User login
Sport-related concussion: How best to help young athletes
› Require athletes who sustain a concussion to wait a minimum of 7 to 10 days before returning to full unrestricted activity. C
› Ensure that any player diagnosed with concussion follows a guided return-to-play progression, supervised by an athletic trainer or physical therapist experienced in post-concussion care. C
› Advise patients who are old enough to drive not to do so for at least 24 hours after a concussion. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Each year in the United States, more than 44 million young people participate in sports activities.1 Yet the number of concussions incurred annually by children and adolescents engaged in sports and recreational play has been underestimated for years, and largely unknown.1,2
Some estimates were based solely on the number of young athletes treated in emergency departments or sports concussion clinics. Others focused only on team players of middle school or high school age, excluding younger children who were hit in the head on playgrounds or during other recreational activities. What’s more, large numbers of concussions—as many as 4 in 10 incurred by high school athletes—were never reported to a coach or medical professional.3
In a new study published in the journal Pediatrics in June, researchers used national databases and current literature to provide what they believe to be “the most accurate and precise estimate of youth concussion” thus far: Between 1.1 and 1.9 million sports- and recreation-related concussions occur among US youth ages 18 or younger annually.1
Among young people playing team sports, concussions are between 2 and 7 times more likely to occur during competitive games than in practice sessions.4-7 Boys on football and ice hockey teams have the highest rates of concussion in young athletes.For overall number of concussions, however, girls on soccer teams are second only to football players.4 Female soccer players are more likely than male soccer players to sustain concussions during equal number of hours of play.4,7
An increase in incidence. The incidence of concussion among young athletes appears to have increased in the past decade, a likely result of greater involvement in team sports, an increasing focus on safeguarding young people from the potential dangers associated with a blow to the brain, and better diagnostic techniques.4,8-10 And a recent study based on data from electronic medical records at a large regional pediatric health care network found that more than three-quarters of young people with sports-related concussions were first seen in a primary care setting.2
With this in mind, we present a comprehensive update of the evidence regarding the diagnosis and management of sport-related concussion. The recommendations we include are consistent with professional association guidelines.8-10 Although we focus on concussion in children and adolescents involved in athletic activities, the principles generally apply to patients of all ages and to concussions that may not be sports related.
Removal from play: A vital first step
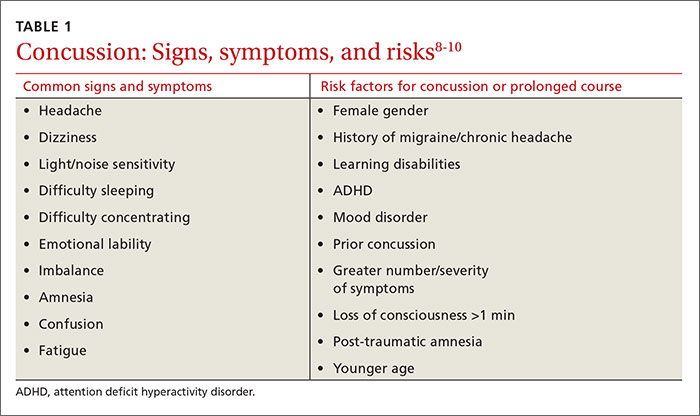
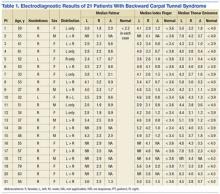
Whenever you conduct a physical exam for a young athlete, remind him or her—and the patient’s parents—that after a blow to the head, immediate removal from play is critical. Concussion is caused by a direct or indirect force to the brain that results in a transient disturbance in brain function,8-10 manifested by alterations in neurocognitive and motor function. While the signs and symptoms (TABLE 1)8-10 resolve within 10 days of injury in about 90% of cases, those who incur additional head impact within 24 hours have a higher symptom burden and prolonged recovery period.11 Even without repetitive impact, younger athletes may take longer to recover.8-10
The initial assessment
A child or adolescent who sustains a suspected concussion should be seen by a physician within 24 to 48 hours. Whether the initial assessment occurs in your office or on the sidelines of a game, it is important to confirm the time the incident occurred and the mechanism of injury.
Concussion is diagnosed by a combination of history, physical exam, and objective testing when symptoms or exam findings associated with mild brain trauma—headache, dizziness, light and/or noise sensitivity, among others—closely follow a head injury.8-10 Certain maneuvers—assessing eye movements by asking the athlete to look in various directions, for instance, then to follow a pen or finger as you move it closer to his or her face—may provoke dizziness, headache, or other symptoms of concussion that were not apparent initially.
The differential diagnosis includes cervical musculoskeletal injury, craniofacial injury, epidural and subdural hematoma, heat-related illness, uncomplicated headache and migraine, upper respiratory infection, and vertigo.8-10
Tools aid in diagnosis
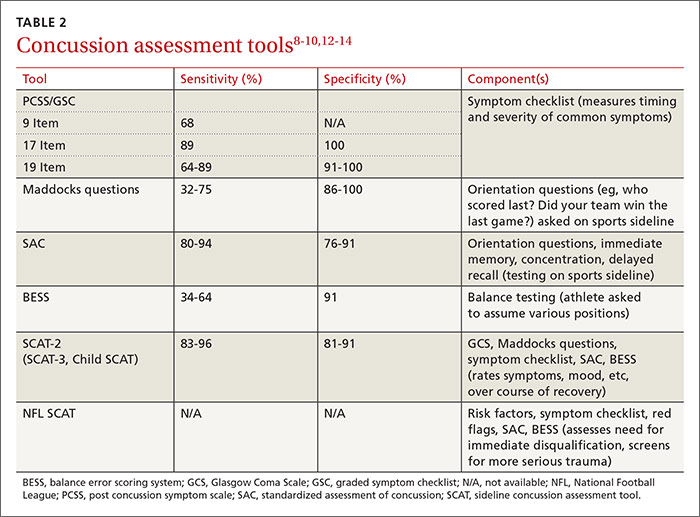
Many clinical assessment tools exist to aid in the diagnosis of concussion (TABLE 2).8-10,12-14 Any one of these tools, many of which use combinations of symptom checklists, balance exams, and cognitive assessments, may be included in your evaluation. No single tool has been found to be superior to any other.8-10 A combination of tools may improve diagnostic accuracy, but assessment tools should not be the sole basis used to diagnose or rule out concussion.
Any child or adolescent who had a blow to the head and at least one sign or symptom of concussion should be evaluated as soon as possible and assessed again later that day or the next day if any reason for concern remains.
Neuropsychological (NP) testing may involve computerized tests developed specifically for athletes. Patients may be required to react to objects that appear on a screen, for example, in a way that tests memory, performance, and reaction time. Because cognitive recovery often lags behind symptom resolution, NP testing may identify subtle brain deficits even in athletes who are asymptomatic at rest or with exercise. In general, NP testing has a sensitivity of 71% to 88% for athletes with concussion,10 but it is most beneficial when baseline test results are available. Interpretation of NP testing should be done only by qualified clinicians.
While NP testing may provide additional prognostic information, it should not alter the management of athletes who are symptomatic either at rest or with exercise.15 Nor is NP testing vital, as concussion can be accurately diagnosed and adequately managed without it.
Neuroimaging, including computed tomography (CT) and magnetic resonance imaging (MRI), is often used unnecessarily in the initial assessment of a patient who sustained a possible concussion.8-10 In fact, neuroimaging should be reserved for cases in which it is necessary to rule out more serious pathology: intracranial or subdural hematoma or a craniofacial injury, for example, in patients with clinical findings that are red flags. These red flags include focal neurologic deficits, continuing nausea/vomiting, or persistent disorientation (TABLE 3),8-10 or symptoms that worsen or persist beyond a few weeks. In such cases, further evaluation—with MRI of the brain, formal NP testing, and/or referral to a neurologist, physiatrist, or other physician who specializes in concussion care—is indicated.
Concussion management: Rest is key
While there is a dearth of high-quality studies on the management of sport-related concussion across all age groups, standardized protocols for both children and adults have been adopted in most clinical settings.8-10,16,17 The protocols provide a framework for an individualized treatment plan. Yet their use among primary care physicians is inconsistent.18-20
Traditionally, concussion management begins with relative physical and cognitive rest to allow the brain time to recover.8-10 Recent randomized controlled trials have challenged this premise by suggesting that mild to moderate physical activity for post-concussion patients who are mildly symptomatic does not adversely affect recovery.21,22 These studies have significant limitations, however, and further research is needed to provide specific guidance on this aspect of concussion management before it is adopted.
Physical restrictions include organized sports, recreational activity, recess, and physical education classes. Walking is permitted unless it exacerbates symptoms. These restrictions should continue until the patient is symptom-free.
Cognitive restrictions include modifications at school and at home. Once an athlete is able to concentrate and tolerate visual and auditory stimuli, he or she may return to school. But classroom modifications should be considered, possibly including shortened school days, extra time for testing and homework, help with note taking, and restrictions from classes likely to provoke symptoms, such as computer science or music. Limiting use of mobile devices, television viewing, noisy environments, and other possible provocations may help speed symptom resolution. These restrictions, too, should remain in place until the patient is symptom-free.
Driving is often not addressed by physicians managing the care of athletes with concussion, but evidence suggests it should be. A study of patients presenting to the emergency department found that within 24 hours of a concussion diagnosis, individuals had an impaired response to traffic hazards.23,24 And Canadian clinical practice guidelines recommend that athletes with mild traumatic brain injury (TBI) avoid driving within the first 24 hours.25
While American guidelines are silent on the question of driving for this patient population, we recommend that athletes with concussion be restricted from driving and engaging in other risky complex tasks, such as welding or shop class, for at least 24 hours. For many athletes diagnosed with concussion, driving restrictions of longer duration may be necessary based on their symptom profile and neurocognitive test results. Continued dizziness or visual deficits would pose a greater risk than fatigue or short-term memory loss, for example.
Overseeing the return to play
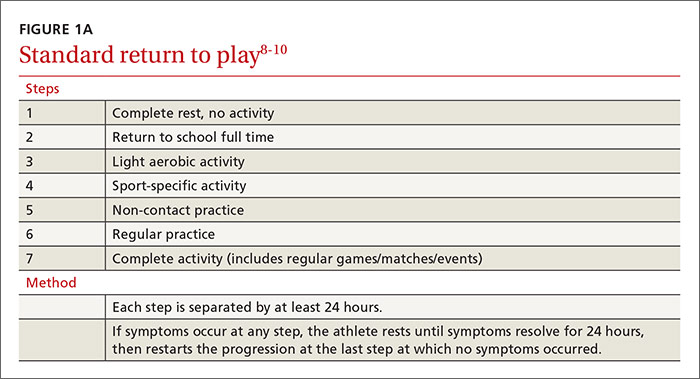
Return-to-activity progression follows a stepwise protocol, with 6 steps that the injured athlete must complete before resuming full activity (FIGURE 1A).8-10 This stepwise progression begins only when athletes are symptom free, even during provocative maneuvers; have had a normal neurologic exam, are back to school full time with no restriction; are off any medications prescribed for concussion symptoms (TABLE 4),8-10 and when neurocognitive testing, if performed, is back to baseline. If an athlete develops symptoms at any stage of the progression, rest is required until he or she remains asymptomatic for at least 24 hours. The progression is then restarted at the last stage at which the patient was symptom free.
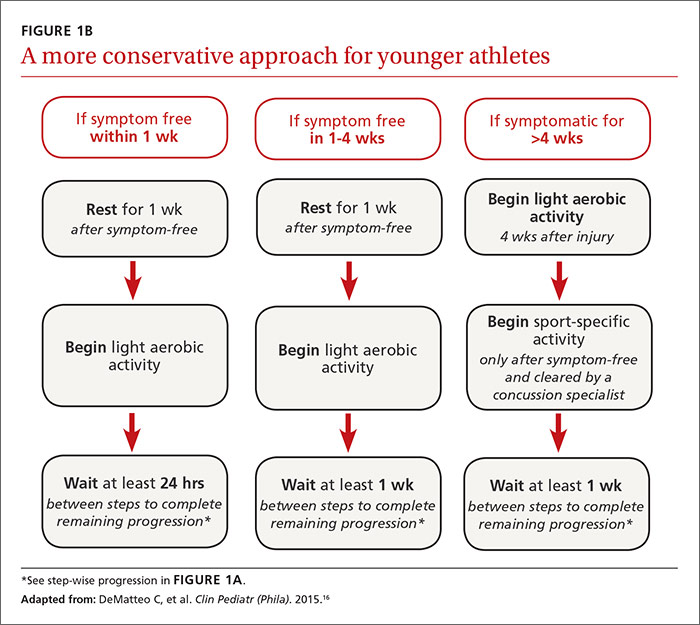
Some individualization, of course, is recommended here, too. Younger athletes and those with a prior history of concussion may require 10 days or more to complete all the steps, allowing an extra day at various steps. Neurologic maturation affects recovery time, and for younger individuals, a more conservative return-to-play protocol based on initial concussion symptom duration has been proposed (FIGURE 1B).16
Return to activity is often supervised by a certified athletic trainer at the athlete’s school. In the event that no athletic trainer is available, patients may be referred to physical therapists with experience in monitoring injured athletes.26 Anyone involved in the patient’s care, including the athlete himself, may use a symptom checklist to monitor recovery.
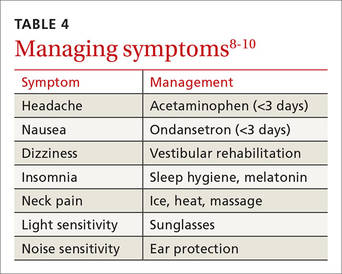
Although there is no evidence that the ongoing use of a symptom checklist affects the course of recovery, its use is often helpful in identifying specific symptoms that can be managed by means other than physical and cognitive rest—a sleep hygiene program for an individual with lingering difficulty sleeping, for example, or the continued application of ice, heat, and massage for persistent neck pain.
Checklist monitoring may be especially helpful for athletes whose symptoms extend beyond 10 days or who have multiple symptoms. Final clearance once all the steps have been completed requires follow-up with a health care provider.
Is a symptom-free waiting period necessary?
There is no evidence suggesting a need for a symptom-free waiting period before starting the return-to-play protocol.10,27 Because a repeat concussion is most likely within 7 to 10 days of the initial injury,8,9 however, most athletes should not return to contact play during that time frame, regardless of symptom resolution.
It is helpful to have asymptomatic athletes participate in non-contact activity before the 7 to 10 days are up, however. Doing so can help prevent deconditioning and injury upon return to contact sport, as there is evidence of increased risk of lower-extremity injury in the 90 days after concussion.28
What to tell athletes—and parents—about repetitive head trauma
There is growing concern about the long-term risks of concussion and repetitive head impact that may manifest as chronic traumatic encephalopathy (CTE) and chronic neurocognitive impairment (CNI) later in life. Indeed, some data strongly suggest—but do not definitively prove—a relationship between repetitive head injury and chronic neurodegenerative disease.8-10 You can tell worried patients or parents, however, that the majority of research on CTE and CNI has been based on professional football players.
Studies of long-term effects of soccer heading have shown conflicting results, with some finding cognitive impairment, altered postural control, and anatomic changes of the brain, while others found no effect on encephalopathy, concussion symptoms, or neurocognitive performance.29-36Here, too, most studies showing negative effects of soccer heading involved professional athletes.
Repetitive sub-concussive impact in high school football athletes has been found to induce biochemical changes to the brain,37 but the long-term effects are unknown. And, while concussion in high school athletes has been associated with short-term cognitive impairment, altered neurochemistry, and evidence of increased symptoms on baseline neurocognitive testing,8-10,38 no studies have linked concussion during middle school or high school with CNI. What’s more, a long-term (50-year) follow-up study of individuals who played football in high school found no difference in rates of neurodegenerative disease compared with age-matched controls.39
A new study of high school and college football players (mean age: 17.4 years) presented at the American Academy of Neurology 2016 Sports Concussion Conference in Chicago in July, however, found significant alterations in white matter 6 months post injury.40 The researchers compared 17 athletes with sport-related concussion with matched controls, using diffusion tensor imaging and diffusion kurtosis tensor imaging as biomarkers of brain recovery. The concussed athletes underwent MRI and symptom assessment at 24 hours, 8 days, and 6 months. The controls followed identical protocols.
At the 6-month assessment, there were no differences between the concussed group and the controls in terms of self-reported concussion symptoms, cognition, or balance. However, the concussed athletes had widespread decreased mean diffusivity compared with the controls. Despite the lack of clinical symptoms, the concussed athletes showed significant alterations in white matter “that were related to initial symptom severity ratings,” the authors concluded. These findings have implications both for determination of recovery from concussion and concussion management, they added.40
Although there is no way to eliminate all concussions, limited evidence suggests that improving athletic technique, limiting contact at practices, better enforcement of game rules, and rule changes regarding physical contact may decrease concussion risk.41-43 Many youth sports organizations have developed policies placing restrictions on head impact during practices and games. Studies are ongoing, too, to see if better headgear—or requiring helmets for soccer players—makes a difference.
CORRESPONDENCE
Ryan A. Sprouse, MD, CAQSM, 203 East Fourth Avenue, Ranson, WV 25438; [email protected].
1. Bryan MA, Rowhani-Rahbar A, Comstock RD, et al. Sports- and recreation-related concussions in US youth. Pediatrics. 2016; June 20 [Epub ahead of print].
2. Arbogast KB, Curry AE, Pfeiffer MR, et al. Point of health care entry for youth with concussion within a large pediatric care network. JAMA Pediatr. 2016; May 31 [Epub ahead of print].
3. Mihalik JK, Guskiewicz KM, Valovich McLeod TC, et al. Knowledge, attitude, and concussion-reporting behaviors among high school athletes: a preliminary study. J Ath Tr. 2013;48:645-653.
4. Marar M, McIlvain NM, Fields SK, et al. Epidemiology of concussions among United States high school athletes in 20 sports. Am J Sports Med. 2012;40:747.
5. Kontos AP, Elbin RJ, Fazio-Sumrock VC. Incidence of sports-related concussion among youth football players aged 8-12 years. J Pediatr. 2013;163:717-720.
6. Dompier TP, Kerr ZY, Marshall SW, et al. Incidence of concussion during practice and games in youth, high school, and collegiate American football players. JAMA Pediatr. 2015;169:659-665.
7. Comstock RD, Currie DW, Pierpont LA, et al. An evidence-based discussion of heading the ball and concussions in high school soccer. JAMA Pediatr. 2015;169:830-837.
8. Harmon KG, Drezner JA, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Br J Sports Med. 2013;47:15-26.
9. McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47:250-258.
10. Giza CC, Kutcher JS, Ashwal S, et al. Summary of the evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80:2250-2257.
11. Terwilliger VK, Pratson L, Vaughan CG, et al. Additional post-concussion impact exposure may affect recovery in adolescent athletes. J Neurotrauma. 2016;33:761-765.
12. Putukian M, Echemendia R, Dettwiler-Danspeckgruber A. Prospective clinical assessment using Sideline Concussion Assessment Tool-2 testing in the evaluation of sport-related concussion in college athletes. Clin J Sport Med. 2015;25:36-42.
13. Broglio SP, Macciocchi SN, Ferrara MS. Sensitivity of the concussion assessment battery. Neurosurgery. 2007;60:1050-1057.
14. Randolph C, McCrea M, Barr WB. Is neuropsychological testing useful in the management of sport-related concussion? J Athl Train. 2005;40:139-152.
15. Shrier I. Neuropsychological testing and concussions: a reasoned approach. Clin J Sport Med. 2012;22:211-213.
16. DeMatteo C, Stazyk K, Singh SK, et al. Development of a conservative protocol to return children and youth to activity following concussive injury. Clin Pediatr (Phila). 2015;54:152-163.
17. Broglio SP, Cantu RC, Gioia GA, et al. National Athletic Trainers Association position statement: management of sport concussion. J Athl Train. 2014;49:245-265.
18. Stoller J, Carson JD, Garel A, et al. Do family physicians, emergency department physicians, and pediatricians give consistent sport-related concussion management advice? Can Fam Physician. 2014;60:548, 550-552.
19. Lebrun CM, Mrazik M, Prasad AS, et al. Sport concussion knowledge base, clinical practices and needs for continuing medical education: a survey of family physicians and cross-border comparison. Br J Sports Med. 2013;47:54-59.
20. Zemek R, Eady K, Moreau K, et al. Knowledge of paediatric concussion among front-line primary care providers. Paediatr Child Health. 2014;19:475-480.
21. Maerlender A, Rieman W, Lichtenstein J, et al. Programmed physical exertion in recovery from sports-related concussion: a randomized pilot study. Dev Neuropsychol. 2015;40:273-278.
22. Buckley TA, Munkasy BA, Clouse BP. Acute cognitive and physical rest may not improve concussion recovery time. J Head Trauma Rehabil. 2015; July 24 [Epub ahead of print].
23. Preece MH, Horswill MS, Langlois JA, et al. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375-378.
24. Baker A, Unsworth CA, Lannin NA. Fitness-to-drive after mild traumatic brain injury: mapping the time trajectory of recovery in the acute stages post injury. Accid Anal Prev. 2015;79:50-55.
25. Marshall S, Bayley M, McCullagh S, et al. Clinical practice guidelines for mild traumatic brain injury and persistent symptoms. Can Fam Physician. 2012;58:257-267.
26. Yorke AM, Littleton S, Alsalaheen BA. Concussion attitudes and beliefs, knowledge, and clinical practice: a survey of physical therapists. Phys Ther. Available at: http://dx.doi.org/10.2522/ptj.20140598. Accessed January 21, 2016.
27. McCrea M, Guskiewicz K, Randolph C, et al. Effects of a symptom-free waiting period on clinical outcome and risk of reinjury after sport-related concussion. Neurosurgery. 2009;65:876-883.
28. Brooks MA, Peterson K, Biese K, et al. Concussion increases odds of sustaining a lower extremity musculoskeletal injury after return to play among collegiate athletes. Am J Sports Med. 2016;44:742-747.
29. Witol AD, Webbe FM. Soccer heading frequency predicts neuropsychological deficits. Arch Clin Neuropsychol. 2003;18:397-417.
30. Haran FJ, Tierney R, Wright WG, et al. Acute changes in postural control after soccer heading. Int J Sports Med. 2013;34:350-354.
31. Lipton ML, Kim N, Zimmerman ME, et al. Soccer heading is associated with white matter microstructural and cognitive abnormalities. Radiology. 2013;268:850-857.
32. Jordan SE, Green GA, Galanty HL, et al. Acute and chronic brain injury in United States national team soccer players. Am J Sports Med. 1996;24:205-210.
33. Kontos AP, Dolese A, Elbin RJ, et al. Relationship of soccer heading to computerized neurocognitive performance and symptoms among female and male youth soccer players. Brain Inj. 2011;25:1234-1241.
34. Straume-Naesheim TM, Andersen TE, Dvorak J, et al. Effects of heading exposure and previous concussions on neuropsychological performance among Norwegian elite footballers. Br J Sports Med. 2005;39:70-77.
35. Stephens R, Rutherford A, Potter D, et al. Neuropsychological impairment as a consequence of football (soccer) play and football heading: a preliminary analysis and report on school students (13-16 years). Child Neuropsychol. 2005;11:513-526.
36. Stephens R, Rutherford A, Potter D, et al. Neuropsychological consequence of soccer play in adolescent UK school team soccer players. J Neuropsychiatry Clin Neurosci. 2010;22:295-303.
37. Poole VN, Breedlove EL, Shenk TE, et al. Sub-concussive hit characteristics predict deviant brain metabolism in football athletes. Dev Neuropsychol. 2015;40:12-17.
38. Mannix R, Iverson GL, Maxwell B, et al. Multiple prior concussions are associated with symptoms in high school athletes. Ann Clin Trans Neurol. 2014;1:433-438.
39. Savica R, Parisi JE, Wold LE, et al. High school football and risk of neurodegeneration: a community-based study. Mayo Clin Proc. 2012;87:335-340.
40. Lancaster M, Muftuler T, Olson D, et al. Chronic white matter changes following sport-related concussion measured by diffusion tensor and diffusion kurtosis imaging. Paper presented at: American Academy of Neurology 2016 Sports Concussion Conference; July 8-10, 2016; Chicago, Ill.
41. Kerr ZY, Yeargin SW, Valovich McLeod TC, et al. Comprehensive coach education reduces head impact exposures in American youth football. Orthop J Sports Med. 2015;3(ecollection):e232596711561545.
42. Black AM, Macpherson AK, Hagel BE, et al. Policy change eliminating body checking in non-elite ice hockey leads to a threefold reduction in injury and concussion risk in 11- and 12-year-old players. Br J Sports Med. 2016;50:55-61.
43. Council on Sports Medicine and Fitness. Tackling in youth football. Policy Statement of the American Academy of Pediatrics. Pediatrics. 2015;136:e1419-e1430.
› Require athletes who sustain a concussion to wait a minimum of 7 to 10 days before returning to full unrestricted activity. C
› Ensure that any player diagnosed with concussion follows a guided return-to-play progression, supervised by an athletic trainer or physical therapist experienced in post-concussion care. C
› Advise patients who are old enough to drive not to do so for at least 24 hours after a concussion. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Each year in the United States, more than 44 million young people participate in sports activities.1 Yet the number of concussions incurred annually by children and adolescents engaged in sports and recreational play has been underestimated for years, and largely unknown.1,2
Some estimates were based solely on the number of young athletes treated in emergency departments or sports concussion clinics. Others focused only on team players of middle school or high school age, excluding younger children who were hit in the head on playgrounds or during other recreational activities. What’s more, large numbers of concussions—as many as 4 in 10 incurred by high school athletes—were never reported to a coach or medical professional.3
In a new study published in the journal Pediatrics in June, researchers used national databases and current literature to provide what they believe to be “the most accurate and precise estimate of youth concussion” thus far: Between 1.1 and 1.9 million sports- and recreation-related concussions occur among US youth ages 18 or younger annually.1
Among young people playing team sports, concussions are between 2 and 7 times more likely to occur during competitive games than in practice sessions.4-7 Boys on football and ice hockey teams have the highest rates of concussion in young athletes.For overall number of concussions, however, girls on soccer teams are second only to football players.4 Female soccer players are more likely than male soccer players to sustain concussions during equal number of hours of play.4,7
An increase in incidence. The incidence of concussion among young athletes appears to have increased in the past decade, a likely result of greater involvement in team sports, an increasing focus on safeguarding young people from the potential dangers associated with a blow to the brain, and better diagnostic techniques.4,8-10 And a recent study based on data from electronic medical records at a large regional pediatric health care network found that more than three-quarters of young people with sports-related concussions were first seen in a primary care setting.2
With this in mind, we present a comprehensive update of the evidence regarding the diagnosis and management of sport-related concussion. The recommendations we include are consistent with professional association guidelines.8-10 Although we focus on concussion in children and adolescents involved in athletic activities, the principles generally apply to patients of all ages and to concussions that may not be sports related.
Removal from play: A vital first step
Whenever you conduct a physical exam for a young athlete, remind him or her—and the patient’s parents—that after a blow to the head, immediate removal from play is critical. Concussion is caused by a direct or indirect force to the brain that results in a transient disturbance in brain function,8-10 manifested by alterations in neurocognitive and motor function. While the signs and symptoms (TABLE 1)8-10 resolve within 10 days of injury in about 90% of cases, those who incur additional head impact within 24 hours have a higher symptom burden and prolonged recovery period.11 Even without repetitive impact, younger athletes may take longer to recover.8-10
The initial assessment
A child or adolescent who sustains a suspected concussion should be seen by a physician within 24 to 48 hours. Whether the initial assessment occurs in your office or on the sidelines of a game, it is important to confirm the time the incident occurred and the mechanism of injury.
Concussion is diagnosed by a combination of history, physical exam, and objective testing when symptoms or exam findings associated with mild brain trauma—headache, dizziness, light and/or noise sensitivity, among others—closely follow a head injury.8-10 Certain maneuvers—assessing eye movements by asking the athlete to look in various directions, for instance, then to follow a pen or finger as you move it closer to his or her face—may provoke dizziness, headache, or other symptoms of concussion that were not apparent initially.
The differential diagnosis includes cervical musculoskeletal injury, craniofacial injury, epidural and subdural hematoma, heat-related illness, uncomplicated headache and migraine, upper respiratory infection, and vertigo.8-10
Tools aid in diagnosis
Many clinical assessment tools exist to aid in the diagnosis of concussion (TABLE 2).8-10,12-14 Any one of these tools, many of which use combinations of symptom checklists, balance exams, and cognitive assessments, may be included in your evaluation. No single tool has been found to be superior to any other.8-10 A combination of tools may improve diagnostic accuracy, but assessment tools should not be the sole basis used to diagnose or rule out concussion.
Any child or adolescent who had a blow to the head and at least one sign or symptom of concussion should be evaluated as soon as possible and assessed again later that day or the next day if any reason for concern remains.
Neuropsychological (NP) testing may involve computerized tests developed specifically for athletes. Patients may be required to react to objects that appear on a screen, for example, in a way that tests memory, performance, and reaction time. Because cognitive recovery often lags behind symptom resolution, NP testing may identify subtle brain deficits even in athletes who are asymptomatic at rest or with exercise. In general, NP testing has a sensitivity of 71% to 88% for athletes with concussion,10 but it is most beneficial when baseline test results are available. Interpretation of NP testing should be done only by qualified clinicians.
While NP testing may provide additional prognostic information, it should not alter the management of athletes who are symptomatic either at rest or with exercise.15 Nor is NP testing vital, as concussion can be accurately diagnosed and adequately managed without it.
Neuroimaging, including computed tomography (CT) and magnetic resonance imaging (MRI), is often used unnecessarily in the initial assessment of a patient who sustained a possible concussion.8-10 In fact, neuroimaging should be reserved for cases in which it is necessary to rule out more serious pathology: intracranial or subdural hematoma or a craniofacial injury, for example, in patients with clinical findings that are red flags. These red flags include focal neurologic deficits, continuing nausea/vomiting, or persistent disorientation (TABLE 3),8-10 or symptoms that worsen or persist beyond a few weeks. In such cases, further evaluation—with MRI of the brain, formal NP testing, and/or referral to a neurologist, physiatrist, or other physician who specializes in concussion care—is indicated.
Concussion management: Rest is key
While there is a dearth of high-quality studies on the management of sport-related concussion across all age groups, standardized protocols for both children and adults have been adopted in most clinical settings.8-10,16,17 The protocols provide a framework for an individualized treatment plan. Yet their use among primary care physicians is inconsistent.18-20
Traditionally, concussion management begins with relative physical and cognitive rest to allow the brain time to recover.8-10 Recent randomized controlled trials have challenged this premise by suggesting that mild to moderate physical activity for post-concussion patients who are mildly symptomatic does not adversely affect recovery.21,22 These studies have significant limitations, however, and further research is needed to provide specific guidance on this aspect of concussion management before it is adopted.
Physical restrictions include organized sports, recreational activity, recess, and physical education classes. Walking is permitted unless it exacerbates symptoms. These restrictions should continue until the patient is symptom-free.
Cognitive restrictions include modifications at school and at home. Once an athlete is able to concentrate and tolerate visual and auditory stimuli, he or she may return to school. But classroom modifications should be considered, possibly including shortened school days, extra time for testing and homework, help with note taking, and restrictions from classes likely to provoke symptoms, such as computer science or music. Limiting use of mobile devices, television viewing, noisy environments, and other possible provocations may help speed symptom resolution. These restrictions, too, should remain in place until the patient is symptom-free.
Driving is often not addressed by physicians managing the care of athletes with concussion, but evidence suggests it should be. A study of patients presenting to the emergency department found that within 24 hours of a concussion diagnosis, individuals had an impaired response to traffic hazards.23,24 And Canadian clinical practice guidelines recommend that athletes with mild traumatic brain injury (TBI) avoid driving within the first 24 hours.25
While American guidelines are silent on the question of driving for this patient population, we recommend that athletes with concussion be restricted from driving and engaging in other risky complex tasks, such as welding or shop class, for at least 24 hours. For many athletes diagnosed with concussion, driving restrictions of longer duration may be necessary based on their symptom profile and neurocognitive test results. Continued dizziness or visual deficits would pose a greater risk than fatigue or short-term memory loss, for example.
Overseeing the return to play
Return-to-activity progression follows a stepwise protocol, with 6 steps that the injured athlete must complete before resuming full activity (FIGURE 1A).8-10 This stepwise progression begins only when athletes are symptom free, even during provocative maneuvers; have had a normal neurologic exam, are back to school full time with no restriction; are off any medications prescribed for concussion symptoms (TABLE 4),8-10 and when neurocognitive testing, if performed, is back to baseline. If an athlete develops symptoms at any stage of the progression, rest is required until he or she remains asymptomatic for at least 24 hours. The progression is then restarted at the last stage at which the patient was symptom free.
Some individualization, of course, is recommended here, too. Younger athletes and those with a prior history of concussion may require 10 days or more to complete all the steps, allowing an extra day at various steps. Neurologic maturation affects recovery time, and for younger individuals, a more conservative return-to-play protocol based on initial concussion symptom duration has been proposed (FIGURE 1B).16
Return to activity is often supervised by a certified athletic trainer at the athlete’s school. In the event that no athletic trainer is available, patients may be referred to physical therapists with experience in monitoring injured athletes.26 Anyone involved in the patient’s care, including the athlete himself, may use a symptom checklist to monitor recovery.
Although there is no evidence that the ongoing use of a symptom checklist affects the course of recovery, its use is often helpful in identifying specific symptoms that can be managed by means other than physical and cognitive rest—a sleep hygiene program for an individual with lingering difficulty sleeping, for example, or the continued application of ice, heat, and massage for persistent neck pain.
Checklist monitoring may be especially helpful for athletes whose symptoms extend beyond 10 days or who have multiple symptoms. Final clearance once all the steps have been completed requires follow-up with a health care provider.
Is a symptom-free waiting period necessary?
There is no evidence suggesting a need for a symptom-free waiting period before starting the return-to-play protocol.10,27 Because a repeat concussion is most likely within 7 to 10 days of the initial injury,8,9 however, most athletes should not return to contact play during that time frame, regardless of symptom resolution.
It is helpful to have asymptomatic athletes participate in non-contact activity before the 7 to 10 days are up, however. Doing so can help prevent deconditioning and injury upon return to contact sport, as there is evidence of increased risk of lower-extremity injury in the 90 days after concussion.28
What to tell athletes—and parents—about repetitive head trauma
There is growing concern about the long-term risks of concussion and repetitive head impact that may manifest as chronic traumatic encephalopathy (CTE) and chronic neurocognitive impairment (CNI) later in life. Indeed, some data strongly suggest—but do not definitively prove—a relationship between repetitive head injury and chronic neurodegenerative disease.8-10 You can tell worried patients or parents, however, that the majority of research on CTE and CNI has been based on professional football players.
Studies of long-term effects of soccer heading have shown conflicting results, with some finding cognitive impairment, altered postural control, and anatomic changes of the brain, while others found no effect on encephalopathy, concussion symptoms, or neurocognitive performance.29-36Here, too, most studies showing negative effects of soccer heading involved professional athletes.
Repetitive sub-concussive impact in high school football athletes has been found to induce biochemical changes to the brain,37 but the long-term effects are unknown. And, while concussion in high school athletes has been associated with short-term cognitive impairment, altered neurochemistry, and evidence of increased symptoms on baseline neurocognitive testing,8-10,38 no studies have linked concussion during middle school or high school with CNI. What’s more, a long-term (50-year) follow-up study of individuals who played football in high school found no difference in rates of neurodegenerative disease compared with age-matched controls.39
A new study of high school and college football players (mean age: 17.4 years) presented at the American Academy of Neurology 2016 Sports Concussion Conference in Chicago in July, however, found significant alterations in white matter 6 months post injury.40 The researchers compared 17 athletes with sport-related concussion with matched controls, using diffusion tensor imaging and diffusion kurtosis tensor imaging as biomarkers of brain recovery. The concussed athletes underwent MRI and symptom assessment at 24 hours, 8 days, and 6 months. The controls followed identical protocols.
At the 6-month assessment, there were no differences between the concussed group and the controls in terms of self-reported concussion symptoms, cognition, or balance. However, the concussed athletes had widespread decreased mean diffusivity compared with the controls. Despite the lack of clinical symptoms, the concussed athletes showed significant alterations in white matter “that were related to initial symptom severity ratings,” the authors concluded. These findings have implications both for determination of recovery from concussion and concussion management, they added.40
Although there is no way to eliminate all concussions, limited evidence suggests that improving athletic technique, limiting contact at practices, better enforcement of game rules, and rule changes regarding physical contact may decrease concussion risk.41-43 Many youth sports organizations have developed policies placing restrictions on head impact during practices and games. Studies are ongoing, too, to see if better headgear—or requiring helmets for soccer players—makes a difference.
CORRESPONDENCE
Ryan A. Sprouse, MD, CAQSM, 203 East Fourth Avenue, Ranson, WV 25438; [email protected].
› Require athletes who sustain a concussion to wait a minimum of 7 to 10 days before returning to full unrestricted activity. C
› Ensure that any player diagnosed with concussion follows a guided return-to-play progression, supervised by an athletic trainer or physical therapist experienced in post-concussion care. C
› Advise patients who are old enough to drive not to do so for at least 24 hours after a concussion. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Each year in the United States, more than 44 million young people participate in sports activities.1 Yet the number of concussions incurred annually by children and adolescents engaged in sports and recreational play has been underestimated for years, and largely unknown.1,2
Some estimates were based solely on the number of young athletes treated in emergency departments or sports concussion clinics. Others focused only on team players of middle school or high school age, excluding younger children who were hit in the head on playgrounds or during other recreational activities. What’s more, large numbers of concussions—as many as 4 in 10 incurred by high school athletes—were never reported to a coach or medical professional.3
In a new study published in the journal Pediatrics in June, researchers used national databases and current literature to provide what they believe to be “the most accurate and precise estimate of youth concussion” thus far: Between 1.1 and 1.9 million sports- and recreation-related concussions occur among US youth ages 18 or younger annually.1
Among young people playing team sports, concussions are between 2 and 7 times more likely to occur during competitive games than in practice sessions.4-7 Boys on football and ice hockey teams have the highest rates of concussion in young athletes.For overall number of concussions, however, girls on soccer teams are second only to football players.4 Female soccer players are more likely than male soccer players to sustain concussions during equal number of hours of play.4,7
An increase in incidence. The incidence of concussion among young athletes appears to have increased in the past decade, a likely result of greater involvement in team sports, an increasing focus on safeguarding young people from the potential dangers associated with a blow to the brain, and better diagnostic techniques.4,8-10 And a recent study based on data from electronic medical records at a large regional pediatric health care network found that more than three-quarters of young people with sports-related concussions were first seen in a primary care setting.2
With this in mind, we present a comprehensive update of the evidence regarding the diagnosis and management of sport-related concussion. The recommendations we include are consistent with professional association guidelines.8-10 Although we focus on concussion in children and adolescents involved in athletic activities, the principles generally apply to patients of all ages and to concussions that may not be sports related.
Removal from play: A vital first step
Whenever you conduct a physical exam for a young athlete, remind him or her—and the patient’s parents—that after a blow to the head, immediate removal from play is critical. Concussion is caused by a direct or indirect force to the brain that results in a transient disturbance in brain function,8-10 manifested by alterations in neurocognitive and motor function. While the signs and symptoms (TABLE 1)8-10 resolve within 10 days of injury in about 90% of cases, those who incur additional head impact within 24 hours have a higher symptom burden and prolonged recovery period.11 Even without repetitive impact, younger athletes may take longer to recover.8-10
The initial assessment
A child or adolescent who sustains a suspected concussion should be seen by a physician within 24 to 48 hours. Whether the initial assessment occurs in your office or on the sidelines of a game, it is important to confirm the time the incident occurred and the mechanism of injury.
Concussion is diagnosed by a combination of history, physical exam, and objective testing when symptoms or exam findings associated with mild brain trauma—headache, dizziness, light and/or noise sensitivity, among others—closely follow a head injury.8-10 Certain maneuvers—assessing eye movements by asking the athlete to look in various directions, for instance, then to follow a pen or finger as you move it closer to his or her face—may provoke dizziness, headache, or other symptoms of concussion that were not apparent initially.
The differential diagnosis includes cervical musculoskeletal injury, craniofacial injury, epidural and subdural hematoma, heat-related illness, uncomplicated headache and migraine, upper respiratory infection, and vertigo.8-10
Tools aid in diagnosis
Many clinical assessment tools exist to aid in the diagnosis of concussion (TABLE 2).8-10,12-14 Any one of these tools, many of which use combinations of symptom checklists, balance exams, and cognitive assessments, may be included in your evaluation. No single tool has been found to be superior to any other.8-10 A combination of tools may improve diagnostic accuracy, but assessment tools should not be the sole basis used to diagnose or rule out concussion.
Any child or adolescent who had a blow to the head and at least one sign or symptom of concussion should be evaluated as soon as possible and assessed again later that day or the next day if any reason for concern remains.
Neuropsychological (NP) testing may involve computerized tests developed specifically for athletes. Patients may be required to react to objects that appear on a screen, for example, in a way that tests memory, performance, and reaction time. Because cognitive recovery often lags behind symptom resolution, NP testing may identify subtle brain deficits even in athletes who are asymptomatic at rest or with exercise. In general, NP testing has a sensitivity of 71% to 88% for athletes with concussion,10 but it is most beneficial when baseline test results are available. Interpretation of NP testing should be done only by qualified clinicians.
While NP testing may provide additional prognostic information, it should not alter the management of athletes who are symptomatic either at rest or with exercise.15 Nor is NP testing vital, as concussion can be accurately diagnosed and adequately managed without it.
Neuroimaging, including computed tomography (CT) and magnetic resonance imaging (MRI), is often used unnecessarily in the initial assessment of a patient who sustained a possible concussion.8-10 In fact, neuroimaging should be reserved for cases in which it is necessary to rule out more serious pathology: intracranial or subdural hematoma or a craniofacial injury, for example, in patients with clinical findings that are red flags. These red flags include focal neurologic deficits, continuing nausea/vomiting, or persistent disorientation (TABLE 3),8-10 or symptoms that worsen or persist beyond a few weeks. In such cases, further evaluation—with MRI of the brain, formal NP testing, and/or referral to a neurologist, physiatrist, or other physician who specializes in concussion care—is indicated.
Concussion management: Rest is key
While there is a dearth of high-quality studies on the management of sport-related concussion across all age groups, standardized protocols for both children and adults have been adopted in most clinical settings.8-10,16,17 The protocols provide a framework for an individualized treatment plan. Yet their use among primary care physicians is inconsistent.18-20
Traditionally, concussion management begins with relative physical and cognitive rest to allow the brain time to recover.8-10 Recent randomized controlled trials have challenged this premise by suggesting that mild to moderate physical activity for post-concussion patients who are mildly symptomatic does not adversely affect recovery.21,22 These studies have significant limitations, however, and further research is needed to provide specific guidance on this aspect of concussion management before it is adopted.
Physical restrictions include organized sports, recreational activity, recess, and physical education classes. Walking is permitted unless it exacerbates symptoms. These restrictions should continue until the patient is symptom-free.
Cognitive restrictions include modifications at school and at home. Once an athlete is able to concentrate and tolerate visual and auditory stimuli, he or she may return to school. But classroom modifications should be considered, possibly including shortened school days, extra time for testing and homework, help with note taking, and restrictions from classes likely to provoke symptoms, such as computer science or music. Limiting use of mobile devices, television viewing, noisy environments, and other possible provocations may help speed symptom resolution. These restrictions, too, should remain in place until the patient is symptom-free.
Driving is often not addressed by physicians managing the care of athletes with concussion, but evidence suggests it should be. A study of patients presenting to the emergency department found that within 24 hours of a concussion diagnosis, individuals had an impaired response to traffic hazards.23,24 And Canadian clinical practice guidelines recommend that athletes with mild traumatic brain injury (TBI) avoid driving within the first 24 hours.25
While American guidelines are silent on the question of driving for this patient population, we recommend that athletes with concussion be restricted from driving and engaging in other risky complex tasks, such as welding or shop class, for at least 24 hours. For many athletes diagnosed with concussion, driving restrictions of longer duration may be necessary based on their symptom profile and neurocognitive test results. Continued dizziness or visual deficits would pose a greater risk than fatigue or short-term memory loss, for example.
Overseeing the return to play
Return-to-activity progression follows a stepwise protocol, with 6 steps that the injured athlete must complete before resuming full activity (FIGURE 1A).8-10 This stepwise progression begins only when athletes are symptom free, even during provocative maneuvers; have had a normal neurologic exam, are back to school full time with no restriction; are off any medications prescribed for concussion symptoms (TABLE 4),8-10 and when neurocognitive testing, if performed, is back to baseline. If an athlete develops symptoms at any stage of the progression, rest is required until he or she remains asymptomatic for at least 24 hours. The progression is then restarted at the last stage at which the patient was symptom free.
Some individualization, of course, is recommended here, too. Younger athletes and those with a prior history of concussion may require 10 days or more to complete all the steps, allowing an extra day at various steps. Neurologic maturation affects recovery time, and for younger individuals, a more conservative return-to-play protocol based on initial concussion symptom duration has been proposed (FIGURE 1B).16
Return to activity is often supervised by a certified athletic trainer at the athlete’s school. In the event that no athletic trainer is available, patients may be referred to physical therapists with experience in monitoring injured athletes.26 Anyone involved in the patient’s care, including the athlete himself, may use a symptom checklist to monitor recovery.
Although there is no evidence that the ongoing use of a symptom checklist affects the course of recovery, its use is often helpful in identifying specific symptoms that can be managed by means other than physical and cognitive rest—a sleep hygiene program for an individual with lingering difficulty sleeping, for example, or the continued application of ice, heat, and massage for persistent neck pain.
Checklist monitoring may be especially helpful for athletes whose symptoms extend beyond 10 days or who have multiple symptoms. Final clearance once all the steps have been completed requires follow-up with a health care provider.
Is a symptom-free waiting period necessary?
There is no evidence suggesting a need for a symptom-free waiting period before starting the return-to-play protocol.10,27 Because a repeat concussion is most likely within 7 to 10 days of the initial injury,8,9 however, most athletes should not return to contact play during that time frame, regardless of symptom resolution.
It is helpful to have asymptomatic athletes participate in non-contact activity before the 7 to 10 days are up, however. Doing so can help prevent deconditioning and injury upon return to contact sport, as there is evidence of increased risk of lower-extremity injury in the 90 days after concussion.28
What to tell athletes—and parents—about repetitive head trauma
There is growing concern about the long-term risks of concussion and repetitive head impact that may manifest as chronic traumatic encephalopathy (CTE) and chronic neurocognitive impairment (CNI) later in life. Indeed, some data strongly suggest—but do not definitively prove—a relationship between repetitive head injury and chronic neurodegenerative disease.8-10 You can tell worried patients or parents, however, that the majority of research on CTE and CNI has been based on professional football players.
Studies of long-term effects of soccer heading have shown conflicting results, with some finding cognitive impairment, altered postural control, and anatomic changes of the brain, while others found no effect on encephalopathy, concussion symptoms, or neurocognitive performance.29-36Here, too, most studies showing negative effects of soccer heading involved professional athletes.
Repetitive sub-concussive impact in high school football athletes has been found to induce biochemical changes to the brain,37 but the long-term effects are unknown. And, while concussion in high school athletes has been associated with short-term cognitive impairment, altered neurochemistry, and evidence of increased symptoms on baseline neurocognitive testing,8-10,38 no studies have linked concussion during middle school or high school with CNI. What’s more, a long-term (50-year) follow-up study of individuals who played football in high school found no difference in rates of neurodegenerative disease compared with age-matched controls.39
A new study of high school and college football players (mean age: 17.4 years) presented at the American Academy of Neurology 2016 Sports Concussion Conference in Chicago in July, however, found significant alterations in white matter 6 months post injury.40 The researchers compared 17 athletes with sport-related concussion with matched controls, using diffusion tensor imaging and diffusion kurtosis tensor imaging as biomarkers of brain recovery. The concussed athletes underwent MRI and symptom assessment at 24 hours, 8 days, and 6 months. The controls followed identical protocols.
At the 6-month assessment, there were no differences between the concussed group and the controls in terms of self-reported concussion symptoms, cognition, or balance. However, the concussed athletes had widespread decreased mean diffusivity compared with the controls. Despite the lack of clinical symptoms, the concussed athletes showed significant alterations in white matter “that were related to initial symptom severity ratings,” the authors concluded. These findings have implications both for determination of recovery from concussion and concussion management, they added.40
Although there is no way to eliminate all concussions, limited evidence suggests that improving athletic technique, limiting contact at practices, better enforcement of game rules, and rule changes regarding physical contact may decrease concussion risk.41-43 Many youth sports organizations have developed policies placing restrictions on head impact during practices and games. Studies are ongoing, too, to see if better headgear—or requiring helmets for soccer players—makes a difference.
CORRESPONDENCE
Ryan A. Sprouse, MD, CAQSM, 203 East Fourth Avenue, Ranson, WV 25438; [email protected].
1. Bryan MA, Rowhani-Rahbar A, Comstock RD, et al. Sports- and recreation-related concussions in US youth. Pediatrics. 2016; June 20 [Epub ahead of print].
2. Arbogast KB, Curry AE, Pfeiffer MR, et al. Point of health care entry for youth with concussion within a large pediatric care network. JAMA Pediatr. 2016; May 31 [Epub ahead of print].
3. Mihalik JK, Guskiewicz KM, Valovich McLeod TC, et al. Knowledge, attitude, and concussion-reporting behaviors among high school athletes: a preliminary study. J Ath Tr. 2013;48:645-653.
4. Marar M, McIlvain NM, Fields SK, et al. Epidemiology of concussions among United States high school athletes in 20 sports. Am J Sports Med. 2012;40:747.
5. Kontos AP, Elbin RJ, Fazio-Sumrock VC. Incidence of sports-related concussion among youth football players aged 8-12 years. J Pediatr. 2013;163:717-720.
6. Dompier TP, Kerr ZY, Marshall SW, et al. Incidence of concussion during practice and games in youth, high school, and collegiate American football players. JAMA Pediatr. 2015;169:659-665.
7. Comstock RD, Currie DW, Pierpont LA, et al. An evidence-based discussion of heading the ball and concussions in high school soccer. JAMA Pediatr. 2015;169:830-837.
8. Harmon KG, Drezner JA, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Br J Sports Med. 2013;47:15-26.
9. McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47:250-258.
10. Giza CC, Kutcher JS, Ashwal S, et al. Summary of the evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80:2250-2257.
11. Terwilliger VK, Pratson L, Vaughan CG, et al. Additional post-concussion impact exposure may affect recovery in adolescent athletes. J Neurotrauma. 2016;33:761-765.
12. Putukian M, Echemendia R, Dettwiler-Danspeckgruber A. Prospective clinical assessment using Sideline Concussion Assessment Tool-2 testing in the evaluation of sport-related concussion in college athletes. Clin J Sport Med. 2015;25:36-42.
13. Broglio SP, Macciocchi SN, Ferrara MS. Sensitivity of the concussion assessment battery. Neurosurgery. 2007;60:1050-1057.
14. Randolph C, McCrea M, Barr WB. Is neuropsychological testing useful in the management of sport-related concussion? J Athl Train. 2005;40:139-152.
15. Shrier I. Neuropsychological testing and concussions: a reasoned approach. Clin J Sport Med. 2012;22:211-213.
16. DeMatteo C, Stazyk K, Singh SK, et al. Development of a conservative protocol to return children and youth to activity following concussive injury. Clin Pediatr (Phila). 2015;54:152-163.
17. Broglio SP, Cantu RC, Gioia GA, et al. National Athletic Trainers Association position statement: management of sport concussion. J Athl Train. 2014;49:245-265.
18. Stoller J, Carson JD, Garel A, et al. Do family physicians, emergency department physicians, and pediatricians give consistent sport-related concussion management advice? Can Fam Physician. 2014;60:548, 550-552.
19. Lebrun CM, Mrazik M, Prasad AS, et al. Sport concussion knowledge base, clinical practices and needs for continuing medical education: a survey of family physicians and cross-border comparison. Br J Sports Med. 2013;47:54-59.
20. Zemek R, Eady K, Moreau K, et al. Knowledge of paediatric concussion among front-line primary care providers. Paediatr Child Health. 2014;19:475-480.
21. Maerlender A, Rieman W, Lichtenstein J, et al. Programmed physical exertion in recovery from sports-related concussion: a randomized pilot study. Dev Neuropsychol. 2015;40:273-278.
22. Buckley TA, Munkasy BA, Clouse BP. Acute cognitive and physical rest may not improve concussion recovery time. J Head Trauma Rehabil. 2015; July 24 [Epub ahead of print].
23. Preece MH, Horswill MS, Langlois JA, et al. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375-378.
24. Baker A, Unsworth CA, Lannin NA. Fitness-to-drive after mild traumatic brain injury: mapping the time trajectory of recovery in the acute stages post injury. Accid Anal Prev. 2015;79:50-55.
25. Marshall S, Bayley M, McCullagh S, et al. Clinical practice guidelines for mild traumatic brain injury and persistent symptoms. Can Fam Physician. 2012;58:257-267.
26. Yorke AM, Littleton S, Alsalaheen BA. Concussion attitudes and beliefs, knowledge, and clinical practice: a survey of physical therapists. Phys Ther. Available at: http://dx.doi.org/10.2522/ptj.20140598. Accessed January 21, 2016.
27. McCrea M, Guskiewicz K, Randolph C, et al. Effects of a symptom-free waiting period on clinical outcome and risk of reinjury after sport-related concussion. Neurosurgery. 2009;65:876-883.
28. Brooks MA, Peterson K, Biese K, et al. Concussion increases odds of sustaining a lower extremity musculoskeletal injury after return to play among collegiate athletes. Am J Sports Med. 2016;44:742-747.
29. Witol AD, Webbe FM. Soccer heading frequency predicts neuropsychological deficits. Arch Clin Neuropsychol. 2003;18:397-417.
30. Haran FJ, Tierney R, Wright WG, et al. Acute changes in postural control after soccer heading. Int J Sports Med. 2013;34:350-354.
31. Lipton ML, Kim N, Zimmerman ME, et al. Soccer heading is associated with white matter microstructural and cognitive abnormalities. Radiology. 2013;268:850-857.
32. Jordan SE, Green GA, Galanty HL, et al. Acute and chronic brain injury in United States national team soccer players. Am J Sports Med. 1996;24:205-210.
33. Kontos AP, Dolese A, Elbin RJ, et al. Relationship of soccer heading to computerized neurocognitive performance and symptoms among female and male youth soccer players. Brain Inj. 2011;25:1234-1241.
34. Straume-Naesheim TM, Andersen TE, Dvorak J, et al. Effects of heading exposure and previous concussions on neuropsychological performance among Norwegian elite footballers. Br J Sports Med. 2005;39:70-77.
35. Stephens R, Rutherford A, Potter D, et al. Neuropsychological impairment as a consequence of football (soccer) play and football heading: a preliminary analysis and report on school students (13-16 years). Child Neuropsychol. 2005;11:513-526.
36. Stephens R, Rutherford A, Potter D, et al. Neuropsychological consequence of soccer play in adolescent UK school team soccer players. J Neuropsychiatry Clin Neurosci. 2010;22:295-303.
37. Poole VN, Breedlove EL, Shenk TE, et al. Sub-concussive hit characteristics predict deviant brain metabolism in football athletes. Dev Neuropsychol. 2015;40:12-17.
38. Mannix R, Iverson GL, Maxwell B, et al. Multiple prior concussions are associated with symptoms in high school athletes. Ann Clin Trans Neurol. 2014;1:433-438.
39. Savica R, Parisi JE, Wold LE, et al. High school football and risk of neurodegeneration: a community-based study. Mayo Clin Proc. 2012;87:335-340.
40. Lancaster M, Muftuler T, Olson D, et al. Chronic white matter changes following sport-related concussion measured by diffusion tensor and diffusion kurtosis imaging. Paper presented at: American Academy of Neurology 2016 Sports Concussion Conference; July 8-10, 2016; Chicago, Ill.
41. Kerr ZY, Yeargin SW, Valovich McLeod TC, et al. Comprehensive coach education reduces head impact exposures in American youth football. Orthop J Sports Med. 2015;3(ecollection):e232596711561545.
42. Black AM, Macpherson AK, Hagel BE, et al. Policy change eliminating body checking in non-elite ice hockey leads to a threefold reduction in injury and concussion risk in 11- and 12-year-old players. Br J Sports Med. 2016;50:55-61.
43. Council on Sports Medicine and Fitness. Tackling in youth football. Policy Statement of the American Academy of Pediatrics. Pediatrics. 2015;136:e1419-e1430.
1. Bryan MA, Rowhani-Rahbar A, Comstock RD, et al. Sports- and recreation-related concussions in US youth. Pediatrics. 2016; June 20 [Epub ahead of print].
2. Arbogast KB, Curry AE, Pfeiffer MR, et al. Point of health care entry for youth with concussion within a large pediatric care network. JAMA Pediatr. 2016; May 31 [Epub ahead of print].
3. Mihalik JK, Guskiewicz KM, Valovich McLeod TC, et al. Knowledge, attitude, and concussion-reporting behaviors among high school athletes: a preliminary study. J Ath Tr. 2013;48:645-653.
4. Marar M, McIlvain NM, Fields SK, et al. Epidemiology of concussions among United States high school athletes in 20 sports. Am J Sports Med. 2012;40:747.
5. Kontos AP, Elbin RJ, Fazio-Sumrock VC. Incidence of sports-related concussion among youth football players aged 8-12 years. J Pediatr. 2013;163:717-720.
6. Dompier TP, Kerr ZY, Marshall SW, et al. Incidence of concussion during practice and games in youth, high school, and collegiate American football players. JAMA Pediatr. 2015;169:659-665.
7. Comstock RD, Currie DW, Pierpont LA, et al. An evidence-based discussion of heading the ball and concussions in high school soccer. JAMA Pediatr. 2015;169:830-837.
8. Harmon KG, Drezner JA, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Br J Sports Med. 2013;47:15-26.
9. McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47:250-258.
10. Giza CC, Kutcher JS, Ashwal S, et al. Summary of the evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80:2250-2257.
11. Terwilliger VK, Pratson L, Vaughan CG, et al. Additional post-concussion impact exposure may affect recovery in adolescent athletes. J Neurotrauma. 2016;33:761-765.
12. Putukian M, Echemendia R, Dettwiler-Danspeckgruber A. Prospective clinical assessment using Sideline Concussion Assessment Tool-2 testing in the evaluation of sport-related concussion in college athletes. Clin J Sport Med. 2015;25:36-42.
13. Broglio SP, Macciocchi SN, Ferrara MS. Sensitivity of the concussion assessment battery. Neurosurgery. 2007;60:1050-1057.
14. Randolph C, McCrea M, Barr WB. Is neuropsychological testing useful in the management of sport-related concussion? J Athl Train. 2005;40:139-152.
15. Shrier I. Neuropsychological testing and concussions: a reasoned approach. Clin J Sport Med. 2012;22:211-213.
16. DeMatteo C, Stazyk K, Singh SK, et al. Development of a conservative protocol to return children and youth to activity following concussive injury. Clin Pediatr (Phila). 2015;54:152-163.
17. Broglio SP, Cantu RC, Gioia GA, et al. National Athletic Trainers Association position statement: management of sport concussion. J Athl Train. 2014;49:245-265.
18. Stoller J, Carson JD, Garel A, et al. Do family physicians, emergency department physicians, and pediatricians give consistent sport-related concussion management advice? Can Fam Physician. 2014;60:548, 550-552.
19. Lebrun CM, Mrazik M, Prasad AS, et al. Sport concussion knowledge base, clinical practices and needs for continuing medical education: a survey of family physicians and cross-border comparison. Br J Sports Med. 2013;47:54-59.
20. Zemek R, Eady K, Moreau K, et al. Knowledge of paediatric concussion among front-line primary care providers. Paediatr Child Health. 2014;19:475-480.
21. Maerlender A, Rieman W, Lichtenstein J, et al. Programmed physical exertion in recovery from sports-related concussion: a randomized pilot study. Dev Neuropsychol. 2015;40:273-278.
22. Buckley TA, Munkasy BA, Clouse BP. Acute cognitive and physical rest may not improve concussion recovery time. J Head Trauma Rehabil. 2015; July 24 [Epub ahead of print].
23. Preece MH, Horswill MS, Langlois JA, et al. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375-378.
24. Baker A, Unsworth CA, Lannin NA. Fitness-to-drive after mild traumatic brain injury: mapping the time trajectory of recovery in the acute stages post injury. Accid Anal Prev. 2015;79:50-55.
25. Marshall S, Bayley M, McCullagh S, et al. Clinical practice guidelines for mild traumatic brain injury and persistent symptoms. Can Fam Physician. 2012;58:257-267.
26. Yorke AM, Littleton S, Alsalaheen BA. Concussion attitudes and beliefs, knowledge, and clinical practice: a survey of physical therapists. Phys Ther. Available at: http://dx.doi.org/10.2522/ptj.20140598. Accessed January 21, 2016.
27. McCrea M, Guskiewicz K, Randolph C, et al. Effects of a symptom-free waiting period on clinical outcome and risk of reinjury after sport-related concussion. Neurosurgery. 2009;65:876-883.
28. Brooks MA, Peterson K, Biese K, et al. Concussion increases odds of sustaining a lower extremity musculoskeletal injury after return to play among collegiate athletes. Am J Sports Med. 2016;44:742-747.
29. Witol AD, Webbe FM. Soccer heading frequency predicts neuropsychological deficits. Arch Clin Neuropsychol. 2003;18:397-417.
30. Haran FJ, Tierney R, Wright WG, et al. Acute changes in postural control after soccer heading. Int J Sports Med. 2013;34:350-354.
31. Lipton ML, Kim N, Zimmerman ME, et al. Soccer heading is associated with white matter microstructural and cognitive abnormalities. Radiology. 2013;268:850-857.
32. Jordan SE, Green GA, Galanty HL, et al. Acute and chronic brain injury in United States national team soccer players. Am J Sports Med. 1996;24:205-210.
33. Kontos AP, Dolese A, Elbin RJ, et al. Relationship of soccer heading to computerized neurocognitive performance and symptoms among female and male youth soccer players. Brain Inj. 2011;25:1234-1241.
34. Straume-Naesheim TM, Andersen TE, Dvorak J, et al. Effects of heading exposure and previous concussions on neuropsychological performance among Norwegian elite footballers. Br J Sports Med. 2005;39:70-77.
35. Stephens R, Rutherford A, Potter D, et al. Neuropsychological impairment as a consequence of football (soccer) play and football heading: a preliminary analysis and report on school students (13-16 years). Child Neuropsychol. 2005;11:513-526.
36. Stephens R, Rutherford A, Potter D, et al. Neuropsychological consequence of soccer play in adolescent UK school team soccer players. J Neuropsychiatry Clin Neurosci. 2010;22:295-303.
37. Poole VN, Breedlove EL, Shenk TE, et al. Sub-concussive hit characteristics predict deviant brain metabolism in football athletes. Dev Neuropsychol. 2015;40:12-17.
38. Mannix R, Iverson GL, Maxwell B, et al. Multiple prior concussions are associated with symptoms in high school athletes. Ann Clin Trans Neurol. 2014;1:433-438.
39. Savica R, Parisi JE, Wold LE, et al. High school football and risk of neurodegeneration: a community-based study. Mayo Clin Proc. 2012;87:335-340.
40. Lancaster M, Muftuler T, Olson D, et al. Chronic white matter changes following sport-related concussion measured by diffusion tensor and diffusion kurtosis imaging. Paper presented at: American Academy of Neurology 2016 Sports Concussion Conference; July 8-10, 2016; Chicago, Ill.
41. Kerr ZY, Yeargin SW, Valovich McLeod TC, et al. Comprehensive coach education reduces head impact exposures in American youth football. Orthop J Sports Med. 2015;3(ecollection):e232596711561545.
42. Black AM, Macpherson AK, Hagel BE, et al. Policy change eliminating body checking in non-elite ice hockey leads to a threefold reduction in injury and concussion risk in 11- and 12-year-old players. Br J Sports Med. 2016;50:55-61.
43. Council on Sports Medicine and Fitness. Tackling in youth football. Policy Statement of the American Academy of Pediatrics. Pediatrics. 2015;136:e1419-e1430.
From The Journal of Family Practice | 2016;65(8):538-544,546.
Extreme Athlete, 18, With Worsening Cough
IN THIS ARTICLE
- Adverse effects of ciprofloxacin
- Symptoms of common tick-borne diseases
- Symptoms of phase 1 and late-phase disease
- Additional resources
Jane, an 18-year-old college student, presents in early November with a three-week history of worsening cough and sinus congestion. Recently, the cough has been interrupting her sleep and yellow-green nasal drainage and sinus pressure have increased. Ordinarily very fit and athletic, she reports that since she arrived at college two months ago, her body has become “more fragile.”
Further questioning reveals that, over the past two months, the patient’s symptoms have included extreme fatigue, severe unremitting headache, blurred vision, shortness of breath, and a racing heart rate on exertion. Her symptoms make it impossible for her to maintain her demanding exercise routine, a development that compounds her frustration and sadness. She has also been forced to limit her participation in school activities, with significant academic decline as a result.
Aside from depression (well controlled with bupropion HCl extended release, 300 mg/d), Jane’s medical history is unremarkable. She reports having “excellent health” until she arrived at her mid-Atlantic urban college.
A complicated history
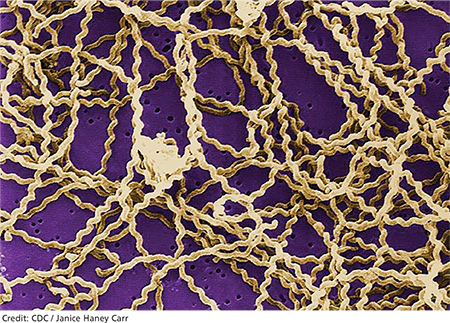
Born and raised in Connecticut, Jane is an avid runner who competes in extreme sports. This past summer, she trained for and participated in two “mud run” events (ie, endurance races of several miles with numerous challenges and obstacles) in Connecticut and New York. Training included endurance runs and sprints, as well as crawling through mud-laden fields and woods.
She also did a three-week summer internship on an oyster farm. There, she was required to shuck oysters and stand in brackish water for six-hour shifts to examine oyster beds. In the process, she sustained numerous cuts and bruises on her hands, arms, and legs.
A week or so after returning to college in late August, Jane developed blisters on both heels, which progressed to infected ulcerations. She was evaluated at the university hospital emergency department (ED) and treated with a 21-day course of ciprofloxacin. When left-sided unilateral knee swelling developed about two weeks later, she underwent arthrocentesis at the university health center, but joint aspirate was not sent for analysis. A two-week course of antibiotic therapy was initiated.
From October to her presentation in early November, Jane has experienced intermittent fevers and chills, with a temperature as high as 101°F. In addition, she complains of fasciculations and weakness in her lower limbs; dyspnea, tachycardia, and dizziness during or after any exertion; unremitting posterior neck pain; and a constant, severe headache located primarily in the bitemporal region. She developed bilateral conjunctivitis, which resolved spontaneously in about one week; persistent blurred vision; a transient petechial chest rash; recurring episodes of syncope; pyelonephritis; a persistent vaginal yeast infection; decreased appetite; and a 7-lb weight loss (5% of her total body weight).
Jane’s academic and athletic performance has been severely impaired. Once a long-distance runner, she can no longer walk any distance without frequent rest. In the four months since the mud runs, the patient reports, she has been seen in the student health center four times and in the ED twice. Additionally, she has undergone thorough examinations by clinicians specializing in infectious disease, pulmonology, neurology, and neuro-ophthalmology. She has undergone lab work, including
• Complete blood cell count with differential
• Comprehensive metabolic panel
• Urinalysis and urine culture
• Lyme antibody and blood polymerase chain reaction (PCR)
• HIV testing
• Rheumatoid factor
• Erythrocyte sedimentation rate (ESR)
• C-reactive protein (CRP)
• Epstein-Barr virus IgM
• Cytomegalovirus (CMV) IgM
• Human granulocytic ehrlichiosis (HGE) antibody and human anaplasma phagocytophilum (HGA)
• HGA PCR
• Rickettsia antibody panel
• Babesia microti antibodies
• Pregnancy testing
• Chest x-ray
• Lumbar puncture
All lab results were within normal range. In light of this, several clinicians have told Jane that her illness is “all in her head.”
Continue for the patient investigates >>
The patient investigates
In mid-December, after she has returned home from college, Jane’s symptoms abruptly worsen. She complains of feeling “shakier,” with weakness in her legs and what she calls “brain fog.” Her headache, blurred vision, and dizziness have worsened. Frightened and concerned, she returns to the ED. Results of a thorough evaluation, including lumbar puncture, reveal no abnormality.
Jane has become extremely frail. She is losing weight, her hair has lost its luster, and her nails are cracking and bleeding. She is unable to walk without concern for falling and cannot climb the 20 steps to her bedroom. Once a healthy and vibrant 18-year-old, she now spends most of her time in a lethargic state on a first-floor living room couch.
Frustrated by her unexplained declining health, she begins to research illnesses associated with extreme sports and prolonged marine exposure. She returns to ask about three possible explanations for her condition:
1. Adverse effects of ciprofloxacin use, which include fever or chills, dizziness, racing heartbeat, headache, and nausea.1
2. A tick-borne disease, possibly contracted during her practice runs in the Connecticut woods (see Table 1).2-4 Each year, she recalls, she has found and removed four or five embedded ticks. In the northeastern United States, the most common tick-borne diseases are borreliosis, babesiosis, and ehrlichiosis.5-7
3. Leptospirosis, contracted through the patient’s exposure to mud and brackish water during her summer activities. According to her research, more than 25 outbreaks and 600 cases of leptospirosis (between 1931 and 1998) have been associated with fresh pond, creek, or river water.8
Based on Jane’s symptoms and history, and in accord with her research, early-phase leptospirosis is identified as a diagnosis of exclusion (with a possible comorbid tick-borne zoonosis).
Continue for discussion >>
DISCUSSION
Leptospirosis develops when humans come into contact with animal urine infected by leptospires—that is, pathogenic spirochetes excreted via the renal tubules of infected host animals.9,10 While host animals include dogs, pigs, cattle, reptiles, and amphibians, the animal most commonly associated with human infection is the brown rat (Rattus norvegicus).11-15
Leptospires enter the human host through mucous membranes, cuts, or abrasions in the skin. Individuals at increased risk for infection include those whose work or other activities expose them “to animal reservoirs or contaminated environments”—including participants in water sports and similar recreation.11-14 As Mwachui et al explain, “recreational exposure to [Leptospira-]contaminated water has become more important for sport enthusiasts, swimmers and travellers from industrialized countries,” whereas flooding is usually involved in infection in undeveloped countries.16
The largest outbreak of leptospirosis reported in the US to date occurred in 1998, when heavy rains preceded a triathlon in Springfield, Illinois. When many participants became ill after the event, researchers from the National Center for Infectious Diseases were able to contact and test 834 of the 876 competing athletes; of these, 98 (12%) reported being ill and 52 (11%) tested positive for leptospirosis. Additionally, 14 of the 248 community residents who were sickened (6%) tested positive.17 According to CDC estimates, between 100 and 200 cases of leptospirosis develop annually in the US, with about half occurring in Hawaii.9
Onset of symptoms, which are described as protean and nonspecific, occurs two days to four weeks after exposure, making leptospirosis difficult to diagnosewithout a high degree of suspicion; zoonotic exposure (as with freshwater or mud sports) or a history of travel to Hawaii, Tahiti, Thailand, Indonesia, the Caribbean, and/or Costa Rica may raise suspicion.12-14,18 In early-phase leptospirosis, symptoms can mimic those of influenza, meningitis, malaria, dengue fever, scrub typhus, rickettsial disease, and typhoid fever (see Table 2).10 Thus, when a patient presents with these symptoms, it is imperative that the clinician consider leptospirosis.19Of note: Flu-like symptoms with conjunctival suffusion are considered pathognomonic for leptospirosis.18
About 10% of patients with early-phase leptospirosis will develop late-phase disease (ie, Weil’s disease), with severe symptoms that include jaundice, meningitis, pulmonary hemorrhage, and acute kidney injury (see Table 3 for a more detailed list).20 The case patient’s history and symptoms were consistent with a diagnosis of early-phase leptospirosis.
Epidemiology
In 2015, leptospirosis was estimated to affect more than 1 million persons worldwide, with 58,900 deaths attributed to the disease each year—making leptospirosis the leading cause of death attributable to zoonotic illness.11 Historically, leptospirosis-associated morbidity and mortality have been greatest in resource-poor countries with tropical climates (eg, southern and Southeast Asia, Central America and tropical Latin America, and East Sub-Saharan Africa).11,12
However, illness resulting from recreational exposures to contaminated water has been linked to increasing travel to exotic destinations, participation in adventure travel, and the growing popularity of extreme sports involving fresh water.9 Recreational mud run events, for example, involve swimming in potentially contaminated waters and crawling through flooded farm fields where animal urine can be present—an ideal environment for Leptospira to thrive and for participants to contract the disease.14,15
Continue for laboratory work-up >>
Laboratory work-up
Diagnosis of leptospirosis is challenging.21 Laboratory tests vary, depending on the timing and stage of infection, and are mostly unavailable in resource-poor countries. Test results for the patient with early-phase leptospirosis may demonstrate renal or hepatic abnormalities.18 However, laboratory confirmation of leptospirosis requires22
• A fourfold increase in antibody titer between acute and convalescent serum samples, as detected by microscopic agglutination testing (MAT) or
• A high MAT titer (> 1:400 to 1:800), in single or paired samples or
• Isolation of pathogenic Leptospira species from a normally sterile site or
• Detection of DNA from pathogenic Leptospira species by PCR
A positive laboratory result is, of course, confirmatory. However, negative laboratory findings must be viewed with healthy skepticism.12 A false-negative result may merely indicate the shortcoming of the testing method to accurately assess the presence of Leptospira.
Treatment options
The high mortality rate associated with severe leptospirosis makes early diagnosis and treatment essential.23 The World Health Organization warns that antibiotic treatment for leptospirosis must be instituted within five days of symptom onset.10
Treatment options for an ambulatory patient with mild symptoms and no organ involvement include oral doxycycline (100 mg bid for 5-7 d) or oral azithromycin (500 mg/d for 5-7 d). For patients with organ involvement, IV penicillin (1.5 million U every 6 h for 7 d), ceftriaxone (1 g/d for 7 d), or cefotaxime (1 g every 6 h for 7 d) may be considered.12,20
OUTCOME FOR THE CASE PATIENT
With leptospirosis as the diagnosis of exclusion, Jane was treated successfully with a 21-day course of oral doxycycline (100 mg bid). She has been symptom free since completing the regimen. After undergoing physical therapy and athletic training, she has been able to resume her full exercise regimen, and her recovery is considered complete.
CONCLUSION
The growing popularity of adventure travel and “extreme sports” events, particularly triathlons and mud runs, may precipitate an increase in associated infections with Leptospira and other zoonotic pathogens. For patients with flulike symptoms who routinely engage in such sports—especially those who present with conjunctival suffusion—leptospirosis should be considered in the differential diagnosis.
REFERENCES
1. Owens RC Jr, Ambrose PG. Antimicrobial safety: focus on fluoroquinolones. Clin Infect Dis. 2005;41(suppl 2):S144-S157.
2. CDC. Signs and symptoms of untreated Lyme disease (2015). www.cdc.gov/lyme/signs_symptoms/index.html. Accessed June 7, 2016.
3. CDC. Parasites: babesiosis (2014). www.cdc.gov/parasites/babesiosis/disease.html. Accessed June 7, 2016.
4. CDC. Ehrlichiosis: symptoms, diagnosis, and treatment (2013). www.cdc.gov/Ehrlichiosis/symptoms/index.html. Accessed June 7, 2016.
5. Pritt BS, Mead PS, Johnson DK, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. 2016 Feb 5. [Epub ahead of print]
6. Choi E, Pyzocha NJ, Maurer DM. Tick-borne illnesses. Curr Sports Med Rep. 2016;15(2):98-104.
7. Chomel B. Lyme disease. Rev Sci Tech. 2015;34(2):569-576.
8. Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14(2):296-326.
9. CDC. Leptospirosis: signs and symptoms (2016). www.cdc.gov/leptospirosis/symptoms/index.html. Accessed June 7, 2016.
10. World Health Organization, International Leptospirosis Society. Human Leptospirosis: Guidance for Diagnosis, Surveillance, and Control (2003). http://apps.who.int/iris/bitstream/10665/42667/1/WHO_CDS_CSR_EPH_2002.23.pdf. Accessed June 7, 2016.
11. Costa F, Hagan JE, Calcagno J, et al. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003898.
12. Haake DA, Levett PN. Leptospirosis in humans. Curr Top Microbiol Immunol. 2015;387:65-97.
13. Picardeau M. Diagnosis and epidemiology of leptospirosis. Médecine et Maladies Infectieuses. 2013;43(1):1-9.
14. Picardeau M. Leptospirosis: updating the global picture of an emerging neglected disease. PLoS Negl Trop Dis. 2015;9(9):e0004039.
15. Zavitsanou A, Babatsikou F. Leptospirosis: epidemiology and preventive measures. Health Sci J. 2008;2(2):75-82.
16. Mwachui MA, Crump L, Hartskeerl R, et al. Environmental and behavioural determinants of leptospirosis transmission: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003843.
17. Morgan J, Bornstein SL, Karpati AM, et al. Outbreak of leptospirosis among triathlon participants and community residents in Springfield, Illinois, 1998. Clin Infect Dis. 2002;34(12):1593-1599.
18. Katz AR, Ansdell VE, Effler PV, et al. Assessment of the clinical presentation and treatment of 353 cases of laboratory-confirmed leptospirosis in Hawaii, 1974-1998. Clin Infect Dis. 2001;33(11):1834-1841.
19. Yaakob Y, Rodrigues KF, John DV. Leptospirosis: recent incidents and available diagnostics—a review. Med J Malaysia. 2015;70(6):351-355.
20. Seguro AC, Andrade L. Pathophysiology of leptospirosis. Shock. 2013;39(suppl 1):17-23.
21. Musso D, La Scola B. Laboratory diagnosis of leptospirosis: a challenge. J Microbiol Immunol Infect. 2013;46(4):245-252.
22. Waggoner JJ, Balassiano I, Mohamed-Hadley A, et al. Reverse-transcriptase PCR detection of Leptospira: absence of agreement with single-specimen microscopic agglutination testing. PLoS One. 2015;10(7):e0132988.
23. Iwasaki H, Chagan-Yasutan H, Leano PS, et al. Combined antibody and DNA detection for early diagnosis of leptospirosis after a disaster. Diagn Microbiol Infect Dis. 2016;84(4):287-291
IN THIS ARTICLE
- Adverse effects of ciprofloxacin
- Symptoms of common tick-borne diseases
- Symptoms of phase 1 and late-phase disease
- Additional resources
Jane, an 18-year-old college student, presents in early November with a three-week history of worsening cough and sinus congestion. Recently, the cough has been interrupting her sleep and yellow-green nasal drainage and sinus pressure have increased. Ordinarily very fit and athletic, she reports that since she arrived at college two months ago, her body has become “more fragile.”
Further questioning reveals that, over the past two months, the patient’s symptoms have included extreme fatigue, severe unremitting headache, blurred vision, shortness of breath, and a racing heart rate on exertion. Her symptoms make it impossible for her to maintain her demanding exercise routine, a development that compounds her frustration and sadness. She has also been forced to limit her participation in school activities, with significant academic decline as a result.
Aside from depression (well controlled with bupropion HCl extended release, 300 mg/d), Jane’s medical history is unremarkable. She reports having “excellent health” until she arrived at her mid-Atlantic urban college.
A complicated history
Born and raised in Connecticut, Jane is an avid runner who competes in extreme sports. This past summer, she trained for and participated in two “mud run” events (ie, endurance races of several miles with numerous challenges and obstacles) in Connecticut and New York. Training included endurance runs and sprints, as well as crawling through mud-laden fields and woods.
