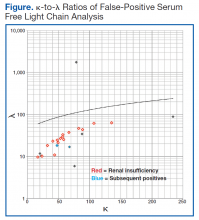
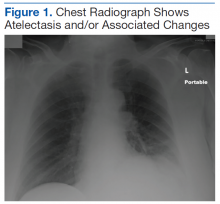
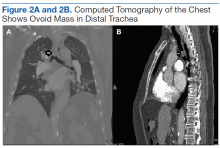
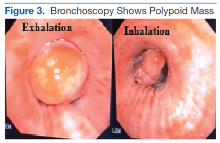
User login
Concussions in American Football
Football is an important component of American culture, with approximately 3 million youth athletes, 1.1 million high school athletes, and 100,000 college athletes participating each year.1 Participation in football provides athletes with physical, social, psychological, and academic benefits. Despite these benefits, widespread focus has been placed on the safety of football due to the risk for sport-related concussion (SRC) and potentially long-term effects; however, little recognition has been given to the advancements in concussion management across time and occurrence of concussions during most life activities. Although it is reasonable for concerns to be presented, it is important to better understand SRC and the current factors leading to prolonged recoveries, increased risk for injury, and potentially long-term effects.
What Is a Concussion?
Concussions occur after sustaining direct or indirect injury to the head or other parts of the body, as long as the injury force is transmitted to the head. Athletes often experience physical, cognitive, emotional, and sleep-related symptoms post-concussion secondary to an “energy crisis” within the brain.2 The energy crisis occurs as the result of transient neurological dysfunction triggered by changes in the brain (eg, release of neurotransmitters, impaired axonal function).2,3 Concussion is undetectable with traditional imaging; however, advanced imaging techniques (eg, diffuse tensor imaging) have shown progress in assessing axonal injury.3 Symptom duration post-concussion is highly variable due to individual differences; a recent study showed recovery took 3 to 4 weeks for memory and symptoms.4,5
Previous Concussion Management
Identification techniques and return-to-play guidelines for concussion have significantly changed across time. In the past, concussion grading scales were utilized for diagnosis and return to play was possible within the same contest.6,7 It has since been recognized that initial concussion severity makes it difficult to predict recovery.3 For example, research revealed memory decline and increased symptoms 36 hours post-injury for athletes with a grade 1 concussion (ie, transient confusion, no loss of consciousness, concussion symptoms or mental status changes that resolve within 15 minutes of injury) compared to baseline.7 Another study found duration of mental status changes to be related to slower symptom resolution and memory impairment 36 hours to 7 days post-injury.6 Consequently, return to play within the same contest was likely too liberal. Guidelines today recommend immediate removal from play with suspected SRC. Nevertheless, the “play through pain” culture has led athletes to continue playing after SRC, contributing to prolonged recoveries and potentially long-term effects.
Current Concussion Management: Continued Concerns and Areas of Improvement
Despite increased awareness of concussions, recent estimates revealed high rates (ie, 27:1 ratio for general players) of underreporting in college football, particularly amongst offensive linemen.8 Researchers have studied recovery implications for remaining in play, with one study revealing a 2.2 times greater risk for prolonged recovery in college athletes with delayed vs immediate removal.9 Another similar study discovered an 8.8 times greater risk for prolonged recovery in adolescent and young adult athletes not removed vs removed from play.10 Further analysis found remaining in play to be the greatest risk factor for prolonged recovery compared to other previously studied risk factors (eg, age, sex, posttraumatic migraine).10 Additionally, significant differences in neurocognitive data were seen between the “removed” and “not removed” groups for verbal memory, visual memory, processing speed, and reaction time at 1 to 7 days and 8 to 30 days.10 The recovery implications of remaining in play and the additional risk for second impact syndrome (SIS), or repeat concussion when recovering from another injury, emphasizes the need for further education efforts amongst athletes to encourage immediate reporting of injury.11
Sideline Assessment
Sideline assessment has become a vital component of concussion management to rule out concussion and/or significant injury other than concussion. Assessment should include observation, cognitive/balance testing, neurologic examination, and possible exertion testing to ensure a comprehensive evaluation of all areas of potential dysfunction.12 Indications for emergency department evaluation include suspicion for cervical spine injury, intracranial hemorrhage, or skull fracture as well as prolonged loss of consciousness, high-risk mechanisms, posttraumatic seizure(s), and/or significant worsening of symptoms.12
Observation
On the sideline, it is important to identify any immediate signs of injury (ie, loss of consciousness, anterograde/retrograde amnesia, and disorientation/confusion). Since immediate signs are not always present, it is important to be aware of the most commonly reported symptoms, including headache, difficulty concentrating, fatigue, drowsiness, and dizziness.13 If symptoms are not reported by the athlete, balance problems, lack of coordination, increased emotionality, and difficulty following instructions may be observed during play.12
On-Field Assessment
Cognitive and balance testing are essential in determining if an athlete has sustained a concussion. Immediate declines in memory, concentration abilities, and balance abilities are common. Given limitations in administering long testing batteries on the sideline, brief standardized tests such as the Standardized Assessment of Concussion (SAC), Balance Error Scoring System (BESS), and Sport Concussion Assessment Tool (SCAT) are commonly utilized. Identification of cognitive and/or balance abnormalities can help the athlete recognize deficits following injury.12 Balance problems are experienced due to abnormalities in sensory organization and generally resolve during the acute recovery period.14,15 Cognitive difficulties typically persist longer than balance problems, though duration varies widely.
Neurologic Evaluation
A neurologic evaluation including cranial nerve testing and evaluation of motor-sensory function (ie, assessment for the strength and sensation of upper and lower extremities) is important to identify focal deficits (ie, sensation changes, loss of fine motor control) indicative of serious intracranial pathology.12 Additionally, clinicians have suggested inclusion of vestibular and oculomotor assessments due to frequent dysfunction post-concussion.12,15,16 Examination of the vestibular/oculomotor systems through tools such as the Vestibular/Ocular Motor Screening (VOMS) assessment (assesses both the vestibular and oculomotor systems) and King-Devick Test (primarily assesses saccadic eye movements) can elicit symptoms that may not present immediately. If assessment appears normal, exertion testing can be utilized to determine if symptoms are provoked through physical exercise that should include cardio, dynamic, and sport-specific activities to stress the vestibular system.12
Risk Factors for Injury and Prolonged Recovery
Medical professionals must consider the presence of risk factors when managing concussion in order to make appropriate treatment recommendations and return-to-play decisions. Research has demonstrated the role of female gender, learning disability, attention-deficit/hyperactivity disorder, psychiatric history, young age, motion sickness, sleep problems, somatization, concussion history, on-field dizziness, posttraumatic migraine, and fogginess in increased risk for injury and/or prolonged recovery.17-25 Additionally, athletes with ongoing symptoms from a previous injury are at risk for sustaining another injury.
Acute Home Concussion Management
Although strict rest has been recommended post-concussion, recent research evaluating strict rest vs usual care for adolescents revealed greater symptom reports and longer recovery periods for the strict rest group.26 Based on these findings and emphasis for regulation within the migraine literature (due to the common pathophysiology between migraine and concussion27), we recommend that athletes follow a regulated daily schedule post-concussion including: 1) regular sleep-wake schedule with avoidance of naps, 2) regular meals, 3) adequate fluid hydration, 4) light noncontact physical activity (ie, walking, with progressions recommended by a physician), and 5) stress management techniques. Use of these strategies immediately can help in preventing against increased symptoms and stress, and decreases the need for medication in select cases. Additionally, over-the-counter medications should be limited to 2 to 3 doses per week to avoid rebound headaches.28
In-Office Concussion Management
Athletes diagnosed with SRC will experience different symptoms based on the injury mechanism, risk factors, and management approach. Comprehensive evaluation should include assessment of risk factors, injury details, symptoms, neurocognitive functioning, vestibular/oculomotor dysfunction, tolerance of physical exertion, balance functioning, and cervical spine integrity (if necessary).29,30 Due to individual differences and the heterogeneous symptom profiles, concussion management must move beyond a “one size fits all” approach to avoid nonspecific treatment strategies and consequently prolonged recoveries.29 Clinicians and researchers at University of Pittsburgh Medical Center have identified 6 concussion clinical profiles (ie, vestibular, ocular, posttraumatic migraine, cervical, anxiety/mood, and cognitive/fatigue) that are generally identifiable 48 hours after injury.29,30 Identification of the clinical profile(s) through a comprehensive evaluation guides the development of individualized treatment plans and targeted rehabilitation strategies.29,30
Vestibular. The vestibular system is responsible for stabilizing vision while the head moves and balance control.15 Athletes can experience central and/or peripheral vestibular dysfunction to include benign paroxysmal positional vertigo (BPPV), visual motion sensitivity, vestibular ocular reflex impairment, and balance impairment.30,31 Symptoms typically include dizziness, impaired balance, blurry vision, difficulty focusing, and environmental sensitivity.15,29,30 Potential treatment options include vestibular rehabilitation, exertion therapy, and school/work accommodations.
Ocular. The oculomotor system is responsible for control of eye movements. Athletes can experience many different posttraumatic vision changes, including convergence problems, eye-tracking difficulties, refractive error, difficulty with pursuits/saccades, and accommodation insufficiency. Symptoms typically include light sensitivity, blurred vision, double vision, headaches, fatigue, and memory difficulties.15,29,30 Potential treatment options include vision therapy, vestibular rehabilitation, and school/work accommodations.32
Posttraumatic Migraine. Headache, the most common post-concussion symptom, can persist and meet criteria for posttraumatic migraine (ie, unilateral headache with accompanying nausea and/or photophobia and phonophobia).29,30,33 Implementation of a routine schedule, daily physical activity, exertion therapy, pharmacologic intervention, and school/work accommodations are potential treatment options.
Cervical. The cervical spine can be injured during whiplash-type injuries. Therefore, determining the location, onset, and typical exacerbations of pain can be helpful in identifying cervical involvement.29,30 Symptoms typically include headaches, neck pain, numbness, and tingling. Evaluation and therapy by a certified physical therapist and pharmacologic intervention (eg, muscle relaxants) are potential treatment options. 29,30
Anxiety/Mood. Anxiety, or worry and fear about everyday situations, is common post-concussion and can sometimes be related to ongoing vestibular impairment. Symptoms typically include ruminative thoughts, avoidance of specific situations, hypervigilance, feelings of being overwhelmed, and difficulty falling asleep.29,30 Potential treatment options include implementation of a routine schedule, exposure to provocative situations, psychotherapy, pharmacologic intervention, and school/work accommodations.34
Cognitive/Fatigue. A global concussion factor (including cognitive, fatigue, and migraine symptoms) has been identified within 1 to 7 days of injury. Although this factor of symptoms generally resolves during the acute recovery period, it persists in select cases.13 Symptoms typically include fatigue, decreased energy levels, nonspecific headaches, potential sleep disruption, increased symptoms towards the end of the day, difficulty concentrating, and increased headache with cognitive activities.29,30,35 Routine schedule, daily physical activity, exertion therapy, pharmacologic intervention (eg, amantadine), and school/work accommodations are potential treatment options.30
Conclusion
Advancements in SRC management warrant change in the conversations regarding concussion in football. Specifically, conversations should address the current understanding of concussion and improvements in the safety of football through stricter concussion guidelines, detailed sideline evaluations, recognition of risk factors, improved acute management, and identification of concussion profiles that help to direct individualized treatment plans and targeted rehabilitation strategies. The biggest concerns related to concussions in football include underreporting of injury, premature return to play, and receiving routine rather than individualized treatment. Therefore, to further improve the safety of football and management of concussion it is essential that future efforts focus on the following 6 areas:
Education: Improved understanding of concussion is imperative to reducing poor outcomes and widespread concerns.
Immediate reporting: Reporting of concussion must be expected and encouraged through consistent responses by coaches to reduce underreporting and fear of reporting in athletes.
Prevention techniques: Athletes must be taught proper form and playing techniques to reduce the risk for concussion. Proper form and technique should be incentivized.
Targeted treatment: Individualized treatment plans and targeted rehabilitation strategies must be developed based on the identified clinical profile(s) to avoid nonspecific treatment recommendations.
Multidisciplinary treatment teams: Given the heterogeneous symptoms profiles and need for care provided by different medical specialties, multidisciplinary teams are essential.
Remain current: With the progress in understanding concussion, providers must remain vigilant of future advances in concussion management to further improve the safety of football.
Am J Orthop. 2016;45(6):352-356. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Dompier TP, Kerr ZY, Marshall SW, et al. Incidence of concussion during practice and games in youth, high school, and collegiate American football players. JAMA Pediatrics. 2015;169(7):659-665.
2. Giza C, Hovda D. The new neurometabolic cascade of concussion
3. Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury - an update. Phys Med Rehabil Clin N Am. 2016;27:373-393.
4. Henry L, Elbin R, Collins M, Marchetti G, Kontos A. Examining recovery trajectories after sport-related concussion with a multimodal clinical assessment approach. Neurosurgery. 2016;78(2):232-241.
5. McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Brit J Sports Med. 2013;47(5):250-258.
6. Lovell MR, Collins MW, Iverson GL, et al. Recovery from mild concussion in high school athletes. J Neurosurg. 2003;98(2):296-301.
7. Lovell MR, Collins MW, Iverson GL, Johnston KM, Bradley JP. Grade 1 or “ding” concussions in high school athletes. Am J Sports Med. 2004;32(1):47-54.
8. Baugh CM, Kroshus E, Daneshvar DH, Filali NA, Hiscox MJ, Glantz LH. Concussion management in United States college sports: compliance with National Collegiate Athletic Association concussion policy and areas for improvement. Am J Sports Med. 2015;43(1):47-56.
9. Asken BM, McCrea MA, Clugston JR, Snyder AR, Houck ZM, Bauer RM. “Playing through it”: Delayed reporting and removal from athletic activity after concussion predicts prolonged recovery. J Athl Train. 2016;51(4):329-335.
10. Elbin RJ, Sufrinko A, Schatz P, et al. Athletes that continue to play with concussion demonstrate worse recovery outcomes than athletes immediately removed from play. J Pediatr. In press.
11. Signoretti S, Lazzarino G, Tavazzi B, Vagnozzi R. The pathophysiology of concussion. PM R. 2011;3(10 Suppl 2):S359-S368.
12. Bloom J, Blount JG. Sideline evaluation of concussion. UpToDate. 2016. http://www.uptodate.com/contents/sideline-evaluation-of-concussion. Accessed July 13, 2016.
13. Kontos AP, Elbin RJ, Schatz P, et al. A revised factor structure for the post-concussion symptom scale: baseline and postconcussion factors. Am J Sports Med. 2012;40(10):2375-2384.
14. Guskiewicz KM, Ross SE, Marshall SW. Postural stability and neuropsychological deficits after concussion in collegiate athletes. J Athl Train. 2001;36(3):263.
15. Mucha A, Collins MW, Elbin R, et al. A brief Vestibular/Ocular Motor Screening (VOMS) assessment to evaluate concussions preliminary findings. Am J Sports Med. 2014;42(10):2479-2486.
16. Bloom J. Vestibular and ocular motor assessments: Important pieces to the concussion puzzle. Athletic Training and Sports Health Care. 2013;5(6):246-248.
17. Covassin T, Elbin R, Harris W, Parker T, Kontos A. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. Am J Sports Med. 2012;40(6):1303-1312.
18. Kontos A, Sufrinko A, Elbin R, Puskar A, Collins M. Reliability and associated risk factors for performance on the Vestibular/Ocular Motor Screening (VOMS) tool in healthy collegiate athletes. Am J Sports Med. 2016;44(6):1400-1406.
19. Guskiewicz KM, McCrea M, Marshall SW, et al. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290(19):2549-2555.
20. Lau B, Lovell MR, Collins MW, Pardini J. Neurocognitive and symptom predictors of recovery in high school athletes. Clin J Sport Med. 2009;19(3):216-221.
21. Lau BC, Kontos AP, Collins MW, Mucha A, Lovell MR. Which on-field signs/symptoms predict protracted recovery from sport-related concussion among high school football players? Am J Sports Med. 2011;39(11):2311-2318.
22. Mihalik JP, Register-Mihalik J, Kerr ZY, Marshall SW, McCrea MC, Guskiewicz KM. Recovery of posttraumatic migraine characteristics in patients after mild traumatic brain injury. Am J Sports Med. 2013;41(7):1490-1496.
23. Covassin T, Moran R, Elbin RJ. Sex differences in reported concussion injury rates and time loss from participation: An update of the National Collegiate Athletic Association injury surveillance program from 2004-2005 through 2008-2009. J Athl Train. 2016;51(3):189-194.
24. Root JM, Zuckerbraun NS, Wang L, et al. History of somatization is associated with prolonged recovery from concussion. J Pediatr. 2016;174:39-44.
25. Sufrinko A, Pearce K, Elbin RJ, et al. The effect of preinjury sleep difficulties on neurocognitive impairment and symptoms after sport-related concussion. Am J Sports Med. 2015;43(4):830-838.
26. Thomas DG, Apps JN, Hoffmann RG, McCrea M, Hammeke T. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics. 2015;135(2):213-223.
27. Choe M, Blume H. Pediatric posttraumatic Headache: a review. J Child Neurol. 2016;31(1):76-85.
28. Tepper SJ, Tepper DE. Breaking the cycle of medication overuse. Cleve Clin J Med. 2010;77(4):236-242.
29. Collins M, Kontos A, Reynolds E, Murawski C, Fu F. A comprehensive, targeted approach to the clinical care of athletes following sport-related concussion. Knee Surg Sports Traumatol Arthrosc. 2014;22(2):235-246.
30. Reynolds E, Collins MW, Mucha A, Troutman-Ensecki C. Establishing a clinical service for the management of sports-related concussions. Neurosurgery. 2014;75 Suppl 4:S71-S81.
31. Broglio SP, Collins MW, Williams RM, Mucha A, Kontos AP. Current and emerging rehabilitation for concussion: a review of the evidence. Clin Sports Med. 2015;34(2):213-231.
32. Master C, Scheiman M, Gallaway M, et al. Vision diagnoses are common after concussion in adolescents. Clin Pediatr (Phila). 2016;55(3):260-267.
33. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629-808.
34. Kontos A, Deitrick JM, Reynolds E. Mental health implication and consequences following sport-related concussion. Brit J Sports Med. 2016;50(3):139-140.
35. Kontos AP, Covassin T, Elbin R, Parker T. Depression and neurocognitive performance after concussion among male and female high school and collegiate athletes. Arch Phys Med Rehabil. 2012;93(10):1751-1756.
Football is an important component of American culture, with approximately 3 million youth athletes, 1.1 million high school athletes, and 100,000 college athletes participating each year.1 Participation in football provides athletes with physical, social, psychological, and academic benefits. Despite these benefits, widespread focus has been placed on the safety of football due to the risk for sport-related concussion (SRC) and potentially long-term effects; however, little recognition has been given to the advancements in concussion management across time and occurrence of concussions during most life activities. Although it is reasonable for concerns to be presented, it is important to better understand SRC and the current factors leading to prolonged recoveries, increased risk for injury, and potentially long-term effects.
What Is a Concussion?
Concussions occur after sustaining direct or indirect injury to the head or other parts of the body, as long as the injury force is transmitted to the head. Athletes often experience physical, cognitive, emotional, and sleep-related symptoms post-concussion secondary to an “energy crisis” within the brain.2 The energy crisis occurs as the result of transient neurological dysfunction triggered by changes in the brain (eg, release of neurotransmitters, impaired axonal function).2,3 Concussion is undetectable with traditional imaging; however, advanced imaging techniques (eg, diffuse tensor imaging) have shown progress in assessing axonal injury.3 Symptom duration post-concussion is highly variable due to individual differences; a recent study showed recovery took 3 to 4 weeks for memory and symptoms.4,5
Previous Concussion Management
Identification techniques and return-to-play guidelines for concussion have significantly changed across time. In the past, concussion grading scales were utilized for diagnosis and return to play was possible within the same contest.6,7 It has since been recognized that initial concussion severity makes it difficult to predict recovery.3 For example, research revealed memory decline and increased symptoms 36 hours post-injury for athletes with a grade 1 concussion (ie, transient confusion, no loss of consciousness, concussion symptoms or mental status changes that resolve within 15 minutes of injury) compared to baseline.7 Another study found duration of mental status changes to be related to slower symptom resolution and memory impairment 36 hours to 7 days post-injury.6 Consequently, return to play within the same contest was likely too liberal. Guidelines today recommend immediate removal from play with suspected SRC. Nevertheless, the “play through pain” culture has led athletes to continue playing after SRC, contributing to prolonged recoveries and potentially long-term effects.
Current Concussion Management: Continued Concerns and Areas of Improvement
Despite increased awareness of concussions, recent estimates revealed high rates (ie, 27:1 ratio for general players) of underreporting in college football, particularly amongst offensive linemen.8 Researchers have studied recovery implications for remaining in play, with one study revealing a 2.2 times greater risk for prolonged recovery in college athletes with delayed vs immediate removal.9 Another similar study discovered an 8.8 times greater risk for prolonged recovery in adolescent and young adult athletes not removed vs removed from play.10 Further analysis found remaining in play to be the greatest risk factor for prolonged recovery compared to other previously studied risk factors (eg, age, sex, posttraumatic migraine).10 Additionally, significant differences in neurocognitive data were seen between the “removed” and “not removed” groups for verbal memory, visual memory, processing speed, and reaction time at 1 to 7 days and 8 to 30 days.10 The recovery implications of remaining in play and the additional risk for second impact syndrome (SIS), or repeat concussion when recovering from another injury, emphasizes the need for further education efforts amongst athletes to encourage immediate reporting of injury.11
Sideline Assessment
Sideline assessment has become a vital component of concussion management to rule out concussion and/or significant injury other than concussion. Assessment should include observation, cognitive/balance testing, neurologic examination, and possible exertion testing to ensure a comprehensive evaluation of all areas of potential dysfunction.12 Indications for emergency department evaluation include suspicion for cervical spine injury, intracranial hemorrhage, or skull fracture as well as prolonged loss of consciousness, high-risk mechanisms, posttraumatic seizure(s), and/or significant worsening of symptoms.12
Observation
On the sideline, it is important to identify any immediate signs of injury (ie, loss of consciousness, anterograde/retrograde amnesia, and disorientation/confusion). Since immediate signs are not always present, it is important to be aware of the most commonly reported symptoms, including headache, difficulty concentrating, fatigue, drowsiness, and dizziness.13 If symptoms are not reported by the athlete, balance problems, lack of coordination, increased emotionality, and difficulty following instructions may be observed during play.12
On-Field Assessment
Cognitive and balance testing are essential in determining if an athlete has sustained a concussion. Immediate declines in memory, concentration abilities, and balance abilities are common. Given limitations in administering long testing batteries on the sideline, brief standardized tests such as the Standardized Assessment of Concussion (SAC), Balance Error Scoring System (BESS), and Sport Concussion Assessment Tool (SCAT) are commonly utilized. Identification of cognitive and/or balance abnormalities can help the athlete recognize deficits following injury.12 Balance problems are experienced due to abnormalities in sensory organization and generally resolve during the acute recovery period.14,15 Cognitive difficulties typically persist longer than balance problems, though duration varies widely.
Neurologic Evaluation
A neurologic evaluation including cranial nerve testing and evaluation of motor-sensory function (ie, assessment for the strength and sensation of upper and lower extremities) is important to identify focal deficits (ie, sensation changes, loss of fine motor control) indicative of serious intracranial pathology.12 Additionally, clinicians have suggested inclusion of vestibular and oculomotor assessments due to frequent dysfunction post-concussion.12,15,16 Examination of the vestibular/oculomotor systems through tools such as the Vestibular/Ocular Motor Screening (VOMS) assessment (assesses both the vestibular and oculomotor systems) and King-Devick Test (primarily assesses saccadic eye movements) can elicit symptoms that may not present immediately. If assessment appears normal, exertion testing can be utilized to determine if symptoms are provoked through physical exercise that should include cardio, dynamic, and sport-specific activities to stress the vestibular system.12
Risk Factors for Injury and Prolonged Recovery
Medical professionals must consider the presence of risk factors when managing concussion in order to make appropriate treatment recommendations and return-to-play decisions. Research has demonstrated the role of female gender, learning disability, attention-deficit/hyperactivity disorder, psychiatric history, young age, motion sickness, sleep problems, somatization, concussion history, on-field dizziness, posttraumatic migraine, and fogginess in increased risk for injury and/or prolonged recovery.17-25 Additionally, athletes with ongoing symptoms from a previous injury are at risk for sustaining another injury.
Acute Home Concussion Management
Although strict rest has been recommended post-concussion, recent research evaluating strict rest vs usual care for adolescents revealed greater symptom reports and longer recovery periods for the strict rest group.26 Based on these findings and emphasis for regulation within the migraine literature (due to the common pathophysiology between migraine and concussion27), we recommend that athletes follow a regulated daily schedule post-concussion including: 1) regular sleep-wake schedule with avoidance of naps, 2) regular meals, 3) adequate fluid hydration, 4) light noncontact physical activity (ie, walking, with progressions recommended by a physician), and 5) stress management techniques. Use of these strategies immediately can help in preventing against increased symptoms and stress, and decreases the need for medication in select cases. Additionally, over-the-counter medications should be limited to 2 to 3 doses per week to avoid rebound headaches.28
In-Office Concussion Management
Athletes diagnosed with SRC will experience different symptoms based on the injury mechanism, risk factors, and management approach. Comprehensive evaluation should include assessment of risk factors, injury details, symptoms, neurocognitive functioning, vestibular/oculomotor dysfunction, tolerance of physical exertion, balance functioning, and cervical spine integrity (if necessary).29,30 Due to individual differences and the heterogeneous symptom profiles, concussion management must move beyond a “one size fits all” approach to avoid nonspecific treatment strategies and consequently prolonged recoveries.29 Clinicians and researchers at University of Pittsburgh Medical Center have identified 6 concussion clinical profiles (ie, vestibular, ocular, posttraumatic migraine, cervical, anxiety/mood, and cognitive/fatigue) that are generally identifiable 48 hours after injury.29,30 Identification of the clinical profile(s) through a comprehensive evaluation guides the development of individualized treatment plans and targeted rehabilitation strategies.29,30
Vestibular. The vestibular system is responsible for stabilizing vision while the head moves and balance control.15 Athletes can experience central and/or peripheral vestibular dysfunction to include benign paroxysmal positional vertigo (BPPV), visual motion sensitivity, vestibular ocular reflex impairment, and balance impairment.30,31 Symptoms typically include dizziness, impaired balance, blurry vision, difficulty focusing, and environmental sensitivity.15,29,30 Potential treatment options include vestibular rehabilitation, exertion therapy, and school/work accommodations.
Ocular. The oculomotor system is responsible for control of eye movements. Athletes can experience many different posttraumatic vision changes, including convergence problems, eye-tracking difficulties, refractive error, difficulty with pursuits/saccades, and accommodation insufficiency. Symptoms typically include light sensitivity, blurred vision, double vision, headaches, fatigue, and memory difficulties.15,29,30 Potential treatment options include vision therapy, vestibular rehabilitation, and school/work accommodations.32
Posttraumatic Migraine. Headache, the most common post-concussion symptom, can persist and meet criteria for posttraumatic migraine (ie, unilateral headache with accompanying nausea and/or photophobia and phonophobia).29,30,33 Implementation of a routine schedule, daily physical activity, exertion therapy, pharmacologic intervention, and school/work accommodations are potential treatment options.
Cervical. The cervical spine can be injured during whiplash-type injuries. Therefore, determining the location, onset, and typical exacerbations of pain can be helpful in identifying cervical involvement.29,30 Symptoms typically include headaches, neck pain, numbness, and tingling. Evaluation and therapy by a certified physical therapist and pharmacologic intervention (eg, muscle relaxants) are potential treatment options. 29,30
Anxiety/Mood. Anxiety, or worry and fear about everyday situations, is common post-concussion and can sometimes be related to ongoing vestibular impairment. Symptoms typically include ruminative thoughts, avoidance of specific situations, hypervigilance, feelings of being overwhelmed, and difficulty falling asleep.29,30 Potential treatment options include implementation of a routine schedule, exposure to provocative situations, psychotherapy, pharmacologic intervention, and school/work accommodations.34
Cognitive/Fatigue. A global concussion factor (including cognitive, fatigue, and migraine symptoms) has been identified within 1 to 7 days of injury. Although this factor of symptoms generally resolves during the acute recovery period, it persists in select cases.13 Symptoms typically include fatigue, decreased energy levels, nonspecific headaches, potential sleep disruption, increased symptoms towards the end of the day, difficulty concentrating, and increased headache with cognitive activities.29,30,35 Routine schedule, daily physical activity, exertion therapy, pharmacologic intervention (eg, amantadine), and school/work accommodations are potential treatment options.30
Conclusion
Advancements in SRC management warrant change in the conversations regarding concussion in football. Specifically, conversations should address the current understanding of concussion and improvements in the safety of football through stricter concussion guidelines, detailed sideline evaluations, recognition of risk factors, improved acute management, and identification of concussion profiles that help to direct individualized treatment plans and targeted rehabilitation strategies. The biggest concerns related to concussions in football include underreporting of injury, premature return to play, and receiving routine rather than individualized treatment. Therefore, to further improve the safety of football and management of concussion it is essential that future efforts focus on the following 6 areas:
Education: Improved understanding of concussion is imperative to reducing poor outcomes and widespread concerns.
Immediate reporting: Reporting of concussion must be expected and encouraged through consistent responses by coaches to reduce underreporting and fear of reporting in athletes.
Prevention techniques: Athletes must be taught proper form and playing techniques to reduce the risk for concussion. Proper form and technique should be incentivized.
Targeted treatment: Individualized treatment plans and targeted rehabilitation strategies must be developed based on the identified clinical profile(s) to avoid nonspecific treatment recommendations.
Multidisciplinary treatment teams: Given the heterogeneous symptoms profiles and need for care provided by different medical specialties, multidisciplinary teams are essential.
Remain current: With the progress in understanding concussion, providers must remain vigilant of future advances in concussion management to further improve the safety of football.
Am J Orthop. 2016;45(6):352-356. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Football is an important component of American culture, with approximately 3 million youth athletes, 1.1 million high school athletes, and 100,000 college athletes participating each year.1 Participation in football provides athletes with physical, social, psychological, and academic benefits. Despite these benefits, widespread focus has been placed on the safety of football due to the risk for sport-related concussion (SRC) and potentially long-term effects; however, little recognition has been given to the advancements in concussion management across time and occurrence of concussions during most life activities. Although it is reasonable for concerns to be presented, it is important to better understand SRC and the current factors leading to prolonged recoveries, increased risk for injury, and potentially long-term effects.
What Is a Concussion?
Concussions occur after sustaining direct or indirect injury to the head or other parts of the body, as long as the injury force is transmitted to the head. Athletes often experience physical, cognitive, emotional, and sleep-related symptoms post-concussion secondary to an “energy crisis” within the brain.2 The energy crisis occurs as the result of transient neurological dysfunction triggered by changes in the brain (eg, release of neurotransmitters, impaired axonal function).2,3 Concussion is undetectable with traditional imaging; however, advanced imaging techniques (eg, diffuse tensor imaging) have shown progress in assessing axonal injury.3 Symptom duration post-concussion is highly variable due to individual differences; a recent study showed recovery took 3 to 4 weeks for memory and symptoms.4,5
Previous Concussion Management
Identification techniques and return-to-play guidelines for concussion have significantly changed across time. In the past, concussion grading scales were utilized for diagnosis and return to play was possible within the same contest.6,7 It has since been recognized that initial concussion severity makes it difficult to predict recovery.3 For example, research revealed memory decline and increased symptoms 36 hours post-injury for athletes with a grade 1 concussion (ie, transient confusion, no loss of consciousness, concussion symptoms or mental status changes that resolve within 15 minutes of injury) compared to baseline.7 Another study found duration of mental status changes to be related to slower symptom resolution and memory impairment 36 hours to 7 days post-injury.6 Consequently, return to play within the same contest was likely too liberal. Guidelines today recommend immediate removal from play with suspected SRC. Nevertheless, the “play through pain” culture has led athletes to continue playing after SRC, contributing to prolonged recoveries and potentially long-term effects.
Current Concussion Management: Continued Concerns and Areas of Improvement
Despite increased awareness of concussions, recent estimates revealed high rates (ie, 27:1 ratio for general players) of underreporting in college football, particularly amongst offensive linemen.8 Researchers have studied recovery implications for remaining in play, with one study revealing a 2.2 times greater risk for prolonged recovery in college athletes with delayed vs immediate removal.9 Another similar study discovered an 8.8 times greater risk for prolonged recovery in adolescent and young adult athletes not removed vs removed from play.10 Further analysis found remaining in play to be the greatest risk factor for prolonged recovery compared to other previously studied risk factors (eg, age, sex, posttraumatic migraine).10 Additionally, significant differences in neurocognitive data were seen between the “removed” and “not removed” groups for verbal memory, visual memory, processing speed, and reaction time at 1 to 7 days and 8 to 30 days.10 The recovery implications of remaining in play and the additional risk for second impact syndrome (SIS), or repeat concussion when recovering from another injury, emphasizes the need for further education efforts amongst athletes to encourage immediate reporting of injury.11
Sideline Assessment
Sideline assessment has become a vital component of concussion management to rule out concussion and/or significant injury other than concussion. Assessment should include observation, cognitive/balance testing, neurologic examination, and possible exertion testing to ensure a comprehensive evaluation of all areas of potential dysfunction.12 Indications for emergency department evaluation include suspicion for cervical spine injury, intracranial hemorrhage, or skull fracture as well as prolonged loss of consciousness, high-risk mechanisms, posttraumatic seizure(s), and/or significant worsening of symptoms.12
Observation
On the sideline, it is important to identify any immediate signs of injury (ie, loss of consciousness, anterograde/retrograde amnesia, and disorientation/confusion). Since immediate signs are not always present, it is important to be aware of the most commonly reported symptoms, including headache, difficulty concentrating, fatigue, drowsiness, and dizziness.13 If symptoms are not reported by the athlete, balance problems, lack of coordination, increased emotionality, and difficulty following instructions may be observed during play.12
On-Field Assessment
Cognitive and balance testing are essential in determining if an athlete has sustained a concussion. Immediate declines in memory, concentration abilities, and balance abilities are common. Given limitations in administering long testing batteries on the sideline, brief standardized tests such as the Standardized Assessment of Concussion (SAC), Balance Error Scoring System (BESS), and Sport Concussion Assessment Tool (SCAT) are commonly utilized. Identification of cognitive and/or balance abnormalities can help the athlete recognize deficits following injury.12 Balance problems are experienced due to abnormalities in sensory organization and generally resolve during the acute recovery period.14,15 Cognitive difficulties typically persist longer than balance problems, though duration varies widely.
Neurologic Evaluation
A neurologic evaluation including cranial nerve testing and evaluation of motor-sensory function (ie, assessment for the strength and sensation of upper and lower extremities) is important to identify focal deficits (ie, sensation changes, loss of fine motor control) indicative of serious intracranial pathology.12 Additionally, clinicians have suggested inclusion of vestibular and oculomotor assessments due to frequent dysfunction post-concussion.12,15,16 Examination of the vestibular/oculomotor systems through tools such as the Vestibular/Ocular Motor Screening (VOMS) assessment (assesses both the vestibular and oculomotor systems) and King-Devick Test (primarily assesses saccadic eye movements) can elicit symptoms that may not present immediately. If assessment appears normal, exertion testing can be utilized to determine if symptoms are provoked through physical exercise that should include cardio, dynamic, and sport-specific activities to stress the vestibular system.12
Risk Factors for Injury and Prolonged Recovery
Medical professionals must consider the presence of risk factors when managing concussion in order to make appropriate treatment recommendations and return-to-play decisions. Research has demonstrated the role of female gender, learning disability, attention-deficit/hyperactivity disorder, psychiatric history, young age, motion sickness, sleep problems, somatization, concussion history, on-field dizziness, posttraumatic migraine, and fogginess in increased risk for injury and/or prolonged recovery.17-25 Additionally, athletes with ongoing symptoms from a previous injury are at risk for sustaining another injury.
Acute Home Concussion Management
Although strict rest has been recommended post-concussion, recent research evaluating strict rest vs usual care for adolescents revealed greater symptom reports and longer recovery periods for the strict rest group.26 Based on these findings and emphasis for regulation within the migraine literature (due to the common pathophysiology between migraine and concussion27), we recommend that athletes follow a regulated daily schedule post-concussion including: 1) regular sleep-wake schedule with avoidance of naps, 2) regular meals, 3) adequate fluid hydration, 4) light noncontact physical activity (ie, walking, with progressions recommended by a physician), and 5) stress management techniques. Use of these strategies immediately can help in preventing against increased symptoms and stress, and decreases the need for medication in select cases. Additionally, over-the-counter medications should be limited to 2 to 3 doses per week to avoid rebound headaches.28
In-Office Concussion Management
Athletes diagnosed with SRC will experience different symptoms based on the injury mechanism, risk factors, and management approach. Comprehensive evaluation should include assessment of risk factors, injury details, symptoms, neurocognitive functioning, vestibular/oculomotor dysfunction, tolerance of physical exertion, balance functioning, and cervical spine integrity (if necessary).29,30 Due to individual differences and the heterogeneous symptom profiles, concussion management must move beyond a “one size fits all” approach to avoid nonspecific treatment strategies and consequently prolonged recoveries.29 Clinicians and researchers at University of Pittsburgh Medical Center have identified 6 concussion clinical profiles (ie, vestibular, ocular, posttraumatic migraine, cervical, anxiety/mood, and cognitive/fatigue) that are generally identifiable 48 hours after injury.29,30 Identification of the clinical profile(s) through a comprehensive evaluation guides the development of individualized treatment plans and targeted rehabilitation strategies.29,30
Vestibular. The vestibular system is responsible for stabilizing vision while the head moves and balance control.15 Athletes can experience central and/or peripheral vestibular dysfunction to include benign paroxysmal positional vertigo (BPPV), visual motion sensitivity, vestibular ocular reflex impairment, and balance impairment.30,31 Symptoms typically include dizziness, impaired balance, blurry vision, difficulty focusing, and environmental sensitivity.15,29,30 Potential treatment options include vestibular rehabilitation, exertion therapy, and school/work accommodations.
Ocular. The oculomotor system is responsible for control of eye movements. Athletes can experience many different posttraumatic vision changes, including convergence problems, eye-tracking difficulties, refractive error, difficulty with pursuits/saccades, and accommodation insufficiency. Symptoms typically include light sensitivity, blurred vision, double vision, headaches, fatigue, and memory difficulties.15,29,30 Potential treatment options include vision therapy, vestibular rehabilitation, and school/work accommodations.32
Posttraumatic Migraine. Headache, the most common post-concussion symptom, can persist and meet criteria for posttraumatic migraine (ie, unilateral headache with accompanying nausea and/or photophobia and phonophobia).29,30,33 Implementation of a routine schedule, daily physical activity, exertion therapy, pharmacologic intervention, and school/work accommodations are potential treatment options.
Cervical. The cervical spine can be injured during whiplash-type injuries. Therefore, determining the location, onset, and typical exacerbations of pain can be helpful in identifying cervical involvement.29,30 Symptoms typically include headaches, neck pain, numbness, and tingling. Evaluation and therapy by a certified physical therapist and pharmacologic intervention (eg, muscle relaxants) are potential treatment options. 29,30
Anxiety/Mood. Anxiety, or worry and fear about everyday situations, is common post-concussion and can sometimes be related to ongoing vestibular impairment. Symptoms typically include ruminative thoughts, avoidance of specific situations, hypervigilance, feelings of being overwhelmed, and difficulty falling asleep.29,30 Potential treatment options include implementation of a routine schedule, exposure to provocative situations, psychotherapy, pharmacologic intervention, and school/work accommodations.34
Cognitive/Fatigue. A global concussion factor (including cognitive, fatigue, and migraine symptoms) has been identified within 1 to 7 days of injury. Although this factor of symptoms generally resolves during the acute recovery period, it persists in select cases.13 Symptoms typically include fatigue, decreased energy levels, nonspecific headaches, potential sleep disruption, increased symptoms towards the end of the day, difficulty concentrating, and increased headache with cognitive activities.29,30,35 Routine schedule, daily physical activity, exertion therapy, pharmacologic intervention (eg, amantadine), and school/work accommodations are potential treatment options.30
Conclusion
Advancements in SRC management warrant change in the conversations regarding concussion in football. Specifically, conversations should address the current understanding of concussion and improvements in the safety of football through stricter concussion guidelines, detailed sideline evaluations, recognition of risk factors, improved acute management, and identification of concussion profiles that help to direct individualized treatment plans and targeted rehabilitation strategies. The biggest concerns related to concussions in football include underreporting of injury, premature return to play, and receiving routine rather than individualized treatment. Therefore, to further improve the safety of football and management of concussion it is essential that future efforts focus on the following 6 areas:
Education: Improved understanding of concussion is imperative to reducing poor outcomes and widespread concerns.
Immediate reporting: Reporting of concussion must be expected and encouraged through consistent responses by coaches to reduce underreporting and fear of reporting in athletes.
Prevention techniques: Athletes must be taught proper form and playing techniques to reduce the risk for concussion. Proper form and technique should be incentivized.
Targeted treatment: Individualized treatment plans and targeted rehabilitation strategies must be developed based on the identified clinical profile(s) to avoid nonspecific treatment recommendations.
Multidisciplinary treatment teams: Given the heterogeneous symptoms profiles and need for care provided by different medical specialties, multidisciplinary teams are essential.
Remain current: With the progress in understanding concussion, providers must remain vigilant of future advances in concussion management to further improve the safety of football.
Am J Orthop. 2016;45(6):352-356. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Dompier TP, Kerr ZY, Marshall SW, et al. Incidence of concussion during practice and games in youth, high school, and collegiate American football players. JAMA Pediatrics. 2015;169(7):659-665.
2. Giza C, Hovda D. The new neurometabolic cascade of concussion
3. Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury - an update. Phys Med Rehabil Clin N Am. 2016;27:373-393.
4. Henry L, Elbin R, Collins M, Marchetti G, Kontos A. Examining recovery trajectories after sport-related concussion with a multimodal clinical assessment approach. Neurosurgery. 2016;78(2):232-241.
5. McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Brit J Sports Med. 2013;47(5):250-258.
6. Lovell MR, Collins MW, Iverson GL, et al. Recovery from mild concussion in high school athletes. J Neurosurg. 2003;98(2):296-301.
7. Lovell MR, Collins MW, Iverson GL, Johnston KM, Bradley JP. Grade 1 or “ding” concussions in high school athletes. Am J Sports Med. 2004;32(1):47-54.
8. Baugh CM, Kroshus E, Daneshvar DH, Filali NA, Hiscox MJ, Glantz LH. Concussion management in United States college sports: compliance with National Collegiate Athletic Association concussion policy and areas for improvement. Am J Sports Med. 2015;43(1):47-56.
9. Asken BM, McCrea MA, Clugston JR, Snyder AR, Houck ZM, Bauer RM. “Playing through it”: Delayed reporting and removal from athletic activity after concussion predicts prolonged recovery. J Athl Train. 2016;51(4):329-335.
10. Elbin RJ, Sufrinko A, Schatz P, et al. Athletes that continue to play with concussion demonstrate worse recovery outcomes than athletes immediately removed from play. J Pediatr. In press.
11. Signoretti S, Lazzarino G, Tavazzi B, Vagnozzi R. The pathophysiology of concussion. PM R. 2011;3(10 Suppl 2):S359-S368.
12. Bloom J, Blount JG. Sideline evaluation of concussion. UpToDate. 2016. http://www.uptodate.com/contents/sideline-evaluation-of-concussion. Accessed July 13, 2016.
13. Kontos AP, Elbin RJ, Schatz P, et al. A revised factor structure for the post-concussion symptom scale: baseline and postconcussion factors. Am J Sports Med. 2012;40(10):2375-2384.
14. Guskiewicz KM, Ross SE, Marshall SW. Postural stability and neuropsychological deficits after concussion in collegiate athletes. J Athl Train. 2001;36(3):263.
15. Mucha A, Collins MW, Elbin R, et al. A brief Vestibular/Ocular Motor Screening (VOMS) assessment to evaluate concussions preliminary findings. Am J Sports Med. 2014;42(10):2479-2486.
16. Bloom J. Vestibular and ocular motor assessments: Important pieces to the concussion puzzle. Athletic Training and Sports Health Care. 2013;5(6):246-248.
17. Covassin T, Elbin R, Harris W, Parker T, Kontos A. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. Am J Sports Med. 2012;40(6):1303-1312.
18. Kontos A, Sufrinko A, Elbin R, Puskar A, Collins M. Reliability and associated risk factors for performance on the Vestibular/Ocular Motor Screening (VOMS) tool in healthy collegiate athletes. Am J Sports Med. 2016;44(6):1400-1406.
19. Guskiewicz KM, McCrea M, Marshall SW, et al. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290(19):2549-2555.
20. Lau B, Lovell MR, Collins MW, Pardini J. Neurocognitive and symptom predictors of recovery in high school athletes. Clin J Sport Med. 2009;19(3):216-221.
21. Lau BC, Kontos AP, Collins MW, Mucha A, Lovell MR. Which on-field signs/symptoms predict protracted recovery from sport-related concussion among high school football players? Am J Sports Med. 2011;39(11):2311-2318.
22. Mihalik JP, Register-Mihalik J, Kerr ZY, Marshall SW, McCrea MC, Guskiewicz KM. Recovery of posttraumatic migraine characteristics in patients after mild traumatic brain injury. Am J Sports Med. 2013;41(7):1490-1496.
23. Covassin T, Moran R, Elbin RJ. Sex differences in reported concussion injury rates and time loss from participation: An update of the National Collegiate Athletic Association injury surveillance program from 2004-2005 through 2008-2009. J Athl Train. 2016;51(3):189-194.
24. Root JM, Zuckerbraun NS, Wang L, et al. History of somatization is associated with prolonged recovery from concussion. J Pediatr. 2016;174:39-44.
25. Sufrinko A, Pearce K, Elbin RJ, et al. The effect of preinjury sleep difficulties on neurocognitive impairment and symptoms after sport-related concussion. Am J Sports Med. 2015;43(4):830-838.
26. Thomas DG, Apps JN, Hoffmann RG, McCrea M, Hammeke T. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics. 2015;135(2):213-223.
27. Choe M, Blume H. Pediatric posttraumatic Headache: a review. J Child Neurol. 2016;31(1):76-85.
28. Tepper SJ, Tepper DE. Breaking the cycle of medication overuse. Cleve Clin J Med. 2010;77(4):236-242.
29. Collins M, Kontos A, Reynolds E, Murawski C, Fu F. A comprehensive, targeted approach to the clinical care of athletes following sport-related concussion. Knee Surg Sports Traumatol Arthrosc. 2014;22(2):235-246.
30. Reynolds E, Collins MW, Mucha A, Troutman-Ensecki C. Establishing a clinical service for the management of sports-related concussions. Neurosurgery. 2014;75 Suppl 4:S71-S81.
31. Broglio SP, Collins MW, Williams RM, Mucha A, Kontos AP. Current and emerging rehabilitation for concussion: a review of the evidence. Clin Sports Med. 2015;34(2):213-231.
32. Master C, Scheiman M, Gallaway M, et al. Vision diagnoses are common after concussion in adolescents. Clin Pediatr (Phila). 2016;55(3):260-267.
33. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629-808.
