User login
A Call for More Autopsies
In the past 50 years, the number of autopsies at most U.S. hospitals has dropped drastically. The decline is due in part to a “widespread perception” that new imaging techniques and laboratory tests have improved diagnostic accuracy to the extent that an autopsy is considered obsolete, say researchers from Temple University Hospital in Philadelphia, Pennsylvania. But the researchers claim that autopsies are still relevant and “a valuable tool to evaluate diagnostic accuracy.”
According to the researchers, autopsy studies continue to show that the proportions of clinical misdiagnosis have remained largely unchanged. They also found that autopsies performed over 10 years of 821 adults showed 8% had clinically undiagnosed malignancies—similar to the numbers found in studies done 10 years earlier. Out of 66 cases, 26 revealed undiagnosed malignancies directly related to the primary cause of death. In 16 autopsies, there was no clinical suspicion of malignancy, but the primary cause of death (such as acute bronchopneumonia and gastrointestinal perforation) was directly related to an undiagnosed neoplasm. In 10 cases, there was clinical suspicion of malignancy based on history, radiologic studies, and laboratory tests but without definite tissue diagnosis.
The researchers note that some studies have suggested a link between short hospital stays and missed diagnoses. But in the current study, length of stay had no bearing on a patient’s having an unsuspected malignancy. “Ironically,” the cases with no clinical suspicion involved longer hospital stays. In at least 5 cases the hospital stay was for more than 5 days. Moreover, CT scans of the thorax/abdomen raised no suspicion of cancer.
The researchers say their findings make a “strong case for a vigorous hospital autopsy program,” and for using autopsy data to improve performance in clinical, radiologic, and laboratory services. Their study “reiterates the value of the hospital autopsy as an auditing tool for diagnostic accuracy.”
Source: Parajuli S, Aneja A, Mukherjee A. Hum Pathol. 2016; 48:32-36.
doi: 10.1016/j.humpath.2015.09.040.
In the past 50 years, the number of autopsies at most U.S. hospitals has dropped drastically. The decline is due in part to a “widespread perception” that new imaging techniques and laboratory tests have improved diagnostic accuracy to the extent that an autopsy is considered obsolete, say researchers from Temple University Hospital in Philadelphia, Pennsylvania. But the researchers claim that autopsies are still relevant and “a valuable tool to evaluate diagnostic accuracy.”
According to the researchers, autopsy studies continue to show that the proportions of clinical misdiagnosis have remained largely unchanged. They also found that autopsies performed over 10 years of 821 adults showed 8% had clinically undiagnosed malignancies—similar to the numbers found in studies done 10 years earlier. Out of 66 cases, 26 revealed undiagnosed malignancies directly related to the primary cause of death. In 16 autopsies, there was no clinical suspicion of malignancy, but the primary cause of death (such as acute bronchopneumonia and gastrointestinal perforation) was directly related to an undiagnosed neoplasm. In 10 cases, there was clinical suspicion of malignancy based on history, radiologic studies, and laboratory tests but without definite tissue diagnosis.
The researchers note that some studies have suggested a link between short hospital stays and missed diagnoses. But in the current study, length of stay had no bearing on a patient’s having an unsuspected malignancy. “Ironically,” the cases with no clinical suspicion involved longer hospital stays. In at least 5 cases the hospital stay was for more than 5 days. Moreover, CT scans of the thorax/abdomen raised no suspicion of cancer.
The researchers say their findings make a “strong case for a vigorous hospital autopsy program,” and for using autopsy data to improve performance in clinical, radiologic, and laboratory services. Their study “reiterates the value of the hospital autopsy as an auditing tool for diagnostic accuracy.”
Source: Parajuli S, Aneja A, Mukherjee A. Hum Pathol. 2016; 48:32-36.
doi: 10.1016/j.humpath.2015.09.040.
In the past 50 years, the number of autopsies at most U.S. hospitals has dropped drastically. The decline is due in part to a “widespread perception” that new imaging techniques and laboratory tests have improved diagnostic accuracy to the extent that an autopsy is considered obsolete, say researchers from Temple University Hospital in Philadelphia, Pennsylvania. But the researchers claim that autopsies are still relevant and “a valuable tool to evaluate diagnostic accuracy.”
According to the researchers, autopsy studies continue to show that the proportions of clinical misdiagnosis have remained largely unchanged. They also found that autopsies performed over 10 years of 821 adults showed 8% had clinically undiagnosed malignancies—similar to the numbers found in studies done 10 years earlier. Out of 66 cases, 26 revealed undiagnosed malignancies directly related to the primary cause of death. In 16 autopsies, there was no clinical suspicion of malignancy, but the primary cause of death (such as acute bronchopneumonia and gastrointestinal perforation) was directly related to an undiagnosed neoplasm. In 10 cases, there was clinical suspicion of malignancy based on history, radiologic studies, and laboratory tests but without definite tissue diagnosis.
The researchers note that some studies have suggested a link between short hospital stays and missed diagnoses. But in the current study, length of stay had no bearing on a patient’s having an unsuspected malignancy. “Ironically,” the cases with no clinical suspicion involved longer hospital stays. In at least 5 cases the hospital stay was for more than 5 days. Moreover, CT scans of the thorax/abdomen raised no suspicion of cancer.
The researchers say their findings make a “strong case for a vigorous hospital autopsy program,” and for using autopsy data to improve performance in clinical, radiologic, and laboratory services. Their study “reiterates the value of the hospital autopsy as an auditing tool for diagnostic accuracy.”
Source: Parajuli S, Aneja A, Mukherjee A. Hum Pathol. 2016; 48:32-36.
doi: 10.1016/j.humpath.2015.09.040.
Zika virus: Counseling considerations for this emerging perinatal threat
Zika virus infection in the news
- CDC: Zika virus disease cases by US state or territory, updated periodically
- CDC: Q&As for ObGyns on pregnant women and Zika virus, 2/9/16
- CDC: Zika virus infection among US pregnant travelers, 2/26/16
- CDC: Interim guidelines for health care providers caring for infants and children with possible Zika virus infection, 2/19/16
- SMFM statement: Ultrasound screening for fetal microcephaly following Zika virus exposure, 2/16/16
- FDA approves first Zika diagnostic test for commercial use. Newsweek, 2/26/16
- NIH accelerates timeline for human trials of Zika vaccine. The Washington Post, 2/17/16
- Patient resource: Zika virus and pregnancy fact sheet from MotherToBaby.org
- Zika virus article collection from New England Journal of Medicine
- Zika infection diagnosed in 18 pregnant US women who traveled to Zika-affected areas
- FDA grants emergency approval to new 3-in-1 lab test for Zika
- ACOG Practice Advisory: Updated interim guidance for care of women of reproductive age during a Zika virus outbreak, 3/31/16
- MMWR: Patterns in Zika virus testing and infection, 4/22/16
- What insect repellents are safe during pregnancy? 5/19/16
- Zika virus and complications: Q&A from WHO, 5/31/16
- WHO strengthens guidelines to prevent sexual transmission of Zika virus, 5/31/16
- Ultrasound screening for fetal microcephaly following Zika virus exposure (from AJOG), 6/1/16
- CDC: Interim guidance for interpretation of Zika virus antibody test results, 6/3/16
- First Zika vaccine to begin testing in human trials, The Washington Post, 6/20/16
- NIH launches the Zika in Infants and Pregnancy (ZIP) international study, 6/21/16
CASE 1: Pregnant traveler asks: Should I be tested for Zika virus?
A 28-year-old Hispanic woman (G3P2) at 15 weeks’ gestation visits your office for a routine prenatal care appointment. She reports having returned from a 3-week holiday in Brazil 2 days ago, and she is concerned about having experienced fever, malaise, arthralgias, and a disseminated erythematous rash. She has since heard about the Zika virus and asks you if she and her baby are in danger and whether she should be tested for the disease.
What should you tell this patient?
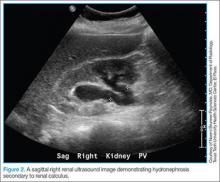
The Zika virus is an RNA Flavivirus, transmitted primarily by the Aedes aegypti mosquito.1 This virus is closely related to the organisms that cause dengue fever, yellow fever, chikungunya infection, and West Nile infection. By feeding on infected prey, mosquitoes can transmit the virus to humans through bites. They breed near pools of stagnant water, can survive both indoors and outdoors, and prefer to be near people. These mosquitoes bite mostly during daylight hours, so it is essential that people use insect repellent throughout the day while in endemic areas.2 These mosquitoes live only in tropical regions; however, the Aedes albopictus mosquito, also known as the Asian tiger mosquito, lives in temperate regions and can transmit the Zika virus as well3 (FIGURE 1).
| FIGURE 1 Aedes aegypti and Aedes albopictus mosquitoes | ||

| 
| |
Aedes aegypti (left) and Aedes albopictus (right) mosquitoes. Aedes mosquitoes are the main transmission vector for the Zika virus. | ||
|
The Zika virus was first discovered in 1947 when it was isolated from a rhesus monkey in Uganda. It subsequently spread to Southeast Asia and eventually caused major outbreaks in the Yap Islands of Micronesia (2007)4 and French Polynesia (2013).5 In 2015, local transmission of the Zika virus infection was noted in Brazil, and, most recently, a pandemic of Zika virus infection has occurred throughout South America, Central America, and the Caribbean islands. To date, local mosquito-borne virus transmission has not occurred in the continental United States, although at least 82 cases acquired during travel to infected areas have been reported.6
Additionally, there have been rare cases involving spread of this virus from infected blood transfusions and through sexual contact.7 In February 2016, the first case of locally acquired Zika virus infection was reported in Texas following sexual transmission of the disease.8
Clinical manifestations of Zika virus infection
Eighty percent of patients infected with Zika virus remain asymptomatic. The illness is short-lived, occurring 2 to 12 days following the mosquito bite, and infected individuals usually do not require hospitalization or experience serious morbidity. When symptoms are present, they typically include low-grade fever (37.8° to 38.5°C), maculopapular rash, arthralgias of the hands and feet, and nonpurulent conjunctivitis. Patients also may experience headache, retro-orbital pain, myalgia, and, rarely, abdominal pain, nausea, vomiting, diarrhea, ulcerations of mucous membranes, and pruritus.9 Guillain-Barré syndrome has been reported in association with Zika virus infection10; however, a definitive cause-effect relationship has not been proven.
If a pregnant woman is infected with the Zika virus, perinatal transmission can occur, either through uteroplacental transmission or vertically from mother to child at the time of delivery. Zika virus RNA has been detected in blood, amniotic fluid, semen, saliva, cerebrospinal fluid, urine, and breast milk. Although the virus has been shown to be present in breast milk, there has been no evidence of viral replication in milk or reported transmission in breastfed infants.11 Pregnant women are not known to have increased susceptibility to Zika virus infection when compared with the general population, and there is no evidence to suggest pregnant women will have a more serious illness if infected.
The Zika virus has been strongly associated with congenital microcephaly and fetal loss among women infected during pregnancy.12 Following the recent large outbreak in Brazil, an alarmingly high number of Brazilian newborns with microcephaly have been observed. The total now exceeds 4,000. Because of these ominous findings, fetuses and neonates born to women with a history of infection should be evaluated for adverse effects of congenital infection.
Management strategies for Zika virus exposure during pregnancy
The incidence of Zika virus infection during pregnancy remains unknown. However, a pregnant woman may be infected in any trimester, and maternal-fetal transmission of the virus can occur throughout pregnancy. If a patient is pregnant and has travelled to areas of Zika virus transmission, or has had unprotected sexual contact with a partner who has had exposure, she should be carefully screened with a detailed review of systems and ultrasonography to evaluate for fetal microcephaly or intracranial calcifications. The US Centers for Disease Control and Prevention (CDC) initially recommended that, if a patient exhibited 2 or more symptoms consistent with Zika virus infection within 2 weeks of exposure or if sonographic evidence revealed fetal microcephaly or intracranial calcifications, she should be tested for Zika virus infection.11
More recently, the CDC issued new guidelines recommending that even asymptomatic women with exposure have serologic testing for infection and that all exposed women undergo serial ultrasound assessments.13 The CDC also recommends offering retesting in the mid second trimester for women who were exposed very early in gestation.
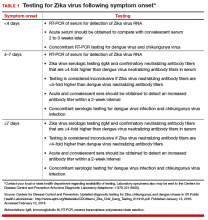
The best diagnostic test for infection is reverse transcriptase-polymerase chain reaction (RT-PCR), and, ideally, it should be completed within 4 days of symptom onset. Beyond 4 days after symptom onset, testing for Zika virus immunoglobulin M (IgM)-specific antibody and neutralizing antibody should be performed in addition to the RT-PCR test. At times, interpretation of antibody testing can be problematic because cross-reaction with related arboviruses is common. Moreover, Zika viremia decreases rapidly over time; therefore, if serum is collected even 5 to 7 days after symptom onset, a negative test does not definitively exclude infection (TABLE 1).
In the United States, local health departments should be contacted to facilitate testing, as the tests described above are not currently commercially available. If the local health department is unable to perform this testing, clinicians should contact the CDC’s Division of Vector-Borne Diseases (telephone: 1-970-221-6400) or visit their website (http://www.cdc.gov/ncezid/dvbd/specimensub/arboviral-shipping.html) for detailed instructions on specimen submission.
Testing is not indicated for women without a history of travel to areas where Zika virus infection is endemic or without a history of unprotected sexual contact with someone who has been exposed to the infection.
Following the delivery of a live infant to an infected or exposed mother, detailed histopathologic evaluation of the placenta and umbilical cord should be performed. Frozen sections of placental and cord tissue should be tested for Zika virus RNA, and cord serum should be tested for Zika and dengue virus IgM and neutralizing antibodies. In cases of fetal loss in the setting of relevant travel history or exposure (particularly maternal symptoms or sonographic evidence of microcephaly), RT-PCR testing and immunohistochemistry should be completed on fetal tissues, umbilical cord, and placenta.2
Treatment is supportive
At present, there is no vaccine for the Zika virus, and no hyperimmune globulin or anti‑ viral chemotherapy is available. Treatment is therefore supportive. Patients should be encouraged to rest and maintain hydration. The preferred antipyretic and analgesic is acetaminophen (650 mg orally every 6 hours or 1,000 mg orally every 8 hours). Aspirin should be avoided until dengue infection has been ruled out because of the related risk of bleeding with hemorrhagic fever. Nonsteroidal anti-inflammatory drugs should be avoided in the second half of pregnancy because of their effect on fetal renal blood flow (oligohydramnios) and stricture of the ductus arteriosus.
CASE 1 Continued
Given this patient’s recent travel, exposure to mosquito-borne illness, and clinical manifestations of malaise, rash, and joint pain, you proceed with serologic testing. The RT-PCR test is positive for Zika virus.
What should be the next step in the management of this patient?
Prenatal diagnosis and fetal surveillance
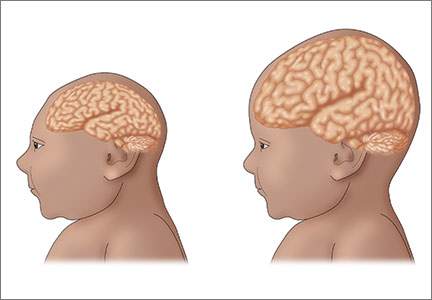
The recent epidemic of microcephaly and poor pregnancy outcomes reported in Brazil has been alarming and demonstrates an almost 20-fold increase in incidence of this condition between 2014–2015.14 Careful surveillance is needed for this birth defect and other poor pregnancy outcomes in association with the Zika virus. To date, a direct causal relationship between Zika virus infection and microcephaly has not been unequivocally established15; however; these microcephaly cases have yet to be attributed to any other cause (FIGURE 2)
| FIGURE 2 Microcephaly: associated with Zika virus infection in pregnancy |

|
Illustration depicts a child with congenital microcephaly (left) and one with head circumference within the mean SD (right). |
Following the outbreak in Brazil, a task force and registry were established to investigate microcephaly and other birth defects associated with Zika virus infection. In one small investigation, 35 cases of microcephaly were reported, and 71% of the infants were seriously affected (head circumference >3 SD below the mean). Fifty percent of babies had at least one neurologic abnormality, and, of the 27 patients who had neuroimaging studies, all had distinct abnormalities, including widespread brain calcifications and cell migration abnormalities, such as lissencephaly, pachgyria, and ventriculomegaly due to cortical atrophy.16
In addition to microcephaly, fetal ultrasound monitoring has revealed focal brain abnormalities, such as asymmetric cerebral hemispheres, ventriculomegaly, displacement of the midline, failure to visualize the corpus callosum, failure of thalamic development, and the presence of intraocular and brain calcifications.17
In collaboration with the CDC, the American College of Obstetricians and Gynecologists and the Society for Maternal Fetal-Medicine have developed guidelines to monitor fetal growth in women with laboratory evidence of Zika virus infection.18 Recommendations include having a detailed anatomy ultrasound and serial growth sonograms every 3 to 4 weeks, along with referral to a maternal-fetal medicine or infectious disease specialist.
If the pregnancy is beyond 15 weeks’ gestational age, an amniocentesis should be performed in symptomatic patients and in those with abnormal ultrasound findings. Amniotic fluid should be tested for Zika virus with RT-PCR (FIGURE 3).12 The sensitivity and specificity of amniotic fluid RT-PCR in detecting congenital infection, as well as the predictive value of a fetal anomaly, remain unknown at this time. For this reason, a patient must be counseled carefully regarding the benefits of confirming intrauterine infection versus the slight risks of premature rupture of membranes, infection, and pregnancy loss related to amniocentesis.
Once diagnosed, microcephaly cannot be “fixed.” However, pregnancy termination is an option that some parents may choose once they are aware of the diagnosis and prognosis of microcephaly. Moreover, even for parents who would not choose abortion, there may be considerable value in being prepared for the care of a severely disabled child. Microcephaly has many possible causes, Zika virus infection being just one. Others include genetic syndromes and other congenital infections, such as cytomegalovirus (CMV) infection and toxoplasmosis. Amniocentesis therefore may help the clinician sort through these causes. For both CMV infection and toxoplasmosis, certain antenatal treatments may be helpful in lessening the severity of fetal injury.
CASE 2 Pregnant patient has travel plans
A 34-year-old woman (G1P0) presents to you for her first prenatal visit. She mentions she plans to take a cruise through the Eastern Caribbean in 2 weeks. Following the history and physical examination, what should you tell this patient?
Perinatal counseling: Limiting exposure is best
As mentioned, there is currently no treatment, prophylactic medication, or vaccination for Zika virus infection. Because of the virus’s significant associations with adverse pregnancy outcomes, birth defects, and fetal loss, the CDC has issued a travel advisory urging pregnant women to avoid travel to areas when Zika virus infection is prevalent. Currently, Zika virus outbreaks are occurring throughout South and Central America, the Pacific Islands, and Africa, and the infection is expected to spread, mainly due to international air travel. If travel to these areas is inevitable, women should take rigorous precautions to avoid exposure to mosquito bites and infection (TABLE 2).
If a woman was infected with laboratory-confirmed Zika virus infection in a prior pregnancy, she should not be at risk for congenital infection during her next pregnancy. This is mainly because the period of viremia is short-lived and lasts approximately 5 to 7 days.2
Further, based on documented sexual transmission of the virus, pregnant women should abstain from sexual activity or should consistently and correctly use condoms with partners who have Zika virus infection or exposure to the virus until further evidence is available.
Stay informed
Zika virus infection is now pandemic; it has evolved from an isolated disease of the tropics to one that is sweeping the Western hemisphere. It is being reported daily in new locations around the world. Given the unsettling association of Zika virus infection with birth defects, careful obstetric surveillance of exposed or symptomatic patients is imperative. Clinicians must carefully screen patients with potential risk of exposure and be prepared to offer appropriate perinatal counseling and diagnostic testing during pregnancy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Dyer O. Zika virus spreads across Americas as concerns mount over birth defects. BMJ. 2015;351:h6983.
- Centers for Disease Control and Prevention. Zika virus. Atlanta, GA: US Dept of Health and Human Services; 2015. http://www.cdc.gov/zika/index.html. Accessed February 12, 2016.
- Bogoch II, Brady OJ, Kraemer MU, et al. Anticipating the international spread of Zika virus from Brazil. Lancet. 2016;387(10016):335–336.
- Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536–2543.
- Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill. 2014;19(13):pii:20751.
- Centers for Disease Control and Prevention. Zika virus disease in the United States, 2015–2016. http://www.cdc.gov/zika/geo/united-states.html. Accessed February 12, 2016.
- Foy BD, Kobylinski KC, Chilson Foy JL, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17(5):880–882.
- Dallas County Health and Human Services. DCHHS reports first Zika virus case in Dallas County acquired through sexual transmission. http://www.dallascounty.org/department/hhs /press/documents/PR2-2-16DCHHSReportsFirstCaseofZikaVirusThroughSexualTransmission.pdf. Accessed February 3, 2016.
- Ministry of Health, Manuatu Hauora. Zika virus. http://www.health.govt.nz/our-work/diseases-and-conditions/zika -virus. Accessed January 13, 2016.
- Oehler E, Watrin L, Larre P, et al. Zika virus infection complicated by Guillain-Barre syndrome—case report, French Polynesia, December 2013. Euro Surveill. 2014;19:4–6.
- Centers for Disease Control and Prevention. Zika virus: transmission. http://www.cdc.gov/zika/transmission/index.html. Accessed January 20, 2016.
- Petersen EE, Staples JE, Meaney-Delamn, D et al. Interim guidelines for pregnant women during a Zika virus outbreak—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30–33.
- Oduyebo T, Petersen EE, Rasmussen SA, et al. Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(5):122–127.
- Pan American Health Organization, World Health Organization. Epidemiological alert: neurological syndrome, congenital malformations, and Zika virus infection. Implications for public health in the Americas. December 1,2015. http://www.paho.org/hq/index.php?option=com_doc man&task=doc_view&Itemid=270&gid=32405&lang=en. Accessed January 13, 2016.
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus epidemic in the Americas: potential associations with microcephaly and Guillain-Barré syndrome. December 10, 2015. http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association -with-microcephaly-rapid-risk-assessment.pdf. Accessed January 13, 2016.
- Schuler-Faccini L, Ribeiro EM, Feitosa IM, et al; Brazilian Medical Genetics Society—Zika Embryopathy Task Force. Possible association between Zika virus infection and microcephaly—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(3):59–62.
- Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol. 2016;47(1):6–7.
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus epidemic in the Americas: potential associations with microcephaly and Guillain-Barré syndrome. December 10, 2015. http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association.
Zika virus infection in the news
- CDC: Zika virus disease cases by US state or territory, updated periodically
- CDC: Q&As for ObGyns on pregnant women and Zika virus, 2/9/16
- CDC: Zika virus infection among US pregnant travelers, 2/26/16
- CDC: Interim guidelines for health care providers caring for infants and children with possible Zika virus infection, 2/19/16
- SMFM statement: Ultrasound screening for fetal microcephaly following Zika virus exposure, 2/16/16
- FDA approves first Zika diagnostic test for commercial use. Newsweek, 2/26/16
- NIH accelerates timeline for human trials of Zika vaccine. The Washington Post, 2/17/16
- Patient resource: Zika virus and pregnancy fact sheet from MotherToBaby.org
- Zika virus article collection from New England Journal of Medicine
- Zika infection diagnosed in 18 pregnant US women who traveled to Zika-affected areas
- FDA grants emergency approval to new 3-in-1 lab test for Zika
- ACOG Practice Advisory: Updated interim guidance for care of women of reproductive age during a Zika virus outbreak, 3/31/16
- MMWR: Patterns in Zika virus testing and infection, 4/22/16
- What insect repellents are safe during pregnancy? 5/19/16
- Zika virus and complications: Q&A from WHO, 5/31/16
- WHO strengthens guidelines to prevent sexual transmission of Zika virus, 5/31/16
- Ultrasound screening for fetal microcephaly following Zika virus exposure (from AJOG), 6/1/16
- CDC: Interim guidance for interpretation of Zika virus antibody test results, 6/3/16
- First Zika vaccine to begin testing in human trials, The Washington Post, 6/20/16
- NIH launches the Zika in Infants and Pregnancy (ZIP) international study, 6/21/16
CASE 1: Pregnant traveler asks: Should I be tested for Zika virus?
A 28-year-old Hispanic woman (G3P2) at 15 weeks’ gestation visits your office for a routine prenatal care appointment. She reports having returned from a 3-week holiday in Brazil 2 days ago, and she is concerned about having experienced fever, malaise, arthralgias, and a disseminated erythematous rash. She has since heard about the Zika virus and asks you if she and her baby are in danger and whether she should be tested for the disease.
What should you tell this patient?
The Zika virus is an RNA Flavivirus, transmitted primarily by the Aedes aegypti mosquito.1 This virus is closely related to the organisms that cause dengue fever, yellow fever, chikungunya infection, and West Nile infection. By feeding on infected prey, mosquitoes can transmit the virus to humans through bites. They breed near pools of stagnant water, can survive both indoors and outdoors, and prefer to be near people. These mosquitoes bite mostly during daylight hours, so it is essential that people use insect repellent throughout the day while in endemic areas.2 These mosquitoes live only in tropical regions; however, the Aedes albopictus mosquito, also known as the Asian tiger mosquito, lives in temperate regions and can transmit the Zika virus as well3 (FIGURE 1).
| FIGURE 1 Aedes aegypti and Aedes albopictus mosquitoes | ||

| 
| |
Aedes aegypti (left) and Aedes albopictus (right) mosquitoes. Aedes mosquitoes are the main transmission vector for the Zika virus. | ||
|
The Zika virus was first discovered in 1947 when it was isolated from a rhesus monkey in Uganda. It subsequently spread to Southeast Asia and eventually caused major outbreaks in the Yap Islands of Micronesia (2007)4 and French Polynesia (2013).5 In 2015, local transmission of the Zika virus infection was noted in Brazil, and, most recently, a pandemic of Zika virus infection has occurred throughout South America, Central America, and the Caribbean islands. To date, local mosquito-borne virus transmission has not occurred in the continental United States, although at least 82 cases acquired during travel to infected areas have been reported.6
Additionally, there have been rare cases involving spread of this virus from infected blood transfusions and through sexual contact.7 In February 2016, the first case of locally acquired Zika virus infection was reported in Texas following sexual transmission of the disease.8
Clinical manifestations of Zika virus infection
Eighty percent of patients infected with Zika virus remain asymptomatic. The illness is short-lived, occurring 2 to 12 days following the mosquito bite, and infected individuals usually do not require hospitalization or experience serious morbidity. When symptoms are present, they typically include low-grade fever (37.8° to 38.5°C), maculopapular rash, arthralgias of the hands and feet, and nonpurulent conjunctivitis. Patients also may experience headache, retro-orbital pain, myalgia, and, rarely, abdominal pain, nausea, vomiting, diarrhea, ulcerations of mucous membranes, and pruritus.9 Guillain-Barré syndrome has been reported in association with Zika virus infection10; however, a definitive cause-effect relationship has not been proven.
If a pregnant woman is infected with the Zika virus, perinatal transmission can occur, either through uteroplacental transmission or vertically from mother to child at the time of delivery. Zika virus RNA has been detected in blood, amniotic fluid, semen, saliva, cerebrospinal fluid, urine, and breast milk. Although the virus has been shown to be present in breast milk, there has been no evidence of viral replication in milk or reported transmission in breastfed infants.11 Pregnant women are not known to have increased susceptibility to Zika virus infection when compared with the general population, and there is no evidence to suggest pregnant women will have a more serious illness if infected.
The Zika virus has been strongly associated with congenital microcephaly and fetal loss among women infected during pregnancy.12 Following the recent large outbreak in Brazil, an alarmingly high number of Brazilian newborns with microcephaly have been observed. The total now exceeds 4,000. Because of these ominous findings, fetuses and neonates born to women with a history of infection should be evaluated for adverse effects of congenital infection.
Management strategies for Zika virus exposure during pregnancy
The incidence of Zika virus infection during pregnancy remains unknown. However, a pregnant woman may be infected in any trimester, and maternal-fetal transmission of the virus can occur throughout pregnancy. If a patient is pregnant and has travelled to areas of Zika virus transmission, or has had unprotected sexual contact with a partner who has had exposure, she should be carefully screened with a detailed review of systems and ultrasonography to evaluate for fetal microcephaly or intracranial calcifications. The US Centers for Disease Control and Prevention (CDC) initially recommended that, if a patient exhibited 2 or more symptoms consistent with Zika virus infection within 2 weeks of exposure or if sonographic evidence revealed fetal microcephaly or intracranial calcifications, she should be tested for Zika virus infection.11
More recently, the CDC issued new guidelines recommending that even asymptomatic women with exposure have serologic testing for infection and that all exposed women undergo serial ultrasound assessments.13 The CDC also recommends offering retesting in the mid second trimester for women who were exposed very early in gestation.
The best diagnostic test for infection is reverse transcriptase-polymerase chain reaction (RT-PCR), and, ideally, it should be completed within 4 days of symptom onset. Beyond 4 days after symptom onset, testing for Zika virus immunoglobulin M (IgM)-specific antibody and neutralizing antibody should be performed in addition to the RT-PCR test. At times, interpretation of antibody testing can be problematic because cross-reaction with related arboviruses is common. Moreover, Zika viremia decreases rapidly over time; therefore, if serum is collected even 5 to 7 days after symptom onset, a negative test does not definitively exclude infection (TABLE 1).
In the United States, local health departments should be contacted to facilitate testing, as the tests described above are not currently commercially available. If the local health department is unable to perform this testing, clinicians should contact the CDC’s Division of Vector-Borne Diseases (telephone: 1-970-221-6400) or visit their website (http://www.cdc.gov/ncezid/dvbd/specimensub/arboviral-shipping.html) for detailed instructions on specimen submission.
Testing is not indicated for women without a history of travel to areas where Zika virus infection is endemic or without a history of unprotected sexual contact with someone who has been exposed to the infection.
Following the delivery of a live infant to an infected or exposed mother, detailed histopathologic evaluation of the placenta and umbilical cord should be performed. Frozen sections of placental and cord tissue should be tested for Zika virus RNA, and cord serum should be tested for Zika and dengue virus IgM and neutralizing antibodies. In cases of fetal loss in the setting of relevant travel history or exposure (particularly maternal symptoms or sonographic evidence of microcephaly), RT-PCR testing and immunohistochemistry should be completed on fetal tissues, umbilical cord, and placenta.2
Treatment is supportive
At present, there is no vaccine for the Zika virus, and no hyperimmune globulin or anti‑ viral chemotherapy is available. Treatment is therefore supportive. Patients should be encouraged to rest and maintain hydration. The preferred antipyretic and analgesic is acetaminophen (650 mg orally every 6 hours or 1,000 mg orally every 8 hours). Aspirin should be avoided until dengue infection has been ruled out because of the related risk of bleeding with hemorrhagic fever. Nonsteroidal anti-inflammatory drugs should be avoided in the second half of pregnancy because of their effect on fetal renal blood flow (oligohydramnios) and stricture of the ductus arteriosus.
CASE 1 Continued
Given this patient’s recent travel, exposure to mosquito-borne illness, and clinical manifestations of malaise, rash, and joint pain, you proceed with serologic testing. The RT-PCR test is positive for Zika virus.
What should be the next step in the management of this patient?
Prenatal diagnosis and fetal surveillance
The recent epidemic of microcephaly and poor pregnancy outcomes reported in Brazil has been alarming and demonstrates an almost 20-fold increase in incidence of this condition between 2014–2015.14 Careful surveillance is needed for this birth defect and other poor pregnancy outcomes in association with the Zika virus. To date, a direct causal relationship between Zika virus infection and microcephaly has not been unequivocally established15; however; these microcephaly cases have yet to be attributed to any other cause (FIGURE 2)
| FIGURE 2 Microcephaly: associated with Zika virus infection in pregnancy |

|
Illustration depicts a child with congenital microcephaly (left) and one with head circumference within the mean SD (right). |
Following the outbreak in Brazil, a task force and registry were established to investigate microcephaly and other birth defects associated with Zika virus infection. In one small investigation, 35 cases of microcephaly were reported, and 71% of the infants were seriously affected (head circumference >3 SD below the mean). Fifty percent of babies had at least one neurologic abnormality, and, of the 27 patients who had neuroimaging studies, all had distinct abnormalities, including widespread brain calcifications and cell migration abnormalities, such as lissencephaly, pachgyria, and ventriculomegaly due to cortical atrophy.16
In addition to microcephaly, fetal ultrasound monitoring has revealed focal brain abnormalities, such as asymmetric cerebral hemispheres, ventriculomegaly, displacement of the midline, failure to visualize the corpus callosum, failure of thalamic development, and the presence of intraocular and brain calcifications.17
In collaboration with the CDC, the American College of Obstetricians and Gynecologists and the Society for Maternal Fetal-Medicine have developed guidelines to monitor fetal growth in women with laboratory evidence of Zika virus infection.18 Recommendations include having a detailed anatomy ultrasound and serial growth sonograms every 3 to 4 weeks, along with referral to a maternal-fetal medicine or infectious disease specialist.
If the pregnancy is beyond 15 weeks’ gestational age, an amniocentesis should be performed in symptomatic patients and in those with abnormal ultrasound findings. Amniotic fluid should be tested for Zika virus with RT-PCR (FIGURE 3).12 The sensitivity and specificity of amniotic fluid RT-PCR in detecting congenital infection, as well as the predictive value of a fetal anomaly, remain unknown at this time. For this reason, a patient must be counseled carefully regarding the benefits of confirming intrauterine infection versus the slight risks of premature rupture of membranes, infection, and pregnancy loss related to amniocentesis.
Once diagnosed, microcephaly cannot be “fixed.” However, pregnancy termination is an option that some parents may choose once they are aware of the diagnosis and prognosis of microcephaly. Moreover, even for parents who would not choose abortion, there may be considerable value in being prepared for the care of a severely disabled child. Microcephaly has many possible causes, Zika virus infection being just one. Others include genetic syndromes and other congenital infections, such as cytomegalovirus (CMV) infection and toxoplasmosis. Amniocentesis therefore may help the clinician sort through these causes. For both CMV infection and toxoplasmosis, certain antenatal treatments may be helpful in lessening the severity of fetal injury.
CASE 2 Pregnant patient has travel plans
A 34-year-old woman (G1P0) presents to you for her first prenatal visit. She mentions she plans to take a cruise through the Eastern Caribbean in 2 weeks. Following the history and physical examination, what should you tell this patient?
Perinatal counseling: Limiting exposure is best
As mentioned, there is currently no treatment, prophylactic medication, or vaccination for Zika virus infection. Because of the virus’s significant associations with adverse pregnancy outcomes, birth defects, and fetal loss, the CDC has issued a travel advisory urging pregnant women to avoid travel to areas when Zika virus infection is prevalent. Currently, Zika virus outbreaks are occurring throughout South and Central America, the Pacific Islands, and Africa, and the infection is expected to spread, mainly due to international air travel. If travel to these areas is inevitable, women should take rigorous precautions to avoid exposure to mosquito bites and infection (TABLE 2).
If a woman was infected with laboratory-confirmed Zika virus infection in a prior pregnancy, she should not be at risk for congenital infection during her next pregnancy. This is mainly because the period of viremia is short-lived and lasts approximately 5 to 7 days.2
Further, based on documented sexual transmission of the virus, pregnant women should abstain from sexual activity or should consistently and correctly use condoms with partners who have Zika virus infection or exposure to the virus until further evidence is available.
Stay informed
Zika virus infection is now pandemic; it has evolved from an isolated disease of the tropics to one that is sweeping the Western hemisphere. It is being reported daily in new locations around the world. Given the unsettling association of Zika virus infection with birth defects, careful obstetric surveillance of exposed or symptomatic patients is imperative. Clinicians must carefully screen patients with potential risk of exposure and be prepared to offer appropriate perinatal counseling and diagnostic testing during pregnancy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Zika virus infection in the news
- CDC: Zika virus disease cases by US state or territory, updated periodically
- CDC: Q&As for ObGyns on pregnant women and Zika virus, 2/9/16
- CDC: Zika virus infection among US pregnant travelers, 2/26/16
- CDC: Interim guidelines for health care providers caring for infants and children with possible Zika virus infection, 2/19/16
- SMFM statement: Ultrasound screening for fetal microcephaly following Zika virus exposure, 2/16/16
- FDA approves first Zika diagnostic test for commercial use. Newsweek, 2/26/16
- NIH accelerates timeline for human trials of Zika vaccine. The Washington Post, 2/17/16
- Patient resource: Zika virus and pregnancy fact sheet from MotherToBaby.org
- Zika virus article collection from New England Journal of Medicine
- Zika infection diagnosed in 18 pregnant US women who traveled to Zika-affected areas
- FDA grants emergency approval to new 3-in-1 lab test for Zika
- ACOG Practice Advisory: Updated interim guidance for care of women of reproductive age during a Zika virus outbreak, 3/31/16
- MMWR: Patterns in Zika virus testing and infection, 4/22/16
- What insect repellents are safe during pregnancy? 5/19/16
- Zika virus and complications: Q&A from WHO, 5/31/16
- WHO strengthens guidelines to prevent sexual transmission of Zika virus, 5/31/16
- Ultrasound screening for fetal microcephaly following Zika virus exposure (from AJOG), 6/1/16
- CDC: Interim guidance for interpretation of Zika virus antibody test results, 6/3/16
- First Zika vaccine to begin testing in human trials, The Washington Post, 6/20/16
- NIH launches the Zika in Infants and Pregnancy (ZIP) international study, 6/21/16
CASE 1: Pregnant traveler asks: Should I be tested for Zika virus?
A 28-year-old Hispanic woman (G3P2) at 15 weeks’ gestation visits your office for a routine prenatal care appointment. She reports having returned from a 3-week holiday in Brazil 2 days ago, and she is concerned about having experienced fever, malaise, arthralgias, and a disseminated erythematous rash. She has since heard about the Zika virus and asks you if she and her baby are in danger and whether she should be tested for the disease.
What should you tell this patient?
The Zika virus is an RNA Flavivirus, transmitted primarily by the Aedes aegypti mosquito.1 This virus is closely related to the organisms that cause dengue fever, yellow fever, chikungunya infection, and West Nile infection. By feeding on infected prey, mosquitoes can transmit the virus to humans through bites. They breed near pools of stagnant water, can survive both indoors and outdoors, and prefer to be near people. These mosquitoes bite mostly during daylight hours, so it is essential that people use insect repellent throughout the day while in endemic areas.2 These mosquitoes live only in tropical regions; however, the Aedes albopictus mosquito, also known as the Asian tiger mosquito, lives in temperate regions and can transmit the Zika virus as well3 (FIGURE 1).
| FIGURE 1 Aedes aegypti and Aedes albopictus mosquitoes | ||

| 
| |
Aedes aegypti (left) and Aedes albopictus (right) mosquitoes. Aedes mosquitoes are the main transmission vector for the Zika virus. | ||
|
The Zika virus was first discovered in 1947 when it was isolated from a rhesus monkey in Uganda. It subsequently spread to Southeast Asia and eventually caused major outbreaks in the Yap Islands of Micronesia (2007)4 and French Polynesia (2013).5 In 2015, local transmission of the Zika virus infection was noted in Brazil, and, most recently, a pandemic of Zika virus infection has occurred throughout South America, Central America, and the Caribbean islands. To date, local mosquito-borne virus transmission has not occurred in the continental United States, although at least 82 cases acquired during travel to infected areas have been reported.6
Additionally, there have been rare cases involving spread of this virus from infected blood transfusions and through sexual contact.7 In February 2016, the first case of locally acquired Zika virus infection was reported in Texas following sexual transmission of the disease.8
Clinical manifestations of Zika virus infection
Eighty percent of patients infected with Zika virus remain asymptomatic. The illness is short-lived, occurring 2 to 12 days following the mosquito bite, and infected individuals usually do not require hospitalization or experience serious morbidity. When symptoms are present, they typically include low-grade fever (37.8° to 38.5°C), maculopapular rash, arthralgias of the hands and feet, and nonpurulent conjunctivitis. Patients also may experience headache, retro-orbital pain, myalgia, and, rarely, abdominal pain, nausea, vomiting, diarrhea, ulcerations of mucous membranes, and pruritus.9 Guillain-Barré syndrome has been reported in association with Zika virus infection10; however, a definitive cause-effect relationship has not been proven.
If a pregnant woman is infected with the Zika virus, perinatal transmission can occur, either through uteroplacental transmission or vertically from mother to child at the time of delivery. Zika virus RNA has been detected in blood, amniotic fluid, semen, saliva, cerebrospinal fluid, urine, and breast milk. Although the virus has been shown to be present in breast milk, there has been no evidence of viral replication in milk or reported transmission in breastfed infants.11 Pregnant women are not known to have increased susceptibility to Zika virus infection when compared with the general population, and there is no evidence to suggest pregnant women will have a more serious illness if infected.
The Zika virus has been strongly associated with congenital microcephaly and fetal loss among women infected during pregnancy.12 Following the recent large outbreak in Brazil, an alarmingly high number of Brazilian newborns with microcephaly have been observed. The total now exceeds 4,000. Because of these ominous findings, fetuses and neonates born to women with a history of infection should be evaluated for adverse effects of congenital infection.
Management strategies for Zika virus exposure during pregnancy
The incidence of Zika virus infection during pregnancy remains unknown. However, a pregnant woman may be infected in any trimester, and maternal-fetal transmission of the virus can occur throughout pregnancy. If a patient is pregnant and has travelled to areas of Zika virus transmission, or has had unprotected sexual contact with a partner who has had exposure, she should be carefully screened with a detailed review of systems and ultrasonography to evaluate for fetal microcephaly or intracranial calcifications. The US Centers for Disease Control and Prevention (CDC) initially recommended that, if a patient exhibited 2 or more symptoms consistent with Zika virus infection within 2 weeks of exposure or if sonographic evidence revealed fetal microcephaly or intracranial calcifications, she should be tested for Zika virus infection.11
More recently, the CDC issued new guidelines recommending that even asymptomatic women with exposure have serologic testing for infection and that all exposed women undergo serial ultrasound assessments.13 The CDC also recommends offering retesting in the mid second trimester for women who were exposed very early in gestation.
The best diagnostic test for infection is reverse transcriptase-polymerase chain reaction (RT-PCR), and, ideally, it should be completed within 4 days of symptom onset. Beyond 4 days after symptom onset, testing for Zika virus immunoglobulin M (IgM)-specific antibody and neutralizing antibody should be performed in addition to the RT-PCR test. At times, interpretation of antibody testing can be problematic because cross-reaction with related arboviruses is common. Moreover, Zika viremia decreases rapidly over time; therefore, if serum is collected even 5 to 7 days after symptom onset, a negative test does not definitively exclude infection (TABLE 1).
In the United States, local health departments should be contacted to facilitate testing, as the tests described above are not currently commercially available. If the local health department is unable to perform this testing, clinicians should contact the CDC’s Division of Vector-Borne Diseases (telephone: 1-970-221-6400) or visit their website (http://www.cdc.gov/ncezid/dvbd/specimensub/arboviral-shipping.html) for detailed instructions on specimen submission.
Testing is not indicated for women without a history of travel to areas where Zika virus infection is endemic or without a history of unprotected sexual contact with someone who has been exposed to the infection.
Following the delivery of a live infant to an infected or exposed mother, detailed histopathologic evaluation of the placenta and umbilical cord should be performed. Frozen sections of placental and cord tissue should be tested for Zika virus RNA, and cord serum should be tested for Zika and dengue virus IgM and neutralizing antibodies. In cases of fetal loss in the setting of relevant travel history or exposure (particularly maternal symptoms or sonographic evidence of microcephaly), RT-PCR testing and immunohistochemistry should be completed on fetal tissues, umbilical cord, and placenta.2
Treatment is supportive
At present, there is no vaccine for the Zika virus, and no hyperimmune globulin or anti‑ viral chemotherapy is available. Treatment is therefore supportive. Patients should be encouraged to rest and maintain hydration. The preferred antipyretic and analgesic is acetaminophen (650 mg orally every 6 hours or 1,000 mg orally every 8 hours). Aspirin should be avoided until dengue infection has been ruled out because of the related risk of bleeding with hemorrhagic fever. Nonsteroidal anti-inflammatory drugs should be avoided in the second half of pregnancy because of their effect on fetal renal blood flow (oligohydramnios) and stricture of the ductus arteriosus.
CASE 1 Continued
Given this patient’s recent travel, exposure to mosquito-borne illness, and clinical manifestations of malaise, rash, and joint pain, you proceed with serologic testing. The RT-PCR test is positive for Zika virus.
What should be the next step in the management of this patient?
Prenatal diagnosis and fetal surveillance
The recent epidemic of microcephaly and poor pregnancy outcomes reported in Brazil has been alarming and demonstrates an almost 20-fold increase in incidence of this condition between 2014–2015.14 Careful surveillance is needed for this birth defect and other poor pregnancy outcomes in association with the Zika virus. To date, a direct causal relationship between Zika virus infection and microcephaly has not been unequivocally established15; however; these microcephaly cases have yet to be attributed to any other cause (FIGURE 2)
| FIGURE 2 Microcephaly: associated with Zika virus infection in pregnancy |

|
Illustration depicts a child with congenital microcephaly (left) and one with head circumference within the mean SD (right). |
Following the outbreak in Brazil, a task force and registry were established to investigate microcephaly and other birth defects associated with Zika virus infection. In one small investigation, 35 cases of microcephaly were reported, and 71% of the infants were seriously affected (head circumference >3 SD below the mean). Fifty percent of babies had at least one neurologic abnormality, and, of the 27 patients who had neuroimaging studies, all had distinct abnormalities, including widespread brain calcifications and cell migration abnormalities, such as lissencephaly, pachgyria, and ventriculomegaly due to cortical atrophy.16
In addition to microcephaly, fetal ultrasound monitoring has revealed focal brain abnormalities, such as asymmetric cerebral hemispheres, ventriculomegaly, displacement of the midline, failure to visualize the corpus callosum, failure of thalamic development, and the presence of intraocular and brain calcifications.17
In collaboration with the CDC, the American College of Obstetricians and Gynecologists and the Society for Maternal Fetal-Medicine have developed guidelines to monitor fetal growth in women with laboratory evidence of Zika virus infection.18 Recommendations include having a detailed anatomy ultrasound and serial growth sonograms every 3 to 4 weeks, along with referral to a maternal-fetal medicine or infectious disease specialist.
If the pregnancy is beyond 15 weeks’ gestational age, an amniocentesis should be performed in symptomatic patients and in those with abnormal ultrasound findings. Amniotic fluid should be tested for Zika virus with RT-PCR (FIGURE 3).12 The sensitivity and specificity of amniotic fluid RT-PCR in detecting congenital infection, as well as the predictive value of a fetal anomaly, remain unknown at this time. For this reason, a patient must be counseled carefully regarding the benefits of confirming intrauterine infection versus the slight risks of premature rupture of membranes, infection, and pregnancy loss related to amniocentesis.
Once diagnosed, microcephaly cannot be “fixed.” However, pregnancy termination is an option that some parents may choose once they are aware of the diagnosis and prognosis of microcephaly. Moreover, even for parents who would not choose abortion, there may be considerable value in being prepared for the care of a severely disabled child. Microcephaly has many possible causes, Zika virus infection being just one. Others include genetic syndromes and other congenital infections, such as cytomegalovirus (CMV) infection and toxoplasmosis. Amniocentesis therefore may help the clinician sort through these causes. For both CMV infection and toxoplasmosis, certain antenatal treatments may be helpful in lessening the severity of fetal injury.
CASE 2 Pregnant patient has travel plans
A 34-year-old woman (G1P0) presents to you for her first prenatal visit. She mentions she plans to take a cruise through the Eastern Caribbean in 2 weeks. Following the history and physical examination, what should you tell this patient?
Perinatal counseling: Limiting exposure is best
As mentioned, there is currently no treatment, prophylactic medication, or vaccination for Zika virus infection. Because of the virus’s significant associations with adverse pregnancy outcomes, birth defects, and fetal loss, the CDC has issued a travel advisory urging pregnant women to avoid travel to areas when Zika virus infection is prevalent. Currently, Zika virus outbreaks are occurring throughout South and Central America, the Pacific Islands, and Africa, and the infection is expected to spread, mainly due to international air travel. If travel to these areas is inevitable, women should take rigorous precautions to avoid exposure to mosquito bites and infection (TABLE 2).
If a woman was infected with laboratory-confirmed Zika virus infection in a prior pregnancy, she should not be at risk for congenital infection during her next pregnancy. This is mainly because the period of viremia is short-lived and lasts approximately 5 to 7 days.2
Further, based on documented sexual transmission of the virus, pregnant women should abstain from sexual activity or should consistently and correctly use condoms with partners who have Zika virus infection or exposure to the virus until further evidence is available.
Stay informed
Zika virus infection is now pandemic; it has evolved from an isolated disease of the tropics to one that is sweeping the Western hemisphere. It is being reported daily in new locations around the world. Given the unsettling association of Zika virus infection with birth defects, careful obstetric surveillance of exposed or symptomatic patients is imperative. Clinicians must carefully screen patients with potential risk of exposure and be prepared to offer appropriate perinatal counseling and diagnostic testing during pregnancy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Dyer O. Zika virus spreads across Americas as concerns mount over birth defects. BMJ. 2015;351:h6983.
- Centers for Disease Control and Prevention. Zika virus. Atlanta, GA: US Dept of Health and Human Services; 2015. http://www.cdc.gov/zika/index.html. Accessed February 12, 2016.
- Bogoch II, Brady OJ, Kraemer MU, et al. Anticipating the international spread of Zika virus from Brazil. Lancet. 2016;387(10016):335–336.
- Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536–2543.
- Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill. 2014;19(13):pii:20751.
- Centers for Disease Control and Prevention. Zika virus disease in the United States, 2015–2016. http://www.cdc.gov/zika/geo/united-states.html. Accessed February 12, 2016.
- Foy BD, Kobylinski KC, Chilson Foy JL, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17(5):880–882.
- Dallas County Health and Human Services. DCHHS reports first Zika virus case in Dallas County acquired through sexual transmission. http://www.dallascounty.org/department/hhs /press/documents/PR2-2-16DCHHSReportsFirstCaseofZikaVirusThroughSexualTransmission.pdf. Accessed February 3, 2016.
- Ministry of Health, Manuatu Hauora. Zika virus. http://www.health.govt.nz/our-work/diseases-and-conditions/zika -virus. Accessed January 13, 2016.
- Oehler E, Watrin L, Larre P, et al. Zika virus infection complicated by Guillain-Barre syndrome—case report, French Polynesia, December 2013. Euro Surveill. 2014;19:4–6.
- Centers for Disease Control and Prevention. Zika virus: transmission. http://www.cdc.gov/zika/transmission/index.html. Accessed January 20, 2016.
- Petersen EE, Staples JE, Meaney-Delamn, D et al. Interim guidelines for pregnant women during a Zika virus outbreak—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30–33.
- Oduyebo T, Petersen EE, Rasmussen SA, et al. Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(5):122–127.
- Pan American Health Organization, World Health Organization. Epidemiological alert: neurological syndrome, congenital malformations, and Zika virus infection. Implications for public health in the Americas. December 1,2015. http://www.paho.org/hq/index.php?option=com_doc man&task=doc_view&Itemid=270&gid=32405&lang=en. Accessed January 13, 2016.
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus epidemic in the Americas: potential associations with microcephaly and Guillain-Barré syndrome. December 10, 2015. http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association -with-microcephaly-rapid-risk-assessment.pdf. Accessed January 13, 2016.
- Schuler-Faccini L, Ribeiro EM, Feitosa IM, et al; Brazilian Medical Genetics Society—Zika Embryopathy Task Force. Possible association between Zika virus infection and microcephaly—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(3):59–62.
- Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol. 2016;47(1):6–7.
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus epidemic in the Americas: potential associations with microcephaly and Guillain-Barré syndrome. December 10, 2015. http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association.
- Dyer O. Zika virus spreads across Americas as concerns mount over birth defects. BMJ. 2015;351:h6983.
- Centers for Disease Control and Prevention. Zika virus. Atlanta, GA: US Dept of Health and Human Services; 2015. http://www.cdc.gov/zika/index.html. Accessed February 12, 2016.
- Bogoch II, Brady OJ, Kraemer MU, et al. Anticipating the international spread of Zika virus from Brazil. Lancet. 2016;387(10016):335–336.
- Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536–2543.
- Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill. 2014;19(13):pii:20751.
- Centers for Disease Control and Prevention. Zika virus disease in the United States, 2015–2016. http://www.cdc.gov/zika/geo/united-states.html. Accessed February 12, 2016.
- Foy BD, Kobylinski KC, Chilson Foy JL, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17(5):880–882.
- Dallas County Health and Human Services. DCHHS reports first Zika virus case in Dallas County acquired through sexual transmission. http://www.dallascounty.org/department/hhs /press/documents/PR2-2-16DCHHSReportsFirstCaseofZikaVirusThroughSexualTransmission.pdf. Accessed February 3, 2016.
- Ministry of Health, Manuatu Hauora. Zika virus. http://www.health.govt.nz/our-work/diseases-and-conditions/zika -virus. Accessed January 13, 2016.
- Oehler E, Watrin L, Larre P, et al. Zika virus infection complicated by Guillain-Barre syndrome—case report, French Polynesia, December 2013. Euro Surveill. 2014;19:4–6.
- Centers for Disease Control and Prevention. Zika virus: transmission. http://www.cdc.gov/zika/transmission/index.html. Accessed January 20, 2016.
- Petersen EE, Staples JE, Meaney-Delamn, D et al. Interim guidelines for pregnant women during a Zika virus outbreak—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30–33.
- Oduyebo T, Petersen EE, Rasmussen SA, et al. Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(5):122–127.
- Pan American Health Organization, World Health Organization. Epidemiological alert: neurological syndrome, congenital malformations, and Zika virus infection. Implications for public health in the Americas. December 1,2015. http://www.paho.org/hq/index.php?option=com_doc man&task=doc_view&Itemid=270&gid=32405&lang=en. Accessed January 13, 2016.
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus epidemic in the Americas: potential associations with microcephaly and Guillain-Barré syndrome. December 10, 2015. http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association -with-microcephaly-rapid-risk-assessment.pdf. Accessed January 13, 2016.
- Schuler-Faccini L, Ribeiro EM, Feitosa IM, et al; Brazilian Medical Genetics Society—Zika Embryopathy Task Force. Possible association between Zika virus infection and microcephaly—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(3):59–62.
- Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol. 2016;47(1):6–7.
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus epidemic in the Americas: potential associations with microcephaly and Guillain-Barré syndrome. December 10, 2015. http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association.
In this Article
- Management strategies for pregnant patients with Zika virus exposure
- Fetal surveillance
- Perinatal counseling on exposure prevention
- Algorithm for evaluation and management
Cardiorenal Syndrome Type 1: Renal Dysfunction in Acute Decompensated Heart Failure
From the Cardiovascular Division, Department of Internal Medicine, University of Minnesota, Minneapolis, MN.
Abstract
- Objective: To present a review of cardiorenal syndrome type 1 (CRS1).
- Methods: Review of the literature.
- Results: Acute kidney injury occurs in approximately one-third of patients with acute decompensated heart failure (ADHF) and the resultant condition was named CRS1. A growing body of literature shows CRS1 patients are at high risk for poor outcomes, and thus there is an urgent need to understand the pathophysiology and subsequently develop effective treatments. In this review we discuss prevalence, proposed pathophysiology including hemodynamic and nonhemodynamic factors, prognosticating variables, data for different treatment strategies, and ongoing clinical trials and highlight questions and problems physicians will face moving forward with this common and challenging condition.
- Conclusion: Further research is needed to understand the pathophysiology of this complex clinical entity and to develop effective treatments.
Acute decompensated heart failure (ADHF) is an epidemic facing physicians throughout the world. In the United States alone, ADHF accounts for over 1 million hospitalizations annually, with costs in 2012 reaching $30.7 billion [1]. Despite the advances in chronic heart failure management, ADHF continues to be associated with poor outcomes as exemplified by 30-day readmission rates of over 20% and in-hospital mortality rates of 5% to 6%, both of which have not significantly improved over the past 20 years [2,3]. One of the strongest predictors of adverse outcomes in ADHF is renal dysfunction. An analysis from the Acute Decompensated Heart Failure National Registry (ADHERE) revealed the combination of renal dysfunction (creatinine > 2.75 mg/dL and blood urea nitrogen (BUN) > 43 mg/dL) and hypotension (systolic blood pressure (SBP) < 115 mm Hg) upon admission was associated with an in-hospital mortality of > 20% [4]. The Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) registry documented a 16.3% in-hospital mortality when patients had a SBP < 100 mm Hg and creatinine > 2.0 mg/dL at admission [5].
The presence of acute kidney injury in the setting of ADHF is a very common occurrence and was termed cardiorenal syndrome type 1 (CRS1) [6]. The prevalence of CRS1 in single-centered studies ranged from 32% to 40% of all ADHF admissions [7,8]. If this estimate holds true throughout the United States, there would be 320,000 to 400,000 hospitalizations for CRS1 annually, highlighting the magnitude of this problem. Moreover, with the number of patients with heart failure expected to continue to rise, CRS1 will only become more prevalent in the future. In this review we discuss the prevalence, proposed pathophysiology including hemodynamic and nonhemodynamic factors, prognosticating variables, data for different treatment strategies, ongoing clinical trials, and highlight questions and problems physicians will face moving forward in this common and challenging condition.
Pathogenesis of CRS1
Hemodynamic Effects
The early hypothesis for renal dysfunction in ADHF centered on hemodynamics, as reduced cardiac output was believed to decrease renal perfusion. However, analysis of invasive hemodynamics from patients with ADHF suggested that central venous pressure (CVP) was actually a better predictor of the development of CRS1 than cardiac output. In a single-center study conducted at the Cleveland Clinic, hemodynamics from 145 patients with ADHF were evaluated and surprisingly baseline cardiac index was greater in the patients with CRS1 than patients without renal dysfunction (2.0 ± 0.8 L/min/m2 vs 1.8 ± 0.4 L/min/m2; P = 0.008). However, baseline CVP was higher in the CRS1 group (18 ± 7 mm Hg vs 12 ± 6 mm Hg; P = 0.001), and there was a heightened risk of developing CRS1 as CVP increased. In fact, 75% of the patients with a CVP of > 24 mm Hg developed renal impairment [9]. In a retrospective study of the Evaluation Study of Congestive Heart Failure and Pulmonary Arterial Catheter Effectiveness (ESCAPE) trial, the only hemodynamic parameter that correlated with baseline creatinine was CVP. However, no invasive measures predicted worsening renal function during hospitalization [10]. Finally, an experiment that used isolated canine kidneys showed increased venous pressure acutely reduced urine production. Interestingly, this relationship was dependent on arterial pressure; as arterial flow decreased smaller increases in CVP were needed to reduce urine output [11]. Together, these data suggest increased CVP plays an important role in CRS1, but imply hemodynamics alone may not fully explain the pathophysiology of CRS1.
Inflammation
As information about how hemodynamics incompletely predict renal dysfunction in ADHF became available, alternative hypotheses were investigated to gain a deeper understanding of the pathophysiology underlying CRS1. A pathological role of inflammation in CRS1 has gained attention due to recent publications. First of all, serum levels of the pro-inflammatory cytokines TNF-a and IL-6 were elevated in patients with CRS1 when compared to health controls [12]. Interestingly, Virzi et al showed that the median value of IL-6 was 5 times higher in CRS1 patients when compared to ADHF patients without renal dysfunction [13]. The negative consequences of elevated serum cytokines were demonstrated when incubation of a human cell line of monocytes with serum from CRS1 patients induced apoptosis in 81% of cells compared to just 11% of cells with control serum [12]. It is possible that cytokine-induced apoptosis could occur in other cell types in different organs in patients with CRS1, which may contribute to both cardiac and renal dysfunction. Finally, analysis from a rat model of CRS1 revealed macrophage infiltration into the kidneys and increased numbers of activated monocytes in the peripheral blood. Interestingly, monocyte/macrophage depletion using liposome clodronate prevented chronic renal dysfunction in the rat model [14]. In summary, these data suggest inflammation contributes to CRS1 pathophysiology, but more experimental data is needed to determine if there is a causal relationship.
Oxidative Stress
Very recently, oxidative stress was proposed to play a role in CRS1. Virzi et al analyzed serum levels of markers of oxidative stress and compared ADHF patients without renal impairment to CRS1 patients. The markers of oxidative stress, which included myeloperoxidase, nitric oxide, copper/zinc superoxide dismutase, and endogenous peroxidase, were all significantly higher in CRS1 patients [13]. While provocative, the tissues responsible for the generation of these molecules and the subsequent effects have not yet been fully elucidated.
Prognostication
Severity of Acute Kidney Injury
Initial publications did not document a strong link between kidney injury and poor outcomes in ADHF. Firstly, Ather et al performed a single-centered study that investigated how change in renal function defined by change in creatinine, estimated GFR, and BUN affected outcomes one year post admission for ADHF. Kidney injury defined by a change in creatinine or in estimated GFR was not associated with increased risk of mortality, but a change in BUN was associated with increased mortality in a univariate analysis [15]. Testani et al retrospectively analyzed patients from the ESCAPE trial and found worsening renal function defined by a ≥ 20% reduction in estimated GFR was not significantly associated with 180-day mortality, but there was a trend towards higher mortality (hazard ration 1.4; P = 0.11) [16]. Importantly, neither of 2 these studies assessed how severity of AKI impacted outcomes, which may have contributed to the weak relationships observed.
Diuretic Responsiveness
Voors et al performed a retrospective analysis of diuretic responsiveness in 1161 patients from the Relaxin in Acute Heart Failure (RELAX-AHF) trial. Diuretic responsiveness was defined as weight change (kg) per diuretic dose (IV furosemide and PO furosemide) over 5 days and then patients were separated into tertiles. The lowest tertile group had an approximate 20% incidence of 60-day combined end-point of death, heart failure or renal failure readmission compared to less than 10% incidence in the middle and upper tertiles. Interestingly, when the effects of worsening renal function (WRF), defined as creatinine change of ≥ 0.3 mg/dL, were examined in patients stratified by diuretic response, WRF did not offer additional prognostic information [19].
Finally, Valenete et al analyzed diuretic response in 1745 patients from the PROTECT trial (Placebo-Controlled Randomized Study of the Selective A1-Adenosine Receptor Antagonist Rolofylline for Patients Hospitalized with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function). Diuretic response was calculated using the weight change per 40 mg of furosemide, and as diuretic response declined there was increasing risk of 60-day rehospitalization and 180-day mortality rates. In fact, the lowest quintile responders had a 25% mortality rate at 180 days [20].
Emerging Biomarkers
Urine Neutrophil Gelatinase-Associated Lipocalin
Because previous studies showed urinary levels of NGAL was an earlier and more reliable marker of renal dysfunction than creatinine in AKI [21], it was studied as a possible biomarker for the development of CRS1 in ADHF. A single-centered study quantified levels of urine NGAL in 100 patients admitted with heart failure and then tracked the rates of acute kidney injury. Urine NGAL was elevated in patients that developed AKI and a cut-off value 12 ng/mL had a sensitivity of 79% and specificity of 67% for predicting CRS1 [22]. While promising, further studies are needed to better define the role of NGAL in CRS1.
Cystatin C
Cystatin C is a ubiquitously expressed cysteine protease that has a constant production rate and is freely filtered by the glomerulus without being secreted into the tubules, and has effectively prognosticated outcomes in ADHF [23]. Lassus et al showed an adjusted hazard ratio of 3.2 (2.0–5.3) for 12-month mortality when cystatin C levels were elevated. Moreover, patients with the highest tertitle of NT-proBNP and cystatin C had a 48.7% 1-year mortality. Interestingly, patients with an elevated cystatin C but normal creatinine had a 40.6% 1-year mortality compared to 12.6% for those with normal cystatin C and creatinine [24]. Furthermore, Arimoto et al showed elevated cystatin C predicted death or rehospitalization in a small cohort of ADHF patients in Japan [25]. Also, Naruse et al showed cystatin C was a better predictor of cardiac death than estimated GFR by the Modification of Diet in Renal Disease Study (MDRD) equation [26]. Finally, Manzano-Fernandez et al showed the highest tertile of cystatin C was a significant independent risk factor for 2-year death or rehospitalization while creatinine and MDRD estimates of GFR were not [27]. In agreement with Lassus et al, elevations in either 2 or 3 of cystatin C, troponin, and NT-proBNP predicted death or rehospitalization when compared to those with normal levels of these 3 markers [27]. In conclusion, cystatin C either alone or in combination with other biomarkers identifies high-risk patients.
Kidney Injury Molecule 1
Kidney injury molecule 1 (KIM-1) is a type-1 cell membrane glycoprotein expressed in regenerating proximal tubular cells but not under normal conditions [28]. Although associated with increased risk of hospitalization and mortality in chronic heart failure [29,30], elevated levels of urinary KIM-1 did not predict mortality in ADHF [31]. Further studies are needed to elucidate the utility of KIM-1 in CRS1.
Treatment Approaches
Diuretics
Loop diuretics are the main treatment for decongestion of patients with CRS1. To date, no clinical trial has compared the different loop diuretics (furosemide, bumetanide, torsemide, or ethacrynic acid) to each other, so there is no clear choice of which loop diuretic is the best. However, dosing scheme was investigated in the Dose Optimization Strategies Evaluation (DOSE) trial. In this trial, 308 patients were randomized in a 1:1:1:1 design in which patients were placed in groups with low-dose (equivalent to oral dose) or high-dose (2.5 times oral dose) intermittent parental therapy or alternatively low-dose or high-dose continuous drip therapy. There were no differences in dyspnea, fluid changes, change in creatinine, hospital length stay, or rehospitalization and death rates when the intermittent and drip approaches were compared. However, the high-dose arm had decreased dyspnea, increased volume removal, but there were more occurrences of AKIs when compared to the low-dose arm [32].
In clinical practice, if loop diuretic treatment does not result in the desired urine output, a second-site diuretic may be added to potentiate diuresis. Unfortunately, there is little data on this common clinical practice and thus the optimal choice of second site agent (chlorthiazide or metolazone) is unknown. Frequently, the deciding factor is based upon cost or concern that oral absorption of metolazone will be ineffective. However, Moranville et al recently performed a retrospective assessment comparing chlorthiazide (22 patients) to metolazone (33 patients) in ADHF patients with renal dysfunction defined by a creatinine clearance of 15–50 mL/min. There was a nonsignificant trend towards increased urine output in the metolazone group, no differences in the rates of adverse events, and the chlorthiazide group actually had a longer hospital stay [33]. While potentially promising results, the retrospective nature of the study made it difficult to determine if the differences were due to treatment approach or dissimilarities of patient illness. Nonetheless, physicians must remain vigilant when implementing the second-site diuretic approach because it can lead to marked diuretic response leading to metabolic derangements including hypokalemia, hyponatremia, hypomagnesaemia, and metabolic alkalosis.
Inotropes
The use of inotropic agents such as dobutamine or milrinone can be used to augment cardiac function when there is a known low-output state for better renal perfusion in CRS1. Unfortunately, there is little objective data available about the utility of this widely implemented approach. The Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of a Chronic Heart Failure (OPTIME-HF) trial did not show improved renal function with milrinone treatment [34]. The use of levosimendan, a cardiac calcium sensitizer that increases contractility not currently approved in the United States, was compared to dobutamine in the Survival of Patients With Acute Heart Failure in Need of Intravenous Inotropic Support (SURVIVE) trial, and there were no differences in rates of renal failure when the 2 groups were compared [35]. Nonetheless, if cardiac output is severely compromised, inotropes can be used for CRS1 treatment, but they should be used cautiously due the increased risks of lethal arrhythmias.
Dopamine
Use of low-dose dopamine to stimulate D1 and D2 receptors as a way to increase renal blood flow and promote increased glomerular filtration and urine production was extensively studied in ADHF. A small trial showed use of low dose dopamine had renal protective effects in a total of 20 patients [36]. However, when larger trials were conducted, such beneficial results were not consistently observed. The Dopamine in Acute Decompensated Heart Failure (DAD-HF I) trial compared low-dose furosemide plus low-dose dopamine (5 µg/kg/min) to high-dose furosemide alone in 60 patients. There were no differences in total diuresis, hospital stay, and 60-day mortality or rehospitalization rates, but there was a reduction in the renal dysfunction at the 24-hour time point in the dopamine-treated arm (6.7% versus 30%) [37]. The Dopamine in Acute Decompensated Heart Failure II trial randomized 161 ADHF patients to high-dose furosemide, low-dose furosemide and lose dose dopamine (5 µg/kg/min), or low-dose furosemide and assessed dyspnea, worsening renal function, length of stay, 60-day and one-year all-cause mortality and hospitalization for heart failure. Dopamine treatment did not improve any of the outcomes measured [38]. Finally, the most recent trial to examine the effects of dopamine was the Renal Optimization Strategies Evaluation (ROSE) trial. In this trial, there were 360 patients with ADHF randomized to nesiritide or dopamine versus placebo in a 2:1 design. When comparing dopamine (111 patients) treatment to placebo (115 patients), there were no differences in urine output, renal function as determined by cystatin C levels, or symptomatic improvements. However, there was more tachycardia in the dopamine group [39]. Currently, there is not strong evidence supporting routine use of dopamine in CRS1.
Nesiritide
Use of nesiritide, recombinant brain natriuretic peptide, was also investigated as a way to enhance urine production through the natriuretic effects of the peptide. The first attempt to explore this hypothesis was the B-Type Natriuretic Peptide in Cardiorenal Decompensation Syndrome (BNP-CARDS) trial. BNP-CARDS showed a 48-hour infusion of nesiritide (39 patients) or placebo (36 patients) in patients with ADHF and renal dysfunction (estimated GFR between 15–60 mL/min) did not reduce the incidence of worsening renal function as defined by a rise in serum creatinine by 20% [40]. A similar approach was implemented in the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND-HF) trial which examined over 7000 patients with ADHF. 3496 patients were treated with nesiritide and 3511 patients were treated with placebo for 24 hours and up to 7 days. Nesiritide treatment did not alter dyspnea at 6 and 24 hours, improve renal function as determined by creatinine change, or alter the combined end-point of rehospitalization or death 30 at days [41]. The ROSE trial examined the effects of nesiritide (117 patients) versus placebo (115 patients) for urine production, change in renal function as defined by change in cystatin C, and decongestion (urinary sodium excretion, weight change, and change in NT-proBNP) at 72 hours. Nesiritide did not alter any of the outcomes investigated [39]. Finally, a single-centered study conducted at the Mayo Clinic examined the effects of nesiritide (37 patients) or placebo (35 patients) with ADHF and pre-existing renal dysfunction (estimated GFR between 20 and 60 mL/min). These investigators found nesiritide treatment resulted in less renal dysfunction as measured by creatinine and BUN, but no changes in diuretic responsiveness, duration of hospitalization, or rehospitalization rates. Nesiritide did reduce serum endothelin levels, but had no effect on ANP, NT-pro BNP, renin, angiotensin II, or aldosterone [42]. In summary, nesiritide does not appear to have significant renal protective effects in ADHF.
Adenosine A1 Receptor Antagonists
The use of adenosine receptor antagonists to prevent adenosine-mediated vasoconstriction of renal vasculature in ADHF has also been examined. The first study conducted was a small double-blind randomized-controlled trial that investigated the effects of rolofylline, an adenosine A-1 antagonist, in patients with ADHF and an estimated creatinine clearance of 20-80 mL/min. The study had 27 patients in the placebo arm, 29 patients that received 2.5 mg of rolofylline, 31 patients received 15 mg of rolofylline, 30 patients received 30 mg of rolofylline, and 29 patients received 60 mg of rolofylline, all of which was daily for up to 3 days. Rolofylline treatment increased urine output on day 1 and improved renal function on day 2 [43]. These positive results led to the Placebo-Controlled Randomized Study of Selective Adenosine A1 Receptor Antagonist Rolofylline for Patients with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function (PROTECT) Trial. PROTECT assessed the effects of rolofylline (1356) or placebo (677) in patients with ADHF and an estimated creatinine clearance between 20 and 80 mL/min. There were no significant differences in renal function out to 14 days, but rolofylline led to more weight loss than placebo [44,45]. In a subgroup analysis of patients with severe baseline renal dysfunction (creatinine clearance of less than 30 mL/min), rolofylline reduced the combined 60-day end-point of hospitalization due to cardiovascular or renal cause and death [45]. Finally, the Effects of KW-3902 Injectable Emulsion on Heart Failure Signs and Symptoms, Diuresis, Renal Function, and Clinical Outcomes in Subjects Hospitalized with Worsening Renal Function and Heart Failure Requiring Intravenous Therapy (REACH-UP) trial probed the effects of rolofylline (36 patients) or placebo (40 patients) in patients with ADHF and renal impairment (creatinine clearance of 20-60 mL/min). Rolofylline treatment did not alter renal function, but there was a nonsignificant trend towards reduction in 60-day combined end-point of hospitalization due to renal or cardiovascular causes or death [46]. In summary, the use of rolofylline has not been conclusively associated with improved outcomes in CRS1.
Vasopressin Antagonists
The use of vasopressin antagonists to induce aquaphoresis and combat hyponatremia was studied in ADHF. Vasopressin antagonists were first investigated in the Acute and Chronic Therapeutic Impact of a Vasopressin Antagonist (ACTIV) trial. ACTIV involved 3 doses of tolvaptan (78 patients received 30 mg, 84 patients received 60 mg, and 77 patients received 90 mg) versus placebo (80 patients), and tolvaptan increased urine production and decreased body weight compared to placebo without compromising renal function. A post-hoc analysis of patients with renal dysfunction (BUN > 29 mg/dL) and severe volume overload revealed a survival benefit at 60 days [47]. The Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVERST) trial compared placebo (2061 patients) versus 30 mg/day of tolvaptan (2072 patients) within 48 hours after admission in an identical 2-trial design. Tolvaptan increased weight loss and reduced dyspnea acutely but did not alter all-cause mortality or cardiovascular or heart failure hospitalization rates out to 24 months post index hospitalization [48,49]. These data suggest vasopressin antagonists may potentiate diuresis acutely but likely do not improve long-term outcomes.
Corticosteroids
The use of corticosteroids in ADHF has been controversial as there were initial concerns that corticosteroids would increase fluid retention. However, corticosteroids augmented diuretic response and improved renal function in 13 ADHF patients who had inadequate response to sequential nephron blockage [50]. Furthermore, Zhang et al showed that prednisone treatment in 35 patients admitted with ADHF increased urinary volume, reduced dyspnea, reduced uric acid, and improved renal function [51]. These promising results led to the Cardiac Outcome Prevention Effectiveness of Glucocorticoids in Acute Decompensated Heart Failure (COPE-ADHF) trial. In this single-centered study, 102 patients with ADHF were randomized to either placebo [51] or corticosteroids [51] and the outcomes recorded included urinary volume, change in creatinine, and cardiovascular death at 30 days. Use of corticosteroids improved renal function, increased urine output, and reduced mortality (3/51 in corticosteroid group versus 10/51 in the placebo group) [52]. The mechanisms underlying the improvements with corticosteroids were not determined, but were hypothesized to be facilitation of natriuretic peptides or dilation of renal vasculature through activation of nitric oxide pathway or dopaminergic system.
Serelaxin
Serelaxin is a recombinantly expressed human relaxin-2, a peptide hormone present during pregnancy which facilitates physiological cardiovascular and renal adaptations [53–55], which showed potential benefits in CRS1. Analysis of the RELAX-AHF trial revealed serelaxin reduced incidence of worsening renal function at day 2 of treatment as defined by changes in serum creatinine, cystatin C, and BUN. Importantly, worsening renal function defined by cystatin C changes was associated with increased 180-day mortality in this analysis [56]. The mechanisms by which serelaxin prevented renal dysfunction are currently unknown as serelaxin treatment did not improve diuretic efficiency [19].
Ultrafiltration
Another treatment choice in CRS1 is mechanical removal of salt and water via ultrafiltration. Ultrafiltration showed early promise in Ultrafiltration Versus Intravenous Diuretics for Patients Hospitalized for Acute Decompensated Heart Failure trial (UNLOAD) trial. In this study, 200 patients with ADHF were randomized to either ultrafiltration or medical management with loop diuretics. Use of ultrafiltration increased volume removal without any differences in renal function and reduced rehospitalization rates at 90 days [57].
However, when ultrafiltration was employed specifically in CRS1 patients in the Cardiorenal Rescue Study in Acute Decompensated Heart Failure trial (CARESS-HF), UF was not superior to medical treatment. There were 188 patients studied in CARESS-HF, and in the ultrafiltration arm there was increased risk of renal dysfunction, no differences in volume removal, and no change in rehospitalization rates at 90 days [58]. When trying to reconcile UNLOAD and CARESS-HF, the medical treatment arm in CARESS-HF was much more standardized and aggressive and UNLOAD was earlier implementation of ultrafiltration, which may have explained the differences. Interestingly, ultrafiltration was hypothesized to be advantageous over diuretic therapy through reduced activation of the renin-angiotensin-aldosterone system, but analysis of the patients from CARESS-HF showed higher levels of plasma renin activity and no difference in aldosterone levels in ultrafiltration patients [59].
Two meta-analyses have examined the use of ultrafiltration versus medical management in ADHF and both showed ultrafiltration was more effective in volume removal than medical therapy but did not improve rehospitalization or mortality rates [60,61]. This fact combined with the risks of vascular access placement and bleeding from anticoagulation limits to routine use of ultrafiltration in CRS1.
Continuous Renal Replacement Therapy
Once renal function deteriorates to the point that renal replacement therapy is needed for both volume removal and solute clearance in CRS1, continuous renal replacement therapy (CRRT) may be implemented. Unfortunately, there are few available data for this group of advanced CRS1 patients to guide physicians. There was a single-centered study conducted in Egypt that randomized 40 ADHF patients to either IV furosemide or CRRT. The patients treated with CRRT had greater weight loss and decreased length of stay in the ICU, but there were no differences in dialysis dependence rates or 30-day mortality [62]. Two single-centered studies reported outcomes associated with advanced CRS1 requiring CRRT. In a study conducted at the Cleveland Clinic, 63 patients with CRS1 were treated with ultrafiltration, of which 37 were converted to CRRT due to worsening renal function. Of the 37 patients treated with CRRT, 16 died in the hospital and 4 were discharged with hospice care [63]. In another retrospective study performed at the University of Alabama-Birmingham, use of rescue CRRT in advanced CRS1 was examined in 37 patients. 23 patients died during hospitalization and 2 were discharged to hospice care [64]. Combination of the Cleveland Clinic and University of Alabama-Birmingham studies revealed patients requiring CRRT in the setting of advanced CRS1 had an in-hospital mortality or palliative discharge rate of 60.8% (45/74). Clearly, this population needs further investigation to prevent such poor outcomes.
Future Treatment Options
Ongoing and Unreported Clinical Trials
Unfortunately, none of the current treatments for CRS1 have definitive improvements in outcomes, but there are several ongoing clinical trials which will hopefully identify novel treatment strategies. First of all, the Acetazolamide and Spironolactone to Increase Natriuresis in Congestive Heart Failure (Diuresis-CHF) trial is being conducted in Belgium. This study will examine the effects of acetazolamide with low dose diuretic versus high dose diuretics in one aim and the effects of upfront spironolactone in another. The outcomes analyzed will include total natriuresis, potassium homeostasis, NT-proBNP changes, change in renal function, peak serum levels of renin and aldosterone, weight change, urine volume, and change in edema (NCT01973335). The Protocolized Diuretic Strategy in Cardiorenal Failure (ProDius) trial is being performed at the University of Pittsburgh, and will determine the effects of a protocolized diuretic approach to target 3-5 liters of urine production a day versus standard therapy and will track the change in body weight, length of hospitalization, reshospitalization rates, mortality rates, venous compliance of internal jugular vein, clinical decongestion, change in renal function, and urine output (NCT01921829). The Levosimendan versus Dobutamine for Renal Function in Heart Failure (ELDOR) study is ongoing in Sweden and will probe the acute effects of levosimendan and dobutamine on renal perfusion. The endpoints will include changes in renal blood flow, GFR, renal vascular resistance, central hemodynamics, renal oxygen extraction and consumptions, and filtration fraction (NCT02133105). Finally, the Safety and Efficacy of Low Dose Hypertonic Saline and High Dose Furosemide for Congestive Heart Failure (REaCH) trial probed the effects of combination of hypertonic saline and furosemide versus furosemide in patients with ADHF and renal impairment defined by a GFR<60 mL/min. The outcomes were change in renal function, diuretic response, length of hospital stay, readmission rates, weight loss, BNP levels, and included a cost analysis. The study was completed but results are not currently available (NCT01028170)
Should Inflammation Be Targeted in CRS1?
Although proposed to play a role in the pathophysiology of CRS1, inflammation has not been explicitly targeted as a treatment for CRS1. One possible way to combat inflammation could be inhibition of the IL-6 pathway, which is support by preclinical work as previous studies showed IL-6 knockout mice were resistant to HgCl2-induced renal injury and death [65] and IL-6 has negative inotropic effects in both isolated cardiomyocytes [66] and intact animals [67]. Thus, IL-6 antagonism may improve both cardiac and renal function, an ideal scenario for CRS1 patients. The availability of tocilizumab, an FDA-approved humanized antibody to the IL-6 receptor, may allow for investigation of this hypothesis in the future. Although not examined in the COPE-ADHF trial, an alternative explanation for the improvements associated with corticosteroids treatment were the anti-inflammatory effects. If this were true, corticosteroids would represent a relatively cheap treatment option for CRS1 patients, but more studies need to be conducted before this approach is widely implemented. Finally, use of cytokine profiling may be used to enrich a population of CRS1 patients that could be investigated in future clinical trials using anti-inflammatory medications.
Unanswered Questions Moving Forward
Severity of AKI and Treatment Effects
An important unknown that warrants further investigation is if the severity of AKI should dictate treatment choice in CRS1. As discussed above, increasing severity of AKI resulted in elevated risk of adverse events, but it remains unknown whether different treatments offer benefits for more or less severe renal impairment. Perhaps, future studies aimed at defining outcomes from different treatment strategies stratified by severity of renal dysfunction may reveal which patients benefit from the various treatment options for CRS1.
How Do We Best Define Renal Dysfunction in CRS1?
Currently, there is no accepted definition of renal dysfunction in CRS1. As discussed above, using the AKIN, KDIGO, or RIFLE scoring systems or diuretic responsiveness effectively differentiated outcomes in patients with CRS1. However, an agreed-upon definition would likely benefit the field going forward so this population could be systematically investigated in future studies.
Conclusion
In summary, CRS1 is a common clinical entity associated with poor patient outcomes. A complex pathophysiology marked by reduced cardiac output, increased central venous pressure, inflammation, and oxidative stress underlies the disease process. Unfortunately, no current treatment approach shows consistent improvements in outcomes, highlighting the urgent need for further research to reduce the burden that CRS1 imposes.
Corresponding author: Kurt W. Prins, MD, PhD, MMC 580 Mayo, 420 Delaware St SE, Minneapolis, MN 55455, [email protected].
Funding/support: Dr. Prins is funded by NIH F32 grant HL129554 and Dr. Thenappen is funded by AHA Scientist Development Grant 15SDG25560048.
1. Mozaffarian D, Benjamin EJ, Go AS,et al. Heart disease and stroke statistics--2015 update: A report from the american heart association. Circulation 2015;131:e29–322.
2. Gheorghiade M, Vaduganathan M, Fonarow GC, Bonow RO. Rehospitalization for heart failure: problems and perspectives. J Am Coll Cardiol 2013;61:391–403.
3. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the medicare fee-for-service program. N Engl J Med 2009;360:1418–28.
4. Fonarow GC, Adams KF Jr, Abraham WT, et al and ADHERE Scientific Advisory Committee, Study Group, and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: Classification and regression tree analysis. JAMA 2005;293:572–80.
5. Abraham WT, Fonarow GC, Albert NM, et al. Predictors of in-hospital mortality in patients hospitalized for heart failure: Insights from the organized program to initiate lifesaving treatment in hospitalized patients with heart failure (OPTIMIZE-HF). J Am Coll Cardiol 2008;52:347–56.
6. Ronco C, Haapio M, House AA, et al. Cardiorenal syndrome. J Am Coll Cardiol 2008:52:1527–39.
7. Roy AK, Mc Gorrian C, Treacy C, et al. A comparison of traditional and novel definitions (RIFLE, AKIN, and KDIGO) of acute kidney injury for the prediction of outcomes in acute decompensated heart failure. Cardiorenal Med 2013;3:26–37.
8. Li Z, Cai L, Liang X, et al. Identification and predicting short-term prognosis of early cardiorenal syndrome type 1: KDIGO is superior to RIFLE or AKIN. PLoS One 2014;9:e114369.
9. Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009:53:589–96.
10. Nohria A, Hasselblad V, Stebbins A, et al. Cardiorenal interactions: Insights from the ESCAPE trial. J Am Coll Cardiol 2008:51:1268–74.
11. Winton FR. The influence of venous pressure on the isolated mammalian kidney. J Physiol 1931;72:49–61.
12. Virzi GM, Torregrossa R, Cruz DN, et al. Cardiorenal syndrome type 1 may be immunologically mediated: A pilot evaluation of monocyte apoptosis. Cardiorenal Med 2012;2:33–42.
13. Virzi GM, Clementi A, de Cal M, et al. Oxidative stress: Dual pathway induction in cardiorenal syndrome type 1 pathogenesis. Oxid Med Cell Longev 2015;391790.
14. Cho E, Kim M, Ko YS, et al. Role of inflammation in the pathogenesis of cardiorenal syndrome in a rat myocardial infarction model. Nephrol Dial Transplant 2013;28:2766–78.
15. Ather S, Bavishi C, McCauley MD, et al. Worsening renal function is not associated with response to treatment in acute heart failure. Int J Cardiol 2013;167:1912–7.
16. Testani JM, McCauley BD, Kimmel SE, Shannon RP. Characteristics of patients with improvement or worsening in renal function during treatment of acute decompensated heart failure. Am J Cardiol 2010;106:1763–69.
17. Hata N, Yokoyama S, Shinada T, et al. Acute kidney injury and outcomes in acute decompensated heart failure: Evaluation of the RIFLE criteria in an acutely ill heart failure population. Eur J Heart Fail 2010;12:32–7.
18. Testani JM, Brisco MA, Turner JM, et al. Loop diuretic efficiency: A metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ Heart Fail 2014;7:261–70.
19. Voors AA, Davison BA, Teerlink JR, et al. Diuretic response in patients with acute decompensated heart failure: Characteristics and clinical outcome--an analysis from RELAX-AHF. Eur J Heart Fail 2014;16:1230–40.
20. Valente MA, Voors AA, Damman K, et al. Diuretic response in acute heart failure: Clinical characteristics and prognostic significance. Eur Heart J 2014;35:1284–93.
21. Devarajan P. Neutrophil gelatinase-associated lipocalin: A troponin-like biomarker for human acute kidney injury. Nephrology (Carlton) 2010;15:419–28.
22. Soyler C, Tanriover MD, Ascioglu S, et al. Urine neutrophil gelatinase-associated lipocalin levels predict acute kidney injury in acute decompensated heart failure patients. Ren Fail 2015;5.
23. Brisco MA,Testani JM. Novel renal biomarkers to assess cardiorenal syndrome. Curr Heart Fail Rep 2014;11;485–99.
24. Lassus J, Harjola VP, Sund R, et al. and FINN-AKVA Study group. Prognostic value of cystatin C in acute heart failure in relation to other markers of renal function and NT-proBNP. Eur Heart J 2007;28:1841–7.
25. Arimoto T, Takeishi Y, Niizeki T, et al. Cystatin C, a novel measure of renal function, is an independent predictor of cardiac events in patients with heart failure. J Card Fail 2005;11:595–601.
26. Naruse H, Ishii J, Kawai T, et al. Cystatin C in acute heart failure without advanced renal impairment. Am J Med 2009;122:566–73.
27. Manzano-Fernandez S, Boronat-Garcia M, Albaladejo-Oton MD, et al. Complementary prognostic value of cystatin C, N-terminal pro-B-type natriuretic peptide and cardiac troponin T in patients with acute heart failure. Am J Cardiol 2009;103:1753–9.
28. Bonventre JV, Yang L. Kidney injury molecule-1. Curr Opin Crit Care 2010;16:556–61.
29. Damman K, Van Veldhuisen DJ, Navis G, et al. Tubular damage in chronic systolic heart failure is associated with reduced survival independent of glomerular filtration rate. Heart 2010;96:1297–302.
30. Jungbauer CG, Birner C, Jung B, et al. Kidney injury molecule-1 and N-acetyl-beta-D-glucosaminidase in chronic heart failure: Possible biomarkers of cardiorenal syndrome. Eur J Heart Fail 2011;13:1104–10.
31. Verbrugge FH, Dupont M, Shao Z, et al. Novel urinary biomarkers in detecting acute kidney injury, persistent renal impairment, and all-cause mortality following decongestive therapy in acute decompensated heart failure. J Card Fail 2013;19:621–8.
32. Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011;364:797–805.
33. Moranville MP, Choi S, Hogg J, et al. Comparison of metolazone versus chlorothiazide in acute decompensated heart failure with diuretic resistance. Cardiovasc Ther 2015;33;42–9.
34. Cuffe MS, Califf RM, Adams KF Jr, et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: A randomized controlled trial. JAMA 2002;287:1541–7.
35. Mebazaa A, Nieminen MS, Packer M, et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: The SURVIVE randomized trial. JAMA 2007;297:1883–91.
36. Varriale P, Mossavi A. The benefit of low-dose dopamine during vigorous diuresis for congestive heart failure associated with renal insufficiency: Does it protect renal function? Clin Cardiol 1997;20:627–30.
37. Giamouzis G, Butler J, Starling RC, et al. Impact of dopamine infusion on renal function in hospitalized heart failure patients: Results of the dopamine in acute decompensated heart failure (DAD-HF) trial. J Card Fail 2010;16:922–30.
38. Triposkiadis FK, Butler J, Karayannis G, et al. Efficacy and safety of high dose versus low dose furosemide with or without dopamine infusion: The dopamine in acute decompensated heart failure II (DAD-HF II) trial. Int J Cardiol 2014;172:115–21.
39. Chen HH, Anstrom KJ, Givertz MM, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: The ROSE acute heart failure randomized trial. JAMA 2013;310:2533–43.
40. Witteles RM, Kao D, Christopherson D, et al. Impact of nesiritide on renal function in patients with acute decompensated heart failure and pre-existing renal dysfunction a randomized, double-blind, placebo-controlled clinical trial. J Am Coll Cardiol 2007;50:1835–40.
41 O'Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med 2011;365:32–43.
42. Owan TE, Chen HH, Frantz RP, et al. The effects of nesiritide on renal function and diuretic responsiveness in acutely decompensated heart failure patients with renal dysfunction. J Card Fail 2008;14:267–75.
43. Givertz MM, Massie BM, Fields TK, et al and CKI-201 and CKI-202 Investigators. The effects of KW-3902, an adenosine A1-receptor antagonist,on diuresis and renal function in patients with acute decompensated heart failure and renal impairment or diuretic resistance. J Am Coll Cardiol 2007;50:1551–60.
44. Massie BM, O'Connor CM, Metra M, et al. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N Engl J Med 2010;363:1419–28.
45. Voors AA, Dittrich HC, Massie BM, et al. Effects of the adenosine A1 receptor antagonist rolofylline on renal function in patients with acute heart failure and renal dysfunction: Results from PROTECT (placebo-controlled randomized study of the selective adenosine A1 receptor antagonist rolofylline for patients hospitalized with acute decompensated heart failure and volume overload to assess treatment effect on congestion and renal function). J Am Coll Cardiol 2011;57:1899–907.
46. Gottlieb SS, Givertz MM, Metra M, et al. The effects of adenosine A(1) receptor antagonism in patients with acute decompensated heart failure and worsening renal function: The REACH UP study. J Card Fail 2010;16:714–9.
47. Gheorghiade M, Gattis WA, O'Connor CM, et al. Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: A randomized controlled trial. JAMA 2004;291:1963–71.
48. Gheorghiade M, Konstam MA, Burnett JC Jr, et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: The EVEREST clinical status trials. JAMA 2007;297:1332–43.
49. Konstam MA, Gheorghiade M, Burnett JC Jr, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: The EVEREST outcome trial. JAMA 2007;297:1319–31.
50. Liu C, Liu G, Zhou C, et al. Potent diuretic effects of prednisone in heart failure patients with refractory diuretic resistance. Can J Cardiol 2007;23:865–8.
51. Zhang H, Liu C, Ji Z, et al. Prednisone adding to usual care treatment for refractory decompensated congestive heart failure. Int Heart J 2008;49:587–95.
52. Liu C, Liu K and COPE-ADHF Study Group. Cardiac outcome prevention effectiveness of glucocorticoids in acute decompensated heart failure: COPE-ADHF study. J Cardiovasc Pharmacol 2014;63:333–8.
53. Teichman SL, Unemori E, Teerlink JR, et al. Relaxin: Review of biology and potential role in treating heart failure. Curr Heart Fail Rep 2010;7:75–82.
54. Conrad KP, Shroff SG. Effects of relaxin on arterial dilation, remodeling, and mechanical properties. Curr Hypertens Rep 2011;13:409–20.
55. Du XJ, Bathgate RA, Samuel CS, et al. Cardiovascular effects of relaxin: From basic science to clinical therapy. Nat Rev Cardiol 2010;7:48–58.
56. Metra M, Cotter G, Davison BA, et al. Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the relaxin in acute heart failure (RELAX-AHF) development program: Correlation with outcomes. J Am Coll Cardiol 2013;61:196-206.
57. Costanzo MR, Guglin ME, Saltzberg MT, et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol 2007;49:675–83.
58. Bart BA, Goldsmith SR, Lee KL, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012;367:2296–304.
59. Mentz RJ, Stevens SR, DeVore AD, et al. Decongestion strategies and renin-angiotensin-aldosterone system activation in acute heart failure. JACC Heart Fail 2015;3:97–107.
60. Ebrahim B, Sindhura K, Okoroh J, et al. Meta-analysis of ultrafiltration versus diuretics treatment option for overload volume reduction in patients with acute decompensated heart failure. Arq Bras Cardiol 2015;104:417–25.
61. Kwong JS, Yu CM. Ultrafiltration for acute decompensated heart failure: A systematic review and meta-analysis of randomized controlled trials. Int J Cardiol 2014;172:395–402.
62. Badawy SS, Fahmy A. Efficacy and cardiovascular tolerability of continuous veno-venous hemodiafiltration in acute decompensated heart failure: A randomized comparative study. J Crit Care 2012;27:106.e7-106.13.
63. Patarroyo M, Wehbe E, Hanna M, et al. Cardiorenal outcomes after slow continuous ultrafiltration therapy in refractory patients with advanced decompensated heart failure. J Am Coll Cardiol 2012;60:1906–12.
64. Prins KW, Wille KM, Tallaj JA, Tolwani AJ. Assessing continuous renal replacement therapy as a rescue strategy in cardiorenal syndrome 1. Clin Kidney J 2015;8:87–92.
65. Nechemia-Arbely Y, Barkan D, Pizov G, et al. IL-6/IL-6R axis plays a critical role in acute kidney injury. J Am Soc Nephrol 2008;19:1106–15.
66. Pathan N, Franklin JL, Eleftherohorinou H, et al. Myocardial depressant effects of interleukin 6 in meningococcal sepsis are regulated by p38 mitogen-activated protein kinase. Crit Care Med 2011;39:1692–711.
67. Janssen SP, Gayan-Ramirez G, Van den Bergh A, et al. Interleukin-6 causes myocardial failure and skeletal muscle atrophy in rats. Circulation 2005;111:996–1005.
68. Bellomo R, Ronco C, Kellum JA and Acute Dialysis Quality Initiative workgroup. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: The second international consensus conference of the acute dialysis quality initiative (ADQI) group. Crit Care 2004;8:R204-12.
69. Mehta RL, Kellum JA, Shah SV, et al and Acute Kidney Injury Network. Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31.
70. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guidelines for acute kidney injury. Kidney Inter Suppl 2012;2:19–36.
From the Cardiovascular Division, Department of Internal Medicine, University of Minnesota, Minneapolis, MN.
Abstract
- Objective: To present a review of cardiorenal syndrome type 1 (CRS1).
- Methods: Review of the literature.
- Results: Acute kidney injury occurs in approximately one-third of patients with acute decompensated heart failure (ADHF) and the resultant condition was named CRS1. A growing body of literature shows CRS1 patients are at high risk for poor outcomes, and thus there is an urgent need to understand the pathophysiology and subsequently develop effective treatments. In this review we discuss prevalence, proposed pathophysiology including hemodynamic and nonhemodynamic factors, prognosticating variables, data for different treatment strategies, and ongoing clinical trials and highlight questions and problems physicians will face moving forward with this common and challenging condition.
- Conclusion: Further research is needed to understand the pathophysiology of this complex clinical entity and to develop effective treatments.
Acute decompensated heart failure (ADHF) is an epidemic facing physicians throughout the world. In the United States alone, ADHF accounts for over 1 million hospitalizations annually, with costs in 2012 reaching $30.7 billion [1]. Despite the advances in chronic heart failure management, ADHF continues to be associated with poor outcomes as exemplified by 30-day readmission rates of over 20% and in-hospital mortality rates of 5% to 6%, both of which have not significantly improved over the past 20 years [2,3]. One of the strongest predictors of adverse outcomes in ADHF is renal dysfunction. An analysis from the Acute Decompensated Heart Failure National Registry (ADHERE) revealed the combination of renal dysfunction (creatinine > 2.75 mg/dL and blood urea nitrogen (BUN) > 43 mg/dL) and hypotension (systolic blood pressure (SBP) < 115 mm Hg) upon admission was associated with an in-hospital mortality of > 20% [4]. The Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) registry documented a 16.3% in-hospital mortality when patients had a SBP < 100 mm Hg and creatinine > 2.0 mg/dL at admission [5].
The presence of acute kidney injury in the setting of ADHF is a very common occurrence and was termed cardiorenal syndrome type 1 (CRS1) [6]. The prevalence of CRS1 in single-centered studies ranged from 32% to 40% of all ADHF admissions [7,8]. If this estimate holds true throughout the United States, there would be 320,000 to 400,000 hospitalizations for CRS1 annually, highlighting the magnitude of this problem. Moreover, with the number of patients with heart failure expected to continue to rise, CRS1 will only become more prevalent in the future. In this review we discuss the prevalence, proposed pathophysiology including hemodynamic and nonhemodynamic factors, prognosticating variables, data for different treatment strategies, ongoing clinical trials, and highlight questions and problems physicians will face moving forward in this common and challenging condition.
Pathogenesis of CRS1
Hemodynamic Effects
The early hypothesis for renal dysfunction in ADHF centered on hemodynamics, as reduced cardiac output was believed to decrease renal perfusion. However, analysis of invasive hemodynamics from patients with ADHF suggested that central venous pressure (CVP) was actually a better predictor of the development of CRS1 than cardiac output. In a single-center study conducted at the Cleveland Clinic, hemodynamics from 145 patients with ADHF were evaluated and surprisingly baseline cardiac index was greater in the patients with CRS1 than patients without renal dysfunction (2.0 ± 0.8 L/min/m2 vs 1.8 ± 0.4 L/min/m2; P = 0.008). However, baseline CVP was higher in the CRS1 group (18 ± 7 mm Hg vs 12 ± 6 mm Hg; P = 0.001), and there was a heightened risk of developing CRS1 as CVP increased. In fact, 75% of the patients with a CVP of > 24 mm Hg developed renal impairment [9]. In a retrospective study of the Evaluation Study of Congestive Heart Failure and Pulmonary Arterial Catheter Effectiveness (ESCAPE) trial, the only hemodynamic parameter that correlated with baseline creatinine was CVP. However, no invasive measures predicted worsening renal function during hospitalization [10]. Finally, an experiment that used isolated canine kidneys showed increased venous pressure acutely reduced urine production. Interestingly, this relationship was dependent on arterial pressure; as arterial flow decreased smaller increases in CVP were needed to reduce urine output [11]. Together, these data suggest increased CVP plays an important role in CRS1, but imply hemodynamics alone may not fully explain the pathophysiology of CRS1.
Inflammation
As information about how hemodynamics incompletely predict renal dysfunction in ADHF became available, alternative hypotheses were investigated to gain a deeper understanding of the pathophysiology underlying CRS1. A pathological role of inflammation in CRS1 has gained attention due to recent publications. First of all, serum levels of the pro-inflammatory cytokines TNF-a and IL-6 were elevated in patients with CRS1 when compared to health controls [12]. Interestingly, Virzi et al showed that the median value of IL-6 was 5 times higher in CRS1 patients when compared to ADHF patients without renal dysfunction [13]. The negative consequences of elevated serum cytokines were demonstrated when incubation of a human cell line of monocytes with serum from CRS1 patients induced apoptosis in 81% of cells compared to just 11% of cells with control serum [12]. It is possible that cytokine-induced apoptosis could occur in other cell types in different organs in patients with CRS1, which may contribute to both cardiac and renal dysfunction. Finally, analysis from a rat model of CRS1 revealed macrophage infiltration into the kidneys and increased numbers of activated monocytes in the peripheral blood. Interestingly, monocyte/macrophage depletion using liposome clodronate prevented chronic renal dysfunction in the rat model [14]. In summary, these data suggest inflammation contributes to CRS1 pathophysiology, but more experimental data is needed to determine if there is a causal relationship.
Oxidative Stress
Very recently, oxidative stress was proposed to play a role in CRS1. Virzi et al analyzed serum levels of markers of oxidative stress and compared ADHF patients without renal impairment to CRS1 patients. The markers of oxidative stress, which included myeloperoxidase, nitric oxide, copper/zinc superoxide dismutase, and endogenous peroxidase, were all significantly higher in CRS1 patients [13]. While provocative, the tissues responsible for the generation of these molecules and the subsequent effects have not yet been fully elucidated.
Prognostication
Severity of Acute Kidney Injury
Initial publications did not document a strong link between kidney injury and poor outcomes in ADHF. Firstly, Ather et al performed a single-centered study that investigated how change in renal function defined by change in creatinine, estimated GFR, and BUN affected outcomes one year post admission for ADHF. Kidney injury defined by a change in creatinine or in estimated GFR was not associated with increased risk of mortality, but a change in BUN was associated with increased mortality in a univariate analysis [15]. Testani et al retrospectively analyzed patients from the ESCAPE trial and found worsening renal function defined by a ≥ 20% reduction in estimated GFR was not significantly associated with 180-day mortality, but there was a trend towards higher mortality (hazard ration 1.4; P = 0.11) [16]. Importantly, neither of 2 these studies assessed how severity of AKI impacted outcomes, which may have contributed to the weak relationships observed.
Diuretic Responsiveness
Voors et al performed a retrospective analysis of diuretic responsiveness in 1161 patients from the Relaxin in Acute Heart Failure (RELAX-AHF) trial. Diuretic responsiveness was defined as weight change (kg) per diuretic dose (IV furosemide and PO furosemide) over 5 days and then patients were separated into tertiles. The lowest tertile group had an approximate 20% incidence of 60-day combined end-point of death, heart failure or renal failure readmission compared to less than 10% incidence in the middle and upper tertiles. Interestingly, when the effects of worsening renal function (WRF), defined as creatinine change of ≥ 0.3 mg/dL, were examined in patients stratified by diuretic response, WRF did not offer additional prognostic information [19].
Finally, Valenete et al analyzed diuretic response in 1745 patients from the PROTECT trial (Placebo-Controlled Randomized Study of the Selective A1-Adenosine Receptor Antagonist Rolofylline for Patients Hospitalized with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function). Diuretic response was calculated using the weight change per 40 mg of furosemide, and as diuretic response declined there was increasing risk of 60-day rehospitalization and 180-day mortality rates. In fact, the lowest quintile responders had a 25% mortality rate at 180 days [20].
Emerging Biomarkers
Urine Neutrophil Gelatinase-Associated Lipocalin
Because previous studies showed urinary levels of NGAL was an earlier and more reliable marker of renal dysfunction than creatinine in AKI [21], it was studied as a possible biomarker for the development of CRS1 in ADHF. A single-centered study quantified levels of urine NGAL in 100 patients admitted with heart failure and then tracked the rates of acute kidney injury. Urine NGAL was elevated in patients that developed AKI and a cut-off value 12 ng/mL had a sensitivity of 79% and specificity of 67% for predicting CRS1 [22]. While promising, further studies are needed to better define the role of NGAL in CRS1.
