User login
Brief Review of Major Depressive Disorder for Primary Care Providers
Although a common condition in the general population, major depressive disorder (MDD) is even more prevalent among patients receiving medical care. Its early recognition and successful treatment have significant implications in all fields of medicine, and the predominant burden of treatment falls on primary care providers (PCPs). The prevalence of MDD over 12 months is about 6.6%; lifetime risk is about 16.6%.1,2 About 50% of depressive episodes are rated severe or higher level. Of those having a depressive episode lasting 1 year, only 51% will seek medical help, and only 42% of these will receive adequate treatment.3 The illness is present across cultures and socioeconomic strata, although the actual rates vary. Women have about a 2-fold higher rate of MDD than do men.4
The illness can vary from mild to severe. Primary care providers usually provide treatment for patients who are at the mild-to-moderate end of the spectrum; more severely ill patients are likely to require consulting with mental health specialists. Patients who have 1 episode of MDD have a 50% chance of having a second episode during their lifetime. After a second episode, the risk of another episode rises to about 80%.5 After a third episode, it is presumed patients will continue to have subsequent episodes, because the risk rises to 90%. Among patients with MDD, about 35% will have a chronic, relapsing pattern of illness.6 Because of a relative shortage of specialty mental health providers in many areas served by federal practitioners, it is important to maximize the impact of a patient’s primary care team in treating this common and persistent illness.
Morbidity from MDD includes dysfunction in all spheres of life: Difficulties with work, home life, self-care, medication adherence, and mental health are all encountered. Although a minority of patients with depression will attempt or complete suicide, depression is a major predictor of suicide risk. However, it is a modifiable risk factor with successful treatment. Among the general population, the reported risk of suicide is about 11.5 per 100,000 per year.7 Among patients with psychiatric illness, the rate is higher.
Many patients treated through the federal system have been identified as having a risk of suicide higher than that of the general population. These populations include veterans (41% to 61% above the national average); Native Americans aged 18 to 24 years (about 2 to 3 times the national average); and active-duty military (18.7 per 100,000 per year, 50% above the national average).8-10
Diagnosis
Several subtypes of MDD and associated depressive disorders exist. The diagnosis requires a constellation of criteria for diagnosis. Using the full criteria to make an MDD diagnosis may seem excessive, but its use is critical to guide sound treatment decisions (ie, past presence of manic or hypomanic episode, which would signify bipolar disorder, or psychotic symptoms that would dictate a different path and a referral to a psychiatrist). For example, an antidepressant given to a patient with a history of hypomania or mania can trigger a full manic episode with all the potential morbidity that mania entails. The presence of psychotic symptoms leads down a different treatment path that requires antipsychotic medication.
A major depressive episode includes the sine qua non of depressed mood or anhedonia (lack of experiencing or seeking pleasurable activities) and 3 to 4 associated criteria, including poor energy (often noted as “I just can’t seem to get moving”), insomnia/hypersomnia (usually middle or late insomnia) with difficulty concentrating (often seen as problems making decisions), increased sense of guilt or worthlessness, psychomotor agitation or retardation (often noted by the patient’s spouse), significant weight loss or gain (5%), and thoughts of death/dying or suicidal ideation. These must be present most days over the previous 2 weeks.1 The time duration criteria are important because some patients may seem very distressed during a visit but do not meet the criteria for depression or have the chronic depressive symptoms of MDD. Symptoms not meeting the full criteria may likely be noted as unspecified depressive disorder or dysthymia (now termed persistent depressive disorder in Diagnostic and Statistical Manual of Mental Disorders, 5th Edition [DSM 5]).1 There are no FDA-approved treatments for these disorders; however, in cases requiring treatment, the standard of practice would be the same as for MDD.