34. Kontos A, Deitrick JM, Reynolds E. Mental health implication and consequences following sport-related concussion. Brit J Sports Med. 2016;50(3):139-140.
35. Kontos AP, Covassin T, Elbin R, Parker T. Depression and neurocognitive performance after concussion among male and female high school and collegiate athletes. Arch Phys Med Rehabil. 2012;93(10):1751-1756.
1. Dompier TP, Kerr ZY, Marshall SW, et al. Incidence of concussion during practice and games in youth, high school, and collegiate American football players. JAMA Pediatrics. 2015;169(7):659-665.
2. Giza C, Hovda D. The new neurometabolic cascade of concussion
3. Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury - an update. Phys Med Rehabil Clin N Am. 2016;27:373-393.
4. Henry L, Elbin R, Collins M, Marchetti G, Kontos A. Examining recovery trajectories after sport-related concussion with a multimodal clinical assessment approach. Neurosurgery. 2016;78(2):232-241.
5. McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Brit J Sports Med. 2013;47(5):250-258.
6. Lovell MR, Collins MW, Iverson GL, et al. Recovery from mild concussion in high school athletes. J Neurosurg. 2003;98(2):296-301.
7. Lovell MR, Collins MW, Iverson GL, Johnston KM, Bradley JP. Grade 1 or “ding” concussions in high school athletes. Am J Sports Med. 2004;32(1):47-54.
8. Baugh CM, Kroshus E, Daneshvar DH, Filali NA, Hiscox MJ, Glantz LH. Concussion management in United States college sports: compliance with National Collegiate Athletic Association concussion policy and areas for improvement. Am J Sports Med. 2015;43(1):47-56.
9. Asken BM, McCrea MA, Clugston JR, Snyder AR, Houck ZM, Bauer RM. “Playing through it”: Delayed reporting and removal from athletic activity after concussion predicts prolonged recovery. J Athl Train. 2016;51(4):329-335.
10. Elbin RJ, Sufrinko A, Schatz P, et al. Athletes that continue to play with concussion demonstrate worse recovery outcomes than athletes immediately removed from play. J Pediatr. In press.
11. Signoretti S, Lazzarino G, Tavazzi B, Vagnozzi R. The pathophysiology of concussion. PM R. 2011;3(10 Suppl 2):S359-S368.
12. Bloom J, Blount JG. Sideline evaluation of concussion. UpToDate. 2016. http://www.uptodate.com/contents/sideline-evaluation-of-concussion. Accessed July 13, 2016.
13. Kontos AP, Elbin RJ, Schatz P, et al. A revised factor structure for the post-concussion symptom scale: baseline and postconcussion factors. Am J Sports Med. 2012;40(10):2375-2384.
14. Guskiewicz KM, Ross SE, Marshall SW. Postural stability and neuropsychological deficits after concussion in collegiate athletes. J Athl Train. 2001;36(3):263.
15. Mucha A, Collins MW, Elbin R, et al. A brief Vestibular/Ocular Motor Screening (VOMS) assessment to evaluate concussions preliminary findings. Am J Sports Med. 2014;42(10):2479-2486.
16. Bloom J. Vestibular and ocular motor assessments: Important pieces to the concussion puzzle. Athletic Training and Sports Health Care. 2013;5(6):246-248.
17. Covassin T, Elbin R, Harris W, Parker T, Kontos A. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. Am J Sports Med. 2012;40(6):1303-1312.
18. Kontos A, Sufrinko A, Elbin R, Puskar A, Collins M. Reliability and associated risk factors for performance on the Vestibular/Ocular Motor Screening (VOMS) tool in healthy collegiate athletes. Am J Sports Med. 2016;44(6):1400-1406.
19. Guskiewicz KM, McCrea M, Marshall SW, et al. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290(19):2549-2555.
20. Lau B, Lovell MR, Collins MW, Pardini J. Neurocognitive and symptom predictors of recovery in high school athletes. Clin J Sport Med. 2009;19(3):216-221.
21. Lau BC, Kontos AP, Collins MW, Mucha A, Lovell MR. Which on-field signs/symptoms predict protracted recovery from sport-related concussion among high school football players? Am J Sports Med. 2011;39(11):2311-2318.
22. Mihalik JP, Register-Mihalik J, Kerr ZY, Marshall SW, McCrea MC, Guskiewicz KM. Recovery of posttraumatic migraine characteristics in patients after mild traumatic brain injury. Am J Sports Med. 2013;41(7):1490-1496.
23. Covassin T, Moran R, Elbin RJ. Sex differences in reported concussion injury rates and time loss from participation: An update of the National Collegiate Athletic Association injury surveillance program from 2004-2005 through 2008-2009. J Athl Train. 2016;51(3):189-194.
24. Root JM, Zuckerbraun NS, Wang L, et al. History of somatization is associated with prolonged recovery from concussion. J Pediatr. 2016;174:39-44.
25. Sufrinko A, Pearce K, Elbin RJ, et al. The effect of preinjury sleep difficulties on neurocognitive impairment and symptoms after sport-related concussion. Am J Sports Med. 2015;43(4):830-838.
26. Thomas DG, Apps JN, Hoffmann RG, McCrea M, Hammeke T. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics. 2015;135(2):213-223.
27. Choe M, Blume H. Pediatric posttraumatic Headache: a review. J Child Neurol. 2016;31(1):76-85.
28. Tepper SJ, Tepper DE. Breaking the cycle of medication overuse. Cleve Clin J Med. 2010;77(4):236-242.
29. Collins M, Kontos A, Reynolds E, Murawski C, Fu F. A comprehensive, targeted approach to the clinical care of athletes following sport-related concussion. Knee Surg Sports Traumatol Arthrosc. 2014;22(2):235-246.
30. Reynolds E, Collins MW, Mucha A, Troutman-Ensecki C. Establishing a clinical service for the management of sports-related concussions. Neurosurgery. 2014;75 Suppl 4:S71-S81.
31. Broglio SP, Collins MW, Williams RM, Mucha A, Kontos AP. Current and emerging rehabilitation for concussion: a review of the evidence. Clin Sports Med. 2015;34(2):213-231.
32. Master C, Scheiman M, Gallaway M, et al. Vision diagnoses are common after concussion in adolescents. Clin Pediatr (Phila). 2016;55(3):260-267.
33. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629-808.
34. Kontos A, Deitrick JM, Reynolds E. Mental health implication and consequences following sport-related concussion. Brit J Sports Med. 2016;50(3):139-140.
35. Kontos AP, Covassin T, Elbin R, Parker T. Depression and neurocognitive performance after concussion among male and female high school and collegiate athletes. Arch Phys Med Rehabil. 2012;93(10):1751-1756.
Exertional Heat Stroke and American Football: What the Team Physician Needs to Know
Football, one of the most popular sports in the United States, is additionally recognized as a leading contributor to sports injury secondary to the contact collision nature of the endeavor. There are an estimated 1.1 million high school football players with another 100,000 participants combined in the National Football League (NFL), college, junior college, Arena Football League, and semipro levels of play.1 USA Football estimates that an additional 3 million youth participate in community football leagues.1 The National Center for Catastrophic Sports Injury Research recently calculated a fatality rate of 0.14 per 100,000 participants in 2014 for the 4.2 million who play football at all levels—and 0.45 per 100,000 in high school.1 While direct deaths from head and spine injury remain a significant contributor to the number of catastrophic injuries, indirect deaths (systemic failure) predominate. Exertional heat stroke (EHS) has emerged as one of the leading indirect causes of death in high school and collegiate football. Boden and colleagues2 reported that high school and college football players sustain approximately 12 fatalities annually, with indirect systemic causes being twice as common as direct blunt trauma.2The most common indirect causes identified included cardiac failure, heat illness, and complications of sickle cell trait (SCT). It was also noted that the risk of SCT, heat-related, and cardiac deaths increased during the second decade of the study, indicating these conditions may require a greater emphasis on diagnosis, treatment, and prevention. This review details for the team physician the unique challenge of exercising in the heat to the football player, and the prevention, diagnosis, management and return-to-play issues pertinent to exertional heat illness (EHI).
The Challenge
EHS represents the most severe manifestation of EHI—a gamut of diseases commonly encountered during the hot summer months when American football season begins. The breadth of EHI includes several important clinical diagnoses: exercise-associated muscle cramps (heat cramps); heat exhaustion with and without syncope; heat injury with evidence of end organ injury (eg, rhabdomyolysis); and EHS. EHS is defined as “a form of hyperthermia associated with a systemic inflammatory response leading to a syndrome of multi-organ dysfunction in which encephalopathy predominates.”3 EHS, if left untreated, or even if clinical treatment is delayed, may result in significant end organ morbidity and/or mortality.
During exercise, the human thermoregulatory system mitigates heat gain by increasing skin blood flow and sweating, causing an increased dissipation of heat to the surrounding environment by leveraging conduction, convection, and evaporation.4,5 Elevated environmental temperatures, increased humidity, and dehydration can impede the body’s ability to dissipate heat at a rate needed to maintain thermoregulation. This imbalance can result in hyperthermia secondary to uncompensated heat stress,5 which in turn can lead to EHI. Football players have unique challenges that make them particularly vulnerable to EHI. The summer heat during early-season participation and the requirement for equipment that covers nearly 60% of body surfaces pose increased risk of volume losses and hyperthermia that trigger the onset of EHI.6 Football athletes’ body compositions and physical size are additional contributing risk factors; the relatively high muscle and fat content increase thermogenicity, which require their bodies to dissipate more heat.7
An estimated 9000 cases of EHI occur annually across all high school sports,8 with an incidence of 1.6:100,000 athlete-exposures.8,9 Studies have demonstrated, however, that EHI occurs in football 11.4 times more often than in all other high school sports combined.10 The incidence of nonfatal EHI in all levels of football is 4.42-5:100,000.8,9 Between 2000 and 2014, 41 football players died from EHS.1 In football, approximately 75% of all EHI events occurred during practices, while only 25% of incidents occurred during games.8
Given these potentially deadly consequences, it is important that football team physicians are not only alert to the early symptoms of heat illness and prepared to intervene to prevent the progression to EHS, but are critical leaders in educating coaches and players in evidence-based EHI prevention practices and policies.
Prevention
EHS is a preventable condition, arguably the most common cause of preventable nontraumatic exertional death in young athletes in the United States. Close attention to mitigating risk factors should begin prior to the onset of preseason practice and continue through the early season, where athletes are at the highest risk of developing heat illness.
Primary Prevention
Primary prevention is fundamental to minimizing the occurrences of EHI. It focuses on the following methods: recognition of inherent risk factors, acclimatization, hydration, and avoidance of inciting substances (including supplements).
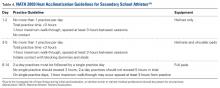
Pre-Participation Examination. The purpose of the pre-participation examination (PPE) is to maximize an athlete’s safety by identifying medical conditions that place the athlete at risk.11,12 The Preparticipation Physical Evaluation, 4th edition, the most widely used consensus publication, specifically queries if an athlete has a previous history of heat injury. However, it only indirectly addresses intrinsic risk factors that may predispose an athlete to EHI who has never had an EHI before. Therefore, providers should take the opportunity of the PPE to inquire about additional risk factors that may make an athlete high risk for sustaining a heat injury. Common risk factors for EHI are listed in Table 1.
Heat Acclimatization. The risk of EHI escalates significantly when athletes are subjected to multiple stressors during periods of heat exposure, such as sudden increases in intensity or duration of exercise; prolonged new exposures to heat; dehydration; and sleep loss.5 When football season begins in late summer, athletes are least conditioned as temperatures reach their seasonal peak, causing increased risk of EHI.15 Planning for heat acclimatization is vital for all athletes who exercise in hot environments. Acclimatization procedures place progressively mounting physiologic strains on the body to improve athletes’ ability to dissipate heat, diminishing thermoregulatory and cardiovascular exertion.4,5 Acclimatization begins with expansion of plasma volume on days 3 to 6, causing improvements in cardiac efficiency and resulting in an overall decrease in basal internal body temperature.4,5,15 This process results in improvements in heat tolerance and exercise performance, evolving over 10 to 14 days of gradual escalation of exercise intensity and duration.5,10,11,16 However, poor fitness levels and extreme temperatures can prolong this period, requiring up to 2 to 3 months to fully take effect.5,7
The National Athletic Trainers Association (NATA) and National Collegiate Athletic Association (NCAA) have released consensus guidelines regarding heat acclimatization protocols for football athletes at the high school and college levels (Tables 3 and 4). Each of these guidelines involves an initial period without use of protective equipment, followed by a gradual addition of further equipment.11,16
Secondary Prevention
Despite physicians’ best efforts to prevent all cases of EHI, athletes will still experience the effects of exercise-induced hyperthermia. The goal of secondary prevention is to slow the progression of this hyperthermia so that it does not progress to more dangerous EHI.
Hydration. Dehydration is an important risk factor for EHI. Sweat maintains thermoregulation by dissipating heat generated during exercise; however, it also contributes to body water losses. Furthermore, intravascular depletion decreases stroke volume, thereby increasing cardiovascular strain. It is estimated that for every 1% loss in body mass from dehydration, body temperature rises 0.22°C in comparison to a euhydrated state.6 Dehydration occurs more rapidly in hot environments, as fluid is lost through increased sweat production.7 After approximately 6% to 10% body weight volume loss, cardiac output cannot be maintained, diminishing sweat production and blood flow to both skin and muscle and causing diminished performance and a significant risk of heat exhaustion.7 If left unchecked, these physiologic changes result in further elevations in body temperature and increased cardiovascular strain, ultimately placing the athlete at significant risk for development of EHS.
Adequate hydration to maintain euvolemia is an important step in avoiding possible EHI. Multiple studies have shown that football players experience a baseline hypovolemia during their competition season,6 a deficit that is most marked during the first week of practices.17 This deficit is multifactorial, as football players expend a significant amount of fluid through sweat, are not able to adequately replace these losses during practice, and do not appropriately hydrate off the field.6,18 Some players, especially linemen, sweat at a higher rate than their teammates, posing a possible risk of significant dehydration.6 Coaches and players alike should be educated on the importance of adequate hydration to meet their fluid needs.
The goal of hydration during exercise is to prevent large fluid losses that can adversely affect performance and increase risk of EHI;6 it may be unrealistic to replace all fluid losses during the practice period. Instead, athletes should target complete volume replacement over the post-exercise period.6 Some recommend hydrating based upon thirst drive; however, thirst is activated following a volume loss of approximately 2% body mass, the same degree of losses that place athletes at an increased risk for performance impairment and EHI.4,6,11,12 Individuals should have access to fluids throughout practice and competition and be encouraged to hydrate as needed.6,12,15 Furthermore, staff should modify their practices based upon WBGT and acclimatization status to provide more frequent hydration breaks.
Hyperhydration and Salt Intake. Of note, there are inherent risks to hyperhydration. Athletes with low sweat rates have an increased risk of overhydration and the development of exercise-associated hyponatremia (EAH),6 a condition whose presentation is very similar to EHS. In addition, inadequate sodium intake and excessive sweating can also contribute to the development of EAH. EAH has been implicated in the deaths of 2 football players in 2014.1,6 Establishing team hydration guidelines and educating players and staff on appropriate hydration and dietary salt intake is essential to reduce the risk of both dehydration and hyperhydration and their complications.6Intra-Event Cooling. During exercise, team physicians can employ strategies for cooling athletes during exertion to mitigate their risk of EHI by decreasing thermal and cardiovascular strain.4,19 Cooling during exercise is hypothesized to allow for accelerated heat dissipation, where heat is lost from the body more effectively. This accelerated loss enables athletes to maintain a higher heat storage capacity over the duration of exercise, avoiding uncompensated heat stresses that ultimately cause EHI.19
Some intra-event cooling strategies include the use of cooling garments, cooling packs, and cold water/slurry ingestion. Cooling garments lower skin temperature, which in turn can decrease thermoregulatory strains;4 a recent meta-analysis of intra-event cooling modalities revealed that wearing an ice vest during exercise resulted in the greatest decrease in thermal heat strain.19 Internal cooling strategies—namely ingestion of cold fluids/ice slurry—have shown some mild benefit in decreasing internal temperatures; however, some studies have demonstrated some decrease in sweat production associated with cold oral intake used in isolation.19 Overall, studies have shown that combining external (cooling clothing, ice packs, fanning) and internal (cold water, ice slurry) cooling methods result in a greater cooling effect than a use of a single method.4
Tertiary Prevention
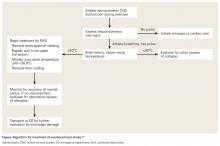
The goal of tertiary prevention is to mitigate the risk of long-term adverse outcomes following an EHS event. The most effective means of reducing risk for morbidity and mortality is rapid identification and treatment of EHS as well as close evaluation of an athlete’s return to activity in heat. This process is spearheaded by an effective and well-rehearsed emergency action plan.
Diagnosis and Management
Rapid identification and treatment of EHS is crucial to minimizing the risk of poor outcomes.7 Any delay in the treatment of EHS can dramatically increase the likelihood of associated morbidity and mortality.20
EHS is diagnosed by an elevated rectal temperature ≥40°C (104°F) and associated central nervous system (CNS) dysfunction.21 EHS should be strongly suspected in any athlete exercising in heat who exhibits signs of CNS dysfunction, including disorientation, confusion, dizziness, erratic behavior, irritability, headache, loss of coordination, delirium, collapse, or seizures.7,12,15 EHS may also present with symptoms of heat exhaustion, including fatigue, hyperventilation, tachycardia, vomiting, diarrhea, and hypotension.7,12,15
Rectal temperature should be taken for any athlete with suspected EHS, as other modalities—oral, skin, axillary, and aural—can be inaccurate and easily modified by ambient confounders such as ambient and skin temperature, athlete hyperventilation, and consumption of liquids.7,11,12 Athletes exhibiting CNS symptoms with moderately elevated rectal temperatures that do not exceed 40°C should also be assumed to be suffering from EHS and treated with rapid cooling.11 On the other hand, athletes with CNS symptoms who are normothermic should be assumed to have EAH until ruled out by electrolyte assessment; IV fluids should be at no more than keep vein open (KVO) pending this determination.11 In some cases, an athlete may initially present with altered mental status but return to “normal.” However, this improvement may represent a “lucid period”; evaluation should continue with rectal temperature and treatment, as EHS in these cases may progress quickly.15
Treatment is centered on rapid, whole body cooling initiated at the first sign of heat illness.7,22 The goal of treatment is to achieve a rectal temperature <38.9°C within 30 minutes of the onset of EHS.15 Upon diagnosis, the athlete should be quickly placed in a tub of ice water to facilitate cold water immersion (CWI) therapy. Some guidelines suggest the athlete’s clothing be removed to potentiate evaporative cooling during CWI;12 however, cooling should not be delayed due to difficulties in removing equipment. CWI, where a heat stroke victim is submerged in ice water up to their neck while water is continuously circulated, is generally considered to be the gold standard treatment as it is the modality with the highest recorded cooling rates and the lowest rate of morbidity and mortality.7,20,21 Multiple studies of CWI have shown that survival nears 100% when aggressive cooling starts within 5 minutes of collapse or identification of EHS.20,21,22
If whole body CWI is unavailable, alternative methods of rapid cooling should be employed. Partial CWI, with torso immersion being preferable to the extremities, has been shown to achieve an acceptable rate of cooling to achieve sufficient drops in internal body temperature.20,23 However, one popular treatment—applying ice packs to the whole body, in particular to the groin and axillae—has not been shown to be sufficient to achieve standard cooling goals.20 None of these methods have been shown to be as effective as CWI.23
Intravenous access should be initiated with fluid resuscitation dictated by the provider’s assessment. Normal saline is recommended as the resuscitative fluid of choice, with the rate dictated by clinical judgment and adjusted as guided by electrolyte determination and clinical response. It cannot be overstated that in normothermic patients with confusion, EAH is the diagnosis of exclusion and aggressive fluid resuscitation should be withheld until electrolyte determination.
Once rectal temperature descends appropriately (~38.9°C), the cooling process should stop and the individual should be transported to a hospital for further observation20 and evaluation of possible sequelae, including rhabdomyolysis and renal injury, cardiac dysfunction and arrhythmia, severe electrolyte abnormalities, acute respiratory distress syndrome, lactic acidosis, and other forms of end-organ failure (Figure).
Rapid cooling is more crucial than transport; transport poses a risk of delayed cooling, which can dramatically increase an individual’s risk of morbidity and mortality.20,23 In situations where a patient can be cooled on-site, physicians should pursue cooling before transporting the patient to a medical treatment facility.
Emergency Action Plan
Team physicians should be proactive in developing an emergency action plan to address possible EHS events. These plans should be site-specific, addressing procedures for all practice and home competition locations.12 All competition venues should have a CWI tub on-site in events where there is an increased risk of EHS.12,15,20 This tub should be set up and functional for all high-risk activities, including practices.12
Following recognition of a potential case of EHS, treatment teams should have procedures in place to transport athletes to the treatment area, obtain rectal temperature, initiate rapid cooling, and stabilize the athlete for transport to an emergency department (ED) for further evaluation.12,15 A written record of treatments and medications provided during athlete stabilization should be maintained and transported with the athlete to the ED.15 A list of helpful equipment and supplies for treatment of EHS can be found in Table 5.
EHS is a unique life-threatening situation where it is best to treat the patient on the sideline before transport.15 Those athletes transported before cooling risk spending an increased amount of time above critical temperatures for cell damage, which has been associated with increased morbidity and mortality. This mantra of “cool first, transport second” cannot be overemphasized, as those individuals with EHS who present to the ED with a persisting rectal temperature >41°F may risk up to an 80% mortality rate.24 Conversely, a recent large, retrospective study of 274 EHS events sustained during the Falmouth Road Race found a 100% survival rate when athletes were rapidly identified via rectal thermometry and treated with aggressive, rapid cooling through CWI.21
Return to Play
Perhaps the most challenging and important role the team physician has is determining an athlete’s return to play following EHI, as there currently are no evidence-based guidelines for return to activity for these athletes.7 The decisions surrounding return to play are highly individualized, as recovery from EHS and heat injury is associated with the duration of internal body temperature elevation above the critical level (40°C).7,20 Guidelines for return to activity following recovery from EHI differ among experts and institutions.7,25 The general consensus from these guidelines is that, at minimum, athletes should not participate in any physical activity until they are asymptomatic and all blood tests have normalized.11 Following this asymptomatic period, most guidelines advocate for a slow, deliberate return to activity.11 The American College of Sports Medicine (ACSM) offers one reasonable approach to the returning athlete following EHS:7
- No exercise for at least 7 days following release from medical care.
- Follow-up with a physician 1 week after release from medical care for physical examination and any warranted lab or radiologic studies (based upon organ systems affected during EHS).
- Once cleared to return to activity, the athlete begins exercise in a cool environment, gradually increasing the duration, intensity, and heat exposure over 2 weeks to demonstrate heat tolerance and acclimatization.
- Athletes who cannot resume vigorous activity due to recurrent symptoms (eg, excessive fatigue) should be reevaluated after 4 weeks. Laboratory exercise-heat tolerance testing may be useful in this setting.
- The athlete may resume full competition once they are able to participate in full training in the heat for 2 to 4 weeks without adverse effects.
Heat tolerance testing (HTT) in these athletes remains controversial.5 26 The ACSM recommends that HTT be considered only for those unable to return to vigorous activity after a suitable period (approximately 4 weeks). In contrast, the Israeli Defense Force (IDF) uses HTT to evaluate soldiers following EHS to guide decision-making about return to duty.27 The IDF HTT assumes that individuals will respond differently to heat stresses. They identify individuals who are “heat intolerant” as being unable to tolerate specific heat challenges, indicated by increases in body temperature occurring more rapidly than normal responders under identical environmental and exercise conditions. However, despite being used for more than 30 years, there is no clear evidence that HTT adequately predicts who will experience subsequent episodes of EHS.
Conclusion
While the recognized cornerstone of being a team physician is the provision of medical care, the ACSM Team Physician Consensus Statement28 further delineates the medical and administrative responsibilities as both (1) understanding medical management and prevention of injury and illness in athletes; and (2) awareness of or involvement in the development and rehearsal of an emergency action plan. These tenets are critical for the team physician who accepts the responsibility to cover sports at the high school level or higher. Football team physicians play an essential role in mitigating risk of EHI in their athletes. Through development and execution of both comprehensive prevention strategies and emergency action plans, physicians can work to minimize athletes’ risk of both developing and experiencing significant adverse outcomes from an EHI.
Am J Orthop. 2016;45(6):340-348. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Kucera KL, Klossner D, Colgate B, Cantu RC. Annual Survey of Football Injury Research: 1931-2014. National Center for Catastrophic Sport Injury Research Web site. https://nccsir.unc.edu/files/2013/10/Annual-Football-2014-Fatalities-Final.pdf. Accessed May 31, 2016.
2. Boden BP, Breit I, Beachler JA, Williams A, Mueller FO. Fatalities in high school and college football players. Am J Sports Med. 2013;41(5):1108-1116.
3. Bouchama A, Knochel JP. Heat stroke. N Engl J Med. 2002;346(25):1978-1988.
4. Racinais S, Alonso JM, Coutts AJ, et al. Consensus recommendations on training and competing in the heat. Scand J Med Sci Sports. 2015;25 Suppl 1:6-19.
5. Pryor RR, Casa DJ, Adams WM, et al. Maximizing athletic performance in the heat. Strength Cond J. 2013;35(6):24-33.
6. Adams WM, Casa DJ. Hydration for football athletes. Sports Sci Exchange. 2015;28(141):1-5.
7. American College of Sports Medicine, Armstrong LE, Casa DJ, et al. American College of Sports Medicine position stand. Exertional heat illness during training and competition. Med Sci Sports Exerc. 2007;39(3):556-572.
8. Yard EE, Gilchrist J, Haileyesus T, et al. Heat illness among high school athletes--United States, 2005-2009. J Safety Res. 2010;41(6):471-474.
9. Huffman EA, Yard EE, Fields SK, Collins CL, Comstock RD. Epidemiology of rare injuries and conditions among United States high school athletes during the 2005-2006 and 2006-2007 school years. J Athl Train. 2008;43(6):624-630.
10. Kerr ZY, Casa DJ, Marshall SW, Comstock RD. Epidemiology of exertional heat illness among U.S. high school athletes. Am J Prev Med. 2013;44(1):8-14.
11. Casa DJ, DeMartini JK, Bergeron MF, et al. National Athletic Trainers’ Association position statement: exertional heat illnesses. J Athl Train. 2015;50(9):986-1000.
12. Casa DJ, Almquist J, Anderson SA. The inter-association task force for preventing sudden death in secondary school athletics programs: best-practices recommendations. J Athl Train. 2013;48(4):546-553.
13. Gardner JW, Kark JA, Karnei K, et al. Risk factors predicting exertional heat illness in male Marine Corps recruits. Med Sci Sports Exerc. 1996;28(8):939-944.
14. Gundstein AJ, Ramseyer C, Zhao F, et al. A retrospective analysis of American football hyperthermia deaths in the United States. Int J Biometerol. 2012;56(1):11-20.
15. Armstrong LE, Johnson EC, Casa DJ, et al. The American football uniform: uncompensable heat stress and hyperthermic exhaustion. J Athl Train. 2010;45(2):117-127.
16. Casa DJ, Csillan D; Inter-Association Task Force for Preseason Secondary School Athletics Participants, et al. Preseason heat-acclimatization guidelines for secondary school athletics. J Athl Train. 2009;44(3):332-333.
17. Godek SF, Godek JJ, Bartolozzi AR. Hydration status in college football players during consecutive days of twice-a-day preseason practices. Am J Sports Med. 2005;33(6):843-851.
18. Stover EA, Zachwieja J, Stofan J, Murray R, Horswill CA. Consistently high urine specific gravity in adolescent American football players and the impact of an acute drinking strategy. Int J Sports Med. 2006;27(4):330-335.
19. Bongers CC, Thijssen DH, Veltmeijer MTW, Hopman MT, Eijsvogels TM. Precooling and percooling (cooling during exercise) both improve performance in the heat: a meta-analytical review. Br J Sports Med. 2015;49(6):377-384.
20. Casa DJ, McDermott BP, Lee EC, Yeargin SW, Armstrong LE, Maresh CM. Cold water immersion: the gold standard for exertional heatstroke treatment. Exerc Sport Sci Rev. 2007;35(3):141-149.
21. DeMartini JK, Casa DJ, Stearns R, et al. Effectiveness of cold water immersion in the treatment of exertional heat stroke at the Falmouth Road Race. Med Sci Sports Exerc. 2015;47(2):240-245.
22. Casa DJ, Kenny GP, Taylor NA. Immersion treatment for exertional hyperthermia: cold or temperate water? Med Sci Sports Exerc. 2010;42(7):1246-1252.
23. Casa DJ, Armstrong LE, Kenny GP, O’Connor FG, Huggins RA. Exertional heat stroke: new concepts regarding cause and care. Curr Sports Med Rep. 2012;11(3):115-122.
24. Argaud L, Ferry T, Le QH, et al. Short- and long-term outcomes of heat stroke following the 2003 heat wave in Lyon, France. Arch Intern Med. 2007;167(20):2177-2183.
25. O’Connor FG, Casa DJ, Bergeron MF, et al. American College of Sports Medicine Roundtable on exertional heat stroke--return to duty/return to play: conference proceedings. Curr Sports Med Rep. 2010;9(5):314-321.
26. Kazman JB, Heled Y, Lisman PJ, Druyan A, Deuster PA, O’Connor FG. Exertional heat illness: the role of heat tolerance testing. Curr Sports Med Rep. 2013;12(2):101-105.
27. Moran DS, Heled Y, Still L, Laor A, Shapiro Y. Assessment of heat tolerance for post exertional heat stroke individuals. Med Sci Monit. 2004;10(6):CR252-CR257.
28. Herring SA, Kibler WB, Putukian M. Team Physician Consensus Statement: 2013 update. Med Sci Sports Exerc. 2013;45(8):1618-1622.
29. Heat stroke treatment. Korey Stringer Institute University of Connecticut Web site. http://ksi.uconn.edu/emergency-conditions/heat-illnesses/exertional-heat-stroke/heat-stroke-treatment/. Accessed June 14, 2016.
30. Headquarters, Department of the Army and the Air Force. Heat Stress Control and Heat Casualty Management. Technical Bulletin Medical 507. http://www.dir.ca.gov/oshsb/documents/Heat_illness_prevention_tbmed507.pdf. Published March 7, 2003. Accessed June 14, 2016.
Football, one of the most popular sports in the United States, is additionally recognized as a leading contributor to sports injury secondary to the contact collision nature of the endeavor. There are an estimated 1.1 million high school football players with another 100,000 participants combined in the National Football League (NFL), college, junior college, Arena Football League, and semipro levels of play.1 USA Football estimates that an additional 3 million youth participate in community football leagues.1 The National Center for Catastrophic Sports Injury Research recently calculated a fatality rate of 0.14 per 100,000 participants in 2014 for the 4.2 million who play football at all levels—and 0.45 per 100,000 in high school.1 While direct deaths from head and spine injury remain a significant contributor to the number of catastrophic injuries, indirect deaths (systemic failure) predominate. Exertional heat stroke (EHS) has emerged as one of the leading indirect causes of death in high school and collegiate football. Boden and colleagues2 reported that high school and college football players sustain approximately 12 fatalities annually, with indirect systemic causes being twice as common as direct blunt trauma.2The most common indirect causes identified included cardiac failure, heat illness, and complications of sickle cell trait (SCT). It was also noted that the risk of SCT, heat-related, and cardiac deaths increased during the second decade of the study, indicating these conditions may require a greater emphasis on diagnosis, treatment, and prevention. This review details for the team physician the unique challenge of exercising in the heat to the football player, and the prevention, diagnosis, management and return-to-play issues pertinent to exertional heat illness (EHI).
The Challenge
EHS represents the most severe manifestation of EHI—a gamut of diseases commonly encountered during the hot summer months when American football season begins. The breadth of EHI includes several important clinical diagnoses: exercise-associated muscle cramps (heat cramps); heat exhaustion with and without syncope; heat injury with evidence of end organ injury (eg, rhabdomyolysis); and EHS. EHS is defined as “a form of hyperthermia associated with a systemic inflammatory response leading to a syndrome of multi-organ dysfunction in which encephalopathy predominates.”3 EHS, if left untreated, or even if clinical treatment is delayed, may result in significant end organ morbidity and/or mortality.
During exercise, the human thermoregulatory system mitigates heat gain by increasing skin blood flow and sweating, causing an increased dissipation of heat to the surrounding environment by leveraging conduction, convection, and evaporation.4,5 Elevated environmental temperatures, increased humidity, and dehydration can impede the body’s ability to dissipate heat at a rate needed to maintain thermoregulation. This imbalance can result in hyperthermia secondary to uncompensated heat stress,5 which in turn can lead to EHI. Football players have unique challenges that make them particularly vulnerable to EHI. The summer heat during early-season participation and the requirement for equipment that covers nearly 60% of body surfaces pose increased risk of volume losses and hyperthermia that trigger the onset of EHI.6 Football athletes’ body compositions and physical size are additional contributing risk factors; the relatively high muscle and fat content increase thermogenicity, which require their bodies to dissipate more heat.7
An estimated 9000 cases of EHI occur annually across all high school sports,8 with an incidence of 1.6:100,000 athlete-exposures.8,9 Studies have demonstrated, however, that EHI occurs in football 11.4 times more often than in all other high school sports combined.10 The incidence of nonfatal EHI in all levels of football is 4.42-5:100,000.8,9 Between 2000 and 2014, 41 football players died from EHS.1 In football, approximately 75% of all EHI events occurred during practices, while only 25% of incidents occurred during games.8
Given these potentially deadly consequences, it is important that football team physicians are not only alert to the early symptoms of heat illness and prepared to intervene to prevent the progression to EHS, but are critical leaders in educating coaches and players in evidence-based EHI prevention practices and policies.
Prevention
EHS is a preventable condition, arguably the most common cause of preventable nontraumatic exertional death in young athletes in the United States. Close attention to mitigating risk factors should begin prior to the onset of preseason practice and continue through the early season, where athletes are at the highest risk of developing heat illness.
Primary Prevention
Primary prevention is fundamental to minimizing the occurrences of EHI. It focuses on the following methods: recognition of inherent risk factors, acclimatization, hydration, and avoidance of inciting substances (including supplements).
Pre-Participation Examination. The purpose of the pre-participation examination (PPE) is to maximize an athlete’s safety by identifying medical conditions that place the athlete at risk.11,12 The Preparticipation Physical Evaluation, 4th edition, the most widely used consensus publication, specifically queries if an athlete has a previous history of heat injury. However, it only indirectly addresses intrinsic risk factors that may predispose an athlete to EHI who has never had an EHI before. Therefore, providers should take the opportunity of the PPE to inquire about additional risk factors that may make an athlete high risk for sustaining a heat injury. Common risk factors for EHI are listed in Table 1.
Heat Acclimatization. The risk of EHI escalates significantly when athletes are subjected to multiple stressors during periods of heat exposure, such as sudden increases in intensity or duration of exercise; prolonged new exposures to heat; dehydration; and sleep loss.5 When football season begins in late summer, athletes are least conditioned as temperatures reach their seasonal peak, causing increased risk of EHI.15 Planning for heat acclimatization is vital for all athletes who exercise in hot environments. Acclimatization procedures place progressively mounting physiologic strains on the body to improve athletes’ ability to dissipate heat, diminishing thermoregulatory and cardiovascular exertion.4,5 Acclimatization begins with expansion of plasma volume on days 3 to 6, causing improvements in cardiac efficiency and resulting in an overall decrease in basal internal body temperature.4,5,15 This process results in improvements in heat tolerance and exercise performance, evolving over 10 to 14 days of gradual escalation of exercise intensity and duration.5,10,11,16 However, poor fitness levels and extreme temperatures can prolong this period, requiring up to 2 to 3 months to fully take effect.5,7
The National Athletic Trainers Association (NATA) and National Collegiate Athletic Association (NCAA) have released consensus guidelines regarding heat acclimatization protocols for football athletes at the high school and college levels (Tables 3 and 4). Each of these guidelines involves an initial period without use of protective equipment, followed by a gradual addition of further equipment.11,16
Secondary Prevention
Despite physicians’ best efforts to prevent all cases of EHI, athletes will still experience the effects of exercise-induced hyperthermia. The goal of secondary prevention is to slow the progression of this hyperthermia so that it does not progress to more dangerous EHI.
Hydration. Dehydration is an important risk factor for EHI. Sweat maintains thermoregulation by dissipating heat generated during exercise; however, it also contributes to body water losses. Furthermore, intravascular depletion decreases stroke volume, thereby increasing cardiovascular strain. It is estimated that for every 1% loss in body mass from dehydration, body temperature rises 0.22°C in comparison to a euhydrated state.6 Dehydration occurs more rapidly in hot environments, as fluid is lost through increased sweat production.7 After approximately 6% to 10% body weight volume loss, cardiac output cannot be maintained, diminishing sweat production and blood flow to both skin and muscle and causing diminished performance and a significant risk of heat exhaustion.7 If left unchecked, these physiologic changes result in further elevations in body temperature and increased cardiovascular strain, ultimately placing the athlete at significant risk for development of EHS.
Adequate hydration to maintain euvolemia is an important step in avoiding possible EHI. Multiple studies have shown that football players experience a baseline hypovolemia during their competition season,6 a deficit that is most marked during the first week of practices.17 This deficit is multifactorial, as football players expend a significant amount of fluid through sweat, are not able to adequately replace these losses during practice, and do not appropriately hydrate off the field.6,18 Some players, especially linemen, sweat at a higher rate than their teammates, posing a possible risk of significant dehydration.6 Coaches and players alike should be educated on the importance of adequate hydration to meet their fluid needs.
The goal of hydration during exercise is to prevent large fluid losses that can adversely affect performance and increase risk of EHI;6 it may be unrealistic to replace all fluid losses during the practice period. Instead, athletes should target complete volume replacement over the post-exercise period.6 Some recommend hydrating based upon thirst drive; however, thirst is activated following a volume loss of approximately 2% body mass, the same degree of losses that place athletes at an increased risk for performance impairment and EHI.4,6,11,12 Individuals should have access to fluids throughout practice and competition and be encouraged to hydrate as needed.6,12,15 Furthermore, staff should modify their practices based upon WBGT and acclimatization status to provide more frequent hydration breaks.
Hyperhydration and Salt Intake. Of note, there are inherent risks to hyperhydration. Athletes with low sweat rates have an increased risk of overhydration and the development of exercise-associated hyponatremia (EAH),6 a condition whose presentation is very similar to EHS. In addition, inadequate sodium intake and excessive sweating can also contribute to the development of EAH. EAH has been implicated in the deaths of 2 football players in 2014.1,6 Establishing team hydration guidelines and educating players and staff on appropriate hydration and dietary salt intake is essential to reduce the risk of both dehydration and hyperhydration and their complications.6Intra-Event Cooling. During exercise, team physicians can employ strategies for cooling athletes during exertion to mitigate their risk of EHI by decreasing thermal and cardiovascular strain.4,19 Cooling during exercise is hypothesized to allow for accelerated heat dissipation, where heat is lost from the body more effectively. This accelerated loss enables athletes to maintain a higher heat storage capacity over the duration of exercise, avoiding uncompensated heat stresses that ultimately cause EHI.19
Some intra-event cooling strategies include the use of cooling garments, cooling packs, and cold water/slurry ingestion. Cooling garments lower skin temperature, which in turn can decrease thermoregulatory strains;4 a recent meta-analysis of intra-event cooling modalities revealed that wearing an ice vest during exercise resulted in the greatest decrease in thermal heat strain.19 Internal cooling strategies—namely ingestion of cold fluids/ice slurry—have shown some mild benefit in decreasing internal temperatures; however, some studies have demonstrated some decrease in sweat production associated with cold oral intake used in isolation.19 Overall, studies have shown that combining external (cooling clothing, ice packs, fanning) and internal (cold water, ice slurry) cooling methods result in a greater cooling effect than a use of a single method.4
Tertiary Prevention
The goal of tertiary prevention is to mitigate the risk of long-term adverse outcomes following an EHS event. The most effective means of reducing risk for morbidity and mortality is rapid identification and treatment of EHS as well as close evaluation of an athlete’s return to activity in heat. This process is spearheaded by an effective and well-rehearsed emergency action plan.
Diagnosis and Management
Rapid identification and treatment of EHS is crucial to minimizing the risk of poor outcomes.7 Any delay in the treatment of EHS can dramatically increase the likelihood of associated morbidity and mortality.20
EHS is diagnosed by an elevated rectal temperature ≥40°C (104°F) and associated central nervous system (CNS) dysfunction.21 EHS should be strongly suspected in any athlete exercising in heat who exhibits signs of CNS dysfunction, including disorientation, confusion, dizziness, erratic behavior, irritability, headache, loss of coordination, delirium, collapse, or seizures.7,12,15 EHS may also present with symptoms of heat exhaustion, including fatigue, hyperventilation, tachycardia, vomiting, diarrhea, and hypotension.7,12,15
Rectal temperature should be taken for any athlete with suspected EHS, as other modalities—oral, skin, axillary, and aural—can be inaccurate and easily modified by ambient confounders such as ambient and skin temperature, athlete hyperventilation, and consumption of liquids.7,11,12 Athletes exhibiting CNS symptoms with moderately elevated rectal temperatures that do not exceed 40°C should also be assumed to be suffering from EHS and treated with rapid cooling.11 On the other hand, athletes with CNS symptoms who are normothermic should be assumed to have EAH until ruled out by electrolyte assessment; IV fluids should be at no more than keep vein open (KVO) pending this determination.11 In some cases, an athlete may initially present with altered mental status but return to “normal.” However, this improvement may represent a “lucid period”; evaluation should continue with rectal temperature and treatment, as EHS in these cases may progress quickly.15
Treatment is centered on rapid, whole body cooling initiated at the first sign of heat illness.7,22 The goal of treatment is to achieve a rectal temperature <38.9°C within 30 minutes of the onset of EHS.15 Upon diagnosis, the athlete should be quickly placed in a tub of ice water to facilitate cold water immersion (CWI) therapy. Some guidelines suggest the athlete’s clothing be removed to potentiate evaporative cooling during CWI;12 however, cooling should not be delayed due to difficulties in removing equipment. CWI, where a heat stroke victim is submerged in ice water up to their neck while water is continuously circulated, is generally considered to be the gold standard treatment as it is the modality with the highest recorded cooling rates and the lowest rate of morbidity and mortality.7,20,21 Multiple studies of CWI have shown that survival nears 100% when aggressive cooling starts within 5 minutes of collapse or identification of EHS.20,21,22
If whole body CWI is unavailable, alternative methods of rapid cooling should be employed. Partial CWI, with torso immersion being preferable to the extremities, has been shown to achieve an acceptable rate of cooling to achieve sufficient drops in internal body temperature.20,23 However, one popular treatment—applying ice packs to the whole body, in particular to the groin and axillae—has not been shown to be sufficient to achieve standard cooling goals.20 None of these methods have been shown to be as effective as CWI.23
Intravenous access should be initiated with fluid resuscitation dictated by the provider’s assessment. Normal saline is recommended as the resuscitative fluid of choice, with the rate dictated by clinical judgment and adjusted as guided by electrolyte determination and clinical response. It cannot be overstated that in normothermic patients with confusion, EAH is the diagnosis of exclusion and aggressive fluid resuscitation should be withheld until electrolyte determination.
Once rectal temperature descends appropriately (~38.9°C), the cooling process should stop and the individual should be transported to a hospital for further observation20 and evaluation of possible sequelae, including rhabdomyolysis and renal injury, cardiac dysfunction and arrhythmia, severe electrolyte abnormalities, acute respiratory distress syndrome, lactic acidosis, and other forms of end-organ failure (Figure).
Rapid cooling is more crucial than transport; transport poses a risk of delayed cooling, which can dramatically increase an individual’s risk of morbidity and mortality.20,23 In situations where a patient can be cooled on-site, physicians should pursue cooling before transporting the patient to a medical treatment facility.
Emergency Action Plan
Team physicians should be proactive in developing an emergency action plan to address possible EHS events. These plans should be site-specific, addressing procedures for all practice and home competition locations.12 All competition venues should have a CWI tub on-site in events where there is an increased risk of EHS.12,15,20 This tub should be set up and functional for all high-risk activities, including practices.12
Following recognition of a potential case of EHS, treatment teams should have procedures in place to transport athletes to the treatment area, obtain rectal temperature, initiate rapid cooling, and stabilize the athlete for transport to an emergency department (ED) for further evaluation.12,15 A written record of treatments and medications provided during athlete stabilization should be maintained and transported with the athlete to the ED.15 A list of helpful equipment and supplies for treatment of EHS can be found in Table 5.
EHS is a unique life-threatening situation where it is best to treat the patient on the sideline before transport.15 Those athletes transported before cooling risk spending an increased amount of time above critical temperatures for cell damage, which has been associated with increased morbidity and mortality. This mantra of “cool first, transport second” cannot be overemphasized, as those individuals with EHS who present to the ED with a persisting rectal temperature >41°F may risk up to an 80% mortality rate.24 Conversely, a recent large, retrospective study of 274 EHS events sustained during the Falmouth Road Race found a 100% survival rate when athletes were rapidly identified via rectal thermometry and treated with aggressive, rapid cooling through CWI.21
Return to Play
Perhaps the most challenging and important role the team physician has is determining an athlete’s return to play following EHI, as there currently are no evidence-based guidelines for return to activity for these athletes.7 The decisions surrounding return to play are highly individualized, as recovery from EHS and heat injury is associated with the duration of internal body temperature elevation above the critical level (40°C).7,20 Guidelines for return to activity following recovery from EHI differ among experts and institutions.7,25 The general consensus from these guidelines is that, at minimum, athletes should not participate in any physical activity until they are asymptomatic and all blood tests have normalized.11 Following this asymptomatic period, most guidelines advocate for a slow, deliberate return to activity.11 The American College of Sports Medicine (ACSM) offers one reasonable approach to the returning athlete following EHS:7
- No exercise for at least 7 days following release from medical care.
- Follow-up with a physician 1 week after release from medical care for physical examination and any warranted lab or radiologic studies (based upon organ systems affected during EHS).
- Once cleared to return to activity, the athlete begins exercise in a cool environment, gradually increasing the duration, intensity, and heat exposure over 2 weeks to demonstrate heat tolerance and acclimatization.
- Athletes who cannot resume vigorous activity due to recurrent symptoms (eg, excessive fatigue) should be reevaluated after 4 weeks. Laboratory exercise-heat tolerance testing may be useful in this setting.
- The athlete may resume full competition once they are able to participate in full training in the heat for 2 to 4 weeks without adverse effects.
Heat tolerance testing (HTT) in these athletes remains controversial.5 26 The ACSM recommends that HTT be considered only for those unable to return to vigorous activity after a suitable period (approximately 4 weeks). In contrast, the Israeli Defense Force (IDF) uses HTT to evaluate soldiers following EHS to guide decision-making about return to duty.27 The IDF HTT assumes that individuals will respond differently to heat stresses. They identify individuals who are “heat intolerant” as being unable to tolerate specific heat challenges, indicated by increases in body temperature occurring more rapidly than normal responders under identical environmental and exercise conditions. However, despite being used for more than 30 years, there is no clear evidence that HTT adequately predicts who will experience subsequent episodes of EHS.