Cystatin C
Cystatin C is a ubiquitously expressed cysteine protease that has a constant production rate and is freely filtered by the glomerulus without being secreted into the tubules, and has effectively prognosticated outcomes in ADHF [23]. Lassus et al showed an adjusted hazard ratio of 3.2 (2.0–5.3) for 12-month mortality when cystatin C levels were elevated. Moreover, patients with the highest tertitle of NT-proBNP and cystatin C had a 48.7% 1-year mortality. Interestingly, patients with an elevated cystatin C but normal creatinine had a 40.6% 1-year mortality compared to 12.6% for those with normal cystatin C and creatinine [24]. Furthermore, Arimoto et al showed elevated cystatin C predicted death or rehospitalization in a small cohort of ADHF patients in Japan [25]. Also, Naruse et al showed cystatin C was a better predictor of cardiac death than estimated GFR by the Modification of Diet in Renal Disease Study (MDRD) equation [26]. Finally, Manzano-Fernandez et al showed the highest tertile of cystatin C was a significant independent risk factor for 2-year death or rehospitalization while creatinine and MDRD estimates of GFR were not [27]. In agreement with Lassus et al, elevations in either 2 or 3 of cystatin C, troponin, and NT-proBNP predicted death or rehospitalization when compared to those with normal levels of these 3 markers [27]. In conclusion, cystatin C either alone or in combination with other biomarkers identifies high-risk patients.
Kidney Injury Molecule 1
Kidney injury molecule 1 (KIM-1) is a type-1 cell membrane glycoprotein expressed in regenerating proximal tubular cells but not under normal conditions [28]. Although associated with increased risk of hospitalization and mortality in chronic heart failure [29,30], elevated levels of urinary KIM-1 did not predict mortality in ADHF [31]. Further studies are needed to elucidate the utility of KIM-1 in CRS1.
Treatment Approaches
Diuretics
Loop diuretics are the main treatment for decongestion of patients with CRS1. To date, no clinical trial has compared the different loop diuretics (furosemide, bumetanide, torsemide, or ethacrynic acid) to each other, so there is no clear choice of which loop diuretic is the best. However, dosing scheme was investigated in the Dose Optimization Strategies Evaluation (DOSE) trial. In this trial, 308 patients were randomized in a 1:1:1:1 design in which patients were placed in groups with low-dose (equivalent to oral dose) or high-dose (2.5 times oral dose) intermittent parental therapy or alternatively low-dose or high-dose continuous drip therapy. There were no differences in dyspnea, fluid changes, change in creatinine, hospital length stay, or rehospitalization and death rates when the intermittent and drip approaches were compared. However, the high-dose arm had decreased dyspnea, increased volume removal, but there were more occurrences of AKIs when compared to the low-dose arm [32].
In clinical practice, if loop diuretic treatment does not result in the desired urine output, a second-site diuretic may be added to potentiate diuresis. Unfortunately, there is little data on this common clinical practice and thus the optimal choice of second site agent (chlorthiazide or metolazone) is unknown. Frequently, the deciding factor is based upon cost or concern that oral absorption of metolazone will be ineffective. However, Moranville et al recently performed a retrospective assessment comparing chlorthiazide (22 patients) to metolazone (33 patients) in ADHF patients with renal dysfunction defined by a creatinine clearance of 15–50 mL/min. There was a nonsignificant trend towards increased urine output in the metolazone group, no differences in the rates of adverse events, and the chlorthiazide group actually had a longer hospital stay [33]. While potentially promising results, the retrospective nature of the study made it difficult to determine if the differences were due to treatment approach or dissimilarities of patient illness. Nonetheless, physicians must remain vigilant when implementing the second-site diuretic approach because it can lead to marked diuretic response leading to metabolic derangements including hypokalemia, hyponatremia, hypomagnesaemia, and metabolic alkalosis.
Inotropes
The use of inotropic agents such as dobutamine or milrinone can be used to augment cardiac function when there is a known low-output state for better renal perfusion in CRS1. Unfortunately, there is little objective data available about the utility of this widely implemented approach. The Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of a Chronic Heart Failure (OPTIME-HF) trial did not show improved renal function with milrinone treatment [34]. The use of levosimendan, a cardiac calcium sensitizer that increases contractility not currently approved in the United States, was compared to dobutamine in the Survival of Patients With Acute Heart Failure in Need of Intravenous Inotropic Support (SURVIVE) trial, and there were no differences in rates of renal failure when the 2 groups were compared [35]. Nonetheless, if cardiac output is severely compromised, inotropes can be used for CRS1 treatment, but they should be used cautiously due the increased risks of lethal arrhythmias.
Dopamine
Use of low-dose dopamine to stimulate D1 and D2 receptors as a way to increase renal blood flow and promote increased glomerular filtration and urine production was extensively studied in ADHF. A small trial showed use of low dose dopamine had renal protective effects in a total of 20 patients [36]. However, when larger trials were conducted, such beneficial results were not consistently observed. The Dopamine in Acute Decompensated Heart Failure (DAD-HF I) trial compared low-dose furosemide plus low-dose dopamine (5 µg/kg/min) to high-dose furosemide alone in 60 patients. There were no differences in total diuresis, hospital stay, and 60-day mortality or rehospitalization rates, but there was a reduction in the renal dysfunction at the 24-hour time point in the dopamine-treated arm (6.7% versus 30%) [37]. The Dopamine in Acute Decompensated Heart Failure II trial randomized 161 ADHF patients to high-dose furosemide, low-dose furosemide and lose dose dopamine (5 µg/kg/min), or low-dose furosemide and assessed dyspnea, worsening renal function, length of stay, 60-day and one-year all-cause mortality and hospitalization for heart failure. Dopamine treatment did not improve any of the outcomes measured [38]. Finally, the most recent trial to examine the effects of dopamine was the Renal Optimization Strategies Evaluation (ROSE) trial. In this trial, there were 360 patients with ADHF randomized to nesiritide or dopamine versus placebo in a 2:1 design. When comparing dopamine (111 patients) treatment to placebo (115 patients), there were no differences in urine output, renal function as determined by cystatin C levels, or symptomatic improvements. However, there was more tachycardia in the dopamine group [39]. Currently, there is not strong evidence supporting routine use of dopamine in CRS1.
Nesiritide
Use of nesiritide, recombinant brain natriuretic peptide, was also investigated as a way to enhance urine production through the natriuretic effects of the peptide. The first attempt to explore this hypothesis was the B-Type Natriuretic Peptide in Cardiorenal Decompensation Syndrome (BNP-CARDS) trial. BNP-CARDS showed a 48-hour infusion of nesiritide (39 patients) or placebo (36 patients) in patients with ADHF and renal dysfunction (estimated GFR between 15–60 mL/min) did not reduce the incidence of worsening renal function as defined by a rise in serum creatinine by 20% [40]. A similar approach was implemented in the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND-HF) trial which examined over 7000 patients with ADHF. 3496 patients were treated with nesiritide and 3511 patients were treated with placebo for 24 hours and up to 7 days. Nesiritide treatment did not alter dyspnea at 6 and 24 hours, improve renal function as determined by creatinine change, or alter the combined end-point of rehospitalization or death 30 at days [41]. The ROSE trial examined the effects of nesiritide (117 patients) versus placebo (115 patients) for urine production, change in renal function as defined by change in cystatin C, and decongestion (urinary sodium excretion, weight change, and change in NT-proBNP) at 72 hours. Nesiritide did not alter any of the outcomes investigated [39]. Finally, a single-centered study conducted at the Mayo Clinic examined the effects of nesiritide (37 patients) or placebo (35 patients) with ADHF and pre-existing renal dysfunction (estimated GFR between 20 and 60 mL/min). These investigators found nesiritide treatment resulted in less renal dysfunction as measured by creatinine and BUN, but no changes in diuretic responsiveness, duration of hospitalization, or rehospitalization rates. Nesiritide did reduce serum endothelin levels, but had no effect on ANP, NT-pro BNP, renin, angiotensin II, or aldosterone [42]. In summary, nesiritide does not appear to have significant renal protective effects in ADHF.
Adenosine A1 Receptor Antagonists
The use of adenosine receptor antagonists to prevent adenosine-mediated vasoconstriction of renal vasculature in ADHF has also been examined. The first study conducted was a small double-blind randomized-controlled trial that investigated the effects of rolofylline, an adenosine A-1 antagonist, in patients with ADHF and an estimated creatinine clearance of 20-80 mL/min. The study had 27 patients in the placebo arm, 29 patients that received 2.5 mg of rolofylline, 31 patients received 15 mg of rolofylline, 30 patients received 30 mg of rolofylline, and 29 patients received 60 mg of rolofylline, all of which was daily for up to 3 days. Rolofylline treatment increased urine output on day 1 and improved renal function on day 2 [43]. These positive results led to the Placebo-Controlled Randomized Study of Selective Adenosine A1 Receptor Antagonist Rolofylline for Patients with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function (PROTECT) Trial. PROTECT assessed the effects of rolofylline (1356) or placebo (677) in patients with ADHF and an estimated creatinine clearance between 20 and 80 mL/min. There were no significant differences in renal function out to 14 days, but rolofylline led to more weight loss than placebo [44,45]. In a subgroup analysis of patients with severe baseline renal dysfunction (creatinine clearance of less than 30 mL/min), rolofylline reduced the combined 60-day end-point of hospitalization due to cardiovascular or renal cause and death [45]. Finally, the Effects of KW-3902 Injectable Emulsion on Heart Failure Signs and Symptoms, Diuresis, Renal Function, and Clinical Outcomes in Subjects Hospitalized with Worsening Renal Function and Heart Failure Requiring Intravenous Therapy (REACH-UP) trial probed the effects of rolofylline (36 patients) or placebo (40 patients) in patients with ADHF and renal impairment (creatinine clearance of 20-60 mL/min). Rolofylline treatment did not alter renal function, but there was a nonsignificant trend towards reduction in 60-day combined end-point of hospitalization due to renal or cardiovascular causes or death [46]. In summary, the use of rolofylline has not been conclusively associated with improved outcomes in CRS1.
Vasopressin Antagonists
The use of vasopressin antagonists to induce aquaphoresis and combat hyponatremia was studied in ADHF. Vasopressin antagonists were first investigated in the Acute and Chronic Therapeutic Impact of a Vasopressin Antagonist (ACTIV) trial. ACTIV involved 3 doses of tolvaptan (78 patients received 30 mg, 84 patients received 60 mg, and 77 patients received 90 mg) versus placebo (80 patients), and tolvaptan increased urine production and decreased body weight compared to placebo without compromising renal function. A post-hoc analysis of patients with renal dysfunction (BUN > 29 mg/dL) and severe volume overload revealed a survival benefit at 60 days [47]. The Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVERST) trial compared placebo (2061 patients) versus 30 mg/day of tolvaptan (2072 patients) within 48 hours after admission in an identical 2-trial design. Tolvaptan increased weight loss and reduced dyspnea acutely but did not alter all-cause mortality or cardiovascular or heart failure hospitalization rates out to 24 months post index hospitalization [48,49]. These data suggest vasopressin antagonists may potentiate diuresis acutely but likely do not improve long-term outcomes.
Corticosteroids
The use of corticosteroids in ADHF has been controversial as there were initial concerns that corticosteroids would increase fluid retention. However, corticosteroids augmented diuretic response and improved renal function in 13 ADHF patients who had inadequate response to sequential nephron blockage [50]. Furthermore, Zhang et al showed that prednisone treatment in 35 patients admitted with ADHF increased urinary volume, reduced dyspnea, reduced uric acid, and improved renal function [51]. These promising results led to the Cardiac Outcome Prevention Effectiveness of Glucocorticoids in Acute Decompensated Heart Failure (COPE-ADHF) trial. In this single-centered study, 102 patients with ADHF were randomized to either placebo [51] or corticosteroids [51] and the outcomes recorded included urinary volume, change in creatinine, and cardiovascular death at 30 days. Use of corticosteroids improved renal function, increased urine output, and reduced mortality (3/51 in corticosteroid group versus 10/51 in the placebo group) [52]. The mechanisms underlying the improvements with corticosteroids were not determined, but were hypothesized to be facilitation of natriuretic peptides or dilation of renal vasculature through activation of nitric oxide pathway or dopaminergic system.
Serelaxin
Serelaxin is a recombinantly expressed human relaxin-2, a peptide hormone present during pregnancy which facilitates physiological cardiovascular and renal adaptations [53–55], which showed potential benefits in CRS1. Analysis of the RELAX-AHF trial revealed serelaxin reduced incidence of worsening renal function at day 2 of treatment as defined by changes in serum creatinine, cystatin C, and BUN. Importantly, worsening renal function defined by cystatin C changes was associated with increased 180-day mortality in this analysis [56]. The mechanisms by which serelaxin prevented renal dysfunction are currently unknown as serelaxin treatment did not improve diuretic efficiency [19].
Ultrafiltration
Another treatment choice in CRS1 is mechanical removal of salt and water via ultrafiltration. Ultrafiltration showed early promise in Ultrafiltration Versus Intravenous Diuretics for Patients Hospitalized for Acute Decompensated Heart Failure trial (UNLOAD) trial. In this study, 200 patients with ADHF were randomized to either ultrafiltration or medical management with loop diuretics. Use of ultrafiltration increased volume removal without any differences in renal function and reduced rehospitalization rates at 90 days [57].
However, when ultrafiltration was employed specifically in CRS1 patients in the Cardiorenal Rescue Study in Acute Decompensated Heart Failure trial (CARESS-HF), UF was not superior to medical treatment. There were 188 patients studied in CARESS-HF, and in the ultrafiltration arm there was increased risk of renal dysfunction, no differences in volume removal, and no change in rehospitalization rates at 90 days [58]. When trying to reconcile UNLOAD and CARESS-HF, the medical treatment arm in CARESS-HF was much more standardized and aggressive and UNLOAD was earlier implementation of ultrafiltration, which may have explained the differences. Interestingly, ultrafiltration was hypothesized to be advantageous over diuretic therapy through reduced activation of the renin-angiotensin-aldosterone system, but analysis of the patients from CARESS-HF showed higher levels of plasma renin activity and no difference in aldosterone levels in ultrafiltration patients [59].
Two meta-analyses have examined the use of ultrafiltration versus medical management in ADHF and both showed ultrafiltration was more effective in volume removal than medical therapy but did not improve rehospitalization or mortality rates [60,61]. This fact combined with the risks of vascular access placement and bleeding from anticoagulation limits to routine use of ultrafiltration in CRS1.
Continuous Renal Replacement Therapy
Once renal function deteriorates to the point that renal replacement therapy is needed for both volume removal and solute clearance in CRS1, continuous renal replacement therapy (CRRT) may be implemented. Unfortunately, there are few available data for this group of advanced CRS1 patients to guide physicians. There was a single-centered study conducted in Egypt that randomized 40 ADHF patients to either IV furosemide or CRRT. The patients treated with CRRT had greater weight loss and decreased length of stay in the ICU, but there were no differences in dialysis dependence rates or 30-day mortality [62]. Two single-centered studies reported outcomes associated with advanced CRS1 requiring CRRT. In a study conducted at the Cleveland Clinic, 63 patients with CRS1 were treated with ultrafiltration, of which 37 were converted to CRRT due to worsening renal function. Of the 37 patients treated with CRRT, 16 died in the hospital and 4 were discharged with hospice care [63]. In another retrospective study performed at the University of Alabama-Birmingham, use of rescue CRRT in advanced CRS1 was examined in 37 patients. 23 patients died during hospitalization and 2 were discharged to hospice care [64]. Combination of the Cleveland Clinic and University of Alabama-Birmingham studies revealed patients requiring CRRT in the setting of advanced CRS1 had an in-hospital mortality or palliative discharge rate of 60.8% (45/74). Clearly, this population needs further investigation to prevent such poor outcomes.
Future Treatment Options
Ongoing and Unreported Clinical Trials
Unfortunately, none of the current treatments for CRS1 have definitive improvements in outcomes, but there are several ongoing clinical trials which will hopefully identify novel treatment strategies. First of all, the Acetazolamide and Spironolactone to Increase Natriuresis in Congestive Heart Failure (Diuresis-CHF) trial is being conducted in Belgium. This study will examine the effects of acetazolamide with low dose diuretic versus high dose diuretics in one aim and the effects of upfront spironolactone in another. The outcomes analyzed will include total natriuresis, potassium homeostasis, NT-proBNP changes, change in renal function, peak serum levels of renin and aldosterone, weight change, urine volume, and change in edema (NCT01973335). The Protocolized Diuretic Strategy in Cardiorenal Failure (ProDius) trial is being performed at the University of Pittsburgh, and will determine the effects of a protocolized diuretic approach to target 3-5 liters of urine production a day versus standard therapy and will track the change in body weight, length of hospitalization, reshospitalization rates, mortality rates, venous compliance of internal jugular vein, clinical decongestion, change in renal function, and urine output (NCT01921829). The Levosimendan versus Dobutamine for Renal Function in Heart Failure (ELDOR) study is ongoing in Sweden and will probe the acute effects of levosimendan and dobutamine on renal perfusion. The endpoints will include changes in renal blood flow, GFR, renal vascular resistance, central hemodynamics, renal oxygen extraction and consumptions, and filtration fraction (NCT02133105). Finally, the Safety and Efficacy of Low Dose Hypertonic Saline and High Dose Furosemide for Congestive Heart Failure (REaCH) trial probed the effects of combination of hypertonic saline and furosemide versus furosemide in patients with ADHF and renal impairment defined by a GFR<60 mL/min. The outcomes were change in renal function, diuretic response, length of hospital stay, readmission rates, weight loss, BNP levels, and included a cost analysis. The study was completed but results are not currently available (NCT01028170)
Should Inflammation Be Targeted in CRS1?
Although proposed to play a role in the pathophysiology of CRS1, inflammation has not been explicitly targeted as a treatment for CRS1. One possible way to combat inflammation could be inhibition of the IL-6 pathway, which is support by preclinical work as previous studies showed IL-6 knockout mice were resistant to HgCl2-induced renal injury and death [65] and IL-6 has negative inotropic effects in both isolated cardiomyocytes [66] and intact animals [67]. Thus, IL-6 antagonism may improve both cardiac and renal function, an ideal scenario for CRS1 patients. The availability of tocilizumab, an FDA-approved humanized antibody to the IL-6 receptor, may allow for investigation of this hypothesis in the future. Although not examined in the COPE-ADHF trial, an alternative explanation for the improvements associated with corticosteroids treatment were the anti-inflammatory effects. If this were true, corticosteroids would represent a relatively cheap treatment option for CRS1 patients, but more studies need to be conducted before this approach is widely implemented. Finally, use of cytokine profiling may be used to enrich a population of CRS1 patients that could be investigated in future clinical trials using anti-inflammatory medications.
Unanswered Questions Moving Forward
Severity of AKI and Treatment Effects
An important unknown that warrants further investigation is if the severity of AKI should dictate treatment choice in CRS1. As discussed above, increasing severity of AKI resulted in elevated risk of adverse events, but it remains unknown whether different treatments offer benefits for more or less severe renal impairment. Perhaps, future studies aimed at defining outcomes from different treatment strategies stratified by severity of renal dysfunction may reveal which patients benefit from the various treatment options for CRS1.
How Do We Best Define Renal Dysfunction in CRS1?
Currently, there is no accepted definition of renal dysfunction in CRS1. As discussed above, using the AKIN, KDIGO, or RIFLE scoring systems or diuretic responsiveness effectively differentiated outcomes in patients with CRS1. However, an agreed-upon definition would likely benefit the field going forward so this population could be systematically investigated in future studies.
Conclusion
In summary, CRS1 is a common clinical entity associated with poor patient outcomes. A complex pathophysiology marked by reduced cardiac output, increased central venous pressure, inflammation, and oxidative stress underlies the disease process. Unfortunately, no current treatment approach shows consistent improvements in outcomes, highlighting the urgent need for further research to reduce the burden that CRS1 imposes.
Corresponding author: Kurt W. Prins, MD, PhD, MMC 580 Mayo, 420 Delaware St SE, Minneapolis, MN 55455, [email protected].
Funding/support: Dr. Prins is funded by NIH F32 grant HL129554 and Dr. Thenappen is funded by AHA Scientist Development Grant 15SDG25560048.
From the Cardiovascular Division, Department of Internal Medicine, University of Minnesota, Minneapolis, MN.
Abstract
- Objective: To present a review of cardiorenal syndrome type 1 (CRS1).
- Methods: Review of the literature.
- Results: Acute kidney injury occurs in approximately one-third of patients with acute decompensated heart failure (ADHF) and the resultant condition was named CRS1. A growing body of literature shows CRS1 patients are at high risk for poor outcomes, and thus there is an urgent need to understand the pathophysiology and subsequently develop effective treatments. In this review we discuss prevalence, proposed pathophysiology including hemodynamic and nonhemodynamic factors, prognosticating variables, data for different treatment strategies, and ongoing clinical trials and highlight questions and problems physicians will face moving forward with this common and challenging condition.
- Conclusion: Further research is needed to understand the pathophysiology of this complex clinical entity and to develop effective treatments.
Acute decompensated heart failure (ADHF) is an epidemic facing physicians throughout the world. In the United States alone, ADHF accounts for over 1 million hospitalizations annually, with costs in 2012 reaching $30.7 billion [1]. Despite the advances in chronic heart failure management, ADHF continues to be associated with poor outcomes as exemplified by 30-day readmission rates of over 20% and in-hospital mortality rates of 5% to 6%, both of which have not significantly improved over the past 20 years [2,3]. One of the strongest predictors of adverse outcomes in ADHF is renal dysfunction. An analysis from the Acute Decompensated Heart Failure National Registry (ADHERE) revealed the combination of renal dysfunction (creatinine > 2.75 mg/dL and blood urea nitrogen (BUN) > 43 mg/dL) and hypotension (systolic blood pressure (SBP) < 115 mm Hg) upon admission was associated with an in-hospital mortality of > 20% [4]. The Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) registry documented a 16.3% in-hospital mortality when patients had a SBP < 100 mm Hg and creatinine > 2.0 mg/dL at admission [5].
The presence of acute kidney injury in the setting of ADHF is a very common occurrence and was termed cardiorenal syndrome type 1 (CRS1) [6]. The prevalence of CRS1 in single-centered studies ranged from 32% to 40% of all ADHF admissions [7,8]. If this estimate holds true throughout the United States, there would be 320,000 to 400,000 hospitalizations for CRS1 annually, highlighting the magnitude of this problem. Moreover, with the number of patients with heart failure expected to continue to rise, CRS1 will only become more prevalent in the future. In this review we discuss the prevalence, proposed pathophysiology including hemodynamic and nonhemodynamic factors, prognosticating variables, data for different treatment strategies, ongoing clinical trials, and highlight questions and problems physicians will face moving forward in this common and challenging condition.
Pathogenesis of CRS1
Hemodynamic Effects
The early hypothesis for renal dysfunction in ADHF centered on hemodynamics, as reduced cardiac output was believed to decrease renal perfusion. However, analysis of invasive hemodynamics from patients with ADHF suggested that central venous pressure (CVP) was actually a better predictor of the development of CRS1 than cardiac output. In a single-center study conducted at the Cleveland Clinic, hemodynamics from 145 patients with ADHF were evaluated and surprisingly baseline cardiac index was greater in the patients with CRS1 than patients without renal dysfunction (2.0 ± 0.8 L/min/m2 vs 1.8 ± 0.4 L/min/m2; P = 0.008). However, baseline CVP was higher in the CRS1 group (18 ± 7 mm Hg vs 12 ± 6 mm Hg; P = 0.001), and there was a heightened risk of developing CRS1 as CVP increased. In fact, 75% of the patients with a CVP of > 24 mm Hg developed renal impairment [9]. In a retrospective study of the Evaluation Study of Congestive Heart Failure and Pulmonary Arterial Catheter Effectiveness (ESCAPE) trial, the only hemodynamic parameter that correlated with baseline creatinine was CVP. However, no invasive measures predicted worsening renal function during hospitalization [10]. Finally, an experiment that used isolated canine kidneys showed increased venous pressure acutely reduced urine production. Interestingly, this relationship was dependent on arterial pressure; as arterial flow decreased smaller increases in CVP were needed to reduce urine output [11]. Together, these data suggest increased CVP plays an important role in CRS1, but imply hemodynamics alone may not fully explain the pathophysiology of CRS1.
Inflammation
As information about how hemodynamics incompletely predict renal dysfunction in ADHF became available, alternative hypotheses were investigated to gain a deeper understanding of the pathophysiology underlying CRS1. A pathological role of inflammation in CRS1 has gained attention due to recent publications. First of all, serum levels of the pro-inflammatory cytokines TNF-a and IL-6 were elevated in patients with CRS1 when compared to health controls [12]. Interestingly, Virzi et al showed that the median value of IL-6 was 5 times higher in CRS1 patients when compared to ADHF patients without renal dysfunction [13]. The negative consequences of elevated serum cytokines were demonstrated when incubation of a human cell line of monocytes with serum from CRS1 patients induced apoptosis in 81% of cells compared to just 11% of cells with control serum [12]. It is possible that cytokine-induced apoptosis could occur in other cell types in different organs in patients with CRS1, which may contribute to both cardiac and renal dysfunction. Finally, analysis from a rat model of CRS1 revealed macrophage infiltration into the kidneys and increased numbers of activated monocytes in the peripheral blood. Interestingly, monocyte/macrophage depletion using liposome clodronate prevented chronic renal dysfunction in the rat model [14]. In summary, these data suggest inflammation contributes to CRS1 pathophysiology, but more experimental data is needed to determine if there is a causal relationship.
Oxidative Stress
Very recently, oxidative stress was proposed to play a role in CRS1. Virzi et al analyzed serum levels of markers of oxidative stress and compared ADHF patients without renal impairment to CRS1 patients. The markers of oxidative stress, which included myeloperoxidase, nitric oxide, copper/zinc superoxide dismutase, and endogenous peroxidase, were all significantly higher in CRS1 patients [13]. While provocative, the tissues responsible for the generation of these molecules and the subsequent effects have not yet been fully elucidated.
Prognostication
Severity of Acute Kidney Injury
Initial publications did not document a strong link between kidney injury and poor outcomes in ADHF. Firstly, Ather et al performed a single-centered study that investigated how change in renal function defined by change in creatinine, estimated GFR, and BUN affected outcomes one year post admission for ADHF. Kidney injury defined by a change in creatinine or in estimated GFR was not associated with increased risk of mortality, but a change in BUN was associated with increased mortality in a univariate analysis [15]. Testani et al retrospectively analyzed patients from the ESCAPE trial and found worsening renal function defined by a ≥ 20% reduction in estimated GFR was not significantly associated with 180-day mortality, but there was a trend towards higher mortality (hazard ration 1.4; P = 0.11) [16]. Importantly, neither of 2 these studies assessed how severity of AKI impacted outcomes, which may have contributed to the weak relationships observed.
Diuretic Responsiveness
Voors et al performed a retrospective analysis of diuretic responsiveness in 1161 patients from the Relaxin in Acute Heart Failure (RELAX-AHF) trial. Diuretic responsiveness was defined as weight change (kg) per diuretic dose (IV furosemide and PO furosemide) over 5 days and then patients were separated into tertiles. The lowest tertile group had an approximate 20% incidence of 60-day combined end-point of death, heart failure or renal failure readmission compared to less than 10% incidence in the middle and upper tertiles. Interestingly, when the effects of worsening renal function (WRF), defined as creatinine change of ≥ 0.3 mg/dL, were examined in patients stratified by diuretic response, WRF did not offer additional prognostic information [19].
Finally, Valenete et al analyzed diuretic response in 1745 patients from the PROTECT trial (Placebo-Controlled Randomized Study of the Selective A1-Adenosine Receptor Antagonist Rolofylline for Patients Hospitalized with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function). Diuretic response was calculated using the weight change per 40 mg of furosemide, and as diuretic response declined there was increasing risk of 60-day rehospitalization and 180-day mortality rates. In fact, the lowest quintile responders had a 25% mortality rate at 180 days [20].
Emerging Biomarkers
Urine Neutrophil Gelatinase-Associated Lipocalin
Because previous studies showed urinary levels of NGAL was an earlier and more reliable marker of renal dysfunction than creatinine in AKI [21], it was studied as a possible biomarker for the development of CRS1 in ADHF. A single-centered study quantified levels of urine NGAL in 100 patients admitted with heart failure and then tracked the rates of acute kidney injury. Urine NGAL was elevated in patients that developed AKI and a cut-off value 12 ng/mL had a sensitivity of 79% and specificity of 67% for predicting CRS1 [22]. While promising, further studies are needed to better define the role of NGAL in CRS1.
Cystatin C
Cystatin C is a ubiquitously expressed cysteine protease that has a constant production rate and is freely filtered by the glomerulus without being secreted into the tubules, and has effectively prognosticated outcomes in ADHF [23]. Lassus et al showed an adjusted hazard ratio of 3.2 (2.0–5.3) for 12-month mortality when cystatin C levels were elevated. Moreover, patients with the highest tertitle of NT-proBNP and cystatin C had a 48.7% 1-year mortality. Interestingly, patients with an elevated cystatin C but normal creatinine had a 40.6% 1-year mortality compared to 12.6% for those with normal cystatin C and creatinine [24]. Furthermore, Arimoto et al showed elevated cystatin C predicted death or rehospitalization in a small cohort of ADHF patients in Japan [25]. Also, Naruse et al showed cystatin C was a better predictor of cardiac death than estimated GFR by the Modification of Diet in Renal Disease Study (MDRD) equation [26]. Finally, Manzano-Fernandez et al showed the highest tertile of cystatin C was a significant independent risk factor for 2-year death or rehospitalization while creatinine and MDRD estimates of GFR were not [27]. In agreement with Lassus et al, elevations in either 2 or 3 of cystatin C, troponin, and NT-proBNP predicted death or rehospitalization when compared to those with normal levels of these 3 markers [27]. In conclusion, cystatin C either alone or in combination with other biomarkers identifies high-risk patients.
Kidney Injury Molecule 1
Kidney injury molecule 1 (KIM-1) is a type-1 cell membrane glycoprotein expressed in regenerating proximal tubular cells but not under normal conditions [28]. Although associated with increased risk of hospitalization and mortality in chronic heart failure [29,30], elevated levels of urinary KIM-1 did not predict mortality in ADHF [31]. Further studies are needed to elucidate the utility of KIM-1 in CRS1.
Treatment Approaches
Diuretics
Loop diuretics are the main treatment for decongestion of patients with CRS1. To date, no clinical trial has compared the different loop diuretics (furosemide, bumetanide, torsemide, or ethacrynic acid) to each other, so there is no clear choice of which loop diuretic is the best. However, dosing scheme was investigated in the Dose Optimization Strategies Evaluation (DOSE) trial. In this trial, 308 patients were randomized in a 1:1:1:1 design in which patients were placed in groups with low-dose (equivalent to oral dose) or high-dose (2.5 times oral dose) intermittent parental therapy or alternatively low-dose or high-dose continuous drip therapy. There were no differences in dyspnea, fluid changes, change in creatinine, hospital length stay, or rehospitalization and death rates when the intermittent and drip approaches were compared. However, the high-dose arm had decreased dyspnea, increased volume removal, but there were more occurrences of AKIs when compared to the low-dose arm [32].
In clinical practice, if loop diuretic treatment does not result in the desired urine output, a second-site diuretic may be added to potentiate diuresis. Unfortunately, there is little data on this common clinical practice and thus the optimal choice of second site agent (chlorthiazide or metolazone) is unknown. Frequently, the deciding factor is based upon cost or concern that oral absorption of metolazone will be ineffective. However, Moranville et al recently performed a retrospective assessment comparing chlorthiazide (22 patients) to metolazone (33 patients) in ADHF patients with renal dysfunction defined by a creatinine clearance of 15–50 mL/min. There was a nonsignificant trend towards increased urine output in the metolazone group, no differences in the rates of adverse events, and the chlorthiazide group actually had a longer hospital stay [33]. While potentially promising results, the retrospective nature of the study made it difficult to determine if the differences were due to treatment approach or dissimilarities of patient illness. Nonetheless, physicians must remain vigilant when implementing the second-site diuretic approach because it can lead to marked diuretic response leading to metabolic derangements including hypokalemia, hyponatremia, hypomagnesaemia, and metabolic alkalosis.
Inotropes
The use of inotropic agents such as dobutamine or milrinone can be used to augment cardiac function when there is a known low-output state for better renal perfusion in CRS1. Unfortunately, there is little objective data available about the utility of this widely implemented approach. The Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of a Chronic Heart Failure (OPTIME-HF) trial did not show improved renal function with milrinone treatment [34]. The use of levosimendan, a cardiac calcium sensitizer that increases contractility not currently approved in the United States, was compared to dobutamine in the Survival of Patients With Acute Heart Failure in Need of Intravenous Inotropic Support (SURVIVE) trial, and there were no differences in rates of renal failure when the 2 groups were compared [35]. Nonetheless, if cardiac output is severely compromised, inotropes can be used for CRS1 treatment, but they should be used cautiously due the increased risks of lethal arrhythmias.
Dopamine
Use of low-dose dopamine to stimulate D1 and D2 receptors as a way to increase renal blood flow and promote increased glomerular filtration and urine production was extensively studied in ADHF. A small trial showed use of low dose dopamine had renal protective effects in a total of 20 patients [36]. However, when larger trials were conducted, such beneficial results were not consistently observed. The Dopamine in Acute Decompensated Heart Failure (DAD-HF I) trial compared low-dose furosemide plus low-dose dopamine (5 µg/kg/min) to high-dose furosemide alone in 60 patients. There were no differences in total diuresis, hospital stay, and 60-day mortality or rehospitalization rates, but there was a reduction in the renal dysfunction at the 24-hour time point in the dopamine-treated arm (6.7% versus 30%) [37]. The Dopamine in Acute Decompensated Heart Failure II trial randomized 161 ADHF patients to high-dose furosemide, low-dose furosemide and lose dose dopamine (5 µg/kg/min), or low-dose furosemide and assessed dyspnea, worsening renal function, length of stay, 60-day and one-year all-cause mortality and hospitalization for heart failure. Dopamine treatment did not improve any of the outcomes measured [38]. Finally, the most recent trial to examine the effects of dopamine was the Renal Optimization Strategies Evaluation (ROSE) trial. In this trial, there were 360 patients with ADHF randomized to nesiritide or dopamine versus placebo in a 2:1 design. When comparing dopamine (111 patients) treatment to placebo (115 patients), there were no differences in urine output, renal function as determined by cystatin C levels, or symptomatic improvements. However, there was more tachycardia in the dopamine group [39]. Currently, there is not strong evidence supporting routine use of dopamine in CRS1.
Nesiritide
Use of nesiritide, recombinant brain natriuretic peptide, was also investigated as a way to enhance urine production through the natriuretic effects of the peptide. The first attempt to explore this hypothesis was the B-Type Natriuretic Peptide in Cardiorenal Decompensation Syndrome (BNP-CARDS) trial. BNP-CARDS showed a 48-hour infusion of nesiritide (39 patients) or placebo (36 patients) in patients with ADHF and renal dysfunction (estimated GFR between 15–60 mL/min) did not reduce the incidence of worsening renal function as defined by a rise in serum creatinine by 20% [40]. A similar approach was implemented in the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND-HF) trial which examined over 7000 patients with ADHF. 3496 patients were treated with nesiritide and 3511 patients were treated with placebo for 24 hours and up to 7 days. Nesiritide treatment did not alter dyspnea at 6 and 24 hours, improve renal function as determined by creatinine change, or alter the combined end-point of rehospitalization or death 30 at days [41]. The ROSE trial examined the effects of nesiritide (117 patients) versus placebo (115 patients) for urine production, change in renal function as defined by change in cystatin C, and decongestion (urinary sodium excretion, weight change, and change in NT-proBNP) at 72 hours. Nesiritide did not alter any of the outcomes investigated [39]. Finally, a single-centered study conducted at the Mayo Clinic examined the effects of nesiritide (37 patients) or placebo (35 patients) with ADHF and pre-existing renal dysfunction (estimated GFR between 20 and 60 mL/min). These investigators found nesiritide treatment resulted in less renal dysfunction as measured by creatinine and BUN, but no changes in diuretic responsiveness, duration of hospitalization, or rehospitalization rates. Nesiritide did reduce serum endothelin levels, but had no effect on ANP, NT-pro BNP, renin, angiotensin II, or aldosterone [42]. In summary, nesiritide does not appear to have significant renal protective effects in ADHF.
Adenosine A1 Receptor Antagonists
The use of adenosine receptor antagonists to prevent adenosine-mediated vasoconstriction of renal vasculature in ADHF has also been examined. The first study conducted was a small double-blind randomized-controlled trial that investigated the effects of rolofylline, an adenosine A-1 antagonist, in patients with ADHF and an estimated creatinine clearance of 20-80 mL/min. The study had 27 patients in the placebo arm, 29 patients that received 2.5 mg of rolofylline, 31 patients received 15 mg of rolofylline, 30 patients received 30 mg of rolofylline, and 29 patients received 60 mg of rolofylline, all of which was daily for up to 3 days. Rolofylline treatment increased urine output on day 1 and improved renal function on day 2 [43]. These positive results led to the Placebo-Controlled Randomized Study of Selective Adenosine A1 Receptor Antagonist Rolofylline for Patients with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function (PROTECT) Trial. PROTECT assessed the effects of rolofylline (1356) or placebo (677) in patients with ADHF and an estimated creatinine clearance between 20 and 80 mL/min. There were no significant differences in renal function out to 14 days, but rolofylline led to more weight loss than placebo [44,45]. In a subgroup analysis of patients with severe baseline renal dysfunction (creatinine clearance of less than 30 mL/min), rolofylline reduced the combined 60-day end-point of hospitalization due to cardiovascular or renal cause and death [45]. Finally, the Effects of KW-3902 Injectable Emulsion on Heart Failure Signs and Symptoms, Diuresis, Renal Function, and Clinical Outcomes in Subjects Hospitalized with Worsening Renal Function and Heart Failure Requiring Intravenous Therapy (REACH-UP) trial probed the effects of rolofylline (36 patients) or placebo (40 patients) in patients with ADHF and renal impairment (creatinine clearance of 20-60 mL/min). Rolofylline treatment did not alter renal function, but there was a nonsignificant trend towards reduction in 60-day combined end-point of hospitalization due to renal or cardiovascular causes or death [46]. In summary, the use of rolofylline has not been conclusively associated with improved outcomes in CRS1.
Vasopressin Antagonists
The use of vasopressin antagonists to induce aquaphoresis and combat hyponatremia was studied in ADHF. Vasopressin antagonists were first investigated in the Acute and Chronic Therapeutic Impact of a Vasopressin Antagonist (ACTIV) trial. ACTIV involved 3 doses of tolvaptan (78 patients received 30 mg, 84 patients received 60 mg, and 77 patients received 90 mg) versus placebo (80 patients), and tolvaptan increased urine production and decreased body weight compared to placebo without compromising renal function. A post-hoc analysis of patients with renal dysfunction (BUN > 29 mg/dL) and severe volume overload revealed a survival benefit at 60 days [47]. The Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVERST) trial compared placebo (2061 patients) versus 30 mg/day of tolvaptan (2072 patients) within 48 hours after admission in an identical 2-trial design. Tolvaptan increased weight loss and reduced dyspnea acutely but did not alter all-cause mortality or cardiovascular or heart failure hospitalization rates out to 24 months post index hospitalization [48,49]. These data suggest vasopressin antagonists may potentiate diuresis acutely but likely do not improve long-term outcomes.
Corticosteroids
The use of corticosteroids in ADHF has been controversial as there were initial concerns that corticosteroids would increase fluid retention. However, corticosteroids augmented diuretic response and improved renal function in 13 ADHF patients who had inadequate response to sequential nephron blockage [50]. Furthermore, Zhang et al showed that prednisone treatment in 35 patients admitted with ADHF increased urinary volume, reduced dyspnea, reduced uric acid, and improved renal function [51]. These promising results led to the Cardiac Outcome Prevention Effectiveness of Glucocorticoids in Acute Decompensated Heart Failure (COPE-ADHF) trial. In this single-centered study, 102 patients with ADHF were randomized to either placebo [51] or corticosteroids [51] and the outcomes recorded included urinary volume, change in creatinine, and cardiovascular death at 30 days. Use of corticosteroids improved renal function, increased urine output, and reduced mortality (3/51 in corticosteroid group versus 10/51 in the placebo group) [52]. The mechanisms underlying the improvements with corticosteroids were not determined, but were hypothesized to be facilitation of natriuretic peptides or dilation of renal vasculature through activation of nitric oxide pathway or dopaminergic system.
Serelaxin
Serelaxin is a recombinantly expressed human relaxin-2, a peptide hormone present during pregnancy which facilitates physiological cardiovascular and renal adaptations [53–55], which showed potential benefits in CRS1. Analysis of the RELAX-AHF trial revealed serelaxin reduced incidence of worsening renal function at day 2 of treatment as defined by changes in serum creatinine, cystatin C, and BUN. Importantly, worsening renal function defined by cystatin C changes was associated with increased 180-day mortality in this analysis [56]. The mechanisms by which serelaxin prevented renal dysfunction are currently unknown as serelaxin treatment did not improve diuretic efficiency [19].
Ultrafiltration
Another treatment choice in CRS1 is mechanical removal of salt and water via ultrafiltration. Ultrafiltration showed early promise in Ultrafiltration Versus Intravenous Diuretics for Patients Hospitalized for Acute Decompensated Heart Failure trial (UNLOAD) trial. In this study, 200 patients with ADHF were randomized to either ultrafiltration or medical management with loop diuretics. Use of ultrafiltration increased volume removal without any differences in renal function and reduced rehospitalization rates at 90 days [57].
However, when ultrafiltration was employed specifically in CRS1 patients in the Cardiorenal Rescue Study in Acute Decompensated Heart Failure trial (CARESS-HF), UF was not superior to medical treatment. There were 188 patients studied in CARESS-HF, and in the ultrafiltration arm there was increased risk of renal dysfunction, no differences in volume removal, and no change in rehospitalization rates at 90 days [58]. When trying to reconcile UNLOAD and CARESS-HF, the medical treatment arm in CARESS-HF was much more standardized and aggressive and UNLOAD was earlier implementation of ultrafiltration, which may have explained the differences. Interestingly, ultrafiltration was hypothesized to be advantageous over diuretic therapy through reduced activation of the renin-angiotensin-aldosterone system, but analysis of the patients from CARESS-HF showed higher levels of plasma renin activity and no difference in aldosterone levels in ultrafiltration patients [59].
Two meta-analyses have examined the use of ultrafiltration versus medical management in ADHF and both showed ultrafiltration was more effective in volume removal than medical therapy but did not improve rehospitalization or mortality rates [60,61]. This fact combined with the risks of vascular access placement and bleeding from anticoagulation limits to routine use of ultrafiltration in CRS1.
Continuous Renal Replacement Therapy
Once renal function deteriorates to the point that renal replacement therapy is needed for both volume removal and solute clearance in CRS1, continuous renal replacement therapy (CRRT) may be implemented. Unfortunately, there are few available data for this group of advanced CRS1 patients to guide physicians. There was a single-centered study conducted in Egypt that randomized 40 ADHF patients to either IV furosemide or CRRT. The patients treated with CRRT had greater weight loss and decreased length of stay in the ICU, but there were no differences in dialysis dependence rates or 30-day mortality [62]. Two single-centered studies reported outcomes associated with advanced CRS1 requiring CRRT. In a study conducted at the Cleveland Clinic, 63 patients with CRS1 were treated with ultrafiltration, of which 37 were converted to CRRT due to worsening renal function. Of the 37 patients treated with CRRT, 16 died in the hospital and 4 were discharged with hospice care [63]. In another retrospective study performed at the University of Alabama-Birmingham, use of rescue CRRT in advanced CRS1 was examined in 37 patients. 23 patients died during hospitalization and 2 were discharged to hospice care [64]. Combination of the Cleveland Clinic and University of Alabama-Birmingham studies revealed patients requiring CRRT in the setting of advanced CRS1 had an in-hospital mortality or palliative discharge rate of 60.8% (45/74). Clearly, this population needs further investigation to prevent such poor outcomes.
Future Treatment Options
Ongoing and Unreported Clinical Trials
Unfortunately, none of the current treatments for CRS1 have definitive improvements in outcomes, but there are several ongoing clinical trials which will hopefully identify novel treatment strategies. First of all, the Acetazolamide and Spironolactone to Increase Natriuresis in Congestive Heart Failure (Diuresis-CHF) trial is being conducted in Belgium. This study will examine the effects of acetazolamide with low dose diuretic versus high dose diuretics in one aim and the effects of upfront spironolactone in another. The outcomes analyzed will include total natriuresis, potassium homeostasis, NT-proBNP changes, change in renal function, peak serum levels of renin and aldosterone, weight change, urine volume, and change in edema (NCT01973335). The Protocolized Diuretic Strategy in Cardiorenal Failure (ProDius) trial is being performed at the University of Pittsburgh, and will determine the effects of a protocolized diuretic approach to target 3-5 liters of urine production a day versus standard therapy and will track the change in body weight, length of hospitalization, reshospitalization rates, mortality rates, venous compliance of internal jugular vein, clinical decongestion, change in renal function, and urine output (NCT01921829). The Levosimendan versus Dobutamine for Renal Function in Heart Failure (ELDOR) study is ongoing in Sweden and will probe the acute effects of levosimendan and dobutamine on renal perfusion. The endpoints will include changes in renal blood flow, GFR, renal vascular resistance, central hemodynamics, renal oxygen extraction and consumptions, and filtration fraction (NCT02133105). Finally, the Safety and Efficacy of Low Dose Hypertonic Saline and High Dose Furosemide for Congestive Heart Failure (REaCH) trial probed the effects of combination of hypertonic saline and furosemide versus furosemide in patients with ADHF and renal impairment defined by a GFR<60 mL/min. The outcomes were change in renal function, diuretic response, length of hospital stay, readmission rates, weight loss, BNP levels, and included a cost analysis. The study was completed but results are not currently available (NCT01028170)
Should Inflammation Be Targeted in CRS1?
Although proposed to play a role in the pathophysiology of CRS1, inflammation has not been explicitly targeted as a treatment for CRS1. One possible way to combat inflammation could be inhibition of the IL-6 pathway, which is support by preclinical work as previous studies showed IL-6 knockout mice were resistant to HgCl2-induced renal injury and death [65] and IL-6 has negative inotropic effects in both isolated cardiomyocytes [66] and intact animals [67]. Thus, IL-6 antagonism may improve both cardiac and renal function, an ideal scenario for CRS1 patients. The availability of tocilizumab, an FDA-approved humanized antibody to the IL-6 receptor, may allow for investigation of this hypothesis in the future. Although not examined in the COPE-ADHF trial, an alternative explanation for the improvements associated with corticosteroids treatment were the anti-inflammatory effects. If this were true, corticosteroids would represent a relatively cheap treatment option for CRS1 patients, but more studies need to be conducted before this approach is widely implemented. Finally, use of cytokine profiling may be used to enrich a population of CRS1 patients that could be investigated in future clinical trials using anti-inflammatory medications.
Unanswered Questions Moving Forward
Severity of AKI and Treatment Effects
An important unknown that warrants further investigation is if the severity of AKI should dictate treatment choice in CRS1. As discussed above, increasing severity of AKI resulted in elevated risk of adverse events, but it remains unknown whether different treatments offer benefits for more or less severe renal impairment. Perhaps, future studies aimed at defining outcomes from different treatment strategies stratified by severity of renal dysfunction may reveal which patients benefit from the various treatment options for CRS1.
How Do We Best Define Renal Dysfunction in CRS1?
Currently, there is no accepted definition of renal dysfunction in CRS1. As discussed above, using the AKIN, KDIGO, or RIFLE scoring systems or diuretic responsiveness effectively differentiated outcomes in patients with CRS1. However, an agreed-upon definition would likely benefit the field going forward so this population could be systematically investigated in future studies.
Conclusion
In summary, CRS1 is a common clinical entity associated with poor patient outcomes. A complex pathophysiology marked by reduced cardiac output, increased central venous pressure, inflammation, and oxidative stress underlies the disease process. Unfortunately, no current treatment approach shows consistent improvements in outcomes, highlighting the urgent need for further research to reduce the burden that CRS1 imposes.
Corresponding author: Kurt W. Prins, MD, PhD, MMC 580 Mayo, 420 Delaware St SE, Minneapolis, MN 55455, [email protected].
Funding/support: Dr. Prins is funded by NIH F32 grant HL129554 and Dr. Thenappen is funded by AHA Scientist Development Grant 15SDG25560048.
1. Mozaffarian D, Benjamin EJ, Go AS,et al. Heart disease and stroke statistics--2015 update: A report from the american heart association. Circulation 2015;131:e29–322.
2. Gheorghiade M, Vaduganathan M, Fonarow GC, Bonow RO. Rehospitalization for heart failure: problems and perspectives. J Am Coll Cardiol 2013;61:391–403.
3. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the medicare fee-for-service program. N Engl J Med 2009;360:1418–28.
4. Fonarow GC, Adams KF Jr, Abraham WT, et al and ADHERE Scientific Advisory Committee, Study Group, and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: Classification and regression tree analysis. JAMA 2005;293:572–80.
5. Abraham WT, Fonarow GC, Albert NM, et al. Predictors of in-hospital mortality in patients hospitalized for heart failure: Insights from the organized program to initiate lifesaving treatment in hospitalized patients with heart failure (OPTIMIZE-HF). J Am Coll Cardiol 2008;52:347–56.
6. Ronco C, Haapio M, House AA, et al. Cardiorenal syndrome. J Am Coll Cardiol 2008:52:1527–39.
7. Roy AK, Mc Gorrian C, Treacy C, et al. A comparison of traditional and novel definitions (RIFLE, AKIN, and KDIGO) of acute kidney injury for the prediction of outcomes in acute decompensated heart failure. Cardiorenal Med 2013;3:26–37.
8. Li Z, Cai L, Liang X, et al. Identification and predicting short-term prognosis of early cardiorenal syndrome type 1: KDIGO is superior to RIFLE or AKIN. PLoS One 2014;9:e114369.
9. Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009:53:589–96.
10. Nohria A, Hasselblad V, Stebbins A, et al. Cardiorenal interactions: Insights from the ESCAPE trial. J Am Coll Cardiol 2008:51:1268–74.
11. Winton FR. The influence of venous pressure on the isolated mammalian kidney. J Physiol 1931;72:49–61.
12. Virzi GM, Torregrossa R, Cruz DN, et al. Cardiorenal syndrome type 1 may be immunologically mediated: A pilot evaluation of monocyte apoptosis. Cardiorenal Med 2012;2:33–42.
13. Virzi GM, Clementi A, de Cal M, et al. Oxidative stress: Dual pathway induction in cardiorenal syndrome type 1 pathogenesis. Oxid Med Cell Longev 2015;391790.
14. Cho E, Kim M, Ko YS, et al. Role of inflammation in the pathogenesis of cardiorenal syndrome in a rat myocardial infarction model. Nephrol Dial Transplant 2013;28:2766–78.
15. Ather S, Bavishi C, McCauley MD, et al. Worsening renal function is not associated with response to treatment in acute heart failure. Int J Cardiol 2013;167:1912–7.
16. Testani JM, McCauley BD, Kimmel SE, Shannon RP. Characteristics of patients with improvement or worsening in renal function during treatment of acute decompensated heart failure. Am J Cardiol 2010;106:1763–69.
17. Hata N, Yokoyama S, Shinada T, et al. Acute kidney injury and outcomes in acute decompensated heart failure: Evaluation of the RIFLE criteria in an acutely ill heart failure population. Eur J Heart Fail 2010;12:32–7.
18. Testani JM, Brisco MA, Turner JM, et al. Loop diuretic efficiency: A metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ Heart Fail 2014;7:261–70.
19. Voors AA, Davison BA, Teerlink JR, et al. Diuretic response in patients with acute decompensated heart failure: Characteristics and clinical outcome--an analysis from RELAX-AHF. Eur J Heart Fail 2014;16:1230–40.
