MRI or ultrasound are mostly small invasive cancers (average size, approximately 1 cm) that are mostly node negative.12,13 MRI also finds some ductal carcinoma in situ (DCIS).
These results suggest that there is a benefit to finding additional cancers with supplemental screening, though it is certainly possible that, as with mammography, some of the cancers found with supplemental screening are slow growing and may never have caused a woman harm even if left untreated.
Dense breasts: Medically sourced resources
Educational Web site
DenseBreast-info.org. This site is a collaborative, multidisciplinary educational resource. It features content for both patients and health care providers with separate data streams for each and includes:
a comprehensive list of FAQs; screening flow charts; a Patient Risk Checklist; an explanation of risks, risk assessment, and links to risk assessment tools; an illustrated round-up of technologies commonly used in screening; and state-by-state legislative analysis of density inform laws across the country.
State-specific Web sites
BreastDensity.info. This site was created by the California Breast Density Information Group (CBDIG), a working group of breast radiologists and breast cancer risk specialists. The content is primarily for health care providers and features screening scenarios as well as FAQs about breast density, breast cancer risk, and the breast density notification law in California.
MIdensebreasts.org. This is a Web-based education resource created for primary care providers by the University of Michigan Health System and the Michigan Department of Health and Human Services. It includes continuing medical education credit.
Medical society materials
American Cancer Society offers Breast Density and Your Mammogram Report for patients: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-039989.pdf
American College of Obstetricians and Gynecologists’ 2015 Density Policy statement is available online: http://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Gynecologic-Practice/Management-of-Women-With-Dense-Breasts-Diagnosed-by-Mammography
American College of Radiology patient brochure details basic information about breast density and can be customized with your center’s information: http://www.acr.org/News-Publications/~/media/180321AF51AF4EA38FEC091461F5B695.pdf
What additional screening tests are available after a 2D mammogram for a woman with dense breasts?
Depending on the patient’s age, risk level, and breast density, additional screening tools—such as tomosynthesis (also known as 3D mammography), ultrasonography, or MRI—may be recommended in addition to mammography. Indeed, in some centers, tomosynthesis is performed alone and the radiologist also reviews computer-generated 2D mammograms.
The addition of another imaging tool after a mammogram will find more cancers than mammography alone (TABLE).14−17 Women at high risk for breast cancer, such as those with pathogenic BRCA mutations, and those who were treated with radiation therapy to their chest (typically for Hodgkin disease) before age 30 and at least 8 years earlier, should be referred for annual MRI in addition to mammography (see Screening Decision Support Tool FIGURE above). If tomosynthesis is performed, the added benefit of ultrasound will be lower; further study on the actual benefit of supplemental ultrasound screening after 3D mammography is needed.
Will insurance cover supplemental screening beyond mammography?
The answer depends on the type of screening, the patient’s insurance and risk factors, the state in which you practice, and whether or not a law is in effect requiring insurance coverage for additional screening. In Illinois, for example, a woman with dense breasts can receive a screening ultrasound without a copay or deductible if it is ordered by a physician. In Connecticut, an ultrasound copay for screening dense breasts cannot exceed $20. Generally, in other states, an ultrasound will be covered if ordered by a physician, but it is subject to the copay and deductible of an individual health plan. In New Jersey, insurance coverage is provided for additional testing if a woman has extremely dense breasts.
Regardless of state, an MRI generally will be covered by insurance (subject to copay and deductible) if the patient meets high-risk criteria. In Michigan, at least one insurance company will cover a screening MRI for normal-risk women with dense breasts at a cost that matches the copay and deductible of a screening mammogram. It is important for patients to check with their insurance carrier prior to having an MRI.
Should women with dense breasts still have mammography screening?
Yes. Mammography is the first step in screening for most women (except for those who are pregnant or breastfeeding, in which case ultrasound can be performed but is usually deferred until several months after the patient is no longer pregnant or breastfeeding). While additional screening may be recommended for women with dense breasts, and for women at high risk for developing breast cancer, there are still some cancers and precancerous changes that will show on a mammogram better than on ultrasound or MRI. Wherever possible, women with dense breasts should have digital mammography rather than film mammography, due to slightly improved cancer detection using digital mammography.18
Does tomosynthesis solve the problem of screening dense breasts?
Tomosynthesis (3D mammography) slightly improves detection of cancers compared with standard digital mammography, but some cancers will remain hidden by overlapping dense tissue. We do not yet know the benefit of annual screening tomosynthesis. Without question, women at high risk for breast cancer still should have MRI if they are able to tolerate it, even if they have had tomosynthesis.
If a patient with dense breasts undergoes screening tomosynthesis, will she also need a screening ultrasound?
Preliminary studies not yet published suggest that, for women with dense breasts, ultrasound does find another 2 to 3 invasive cancers per 1,000 women screened that are not found on tomosynthesis, but further study of this issue is needed.
If recommended for additional screening with ultrasound or MRI, will a patient need that screening every year?
Usually, yes, though age and other medical conditions will change a patient’s personal risk and benefit considerations. Therefore, screening recommendations may change from one year to the next. With technology advancements and evolving guidelines, additional screening recommendations will change in the future.
Prepare yourself and your patients will benefit
The foundation of a successful screening program involves buy-in from both patient and clinician. Patients confused as to what their density notification means may have little understanding as to what next steps should be considered. To allay confusion, your patient will be best served by being provided understandable and actionable information. Advanced preparation for these conversations about the implications of dense tissue will make for more effective patient engagement.
Acknowledgment
Much of the material within this article has been sourced from the educational Web site DenseBreast-info.org. For comprehensive lists of both patient and health care provider frequently asked questions, visit http://www.DenseBreast-info.org.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Khong KA, Hargreaves J, Aminololama-Shakeri S, Lindfors KK. Impact of the California breast density law on primary care physicians. J Am Coll Radiol. 2015;12(3):256–260.
- Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS Mammography. In: ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013.
- Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106(10).
- American Cancer Society. Breast Cancer Facts & Figures 2013–2014. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-042725.pdf. Published 2013. Accessed September 15, 2015.
- Harvey JA, Bovbjerg VE. Quantitative assessment of mammographic breast density: relationship with breast cancer risk. Radiology. 2004;230(1):29–41.
- Kerlikowske K, Cook AJ, Buist DS, et al. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol. 2010;28(24):3830–3837.
- Arora N, King TA, Jacks LM, et al. Impact of breast density on the presenting features of malignancy. Ann Surg Oncol. 2010;17(suppl 3):211–218.
- Smith RA, Duffy SW, Gabe R, Tabar L, Yen AM, Chen TH. The randomized trials of breast cancer screening: what have we learned? Radiol Clin North Am. 2004;42(5):793–806, v.
- Warner E, Hill K, Causer P, et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol. 2011;29(13):1664–1669.
- Corsetti V, Houssami N, Ghirardi M, et al. Evidence of the effect of adjunct ultrasound screening in women with mammography-negative dense breasts: interval breast cancers at 1 year follow-up. Eur J Cancer. 2011;47(7): 1021–1026.
- Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307(13):1394–1404.
- Berg WA. Tailored supplemental screening for breast cancer: what now and what next? AJR Am J Roentgenol. 2009;192(2):390–399.
- Brem RF, Lenihan MJ, Lieberman J, Torrente J. Screening breast ultrasound: past, present, and future. AJR Am J Roentgenol. 2015;204(2):234–240.
- Hooley R. Tomosynthesis. In: Berg WA, Yang WT, eds. Diagnostic Imaging: Breast. 2nd ed. Salt Lake City, UT: Amirsys; 2014:2–19.
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499–2507.
- Berg WA. Screening Ultrasound. In: Berg WA, Yang WT, eds. Diagnostic Imaging: Breast. 2nd ed. Salt Lake City, UT: Amirsys; 2014:9–43.
- Berg WA. Screening MRI. In: Berg WA, Yang WT, eds. Diagnostic Imaging: Breast. 2nd ed. Salt Lake City, UT: Amirsys; 2014:9–49.
- Hooley R. Tomosynthesis. In: Berg WA, Yang WT, eds.Berg WA. Screening Ultrasound. In: Berg WA, Yang WT, eds.Berg WA. Screening MRI. In: Berg WA, Yang WT, eds.Pisano ED, Gatsonis C, Hendrick E, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005;353(17):1773–1783.
It’s a movement that shows no signs of abating. Women in 24 states, encompassing 67% of American women, now receive some level of notification after their mammogram about breast density. Individual patient advocates continue to push for notification, and states are likely to continue to draft bills. On the national level, a federal standard is being pursued through both federal legislation and federal regulation. Clinicians practicing in states with an “inform” law, either already in effect or imminent, will be tasked with engaging in new patient conversations arising from density notification. Are you ready to answer your patients’ questions?
Navigating inconsistent data and expert comments about density and discerning which patients may benefit from additional screening can create challenges in addressing a patient’s questions about the implications of her dense tissue. If you feel less than equipped to address these issues, you are not alone. A recent survey of clinicians, con- ducted after California’s breast density notification law went into effect, showed that only 6% were comfortable answering patients’ questions relating to breast density. Seventy-five percent of respondents indicated they wanted more education on the topic.1
For women having mammography, dense breast tissue is most important because it can mask detection of cancers, and women may want to have additional screening beyond mammography. Women with dense breasts are also at increased risk for developing breast cancer. For clinicians who are on the front lines of care for women undergoing screening, the most important action items are:
- identifying who needs more screening
- weighing the risks and benefits of such additional screening.
To assist you in informing patient discussions, in this article we answer some of the most frequently asked questions of ObGyns.
Which breasts are considered dense?
The American College of Radiology recommends that density be reported in 1 of 4 categories depending on the relative amounts of fat and fibroglandular tissue2:
- almost entirely fatty—on mammography most of the tissue appears dark gray while small amounts of dense (or fibroglandular) tissue display as light gray or white.
- scattered fibroglandular density—scattered areas of dense tissue mixed with fat. Even in breasts with scattered areas of breast tissue, cancers sometimes can be missed when they resemble areas of normal tissue or are within an area of denser tissue.
- heterogeneously dense—there are large portions of the breast where dense tissue could hide masses.
- extremely dense—most of the breast appears to consist of dense tissue, creating a “white out” situation and making it extremely difficult to see through.
Breasts that are either heterogeneously dense or extremely dense are considered “dense.” About 40% of women older than age 40 have dense breasts.3
Case study: Imaging of a cancerous breast mass in a 48-year-old woman with dense breasts
This patient has heterogeneously dense breast tissue. Standard 2D mediolateral oblique (MLO) digital mammogram (A) and MLO tomosynthesis 1-mm slice (B) reveal subtle possible distortion (arrow) in the upper right breast. On tomosynthesis, the distortion is better seen, as is the underlying irregular mass (red circle).
Ultrasound (US) directed to the mass (C) shows an irregular hypoechoic (dark gray) mass (marked by calipers), compatible with cancer. US-guided core needle biopsy revealed grade 2 invasive ductal cancer with associated ductal carcinoma in situ.