The more severe types of MDD are usually not subtle and would likely require an immediate referral to a mental health professional. These include the presence of melancholic features, such as severe anhedonia, or loss of reactivity to normally pleasurable things, and at least 3 of the following: (1) distinct quality of depressed mood that is characterized as different from serious loss (ie, death of a loved one); (2) worse depression in the morning; (3) late insomnia (waking 2+ hours early); (4) marked psychomotor agitation or retardation; (5) significant anorexia or weight loss; and (6) excessive and inappropriate guilt; or the presence of psychotic features, such as delusions or hallucinations. There are other subtypes of MDD that can be found in DSM 5.
Treatment
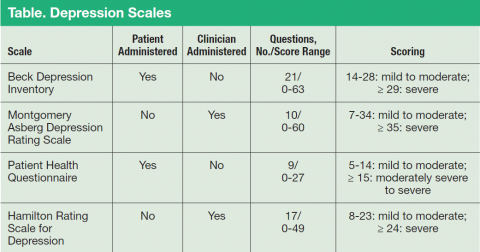
Major depressive disorder can be viewed as analogous to common illnesses, such as hypertension or diabetes, which are treated by a PCP; screening for depression should be systematically included as part of primary care services. The clear goal of treatment for any illness is elimination or reduction of symptoms so they no longer cause any significant problem for the patient. In MDD remission is the complete resolution of depressive symptoms. Response is considered a 50% reduction of MDD symptom severity as rated on various depression scales (Table). Because no objective physical measurements exist for assessing a patient’s depression, using a rating scale is necessary to monitor the severity of depressive symptoms and a patient’s response to treatment.
There are many validated depression scales, both clinician and patient administered. A patient-administered scale can save time and provide needed information; however, the questions of a clinician-administered scale can help screen for depression and improve a clinician’s sensitivity to a patient’s depression even if the patient does not bring up the subject. These scales are available on the Internet and include instructions for proper use. Benefits of the scales include giving clinicians data to discuss with patients and helping patients track their progress. For example, patients do not always recall how they were doing before they started taking medications, so the scales can help them measure the improvement.
Psychotherapies
As first-line treatment for patients with MDD, no clear benefit exists for medication over psychological therapy, specifically evidence-based therapies (EBTs).11,12 The best studied and known EBTs are cognitive behavioral therapy for depression (CBT-D) and interpersonal therapy (IPT). Both are brief, targeted therapies with clear rules and expectations as opposed to more traditional long-term therapies, such as insight-oriented psychotherapy. These therapies generally involve weekly meetings with a therapist for about 12 weeks. They both require the patient to do homework during the therapy period; therefore, it is important for the patient to be a willing participant in these treatments to receive the maximum benefit. Many patients prefer not taking medication for depression, so psychotherapy is an excellent option. It is widely believed, although without clear evidence at this time, that the combination of medications and EBT offers improved outcomes. There are no contraindications to combining antidepressant medication and EBT.
Medications
Given the realities of practice settings and patient preferences, medication is often the most practical first-line treatment choice. The newer antidepressant medications are the most likely choices in the primary care setting for treating depression, because they offer effective treatment with less severe adverse effects (AEs) and are much safer than the early tricyclic antidepressant and monoamine oxidase inhibitor medications. There are numerous metaanalyses comparing the effectiveness of the commonly prescribed newer antidepressants that consistently show there is no absolutely best choice antidepressant. A number of studies have tried to identify predictors of response for a particular antidepressant, but these have not yielded clinically significant results. Additionally, there is little difference between an antidepressant’s rate of response and tolerability on a population scale.13 Therefore, it is the AE profile and alternate uses that usually drive the choice of which to use for a particular patient.