20. Valente MA, Voors AA, Damman K, et al. Diuretic response in acute heart failure: Clinical characteristics and prognostic significance. Eur Heart J 2014;35:1284–93.
21. Devarajan P. Neutrophil gelatinase-associated lipocalin: A troponin-like biomarker for human acute kidney injury. Nephrology (Carlton) 2010;15:419–28.
22. Soyler C, Tanriover MD, Ascioglu S, et al. Urine neutrophil gelatinase-associated lipocalin levels predict acute kidney injury in acute decompensated heart failure patients. Ren Fail 2015;5.
23. Brisco MA,Testani JM. Novel renal biomarkers to assess cardiorenal syndrome. Curr Heart Fail Rep 2014;11;485–99.
24. Lassus J, Harjola VP, Sund R, et al. and FINN-AKVA Study group. Prognostic value of cystatin C in acute heart failure in relation to other markers of renal function and NT-proBNP. Eur Heart J 2007;28:1841–7.
25. Arimoto T, Takeishi Y, Niizeki T, et al. Cystatin C, a novel measure of renal function, is an independent predictor of cardiac events in patients with heart failure. J Card Fail 2005;11:595–601.
26. Naruse H, Ishii J, Kawai T, et al. Cystatin C in acute heart failure without advanced renal impairment. Am J Med 2009;122:566–73.
27. Manzano-Fernandez S, Boronat-Garcia M, Albaladejo-Oton MD, et al. Complementary prognostic value of cystatin C, N-terminal pro-B-type natriuretic peptide and cardiac troponin T in patients with acute heart failure. Am J Cardiol 2009;103:1753–9.
28. Bonventre JV, Yang L. Kidney injury molecule-1. Curr Opin Crit Care 2010;16:556–61.
29. Damman K, Van Veldhuisen DJ, Navis G, et al. Tubular damage in chronic systolic heart failure is associated with reduced survival independent of glomerular filtration rate. Heart 2010;96:1297–302.
30. Jungbauer CG, Birner C, Jung B, et al. Kidney injury molecule-1 and N-acetyl-beta-D-glucosaminidase in chronic heart failure: Possible biomarkers of cardiorenal syndrome. Eur J Heart Fail 2011;13:1104–10.
31. Verbrugge FH, Dupont M, Shao Z, et al. Novel urinary biomarkers in detecting acute kidney injury, persistent renal impairment, and all-cause mortality following decongestive therapy in acute decompensated heart failure. J Card Fail 2013;19:621–8.
32. Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011;364:797–805.
33. Moranville MP, Choi S, Hogg J, et al. Comparison of metolazone versus chlorothiazide in acute decompensated heart failure with diuretic resistance. Cardiovasc Ther 2015;33;42–9.
34. Cuffe MS, Califf RM, Adams KF Jr, et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: A randomized controlled trial. JAMA 2002;287:1541–7.
35. Mebazaa A, Nieminen MS, Packer M, et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: The SURVIVE randomized trial. JAMA 2007;297:1883–91.
36. Varriale P, Mossavi A. The benefit of low-dose dopamine during vigorous diuresis for congestive heart failure associated with renal insufficiency: Does it protect renal function? Clin Cardiol 1997;20:627–30.
37. Giamouzis G, Butler J, Starling RC, et al. Impact of dopamine infusion on renal function in hospitalized heart failure patients: Results of the dopamine in acute decompensated heart failure (DAD-HF) trial. J Card Fail 2010;16:922–30.
38. Triposkiadis FK, Butler J, Karayannis G, et al. Efficacy and safety of high dose versus low dose furosemide with or without dopamine infusion: The dopamine in acute decompensated heart failure II (DAD-HF II) trial. Int J Cardiol 2014;172:115–21.
39. Chen HH, Anstrom KJ, Givertz MM, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: The ROSE acute heart failure randomized trial. JAMA 2013;310:2533–43.
40. Witteles RM, Kao D, Christopherson D, et al. Impact of nesiritide on renal function in patients with acute decompensated heart failure and pre-existing renal dysfunction a randomized, double-blind, placebo-controlled clinical trial. J Am Coll Cardiol 2007;50:1835–40.
41 O'Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med 2011;365:32–43.
42. Owan TE, Chen HH, Frantz RP, et al. The effects of nesiritide on renal function and diuretic responsiveness in acutely decompensated heart failure patients with renal dysfunction. J Card Fail 2008;14:267–75.
43. Givertz MM, Massie BM, Fields TK, et al and CKI-201 and CKI-202 Investigators. The effects of KW-3902, an adenosine A1-receptor antagonist,on diuresis and renal function in patients with acute decompensated heart failure and renal impairment or diuretic resistance. J Am Coll Cardiol 2007;50:1551–60.
44. Massie BM, O'Connor CM, Metra M, et al. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N Engl J Med 2010;363:1419–28.
45. Voors AA, Dittrich HC, Massie BM, et al. Effects of the adenosine A1 receptor antagonist rolofylline on renal function in patients with acute heart failure and renal dysfunction: Results from PROTECT (placebo-controlled randomized study of the selective adenosine A1 receptor antagonist rolofylline for patients hospitalized with acute decompensated heart failure and volume overload to assess treatment effect on congestion and renal function). J Am Coll Cardiol 2011;57:1899–907.
46. Gottlieb SS, Givertz MM, Metra M, et al. The effects of adenosine A(1) receptor antagonism in patients with acute decompensated heart failure and worsening renal function: The REACH UP study. J Card Fail 2010;16:714–9.
47. Gheorghiade M, Gattis WA, O'Connor CM, et al. Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: A randomized controlled trial. JAMA 2004;291:1963–71.
48. Gheorghiade M, Konstam MA, Burnett JC Jr, et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: The EVEREST clinical status trials. JAMA 2007;297:1332–43.
49. Konstam MA, Gheorghiade M, Burnett JC Jr, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: The EVEREST outcome trial. JAMA 2007;297:1319–31.
50. Liu C, Liu G, Zhou C, et al. Potent diuretic effects of prednisone in heart failure patients with refractory diuretic resistance. Can J Cardiol 2007;23:865–8.
51. Zhang H, Liu C, Ji Z, et al. Prednisone adding to usual care treatment for refractory decompensated congestive heart failure. Int Heart J 2008;49:587–95.
52. Liu C, Liu K and COPE-ADHF Study Group. Cardiac outcome prevention effectiveness of glucocorticoids in acute decompensated heart failure: COPE-ADHF study. J Cardiovasc Pharmacol 2014;63:333–8.
53. Teichman SL, Unemori E, Teerlink JR, et al. Relaxin: Review of biology and potential role in treating heart failure. Curr Heart Fail Rep 2010;7:75–82.
54. Conrad KP, Shroff SG. Effects of relaxin on arterial dilation, remodeling, and mechanical properties. Curr Hypertens Rep 2011;13:409–20.
55. Du XJ, Bathgate RA, Samuel CS, et al. Cardiovascular effects of relaxin: From basic science to clinical therapy. Nat Rev Cardiol 2010;7:48–58.
56. Metra M, Cotter G, Davison BA, et al. Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the relaxin in acute heart failure (RELAX-AHF) development program: Correlation with outcomes. J Am Coll Cardiol 2013;61:196-206.
57. Costanzo MR, Guglin ME, Saltzberg MT, et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol 2007;49:675–83.
58. Bart BA, Goldsmith SR, Lee KL, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012;367:2296–304.
59. Mentz RJ, Stevens SR, DeVore AD, et al. Decongestion strategies and renin-angiotensin-aldosterone system activation in acute heart failure. JACC Heart Fail 2015;3:97–107.
60. Ebrahim B, Sindhura K, Okoroh J, et al. Meta-analysis of ultrafiltration versus diuretics treatment option for overload volume reduction in patients with acute decompensated heart failure. Arq Bras Cardiol 2015;104:417–25.
61. Kwong JS, Yu CM. Ultrafiltration for acute decompensated heart failure: A systematic review and meta-analysis of randomized controlled trials. Int J Cardiol 2014;172:395–402.
62. Badawy SS, Fahmy A. Efficacy and cardiovascular tolerability of continuous veno-venous hemodiafiltration in acute decompensated heart failure: A randomized comparative study. J Crit Care 2012;27:106.e7-106.13.
63. Patarroyo M, Wehbe E, Hanna M, et al. Cardiorenal outcomes after slow continuous ultrafiltration therapy in refractory patients with advanced decompensated heart failure. J Am Coll Cardiol 2012;60:1906–12.
64. Prins KW, Wille KM, Tallaj JA, Tolwani AJ. Assessing continuous renal replacement therapy as a rescue strategy in cardiorenal syndrome 1. Clin Kidney J 2015;8:87–92.
65. Nechemia-Arbely Y, Barkan D, Pizov G, et al. IL-6/IL-6R axis plays a critical role in acute kidney injury. J Am Soc Nephrol 2008;19:1106–15.
66. Pathan N, Franklin JL, Eleftherohorinou H, et al. Myocardial depressant effects of interleukin 6 in meningococcal sepsis are regulated by p38 mitogen-activated protein kinase. Crit Care Med 2011;39:1692–711.
67. Janssen SP, Gayan-Ramirez G, Van den Bergh A, et al. Interleukin-6 causes myocardial failure and skeletal muscle atrophy in rats. Circulation 2005;111:996–1005.
68. Bellomo R, Ronco C, Kellum JA and Acute Dialysis Quality Initiative workgroup. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: The second international consensus conference of the acute dialysis quality initiative (ADQI) group. Crit Care 2004;8:R204-12.
69. Mehta RL, Kellum JA, Shah SV, et al and Acute Kidney Injury Network. Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31.
70. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guidelines for acute kidney injury. Kidney Inter Suppl 2012;2:19–36.
1. Mozaffarian D, Benjamin EJ, Go AS,et al. Heart disease and stroke statistics--2015 update: A report from the american heart association. Circulation 2015;131:e29–322.
2. Gheorghiade M, Vaduganathan M, Fonarow GC, Bonow RO. Rehospitalization for heart failure: problems and perspectives. J Am Coll Cardiol 2013;61:391–403.
3. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the medicare fee-for-service program. N Engl J Med 2009;360:1418–28.
4. Fonarow GC, Adams KF Jr, Abraham WT, et al and ADHERE Scientific Advisory Committee, Study Group, and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: Classification and regression tree analysis. JAMA 2005;293:572–80.
5. Abraham WT, Fonarow GC, Albert NM, et al. Predictors of in-hospital mortality in patients hospitalized for heart failure: Insights from the organized program to initiate lifesaving treatment in hospitalized patients with heart failure (OPTIMIZE-HF). J Am Coll Cardiol 2008;52:347–56.
6. Ronco C, Haapio M, House AA, et al. Cardiorenal syndrome. J Am Coll Cardiol 2008:52:1527–39.
7. Roy AK, Mc Gorrian C, Treacy C, et al. A comparison of traditional and novel definitions (RIFLE, AKIN, and KDIGO) of acute kidney injury for the prediction of outcomes in acute decompensated heart failure. Cardiorenal Med 2013;3:26–37.
8. Li Z, Cai L, Liang X, et al. Identification and predicting short-term prognosis of early cardiorenal syndrome type 1: KDIGO is superior to RIFLE or AKIN. PLoS One 2014;9:e114369.
9. Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009:53:589–96.
10. Nohria A, Hasselblad V, Stebbins A, et al. Cardiorenal interactions: Insights from the ESCAPE trial. J Am Coll Cardiol 2008:51:1268–74.
11. Winton FR. The influence of venous pressure on the isolated mammalian kidney. J Physiol 1931;72:49–61.
12. Virzi GM, Torregrossa R, Cruz DN, et al. Cardiorenal syndrome type 1 may be immunologically mediated: A pilot evaluation of monocyte apoptosis. Cardiorenal Med 2012;2:33–42.
13. Virzi GM, Clementi A, de Cal M, et al. Oxidative stress: Dual pathway induction in cardiorenal syndrome type 1 pathogenesis. Oxid Med Cell Longev 2015;391790.
14. Cho E, Kim M, Ko YS, et al. Role of inflammation in the pathogenesis of cardiorenal syndrome in a rat myocardial infarction model. Nephrol Dial Transplant 2013;28:2766–78.
15. Ather S, Bavishi C, McCauley MD, et al. Worsening renal function is not associated with response to treatment in acute heart failure. Int J Cardiol 2013;167:1912–7.
16. Testani JM, McCauley BD, Kimmel SE, Shannon RP. Characteristics of patients with improvement or worsening in renal function during treatment of acute decompensated heart failure. Am J Cardiol 2010;106:1763–69.
17. Hata N, Yokoyama S, Shinada T, et al. Acute kidney injury and outcomes in acute decompensated heart failure: Evaluation of the RIFLE criteria in an acutely ill heart failure population. Eur J Heart Fail 2010;12:32–7.
18. Testani JM, Brisco MA, Turner JM, et al. Loop diuretic efficiency: A metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ Heart Fail 2014;7:261–70.
19. Voors AA, Davison BA, Teerlink JR, et al. Diuretic response in patients with acute decompensated heart failure: Characteristics and clinical outcome--an analysis from RELAX-AHF. Eur J Heart Fail 2014;16:1230–40.
20. Valente MA, Voors AA, Damman K, et al. Diuretic response in acute heart failure: Clinical characteristics and prognostic significance. Eur Heart J 2014;35:1284–93.
21. Devarajan P. Neutrophil gelatinase-associated lipocalin: A troponin-like biomarker for human acute kidney injury. Nephrology (Carlton) 2010;15:419–28.
22. Soyler C, Tanriover MD, Ascioglu S, et al. Urine neutrophil gelatinase-associated lipocalin levels predict acute kidney injury in acute decompensated heart failure patients. Ren Fail 2015;5.
23. Brisco MA,Testani JM. Novel renal biomarkers to assess cardiorenal syndrome. Curr Heart Fail Rep 2014;11;485–99.
24. Lassus J, Harjola VP, Sund R, et al. and FINN-AKVA Study group. Prognostic value of cystatin C in acute heart failure in relation to other markers of renal function and NT-proBNP. Eur Heart J 2007;28:1841–7.
25. Arimoto T, Takeishi Y, Niizeki T, et al. Cystatin C, a novel measure of renal function, is an independent predictor of cardiac events in patients with heart failure. J Card Fail 2005;11:595–601.
26. Naruse H, Ishii J, Kawai T, et al. Cystatin C in acute heart failure without advanced renal impairment. Am J Med 2009;122:566–73.
27. Manzano-Fernandez S, Boronat-Garcia M, Albaladejo-Oton MD, et al. Complementary prognostic value of cystatin C, N-terminal pro-B-type natriuretic peptide and cardiac troponin T in patients with acute heart failure. Am J Cardiol 2009;103:1753–9.
28. Bonventre JV, Yang L. Kidney injury molecule-1. Curr Opin Crit Care 2010;16:556–61.
29. Damman K, Van Veldhuisen DJ, Navis G, et al. Tubular damage in chronic systolic heart failure is associated with reduced survival independent of glomerular filtration rate. Heart 2010;96:1297–302.
30. Jungbauer CG, Birner C, Jung B, et al. Kidney injury molecule-1 and N-acetyl-beta-D-glucosaminidase in chronic heart failure: Possible biomarkers of cardiorenal syndrome. Eur J Heart Fail 2011;13:1104–10.
31. Verbrugge FH, Dupont M, Shao Z, et al. Novel urinary biomarkers in detecting acute kidney injury, persistent renal impairment, and all-cause mortality following decongestive therapy in acute decompensated heart failure. J Card Fail 2013;19:621–8.
32. Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011;364:797–805.
33. Moranville MP, Choi S, Hogg J, et al. Comparison of metolazone versus chlorothiazide in acute decompensated heart failure with diuretic resistance. Cardiovasc Ther 2015;33;42–9.
34. Cuffe MS, Califf RM, Adams KF Jr, et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: A randomized controlled trial. JAMA 2002;287:1541–7.
35. Mebazaa A, Nieminen MS, Packer M, et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: The SURVIVE randomized trial. JAMA 2007;297:1883–91.
36. Varriale P, Mossavi A. The benefit of low-dose dopamine during vigorous diuresis for congestive heart failure associated with renal insufficiency: Does it protect renal function? Clin Cardiol 1997;20:627–30.
37. Giamouzis G, Butler J, Starling RC, et al. Impact of dopamine infusion on renal function in hospitalized heart failure patients: Results of the dopamine in acute decompensated heart failure (DAD-HF) trial. J Card Fail 2010;16:922–30.
38. Triposkiadis FK, Butler J, Karayannis G, et al. Efficacy and safety of high dose versus low dose furosemide with or without dopamine infusion: The dopamine in acute decompensated heart failure II (DAD-HF II) trial. Int J Cardiol 2014;172:115–21.
39. Chen HH, Anstrom KJ, Givertz MM, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: The ROSE acute heart failure randomized trial. JAMA 2013;310:2533–43.
40. Witteles RM, Kao D, Christopherson D, et al. Impact of nesiritide on renal function in patients with acute decompensated heart failure and pre-existing renal dysfunction a randomized, double-blind, placebo-controlled clinical trial. J Am Coll Cardiol 2007;50:1835–40.
41 O'Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med 2011;365:32–43.
42. Owan TE, Chen HH, Frantz RP, et al. The effects of nesiritide on renal function and diuretic responsiveness in acutely decompensated heart failure patients with renal dysfunction. J Card Fail 2008;14:267–75.
43. Givertz MM, Massie BM, Fields TK, et al and CKI-201 and CKI-202 Investigators. The effects of KW-3902, an adenosine A1-receptor antagonist,on diuresis and renal function in patients with acute decompensated heart failure and renal impairment or diuretic resistance. J Am Coll Cardiol 2007;50:1551–60.
44. Massie BM, O'Connor CM, Metra M, et al. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N Engl J Med 2010;363:1419–28.
45. Voors AA, Dittrich HC, Massie BM, et al. Effects of the adenosine A1 receptor antagonist rolofylline on renal function in patients with acute heart failure and renal dysfunction: Results from PROTECT (placebo-controlled randomized study of the selective adenosine A1 receptor antagonist rolofylline for patients hospitalized with acute decompensated heart failure and volume overload to assess treatment effect on congestion and renal function). J Am Coll Cardiol 2011;57:1899–907.
46. Gottlieb SS, Givertz MM, Metra M, et al. The effects of adenosine A(1) receptor antagonism in patients with acute decompensated heart failure and worsening renal function: The REACH UP study. J Card Fail 2010;16:714–9.
47. Gheorghiade M, Gattis WA, O'Connor CM, et al. Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: A randomized controlled trial. JAMA 2004;291:1963–71.
48. Gheorghiade M, Konstam MA, Burnett JC Jr, et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: The EVEREST clinical status trials. JAMA 2007;297:1332–43.
49. Konstam MA, Gheorghiade M, Burnett JC Jr, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: The EVEREST outcome trial. JAMA 2007;297:1319–31.
50. Liu C, Liu G, Zhou C, et al. Potent diuretic effects of prednisone in heart failure patients with refractory diuretic resistance. Can J Cardiol 2007;23:865–8.
51. Zhang H, Liu C, Ji Z, et al. Prednisone adding to usual care treatment for refractory decompensated congestive heart failure. Int Heart J 2008;49:587–95.
52. Liu C, Liu K and COPE-ADHF Study Group. Cardiac outcome prevention effectiveness of glucocorticoids in acute decompensated heart failure: COPE-ADHF study. J Cardiovasc Pharmacol 2014;63:333–8.
53. Teichman SL, Unemori E, Teerlink JR, et al. Relaxin: Review of biology and potential role in treating heart failure. Curr Heart Fail Rep 2010;7:75–82.
54. Conrad KP, Shroff SG. Effects of relaxin on arterial dilation, remodeling, and mechanical properties. Curr Hypertens Rep 2011;13:409–20.
55. Du XJ, Bathgate RA, Samuel CS, et al. Cardiovascular effects of relaxin: From basic science to clinical therapy. Nat Rev Cardiol 2010;7:48–58.
56. Metra M, Cotter G, Davison BA, et al. Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the relaxin in acute heart failure (RELAX-AHF) development program: Correlation with outcomes. J Am Coll Cardiol 2013;61:196-206.
57. Costanzo MR, Guglin ME, Saltzberg MT, et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol 2007;49:675–83.
58. Bart BA, Goldsmith SR, Lee KL, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012;367:2296–304.
59. Mentz RJ, Stevens SR, DeVore AD, et al. Decongestion strategies and renin-angiotensin-aldosterone system activation in acute heart failure. JACC Heart Fail 2015;3:97–107.
60. Ebrahim B, Sindhura K, Okoroh J, et al. Meta-analysis of ultrafiltration versus diuretics treatment option for overload volume reduction in patients with acute decompensated heart failure. Arq Bras Cardiol 2015;104:417–25.
61. Kwong JS, Yu CM. Ultrafiltration for acute decompensated heart failure: A systematic review and meta-analysis of randomized controlled trials. Int J Cardiol 2014;172:395–402.
62. Badawy SS, Fahmy A. Efficacy and cardiovascular tolerability of continuous veno-venous hemodiafiltration in acute decompensated heart failure: A randomized comparative study. J Crit Care 2012;27:106.e7-106.13.
63. Patarroyo M, Wehbe E, Hanna M, et al. Cardiorenal outcomes after slow continuous ultrafiltration therapy in refractory patients with advanced decompensated heart failure. J Am Coll Cardiol 2012;60:1906–12.
64. Prins KW, Wille KM, Tallaj JA, Tolwani AJ. Assessing continuous renal replacement therapy as a rescue strategy in cardiorenal syndrome 1. Clin Kidney J 2015;8:87–92.
65. Nechemia-Arbely Y, Barkan D, Pizov G, et al. IL-6/IL-6R axis plays a critical role in acute kidney injury. J Am Soc Nephrol 2008;19:1106–15.
66. Pathan N, Franklin JL, Eleftherohorinou H, et al. Myocardial depressant effects of interleukin 6 in meningococcal sepsis are regulated by p38 mitogen-activated protein kinase. Crit Care Med 2011;39:1692–711.
67. Janssen SP, Gayan-Ramirez G, Van den Bergh A, et al. Interleukin-6 causes myocardial failure and skeletal muscle atrophy in rats. Circulation 2005;111:996–1005.
68. Bellomo R, Ronco C, Kellum JA and Acute Dialysis Quality Initiative workgroup. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: The second international consensus conference of the acute dialysis quality initiative (ADQI) group. Crit Care 2004;8:R204-12.
69. Mehta RL, Kellum JA, Shah SV, et al and Acute Kidney Injury Network. Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31.
70. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guidelines for acute kidney injury. Kidney Inter Suppl 2012;2:19–36.
Tracking a Tumor
Is there a universal cancer fingerprint? Researchers at the National Institutes of Health believe they may have found a potential common biomarker for 5 different tumor types. The clue is a “methylation signature”—evidence of a chemical modification of DNA. Methylation controls the expression of genes, and higher amounts of DNA methylation reduce a gene’s activity, like a dimmer switch on a light fixture.
In an earlier study using DNA taken from solid tumors, the researchers found a methylation signature in 15 tumor types in 13 different organs around the gene called ZNF154. In the new study, the researchers uncovered methylation in colon, lung, breast, stomach, and endometrial cancers. All the tumor types and subtypes consistently produced the same methylation mark around ZNF154.
Researchers developed a computer program that looked at methylation marks in the DNA of people with and without cancer and were able to predict a threshold for detecting tumor DNA. Because tumors often shed DNA into the bloodstream, the researchers were able to calculate the proportions of circulating tumor DNA. The researchers hope their results lead to a blood test that can diagnose cancers at early stages.
Currently, blood tests are specific to a known tumor type. Clinicians must first find the tumor and then sequence a sample from it before they can track the tumor-specific mutations in the blood. By contrast, a method derived from the methylation signatures would mean no prior knowledge of the cancer was required. The tests would be less intrusive than that of other screening methods and could be used to follow high-risk patients or monitor the activity of a tumor during treatment.
Source:
National Institutes of Health. NIH researchers identify striking genomic signature shared by five types of cancer [news release]. National Institutes of Health Website. http://www.nih.gov/news-events/news-releases/nih-researchers-identify-striking-genomic-signature-shared-five-types-cancer. Published February 5, 2016. Accessed February 29, 2016.
Is there a universal cancer fingerprint? Researchers at the National Institutes of Health believe they may have found a potential common biomarker for 5 different tumor types. The clue is a “methylation signature”—evidence of a chemical modification of DNA. Methylation controls the expression of genes, and higher amounts of DNA methylation reduce a gene’s activity, like a dimmer switch on a light fixture.
In an earlier study using DNA taken from solid tumors, the researchers found a methylation signature in 15 tumor types in 13 different organs around the gene called ZNF154. In the new study, the researchers uncovered methylation in colon, lung, breast, stomach, and endometrial cancers. All the tumor types and subtypes consistently produced the same methylation mark around ZNF154.
Researchers developed a computer program that looked at methylation marks in the DNA of people with and without cancer and were able to predict a threshold for detecting tumor DNA. Because tumors often shed DNA into the bloodstream, the researchers were able to calculate the proportions of circulating tumor DNA. The researchers hope their results lead to a blood test that can diagnose cancers at early stages.
Currently, blood tests are specific to a known tumor type. Clinicians must first find the tumor and then sequence a sample from it before they can track the tumor-specific mutations in the blood. By contrast, a method derived from the methylation signatures would mean no prior knowledge of the cancer was required. The tests would be less intrusive than that of other screening methods and could be used to follow high-risk patients or monitor the activity of a tumor during treatment.
Source:
National Institutes of Health. NIH researchers identify striking genomic signature shared by five types of cancer [news release]. National Institutes of Health Website. http://www.nih.gov/news-events/news-releases/nih-researchers-identify-striking-genomic-signature-shared-five-types-cancer. Published February 5, 2016. Accessed February 29, 2016.
Is there a universal cancer fingerprint? Researchers at the National Institutes of Health believe they may have found a potential common biomarker for 5 different tumor types. The clue is a “methylation signature”—evidence of a chemical modification of DNA. Methylation controls the expression of genes, and higher amounts of DNA methylation reduce a gene’s activity, like a dimmer switch on a light fixture.
In an earlier study using DNA taken from solid tumors, the researchers found a methylation signature in 15 tumor types in 13 different organs around the gene called ZNF154. In the new study, the researchers uncovered methylation in colon, lung, breast, stomach, and endometrial cancers. All the tumor types and subtypes consistently produced the same methylation mark around ZNF154.
Researchers developed a computer program that looked at methylation marks in the DNA of people with and without cancer and were able to predict a threshold for detecting tumor DNA. Because tumors often shed DNA into the bloodstream, the researchers were able to calculate the proportions of circulating tumor DNA. The researchers hope their results lead to a blood test that can diagnose cancers at early stages.
Currently, blood tests are specific to a known tumor type. Clinicians must first find the tumor and then sequence a sample from it before they can track the tumor-specific mutations in the blood. By contrast, a method derived from the methylation signatures would mean no prior knowledge of the cancer was required. The tests would be less intrusive than that of other screening methods and could be used to follow high-risk patients or monitor the activity of a tumor during treatment.
Source:
National Institutes of Health. NIH researchers identify striking genomic signature shared by five types of cancer [news release]. National Institutes of Health Website. http://www.nih.gov/news-events/news-releases/nih-researchers-identify-striking-genomic-signature-shared-five-types-cancer. Published February 5, 2016. Accessed February 29, 2016.
Current Management of Nephrolithiasis
Case
A 39-year-old woman presented to the ED with a chief complaint of intermittent right flank pain that radiated into her groin area. She stated the pain had begun suddenly, 4 hours prior to arrival, and was accompanied by nausea and vomiting. The patient said that she had taken acetaminophen for the pain, but had received no relief. Regarding history, according to the patient, her last menstrual period ended 2 days earlier. She denied any urinary symptoms, diarrhea, or constipation. She had no history of abdominal surgery and was currently not on any medications.
The patient’s vital signs at presentation were: temperature 98.7°F; blood pressure, 130/90 mm Hg; heart rate, 110 beats/minute; and respiratory rate, 18 breaths/minute. Oxygen saturation was 98% on room air. On physical examination, she appeared to be in mild distress, pacing around the room. There was moderate right costovertebral tenderness on percussion; the abdomen was soft and nontender.
Incidence
As ED visits for nephrolithiasis are increasing, so too are the health-care costs associated with this condition. Between 1992 and 2009, emergent-care presentations for nephrolithiasis rose from 178 to 340 visits per 100,000 individuals.1 Approximately 1 in 11 people in the United States will be affected by nephrolithiasis during their lifetime.2 Estimated health-care costs associated with these complaints were roughly $2 billion in 2000—an increase of 50% since 1994.2
Evaluation and Diagnosis
Laboratory Evaluation
Urinalysis is one of the initial studies for patients with suspected nephrolithiasis. Although hematuria is a classic finding associated with renal calculi, its sensitivity on microscopic analysis is around 84%. Therefore, the absence of hematuria does not exclude renal colic in the differential diagnosis.3
In addition to detecting hematuria, urinalysis can also reveal an underlying infection. One study by Abrahamian et al4 found that roughly 8% of patients presenting with acute nephrolithiasis had a urinary tract infection (UTI)—many without any clinical findings of infection. The presence of pyuria, however, has only moderate accuracy in identifying UTIs in patients with kidney stones.4 If an infected stone cannot be excluded clinically, computed tomography (CT) is indicated.
Mild leukocytosis (ie, <15,000 cells/mcL) is another common finding in patients with acute renal colic.5 A leukocyte count >15,000 cells/mcL is suspicious for infection or other pathology. A blood-chemistry panel to evaluate renal function is appropriate as a baseline—particularly for patients in whom treatment with a nonsteroidal anti-inflammatory (NSAID) drug is anticipated.
With the ability to visualize renal calculi (Figure 1), the use of noncontrast CT has become a standard initial imaging modality in assessing patients with renal colic. Between 1992 and 2009, the use of CT to evaluate patients presenting with flank pain for suspected renal colic more than tripled from 21% to 71%.6 An analysis performed by the American College of National Radiology Data Registry7 shows the mean radiation dose given by institutions for renal colic CT is unnecessarily high, and that few institutions follow CT-stone protocols aimed at minimizing radiation exposure while still maintaining proper diagnostic accuracy. A typical CT of the abdomen and pelvis is equivalent to over 100 two-view chest X-rays.8 Though controversial, data from a white paper by the American College of Radiology suggest that the ionizing radiation exposure from just one CT for renal colic causes an increase in lifetime cancer risk.9
Despite the increase in CT imaging to evaluate patients presenting to the ED with nephrolithiasis/flank pain, the proportion of patients diagnosed with a kidney stone remained the same between 2000 and 2008, with no significant change in outcomes.10-12 Moreover, the use of CT as an initial imaging modality in patients presenting with flank pain—but with no sign of infection—is unlikely to reveal important alternative findings.13
Regarding the sensitivity of CT in detecting nephrolithiasis, one study demonstrates a sensitivity of 100% and a specificity of 94% for noncontrast CT.14 Controversy, however, still exists regarding the necessity and utility of CT in diagnosing nephrolithiasis,15 and CT is one of the top 10 tests included in the American College of Emergency Physicians (ACEP) 2014 Choosing Wisely campaign. In this campaign, ACEP recommended emergency physicians (EPs) avoid abdominal and pelvic CT in otherwise healthy patients younger than age 50 years who present with symptoms consistent with uncomplicated renal colic and who have a known history of nephrolithiasis or ureterolithiasis.15 The ACEP also noted that CTs in this context do not often change treatment decisions and are associated with unnecessary radiation exposure and cost.15
While keeping the aforementioned recommendations in mind, if an EP intends to refer a renal colic patient to a urologist a CT scan is necessary either in the ED or as an outpatient. In all cases (except perhaps in patients in whom there is a history of renal stones), the urologist will need this study to determine the size and location of the stone in order to provide recommendations for management.
Ultrasound
Clinical Decision Score
Moore et al,17 authors of the Size, Topography, Location, Obstruction, Number of stones, and Evaluation (STONE) scoring system, developed a classification system for patients with suspected nephrolithiasis. This system places patients into low-, moderate-, and high-score groups, with corresponding probabilities of ureteral stone based on symptoms and epidemiological classifications.
The intent of the STONE system is to accurately predict, based on classification, the likelihood of a patient having a simple ureteral stone versus a more significant, complicated stone and to help guide which, if any, imaging studies are indicated. For example, a lower STONE score would help guide the decision to defer advanced imaging studies that would be unlikely to reveal an alternate serious diagnosis. Likewise, an individual with a high STONE score could potentially receive ultrasonography, reduced-dose CT, or no further imaging.
The STONE score performs fairly well and appears to be superior to physician gestalt, with an area under the receiver operating characteristic curve (AUC) of .78 compared to .68 with physician gestalt. This system, however, is not always accurate in its classification and has been shown to have 87% specificity at the high end to rule in stone and 96% sensitivity rate at the low end to rule out a stone. Of course, when using a clinical decision rule to rule in or rule out a stone, a tool with a very high specificity is preferred. Although the STONE scoring system does show promise, further studies are needed before it can be applied clinically.17
Treatment
Analgesia
By inhibiting prostaglandin synthesis, NSAIDs reduce inflammation and ureteral muscular hyperactivity.18 A recent Cochrane review of over 50 studies concluded that NSAIDs were effective in relieving acute renal colic pain.19 A systematic review by Holdgate and Pollock20 shows that patients treated with NSAIDs achieve greater reductions in pain scores and are less likely to require additional analgesia in the short term compared to patients treated with opioids. Although opioid medications are effective in relieving pain associated with nephrolithiasis, this class of drugs can exacerbate the nausea often associated with this condition. This same study also showed that patients who were prescribed NSAIDs following an ED visit for renal colic required less medication for pain control, experienced less nausea, and had greater improvements in their pain.20
Nevertheless, the utility of opiates as an adjunct therapy should not be overlooked. For example, in patients with renal colic, numerous studies show treatment with a combination of an NSAID and opiate provides superior pain relief compared to either treatment modality in isolation.21 Opioid analgesia may be indicated in patients in whom NSAIDs are not recommended or contraindicated (eg, elderly patients, patients with renal disease). While NSAIDs address the underlying pathophysiology associated with renal colic, they are sometimes not the best treatment option. Depending on the situation, treatment with an opioid should instead be considered.
Intravenous Fluid Therapy
A 2012 Cochrane Review of randomized control trials (RCT) on intravenous (IV) fluid therapy hydration/diuretic use concluded that there was “no reliable evidence in the literature to support the use of diuretics and high-volume fluid therapy for people with acute ureteric colic.” The review, however, did note that further investigation is warranted for a definitive answer.22 Another study by Springhart et al23 showed no difference in pain or stone expulsion between large-volume (2 L IV fluids over 2 hours) and small-volume fluid administration (20 mL/h). Regarding administration, the use of IV fluids in renal colic is no different than the usual indications for fluid therapy in the ED and should be restricted to patients with signs of dehydration or kidney injury.
Many patients with renal colic will have decreased oral intake from the pain and nausea associated with the stone and may be vomiting. Under these circumstances, it is reasonable to rehydrate the patients, even though large-volume hydration with the intent of aiding stone expulsion or improving pain has not been shown efficacious. Conversely, in addition to the perceived benefit of rehydrating patients, a small amount of fluid hydration may improve the visualization of hydronephrosis on ultrasound.24
Medical Expulsive Therapy
For many years, clinicians have considered the use of tamsulosin, an α1-receptor blocker, as well as nifedipine, a calcium channel blocker, in treating renal colic due to the theoretical benefit of reducing ureteral smooth muscle spasm/constriction thus expediting stone passage. Over the years, dozens of studies showed positive benefit in the use of medical expulsive therapy (MET). A 2014 Cochrane Review demonstrated that patients treated with α1-blockers experienced a higher stone-free rate and shorter time to stone expulsion, and concluded that α1-blockers should be offered as one of the primary treatment modalities in MET.25 This review, however, has been criticized for using a number of studies with very small patient samples, non-peer-reviewed abstracts, and low-quality study designs.26
More recently, in April 2015, Lancet published a large RCT from 24 hospitals in the United Kingdom, comparing placebo versus 400 mcg tamsulosin and 30 mg nifedipine. The authors concluded that “tamsulosin 400 mcg and nifedipine 30 mg are not effective at decreasing the need for further treatment to achieve stone clearance in 4 weeks for patients with expectantly managed ureteric colic.”27 Another large double-blind, placebo-controlled, randomized, multicenter trial by Furyk et al28 in July 2015 went a step further and evaluated distal stones, which have historically caused complications requiring intervention. They concluded that there was “no benefit overall of 0.4 mg of tamsulosin daily for patients with distal ureteric calculi less than or equal to 10 mm in terms of spontaneous passage, time to stone passage, pain, or analgesia requirements. In the subgroup with large stones (5 to 10 mm), tamsulosin did increase passage and should be considered.”28 Based on these recent studies, the use of tamsulosin in patients with stones larger than 5 mm—but not those with smaller stones—appears to be an appropriate treatment option.
Patient Disposition
The American Urological Association cited indications for urgent/emergent urological interventions necessitating the need for inpatient admission and further workup.29 Patients who do not fall into any of the categories outlined in the Table may be seen on an outpatient basis. These patients may be treated symptomatically until they can follow up with a urologist, who will determine expectant management versus intervention.
Prognosis
The majority of stones <5mm will pass spontaneously.30 Larger stones may still pass spontaneously but are more likely to require lithotripsy or other urologic intervention; therefore, patients with stones >5 mm should be referred to urology services.30
Recurrence
Patients with a first-time kidney stone have a 30% to 50% chance of disease recurrence within 5 years,31 and a 60% to 80% chance of recurrence during their lifetime.32 Those with a family history of nephrolithiasis are likely to develop an earlier onset of stones as well as experience more frequent recurrent episodes.33 Patients with recurrent disease should undergo outpatient risk stratification, including stone-composition analysis and assessment for modifiable risk factors.
Case Conclusion
The patient’s urinalysis demonstrated microscopic hematuria; blood urea nitrogen and creatinine levels were within normal limits. As the patient was tachycardic and appeared mildly dehydrated, an IV infusion of 1 L normal saline was initiated, along with ketorolac and ondansetron for symptomatic relief. A POC ultrasound of the right kidney revealed mild-to-moderate hydronephrosis; the left kidney appeared sonographically normal. Since this patient had no history of nephrolithiasis, a nonenhanced CT of the abdomen was obtained, which revealed moderate, right-sided hydronephrosis and a 3-mm distal ureteral stone. Once the patient’s symptoms were controlled, she was discharged home with a prescription for ibuprofen for symptomatic relief and instructions to follow up with her PCP.
Conclusion
The evaluation and treatment of nephrolithiasis is important due to its increasing prevalence, as well as implications on costs to the health-care system and to patients themselves. The workup and treatment of nephrolithiasis has been and continues to be the subject of much controversy. Until very recently, treatment recommendations were founded on physiological theories more so than robust research. In an era where improved imaging technology is becoming more readily available in the ED, EPs should weigh the pros and cons of its utilization for common ED complaints such as nephrolithiasis.
Dr Parsa is an assistant professor in the department of emergency medicine, Texas Tech University Health Sciences Center, El Paso. Dr Khafi is a resident in the department of emergency medicine, Texas Tech University Health Sciences Center, El Paso.
- Scales CD Jr, Smith AC, Hanley JM, Saigal CS; Urologic Diseases in America Project. Prevalence of kidney stones in the United States. Eur Urol. 2012;62(1):160-165.
- Pearle MS, Calhoun EA, Curhan GC; Urologic Diseases of America Project: urolithiasis. J Urol. 2005;173(3):848-857.
- Luchs JS, Katz DS, Lane MJ et al. Utility of hematuria testing in patients with suspected renal colic: correlation with unenhanced helical CT results. Urology. 2002;59(6):839-842.
- Abrahamian FM, Krishnadasan A, Mower WR, Moran GJ, Talan DA. Association of pyuria and clinical characteristics with the presence of urinary tract infection among patients with acute nephrolithiasis. Ann Emerg Med. 2013;62(5):526-533.
- Yilmaz S, Pekdemir M, Aksu NM, Koyuncu N, Cinar O, Akpinar E. A multicenter case–control study of diagnostic tests for urinary tract infection in the presence of urolithiasis. Urol Res. 2011;40(1):61-65. doi:10.1007/s00240-011-0402-x.
- Fwu CW, Eggers PW, Kimmel PL, Kusek JW, Kirkali Z. Emergency department visits, use of imaging, and drugs for urolithiasis have increased in the United States. Kidney Int. 2013;83(3):479-486. doi:10.1038/ki.2012.419.
- Lukasiewicz A, Bhargavan-Chatfield M, Coombs L, et al. Radiation dose index of renal colic protocol CT studies in the United States: a report from the American College of Radiology National Radiology Data Registry. Radiology. 2014;271(2):445-451. doi:10.1148/radiol.14131601.
- Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 248(1):254-263.
- Amis ES Jr, Butler PF, Applegate KE, et al; American College of Radiology. American College of Radiology white paper on radiation dose in medicine. J Am Coll Radiol. 2007;4(5):272-284.
- Hyams ES, Korley FK, Pham JC, Matlaga BR. Trends in imaging use during the emergency department evaluation of flank pain. J Urol. 2011;186(6):2270-2274. doi:10.1016/j.juro.2011.07.079.
- Ripollés T, Agramunt M, Errando J, Martínez MJ, Coronel B, Morales M. Suspected ureteral colic: plain film and sonography vs unenhanced helical CT. A prospective study in 66 patients. Eur Radiol. 2004;14(1):129-36. doi:10.1007/s00330-003-1924-1926.
- Westphalen AC, Hsia RY, Maselli JH, Wang R, Gonzales R. Radiological imaging of patients with suspected urinary tract stones: national trends, diagnoses, and predictors. Acad Emerg Med. 2011;18(7):699-707. doi:10.1111/j.1553-2712.2011.01103.x.
- Moore CL, Daniels B, Singh D, Luty S, Molinaro A. Prevalence and clinical importance of alternative causes of symptoms using a renal colic computed tomography protocol in patients with flank or back pain and absence of pyuria. Acad Emerg Med. 2013;20(5):470-478. doi:10.1111/acem.12127.
- Chen MY, Zagoria RJ. Can noncontrast helical computed tomography replace intravenous urography for evaluation of patients with acute urinary tract colic? J Emerg Med. 1999;17(2):299-303.
- American College of Emergency Physicians. Five things physicians and patients should question. Choosing Wisely Web site. 2013;10:1-5. Available at: http://www.choosingwisely.org/societies/american-college-of-emergency-physicians/. Accessed February 10, 2016.
- Smith-Bindman R, Aubin C, Bailitz J, et al. Ultrasonography versus computed tomography for suspected nephrolithiasis. N Engl J Med. 2014;371(12):1100-1110. doi:10.1056/nejmoa1404446.
- Moore CL, Bomann S, Daniels B, et al. Derivation and validation of a clinical prediction rule for uncomplicated ureteral stone—the STONE score: retrospective and prospective observational cohort studies. BMJ. 2014;348:g2191. doi:10.1136/bmj.g2191.
- Cole RS, Fry CH, Shuttleworth KE. The action of the prostaglandins on isolated human ureteric smooth muscle. Br J Urol. 1988;61(1):19-26.
- Afshar K, Jafari S, Marks AJ, Eftekhari R, McNeily AE. Nonsteroidal anti-inflammatory drugs (NSAIDs) and non-opioids for acute renal colic. Cochrane Database Syst Rev. 2015;6:CD006027. doi:10.1002/14651858.CD006027.pub2.
- Holdgate A, Pollock T. Systematic review of the relative efficacy of non-steroidal anti-inflammatory drugs and opioids in the treatment of acute renal colic. BMJ. 2004;328(7453):1401. doi:10.1136/bmj.38119.581991.55.
- Safdar B, Degutis LC, Landry K, Vedere SR, Moscovitz HC, D’Onofrio G. Intravenous morphine plus ketorolac is superior to either drug alone for treatment of acute renal colic. Ann Emerg Med. 2006;48(2):173-181, 181.e1. doi:10.1016/j.annemergmed.2006.03.013.
- Worster AS, Bhanich Supapol W. Fluids and diuretics for acute ureteric colic. Cochrane Database Syst Rev. 2012;15;2:CD004926. doi:10.1002/14651858.CD004926.pub3.
- Springhart WP, Marguet CG, Sur RL, et al. Forced versus minimal intravenous hydration in the management of acute renal colic: a randomized trial. J. Endourol. 2006;20(10):713-716. doi:10.1089/end.2006.20.713.
- Morse JW, Hill R, Greissinger WP, Patterson JW, Melanson SW, Heller MB. Rapid oral hydration results in hydronephrosis as demonstrated by bedside ultrasound. Ann Emerg Med. 1999;34(2):134-140. doi:10.1016/s0196-0644(99)70221-0.
- Campschroer T, Zhu Y, Duijvesz D, Grobbee DE, Lock MT. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014;4:CD008509. doi:10.1002/14651858.CD008509.pub2.
- Radecki R. Sadly inadequate Cochrane review of renal colic. Emergency Medicine Literature of Note. 2014. Available at: http://www.emlitofnote.com/2014/04/sadly-inadequate-cochrane-review-of.html. Accessed February 10, 2016.
- Pickard R, Starr K, MacLennan G, et al. Medical expulsive therapy in adults with ureteric colic: a multicentre, randomised, placebo-controlled trial. Lancet. 2015;386(9991):341-349. doi:10.1016/S0140-6736(15)60933-3.
- Furyk JS, Chu K, Banks C, et al. Distal ureteric stones and tamsulosin: a double-blind, placebo-controlled, randomized, multicenter trial. Ann Emerg Med. 2016;67(1):86-95.e2. doi:10.1016/j.annemergmed.2015.06.001.
- Kidney stones. American Urological Association Web site. 2016. Available at: https://www.auanet.org/education/kidney-stones.cfm. Accessed February 10, 2016.
- Miller OF, Kane CJ. Time to stone passage for observed ureteral calculi: a guide for patient education. J Urol. 1999;162(3 Pt 1):688-690.
- Lipkin ME, Preminger GM. Demystifying the medical management of nephrolithiasis. Rev Urol. 2011;13(1):34-38.
- Morton AR, Iliescu EA, Wilson JW. Nephrology: 1. Investigation and treatment of recurrent kidney stones. CMAJ. 2002;166(2):213-218.
- Koyuncu HH, Yencilek F, Eryildirim B, Sarica K. Family history in stone disease: how important is it for the onset of the disease and the incidence of recurrence? Urol Res. 2010;38(2):105-109. doi:10.1007/s00240-009-0249-6.
Case
A 39-year-old woman presented to the ED with a chief complaint of intermittent right flank pain that radiated into her groin area. She stated the pain had begun suddenly, 4 hours prior to arrival, and was accompanied by nausea and vomiting. The patient said that she had taken acetaminophen for the pain, but had received no relief. Regarding history, according to the patient, her last menstrual period ended 2 days earlier. She denied any urinary symptoms, diarrhea, or constipation. She had no history of abdominal surgery and was currently not on any medications.
The patient’s vital signs at presentation were: temperature 98.7°F; blood pressure, 130/90 mm Hg; heart rate, 110 beats/minute; and respiratory rate, 18 breaths/minute. Oxygen saturation was 98% on room air. On physical examination, she appeared to be in mild distress, pacing around the room. There was moderate right costovertebral tenderness on percussion; the abdomen was soft and nontender.
Incidence
As ED visits for nephrolithiasis are increasing, so too are the health-care costs associated with this condition. Between 1992 and 2009, emergent-care presentations for nephrolithiasis rose from 178 to 340 visits per 100,000 individuals.1 Approximately 1 in 11 people in the United States will be affected by nephrolithiasis during their lifetime.2 Estimated health-care costs associated with these complaints were roughly $2 billion in 2000—an increase of 50% since 1994.2
Evaluation and Diagnosis
Laboratory Evaluation
Urinalysis is one of the initial studies for patients with suspected nephrolithiasis. Although hematuria is a classic finding associated with renal calculi, its sensitivity on microscopic analysis is around 84%. Therefore, the absence of hematuria does not exclude renal colic in the differential diagnosis.3
In addition to detecting hematuria, urinalysis can also reveal an underlying infection. One study by Abrahamian et al4 found that roughly 8% of patients presenting with acute nephrolithiasis had a urinary tract infection (UTI)—many without any clinical findings of infection. The presence of pyuria, however, has only moderate accuracy in identifying UTIs in patients with kidney stones.4 If an infected stone cannot be excluded clinically, computed tomography (CT) is indicated.
Mild leukocytosis (ie, <15,000 cells/mcL) is another common finding in patients with acute renal colic.5 A leukocyte count >15,000 cells/mcL is suspicious for infection or other pathology. A blood-chemistry panel to evaluate renal function is appropriate as a baseline—particularly for patients in whom treatment with a nonsteroidal anti-inflammatory (NSAID) drug is anticipated.
With the ability to visualize renal calculi (Figure 1), the use of noncontrast CT has become a standard initial imaging modality in assessing patients with renal colic. Between 1992 and 2009, the use of CT to evaluate patients presenting with flank pain for suspected renal colic more than tripled from 21% to 71%.6 An analysis performed by the American College of National Radiology Data Registry7 shows the mean radiation dose given by institutions for renal colic CT is unnecessarily high, and that few institutions follow CT-stone protocols aimed at minimizing radiation exposure while still maintaining proper diagnostic accuracy. A typical CT of the abdomen and pelvis is equivalent to over 100 two-view chest X-rays.8 Though controversial, data from a white paper by the American College of Radiology suggest that the ionizing radiation exposure from just one CT for renal colic causes an increase in lifetime cancer risk.9
Despite the increase in CT imaging to evaluate patients presenting to the ED with nephrolithiasis/flank pain, the proportion of patients diagnosed with a kidney stone remained the same between 2000 and 2008, with no significant change in outcomes.10-12 Moreover, the use of CT as an initial imaging modality in patients presenting with flank pain—but with no sign of infection—is unlikely to reveal important alternative findings.13
Regarding the sensitivity of CT in detecting nephrolithiasis, one study demonstrates a sensitivity of 100% and a specificity of 94% for noncontrast CT.14 Controversy, however, still exists regarding the necessity and utility of CT in diagnosing nephrolithiasis,15 and CT is one of the top 10 tests included in the American College of Emergency Physicians (ACEP) 2014 Choosing Wisely campaign. In this campaign, ACEP recommended emergency physicians (EPs) avoid abdominal and pelvic CT in otherwise healthy patients younger than age 50 years who present with symptoms consistent with uncomplicated renal colic and who have a known history of nephrolithiasis or ureterolithiasis.15 The ACEP also noted that CTs in this context do not often change treatment decisions and are associated with unnecessary radiation exposure and cost.15
While keeping the aforementioned recommendations in mind, if an EP intends to refer a renal colic patient to a urologist a CT scan is necessary either in the ED or as an outpatient. In all cases (except perhaps in patients in whom there is a history of renal stones), the urologist will need this study to determine the size and location of the stone in order to provide recommendations for management.
Ultrasound
Clinical Decision Score
Moore et al,17 authors of the Size, Topography, Location, Obstruction, Number of stones, and Evaluation (STONE) scoring system, developed a classification system for patients with suspected nephrolithiasis. This system places patients into low-, moderate-, and high-score groups, with corresponding probabilities of ureteral stone based on symptoms and epidemiological classifications.
The intent of the STONE system is to accurately predict, based on classification, the likelihood of a patient having a simple ureteral stone versus a more significant, complicated stone and to help guide which, if any, imaging studies are indicated. For example, a lower STONE score would help guide the decision to defer advanced imaging studies that would be unlikely to reveal an alternate serious diagnosis. Likewise, an individual with a high STONE score could potentially receive ultrasonography, reduced-dose CT, or no further imaging.