Conclusion
While the recognized cornerstone of being a team physician is the provision of medical care, the ACSM Team Physician Consensus Statement28 further delineates the medical and administrative responsibilities as both (1) understanding medical management and prevention of injury and illness in athletes; and (2) awareness of or involvement in the development and rehearsal of an emergency action plan. These tenets are critical for the team physician who accepts the responsibility to cover sports at the high school level or higher. Football team physicians play an essential role in mitigating risk of EHI in their athletes. Through development and execution of both comprehensive prevention strategies and emergency action plans, physicians can work to minimize athletes’ risk of both developing and experiencing significant adverse outcomes from an EHI.
Am J Orthop. 2016;45(6):340-348. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Football, one of the most popular sports in the United States, is additionally recognized as a leading contributor to sports injury secondary to the contact collision nature of the endeavor. There are an estimated 1.1 million high school football players with another 100,000 participants combined in the National Football League (NFL), college, junior college, Arena Football League, and semipro levels of play.1 USA Football estimates that an additional 3 million youth participate in community football leagues.1 The National Center for Catastrophic Sports Injury Research recently calculated a fatality rate of 0.14 per 100,000 participants in 2014 for the 4.2 million who play football at all levels—and 0.45 per 100,000 in high school.1 While direct deaths from head and spine injury remain a significant contributor to the number of catastrophic injuries, indirect deaths (systemic failure) predominate. Exertional heat stroke (EHS) has emerged as one of the leading indirect causes of death in high school and collegiate football. Boden and colleagues2 reported that high school and college football players sustain approximately 12 fatalities annually, with indirect systemic causes being twice as common as direct blunt trauma.2The most common indirect causes identified included cardiac failure, heat illness, and complications of sickle cell trait (SCT). It was also noted that the risk of SCT, heat-related, and cardiac deaths increased during the second decade of the study, indicating these conditions may require a greater emphasis on diagnosis, treatment, and prevention. This review details for the team physician the unique challenge of exercising in the heat to the football player, and the prevention, diagnosis, management and return-to-play issues pertinent to exertional heat illness (EHI).
The Challenge
EHS represents the most severe manifestation of EHI—a gamut of diseases commonly encountered during the hot summer months when American football season begins. The breadth of EHI includes several important clinical diagnoses: exercise-associated muscle cramps (heat cramps); heat exhaustion with and without syncope; heat injury with evidence of end organ injury (eg, rhabdomyolysis); and EHS. EHS is defined as “a form of hyperthermia associated with a systemic inflammatory response leading to a syndrome of multi-organ dysfunction in which encephalopathy predominates.”3 EHS, if left untreated, or even if clinical treatment is delayed, may result in significant end organ morbidity and/or mortality.
During exercise, the human thermoregulatory system mitigates heat gain by increasing skin blood flow and sweating, causing an increased dissipation of heat to the surrounding environment by leveraging conduction, convection, and evaporation.4,5 Elevated environmental temperatures, increased humidity, and dehydration can impede the body’s ability to dissipate heat at a rate needed to maintain thermoregulation. This imbalance can result in hyperthermia secondary to uncompensated heat stress,5 which in turn can lead to EHI. Football players have unique challenges that make them particularly vulnerable to EHI. The summer heat during early-season participation and the requirement for equipment that covers nearly 60% of body surfaces pose increased risk of volume losses and hyperthermia that trigger the onset of EHI.6 Football athletes’ body compositions and physical size are additional contributing risk factors; the relatively high muscle and fat content increase thermogenicity, which require their bodies to dissipate more heat.7
An estimated 9000 cases of EHI occur annually across all high school sports,8 with an incidence of 1.6:100,000 athlete-exposures.8,9 Studies have demonstrated, however, that EHI occurs in football 11.4 times more often than in all other high school sports combined.10 The incidence of nonfatal EHI in all levels of football is 4.42-5:100,000.8,9 Between 2000 and 2014, 41 football players died from EHS.1 In football, approximately 75% of all EHI events occurred during practices, while only 25% of incidents occurred during games.8
Given these potentially deadly consequences, it is important that football team physicians are not only alert to the early symptoms of heat illness and prepared to intervene to prevent the progression to EHS, but are critical leaders in educating coaches and players in evidence-based EHI prevention practices and policies.
Prevention
EHS is a preventable condition, arguably the most common cause of preventable nontraumatic exertional death in young athletes in the United States. Close attention to mitigating risk factors should begin prior to the onset of preseason practice and continue through the early season, where athletes are at the highest risk of developing heat illness.
Primary Prevention
Primary prevention is fundamental to minimizing the occurrences of EHI. It focuses on the following methods: recognition of inherent risk factors, acclimatization, hydration, and avoidance of inciting substances (including supplements).
Pre-Participation Examination. The purpose of the pre-participation examination (PPE) is to maximize an athlete’s safety by identifying medical conditions that place the athlete at risk.11,12 The Preparticipation Physical Evaluation, 4th edition, the most widely used consensus publication, specifically queries if an athlete has a previous history of heat injury. However, it only indirectly addresses intrinsic risk factors that may predispose an athlete to EHI who has never had an EHI before. Therefore, providers should take the opportunity of the PPE to inquire about additional risk factors that may make an athlete high risk for sustaining a heat injury. Common risk factors for EHI are listed in Table 1.
Heat Acclimatization. The risk of EHI escalates significantly when athletes are subjected to multiple stressors during periods of heat exposure, such as sudden increases in intensity or duration of exercise; prolonged new exposures to heat; dehydration; and sleep loss.5 When football season begins in late summer, athletes are least conditioned as temperatures reach their seasonal peak, causing increased risk of EHI.15 Planning for heat acclimatization is vital for all athletes who exercise in hot environments. Acclimatization procedures place progressively mounting physiologic strains on the body to improve athletes’ ability to dissipate heat, diminishing thermoregulatory and cardiovascular exertion.4,5 Acclimatization begins with expansion of plasma volume on days 3 to 6, causing improvements in cardiac efficiency and resulting in an overall decrease in basal internal body temperature.4,5,15 This process results in improvements in heat tolerance and exercise performance, evolving over 10 to 14 days of gradual escalation of exercise intensity and duration.5,10,11,16 However, poor fitness levels and extreme temperatures can prolong this period, requiring up to 2 to 3 months to fully take effect.5,7
The National Athletic Trainers Association (NATA) and National Collegiate Athletic Association (NCAA) have released consensus guidelines regarding heat acclimatization protocols for football athletes at the high school and college levels (Tables 3 and 4). Each of these guidelines involves an initial period without use of protective equipment, followed by a gradual addition of further equipment.11,16
Secondary Prevention
Despite physicians’ best efforts to prevent all cases of EHI, athletes will still experience the effects of exercise-induced hyperthermia. The goal of secondary prevention is to slow the progression of this hyperthermia so that it does not progress to more dangerous EHI.
Hydration. Dehydration is an important risk factor for EHI. Sweat maintains thermoregulation by dissipating heat generated during exercise; however, it also contributes to body water losses. Furthermore, intravascular depletion decreases stroke volume, thereby increasing cardiovascular strain. It is estimated that for every 1% loss in body mass from dehydration, body temperature rises 0.22°C in comparison to a euhydrated state.6 Dehydration occurs more rapidly in hot environments, as fluid is lost through increased sweat production.7 After approximately 6% to 10% body weight volume loss, cardiac output cannot be maintained, diminishing sweat production and blood flow to both skin and muscle and causing diminished performance and a significant risk of heat exhaustion.7 If left unchecked, these physiologic changes result in further elevations in body temperature and increased cardiovascular strain, ultimately placing the athlete at significant risk for development of EHS.
Adequate hydration to maintain euvolemia is an important step in avoiding possible EHI. Multiple studies have shown that football players experience a baseline hypovolemia during their competition season,6 a deficit that is most marked during the first week of practices.17 This deficit is multifactorial, as football players expend a significant amount of fluid through sweat, are not able to adequately replace these losses during practice, and do not appropriately hydrate off the field.6,18 Some players, especially linemen, sweat at a higher rate than their teammates, posing a possible risk of significant dehydration.6 Coaches and players alike should be educated on the importance of adequate hydration to meet their fluid needs.
The goal of hydration during exercise is to prevent large fluid losses that can adversely affect performance and increase risk of EHI;6 it may be unrealistic to replace all fluid losses during the practice period. Instead, athletes should target complete volume replacement over the post-exercise period.6 Some recommend hydrating based upon thirst drive; however, thirst is activated following a volume loss of approximately 2% body mass, the same degree of losses that place athletes at an increased risk for performance impairment and EHI.4,6,11,12 Individuals should have access to fluids throughout practice and competition and be encouraged to hydrate as needed.6,12,15 Furthermore, staff should modify their practices based upon WBGT and acclimatization status to provide more frequent hydration breaks.
Hyperhydration and Salt Intake. Of note, there are inherent risks to hyperhydration. Athletes with low sweat rates have an increased risk of overhydration and the development of exercise-associated hyponatremia (EAH),6 a condition whose presentation is very similar to EHS. In addition, inadequate sodium intake and excessive sweating can also contribute to the development of EAH. EAH has been implicated in the deaths of 2 football players in 2014.1,6 Establishing team hydration guidelines and educating players and staff on appropriate hydration and dietary salt intake is essential to reduce the risk of both dehydration and hyperhydration and their complications.6Intra-Event Cooling. During exercise, team physicians can employ strategies for cooling athletes during exertion to mitigate their risk of EHI by decreasing thermal and cardiovascular strain.4,19 Cooling during exercise is hypothesized to allow for accelerated heat dissipation, where heat is lost from the body more effectively. This accelerated loss enables athletes to maintain a higher heat storage capacity over the duration of exercise, avoiding uncompensated heat stresses that ultimately cause EHI.19
Some intra-event cooling strategies include the use of cooling garments, cooling packs, and cold water/slurry ingestion. Cooling garments lower skin temperature, which in turn can decrease thermoregulatory strains;4 a recent meta-analysis of intra-event cooling modalities revealed that wearing an ice vest during exercise resulted in the greatest decrease in thermal heat strain.19 Internal cooling strategies—namely ingestion of cold fluids/ice slurry—have shown some mild benefit in decreasing internal temperatures; however, some studies have demonstrated some decrease in sweat production associated with cold oral intake used in isolation.19 Overall, studies have shown that combining external (cooling clothing, ice packs, fanning) and internal (cold water, ice slurry) cooling methods result in a greater cooling effect than a use of a single method.4
Tertiary Prevention
The goal of tertiary prevention is to mitigate the risk of long-term adverse outcomes following an EHS event. The most effective means of reducing risk for morbidity and mortality is rapid identification and treatment of EHS as well as close evaluation of an athlete’s return to activity in heat. This process is spearheaded by an effective and well-rehearsed emergency action plan.
Diagnosis and Management
Rapid identification and treatment of EHS is crucial to minimizing the risk of poor outcomes.7 Any delay in the treatment of EHS can dramatically increase the likelihood of associated morbidity and mortality.20
EHS is diagnosed by an elevated rectal temperature ≥40°C (104°F) and associated central nervous system (CNS) dysfunction.21 EHS should be strongly suspected in any athlete exercising in heat who exhibits signs of CNS dysfunction, including disorientation, confusion, dizziness, erratic behavior, irritability, headache, loss of coordination, delirium, collapse, or seizures.7,12,15 EHS may also present with symptoms of heat exhaustion, including fatigue, hyperventilation, tachycardia, vomiting, diarrhea, and hypotension.7,12,15
Rectal temperature should be taken for any athlete with suspected EHS, as other modalities—oral, skin, axillary, and aural—can be inaccurate and easily modified by ambient confounders such as ambient and skin temperature, athlete hyperventilation, and consumption of liquids.7,11,12 Athletes exhibiting CNS symptoms with moderately elevated rectal temperatures that do not exceed 40°C should also be assumed to be suffering from EHS and treated with rapid cooling.11 On the other hand, athletes with CNS symptoms who are normothermic should be assumed to have EAH until ruled out by electrolyte assessment; IV fluids should be at no more than keep vein open (KVO) pending this determination.11 In some cases, an athlete may initially present with altered mental status but return to “normal.” However, this improvement may represent a “lucid period”; evaluation should continue with rectal temperature and treatment, as EHS in these cases may progress quickly.15
Treatment is centered on rapid, whole body cooling initiated at the first sign of heat illness.7,22 The goal of treatment is to achieve a rectal temperature <38.9°C within 30 minutes of the onset of EHS.15 Upon diagnosis, the athlete should be quickly placed in a tub of ice water to facilitate cold water immersion (CWI) therapy. Some guidelines suggest the athlete’s clothing be removed to potentiate evaporative cooling during CWI;12 however, cooling should not be delayed due to difficulties in removing equipment. CWI, where a heat stroke victim is submerged in ice water up to their neck while water is continuously circulated, is generally considered to be the gold standard treatment as it is the modality with the highest recorded cooling rates and the lowest rate of morbidity and mortality.7,20,21 Multiple studies of CWI have shown that survival nears 100% when aggressive cooling starts within 5 minutes of collapse or identification of EHS.20,21,22
If whole body CWI is unavailable, alternative methods of rapid cooling should be employed. Partial CWI, with torso immersion being preferable to the extremities, has been shown to achieve an acceptable rate of cooling to achieve sufficient drops in internal body temperature.20,23 However, one popular treatment—applying ice packs to the whole body, in particular to the groin and axillae—has not been shown to be sufficient to achieve standard cooling goals.20 None of these methods have been shown to be as effective as CWI.23
Intravenous access should be initiated with fluid resuscitation dictated by the provider’s assessment. Normal saline is recommended as the resuscitative fluid of choice, with the rate dictated by clinical judgment and adjusted as guided by electrolyte determination and clinical response. It cannot be overstated that in normothermic patients with confusion, EAH is the diagnosis of exclusion and aggressive fluid resuscitation should be withheld until electrolyte determination.
Once rectal temperature descends appropriately (~38.9°C), the cooling process should stop and the individual should be transported to a hospital for further observation20 and evaluation of possible sequelae, including rhabdomyolysis and renal injury, cardiac dysfunction and arrhythmia, severe electrolyte abnormalities, acute respiratory distress syndrome, lactic acidosis, and other forms of end-organ failure (Figure).
Rapid cooling is more crucial than transport; transport poses a risk of delayed cooling, which can dramatically increase an individual’s risk of morbidity and mortality.20,23 In situations where a patient can be cooled on-site, physicians should pursue cooling before transporting the patient to a medical treatment facility.
Emergency Action Plan
Team physicians should be proactive in developing an emergency action plan to address possible EHS events. These plans should be site-specific, addressing procedures for all practice and home competition locations.12 All competition venues should have a CWI tub on-site in events where there is an increased risk of EHS.12,15,20 This tub should be set up and functional for all high-risk activities, including practices.12
Following recognition of a potential case of EHS, treatment teams should have procedures in place to transport athletes to the treatment area, obtain rectal temperature, initiate rapid cooling, and stabilize the athlete for transport to an emergency department (ED) for further evaluation.12,15 A written record of treatments and medications provided during athlete stabilization should be maintained and transported with the athlete to the ED.15 A list of helpful equipment and supplies for treatment of EHS can be found in Table 5.
EHS is a unique life-threatening situation where it is best to treat the patient on the sideline before transport.15 Those athletes transported before cooling risk spending an increased amount of time above critical temperatures for cell damage, which has been associated with increased morbidity and mortality. This mantra of “cool first, transport second” cannot be overemphasized, as those individuals with EHS who present to the ED with a persisting rectal temperature >41°F may risk up to an 80% mortality rate.24 Conversely, a recent large, retrospective study of 274 EHS events sustained during the Falmouth Road Race found a 100% survival rate when athletes were rapidly identified via rectal thermometry and treated with aggressive, rapid cooling through CWI.21
Return to Play
Perhaps the most challenging and important role the team physician has is determining an athlete’s return to play following EHI, as there currently are no evidence-based guidelines for return to activity for these athletes.7 The decisions surrounding return to play are highly individualized, as recovery from EHS and heat injury is associated with the duration of internal body temperature elevation above the critical level (40°C).7,20 Guidelines for return to activity following recovery from EHI differ among experts and institutions.7,25 The general consensus from these guidelines is that, at minimum, athletes should not participate in any physical activity until they are asymptomatic and all blood tests have normalized.11 Following this asymptomatic period, most guidelines advocate for a slow, deliberate return to activity.11 The American College of Sports Medicine (ACSM) offers one reasonable approach to the returning athlete following EHS:7
- No exercise for at least 7 days following release from medical care.
- Follow-up with a physician 1 week after release from medical care for physical examination and any warranted lab or radiologic studies (based upon organ systems affected during EHS).
- Once cleared to return to activity, the athlete begins exercise in a cool environment, gradually increasing the duration, intensity, and heat exposure over 2 weeks to demonstrate heat tolerance and acclimatization.
- Athletes who cannot resume vigorous activity due to recurrent symptoms (eg, excessive fatigue) should be reevaluated after 4 weeks. Laboratory exercise-heat tolerance testing may be useful in this setting.
- The athlete may resume full competition once they are able to participate in full training in the heat for 2 to 4 weeks without adverse effects.
Heat tolerance testing (HTT) in these athletes remains controversial.5 26 The ACSM recommends that HTT be considered only for those unable to return to vigorous activity after a suitable period (approximately 4 weeks). In contrast, the Israeli Defense Force (IDF) uses HTT to evaluate soldiers following EHS to guide decision-making about return to duty.27 The IDF HTT assumes that individuals will respond differently to heat stresses. They identify individuals who are “heat intolerant” as being unable to tolerate specific heat challenges, indicated by increases in body temperature occurring more rapidly than normal responders under identical environmental and exercise conditions. However, despite being used for more than 30 years, there is no clear evidence that HTT adequately predicts who will experience subsequent episodes of EHS.
Conclusion
While the recognized cornerstone of being a team physician is the provision of medical care, the ACSM Team Physician Consensus Statement28 further delineates the medical and administrative responsibilities as both (1) understanding medical management and prevention of injury and illness in athletes; and (2) awareness of or involvement in the development and rehearsal of an emergency action plan. These tenets are critical for the team physician who accepts the responsibility to cover sports at the high school level or higher. Football team physicians play an essential role in mitigating risk of EHI in their athletes. Through development and execution of both comprehensive prevention strategies and emergency action plans, physicians can work to minimize athletes’ risk of both developing and experiencing significant adverse outcomes from an EHI.
Am J Orthop. 2016;45(6):340-348. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Kucera KL, Klossner D, Colgate B, Cantu RC. Annual Survey of Football Injury Research: 1931-2014. National Center for Catastrophic Sport Injury Research Web site. https://nccsir.unc.edu/files/2013/10/Annual-Football-2014-Fatalities-Final.pdf. Accessed May 31, 2016.
2. Boden BP, Breit I, Beachler JA, Williams A, Mueller FO. Fatalities in high school and college football players. Am J Sports Med. 2013;41(5):1108-1116.
3. Bouchama A, Knochel JP. Heat stroke. N Engl J Med. 2002;346(25):1978-1988.
4. Racinais S, Alonso JM, Coutts AJ, et al. Consensus recommendations on training and competing in the heat. Scand J Med Sci Sports. 2015;25 Suppl 1:6-19.
5. Pryor RR, Casa DJ, Adams WM, et al. Maximizing athletic performance in the heat. Strength Cond J. 2013;35(6):24-33.
6. Adams WM, Casa DJ. Hydration for football athletes. Sports Sci Exchange. 2015;28(141):1-5.
7. American College of Sports Medicine, Armstrong LE, Casa DJ, et al. American College of Sports Medicine position stand. Exertional heat illness during training and competition. Med Sci Sports Exerc. 2007;39(3):556-572.
8. Yard EE, Gilchrist J, Haileyesus T, et al. Heat illness among high school athletes--United States, 2005-2009. J Safety Res. 2010;41(6):471-474.
9. Huffman EA, Yard EE, Fields SK, Collins CL, Comstock RD. Epidemiology of rare injuries and conditions among United States high school athletes during the 2005-2006 and 2006-2007 school years. J Athl Train. 2008;43(6):624-630.
10. Kerr ZY, Casa DJ, Marshall SW, Comstock RD. Epidemiology of exertional heat illness among U.S. high school athletes. Am J Prev Med. 2013;44(1):8-14.
11. Casa DJ, DeMartini JK, Bergeron MF, et al. National Athletic Trainers’ Association position statement: exertional heat illnesses. J Athl Train. 2015;50(9):986-1000.
12. Casa DJ, Almquist J, Anderson SA. The inter-association task force for preventing sudden death in secondary school athletics programs: best-practices recommendations. J Athl Train. 2013;48(4):546-553.
13. Gardner JW, Kark JA, Karnei K, et al. Risk factors predicting exertional heat illness in male Marine Corps recruits. Med Sci Sports Exerc. 1996;28(8):939-944.
14. Gundstein AJ, Ramseyer C, Zhao F, et al. A retrospective analysis of American football hyperthermia deaths in the United States. Int J Biometerol. 2012;56(1):11-20.
15. Armstrong LE, Johnson EC, Casa DJ, et al. The American football uniform: uncompensable heat stress and hyperthermic exhaustion. J Athl Train. 2010;45(2):117-127.
16. Casa DJ, Csillan D; Inter-Association Task Force for Preseason Secondary School Athletics Participants, et al. Preseason heat-acclimatization guidelines for secondary school athletics. J Athl Train. 2009;44(3):332-333.
17. Godek SF, Godek JJ, Bartolozzi AR. Hydration status in college football players during consecutive days of twice-a-day preseason practices. Am J Sports Med. 2005;33(6):843-851.
18. Stover EA, Zachwieja J, Stofan J, Murray R, Horswill CA. Consistently high urine specific gravity in adolescent American football players and the impact of an acute drinking strategy. Int J Sports Med. 2006;27(4):330-335.
19. Bongers CC, Thijssen DH, Veltmeijer MTW, Hopman MT, Eijsvogels TM. Precooling and percooling (cooling during exercise) both improve performance in the heat: a meta-analytical review. Br J Sports Med. 2015;49(6):377-384.
20. Casa DJ, McDermott BP, Lee EC, Yeargin SW, Armstrong LE, Maresh CM. Cold water immersion: the gold standard for exertional heatstroke treatment. Exerc Sport Sci Rev. 2007;35(3):141-149.
21. DeMartini JK, Casa DJ, Stearns R, et al. Effectiveness of cold water immersion in the treatment of exertional heat stroke at the Falmouth Road Race. Med Sci Sports Exerc. 2015;47(2):240-245.
22. Casa DJ, Kenny GP, Taylor NA. Immersion treatment for exertional hyperthermia: cold or temperate water? Med Sci Sports Exerc. 2010;42(7):1246-1252.
23. Casa DJ, Armstrong LE, Kenny GP, O’Connor FG, Huggins RA. Exertional heat stroke: new concepts regarding cause and care. Curr Sports Med Rep. 2012;11(3):115-122.
24. Argaud L, Ferry T, Le QH, et al. Short- and long-term outcomes of heat stroke following the 2003 heat wave in Lyon, France. Arch Intern Med. 2007;167(20):2177-2183.
25. O’Connor FG, Casa DJ, Bergeron MF, et al. American College of Sports Medicine Roundtable on exertional heat stroke--return to duty/return to play: conference proceedings. Curr Sports Med Rep. 2010;9(5):314-321.
26. Kazman JB, Heled Y, Lisman PJ, Druyan A, Deuster PA, O’Connor FG. Exertional heat illness: the role of heat tolerance testing. Curr Sports Med Rep. 2013;12(2):101-105.
27. Moran DS, Heled Y, Still L, Laor A, Shapiro Y. Assessment of heat tolerance for post exertional heat stroke individuals. Med Sci Monit. 2004;10(6):CR252-CR257.
28. Herring SA, Kibler WB, Putukian M. Team Physician Consensus Statement: 2013 update. Med Sci Sports Exerc. 2013;45(8):1618-1622.
29. Heat stroke treatment. Korey Stringer Institute University of Connecticut Web site. http://ksi.uconn.edu/emergency-conditions/heat-illnesses/exertional-heat-stroke/heat-stroke-treatment/. Accessed June 14, 2016.
30. Headquarters, Department of the Army and the Air Force. Heat Stress Control and Heat Casualty Management. Technical Bulletin Medical 507. http://www.dir.ca.gov/oshsb/documents/Heat_illness_prevention_tbmed507.pdf. Published March 7, 2003. Accessed June 14, 2016.
1. Kucera KL, Klossner D, Colgate B, Cantu RC. Annual Survey of Football Injury Research: 1931-2014. National Center for Catastrophic Sport Injury Research Web site. https://nccsir.unc.edu/files/2013/10/Annual-Football-2014-Fatalities-Final.pdf. Accessed May 31, 2016.
2. Boden BP, Breit I, Beachler JA, Williams A, Mueller FO. Fatalities in high school and college football players. Am J Sports Med. 2013;41(5):1108-1116.
3. Bouchama A, Knochel JP. Heat stroke. N Engl J Med. 2002;346(25):1978-1988.
4. Racinais S, Alonso JM, Coutts AJ, et al. Consensus recommendations on training and competing in the heat. Scand J Med Sci Sports. 2015;25 Suppl 1:6-19.
5. Pryor RR, Casa DJ, Adams WM, et al. Maximizing athletic performance in the heat. Strength Cond J. 2013;35(6):24-33.
6. Adams WM, Casa DJ. Hydration for football athletes. Sports Sci Exchange. 2015;28(141):1-5.
7. American College of Sports Medicine, Armstrong LE, Casa DJ, et al. American College of Sports Medicine position stand. Exertional heat illness during training and competition. Med Sci Sports Exerc. 2007;39(3):556-572.
8. Yard EE, Gilchrist J, Haileyesus T, et al. Heat illness among high school athletes--United States, 2005-2009. J Safety Res. 2010;41(6):471-474.
9. Huffman EA, Yard EE, Fields SK, Collins CL, Comstock RD. Epidemiology of rare injuries and conditions among United States high school athletes during the 2005-2006 and 2006-2007 school years. J Athl Train. 2008;43(6):624-630.
10. Kerr ZY, Casa DJ, Marshall SW, Comstock RD. Epidemiology of exertional heat illness among U.S. high school athletes. Am J Prev Med. 2013;44(1):8-14.
11. Casa DJ, DeMartini JK, Bergeron MF, et al. National Athletic Trainers’ Association position statement: exertional heat illnesses. J Athl Train. 2015;50(9):986-1000.
12. Casa DJ, Almquist J, Anderson SA. The inter-association task force for preventing sudden death in secondary school athletics programs: best-practices recommendations. J Athl Train. 2013;48(4):546-553.
13. Gardner JW, Kark JA, Karnei K, et al. Risk factors predicting exertional heat illness in male Marine Corps recruits. Med Sci Sports Exerc. 1996;28(8):939-944.
14. Gundstein AJ, Ramseyer C, Zhao F, et al. A retrospective analysis of American football hyperthermia deaths in the United States. Int J Biometerol. 2012;56(1):11-20.
15. Armstrong LE, Johnson EC, Casa DJ, et al. The American football uniform: uncompensable heat stress and hyperthermic exhaustion. J Athl Train. 2010;45(2):117-127.
16. Casa DJ, Csillan D; Inter-Association Task Force for Preseason Secondary School Athletics Participants, et al. Preseason heat-acclimatization guidelines for secondary school athletics. J Athl Train. 2009;44(3):332-333.
17. Godek SF, Godek JJ, Bartolozzi AR. Hydration status in college football players during consecutive days of twice-a-day preseason practices. Am J Sports Med. 2005;33(6):843-851.
18. Stover EA, Zachwieja J, Stofan J, Murray R, Horswill CA. Consistently high urine specific gravity in adolescent American football players and the impact of an acute drinking strategy. Int J Sports Med. 2006;27(4):330-335.
19. Bongers CC, Thijssen DH, Veltmeijer MTW, Hopman MT, Eijsvogels TM. Precooling and percooling (cooling during exercise) both improve performance in the heat: a meta-analytical review. Br J Sports Med. 2015;49(6):377-384.
20. Casa DJ, McDermott BP, Lee EC, Yeargin SW, Armstrong LE, Maresh CM. Cold water immersion: the gold standard for exertional heatstroke treatment. Exerc Sport Sci Rev. 2007;35(3):141-149.
21. DeMartini JK, Casa DJ, Stearns R, et al. Effectiveness of cold water immersion in the treatment of exertional heat stroke at the Falmouth Road Race. Med Sci Sports Exerc. 2015;47(2):240-245.
22. Casa DJ, Kenny GP, Taylor NA. Immersion treatment for exertional hyperthermia: cold or temperate water? Med Sci Sports Exerc. 2010;42(7):1246-1252.
23. Casa DJ, Armstrong LE, Kenny GP, O’Connor FG, Huggins RA. Exertional heat stroke: new concepts regarding cause and care. Curr Sports Med Rep. 2012;11(3):115-122.
24. Argaud L, Ferry T, Le QH, et al. Short- and long-term outcomes of heat stroke following the 2003 heat wave in Lyon, France. Arch Intern Med. 2007;167(20):2177-2183.
25. O’Connor FG, Casa DJ, Bergeron MF, et al. American College of Sports Medicine Roundtable on exertional heat stroke--return to duty/return to play: conference proceedings. Curr Sports Med Rep. 2010;9(5):314-321.
26. Kazman JB, Heled Y, Lisman PJ, Druyan A, Deuster PA, O’Connor FG. Exertional heat illness: the role of heat tolerance testing. Curr Sports Med Rep. 2013;12(2):101-105.
27. Moran DS, Heled Y, Still L, Laor A, Shapiro Y. Assessment of heat tolerance for post exertional heat stroke individuals. Med Sci Monit. 2004;10(6):CR252-CR257.
28. Herring SA, Kibler WB, Putukian M. Team Physician Consensus Statement: 2013 update. Med Sci Sports Exerc. 2013;45(8):1618-1622.
29. Heat stroke treatment. Korey Stringer Institute University of Connecticut Web site. http://ksi.uconn.edu/emergency-conditions/heat-illnesses/exertional-heat-stroke/heat-stroke-treatment/. Accessed June 14, 2016.
30. Headquarters, Department of the Army and the Air Force. Heat Stress Control and Heat Casualty Management. Technical Bulletin Medical 507. http://www.dir.ca.gov/oshsb/documents/Heat_illness_prevention_tbmed507.pdf. Published March 7, 2003. Accessed June 14, 2016.
2016 Update on female sexual dysfunction
The age-adjusted prevalence of any sexual problem is 43% among US women. A full 22% of these women experience sexually related personal distress.1 With publication of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition2 has come a shift in classification and, at times, management approach for reported female sexual dysfunction. When women report to their clinicians decreased sexual desire or arousal or pain at penetration, the management is no longer guided by a linear model of sexual response (excitation, plateau, orgasm, and resolution) but rather by a more nuanced and complex biopsychosocial approach. In this model, diagnosis and management strategies to address bothersome sexual concerns consider the whole woman in the context of her physical and psychosocial health. The patient’s age, medical history, and relationship status are among the factors that could affect management of the problem. In an effort to explore this management approach, I used this Update on Female Sexual Dysfunction as an opportunity to convene a roundtable of several experts, representing varying backgrounds and practice vantage points, to discuss 5 cases of sexual problems that you as a busy clinician may encounter in your practice.

Genital atrophy in a sexually inactive 61-year-old woman
Barbara S. Levy, MD: Two years after her husband's death, which followed several years of illness, your 61-year-old patient mentions at her well woman visit that she anticipates becoming sexually active again. She has not used systemic or vaginal hormone therapy. During pelvic examination, atrophic external genital changes are present, and use of an ultrathin (thinner than a Pederson) speculum reveals vaginal epithelial atrophic changes. A single-digit bimanual exam can be performed with moderate patient discomfort; the patient cannot tolerate a 2-digit bimanual exam. She expresses concern about being able to engage in penile/vaginal sexual intercourse.
Dr. Kaunitz, what is important for you to ask this patient, and what concerns you most on her physical exam?
Andrew M. Kaunitz, MD: First, it is important to recognize the patient's expectations and desires. As the case suggests, but further questioning could clarify, she would like to be able to comfortably engage in sexual intercourse with a new partner, but penetration may be difficult (and definitely painful for her) unless treatment is pursued. This combination of mucosal and vestibular atrophic changes (genitourinary syndrome of menopause [GSM], or vulvovaginal atrophy [VVA]) plus the absence of penetration for many years can be a double whammy situation for menopausal women. In this case it has led to extensive contracture of the introitus, and if it is not addressed will cause sexual dysfunction.
Dr. Levy: In addition we need to clarify whether or not a history of breast cancer or some other thing may impact the care we provide. How would you approach talking with this patient in order to manage her care?
Dr. Kaunitz: One step is to see how motivated she is to address this, as it is not something that, as gynecologists, we can snap our fingers and the situation will be resolved. If the patient is motivated to treat the atrophic changes with medical treatment, in the form of low-dose vaginal estrogen, and dilation, either on her own if she's highly motivated to do so, or in my practice more commonly with the support of a women's physical therapist, over time she should be able to comfortably engage in sexual intercourse with penetration. If this is what she wants, we can help steer her in the right direction.
Sheryl Kingsberg, PhD: You know that this woman is motivated by virtue of her initiating the topic herself. Patients are often embarrassed talking about sexual issues, or they are not sure that their gynecologist is comfortable with it. After all, they think, if this is the right place to discuss sexual problems, why didn't he or she ask me? Clinicians must be aware that it is their responsibility to ask about sexual function and not leave it for the patient to open the door.
Dr. Kaunitz: Great point.
Cheryl B. Iglesia, MD: Gratefully, a lot of the atrophic changes this patient demonstrates are reversible. However, other autoimmune diseases (eg, lichen planus, which can affect the vaginal epithelium, or lichen sclerosus, which can affect the clitoris, labia, and vulva) can also cause constriction, and in severe cases, complete obliteration of the vagina and introitus. Women may not be sexually active, and for each annual exam their clinician uses a smaller and smaller speculum--to the point that they cannot even access the cervix anymore--and the vagina can close off. Clinicians may not realize that you need something other than estrogen; with lichen planus you need steroid suppository treatment, and with lichen sclerosus you need topical steroid treatment. So these autoimmune conditions should also be in the differential and, with appropriate treatment, the sexual effects can be reversible.
Michael Krychman, MD: I agree. The vulva can be a great mimicker and, according to the history and physical exam, at some point a vulvoscopy, and even potential biopsies, may be warranted as clinically indicated.
The concept of a comprehensive approach, as Dr. Kingsberg had previously mentioned, involves not only sexual medicine but also evaluating the patient's biopsychosocial variables that may impact her condition. We also need to set realistic expectations. Some women may benefit from off-label use of medications besides estrogen, including topical testosterone. Informed consent is very important with these treatments. I also have had much clinical success with intravaginal diazepam/lorazepam for pelvic floor hypertonus.
In addition, certainly I agree that pelvic floor physical therapy (PT) is a vital treatment component for this patient and, not to diminish its importance, but many women cannot afford, nor do they have the time or opportunity, to go to pelvic floor PT. As clinicians, we can develop and implement effective programs, even in the office, to educate the patient to help herself as well.
Dr. Kaunitz: Absolutely. Also, if, in a clinical setting consistent with atrophic changes, an ObGyn physician is comfortable that vulvovaginal changes noted on exam represent GSM/atrophic changes, I do not feel vulvoscopy is warranted.
Dr. Levy: In conclusion, we need to be aware that pelvic floor PT may not be available everywhere and that a woman's own digits and her partner can also be incorporated into this treatment.
Something that we have all talked about in other venues, but have not looked at in the larger sphere here, is whether there is value to seeing women annually and performing pelvic exams. As Dr. Kingsberg mentioned, this is a highly motivated patient. We have many patients out there who are silent sufferers. The physical exam is an opportunity for us to recognize and address this problem.
Dyspareunia and low sexual desire in a breast cancer survivor
Dr. Levy: In this case, a 36-year-old woman with BRCA1−positive breast cancer has vaginal dryness, painful intercourse, and lowered sexual interest since her treatment, which included chemotherapy after bilateral mastectomies. She has a bilateral salpingo-oophorectomy(BSO) scheduled for primary prevention of her ovarian cancer risk.
Dr. Kingsberg, what is important for you to know to help guide case management?
Dr. Kingsberg: This woman is actually presenting with 2 sexual problems: dyspareunia, which is probably secondary to VVA or GSM, and low sexual desire. Key questions are: 1) When was symptom onset--acquired after treatment or lifelong? 2) Did she develop the dyspareunia and as a result of having pain during sex lost desire to have sex? Or, did she lose desire and then, without it, had no arousal and therefore pain with penetration developed? It also could be that she has 2 distinct problems, VVA and hypoactive sexual desire disorder (HSDD), in which case you need to think about treating both. Finally, we do not actually know if she is having penetrative intercourse or even if she has a partner.
A vulvovaginal exam would give clues as to whether she has VVA, and hormone levels would indicate if she now has chemo-induced menopause. If she is not in menopause now, she certainly is going to be with her BSO. The hormonal changes due to menopause actually can be primarily responsible for both the dyspareunia and HSDD. Management of both symptoms really needs to be based on shared decision making with the patient--with which treatment for which conditions coming first, based on what is causing her the most distress.
I would encourage this woman to treat her VVA since GSM does have long-term physiologic consequences if untreated. The American College of Obstetricians and Gynecologists (ACOG) recommends nonhormonal treatments as first-line treatments, with vaginal estrogen considered if these therapies fail.3 If lubricants and moisturizers and other nonhormonal options are not sufficient, you could consider local estrogen, even though she is a breast cancer survivor, as well as ospemiphene.
If she is distressed by her loss of sexual desire, you can choose to treat her for HSDD. Flibanserin is the first FDA-approved treatment for HSDD. It is only approved in premenopausal women, so it would be considered off-label use if she is postmenopausal (even though she is quite young). You also could consider exogenous testosterone off-label, after consulting with her oncologist.
In addition to the obvious physiologic etiology of her pain and her low desire, the biopsychosocial aspects to consider are: 1) changes to her body image, as she has had bilateral mastectomies, 2) her anxiety about the cancer diagnosis, and 3) concerns about her relationship if she has one--her partner's reactions to her illness and the quality of the relationship outside the bedroom.
Dr. Iglesia: I am seeing here in our nation's capital a lot of advertisements for laser therapy for GSM. I caution women about this because providers are charging a lot of money for this therapy when we do not have long-term safety and effectiveness data for it.
Our group is currently conducting a randomized controlled trial, looking at vaginal estrogen cream versus laser therapy for GSM here at Medstar Health--one of the first in the country as part of a multisite trial. But the North American Menopause Society (NAMS) has come out with a pretty strong statement,4 as has ACOG,5 on this therapy, and I caution people about overzealously offering a very costly procedure targeted to a very vulnerable population, especially to women with personal histories of estrogen-sensitive cancers.
Dr. Krychman: I agree. Very often cancer patients are preyed upon by those offering emerging unproven technologies or medications. We have to work as a coordinated comprehensive team, whether it's a sexual medicine expert, psychologist, urogynecologist, gynecologist, or oncologist, and incorporate the patient's needs and expectations and risk tolerance coupled with treatment efficacy and safety.
Dr. Levy: This was a complex case. The biopsychosocial model is critical here. It's important that we are not siloes in our medical management approach and that we try to help this patient embrace the complexity of her situation. It's not only that she has cancer at age 36; there is a possible guilt factor if she has children and passed that gene on.
Another point that we began to talk about is the fact that in this country we tend to be early adopters of new technology. In our discussion with patients, we should focus on what we know and the risk of the unknowns related to some of the treatment options. But let's discuss lasers a little more later on.
Diminished arousal and orgasmic intensity in a patient taking SSRIs
Dr. Levy: In this next case, a 44-year-old woman in a 15-year marriage notices a change in her orgasmic intensity and latency. She has a supportive husband, and they are attentive to each other's sexual needs. However, she notices a change in her arousal and orgasmic intensity, which has diminished over the last year. She reports that the time to orgasm or latency has increased and both she and her partner are frustrated and getting concerned. She has a history of depression that has been managed by selective serotonin reuptake inhibitors for the past 5 years and has no depressive symptoms currently.
Dr. Krychman, what are you considering before beginning to talk with this patient?
Dr. Krychman: My approach really is a comprehensive one, looking not only at the underlying medical issues but also at the psychological and dynamic relationship facets. We of course also want to look at medications: Has she changed her dose or the timing of when she takes it? Is this a new onset? Finally, we want to know why this is coming to the forefront now. Is it because it is getting worse, or is it because there is some significant issue that is going on in the relationship?
Regarding the physical exam, it is important to rule out underlying genital pelvic pathology. Young women can get changes in the integrity of the pelvic floor, in what I would call the orgasmic matrix--the clitoral tissue, the body, the crura (or arms of the clitoris)--we want to examine and be reassured that her genital anatomy is normal and that there is no underlying pathology that could signal an underlying abnormal hormonal profile. Young women certainly can get lowered estrogen effects at the genital/pelvic tissues (including the labia and vulva), and intravaginally as well. Sometimes women will have pelvic floor hypertonus, as we see with other urinary issues. A thorough pelvic exam is quite vital.
Let's not forget the body that is attached to the genitals; we want to rule out chronic medical disease that may impact her: hypertension, diabetes, or hypercholesterolemia. Untreated, these conditions may directly impact the arousal physiologic mechanisms.
Dr. Levy: In doing this patient's physical exam I would be looking for significant weight gain, and even asking about her partner's weight. Body image can be a huge issue. If she has a history of depression, if she is suffering from a body image problem, she can be feeling unattractive. In my experience this can be a common thing to affect women in their mid-40s.
How would you manage this case?
Dr. Krychman: It is important to divide it up in terms of a conservative to aggressive approach. We want to find out about the relationship. For instance, is the sexual dynamic scripted (ie, boring and predictable)? Is she distracted and frustrated or is she getting enough of the type of stimulation that she likes and enjoys? There certainly are a lot of new devices that are available, whether a self-stimulator or vibrator, the Fiera, or other stimulating devices, that may be important to incorporate into the sexual repertoire. If there is underlying pathology, we want to evaluate and treat that. She may need to be primed, so to speak, with systemic hormones. And does she have issues related to other effects of hormonal deprivation, even local effects? Does she have clitoral atrophy?
There are neutraceuticals that are currently available, whether topical arousal gels or ointments, and we as clinicians need to be critical and evaluate their benefit/risk and look at the data concerning these products. In addition, women who have changes in arousal and in orgasmic intensity and latency may be very frustrated. They describe it as climbing up to a peak but never getting over the top, and this frustration may lead to participant spectatoring, so incorporating a certified sex therapist or counselor is sometimes very critical.
Finally, there are a lot of snake oils, charmers, and charlatan unproven procedures--injecting fillers or other substances into the clitoris are a few examples. I would be a critical clinician, examine the evidence, look at the benefit/risk before advocating an intervention that does not have good clinical data to support its use--a comprehensive approach of sexual medicine as well as sexual psychology.
Dr. Kingsberg: Additionally, we know she is in a long-term relationship--15 years; we want to acknowledge the partner. We talked about the partner's weight, but what about his erectile function? Does he have changes in sexual function that are affecting her, and she is the one carrying the "symptom"?
Looking at each piece separately helps a clinician from getting overwhelmed by the patient who comes in reporting distress with orgasmic dysfunction. We have no pharmacologic FDA-approved treatments, so it can feel off-putting for a clinician to try to fix the reported issue. Looking at each component to help her figure out the underlying cause can be helpful.
Dr. Iglesia: With aging, there can be changes in blood flow, not to mention the hormonal and even peripheral nerve changes, that require more stimulation in order to achieve the desired response. I echo concern about expensive procedures being offered with no evidence, such as the "O" or "G" shot, that can cost up to thousands of dollars.
The other procedure that gives me a lot of angst is clitoral unhooding. The 3 parts of the clitoris are sensitive in terms of innervation and blood flow, and cutting around that delicate tissue goes against the surgical principles required for preserving nerves and blood flow.
New onset pain post−prolapse surgery with TOT sling placement
Dr. Levy: For this case, let's consider a 42-year-old woman (P3) who is 6 months post− vaginal hysterectomy. The surgery included ovarian preservation combined with anterior and posterior repair for prolapse as well as apical uterosacral ligament suspension for stage 2 uterovaginal prolapse. A transobturator sling was used.
Extensive preop evaluation was performed, with confirmed symptomatic prolapse. She had no stress incontinence symptoms but did have confirmed occult stress incontinence.
Surgery was uneventful. She resumed intercourse at 8 weeks, but she now has pain with both initial entry and deep penetration. Lubricants and changes in position have not helped. She is in a stable relationship with her husband of 17 years, and she is worried that the sling mesh might be the culprit. On exam, she has no atrophy, pH is 4.5, vaginal length is 8 cm, and there is no prolapse. There is no mesh exposure noted, although she reports slight tenderness with palpation of the right sling arm beneath the right pubic bone.
Dr. Iglesia, what are the patient history questions important to ask here?
Dr. Iglesia: This is not an uncommon scenario--elective surgical correction of occult or latent stress incontinence after surgical correction for pelvic organ prolapse. Now this patient here has no more prolapse complaints; however, she has a new symptom. There are many different causes of dyspareunia; we cannot just assume it is the sling mesh (although with all the legal representation advertisements for those who have had mesh placed, it can certainly be at the top of the patient's mind, causing anxiety and fear).
Multiple trials have looked at prophylactic surgery for incontinence at the time of prolapse repairs. This woman happened to be one of those patients who did not have incontinence symptoms, and they put a sling in. A recent large trial examined women with vaginal prolapse who underwent hysterectomy and suspension.6 (They compared 2 different suspensions.) What is interesting is that 25% of women with prolapse do have baseline pain. However, at 24 months, de novo pain can occur in 10% of women--just from the apical suspension. So, here, it could be the prolapse suspension. Or, in terms of the transobturator sling, long-term data do tell us that the de novo dyspareunia rate ranges on the magnitude of 1% to 9%.7 What is important here is figuring out the cause of the dyspareunia.
Dr. Levy: One of the important points you raised already was that 25% of these women have preoperative pain. So figuring out what her functioning was before surgery and incorporating that into our assessment postop would be pretty important I would think.
Dr. Iglesia: Yes, you need to understand what her typical encounter was before the surgery and how things have changed now that the prolapse is not in the way. Changes obviously can occur with scar tissue, which over time will improve. If she is perimenopausal and starts to get epithelial changes, we can fix that. The question then becomes, "Is the pain emanating from the mesh?"
When examining this patient, it is not uncommon for me to be able to feel "banjo" strings if the mesh is too tight or close to the surface. It is not exposed but it's palpable, and the patient may feel a ridge during penetration. You can ask the patient if pain occurs with different penetration positions. In addition, explore associated neurologic symptoms (numbness or muscle pain in the thigh).
Dr. Kingsberg: There were 2 different sources of pain--on initial entry and at deep penetration. You want to make sure you address both. Importantly, did one precede the other? For instance, if women have pain with penetration they can then end up with an arousal disorder (the length of the vagina cannot increase as much as it might otherwise) and dystonia secondary to the pain with penetration. The timing of the pain--did it all happen at the same time, or did she start out with pain at one point and did it move to something else--is another critical piece of the history.
Dr. Iglesia: It does take a detailed history and physical exam to identify myofascial trigger points. In this case there seems to be pinpoint tenderness directly on the part of the mesh that is not exposed on the right. There are people who feel you should remove the whole mesh, including the arms. Others feel, okay, we can work on these trigger points, with injections, physical therapy, extra lubrication, and neuromodulatory medications. Only then would they think about potentially excising the sling or a portion thereof.