Before discussing antidepressant medications with patients, it is important to note the FDA-required black box warning for increased risk of suicidal ideation in young people aged ≤ 24 years. The data that led to this warning did not show an increased risk of suicide.14 In patients aged > 24 years, there is no difference in risk of suicidal ideation, and in patients aged > 65 years, the risk of suicidal ideation decreases with the start of antidepressant medication.1
SSRIs
Selective serotonin reuptake inhibitors (SSRIs) are a common first-line treatment for MDD. Of interest, all SSRIs share the same mechanism of action (MOA), so failure of a single agent does not preclude a trial of a second agent of the same class, because the chance of response is essentially the same as switching the patient to another class. However, after a second failure, there is less chance of response to another SSRI.5 These agents are used for depression, anxiety (long term not acute), posttraumatic stress disorder (PTSD), and obsessive-compulsive disorder. The AEs include nausea, headache, insomnia, dry mouth, and loss of emotion. Other than feeling emotionally blunted, most of these AEs are usually temporary and resolve in days. Sexual dysfunction is often the AE of SSRIs that draws primary concern from patients. The most frequently experienced sexual AE is delayed orgasm. A review of FDA package inserts showed rates from 7% (sertraline) to as high as 28% (high-dose paroxetine).16,17 Impotence or decreased libido is often more concerning for patients and occurs at 3% to 6%. A common concern for patients is weight gain, and SSRIs are considered weight neutral. There has been no demonstrated benefit from combining these medications or using them with selective serotonin norepinephrine reuptake inhibitors (SNRIs), which only exposes patients to increased risk of AEs.
Other Medications
Often used as first-line medications for MDD, SNRIs are also useful for MDD, anxiety (long term not acute), and PTSD. Duloxetine also has indications for diabetic peripheral neuropathy, fibromyalgia, and chronic musculoskeletal pain. The SNRIs share the same AEs as SSRIs; however, some patients show an increase in blood pressure with venlafaxine. There has been no demonstrated benefit from combining these medications or with SSRIs. These are also considered weight neutral.
Mirtazapine, bupropion, and trazodone are medications with MOAs that do not fit within the abovementioned categories and differ from one another as well. These carry FDA indications for MDD. Bupropion also has indications of smoking cessation and seasonal affective disorder for the extended-release form. The AEs are varied and unique to each medication. Mirtazapine may show a rapid improvement in mood within 2 weeks and very low risk of sexual AEs.18 Associated with some weight gain and sedation, mirtazapine is useful for patients who have sleep problems or who are experiencing weight loss. It is noted in practice to be more sedating at lower doses.
Bupropion is considered an activating medication that can lead to jitteriness and increased anxiety for some patients. Bupropion also is associated with some weight loss and will disrupt sleep if taken later in the day. Therefore, bupropion can be useful for overweight patients or those with significant energy problems. It has few sexual AEs.
Although not widely used as a primary agent for MDD, trazodone is commonly used at low doses to treat insomnia. Sedation and dry mouth are the primary AEs with some sexual AEs. These medications can be used alone, in combination with SSRIs or SNRIs, or with one another in what is commonly called rational pharmacology.
Patient Response
There is a significant portion of patients who are treated for MDD who will not have an adequate response to their first medication, somewhat analogous to patients who are treated for hypertension or diabetes. If patients are showing a response at 6 to 8 weeks but not remission, the medication trial should continue for 12 or more weeks, increasing the dose as tolerated. If the dose has been raised to the maximum and if there are still significant depressive symptoms, some combination or augmentation strategy should be tried. If patients show no improvement at 6 to 8 weeks, it is appropriate to discontinue the current treatment and trial another medication.
There are no clear guidelines for the best way to switch medications. Some practitioners cross taper; some discontinue one medication prior to starting another; and some abruptly stop one and start another (ie, changing between SSRIs, or from a SSRI to a SNRI). In all cases, some caution is recommended to avoid discontinuation syndromes, which often drive the rate of discontinuation of the medication: Many people have no problem; others are very sensitive to dosage changes.
If a patient has achieved remission with the medication, the next step is easy—continue the medication. It is generally accepted that 6 to 12 months of medication treatment after remission is best, to avoid relapse to another episode of MDD. If a patient desires to stop medication after that period, it is best to slowly titrate off over a month or more, as the patient tolerates, to avoid the discontinuation syndrome and lessen the risk of relapse. Remission rates for a single trial of medication are 35% to 45% and up to 65% with repeated medication changes.19 Therefore, 35% of patients are inadequately relieved of illness, referred to as treatment-resistant illness.