She also did a three-week summer internship on an oyster farm. There, she was required to shuck oysters and stand in brackish water for six-hour shifts to examine oyster beds. In the process, she sustained numerous cuts and bruises on her hands, arms, and legs.
A week or so after returning to college in late August, Jane developed blisters on both heels, which progressed to infected ulcerations. She was evaluated at the university hospital emergency department (ED) and treated with a 21-day course of ciprofloxacin. When left-sided unilateral knee swelling developed about two weeks later, she underwent arthrocentesis at the university health center, but joint aspirate was not sent for analysis. A two-week course of antibiotic therapy was initiated.
From October to her presentation in early November, Jane has experienced intermittent fevers and chills, with a temperature as high as 101°F. In addition, she complains of fasciculations and weakness in her lower limbs; dyspnea, tachycardia, and dizziness during or after any exertion; unremitting posterior neck pain; and a constant, severe headache located primarily in the bitemporal region. She developed bilateral conjunctivitis, which resolved spontaneously in about one week; persistent blurred vision; a transient petechial chest rash; recurring episodes of syncope; pyelonephritis; a persistent vaginal yeast infection; decreased appetite; and a 7-lb weight loss (5% of her total body weight).
Jane’s academic and athletic performance has been severely impaired. Once a long-distance runner, she can no longer walk any distance without frequent rest. In the four months since the mud runs, the patient reports, she has been seen in the student health center four times and in the ED twice. Additionally, she has undergone thorough examinations by clinicians specializing in infectious disease, pulmonology, neurology, and neuro-ophthalmology. She has undergone lab work, including
• Complete blood cell count with differential
• Comprehensive metabolic panel
• Urinalysis and urine culture
• Lyme antibody and blood polymerase chain reaction (PCR)
• HIV testing
• Rheumatoid factor
• Erythrocyte sedimentation rate (ESR)
• C-reactive protein (CRP)
• Epstein-Barr virus IgM
• Cytomegalovirus (CMV) IgM
• Human granulocytic ehrlichiosis (HGE) antibody and human anaplasma phagocytophilum (HGA)
• HGA PCR
• Rickettsia antibody panel
• Babesia microti antibodies
• Pregnancy testing
• Chest x-ray
• Lumbar puncture
All lab results were within normal range. In light of this, several clinicians have told Jane that her illness is “all in her head.”
Continue for the patient investigates >>
The patient investigates
In mid-December, after she has returned home from college, Jane’s symptoms abruptly worsen. She complains of feeling “shakier,” with weakness in her legs and what she calls “brain fog.” Her headache, blurred vision, and dizziness have worsened. Frightened and concerned, she returns to the ED. Results of a thorough evaluation, including lumbar puncture, reveal no abnormality.
Jane has become extremely frail. She is losing weight, her hair has lost its luster, and her nails are cracking and bleeding. She is unable to walk without concern for falling and cannot climb the 20 steps to her bedroom. Once a healthy and vibrant 18-year-old, she now spends most of her time in a lethargic state on a first-floor living room couch.
Frustrated by her unexplained declining health, she begins to research illnesses associated with extreme sports and prolonged marine exposure. She returns to ask about three possible explanations for her condition:
1. Adverse effects of ciprofloxacin use, which include fever or chills, dizziness, racing heartbeat, headache, and nausea.1
2. A tick-borne disease, possibly contracted during her practice runs in the Connecticut woods (see Table 1).2-4 Each year, she recalls, she has found and removed four or five embedded ticks. In the northeastern United States, the most common tick-borne diseases are borreliosis, babesiosis, and ehrlichiosis.5-7
3. Leptospirosis, contracted through the patient’s exposure to mud and brackish water during her summer activities. According to her research, more than 25 outbreaks and 600 cases of leptospirosis (between 1931 and 1998) have been associated with fresh pond, creek, or river water.8
Based on Jane’s symptoms and history, and in accord with her research, early-phase leptospirosis is identified as a diagnosis of exclusion (with a possible comorbid tick-borne zoonosis).
Continue for discussion >>
DISCUSSION
Leptospirosis develops when humans come into contact with animal urine infected by leptospires—that is, pathogenic spirochetes excreted via the renal tubules of infected host animals.9,10 While host animals include dogs, pigs, cattle, reptiles, and amphibians, the animal most commonly associated with human infection is the brown rat (Rattus norvegicus).11-15
Leptospires enter the human host through mucous membranes, cuts, or abrasions in the skin. Individuals at increased risk for infection include those whose work or other activities expose them “to animal reservoirs or contaminated environments”—including participants in water sports and similar recreation.11-14 As Mwachui et al explain, “recreational exposure to [Leptospira-]contaminated water has become more important for sport enthusiasts, swimmers and travellers from industrialized countries,” whereas flooding is usually involved in infection in undeveloped countries.16
The largest outbreak of leptospirosis reported in the US to date occurred in 1998, when heavy rains preceded a triathlon in Springfield, Illinois. When many participants became ill after the event, researchers from the National Center for Infectious Diseases were able to contact and test 834 of the 876 competing athletes; of these, 98 (12%) reported being ill and 52 (11%) tested positive for leptospirosis. Additionally, 14 of the 248 community residents who were sickened (6%) tested positive.17 According to CDC estimates, between 100 and 200 cases of leptospirosis develop annually in the US, with about half occurring in Hawaii.9
Onset of symptoms, which are described as protean and nonspecific, occurs two days to four weeks after exposure, making leptospirosis difficult to diagnosewithout a high degree of suspicion; zoonotic exposure (as with freshwater or mud sports) or a history of travel to Hawaii, Tahiti, Thailand, Indonesia, the Caribbean, and/or Costa Rica may raise suspicion.12-14,18 In early-phase leptospirosis, symptoms can mimic those of influenza, meningitis, malaria, dengue fever, scrub typhus, rickettsial disease, and typhoid fever (see Table 2).10 Thus, when a patient presents with these symptoms, it is imperative that the clinician consider leptospirosis.19Of note: Flu-like symptoms with conjunctival suffusion are considered pathognomonic for leptospirosis.18
About 10% of patients with early-phase leptospirosis will develop late-phase disease (ie, Weil’s disease), with severe symptoms that include jaundice, meningitis, pulmonary hemorrhage, and acute kidney injury (see Table 3 for a more detailed list).20 The case patient’s history and symptoms were consistent with a diagnosis of early-phase leptospirosis.
Epidemiology
In 2015, leptospirosis was estimated to affect more than 1 million persons worldwide, with 58,900 deaths attributed to the disease each year—making leptospirosis the leading cause of death attributable to zoonotic illness.11 Historically, leptospirosis-associated morbidity and mortality have been greatest in resource-poor countries with tropical climates (eg, southern and Southeast Asia, Central America and tropical Latin America, and East Sub-Saharan Africa).11,12
However, illness resulting from recreational exposures to contaminated water has been linked to increasing travel to exotic destinations, participation in adventure travel, and the growing popularity of extreme sports involving fresh water.9 Recreational mud run events, for example, involve swimming in potentially contaminated waters and crawling through flooded farm fields where animal urine can be present—an ideal environment for Leptospira to thrive and for participants to contract the disease.14,15
Continue for laboratory work-up >>
Laboratory work-up
Diagnosis of leptospirosis is challenging.21 Laboratory tests vary, depending on the timing and stage of infection, and are mostly unavailable in resource-poor countries. Test results for the patient with early-phase leptospirosis may demonstrate renal or hepatic abnormalities.18 However, laboratory confirmation of leptospirosis requires22
• A fourfold increase in antibody titer between acute and convalescent serum samples, as detected by microscopic agglutination testing (MAT) or
• A high MAT titer (> 1:400 to 1:800), in single or paired samples or
• Isolation of pathogenic Leptospira species from a normally sterile site or
• Detection of DNA from pathogenic Leptospira species by PCR
A positive laboratory result is, of course, confirmatory. However, negative laboratory findings must be viewed with healthy skepticism.12 A false-negative result may merely indicate the shortcoming of the testing method to accurately assess the presence of Leptospira.
Treatment options
The high mortality rate associated with severe leptospirosis makes early diagnosis and treatment essential.23 The World Health Organization warns that antibiotic treatment for leptospirosis must be instituted within five days of symptom onset.10
Treatment options for an ambulatory patient with mild symptoms and no organ involvement include oral doxycycline (100 mg bid for 5-7 d) or oral azithromycin (500 mg/d for 5-7 d). For patients with organ involvement, IV penicillin (1.5 million U every 6 h for 7 d), ceftriaxone (1 g/d for 7 d), or cefotaxime (1 g every 6 h for 7 d) may be considered.12,20
OUTCOME FOR THE CASE PATIENT
With leptospirosis as the diagnosis of exclusion, Jane was treated successfully with a 21-day course of oral doxycycline (100 mg bid). She has been symptom free since completing the regimen. After undergoing physical therapy and athletic training, she has been able to resume her full exercise regimen, and her recovery is considered complete.
CONCLUSION
The growing popularity of adventure travel and “extreme sports” events, particularly triathlons and mud runs, may precipitate an increase in associated infections with Leptospira and other zoonotic pathogens. For patients with flulike symptoms who routinely engage in such sports—especially those who present with conjunctival suffusion—leptospirosis should be considered in the differential diagnosis.
REFERENCES
1. Owens RC Jr, Ambrose PG. Antimicrobial safety: focus on fluoroquinolones. Clin Infect Dis. 2005;41(suppl 2):S144-S157.
2. CDC. Signs and symptoms of untreated Lyme disease (2015). www.cdc.gov/lyme/signs_symptoms/index.html. Accessed June 7, 2016.
3. CDC. Parasites: babesiosis (2014). www.cdc.gov/parasites/babesiosis/disease.html. Accessed June 7, 2016.
4. CDC. Ehrlichiosis: symptoms, diagnosis, and treatment (2013). www.cdc.gov/Ehrlichiosis/symptoms/index.html. Accessed June 7, 2016.
5. Pritt BS, Mead PS, Johnson DK, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. 2016 Feb 5. [Epub ahead of print]
6. Choi E, Pyzocha NJ, Maurer DM. Tick-borne illnesses. Curr Sports Med Rep. 2016;15(2):98-104.
7. Chomel B. Lyme disease. Rev Sci Tech. 2015;34(2):569-576.
8. Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14(2):296-326.
9. CDC. Leptospirosis: signs and symptoms (2016). www.cdc.gov/leptospirosis/symptoms/index.html. Accessed June 7, 2016.
10. World Health Organization, International Leptospirosis Society. Human Leptospirosis: Guidance for Diagnosis, Surveillance, and Control (2003). http://apps.who.int/iris/bitstream/10665/42667/1/WHO_CDS_CSR_EPH_2002.23.pdf. Accessed June 7, 2016.
11. Costa F, Hagan JE, Calcagno J, et al. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003898.
12. Haake DA, Levett PN. Leptospirosis in humans. Curr Top Microbiol Immunol. 2015;387:65-97.
13. Picardeau M. Diagnosis and epidemiology of leptospirosis. Médecine et Maladies Infectieuses. 2013;43(1):1-9.
14. Picardeau M. Leptospirosis: updating the global picture of an emerging neglected disease. PLoS Negl Trop Dis. 2015;9(9):e0004039.
15. Zavitsanou A, Babatsikou F. Leptospirosis: epidemiology and preventive measures. Health Sci J. 2008;2(2):75-82.
16. Mwachui MA, Crump L, Hartskeerl R, et al. Environmental and behavioural determinants of leptospirosis transmission: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003843.
17. Morgan J, Bornstein SL, Karpati AM, et al. Outbreak of leptospirosis among triathlon participants and community residents in Springfield, Illinois, 1998. Clin Infect Dis. 2002;34(12):1593-1599.
18. Katz AR, Ansdell VE, Effler PV, et al. Assessment of the clinical presentation and treatment of 353 cases of laboratory-confirmed leptospirosis in Hawaii, 1974-1998. Clin Infect Dis. 2001;33(11):1834-1841.
19. Yaakob Y, Rodrigues KF, John DV. Leptospirosis: recent incidents and available diagnostics—a review. Med J Malaysia. 2015;70(6):351-355.
20. Seguro AC, Andrade L. Pathophysiology of leptospirosis. Shock. 2013;39(suppl 1):17-23.
21. Musso D, La Scola B. Laboratory diagnosis of leptospirosis: a challenge. J Microbiol Immunol Infect. 2013;46(4):245-252.
22. Waggoner JJ, Balassiano I, Mohamed-Hadley A, et al. Reverse-transcriptase PCR detection of Leptospira: absence of agreement with single-specimen microscopic agglutination testing. PLoS One. 2015;10(7):e0132988.
23. Iwasaki H, Chagan-Yasutan H, Leano PS, et al. Combined antibody and DNA detection for early diagnosis of leptospirosis after a disaster. Diagn Microbiol Infect Dis. 2016;84(4):287-291
IN THIS ARTICLE
- Adverse effects of ciprofloxacin
- Symptoms of common tick-borne diseases
- Symptoms of phase 1 and late-phase disease
- Additional resources
Jane, an 18-year-old college student, presents in early November with a three-week history of worsening cough and sinus congestion. Recently, the cough has been interrupting her sleep and yellow-green nasal drainage and sinus pressure have increased. Ordinarily very fit and athletic, she reports that since she arrived at college two months ago, her body has become “more fragile.”
Further questioning reveals that, over the past two months, the patient’s symptoms have included extreme fatigue, severe unremitting headache, blurred vision, shortness of breath, and a racing heart rate on exertion. Her symptoms make it impossible for her to maintain her demanding exercise routine, a development that compounds her frustration and sadness. She has also been forced to limit her participation in school activities, with significant academic decline as a result.
Aside from depression (well controlled with bupropion HCl extended release, 300 mg/d), Jane’s medical history is unremarkable. She reports having “excellent health” until she arrived at her mid-Atlantic urban college.
A complicated history
Born and raised in Connecticut, Jane is an avid runner who competes in extreme sports. This past summer, she trained for and participated in two “mud run” events (ie, endurance races of several miles with numerous challenges and obstacles) in Connecticut and New York. Training included endurance runs and sprints, as well as crawling through mud-laden fields and woods.
She also did a three-week summer internship on an oyster farm. There, she was required to shuck oysters and stand in brackish water for six-hour shifts to examine oyster beds. In the process, she sustained numerous cuts and bruises on her hands, arms, and legs.
A week or so after returning to college in late August, Jane developed blisters on both heels, which progressed to infected ulcerations. She was evaluated at the university hospital emergency department (ED) and treated with a 21-day course of ciprofloxacin. When left-sided unilateral knee swelling developed about two weeks later, she underwent arthrocentesis at the university health center, but joint aspirate was not sent for analysis. A two-week course of antibiotic therapy was initiated.
From October to her presentation in early November, Jane has experienced intermittent fevers and chills, with a temperature as high as 101°F. In addition, she complains of fasciculations and weakness in her lower limbs; dyspnea, tachycardia, and dizziness during or after any exertion; unremitting posterior neck pain; and a constant, severe headache located primarily in the bitemporal region. She developed bilateral conjunctivitis, which resolved spontaneously in about one week; persistent blurred vision; a transient petechial chest rash; recurring episodes of syncope; pyelonephritis; a persistent vaginal yeast infection; decreased appetite; and a 7-lb weight loss (5% of her total body weight).
Jane’s academic and athletic performance has been severely impaired. Once a long-distance runner, she can no longer walk any distance without frequent rest. In the four months since the mud runs, the patient reports, she has been seen in the student health center four times and in the ED twice. Additionally, she has undergone thorough examinations by clinicians specializing in infectious disease, pulmonology, neurology, and neuro-ophthalmology. She has undergone lab work, including
• Complete blood cell count with differential
• Comprehensive metabolic panel
• Urinalysis and urine culture
• Lyme antibody and blood polymerase chain reaction (PCR)
• HIV testing
• Rheumatoid factor
• Erythrocyte sedimentation rate (ESR)
• C-reactive protein (CRP)
• Epstein-Barr virus IgM
• Cytomegalovirus (CMV) IgM
• Human granulocytic ehrlichiosis (HGE) antibody and human anaplasma phagocytophilum (HGA)
• HGA PCR
• Rickettsia antibody panel
• Babesia microti antibodies
• Pregnancy testing
• Chest x-ray
• Lumbar puncture
All lab results were within normal range. In light of this, several clinicians have told Jane that her illness is “all in her head.”
Continue for the patient investigates >>
The patient investigates
In mid-December, after she has returned home from college, Jane’s symptoms abruptly worsen. She complains of feeling “shakier,” with weakness in her legs and what she calls “brain fog.” Her headache, blurred vision, and dizziness have worsened. Frightened and concerned, she returns to the ED. Results of a thorough evaluation, including lumbar puncture, reveal no abnormality.
Jane has become extremely frail. She is losing weight, her hair has lost its luster, and her nails are cracking and bleeding. She is unable to walk without concern for falling and cannot climb the 20 steps to her bedroom. Once a healthy and vibrant 18-year-old, she now spends most of her time in a lethargic state on a first-floor living room couch.
Frustrated by her unexplained declining health, she begins to research illnesses associated with extreme sports and prolonged marine exposure. She returns to ask about three possible explanations for her condition:
1. Adverse effects of ciprofloxacin use, which include fever or chills, dizziness, racing heartbeat, headache, and nausea.1
2. A tick-borne disease, possibly contracted during her practice runs in the Connecticut woods (see Table 1).2-4 Each year, she recalls, she has found and removed four or five embedded ticks. In the northeastern United States, the most common tick-borne diseases are borreliosis, babesiosis, and ehrlichiosis.5-7
3. Leptospirosis, contracted through the patient’s exposure to mud and brackish water during her summer activities. According to her research, more than 25 outbreaks and 600 cases of leptospirosis (between 1931 and 1998) have been associated with fresh pond, creek, or river water.8
Based on Jane’s symptoms and history, and in accord with her research, early-phase leptospirosis is identified as a diagnosis of exclusion (with a possible comorbid tick-borne zoonosis).
Continue for discussion >>
DISCUSSION
Leptospirosis develops when humans come into contact with animal urine infected by leptospires—that is, pathogenic spirochetes excreted via the renal tubules of infected host animals.9,10 While host animals include dogs, pigs, cattle, reptiles, and amphibians, the animal most commonly associated with human infection is the brown rat (Rattus norvegicus).11-15
Leptospires enter the human host through mucous membranes, cuts, or abrasions in the skin. Individuals at increased risk for infection include those whose work or other activities expose them “to animal reservoirs or contaminated environments”—including participants in water sports and similar recreation.11-14 As Mwachui et al explain, “recreational exposure to [Leptospira-]contaminated water has become more important for sport enthusiasts, swimmers and travellers from industrialized countries,” whereas flooding is usually involved in infection in undeveloped countries.16
The largest outbreak of leptospirosis reported in the US to date occurred in 1998, when heavy rains preceded a triathlon in Springfield, Illinois. When many participants became ill after the event, researchers from the National Center for Infectious Diseases were able to contact and test 834 of the 876 competing athletes; of these, 98 (12%) reported being ill and 52 (11%) tested positive for leptospirosis. Additionally, 14 of the 248 community residents who were sickened (6%) tested positive.17 According to CDC estimates, between 100 and 200 cases of leptospirosis develop annually in the US, with about half occurring in Hawaii.9
Onset of symptoms, which are described as protean and nonspecific, occurs two days to four weeks after exposure, making leptospirosis difficult to diagnosewithout a high degree of suspicion; zoonotic exposure (as with freshwater or mud sports) or a history of travel to Hawaii, Tahiti, Thailand, Indonesia, the Caribbean, and/or Costa Rica may raise suspicion.12-14,18 In early-phase leptospirosis, symptoms can mimic those of influenza, meningitis, malaria, dengue fever, scrub typhus, rickettsial disease, and typhoid fever (see Table 2).10 Thus, when a patient presents with these symptoms, it is imperative that the clinician consider leptospirosis.19Of note: Flu-like symptoms with conjunctival suffusion are considered pathognomonic for leptospirosis.18
About 10% of patients with early-phase leptospirosis will develop late-phase disease (ie, Weil’s disease), with severe symptoms that include jaundice, meningitis, pulmonary hemorrhage, and acute kidney injury (see Table 3 for a more detailed list).20 The case patient’s history and symptoms were consistent with a diagnosis of early-phase leptospirosis.
Epidemiology
In 2015, leptospirosis was estimated to affect more than 1 million persons worldwide, with 58,900 deaths attributed to the disease each year—making leptospirosis the leading cause of death attributable to zoonotic illness.11 Historically, leptospirosis-associated morbidity and mortality have been greatest in resource-poor countries with tropical climates (eg, southern and Southeast Asia, Central America and tropical Latin America, and East Sub-Saharan Africa).11,12
However, illness resulting from recreational exposures to contaminated water has been linked to increasing travel to exotic destinations, participation in adventure travel, and the growing popularity of extreme sports involving fresh water.9 Recreational mud run events, for example, involve swimming in potentially contaminated waters and crawling through flooded farm fields where animal urine can be present—an ideal environment for Leptospira to thrive and for participants to contract the disease.14,15
Continue for laboratory work-up >>
Laboratory work-up
Diagnosis of leptospirosis is challenging.21 Laboratory tests vary, depending on the timing and stage of infection, and are mostly unavailable in resource-poor countries. Test results for the patient with early-phase leptospirosis may demonstrate renal or hepatic abnormalities.18 However, laboratory confirmation of leptospirosis requires22
• A fourfold increase in antibody titer between acute and convalescent serum samples, as detected by microscopic agglutination testing (MAT) or
• A high MAT titer (> 1:400 to 1:800), in single or paired samples or
• Isolation of pathogenic Leptospira species from a normally sterile site or
• Detection of DNA from pathogenic Leptospira species by PCR
A positive laboratory result is, of course, confirmatory. However, negative laboratory findings must be viewed with healthy skepticism.12 A false-negative result may merely indicate the shortcoming of the testing method to accurately assess the presence of Leptospira.
Treatment options
The high mortality rate associated with severe leptospirosis makes early diagnosis and treatment essential.23 The World Health Organization warns that antibiotic treatment for leptospirosis must be instituted within five days of symptom onset.10
Treatment options for an ambulatory patient with mild symptoms and no organ involvement include oral doxycycline (100 mg bid for 5-7 d) or oral azithromycin (500 mg/d for 5-7 d). For patients with organ involvement, IV penicillin (1.5 million U every 6 h for 7 d), ceftriaxone (1 g/d for 7 d), or cefotaxime (1 g every 6 h for 7 d) may be considered.12,20
OUTCOME FOR THE CASE PATIENT
With leptospirosis as the diagnosis of exclusion, Jane was treated successfully with a 21-day course of oral doxycycline (100 mg bid). She has been symptom free since completing the regimen. After undergoing physical therapy and athletic training, she has been able to resume her full exercise regimen, and her recovery is considered complete.
CONCLUSION
The growing popularity of adventure travel and “extreme sports” events, particularly triathlons and mud runs, may precipitate an increase in associated infections with Leptospira and other zoonotic pathogens. For patients with flulike symptoms who routinely engage in such sports—especially those who present with conjunctival suffusion—leptospirosis should be considered in the differential diagnosis.
REFERENCES
1. Owens RC Jr, Ambrose PG. Antimicrobial safety: focus on fluoroquinolones. Clin Infect Dis. 2005;41(suppl 2):S144-S157.
2. CDC. Signs and symptoms of untreated Lyme disease (2015). www.cdc.gov/lyme/signs_symptoms/index.html. Accessed June 7, 2016.
3. CDC. Parasites: babesiosis (2014). www.cdc.gov/parasites/babesiosis/disease.html. Accessed June 7, 2016.
4. CDC. Ehrlichiosis: symptoms, diagnosis, and treatment (2013). www.cdc.gov/Ehrlichiosis/symptoms/index.html. Accessed June 7, 2016.
5. Pritt BS, Mead PS, Johnson DK, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. 2016 Feb 5. [Epub ahead of print]
6. Choi E, Pyzocha NJ, Maurer DM. Tick-borne illnesses. Curr Sports Med Rep. 2016;15(2):98-104.
7. Chomel B. Lyme disease. Rev Sci Tech. 2015;34(2):569-576.
8. Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14(2):296-326.
9. CDC. Leptospirosis: signs and symptoms (2016). www.cdc.gov/leptospirosis/symptoms/index.html. Accessed June 7, 2016.
10. World Health Organization, International Leptospirosis Society. Human Leptospirosis: Guidance for Diagnosis, Surveillance, and Control (2003). http://apps.who.int/iris/bitstream/10665/42667/1/WHO_CDS_CSR_EPH_2002.23.pdf. Accessed June 7, 2016.
11. Costa F, Hagan JE, Calcagno J, et al. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003898.
12. Haake DA, Levett PN. Leptospirosis in humans. Curr Top Microbiol Immunol. 2015;387:65-97.
13. Picardeau M. Diagnosis and epidemiology of leptospirosis. Médecine et Maladies Infectieuses. 2013;43(1):1-9.
14. Picardeau M. Leptospirosis: updating the global picture of an emerging neglected disease. PLoS Negl Trop Dis. 2015;9(9):e0004039.
15. Zavitsanou A, Babatsikou F. Leptospirosis: epidemiology and preventive measures. Health Sci J. 2008;2(2):75-82.
16. Mwachui MA, Crump L, Hartskeerl R, et al. Environmental and behavioural determinants of leptospirosis transmission: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003843.
17. Morgan J, Bornstein SL, Karpati AM, et al. Outbreak of leptospirosis among triathlon participants and community residents in Springfield, Illinois, 1998. Clin Infect Dis. 2002;34(12):1593-1599.
18. Katz AR, Ansdell VE, Effler PV, et al. Assessment of the clinical presentation and treatment of 353 cases of laboratory-confirmed leptospirosis in Hawaii, 1974-1998. Clin Infect Dis. 2001;33(11):1834-1841.
19. Yaakob Y, Rodrigues KF, John DV. Leptospirosis: recent incidents and available diagnostics—a review. Med J Malaysia. 2015;70(6):351-355.
20. Seguro AC, Andrade L. Pathophysiology of leptospirosis. Shock. 2013;39(suppl 1):17-23.
21. Musso D, La Scola B. Laboratory diagnosis of leptospirosis: a challenge. J Microbiol Immunol Infect. 2013;46(4):245-252.
22. Waggoner JJ, Balassiano I, Mohamed-Hadley A, et al. Reverse-transcriptase PCR detection of Leptospira: absence of agreement with single-specimen microscopic agglutination testing. PLoS One. 2015;10(7):e0132988.
23. Iwasaki H, Chagan-Yasutan H, Leano PS, et al. Combined antibody and DNA detection for early diagnosis of leptospirosis after a disaster. Diagn Microbiol Infect Dis. 2016;84(4):287-291
The Relationship Between Sustained Gripping and the Development of Carpal Tunnel Syndrome
The dominant limb is the limb preferred for performing an activity that requires one hand or for performing the more demanding part of an activity that requires both hands. For example, most playing card dealers use their dominant limb to distribute cards (the more demanding part of the activity) and their nondominant limb to hold the rest of the pack (the less demanding activity). Although a relationship between nocturnal hand paresthesias and daily hand activities has been known for more than a century, it was not until more recently that it was recognized that unilateral carpal tunnel syndrome (CTS) more commonly involves the dominant limb.1,2
Among people with CTS, the dominant limb tends to be affected earlier and, in the setting of bilateral involvement, more severely.3,4 This relationship, however, is not absolute. In 1983, Falck and Aarnio reported that CTS could be more pronounced on the nondominant side whenever upper extremity usage requirements, especially occupational requirements, stressed that limb to a greater extent than they stressed the dominant limb.5
Regarding occupation, particular CTS risk factors and associations have been reported. One study found that the most common work-related risk factor was repetitive bending and twisting of the hands and wrists.6 In another study, the incidence of CTS was almost 10-fold higher among workers performing high force, high repetition jobs than among those performing low force, low repetition jobs.7-10 A meta-analysis identified a strong causal relationship between forceful, repetitive work and development of CTS.11 A more recent and controversial study found no association between heavy use of computers and CTS.12 In 1911, Hart reported an association between repetitive gripping and thenar atrophy.13 Although he misattributed the association to trauma of the recurrent thenar motor branch, 2 of the 3 described patients reported a period of episodic hand paresthesias preceding the development of thenar eminence atrophy and thus more likely had typical CTS.
Background
The present study was prompted by the clinical and electrodiagnostic (EDX) features of a 27-year-old right-hand–dominant man who presented to the EDX laboratory for assessment of bilateral hand paresthesias. The patient reported episodic bilateral hand tingling that was much more pronounced on the left (nondominant) side. Consistent with his report, EDX assessment revealed bilateral CTS that involved the nondominant limb to a much greater extent than that of the dominant limb. As a blackjack dealer, the patient was using his nondominant hand to “tightly grip 2 decks of cards” and the dominant hand to distribute those cards.
Similar history and EDX patterns (bilateral CTS more pronounced on nondominant side) were subsequently noted in 2 other patients, both of whom were using their nondominant limb to perform an activity that required sustained gripping. One of these patients was a minnow counter. He was using his nondominant hand to firmly grip the top of a bucket and the dominant hand to “deal” the fish into separate tanks. The other patient was a mason. He was using his nondominant hand to firmly hold a brick or stone in place and the dominant hand to apply cement. The clinical and EDX features of these 3 patients suggested that sustained gripping might be a significant risk factor for development of CTS. That all 3 of these patients were using their dominant hand for a repetitive activity (dealing) further suggested that, compared with repetitive activity, sustained gripping was more significant as a risk factor for development of CTS.
As unilateral CTS typically occurs on the dominant side, and bilateral CTS typically is more pronounced on the dominant side, the term backward CTS is applied to cases in which unilateral CTS occurs on the nondominant side or bilateral CTS involves the nondominant side to a greater extent than the dominant side.
Although many investigators have purported an association between CTS and a particular upper extremity activity, their conclusions are limited by use of poorly validated symptom surveys, use of faulty epidemiologic methods, selection of a specific basis for clinical diagnosis (eg, isolated hand pain), or lack of EDX confirmation. Associations between a particular activity and development of CTS are best addressed by studies that include both clinical and EDX assessments and that fully characterize the individual hand usage patterns.
Methods
This study identified the upper extremity usage patterns associated with development of CTS among patients found in the EDX laboratory to have backward CTS (unilateral CTS in nondominant limb or bilateral CTS involving nondominant limb more than dominant limb). Thus, whenever patients who were referred to the EDX laboratory for upper extremity studies were noted to have backward CTS, an extensive upper extremity usage assessment was immediately performed. Both the EDX studies and the upper extremity usage assessments were performed by the author during the same encounter.
All patients had initial screening sensory and motor nerve conduction studies performed: median sensory, recording the second digit; ulnar sensory, recording the fifth digit; superficial radial, recording the dorsum of hand; median motor, recording the thenar eminence; and ulnar motor, recording the hypothenar eminence. As CTS was suspected in all cases, median and ulnar palmar nerve conduction studies were performed as well. All these studies were performed using previously reported techniques, and all collected values were compared with EMG laboratory control values.14,15 In all patients, the median nerve conduction studies were performed bilaterally. Approval from an ethics board or an institutional review board was not needed because this study did not involve personal information or identifiable images.
To avoid identifying small, chance asymmetries related to hypothyroidism and other conditions that produce bilateral CTS, the author predefined the degree of asymmetry required for study inclusion to identify only large asymmetries. Because the EDX manifestations of CTS typically reflect features of demyelination before those of axon loss, the required asymmetries were predefined using peak sensory and distal motor latency values. For study inclusion, the median nerve latency value recorded from the nondominant limb needed to exceed the value recorded from the dominant limb by 0.6 msec for the median palmar responses, 1.0 msec for the median digital sensory responses, or 1.0 msec for the median motor responses.
Excluded from the study were patients who reported being ambidextrous, those who had changed hand dominance at any age and for any reason, those with a history of upper extremity trauma or surgery, and those with EDX findings indicating a concomitant neuromuscular disorder. In addition, patients with diabetes mellitus or any other condition associated with bilateral CTS were excluded.
Results
From the approximately 2,000 upper extremity EDX studies performed over a 30-month period, the author identified 21 patients who met the inclusion criteria (Table 1). Of these 21 patients, 15 (71%) had bilateral CTS and 6 (29%) had unilateral CTS. Sixteen of the 21 patients used their nondominant hand, through a significant portion of the day, to perform an activity that required sustained gripping (Table 2).
Of these 16 patients, 14 reported that the sustained gripping activity was related to their occupation: pipe fitter (4 patients), card dealer (4), professional driver (2), grocery store clerk (1), wire stripper (1), bakery worker (1), and motel room cleaner (1). In their jobs, the pipe fitters were continually cutting pipe during their entire 8-hour shift—using the nondominant hand to tightly grip a pipe while using the dominant hand to direct an electrically powered blade through it. Of the card dealers, 1 was a professional playing card dealer (not the dealer whose case prompted this study), 1 distributed store coupons into containers, and 2 distributed pieces of mail into bins (referred to as casing the mail). All the card dealers used their nondominant hand to tightly grip items that the dominant limb distributed. The professional drivers used their nondominant hand to grip the steering wheel. The grocery store clerk used her nondominant hand to grip shopping items while moving them across a barcode detector. The wire stripper used her nondominant hand to tightly grip bundles of wire while holding a tool in the dominant hand to snip or strip them. The bakery worker continually used her nondominant hand to squeeze off pieces of dough from a mound. And the motel room cleaner used her nondominant hand to grip the side of a bathtub while scrubbing the tub with her dominant hand (she estimated she cleaned bathtubs for about 25% of her 8-hour shift).
Of the 2 patients who reported sustained gripping unrelated to occupation, 1 was baby-sitting her grandson 5 days per week. She carried him, grasping his buttock with her nondominant hand, while performing her daily activities. She estimated she carried the child a minimum of 2 hours a day. After several weeks, she noted episodic tingling in the nondominant hand, yet she continued carrying him for another 7 months, at which point she sought medical care. The other patient, a student in a stress relief class, was instructed to repetitively open and tightly close her nondominant hand for 10 minutes 4 or more times per day. After several weeks, she noted episodic tingling in the exercised, nondominant hand.
Of the 5 patients who denied performing an activity that required sustained gripping, 2 used their nondominant limb to enter data into a computer while turning pages with the dominant limb. A piano teacher, used her nondominant limb to strike piano keys while sitting to the right of her pupils; and a typist, consistently slept with the dorsal aspect of the nondominant hand pressed into her cheek, resulting in sustained wrist flexion throughout the night. One patient could not identify an activity performed with her nondominant limb both frequently and for prolonged periods.
Discussion
As with other syndromic disorders, CTS is associated with several clinical features, the presence of which correlates with the severity of median nerve involvement. During the earliest stage of CTS, episodic hand tingling (a positive symptom) is commonly reported. This tingling typically is more pronounced at night and during relaxation. In addition, many patients come to recognize that their hand tingling is precipitated by activities that involve sustained upper extremity elevation (eg, driving with a limb resting on upper portion of steering wheel; reading with upper extremities maintained in forward abduction) and that lowering a symptomatic limb relieves its tingling.
With progression, negative symptoms appear (eg, numbness and then weakness and wasting). Unfortunately, as the negative symptoms replace the positive ones, affected individuals may become less symptomatic and mistakenly believe their condition is improving. Features of autonomic fiber involvement may also be present but are less reliably elicited. Isolated hand pain is an uncommon manifestation of CTS because pain more commonly occurs later in the course and for this reason tends to be accompanied by other features of CTS.
The clinical features of CTS correlate with its underlying pathology. As demyelination precedes axon disruption pathologically, the clinical features of demyelination (episodic paresthesias) precede those of axon loss (numbness, weakness, wasting). However, clinical features may go unrecognized or be dismissed by the patient. Moreover, there is substantial variation in type, intensity, and frequency of symptoms.16,17
The EDX features of CTS correlate with its underlying pathology and pathophysiology. As demyelination (loss of insulation) increases the capacitance of the membrane and increases internodal current leakage, conduction velocity is reduced. As severity worsens and pathology changes from predominantly demyelination to predominantly axon loss, the individual nerve fiber action potentials, which make up the compound responses being recorded, are lost. As a result the amplitude and negative area under the curve values decrease. Thus, the EDX features of demyelination (eg, prolonged latencies) precede those of axon loss (eg, amplitude, negative area under the curve reduction).
As with other focal mononeuropathies, the sensory responses tend to be affected earlier and to a greater degree than do the motor responses. Consequently, the EDX features of CTS typically follow a standard progression. The median palmar responses are involved sooner and to a greater degree than the median sensory responses recorded from the digits, which in turn tend to be involved earlier and to a greater degree than are the median motor responses.
Awareness of this relationship dictates the severity of the lesion and helps in the recognition of a cool limb and in the avoidance of a false-positive study interpretation. In a cool limb, the fingers are cooler than the wrists. Thus, the peak latency of the median digital sensory response is delayed to a greater extent than the ipsilateral median palmar response (the opposite of the CTS pattern). Accordingly, whenever this pattern is identified, the hand must be warmed or rewarmed and the studies repeated. The hand is also warmed or rewarmed whenever the median motor response is delayed out of proportion to that of the median palmar response.
Conclusion
Cases of CTS mainly in the nondominant limb provide an opportunity to identify particular limb usage patterns that might be associated with CTS. Of the present study’s 21 affected patients, 16 were using their nondominant limb to perform activities that required sustained gripping. Fourteen of the 16 activities were related to occupation. These findings strongly suggest an association between activities that require sustained gripping and development of CTS.
That the card dealers simultaneously used their nondominant hand for sustained gripping and the dominant hand for the repetitive activity of dealing suggests that sustained gripping is a stronger risk factor than repetitive activity for the development of CTS—an unanticipated finding. Interestingly, in a 2001 study that suggested repetitive activity might not be a CTS risk factor, there was a higher incidence of CTS among computer users working with a mouse—an activity that requires sustained gripping.12
Episodic hand tingling during mouse use likely reflects impaired blood flow to the median nerve, which occurs when carpal tunnel pressure approaches or exceeds 20 to 30 mm Hg.18 Placement of a hand on a mouse increases intracarpal pressure from 3 to 5 mm Hg (wrist in neutral position) to 16 to 21 mm Hg, whereas mouse use increases intracarpal pressure to 28 to 33 mm Hg.18-20
1. Ormerod JA. On a peculiar numbness and paresis of the hands. St Barts Hosp Rep. 1883;19:17-26.
2. Rosenbaum RB, Ochoa JL. Carpal Tunnel Syndrome and Other Disorders of the Median Nerve. 2nd ed. Boston, MA: Butterworth-Heineman; 2002.
3. Gainer JV Jr, Nugent GR. Carpal tunnel syndrome: report of 430 operations. South Med J. 1977;70(3):325-328.
4. Reinstein L. Hand dominance in carpal tunnel syndrome. Arch Phys Med Rehabil. 1981;62(5):202-203.
5. Falck B, Aarnio P. Left-sided carpal tunnel syndrome in butchers. Scand J Work Environ Health. 1983;9(3):291-297.
6. Tanaka S, Wild DK, Seligman PJ, Halperin WE, Behrens VJ, Putz-Anerson V. Prevalence and work-relatedness of self-reported carpal tunnel syndrome among U.S. workers: analysis of the Occupational Health Supplement data of 1988 National Health Interview Survey. Am J Ind Med. 1995;27(4):451-470.
7. Silverstein BA, Fine LJ, Armstrong TJ. Occupational factors and carpal tunnel syndrome. Am J Ind Med. 1987;11(3):343-358.
8. de Krom MC, Kester AD, Knipschild PG, Spaans F. Risk factors for carpal tunnel syndrome. Am J Epidemiol. 1990;132(6):1102-1110.
9. Hales TR, Bernard BP. Epidemiology of work-related musculoskeletal disorders. Orthop Clin North Am. 1996;27(4):679-709.
10. Roquelaure Y, Ha C, Pelier-Cady MC, et al. Work increases the incidence of carpal tunnel syndrome in the general population. Muscle Nerve. 2008;37(4):477-482.
11. Stock SR. Workplace ergonomic factors and the development of musculoskeletal disorders of the neck and upper limbs: a meta-analysis. Am J Ind Med. 1991;19(1):87-107.
12. Stevens JC, Witt JC, Smith BE, Weaver AL. The frequency of carpal tunnel syndrome in computer users at a medical facility. Neurology. 2001;56(11):1568-1570.
13. Hart JR. The thenar and hypothenar types of neural atrophy of the hand. Am J Med Sci. 1911;141:224-241.
14. Ferrante MA, Parry GJ, Wilbourn AJ. Sensory nerve conduction studies. Paper presented at: 51st Annual Meeting of the American Academy of Neurology; April 1999; Toronto, Canada.
15. Litchy WJ, Miller RG, Shields RW. Motor nerve conduction studies. Paper presented at: 51st Annual Meeting of the American Academy of Neurology; April 1999; Toronto, Canada.
16. Nunez F, Vranceanu AM, Ring D. Determinants of pain in patients with carpal tunnel syndrome. Clin Orthop Relat Res. 2010;468(12):3328-3332.
17. van Suchtelen M, Beck SJ, Gruber JS, Ring D. Progression of carpal tunnel syndrome according to electrodiagnostic testing in nonoperatively treated patients. Arch Bone Jt Surg. 2014;2(3):185-191.
18. Ghasemi-Rad M, Nosair E, Vegh A, et al. A handy review of carpal tunnel syndrome: from anatomy to diagnosis and treatment. World J Radiol. 2014;6(6):284-300.
19. Rydevik B, Lundborg G, Bagge U. Effects of graded compression on intraneural blood flow. An in vivo study on rabbit tibial nerve. J Hand Surg Am. 1981;6(1):3-12.
20. Keir PJ, Bach JM, Rempel D. Effects of computer mouse design and task on carpal tunnel pressure. Ergonomics. 1999;42(10):1350-1360.=
The dominant limb is the limb preferred for performing an activity that requires one hand or for performing the more demanding part of an activity that requires both hands. For example, most playing card dealers use their dominant limb to distribute cards (the more demanding part of the activity) and their nondominant limb to hold the rest of the pack (the less demanding activity). Although a relationship between nocturnal hand paresthesias and daily hand activities has been known for more than a century, it was not until more recently that it was recognized that unilateral carpal tunnel syndrome (CTS) more commonly involves the dominant limb.1,2
Among people with CTS, the dominant limb tends to be affected earlier and, in the setting of bilateral involvement, more severely.3,4 This relationship, however, is not absolute. In 1983, Falck and Aarnio reported that CTS could be more pronounced on the nondominant side whenever upper extremity usage requirements, especially occupational requirements, stressed that limb to a greater extent than they stressed the dominant limb.5
Regarding occupation, particular CTS risk factors and associations have been reported. One study found that the most common work-related risk factor was repetitive bending and twisting of the hands and wrists.6 In another study, the incidence of CTS was almost 10-fold higher among workers performing high force, high repetition jobs than among those performing low force, low repetition jobs.7-10 A meta-analysis identified a strong causal relationship between forceful, repetitive work and development of CTS.11 A more recent and controversial study found no association between heavy use of computers and CTS.12 In 1911, Hart reported an association between repetitive gripping and thenar atrophy.13 Although he misattributed the association to trauma of the recurrent thenar motor branch, 2 of the 3 described patients reported a period of episodic hand paresthesias preceding the development of thenar eminence atrophy and thus more likely had typical CTS.
Background
The present study was prompted by the clinical and electrodiagnostic (EDX) features of a 27-year-old right-hand–dominant man who presented to the EDX laboratory for assessment of bilateral hand paresthesias. The patient reported episodic bilateral hand tingling that was much more pronounced on the left (nondominant) side. Consistent with his report, EDX assessment revealed bilateral CTS that involved the nondominant limb to a much greater extent than that of the dominant limb. As a blackjack dealer, the patient was using his nondominant hand to “tightly grip 2 decks of cards” and the dominant hand to distribute those cards.
Similar history and EDX patterns (bilateral CTS more pronounced on nondominant side) were subsequently noted in 2 other patients, both of whom were using their nondominant limb to perform an activity that required sustained gripping. One of these patients was a minnow counter. He was using his nondominant hand to firmly grip the top of a bucket and the dominant hand to “deal” the fish into separate tanks. The other patient was a mason. He was using his nondominant hand to firmly hold a brick or stone in place and the dominant hand to apply cement. The clinical and EDX features of these 3 patients suggested that sustained gripping might be a significant risk factor for development of CTS. That all 3 of these patients were using their dominant hand for a repetitive activity (dealing) further suggested that, compared with repetitive activity, sustained gripping was more significant as a risk factor for development of CTS.
As unilateral CTS typically occurs on the dominant side, and bilateral CTS typically is more pronounced on the dominant side, the term backward CTS is applied to cases in which unilateral CTS occurs on the nondominant side or bilateral CTS involves the nondominant side to a greater extent than the dominant side.
Although many investigators have purported an association between CTS and a particular upper extremity activity, their conclusions are limited by use of poorly validated symptom surveys, use of faulty epidemiologic methods, selection of a specific basis for clinical diagnosis (eg, isolated hand pain), or lack of EDX confirmation. Associations between a particular activity and development of CTS are best addressed by studies that include both clinical and EDX assessments and that fully characterize the individual hand usage patterns.
Methods
This study identified the upper extremity usage patterns associated with development of CTS among patients found in the EDX laboratory to have backward CTS (unilateral CTS in nondominant limb or bilateral CTS involving nondominant limb more than dominant limb). Thus, whenever patients who were referred to the EDX laboratory for upper extremity studies were noted to have backward CTS, an extensive upper extremity usage assessment was immediately performed. Both the EDX studies and the upper extremity usage assessments were performed by the author during the same encounter.
All patients had initial screening sensory and motor nerve conduction studies performed: median sensory, recording the second digit; ulnar sensory, recording the fifth digit; superficial radial, recording the dorsum of hand; median motor, recording the thenar eminence; and ulnar motor, recording the hypothenar eminence. As CTS was suspected in all cases, median and ulnar palmar nerve conduction studies were performed as well. All these studies were performed using previously reported techniques, and all collected values were compared with EMG laboratory control values.14,15 In all patients, the median nerve conduction studies were performed bilaterally. Approval from an ethics board or an institutional review board was not needed because this study did not involve personal information or identifiable images.
To avoid identifying small, chance asymmetries related to hypothyroidism and other conditions that produce bilateral CTS, the author predefined the degree of asymmetry required for study inclusion to identify only large asymmetries. Because the EDX manifestations of CTS typically reflect features of demyelination before those of axon loss, the required asymmetries were predefined using peak sensory and distal motor latency values. For study inclusion, the median nerve latency value recorded from the nondominant limb needed to exceed the value recorded from the dominant limb by 0.6 msec for the median palmar responses, 1.0 msec for the median digital sensory responses, or 1.0 msec for the median motor responses.
Excluded from the study were patients who reported being ambidextrous, those who had changed hand dominance at any age and for any reason, those with a history of upper extremity trauma or surgery, and those with EDX findings indicating a concomitant neuromuscular disorder. In addition, patients with diabetes mellitus or any other condition associated with bilateral CTS were excluded.
Results
From the approximately 2,000 upper extremity EDX studies performed over a 30-month period, the author identified 21 patients who met the inclusion criteria (Table 1). Of these 21 patients, 15 (71%) had bilateral CTS and 6 (29%) had unilateral CTS. Sixteen of the 21 patients used their nondominant hand, through a significant portion of the day, to perform an activity that required sustained gripping (Table 2).
Of these 16 patients, 14 reported that the sustained gripping activity was related to their occupation: pipe fitter (4 patients), card dealer (4), professional driver (2), grocery store clerk (1), wire stripper (1), bakery worker (1), and motel room cleaner (1). In their jobs, the pipe fitters were continually cutting pipe during their entire 8-hour shift—using the nondominant hand to tightly grip a pipe while using the dominant hand to direct an electrically powered blade through it. Of the card dealers, 1 was a professional playing card dealer (not the dealer whose case prompted this study), 1 distributed store coupons into containers, and 2 distributed pieces of mail into bins (referred to as casing the mail). All the card dealers used their nondominant hand to tightly grip items that the dominant limb distributed. The professional drivers used their nondominant hand to grip the steering wheel. The grocery store clerk used her nondominant hand to grip shopping items while moving them across a barcode detector. The wire stripper used her nondominant hand to tightly grip bundles of wire while holding a tool in the dominant hand to snip or strip them. The bakery worker continually used her nondominant hand to squeeze off pieces of dough from a mound. And the motel room cleaner used her nondominant hand to grip the side of a bathtub while scrubbing the tub with her dominant hand (she estimated she cleaned bathtubs for about 25% of her 8-hour shift).
Of the 2 patients who reported sustained gripping unrelated to occupation, 1 was baby-sitting her grandson 5 days per week. She carried him, grasping his buttock with her nondominant hand, while performing her daily activities. She estimated she carried the child a minimum of 2 hours a day. After several weeks, she noted episodic tingling in the nondominant hand, yet she continued carrying him for another 7 months, at which point she sought medical care. The other patient, a student in a stress relief class, was instructed to repetitively open and tightly close her nondominant hand for 10 minutes 4 or more times per day. After several weeks, she noted episodic tingling in the exercised, nondominant hand.
Of the 5 patients who denied performing an activity that required sustained gripping, 2 used their nondominant limb to enter data into a computer while turning pages with the dominant limb. A piano teacher, used her nondominant limb to strike piano keys while sitting to the right of her pupils; and a typist, consistently slept with the dorsal aspect of the nondominant hand pressed into her cheek, resulting in sustained wrist flexion throughout the night. One patient could not identify an activity performed with her nondominant limb both frequently and for prolonged periods.
Discussion
As with other syndromic disorders, CTS is associated with several clinical features, the presence of which correlates with the severity of median nerve involvement. During the earliest stage of CTS, episodic hand tingling (a positive symptom) is commonly reported. This tingling typically is more pronounced at night and during relaxation. In addition, many patients come to recognize that their hand tingling is precipitated by activities that involve sustained upper extremity elevation (eg, driving with a limb resting on upper portion of steering wheel; reading with upper extremities maintained in forward abduction) and that lowering a symptomatic limb relieves its tingling.
With progression, negative symptoms appear (eg, numbness and then weakness and wasting). Unfortunately, as the negative symptoms replace the positive ones, affected individuals may become less symptomatic and mistakenly believe their condition is improving. Features of autonomic fiber involvement may also be present but are less reliably elicited. Isolated hand pain is an uncommon manifestation of CTS because pain more commonly occurs later in the course and for this reason tends to be accompanied by other features of CTS.
The clinical features of CTS correlate with its underlying pathology. As demyelination precedes axon disruption pathologically, the clinical features of demyelination (episodic paresthesias) precede those of axon loss (numbness, weakness, wasting). However, clinical features may go unrecognized or be dismissed by the patient. Moreover, there is substantial variation in type, intensity, and frequency of symptoms.16,17
The EDX features of CTS correlate with its underlying pathology and pathophysiology. As demyelination (loss of insulation) increases the capacitance of the membrane and increases internodal current leakage, conduction velocity is reduced. As severity worsens and pathology changes from predominantly demyelination to predominantly axon loss, the individual nerve fiber action potentials, which make up the compound responses being recorded, are lost. As a result the amplitude and negative area under the curve values decrease. Thus, the EDX features of demyelination (eg, prolonged latencies) precede those of axon loss (eg, amplitude, negative area under the curve reduction).
As with other focal mononeuropathies, the sensory responses tend to be affected earlier and to a greater degree than do the motor responses. Consequently, the EDX features of CTS typically follow a standard progression. The median palmar responses are involved sooner and to a greater degree than the median sensory responses recorded from the digits, which in turn tend to be involved earlier and to a greater degree than are the median motor responses.
Awareness of this relationship dictates the severity of the lesion and helps in the recognition of a cool limb and in the avoidance of a false-positive study interpretation. In a cool limb, the fingers are cooler than the wrists. Thus, the peak latency of the median digital sensory response is delayed to a greater extent than the ipsilateral median palmar response (the opposite of the CTS pattern). Accordingly, whenever this pattern is identified, the hand must be warmed or rewarmed and the studies repeated. The hand is also warmed or rewarmed whenever the median motor response is delayed out of proportion to that of the median palmar response.
Conclusion
Cases of CTS mainly in the nondominant limb provide an opportunity to identify particular limb usage patterns that might be associated with CTS. Of the present study’s 21 affected patients, 16 were using their nondominant limb to perform activities that required sustained gripping. Fourteen of the 16 activities were related to occupation. These findings strongly suggest an association between activities that require sustained gripping and development of CTS.
That the card dealers simultaneously used their nondominant hand for sustained gripping and the dominant hand for the repetitive activity of dealing suggests that sustained gripping is a stronger risk factor than repetitive activity for the development of CTS—an unanticipated finding. Interestingly, in a 2001 study that suggested repetitive activity might not be a CTS risk factor, there was a higher incidence of CTS among computer users working with a mouse—an activity that requires sustained gripping.12
Episodic hand tingling during mouse use likely reflects impaired blood flow to the median nerve, which occurs when carpal tunnel pressure approaches or exceeds 20 to 30 mm Hg.18 Placement of a hand on a mouse increases intracarpal pressure from 3 to 5 mm Hg (wrist in neutral position) to 16 to 21 mm Hg, whereas mouse use increases intracarpal pressure to 28 to 33 mm Hg.18-20
The dominant limb is the limb preferred for performing an activity that requires one hand or for performing the more demanding part of an activity that requires both hands. For example, most playing card dealers use their dominant limb to distribute cards (the more demanding part of the activity) and their nondominant limb to hold the rest of the pack (the less demanding activity). Although a relationship between nocturnal hand paresthesias and daily hand activities has been known for more than a century, it was not until more recently that it was recognized that unilateral carpal tunnel syndrome (CTS) more commonly involves the dominant limb.1,2
Among people with CTS, the dominant limb tends to be affected earlier and, in the setting of bilateral involvement, more severely.3,4 This relationship, however, is not absolute. In 1983, Falck and Aarnio reported that CTS could be more pronounced on the nondominant side whenever upper extremity usage requirements, especially occupational requirements, stressed that limb to a greater extent than they stressed the dominant limb.5
Regarding occupation, particular CTS risk factors and associations have been reported. One study found that the most common work-related risk factor was repetitive bending and twisting of the hands and wrists.6 In another study, the incidence of CTS was almost 10-fold higher among workers performing high force, high repetition jobs than among those performing low force, low repetition jobs.7-10 A meta-analysis identified a strong causal relationship between forceful, repetitive work and development of CTS.11 A more recent and controversial study found no association between heavy use of computers and CTS.12 In 1911, Hart reported an association between repetitive gripping and thenar atrophy.13 Although he misattributed the association to trauma of the recurrent thenar motor branch, 2 of the 3 described patients reported a period of episodic hand paresthesias preceding the development of thenar eminence atrophy and thus more likely had typical CTS.
Background
The present study was prompted by the clinical and electrodiagnostic (EDX) features of a 27-year-old right-hand–dominant man who presented to the EDX laboratory for assessment of bilateral hand paresthesias. The patient reported episodic bilateral hand tingling that was much more pronounced on the left (nondominant) side. Consistent with his report, EDX assessment revealed bilateral CTS that involved the nondominant limb to a much greater extent than that of the dominant limb. As a blackjack dealer, the patient was using his nondominant hand to “tightly grip 2 decks of cards” and the dominant hand to distribute those cards.
Similar history and EDX patterns (bilateral CTS more pronounced on nondominant side) were subsequently noted in 2 other patients, both of whom were using their nondominant limb to perform an activity that required sustained gripping. One of these patients was a minnow counter. He was using his nondominant hand to firmly grip the top of a bucket and the dominant hand to “deal” the fish into separate tanks. The other patient was a mason. He was using his nondominant hand to firmly hold a brick or stone in place and the dominant hand to apply cement. The clinical and EDX features of these 3 patients suggested that sustained gripping might be a significant risk factor for development of CTS. That all 3 of these patients were using their dominant hand for a repetitive activity (dealing) further suggested that, compared with repetitive activity, sustained gripping was more significant as a risk factor for development of CTS.
As unilateral CTS typically occurs on the dominant side, and bilateral CTS typically is more pronounced on the dominant side, the term backward CTS is applied to cases in which unilateral CTS occurs on the nondominant side or bilateral CTS involves the nondominant side to a greater extent than the dominant side.
Although many investigators have purported an association between CTS and a particular upper extremity activity, their conclusions are limited by use of poorly validated symptom surveys, use of faulty epidemiologic methods, selection of a specific basis for clinical diagnosis (eg, isolated hand pain), or lack of EDX confirmation. Associations between a particular activity and development of CTS are best addressed by studies that include both clinical and EDX assessments and that fully characterize the individual hand usage patterns.
Methods
This study identified the upper extremity usage patterns associated with development of CTS among patients found in the EDX laboratory to have backward CTS (unilateral CTS in nondominant limb or bilateral CTS involving nondominant limb more than dominant limb). Thus, whenever patients who were referred to the EDX laboratory for upper extremity studies were noted to have backward CTS, an extensive upper extremity usage assessment was immediately performed. Both the EDX studies and the upper extremity usage assessments were performed by the author during the same encounter.
All patients had initial screening sensory and motor nerve conduction studies performed: median sensory, recording the second digit; ulnar sensory, recording the fifth digit; superficial radial, recording the dorsum of hand; median motor, recording the thenar eminence; and ulnar motor, recording the hypothenar eminence. As CTS was suspected in all cases, median and ulnar palmar nerve conduction studies were performed as well. All these studies were performed using previously reported techniques, and all collected values were compared with EMG laboratory control values.14,15 In all patients, the median nerve conduction studies were performed bilaterally. Approval from an ethics board or an institutional review board was not needed because this study did not involve personal information or identifiable images.
To avoid identifying small, chance asymmetries related to hypothyroidism and other conditions that produce bilateral CTS, the author predefined the degree of asymmetry required for study inclusion to identify only large asymmetries. Because the EDX manifestations of CTS typically reflect features of demyelination before those of axon loss, the required asymmetries were predefined using peak sensory and distal motor latency values. For study inclusion, the median nerve latency value recorded from the nondominant limb needed to exceed the value recorded from the dominant limb by 0.6 msec for the median palmar responses, 1.0 msec for the median digital sensory responses, or 1.0 msec for the median motor responses.
Excluded from the study were patients who reported being ambidextrous, those who had changed hand dominance at any age and for any reason, those with a history of upper extremity trauma or surgery, and those with EDX findings indicating a concomitant neuromuscular disorder. In addition, patients with diabetes mellitus or any other condition associated with bilateral CTS were excluded.
Results
From the approximately 2,000 upper extremity EDX studies performed over a 30-month period, the author identified 21 patients who met the inclusion criteria (Table 1). Of these 21 patients, 15 (71%) had bilateral CTS and 6 (29%) had unilateral CTS. Sixteen of the 21 patients used their nondominant hand, through a significant portion of the day, to perform an activity that required sustained gripping (Table 2).
Of these 16 patients, 14 reported that the sustained gripping activity was related to their occupation: pipe fitter (4 patients), card dealer (4), professional driver (2), grocery store clerk (1), wire stripper (1), bakery worker (1), and motel room cleaner (1). In their jobs, the pipe fitters were continually cutting pipe during their entire 8-hour shift—using the nondominant hand to tightly grip a pipe while using the dominant hand to direct an electrically powered blade through it. Of the card dealers, 1 was a professional playing card dealer (not the dealer whose case prompted this study), 1 distributed store coupons into containers, and 2 distributed pieces of mail into bins (referred to as casing the mail). All the card dealers used their nondominant hand to tightly grip items that the dominant limb distributed. The professional drivers used their nondominant hand to grip the steering wheel. The grocery store clerk used her nondominant hand to grip shopping items while moving them across a barcode detector. The wire stripper used her nondominant hand to tightly grip bundles of wire while holding a tool in the dominant hand to snip or strip them. The bakery worker continually used her nondominant hand to squeeze off pieces of dough from a mound. And the motel room cleaner used her nondominant hand to grip the side of a bathtub while scrubbing the tub with her dominant hand (she estimated she cleaned bathtubs for about 25% of her 8-hour shift).
Of the 2 patients who reported sustained gripping unrelated to occupation, 1 was baby-sitting her grandson 5 days per week. She carried him, grasping his buttock with her nondominant hand, while performing her daily activities. She estimated she carried the child a minimum of 2 hours a day. After several weeks, she noted episodic tingling in the nondominant hand, yet she continued carrying him for another 7 months, at which point she sought medical care. The other patient, a student in a stress relief class, was instructed to repetitively open and tightly close her nondominant hand for 10 minutes 4 or more times per day. After several weeks, she noted episodic tingling in the exercised, nondominant hand.
Of the 5 patients who denied performing an activity that required sustained gripping, 2 used their nondominant limb to enter data into a computer while turning pages with the dominant limb. A piano teacher, used her nondominant limb to strike piano keys while sitting to the right of her pupils; and a typist, consistently slept with the dorsal aspect of the nondominant hand pressed into her cheek, resulting in sustained wrist flexion throughout the night. One patient could not identify an activity performed with her nondominant limb both frequently and for prolonged periods.
Discussion
As with other syndromic disorders, CTS is associated with several clinical features, the presence of which correlates with the severity of median nerve involvement. During the earliest stage of CTS, episodic hand tingling (a positive symptom) is commonly reported. This tingling typically is more pronounced at night and during relaxation. In addition, many patients come to recognize that their hand tingling is precipitated by activities that involve sustained upper extremity elevation (eg, driving with a limb resting on upper portion of steering wheel; reading with upper extremities maintained in forward abduction) and that lowering a symptomatic limb relieves its tingling.
With progression, negative symptoms appear (eg, numbness and then weakness and wasting). Unfortunately, as the negative symptoms replace the positive ones, affected individuals may become less symptomatic and mistakenly believe their condition is improving. Features of autonomic fiber involvement may also be present but are less reliably elicited. Isolated hand pain is an uncommon manifestation of CTS because pain more commonly occurs later in the course and for this reason tends to be accompanied by other features of CTS.
The clinical features of CTS correlate with its underlying pathology. As demyelination precedes axon disruption pathologically, the clinical features of demyelination (episodic paresthesias) precede those of axon loss (numbness, weakness, wasting). However, clinical features may go unrecognized or be dismissed by the patient. Moreover, there is substantial variation in type, intensity, and frequency of symptoms.16,17
The EDX features of CTS correlate with its underlying pathology and pathophysiology. As demyelination (loss of insulation) increases the capacitance of the membrane and increases internodal current leakage, conduction velocity is reduced. As severity worsens and pathology changes from predominantly demyelination to predominantly axon loss, the individual nerve fiber action potentials, which make up the compound responses being recorded, are lost. As a result the amplitude and negative area under the curve values decrease. Thus, the EDX features of demyelination (eg, prolonged latencies) precede those of axon loss (eg, amplitude, negative area under the curve reduction).
As with other focal mononeuropathies, the sensory responses tend to be affected earlier and to a greater degree than do the motor responses. Consequently, the EDX features of CTS typically follow a standard progression. The median palmar responses are involved sooner and to a greater degree than the median sensory responses recorded from the digits, which in turn tend to be involved earlier and to a greater degree than are the median motor responses.
Awareness of this relationship dictates the severity of the lesion and helps in the recognition of a cool limb and in the avoidance of a false-positive study interpretation. In a cool limb, the fingers are cooler than the wrists. Thus, the peak latency of the median digital sensory response is delayed to a greater extent than the ipsilateral median palmar response (the opposite of the CTS pattern). Accordingly, whenever this pattern is identified, the hand must be warmed or rewarmed and the studies repeated. The hand is also warmed or rewarmed whenever the median motor response is delayed out of proportion to that of the median palmar response.
Conclusion
Cases of CTS mainly in the nondominant limb provide an opportunity to identify particular limb usage patterns that might be associated with CTS. Of the present study’s 21 affected patients, 16 were using their nondominant limb to perform activities that required sustained gripping. Fourteen of the 16 activities were related to occupation. These findings strongly suggest an association between activities that require sustained gripping and development of CTS.
That the card dealers simultaneously used their nondominant hand for sustained gripping and the dominant hand for the repetitive activity of dealing suggests that sustained gripping is a stronger risk factor than repetitive activity for the development of CTS—an unanticipated finding. Interestingly, in a 2001 study that suggested repetitive activity might not be a CTS risk factor, there was a higher incidence of CTS among computer users working with a mouse—an activity that requires sustained gripping.12
Episodic hand tingling during mouse use likely reflects impaired blood flow to the median nerve, which occurs when carpal tunnel pressure approaches or exceeds 20 to 30 mm Hg.18 Placement of a hand on a mouse increases intracarpal pressure from 3 to 5 mm Hg (wrist in neutral position) to 16 to 21 mm Hg, whereas mouse use increases intracarpal pressure to 28 to 33 mm Hg.18-20
1. Ormerod JA. On a peculiar numbness and paresis of the hands. St Barts Hosp Rep. 1883;19:17-26.
2. Rosenbaum RB, Ochoa JL. Carpal Tunnel Syndrome and Other Disorders of the Median Nerve. 2nd ed. Boston, MA: Butterworth-Heineman; 2002.
3. Gainer JV Jr, Nugent GR. Carpal tunnel syndrome: report of 430 operations. South Med J. 1977;70(3):325-328.
4. Reinstein L. Hand dominance in carpal tunnel syndrome. Arch Phys Med Rehabil. 1981;62(5):202-203.
5. Falck B, Aarnio P. Left-sided carpal tunnel syndrome in butchers. Scand J Work Environ Health. 1983;9(3):291-297.
6. Tanaka S, Wild DK, Seligman PJ, Halperin WE, Behrens VJ, Putz-Anerson V. Prevalence and work-relatedness of self-reported carpal tunnel syndrome among U.S. workers: analysis of the Occupational Health Supplement data of 1988 National Health Interview Survey. Am J Ind Med. 1995;27(4):451-470.
7. Silverstein BA, Fine LJ, Armstrong TJ. Occupational factors and carpal tunnel syndrome. Am J Ind Med. 1987;11(3):343-358.
8. de Krom MC, Kester AD, Knipschild PG, Spaans F. Risk factors for carpal tunnel syndrome. Am J Epidemiol. 1990;132(6):1102-1110.
9. Hales TR, Bernard BP. Epidemiology of work-related musculoskeletal disorders. Orthop Clin North Am. 1996;27(4):679-709.
10. Roquelaure Y, Ha C, Pelier-Cady MC, et al. Work increases the incidence of carpal tunnel syndrome in the general population. Muscle Nerve. 2008;37(4):477-482.
11. Stock SR. Workplace ergonomic factors and the development of musculoskeletal disorders of the neck and upper limbs: a meta-analysis. Am J Ind Med. 1991;19(1):87-107.
12. Stevens JC, Witt JC, Smith BE, Weaver AL. The frequency of carpal tunnel syndrome in computer users at a medical facility. Neurology. 2001;56(11):1568-1570.
13. Hart JR. The thenar and hypothenar types of neural atrophy of the hand. Am J Med Sci. 1911;141:224-241.
14. Ferrante MA, Parry GJ, Wilbourn AJ. Sensory nerve conduction studies. Paper presented at: 51st Annual Meeting of the American Academy of Neurology; April 1999; Toronto, Canada.
15. Litchy WJ, Miller RG, Shields RW. Motor nerve conduction studies. Paper presented at: 51st Annual Meeting of the American Academy of Neurology; April 1999; Toronto, Canada.
16. Nunez F, Vranceanu AM, Ring D. Determinants of pain in patients with carpal tunnel syndrome. Clin Orthop Relat Res. 2010;468(12):3328-3332.
17. van Suchtelen M, Beck SJ, Gruber JS, Ring D. Progression of carpal tunnel syndrome according to electrodiagnostic testing in nonoperatively treated patients. Arch Bone Jt Surg. 2014;2(3):185-191.
18. Ghasemi-Rad M, Nosair E, Vegh A, et al. A handy review of carpal tunnel syndrome: from anatomy to diagnosis and treatment. World J Radiol. 2014;6(6):284-300.
19. Rydevik B, Lundborg G, Bagge U. Effects of graded compression on intraneural blood flow. An in vivo study on rabbit tibial nerve. J Hand Surg Am. 1981;6(1):3-12.
20. Keir PJ, Bach JM, Rempel D. Effects of computer mouse design and task on carpal tunnel pressure. Ergonomics. 1999;42(10):1350-1360.=
1. Ormerod JA. On a peculiar numbness and paresis of the hands. St Barts Hosp Rep. 1883;19:17-26.
2. Rosenbaum RB, Ochoa JL. Carpal Tunnel Syndrome and Other Disorders of the Median Nerve. 2nd ed. Boston, MA: Butterworth-Heineman; 2002.
3. Gainer JV Jr, Nugent GR. Carpal tunnel syndrome: report of 430 operations. South Med J. 1977;70(3):325-328.
4. Reinstein L. Hand dominance in carpal tunnel syndrome. Arch Phys Med Rehabil. 1981;62(5):202-203.
5. Falck B, Aarnio P. Left-sided carpal tunnel syndrome in butchers. Scand J Work Environ Health. 1983;9(3):291-297.
6. Tanaka S, Wild DK, Seligman PJ, Halperin WE, Behrens VJ, Putz-Anerson V. Prevalence and work-relatedness of self-reported carpal tunnel syndrome among U.S. workers: analysis of the Occupational Health Supplement data of 1988 National Health Interview Survey. Am J Ind Med. 1995;27(4):451-470.
7. Silverstein BA, Fine LJ, Armstrong TJ. Occupational factors and carpal tunnel syndrome. Am J Ind Med. 1987;11(3):343-358.
8. de Krom MC, Kester AD, Knipschild PG, Spaans F. Risk factors for carpal tunnel syndrome. Am J Epidemiol. 1990;132(6):1102-1110.
9. Hales TR, Bernard BP. Epidemiology of work-related musculoskeletal disorders. Orthop Clin North Am. 1996;27(4):679-709.
10. Roquelaure Y, Ha C, Pelier-Cady MC, et al. Work increases the incidence of carpal tunnel syndrome in the general population. Muscle Nerve. 2008;37(4):477-482.
11. Stock SR. Workplace ergonomic factors and the development of musculoskeletal disorders of the neck and upper limbs: a meta-analysis. Am J Ind Med. 1991;19(1):87-107.
12. Stevens JC, Witt JC, Smith BE, Weaver AL. The frequency of carpal tunnel syndrome in computer users at a medical facility. Neurology. 2001;56(11):1568-1570.
13. Hart JR. The thenar and hypothenar types of neural atrophy of the hand. Am J Med Sci. 1911;141:224-241.
14. Ferrante MA, Parry GJ, Wilbourn AJ. Sensory nerve conduction studies. Paper presented at: 51st Annual Meeting of the American Academy of Neurology; April 1999; Toronto, Canada.
15. Litchy WJ, Miller RG, Shields RW. Motor nerve conduction studies. Paper presented at: 51st Annual Meeting of the American Academy of Neurology; April 1999; Toronto, Canada.
16. Nunez F, Vranceanu AM, Ring D. Determinants of pain in patients with carpal tunnel syndrome. Clin Orthop Relat Res. 2010;468(12):3328-3332.
17. van Suchtelen M, Beck SJ, Gruber JS, Ring D. Progression of carpal tunnel syndrome according to electrodiagnostic testing in nonoperatively treated patients. Arch Bone Jt Surg. 2014;2(3):185-191.
18. Ghasemi-Rad M, Nosair E, Vegh A, et al. A handy review of carpal tunnel syndrome: from anatomy to diagnosis and treatment. World J Radiol. 2014;6(6):284-300.
19. Rydevik B, Lundborg G, Bagge U. Effects of graded compression on intraneural blood flow. An in vivo study on rabbit tibial nerve. J Hand Surg Am. 1981;6(1):3-12.
20. Keir PJ, Bach JM, Rempel D. Effects of computer mouse design and task on carpal tunnel pressure. Ergonomics. 1999;42(10):1350-1360.=
The Cost of Oncology Drugs: A Pharmacy Perspective, Part 2
The Cost of Oncology Drugs: A Pharmacy Perspective, Part 1, appeared in the Federal Practitioner February 2016 special issue “Best Practices in Hematology and Oncology” and can be accessed here.
Health care costs are the fastest growing financial segment of the U.S. economy. The cost of medications, especially those for treating cancer, is the leading cause of increased health care spending.1 Until recently, the discussion of the high costs of cancer treatment was rarely made public.
Part 1 of this article focused on the emerging discussion of the financial impact of high-cost drugs in the U.S. Part 2 will focus on the drivers of increasing oncology drug costs and the challenges high-cost medications pose for the VA. The article also will review the role of the VA Pharmacy Benefits Management Service (PBM) in evaluating new oncology agents. Also presented are the clinical guidance tools designed to aid the clinician in the cost-effective use of these agents and results of a nationwide survey of VA oncology pharmacists regarding the use of cost-containment strategies.
Cost Drivers
Many factors are driving increased oncology drug costs within the VA. Although the cost of individual drugs has the largest impact on the accelerating cost of treating each patient, other clinical and social factors may play a role.
Increasing Cost of Individual Drugs
Drug pricing is not announced until after FDA approval. Oncology drugs at the high end of the cost spectrum are rarely curative and often add only weeks or months to overall survival (OS), the gold standard. Current clinical trial design often uses progression free survival (PFS) as the primary endpoint, which makes the use of traditional pharmacoeconomic determinations of value difficult. In addition, many new drugs are first in class and/or have narrow indications that preclude competition from other drugs. Although addressing the issue of the market price for drugs seems to be one that is not controllable, there is increasing demand for drug pricing reform.2
Many believe drug prices should be linked directly to clinical benefit. In a recent article, Goldstein and colleagues proposed establishing a value-based price for necitumumab based on clinical benefit, prior to FDA approval.3 When this analysis was done, necitumumab was pending FDA approval in combination with cisplatin and gemcitabine for the treatment of squamous carcinoma of the lung. Using clinical data from the SQUIRE trial on which FDA approval was based, the addition of necitumumab to the chemotherapy regimen led to an incremental survival benefit of 0.15 life-years and 0.11 quality-adjusted life-years (QALY).4 Using a Markov model to evaluate cost-effectiveness, these authors established that the price of necitumumab should be from $563 to $1,309 per cycle. Necitumumab was approved by the FDA on November 24, 2015, with the VA acquisition cost, as of May 2016, at $6,100 per cycle.
Lack of Generic Products
The approval of generic alternatives for targeted oncology agents should reduce the cost of treating oncology patients. However, since imatinib was approved in May 2001, no single targeted agent had become available as a generic until February 1, 2016, when generic imatinib was made available in the U.S. following approval by the FDA. Currently, generic imatinib is not used in the VA due to lack of Federal Supply Schedule (FSS) contract pricing, which leads to a generic cost that is much higher than the brand-name drug, Gleevec ($6,127 per month vs $9,472 per month for the generic). The reality is that many older agents have steadily increased in price, outpacing inflation (Table 1).5
Aging U.S. Population
Advancing age is the most common risk factor for cancer, leading to an increase in the incidence and treatment of cancer. Because many newer agents are considered easier to tolerate than are traditional cytotoxic chemotherapy, clinicians have become more comfortable treating elderly patients, and geriatric oncology has become an established subspecialty within oncology.
Changing Treatment Paradigms
The use of targeted therapies is changing the paradigm from the acute treatment of cancer to chronic cancer management. Most targeted therapies are continued until disease progression or toxicity, leading to chronic, open-ended treatment. This approach is in contrast to older treatment approaches such as chemotherapy, which is often given for a limited duration followed by observation. When successful, chronic treatment with targeted agents can lead to unanticipated high costs. The following current cases at the VA San Diego Healthcare System illustrate this point:
- Renal cell carcinoma: 68-year-old man diagnosed in 2005 with a recurrence in 2012
- High-dose interleukin-2 (2 cycles); sunitinib (3.3 years); pazopanib (2 months); everolimus (2 months); sorafenib (3 months); axitinib (7 months)
- Cutaneous T-cell lymphoma: 68-year-old man started romidepsin September 22, 2010
The rate of FDA approval for oncology drugs has been accelerating rapidly in the past 15 years. Sequential therapies beyond second-line therapy are common as more agents become available. Table 2 shows FDA approval for all cancer drugs by decade.
As researchers continue to better understand the many pathways involved with the development and progression of cancer, they are beginning to combine multiple targeted agents to augment response rates, prolong survival, and reduce the potential for resistance. Recent combination regimens approved by the FDA include dabrafenib plus trametinib (January 2014), and ipilimumab plus nivolumab (October 2015), both for the treatment of melanoma. In November 2015, ixazomib was FDA approved to be used in combination with lenalidomide for multiple myeloma. Many more combination regimens are currently in clinical trials, and more combinations are expected to receive FDA approval. It is easy to see how the combination of multiple expensive agents with the prospect of prolonged therapy has the potential to increase the cost of many regimens to well over $100,000 per year.
Maintenance therapy is used to prolong PFS for patients receiving an excellent response to primary therapy. For example, VA costs for maintenance regimens include lenalidomide 10 mg daily: $8,314 for 28 days equals $216,177 for 2 years; bortezomib 1.3 mg/m2 (2.6 mg) q: 2 weeks equals $60,730 for 2 years (includes waste as bortezomib 3.5-mg vials do not a contain preservative and must be discarded within 8 hours of preparation); and rituximab 800 mg q: 2 months equals $47,635 for 2 years.
Until recently, immunotherapy for cancer was limited to melanoma and renal cell carcinoma using interleukin-2 (aldesleukin) and interferon alfa. However, the immergence of new immunotherapies, such as anti-PD-1 and anti-CTLA-4 monoclonal antibodies, have expanded the role of immunotherapy to many other, more common, malignancies, such as lung cancer, breast cancer, prostate cancer, head and neck cancer, and many more.
Most randomized clinical trials study drugs as second- or occasionally third-line therapy. However, many patients continue to be treated beyond the third-line setting, often without evidence-based data to support potential benefit. Patients often place value on treatments unlikely to work so as not to give up hope. These “hopeful gambles,” even with the potential of significant toxicity and decreased quality of life (QOL), are common in cancer treatment.6 In addition, oncologists often overestimate the clinical benefit when considering additional therapy in this setting.7
Influx of New Patients
Outside the VHA setting, the financial burden of cancer treatment has led to an influx of new patients transferring care to the VHA to reduce out-of-pocket expenses. Because private insurance copays for oral agents are increasing, many reaching 20% to 30%, out-of-pocket expenses for medications can reach several thousand dollars per month. Patients often change insurance plans due to changing jobs or to decrease cost, or employers may change plans to save money, which may significantly alter or discontinue coverage. Patients often request that the VA provide medication while continuing to see only their private oncologist. This practice should be discouraged because the VA, without clinical involvement, may supply drugs for inappropriate indications. In addition, VA providers writing prescriptions for medications without personally following patients may be liable for poor outcomes.
VA PBM Services
Prior to 1995, the VA was a much criticized and poorly performing health care system that had experienced significant budget cuts, forcing many veterans to seek care outside the VA. Then beginning in 1995, a remarkable transformation occurred, which modernized and improved the VA into a system that consistently outperforms the private sector in quality of care, patient safety, patient satisfaction, all at a lower cost.8 The story of the VA’s transformation has been well chronicled by Phillip Longman.9
Under the direction of VA Under Secretary for Health Kenneth Kizer, MD, MPH, VA established PBM Clinical Services to develop and maintain the National Drug Formulary, create clinical guidance documents, and manage drug costs and utilization. A recent article by Heron and Geraci examined the functions and role of the VA PBM in controlling oncology drug costs.10 The following is a brief review of several documents and VA PBM responsibilities as reviewed by Heron and Geraci.
VA National Formulary
Prior to the establishment of the VA National Formulary in 1995, each VA maintained its own formulary, which led to extreme variability in drug access across the country. When a patient accessed care at different VAMCs, it was common for the patient’s medications to be changed based on the specific facility formulary. This practice led to many potential problems, such as lack of clinical benefit and potentially increased or new toxicities, and led to extra hospital visits for monitoring and adjustment of medications.