The STONE score performs fairly well and appears to be superior to physician gestalt, with an area under the receiver operating characteristic curve (AUC) of .78 compared to .68 with physician gestalt. This system, however, is not always accurate in its classification and has been shown to have 87% specificity at the high end to rule in stone and 96% sensitivity rate at the low end to rule out a stone. Of course, when using a clinical decision rule to rule in or rule out a stone, a tool with a very high specificity is preferred. Although the STONE scoring system does show promise, further studies are needed before it can be applied clinically.17
Treatment
Analgesia
By inhibiting prostaglandin synthesis, NSAIDs reduce inflammation and ureteral muscular hyperactivity.18 A recent Cochrane review of over 50 studies concluded that NSAIDs were effective in relieving acute renal colic pain.19 A systematic review by Holdgate and Pollock20 shows that patients treated with NSAIDs achieve greater reductions in pain scores and are less likely to require additional analgesia in the short term compared to patients treated with opioids. Although opioid medications are effective in relieving pain associated with nephrolithiasis, this class of drugs can exacerbate the nausea often associated with this condition. This same study also showed that patients who were prescribed NSAIDs following an ED visit for renal colic required less medication for pain control, experienced less nausea, and had greater improvements in their pain.20
Nevertheless, the utility of opiates as an adjunct therapy should not be overlooked. For example, in patients with renal colic, numerous studies show treatment with a combination of an NSAID and opiate provides superior pain relief compared to either treatment modality in isolation.21 Opioid analgesia may be indicated in patients in whom NSAIDs are not recommended or contraindicated (eg, elderly patients, patients with renal disease). While NSAIDs address the underlying pathophysiology associated with renal colic, they are sometimes not the best treatment option. Depending on the situation, treatment with an opioid should instead be considered.
Intravenous Fluid Therapy
A 2012 Cochrane Review of randomized control trials (RCT) on intravenous (IV) fluid therapy hydration/diuretic use concluded that there was “no reliable evidence in the literature to support the use of diuretics and high-volume fluid therapy for people with acute ureteric colic.” The review, however, did note that further investigation is warranted for a definitive answer.22 Another study by Springhart et al23 showed no difference in pain or stone expulsion between large-volume (2 L IV fluids over 2 hours) and small-volume fluid administration (20 mL/h). Regarding administration, the use of IV fluids in renal colic is no different than the usual indications for fluid therapy in the ED and should be restricted to patients with signs of dehydration or kidney injury.
Many patients with renal colic will have decreased oral intake from the pain and nausea associated with the stone and may be vomiting. Under these circumstances, it is reasonable to rehydrate the patients, even though large-volume hydration with the intent of aiding stone expulsion or improving pain has not been shown efficacious. Conversely, in addition to the perceived benefit of rehydrating patients, a small amount of fluid hydration may improve the visualization of hydronephrosis on ultrasound.24
Medical Expulsive Therapy
For many years, clinicians have considered the use of tamsulosin, an α1-receptor blocker, as well as nifedipine, a calcium channel blocker, in treating renal colic due to the theoretical benefit of reducing ureteral smooth muscle spasm/constriction thus expediting stone passage. Over the years, dozens of studies showed positive benefit in the use of medical expulsive therapy (MET). A 2014 Cochrane Review demonstrated that patients treated with α1-blockers experienced a higher stone-free rate and shorter time to stone expulsion, and concluded that α1-blockers should be offered as one of the primary treatment modalities in MET.25 This review, however, has been criticized for using a number of studies with very small patient samples, non-peer-reviewed abstracts, and low-quality study designs.26
More recently, in April 2015, Lancet published a large RCT from 24 hospitals in the United Kingdom, comparing placebo versus 400 mcg tamsulosin and 30 mg nifedipine. The authors concluded that “tamsulosin 400 mcg and nifedipine 30 mg are not effective at decreasing the need for further treatment to achieve stone clearance in 4 weeks for patients with expectantly managed ureteric colic.”27 Another large double-blind, placebo-controlled, randomized, multicenter trial by Furyk et al28 in July 2015 went a step further and evaluated distal stones, which have historically caused complications requiring intervention. They concluded that there was “no benefit overall of 0.4 mg of tamsulosin daily for patients with distal ureteric calculi less than or equal to 10 mm in terms of spontaneous passage, time to stone passage, pain, or analgesia requirements. In the subgroup with large stones (5 to 10 mm), tamsulosin did increase passage and should be considered.”28 Based on these recent studies, the use of tamsulosin in patients with stones larger than 5 mm—but not those with smaller stones—appears to be an appropriate treatment option.
Patient Disposition
The American Urological Association cited indications for urgent/emergent urological interventions necessitating the need for inpatient admission and further workup.29 Patients who do not fall into any of the categories outlined in the Table may be seen on an outpatient basis. These patients may be treated symptomatically until they can follow up with a urologist, who will determine expectant management versus intervention.
Prognosis
The majority of stones <5mm will pass spontaneously.30 Larger stones may still pass spontaneously but are more likely to require lithotripsy or other urologic intervention; therefore, patients with stones >5 mm should be referred to urology services.30
Recurrence
Patients with a first-time kidney stone have a 30% to 50% chance of disease recurrence within 5 years,31 and a 60% to 80% chance of recurrence during their lifetime.32 Those with a family history of nephrolithiasis are likely to develop an earlier onset of stones as well as experience more frequent recurrent episodes.33 Patients with recurrent disease should undergo outpatient risk stratification, including stone-composition analysis and assessment for modifiable risk factors.
Case Conclusion
The patient’s urinalysis demonstrated microscopic hematuria; blood urea nitrogen and creatinine levels were within normal limits. As the patient was tachycardic and appeared mildly dehydrated, an IV infusion of 1 L normal saline was initiated, along with ketorolac and ondansetron for symptomatic relief. A POC ultrasound of the right kidney revealed mild-to-moderate hydronephrosis; the left kidney appeared sonographically normal. Since this patient had no history of nephrolithiasis, a nonenhanced CT of the abdomen was obtained, which revealed moderate, right-sided hydronephrosis and a 3-mm distal ureteral stone. Once the patient’s symptoms were controlled, she was discharged home with a prescription for ibuprofen for symptomatic relief and instructions to follow up with her PCP.
Conclusion
The evaluation and treatment of nephrolithiasis is important due to its increasing prevalence, as well as implications on costs to the health-care system and to patients themselves. The workup and treatment of nephrolithiasis has been and continues to be the subject of much controversy. Until very recently, treatment recommendations were founded on physiological theories more so than robust research. In an era where improved imaging technology is becoming more readily available in the ED, EPs should weigh the pros and cons of its utilization for common ED complaints such as nephrolithiasis.
Dr Parsa is an assistant professor in the department of emergency medicine, Texas Tech University Health Sciences Center, El Paso. Dr Khafi is a resident in the department of emergency medicine, Texas Tech University Health Sciences Center, El Paso.
Case
A 39-year-old woman presented to the ED with a chief complaint of intermittent right flank pain that radiated into her groin area. She stated the pain had begun suddenly, 4 hours prior to arrival, and was accompanied by nausea and vomiting. The patient said that she had taken acetaminophen for the pain, but had received no relief. Regarding history, according to the patient, her last menstrual period ended 2 days earlier. She denied any urinary symptoms, diarrhea, or constipation. She had no history of abdominal surgery and was currently not on any medications.
The patient’s vital signs at presentation were: temperature 98.7°F; blood pressure, 130/90 mm Hg; heart rate, 110 beats/minute; and respiratory rate, 18 breaths/minute. Oxygen saturation was 98% on room air. On physical examination, she appeared to be in mild distress, pacing around the room. There was moderate right costovertebral tenderness on percussion; the abdomen was soft and nontender.
Incidence
As ED visits for nephrolithiasis are increasing, so too are the health-care costs associated with this condition. Between 1992 and 2009, emergent-care presentations for nephrolithiasis rose from 178 to 340 visits per 100,000 individuals.1 Approximately 1 in 11 people in the United States will be affected by nephrolithiasis during their lifetime.2 Estimated health-care costs associated with these complaints were roughly $2 billion in 2000—an increase of 50% since 1994.2
Evaluation and Diagnosis
Laboratory Evaluation
Urinalysis is one of the initial studies for patients with suspected nephrolithiasis. Although hematuria is a classic finding associated with renal calculi, its sensitivity on microscopic analysis is around 84%. Therefore, the absence of hematuria does not exclude renal colic in the differential diagnosis.3
In addition to detecting hematuria, urinalysis can also reveal an underlying infection. One study by Abrahamian et al4 found that roughly 8% of patients presenting with acute nephrolithiasis had a urinary tract infection (UTI)—many without any clinical findings of infection. The presence of pyuria, however, has only moderate accuracy in identifying UTIs in patients with kidney stones.4 If an infected stone cannot be excluded clinically, computed tomography (CT) is indicated.
Mild leukocytosis (ie, <15,000 cells/mcL) is another common finding in patients with acute renal colic.5 A leukocyte count >15,000 cells/mcL is suspicious for infection or other pathology. A blood-chemistry panel to evaluate renal function is appropriate as a baseline—particularly for patients in whom treatment with a nonsteroidal anti-inflammatory (NSAID) drug is anticipated.
With the ability to visualize renal calculi (Figure 1), the use of noncontrast CT has become a standard initial imaging modality in assessing patients with renal colic. Between 1992 and 2009, the use of CT to evaluate patients presenting with flank pain for suspected renal colic more than tripled from 21% to 71%.6 An analysis performed by the American College of National Radiology Data Registry7 shows the mean radiation dose given by institutions for renal colic CT is unnecessarily high, and that few institutions follow CT-stone protocols aimed at minimizing radiation exposure while still maintaining proper diagnostic accuracy. A typical CT of the abdomen and pelvis is equivalent to over 100 two-view chest X-rays.8 Though controversial, data from a white paper by the American College of Radiology suggest that the ionizing radiation exposure from just one CT for renal colic causes an increase in lifetime cancer risk.9
Despite the increase in CT imaging to evaluate patients presenting to the ED with nephrolithiasis/flank pain, the proportion of patients diagnosed with a kidney stone remained the same between 2000 and 2008, with no significant change in outcomes.10-12 Moreover, the use of CT as an initial imaging modality in patients presenting with flank pain—but with no sign of infection—is unlikely to reveal important alternative findings.13
Regarding the sensitivity of CT in detecting nephrolithiasis, one study demonstrates a sensitivity of 100% and a specificity of 94% for noncontrast CT.14 Controversy, however, still exists regarding the necessity and utility of CT in diagnosing nephrolithiasis,15 and CT is one of the top 10 tests included in the American College of Emergency Physicians (ACEP) 2014 Choosing Wisely campaign. In this campaign, ACEP recommended emergency physicians (EPs) avoid abdominal and pelvic CT in otherwise healthy patients younger than age 50 years who present with symptoms consistent with uncomplicated renal colic and who have a known history of nephrolithiasis or ureterolithiasis.15 The ACEP also noted that CTs in this context do not often change treatment decisions and are associated with unnecessary radiation exposure and cost.15
While keeping the aforementioned recommendations in mind, if an EP intends to refer a renal colic patient to a urologist a CT scan is necessary either in the ED or as an outpatient. In all cases (except perhaps in patients in whom there is a history of renal stones), the urologist will need this study to determine the size and location of the stone in order to provide recommendations for management.
Ultrasound
Clinical Decision Score
Moore et al,17 authors of the Size, Topography, Location, Obstruction, Number of stones, and Evaluation (STONE) scoring system, developed a classification system for patients with suspected nephrolithiasis. This system places patients into low-, moderate-, and high-score groups, with corresponding probabilities of ureteral stone based on symptoms and epidemiological classifications.
The intent of the STONE system is to accurately predict, based on classification, the likelihood of a patient having a simple ureteral stone versus a more significant, complicated stone and to help guide which, if any, imaging studies are indicated. For example, a lower STONE score would help guide the decision to defer advanced imaging studies that would be unlikely to reveal an alternate serious diagnosis. Likewise, an individual with a high STONE score could potentially receive ultrasonography, reduced-dose CT, or no further imaging.
The STONE score performs fairly well and appears to be superior to physician gestalt, with an area under the receiver operating characteristic curve (AUC) of .78 compared to .68 with physician gestalt. This system, however, is not always accurate in its classification and has been shown to have 87% specificity at the high end to rule in stone and 96% sensitivity rate at the low end to rule out a stone. Of course, when using a clinical decision rule to rule in or rule out a stone, a tool with a very high specificity is preferred. Although the STONE scoring system does show promise, further studies are needed before it can be applied clinically.17
Treatment
Analgesia
By inhibiting prostaglandin synthesis, NSAIDs reduce inflammation and ureteral muscular hyperactivity.18 A recent Cochrane review of over 50 studies concluded that NSAIDs were effective in relieving acute renal colic pain.19 A systematic review by Holdgate and Pollock20 shows that patients treated with NSAIDs achieve greater reductions in pain scores and are less likely to require additional analgesia in the short term compared to patients treated with opioids. Although opioid medications are effective in relieving pain associated with nephrolithiasis, this class of drugs can exacerbate the nausea often associated with this condition. This same study also showed that patients who were prescribed NSAIDs following an ED visit for renal colic required less medication for pain control, experienced less nausea, and had greater improvements in their pain.20
Nevertheless, the utility of opiates as an adjunct therapy should not be overlooked. For example, in patients with renal colic, numerous studies show treatment with a combination of an NSAID and opiate provides superior pain relief compared to either treatment modality in isolation.21 Opioid analgesia may be indicated in patients in whom NSAIDs are not recommended or contraindicated (eg, elderly patients, patients with renal disease). While NSAIDs address the underlying pathophysiology associated with renal colic, they are sometimes not the best treatment option. Depending on the situation, treatment with an opioid should instead be considered.
Intravenous Fluid Therapy
A 2012 Cochrane Review of randomized control trials (RCT) on intravenous (IV) fluid therapy hydration/diuretic use concluded that there was “no reliable evidence in the literature to support the use of diuretics and high-volume fluid therapy for people with acute ureteric colic.” The review, however, did note that further investigation is warranted for a definitive answer.22 Another study by Springhart et al23 showed no difference in pain or stone expulsion between large-volume (2 L IV fluids over 2 hours) and small-volume fluid administration (20 mL/h). Regarding administration, the use of IV fluids in renal colic is no different than the usual indications for fluid therapy in the ED and should be restricted to patients with signs of dehydration or kidney injury.
Many patients with renal colic will have decreased oral intake from the pain and nausea associated with the stone and may be vomiting. Under these circumstances, it is reasonable to rehydrate the patients, even though large-volume hydration with the intent of aiding stone expulsion or improving pain has not been shown efficacious. Conversely, in addition to the perceived benefit of rehydrating patients, a small amount of fluid hydration may improve the visualization of hydronephrosis on ultrasound.24
Medical Expulsive Therapy
For many years, clinicians have considered the use of tamsulosin, an α1-receptor blocker, as well as nifedipine, a calcium channel blocker, in treating renal colic due to the theoretical benefit of reducing ureteral smooth muscle spasm/constriction thus expediting stone passage. Over the years, dozens of studies showed positive benefit in the use of medical expulsive therapy (MET). A 2014 Cochrane Review demonstrated that patients treated with α1-blockers experienced a higher stone-free rate and shorter time to stone expulsion, and concluded that α1-blockers should be offered as one of the primary treatment modalities in MET.25 This review, however, has been criticized for using a number of studies with very small patient samples, non-peer-reviewed abstracts, and low-quality study designs.26
More recently, in April 2015, Lancet published a large RCT from 24 hospitals in the United Kingdom, comparing placebo versus 400 mcg tamsulosin and 30 mg nifedipine. The authors concluded that “tamsulosin 400 mcg and nifedipine 30 mg are not effective at decreasing the need for further treatment to achieve stone clearance in 4 weeks for patients with expectantly managed ureteric colic.”27 Another large double-blind, placebo-controlled, randomized, multicenter trial by Furyk et al28 in July 2015 went a step further and evaluated distal stones, which have historically caused complications requiring intervention. They concluded that there was “no benefit overall of 0.4 mg of tamsulosin daily for patients with distal ureteric calculi less than or equal to 10 mm in terms of spontaneous passage, time to stone passage, pain, or analgesia requirements. In the subgroup with large stones (5 to 10 mm), tamsulosin did increase passage and should be considered.”28 Based on these recent studies, the use of tamsulosin in patients with stones larger than 5 mm—but not those with smaller stones—appears to be an appropriate treatment option.
Patient Disposition
The American Urological Association cited indications for urgent/emergent urological interventions necessitating the need for inpatient admission and further workup.29 Patients who do not fall into any of the categories outlined in the Table may be seen on an outpatient basis. These patients may be treated symptomatically until they can follow up with a urologist, who will determine expectant management versus intervention.
Prognosis
The majority of stones <5mm will pass spontaneously.30 Larger stones may still pass spontaneously but are more likely to require lithotripsy or other urologic intervention; therefore, patients with stones >5 mm should be referred to urology services.30
Recurrence
Patients with a first-time kidney stone have a 30% to 50% chance of disease recurrence within 5 years,31 and a 60% to 80% chance of recurrence during their lifetime.32 Those with a family history of nephrolithiasis are likely to develop an earlier onset of stones as well as experience more frequent recurrent episodes.33 Patients with recurrent disease should undergo outpatient risk stratification, including stone-composition analysis and assessment for modifiable risk factors.
Case Conclusion
The patient’s urinalysis demonstrated microscopic hematuria; blood urea nitrogen and creatinine levels were within normal limits. As the patient was tachycardic and appeared mildly dehydrated, an IV infusion of 1 L normal saline was initiated, along with ketorolac and ondansetron for symptomatic relief. A POC ultrasound of the right kidney revealed mild-to-moderate hydronephrosis; the left kidney appeared sonographically normal. Since this patient had no history of nephrolithiasis, a nonenhanced CT of the abdomen was obtained, which revealed moderate, right-sided hydronephrosis and a 3-mm distal ureteral stone. Once the patient’s symptoms were controlled, she was discharged home with a prescription for ibuprofen for symptomatic relief and instructions to follow up with her PCP.
Conclusion
The evaluation and treatment of nephrolithiasis is important due to its increasing prevalence, as well as implications on costs to the health-care system and to patients themselves. The workup and treatment of nephrolithiasis has been and continues to be the subject of much controversy. Until very recently, treatment recommendations were founded on physiological theories more so than robust research. In an era where improved imaging technology is becoming more readily available in the ED, EPs should weigh the pros and cons of its utilization for common ED complaints such as nephrolithiasis.
Dr Parsa is an assistant professor in the department of emergency medicine, Texas Tech University Health Sciences Center, El Paso. Dr Khafi is a resident in the department of emergency medicine, Texas Tech University Health Sciences Center, El Paso.
- Scales CD Jr, Smith AC, Hanley JM, Saigal CS; Urologic Diseases in America Project. Prevalence of kidney stones in the United States. Eur Urol. 2012;62(1):160-165.
- Pearle MS, Calhoun EA, Curhan GC; Urologic Diseases of America Project: urolithiasis. J Urol. 2005;173(3):848-857.
- Luchs JS, Katz DS, Lane MJ et al. Utility of hematuria testing in patients with suspected renal colic: correlation with unenhanced helical CT results. Urology. 2002;59(6):839-842.
- Abrahamian FM, Krishnadasan A, Mower WR, Moran GJ, Talan DA. Association of pyuria and clinical characteristics with the presence of urinary tract infection among patients with acute nephrolithiasis. Ann Emerg Med. 2013;62(5):526-533.
- Yilmaz S, Pekdemir M, Aksu NM, Koyuncu N, Cinar O, Akpinar E. A multicenter case–control study of diagnostic tests for urinary tract infection in the presence of urolithiasis. Urol Res. 2011;40(1):61-65. doi:10.1007/s00240-011-0402-x.
- Fwu CW, Eggers PW, Kimmel PL, Kusek JW, Kirkali Z. Emergency department visits, use of imaging, and drugs for urolithiasis have increased in the United States. Kidney Int. 2013;83(3):479-486. doi:10.1038/ki.2012.419.
- Lukasiewicz A, Bhargavan-Chatfield M, Coombs L, et al. Radiation dose index of renal colic protocol CT studies in the United States: a report from the American College of Radiology National Radiology Data Registry. Radiology. 2014;271(2):445-451. doi:10.1148/radiol.14131601.
- Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 248(1):254-263.
- Amis ES Jr, Butler PF, Applegate KE, et al; American College of Radiology. American College of Radiology white paper on radiation dose in medicine. J Am Coll Radiol. 2007;4(5):272-284.
- Hyams ES, Korley FK, Pham JC, Matlaga BR. Trends in imaging use during the emergency department evaluation of flank pain. J Urol. 2011;186(6):2270-2274. doi:10.1016/j.juro.2011.07.079.
- Ripollés T, Agramunt M, Errando J, Martínez MJ, Coronel B, Morales M. Suspected ureteral colic: plain film and sonography vs unenhanced helical CT. A prospective study in 66 patients. Eur Radiol. 2004;14(1):129-36. doi:10.1007/s00330-003-1924-1926.
- Westphalen AC, Hsia RY, Maselli JH, Wang R, Gonzales R. Radiological imaging of patients with suspected urinary tract stones: national trends, diagnoses, and predictors. Acad Emerg Med. 2011;18(7):699-707. doi:10.1111/j.1553-2712.2011.01103.x.
- Moore CL, Daniels B, Singh D, Luty S, Molinaro A. Prevalence and clinical importance of alternative causes of symptoms using a renal colic computed tomography protocol in patients with flank or back pain and absence of pyuria. Acad Emerg Med. 2013;20(5):470-478. doi:10.1111/acem.12127.
- Chen MY, Zagoria RJ. Can noncontrast helical computed tomography replace intravenous urography for evaluation of patients with acute urinary tract colic? J Emerg Med. 1999;17(2):299-303.
- American College of Emergency Physicians. Five things physicians and patients should question. Choosing Wisely Web site. 2013;10:1-5. Available at: http://www.choosingwisely.org/societies/american-college-of-emergency-physicians/. Accessed February 10, 2016.
- Smith-Bindman R, Aubin C, Bailitz J, et al. Ultrasonography versus computed tomography for suspected nephrolithiasis. N Engl J Med. 2014;371(12):1100-1110. doi:10.1056/nejmoa1404446.
- Moore CL, Bomann S, Daniels B, et al. Derivation and validation of a clinical prediction rule for uncomplicated ureteral stone—the STONE score: retrospective and prospective observational cohort studies. BMJ. 2014;348:g2191. doi:10.1136/bmj.g2191.
- Cole RS, Fry CH, Shuttleworth KE. The action of the prostaglandins on isolated human ureteric smooth muscle. Br J Urol. 1988;61(1):19-26.
- Afshar K, Jafari S, Marks AJ, Eftekhari R, McNeily AE. Nonsteroidal anti-inflammatory drugs (NSAIDs) and non-opioids for acute renal colic. Cochrane Database Syst Rev. 2015;6:CD006027. doi:10.1002/14651858.CD006027.pub2.
- Holdgate A, Pollock T. Systematic review of the relative efficacy of non-steroidal anti-inflammatory drugs and opioids in the treatment of acute renal colic. BMJ. 2004;328(7453):1401. doi:10.1136/bmj.38119.581991.55.
- Safdar B, Degutis LC, Landry K, Vedere SR, Moscovitz HC, D’Onofrio G. Intravenous morphine plus ketorolac is superior to either drug alone for treatment of acute renal colic. Ann Emerg Med. 2006;48(2):173-181, 181.e1. doi:10.1016/j.annemergmed.2006.03.013.
- Worster AS, Bhanich Supapol W. Fluids and diuretics for acute ureteric colic. Cochrane Database Syst Rev. 2012;15;2:CD004926. doi:10.1002/14651858.CD004926.pub3.
- Springhart WP, Marguet CG, Sur RL, et al. Forced versus minimal intravenous hydration in the management of acute renal colic: a randomized trial. J. Endourol. 2006;20(10):713-716. doi:10.1089/end.2006.20.713.
- Morse JW, Hill R, Greissinger WP, Patterson JW, Melanson SW, Heller MB. Rapid oral hydration results in hydronephrosis as demonstrated by bedside ultrasound. Ann Emerg Med. 1999;34(2):134-140. doi:10.1016/s0196-0644(99)70221-0.
- Campschroer T, Zhu Y, Duijvesz D, Grobbee DE, Lock MT. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014;4:CD008509. doi:10.1002/14651858.CD008509.pub2.
- Radecki R. Sadly inadequate Cochrane review of renal colic. Emergency Medicine Literature of Note. 2014. Available at: http://www.emlitofnote.com/2014/04/sadly-inadequate-cochrane-review-of.html. Accessed February 10, 2016.
- Pickard R, Starr K, MacLennan G, et al. Medical expulsive therapy in adults with ureteric colic: a multicentre, randomised, placebo-controlled trial. Lancet. 2015;386(9991):341-349. doi:10.1016/S0140-6736(15)60933-3.
- Furyk JS, Chu K, Banks C, et al. Distal ureteric stones and tamsulosin: a double-blind, placebo-controlled, randomized, multicenter trial. Ann Emerg Med. 2016;67(1):86-95.e2. doi:10.1016/j.annemergmed.2015.06.001.
- Kidney stones. American Urological Association Web site. 2016. Available at: https://www.auanet.org/education/kidney-stones.cfm. Accessed February 10, 2016.
- Miller OF, Kane CJ. Time to stone passage for observed ureteral calculi: a guide for patient education. J Urol. 1999;162(3 Pt 1):688-690.
- Lipkin ME, Preminger GM. Demystifying the medical management of nephrolithiasis. Rev Urol. 2011;13(1):34-38.
- Morton AR, Iliescu EA, Wilson JW. Nephrology: 1. Investigation and treatment of recurrent kidney stones. CMAJ. 2002;166(2):213-218.
- Koyuncu HH, Yencilek F, Eryildirim B, Sarica K. Family history in stone disease: how important is it for the onset of the disease and the incidence of recurrence? Urol Res. 2010;38(2):105-109. doi:10.1007/s00240-009-0249-6.
- Scales CD Jr, Smith AC, Hanley JM, Saigal CS; Urologic Diseases in America Project. Prevalence of kidney stones in the United States. Eur Urol. 2012;62(1):160-165.
- Pearle MS, Calhoun EA, Curhan GC; Urologic Diseases of America Project: urolithiasis. J Urol. 2005;173(3):848-857.
- Luchs JS, Katz DS, Lane MJ et al. Utility of hematuria testing in patients with suspected renal colic: correlation with unenhanced helical CT results. Urology. 2002;59(6):839-842.
- Abrahamian FM, Krishnadasan A, Mower WR, Moran GJ, Talan DA. Association of pyuria and clinical characteristics with the presence of urinary tract infection among patients with acute nephrolithiasis. Ann Emerg Med. 2013;62(5):526-533.
- Yilmaz S, Pekdemir M, Aksu NM, Koyuncu N, Cinar O, Akpinar E. A multicenter case–control study of diagnostic tests for urinary tract infection in the presence of urolithiasis. Urol Res. 2011;40(1):61-65. doi:10.1007/s00240-011-0402-x.
- Fwu CW, Eggers PW, Kimmel PL, Kusek JW, Kirkali Z. Emergency department visits, use of imaging, and drugs for urolithiasis have increased in the United States. Kidney Int. 2013;83(3):479-486. doi:10.1038/ki.2012.419.
- Lukasiewicz A, Bhargavan-Chatfield M, Coombs L, et al. Radiation dose index of renal colic protocol CT studies in the United States: a report from the American College of Radiology National Radiology Data Registry. Radiology. 2014;271(2):445-451. doi:10.1148/radiol.14131601.
- Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 248(1):254-263.
- Amis ES Jr, Butler PF, Applegate KE, et al; American College of Radiology. American College of Radiology white paper on radiation dose in medicine. J Am Coll Radiol. 2007;4(5):272-284.
- Hyams ES, Korley FK, Pham JC, Matlaga BR. Trends in imaging use during the emergency department evaluation of flank pain. J Urol. 2011;186(6):2270-2274. doi:10.1016/j.juro.2011.07.079.
- Ripollés T, Agramunt M, Errando J, Martínez MJ, Coronel B, Morales M. Suspected ureteral colic: plain film and sonography vs unenhanced helical CT. A prospective study in 66 patients. Eur Radiol. 2004;14(1):129-36. doi:10.1007/s00330-003-1924-1926.
- Westphalen AC, Hsia RY, Maselli JH, Wang R, Gonzales R. Radiological imaging of patients with suspected urinary tract stones: national trends, diagnoses, and predictors. Acad Emerg Med. 2011;18(7):699-707. doi:10.1111/j.1553-2712.2011.01103.x.
- Moore CL, Daniels B, Singh D, Luty S, Molinaro A. Prevalence and clinical importance of alternative causes of symptoms using a renal colic computed tomography protocol in patients with flank or back pain and absence of pyuria. Acad Emerg Med. 2013;20(5):470-478. doi:10.1111/acem.12127.
- Chen MY, Zagoria RJ. Can noncontrast helical computed tomography replace intravenous urography for evaluation of patients with acute urinary tract colic? J Emerg Med. 1999;17(2):299-303.
- American College of Emergency Physicians. Five things physicians and patients should question. Choosing Wisely Web site. 2013;10:1-5. Available at: http://www.choosingwisely.org/societies/american-college-of-emergency-physicians/. Accessed February 10, 2016.
- Smith-Bindman R, Aubin C, Bailitz J, et al. Ultrasonography versus computed tomography for suspected nephrolithiasis. N Engl J Med. 2014;371(12):1100-1110. doi:10.1056/nejmoa1404446.
- Moore CL, Bomann S, Daniels B, et al. Derivation and validation of a clinical prediction rule for uncomplicated ureteral stone—the STONE score: retrospective and prospective observational cohort studies. BMJ. 2014;348:g2191. doi:10.1136/bmj.g2191.
- Cole RS, Fry CH, Shuttleworth KE. The action of the prostaglandins on isolated human ureteric smooth muscle. Br J Urol. 1988;61(1):19-26.
- Afshar K, Jafari S, Marks AJ, Eftekhari R, McNeily AE. Nonsteroidal anti-inflammatory drugs (NSAIDs) and non-opioids for acute renal colic. Cochrane Database Syst Rev. 2015;6:CD006027. doi:10.1002/14651858.CD006027.pub2.
- Holdgate A, Pollock T. Systematic review of the relative efficacy of non-steroidal anti-inflammatory drugs and opioids in the treatment of acute renal colic. BMJ. 2004;328(7453):1401. doi:10.1136/bmj.38119.581991.55.
- Safdar B, Degutis LC, Landry K, Vedere SR, Moscovitz HC, D’Onofrio G. Intravenous morphine plus ketorolac is superior to either drug alone for treatment of acute renal colic. Ann Emerg Med. 2006;48(2):173-181, 181.e1. doi:10.1016/j.annemergmed.2006.03.013.
- Worster AS, Bhanich Supapol W. Fluids and diuretics for acute ureteric colic. Cochrane Database Syst Rev. 2012;15;2:CD004926. doi:10.1002/14651858.CD004926.pub3.
- Springhart WP, Marguet CG, Sur RL, et al. Forced versus minimal intravenous hydration in the management of acute renal colic: a randomized trial. J. Endourol. 2006;20(10):713-716. doi:10.1089/end.2006.20.713.
- Morse JW, Hill R, Greissinger WP, Patterson JW, Melanson SW, Heller MB. Rapid oral hydration results in hydronephrosis as demonstrated by bedside ultrasound. Ann Emerg Med. 1999;34(2):134-140. doi:10.1016/s0196-0644(99)70221-0.
- Campschroer T, Zhu Y, Duijvesz D, Grobbee DE, Lock MT. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014;4:CD008509. doi:10.1002/14651858.CD008509.pub2.
- Radecki R. Sadly inadequate Cochrane review of renal colic. Emergency Medicine Literature of Note. 2014. Available at: http://www.emlitofnote.com/2014/04/sadly-inadequate-cochrane-review-of.html. Accessed February 10, 2016.
- Pickard R, Starr K, MacLennan G, et al. Medical expulsive therapy in adults with ureteric colic: a multicentre, randomised, placebo-controlled trial. Lancet. 2015;386(9991):341-349. doi:10.1016/S0140-6736(15)60933-3.
- Furyk JS, Chu K, Banks C, et al. Distal ureteric stones and tamsulosin: a double-blind, placebo-controlled, randomized, multicenter trial. Ann Emerg Med. 2016;67(1):86-95.e2. doi:10.1016/j.annemergmed.2015.06.001.
- Kidney stones. American Urological Association Web site. 2016. Available at: https://www.auanet.org/education/kidney-stones.cfm. Accessed February 10, 2016.
- Miller OF, Kane CJ. Time to stone passage for observed ureteral calculi: a guide for patient education. J Urol. 1999;162(3 Pt 1):688-690.
- Lipkin ME, Preminger GM. Demystifying the medical management of nephrolithiasis. Rev Urol. 2011;13(1):34-38.
- Morton AR, Iliescu EA, Wilson JW. Nephrology: 1. Investigation and treatment of recurrent kidney stones. CMAJ. 2002;166(2):213-218.
- Koyuncu HH, Yencilek F, Eryildirim B, Sarica K. Family history in stone disease: how important is it for the onset of the disease and the incidence of recurrence? Urol Res. 2010;38(2):105-109. doi:10.1007/s00240-009-0249-6.
Allegations: Current Trends in Medical Malpractice, Part 1
We’re lost, but we’re making good time.
-Yogi Berra
As Yogi Berra alludes, it is very easy to get caught up “in the flow” and continue to move along at a good pace, even when one does not know where he or she is ultimately headed. Similarly, in terms of medical malpractice, everyone seems to have an opinion on what should be done to improve the climate of medical malpractice for both providers and patients. Yet, there are many differences in opinions on how to solve these issues, and the “cure” for what “ails” in the system are many—with an undetermined endpoint.
Tort reform is often conjured as the communal fix; yet each state in the Union has its own medical malpractice tort laws, which begs the question of how an issue with so many different facets can be resolved. Additionally, the risk alone of medical malpractice continues to be an important area of concern to emergency physicians (EPs), not only because of the looming threat of malpractice litigation—both real and perceived—but also because of its influence on practice patterns, resource utilization, and patient care in the ED.1,2
Physician Perception
Over the course of a career, an EP faces at least one claim, further perpetuating a common physician perception that the occurrence of a suit is not a condition of “if” but rather of “when.”3 This anxiety and fear among physicians in general are further provoked by the many headlines highlighting massive jury verdicts that dominate the news cycle.4
In addition, the EP’s work and practice are increasingly affected by the impositions of multiple nationally reported quality metrics, institutional throughput goals, and process-improvement efforts. Each of these in turn has the effect of increasing the pace of care and can challenge one’s real-time ability to recognize the dangers of inherent biases, to appreciate and act upon subtle clinical clues, and to rescue patient-experience misadventures. Accordingly, medical malpractice is a frequent topic of discussion for policy proposals among physicians and legislators.
Defensive Medicine and Tort Reform
As spending on health care in the United States topped $3 trillion, or 17.5% of the US gross domestic product in 2014, strategies for cost-containment have become a primary concern across all sectors of the health-care industry.5,6 With defensive medicine proposed by some as a substantial driver of health-care costs, many physicians have focused on tort reform as an avenue to curb defensive testing. This has resulted in substantial policy shifts in a number of jurisdictions.7,8 Some of the policy changes that have taken place over the past few decades have included state-imposed caps on medical malpractice awards and noneconomic damages, caps on attorney fees, and shortened statutes of limitations that require more timely filing of malpractice suits.
Defining Malpractice and Imposing Caps
In 2003, Texas changed its definition of the medical malpractice standard to “willful and wanton negligence”; in Georgia (2005) and South Carolina (2005) the definition was changed to “gross negligence.” Both of these revised definitions are essentially synonymous in a legal sense and are intended to protect physicians working in a high-risk, limited-information, high-intensity environment (eg, the ED) by raising the plaintiff’s responsibility to prove that the defendant physician was aware of the likelihood of serious injury but proceeded with “conscious indifference.”9
It seems, however, that such efforts have been not been entirely effective in reigning in costs of care, decreasing insurance costs, and limiting defensive medicine, particularly in the ED.9 A study by Paik et al10 on the effect of caps on malpractice claims and payouts found that in states with caps, both claims and payouts were effectively reduced, with a large impact on payout per physician and a drop in claims for those cases with larger payouts. While stricter caps had larger effects, the authors did not examine the impact of caps on “defensive medicine.”10 Furthermore, many physicians, health systems, and patient advocacy groups have been exploring and implementing alternative models of claim resolution outside of the legal process.11
Alternative Compensation Models
In the state legislatures of Georgia and Florida, alternative patient-compensation models are currently under proposal. Both models are designed to eliminate the current medical tort system and replace it with an administrative system to compensate patients for medical errors that have caused them harm.12 These proposals are similar to the existing Birth-Related Neurological Injury Compensation Programs (BRNICP) in effect in both Florida and Virginia. The BRNICP in each of these states serves as an administrative system to provide monetary compensation to patients who have clearly suffered only birth-related medical injuries, thus keeping this type of liability out of the court system.
Program Structure
Compensation programs such as the BRNICP in Florida and Virginia would replace traditional tort law. In this system, physicians would pay annually into a compensation fund (as do the physicians in Virginia and Florida), with amounts prorated to liability risk based on practice specialty. A patient harmed by a claimed medical injury that was allegedly caused by the proximate treatment rendered, would apply to the patient compensation system via a designated patient advocate. The advocate would initiate the claim process on behalf of the patient, after which the claim would be reviewed by a panel of medical experts in the appropriate field. If the panel finds the injury was preventable or avoidable, the case would then proceed to a compensation committee to render payment to the injured individual.
This compensation model not only eliminates the need for legal counsel for the patient, but also the need for medical malpractice liability insurance and defense counsel for the physician. Unlike traditional tort law, this alternate process encourages a system of transparency that supports appropriate disclosure of medical error rather than delaying late discovery of error and increased angst both for the patient and the physician.
Potential Benefits
One would anticipate that an alternate compensation model such as the BRNCIP that eliminates the fear of a lawsuit (ie, if patients no longer sued physicians for medical malpractice) would have a significant impact on defensive medicine and its associated costs. A study conducted by Emory University concluded that as much as $7 billion in the state of Georgia could be saved each year if such a program was enacted.13 In addition to the financial benefits, the care of all patients would improve through increased efficiency and better appropriation of finite resources. Moreover, patients harmed in a medical mishap would have a more direct, expedited, and less expensive mechanism of compensation compared to traditional tort systems.
The alternate compensation model would also benefit patients by negating the need for legal counsel. In the current tort system, many cases go unaddressed either because the patient does not have the means to hire counsel or the case seems too inconsequential for a lawyer to accept it. The compensation system would improve access for patients with valid claims, from egregious high impact errors to the lower impact errors, which are still significant.
There are also public health benefits to the alternate compensation model, including advances in patient safety as a result of the transparency of medical error and addressing medical mishaps in a timelier manner, providing an opportunity to improve knowledge and system gaps closer to real-time events. No longer would a patient have to forge an adversarial offensive on a physician. The panel of experts, who becomes the peer of the physician, can fairly assess the conditions of the case and bring forth an impartial recommendation to either reimburse or not reimburse the patient.
By eliminating the punitive nature of tort law upon the physician, and because this system compensates through a state-based compensation program, there is no indelible report made naming the physician to the National Practitioner Data Bank. Further, if a provider is identified as a significant risk to the public, the panel of medical experts can report that physician to the state licensing board immediately, which would prove more effective and efficient than the traditional method of data collection and referral currently in place in most states.
Challenges
Challenges to these bills include resistance from those who may be adversely affected by such legislation—mainly medical malpractice trial lawyers (both plaintiff and defendant) and medical malpractice insurance companies.
Conclusion
In consideration of innovative solutions to medical malpractice reform, the efforts in the states of Georgia and Florida clearly think outside the box. Neither of these proposed solutions is currently operational, but certainly if they become state statutes, they will create a very interesting environment to observe while the effects of such systems play out. The operations of the birth-related injury funds have been successful in states that have already implemented such programs. In the meantime, pending such changes in policy and legislation, EPs can mitigate malpractice risk by maintaining board certification and specialty training requirements, and by employing the following:
Follow the basic principles for every patient. Vital signs are vital for a reason, and all abnormal data must be accounted for;
Maintain open communication with patients—a paramount component in reducing the risk of a malpractice allegation;
Ensure that all members of the care team engender an environment that is focused on patient safety, including open communication with nursing staff and technical support;
Be aware of inherent biases in medical decision-making, which helps to maintain mindfulness in the routine practice of emergency medicine (EM);
Make sure departmental policies and procedures are designed to identify and address all late resulting laboratory results, radiology reading discrepancies and culture results in a timely and uniform manner; and
Provide clear and concise at-home care instructions to patients—prior to discharge—and in a manner the patient can understand.
Part 2 will discuss each of these recommendations in detail and will consider recent trends in medical malpractice as they relate to EM, explore areas of risk, and discuss strategies to reduce medical malpractice risk in the ED.
- Charles SC. Coping with a medical malpractice suit. West J Med. 2001;174(1): 55-58.
- Katz DA, Williams GC, Brown RL, et al. Emergency physicians’ fear of malpractice in evaluating patient with possible acute cardiac ischemia. Ann Emerg Med. 2005;46(6): 525-533.
- Jena AB, Seabury S, Lakdawalla D, Chandra A. Malpractice risk according to physician specialty. N Engl J Med. 2011; 365(7):629-636.
- Moran B. $6.41 million verdict in Temple malpractice lawsuit. Philadelphia Inquirer. 2012, June 2. http://articles.philly.com/2012-06-02/news/31960243_1_million-verdict-malpractice-massive-heart-attack. Accessed March 1, 2016.
- Martin AB, Hartman M, Benson J, Catlin A; National Health Expenditure Accounts Team. National health spending in 2014: faster growth driven by coverage expansion and prescription drug spending. Health Aff (Millwood). 2015;35(1):150-160.
- Emanuel E, Tanden N, Altman S, et al. A systemic approach to containing health care spending. N Engl J Med. 2012;367(10):949-954.
- Jost TS. Health care reform requires law reform. Health Aff (Millwood). 2009; 28(5): w761-w769.
- Roslund G. The medical malpractice rundown: a state-by-state report card. Emerg Phys Monthly. 2014;July 21. Available at http://epmonthly.com/article/the-medical-malpractice-rundown-a-state-by-state-report-card/. Accessed March 1, 2016.
- Waxman DA, Greenberg MD, Ridgely MS, Kellermann AL, Heaton P. The effect of malpractice reform on emergency department care. N Engl J Med. 2014;371(16):1518-1525.
- Paik M, Black BS, Hyma DA. The receding tide of medical malpractice litigation part 2: effect of damage caps. J Empirical Leg Stud. 2013;10(4):639-669.
- Stamm JA, Korzick KA, Beech K, Wood KE. Medical malpractice: reform for today’s patients and clinicians. Am J Med. 2016;129(1):20-25.
- Segal J. Finally: an end to malpractice litigation? Medscape. Available at http://www.medscape.com/viewarticle/840337_1. March 5,2015. Accessed March 1, 2016.
- Shinkman R. Patient compensation system could replace malpractice torts. Fierce Health Finance Web site. November 9, 2014. Available at http://www.fiercehealthfinance.com/story/patient-compensation-system-could-replace-malpractice-torts/2014-11-09. Accessed March 1, 2016.
We’re lost, but we’re making good time.
-Yogi Berra
As Yogi Berra alludes, it is very easy to get caught up “in the flow” and continue to move along at a good pace, even when one does not know where he or she is ultimately headed. Similarly, in terms of medical malpractice, everyone seems to have an opinion on what should be done to improve the climate of medical malpractice for both providers and patients. Yet, there are many differences in opinions on how to solve these issues, and the “cure” for what “ails” in the system are many—with an undetermined endpoint.
Tort reform is often conjured as the communal fix; yet each state in the Union has its own medical malpractice tort laws, which begs the question of how an issue with so many different facets can be resolved. Additionally, the risk alone of medical malpractice continues to be an important area of concern to emergency physicians (EPs), not only because of the looming threat of malpractice litigation—both real and perceived—but also because of its influence on practice patterns, resource utilization, and patient care in the ED.1,2
Physician Perception
Over the course of a career, an EP faces at least one claim, further perpetuating a common physician perception that the occurrence of a suit is not a condition of “if” but rather of “when.”3 This anxiety and fear among physicians in general are further provoked by the many headlines highlighting massive jury verdicts that dominate the news cycle.4
In addition, the EP’s work and practice are increasingly affected by the impositions of multiple nationally reported quality metrics, institutional throughput goals, and process-improvement efforts. Each of these in turn has the effect of increasing the pace of care and can challenge one’s real-time ability to recognize the dangers of inherent biases, to appreciate and act upon subtle clinical clues, and to rescue patient-experience misadventures. Accordingly, medical malpractice is a frequent topic of discussion for policy proposals among physicians and legislators.
Defensive Medicine and Tort Reform
As spending on health care in the United States topped $3 trillion, or 17.5% of the US gross domestic product in 2014, strategies for cost-containment have become a primary concern across all sectors of the health-care industry.5,6 With defensive medicine proposed by some as a substantial driver of health-care costs, many physicians have focused on tort reform as an avenue to curb defensive testing. This has resulted in substantial policy shifts in a number of jurisdictions.7,8 Some of the policy changes that have taken place over the past few decades have included state-imposed caps on medical malpractice awards and noneconomic damages, caps on attorney fees, and shortened statutes of limitations that require more timely filing of malpractice suits.
Defining Malpractice and Imposing Caps
In 2003, Texas changed its definition of the medical malpractice standard to “willful and wanton negligence”; in Georgia (2005) and South Carolina (2005) the definition was changed to “gross negligence.” Both of these revised definitions are essentially synonymous in a legal sense and are intended to protect physicians working in a high-risk, limited-information, high-intensity environment (eg, the ED) by raising the plaintiff’s responsibility to prove that the defendant physician was aware of the likelihood of serious injury but proceeded with “conscious indifference.”9
It seems, however, that such efforts have been not been entirely effective in reigning in costs of care, decreasing insurance costs, and limiting defensive medicine, particularly in the ED.9 A study by Paik et al10 on the effect of caps on malpractice claims and payouts found that in states with caps, both claims and payouts were effectively reduced, with a large impact on payout per physician and a drop in claims for those cases with larger payouts. While stricter caps had larger effects, the authors did not examine the impact of caps on “defensive medicine.”10 Furthermore, many physicians, health systems, and patient advocacy groups have been exploring and implementing alternative models of claim resolution outside of the legal process.11
Alternative Compensation Models
In the state legislatures of Georgia and Florida, alternative patient-compensation models are currently under proposal. Both models are designed to eliminate the current medical tort system and replace it with an administrative system to compensate patients for medical errors that have caused them harm.12 These proposals are similar to the existing Birth-Related Neurological Injury Compensation Programs (BRNICP) in effect in both Florida and Virginia. The BRNICP in each of these states serves as an administrative system to provide monetary compensation to patients who have clearly suffered only birth-related medical injuries, thus keeping this type of liability out of the court system.
Program Structure
Compensation programs such as the BRNICP in Florida and Virginia would replace traditional tort law. In this system, physicians would pay annually into a compensation fund (as do the physicians in Virginia and Florida), with amounts prorated to liability risk based on practice specialty. A patient harmed by a claimed medical injury that was allegedly caused by the proximate treatment rendered, would apply to the patient compensation system via a designated patient advocate. The advocate would initiate the claim process on behalf of the patient, after which the claim would be reviewed by a panel of medical experts in the appropriate field. If the panel finds the injury was preventable or avoidable, the case would then proceed to a compensation committee to render payment to the injured individual.
This compensation model not only eliminates the need for legal counsel for the patient, but also the need for medical malpractice liability insurance and defense counsel for the physician. Unlike traditional tort law, this alternate process encourages a system of transparency that supports appropriate disclosure of medical error rather than delaying late discovery of error and increased angst both for the patient and the physician.
Potential Benefits
One would anticipate that an alternate compensation model such as the BRNCIP that eliminates the fear of a lawsuit (ie, if patients no longer sued physicians for medical malpractice) would have a significant impact on defensive medicine and its associated costs. A study conducted by Emory University concluded that as much as $7 billion in the state of Georgia could be saved each year if such a program was enacted.13 In addition to the financial benefits, the care of all patients would improve through increased efficiency and better appropriation of finite resources. Moreover, patients harmed in a medical mishap would have a more direct, expedited, and less expensive mechanism of compensation compared to traditional tort systems.
The alternate compensation model would also benefit patients by negating the need for legal counsel. In the current tort system, many cases go unaddressed either because the patient does not have the means to hire counsel or the case seems too inconsequential for a lawyer to accept it. The compensation system would improve access for patients with valid claims, from egregious high impact errors to the lower impact errors, which are still significant.
There are also public health benefits to the alternate compensation model, including advances in patient safety as a result of the transparency of medical error and addressing medical mishaps in a timelier manner, providing an opportunity to improve knowledge and system gaps closer to real-time events. No longer would a patient have to forge an adversarial offensive on a physician. The panel of experts, who becomes the peer of the physician, can fairly assess the conditions of the case and bring forth an impartial recommendation to either reimburse or not reimburse the patient.
By eliminating the punitive nature of tort law upon the physician, and because this system compensates through a state-based compensation program, there is no indelible report made naming the physician to the National Practitioner Data Bank. Further, if a provider is identified as a significant risk to the public, the panel of medical experts can report that physician to the state licensing board immediately, which would prove more effective and efficient than the traditional method of data collection and referral currently in place in most states.
Challenges
Challenges to these bills include resistance from those who may be adversely affected by such legislation—mainly medical malpractice trial lawyers (both plaintiff and defendant) and medical malpractice insurance companies.
Conclusion
In consideration of innovative solutions to medical malpractice reform, the efforts in the states of Georgia and Florida clearly think outside the box. Neither of these proposed solutions is currently operational, but certainly if they become state statutes, they will create a very interesting environment to observe while the effects of such systems play out. The operations of the birth-related injury funds have been successful in states that have already implemented such programs. In the meantime, pending such changes in policy and legislation, EPs can mitigate malpractice risk by maintaining board certification and specialty training requirements, and by employing the following:
Follow the basic principles for every patient. Vital signs are vital for a reason, and all abnormal data must be accounted for;
Maintain open communication with patients—a paramount component in reducing the risk of a malpractice allegation;
Ensure that all members of the care team engender an environment that is focused on patient safety, including open communication with nursing staff and technical support;
Be aware of inherent biases in medical decision-making, which helps to maintain mindfulness in the routine practice of emergency medicine (EM);
Make sure departmental policies and procedures are designed to identify and address all late resulting laboratory results, radiology reading discrepancies and culture results in a timely and uniform manner; and
Provide clear and concise at-home care instructions to patients—prior to discharge—and in a manner the patient can understand.
Part 2 will discuss each of these recommendations in detail and will consider recent trends in medical malpractice as they relate to EM, explore areas of risk, and discuss strategies to reduce medical malpractice risk in the ED.
We’re lost, but we’re making good time.
-Yogi Berra
As Yogi Berra alludes, it is very easy to get caught up “in the flow” and continue to move along at a good pace, even when one does not know where he or she is ultimately headed. Similarly, in terms of medical malpractice, everyone seems to have an opinion on what should be done to improve the climate of medical malpractice for both providers and patients. Yet, there are many differences in opinions on how to solve these issues, and the “cure” for what “ails” in the system are many—with an undetermined endpoint.
Tort reform is often conjured as the communal fix; yet each state in the Union has its own medical malpractice tort laws, which begs the question of how an issue with so many different facets can be resolved. Additionally, the risk alone of medical malpractice continues to be an important area of concern to emergency physicians (EPs), not only because of the looming threat of malpractice litigation—both real and perceived—but also because of its influence on practice patterns, resource utilization, and patient care in the ED.1,2
Physician Perception
Over the course of a career, an EP faces at least one claim, further perpetuating a common physician perception that the occurrence of a suit is not a condition of “if” but rather of “when.”3 This anxiety and fear among physicians in general are further provoked by the many headlines highlighting massive jury verdicts that dominate the news cycle.4
In addition, the EP’s work and practice are increasingly affected by the impositions of multiple nationally reported quality metrics, institutional throughput goals, and process-improvement efforts. Each of these in turn has the effect of increasing the pace of care and can challenge one’s real-time ability to recognize the dangers of inherent biases, to appreciate and act upon subtle clinical clues, and to rescue patient-experience misadventures. Accordingly, medical malpractice is a frequent topic of discussion for policy proposals among physicians and legislators.