Dr. Krychman: Keep in mind that, even if you do remove the sling, her pain may not subside, if it is secondary to an underlying issue. Because of media sensationalism, she could be focusing on the sling. It is important to set realistic expectations. I often see vulvar pathology or even provoked vestibulodynia that can present with a deep dyspareunia. The concept of collision dyspareunia or introital discomfort or pain on insertion has far reaching implications. We need to look at the patient in totality, ruling out underlying issues related to the bladder, even the colon.
Patient inquires about the benefits of laser treatment for vaginal health
Dr. Levy: Let's move to this last case: A 47-year-old patient who reports lack of sexual satisfaction and attributes it to a loose vagina says, "I've heard about surgery and laser treatment and radiofrequency devices for vaginal health. What are the benefits and risks of these procedures, and will they correct the issues that I'm experiencing?"
How do you approach this patient?
Dr. Krychman: I want to know why this is of paramount importance to her. Is this her actual complaint or is it society's unrealistic expectation of sexual pleasure and performance placed upon her? Or is this a relationship issue surfacing, compounded by physiologic changes? With good communication techniques, like "ask, tell, ask" or effective use of silence (not interrupting), patients will lead us to the reason and the rationale.
Dr. Levy: I have seen a lot of advertising to women, now showing pictures of genitalia, perhaps creating an expectation that we should look infantile in some way. We are creating a sense of beauty and acceptablevisualization of the vagina and vulva that are completely unrealistic. It's fascinating on one hand but it is also disturbing in that some of the direct-to-consumer marketing going on is creating a sense of unease in women who are otherwise perfectly satisfied. Now they take a look at their sagging skin, maybe after having 2 or 3 children, and although they may not look the same they function not so badly perhaps. I think we are creating a distress and an illness model that is interesting to discuss.
Dr. Iglesia, you are in the midst of a randomized trial, giving an informed consent to participants about the expectations for this potential intervention. How do you explain to women what these laser and radiofrequency devices are expected to do and why they might or might not work?
Dr. Iglesia: We are doing a randomized trial for menopausal women who have GSM. We are comparing estrogen cream with fractional CO2 laser therapy. I also am involved in another randomized trial for lichen sclerosus. I am not involved in the cosmetic use of a laser for people who feel their vaginas are just "loose." Like you, Dr. Levy, I am very concerned about the images that women are seeing of the idealized vulva and vagina, and about the rise of cosmetic gynecology, much of which is being performed by plastic surgeons or dermatologists.
A recent article looked at the number of women who are doing pubic hair grooming; the prevalance is about 80% here in America.8 So people have a clear view of what is down there, and then they compare it to what they see in pornographic images on the Internet and want to look like a Barbie doll. That is disturbing because women, particularly young women, do not realize what happens with GSM changes to the vulva and vagina. On the other hand, these laser machines are very expensive, and some doctors are charging thousands of dollars and promising cosmetic and functional results for which we lack long-term, comparative data.
The laser that we are studying is one by Cynosure called Mona Lisa, which works with fractional CO2 and has very low depth of penetration. The concept is that, with microdot therapy (on the order of micrometers), pinpoint destruction will foster regeneration of new collagen and blood flow to the vagina and vulva. We are still in the midst of analyzing this.
Dr. Krychman: I caution people not to lump devices together. There is a significant difference between laser and radiofrequency--especially in the depth of tissue penetration, and level of evidence as well. There are companies performing randomized clinical trials, with well designed sham controls, and have demonstrated clinical efficacy. We need to be cautious of a procedure that is saying it's the best thing since sliced bread, curing interstitial cystitis, dyspareunia, and lichen sclerosus and improving orgasm, lubrication, and arousal. These far reaching, off-label claims are concerning and misleading.
Dr. Levy: I think the important things are 1) shared decision making with the patient and 2) disclosure of what we do not know, which are the long-term results and outcomes and possible downstream negative effects of some of these treatments, since the data we have are so short term.
Dr. Kingsberg: Basics are important. You talked about the pressure for cosmetic appearance, but is that really what is going on for this particular patient? Is she describing a sexual dysfunction when she talks about lack of satisfaction? You need to operationally define that term. Does she have problems with arousal or orgasm or desire and those are what underlie her lack of satisfaction? Is the key to management helping her come to terms with body image issues or to treat a sexual dysfunction? If it truly is a sexual dysfunction, then you can have the shared decision making on preferred treatment approach.
Dr. Levy: This has been an enlightening discussion. Thank all of you for your expertise and clinical acumen.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM), Fifth Edition. American Psychiatric Association Publishing: Arlington, VA; 2013.
- American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: The Use of Vaginal Estrogen in Women With a History of Estrogen-Dependent Breast Cancer. Obstet Gynecol. 2016;127(3):e93−e96.
- Krychman ML, Shifren JL, Liu JH, Kingsberg SL, Utian WH. The North American Menopause Society (NAMS). NAMS Menopause e-Consult: Laser treatment safe for vulvovaginal atrophy? 2015;11(3). http://www.medscape.com/viewarticle/846960. Accessed August 17, 2016.
- The American College of Obstetricians and Gynecologists and The American Congress of Obstetricians and Gynecologists. Fractional laser treatment of vulvovaginal atrophy and U.S. Food and Drug Administration clearance: Position Statement. http://www.acog.org/Resources-And-Publications/Position-Statements/Fractional-Laser-Treatment-of-Vulvovaginal-Atrophy-and-US-Food-and-Drug-Administration-Clearance. Published May 2016. Accessed August 17, 2016.
- Lukacz ES, Warren LK, Richter HE, et al. Quality of life and sexual function 2 years after vaginal surgery for prolapse. Obstet Gynecol. 2016;127(6):1071−1079.
- Brubaker L, Chiang S, Zyczynski H, et al. The impact of stress incontinence surgery on female sexual function. Am J Obstet Gynecol. 2009;200(5):562.e1.
- Rowen TS, Gaither TW, Awad MA, Osterberg EC, Shindel AW, Breyer BN. Pubic hair grooming prevalence and motivation among women in the United States [published online ahead of print June 29, 2016]. JAMA Dermatol. doi:10.1001/jamader matol.2016.2154.
The age-adjusted prevalence of any sexual problem is 43% among US women. A full 22% of these women experience sexually related personal distress.1 With publication of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition2 has come a shift in classification and, at times, management approach for reported female sexual dysfunction. When women report to their clinicians decreased sexual desire or arousal or pain at penetration, the management is no longer guided by a linear model of sexual response (excitation, plateau, orgasm, and resolution) but rather by a more nuanced and complex biopsychosocial approach. In this model, diagnosis and management strategies to address bothersome sexual concerns consider the whole woman in the context of her physical and psychosocial health. The patient’s age, medical history, and relationship status are among the factors that could affect management of the problem. In an effort to explore this management approach, I used this Update on Female Sexual Dysfunction as an opportunity to convene a roundtable of several experts, representing varying backgrounds and practice vantage points, to discuss 5 cases of sexual problems that you as a busy clinician may encounter in your practice.

Genital atrophy in a sexually inactive 61-year-old woman
Barbara S. Levy, MD: Two years after her husband's death, which followed several years of illness, your 61-year-old patient mentions at her well woman visit that she anticipates becoming sexually active again. She has not used systemic or vaginal hormone therapy. During pelvic examination, atrophic external genital changes are present, and use of an ultrathin (thinner than a Pederson) speculum reveals vaginal epithelial atrophic changes. A single-digit bimanual exam can be performed with moderate patient discomfort; the patient cannot tolerate a 2-digit bimanual exam. She expresses concern about being able to engage in penile/vaginal sexual intercourse.
Dr. Kaunitz, what is important for you to ask this patient, and what concerns you most on her physical exam?
Andrew M. Kaunitz, MD: First, it is important to recognize the patient's expectations and desires. As the case suggests, but further questioning could clarify, she would like to be able to comfortably engage in sexual intercourse with a new partner, but penetration may be difficult (and definitely painful for her) unless treatment is pursued. This combination of mucosal and vestibular atrophic changes (genitourinary syndrome of menopause [GSM], or vulvovaginal atrophy [VVA]) plus the absence of penetration for many years can be a double whammy situation for menopausal women. In this case it has led to extensive contracture of the introitus, and if it is not addressed will cause sexual dysfunction.
Dr. Levy: In addition we need to clarify whether or not a history of breast cancer or some other thing may impact the care we provide. How would you approach talking with this patient in order to manage her care?
Dr. Kaunitz: One step is to see how motivated she is to address this, as it is not something that, as gynecologists, we can snap our fingers and the situation will be resolved. If the patient is motivated to treat the atrophic changes with medical treatment, in the form of low-dose vaginal estrogen, and dilation, either on her own if she's highly motivated to do so, or in my practice more commonly with the support of a women's physical therapist, over time she should be able to comfortably engage in sexual intercourse with penetration. If this is what she wants, we can help steer her in the right direction.
Sheryl Kingsberg, PhD: You know that this woman is motivated by virtue of her initiating the topic herself. Patients are often embarrassed talking about sexual issues, or they are not sure that their gynecologist is comfortable with it. After all, they think, if this is the right place to discuss sexual problems, why didn't he or she ask me? Clinicians must be aware that it is their responsibility to ask about sexual function and not leave it for the patient to open the door.
Dr. Kaunitz: Great point.
Cheryl B. Iglesia, MD: Gratefully, a lot of the atrophic changes this patient demonstrates are reversible. However, other autoimmune diseases (eg, lichen planus, which can affect the vaginal epithelium, or lichen sclerosus, which can affect the clitoris, labia, and vulva) can also cause constriction, and in severe cases, complete obliteration of the vagina and introitus. Women may not be sexually active, and for each annual exam their clinician uses a smaller and smaller speculum--to the point that they cannot even access the cervix anymore--and the vagina can close off. Clinicians may not realize that you need something other than estrogen; with lichen planus you need steroid suppository treatment, and with lichen sclerosus you need topical steroid treatment. So these autoimmune conditions should also be in the differential and, with appropriate treatment, the sexual effects can be reversible.
Michael Krychman, MD: I agree. The vulva can be a great mimicker and, according to the history and physical exam, at some point a vulvoscopy, and even potential biopsies, may be warranted as clinically indicated.
The concept of a comprehensive approach, as Dr. Kingsberg had previously mentioned, involves not only sexual medicine but also evaluating the patient's biopsychosocial variables that may impact her condition. We also need to set realistic expectations. Some women may benefit from off-label use of medications besides estrogen, including topical testosterone. Informed consent is very important with these treatments. I also have had much clinical success with intravaginal diazepam/lorazepam for pelvic floor hypertonus.
In addition, certainly I agree that pelvic floor physical therapy (PT) is a vital treatment component for this patient and, not to diminish its importance, but many women cannot afford, nor do they have the time or opportunity, to go to pelvic floor PT. As clinicians, we can develop and implement effective programs, even in the office, to educate the patient to help herself as well.
Dr. Kaunitz: Absolutely. Also, if, in a clinical setting consistent with atrophic changes, an ObGyn physician is comfortable that vulvovaginal changes noted on exam represent GSM/atrophic changes, I do not feel vulvoscopy is warranted.
Dr. Levy: In conclusion, we need to be aware that pelvic floor PT may not be available everywhere and that a woman's own digits and her partner can also be incorporated into this treatment.
Something that we have all talked about in other venues, but have not looked at in the larger sphere here, is whether there is value to seeing women annually and performing pelvic exams. As Dr. Kingsberg mentioned, this is a highly motivated patient. We have many patients out there who are silent sufferers. The physical exam is an opportunity for us to recognize and address this problem.

Dyspareunia and low sexual desire in a breast cancer survivor
Dr. Levy: In this case, a 36-year-old woman with BRCA1−positive breast cancer has vaginal dryness, painful intercourse, and lowered sexual interest since her treatment, which included chemotherapy after bilateral mastectomies. She has a bilateral salpingo-oophorectomy(BSO) scheduled for primary prevention of her ovarian cancer risk.
Dr. Kingsberg, what is important for you to know to help guide case management?
Dr. Kingsberg: This woman is actually presenting with 2 sexual problems: dyspareunia, which is probably secondary to VVA or GSM, and low sexual desire. Key questions are: 1) When was symptom onset--acquired after treatment or lifelong? 2) Did she develop the dyspareunia and as a result of having pain during sex lost desire to have sex? Or, did she lose desire and then, without it, had no arousal and therefore pain with penetration developed? It also could be that she has 2 distinct problems, VVA and hypoactive sexual desire disorder (HSDD), in which case you need to think about treating both. Finally, we do not actually know if she is having penetrative intercourse or even if she has a partner.
A vulvovaginal exam would give clues as to whether she has VVA, and hormone levels would indicate if she now has chemo-induced menopause. If she is not in menopause now, she certainly is going to be with her BSO. The hormonal changes due to menopause actually can be primarily responsible for both the dyspareunia and HSDD. Management of both symptoms really needs to be based on shared decision making with the patient--with which treatment for which conditions coming first, based on what is causing her the most distress.
I would encourage this woman to treat her VVA since GSM does have long-term physiologic consequences if untreated. The American College of Obstetricians and Gynecologists (ACOG) recommends nonhormonal treatments as first-line treatments, with vaginal estrogen considered if these therapies fail.3 If lubricants and moisturizers and other nonhormonal options are not sufficient, you could consider local estrogen, even though she is a breast cancer survivor, as well as ospemiphene.
If she is distressed by her loss of sexual desire, you can choose to treat her for HSDD. Flibanserin is the first FDA-approved treatment for HSDD. It is only approved in premenopausal women, so it would be considered off-label use if she is postmenopausal (even though she is quite young). You also could consider exogenous testosterone off-label, after consulting with her oncologist.
In addition to the obvious physiologic etiology of her pain and her low desire, the biopsychosocial aspects to consider are: 1) changes to her body image, as she has had bilateral mastectomies, 2) her anxiety about the cancer diagnosis, and 3) concerns about her relationship if she has one--her partner's reactions to her illness and the quality of the relationship outside the bedroom.
Dr. Iglesia: I am seeing here in our nation's capital a lot of advertisements for laser therapy for GSM. I caution women about this because providers are charging a lot of money for this therapy when we do not have long-term safety and effectiveness data for it.
Our group is currently conducting a randomized controlled trial, looking at vaginal estrogen cream versus laser therapy for GSM here at Medstar Health--one of the first in the country as part of a multisite trial. But the North American Menopause Society (NAMS) has come out with a pretty strong statement,4 as has ACOG,5 on this therapy, and I caution people about overzealously offering a very costly procedure targeted to a very vulnerable population, especially to women with personal histories of estrogen-sensitive cancers.
Dr. Krychman: I agree. Very often cancer patients are preyed upon by those offering emerging unproven technologies or medications. We have to work as a coordinated comprehensive team, whether it's a sexual medicine expert, psychologist, urogynecologist, gynecologist, or oncologist, and incorporate the patient's needs and expectations and risk tolerance coupled with treatment efficacy and safety.
Dr. Levy: This was a complex case. The biopsychosocial model is critical here. It's important that we are not siloes in our medical management approach and that we try to help this patient embrace the complexity of her situation. It's not only that she has cancer at age 36; there is a possible guilt factor if she has children and passed that gene on.
Another point that we began to talk about is the fact that in this country we tend to be early adopters of new technology. In our discussion with patients, we should focus on what we know and the risk of the unknowns related to some of the treatment options. But let's discuss lasers a little more later on.
Diminished arousal and orgasmic intensity in a patient taking SSRIs
Dr. Levy: In this next case, a 44-year-old woman in a 15-year marriage notices a change in her orgasmic intensity and latency. She has a supportive husband, and they are attentive to each other's sexual needs. However, she notices a change in her arousal and orgasmic intensity, which has diminished over the last year. She reports that the time to orgasm or latency has increased and both she and her partner are frustrated and getting concerned. She has a history of depression that has been managed by selective serotonin reuptake inhibitors for the past 5 years and has no depressive symptoms currently.
Dr. Krychman, what are you considering before beginning to talk with this patient?
Dr. Krychman: My approach really is a comprehensive one, looking not only at the underlying medical issues but also at the psychological and dynamic relationship facets. We of course also want to look at medications: Has she changed her dose or the timing of when she takes it? Is this a new onset? Finally, we want to know why this is coming to the forefront now. Is it because it is getting worse, or is it because there is some significant issue that is going on in the relationship?
Regarding the physical exam, it is important to rule out underlying genital pelvic pathology. Young women can get changes in the integrity of the pelvic floor, in what I would call the orgasmic matrix--the clitoral tissue, the body, the crura (or arms of the clitoris)--we want to examine and be reassured that her genital anatomy is normal and that there is no underlying pathology that could signal an underlying abnormal hormonal profile. Young women certainly can get lowered estrogen effects at the genital/pelvic tissues (including the labia and vulva), and intravaginally as well. Sometimes women will have pelvic floor hypertonus, as we see with other urinary issues. A thorough pelvic exam is quite vital.
Let's not forget the body that is attached to the genitals; we want to rule out chronic medical disease that may impact her: hypertension, diabetes, or hypercholesterolemia. Untreated, these conditions may directly impact the arousal physiologic mechanisms.
Dr. Levy: In doing this patient's physical exam I would be looking for significant weight gain, and even asking about her partner's weight. Body image can be a huge issue. If she has a history of depression, if she is suffering from a body image problem, she can be feeling unattractive. In my experience this can be a common thing to affect women in their mid-40s.
How would you manage this case?
Dr. Krychman: It is important to divide it up in terms of a conservative to aggressive approach. We want to find out about the relationship. For instance, is the sexual dynamic scripted (ie, boring and predictable)? Is she distracted and frustrated or is she getting enough of the type of stimulation that she likes and enjoys? There certainly are a lot of new devices that are available, whether a self-stimulator or vibrator, the Fiera, or other stimulating devices, that may be important to incorporate into the sexual repertoire. If there is underlying pathology, we want to evaluate and treat that. She may need to be primed, so to speak, with systemic hormones. And does she have issues related to other effects of hormonal deprivation, even local effects? Does she have clitoral atrophy?
There are neutraceuticals that are currently available, whether topical arousal gels or ointments, and we as clinicians need to be critical and evaluate their benefit/risk and look at the data concerning these products. In addition, women who have changes in arousal and in orgasmic intensity and latency may be very frustrated. They describe it as climbing up to a peak but never getting over the top, and this frustration may lead to participant spectatoring, so incorporating a certified sex therapist or counselor is sometimes very critical.
Finally, there are a lot of snake oils, charmers, and charlatan unproven procedures--injecting fillers or other substances into the clitoris are a few examples. I would be a critical clinician, examine the evidence, look at the benefit/risk before advocating an intervention that does not have good clinical data to support its use--a comprehensive approach of sexual medicine as well as sexual psychology.
Dr. Kingsberg: Additionally, we know she is in a long-term relationship--15 years; we want to acknowledge the partner. We talked about the partner's weight, but what about his erectile function? Does he have changes in sexual function that are affecting her, and she is the one carrying the "symptom"?
Looking at each piece separately helps a clinician from getting overwhelmed by the patient who comes in reporting distress with orgasmic dysfunction. We have no pharmacologic FDA-approved treatments, so it can feel off-putting for a clinician to try to fix the reported issue. Looking at each component to help her figure out the underlying cause can be helpful.
Dr. Iglesia: With aging, there can be changes in blood flow, not to mention the hormonal and even peripheral nerve changes, that require more stimulation in order to achieve the desired response. I echo concern about expensive procedures being offered with no evidence, such as the "O" or "G" shot, that can cost up to thousands of dollars.
The other procedure that gives me a lot of angst is clitoral unhooding. The 3 parts of the clitoris are sensitive in terms of innervation and blood flow, and cutting around that delicate tissue goes against the surgical principles required for preserving nerves and blood flow.
New onset pain post−prolapse surgery with TOT sling placement
Dr. Levy: For this case, let's consider a 42-year-old woman (P3) who is 6 months post− vaginal hysterectomy. The surgery included ovarian preservation combined with anterior and posterior repair for prolapse as well as apical uterosacral ligament suspension for stage 2 uterovaginal prolapse. A transobturator sling was used.
Extensive preop evaluation was performed, with confirmed symptomatic prolapse. She had no stress incontinence symptoms but did have confirmed occult stress incontinence.
Surgery was uneventful. She resumed intercourse at 8 weeks, but she now has pain with both initial entry and deep penetration. Lubricants and changes in position have not helped. She is in a stable relationship with her husband of 17 years, and she is worried that the sling mesh might be the culprit. On exam, she has no atrophy, pH is 4.5, vaginal length is 8 cm, and there is no prolapse. There is no mesh exposure noted, although she reports slight tenderness with palpation of the right sling arm beneath the right pubic bone.
Dr. Iglesia, what are the patient history questions important to ask here?
Dr. Iglesia: This is not an uncommon scenario--elective surgical correction of occult or latent stress incontinence after surgical correction for pelvic organ prolapse. Now this patient here has no more prolapse complaints; however, she has a new symptom. There are many different causes of dyspareunia; we cannot just assume it is the sling mesh (although with all the legal representation advertisements for those who have had mesh placed, it can certainly be at the top of the patient's mind, causing anxiety and fear).
Multiple trials have looked at prophylactic surgery for incontinence at the time of prolapse repairs. This woman happened to be one of those patients who did not have incontinence symptoms, and they put a sling in. A recent large trial examined women with vaginal prolapse who underwent hysterectomy and suspension.6 (They compared 2 different suspensions.) What is interesting is that 25% of women with prolapse do have baseline pain. However, at 24 months, de novo pain can occur in 10% of women--just from the apical suspension. So, here, it could be the prolapse suspension. Or, in terms of the transobturator sling, long-term data do tell us that the de novo dyspareunia rate ranges on the magnitude of 1% to 9%.7 What is important here is figuring out the cause of the dyspareunia.
Dr. Levy: One of the important points you raised already was that 25% of these women have preoperative pain. So figuring out what her functioning was before surgery and incorporating that into our assessment postop would be pretty important I would think.
Dr. Iglesia: Yes, you need to understand what her typical encounter was before the surgery and how things have changed now that the prolapse is not in the way. Changes obviously can occur with scar tissue, which over time will improve. If she is perimenopausal and starts to get epithelial changes, we can fix that. The question then becomes, "Is the pain emanating from the mesh?"
When examining this patient, it is not uncommon for me to be able to feel "banjo" strings if the mesh is too tight or close to the surface. It is not exposed but it's palpable, and the patient may feel a ridge during penetration. You can ask the patient if pain occurs with different penetration positions. In addition, explore associated neurologic symptoms (numbness or muscle pain in the thigh).
Dr. Kingsberg: There were 2 different sources of pain--on initial entry and at deep penetration. You want to make sure you address both. Importantly, did one precede the other? For instance, if women have pain with penetration they can then end up with an arousal disorder (the length of the vagina cannot increase as much as it might otherwise) and dystonia secondary to the pain with penetration. The timing of the pain--did it all happen at the same time, or did she start out with pain at one point and did it move to something else--is another critical piece of the history.
Dr. Iglesia: It does take a detailed history and physical exam to identify myofascial trigger points. In this case there seems to be pinpoint tenderness directly on the part of the mesh that is not exposed on the right. There are people who feel you should remove the whole mesh, including the arms. Others feel, okay, we can work on these trigger points, with injections, physical therapy, extra lubrication, and neuromodulatory medications. Only then would they think about potentially excising the sling or a portion thereof.
Dr. Krychman: Keep in mind that, even if you do remove the sling, her pain may not subside, if it is secondary to an underlying issue. Because of media sensationalism, she could be focusing on the sling. It is important to set realistic expectations. I often see vulvar pathology or even provoked vestibulodynia that can present with a deep dyspareunia. The concept of collision dyspareunia or introital discomfort or pain on insertion has far reaching implications. We need to look at the patient in totality, ruling out underlying issues related to the bladder, even the colon.
Patient inquires about the benefits of laser treatment for vaginal health
Dr. Levy: Let's move to this last case: A 47-year-old patient who reports lack of sexual satisfaction and attributes it to a loose vagina says, "I've heard about surgery and laser treatment and radiofrequency devices for vaginal health. What are the benefits and risks of these procedures, and will they correct the issues that I'm experiencing?"
How do you approach this patient?
Dr. Krychman: I want to know why this is of paramount importance to her. Is this her actual complaint or is it society's unrealistic expectation of sexual pleasure and performance placed upon her? Or is this a relationship issue surfacing, compounded by physiologic changes? With good communication techniques, like "ask, tell, ask" or effective use of silence (not interrupting), patients will lead us to the reason and the rationale.
Dr. Levy: I have seen a lot of advertising to women, now showing pictures of genitalia, perhaps creating an expectation that we should look infantile in some way. We are creating a sense of beauty and acceptablevisualization of the vagina and vulva that are completely unrealistic. It's fascinating on one hand but it is also disturbing in that some of the direct-to-consumer marketing going on is creating a sense of unease in women who are otherwise perfectly satisfied. Now they take a look at their sagging skin, maybe after having 2 or 3 children, and although they may not look the same they function not so badly perhaps. I think we are creating a distress and an illness model that is interesting to discuss.
Dr. Iglesia, you are in the midst of a randomized trial, giving an informed consent to participants about the expectations for this potential intervention. How do you explain to women what these laser and radiofrequency devices are expected to do and why they might or might not work?
Dr. Iglesia: We are doing a randomized trial for menopausal women who have GSM. We are comparing estrogen cream with fractional CO2 laser therapy. I also am involved in another randomized trial for lichen sclerosus. I am not involved in the cosmetic use of a laser for people who feel their vaginas are just "loose." Like you, Dr. Levy, I am very concerned about the images that women are seeing of the idealized vulva and vagina, and about the rise of cosmetic gynecology, much of which is being performed by plastic surgeons or dermatologists.
A recent article looked at the number of women who are doing pubic hair grooming; the prevalance is about 80% here in America.8 So people have a clear view of what is down there, and then they compare it to what they see in pornographic images on the Internet and want to look like a Barbie doll. That is disturbing because women, particularly young women, do not realize what happens with GSM changes to the vulva and vagina. On the other hand, these laser machines are very expensive, and some doctors are charging thousands of dollars and promising cosmetic and functional results for which we lack long-term, comparative data.
The laser that we are studying is one by Cynosure called Mona Lisa, which works with fractional CO2 and has very low depth of penetration. The concept is that, with microdot therapy (on the order of micrometers), pinpoint destruction will foster regeneration of new collagen and blood flow to the vagina and vulva. We are still in the midst of analyzing this.
Dr. Krychman: I caution people not to lump devices together. There is a significant difference between laser and radiofrequency--especially in the depth of tissue penetration, and level of evidence as well. There are companies performing randomized clinical trials, with well designed sham controls, and have demonstrated clinical efficacy. We need to be cautious of a procedure that is saying it's the best thing since sliced bread, curing interstitial cystitis, dyspareunia, and lichen sclerosus and improving orgasm, lubrication, and arousal. These far reaching, off-label claims are concerning and misleading.
Dr. Levy: I think the important things are 1) shared decision making with the patient and 2) disclosure of what we do not know, which are the long-term results and outcomes and possible downstream negative effects of some of these treatments, since the data we have are so short term.
Dr. Kingsberg: Basics are important. You talked about the pressure for cosmetic appearance, but is that really what is going on for this particular patient? Is she describing a sexual dysfunction when she talks about lack of satisfaction? You need to operationally define that term. Does she have problems with arousal or orgasm or desire and those are what underlie her lack of satisfaction? Is the key to management helping her come to terms with body image issues or to treat a sexual dysfunction? If it truly is a sexual dysfunction, then you can have the shared decision making on preferred treatment approach.
Dr. Levy: This has been an enlightening discussion. Thank all of you for your expertise and clinical acumen.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The age-adjusted prevalence of any sexual problem is 43% among US women. A full 22% of these women experience sexually related personal distress.1 With publication of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition2 has come a shift in classification and, at times, management approach for reported female sexual dysfunction. When women report to their clinicians decreased sexual desire or arousal or pain at penetration, the management is no longer guided by a linear model of sexual response (excitation, plateau, orgasm, and resolution) but rather by a more nuanced and complex biopsychosocial approach. In this model, diagnosis and management strategies to address bothersome sexual concerns consider the whole woman in the context of her physical and psychosocial health. The patient’s age, medical history, and relationship status are among the factors that could affect management of the problem. In an effort to explore this management approach, I used this Update on Female Sexual Dysfunction as an opportunity to convene a roundtable of several experts, representing varying backgrounds and practice vantage points, to discuss 5 cases of sexual problems that you as a busy clinician may encounter in your practice.

Genital atrophy in a sexually inactive 61-year-old woman
Barbara S. Levy, MD: Two years after her husband's death, which followed several years of illness, your 61-year-old patient mentions at her well woman visit that she anticipates becoming sexually active again. She has not used systemic or vaginal hormone therapy. During pelvic examination, atrophic external genital changes are present, and use of an ultrathin (thinner than a Pederson) speculum reveals vaginal epithelial atrophic changes. A single-digit bimanual exam can be performed with moderate patient discomfort; the patient cannot tolerate a 2-digit bimanual exam. She expresses concern about being able to engage in penile/vaginal sexual intercourse.
Dr. Kaunitz, what is important for you to ask this patient, and what concerns you most on her physical exam?
Andrew M. Kaunitz, MD: First, it is important to recognize the patient's expectations and desires. As the case suggests, but further questioning could clarify, she would like to be able to comfortably engage in sexual intercourse with a new partner, but penetration may be difficult (and definitely painful for her) unless treatment is pursued. This combination of mucosal and vestibular atrophic changes (genitourinary syndrome of menopause [GSM], or vulvovaginal atrophy [VVA]) plus the absence of penetration for many years can be a double whammy situation for menopausal women. In this case it has led to extensive contracture of the introitus, and if it is not addressed will cause sexual dysfunction.
Dr. Levy: In addition we need to clarify whether or not a history of breast cancer or some other thing may impact the care we provide. How would you approach talking with this patient in order to manage her care?
Dr. Kaunitz: One step is to see how motivated she is to address this, as it is not something that, as gynecologists, we can snap our fingers and the situation will be resolved. If the patient is motivated to treat the atrophic changes with medical treatment, in the form of low-dose vaginal estrogen, and dilation, either on her own if she's highly motivated to do so, or in my practice more commonly with the support of a women's physical therapist, over time she should be able to comfortably engage in sexual intercourse with penetration. If this is what she wants, we can help steer her in the right direction.
Sheryl Kingsberg, PhD: You know that this woman is motivated by virtue of her initiating the topic herself. Patients are often embarrassed talking about sexual issues, or they are not sure that their gynecologist is comfortable with it. After all, they think, if this is the right place to discuss sexual problems, why didn't he or she ask me? Clinicians must be aware that it is their responsibility to ask about sexual function and not leave it for the patient to open the door.
Dr. Kaunitz: Great point.
Cheryl B. Iglesia, MD: Gratefully, a lot of the atrophic changes this patient demonstrates are reversible. However, other autoimmune diseases (eg, lichen planus, which can affect the vaginal epithelium, or lichen sclerosus, which can affect the clitoris, labia, and vulva) can also cause constriction, and in severe cases, complete obliteration of the vagina and introitus. Women may not be sexually active, and for each annual exam their clinician uses a smaller and smaller speculum--to the point that they cannot even access the cervix anymore--and the vagina can close off. Clinicians may not realize that you need something other than estrogen; with lichen planus you need steroid suppository treatment, and with lichen sclerosus you need topical steroid treatment. So these autoimmune conditions should also be in the differential and, with appropriate treatment, the sexual effects can be reversible.
Michael Krychman, MD: I agree. The vulva can be a great mimicker and, according to the history and physical exam, at some point a vulvoscopy, and even potential biopsies, may be warranted as clinically indicated.
The concept of a comprehensive approach, as Dr. Kingsberg had previously mentioned, involves not only sexual medicine but also evaluating the patient's biopsychosocial variables that may impact her condition. We also need to set realistic expectations. Some women may benefit from off-label use of medications besides estrogen, including topical testosterone. Informed consent is very important with these treatments. I also have had much clinical success with intravaginal diazepam/lorazepam for pelvic floor hypertonus.
In addition, certainly I agree that pelvic floor physical therapy (PT) is a vital treatment component for this patient and, not to diminish its importance, but many women cannot afford, nor do they have the time or opportunity, to go to pelvic floor PT. As clinicians, we can develop and implement effective programs, even in the office, to educate the patient to help herself as well.
Dr. Kaunitz: Absolutely. Also, if, in a clinical setting consistent with atrophic changes, an ObGyn physician is comfortable that vulvovaginal changes noted on exam represent GSM/atrophic changes, I do not feel vulvoscopy is warranted.
Dr. Levy: In conclusion, we need to be aware that pelvic floor PT may not be available everywhere and that a woman's own digits and her partner can also be incorporated into this treatment.
Something that we have all talked about in other venues, but have not looked at in the larger sphere here, is whether there is value to seeing women annually and performing pelvic exams. As Dr. Kingsberg mentioned, this is a highly motivated patient. We have many patients out there who are silent sufferers. The physical exam is an opportunity for us to recognize and address this problem.

Dyspareunia and low sexual desire in a breast cancer survivor
Dr. Levy: In this case, a 36-year-old woman with BRCA1−positive breast cancer has vaginal dryness, painful intercourse, and lowered sexual interest since her treatment, which included chemotherapy after bilateral mastectomies. She has a bilateral salpingo-oophorectomy(BSO) scheduled for primary prevention of her ovarian cancer risk.
Dr. Kingsberg, what is important for you to know to help guide case management?
Dr. Kingsberg: This woman is actually presenting with 2 sexual problems: dyspareunia, which is probably secondary to VVA or GSM, and low sexual desire. Key questions are: 1) When was symptom onset--acquired after treatment or lifelong? 2) Did she develop the dyspareunia and as a result of having pain during sex lost desire to have sex? Or, did she lose desire and then, without it, had no arousal and therefore pain with penetration developed? It also could be that she has 2 distinct problems, VVA and hypoactive sexual desire disorder (HSDD), in which case you need to think about treating both. Finally, we do not actually know if she is having penetrative intercourse or even if she has a partner.
A vulvovaginal exam would give clues as to whether she has VVA, and hormone levels would indicate if she now has chemo-induced menopause. If she is not in menopause now, she certainly is going to be with her BSO. The hormonal changes due to menopause actually can be primarily responsible for both the dyspareunia and HSDD. Management of both symptoms really needs to be based on shared decision making with the patient--with which treatment for which conditions coming first, based on what is causing her the most distress.
I would encourage this woman to treat her VVA since GSM does have long-term physiologic consequences if untreated. The American College of Obstetricians and Gynecologists (ACOG) recommends nonhormonal treatments as first-line treatments, with vaginal estrogen considered if these therapies fail.3 If lubricants and moisturizers and other nonhormonal options are not sufficient, you could consider local estrogen, even though she is a breast cancer survivor, as well as ospemiphene.
If she is distressed by her loss of sexual desire, you can choose to treat her for HSDD. Flibanserin is the first FDA-approved treatment for HSDD. It is only approved in premenopausal women, so it would be considered off-label use if she is postmenopausal (even though she is quite young). You also could consider exogenous testosterone off-label, after consulting with her oncologist.
In addition to the obvious physiologic etiology of her pain and her low desire, the biopsychosocial aspects to consider are: 1) changes to her body image, as she has had bilateral mastectomies, 2) her anxiety about the cancer diagnosis, and 3) concerns about her relationship if she has one--her partner's reactions to her illness and the quality of the relationship outside the bedroom.
Dr. Iglesia: I am seeing here in our nation's capital a lot of advertisements for laser therapy for GSM. I caution women about this because providers are charging a lot of money for this therapy when we do not have long-term safety and effectiveness data for it.
Our group is currently conducting a randomized controlled trial, looking at vaginal estrogen cream versus laser therapy for GSM here at Medstar Health--one of the first in the country as part of a multisite trial. But the North American Menopause Society (NAMS) has come out with a pretty strong statement,4 as has ACOG,5 on this therapy, and I caution people about overzealously offering a very costly procedure targeted to a very vulnerable population, especially to women with personal histories of estrogen-sensitive cancers.
Dr. Krychman: I agree. Very often cancer patients are preyed upon by those offering emerging unproven technologies or medications. We have to work as a coordinated comprehensive team, whether it's a sexual medicine expert, psychologist, urogynecologist, gynecologist, or oncologist, and incorporate the patient's needs and expectations and risk tolerance coupled with treatment efficacy and safety.
Dr. Levy: This was a complex case. The biopsychosocial model is critical here. It's important that we are not siloes in our medical management approach and that we try to help this patient embrace the complexity of her situation. It's not only that she has cancer at age 36; there is a possible guilt factor if she has children and passed that gene on.
Another point that we began to talk about is the fact that in this country we tend to be early adopters of new technology. In our discussion with patients, we should focus on what we know and the risk of the unknowns related to some of the treatment options. But let's discuss lasers a little more later on.
Diminished arousal and orgasmic intensity in a patient taking SSRIs
Dr. Levy: In this next case, a 44-year-old woman in a 15-year marriage notices a change in her orgasmic intensity and latency. She has a supportive husband, and they are attentive to each other's sexual needs. However, she notices a change in her arousal and orgasmic intensity, which has diminished over the last year. She reports that the time to orgasm or latency has increased and both she and her partner are frustrated and getting concerned. She has a history of depression that has been managed by selective serotonin reuptake inhibitors for the past 5 years and has no depressive symptoms currently.
Dr. Krychman, what are you considering before beginning to talk with this patient?
Dr. Krychman: My approach really is a comprehensive one, looking not only at the underlying medical issues but also at the psychological and dynamic relationship facets. We of course also want to look at medications: Has she changed her dose or the timing of when she takes it? Is this a new onset? Finally, we want to know why this is coming to the forefront now. Is it because it is getting worse, or is it because there is some significant issue that is going on in the relationship?
Regarding the physical exam, it is important to rule out underlying genital pelvic pathology. Young women can get changes in the integrity of the pelvic floor, in what I would call the orgasmic matrix--the clitoral tissue, the body, the crura (or arms of the clitoris)--we want to examine and be reassured that her genital anatomy is normal and that there is no underlying pathology that could signal an underlying abnormal hormonal profile. Young women certainly can get lowered estrogen effects at the genital/pelvic tissues (including the labia and vulva), and intravaginally as well. Sometimes women will have pelvic floor hypertonus, as we see with other urinary issues. A thorough pelvic exam is quite vital.
Let's not forget the body that is attached to the genitals; we want to rule out chronic medical disease that may impact her: hypertension, diabetes, or hypercholesterolemia. Untreated, these conditions may directly impact the arousal physiologic mechanisms.
Dr. Levy: In doing this patient's physical exam I would be looking for significant weight gain, and even asking about her partner's weight. Body image can be a huge issue. If she has a history of depression, if she is suffering from a body image problem, she can be feeling unattractive. In my experience this can be a common thing to affect women in their mid-40s.
How would you manage this case?
Dr. Krychman: It is important to divide it up in terms of a conservative to aggressive approach. We want to find out about the relationship. For instance, is the sexual dynamic scripted (ie, boring and predictable)? Is she distracted and frustrated or is she getting enough of the type of stimulation that she likes and enjoys? There certainly are a lot of new devices that are available, whether a self-stimulator or vibrator, the Fiera, or other stimulating devices, that may be important to incorporate into the sexual repertoire. If there is underlying pathology, we want to evaluate and treat that. She may need to be primed, so to speak, with systemic hormones. And does she have issues related to other effects of hormonal deprivation, even local effects? Does she have clitoral atrophy?
There are neutraceuticals that are currently available, whether topical arousal gels or ointments, and we as clinicians need to be critical and evaluate their benefit/risk and look at the data concerning these products. In addition, women who have changes in arousal and in orgasmic intensity and latency may be very frustrated. They describe it as climbing up to a peak but never getting over the top, and this frustration may lead to participant spectatoring, so incorporating a certified sex therapist or counselor is sometimes very critical.
Finally, there are a lot of snake oils, charmers, and charlatan unproven procedures--injecting fillers or other substances into the clitoris are a few examples. I would be a critical clinician, examine the evidence, look at the benefit/risk before advocating an intervention that does not have good clinical data to support its use--a comprehensive approach of sexual medicine as well as sexual psychology.
Dr. Kingsberg: Additionally, we know she is in a long-term relationship--15 years; we want to acknowledge the partner. We talked about the partner's weight, but what about his erectile function? Does he have changes in sexual function that are affecting her, and she is the one carrying the "symptom"?
Looking at each piece separately helps a clinician from getting overwhelmed by the patient who comes in reporting distress with orgasmic dysfunction. We have no pharmacologic FDA-approved treatments, so it can feel off-putting for a clinician to try to fix the reported issue. Looking at each component to help her figure out the underlying cause can be helpful.
Dr. Iglesia: With aging, there can be changes in blood flow, not to mention the hormonal and even peripheral nerve changes, that require more stimulation in order to achieve the desired response. I echo concern about expensive procedures being offered with no evidence, such as the "O" or "G" shot, that can cost up to thousands of dollars.
The other procedure that gives me a lot of angst is clitoral unhooding. The 3 parts of the clitoris are sensitive in terms of innervation and blood flow, and cutting around that delicate tissue goes against the surgical principles required for preserving nerves and blood flow.
New onset pain post−prolapse surgery with TOT sling placement
Dr. Levy: For this case, let's consider a 42-year-old woman (P3) who is 6 months post− vaginal hysterectomy. The surgery included ovarian preservation combined with anterior and posterior repair for prolapse as well as apical uterosacral ligament suspension for stage 2 uterovaginal prolapse. A transobturator sling was used.
Extensive preop evaluation was performed, with confirmed symptomatic prolapse. She had no stress incontinence symptoms but did have confirmed occult stress incontinence.
Surgery was uneventful. She resumed intercourse at 8 weeks, but she now has pain with both initial entry and deep penetration. Lubricants and changes in position have not helped. She is in a stable relationship with her husband of 17 years, and she is worried that the sling mesh might be the culprit. On exam, she has no atrophy, pH is 4.5, vaginal length is 8 cm, and there is no prolapse. There is no mesh exposure noted, although she reports slight tenderness with palpation of the right sling arm beneath the right pubic bone.
Dr. Iglesia, what are the patient history questions important to ask here?
Dr. Iglesia: This is not an uncommon scenario--elective surgical correction of occult or latent stress incontinence after surgical correction for pelvic organ prolapse. Now this patient here has no more prolapse complaints; however, she has a new symptom. There are many different causes of dyspareunia; we cannot just assume it is the sling mesh (although with all the legal representation advertisements for those who have had mesh placed, it can certainly be at the top of the patient's mind, causing anxiety and fear).
Multiple trials have looked at prophylactic surgery for incontinence at the time of prolapse repairs. This woman happened to be one of those patients who did not have incontinence symptoms, and they put a sling in. A recent large trial examined women with vaginal prolapse who underwent hysterectomy and suspension.6 (They compared 2 different suspensions.) What is interesting is that 25% of women with prolapse do have baseline pain. However, at 24 months, de novo pain can occur in 10% of women--just from the apical suspension. So, here, it could be the prolapse suspension. Or, in terms of the transobturator sling, long-term data do tell us that the de novo dyspareunia rate ranges on the magnitude of 1% to 9%.7 What is important here is figuring out the cause of the dyspareunia.
Dr. Levy: One of the important points you raised already was that 25% of these women have preoperative pain. So figuring out what her functioning was before surgery and incorporating that into our assessment postop would be pretty important I would think.
Dr. Iglesia: Yes, you need to understand what her typical encounter was before the surgery and how things have changed now that the prolapse is not in the way. Changes obviously can occur with scar tissue, which over time will improve. If she is perimenopausal and starts to get epithelial changes, we can fix that. The question then becomes, "Is the pain emanating from the mesh?"
When examining this patient, it is not uncommon for me to be able to feel "banjo" strings if the mesh is too tight or close to the surface. It is not exposed but it's palpable, and the patient may feel a ridge during penetration. You can ask the patient if pain occurs with different penetration positions. In addition, explore associated neurologic symptoms (numbness or muscle pain in the thigh).
Dr. Kingsberg: There were 2 different sources of pain--on initial entry and at deep penetration. You want to make sure you address both. Importantly, did one precede the other? For instance, if women have pain with penetration they can then end up with an arousal disorder (the length of the vagina cannot increase as much as it might otherwise) and dystonia secondary to the pain with penetration. The timing of the pain--did it all happen at the same time, or did she start out with pain at one point and did it move to something else--is another critical piece of the history.
Dr. Iglesia: It does take a detailed history and physical exam to identify myofascial trigger points. In this case there seems to be pinpoint tenderness directly on the part of the mesh that is not exposed on the right. There are people who feel you should remove the whole mesh, including the arms. Others feel, okay, we can work on these trigger points, with injections, physical therapy, extra lubrication, and neuromodulatory medications. Only then would they think about potentially excising the sling or a portion thereof.
Dr. Krychman: Keep in mind that, even if you do remove the sling, her pain may not subside, if it is secondary to an underlying issue. Because of media sensationalism, she could be focusing on the sling. It is important to set realistic expectations. I often see vulvar pathology or even provoked vestibulodynia that can present with a deep dyspareunia. The concept of collision dyspareunia or introital discomfort or pain on insertion has far reaching implications. We need to look at the patient in totality, ruling out underlying issues related to the bladder, even the colon.
Patient inquires about the benefits of laser treatment for vaginal health
Dr. Levy: Let's move to this last case: A 47-year-old patient who reports lack of sexual satisfaction and attributes it to a loose vagina says, "I've heard about surgery and laser treatment and radiofrequency devices for vaginal health. What are the benefits and risks of these procedures, and will they correct the issues that I'm experiencing?"
How do you approach this patient?
Dr. Krychman: I want to know why this is of paramount importance to her. Is this her actual complaint or is it society's unrealistic expectation of sexual pleasure and performance placed upon her? Or is this a relationship issue surfacing, compounded by physiologic changes? With good communication techniques, like "ask, tell, ask" or effective use of silence (not interrupting), patients will lead us to the reason and the rationale.
Dr. Levy: I have seen a lot of advertising to women, now showing pictures of genitalia, perhaps creating an expectation that we should look infantile in some way. We are creating a sense of beauty and acceptablevisualization of the vagina and vulva that are completely unrealistic. It's fascinating on one hand but it is also disturbing in that some of the direct-to-consumer marketing going on is creating a sense of unease in women who are otherwise perfectly satisfied. Now they take a look at their sagging skin, maybe after having 2 or 3 children, and although they may not look the same they function not so badly perhaps. I think we are creating a distress and an illness model that is interesting to discuss.
Dr. Iglesia, you are in the midst of a randomized trial, giving an informed consent to participants about the expectations for this potential intervention. How do you explain to women what these laser and radiofrequency devices are expected to do and why they might or might not work?
Dr. Iglesia: We are doing a randomized trial for menopausal women who have GSM. We are comparing estrogen cream with fractional CO2 laser therapy. I also am involved in another randomized trial for lichen sclerosus. I am not involved in the cosmetic use of a laser for people who feel their vaginas are just "loose." Like you, Dr. Levy, I am very concerned about the images that women are seeing of the idealized vulva and vagina, and about the rise of cosmetic gynecology, much of which is being performed by plastic surgeons or dermatologists.
A recent article looked at the number of women who are doing pubic hair grooming; the prevalance is about 80% here in America.8 So people have a clear view of what is down there, and then they compare it to what they see in pornographic images on the Internet and want to look like a Barbie doll. That is disturbing because women, particularly young women, do not realize what happens with GSM changes to the vulva and vagina. On the other hand, these laser machines are very expensive, and some doctors are charging thousands of dollars and promising cosmetic and functional results for which we lack long-term, comparative data.