In contrast, the VA National Formulary now offers a uniform pharmacy benefit to all veterans by reducing variation in access to drugs. In addition, using preferred agents in each drug class provides VA with additional leverage when contracting with drug suppliers to reduce prices across the entire VA system.
Many oncology agents are not included on the VA National Formulary due to cost and the potential for off-label use. However, the formulary status of oncology agents in no way limits access or the availability of any oncology drug for appropriate patients. In fact, nonformulary approval requests work as a mechanism for review to ensure that these agents are used properly in the subset of patients who are most likely to benefit.
The PBM assesses all new oncology drugs for value and potential use within the VA, as well as cost impact. Following this assessment, various clinical guidance documents may be developed that are intended to guide clinicians in the proper use of medications for veterans. All documents prepared by the PBM undergo an extensive peer review by the Medical Advisory Panel and other experts in the field.
Drug Monographs
A drug monograph is a comprehensive, evidence-based drug review that summarizes efficacy and safety based on clinical trial data published in peer-reviewed journals, abstracts, and/or FDA Medical Review transcripts. Cost-effectiveness analysis is included if available.
Criteria for Use
Criteria for Use (CFU) are developed for drugs considered to be at high risk for inappropriate use or with safety concerns. The purpose of the CFU is to select patients most likely to benefit from these agents by using clinical criteria, which may qualify or eliminate a patient for treatment. National CFUs are available on the national PBM website. Local CFUs are often written and shared among oncology pharmacists via the VA oncology pharmacist listserv.
Abbreviated Reviews
Similar to drug monographs, abbreviated reviews are much shorter and focus on the relevant clinical sections of the drug monograph necessary for clinical or formulary decision making.
National Acquisition Center
The National Acquisition Center (NAC) is the pharmaceutical contracting mechanism for the VA and works closely with the PBM.5 The NAC pursues significant drug price reductions for the VA based on many strategies. Public Law 102-585 ensures that certain government agencies, including the VA, receive special discounts on pharmaceuticals, which is at least a 24% discount from the nonfederal Average Manufacturer Price. This is known as the Federal Supply Schedule (FSS) and/or Big 4 pricing. In addition, bulk purchases and performance-based incentive agreements can lead to substantial local discounts. By working with specific drug distribution and warehouse contractors, the NAC assures ready access to drugs for VA patients. The NAC also allows for an efficient drug inventory process, thus reducing inventory management costs.
Guidance Documents
In 2012, the VA Oncology Field Advisory Committee (FAC) created the High Cost Oncology Drug Work Group to address the impact of high-cost oncology drugs within the VA.11 This work group was composed of VA oncologists and pharmacists whose efforts resulted in 5 guidance documents designed to reduce drug costs by optimizing therapy and reducing waste: (1) Dose Rounding in Oncology; (2) Oral Anticancer Drugs Dispensing and Monitoring; (3) Oncology Drug Table: Recommended Dispensing and Monitoring; (4) Chemotherapy Review Committee Process; and (5) Determining Clinical Benefit of High Cost Oncology Drugs. Reviews of 2 of these documents follows.
Determining Clinical Benefit of High Cost Oncology Drugs provides a decision tool to aid members of the oncology health care team in optimizing patient outcomes while attempting to obtain the greatest value from innovative therapies. When a high-cost or off-label request is made for a particular patient, using this process encourages thoughtful and evidence-based use of the drug by considering all clinical evidence in addition to the FDA-approved indication. Finally, a drug’s safety profile in relation to the indication, therapeutic goal, and specific patient characteristics and desires are integrated into a final decision to determine the appropriateness of the therapeutic intervention for the patient.
Oncology Drug Table: Recommended Dispensing and Monitoring contains a list of oral oncology drugs and includes recommendations for dispensing amount, adverse effects, laboratory monitoring, formulary status, approval requirements, and monthly cost of each agent based on the current NAC pricing.5 Cost awareness is critical when comparing alternative treatment options to minimize cost when treatments with similar benefits are considered. Most VA oncologists do not have easy access to the cost of various treatments and can be surprised about how expensive many common regimens cost. The costs listed in this document are updated about every 3 months.
Conclusion
Using newer, expensive targeted oncology agents in a cost-effective manner must be a proactive, collaborative, and multidisciplinary process. Pharmacists should not be solely responsible for monitoring and controlling high-cost treatments. Well-informed, evidence-based decisions are needed to ensure expensive agents are used in the subset of patients who are most likely to benefit. Clinical tools addressing value should be used to aid in appropriate and cost-effective treatment plans using drug monographs and CFUs, VHA Guidance on Determining Clinical Benefit of High Cost Oncology Drugs, and the Oral Chemotherapy Dispensing and Monitoring Reference, among other resources. Due to the subjective nature of value in medicine, agreeing on policy will have many challenges, such as how to place a value on various gains in overall survival, progression free survival, response rates, and QOL.
eAppendix
1. Bach PB. Limits on Medicare's ability to control rising spending on cancer drugs. N Engl J Med. 2009;360(6):626-633.
2. Kantarjian H, Steensma D, Rius Sanjuan J, Eishaug A, Light D. High cancer drug prices in the United States: reasons and proposed solutions. J Oncol Pract. 2014;10(4):e208-e211.
3. Goldstein DA, Chen Q, Ayer T, et al. Necitumumab in metastatic squamous cell lung cancer: establishing a value-based cost. JAMA Oncol. 2015;1(9):1293-1300.
4. Thatcher N, Hirsch FR, Luft AV, et al; SQUIRE Investigators. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2015;16(7):763-774.
5. U.S. Department of Veterans Affairs, National Acquisition Center, Pharmaceutical Catalog Search. U.S. Department of Veterans Affairs, National Acquisition Center website. http://www1.va.gov/nac/index.cfm?template=Search_Pharmaceutical_Catalog. Updated June 13, 2016. Accessed June 13, 2016.
6. Lakdawalla DN, Romley JA, Sanchez Y, Maclean JR, Penrod JR, Philipson T. How cancer patients value hope and the implications for cost-effectiveness assessments of high-cost cancer therapies. Health Aff (Millwood). 2012;31(4):676-682.
7. Ubel PA, Berry SR, Nadler E, et al. In a survey, marked inconsistency in how oncologists judged value of high-cost cancer drugs in relation to gains in survival. Health Aff (Millwood). 2012;31(4):709-717.
8. Asch SM, McGlynn EA, Hogan MM, et al. Comparison of quality of care for patients in the Veterans Health Administration and patients in a national sample. Ann Intern Med. 2004;141(12):938-945. 9. Longman P. Best Care Anywhere: Why VA Health Care Would Work for Everyone. 3rd ed. San Francisco, CA: Berrett-Koehler Publishers; 2012. 10. Heron BB, Geraci MC. Controlling the cost of oncology drugs within the VA: a national perspective. Fed Pract. 2015;32(suppl 1):18S-22S.
11. U.S. Department of Veterans Affairs. Pharmacy Benefits Management Services Intranet, Documents and Lists. https://vaww.cmopnational.va.gov/cmop/PBM/Clinical%20Guidance/Forms/AllItems.aspx. Accessed May 19, 2016.
The Cost of Oncology Drugs: A Pharmacy Perspective, Part 1, appeared in the Federal Practitioner February 2016 special issue “Best Practices in Hematology and Oncology” and can be accessed here.
Health care costs are the fastest growing financial segment of the U.S. economy. The cost of medications, especially those for treating cancer, is the leading cause of increased health care spending.1 Until recently, the discussion of the high costs of cancer treatment was rarely made public.
Part 1 of this article focused on the emerging discussion of the financial impact of high-cost drugs in the U.S. Part 2 will focus on the drivers of increasing oncology drug costs and the challenges high-cost medications pose for the VA. The article also will review the role of the VA Pharmacy Benefits Management Service (PBM) in evaluating new oncology agents. Also presented are the clinical guidance tools designed to aid the clinician in the cost-effective use of these agents and results of a nationwide survey of VA oncology pharmacists regarding the use of cost-containment strategies.
Cost Drivers
Many factors are driving increased oncology drug costs within the VA. Although the cost of individual drugs has the largest impact on the accelerating cost of treating each patient, other clinical and social factors may play a role.
Increasing Cost of Individual Drugs
Drug pricing is not announced until after FDA approval. Oncology drugs at the high end of the cost spectrum are rarely curative and often add only weeks or months to overall survival (OS), the gold standard. Current clinical trial design often uses progression free survival (PFS) as the primary endpoint, which makes the use of traditional pharmacoeconomic determinations of value difficult. In addition, many new drugs are first in class and/or have narrow indications that preclude competition from other drugs. Although addressing the issue of the market price for drugs seems to be one that is not controllable, there is increasing demand for drug pricing reform.2
Many believe drug prices should be linked directly to clinical benefit. In a recent article, Goldstein and colleagues proposed establishing a value-based price for necitumumab based on clinical benefit, prior to FDA approval.3 When this analysis was done, necitumumab was pending FDA approval in combination with cisplatin and gemcitabine for the treatment of squamous carcinoma of the lung. Using clinical data from the SQUIRE trial on which FDA approval was based, the addition of necitumumab to the chemotherapy regimen led to an incremental survival benefit of 0.15 life-years and 0.11 quality-adjusted life-years (QALY).4 Using a Markov model to evaluate cost-effectiveness, these authors established that the price of necitumumab should be from $563 to $1,309 per cycle. Necitumumab was approved by the FDA on November 24, 2015, with the VA acquisition cost, as of May 2016, at $6,100 per cycle.
Lack of Generic Products
The approval of generic alternatives for targeted oncology agents should reduce the cost of treating oncology patients. However, since imatinib was approved in May 2001, no single targeted agent had become available as a generic until February 1, 2016, when generic imatinib was made available in the U.S. following approval by the FDA. Currently, generic imatinib is not used in the VA due to lack of Federal Supply Schedule (FSS) contract pricing, which leads to a generic cost that is much higher than the brand-name drug, Gleevec ($6,127 per month vs $9,472 per month for the generic). The reality is that many older agents have steadily increased in price, outpacing inflation (Table 1).5
Aging U.S. Population
Advancing age is the most common risk factor for cancer, leading to an increase in the incidence and treatment of cancer. Because many newer agents are considered easier to tolerate than are traditional cytotoxic chemotherapy, clinicians have become more comfortable treating elderly patients, and geriatric oncology has become an established subspecialty within oncology.
Changing Treatment Paradigms
The use of targeted therapies is changing the paradigm from the acute treatment of cancer to chronic cancer management. Most targeted therapies are continued until disease progression or toxicity, leading to chronic, open-ended treatment. This approach is in contrast to older treatment approaches such as chemotherapy, which is often given for a limited duration followed by observation. When successful, chronic treatment with targeted agents can lead to unanticipated high costs. The following current cases at the VA San Diego Healthcare System illustrate this point:
- Renal cell carcinoma: 68-year-old man diagnosed in 2005 with a recurrence in 2012
- High-dose interleukin-2 (2 cycles); sunitinib (3.3 years); pazopanib (2 months); everolimus (2 months); sorafenib (3 months); axitinib (7 months)
- Cutaneous T-cell lymphoma: 68-year-old man started romidepsin September 22, 2010
The rate of FDA approval for oncology drugs has been accelerating rapidly in the past 15 years. Sequential therapies beyond second-line therapy are common as more agents become available. Table 2 shows FDA approval for all cancer drugs by decade.
As researchers continue to better understand the many pathways involved with the development and progression of cancer, they are beginning to combine multiple targeted agents to augment response rates, prolong survival, and reduce the potential for resistance. Recent combination regimens approved by the FDA include dabrafenib plus trametinib (January 2014), and ipilimumab plus nivolumab (October 2015), both for the treatment of melanoma. In November 2015, ixazomib was FDA approved to be used in combination with lenalidomide for multiple myeloma. Many more combination regimens are currently in clinical trials, and more combinations are expected to receive FDA approval. It is easy to see how the combination of multiple expensive agents with the prospect of prolonged therapy has the potential to increase the cost of many regimens to well over $100,000 per year.
Maintenance therapy is used to prolong PFS for patients receiving an excellent response to primary therapy. For example, VA costs for maintenance regimens include lenalidomide 10 mg daily: $8,314 for 28 days equals $216,177 for 2 years; bortezomib 1.3 mg/m2 (2.6 mg) q: 2 weeks equals $60,730 for 2 years (includes waste as bortezomib 3.5-mg vials do not a contain preservative and must be discarded within 8 hours of preparation); and rituximab 800 mg q: 2 months equals $47,635 for 2 years.
Until recently, immunotherapy for cancer was limited to melanoma and renal cell carcinoma using interleukin-2 (aldesleukin) and interferon alfa. However, the immergence of new immunotherapies, such as anti-PD-1 and anti-CTLA-4 monoclonal antibodies, have expanded the role of immunotherapy to many other, more common, malignancies, such as lung cancer, breast cancer, prostate cancer, head and neck cancer, and many more.
Most randomized clinical trials study drugs as second- or occasionally third-line therapy. However, many patients continue to be treated beyond the third-line setting, often without evidence-based data to support potential benefit. Patients often place value on treatments unlikely to work so as not to give up hope. These “hopeful gambles,” even with the potential of significant toxicity and decreased quality of life (QOL), are common in cancer treatment.6 In addition, oncologists often overestimate the clinical benefit when considering additional therapy in this setting.7
Influx of New Patients
Outside the VHA setting, the financial burden of cancer treatment has led to an influx of new patients transferring care to the VHA to reduce out-of-pocket expenses. Because private insurance copays for oral agents are increasing, many reaching 20% to 30%, out-of-pocket expenses for medications can reach several thousand dollars per month. Patients often change insurance plans due to changing jobs or to decrease cost, or employers may change plans to save money, which may significantly alter or discontinue coverage. Patients often request that the VA provide medication while continuing to see only their private oncologist. This practice should be discouraged because the VA, without clinical involvement, may supply drugs for inappropriate indications. In addition, VA providers writing prescriptions for medications without personally following patients may be liable for poor outcomes.
VA PBM Services
Prior to 1995, the VA was a much criticized and poorly performing health care system that had experienced significant budget cuts, forcing many veterans to seek care outside the VA. Then beginning in 1995, a remarkable transformation occurred, which modernized and improved the VA into a system that consistently outperforms the private sector in quality of care, patient safety, patient satisfaction, all at a lower cost.8 The story of the VA’s transformation has been well chronicled by Phillip Longman.9
Under the direction of VA Under Secretary for Health Kenneth Kizer, MD, MPH, VA established PBM Clinical Services to develop and maintain the National Drug Formulary, create clinical guidance documents, and manage drug costs and utilization. A recent article by Heron and Geraci examined the functions and role of the VA PBM in controlling oncology drug costs.10 The following is a brief review of several documents and VA PBM responsibilities as reviewed by Heron and Geraci.
VA National Formulary
Prior to the establishment of the VA National Formulary in 1995, each VA maintained its own formulary, which led to extreme variability in drug access across the country. When a patient accessed care at different VAMCs, it was common for the patient’s medications to be changed based on the specific facility formulary. This practice led to many potential problems, such as lack of clinical benefit and potentially increased or new toxicities, and led to extra hospital visits for monitoring and adjustment of medications.
In contrast, the VA National Formulary now offers a uniform pharmacy benefit to all veterans by reducing variation in access to drugs. In addition, using preferred agents in each drug class provides VA with additional leverage when contracting with drug suppliers to reduce prices across the entire VA system.
Many oncology agents are not included on the VA National Formulary due to cost and the potential for off-label use. However, the formulary status of oncology agents in no way limits access or the availability of any oncology drug for appropriate patients. In fact, nonformulary approval requests work as a mechanism for review to ensure that these agents are used properly in the subset of patients who are most likely to benefit.
The PBM assesses all new oncology drugs for value and potential use within the VA, as well as cost impact. Following this assessment, various clinical guidance documents may be developed that are intended to guide clinicians in the proper use of medications for veterans. All documents prepared by the PBM undergo an extensive peer review by the Medical Advisory Panel and other experts in the field.
Drug Monographs
A drug monograph is a comprehensive, evidence-based drug review that summarizes efficacy and safety based on clinical trial data published in peer-reviewed journals, abstracts, and/or FDA Medical Review transcripts. Cost-effectiveness analysis is included if available.
Criteria for Use
Criteria for Use (CFU) are developed for drugs considered to be at high risk for inappropriate use or with safety concerns. The purpose of the CFU is to select patients most likely to benefit from these agents by using clinical criteria, which may qualify or eliminate a patient for treatment. National CFUs are available on the national PBM website. Local CFUs are often written and shared among oncology pharmacists via the VA oncology pharmacist listserv.
Abbreviated Reviews
Similar to drug monographs, abbreviated reviews are much shorter and focus on the relevant clinical sections of the drug monograph necessary for clinical or formulary decision making.
National Acquisition Center
The National Acquisition Center (NAC) is the pharmaceutical contracting mechanism for the VA and works closely with the PBM.5 The NAC pursues significant drug price reductions for the VA based on many strategies. Public Law 102-585 ensures that certain government agencies, including the VA, receive special discounts on pharmaceuticals, which is at least a 24% discount from the nonfederal Average Manufacturer Price. This is known as the Federal Supply Schedule (FSS) and/or Big 4 pricing. In addition, bulk purchases and performance-based incentive agreements can lead to substantial local discounts. By working with specific drug distribution and warehouse contractors, the NAC assures ready access to drugs for VA patients. The NAC also allows for an efficient drug inventory process, thus reducing inventory management costs.
Guidance Documents
In 2012, the VA Oncology Field Advisory Committee (FAC) created the High Cost Oncology Drug Work Group to address the impact of high-cost oncology drugs within the VA.11 This work group was composed of VA oncologists and pharmacists whose efforts resulted in 5 guidance documents designed to reduce drug costs by optimizing therapy and reducing waste: (1) Dose Rounding in Oncology; (2) Oral Anticancer Drugs Dispensing and Monitoring; (3) Oncology Drug Table: Recommended Dispensing and Monitoring; (4) Chemotherapy Review Committee Process; and (5) Determining Clinical Benefit of High Cost Oncology Drugs. Reviews of 2 of these documents follows.
Determining Clinical Benefit of High Cost Oncology Drugs provides a decision tool to aid members of the oncology health care team in optimizing patient outcomes while attempting to obtain the greatest value from innovative therapies. When a high-cost or off-label request is made for a particular patient, using this process encourages thoughtful and evidence-based use of the drug by considering all clinical evidence in addition to the FDA-approved indication. Finally, a drug’s safety profile in relation to the indication, therapeutic goal, and specific patient characteristics and desires are integrated into a final decision to determine the appropriateness of the therapeutic intervention for the patient.
Oncology Drug Table: Recommended Dispensing and Monitoring contains a list of oral oncology drugs and includes recommendations for dispensing amount, adverse effects, laboratory monitoring, formulary status, approval requirements, and monthly cost of each agent based on the current NAC pricing.5 Cost awareness is critical when comparing alternative treatment options to minimize cost when treatments with similar benefits are considered. Most VA oncologists do not have easy access to the cost of various treatments and can be surprised about how expensive many common regimens cost. The costs listed in this document are updated about every 3 months.
Conclusion
Using newer, expensive targeted oncology agents in a cost-effective manner must be a proactive, collaborative, and multidisciplinary process. Pharmacists should not be solely responsible for monitoring and controlling high-cost treatments. Well-informed, evidence-based decisions are needed to ensure expensive agents are used in the subset of patients who are most likely to benefit. Clinical tools addressing value should be used to aid in appropriate and cost-effective treatment plans using drug monographs and CFUs, VHA Guidance on Determining Clinical Benefit of High Cost Oncology Drugs, and the Oral Chemotherapy Dispensing and Monitoring Reference, among other resources. Due to the subjective nature of value in medicine, agreeing on policy will have many challenges, such as how to place a value on various gains in overall survival, progression free survival, response rates, and QOL.
eAppendix
The Cost of Oncology Drugs: A Pharmacy Perspective, Part 1, appeared in the Federal Practitioner February 2016 special issue “Best Practices in Hematology and Oncology” and can be accessed here.
Health care costs are the fastest growing financial segment of the U.S. economy. The cost of medications, especially those for treating cancer, is the leading cause of increased health care spending.1 Until recently, the discussion of the high costs of cancer treatment was rarely made public.
Part 1 of this article focused on the emerging discussion of the financial impact of high-cost drugs in the U.S. Part 2 will focus on the drivers of increasing oncology drug costs and the challenges high-cost medications pose for the VA. The article also will review the role of the VA Pharmacy Benefits Management Service (PBM) in evaluating new oncology agents. Also presented are the clinical guidance tools designed to aid the clinician in the cost-effective use of these agents and results of a nationwide survey of VA oncology pharmacists regarding the use of cost-containment strategies.
Cost Drivers
Many factors are driving increased oncology drug costs within the VA. Although the cost of individual drugs has the largest impact on the accelerating cost of treating each patient, other clinical and social factors may play a role.
Increasing Cost of Individual Drugs
Drug pricing is not announced until after FDA approval. Oncology drugs at the high end of the cost spectrum are rarely curative and often add only weeks or months to overall survival (OS), the gold standard. Current clinical trial design often uses progression free survival (PFS) as the primary endpoint, which makes the use of traditional pharmacoeconomic determinations of value difficult. In addition, many new drugs are first in class and/or have narrow indications that preclude competition from other drugs. Although addressing the issue of the market price for drugs seems to be one that is not controllable, there is increasing demand for drug pricing reform.2
Many believe drug prices should be linked directly to clinical benefit. In a recent article, Goldstein and colleagues proposed establishing a value-based price for necitumumab based on clinical benefit, prior to FDA approval.3 When this analysis was done, necitumumab was pending FDA approval in combination with cisplatin and gemcitabine for the treatment of squamous carcinoma of the lung. Using clinical data from the SQUIRE trial on which FDA approval was based, the addition of necitumumab to the chemotherapy regimen led to an incremental survival benefit of 0.15 life-years and 0.11 quality-adjusted life-years (QALY).4 Using a Markov model to evaluate cost-effectiveness, these authors established that the price of necitumumab should be from $563 to $1,309 per cycle. Necitumumab was approved by the FDA on November 24, 2015, with the VA acquisition cost, as of May 2016, at $6,100 per cycle.
Lack of Generic Products
The approval of generic alternatives for targeted oncology agents should reduce the cost of treating oncology patients. However, since imatinib was approved in May 2001, no single targeted agent had become available as a generic until February 1, 2016, when generic imatinib was made available in the U.S. following approval by the FDA. Currently, generic imatinib is not used in the VA due to lack of Federal Supply Schedule (FSS) contract pricing, which leads to a generic cost that is much higher than the brand-name drug, Gleevec ($6,127 per month vs $9,472 per month for the generic). The reality is that many older agents have steadily increased in price, outpacing inflation (Table 1).5
Aging U.S. Population
Advancing age is the most common risk factor for cancer, leading to an increase in the incidence and treatment of cancer. Because many newer agents are considered easier to tolerate than are traditional cytotoxic chemotherapy, clinicians have become more comfortable treating elderly patients, and geriatric oncology has become an established subspecialty within oncology.
Changing Treatment Paradigms
The use of targeted therapies is changing the paradigm from the acute treatment of cancer to chronic cancer management. Most targeted therapies are continued until disease progression or toxicity, leading to chronic, open-ended treatment. This approach is in contrast to older treatment approaches such as chemotherapy, which is often given for a limited duration followed by observation. When successful, chronic treatment with targeted agents can lead to unanticipated high costs. The following current cases at the VA San Diego Healthcare System illustrate this point:
- Renal cell carcinoma: 68-year-old man diagnosed in 2005 with a recurrence in 2012
- High-dose interleukin-2 (2 cycles); sunitinib (3.3 years); pazopanib (2 months); everolimus (2 months); sorafenib (3 months); axitinib (7 months)
- Cutaneous T-cell lymphoma: 68-year-old man started romidepsin September 22, 2010
The rate of FDA approval for oncology drugs has been accelerating rapidly in the past 15 years. Sequential therapies beyond second-line therapy are common as more agents become available. Table 2 shows FDA approval for all cancer drugs by decade.
As researchers continue to better understand the many pathways involved with the development and progression of cancer, they are beginning to combine multiple targeted agents to augment response rates, prolong survival, and reduce the potential for resistance. Recent combination regimens approved by the FDA include dabrafenib plus trametinib (January 2014), and ipilimumab plus nivolumab (October 2015), both for the treatment of melanoma. In November 2015, ixazomib was FDA approved to be used in combination with lenalidomide for multiple myeloma. Many more combination regimens are currently in clinical trials, and more combinations are expected to receive FDA approval. It is easy to see how the combination of multiple expensive agents with the prospect of prolonged therapy has the potential to increase the cost of many regimens to well over $100,000 per year.
Maintenance therapy is used to prolong PFS for patients receiving an excellent response to primary therapy. For example, VA costs for maintenance regimens include lenalidomide 10 mg daily: $8,314 for 28 days equals $216,177 for 2 years; bortezomib 1.3 mg/m2 (2.6 mg) q: 2 weeks equals $60,730 for 2 years (includes waste as bortezomib 3.5-mg vials do not a contain preservative and must be discarded within 8 hours of preparation); and rituximab 800 mg q: 2 months equals $47,635 for 2 years.
Until recently, immunotherapy for cancer was limited to melanoma and renal cell carcinoma using interleukin-2 (aldesleukin) and interferon alfa. However, the immergence of new immunotherapies, such as anti-PD-1 and anti-CTLA-4 monoclonal antibodies, have expanded the role of immunotherapy to many other, more common, malignancies, such as lung cancer, breast cancer, prostate cancer, head and neck cancer, and many more.
Most randomized clinical trials study drugs as second- or occasionally third-line therapy. However, many patients continue to be treated beyond the third-line setting, often without evidence-based data to support potential benefit. Patients often place value on treatments unlikely to work so as not to give up hope. These “hopeful gambles,” even with the potential of significant toxicity and decreased quality of life (QOL), are common in cancer treatment.6 In addition, oncologists often overestimate the clinical benefit when considering additional therapy in this setting.7
Influx of New Patients
Outside the VHA setting, the financial burden of cancer treatment has led to an influx of new patients transferring care to the VHA to reduce out-of-pocket expenses. Because private insurance copays for oral agents are increasing, many reaching 20% to 30%, out-of-pocket expenses for medications can reach several thousand dollars per month. Patients often change insurance plans due to changing jobs or to decrease cost, or employers may change plans to save money, which may significantly alter or discontinue coverage. Patients often request that the VA provide medication while continuing to see only their private oncologist. This practice should be discouraged because the VA, without clinical involvement, may supply drugs for inappropriate indications. In addition, VA providers writing prescriptions for medications without personally following patients may be liable for poor outcomes.
VA PBM Services
Prior to 1995, the VA was a much criticized and poorly performing health care system that had experienced significant budget cuts, forcing many veterans to seek care outside the VA. Then beginning in 1995, a remarkable transformation occurred, which modernized and improved the VA into a system that consistently outperforms the private sector in quality of care, patient safety, patient satisfaction, all at a lower cost.8 The story of the VA’s transformation has been well chronicled by Phillip Longman.9
Under the direction of VA Under Secretary for Health Kenneth Kizer, MD, MPH, VA established PBM Clinical Services to develop and maintain the National Drug Formulary, create clinical guidance documents, and manage drug costs and utilization. A recent article by Heron and Geraci examined the functions and role of the VA PBM in controlling oncology drug costs.10 The following is a brief review of several documents and VA PBM responsibilities as reviewed by Heron and Geraci.
VA National Formulary
Prior to the establishment of the VA National Formulary in 1995, each VA maintained its own formulary, which led to extreme variability in drug access across the country. When a patient accessed care at different VAMCs, it was common for the patient’s medications to be changed based on the specific facility formulary. This practice led to many potential problems, such as lack of clinical benefit and potentially increased or new toxicities, and led to extra hospital visits for monitoring and adjustment of medications.
In contrast, the VA National Formulary now offers a uniform pharmacy benefit to all veterans by reducing variation in access to drugs. In addition, using preferred agents in each drug class provides VA with additional leverage when contracting with drug suppliers to reduce prices across the entire VA system.
Many oncology agents are not included on the VA National Formulary due to cost and the potential for off-label use. However, the formulary status of oncology agents in no way limits access or the availability of any oncology drug for appropriate patients. In fact, nonformulary approval requests work as a mechanism for review to ensure that these agents are used properly in the subset of patients who are most likely to benefit.
The PBM assesses all new oncology drugs for value and potential use within the VA, as well as cost impact. Following this assessment, various clinical guidance documents may be developed that are intended to guide clinicians in the proper use of medications for veterans. All documents prepared by the PBM undergo an extensive peer review by the Medical Advisory Panel and other experts in the field.
Drug Monographs
A drug monograph is a comprehensive, evidence-based drug review that summarizes efficacy and safety based on clinical trial data published in peer-reviewed journals, abstracts, and/or FDA Medical Review transcripts. Cost-effectiveness analysis is included if available.
Criteria for Use
Criteria for Use (CFU) are developed for drugs considered to be at high risk for inappropriate use or with safety concerns. The purpose of the CFU is to select patients most likely to benefit from these agents by using clinical criteria, which may qualify or eliminate a patient for treatment. National CFUs are available on the national PBM website. Local CFUs are often written and shared among oncology pharmacists via the VA oncology pharmacist listserv.
Abbreviated Reviews
Similar to drug monographs, abbreviated reviews are much shorter and focus on the relevant clinical sections of the drug monograph necessary for clinical or formulary decision making.
National Acquisition Center
The National Acquisition Center (NAC) is the pharmaceutical contracting mechanism for the VA and works closely with the PBM.5 The NAC pursues significant drug price reductions for the VA based on many strategies. Public Law 102-585 ensures that certain government agencies, including the VA, receive special discounts on pharmaceuticals, which is at least a 24% discount from the nonfederal Average Manufacturer Price. This is known as the Federal Supply Schedule (FSS) and/or Big 4 pricing. In addition, bulk purchases and performance-based incentive agreements can lead to substantial local discounts. By working with specific drug distribution and warehouse contractors, the NAC assures ready access to drugs for VA patients. The NAC also allows for an efficient drug inventory process, thus reducing inventory management costs.
Guidance Documents
In 2012, the VA Oncology Field Advisory Committee (FAC) created the High Cost Oncology Drug Work Group to address the impact of high-cost oncology drugs within the VA.11 This work group was composed of VA oncologists and pharmacists whose efforts resulted in 5 guidance documents designed to reduce drug costs by optimizing therapy and reducing waste: (1) Dose Rounding in Oncology; (2) Oral Anticancer Drugs Dispensing and Monitoring; (3) Oncology Drug Table: Recommended Dispensing and Monitoring; (4) Chemotherapy Review Committee Process; and (5) Determining Clinical Benefit of High Cost Oncology Drugs. Reviews of 2 of these documents follows.
Determining Clinical Benefit of High Cost Oncology Drugs provides a decision tool to aid members of the oncology health care team in optimizing patient outcomes while attempting to obtain the greatest value from innovative therapies. When a high-cost or off-label request is made for a particular patient, using this process encourages thoughtful and evidence-based use of the drug by considering all clinical evidence in addition to the FDA-approved indication. Finally, a drug’s safety profile in relation to the indication, therapeutic goal, and specific patient characteristics and desires are integrated into a final decision to determine the appropriateness of the therapeutic intervention for the patient.
Oncology Drug Table: Recommended Dispensing and Monitoring contains a list of oral oncology drugs and includes recommendations for dispensing amount, adverse effects, laboratory monitoring, formulary status, approval requirements, and monthly cost of each agent based on the current NAC pricing.5 Cost awareness is critical when comparing alternative treatment options to minimize cost when treatments with similar benefits are considered. Most VA oncologists do not have easy access to the cost of various treatments and can be surprised about how expensive many common regimens cost. The costs listed in this document are updated about every 3 months.
Conclusion
Using newer, expensive targeted oncology agents in a cost-effective manner must be a proactive, collaborative, and multidisciplinary process. Pharmacists should not be solely responsible for monitoring and controlling high-cost treatments. Well-informed, evidence-based decisions are needed to ensure expensive agents are used in the subset of patients who are most likely to benefit. Clinical tools addressing value should be used to aid in appropriate and cost-effective treatment plans using drug monographs and CFUs, VHA Guidance on Determining Clinical Benefit of High Cost Oncology Drugs, and the Oral Chemotherapy Dispensing and Monitoring Reference, among other resources. Due to the subjective nature of value in medicine, agreeing on policy will have many challenges, such as how to place a value on various gains in overall survival, progression free survival, response rates, and QOL.
eAppendix
1. Bach PB. Limits on Medicare's ability to control rising spending on cancer drugs. N Engl J Med. 2009;360(6):626-633.
2. Kantarjian H, Steensma D, Rius Sanjuan J, Eishaug A, Light D. High cancer drug prices in the United States: reasons and proposed solutions. J Oncol Pract. 2014;10(4):e208-e211.
3. Goldstein DA, Chen Q, Ayer T, et al. Necitumumab in metastatic squamous cell lung cancer: establishing a value-based cost. JAMA Oncol. 2015;1(9):1293-1300.
4. Thatcher N, Hirsch FR, Luft AV, et al; SQUIRE Investigators. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2015;16(7):763-774.
5. U.S. Department of Veterans Affairs, National Acquisition Center, Pharmaceutical Catalog Search. U.S. Department of Veterans Affairs, National Acquisition Center website. http://www1.va.gov/nac/index.cfm?template=Search_Pharmaceutical_Catalog. Updated June 13, 2016. Accessed June 13, 2016.
6. Lakdawalla DN, Romley JA, Sanchez Y, Maclean JR, Penrod JR, Philipson T. How cancer patients value hope and the implications for cost-effectiveness assessments of high-cost cancer therapies. Health Aff (Millwood). 2012;31(4):676-682.
7. Ubel PA, Berry SR, Nadler E, et al. In a survey, marked inconsistency in how oncologists judged value of high-cost cancer drugs in relation to gains in survival. Health Aff (Millwood). 2012;31(4):709-717.
8. Asch SM, McGlynn EA, Hogan MM, et al. Comparison of quality of care for patients in the Veterans Health Administration and patients in a national sample. Ann Intern Med. 2004;141(12):938-945. 9. Longman P. Best Care Anywhere: Why VA Health Care Would Work for Everyone. 3rd ed. San Francisco, CA: Berrett-Koehler Publishers; 2012. 10. Heron BB, Geraci MC. Controlling the cost of oncology drugs within the VA: a national perspective. Fed Pract. 2015;32(suppl 1):18S-22S.
11. U.S. Department of Veterans Affairs. Pharmacy Benefits Management Services Intranet, Documents and Lists. https://vaww.cmopnational.va.gov/cmop/PBM/Clinical%20Guidance/Forms/AllItems.aspx. Accessed May 19, 2016.
1. Bach PB. Limits on Medicare's ability to control rising spending on cancer drugs. N Engl J Med. 2009;360(6):626-633.
2. Kantarjian H, Steensma D, Rius Sanjuan J, Eishaug A, Light D. High cancer drug prices in the United States: reasons and proposed solutions. J Oncol Pract. 2014;10(4):e208-e211.
3. Goldstein DA, Chen Q, Ayer T, et al. Necitumumab in metastatic squamous cell lung cancer: establishing a value-based cost. JAMA Oncol. 2015;1(9):1293-1300.
4. Thatcher N, Hirsch FR, Luft AV, et al; SQUIRE Investigators. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2015;16(7):763-774.
5. U.S. Department of Veterans Affairs, National Acquisition Center, Pharmaceutical Catalog Search. U.S. Department of Veterans Affairs, National Acquisition Center website. http://www1.va.gov/nac/index.cfm?template=Search_Pharmaceutical_Catalog. Updated June 13, 2016. Accessed June 13, 2016.
6. Lakdawalla DN, Romley JA, Sanchez Y, Maclean JR, Penrod JR, Philipson T. How cancer patients value hope and the implications for cost-effectiveness assessments of high-cost cancer therapies. Health Aff (Millwood). 2012;31(4):676-682.
7. Ubel PA, Berry SR, Nadler E, et al. In a survey, marked inconsistency in how oncologists judged value of high-cost cancer drugs in relation to gains in survival. Health Aff (Millwood). 2012;31(4):709-717.
8. Asch SM, McGlynn EA, Hogan MM, et al. Comparison of quality of care for patients in the Veterans Health Administration and patients in a national sample. Ann Intern Med. 2004;141(12):938-945. 9. Longman P. Best Care Anywhere: Why VA Health Care Would Work for Everyone. 3rd ed. San Francisco, CA: Berrett-Koehler Publishers; 2012. 10. Heron BB, Geraci MC. Controlling the cost of oncology drugs within the VA: a national perspective. Fed Pract. 2015;32(suppl 1):18S-22S.
11. U.S. Department of Veterans Affairs. Pharmacy Benefits Management Services Intranet, Documents and Lists. https://vaww.cmopnational.va.gov/cmop/PBM/Clinical%20Guidance/Forms/AllItems.aspx. Accessed May 19, 2016.
Diabetic Peripheral Neuropathy: The Learning Curve
When R was a 19-year-old sailor heading out to sea, he had no idea of the forthcoming medical diagnosis that would change his life. R was like any other young seaman: ready to do his assigned tasks and ready to serve his country. He was stationed on a Los Angeles class, nuclear-powered, fast attack submarine. R was living his lifelong dream of serving in the U.S. Navy. The submarine was conducting sound trials and tactical readiness exams off the southeast U.S. coast near Bermuda. The days were long, but he loved what he was doing, so he ignored his symptoms of fatigue, attributing it to his busy schedule. He was enjoying his time in the navy and looking forward to a long career.
Diagnosis
R was assigned watch duty during the day, but he couldn’t understand why he felt so fatigued during his watch or his ability to fall asleep while standing. R didn’t complain because he knew everyone was working hard, long hours. He knew he was not sleeping well, mainly due to the frequent trips to the bathroom to urinate, and at first attributed it to drinking large amounts of coffee and sugar to stay awake during the drills. He also knew he was constantly hungry, thirsty, and tired. After falling asleep while on duty during the exercise, R found himself facing possible disciplinary action. He had no idea what was happening but realized it was not in his nature to fail at a task and certainly not to fall asleep on duty. Having a chronic disease that would affect him for the rest of his life was certainly not on his mind. He recalled, “At the time I didn’t even know what diabetes was.”
R finally admitted his array of symptoms to one of the corpsman. He often urinated every 20 minutes and at times did not make it to the bathroom. His vision was blurred to the point he could not make out faces just a few feet away from him, and the lethargy was overwhelming.
The corpsman immediately knew something was wrong with R and instructed R to report to the boat’s sick bay. Fortunately for R, the classic symptoms of hunger, thirst, frequent urination, and fatigue struck a chord with the corpsman who also noticed that R had lost a great amount of weight, a fact R had not noticed. Labs were drawn, and a urine specimen was obtained. R had a blood glucose level of > 1,000 mg/dL, was in severe ketoacidosis, and was diagnosed with type 1 diabetes mellitus (T1DM). The corpsman was surprised he was even coherent at this point. He was given IV infusions in both arms. The boat’s mission was halted. The immediate thought was to send for a medical evacuation helicopter. The weather conditions were too severe at the time to arrange for air evacuation, so the captain decided to head back to port and transfer R to the Portsmouth Naval Medical Center. R will never forget that day; however, the days and weeks following became somewhat of a blur. R recalls, “Time seemed to standstill some days, then others were on fast forward.” He was hospitalized for the next 2 weeks. His condition was stabilized, and he learned how to care for himself.
Learning About Diabetes
The following weeks and months while on medical hold and being processed for discharge, R was assigned a variety of duties. He felt well prepared to manage his disease on a daily basis and at first had hopes of continuing his navy career. He recalls now, he had no clue what the diagnosis would mean in the years to come. R learned he would eventually be medically retired from the navy and rejoined civilian life.
Initial Complications
After leaving the navy, R decided to become a law enforcement officer. He joined a local police department and quickly rose through the ranks. He began to settle into a routine, learning to manage his insulin, control his diet, and enjoy his new career. For the next several years, he experienced few complications, although he never regained the 50 pounds he had lost when he was first diagnosed. Around 25 years old, he began to notice pain in the bottom of his feet. He was still able to run, had great balance, and didn’t think his symptoms of sore feet were attributable to his diabetes. He did notice that without shoes on, his feet were extremely sensitive to any texture.
Over the next year, R experienced worsening pain and increased sensitivity in his feet. He started to spend more time in his patrol car instead of on foot patrol because of the pain. He was no longer able to enjoy one of his favorite pastimes, walking barefoot on the beach. During the next several years, R would gradually begin to realize he had no sensation in his feet. He noted this affected his balance and gait. He loved his career in law enforcement, but often the complications of his disease would impact his daily work. He felt he was no longer fulfilling his responsibilities as an officer because of his inability to complete daily assignments due to the neuropathy in his feet. He left his law enforcement career and spent most of his time in an office, which was much less taxing on his body.
Foot Ulcers
In 2011, 15 years after the T1DM diagnosis, R experienced his first foot wound. After a day of hiking and walking in creek beds, he realized he had essentially rubbed off the skin on the ball of his foot. He cleaned it like he normally would; however, the area failed to heal. He developed a hard callus around the wound, but the center remained open. At the time, he did not realize the significance of this type of wound for a diabetic patient.
The foot ulcer was discovered while in the emergency department for an unrelated issue. It was then he was referred to the Greenville VA Outpatient Clinic wound healing center in South Carolina for further treatment. At 36 years old, he was far younger than most of the veterans being treated for diabetic foot ulcers. Per the CDC Report Card, about 90% to 95% of patients with diabetes have type 2 diabetes mellitus (T2DM).1,2 Most persons diagnosed with diabetes are in the fifth and sixth decades of life.1,2 For R, patient education had consisted of learning to manage his diet and insulin therapy. He has no recollection of education about future complications and reported feeling “clueless” about the potential complications of foot ulcerations.
During the patient’s first visit to the wound healing center, R was educated about diabetic foot health, complications, the healing process, and the importance of diabetes management. The center is staffed by a nurse practitioner (NP) certified in wound care with extensive experience in diabetic foot ulcers and by several wound care nurses. Each staff member incorporates patient education and positive reinforcement into every patient visit. According to Jeffrey Frenchman, DPM, director of limb preservation at the Atlanta VAMC in Georgia, “Patient education and positive reinforcement cost nothing to provide and offer great return on patient adherence.” (Jeffrey Frenchman, April 12, 2014, oral interview).
R visited the center once or twice weekly, depending on the appearance of the wound and the type of treatment he was receiving. He noted that having frequent contact with the wound center staff made him feel as though he was making progress. For the staff, ensuring R could adhere to the treatment regimen was paramount. If a patient is unable to follow home care instructions or lacks understanding of the importance of following wound care instructions, then the likelihood of adherence is less.
Continued Complications
R was unprepared for the months of healing. He learned about the importance of offloading (the reduction of pressure), noting that during the weeks he spent more time on his feet, ulcer healing failed to progress or worsened.3 Eventually, the ulcer healed, and he felt better prepared to prevent future problems as a result of having been educated about foot care. Unfortunately, he experienced his next complication a few months later after wearing new boots. When removing his boots at the end of the workday, he noticed blood on his sock. He realized the boots had caused blisters that had ruptured on the third, fourth, and fifth toes. Once again, having T1DM and totally insensate feet caused further problems with delayed healing. Since his first foot ulcer in 2011, R continued to have problems with foot ulcers. Some ulcers were caused by shoe pressure, blisters from hot beach sand, or from a typical neuropathic foot ulcer, which first develops as a preulcerative callus and rapidly progresses to an ulcer. Despite his daily astute monitoring of his feet he noted, “Problems just seem to occur overnight.”
Quality of Life
The greatest impact of diabetes for R was on his quality of life (QOL). He noted that the frustrations of dealing with foot wounds had a profound negative impact on QOL. As an avid outdoor enthusiast, the months he spent on crutches, wearing off-loading shoes, attending numerous wound clinic visits, and being unable to take part in the activities he loved greatly impacted his mental and physical well-being. “Having to change my daily routine such as bathing, driving, and even going out to dinner is hard enough. Having to give up hiking, camping, and swimming changes my entire outlook on life.” R also noted the unintended isolation from friends had a profound impact on his feelings. “They want to include you, but know they can’t. You want to go, but know you can’t keep up. Sometimes being alone is the worst feeling.”
Receiving care from wound care professionals offered R hope that his wounds would heal and he would return to the activities he enjoyed. He noted that the education and support he received from the wound center staff made him feel more confident not only in caring for current wounds, but also in preventing wounds in the future. He also realized that prompt treatment for even the smallest of wounds was essential.
R was able to contact the wound center staff either by phone or by secure messaging e-mail anytime he had a concern or question. When he developed new foot wounds, he could contact the staff and be evaluated within 72 hours of notification. He noted that being able to talk with the staff as soon as a problem developed offered him reassurance that he was properly taking care of his feet.
During his treatment, R needed to wear offloading shoes to minimize the weight-bearing pressure.3 The wound center staff took care to ensure that R could ambulate safely with these shoes and avoid further injury. They also reinforced the importance of wearing these shoes, despite their unfashionable appearance.
Given the depth of some of R’s foot ulcers, the staff used negative pressure dressings to enhance healing. Negative pressure dressings provide a vacuum source to create continuous or intermittent negative pressure inside a wound to remove fluid, exudates, and infectious materials and prepare the wound for healing and closure. A mechanically powered, negative pressure dressing with a 125 mm Hg cartridge device was used during R’s treatment. This type of negative pressure dressing offered the benefit of dressing changes twice weekly vs 3 times weekly with other electric-powered negative pressure devices.4
Another important aspect of R’s care was the use of human amniotic tissue allografts. When R’s wounds did not show healing progression during the first 4 weeks of traditional treatment, amniotic tissue allografts were added to his plan of care. This type of product for the wound bed provides critical growth factors and collagen to promote effective, enhanced wound healing. Patient education again is critical when using human amniotic tissue allografts so that the patient learns to keep dressings intact and undisturbed.5
Future Implications
Diabetic foot ulcers are a preventable complication of diabetic peripheral neuropathy. Patient education about foot health should not only be incorporated in diabetic education, but also reinforced by the health care staff at each visit. When a patient presents with a diabetic foot ulcer, early, prompt treatment is vital to ensure a favorable outcome.
For health care providers, cognizance of the impact that wounds have on patients’ QOL is an essential aspect of care. Identification of factors that promote expedient and effective wound healing is vital. Patient education that is focused on engaging the patient to actively participate in the healing process is paramount. Involving R in every aspect of his care was the focus of the wound center staff. Explaining the purpose of each product used and why it was chosen was not only interesting for R, but also allowed him to actively participate in his appointments and care. As the leader of the wound treatment team, the NP may order, guide, and direct care, but empowering patients to be active participants in their care enhances adherence to the plan of care.6
Conclusion
Focusing on these critical aspects of patient-centered wound healing must be at the forefront when treating patients with diabetic foot ulcers. Although a price cannot be placed on QOL, the cost of diabetic foot ulcers and its complications is astounding. In 2007, nearly $116 billion was spent on diabetes treatment, and more than one-third was for the care of diabetic foot ulcers and complications from those ulcers.7
Finally, the incidence of T2DM is rising: The average age of patients at the onset of T2DM is becoming younger, the development of T2DM in children is rising, and treatment costs are rising.1 Given the alarming statistics of T2DM and its complications in the U.S., focusing on prevention, patient education, and effective treatment of diabetic foot ulcers is important.
1. Diabetes Report Card 2014. Centers for Disease Control and Prevention website. http://www.cdc.gov/diabetes/pdfs/library/diabetesreportcard2014.pdf. Published 2012. Accessed June 1, 2016.
2. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. Centers for Disease Control and Prevention website. http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Published 2012. Accessed June 1, 2016.
3. Wu SC, Jensen JL, Weber AK, Robinson DE, Armstrong DG. Use of pressure offloading devices in diabetic foot ulcers: do we practice what we preach? Diabetes Care. 2008;31(11):2118-2119. Armstrong DG, Marston WA, Reyzelman AM. Kirsner RS.
4. Comparative effectiveness of mechanically and electrically powered negative pressure wound therapy devices: a multicenter randomized controlled trial. Wound Repair Regen. 2012;20(3):332-341.
5. Zelen CM, Serena TE, Fetterolf DE. Dehydrated human amnion/chorion membrane allografts in patients with chronic diabetic foot ulcers: a long-term follow-up study. Wound Med. 2014;4:1-4.
6. Boulton AJ, Kirsner RS, Vileikyte L. Clinical Practice. Neuropathic diabetic foot ulcers. N Engl J Med. 2004;351(1):48-55.
7. Driver VR, Fabbi M, Lavery LA, Gibbons G. The costs of diabetic foot: the economic case for the limb salvage team. J Vasc Surg. 2010;52(3)(suppl):17S-22S.
When R was a 19-year-old sailor heading out to sea, he had no idea of the forthcoming medical diagnosis that would change his life. R was like any other young seaman: ready to do his assigned tasks and ready to serve his country. He was stationed on a Los Angeles class, nuclear-powered, fast attack submarine. R was living his lifelong dream of serving in the U.S. Navy. The submarine was conducting sound trials and tactical readiness exams off the southeast U.S. coast near Bermuda. The days were long, but he loved what he was doing, so he ignored his symptoms of fatigue, attributing it to his busy schedule. He was enjoying his time in the navy and looking forward to a long career.
Diagnosis
R was assigned watch duty during the day, but he couldn’t understand why he felt so fatigued during his watch or his ability to fall asleep while standing. R didn’t complain because he knew everyone was working hard, long hours. He knew he was not sleeping well, mainly due to the frequent trips to the bathroom to urinate, and at first attributed it to drinking large amounts of coffee and sugar to stay awake during the drills. He also knew he was constantly hungry, thirsty, and tired. After falling asleep while on duty during the exercise, R found himself facing possible disciplinary action. He had no idea what was happening but realized it was not in his nature to fail at a task and certainly not to fall asleep on duty. Having a chronic disease that would affect him for the rest of his life was certainly not on his mind. He recalled, “At the time I didn’t even know what diabetes was.”
R finally admitted his array of symptoms to one of the corpsman. He often urinated every 20 minutes and at times did not make it to the bathroom. His vision was blurred to the point he could not make out faces just a few feet away from him, and the lethargy was overwhelming.
The corpsman immediately knew something was wrong with R and instructed R to report to the boat’s sick bay. Fortunately for R, the classic symptoms of hunger, thirst, frequent urination, and fatigue struck a chord with the corpsman who also noticed that R had lost a great amount of weight, a fact R had not noticed. Labs were drawn, and a urine specimen was obtained. R had a blood glucose level of > 1,000 mg/dL, was in severe ketoacidosis, and was diagnosed with type 1 diabetes mellitus (T1DM). The corpsman was surprised he was even coherent at this point. He was given IV infusions in both arms. The boat’s mission was halted. The immediate thought was to send for a medical evacuation helicopter. The weather conditions were too severe at the time to arrange for air evacuation, so the captain decided to head back to port and transfer R to the Portsmouth Naval Medical Center. R will never forget that day; however, the days and weeks following became somewhat of a blur. R recalls, “Time seemed to standstill some days, then others were on fast forward.” He was hospitalized for the next 2 weeks. His condition was stabilized, and he learned how to care for himself.
Learning About Diabetes
The following weeks and months while on medical hold and being processed for discharge, R was assigned a variety of duties. He felt well prepared to manage his disease on a daily basis and at first had hopes of continuing his navy career. He recalls now, he had no clue what the diagnosis would mean in the years to come. R learned he would eventually be medically retired from the navy and rejoined civilian life.
Initial Complications
After leaving the navy, R decided to become a law enforcement officer. He joined a local police department and quickly rose through the ranks. He began to settle into a routine, learning to manage his insulin, control his diet, and enjoy his new career. For the next several years, he experienced few complications, although he never regained the 50 pounds he had lost when he was first diagnosed. Around 25 years old, he began to notice pain in the bottom of his feet. He was still able to run, had great balance, and didn’t think his symptoms of sore feet were attributable to his diabetes. He did notice that without shoes on, his feet were extremely sensitive to any texture.
Over the next year, R experienced worsening pain and increased sensitivity in his feet. He started to spend more time in his patrol car instead of on foot patrol because of the pain. He was no longer able to enjoy one of his favorite pastimes, walking barefoot on the beach. During the next several years, R would gradually begin to realize he had no sensation in his feet. He noted this affected his balance and gait. He loved his career in law enforcement, but often the complications of his disease would impact his daily work. He felt he was no longer fulfilling his responsibilities as an officer because of his inability to complete daily assignments due to the neuropathy in his feet. He left his law enforcement career and spent most of his time in an office, which was much less taxing on his body.
Foot Ulcers
In 2011, 15 years after the T1DM diagnosis, R experienced his first foot wound. After a day of hiking and walking in creek beds, he realized he had essentially rubbed off the skin on the ball of his foot. He cleaned it like he normally would; however, the area failed to heal. He developed a hard callus around the wound, but the center remained open. At the time, he did not realize the significance of this type of wound for a diabetic patient.
The foot ulcer was discovered while in the emergency department for an unrelated issue. It was then he was referred to the Greenville VA Outpatient Clinic wound healing center in South Carolina for further treatment. At 36 years old, he was far younger than most of the veterans being treated for diabetic foot ulcers. Per the CDC Report Card, about 90% to 95% of patients with diabetes have type 2 diabetes mellitus (T2DM).1,2 Most persons diagnosed with diabetes are in the fifth and sixth decades of life.1,2 For R, patient education had consisted of learning to manage his diet and insulin therapy. He has no recollection of education about future complications and reported feeling “clueless” about the potential complications of foot ulcerations.
During the patient’s first visit to the wound healing center, R was educated about diabetic foot health, complications, the healing process, and the importance of diabetes management. The center is staffed by a nurse practitioner (NP) certified in wound care with extensive experience in diabetic foot ulcers and by several wound care nurses. Each staff member incorporates patient education and positive reinforcement into every patient visit. According to Jeffrey Frenchman, DPM, director of limb preservation at the Atlanta VAMC in Georgia, “Patient education and positive reinforcement cost nothing to provide and offer great return on patient adherence.” (Jeffrey Frenchman, April 12, 2014, oral interview).
R visited the center once or twice weekly, depending on the appearance of the wound and the type of treatment he was receiving. He noted that having frequent contact with the wound center staff made him feel as though he was making progress. For the staff, ensuring R could adhere to the treatment regimen was paramount. If a patient is unable to follow home care instructions or lacks understanding of the importance of following wound care instructions, then the likelihood of adherence is less.
Continued Complications
R was unprepared for the months of healing. He learned about the importance of offloading (the reduction of pressure), noting that during the weeks he spent more time on his feet, ulcer healing failed to progress or worsened.3 Eventually, the ulcer healed, and he felt better prepared to prevent future problems as a result of having been educated about foot care. Unfortunately, he experienced his next complication a few months later after wearing new boots. When removing his boots at the end of the workday, he noticed blood on his sock. He realized the boots had caused blisters that had ruptured on the third, fourth, and fifth toes. Once again, having T1DM and totally insensate feet caused further problems with delayed healing. Since his first foot ulcer in 2011, R continued to have problems with foot ulcers. Some ulcers were caused by shoe pressure, blisters from hot beach sand, or from a typical neuropathic foot ulcer, which first develops as a preulcerative callus and rapidly progresses to an ulcer. Despite his daily astute monitoring of his feet he noted, “Problems just seem to occur overnight.”
Quality of Life
The greatest impact of diabetes for R was on his quality of life (QOL). He noted that the frustrations of dealing with foot wounds had a profound negative impact on QOL. As an avid outdoor enthusiast, the months he spent on crutches, wearing off-loading shoes, attending numerous wound clinic visits, and being unable to take part in the activities he loved greatly impacted his mental and physical well-being. “Having to change my daily routine such as bathing, driving, and even going out to dinner is hard enough. Having to give up hiking, camping, and swimming changes my entire outlook on life.” R also noted the unintended isolation from friends had a profound impact on his feelings. “They want to include you, but know they can’t. You want to go, but know you can’t keep up. Sometimes being alone is the worst feeling.”
Receiving care from wound care professionals offered R hope that his wounds would heal and he would return to the activities he enjoyed. He noted that the education and support he received from the wound center staff made him feel more confident not only in caring for current wounds, but also in preventing wounds in the future. He also realized that prompt treatment for even the smallest of wounds was essential.
R was able to contact the wound center staff either by phone or by secure messaging e-mail anytime he had a concern or question. When he developed new foot wounds, he could contact the staff and be evaluated within 72 hours of notification. He noted that being able to talk with the staff as soon as a problem developed offered him reassurance that he was properly taking care of his feet.
During his treatment, R needed to wear offloading shoes to minimize the weight-bearing pressure.3 The wound center staff took care to ensure that R could ambulate safely with these shoes and avoid further injury. They also reinforced the importance of wearing these shoes, despite their unfashionable appearance.
Given the depth of some of R’s foot ulcers, the staff used negative pressure dressings to enhance healing. Negative pressure dressings provide a vacuum source to create continuous or intermittent negative pressure inside a wound to remove fluid, exudates, and infectious materials and prepare the wound for healing and closure. A mechanically powered, negative pressure dressing with a 125 mm Hg cartridge device was used during R’s treatment. This type of negative pressure dressing offered the benefit of dressing changes twice weekly vs 3 times weekly with other electric-powered negative pressure devices.4
Another important aspect of R’s care was the use of human amniotic tissue allografts. When R’s wounds did not show healing progression during the first 4 weeks of traditional treatment, amniotic tissue allografts were added to his plan of care. This type of product for the wound bed provides critical growth factors and collagen to promote effective, enhanced wound healing. Patient education again is critical when using human amniotic tissue allografts so that the patient learns to keep dressings intact and undisturbed.5
Future Implications
Diabetic foot ulcers are a preventable complication of diabetic peripheral neuropathy. Patient education about foot health should not only be incorporated in diabetic education, but also reinforced by the health care staff at each visit. When a patient presents with a diabetic foot ulcer, early, prompt treatment is vital to ensure a favorable outcome.
For health care providers, cognizance of the impact that wounds have on patients’ QOL is an essential aspect of care. Identification of factors that promote expedient and effective wound healing is vital. Patient education that is focused on engaging the patient to actively participate in the healing process is paramount. Involving R in every aspect of his care was the focus of the wound center staff. Explaining the purpose of each product used and why it was chosen was not only interesting for R, but also allowed him to actively participate in his appointments and care. As the leader of the wound treatment team, the NP may order, guide, and direct care, but empowering patients to be active participants in their care enhances adherence to the plan of care.6
Conclusion
Focusing on these critical aspects of patient-centered wound healing must be at the forefront when treating patients with diabetic foot ulcers. Although a price cannot be placed on QOL, the cost of diabetic foot ulcers and its complications is astounding. In 2007, nearly $116 billion was spent on diabetes treatment, and more than one-third was for the care of diabetic foot ulcers and complications from those ulcers.7
Finally, the incidence of T2DM is rising: The average age of patients at the onset of T2DM is becoming younger, the development of T2DM in children is rising, and treatment costs are rising.1 Given the alarming statistics of T2DM and its complications in the U.S., focusing on prevention, patient education, and effective treatment of diabetic foot ulcers is important.
When R was a 19-year-old sailor heading out to sea, he had no idea of the forthcoming medical diagnosis that would change his life. R was like any other young seaman: ready to do his assigned tasks and ready to serve his country. He was stationed on a Los Angeles class, nuclear-powered, fast attack submarine. R was living his lifelong dream of serving in the U.S. Navy. The submarine was conducting sound trials and tactical readiness exams off the southeast U.S. coast near Bermuda. The days were long, but he loved what he was doing, so he ignored his symptoms of fatigue, attributing it to his busy schedule. He was enjoying his time in the navy and looking forward to a long career.
Diagnosis
R was assigned watch duty during the day, but he couldn’t understand why he felt so fatigued during his watch or his ability to fall asleep while standing. R didn’t complain because he knew everyone was working hard, long hours. He knew he was not sleeping well, mainly due to the frequent trips to the bathroom to urinate, and at first attributed it to drinking large amounts of coffee and sugar to stay awake during the drills. He also knew he was constantly hungry, thirsty, and tired. After falling asleep while on duty during the exercise, R found himself facing possible disciplinary action. He had no idea what was happening but realized it was not in his nature to fail at a task and certainly not to fall asleep on duty. Having a chronic disease that would affect him for the rest of his life was certainly not on his mind. He recalled, “At the time I didn’t even know what diabetes was.”
R finally admitted his array of symptoms to one of the corpsman. He often urinated every 20 minutes and at times did not make it to the bathroom. His vision was blurred to the point he could not make out faces just a few feet away from him, and the lethargy was overwhelming.
The corpsman immediately knew something was wrong with R and instructed R to report to the boat’s sick bay. Fortunately for R, the classic symptoms of hunger, thirst, frequent urination, and fatigue struck a chord with the corpsman who also noticed that R had lost a great amount of weight, a fact R had not noticed. Labs were drawn, and a urine specimen was obtained. R had a blood glucose level of > 1,000 mg/dL, was in severe ketoacidosis, and was diagnosed with type 1 diabetes mellitus (T1DM). The corpsman was surprised he was even coherent at this point. He was given IV infusions in both arms. The boat’s mission was halted. The immediate thought was to send for a medical evacuation helicopter. The weather conditions were too severe at the time to arrange for air evacuation, so the captain decided to head back to port and transfer R to the Portsmouth Naval Medical Center. R will never forget that day; however, the days and weeks following became somewhat of a blur. R recalls, “Time seemed to standstill some days, then others were on fast forward.” He was hospitalized for the next 2 weeks. His condition was stabilized, and he learned how to care for himself.
Learning About Diabetes
The following weeks and months while on medical hold and being processed for discharge, R was assigned a variety of duties. He felt well prepared to manage his disease on a daily basis and at first had hopes of continuing his navy career. He recalls now, he had no clue what the diagnosis would mean in the years to come. R learned he would eventually be medically retired from the navy and rejoined civilian life.
Initial Complications
After leaving the navy, R decided to become a law enforcement officer. He joined a local police department and quickly rose through the ranks. He began to settle into a routine, learning to manage his insulin, control his diet, and enjoy his new career. For the next several years, he experienced few complications, although he never regained the 50 pounds he had lost when he was first diagnosed. Around 25 years old, he began to notice pain in the bottom of his feet. He was still able to run, had great balance, and didn’t think his symptoms of sore feet were attributable to his diabetes. He did notice that without shoes on, his feet were extremely sensitive to any texture.
Over the next year, R experienced worsening pain and increased sensitivity in his feet. He started to spend more time in his patrol car instead of on foot patrol because of the pain. He was no longer able to enjoy one of his favorite pastimes, walking barefoot on the beach. During the next several years, R would gradually begin to realize he had no sensation in his feet. He noted this affected his balance and gait. He loved his career in law enforcement, but often the complications of his disease would impact his daily work. He felt he was no longer fulfilling his responsibilities as an officer because of his inability to complete daily assignments due to the neuropathy in his feet. He left his law enforcement career and spent most of his time in an office, which was much less taxing on his body.
Foot Ulcers
In 2011, 15 years after the T1DM diagnosis, R experienced his first foot wound. After a day of hiking and walking in creek beds, he realized he had essentially rubbed off the skin on the ball of his foot. He cleaned it like he normally would; however, the area failed to heal. He developed a hard callus around the wound, but the center remained open. At the time, he did not realize the significance of this type of wound for a diabetic patient.
The foot ulcer was discovered while in the emergency department for an unrelated issue. It was then he was referred to the Greenville VA Outpatient Clinic wound healing center in South Carolina for further treatment. At 36 years old, he was far younger than most of the veterans being treated for diabetic foot ulcers. Per the CDC Report Card, about 90% to 95% of patients with diabetes have type 2 diabetes mellitus (T2DM).1,2 Most persons diagnosed with diabetes are in the fifth and sixth decades of life.1,2 For R, patient education had consisted of learning to manage his diet and insulin therapy. He has no recollection of education about future complications and reported feeling “clueless” about the potential complications of foot ulcerations.
During the patient’s first visit to the wound healing center, R was educated about diabetic foot health, complications, the healing process, and the importance of diabetes management. The center is staffed by a nurse practitioner (NP) certified in wound care with extensive experience in diabetic foot ulcers and by several wound care nurses. Each staff member incorporates patient education and positive reinforcement into every patient visit. According to Jeffrey Frenchman, DPM, director of limb preservation at the Atlanta VAMC in Georgia, “Patient education and positive reinforcement cost nothing to provide and offer great return on patient adherence.” (Jeffrey Frenchman, April 12, 2014, oral interview).
R visited the center once or twice weekly, depending on the appearance of the wound and the type of treatment he was receiving. He noted that having frequent contact with the wound center staff made him feel as though he was making progress. For the staff, ensuring R could adhere to the treatment regimen was paramount. If a patient is unable to follow home care instructions or lacks understanding of the importance of following wound care instructions, then the likelihood of adherence is less.
Continued Complications
R was unprepared for the months of healing. He learned about the importance of offloading (the reduction of pressure), noting that during the weeks he spent more time on his feet, ulcer healing failed to progress or worsened.3 Eventually, the ulcer healed, and he felt better prepared to prevent future problems as a result of having been educated about foot care. Unfortunately, he experienced his next complication a few months later after wearing new boots. When removing his boots at the end of the workday, he noticed blood on his sock. He realized the boots had caused blisters that had ruptured on the third, fourth, and fifth toes. Once again, having T1DM and totally insensate feet caused further problems with delayed healing. Since his first foot ulcer in 2011, R continued to have problems with foot ulcers. Some ulcers were caused by shoe pressure, blisters from hot beach sand, or from a typical neuropathic foot ulcer, which first develops as a preulcerative callus and rapidly progresses to an ulcer. Despite his daily astute monitoring of his feet he noted, “Problems just seem to occur overnight.”
Quality of Life
The greatest impact of diabetes for R was on his quality of life (QOL). He noted that the frustrations of dealing with foot wounds had a profound negative impact on QOL. As an avid outdoor enthusiast, the months he spent on crutches, wearing off-loading shoes, attending numerous wound clinic visits, and being unable to take part in the activities he loved greatly impacted his mental and physical well-being. “Having to change my daily routine such as bathing, driving, and even going out to dinner is hard enough. Having to give up hiking, camping, and swimming changes my entire outlook on life.” R also noted the unintended isolation from friends had a profound impact on his feelings. “They want to include you, but know they can’t. You want to go, but know you can’t keep up. Sometimes being alone is the worst feeling.”
Receiving care from wound care professionals offered R hope that his wounds would heal and he would return to the activities he enjoyed. He noted that the education and support he received from the wound center staff made him feel more confident not only in caring for current wounds, but also in preventing wounds in the future. He also realized that prompt treatment for even the smallest of wounds was essential.
R was able to contact the wound center staff either by phone or by secure messaging e-mail anytime he had a concern or question. When he developed new foot wounds, he could contact the staff and be evaluated within 72 hours of notification. He noted that being able to talk with the staff as soon as a problem developed offered him reassurance that he was properly taking care of his feet.
During his treatment, R needed to wear offloading shoes to minimize the weight-bearing pressure.3 The wound center staff took care to ensure that R could ambulate safely with these shoes and avoid further injury. They also reinforced the importance of wearing these shoes, despite their unfashionable appearance.
Given the depth of some of R’s foot ulcers, the staff used negative pressure dressings to enhance healing. Negative pressure dressings provide a vacuum source to create continuous or intermittent negative pressure inside a wound to remove fluid, exudates, and infectious materials and prepare the wound for healing and closure. A mechanically powered, negative pressure dressing with a 125 mm Hg cartridge device was used during R’s treatment. This type of negative pressure dressing offered the benefit of dressing changes twice weekly vs 3 times weekly with other electric-powered negative pressure devices.4
Another important aspect of R’s care was the use of human amniotic tissue allografts. When R’s wounds did not show healing progression during the first 4 weeks of traditional treatment, amniotic tissue allografts were added to his plan of care. This type of product for the wound bed provides critical growth factors and collagen to promote effective, enhanced wound healing. Patient education again is critical when using human amniotic tissue allografts so that the patient learns to keep dressings intact and undisturbed.5
Future Implications
Diabetic foot ulcers are a preventable complication of diabetic peripheral neuropathy. Patient education about foot health should not only be incorporated in diabetic education, but also reinforced by the health care staff at each visit. When a patient presents with a diabetic foot ulcer, early, prompt treatment is vital to ensure a favorable outcome.
For health care providers, cognizance of the impact that wounds have on patients’ QOL is an essential aspect of care. Identification of factors that promote expedient and effective wound healing is vital. Patient education that is focused on engaging the patient to actively participate in the healing process is paramount. Involving R in every aspect of his care was the focus of the wound center staff. Explaining the purpose of each product used and why it was chosen was not only interesting for R, but also allowed him to actively participate in his appointments and care. As the leader of the wound treatment team, the NP may order, guide, and direct care, but empowering patients to be active participants in their care enhances adherence to the plan of care.6
Conclusion
Focusing on these critical aspects of patient-centered wound healing must be at the forefront when treating patients with diabetic foot ulcers. Although a price cannot be placed on QOL, the cost of diabetic foot ulcers and its complications is astounding. In 2007, nearly $116 billion was spent on diabetes treatment, and more than one-third was for the care of diabetic foot ulcers and complications from those ulcers.7
Finally, the incidence of T2DM is rising: The average age of patients at the onset of T2DM is becoming younger, the development of T2DM in children is rising, and treatment costs are rising.1 Given the alarming statistics of T2DM and its complications in the U.S., focusing on prevention, patient education, and effective treatment of diabetic foot ulcers is important.
1. Diabetes Report Card 2014. Centers for Disease Control and Prevention website. http://www.cdc.gov/diabetes/pdfs/library/diabetesreportcard2014.pdf. Published 2012. Accessed June 1, 2016.
2. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. Centers for Disease Control and Prevention website. http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Published 2012. Accessed June 1, 2016.
3. Wu SC, Jensen JL, Weber AK, Robinson DE, Armstrong DG. Use of pressure offloading devices in diabetic foot ulcers: do we practice what we preach? Diabetes Care. 2008;31(11):2118-2119. Armstrong DG, Marston WA, Reyzelman AM. Kirsner RS.
4. Comparative effectiveness of mechanically and electrically powered negative pressure wound therapy devices: a multicenter randomized controlled trial. Wound Repair Regen. 2012;20(3):332-341.
5. Zelen CM, Serena TE, Fetterolf DE. Dehydrated human amnion/chorion membrane allografts in patients with chronic diabetic foot ulcers: a long-term follow-up study. Wound Med. 2014;4:1-4.
6. Boulton AJ, Kirsner RS, Vileikyte L. Clinical Practice. Neuropathic diabetic foot ulcers. N Engl J Med. 2004;351(1):48-55.
7. Driver VR, Fabbi M, Lavery LA, Gibbons G. The costs of diabetic foot: the economic case for the limb salvage team. J Vasc Surg. 2010;52(3)(suppl):17S-22S.
1. Diabetes Report Card 2014. Centers for Disease Control and Prevention website. http://www.cdc.gov/diabetes/pdfs/library/diabetesreportcard2014.pdf. Published 2012. Accessed June 1, 2016.
2. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. Centers for Disease Control and Prevention website. http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Published 2012. Accessed June 1, 2016.
3. Wu SC, Jensen JL, Weber AK, Robinson DE, Armstrong DG. Use of pressure offloading devices in diabetic foot ulcers: do we practice what we preach? Diabetes Care. 2008;31(11):2118-2119. Armstrong DG, Marston WA, Reyzelman AM. Kirsner RS.
4. Comparative effectiveness of mechanically and electrically powered negative pressure wound therapy devices: a multicenter randomized controlled trial. Wound Repair Regen. 2012;20(3):332-341.
5. Zelen CM, Serena TE, Fetterolf DE. Dehydrated human amnion/chorion membrane allografts in patients with chronic diabetic foot ulcers: a long-term follow-up study. Wound Med. 2014;4:1-4.
6. Boulton AJ, Kirsner RS, Vileikyte L. Clinical Practice. Neuropathic diabetic foot ulcers. N Engl J Med. 2004;351(1):48-55.
7. Driver VR, Fabbi M, Lavery LA, Gibbons G. The costs of diabetic foot: the economic case for the limb salvage team. J Vasc Surg. 2010;52(3)(suppl):17S-22S.
The Most Expensive Drug in the World: To Continue or Discontinue, That Is the Question
A 59-year-old man with a 20-year history (1994) of HIV well controlled on highly active antiretroviral therapy (HAART) therapy (baseline viral load undetectable, CD4+ cell count 781), presented to a community hospital (May 7, 2014) with abdominal pain. The patient’s girlfriend reported unusual behavior for 1 week before admission, including decreased appetite, binge drinking, and nonadherence to HAART therapy.
There was no history of fever, illegal medication use, or diarrhea. In addition to HIV, his past history was remarkable for hepatitis B, hypertension, and left lower extremity amputation secondary to a motor vehicle accident. He had a remote history of cocaine, PCP (phencyclidine), LSD (lysergic acid diethylamide), marijuana, and alcohol misuse and a 50 pack-year smoking history. His family history was remarkable for a mother who died of pancreatic cancer.
During his hospitalization, he developed pronounced expressive aphasia and lethargy but was able to follow simple commands. A computed tomography (CT) scan of the head revealed a left lacunar infarction, and he was transferred to the VA Long Beach Healthcare System in California for further care of a possible stroke.
Shortly after arrival, he developed a fever of 100.9º F. His pulse was 100 bpm and regular, blood pressure was 164/92 mm Hg, and respiratory rate was 14 breaths per minute. A physical examination was remarkable for somnolence, disorientation, and aphasia. He was grimacing to light palpation in all 4 quadrants of the abdomen and had diffuse purpura on skin examination. Laboratory results showed worsening thrombocytopenia, acute kidney injury with proteinuria and hemoglobinuria, and hemolysis (schistocytes, low haptoglobin level, and elevated lactate dehydrogenase [LDH]).
The patient’s changes in baseline laboratory results were platelet count 206,000 mm3 to 64,000 mm3, serum creatinine level 0.98 mg/dL to 1.55 mg/dL. His hemogram showed normochromic normocytic anemia (hemoglobin [Hb] level 10.2 g/dL) with schistocytes. Serum samples were initially unreportable by the laboratory due to severe hemolysis, but his haptoglobin level was found to be low and, conversely, LDH remarkably high. Fifteen days after admission, his CD4+ cell count was 141. An abdominal CT scan showed right lower quadrant abdominal free fluid and thickening of the terminal ileum with surrounding stranding, suggestive of terminal ileitis, and he was started on piperacillin-tazobactam. A lumbar puncture was unremarkable, and HAART medications were resumed. The patient required intubation and a ventilator for acute respiratory failure.
Empiric treatment for presumed thrombotic thrombocytopenic purpura (TTP) with plasmapheresis and methylprednisolone was ineffective, and the patient required mechanical ventilation and hemodialysis.
In refractory cases of TTP-hemolytic uremic syndrome, rituximab, a monoclonal antibody directed at CD20 present on B lymphocytes, is added empirically as effective salvage therapy and was therefore tried in this case.1
However, the addition of rituximab failed to improve the patient’s condition, and he developed further seizure activity and evidence of new lacunar infarctions as seen on magnetic resonance imaging of the brain. His hospital course was complicated by recurrent hemoptysis and respiratory failure, requiring assisted ventilation and eventually tracheostomy.
A normal ADAMTS13 level (72%) and negative Shiga toxin test changed the diagnosis to atypical hemolytic uremic syndrome (aHUS). Mean complement C3 (74 mg/dL) and C4 (9 mg/dL) levels were low. Plasmapheresis was discontinued, and treatment with eculizumab (Soliris, Alexion Pharmaceuticals) was initiated. Meningococcal vaccine was administered post-eculizumab, aimed at reducing but not eliminating the risk of meningococcemia.2 Two weeks later, the patient’s platelet count normalized, renal function improved, hemolysis resolved, and the patient regained full mental status. Eight weeks after initiating eculizumab, he no longer required dialysis.
Discussion
Generalized thrombosis of smaller blood vessels (thrombotic microangiopathy [TMA]) occurs in 3 uncommon syndromes—TTP, HUS, and aHUS—all with similar clinical presentations but distinct pathologic etiologies and treatment. These syndromes share a clinical picture of thrombocytopenia, hemolytic anemia, and renal failure. Hemolysis in these conditions is manifested by schistocytes, elevated lactate dehydrogenase from damaged cells, decreased haptoglobin, anemia, and hemoglobinuria.
Thrombotic Thrombocytopenic Purpura
Thrombocytopenic purpura occurs in about 3 cases per 1,000,000 adults per year.3 It occurs when the metalloproteinase enzyme ADAMTS13 activity is impaired, interrupting its function to cleave large sticky von Willebrand factor (vWF) multimers, resulting in coagulation in microvasculature by increased platelet aggregation, hemolysis from shearing of red blood cells, and compromised circulation to the highly vascularized kidney and other vital organs.4 The hallmark of TTP is a severely decreased ADAMTS13 activity (< 5% of normal) secondary to coexisting conditions, such as cancer, pregnancy, HIV infection, adverse effects (AEs), or antibodies to ADAMTS13.5
The TTP pentad of thrombocytopenia, hemolytic anemia, neurologic symptoms, renal failure, and fever were present in our patient. The patient had a known HIV infection but no exposure to medications associated with TTP (such as acyclovir, quinine, oxymorphone, platelet aggregation inhibitors, or immunosuppressants). Prior to obtaining ADAMTS13 level, the patient was treated empirically for TTP with early and daily plasma exchange to remove the inhibitor of ADAMTS13 and replace it with fresh frozen plasma. Rituximab also was used to inhibit production of antibodies to ADAMTS13 from CD20 B lymphocytes. These empiric clinical measures were not effective in stopping his decline in renal and neurologic functions.
Hemolytic Uremic Syndrome
Like TTP, HUS is also a consequence of thrombotic microangiopathy. However, in contrast to TTP, which is more commonly seen in adults,3 HUS is usually seen in young children secondary to Shiga toxin-producing Escherichia coli (STEC).6 Hemolytic uremic syndrome, also referred to as STEC-HUS or typical HUS, is a rare disease affecting 10 to 20 people per million annually. About 10% of these patients are classified as having aHUS because STEC is not implicated in their disease. It is of interest that, unlike aHUS, STEC-HUS is usually a self-limited disease of children, the majority of whom recover without relapse, and evidence that eculizumab improves prognosis in STEC-HUS is not compelling.5
Atypical Hemolytic Uremic Syndrome
Atypical HUS is a complement-mediated disease. The usual function of complement proteins is to destroy foreign cells and activate immune cells. However, in aHUS this protective defense system goes awry resulting in a pathologic thrombotic milieu. Specifically, aHUS is a continuous complement mediated attack on vascular endothelial beds due to the failure of protein regulators to terminate the complement cascade. Unlike typical HUS, which is usually associated with a Shiga-toxin producing gastrointestinal infection, the trigger in aHUS is unknown and thought to be associated with a genetic predisposition.
Atypical HUS distinguishes itself from TTP and HUS in that it does not respond to plasma exchange, corticosteroids, rituximab, or other immunosupressants. This is due to the distinct underlying pathophysiology of aHUS in which the problem is the unbridled activation of the alternate arm of the complement system.
The complement system is part of the innate immune system, which acts with or without the adaptive immune system it “complements” by amplifying a cascade of responses to eliminate the trigger pathogen. There are 3 complement pathways—classical, lectin, and alternate. The alternate complement pathway, whose activation generates C5a complement (anaphylotoxin), was most pertinent to this case. This precipitates a number of downstream protein cleaving events that lead to the cell lysing membrane attack complex (MAC), which creates a pore in the cell membrane of pathogens seen as foreign. In aHUS, the patient’s own cells come under attack by their own complement, which is no longer inhibited due to mutations in regulatory proteins of the alternate pathway.