In the patient who has experienced some benefit but not remission after taking the dose to the highest approved or tolerated dose, the question is whether to switch from a medication that has shown some efficacy to another that may be better or to add another medication to augment the efficacy of the first treatment. There are many opinions regarding these decisions and about how to proceed.
Augmentation and Combination Treatments
Augmentation is the use of a nonantidepressant medication in addition to the antidepressant to improve the efficacy of the antidepressant medication. Combination treatments use 2 antidepressant medications to improve efficacy. Although there is clear evidence of benefit from a number of augmentation strategies, including some that are FDA approved, combination treatments have conflicting
published evidence of efficacy.20,21
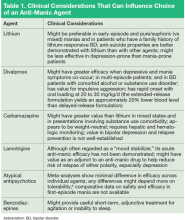
The FDA-approved medications for augmenting antidepressant medications in treatment-resistant MDD are aripiprazole and quetiapine XR. Both are atypical antipsychotic medications with the intrinsic class risks associated with them. Only psychiatrists usually use them, because most systems require the approval of a specialist to use these agents. Of particular concern is the longterm risks of tardive dyskinesia, neuroleptic malignant syndrome, weight gain, metabolic syndrome, diabetes, and sudden death in geriatric patients with dementia that warranted a black box warning. Other well-established augmentation strategies used by psychiatrists include the usage of lithium or T3 hormone. Using these agents requires collaborative monitoring with the PCP to prevent potential renal, cardiac, and thyroid abnormalities.
Conclusions
Successful treatment of depression in medical settings can have a positive impact on medical conditions, such as potentially improving outcomes in the treatment of diabetes, cardiovascular problems, and pain. Active screening for depression followed by a safe and well-tolerated antidepressant may relieve symptoms in 6 to 8 weeks in about half of treated patients.
Tracking responses with one of the aforementioned scales is an excellent way to guide treatment decisions. Lack of tolerability or response should not discourage physicians from trying a different antidepressant. The successful treatment of depression requires patience but can make a big difference in the patient’s quality of life.
Click here to read the digital editition.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM5). 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
2. Kessler R, Berglund P, Demler O, Jin R, Merikangas K, Walters E. Lifetime prevalence and age-of-onset distributions of DSM-IV Disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
3. Kessler RC, Berglund P, Demler O, et al; National Comorbidity Survey Replication. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289(23):3095-3105.
4. Weissman MM, Bland RC, Canino GJ, et al. Cross-national epidemiology of major depressive and bipolar disorder. JAMA. 1996;276(4):293-299.
5. Burcusa SL, Iacono WG. Risk for recurrence in depression. Clin Psych Rev. 2007;279(8):959-985.
6. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. 3rd ed. Psychiatry Online Website. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published October 2010. Accessed February 17, 2016.
7. American Association of Suicidology. U.S.A. suicide: 2014 official final data. American Association of Suicidology Website. http://www.suicidology.org /Portals/14/docs/Resources/FactSheets/2014/2014datapgsv1b.pdf. Revised December 22, 2015. Accessed February 17, 2016.
8. Kang HK, Bullman TA, Smolenski DJ, Skopp NA, Gahm GA, Reger MA. Suicide risk among 1.3 million veterans who were on active duty during the Iraq and Afghanistan
wars. Ann Epidemiol. 2015;25(2):96-100.
9. Jiang C, Mitran A, Miniño A, Ni H. Racial and gender disparities in suicide among young adults aged 18-24: United States, 2009-2013. Centers for Disease Control and Prevention Website. http://www.cdc.gov/nchs/data/hestat/suicide/racial_and_gender_2009_2013.htm. Updated September 30, 2015. Accessed February 9, 2016.