Axial magnetic resonance imaging of the right breast obtained after contrast injection, and after computer subtraction of nonenhanced image (D), shows irregular spiculated enhancing (white) mass (arrow) due to the known carcinoma.
Images: Courtesy Wendie Berg, MD, PhD
Who needs more screening?
The FIGURE is a screening decision support tool representing the consensus opinion of several medical experts based on the best available scientific evidence to optimize breast cancer detection.
Do dense breasts affect the risk of developing breast cancer?
Yes. Dense breasts are a risk factor for breast cancer. According to the American Cancer Society’s Breast Cancer Facts & Figures 2013−2014, “The risk of breast cancer in-creases with increasing breast density; women with very high breast density have a 4- to 6-fold increased risk of breast cancer compared to women with the least dense breasts.”4,5
There are several reasons that dense tissue increases risk. First, the glands tend to be made up of relatively actively dividing cells that can mutate and become cancerous (the more glandular tissue present, the greater the risk). Second, the local environment around the glands may produce certain growth hormones that stimulate cells to divide, and this seems to occur more in fibrous tissue than in fatty tissue.
Most women have breast density somewhere in the middle range, with their risk for breast cancer falling in between those with extremely dense breasts and those with fatty breasts.6 The risk for developing breast cancer is influenced by a combination of many different factors, including age, family history of cancer (particularly breast or ovarian cancer), and prior atypical breast biopsies. There currently is no reliable way to fully account for the interplay of breast density, family history, prior biopsy results, and other factors in determining overall risk. Importantly, more than half of all women who develop breast cancer have no known risk factors other than being female and aging.
Is your medical support staff “density ready?”
We’re all familiar with the adage that a picture is worth a thousand words. While the medical support personnel in your office are likely quite familiar with imaging reports and the terminology used in describing dense breasts, they may be quite unfamiliar with what a fatty versus dense breast actually looks like on a mammogram, and how cancer may display in each. Illustrated examples, as seen here, are useful for reference.
In the fatty breast (A), a small cancer (arrow) is seen easily. In a breast categorized as scattered fibroglandular density (B), a large cancer is easily seen (arrow) in the relatively fatty portion of the breast, though a small cancer could have been hidden in areas with normal glandular tissue.
In a breast categorized as heterogeneously dense (C), a 4-cm (about 1.5-inch) cancer (arrows) is hidden by the dense breast tissue. This cancer also has spread to a lymph node under the arm (curved arrow).
In an extremely dense breast (D), a cancer is seen because part of it is located in the back of the breast where there is a small amount of dark fat making it easier to see (arrow and triangle marker indicating lump). If this cancer had been located near the nipple and completely surrounded by white (dense) tissue, it probably would not have been seen on mammography.
Image: Courtesy of Dr. Regina Hooley and DenseBreast-info.org
Are screening mammography outcomes different for women with dense versus fatty breasts?
Yes. Cancer is more likely to be clinically detected in the interval between mammography screens (defined as interval cancer) in women with dense breasts. Such interval cancers tend to be more aggressive and have worse outcomes. Compared with those in fatty breasts, cancers found in dense breasts more often7:
- are locally advanced (stage IIb and III)
- are multifocal or multicentric
- require a mastectomy (rather than a lumpectomy).
Does supplemental screening beyond mammography save lives?
Mammography is the only imaging screening modality that has been studied by multiple randomized controlled trials with mortality as an endpoint. Across those trials, mammography has been shown to reduce deaths due to breast cancer. The randomized trials that show a benefit from mammography are those in which mammography increased detection of invasive breast cancers before they spread to lymph nodes.8
No randomized controlled trial has yet been reported on any other imaging screening modality, but it is expected that other screening tests that increase detection of node-negative invasive breast cancers beyond mammography should further reduce breast cancer mortality.
Proving the mortality benefit of any supplemental screening modality would require a very large, very expensive randomized controlled trial with 15 to 20 years of follow-up. Given the speed of technologic developments, any results likely would be obsolete by the trial’s conclusion. What we do know is that women at high risk for breast cancer who undergo annual magnetic resonance imaging (MRI) screening are less likely to have advanced breast cancer than their counterparts who were not screened with MRI.9
We also know that average-risk women who are screened with ultrasonography in addition to mammography are unlikely to have palpable cancer in the interval between screens,10,11 with the rates of such interval cancers similar to women with fatty breasts screened only with mammography. The cancers found only on MRI or ultrasound are mostly small invasive cancers (average size, approximately 1 cm) that are mostly node negative.12,13 MRI also finds some ductal carcinoma in situ (DCIS).
These results suggest that there is a benefit to finding additional cancers with supplemental screening, though it is certainly possible that, as with mammography, some of the cancers found with supplemental screening are slow growing and may never have caused a woman harm even if left untreated.
Dense breasts: Medically sourced resources
Educational Web site
DenseBreast-info.org. This site is a collaborative, multidisciplinary educational resource. It features content for both patients and health care providers with separate data streams for each and includes:
a comprehensive list of FAQs; screening flow charts; a Patient Risk Checklist; an explanation of risks, risk assessment, and links to risk assessment tools; an illustrated round-up of technologies commonly used in screening; and state-by-state legislative analysis of density inform laws across the country.
State-specific Web sites
BreastDensity.info. This site was created by the California Breast Density Information Group (CBDIG), a working group of breast radiologists and breast cancer risk specialists. The content is primarily for health care providers and features screening scenarios as well as FAQs about breast density, breast cancer risk, and the breast density notification law in California.
MIdensebreasts.org. This is a Web-based education resource created for primary care providers by the University of Michigan Health System and the Michigan Department of Health and Human Services. It includes continuing medical education credit.
Medical society materials
American Cancer Society offers Breast Density and Your Mammogram Report for patients: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-039989.pdf
American College of Obstetricians and Gynecologists’ 2015 Density Policy statement is available online: http://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Gynecologic-Practice/Management-of-Women-With-Dense-Breasts-Diagnosed-by-Mammography
American College of Radiology patient brochure details basic information about breast density and can be customized with your center’s information: http://www.acr.org/News-Publications/~/media/180321AF51AF4EA38FEC091461F5B695.pdf
What additional screening tests are available after a 2D mammogram for a woman with dense breasts?
Depending on the patient’s age, risk level, and breast density, additional screening tools—such as tomosynthesis (also known as 3D mammography), ultrasonography, or MRI—may be recommended in addition to mammography. Indeed, in some centers, tomosynthesis is performed alone and the radiologist also reviews computer-generated 2D mammograms.
The addition of another imaging tool after a mammogram will find more cancers than mammography alone (TABLE).14−17 Women at high risk for breast cancer, such as those with pathogenic BRCA mutations, and those who were treated with radiation therapy to their chest (typically for Hodgkin disease) before age 30 and at least 8 years earlier, should be referred for annual MRI in addition to mammography (see Screening Decision Support Tool FIGURE above). If tomosynthesis is performed, the added benefit of ultrasound will be lower; further study on the actual benefit of supplemental ultrasound screening after 3D mammography is needed.
Will insurance cover supplemental screening beyond mammography?
The answer depends on the type of screening, the patient’s insurance and risk factors, the state in which you practice, and whether or not a law is in effect requiring insurance coverage for additional screening. In Illinois, for example, a woman with dense breasts can receive a screening ultrasound without a copay or deductible if it is ordered by a physician. In Connecticut, an ultrasound copay for screening dense breasts cannot exceed $20. Generally, in other states, an ultrasound will be covered if ordered by a physician, but it is subject to the copay and deductible of an individual health plan. In New Jersey, insurance coverage is provided for additional testing if a woman has extremely dense breasts.
Regardless of state, an MRI generally will be covered by insurance (subject to copay and deductible) if the patient meets high-risk criteria. In Michigan, at least one insurance company will cover a screening MRI for normal-risk women with dense breasts at a cost that matches the copay and deductible of a screening mammogram. It is important for patients to check with their insurance carrier prior to having an MRI.
Should women with dense breasts still have mammography screening?
Yes. Mammography is the first step in screening for most women (except for those who are pregnant or breastfeeding, in which case ultrasound can be performed but is usually deferred until several months after the patient is no longer pregnant or breastfeeding). While additional screening may be recommended for women with dense breasts, and for women at high risk for developing breast cancer, there are still some cancers and precancerous changes that will show on a mammogram better than on ultrasound or MRI. Wherever possible, women with dense breasts should have digital mammography rather than film mammography, due to slightly improved cancer detection using digital mammography.18
Does tomosynthesis solve the problem of screening dense breasts?
Tomosynthesis (3D mammography) slightly improves detection of cancers compared with standard digital mammography, but some cancers will remain hidden by overlapping dense tissue. We do not yet know the benefit of annual screening tomosynthesis. Without question, women at high risk for breast cancer still should have MRI if they are able to tolerate it, even if they have had tomosynthesis.
If a patient with dense breasts undergoes screening tomosynthesis, will she also need a screening ultrasound?
Preliminary studies not yet published suggest that, for women with dense breasts, ultrasound does find another 2 to 3 invasive cancers per 1,000 women screened that are not found on tomosynthesis, but further study of this issue is needed.
If recommended for additional screening with ultrasound or MRI, will a patient need that screening every year?
Usually, yes, though age and other medical conditions will change a patient’s personal risk and benefit considerations. Therefore, screening recommendations may change from one year to the next. With technology advancements and evolving guidelines, additional screening recommendations will change in the future.
Prepare yourself and your patients will benefit
The foundation of a successful screening program involves buy-in from both patient and clinician. Patients confused as to what their density notification means may have little understanding as to what next steps should be considered. To allay confusion, your patient will be best served by being provided understandable and actionable information. Advanced preparation for these conversations about the implications of dense tissue will make for more effective patient engagement.
Acknowledgment
Much of the material within this article has been sourced from the educational Web site DenseBreast-info.org. For comprehensive lists of both patient and health care provider frequently asked questions, visit http://www.DenseBreast-info.org.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
It’s a movement that shows no signs of abating. Women in 24 states, encompassing 67% of American women, now receive some level of notification after their mammogram about breast density. Individual patient advocates continue to push for notification, and states are likely to continue to draft bills. On the national level, a federal standard is being pursued through both federal legislation and federal regulation. Clinicians practicing in states with an “inform” law, either already in effect or imminent, will be tasked with engaging in new patient conversations arising from density notification. Are you ready to answer your patients’ questions?
Navigating inconsistent data and expert comments about density and discerning which patients may benefit from additional screening can create challenges in addressing a patient’s questions about the implications of her dense tissue. If you feel less than equipped to address these issues, you are not alone. A recent survey of clinicians, con- ducted after California’s breast density notification law went into effect, showed that only 6% were comfortable answering patients’ questions relating to breast density. Seventy-five percent of respondents indicated they wanted more education on the topic.1
For women having mammography, dense breast tissue is most important because it can mask detection of cancers, and women may want to have additional screening beyond mammography. Women with dense breasts are also at increased risk for developing breast cancer. For clinicians who are on the front lines of care for women undergoing screening, the most important action items are:
- identifying who needs more screening
- weighing the risks and benefits of such additional screening.