Defensive Medicine and Tort Reform
As spending on health care in the United States topped $3 trillion, or 17.5% of the US gross domestic product in 2014, strategies for cost-containment have become a primary concern across all sectors of the health-care industry.5,6 With defensive medicine proposed by some as a substantial driver of health-care costs, many physicians have focused on tort reform as an avenue to curb defensive testing. This has resulted in substantial policy shifts in a number of jurisdictions.7,8 Some of the policy changes that have taken place over the past few decades have included state-imposed caps on medical malpractice awards and noneconomic damages, caps on attorney fees, and shortened statutes of limitations that require more timely filing of malpractice suits.
Defining Malpractice and Imposing Caps
In 2003, Texas changed its definition of the medical malpractice standard to “willful and wanton negligence”; in Georgia (2005) and South Carolina (2005) the definition was changed to “gross negligence.” Both of these revised definitions are essentially synonymous in a legal sense and are intended to protect physicians working in a high-risk, limited-information, high-intensity environment (eg, the ED) by raising the plaintiff’s responsibility to prove that the defendant physician was aware of the likelihood of serious injury but proceeded with “conscious indifference.”9
It seems, however, that such efforts have been not been entirely effective in reigning in costs of care, decreasing insurance costs, and limiting defensive medicine, particularly in the ED.9 A study by Paik et al10 on the effect of caps on malpractice claims and payouts found that in states with caps, both claims and payouts were effectively reduced, with a large impact on payout per physician and a drop in claims for those cases with larger payouts. While stricter caps had larger effects, the authors did not examine the impact of caps on “defensive medicine.”10 Furthermore, many physicians, health systems, and patient advocacy groups have been exploring and implementing alternative models of claim resolution outside of the legal process.11
Alternative Compensation Models
In the state legislatures of Georgia and Florida, alternative patient-compensation models are currently under proposal. Both models are designed to eliminate the current medical tort system and replace it with an administrative system to compensate patients for medical errors that have caused them harm.12 These proposals are similar to the existing Birth-Related Neurological Injury Compensation Programs (BRNICP) in effect in both Florida and Virginia. The BRNICP in each of these states serves as an administrative system to provide monetary compensation to patients who have clearly suffered only birth-related medical injuries, thus keeping this type of liability out of the court system.
Program Structure
Compensation programs such as the BRNICP in Florida and Virginia would replace traditional tort law. In this system, physicians would pay annually into a compensation fund (as do the physicians in Virginia and Florida), with amounts prorated to liability risk based on practice specialty. A patient harmed by a claimed medical injury that was allegedly caused by the proximate treatment rendered, would apply to the patient compensation system via a designated patient advocate. The advocate would initiate the claim process on behalf of the patient, after which the claim would be reviewed by a panel of medical experts in the appropriate field. If the panel finds the injury was preventable or avoidable, the case would then proceed to a compensation committee to render payment to the injured individual.
This compensation model not only eliminates the need for legal counsel for the patient, but also the need for medical malpractice liability insurance and defense counsel for the physician. Unlike traditional tort law, this alternate process encourages a system of transparency that supports appropriate disclosure of medical error rather than delaying late discovery of error and increased angst both for the patient and the physician.
Potential Benefits
One would anticipate that an alternate compensation model such as the BRNCIP that eliminates the fear of a lawsuit (ie, if patients no longer sued physicians for medical malpractice) would have a significant impact on defensive medicine and its associated costs. A study conducted by Emory University concluded that as much as $7 billion in the state of Georgia could be saved each year if such a program was enacted.13 In addition to the financial benefits, the care of all patients would improve through increased efficiency and better appropriation of finite resources. Moreover, patients harmed in a medical mishap would have a more direct, expedited, and less expensive mechanism of compensation compared to traditional tort systems.
The alternate compensation model would also benefit patients by negating the need for legal counsel. In the current tort system, many cases go unaddressed either because the patient does not have the means to hire counsel or the case seems too inconsequential for a lawyer to accept it. The compensation system would improve access for patients with valid claims, from egregious high impact errors to the lower impact errors, which are still significant.
There are also public health benefits to the alternate compensation model, including advances in patient safety as a result of the transparency of medical error and addressing medical mishaps in a timelier manner, providing an opportunity to improve knowledge and system gaps closer to real-time events. No longer would a patient have to forge an adversarial offensive on a physician. The panel of experts, who becomes the peer of the physician, can fairly assess the conditions of the case and bring forth an impartial recommendation to either reimburse or not reimburse the patient.
By eliminating the punitive nature of tort law upon the physician, and because this system compensates through a state-based compensation program, there is no indelible report made naming the physician to the National Practitioner Data Bank. Further, if a provider is identified as a significant risk to the public, the panel of medical experts can report that physician to the state licensing board immediately, which would prove more effective and efficient than the traditional method of data collection and referral currently in place in most states.
Challenges
Challenges to these bills include resistance from those who may be adversely affected by such legislation—mainly medical malpractice trial lawyers (both plaintiff and defendant) and medical malpractice insurance companies.
Conclusion
In consideration of innovative solutions to medical malpractice reform, the efforts in the states of Georgia and Florida clearly think outside the box. Neither of these proposed solutions is currently operational, but certainly if they become state statutes, they will create a very interesting environment to observe while the effects of such systems play out. The operations of the birth-related injury funds have been successful in states that have already implemented such programs. In the meantime, pending such changes in policy and legislation, EPs can mitigate malpractice risk by maintaining board certification and specialty training requirements, and by employing the following:
Follow the basic principles for every patient. Vital signs are vital for a reason, and all abnormal data must be accounted for;
Maintain open communication with patients—a paramount component in reducing the risk of a malpractice allegation;
Ensure that all members of the care team engender an environment that is focused on patient safety, including open communication with nursing staff and technical support;
Be aware of inherent biases in medical decision-making, which helps to maintain mindfulness in the routine practice of emergency medicine (EM);
Make sure departmental policies and procedures are designed to identify and address all late resulting laboratory results, radiology reading discrepancies and culture results in a timely and uniform manner; and
Provide clear and concise at-home care instructions to patients—prior to discharge—and in a manner the patient can understand.
Part 2 will discuss each of these recommendations in detail and will consider recent trends in medical malpractice as they relate to EM, explore areas of risk, and discuss strategies to reduce medical malpractice risk in the ED.
- Charles SC. Coping with a medical malpractice suit. West J Med. 2001;174(1): 55-58.
- Katz DA, Williams GC, Brown RL, et al. Emergency physicians’ fear of malpractice in evaluating patient with possible acute cardiac ischemia. Ann Emerg Med. 2005;46(6): 525-533.
- Jena AB, Seabury S, Lakdawalla D, Chandra A. Malpractice risk according to physician specialty. N Engl J Med. 2011; 365(7):629-636.
- Moran B. $6.41 million verdict in Temple malpractice lawsuit. Philadelphia Inquirer. 2012, June 2. http://articles.philly.com/2012-06-02/news/31960243_1_million-verdict-malpractice-massive-heart-attack. Accessed March 1, 2016.
- Martin AB, Hartman M, Benson J, Catlin A; National Health Expenditure Accounts Team. National health spending in 2014: faster growth driven by coverage expansion and prescription drug spending. Health Aff (Millwood). 2015;35(1):150-160.
- Emanuel E, Tanden N, Altman S, et al. A systemic approach to containing health care spending. N Engl J Med. 2012;367(10):949-954.
- Jost TS. Health care reform requires law reform. Health Aff (Millwood). 2009; 28(5): w761-w769.
- Roslund G. The medical malpractice rundown: a state-by-state report card. Emerg Phys Monthly. 2014;July 21. Available at http://epmonthly.com/article/the-medical-malpractice-rundown-a-state-by-state-report-card/. Accessed March 1, 2016.
- Waxman DA, Greenberg MD, Ridgely MS, Kellermann AL, Heaton P. The effect of malpractice reform on emergency department care. N Engl J Med. 2014;371(16):1518-1525.
- Paik M, Black BS, Hyma DA. The receding tide of medical malpractice litigation part 2: effect of damage caps. J Empirical Leg Stud. 2013;10(4):639-669.
- Stamm JA, Korzick KA, Beech K, Wood KE. Medical malpractice: reform for today’s patients and clinicians. Am J Med. 2016;129(1):20-25.
- Segal J. Finally: an end to malpractice litigation? Medscape. Available at http://www.medscape.com/viewarticle/840337_1. March 5,2015. Accessed March 1, 2016.
- Shinkman R. Patient compensation system could replace malpractice torts. Fierce Health Finance Web site. November 9, 2014. Available at http://www.fiercehealthfinance.com/story/patient-compensation-system-could-replace-malpractice-torts/2014-11-09. Accessed March 1, 2016.
- Charles SC. Coping with a medical malpractice suit. West J Med. 2001;174(1): 55-58.
- Katz DA, Williams GC, Brown RL, et al. Emergency physicians’ fear of malpractice in evaluating patient with possible acute cardiac ischemia. Ann Emerg Med. 2005;46(6): 525-533.
- Jena AB, Seabury S, Lakdawalla D, Chandra A. Malpractice risk according to physician specialty. N Engl J Med. 2011; 365(7):629-636.
- Moran B. $6.41 million verdict in Temple malpractice lawsuit. Philadelphia Inquirer. 2012, June 2. http://articles.philly.com/2012-06-02/news/31960243_1_million-verdict-malpractice-massive-heart-attack. Accessed March 1, 2016.
- Martin AB, Hartman M, Benson J, Catlin A; National Health Expenditure Accounts Team. National health spending in 2014: faster growth driven by coverage expansion and prescription drug spending. Health Aff (Millwood). 2015;35(1):150-160.
- Emanuel E, Tanden N, Altman S, et al. A systemic approach to containing health care spending. N Engl J Med. 2012;367(10):949-954.
- Jost TS. Health care reform requires law reform. Health Aff (Millwood). 2009; 28(5): w761-w769.
- Roslund G. The medical malpractice rundown: a state-by-state report card. Emerg Phys Monthly. 2014;July 21. Available at http://epmonthly.com/article/the-medical-malpractice-rundown-a-state-by-state-report-card/. Accessed March 1, 2016.
- Waxman DA, Greenberg MD, Ridgely MS, Kellermann AL, Heaton P. The effect of malpractice reform on emergency department care. N Engl J Med. 2014;371(16):1518-1525.
- Paik M, Black BS, Hyma DA. The receding tide of medical malpractice litigation part 2: effect of damage caps. J Empirical Leg Stud. 2013;10(4):639-669.
- Stamm JA, Korzick KA, Beech K, Wood KE. Medical malpractice: reform for today’s patients and clinicians. Am J Med. 2016;129(1):20-25.
- Segal J. Finally: an end to malpractice litigation? Medscape. Available at http://www.medscape.com/viewarticle/840337_1. March 5,2015. Accessed March 1, 2016.
- Shinkman R. Patient compensation system could replace malpractice torts. Fierce Health Finance Web site. November 9, 2014. Available at http://www.fiercehealthfinance.com/story/patient-compensation-system-could-replace-malpractice-torts/2014-11-09. Accessed March 1, 2016.
Diagnosis at a Glance: Bedside Ultrasound Diagnosis of Acute Angle Closure Glaucoma
Case
An 82-year-old woman presented to the ED for evaluation of left eye pain. She stated the pain began earlier in the day as a mild discomfort but progressed and acutely worsened 2 hours prior to presentation. She rated the pain as a “9” out of “10” on a pain scale; she described the pain as constant, with throbbing behind her left eye. There was no pain associated with extraocular movements. Photosensitivity and increased lacrimation of the left eye were present, along with associated nausea. The patient denied any ocular trauma or previous surgery.
On examination, the patient’s left pupil measured 4 mm, was oval in shape, and was nonreactive with surrounding scleral edema. Visual acuity on the right eye was 20/50, but on the left eye, she had only finger-counting at 2 feet. Since tonometry was unavailable, bedside ultrasound images of the affected eye (Figure 1) and a comparison image of the patient’s normal, unaffected eye (Figure 2) were taken, revealing acute angle closure glaucoma (AACG) in the patient’s left eye.
Ocular Ultrasound
Diagnosis of AACG in the ED is generally made through clinical examination and tonometry. Tonometry, however, may be either unavailable or malfunctioning. In such cases, bedside ultrasound can serve as an alternative diagnostic tool. Ocular ultrasound is also beneficial in diagnosing AACG in patients who do not present with classic signs and symptoms of the condition. The abnormal bedside ultrasound can prompt earlier specialist consultation, which may decrease negative long-term sequelae.
Dr Rose is ultrasound fellow and clinical instructor in the department of emergency medicine, University of Kentucky, Lexington. Dr Cuevas is a resident in the department of emergency medicine, University of Kentucky, Lexington. Dr Dawson is an associate professor, director of ultrasound fellowship, and director of point-of-care ultrasound in the department of emergency medicine, University of Kentucky, Lexington.
- Rippey, J. Ultrasound of Acute angle closure glaucoma. The SonoCave Web site. Available at: http://thesonocave.com/2013/04/ultrasound-of-acute-angle-closure-glaucoma. Accessed February 23, 2016.
- Feng MT, Belin MW, Ambrósio R Jr, et al. Anterior chamber depth in normal subjects by rotating scheimpflug imaging. Saudi J Ophthalmol. 2011;25(3):255-259. doi:10.1016/j.sjopt.2011.04.005.
Case
An 82-year-old woman presented to the ED for evaluation of left eye pain. She stated the pain began earlier in the day as a mild discomfort but progressed and acutely worsened 2 hours prior to presentation. She rated the pain as a “9” out of “10” on a pain scale; she described the pain as constant, with throbbing behind her left eye. There was no pain associated with extraocular movements. Photosensitivity and increased lacrimation of the left eye were present, along with associated nausea. The patient denied any ocular trauma or previous surgery.
On examination, the patient’s left pupil measured 4 mm, was oval in shape, and was nonreactive with surrounding scleral edema. Visual acuity on the right eye was 20/50, but on the left eye, she had only finger-counting at 2 feet. Since tonometry was unavailable, bedside ultrasound images of the affected eye (Figure 1) and a comparison image of the patient’s normal, unaffected eye (Figure 2) were taken, revealing acute angle closure glaucoma (AACG) in the patient’s left eye.
Ocular Ultrasound
Diagnosis of AACG in the ED is generally made through clinical examination and tonometry. Tonometry, however, may be either unavailable or malfunctioning. In such cases, bedside ultrasound can serve as an alternative diagnostic tool. Ocular ultrasound is also beneficial in diagnosing AACG in patients who do not present with classic signs and symptoms of the condition. The abnormal bedside ultrasound can prompt earlier specialist consultation, which may decrease negative long-term sequelae.
Dr Rose is ultrasound fellow and clinical instructor in the department of emergency medicine, University of Kentucky, Lexington. Dr Cuevas is a resident in the department of emergency medicine, University of Kentucky, Lexington. Dr Dawson is an associate professor, director of ultrasound fellowship, and director of point-of-care ultrasound in the department of emergency medicine, University of Kentucky, Lexington.
Case
An 82-year-old woman presented to the ED for evaluation of left eye pain. She stated the pain began earlier in the day as a mild discomfort but progressed and acutely worsened 2 hours prior to presentation. She rated the pain as a “9” out of “10” on a pain scale; she described the pain as constant, with throbbing behind her left eye. There was no pain associated with extraocular movements. Photosensitivity and increased lacrimation of the left eye were present, along with associated nausea. The patient denied any ocular trauma or previous surgery.
On examination, the patient’s left pupil measured 4 mm, was oval in shape, and was nonreactive with surrounding scleral edema. Visual acuity on the right eye was 20/50, but on the left eye, she had only finger-counting at 2 feet. Since tonometry was unavailable, bedside ultrasound images of the affected eye (Figure 1) and a comparison image of the patient’s normal, unaffected eye (Figure 2) were taken, revealing acute angle closure glaucoma (AACG) in the patient’s left eye.
Ocular Ultrasound
Diagnosis of AACG in the ED is generally made through clinical examination and tonometry. Tonometry, however, may be either unavailable or malfunctioning. In such cases, bedside ultrasound can serve as an alternative diagnostic tool. Ocular ultrasound is also beneficial in diagnosing AACG in patients who do not present with classic signs and symptoms of the condition. The abnormal bedside ultrasound can prompt earlier specialist consultation, which may decrease negative long-term sequelae.
Dr Rose is ultrasound fellow and clinical instructor in the department of emergency medicine, University of Kentucky, Lexington. Dr Cuevas is a resident in the department of emergency medicine, University of Kentucky, Lexington. Dr Dawson is an associate professor, director of ultrasound fellowship, and director of point-of-care ultrasound in the department of emergency medicine, University of Kentucky, Lexington.
- Rippey, J. Ultrasound of Acute angle closure glaucoma. The SonoCave Web site. Available at: http://thesonocave.com/2013/04/ultrasound-of-acute-angle-closure-glaucoma. Accessed February 23, 2016.
- Feng MT, Belin MW, Ambrósio R Jr, et al. Anterior chamber depth in normal subjects by rotating scheimpflug imaging. Saudi J Ophthalmol. 2011;25(3):255-259. doi:10.1016/j.sjopt.2011.04.005.
- Rippey, J. Ultrasound of Acute angle closure glaucoma. The SonoCave Web site. Available at: http://thesonocave.com/2013/04/ultrasound-of-acute-angle-closure-glaucoma. Accessed February 23, 2016.
- Feng MT, Belin MW, Ambrósio R Jr, et al. Anterior chamber depth in normal subjects by rotating scheimpflug imaging. Saudi J Ophthalmol. 2011;25(3):255-259. doi:10.1016/j.sjopt.2011.04.005.
The Challenges of Type 1 Diabetes: A Case-based Review
IN THIS ARTICLE
- Progress and treatment timeline with long- and rapid-acting insulin
- Progress and treatment timeline with continuous subcutaneous insulin infusion
- American Diabetes Association criteria for diagnosis of diabetes
- Blood glucose and A1C goals for type 1 diabetes by age-group
A 5-year-old Caucasian girl presents to the primary care practitioner’s office with chief complaints of polydipsia, polyuria with nocturia, polyphagia, and weight loss over the past three weeks. Her medical history includes a four-year history of keratosis pilaris (KP). The child experienced a KP flare-up two weeks ago; application of triamcinolone acetonide cream yielded no improvement. She also has xerosis, which is treated daily with OTC moisturizing lotion. She was born vaginally and breast-fed and is up to date on her immunizations. There is no family history of diabetes or autoimmune diseases.
Physical examination reveals a weight of 54 lb (95th percentile); height, 47 in (97th percentile); and BMI, 17.2. Vital signs include a blood pressure of 105/55 mm Hg; pulse, 85 beats/min and regular; temperature, 98.2°F; and respiratory rate, 22 breaths/min. KP is noted on the patient’s eyebrows, bilateral upper arms, and bilateral cheeks; the affected skin is erythemic and rough to the touch. Her physical examination findings are otherwise unremarkable.
The child’s urine is tested in the office for glucose and ketones, with results of 4+ glucose and 3+ ketones. These results and the child’s history prompt her admission to the pediatric ICU at a nearby hospital for further treatment with a diagnosis of new-onset type 1 diabetes (T1D) and diabetic ketoacidosis (DKA).
The diagnosis is confirmed at the hospital with laboratory results that include venous glucose, 418 mg/dL (normal range, 70 to 100 mg/dL) and A1C, 10.5% (range, 4.0% to 5.6%). Venous blood gas results include pH, 7.278 (7.32 to 7.42); PCO2, 29.6 mm Hg (39 to 54 mm Hg); HCO3, 13.8 mEq/L (19 to 25 mEq/L); base excess, –12 mmol/L (–4 to +2 mmol/L); beta hydroxybutyrate, 6.0 mmol/L (0.4 to 0.5 mmol/L); insulin antibody, 0.9 U/mL (< 0.4 U/mL); glutamic acid decarboxylase, 166 U/mL (< 0.5 U/mL); and venous lactate, 1.79 mmol/L (0.69 to 2.75 mmol/L).
The child is treated initially with an IV insulin infusion for 24 hours, then transitioned to subcutaneous insulin therapy once the DKA resolves and glucose levels are within normal limits. The child remains hospitalized for four days. Discharge medications include insulin glargine, 8 U/d, and insulin lispro before each meal, at bedtime, and at 0200 hours, with dosing based on sliding scales. Dietary orders include 45 to 60 g carbohydrates per meal, along with two snacks of 15 g carbohydrates.
The child is instructed to exercise at least 30 min/d (unless hypoglycemic events occur more than once per week or ketones are found in the blood or urine), drink plenty of water, and avoid concentrated sweets. Education is provided via the Diabetes Educator; the family takes home the beginner T1D booklet and is instructed to log the child’s blood glucose levels and return with this information in two weeks.
In the first three months, the patient experiences eight asymptomatic hypoglycemic events; for the next seven months, after dosing changes, she remains hyperglycemic most of the time (see Table 1). Insulin doses are adjusted, ranging from weekly to every three months, but glycemic goals are not achieved with the subcutaneous insulin injections. Use of continuous subcutaneous insulin infusion, the “insulin pump,” is then considered. Ten months postdiagnosis, the child begins a five-day-long saline (placebo) pump trial to determine whether the pump is appropriate for her and her lifestyle. After the trial, the decision is made to move forward with the insulin pump, initiated 11 months postdiagnosis.
The practitioner remains in frequent communication with the child’s mother in an effort to maintain glycemic control. After three months on the insulin pump, the child’s A1C is reduced to 7.9%, which is within the target range for her age-group (see Table 2). The child is now maintaining glycemic goals with the use of the insulin pump and close monitoring by the practitioner.
Continue for the discussion >>
DISCUSSION
According to the Juvenile Diabetes Research Foundation, as many as 1.25 million Americans are currently living with T1D; from 2001 to 2009, the prevalence of T1D in people younger than 20 increased by 23%.1 The overall prevalence of diabetes (both types 1 and 2) is predicted to be one in every three people by 2050 if current trends continue.2 According to the American Diabetes Association (ADA), 18,436 US youths are diagnosed with T1D every year, and T1D accounts for about 5% of diabetes cases in the US population.2
Diagnosis
Diabetes is diagnosed based on blood test results that fall within the parameters set by the ADA diagnostic criteria (see Table 3).3 In addition to diagnostic testing for diabetes recommended by the ADA guidelines, blood tests are ordered for autoantibodies that are associated with T1D, to distinguish between type 1 and type 2 diabetes. (T1D results from cellular-mediated autoimmune destruction of the insulin-producing beta cells in the pancreas.4) Upon initial diagnosis, about 85% to 90% of T1D patients have one or more autoantibodies present in blood work, such as autoantibodies to islet cells or to insulin, glutamic acid decarboxylase (GAD65), or tyrosine phosphatases IA-2 and IA-2B.4
In this case study, the child had an elevated GAD65 value and a positive screening for an insulin autoantibody, which explained the destruction of her beta cells. The patient also had KP and xerosis, which are clinical manifestations commonly seen in T1D. In one study of children with T1D, 22% had xerosis, compared with 3% of healthy, age-matched controls, and KP was also significantly more common in T1D patients than in controls (12% vs 1.5%).5
The presence of ketones in the case patient’s urine also suggests T1D, rather than type 2.4 The differential diagnosis for T1D includes type 2 diabetes mellitus, monogenic diabetes mellitus (formerly known as maturity-onset diabetes of the young), secondary hyperglycemia, and other endocrine disorders.6
Acute complications associated with T1D include hypoglycemia, hyperglycemia, and DKA. Long-term complications may include diabetic retinopathy, cataracts, gastroparesis, hypertension, renal failure, coronary artery disease, peripheral vascular disease, diabetic neuropathy, and increased risk for infection.7 These complications can likely be prevented by good glycemic control, proper diet, exercise, and avoidance of nicotine.7
Unfortunately, T1D cannot currently be prevented, although research studies are under way. TrialNet is currently conducting a “Pathway to Prevention” trial; the researchers are testing ways to delay and prevent T1D, as well as slow its progression after diagnosis.8 Potential participants (family members of a T1D patient) are screened for T1D autoantibodies. If test results are positive, these participants are included in the prevention pathway study.
Continue for management >>
Management
Most cases of T1D are diagnosed in patients younger than 18.9 Management of the child with T1D involves many challenges. The patient will experience an initial honeymoon period, that is, a brief remission during which the pancreas begins to secrete some insulin again and exogenous insulin demands are lower. However, this period is temporary, lasting only a few weeks, months, or years. Once pancreatic insulin secretion stops (as a result of complete beta-cell destruction), the exogenous insulin demands increase. In the case study, the child’s insulin demands were initially low, and she experienced hypoglycemia. Once she transitioned out of the honeymoon period, however, her blood glucose levels rose because her pancreas was producing little to no insulin.
As the patient ages, physical growth and hormone changes also alter the demand for insulin. A key factor to keep in mind is lifestyle changes: The child may need age-appropriate supervision and adjustments in exercise, diet, and diabetes education regimens when school routines and self-care capacities change. The child with T1D can only be educated as far as his or her cognitive ability will allow, but autonomy should increase with age.
Helping the patient reach glycemic goals requires special consideration, based on the child’s age. Whereas the target A1C for an adult with diabetes is below 7%, that for a young child is either < 7.5% or < 8.5%, depending on age (see Table 4).9 According to Danne et al, approximately 60% of children younger than 6 years have an imperfect awareness of hypoglycemia. 10 Because the risk for a hypoglycemic event is increased in this age-group, their target A1C is higher.10
This is also an important age for brain development: The metabolism of glucose in the brain of a young child occurs at double the rate of that in an adult brain.11 Between ages 1 and 6 years, the brain increases in size dramatically, reaching 90% of its adult volume by age 6.11 In retrospective studies reviewed by Arbelaez et al,data show that frequent, severe hypoglycemic and hyperglycemic events are associated with poor cognitive function, particularly memory and attention.11 Due to the timing of brain development and the risk for glycemic extremes in young children, practitioners are advised to follow the ADA recommendations shown in Table 4.9
Continue for the conclusion >>
CONCLUSION
T1D is the most common chronic, serious, potentially life-threatening disease among children and adolescents. This lifelong illness is challenging to control, especially when managing the honeymoon period and addressing the increasing insulin demands in a growing child. Once a diagnosis is confirmed, the challenges persist, as each patient needs an individualized treatment regimen with ongoing adjustments. Knowledge of the ADA guidelines for age-appropriate A1C goals is essential for the practitioner who manages a growing child with T1D in order to achieve glycemic control while avoiding hypoglycemia. Preventing hypoglycemia is of the utmost importance, especially in a child too young to recognize symptoms.
Considering all the changes that a child with T1D is likely to experience, it is also important to remember that the foremost goal is for this child to live a healthy life. Thus, practitioners must educate both patients and parents regarding the complications that can arise with poor glycemic control and encourage adherence to the insulin therapy.
T1D requires vigilant monitoring and ongoing adjusted insulin therapy. Understanding age-appropriate treatment and maintaining good communications with patients and their parents are key to successful management of this disease.
REFERENCES
1. Juvenile Diabetes Research Foundation. Type 1 diabetes facts (2014). http://jdrf.org/about-jdrf/fact-sheets/type-1-diabetes-facts/. Accessed February 8, 2016.
2. American Diabetes Association. Fast facts: data and statistics about diabetes (2015). http://professional2.diabetes.org/admin/UserFiles/0%20-%20Sean/Documents/Fast_Facts_12-2015a.pdf. Accessed February 8, 2016.
3. American Diabetes Association. Executive summary: standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S5-S13.
4. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(1):81-90.
5. Pavlovic MD, Milenkovic T, Dinic M, et al. The prevalence of cutaneous manifestations in young patients with type 1 diabetes. Diabetes Care. 2007;30(8):1964-1967.
6. Khardori R. Type 1 diabetes mellitus differential diagnosis (updated 2015). http://emedicine.medscape.com/article/117739-differential. Accessed February 8, 2016.
7. Lamb WH. Pediatric type 1 diabetes mellitus (updated 2015). http://emedicine.medscape.com/article/919999-overview. Accessed February 8, 2016.
8. Type 1 Diabetes TrialNet. TrialNet Pathway to Prevention (2014). www.pathway2prevention.org/study/. Accessed February 8, 2016.
9. American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(suppl 1):S11-S66. VIII. Diabetes care in specific populations. http://care.diabetesjournals.org/content/36/Supplement_1/S11.full#sec-128. Accessed February 8, 2016.
10. Danne T, Philotheou A, Goldman D, et al. A randomized trial comparing the rate of hypoglycemia—assessed using continuous glucose monitoring—in 125 preschool children with type 1 diabetes treated with insulin glargine or NPH insulin (the PRESCHOOL study). Pediatr Diabetes. 2013;14(8):593-601.
11. Arbelaez AM, Semenkovich K, Hershey T. Glycemic extremes in youth with T1DM: the structural and functional integrity of the developing brain. Pediatr Diabetes. 2013;14(8):541-553.
IN THIS ARTICLE
- Progress and treatment timeline with long- and rapid-acting insulin
- Progress and treatment timeline with continuous subcutaneous insulin infusion
- American Diabetes Association criteria for diagnosis of diabetes
- Blood glucose and A1C goals for type 1 diabetes by age-group
A 5-year-old Caucasian girl presents to the primary care practitioner’s office with chief complaints of polydipsia, polyuria with nocturia, polyphagia, and weight loss over the past three weeks. Her medical history includes a four-year history of keratosis pilaris (KP). The child experienced a KP flare-up two weeks ago; application of triamcinolone acetonide cream yielded no improvement. She also has xerosis, which is treated daily with OTC moisturizing lotion. She was born vaginally and breast-fed and is up to date on her immunizations. There is no family history of diabetes or autoimmune diseases.
Physical examination reveals a weight of 54 lb (95th percentile); height, 47 in (97th percentile); and BMI, 17.2. Vital signs include a blood pressure of 105/55 mm Hg; pulse, 85 beats/min and regular; temperature, 98.2°F; and respiratory rate, 22 breaths/min. KP is noted on the patient’s eyebrows, bilateral upper arms, and bilateral cheeks; the affected skin is erythemic and rough to the touch. Her physical examination findings are otherwise unremarkable.
The child’s urine is tested in the office for glucose and ketones, with results of 4+ glucose and 3+ ketones. These results and the child’s history prompt her admission to the pediatric ICU at a nearby hospital for further treatment with a diagnosis of new-onset type 1 diabetes (T1D) and diabetic ketoacidosis (DKA).
The diagnosis is confirmed at the hospital with laboratory results that include venous glucose, 418 mg/dL (normal range, 70 to 100 mg/dL) and A1C, 10.5% (range, 4.0% to 5.6%). Venous blood gas results include pH, 7.278 (7.32 to 7.42); PCO2, 29.6 mm Hg (39 to 54 mm Hg); HCO3, 13.8 mEq/L (19 to 25 mEq/L); base excess, –12 mmol/L (–4 to +2 mmol/L); beta hydroxybutyrate, 6.0 mmol/L (0.4 to 0.5 mmol/L); insulin antibody, 0.9 U/mL (< 0.4 U/mL); glutamic acid decarboxylase, 166 U/mL (< 0.5 U/mL); and venous lactate, 1.79 mmol/L (0.69 to 2.75 mmol/L).
The child is treated initially with an IV insulin infusion for 24 hours, then transitioned to subcutaneous insulin therapy once the DKA resolves and glucose levels are within normal limits. The child remains hospitalized for four days. Discharge medications include insulin glargine, 8 U/d, and insulin lispro before each meal, at bedtime, and at 0200 hours, with dosing based on sliding scales. Dietary orders include 45 to 60 g carbohydrates per meal, along with two snacks of 15 g carbohydrates.
The child is instructed to exercise at least 30 min/d (unless hypoglycemic events occur more than once per week or ketones are found in the blood or urine), drink plenty of water, and avoid concentrated sweets. Education is provided via the Diabetes Educator; the family takes home the beginner T1D booklet and is instructed to log the child’s blood glucose levels and return with this information in two weeks.
In the first three months, the patient experiences eight asymptomatic hypoglycemic events; for the next seven months, after dosing changes, she remains hyperglycemic most of the time (see Table 1). Insulin doses are adjusted, ranging from weekly to every three months, but glycemic goals are not achieved with the subcutaneous insulin injections. Use of continuous subcutaneous insulin infusion, the “insulin pump,” is then considered. Ten months postdiagnosis, the child begins a five-day-long saline (placebo) pump trial to determine whether the pump is appropriate for her and her lifestyle. After the trial, the decision is made to move forward with the insulin pump, initiated 11 months postdiagnosis.
The practitioner remains in frequent communication with the child’s mother in an effort to maintain glycemic control. After three months on the insulin pump, the child’s A1C is reduced to 7.9%, which is within the target range for her age-group (see Table 2). The child is now maintaining glycemic goals with the use of the insulin pump and close monitoring by the practitioner.
Continue for the discussion >>
DISCUSSION
According to the Juvenile Diabetes Research Foundation, as many as 1.25 million Americans are currently living with T1D; from 2001 to 2009, the prevalence of T1D in people younger than 20 increased by 23%.1 The overall prevalence of diabetes (both types 1 and 2) is predicted to be one in every three people by 2050 if current trends continue.2 According to the American Diabetes Association (ADA), 18,436 US youths are diagnosed with T1D every year, and T1D accounts for about 5% of diabetes cases in the US population.2
Diagnosis
Diabetes is diagnosed based on blood test results that fall within the parameters set by the ADA diagnostic criteria (see Table 3).3 In addition to diagnostic testing for diabetes recommended by the ADA guidelines, blood tests are ordered for autoantibodies that are associated with T1D, to distinguish between type 1 and type 2 diabetes. (T1D results from cellular-mediated autoimmune destruction of the insulin-producing beta cells in the pancreas.4) Upon initial diagnosis, about 85% to 90% of T1D patients have one or more autoantibodies present in blood work, such as autoantibodies to islet cells or to insulin, glutamic acid decarboxylase (GAD65), or tyrosine phosphatases IA-2 and IA-2B.4
In this case study, the child had an elevated GAD65 value and a positive screening for an insulin autoantibody, which explained the destruction of her beta cells. The patient also had KP and xerosis, which are clinical manifestations commonly seen in T1D. In one study of children with T1D, 22% had xerosis, compared with 3% of healthy, age-matched controls, and KP was also significantly more common in T1D patients than in controls (12% vs 1.5%).5
The presence of ketones in the case patient’s urine also suggests T1D, rather than type 2.4 The differential diagnosis for T1D includes type 2 diabetes mellitus, monogenic diabetes mellitus (formerly known as maturity-onset diabetes of the young), secondary hyperglycemia, and other endocrine disorders.6
Acute complications associated with T1D include hypoglycemia, hyperglycemia, and DKA. Long-term complications may include diabetic retinopathy, cataracts, gastroparesis, hypertension, renal failure, coronary artery disease, peripheral vascular disease, diabetic neuropathy, and increased risk for infection.7 These complications can likely be prevented by good glycemic control, proper diet, exercise, and avoidance of nicotine.7
Unfortunately, T1D cannot currently be prevented, although research studies are under way. TrialNet is currently conducting a “Pathway to Prevention” trial; the researchers are testing ways to delay and prevent T1D, as well as slow its progression after diagnosis.8 Potential participants (family members of a T1D patient) are screened for T1D autoantibodies. If test results are positive, these participants are included in the prevention pathway study.
Continue for management >>
Management
Most cases of T1D are diagnosed in patients younger than 18.9 Management of the child with T1D involves many challenges. The patient will experience an initial honeymoon period, that is, a brief remission during which the pancreas begins to secrete some insulin again and exogenous insulin demands are lower. However, this period is temporary, lasting only a few weeks, months, or years. Once pancreatic insulin secretion stops (as a result of complete beta-cell destruction), the exogenous insulin demands increase. In the case study, the child’s insulin demands were initially low, and she experienced hypoglycemia. Once she transitioned out of the honeymoon period, however, her blood glucose levels rose because her pancreas was producing little to no insulin.
As the patient ages, physical growth and hormone changes also alter the demand for insulin. A key factor to keep in mind is lifestyle changes: The child may need age-appropriate supervision and adjustments in exercise, diet, and diabetes education regimens when school routines and self-care capacities change. The child with T1D can only be educated as far as his or her cognitive ability will allow, but autonomy should increase with age.
Helping the patient reach glycemic goals requires special consideration, based on the child’s age. Whereas the target A1C for an adult with diabetes is below 7%, that for a young child is either < 7.5% or < 8.5%, depending on age (see Table 4).9 According to Danne et al, approximately 60% of children younger than 6 years have an imperfect awareness of hypoglycemia. 10 Because the risk for a hypoglycemic event is increased in this age-group, their target A1C is higher.10
This is also an important age for brain development: The metabolism of glucose in the brain of a young child occurs at double the rate of that in an adult brain.11 Between ages 1 and 6 years, the brain increases in size dramatically, reaching 90% of its adult volume by age 6.11 In retrospective studies reviewed by Arbelaez et al,data show that frequent, severe hypoglycemic and hyperglycemic events are associated with poor cognitive function, particularly memory and attention.11 Due to the timing of brain development and the risk for glycemic extremes in young children, practitioners are advised to follow the ADA recommendations shown in Table 4.9
Continue for the conclusion >>
CONCLUSION
T1D is the most common chronic, serious, potentially life-threatening disease among children and adolescents. This lifelong illness is challenging to control, especially when managing the honeymoon period and addressing the increasing insulin demands in a growing child. Once a diagnosis is confirmed, the challenges persist, as each patient needs an individualized treatment regimen with ongoing adjustments. Knowledge of the ADA guidelines for age-appropriate A1C goals is essential for the practitioner who manages a growing child with T1D in order to achieve glycemic control while avoiding hypoglycemia. Preventing hypoglycemia is of the utmost importance, especially in a child too young to recognize symptoms.
Considering all the changes that a child with T1D is likely to experience, it is also important to remember that the foremost goal is for this child to live a healthy life. Thus, practitioners must educate both patients and parents regarding the complications that can arise with poor glycemic control and encourage adherence to the insulin therapy.
T1D requires vigilant monitoring and ongoing adjusted insulin therapy. Understanding age-appropriate treatment and maintaining good communications with patients and their parents are key to successful management of this disease.
REFERENCES
1. Juvenile Diabetes Research Foundation. Type 1 diabetes facts (2014). http://jdrf.org/about-jdrf/fact-sheets/type-1-diabetes-facts/. Accessed February 8, 2016.
2. American Diabetes Association. Fast facts: data and statistics about diabetes (2015). http://professional2.diabetes.org/admin/UserFiles/0%20-%20Sean/Documents/Fast_Facts_12-2015a.pdf. Accessed February 8, 2016.
3. American Diabetes Association. Executive summary: standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S5-S13.
4. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(1):81-90.
5. Pavlovic MD, Milenkovic T, Dinic M, et al. The prevalence of cutaneous manifestations in young patients with type 1 diabetes. Diabetes Care. 2007;30(8):1964-1967.
6. Khardori R. Type 1 diabetes mellitus differential diagnosis (updated 2015). http://emedicine.medscape.com/article/117739-differential. Accessed February 8, 2016.
7. Lamb WH. Pediatric type 1 diabetes mellitus (updated 2015). http://emedicine.medscape.com/article/919999-overview. Accessed February 8, 2016.
8. Type 1 Diabetes TrialNet. TrialNet Pathway to Prevention (2014). www.pathway2prevention.org/study/. Accessed February 8, 2016.
9. American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(suppl 1):S11-S66. VIII. Diabetes care in specific populations. http://care.diabetesjournals.org/content/36/Supplement_1/S11.full#sec-128. Accessed February 8, 2016.
10. Danne T, Philotheou A, Goldman D, et al. A randomized trial comparing the rate of hypoglycemia—assessed using continuous glucose monitoring—in 125 preschool children with type 1 diabetes treated with insulin glargine or NPH insulin (the PRESCHOOL study). Pediatr Diabetes. 2013;14(8):593-601.
11. Arbelaez AM, Semenkovich K, Hershey T. Glycemic extremes in youth with T1DM: the structural and functional integrity of the developing brain. Pediatr Diabetes. 2013;14(8):541-553.
IN THIS ARTICLE
- Progress and treatment timeline with long- and rapid-acting insulin
- Progress and treatment timeline with continuous subcutaneous insulin infusion
- American Diabetes Association criteria for diagnosis of diabetes
- Blood glucose and A1C goals for type 1 diabetes by age-group
A 5-year-old Caucasian girl presents to the primary care practitioner’s office with chief complaints of polydipsia, polyuria with nocturia, polyphagia, and weight loss over the past three weeks. Her medical history includes a four-year history of keratosis pilaris (KP). The child experienced a KP flare-up two weeks ago; application of triamcinolone acetonide cream yielded no improvement. She also has xerosis, which is treated daily with OTC moisturizing lotion. She was born vaginally and breast-fed and is up to date on her immunizations. There is no family history of diabetes or autoimmune diseases.
Physical examination reveals a weight of 54 lb (95th percentile); height, 47 in (97th percentile); and BMI, 17.2. Vital signs include a blood pressure of 105/55 mm Hg; pulse, 85 beats/min and regular; temperature, 98.2°F; and respiratory rate, 22 breaths/min. KP is noted on the patient’s eyebrows, bilateral upper arms, and bilateral cheeks; the affected skin is erythemic and rough to the touch. Her physical examination findings are otherwise unremarkable.
The child’s urine is tested in the office for glucose and ketones, with results of 4+ glucose and 3+ ketones. These results and the child’s history prompt her admission to the pediatric ICU at a nearby hospital for further treatment with a diagnosis of new-onset type 1 diabetes (T1D) and diabetic ketoacidosis (DKA).
The diagnosis is confirmed at the hospital with laboratory results that include venous glucose, 418 mg/dL (normal range, 70 to 100 mg/dL) and A1C, 10.5% (range, 4.0% to 5.6%). Venous blood gas results include pH, 7.278 (7.32 to 7.42); PCO2, 29.6 mm Hg (39 to 54 mm Hg); HCO3, 13.8 mEq/L (19 to 25 mEq/L); base excess, –12 mmol/L (–4 to +2 mmol/L); beta hydroxybutyrate, 6.0 mmol/L (0.4 to 0.5 mmol/L); insulin antibody, 0.9 U/mL (< 0.4 U/mL); glutamic acid decarboxylase, 166 U/mL (< 0.5 U/mL); and venous lactate, 1.79 mmol/L (0.69 to 2.75 mmol/L).
The child is treated initially with an IV insulin infusion for 24 hours, then transitioned to subcutaneous insulin therapy once the DKA resolves and glucose levels are within normal limits. The child remains hospitalized for four days. Discharge medications include insulin glargine, 8 U/d, and insulin lispro before each meal, at bedtime, and at 0200 hours, with dosing based on sliding scales. Dietary orders include 45 to 60 g carbohydrates per meal, along with two snacks of 15 g carbohydrates.
The child is instructed to exercise at least 30 min/d (unless hypoglycemic events occur more than once per week or ketones are found in the blood or urine), drink plenty of water, and avoid concentrated sweets. Education is provided via the Diabetes Educator; the family takes home the beginner T1D booklet and is instructed to log the child’s blood glucose levels and return with this information in two weeks.
In the first three months, the patient experiences eight asymptomatic hypoglycemic events; for the next seven months, after dosing changes, she remains hyperglycemic most of the time (see Table 1). Insulin doses are adjusted, ranging from weekly to every three months, but glycemic goals are not achieved with the subcutaneous insulin injections. Use of continuous subcutaneous insulin infusion, the “insulin pump,” is then considered. Ten months postdiagnosis, the child begins a five-day-long saline (placebo) pump trial to determine whether the pump is appropriate for her and her lifestyle. After the trial, the decision is made to move forward with the insulin pump, initiated 11 months postdiagnosis.
The practitioner remains in frequent communication with the child’s mother in an effort to maintain glycemic control. After three months on the insulin pump, the child’s A1C is reduced to 7.9%, which is within the target range for her age-group (see Table 2). The child is now maintaining glycemic goals with the use of the insulin pump and close monitoring by the practitioner.
Continue for the discussion >>
DISCUSSION
According to the Juvenile Diabetes Research Foundation, as many as 1.25 million Americans are currently living with T1D; from 2001 to 2009, the prevalence of T1D in people younger than 20 increased by 23%.1 The overall prevalence of diabetes (both types 1 and 2) is predicted to be one in every three people by 2050 if current trends continue.2 According to the American Diabetes Association (ADA), 18,436 US youths are diagnosed with T1D every year, and T1D accounts for about 5% of diabetes cases in the US population.2
Diagnosis
Diabetes is diagnosed based on blood test results that fall within the parameters set by the ADA diagnostic criteria (see Table 3).3 In addition to diagnostic testing for diabetes recommended by the ADA guidelines, blood tests are ordered for autoantibodies that are associated with T1D, to distinguish between type 1 and type 2 diabetes. (T1D results from cellular-mediated autoimmune destruction of the insulin-producing beta cells in the pancreas.4) Upon initial diagnosis, about 85% to 90% of T1D patients have one or more autoantibodies present in blood work, such as autoantibodies to islet cells or to insulin, glutamic acid decarboxylase (GAD65), or tyrosine phosphatases IA-2 and IA-2B.4
In this case study, the child had an elevated GAD65 value and a positive screening for an insulin autoantibody, which explained the destruction of her beta cells. The patient also had KP and xerosis, which are clinical manifestations commonly seen in T1D. In one study of children with T1D, 22% had xerosis, compared with 3% of healthy, age-matched controls, and KP was also significantly more common in T1D patients than in controls (12% vs 1.5%).5
The presence of ketones in the case patient’s urine also suggests T1D, rather than type 2.4 The differential diagnosis for T1D includes type 2 diabetes mellitus, monogenic diabetes mellitus (formerly known as maturity-onset diabetes of the young), secondary hyperglycemia, and other endocrine disorders.6
Acute complications associated with T1D include hypoglycemia, hyperglycemia, and DKA. Long-term complications may include diabetic retinopathy, cataracts, gastroparesis, hypertension, renal failure, coronary artery disease, peripheral vascular disease, diabetic neuropathy, and increased risk for infection.7 These complications can likely be prevented by good glycemic control, proper diet, exercise, and avoidance of nicotine.7
Unfortunately, T1D cannot currently be prevented, although research studies are under way. TrialNet is currently conducting a “Pathway to Prevention” trial; the researchers are testing ways to delay and prevent T1D, as well as slow its progression after diagnosis.8 Potential participants (family members of a T1D patient) are screened for T1D autoantibodies. If test results are positive, these participants are included in the prevention pathway study.
Continue for management >>
Management
Most cases of T1D are diagnosed in patients younger than 18.9 Management of the child with T1D involves many challenges. The patient will experience an initial honeymoon period, that is, a brief remission during which the pancreas begins to secrete some insulin again and exogenous insulin demands are lower. However, this period is temporary, lasting only a few weeks, months, or years. Once pancreatic insulin secretion stops (as a result of complete beta-cell destruction), the exogenous insulin demands increase. In the case study, the child’s insulin demands were initially low, and she experienced hypoglycemia. Once she transitioned out of the honeymoon period, however, her blood glucose levels rose because her pancreas was producing little to no insulin.
As the patient ages, physical growth and hormone changes also alter the demand for insulin. A key factor to keep in mind is lifestyle changes: The child may need age-appropriate supervision and adjustments in exercise, diet, and diabetes education regimens when school routines and self-care capacities change. The child with T1D can only be educated as far as his or her cognitive ability will allow, but autonomy should increase with age.
Helping the patient reach glycemic goals requires special consideration, based on the child’s age. Whereas the target A1C for an adult with diabetes is below 7%, that for a young child is either < 7.5% or < 8.5%, depending on age (see Table 4).9 According to Danne et al, approximately 60% of children younger than 6 years have an imperfect awareness of hypoglycemia. 10 Because the risk for a hypoglycemic event is increased in this age-group, their target A1C is higher.10
This is also an important age for brain development: The metabolism of glucose in the brain of a young child occurs at double the rate of that in an adult brain.11 Between ages 1 and 6 years, the brain increases in size dramatically, reaching 90% of its adult volume by age 6.11 In retrospective studies reviewed by Arbelaez et al,data show that frequent, severe hypoglycemic and hyperglycemic events are associated with poor cognitive function, particularly memory and attention.11 Due to the timing of brain development and the risk for glycemic extremes in young children, practitioners are advised to follow the ADA recommendations shown in Table 4.9
Continue for the conclusion >>
CONCLUSION
T1D is the most common chronic, serious, potentially life-threatening disease among children and adolescents. This lifelong illness is challenging to control, especially when managing the honeymoon period and addressing the increasing insulin demands in a growing child. Once a diagnosis is confirmed, the challenges persist, as each patient needs an individualized treatment regimen with ongoing adjustments. Knowledge of the ADA guidelines for age-appropriate A1C goals is essential for the practitioner who manages a growing child with T1D in order to achieve glycemic control while avoiding hypoglycemia. Preventing hypoglycemia is of the utmost importance, especially in a child too young to recognize symptoms.
Considering all the changes that a child with T1D is likely to experience, it is also important to remember that the foremost goal is for this child to live a healthy life. Thus, practitioners must educate both patients and parents regarding the complications that can arise with poor glycemic control and encourage adherence to the insulin therapy.
T1D requires vigilant monitoring and ongoing adjusted insulin therapy. Understanding age-appropriate treatment and maintaining good communications with patients and their parents are key to successful management of this disease.
REFERENCES
1. Juvenile Diabetes Research Foundation. Type 1 diabetes facts (2014). http://jdrf.org/about-jdrf/fact-sheets/type-1-diabetes-facts/. Accessed February 8, 2016.
2. American Diabetes Association. Fast facts: data and statistics about diabetes (2015). http://professional2.diabetes.org/admin/UserFiles/0%20-%20Sean/Documents/Fast_Facts_12-2015a.pdf. Accessed February 8, 2016.
3. American Diabetes Association. Executive summary: standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S5-S13.
4. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(1):81-90.
5. Pavlovic MD, Milenkovic T, Dinic M, et al. The prevalence of cutaneous manifestations in young patients with type 1 diabetes. Diabetes Care. 2007;30(8):1964-1967.
6. Khardori R. Type 1 diabetes mellitus differential diagnosis (updated 2015). http://emedicine.medscape.com/article/117739-differential. Accessed February 8, 2016.
7. Lamb WH. Pediatric type 1 diabetes mellitus (updated 2015). http://emedicine.medscape.com/article/919999-overview. Accessed February 8, 2016.
8. Type 1 Diabetes TrialNet. TrialNet Pathway to Prevention (2014). www.pathway2prevention.org/study/. Accessed February 8, 2016.
9. American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(suppl 1):S11-S66. VIII. Diabetes care in specific populations. http://care.diabetesjournals.org/content/36/Supplement_1/S11.full#sec-128. Accessed February 8, 2016.
10. Danne T, Philotheou A, Goldman D, et al. A randomized trial comparing the rate of hypoglycemia—assessed using continuous glucose monitoring—in 125 preschool children with type 1 diabetes treated with insulin glargine or NPH insulin (the PRESCHOOL study). Pediatr Diabetes. 2013;14(8):593-601.
11. Arbelaez AM, Semenkovich K, Hershey T. Glycemic extremes in youth with T1DM: the structural and functional integrity of the developing brain. Pediatr Diabetes. 2013;14(8):541-553.
Neurocognitive Deficits and Cerebral Desaturation During Shoulder Arthroscopy With Patient in Beach-Chair Position: A Review of the Current Literature
The beach-chair position (BCP) is commonly used for both arthroscopic and open shoulder surgery. This technique positions the shoulder in an anatomical upright position, facilitating shoulder access and visualization.1 Compared with the lateral decubitus position, the BCP also improves airway access, reduces bleeding, and lessens the risk for brachial plexus injury.2
Despite the advantages of using the BCP, there have been multiple reports of catastrophic neurologic complications, including severe brain damage and death, in relatively healthy patients without any known risk factors.3-6 The definitive etiology of these complications remains unclear, but it has been hypothesized that BCP use may be an independent risk factor for cerebrovascular ischemia,1,5-16 as the upright position can cause hypotension leading to increased risk for cerebral hypoperfusion.7-11,17 Reducing cerebral perfusion pressure below critical thresholds may result in permanent neurologic injury.4-6,14 Therefore, monitoring of cerebral perfusion and optimization of intraoperative cerebral oxygenation have been recommended to help avoid potential neurologic complications. However, a direct relationship between intraoperative cerebral desaturation events (CDEs) and postoperative neurocognitive deficits has not been definitively established.1,9-12
To put into perspective the importance of detecting and preventing CDEs and neurologic complications, we can consider the incidence of fatal pulmonary embolism associated with total joint arthroplasty. Although the incidence is very low, about 0.1% to 2.0%, some form of venous thromboembolism prophylaxis is the standard of care for helping prevent this serious complication. Similarly, catastrophic neurologic complications of upright shoulder arthroscopy are very rare, but it is still important to consider measures that help minimize them.