10. Smolenski DJ, Reger MA, Bush NE, Skopp NA, Zhang Y, Campise RL. Department of Defense suicide event report 2013 annual report. National Center for Telehealth & Technology Website. http://t2health.dcoe.mil/sites/default/files/DoDSER-2013-Jan-13-2015-Final.pdf. Accessed February 9, 2016.
11. DeRubeis RJ, Hollon SD, Amsterdam JD, et al. Cognitive therapy vs medications in the treatment of moderate to severe depression. Arch Gen Psychiatry. 2005;62(4):409-416.
12. Elkin I, Shea MT, Watkins JT, et al. National Institute of Mental Health Treatment of Depression Collaborative Research Program. General effectiveness of treatments. Arch Gen Psychiatry. 1989;46(11):971-978.
13. Gartlehner G, Hansen RA, Reichenpfader U, et al. Drug class review: secondgeneration antidepressants final update 5 report. National Center for Biotechnology Information Website. http://www.ncbi.nlm.nih.gov/books/NBK54355/pdf/Bookshelf_NBK54355.pdf. Updated March 11, 2011. Accessed February 17, 2016.
14. Friedman RA. Antidepressants’ black-box warning—10 years later. N Engl J Med. 2014;371(18):1666-1668.
15. U.S. Food and Drug Administration. Antidepressant use in children, adolescents, and adults. Revisions to product labeling. U.S. Food and Drug Administration Website. http://www.fda.gov/downloads/Drugs/DrugSafety/InformationbyDrug-Class/UCM173233.pdf. Updated December 23, 2014. Accessed February 17, 2016.
16. Zoloft [package insert]. New York, NY: Pfizer; 2014.
17. Paxil [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2012.
18. Lavergne F, Berlin I, Gamma A, Stassen H, Angst J. Onset of improvement and response to mirtazapine in depression: a multicenter naturalistic study of 4771 patients. Neuropsychiatr Dis Treat. 2005;1(1):59-68.
19. Rush JA, Trivedi MH, Wisniewski SR, et al. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12): 1231-1242.
20. Rush AJ, Trivedi MH, Stewart JW, et al. Combining medications to enhance depression outcomes (CO-MED): acute and long-term outcomes of a single-blind randomized study. Am J Psychiatry. 2011;168(7):689-701.
21. Blier P, Ward HE, Tremblay P, Laberge L, Hébert C, Bergeron R. Combination of antidepressant medications from treatment initiation for major depressive disorder: a double-blind randomized study. Am J Psychiatry. 2010;167(3):281-288.
Note: Page numbers differ between the print issue and digital edition.
Although a common condition in the general population, major depressive disorder (MDD) is even more prevalent among patients receiving medical care. Its early recognition and successful treatment have significant implications in all fields of medicine, and the predominant burden of treatment falls on primary care providers (PCPs). The prevalence of MDD over 12 months is about 6.6%; lifetime risk is about 16.6%.1,2 About 50% of depressive episodes are rated severe or higher level. Of those having a depressive episode lasting 1 year, only 51% will seek medical help, and only 42% of these will receive adequate treatment.3 The illness is present across cultures and socioeconomic strata, although the actual rates vary. Women have about a 2-fold higher rate of MDD than do men.4
The illness can vary from mild to severe. Primary care providers usually provide treatment for patients who are at the mild-to-moderate end of the spectrum; more severely ill patients are likely to require consulting with mental health specialists. Patients who have 1 episode of MDD have a 50% chance of having a second episode during their lifetime. After a second episode, the risk of another episode rises to about 80%.5 After a third episode, it is presumed patients will continue to have subsequent episodes, because the risk rises to 90%. Among patients with MDD, about 35% will have a chronic, relapsing pattern of illness.6 Because of a relative shortage of specialty mental health providers in many areas served by federal practitioners, it is important to maximize the impact of a patient’s primary care team in treating this common and persistent illness.