To assist you in informing patient discussions, in this article we answer some of the most frequently asked questions of ObGyns.
Which breasts are considered dense?
The American College of Radiology recommends that density be reported in 1 of 4 categories depending on the relative amounts of fat and fibroglandular tissue2:
- almost entirely fatty—on mammography most of the tissue appears dark gray while small amounts of dense (or fibroglandular) tissue display as light gray or white.
- scattered fibroglandular density—scattered areas of dense tissue mixed with fat. Even in breasts with scattered areas of breast tissue, cancers sometimes can be missed when they resemble areas of normal tissue or are within an area of denser tissue.
- heterogeneously dense—there are large portions of the breast where dense tissue could hide masses.
- extremely dense—most of the breast appears to consist of dense tissue, creating a “white out” situation and making it extremely difficult to see through.
Breasts that are either heterogeneously dense or extremely dense are considered “dense.” About 40% of women older than age 40 have dense breasts.3
Case study: Imaging of a cancerous breast mass in a 48-year-old woman with dense breasts
This patient has heterogeneously dense breast tissue. Standard 2D mediolateral oblique (MLO) digital mammogram (A) and MLO tomosynthesis 1-mm slice (B) reveal subtle possible distortion (arrow) in the upper right breast. On tomosynthesis, the distortion is better seen, as is the underlying irregular mass (red circle).
Ultrasound (US) directed to the mass (C) shows an irregular hypoechoic (dark gray) mass (marked by calipers), compatible with cancer. US-guided core needle biopsy revealed grade 2 invasive ductal cancer with associated ductal carcinoma in situ.
Axial magnetic resonance imaging of the right breast obtained after contrast injection, and after computer subtraction of nonenhanced image (D), shows irregular spiculated enhancing (white) mass (arrow) due to the known carcinoma.
Images: Courtesy Wendie Berg, MD, PhD
Who needs more screening?
The FIGURE is a screening decision support tool representing the consensus opinion of several medical experts based on the best available scientific evidence to optimize breast cancer detection.
Do dense breasts affect the risk of developing breast cancer?
Yes. Dense breasts are a risk factor for breast cancer. According to the American Cancer Society’s Breast Cancer Facts & Figures 2013−2014, “The risk of breast cancer in-creases with increasing breast density; women with very high breast density have a 4- to 6-fold increased risk of breast cancer compared to women with the least dense breasts.”4,5
There are several reasons that dense tissue increases risk. First, the glands tend to be made up of relatively actively dividing cells that can mutate and become cancerous (the more glandular tissue present, the greater the risk). Second, the local environment around the glands may produce certain growth hormones that stimulate cells to divide, and this seems to occur more in fibrous tissue than in fatty tissue.
Most women have breast density somewhere in the middle range, with their risk for breast cancer falling in between those with extremely dense breasts and those with fatty breasts.6 The risk for developing breast cancer is influenced by a combination of many different factors, including age, family history of cancer (particularly breast or ovarian cancer), and prior atypical breast biopsies. There currently is no reliable way to fully account for the interplay of breast density, family history, prior biopsy results, and other factors in determining overall risk. Importantly, more than half of all women who develop breast cancer have no known risk factors other than being female and aging.
Is your medical support staff “density ready?”
We’re all familiar with the adage that a picture is worth a thousand words. While the medical support personnel in your office are likely quite familiar with imaging reports and the terminology used in describing dense breasts, they may be quite unfamiliar with what a fatty versus dense breast actually looks like on a mammogram, and how cancer may display in each. Illustrated examples, as seen here, are useful for reference.
In the fatty breast (A), a small cancer (arrow) is seen easily. In a breast categorized as scattered fibroglandular density (B), a large cancer is easily seen (arrow) in the relatively fatty portion of the breast, though a small cancer could have been hidden in areas with normal glandular tissue.
In a breast categorized as heterogeneously dense (C), a 4-cm (about 1.5-inch) cancer (arrows) is hidden by the dense breast tissue. This cancer also has spread to a lymph node under the arm (curved arrow).
In an extremely dense breast (D), a cancer is seen because part of it is located in the back of the breast where there is a small amount of dark fat making it easier to see (arrow and triangle marker indicating lump). If this cancer had been located near the nipple and completely surrounded by white (dense) tissue, it probably would not have been seen on mammography.
Image: Courtesy of Dr. Regina Hooley and DenseBreast-info.org
Are screening mammography outcomes different for women with dense versus fatty breasts?
Yes. Cancer is more likely to be clinically detected in the interval between mammography screens (defined as interval cancer) in women with dense breasts. Such interval cancers tend to be more aggressive and have worse outcomes. Compared with those in fatty breasts, cancers found in dense breasts more often7:
- are locally advanced (stage IIb and III)
- are multifocal or multicentric
- require a mastectomy (rather than a lumpectomy).
Does supplemental screening beyond mammography save lives?
Mammography is the only imaging screening modality that has been studied by multiple randomized controlled trials with mortality as an endpoint. Across those trials, mammography has been shown to reduce deaths due to breast cancer. The randomized trials that show a benefit from mammography are those in which mammography increased detection of invasive breast cancers before they spread to lymph nodes.8
No randomized controlled trial has yet been reported on any other imaging screening modality, but it is expected that other screening tests that increase detection of node-negative invasive breast cancers beyond mammography should further reduce breast cancer mortality.
Proving the mortality benefit of any supplemental screening modality would require a very large, very expensive randomized controlled trial with 15 to 20 years of follow-up. Given the speed of technologic developments, any results likely would be obsolete by the trial’s conclusion. What we do know is that women at high risk for breast cancer who undergo annual magnetic resonance imaging (MRI) screening are less likely to have advanced breast cancer than their counterparts who were not screened with MRI.9
We also know that average-risk women who are screened with ultrasonography in addition to mammography are unlikely to have palpable cancer in the interval between screens,10,11 with the rates of such interval cancers similar to women with fatty breasts screened only with mammography. The cancers found only on MRI or ultrasound are mostly small invasive cancers (average size, approximately 1 cm) that are mostly node negative.12,13 MRI also finds some ductal carcinoma in situ (DCIS).
These results suggest that there is a benefit to finding additional cancers with supplemental screening, though it is certainly possible that, as with mammography, some of the cancers found with supplemental screening are slow growing and may never have caused a woman harm even if left untreated.
Dense breasts: Medically sourced resources
Educational Web site
DenseBreast-info.org. This site is a collaborative, multidisciplinary educational resource. It features content for both patients and health care providers with separate data streams for each and includes:
a comprehensive list of FAQs; screening flow charts; a Patient Risk Checklist; an explanation of risks, risk assessment, and links to risk assessment tools; an illustrated round-up of technologies commonly used in screening; and state-by-state legislative analysis of density inform laws across the country.
State-specific Web sites
BreastDensity.info. This site was created by the California Breast Density Information Group (CBDIG), a working group of breast radiologists and breast cancer risk specialists. The content is primarily for health care providers and features screening scenarios as well as FAQs about breast density, breast cancer risk, and the breast density notification law in California.
MIdensebreasts.org. This is a Web-based education resource created for primary care providers by the University of Michigan Health System and the Michigan Department of Health and Human Services. It includes continuing medical education credit.
Medical society materials
American Cancer Society offers Breast Density and Your Mammogram Report for patients: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-039989.pdf
American College of Obstetricians and Gynecologists’ 2015 Density Policy statement is available online: http://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Gynecologic-Practice/Management-of-Women-With-Dense-Breasts-Diagnosed-by-Mammography
American College of Radiology patient brochure details basic information about breast density and can be customized with your center’s information: http://www.acr.org/News-Publications/~/media/180321AF51AF4EA38FEC091461F5B695.pdf
What additional screening tests are available after a 2D mammogram for a woman with dense breasts?
Depending on the patient’s age, risk level, and breast density, additional screening tools—such as tomosynthesis (also known as 3D mammography), ultrasonography, or MRI—may be recommended in addition to mammography. Indeed, in some centers, tomosynthesis is performed alone and the radiologist also reviews computer-generated 2D mammograms.
The addition of another imaging tool after a mammogram will find more cancers than mammography alone (TABLE).14−17 Women at high risk for breast cancer, such as those with pathogenic BRCA mutations, and those who were treated with radiation therapy to their chest (typically for Hodgkin disease) before age 30 and at least 8 years earlier, should be referred for annual MRI in addition to mammography (see Screening Decision Support Tool FIGURE above). If tomosynthesis is performed, the added benefit of ultrasound will be lower; further study on the actual benefit of supplemental ultrasound screening after 3D mammography is needed.
Will insurance cover supplemental screening beyond mammography?
The answer depends on the type of screening, the patient’s insurance and risk factors, the state in which you practice, and whether or not a law is in effect requiring insurance coverage for additional screening. In Illinois, for example, a woman with dense breasts can receive a screening ultrasound without a copay or deductible if it is ordered by a physician. In Connecticut, an ultrasound copay for screening dense breasts cannot exceed $20. Generally, in other states, an ultrasound will be covered if ordered by a physician, but it is subject to the copay and deductible of an individual health plan. In New Jersey, insurance coverage is provided for additional testing if a woman has extremely dense breasts.
Regardless of state, an MRI generally will be covered by insurance (subject to copay and deductible) if the patient meets high-risk criteria. In Michigan, at least one insurance company will cover a screening MRI for normal-risk women with dense breasts at a cost that matches the copay and deductible of a screening mammogram. It is important for patients to check with their insurance carrier prior to having an MRI.
Should women with dense breasts still have mammography screening?
Yes. Mammography is the first step in screening for most women (except for those who are pregnant or breastfeeding, in which case ultrasound can be performed but is usually deferred until several months after the patient is no longer pregnant or breastfeeding). While additional screening may be recommended for women with dense breasts, and for women at high risk for developing breast cancer, there are still some cancers and precancerous changes that will show on a mammogram better than on ultrasound or MRI. Wherever possible, women with dense breasts should have digital mammography rather than film mammography, due to slightly improved cancer detection using digital mammography.18
Does tomosynthesis solve the problem of screening dense breasts?
Tomosynthesis (3D mammography) slightly improves detection of cancers compared with standard digital mammography, but some cancers will remain hidden by overlapping dense tissue. We do not yet know the benefit of annual screening tomosynthesis. Without question, women at high risk for breast cancer still should have MRI if they are able to tolerate it, even if they have had tomosynthesis.
If a patient with dense breasts undergoes screening tomosynthesis, will she also need a screening ultrasound?
Preliminary studies not yet published suggest that, for women with dense breasts, ultrasound does find another 2 to 3 invasive cancers per 1,000 women screened that are not found on tomosynthesis, but further study of this issue is needed.
If recommended for additional screening with ultrasound or MRI, will a patient need that screening every year?
Usually, yes, though age and other medical conditions will change a patient’s personal risk and benefit considerations. Therefore, screening recommendations may change from one year to the next. With technology advancements and evolving guidelines, additional screening recommendations will change in the future.