With the foot off the complement brake (the hallmark feature underlying aHUS), endothelial cells, leukocytes, and platelets become hyperactive and thrombogenic, thereby resulting in microangiopathy and ischemia of involved organs.7 These mutations may be sporadic or familial and occur in a genetically susceptible host.8 It should be emphasized that genetic testing in complement mediated HUS is a specialized and slow process (weeks); the initial clinical diagnosis is one of exclusion and does not rest on genetic testing. Furthermore, serum complement levels may be normal in cases of complement mediated aHUS.9-11
This patient had a life-threatening condition that required distinguishing it from 2 rare diseases with very similar presentations; failure to do so in a timely fashion could easily have resulted in his demise. TTP or HUS was the important question, and ADAMTS13 level was one of the determining diagnostic tests. The usual interventions for TTP and HUS (plasmapheresis/plasma exchange and, in some cases, rituximab) were ineffective in this patient with aHUS. The patient achieved full recovery of neurologic, renal, and hematologic impairments after treatment with eculizumab, the recombinant humanized monoclonal antibody that binds to the complement protein C5 brake and inhibits its enzymatic cleavage, thereby interfering with the production of the MAC and cell lysis.
Although the patient did not have an identifiable mutation in the panel of complement regulatory genes tested, the rather dramatic efficacy of the orphan drug eculizumab was in a sense confirmation of his complement related hemolytic uremia. Left undecided are the questions of how long to continue eculizumab, the potential risk of relapse with discontinuation, and the ethical dilemma of proper length of treatment with the most expensive medication in the world given its total cost and no clear discontinuation criteria.12-15 The cost of medications for rare and ultra-rare orphan drugs have approached unsustainable levels, posing ethical challenges to many developed countries.16
Eculizumab and Orphan Drugs
Several months before his assassination, President Kennedy awarded Frances Kathleen Oldham Kelsey, MD, PhD, the President’s Award for Distinguished Federal Civilian Service (August 7, 1962) for her insistence that more safety evidence for thalidomide be presented before she would approve its use in the U.S. As a result of the thalidomide tragedy, the Kefauver Harris amendment was passed unanimously by Congress and signed into law by President Kennedy on October 10, 1962. It required stringent evidence of safety and efficacy for FDA approval of a new medication, reporting of AEs to the FDA, truth in drug advertising, rules governing generic drugs, and informed consent from patients participating in clinical trials.
An unintended consequence was that the development of medications for uncommon diseases became fiscally unattractive to the pharmaceutical industry, ie, “orphaned.” The Orphan Drug Act was enacted by Congress in 1983 to encourage development of drugs to treat less common diseases (diseases/disorders affecting fewer than 200,000 people in the U.S.) through incentives such as exclusive use approval for 7 years, reduced taxes, grants, and favorable laws. Ironically, thalidomide was designated an orphan drug on October 14, 1998 for treatment of multiple myeloma. Since its enactment in 1983, more than 400 orphan drugs and biologic products have been marketed. There may be as many as 7,000 orphan diseases to target for drug therapy, and the 17 of the 20 most expensive drugs in the world in 2013 were for rare orphan diseases.16
Paroxysmal nocturnal hemoglobinuria (PNH) is one of those rare diseases. Mutations of hematopoietic stem cells produce red blood cell membranes deficient in the glycoprotein to which signaling proteins attach (glycosyl phosphatidylinositol) and serve to inhibit complement-induced lysis. This results in intravascular hemolysis (increased LDH and decreased haptoglobin) and increased thrombosis. The FDA approved the orphan drug eculizumab for the treatment of the orphan disease PNH on March 16, 2007.
Eculizumab is a humanized mouse monoclonal antibody that gained FDA orphan drug approval (and exclusivity rights until 2019) for the treatment of aHUS on September 23, 2011, based on 2 industry-sponsored small trials of 17 and 20 patients, and it remains the primary and only known effective treatment for this disease.17-19
Eculizumab has raised many interesting questions. Its mechanism of action wets the appetite of pharmacologists and unveils more basic science questions regarding other related mechanisms of disease, recognition of foreign vs self, genetic influences, virulence of organisms, and more. National and international dilemmas have arisen because of the extreme cost of eculizumab, its position as the only effective treatment for this rare and often fatal disease, and the manufacturer’s recommendation and promotion that it be continued indefinitely. How should the price of a drug, developed in large part by government-supported research and tax incentives, and without competition, be determined and justified?
Pharmaceutical Inflation
A marketplace for pharmaceuticals is simply not analogous to other industries. Advances in pharmacotherapy, some miraculous, have come at a substantial cost. The high cost of drugs became newsworthy with the AIDS pandemic and the approval of the lifesaving azidothymidine (AZT) in 1989 (Burroughs Wellcome–also the developer of pyrimethamine [Daraprim]) and its then record price. Cancer treatment that used to cost $10,000 per year now costs $10,000 per month while the oncology community extolls a 2- or 3-month progression free survival benefit. Patients must now deal with the shock of a cancer diagnosis followed by the shock of an exorbitant copayment.
Recent media attention focused derision on Martin Shkreli, chief executive officer of Turing Pharmaceuticals, for purchasing pyrimethamine and then raising the price of the 62-year-old treatment for protozoan infection toxoplasmosis from $13.50 to $750 per pill. The debacle may also serve to highlight the complexities and ethical issues involved when profit intersects with health care. Some drug costs have dramatically increased in the U.S. because of greed, a belief that a marketplace can control costs, and the lack of regulation. The usual suspects, such as cost of research, length of development, stimulus to innovation, and return on investment, are difficult to apply to old medications whose marketing rights were acquired by purchase of another company. Can marketplace economics be applied in health situations where there is no competition, legal protections afforded manufacturers, consumers unable to make an informed decision?
While pharmacy and therapeutics committees were debating treatment of hepatitis C with either of 2 drugs approved in 2011, boceprevir (Victrelis, Merck) or telapravir (Incivek, Vertex and Johnson & Johnson), Gilead Pharmaceuticals acquired Pharmasset Inc. and its hepatitis C drug sofosbuvir (Sovaldi) for a whopping $11.2 billion in 2012. It received FDA approval April 8, 2013, under Breakthrough Therapy Designation.
While economists argued over the wisdom of such a high-cost acquisition, Gilead generated $9 billion in sales during the first 3 quarters of 2014, surpassing adalimumab (Humira), which had been the highest earning drug in 2014. Hepatitis C could now be quickly treated with truly unprecedented efficacy and without the AEs of interferon. The oft-quoted cost $1,000 per pill or about $84,000 per treatment in the U.S. drew international attention. Prior options for hepatitis C treatment, which preceded sofosbuvir by a mere couple of years, fell into pharmaceutical extinction. Telapravir succumbed to competition and ceased to be manufactured on August 12, 2014. Shortly after approval of sofosbuvir, Gilead also gained approval of its combination product for hepatitis C ledipasvir 90 mg/sofosbuvir 400 mg (Harvoni) on October 10, 2014.
The Most Expensive Drug in the World
Although there are no shortages of contenders for the coveted most expensive title, eculizumab is the current champion. Drugs that offer a cure, such as antibiotics, usually involve a relatively short, onetime course. Lack of return on the cost of development of curative agents may have reduced industrial incentives to develop antibiotics. On the other hand, the extent of infectious diseases, such as malaria, Ebola, tuberculosis, HIV, hepatitis, and the proliferation of drug-resistant organisms, continues to fuel industrial interest for this lifesaving class of medications.
Cancer medications touting a brief interruption of the race to death have raised questions of affordability, equitable access, and quality vs quantity of life. The $11,000 per month endothelial growth factor inhibitor aflibercept (Zaltrap, Regeneron, and Sanofi) was approved by the FDA in November 2012 for colorectal cancer and was followed by a historic rejection by Memorial Sloan-Kettering Cancer Center, since its cost was nearly double that of a similar medication bevacizumab (Avastin) with similar meager benefit of a median progression free survival of 1.4 months.20 Moreover Medicare is mandated to cover the price the manufacturers charge plus a 6% cushion for any cancer drug that the FDA approves.21 Patients with private insurance, often elderly and on a fixed income, are burdened with a copayment requirement of 20% of the cost of the drug. The nonnegotiation clause of Medicare has not reduced cost of medications, particularly for cancer, which many of the elderly will likely face.
The VA, a single-payer system distinguished by bipartisan congressional support, can directly negotiate with pharmaceutical companies, resulting in lower drug prices than discounts guaranteed by federal law; but what if there is no competitor? Biosimilar drugs are currently being debated by those seeking to prolong their patent protection. Stem cell therapies that offer a cure for some rare diseases or hope for common diseases are certain to command astronomical prices. Gene therapy offers hope for cure of both rare and common afflictions but at astounding prices.
The medication alipogene tiparvovec (Glybera, UniQure) delivered by adenovirus, for example, has been approved for use in the rare disorder lipoprotein lipase deficiency and is anticipated to cost $1.6 million for a onetime curative treatment. The pharmaceutical industry has joined the gene therapy race. While this is indeed a record acquisition sum for alipogene tiparvovec, at least it offers a cure. Eculizumab, although unique and effective, offers indefinite administration at a cost exceeding $600,000 per year, every year, for life. In 2014, sales of eculizumab climbed 44% to $2.234 billion.
To Continue or Discontinue Treatment
The duration of treatment with eculizumab poses a challenging dilemma for patients, clinicians, and health care providers. Eculizumab is the only effective treatment for a life-threatening condition, and the manufacturer, Alexion, recommends lifelong therapy of its product that has no competitors. Our patient was treated with 47 fixed-dose infusions of eculizumab at 2-week intervals from May 31, 2014, to February 18, 2016, at a cost of $737,957.80. The commercial cost outside the VA would be about 1.8 times this amount ($1.3 million). This extraordinary cost is the basic ethical issue. Without competition there is little to negotiate.
Need the treatment be lifelong? The AEs of eculizumab are not trivial, and some clinicians felt evidence for indefinite use in this patient was not compelling. Our patient’s initial critical and unstable condition had completely resolved after 2 months of eculizumab. The initial unknown precipitating event triggering the patient’s aHUS probably had resolved. His genetic testing did not disclose any HUS-related mutation. The patient’s serum was sent to Cincinnati Children’s Hospital (CCH) Clinical Laboratory Service to determine his eculizumab level and complement inhibition. His complement inhibition, as measured by CH50 activity, was adequately suppressed at 6% on eculizumab (target of < 10%) in spite of a free serum eculizumab level (81 mg/mL) that was somewhat below the therapeutic range of > 100 mg/mL.
Arguments for lifelong eculizumab therapy are based in part on the theoretical development of anti-eculizumab antibodies that could render reinstitution of eculizumab ineffective.22 Monitoring patients for relapse of their aHUS involves following markers of disease activity (levels of creatinine, LDH, haptoglobin, platelet counts, and Hb in urine). A report of 10 adult patients with aHUS who were treated effectively with eculizumab supports a trial of discontinuation.23 Seven of the 10 patients did not relapse following discontinuation of eculizumab. Three of 10 patients experienced a relapse when monitored for a cumulative 95 months, but all 3 had immediate and complete recovery after resuming therapy. All 3 patients who experienced relapse carried a complement factor H mutation. Their relapses occurred within 6 weeks from the last dose and were detected simply by performing home urine dipstick monitoring for haptoglobin 3 times per week. The 3 patients who relapsed promptly responded to eculizumab reinstitution with return of their labs to baseline.
Monitoring of complement function in patients with aHUS can guide clinically appropriate dosing intervals without changing disease activity markers.24 The half-life of eculizumab is about 11 days, and dosing intervals may be safely extended beyond 2 weeks in select patients.25 The target minimum inhibitory serum eculizumab level necessary to inhibit complement-mediated hemolysis is 50 µg/mL and 35 mg/mL for aHUS and PNH, respectively. In a small pharmacokinetic pilot study, Gatault and colleagues noted that trough levels during eculizumab maintenance by enzyme-linked immunoabsorbent assay (ELISA) of 44 mg/mL to 59 mg/mL inhibited the complement cascade.26 We suggested that weight-based dosing aiming at a trough > 50 mg/mL (rather than fixed dosing at a fixed interval) would be a better maintenance strategy.
In select patients, a trial of gradual discontinuation by lengthening the dosing interval of eculizumab seems a reasonable and safe alternative to indefinite continuation of the drug. After a patient’s successful recovery, the initial and unknown trigger of aHUS may no longer play a role. Improvement in the patient’s medical condition may permit the restoration of the patient’s defenses to once again function normally. Eculizumab seemed to retain its efficacy in the small number of patients who relapsed. Those who relapsed had positive genetic markers.
Further arguments favoring trial discontinuation in patients without known genetic predisposition are that continuation is not without risk, particularly of meningococcal infection, necessity for infusion every 2 weeks for life, little is known regarding long-term risk of the drug, and a lot is known of its extreme cost. Suppression of C5 inhibitory effect can lead to increased susceptibility to infections, whereas increased C5 activity may lead to a continued autoimmune attack on native cells.
As proposed by others, we suggest that this decision be made on a case by case basis, tailoring treatment based on an individual’s genetics and medical history.27 Although the European Medicines Agency has approved lifelong therapy for aHUS, this may be appropriate only for patients who have aHUS complement mutations associated with poor outcomes.27 This approach may not be warranted, however, in a patient, such as the one presented with no genetic mutations, or in those with mutations of uncertain clinical consequence. In such cases, given that 90% of adults who have a relapse experience within the first year after an aHUS episode, a reasonable alternative may be a trial discontinuation.27,28 After 1 year of eculizumab therapy, a trial of discontinuation with urine dipstick monitoring for Hb (Hemastix) 3 times a week for relapse may avoid the unnecessary expense and risk for infection posed by lifelong therapy, and eculizumab may be effectively restarted in case of relapse.
We propose that in these cases it would be reasonable to perform a trial of discontinuation after 1 year of therapy with urine dipstick monitoring for relapse, as lifelong therapy may pose unnecessary expense and risk for infection. In fact, given the financial burden of prolonged therapy on society, we believe it is unethical to continue treatment in a patient with unknown risk for relapse without a trial of discontinuation, as evidence has shown good response to re-initiation of therapy in the event of relapse.28 Agencies that have negotiated or attempted to regulate the cost of eculizumab have been met with public media campaigns featuring afflicted children at risk of death without eculizumab. The public relations company behind these efforts received support from Alexion.29-31
Conclusion
Given the formidable cost and the international monopoly status of eculizumab for a life-threatening condition, prospective discontinuation trials supported by the manufacturer would seem warranted in select cases. Delineating which patients will have a chronic relapsing course and those who will not should be one of these clinical trials. For now, one can only wonder: What’s worse than having a rare disease like aHUS? Perhaps the cost of treatment for a potentially indefinite period of time—now that’s a “bitter pill.”32
1. Caramazza D, Quintini G, Abbene I, et al. Relapsing or refractory idiopathic thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: the role of rituximab. Transfusion. 2010;50(12):2753-2760.
2. Köse O, Zimmerhackl LB, Jungraithmayr T, Mache C, Nürnberger J. New treatment options for atypical hemolytic uremic syndrome with the complement inhibitor eculizumab. Semin Thromb Hemost. 2010;36(6):669-672.
3. Reese JA, Muthurajah DS, Kremer Hovinga JA, Vesely SK, Terrell DR, George JN. Children and adults with thrombotic thrombocytopenic purpura associated with severe, acquired Adamts13 deficiency: comparison of incidence, demographic and clinical features. Pediatr Blood Cancer. 2013;60(10):1676-1682.
4. Sadler JE. Von Willebrand factor, ADAMTS13, and thrombotic thrombocytopenic purpura. Blood. 2008;112(1):11-18.
5. Tsai H-M. Pathophysiology of thrombotic thrombocytopenic purpura. Int J Hematol. 2010;91(1):1-19.
6. Noris M, Remuzzi G. Hemolytic uremic syndrome. J Am Soc Nephrol. 2005;16(4):1035-1050.
7. Liszewski MK, Atkinson JP. Exploring the complement system in human disease. The Rheumatologist website. http://www.the-rheumatologist.org/article/exploring-the-complement-system-in-human-disease. Published February 1, 2010. Accessed May 9, 2016.
8. Mayer CL, Leibowitz CS, Kurosawa S, Stearns-Kurosawa DJ. Shiga toxins and the pathophysiology of hemolytic uremic syndrome in humans and animals. Toxins (Basel). 2012;4(11):1261-1287.
9. Noris M, Mescia F, Remuzzi G. STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat Rev Nephrol. 2012;8(11):622-633.
10. Geerdink LM, Westra D, van Wijk JA, et al. Atypical hemolytic uremic syndrome in children: complement mutations and clinical characteristics. Pediatr Nephrol. 2012;27(8):1283-1291.
11. Sellier-Leclerc AL, Frémeaux-Bacchi V, Dragon-Durey MA, et al; French Society of Pediatric Nephrology. Differential impact of complement mutations on clinical characteristics in atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2007;18(8):2392-2400.
12. Herper M. The worlds most expensive drugs. Forbes. February 22, 2010.
13. Nordrum A. Drug prices: world's most expensive medicine costs $440,000 a year, but is it worth the expense? International Business Times website. http://www.ibtimes.com/drug-prices-worlds-most-expensive-medicine-costs-440000-year-it-worth-expense-2302609. Updated February 13, 2016. Published June 24, 2015. Accessed June 15, 2016.
14. CBC News. The real cost of the world's most expensive drug [video]. CBC/Radio Canada website. http://www.cbc.ca/player/play/2670383596. Accessed June 15, 2016.
15. EvaluatePharma. Orphan Drug Report 2014. EvaluatePharma website. http://www.evaluategroup.com/orphandrug2014. Published 2014. Accessed June 15, 2016.
16. Isaacs D. Ethical dilemmas about orphan drugs for orphan diseases. J Paediatr Child Health. 2014;50(4):249-250.
17. Licht C, Muus P, Legendre CM, et al. Eculizumab (ECU) safety and efficacy in atypical hemolytic uremic syndrome (aHUS) patients with long disease duration and chronic kidney disease (CKD): 2-year results. Poster presented at: 54th Annual Meeting of the American Society of Hematology; December 8-12, 2012; Atlanta, GA.
18. Greenbaum L, Legendre CM, Babu S, et al. Eculizumab (ECU) in atypical hemolytic uremic syndrome (aHUS) patients with progressing thrombotic microangiopathy (TMA): 2-year data. Poster presented at: 54th Annual Meeting of the American Society of Hematology; December 8-12, 2012; Atlanta, GA.
19. Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Eng J Med. 2013;368(23):2169-2181.
20. Bach PB, Saltz LB, Wittes RE. In cancer care, cost matters. New York Times. October 14, 2012:Opinion Pages.
21. Medicare Prescription Drug, Improvement, and Modernization Act of 2003, Public Law 108-173;117 Stat 2066. U.S. Government Printing Office website. https://www.gpo.gov/fdsys/pkg/PLAW-108publ173/pdf/PLAW-108publ173.pdf. Approved December 8, 2003. Accessed June 6, 2016.
22. Jodele S, Fukuda T, Vinks A, et al. Eculizumab therapy in children with severe hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Biol Blood Marrow Transplant. 2014;20(4):518-525.
23. Ardissino G, Testa S, Possenti I, et al. Discontinuation of eculizumab maintenance treatment for atypical hemolytic uremic syndrome: a report of 10 cases. Am J Kidney Dis. 2014;64(4):633-637.
24. Cugno M, Gualtierotti R, Possenti I, et al. Complement functional tests for monitoring eculizumab treatment in patients with atypical hemolytic uremic syndrome. J Thromb Haemost. 2014;12(9):1440-1448.
25. Waters AM, Licht C. aHUS caused by complement dysregulation: new therapies on the horizon. Pediatr Nephrol. 2011;26(1):41-57.
26. Gatault P, Brachet G, Ternant D, et al. Therapeutic drug monitoring of eculizumab: rational for an individualized dosing schedule. MAbs. 2015;7(6):1205-1211.
27. Zuber J, Fakhouri F, Roumenina LT, Loirat C, Frémeaux-Bacchi V; French Study Group for aHUS/C3G. Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nat Rev Nephrol. 2012;8(11):643-657.
28. Fakhouri F, Frémeaux-Bacchi V, Loirat C. Atypical hemolytic uremic syndrome: from the rediscovery of complement to targeted therapy. Eur J Intern Med. 2013;24(6):492-495.
29. Crowe K. Analysis: how pharmaceutical company Alexion set the price of the world's most expensive drug. CBC/Radio Canada website. http://www.cbc.ca/news/health/how-pharmaceutical-company-alexion-set-the-price-of-the-world-s-most-expensive-drug-1.3125251 Updated June 25, 2015. Accessed June 21, 2016.
30. Drug campaign for sick child was a PR stunt. FlandersToday website. http://www.flanderstoday.eu/business/drug-campaign-sick-child-was-pr-stunt. Published May 8, 2013. Accessed June 21, 2016.
31. Herald on Sunday editorial: miracle cure, morally derelect. New Zealand Herald website. http://www.nzherald.co.nz/opinion/news/article.cfm?c_id=466&objectid=10861630. Published January 27, 2013. Accessed June 21, 2016.
32. Brill S. Bitter pill: why medical bills are killing us. http://healthland.time.com/2013/02/20/bitter-pill-why-medical-bills-are-killing-us/print/[2/26/2013. Time website. Published February 20, 2013. Accessed June 6, 2016.
A 59-year-old man with a 20-year history (1994) of HIV well controlled on highly active antiretroviral therapy (HAART) therapy (baseline viral load undetectable, CD4+ cell count 781), presented to a community hospital (May 7, 2014) with abdominal pain. The patient’s girlfriend reported unusual behavior for 1 week before admission, including decreased appetite, binge drinking, and nonadherence to HAART therapy.
There was no history of fever, illegal medication use, or diarrhea. In addition to HIV, his past history was remarkable for hepatitis B, hypertension, and left lower extremity amputation secondary to a motor vehicle accident. He had a remote history of cocaine, PCP (phencyclidine), LSD (lysergic acid diethylamide), marijuana, and alcohol misuse and a 50 pack-year smoking history. His family history was remarkable for a mother who died of pancreatic cancer.
During his hospitalization, he developed pronounced expressive aphasia and lethargy but was able to follow simple commands. A computed tomography (CT) scan of the head revealed a left lacunar infarction, and he was transferred to the VA Long Beach Healthcare System in California for further care of a possible stroke.
Shortly after arrival, he developed a fever of 100.9º F. His pulse was 100 bpm and regular, blood pressure was 164/92 mm Hg, and respiratory rate was 14 breaths per minute. A physical examination was remarkable for somnolence, disorientation, and aphasia. He was grimacing to light palpation in all 4 quadrants of the abdomen and had diffuse purpura on skin examination. Laboratory results showed worsening thrombocytopenia, acute kidney injury with proteinuria and hemoglobinuria, and hemolysis (schistocytes, low haptoglobin level, and elevated lactate dehydrogenase [LDH]).
The patient’s changes in baseline laboratory results were platelet count 206,000 mm3 to 64,000 mm3, serum creatinine level 0.98 mg/dL to 1.55 mg/dL. His hemogram showed normochromic normocytic anemia (hemoglobin [Hb] level 10.2 g/dL) with schistocytes. Serum samples were initially unreportable by the laboratory due to severe hemolysis, but his haptoglobin level was found to be low and, conversely, LDH remarkably high. Fifteen days after admission, his CD4+ cell count was 141. An abdominal CT scan showed right lower quadrant abdominal free fluid and thickening of the terminal ileum with surrounding stranding, suggestive of terminal ileitis, and he was started on piperacillin-tazobactam. A lumbar puncture was unremarkable, and HAART medications were resumed. The patient required intubation and a ventilator for acute respiratory failure.
Empiric treatment for presumed thrombotic thrombocytopenic purpura (TTP) with plasmapheresis and methylprednisolone was ineffective, and the patient required mechanical ventilation and hemodialysis.
In refractory cases of TTP-hemolytic uremic syndrome, rituximab, a monoclonal antibody directed at CD20 present on B lymphocytes, is added empirically as effective salvage therapy and was therefore tried in this case.1
However, the addition of rituximab failed to improve the patient’s condition, and he developed further seizure activity and evidence of new lacunar infarctions as seen on magnetic resonance imaging of the brain. His hospital course was complicated by recurrent hemoptysis and respiratory failure, requiring assisted ventilation and eventually tracheostomy.
A normal ADAMTS13 level (72%) and negative Shiga toxin test changed the diagnosis to atypical hemolytic uremic syndrome (aHUS). Mean complement C3 (74 mg/dL) and C4 (9 mg/dL) levels were low. Plasmapheresis was discontinued, and treatment with eculizumab (Soliris, Alexion Pharmaceuticals) was initiated. Meningococcal vaccine was administered post-eculizumab, aimed at reducing but not eliminating the risk of meningococcemia.2 Two weeks later, the patient’s platelet count normalized, renal function improved, hemolysis resolved, and the patient regained full mental status. Eight weeks after initiating eculizumab, he no longer required dialysis.
Discussion
Generalized thrombosis of smaller blood vessels (thrombotic microangiopathy [TMA]) occurs in 3 uncommon syndromes—TTP, HUS, and aHUS—all with similar clinical presentations but distinct pathologic etiologies and treatment. These syndromes share a clinical picture of thrombocytopenia, hemolytic anemia, and renal failure. Hemolysis in these conditions is manifested by schistocytes, elevated lactate dehydrogenase from damaged cells, decreased haptoglobin, anemia, and hemoglobinuria.
Thrombotic Thrombocytopenic Purpura
Thrombocytopenic purpura occurs in about 3 cases per 1,000,000 adults per year.3 It occurs when the metalloproteinase enzyme ADAMTS13 activity is impaired, interrupting its function to cleave large sticky von Willebrand factor (vWF) multimers, resulting in coagulation in microvasculature by increased platelet aggregation, hemolysis from shearing of red blood cells, and compromised circulation to the highly vascularized kidney and other vital organs.4 The hallmark of TTP is a severely decreased ADAMTS13 activity (< 5% of normal) secondary to coexisting conditions, such as cancer, pregnancy, HIV infection, adverse effects (AEs), or antibodies to ADAMTS13.5
The TTP pentad of thrombocytopenia, hemolytic anemia, neurologic symptoms, renal failure, and fever were present in our patient. The patient had a known HIV infection but no exposure to medications associated with TTP (such as acyclovir, quinine, oxymorphone, platelet aggregation inhibitors, or immunosuppressants). Prior to obtaining ADAMTS13 level, the patient was treated empirically for TTP with early and daily plasma exchange to remove the inhibitor of ADAMTS13 and replace it with fresh frozen plasma. Rituximab also was used to inhibit production of antibodies to ADAMTS13 from CD20 B lymphocytes. These empiric clinical measures were not effective in stopping his decline in renal and neurologic functions.
Hemolytic Uremic Syndrome
Like TTP, HUS is also a consequence of thrombotic microangiopathy. However, in contrast to TTP, which is more commonly seen in adults,3 HUS is usually seen in young children secondary to Shiga toxin-producing Escherichia coli (STEC).6 Hemolytic uremic syndrome, also referred to as STEC-HUS or typical HUS, is a rare disease affecting 10 to 20 people per million annually. About 10% of these patients are classified as having aHUS because STEC is not implicated in their disease. It is of interest that, unlike aHUS, STEC-HUS is usually a self-limited disease of children, the majority of whom recover without relapse, and evidence that eculizumab improves prognosis in STEC-HUS is not compelling.5
Atypical Hemolytic Uremic Syndrome
Atypical HUS is a complement-mediated disease. The usual function of complement proteins is to destroy foreign cells and activate immune cells. However, in aHUS this protective defense system goes awry resulting in a pathologic thrombotic milieu. Specifically, aHUS is a continuous complement mediated attack on vascular endothelial beds due to the failure of protein regulators to terminate the complement cascade. Unlike typical HUS, which is usually associated with a Shiga-toxin producing gastrointestinal infection, the trigger in aHUS is unknown and thought to be associated with a genetic predisposition.
Atypical HUS distinguishes itself from TTP and HUS in that it does not respond to plasma exchange, corticosteroids, rituximab, or other immunosupressants. This is due to the distinct underlying pathophysiology of aHUS in which the problem is the unbridled activation of the alternate arm of the complement system.
The complement system is part of the innate immune system, which acts with or without the adaptive immune system it “complements” by amplifying a cascade of responses to eliminate the trigger pathogen. There are 3 complement pathways—classical, lectin, and alternate. The alternate complement pathway, whose activation generates C5a complement (anaphylotoxin), was most pertinent to this case. This precipitates a number of downstream protein cleaving events that lead to the cell lysing membrane attack complex (MAC), which creates a pore in the cell membrane of pathogens seen as foreign. In aHUS, the patient’s own cells come under attack by their own complement, which is no longer inhibited due to mutations in regulatory proteins of the alternate pathway.
With the foot off the complement brake (the hallmark feature underlying aHUS), endothelial cells, leukocytes, and platelets become hyperactive and thrombogenic, thereby resulting in microangiopathy and ischemia of involved organs.7 These mutations may be sporadic or familial and occur in a genetically susceptible host.8 It should be emphasized that genetic testing in complement mediated HUS is a specialized and slow process (weeks); the initial clinical diagnosis is one of exclusion and does not rest on genetic testing. Furthermore, serum complement levels may be normal in cases of complement mediated aHUS.9-11
This patient had a life-threatening condition that required distinguishing it from 2 rare diseases with very similar presentations; failure to do so in a timely fashion could easily have resulted in his demise. TTP or HUS was the important question, and ADAMTS13 level was one of the determining diagnostic tests. The usual interventions for TTP and HUS (plasmapheresis/plasma exchange and, in some cases, rituximab) were ineffective in this patient with aHUS. The patient achieved full recovery of neurologic, renal, and hematologic impairments after treatment with eculizumab, the recombinant humanized monoclonal antibody that binds to the complement protein C5 brake and inhibits its enzymatic cleavage, thereby interfering with the production of the MAC and cell lysis.
Although the patient did not have an identifiable mutation in the panel of complement regulatory genes tested, the rather dramatic efficacy of the orphan drug eculizumab was in a sense confirmation of his complement related hemolytic uremia. Left undecided are the questions of how long to continue eculizumab, the potential risk of relapse with discontinuation, and the ethical dilemma of proper length of treatment with the most expensive medication in the world given its total cost and no clear discontinuation criteria.12-15 The cost of medications for rare and ultra-rare orphan drugs have approached unsustainable levels, posing ethical challenges to many developed countries.16
Eculizumab and Orphan Drugs
Several months before his assassination, President Kennedy awarded Frances Kathleen Oldham Kelsey, MD, PhD, the President’s Award for Distinguished Federal Civilian Service (August 7, 1962) for her insistence that more safety evidence for thalidomide be presented before she would approve its use in the U.S. As a result of the thalidomide tragedy, the Kefauver Harris amendment was passed unanimously by Congress and signed into law by President Kennedy on October 10, 1962. It required stringent evidence of safety and efficacy for FDA approval of a new medication, reporting of AEs to the FDA, truth in drug advertising, rules governing generic drugs, and informed consent from patients participating in clinical trials.
An unintended consequence was that the development of medications for uncommon diseases became fiscally unattractive to the pharmaceutical industry, ie, “orphaned.” The Orphan Drug Act was enacted by Congress in 1983 to encourage development of drugs to treat less common diseases (diseases/disorders affecting fewer than 200,000 people in the U.S.) through incentives such as exclusive use approval for 7 years, reduced taxes, grants, and favorable laws. Ironically, thalidomide was designated an orphan drug on October 14, 1998 for treatment of multiple myeloma. Since its enactment in 1983, more than 400 orphan drugs and biologic products have been marketed. There may be as many as 7,000 orphan diseases to target for drug therapy, and the 17 of the 20 most expensive drugs in the world in 2013 were for rare orphan diseases.16
Paroxysmal nocturnal hemoglobinuria (PNH) is one of those rare diseases. Mutations of hematopoietic stem cells produce red blood cell membranes deficient in the glycoprotein to which signaling proteins attach (glycosyl phosphatidylinositol) and serve to inhibit complement-induced lysis. This results in intravascular hemolysis (increased LDH and decreased haptoglobin) and increased thrombosis. The FDA approved the orphan drug eculizumab for the treatment of the orphan disease PNH on March 16, 2007.
Eculizumab is a humanized mouse monoclonal antibody that gained FDA orphan drug approval (and exclusivity rights until 2019) for the treatment of aHUS on September 23, 2011, based on 2 industry-sponsored small trials of 17 and 20 patients, and it remains the primary and only known effective treatment for this disease.17-19
Eculizumab has raised many interesting questions. Its mechanism of action wets the appetite of pharmacologists and unveils more basic science questions regarding other related mechanisms of disease, recognition of foreign vs self, genetic influences, virulence of organisms, and more. National and international dilemmas have arisen because of the extreme cost of eculizumab, its position as the only effective treatment for this rare and often fatal disease, and the manufacturer’s recommendation and promotion that it be continued indefinitely. How should the price of a drug, developed in large part by government-supported research and tax incentives, and without competition, be determined and justified?
Pharmaceutical Inflation
A marketplace for pharmaceuticals is simply not analogous to other industries. Advances in pharmacotherapy, some miraculous, have come at a substantial cost. The high cost of drugs became newsworthy with the AIDS pandemic and the approval of the lifesaving azidothymidine (AZT) in 1989 (Burroughs Wellcome–also the developer of pyrimethamine [Daraprim]) and its then record price. Cancer treatment that used to cost $10,000 per year now costs $10,000 per month while the oncology community extolls a 2- or 3-month progression free survival benefit. Patients must now deal with the shock of a cancer diagnosis followed by the shock of an exorbitant copayment.
Recent media attention focused derision on Martin Shkreli, chief executive officer of Turing Pharmaceuticals, for purchasing pyrimethamine and then raising the price of the 62-year-old treatment for protozoan infection toxoplasmosis from $13.50 to $750 per pill. The debacle may also serve to highlight the complexities and ethical issues involved when profit intersects with health care. Some drug costs have dramatically increased in the U.S. because of greed, a belief that a marketplace can control costs, and the lack of regulation. The usual suspects, such as cost of research, length of development, stimulus to innovation, and return on investment, are difficult to apply to old medications whose marketing rights were acquired by purchase of another company. Can marketplace economics be applied in health situations where there is no competition, legal protections afforded manufacturers, consumers unable to make an informed decision?
While pharmacy and therapeutics committees were debating treatment of hepatitis C with either of 2 drugs approved in 2011, boceprevir (Victrelis, Merck) or telapravir (Incivek, Vertex and Johnson & Johnson), Gilead Pharmaceuticals acquired Pharmasset Inc. and its hepatitis C drug sofosbuvir (Sovaldi) for a whopping $11.2 billion in 2012. It received FDA approval April 8, 2013, under Breakthrough Therapy Designation.
While economists argued over the wisdom of such a high-cost acquisition, Gilead generated $9 billion in sales during the first 3 quarters of 2014, surpassing adalimumab (Humira), which had been the highest earning drug in 2014. Hepatitis C could now be quickly treated with truly unprecedented efficacy and without the AEs of interferon. The oft-quoted cost $1,000 per pill or about $84,000 per treatment in the U.S. drew international attention. Prior options for hepatitis C treatment, which preceded sofosbuvir by a mere couple of years, fell into pharmaceutical extinction. Telapravir succumbed to competition and ceased to be manufactured on August 12, 2014. Shortly after approval of sofosbuvir, Gilead also gained approval of its combination product for hepatitis C ledipasvir 90 mg/sofosbuvir 400 mg (Harvoni) on October 10, 2014.
The Most Expensive Drug in the World
Although there are no shortages of contenders for the coveted most expensive title, eculizumab is the current champion. Drugs that offer a cure, such as antibiotics, usually involve a relatively short, onetime course. Lack of return on the cost of development of curative agents may have reduced industrial incentives to develop antibiotics. On the other hand, the extent of infectious diseases, such as malaria, Ebola, tuberculosis, HIV, hepatitis, and the proliferation of drug-resistant organisms, continues to fuel industrial interest for this lifesaving class of medications.
Cancer medications touting a brief interruption of the race to death have raised questions of affordability, equitable access, and quality vs quantity of life. The $11,000 per month endothelial growth factor inhibitor aflibercept (Zaltrap, Regeneron, and Sanofi) was approved by the FDA in November 2012 for colorectal cancer and was followed by a historic rejection by Memorial Sloan-Kettering Cancer Center, since its cost was nearly double that of a similar medication bevacizumab (Avastin) with similar meager benefit of a median progression free survival of 1.4 months.20 Moreover Medicare is mandated to cover the price the manufacturers charge plus a 6% cushion for any cancer drug that the FDA approves.21 Patients with private insurance, often elderly and on a fixed income, are burdened with a copayment requirement of 20% of the cost of the drug. The nonnegotiation clause of Medicare has not reduced cost of medications, particularly for cancer, which many of the elderly will likely face.
The VA, a single-payer system distinguished by bipartisan congressional support, can directly negotiate with pharmaceutical companies, resulting in lower drug prices than discounts guaranteed by federal law; but what if there is no competitor? Biosimilar drugs are currently being debated by those seeking to prolong their patent protection. Stem cell therapies that offer a cure for some rare diseases or hope for common diseases are certain to command astronomical prices. Gene therapy offers hope for cure of both rare and common afflictions but at astounding prices.
The medication alipogene tiparvovec (Glybera, UniQure) delivered by adenovirus, for example, has been approved for use in the rare disorder lipoprotein lipase deficiency and is anticipated to cost $1.6 million for a onetime curative treatment. The pharmaceutical industry has joined the gene therapy race. While this is indeed a record acquisition sum for alipogene tiparvovec, at least it offers a cure. Eculizumab, although unique and effective, offers indefinite administration at a cost exceeding $600,000 per year, every year, for life. In 2014, sales of eculizumab climbed 44% to $2.234 billion.
To Continue or Discontinue Treatment
The duration of treatment with eculizumab poses a challenging dilemma for patients, clinicians, and health care providers. Eculizumab is the only effective treatment for a life-threatening condition, and the manufacturer, Alexion, recommends lifelong therapy of its product that has no competitors. Our patient was treated with 47 fixed-dose infusions of eculizumab at 2-week intervals from May 31, 2014, to February 18, 2016, at a cost of $737,957.80. The commercial cost outside the VA would be about 1.8 times this amount ($1.3 million). This extraordinary cost is the basic ethical issue. Without competition there is little to negotiate.
Need the treatment be lifelong? The AEs of eculizumab are not trivial, and some clinicians felt evidence for indefinite use in this patient was not compelling. Our patient’s initial critical and unstable condition had completely resolved after 2 months of eculizumab. The initial unknown precipitating event triggering the patient’s aHUS probably had resolved. His genetic testing did not disclose any HUS-related mutation. The patient’s serum was sent to Cincinnati Children’s Hospital (CCH) Clinical Laboratory Service to determine his eculizumab level and complement inhibition. His complement inhibition, as measured by CH50 activity, was adequately suppressed at 6% on eculizumab (target of < 10%) in spite of a free serum eculizumab level (81 mg/mL) that was somewhat below the therapeutic range of > 100 mg/mL.
Arguments for lifelong eculizumab therapy are based in part on the theoretical development of anti-eculizumab antibodies that could render reinstitution of eculizumab ineffective.22 Monitoring patients for relapse of their aHUS involves following markers of disease activity (levels of creatinine, LDH, haptoglobin, platelet counts, and Hb in urine). A report of 10 adult patients with aHUS who were treated effectively with eculizumab supports a trial of discontinuation.23 Seven of the 10 patients did not relapse following discontinuation of eculizumab. Three of 10 patients experienced a relapse when monitored for a cumulative 95 months, but all 3 had immediate and complete recovery after resuming therapy. All 3 patients who experienced relapse carried a complement factor H mutation. Their relapses occurred within 6 weeks from the last dose and were detected simply by performing home urine dipstick monitoring for haptoglobin 3 times per week. The 3 patients who relapsed promptly responded to eculizumab reinstitution with return of their labs to baseline.
Monitoring of complement function in patients with aHUS can guide clinically appropriate dosing intervals without changing disease activity markers.24 The half-life of eculizumab is about 11 days, and dosing intervals may be safely extended beyond 2 weeks in select patients.25 The target minimum inhibitory serum eculizumab level necessary to inhibit complement-mediated hemolysis is 50 µg/mL and 35 mg/mL for aHUS and PNH, respectively. In a small pharmacokinetic pilot study, Gatault and colleagues noted that trough levels during eculizumab maintenance by enzyme-linked immunoabsorbent assay (ELISA) of 44 mg/mL to 59 mg/mL inhibited the complement cascade.26 We suggested that weight-based dosing aiming at a trough > 50 mg/mL (rather than fixed dosing at a fixed interval) would be a better maintenance strategy.
In select patients, a trial of gradual discontinuation by lengthening the dosing interval of eculizumab seems a reasonable and safe alternative to indefinite continuation of the drug. After a patient’s successful recovery, the initial and unknown trigger of aHUS may no longer play a role. Improvement in the patient’s medical condition may permit the restoration of the patient’s defenses to once again function normally. Eculizumab seemed to retain its efficacy in the small number of patients who relapsed. Those who relapsed had positive genetic markers.
Further arguments favoring trial discontinuation in patients without known genetic predisposition are that continuation is not without risk, particularly of meningococcal infection, necessity for infusion every 2 weeks for life, little is known regarding long-term risk of the drug, and a lot is known of its extreme cost. Suppression of C5 inhibitory effect can lead to increased susceptibility to infections, whereas increased C5 activity may lead to a continued autoimmune attack on native cells.
As proposed by others, we suggest that this decision be made on a case by case basis, tailoring treatment based on an individual’s genetics and medical history.27 Although the European Medicines Agency has approved lifelong therapy for aHUS, this may be appropriate only for patients who have aHUS complement mutations associated with poor outcomes.27 This approach may not be warranted, however, in a patient, such as the one presented with no genetic mutations, or in those with mutations of uncertain clinical consequence. In such cases, given that 90% of adults who have a relapse experience within the first year after an aHUS episode, a reasonable alternative may be a trial discontinuation.27,28 After 1 year of eculizumab therapy, a trial of discontinuation with urine dipstick monitoring for Hb (Hemastix) 3 times a week for relapse may avoid the unnecessary expense and risk for infection posed by lifelong therapy, and eculizumab may be effectively restarted in case of relapse.
We propose that in these cases it would be reasonable to perform a trial of discontinuation after 1 year of therapy with urine dipstick monitoring for relapse, as lifelong therapy may pose unnecessary expense and risk for infection. In fact, given the financial burden of prolonged therapy on society, we believe it is unethical to continue treatment in a patient with unknown risk for relapse without a trial of discontinuation, as evidence has shown good response to re-initiation of therapy in the event of relapse.28 Agencies that have negotiated or attempted to regulate the cost of eculizumab have been met with public media campaigns featuring afflicted children at risk of death without eculizumab. The public relations company behind these efforts received support from Alexion.29-31
Conclusion
Given the formidable cost and the international monopoly status of eculizumab for a life-threatening condition, prospective discontinuation trials supported by the manufacturer would seem warranted in select cases. Delineating which patients will have a chronic relapsing course and those who will not should be one of these clinical trials. For now, one can only wonder: What’s worse than having a rare disease like aHUS? Perhaps the cost of treatment for a potentially indefinite period of time—now that’s a “bitter pill.”32
A 59-year-old man with a 20-year history (1994) of HIV well controlled on highly active antiretroviral therapy (HAART) therapy (baseline viral load undetectable, CD4+ cell count 781), presented to a community hospital (May 7, 2014) with abdominal pain. The patient’s girlfriend reported unusual behavior for 1 week before admission, including decreased appetite, binge drinking, and nonadherence to HAART therapy.
There was no history of fever, illegal medication use, or diarrhea. In addition to HIV, his past history was remarkable for hepatitis B, hypertension, and left lower extremity amputation secondary to a motor vehicle accident. He had a remote history of cocaine, PCP (phencyclidine), LSD (lysergic acid diethylamide), marijuana, and alcohol misuse and a 50 pack-year smoking history. His family history was remarkable for a mother who died of pancreatic cancer.
During his hospitalization, he developed pronounced expressive aphasia and lethargy but was able to follow simple commands. A computed tomography (CT) scan of the head revealed a left lacunar infarction, and he was transferred to the VA Long Beach Healthcare System in California for further care of a possible stroke.
Shortly after arrival, he developed a fever of 100.9º F. His pulse was 100 bpm and regular, blood pressure was 164/92 mm Hg, and respiratory rate was 14 breaths per minute. A physical examination was remarkable for somnolence, disorientation, and aphasia. He was grimacing to light palpation in all 4 quadrants of the abdomen and had diffuse purpura on skin examination. Laboratory results showed worsening thrombocytopenia, acute kidney injury with proteinuria and hemoglobinuria, and hemolysis (schistocytes, low haptoglobin level, and elevated lactate dehydrogenase [LDH]).
The patient’s changes in baseline laboratory results were platelet count 206,000 mm3 to 64,000 mm3, serum creatinine level 0.98 mg/dL to 1.55 mg/dL. His hemogram showed normochromic normocytic anemia (hemoglobin [Hb] level 10.2 g/dL) with schistocytes. Serum samples were initially unreportable by the laboratory due to severe hemolysis, but his haptoglobin level was found to be low and, conversely, LDH remarkably high. Fifteen days after admission, his CD4+ cell count was 141. An abdominal CT scan showed right lower quadrant abdominal free fluid and thickening of the terminal ileum with surrounding stranding, suggestive of terminal ileitis, and he was started on piperacillin-tazobactam. A lumbar puncture was unremarkable, and HAART medications were resumed. The patient required intubation and a ventilator for acute respiratory failure.
Empiric treatment for presumed thrombotic thrombocytopenic purpura (TTP) with plasmapheresis and methylprednisolone was ineffective, and the patient required mechanical ventilation and hemodialysis.
In refractory cases of TTP-hemolytic uremic syndrome, rituximab, a monoclonal antibody directed at CD20 present on B lymphocytes, is added empirically as effective salvage therapy and was therefore tried in this case.1
However, the addition of rituximab failed to improve the patient’s condition, and he developed further seizure activity and evidence of new lacunar infarctions as seen on magnetic resonance imaging of the brain. His hospital course was complicated by recurrent hemoptysis and respiratory failure, requiring assisted ventilation and eventually tracheostomy.
A normal ADAMTS13 level (72%) and negative Shiga toxin test changed the diagnosis to atypical hemolytic uremic syndrome (aHUS). Mean complement C3 (74 mg/dL) and C4 (9 mg/dL) levels were low. Plasmapheresis was discontinued, and treatment with eculizumab (Soliris, Alexion Pharmaceuticals) was initiated. Meningococcal vaccine was administered post-eculizumab, aimed at reducing but not eliminating the risk of meningococcemia.2 Two weeks later, the patient’s platelet count normalized, renal function improved, hemolysis resolved, and the patient regained full mental status. Eight weeks after initiating eculizumab, he no longer required dialysis.
Discussion
Generalized thrombosis of smaller blood vessels (thrombotic microangiopathy [TMA]) occurs in 3 uncommon syndromes—TTP, HUS, and aHUS—all with similar clinical presentations but distinct pathologic etiologies and treatment. These syndromes share a clinical picture of thrombocytopenia, hemolytic anemia, and renal failure. Hemolysis in these conditions is manifested by schistocytes, elevated lactate dehydrogenase from damaged cells, decreased haptoglobin, anemia, and hemoglobinuria.
Thrombotic Thrombocytopenic Purpura
Thrombocytopenic purpura occurs in about 3 cases per 1,000,000 adults per year.3 It occurs when the metalloproteinase enzyme ADAMTS13 activity is impaired, interrupting its function to cleave large sticky von Willebrand factor (vWF) multimers, resulting in coagulation in microvasculature by increased platelet aggregation, hemolysis from shearing of red blood cells, and compromised circulation to the highly vascularized kidney and other vital organs.4 The hallmark of TTP is a severely decreased ADAMTS13 activity (< 5% of normal) secondary to coexisting conditions, such as cancer, pregnancy, HIV infection, adverse effects (AEs), or antibodies to ADAMTS13.5
The TTP pentad of thrombocytopenia, hemolytic anemia, neurologic symptoms, renal failure, and fever were present in our patient. The patient had a known HIV infection but no exposure to medications associated with TTP (such as acyclovir, quinine, oxymorphone, platelet aggregation inhibitors, or immunosuppressants). Prior to obtaining ADAMTS13 level, the patient was treated empirically for TTP with early and daily plasma exchange to remove the inhibitor of ADAMTS13 and replace it with fresh frozen plasma. Rituximab also was used to inhibit production of antibodies to ADAMTS13 from CD20 B lymphocytes. These empiric clinical measures were not effective in stopping his decline in renal and neurologic functions.
Hemolytic Uremic Syndrome
Like TTP, HUS is also a consequence of thrombotic microangiopathy. However, in contrast to TTP, which is more commonly seen in adults,3 HUS is usually seen in young children secondary to Shiga toxin-producing Escherichia coli (STEC).6 Hemolytic uremic syndrome, also referred to as STEC-HUS or typical HUS, is a rare disease affecting 10 to 20 people per million annually. About 10% of these patients are classified as having aHUS because STEC is not implicated in their disease. It is of interest that, unlike aHUS, STEC-HUS is usually a self-limited disease of children, the majority of whom recover without relapse, and evidence that eculizumab improves prognosis in STEC-HUS is not compelling.5
Atypical Hemolytic Uremic Syndrome
Atypical HUS is a complement-mediated disease. The usual function of complement proteins is to destroy foreign cells and activate immune cells. However, in aHUS this protective defense system goes awry resulting in a pathologic thrombotic milieu. Specifically, aHUS is a continuous complement mediated attack on vascular endothelial beds due to the failure of protein regulators to terminate the complement cascade. Unlike typical HUS, which is usually associated with a Shiga-toxin producing gastrointestinal infection, the trigger in aHUS is unknown and thought to be associated with a genetic predisposition.
Atypical HUS distinguishes itself from TTP and HUS in that it does not respond to plasma exchange, corticosteroids, rituximab, or other immunosupressants. This is due to the distinct underlying pathophysiology of aHUS in which the problem is the unbridled activation of the alternate arm of the complement system.
The complement system is part of the innate immune system, which acts with or without the adaptive immune system it “complements” by amplifying a cascade of responses to eliminate the trigger pathogen. There are 3 complement pathways—classical, lectin, and alternate. The alternate complement pathway, whose activation generates C5a complement (anaphylotoxin), was most pertinent to this case. This precipitates a number of downstream protein cleaving events that lead to the cell lysing membrane attack complex (MAC), which creates a pore in the cell membrane of pathogens seen as foreign. In aHUS, the patient’s own cells come under attack by their own complement, which is no longer inhibited due to mutations in regulatory proteins of the alternate pathway.
With the foot off the complement brake (the hallmark feature underlying aHUS), endothelial cells, leukocytes, and platelets become hyperactive and thrombogenic, thereby resulting in microangiopathy and ischemia of involved organs.7 These mutations may be sporadic or familial and occur in a genetically susceptible host.8 It should be emphasized that genetic testing in complement mediated HUS is a specialized and slow process (weeks); the initial clinical diagnosis is one of exclusion and does not rest on genetic testing. Furthermore, serum complement levels may be normal in cases of complement mediated aHUS.9-11
This patient had a life-threatening condition that required distinguishing it from 2 rare diseases with very similar presentations; failure to do so in a timely fashion could easily have resulted in his demise. TTP or HUS was the important question, and ADAMTS13 level was one of the determining diagnostic tests. The usual interventions for TTP and HUS (plasmapheresis/plasma exchange and, in some cases, rituximab) were ineffective in this patient with aHUS. The patient achieved full recovery of neurologic, renal, and hematologic impairments after treatment with eculizumab, the recombinant humanized monoclonal antibody that binds to the complement protein C5 brake and inhibits its enzymatic cleavage, thereby interfering with the production of the MAC and cell lysis.
Although the patient did not have an identifiable mutation in the panel of complement regulatory genes tested, the rather dramatic efficacy of the orphan drug eculizumab was in a sense confirmation of his complement related hemolytic uremia. Left undecided are the questions of how long to continue eculizumab, the potential risk of relapse with discontinuation, and the ethical dilemma of proper length of treatment with the most expensive medication in the world given its total cost and no clear discontinuation criteria.12-15 The cost of medications for rare and ultra-rare orphan drugs have approached unsustainable levels, posing ethical challenges to many developed countries.16
Eculizumab and Orphan Drugs
Several months before his assassination, President Kennedy awarded Frances Kathleen Oldham Kelsey, MD, PhD, the President’s Award for Distinguished Federal Civilian Service (August 7, 1962) for her insistence that more safety evidence for thalidomide be presented before she would approve its use in the U.S. As a result of the thalidomide tragedy, the Kefauver Harris amendment was passed unanimously by Congress and signed into law by President Kennedy on October 10, 1962. It required stringent evidence of safety and efficacy for FDA approval of a new medication, reporting of AEs to the FDA, truth in drug advertising, rules governing generic drugs, and informed consent from patients participating in clinical trials.
An unintended consequence was that the development of medications for uncommon diseases became fiscally unattractive to the pharmaceutical industry, ie, “orphaned.” The Orphan Drug Act was enacted by Congress in 1983 to encourage development of drugs to treat less common diseases (diseases/disorders affecting fewer than 200,000 people in the U.S.) through incentives such as exclusive use approval for 7 years, reduced taxes, grants, and favorable laws. Ironically, thalidomide was designated an orphan drug on October 14, 1998 for treatment of multiple myeloma. Since its enactment in 1983, more than 400 orphan drugs and biologic products have been marketed. There may be as many as 7,000 orphan diseases to target for drug therapy, and the 17 of the 20 most expensive drugs in the world in 2013 were for rare orphan diseases.16
Paroxysmal nocturnal hemoglobinuria (PNH) is one of those rare diseases. Mutations of hematopoietic stem cells produce red blood cell membranes deficient in the glycoprotein to which signaling proteins attach (glycosyl phosphatidylinositol) and serve to inhibit complement-induced lysis. This results in intravascular hemolysis (increased LDH and decreased haptoglobin) and increased thrombosis. The FDA approved the orphan drug eculizumab for the treatment of the orphan disease PNH on March 16, 2007.
Eculizumab is a humanized mouse monoclonal antibody that gained FDA orphan drug approval (and exclusivity rights until 2019) for the treatment of aHUS on September 23, 2011, based on 2 industry-sponsored small trials of 17 and 20 patients, and it remains the primary and only known effective treatment for this disease.17-19
Eculizumab has raised many interesting questions. Its mechanism of action wets the appetite of pharmacologists and unveils more basic science questions regarding other related mechanisms of disease, recognition of foreign vs self, genetic influences, virulence of organisms, and more. National and international dilemmas have arisen because of the extreme cost of eculizumab, its position as the only effective treatment for this rare and often fatal disease, and the manufacturer’s recommendation and promotion that it be continued indefinitely. How should the price of a drug, developed in large part by government-supported research and tax incentives, and without competition, be determined and justified?
Pharmaceutical Inflation
A marketplace for pharmaceuticals is simply not analogous to other industries. Advances in pharmacotherapy, some miraculous, have come at a substantial cost. The high cost of drugs became newsworthy with the AIDS pandemic and the approval of the lifesaving azidothymidine (AZT) in 1989 (Burroughs Wellcome–also the developer of pyrimethamine [Daraprim]) and its then record price. Cancer treatment that used to cost $10,000 per year now costs $10,000 per month while the oncology community extolls a 2- or 3-month progression free survival benefit. Patients must now deal with the shock of a cancer diagnosis followed by the shock of an exorbitant copayment.
Recent media attention focused derision on Martin Shkreli, chief executive officer of Turing Pharmaceuticals, for purchasing pyrimethamine and then raising the price of the 62-year-old treatment for protozoan infection toxoplasmosis from $13.50 to $750 per pill. The debacle may also serve to highlight the complexities and ethical issues involved when profit intersects with health care. Some drug costs have dramatically increased in the U.S. because of greed, a belief that a marketplace can control costs, and the lack of regulation. The usual suspects, such as cost of research, length of development, stimulus to innovation, and return on investment, are difficult to apply to old medications whose marketing rights were acquired by purchase of another company. Can marketplace economics be applied in health situations where there is no competition, legal protections afforded manufacturers, consumers unable to make an informed decision?
While pharmacy and therapeutics committees were debating treatment of hepatitis C with either of 2 drugs approved in 2011, boceprevir (Victrelis, Merck) or telapravir (Incivek, Vertex and Johnson & Johnson), Gilead Pharmaceuticals acquired Pharmasset Inc. and its hepatitis C drug sofosbuvir (Sovaldi) for a whopping $11.2 billion in 2012. It received FDA approval April 8, 2013, under Breakthrough Therapy Designation.
While economists argued over the wisdom of such a high-cost acquisition, Gilead generated $9 billion in sales during the first 3 quarters of 2014, surpassing adalimumab (Humira), which had been the highest earning drug in 2014. Hepatitis C could now be quickly treated with truly unprecedented efficacy and without the AEs of interferon. The oft-quoted cost $1,000 per pill or about $84,000 per treatment in the U.S. drew international attention. Prior options for hepatitis C treatment, which preceded sofosbuvir by a mere couple of years, fell into pharmaceutical extinction. Telapravir succumbed to competition and ceased to be manufactured on August 12, 2014. Shortly after approval of sofosbuvir, Gilead also gained approval of its combination product for hepatitis C ledipasvir 90 mg/sofosbuvir 400 mg (Harvoni) on October 10, 2014.
The Most Expensive Drug in the World
Although there are no shortages of contenders for the coveted most expensive title, eculizumab is the current champion. Drugs that offer a cure, such as antibiotics, usually involve a relatively short, onetime course. Lack of return on the cost of development of curative agents may have reduced industrial incentives to develop antibiotics. On the other hand, the extent of infectious diseases, such as malaria, Ebola, tuberculosis, HIV, hepatitis, and the proliferation of drug-resistant organisms, continues to fuel industrial interest for this lifesaving class of medications.
Cancer medications touting a brief interruption of the race to death have raised questions of affordability, equitable access, and quality vs quantity of life. The $11,000 per month endothelial growth factor inhibitor aflibercept (Zaltrap, Regeneron, and Sanofi) was approved by the FDA in November 2012 for colorectal cancer and was followed by a historic rejection by Memorial Sloan-Kettering Cancer Center, since its cost was nearly double that of a similar medication bevacizumab (Avastin) with similar meager benefit of a median progression free survival of 1.4 months.20 Moreover Medicare is mandated to cover the price the manufacturers charge plus a 6% cushion for any cancer drug that the FDA approves.21 Patients with private insurance, often elderly and on a fixed income, are burdened with a copayment requirement of 20% of the cost of the drug. The nonnegotiation clause of Medicare has not reduced cost of medications, particularly for cancer, which many of the elderly will likely face.
The VA, a single-payer system distinguished by bipartisan congressional support, can directly negotiate with pharmaceutical companies, resulting in lower drug prices than discounts guaranteed by federal law; but what if there is no competitor? Biosimilar drugs are currently being debated by those seeking to prolong their patent protection. Stem cell therapies that offer a cure for some rare diseases or hope for common diseases are certain to command astronomical prices. Gene therapy offers hope for cure of both rare and common afflictions but at astounding prices.
The medication alipogene tiparvovec (Glybera, UniQure) delivered by adenovirus, for example, has been approved for use in the rare disorder lipoprotein lipase deficiency and is anticipated to cost $1.6 million for a onetime curative treatment. The pharmaceutical industry has joined the gene therapy race. While this is indeed a record acquisition sum for alipogene tiparvovec, at least it offers a cure. Eculizumab, although unique and effective, offers indefinite administration at a cost exceeding $600,000 per year, every year, for life. In 2014, sales of eculizumab climbed 44% to $2.234 billion.
To Continue or Discontinue Treatment
The duration of treatment with eculizumab poses a challenging dilemma for patients, clinicians, and health care providers. Eculizumab is the only effective treatment for a life-threatening condition, and the manufacturer, Alexion, recommends lifelong therapy of its product that has no competitors. Our patient was treated with 47 fixed-dose infusions of eculizumab at 2-week intervals from May 31, 2014, to February 18, 2016, at a cost of $737,957.80. The commercial cost outside the VA would be about 1.8 times this amount ($1.3 million). This extraordinary cost is the basic ethical issue. Without competition there is little to negotiate.
Need the treatment be lifelong? The AEs of eculizumab are not trivial, and some clinicians felt evidence for indefinite use in this patient was not compelling. Our patient’s initial critical and unstable condition had completely resolved after 2 months of eculizumab. The initial unknown precipitating event triggering the patient’s aHUS probably had resolved. His genetic testing did not disclose any HUS-related mutation. The patient’s serum was sent to Cincinnati Children’s Hospital (CCH) Clinical Laboratory Service to determine his eculizumab level and complement inhibition. His complement inhibition, as measured by CH50 activity, was adequately suppressed at 6% on eculizumab (target of < 10%) in spite of a free serum eculizumab level (81 mg/mL) that was somewhat below the therapeutic range of > 100 mg/mL.
Arguments for lifelong eculizumab therapy are based in part on the theoretical development of anti-eculizumab antibodies that could render reinstitution of eculizumab ineffective.22 Monitoring patients for relapse of their aHUS involves following markers of disease activity (levels of creatinine, LDH, haptoglobin, platelet counts, and Hb in urine). A report of 10 adult patients with aHUS who were treated effectively with eculizumab supports a trial of discontinuation.23 Seven of the 10 patients did not relapse following discontinuation of eculizumab. Three of 10 patients experienced a relapse when monitored for a cumulative 95 months, but all 3 had immediate and complete recovery after resuming therapy. All 3 patients who experienced relapse carried a complement factor H mutation. Their relapses occurred within 6 weeks from the last dose and were detected simply by performing home urine dipstick monitoring for haptoglobin 3 times per week. The 3 patients who relapsed promptly responded to eculizumab reinstitution with return of their labs to baseline.
Monitoring of complement function in patients with aHUS can guide clinically appropriate dosing intervals without changing disease activity markers.24 The half-life of eculizumab is about 11 days, and dosing intervals may be safely extended beyond 2 weeks in select patients.25 The target minimum inhibitory serum eculizumab level necessary to inhibit complement-mediated hemolysis is 50 µg/mL and 35 mg/mL for aHUS and PNH, respectively. In a small pharmacokinetic pilot study, Gatault and colleagues noted that trough levels during eculizumab maintenance by enzyme-linked immunoabsorbent assay (ELISA) of 44 mg/mL to 59 mg/mL inhibited the complement cascade.26 We suggested that weight-based dosing aiming at a trough > 50 mg/mL (rather than fixed dosing at a fixed interval) would be a better maintenance strategy.
In select patients, a trial of gradual discontinuation by lengthening the dosing interval of eculizumab seems a reasonable and safe alternative to indefinite continuation of the drug. After a patient’s successful recovery, the initial and unknown trigger of aHUS may no longer play a role. Improvement in the patient’s medical condition may permit the restoration of the patient’s defenses to once again function normally. Eculizumab seemed to retain its efficacy in the small number of patients who relapsed. Those who relapsed had positive genetic markers.
Further arguments favoring trial discontinuation in patients without known genetic predisposition are that continuation is not without risk, particularly of meningococcal infection, necessity for infusion every 2 weeks for life, little is known regarding long-term risk of the drug, and a lot is known of its extreme cost. Suppression of C5 inhibitory effect can lead to increased susceptibility to infections, whereas increased C5 activity may lead to a continued autoimmune attack on native cells.
As proposed by others, we suggest that this decision be made on a case by case basis, tailoring treatment based on an individual’s genetics and medical history.27 Although the European Medicines Agency has approved lifelong therapy for aHUS, this may be appropriate only for patients who have aHUS complement mutations associated with poor outcomes.27 This approach may not be warranted, however, in a patient, such as the one presented with no genetic mutations, or in those with mutations of uncertain clinical consequence. In such cases, given that 90% of adults who have a relapse experience within the first year after an aHUS episode, a reasonable alternative may be a trial discontinuation.27,28 After 1 year of eculizumab therapy, a trial of discontinuation with urine dipstick monitoring for Hb (Hemastix) 3 times a week for relapse may avoid the unnecessary expense and risk for infection posed by lifelong therapy, and eculizumab may be effectively restarted in case of relapse.
We propose that in these cases it would be reasonable to perform a trial of discontinuation after 1 year of therapy with urine dipstick monitoring for relapse, as lifelong therapy may pose unnecessary expense and risk for infection. In fact, given the financial burden of prolonged therapy on society, we believe it is unethical to continue treatment in a patient with unknown risk for relapse without a trial of discontinuation, as evidence has shown good response to re-initiation of therapy in the event of relapse.28 Agencies that have negotiated or attempted to regulate the cost of eculizumab have been met with public media campaigns featuring afflicted children at risk of death without eculizumab. The public relations company behind these efforts received support from Alexion.29-31
Conclusion
Given the formidable cost and the international monopoly status of eculizumab for a life-threatening condition, prospective discontinuation trials supported by the manufacturer would seem warranted in select cases. Delineating which patients will have a chronic relapsing course and those who will not should be one of these clinical trials. For now, one can only wonder: What’s worse than having a rare disease like aHUS? Perhaps the cost of treatment for a potentially indefinite period of time—now that’s a “bitter pill.”32
1. Caramazza D, Quintini G, Abbene I, et al. Relapsing or refractory idiopathic thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: the role of rituximab. Transfusion. 2010;50(12):2753-2760.
2. Köse O, Zimmerhackl LB, Jungraithmayr T, Mache C, Nürnberger J. New treatment options for atypical hemolytic uremic syndrome with the complement inhibitor eculizumab. Semin Thromb Hemost. 2010;36(6):669-672.
3. Reese JA, Muthurajah DS, Kremer Hovinga JA, Vesely SK, Terrell DR, George JN. Children and adults with thrombotic thrombocytopenic purpura associated with severe, acquired Adamts13 deficiency: comparison of incidence, demographic and clinical features. Pediatr Blood Cancer. 2013;60(10):1676-1682.
4. Sadler JE. Von Willebrand factor, ADAMTS13, and thrombotic thrombocytopenic purpura. Blood. 2008;112(1):11-18.
5. Tsai H-M. Pathophysiology of thrombotic thrombocytopenic purpura. Int J Hematol. 2010;91(1):1-19.
6. Noris M, Remuzzi G. Hemolytic uremic syndrome. J Am Soc Nephrol. 2005;16(4):1035-1050.
7. Liszewski MK, Atkinson JP. Exploring the complement system in human disease. The Rheumatologist website. http://www.the-rheumatologist.org/article/exploring-the-complement-system-in-human-disease. Published February 1, 2010. Accessed May 9, 2016.
8. Mayer CL, Leibowitz CS, Kurosawa S, Stearns-Kurosawa DJ. Shiga toxins and the pathophysiology of hemolytic uremic syndrome in humans and animals. Toxins (Basel). 2012;4(11):1261-1287.
9. Noris M, Mescia F, Remuzzi G. STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat Rev Nephrol. 2012;8(11):622-633.
10. Geerdink LM, Westra D, van Wijk JA, et al. Atypical hemolytic uremic syndrome in children: complement mutations and clinical characteristics. Pediatr Nephrol. 2012;27(8):1283-1291.
11. Sellier-Leclerc AL, Frémeaux-Bacchi V, Dragon-Durey MA, et al; French Society of Pediatric Nephrology. Differential impact of complement mutations on clinical characteristics in atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2007;18(8):2392-2400.
12. Herper M. The worlds most expensive drugs. Forbes. February 22, 2010.
13. Nordrum A. Drug prices: world's most expensive medicine costs $440,000 a year, but is it worth the expense? International Business Times website. http://www.ibtimes.com/drug-prices-worlds-most-expensive-medicine-costs-440000-year-it-worth-expense-2302609. Updated February 13, 2016. Published June 24, 2015. Accessed June 15, 2016.
14. CBC News. The real cost of the world's most expensive drug [video]. CBC/Radio Canada website. http://www.cbc.ca/player/play/2670383596. Accessed June 15, 2016.
15. EvaluatePharma. Orphan Drug Report 2014. EvaluatePharma website. http://www.evaluategroup.com/orphandrug2014. Published 2014. Accessed June 15, 2016.
16. Isaacs D. Ethical dilemmas about orphan drugs for orphan diseases. J Paediatr Child Health. 2014;50(4):249-250.
17. Licht C, Muus P, Legendre CM, et al. Eculizumab (ECU) safety and efficacy in atypical hemolytic uremic syndrome (aHUS) patients with long disease duration and chronic kidney disease (CKD): 2-year results. Poster presented at: 54th Annual Meeting of the American Society of Hematology; December 8-12, 2012; Atlanta, GA.
18. Greenbaum L, Legendre CM, Babu S, et al. Eculizumab (ECU) in atypical hemolytic uremic syndrome (aHUS) patients with progressing thrombotic microangiopathy (TMA): 2-year data. Poster presented at: 54th Annual Meeting of the American Society of Hematology; December 8-12, 2012; Atlanta, GA.
19. Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Eng J Med. 2013;368(23):2169-2181.
20. Bach PB, Saltz LB, Wittes RE. In cancer care, cost matters. New York Times. October 14, 2012:Opinion Pages.
21. Medicare Prescription Drug, Improvement, and Modernization Act of 2003, Public Law 108-173;117 Stat 2066. U.S. Government Printing Office website. https://www.gpo.gov/fdsys/pkg/PLAW-108publ173/pdf/PLAW-108publ173.pdf. Approved December 8, 2003. Accessed June 6, 2016.
22. Jodele S, Fukuda T, Vinks A, et al. Eculizumab therapy in children with severe hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Biol Blood Marrow Transplant. 2014;20(4):518-525.
23. Ardissino G, Testa S, Possenti I, et al. Discontinuation of eculizumab maintenance treatment for atypical hemolytic uremic syndrome: a report of 10 cases. Am J Kidney Dis. 2014;64(4):633-637.
24. Cugno M, Gualtierotti R, Possenti I, et al. Complement functional tests for monitoring eculizumab treatment in patients with atypical hemolytic uremic syndrome. J Thromb Haemost. 2014;12(9):1440-1448.
25. Waters AM, Licht C. aHUS caused by complement dysregulation: new therapies on the horizon. Pediatr Nephrol. 2011;26(1):41-57.
26. Gatault P, Brachet G, Ternant D, et al. Therapeutic drug monitoring of eculizumab: rational for an individualized dosing schedule. MAbs. 2015;7(6):1205-1211.
27. Zuber J, Fakhouri F, Roumenina LT, Loirat C, Frémeaux-Bacchi V; French Study Group for aHUS/C3G. Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nat Rev Nephrol. 2012;8(11):643-657.
28. Fakhouri F, Frémeaux-Bacchi V, Loirat C. Atypical hemolytic uremic syndrome: from the rediscovery of complement to targeted therapy. Eur J Intern Med. 2013;24(6):492-495.
29. Crowe K. Analysis: how pharmaceutical company Alexion set the price of the world's most expensive drug. CBC/Radio Canada website. http://www.cbc.ca/news/health/how-pharmaceutical-company-alexion-set-the-price-of-the-world-s-most-expensive-drug-1.3125251 Updated June 25, 2015. Accessed June 21, 2016.
30. Drug campaign for sick child was a PR stunt. FlandersToday website. http://www.flanderstoday.eu/business/drug-campaign-sick-child-was-pr-stunt. Published May 8, 2013. Accessed June 21, 2016.
31. Herald on Sunday editorial: miracle cure, morally derelect. New Zealand Herald website. http://www.nzherald.co.nz/opinion/news/article.cfm?c_id=466&objectid=10861630. Published January 27, 2013. Accessed June 21, 2016.
32. Brill S. Bitter pill: why medical bills are killing us. http://healthland.time.com/2013/02/20/bitter-pill-why-medical-bills-are-killing-us/print/[2/26/2013. Time website. Published February 20, 2013. Accessed June 6, 2016.
1. Caramazza D, Quintini G, Abbene I, et al. Relapsing or refractory idiopathic thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: the role of rituximab. Transfusion. 2010;50(12):2753-2760.
2. Köse O, Zimmerhackl LB, Jungraithmayr T, Mache C, Nürnberger J. New treatment options for atypical hemolytic uremic syndrome with the complement inhibitor eculizumab. Semin Thromb Hemost. 2010;36(6):669-672.
3. Reese JA, Muthurajah DS, Kremer Hovinga JA, Vesely SK, Terrell DR, George JN. Children and adults with thrombotic thrombocytopenic purpura associated with severe, acquired Adamts13 deficiency: comparison of incidence, demographic and clinical features. Pediatr Blood Cancer. 2013;60(10):1676-1682.
4. Sadler JE. Von Willebrand factor, ADAMTS13, and thrombotic thrombocytopenic purpura. Blood. 2008;112(1):11-18.
5. Tsai H-M. Pathophysiology of thrombotic thrombocytopenic purpura. Int J Hematol. 2010;91(1):1-19.
6. Noris M, Remuzzi G. Hemolytic uremic syndrome. J Am Soc Nephrol. 2005;16(4):1035-1050.
7. Liszewski MK, Atkinson JP. Exploring the complement system in human disease. The Rheumatologist website. http://www.the-rheumatologist.org/article/exploring-the-complement-system-in-human-disease. Published February 1, 2010. Accessed May 9, 2016.
8. Mayer CL, Leibowitz CS, Kurosawa S, Stearns-Kurosawa DJ. Shiga toxins and the pathophysiology of hemolytic uremic syndrome in humans and animals. Toxins (Basel). 2012;4(11):1261-1287.
9. Noris M, Mescia F, Remuzzi G. STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat Rev Nephrol. 2012;8(11):622-633.
10. Geerdink LM, Westra D, van Wijk JA, et al. Atypical hemolytic uremic syndrome in children: complement mutations and clinical characteristics. Pediatr Nephrol. 2012;27(8):1283-1291.
11. Sellier-Leclerc AL, Frémeaux-Bacchi V, Dragon-Durey MA, et al; French Society of Pediatric Nephrology. Differential impact of complement mutations on clinical characteristics in atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2007;18(8):2392-2400.
12. Herper M. The worlds most expensive drugs. Forbes. February 22, 2010.
13. Nordrum A. Drug prices: world's most expensive medicine costs $440,000 a year, but is it worth the expense? International Business Times website. http://www.ibtimes.com/drug-prices-worlds-most-expensive-medicine-costs-440000-year-it-worth-expense-2302609. Updated February 13, 2016. Published June 24, 2015. Accessed June 15, 2016.
14. CBC News. The real cost of the world's most expensive drug [video]. CBC/Radio Canada website. http://www.cbc.ca/player/play/2670383596. Accessed June 15, 2016.
15. EvaluatePharma. Orphan Drug Report 2014. EvaluatePharma website. http://www.evaluategroup.com/orphandrug2014. Published 2014. Accessed June 15, 2016.
16. Isaacs D. Ethical dilemmas about orphan drugs for orphan diseases. J Paediatr Child Health. 2014;50(4):249-250.
17. Licht C, Muus P, Legendre CM, et al. Eculizumab (ECU) safety and efficacy in atypical hemolytic uremic syndrome (aHUS) patients with long disease duration and chronic kidney disease (CKD): 2-year results. Poster presented at: 54th Annual Meeting of the American Society of Hematology; December 8-12, 2012; Atlanta, GA.
18. Greenbaum L, Legendre CM, Babu S, et al. Eculizumab (ECU) in atypical hemolytic uremic syndrome (aHUS) patients with progressing thrombotic microangiopathy (TMA): 2-year data. Poster presented at: 54th Annual Meeting of the American Society of Hematology; December 8-12, 2012; Atlanta, GA.
19. Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Eng J Med. 2013;368(23):2169-2181.
20. Bach PB, Saltz LB, Wittes RE. In cancer care, cost matters. New York Times. October 14, 2012:Opinion Pages.
21. Medicare Prescription Drug, Improvement, and Modernization Act of 2003, Public Law 108-173;117 Stat 2066. U.S. Government Printing Office website. https://www.gpo.gov/fdsys/pkg/PLAW-108publ173/pdf/PLAW-108publ173.pdf. Approved December 8, 2003. Accessed June 6, 2016.
22. Jodele S, Fukuda T, Vinks A, et al. Eculizumab therapy in children with severe hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Biol Blood Marrow Transplant. 2014;20(4):518-525.
23. Ardissino G, Testa S, Possenti I, et al. Discontinuation of eculizumab maintenance treatment for atypical hemolytic uremic syndrome: a report of 10 cases. Am J Kidney Dis. 2014;64(4):633-637.
24. Cugno M, Gualtierotti R, Possenti I, et al. Complement functional tests for monitoring eculizumab treatment in patients with atypical hemolytic uremic syndrome. J Thromb Haemost. 2014;12(9):1440-1448.
25. Waters AM, Licht C. aHUS caused by complement dysregulation: new therapies on the horizon. Pediatr Nephrol. 2011;26(1):41-57.
26. Gatault P, Brachet G, Ternant D, et al. Therapeutic drug monitoring of eculizumab: rational for an individualized dosing schedule. MAbs. 2015;7(6):1205-1211.
27. Zuber J, Fakhouri F, Roumenina LT, Loirat C, Frémeaux-Bacchi V; French Study Group for aHUS/C3G. Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nat Rev Nephrol. 2012;8(11):643-657.
28. Fakhouri F, Frémeaux-Bacchi V, Loirat C. Atypical hemolytic uremic syndrome: from the rediscovery of complement to targeted therapy. Eur J Intern Med. 2013;24(6):492-495.
29. Crowe K. Analysis: how pharmaceutical company Alexion set the price of the world's most expensive drug. CBC/Radio Canada website. http://www.cbc.ca/news/health/how-pharmaceutical-company-alexion-set-the-price-of-the-world-s-most-expensive-drug-1.3125251 Updated June 25, 2015. Accessed June 21, 2016.
30. Drug campaign for sick child was a PR stunt. FlandersToday website. http://www.flanderstoday.eu/business/drug-campaign-sick-child-was-pr-stunt. Published May 8, 2013. Accessed June 21, 2016.
31. Herald on Sunday editorial: miracle cure, morally derelect. New Zealand Herald website. http://www.nzherald.co.nz/opinion/news/article.cfm?c_id=466&objectid=10861630. Published January 27, 2013. Accessed June 21, 2016.
32. Brill S. Bitter pill: why medical bills are killing us. http://healthland.time.com/2013/02/20/bitter-pill-why-medical-bills-are-killing-us/print/[2/26/2013. Time website. Published February 20, 2013. Accessed June 6, 2016.
Prevention of Periprosthetic Joint Infections of the Hip and Knee
Nearly 2% of patients who undergo total knee arthroplasty (TKA) or total hip arthroplasty (THA) develop a periprosthetic joint infection (PJI) within 20 years of surgery, and 41% of these infections occur within the first 2 years.1 PJI is the most common cause of TKA failure and the third leading complication of THA.2 The estimated total hospital cost of treating PJI increased from $320 million in 2001 to $566 million in 2009, which can be extrapolated to $1.62 billion in 2020.3 By 2030, the projected increase in demand for TKA and THA will be 673% and 174% of what it was in 2005, respectively.4 Treatment of PJI of the knee is estimated to cost 3 to 4 times more than a primary TKA, and the cost of revision THA for PJI is almost $6000 more than that of revision TKA for PJI.3
In this article, we review the numerous preoperative, intraoperative, and postoperative methods of decreasing PJI incidence after total joint arthroplasty (TJA).
Preoperative Risk Prevention
Medical Comorbidities
Preoperative medical optimization is a key element in PJI prevention (Table 1). An American Society of Anesthesiologists classification score of 3 or more has been associated with doubled risk for surgical site infections (SSIs) after THA.5 Autoimmune conditions confer a particularly higher risk. In a retrospective double-cohort study of 924 subjects, Bongartz and colleagues6 found that, compared with osteoarthritis, rheumatoid arthritis tripled the risk of PJI. Small case series originally suggested a higher risk of PJI in patients with psoriasis,7,8 but more recent studies have contradicted that finding.9,10 Nevertheless, psoriatic plaques have elevated bacterial counts,11 and planned incisions should circumvent these areas.
Diabetes mellitus is a clear risk factor for PJI.12-16 Regarding whether preoperative glucose control affects risk, findings have been mixed. Mraovic and colleagues17 showed preoperative hyperglycemia to be an independent risk factor; Jämsen and colleagues,15 in a single-center analysis of more than 7000 TJAs, suggested preoperative blood glucose levels were not independently associated with PJI; and Iorio and colleagues16 found no association between surgical infections and hemoglobin A1c levels.