The laser that we are studying is one by Cynosure called Mona Lisa, which works with fractional CO2 and has very low depth of penetration. The concept is that, with microdot therapy (on the order of micrometers), pinpoint destruction will foster regeneration of new collagen and blood flow to the vagina and vulva. We are still in the midst of analyzing this.
Dr. Krychman: I caution people not to lump devices together. There is a significant difference between laser and radiofrequency--especially in the depth of tissue penetration, and level of evidence as well. There are companies performing randomized clinical trials, with well designed sham controls, and have demonstrated clinical efficacy. We need to be cautious of a procedure that is saying it's the best thing since sliced bread, curing interstitial cystitis, dyspareunia, and lichen sclerosus and improving orgasm, lubrication, and arousal. These far reaching, off-label claims are concerning and misleading.
Dr. Levy: I think the important things are 1) shared decision making with the patient and 2) disclosure of what we do not know, which are the long-term results and outcomes and possible downstream negative effects of some of these treatments, since the data we have are so short term.
Dr. Kingsberg: Basics are important. You talked about the pressure for cosmetic appearance, but is that really what is going on for this particular patient? Is she describing a sexual dysfunction when she talks about lack of satisfaction? You need to operationally define that term. Does she have problems with arousal or orgasm or desire and those are what underlie her lack of satisfaction? Is the key to management helping her come to terms with body image issues or to treat a sexual dysfunction? If it truly is a sexual dysfunction, then you can have the shared decision making on preferred treatment approach.
Dr. Levy: This has been an enlightening discussion. Thank all of you for your expertise and clinical acumen.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM), Fifth Edition. American Psychiatric Association Publishing: Arlington, VA; 2013.
- American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: The Use of Vaginal Estrogen in Women With a History of Estrogen-Dependent Breast Cancer. Obstet Gynecol. 2016;127(3):e93−e96.
- Krychman ML, Shifren JL, Liu JH, Kingsberg SL, Utian WH. The North American Menopause Society (NAMS). NAMS Menopause e-Consult: Laser treatment safe for vulvovaginal atrophy? 2015;11(3). http://www.medscape.com/viewarticle/846960. Accessed August 17, 2016.
- The American College of Obstetricians and Gynecologists and The American Congress of Obstetricians and Gynecologists. Fractional laser treatment of vulvovaginal atrophy and U.S. Food and Drug Administration clearance: Position Statement. http://www.acog.org/Resources-And-Publications/Position-Statements/Fractional-Laser-Treatment-of-Vulvovaginal-Atrophy-and-US-Food-and-Drug-Administration-Clearance. Published May 2016. Accessed August 17, 2016.
- Lukacz ES, Warren LK, Richter HE, et al. Quality of life and sexual function 2 years after vaginal surgery for prolapse. Obstet Gynecol. 2016;127(6):1071−1079.
- Brubaker L, Chiang S, Zyczynski H, et al. The impact of stress incontinence surgery on female sexual function. Am J Obstet Gynecol. 2009;200(5):562.e1.
- Rowen TS, Gaither TW, Awad MA, Osterberg EC, Shindel AW, Breyer BN. Pubic hair grooming prevalence and motivation among women in the United States [published online ahead of print June 29, 2016]. JAMA Dermatol. doi:10.1001/jamader matol.2016.2154.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM), Fifth Edition. American Psychiatric Association Publishing: Arlington, VA; 2013.
- American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: The Use of Vaginal Estrogen in Women With a History of Estrogen-Dependent Breast Cancer. Obstet Gynecol. 2016;127(3):e93−e96.
- Krychman ML, Shifren JL, Liu JH, Kingsberg SL, Utian WH. The North American Menopause Society (NAMS). NAMS Menopause e-Consult: Laser treatment safe for vulvovaginal atrophy? 2015;11(3). http://www.medscape.com/viewarticle/846960. Accessed August 17, 2016.
- The American College of Obstetricians and Gynecologists and The American Congress of Obstetricians and Gynecologists. Fractional laser treatment of vulvovaginal atrophy and U.S. Food and Drug Administration clearance: Position Statement. http://www.acog.org/Resources-And-Publications/Position-Statements/Fractional-Laser-Treatment-of-Vulvovaginal-Atrophy-and-US-Food-and-Drug-Administration-Clearance. Published May 2016. Accessed August 17, 2016.
- Lukacz ES, Warren LK, Richter HE, et al. Quality of life and sexual function 2 years after vaginal surgery for prolapse. Obstet Gynecol. 2016;127(6):1071−1079.
- Brubaker L, Chiang S, Zyczynski H, et al. The impact of stress incontinence surgery on female sexual function. Am J Obstet Gynecol. 2009;200(5):562.e1.
- Rowen TS, Gaither TW, Awad MA, Osterberg EC, Shindel AW, Breyer BN. Pubic hair grooming prevalence and motivation among women in the United States [published online ahead of print June 29, 2016]. JAMA Dermatol. doi:10.1001/jamader matol.2016.2154.
In This Article
- This roundtable's expert panel
- Dyspareunia and low sexual desire in a breast cancer survivor
- Laser treatment and vaginal health
Can Serum Free Light Chains Be Used for the Early Diagnosis of Monoclonal Immunoglobulin-Secreting B-Cell and Plasma-Cell Diseases? (FULL)
Patients who are undergoing multiple myeloma screening with serum protein electrophoresis and immunofixation, especially those with renal failure, also should receive serum free light chain testing to increase specificity and reduce false-negatives.
Multiple myeloma (MM) is a devastating disease with an estimated 26,850 new cases in 2015 according to Surveillance, Epidemiology, and End Results data and no definitive chemotherapeutic cure.1 In 97% of cases, MM is defined by monoclonal hypergammaglobulinemia, in which a malignant plasma cell clone secretes a monoclonal globulin; the remaining cases are nonsecretors.2 Each pathologically produced clonal globulin contains 2 heavy chains attached by disulfide linkage and 2 light chains. Unchecked plasma cell production is what later causes the symptoms of renal failure, bone destruction, and anemia.
The rate of MM is disproportionately high in the veteran population, and the VA health care system provides care for many of these patients. The higher rate is likely secondary to the predominantly male population, which has higher MM rates, and has been linked to Agent Orange exposure in Vietnam. As MM is not easy to diagnose, any algorithm or testing method would be of great benefit to this population.
The gold standard for MM detection remains serum protein electrophoresis (SPEP) with immunofixation (IFE), but other detection methods have been emerging. The method of serum free light chain (SFLC) assay has become more readily available, and its incorporation into diagnostic guidelines has become more apparent but is not universal.3
In the case series reported in this article, SPEP/IFE and SFLC assays were used to test 207 patients from the VA New York Harbor Healthcare System (VANYHHS). All these patients had a clinical context for MM testing.
Methods
In this retrospective study, the authors reviewed the charts of VANYHHS patients who were being treated for conditions that prompted SPEP/IFE and λ and κ SFLC analysis between December 2013 and March 2014. The study was exempt from institutional review board approval.
The SPEP/IFE analysis was performed with an automated electrophoresis machine (Sebia Electrophoresis), and the SFLC analysis was performed with an automated SFLC assay (Freelite). Sensitivity, specificity, and positive and negative predictive values were calculated using SPEP/IFE as the gold standard and SFLC κ-to-λ ratio asthe test method. Patients with a positive κ-to-λ ratio but negative SPEP were considered false-positives. These patients’ SFLC analyses were further analyzed in an effort to evaluate use of the κ-to-λ ratio as an early tumor marker.
The κ reference range used was 3.3 to 19.4 mg/L, and the λ reference range used was 5.7 to 26.3 mg/L.4 The traditional reference range for the κ-to-λ ratio is 0.26 to 1.65.5
Results
Of the 207 patients in this study, 205 were men. Mean age was 69 years (range, 28-97 years). Mean serum urea nitrogen level was 8.75 mmol/L (range, 2.86-38.21 mmol/L), and mean creatinine level was 140.59 μmol/L (range, 44.21-1503.14 μmol/L). Mean κ was 49.82 mg/L (range, 4.6-700.96 mg/L), and mean λ was 54.27 mg/L (range, 3-1,750 mg/L). Table 1 compares the SPEP and SFLC data. Sensitivity was 67%, specificity was 85%, positive predictive value was 58%, and negative predictive value was 89%. Concordance of the 2 methods was 80%. The false-positive group was followed up 16 months later to check for diagnosis of disease. Two of the 24 patients in this quadrant were later diagnosed with MM (Table 1).
One of the patients with MM was an 82-year-old African American man with a history of hypertension, diabetes, and prostate cancer (Gleason 4 + 4 = 8/10). He presented to VANYHHS after a fall in which he sustained a pathologic fracture of the left acromion. Recurrent prostate cancer was initially suspected, and nuclear bone scintigraphy revealed increased uptake in the left shoulder and the posterior ninth rib. Results of computed tomography-guided biopsy showed the rib lesion packed with plasma cells and consistent with MM. Immunohistochemical analysis was positive for CD138 and κ in the malignant plasma cells. Initial SPEP performed before the biopsy showed an acute phase reaction with hypogammaglobulinemia, and SPEP after the biopsy showed an increased α-2 band but no monoclonal gammaglobulinopathy. The initial κ of 42.18 mg/L (κ-to-λ ratio, 4.01) was up to 67.53 mg/L 4 months later.
The other patient with MM was a 91-year-old man who had coronary artery disease after undergoing coronary artery bypass grafting in 1993, sick sinus syndrome after pacemaker implantation, hypertension, and anemia. He initially presented to the geriatrics clinic with polyneuropathy, which prompted SPEP and SFLC analysis. SPEP results showed a normal electrophoretic pattern, but κ increased to 47.52 mg/L (κ-to-λ ratio, 2.63). The decision was made to monitor the patient in the hematology clinic. Subsequent κ chain analysis revealed an increase to 59.50 mg/L. A repeat SPEP, performed 1 year after the first SPEP, revealed monoclonal immunoglobulin A on IFE.
Of the 24 patients with false-positive results, 16 had moderate-to-severe kidney disease (stage IIIa-IV).6All patients in this quadrant were men; their mean age was 75 years, and their mean creatinine level was 182.15 μmol/L. Further laboratory data are listed in Table 2.
The patient whose biopsy results led to an MM diagnosis and the patient whose IFE led to a gammopathy diagnosis both maintained a glomerular filtration rate within normal limits. The Figure shows the κ-to-λ ratios of this quadrant logarithmically.
Discussion
Use of SFLC analysis as a supplement to serum and urine protein electrophoresis has been investigated and accepted in the recent literature.3,4,7,8 Use of light chains as a method of earlier or alternative detection has not been proved. In the present study of 207 patients, comparisons showed that more traditional MM detection methods and SFLC analysis are largely concordant. The 2 patients with MM and negative electrophoretic patterns provided a clear indication of the potential benefit of SFLC analysis in the diagnosis of secretory and nonsecretory myeloma.
In 2014, Kim and colleagues compared 2 SFLC assays (Freelite, N Latex) to each other and to SPEP in a 120-patient population.9 The Freelite results in their study correlated closely with VA population findings (κ-to-λ ratio sensitivity and specificity: 72.2% and 93.6%, respectively). N Latex, the newer SFLC assay, had lower sensitivity (64.6%) and higher specificity (100%). With application of the extended reference range (0.37-3.1) proposed by Hutchison and colleagues for use in patients with renal failure, SFLC becomes a more statistically powerful tool.5
The patients who tested false-positive had higher mean creatinine levels, and 16 had renal insufficiency. The 2 false-positive patients were later found to have clinical myeloma and were within the normal range of renal function. Of the 16 patients with an abnormal κ-to-λ ratio and renal failure, 15 would be within the revised normal reference range, leaving 9 false-positives, 2 of whom eventually were found to have disease. With the application of the extended light chain range (as per Hutchison) for those patients with renal failure, 15 of the original 24 false-positives became true-negatives. Two of the false-positives become true-positives after they were subsequently diagnosed. Therefore, SFLC analysis detected disease in 22% of the revised false-positives when SPEP could not.
Table 2 lists the revised data after follow-up and renal failure correction. The strongest aspect of SFLC analysis remains its 95% specificity; its 69% sensitivity remains relatively constant. The test’s positive predictive value is 84%, and its negative predictive value is 90%. In veteran and other at-risk populations, SFLC analysis proves to be a very powerful tool on its own.
Conclusion
Both patient cases described in this article demonstrate the usefulness of SFLC analysis as an adjunct to SPEP. The authors propose SFLC testing for all patients who are undergoing MM screening with SPEP/IFE. In patients with renal failure, the expanded reference range seems to reduce erroneous false-positive results. Patients who have abnormal ratios should be followed up in clinic with repeat MM testing. It seems clear that, at the very least, SFLC analysis is a necessary adjunct to SPEP testing. However, SFLC stands on its own merit as well.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. National Cancer Institute, Surveillance, Epidemiology, and End Results (SEER) Program. SEER website. http://seer.cancer.gov/statfacts/html/mulmy.html. Accessed July 11, 2016.
2. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33.
3. Dimopoulos M, Kyle R, Fermand JP, et al; International Myeloma Workshop Consensus Panel 3. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117(18):4701-4705.
4. Katzmann JA, Clark RJ, Abraham RS, et al. Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem. 2002;48(9):1437-1444.
5. Hutchison CA, Plant T, Drayson M, et al. Serum free light chain measurement
aids the diagnosis of myeloma in patients with severe renal failure.
BMC Nephrol. 2008;9:11.
6. Levey AS, Stevens LA, Schmid CH, et al; CKD-EPI (Chronic Kidney Disease
Epidemiology Collaboration). A new equation to estimate glomerular filtration
rate. Ann Intern Med. 2009;150(9):604-612.
7. McTaggart MP, Lindsay J, Kearney EM. Replacing urine protein electrophoresis
with serum free light chain analysis as a first-line test for detecting plasma
cell disorders offers increased diagnostic accuracy and potential health benefit
to patients. Am J Clin Pathol. 2013;140(6):890-897.
8. Abadie JM, Bankson DD. Assessment of serum free light chain assays for
plasma cell disorder screening in a Veterans Affairs population. Ann Clin Lab
Sci. 2006;36(2):157-162.
9. Kim HS, Kim HS, Shin KS, et al. Clinical comparisons of two free light chain
assays to immunofixation electrophoresis for detecting monoclonal gammopathy.
Biomed Res Int. 2014;2014:647238.
Note: Page numbers differ between the print issue and digital edition.
Patients who are undergoing multiple myeloma screening with serum protein electrophoresis and immunofixation, especially those with renal failure, also should receive serum free light chain testing to increase specificity and reduce false-negatives.
Multiple myeloma (MM) is a devastating disease with an estimated 26,850 new cases in 2015 according to Surveillance, Epidemiology, and End Results data and no definitive chemotherapeutic cure.1 In 97% of cases, MM is defined by monoclonal hypergammaglobulinemia, in which a malignant plasma cell clone secretes a monoclonal globulin; the remaining cases are nonsecretors.2 Each pathologically produced clonal globulin contains 2 heavy chains attached by disulfide linkage and 2 light chains. Unchecked plasma cell production is what later causes the symptoms of renal failure, bone destruction, and anemia.
The rate of MM is disproportionately high in the veteran population, and the VA health care system provides care for many of these patients. The higher rate is likely secondary to the predominantly male population, which has higher MM rates, and has been linked to Agent Orange exposure in Vietnam. As MM is not easy to diagnose, any algorithm or testing method would be of great benefit to this population.
The gold standard for MM detection remains serum protein electrophoresis (SPEP) with immunofixation (IFE), but other detection methods have been emerging. The method of serum free light chain (SFLC) assay has become more readily available, and its incorporation into diagnostic guidelines has become more apparent but is not universal.3
In the case series reported in this article, SPEP/IFE and SFLC assays were used to test 207 patients from the VA New York Harbor Healthcare System (VANYHHS). All these patients had a clinical context for MM testing.
Methods
In this retrospective study, the authors reviewed the charts of VANYHHS patients who were being treated for conditions that prompted SPEP/IFE and λ and κ SFLC analysis between December 2013 and March 2014. The study was exempt from institutional review board approval.
The SPEP/IFE analysis was performed with an automated electrophoresis machine (Sebia Electrophoresis), and the SFLC analysis was performed with an automated SFLC assay (Freelite). Sensitivity, specificity, and positive and negative predictive values were calculated using SPEP/IFE as the gold standard and SFLC κ-to-λ ratio asthe test method. Patients with a positive κ-to-λ ratio but negative SPEP were considered false-positives. These patients’ SFLC analyses were further analyzed in an effort to evaluate use of the κ-to-λ ratio as an early tumor marker.
The κ reference range used was 3.3 to 19.4 mg/L, and the λ reference range used was 5.7 to 26.3 mg/L.4 The traditional reference range for the κ-to-λ ratio is 0.26 to 1.65.5
Results
Of the 207 patients in this study, 205 were men. Mean age was 69 years (range, 28-97 years). Mean serum urea nitrogen level was 8.75 mmol/L (range, 2.86-38.21 mmol/L), and mean creatinine level was 140.59 μmol/L (range, 44.21-1503.14 μmol/L). Mean κ was 49.82 mg/L (range, 4.6-700.96 mg/L), and mean λ was 54.27 mg/L (range, 3-1,750 mg/L). Table 1 compares the SPEP and SFLC data. Sensitivity was 67%, specificity was 85%, positive predictive value was 58%, and negative predictive value was 89%. Concordance of the 2 methods was 80%. The false-positive group was followed up 16 months later to check for diagnosis of disease. Two of the 24 patients in this quadrant were later diagnosed with MM (Table 1).
One of the patients with MM was an 82-year-old African American man with a history of hypertension, diabetes, and prostate cancer (Gleason 4 + 4 = 8/10). He presented to VANYHHS after a fall in which he sustained a pathologic fracture of the left acromion. Recurrent prostate cancer was initially suspected, and nuclear bone scintigraphy revealed increased uptake in the left shoulder and the posterior ninth rib. Results of computed tomography-guided biopsy showed the rib lesion packed with plasma cells and consistent with MM. Immunohistochemical analysis was positive for CD138 and κ in the malignant plasma cells. Initial SPEP performed before the biopsy showed an acute phase reaction with hypogammaglobulinemia, and SPEP after the biopsy showed an increased α-2 band but no monoclonal gammaglobulinopathy. The initial κ of 42.18 mg/L (κ-to-λ ratio, 4.01) was up to 67.53 mg/L 4 months later.
The other patient with MM was a 91-year-old man who had coronary artery disease after undergoing coronary artery bypass grafting in 1993, sick sinus syndrome after pacemaker implantation, hypertension, and anemia. He initially presented to the geriatrics clinic with polyneuropathy, which prompted SPEP and SFLC analysis. SPEP results showed a normal electrophoretic pattern, but κ increased to 47.52 mg/L (κ-to-λ ratio, 2.63). The decision was made to monitor the patient in the hematology clinic. Subsequent κ chain analysis revealed an increase to 59.50 mg/L. A repeat SPEP, performed 1 year after the first SPEP, revealed monoclonal immunoglobulin A on IFE.
Of the 24 patients with false-positive results, 16 had moderate-to-severe kidney disease (stage IIIa-IV).6All patients in this quadrant were men; their mean age was 75 years, and their mean creatinine level was 182.15 μmol/L. Further laboratory data are listed in Table 2.
The patient whose biopsy results led to an MM diagnosis and the patient whose IFE led to a gammopathy diagnosis both maintained a glomerular filtration rate within normal limits. The Figure shows the κ-to-λ ratios of this quadrant logarithmically.
Discussion
Use of SFLC analysis as a supplement to serum and urine protein electrophoresis has been investigated and accepted in the recent literature.3,4,7,8 Use of light chains as a method of earlier or alternative detection has not been proved. In the present study of 207 patients, comparisons showed that more traditional MM detection methods and SFLC analysis are largely concordant. The 2 patients with MM and negative electrophoretic patterns provided a clear indication of the potential benefit of SFLC analysis in the diagnosis of secretory and nonsecretory myeloma.
In 2014, Kim and colleagues compared 2 SFLC assays (Freelite, N Latex) to each other and to SPEP in a 120-patient population.9 The Freelite results in their study correlated closely with VA population findings (κ-to-λ ratio sensitivity and specificity: 72.2% and 93.6%, respectively). N Latex, the newer SFLC assay, had lower sensitivity (64.6%) and higher specificity (100%). With application of the extended reference range (0.37-3.1) proposed by Hutchison and colleagues for use in patients with renal failure, SFLC becomes a more statistically powerful tool.5
The patients who tested false-positive had higher mean creatinine levels, and 16 had renal insufficiency. The 2 false-positive patients were later found to have clinical myeloma and were within the normal range of renal function. Of the 16 patients with an abnormal κ-to-λ ratio and renal failure, 15 would be within the revised normal reference range, leaving 9 false-positives, 2 of whom eventually were found to have disease. With the application of the extended light chain range (as per Hutchison) for those patients with renal failure, 15 of the original 24 false-positives became true-negatives. Two of the false-positives become true-positives after they were subsequently diagnosed. Therefore, SFLC analysis detected disease in 22% of the revised false-positives when SPEP could not.
Table 2 lists the revised data after follow-up and renal failure correction. The strongest aspect of SFLC analysis remains its 95% specificity; its 69% sensitivity remains relatively constant. The test’s positive predictive value is 84%, and its negative predictive value is 90%. In veteran and other at-risk populations, SFLC analysis proves to be a very powerful tool on its own.
Conclusion
Both patient cases described in this article demonstrate the usefulness of SFLC analysis as an adjunct to SPEP. The authors propose SFLC testing for all patients who are undergoing MM screening with SPEP/IFE. In patients with renal failure, the expanded reference range seems to reduce erroneous false-positive results. Patients who have abnormal ratios should be followed up in clinic with repeat MM testing. It seems clear that, at the very least, SFLC analysis is a necessary adjunct to SPEP testing. However, SFLC stands on its own merit as well.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
Patients who are undergoing multiple myeloma screening with serum protein electrophoresis and immunofixation, especially those with renal failure, also should receive serum free light chain testing to increase specificity and reduce false-negatives.
Multiple myeloma (MM) is a devastating disease with an estimated 26,850 new cases in 2015 according to Surveillance, Epidemiology, and End Results data and no definitive chemotherapeutic cure.1 In 97% of cases, MM is defined by monoclonal hypergammaglobulinemia, in which a malignant plasma cell clone secretes a monoclonal globulin; the remaining cases are nonsecretors.2 Each pathologically produced clonal globulin contains 2 heavy chains attached by disulfide linkage and 2 light chains. Unchecked plasma cell production is what later causes the symptoms of renal failure, bone destruction, and anemia.
The rate of MM is disproportionately high in the veteran population, and the VA health care system provides care for many of these patients. The higher rate is likely secondary to the predominantly male population, which has higher MM rates, and has been linked to Agent Orange exposure in Vietnam. As MM is not easy to diagnose, any algorithm or testing method would be of great benefit to this population.
The gold standard for MM detection remains serum protein electrophoresis (SPEP) with immunofixation (IFE), but other detection methods have been emerging. The method of serum free light chain (SFLC) assay has become more readily available, and its incorporation into diagnostic guidelines has become more apparent but is not universal.3
In the case series reported in this article, SPEP/IFE and SFLC assays were used to test 207 patients from the VA New York Harbor Healthcare System (VANYHHS). All these patients had a clinical context for MM testing.
Methods
In this retrospective study, the authors reviewed the charts of VANYHHS patients who were being treated for conditions that prompted SPEP/IFE and λ and κ SFLC analysis between December 2013 and March 2014. The study was exempt from institutional review board approval.
The SPEP/IFE analysis was performed with an automated electrophoresis machine (Sebia Electrophoresis), and the SFLC analysis was performed with an automated SFLC assay (Freelite). Sensitivity, specificity, and positive and negative predictive values were calculated using SPEP/IFE as the gold standard and SFLC κ-to-λ ratio asthe test method. Patients with a positive κ-to-λ ratio but negative SPEP were considered false-positives. These patients’ SFLC analyses were further analyzed in an effort to evaluate use of the κ-to-λ ratio as an early tumor marker.
The κ reference range used was 3.3 to 19.4 mg/L, and the λ reference range used was 5.7 to 26.3 mg/L.4 The traditional reference range for the κ-to-λ ratio is 0.26 to 1.65.5
Results
Of the 207 patients in this study, 205 were men. Mean age was 69 years (range, 28-97 years). Mean serum urea nitrogen level was 8.75 mmol/L (range, 2.86-38.21 mmol/L), and mean creatinine level was 140.59 μmol/L (range, 44.21-1503.14 μmol/L). Mean κ was 49.82 mg/L (range, 4.6-700.96 mg/L), and mean λ was 54.27 mg/L (range, 3-1,750 mg/L). Table 1 compares the SPEP and SFLC data. Sensitivity was 67%, specificity was 85%, positive predictive value was 58%, and negative predictive value was 89%. Concordance of the 2 methods was 80%. The false-positive group was followed up 16 months later to check for diagnosis of disease. Two of the 24 patients in this quadrant were later diagnosed with MM (Table 1).
One of the patients with MM was an 82-year-old African American man with a history of hypertension, diabetes, and prostate cancer (Gleason 4 + 4 = 8/10). He presented to VANYHHS after a fall in which he sustained a pathologic fracture of the left acromion. Recurrent prostate cancer was initially suspected, and nuclear bone scintigraphy revealed increased uptake in the left shoulder and the posterior ninth rib. Results of computed tomography-guided biopsy showed the rib lesion packed with plasma cells and consistent with MM. Immunohistochemical analysis was positive for CD138 and κ in the malignant plasma cells. Initial SPEP performed before the biopsy showed an acute phase reaction with hypogammaglobulinemia, and SPEP after the biopsy showed an increased α-2 band but no monoclonal gammaglobulinopathy. The initial κ of 42.18 mg/L (κ-to-λ ratio, 4.01) was up to 67.53 mg/L 4 months later.
The other patient with MM was a 91-year-old man who had coronary artery disease after undergoing coronary artery bypass grafting in 1993, sick sinus syndrome after pacemaker implantation, hypertension, and anemia. He initially presented to the geriatrics clinic with polyneuropathy, which prompted SPEP and SFLC analysis. SPEP results showed a normal electrophoretic pattern, but κ increased to 47.52 mg/L (κ-to-λ ratio, 2.63). The decision was made to monitor the patient in the hematology clinic. Subsequent κ chain analysis revealed an increase to 59.50 mg/L. A repeat SPEP, performed 1 year after the first SPEP, revealed monoclonal immunoglobulin A on IFE.
Of the 24 patients with false-positive results, 16 had moderate-to-severe kidney disease (stage IIIa-IV).6All patients in this quadrant were men; their mean age was 75 years, and their mean creatinine level was 182.15 μmol/L. Further laboratory data are listed in Table 2.
The patient whose biopsy results led to an MM diagnosis and the patient whose IFE led to a gammopathy diagnosis both maintained a glomerular filtration rate within normal limits. The Figure shows the κ-to-λ ratios of this quadrant logarithmically.
Discussion
Use of SFLC analysis as a supplement to serum and urine protein electrophoresis has been investigated and accepted in the recent literature.3,4,7,8 Use of light chains as a method of earlier or alternative detection has not been proved. In the present study of 207 patients, comparisons showed that more traditional MM detection methods and SFLC analysis are largely concordant. The 2 patients with MM and negative electrophoretic patterns provided a clear indication of the potential benefit of SFLC analysis in the diagnosis of secretory and nonsecretory myeloma.
In 2014, Kim and colleagues compared 2 SFLC assays (Freelite, N Latex) to each other and to SPEP in a 120-patient population.9 The Freelite results in their study correlated closely with VA population findings (κ-to-λ ratio sensitivity and specificity: 72.2% and 93.6%, respectively). N Latex, the newer SFLC assay, had lower sensitivity (64.6%) and higher specificity (100%). With application of the extended reference range (0.37-3.1) proposed by Hutchison and colleagues for use in patients with renal failure, SFLC becomes a more statistically powerful tool.5
The patients who tested false-positive had higher mean creatinine levels, and 16 had renal insufficiency. The 2 false-positive patients were later found to have clinical myeloma and were within the normal range of renal function. Of the 16 patients with an abnormal κ-to-λ ratio and renal failure, 15 would be within the revised normal reference range, leaving 9 false-positives, 2 of whom eventually were found to have disease. With the application of the extended light chain range (as per Hutchison) for those patients with renal failure, 15 of the original 24 false-positives became true-negatives. Two of the false-positives become true-positives after they were subsequently diagnosed. Therefore, SFLC analysis detected disease in 22% of the revised false-positives when SPEP could not.
Table 2 lists the revised data after follow-up and renal failure correction. The strongest aspect of SFLC analysis remains its 95% specificity; its 69% sensitivity remains relatively constant. The test’s positive predictive value is 84%, and its negative predictive value is 90%. In veteran and other at-risk populations, SFLC analysis proves to be a very powerful tool on its own.
Conclusion
Both patient cases described in this article demonstrate the usefulness of SFLC analysis as an adjunct to SPEP. The authors propose SFLC testing for all patients who are undergoing MM screening with SPEP/IFE. In patients with renal failure, the expanded reference range seems to reduce erroneous false-positive results. Patients who have abnormal ratios should be followed up in clinic with repeat MM testing. It seems clear that, at the very least, SFLC analysis is a necessary adjunct to SPEP testing. However, SFLC stands on its own merit as well.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. National Cancer Institute, Surveillance, Epidemiology, and End Results (SEER) Program. SEER website. http://seer.cancer.gov/statfacts/html/mulmy.html. Accessed July 11, 2016.
2. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33.
3. Dimopoulos M, Kyle R, Fermand JP, et al; International Myeloma Workshop Consensus Panel 3. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117(18):4701-4705.
4. Katzmann JA, Clark RJ, Abraham RS, et al. Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem. 2002;48(9):1437-1444.
5. Hutchison CA, Plant T, Drayson M, et al. Serum free light chain measurement
aids the diagnosis of myeloma in patients with severe renal failure.
BMC Nephrol. 2008;9:11.
6. Levey AS, Stevens LA, Schmid CH, et al; CKD-EPI (Chronic Kidney Disease
Epidemiology Collaboration). A new equation to estimate glomerular filtration
rate. Ann Intern Med. 2009;150(9):604-612.
7. McTaggart MP, Lindsay J, Kearney EM. Replacing urine protein electrophoresis
with serum free light chain analysis as a first-line test for detecting plasma
cell disorders offers increased diagnostic accuracy and potential health benefit
to patients. Am J Clin Pathol. 2013;140(6):890-897.
8. Abadie JM, Bankson DD. Assessment of serum free light chain assays for
plasma cell disorder screening in a Veterans Affairs population. Ann Clin Lab
Sci. 2006;36(2):157-162.
9. Kim HS, Kim HS, Shin KS, et al. Clinical comparisons of two free light chain
assays to immunofixation electrophoresis for detecting monoclonal gammopathy.
Biomed Res Int. 2014;2014:647238.
Note: Page numbers differ between the print issue and digital edition.
1. National Cancer Institute, Surveillance, Epidemiology, and End Results (SEER) Program. SEER website. http://seer.cancer.gov/statfacts/html/mulmy.html. Accessed July 11, 2016.
2. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33.
3. Dimopoulos M, Kyle R, Fermand JP, et al; International Myeloma Workshop Consensus Panel 3. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117(18):4701-4705.
4. Katzmann JA, Clark RJ, Abraham RS, et al. Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem. 2002;48(9):1437-1444.
5. Hutchison CA, Plant T, Drayson M, et al. Serum free light chain measurement
aids the diagnosis of myeloma in patients with severe renal failure.
BMC Nephrol. 2008;9:11.
6. Levey AS, Stevens LA, Schmid CH, et al; CKD-EPI (Chronic Kidney Disease
Epidemiology Collaboration). A new equation to estimate glomerular filtration
rate. Ann Intern Med. 2009;150(9):604-612.
7. McTaggart MP, Lindsay J, Kearney EM. Replacing urine protein electrophoresis
with serum free light chain analysis as a first-line test for detecting plasma
cell disorders offers increased diagnostic accuracy and potential health benefit
to patients. Am J Clin Pathol. 2013;140(6):890-897.
8. Abadie JM, Bankson DD. Assessment of serum free light chain assays for
plasma cell disorder screening in a Veterans Affairs population. Ann Clin Lab
Sci. 2006;36(2):157-162.
9. Kim HS, Kim HS, Shin KS, et al. Clinical comparisons of two free light chain
assays to immunofixation electrophoresis for detecting monoclonal gammopathy.
Biomed Res Int. 2014;2014:647238.
Note: Page numbers differ between the print issue and digital edition.
An Unusual Cause of Shortness of Breath: Primary Tracheal Basal Cell Adenocarcinoma
Salivary gland lung tumors are extremely rare intrathoracic malignancies, accounting for only 0.2% of all lung tumors.1 It has been postulated that these lung tumors arise from pluripotential cells in the epithelium of the submucosal bronchial glands and usually present as endoluminal lesions. The cause of salivary gland tumors is unclear. They seem to be unrelated to exposure to smoking, air pollutants, or other chemicals.2 Associated symptoms are generally related to endoluminal obstruction by the tumors, which are centrally located. Thus, presenting symptoms commonly include chronic cough, progressive dyspnea, hoarseness, wheezing, and occasional hemoptysis.3 Chest radiographs seem normal in most cases except those in which obstruction is present. Computed tomography usually shows well-defined endotracheal or endobronchial lesions that are lobulated, polypoid, or smooth, without infiltration into surrounding tissues.
Although basal cell adenocarcinoma (BCAC) was included in World Health Organization’s (WHO) Pathology and Genetics of Head and Neck Tumours in 1991, its definition—“an epithelial neoplasm that has cytological characteristics of basal cell adenoma (BCA), but a morphologic growth pattern indicative of malignancy”—did not appear until 2005.4 Basal cell adenocarcinoma generally is classified as a low-grade malignancy with a good long-term prognosis. It usually affects parotid and submandibular minor salivary glands.5
Histologic differentiation of BCAC and BCA is difficult and depends on whether local structures have been invaded or on which histologic features of perineural or vascular invasion are present.6 Basal cell adenocarcinoma also shows strong immunoreactivity to cytokeratin 7 (CK-7) and variable myoepithelial staining with S100.7 Because of the prognosis and potential treatment differences involved, BCAC must be differentiated from other basaloid cell tumors, such as BCA, adenoid cystic carcinoma, polymorphous low-grade adenocarcinoma, myoepithelial tumor, epithelial-myoepithelial carcinoma, and basaloid squamous cell carcinoma (SCC).8 Surgical excision is the primary treatment of choice.5 Rare in the salivary glands, BCA and BCAC are even rarer in salivary gland tissue outside the head and neck region. The authors report on a case of BCAC of the salivary gland tissue in the trachea.
Case Report
An 84-year-old man with diabetes mellitus and hypertension and a nonsmoker presented to the emergency department of the VA Caribbean Healthcare System in San Juan, Puerto Rico, with a dry cough and shortness of breath lasting 1 week, which worsened the day before
presentation. On physical examination, the patient was afebrile and in no respiratory distress, and his vital signs were within normal limits. There was no barrel chest, no prolonged expiratory phase, and occasional wheezing more prominent on the left side. A chest radiograph showed atelectasis and/or associated changes (Figure 1).
After the initial biopsy results led to a provisional diagnosis of basaloid neoplasm with squamous features, the patient underwent rigid bronchoscopic tracheal tumor debridement followed by cryotherapy at the base of the tumor.
Discussion
Primary tracheal tumors are rare, accounting for < 1% of all malignancies.9,10 According to the National Cancer Institute Surveillance, Epidemiology, and End Results database, the rate of new cases of primary carcinoma of the trachea was 2.6 per 1 million people per year.11 Of all primary tumors of the trachea, 80% are malignant9,10; the rest vary widely and include both malignant and benign histotypes.11
The abundant minor salivary gland tissue in the upper respiratory tract can potentially develop neoplasia the same as the wide spectrum seen in head and neck salivary gland tumors. The most recent (2004) WHO Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart includes a brief section on salivary gland tumors and mentions only mucoepidermoid carcinoma, adenoid cystic carcinoma, myoepithelial carcinoma, epithelial-myoepithelial carcinoma, pleomorphic adenoma, and carcinoma ex-pleomorphic adenoma.12 However, there are reports of other salivary gland tumors in the tracheopulmonary location.
García and colleagues recently described a case of hyalinizing clear cell carcinoma with its defining EWSR1-ATF1 (Ewing sarcoma breakpoint region 1–activating transcription factor 1) fusion transcript.13 Similarly, no tracheopulmonary BCAC cases have been reported in the English-language literature since 2004, when Damiani and colleagues described a case of basal adenocarcinoma of the lung.14 In 2012, Yamada and colleagues reported a case of “basaloid carcinoma with central cavitation,”15 which showed a peculiar immunohistochemical profile similar
to the tumor described in the present article—suggestive of a myoepithelial origin if the morphologic architectural features match.
Salivary gland tumors are prone to manifest the squamous phenotype. Depending on the biopsy modality, these squamous areas (squamous eddies) can be sampled and can lead to the erroneous diagnosis of SCC. However, the histologic features of squamous malignancy are
lacking in these squamous components, and the pathologist should be able to distinguish additional phenotypes that can generate a wider differential diagnosis.
On follow-up, this patient has maintained satisfactory respiratory function. Starting 2 years after the initial resection, he has had annual checkups. Recent imaging and bronchoscopy have revealed tumor recurrence in the same site. Because of the patient’s extensive comorbidities, surgical intervention has been deferred in favor of cryotherapy, a less aggressive therapy.
Author disclosure
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Moran CA. Primary salivary gland-type tumors of the lung. Semin Diagn Pathol.
1995;12(2):106-122.
2. Molina JR, Aubry MC, Lewis JE, et al. Primary salivary gland-type lung cancer: spectrum of clinical presentation, histopathologic and prognostic factors. Cancer. 2007;110(10):2253-2259.
3. Brutinel WM, Cortese DA, McDougall JC, Gillio RG, Bergstralh EJ. A two-year experience with the neodymium-YAG laser in endobronchial obstruction. Chest. 1987;91(2):159-165.
4. Barnes L, Eveson JW, Reichart P, Sidransky D, eds. World Health Organization Classification of Tumours. Pathology and Genetics of Head and Neck Tumours. Lyon, France: IARC Press; 2005. International Agency for Research on Cancer website. https://www.iarc.fr/en/publications/pdfs-online/pat-gen/bb9/BB9.pdf. Accessed June 16, 2016.
5. Sarath PV, Kannan N, Patil R, Manne RK, Swapna B, Suneel Kumar KV. Basal cell adenocarcinoma of the minor salivary glands involving palate and maxillary sinus. J Clin Imaging Sci. 2013;3(suppl 1):4.
6. Farrell T, Chang YL. Basal cell adenocarcinoma of minor salivary glands. Arch Pathol Lab Med. 2007;131(10):1602-1604.
7. Jung MJ, Roh JL, Choi SH, et al. Basal cell adenocarcinoma of the salivary gland: a morphological and immunohistochemical comparison with basal cell adenoma with and without capsular invasion. Diagn Pathol. 2013;8:171.
8. Jäkel KT, Löning T. Differential diagnosis of basaloid salivary gland tumors [in German]. Pathologe. 2004;25(1):46-55.
9. Urdaneta AI, Yu JB, Wilson LD. Population based cancer registry analysis of primary tracheal carcinoma. Am J Clin Oncol. 2011;34(1):32-37.
10. Varadhachary GR, Rabe MN. Cancer of unknown primary site. N Engl J Med. 2014;371(8):757-765.
11. Pelosi G, Fraggetta F, Maffini F, Solli P, Cavallon A, Viale G. Pulmonary epithelialmyoepithelial tumor of unproven malignant potential: report of a case and review
of the literature. Mod Pathol. 2001;14(5):521-526.
12. Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC, eds. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon, France: IARC Press; 2004. International Agency for Research on Cancer website. https://www.iarc.fr/en/publications/pdfs-online /pat-gen/bb10/BB10.pdf. Accessed June 29, 2016.
13. García JJ, Jin L, Jackson SB, et al. Primary pulmonary hyalinizing clear cell carcinoma of bronchial submucosal gland origin. Hum Pathol. 2015;46(3):471-475.
14. Damiani S, Magrini E, Farnedi A, Pession A. Basal cell (myoepithelial) adenocarcinoma of the lung. First case with cytogenetic findings. Histopathology. 2004;45(4):422-424.
15. Yamada S, Noguchi H, Nabeshima A, et al. Basaloid carcinoma of the lung associated with central cavitation: a unique surgical case focusing on cytological and immunohistochemical findings. Diagn Pathol. 2012;7:175-180.
Note: Page numbers differ between the print issue and digital edition.
Salivary gland lung tumors are extremely rare intrathoracic malignancies, accounting for only 0.2% of all lung tumors.1 It has been postulated that these lung tumors arise from pluripotential cells in the epithelium of the submucosal bronchial glands and usually present as endoluminal lesions. The cause of salivary gland tumors is unclear. They seem to be unrelated to exposure to smoking, air pollutants, or other chemicals.2 Associated symptoms are generally related to endoluminal obstruction by the tumors, which are centrally located. Thus, presenting symptoms commonly include chronic cough, progressive dyspnea, hoarseness, wheezing, and occasional hemoptysis.3 Chest radiographs seem normal in most cases except those in which obstruction is present. Computed tomography usually shows well-defined endotracheal or endobronchial lesions that are lobulated, polypoid, or smooth, without infiltration into surrounding tissues.
Although basal cell adenocarcinoma (BCAC) was included in World Health Organization’s (WHO) Pathology and Genetics of Head and Neck Tumours in 1991, its definition—“an epithelial neoplasm that has cytological characteristics of basal cell adenoma (BCA), but a morphologic growth pattern indicative of malignancy”—did not appear until 2005.4 Basal cell adenocarcinoma generally is classified as a low-grade malignancy with a good long-term prognosis. It usually affects parotid and submandibular minor salivary glands.5
Histologic differentiation of BCAC and BCA is difficult and depends on whether local structures have been invaded or on which histologic features of perineural or vascular invasion are present.6 Basal cell adenocarcinoma also shows strong immunoreactivity to cytokeratin 7 (CK-7) and variable myoepithelial staining with S100.7 Because of the prognosis and potential treatment differences involved, BCAC must be differentiated from other basaloid cell tumors, such as BCA, adenoid cystic carcinoma, polymorphous low-grade adenocarcinoma, myoepithelial tumor, epithelial-myoepithelial carcinoma, and basaloid squamous cell carcinoma (SCC).8 Surgical excision is the primary treatment of choice.5 Rare in the salivary glands, BCA and BCAC are even rarer in salivary gland tissue outside the head and neck region. The authors report on a case of BCAC of the salivary gland tissue in the trachea.
Case Report
An 84-year-old man with diabetes mellitus and hypertension and a nonsmoker presented to the emergency department of the VA Caribbean Healthcare System in San Juan, Puerto Rico, with a dry cough and shortness of breath lasting 1 week, which worsened the day before
presentation. On physical examination, the patient was afebrile and in no respiratory distress, and his vital signs were within normal limits. There was no barrel chest, no prolonged expiratory phase, and occasional wheezing more prominent on the left side. A chest radiograph showed atelectasis and/or associated changes (Figure 1).
After the initial biopsy results led to a provisional diagnosis of basaloid neoplasm with squamous features, the patient underwent rigid bronchoscopic tracheal tumor debridement followed by cryotherapy at the base of the tumor.
Discussion
Primary tracheal tumors are rare, accounting for < 1% of all malignancies.9,10 According to the National Cancer Institute Surveillance, Epidemiology, and End Results database, the rate of new cases of primary carcinoma of the trachea was 2.6 per 1 million people per year.11 Of all primary tumors of the trachea, 80% are malignant9,10; the rest vary widely and include both malignant and benign histotypes.11
The abundant minor salivary gland tissue in the upper respiratory tract can potentially develop neoplasia the same as the wide spectrum seen in head and neck salivary gland tumors. The most recent (2004) WHO Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart includes a brief section on salivary gland tumors and mentions only mucoepidermoid carcinoma, adenoid cystic carcinoma, myoepithelial carcinoma, epithelial-myoepithelial carcinoma, pleomorphic adenoma, and carcinoma ex-pleomorphic adenoma.12 However, there are reports of other salivary gland tumors in the tracheopulmonary location.
García and colleagues recently described a case of hyalinizing clear cell carcinoma with its defining EWSR1-ATF1 (Ewing sarcoma breakpoint region 1–activating transcription factor 1) fusion transcript.13 Similarly, no tracheopulmonary BCAC cases have been reported in the English-language literature since 2004, when Damiani and colleagues described a case of basal adenocarcinoma of the lung.14 In 2012, Yamada and colleagues reported a case of “basaloid carcinoma with central cavitation,”15 which showed a peculiar immunohistochemical profile similar
to the tumor described in the present article—suggestive of a myoepithelial origin if the morphologic architectural features match.
Salivary gland tumors are prone to manifest the squamous phenotype. Depending on the biopsy modality, these squamous areas (squamous eddies) can be sampled and can lead to the erroneous diagnosis of SCC. However, the histologic features of squamous malignancy are
lacking in these squamous components, and the pathologist should be able to distinguish additional phenotypes that can generate a wider differential diagnosis.
On follow-up, this patient has maintained satisfactory respiratory function. Starting 2 years after the initial resection, he has had annual checkups. Recent imaging and bronchoscopy have revealed tumor recurrence in the same site. Because of the patient’s extensive comorbidities, surgical intervention has been deferred in favor of cryotherapy, a less aggressive therapy.
Author disclosure
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
Salivary gland lung tumors are extremely rare intrathoracic malignancies, accounting for only 0.2% of all lung tumors.1 It has been postulated that these lung tumors arise from pluripotential cells in the epithelium of the submucosal bronchial glands and usually present as endoluminal lesions. The cause of salivary gland tumors is unclear. They seem to be unrelated to exposure to smoking, air pollutants, or other chemicals.2 Associated symptoms are generally related to endoluminal obstruction by the tumors, which are centrally located. Thus, presenting symptoms commonly include chronic cough, progressive dyspnea, hoarseness, wheezing, and occasional hemoptysis.3 Chest radiographs seem normal in most cases except those in which obstruction is present. Computed tomography usually shows well-defined endotracheal or endobronchial lesions that are lobulated, polypoid, or smooth, without infiltration into surrounding tissues.
Although basal cell adenocarcinoma (BCAC) was included in World Health Organization’s (WHO) Pathology and Genetics of Head and Neck Tumours in 1991, its definition—“an epithelial neoplasm that has cytological characteristics of basal cell adenoma (BCA), but a morphologic growth pattern indicative of malignancy”—did not appear until 2005.4 Basal cell adenocarcinoma generally is classified as a low-grade malignancy with a good long-term prognosis. It usually affects parotid and submandibular minor salivary glands.5
Histologic differentiation of BCAC and BCA is difficult and depends on whether local structures have been invaded or on which histologic features of perineural or vascular invasion are present.6 Basal cell adenocarcinoma also shows strong immunoreactivity to cytokeratin 7 (CK-7) and variable myoepithelial staining with S100.7 Because of the prognosis and potential treatment differences involved, BCAC must be differentiated from other basaloid cell tumors, such as BCA, adenoid cystic carcinoma, polymorphous low-grade adenocarcinoma, myoepithelial tumor, epithelial-myoepithelial carcinoma, and basaloid squamous cell carcinoma (SCC).8 Surgical excision is the primary treatment of choice.5 Rare in the salivary glands, BCA and BCAC are even rarer in salivary gland tissue outside the head and neck region. The authors report on a case of BCAC of the salivary gland tissue in the trachea.