Prepare yourself and your patients will benefit
The foundation of a successful screening program involves buy-in from both patient and clinician. Patients confused as to what their density notification means may have little understanding as to what next steps should be considered. To allay confusion, your patient will be best served by being provided understandable and actionable information. Advanced preparation for these conversations about the implications of dense tissue will make for more effective patient engagement.
Acknowledgment
Much of the material within this article has been sourced from the educational Web site DenseBreast-info.org. For comprehensive lists of both patient and health care provider frequently asked questions, visit http://www.DenseBreast-info.org.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Khong KA, Hargreaves J, Aminololama-Shakeri S, Lindfors KK. Impact of the California breast density law on primary care physicians. J Am Coll Radiol. 2015;12(3):256–260.
- Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS Mammography. In: ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013.
- Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106(10).
- American Cancer Society. Breast Cancer Facts & Figures 2013–2014. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-042725.pdf. Published 2013. Accessed September 15, 2015.
- Harvey JA, Bovbjerg VE. Quantitative assessment of mammographic breast density: relationship with breast cancer risk. Radiology. 2004;230(1):29–41.
- Kerlikowske K, Cook AJ, Buist DS, et al. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol. 2010;28(24):3830–3837.
- Arora N, King TA, Jacks LM, et al. Impact of breast density on the presenting features of malignancy. Ann Surg Oncol. 2010;17(suppl 3):211–218.
- Smith RA, Duffy SW, Gabe R, Tabar L, Yen AM, Chen TH. The randomized trials of breast cancer screening: what have we learned? Radiol Clin North Am. 2004;42(5):793–806, v.
- Warner E, Hill K, Causer P, et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol. 2011;29(13):1664–1669.
- Corsetti V, Houssami N, Ghirardi M, et al. Evidence of the effect of adjunct ultrasound screening in women with mammography-negative dense breasts: interval breast cancers at 1 year follow-up. Eur J Cancer. 2011;47(7): 1021–1026.
- Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307(13):1394–1404.
- Berg WA. Tailored supplemental screening for breast cancer: what now and what next? AJR Am J Roentgenol. 2009;192(2):390–399.
- Brem RF, Lenihan MJ, Lieberman J, Torrente J. Screening breast ultrasound: past, present, and future. AJR Am J Roentgenol. 2015;204(2):234–240.
- Hooley R. Tomosynthesis. In: Berg WA, Yang WT, eds. Diagnostic Imaging: Breast. 2nd ed. Salt Lake City, UT: Amirsys; 2014:2–19.
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499–2507.
- Berg WA. Screening Ultrasound. In: Berg WA, Yang WT, eds. Diagnostic Imaging: Breast. 2nd ed. Salt Lake City, UT: Amirsys; 2014:9–43.
- Berg WA. Screening MRI. In: Berg WA, Yang WT, eds. Diagnostic Imaging: Breast. 2nd ed. Salt Lake City, UT: Amirsys; 2014:9–49.
- Hooley R. Tomosynthesis. In: Berg WA, Yang WT, eds.Berg WA. Screening Ultrasound. In: Berg WA, Yang WT, eds.Berg WA. Screening MRI. In: Berg WA, Yang WT, eds.Pisano ED, Gatsonis C, Hendrick E, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005;353(17):1773–1783.
- Khong KA, Hargreaves J, Aminololama-Shakeri S, Lindfors KK. Impact of the California breast density law on primary care physicians. J Am Coll Radiol. 2015;12(3):256–260.
- Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS Mammography. In: ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013.
- Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106(10).
- American Cancer Society. Breast Cancer Facts & Figures 2013–2014. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-042725.pdf. Published 2013. Accessed September 15, 2015.
- Harvey JA, Bovbjerg VE. Quantitative assessment of mammographic breast density: relationship with breast cancer risk. Radiology. 2004;230(1):29–41.
- Kerlikowske K, Cook AJ, Buist DS, et al. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol. 2010;28(24):3830–3837.
- Arora N, King TA, Jacks LM, et al. Impact of breast density on the presenting features of malignancy. Ann Surg Oncol. 2010;17(suppl 3):211–218.
- Smith RA, Duffy SW, Gabe R, Tabar L, Yen AM, Chen TH. The randomized trials of breast cancer screening: what have we learned? Radiol Clin North Am. 2004;42(5):793–806, v.
- Warner E, Hill K, Causer P, et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol. 2011;29(13):1664–1669.
- Corsetti V, Houssami N, Ghirardi M, et al. Evidence of the effect of adjunct ultrasound screening in women with mammography-negative dense breasts: interval breast cancers at 1 year follow-up. Eur J Cancer. 2011;47(7): 1021–1026.
- Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307(13):1394–1404.
- Berg WA. Tailored supplemental screening for breast cancer: what now and what next? AJR Am J Roentgenol. 2009;192(2):390–399.
- Brem RF, Lenihan MJ, Lieberman J, Torrente J. Screening breast ultrasound: past, present, and future. AJR Am J Roentgenol. 2015;204(2):234–240.
- Hooley R. Tomosynthesis. In: Berg WA, Yang WT, eds. Diagnostic Imaging: Breast. 2nd ed. Salt Lake City, UT: Amirsys; 2014:2–19.
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499–2507.
- Berg WA. Screening Ultrasound. In: Berg WA, Yang WT, eds. Diagnostic Imaging: Breast. 2nd ed. Salt Lake City, UT: Amirsys; 2014:9–43.
- Berg WA. Screening MRI. In: Berg WA, Yang WT, eds. Diagnostic Imaging: Breast. 2nd ed. Salt Lake City, UT: Amirsys; 2014:9–49.
- Hooley R. Tomosynthesis. In: Berg WA, Yang WT, eds.Berg WA. Screening Ultrasound. In: Berg WA, Yang WT, eds.Berg WA. Screening MRI. In: Berg WA, Yang WT, eds.Pisano ED, Gatsonis C, Hendrick E, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005;353(17):1773–1783.
In this Article
- Breast mass imaging case study
- Screening decision support tool
- Is your support staff “density” ready?
2015 Update on cancer
As the proportion of the elderly in the US population continues to increase, with life expectancy trending upward, we can expect to see more gynecologic cancers in our patients.1,2 At present, the most effective approach to these cancers commonly includes aggressive surgical resection with chemotherapy and, in some cases, radiation. It remains unclear whether elderly patients should be managed the same as younger patients, with minimal data to guide physicians. Some evidence suggests an increased risk of surgical complications in older adults.3
To optimize surgical care in our elderly patients, we need to understand the risks of perioperative mortality and morbidity in this population. For example, the current standard of care for advanced epithelial ovarian cancer is aggressive cytoreductive surgery followed by adjuvant chemotherapy,4 although neoadjuvant chemotherapy is gaining utility and popularity in certain circumstances. During pretreatment counseling, it is imperative that we communicate patient-specific outcomes so that patients and their families can make educated decisions in line with their goals. What should we know about age-dependent outcomes when counseling our patients?
To optimize surgical care in this population, we also need to develop and use new methods of surgical decision making. Although some data suggest that age is an independent risk factor for postoperative complications, not all elderly patients are the same in terms of comorbidities and functional status. In order to truly assess risks, we need to identify additional preoperative risk factors. Are there accurate scoring tools or predictors of outcomes available to help us assess the risks of postoperative mortality and morbidity?
In this article, we highlight recent developments in surgical treatment of the elderly, focusing on:
- postoperative mortality and morbidity in patients older than 80 years
- adjuncts to preoperative assessment for oncogeriatric surgical patients.
Risks rise sharply in older patients undergoing treatment for ovarian Ca
Moore KN, Reid MS, Fong DN, et al. Ovarian cancer in the octogenarian: does the paradigm of aggressive cytoreductive surgery and chemotherapy still apply? Gynecol Oncol. 2008;110(2):133–139.
Mahdi H, Wiechert A, Lockhart D, Rose PG. Impact of age on 30-day mortality and morbidity in patients undergoing surgery for ovarian cancer. Int J Gynecol Cancer. 2015;25(7):1216–1223.
The cornerstone of optimal survival from certain gynecologic cancers, such as advanced ovarian cancer, is aggressive debulking surgery. However, older adults are classically under-represented in clinical trials that guide this standard of care.
To determine whether patients aged 80 years or older respond differently from younger patients to conventional ovarian cancer management, Moore and colleagues retrospectively reviewed their institutional experience. They found that postoperative mortality increased from 5.4% in patients aged 80 to 84 years to 9.1% in those aged 85 to 89 and 14.4% in those older than 90. The rates for younger patients were 0.6% for patients younger than 60 years, 2.8% for those aged 60 to 69 years, and 2.5% for those aged 70 to 79 years (P<.001).
Notably, 13% of patients aged 80 years or older who underwent primary surgery died during their primary hospitalization. Of those who survived, 50% were discharged to skilled nursing facilities. Of patients who underwent cytoreductive surgery, 13% were unable to undergo any intended adjuvant therapy, and only 57% completed more than 3 cycles of chemotherapy, either due to demise or toxicities. Two-month survival for patients 80 years or older was comparable between patients who underwent primary surgery and those who had primary chemotherapy (20% and 26%, respectively).
With a similar objective, Mahdi and colleagues identified 2,087 patients with ovarian cancer who underwent surgery. After adjusting for confounders with multivariable analyses, they found that octogenarians whose initial management was surgery were 9 times more likely than younger patients to die and 70% more likely to develop complications within 30 days. Among patients who underwent neoadjuvant chemotherapy, there were no significant differences between older and younger patients in 30-day postoperative mortality or morbidity.
When evaluating elderly patients for surgery, the use of multiple risk-assessment strategies may improve accuracy
Huisman MG, Audisio RA, Ugolini G, et al. Screening for predictors of adverse outcome in onco-geriatric surgical patients: a multicenter prospective cohort study. Eur J Surg Oncol. 2015;41(7):844–851.
Uppal S, Igwe E, Rice L, Spencer R, Rose SL. Frailty index predicts severe complications in gynecologic oncology patients. Gynecol Oncol. 2015;137(1):98–101.
The National Comprehensive Cancer Network recommends that clinicians determine baseline life expectancy for older adults with cancer to aid in management decision making. The use of tools such as www.eprognosis.com, developed to determine anticipated life expectancy independent of cancer, can prove useful in determining a patient’s risk of dying or suffering from their cancer before dying of another cause.5
When it comes to the determination of risk related to a patient’s cancer diagnosis and selection of potential management options, many argue that the subgroup of elderly patients is not homogenous and that the use of age alone to guide management decisions may be unfair. Preoperative evaluation ideally should incorporate a global assessment of predictive risk factors.
Three assessment tools are especially useful
Huisman and colleagues set out to identify accurate preoperative assessment methods in elderly patients undergoing oncologic surgery. They prospectively recruited 328 patients aged 70 years or older and evaluated patients preoperatively using 11 well-known geriatric screening tools. They compared these evaluations with outcomes to determine which tools best predict the occurrence of major postoperative complications. They found the strongest correlation with outcomes when combining gender and type of surgery with the following 3 assessment tools:
- Timed Up and Go (TUG)—a walking test to measure functional status
- American Society of Anesthesiologists scale—a scoring system that quantifies preoperative physical status and estimates anesthetic risk
- Nutritional Risk Screening—an assessment of nutritional risk based on recent weight loss, overall condition, and reduction of food intake.