TJA incidence is higher in patients with chronic kidney disease (CKD) than in the general population.18 Dialysis users have a post-THA PJI rate as high as 13% to 19%.19,20 Early clinical data suggested that outcomes are improved in dialysis users who undergo renal transplant, but this finding recently has been questioned.19,21 Deegan and colleagues22 found an increased PJA rate of 3.5% even in low-level CKD (stage 1, 2, or 3), but this may be confounded by the increased association of CKD with other PJI-predisposing comorbidities.
Given a higher incidence of urinary tract infections (UTIs) among patients with PJI, some surgeons think UTIs predispose to PJIs by hematogenous seeding.12,23,24 Symptomatic UTIs should be cleared before surgery and confirmed on urinalysis. Obstructive symptoms should prompt urologic evaluation. As asymptomatic pyuria and bacteriuria (colony counts, >1 × 105/mL) do not predispose to PJI, patients without symptoms do not require intervention.25,26 Past history of malignancy may also have a role in PJI. In a case-control study of the Mayo Clinic arthroplasty experience from 1969 to 1991, Berbari and colleagues1 found an association between malignancy and PJI (odds ratio, 2.4). They theorized the immunosuppressive effects of cancer treatment might be responsible for this increased risk.
Immunocompromising Medications
Immunocompromising medications are modifiable and should be adjusted before surgery. Stopping any disease-modifying antirheumatic drug (DMARD) more than 4 weeks before surgery is not recommended.27
Corticosteroid use can lead to immunosuppression and increased protein catabolism, which impairs soft-tissue healing. To avoid flares or adrenal insufficiency, however, chronic corticosteroid users should continue their regular doses perioperatively.28 On the day of surgery, they should also receive a stress dose of hydrocortisone 50 to 75 mg (for primary arthroplasty) or 100 to 150 mg (for revision arthroplasty), followed by expeditious tapering over 1 to 2 days.29 DMARDs are increasingly used by rheumatologists. One of the most effective DMARDs is methotrexate. Despite its immunocompromising activity, methotrexate should be continued perioperatively, as stopping for even 2 days may increase flare-related complications.30 Hydroxychloroquine can be continued perioperatively and has even been shown, by Johnson and Charnley,31 to prevent deep vein thromboses. Sulfasalazine can also be continued perioperatively—but with caution, as it may elevate international normalized ratio (INR) levels in patients receiving warfarin.29 Most other DMARDs should be temporarily discontinued. Leflunomide and interleukin 1 antagonists, such as anakinra, should be stopped 1 to 2 days before surgery and restarted 10 to 14 days after surgery.29 Rituximab should be stopped 1 week before surgery and restarted 10 to 14 days after surgery. Tumor necrosis factor α inhibitors should be discontinued for 2 half-lives before and after surgery.32 Etanercept has a half-life of 3 to 5 days; infliximab, 8 to 10 days; and adalimumab, 10 to 13 days. Most surgeons schedule surgery for the end of a dosing cycle and discontinue these biologic agents for another 10 to 14 days after surgery.
Metabolic Factors
Obese patients are susceptible to longer surgeries, more extensive dissection, poorly vascularized subcutaneous tissue, and higher requirements of weight-adjusted antibiotic dosing.13 Body mass index (BMI) of 40 kg/m2 or more (morbid obesity) and BMI over 50 kg/m2 have been associated with 9 times and 21.3 times increased risk of PJI, respectively.13,14 Delaying surgery with dietary consultation has been suggested,33,34 and bariatric surgery before TKA may decrease infection rates by 3.5 times.35
Nutritional markers are considered before arthroplasty. According to most laboratories, a serum transferrin level under 200 mg/dL, albumin level under 3.5 g/dL, and total lymphocyte count under 1500 cells/mm3 indicate malnourishment, which can increase the incidence of wound complications by 5 to 7 times.36 Patients should also have sufficient protein, vitamin, and mineral supplementation, particularly vitamins A and C, zinc, and copper.37Smokers who cease smoking at least 4 to 6 weeks before surgery lower their wound complication rate by up to 26%.38,39 When nicotine leaves the bloodstream, vasodilation occurs, oxygenation improves, and the immune system recovers.39 Studies have found more SSIs in patients who abuse alcohol,40 and numerous authors have confirmed this finding in the arthroplasty population.24,41,42 Alcohol inhibits platelet function and may predispose to a postoperative hematoma. In contrast to smoking cessation evidence, evidence regarding alcohol interventions in preventing postoperative infections is less conclusive.43,44
MRSA Colonization
Methicillin-resistant Staphylococcus aureus (MRSA) is a particularly difficult bacterium to eradicate in PJI. As the mean cost of treating a single case of MRSA-related prosthetic infection is $107,264 vs $68,053 for susceptible strains,45,46 many infection-containment strategies focus on addressing benign MRSA colonization before surgery.
MRSA is present in the nares of 25 million people in the United States. Nasal colonization increases the risk of bacteremia 4-fold47 and SSI 2- to 9-fold.48,49 Nasal swabs are analyzed with either a rapid polymerase chain reaction (PCR) test, which provides results in 2 hours, or a bacterial culture, which provides results in 1 to 4 days. The PCR test is more expensive.
Eradication of MRSA colonization is increasingly prevalent. Several Scandinavian countries have instituted strict practices by which patients are denied elective surgery until negative nasal swabs are obtained.49 Nasal decontamination is one method of colonization reduction. Topical mupirocin, which yields eradication in 91% of nasal carriers immediately after treatment and in 87% after 4 weeks,50 is effective in reducing SSI rates only when used in conjunction with a body wash, which is used to clean the axilla and groin.51 There is no consensus on optimal timing, but Bode and colleagues52 found a significant decrease in deep SSIs when decontamination occurred just 24 hours before surgery.
Povidone-iodine showers went out of favor with the realization that chlorhexidine gluconate acts longer on the skin surface.53,54 Preoperative showers involve rinsing with liquid chlorhexidine soap 24 to 48 hours before surgery. However, chlorhexidine binds preferentially to the cotton in washcloths instead of the skin. Edmiston and colleagues54,55 found that 4% chlorhexidine liquid soaps achieve much lower skin chlorhexidine concentrations than 2% polyester cloths do. Use of these “chlorhexidine wipes” the night before and the day of surgery has decreased PJI after TKA from 2.2% to 0.6%.56,57
Intraoperative Risk Prevention
Preparation
Which preoperative antibiotic to use is one of the first operative considerations in PJI prophylaxis (Table 2). Cefazolin is recommended as a first-line agent for its excellent soft-tissue penetration, long half-life, and activity against gram-positive bacteria such as skin flora.58 Clindamycin may be considered for patients allergic to β-lactam antibiotics. Vancomycin may be considered for adjunctive use with cephalosporins in cases of known MRSA colonization. Vancomycin infusion should be started earlier than infusion with other antibiotics, as vancomycin must be infused slowly and takes longer to become therapeutic.
Antibiotic dosing should be based on local antibiograms, adjusted dosing weight, or BMI.59 For revision arthroplasty, preoperative prophylaxis should not be stopped out of fear of affecting operative cultures.60 Some surgeons pause antibiotic use if a preoperative joint aspirate has not been obtained. Infusion within 1 hour of incision is part of the pay-for-performance guidelines established by the US Centers for Medicare & Medicaid Services.61 An antibiotic should be redosed if the operation will take longer than 2 half-lives of the drug.59 Surgeons should consider administering a dose every 4 hours or whenever blood loss exceeds 1000 mL.62 Engesæter and colleagues63 found that antibiotic prophylaxis was most effective given 4 times perioperatively (1 time before surgery, 3 times after surgery). Postoperative antibiotics should not be administered longer than 24 hours, as prolonged dosing confers no benefit.58 Operating room conditions must be optimized for prophylaxis. More people and operating room traffic in nonsterile corridors increase contamination of instruments open to air.64 Laminar airflow systems are commonly used. Although there is little dispute that laminar flow decreases the bacterial load of air, there are mixed results regarding its benefit in preventing PJI.65-68 Skin preparation may address patient risk factors. Hair clipping is preferred to shaving, which may cause microabrasions and increased susceptibility to skin flora.69 Patients should be prepared with antiseptic solution. One randomized controlled trial found that 2% chlorhexidine gluconate mixed with 70% isopropyl alcohol was superior to 10% povidone-iodine in preventing SSIs.70 However, a recent cohort study showed a lower rate of superficial wound infections when 1% povidone-iodine (vs 0.5% chlorhexidine) was used with alcohol.71 This finding may indicate the need for alcohol preparation, higher concentrations of chlorhexidine, or both.
Proper scrubbing and protective gear are needed to reduce surgeon risk factors. Hand washing is a routine part of any surgery. Alcohol-based hand scrubs are as effective as hand scrubbing.65 They reduce local skin flora by 95% immediately and by 99% with repeated applications.72 Lidwell and colleagues73 found a 75% reduction in infection when body exhaust suits were used in combination with laminar flow in a multicenter randomized controlled trial of 8052 patients. Sterile draping with impermeable drapes should be done over properly prepared skin. Ioban drapes (3M) are often used as a protective barrier. Interestingly, a Cochrane review found no benefit in using plastic adhesives impregnated with iodine over sterilely prepared skin.74
Operative Considerations
Surgical gloves become contaminated in almost one third of cases, half the time during draping.75 For this reason, many surgeons change gloves after draping. In addition, double gloving prevents a breech of aseptic technique should the outer glove become perforated.76 Demircay and colleagues77 assessed double latex gloving in arthroplasty and found the outer and inner gloves perforated in 18.4% and 8.4% of cases, respectively. Punctures are most common along the nondominant index finger, and then the dominant thumb.77,78 Perforation is more common when 2 latex gloves are worn—vs 1 latex glove plus an outer cloth glove—and the chance of perforation increases with surgery duration. The inner glove may become punctured in up to 100% of operations that last over 3 hours.79 Although Dodds and colleagues80 found no change in bacterial counts on surgeons’ hands or gloves after perforation, precautions are still recommended. Al-Maiyah and colleagues81 went as far as to recommend glove changes at 20-minute intervals and before cementation.
Surgical instruments can be sources of contamination. Some authors change the suction tip every hour to minimize the risk of deep wound infection.82-85 Others change it before femoral canal preparation and prosthesis insertion during THA.86 The splash basin is frequently contaminated, and instruments placed in it should not be returned to the operative field.87 Hargrove and colleagues88 suggested pulsatile lavage decreases PJI more than bulb syringe irrigation does, whereas others argued that high-pressure lavage allows bacteria to penetrate more deeply, which could lead to retention of more bacteria.89 Minimizing operating room time was found by Kurtz and colleagues90 and Peersman and colleagues91 to decrease PJI incidence. Carroll and colleagues71 correlated longer tourniquet use with a higher rate of infection after TKA; proposed mechanisms include local tissue hypoxia and lowered concentrations of prophylactic antibiotics.
Similarly, minimizing blood loss and transfusion needs is another strategy for preventing infection. Allogenic transfusion may increase the risk of PJI 2 times.23,71,92 The mechanism seems to be immune system modulation by allogenic blood, which impairs microcirculation and oxygen delivery at the surgical site.23,75 Transfusions should be approached with caution, and consideration given to preoperative optimization and autologous blood donation. Cherian and colleagues93 reviewed different blood management strategies and found preoperative iron therapy, intravenous erythropoietin, and autologous blood donation to be equally effective in reducing the need for allogenic transfusions. Numerous studies of tranexamic acid, thrombin-based hemostatic matrix (Floseal; Baxter Inc), and bipolar sealer with radiofrequency ablation (Aquamantys; Medtronic Inc) have found no alterations in infection rates, but most have used calculated blood loss, not PJI, as the primary endpoint.94-105 Antibiotic cement also can be used to block infection.63,106-110 Although liquid gentamicin may weaken bone cement,111 most antibiotics, including powdered tobramycin and vancomycin, do not weaken its fatigue strength.111-114 A recent meta-analysis by Parvizi and colleagues115 revealed that deep infection rates dropped from 2.3% to 1.2% with use of antibiotic cement for primary THAs. Cummins and colleagues,116 however, reported the limited cost-effectiveness of antibiotic cement in primary arthroplasty. Performing povidone-iodine lavage at the end of the case may be a more inexpensive alternative. Brown and colleagues117 found that rinsing with dilute povidone-iodine (.35%) for 3 minutes significantly decreased the incidence of PJI.
Closure techniques and sutures have been a focus of much of the recent literature. Winiarsky and colleagues34 advocated using a longer incision for obese patients and augmenting closure in fattier areas with vertical mattress retention sutures, which are removed after 5 days. A barbed monofilament suture (Quill; Angiotech Inc) is gaining in popularity. Laboratory research has shown that bacteria adhere less to barbed monofilament sutures than to braided sutures.118 Smith and colleagues119 found a statistically nonsignificant higher rate of wound complications with barbed monofilament sutures, whereas Ting and colleagues120 found no difference in complications. These studies were powered to detect differences in time and cost, not postoperative complications. Skin adhesive (Dermabond; Ethicon Inc), also used in closure, may be superior to staples in avoiding superficial skin abscesses.121 Although expensive, silver-impregnated dressing has antimicrobial activity that reduces PJI incidence by up to 74%.122 One brand of this dressing (Aquacel; ConvaTec Inc) has a polyurethane waterproof barrier that allows it to be worn for 7 days.
Three factors commonly mentioned in PJI prevention show little supporting evidence. Drains, which are often used, may create a passage for postoperative infection and are associated with increased transfusion needs.123,124 Adding antibiotics to irrigation solution125 and routinely changing scalpel blades126-129 also have little supporting evidence. In 2014, the utility of changing scalpel blades after incision was studied by Lee and colleagues,130 who reported persistence of Propionibacterium acnes in the dermal layer after skin preparation. Their study, however, was isolated to the upper back region, not the hip or knee.
Postoperative Risk Prevention
Most arthroplasty patients receive anticoagulation after surgery, but it must be used with caution. Large hematomas can predispose to wound complications. Parvizi and colleagues131 associated wound drainage, hematoma, and subsequent PJI with an INR above 1.5 in the early postoperative period. Therefore, balanced anticoagulation is crucial. Postoperative glucose control is also essential, particularly for patients with diabetes. Although preoperative blood glucose levels may or may not affect PJI risk,15,17,132 postoperative blood glucose levels of 126 mg/dL or higher are strongly associated with joint infections.133 Even nondiabetic patients with postoperative morning levels over 140 mg/dL are 3 times more likely to develop an infection.17
Efforts should be made to discharge patients as soon as it is safe to do so. With longer hospital stays, patients are more exposed to nosocomial organisms and increased antibiotic resistance.5,23,134 Outpatient antibiotics should be considered for dental, gastrointestinal, and genitourinary procedures. Oral antibiotic prophylaxis is controversial, as there is some evidence that dental procedures increase the risk of PJI only minimally.10,135-138
Conclusion
PJI is a potentially devastating complication of TJA. For this reason, much research has been devoted to proper diagnosis and treatment. Although the literature on PJI prophylaxis is abundant, there is relatively little consensus on appropriate PJI precautions. Preoperative considerations should include medical comorbidities, use of immunocompromising medications, obesity, nutritional factors, smoking, alcohol use, and MRSA colonization. Surgeons must have a consistent intraoperative method of antibiotic administration, skin preparation, scrubbing, draping, gloving, instrument exchange, blood loss management, cementing, and closure. In addition, monitoring of postoperative anticoagulation and blood glucose management is important. Having a thorough understanding of PJI risk factors may help reduce the incidence of this devastating complication.
1. Berbari EF, Hanssen AD, Duffy MC, et al. Risk factors for prosthetic joint infection: case–control study. Clin Infect Dis. 1998;27(5):1247-1254.
2. Adeli B, Parvizi J. Strategies for the prevention of periprosthetic joint infection. J Bone Joint Surg Br. 2012;94(11 suppl A):42-46.
3. Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8 suppl):61-65.e1.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Ridgeway S. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005;87(6):844-850.
6. Bongartz T, Halligan CS, Osmon DR, et al. Incidence and risk factors of prosthetic joint infection after total hip or knee replacement in patients with rheumatoid arthritis. Arthritis Rheum. 2008;59(12):1713-1720.
7. Menon TJ, Wroblewski BM. Charnley low-friction arthroplasty in patients with psoriasis. Clin Orthop Relat Res. 1983;(176):127-128.
8. Stern SH, Insall JN, Windsor RE, Inglis AE, Dines DM. Total knee arthroplasty in patients with psoriasis. Clin Orthop Relat Res. 1989;(248):108-100.
9. Beyer CA, Hanssen AD, Lewallen DG, Pittelkow MR. Primary total knee arthroplasty in patients with psoriasis. J Bone Joint Surg Br. 1991;73(2):258-259.
10. Berbari EF, Osmon DR, Carr A, et al. Dental procedures as risk factors for prosthetic hip or knee infection: a hospital-based prospective case–control study. Clin Infect Dis. 2010;50(1):8-16.
11. Singh G, Rao DJ. Bacteriology of psoriatic plaques. Dermatologica. 1978;157(1):21-27.
12. Bozic KJ, Ong K, Lau E, et al. Estimating risk in Medicare patients with THA: an electronic risk calculator for periprosthetic joint infection and mortality. Clin Orthop Relat Res. 2013;471(2):574-583.
13. Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009;24(6 suppl):84-88.
14. Dowsey MM, Choong PFM. Obese diabetic patients are at substantial risk for deep infection after primary TKA. Clin Orthop Relat Res. 2009;467(6):1577-1581.
15. Jämsen E, Nevalainen P, Eskelinen A, Huotari K, Kalliovalkama J, Moilanen T. Obesity, diabetes, and preoperative hyperglycemia as predictors of periprosthetic joint infection: a single-center analysis of 7181 primary hip and knee replacements for osteoarthritis. J Bone Joint Surg Am. 2012;94(14):e101.
16. Iorio R, Williams KM, Marcantonio AJ, Specht LM, Tilzey JF, Healy WL. Diabetes mellitus, hemoglobin A1C, and the incidence of total joint arthroplasty infection. J Arthroplasty. 2012;27(5):726-729.e1.
17. Mraovic B, Suh D, Jacovides C. Perioperative hyperglycemia and postoperative infection after lower limb arthroplasty. J Diabetes Sci Technol. 2011;5(2):412-418.
18. Abbott KC, Bucci JR, Agodoa LY. Total hip arthroplasty in chronic dialysis patients in the United States. J Nephrol. 2003;16(1):34-39.
19. Lieberman JR, Fuchs MD, Haas SB, et al. Hip arthroplasty in patients with chronic renal failure. J Arthroplasty. 1995;10(2):191-195.
20. Sakalkale DP, Hozack WJ, Rothman RH. Total hip arthroplasty in patients on long-term renal dialysis. J Arthroplasty. 1999;14(5):571-575.
21. Shrader MW, Schall D, Parvizi J, McCarthy JT, Lewallen DG. Total hip arthroplasty in patients with renal failure: a comparison between transplant and dialysis patients. J Arthroplasty. 2006;21(3):324-329.
22. Deegan BF, Richard RD, Bowen TR, Perkins RM, Graham JH, Foltzer MA. Impact of chronic kidney disease stage on lower-extremity arthroplasty. Orthopedics. 2014;37(7):e613-e618.
23. Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466(7):1710-1715.
24. Tomás T. Patient-related risk factors for infected total arthroplasty. Acta Chir Orthop. 2008;75(6):451-456.
25. Ritter MA, Fechtman RW. Urinary tract sequelae: possible influence on joint infections following total joint replacement. Orthopedics. 1987;10(3):467-469.
26. Gou W, Chen J, Jia Y, Wang Y. Preoperative asymptomatic leucocyturia and early prosthetic joint infections in patients undergoing joint arthroplasty. J Arthroplasty. 2014;29(3):473-476.
27. Goodman SM, Paget S. Perioperative drug safety in patients with rheumatoid arthritis. Rheum Dis Clin North Am. 2012;38(4):747-759.
28. Salem M, Tainsh RE Jr, Bromberg J, Loriaux DL, Chernow B. Perioperative glucocorticoid coverage. A reassessment 42 years after emergence of a problem. Ann Surg. 1994;219(4):416-425.
29. Howe CR, Gardner GC, Kadel NJ. Perioperative medication management for the patient with rheumatoid arthritis. J Am Acad Orthop Surg. 2006;14(9):544-551.
30. Grennan DM. Methotrexate and early postoperative complications in patients with rheumatoid arthritis undergoing elective orthopaedic surgery. Ann Rheum Dis. 2001;60(3):214-217.
31. Johnson R, Charnley J. Hydroxychloroquine in prophylaxis of pulmonary embolism following hip arthroplasty. Clin Orthop Relat Res. 1979;(144):174-177.
32. Mushtaq S, Goodman SM, Scanzello CR. Perioperative management of biologic agents used in treatment of rheumatoid arthritis. Am J Ther. 2011;18(5):426-434.
33. Namba RS, Paxton L, Fithian DC, Stone ML. Obesity and perioperative morbidity in total hip and total knee arthroplasty patients. J Arthroplasty. 2005;20(7 suppl 3):46-50.
34. Winiarsky R, Barth P, Lotke PA. Total knee arthroplasty in morbidly obese patients. J Bone Joint Surg Am. 1998;80(12):1770-1774.
35. Kulkarni A, Jameson SS, James P, Woodcock S, Muller S, Reed MR. Does bariatric surgery prior to lower limb joint replacement reduce complications? Surgeon. 2011;9(1):18-21.
36. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients. J Arthroplasty. 1991;6(4):321-325.
37. Fairfield KM, Fletcher RH. Vitamins for chronic disease prevention in adults. JAMA. 2002;287(23):3116.
38. Kwiatkowski TC, Hanley EN Jr, Ramp WK. Cigarette smoking and its orthopedic consequences. Am J Orthop. 1996;25(9):590-597.
39. Møller AM, Villebro N, Pedersen T, Tønnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet. 2002;359(9301):114-117.
40. Rantala A, Lehtonen OP, Niinikoski J. Alcohol abuse: a risk factor for surgical wound infections? Am J Infect Control. 1997;25(5):381-386.
41. Wu C, Qu X, Liu F, Li H, Mao Y, Zhu Z. Risk factors for periprosthetic joint infection after total hip arthroplasty and total knee arthroplasty in Chinese patients. PLoS One. 2014;9(4):e95300.
42. Cordero-Ampuero J, de Dios M. What are the risk factors for infection in hemiarthroplasties and total hip arthroplasties? Clin Orthop Relat Res. 2010;468(12):3268-3277.
43. Tønnesen H, Rosenberg J, Nielsen HJ, et al. Effect of preoperative abstinence on poor postoperative outcome in alcohol misusers: randomised controlled trial. BMJ. 1999;318(7194):1311-1316.
44. Shourie S, Conigrave KM, Proude EM, Ward JE, Wutzke SE, Haber PS. The effectiveness of a tailored intervention for excessive alcohol consumption prior to elective surgery. Alcohol Alcohol. 2006;41(6):643-649.
45. Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128-133.
46. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
47. Safdar N, Bradley EA. The risk of infection after nasal colonization with Staphylococcus aureus. Am J Med. 2008;121(4):310-315.
48. American Academy of Orthopaedic Surgeons Patient Safety Committee, Evans RP. Surgical site infection prevention and control: an emerging paradigm. J Bone Joint Surg Am. 2009;91(suppl 6):2-9.
49. Goyal N, Aggarwal V, Parvizi J. Methicillin-resistant Staphylococcus aureus screening in total joint arthroplasty: a worthwhile endeavor. J Knee Surg. 2012;25(1):37-43.
50. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10(3):505-520.
51. Wilcox MH, Hall J, Pike H, et al. Use of perioperative mupirocin to prevent methicillin-resistant Staphylococcus aureus (MRSA) orthopaedic surgical site infections. J Hosp Infect. 2003;54(3):196-201.
52. Bode LG, Kluytmans JA, Wertheim HF, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010;362(1):9-17.
53. Association of Operating Room Nurses. Recommended practices for skin preparation of patients. AORN J. 2002;75(1):184-187.
54. Edmiston CE Jr, Seabrook GR, Johnson CP, Paulson DS, Beausoleil CM. Comparative of a new and innovative 2% chlorhexidine gluconate–impregnated cloth with 4% chlorhexidine gluconate as topical antiseptic for preparation of the skin prior to surgery. Am J Infect Control. 2007;35(2):89-96.
55. Edmiston CE Jr, Krepel CJ, Seabrook GR, Lewis BD, Brown KR, Towne JB. Preoperative shower revisited: can high topical antiseptic levels be achieved on the skin surface before surgical admission? J Am Coll Surg. 2008;207(2):233-239.
56. Johnson AJ, Kapadia BH, Daley JA, Molina CB, Mont MA. Chlorhexidine reduces infections in knee arthroplasty. J Knee Surg. 2013;26(3):213-218.
57. Johnson AJ, Daley JA, Zywiel MG, Delanois RE, Mont MA. Preoperative chlorhexidine preparation and the incidence of surgical site infections after hip arthroplasty. J Arthroplasty. 2010;25(6 suppl):98-102.
58. Mauerhan DR, Nelson CL, Smith DL, et al. Prophylaxis against infection in total joint arthroplasty. One day of cefuroxime compared with three days of cefazolin. J Bone Joint Surg Am. 1994;76(1):39-45.
59. Bratzler DW, Houck PM; Surgical Infection Prevention Guideline Writers Workgroup. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Am J Surg. 2005;189(4):395-404.
60. Tetreault MW, Wetters NG, Aggarwal V, Mont M, Parvizi J, Della Valle CJ. The Chitranjan Ranawat Award: should prophylactic antibiotics be withheld before revision surgery to obtain appropriate cultures? Clin Orthop Relat Res. 2014;472(1):52-56.
61. Illingworth KD, Mihalko WM, Parvizi J, et al. How to minimize infection and thereby maximize patient outcomes in total joint arthroplasty: a multicenter approach: AAOS exhibit selection. J Bone Joint Surg Am. 2013;95(8):e50.
62. Bannister GC, Auchincloss JM, Johnson DP, Newman JH. The timing of tourniquet application in relation to prophylactic antibiotic administration. J Bone Joint Surg Br. 1988;70(2):322-324.
63. Engesæter LB, Lie SA, Espehaug B, Furnes O, Vollset SE, Havelin LI. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand. 2003;74(6):644-651.
64. Ritter MA. Operating room environment. Clin Orthop Relat Res. 1999;(369):103-109.
65. Brandt C, Hott U, Sohr D, Daschner F, Gastmeier P, Rüden H. Operating room ventilation with laminar airflow shows no protective effect on the surgical site infection rate in orthopedic and abdominal surgery. Ann Surg. 2008;248(5):695-700.
66. Dharan S, Pittet D. Environmental controls in operating theatres. J Hosp Infect. 2002;51(2):79-84.
67. Hamilton HW, Booth AD, Lone FJ, Clark N. Penetration of gown material by organisms from the surgical team. Clin Orthop Relat Res. 1979;(141):237-246.
68. Da Costa AR, Kothari A, Bannister GC, Blom AW. Investigating bacterial growth in surgical theatres: establishing the effect of laminar airflow on bacterial growth on plastic, metal and wood surfaces. Ann R Coll Surg Engl. 2008;90(5):417-419.
69. Tanner J, Woodings D, Moncaster K. Preoperative hair removal to reduce surgical site infection. Cochrane Database Syst Rev. 2006;(2):CD004122.
70. Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18-26.
71. Carroll K, Dowsey M, Choong P, Peel T. Risk factors for superficial wound complications in hip and knee arthroplasty. Clin Microbiol Infect. 2013;20(2):130-135.
72. Ayliffe GA. Surgical scrub and skin disinfection. Infect Control. 1984;5(1):23-27.
73. Lidwell OM, Lowbury EJ, Whyte W, Blowers R, Lowe D. Extended follow-up of patients suspected of having joint sepsis after total joint replacement. J Hyg (Lond). 1985;95(3):655-664.
74. Webster J, Alghamdi AA. Use of plastic adhesive drapes during surgery for preventing surgical site infection. Cochrane Database Syst Rev. 2007;(4):CD006353.
75. Alijanipour P, Heller S, Parvizi J. Prevention of periprosthetic joint infection: what are the effective strategies? J Knee Surg. 2014;27(4):251-258.
76. Tanner J, Parkinson H. Double gloving to reduce surgical cross-infection. Cochrane Database Syst Rev. 2002;(3):CD003087.
77. Demircay E, Unay K, Bilgili MG, Alataca G. Glove perforation in hip and knee arthroplasty. J Orthop Sci. 2010;15(6):790-794.
78. Ersozlu S, Sahin O, Ozgur AF, Akkaya T, Tuncay C. Glove punctures in major and minor orthopaedic surgery with double gloving. Acta Orthop Belg. 2007;73(6):760-764.
79. Sanders R, Fortin P, Ross E, Helfet D. Outer gloves in orthopaedic procedures. Cloth compared with latex. J Bone Joint Surg Am. 1990;72(6):914-917.
80. Dodds RD, Guy PJ, Peacock AM, Duffy SR, Barker SG, Thomas MH. Surgical glove perforation. Br J Surg. 1988;75(10):966-968.
81. Al-Maiyah M, Bajwa A, Mackenney P, et al. Glove perforation and contamination in primary total hip arthroplasty. J Bone Joint Surg Br. 2005;87(4):556-559.
82. Insull PJ, Hudson J. Suction tip: a potential source of infection in clean orthopaedic procedures. ANZ J Surg. 2012;82(3):185-186.
83. Givissis P, Karataglis D, Antonarakos P, Symeonidis PD, Christodoulou A. Suction during orthopaedic surgery. How safe is the suction tip? Acta Orthop Belg. 2008;74(4):531-533.
84. Meals RA, Knoke L. The surgical suction top—a contaminated instrument. J Bone Joint Surg Am. 1978;60(3):409-410.
85. Strange-Vognsen MH, Klareskov B. Bacteriologic contamination of suction tips during hip arthroplasty. Acta Orthop Scand. 1988;59(4):410-411.
86. Greenough CG. An investigation into contamination of operative suction. J Bone Joint Surg Br. 1986;68(1):151-153.
87. Baird RA, Nickel FR, Thrupp LD, Rucker S, Hawkins B. Splash basin contamination in orthopaedic surgery. Clin Orthop Relat Res. 1984;(187):129-133.
88. Hargrove R, Ridgeway S, Russell R, Norris M, Packham I, Levy B. Does pulse lavage reduce hip hemiarthroplasty infection rates? J Hosp Infect. 2006;62(4):446-449.
89. Hassinger SM, Harding G, Wongworawat MD. High-pressure pulsatile lavage propagates bacteria into soft tissue. Clin Orthop Relat Res. 2005;(439):27-31.
90. Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res. 2010;468(1):52-56.
91. Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement. Clin Orthop Relat Res. 2001;(392):15-23.
92. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
93. Cherian JJ, Kapadia BH, Issa K, et al. Preoperative blood management strategies for total hip arthroplasty. Surg Technol Int. 2013;23:261-266.
94. Issa K, Banerjee S, Rifai A, et al. Blood management strategies in primary and revision total knee arthroplasty for Jehovah’s Witness patients. J Knee Surg. 2013;26(6):401-404.
95. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2010;93(1):39-46.
96. Berger V, Alperson S. A general framework for the evaluation of clinical trial quality. Rev Recent Clin Trials. 2009;4(2):79-88.
97. Chimento GF, Huff T, Ochsner JL, Meyer M, Brandner L, Babin S. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 suppl):74-77.
98. Karam JA, Bloomfield MR, DiIorio TM, Irizarry AM, Sharkey PF. Evaluation of the efficacy and safety of tranexamic acid for reducing blood loss in bilateral total knee arthroplasty. J Arthroplasty. 2014;29(3):501-503.
99. Kim HJ, Fraser MR, Kahn B, Lyman S, Figgie MP. The efficacy of a thrombin-based hemostatic agent in unilateral total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(13):1160-1165.
100. Suarez JC, Slotkin EM, Alvarez AM, Szubski CR, Barsoum WK, Patel PD. Prospective, randomized trial to evaluate efficacy of a thrombin-based hemostatic agent in total knee arthroplasty. J Arthroplasty. 2014;29(10):1950-1955.
101. Romanò CL, Monti L, Logoluso N, Romanò D, Drago L. Does a thrombin-based topical haemostatic agent reduce blood loss and transfusion requirements after total knee revision surgery? A randomized, controlled trial. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3337-3342.
102. Falez F, Meo A, Panegrossi G, Favetti F, Cava F, Casella F. Blood loss reduction in cementless total hip replacement with fibrin spray or bipolar sealer: a randomised controlled trial on ninety five patients. Int Orthop. 2013;37(7):1213-1217.
103. Morris MJ, Barrett M, Lombardi AV, Tucker TL, Berend KR. Randomized blinded study comparing a bipolar sealer and standard electrocautery in reducing transfusion requirements in anterior supine intermuscular total hip arthroplasty. J Arthroplasty. 2013;28(9):1614-1617.
104. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518.
105. Zeh A, Messer J, Davis J, Vasarhelyi A, Wohlrab D. The Aquamantys system—an alternative to reduce blood loss in primary total hip arthroplasty? J Arthroplasty. 2010;25(7):1072-1077.
106. Heck D, Rosenberg A, Schink-Ascani M, Garbus S, Kiewitt T. Use of antibiotic-impregnated cement during hip and knee arthroplasty in the United States. J Arthroplasty. 1995;10(4):470-475.
107. Srivastav A, Nadkarni B, Srivastav S, Mittal V, Agarwal S. Prophylactic use of antibiotic-loaded bone cement in primary total knee arthroplasty: justified or not? Indian J Orthop. 2009;43(3):259-263.
108. Dunbar MJ. Antibiotic bone cements: their use in routine primary total joint arthroplasty is justified. Orthopedics. 2009;32(9).
109. Merollini KM, Zheng H, Graves N. Most relevant strategies for preventing surgical site infection after total hip arthroplasty: guideline recommendations and expert opinion. Am J Infect Control. 2013;41(3):221-226.
110. Jämsen E, Huhtala H, Puolakka T, Moilanen T. Risk factors for infection after knee arthroplasty. A register-based analysis of 43,149 cases. J Bone Joint Surg Am. 2009;91(1):38-47.
111. Seldes RM, Winiarsky R, Jordan LC, et al. Liquid gentamicin in bone cement: a laboratory study of a potentially more cost-effective cement spacer. J Bone Joint Surg Am. 2005;87(2):268-272.
112. Wright TM, Sullivan DJ, Arnoczky SP. The effect of antibiotic additions on the fracture properties of bone cements. Acta Orthop Scand. 1984;55(4):414-418.
113. Baleani M, Persson C, Zolezzi C, Andollina A, Borrelli AM, Tigani D. Biological and biomechanical effects of vancomycin and meropenem in acrylic bone cement. J Arthroplasty. 2008;23(8):1232-1238.
114. Baleani M, Cristofolini L, Minari C, Toni A. Fatigue strength of PMMA bone cement mixed with gentamicin and barium sulphate vs pure PMMA. Proc Inst Mech Eng H. 2005;217(1):9-12.
115. Parvizi J, Saleh KJ, Ragland PS, Pour AE, Mont MA. Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthop Scand. 2008;79(3):335-341.
116. Cummins JS, Tomek IM, Kantor SR, Furnes O, Engesæter LB, Finlayson SRG. Cost-effectiveness of antibiotic-impregnated bone cement used in primary total hip arthroplasty. J Bone Joint Surg Am. 2009;91(3):634-641.
117. Brown NM, Cipriano CA, Moric M, Sporer SM, Della Valle CJ. Dilute Betadine lavage before closure for the prevention of acute postoperative deep periprosthetic joint infection. J Arthroplasty. 2012;27(1):27-30.
118. Fowler JR, Perkins TA, Buttaro BA, Truant AL. Bacteria adhere less to barbed monofilament than braided sutures in a contaminated wound model. Clin Orthop Relat Res. 2013;471(2):665-671.
119. Smith EL, DiSegna ST, Shukla PY, Matzkin EG. Barbed versus traditional sutures: closure time, cost, and wound related outcomes in total joint arthroplasty. J Arthroplasty. 2014;29(2):283-287.
120. Ting NT, Moric MM, Della Valle CJ, Levine BR. Use of knotless suture for closure of total hip and knee arthroplasties: a prospective, randomized clinical trial. J Arthroplasty. 2012;27(10):1783-1788.
121. Miller AG, Swank ML. Dermabond efficacy in total joint arthroplasty wounds. Am J Orthop. 2010;39(10):476-478.
122. Cai J, Karam JA, Parvizi J, Smith EB, Sharkey PF. Aquacel surgical dressing reduces the rate of acute PJI following total joint arthroplasty: a case–control study. J Arthroplasty. 2014;29(6):1098-1100.
123. Drinkwater CJ, Neil MJ. Optimal timing of wound drain removal following total joint arthroplasty. J Arthroplasty. 1995;10(2):185-189.
124. Parker MJ, Roberts CP, Hay D. Closed suction drainage for hip and knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2004;86(6):1146-1152.
125. Matar WY, Jafari SM, Restrepo C, Austin M, Purtill JJ, Parvizi J. Preventing infection in total joint arthroplasty. J Bone Joint Surg Am. 2010;92(suppl 2):36-46.
126. Ritter MA, French ML, Eitzen HE. Bacterial contamination of the surgical knife. Clin Orthop Relat Res. 1975;(108):158-160.
127. Fairclough JA, Mackie IG, Mintowt-Czyz W, Phillips GE. The contaminated skin-knife. A surgical myth. J Bone Joint Surg Br. 1983;65(2):210.
128. Ramón R, García S, Combalía A, Puig de la Bellacasa J, Segur JM. Bacteriological study of surgical knives: is the use of two blades necessary? Arch Orthop Trauma Surg. 1994;113(3):157-158.
129. Hasselgren PO, Hagberg E, Malmer H, Säljö A, Seeman T. One instead of two knives for surgical incision. Does it increase the risk of postoperative wound infection? Arch Surg. 1984;119(8):917-920.
130. Lee MJ, Pottinger PS, Butler-Wu S, Bumgarner RE, Russ SM, Matsen FA 3rd. Propionibacterium persists in the skin despite standard surgical preparation. J Bone Joint Surg Am. 2014;96(17):1447-1450.
131. Parvizi J, Ghanem E, Joshi A, Sharkey PF, Hozack WJ, Rothman RH. Does “excessive” anticoagulation predispose to periprosthetic infection? J Arthroplasty. 2007;22(6 suppl 2):24-28.
132. Marchant MH, Viens NA, Cook C, Vail TP, Bolognesi MP. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. J Bone Joint Surg Am. 2009;91(7):1621-1629.
133. Reátegui D, Sanchez-Etayo G, Núñez E, et al. Perioperative hyperglycaemia and incidence of post-operative complications in patients undergoing total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2026-2031.
134. Urquhart DM, Hanna FS, Brennan SL, et al. Incidence and risk factors for deep surgical site infection after primary total hip arthroplasty: a systematic review. J Arthroplasty. 2010;25(8):1216-1222.e1-e3.
135. Friedlander AH. Oral cavity staphylococci are a potential source of prosthetic joint infection. Clin Infect Dis. 2010;50(12):1682-1683.
136. Zimmerli W, Sendi P. Antibiotics for prevention of periprosthetic joint infection following dentistry: time to focus on data. Clin Infect Dis. 2010;50(1):17-19.
137. Young H, Hirsh J, Hammerberg EM, Price CS. Dental disease and periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(2):162-168.
138. Simmons NA, Ball AP, Cawson RA, et al. Case against antibiotic prophylaxis for dental treatment of
Nearly 2% of patients who undergo total knee arthroplasty (TKA) or total hip arthroplasty (THA) develop a periprosthetic joint infection (PJI) within 20 years of surgery, and 41% of these infections occur within the first 2 years.1 PJI is the most common cause of TKA failure and the third leading complication of THA.2 The estimated total hospital cost of treating PJI increased from $320 million in 2001 to $566 million in 2009, which can be extrapolated to $1.62 billion in 2020.3 By 2030, the projected increase in demand for TKA and THA will be 673% and 174% of what it was in 2005, respectively.4 Treatment of PJI of the knee is estimated to cost 3 to 4 times more than a primary TKA, and the cost of revision THA for PJI is almost $6000 more than that of revision TKA for PJI.3
In this article, we review the numerous preoperative, intraoperative, and postoperative methods of decreasing PJI incidence after total joint arthroplasty (TJA).
Preoperative Risk Prevention
Medical Comorbidities
Preoperative medical optimization is a key element in PJI prevention (Table 1). An American Society of Anesthesiologists classification score of 3 or more has been associated with doubled risk for surgical site infections (SSIs) after THA.5 Autoimmune conditions confer a particularly higher risk. In a retrospective double-cohort study of 924 subjects, Bongartz and colleagues6 found that, compared with osteoarthritis, rheumatoid arthritis tripled the risk of PJI. Small case series originally suggested a higher risk of PJI in patients with psoriasis,7,8 but more recent studies have contradicted that finding.9,10 Nevertheless, psoriatic plaques have elevated bacterial counts,11 and planned incisions should circumvent these areas.
Diabetes mellitus is a clear risk factor for PJI.12-16 Regarding whether preoperative glucose control affects risk, findings have been mixed. Mraovic and colleagues17 showed preoperative hyperglycemia to be an independent risk factor; Jämsen and colleagues,15 in a single-center analysis of more than 7000 TJAs, suggested preoperative blood glucose levels were not independently associated with PJI; and Iorio and colleagues16 found no association between surgical infections and hemoglobin A1c levels.
TJA incidence is higher in patients with chronic kidney disease (CKD) than in the general population.18 Dialysis users have a post-THA PJI rate as high as 13% to 19%.19,20 Early clinical data suggested that outcomes are improved in dialysis users who undergo renal transplant, but this finding recently has been questioned.19,21 Deegan and colleagues22 found an increased PJA rate of 3.5% even in low-level CKD (stage 1, 2, or 3), but this may be confounded by the increased association of CKD with other PJI-predisposing comorbidities.
Given a higher incidence of urinary tract infections (UTIs) among patients with PJI, some surgeons think UTIs predispose to PJIs by hematogenous seeding.12,23,24 Symptomatic UTIs should be cleared before surgery and confirmed on urinalysis. Obstructive symptoms should prompt urologic evaluation. As asymptomatic pyuria and bacteriuria (colony counts, >1 × 105/mL) do not predispose to PJI, patients without symptoms do not require intervention.25,26 Past history of malignancy may also have a role in PJI. In a case-control study of the Mayo Clinic arthroplasty experience from 1969 to 1991, Berbari and colleagues1 found an association between malignancy and PJI (odds ratio, 2.4). They theorized the immunosuppressive effects of cancer treatment might be responsible for this increased risk.
Immunocompromising Medications
Immunocompromising medications are modifiable and should be adjusted before surgery. Stopping any disease-modifying antirheumatic drug (DMARD) more than 4 weeks before surgery is not recommended.27
Corticosteroid use can lead to immunosuppression and increased protein catabolism, which impairs soft-tissue healing. To avoid flares or adrenal insufficiency, however, chronic corticosteroid users should continue their regular doses perioperatively.28 On the day of surgery, they should also receive a stress dose of hydrocortisone 50 to 75 mg (for primary arthroplasty) or 100 to 150 mg (for revision arthroplasty), followed by expeditious tapering over 1 to 2 days.29 DMARDs are increasingly used by rheumatologists. One of the most effective DMARDs is methotrexate. Despite its immunocompromising activity, methotrexate should be continued perioperatively, as stopping for even 2 days may increase flare-related complications.30 Hydroxychloroquine can be continued perioperatively and has even been shown, by Johnson and Charnley,31 to prevent deep vein thromboses. Sulfasalazine can also be continued perioperatively—but with caution, as it may elevate international normalized ratio (INR) levels in patients receiving warfarin.29 Most other DMARDs should be temporarily discontinued. Leflunomide and interleukin 1 antagonists, such as anakinra, should be stopped 1 to 2 days before surgery and restarted 10 to 14 days after surgery.29 Rituximab should be stopped 1 week before surgery and restarted 10 to 14 days after surgery. Tumor necrosis factor α inhibitors should be discontinued for 2 half-lives before and after surgery.32 Etanercept has a half-life of 3 to 5 days; infliximab, 8 to 10 days; and adalimumab, 10 to 13 days. Most surgeons schedule surgery for the end of a dosing cycle and discontinue these biologic agents for another 10 to 14 days after surgery.
Metabolic Factors
Obese patients are susceptible to longer surgeries, more extensive dissection, poorly vascularized subcutaneous tissue, and higher requirements of weight-adjusted antibiotic dosing.13 Body mass index (BMI) of 40 kg/m2 or more (morbid obesity) and BMI over 50 kg/m2 have been associated with 9 times and 21.3 times increased risk of PJI, respectively.13,14 Delaying surgery with dietary consultation has been suggested,33,34 and bariatric surgery before TKA may decrease infection rates by 3.5 times.35
Nutritional markers are considered before arthroplasty. According to most laboratories, a serum transferrin level under 200 mg/dL, albumin level under 3.5 g/dL, and total lymphocyte count under 1500 cells/mm3 indicate malnourishment, which can increase the incidence of wound complications by 5 to 7 times.36 Patients should also have sufficient protein, vitamin, and mineral supplementation, particularly vitamins A and C, zinc, and copper.37Smokers who cease smoking at least 4 to 6 weeks before surgery lower their wound complication rate by up to 26%.38,39 When nicotine leaves the bloodstream, vasodilation occurs, oxygenation improves, and the immune system recovers.39 Studies have found more SSIs in patients who abuse alcohol,40 and numerous authors have confirmed this finding in the arthroplasty population.24,41,42 Alcohol inhibits platelet function and may predispose to a postoperative hematoma. In contrast to smoking cessation evidence, evidence regarding alcohol interventions in preventing postoperative infections is less conclusive.43,44
MRSA Colonization
Methicillin-resistant Staphylococcus aureus (MRSA) is a particularly difficult bacterium to eradicate in PJI. As the mean cost of treating a single case of MRSA-related prosthetic infection is $107,264 vs $68,053 for susceptible strains,45,46 many infection-containment strategies focus on addressing benign MRSA colonization before surgery.
MRSA is present in the nares of 25 million people in the United States. Nasal colonization increases the risk of bacteremia 4-fold47 and SSI 2- to 9-fold.48,49 Nasal swabs are analyzed with either a rapid polymerase chain reaction (PCR) test, which provides results in 2 hours, or a bacterial culture, which provides results in 1 to 4 days. The PCR test is more expensive.
Eradication of MRSA colonization is increasingly prevalent. Several Scandinavian countries have instituted strict practices by which patients are denied elective surgery until negative nasal swabs are obtained.49 Nasal decontamination is one method of colonization reduction. Topical mupirocin, which yields eradication in 91% of nasal carriers immediately after treatment and in 87% after 4 weeks,50 is effective in reducing SSI rates only when used in conjunction with a body wash, which is used to clean the axilla and groin.51 There is no consensus on optimal timing, but Bode and colleagues52 found a significant decrease in deep SSIs when decontamination occurred just 24 hours before surgery.
Povidone-iodine showers went out of favor with the realization that chlorhexidine gluconate acts longer on the skin surface.53,54 Preoperative showers involve rinsing with liquid chlorhexidine soap 24 to 48 hours before surgery. However, chlorhexidine binds preferentially to the cotton in washcloths instead of the skin. Edmiston and colleagues54,55 found that 4% chlorhexidine liquid soaps achieve much lower skin chlorhexidine concentrations than 2% polyester cloths do. Use of these “chlorhexidine wipes” the night before and the day of surgery has decreased PJI after TKA from 2.2% to 0.6%.56,57
Intraoperative Risk Prevention
Preparation
Which preoperative antibiotic to use is one of the first operative considerations in PJI prophylaxis (Table 2). Cefazolin is recommended as a first-line agent for its excellent soft-tissue penetration, long half-life, and activity against gram-positive bacteria such as skin flora.58 Clindamycin may be considered for patients allergic to β-lactam antibiotics. Vancomycin may be considered for adjunctive use with cephalosporins in cases of known MRSA colonization. Vancomycin infusion should be started earlier than infusion with other antibiotics, as vancomycin must be infused slowly and takes longer to become therapeutic.
Antibiotic dosing should be based on local antibiograms, adjusted dosing weight, or BMI.59 For revision arthroplasty, preoperative prophylaxis should not be stopped out of fear of affecting operative cultures.60 Some surgeons pause antibiotic use if a preoperative joint aspirate has not been obtained. Infusion within 1 hour of incision is part of the pay-for-performance guidelines established by the US Centers for Medicare & Medicaid Services.61 An antibiotic should be redosed if the operation will take longer than 2 half-lives of the drug.59 Surgeons should consider administering a dose every 4 hours or whenever blood loss exceeds 1000 mL.62 Engesæter and colleagues63 found that antibiotic prophylaxis was most effective given 4 times perioperatively (1 time before surgery, 3 times after surgery). Postoperative antibiotics should not be administered longer than 24 hours, as prolonged dosing confers no benefit.58 Operating room conditions must be optimized for prophylaxis. More people and operating room traffic in nonsterile corridors increase contamination of instruments open to air.64 Laminar airflow systems are commonly used. Although there is little dispute that laminar flow decreases the bacterial load of air, there are mixed results regarding its benefit in preventing PJI.65-68 Skin preparation may address patient risk factors. Hair clipping is preferred to shaving, which may cause microabrasions and increased susceptibility to skin flora.69 Patients should be prepared with antiseptic solution. One randomized controlled trial found that 2% chlorhexidine gluconate mixed with 70% isopropyl alcohol was superior to 10% povidone-iodine in preventing SSIs.70 However, a recent cohort study showed a lower rate of superficial wound infections when 1% povidone-iodine (vs 0.5% chlorhexidine) was used with alcohol.71 This finding may indicate the need for alcohol preparation, higher concentrations of chlorhexidine, or both.
Proper scrubbing and protective gear are needed to reduce surgeon risk factors. Hand washing is a routine part of any surgery. Alcohol-based hand scrubs are as effective as hand scrubbing.65 They reduce local skin flora by 95% immediately and by 99% with repeated applications.72 Lidwell and colleagues73 found a 75% reduction in infection when body exhaust suits were used in combination with laminar flow in a multicenter randomized controlled trial of 8052 patients. Sterile draping with impermeable drapes should be done over properly prepared skin. Ioban drapes (3M) are often used as a protective barrier. Interestingly, a Cochrane review found no benefit in using plastic adhesives impregnated with iodine over sterilely prepared skin.74
Operative Considerations
Surgical gloves become contaminated in almost one third of cases, half the time during draping.75 For this reason, many surgeons change gloves after draping. In addition, double gloving prevents a breech of aseptic technique should the outer glove become perforated.76 Demircay and colleagues77 assessed double latex gloving in arthroplasty and found the outer and inner gloves perforated in 18.4% and 8.4% of cases, respectively. Punctures are most common along the nondominant index finger, and then the dominant thumb.77,78 Perforation is more common when 2 latex gloves are worn—vs 1 latex glove plus an outer cloth glove—and the chance of perforation increases with surgery duration. The inner glove may become punctured in up to 100% of operations that last over 3 hours.79 Although Dodds and colleagues80 found no change in bacterial counts on surgeons’ hands or gloves after perforation, precautions are still recommended. Al-Maiyah and colleagues81 went as far as to recommend glove changes at 20-minute intervals and before cementation.
Surgical instruments can be sources of contamination. Some authors change the suction tip every hour to minimize the risk of deep wound infection.82-85 Others change it before femoral canal preparation and prosthesis insertion during THA.86 The splash basin is frequently contaminated, and instruments placed in it should not be returned to the operative field.87 Hargrove and colleagues88 suggested pulsatile lavage decreases PJI more than bulb syringe irrigation does, whereas others argued that high-pressure lavage allows bacteria to penetrate more deeply, which could lead to retention of more bacteria.89 Minimizing operating room time was found by Kurtz and colleagues90 and Peersman and colleagues91 to decrease PJI incidence. Carroll and colleagues71 correlated longer tourniquet use with a higher rate of infection after TKA; proposed mechanisms include local tissue hypoxia and lowered concentrations of prophylactic antibiotics.
Similarly, minimizing blood loss and transfusion needs is another strategy for preventing infection. Allogenic transfusion may increase the risk of PJI 2 times.23,71,92 The mechanism seems to be immune system modulation by allogenic blood, which impairs microcirculation and oxygen delivery at the surgical site.23,75 Transfusions should be approached with caution, and consideration given to preoperative optimization and autologous blood donation. Cherian and colleagues93 reviewed different blood management strategies and found preoperative iron therapy, intravenous erythropoietin, and autologous blood donation to be equally effective in reducing the need for allogenic transfusions. Numerous studies of tranexamic acid, thrombin-based hemostatic matrix (Floseal; Baxter Inc), and bipolar sealer with radiofrequency ablation (Aquamantys; Medtronic Inc) have found no alterations in infection rates, but most have used calculated blood loss, not PJI, as the primary endpoint.94-105 Antibiotic cement also can be used to block infection.63,106-110 Although liquid gentamicin may weaken bone cement,111 most antibiotics, including powdered tobramycin and vancomycin, do not weaken its fatigue strength.111-114 A recent meta-analysis by Parvizi and colleagues115 revealed that deep infection rates dropped from 2.3% to 1.2% with use of antibiotic cement for primary THAs. Cummins and colleagues,116 however, reported the limited cost-effectiveness of antibiotic cement in primary arthroplasty. Performing povidone-iodine lavage at the end of the case may be a more inexpensive alternative. Brown and colleagues117 found that rinsing with dilute povidone-iodine (.35%) for 3 minutes significantly decreased the incidence of PJI.
Closure techniques and sutures have been a focus of much of the recent literature. Winiarsky and colleagues34 advocated using a longer incision for obese patients and augmenting closure in fattier areas with vertical mattress retention sutures, which are removed after 5 days. A barbed monofilament suture (Quill; Angiotech Inc) is gaining in popularity. Laboratory research has shown that bacteria adhere less to barbed monofilament sutures than to braided sutures.118 Smith and colleagues119 found a statistically nonsignificant higher rate of wound complications with barbed monofilament sutures, whereas Ting and colleagues120 found no difference in complications. These studies were powered to detect differences in time and cost, not postoperative complications. Skin adhesive (Dermabond; Ethicon Inc), also used in closure, may be superior to staples in avoiding superficial skin abscesses.121 Although expensive, silver-impregnated dressing has antimicrobial activity that reduces PJI incidence by up to 74%.122 One brand of this dressing (Aquacel; ConvaTec Inc) has a polyurethane waterproof barrier that allows it to be worn for 7 days.
Three factors commonly mentioned in PJI prevention show little supporting evidence. Drains, which are often used, may create a passage for postoperative infection and are associated with increased transfusion needs.123,124 Adding antibiotics to irrigation solution125 and routinely changing scalpel blades126-129 also have little supporting evidence. In 2014, the utility of changing scalpel blades after incision was studied by Lee and colleagues,130 who reported persistence of Propionibacterium acnes in the dermal layer after skin preparation. Their study, however, was isolated to the upper back region, not the hip or knee.
Postoperative Risk Prevention
Most arthroplasty patients receive anticoagulation after surgery, but it must be used with caution. Large hematomas can predispose to wound complications. Parvizi and colleagues131 associated wound drainage, hematoma, and subsequent PJI with an INR above 1.5 in the early postoperative period. Therefore, balanced anticoagulation is crucial. Postoperative glucose control is also essential, particularly for patients with diabetes. Although preoperative blood glucose levels may or may not affect PJI risk,15,17,132 postoperative blood glucose levels of 126 mg/dL or higher are strongly associated with joint infections.133 Even nondiabetic patients with postoperative morning levels over 140 mg/dL are 3 times more likely to develop an infection.17
Efforts should be made to discharge patients as soon as it is safe to do so. With longer hospital stays, patients are more exposed to nosocomial organisms and increased antibiotic resistance.5,23,134 Outpatient antibiotics should be considered for dental, gastrointestinal, and genitourinary procedures. Oral antibiotic prophylaxis is controversial, as there is some evidence that dental procedures increase the risk of PJI only minimally.10,135-138
Conclusion
PJI is a potentially devastating complication of TJA. For this reason, much research has been devoted to proper diagnosis and treatment. Although the literature on PJI prophylaxis is abundant, there is relatively little consensus on appropriate PJI precautions. Preoperative considerations should include medical comorbidities, use of immunocompromising medications, obesity, nutritional factors, smoking, alcohol use, and MRSA colonization. Surgeons must have a consistent intraoperative method of antibiotic administration, skin preparation, scrubbing, draping, gloving, instrument exchange, blood loss management, cementing, and closure. In addition, monitoring of postoperative anticoagulation and blood glucose management is important. Having a thorough understanding of PJI risk factors may help reduce the incidence of this devastating complication.
Nearly 2% of patients who undergo total knee arthroplasty (TKA) or total hip arthroplasty (THA) develop a periprosthetic joint infection (PJI) within 20 years of surgery, and 41% of these infections occur within the first 2 years.1 PJI is the most common cause of TKA failure and the third leading complication of THA.2 The estimated total hospital cost of treating PJI increased from $320 million in 2001 to $566 million in 2009, which can be extrapolated to $1.62 billion in 2020.3 By 2030, the projected increase in demand for TKA and THA will be 673% and 174% of what it was in 2005, respectively.4 Treatment of PJI of the knee is estimated to cost 3 to 4 times more than a primary TKA, and the cost of revision THA for PJI is almost $6000 more than that of revision TKA for PJI.3
In this article, we review the numerous preoperative, intraoperative, and postoperative methods of decreasing PJI incidence after total joint arthroplasty (TJA).
Preoperative Risk Prevention
Medical Comorbidities
Preoperative medical optimization is a key element in PJI prevention (Table 1). An American Society of Anesthesiologists classification score of 3 or more has been associated with doubled risk for surgical site infections (SSIs) after THA.5 Autoimmune conditions confer a particularly higher risk. In a retrospective double-cohort study of 924 subjects, Bongartz and colleagues6 found that, compared with osteoarthritis, rheumatoid arthritis tripled the risk of PJI. Small case series originally suggested a higher risk of PJI in patients with psoriasis,7,8 but more recent studies have contradicted that finding.9,10 Nevertheless, psoriatic plaques have elevated bacterial counts,11 and planned incisions should circumvent these areas.
Diabetes mellitus is a clear risk factor for PJI.12-16 Regarding whether preoperative glucose control affects risk, findings have been mixed. Mraovic and colleagues17 showed preoperative hyperglycemia to be an independent risk factor; Jämsen and colleagues,15 in a single-center analysis of more than 7000 TJAs, suggested preoperative blood glucose levels were not independently associated with PJI; and Iorio and colleagues16 found no association between surgical infections and hemoglobin A1c levels.
TJA incidence is higher in patients with chronic kidney disease (CKD) than in the general population.18 Dialysis users have a post-THA PJI rate as high as 13% to 19%.19,20 Early clinical data suggested that outcomes are improved in dialysis users who undergo renal transplant, but this finding recently has been questioned.19,21 Deegan and colleagues22 found an increased PJA rate of 3.5% even in low-level CKD (stage 1, 2, or 3), but this may be confounded by the increased association of CKD with other PJI-predisposing comorbidities.
Given a higher incidence of urinary tract infections (UTIs) among patients with PJI, some surgeons think UTIs predispose to PJIs by hematogenous seeding.12,23,24 Symptomatic UTIs should be cleared before surgery and confirmed on urinalysis. Obstructive symptoms should prompt urologic evaluation. As asymptomatic pyuria and bacteriuria (colony counts, >1 × 105/mL) do not predispose to PJI, patients without symptoms do not require intervention.25,26 Past history of malignancy may also have a role in PJI. In a case-control study of the Mayo Clinic arthroplasty experience from 1969 to 1991, Berbari and colleagues1 found an association between malignancy and PJI (odds ratio, 2.4). They theorized the immunosuppressive effects of cancer treatment might be responsible for this increased risk.
Immunocompromising Medications
Immunocompromising medications are modifiable and should be adjusted before surgery. Stopping any disease-modifying antirheumatic drug (DMARD) more than 4 weeks before surgery is not recommended.27
Corticosteroid use can lead to immunosuppression and increased protein catabolism, which impairs soft-tissue healing. To avoid flares or adrenal insufficiency, however, chronic corticosteroid users should continue their regular doses perioperatively.28 On the day of surgery, they should also receive a stress dose of hydrocortisone 50 to 75 mg (for primary arthroplasty) or 100 to 150 mg (for revision arthroplasty), followed by expeditious tapering over 1 to 2 days.29 DMARDs are increasingly used by rheumatologists. One of the most effective DMARDs is methotrexate. Despite its immunocompromising activity, methotrexate should be continued perioperatively, as stopping for even 2 days may increase flare-related complications.30 Hydroxychloroquine can be continued perioperatively and has even been shown, by Johnson and Charnley,31 to prevent deep vein thromboses. Sulfasalazine can also be continued perioperatively—but with caution, as it may elevate international normalized ratio (INR) levels in patients receiving warfarin.29 Most other DMARDs should be temporarily discontinued. Leflunomide and interleukin 1 antagonists, such as anakinra, should be stopped 1 to 2 days before surgery and restarted 10 to 14 days after surgery.29 Rituximab should be stopped 1 week before surgery and restarted 10 to 14 days after surgery. Tumor necrosis factor α inhibitors should be discontinued for 2 half-lives before and after surgery.32 Etanercept has a half-life of 3 to 5 days; infliximab, 8 to 10 days; and adalimumab, 10 to 13 days. Most surgeons schedule surgery for the end of a dosing cycle and discontinue these biologic agents for another 10 to 14 days after surgery.
Metabolic Factors
Obese patients are susceptible to longer surgeries, more extensive dissection, poorly vascularized subcutaneous tissue, and higher requirements of weight-adjusted antibiotic dosing.13 Body mass index (BMI) of 40 kg/m2 or more (morbid obesity) and BMI over 50 kg/m2 have been associated with 9 times and 21.3 times increased risk of PJI, respectively.13,14 Delaying surgery with dietary consultation has been suggested,33,34 and bariatric surgery before TKA may decrease infection rates by 3.5 times.35
Nutritional markers are considered before arthroplasty. According to most laboratories, a serum transferrin level under 200 mg/dL, albumin level under 3.5 g/dL, and total lymphocyte count under 1500 cells/mm3 indicate malnourishment, which can increase the incidence of wound complications by 5 to 7 times.36 Patients should also have sufficient protein, vitamin, and mineral supplementation, particularly vitamins A and C, zinc, and copper.37Smokers who cease smoking at least 4 to 6 weeks before surgery lower their wound complication rate by up to 26%.38,39 When nicotine leaves the bloodstream, vasodilation occurs, oxygenation improves, and the immune system recovers.39 Studies have found more SSIs in patients who abuse alcohol,40 and numerous authors have confirmed this finding in the arthroplasty population.24,41,42 Alcohol inhibits platelet function and may predispose to a postoperative hematoma. In contrast to smoking cessation evidence, evidence regarding alcohol interventions in preventing postoperative infections is less conclusive.43,44
MRSA Colonization
Methicillin-resistant Staphylococcus aureus (MRSA) is a particularly difficult bacterium to eradicate in PJI. As the mean cost of treating a single case of MRSA-related prosthetic infection is $107,264 vs $68,053 for susceptible strains,45,46 many infection-containment strategies focus on addressing benign MRSA colonization before surgery.
MRSA is present in the nares of 25 million people in the United States. Nasal colonization increases the risk of bacteremia 4-fold47 and SSI 2- to 9-fold.48,49 Nasal swabs are analyzed with either a rapid polymerase chain reaction (PCR) test, which provides results in 2 hours, or a bacterial culture, which provides results in 1 to 4 days. The PCR test is more expensive.
Eradication of MRSA colonization is increasingly prevalent. Several Scandinavian countries have instituted strict practices by which patients are denied elective surgery until negative nasal swabs are obtained.49 Nasal decontamination is one method of colonization reduction. Topical mupirocin, which yields eradication in 91% of nasal carriers immediately after treatment and in 87% after 4 weeks,50 is effective in reducing SSI rates only when used in conjunction with a body wash, which is used to clean the axilla and groin.51 There is no consensus on optimal timing, but Bode and colleagues52 found a significant decrease in deep SSIs when decontamination occurred just 24 hours before surgery.
Povidone-iodine showers went out of favor with the realization that chlorhexidine gluconate acts longer on the skin surface.53,54 Preoperative showers involve rinsing with liquid chlorhexidine soap 24 to 48 hours before surgery. However, chlorhexidine binds preferentially to the cotton in washcloths instead of the skin. Edmiston and colleagues54,55 found that 4% chlorhexidine liquid soaps achieve much lower skin chlorhexidine concentrations than 2% polyester cloths do. Use of these “chlorhexidine wipes” the night before and the day of surgery has decreased PJI after TKA from 2.2% to 0.6%.56,57
Intraoperative Risk Prevention
Preparation
Which preoperative antibiotic to use is one of the first operative considerations in PJI prophylaxis (Table 2). Cefazolin is recommended as a first-line agent for its excellent soft-tissue penetration, long half-life, and activity against gram-positive bacteria such as skin flora.58 Clindamycin may be considered for patients allergic to β-lactam antibiotics. Vancomycin may be considered for adjunctive use with cephalosporins in cases of known MRSA colonization. Vancomycin infusion should be started earlier than infusion with other antibiotics, as vancomycin must be infused slowly and takes longer to become therapeutic.
Antibiotic dosing should be based on local antibiograms, adjusted dosing weight, or BMI.59 For revision arthroplasty, preoperative prophylaxis should not be stopped out of fear of affecting operative cultures.60 Some surgeons pause antibiotic use if a preoperative joint aspirate has not been obtained. Infusion within 1 hour of incision is part of the pay-for-performance guidelines established by the US Centers for Medicare & Medicaid Services.61 An antibiotic should be redosed if the operation will take longer than 2 half-lives of the drug.59 Surgeons should consider administering a dose every 4 hours or whenever blood loss exceeds 1000 mL.62 Engesæter and colleagues63 found that antibiotic prophylaxis was most effective given 4 times perioperatively (1 time before surgery, 3 times after surgery). Postoperative antibiotics should not be administered longer than 24 hours, as prolonged dosing confers no benefit.58 Operating room conditions must be optimized for prophylaxis. More people and operating room traffic in nonsterile corridors increase contamination of instruments open to air.64 Laminar airflow systems are commonly used. Although there is little dispute that laminar flow decreases the bacterial load of air, there are mixed results regarding its benefit in preventing PJI.65-68 Skin preparation may address patient risk factors. Hair clipping is preferred to shaving, which may cause microabrasions and increased susceptibility to skin flora.69 Patients should be prepared with antiseptic solution. One randomized controlled trial found that 2% chlorhexidine gluconate mixed with 70% isopropyl alcohol was superior to 10% povidone-iodine in preventing SSIs.70 However, a recent cohort study showed a lower rate of superficial wound infections when 1% povidone-iodine (vs 0.5% chlorhexidine) was used with alcohol.71 This finding may indicate the need for alcohol preparation, higher concentrations of chlorhexidine, or both.
Proper scrubbing and protective gear are needed to reduce surgeon risk factors. Hand washing is a routine part of any surgery. Alcohol-based hand scrubs are as effective as hand scrubbing.65 They reduce local skin flora by 95% immediately and by 99% with repeated applications.72 Lidwell and colleagues73 found a 75% reduction in infection when body exhaust suits were used in combination with laminar flow in a multicenter randomized controlled trial of 8052 patients. Sterile draping with impermeable drapes should be done over properly prepared skin. Ioban drapes (3M) are often used as a protective barrier. Interestingly, a Cochrane review found no benefit in using plastic adhesives impregnated with iodine over sterilely prepared skin.74
Operative Considerations
Surgical gloves become contaminated in almost one third of cases, half the time during draping.75 For this reason, many surgeons change gloves after draping. In addition, double gloving prevents a breech of aseptic technique should the outer glove become perforated.76 Demircay and colleagues77 assessed double latex gloving in arthroplasty and found the outer and inner gloves perforated in 18.4% and 8.4% of cases, respectively. Punctures are most common along the nondominant index finger, and then the dominant thumb.77,78 Perforation is more common when 2 latex gloves are worn—vs 1 latex glove plus an outer cloth glove—and the chance of perforation increases with surgery duration. The inner glove may become punctured in up to 100% of operations that last over 3 hours.79 Although Dodds and colleagues80 found no change in bacterial counts on surgeons’ hands or gloves after perforation, precautions are still recommended. Al-Maiyah and colleagues81 went as far as to recommend glove changes at 20-minute intervals and before cementation.
Surgical instruments can be sources of contamination. Some authors change the suction tip every hour to minimize the risk of deep wound infection.82-85 Others change it before femoral canal preparation and prosthesis insertion during THA.86 The splash basin is frequently contaminated, and instruments placed in it should not be returned to the operative field.87 Hargrove and colleagues88 suggested pulsatile lavage decreases PJI more than bulb syringe irrigation does, whereas others argued that high-pressure lavage allows bacteria to penetrate more deeply, which could lead to retention of more bacteria.89 Minimizing operating room time was found by Kurtz and colleagues90 and Peersman and colleagues91 to decrease PJI incidence. Carroll and colleagues71 correlated longer tourniquet use with a higher rate of infection after TKA; proposed mechanisms include local tissue hypoxia and lowered concentrations of prophylactic antibiotics.
Similarly, minimizing blood loss and transfusion needs is another strategy for preventing infection. Allogenic transfusion may increase the risk of PJI 2 times.23,71,92 The mechanism seems to be immune system modulation by allogenic blood, which impairs microcirculation and oxygen delivery at the surgical site.23,75 Transfusions should be approached with caution, and consideration given to preoperative optimization and autologous blood donation. Cherian and colleagues93 reviewed different blood management strategies and found preoperative iron therapy, intravenous erythropoietin, and autologous blood donation to be equally effective in reducing the need for allogenic transfusions. Numerous studies of tranexamic acid, thrombin-based hemostatic matrix (Floseal; Baxter Inc), and bipolar sealer with radiofrequency ablation (Aquamantys; Medtronic Inc) have found no alterations in infection rates, but most have used calculated blood loss, not PJI, as the primary endpoint.94-105 Antibiotic cement also can be used to block infection.63,106-110 Although liquid gentamicin may weaken bone cement,111 most antibiotics, including powdered tobramycin and vancomycin, do not weaken its fatigue strength.111-114 A recent meta-analysis by Parvizi and colleagues115 revealed that deep infection rates dropped from 2.3% to 1.2% with use of antibiotic cement for primary THAs. Cummins and colleagues,116 however, reported the limited cost-effectiveness of antibiotic cement in primary arthroplasty. Performing povidone-iodine lavage at the end of the case may be a more inexpensive alternative. Brown and colleagues117 found that rinsing with dilute povidone-iodine (.35%) for 3 minutes significantly decreased the incidence of PJI.
Closure techniques and sutures have been a focus of much of the recent literature. Winiarsky and colleagues34 advocated using a longer incision for obese patients and augmenting closure in fattier areas with vertical mattress retention sutures, which are removed after 5 days. A barbed monofilament suture (Quill; Angiotech Inc) is gaining in popularity. Laboratory research has shown that bacteria adhere less to barbed monofilament sutures than to braided sutures.118 Smith and colleagues119 found a statistically nonsignificant higher rate of wound complications with barbed monofilament sutures, whereas Ting and colleagues120 found no difference in complications. These studies were powered to detect differences in time and cost, not postoperative complications. Skin adhesive (Dermabond; Ethicon Inc), also used in closure, may be superior to staples in avoiding superficial skin abscesses.121 Although expensive, silver-impregnated dressing has antimicrobial activity that reduces PJI incidence by up to 74%.122 One brand of this dressing (Aquacel; ConvaTec Inc) has a polyurethane waterproof barrier that allows it to be worn for 7 days.
Three factors commonly mentioned in PJI prevention show little supporting evidence. Drains, which are often used, may create a passage for postoperative infection and are associated with increased transfusion needs.123,124 Adding antibiotics to irrigation solution125 and routinely changing scalpel blades126-129 also have little supporting evidence. In 2014, the utility of changing scalpel blades after incision was studied by Lee and colleagues,130 who reported persistence of Propionibacterium acnes in the dermal layer after skin preparation. Their study, however, was isolated to the upper back region, not the hip or knee.
Postoperative Risk Prevention
Most arthroplasty patients receive anticoagulation after surgery, but it must be used with caution. Large hematomas can predispose to wound complications. Parvizi and colleagues131 associated wound drainage, hematoma, and subsequent PJI with an INR above 1.5 in the early postoperative period. Therefore, balanced anticoagulation is crucial. Postoperative glucose control is also essential, particularly for patients with diabetes. Although preoperative blood glucose levels may or may not affect PJI risk,15,17,132 postoperative blood glucose levels of 126 mg/dL or higher are strongly associated with joint infections.133 Even nondiabetic patients with postoperative morning levels over 140 mg/dL are 3 times more likely to develop an infection.17
Efforts should be made to discharge patients as soon as it is safe to do so. With longer hospital stays, patients are more exposed to nosocomial organisms and increased antibiotic resistance.5,23,134 Outpatient antibiotics should be considered for dental, gastrointestinal, and genitourinary procedures. Oral antibiotic prophylaxis is controversial, as there is some evidence that dental procedures increase the risk of PJI only minimally.10,135-138
Conclusion
PJI is a potentially devastating complication of TJA. For this reason, much research has been devoted to proper diagnosis and treatment. Although the literature on PJI prophylaxis is abundant, there is relatively little consensus on appropriate PJI precautions. Preoperative considerations should include medical comorbidities, use of immunocompromising medications, obesity, nutritional factors, smoking, alcohol use, and MRSA colonization. Surgeons must have a consistent intraoperative method of antibiotic administration, skin preparation, scrubbing, draping, gloving, instrument exchange, blood loss management, cementing, and closure. In addition, monitoring of postoperative anticoagulation and blood glucose management is important. Having a thorough understanding of PJI risk factors may help reduce the incidence of this devastating complication.
1. Berbari EF, Hanssen AD, Duffy MC, et al. Risk factors for prosthetic joint infection: case–control study. Clin Infect Dis. 1998;27(5):1247-1254.
2. Adeli B, Parvizi J. Strategies for the prevention of periprosthetic joint infection. J Bone Joint Surg Br. 2012;94(11 suppl A):42-46.
3. Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8 suppl):61-65.e1.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Ridgeway S. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005;87(6):844-850.
6. Bongartz T, Halligan CS, Osmon DR, et al. Incidence and risk factors of prosthetic joint infection after total hip or knee replacement in patients with rheumatoid arthritis. Arthritis Rheum. 2008;59(12):1713-1720.
7. Menon TJ, Wroblewski BM. Charnley low-friction arthroplasty in patients with psoriasis. Clin Orthop Relat Res. 1983;(176):127-128.
8. Stern SH, Insall JN, Windsor RE, Inglis AE, Dines DM. Total knee arthroplasty in patients with psoriasis. Clin Orthop Relat Res. 1989;(248):108-100.
9. Beyer CA, Hanssen AD, Lewallen DG, Pittelkow MR. Primary total knee arthroplasty in patients with psoriasis. J Bone Joint Surg Br. 1991;73(2):258-259.
10. Berbari EF, Osmon DR, Carr A, et al. Dental procedures as risk factors for prosthetic hip or knee infection: a hospital-based prospective case–control study. Clin Infect Dis. 2010;50(1):8-16.
11. Singh G, Rao DJ. Bacteriology of psoriatic plaques. Dermatologica. 1978;157(1):21-27.
12. Bozic KJ, Ong K, Lau E, et al. Estimating risk in Medicare patients with THA: an electronic risk calculator for periprosthetic joint infection and mortality. Clin Orthop Relat Res. 2013;471(2):574-583.
13. Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009;24(6 suppl):84-88.
14. Dowsey MM, Choong PFM. Obese diabetic patients are at substantial risk for deep infection after primary TKA. Clin Orthop Relat Res. 2009;467(6):1577-1581.
15. Jämsen E, Nevalainen P, Eskelinen A, Huotari K, Kalliovalkama J, Moilanen T. Obesity, diabetes, and preoperative hyperglycemia as predictors of periprosthetic joint infection: a single-center analysis of 7181 primary hip and knee replacements for osteoarthritis. J Bone Joint Surg Am. 2012;94(14):e101.
16. Iorio R, Williams KM, Marcantonio AJ, Specht LM, Tilzey JF, Healy WL. Diabetes mellitus, hemoglobin A1C, and the incidence of total joint arthroplasty infection. J Arthroplasty. 2012;27(5):726-729.e1.
17. Mraovic B, Suh D, Jacovides C. Perioperative hyperglycemia and postoperative infection after lower limb arthroplasty. J Diabetes Sci Technol. 2011;5(2):412-418.
18. Abbott KC, Bucci JR, Agodoa LY. Total hip arthroplasty in chronic dialysis patients in the United States. J Nephrol. 2003;16(1):34-39.
19. Lieberman JR, Fuchs MD, Haas SB, et al. Hip arthroplasty in patients with chronic renal failure. J Arthroplasty. 1995;10(2):191-195.
20. Sakalkale DP, Hozack WJ, Rothman RH. Total hip arthroplasty in patients on long-term renal dialysis. J Arthroplasty. 1999;14(5):571-575.
21. Shrader MW, Schall D, Parvizi J, McCarthy JT, Lewallen DG. Total hip arthroplasty in patients with renal failure: a comparison between transplant and dialysis patients. J Arthroplasty. 2006;21(3):324-329.
22. Deegan BF, Richard RD, Bowen TR, Perkins RM, Graham JH, Foltzer MA. Impact of chronic kidney disease stage on lower-extremity arthroplasty. Orthopedics. 2014;37(7):e613-e618.
23. Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466(7):1710-1715.
24. Tomás T. Patient-related risk factors for infected total arthroplasty. Acta Chir Orthop. 2008;75(6):451-456.
25. Ritter MA, Fechtman RW. Urinary tract sequelae: possible influence on joint infections following total joint replacement. Orthopedics. 1987;10(3):467-469.
26. Gou W, Chen J, Jia Y, Wang Y. Preoperative asymptomatic leucocyturia and early prosthetic joint infections in patients undergoing joint arthroplasty. J Arthroplasty. 2014;29(3):473-476.
27. Goodman SM, Paget S. Perioperative drug safety in patients with rheumatoid arthritis. Rheum Dis Clin North Am. 2012;38(4):747-759.
28. Salem M, Tainsh RE Jr, Bromberg J, Loriaux DL, Chernow B. Perioperative glucocorticoid coverage. A reassessment 42 years after emergence of a problem. Ann Surg. 1994;219(4):416-425.
29. Howe CR, Gardner GC, Kadel NJ. Perioperative medication management for the patient with rheumatoid arthritis. J Am Acad Orthop Surg. 2006;14(9):544-551.
30. Grennan DM. Methotrexate and early postoperative complications in patients with rheumatoid arthritis undergoing elective orthopaedic surgery. Ann Rheum Dis. 2001;60(3):214-217.
31. Johnson R, Charnley J. Hydroxychloroquine in prophylaxis of pulmonary embolism following hip arthroplasty. Clin Orthop Relat Res. 1979;(144):174-177.
32. Mushtaq S, Goodman SM, Scanzello CR. Perioperative management of biologic agents used in treatment of rheumatoid arthritis. Am J Ther. 2011;18(5):426-434.
33. Namba RS, Paxton L, Fithian DC, Stone ML. Obesity and perioperative morbidity in total hip and total knee arthroplasty patients. J Arthroplasty. 2005;20(7 suppl 3):46-50.
34. Winiarsky R, Barth P, Lotke PA. Total knee arthroplasty in morbidly obese patients. J Bone Joint Surg Am. 1998;80(12):1770-1774.
35. Kulkarni A, Jameson SS, James P, Woodcock S, Muller S, Reed MR. Does bariatric surgery prior to lower limb joint replacement reduce complications? Surgeon. 2011;9(1):18-21.
36. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients. J Arthroplasty. 1991;6(4):321-325.