We reviewed the literature for the incidence of postoperative neurocognitive deficits, number of reported neurocognitive complications, and incidence of intraoperative CDEs in patients who underwent arthroscopic shoulder surgery in the BCP.
Methods
Dr. Salazar and Dr. Hazel independently searched the Medline, Cochrane, and Embase databases for case series, prospective studies, and cohort studies that reported neurocognitive complications associated with the BCP and the incidence of intraoperative CDEs. The authors used beach chair, desaturation, near infrared spectroscopy, and shoulder as medical subject headings (MeSH). In addition, bibliographies of retrieved articles were checked for studies that the search terms may have missed. Eighty-one publications were identified and reviewed for possible inclusion.
Next, the same 2 authors reviewed the titles and abstracts for relevance and determined which articles had potential to contribute to the study. Only English-language publications were considered for inclusion. To review the incidence of postoperative neurocognitive deficits, we included only those studies with more than 25 patients, documentation of postoperative complications, and arthroscopic shoulder surgery performed with the patient in the seated, semi-upright, or BCP. Only studies with at least 25 patients were used in order to increase the power and improve the level of evidence. To review reported cases of neurocognitive complications, we included all relevant case reports and case series. To review the incidence of intraoperative CDEs and investigate their relationship with postoperative neurocognitive deficits, we included studies that reported on use of intraoperative cerebral perfusion monitoring. Modalities used in these studies included near infrared spectroscopy, electroencephalography, and invasive blood pressure monitoring calculated at the brain level. Studies were excluded if they did not involve arthroscopic shoulder surgery or were not conducted with human subjects.
Information recorded for each study included general information such as author and publication year, type of study, number of patients enrolled, type of intraoperative monitoring, anesthesia protocol, number of patients with CDEs, and number of patients with neurocognitive complications after surgery.
Results
Our search identified 81 publications for potential inclusion. Our first aim was to identify the overall incidence of reported neurocognitive deficits after arthroscopic shoulder surgery with the patient in the BCP. We identified 10 studies (Table 1) that met the inclusion criteria. Among the 24,701 patients in these 10 studies, there was only 1 reported case of neurocognitive deficit after surgery, in a mixed prospective-retrospective study of 15,014 cases by Rohrbaugh and colleagues.18 The deficit they reported was an ischemic cerebral vascular accident. The 0.0067% incidence in their study demonstrates how rare the complication is. Two large retrospective studies (Ns = 4169 and 5177 patients) found no postoperative neurocognitive complications.19,20 Only 3 studies performed formal postoperative cognitive testing. Salazar and colleagues21 used the Repeatable Battery for the Assessment of Neuropsychological Status before and after surgery, and Gillespie and colleagues8 and Lee and colleagues10 used the Mini–Mental State Examination before and after surgery. Total incidence of reported neurocognitive deficits from our review was 0.004% (1/24,701).
Our second aim was to review all reported cases of neurocognitive complications after arthroscopic shoulder surgery with the patient in the BCP. We identified 4 publications that fit our inclusion criteria (Table 2). Pohl and Cullen6 described 4 cases of ischemic brain injury after arthroscopic shoulder surgery with the patient in the BCP. Age range was 47 to 57 years. Specific intraoperative cerebral monitoring was not used. However, these patients had several episodes of intraoperative hypotension (systolic blood pressures, 80-90 mm Hg), measured with a traditional blood pressure cuff on the arm. In general, these patients had minimal cerebrovascular risk factors and no known preexisting cerebrovascular disease. Drummond and colleagues22 described an ischemic stroke in a 50-year-old man after arthroscopic subacromial decompression and open rotator cuff repair that resulted in unresolved right hemiplegia. Subsequent diagnostic investigation revealed an asymmetry of the circle of Willis resulting in limited flow to the left anterior and middle cerebral artery distributions. Bhatti and Enneking3 reported the case of a 64-year-old man who lost vision in the right eye immediately after arthroscopic rotator cuff repair. His vision improved spontaneously the next morning and continued to improve over the next 6 months—he regained 20/20 vision with some residual optic neuropathy.
Our third aim was to determine the incidence of intraoperative CDEs during arthroscopic shoulder surgery with the patient in the BCP. Incidence of CDEs varied widely among the 7 studies reviewed (Table 3). Minimum incidence of intraoperative CDE was 0% in a cohort of 30 patients,1 and maximum incidence was 80% in a study of 61 patients,12 all of whom underwent elective arthroscopic shoulder surgery in the BCP. Although there was wide variability in CDE incidence, the studies were consistent with respect to their definition of a CDE. Most authors used a decrease in regional cerebral tissue oxygen saturation of 20% or more from baseline, or an absolute value up to 55%, to define a CDE. None of the 7 studies reviewed reported a clinically significant adverse neurocognitive event.
Discussion
Of concern, there have been several surveys, case reports, and small case series of previously healthy patients who had no known risk factors, underwent arthroscopic shoulder surgery in the BCP, and developed unanticipated postoperative neurologic complications.4-6,14 Beach-chair positioning during surgical procedures has been implicated as a contributing factor leading to cerebral hypoperfusion with potential for cerebral ischemia.1,12,23 These changes in cerebral perfusion pressure are thought to be the major determinant of poor neurologic outcomes. Such reports have exposed the potential need for heightened vigilance, alternative anesthesia techniques, and improved monitoring, though the exact etiology of the central nervous system injuries in this patient population is incompletely understood and is likely multifactorial. Therefore, in this study we wanted to determine the incidence of postoperative neurocognitive deficits and review all reported cases of neurocognitive complications in patients who have undergone arthroscopic shoulder surgery in the BCP. In addition, we wanted to define the incidence of intraoperative CDEs and investigate their relationship with postoperative neurocognitive complications.
According to our review, the incidence of postoperative neurocognitive complications after surgery in the BCP is 0.004% (1/24,701). However, this finding is based only on what has been reported; the true incidence is not known. It is also important to note that the incidence of neurocognitive deficits after many other types of surgery is not known and that surgery itself may be a risk factor for postoperative neurocognitive deficits.24 In their retrospective review of 15,014 patients who underwent arthroscopic shoulder surgery in the BCP at a single institution over an 11-year period, Rohrbaugh and colleagues18 found an overall postoperative complication rate of 0.37% and a 0.0067% incidence of neurocognitive deficits. One patient in the series was given a diagnosis of ischemic stroke on the basis of neurologic deficits that occurred 24 hours after surgery. Yadeau and colleagues20 found no postoperative neurocognitive complications in a mixed prospective-retrospective study of 4169 patients—3000 identified retrospectively, 1169 prospectively—who underwent arthroscopic shoulder surgery in the BCP at an ambulatory surgery center. Pin-on and colleagues19 reported on a series of 5177 orthopedic and neurosurgical patients who underwent surgery in the BCP. In those who had arthroscopic shoulder surgery, intraoperative systolic blood pressures obtained from an arterial line referenced to heart level decreased a mean (SD) of 14.4% (12.7%), whereas in those whose pressures were obtained from a noninvasive blood pressure cuff referenced to heart level decreased 19.3% (12.6%). However, the authors reported no incidence of postoperative stroke or neurologic deficits.
Although uncommon, perioperative cerebral ischemic accidents are potentially devastating for patients, their families, and the health care professionals involved. These events have tremendous economic, social, professional, and medicolegal implications, with perioperative stroke being particularly morbid. Perioperative stroke has a mortality rate of 60%, versus 15% to 46% for stroke in general.25,26 In 2005, Pohl and Cullen6 published a landmark article on a series of 4 relatively healthy middle-aged patients who were at low risk for stroke but had catastrophic neurocognitive complications (including 1 death) after arthroscopic shoulder surgery in the BCP. Bhatti and Enneking3 described a case of acute postoperative vision loss and ophthalmoplegia attributed to intraoperative hypotension leading to ischemia in a patient who underwent an elective shoulder arthroscopic procedure in the BCP. These reports prompted multiple investigations into the physiologic hemodynamic changes associated with surgery in the BCP and the treatment strategies used to improve patient safety.
In the normal physiologic state, the sympathetic nervous system is activated when a person assumes the seated position. The result is increased systemic vascular resistance and heart rate alterations to maintain cardiac output and mean arterial pressure. In anesthetized patients, this response is blunted by the vasodilatory effects of intravenous and volatile anesthetics. Multiple studies have demonstrated substantial hemodynamic changes in both awake and anesthetized patients during the maneuver from the supine position to the seated position1,27,28; these changes include diminished cardiac index, stroke volume, and arterial pressure.17 The data underscore the need for attentiveness and accurate monitoring of cerebral perfusion when the transition is made from the supine position to the BCP, particularly in the early phase of surgery and in high-risk patients.
Knowledge of these hemodynamic changes has led several authors to recommend additional intraoperative monitoring of cerebral perfusion. Monitoring techniques have included use of invasive blood pressure monitoring adjusted to brain level, cerebral oximetry using near infrared spectroscopy, and electroencephalography. However, the clinical relevance of intraoperative CDEs in isolation is not well understood.1,6,7,23 In addition, cost and availability of additional advanced monitoring likely factor into why it is not more commonly used. For this patient population, the severity, frequency, and duration of desaturation that causes cerebral ischemia and the relationship with postoperative neurocognitive deficits remain undefined.
The incidence of CDEs in patients being monitored with near infrared spectroscopy while undergoing elective arthroscopic shoulder surgery in the BCP varies widely, from 0% to 80% (mean, 41%).1,4,7,10,12,21 Magnitude and duration of cerebral ischemia required to produce neurocognitive dysfunction in this patient population remain unidentified as well. In conscious patients, a 20% reduction in frontal lobe oxygenation is associated with clinical manifestations of cerebral hypoperfusion, such as syncope.15,29 As none of the patients in the studies we reviewed experienced any sort of deficit, we cannot definitively state there is a correlation between CDE occurrence and neurocognitive deficit.
One limitation of our investigation is that it was a systemic review, and thus there was substantial heterogeneity in the methods and designs of the studies included in the analysis. Among the different series, there was variability in multiple aspects of the study design, including type of anesthetic, patient inclusion criteria, type of surgery, type of intraoperative cerebral perfusion monitoring, and type of neurocognitive testing. As a result, comparing the groups was difficult, and the generalizability of our findings may be limited. In addition, it is difficult to accurately establish incidence and comprehensively review these events because of the paucity of literature.
Conclusion
Neurocognitive complications after shoulder arthroscopy with the patient in the BCP are extremely rare but potentially devastating events that can affect healthy patients with no preexisting cerebrovascular risk factors. Our review indicated the incidence of permanent neurologic deficit after arthroscopy in the BCP may be as low as 0.004%. The exact etiology of such complications is not clear. Basic science research and large prospective studies are needed to identify the clinically relevant thresholds of magnitude, duration, and frequency of intraoperative CDEs in order to establish their relationship with postoperative neurocognitive complications. Such large studies may also elucidate modifiable patient-specific risk factors and establish the most sensitive, safe, and cost-effective intraoperative monitoring tools. Current literature suggests that accurate intraoperative monitoring of cerebral perfusion, alternatives to general anesthesia, and prudent use of intraoperative blood pressure control may improve patient safety.
1. Tange K, Kinoshita H, Minonishi T, et al. Cerebral oxygenation in the beach chair position before and during general anesthesia. Minerva Anestesiol. 2010;76(7):485-490.
2. Skyhar MJ, Altchek DW, Warren RF, Wickiewicz TL, O’Brien SJ. Shoulder arthroscopy with the patient in the beach-chair position. Arthroscopy. 1988;4(4):256-259.
3. Bhatti MT, Enneking FK. Visual loss and ophthalmoplegia after shoulder surgery. Anesth Analg. 2003;96(3):899-902.
4. Friedman DJ, Parnes NZ, Zimmer Z, Higgins LD, Warner JJ. Prevalence of cerebrovascular events during shoulder surgery and association with patient position. Orthopedics. 2009;32(4).
5. Papadonikolakis A, Wiesler ER, Olympio MA, Poehling GG. Avoiding catastrophic complications of stroke and death related to shoulder surgery in the sitting position. Arthroscopy. 2008;24(4):481-482.
6. Pohl A, Cullen DJ. Cerebral ischemia during shoulder surgery in the upright position: a case series. J Clin Anesth. 2005;17(6):463-469.
7. Dippmann C, Winge S, Nielsen HB. Severe cerebral desaturation during shoulder arthroscopy in the beach-chair position. Arthroscopy. 2010;26(9 suppl):S148-S150.
8. Gillespie R, Shishani Y, Streit J, et al. The safety of controlled hypotension for shoulder arthroscopy in the beach-chair position. J Bone Joint Surg Am. 2012;94(14):1284-1290.
9. Jeong H, Lee SH, Jang EA, Chung SS, Lee J, Yoo KY. Haemodynamics and cerebral oxygenation during arthroscopic shoulder surgery in beach chair position under general anaesthesia. Acta Anaesthesiol Scand. 2012;56(7):872-879.
10. Lee JH, Min KT, Chun YM, Kim EJ, Choi SH. Effects of beach-chair position and induced hypotension on cerebral oxygen saturation in patients undergoing arthroscopic shoulder surgery. Arthroscopy. 2011;27(7):889-894.
11. Moerman AT, De Hert SG, Jacobs TF, De Wilde LF, Wouters PF. Cerebral oxygen desaturation during beach chair position. Eur J Anaesthesiol. 2012;29(2):82-87.
12. Murphy GS, Szokol JW, Marymont JH, et al. Cerebral oxygen desaturation events assessed by near-infrared spectroscopy during shoulder arthroscopy in the beach chair and lateral decubitus positions. Anesth Analg. 2010;111(2):496-505.
13. Peruto CM, Ciccotti MG, Cohen SB. Shoulder arthroscopy positioning: lateral decubitus versus beach chair. Arthroscopy. 2009;25(8):891-896.
14. Rains DD, Rooke GA, Wahl CJ. Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthroscopy. 2011;27(4):532-541.
15. Samra SK, Dy EA, Welch K, Dorje P, Zelenock GB, Stanley JC. Evaluation of a cerebral oximeter as a monitor of cerebral ischemia during carotid endarterectomy. Anesthesiology. 2000;93(4):964-970.
16. Smythe PR, Samra SK. Monitors of cerebral oxygenation. Anesthesiol Clin North Am. 2002;20(2):293-313.
17. Smith JJ, Porth CM, Erickson M. Hemodynamic response to the upright posture. J Clin Pharmacol. 1994;34(5):375-386.
18. Rohrbaugh M, Kentor ML, Orebaugh SL, Williams B. Outcomes of shoulder surgery in the sitting position with interscalene nerve block: a single-center series. Reg Anesth Pain Med. 2013;38(1):28-33.
19. Pin-on P, Schroeder D, Munis J. The hemodynamic management of 5177 neurosurgical and orthopedic patients who underwent surgery in the sitting or “beach chair” position without incidence of adverse neurologic events. Anesth Analg. 2013;116(6):1317-1324.
20. Yadeau JT, Casciano M, Liu SS, et al. Stroke, regional anesthesia in the sitting position, and hypotension: a review of 4169 ambulatory surgery patients. Reg Anesth Pain Med. 2011;36(5):430-435.
21. Salazar D, Sears BW, Aghdasi B, et al. Cerebral desaturation events during shoulder arthroscopy in the beach chair position: patient risk factors and neurocognitive effects. J Shoulder Elbow Surg. 2013;22(9):1228-1235.
22. Drummond JC, Lee RR, Howell JP Jr. Focal cerebral ischemia after surgery in the “beach chair” position: the role of a congenital variation of circle of Willis anatomy. Anesth Analg. 2012;114(6):1301-1303.
23. Fischer GW, Torrillo TM, Weiner MM, Rosenblatt MA. The use of cerebral oximetry as a monitor of the adequacy of cerebral perfusion in a patient undergoing shoulder surgery in the beach chair position. Pain Pract. 2009;9(4):304-307.
24. Wong GY, Warner DO, Schroeder DR, et al. Risk of surgery and anesthesia for ischemic stroke. Anesthesiology. 2000;92(2):425-432.
25. Knapp RB, Topkins MJ, Artusio JF Jr. The cerebrovascular accident and coronary occlusion in anesthesia. JAMA. 1962;182:332-334.
26. Landercasper J, Merz BJ, Cogbill TH, et al. Perioperative stroke risk in 173 consecutive patients with a past history of stroke. Arch Surg. 1990;125(8):986-989.
27. Fuchs G, Schwarz G, Kulier A, Litscher G. The influence of positioning on spectroscopic measurements of brain oxygenation. J Neurosurg Anesthesiol. 2000;12(2):75-80.
28. Lovell AT, Owen-Reece H, Elwell CE, Smith M, Goldstone JC. Continuous measurement of cerebral oxygenation by near infrared spectroscopy during induction of anesthesia. Anesth Analg. 1999;88(3):554-558.
29. Madsen PL, Secher NH. Near-infrared oximetry of the brain. Prog Neurobiol. 1999;58(6):541-560.
30. Koh JL, Levin SD, Chehab EL, Murphy GS. Neer award 2012: cerebral oxygenation in the beach chair position: a prospective study on the effect of general anesthesia compared with regional anesthesia and sedation. J Shoulder Elbow Surg. 2013;22:1325-1331.
The beach-chair position (BCP) is commonly used for both arthroscopic and open shoulder surgery. This technique positions the shoulder in an anatomical upright position, facilitating shoulder access and visualization.1 Compared with the lateral decubitus position, the BCP also improves airway access, reduces bleeding, and lessens the risk for brachial plexus injury.2
Despite the advantages of using the BCP, there have been multiple reports of catastrophic neurologic complications, including severe brain damage and death, in relatively healthy patients without any known risk factors.3-6 The definitive etiology of these complications remains unclear, but it has been hypothesized that BCP use may be an independent risk factor for cerebrovascular ischemia,1,5-16 as the upright position can cause hypotension leading to increased risk for cerebral hypoperfusion.7-11,17 Reducing cerebral perfusion pressure below critical thresholds may result in permanent neurologic injury.4-6,14 Therefore, monitoring of cerebral perfusion and optimization of intraoperative cerebral oxygenation have been recommended to help avoid potential neurologic complications. However, a direct relationship between intraoperative cerebral desaturation events (CDEs) and postoperative neurocognitive deficits has not been definitively established.1,9-12
To put into perspective the importance of detecting and preventing CDEs and neurologic complications, we can consider the incidence of fatal pulmonary embolism associated with total joint arthroplasty. Although the incidence is very low, about 0.1% to 2.0%, some form of venous thromboembolism prophylaxis is the standard of care for helping prevent this serious complication. Similarly, catastrophic neurologic complications of upright shoulder arthroscopy are very rare, but it is still important to consider measures that help minimize them.
We reviewed the literature for the incidence of postoperative neurocognitive deficits, number of reported neurocognitive complications, and incidence of intraoperative CDEs in patients who underwent arthroscopic shoulder surgery in the BCP.
Methods
Dr. Salazar and Dr. Hazel independently searched the Medline, Cochrane, and Embase databases for case series, prospective studies, and cohort studies that reported neurocognitive complications associated with the BCP and the incidence of intraoperative CDEs. The authors used beach chair, desaturation, near infrared spectroscopy, and shoulder as medical subject headings (MeSH). In addition, bibliographies of retrieved articles were checked for studies that the search terms may have missed. Eighty-one publications were identified and reviewed for possible inclusion.
Next, the same 2 authors reviewed the titles and abstracts for relevance and determined which articles had potential to contribute to the study. Only English-language publications were considered for inclusion. To review the incidence of postoperative neurocognitive deficits, we included only those studies with more than 25 patients, documentation of postoperative complications, and arthroscopic shoulder surgery performed with the patient in the seated, semi-upright, or BCP. Only studies with at least 25 patients were used in order to increase the power and improve the level of evidence. To review reported cases of neurocognitive complications, we included all relevant case reports and case series. To review the incidence of intraoperative CDEs and investigate their relationship with postoperative neurocognitive deficits, we included studies that reported on use of intraoperative cerebral perfusion monitoring. Modalities used in these studies included near infrared spectroscopy, electroencephalography, and invasive blood pressure monitoring calculated at the brain level. Studies were excluded if they did not involve arthroscopic shoulder surgery or were not conducted with human subjects.
Information recorded for each study included general information such as author and publication year, type of study, number of patients enrolled, type of intraoperative monitoring, anesthesia protocol, number of patients with CDEs, and number of patients with neurocognitive complications after surgery.
Results
Our search identified 81 publications for potential inclusion. Our first aim was to identify the overall incidence of reported neurocognitive deficits after arthroscopic shoulder surgery with the patient in the BCP. We identified 10 studies (Table 1) that met the inclusion criteria. Among the 24,701 patients in these 10 studies, there was only 1 reported case of neurocognitive deficit after surgery, in a mixed prospective-retrospective study of 15,014 cases by Rohrbaugh and colleagues.18 The deficit they reported was an ischemic cerebral vascular accident. The 0.0067% incidence in their study demonstrates how rare the complication is. Two large retrospective studies (Ns = 4169 and 5177 patients) found no postoperative neurocognitive complications.19,20 Only 3 studies performed formal postoperative cognitive testing. Salazar and colleagues21 used the Repeatable Battery for the Assessment of Neuropsychological Status before and after surgery, and Gillespie and colleagues8 and Lee and colleagues10 used the Mini–Mental State Examination before and after surgery. Total incidence of reported neurocognitive deficits from our review was 0.004% (1/24,701).
Our second aim was to review all reported cases of neurocognitive complications after arthroscopic shoulder surgery with the patient in the BCP. We identified 4 publications that fit our inclusion criteria (Table 2). Pohl and Cullen6 described 4 cases of ischemic brain injury after arthroscopic shoulder surgery with the patient in the BCP. Age range was 47 to 57 years. Specific intraoperative cerebral monitoring was not used. However, these patients had several episodes of intraoperative hypotension (systolic blood pressures, 80-90 mm Hg), measured with a traditional blood pressure cuff on the arm. In general, these patients had minimal cerebrovascular risk factors and no known preexisting cerebrovascular disease. Drummond and colleagues22 described an ischemic stroke in a 50-year-old man after arthroscopic subacromial decompression and open rotator cuff repair that resulted in unresolved right hemiplegia. Subsequent diagnostic investigation revealed an asymmetry of the circle of Willis resulting in limited flow to the left anterior and middle cerebral artery distributions. Bhatti and Enneking3 reported the case of a 64-year-old man who lost vision in the right eye immediately after arthroscopic rotator cuff repair. His vision improved spontaneously the next morning and continued to improve over the next 6 months—he regained 20/20 vision with some residual optic neuropathy.
Our third aim was to determine the incidence of intraoperative CDEs during arthroscopic shoulder surgery with the patient in the BCP. Incidence of CDEs varied widely among the 7 studies reviewed (Table 3). Minimum incidence of intraoperative CDE was 0% in a cohort of 30 patients,1 and maximum incidence was 80% in a study of 61 patients,12 all of whom underwent elective arthroscopic shoulder surgery in the BCP. Although there was wide variability in CDE incidence, the studies were consistent with respect to their definition of a CDE. Most authors used a decrease in regional cerebral tissue oxygen saturation of 20% or more from baseline, or an absolute value up to 55%, to define a CDE. None of the 7 studies reviewed reported a clinically significant adverse neurocognitive event.
Discussion
Of concern, there have been several surveys, case reports, and small case series of previously healthy patients who had no known risk factors, underwent arthroscopic shoulder surgery in the BCP, and developed unanticipated postoperative neurologic complications.4-6,14 Beach-chair positioning during surgical procedures has been implicated as a contributing factor leading to cerebral hypoperfusion with potential for cerebral ischemia.1,12,23 These changes in cerebral perfusion pressure are thought to be the major determinant of poor neurologic outcomes. Such reports have exposed the potential need for heightened vigilance, alternative anesthesia techniques, and improved monitoring, though the exact etiology of the central nervous system injuries in this patient population is incompletely understood and is likely multifactorial. Therefore, in this study we wanted to determine the incidence of postoperative neurocognitive deficits and review all reported cases of neurocognitive complications in patients who have undergone arthroscopic shoulder surgery in the BCP. In addition, we wanted to define the incidence of intraoperative CDEs and investigate their relationship with postoperative neurocognitive complications.
According to our review, the incidence of postoperative neurocognitive complications after surgery in the BCP is 0.004% (1/24,701). However, this finding is based only on what has been reported; the true incidence is not known. It is also important to note that the incidence of neurocognitive deficits after many other types of surgery is not known and that surgery itself may be a risk factor for postoperative neurocognitive deficits.24 In their retrospective review of 15,014 patients who underwent arthroscopic shoulder surgery in the BCP at a single institution over an 11-year period, Rohrbaugh and colleagues18 found an overall postoperative complication rate of 0.37% and a 0.0067% incidence of neurocognitive deficits. One patient in the series was given a diagnosis of ischemic stroke on the basis of neurologic deficits that occurred 24 hours after surgery. Yadeau and colleagues20 found no postoperative neurocognitive complications in a mixed prospective-retrospective study of 4169 patients—3000 identified retrospectively, 1169 prospectively—who underwent arthroscopic shoulder surgery in the BCP at an ambulatory surgery center. Pin-on and colleagues19 reported on a series of 5177 orthopedic and neurosurgical patients who underwent surgery in the BCP. In those who had arthroscopic shoulder surgery, intraoperative systolic blood pressures obtained from an arterial line referenced to heart level decreased a mean (SD) of 14.4% (12.7%), whereas in those whose pressures were obtained from a noninvasive blood pressure cuff referenced to heart level decreased 19.3% (12.6%). However, the authors reported no incidence of postoperative stroke or neurologic deficits.
Although uncommon, perioperative cerebral ischemic accidents are potentially devastating for patients, their families, and the health care professionals involved. These events have tremendous economic, social, professional, and medicolegal implications, with perioperative stroke being particularly morbid. Perioperative stroke has a mortality rate of 60%, versus 15% to 46% for stroke in general.25,26 In 2005, Pohl and Cullen6 published a landmark article on a series of 4 relatively healthy middle-aged patients who were at low risk for stroke but had catastrophic neurocognitive complications (including 1 death) after arthroscopic shoulder surgery in the BCP. Bhatti and Enneking3 described a case of acute postoperative vision loss and ophthalmoplegia attributed to intraoperative hypotension leading to ischemia in a patient who underwent an elective shoulder arthroscopic procedure in the BCP. These reports prompted multiple investigations into the physiologic hemodynamic changes associated with surgery in the BCP and the treatment strategies used to improve patient safety.
In the normal physiologic state, the sympathetic nervous system is activated when a person assumes the seated position. The result is increased systemic vascular resistance and heart rate alterations to maintain cardiac output and mean arterial pressure. In anesthetized patients, this response is blunted by the vasodilatory effects of intravenous and volatile anesthetics. Multiple studies have demonstrated substantial hemodynamic changes in both awake and anesthetized patients during the maneuver from the supine position to the seated position1,27,28; these changes include diminished cardiac index, stroke volume, and arterial pressure.17 The data underscore the need for attentiveness and accurate monitoring of cerebral perfusion when the transition is made from the supine position to the BCP, particularly in the early phase of surgery and in high-risk patients.
Knowledge of these hemodynamic changes has led several authors to recommend additional intraoperative monitoring of cerebral perfusion. Monitoring techniques have included use of invasive blood pressure monitoring adjusted to brain level, cerebral oximetry using near infrared spectroscopy, and electroencephalography. However, the clinical relevance of intraoperative CDEs in isolation is not well understood.1,6,7,23 In addition, cost and availability of additional advanced monitoring likely factor into why it is not more commonly used. For this patient population, the severity, frequency, and duration of desaturation that causes cerebral ischemia and the relationship with postoperative neurocognitive deficits remain undefined.
The incidence of CDEs in patients being monitored with near infrared spectroscopy while undergoing elective arthroscopic shoulder surgery in the BCP varies widely, from 0% to 80% (mean, 41%).1,4,7,10,12,21 Magnitude and duration of cerebral ischemia required to produce neurocognitive dysfunction in this patient population remain unidentified as well. In conscious patients, a 20% reduction in frontal lobe oxygenation is associated with clinical manifestations of cerebral hypoperfusion, such as syncope.15,29 As none of the patients in the studies we reviewed experienced any sort of deficit, we cannot definitively state there is a correlation between CDE occurrence and neurocognitive deficit.
One limitation of our investigation is that it was a systemic review, and thus there was substantial heterogeneity in the methods and designs of the studies included in the analysis. Among the different series, there was variability in multiple aspects of the study design, including type of anesthetic, patient inclusion criteria, type of surgery, type of intraoperative cerebral perfusion monitoring, and type of neurocognitive testing. As a result, comparing the groups was difficult, and the generalizability of our findings may be limited. In addition, it is difficult to accurately establish incidence and comprehensively review these events because of the paucity of literature.
Conclusion
Neurocognitive complications after shoulder arthroscopy with the patient in the BCP are extremely rare but potentially devastating events that can affect healthy patients with no preexisting cerebrovascular risk factors. Our review indicated the incidence of permanent neurologic deficit after arthroscopy in the BCP may be as low as 0.004%. The exact etiology of such complications is not clear. Basic science research and large prospective studies are needed to identify the clinically relevant thresholds of magnitude, duration, and frequency of intraoperative CDEs in order to establish their relationship with postoperative neurocognitive complications. Such large studies may also elucidate modifiable patient-specific risk factors and establish the most sensitive, safe, and cost-effective intraoperative monitoring tools. Current literature suggests that accurate intraoperative monitoring of cerebral perfusion, alternatives to general anesthesia, and prudent use of intraoperative blood pressure control may improve patient safety.
The beach-chair position (BCP) is commonly used for both arthroscopic and open shoulder surgery. This technique positions the shoulder in an anatomical upright position, facilitating shoulder access and visualization.1 Compared with the lateral decubitus position, the BCP also improves airway access, reduces bleeding, and lessens the risk for brachial plexus injury.2
Despite the advantages of using the BCP, there have been multiple reports of catastrophic neurologic complications, including severe brain damage and death, in relatively healthy patients without any known risk factors.3-6 The definitive etiology of these complications remains unclear, but it has been hypothesized that BCP use may be an independent risk factor for cerebrovascular ischemia,1,5-16 as the upright position can cause hypotension leading to increased risk for cerebral hypoperfusion.7-11,17 Reducing cerebral perfusion pressure below critical thresholds may result in permanent neurologic injury.4-6,14 Therefore, monitoring of cerebral perfusion and optimization of intraoperative cerebral oxygenation have been recommended to help avoid potential neurologic complications. However, a direct relationship between intraoperative cerebral desaturation events (CDEs) and postoperative neurocognitive deficits has not been definitively established.1,9-12
To put into perspective the importance of detecting and preventing CDEs and neurologic complications, we can consider the incidence of fatal pulmonary embolism associated with total joint arthroplasty. Although the incidence is very low, about 0.1% to 2.0%, some form of venous thromboembolism prophylaxis is the standard of care for helping prevent this serious complication. Similarly, catastrophic neurologic complications of upright shoulder arthroscopy are very rare, but it is still important to consider measures that help minimize them.
We reviewed the literature for the incidence of postoperative neurocognitive deficits, number of reported neurocognitive complications, and incidence of intraoperative CDEs in patients who underwent arthroscopic shoulder surgery in the BCP.
Methods
Dr. Salazar and Dr. Hazel independently searched the Medline, Cochrane, and Embase databases for case series, prospective studies, and cohort studies that reported neurocognitive complications associated with the BCP and the incidence of intraoperative CDEs. The authors used beach chair, desaturation, near infrared spectroscopy, and shoulder as medical subject headings (MeSH). In addition, bibliographies of retrieved articles were checked for studies that the search terms may have missed. Eighty-one publications were identified and reviewed for possible inclusion.
Next, the same 2 authors reviewed the titles and abstracts for relevance and determined which articles had potential to contribute to the study. Only English-language publications were considered for inclusion. To review the incidence of postoperative neurocognitive deficits, we included only those studies with more than 25 patients, documentation of postoperative complications, and arthroscopic shoulder surgery performed with the patient in the seated, semi-upright, or BCP. Only studies with at least 25 patients were used in order to increase the power and improve the level of evidence. To review reported cases of neurocognitive complications, we included all relevant case reports and case series. To review the incidence of intraoperative CDEs and investigate their relationship with postoperative neurocognitive deficits, we included studies that reported on use of intraoperative cerebral perfusion monitoring. Modalities used in these studies included near infrared spectroscopy, electroencephalography, and invasive blood pressure monitoring calculated at the brain level. Studies were excluded if they did not involve arthroscopic shoulder surgery or were not conducted with human subjects.
Information recorded for each study included general information such as author and publication year, type of study, number of patients enrolled, type of intraoperative monitoring, anesthesia protocol, number of patients with CDEs, and number of patients with neurocognitive complications after surgery.
Results
Our search identified 81 publications for potential inclusion. Our first aim was to identify the overall incidence of reported neurocognitive deficits after arthroscopic shoulder surgery with the patient in the BCP. We identified 10 studies (Table 1) that met the inclusion criteria. Among the 24,701 patients in these 10 studies, there was only 1 reported case of neurocognitive deficit after surgery, in a mixed prospective-retrospective study of 15,014 cases by Rohrbaugh and colleagues.18 The deficit they reported was an ischemic cerebral vascular accident. The 0.0067% incidence in their study demonstrates how rare the complication is. Two large retrospective studies (Ns = 4169 and 5177 patients) found no postoperative neurocognitive complications.19,20 Only 3 studies performed formal postoperative cognitive testing. Salazar and colleagues21 used the Repeatable Battery for the Assessment of Neuropsychological Status before and after surgery, and Gillespie and colleagues8 and Lee and colleagues10 used the Mini–Mental State Examination before and after surgery. Total incidence of reported neurocognitive deficits from our review was 0.004% (1/24,701).
Our second aim was to review all reported cases of neurocognitive complications after arthroscopic shoulder surgery with the patient in the BCP. We identified 4 publications that fit our inclusion criteria (Table 2). Pohl and Cullen6 described 4 cases of ischemic brain injury after arthroscopic shoulder surgery with the patient in the BCP. Age range was 47 to 57 years. Specific intraoperative cerebral monitoring was not used. However, these patients had several episodes of intraoperative hypotension (systolic blood pressures, 80-90 mm Hg), measured with a traditional blood pressure cuff on the arm. In general, these patients had minimal cerebrovascular risk factors and no known preexisting cerebrovascular disease. Drummond and colleagues22 described an ischemic stroke in a 50-year-old man after arthroscopic subacromial decompression and open rotator cuff repair that resulted in unresolved right hemiplegia. Subsequent diagnostic investigation revealed an asymmetry of the circle of Willis resulting in limited flow to the left anterior and middle cerebral artery distributions. Bhatti and Enneking3 reported the case of a 64-year-old man who lost vision in the right eye immediately after arthroscopic rotator cuff repair. His vision improved spontaneously the next morning and continued to improve over the next 6 months—he regained 20/20 vision with some residual optic neuropathy.
Our third aim was to determine the incidence of intraoperative CDEs during arthroscopic shoulder surgery with the patient in the BCP. Incidence of CDEs varied widely among the 7 studies reviewed (Table 3). Minimum incidence of intraoperative CDE was 0% in a cohort of 30 patients,1 and maximum incidence was 80% in a study of 61 patients,12 all of whom underwent elective arthroscopic shoulder surgery in the BCP. Although there was wide variability in CDE incidence, the studies were consistent with respect to their definition of a CDE. Most authors used a decrease in regional cerebral tissue oxygen saturation of 20% or more from baseline, or an absolute value up to 55%, to define a CDE. None of the 7 studies reviewed reported a clinically significant adverse neurocognitive event.
Discussion
Of concern, there have been several surveys, case reports, and small case series of previously healthy patients who had no known risk factors, underwent arthroscopic shoulder surgery in the BCP, and developed unanticipated postoperative neurologic complications.4-6,14 Beach-chair positioning during surgical procedures has been implicated as a contributing factor leading to cerebral hypoperfusion with potential for cerebral ischemia.1,12,23 These changes in cerebral perfusion pressure are thought to be the major determinant of poor neurologic outcomes. Such reports have exposed the potential need for heightened vigilance, alternative anesthesia techniques, and improved monitoring, though the exact etiology of the central nervous system injuries in this patient population is incompletely understood and is likely multifactorial. Therefore, in this study we wanted to determine the incidence of postoperative neurocognitive deficits and review all reported cases of neurocognitive complications in patients who have undergone arthroscopic shoulder surgery in the BCP. In addition, we wanted to define the incidence of intraoperative CDEs and investigate their relationship with postoperative neurocognitive complications.
According to our review, the incidence of postoperative neurocognitive complications after surgery in the BCP is 0.004% (1/24,701). However, this finding is based only on what has been reported; the true incidence is not known. It is also important to note that the incidence of neurocognitive deficits after many other types of surgery is not known and that surgery itself may be a risk factor for postoperative neurocognitive deficits.24 In their retrospective review of 15,014 patients who underwent arthroscopic shoulder surgery in the BCP at a single institution over an 11-year period, Rohrbaugh and colleagues18 found an overall postoperative complication rate of 0.37% and a 0.0067% incidence of neurocognitive deficits. One patient in the series was given a diagnosis of ischemic stroke on the basis of neurologic deficits that occurred 24 hours after surgery. Yadeau and colleagues20 found no postoperative neurocognitive complications in a mixed prospective-retrospective study of 4169 patients—3000 identified retrospectively, 1169 prospectively—who underwent arthroscopic shoulder surgery in the BCP at an ambulatory surgery center. Pin-on and colleagues19 reported on a series of 5177 orthopedic and neurosurgical patients who underwent surgery in the BCP. In those who had arthroscopic shoulder surgery, intraoperative systolic blood pressures obtained from an arterial line referenced to heart level decreased a mean (SD) of 14.4% (12.7%), whereas in those whose pressures were obtained from a noninvasive blood pressure cuff referenced to heart level decreased 19.3% (12.6%). However, the authors reported no incidence of postoperative stroke or neurologic deficits.
Although uncommon, perioperative cerebral ischemic accidents are potentially devastating for patients, their families, and the health care professionals involved. These events have tremendous economic, social, professional, and medicolegal implications, with perioperative stroke being particularly morbid. Perioperative stroke has a mortality rate of 60%, versus 15% to 46% for stroke in general.25,26 In 2005, Pohl and Cullen6 published a landmark article on a series of 4 relatively healthy middle-aged patients who were at low risk for stroke but had catastrophic neurocognitive complications (including 1 death) after arthroscopic shoulder surgery in the BCP. Bhatti and Enneking3 described a case of acute postoperative vision loss and ophthalmoplegia attributed to intraoperative hypotension leading to ischemia in a patient who underwent an elective shoulder arthroscopic procedure in the BCP. These reports prompted multiple investigations into the physiologic hemodynamic changes associated with surgery in the BCP and the treatment strategies used to improve patient safety.
In the normal physiologic state, the sympathetic nervous system is activated when a person assumes the seated position. The result is increased systemic vascular resistance and heart rate alterations to maintain cardiac output and mean arterial pressure. In anesthetized patients, this response is blunted by the vasodilatory effects of intravenous and volatile anesthetics. Multiple studies have demonstrated substantial hemodynamic changes in both awake and anesthetized patients during the maneuver from the supine position to the seated position1,27,28; these changes include diminished cardiac index, stroke volume, and arterial pressure.17 The data underscore the need for attentiveness and accurate monitoring of cerebral perfusion when the transition is made from the supine position to the BCP, particularly in the early phase of surgery and in high-risk patients.
Knowledge of these hemodynamic changes has led several authors to recommend additional intraoperative monitoring of cerebral perfusion. Monitoring techniques have included use of invasive blood pressure monitoring adjusted to brain level, cerebral oximetry using near infrared spectroscopy, and electroencephalography. However, the clinical relevance of intraoperative CDEs in isolation is not well understood.1,6,7,23 In addition, cost and availability of additional advanced monitoring likely factor into why it is not more commonly used. For this patient population, the severity, frequency, and duration of desaturation that causes cerebral ischemia and the relationship with postoperative neurocognitive deficits remain undefined.
The incidence of CDEs in patients being monitored with near infrared spectroscopy while undergoing elective arthroscopic shoulder surgery in the BCP varies widely, from 0% to 80% (mean, 41%).1,4,7,10,12,21 Magnitude and duration of cerebral ischemia required to produce neurocognitive dysfunction in this patient population remain unidentified as well. In conscious patients, a 20% reduction in frontal lobe oxygenation is associated with clinical manifestations of cerebral hypoperfusion, such as syncope.15,29 As none of the patients in the studies we reviewed experienced any sort of deficit, we cannot definitively state there is a correlation between CDE occurrence and neurocognitive deficit.
One limitation of our investigation is that it was a systemic review, and thus there was substantial heterogeneity in the methods and designs of the studies included in the analysis. Among the different series, there was variability in multiple aspects of the study design, including type of anesthetic, patient inclusion criteria, type of surgery, type of intraoperative cerebral perfusion monitoring, and type of neurocognitive testing. As a result, comparing the groups was difficult, and the generalizability of our findings may be limited. In addition, it is difficult to accurately establish incidence and comprehensively review these events because of the paucity of literature.
Conclusion
Neurocognitive complications after shoulder arthroscopy with the patient in the BCP are extremely rare but potentially devastating events that can affect healthy patients with no preexisting cerebrovascular risk factors. Our review indicated the incidence of permanent neurologic deficit after arthroscopy in the BCP may be as low as 0.004%. The exact etiology of such complications is not clear. Basic science research and large prospective studies are needed to identify the clinically relevant thresholds of magnitude, duration, and frequency of intraoperative CDEs in order to establish their relationship with postoperative neurocognitive complications. Such large studies may also elucidate modifiable patient-specific risk factors and establish the most sensitive, safe, and cost-effective intraoperative monitoring tools. Current literature suggests that accurate intraoperative monitoring of cerebral perfusion, alternatives to general anesthesia, and prudent use of intraoperative blood pressure control may improve patient safety.
1. Tange K, Kinoshita H, Minonishi T, et al. Cerebral oxygenation in the beach chair position before and during general anesthesia. Minerva Anestesiol. 2010;76(7):485-490.
2. Skyhar MJ, Altchek DW, Warren RF, Wickiewicz TL, O’Brien SJ. Shoulder arthroscopy with the patient in the beach-chair position. Arthroscopy. 1988;4(4):256-259.
3. Bhatti MT, Enneking FK. Visual loss and ophthalmoplegia after shoulder surgery. Anesth Analg. 2003;96(3):899-902.
4. Friedman DJ, Parnes NZ, Zimmer Z, Higgins LD, Warner JJ. Prevalence of cerebrovascular events during shoulder surgery and association with patient position. Orthopedics. 2009;32(4).
5. Papadonikolakis A, Wiesler ER, Olympio MA, Poehling GG. Avoiding catastrophic complications of stroke and death related to shoulder surgery in the sitting position. Arthroscopy. 2008;24(4):481-482.
6. Pohl A, Cullen DJ. Cerebral ischemia during shoulder surgery in the upright position: a case series. J Clin Anesth. 2005;17(6):463-469.
7. Dippmann C, Winge S, Nielsen HB. Severe cerebral desaturation during shoulder arthroscopy in the beach-chair position. Arthroscopy. 2010;26(9 suppl):S148-S150.
8. Gillespie R, Shishani Y, Streit J, et al. The safety of controlled hypotension for shoulder arthroscopy in the beach-chair position. J Bone Joint Surg Am. 2012;94(14):1284-1290.
9. Jeong H, Lee SH, Jang EA, Chung SS, Lee J, Yoo KY. Haemodynamics and cerebral oxygenation during arthroscopic shoulder surgery in beach chair position under general anaesthesia. Acta Anaesthesiol Scand. 2012;56(7):872-879.
10. Lee JH, Min KT, Chun YM, Kim EJ, Choi SH. Effects of beach-chair position and induced hypotension on cerebral oxygen saturation in patients undergoing arthroscopic shoulder surgery. Arthroscopy. 2011;27(7):889-894.
11. Moerman AT, De Hert SG, Jacobs TF, De Wilde LF, Wouters PF. Cerebral oxygen desaturation during beach chair position. Eur J Anaesthesiol. 2012;29(2):82-87.
12. Murphy GS, Szokol JW, Marymont JH, et al. Cerebral oxygen desaturation events assessed by near-infrared spectroscopy during shoulder arthroscopy in the beach chair and lateral decubitus positions. Anesth Analg. 2010;111(2):496-505.
13. Peruto CM, Ciccotti MG, Cohen SB. Shoulder arthroscopy positioning: lateral decubitus versus beach chair. Arthroscopy. 2009;25(8):891-896.
14. Rains DD, Rooke GA, Wahl CJ. Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthroscopy. 2011;27(4):532-541.
15. Samra SK, Dy EA, Welch K, Dorje P, Zelenock GB, Stanley JC. Evaluation of a cerebral oximeter as a monitor of cerebral ischemia during carotid endarterectomy. Anesthesiology. 2000;93(4):964-970.
16. Smythe PR, Samra SK. Monitors of cerebral oxygenation. Anesthesiol Clin North Am. 2002;20(2):293-313.
17. Smith JJ, Porth CM, Erickson M. Hemodynamic response to the upright posture. J Clin Pharmacol. 1994;34(5):375-386.
18. Rohrbaugh M, Kentor ML, Orebaugh SL, Williams B. Outcomes of shoulder surgery in the sitting position with interscalene nerve block: a single-center series. Reg Anesth Pain Med. 2013;38(1):28-33.
19. Pin-on P, Schroeder D, Munis J. The hemodynamic management of 5177 neurosurgical and orthopedic patients who underwent surgery in the sitting or “beach chair” position without incidence of adverse neurologic events. Anesth Analg. 2013;116(6):1317-1324.
20. Yadeau JT, Casciano M, Liu SS, et al. Stroke, regional anesthesia in the sitting position, and hypotension: a review of 4169 ambulatory surgery patients. Reg Anesth Pain Med. 2011;36(5):430-435.
21. Salazar D, Sears BW, Aghdasi B, et al. Cerebral desaturation events during shoulder arthroscopy in the beach chair position: patient risk factors and neurocognitive effects. J Shoulder Elbow Surg. 2013;22(9):1228-1235.
22. Drummond JC, Lee RR, Howell JP Jr. Focal cerebral ischemia after surgery in the “beach chair” position: the role of a congenital variation of circle of Willis anatomy. Anesth Analg. 2012;114(6):1301-1303.
23. Fischer GW, Torrillo TM, Weiner MM, Rosenblatt MA. The use of cerebral oximetry as a monitor of the adequacy of cerebral perfusion in a patient undergoing shoulder surgery in the beach chair position. Pain Pract. 2009;9(4):304-307.
24. Wong GY, Warner DO, Schroeder DR, et al. Risk of surgery and anesthesia for ischemic stroke. Anesthesiology. 2000;92(2):425-432.
25. Knapp RB, Topkins MJ, Artusio JF Jr. The cerebrovascular accident and coronary occlusion in anesthesia. JAMA. 1962;182:332-334.
26. Landercasper J, Merz BJ, Cogbill TH, et al. Perioperative stroke risk in 173 consecutive patients with a past history of stroke. Arch Surg. 1990;125(8):986-989.
27. Fuchs G, Schwarz G, Kulier A, Litscher G. The influence of positioning on spectroscopic measurements of brain oxygenation. J Neurosurg Anesthesiol. 2000;12(2):75-80.
28. Lovell AT, Owen-Reece H, Elwell CE, Smith M, Goldstone JC. Continuous measurement of cerebral oxygenation by near infrared spectroscopy during induction of anesthesia. Anesth Analg. 1999;88(3):554-558.
29. Madsen PL, Secher NH. Near-infrared oximetry of the brain. Prog Neurobiol. 1999;58(6):541-560.
30. Koh JL, Levin SD, Chehab EL, Murphy GS. Neer award 2012: cerebral oxygenation in the beach chair position: a prospective study on the effect of general anesthesia compared with regional anesthesia and sedation. J Shoulder Elbow Surg. 2013;22:1325-1331.
1. Tange K, Kinoshita H, Minonishi T, et al. Cerebral oxygenation in the beach chair position before and during general anesthesia. Minerva Anestesiol. 2010;76(7):485-490.
2. Skyhar MJ, Altchek DW, Warren RF, Wickiewicz TL, O’Brien SJ. Shoulder arthroscopy with the patient in the beach-chair position. Arthroscopy. 1988;4(4):256-259.
3. Bhatti MT, Enneking FK. Visual loss and ophthalmoplegia after shoulder surgery. Anesth Analg. 2003;96(3):899-902.
4. Friedman DJ, Parnes NZ, Zimmer Z, Higgins LD, Warner JJ. Prevalence of cerebrovascular events during shoulder surgery and association with patient position. Orthopedics. 2009;32(4).
5. Papadonikolakis A, Wiesler ER, Olympio MA, Poehling GG. Avoiding catastrophic complications of stroke and death related to shoulder surgery in the sitting position. Arthroscopy. 2008;24(4):481-482.
6. Pohl A, Cullen DJ. Cerebral ischemia during shoulder surgery in the upright position: a case series. J Clin Anesth. 2005;17(6):463-469.
7. Dippmann C, Winge S, Nielsen HB. Severe cerebral desaturation during shoulder arthroscopy in the beach-chair position. Arthroscopy. 2010;26(9 suppl):S148-S150.
8. Gillespie R, Shishani Y, Streit J, et al. The safety of controlled hypotension for shoulder arthroscopy in the beach-chair position. J Bone Joint Surg Am. 2012;94(14):1284-1290.
9. Jeong H, Lee SH, Jang EA, Chung SS, Lee J, Yoo KY. Haemodynamics and cerebral oxygenation during arthroscopic shoulder surgery in beach chair position under general anaesthesia. Acta Anaesthesiol Scand. 2012;56(7):872-879.
10. Lee JH, Min KT, Chun YM, Kim EJ, Choi SH. Effects of beach-chair position and induced hypotension on cerebral oxygen saturation in patients undergoing arthroscopic shoulder surgery. Arthroscopy. 2011;27(7):889-894.
11. Moerman AT, De Hert SG, Jacobs TF, De Wilde LF, Wouters PF. Cerebral oxygen desaturation during beach chair position. Eur J Anaesthesiol. 2012;29(2):82-87.
12. Murphy GS, Szokol JW, Marymont JH, et al. Cerebral oxygen desaturation events assessed by near-infrared spectroscopy during shoulder arthroscopy in the beach chair and lateral decubitus positions. Anesth Analg. 2010;111(2):496-505.
13. Peruto CM, Ciccotti MG, Cohen SB. Shoulder arthroscopy positioning: lateral decubitus versus beach chair. Arthroscopy. 2009;25(8):891-896.
14. Rains DD, Rooke GA, Wahl CJ. Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthroscopy. 2011;27(4):532-541.
15. Samra SK, Dy EA, Welch K, Dorje P, Zelenock GB, Stanley JC. Evaluation of a cerebral oximeter as a monitor of cerebral ischemia during carotid endarterectomy. Anesthesiology. 2000;93(4):964-970.
16. Smythe PR, Samra SK. Monitors of cerebral oxygenation. Anesthesiol Clin North Am. 2002;20(2):293-313.
17. Smith JJ, Porth CM, Erickson M. Hemodynamic response to the upright posture. J Clin Pharmacol. 1994;34(5):375-386.
18. Rohrbaugh M, Kentor ML, Orebaugh SL, Williams B. Outcomes of shoulder surgery in the sitting position with interscalene nerve block: a single-center series. Reg Anesth Pain Med. 2013;38(1):28-33.
19. Pin-on P, Schroeder D, Munis J. The hemodynamic management of 5177 neurosurgical and orthopedic patients who underwent surgery in the sitting or “beach chair” position without incidence of adverse neurologic events. Anesth Analg. 2013;116(6):1317-1324.
20. Yadeau JT, Casciano M, Liu SS, et al. Stroke, regional anesthesia in the sitting position, and hypotension: a review of 4169 ambulatory surgery patients. Reg Anesth Pain Med. 2011;36(5):430-435.