All 3 are simple and short screening tools. When used together, they can provide clinicians with accurate risk estimations.
The findings of Huisman and colleagues reinforce the importance of a global assessment of the patient’s comorbidities, functional status, and nutritional status when determining candidacy for oncologic surgery.
Functional index predicts need for postoperative ICU care and risk of death
Uppal and colleagues set out to quantify the predictive value of the modified Functional Index (mFI) in assessing the need for postoperative critical care support and/or the risk of death within 30 days after gynecologic cancer surgery. The mFI can be calculated by adding 1 point for each variable listed in the TABLE, with a score of 4 or higher representing a high-frailty cohort.
Of 6,551 patients who underwent gynecologic surgery, 188 were admitted to the intensive care unit (ICU) or died within 30 days after surgery. The mFI was calculated, with multivariate analyses of additional variables. An mFI score of 3 or higher was predictive of the need for critical care support and the risk of 30-day mortality and was associated with a significantly higher number of complications (P<.001).
Predictors significant for postoperative critical care support or death were:
- preoperative albumin level less than 3 g/dL (odds ratio [OR] = 6.5)
- operative time (OR = 1.003 per minute of increase)
- nonlaparoscopic surgery (OR = 3.3)
- mFI score, with a score of 0 serving as the reference (OR for a score of 1 = 1.26; score of 2 = 1.9; score of 3 = 2.33; and score of 4 or higher = 12.5).
When they combined the mFI and albumin scores—both readily available in the preoperative setting—Uppal and colleagues were able to develop an algorithm to determine patients who were at “low risk” versus “high risk” for ICU admission and/or death postoperatively (FIGURE).
[[{"fid":"79834","view_mode":"medstat_image_full_text","fields":{"format":"medstat_image_full_text","field_file_image_alt_text[und][0][value]":"","field_file_image_title_text[und][0][value]":"","field_file_image_caption[und][0][value]":"Postoperative risk stratification based on preoperative albumin level
and modified Functional Index","field_file_image_credit[und][0][value]":"6"},"type":"media","attributes":{"height":"316","width":"665","class":"media-element file-medstat-image-full-text"}}]]
Bottom line
Older patients are more commonly affected by multiple medical comorbidities, as well as functional, cognitive, and nutritional deficiencies, which contribute to their increased risk of morbidity and mortality after surgery. The elderly experience greater morbidity with noncardiac surgery in general.
Clearly, the decision to operate on an elderly patient should be approached with caution, and a critical assessment of the patient’s risk factors should be performed to inform counseling about the patient’s management options. Future randomized prospective data will help us better understand the relationship between age and surgical outcomes.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- US Census Bureau. Population Projections: Projections of the Population by Sex and Selected Age Groups for the United States: 2015 to 2060. https://www.census.gov/population/projections/data/national/2014/summarytables.html. Published December 2014. Accessed August 31, 2015.
- US Census Bureau. Population Projections: Percent Distribution of the Projected Population by Sex and Selected Age Groups for the United States: 2015 to 2060. https://www.census.gov/population/projections/data/national/2014/summarytables.html. Published December 2014. Accessed August 31, 2015.
- Polanczyk CA, Marcantonio E, Goldman L, et al. Impact of age on perioperative complications and length of stay in patients undergoing noncardiac surgery. Ann Intern Med. 2001;134(8):637–643.
- Aletti G, Dowdy SC, Gostout BS, et al. Aggressive surgical effort and improved survival in advanced stage ovarian cancer. Obstet Gynecol. 2006;107(1):77–85.
- National Comprehensive Cancer Network. NCCN Guidelines for Age-Related Recommendations: Older Adult Oncology. . Published 2015. Accessed August 31, 2015.
- Uppal S, Igwe E, Rice L, Spencer R, Rose SL. Frailty index predicts severe complications in gynecologic oncology patients. Gynecol Oncol. 2015;137(1):98–101.
As the proportion of the elderly in the US population continues to increase, with life expectancy trending upward, we can expect to see more gynecologic cancers in our patients.1,2 At present, the most effective approach to these cancers commonly includes aggressive surgical resection with chemotherapy and, in some cases, radiation. It remains unclear whether elderly patients should be managed the same as younger patients, with minimal data to guide physicians. Some evidence suggests an increased risk of surgical complications in older adults.3
To optimize surgical care in our elderly patients, we need to understand the risks of perioperative mortality and morbidity in this population. For example, the current standard of care for advanced epithelial ovarian cancer is aggressive cytoreductive surgery followed by adjuvant chemotherapy,4 although neoadjuvant chemotherapy is gaining utility and popularity in certain circumstances. During pretreatment counseling, it is imperative that we communicate patient-specific outcomes so that patients and their families can make educated decisions in line with their goals. What should we know about age-dependent outcomes when counseling our patients?
To optimize surgical care in this population, we also need to develop and use new methods of surgical decision making. Although some data suggest that age is an independent risk factor for postoperative complications, not all elderly patients are the same in terms of comorbidities and functional status. In order to truly assess risks, we need to identify additional preoperative risk factors. Are there accurate scoring tools or predictors of outcomes available to help us assess the risks of postoperative mortality and morbidity?
In this article, we highlight recent developments in surgical treatment of the elderly, focusing on:
- postoperative mortality and morbidity in patients older than 80 years
- adjuncts to preoperative assessment for oncogeriatric surgical patients.
Risks rise sharply in older patients undergoing treatment for ovarian Ca
Moore KN, Reid MS, Fong DN, et al. Ovarian cancer in the octogenarian: does the paradigm of aggressive cytoreductive surgery and chemotherapy still apply? Gynecol Oncol. 2008;110(2):133–139.
Mahdi H, Wiechert A, Lockhart D, Rose PG. Impact of age on 30-day mortality and morbidity in patients undergoing surgery for ovarian cancer. Int J Gynecol Cancer. 2015;25(7):1216–1223.
The cornerstone of optimal survival from certain gynecologic cancers, such as advanced ovarian cancer, is aggressive debulking surgery. However, older adults are classically under-represented in clinical trials that guide this standard of care.
To determine whether patients aged 80 years or older respond differently from younger patients to conventional ovarian cancer management, Moore and colleagues retrospectively reviewed their institutional experience. They found that postoperative mortality increased from 5.4% in patients aged 80 to 84 years to 9.1% in those aged 85 to 89 and 14.4% in those older than 90. The rates for younger patients were 0.6% for patients younger than 60 years, 2.8% for those aged 60 to 69 years, and 2.5% for those aged 70 to 79 years (P<.001).
Notably, 13% of patients aged 80 years or older who underwent primary surgery died during their primary hospitalization. Of those who survived, 50% were discharged to skilled nursing facilities. Of patients who underwent cytoreductive surgery, 13% were unable to undergo any intended adjuvant therapy, and only 57% completed more than 3 cycles of chemotherapy, either due to demise or toxicities. Two-month survival for patients 80 years or older was comparable between patients who underwent primary surgery and those who had primary chemotherapy (20% and 26%, respectively).
With a similar objective, Mahdi and colleagues identified 2,087 patients with ovarian cancer who underwent surgery. After adjusting for confounders with multivariable analyses, they found that octogenarians whose initial management was surgery were 9 times more likely than younger patients to die and 70% more likely to develop complications within 30 days. Among patients who underwent neoadjuvant chemotherapy, there were no significant differences between older and younger patients in 30-day postoperative mortality or morbidity.
When evaluating elderly patients for surgery, the use of multiple risk-assessment strategies may improve accuracy
Huisman MG, Audisio RA, Ugolini G, et al. Screening for predictors of adverse outcome in onco-geriatric surgical patients: a multicenter prospective cohort study. Eur J Surg Oncol. 2015;41(7):844–851.
Uppal S, Igwe E, Rice L, Spencer R, Rose SL. Frailty index predicts severe complications in gynecologic oncology patients. Gynecol Oncol. 2015;137(1):98–101.
The National Comprehensive Cancer Network recommends that clinicians determine baseline life expectancy for older adults with cancer to aid in management decision making. The use of tools such as www.eprognosis.com, developed to determine anticipated life expectancy independent of cancer, can prove useful in determining a patient’s risk of dying or suffering from their cancer before dying of another cause.5
When it comes to the determination of risk related to a patient’s cancer diagnosis and selection of potential management options, many argue that the subgroup of elderly patients is not homogenous and that the use of age alone to guide management decisions may be unfair. Preoperative evaluation ideally should incorporate a global assessment of predictive risk factors.
Three assessment tools are especially useful
Huisman and colleagues set out to identify accurate preoperative assessment methods in elderly patients undergoing oncologic surgery. They prospectively recruited 328 patients aged 70 years or older and evaluated patients preoperatively using 11 well-known geriatric screening tools. They compared these evaluations with outcomes to determine which tools best predict the occurrence of major postoperative complications. They found the strongest correlation with outcomes when combining gender and type of surgery with the following 3 assessment tools:
- Timed Up and Go (TUG)—a walking test to measure functional status
- American Society of Anesthesiologists scale—a scoring system that quantifies preoperative physical status and estimates anesthetic risk
- Nutritional Risk Screening—an assessment of nutritional risk based on recent weight loss, overall condition, and reduction of food intake.
All 3 are simple and short screening tools. When used together, they can provide clinicians with accurate risk estimations.
The findings of Huisman and colleagues reinforce the importance of a global assessment of the patient’s comorbidities, functional status, and nutritional status when determining candidacy for oncologic surgery.
Functional index predicts need for postoperative ICU care and risk of death
Uppal and colleagues set out to quantify the predictive value of the modified Functional Index (mFI) in assessing the need for postoperative critical care support and/or the risk of death within 30 days after gynecologic cancer surgery. The mFI can be calculated by adding 1 point for each variable listed in the TABLE, with a score of 4 or higher representing a high-frailty cohort.
Of 6,551 patients who underwent gynecologic surgery, 188 were admitted to the intensive care unit (ICU) or died within 30 days after surgery. The mFI was calculated, with multivariate analyses of additional variables. An mFI score of 3 or higher was predictive of the need for critical care support and the risk of 30-day mortality and was associated with a significantly higher number of complications (P<.001).
Predictors significant for postoperative critical care support or death were:
- preoperative albumin level less than 3 g/dL (odds ratio [OR] = 6.5)
- operative time (OR = 1.003 per minute of increase)
- nonlaparoscopic surgery (OR = 3.3)
- mFI score, with a score of 0 serving as the reference (OR for a score of 1 = 1.26; score of 2 = 1.9; score of 3 = 2.33; and score of 4 or higher = 12.5).
When they combined the mFI and albumin scores—both readily available in the preoperative setting—Uppal and colleagues were able to develop an algorithm to determine patients who were at “low risk” versus “high risk” for ICU admission and/or death postoperatively (FIGURE).