37. Fairfield KM, Fletcher RH. Vitamins for chronic disease prevention in adults. JAMA. 2002;287(23):3116.
38. Kwiatkowski TC, Hanley EN Jr, Ramp WK. Cigarette smoking and its orthopedic consequences. Am J Orthop. 1996;25(9):590-597.
39. Møller AM, Villebro N, Pedersen T, Tønnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet. 2002;359(9301):114-117.
40. Rantala A, Lehtonen OP, Niinikoski J. Alcohol abuse: a risk factor for surgical wound infections? Am J Infect Control. 1997;25(5):381-386.
41. Wu C, Qu X, Liu F, Li H, Mao Y, Zhu Z. Risk factors for periprosthetic joint infection after total hip arthroplasty and total knee arthroplasty in Chinese patients. PLoS One. 2014;9(4):e95300.
42. Cordero-Ampuero J, de Dios M. What are the risk factors for infection in hemiarthroplasties and total hip arthroplasties? Clin Orthop Relat Res. 2010;468(12):3268-3277.
43. Tønnesen H, Rosenberg J, Nielsen HJ, et al. Effect of preoperative abstinence on poor postoperative outcome in alcohol misusers: randomised controlled trial. BMJ. 1999;318(7194):1311-1316.
44. Shourie S, Conigrave KM, Proude EM, Ward JE, Wutzke SE, Haber PS. The effectiveness of a tailored intervention for excessive alcohol consumption prior to elective surgery. Alcohol Alcohol. 2006;41(6):643-649.
45. Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128-133.
46. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
47. Safdar N, Bradley EA. The risk of infection after nasal colonization with Staphylococcus aureus. Am J Med. 2008;121(4):310-315.
48. American Academy of Orthopaedic Surgeons Patient Safety Committee, Evans RP. Surgical site infection prevention and control: an emerging paradigm. J Bone Joint Surg Am. 2009;91(suppl 6):2-9.
49. Goyal N, Aggarwal V, Parvizi J. Methicillin-resistant Staphylococcus aureus screening in total joint arthroplasty: a worthwhile endeavor. J Knee Surg. 2012;25(1):37-43.
50. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10(3):505-520.
51. Wilcox MH, Hall J, Pike H, et al. Use of perioperative mupirocin to prevent methicillin-resistant Staphylococcus aureus (MRSA) orthopaedic surgical site infections. J Hosp Infect. 2003;54(3):196-201.
52. Bode LG, Kluytmans JA, Wertheim HF, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010;362(1):9-17.
53. Association of Operating Room Nurses. Recommended practices for skin preparation of patients. AORN J. 2002;75(1):184-187.
54. Edmiston CE Jr, Seabrook GR, Johnson CP, Paulson DS, Beausoleil CM. Comparative of a new and innovative 2% chlorhexidine gluconate–impregnated cloth with 4% chlorhexidine gluconate as topical antiseptic for preparation of the skin prior to surgery. Am J Infect Control. 2007;35(2):89-96.
55. Edmiston CE Jr, Krepel CJ, Seabrook GR, Lewis BD, Brown KR, Towne JB. Preoperative shower revisited: can high topical antiseptic levels be achieved on the skin surface before surgical admission? J Am Coll Surg. 2008;207(2):233-239.
56. Johnson AJ, Kapadia BH, Daley JA, Molina CB, Mont MA. Chlorhexidine reduces infections in knee arthroplasty. J Knee Surg. 2013;26(3):213-218.
57. Johnson AJ, Daley JA, Zywiel MG, Delanois RE, Mont MA. Preoperative chlorhexidine preparation and the incidence of surgical site infections after hip arthroplasty. J Arthroplasty. 2010;25(6 suppl):98-102.
58. Mauerhan DR, Nelson CL, Smith DL, et al. Prophylaxis against infection in total joint arthroplasty. One day of cefuroxime compared with three days of cefazolin. J Bone Joint Surg Am. 1994;76(1):39-45.
59. Bratzler DW, Houck PM; Surgical Infection Prevention Guideline Writers Workgroup. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Am J Surg. 2005;189(4):395-404.
60. Tetreault MW, Wetters NG, Aggarwal V, Mont M, Parvizi J, Della Valle CJ. The Chitranjan Ranawat Award: should prophylactic antibiotics be withheld before revision surgery to obtain appropriate cultures? Clin Orthop Relat Res. 2014;472(1):52-56.
61. Illingworth KD, Mihalko WM, Parvizi J, et al. How to minimize infection and thereby maximize patient outcomes in total joint arthroplasty: a multicenter approach: AAOS exhibit selection. J Bone Joint Surg Am. 2013;95(8):e50.
62. Bannister GC, Auchincloss JM, Johnson DP, Newman JH. The timing of tourniquet application in relation to prophylactic antibiotic administration. J Bone Joint Surg Br. 1988;70(2):322-324.
63. Engesæter LB, Lie SA, Espehaug B, Furnes O, Vollset SE, Havelin LI. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand. 2003;74(6):644-651.
64. Ritter MA. Operating room environment. Clin Orthop Relat Res. 1999;(369):103-109.
65. Brandt C, Hott U, Sohr D, Daschner F, Gastmeier P, Rüden H. Operating room ventilation with laminar airflow shows no protective effect on the surgical site infection rate in orthopedic and abdominal surgery. Ann Surg. 2008;248(5):695-700.
66. Dharan S, Pittet D. Environmental controls in operating theatres. J Hosp Infect. 2002;51(2):79-84.
67. Hamilton HW, Booth AD, Lone FJ, Clark N. Penetration of gown material by organisms from the surgical team. Clin Orthop Relat Res. 1979;(141):237-246.
68. Da Costa AR, Kothari A, Bannister GC, Blom AW. Investigating bacterial growth in surgical theatres: establishing the effect of laminar airflow on bacterial growth on plastic, metal and wood surfaces. Ann R Coll Surg Engl. 2008;90(5):417-419.
69. Tanner J, Woodings D, Moncaster K. Preoperative hair removal to reduce surgical site infection. Cochrane Database Syst Rev. 2006;(2):CD004122.
70. Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18-26.
71. Carroll K, Dowsey M, Choong P, Peel T. Risk factors for superficial wound complications in hip and knee arthroplasty. Clin Microbiol Infect. 2013;20(2):130-135.
72. Ayliffe GA. Surgical scrub and skin disinfection. Infect Control. 1984;5(1):23-27.
73. Lidwell OM, Lowbury EJ, Whyte W, Blowers R, Lowe D. Extended follow-up of patients suspected of having joint sepsis after total joint replacement. J Hyg (Lond). 1985;95(3):655-664.
74. Webster J, Alghamdi AA. Use of plastic adhesive drapes during surgery for preventing surgical site infection. Cochrane Database Syst Rev. 2007;(4):CD006353.
75. Alijanipour P, Heller S, Parvizi J. Prevention of periprosthetic joint infection: what are the effective strategies? J Knee Surg. 2014;27(4):251-258.
76. Tanner J, Parkinson H. Double gloving to reduce surgical cross-infection. Cochrane Database Syst Rev. 2002;(3):CD003087.
77. Demircay E, Unay K, Bilgili MG, Alataca G. Glove perforation in hip and knee arthroplasty. J Orthop Sci. 2010;15(6):790-794.
78. Ersozlu S, Sahin O, Ozgur AF, Akkaya T, Tuncay C. Glove punctures in major and minor orthopaedic surgery with double gloving. Acta Orthop Belg. 2007;73(6):760-764.
79. Sanders R, Fortin P, Ross E, Helfet D. Outer gloves in orthopaedic procedures. Cloth compared with latex. J Bone Joint Surg Am. 1990;72(6):914-917.
80. Dodds RD, Guy PJ, Peacock AM, Duffy SR, Barker SG, Thomas MH. Surgical glove perforation. Br J Surg. 1988;75(10):966-968.
81. Al-Maiyah M, Bajwa A, Mackenney P, et al. Glove perforation and contamination in primary total hip arthroplasty. J Bone Joint Surg Br. 2005;87(4):556-559.
82. Insull PJ, Hudson J. Suction tip: a potential source of infection in clean orthopaedic procedures. ANZ J Surg. 2012;82(3):185-186.
83. Givissis P, Karataglis D, Antonarakos P, Symeonidis PD, Christodoulou A. Suction during orthopaedic surgery. How safe is the suction tip? Acta Orthop Belg. 2008;74(4):531-533.
84. Meals RA, Knoke L. The surgical suction top—a contaminated instrument. J Bone Joint Surg Am. 1978;60(3):409-410.
85. Strange-Vognsen MH, Klareskov B. Bacteriologic contamination of suction tips during hip arthroplasty. Acta Orthop Scand. 1988;59(4):410-411.
86. Greenough CG. An investigation into contamination of operative suction. J Bone Joint Surg Br. 1986;68(1):151-153.
87. Baird RA, Nickel FR, Thrupp LD, Rucker S, Hawkins B. Splash basin contamination in orthopaedic surgery. Clin Orthop Relat Res. 1984;(187):129-133.
88. Hargrove R, Ridgeway S, Russell R, Norris M, Packham I, Levy B. Does pulse lavage reduce hip hemiarthroplasty infection rates? J Hosp Infect. 2006;62(4):446-449.
89. Hassinger SM, Harding G, Wongworawat MD. High-pressure pulsatile lavage propagates bacteria into soft tissue. Clin Orthop Relat Res. 2005;(439):27-31.
90. Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res. 2010;468(1):52-56.
91. Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement. Clin Orthop Relat Res. 2001;(392):15-23.
92. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
93. Cherian JJ, Kapadia BH, Issa K, et al. Preoperative blood management strategies for total hip arthroplasty. Surg Technol Int. 2013;23:261-266.
94. Issa K, Banerjee S, Rifai A, et al. Blood management strategies in primary and revision total knee arthroplasty for Jehovah’s Witness patients. J Knee Surg. 2013;26(6):401-404.
95. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2010;93(1):39-46.
96. Berger V, Alperson S. A general framework for the evaluation of clinical trial quality. Rev Recent Clin Trials. 2009;4(2):79-88.
97. Chimento GF, Huff T, Ochsner JL, Meyer M, Brandner L, Babin S. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 suppl):74-77.
98. Karam JA, Bloomfield MR, DiIorio TM, Irizarry AM, Sharkey PF. Evaluation of the efficacy and safety of tranexamic acid for reducing blood loss in bilateral total knee arthroplasty. J Arthroplasty. 2014;29(3):501-503.
99. Kim HJ, Fraser MR, Kahn B, Lyman S, Figgie MP. The efficacy of a thrombin-based hemostatic agent in unilateral total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(13):1160-1165.
100. Suarez JC, Slotkin EM, Alvarez AM, Szubski CR, Barsoum WK, Patel PD. Prospective, randomized trial to evaluate efficacy of a thrombin-based hemostatic agent in total knee arthroplasty. J Arthroplasty. 2014;29(10):1950-1955.
101. Romanò CL, Monti L, Logoluso N, Romanò D, Drago L. Does a thrombin-based topical haemostatic agent reduce blood loss and transfusion requirements after total knee revision surgery? A randomized, controlled trial. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3337-3342.
102. Falez F, Meo A, Panegrossi G, Favetti F, Cava F, Casella F. Blood loss reduction in cementless total hip replacement with fibrin spray or bipolar sealer: a randomised controlled trial on ninety five patients. Int Orthop. 2013;37(7):1213-1217.
103. Morris MJ, Barrett M, Lombardi AV, Tucker TL, Berend KR. Randomized blinded study comparing a bipolar sealer and standard electrocautery in reducing transfusion requirements in anterior supine intermuscular total hip arthroplasty. J Arthroplasty. 2013;28(9):1614-1617.
104. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518.
105. Zeh A, Messer J, Davis J, Vasarhelyi A, Wohlrab D. The Aquamantys system—an alternative to reduce blood loss in primary total hip arthroplasty? J Arthroplasty. 2010;25(7):1072-1077.
106. Heck D, Rosenberg A, Schink-Ascani M, Garbus S, Kiewitt T. Use of antibiotic-impregnated cement during hip and knee arthroplasty in the United States. J Arthroplasty. 1995;10(4):470-475.
107. Srivastav A, Nadkarni B, Srivastav S, Mittal V, Agarwal S. Prophylactic use of antibiotic-loaded bone cement in primary total knee arthroplasty: justified or not? Indian J Orthop. 2009;43(3):259-263.
108. Dunbar MJ. Antibiotic bone cements: their use in routine primary total joint arthroplasty is justified. Orthopedics. 2009;32(9).
109. Merollini KM, Zheng H, Graves N. Most relevant strategies for preventing surgical site infection after total hip arthroplasty: guideline recommendations and expert opinion. Am J Infect Control. 2013;41(3):221-226.
110. Jämsen E, Huhtala H, Puolakka T, Moilanen T. Risk factors for infection after knee arthroplasty. A register-based analysis of 43,149 cases. J Bone Joint Surg Am. 2009;91(1):38-47.
111. Seldes RM, Winiarsky R, Jordan LC, et al. Liquid gentamicin in bone cement: a laboratory study of a potentially more cost-effective cement spacer. J Bone Joint Surg Am. 2005;87(2):268-272.
112. Wright TM, Sullivan DJ, Arnoczky SP. The effect of antibiotic additions on the fracture properties of bone cements. Acta Orthop Scand. 1984;55(4):414-418.
113. Baleani M, Persson C, Zolezzi C, Andollina A, Borrelli AM, Tigani D. Biological and biomechanical effects of vancomycin and meropenem in acrylic bone cement. J Arthroplasty. 2008;23(8):1232-1238.
114. Baleani M, Cristofolini L, Minari C, Toni A. Fatigue strength of PMMA bone cement mixed with gentamicin and barium sulphate vs pure PMMA. Proc Inst Mech Eng H. 2005;217(1):9-12.
115. Parvizi J, Saleh KJ, Ragland PS, Pour AE, Mont MA. Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthop Scand. 2008;79(3):335-341.
116. Cummins JS, Tomek IM, Kantor SR, Furnes O, Engesæter LB, Finlayson SRG. Cost-effectiveness of antibiotic-impregnated bone cement used in primary total hip arthroplasty. J Bone Joint Surg Am. 2009;91(3):634-641.
117. Brown NM, Cipriano CA, Moric M, Sporer SM, Della Valle CJ. Dilute Betadine lavage before closure for the prevention of acute postoperative deep periprosthetic joint infection. J Arthroplasty. 2012;27(1):27-30.
118. Fowler JR, Perkins TA, Buttaro BA, Truant AL. Bacteria adhere less to barbed monofilament than braided sutures in a contaminated wound model. Clin Orthop Relat Res. 2013;471(2):665-671.
119. Smith EL, DiSegna ST, Shukla PY, Matzkin EG. Barbed versus traditional sutures: closure time, cost, and wound related outcomes in total joint arthroplasty. J Arthroplasty. 2014;29(2):283-287.
120. Ting NT, Moric MM, Della Valle CJ, Levine BR. Use of knotless suture for closure of total hip and knee arthroplasties: a prospective, randomized clinical trial. J Arthroplasty. 2012;27(10):1783-1788.
121. Miller AG, Swank ML. Dermabond efficacy in total joint arthroplasty wounds. Am J Orthop. 2010;39(10):476-478.
122. Cai J, Karam JA, Parvizi J, Smith EB, Sharkey PF. Aquacel surgical dressing reduces the rate of acute PJI following total joint arthroplasty: a case–control study. J Arthroplasty. 2014;29(6):1098-1100.
123. Drinkwater CJ, Neil MJ. Optimal timing of wound drain removal following total joint arthroplasty. J Arthroplasty. 1995;10(2):185-189.
124. Parker MJ, Roberts CP, Hay D. Closed suction drainage for hip and knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2004;86(6):1146-1152.
125. Matar WY, Jafari SM, Restrepo C, Austin M, Purtill JJ, Parvizi J. Preventing infection in total joint arthroplasty. J Bone Joint Surg Am. 2010;92(suppl 2):36-46.
126. Ritter MA, French ML, Eitzen HE. Bacterial contamination of the surgical knife. Clin Orthop Relat Res. 1975;(108):158-160.
127. Fairclough JA, Mackie IG, Mintowt-Czyz W, Phillips GE. The contaminated skin-knife. A surgical myth. J Bone Joint Surg Br. 1983;65(2):210.
128. Ramón R, García S, Combalía A, Puig de la Bellacasa J, Segur JM. Bacteriological study of surgical knives: is the use of two blades necessary? Arch Orthop Trauma Surg. 1994;113(3):157-158.
129. Hasselgren PO, Hagberg E, Malmer H, Säljö A, Seeman T. One instead of two knives for surgical incision. Does it increase the risk of postoperative wound infection? Arch Surg. 1984;119(8):917-920.
130. Lee MJ, Pottinger PS, Butler-Wu S, Bumgarner RE, Russ SM, Matsen FA 3rd. Propionibacterium persists in the skin despite standard surgical preparation. J Bone Joint Surg Am. 2014;96(17):1447-1450.
131. Parvizi J, Ghanem E, Joshi A, Sharkey PF, Hozack WJ, Rothman RH. Does “excessive” anticoagulation predispose to periprosthetic infection? J Arthroplasty. 2007;22(6 suppl 2):24-28.
132. Marchant MH, Viens NA, Cook C, Vail TP, Bolognesi MP. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. J Bone Joint Surg Am. 2009;91(7):1621-1629.
133. Reátegui D, Sanchez-Etayo G, Núñez E, et al. Perioperative hyperglycaemia and incidence of post-operative complications in patients undergoing total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2026-2031.
134. Urquhart DM, Hanna FS, Brennan SL, et al. Incidence and risk factors for deep surgical site infection after primary total hip arthroplasty: a systematic review. J Arthroplasty. 2010;25(8):1216-1222.e1-e3.
135. Friedlander AH. Oral cavity staphylococci are a potential source of prosthetic joint infection. Clin Infect Dis. 2010;50(12):1682-1683.
136. Zimmerli W, Sendi P. Antibiotics for prevention of periprosthetic joint infection following dentistry: time to focus on data. Clin Infect Dis. 2010;50(1):17-19.
137. Young H, Hirsh J, Hammerberg EM, Price CS. Dental disease and periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(2):162-168.
138. Simmons NA, Ball AP, Cawson RA, et al. Case against antibiotic prophylaxis for dental treatment of
1. Berbari EF, Hanssen AD, Duffy MC, et al. Risk factors for prosthetic joint infection: case–control study. Clin Infect Dis. 1998;27(5):1247-1254.
2. Adeli B, Parvizi J. Strategies for the prevention of periprosthetic joint infection. J Bone Joint Surg Br. 2012;94(11 suppl A):42-46.
3. Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8 suppl):61-65.e1.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Ridgeway S. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005;87(6):844-850.
6. Bongartz T, Halligan CS, Osmon DR, et al. Incidence and risk factors of prosthetic joint infection after total hip or knee replacement in patients with rheumatoid arthritis. Arthritis Rheum. 2008;59(12):1713-1720.
7. Menon TJ, Wroblewski BM. Charnley low-friction arthroplasty in patients with psoriasis. Clin Orthop Relat Res. 1983;(176):127-128.
8. Stern SH, Insall JN, Windsor RE, Inglis AE, Dines DM. Total knee arthroplasty in patients with psoriasis. Clin Orthop Relat Res. 1989;(248):108-100.
9. Beyer CA, Hanssen AD, Lewallen DG, Pittelkow MR. Primary total knee arthroplasty in patients with psoriasis. J Bone Joint Surg Br. 1991;73(2):258-259.
10. Berbari EF, Osmon DR, Carr A, et al. Dental procedures as risk factors for prosthetic hip or knee infection: a hospital-based prospective case–control study. Clin Infect Dis. 2010;50(1):8-16.
11. Singh G, Rao DJ. Bacteriology of psoriatic plaques. Dermatologica. 1978;157(1):21-27.
12. Bozic KJ, Ong K, Lau E, et al. Estimating risk in Medicare patients with THA: an electronic risk calculator for periprosthetic joint infection and mortality. Clin Orthop Relat Res. 2013;471(2):574-583.
13. Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009;24(6 suppl):84-88.
14. Dowsey MM, Choong PFM. Obese diabetic patients are at substantial risk for deep infection after primary TKA. Clin Orthop Relat Res. 2009;467(6):1577-1581.
15. Jämsen E, Nevalainen P, Eskelinen A, Huotari K, Kalliovalkama J, Moilanen T. Obesity, diabetes, and preoperative hyperglycemia as predictors of periprosthetic joint infection: a single-center analysis of 7181 primary hip and knee replacements for osteoarthritis. J Bone Joint Surg Am. 2012;94(14):e101.
16. Iorio R, Williams KM, Marcantonio AJ, Specht LM, Tilzey JF, Healy WL. Diabetes mellitus, hemoglobin A1C, and the incidence of total joint arthroplasty infection. J Arthroplasty. 2012;27(5):726-729.e1.
17. Mraovic B, Suh D, Jacovides C. Perioperative hyperglycemia and postoperative infection after lower limb arthroplasty. J Diabetes Sci Technol. 2011;5(2):412-418.
18. Abbott KC, Bucci JR, Agodoa LY. Total hip arthroplasty in chronic dialysis patients in the United States. J Nephrol. 2003;16(1):34-39.
19. Lieberman JR, Fuchs MD, Haas SB, et al. Hip arthroplasty in patients with chronic renal failure. J Arthroplasty. 1995;10(2):191-195.
20. Sakalkale DP, Hozack WJ, Rothman RH. Total hip arthroplasty in patients on long-term renal dialysis. J Arthroplasty. 1999;14(5):571-575.
21. Shrader MW, Schall D, Parvizi J, McCarthy JT, Lewallen DG. Total hip arthroplasty in patients with renal failure: a comparison between transplant and dialysis patients. J Arthroplasty. 2006;21(3):324-329.
22. Deegan BF, Richard RD, Bowen TR, Perkins RM, Graham JH, Foltzer MA. Impact of chronic kidney disease stage on lower-extremity arthroplasty. Orthopedics. 2014;37(7):e613-e618.
23. Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466(7):1710-1715.
24. Tomás T. Patient-related risk factors for infected total arthroplasty. Acta Chir Orthop. 2008;75(6):451-456.
25. Ritter MA, Fechtman RW. Urinary tract sequelae: possible influence on joint infections following total joint replacement. Orthopedics. 1987;10(3):467-469.
26. Gou W, Chen J, Jia Y, Wang Y. Preoperative asymptomatic leucocyturia and early prosthetic joint infections in patients undergoing joint arthroplasty. J Arthroplasty. 2014;29(3):473-476.
27. Goodman SM, Paget S. Perioperative drug safety in patients with rheumatoid arthritis. Rheum Dis Clin North Am. 2012;38(4):747-759.
28. Salem M, Tainsh RE Jr, Bromberg J, Loriaux DL, Chernow B. Perioperative glucocorticoid coverage. A reassessment 42 years after emergence of a problem. Ann Surg. 1994;219(4):416-425.
29. Howe CR, Gardner GC, Kadel NJ. Perioperative medication management for the patient with rheumatoid arthritis. J Am Acad Orthop Surg. 2006;14(9):544-551.
30. Grennan DM. Methotrexate and early postoperative complications in patients with rheumatoid arthritis undergoing elective orthopaedic surgery. Ann Rheum Dis. 2001;60(3):214-217.
31. Johnson R, Charnley J. Hydroxychloroquine in prophylaxis of pulmonary embolism following hip arthroplasty. Clin Orthop Relat Res. 1979;(144):174-177.
32. Mushtaq S, Goodman SM, Scanzello CR. Perioperative management of biologic agents used in treatment of rheumatoid arthritis. Am J Ther. 2011;18(5):426-434.
33. Namba RS, Paxton L, Fithian DC, Stone ML. Obesity and perioperative morbidity in total hip and total knee arthroplasty patients. J Arthroplasty. 2005;20(7 suppl 3):46-50.
34. Winiarsky R, Barth P, Lotke PA. Total knee arthroplasty in morbidly obese patients. J Bone Joint Surg Am. 1998;80(12):1770-1774.
35. Kulkarni A, Jameson SS, James P, Woodcock S, Muller S, Reed MR. Does bariatric surgery prior to lower limb joint replacement reduce complications? Surgeon. 2011;9(1):18-21.
36. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients. J Arthroplasty. 1991;6(4):321-325.
37. Fairfield KM, Fletcher RH. Vitamins for chronic disease prevention in adults. JAMA. 2002;287(23):3116.
38. Kwiatkowski TC, Hanley EN Jr, Ramp WK. Cigarette smoking and its orthopedic consequences. Am J Orthop. 1996;25(9):590-597.
39. Møller AM, Villebro N, Pedersen T, Tønnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet. 2002;359(9301):114-117.
40. Rantala A, Lehtonen OP, Niinikoski J. Alcohol abuse: a risk factor for surgical wound infections? Am J Infect Control. 1997;25(5):381-386.
41. Wu C, Qu X, Liu F, Li H, Mao Y, Zhu Z. Risk factors for periprosthetic joint infection after total hip arthroplasty and total knee arthroplasty in Chinese patients. PLoS One. 2014;9(4):e95300.
42. Cordero-Ampuero J, de Dios M. What are the risk factors for infection in hemiarthroplasties and total hip arthroplasties? Clin Orthop Relat Res. 2010;468(12):3268-3277.
43. Tønnesen H, Rosenberg J, Nielsen HJ, et al. Effect of preoperative abstinence on poor postoperative outcome in alcohol misusers: randomised controlled trial. BMJ. 1999;318(7194):1311-1316.
44. Shourie S, Conigrave KM, Proude EM, Ward JE, Wutzke SE, Haber PS. The effectiveness of a tailored intervention for excessive alcohol consumption prior to elective surgery. Alcohol Alcohol. 2006;41(6):643-649.
45. Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128-133.
46. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
47. Safdar N, Bradley EA. The risk of infection after nasal colonization with Staphylococcus aureus. Am J Med. 2008;121(4):310-315.
48. American Academy of Orthopaedic Surgeons Patient Safety Committee, Evans RP. Surgical site infection prevention and control: an emerging paradigm. J Bone Joint Surg Am. 2009;91(suppl 6):2-9.
49. Goyal N, Aggarwal V, Parvizi J. Methicillin-resistant Staphylococcus aureus screening in total joint arthroplasty: a worthwhile endeavor. J Knee Surg. 2012;25(1):37-43.
50. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10(3):505-520.
51. Wilcox MH, Hall J, Pike H, et al. Use of perioperative mupirocin to prevent methicillin-resistant Staphylococcus aureus (MRSA) orthopaedic surgical site infections. J Hosp Infect. 2003;54(3):196-201.
52. Bode LG, Kluytmans JA, Wertheim HF, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010;362(1):9-17.
53. Association of Operating Room Nurses. Recommended practices for skin preparation of patients. AORN J. 2002;75(1):184-187.
54. Edmiston CE Jr, Seabrook GR, Johnson CP, Paulson DS, Beausoleil CM. Comparative of a new and innovative 2% chlorhexidine gluconate–impregnated cloth with 4% chlorhexidine gluconate as topical antiseptic for preparation of the skin prior to surgery. Am J Infect Control. 2007;35(2):89-96.
55. Edmiston CE Jr, Krepel CJ, Seabrook GR, Lewis BD, Brown KR, Towne JB. Preoperative shower revisited: can high topical antiseptic levels be achieved on the skin surface before surgical admission? J Am Coll Surg. 2008;207(2):233-239.
56. Johnson AJ, Kapadia BH, Daley JA, Molina CB, Mont MA. Chlorhexidine reduces infections in knee arthroplasty. J Knee Surg. 2013;26(3):213-218.
57. Johnson AJ, Daley JA, Zywiel MG, Delanois RE, Mont MA. Preoperative chlorhexidine preparation and the incidence of surgical site infections after hip arthroplasty. J Arthroplasty. 2010;25(6 suppl):98-102.
58. Mauerhan DR, Nelson CL, Smith DL, et al. Prophylaxis against infection in total joint arthroplasty. One day of cefuroxime compared with three days of cefazolin. J Bone Joint Surg Am. 1994;76(1):39-45.
59. Bratzler DW, Houck PM; Surgical Infection Prevention Guideline Writers Workgroup. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Am J Surg. 2005;189(4):395-404.
60. Tetreault MW, Wetters NG, Aggarwal V, Mont M, Parvizi J, Della Valle CJ. The Chitranjan Ranawat Award: should prophylactic antibiotics be withheld before revision surgery to obtain appropriate cultures? Clin Orthop Relat Res. 2014;472(1):52-56.
61. Illingworth KD, Mihalko WM, Parvizi J, et al. How to minimize infection and thereby maximize patient outcomes in total joint arthroplasty: a multicenter approach: AAOS exhibit selection. J Bone Joint Surg Am. 2013;95(8):e50.
62. Bannister GC, Auchincloss JM, Johnson DP, Newman JH. The timing of tourniquet application in relation to prophylactic antibiotic administration. J Bone Joint Surg Br. 1988;70(2):322-324.
63. Engesæter LB, Lie SA, Espehaug B, Furnes O, Vollset SE, Havelin LI. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand. 2003;74(6):644-651.
64. Ritter MA. Operating room environment. Clin Orthop Relat Res. 1999;(369):103-109.
65. Brandt C, Hott U, Sohr D, Daschner F, Gastmeier P, Rüden H. Operating room ventilation with laminar airflow shows no protective effect on the surgical site infection rate in orthopedic and abdominal surgery. Ann Surg. 2008;248(5):695-700.
66. Dharan S, Pittet D. Environmental controls in operating theatres. J Hosp Infect. 2002;51(2):79-84.
67. Hamilton HW, Booth AD, Lone FJ, Clark N. Penetration of gown material by organisms from the surgical team. Clin Orthop Relat Res. 1979;(141):237-246.
68. Da Costa AR, Kothari A, Bannister GC, Blom AW. Investigating bacterial growth in surgical theatres: establishing the effect of laminar airflow on bacterial growth on plastic, metal and wood surfaces. Ann R Coll Surg Engl. 2008;90(5):417-419.
69. Tanner J, Woodings D, Moncaster K. Preoperative hair removal to reduce surgical site infection. Cochrane Database Syst Rev. 2006;(2):CD004122.
70. Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18-26.
71. Carroll K, Dowsey M, Choong P, Peel T. Risk factors for superficial wound complications in hip and knee arthroplasty. Clin Microbiol Infect. 2013;20(2):130-135.
72. Ayliffe GA. Surgical scrub and skin disinfection. Infect Control. 1984;5(1):23-27.
73. Lidwell OM, Lowbury EJ, Whyte W, Blowers R, Lowe D. Extended follow-up of patients suspected of having joint sepsis after total joint replacement. J Hyg (Lond). 1985;95(3):655-664.
74. Webster J, Alghamdi AA. Use of plastic adhesive drapes during surgery for preventing surgical site infection. Cochrane Database Syst Rev. 2007;(4):CD006353.
75. Alijanipour P, Heller S, Parvizi J. Prevention of periprosthetic joint infection: what are the effective strategies? J Knee Surg. 2014;27(4):251-258.
76. Tanner J, Parkinson H. Double gloving to reduce surgical cross-infection. Cochrane Database Syst Rev. 2002;(3):CD003087.
77. Demircay E, Unay K, Bilgili MG, Alataca G. Glove perforation in hip and knee arthroplasty. J Orthop Sci. 2010;15(6):790-794.
78. Ersozlu S, Sahin O, Ozgur AF, Akkaya T, Tuncay C. Glove punctures in major and minor orthopaedic surgery with double gloving. Acta Orthop Belg. 2007;73(6):760-764.
79. Sanders R, Fortin P, Ross E, Helfet D. Outer gloves in orthopaedic procedures. Cloth compared with latex. J Bone Joint Surg Am. 1990;72(6):914-917.
80. Dodds RD, Guy PJ, Peacock AM, Duffy SR, Barker SG, Thomas MH. Surgical glove perforation. Br J Surg. 1988;75(10):966-968.
81. Al-Maiyah M, Bajwa A, Mackenney P, et al. Glove perforation and contamination in primary total hip arthroplasty. J Bone Joint Surg Br. 2005;87(4):556-559.
82. Insull PJ, Hudson J. Suction tip: a potential source of infection in clean orthopaedic procedures. ANZ J Surg. 2012;82(3):185-186.
83. Givissis P, Karataglis D, Antonarakos P, Symeonidis PD, Christodoulou A. Suction during orthopaedic surgery. How safe is the suction tip? Acta Orthop Belg. 2008;74(4):531-533.
84. Meals RA, Knoke L. The surgical suction top—a contaminated instrument. J Bone Joint Surg Am. 1978;60(3):409-410.
85. Strange-Vognsen MH, Klareskov B. Bacteriologic contamination of suction tips during hip arthroplasty. Acta Orthop Scand. 1988;59(4):410-411.
86. Greenough CG. An investigation into contamination of operative suction. J Bone Joint Surg Br. 1986;68(1):151-153.
87. Baird RA, Nickel FR, Thrupp LD, Rucker S, Hawkins B. Splash basin contamination in orthopaedic surgery. Clin Orthop Relat Res. 1984;(187):129-133.
88. Hargrove R, Ridgeway S, Russell R, Norris M, Packham I, Levy B. Does pulse lavage reduce hip hemiarthroplasty infection rates? J Hosp Infect. 2006;62(4):446-449.
89. Hassinger SM, Harding G, Wongworawat MD. High-pressure pulsatile lavage propagates bacteria into soft tissue. Clin Orthop Relat Res. 2005;(439):27-31.
90. Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res. 2010;468(1):52-56.
91. Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement. Clin Orthop Relat Res. 2001;(392):15-23.
92. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
93. Cherian JJ, Kapadia BH, Issa K, et al. Preoperative blood management strategies for total hip arthroplasty. Surg Technol Int. 2013;23:261-266.
94. Issa K, Banerjee S, Rifai A, et al. Blood management strategies in primary and revision total knee arthroplasty for Jehovah’s Witness patients. J Knee Surg. 2013;26(6):401-404.
95. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2010;93(1):39-46.
96. Berger V, Alperson S. A general framework for the evaluation of clinical trial quality. Rev Recent Clin Trials. 2009;4(2):79-88.
97. Chimento GF, Huff T, Ochsner JL, Meyer M, Brandner L, Babin S. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 suppl):74-77.
98. Karam JA, Bloomfield MR, DiIorio TM, Irizarry AM, Sharkey PF. Evaluation of the efficacy and safety of tranexamic acid for reducing blood loss in bilateral total knee arthroplasty. J Arthroplasty. 2014;29(3):501-503.
99. Kim HJ, Fraser MR, Kahn B, Lyman S, Figgie MP. The efficacy of a thrombin-based hemostatic agent in unilateral total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(13):1160-1165.
100. Suarez JC, Slotkin EM, Alvarez AM, Szubski CR, Barsoum WK, Patel PD. Prospective, randomized trial to evaluate efficacy of a thrombin-based hemostatic agent in total knee arthroplasty. J Arthroplasty. 2014;29(10):1950-1955.
101. Romanò CL, Monti L, Logoluso N, Romanò D, Drago L. Does a thrombin-based topical haemostatic agent reduce blood loss and transfusion requirements after total knee revision surgery? A randomized, controlled trial. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3337-3342.
102. Falez F, Meo A, Panegrossi G, Favetti F, Cava F, Casella F. Blood loss reduction in cementless total hip replacement with fibrin spray or bipolar sealer: a randomised controlled trial on ninety five patients. Int Orthop. 2013;37(7):1213-1217.
103. Morris MJ, Barrett M, Lombardi AV, Tucker TL, Berend KR. Randomized blinded study comparing a bipolar sealer and standard electrocautery in reducing transfusion requirements in anterior supine intermuscular total hip arthroplasty. J Arthroplasty. 2013;28(9):1614-1617.
104. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518.
105. Zeh A, Messer J, Davis J, Vasarhelyi A, Wohlrab D. The Aquamantys system—an alternative to reduce blood loss in primary total hip arthroplasty? J Arthroplasty. 2010;25(7):1072-1077.
106. Heck D, Rosenberg A, Schink-Ascani M, Garbus S, Kiewitt T. Use of antibiotic-impregnated cement during hip and knee arthroplasty in the United States. J Arthroplasty. 1995;10(4):470-475.
107. Srivastav A, Nadkarni B, Srivastav S, Mittal V, Agarwal S. Prophylactic use of antibiotic-loaded bone cement in primary total knee arthroplasty: justified or not? Indian J Orthop. 2009;43(3):259-263.
108. Dunbar MJ. Antibiotic bone cements: their use in routine primary total joint arthroplasty is justified. Orthopedics. 2009;32(9).
109. Merollini KM, Zheng H, Graves N. Most relevant strategies for preventing surgical site infection after total hip arthroplasty: guideline recommendations and expert opinion. Am J Infect Control. 2013;41(3):221-226.
110. Jämsen E, Huhtala H, Puolakka T, Moilanen T. Risk factors for infection after knee arthroplasty. A register-based analysis of 43,149 cases. J Bone Joint Surg Am. 2009;91(1):38-47.
111. Seldes RM, Winiarsky R, Jordan LC, et al. Liquid gentamicin in bone cement: a laboratory study of a potentially more cost-effective cement spacer. J Bone Joint Surg Am. 2005;87(2):268-272.
112. Wright TM, Sullivan DJ, Arnoczky SP. The effect of antibiotic additions on the fracture properties of bone cements. Acta Orthop Scand. 1984;55(4):414-418.
113. Baleani M, Persson C, Zolezzi C, Andollina A, Borrelli AM, Tigani D. Biological and biomechanical effects of vancomycin and meropenem in acrylic bone cement. J Arthroplasty. 2008;23(8):1232-1238.
114. Baleani M, Cristofolini L, Minari C, Toni A. Fatigue strength of PMMA bone cement mixed with gentamicin and barium sulphate vs pure PMMA. Proc Inst Mech Eng H. 2005;217(1):9-12.
115. Parvizi J, Saleh KJ, Ragland PS, Pour AE, Mont MA. Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthop Scand. 2008;79(3):335-341.
116. Cummins JS, Tomek IM, Kantor SR, Furnes O, Engesæter LB, Finlayson SRG. Cost-effectiveness of antibiotic-impregnated bone cement used in primary total hip arthroplasty. J Bone Joint Surg Am. 2009;91(3):634-641.
117. Brown NM, Cipriano CA, Moric M, Sporer SM, Della Valle CJ. Dilute Betadine lavage before closure for the prevention of acute postoperative deep periprosthetic joint infection. J Arthroplasty. 2012;27(1):27-30.
118. Fowler JR, Perkins TA, Buttaro BA, Truant AL. Bacteria adhere less to barbed monofilament than braided sutures in a contaminated wound model. Clin Orthop Relat Res. 2013;471(2):665-671.
119. Smith EL, DiSegna ST, Shukla PY, Matzkin EG. Barbed versus traditional sutures: closure time, cost, and wound related outcomes in total joint arthroplasty. J Arthroplasty. 2014;29(2):283-287.
120. Ting NT, Moric MM, Della Valle CJ, Levine BR. Use of knotless suture for closure of total hip and knee arthroplasties: a prospective, randomized clinical trial. J Arthroplasty. 2012;27(10):1783-1788.
121. Miller AG, Swank ML. Dermabond efficacy in total joint arthroplasty wounds. Am J Orthop. 2010;39(10):476-478.
122. Cai J, Karam JA, Parvizi J, Smith EB, Sharkey PF. Aquacel surgical dressing reduces the rate of acute PJI following total joint arthroplasty: a case–control study. J Arthroplasty. 2014;29(6):1098-1100.
123. Drinkwater CJ, Neil MJ. Optimal timing of wound drain removal following total joint arthroplasty. J Arthroplasty. 1995;10(2):185-189.
124. Parker MJ, Roberts CP, Hay D. Closed suction drainage for hip and knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2004;86(6):1146-1152.
125. Matar WY, Jafari SM, Restrepo C, Austin M, Purtill JJ, Parvizi J. Preventing infection in total joint arthroplasty. J Bone Joint Surg Am. 2010;92(suppl 2):36-46.
126. Ritter MA, French ML, Eitzen HE. Bacterial contamination of the surgical knife. Clin Orthop Relat Res. 1975;(108):158-160.
127. Fairclough JA, Mackie IG, Mintowt-Czyz W, Phillips GE. The contaminated skin-knife. A surgical myth. J Bone Joint Surg Br. 1983;65(2):210.
128. Ramón R, García S, Combalía A, Puig de la Bellacasa J, Segur JM. Bacteriological study of surgical knives: is the use of two blades necessary? Arch Orthop Trauma Surg. 1994;113(3):157-158.
129. Hasselgren PO, Hagberg E, Malmer H, Säljö A, Seeman T. One instead of two knives for surgical incision. Does it increase the risk of postoperative wound infection? Arch Surg. 1984;119(8):917-920.
130. Lee MJ, Pottinger PS, Butler-Wu S, Bumgarner RE, Russ SM, Matsen FA 3rd. Propionibacterium persists in the skin despite standard surgical preparation. J Bone Joint Surg Am. 2014;96(17):1447-1450.
131. Parvizi J, Ghanem E, Joshi A, Sharkey PF, Hozack WJ, Rothman RH. Does “excessive” anticoagulation predispose to periprosthetic infection? J Arthroplasty. 2007;22(6 suppl 2):24-28.
132. Marchant MH, Viens NA, Cook C, Vail TP, Bolognesi MP. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. J Bone Joint Surg Am. 2009;91(7):1621-1629.
133. Reátegui D, Sanchez-Etayo G, Núñez E, et al. Perioperative hyperglycaemia and incidence of post-operative complications in patients undergoing total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2026-2031.
134. Urquhart DM, Hanna FS, Brennan SL, et al. Incidence and risk factors for deep surgical site infection after primary total hip arthroplasty: a systematic review. J Arthroplasty. 2010;25(8):1216-1222.e1-e3.
135. Friedlander AH. Oral cavity staphylococci are a potential source of prosthetic joint infection. Clin Infect Dis. 2010;50(12):1682-1683.
136. Zimmerli W, Sendi P. Antibiotics for prevention of periprosthetic joint infection following dentistry: time to focus on data. Clin Infect Dis. 2010;50(1):17-19.
137. Young H, Hirsh J, Hammerberg EM, Price CS. Dental disease and periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(2):162-168.
138. Simmons NA, Ball AP, Cawson RA, et al. Case against antibiotic prophylaxis for dental treatment of
Quality and Quantity of the Elbow Arthroscopy Literature: A Systematic Review and Meta-Analysis
Although elbow arthroscopy was first described in the 1930s, it has become increasingly popular in the last 30 years.1 While initially considered as a tool for diagnosis and loose body removal, indications have expanded to include treatment of osteochondritis dissecans (OCD), treatment of lateral epicondylitis, fixation of fractures, and others.2-5 Miyake and colleagues6 found a significant improvement in range of motion, both flexion and extension, and outcome scores when elbow arthroscopy was used to remove impinging osteophytes. Babaqi and colleagues7 found significant improvement in pain, satisfaction, and outcome scores in 31 patients who underwent elbow arthroscopy for lateral epicondylitis refractory to nonsurgical management. The technical difficulty of the procedure, lower frequency of pathology amenable to arthroscopic intervention, and potential neurovascular complications make the elbow less frequently evaluated with the arthroscope vs other joints, such as the knee and shoulder.2,8,9
Geographic distribution of subjects undergoing elbow arthroscopy, the indications used, surgical techniques being performed, and their associated clinical outcomes have received little to no recognition in the peer-reviewed literature.10 Differences in the elbow arthroscopy literature include characteristics related to the patient (age, gender, hand dominance, duration of symptoms), study (level of evidence, number of subjects, number of participating centers, design), indication (lateral epicondylitis, loose bodies, olecranon osteophytes, OCD), surgical technique, and outcome. Evidence-based medicine and clinical practice guidelines direct surgeons in clinical decision-making. Payers investigate the cost of surgical interventions and the value that surgery may provide, while following trends in different surgical techniques. Regulatory agencies and associations emphasize subjective patient-reported outcomes as the primary outcome measured in high-quality trials. Thus, in discussion of complex surgical interventions such as elbow arthroscopy, it is important to characterize the studies, subjects, and surgeries across the world to understand the geographic similarities and differences to optimize care in this clinical situation.
The goal of this study was to perform a systematic review and meta-analysis of elbow arthroscopy literature to identify and compare the characteristics of the studies published, the subjects analyzed, and surgical techniques performed across continents and countries to answer these questions: “Across the world, what demographic of patients are undergoing elbow arthroscopy, what are the most common indications for elbow arthroscopy, and how good is the evidence?” The authors hypothesized that patients who undergo elbow arthroscopy will be largely age <40 years, the most common indication for elbow arthroscopy will be a release/débridement, and the evidence for elbow arthroscopy will be poor. Also, no significant differences will exist in elbow arthroscopy publications, subjects, outcomes, and techniques based on continent/country of publication.
Methods
A systematic review was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines using a PRISMA checklist.11 Systematic review registration was performed using the International Prospective Register of Ongoing Systematic Reviews (PROSPERO; registration number, CRD42014010580; registration date, July 15, 2014).12 Two study authors independently conducted the search on June 23, 2014 using the following databases: Medline, Cochrane Central Register of Controlled Trials, SportDiscus, and CINAHL. The electronic search citation algorithm used was: (elbow) AND arthroscopy) NOT shoulder) NOT knee) NOT ankle) NOT wrist) NOT hip) NOT dog) NOT cadaver). English language Level I-IV evidence (2012 update by the Oxford Centre for Evidence-Based Medicine13) clinical studies were eligible for inclusion into this study. Abstracts were ineligible for inclusion. All references in selected studies were cross-referenced for inclusion if they were missed during the initial search. Duplicate subject publications within separate unique studies were not reported twice. The study with longer duration follow-up, higher level of evidence, greater number of subjects, or more detailed subject, surgical technique, or outcome reporting was retained for inclusion. Level V evidence reviews, expert opinion articles, letters to the editor, basic science, biomechanical studies, open elbow surgery, imaging, surgical technique, and classification studies were excluded.
All included patients underwent elbow arthroscopy for either intra- or extra-articular elbow pathology (ulnotrochlear osteoarthritis, lateral epicondylitis, rheumatoid arthritis, post-traumatic contracture, osteonecrosis of the capitellum or radial head, osteoid osteoma, and others). There was no minimum follow-up duration or rehabilitation requirement. The study and subject demographic parameters that we analyzed included year of publication, years of subject enrollment, presence of study financial conflict of interest, number of subjects and elbows, elbow dominance, gender, age, body mass index, diagnoses treated, type of anesthesia (block or general), and surgical positioning. Postoperative splint application and pain management, and whether a continuous passive motion machine was used and whether a drain was placed were recorded. Clinical outcome scores were DASH (Disability of the Arm, Shoulder, and Hand), Morrey score, MEPS (Mayo Elbow Performance Score), Andrews-Carson score, Timmerman-Andrews score, LES (Liverpool Elbow Score), Tegner score, HSS (Hospital for Special Surgery Score), VAS (Visual Analog Scale), EFA (Elbow Functional Assessment), Short Form-12 (SF-12), Short Form-36 (SF-36), Kerlan-Jobe Orthopaedic Clinic (KJOC) Shoulder and Elbow Questionnaire, and MAESS (Modified Andrews Elbow Scoring System). Radiographs, computed tomography (CT), computed tomography arthrography (CTA), magnetic resonance imaging (MRI), and magnetic resonance arthrography (MRA) data were extracted when available. Range of motion (flexion, extension, supination, and pronation) and grip strength data, both preoperative and postoperative, were extracted when available. Study methodological quality was evaluated using the Modified Coleman Methodology Score (MCMS).14
Statistical Analysis
Study descriptive statistics were calculated. Continuous variable data were reported as weighted means ± weighted standard deviations. Categorical variable data were reported as frequencies with percentages. For all statistical analysis either measured and calculated from study data extraction or directly reported from the individual studies, P < .05 was considered statistically significant. Study, subject, and surgical outcomes data were compared using 1-way analysis of variance (ANOVA) tests. Where applicable, study, subject, and surgical outcomes data were also compared using 2-sample and 2-proportion Z-test calculators with α .05 because of the difference in sample sizes between compared groups. To examine trends over time, Pearson’s correlation coefficients were calculated. For the purposes of analysis, the indications of “osteoarthritis,” “arthrofibrosis,” “loose body removal,” “ulnotrochlear osteoarthritis causing stiffness,” “post-traumatic contracture/stiffness,” and “post-operative elbow contracture” were combined into the indication “release and débridement.” For the 3 most common indications for arthroscopy (OCD, lateral epicondylitis, and release and débridement) data were combined into 5-year increments to overcome the smaller sample size within each of these categories, and Pearson’s correlation coefficients were calculated to determine if number of reported cases covaried with year period. Within these 3 diagnoses, ANOVA analyses were performed to determine whether the number of cases differed between continents and countries.
Results
A total of 353 studies were located, and, after implementation of the exclusion criteria, 112 studies were included in the final analysis (Figure 1; 3093 subjects; 3168 elbows; 64% male; mean age, 34.9 ± 14.68 years). There was a mean of 33.4 ± 26.02 months of follow-up, and 75% of surgeries involved the dominant elbow (Table 1). Most studies were level IV evidence (94.6%), had a low MCMS (mean 28.1 ± 8.06; poor rating), and were single-center investigations (94.6%). Most studies did not report financial conflicts of interest (56.3%) (Tables 1 and 2). From 1985 through 2014, the number of publications significantly increased with time (P = .004) among all continents. The MCMS was unchanged over time (P = .247) (Figure 2A), as was the level of evidence (P = .094) (Figure 2B). Conflicts of interest significantly increased with time (P = .025) (Figure 3).
Among continents, North America published the largest number of studies (54), and had the largest number of patients (1395) and elbow surgeries (1425) (Table 1). The United States published the largest number of studies (43%). There were no significant differences between age (P = .331), length of follow-up (P = .403), MCMS (P = .123), and level of evidence (P = .288) between continents. Of the 32 studies that reported the use of preoperative MRI, studies from Asia reported significantly more MRI scans than those from other continents (P = .040); there were no other significant differences between continents in reference to preoperative imaging studies or other demographic information.
The most common surgical indications were OCD (Figure 4), lateral epicondylitis (Figure 5), and release and débridement (Figure 6, Table 3; all studies listed indications). The number of reported cases for these 3 indications significantly increased over time (OCD P = .005, lateral epicondylitis P = .044, release and débridement P = .042) but did not significantly differ between regions (P > .05 in all cases).
Thirty-two (28.6%) studies reported the use of outcome measures (16 different outcome scores were used by the included studies). Asia reported outcome measures in 9 of 23 studies (39%), Europe in 12 of 35 studies (34%) and North America in 11 of 54 (20%) of studies. The MEPS was the most frequently used outcome score in 9.8% of studies, followed by VAS for pain in 5.3% of cases. North American studies reported a significantly higher increase in extension after elbow arthroscopy than Asia (P = .0432) (Figure 7), with no differences in flexion (P = .699), pronation (P = .376), or supination (P = .408). No significant differences were observed between continents in the type of anesthesia chosen (general anesthesia [P = .94] or regional anesthesia [P = .85]). Asia and Europe performed elbow arthroscopy most frequently in the lateral decubitus position, while North American studies most often used the supine position (Table 4).
Twenty (17.9%) studies reported the use of a postoperative splint, 12 (10.7%) studies reported use of a drain, 2 (1.79%) studies reported use of a hinged elbow brace, 9 (8.03%) studies reported use of a continuous passive motion machine postoperatively, and 3 (2.68%) studies reported use of an indwelling axillary catheter for postoperative pain management. Of 130 reported surgical complications (4.1%), the most frequent complication was transient sensory ulnar nerve palsy (1.5%), followed by persistent wound drainage (.76%), and transient sensory radial nerve palsy (.38%). Other reported complications included infection (.22%), transient sensory palsy of the median nerve (.19%), heterotopic ossification (.13%), complete transection of the ulnar nerve (.10%), loose body formation (.06%), hematoma formation (.06%), transient sensory palsy of the posterior interosseous (.06%), or anterior interosseous nerve (.03%), and complete transection of the radial (.03%), or median nerve (.03%).
Discussion
Elbow arthroscopy is an evolving surgical procedure that is used to treat intra- and extra-articular pathologies of the elbow. Outcomes of elbow arthroscopy for certain conditions have generally been reported as good, with improvements seen in pain, functional scores, and range of motion.6,15-17 The authors’ hypotheses were mostly confirmed in that the average age of patients undergoing elbow arthroscopy was <40 years, release/débridement was one of the most common indications (along with lateral epicondylitis and OCD), and the general evidence for elbow arthroscopy was poor. Also, there were almost no differences between continents/countries related to patient indications, preoperative imaging, anesthesia choice, indications, postoperative protocols, and outcomes (although the number of studies that reported outcomes was low and could have skewed the results), with the exception of a higher number of preoperative MRI scans in Asia. Some of the notable findings of this study included: 1) the number of studies published on elbow arthroscopy is significantly increasing with time, despite a lack of improvement in the level of evidence; 2) the majority of studies on elbow arthroscopy do not report a surgical outcome score; and 3) the number of reported cases for the 3 most common indications significantly increased over time (OCD, P = .005; lateral epicondylitis, P = .044; release and débridement, P = .042) but did not differ between regions (P > .05 in all cases).
The indications for elbow arthroscopy have grown dramatically in the past 2 decades to include both intra- and extra-articular pathologies.18 Despite this increase in the number of indications for elbow arthroscopy, the study did not find a significant difference between countries/continents in the indications each used for elbow arthroscopy patients. There was a trend towards an increase in OCD cases in all continents, especially Asia (Figure 4), with time. Interestingly, while not statistically significant, there was variation among countries for surgical indications. In North America, removal of loose bodies accounts for 18% of patients, while in Europe this accounted for only 9% and in Asia for 1%. Post-traumatic stiffness was the indication for elbow arthroscopy in Europe in 19% of patients vs 7% in North America and 10% in Asia. In Asia, OCD accounts for 40% of arthroscopies, 7% in Europe, and 14% in North America (Figure 4) (Table 3).
This study demonstrated that the mean increase in elbow extension gained after surgery in North America was significantly greater when compared with studies from Asia, but the gain in flexion, pronation, and supination was similar across continents. The underlying cause of this difference in improvement in elbow extension between nations is unclear, although differences in diagnosis could account for some variation. This study did not examine differences in rehabilitation protocols, and certainly, it is plausible that protocol variations by country could account for some discrepancy. Furthermore, differences in functional needs may vary by continent and could have driven this result.
This study found no routine reporting of outcome scores by elbow arthroscopy studies from any continent, and that when outcome scores are reported, there is substantial inconsistency with regard to the actual scoring system used. No continent reported outcome scores in more than 40% of the studies published from that area, and the variation of outcome scores used, even from a single region, was large. This makes comparing clinical outcomes between studies difficult, even when performing identical procedures for identical indications, because there is no standardized method of reporting outcomes. To allow comparison of studies and generalizability of the results to different populations, a more standardized approach to outcome reporting needs to be instituted in the elbow arthroscopy literature. To date, there is no standardized score that has been validated for reporting clinical outcomes after elbow arthroscopy.19 Hence, it is not surprising that there were 16 different outcome scores reported throughout the 112 studies analyzed in this review, with the most frequent score, the MEPS, reported in a total of 10 studies. As medicine moves towards pay scales that are based on patient outcomes, it will become more important to define a clear outcome score that can be used to assess these patients, and reliably report scores. This will allow comparison of patients across nations to determine the best surgical treatment for different clinical problems. A validation study comparing these outcome scores to determine which score best summarizes the patient’s level of pain and function after surgery would be beneficial, because this could identify 1 score that could be standardized to allow comparison among reported outcomes.
Limitations
This study had several limitations. Despite having 2 authors search independently for studies, some studies could have been missed during the search process, introducing possible selection bias. Including only published studies could have introduced publication bias. Numerous studies did not report all the variables the authors examined. This could have skewed some results, and had additional variables been reported, could have altered the data to show significant differences in some measured variables. Because this study did not compare outcome measures for varying pathologies, conclusions cannot be drawn on the best treatment options for different indications. Case reports could have lowered the MCMS score and the average in studies reporting outcomes. Furthermore, the poor quality of the underlying data used in this study could limit the validity/generalizability of the results because this is a systematic review, and its level of evidence is only as high as the studies it includes. Because the primary aim was to report on demographics, this study did not examine concomitant pathology at the time of surgery or rehabilitation protocols.
Conclusion
The quantity, but not the quality, of arthroscopic elbow publications has significantly increased over time. Most patients undergo elbow arthroscopy for lateral epicondylitis, OCD, and release and débridement. Pathology and indications do not appear to differ geographically with more men undergoing elbow arthroscopy than women.
1. Khanchandani P. Elbow arthroscopy: review of the literature and case reports. Case Rep Orthop. 2012;2012:478214.
2. Dodson CC, Nho SJ, Williams RJ 3rd, Altchek DW. Elbow arthroscopy. J Am Acad Orthop Surg. 2008;16(10):574-585.
3. Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 Pt 1):47-62.
4. Kelly EW, Morrey BF, O’Driscoll SW. Complications of elbow arthroscopy. J Bone Joint Surg Am. 2001;83-A(1):25-34.
5. Rajeev A, Pooley J. Lateral compartment cartilage changes and lateral elbow pain. Acta Orthop Belg. 2009;75(1):37-40.
6. Miyake J, Shimada K, Oka K, et al. Arthroscopic debridement in the treatment of patients with osteoarthritis of the elbow, based on computer simulation. Bone Joint J. 2014;96-B(2):237-241.
7. Babaqi AA, Kotb MM, Said HG, AbdelHamid MM, ElKady HA, ElAssal MA. Short-term evaluation of arthroscopic management of tennis elbow; including resection of radio-capitellar capsular complex. J Orthop. 2014;11(2):82-86.
8. Gay DM, Raphael BS, Weiland AJ. Revision arthroscopic contracture release in the elbow resulting in an ulnar nerve transection: a case report. J Bone Joint Surg Am. 2010;92(5):1246-1249.
9. Haapaniemi T, Berggren M, Adolfsson L. Complete transection of the median and radial nerves during arthroscopic release of post-traumatic elbow contracture. Arthroscopy. 1999;15(7):784-787.
10. Yeoh KM, King GJ, Faber KJ, Glazebrook MA, Athwal GS. Evidence-based indications for elbow arthroscopy. Arthroscopy. 2012;28(2):272-282.
11. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. BMJ. 2009;339:b2700.
12. PROSPERO. International Prospective Register of Ongoing Systematic Reviews. The University of York CfRaDP-Iprosr-v. 2013 [cited 2014]. http://www.crd.york.ac.uk/PROSPERO/. Accessed March 17, 2016.
13. Oxford Centre for Evidence-Based Medicine - levels of evidence (March 2009). Centre for Evidence-Based Medicine Web site. http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed July 6, 2016.
14. Cowan J, Lozano-Calderόn S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
15. Jones GS, Savoie FH 3rd. Arthroscopic capsular release of flexion contractures (arthrofibrosis) of the elbow. Arthroscopy. 1993;9(3):277-283.
16. O’Brien MJ, Lee Murphy R, Savoie FH 3rd. A preliminary report of acute and subacute arthroscopic repair of the radial ulnohumeral ligament after elbow dislocation in the high-demand patient. Arthroscopy. 2014;30(6):679-687.
17. Rhyou IH, Kim KW. Is posterior synovial plica excision necessary for refractory lateral epicondylitis of the elbow? Clin Orthop Relat Res. 2013;471(1):284-290.
18. Jerosch J, Schunck J. Arthroscopic treatment of lateral epicondylitis: indication, technique and early results. Knee Surg Sports Traumatol Arthrosc. 2006;14(4):379-382.
19. Tijssen M, van Cingel R, van Melick N, de Visser E. Patient-Reported Outcome questionnaires for hip arthroscopy: a systematic review of the psychometric evidence. BMC Musculoskelet Disord. 2011;12:117.
Although elbow arthroscopy was first described in the 1930s, it has become increasingly popular in the last 30 years.1 While initially considered as a tool for diagnosis and loose body removal, indications have expanded to include treatment of osteochondritis dissecans (OCD), treatment of lateral epicondylitis, fixation of fractures, and others.2-5 Miyake and colleagues6 found a significant improvement in range of motion, both flexion and extension, and outcome scores when elbow arthroscopy was used to remove impinging osteophytes. Babaqi and colleagues7 found significant improvement in pain, satisfaction, and outcome scores in 31 patients who underwent elbow arthroscopy for lateral epicondylitis refractory to nonsurgical management. The technical difficulty of the procedure, lower frequency of pathology amenable to arthroscopic intervention, and potential neurovascular complications make the elbow less frequently evaluated with the arthroscope vs other joints, such as the knee and shoulder.2,8,9
Geographic distribution of subjects undergoing elbow arthroscopy, the indications used, surgical techniques being performed, and their associated clinical outcomes have received little to no recognition in the peer-reviewed literature.10 Differences in the elbow arthroscopy literature include characteristics related to the patient (age, gender, hand dominance, duration of symptoms), study (level of evidence, number of subjects, number of participating centers, design), indication (lateral epicondylitis, loose bodies, olecranon osteophytes, OCD), surgical technique, and outcome. Evidence-based medicine and clinical practice guidelines direct surgeons in clinical decision-making. Payers investigate the cost of surgical interventions and the value that surgery may provide, while following trends in different surgical techniques. Regulatory agencies and associations emphasize subjective patient-reported outcomes as the primary outcome measured in high-quality trials. Thus, in discussion of complex surgical interventions such as elbow arthroscopy, it is important to characterize the studies, subjects, and surgeries across the world to understand the geographic similarities and differences to optimize care in this clinical situation.
The goal of this study was to perform a systematic review and meta-analysis of elbow arthroscopy literature to identify and compare the characteristics of the studies published, the subjects analyzed, and surgical techniques performed across continents and countries to answer these questions: “Across the world, what demographic of patients are undergoing elbow arthroscopy, what are the most common indications for elbow arthroscopy, and how good is the evidence?” The authors hypothesized that patients who undergo elbow arthroscopy will be largely age <40 years, the most common indication for elbow arthroscopy will be a release/débridement, and the evidence for elbow arthroscopy will be poor. Also, no significant differences will exist in elbow arthroscopy publications, subjects, outcomes, and techniques based on continent/country of publication.
Methods
A systematic review was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines using a PRISMA checklist.11 Systematic review registration was performed using the International Prospective Register of Ongoing Systematic Reviews (PROSPERO; registration number, CRD42014010580; registration date, July 15, 2014).12 Two study authors independently conducted the search on June 23, 2014 using the following databases: Medline, Cochrane Central Register of Controlled Trials, SportDiscus, and CINAHL. The electronic search citation algorithm used was: (elbow) AND arthroscopy) NOT shoulder) NOT knee) NOT ankle) NOT wrist) NOT hip) NOT dog) NOT cadaver). English language Level I-IV evidence (2012 update by the Oxford Centre for Evidence-Based Medicine13) clinical studies were eligible for inclusion into this study. Abstracts were ineligible for inclusion. All references in selected studies were cross-referenced for inclusion if they were missed during the initial search. Duplicate subject publications within separate unique studies were not reported twice. The study with longer duration follow-up, higher level of evidence, greater number of subjects, or more detailed subject, surgical technique, or outcome reporting was retained for inclusion. Level V evidence reviews, expert opinion articles, letters to the editor, basic science, biomechanical studies, open elbow surgery, imaging, surgical technique, and classification studies were excluded.
All included patients underwent elbow arthroscopy for either intra- or extra-articular elbow pathology (ulnotrochlear osteoarthritis, lateral epicondylitis, rheumatoid arthritis, post-traumatic contracture, osteonecrosis of the capitellum or radial head, osteoid osteoma, and others). There was no minimum follow-up duration or rehabilitation requirement. The study and subject demographic parameters that we analyzed included year of publication, years of subject enrollment, presence of study financial conflict of interest, number of subjects and elbows, elbow dominance, gender, age, body mass index, diagnoses treated, type of anesthesia (block or general), and surgical positioning. Postoperative splint application and pain management, and whether a continuous passive motion machine was used and whether a drain was placed were recorded. Clinical outcome scores were DASH (Disability of the Arm, Shoulder, and Hand), Morrey score, MEPS (Mayo Elbow Performance Score), Andrews-Carson score, Timmerman-Andrews score, LES (Liverpool Elbow Score), Tegner score, HSS (Hospital for Special Surgery Score), VAS (Visual Analog Scale), EFA (Elbow Functional Assessment), Short Form-12 (SF-12), Short Form-36 (SF-36), Kerlan-Jobe Orthopaedic Clinic (KJOC) Shoulder and Elbow Questionnaire, and MAESS (Modified Andrews Elbow Scoring System). Radiographs, computed tomography (CT), computed tomography arthrography (CTA), magnetic resonance imaging (MRI), and magnetic resonance arthrography (MRA) data were extracted when available. Range of motion (flexion, extension, supination, and pronation) and grip strength data, both preoperative and postoperative, were extracted when available. Study methodological quality was evaluated using the Modified Coleman Methodology Score (MCMS).14
Statistical Analysis
Study descriptive statistics were calculated. Continuous variable data were reported as weighted means ± weighted standard deviations. Categorical variable data were reported as frequencies with percentages. For all statistical analysis either measured and calculated from study data extraction or directly reported from the individual studies, P < .05 was considered statistically significant. Study, subject, and surgical outcomes data were compared using 1-way analysis of variance (ANOVA) tests. Where applicable, study, subject, and surgical outcomes data were also compared using 2-sample and 2-proportion Z-test calculators with α .05 because of the difference in sample sizes between compared groups. To examine trends over time, Pearson’s correlation coefficients were calculated. For the purposes of analysis, the indications of “osteoarthritis,” “arthrofibrosis,” “loose body removal,” “ulnotrochlear osteoarthritis causing stiffness,” “post-traumatic contracture/stiffness,” and “post-operative elbow contracture” were combined into the indication “release and débridement.” For the 3 most common indications for arthroscopy (OCD, lateral epicondylitis, and release and débridement) data were combined into 5-year increments to overcome the smaller sample size within each of these categories, and Pearson’s correlation coefficients were calculated to determine if number of reported cases covaried with year period. Within these 3 diagnoses, ANOVA analyses were performed to determine whether the number of cases differed between continents and countries.
Results
A total of 353 studies were located, and, after implementation of the exclusion criteria, 112 studies were included in the final analysis (Figure 1; 3093 subjects; 3168 elbows; 64% male; mean age, 34.9 ± 14.68 years). There was a mean of 33.4 ± 26.02 months of follow-up, and 75% of surgeries involved the dominant elbow (Table 1). Most studies were level IV evidence (94.6%), had a low MCMS (mean 28.1 ± 8.06; poor rating), and were single-center investigations (94.6%). Most studies did not report financial conflicts of interest (56.3%) (Tables 1 and 2). From 1985 through 2014, the number of publications significantly increased with time (P = .004) among all continents. The MCMS was unchanged over time (P = .247) (Figure 2A), as was the level of evidence (P = .094) (Figure 2B). Conflicts of interest significantly increased with time (P = .025) (Figure 3).
Among continents, North America published the largest number of studies (54), and had the largest number of patients (1395) and elbow surgeries (1425) (Table 1). The United States published the largest number of studies (43%). There were no significant differences between age (P = .331), length of follow-up (P = .403), MCMS (P = .123), and level of evidence (P = .288) between continents. Of the 32 studies that reported the use of preoperative MRI, studies from Asia reported significantly more MRI scans than those from other continents (P = .040); there were no other significant differences between continents in reference to preoperative imaging studies or other demographic information.
The most common surgical indications were OCD (Figure 4), lateral epicondylitis (Figure 5), and release and débridement (Figure 6, Table 3; all studies listed indications). The number of reported cases for these 3 indications significantly increased over time (OCD P = .005, lateral epicondylitis P = .044, release and débridement P = .042) but did not significantly differ between regions (P > .05 in all cases).
Thirty-two (28.6%) studies reported the use of outcome measures (16 different outcome scores were used by the included studies). Asia reported outcome measures in 9 of 23 studies (39%), Europe in 12 of 35 studies (34%) and North America in 11 of 54 (20%) of studies. The MEPS was the most frequently used outcome score in 9.8% of studies, followed by VAS for pain in 5.3% of cases. North American studies reported a significantly higher increase in extension after elbow arthroscopy than Asia (P = .0432) (Figure 7), with no differences in flexion (P = .699), pronation (P = .376), or supination (P = .408). No significant differences were observed between continents in the type of anesthesia chosen (general anesthesia [P = .94] or regional anesthesia [P = .85]). Asia and Europe performed elbow arthroscopy most frequently in the lateral decubitus position, while North American studies most often used the supine position (Table 4).
Twenty (17.9%) studies reported the use of a postoperative splint, 12 (10.7%) studies reported use of a drain, 2 (1.79%) studies reported use of a hinged elbow brace, 9 (8.03%) studies reported use of a continuous passive motion machine postoperatively, and 3 (2.68%) studies reported use of an indwelling axillary catheter for postoperative pain management. Of 130 reported surgical complications (4.1%), the most frequent complication was transient sensory ulnar nerve palsy (1.5%), followed by persistent wound drainage (.76%), and transient sensory radial nerve palsy (.38%). Other reported complications included infection (.22%), transient sensory palsy of the median nerve (.19%), heterotopic ossification (.13%), complete transection of the ulnar nerve (.10%), loose body formation (.06%), hematoma formation (.06%), transient sensory palsy of the posterior interosseous (.06%), or anterior interosseous nerve (.03%), and complete transection of the radial (.03%), or median nerve (.03%).
Discussion
Elbow arthroscopy is an evolving surgical procedure that is used to treat intra- and extra-articular pathologies of the elbow. Outcomes of elbow arthroscopy for certain conditions have generally been reported as good, with improvements seen in pain, functional scores, and range of motion.6,15-17 The authors’ hypotheses were mostly confirmed in that the average age of patients undergoing elbow arthroscopy was <40 years, release/débridement was one of the most common indications (along with lateral epicondylitis and OCD), and the general evidence for elbow arthroscopy was poor. Also, there were almost no differences between continents/countries related to patient indications, preoperative imaging, anesthesia choice, indications, postoperative protocols, and outcomes (although the number of studies that reported outcomes was low and could have skewed the results), with the exception of a higher number of preoperative MRI scans in Asia. Some of the notable findings of this study included: 1) the number of studies published on elbow arthroscopy is significantly increasing with time, despite a lack of improvement in the level of evidence; 2) the majority of studies on elbow arthroscopy do not report a surgical outcome score; and 3) the number of reported cases for the 3 most common indications significantly increased over time (OCD, P = .005; lateral epicondylitis, P = .044; release and débridement, P = .042) but did not differ between regions (P > .05 in all cases).
The indications for elbow arthroscopy have grown dramatically in the past 2 decades to include both intra- and extra-articular pathologies.18 Despite this increase in the number of indications for elbow arthroscopy, the study did not find a significant difference between countries/continents in the indications each used for elbow arthroscopy patients. There was a trend towards an increase in OCD cases in all continents, especially Asia (Figure 4), with time. Interestingly, while not statistically significant, there was variation among countries for surgical indications. In North America, removal of loose bodies accounts for 18% of patients, while in Europe this accounted for only 9% and in Asia for 1%. Post-traumatic stiffness was the indication for elbow arthroscopy in Europe in 19% of patients vs 7% in North America and 10% in Asia. In Asia, OCD accounts for 40% of arthroscopies, 7% in Europe, and 14% in North America (Figure 4) (Table 3).
This study demonstrated that the mean increase in elbow extension gained after surgery in North America was significantly greater when compared with studies from Asia, but the gain in flexion, pronation, and supination was similar across continents. The underlying cause of this difference in improvement in elbow extension between nations is unclear, although differences in diagnosis could account for some variation. This study did not examine differences in rehabilitation protocols, and certainly, it is plausible that protocol variations by country could account for some discrepancy. Furthermore, differences in functional needs may vary by continent and could have driven this result.
This study found no routine reporting of outcome scores by elbow arthroscopy studies from any continent, and that when outcome scores are reported, there is substantial inconsistency with regard to the actual scoring system used. No continent reported outcome scores in more than 40% of the studies published from that area, and the variation of outcome scores used, even from a single region, was large. This makes comparing clinical outcomes between studies difficult, even when performing identical procedures for identical indications, because there is no standardized method of reporting outcomes. To allow comparison of studies and generalizability of the results to different populations, a more standardized approach to outcome reporting needs to be instituted in the elbow arthroscopy literature. To date, there is no standardized score that has been validated for reporting clinical outcomes after elbow arthroscopy.19 Hence, it is not surprising that there were 16 different outcome scores reported throughout the 112 studies analyzed in this review, with the most frequent score, the MEPS, reported in a total of 10 studies. As medicine moves towards pay scales that are based on patient outcomes, it will become more important to define a clear outcome score that can be used to assess these patients, and reliably report scores. This will allow comparison of patients across nations to determine the best surgical treatment for different clinical problems. A validation study comparing these outcome scores to determine which score best summarizes the patient’s level of pain and function after surgery would be beneficial, because this could identify 1 score that could be standardized to allow comparison among reported outcomes.
Limitations
This study had several limitations. Despite having 2 authors search independently for studies, some studies could have been missed during the search process, introducing possible selection bias. Including only published studies could have introduced publication bias. Numerous studies did not report all the variables the authors examined. This could have skewed some results, and had additional variables been reported, could have altered the data to show significant differences in some measured variables. Because this study did not compare outcome measures for varying pathologies, conclusions cannot be drawn on the best treatment options for different indications. Case reports could have lowered the MCMS score and the average in studies reporting outcomes. Furthermore, the poor quality of the underlying data used in this study could limit the validity/generalizability of the results because this is a systematic review, and its level of evidence is only as high as the studies it includes. Because the primary aim was to report on demographics, this study did not examine concomitant pathology at the time of surgery or rehabilitation protocols.
Conclusion
The quantity, but not the quality, of arthroscopic elbow publications has significantly increased over time. Most patients undergo elbow arthroscopy for lateral epicondylitis, OCD, and release and débridement. Pathology and indications do not appear to differ geographically with more men undergoing elbow arthroscopy than women.
Although elbow arthroscopy was first described in the 1930s, it has become increasingly popular in the last 30 years.1 While initially considered as a tool for diagnosis and loose body removal, indications have expanded to include treatment of osteochondritis dissecans (OCD), treatment of lateral epicondylitis, fixation of fractures, and others.2-5 Miyake and colleagues6 found a significant improvement in range of motion, both flexion and extension, and outcome scores when elbow arthroscopy was used to remove impinging osteophytes. Babaqi and colleagues7 found significant improvement in pain, satisfaction, and outcome scores in 31 patients who underwent elbow arthroscopy for lateral epicondylitis refractory to nonsurgical management. The technical difficulty of the procedure, lower frequency of pathology amenable to arthroscopic intervention, and potential neurovascular complications make the elbow less frequently evaluated with the arthroscope vs other joints, such as the knee and shoulder.2,8,9
Geographic distribution of subjects undergoing elbow arthroscopy, the indications used, surgical techniques being performed, and their associated clinical outcomes have received little to no recognition in the peer-reviewed literature.10 Differences in the elbow arthroscopy literature include characteristics related to the patient (age, gender, hand dominance, duration of symptoms), study (level of evidence, number of subjects, number of participating centers, design), indication (lateral epicondylitis, loose bodies, olecranon osteophytes, OCD), surgical technique, and outcome. Evidence-based medicine and clinical practice guidelines direct surgeons in clinical decision-making. Payers investigate the cost of surgical interventions and the value that surgery may provide, while following trends in different surgical techniques. Regulatory agencies and associations emphasize subjective patient-reported outcomes as the primary outcome measured in high-quality trials. Thus, in discussion of complex surgical interventions such as elbow arthroscopy, it is important to characterize the studies, subjects, and surgeries across the world to understand the geographic similarities and differences to optimize care in this clinical situation.
The goal of this study was to perform a systematic review and meta-analysis of elbow arthroscopy literature to identify and compare the characteristics of the studies published, the subjects analyzed, and surgical techniques performed across continents and countries to answer these questions: “Across the world, what demographic of patients are undergoing elbow arthroscopy, what are the most common indications for elbow arthroscopy, and how good is the evidence?” The authors hypothesized that patients who undergo elbow arthroscopy will be largely age <40 years, the most common indication for elbow arthroscopy will be a release/débridement, and the evidence for elbow arthroscopy will be poor. Also, no significant differences will exist in elbow arthroscopy publications, subjects, outcomes, and techniques based on continent/country of publication.
Methods
A systematic review was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines using a PRISMA checklist.11 Systematic review registration was performed using the International Prospective Register of Ongoing Systematic Reviews (PROSPERO; registration number, CRD42014010580; registration date, July 15, 2014).12 Two study authors independently conducted the search on June 23, 2014 using the following databases: Medline, Cochrane Central Register of Controlled Trials, SportDiscus, and CINAHL. The electronic search citation algorithm used was: (elbow) AND arthroscopy) NOT shoulder) NOT knee) NOT ankle) NOT wrist) NOT hip) NOT dog) NOT cadaver). English language Level I-IV evidence (2012 update by the Oxford Centre for Evidence-Based Medicine13) clinical studies were eligible for inclusion into this study. Abstracts were ineligible for inclusion. All references in selected studies were cross-referenced for inclusion if they were missed during the initial search. Duplicate subject publications within separate unique studies were not reported twice. The study with longer duration follow-up, higher level of evidence, greater number of subjects, or more detailed subject, surgical technique, or outcome reporting was retained for inclusion. Level V evidence reviews, expert opinion articles, letters to the editor, basic science, biomechanical studies, open elbow surgery, imaging, surgical technique, and classification studies were excluded.
All included patients underwent elbow arthroscopy for either intra- or extra-articular elbow pathology (ulnotrochlear osteoarthritis, lateral epicondylitis, rheumatoid arthritis, post-traumatic contracture, osteonecrosis of the capitellum or radial head, osteoid osteoma, and others). There was no minimum follow-up duration or rehabilitation requirement. The study and subject demographic parameters that we analyzed included year of publication, years of subject enrollment, presence of study financial conflict of interest, number of subjects and elbows, elbow dominance, gender, age, body mass index, diagnoses treated, type of anesthesia (block or general), and surgical positioning. Postoperative splint application and pain management, and whether a continuous passive motion machine was used and whether a drain was placed were recorded. Clinical outcome scores were DASH (Disability of the Arm, Shoulder, and Hand), Morrey score, MEPS (Mayo Elbow Performance Score), Andrews-Carson score, Timmerman-Andrews score, LES (Liverpool Elbow Score), Tegner score, HSS (Hospital for Special Surgery Score), VAS (Visual Analog Scale), EFA (Elbow Functional Assessment), Short Form-12 (SF-12), Short Form-36 (SF-36), Kerlan-Jobe Orthopaedic Clinic (KJOC) Shoulder and Elbow Questionnaire, and MAESS (Modified Andrews Elbow Scoring System). Radiographs, computed tomography (CT), computed tomography arthrography (CTA), magnetic resonance imaging (MRI), and magnetic resonance arthrography (MRA) data were extracted when available. Range of motion (flexion, extension, supination, and pronation) and grip strength data, both preoperative and postoperative, were extracted when available. Study methodological quality was evaluated using the Modified Coleman Methodology Score (MCMS).14
Statistical Analysis
Study descriptive statistics were calculated. Continuous variable data were reported as weighted means ± weighted standard deviations. Categorical variable data were reported as frequencies with percentages. For all statistical analysis either measured and calculated from study data extraction or directly reported from the individual studies, P < .05 was considered statistically significant. Study, subject, and surgical outcomes data were compared using 1-way analysis of variance (ANOVA) tests. Where applicable, study, subject, and surgical outcomes data were also compared using 2-sample and 2-proportion Z-test calculators with α .05 because of the difference in sample sizes between compared groups. To examine trends over time, Pearson’s correlation coefficients were calculated. For the purposes of analysis, the indications of “osteoarthritis,” “arthrofibrosis,” “loose body removal,” “ulnotrochlear osteoarthritis causing stiffness,” “post-traumatic contracture/stiffness,” and “post-operative elbow contracture” were combined into the indication “release and débridement.” For the 3 most common indications for arthroscopy (OCD, lateral epicondylitis, and release and débridement) data were combined into 5-year increments to overcome the smaller sample size within each of these categories, and Pearson’s correlation coefficients were calculated to determine if number of reported cases covaried with year period. Within these 3 diagnoses, ANOVA analyses were performed to determine whether the number of cases differed between continents and countries.