Case Report
An 84-year-old man with diabetes mellitus and hypertension and a nonsmoker presented to the emergency department of the VA Caribbean Healthcare System in San Juan, Puerto Rico, with a dry cough and shortness of breath lasting 1 week, which worsened the day before
presentation. On physical examination, the patient was afebrile and in no respiratory distress, and his vital signs were within normal limits. There was no barrel chest, no prolonged expiratory phase, and occasional wheezing more prominent on the left side. A chest radiograph showed atelectasis and/or associated changes (Figure 1).
After the initial biopsy results led to a provisional diagnosis of basaloid neoplasm with squamous features, the patient underwent rigid bronchoscopic tracheal tumor debridement followed by cryotherapy at the base of the tumor.
Discussion
Primary tracheal tumors are rare, accounting for < 1% of all malignancies.9,10 According to the National Cancer Institute Surveillance, Epidemiology, and End Results database, the rate of new cases of primary carcinoma of the trachea was 2.6 per 1 million people per year.11 Of all primary tumors of the trachea, 80% are malignant9,10; the rest vary widely and include both malignant and benign histotypes.11
The abundant minor salivary gland tissue in the upper respiratory tract can potentially develop neoplasia the same as the wide spectrum seen in head and neck salivary gland tumors. The most recent (2004) WHO Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart includes a brief section on salivary gland tumors and mentions only mucoepidermoid carcinoma, adenoid cystic carcinoma, myoepithelial carcinoma, epithelial-myoepithelial carcinoma, pleomorphic adenoma, and carcinoma ex-pleomorphic adenoma.12 However, there are reports of other salivary gland tumors in the tracheopulmonary location.
García and colleagues recently described a case of hyalinizing clear cell carcinoma with its defining EWSR1-ATF1 (Ewing sarcoma breakpoint region 1–activating transcription factor 1) fusion transcript.13 Similarly, no tracheopulmonary BCAC cases have been reported in the English-language literature since 2004, when Damiani and colleagues described a case of basal adenocarcinoma of the lung.14 In 2012, Yamada and colleagues reported a case of “basaloid carcinoma with central cavitation,”15 which showed a peculiar immunohistochemical profile similar
to the tumor described in the present article—suggestive of a myoepithelial origin if the morphologic architectural features match.
Salivary gland tumors are prone to manifest the squamous phenotype. Depending on the biopsy modality, these squamous areas (squamous eddies) can be sampled and can lead to the erroneous diagnosis of SCC. However, the histologic features of squamous malignancy are
lacking in these squamous components, and the pathologist should be able to distinguish additional phenotypes that can generate a wider differential diagnosis.
On follow-up, this patient has maintained satisfactory respiratory function. Starting 2 years after the initial resection, he has had annual checkups. Recent imaging and bronchoscopy have revealed tumor recurrence in the same site. Because of the patient’s extensive comorbidities, surgical intervention has been deferred in favor of cryotherapy, a less aggressive therapy.
Author disclosure
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Moran CA. Primary salivary gland-type tumors of the lung. Semin Diagn Pathol.
1995;12(2):106-122.
2. Molina JR, Aubry MC, Lewis JE, et al. Primary salivary gland-type lung cancer: spectrum of clinical presentation, histopathologic and prognostic factors. Cancer. 2007;110(10):2253-2259.
3. Brutinel WM, Cortese DA, McDougall JC, Gillio RG, Bergstralh EJ. A two-year experience with the neodymium-YAG laser in endobronchial obstruction. Chest. 1987;91(2):159-165.
4. Barnes L, Eveson JW, Reichart P, Sidransky D, eds. World Health Organization Classification of Tumours. Pathology and Genetics of Head and Neck Tumours. Lyon, France: IARC Press; 2005. International Agency for Research on Cancer website. https://www.iarc.fr/en/publications/pdfs-online/pat-gen/bb9/BB9.pdf. Accessed June 16, 2016.
5. Sarath PV, Kannan N, Patil R, Manne RK, Swapna B, Suneel Kumar KV. Basal cell adenocarcinoma of the minor salivary glands involving palate and maxillary sinus. J Clin Imaging Sci. 2013;3(suppl 1):4.
6. Farrell T, Chang YL. Basal cell adenocarcinoma of minor salivary glands. Arch Pathol Lab Med. 2007;131(10):1602-1604.
7. Jung MJ, Roh JL, Choi SH, et al. Basal cell adenocarcinoma of the salivary gland: a morphological and immunohistochemical comparison with basal cell adenoma with and without capsular invasion. Diagn Pathol. 2013;8:171.
8. Jäkel KT, Löning T. Differential diagnosis of basaloid salivary gland tumors [in German]. Pathologe. 2004;25(1):46-55.
9. Urdaneta AI, Yu JB, Wilson LD. Population based cancer registry analysis of primary tracheal carcinoma. Am J Clin Oncol. 2011;34(1):32-37.
10. Varadhachary GR, Rabe MN. Cancer of unknown primary site. N Engl J Med. 2014;371(8):757-765.
11. Pelosi G, Fraggetta F, Maffini F, Solli P, Cavallon A, Viale G. Pulmonary epithelialmyoepithelial tumor of unproven malignant potential: report of a case and review
of the literature. Mod Pathol. 2001;14(5):521-526.
12. Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC, eds. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon, France: IARC Press; 2004. International Agency for Research on Cancer website. https://www.iarc.fr/en/publications/pdfs-online /pat-gen/bb10/BB10.pdf. Accessed June 29, 2016.
13. García JJ, Jin L, Jackson SB, et al. Primary pulmonary hyalinizing clear cell carcinoma of bronchial submucosal gland origin. Hum Pathol. 2015;46(3):471-475.
14. Damiani S, Magrini E, Farnedi A, Pession A. Basal cell (myoepithelial) adenocarcinoma of the lung. First case with cytogenetic findings. Histopathology. 2004;45(4):422-424.
15. Yamada S, Noguchi H, Nabeshima A, et al. Basaloid carcinoma of the lung associated with central cavitation: a unique surgical case focusing on cytological and immunohistochemical findings. Diagn Pathol. 2012;7:175-180.
Note: Page numbers differ between the print issue and digital edition.
1. Moran CA. Primary salivary gland-type tumors of the lung. Semin Diagn Pathol.
1995;12(2):106-122.
2. Molina JR, Aubry MC, Lewis JE, et al. Primary salivary gland-type lung cancer: spectrum of clinical presentation, histopathologic and prognostic factors. Cancer. 2007;110(10):2253-2259.
3. Brutinel WM, Cortese DA, McDougall JC, Gillio RG, Bergstralh EJ. A two-year experience with the neodymium-YAG laser in endobronchial obstruction. Chest. 1987;91(2):159-165.
4. Barnes L, Eveson JW, Reichart P, Sidransky D, eds. World Health Organization Classification of Tumours. Pathology and Genetics of Head and Neck Tumours. Lyon, France: IARC Press; 2005. International Agency for Research on Cancer website. https://www.iarc.fr/en/publications/pdfs-online/pat-gen/bb9/BB9.pdf. Accessed June 16, 2016.
5. Sarath PV, Kannan N, Patil R, Manne RK, Swapna B, Suneel Kumar KV. Basal cell adenocarcinoma of the minor salivary glands involving palate and maxillary sinus. J Clin Imaging Sci. 2013;3(suppl 1):4.
6. Farrell T, Chang YL. Basal cell adenocarcinoma of minor salivary glands. Arch Pathol Lab Med. 2007;131(10):1602-1604.
7. Jung MJ, Roh JL, Choi SH, et al. Basal cell adenocarcinoma of the salivary gland: a morphological and immunohistochemical comparison with basal cell adenoma with and without capsular invasion. Diagn Pathol. 2013;8:171.
8. Jäkel KT, Löning T. Differential diagnosis of basaloid salivary gland tumors [in German]. Pathologe. 2004;25(1):46-55.
9. Urdaneta AI, Yu JB, Wilson LD. Population based cancer registry analysis of primary tracheal carcinoma. Am J Clin Oncol. 2011;34(1):32-37.
10. Varadhachary GR, Rabe MN. Cancer of unknown primary site. N Engl J Med. 2014;371(8):757-765.
11. Pelosi G, Fraggetta F, Maffini F, Solli P, Cavallon A, Viale G. Pulmonary epithelialmyoepithelial tumor of unproven malignant potential: report of a case and review
of the literature. Mod Pathol. 2001;14(5):521-526.
12. Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC, eds. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon, France: IARC Press; 2004. International Agency for Research on Cancer website. https://www.iarc.fr/en/publications/pdfs-online /pat-gen/bb10/BB10.pdf. Accessed June 29, 2016.
13. García JJ, Jin L, Jackson SB, et al. Primary pulmonary hyalinizing clear cell carcinoma of bronchial submucosal gland origin. Hum Pathol. 2015;46(3):471-475.
14. Damiani S, Magrini E, Farnedi A, Pession A. Basal cell (myoepithelial) adenocarcinoma of the lung. First case with cytogenetic findings. Histopathology. 2004;45(4):422-424.
15. Yamada S, Noguchi H, Nabeshima A, et al. Basaloid carcinoma of the lung associated with central cavitation: a unique surgical case focusing on cytological and immunohistochemical findings. Diagn Pathol. 2012;7:175-180.
Note: Page numbers differ between the print issue and digital edition.
Long-Term Survival of a Patient With Late-Stage Non-Small Cell Lung Cancer
Lung cancer is the leading cause of cancer death in the world, with non-small cell lung cancer (NSCLC) a significant component of those deaths.1,2 Treatments for advanced-stage NSCLC, however, are limited. Erlotinib, a small-molecule tyrosine kinase inhibitor of epidermal growth factor receptor (EGFR), has aided in advancing NSCLC therapy. Erlotinib has been shown to increase survival by 2 months compared with placebo in a phase 3, randomized controlled trial when used as second- or third-line therapy.3 The authors present a case of a man surviving almost 8 years with late-stage NSCLC on treatment with erlotinib at the VA West Los Angeles Medical Center (WLAMC).
Case Report
Mr. J is a 59-year-old man with a medical history of hepatitis C. He smoked 2 packs of cigarettes a day for 25 years and quit in 2003. He also had a known history of IV drug use. He was unaware of his family history because he was adopted, but his twin sister, who has no known medical problems, is also a smoker. In 2005, when Mr. J was evaluated for treatment options of long-standing hepatitis C with liver ultrasound, a large, irregular right adrenal mass was found, measuring 6.4 x 3.5 cm. A subsequent positron emission tomography (PET) scan identified a lung nodule measuring 3.8 x 3.6 x 3.3 cm. A biopsy guided by computed tomography (CT) showed NSCLC. Subsequently, metastases to the liver and adrenal glands were noted, and Mr. J was started on chemotherapy. He received 4 cycles of carboplatin 725 mg and gemcitabine 2,000 mg as well as a thoracotomy and left upper lung wedge resection in December 2005. His pain was controlled with slow-release morphine 15 mg 2 times per day and oxycodone 5 mg and acetaminophen 325 mg 4 times per day as needed for breakthrough pain.
In 2006, after 4 cycles of chemotherapy, the size of the adrenal mass and the lung mass had decreased; however, he developed new abdominal pain. A CT scan showed new intrahepatic and extrahepatic biliary dilatation and worsening pancreatic function. He could not tolerate the recommended endoscopic ultrasound and left WLAMC, later presenting to an outside hospital for his abdominal pain.
At the outside hospital, 2 masses that were surgically removed from the head of the pancreas were confirmed to be EGFR-positive NSCLC, and he was given 4 cycles of cisplatin and irinotecan at unknown doses. The only adverse effects (AEs) Mr. J reported during this period were nausea and vomiting immediately after chemotherapy. He failed to respond to this treatment and was started on bevacizumab, also at an unknown dose. The patient again did not respond and was transitioned to erlotinib 150 mg daily. The patient showed remarkable response, with lesions decreasing in size.
The patient returned to the WLAMC with multiple ulcerated lesions on his face, chest, back, and extremities and hair loss, which he reported all began within weeks of starting erlotinib. Later, he also developed trichomegaly, also presumed to be a consequence of erlotinib. Despite these AEs, erlotinib was continued at the same dose, given his impressive response to this treatment, the absence of response to other therapy, and the patient’s insistence on continuing the medication.
Of note, after his transition to the outside hospital, Mr. J and his family paid all his medical expenses because he had no insurance. His family was very supportive, and the patient described their motivation and support as paramount in his receiving treatment.
In 2008, Mr. J presented to a dermatologist and was treated with cleocin solution. Although this helped to control his symptoms, the rash persisted. As a complication of these lesions, he also experienced several superinfections for which he was treated with cephalexin. At this same time, a PET scan showed no evidence of disease. He presented to the pain service for persistent chest wall pain around the surgical site, and his pain regimen was changed to slow-release morphine 200 mg 3 times per day and morphine sulfate solution 20 mg/mL 80 mg every 4 hours for breakthrough pain.
The PET scans, which were repeated every 3 months after Mr. J resumed treatment at WLAMC, showed continued absence of disease. In 2009, when he presented to the hospital with pneumonia, a PET scan showed 2 new areas of tracer uptake measuring 1 cm. His chest wall was irradiated, but radiation therapy was stopped after the biopsy returned benign. In 2015, an annual PET scan showed only evidence of postsurgical changes.
Discussion
The benefits of EGFR therapy have been established for treatment of late-stage NSCLC, but such therapy has limitations. For advanced-stage NSCLC, erlotinib has been shown to improve disease-free progression by 2.7 to 3.25 months and overall survival by 6.7 to 7.9 months.4-6 However, 1-year survival estimates remain as low as 35.0 to 37.7%,5,6 and its utility as first-line therapy has been questioned; randomized control trials have shown EGFR therapy to be of benefit only as secondor third-line therapy, when used with platinum-based chemotherapeutics.3,4 The few reports of complete response, however, have not included a definition of duration of survival.5,6
Occasionally, there have been reports of patients surviving for significantly longer periods, including 1 report of a patient who survived with complete remission for 2 years.7 In another case report, a patient experienced partial remission for more than 1 year with erlotinib as a third-line therapy.8 Although several reports indicated prolonged survival with erlotinib, or induction of complete remission of metastasis, survival has not been longer than 2 years.9-12
Important considerations for use of erlotinib are factors that predict a positive treatment response, including female sex, no previous exposure to tobacco, Asian origin, and adenocarcinoma on histologic examination.3,13 Mr. J did not meet any of these criteria. Interestingly, one study examining characteristics predictive of a positive response to erlotinib did not show that EGFR gene mutations were associated with response, although other studies have shown this to be a significant predictor of response.3,14,15
In this patient, his impressive response to erlotinib was most likely augmented by the presence of the EGFR mutation. Additionally, some reports indicate that pretreatment with platinum-based therapy can induce genetic changes resulting in EGFR mutations, thus enabling the benefit of erlotinib.10 Given that his biopsy results were not tested for the EGFR mutation prior to initiating carboplatin, this is a possibility.
Other factors specific to Mr. J that may have influenced his response to therapy include his personal wealth, which may have given him direct access to physicians outside the VA. His family support also may have motivated him to pursue and continue treatment, thus augmenting his survival. This support likely contributed in large part to his continuing erlotinib therapy despite the severe rash, hair loss, and trichomegaly. Other AEs associated with long-term erlotinib therapy include folliculitis, diarrhea, fatigue, and paronychia,although Mr. J did not experience these.16
Conclusion
Mr. J continues to follow up regularly at WLAMC. To the authors’ knowledge, this patient’s 8 year survival is the longest length of survival for any patient with NSCLC on erlotinib therapy. While the therapeutic benefits of erlotinib as a second-line therapy have been shown, EGFR therapy may be more effective than previously thought. Further research is needed to fully understand the benefits of erlotinib.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Ridge CA, McErlean AM, Ginsberg MS. Epidemiology of lung cancer. Semin Intervent Radiol. 2013;30(2):93-98.
2. Gridelli C, Bareschino MA, Schettino C, Rossi A, Maione P, Ciardiello F. Erlotinib in non-small cell lung cancer treatment: current status and future development. Oncologist. 2007;12(7):840-849.
3. Shepherd FA, Pereira JR, Ciuleanu T, et al; National Cancer Institute of Canada Clinical Trials Group. Erlotinib in previously treated non–small-cell lung cancer. N Engl J Med. 2005;353(2):123-132.
4. Smith J. Erlotinib: small-molecule targeted therapy in the treatment of non-smallcell lung cancer. Clin Ther. 2005;27(10):1513-1534.
5. Boyer M, Horwood K, Pavlakis N, et al. Efficacy of erlotinib in patients with advanced non-small-cell lung cancer (NSCLC): analysis of the Australian subpopulation of the TRUST study. Asia Pac J Clin Oncol. 2012;8(3):248-254.
6. Reck M, van Zandwijk N, Gridelli C, et al. Erlotinib in advanced non-small cell lung cancer: efficacy and safety findings of the global phase IV Tarceva Lung Cancer Survival Treatment study. J Thorac Oncol. 2010;5(10):1616-1622.
7. Vitale MG, Riccardi F, Mocerino C, et al. Erlotinib-induced complete response in a patient with epidermal growth factor receptor wild-type lung adenocarcinoma after chemotherapy failure: a case report. J Med Case Rep. 2014;8:102.
8. Duchnowska R, Siemiatkowska A, Grala B, Smoter M. Long-term remission after erlotinib therapy in an elderly patient with advanced non-small-cell lung cancer. Case report and conclusions for clinical practice [in Polish]. Pneumonol Alergol Pol. 2008;76(6):451-455.
9. Lai CSL, Boshoff C, Falzon M, Lee SM. Complete response to erlotinib treatment in brain metastases from recurrent NSCLC. Thorax. 2006;61(1):91.
10. Karam I, Melosky B. Response to second-line erlotinib in an EGFR mutationnegative patient with non-small-cell lung cancer: make no assumptions. Curr Oncol. 2012;19(1):42-46.
11. Kobayashi T, Koizumi T, Agatsuma T, et al. A phase II trial of erlotinib in patients with EGFR wild-type advanced non-small-cell lung cancer. Cancer Chemother Pharmacol. 2012;69(5):1241-1246.
12. Gridelli C, Maione P, Galetta D, et al. Three cases of long-lasting tumor control with erlotinib after progression with gefitinib in advanced non-small cell lung cancer. J Thorac Oncol. 2007;2(8):758-761.
13. Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol. 2003;21(12):2237-2246.
14. Tsao MS, Sakurada A, Cutz JC, et al. Erlotinib in lung cancer—molecular and clinical predictors of outcome. N Engl J Med. 2005;353(2):133-144.
15. Sequist LV, Bell DW, Lynch TJ, Haber DA. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol. 2007;25(5):587-595.
16. Becker A, van Wijk A, Smit EF, Postmus PE. Side-effects of long-term administration of erlotinib in patients with non-small cell lung cancer. J Thorac Oncol. 2010;5(9):1477-1480.
Note: Page numbers differ between the print issue and digital edition.
Lung cancer is the leading cause of cancer death in the world, with non-small cell lung cancer (NSCLC) a significant component of those deaths.1,2 Treatments for advanced-stage NSCLC, however, are limited. Erlotinib, a small-molecule tyrosine kinase inhibitor of epidermal growth factor receptor (EGFR), has aided in advancing NSCLC therapy. Erlotinib has been shown to increase survival by 2 months compared with placebo in a phase 3, randomized controlled trial when used as second- or third-line therapy.3 The authors present a case of a man surviving almost 8 years with late-stage NSCLC on treatment with erlotinib at the VA West Los Angeles Medical Center (WLAMC).
Case Report
Mr. J is a 59-year-old man with a medical history of hepatitis C. He smoked 2 packs of cigarettes a day for 25 years and quit in 2003. He also had a known history of IV drug use. He was unaware of his family history because he was adopted, but his twin sister, who has no known medical problems, is also a smoker. In 2005, when Mr. J was evaluated for treatment options of long-standing hepatitis C with liver ultrasound, a large, irregular right adrenal mass was found, measuring 6.4 x 3.5 cm. A subsequent positron emission tomography (PET) scan identified a lung nodule measuring 3.8 x 3.6 x 3.3 cm. A biopsy guided by computed tomography (CT) showed NSCLC. Subsequently, metastases to the liver and adrenal glands were noted, and Mr. J was started on chemotherapy. He received 4 cycles of carboplatin 725 mg and gemcitabine 2,000 mg as well as a thoracotomy and left upper lung wedge resection in December 2005. His pain was controlled with slow-release morphine 15 mg 2 times per day and oxycodone 5 mg and acetaminophen 325 mg 4 times per day as needed for breakthrough pain.
In 2006, after 4 cycles of chemotherapy, the size of the adrenal mass and the lung mass had decreased; however, he developed new abdominal pain. A CT scan showed new intrahepatic and extrahepatic biliary dilatation and worsening pancreatic function. He could not tolerate the recommended endoscopic ultrasound and left WLAMC, later presenting to an outside hospital for his abdominal pain.
At the outside hospital, 2 masses that were surgically removed from the head of the pancreas were confirmed to be EGFR-positive NSCLC, and he was given 4 cycles of cisplatin and irinotecan at unknown doses. The only adverse effects (AEs) Mr. J reported during this period were nausea and vomiting immediately after chemotherapy. He failed to respond to this treatment and was started on bevacizumab, also at an unknown dose. The patient again did not respond and was transitioned to erlotinib 150 mg daily. The patient showed remarkable response, with lesions decreasing in size.
The patient returned to the WLAMC with multiple ulcerated lesions on his face, chest, back, and extremities and hair loss, which he reported all began within weeks of starting erlotinib. Later, he also developed trichomegaly, also presumed to be a consequence of erlotinib. Despite these AEs, erlotinib was continued at the same dose, given his impressive response to this treatment, the absence of response to other therapy, and the patient’s insistence on continuing the medication.
Of note, after his transition to the outside hospital, Mr. J and his family paid all his medical expenses because he had no insurance. His family was very supportive, and the patient described their motivation and support as paramount in his receiving treatment.
In 2008, Mr. J presented to a dermatologist and was treated with cleocin solution. Although this helped to control his symptoms, the rash persisted. As a complication of these lesions, he also experienced several superinfections for which he was treated with cephalexin. At this same time, a PET scan showed no evidence of disease. He presented to the pain service for persistent chest wall pain around the surgical site, and his pain regimen was changed to slow-release morphine 200 mg 3 times per day and morphine sulfate solution 20 mg/mL 80 mg every 4 hours for breakthrough pain.
The PET scans, which were repeated every 3 months after Mr. J resumed treatment at WLAMC, showed continued absence of disease. In 2009, when he presented to the hospital with pneumonia, a PET scan showed 2 new areas of tracer uptake measuring 1 cm. His chest wall was irradiated, but radiation therapy was stopped after the biopsy returned benign. In 2015, an annual PET scan showed only evidence of postsurgical changes.
Discussion
The benefits of EGFR therapy have been established for treatment of late-stage NSCLC, but such therapy has limitations. For advanced-stage NSCLC, erlotinib has been shown to improve disease-free progression by 2.7 to 3.25 months and overall survival by 6.7 to 7.9 months.4-6 However, 1-year survival estimates remain as low as 35.0 to 37.7%,5,6 and its utility as first-line therapy has been questioned; randomized control trials have shown EGFR therapy to be of benefit only as secondor third-line therapy, when used with platinum-based chemotherapeutics.3,4 The few reports of complete response, however, have not included a definition of duration of survival.5,6
Occasionally, there have been reports of patients surviving for significantly longer periods, including 1 report of a patient who survived with complete remission for 2 years.7 In another case report, a patient experienced partial remission for more than 1 year with erlotinib as a third-line therapy.8 Although several reports indicated prolonged survival with erlotinib, or induction of complete remission of metastasis, survival has not been longer than 2 years.9-12
Important considerations for use of erlotinib are factors that predict a positive treatment response, including female sex, no previous exposure to tobacco, Asian origin, and adenocarcinoma on histologic examination.3,13 Mr. J did not meet any of these criteria. Interestingly, one study examining characteristics predictive of a positive response to erlotinib did not show that EGFR gene mutations were associated with response, although other studies have shown this to be a significant predictor of response.3,14,15
In this patient, his impressive response to erlotinib was most likely augmented by the presence of the EGFR mutation. Additionally, some reports indicate that pretreatment with platinum-based therapy can induce genetic changes resulting in EGFR mutations, thus enabling the benefit of erlotinib.10 Given that his biopsy results were not tested for the EGFR mutation prior to initiating carboplatin, this is a possibility.
Other factors specific to Mr. J that may have influenced his response to therapy include his personal wealth, which may have given him direct access to physicians outside the VA. His family support also may have motivated him to pursue and continue treatment, thus augmenting his survival. This support likely contributed in large part to his continuing erlotinib therapy despite the severe rash, hair loss, and trichomegaly. Other AEs associated with long-term erlotinib therapy include folliculitis, diarrhea, fatigue, and paronychia,although Mr. J did not experience these.16
Conclusion
Mr. J continues to follow up regularly at WLAMC. To the authors’ knowledge, this patient’s 8 year survival is the longest length of survival for any patient with NSCLC on erlotinib therapy. While the therapeutic benefits of erlotinib as a second-line therapy have been shown, EGFR therapy may be more effective than previously thought. Further research is needed to fully understand the benefits of erlotinib.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
Lung cancer is the leading cause of cancer death in the world, with non-small cell lung cancer (NSCLC) a significant component of those deaths.1,2 Treatments for advanced-stage NSCLC, however, are limited. Erlotinib, a small-molecule tyrosine kinase inhibitor of epidermal growth factor receptor (EGFR), has aided in advancing NSCLC therapy. Erlotinib has been shown to increase survival by 2 months compared with placebo in a phase 3, randomized controlled trial when used as second- or third-line therapy.3 The authors present a case of a man surviving almost 8 years with late-stage NSCLC on treatment with erlotinib at the VA West Los Angeles Medical Center (WLAMC).
Case Report
Mr. J is a 59-year-old man with a medical history of hepatitis C. He smoked 2 packs of cigarettes a day for 25 years and quit in 2003. He also had a known history of IV drug use. He was unaware of his family history because he was adopted, but his twin sister, who has no known medical problems, is also a smoker. In 2005, when Mr. J was evaluated for treatment options of long-standing hepatitis C with liver ultrasound, a large, irregular right adrenal mass was found, measuring 6.4 x 3.5 cm. A subsequent positron emission tomography (PET) scan identified a lung nodule measuring 3.8 x 3.6 x 3.3 cm. A biopsy guided by computed tomography (CT) showed NSCLC. Subsequently, metastases to the liver and adrenal glands were noted, and Mr. J was started on chemotherapy. He received 4 cycles of carboplatin 725 mg and gemcitabine 2,000 mg as well as a thoracotomy and left upper lung wedge resection in December 2005. His pain was controlled with slow-release morphine 15 mg 2 times per day and oxycodone 5 mg and acetaminophen 325 mg 4 times per day as needed for breakthrough pain.
In 2006, after 4 cycles of chemotherapy, the size of the adrenal mass and the lung mass had decreased; however, he developed new abdominal pain. A CT scan showed new intrahepatic and extrahepatic biliary dilatation and worsening pancreatic function. He could not tolerate the recommended endoscopic ultrasound and left WLAMC, later presenting to an outside hospital for his abdominal pain.
At the outside hospital, 2 masses that were surgically removed from the head of the pancreas were confirmed to be EGFR-positive NSCLC, and he was given 4 cycles of cisplatin and irinotecan at unknown doses. The only adverse effects (AEs) Mr. J reported during this period were nausea and vomiting immediately after chemotherapy. He failed to respond to this treatment and was started on bevacizumab, also at an unknown dose. The patient again did not respond and was transitioned to erlotinib 150 mg daily. The patient showed remarkable response, with lesions decreasing in size.
The patient returned to the WLAMC with multiple ulcerated lesions on his face, chest, back, and extremities and hair loss, which he reported all began within weeks of starting erlotinib. Later, he also developed trichomegaly, also presumed to be a consequence of erlotinib. Despite these AEs, erlotinib was continued at the same dose, given his impressive response to this treatment, the absence of response to other therapy, and the patient’s insistence on continuing the medication.
Of note, after his transition to the outside hospital, Mr. J and his family paid all his medical expenses because he had no insurance. His family was very supportive, and the patient described their motivation and support as paramount in his receiving treatment.
In 2008, Mr. J presented to a dermatologist and was treated with cleocin solution. Although this helped to control his symptoms, the rash persisted. As a complication of these lesions, he also experienced several superinfections for which he was treated with cephalexin. At this same time, a PET scan showed no evidence of disease. He presented to the pain service for persistent chest wall pain around the surgical site, and his pain regimen was changed to slow-release morphine 200 mg 3 times per day and morphine sulfate solution 20 mg/mL 80 mg every 4 hours for breakthrough pain.
The PET scans, which were repeated every 3 months after Mr. J resumed treatment at WLAMC, showed continued absence of disease. In 2009, when he presented to the hospital with pneumonia, a PET scan showed 2 new areas of tracer uptake measuring 1 cm. His chest wall was irradiated, but radiation therapy was stopped after the biopsy returned benign. In 2015, an annual PET scan showed only evidence of postsurgical changes.
Discussion
The benefits of EGFR therapy have been established for treatment of late-stage NSCLC, but such therapy has limitations. For advanced-stage NSCLC, erlotinib has been shown to improve disease-free progression by 2.7 to 3.25 months and overall survival by 6.7 to 7.9 months.4-6 However, 1-year survival estimates remain as low as 35.0 to 37.7%,5,6 and its utility as first-line therapy has been questioned; randomized control trials have shown EGFR therapy to be of benefit only as secondor third-line therapy, when used with platinum-based chemotherapeutics.3,4 The few reports of complete response, however, have not included a definition of duration of survival.5,6
Occasionally, there have been reports of patients surviving for significantly longer periods, including 1 report of a patient who survived with complete remission for 2 years.7 In another case report, a patient experienced partial remission for more than 1 year with erlotinib as a third-line therapy.8 Although several reports indicated prolonged survival with erlotinib, or induction of complete remission of metastasis, survival has not been longer than 2 years.9-12
Important considerations for use of erlotinib are factors that predict a positive treatment response, including female sex, no previous exposure to tobacco, Asian origin, and adenocarcinoma on histologic examination.3,13 Mr. J did not meet any of these criteria. Interestingly, one study examining characteristics predictive of a positive response to erlotinib did not show that EGFR gene mutations were associated with response, although other studies have shown this to be a significant predictor of response.3,14,15
In this patient, his impressive response to erlotinib was most likely augmented by the presence of the EGFR mutation. Additionally, some reports indicate that pretreatment with platinum-based therapy can induce genetic changes resulting in EGFR mutations, thus enabling the benefit of erlotinib.10 Given that his biopsy results were not tested for the EGFR mutation prior to initiating carboplatin, this is a possibility.
Other factors specific to Mr. J that may have influenced his response to therapy include his personal wealth, which may have given him direct access to physicians outside the VA. His family support also may have motivated him to pursue and continue treatment, thus augmenting his survival. This support likely contributed in large part to his continuing erlotinib therapy despite the severe rash, hair loss, and trichomegaly. Other AEs associated with long-term erlotinib therapy include folliculitis, diarrhea, fatigue, and paronychia,although Mr. J did not experience these.16
Conclusion
Mr. J continues to follow up regularly at WLAMC. To the authors’ knowledge, this patient’s 8 year survival is the longest length of survival for any patient with NSCLC on erlotinib therapy. While the therapeutic benefits of erlotinib as a second-line therapy have been shown, EGFR therapy may be more effective than previously thought. Further research is needed to fully understand the benefits of erlotinib.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Ridge CA, McErlean AM, Ginsberg MS. Epidemiology of lung cancer. Semin Intervent Radiol. 2013;30(2):93-98.
2. Gridelli C, Bareschino MA, Schettino C, Rossi A, Maione P, Ciardiello F. Erlotinib in non-small cell lung cancer treatment: current status and future development. Oncologist. 2007;12(7):840-849.
3. Shepherd FA, Pereira JR, Ciuleanu T, et al; National Cancer Institute of Canada Clinical Trials Group. Erlotinib in previously treated non–small-cell lung cancer. N Engl J Med. 2005;353(2):123-132.
4. Smith J. Erlotinib: small-molecule targeted therapy in the treatment of non-smallcell lung cancer. Clin Ther. 2005;27(10):1513-1534.
5. Boyer M, Horwood K, Pavlakis N, et al. Efficacy of erlotinib in patients with advanced non-small-cell lung cancer (NSCLC): analysis of the Australian subpopulation of the TRUST study. Asia Pac J Clin Oncol. 2012;8(3):248-254.
6. Reck M, van Zandwijk N, Gridelli C, et al. Erlotinib in advanced non-small cell lung cancer: efficacy and safety findings of the global phase IV Tarceva Lung Cancer Survival Treatment study. J Thorac Oncol. 2010;5(10):1616-1622.
7. Vitale MG, Riccardi F, Mocerino C, et al. Erlotinib-induced complete response in a patient with epidermal growth factor receptor wild-type lung adenocarcinoma after chemotherapy failure: a case report. J Med Case Rep. 2014;8:102.
8. Duchnowska R, Siemiatkowska A, Grala B, Smoter M. Long-term remission after erlotinib therapy in an elderly patient with advanced non-small-cell lung cancer. Case report and conclusions for clinical practice [in Polish]. Pneumonol Alergol Pol. 2008;76(6):451-455.
9. Lai CSL, Boshoff C, Falzon M, Lee SM. Complete response to erlotinib treatment in brain metastases from recurrent NSCLC. Thorax. 2006;61(1):91.
10. Karam I, Melosky B. Response to second-line erlotinib in an EGFR mutationnegative patient with non-small-cell lung cancer: make no assumptions. Curr Oncol. 2012;19(1):42-46.
11. Kobayashi T, Koizumi T, Agatsuma T, et al. A phase II trial of erlotinib in patients with EGFR wild-type advanced non-small-cell lung cancer. Cancer Chemother Pharmacol. 2012;69(5):1241-1246.
12. Gridelli C, Maione P, Galetta D, et al. Three cases of long-lasting tumor control with erlotinib after progression with gefitinib in advanced non-small cell lung cancer. J Thorac Oncol. 2007;2(8):758-761.
13. Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol. 2003;21(12):2237-2246.
14. Tsao MS, Sakurada A, Cutz JC, et al. Erlotinib in lung cancer—molecular and clinical predictors of outcome. N Engl J Med. 2005;353(2):133-144.
15. Sequist LV, Bell DW, Lynch TJ, Haber DA. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol. 2007;25(5):587-595.
16. Becker A, van Wijk A, Smit EF, Postmus PE. Side-effects of long-term administration of erlotinib in patients with non-small cell lung cancer. J Thorac Oncol. 2010;5(9):1477-1480.
Note: Page numbers differ between the print issue and digital edition.
1. Ridge CA, McErlean AM, Ginsberg MS. Epidemiology of lung cancer. Semin Intervent Radiol. 2013;30(2):93-98.
2. Gridelli C, Bareschino MA, Schettino C, Rossi A, Maione P, Ciardiello F. Erlotinib in non-small cell lung cancer treatment: current status and future development. Oncologist. 2007;12(7):840-849.
3. Shepherd FA, Pereira JR, Ciuleanu T, et al; National Cancer Institute of Canada Clinical Trials Group. Erlotinib in previously treated non–small-cell lung cancer. N Engl J Med. 2005;353(2):123-132.
4. Smith J. Erlotinib: small-molecule targeted therapy in the treatment of non-smallcell lung cancer. Clin Ther. 2005;27(10):1513-1534.
5. Boyer M, Horwood K, Pavlakis N, et al. Efficacy of erlotinib in patients with advanced non-small-cell lung cancer (NSCLC): analysis of the Australian subpopulation of the TRUST study. Asia Pac J Clin Oncol. 2012;8(3):248-254.
6. Reck M, van Zandwijk N, Gridelli C, et al. Erlotinib in advanced non-small cell lung cancer: efficacy and safety findings of the global phase IV Tarceva Lung Cancer Survival Treatment study. J Thorac Oncol. 2010;5(10):1616-1622.
7. Vitale MG, Riccardi F, Mocerino C, et al. Erlotinib-induced complete response in a patient with epidermal growth factor receptor wild-type lung adenocarcinoma after chemotherapy failure: a case report. J Med Case Rep. 2014;8:102.
8. Duchnowska R, Siemiatkowska A, Grala B, Smoter M. Long-term remission after erlotinib therapy in an elderly patient with advanced non-small-cell lung cancer. Case report and conclusions for clinical practice [in Polish]. Pneumonol Alergol Pol. 2008;76(6):451-455.
9. Lai CSL, Boshoff C, Falzon M, Lee SM. Complete response to erlotinib treatment in brain metastases from recurrent NSCLC. Thorax. 2006;61(1):91.
10. Karam I, Melosky B. Response to second-line erlotinib in an EGFR mutationnegative patient with non-small-cell lung cancer: make no assumptions. Curr Oncol. 2012;19(1):42-46.
11. Kobayashi T, Koizumi T, Agatsuma T, et al. A phase II trial of erlotinib in patients with EGFR wild-type advanced non-small-cell lung cancer. Cancer Chemother Pharmacol. 2012;69(5):1241-1246.
12. Gridelli C, Maione P, Galetta D, et al. Three cases of long-lasting tumor control with erlotinib after progression with gefitinib in advanced non-small cell lung cancer. J Thorac Oncol. 2007;2(8):758-761.
13. Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol. 2003;21(12):2237-2246.
14. Tsao MS, Sakurada A, Cutz JC, et al. Erlotinib in lung cancer—molecular and clinical predictors of outcome. N Engl J Med. 2005;353(2):133-144.
15. Sequist LV, Bell DW, Lynch TJ, Haber DA. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol. 2007;25(5):587-595.
16. Becker A, van Wijk A, Smit EF, Postmus PE. Side-effects of long-term administration of erlotinib in patients with non-small cell lung cancer. J Thorac Oncol. 2010;5(9):1477-1480.
Note: Page numbers differ between the print issue and digital edition.
New Vaccination Data & Trends
For many diseases, prevention may be the most effective treatment plan. Vaccination is considered a safe medical procedure that not only protects those who receive the immunization, but also helps to prevent the spread of communicable diseases. Or more simply, vaccines are the most effective tool we have to prevent infectious diseases, according to the National Vaccine Program Office.
When the measles vaccine was licensed in 1962, there were more than 400,000 cases reported in the U.S. In 2012, there were just 55, according to the CDC. In fact today, a single outbreak, such as the one experienced by visitors to Disneyland in 2015 creates national headlines.
The CDC offers a tool that can help veterans determine which age-appropriate vaccinations they need to protect their health group. A short quiz, available at http://www2a.cdc.gov/nip/adultImmSched/ can help people determine which vaccinations are most crucial for them to receive. At a minimum the VA recommends that all veterans consider receiving vaccinations for influenza; pneumococcal; hepatitis A and B; measles, mumps and rubella (MMR); chickenpox (varicella); shingles (herpes zoster); tetanus, diphtheria, and pertussis vaccines. In addition, international travelers may be eligible to receive vaccinations that protect against diseases common in the countries being visited. Vaccination needs can change based on age, so the VA advises veterans to speak with their health care team about additional immunizations that might be wise to consider.
Click here to read the digital edition.
For many diseases, prevention may be the most effective treatment plan. Vaccination is considered a safe medical procedure that not only protects those who receive the immunization, but also helps to prevent the spread of communicable diseases. Or more simply, vaccines are the most effective tool we have to prevent infectious diseases, according to the National Vaccine Program Office.
When the measles vaccine was licensed in 1962, there were more than 400,000 cases reported in the U.S. In 2012, there were just 55, according to the CDC. In fact today, a single outbreak, such as the one experienced by visitors to Disneyland in 2015 creates national headlines.
The CDC offers a tool that can help veterans determine which age-appropriate vaccinations they need to protect their health group. A short quiz, available at http://www2a.cdc.gov/nip/adultImmSched/ can help people determine which vaccinations are most crucial for them to receive. At a minimum the VA recommends that all veterans consider receiving vaccinations for influenza; pneumococcal; hepatitis A and B; measles, mumps and rubella (MMR); chickenpox (varicella); shingles (herpes zoster); tetanus, diphtheria, and pertussis vaccines. In addition, international travelers may be eligible to receive vaccinations that protect against diseases common in the countries being visited. Vaccination needs can change based on age, so the VA advises veterans to speak with their health care team about additional immunizations that might be wise to consider.
Click here to read the digital edition.
For many diseases, prevention may be the most effective treatment plan. Vaccination is considered a safe medical procedure that not only protects those who receive the immunization, but also helps to prevent the spread of communicable diseases. Or more simply, vaccines are the most effective tool we have to prevent infectious diseases, according to the National Vaccine Program Office.
When the measles vaccine was licensed in 1962, there were more than 400,000 cases reported in the U.S. In 2012, there were just 55, according to the CDC. In fact today, a single outbreak, such as the one experienced by visitors to Disneyland in 2015 creates national headlines.
The CDC offers a tool that can help veterans determine which age-appropriate vaccinations they need to protect their health group. A short quiz, available at http://www2a.cdc.gov/nip/adultImmSched/ can help people determine which vaccinations are most crucial for them to receive. At a minimum the VA recommends that all veterans consider receiving vaccinations for influenza; pneumococcal; hepatitis A and B; measles, mumps and rubella (MMR); chickenpox (varicella); shingles (herpes zoster); tetanus, diphtheria, and pertussis vaccines. In addition, international travelers may be eligible to receive vaccinations that protect against diseases common in the countries being visited. Vaccination needs can change based on age, so the VA advises veterans to speak with their health care team about additional immunizations that might be wise to consider.
Click here to read the digital edition.
A Motivational Interviewing Training Program for Tobacco Cessation Counseling in Primary Care
Primary care providers (PCPs) need effective tools for activating health behavior change for the 125 million Americans living with a chronic condition.1 Smoking is an important and difficult behavior to change, and a motivator for quitting is tobacco cessation advice from a PCP.2,3 However, few PCPs provide comprehensive tobacco cessation counseling as part of routine care.4,5 One perceived barrier that providers report is their lack of training to be effective tobacco cessation advocates.4,6-8
Motivational interviewing (MI) promotes behavior change by using a nonadversarial approach aimed at resolving patient ambivalence. Motivational interviewing tools, such as asking open-ended questions, providing summary statements of what the patient expresses, reflective listening, and affirmations, are used to spur an intrinsic drive to change. These techniques have been applied to a broad range of health behaviors with positive outcomes and demonstrated efficacy.9-11 Furthermore, MI can be used in primary care for changing tobacco use, alcohol consumption, physical activity, and diet.12-14
Despite its efficacy, MI can be time-intensive to learn. Fortunately, even abbreviated MI can influence patient behavior.15,16 Rollnick and others have developed MI interventions that are deliverable in 5 to 10 minutes.17,18 These brief interventions focus on performing a rapid assessment of patients’ perceived importance and self-efficacy for change.17,18
There is increased interest in training health care professionals (HCPs) in MI, yet there is no consensus on the most effective training approach.19,20 Practitioners with many competing priorities often like to learn new skills through self-study or onetime workshops. Yet evidence suggests that these are not effective methods for gaining MI proficiency. Instead, MI training sessions that offer feedback and coaching are more effective in helping participants retain MI skills over time.21,22
The authors developed and successfully pilot-tested an MI training program called the Motivational Interviewing Smoking Treatment Enhancement Program (MI-STEP) for HCPs. This program was designed to facilitate tobacco cessation care in the VHA primary care patient centered medical home, which VHA calls patient aligned care teams (PACTs).23 The main conclusions of this pilot study have been reported elsewhere.24
The objective of this article is to describe the process evaluation the authors conducted during the MI-STEP study to gain a better understanding of how the implementation of the MI training program could be improved. The authors identified barriers and facilitators from the perspectives of MI champions and PACT practitioners.
Methods
Thirty-four PACT practitioners (physicians, nurse practitioners, registered nurses, licensed practical nurses, and pharmacists) at 2 VA medical centers were randomly assigned to a high- or moderate-intensity MI training program during the summer of 2012. This training was delivered by “MI champions,” who were recruited from PACTs and who attended a 3-day advanced training class on MI. The training included MI skills practice, group case analysis, various role-play exercises, and didactics adapted from the Rx for Change program.25 The curriculum also addressed tobacco cessation counseling using the national tobacco cessation guideline.2 Each site’s health behavior coordinator (HBC) also was recruited to be an MI champion. The HBCs are typically psychologists who have received prior training in MI as well as facilitator and clinician coaching. At the VA, HBCs are charged with integrating preventive services into care. The participating sites’ institutional review boards approved all study procedures.
MI-STEP Training Program
All 34 practitioners attended a half-day on-site MI training workshop led by the site’s HBC. This training covered the basics of MI and used interactive learning methods such as role-play (Table 1). The study practitioners also received self-study materials, and throughout the study period had access to the MI champions. Practitioners who were randomized to high-intensity MI training also attended 6 supplemental 1-hour “booster sessions” to enhance specific MI skills. The MI champions led 3 of the 1-hour booster sessions with a standard agenda, including patient cases and MI exercises. During the other 3 booster sessions, participants used patient cases to interact with a standardized patient over the telephone, and the MI champions provided feedback and coaching.
Process Evaluation
Six months after the program’s completion, investigators conducted an evaluation of the MI-STEP training program with MI champions and study practitioners. One-hour focus group sessions (2 in Minneapolis; 1 in Denver) were conducted with the MI champions by a co-investigator in Minneapolis and a facilitator in Denver. Notes were taken during the sessions. MI champions were asked about the quality of their training sessions, challenges to getting PACT members to participate in the site training, challenges to teaching MI, and how they felt MI fit within VA health care philosophy.
Ten training study practitioners were randomly selected and stratified based on group intensity assignment, discipline, and site to participate in in-depth interviews. The interviews lasted about 30 minutes, and Minneapolis study investigators conducted in-person interviews with local participants and telephone interviews with Denver participants. The interviews focused on experiences with both high- and moderate-intensity MI training programs, how MI was used in their practice, barriers to implementing MI, impressions of the MI training program, and their interactions with MI champions.
Focus group leaders were experienced interviewers who had not previously interacted with MI champions in the context of this study. Investigators conducting study practitioner interviews were blinded to group assignment. All interviews were audio-recorded and transcribed verbatim. Study investigators reviewed the focus group notes and interview transcripts, identified themes independently, and then discussed group themes. The most salient themes were selected to inform implementation of a larger scale MI training program.
Results
Nine MI champions participated in the focus groups, and 8 study practitioners from both sites representing all clinical disciplines completed in-depth interviews. Table 2 identifies the characteristics of each population.
MI Champion Focus Group Themes
The champions were asked to discuss all aspects of the program, including their training as champions, role as trainers, attitudes about using MI during patient encounters, and participation in the training program. Themes from the MI champion focus groups were placed in the following categories based on the authors’ analytic approach: training MI champions, training study practitioners, and attitudes about MI.
Training MI champions. The champions identified role-play exercises and receiving feedback as strengths of the training program. The champions also expressed the desire for more hands-on practice, especially in small groups. They wanted additional training on teaching MI and facilitating the booster sessions. The champions wanted an expert to train them on how to give feedback and how to best coach practitioners in their use of MI. Champions expressed a desire to have follow-up training sessions with the standardized patient to help them hone their newly acquired coaching skills.