[[{"fid":"79834","view_mode":"medstat_image_full_text","fields":{"format":"medstat_image_full_text","field_file_image_alt_text[und][0][value]":"","field_file_image_title_text[und][0][value]":"","field_file_image_caption[und][0][value]":"Postoperative risk stratification based on preoperative albumin level
and modified Functional Index","field_file_image_credit[und][0][value]":"6"},"type":"media","attributes":{"height":"316","width":"665","class":"media-element file-medstat-image-full-text"}}]]
Bottom line
Older patients are more commonly affected by multiple medical comorbidities, as well as functional, cognitive, and nutritional deficiencies, which contribute to their increased risk of morbidity and mortality after surgery. The elderly experience greater morbidity with noncardiac surgery in general.
Clearly, the decision to operate on an elderly patient should be approached with caution, and a critical assessment of the patient’s risk factors should be performed to inform counseling about the patient’s management options. Future randomized prospective data will help us better understand the relationship between age and surgical outcomes.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
As the proportion of the elderly in the US population continues to increase, with life expectancy trending upward, we can expect to see more gynecologic cancers in our patients.1,2 At present, the most effective approach to these cancers commonly includes aggressive surgical resection with chemotherapy and, in some cases, radiation. It remains unclear whether elderly patients should be managed the same as younger patients, with minimal data to guide physicians. Some evidence suggests an increased risk of surgical complications in older adults.3
To optimize surgical care in our elderly patients, we need to understand the risks of perioperative mortality and morbidity in this population. For example, the current standard of care for advanced epithelial ovarian cancer is aggressive cytoreductive surgery followed by adjuvant chemotherapy,4 although neoadjuvant chemotherapy is gaining utility and popularity in certain circumstances. During pretreatment counseling, it is imperative that we communicate patient-specific outcomes so that patients and their families can make educated decisions in line with their goals. What should we know about age-dependent outcomes when counseling our patients?
To optimize surgical care in this population, we also need to develop and use new methods of surgical decision making. Although some data suggest that age is an independent risk factor for postoperative complications, not all elderly patients are the same in terms of comorbidities and functional status. In order to truly assess risks, we need to identify additional preoperative risk factors. Are there accurate scoring tools or predictors of outcomes available to help us assess the risks of postoperative mortality and morbidity?
In this article, we highlight recent developments in surgical treatment of the elderly, focusing on:
- postoperative mortality and morbidity in patients older than 80 years
- adjuncts to preoperative assessment for oncogeriatric surgical patients.
Risks rise sharply in older patients undergoing treatment for ovarian Ca
Moore KN, Reid MS, Fong DN, et al. Ovarian cancer in the octogenarian: does the paradigm of aggressive cytoreductive surgery and chemotherapy still apply? Gynecol Oncol. 2008;110(2):133–139.
Mahdi H, Wiechert A, Lockhart D, Rose PG. Impact of age on 30-day mortality and morbidity in patients undergoing surgery for ovarian cancer. Int J Gynecol Cancer. 2015;25(7):1216–1223.
The cornerstone of optimal survival from certain gynecologic cancers, such as advanced ovarian cancer, is aggressive debulking surgery. However, older adults are classically under-represented in clinical trials that guide this standard of care.
To determine whether patients aged 80 years or older respond differently from younger patients to conventional ovarian cancer management, Moore and colleagues retrospectively reviewed their institutional experience. They found that postoperative mortality increased from 5.4% in patients aged 80 to 84 years to 9.1% in those aged 85 to 89 and 14.4% in those older than 90. The rates for younger patients were 0.6% for patients younger than 60 years, 2.8% for those aged 60 to 69 years, and 2.5% for those aged 70 to 79 years (P<.001).
Notably, 13% of patients aged 80 years or older who underwent primary surgery died during their primary hospitalization. Of those who survived, 50% were discharged to skilled nursing facilities. Of patients who underwent cytoreductive surgery, 13% were unable to undergo any intended adjuvant therapy, and only 57% completed more than 3 cycles of chemotherapy, either due to demise or toxicities. Two-month survival for patients 80 years or older was comparable between patients who underwent primary surgery and those who had primary chemotherapy (20% and 26%, respectively).
With a similar objective, Mahdi and colleagues identified 2,087 patients with ovarian cancer who underwent surgery. After adjusting for confounders with multivariable analyses, they found that octogenarians whose initial management was surgery were 9 times more likely than younger patients to die and 70% more likely to develop complications within 30 days. Among patients who underwent neoadjuvant chemotherapy, there were no significant differences between older and younger patients in 30-day postoperative mortality or morbidity.
When evaluating elderly patients for surgery, the use of multiple risk-assessment strategies may improve accuracy
Huisman MG, Audisio RA, Ugolini G, et al. Screening for predictors of adverse outcome in onco-geriatric surgical patients: a multicenter prospective cohort study. Eur J Surg Oncol. 2015;41(7):844–851.
Uppal S, Igwe E, Rice L, Spencer R, Rose SL. Frailty index predicts severe complications in gynecologic oncology patients. Gynecol Oncol. 2015;137(1):98–101.
The National Comprehensive Cancer Network recommends that clinicians determine baseline life expectancy for older adults with cancer to aid in management decision making. The use of tools such as www.eprognosis.com, developed to determine anticipated life expectancy independent of cancer, can prove useful in determining a patient’s risk of dying or suffering from their cancer before dying of another cause.5
When it comes to the determination of risk related to a patient’s cancer diagnosis and selection of potential management options, many argue that the subgroup of elderly patients is not homogenous and that the use of age alone to guide management decisions may be unfair. Preoperative evaluation ideally should incorporate a global assessment of predictive risk factors.
Three assessment tools are especially useful
Huisman and colleagues set out to identify accurate preoperative assessment methods in elderly patients undergoing oncologic surgery. They prospectively recruited 328 patients aged 70 years or older and evaluated patients preoperatively using 11 well-known geriatric screening tools. They compared these evaluations with outcomes to determine which tools best predict the occurrence of major postoperative complications. They found the strongest correlation with outcomes when combining gender and type of surgery with the following 3 assessment tools:
- Timed Up and Go (TUG)—a walking test to measure functional status
- American Society of Anesthesiologists scale—a scoring system that quantifies preoperative physical status and estimates anesthetic risk
- Nutritional Risk Screening—an assessment of nutritional risk based on recent weight loss, overall condition, and reduction of food intake.
All 3 are simple and short screening tools. When used together, they can provide clinicians with accurate risk estimations.
The findings of Huisman and colleagues reinforce the importance of a global assessment of the patient’s comorbidities, functional status, and nutritional status when determining candidacy for oncologic surgery.
Functional index predicts need for postoperative ICU care and risk of death
Uppal and colleagues set out to quantify the predictive value of the modified Functional Index (mFI) in assessing the need for postoperative critical care support and/or the risk of death within 30 days after gynecologic cancer surgery. The mFI can be calculated by adding 1 point for each variable listed in the TABLE, with a score of 4 or higher representing a high-frailty cohort.
Of 6,551 patients who underwent gynecologic surgery, 188 were admitted to the intensive care unit (ICU) or died within 30 days after surgery. The mFI was calculated, with multivariate analyses of additional variables. An mFI score of 3 or higher was predictive of the need for critical care support and the risk of 30-day mortality and was associated with a significantly higher number of complications (P<.001).
Predictors significant for postoperative critical care support or death were:
- preoperative albumin level less than 3 g/dL (odds ratio [OR] = 6.5)
- operative time (OR = 1.003 per minute of increase)
- nonlaparoscopic surgery (OR = 3.3)
- mFI score, with a score of 0 serving as the reference (OR for a score of 1 = 1.26; score of 2 = 1.9; score of 3 = 2.33; and score of 4 or higher = 12.5).
When they combined the mFI and albumin scores—both readily available in the preoperative setting—Uppal and colleagues were able to develop an algorithm to determine patients who were at “low risk” versus “high risk” for ICU admission and/or death postoperatively (FIGURE).
[[{"fid":"79834","view_mode":"medstat_image_full_text","fields":{"format":"medstat_image_full_text","field_file_image_alt_text[und][0][value]":"","field_file_image_title_text[und][0][value]":"","field_file_image_caption[und][0][value]":"Postoperative risk stratification based on preoperative albumin level
and modified Functional Index","field_file_image_credit[und][0][value]":"6"},"type":"media","attributes":{"height":"316","width":"665","class":"media-element file-medstat-image-full-text"}}]]
Bottom line
Older patients are more commonly affected by multiple medical comorbidities, as well as functional, cognitive, and nutritional deficiencies, which contribute to their increased risk of morbidity and mortality after surgery. The elderly experience greater morbidity with noncardiac surgery in general.
Clearly, the decision to operate on an elderly patient should be approached with caution, and a critical assessment of the patient’s risk factors should be performed to inform counseling about the patient’s management options. Future randomized prospective data will help us better understand the relationship between age and surgical outcomes.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- US Census Bureau. Population Projections: Projections of the Population by Sex and Selected Age Groups for the United States: 2015 to 2060. https://www.census.gov/population/projections/data/national/2014/summarytables.html. Published December 2014. Accessed August 31, 2015.
- US Census Bureau. Population Projections: Percent Distribution of the Projected Population by Sex and Selected Age Groups for the United States: 2015 to 2060. https://www.census.gov/population/projections/data/national/2014/summarytables.html. Published December 2014. Accessed August 31, 2015.
- Polanczyk CA, Marcantonio E, Goldman L, et al. Impact of age on perioperative complications and length of stay in patients undergoing noncardiac surgery. Ann Intern Med. 2001;134(8):637–643.
- Aletti G, Dowdy SC, Gostout BS, et al. Aggressive surgical effort and improved survival in advanced stage ovarian cancer. Obstet Gynecol. 2006;107(1):77–85.
- National Comprehensive Cancer Network. NCCN Guidelines for Age-Related Recommendations: Older Adult Oncology. . Published 2015. Accessed August 31, 2015.
- Uppal S, Igwe E, Rice L, Spencer R, Rose SL. Frailty index predicts severe complications in gynecologic oncology patients. Gynecol Oncol. 2015;137(1):98–101.
- US Census Bureau. Population Projections: Projections of the Population by Sex and Selected Age Groups for the United States: 2015 to 2060. https://www.census.gov/population/projections/data/national/2014/summarytables.html. Published December 2014. Accessed August 31, 2015.
- US Census Bureau. Population Projections: Percent Distribution of the Projected Population by Sex and Selected Age Groups for the United States: 2015 to 2060. https://www.census.gov/population/projections/data/national/2014/summarytables.html. Published December 2014. Accessed August 31, 2015.
- Polanczyk CA, Marcantonio E, Goldman L, et al. Impact of age on perioperative complications and length of stay in patients undergoing noncardiac surgery. Ann Intern Med. 2001;134(8):637–643.
- Aletti G, Dowdy SC, Gostout BS, et al. Aggressive surgical effort and improved survival in advanced stage ovarian cancer. Obstet Gynecol. 2006;107(1):77–85.
- National Comprehensive Cancer Network. NCCN Guidelines for Age-Related Recommendations: Older Adult Oncology. . Published 2015. Accessed August 31, 2015.