Results
A total of 353 studies were located, and, after implementation of the exclusion criteria, 112 studies were included in the final analysis (Figure 1; 3093 subjects; 3168 elbows; 64% male; mean age, 34.9 ± 14.68 years). There was a mean of 33.4 ± 26.02 months of follow-up, and 75% of surgeries involved the dominant elbow (Table 1). Most studies were level IV evidence (94.6%), had a low MCMS (mean 28.1 ± 8.06; poor rating), and were single-center investigations (94.6%). Most studies did not report financial conflicts of interest (56.3%) (Tables 1 and 2). From 1985 through 2014, the number of publications significantly increased with time (P = .004) among all continents. The MCMS was unchanged over time (P = .247) (Figure 2A), as was the level of evidence (P = .094) (Figure 2B). Conflicts of interest significantly increased with time (P = .025) (Figure 3).
Among continents, North America published the largest number of studies (54), and had the largest number of patients (1395) and elbow surgeries (1425) (Table 1). The United States published the largest number of studies (43%). There were no significant differences between age (P = .331), length of follow-up (P = .403), MCMS (P = .123), and level of evidence (P = .288) between continents. Of the 32 studies that reported the use of preoperative MRI, studies from Asia reported significantly more MRI scans than those from other continents (P = .040); there were no other significant differences between continents in reference to preoperative imaging studies or other demographic information.
The most common surgical indications were OCD (Figure 4), lateral epicondylitis (Figure 5), and release and débridement (Figure 6, Table 3; all studies listed indications). The number of reported cases for these 3 indications significantly increased over time (OCD P = .005, lateral epicondylitis P = .044, release and débridement P = .042) but did not significantly differ between regions (P > .05 in all cases).
Thirty-two (28.6%) studies reported the use of outcome measures (16 different outcome scores were used by the included studies). Asia reported outcome measures in 9 of 23 studies (39%), Europe in 12 of 35 studies (34%) and North America in 11 of 54 (20%) of studies. The MEPS was the most frequently used outcome score in 9.8% of studies, followed by VAS for pain in 5.3% of cases. North American studies reported a significantly higher increase in extension after elbow arthroscopy than Asia (P = .0432) (Figure 7), with no differences in flexion (P = .699), pronation (P = .376), or supination (P = .408). No significant differences were observed between continents in the type of anesthesia chosen (general anesthesia [P = .94] or regional anesthesia [P = .85]). Asia and Europe performed elbow arthroscopy most frequently in the lateral decubitus position, while North American studies most often used the supine position (Table 4).
Twenty (17.9%) studies reported the use of a postoperative splint, 12 (10.7%) studies reported use of a drain, 2 (1.79%) studies reported use of a hinged elbow brace, 9 (8.03%) studies reported use of a continuous passive motion machine postoperatively, and 3 (2.68%) studies reported use of an indwelling axillary catheter for postoperative pain management. Of 130 reported surgical complications (4.1%), the most frequent complication was transient sensory ulnar nerve palsy (1.5%), followed by persistent wound drainage (.76%), and transient sensory radial nerve palsy (.38%). Other reported complications included infection (.22%), transient sensory palsy of the median nerve (.19%), heterotopic ossification (.13%), complete transection of the ulnar nerve (.10%), loose body formation (.06%), hematoma formation (.06%), transient sensory palsy of the posterior interosseous (.06%), or anterior interosseous nerve (.03%), and complete transection of the radial (.03%), or median nerve (.03%).
Discussion
Elbow arthroscopy is an evolving surgical procedure that is used to treat intra- and extra-articular pathologies of the elbow. Outcomes of elbow arthroscopy for certain conditions have generally been reported as good, with improvements seen in pain, functional scores, and range of motion.6,15-17 The authors’ hypotheses were mostly confirmed in that the average age of patients undergoing elbow arthroscopy was <40 years, release/débridement was one of the most common indications (along with lateral epicondylitis and OCD), and the general evidence for elbow arthroscopy was poor. Also, there were almost no differences between continents/countries related to patient indications, preoperative imaging, anesthesia choice, indications, postoperative protocols, and outcomes (although the number of studies that reported outcomes was low and could have skewed the results), with the exception of a higher number of preoperative MRI scans in Asia. Some of the notable findings of this study included: 1) the number of studies published on elbow arthroscopy is significantly increasing with time, despite a lack of improvement in the level of evidence; 2) the majority of studies on elbow arthroscopy do not report a surgical outcome score; and 3) the number of reported cases for the 3 most common indications significantly increased over time (OCD, P = .005; lateral epicondylitis, P = .044; release and débridement, P = .042) but did not differ between regions (P > .05 in all cases).
The indications for elbow arthroscopy have grown dramatically in the past 2 decades to include both intra- and extra-articular pathologies.18 Despite this increase in the number of indications for elbow arthroscopy, the study did not find a significant difference between countries/continents in the indications each used for elbow arthroscopy patients. There was a trend towards an increase in OCD cases in all continents, especially Asia (Figure 4), with time. Interestingly, while not statistically significant, there was variation among countries for surgical indications. In North America, removal of loose bodies accounts for 18% of patients, while in Europe this accounted for only 9% and in Asia for 1%. Post-traumatic stiffness was the indication for elbow arthroscopy in Europe in 19% of patients vs 7% in North America and 10% in Asia. In Asia, OCD accounts for 40% of arthroscopies, 7% in Europe, and 14% in North America (Figure 4) (Table 3).
This study demonstrated that the mean increase in elbow extension gained after surgery in North America was significantly greater when compared with studies from Asia, but the gain in flexion, pronation, and supination was similar across continents. The underlying cause of this difference in improvement in elbow extension between nations is unclear, although differences in diagnosis could account for some variation. This study did not examine differences in rehabilitation protocols, and certainly, it is plausible that protocol variations by country could account for some discrepancy. Furthermore, differences in functional needs may vary by continent and could have driven this result.
This study found no routine reporting of outcome scores by elbow arthroscopy studies from any continent, and that when outcome scores are reported, there is substantial inconsistency with regard to the actual scoring system used. No continent reported outcome scores in more than 40% of the studies published from that area, and the variation of outcome scores used, even from a single region, was large. This makes comparing clinical outcomes between studies difficult, even when performing identical procedures for identical indications, because there is no standardized method of reporting outcomes. To allow comparison of studies and generalizability of the results to different populations, a more standardized approach to outcome reporting needs to be instituted in the elbow arthroscopy literature. To date, there is no standardized score that has been validated for reporting clinical outcomes after elbow arthroscopy.19 Hence, it is not surprising that there were 16 different outcome scores reported throughout the 112 studies analyzed in this review, with the most frequent score, the MEPS, reported in a total of 10 studies. As medicine moves towards pay scales that are based on patient outcomes, it will become more important to define a clear outcome score that can be used to assess these patients, and reliably report scores. This will allow comparison of patients across nations to determine the best surgical treatment for different clinical problems. A validation study comparing these outcome scores to determine which score best summarizes the patient’s level of pain and function after surgery would be beneficial, because this could identify 1 score that could be standardized to allow comparison among reported outcomes.
Limitations
This study had several limitations. Despite having 2 authors search independently for studies, some studies could have been missed during the search process, introducing possible selection bias. Including only published studies could have introduced publication bias. Numerous studies did not report all the variables the authors examined. This could have skewed some results, and had additional variables been reported, could have altered the data to show significant differences in some measured variables. Because this study did not compare outcome measures for varying pathologies, conclusions cannot be drawn on the best treatment options for different indications. Case reports could have lowered the MCMS score and the average in studies reporting outcomes. Furthermore, the poor quality of the underlying data used in this study could limit the validity/generalizability of the results because this is a systematic review, and its level of evidence is only as high as the studies it includes. Because the primary aim was to report on demographics, this study did not examine concomitant pathology at the time of surgery or rehabilitation protocols.
Conclusion
The quantity, but not the quality, of arthroscopic elbow publications has significantly increased over time. Most patients undergo elbow arthroscopy for lateral epicondylitis, OCD, and release and débridement. Pathology and indications do not appear to differ geographically with more men undergoing elbow arthroscopy than women.
1. Khanchandani P. Elbow arthroscopy: review of the literature and case reports. Case Rep Orthop. 2012;2012:478214.
2. Dodson CC, Nho SJ, Williams RJ 3rd, Altchek DW. Elbow arthroscopy. J Am Acad Orthop Surg. 2008;16(10):574-585.
3. Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 Pt 1):47-62.
4. Kelly EW, Morrey BF, O’Driscoll SW. Complications of elbow arthroscopy. J Bone Joint Surg Am. 2001;83-A(1):25-34.
5. Rajeev A, Pooley J. Lateral compartment cartilage changes and lateral elbow pain. Acta Orthop Belg. 2009;75(1):37-40.
6. Miyake J, Shimada K, Oka K, et al. Arthroscopic debridement in the treatment of patients with osteoarthritis of the elbow, based on computer simulation. Bone Joint J. 2014;96-B(2):237-241.
7. Babaqi AA, Kotb MM, Said HG, AbdelHamid MM, ElKady HA, ElAssal MA. Short-term evaluation of arthroscopic management of tennis elbow; including resection of radio-capitellar capsular complex. J Orthop. 2014;11(2):82-86.
8. Gay DM, Raphael BS, Weiland AJ. Revision arthroscopic contracture release in the elbow resulting in an ulnar nerve transection: a case report. J Bone Joint Surg Am. 2010;92(5):1246-1249.
9. Haapaniemi T, Berggren M, Adolfsson L. Complete transection of the median and radial nerves during arthroscopic release of post-traumatic elbow contracture. Arthroscopy. 1999;15(7):784-787.
10. Yeoh KM, King GJ, Faber KJ, Glazebrook MA, Athwal GS. Evidence-based indications for elbow arthroscopy. Arthroscopy. 2012;28(2):272-282.
11. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. BMJ. 2009;339:b2700.
12. PROSPERO. International Prospective Register of Ongoing Systematic Reviews. The University of York CfRaDP-Iprosr-v. 2013 [cited 2014]. http://www.crd.york.ac.uk/PROSPERO/. Accessed March 17, 2016.
13. Oxford Centre for Evidence-Based Medicine - levels of evidence (March 2009). Centre for Evidence-Based Medicine Web site. http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed July 6, 2016.
14. Cowan J, Lozano-Calderόn S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
15. Jones GS, Savoie FH 3rd. Arthroscopic capsular release of flexion contractures (arthrofibrosis) of the elbow. Arthroscopy. 1993;9(3):277-283.
16. O’Brien MJ, Lee Murphy R, Savoie FH 3rd. A preliminary report of acute and subacute arthroscopic repair of the radial ulnohumeral ligament after elbow dislocation in the high-demand patient. Arthroscopy. 2014;30(6):679-687.
17. Rhyou IH, Kim KW. Is posterior synovial plica excision necessary for refractory lateral epicondylitis of the elbow? Clin Orthop Relat Res. 2013;471(1):284-290.
18. Jerosch J, Schunck J. Arthroscopic treatment of lateral epicondylitis: indication, technique and early results. Knee Surg Sports Traumatol Arthrosc. 2006;14(4):379-382.
19. Tijssen M, van Cingel R, van Melick N, de Visser E. Patient-Reported Outcome questionnaires for hip arthroscopy: a systematic review of the psychometric evidence. BMC Musculoskelet Disord. 2011;12:117.
1. Khanchandani P. Elbow arthroscopy: review of the literature and case reports. Case Rep Orthop. 2012;2012:478214.
2. Dodson CC, Nho SJ, Williams RJ 3rd, Altchek DW. Elbow arthroscopy. J Am Acad Orthop Surg. 2008;16(10):574-585.
3. Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 Pt 1):47-62.
4. Kelly EW, Morrey BF, O’Driscoll SW. Complications of elbow arthroscopy. J Bone Joint Surg Am. 2001;83-A(1):25-34.
5. Rajeev A, Pooley J. Lateral compartment cartilage changes and lateral elbow pain. Acta Orthop Belg. 2009;75(1):37-40.
6. Miyake J, Shimada K, Oka K, et al. Arthroscopic debridement in the treatment of patients with osteoarthritis of the elbow, based on computer simulation. Bone Joint J. 2014;96-B(2):237-241.
7. Babaqi AA, Kotb MM, Said HG, AbdelHamid MM, ElKady HA, ElAssal MA. Short-term evaluation of arthroscopic management of tennis elbow; including resection of radio-capitellar capsular complex. J Orthop. 2014;11(2):82-86.
8. Gay DM, Raphael BS, Weiland AJ. Revision arthroscopic contracture release in the elbow resulting in an ulnar nerve transection: a case report. J Bone Joint Surg Am. 2010;92(5):1246-1249.
9. Haapaniemi T, Berggren M, Adolfsson L. Complete transection of the median and radial nerves during arthroscopic release of post-traumatic elbow contracture. Arthroscopy. 1999;15(7):784-787.
10. Yeoh KM, King GJ, Faber KJ, Glazebrook MA, Athwal GS. Evidence-based indications for elbow arthroscopy. Arthroscopy. 2012;28(2):272-282.
11. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. BMJ. 2009;339:b2700.
12. PROSPERO. International Prospective Register of Ongoing Systematic Reviews. The University of York CfRaDP-Iprosr-v. 2013 [cited 2014]. http://www.crd.york.ac.uk/PROSPERO/. Accessed March 17, 2016.
13. Oxford Centre for Evidence-Based Medicine - levels of evidence (March 2009). Centre for Evidence-Based Medicine Web site. http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed July 6, 2016.
14. Cowan J, Lozano-Calderόn S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
15. Jones GS, Savoie FH 3rd. Arthroscopic capsular release of flexion contractures (arthrofibrosis) of the elbow. Arthroscopy. 1993;9(3):277-283.
16. O’Brien MJ, Lee Murphy R, Savoie FH 3rd. A preliminary report of acute and subacute arthroscopic repair of the radial ulnohumeral ligament after elbow dislocation in the high-demand patient. Arthroscopy. 2014;30(6):679-687.
17. Rhyou IH, Kim KW. Is posterior synovial plica excision necessary for refractory lateral epicondylitis of the elbow? Clin Orthop Relat Res. 2013;471(1):284-290.
18. Jerosch J, Schunck J. Arthroscopic treatment of lateral epicondylitis: indication, technique and early results. Knee Surg Sports Traumatol Arthrosc. 2006;14(4):379-382.
19. Tijssen M, van Cingel R, van Melick N, de Visser E. Patient-Reported Outcome questionnaires for hip arthroscopy: a systematic review of the psychometric evidence. BMC Musculoskelet Disord. 2011;12:117.
Medical Therapy for Osteoporosis and Approaches to Improving Adherence
From the Division of Endocrinology, Diabetes, and Metabolism, University of Alabama at Birmingham, Birmingham, Alabama.
Abstract
- Objective: To review the treatment of osteoporosis, challenges to treatment adherence, and factors associated with improved adherence.
- Methods: Review of the literature.
- Results: With the growing aging population, there is an increased number of people at risk of osteoporosis and fracture. Several medications are available that reduce the risk of fracture. However, adherence to osteoporosis medications is suboptimal. Factors related to nonadherence include dosing frequency, real side effects, and concern about potential side effects. Interventions that may improve adherence include clinician and patient education, less frequent and less complex dosing regimens, medication reminders, and adherence counseling.
- Conclusions: Improving adherence to osteoporosis medications is a complex and challenging issue. Considering and implementing strategies to improve adherence tailored to patient preferences may enhance long-term outcomes for patients with osteoporosis.
Osteoporosis is a chronic but asymptomatic disease that is characterized by an increased fragility of bones and increased risk of fractures. Hip and vertebral fractures are associated with the greatest morbidity and mortality. The prevalence of osteoporosis is estimated to be 10.3% in the US, with approximately 10.2 million adults over the age of 50 having osteoporosis based on 2010 census data and results from the National Health and Nutrition Examination Survey (NHANES) [1].
Several drugs are currently available for the treatment of osteoporosis, but adherence to treatment is low. Understanding the factors associated with low adherence and actions that can be taken to improve adherence to treatment is important given the large number of individuals with osteoporosis and the need to reduce the burden caused by fragility fracture. In this article, we review the treatment of osteoporosis, challenges to treatment adherence, and factors associated with improved adherence.
Nonprescription Medications
Calcium
There have been several published studies over the last decade evaluating calcium supplementation and its efficacy in reducing fractures. Although these studies showed that calcium reduces bone turnover by 20% and slowed postmenopausal bone loss by one third [2,3], none of these studies or a recent systematic review [4] showed any degree of fracture risk reduction with calcium supplements alone.
Although some calcium intake may be good, too much calcium has the potential to cause harm, including an increased risk of nephrolithiasis and constipation/bloating. An analysis of the Women’s Health Initiative (WHI) study reported a 17% increase in renal calculi in women who received calcium and vitamin D supplements [5]. Another recently published meta-analysis showed a 43% increase in gastrointestinal complaints in patients who were taking calcium supplements [6]. The potential for increased cardiovascular risk with calcium supplements is controversial [7]. The WHI study did not show an increased occurrence of cardiovascular events among those taking calcium supplements [8]. In a different population, men who consumed more than 1000 mg per day of supplemental calcium had higher all-cause and cardiovascular disease-specific mortality [9]. Large, well-conducted randomized controlled trials will be needed to further elucidate the question of calcium supplementation and risk of cardiovascular disease.
Vitamin D
Deficiency of vitamin D is common with one study finding more than 90% of older adults deficient in vitamin D [10]. Vitamin D is essential for proper calcium metabolism and deficiency is known to induce secondary hyperparathyroidism. Studies in mouse models have also shown that normal vitamin D receptors in enterocytes are essential for normal bone mineralization [11,12]. A systematic Cochrane database review showed that vitamin D3 supplementation decreased mortality in elderly people living independently or in institutional care [13]. Vitamin D was administered for a weighted mean of 4.4 years. Vitamin D2, alfacalcidol, and calcitriol had no statistically significant beneficial effects on mortality. Vitamin D3 combined with calcium was associated with an increased risk of nephrolithiasis during a follow-up period of 1.25 to 7 years (relative risk [RR] 1.17, 95% confidence interval (CI) 1.02–1.34) [13]. The inconsistencies of published reports looking at benefits of vitamin D supplementation may be due in part to variability in compliance with taking the supplements and baseline vitamin D levels.
Two randomized controlled trials have shown that low vitamin D appears to be an independent predictor of fall risk, and vitamin D supplementation has been found to reduce this risk of falls, through improved musculo-skeletal function [14–16]. Thus, vitamin D may play a role in fracture risk reduction beyond direct bone effects.
Prescription Osteoporosis Treatments
Bisphosphonates
Bisphosphonates are the most commonly prescribed medication for osteoporosis. The efficacy of bisphosphonates to reduce fractures is well established. There are oral bisphosphonates, which can be dosed daily, weekly, or monthly, and intravenous bisphosphonates, which can be given every 3 months or annually. Side effects with this class of medications include gastrointestinal effects with the oral options in up to 20% to 30% of users [17]. With intravenous bisphosphonates, the greatest risk is an acute phase response, which can occur in up to 42% of patients [18]. The risk of an acute phase reaction is much lower with doses beyond the first dose and lower if patients have ever previously taken an oral bisphosphonate and/or receive acetaminophen prior to the infusion. Other potential side effects with all bisphosphonates include osteonecrosis of jaw (ONJ) and atypical subtrochanteric fractures. Post marketing studies have indicated that the incidence of ONJ is less than 2 per 100,000 patient-years among those taking bisphosphonates [19,20]. A number of database analyses have shown that ONJ-like lesions can also occur in older individuals with osteoporosis who have never been exposed to bisphosphonates [21]. A case series of an osteoporotic population showed that ONJ-like lesions are lower grade than those typically seen in cancer patients who usually are exposed to higher doses of bisphosphonates [22]. A study of Swedish older men and women reported that long-term use of bisphosphonates (4 years or more) was associated with an increased incidence of atypical fractures. The RR for women was 126.0 (95% CI, 55.1–288.1) after 4 years of bisphosphonates [23]. A U.S. health care database analysis reported that 90% of those with atypical fractures were bisphosphonate users, almost half were Asian (49%), and use beyond 6 years showed the greatest risk [24].
Non-Bisphosphonate Medications
Other osteoporosis medications include denosumab, raloxifene, estrogen, and teriparatide (calcitonin will not be discussed here). Newer options currently under study, including cathepsin K inhibitors and anti-sclerostin therapies, are not available in the United States.
Denosumab is a monoclonal antibody that interferes with the receptor activator of nuclear kappa B ligand (RANK-L), which is the principal stimulus for osteoclastogenesis. Denosumab is administered once every 6 months subcutaneously. Phase III trials of denosumab demonstrated a 68% reduction in vertebral fractures and 40% and 20% reduction in fractures at hip and non-vertebral sites, respectively [25]. Similar to bisphosphonates, other risks include atypical femoral fractures and ONJ. In addition, hypocalcemia, including severe, symptomatic hypocalcemia, has been reported at rates higher than initially reported in the original clinical trials [26]. Hypocalcemia can be severe, especially in patients who are deficient in vitamin D [10,27].
Estrogen is effective in reducing the risk of vertebral fractures. Selective estrogen receptor modulators (SERMs) have both estrogen agonist and antagonist effects. The SERM, raloxifene, has been used in osteoporosis for its antiresorptive effects through the estrogen receptor [28,29]. A newer SERM, bazedoxifene, has been studied in combination with conjugated estrogen and has been reported to improve bone mineral density and other symptoms of menopause, like vasomotor symptoms and vulvo-vaginal atrophy, but its efficacy in reducing fracture risk has not been demonstrated [30,31].
Teriparatide is an anabolic agent that works by stimulating osteoblastic bone formation which results in an increase in bone density and reduction in both vertebral and non-vertebral fracture risk. In women with postmenopausal osteoporosis, it is typically reserved for those with very low bone mineral density (BMD) or those who continue to have fractures despite a bisphosphonate [32]. Barriers to use of teriparatide include high cost, the need for daily injections, and approved use for a total of two years in a lifetime. There is also a theoretical risk of osteosarcoma shown in animal studies but human cases have not been reported when used for postmenopausal osteoporosis. Published studies have shown that combination zolendronate and teriparatide have additive benefits to spine and hip BMD [33]. Another study reported that the combination of denosumab and teriparatide resulted in additive effects, ie, an increase in lumbar, hip, and femoral neck BMD [34]. These combinations have not been studied in populations large enough or for long enough duration to evaluate fracture risk reduction.
Adherence to Osteoporosis Medications
Treatment of osteoporosis reduces risk of fracture, but the benefit of osteoporosis medications is dependent on adherence. Adherence is associated with improved clinical outcomes [35,36] as well as reduced costs and utilization [37,38]; however, adherence to osteoporosis medications is poor. In a meta-analysis of 24 observational studies conducted in large populations, overall adherence for all osteoporosis therapies ranged from approximately 40% to 70% [39]. A recent retrospective claims database analysis in the U.S. reported a 60% noncompliance rate among the 57,913 postmenopausal women prescribed bisphosphonates over 1 year [40]. Another administrative database analysis from a managed care population compared the 3 oral bisphosphonates (risedronate, ibandronate, and alendronate) and found a mean medication possession ratio (MPR) between 0.57–0.58 at 12 months, which dropped to 0.47–0.50 after 24 months and 0.44–0.47 after 36 months [41]. In an observational study of 3200 older women in the U.K. low adherence was self-reported in 8.5%, and 21.6% self-discontinued treatment within 2 years [42]. In a study of Medicare Advantage prescription drug plan members, a small but significant increase in adherence was seen after osteo-porosis treatment change but overall adherence remained low (51% MPR in the change cohort and vs. 44% in the no-change cohort at 24 months, P < 0.01) [43].
Some patients restart osteoporosis therapy after a prolonged lapse in medication use. In one study, re-initiation rates for bisphosphonate therapy among persons who discontinued were as high as 30% within 6 months and 50% within 2 years [44]. Predictors of treatment re-initiation included younger age, female sex, history of fracture, recent hip fracture, nursing home discharge, and BMD testing [44].
Factors that Impact Adherence
Understanding which patients are most likely to be compliant with medications can aid physicians when monitoring osteoporosis treatment responses. In a retro-spective claims analysis, older age was found to be a predictor of compliance: women 65 years and older were more likely to be compliant than younger patients (P = 0.012) [45]. Among women receiving denosumab, improved adherence was found among women with a family history of a parent with a hip fracture, and lower adherence was seen in those with higher age, decreased mobility, and further distance from the clinic where the medication was provided [46].
Major reasons for nonadherence include a fear of potential side effects, occurrence of real side effects, the complicated dosing regimens, and perceived lack of benefit from the medications due to the asymptomatic nature of osteoporosis. In the above noted observational study from the U.K., more than half of the nonadherent patients attributed their nonadherence to side effects (53.9%), with a smaller proportion reporting fear of potential side effects (20.5%) or trouble with the dosing regimen (8.0%)[42].
Patients may also be unwilling to continue to take an osteoporosis medication if a fracture develops while on it and if they are not otherwise provided evidence that the medication is working. In a study by Costa Paiva et al, an understanding and knowledge to osteoporosis was a prerequisite to adherence and the strongest predictor of knowledge was higher education level [47]. Factors that impaired adherence were lower socioeconomic status and presence of comorbidities [47]. In a phenomenological qualitative study, trust in a health care provider was the most common reason for patients’ decision to accept an osteoporosis medication, emphasizing the importance of physician-patient communication [48].
Interventions to Enhance Adherence
Current methods of improving adherence for chronic health problems are mostly complex and not very effective [49]. In a systematic review of interventions to improve medication adherence, only 37 out of 81 studies reported improved adherence in the treatment of chronic diseases, and multifaceted treatments were more likely to succeed [49]. Improving adherence to osteoporosis medications is a complex issue, and a number of interventions evaluated in systematic reviews have shown limited efficacy [50,51]. Simplification of dosing regimens have been found to have a significant impact in chronic disease management [52,53] as well as in some studies of osteoporosis medications.
Simplification of Dosing
Among women prescribed daily vs. weekly bisphosphonates, those on the weekly regimen had significantly higher compliance [54]. However, rates were suboptimal in both groups and more than 50% of women discontinued at 1 year [54]. In addition, in a meta-analysis of osteoporosis medication adherence, a nearly two-fold higher odds of discontinuation with daily vs. weekly bisphosphonates was seen (odds ratio 1.90, 95% CI 1.81–2.00) [55]. Likewise, in a retrospective study in Spain, nearly 85% of those started on a daily bisphosphonate stopped within a year [56], while discontinuation was significantly lower in those prescribed a weekly or monthly bisphosphonate or daily teriparatide; however, discontinuation was still nearly 50% in these groups [56].
Once monthly dosing may be preferred by some patients as there is less time involved in thinking about the disease being treated and a perception of lower likelihood of side effects. In one study, postmenopausal women who had previously stopped oral bisphosphonates due to GI side effects had high adherence rates after self-selecting either monthly oral or quarterly intravenous ibandronate therapy [57]. However, not all studies show significant differences in adherence between weekly and monthly preparations [58–60].
The newer parenteral treatment options that can be given every 6 months or once yearly have the potential to significantly improve adherence. Once a year parenteral administration of a bisphosphonate was preferred over once-weekly oral administration, according to a 1-year study in patients with low bone density previously treated with alendronate [61]. A recent study that looked at persistence with an infusion of zolendronic acid in Taiwanese patients for 48 months found that 85% of patients received at least 2 infusions [62]. In patients treated with denosumab in 4 European countries, adherence and persistence at 12 months were consistently > 80% [46]. Persistence in this study was defined as receiving the subsequent injection within 6 months ± 8 weeks of the previous injection; adherence was defined as receiving 2 consecutive injections within 6 months ± 4 weeks of each other [46].
In a study by Cramer et al, increased adherence and persistence was seen with weekly alendronate compared daily alendronate at the end of 12 months [54]. Similar results were seen in a large longitudinal cohort study of weekly vs. daily bisphosphonates but less than 50% of patients were adherent with the weekly regimen [63]. When once monthly preparations of bisphosphonates became available, studies continued to support a patient preference for less frequently dosed bisphosphonates, with the majority of patients preferring monthly over weekly dosed medications [64–66].
The availability of quarterly ibandronate and yearly zoledronic acid infusions have further simplified dosing. In large, randomized, multicenter studies, patients consistently expressed a preference for yearly infusions over a weekly oral medication [61,67]. Adherence and persistence to osteoporosis medications was also greater in women receiving intravenous ibandronate compared to those receiving oral alendronate [68,69]. However, a study by Curtis et al showed low persistence with intravenous bisphosphonates in a Medicare population [70]. A possible reason for the lower adherence in this population was postulated to include the provision of the infusions at an outpatient center rather than a physician office. Automated nursing reminders with either phone calls or emails have the potential to mitigate the problem of persistence with this less frequent regimen [71,72]. In a review of patient preferences, less frequent dosing of medications was a common desire, but further generalizability were limited, emphasizing the need to individualize treatment [73].
Patient-Provider Communication
Individualizing treatment with better patient-provider communication and identification of potential barriers may increase compliance [74]. In one study, increasing patient participation in determining the treatment option was associated with improved patient adherence [57]. A systematic review of literature on interventions to improve adherence found that periodic follow-up interaction between patients and their health professionals also improved adherence [50]. Positive reinforcement via physician-patient discussion of either bone turnover markers or bone mineral density test results has also been found to improve long-term adherence with osteoporosis medications [71,75].
Better perceived physician knowledge may help with patient adherence. A study by Pickney et al reported that the patient confidence in their health care providers has influence on improved adherence, and patients were more likely to comply when the medications were prescribed by a specialist rather than a general practitioner [76].
Education, Reminders, Phone-Based
Improving patient knowledge of osteoporosis, especially with education using visual aids, may help with improving adherence [47]. In a randomized controlled trial at a single health management organization, an interactive voice response phone call plus a letter 1 week later increased the rate of obtaining a prescribed oral bisphosphonate in the intervention group (48.8% vs. 30.5% control; OR 2.3, 95% CI 1.34–3.94) when adjusted for age, sex, prior BMD, and fracture [77]. Use of an encounter decision aid also improved knowledge of osteoporosis medication options and led to a doubling of medication prescription attainment. However, adherence at 6 months was not improved [78].
Pill reminders in the form of text messages, paging systems on medication devices, and alarm beeps have been studied in patients with chronic diseases, and these technologies could be utilized for osteoporosis treatment [79–81]. A study of smart phone applications showed that many apps help with adherence, especially in noncompliant patients [82]. The researchers reported that of apps studied, MyMedSchedule, MyMeds, and RxmindMe were among the most highly rated due to their ease of use and enhanced functions. Solomon et all studied the effectiveness of a telephone-based counseling program using motivational interviewing in a large randomized study. They found no significant improvement in adherence to an osteoporosis regimen with the telephonic motivational interview compared to mailed educational materials (control group) (P = 0.07) [83]. In a 12-month multicenter, prospective randomized study, Bianchi et al examined the effectiveness of an intervention of reminders or reminders plus phone calls and meetings at the referral center in postmenopausal women initiating an oral osteoporosis prescription. No significant difference was seen in adherence at 12 months compared to standard care [84]. Adherence among the entire cohort, however, was very high [84]
Pharmacist-Based
The role of pharmacists in the treatment of chronic diseases, including osteoporosis, has been studied and shown to be cost-effective. In a study by van Boven et al, an algorithm was designed to detect patients with nonadherence and then tailor an intervention that consisted of structured counseling and active monitoring by pharmacists in initial and continuous phases [85]. This effort-intensive intervention resulted in reduced discontinuation of bisphosphonates after 12 months (reduction from 31.7% to 16.2% at 12 months) [85]. Despite the effort required, findings from the study support overall cost-effectiveness of this intervention [85]. A randomized controlled study by Lai et al showed that pharmacists can play a role in improving medication adherence through counseling patients on the importance of adherence, side effects, and goals of therapy [86]. The same authors also showed that involvement of a clinical pharmacist in the care of patients helped to further improve patient knowledge of medications and osteoporosis treatments, resolve medication-related concerns, and improve overall quality of life [87]. Such pharmacist-led interventions would require pharmacists to understand their role and the potential for drug holidays in the course of osteoporosis treatments and not mislabel patients as nonadherent when in fact purposefully holding osteoporosis medications [88].
Conclusion
Osteoporosis is a growing problem with increasing numbers of patients at risk for osteoporosis and related fractures. Currently available osteoporosis medications have shown clear benefit in reducing fracture risk; however, adherence to these therapies is required to obtain benefit. Unfortunately, osteoporosis medications have several limitations to full compliance, particularly the oral treatment options, including known possible side effects acutely and chronically, potential/feared side effects, irregular dosing intervals, complicated dosing instructions, and absence of an immediate recognizable benefit/effect. Improving adherence is complex [89] and tailoring to individual patients is of importance. Successful techniques for improving adherence may include a focus on physician-patient communication, use of the less frequently dosed medications, various medication reminders, use of available technology, and use of pharmacists for patient counseling and monitoring. Recognition of this common problem by clinicians is of utmost importance.
Corresponding author: Amy H. Warriner, MD, The University of Alabama at Birmingham, Division of Endocrinology, Diabetes and Metabolism, 702 Faculty Office Tower, 510 20th Street South, Birmingham, AL 35294, [email protected].
Financial disclosures: None.
1. Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520–6.
2. Reid IR, Mason B, Horne A, et al. Randomized controlled trial of calcium in healthy older women. Am J Med. 2006;119(9):777–85.
3. Reid IR, Ames R, Mason B, et al. Randomized controlled trial of calcium supplementation in healthy, nonosteoporotic, older men. Arch Intern Med. 2008;168(20):2276–82.
4. Murad MH, Drake MT, Mullan RJ, et al. Clinical review. Comparative effectiveness of drug treatments to prevent fragility fractures: a systematic review and network meta-analysis. J Clin Endocrinol Metab. 2012;97(6):1871–80.
5. Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669–83.
6. Lewis JR, Zhu K, Prince RL. Adverse events from calcium supplementation: relationship to errors in myocardial infarction self-reporting in randomized controlled trials of calcium supplementation. J Bone Miner Res. 2012;27(3):719–22.
7. Challoumas D, Stavrou A, Pericleous A, Dimitrakakis G. Effects of combined vitamin D--calcium supplements on the cardiovascular system: should we be cautious? Atherosclerosis. 2015;238(2):388–98.
8. Hsia J, Heiss G, Ren H, et al. Calcium/vitamin D supplementation and cardiovascular events. Circulation. 2007;115(7):846–54.
9. Yang B, Campbell PT, Gapstur SM, et al. Calcium intake and mortality from all causes, cancer, and cardiovascular disease: the Cancer Prevention Study II Nutrition Cohort. Am J Clin Nutr. 2016;103(3):886–94.
10. Reid IR. Efficacy, effectiveness and side effects of medications used to prevent fractures. J Intern Med. 2015;277(6):690–706.
11. Xue Y, Fleet JC. Intestinal vitamin D receptor is required for normal calcium and bone metabolism in mice. Gastroenterology. 2009;136(4):1317-27, e1–2.
12. Lieben L, Masuyama R, Torrekens S, et al. Normocalcemia is maintained in mice under conditions of calcium malabsorption by vitamin D-induced inhibition of bone mineralization. J Clin Invest. 2012;122(5):1803–15.
13. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2014;1:CD007470.
14. Flicker L, Mead K, MacInnis RJ, et al. Serum vitamin D and falls in older women in residential care in Australia. J Am Geriatr Soc. 2003;51(11):1533–8.
15. Bischoff-Ferrari HA, Orav EJ, Dawson-Hughes B. Effect of cholecalciferol plus calcium on falling in ambulatory older men and women: a 3-year randomized controlled trial. Arch Intern Med. 2006;166(4):424–30.
16. Flicker L, MacInnis RJ, Stein MS, et al. Should older people in residential care receive vitamin D to prevent falls? Results of a randomized trial. J Am Geriatr Soc. 2005;53(11):1881–8.
17. Biswas PN, Wilton LV, Shakir SA. Pharmacovigilance study of alendronate in England. Osteoporos Int. 2003;14(6):507–14.
18. Reid IR, Gamble GD, Mesenbrink P, et al. Characterization of and risk factors for the acute-phase response after zoledronic acid. J Clin Endocrinol Metab. 2010;95(9):4380–7.
19. Grbic JT, Landesberg R, Lin SQ, et al. Incidence of osteonecrosis of the jaw in women with postmenopausal osteoporosis in the health outcomes and reduced incidence with zoledronic acid once yearly pivotal fracture trial. J Am Dent Assoc. 2008;139(1):32–40.
20. Grbic JT, Black DM, Lyles KW, et al. The incidence of osteonecrosis of the jaw in patients receiving 5 milligrams of zoledronic acid: data from the health outcomes and reduced incidence with zoledronic acid once yearly clinical trials program. J Am Dent Assoc. 2010;141(11):1365–70.
21. Lin TC, Yang CY, Kao Yang YH, Lin SJ. Incidence and risk of osteonecrosis of the jaw among the Taiwan osteoporosis population. Osteoporos Int. 2014;25(5):1503–11.
22. Assael LA. Oral bisphosphonates as a cause of bisphosphonate-related osteonecrosis of the jaws: clinical findings, assessment of risks, and preventive strategies. J Oral Maxillofac Surg. 2009;67(5 Suppl):35–43.
23. Schilcher J, Koeppen V, Aspenberg P, Michaelsson K. Risk of atypical femoral fracture during and after bisphosphonate use. N Engl J Med. 2014;371(10):974–6.
24. Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res. 2012;27(12):2544–50.
25. Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–65.
26. Yerram P, Kansagra S, Abdelghany O. Incidence of hypocalcemia in patients receiving denosumab for prevention of skeletal-related events in bone metastasis. J Oncol Pharm Pract. 2016.
27. Reid IR. Short-term and long-term effects of osteoporosis therapies. Nat Rev Endocrinol. 2015;11(7):418–28.
28. Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282(7):637–45.
29. Reid IR, Eastell R, Fogelman I, et al. A comparison of the effects of raloxifene and conjugated equine estrogen on bone and lipids in healthy postmenopausal women. Arch Intern Med. 2004;164(8):871–9.
30. Sharifi M, Lewiecki EM. Conjugated estrogens combined with bazedoxifene: the first approved tissue selective estrogen complex therapy. Expert Rev Clin Pharmacol. 2014;7(3):281–91.
31. Mirkin S, Ryan KA, Chandran AB, Komm BS. Bazedoxifene/conjugated estrogens for managing the burden of estrogen deficiency symptoms. Maturitas. 2014;77(1):24–31.
32. Warriner AH, Saag KG. Prevention and treatment of bone changes associated with exposure to glucocorticoids. Curr Osteoporos Rep. 2013;11(4):341–7.
33. Cosman F, Eriksen EF, Recknor C, et al. Effects of intravenous zoledronic acid plus subcutaneous teriparatide [rhPTH(1-34)] in postmenopausal osteoporosis. J Bone Miner Res. 2011;26(3):503–11.
34. Leder BZ, Tsai JN, Uihlein AV, et al. Two years of Denosumab and teriparatide administration in postmenopausal women with osteoporosis (The DATA Extension Study): a randomized controlled trial. J Clin Endocrinol Metab. 2014;99(5):1694–700.
35. Imaz I, Zegarra P, Gonzalez-Enriquez J, et al. Poor bisphosphonate adherence for treatment of osteoporosis increases fracture risk: systematic review and meta-analysis. Osteoporos Int. 2010;21(11):1943–51.
36. Curtis JR, Westfall AO, Cheng H, et al. Benefit of adherence with bisphosphonates depends on age and fracture type: results from an analysis of 101,038 new bisphosphonate users. J Bone Miner Res. 2008;23(9):1435–41.
37. Strom O, Borgstrom F, Kanis JA, Jonsson B. Incorporating adherence into health economic modelling of osteoporosis. Osteoporos Int. 2009;20(1):23–34.
38. Sunyecz JA, Mucha L, Baser O, et al. Impact of compliance and persistence with bisphosphonate therapy on health care costs and utilization. Osteoporos Int. 2008;19(10):1421–9.
39. Halpern R, Becker L, Iqbal SU, et al. The association of adherence to osteoporosis therapies with fracture, all-cause medical costs, and all-cause hospitalizations: a retrospective claims analysis of female health plan enrollees with osteoporosis. J Manag Care Pharm. 2011;17(1):25–39.
40. Modi A, Siris ES, Tang J, Sen S. Cost and consequences of noncompliance with osteoporosis treatment among women initiating therapy. Curr Med Res Opin. 2015;31(4):757–65.
41. Martin KE, Yu J, Campbell HE, et al. Analysis of the comparative effectiveness of 3 oral bisphosphonates in a large managed care organization: adherence, fracture rates, and all-cause cost. J Manag Care Pharm. 2011;17(8):596–609.
42. Clark EM, Gould VC, Tobias JH, Horne R. Natural history, reasons for, and impact of low/non-adherence to medications for osteoporosis in a cohort of community-dwelling older women already established on medication: a 2-year follow-up study. Osteoporos Int. 2016;27(2):579–90.
43. Ward MA, Xu Y, Viswanathan HN, et al. Association between osteoporosis treatment change and adherence, incident fracture, and total healthcare costs in a Medicare Advantage Prescription Drug plan. Osteoporos Int. 2013;24(4):1195–206.
44. Brookhart MA, Avorn J, Katz JN, et al. Gaps in treatment among users of osteoporosis medications: the dynamics of noncompliance. Am J Med. 2007;120(3):251–6.
45. Eisenberg DF, Placzek H, Gu T, et al. Cost and consequences of noncompliance to oral bisphosphonate treatment. J Manag Care Spec Pharm. 2015;21(1):56–65.
46. Hadji P, Papaioannou N, Gielen E, et al. Persistence, adherence, and medication-taking behavior in women with postmenopausal osteoporosis receiving denosumab in routine practice in Germany, Austria, Greece, and Belgium: 12-month results from a European non-interventional study. Osteoporos Int. 2015;26(10):2479–89.
47. Costa-Paiva L, Gomes DC, Morais SS, et al. Knowledge about osteoporosis in postmenopausal women undergoing antiresorptive treatment. Maturitas. 2011;69(1):81–5.
48. Sale JE, Gignac MA, Hawker G, et al. Decision to take osteoporosis medication in patients who have had a fracture and are ‘high’ risk for future fracture: a qualitative study. BMC Musculoskelet Disord. 2011;12:92.
49. Haynes RB, Yao X, Degani A, et al. Interventions to enhance medication adherence. Cochrane Database Syst Rev. 2005(4):CD000011.
50. Gleeson T, Iversen MD, Avorn J, et al. Interventions to improve adherence and persistence with osteoporosis medications: a systematic literature review. Osteoporos Int. 2009; 20(12):2127–34.
51. Hiligsmann M, Salas M, Hughes DA, et al. Interventions to improve osteoporosis medication adherence and persistence: a systematic review and literature appraisal by the ISPOR Medication Adherence & Persistence Special Interest Group. Osteoporos Int. 2013;24(12):2907–18.
52. Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23(8):1296–310.
53. Richter A, Anton SF, Koch P, Dennett SL. The impact of reducing dose frequency on health outcomes. Clin Ther. 2003;25(8):2307–35; discussion 6.
54. Cramer JA, Amonkar MM, Hebborn A, Altman R. Compliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosis. Curr Med Res Opin. 2005;21(9):1453–60.
55. Iglay K, Cao X, Mavros P, et al. Systematic Literature Review and Meta-analysis of Medication Adherence With Once-weekly Versus Once-daily Therapy. Clin Ther. 2015;37(8):1813–21 e1.
56. Carbonell-Abella C, Pages-Castella A, Javaid MK, et al. Early (1-year) Discontinuation of Different Anti-osteoporosis Medications Compared: A Population-Based Cohort Study. Calcif Tissue Int. 2015;97(6):535–41.
57. Lewiecki EM, Babbitt AM, Piziak VK, et al. Adherence to and gastrointestinal tolerability of monthly oral or quarterly intravenous ibandronate therapy in women with previous intolerance to oral bisphosphonates: a 12-month, open-label, prospective evaluation. Clin Ther. 2008;30(4):605–21.
58. Payer J, Killinger Z, Sulkova I, Celec P. Preferences of patients receiving bisphosphonates--how to influence the therapeutic adherence. Biomed Pharmacother. 2008;62(2):122–4.
59. Weycker D, Macarios D, Edelsberg J, Oster G. Compliance with drug therapy for postmenopausal osteoporosis. Osteoporos Int. 2006;17(11):1645–52.
60. Kastelan D, Lozo P, Stamenkovic D, et al. Preference for weekly and monthly bisphosphonates among patients with postmenopausal osteoporosis: results from the Croatian PROMO Study. Clin Rheumatol. 2009;28(3):321–6.
61. McClung M, Recker R, Miller P, et al. Intravenous zoledronic acid 5 mg in the treatment of postmenopausal women with low bone density previously treated with alendronate. Bone. 2007;41(1):122–8.
62. Hsieh PC. Effectiveness and safety of zoledronic acid in the treatment of osteoporosis. Orthopedics. 2016:1–8.
63. Recker RR, Gallagher R, MacCosbe PE. Effect of dosing frequency on bisphosphonate medication adherence in a large longitudinal cohort of women. Mayo Clin Proc. 2005;80(7):856–61.
64. Emkey R, Koltun W, Beusterien K, et al. Patient preference for once-monthly ibandronate versus once-weekly alendronate in a randomized, open-label, cross-over trial: the Boniva Alendronate Trial in Osteoporosis (BALTO). Curr Med Res Opin. 2005;21(12):1895–903.
65. Hadji P, Minne H, Pfeifer M, et al. Treatment preference for monthly oral ibandronate and weekly oral alendronate in women with postmenopausal osteoporosis: A randomized, crossover study (BALTO II). Joint Bone Spine. 2008;75(3):303–10.
66. Ryzner KL, Burkiewicz JS, Griffin BL, Komperda KE. Survey of bisphosphonate regimen preferences in an urban community health center. Consult Pharm. 2010;25(10):671–5.
67. Saag K, Lindsay R, Kriegman A, et al. A single zoledronic acid infusion reduces bone resorption markers more rapidly than weekly oral alendronate in postmenopausal women with low bone mineral density. Bone. 2007;40(5):1238–43.
68. Hadji P, Felsenberg D, Amling M, et al. The non-interventional BonViva Intravenous Versus Alendronate (VIVA) study: real-world adherence and persistence to medication, efficacy, and safety, in patients with postmenopausal osteoporosis. Osteoporos Int. 2014;25(1):339–47.
69. Ziller V, Kostev K, Kyvernitakis I, et al. Persistence and compliance of medications used in the treatment of osteoporosis--analysis using a large scale, representative, longitudinal German database. Int J Clin Pharmacol Ther. 2012;50(5):315–22.
70. Curtis JR, Yun H, Matthews R, et al. Adherence with intravenous zoledronate and intravenous ibandronate in the United States Medicare population. Arthritis Care Res (Hoboken). 2012;64(7):1054–60.
71. Clowes JA, Peel NF, Eastell R. The impact of monitoring on adherence and persistence with antiresorptive treatment for postmenopausal osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89(3):1117–23.
72. Cooper A, Drake J, Brankin E; PERSIST Investigators. Treatment persistence with once-monthly ibandronate and patient support vs. once-weekly alendronate: results from the PERSIST study. Int J Clin Pract. 2006;60(8):896–905.
73. Hiligsmann M, Bours SP, Boonen A. A review of patient preferences for osteoporosis drug treatment. Curr Rheumatol Rep. 2015;17(9):61.
74. Bond WS, Hussar DA. Detection methods and strategies for improving medication compliance. Am J Hosp Pharm. 1991;48(9):1978–88.
75. Delmas PD, Vrijens B, Eastell R, et al. Effect of monitoring bone turnover markers on persistence with risedronate treatment of postmenopausal osteoporosis. J Clin Endocrinol Metab. 2007;92(4):1296–304.
76. Pickney CS, Arnason JA. Correlation between patient recall of bone densitometry results and subsequent treatment adherence. Osteoporos Int. 2005;16(9):1156–60.
77. Cizmic AD, Heilmann RM, Milchak JL, et al. Impact of interactive voice response technology on primary adherence to bisphosphonate therapy: a randomized controlled trial. Osteoporos Int. 2015;26(8):2131–6.
78. LeBlanc A, Wang AT, Wyatt K, et al. Encounter Decision Aid vs. Clinical Decision Support or Usual Care to Support Patient-Centered Treatment Decisions in Osteoporosis: The Osteoporosis Choice Randomized Trial II. PLoS One. 2015;10(5):e0128063.
79. Tao D, Xie L, Wang T, Wang T. A meta-analysis of the use of electronic reminders for patient adherence to medication in chronic disease care. J Telemed Telecare. 2015;21(1):3–13.
80. Vervloet M, van Dijk L, Santen-Reestman J, et al. SMS reminders improve adherence to oral medication in type 2 diabetes patients who are real time electronically monitored. Int J Med Inform. 2012;81(9):594–604.
81. Vervloet M, Linn AJ, van Weert JC, et al. The effectiveness of interventions using electronic reminders to improve adherence to chronic medication: a systematic review of the literature. J Am Med Inform Assoc. 2012;19(5):696–704.
82. Dayer L, Heldenbrand S, Anderson P, et al. Smartphone medication adherence apps: potential benefits to patients and providers. J Am Pharm Assoc (2003). 2013;53(2):172–81.
83. Solomon DH, Iversen MD, Avorn J, et al. Osteoporosis telephonic intervention to improve medication regimen adherence: a large, pragmatic, randomized controlled trial. Arch Intern Med. 2012;172(6):477–83.
84. Bianchi ML, Duca P, Vai S, et al. Improving adherence to and persistence with oral therapy of osteoporosis. Osteoporos Int. 2015;26(5):1629–38.
85. van Boven JF, Stuurman-Bieze AG, Hiddink EG, et al. Medication monitoring and optimization: a targeted pharmacist program for effective and cost-effective improvement of chronic therapy adherence. J Manag Care Spec Pharm. 2014;20(8):786–92.
86. Lai PS, Chua SS, Chan SP. Pharmaceutical care issues encountered by post-menopausal osteoporotic women prescribed bisphosphonates. J Clin Pharm Ther. 2012;37(5):536–43.
87. Lai PS, Chua SS, Chan SP. Impact of pharmaceutical care on knowledge, quality of life and satisfaction of postmenopausal women with osteoporosis. Int J Clin Pharm. 2013;35(4):629–37.
88. Murphy-Menezes M. Role of the pharmacist in medication therapy management services in patients with osteoporosis. Clin Ther. 2015;37(7):1573–86.
89. Salter C, McDaid L, Bhattacharya D, et al. Abandoned acid? Understanding adherence to bisphosphonate medications for the prevention of osteoporosis among older women: a qualitative longitudinal study. PLoS One. 2014;9(1):e83552.
From the Division of Endocrinology, Diabetes, and Metabolism, University of Alabama at Birmingham, Birmingham, Alabama.
Abstract
- Objective: To review the treatment of osteoporosis, challenges to treatment adherence, and factors associated with improved adherence.
- Methods: Review of the literature.
- Results: With the growing aging population, there is an increased number of people at risk of osteoporosis and fracture. Several medications are available that reduce the risk of fracture. However, adherence to osteoporosis medications is suboptimal. Factors related to nonadherence include dosing frequency, real side effects, and concern about potential side effects. Interventions that may improve adherence include clinician and patient education, less frequent and less complex dosing regimens, medication reminders, and adherence counseling.
- Conclusions: Improving adherence to osteoporosis medications is a complex and challenging issue. Considering and implementing strategies to improve adherence tailored to patient preferences may enhance long-term outcomes for patients with osteoporosis.
Osteoporosis is a chronic but asymptomatic disease that is characterized by an increased fragility of bones and increased risk of fractures. Hip and vertebral fractures are associated with the greatest morbidity and mortality. The prevalence of osteoporosis is estimated to be 10.3% in the US, with approximately 10.2 million adults over the age of 50 having osteoporosis based on 2010 census data and results from the National Health and Nutrition Examination Survey (NHANES) [1].
Several drugs are currently available for the treatment of osteoporosis, but adherence to treatment is low. Understanding the factors associated with low adherence and actions that can be taken to improve adherence to treatment is important given the large number of individuals with osteoporosis and the need to reduce the burden caused by fragility fracture. In this article, we review the treatment of osteoporosis, challenges to treatment adherence, and factors associated with improved adherence.
Nonprescription Medications
Calcium
There have been several published studies over the last decade evaluating calcium supplementation and its efficacy in reducing fractures. Although these studies showed that calcium reduces bone turnover by 20% and slowed postmenopausal bone loss by one third [2,3], none of these studies or a recent systematic review [4] showed any degree of fracture risk reduction with calcium supplements alone.
Although some calcium intake may be good, too much calcium has the potential to cause harm, including an increased risk of nephrolithiasis and constipation/bloating. An analysis of the Women’s Health Initiative (WHI) study reported a 17% increase in renal calculi in women who received calcium and vitamin D supplements [5]. Another recently published meta-analysis showed a 43% increase in gastrointestinal complaints in patients who were taking calcium supplements [6]. The potential for increased cardiovascular risk with calcium supplements is controversial [7]. The WHI study did not show an increased occurrence of cardiovascular events among those taking calcium supplements [8]. In a different population, men who consumed more than 1000 mg per day of supplemental calcium had higher all-cause and cardiovascular disease-specific mortality [9]. Large, well-conducted randomized controlled trials will be needed to further elucidate the question of calcium supplementation and risk of cardiovascular disease.
Vitamin D
Deficiency of vitamin D is common with one study finding more than 90% of older adults deficient in vitamin D [10]. Vitamin D is essential for proper calcium metabolism and deficiency is known to induce secondary hyperparathyroidism. Studies in mouse models have also shown that normal vitamin D receptors in enterocytes are essential for normal bone mineralization [11,12]. A systematic Cochrane database review showed that vitamin D3 supplementation decreased mortality in elderly people living independently or in institutional care [13]. Vitamin D was administered for a weighted mean of 4.4 years. Vitamin D2, alfacalcidol, and calcitriol had no statistically significant beneficial effects on mortality. Vitamin D3 combined with calcium was associated with an increased risk of nephrolithiasis during a follow-up period of 1.25 to 7 years (relative risk [RR] 1.17, 95% confidence interval (CI) 1.02–1.34) [13]. The inconsistencies of published reports looking at benefits of vitamin D supplementation may be due in part to variability in compliance with taking the supplements and baseline vitamin D levels.
Two randomized controlled trials have shown that low vitamin D appears to be an independent predictor of fall risk, and vitamin D supplementation has been found to reduce this risk of falls, through improved musculo-skeletal function [14–16]. Thus, vitamin D may play a role in fracture risk reduction beyond direct bone effects.
Prescription Osteoporosis Treatments
Bisphosphonates
Bisphosphonates are the most commonly prescribed medication for osteoporosis. The efficacy of bisphosphonates to reduce fractures is well established. There are oral bisphosphonates, which can be dosed daily, weekly, or monthly, and intravenous bisphosphonates, which can be given every 3 months or annually. Side effects with this class of medications include gastrointestinal effects with the oral options in up to 20% to 30% of users [17]. With intravenous bisphosphonates, the greatest risk is an acute phase response, which can occur in up to 42% of patients [18]. The risk of an acute phase reaction is much lower with doses beyond the first dose and lower if patients have ever previously taken an oral bisphosphonate and/or receive acetaminophen prior to the infusion. Other potential side effects with all bisphosphonates include osteonecrosis of jaw (ONJ) and atypical subtrochanteric fractures. Post marketing studies have indicated that the incidence of ONJ is less than 2 per 100,000 patient-years among those taking bisphosphonates [19,20]. A number of database analyses have shown that ONJ-like lesions can also occur in older individuals with osteoporosis who have never been exposed to bisphosphonates [21]. A case series of an osteoporotic population showed that ONJ-like lesions are lower grade than those typically seen in cancer patients who usually are exposed to higher doses of bisphosphonates [22]. A study of Swedish older men and women reported that long-term use of bisphosphonates (4 years or more) was associated with an increased incidence of atypical fractures. The RR for women was 126.0 (95% CI, 55.1–288.1) after 4 years of bisphosphonates [23]. A U.S. health care database analysis reported that 90% of those with atypical fractures were bisphosphonate users, almost half were Asian (49%), and use beyond 6 years showed the greatest risk [24].
Non-Bisphosphonate Medications
Other osteoporosis medications include denosumab, raloxifene, estrogen, and teriparatide (calcitonin will not be discussed here). Newer options currently under study, including cathepsin K inhibitors and anti-sclerostin therapies, are not available in the United States.
Denosumab is a monoclonal antibody that interferes with the receptor activator of nuclear kappa B ligand (RANK-L), which is the principal stimulus for osteoclastogenesis. Denosumab is administered once every 6 months subcutaneously. Phase III trials of denosumab demonstrated a 68% reduction in vertebral fractures and 40% and 20% reduction in fractures at hip and non-vertebral sites, respectively [25]. Similar to bisphosphonates, other risks include atypical femoral fractures and ONJ. In addition, hypocalcemia, including severe, symptomatic hypocalcemia, has been reported at rates higher than initially reported in the original clinical trials [26]. Hypocalcemia can be severe, especially in patients who are deficient in vitamin D [10,27].
Estrogen is effective in reducing the risk of vertebral fractures. Selective estrogen receptor modulators (SERMs) have both estrogen agonist and antagonist effects. The SERM, raloxifene, has been used in osteoporosis for its antiresorptive effects through the estrogen receptor [28,29]. A newer SERM, bazedoxifene, has been studied in combination with conjugated estrogen and has been reported to improve bone mineral density and other symptoms of menopause, like vasomotor symptoms and vulvo-vaginal atrophy, but its efficacy in reducing fracture risk has not been demonstrated [30,31].
Teriparatide is an anabolic agent that works by stimulating osteoblastic bone formation which results in an increase in bone density and reduction in both vertebral and non-vertebral fracture risk. In women with postmenopausal osteoporosis, it is typically reserved for those with very low bone mineral density (BMD) or those who continue to have fractures despite a bisphosphonate [32]. Barriers to use of teriparatide include high cost, the need for daily injections, and approved use for a total of two years in a lifetime. There is also a theoretical risk of osteosarcoma shown in animal studies but human cases have not been reported when used for postmenopausal osteoporosis. Published studies have shown that combination zolendronate and teriparatide have additive benefits to spine and hip BMD [33]. Another study reported that the combination of denosumab and teriparatide resulted in additive effects, ie, an increase in lumbar, hip, and femoral neck BMD [34]. These combinations have not been studied in populations large enough or for long enough duration to evaluate fracture risk reduction.
Adherence to Osteoporosis Medications
Treatment of osteoporosis reduces risk of fracture, but the benefit of osteoporosis medications is dependent on adherence. Adherence is associated with improved clinical outcomes [35,36] as well as reduced costs and utilization [37,38]; however, adherence to osteoporosis medications is poor. In a meta-analysis of 24 observational studies conducted in large populations, overall adherence for all osteoporosis therapies ranged from approximately 40% to 70% [39]. A recent retrospective claims database analysis in the U.S. reported a 60% noncompliance rate among the 57,913 postmenopausal women prescribed bisphosphonates over 1 year [40]. Another administrative database analysis from a managed care population compared the 3 oral bisphosphonates (risedronate, ibandronate, and alendronate) and found a mean medication possession ratio (MPR) between 0.57–0.58 at 12 months, which dropped to 0.47–0.50 after 24 months and 0.44–0.47 after 36 months [41]. In an observational study of 3200 older women in the U.K. low adherence was self-reported in 8.5%, and 21.6% self-discontinued treatment within 2 years [42]. In a study of Medicare Advantage prescription drug plan members, a small but significant increase in adherence was seen after osteo-porosis treatment change but overall adherence remained low (51% MPR in the change cohort and vs. 44% in the no-change cohort at 24 months, P < 0.01) [43].
Some patients restart osteoporosis therapy after a prolonged lapse in medication use. In one study, re-initiation rates for bisphosphonate therapy among persons who discontinued were as high as 30% within 6 months and 50% within 2 years [44]. Predictors of treatment re-initiation included younger age, female sex, history of fracture, recent hip fracture, nursing home discharge, and BMD testing [44].
Factors that Impact Adherence
Understanding which patients are most likely to be compliant with medications can aid physicians when monitoring osteoporosis treatment responses. In a retro-spective claims analysis, older age was found to be a predictor of compliance: women 65 years and older were more likely to be compliant than younger patients (P = 0.012) [45]. Among women receiving denosumab, improved adherence was found among women with a family history of a parent with a hip fracture, and lower adherence was seen in those with higher age, decreased mobility, and further distance from the clinic where the medication was provided [46].
Major reasons for nonadherence include a fear of potential side effects, occurrence of real side effects, the complicated dosing regimens, and perceived lack of benefit from the medications due to the asymptomatic nature of osteoporosis. In the above noted observational study from the U.K., more than half of the nonadherent patients attributed their nonadherence to side effects (53.9%), with a smaller proportion reporting fear of potential side effects (20.5%) or trouble with the dosing regimen (8.0%)[42].
Patients may also be unwilling to continue to take an osteoporosis medication if a fracture develops while on it and if they are not otherwise provided evidence that the medication is working. In a study by Costa Paiva et al, an understanding and knowledge to osteoporosis was a prerequisite to adherence and the strongest predictor of knowledge was higher education level [47]. Factors that impaired adherence were lower socioeconomic status and presence of comorbidities [47]. In a phenomenological qualitative study, trust in a health care provider was the most common reason for patients’ decision to accept an osteoporosis medication, emphasizing the importance of physician-patient communication [48].
Interventions to Enhance Adherence
Current methods of improving adherence for chronic health problems are mostly complex and not very effective [49]. In a systematic review of interventions to improve medication adherence, only 37 out of 81 studies reported improved adherence in the treatment of chronic diseases, and multifaceted treatments were more likely to succeed [49]. Improving adherence to osteoporosis medications is a complex issue, and a number of interventions evaluated in systematic reviews have shown limited efficacy [50,51]. Simplification of dosing regimens have been found to have a significant impact in chronic disease management [52,53] as well as in some studies of osteoporosis medications.
Simplification of Dosing
Among women prescribed daily vs. weekly bisphosphonates, those on the weekly regimen had significantly higher compliance [54]. However, rates were suboptimal in both groups and more than 50% of women discontinued at 1 year [54]. In addition, in a meta-analysis of osteoporosis medication adherence, a nearly two-fold higher odds of discontinuation with daily vs. weekly bisphosphonates was seen (odds ratio 1.90, 95% CI 1.81–2.00) [55]. Likewise, in a retrospective study in Spain, nearly 85% of those started on a daily bisphosphonate stopped within a year [56], while discontinuation was significantly lower in those prescribed a weekly or monthly bisphosphonate or daily teriparatide; however, discontinuation was still nearly 50% in these groups [56].
Once monthly dosing may be preferred by some patients as there is less time involved in thinking about the disease being treated and a perception of lower likelihood of side effects. In one study, postmenopausal women who had previously stopped oral bisphosphonates due to GI side effects had high adherence rates after self-selecting either monthly oral or quarterly intravenous ibandronate therapy [57]. However, not all studies show significant differences in adherence between weekly and monthly preparations [58–60].
The newer parenteral treatment options that can be given every 6 months or once yearly have the potential to significantly improve adherence. Once a year parenteral administration of a bisphosphonate was preferred over once-weekly oral administration, according to a 1-year study in patients with low bone density previously treated with alendronate [61]. A recent study that looked at persistence with an infusion of zolendronic acid in Taiwanese patients for 48 months found that 85% of patients received at least 2 infusions [62]. In patients treated with denosumab in 4 European countries, adherence and persistence at 12 months were consistently > 80% [46]. Persistence in this study was defined as receiving the subsequent injection within 6 months ± 8 weeks of the previous injection; adherence was defined as receiving 2 consecutive injections within 6 months ± 4 weeks of each other [46].
In a study by Cramer et al, increased adherence and persistence was seen with weekly alendronate compared daily alendronate at the end of 12 months [54]. Similar results were seen in a large longitudinal cohort study of weekly vs. daily bisphosphonates but less than 50% of patients were adherent with the weekly regimen [63]. When once monthly preparations of bisphosphonates became available, studies continued to support a patient preference for less frequently dosed bisphosphonates, with the majority of patients preferring monthly over weekly dosed medications [64–66].
The availability of quarterly ibandronate and yearly zoledronic acid infusions have further simplified dosing. In large, randomized, multicenter studies, patients consistently expressed a preference for yearly infusions over a weekly oral medication [61,67]. Adherence and persistence to osteoporosis medications was also greater in women receiving intravenous ibandronate compared to those receiving oral alendronate [68,69]. However, a study by Curtis et al showed low persistence with intravenous bisphosphonates in a Medicare population [70]. A possible reason for the lower adherence in this population was postulated to include the provision of the infusions at an outpatient center rather than a physician office. Automated nursing reminders with either phone calls or emails have the potential to mitigate the problem of persistence with this less frequent regimen [71,72]. In a review of patient preferences, less frequent dosing of medications was a common desire, but further generalizability were limited, emphasizing the need to individualize treatment [73].
Patient-Provider Communication
Individualizing treatment with better patient-provider communication and identification of potential barriers may increase compliance [74]. In one study, increasing patient participation in determining the treatment option was associated with improved patient adherence [57]. A systematic review of literature on interventions to improve adherence found that periodic follow-up interaction between patients and their health professionals also improved adherence [50]. Positive reinforcement via physician-patient discussion of either bone turnover markers or bone mineral density test results has also been found to improve long-term adherence with osteoporosis medications [71,75].
Better perceived physician knowledge may help with patient adherence. A study by Pickney et al reported that the patient confidence in their health care providers has influence on improved adherence, and patients were more likely to comply when the medications were prescribed by a specialist rather than a general practitioner [76].
Education, Reminders, Phone-Based
Improving patient knowledge of osteoporosis, especially with education using visual aids, may help with improving adherence [47]. In a randomized controlled trial at a single health management organization, an interactive voice response phone call plus a letter 1 week later increased the rate of obtaining a prescribed oral bisphosphonate in the intervention group (48.8% vs. 30.5% control; OR 2.3, 95% CI 1.34–3.94) when adjusted for age, sex, prior BMD, and fracture [77]. Use of an encounter decision aid also improved knowledge of osteoporosis medication options and led to a doubling of medication prescription attainment. However, adherence at 6 months was not improved [78].
Pill reminders in the form of text messages, paging systems on medication devices, and alarm beeps have been studied in patients with chronic diseases, and these technologies could be utilized for osteoporosis treatment [79–81]. A study of smart phone applications showed that many apps help with adherence, especially in noncompliant patients [82]. The researchers reported that of apps studied, MyMedSchedule, MyMeds, and RxmindMe were among the most highly rated due to their ease of use and enhanced functions. Solomon et all studied the effectiveness of a telephone-based counseling program using motivational interviewing in a large randomized study. They found no significant improvement in adherence to an osteoporosis regimen with the telephonic motivational interview compared to mailed educational materials (control group) (P = 0.07) [83]. In a 12-month multicenter, prospective randomized study, Bianchi et al examined the effectiveness of an intervention of reminders or reminders plus phone calls and meetings at the referral center in postmenopausal women initiating an oral osteoporosis prescription. No significant difference was seen in adherence at 12 months compared to standard care [84]. Adherence among the entire cohort, however, was very high [84]
Pharmacist-Based
The role of pharmacists in the treatment of chronic diseases, including osteoporosis, has been studied and shown to be cost-effective. In a study by van Boven et al, an algorithm was designed to detect patients with nonadherence and then tailor an intervention that consisted of structured counseling and active monitoring by pharmacists in initial and continuous phases [85]. This effort-intensive intervention resulted in reduced discontinuation of bisphosphonates after 12 months (reduction from 31.7% to 16.2% at 12 months) [85]. Despite the effort required, findings from the study support overall cost-effectiveness of this intervention [85]. A randomized controlled study by Lai et al showed that pharmacists can play a role in improving medication adherence through counseling patients on the importance of adherence, side effects, and goals of therapy [86]. The same authors also showed that involvement of a clinical pharmacist in the care of patients helped to further improve patient knowledge of medications and osteoporosis treatments, resolve medication-related concerns, and improve overall quality of life [87]. Such pharmacist-led interventions would require pharmacists to understand their role and the potential for drug holidays in the course of osteoporosis treatments and not mislabel patients as nonadherent when in fact purposefully holding osteoporosis medications [88].
Conclusion
Osteoporosis is a growing problem with increasing numbers of patients at risk for osteoporosis and related fractures. Currently available osteoporosis medications have shown clear benefit in reducing fracture risk; however, adherence to these therapies is required to obtain benefit. Unfortunately, osteoporosis medications have several limitations to full compliance, particularly the oral treatment options, including known possible side effects acutely and chronically, potential/feared side effects, irregular dosing intervals, complicated dosing instructions, and absence of an immediate recognizable benefit/effect. Improving adherence is complex [89] and tailoring to individual patients is of importance. Successful techniques for improving adherence may include a focus on physician-patient communication, use of the less frequently dosed medications, various medication reminders, use of available technology, and use of pharmacists for patient counseling and monitoring. Recognition of this common problem by clinicians is of utmost importance.
Corresponding author: Amy H. Warriner, MD, The University of Alabama at Birmingham, Division of Endocrinology, Diabetes and Metabolism, 702 Faculty Office Tower, 510 20th Street South, Birmingham, AL 35294, [email protected].
Financial disclosures: None.
From the Division of Endocrinology, Diabetes, and Metabolism, University of Alabama at Birmingham, Birmingham, Alabama.
Abstract
- Objective: To review the treatment of osteoporosis, challenges to treatment adherence, and factors associated with improved adherence.
- Methods: Review of the literature.
- Results: With the growing aging population, there is an increased number of people at risk of osteoporosis and fracture. Several medications are available that reduce the risk of fracture. However, adherence to osteoporosis medications is suboptimal. Factors related to nonadherence include dosing frequency, real side effects, and concern about potential side effects. Interventions that may improve adherence include clinician and patient education, less frequent and less complex dosing regimens, medication reminders, and adherence counseling.
- Conclusions: Improving adherence to osteoporosis medications is a complex and challenging issue. Considering and implementing strategies to improve adherence tailored to patient preferences may enhance long-term outcomes for patients with osteoporosis.
Osteoporosis is a chronic but asymptomatic disease that is characterized by an increased fragility of bones and increased risk of fractures. Hip and vertebral fractures are associated with the greatest morbidity and mortality. The prevalence of osteoporosis is estimated to be 10.3% in the US, with approximately 10.2 million adults over the age of 50 having osteoporosis based on 2010 census data and results from the National Health and Nutrition Examination Survey (NHANES) [1].
Several drugs are currently available for the treatment of osteoporosis, but adherence to treatment is low. Understanding the factors associated with low adherence and actions that can be taken to improve adherence to treatment is important given the large number of individuals with osteoporosis and the need to reduce the burden caused by fragility fracture. In this article, we review the treatment of osteoporosis, challenges to treatment adherence, and factors associated with improved adherence.
Nonprescription Medications
Calcium
There have been several published studies over the last decade evaluating calcium supplementation and its efficacy in reducing fractures. Although these studies showed that calcium reduces bone turnover by 20% and slowed postmenopausal bone loss by one third [2,3], none of these studies or a recent systematic review [4] showed any degree of fracture risk reduction with calcium supplements alone.
Although some calcium intake may be good, too much calcium has the potential to cause harm, including an increased risk of nephrolithiasis and constipation/bloating. An analysis of the Women’s Health Initiative (WHI) study reported a 17% increase in renal calculi in women who received calcium and vitamin D supplements [5]. Another recently published meta-analysis showed a 43% increase in gastrointestinal complaints in patients who were taking calcium supplements [6]. The potential for increased cardiovascular risk with calcium supplements is controversial [7]. The WHI study did not show an increased occurrence of cardiovascular events among those taking calcium supplements [8]. In a different population, men who consumed more than 1000 mg per day of supplemental calcium had higher all-cause and cardiovascular disease-specific mortality [9]. Large, well-conducted randomized controlled trials will be needed to further elucidate the question of calcium supplementation and risk of cardiovascular disease.
Vitamin D
Deficiency of vitamin D is common with one study finding more than 90% of older adults deficient in vitamin D [10]. Vitamin D is essential for proper calcium metabolism and deficiency is known to induce secondary hyperparathyroidism. Studies in mouse models have also shown that normal vitamin D receptors in enterocytes are essential for normal bone mineralization [11,12]. A systematic Cochrane database review showed that vitamin D3 supplementation decreased mortality in elderly people living independently or in institutional care [13]. Vitamin D was administered for a weighted mean of 4.4 years. Vitamin D2, alfacalcidol, and calcitriol had no statistically significant beneficial effects on mortality. Vitamin D3 combined with calcium was associated with an increased risk of nephrolithiasis during a follow-up period of 1.25 to 7 years (relative risk [RR] 1.17, 95% confidence interval (CI) 1.02–1.34) [13]. The inconsistencies of published reports looking at benefits of vitamin D supplementation may be due in part to variability in compliance with taking the supplements and baseline vitamin D levels.
Two randomized controlled trials have shown that low vitamin D appears to be an independent predictor of fall risk, and vitamin D supplementation has been found to reduce this risk of falls, through improved musculo-skeletal function [14–16]. Thus, vitamin D may play a role in fracture risk reduction beyond direct bone effects.
Prescription Osteoporosis Treatments
Bisphosphonates
Bisphosphonates are the most commonly prescribed medication for osteoporosis. The efficacy of bisphosphonates to reduce fractures is well established. There are oral bisphosphonates, which can be dosed daily, weekly, or monthly, and intravenous bisphosphonates, which can be given every 3 months or annually. Side effects with this class of medications include gastrointestinal effects with the oral options in up to 20% to 30% of users [17]. With intravenous bisphosphonates, the greatest risk is an acute phase response, which can occur in up to 42% of patients [18]. The risk of an acute phase reaction is much lower with doses beyond the first dose and lower if patients have ever previously taken an oral bisphosphonate and/or receive acetaminophen prior to the infusion. Other potential side effects with all bisphosphonates include osteonecrosis of jaw (ONJ) and atypical subtrochanteric fractures. Post marketing studies have indicated that the incidence of ONJ is less than 2 per 100,000 patient-years among those taking bisphosphonates [19,20]. A number of database analyses have shown that ONJ-like lesions can also occur in older individuals with osteoporosis who have never been exposed to bisphosphonates [21]. A case series of an osteoporotic population showed that ONJ-like lesions are lower grade than those typically seen in cancer patients who usually are exposed to higher doses of bisphosphonates [22]. A study of Swedish older men and women reported that long-term use of bisphosphonates (4 years or more) was associated with an increased incidence of atypical fractures. The RR for women was 126.0 (95% CI, 55.1–288.1) after 4 years of bisphosphonates [23]. A U.S. health care database analysis reported that 90% of those with atypical fractures were bisphosphonate users, almost half were Asian (49%), and use beyond 6 years showed the greatest risk [24].
Non-Bisphosphonate Medications
Other osteoporosis medications include denosumab, raloxifene, estrogen, and teriparatide (calcitonin will not be discussed here). Newer options currently under study, including cathepsin K inhibitors and anti-sclerostin therapies, are not available in the United States.
Denosumab is a monoclonal antibody that interferes with the receptor activator of nuclear kappa B ligand (RANK-L), which is the principal stimulus for osteoclastogenesis. Denosumab is administered once every 6 months subcutaneously. Phase III trials of denosumab demonstrated a 68% reduction in vertebral fractures and 40% and 20% reduction in fractures at hip and non-vertebral sites, respectively [25]. Similar to bisphosphonates, other risks include atypical femoral fractures and ONJ. In addition, hypocalcemia, including severe, symptomatic hypocalcemia, has been reported at rates higher than initially reported in the original clinical trials [26]. Hypocalcemia can be severe, especially in patients who are deficient in vitamin D [10,27].
Estrogen is effective in reducing the risk of vertebral fractures. Selective estrogen receptor modulators (SERMs) have both estrogen agonist and antagonist effects. The SERM, raloxifene, has been used in osteoporosis for its antiresorptive effects through the estrogen receptor [28,29]. A newer SERM, bazedoxifene, has been studied in combination with conjugated estrogen and has been reported to improve bone mineral density and other symptoms of menopause, like vasomotor symptoms and vulvo-vaginal atrophy, but its efficacy in reducing fracture risk has not been demonstrated [30,31].
Teriparatide is an anabolic agent that works by stimulating osteoblastic bone formation which results in an increase in bone density and reduction in both vertebral and non-vertebral fracture risk. In women with postmenopausal osteoporosis, it is typically reserved for those with very low bone mineral density (BMD) or those who continue to have fractures despite a bisphosphonate [32]. Barriers to use of teriparatide include high cost, the need for daily injections, and approved use for a total of two years in a lifetime. There is also a theoretical risk of osteosarcoma shown in animal studies but human cases have not been reported when used for postmenopausal osteoporosis. Published studies have shown that combination zolendronate and teriparatide have additive benefits to spine and hip BMD [33]. Another study reported that the combination of denosumab and teriparatide resulted in additive effects, ie, an increase in lumbar, hip, and femoral neck BMD [34]. These combinations have not been studied in populations large enough or for long enough duration to evaluate fracture risk reduction.
Adherence to Osteoporosis Medications
Treatment of osteoporosis reduces risk of fracture, but the benefit of osteoporosis medications is dependent on adherence. Adherence is associated with improved clinical outcomes [35,36] as well as reduced costs and utilization [37,38]; however, adherence to osteoporosis medications is poor. In a meta-analysis of 24 observational studies conducted in large populations, overall adherence for all osteoporosis therapies ranged from approximately 40% to 70% [39]. A recent retrospective claims database analysis in the U.S. reported a 60% noncompliance rate among the 57,913 postmenopausal women prescribed bisphosphonates over 1 year [40]. Another administrative database analysis from a managed care population compared the 3 oral bisphosphonates (risedronate, ibandronate, and alendronate) and found a mean medication possession ratio (MPR) between 0.57–0.58 at 12 months, which dropped to 0.47–0.50 after 24 months and 0.44–0.47 after 36 months [41]. In an observational study of 3200 older women in the U.K. low adherence was self-reported in 8.5%, and 21.6% self-discontinued treatment within 2 years [42]. In a study of Medicare Advantage prescription drug plan members, a small but significant increase in adherence was seen after osteo-porosis treatment change but overall adherence remained low (51% MPR in the change cohort and vs. 44% in the no-change cohort at 24 months, P < 0.01) [43].
Some patients restart osteoporosis therapy after a prolonged lapse in medication use. In one study, re-initiation rates for bisphosphonate therapy among persons who discontinued were as high as 30% within 6 months and 50% within 2 years [44]. Predictors of treatment re-initiation included younger age, female sex, history of fracture, recent hip fracture, nursing home discharge, and BMD testing [44].
Factors that Impact Adherence
Understanding which patients are most likely to be compliant with medications can aid physicians when monitoring osteoporosis treatment responses. In a retro-spective claims analysis, older age was found to be a predictor of compliance: women 65 years and older were more likely to be compliant than younger patients (P = 0.012) [45]. Among women receiving denosumab, improved adherence was found among women with a family history of a parent with a hip fracture, and lower adherence was seen in those with higher age, decreased mobility, and further distance from the clinic where the medication was provided [46].
Major reasons for nonadherence include a fear of potential side effects, occurrence of real side effects, the complicated dosing regimens, and perceived lack of benefit from the medications due to the asymptomatic nature of osteoporosis. In the above noted observational study from the U.K., more than half of the nonadherent patients attributed their nonadherence to side effects (53.9%), with a smaller proportion reporting fear of potential side effects (20.5%) or trouble with the dosing regimen (8.0%)[42].