Training study practitioners. The champions’ key role was to train local practitioners and lead the booster sessions for the high-intensity MI training group. Champions felt ill-prepared to fully cover the training materials during the initial half-day workshop and 6 booster sessions. Champions identified difficulty coordinating schedules with the practitioners and lack of compensation for participation as significant barriers to implementing the booster sessions. Champions felt that using a standardized patient during the booster sessions was a strength of the program and that making the cases more realistic could have further enhanced the program.
Attitudes about MI. Champions from both sites perceived MI to have a positive impact on patient care. However, all champions noted there were challenges in using MI in practice. Champions felt MI takes time, energy, and practice to gain proficiency. The current primary care system is not set up to support the use of MI. The appointment time slots are fixed, and VHA goals and the spirit of MI are not always compatible. VHA performance measures encourage providers to achieve performance targets with each patient, often requiring use of directives for patients on what to do. In contrast, MI encourages the patient to take the lead on goal setting and prioritizing.
Study Practitioner Interview Themes
The practitioners were asked to discuss MI skills training, using MI skills with patients, integrating MI into daily practice, getting other PACT members involved, booster sessions, interactions with champions, and suggestions for improving the MI program. Themes from the study practitioner interviews were grouped into the following categories: MI skills training, using MI skills, integrating MI into practice, and suggestions for improving MI training (Table 3).
MI skills training. Overall, the MI high-intensity participants stated they learned useful skills. They reported asking more questions that are open-ended and were more aware of the patient’s perspective. Practitioners reported that booster sessions provided a way to reinforce, refine, and practice their MI skills. Practitioners reported that having the champion located in their own PACT was critical for connecting with their champion between sessions. Nurses and doctors reported that not having time to meet with champions was a barrier, while pharmacists reported more flexibility.
The moderate-intensity participants reported that the training had less impact. Half the respondents reported that they did not remember much of the MI training and either forgot or did not use the newly learned MI skills.
Using MI skills. Both high- and moderate-intensity participants reported using open-ended questions, reflections, affirmations, motivation scales, and active listening.
Practitioners reported that MI helped them focus on patient-centered care, since MI is collaborative. Even when a session was not successful in leading to behavior change, practitioners felt more satisfied with the quality of the interaction.
Integrating MI into practice. The high- and moderate-intensity practitioners had different perceptions of using MI in daily practice. High-intensity participants thought MI required an initial time investment, but that would be balanced by a decrease in the number of follow-up visits needed and/or delay the time between visits. The moderate-intensity participants were more likely to report struggling with the amount of time MI took.
Suggestions for improving MI training. Practitioners from both training groups offered suggestions for improving MI training. Supervisor buy-in was deemed critical to getting other PACT members involved. Practitioners suggested providing compensation or making training mandatory to help motivate others to participate in MI training. Also, practitioners were ready to expand the MI training beyond smoking cessation to incorporate other diseases and multiple comorbidities.
The moderate-intensity participants suggested more training, practice, follow-up, and feedback. These participants also suggested boosterlike sessions.
Discussion
Champions and study practitioners reported that learning MI skills was useful. The participants felt that MI was consistent with their personal philosophies regarding patient-centered care and that MI had a positive impact on patient care. Practitioners and MI champions offered several insights for improving the delivery of MI training. First, practitioners and champions highlighted how important practice and feedback were to learning MI. Booster sessions, standardized patients, and critical feedback enhanced learning.
Second, champions reported that they wanted more training in how to teach MI. Third, practitioners and champions repeatedly stated that finding the time needed to become proficient in MI was difficult and that using the MI approach with patients took additional time during clinical sessions. However, participants in the high-intensity group reported more satisfaction with the quality of their patient encounters and the freedom to follow up with patients less often.
There were aspects of the environment and MI training program that facilitated the MI learning process. The high-intensity group cited booster session feedback as being reinforcing; the moderate-intensity group expressed a desire to practice their newly acquired skill and felt feedback and coaching would have enhanced their learning. Practitioners and champions reported that using a standardized patient to enhance experiential learning activities was an asset. Standardized patients have been used successfully in other training programs.21
Implementing an MI training program posed a number of challenges. The biggest barrier was lack of time. PACT members found it difficult to attend a half-day MI workshop, practice MI skills, and incorporate MI routinely into daily practice. However, without the investment of time, even basic MI proficiency is unachievable.22
This study highlighted several ways to improve feedback and coaching. First, the authors would expand the MI champion curriculum to include training to provide effective feedback/coaching. Second, the authors would train the standardized patient on how to provide feedback to the MI learner. As implemented, the standardized patient evaluated the learner only on whether the patient felt “heard” by the learner.
Perhaps most critical to the success of an MI training program is institutional support. There needs to be adequate time and space for the training process as well as support for ongoing learning and feedback as MI skills are refined. Furthermore, sufficient time is needed during patients’ appointments to allow for MI-oriented conversations. Time is an important, valuable, and scarce resource that institutions control. Administrators should realize that the up-front investment is likely to provide a downstream return as providers become proficient in MI.
There is an urgent need to find ways to incorporate training into the daily practice of busy HCPs. Although this study was limited by its small sample, it demonstrated the feasibility of implementing an MI training program for practitioners working in a busy primary care environment. This study offers concrete suggestions for overcoming barriers and enhancing facilitators, which can guide much needed larger studies as they examine MI training effectiveness on patient and clinician outcomes.
Champions and practitioners reported that learning MI was important, but opportunities to practice and receive critical feedback are needed to achieve proficiency and improve confidence. Both champions and study practitioners thought practicing with a standardized patient would enrich their learning. However, dedicated time for learning and practicing MI skills is critical and hard to arrange.
Conclusion
Practitioners can use MI to activate health behavior change in their patients. Training PACT practitioners to use MI is feasible. The results of this evaluation can be used to inform the next iteration of an MI training program for HCPs by highlighting the facilitators of and barriers to training.
Because of the interest in activating patient-centered health behavior change, these findings are important. The educational and practice opportunities were well received. Training with standardized patients and incorporating MI champions into PACTs facilitated training. However, the lack of time was a major barrier to learning and practicing MI skills and will need to be addressed. If effectively implemented, training providers by using an evidence-based approach, such as MI, can promote long-term health.
Acknowledgments
This study was funded by VA Health Services Research & Development (HSR&D) Rapid Response Project 11-019. The Center for Chronic Disease Outcomes Research is supported by the VA, VHA, Office of Research and Development, and HSR&D. Dr. Widome was supported by a VA HSR&D Career Development Award.
1. Anderson G, Horvath J. The growing burden of chronic disease in America. Public Health Rep. 2004;119(3):263-270
2. Fiore MC, Jaen CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: U.S. Dept of Health and Human Services, Public Health Service; 2008.
3. Park E, Eaton CA, Goldstein MG, et al. The development of a decisional balance measure of physician smoking cessation interventions. Prev Med. 2001;33(4):261-267.
4. Ferketich AK, Khan Y, Wewers ME. Are physicians asking about tobacco use and assisting with cessation? Results from the 2001-2004 National Ambulatory Medical Care Survey (NAMCS). Prev Med. 2006;43(6):472-476.
5. Marcy TW, Skelly J, Shiffman RN, Flynn BS. Facilitating adherence to the tobacco use treatment guideline with computer-mediated decision support systems: physician and clinic office manager perspectives. Prev Med. 2005;41(2):479-487.
6. Cabana MD, Rand CS, Powe NR, et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458-1465.
7. Jaén CR, McIlvain H, Pol L, Phillips RL Jr, Flocke S, Crabtree BF. Tailoring tobacco counseling to the competing demands in the clinical encounter. J Fam Pract. 2001;50(10):859-863.
8. Malte CA, McFall M, Chow B, Beckham JC, Carmody TP, Saxon AJ. Survey of providers' attitudes toward integrating smoking cessation treatment into posttraumatic stress disorder care. Psychol Addict Behav. 2013;27(1):249-255.
9. Hettema J, Steele J, Miller WR. Motivational interviewing. Annu Rev Clin Psychol. 2005;1:91-111.
10. Rollnick S, Miller WR, Butler BC. Motivational Interviewing in Health Care: Helping Patients Change Behavior. New York, NY: Guilford Press; 2008.
11. Miller WR. Motivational interviewing with problem drinkers. Behav Psychother. 1983;11(2):147-172.
12. Brodie DA, Inoue A. Motivational interviewing to promote physical activity for people with chronic heart failure. J Adv Nurs. 2005;50(5):518-527.
13. Perry CK, Rosenfeld AG, Bennett JA, Potempa K. Heart-to-Heart: promoting walking in rural women through motivational interviewing and group support. J Cardiovascular Nurs. 2007;22(4):304-312.
14. West DS, DiLillo V, Bursac Z, Gore SA, Greene PG. Motivational interviewing improves weight loss in women with type 2 diabetes. Diabetes Care. 2007;30(5):1081-1087.
15. Fiore MC, Novotny TE, Pierce JP, et al. Trends in cigarette smoking in the United States. JAMA. 1989;261(1):49-55.
16. Lancaster T, Stead L. Physician advice for smoking cessation. Cochrane Database Syst Rev. 2004;18(4):CD000165.
17. Butler C, Rollnick S, Cohen D, Bachmann M, Russell I, Stott N. Motivational counseling versus brief advice for smokers in general practice: a randomized trial. Br J Gen Pract. 1999;49(445):611-616.
18. Rollnick S, Heather N, Bell A. Negotiating behaviour change in medical settings: the development of brief motivational interviewing. J Ment Health. 1992;1(1):25-37.
19. Madson MB, Loignon AC, Lane C. Training in motivational interviewing: a systematic review. J Subst Abuse Treat. 2009;36(1):101-109.
20. Miller WR, Yahne CE, Moyers TB, Martinez J, Pirritano M. A randomized trial of methods to help clinicians learn motivational interviewing. J Consult Clin Psychol. 2004;72(6):1050-1062.
21. Lundahl B, Burke BL. The effectiveness and applicability of motivational interviewing: a practice-friendly review of four meta-analyses. J Clin Pyschol. 2009;65(11):1232-1245.
22. Miller WR, Moyers TB. Eight stages in Learning motivational interviewing. J Teaching Addict. 2006;5(1):13-15.
23. Rosland AM, Nelson K, Sun H, et al. The patient-centered medical home in the Veterans Health Administration. Am J Manag Care. 2013;19(7):e263-e272.
24. Fu S, Roth C, Battaglia CT, et al. Training primary care clinicians in motivational interviewing: a comparison of two models. Patient Educ Couns. 2015;98(1):61-68.
25. School of Pharmacy & Medicine University of California, San Francisco. Rx for change website. http://rxforchange.ucsf.edu/. Accessed May 25, 2016.
Primary care providers (PCPs) need effective tools for activating health behavior change for the 125 million Americans living with a chronic condition.1 Smoking is an important and difficult behavior to change, and a motivator for quitting is tobacco cessation advice from a PCP.2,3 However, few PCPs provide comprehensive tobacco cessation counseling as part of routine care.4,5 One perceived barrier that providers report is their lack of training to be effective tobacco cessation advocates.4,6-8
Motivational interviewing (MI) promotes behavior change by using a nonadversarial approach aimed at resolving patient ambivalence. Motivational interviewing tools, such as asking open-ended questions, providing summary statements of what the patient expresses, reflective listening, and affirmations, are used to spur an intrinsic drive to change. These techniques have been applied to a broad range of health behaviors with positive outcomes and demonstrated efficacy.9-11 Furthermore, MI can be used in primary care for changing tobacco use, alcohol consumption, physical activity, and diet.12-14
Despite its efficacy, MI can be time-intensive to learn. Fortunately, even abbreviated MI can influence patient behavior.15,16 Rollnick and others have developed MI interventions that are deliverable in 5 to 10 minutes.17,18 These brief interventions focus on performing a rapid assessment of patients’ perceived importance and self-efficacy for change.17,18
There is increased interest in training health care professionals (HCPs) in MI, yet there is no consensus on the most effective training approach.19,20 Practitioners with many competing priorities often like to learn new skills through self-study or onetime workshops. Yet evidence suggests that these are not effective methods for gaining MI proficiency. Instead, MI training sessions that offer feedback and coaching are more effective in helping participants retain MI skills over time.21,22
The authors developed and successfully pilot-tested an MI training program called the Motivational Interviewing Smoking Treatment Enhancement Program (MI-STEP) for HCPs. This program was designed to facilitate tobacco cessation care in the VHA primary care patient centered medical home, which VHA calls patient aligned care teams (PACTs).23 The main conclusions of this pilot study have been reported elsewhere.24
The objective of this article is to describe the process evaluation the authors conducted during the MI-STEP study to gain a better understanding of how the implementation of the MI training program could be improved. The authors identified barriers and facilitators from the perspectives of MI champions and PACT practitioners.
Methods
Thirty-four PACT practitioners (physicians, nurse practitioners, registered nurses, licensed practical nurses, and pharmacists) at 2 VA medical centers were randomly assigned to a high- or moderate-intensity MI training program during the summer of 2012. This training was delivered by “MI champions,” who were recruited from PACTs and who attended a 3-day advanced training class on MI. The training included MI skills practice, group case analysis, various role-play exercises, and didactics adapted from the Rx for Change program.25 The curriculum also addressed tobacco cessation counseling using the national tobacco cessation guideline.2 Each site’s health behavior coordinator (HBC) also was recruited to be an MI champion. The HBCs are typically psychologists who have received prior training in MI as well as facilitator and clinician coaching. At the VA, HBCs are charged with integrating preventive services into care. The participating sites’ institutional review boards approved all study procedures.
MI-STEP Training Program
All 34 practitioners attended a half-day on-site MI training workshop led by the site’s HBC. This training covered the basics of MI and used interactive learning methods such as role-play (Table 1). The study practitioners also received self-study materials, and throughout the study period had access to the MI champions. Practitioners who were randomized to high-intensity MI training also attended 6 supplemental 1-hour “booster sessions” to enhance specific MI skills. The MI champions led 3 of the 1-hour booster sessions with a standard agenda, including patient cases and MI exercises. During the other 3 booster sessions, participants used patient cases to interact with a standardized patient over the telephone, and the MI champions provided feedback and coaching.
Process Evaluation
Six months after the program’s completion, investigators conducted an evaluation of the MI-STEP training program with MI champions and study practitioners. One-hour focus group sessions (2 in Minneapolis; 1 in Denver) were conducted with the MI champions by a co-investigator in Minneapolis and a facilitator in Denver. Notes were taken during the sessions. MI champions were asked about the quality of their training sessions, challenges to getting PACT members to participate in the site training, challenges to teaching MI, and how they felt MI fit within VA health care philosophy.
Ten training study practitioners were randomly selected and stratified based on group intensity assignment, discipline, and site to participate in in-depth interviews. The interviews lasted about 30 minutes, and Minneapolis study investigators conducted in-person interviews with local participants and telephone interviews with Denver participants. The interviews focused on experiences with both high- and moderate-intensity MI training programs, how MI was used in their practice, barriers to implementing MI, impressions of the MI training program, and their interactions with MI champions.
Focus group leaders were experienced interviewers who had not previously interacted with MI champions in the context of this study. Investigators conducting study practitioner interviews were blinded to group assignment. All interviews were audio-recorded and transcribed verbatim. Study investigators reviewed the focus group notes and interview transcripts, identified themes independently, and then discussed group themes. The most salient themes were selected to inform implementation of a larger scale MI training program.
Results
Nine MI champions participated in the focus groups, and 8 study practitioners from both sites representing all clinical disciplines completed in-depth interviews. Table 2 identifies the characteristics of each population.
MI Champion Focus Group Themes
The champions were asked to discuss all aspects of the program, including their training as champions, role as trainers, attitudes about using MI during patient encounters, and participation in the training program. Themes from the MI champion focus groups were placed in the following categories based on the authors’ analytic approach: training MI champions, training study practitioners, and attitudes about MI.
Training MI champions. The champions identified role-play exercises and receiving feedback as strengths of the training program. The champions also expressed the desire for more hands-on practice, especially in small groups. They wanted additional training on teaching MI and facilitating the booster sessions. The champions wanted an expert to train them on how to give feedback and how to best coach practitioners in their use of MI. Champions expressed a desire to have follow-up training sessions with the standardized patient to help them hone their newly acquired coaching skills.
Training study practitioners. The champions’ key role was to train local practitioners and lead the booster sessions for the high-intensity MI training group. Champions felt ill-prepared to fully cover the training materials during the initial half-day workshop and 6 booster sessions. Champions identified difficulty coordinating schedules with the practitioners and lack of compensation for participation as significant barriers to implementing the booster sessions. Champions felt that using a standardized patient during the booster sessions was a strength of the program and that making the cases more realistic could have further enhanced the program.
Attitudes about MI. Champions from both sites perceived MI to have a positive impact on patient care. However, all champions noted there were challenges in using MI in practice. Champions felt MI takes time, energy, and practice to gain proficiency. The current primary care system is not set up to support the use of MI. The appointment time slots are fixed, and VHA goals and the spirit of MI are not always compatible. VHA performance measures encourage providers to achieve performance targets with each patient, often requiring use of directives for patients on what to do. In contrast, MI encourages the patient to take the lead on goal setting and prioritizing.
Study Practitioner Interview Themes
The practitioners were asked to discuss MI skills training, using MI skills with patients, integrating MI into daily practice, getting other PACT members involved, booster sessions, interactions with champions, and suggestions for improving the MI program. Themes from the study practitioner interviews were grouped into the following categories: MI skills training, using MI skills, integrating MI into practice, and suggestions for improving MI training (Table 3).
MI skills training. Overall, the MI high-intensity participants stated they learned useful skills. They reported asking more questions that are open-ended and were more aware of the patient’s perspective. Practitioners reported that booster sessions provided a way to reinforce, refine, and practice their MI skills. Practitioners reported that having the champion located in their own PACT was critical for connecting with their champion between sessions. Nurses and doctors reported that not having time to meet with champions was a barrier, while pharmacists reported more flexibility.
The moderate-intensity participants reported that the training had less impact. Half the respondents reported that they did not remember much of the MI training and either forgot or did not use the newly learned MI skills.
Using MI skills. Both high- and moderate-intensity participants reported using open-ended questions, reflections, affirmations, motivation scales, and active listening.
Practitioners reported that MI helped them focus on patient-centered care, since MI is collaborative. Even when a session was not successful in leading to behavior change, practitioners felt more satisfied with the quality of the interaction.
Integrating MI into practice. The high- and moderate-intensity practitioners had different perceptions of using MI in daily practice. High-intensity participants thought MI required an initial time investment, but that would be balanced by a decrease in the number of follow-up visits needed and/or delay the time between visits. The moderate-intensity participants were more likely to report struggling with the amount of time MI took.
Suggestions for improving MI training. Practitioners from both training groups offered suggestions for improving MI training. Supervisor buy-in was deemed critical to getting other PACT members involved. Practitioners suggested providing compensation or making training mandatory to help motivate others to participate in MI training. Also, practitioners were ready to expand the MI training beyond smoking cessation to incorporate other diseases and multiple comorbidities.
The moderate-intensity participants suggested more training, practice, follow-up, and feedback. These participants also suggested boosterlike sessions.
Discussion
Champions and study practitioners reported that learning MI skills was useful. The participants felt that MI was consistent with their personal philosophies regarding patient-centered care and that MI had a positive impact on patient care. Practitioners and MI champions offered several insights for improving the delivery of MI training. First, practitioners and champions highlighted how important practice and feedback were to learning MI. Booster sessions, standardized patients, and critical feedback enhanced learning.
Second, champions reported that they wanted more training in how to teach MI. Third, practitioners and champions repeatedly stated that finding the time needed to become proficient in MI was difficult and that using the MI approach with patients took additional time during clinical sessions. However, participants in the high-intensity group reported more satisfaction with the quality of their patient encounters and the freedom to follow up with patients less often.
There were aspects of the environment and MI training program that facilitated the MI learning process. The high-intensity group cited booster session feedback as being reinforcing; the moderate-intensity group expressed a desire to practice their newly acquired skill and felt feedback and coaching would have enhanced their learning. Practitioners and champions reported that using a standardized patient to enhance experiential learning activities was an asset. Standardized patients have been used successfully in other training programs.21
Implementing an MI training program posed a number of challenges. The biggest barrier was lack of time. PACT members found it difficult to attend a half-day MI workshop, practice MI skills, and incorporate MI routinely into daily practice. However, without the investment of time, even basic MI proficiency is unachievable.22
This study highlighted several ways to improve feedback and coaching. First, the authors would expand the MI champion curriculum to include training to provide effective feedback/coaching. Second, the authors would train the standardized patient on how to provide feedback to the MI learner. As implemented, the standardized patient evaluated the learner only on whether the patient felt “heard” by the learner.
Perhaps most critical to the success of an MI training program is institutional support. There needs to be adequate time and space for the training process as well as support for ongoing learning and feedback as MI skills are refined. Furthermore, sufficient time is needed during patients’ appointments to allow for MI-oriented conversations. Time is an important, valuable, and scarce resource that institutions control. Administrators should realize that the up-front investment is likely to provide a downstream return as providers become proficient in MI.
There is an urgent need to find ways to incorporate training into the daily practice of busy HCPs. Although this study was limited by its small sample, it demonstrated the feasibility of implementing an MI training program for practitioners working in a busy primary care environment. This study offers concrete suggestions for overcoming barriers and enhancing facilitators, which can guide much needed larger studies as they examine MI training effectiveness on patient and clinician outcomes.
Champions and practitioners reported that learning MI was important, but opportunities to practice and receive critical feedback are needed to achieve proficiency and improve confidence. Both champions and study practitioners thought practicing with a standardized patient would enrich their learning. However, dedicated time for learning and practicing MI skills is critical and hard to arrange.
Conclusion
Practitioners can use MI to activate health behavior change in their patients. Training PACT practitioners to use MI is feasible. The results of this evaluation can be used to inform the next iteration of an MI training program for HCPs by highlighting the facilitators of and barriers to training.
Because of the interest in activating patient-centered health behavior change, these findings are important. The educational and practice opportunities were well received. Training with standardized patients and incorporating MI champions into PACTs facilitated training. However, the lack of time was a major barrier to learning and practicing MI skills and will need to be addressed. If effectively implemented, training providers by using an evidence-based approach, such as MI, can promote long-term health.
Acknowledgments
This study was funded by VA Health Services Research & Development (HSR&D) Rapid Response Project 11-019. The Center for Chronic Disease Outcomes Research is supported by the VA, VHA, Office of Research and Development, and HSR&D. Dr. Widome was supported by a VA HSR&D Career Development Award.
Primary care providers (PCPs) need effective tools for activating health behavior change for the 125 million Americans living with a chronic condition.1 Smoking is an important and difficult behavior to change, and a motivator for quitting is tobacco cessation advice from a PCP.2,3 However, few PCPs provide comprehensive tobacco cessation counseling as part of routine care.4,5 One perceived barrier that providers report is their lack of training to be effective tobacco cessation advocates.4,6-8
Motivational interviewing (MI) promotes behavior change by using a nonadversarial approach aimed at resolving patient ambivalence. Motivational interviewing tools, such as asking open-ended questions, providing summary statements of what the patient expresses, reflective listening, and affirmations, are used to spur an intrinsic drive to change. These techniques have been applied to a broad range of health behaviors with positive outcomes and demonstrated efficacy.9-11 Furthermore, MI can be used in primary care for changing tobacco use, alcohol consumption, physical activity, and diet.12-14
Despite its efficacy, MI can be time-intensive to learn. Fortunately, even abbreviated MI can influence patient behavior.15,16 Rollnick and others have developed MI interventions that are deliverable in 5 to 10 minutes.17,18 These brief interventions focus on performing a rapid assessment of patients’ perceived importance and self-efficacy for change.17,18
There is increased interest in training health care professionals (HCPs) in MI, yet there is no consensus on the most effective training approach.19,20 Practitioners with many competing priorities often like to learn new skills through self-study or onetime workshops. Yet evidence suggests that these are not effective methods for gaining MI proficiency. Instead, MI training sessions that offer feedback and coaching are more effective in helping participants retain MI skills over time.21,22
The authors developed and successfully pilot-tested an MI training program called the Motivational Interviewing Smoking Treatment Enhancement Program (MI-STEP) for HCPs. This program was designed to facilitate tobacco cessation care in the VHA primary care patient centered medical home, which VHA calls patient aligned care teams (PACTs).23 The main conclusions of this pilot study have been reported elsewhere.24
The objective of this article is to describe the process evaluation the authors conducted during the MI-STEP study to gain a better understanding of how the implementation of the MI training program could be improved. The authors identified barriers and facilitators from the perspectives of MI champions and PACT practitioners.
Methods
Thirty-four PACT practitioners (physicians, nurse practitioners, registered nurses, licensed practical nurses, and pharmacists) at 2 VA medical centers were randomly assigned to a high- or moderate-intensity MI training program during the summer of 2012. This training was delivered by “MI champions,” who were recruited from PACTs and who attended a 3-day advanced training class on MI. The training included MI skills practice, group case analysis, various role-play exercises, and didactics adapted from the Rx for Change program.25 The curriculum also addressed tobacco cessation counseling using the national tobacco cessation guideline.2 Each site’s health behavior coordinator (HBC) also was recruited to be an MI champion. The HBCs are typically psychologists who have received prior training in MI as well as facilitator and clinician coaching. At the VA, HBCs are charged with integrating preventive services into care. The participating sites’ institutional review boards approved all study procedures.
MI-STEP Training Program
All 34 practitioners attended a half-day on-site MI training workshop led by the site’s HBC. This training covered the basics of MI and used interactive learning methods such as role-play (Table 1). The study practitioners also received self-study materials, and throughout the study period had access to the MI champions. Practitioners who were randomized to high-intensity MI training also attended 6 supplemental 1-hour “booster sessions” to enhance specific MI skills. The MI champions led 3 of the 1-hour booster sessions with a standard agenda, including patient cases and MI exercises. During the other 3 booster sessions, participants used patient cases to interact with a standardized patient over the telephone, and the MI champions provided feedback and coaching.
Process Evaluation
Six months after the program’s completion, investigators conducted an evaluation of the MI-STEP training program with MI champions and study practitioners. One-hour focus group sessions (2 in Minneapolis; 1 in Denver) were conducted with the MI champions by a co-investigator in Minneapolis and a facilitator in Denver. Notes were taken during the sessions. MI champions were asked about the quality of their training sessions, challenges to getting PACT members to participate in the site training, challenges to teaching MI, and how they felt MI fit within VA health care philosophy.
Ten training study practitioners were randomly selected and stratified based on group intensity assignment, discipline, and site to participate in in-depth interviews. The interviews lasted about 30 minutes, and Minneapolis study investigators conducted in-person interviews with local participants and telephone interviews with Denver participants. The interviews focused on experiences with both high- and moderate-intensity MI training programs, how MI was used in their practice, barriers to implementing MI, impressions of the MI training program, and their interactions with MI champions.
Focus group leaders were experienced interviewers who had not previously interacted with MI champions in the context of this study. Investigators conducting study practitioner interviews were blinded to group assignment. All interviews were audio-recorded and transcribed verbatim. Study investigators reviewed the focus group notes and interview transcripts, identified themes independently, and then discussed group themes. The most salient themes were selected to inform implementation of a larger scale MI training program.
Results
Nine MI champions participated in the focus groups, and 8 study practitioners from both sites representing all clinical disciplines completed in-depth interviews. Table 2 identifies the characteristics of each population.
MI Champion Focus Group Themes
The champions were asked to discuss all aspects of the program, including their training as champions, role as trainers, attitudes about using MI during patient encounters, and participation in the training program. Themes from the MI champion focus groups were placed in the following categories based on the authors’ analytic approach: training MI champions, training study practitioners, and attitudes about MI.
Training MI champions. The champions identified role-play exercises and receiving feedback as strengths of the training program. The champions also expressed the desire for more hands-on practice, especially in small groups. They wanted additional training on teaching MI and facilitating the booster sessions. The champions wanted an expert to train them on how to give feedback and how to best coach practitioners in their use of MI. Champions expressed a desire to have follow-up training sessions with the standardized patient to help them hone their newly acquired coaching skills.
Training study practitioners. The champions’ key role was to train local practitioners and lead the booster sessions for the high-intensity MI training group. Champions felt ill-prepared to fully cover the training materials during the initial half-day workshop and 6 booster sessions. Champions identified difficulty coordinating schedules with the practitioners and lack of compensation for participation as significant barriers to implementing the booster sessions. Champions felt that using a standardized patient during the booster sessions was a strength of the program and that making the cases more realistic could have further enhanced the program.
Attitudes about MI. Champions from both sites perceived MI to have a positive impact on patient care. However, all champions noted there were challenges in using MI in practice. Champions felt MI takes time, energy, and practice to gain proficiency. The current primary care system is not set up to support the use of MI. The appointment time slots are fixed, and VHA goals and the spirit of MI are not always compatible. VHA performance measures encourage providers to achieve performance targets with each patient, often requiring use of directives for patients on what to do. In contrast, MI encourages the patient to take the lead on goal setting and prioritizing.
Study Practitioner Interview Themes
The practitioners were asked to discuss MI skills training, using MI skills with patients, integrating MI into daily practice, getting other PACT members involved, booster sessions, interactions with champions, and suggestions for improving the MI program. Themes from the study practitioner interviews were grouped into the following categories: MI skills training, using MI skills, integrating MI into practice, and suggestions for improving MI training (Table 3).
MI skills training. Overall, the MI high-intensity participants stated they learned useful skills. They reported asking more questions that are open-ended and were more aware of the patient’s perspective. Practitioners reported that booster sessions provided a way to reinforce, refine, and practice their MI skills. Practitioners reported that having the champion located in their own PACT was critical for connecting with their champion between sessions. Nurses and doctors reported that not having time to meet with champions was a barrier, while pharmacists reported more flexibility.
The moderate-intensity participants reported that the training had less impact. Half the respondents reported that they did not remember much of the MI training and either forgot or did not use the newly learned MI skills.
Using MI skills. Both high- and moderate-intensity participants reported using open-ended questions, reflections, affirmations, motivation scales, and active listening.
Practitioners reported that MI helped them focus on patient-centered care, since MI is collaborative. Even when a session was not successful in leading to behavior change, practitioners felt more satisfied with the quality of the interaction.
Integrating MI into practice. The high- and moderate-intensity practitioners had different perceptions of using MI in daily practice. High-intensity participants thought MI required an initial time investment, but that would be balanced by a decrease in the number of follow-up visits needed and/or delay the time between visits. The moderate-intensity participants were more likely to report struggling with the amount of time MI took.
Suggestions for improving MI training. Practitioners from both training groups offered suggestions for improving MI training. Supervisor buy-in was deemed critical to getting other PACT members involved. Practitioners suggested providing compensation or making training mandatory to help motivate others to participate in MI training. Also, practitioners were ready to expand the MI training beyond smoking cessation to incorporate other diseases and multiple comorbidities.
The moderate-intensity participants suggested more training, practice, follow-up, and feedback. These participants also suggested boosterlike sessions.
Discussion
Champions and study practitioners reported that learning MI skills was useful. The participants felt that MI was consistent with their personal philosophies regarding patient-centered care and that MI had a positive impact on patient care. Practitioners and MI champions offered several insights for improving the delivery of MI training. First, practitioners and champions highlighted how important practice and feedback were to learning MI. Booster sessions, standardized patients, and critical feedback enhanced learning.
Second, champions reported that they wanted more training in how to teach MI. Third, practitioners and champions repeatedly stated that finding the time needed to become proficient in MI was difficult and that using the MI approach with patients took additional time during clinical sessions. However, participants in the high-intensity group reported more satisfaction with the quality of their patient encounters and the freedom to follow up with patients less often.
There were aspects of the environment and MI training program that facilitated the MI learning process. The high-intensity group cited booster session feedback as being reinforcing; the moderate-intensity group expressed a desire to practice their newly acquired skill and felt feedback and coaching would have enhanced their learning. Practitioners and champions reported that using a standardized patient to enhance experiential learning activities was an asset. Standardized patients have been used successfully in other training programs.21
Implementing an MI training program posed a number of challenges. The biggest barrier was lack of time. PACT members found it difficult to attend a half-day MI workshop, practice MI skills, and incorporate MI routinely into daily practice. However, without the investment of time, even basic MI proficiency is unachievable.22
This study highlighted several ways to improve feedback and coaching. First, the authors would expand the MI champion curriculum to include training to provide effective feedback/coaching. Second, the authors would train the standardized patient on how to provide feedback to the MI learner. As implemented, the standardized patient evaluated the learner only on whether the patient felt “heard” by the learner.
Perhaps most critical to the success of an MI training program is institutional support. There needs to be adequate time and space for the training process as well as support for ongoing learning and feedback as MI skills are refined. Furthermore, sufficient time is needed during patients’ appointments to allow for MI-oriented conversations. Time is an important, valuable, and scarce resource that institutions control. Administrators should realize that the up-front investment is likely to provide a downstream return as providers become proficient in MI.
There is an urgent need to find ways to incorporate training into the daily practice of busy HCPs. Although this study was limited by its small sample, it demonstrated the feasibility of implementing an MI training program for practitioners working in a busy primary care environment. This study offers concrete suggestions for overcoming barriers and enhancing facilitators, which can guide much needed larger studies as they examine MI training effectiveness on patient and clinician outcomes.
Champions and practitioners reported that learning MI was important, but opportunities to practice and receive critical feedback are needed to achieve proficiency and improve confidence. Both champions and study practitioners thought practicing with a standardized patient would enrich their learning. However, dedicated time for learning and practicing MI skills is critical and hard to arrange.
Conclusion
Practitioners can use MI to activate health behavior change in their patients. Training PACT practitioners to use MI is feasible. The results of this evaluation can be used to inform the next iteration of an MI training program for HCPs by highlighting the facilitators of and barriers to training.
Because of the interest in activating patient-centered health behavior change, these findings are important. The educational and practice opportunities were well received. Training with standardized patients and incorporating MI champions into PACTs facilitated training. However, the lack of time was a major barrier to learning and practicing MI skills and will need to be addressed. If effectively implemented, training providers by using an evidence-based approach, such as MI, can promote long-term health.
Acknowledgments
This study was funded by VA Health Services Research & Development (HSR&D) Rapid Response Project 11-019. The Center for Chronic Disease Outcomes Research is supported by the VA, VHA, Office of Research and Development, and HSR&D. Dr. Widome was supported by a VA HSR&D Career Development Award.
1. Anderson G, Horvath J. The growing burden of chronic disease in America. Public Health Rep. 2004;119(3):263-270
2. Fiore MC, Jaen CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: U.S. Dept of Health and Human Services, Public Health Service; 2008.
3. Park E, Eaton CA, Goldstein MG, et al. The development of a decisional balance measure of physician smoking cessation interventions. Prev Med. 2001;33(4):261-267.
4. Ferketich AK, Khan Y, Wewers ME. Are physicians asking about tobacco use and assisting with cessation? Results from the 2001-2004 National Ambulatory Medical Care Survey (NAMCS). Prev Med. 2006;43(6):472-476.
5. Marcy TW, Skelly J, Shiffman RN, Flynn BS. Facilitating adherence to the tobacco use treatment guideline with computer-mediated decision support systems: physician and clinic office manager perspectives. Prev Med. 2005;41(2):479-487.
6. Cabana MD, Rand CS, Powe NR, et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458-1465.
7. Jaén CR, McIlvain H, Pol L, Phillips RL Jr, Flocke S, Crabtree BF. Tailoring tobacco counseling to the competing demands in the clinical encounter. J Fam Pract. 2001;50(10):859-863.
8. Malte CA, McFall M, Chow B, Beckham JC, Carmody TP, Saxon AJ. Survey of providers' attitudes toward integrating smoking cessation treatment into posttraumatic stress disorder care. Psychol Addict Behav. 2013;27(1):249-255.
9. Hettema J, Steele J, Miller WR. Motivational interviewing. Annu Rev Clin Psychol. 2005;1:91-111.
10. Rollnick S, Miller WR, Butler BC. Motivational Interviewing in Health Care: Helping Patients Change Behavior. New York, NY: Guilford Press; 2008.
11. Miller WR. Motivational interviewing with problem drinkers. Behav Psychother. 1983;11(2):147-172.
12. Brodie DA, Inoue A. Motivational interviewing to promote physical activity for people with chronic heart failure. J Adv Nurs. 2005;50(5):518-527.
13. Perry CK, Rosenfeld AG, Bennett JA, Potempa K. Heart-to-Heart: promoting walking in rural women through motivational interviewing and group support. J Cardiovascular Nurs. 2007;22(4):304-312.
14. West DS, DiLillo V, Bursac Z, Gore SA, Greene PG. Motivational interviewing improves weight loss in women with type 2 diabetes. Diabetes Care. 2007;30(5):1081-1087.
15. Fiore MC, Novotny TE, Pierce JP, et al. Trends in cigarette smoking in the United States. JAMA. 1989;261(1):49-55.
16. Lancaster T, Stead L. Physician advice for smoking cessation. Cochrane Database Syst Rev. 2004;18(4):CD000165.
17. Butler C, Rollnick S, Cohen D, Bachmann M, Russell I, Stott N. Motivational counseling versus brief advice for smokers in general practice: a randomized trial. Br J Gen Pract. 1999;49(445):611-616.
18. Rollnick S, Heather N, Bell A. Negotiating behaviour change in medical settings: the development of brief motivational interviewing. J Ment Health. 1992;1(1):25-37.
19. Madson MB, Loignon AC, Lane C. Training in motivational interviewing: a systematic review. J Subst Abuse Treat. 2009;36(1):101-109.
20. Miller WR, Yahne CE, Moyers TB, Martinez J, Pirritano M. A randomized trial of methods to help clinicians learn motivational interviewing. J Consult Clin Psychol. 2004;72(6):1050-1062.
21. Lundahl B, Burke BL. The effectiveness and applicability of motivational interviewing: a practice-friendly review of four meta-analyses. J Clin Pyschol. 2009;65(11):1232-1245.
22. Miller WR, Moyers TB. Eight stages in Learning motivational interviewing. J Teaching Addict. 2006;5(1):13-15.
23. Rosland AM, Nelson K, Sun H, et al. The patient-centered medical home in the Veterans Health Administration. Am J Manag Care. 2013;19(7):e263-e272.
24. Fu S, Roth C, Battaglia CT, et al. Training primary care clinicians in motivational interviewing: a comparison of two models. Patient Educ Couns. 2015;98(1):61-68.
25. School of Pharmacy & Medicine University of California, San Francisco. Rx for change website. http://rxforchange.ucsf.edu/. Accessed May 25, 2016.
1. Anderson G, Horvath J. The growing burden of chronic disease in America. Public Health Rep. 2004;119(3):263-270
2. Fiore MC, Jaen CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: U.S. Dept of Health and Human Services, Public Health Service; 2008.
3. Park E, Eaton CA, Goldstein MG, et al. The development of a decisional balance measure of physician smoking cessation interventions. Prev Med. 2001;33(4):261-267.
4. Ferketich AK, Khan Y, Wewers ME. Are physicians asking about tobacco use and assisting with cessation? Results from the 2001-2004 National Ambulatory Medical Care Survey (NAMCS). Prev Med. 2006;43(6):472-476.
5. Marcy TW, Skelly J, Shiffman RN, Flynn BS. Facilitating adherence to the tobacco use treatment guideline with computer-mediated decision support systems: physician and clinic office manager perspectives. Prev Med. 2005;41(2):479-487.
6. Cabana MD, Rand CS, Powe NR, et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458-1465.
7. Jaén CR, McIlvain H, Pol L, Phillips RL Jr, Flocke S, Crabtree BF. Tailoring tobacco counseling to the competing demands in the clinical encounter. J Fam Pract. 2001;50(10):859-863.
8. Malte CA, McFall M, Chow B, Beckham JC, Carmody TP, Saxon AJ. Survey of providers' attitudes toward integrating smoking cessation treatment into posttraumatic stress disorder care. Psychol Addict Behav. 2013;27(1):249-255.
9. Hettema J, Steele J, Miller WR. Motivational interviewing. Annu Rev Clin Psychol. 2005;1:91-111.
10. Rollnick S, Miller WR, Butler BC. Motivational Interviewing in Health Care: Helping Patients Change Behavior. New York, NY: Guilford Press; 2008.
11. Miller WR. Motivational interviewing with problem drinkers. Behav Psychother. 1983;11(2):147-172.
12. Brodie DA, Inoue A. Motivational interviewing to promote physical activity for people with chronic heart failure. J Adv Nurs. 2005;50(5):518-527.
13. Perry CK, Rosenfeld AG, Bennett JA, Potempa K. Heart-to-Heart: promoting walking in rural women through motivational interviewing and group support. J Cardiovascular Nurs. 2007;22(4):304-312.
14. West DS, DiLillo V, Bursac Z, Gore SA, Greene PG. Motivational interviewing improves weight loss in women with type 2 diabetes. Diabetes Care. 2007;30(5):1081-1087.
15. Fiore MC, Novotny TE, Pierce JP, et al. Trends in cigarette smoking in the United States. JAMA. 1989;261(1):49-55.
16. Lancaster T, Stead L. Physician advice for smoking cessation. Cochrane Database Syst Rev. 2004;18(4):CD000165.
17. Butler C, Rollnick S, Cohen D, Bachmann M, Russell I, Stott N. Motivational counseling versus brief advice for smokers in general practice: a randomized trial. Br J Gen Pract. 1999;49(445):611-616.
18. Rollnick S, Heather N, Bell A. Negotiating behaviour change in medical settings: the development of brief motivational interviewing. J Ment Health. 1992;1(1):25-37.
19. Madson MB, Loignon AC, Lane C. Training in motivational interviewing: a systematic review. J Subst Abuse Treat. 2009;36(1):101-109.
20. Miller WR, Yahne CE, Moyers TB, Martinez J, Pirritano M. A randomized trial of methods to help clinicians learn motivational interviewing. J Consult Clin Psychol. 2004;72(6):1050-1062.
21. Lundahl B, Burke BL. The effectiveness and applicability of motivational interviewing: a practice-friendly review of four meta-analyses. J Clin Pyschol. 2009;65(11):1232-1245.
22. Miller WR, Moyers TB. Eight stages in Learning motivational interviewing. J Teaching Addict. 2006;5(1):13-15.
23. Rosland AM, Nelson K, Sun H, et al. The patient-centered medical home in the Veterans Health Administration. Am J Manag Care. 2013;19(7):e263-e272.
24. Fu S, Roth C, Battaglia CT, et al. Training primary care clinicians in motivational interviewing: a comparison of two models. Patient Educ Couns. 2015;98(1):61-68.
25. School of Pharmacy & Medicine University of California, San Francisco. Rx for change website. http://rxforchange.ucsf.edu/. Accessed May 25, 2016.
Characteristics of High-Functioning Collaborations Between Primary Care and Podiatry in VHA PACTs
The patient centered medical home (PCMH) concept was developed in response to the need to improve the overall health care system in the U.S.1 The episodic/acute care model has not provided high-value health services for the costs incurred. A 2010 Commonwealth Fund report indicated that the U.S. was near the bottom on quality measures of patient safety, care coordination, access, efficiency, overall quality, and healthy life expectancy compared with 6 other western countries.2 The U.S. spends an average of $7,960 per capita, 2.5 times more than the average of the 6 other western countries surveyed, on health care.1 The core principles that define the PCMH include (1) enhanced access; (2) continuity; (3) comprehensiveness; (4) team-based care; (5) care coordination; (6) a systems-based approach to quality and safety; and (7) reimbursement structures consistent with the added value of this system.1
The VHA adapted the PCMH concept to fit its unique integrated health care system. The development and implementation of the patient aligned care teams (PACTs) was designed to advance and expand primary care through increased access, continuity, and coordination of care for veteran patients.3 To accomplish the care coordination component, a set of principals was developed to define its structure, using the PCMH neighbor concept. Recognizing the importance of specialty and subspecialty collaboration with primary care, the American College of Physicians issued a white paper in 2010 to define policies and features of this relationship.4 Those characteristics include bidirectional effective communication, coordination, and integration; appropriate and timely consultations and referrals; efficient, appropriate, and effective information flow; comanagement responsibility; patient-centered care, enhanced care access and high levels of care quality and safety; and whole-person coordination and integration by primary care.5
The purpose of this study was to describe the PCMH characteristics within VHA centers that self-identified as centers with good or fair/poor communication between PACTs and Podiatry. The authors’ prior work showed that higher levels of coordination were associated with lower rates of diabetes-related lower limb amputations at VA centers.6
Methods
The podiatry service chiefs at 107 VHA hospitals were sent an online survey via e-mail on October 2, 2014. Two follow-up e-mails were sent to centers that did not respond after 1 week and then again after 2 weeks. Respondents were not offered rewards or inducements to participate. Centers were chosen at random and represented the diversity of facility complexity groups. The VHA Facility Complexity Model classifies VHA facilities at levels 1a, 1b, 1c, 2, or 3. Level 1a facilities are the most complex and level 3 facilities are the least complex.
The survey was designed to determine the characteristics of high-functioning teams as defined by the joint principles of the PCMH and to assess the operational theories that good functioning teams possess the following characteristics, based on the VHA Handbook 1101.10 PACT Handbook.7
- Good bidirectional communication between PACT and podiatry.
- A working care coordination agreement (CCA) that defines referral processes, e-consult conversion when appropriate, and successful coordination of care.
- Face-to-face meetings to discuss and adjust the CCA and other program components.
The audience for the survey was the chiefs of podiatry at 107 medical centers, representing a combination of medical center complexity groups 1, 2, and 3. The survey consisted of questions designed to assess the self-reported relationship between PACT and Podiatry Service at each reporting medical center (Appendix).
Statistical Analysis
A group level analysis was performed between centers identifying themselves by having good or fair/poor communication between PACT and Podiatry. The Fisher exact test (2-sided) was used to assess for associations. Significance was set at P ≤ .05.
Results
The response rate for this survey was 54% (58/107). The Table describes the frequency of PCMH characteristics in good communicating and fair/poor communicating centers. Thirty-seven centers self-identified as having good communication between PACT and Podiatry, and 21 reported fair/poor communication (P = .015). Frequent bidirectional communication occurred in 68% of good communication centers and 10% in fair/poor communication centers (P < .001). There were no differences between good communicating centers and fair/poor communicating centers for having working care coordination agreements. In good communication centers, 69% of consults were appropriate at least 75% of the time compared with 40% of the time for fair/poor communication centers (P = .032). Active care coordination in most cases occurred in 53% of good communication centers vs 5% of fair/poor communication centers (P < .001).
In the survey, characteristics supported by the joint principles statement for developing a PCMH were assessed.3 Favorable characteristics included good communication between providers (PACT and Podiatry), a high percentage of consults considered appropriate (> 75%), and high levels of coordination. Unfavorable characteristics included poor communication between providers (PACT and Podiatry), low percentage of consults considered inappropriate (< 75%), and poor levels of communication. In the survey, 47% of good communicating centers had 1 or 2 favorable characteristics for a PCMH compared with 80% fair/poor communication centers that had 1 or 2 unfavorable characteristics (P = .025) (Figure 1).
Figure 2 describes the equivocal correlations that were found between fair or poor self-reported centers and high-functioning PACT/Podiatry services with:
- Presence of a signed CCA.
- Multiple positive or negative characteristics.
- Referrals tied to the CCA.
- Provision to convert to an e-consult.
- Face-to-face meetings to review the CCA.
Discussion
The key to high-functioning PACT/Podiatry teams rests with the quality of the communication between providers. Without this basic tenet, CCAs cannot be effective.