21. Salazar D, Sears BW, Aghdasi B, et al. Cerebral desaturation events during shoulder arthroscopy in the beach chair position: patient risk factors and neurocognitive effects. J Shoulder Elbow Surg. 2013;22(9):1228-1235.
22. Drummond JC, Lee RR, Howell JP Jr. Focal cerebral ischemia after surgery in the “beach chair” position: the role of a congenital variation of circle of Willis anatomy. Anesth Analg. 2012;114(6):1301-1303.
23. Fischer GW, Torrillo TM, Weiner MM, Rosenblatt MA. The use of cerebral oximetry as a monitor of the adequacy of cerebral perfusion in a patient undergoing shoulder surgery in the beach chair position. Pain Pract. 2009;9(4):304-307.
24. Wong GY, Warner DO, Schroeder DR, et al. Risk of surgery and anesthesia for ischemic stroke. Anesthesiology. 2000;92(2):425-432.
25. Knapp RB, Topkins MJ, Artusio JF Jr. The cerebrovascular accident and coronary occlusion in anesthesia. JAMA. 1962;182:332-334.
26. Landercasper J, Merz BJ, Cogbill TH, et al. Perioperative stroke risk in 173 consecutive patients with a past history of stroke. Arch Surg. 1990;125(8):986-989.
27. Fuchs G, Schwarz G, Kulier A, Litscher G. The influence of positioning on spectroscopic measurements of brain oxygenation. J Neurosurg Anesthesiol. 2000;12(2):75-80.
28. Lovell AT, Owen-Reece H, Elwell CE, Smith M, Goldstone JC. Continuous measurement of cerebral oxygenation by near infrared spectroscopy during induction of anesthesia. Anesth Analg. 1999;88(3):554-558.
29. Madsen PL, Secher NH. Near-infrared oximetry of the brain. Prog Neurobiol. 1999;58(6):541-560.
30. Koh JL, Levin SD, Chehab EL, Murphy GS. Neer award 2012: cerebral oxygenation in the beach chair position: a prospective study on the effect of general anesthesia compared with regional anesthesia and sedation. J Shoulder Elbow Surg. 2013;22:1325-1331.
Latissimus Dorsi and Teres Major Injuries in Major League Baseball Pitchers: A Systematic Review
Upper extremity injuries are very common in pitchers in amateur and professional baseball. The vast majority involving labral or rotator cuff pathology.1-3 While uncommon, injuries to the latissimus dorsi (LD) (Figure) and teres major (TM) have been reported in Major League Baseball (MLB) pitchers.4 Jobe and colleagues5 demonstrated the role of the LD during the various phases of pitching. The LD is most active during the acceleration phase and remains active during the deceleration phase and follow-through.6 Anatomically, the TM lies posterior to the LD separated by bursal tissue. The tendon fibers converge and unite along their lower borders, leading to a synergistic mechanism of action.
Due to the rarity of LD and TM injuries, literature on the pathology and appropriate treatments for these injuries is limited. The goal of this review is to present the current literature on professional baseball players who have undergone either nonsurgical treatment or surgery for LD and TM strains and/or avulsion injuries. This review will ultimately assist clinicians when deciding on the optimal treatment method for professional baseball players.
Methods
We performed an extensive Medline database search with the following search algorithm: ([latissimus OR latissimus dorsi OR teres major] AND baseball). The search returned 20 citations. Inclusion criteria consisted of clinical studies that focused on professional baseball pitchers with TM and/or LD injuries that underwent either conservative nonsurgical treatment or surgical repair. There was no exclusion based on the type of injury present, such as avulsion vs strain. Any study with amateur athletes or athletes from other sports such as handball or rugby were excluded. Due to the limited amount of data available, the majority of included studies were case reports and case series.
Based on these parameters, 5 articles met criteria for inclusion. Of the 5 included studies, 3 were case reports and 2 were case series. From the eligible articles, the following information was obtained: publication year, sample size, mean age, mean follow-up duration, type of treatment (conservative vs surgical), ability to return to original level of play, time required to return to original form, and complications (Tables 1, 2).
Results
Nonoperative Management
Four of the 5 included studies implemented only conservative therapy for their patients.4,7-9 The average duration these patients were followed for during treatment and rehabilitation was 26.3 months. Malcolm and colleagues7 followed patients for 8 months, the shortest length among the 4 conservative studies in this review. Leland and colleagues8 followed patients for 17 months, and Nagda and colleagues9 had the longest length of observation of 36 months (range 12 to 82 months).Schickendantz and colleagues4 followed patients for >12 months, but the exact duration was not specified. In order to calculate the average duration of observation, each patient was assigned a duration of 12 months.
Of the 30 patients included in this review, 29 were treated conservatively. All of the included studies consisted of male patients. The mean age was 26.8 years (range 22 to 28.1 years). Of the 29 injuries treated conservatively, there were 2 LD tendon avulsions, 4 TM tendon avulsions, 1 LD and TM tendon avulsion, 7 LD intramuscular strains, 9 TM intramuscular strains, and 6 LD and TM intramuscular strains.
Treatment Protocol
The various treatment and rehabilitation programs used for the conservative patient population all followed a similar pathway. After initial injury, a rest period focused on stretching was implemented. Patients were started on steroid or anti-inflammatory medications, cryotherapy, or other therapeutic modalities. Once pain-free and full range of motion was achieved, patients began the strength and throwing components of the rehabilitation program. Reoccurrence of symptoms would halt the throwing component of the rehabilitation program until symptoms improved. Patients were progressed through a return-to-throw program and once they could throw off the mound and achieve their preinjury velocity, strength, and range of motion, they were cleared to return to competitive pitching.
In the senior author’s (MSS) practice, all throwers are managed with the same nonoperative protocol.4 Initial treatment consists of short periods of rest and symptom control via the application of cryotherapy, among other modalities. Restoration of preinjury range of motion is achieved with active-assisted stretching exercises. As range of motion begins approaching pre-injury levels, strength training is initiated with isometric strengthening of the LD and TM progressing to resistance exercises. Exercising the abdominal core, strengthening the lower body, and cardiovascular conditioning are focal points of the rehabilitation period. Once patients regain preinjury shoulder strength and range of motion without pain, they begin a throwing program that consists of 4 weeks of long toss followed by 2 weeks of throwing from the pitching mound. After completion of the throwing program, the patient is allowed to return to competitive pitching. For patients who did not suffer season-ending injury, the average time required to return to play was 99.8 days (range 72.3 to 182.6 days).
Complications and Reinjury
The patients in Leland and colleagues8 and Malcolm and colleagues7 did not suffer any complications or reinjuries. In Schickendantz and colleagues4, all but 3 of the 10 patients were able to return to full speed pitching by 3 months. The other 3 required 4, 6, and 10 months. The patient that required 10 months tore both his LD and TM and the patient that required 6 months tore his TM and was never able to regain his pre-injury throwing velocity. None of the TM tears had a recurrence, while 1 LD tear had a recurrence of injury 6 months after returning to competitive pitching. This patient was successfully treated with 6 weeks of conservative rest and rehabilitation.
In Nagda and colleagues9, 2 athletes suffered injury recurrence. One athlete with a LD strain suffered 2 subsequent LD strains, 4 months and 1 year after initial injury. The other athlete with a LD avulsion suffered a subsequent TM avulsion 13 months after initial injury. One pitcher who had an LD and TM strain suffered a superior labrum anterior and posterior (SLAP) tear and was never able to return to his prior level of play.
Surgical Treatment
Only 1 of the 5 included studies utilized surgical repair for their patient.10 The single patient suffered an avulsion injury of the distal LD tendon and its insertion on the humerus. The LD tendon was retracted approximately 5 cm from the distal humeral insertion. The TM was not involved. Eight days post-injury, the patient underwent surgical repair.11 Postoperatively, the patient started passive range of motion after 2 weeks and active range of motion after 6 weeks. He started throwing at 12 weeks and returned to play at 30 weeks after he had returned to his preinjury form in regards to muscle strength, pitch control, and velocity. The patient was able to resume pitching at a high level in MLB.
Discussion
Overhand throwing athletes, especially professional baseball players, have to constantly deal with a variety of shoulder injuries.12,13 Currently, there is minimal literature on isolated TM and LD injuries. As a result, there is still debate about the optimal treatment method for these injuries, especially in athletes who compete at the highest level. In order to treat isolated injuries of these muscles, it is important to understand their anatomic relationship, as these 2 muscles are intimately associated. The LD originates from the thoracolumbar spine and inserts on the proximal humerus between the pectoralis<hl name="2"/> major and TM tendons. The TM originates from the scapula and, similar to the LD, inserts on the proximal humerus. In an anatomic study, the TM tendon inserted into the LD tendon before its humeral insertion in the majority of cadavers.14,15
The LD is responsible for extension, adduction, and internal rotation of the humerus. The TM, while not as extensively studied, is believed to also contribute to extension, adduction, and internal rotation of the humerus.16 As Jobe and colleagues5 demonstrated, the LD is vital during the acceleration phase of pitching. While they were unable to make any conclusions about the role of the TM during the pitching cycle, it is reasonable to hypothesize that these 2 muscles work together. While it is thought that these 2 muscles work as a unit, it is significant to note that a professional pitcher can sustain an isolated injury to the TM without injury to the LD, and vice versa. This questions whether these 2 muscles work more independently than once thought. One hypothesis is that the physical size of the LD provides protection from injuries that the smaller TM cannot overcome. This is a potential area of further research.
The most common findings in patients with TM injuries include swelling, bruising, tenderness of the proximal arm, and limitations of shoulder range of motion in abduction, flexion, and external rotation. There is also weakness when resistance is applied against internal rotation and extension. Similar to the TM, common findings in patients with LD injuries include pain in the posterior shoulder, bruising, and weakness when resistance is applied against internal rotation of the shoulder. Pitchers are often able to pinpoint the occurrence of their acute pain during a specific time in the game. They commonly experience a pulling sensation and sometimes even feel a “pop” in their shoulder followed by an acute onset of pain and stiffness in the posterior aspect of the axilla. These injuries seem to be associated with the pitcher throwing a “breaking ball,” a pitch that requires greater shoulder rotation since it changes trajectory while traveling towards home plate. Despite the clear role of the LD and hypothesized role of the TM in the pitching sequence, there has been limited research on the optimal treatment of isolated injuries of these muscles in MLB pitchers. The majority of studies in this review opted for conservative treatment for both LD and TM injuries. The only study that presented a surgical option was for a LD avulsion injury.
Athletes undergoing either conservative or surgical treatment required a significant period of recovery and rehabilitation before they were able to compete at the professional level. In Leland and colleagues8, it took about 10 to 12 weeks of rehabilitation for both pitchers to return to pitching against competition. In Schickendantz and colleagues4, barring any complications or injury recurrence, it took patients 12 weeks to return to their preinjury level. In Malcolm and colleagues7, magnetic resonance imaging after 8 weeks showed marked recovery, and shortly after the pitcher was able to return to the pitching rotation. In Nagda and colleagues9, the time lost to injury ranged from 7 weeks to an entire season. Of the 9 pitchers who were lost for the season, 6 had avulsion injuries. The other 3 consisted of an LD strain, TM strain, and LD plus TM strain.9 In this study, it seems that avulsion injuries had a more significant impact on patient recovery. On average, it took 35.6 days after injury for players to begin throwing. In contrast, it took an average of 65.5 days after an avulsion injury for players to begin throwing. Ellman and colleagues10 included the only surgically repaired injury, and it was for an avulsion of the LD tendon. In the surgical case, it took slightly longer for the pitcher to return to preinjury form. It took him 12 to 16 weeks to begin light throwing and his full return to pitching took about 20 to 30 weeks. Since muscle strains and tendon avulsions are significantly different injuries in regards to the type of soft tissue damage and healing potential, they may require different treatment strategies. An avulsion injury may require more aggressive intervention, whereas a strain may only require conservative rehabilitation. Ultimately, there does not seem to be a significant benefit of one treatment option compared to the other. The majority of conservatively managed pitchers were able to return to previous form in a reasonable time frame. While each rehabilitation protocol was slightly different, multiple studies advocated for rehab programs that centered around the following goals: slowly progressing pitchers to light throwing once their pain resolved, followed by long throwing, then throwing off of the mound, and finally returning to competitive pitching. It is important to discuss with patients that rehabilitation generally takes 12 to 16 weeks before they are able to fully return to pitching against competition and that rest should immediately follow any recurrence of pain or stiffness. Once those symptoms resolve, patients may continue the rehabilitation protocol.
As with any form of treatment, there are risks involved. This holds true for both conservative and nonconservative therapy for LD and TM injuries. One risk of nonoperative treatment of an LD avulsion is the development of strength deficits in the muscle.17 While this deficit may go unnoticed in a recreational athlete, it may be more pronounced in a professional athlete, especially since the LD of a professional baseball pitcher is more active on electromyography during the acceleration phase of the pitching cycle compared to a recreational athlete.18 Another risk of conservative treatment of an LD avulsion is jeopardizing the potential for future surgery. As a result, some advocate for early surgical intervention of an acute LD avulsion.19,20 Others, however, recommend conservative management with subsequent surgical intervention if conservative measures fail. One caveat is that surgical intervention to restore the original anatomy may become difficult after a certain period of time due to the buildup of scar tissue. Surgical intervention also has associated risks, such as nerve injury, infection, vascular damage, persistent pain, and the buildup of large amounts of scar tissue. It is important to discuss these risks with patients when deciding on a treatment option.
LD and TM avulsion and tears typically present after an acute event in throwing athletes. There are a number of case reports published that demonstrate successful outcomes with both nonoperative management21 and operative repair of LD injuries in non-throwing athletes such as competitive water skiers,22,23 steer wrestlers,24 professional wrestlers,25 and recreational rock climbers.26 The 5 studies included in this review were the first ones to present LD and TM injuries in MLB pitchers. They discussed the outcomes of mainly conservative and surgical management of LD and TM avulsion and tears. Unfortunately, there remains a limited number of cases on the treatment of these injuries in highly competitive throwing athletes. Further research is required to elucidate the advantages and disadvantages of operative vs nonoperative treatment. The goal of this review is to provide clinicians with a concise summary of the current literature so that they may offer some evidence to their patients when discussing appropriate treatment plans.
1. Conway JE, Arthroscopic repair of partial-thickness rotator cuff tears and SLAP lesions in professional baseball players. Orthop Clin North Am. 2001;32(3):443-456.
2. Mazoue CG, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34(2):182-189.
3. Cerynik DL, Ewald TJ, Sastry A, Amin NH, Liao JG, Tom JA. Outcomes of isolated glenoid labral injuries in professional baseball pitchers. Clin J Sport Med. 2008;18(3):255-258
4. Schickendantz MS, Kaar SG, Meister K, Lund P, Beverley L. Latissimus dorsi and teres major tears in professional baseball pitchers: a case series. Am J Sports Med. 2009;37(10):2016-2020.
5. Jobe FW, Moynes DR, Tibone JE, Perry J. An EMG analysis of the shoulder in pitching. A second report. Am J Sports Med. 1984;12(3):218-220.
6. Glousman R, Jobe F, Tibone J, Moynes D, Antonelli D, Perry J. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am. 1988;70(2):220-226.
7. Malcolm PN, Reinus WR, London SL. Magnetic resonance imaging appearance of teres major tendon injury in a baseball pitcher. Am J Sports Med. 1999;27(1):98-100.
8. Leland JM, Ciccotti MG, Cohen SB, Zoga AC, Frederick RJ. Teres major injuries in two professional baseball pitchers. J Shoulder Elbow Surg. 2009;18(6):e1-e5.
9. Nagda SH, Cohen SB, Noonan TJ, Raasch WG, Ciccotti MG, Yocum LA. Management and outcomes of latissimus dorsi and teres major injuries in professional baseball pitchers. Am J Sports Med. 2011;39(10):2181-2186.
10. Ellman MB, Yanke A, Juhan T, et al. Open repair of an acute latissimus tendon avulsion in a Major League Baseball pitcher. J Shoulder Elbow Surg. 2013;22(7):e19-e23.
11. Ellman MB, Yanke A, Juhan T, et al. Open repair of retracted latissimus dorsi tendon avulsion. Am J Orthop. 2013;42(6):280-285.
12. Altchek DW, Dines DM. Shoulder injuries in the throwing athlete. J Am Acad Orthop Surg. 1995;3(3):159-165.
13. Limpisvasti O, ElAttrache NS, Jobe FW. Understanding shoulder and elbow injuries in baseball. J Am Acad Orthop Surg. 2007;15(3):139-147.
14. Beck PA, Hoffer MM. Latissimus dorsi and teres major tendons: separate or conjoint tendons? J Pediatr Orthop. 1989;9(3):308-309.
15. Morelli M, Nagamori J, Gilbart M, Miniaci A. Latissimus dorsi tendon transfer for massive irreparable cuff tears: an anatomic study. J Shoulder Elbow Surg. 2008;17(1):139-143.
16. Broome HL, Basmajian JV. The function of the teres major muscle: an electromyographic study. Anat Rec. 1971;170(3):309-310.
17. Brumback RJ, McBride MS, Ortolani NC. Functional evaluation of the shoulder after transfer of the vascularized latissimus dorsi muscle. J Bone Joint Surg Am. 1992;74(3):377-382.
18. Gowan ID, Jobe FW, Tibone JE, Perry J, Moynes DR. A comparative electromyographic analysis of the shoulder during pitching. Professional versus amateur pitchers. Am J Sports Med. 1987;15(6):586-590.
19. Park JY, Lhee SH, Keum JS. Rupture of latissimus dorsi muscle in a tennis player. Orthopedics. 2008;31(10).
20. Gregory JM, Harwood DP, Sherman SL, Romeo AA. Surgical repair of a subacute latissimus dorsi tendon rupture. Tech Shoulder Elbow Surg. 2011;12(4):77-79.
21. Butterwick DJ, Mohtadi NG, Meeuwisse WH, Frizzell JB. Rupture of latissimus dorsi in an athlete. Clin J Sport Med. 2003;13(3):189-191.
22. Henry JC, Scerpella TA. Acute traumatic tear of the latissimus dorsi tendon from its insertion. A case report. Am J Sports Med. 2000;28(4):577-579.
23. Lim JK, Tilford ME, Hamersly SF, Sallay PI. Surgical repair of an acute latissimus dorsi tendon avulsion using suture anchors through a single incision. Am J Sports Med. 2006;34(8):1351-1355.
24. Hiemstra LA, Butterwick D, Cooke M, Walker RE. Surgical management of latissimus dorsi rupture in a steer wrestler. Clin J Sport Med. 2007;17(4):316-318.
25. Hapa O, Wijdicks CA, LaPrade RF, Braman JP. Out of the ring and into a sling: acute latissimus dorsi avulsion in a professional wrestler: a case report and review of the literature. Knee Surg Sports Traumatol Arthrosc. 2008;16(12):1146-1150.
26. Livesey J, Brownson P, Wallace WA. Traumatic latissimus dorsi tendon rupture. J Shoulder Elbow Surg. 2002;11(6):642-644.
Upper extremity injuries are very common in pitchers in amateur and professional baseball. The vast majority involving labral or rotator cuff pathology.1-3 While uncommon, injuries to the latissimus dorsi (LD) (Figure) and teres major (TM) have been reported in Major League Baseball (MLB) pitchers.4 Jobe and colleagues5 demonstrated the role of the LD during the various phases of pitching. The LD is most active during the acceleration phase and remains active during the deceleration phase and follow-through.6 Anatomically, the TM lies posterior to the LD separated by bursal tissue. The tendon fibers converge and unite along their lower borders, leading to a synergistic mechanism of action.
Due to the rarity of LD and TM injuries, literature on the pathology and appropriate treatments for these injuries is limited. The goal of this review is to present the current literature on professional baseball players who have undergone either nonsurgical treatment or surgery for LD and TM strains and/or avulsion injuries. This review will ultimately assist clinicians when deciding on the optimal treatment method for professional baseball players.
Methods
We performed an extensive Medline database search with the following search algorithm: ([latissimus OR latissimus dorsi OR teres major] AND baseball). The search returned 20 citations. Inclusion criteria consisted of clinical studies that focused on professional baseball pitchers with TM and/or LD injuries that underwent either conservative nonsurgical treatment or surgical repair. There was no exclusion based on the type of injury present, such as avulsion vs strain. Any study with amateur athletes or athletes from other sports such as handball or rugby were excluded. Due to the limited amount of data available, the majority of included studies were case reports and case series.
Based on these parameters, 5 articles met criteria for inclusion. Of the 5 included studies, 3 were case reports and 2 were case series. From the eligible articles, the following information was obtained: publication year, sample size, mean age, mean follow-up duration, type of treatment (conservative vs surgical), ability to return to original level of play, time required to return to original form, and complications (Tables 1, 2).
Results
Nonoperative Management
Four of the 5 included studies implemented only conservative therapy for their patients.4,7-9 The average duration these patients were followed for during treatment and rehabilitation was 26.3 months. Malcolm and colleagues7 followed patients for 8 months, the shortest length among the 4 conservative studies in this review. Leland and colleagues8 followed patients for 17 months, and Nagda and colleagues9 had the longest length of observation of 36 months (range 12 to 82 months).Schickendantz and colleagues4 followed patients for >12 months, but the exact duration was not specified. In order to calculate the average duration of observation, each patient was assigned a duration of 12 months.
Of the 30 patients included in this review, 29 were treated conservatively. All of the included studies consisted of male patients. The mean age was 26.8 years (range 22 to 28.1 years). Of the 29 injuries treated conservatively, there were 2 LD tendon avulsions, 4 TM tendon avulsions, 1 LD and TM tendon avulsion, 7 LD intramuscular strains, 9 TM intramuscular strains, and 6 LD and TM intramuscular strains.
Treatment Protocol
The various treatment and rehabilitation programs used for the conservative patient population all followed a similar pathway. After initial injury, a rest period focused on stretching was implemented. Patients were started on steroid or anti-inflammatory medications, cryotherapy, or other therapeutic modalities. Once pain-free and full range of motion was achieved, patients began the strength and throwing components of the rehabilitation program. Reoccurrence of symptoms would halt the throwing component of the rehabilitation program until symptoms improved. Patients were progressed through a return-to-throw program and once they could throw off the mound and achieve their preinjury velocity, strength, and range of motion, they were cleared to return to competitive pitching.
In the senior author’s (MSS) practice, all throwers are managed with the same nonoperative protocol.4 Initial treatment consists of short periods of rest and symptom control via the application of cryotherapy, among other modalities. Restoration of preinjury range of motion is achieved with active-assisted stretching exercises. As range of motion begins approaching pre-injury levels, strength training is initiated with isometric strengthening of the LD and TM progressing to resistance exercises. Exercising the abdominal core, strengthening the lower body, and cardiovascular conditioning are focal points of the rehabilitation period. Once patients regain preinjury shoulder strength and range of motion without pain, they begin a throwing program that consists of 4 weeks of long toss followed by 2 weeks of throwing from the pitching mound. After completion of the throwing program, the patient is allowed to return to competitive pitching. For patients who did not suffer season-ending injury, the average time required to return to play was 99.8 days (range 72.3 to 182.6 days).
Complications and Reinjury
The patients in Leland and colleagues8 and Malcolm and colleagues7 did not suffer any complications or reinjuries. In Schickendantz and colleagues4, all but 3 of the 10 patients were able to return to full speed pitching by 3 months. The other 3 required 4, 6, and 10 months. The patient that required 10 months tore both his LD and TM and the patient that required 6 months tore his TM and was never able to regain his pre-injury throwing velocity. None of the TM tears had a recurrence, while 1 LD tear had a recurrence of injury 6 months after returning to competitive pitching. This patient was successfully treated with 6 weeks of conservative rest and rehabilitation.
In Nagda and colleagues9, 2 athletes suffered injury recurrence. One athlete with a LD strain suffered 2 subsequent LD strains, 4 months and 1 year after initial injury. The other athlete with a LD avulsion suffered a subsequent TM avulsion 13 months after initial injury. One pitcher who had an LD and TM strain suffered a superior labrum anterior and posterior (SLAP) tear and was never able to return to his prior level of play.
Surgical Treatment
Only 1 of the 5 included studies utilized surgical repair for their patient.10 The single patient suffered an avulsion injury of the distal LD tendon and its insertion on the humerus. The LD tendon was retracted approximately 5 cm from the distal humeral insertion. The TM was not involved. Eight days post-injury, the patient underwent surgical repair.11 Postoperatively, the patient started passive range of motion after 2 weeks and active range of motion after 6 weeks. He started throwing at 12 weeks and returned to play at 30 weeks after he had returned to his preinjury form in regards to muscle strength, pitch control, and velocity. The patient was able to resume pitching at a high level in MLB.
Discussion
Overhand throwing athletes, especially professional baseball players, have to constantly deal with a variety of shoulder injuries.12,13 Currently, there is minimal literature on isolated TM and LD injuries. As a result, there is still debate about the optimal treatment method for these injuries, especially in athletes who compete at the highest level. In order to treat isolated injuries of these muscles, it is important to understand their anatomic relationship, as these 2 muscles are intimately associated. The LD originates from the thoracolumbar spine and inserts on the proximal humerus between the pectoralis<hl name="2"/> major and TM tendons. The TM originates from the scapula and, similar to the LD, inserts on the proximal humerus. In an anatomic study, the TM tendon inserted into the LD tendon before its humeral insertion in the majority of cadavers.14,15
The LD is responsible for extension, adduction, and internal rotation of the humerus. The TM, while not as extensively studied, is believed to also contribute to extension, adduction, and internal rotation of the humerus.16 As Jobe and colleagues5 demonstrated, the LD is vital during the acceleration phase of pitching. While they were unable to make any conclusions about the role of the TM during the pitching cycle, it is reasonable to hypothesize that these 2 muscles work together. While it is thought that these 2 muscles work as a unit, it is significant to note that a professional pitcher can sustain an isolated injury to the TM without injury to the LD, and vice versa. This questions whether these 2 muscles work more independently than once thought. One hypothesis is that the physical size of the LD provides protection from injuries that the smaller TM cannot overcome. This is a potential area of further research.
The most common findings in patients with TM injuries include swelling, bruising, tenderness of the proximal arm, and limitations of shoulder range of motion in abduction, flexion, and external rotation. There is also weakness when resistance is applied against internal rotation and extension. Similar to the TM, common findings in patients with LD injuries include pain in the posterior shoulder, bruising, and weakness when resistance is applied against internal rotation of the shoulder. Pitchers are often able to pinpoint the occurrence of their acute pain during a specific time in the game. They commonly experience a pulling sensation and sometimes even feel a “pop” in their shoulder followed by an acute onset of pain and stiffness in the posterior aspect of the axilla. These injuries seem to be associated with the pitcher throwing a “breaking ball,” a pitch that requires greater shoulder rotation since it changes trajectory while traveling towards home plate. Despite the clear role of the LD and hypothesized role of the TM in the pitching sequence, there has been limited research on the optimal treatment of isolated injuries of these muscles in MLB pitchers. The majority of studies in this review opted for conservative treatment for both LD and TM injuries. The only study that presented a surgical option was for a LD avulsion injury.
Athletes undergoing either conservative or surgical treatment required a significant period of recovery and rehabilitation before they were able to compete at the professional level. In Leland and colleagues8, it took about 10 to 12 weeks of rehabilitation for both pitchers to return to pitching against competition. In Schickendantz and colleagues4, barring any complications or injury recurrence, it took patients 12 weeks to return to their preinjury level. In Malcolm and colleagues7, magnetic resonance imaging after 8 weeks showed marked recovery, and shortly after the pitcher was able to return to the pitching rotation. In Nagda and colleagues9, the time lost to injury ranged from 7 weeks to an entire season. Of the 9 pitchers who were lost for the season, 6 had avulsion injuries. The other 3 consisted of an LD strain, TM strain, and LD plus TM strain.9 In this study, it seems that avulsion injuries had a more significant impact on patient recovery. On average, it took 35.6 days after injury for players to begin throwing. In contrast, it took an average of 65.5 days after an avulsion injury for players to begin throwing. Ellman and colleagues10 included the only surgically repaired injury, and it was for an avulsion of the LD tendon. In the surgical case, it took slightly longer for the pitcher to return to preinjury form. It took him 12 to 16 weeks to begin light throwing and his full return to pitching took about 20 to 30 weeks. Since muscle strains and tendon avulsions are significantly different injuries in regards to the type of soft tissue damage and healing potential, they may require different treatment strategies. An avulsion injury may require more aggressive intervention, whereas a strain may only require conservative rehabilitation. Ultimately, there does not seem to be a significant benefit of one treatment option compared to the other. The majority of conservatively managed pitchers were able to return to previous form in a reasonable time frame. While each rehabilitation protocol was slightly different, multiple studies advocated for rehab programs that centered around the following goals: slowly progressing pitchers to light throwing once their pain resolved, followed by long throwing, then throwing off of the mound, and finally returning to competitive pitching. It is important to discuss with patients that rehabilitation generally takes 12 to 16 weeks before they are able to fully return to pitching against competition and that rest should immediately follow any recurrence of pain or stiffness. Once those symptoms resolve, patients may continue the rehabilitation protocol.
As with any form of treatment, there are risks involved. This holds true for both conservative and nonconservative therapy for LD and TM injuries. One risk of nonoperative treatment of an LD avulsion is the development of strength deficits in the muscle.17 While this deficit may go unnoticed in a recreational athlete, it may be more pronounced in a professional athlete, especially since the LD of a professional baseball pitcher is more active on electromyography during the acceleration phase of the pitching cycle compared to a recreational athlete.18 Another risk of conservative treatment of an LD avulsion is jeopardizing the potential for future surgery. As a result, some advocate for early surgical intervention of an acute LD avulsion.19,20 Others, however, recommend conservative management with subsequent surgical intervention if conservative measures fail. One caveat is that surgical intervention to restore the original anatomy may become difficult after a certain period of time due to the buildup of scar tissue. Surgical intervention also has associated risks, such as nerve injury, infection, vascular damage, persistent pain, and the buildup of large amounts of scar tissue. It is important to discuss these risks with patients when deciding on a treatment option.
LD and TM avulsion and tears typically present after an acute event in throwing athletes. There are a number of case reports published that demonstrate successful outcomes with both nonoperative management21 and operative repair of LD injuries in non-throwing athletes such as competitive water skiers,22,23 steer wrestlers,24 professional wrestlers,25 and recreational rock climbers.26 The 5 studies included in this review were the first ones to present LD and TM injuries in MLB pitchers. They discussed the outcomes of mainly conservative and surgical management of LD and TM avulsion and tears. Unfortunately, there remains a limited number of cases on the treatment of these injuries in highly competitive throwing athletes. Further research is required to elucidate the advantages and disadvantages of operative vs nonoperative treatment. The goal of this review is to provide clinicians with a concise summary of the current literature so that they may offer some evidence to their patients when discussing appropriate treatment plans.
Upper extremity injuries are very common in pitchers in amateur and professional baseball. The vast majority involving labral or rotator cuff pathology.1-3 While uncommon, injuries to the latissimus dorsi (LD) (Figure) and teres major (TM) have been reported in Major League Baseball (MLB) pitchers.4 Jobe and colleagues5 demonstrated the role of the LD during the various phases of pitching. The LD is most active during the acceleration phase and remains active during the deceleration phase and follow-through.6 Anatomically, the TM lies posterior to the LD separated by bursal tissue. The tendon fibers converge and unite along their lower borders, leading to a synergistic mechanism of action.
Due to the rarity of LD and TM injuries, literature on the pathology and appropriate treatments for these injuries is limited. The goal of this review is to present the current literature on professional baseball players who have undergone either nonsurgical treatment or surgery for LD and TM strains and/or avulsion injuries. This review will ultimately assist clinicians when deciding on the optimal treatment method for professional baseball players.
Methods
We performed an extensive Medline database search with the following search algorithm: ([latissimus OR latissimus dorsi OR teres major] AND baseball). The search returned 20 citations. Inclusion criteria consisted of clinical studies that focused on professional baseball pitchers with TM and/or LD injuries that underwent either conservative nonsurgical treatment or surgical repair. There was no exclusion based on the type of injury present, such as avulsion vs strain. Any study with amateur athletes or athletes from other sports such as handball or rugby were excluded. Due to the limited amount of data available, the majority of included studies were case reports and case series.
Based on these parameters, 5 articles met criteria for inclusion. Of the 5 included studies, 3 were case reports and 2 were case series. From the eligible articles, the following information was obtained: publication year, sample size, mean age, mean follow-up duration, type of treatment (conservative vs surgical), ability to return to original level of play, time required to return to original form, and complications (Tables 1, 2).
Results
Nonoperative Management
Four of the 5 included studies implemented only conservative therapy for their patients.4,7-9 The average duration these patients were followed for during treatment and rehabilitation was 26.3 months. Malcolm and colleagues7 followed patients for 8 months, the shortest length among the 4 conservative studies in this review. Leland and colleagues8 followed patients for 17 months, and Nagda and colleagues9 had the longest length of observation of 36 months (range 12 to 82 months).Schickendantz and colleagues4 followed patients for >12 months, but the exact duration was not specified. In order to calculate the average duration of observation, each patient was assigned a duration of 12 months.
Of the 30 patients included in this review, 29 were treated conservatively. All of the included studies consisted of male patients. The mean age was 26.8 years (range 22 to 28.1 years). Of the 29 injuries treated conservatively, there were 2 LD tendon avulsions, 4 TM tendon avulsions, 1 LD and TM tendon avulsion, 7 LD intramuscular strains, 9 TM intramuscular strains, and 6 LD and TM intramuscular strains.
Treatment Protocol
The various treatment and rehabilitation programs used for the conservative patient population all followed a similar pathway. After initial injury, a rest period focused on stretching was implemented. Patients were started on steroid or anti-inflammatory medications, cryotherapy, or other therapeutic modalities. Once pain-free and full range of motion was achieved, patients began the strength and throwing components of the rehabilitation program. Reoccurrence of symptoms would halt the throwing component of the rehabilitation program until symptoms improved. Patients were progressed through a return-to-throw program and once they could throw off the mound and achieve their preinjury velocity, strength, and range of motion, they were cleared to return to competitive pitching.
In the senior author’s (MSS) practice, all throwers are managed with the same nonoperative protocol.4 Initial treatment consists of short periods of rest and symptom control via the application of cryotherapy, among other modalities. Restoration of preinjury range of motion is achieved with active-assisted stretching exercises. As range of motion begins approaching pre-injury levels, strength training is initiated with isometric strengthening of the LD and TM progressing to resistance exercises. Exercising the abdominal core, strengthening the lower body, and cardiovascular conditioning are focal points of the rehabilitation period. Once patients regain preinjury shoulder strength and range of motion without pain, they begin a throwing program that consists of 4 weeks of long toss followed by 2 weeks of throwing from the pitching mound. After completion of the throwing program, the patient is allowed to return to competitive pitching. For patients who did not suffer season-ending injury, the average time required to return to play was 99.8 days (range 72.3 to 182.6 days).
Complications and Reinjury
The patients in Leland and colleagues8 and Malcolm and colleagues7 did not suffer any complications or reinjuries. In Schickendantz and colleagues4, all but 3 of the 10 patients were able to return to full speed pitching by 3 months. The other 3 required 4, 6, and 10 months. The patient that required 10 months tore both his LD and TM and the patient that required 6 months tore his TM and was never able to regain his pre-injury throwing velocity. None of the TM tears had a recurrence, while 1 LD tear had a recurrence of injury 6 months after returning to competitive pitching. This patient was successfully treated with 6 weeks of conservative rest and rehabilitation.
In Nagda and colleagues9, 2 athletes suffered injury recurrence. One athlete with a LD strain suffered 2 subsequent LD strains, 4 months and 1 year after initial injury. The other athlete with a LD avulsion suffered a subsequent TM avulsion 13 months after initial injury. One pitcher who had an LD and TM strain suffered a superior labrum anterior and posterior (SLAP) tear and was never able to return to his prior level of play.
Surgical Treatment
Only 1 of the 5 included studies utilized surgical repair for their patient.10 The single patient suffered an avulsion injury of the distal LD tendon and its insertion on the humerus. The LD tendon was retracted approximately 5 cm from the distal humeral insertion. The TM was not involved. Eight days post-injury, the patient underwent surgical repair.11 Postoperatively, the patient started passive range of motion after 2 weeks and active range of motion after 6 weeks. He started throwing at 12 weeks and returned to play at 30 weeks after he had returned to his preinjury form in regards to muscle strength, pitch control, and velocity. The patient was able to resume pitching at a high level in MLB.
Discussion
Overhand throwing athletes, especially professional baseball players, have to constantly deal with a variety of shoulder injuries.12,13 Currently, there is minimal literature on isolated TM and LD injuries. As a result, there is still debate about the optimal treatment method for these injuries, especially in athletes who compete at the highest level. In order to treat isolated injuries of these muscles, it is important to understand their anatomic relationship, as these 2 muscles are intimately associated. The LD originates from the thoracolumbar spine and inserts on the proximal humerus between the pectoralis<hl name="2"/> major and TM tendons. The TM originates from the scapula and, similar to the LD, inserts on the proximal humerus. In an anatomic study, the TM tendon inserted into the LD tendon before its humeral insertion in the majority of cadavers.14,15
The LD is responsible for extension, adduction, and internal rotation of the humerus. The TM, while not as extensively studied, is believed to also contribute to extension, adduction, and internal rotation of the humerus.16 As Jobe and colleagues5 demonstrated, the LD is vital during the acceleration phase of pitching. While they were unable to make any conclusions about the role of the TM during the pitching cycle, it is reasonable to hypothesize that these 2 muscles work together. While it is thought that these 2 muscles work as a unit, it is significant to note that a professional pitcher can sustain an isolated injury to the TM without injury to the LD, and vice versa. This questions whether these 2 muscles work more independently than once thought. One hypothesis is that the physical size of the LD provides protection from injuries that the smaller TM cannot overcome. This is a potential area of further research.
The most common findings in patients with TM injuries include swelling, bruising, tenderness of the proximal arm, and limitations of shoulder range of motion in abduction, flexion, and external rotation. There is also weakness when resistance is applied against internal rotation and extension. Similar to the TM, common findings in patients with LD injuries include pain in the posterior shoulder, bruising, and weakness when resistance is applied against internal rotation of the shoulder. Pitchers are often able to pinpoint the occurrence of their acute pain during a specific time in the game. They commonly experience a pulling sensation and sometimes even feel a “pop” in their shoulder followed by an acute onset of pain and stiffness in the posterior aspect of the axilla. These injuries seem to be associated with the pitcher throwing a “breaking ball,” a pitch that requires greater shoulder rotation since it changes trajectory while traveling towards home plate. Despite the clear role of the LD and hypothesized role of the TM in the pitching sequence, there has been limited research on the optimal treatment of isolated injuries of these muscles in MLB pitchers. The majority of studies in this review opted for conservative treatment for both LD and TM injuries. The only study that presented a surgical option was for a LD avulsion injury.
Athletes undergoing either conservative or surgical treatment required a significant period of recovery and rehabilitation before they were able to compete at the professional level. In Leland and colleagues8, it took about 10 to 12 weeks of rehabilitation for both pitchers to return to pitching against competition. In Schickendantz and colleagues4, barring any complications or injury recurrence, it took patients 12 weeks to return to their preinjury level. In Malcolm and colleagues7, magnetic resonance imaging after 8 weeks showed marked recovery, and shortly after the pitcher was able to return to the pitching rotation. In Nagda and colleagues9, the time lost to injury ranged from 7 weeks to an entire season. Of the 9 pitchers who were lost for the season, 6 had avulsion injuries. The other 3 consisted of an LD strain, TM strain, and LD plus TM strain.9 In this study, it seems that avulsion injuries had a more significant impact on patient recovery. On average, it took 35.6 days after injury for players to begin throwing. In contrast, it took an average of 65.5 days after an avulsion injury for players to begin throwing. Ellman and colleagues10 included the only surgically repaired injury, and it was for an avulsion of the LD tendon. In the surgical case, it took slightly longer for the pitcher to return to preinjury form. It took him 12 to 16 weeks to begin light throwing and his full return to pitching took about 20 to 30 weeks. Since muscle strains and tendon avulsions are significantly different injuries in regards to the type of soft tissue damage and healing potential, they may require different treatment strategies. An avulsion injury may require more aggressive intervention, whereas a strain may only require conservative rehabilitation. Ultimately, there does not seem to be a significant benefit of one treatment option compared to the other. The majority of conservatively managed pitchers were able to return to previous form in a reasonable time frame. While each rehabilitation protocol was slightly different, multiple studies advocated for rehab programs that centered around the following goals: slowly progressing pitchers to light throwing once their pain resolved, followed by long throwing, then throwing off of the mound, and finally returning to competitive pitching. It is important to discuss with patients that rehabilitation generally takes 12 to 16 weeks before they are able to fully return to pitching against competition and that rest should immediately follow any recurrence of pain or stiffness. Once those symptoms resolve, patients may continue the rehabilitation protocol.
As with any form of treatment, there are risks involved. This holds true for both conservative and nonconservative therapy for LD and TM injuries. One risk of nonoperative treatment of an LD avulsion is the development of strength deficits in the muscle.17 While this deficit may go unnoticed in a recreational athlete, it may be more pronounced in a professional athlete, especially since the LD of a professional baseball pitcher is more active on electromyography during the acceleration phase of the pitching cycle compared to a recreational athlete.18 Another risk of conservative treatment of an LD avulsion is jeopardizing the potential for future surgery. As a result, some advocate for early surgical intervention of an acute LD avulsion.19,20 Others, however, recommend conservative management with subsequent surgical intervention if conservative measures fail. One caveat is that surgical intervention to restore the original anatomy may become difficult after a certain period of time due to the buildup of scar tissue. Surgical intervention also has associated risks, such as nerve injury, infection, vascular damage, persistent pain, and the buildup of large amounts of scar tissue. It is important to discuss these risks with patients when deciding on a treatment option.
LD and TM avulsion and tears typically present after an acute event in throwing athletes. There are a number of case reports published that demonstrate successful outcomes with both nonoperative management21 and operative repair of LD injuries in non-throwing athletes such as competitive water skiers,22,23 steer wrestlers,24 professional wrestlers,25 and recreational rock climbers.26 The 5 studies included in this review were the first ones to present LD and TM injuries in MLB pitchers. They discussed the outcomes of mainly conservative and surgical management of LD and TM avulsion and tears. Unfortunately, there remains a limited number of cases on the treatment of these injuries in highly competitive throwing athletes. Further research is required to elucidate the advantages and disadvantages of operative vs nonoperative treatment. The goal of this review is to provide clinicians with a concise summary of the current literature so that they may offer some evidence to their patients when discussing appropriate treatment plans.
1. Conway JE, Arthroscopic repair of partial-thickness rotator cuff tears and SLAP lesions in professional baseball players. Orthop Clin North Am. 2001;32(3):443-456.
2. Mazoue CG, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34(2):182-189.
3. Cerynik DL, Ewald TJ, Sastry A, Amin NH, Liao JG, Tom JA. Outcomes of isolated glenoid labral injuries in professional baseball pitchers. Clin J Sport Med. 2008;18(3):255-258
4. Schickendantz MS, Kaar SG, Meister K, Lund P, Beverley L. Latissimus dorsi and teres major tears in professional baseball pitchers: a case series. Am J Sports Med. 2009;37(10):2016-2020.
5. Jobe FW, Moynes DR, Tibone JE, Perry J. An EMG analysis of the shoulder in pitching. A second report. Am J Sports Med. 1984;12(3):218-220.
6. Glousman R, Jobe F, Tibone J, Moynes D, Antonelli D, Perry J. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am. 1988;70(2):220-226.
7. Malcolm PN, Reinus WR, London SL. Magnetic resonance imaging appearance of teres major tendon injury in a baseball pitcher. Am J Sports Med. 1999;27(1):98-100.
8. Leland JM, Ciccotti MG, Cohen SB, Zoga AC, Frederick RJ. Teres major injuries in two professional baseball pitchers. J Shoulder Elbow Surg. 2009;18(6):e1-e5.
9. Nagda SH, Cohen SB, Noonan TJ, Raasch WG, Ciccotti MG, Yocum LA. Management and outcomes of latissimus dorsi and teres major injuries in professional baseball pitchers. Am J Sports Med. 2011;39(10):2181-2186.
10. Ellman MB, Yanke A, Juhan T, et al. Open repair of an acute latissimus tendon avulsion in a Major League Baseball pitcher. J Shoulder Elbow Surg. 2013;22(7):e19-e23.
11. Ellman MB, Yanke A, Juhan T, et al. Open repair of retracted latissimus dorsi tendon avulsion. Am J Orthop. 2013;42(6):280-285.
12. Altchek DW, Dines DM. Shoulder injuries in the throwing athlete. J Am Acad Orthop Surg. 1995;3(3):159-165.
13. Limpisvasti O, ElAttrache NS, Jobe FW. Understanding shoulder and elbow injuries in baseball. J Am Acad Orthop Surg. 2007;15(3):139-147.
14. Beck PA, Hoffer MM. Latissimus dorsi and teres major tendons: separate or conjoint tendons? J Pediatr Orthop. 1989;9(3):308-309.
15. Morelli M, Nagamori J, Gilbart M, Miniaci A. Latissimus dorsi tendon transfer for massive irreparable cuff tears: an anatomic study. J Shoulder Elbow Surg. 2008;17(1):139-143.
16. Broome HL, Basmajian JV. The function of the teres major muscle: an electromyographic study. Anat Rec. 1971;170(3):309-310.
17. Brumback RJ, McBride MS, Ortolani NC. Functional evaluation of the shoulder after transfer of the vascularized latissimus dorsi muscle. J Bone Joint Surg Am. 1992;74(3):377-382.
18. Gowan ID, Jobe FW, Tibone JE, Perry J, Moynes DR. A comparative electromyographic analysis of the shoulder during pitching. Professional versus amateur pitchers. Am J Sports Med. 1987;15(6):586-590.
19. Park JY, Lhee SH, Keum JS. Rupture of latissimus dorsi muscle in a tennis player. Orthopedics. 2008;31(10).
20. Gregory JM, Harwood DP, Sherman SL, Romeo AA. Surgical repair of a subacute latissimus dorsi tendon rupture. Tech Shoulder Elbow Surg. 2011;12(4):77-79.
21. Butterwick DJ, Mohtadi NG, Meeuwisse WH, Frizzell JB. Rupture of latissimus dorsi in an athlete. Clin J Sport Med. 2003;13(3):189-191.
22. Henry JC, Scerpella TA. Acute traumatic tear of the latissimus dorsi tendon from its insertion. A case report. Am J Sports Med. 2000;28(4):577-579.
23. Lim JK, Tilford ME, Hamersly SF, Sallay PI. Surgical repair of an acute latissimus dorsi tendon avulsion using suture anchors through a single incision. Am J Sports Med. 2006;34(8):1351-1355.
24. Hiemstra LA, Butterwick D, Cooke M, Walker RE. Surgical management of latissimus dorsi rupture in a steer wrestler. Clin J Sport Med. 2007;17(4):316-318.
25. Hapa O, Wijdicks CA, LaPrade RF, Braman JP. Out of the ring and into a sling: acute latissimus dorsi avulsion in a professional wrestler: a case report and review of the literature. Knee Surg Sports Traumatol Arthrosc. 2008;16(12):1146-1150.
26. Livesey J, Brownson P, Wallace WA. Traumatic latissimus dorsi tendon rupture. J Shoulder Elbow Surg. 2002;11(6):642-644.
1. Conway JE, Arthroscopic repair of partial-thickness rotator cuff tears and SLAP lesions in professional baseball players. Orthop Clin North Am. 2001;32(3):443-456.
2. Mazoue CG, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34(2):182-189.
3. Cerynik DL, Ewald TJ, Sastry A, Amin NH, Liao JG, Tom JA. Outcomes of isolated glenoid labral injuries in professional baseball pitchers. Clin J Sport Med. 2008;18(3):255-258
4. Schickendantz MS, Kaar SG, Meister K, Lund P, Beverley L. Latissimus dorsi and teres major tears in professional baseball pitchers: a case series. Am J Sports Med. 2009;37(10):2016-2020.
5. Jobe FW, Moynes DR, Tibone JE, Perry J. An EMG analysis of the shoulder in pitching. A second report. Am J Sports Med. 1984;12(3):218-220.
6. Glousman R, Jobe F, Tibone J, Moynes D, Antonelli D, Perry J. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am. 1988;70(2):220-226.
7. Malcolm PN, Reinus WR, London SL. Magnetic resonance imaging appearance of teres major tendon injury in a baseball pitcher. Am J Sports Med. 1999;27(1):98-100.
8. Leland JM, Ciccotti MG, Cohen SB, Zoga AC, Frederick RJ. Teres major injuries in two professional baseball pitchers. J Shoulder Elbow Surg. 2009;18(6):e1-e5.
9. Nagda SH, Cohen SB, Noonan TJ, Raasch WG, Ciccotti MG, Yocum LA. Management and outcomes of latissimus dorsi and teres major injuries in professional baseball pitchers. Am J Sports Med. 2011;39(10):2181-2186.
10. Ellman MB, Yanke A, Juhan T, et al. Open repair of an acute latissimus tendon avulsion in a Major League Baseball pitcher. J Shoulder Elbow Surg. 2013;22(7):e19-e23.
11. Ellman MB, Yanke A, Juhan T, et al. Open repair of retracted latissimus dorsi tendon avulsion. Am J Orthop. 2013;42(6):280-285.
12. Altchek DW, Dines DM. Shoulder injuries in the throwing athlete. J Am Acad Orthop Surg. 1995;3(3):159-165.
13. Limpisvasti O, ElAttrache NS, Jobe FW. Understanding shoulder and elbow injuries in baseball. J Am Acad Orthop Surg. 2007;15(3):139-147.
14. Beck PA, Hoffer MM. Latissimus dorsi and teres major tendons: separate or conjoint tendons? J Pediatr Orthop. 1989;9(3):308-309.
15. Morelli M, Nagamori J, Gilbart M, Miniaci A. Latissimus dorsi tendon transfer for massive irreparable cuff tears: an anatomic study. J Shoulder Elbow Surg. 2008;17(1):139-143.
16. Broome HL, Basmajian JV. The function of the teres major muscle: an electromyographic study. Anat Rec. 1971;170(3):309-310.
17. Brumback RJ, McBride MS, Ortolani NC. Functional evaluation of the shoulder after transfer of the vascularized latissimus dorsi muscle. J Bone Joint Surg Am. 1992;74(3):377-382.
18. Gowan ID, Jobe FW, Tibone JE, Perry J, Moynes DR. A comparative electromyographic analysis of the shoulder during pitching. Professional versus amateur pitchers. Am J Sports Med. 1987;15(6):586-590.
19. Park JY, Lhee SH, Keum JS. Rupture of latissimus dorsi muscle in a tennis player. Orthopedics. 2008;31(10).
20. Gregory JM, Harwood DP, Sherman SL, Romeo AA. Surgical repair of a subacute latissimus dorsi tendon rupture. Tech Shoulder Elbow Surg. 2011;12(4):77-79.
21. Butterwick DJ, Mohtadi NG, Meeuwisse WH, Frizzell JB. Rupture of latissimus dorsi in an athlete. Clin J Sport Med. 2003;13(3):189-191.
22. Henry JC, Scerpella TA. Acute traumatic tear of the latissimus dorsi tendon from its insertion. A case report. Am J Sports Med. 2000;28(4):577-579.
23. Lim JK, Tilford ME, Hamersly SF, Sallay PI. Surgical repair of an acute latissimus dorsi tendon avulsion using suture anchors through a single incision. Am J Sports Med. 2006;34(8):1351-1355.
24. Hiemstra LA, Butterwick D, Cooke M, Walker RE. Surgical management of latissimus dorsi rupture in a steer wrestler. Clin J Sport Med. 2007;17(4):316-318.
25. Hapa O, Wijdicks CA, LaPrade RF, Braman JP. Out of the ring and into a sling: acute latissimus dorsi avulsion in a professional wrestler: a case report and review of the literature. Knee Surg Sports Traumatol Arthrosc. 2008;16(12):1146-1150.
26. Livesey J, Brownson P, Wallace WA. Traumatic latissimus dorsi tendon rupture. J Shoulder Elbow Surg. 2002;11(6):642-644.