Patients may also be unwilling to continue to take an osteoporosis medication if a fracture develops while on it and if they are not otherwise provided evidence that the medication is working. In a study by Costa Paiva et al, an understanding and knowledge to osteoporosis was a prerequisite to adherence and the strongest predictor of knowledge was higher education level [47]. Factors that impaired adherence were lower socioeconomic status and presence of comorbidities [47]. In a phenomenological qualitative study, trust in a health care provider was the most common reason for patients’ decision to accept an osteoporosis medication, emphasizing the importance of physician-patient communication [48].
Interventions to Enhance Adherence
Current methods of improving adherence for chronic health problems are mostly complex and not very effective [49]. In a systematic review of interventions to improve medication adherence, only 37 out of 81 studies reported improved adherence in the treatment of chronic diseases, and multifaceted treatments were more likely to succeed [49]. Improving adherence to osteoporosis medications is a complex issue, and a number of interventions evaluated in systematic reviews have shown limited efficacy [50,51]. Simplification of dosing regimens have been found to have a significant impact in chronic disease management [52,53] as well as in some studies of osteoporosis medications.
Simplification of Dosing
Among women prescribed daily vs. weekly bisphosphonates, those on the weekly regimen had significantly higher compliance [54]. However, rates were suboptimal in both groups and more than 50% of women discontinued at 1 year [54]. In addition, in a meta-analysis of osteoporosis medication adherence, a nearly two-fold higher odds of discontinuation with daily vs. weekly bisphosphonates was seen (odds ratio 1.90, 95% CI 1.81–2.00) [55]. Likewise, in a retrospective study in Spain, nearly 85% of those started on a daily bisphosphonate stopped within a year [56], while discontinuation was significantly lower in those prescribed a weekly or monthly bisphosphonate or daily teriparatide; however, discontinuation was still nearly 50% in these groups [56].
Once monthly dosing may be preferred by some patients as there is less time involved in thinking about the disease being treated and a perception of lower likelihood of side effects. In one study, postmenopausal women who had previously stopped oral bisphosphonates due to GI side effects had high adherence rates after self-selecting either monthly oral or quarterly intravenous ibandronate therapy [57]. However, not all studies show significant differences in adherence between weekly and monthly preparations [58–60].
The newer parenteral treatment options that can be given every 6 months or once yearly have the potential to significantly improve adherence. Once a year parenteral administration of a bisphosphonate was preferred over once-weekly oral administration, according to a 1-year study in patients with low bone density previously treated with alendronate [61]. A recent study that looked at persistence with an infusion of zolendronic acid in Taiwanese patients for 48 months found that 85% of patients received at least 2 infusions [62]. In patients treated with denosumab in 4 European countries, adherence and persistence at 12 months were consistently > 80% [46]. Persistence in this study was defined as receiving the subsequent injection within 6 months ± 8 weeks of the previous injection; adherence was defined as receiving 2 consecutive injections within 6 months ± 4 weeks of each other [46].
In a study by Cramer et al, increased adherence and persistence was seen with weekly alendronate compared daily alendronate at the end of 12 months [54]. Similar results were seen in a large longitudinal cohort study of weekly vs. daily bisphosphonates but less than 50% of patients were adherent with the weekly regimen [63]. When once monthly preparations of bisphosphonates became available, studies continued to support a patient preference for less frequently dosed bisphosphonates, with the majority of patients preferring monthly over weekly dosed medications [64–66].
The availability of quarterly ibandronate and yearly zoledronic acid infusions have further simplified dosing. In large, randomized, multicenter studies, patients consistently expressed a preference for yearly infusions over a weekly oral medication [61,67]. Adherence and persistence to osteoporosis medications was also greater in women receiving intravenous ibandronate compared to those receiving oral alendronate [68,69]. However, a study by Curtis et al showed low persistence with intravenous bisphosphonates in a Medicare population [70]. A possible reason for the lower adherence in this population was postulated to include the provision of the infusions at an outpatient center rather than a physician office. Automated nursing reminders with either phone calls or emails have the potential to mitigate the problem of persistence with this less frequent regimen [71,72]. In a review of patient preferences, less frequent dosing of medications was a common desire, but further generalizability were limited, emphasizing the need to individualize treatment [73].
Patient-Provider Communication
Individualizing treatment with better patient-provider communication and identification of potential barriers may increase compliance [74]. In one study, increasing patient participation in determining the treatment option was associated with improved patient adherence [57]. A systematic review of literature on interventions to improve adherence found that periodic follow-up interaction between patients and their health professionals also improved adherence [50]. Positive reinforcement via physician-patient discussion of either bone turnover markers or bone mineral density test results has also been found to improve long-term adherence with osteoporosis medications [71,75].
Better perceived physician knowledge may help with patient adherence. A study by Pickney et al reported that the patient confidence in their health care providers has influence on improved adherence, and patients were more likely to comply when the medications were prescribed by a specialist rather than a general practitioner [76].
Education, Reminders, Phone-Based
Improving patient knowledge of osteoporosis, especially with education using visual aids, may help with improving adherence [47]. In a randomized controlled trial at a single health management organization, an interactive voice response phone call plus a letter 1 week later increased the rate of obtaining a prescribed oral bisphosphonate in the intervention group (48.8% vs. 30.5% control; OR 2.3, 95% CI 1.34–3.94) when adjusted for age, sex, prior BMD, and fracture [77]. Use of an encounter decision aid also improved knowledge of osteoporosis medication options and led to a doubling of medication prescription attainment. However, adherence at 6 months was not improved [78].
Pill reminders in the form of text messages, paging systems on medication devices, and alarm beeps have been studied in patients with chronic diseases, and these technologies could be utilized for osteoporosis treatment [79–81]. A study of smart phone applications showed that many apps help with adherence, especially in noncompliant patients [82]. The researchers reported that of apps studied, MyMedSchedule, MyMeds, and RxmindMe were among the most highly rated due to their ease of use and enhanced functions. Solomon et all studied the effectiveness of a telephone-based counseling program using motivational interviewing in a large randomized study. They found no significant improvement in adherence to an osteoporosis regimen with the telephonic motivational interview compared to mailed educational materials (control group) (P = 0.07) [83]. In a 12-month multicenter, prospective randomized study, Bianchi et al examined the effectiveness of an intervention of reminders or reminders plus phone calls and meetings at the referral center in postmenopausal women initiating an oral osteoporosis prescription. No significant difference was seen in adherence at 12 months compared to standard care [84]. Adherence among the entire cohort, however, was very high [84]
Pharmacist-Based
The role of pharmacists in the treatment of chronic diseases, including osteoporosis, has been studied and shown to be cost-effective. In a study by van Boven et al, an algorithm was designed to detect patients with nonadherence and then tailor an intervention that consisted of structured counseling and active monitoring by pharmacists in initial and continuous phases [85]. This effort-intensive intervention resulted in reduced discontinuation of bisphosphonates after 12 months (reduction from 31.7% to 16.2% at 12 months) [85]. Despite the effort required, findings from the study support overall cost-effectiveness of this intervention [85]. A randomized controlled study by Lai et al showed that pharmacists can play a role in improving medication adherence through counseling patients on the importance of adherence, side effects, and goals of therapy [86]. The same authors also showed that involvement of a clinical pharmacist in the care of patients helped to further improve patient knowledge of medications and osteoporosis treatments, resolve medication-related concerns, and improve overall quality of life [87]. Such pharmacist-led interventions would require pharmacists to understand their role and the potential for drug holidays in the course of osteoporosis treatments and not mislabel patients as nonadherent when in fact purposefully holding osteoporosis medications [88].
Conclusion
Osteoporosis is a growing problem with increasing numbers of patients at risk for osteoporosis and related fractures. Currently available osteoporosis medications have shown clear benefit in reducing fracture risk; however, adherence to these therapies is required to obtain benefit. Unfortunately, osteoporosis medications have several limitations to full compliance, particularly the oral treatment options, including known possible side effects acutely and chronically, potential/feared side effects, irregular dosing intervals, complicated dosing instructions, and absence of an immediate recognizable benefit/effect. Improving adherence is complex [89] and tailoring to individual patients is of importance. Successful techniques for improving adherence may include a focus on physician-patient communication, use of the less frequently dosed medications, various medication reminders, use of available technology, and use of pharmacists for patient counseling and monitoring. Recognition of this common problem by clinicians is of utmost importance.
Corresponding author: Amy H. Warriner, MD, The University of Alabama at Birmingham, Division of Endocrinology, Diabetes and Metabolism, 702 Faculty Office Tower, 510 20th Street South, Birmingham, AL 35294, [email protected].
Financial disclosures: None.
1. Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520–6.
2. Reid IR, Mason B, Horne A, et al. Randomized controlled trial of calcium in healthy older women. Am J Med. 2006;119(9):777–85.
3. Reid IR, Ames R, Mason B, et al. Randomized controlled trial of calcium supplementation in healthy, nonosteoporotic, older men. Arch Intern Med. 2008;168(20):2276–82.
4. Murad MH, Drake MT, Mullan RJ, et al. Clinical review. Comparative effectiveness of drug treatments to prevent fragility fractures: a systematic review and network meta-analysis. J Clin Endocrinol Metab. 2012;97(6):1871–80.
5. Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669–83.
6. Lewis JR, Zhu K, Prince RL. Adverse events from calcium supplementation: relationship to errors in myocardial infarction self-reporting in randomized controlled trials of calcium supplementation. J Bone Miner Res. 2012;27(3):719–22.
7. Challoumas D, Stavrou A, Pericleous A, Dimitrakakis G. Effects of combined vitamin D--calcium supplements on the cardiovascular system: should we be cautious? Atherosclerosis. 2015;238(2):388–98.
8. Hsia J, Heiss G, Ren H, et al. Calcium/vitamin D supplementation and cardiovascular events. Circulation. 2007;115(7):846–54.
9. Yang B, Campbell PT, Gapstur SM, et al. Calcium intake and mortality from all causes, cancer, and cardiovascular disease: the Cancer Prevention Study II Nutrition Cohort. Am J Clin Nutr. 2016;103(3):886–94.
10. Reid IR. Efficacy, effectiveness and side effects of medications used to prevent fractures. J Intern Med. 2015;277(6):690–706.
11. Xue Y, Fleet JC. Intestinal vitamin D receptor is required for normal calcium and bone metabolism in mice. Gastroenterology. 2009;136(4):1317-27, e1–2.
12. Lieben L, Masuyama R, Torrekens S, et al. Normocalcemia is maintained in mice under conditions of calcium malabsorption by vitamin D-induced inhibition of bone mineralization. J Clin Invest. 2012;122(5):1803–15.
13. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2014;1:CD007470.
14. Flicker L, Mead K, MacInnis RJ, et al. Serum vitamin D and falls in older women in residential care in Australia. J Am Geriatr Soc. 2003;51(11):1533–8.
15. Bischoff-Ferrari HA, Orav EJ, Dawson-Hughes B. Effect of cholecalciferol plus calcium on falling in ambulatory older men and women: a 3-year randomized controlled trial. Arch Intern Med. 2006;166(4):424–30.
16. Flicker L, MacInnis RJ, Stein MS, et al. Should older people in residential care receive vitamin D to prevent falls? Results of a randomized trial. J Am Geriatr Soc. 2005;53(11):1881–8.
17. Biswas PN, Wilton LV, Shakir SA. Pharmacovigilance study of alendronate in England. Osteoporos Int. 2003;14(6):507–14.
18. Reid IR, Gamble GD, Mesenbrink P, et al. Characterization of and risk factors for the acute-phase response after zoledronic acid. J Clin Endocrinol Metab. 2010;95(9):4380–7.
19. Grbic JT, Landesberg R, Lin SQ, et al. Incidence of osteonecrosis of the jaw in women with postmenopausal osteoporosis in the health outcomes and reduced incidence with zoledronic acid once yearly pivotal fracture trial. J Am Dent Assoc. 2008;139(1):32–40.
20. Grbic JT, Black DM, Lyles KW, et al. The incidence of osteonecrosis of the jaw in patients receiving 5 milligrams of zoledronic acid: data from the health outcomes and reduced incidence with zoledronic acid once yearly clinical trials program. J Am Dent Assoc. 2010;141(11):1365–70.
21. Lin TC, Yang CY, Kao Yang YH, Lin SJ. Incidence and risk of osteonecrosis of the jaw among the Taiwan osteoporosis population. Osteoporos Int. 2014;25(5):1503–11.
22. Assael LA. Oral bisphosphonates as a cause of bisphosphonate-related osteonecrosis of the jaws: clinical findings, assessment of risks, and preventive strategies. J Oral Maxillofac Surg. 2009;67(5 Suppl):35–43.
23. Schilcher J, Koeppen V, Aspenberg P, Michaelsson K. Risk of atypical femoral fracture during and after bisphosphonate use. N Engl J Med. 2014;371(10):974–6.
24. Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res. 2012;27(12):2544–50.
25. Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–65.
26. Yerram P, Kansagra S, Abdelghany O. Incidence of hypocalcemia in patients receiving denosumab for prevention of skeletal-related events in bone metastasis. J Oncol Pharm Pract. 2016.
27. Reid IR. Short-term and long-term effects of osteoporosis therapies. Nat Rev Endocrinol. 2015;11(7):418–28.
28. Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282(7):637–45.
29. Reid IR, Eastell R, Fogelman I, et al. A comparison of the effects of raloxifene and conjugated equine estrogen on bone and lipids in healthy postmenopausal women. Arch Intern Med. 2004;164(8):871–9.
30. Sharifi M, Lewiecki EM. Conjugated estrogens combined with bazedoxifene: the first approved tissue selective estrogen complex therapy. Expert Rev Clin Pharmacol. 2014;7(3):281–91.
31. Mirkin S, Ryan KA, Chandran AB, Komm BS. Bazedoxifene/conjugated estrogens for managing the burden of estrogen deficiency symptoms. Maturitas. 2014;77(1):24–31.
32. Warriner AH, Saag KG. Prevention and treatment of bone changes associated with exposure to glucocorticoids. Curr Osteoporos Rep. 2013;11(4):341–7.
33. Cosman F, Eriksen EF, Recknor C, et al. Effects of intravenous zoledronic acid plus subcutaneous teriparatide [rhPTH(1-34)] in postmenopausal osteoporosis. J Bone Miner Res. 2011;26(3):503–11.
34. Leder BZ, Tsai JN, Uihlein AV, et al. Two years of Denosumab and teriparatide administration in postmenopausal women with osteoporosis (The DATA Extension Study): a randomized controlled trial. J Clin Endocrinol Metab. 2014;99(5):1694–700.
35. Imaz I, Zegarra P, Gonzalez-Enriquez J, et al. Poor bisphosphonate adherence for treatment of osteoporosis increases fracture risk: systematic review and meta-analysis. Osteoporos Int. 2010;21(11):1943–51.
36. Curtis JR, Westfall AO, Cheng H, et al. Benefit of adherence with bisphosphonates depends on age and fracture type: results from an analysis of 101,038 new bisphosphonate users. J Bone Miner Res. 2008;23(9):1435–41.
37. Strom O, Borgstrom F, Kanis JA, Jonsson B. Incorporating adherence into health economic modelling of osteoporosis. Osteoporos Int. 2009;20(1):23–34.
38. Sunyecz JA, Mucha L, Baser O, et al. Impact of compliance and persistence with bisphosphonate therapy on health care costs and utilization. Osteoporos Int. 2008;19(10):1421–9.
39. Halpern R, Becker L, Iqbal SU, et al. The association of adherence to osteoporosis therapies with fracture, all-cause medical costs, and all-cause hospitalizations: a retrospective claims analysis of female health plan enrollees with osteoporosis. J Manag Care Pharm. 2011;17(1):25–39.
40. Modi A, Siris ES, Tang J, Sen S. Cost and consequences of noncompliance with osteoporosis treatment among women initiating therapy. Curr Med Res Opin. 2015;31(4):757–65.
41. Martin KE, Yu J, Campbell HE, et al. Analysis of the comparative effectiveness of 3 oral bisphosphonates in a large managed care organization: adherence, fracture rates, and all-cause cost. J Manag Care Pharm. 2011;17(8):596–609.
42. Clark EM, Gould VC, Tobias JH, Horne R. Natural history, reasons for, and impact of low/non-adherence to medications for osteoporosis in a cohort of community-dwelling older women already established on medication: a 2-year follow-up study. Osteoporos Int. 2016;27(2):579–90.
43. Ward MA, Xu Y, Viswanathan HN, et al. Association between osteoporosis treatment change and adherence, incident fracture, and total healthcare costs in a Medicare Advantage Prescription Drug plan. Osteoporos Int. 2013;24(4):1195–206.
44. Brookhart MA, Avorn J, Katz JN, et al. Gaps in treatment among users of osteoporosis medications: the dynamics of noncompliance. Am J Med. 2007;120(3):251–6.
45. Eisenberg DF, Placzek H, Gu T, et al. Cost and consequences of noncompliance to oral bisphosphonate treatment. J Manag Care Spec Pharm. 2015;21(1):56–65.
46. Hadji P, Papaioannou N, Gielen E, et al. Persistence, adherence, and medication-taking behavior in women with postmenopausal osteoporosis receiving denosumab in routine practice in Germany, Austria, Greece, and Belgium: 12-month results from a European non-interventional study. Osteoporos Int. 2015;26(10):2479–89.
47. Costa-Paiva L, Gomes DC, Morais SS, et al. Knowledge about osteoporosis in postmenopausal women undergoing antiresorptive treatment. Maturitas. 2011;69(1):81–5.
48. Sale JE, Gignac MA, Hawker G, et al. Decision to take osteoporosis medication in patients who have had a fracture and are ‘high’ risk for future fracture: a qualitative study. BMC Musculoskelet Disord. 2011;12:92.
49. Haynes RB, Yao X, Degani A, et al. Interventions to enhance medication adherence. Cochrane Database Syst Rev. 2005(4):CD000011.
50. Gleeson T, Iversen MD, Avorn J, et al. Interventions to improve adherence and persistence with osteoporosis medications: a systematic literature review. Osteoporos Int. 2009; 20(12):2127–34.
51. Hiligsmann M, Salas M, Hughes DA, et al. Interventions to improve osteoporosis medication adherence and persistence: a systematic review and literature appraisal by the ISPOR Medication Adherence & Persistence Special Interest Group. Osteoporos Int. 2013;24(12):2907–18.
52. Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23(8):1296–310.
53. Richter A, Anton SF, Koch P, Dennett SL. The impact of reducing dose frequency on health outcomes. Clin Ther. 2003;25(8):2307–35; discussion 6.
54. Cramer JA, Amonkar MM, Hebborn A, Altman R. Compliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosis. Curr Med Res Opin. 2005;21(9):1453–60.
55. Iglay K, Cao X, Mavros P, et al. Systematic Literature Review and Meta-analysis of Medication Adherence With Once-weekly Versus Once-daily Therapy. Clin Ther. 2015;37(8):1813–21 e1.
56. Carbonell-Abella C, Pages-Castella A, Javaid MK, et al. Early (1-year) Discontinuation of Different Anti-osteoporosis Medications Compared: A Population-Based Cohort Study. Calcif Tissue Int. 2015;97(6):535–41.
57. Lewiecki EM, Babbitt AM, Piziak VK, et al. Adherence to and gastrointestinal tolerability of monthly oral or quarterly intravenous ibandronate therapy in women with previous intolerance to oral bisphosphonates: a 12-month, open-label, prospective evaluation. Clin Ther. 2008;30(4):605–21.
58. Payer J, Killinger Z, Sulkova I, Celec P. Preferences of patients receiving bisphosphonates--how to influence the therapeutic adherence. Biomed Pharmacother. 2008;62(2):122–4.
59. Weycker D, Macarios D, Edelsberg J, Oster G. Compliance with drug therapy for postmenopausal osteoporosis. Osteoporos Int. 2006;17(11):1645–52.
60. Kastelan D, Lozo P, Stamenkovic D, et al. Preference for weekly and monthly bisphosphonates among patients with postmenopausal osteoporosis: results from the Croatian PROMO Study. Clin Rheumatol. 2009;28(3):321–6.
61. McClung M, Recker R, Miller P, et al. Intravenous zoledronic acid 5 mg in the treatment of postmenopausal women with low bone density previously treated with alendronate. Bone. 2007;41(1):122–8.
62. Hsieh PC. Effectiveness and safety of zoledronic acid in the treatment of osteoporosis. Orthopedics. 2016:1–8.
63. Recker RR, Gallagher R, MacCosbe PE. Effect of dosing frequency on bisphosphonate medication adherence in a large longitudinal cohort of women. Mayo Clin Proc. 2005;80(7):856–61.
64. Emkey R, Koltun W, Beusterien K, et al. Patient preference for once-monthly ibandronate versus once-weekly alendronate in a randomized, open-label, cross-over trial: the Boniva Alendronate Trial in Osteoporosis (BALTO). Curr Med Res Opin. 2005;21(12):1895–903.
65. Hadji P, Minne H, Pfeifer M, et al. Treatment preference for monthly oral ibandronate and weekly oral alendronate in women with postmenopausal osteoporosis: A randomized, crossover study (BALTO II). Joint Bone Spine. 2008;75(3):303–10.
66. Ryzner KL, Burkiewicz JS, Griffin BL, Komperda KE. Survey of bisphosphonate regimen preferences in an urban community health center. Consult Pharm. 2010;25(10):671–5.
67. Saag K, Lindsay R, Kriegman A, et al. A single zoledronic acid infusion reduces bone resorption markers more rapidly than weekly oral alendronate in postmenopausal women with low bone mineral density. Bone. 2007;40(5):1238–43.
68. Hadji P, Felsenberg D, Amling M, et al. The non-interventional BonViva Intravenous Versus Alendronate (VIVA) study: real-world adherence and persistence to medication, efficacy, and safety, in patients with postmenopausal osteoporosis. Osteoporos Int. 2014;25(1):339–47.
69. Ziller V, Kostev K, Kyvernitakis I, et al. Persistence and compliance of medications used in the treatment of osteoporosis--analysis using a large scale, representative, longitudinal German database. Int J Clin Pharmacol Ther. 2012;50(5):315–22.
70. Curtis JR, Yun H, Matthews R, et al. Adherence with intravenous zoledronate and intravenous ibandronate in the United States Medicare population. Arthritis Care Res (Hoboken). 2012;64(7):1054–60.
71. Clowes JA, Peel NF, Eastell R. The impact of monitoring on adherence and persistence with antiresorptive treatment for postmenopausal osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89(3):1117–23.
72. Cooper A, Drake J, Brankin E; PERSIST Investigators. Treatment persistence with once-monthly ibandronate and patient support vs. once-weekly alendronate: results from the PERSIST study. Int J Clin Pract. 2006;60(8):896–905.
73. Hiligsmann M, Bours SP, Boonen A. A review of patient preferences for osteoporosis drug treatment. Curr Rheumatol Rep. 2015;17(9):61.
74. Bond WS, Hussar DA. Detection methods and strategies for improving medication compliance. Am J Hosp Pharm. 1991;48(9):1978–88.
75. Delmas PD, Vrijens B, Eastell R, et al. Effect of monitoring bone turnover markers on persistence with risedronate treatment of postmenopausal osteoporosis. J Clin Endocrinol Metab. 2007;92(4):1296–304.
76. Pickney CS, Arnason JA. Correlation between patient recall of bone densitometry results and subsequent treatment adherence. Osteoporos Int. 2005;16(9):1156–60.
77. Cizmic AD, Heilmann RM, Milchak JL, et al. Impact of interactive voice response technology on primary adherence to bisphosphonate therapy: a randomized controlled trial. Osteoporos Int. 2015;26(8):2131–6.
78. LeBlanc A, Wang AT, Wyatt K, et al. Encounter Decision Aid vs. Clinical Decision Support or Usual Care to Support Patient-Centered Treatment Decisions in Osteoporosis: The Osteoporosis Choice Randomized Trial II. PLoS One. 2015;10(5):e0128063.
79. Tao D, Xie L, Wang T, Wang T. A meta-analysis of the use of electronic reminders for patient adherence to medication in chronic disease care. J Telemed Telecare. 2015;21(1):3–13.
80. Vervloet M, van Dijk L, Santen-Reestman J, et al. SMS reminders improve adherence to oral medication in type 2 diabetes patients who are real time electronically monitored. Int J Med Inform. 2012;81(9):594–604.
81. Vervloet M, Linn AJ, van Weert JC, et al. The effectiveness of interventions using electronic reminders to improve adherence to chronic medication: a systematic review of the literature. J Am Med Inform Assoc. 2012;19(5):696–704.
82. Dayer L, Heldenbrand S, Anderson P, et al. Smartphone medication adherence apps: potential benefits to patients and providers. J Am Pharm Assoc (2003). 2013;53(2):172–81.
83. Solomon DH, Iversen MD, Avorn J, et al. Osteoporosis telephonic intervention to improve medication regimen adherence: a large, pragmatic, randomized controlled trial. Arch Intern Med. 2012;172(6):477–83.
84. Bianchi ML, Duca P, Vai S, et al. Improving adherence to and persistence with oral therapy of osteoporosis. Osteoporos Int. 2015;26(5):1629–38.
85. van Boven JF, Stuurman-Bieze AG, Hiddink EG, et al. Medication monitoring and optimization: a targeted pharmacist program for effective and cost-effective improvement of chronic therapy adherence. J Manag Care Spec Pharm. 2014;20(8):786–92.
86. Lai PS, Chua SS, Chan SP. Pharmaceutical care issues encountered by post-menopausal osteoporotic women prescribed bisphosphonates. J Clin Pharm Ther. 2012;37(5):536–43.
87. Lai PS, Chua SS, Chan SP. Impact of pharmaceutical care on knowledge, quality of life and satisfaction of postmenopausal women with osteoporosis. Int J Clin Pharm. 2013;35(4):629–37.
88. Murphy-Menezes M. Role of the pharmacist in medication therapy management services in patients with osteoporosis. Clin Ther. 2015;37(7):1573–86.
89. Salter C, McDaid L, Bhattacharya D, et al. Abandoned acid? Understanding adherence to bisphosphonate medications for the prevention of osteoporosis among older women: a qualitative longitudinal study. PLoS One. 2014;9(1):e83552.
1. Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520–6.
2. Reid IR, Mason B, Horne A, et al. Randomized controlled trial of calcium in healthy older women. Am J Med. 2006;119(9):777–85.
3. Reid IR, Ames R, Mason B, et al. Randomized controlled trial of calcium supplementation in healthy, nonosteoporotic, older men. Arch Intern Med. 2008;168(20):2276–82.
4. Murad MH, Drake MT, Mullan RJ, et al. Clinical review. Comparative effectiveness of drug treatments to prevent fragility fractures: a systematic review and network meta-analysis. J Clin Endocrinol Metab. 2012;97(6):1871–80.
5. Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669–83.
6. Lewis JR, Zhu K, Prince RL. Adverse events from calcium supplementation: relationship to errors in myocardial infarction self-reporting in randomized controlled trials of calcium supplementation. J Bone Miner Res. 2012;27(3):719–22.
7. Challoumas D, Stavrou A, Pericleous A, Dimitrakakis G. Effects of combined vitamin D--calcium supplements on the cardiovascular system: should we be cautious? Atherosclerosis. 2015;238(2):388–98.
8. Hsia J, Heiss G, Ren H, et al. Calcium/vitamin D supplementation and cardiovascular events. Circulation. 2007;115(7):846–54.
9. Yang B, Campbell PT, Gapstur SM, et al. Calcium intake and mortality from all causes, cancer, and cardiovascular disease: the Cancer Prevention Study II Nutrition Cohort. Am J Clin Nutr. 2016;103(3):886–94.
10. Reid IR. Efficacy, effectiveness and side effects of medications used to prevent fractures. J Intern Med. 2015;277(6):690–706.
11. Xue Y, Fleet JC. Intestinal vitamin D receptor is required for normal calcium and bone metabolism in mice. Gastroenterology. 2009;136(4):1317-27, e1–2.
12. Lieben L, Masuyama R, Torrekens S, et al. Normocalcemia is maintained in mice under conditions of calcium malabsorption by vitamin D-induced inhibition of bone mineralization. J Clin Invest. 2012;122(5):1803–15.
13. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2014;1:CD007470.
14. Flicker L, Mead K, MacInnis RJ, et al. Serum vitamin D and falls in older women in residential care in Australia. J Am Geriatr Soc. 2003;51(11):1533–8.
15. Bischoff-Ferrari HA, Orav EJ, Dawson-Hughes B. Effect of cholecalciferol plus calcium on falling in ambulatory older men and women: a 3-year randomized controlled trial. Arch Intern Med. 2006;166(4):424–30.
16. Flicker L, MacInnis RJ, Stein MS, et al. Should older people in residential care receive vitamin D to prevent falls? Results of a randomized trial. J Am Geriatr Soc. 2005;53(11):1881–8.
17. Biswas PN, Wilton LV, Shakir SA. Pharmacovigilance study of alendronate in England. Osteoporos Int. 2003;14(6):507–14.
18. Reid IR, Gamble GD, Mesenbrink P, et al. Characterization of and risk factors for the acute-phase response after zoledronic acid. J Clin Endocrinol Metab. 2010;95(9):4380–7.
19. Grbic JT, Landesberg R, Lin SQ, et al. Incidence of osteonecrosis of the jaw in women with postmenopausal osteoporosis in the health outcomes and reduced incidence with zoledronic acid once yearly pivotal fracture trial. J Am Dent Assoc. 2008;139(1):32–40.
20. Grbic JT, Black DM, Lyles KW, et al. The incidence of osteonecrosis of the jaw in patients receiving 5 milligrams of zoledronic acid: data from the health outcomes and reduced incidence with zoledronic acid once yearly clinical trials program. J Am Dent Assoc. 2010;141(11):1365–70.
21. Lin TC, Yang CY, Kao Yang YH, Lin SJ. Incidence and risk of osteonecrosis of the jaw among the Taiwan osteoporosis population. Osteoporos Int. 2014;25(5):1503–11.
22. Assael LA. Oral bisphosphonates as a cause of bisphosphonate-related osteonecrosis of the jaws: clinical findings, assessment of risks, and preventive strategies. J Oral Maxillofac Surg. 2009;67(5 Suppl):35–43.
23. Schilcher J, Koeppen V, Aspenberg P, Michaelsson K. Risk of atypical femoral fracture during and after bisphosphonate use. N Engl J Med. 2014;371(10):974–6.
24. Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res. 2012;27(12):2544–50.
25. Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–65.
26. Yerram P, Kansagra S, Abdelghany O. Incidence of hypocalcemia in patients receiving denosumab for prevention of skeletal-related events in bone metastasis. J Oncol Pharm Pract. 2016.
27. Reid IR. Short-term and long-term effects of osteoporosis therapies. Nat Rev Endocrinol. 2015;11(7):418–28.
28. Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282(7):637–45.
29. Reid IR, Eastell R, Fogelman I, et al. A comparison of the effects of raloxifene and conjugated equine estrogen on bone and lipids in healthy postmenopausal women. Arch Intern Med. 2004;164(8):871–9.
30. Sharifi M, Lewiecki EM. Conjugated estrogens combined with bazedoxifene: the first approved tissue selective estrogen complex therapy. Expert Rev Clin Pharmacol. 2014;7(3):281–91.
31. Mirkin S, Ryan KA, Chandran AB, Komm BS. Bazedoxifene/conjugated estrogens for managing the burden of estrogen deficiency symptoms. Maturitas. 2014;77(1):24–31.
32. Warriner AH, Saag KG. Prevention and treatment of bone changes associated with exposure to glucocorticoids. Curr Osteoporos Rep. 2013;11(4):341–7.
33. Cosman F, Eriksen EF, Recknor C, et al. Effects of intravenous zoledronic acid plus subcutaneous teriparatide [rhPTH(1-34)] in postmenopausal osteoporosis. J Bone Miner Res. 2011;26(3):503–11.
34. Leder BZ, Tsai JN, Uihlein AV, et al. Two years of Denosumab and teriparatide administration in postmenopausal women with osteoporosis (The DATA Extension Study): a randomized controlled trial. J Clin Endocrinol Metab. 2014;99(5):1694–700.
35. Imaz I, Zegarra P, Gonzalez-Enriquez J, et al. Poor bisphosphonate adherence for treatment of osteoporosis increases fracture risk: systematic review and meta-analysis. Osteoporos Int. 2010;21(11):1943–51.
36. Curtis JR, Westfall AO, Cheng H, et al. Benefit of adherence with bisphosphonates depends on age and fracture type: results from an analysis of 101,038 new bisphosphonate users. J Bone Miner Res. 2008;23(9):1435–41.
37. Strom O, Borgstrom F, Kanis JA, Jonsson B. Incorporating adherence into health economic modelling of osteoporosis. Osteoporos Int. 2009;20(1):23–34.
38. Sunyecz JA, Mucha L, Baser O, et al. Impact of compliance and persistence with bisphosphonate therapy on health care costs and utilization. Osteoporos Int. 2008;19(10):1421–9.
39. Halpern R, Becker L, Iqbal SU, et al. The association of adherence to osteoporosis therapies with fracture, all-cause medical costs, and all-cause hospitalizations: a retrospective claims analysis of female health plan enrollees with osteoporosis. J Manag Care Pharm. 2011;17(1):25–39.
40. Modi A, Siris ES, Tang J, Sen S. Cost and consequences of noncompliance with osteoporosis treatment among women initiating therapy. Curr Med Res Opin. 2015;31(4):757–65.
41. Martin KE, Yu J, Campbell HE, et al. Analysis of the comparative effectiveness of 3 oral bisphosphonates in a large managed care organization: adherence, fracture rates, and all-cause cost. J Manag Care Pharm. 2011;17(8):596–609.
42. Clark EM, Gould VC, Tobias JH, Horne R. Natural history, reasons for, and impact of low/non-adherence to medications for osteoporosis in a cohort of community-dwelling older women already established on medication: a 2-year follow-up study. Osteoporos Int. 2016;27(2):579–90.
43. Ward MA, Xu Y, Viswanathan HN, et al. Association between osteoporosis treatment change and adherence, incident fracture, and total healthcare costs in a Medicare Advantage Prescription Drug plan. Osteoporos Int. 2013;24(4):1195–206.
44. Brookhart MA, Avorn J, Katz JN, et al. Gaps in treatment among users of osteoporosis medications: the dynamics of noncompliance. Am J Med. 2007;120(3):251–6.
45. Eisenberg DF, Placzek H, Gu T, et al. Cost and consequences of noncompliance to oral bisphosphonate treatment. J Manag Care Spec Pharm. 2015;21(1):56–65.
46. Hadji P, Papaioannou N, Gielen E, et al. Persistence, adherence, and medication-taking behavior in women with postmenopausal osteoporosis receiving denosumab in routine practice in Germany, Austria, Greece, and Belgium: 12-month results from a European non-interventional study. Osteoporos Int. 2015;26(10):2479–89.
47. Costa-Paiva L, Gomes DC, Morais SS, et al. Knowledge about osteoporosis in postmenopausal women undergoing antiresorptive treatment. Maturitas. 2011;69(1):81–5.
48. Sale JE, Gignac MA, Hawker G, et al. Decision to take osteoporosis medication in patients who have had a fracture and are ‘high’ risk for future fracture: a qualitative study. BMC Musculoskelet Disord. 2011;12:92.
49. Haynes RB, Yao X, Degani A, et al. Interventions to enhance medication adherence. Cochrane Database Syst Rev. 2005(4):CD000011.
50. Gleeson T, Iversen MD, Avorn J, et al. Interventions to improve adherence and persistence with osteoporosis medications: a systematic literature review. Osteoporos Int. 2009; 20(12):2127–34.
51. Hiligsmann M, Salas M, Hughes DA, et al. Interventions to improve osteoporosis medication adherence and persistence: a systematic review and literature appraisal by the ISPOR Medication Adherence & Persistence Special Interest Group. Osteoporos Int. 2013;24(12):2907–18.
52. Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23(8):1296–310.
53. Richter A, Anton SF, Koch P, Dennett SL. The impact of reducing dose frequency on health outcomes. Clin Ther. 2003;25(8):2307–35; discussion 6.
54. Cramer JA, Amonkar MM, Hebborn A, Altman R. Compliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosis. Curr Med Res Opin. 2005;21(9):1453–60.
55. Iglay K, Cao X, Mavros P, et al. Systematic Literature Review and Meta-analysis of Medication Adherence With Once-weekly Versus Once-daily Therapy. Clin Ther. 2015;37(8):1813–21 e1.
56. Carbonell-Abella C, Pages-Castella A, Javaid MK, et al. Early (1-year) Discontinuation of Different Anti-osteoporosis Medications Compared: A Population-Based Cohort Study. Calcif Tissue Int. 2015;97(6):535–41.
57. Lewiecki EM, Babbitt AM, Piziak VK, et al. Adherence to and gastrointestinal tolerability of monthly oral or quarterly intravenous ibandronate therapy in women with previous intolerance to oral bisphosphonates: a 12-month, open-label, prospective evaluation. Clin Ther. 2008;30(4):605–21.
58. Payer J, Killinger Z, Sulkova I, Celec P. Preferences of patients receiving bisphosphonates--how to influence the therapeutic adherence. Biomed Pharmacother. 2008;62(2):122–4.
59. Weycker D, Macarios D, Edelsberg J, Oster G. Compliance with drug therapy for postmenopausal osteoporosis. Osteoporos Int. 2006;17(11):1645–52.
60. Kastelan D, Lozo P, Stamenkovic D, et al. Preference for weekly and monthly bisphosphonates among patients with postmenopausal osteoporosis: results from the Croatian PROMO Study. Clin Rheumatol. 2009;28(3):321–6.
61. McClung M, Recker R, Miller P, et al. Intravenous zoledronic acid 5 mg in the treatment of postmenopausal women with low bone density previously treated with alendronate. Bone. 2007;41(1):122–8.
62. Hsieh PC. Effectiveness and safety of zoledronic acid in the treatment of osteoporosis. Orthopedics. 2016:1–8.
63. Recker RR, Gallagher R, MacCosbe PE. Effect of dosing frequency on bisphosphonate medication adherence in a large longitudinal cohort of women. Mayo Clin Proc. 2005;80(7):856–61.
64. Emkey R, Koltun W, Beusterien K, et al. Patient preference for once-monthly ibandronate versus once-weekly alendronate in a randomized, open-label, cross-over trial: the Boniva Alendronate Trial in Osteoporosis (BALTO). Curr Med Res Opin. 2005;21(12):1895–903.
65. Hadji P, Minne H, Pfeifer M, et al. Treatment preference for monthly oral ibandronate and weekly oral alendronate in women with postmenopausal osteoporosis: A randomized, crossover study (BALTO II). Joint Bone Spine. 2008;75(3):303–10.
66. Ryzner KL, Burkiewicz JS, Griffin BL, Komperda KE. Survey of bisphosphonate regimen preferences in an urban community health center. Consult Pharm. 2010;25(10):671–5.
67. Saag K, Lindsay R, Kriegman A, et al. A single zoledronic acid infusion reduces bone resorption markers more rapidly than weekly oral alendronate in postmenopausal women with low bone mineral density. Bone. 2007;40(5):1238–43.
68. Hadji P, Felsenberg D, Amling M, et al. The non-interventional BonViva Intravenous Versus Alendronate (VIVA) study: real-world adherence and persistence to medication, efficacy, and safety, in patients with postmenopausal osteoporosis. Osteoporos Int. 2014;25(1):339–47.
69. Ziller V, Kostev K, Kyvernitakis I, et al. Persistence and compliance of medications used in the treatment of osteoporosis--analysis using a large scale, representative, longitudinal German database. Int J Clin Pharmacol Ther. 2012;50(5):315–22.
70. Curtis JR, Yun H, Matthews R, et al. Adherence with intravenous zoledronate and intravenous ibandronate in the United States Medicare population. Arthritis Care Res (Hoboken). 2012;64(7):1054–60.
71. Clowes JA, Peel NF, Eastell R. The impact of monitoring on adherence and persistence with antiresorptive treatment for postmenopausal osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89(3):1117–23.
72. Cooper A, Drake J, Brankin E; PERSIST Investigators. Treatment persistence with once-monthly ibandronate and patient support vs. once-weekly alendronate: results from the PERSIST study. Int J Clin Pract. 2006;60(8):896–905.
73. Hiligsmann M, Bours SP, Boonen A. A review of patient preferences for osteoporosis drug treatment. Curr Rheumatol Rep. 2015;17(9):61.
74. Bond WS, Hussar DA. Detection methods and strategies for improving medication compliance. Am J Hosp Pharm. 1991;48(9):1978–88.
75. Delmas PD, Vrijens B, Eastell R, et al. Effect of monitoring bone turnover markers on persistence with risedronate treatment of postmenopausal osteoporosis. J Clin Endocrinol Metab. 2007;92(4):1296–304.
76. Pickney CS, Arnason JA. Correlation between patient recall of bone densitometry results and subsequent treatment adherence. Osteoporos Int. 2005;16(9):1156–60.
77. Cizmic AD, Heilmann RM, Milchak JL, et al. Impact of interactive voice response technology on primary adherence to bisphosphonate therapy: a randomized controlled trial. Osteoporos Int. 2015;26(8):2131–6.
78. LeBlanc A, Wang AT, Wyatt K, et al. Encounter Decision Aid vs. Clinical Decision Support or Usual Care to Support Patient-Centered Treatment Decisions in Osteoporosis: The Osteoporosis Choice Randomized Trial II. PLoS One. 2015;10(5):e0128063.
79. Tao D, Xie L, Wang T, Wang T. A meta-analysis of the use of electronic reminders for patient adherence to medication in chronic disease care. J Telemed Telecare. 2015;21(1):3–13.
80. Vervloet M, van Dijk L, Santen-Reestman J, et al. SMS reminders improve adherence to oral medication in type 2 diabetes patients who are real time electronically monitored. Int J Med Inform. 2012;81(9):594–604.
81. Vervloet M, Linn AJ, van Weert JC, et al. The effectiveness of interventions using electronic reminders to improve adherence to chronic medication: a systematic review of the literature. J Am Med Inform Assoc. 2012;19(5):696–704.
82. Dayer L, Heldenbrand S, Anderson P, et al. Smartphone medication adherence apps: potential benefits to patients and providers. J Am Pharm Assoc (2003). 2013;53(2):172–81.
83. Solomon DH, Iversen MD, Avorn J, et al. Osteoporosis telephonic intervention to improve medication regimen adherence: a large, pragmatic, randomized controlled trial. Arch Intern Med. 2012;172(6):477–83.
84. Bianchi ML, Duca P, Vai S, et al. Improving adherence to and persistence with oral therapy of osteoporosis. Osteoporos Int. 2015;26(5):1629–38.
85. van Boven JF, Stuurman-Bieze AG, Hiddink EG, et al. Medication monitoring and optimization: a targeted pharmacist program for effective and cost-effective improvement of chronic therapy adherence. J Manag Care Spec Pharm. 2014;20(8):786–92.
86. Lai PS, Chua SS, Chan SP. Pharmaceutical care issues encountered by post-menopausal osteoporotic women prescribed bisphosphonates. J Clin Pharm Ther. 2012;37(5):536–43.
87. Lai PS, Chua SS, Chan SP. Impact of pharmaceutical care on knowledge, quality of life and satisfaction of postmenopausal women with osteoporosis. Int J Clin Pharm. 2013;35(4):629–37.
88. Murphy-Menezes M. Role of the pharmacist in medication therapy management services in patients with osteoporosis. Clin Ther. 2015;37(7):1573–86.
89. Salter C, McDaid L, Bhattacharya D, et al. Abandoned acid? Understanding adherence to bisphosphonate medications for the prevention of osteoporosis among older women: a qualitative longitudinal study. PLoS One. 2014;9(1):e83552.
Does Optic Nerve Sheath Diameter Ultrasonography Permit Accurate Detection of Real-Time Changes in ICP?
Case Scenarios
Case 1
While working abroad in a resource-limited environment, a patient was brought in after falling and hitting his head. Initially, the patient was awake and alert, but he gradually became minimally responsive, with a Glasgow Coma Scale score of 9. Your facility did not have computed tomography (CT) or magnetic resonance imaging (MRI), but did have a point-of-care ultrasound (US) machine. You measured the patient’s optic nerve sheath diameter (ONSD) with the US and found a diameter of 4.5 mm in each eye. With this clinical change, you wondered if repeat US scans to detect increasing intracranial pressure (ICP) would represent changes in the patient’s condition.
Case 2
A patient who presented with an intracranial hemorrhage was treated with hypertonic saline and was awaiting neurosurgical placement of an extraventicular drain. During this time, a resident who was on a US rotation asked you if she would be able to detect changes in the patient’s ICP using US rather than placing an invasive device. How do you respond?
In adults, ICP is normally 10 to 15 mm Hg. It may be pathologically increased in several life-threatening conditions, including traumatic brain injury (TBI), subarachnoid hemorrhage, central venous thrombosis, brain tumor, and abscess. It is also increased by nonacute pathology, such as idiopathic intracranial hypertension (IIH), which also is known as pseudotumor cerebri. In patients with acute pathology, ICP above 20 mm Hg is generally considered an indication for treatment.1 Indications for ICP monitoring in TBI include positive CT findings, patient age greater than 40 years, systemic hypotension, or abnormal flexion/extension in response to pain.2 Other reasons to monitor ICP include the management of pseudotumor cerebri or after ventriculoperitoneal shunt surgery.3
Unfortunately, current methods of ICP monitoring have significant drawbacks and limitations. The gold standard of ICP monitoring—measurement using an intraventricular catheter—increases the risks of infection and hemorrhage, requires the skill of a neurosurgeon, and may be contraindicated due to coagulopathy or thrombocytopenia. It also cannot be done in a prehospital setting and only to a limited extent in the ED.4
Computed tomography scans and MRI can assess elevated ICP, but these tests are expensive, may increase patient radiation exposure, require patient transport, and may not always detect raised ICP. In the appropriate clinical context, signs present on physical examination, such as decorticate/decerebrate posturing, papilledema, or fixed/dilated pupils, may be highly suggestive of an increased ICP, but sensitivity and specificity are inadequate. Delay in diagnosis is also a drawback of imaging and physical examination, as findings may not present until ICP has been persistently elevated.
Given the disadvantages of current means of assessing elevated ICP, several noninvasive methods of measuring ICP are being investigated. These include such techniques as transcranial Doppler, electroencephalogram, pupillometry, and ONSD measurements.5 This article reviews current applications of ultrasonography measurements of the ONSD in assessing elevations in ICP.
ONSD US
Assessment of ICP via measurement of the ONSD has attracted increasing attention, particularly in emergency medicine. Measurements of the ONSD are possible with CT, MRI, and US. Of these modalities, ONSD US has attracted the most interest, due to its low cost, wide availability, and rapidity. It does not require patient transport, and does not expose a patient to additional radiation. In addition, ONSD US has been utilized in low-resource settings, and may be particularly useful in prehospital and mass-casualty situations.6
The underlying relationship between ONSD and ICP is a result of the enclosure of the subarachnoid space by the ONS. Increased ICP leads to expansion of the ONS, particularly at 3 mm behind the globe, in the retrobulbar compartment (Figures 1 and 2).7
Unfortunately, it is not possible to precisely determine ICP from an ONSD measurement, because baseline ONSD values and elasticity vary significantly within the population.4,8 As a result, ONSD US has been investigated mostly for its ability to detect qualitative changes—particularly as a screen for elevated ICP. Optic nerve sheath diameter has high discriminative value in its ability to distinguish normal from elevated ICP. In a meta-analysis, Dubourg et al9 showed that the technique had an area under the summary receiver-operating curve of 0.94, signifying excellent test accuracy to diagnose elevated ICPs.
Researchers have attempted to determine a threshold value of ONSD that would serve as a clinically useful predictor of elevated ICP. Currently, this value ranges from 4.8 to 5.9 mm, depending on the study9; 5 mm is commonly used clinically as a threshold.10
Using ONSD US to Monitor Rapid Changes in ICP
While the use of the ONSD technique to screen for elevated ICP is relatively well established, the use of ONSD US to track acute changes in ICP is not as well studied. Serial tracking of acute changes could be useful in a patient at risk for intracranial hypertension secondary to trauma, to monitor the results of treating a patient with IIH, or after ventriculoperitoneal shunt placement.3
In Vivo Data
In 1993, Tamburrelli et al11 performed the first ONSD intrathecal infusion study, using A-scan sonography, and concluded that there was a “direct, biphasic, positive relation between diastolic intracranial pressure and optic nerve diameters” and that the data showed “rapid changes of optic nerve diameters in response to variation of intracranial pressure.”
In 1997, Hansen and Helmke12 recorded ONSD versus ICP data in the first intrathecal infusion test to use B-scan mode sonography. Ultrasonography was performed at 2- to 4-minute intervals. Their data demonstrated a linear relationship between ICP and ONSD over a particular cerebrospinal fluid pressure interval. They noted that “this interval differed between patients: ONS dilation commenced at pressure thresholds between 15 mm Hg and 30 mm Hg and in some patients saturation of the response (constant ONSD) occurred between 30 mm Hg and 40 mm Hg.”
The slope of ONSD versus ICP curve varied considerably by patient, making it impossible to infer an absolute ICP value from an ONSD without prior knowledge of the patient’s ratio. Similar to the data from Tamburrelli et al,11 Hansen and Helmke12 also found that there was no lag in ONSD response to ICP: “Within this interval, no temporal delay of the ONS response was noted.”
The only study comparing real-time ONSD data to gold-standard measurements of rapidly changing ICP in humans was performed by Maissan et al13 in 2015. This study involved a cohort of 18 patients who had suffered TBI and had intraparenchymal probes inserted. Because ICP rises transiently during endotracheal tube suctioning due to irritation of the trachea, the increase and subsequent decrease after suctioning was an ideal time to perform ONSD measurements and compare them to simultaneous gold-standard ICP measurements. The ONSD US measurements were performed 30 to 60 seconds prior to suctioning, during suctioning, and 30 to 60 seconds after suctioning.
Even during this very rapid time course, a strong correlation between ICP and ONSD measurements was demonstrated. The R2 value was 0.80. There was no perceptible “lag” in ONSD change; changes in ICP were immediately reflected in ONSD. Notably, an absolute change of less than 8 to 10 mm Hg in ICP did not affect ONSD, which is consistent with data collected by Hansen and Helmke.12
Therapeutic Lumbar Puncture for IIH
There are two case reports of ONSD US measurements being taken pre- and postlumbar puncture (LP) in patients with IIH. In the first, in 1989 Galetta et al14 used A-scan US to measure pre- and post-LP ONSD in a woman with papilledema secondary to IIH. They found a significant reduction in ONSD bilaterally “within minutes” of performing the LP.14
The second case report was published in 2015 by Singleton et al.15 They recorded ONSD measurements 30 minutes pre- and post-LP in a woman who presented to the ED with symptoms from elevated ICP. After reduction of pressure via LP, they recorded a significant reduction in ONSD bilaterally.15
Cadaver Data
Hansen et al16 evaluated the distensibility and elasticity of the ONS using postmortem optic nerve preparations. The ONSD was recorded 200 seconds after each pressure increase, which was long enough to achieve stable diameters. They found a linear correlation between pressure increases of 5 to 45 mm Hg and ONSD. This would suggest a potential positively correlated change in ONSD with in vivo changes in ICP. However, this still needs further clinical study to better assess measurable changes in living patients.
Conclusion
Published data have consistently demonstrated that changes in ICP are rapidly transmitted to the optic nerve sheath and that there does not appear to be a temporal lag in the ONSD. Based on in vivo data, the relationship between ICP and ONSD appears to be linear only over a range of moderately elevated ICP. According to Hansen and Helmke,12 this range starts at approximately 18 to 30 mm Hg, and ends at approximately 40 to 45 mm Hg. Maissan et al13 observed similar findings: “At low levels, ICP changes (8-10 mm Hg) do not affect the ONSD.”
There is still need for additional research to validate and refine these findings. Only one study has compared gold-standard ICP measurements with ONSD US measurements in real time,13 and the literature on ONSD US in tracking ICP after therapeutic LP in IIH consists of only two case reports.
Thus, with some caveats, ONSD US appears to permit qualitative tracking of ICP in real time. This supports its use in situations where a patient may have rapidly changing ICP, such as close monitoring of patients at risk for elevated ICP in a critical care setting, and response to treatment in patients with IIH.
1. Stocchetti N, Maas AI. Traumatic intracranial hypertension. N Engl J Med. 2014;370(22):2121-2130.
2. Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; et al. Guidelines for the management of severe traumatic brain injury. VI. Indications for intracranial pressure monitoring. J Neurotrauma. 2007;24(Suppl 1):S37-S44.
3. Choi SH, Min KT, Park EK, Kim MS, Jung JH, Kim H. Ultrasonography of the optic nerve sheath to assess intracranial pressure changes after ventriculo-peritoneal shunt surgery in children with hydrocephalus: a prospective observational study. Anaesthesia. 2015;70(11):1268-1273.
4. Kristiansson H, Nissborg E, Bartek J Jr, Andresen M, Reinstrup P, Romner B. Measuring elevated intracranial pressure through noninvasive methods: a review of the literature. J Neurosurg Anesthesiol. 2013;25(4):372-385.
5. Rajajee V, Thyagarajan P, Rajagopalan RE. Optic nerve ultrasonography for detection of raised intracranial pressure when invasive monitoring is unavailable. Neurol India. 2010;58(5):812-813.
6. Robba C, Baciqaluppi S, Cardim D, Donnelly J, Bertuccio A, Czosnyka M. Non-invasive assessment of intracranial pressure. Acta Neurol Scand. 2016;134(1):4-21.
7. Hansen HC, Helmke K. The subarachnoid space surrounding the optic nerves. An ultrasound study of the optic nerve sheath. Surg Radiol Anat. 1996;18(4):323-328.
8. Hansen HC, Lagrèze W, Krueger O, Helmke K. Dependence of the optic nerve sheath diameter on acutely applied subarachnoidal pressure - an experimental ultrasound study. Acta Ophthalmol. 2011;89(6):e528-e532.
9. Dubourg J, Javouhey E, Geeraerts T, Messerer M, Kassai B. Ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure: a systematic review and meta-analysis. Intensive Care Med. 2011;37(7):1059-1068.
10. Kimberly HH, Shah S, Marill K, Noble V. Correlation of optic nerve sheath diameter with direct measurement of intracranial pressure. Acad Emerg Med. 2008;15(2):201-204.
11. Tamburrelli C, Anile C, Mangiola A, Falsini B, Palma P. CSF dynamic parameters and changes of optic nerve diameters measured by standardized echography. In: Till P, ed. Ophthalmic Echography 13: Proceedings of the 13th SIDUO Congress, Vienna, Austria, 1990; vol 55. Dordrecht, Netherlands: Springer Netherlands; 1993:101-109.
12. Hansen HC, Helmke K. Validation of the optic nerve sheath response to changing cerebrospinal fluid pressure: ultrasound findings during intrathecal infusion tests. J Neurosurg. 1997;87(1):34-40.
13. Maissan IM, Dirven PJ, Haitsma IK, Hoeks SE, Gommers D, Stolker RJ. Ultrasonographic measured optic nerve sheath diameter as an accurate and quick monitor for changes in intracranial pressure. J Neurosurg. 2015;123(3)743-747.
14. Galetta S, Byrne SF, Smith JL. Echographic correlation of optic nerve sheath size and cerebrospinal fluid pressure. J Clin Neuroophthalmol. 1989;9(2):79-82.
15. Singleton J, Dagan A, Edlow JA, Hoffmann B. Real-time optic nerve sheath diameter reduction measured with bedside ultrasound after therapeutic lumbar puncture in a patient with idiopathic intracranial hypertension. Am J Emerg Med. 2015;33(6):860.e5-e7.
16. Hansen HC, Lagrèze W, Krueger O, Helmke K. Dependence of the optic nerve sheath diameter on acutely applied subarachnoidal pressure—an experimental ultrasound study. Acta Ophthalmol. 2011;89(6):e528-e532.
Case Scenarios
Case 1
While working abroad in a resource-limited environment, a patient was brought in after falling and hitting his head. Initially, the patient was awake and alert, but he gradually became minimally responsive, with a Glasgow Coma Scale score of 9. Your facility did not have computed tomography (CT) or magnetic resonance imaging (MRI), but did have a point-of-care ultrasound (US) machine. You measured the patient’s optic nerve sheath diameter (ONSD) with the US and found a diameter of 4.5 mm in each eye. With this clinical change, you wondered if repeat US scans to detect increasing intracranial pressure (ICP) would represent changes in the patient’s condition.
Case 2
A patient who presented with an intracranial hemorrhage was treated with hypertonic saline and was awaiting neurosurgical placement of an extraventicular drain. During this time, a resident who was on a US rotation asked you if she would be able to detect changes in the patient’s ICP using US rather than placing an invasive device. How do you respond?
In adults, ICP is normally 10 to 15 mm Hg. It may be pathologically increased in several life-threatening conditions, including traumatic brain injury (TBI), subarachnoid hemorrhage, central venous thrombosis, brain tumor, and abscess. It is also increased by nonacute pathology, such as idiopathic intracranial hypertension (IIH), which also is known as pseudotumor cerebri. In patients with acute pathology, ICP above 20 mm Hg is generally considered an indication for treatment.1 Indications for ICP monitoring in TBI include positive CT findings, patient age greater than 40 years, systemic hypotension, or abnormal flexion/extension in response to pain.2 Other reasons to monitor ICP include the management of pseudotumor cerebri or after ventriculoperitoneal shunt surgery.3
Unfortunately, current methods of ICP monitoring have significant drawbacks and limitations. The gold standard of ICP monitoring—measurement using an intraventricular catheter—increases the risks of infection and hemorrhage, requires the skill of a neurosurgeon, and may be contraindicated due to coagulopathy or thrombocytopenia. It also cannot be done in a prehospital setting and only to a limited extent in the ED.4
Computed tomography scans and MRI can assess elevated ICP, but these tests are expensive, may increase patient radiation exposure, require patient transport, and may not always detect raised ICP. In the appropriate clinical context, signs present on physical examination, such as decorticate/decerebrate posturing, papilledema, or fixed/dilated pupils, may be highly suggestive of an increased ICP, but sensitivity and specificity are inadequate. Delay in diagnosis is also a drawback of imaging and physical examination, as findings may not present until ICP has been persistently elevated.
Given the disadvantages of current means of assessing elevated ICP, several noninvasive methods of measuring ICP are being investigated. These include such techniques as transcranial Doppler, electroencephalogram, pupillometry, and ONSD measurements.5 This article reviews current applications of ultrasonography measurements of the ONSD in assessing elevations in ICP.
ONSD US
Assessment of ICP via measurement of the ONSD has attracted increasing attention, particularly in emergency medicine. Measurements of the ONSD are possible with CT, MRI, and US. Of these modalities, ONSD US has attracted the most interest, due to its low cost, wide availability, and rapidity. It does not require patient transport, and does not expose a patient to additional radiation. In addition, ONSD US has been utilized in low-resource settings, and may be particularly useful in prehospital and mass-casualty situations.6
The underlying relationship between ONSD and ICP is a result of the enclosure of the subarachnoid space by the ONS. Increased ICP leads to expansion of the ONS, particularly at 3 mm behind the globe, in the retrobulbar compartment (Figures 1 and 2).7
Unfortunately, it is not possible to precisely determine ICP from an ONSD measurement, because baseline ONSD values and elasticity vary significantly within the population.4,8 As a result, ONSD US has been investigated mostly for its ability to detect qualitative changes—particularly as a screen for elevated ICP. Optic nerve sheath diameter has high discriminative value in its ability to distinguish normal from elevated ICP. In a meta-analysis, Dubourg et al9 showed that the technique had an area under the summary receiver-operating curve of 0.94, signifying excellent test accuracy to diagnose elevated ICPs.
Researchers have attempted to determine a threshold value of ONSD that would serve as a clinically useful predictor of elevated ICP. Currently, this value ranges from 4.8 to 5.9 mm, depending on the study9; 5 mm is commonly used clinically as a threshold.10
Using ONSD US to Monitor Rapid Changes in ICP
While the use of the ONSD technique to screen for elevated ICP is relatively well established, the use of ONSD US to track acute changes in ICP is not as well studied. Serial tracking of acute changes could be useful in a patient at risk for intracranial hypertension secondary to trauma, to monitor the results of treating a patient with IIH, or after ventriculoperitoneal shunt placement.3
In Vivo Data
In 1993, Tamburrelli et al11 performed the first ONSD intrathecal infusion study, using A-scan sonography, and concluded that there was a “direct, biphasic, positive relation between diastolic intracranial pressure and optic nerve diameters” and that the data showed “rapid changes of optic nerve diameters in response to variation of intracranial pressure.”
In 1997, Hansen and Helmke12 recorded ONSD versus ICP data in the first intrathecal infusion test to use B-scan mode sonography. Ultrasonography was performed at 2- to 4-minute intervals. Their data demonstrated a linear relationship between ICP and ONSD over a particular cerebrospinal fluid pressure interval. They noted that “this interval differed between patients: ONS dilation commenced at pressure thresholds between 15 mm Hg and 30 mm Hg and in some patients saturation of the response (constant ONSD) occurred between 30 mm Hg and 40 mm Hg.”
The slope of ONSD versus ICP curve varied considerably by patient, making it impossible to infer an absolute ICP value from an ONSD without prior knowledge of the patient’s ratio. Similar to the data from Tamburrelli et al,11 Hansen and Helmke12 also found that there was no lag in ONSD response to ICP: “Within this interval, no temporal delay of the ONS response was noted.”
The only study comparing real-time ONSD data to gold-standard measurements of rapidly changing ICP in humans was performed by Maissan et al13 in 2015. This study involved a cohort of 18 patients who had suffered TBI and had intraparenchymal probes inserted. Because ICP rises transiently during endotracheal tube suctioning due to irritation of the trachea, the increase and subsequent decrease after suctioning was an ideal time to perform ONSD measurements and compare them to simultaneous gold-standard ICP measurements. The ONSD US measurements were performed 30 to 60 seconds prior to suctioning, during suctioning, and 30 to 60 seconds after suctioning.
Even during this very rapid time course, a strong correlation between ICP and ONSD measurements was demonstrated. The R2 value was 0.80. There was no perceptible “lag” in ONSD change; changes in ICP were immediately reflected in ONSD. Notably, an absolute change of less than 8 to 10 mm Hg in ICP did not affect ONSD, which is consistent with data collected by Hansen and Helmke.12
Therapeutic Lumbar Puncture for IIH
There are two case reports of ONSD US measurements being taken pre- and postlumbar puncture (LP) in patients with IIH. In the first, in 1989 Galetta et al14 used A-scan US to measure pre- and post-LP ONSD in a woman with papilledema secondary to IIH. They found a significant reduction in ONSD bilaterally “within minutes” of performing the LP.14
The second case report was published in 2015 by Singleton et al.15 They recorded ONSD measurements 30 minutes pre- and post-LP in a woman who presented to the ED with symptoms from elevated ICP. After reduction of pressure via LP, they recorded a significant reduction in ONSD bilaterally.15
Cadaver Data
Hansen et al16 evaluated the distensibility and elasticity of the ONS using postmortem optic nerve preparations. The ONSD was recorded 200 seconds after each pressure increase, which was long enough to achieve stable diameters. They found a linear correlation between pressure increases of 5 to 45 mm Hg and ONSD. This would suggest a potential positively correlated change in ONSD with in vivo changes in ICP. However, this still needs further clinical study to better assess measurable changes in living patients.
Conclusion
Published data have consistently demonstrated that changes in ICP are rapidly transmitted to the optic nerve sheath and that there does not appear to be a temporal lag in the ONSD. Based on in vivo data, the relationship between ICP and ONSD appears to be linear only over a range of moderately elevated ICP. According to Hansen and Helmke,12 this range starts at approximately 18 to 30 mm Hg, and ends at approximately 40 to 45 mm Hg. Maissan et al13 observed similar findings: “At low levels, ICP changes (8-10 mm Hg) do not affect the ONSD.”
There is still need for additional research to validate and refine these findings. Only one study has compared gold-standard ICP measurements with ONSD US measurements in real time,13 and the literature on ONSD US in tracking ICP after therapeutic LP in IIH consists of only two case reports.
Thus, with some caveats, ONSD US appears to permit qualitative tracking of ICP in real time. This supports its use in situations where a patient may have rapidly changing ICP, such as close monitoring of patients at risk for elevated ICP in a critical care setting, and response to treatment in patients with IIH.
Case Scenarios
Case 1
While working abroad in a resource-limited environment, a patient was brought in after falling and hitting his head. Initially, the patient was awake and alert, but he gradually became minimally responsive, with a Glasgow Coma Scale score of 9. Your facility did not have computed tomography (CT) or magnetic resonance imaging (MRI), but did have a point-of-care ultrasound (US) machine. You measured the patient’s optic nerve sheath diameter (ONSD) with the US and found a diameter of 4.5 mm in each eye. With this clinical change, you wondered if repeat US scans to detect increasing intracranial pressure (ICP) would represent changes in the patient’s condition.
Case 2
A patient who presented with an intracranial hemorrhage was treated with hypertonic saline and was awaiting neurosurgical placement of an extraventicular drain. During this time, a resident who was on a US rotation asked you if she would be able to detect changes in the patient’s ICP using US rather than placing an invasive device. How do you respond?
In adults, ICP is normally 10 to 15 mm Hg. It may be pathologically increased in several life-threatening conditions, including traumatic brain injury (TBI), subarachnoid hemorrhage, central venous thrombosis, brain tumor, and abscess. It is also increased by nonacute pathology, such as idiopathic intracranial hypertension (IIH), which also is known as pseudotumor cerebri. In patients with acute pathology, ICP above 20 mm Hg is generally considered an indication for treatment.1 Indications for ICP monitoring in TBI include positive CT findings, patient age greater than 40 years, systemic hypotension, or abnormal flexion/extension in response to pain.2 Other reasons to monitor ICP include the management of pseudotumor cerebri or after ventriculoperitoneal shunt surgery.3
Unfortunately, current methods of ICP monitoring have significant drawbacks and limitations. The gold standard of ICP monitoring—measurement using an intraventricular catheter—increases the risks of infection and hemorrhage, requires the skill of a neurosurgeon, and may be contraindicated due to coagulopathy or thrombocytopenia. It also cannot be done in a prehospital setting and only to a limited extent in the ED.4
Computed tomography scans and MRI can assess elevated ICP, but these tests are expensive, may increase patient radiation exposure, require patient transport, and may not always detect raised ICP. In the appropriate clinical context, signs present on physical examination, such as decorticate/decerebrate posturing, papilledema, or fixed/dilated pupils, may be highly suggestive of an increased ICP, but sensitivity and specificity are inadequate. Delay in diagnosis is also a drawback of imaging and physical examination, as findings may not present until ICP has been persistently elevated.
Given the disadvantages of current means of assessing elevated ICP, several noninvasive methods of measuring ICP are being investigated. These include such techniques as transcranial Doppler, electroencephalogram, pupillometry, and ONSD measurements.5 This article reviews current applications of ultrasonography measurements of the ONSD in assessing elevations in ICP.
ONSD US
Assessment of ICP via measurement of the ONSD has attracted increasing attention, particularly in emergency medicine. Measurements of the ONSD are possible with CT, MRI, and US. Of these modalities, ONSD US has attracted the most interest, due to its low cost, wide availability, and rapidity. It does not require patient transport, and does not expose a patient to additional radiation. In addition, ONSD US has been utilized in low-resource settings, and may be particularly useful in prehospital and mass-casualty situations.6
The underlying relationship between ONSD and ICP is a result of the enclosure of the subarachnoid space by the ONS. Increased ICP leads to expansion of the ONS, particularly at 3 mm behind the globe, in the retrobulbar compartment (Figures 1 and 2).7
Unfortunately, it is not possible to precisely determine ICP from an ONSD measurement, because baseline ONSD values and elasticity vary significantly within the population.4,8 As a result, ONSD US has been investigated mostly for its ability to detect qualitative changes—particularly as a screen for elevated ICP. Optic nerve sheath diameter has high discriminative value in its ability to distinguish normal from elevated ICP. In a meta-analysis, Dubourg et al9 showed that the technique had an area under the summary receiver-operating curve of 0.94, signifying excellent test accuracy to diagnose elevated ICPs.
Researchers have attempted to determine a threshold value of ONSD that would serve as a clinically useful predictor of elevated ICP. Currently, this value ranges from 4.8 to 5.9 mm, depending on the study9; 5 mm is commonly used clinically as a threshold.10
Using ONSD US to Monitor Rapid Changes in ICP
While the use of the ONSD technique to screen for elevated ICP is relatively well established, the use of ONSD US to track acute changes in ICP is not as well studied. Serial tracking of acute changes could be useful in a patient at risk for intracranial hypertension secondary to trauma, to monitor the results of treating a patient with IIH, or after ventriculoperitoneal shunt placement.3
In Vivo Data
In 1993, Tamburrelli et al11 performed the first ONSD intrathecal infusion study, using A-scan sonography, and concluded that there was a “direct, biphasic, positive relation between diastolic intracranial pressure and optic nerve diameters” and that the data showed “rapid changes of optic nerve diameters in response to variation of intracranial pressure.”
In 1997, Hansen and Helmke12 recorded ONSD versus ICP data in the first intrathecal infusion test to use B-scan mode sonography. Ultrasonography was performed at 2- to 4-minute intervals. Their data demonstrated a linear relationship between ICP and ONSD over a particular cerebrospinal fluid pressure interval. They noted that “this interval differed between patients: ONS dilation commenced at pressure thresholds between 15 mm Hg and 30 mm Hg and in some patients saturation of the response (constant ONSD) occurred between 30 mm Hg and 40 mm Hg.”
The slope of ONSD versus ICP curve varied considerably by patient, making it impossible to infer an absolute ICP value from an ONSD without prior knowledge of the patient’s ratio. Similar to the data from Tamburrelli et al,11 Hansen and Helmke12 also found that there was no lag in ONSD response to ICP: “Within this interval, no temporal delay of the ONS response was noted.”
The only study comparing real-time ONSD data to gold-standard measurements of rapidly changing ICP in humans was performed by Maissan et al13 in 2015. This study involved a cohort of 18 patients who had suffered TBI and had intraparenchymal probes inserted. Because ICP rises transiently during endotracheal tube suctioning due to irritation of the trachea, the increase and subsequent decrease after suctioning was an ideal time to perform ONSD measurements and compare them to simultaneous gold-standard ICP measurements. The ONSD US measurements were performed 30 to 60 seconds prior to suctioning, during suctioning, and 30 to 60 seconds after suctioning.
Even during this very rapid time course, a strong correlation between ICP and ONSD measurements was demonstrated. The R2 value was 0.80. There was no perceptible “lag” in ONSD change; changes in ICP were immediately reflected in ONSD. Notably, an absolute change of less than 8 to 10 mm Hg in ICP did not affect ONSD, which is consistent with data collected by Hansen and Helmke.12
Therapeutic Lumbar Puncture for IIH
There are two case reports of ONSD US measurements being taken pre- and postlumbar puncture (LP) in patients with IIH. In the first, in 1989 Galetta et al14 used A-scan US to measure pre- and post-LP ONSD in a woman with papilledema secondary to IIH. They found a significant reduction in ONSD bilaterally “within minutes” of performing the LP.14
The second case report was published in 2015 by Singleton et al.15 They recorded ONSD measurements 30 minutes pre- and post-LP in a woman who presented to the ED with symptoms from elevated ICP. After reduction of pressure via LP, they recorded a significant reduction in ONSD bilaterally.15
Cadaver Data
Hansen et al16 evaluated the distensibility and elasticity of the ONS using postmortem optic nerve preparations. The ONSD was recorded 200 seconds after each pressure increase, which was long enough to achieve stable diameters. They found a linear correlation between pressure increases of 5 to 45 mm Hg and ONSD. This would suggest a potential positively correlated change in ONSD with in vivo changes in ICP. However, this still needs further clinical study to better assess measurable changes in living patients.
Conclusion
Published data have consistently demonstrated that changes in ICP are rapidly transmitted to the optic nerve sheath and that there does not appear to be a temporal lag in the ONSD. Based on in vivo data, the relationship between ICP and ONSD appears to be linear only over a range of moderately elevated ICP. According to Hansen and Helmke,12 this range starts at approximately 18 to 30 mm Hg, and ends at approximately 40 to 45 mm Hg. Maissan et al13 observed similar findings: “At low levels, ICP changes (8-10 mm Hg) do not affect the ONSD.”
There is still need for additional research to validate and refine these findings. Only one study has compared gold-standard ICP measurements with ONSD US measurements in real time,13 and the literature on ONSD US in tracking ICP after therapeutic LP in IIH consists of only two case reports.
Thus, with some caveats, ONSD US appears to permit qualitative tracking of ICP in real time. This supports its use in situations where a patient may have rapidly changing ICP, such as close monitoring of patients at risk for elevated ICP in a critical care setting, and response to treatment in patients with IIH.
1. Stocchetti N, Maas AI. Traumatic intracranial hypertension. N Engl J Med. 2014;370(22):2121-2130.
2. Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; et al. Guidelines for the management of severe traumatic brain injury. VI. Indications for intracranial pressure monitoring. J Neurotrauma. 2007;24(Suppl 1):S37-S44.
3. Choi SH, Min KT, Park EK, Kim MS, Jung JH, Kim H. Ultrasonography of the optic nerve sheath to assess intracranial pressure changes after ventriculo-peritoneal shunt surgery in children with hydrocephalus: a prospective observational study. Anaesthesia. 2015;70(11):1268-1273.
4. Kristiansson H, Nissborg E, Bartek J Jr, Andresen M, Reinstrup P, Romner B. Measuring elevated intracranial pressure through noninvasive methods: a review of the literature. J Neurosurg Anesthesiol. 2013;25(4):372-385.
5. Rajajee V, Thyagarajan P, Rajagopalan RE. Optic nerve ultrasonography for detection of raised intracranial pressure when invasive monitoring is unavailable. Neurol India. 2010;58(5):812-813.
6. Robba C, Baciqaluppi S, Cardim D, Donnelly J, Bertuccio A, Czosnyka M. Non-invasive assessment of intracranial pressure. Acta Neurol Scand. 2016;134(1):4-21.
7. Hansen HC, Helmke K. The subarachnoid space surrounding the optic nerves. An ultrasound study of the optic nerve sheath. Surg Radiol Anat. 1996;18(4):323-328.
8. Hansen HC, Lagrèze W, Krueger O, Helmke K. Dependence of the optic nerve sheath diameter on acutely applied subarachnoidal pressure - an experimental ultrasound study. Acta Ophthalmol. 2011;89(6):e528-e532.
9. Dubourg J, Javouhey E, Geeraerts T, Messerer M, Kassai B. Ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure: a systematic review and meta-analysis. Intensive Care Med. 2011;37(7):1059-1068.
10. Kimberly HH, Shah S, Marill K, Noble V. Correlation of optic nerve sheath diameter with direct measurement of intracranial pressure. Acad Emerg Med. 2008;15(2):201-204.
11. Tamburrelli C, Anile C, Mangiola A, Falsini B, Palma P. CSF dynamic parameters and changes of optic nerve diameters measured by standardized echography. In: Till P, ed. Ophthalmic Echography 13: Proceedings of the 13th SIDUO Congress, Vienna, Austria, 1990; vol 55. Dordrecht, Netherlands: Springer Netherlands; 1993:101-109.
12. Hansen HC, Helmke K. Validation of the optic nerve sheath response to changing cerebrospinal fluid pressure: ultrasound findings during intrathecal infusion tests. J Neurosurg. 1997;87(1):34-40.
13. Maissan IM, Dirven PJ, Haitsma IK, Hoeks SE, Gommers D, Stolker RJ. Ultrasonographic measured optic nerve sheath diameter as an accurate and quick monitor for changes in intracranial pressure. J Neurosurg. 2015;123(3)743-747.
14. Galetta S, Byrne SF, Smith JL. Echographic correlation of optic nerve sheath size and cerebrospinal fluid pressure. J Clin Neuroophthalmol. 1989;9(2):79-82.
15. Singleton J, Dagan A, Edlow JA, Hoffmann B. Real-time optic nerve sheath diameter reduction measured with bedside ultrasound after therapeutic lumbar puncture in a patient with idiopathic intracranial hypertension. Am J Emerg Med. 2015;33(6):860.e5-e7.
16. Hansen HC, Lagrèze W, Krueger O, Helmke K. Dependence of the optic nerve sheath diameter on acutely applied subarachnoidal pressure—an experimental ultrasound study. Acta Ophthalmol. 2011;89(6):e528-e532.
1. Stocchetti N, Maas AI. Traumatic intracranial hypertension. N Engl J Med. 2014;370(22):2121-2130.
2. Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; et al. Guidelines for the management of severe traumatic brain injury. VI. Indications for intracranial pressure monitoring. J Neurotrauma. 2007;24(Suppl 1):S37-S44.
3. Choi SH, Min KT, Park EK, Kim MS, Jung JH, Kim H. Ultrasonography of the optic nerve sheath to assess intracranial pressure changes after ventriculo-peritoneal shunt surgery in children with hydrocephalus: a prospective observational study. Anaesthesia. 2015;70(11):1268-1273.
4. Kristiansson H, Nissborg E, Bartek J Jr, Andresen M, Reinstrup P, Romner B. Measuring elevated intracranial pressure through noninvasive methods: a review of the literature. J Neurosurg Anesthesiol. 2013;25(4):372-385.
5. Rajajee V, Thyagarajan P, Rajagopalan RE. Optic nerve ultrasonography for detection of raised intracranial pressure when invasive monitoring is unavailable. Neurol India. 2010;58(5):812-813.
6. Robba C, Baciqaluppi S, Cardim D, Donnelly J, Bertuccio A, Czosnyka M. Non-invasive assessment of intracranial pressure. Acta Neurol Scand. 2016;134(1):4-21.
7. Hansen HC, Helmke K. The subarachnoid space surrounding the optic nerves. An ultrasound study of the optic nerve sheath. Surg Radiol Anat. 1996;18(4):323-328.
8. Hansen HC, Lagrèze W, Krueger O, Helmke K. Dependence of the optic nerve sheath diameter on acutely applied subarachnoidal pressure - an experimental ultrasound study. Acta Ophthalmol. 2011;89(6):e528-e532.
9. Dubourg J, Javouhey E, Geeraerts T, Messerer M, Kassai B. Ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure: a systematic review and meta-analysis. Intensive Care Med. 2011;37(7):1059-1068.
10. Kimberly HH, Shah S, Marill K, Noble V. Correlation of optic nerve sheath diameter with direct measurement of intracranial pressure. Acad Emerg Med. 2008;15(2):201-204.
11. Tamburrelli C, Anile C, Mangiola A, Falsini B, Palma P. CSF dynamic parameters and changes of optic nerve diameters measured by standardized echography. In: Till P, ed. Ophthalmic Echography 13: Proceedings of the 13th SIDUO Congress, Vienna, Austria, 1990; vol 55. Dordrecht, Netherlands: Springer Netherlands; 1993:101-109.
12. Hansen HC, Helmke K. Validation of the optic nerve sheath response to changing cerebrospinal fluid pressure: ultrasound findings during intrathecal infusion tests. J Neurosurg. 1997;87(1):34-40.
13. Maissan IM, Dirven PJ, Haitsma IK, Hoeks SE, Gommers D, Stolker RJ. Ultrasonographic measured optic nerve sheath diameter as an accurate and quick monitor for changes in intracranial pressure. J Neurosurg. 2015;123(3)743-747.
14. Galetta S, Byrne SF, Smith JL. Echographic correlation of optic nerve sheath size and cerebrospinal fluid pressure. J Clin Neuroophthalmol. 1989;9(2):79-82.
15. Singleton J, Dagan A, Edlow JA, Hoffmann B. Real-time optic nerve sheath diameter reduction measured with bedside ultrasound after therapeutic lumbar puncture in a patient with idiopathic intracranial hypertension. Am J Emerg Med. 2015;33(6):860.e5-e7.
16. Hansen HC, Lagrèze W, Krueger O, Helmke K. Dependence of the optic nerve sheath diameter on acutely applied subarachnoidal pressure—an experimental ultrasound study. Acta Ophthalmol. 2011;89(6):e528-e532.