Conclusion
Self-reporting high-functioning PACT/Podiatry teams depend more on the relationships between providers, the ease of bidirectional communication and coordination of care, and a seemless consult and less on the formal care coordination documents and e-consults that reduce the direct exchanges between providers.
1. Arend J, Tsang-Quinn J, Levine C, Thomas D. The patient-centered medical home: history, components, and review of the evidence. Mt Sinai J Med. 2012;79(4):433-450.
2. Schoen, C, Osborn, R, Squires D, Doty MM, Pierson R, Applebaum S. How health insurance design affects access to care and costs by income, in eleven countries. Health Aff. 2010;29(12):2323;2334.
3. Bein B. AMA delegates adopt AAFP’s joint principles of patient-centered medical home. Ann Fam Med. 2009;7(1):86-87.
4. Kirschner, N, Greenlee, MC, The patient centered medical home neighbor: the interface of the patient centered medical home with specialty/subspecialty practices. Phildelphia, PA: American College of Physicians; 2010.
5. Nelson K, Sun H, Dolan E, et al. Elements of the patient-centered medical home associated with health outcomes among veterans: the role of primary care continuity, expanded access, and care coordination. J Ambul Care Manage. 2014;37(4):331-338.
6. Pogach L, Charns MP, Wrobel JS, et al. Impact of policies and performance measurement on development of organizational coordinating strategies for chronic care delivery. Am J Manag Care. 2014;10(2)(pt 2):171-180.
7. U.S. Department of Veteran Affairs. VHA Handbook 1101.10 PACT Handbook. Affairs, Washington DC: U.S. Department of Veterans Affairs; 2014.
8. Wrobel JS, Charns MP, Diehr P, et al. The relationship between provider coordination and diabetes-related foot outcomes. Diabetes Care. 2003;26(11):3042-3047.
9. Wrobel JS, Robbins JM, Charns MP, Bonacker KM, Reiber GE, Pogach L. Diabetes-related foot care at 10 Veterans Affairs medical centers: must do’s associated with successful microsystems. Jt Comm J Qual Patient Saf. 2006;32(4):206-213.
The patient centered medical home (PCMH) concept was developed in response to the need to improve the overall health care system in the U.S.1 The episodic/acute care model has not provided high-value health services for the costs incurred. A 2010 Commonwealth Fund report indicated that the U.S. was near the bottom on quality measures of patient safety, care coordination, access, efficiency, overall quality, and healthy life expectancy compared with 6 other western countries.2 The U.S. spends an average of $7,960 per capita, 2.5 times more than the average of the 6 other western countries surveyed, on health care.1 The core principles that define the PCMH include (1) enhanced access; (2) continuity; (3) comprehensiveness; (4) team-based care; (5) care coordination; (6) a systems-based approach to quality and safety; and (7) reimbursement structures consistent with the added value of this system.1
The VHA adapted the PCMH concept to fit its unique integrated health care system. The development and implementation of the patient aligned care teams (PACTs) was designed to advance and expand primary care through increased access, continuity, and coordination of care for veteran patients.3 To accomplish the care coordination component, a set of principals was developed to define its structure, using the PCMH neighbor concept. Recognizing the importance of specialty and subspecialty collaboration with primary care, the American College of Physicians issued a white paper in 2010 to define policies and features of this relationship.4 Those characteristics include bidirectional effective communication, coordination, and integration; appropriate and timely consultations and referrals; efficient, appropriate, and effective information flow; comanagement responsibility; patient-centered care, enhanced care access and high levels of care quality and safety; and whole-person coordination and integration by primary care.5
The purpose of this study was to describe the PCMH characteristics within VHA centers that self-identified as centers with good or fair/poor communication between PACTs and Podiatry. The authors’ prior work showed that higher levels of coordination were associated with lower rates of diabetes-related lower limb amputations at VA centers.6
Methods
The podiatry service chiefs at 107 VHA hospitals were sent an online survey via e-mail on October 2, 2014. Two follow-up e-mails were sent to centers that did not respond after 1 week and then again after 2 weeks. Respondents were not offered rewards or inducements to participate. Centers were chosen at random and represented the diversity of facility complexity groups. The VHA Facility Complexity Model classifies VHA facilities at levels 1a, 1b, 1c, 2, or 3. Level 1a facilities are the most complex and level 3 facilities are the least complex.
The survey was designed to determine the characteristics of high-functioning teams as defined by the joint principles of the PCMH and to assess the operational theories that good functioning teams possess the following characteristics, based on the VHA Handbook 1101.10 PACT Handbook.7
- Good bidirectional communication between PACT and podiatry.
- A working care coordination agreement (CCA) that defines referral processes, e-consult conversion when appropriate, and successful coordination of care.
- Face-to-face meetings to discuss and adjust the CCA and other program components.
The audience for the survey was the chiefs of podiatry at 107 medical centers, representing a combination of medical center complexity groups 1, 2, and 3. The survey consisted of questions designed to assess the self-reported relationship between PACT and Podiatry Service at each reporting medical center (Appendix).
Statistical Analysis
A group level analysis was performed between centers identifying themselves by having good or fair/poor communication between PACT and Podiatry. The Fisher exact test (2-sided) was used to assess for associations. Significance was set at P ≤ .05.
Results
The response rate for this survey was 54% (58/107). The Table describes the frequency of PCMH characteristics in good communicating and fair/poor communicating centers. Thirty-seven centers self-identified as having good communication between PACT and Podiatry, and 21 reported fair/poor communication (P = .015). Frequent bidirectional communication occurred in 68% of good communication centers and 10% in fair/poor communication centers (P < .001). There were no differences between good communicating centers and fair/poor communicating centers for having working care coordination agreements. In good communication centers, 69% of consults were appropriate at least 75% of the time compared with 40% of the time for fair/poor communication centers (P = .032). Active care coordination in most cases occurred in 53% of good communication centers vs 5% of fair/poor communication centers (P < .001).
In the survey, characteristics supported by the joint principles statement for developing a PCMH were assessed.3 Favorable characteristics included good communication between providers (PACT and Podiatry), a high percentage of consults considered appropriate (> 75%), and high levels of coordination. Unfavorable characteristics included poor communication between providers (PACT and Podiatry), low percentage of consults considered inappropriate (< 75%), and poor levels of communication. In the survey, 47% of good communicating centers had 1 or 2 favorable characteristics for a PCMH compared with 80% fair/poor communication centers that had 1 or 2 unfavorable characteristics (P = .025) (Figure 1).
Figure 2 describes the equivocal correlations that were found between fair or poor self-reported centers and high-functioning PACT/Podiatry services with:
- Presence of a signed CCA.
- Multiple positive or negative characteristics.
- Referrals tied to the CCA.
- Provision to convert to an e-consult.
- Face-to-face meetings to review the CCA.
Discussion
The key to high-functioning PACT/Podiatry teams rests with the quality of the communication between providers. Without this basic tenet, CCAs cannot be effective.
Conclusion
Self-reporting high-functioning PACT/Podiatry teams depend more on the relationships between providers, the ease of bidirectional communication and coordination of care, and a seemless consult and less on the formal care coordination documents and e-consults that reduce the direct exchanges between providers.
The patient centered medical home (PCMH) concept was developed in response to the need to improve the overall health care system in the U.S.1 The episodic/acute care model has not provided high-value health services for the costs incurred. A 2010 Commonwealth Fund report indicated that the U.S. was near the bottom on quality measures of patient safety, care coordination, access, efficiency, overall quality, and healthy life expectancy compared with 6 other western countries.2 The U.S. spends an average of $7,960 per capita, 2.5 times more than the average of the 6 other western countries surveyed, on health care.1 The core principles that define the PCMH include (1) enhanced access; (2) continuity; (3) comprehensiveness; (4) team-based care; (5) care coordination; (6) a systems-based approach to quality and safety; and (7) reimbursement structures consistent with the added value of this system.1
The VHA adapted the PCMH concept to fit its unique integrated health care system. The development and implementation of the patient aligned care teams (PACTs) was designed to advance and expand primary care through increased access, continuity, and coordination of care for veteran patients.3 To accomplish the care coordination component, a set of principals was developed to define its structure, using the PCMH neighbor concept. Recognizing the importance of specialty and subspecialty collaboration with primary care, the American College of Physicians issued a white paper in 2010 to define policies and features of this relationship.4 Those characteristics include bidirectional effective communication, coordination, and integration; appropriate and timely consultations and referrals; efficient, appropriate, and effective information flow; comanagement responsibility; patient-centered care, enhanced care access and high levels of care quality and safety; and whole-person coordination and integration by primary care.5
The purpose of this study was to describe the PCMH characteristics within VHA centers that self-identified as centers with good or fair/poor communication between PACTs and Podiatry. The authors’ prior work showed that higher levels of coordination were associated with lower rates of diabetes-related lower limb amputations at VA centers.6
Methods
The podiatry service chiefs at 107 VHA hospitals were sent an online survey via e-mail on October 2, 2014. Two follow-up e-mails were sent to centers that did not respond after 1 week and then again after 2 weeks. Respondents were not offered rewards or inducements to participate. Centers were chosen at random and represented the diversity of facility complexity groups. The VHA Facility Complexity Model classifies VHA facilities at levels 1a, 1b, 1c, 2, or 3. Level 1a facilities are the most complex and level 3 facilities are the least complex.
The survey was designed to determine the characteristics of high-functioning teams as defined by the joint principles of the PCMH and to assess the operational theories that good functioning teams possess the following characteristics, based on the VHA Handbook 1101.10 PACT Handbook.7
- Good bidirectional communication between PACT and podiatry.
- A working care coordination agreement (CCA) that defines referral processes, e-consult conversion when appropriate, and successful coordination of care.
- Face-to-face meetings to discuss and adjust the CCA and other program components.
The audience for the survey was the chiefs of podiatry at 107 medical centers, representing a combination of medical center complexity groups 1, 2, and 3. The survey consisted of questions designed to assess the self-reported relationship between PACT and Podiatry Service at each reporting medical center (Appendix).
Statistical Analysis
A group level analysis was performed between centers identifying themselves by having good or fair/poor communication between PACT and Podiatry. The Fisher exact test (2-sided) was used to assess for associations. Significance was set at P ≤ .05.
Results
The response rate for this survey was 54% (58/107). The Table describes the frequency of PCMH characteristics in good communicating and fair/poor communicating centers. Thirty-seven centers self-identified as having good communication between PACT and Podiatry, and 21 reported fair/poor communication (P = .015). Frequent bidirectional communication occurred in 68% of good communication centers and 10% in fair/poor communication centers (P < .001). There were no differences between good communicating centers and fair/poor communicating centers for having working care coordination agreements. In good communication centers, 69% of consults were appropriate at least 75% of the time compared with 40% of the time for fair/poor communication centers (P = .032). Active care coordination in most cases occurred in 53% of good communication centers vs 5% of fair/poor communication centers (P < .001).
In the survey, characteristics supported by the joint principles statement for developing a PCMH were assessed.3 Favorable characteristics included good communication between providers (PACT and Podiatry), a high percentage of consults considered appropriate (> 75%), and high levels of coordination. Unfavorable characteristics included poor communication between providers (PACT and Podiatry), low percentage of consults considered inappropriate (< 75%), and poor levels of communication. In the survey, 47% of good communicating centers had 1 or 2 favorable characteristics for a PCMH compared with 80% fair/poor communication centers that had 1 or 2 unfavorable characteristics (P = .025) (Figure 1).
Figure 2 describes the equivocal correlations that were found between fair or poor self-reported centers and high-functioning PACT/Podiatry services with:
- Presence of a signed CCA.
- Multiple positive or negative characteristics.
- Referrals tied to the CCA.
- Provision to convert to an e-consult.
- Face-to-face meetings to review the CCA.
Discussion
The key to high-functioning PACT/Podiatry teams rests with the quality of the communication between providers. Without this basic tenet, CCAs cannot be effective.
Conclusion
Self-reporting high-functioning PACT/Podiatry teams depend more on the relationships between providers, the ease of bidirectional communication and coordination of care, and a seemless consult and less on the formal care coordination documents and e-consults that reduce the direct exchanges between providers.
1. Arend J, Tsang-Quinn J, Levine C, Thomas D. The patient-centered medical home: history, components, and review of the evidence. Mt Sinai J Med. 2012;79(4):433-450.
2. Schoen, C, Osborn, R, Squires D, Doty MM, Pierson R, Applebaum S. How health insurance design affects access to care and costs by income, in eleven countries. Health Aff. 2010;29(12):2323;2334.
3. Bein B. AMA delegates adopt AAFP’s joint principles of patient-centered medical home. Ann Fam Med. 2009;7(1):86-87.
4. Kirschner, N, Greenlee, MC, The patient centered medical home neighbor: the interface of the patient centered medical home with specialty/subspecialty practices. Phildelphia, PA: American College of Physicians; 2010.
5. Nelson K, Sun H, Dolan E, et al. Elements of the patient-centered medical home associated with health outcomes among veterans: the role of primary care continuity, expanded access, and care coordination. J Ambul Care Manage. 2014;37(4):331-338.
6. Pogach L, Charns MP, Wrobel JS, et al. Impact of policies and performance measurement on development of organizational coordinating strategies for chronic care delivery. Am J Manag Care. 2014;10(2)(pt 2):171-180.
7. U.S. Department of Veteran Affairs. VHA Handbook 1101.10 PACT Handbook. Affairs, Washington DC: U.S. Department of Veterans Affairs; 2014.
8. Wrobel JS, Charns MP, Diehr P, et al. The relationship between provider coordination and diabetes-related foot outcomes. Diabetes Care. 2003;26(11):3042-3047.
9. Wrobel JS, Robbins JM, Charns MP, Bonacker KM, Reiber GE, Pogach L. Diabetes-related foot care at 10 Veterans Affairs medical centers: must do’s associated with successful microsystems. Jt Comm J Qual Patient Saf. 2006;32(4):206-213.
1. Arend J, Tsang-Quinn J, Levine C, Thomas D. The patient-centered medical home: history, components, and review of the evidence. Mt Sinai J Med. 2012;79(4):433-450.
2. Schoen, C, Osborn, R, Squires D, Doty MM, Pierson R, Applebaum S. How health insurance design affects access to care and costs by income, in eleven countries. Health Aff. 2010;29(12):2323;2334.
3. Bein B. AMA delegates adopt AAFP’s joint principles of patient-centered medical home. Ann Fam Med. 2009;7(1):86-87.
4. Kirschner, N, Greenlee, MC, The patient centered medical home neighbor: the interface of the patient centered medical home with specialty/subspecialty practices. Phildelphia, PA: American College of Physicians; 2010.
5. Nelson K, Sun H, Dolan E, et al. Elements of the patient-centered medical home associated with health outcomes among veterans: the role of primary care continuity, expanded access, and care coordination. J Ambul Care Manage. 2014;37(4):331-338.
6. Pogach L, Charns MP, Wrobel JS, et al. Impact of policies and performance measurement on development of organizational coordinating strategies for chronic care delivery. Am J Manag Care. 2014;10(2)(pt 2):171-180.
7. U.S. Department of Veteran Affairs. VHA Handbook 1101.10 PACT Handbook. Affairs, Washington DC: U.S. Department of Veterans Affairs; 2014.
8. Wrobel JS, Charns MP, Diehr P, et al. The relationship between provider coordination and diabetes-related foot outcomes. Diabetes Care. 2003;26(11):3042-3047.
9. Wrobel JS, Robbins JM, Charns MP, Bonacker KM, Reiber GE, Pogach L. Diabetes-related foot care at 10 Veterans Affairs medical centers: must do’s associated with successful microsystems. Jt Comm J Qual Patient Saf. 2006;32(4):206-213.
Integrating Palliative Care in COPD Treatment
The integration of palliative care in cancer care is an emerging trend driven by data on the benefits of palliative care intervention in the care of patients with terminal malignancies. Although studies have shown that patients with end-stage organ disease tend to develop similar symptoms and issues as those of cancer patients, the use of palliative care services among patients with end-stage organ disease seems to be limited.1 The clinical course of terminal malignancy is usually marked by a consistent decline, whereas organ failure is usually marked by periods of exacerbations in relation to decompensation.2 Patients with organ failure often exhibit a gradual and subtle decline over time, making it more challenging to predict the disease course.2
Woo and colleagues studied patients with chronic illnesses and showed that, similar to patients diagnosed with cancer, symptoms of fatigue, pain, and dyspnea were common.3 They also found that caregivers of patients with chronic illness reported suboptimal physical and emotional well-being as well as moderate levels of stress.3 These findings suggest that caregivers for cancer and noncancer patients will benefit from the support inherent in an interdisciplinary approach to palliative care.3 According to the CDC, the second leading cause of death in the U.S. in 2011 was cancer followed by chronic respiratory disease.4
The authors conducted a quality improvement (QI) initiative to explore the benefits of integrating palliative care in the care of patients with chronic obstructive pulmonary disease (COPD) and share outcomes of improved palliative care education at John D. Dingell VAMC (JDDVAMC) in Detroit, Michigan, for care of patients with COPD.
Background
Chronic obstructive pulmonary disease is a progressive, incurable lung disease.5 It also has been referred to as chronic bronchitis, emphysema, or chronic asthma.5 The degree of severity of COPD is determined by measuring the degree of air flow obstruction by conducting a spirometry test.5 Common symptoms associated with COPD include dyspnea, cough, wheezing, recurring respiratory infections, and generalized weakness.5
Compared with terminally ill patients with lung cancer, patients with COPD were found to have a poorer quality of life as well as more anxiety and depression.6 In a study to evaluate for breathlessness among patients with severe COPD and advanced cancer, Bausewein and colleagues found that both groups reported moderately distressing physical symptoms.7 Both groups also reported shortness of breath as their most distressing physical symptom and worrying as the most common psychological symptom.7 The study also identified a 50% commonality among the participants on palliative care needs.7
The common palliative care needs that were identified were the need for symptom management for breathlessness, access to information, ability to share feelings, a sense of wasted time, and assistance with practical matters.7 During the study’s 6-month data collection period, 61% of the patients with cancer and 10% of the patients with COPD died.7 Median survival for both groups showed that the patients with COPD had a significantly longer median survival of 589 days compared with 107 days for the patients with cancer.7
A retrospective review of patient records from 2010 to 2013 showed that providers referred only 5% of patients with COPD for palliative care.8 In the United Kingdom, the 5-year survival rate among patients diagnosed with severe COPD is 24% to 30%.9 Chronic obstructive pulmonary disease is one of the most common causes of hospital admissions, and treatments are aimed toward palliation of symptoms.9 As COPD reaches its end stage, incorporation of end-of-life (EOL) care should be considered. Signs that may indicate EOL care is needed include long-term oxygen therapy, depression, hospitalization for exacerbations at a rate of 2 or more a year, evidence of right-sided heart failure, cortisone treatment for > 6 weeks, and a history of noninvasive ventilation or admission to the intensive care unit (ICU).9
Nguyen and colleagues conducted a study in Montreal, Canada, among patients with moderate-to-severe COPD.10 The participants watched a DVD on EOL topics as well as life support measures and their implications.10 After watching the DVD, the researchers conducted interviews with the participants’ about their beliefs and experiences with regards to advance care planning.10 In conducting advance care planning, the participants identified having a relationship with the medical team and appropriate timing for the discussion as important.10
Crucial topics identified by participants included life expectancy, availability of medications to treat symptoms, different treatment options, stages of disease progression, and quality of EOL care.10 Other findings from the study included the participants’ desire to consider their families in the decision-making process.10 Becoming a burden to their families due to their need for physical and financial assistance and the inability to establish clear health care directives were identified as sources of concern.10 Many of the participants also shared a preference to die rather than to give up quality of life or mental capacity.10 Nguyen and colleagues also found that the severity of illness was not a good predictor of the participants’ readiness to engage in advance care planning.10
In Australia, a study conducted among bereaved and current caregivers for patients with severe COPD showed that > 20% of patients who had died of COPD required hands-on care by their caregivers.11 The caregivers also reported similar concerns as those patients with COPD, which included uncertainty about the future, fear of exacerbations, social isolation, and deteriorating health.11 They also reported competing emotions of loyalty, resentment, guilt, and exhaustion.11 Caregivers identified areas they felt could have improved their ability to provide care, such as availability of adaptive equipment, contingency plans for emergency situations, education on the illness, its symptoms and prognosis, and advance care planning information.11 The caregivers believed that receiving this information might have lessened their stress and plan for the future.11 Although most of the aspects of care that they identified as important are components of palliative care, most of the caregivers were unfamiliar with the term palliative care.11
QI Initiative
To improve palliative care education and use in the ICUs of the VA hospitals, the VHA conducted training, which was made available to intensive care providers on improving EOL care and communication. An attending physician in the ICU who also is a pulmonologist took part in this training in July 2013. To evaluate the outcome of this educational effort, the authors’ reviewed the palliative care referrals from 2012 to 2014.
Results
There were a total of 29 patients with COPD who were referred for palliative care services. Sixteen (55%) were referred by pulmonology. Medical oncology and primary care each referred 4 patients (14%). Acute care referred 5 patients (17%). Emergency department (ED) visits were compared 1 year prior with postpalliative care involvement in the patients’ care (Figure 1). The average ED visit for these patients prepalliative care was 3.2 days, and this dropped to 1.7 days postpalliative care involvement. Of the 29 patients, there were 7 who were never seen in the ED for symptoms of COPD prior to palliative care involvement in their care, and 17 who did not have ED visits after palliative care’s involvement in their care. Of the 29 patients, 3 had frequent visits to the ED (more than 10 days total) prepalliative care, and only 1 had frequent visits to the ED following involvement in the palliative care clinic.
According to the JDDVAMC Managerial Cost Accounting Office (MCAO), the average cost to care for a patient who is presenting to the ED with symptoms related to COPD is $527. The cost of caring for the 22 patients who were seen at least once in the ED for symptoms related to their COPD would be $11,594. With palliative care involvement, only 12 of the 29 patients were seen in the ED for symptoms related to COPD for a total of $6,324, a savings of $5,270 for single ED visits for this set of patients.
Prior to palliative care involvement for the 29 patients, there were 27 admissions, which dropped to 15 admissions after palliative care involvement. According to the MCAO, the average cost to care for a patient who is admitted to the hospital due to an exacerbation of COPD is $20,944. With 27 admissions prior to palliative care involvement, the results total $565,488 compared with $314,160 for 15 admissions with palliative care involvement, showing a cost savings of $251,328.
Fourteen of the 29 patients had advance directive discussions, 9 of which were completed by assigning a durable power of attorney and/or completing a living will. There were 22 (76%) of the 29 patients who had code status discussions, and 18 (62%) elected not to be resuscitated (Figure 2). According to MCAO, the average cost to care for a patient in the ICU who required ventilator support for at least 96 hours is $102,175. For the 18 patients who decided not to pursue cardiopulmonary resuscitation, this results in a potential cost savings of $1,839,150.
Conclusion
The outcome of this QI initiative is congruent with the findings published in the literature on the benefits of palliative care involvement in the care of patients with COPD. Palliative care involvement improved goals of care discussions and resulted in decreased ED visits. Palliative care educational outreach also seems to improve palliative care referrals.
In 2007, the American Thoracic Society issued a policy statement recommending that palliative care should be available at any stage during the course of a progressive or chronic respiratory disease or critical illness when the patient becomes symptomatic.12 Compared with patients with lung cancer, patients with COPD have to cope with symptom burden for a longer period. Breathlessness seems to be the most debilitating physical symptom for COPD and should trigger a palliative care referral.7 Comprehensive respiratory care similar to that for cancer care should be considered for severe COPD and should involve both the palliative care team and the pulmonary care teams for optimal results.
The results of this QI initiative also seem to support the potential benefits of palliative care involvement in the care of patients with other chronic illnesses that are expected to progress over time, leading to a shortened life expectancy.
1. Saini T, Murtagh FE, Dupont PJ, McKinnon PM, Hatfield P, Saunders Y. Comparative pilot study of symptoms and quality of life in cancer patients and patients with end stage renal disease. Palliat Med. 2006;20(6):631-636.
2. Lorenz KA, Lynn J, Dy SM, et al. Evidence for improving palliative care at end of life: a systematic review. Ann Intern Med. 2008;148(2):147-159.
3. Woo J, Lo R, Cheng JO, Wong F, Mak B. Quality of end-of-life care for non-cancer patients in a non-acute hospital. J Clin Nurs. 2011;20(13-14):1834-1841.
4. Hoyert DL, Xu JQ. Deaths: Preliminary Data for 2011. National Vital Statistics Reports. Vol 61. No 6. Hyattsville, MD: National Center for Health Statistics. 2012. Centers for Disease Control and Prevention website. http://www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_06.pdf. Published October 10, 2012. Accessed July 1, 2016.
5. Barnett M. End of life issues in the management of COPD. J Comm Nurs. 2012; 26(3):4-8.
6. Fitzsimons D, Mullan D, Wilson JS, et al. The challenge of patients’ unmet palliative care needs in the final stages of chronic illness. Palliat Med. 2007;21(4):313-322.
7. Bausewein C, Booth S, Gysels M, Kühnbach R, Haberland B, Higginson IJ. Understanding breathlessness: cross-sectional comparison of symptom burden and palliative care needs in chronic obstructive pulmonary disease and cancer. J Palliat Med. 2010;13(9):1109-1118.
8. Schroedl C, Yount S, Szmullowicz E, Rosenberg SR, Kalhan R. Outpatient palliative care for chronic obstructive pulmonary disease: a case series. J Palliat Med. 2014;17(11):1256-1261.
9. Iley K. Improving palliative care for patients with COPD. Nurs Stand. 2012;26(37):40-46.
10. Nguyen M, Chamber-Evans J, Joubert A, Drouin I, Ouellet I. Exploring the advance care planning needs of moderately to severely ill people with COPD. Int J Palliat Nurs. 2013;19(8):389-395.
11. Philip J, Gold M, Brand C, Miller B, Douglass J, Sundararajan V. Facilitating change and adaptation: the experiences of current and bereaved carers of patients with severe chronic obstructive pulmonary disease. J Palliat Med. 2014;17(4):421-427.
12. Lanken PN, Terry PB, DeLisser HM, et al; American Thoracic Society End-of-Life Care Task Force. An official American Thoracic Society clinical policy statement: palliative care for patients with respiratory diseases and critical illness. Am J Respir Crit Care Med. 2008;177(8):912-927.
The integration of palliative care in cancer care is an emerging trend driven by data on the benefits of palliative care intervention in the care of patients with terminal malignancies. Although studies have shown that patients with end-stage organ disease tend to develop similar symptoms and issues as those of cancer patients, the use of palliative care services among patients with end-stage organ disease seems to be limited.1 The clinical course of terminal malignancy is usually marked by a consistent decline, whereas organ failure is usually marked by periods of exacerbations in relation to decompensation.2 Patients with organ failure often exhibit a gradual and subtle decline over time, making it more challenging to predict the disease course.2
Woo and colleagues studied patients with chronic illnesses and showed that, similar to patients diagnosed with cancer, symptoms of fatigue, pain, and dyspnea were common.3 They also found that caregivers of patients with chronic illness reported suboptimal physical and emotional well-being as well as moderate levels of stress.3 These findings suggest that caregivers for cancer and noncancer patients will benefit from the support inherent in an interdisciplinary approach to palliative care.3 According to the CDC, the second leading cause of death in the U.S. in 2011 was cancer followed by chronic respiratory disease.4
The authors conducted a quality improvement (QI) initiative to explore the benefits of integrating palliative care in the care of patients with chronic obstructive pulmonary disease (COPD) and share outcomes of improved palliative care education at John D. Dingell VAMC (JDDVAMC) in Detroit, Michigan, for care of patients with COPD.
Background
Chronic obstructive pulmonary disease is a progressive, incurable lung disease.5 It also has been referred to as chronic bronchitis, emphysema, or chronic asthma.5 The degree of severity of COPD is determined by measuring the degree of air flow obstruction by conducting a spirometry test.5 Common symptoms associated with COPD include dyspnea, cough, wheezing, recurring respiratory infections, and generalized weakness.5
Compared with terminally ill patients with lung cancer, patients with COPD were found to have a poorer quality of life as well as more anxiety and depression.6 In a study to evaluate for breathlessness among patients with severe COPD and advanced cancer, Bausewein and colleagues found that both groups reported moderately distressing physical symptoms.7 Both groups also reported shortness of breath as their most distressing physical symptom and worrying as the most common psychological symptom.7 The study also identified a 50% commonality among the participants on palliative care needs.7
The common palliative care needs that were identified were the need for symptom management for breathlessness, access to information, ability to share feelings, a sense of wasted time, and assistance with practical matters.7 During the study’s 6-month data collection period, 61% of the patients with cancer and 10% of the patients with COPD died.7 Median survival for both groups showed that the patients with COPD had a significantly longer median survival of 589 days compared with 107 days for the patients with cancer.7
A retrospective review of patient records from 2010 to 2013 showed that providers referred only 5% of patients with COPD for palliative care.8 In the United Kingdom, the 5-year survival rate among patients diagnosed with severe COPD is 24% to 30%.9 Chronic obstructive pulmonary disease is one of the most common causes of hospital admissions, and treatments are aimed toward palliation of symptoms.9 As COPD reaches its end stage, incorporation of end-of-life (EOL) care should be considered. Signs that may indicate EOL care is needed include long-term oxygen therapy, depression, hospitalization for exacerbations at a rate of 2 or more a year, evidence of right-sided heart failure, cortisone treatment for > 6 weeks, and a history of noninvasive ventilation or admission to the intensive care unit (ICU).9
Nguyen and colleagues conducted a study in Montreal, Canada, among patients with moderate-to-severe COPD.10 The participants watched a DVD on EOL topics as well as life support measures and their implications.10 After watching the DVD, the researchers conducted interviews with the participants’ about their beliefs and experiences with regards to advance care planning.10 In conducting advance care planning, the participants identified having a relationship with the medical team and appropriate timing for the discussion as important.10
Crucial topics identified by participants included life expectancy, availability of medications to treat symptoms, different treatment options, stages of disease progression, and quality of EOL care.10 Other findings from the study included the participants’ desire to consider their families in the decision-making process.10 Becoming a burden to their families due to their need for physical and financial assistance and the inability to establish clear health care directives were identified as sources of concern.10 Many of the participants also shared a preference to die rather than to give up quality of life or mental capacity.10 Nguyen and colleagues also found that the severity of illness was not a good predictor of the participants’ readiness to engage in advance care planning.10
In Australia, a study conducted among bereaved and current caregivers for patients with severe COPD showed that > 20% of patients who had died of COPD required hands-on care by their caregivers.11 The caregivers also reported similar concerns as those patients with COPD, which included uncertainty about the future, fear of exacerbations, social isolation, and deteriorating health.11 They also reported competing emotions of loyalty, resentment, guilt, and exhaustion.11 Caregivers identified areas they felt could have improved their ability to provide care, such as availability of adaptive equipment, contingency plans for emergency situations, education on the illness, its symptoms and prognosis, and advance care planning information.11 The caregivers believed that receiving this information might have lessened their stress and plan for the future.11 Although most of the aspects of care that they identified as important are components of palliative care, most of the caregivers were unfamiliar with the term palliative care.11
QI Initiative
To improve palliative care education and use in the ICUs of the VA hospitals, the VHA conducted training, which was made available to intensive care providers on improving EOL care and communication. An attending physician in the ICU who also is a pulmonologist took part in this training in July 2013. To evaluate the outcome of this educational effort, the authors’ reviewed the palliative care referrals from 2012 to 2014.
Results
There were a total of 29 patients with COPD who were referred for palliative care services. Sixteen (55%) were referred by pulmonology. Medical oncology and primary care each referred 4 patients (14%). Acute care referred 5 patients (17%). Emergency department (ED) visits were compared 1 year prior with postpalliative care involvement in the patients’ care (Figure 1). The average ED visit for these patients prepalliative care was 3.2 days, and this dropped to 1.7 days postpalliative care involvement. Of the 29 patients, there were 7 who were never seen in the ED for symptoms of COPD prior to palliative care involvement in their care, and 17 who did not have ED visits after palliative care’s involvement in their care. Of the 29 patients, 3 had frequent visits to the ED (more than 10 days total) prepalliative care, and only 1 had frequent visits to the ED following involvement in the palliative care clinic.
According to the JDDVAMC Managerial Cost Accounting Office (MCAO), the average cost to care for a patient who is presenting to the ED with symptoms related to COPD is $527. The cost of caring for the 22 patients who were seen at least once in the ED for symptoms related to their COPD would be $11,594. With palliative care involvement, only 12 of the 29 patients were seen in the ED for symptoms related to COPD for a total of $6,324, a savings of $5,270 for single ED visits for this set of patients.
Prior to palliative care involvement for the 29 patients, there were 27 admissions, which dropped to 15 admissions after palliative care involvement. According to the MCAO, the average cost to care for a patient who is admitted to the hospital due to an exacerbation of COPD is $20,944. With 27 admissions prior to palliative care involvement, the results total $565,488 compared with $314,160 for 15 admissions with palliative care involvement, showing a cost savings of $251,328.
Fourteen of the 29 patients had advance directive discussions, 9 of which were completed by assigning a durable power of attorney and/or completing a living will. There were 22 (76%) of the 29 patients who had code status discussions, and 18 (62%) elected not to be resuscitated (Figure 2). According to MCAO, the average cost to care for a patient in the ICU who required ventilator support for at least 96 hours is $102,175. For the 18 patients who decided not to pursue cardiopulmonary resuscitation, this results in a potential cost savings of $1,839,150.
Conclusion
The outcome of this QI initiative is congruent with the findings published in the literature on the benefits of palliative care involvement in the care of patients with COPD. Palliative care involvement improved goals of care discussions and resulted in decreased ED visits. Palliative care educational outreach also seems to improve palliative care referrals.
In 2007, the American Thoracic Society issued a policy statement recommending that palliative care should be available at any stage during the course of a progressive or chronic respiratory disease or critical illness when the patient becomes symptomatic.12 Compared with patients with lung cancer, patients with COPD have to cope with symptom burden for a longer period. Breathlessness seems to be the most debilitating physical symptom for COPD and should trigger a palliative care referral.7 Comprehensive respiratory care similar to that for cancer care should be considered for severe COPD and should involve both the palliative care team and the pulmonary care teams for optimal results.
The results of this QI initiative also seem to support the potential benefits of palliative care involvement in the care of patients with other chronic illnesses that are expected to progress over time, leading to a shortened life expectancy.
The integration of palliative care in cancer care is an emerging trend driven by data on the benefits of palliative care intervention in the care of patients with terminal malignancies. Although studies have shown that patients with end-stage organ disease tend to develop similar symptoms and issues as those of cancer patients, the use of palliative care services among patients with end-stage organ disease seems to be limited.1 The clinical course of terminal malignancy is usually marked by a consistent decline, whereas organ failure is usually marked by periods of exacerbations in relation to decompensation.2 Patients with organ failure often exhibit a gradual and subtle decline over time, making it more challenging to predict the disease course.2
Woo and colleagues studied patients with chronic illnesses and showed that, similar to patients diagnosed with cancer, symptoms of fatigue, pain, and dyspnea were common.3 They also found that caregivers of patients with chronic illness reported suboptimal physical and emotional well-being as well as moderate levels of stress.3 These findings suggest that caregivers for cancer and noncancer patients will benefit from the support inherent in an interdisciplinary approach to palliative care.3 According to the CDC, the second leading cause of death in the U.S. in 2011 was cancer followed by chronic respiratory disease.4
The authors conducted a quality improvement (QI) initiative to explore the benefits of integrating palliative care in the care of patients with chronic obstructive pulmonary disease (COPD) and share outcomes of improved palliative care education at John D. Dingell VAMC (JDDVAMC) in Detroit, Michigan, for care of patients with COPD.
Background
Chronic obstructive pulmonary disease is a progressive, incurable lung disease.5 It also has been referred to as chronic bronchitis, emphysema, or chronic asthma.5 The degree of severity of COPD is determined by measuring the degree of air flow obstruction by conducting a spirometry test.5 Common symptoms associated with COPD include dyspnea, cough, wheezing, recurring respiratory infections, and generalized weakness.5
Compared with terminally ill patients with lung cancer, patients with COPD were found to have a poorer quality of life as well as more anxiety and depression.6 In a study to evaluate for breathlessness among patients with severe COPD and advanced cancer, Bausewein and colleagues found that both groups reported moderately distressing physical symptoms.7 Both groups also reported shortness of breath as their most distressing physical symptom and worrying as the most common psychological symptom.7 The study also identified a 50% commonality among the participants on palliative care needs.7
The common palliative care needs that were identified were the need for symptom management for breathlessness, access to information, ability to share feelings, a sense of wasted time, and assistance with practical matters.7 During the study’s 6-month data collection period, 61% of the patients with cancer and 10% of the patients with COPD died.7 Median survival for both groups showed that the patients with COPD had a significantly longer median survival of 589 days compared with 107 days for the patients with cancer.7
A retrospective review of patient records from 2010 to 2013 showed that providers referred only 5% of patients with COPD for palliative care.8 In the United Kingdom, the 5-year survival rate among patients diagnosed with severe COPD is 24% to 30%.9 Chronic obstructive pulmonary disease is one of the most common causes of hospital admissions, and treatments are aimed toward palliation of symptoms.9 As COPD reaches its end stage, incorporation of end-of-life (EOL) care should be considered. Signs that may indicate EOL care is needed include long-term oxygen therapy, depression, hospitalization for exacerbations at a rate of 2 or more a year, evidence of right-sided heart failure, cortisone treatment for > 6 weeks, and a history of noninvasive ventilation or admission to the intensive care unit (ICU).9
Nguyen and colleagues conducted a study in Montreal, Canada, among patients with moderate-to-severe COPD.10 The participants watched a DVD on EOL topics as well as life support measures and their implications.10 After watching the DVD, the researchers conducted interviews with the participants’ about their beliefs and experiences with regards to advance care planning.10 In conducting advance care planning, the participants identified having a relationship with the medical team and appropriate timing for the discussion as important.10
Crucial topics identified by participants included life expectancy, availability of medications to treat symptoms, different treatment options, stages of disease progression, and quality of EOL care.10 Other findings from the study included the participants’ desire to consider their families in the decision-making process.10 Becoming a burden to their families due to their need for physical and financial assistance and the inability to establish clear health care directives were identified as sources of concern.10 Many of the participants also shared a preference to die rather than to give up quality of life or mental capacity.10 Nguyen and colleagues also found that the severity of illness was not a good predictor of the participants’ readiness to engage in advance care planning.10
In Australia, a study conducted among bereaved and current caregivers for patients with severe COPD showed that > 20% of patients who had died of COPD required hands-on care by their caregivers.11 The caregivers also reported similar concerns as those patients with COPD, which included uncertainty about the future, fear of exacerbations, social isolation, and deteriorating health.11 They also reported competing emotions of loyalty, resentment, guilt, and exhaustion.11 Caregivers identified areas they felt could have improved their ability to provide care, such as availability of adaptive equipment, contingency plans for emergency situations, education on the illness, its symptoms and prognosis, and advance care planning information.11 The caregivers believed that receiving this information might have lessened their stress and plan for the future.11 Although most of the aspects of care that they identified as important are components of palliative care, most of the caregivers were unfamiliar with the term palliative care.11
QI Initiative
To improve palliative care education and use in the ICUs of the VA hospitals, the VHA conducted training, which was made available to intensive care providers on improving EOL care and communication. An attending physician in the ICU who also is a pulmonologist took part in this training in July 2013. To evaluate the outcome of this educational effort, the authors’ reviewed the palliative care referrals from 2012 to 2014.
Results
There were a total of 29 patients with COPD who were referred for palliative care services. Sixteen (55%) were referred by pulmonology. Medical oncology and primary care each referred 4 patients (14%). Acute care referred 5 patients (17%). Emergency department (ED) visits were compared 1 year prior with postpalliative care involvement in the patients’ care (Figure 1). The average ED visit for these patients prepalliative care was 3.2 days, and this dropped to 1.7 days postpalliative care involvement. Of the 29 patients, there were 7 who were never seen in the ED for symptoms of COPD prior to palliative care involvement in their care, and 17 who did not have ED visits after palliative care’s involvement in their care. Of the 29 patients, 3 had frequent visits to the ED (more than 10 days total) prepalliative care, and only 1 had frequent visits to the ED following involvement in the palliative care clinic.
According to the JDDVAMC Managerial Cost Accounting Office (MCAO), the average cost to care for a patient who is presenting to the ED with symptoms related to COPD is $527. The cost of caring for the 22 patients who were seen at least once in the ED for symptoms related to their COPD would be $11,594. With palliative care involvement, only 12 of the 29 patients were seen in the ED for symptoms related to COPD for a total of $6,324, a savings of $5,270 for single ED visits for this set of patients.
Prior to palliative care involvement for the 29 patients, there were 27 admissions, which dropped to 15 admissions after palliative care involvement. According to the MCAO, the average cost to care for a patient who is admitted to the hospital due to an exacerbation of COPD is $20,944. With 27 admissions prior to palliative care involvement, the results total $565,488 compared with $314,160 for 15 admissions with palliative care involvement, showing a cost savings of $251,328.
Fourteen of the 29 patients had advance directive discussions, 9 of which were completed by assigning a durable power of attorney and/or completing a living will. There were 22 (76%) of the 29 patients who had code status discussions, and 18 (62%) elected not to be resuscitated (Figure 2). According to MCAO, the average cost to care for a patient in the ICU who required ventilator support for at least 96 hours is $102,175. For the 18 patients who decided not to pursue cardiopulmonary resuscitation, this results in a potential cost savings of $1,839,150.
Conclusion
The outcome of this QI initiative is congruent with the findings published in the literature on the benefits of palliative care involvement in the care of patients with COPD. Palliative care involvement improved goals of care discussions and resulted in decreased ED visits. Palliative care educational outreach also seems to improve palliative care referrals.
In 2007, the American Thoracic Society issued a policy statement recommending that palliative care should be available at any stage during the course of a progressive or chronic respiratory disease or critical illness when the patient becomes symptomatic.12 Compared with patients with lung cancer, patients with COPD have to cope with symptom burden for a longer period. Breathlessness seems to be the most debilitating physical symptom for COPD and should trigger a palliative care referral.7 Comprehensive respiratory care similar to that for cancer care should be considered for severe COPD and should involve both the palliative care team and the pulmonary care teams for optimal results.
The results of this QI initiative also seem to support the potential benefits of palliative care involvement in the care of patients with other chronic illnesses that are expected to progress over time, leading to a shortened life expectancy.
1. Saini T, Murtagh FE, Dupont PJ, McKinnon PM, Hatfield P, Saunders Y. Comparative pilot study of symptoms and quality of life in cancer patients and patients with end stage renal disease. Palliat Med. 2006;20(6):631-636.
2. Lorenz KA, Lynn J, Dy SM, et al. Evidence for improving palliative care at end of life: a systematic review. Ann Intern Med. 2008;148(2):147-159.
3. Woo J, Lo R, Cheng JO, Wong F, Mak B. Quality of end-of-life care for non-cancer patients in a non-acute hospital. J Clin Nurs. 2011;20(13-14):1834-1841.
4. Hoyert DL, Xu JQ. Deaths: Preliminary Data for 2011. National Vital Statistics Reports. Vol 61. No 6. Hyattsville, MD: National Center for Health Statistics. 2012. Centers for Disease Control and Prevention website. http://www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_06.pdf. Published October 10, 2012. Accessed July 1, 2016.
5. Barnett M. End of life issues in the management of COPD. J Comm Nurs. 2012; 26(3):4-8.
6. Fitzsimons D, Mullan D, Wilson JS, et al. The challenge of patients’ unmet palliative care needs in the final stages of chronic illness. Palliat Med. 2007;21(4):313-322.
7. Bausewein C, Booth S, Gysels M, Kühnbach R, Haberland B, Higginson IJ. Understanding breathlessness: cross-sectional comparison of symptom burden and palliative care needs in chronic obstructive pulmonary disease and cancer. J Palliat Med. 2010;13(9):1109-1118.
8. Schroedl C, Yount S, Szmullowicz E, Rosenberg SR, Kalhan R. Outpatient palliative care for chronic obstructive pulmonary disease: a case series. J Palliat Med. 2014;17(11):1256-1261.
9. Iley K. Improving palliative care for patients with COPD. Nurs Stand. 2012;26(37):40-46.
10. Nguyen M, Chamber-Evans J, Joubert A, Drouin I, Ouellet I. Exploring the advance care planning needs of moderately to severely ill people with COPD. Int J Palliat Nurs. 2013;19(8):389-395.
11. Philip J, Gold M, Brand C, Miller B, Douglass J, Sundararajan V. Facilitating change and adaptation: the experiences of current and bereaved carers of patients with severe chronic obstructive pulmonary disease. J Palliat Med. 2014;17(4):421-427.
12. Lanken PN, Terry PB, DeLisser HM, et al; American Thoracic Society End-of-Life Care Task Force. An official American Thoracic Society clinical policy statement: palliative care for patients with respiratory diseases and critical illness. Am J Respir Crit Care Med. 2008;177(8):912-927.
1. Saini T, Murtagh FE, Dupont PJ, McKinnon PM, Hatfield P, Saunders Y. Comparative pilot study of symptoms and quality of life in cancer patients and patients with end stage renal disease. Palliat Med. 2006;20(6):631-636.
2. Lorenz KA, Lynn J, Dy SM, et al. Evidence for improving palliative care at end of life: a systematic review. Ann Intern Med. 2008;148(2):147-159.
3. Woo J, Lo R, Cheng JO, Wong F, Mak B. Quality of end-of-life care for non-cancer patients in a non-acute hospital. J Clin Nurs. 2011;20(13-14):1834-1841.
4. Hoyert DL, Xu JQ. Deaths: Preliminary Data for 2011. National Vital Statistics Reports. Vol 61. No 6. Hyattsville, MD: National Center for Health Statistics. 2012. Centers for Disease Control and Prevention website. http://www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_06.pdf. Published October 10, 2012. Accessed July 1, 2016.
5. Barnett M. End of life issues in the management of COPD. J Comm Nurs. 2012; 26(3):4-8.
6. Fitzsimons D, Mullan D, Wilson JS, et al. The challenge of patients’ unmet palliative care needs in the final stages of chronic illness. Palliat Med. 2007;21(4):313-322.
7. Bausewein C, Booth S, Gysels M, Kühnbach R, Haberland B, Higginson IJ. Understanding breathlessness: cross-sectional comparison of symptom burden and palliative care needs in chronic obstructive pulmonary disease and cancer. J Palliat Med. 2010;13(9):1109-1118.
8. Schroedl C, Yount S, Szmullowicz E, Rosenberg SR, Kalhan R. Outpatient palliative care for chronic obstructive pulmonary disease: a case series. J Palliat Med. 2014;17(11):1256-1261.
9. Iley K. Improving palliative care for patients with COPD. Nurs Stand. 2012;26(37):40-46.
10. Nguyen M, Chamber-Evans J, Joubert A, Drouin I, Ouellet I. Exploring the advance care planning needs of moderately to severely ill people with COPD. Int J Palliat Nurs. 2013;19(8):389-395.
11. Philip J, Gold M, Brand C, Miller B, Douglass J, Sundararajan V. Facilitating change and adaptation: the experiences of current and bereaved carers of patients with severe chronic obstructive pulmonary disease. J Palliat Med. 2014;17(4):421-427.
12. Lanken PN, Terry PB, DeLisser HM, et al; American Thoracic Society End-of-Life Care Task Force. An official American Thoracic Society clinical policy statement: palliative care for patients with respiratory diseases and critical illness. Am J Respir Crit Care Med. 2008;177(8):912-927.