- Uppal S, Igwe E, Rice L, Spencer R, Rose SL. Frailty index predicts severe complications in gynecologic oncology patients. Gynecol Oncol. 2015;137(1):98–101.
IN THIS ARTICLE
- Preoperative risk-assessment strategies
- The 11-item modified Functional Index
- Using the Functional Index in practice
Updates on Antidepressant Use
MINDFULNESS-BASED COGNITIVE THERAPY AND ANTIDEPRESSANTS
Kuyken W, Hayes R, Barrett B, et al. Effectiveness and cost-effectiveness of mindfulness-based cognitive therapy compared with maintenance antidepressant treatment in the prevention of depressive relapse or recurrence (PREVENT): a randomised controlled trial. Lancet. 2015;386(9988):63-73. doi:10.1016/S0140-6736(14)62222-4.
Mindfulness-based cognitive therapy—a group-based psychosocial intervention designed to enhance self-management of prodromal symptoms associated with depressive relapse—with support to taper or discontinue antidepressant treatment (MBCT-TS) is neither superior nor inferior to maintenance antidepressant treatment for preventing a depressive relapse, according to the PREVENT trial.
Researchers randomly assigned 424 patients to MBCT-TS or maintenance therapy and found no difference in time to relapse or recurrence of depression between the two groups. Rates of adverse effects were similar in both groups.
The study authors note that both treatments were associated with positive outcomes regarding relapse or recurrence, residual depressive symptoms, and quality of life.
COMMENTARY
Patients with recurrent depression have a 50% to 80% lifetime rate of relapse, making a prevention strategy an important part of their care. Current recommendations suggest long-term continuation of antidepressant treatment decreases recurrence by 50% to 60%.1 However, antidepressant medication only works for as long as you take it, and many people do not want to be on antidepressants long term. A previous study compared MBCT-TS, continuation of antidepressant medication, and placebo; the respective relapse rates of 28%, 27%, and 71% indicate that both MBCT-TS and antidepressant medication substantially decrease the rate of depression relapse.2 This study provides further evidence that MBCT-TS is an excellent alternative to antidepressant medication for decreasing depression relapse.
1. Geddes JR, Carney SM, Davies C, et al. Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet. 2003;361:653-661.
2. Segal ZV, Bieling P, Young T, et al. Antidepressant monotherapy vs sequential pharmacotherapy and mindfulness-based cognitive therapy, or placebo, for relapse prophylaxis in recurrent depression. Arch Gen Psych. 2010;67:1256-1264. doi:10.1001/archgenpsychiatry.2010.168.
Continue for treating preconception depression: To stop SSRIs or not >>
TREATING PRECONCEPTION DEPRESSION: TO STOP SSRIs OR NOT
Andersen JT, Andersen NL, Horwitz H, et al. Exposure to selective serotonin reuptake inhibitors in early pregnancy and the risk of miscarriage. Obstet Gynecol. 2014;124(4):655-661. doi: 10.1097/AOG.0000000000000447.
Miscarriage rates in women taking selective serotonin reuptake inhibitors (SSRIs) in early pregnancy were higher than in those not taking SSRIs but similar to those who discontinued SSRI treatment prior to pregnancy, a Danish cohort study revealed.
Out of 1.3 million pregnancies between 1997 and 2010, researchers identified 22,884 women who were exposed to an SSRI during the first 35 days of pregnancy and found miscarriage rates of 13% in those exposed to the antidepressants, compared to 11% for those not exposed. Investigators also identified 14,016 women who discontinued SSRI treatment three to 12 months prior to conception and found a miscarriage rate of 14%.
The adjusted hazard ratio for miscarriage while taking SSRIs in early pregnancy was 1.27, and for miscarriage after discontinuing SSRIs prior to pregnancy, 1.24. When the data were stratified according to specific SSRIs, rates were lowest among those taking fluoxetine during pregnancy (1.10) and highest among those taking sertraline (1.45). Miscarriage rates among women who stopped SSRIs prior to pregnancy were lowest for fluoxetine (1.2) and highest for escitalopram (1.33).
“Because the risk for miscarriage is elevated in both groups compared with an unexposed population, there is likely no benefit in discontinuing SSRI use before pregnancy to decrease one’s chances of miscarriage,” the study authors conclude.
COMMENTARY
The effects of depression on a woman’s experience during pregnancy are large, as are the effects of depression on pregnancy outcomes. Depression during pregnancy is associated with increased rates of prematurity, low birth weight, and preeclampsia.1 Depression during pregnancy is also an important risk factor for postpartum depression, which affects babies as well as mothers and is associated with maternal suicide.
At the same time, use of SSRIs in pregnancy has been inconsistently associated with miscarriage, cardiac defects, premature birth, and primary pulmonary hypertension in the newborn.2 This study is reassuring in that SSRIs are unlikely to be a significant contributor to miscarriage. But it is important to realize that this article only addresses miscarriage rates, not other potential effects of SSRIs on the fetus. The decision about the use of SSRIs in pregnancy remains a difficult one, balancing risk and benefit. When determining that balance, bear in mind that cognitive behavioral therapy (CBT) has been shown in other studies to be equally effective to medication in treating depression and may also be considered in our range of options for treatment of depression in pregnancy.3,4
The decision about whether to use or continue an SSRI and whether to use or supplement with CBT instead is an important one and always requires detailed discussion with the mother-to-be.
1. Grigoriadis S, VonderPorten EH, Mamisashvili L, et al. The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta-analysis. J Clin Psych. 2013;74:e321-341.
2. Meltzer-Brody S. Treating perinatal depression: risks and stigma. Obstet Gynecol. 2014;124(4):653-654. doi: 10.1097/AOG.0000000000000498.
3. Keller MB, McCullough JP, Klein DN, et al. A comparison of nefazodone, the cognitive behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression [published correction appears in N Engl J Med. 2001;345(3):232]. N Engl J Med. 2000;342(20): 1462-1470.
4. Cuijpers P, Hollon SD, van Straten A, et al. Does cognitive behaviour therapy have an enduring effect that is superior to keeping patients on continuation pharmacotherapy? A meta-analysis. BMJ Open. 2013;3(4). pii: e002542. doi: 10.1136/bmjopen-2012-002542.
Continue for suicide, self-harm rates, and antidepressants >>
SUICIDE, SELF-HARM RATES, AND ANTIDEPRESSANTS
Coupland C, Hill T, Morriss R, et al. Antidepressant use and risk of suicide and attempted suicide or self harm in people aged 20 to 64: cohort study using a primary care database. BMJ. 2015;350:h517. doi: 10.1136/bmj.h517.
In patients with clinical depression, rates of suicide and self-harm are similar among those treated with selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants but significantly higher among those treated with other antidepressants, according to a review of 238,963 patients who were diagnosed with depression.
During an average five years’ follow-up, researchers noted 198 cases of suicide and 5,243 cases of attempted suicide or self-harm. The following hazard ratios (HR) were associated with antidepressant use:
Absolute risk for suicide over one year ranged from 0.02% for amitriptyline to 0.19% for mirtazapine.
COMMENTARY
This large study suggests suicide rates may be greater with non-SSRI antidepressants than with SSRIs. The data are far from solid, though, because of the small number of events and the potential for systematic differences in how these antidepressants are prescribed. For instance, if dual norepinephrine and serotonin agents are prescribed more often to individuals with more severe depression, then the increased suicide risk with use of combined norepinephrine/serotonin agents (eg, venlafaxine) could relate to the severity of the depression treated, not to an effect of the medication. Of importance is that the rate of suicide was increased in the first 28 days after starting an antidepressant and in the 28 days after stopping the antidepressant, times when we should have increased vigilance for suicidal ideation.
MINDFULNESS-BASED COGNITIVE THERAPY AND ANTIDEPRESSANTS
Kuyken W, Hayes R, Barrett B, et al. Effectiveness and cost-effectiveness of mindfulness-based cognitive therapy compared with maintenance antidepressant treatment in the prevention of depressive relapse or recurrence (PREVENT): a randomised controlled trial. Lancet. 2015;386(9988):63-73. doi:10.1016/S0140-6736(14)62222-4.
Mindfulness-based cognitive therapy—a group-based psychosocial intervention designed to enhance self-management of prodromal symptoms associated with depressive relapse—with support to taper or discontinue antidepressant treatment (MBCT-TS) is neither superior nor inferior to maintenance antidepressant treatment for preventing a depressive relapse, according to the PREVENT trial.
Researchers randomly assigned 424 patients to MBCT-TS or maintenance therapy and found no difference in time to relapse or recurrence of depression between the two groups. Rates of adverse effects were similar in both groups.
The study authors note that both treatments were associated with positive outcomes regarding relapse or recurrence, residual depressive symptoms, and quality of life.
COMMENTARY
Patients with recurrent depression have a 50% to 80% lifetime rate of relapse, making a prevention strategy an important part of their care. Current recommendations suggest long-term continuation of antidepressant treatment decreases recurrence by 50% to 60%.1 However, antidepressant medication only works for as long as you take it, and many people do not want to be on antidepressants long term. A previous study compared MBCT-TS, continuation of antidepressant medication, and placebo; the respective relapse rates of 28%, 27%, and 71% indicate that both MBCT-TS and antidepressant medication substantially decrease the rate of depression relapse.2 This study provides further evidence that MBCT-TS is an excellent alternative to antidepressant medication for decreasing depression relapse.
1. Geddes JR, Carney SM, Davies C, et al. Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet. 2003;361:653-661.
2. Segal ZV, Bieling P, Young T, et al. Antidepressant monotherapy vs sequential pharmacotherapy and mindfulness-based cognitive therapy, or placebo, for relapse prophylaxis in recurrent depression. Arch Gen Psych. 2010;67:1256-1264. doi:10.1001/archgenpsychiatry.2010.168.
Continue for treating preconception depression: To stop SSRIs or not >>
TREATING PRECONCEPTION DEPRESSION: TO STOP SSRIs OR NOT
Andersen JT, Andersen NL, Horwitz H, et al. Exposure to selective serotonin reuptake inhibitors in early pregnancy and the risk of miscarriage. Obstet Gynecol. 2014;124(4):655-661. doi: 10.1097/AOG.0000000000000447.
Miscarriage rates in women taking selective serotonin reuptake inhibitors (SSRIs) in early pregnancy were higher than in those not taking SSRIs but similar to those who discontinued SSRI treatment prior to pregnancy, a Danish cohort study revealed.
Out of 1.3 million pregnancies between 1997 and 2010, researchers identified 22,884 women who were exposed to an SSRI during the first 35 days of pregnancy and found miscarriage rates of 13% in those exposed to the antidepressants, compared to 11% for those not exposed. Investigators also identified 14,016 women who discontinued SSRI treatment three to 12 months prior to conception and found a miscarriage rate of 14%.
The adjusted hazard ratio for miscarriage while taking SSRIs in early pregnancy was 1.27, and for miscarriage after discontinuing SSRIs prior to pregnancy, 1.24. When the data were stratified according to specific SSRIs, rates were lowest among those taking fluoxetine during pregnancy (1.10) and highest among those taking sertraline (1.45). Miscarriage rates among women who stopped SSRIs prior to pregnancy were lowest for fluoxetine (1.2) and highest for escitalopram (1.33).
“Because the risk for miscarriage is elevated in both groups compared with an unexposed population, there is likely no benefit in discontinuing SSRI use before pregnancy to decrease one’s chances of miscarriage,” the study authors conclude.
COMMENTARY
The effects of depression on a woman’s experience during pregnancy are large, as are the effects of depression on pregnancy outcomes. Depression during pregnancy is associated with increased rates of prematurity, low birth weight, and preeclampsia.1 Depression during pregnancy is also an important risk factor for postpartum depression, which affects babies as well as mothers and is associated with maternal suicide.
At the same time, use of SSRIs in pregnancy has been inconsistently associated with miscarriage, cardiac defects, premature birth, and primary pulmonary hypertension in the newborn.2 This study is reassuring in that SSRIs are unlikely to be a significant contributor to miscarriage. But it is important to realize that this article only addresses miscarriage rates, not other potential effects of SSRIs on the fetus. The decision about the use of SSRIs in pregnancy remains a difficult one, balancing risk and benefit. When determining that balance, bear in mind that cognitive behavioral therapy (CBT) has been shown in other studies to be equally effective to medication in treating depression and may also be considered in our range of options for treatment of depression in pregnancy.3,4
The decision about whether to use or continue an SSRI and whether to use or supplement with CBT instead is an important one and always requires detailed discussion with the mother-to-be.
1. Grigoriadis S, VonderPorten EH, Mamisashvili L, et al. The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta-analysis. J Clin Psych. 2013;74:e321-341.
2. Meltzer-Brody S. Treating perinatal depression: risks and stigma. Obstet Gynecol. 2014;124(4):653-654. doi: 10.1097/AOG.0000000000000498.
3. Keller MB, McCullough JP, Klein DN, et al. A comparison of nefazodone, the cognitive behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression [published correction appears in N Engl J Med. 2001;345(3):232]. N Engl J Med. 2000;342(20): 1462-1470.
4. Cuijpers P, Hollon SD, van Straten A, et al. Does cognitive behaviour therapy have an enduring effect that is superior to keeping patients on continuation pharmacotherapy? A meta-analysis. BMJ Open. 2013;3(4). pii: e002542. doi: 10.1136/bmjopen-2012-002542.
Continue for suicide, self-harm rates, and antidepressants >>
SUICIDE, SELF-HARM RATES, AND ANTIDEPRESSANTS
Coupland C, Hill T, Morriss R, et al. Antidepressant use and risk of suicide and attempted suicide or self harm in people aged 20 to 64: cohort study using a primary care database. BMJ. 2015;350:h517. doi: 10.1136/bmj.h517.
In patients with clinical depression, rates of suicide and self-harm are similar among those treated with selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants but significantly higher among those treated with other antidepressants, according to a review of 238,963 patients who were diagnosed with depression.
During an average five years’ follow-up, researchers noted 198 cases of suicide and 5,243 cases of attempted suicide or self-harm. The following hazard ratios (HR) were associated with antidepressant use:
Absolute risk for suicide over one year ranged from 0.02% for amitriptyline to 0.19% for mirtazapine.
COMMENTARY
This large study suggests suicide rates may be greater with non-SSRI antidepressants than with SSRIs. The data are far from solid, though, because of the small number of events and the potential for systematic differences in how these antidepressants are prescribed. For instance, if dual norepinephrine and serotonin agents are prescribed more often to individuals with more severe depression, then the increased suicide risk with use of combined norepinephrine/serotonin agents (eg, venlafaxine) could relate to the severity of the depression treated, not to an effect of the medication. Of importance is that the rate of suicide was increased in the first 28 days after starting an antidepressant and in the 28 days after stopping the antidepressant, times when we should have increased vigilance for suicidal ideation.
MINDFULNESS-BASED COGNITIVE THERAPY AND ANTIDEPRESSANTS
Kuyken W, Hayes R, Barrett B, et al. Effectiveness and cost-effectiveness of mindfulness-based cognitive therapy compared with maintenance antidepressant treatment in the prevention of depressive relapse or recurrence (PREVENT): a randomised controlled trial. Lancet. 2015;386(9988):63-73. doi:10.1016/S0140-6736(14)62222-4.
Mindfulness-based cognitive therapy—a group-based psychosocial intervention designed to enhance self-management of prodromal symptoms associated with depressive relapse—with support to taper or discontinue antidepressant treatment (MBCT-TS) is neither superior nor inferior to maintenance antidepressant treatment for preventing a depressive relapse, according to the PREVENT trial.
Researchers randomly assigned 424 patients to MBCT-TS or maintenance therapy and found no difference in time to relapse or recurrence of depression between the two groups. Rates of adverse effects were similar in both groups.
The study authors note that both treatments were associated with positive outcomes regarding relapse or recurrence, residual depressive symptoms, and quality of life.
COMMENTARY
Patients with recurrent depression have a 50% to 80% lifetime rate of relapse, making a prevention strategy an important part of their care. Current recommendations suggest long-term continuation of antidepressant treatment decreases recurrence by 50% to 60%.1 However, antidepressant medication only works for as long as you take it, and many people do not want to be on antidepressants long term. A previous study compared MBCT-TS, continuation of antidepressant medication, and placebo; the respective relapse rates of 28%, 27%, and 71% indicate that both MBCT-TS and antidepressant medication substantially decrease the rate of depression relapse.2 This study provides further evidence that MBCT-TS is an excellent alternative to antidepressant medication for decreasing depression relapse.
1. Geddes JR, Carney SM, Davies C, et al. Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet. 2003;361:653-661.
2. Segal ZV, Bieling P, Young T, et al. Antidepressant monotherapy vs sequential pharmacotherapy and mindfulness-based cognitive therapy, or placebo, for relapse prophylaxis in recurrent depression. Arch Gen Psych. 2010;67:1256-1264. doi:10.1001/archgenpsychiatry.2010.168.
Continue for treating preconception depression: To stop SSRIs or not >>
TREATING PRECONCEPTION DEPRESSION: TO STOP SSRIs OR NOT
Andersen JT, Andersen NL, Horwitz H, et al. Exposure to selective serotonin reuptake inhibitors in early pregnancy and the risk of miscarriage. Obstet Gynecol. 2014;124(4):655-661. doi: 10.1097/AOG.0000000000000447.
Miscarriage rates in women taking selective serotonin reuptake inhibitors (SSRIs) in early pregnancy were higher than in those not taking SSRIs but similar to those who discontinued SSRI treatment prior to pregnancy, a Danish cohort study revealed.
Out of 1.3 million pregnancies between 1997 and 2010, researchers identified 22,884 women who were exposed to an SSRI during the first 35 days of pregnancy and found miscarriage rates of 13% in those exposed to the antidepressants, compared to 11% for those not exposed. Investigators also identified 14,016 women who discontinued SSRI treatment three to 12 months prior to conception and found a miscarriage rate of 14%.
The adjusted hazard ratio for miscarriage while taking SSRIs in early pregnancy was 1.27, and for miscarriage after discontinuing SSRIs prior to pregnancy, 1.24. When the data were stratified according to specific SSRIs, rates were lowest among those taking fluoxetine during pregnancy (1.10) and highest among those taking sertraline (1.45). Miscarriage rates among women who stopped SSRIs prior to pregnancy were lowest for fluoxetine (1.2) and highest for escitalopram (1.33).
“Because the risk for miscarriage is elevated in both groups compared with an unexposed population, there is likely no benefit in discontinuing SSRI use before pregnancy to decrease one’s chances of miscarriage,” the study authors conclude.
COMMENTARY
The effects of depression on a woman’s experience during pregnancy are large, as are the effects of depression on pregnancy outcomes. Depression during pregnancy is associated with increased rates of prematurity, low birth weight, and preeclampsia.1 Depression during pregnancy is also an important risk factor for postpartum depression, which affects babies as well as mothers and is associated with maternal suicide.
At the same time, use of SSRIs in pregnancy has been inconsistently associated with miscarriage, cardiac defects, premature birth, and primary pulmonary hypertension in the newborn.2 This study is reassuring in that SSRIs are unlikely to be a significant contributor to miscarriage. But it is important to realize that this article only addresses miscarriage rates, not other potential effects of SSRIs on the fetus. The decision about the use of SSRIs in pregnancy remains a difficult one, balancing risk and benefit. When determining that balance, bear in mind that cognitive behavioral therapy (CBT) has been shown in other studies to be equally effective to medication in treating depression and may also be considered in our range of options for treatment of depression in pregnancy.3,4
The decision about whether to use or continue an SSRI and whether to use or supplement with CBT instead is an important one and always requires detailed discussion with the mother-to-be.
1. Grigoriadis S, VonderPorten EH, Mamisashvili L, et al. The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta-analysis. J Clin Psych. 2013;74:e321-341.
2. Meltzer-Brody S. Treating perinatal depression: risks and stigma. Obstet Gynecol. 2014;124(4):653-654. doi: 10.1097/AOG.0000000000000498.
3. Keller MB, McCullough JP, Klein DN, et al. A comparison of nefazodone, the cognitive behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression [published correction appears in N Engl J Med. 2001;345(3):232]. N Engl J Med. 2000;342(20): 1462-1470.
4. Cuijpers P, Hollon SD, van Straten A, et al. Does cognitive behaviour therapy have an enduring effect that is superior to keeping patients on continuation pharmacotherapy? A meta-analysis. BMJ Open. 2013;3(4). pii: e002542. doi: 10.1136/bmjopen-2012-002542.
Continue for suicide, self-harm rates, and antidepressants >>
SUICIDE, SELF-HARM RATES, AND ANTIDEPRESSANTS
Coupland C, Hill T, Morriss R, et al. Antidepressant use and risk of suicide and attempted suicide or self harm in people aged 20 to 64: cohort study using a primary care database. BMJ. 2015;350:h517. doi: 10.1136/bmj.h517.
In patients with clinical depression, rates of suicide and self-harm are similar among those treated with selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants but significantly higher among those treated with other antidepressants, according to a review of 238,963 patients who were diagnosed with depression.
During an average five years’ follow-up, researchers noted 198 cases of suicide and 5,243 cases of attempted suicide or self-harm. The following hazard ratios (HR) were associated with antidepressant use:
Absolute risk for suicide over one year ranged from 0.02% for amitriptyline to 0.19% for mirtazapine.
COMMENTARY
This large study suggests suicide rates may be greater with non-SSRI antidepressants than with SSRIs. The data are far from solid, though, because of the small number of events and the potential for systematic differences in how these antidepressants are prescribed. For instance, if dual norepinephrine and serotonin agents are prescribed more often to individuals with more severe depression, then the increased suicide risk with use of combined norepinephrine/serotonin agents (eg, venlafaxine) could relate to the severity of the depression treated, not to an effect of the medication. Of importance is that the rate of suicide was increased in the first 28 days after starting an antidepressant and in the 28 days after stopping the antidepressant, times when we should have increased vigilance for suicidal ideation.