Morbidity from MDD includes dysfunction in all spheres of life: Difficulties with work, home life, self-care, medication adherence, and mental health are all encountered. Although a minority of patients with depression will attempt or complete suicide, depression is a major predictor of suicide risk. However, it is a modifiable risk factor with successful treatment. Among the general population, the reported risk of suicide is about 11.5 per 100,000 per year.7 Among patients with psychiatric illness, the rate is higher.
Many patients treated through the federal system have been identified as having a risk of suicide higher than that of the general population. These populations include veterans (41% to 61% above the national average); Native Americans aged 18 to 24 years (about 2 to 3 times the national average); and active-duty military (18.7 per 100,000 per year, 50% above the national average).8-10
Diagnosis
Several subtypes of MDD and associated depressive disorders exist. The diagnosis requires a constellation of criteria for diagnosis. Using the full criteria to make an MDD diagnosis may seem excessive, but its use is critical to guide sound treatment decisions (ie, past presence of manic or hypomanic episode, which would signify bipolar disorder, or psychotic symptoms that would dictate a different path and a referral to a psychiatrist). For example, an antidepressant given to a patient with a history of hypomania or mania can trigger a full manic episode with all the potential morbidity that mania entails. The presence of psychotic symptoms leads down a different treatment path that requires antipsychotic medication.
A major depressive episode includes the sine qua non of depressed mood or anhedonia (lack of experiencing or seeking pleasurable activities) and 3 to 4 associated criteria, including poor energy (often noted as “I just can’t seem to get moving”), insomnia/hypersomnia (usually middle or late insomnia) with difficulty concentrating (often seen as problems making decisions), increased sense of guilt or worthlessness, psychomotor agitation or retardation (often noted by the patient’s spouse), significant weight loss or gain (5%), and thoughts of death/dying or suicidal ideation. These must be present most days over the previous 2 weeks.1 The time duration criteria are important because some patients may seem very distressed during a visit but do not meet the criteria for depression or have the chronic depressive symptoms of MDD. Symptoms not meeting the full criteria may likely be noted as unspecified depressive disorder or dysthymia (now termed persistent depressive disorder in Diagnostic and Statistical Manual of Mental Disorders, 5th Edition [DSM 5]).1 There are no FDA-approved treatments for these disorders; however, in cases requiring treatment, the standard of practice would be the same as for MDD.
The more severe types of MDD are usually not subtle and would likely require an immediate referral to a mental health professional. These include the presence of melancholic features, such as severe anhedonia, or loss of reactivity to normally pleasurable things, and at least 3 of the following: (1) distinct quality of depressed mood that is characterized as different from serious loss (ie, death of a loved one); (2) worse depression in the morning; (3) late insomnia (waking 2+ hours early); (4) marked psychomotor agitation or retardation; (5) significant anorexia or weight loss; and (6) excessive and inappropriate guilt; or the presence of psychotic features, such as delusions or hallucinations. There are other subtypes of MDD that can be found in DSM 5.
Treatment
Major depressive disorder can be viewed as analogous to common illnesses, such as hypertension or diabetes, which are treated by a PCP; screening for depression should be systematically included as part of primary care services. The clear goal of treatment for any illness is elimination or reduction of symptoms so they no longer cause any significant problem for the patient. In MDD remission is the complete resolution of depressive symptoms. Response is considered a 50% reduction of MDD symptom severity as rated on various depression scales (Table). Because no objective physical measurements exist for assessing a patient’s depression, using a rating scale is necessary to monitor the severity of depressive symptoms and a patient’s response to treatment.
There are many validated depression scales, both clinician and patient administered. A patient-administered scale can save time and provide needed information; however, the questions of a clinician-administered scale can help screen for depression and improve a clinician’s sensitivity to a patient’s depression even if the patient does not bring up the subject. These scales are available on the Internet and include instructions for proper use. Benefits of the scales include giving clinicians data to discuss with patients and helping patients track their progress. For example, patients do not always recall how they were doing before they started taking medications, so the scales can help them measure the improvement.
Psychotherapies
As first-line treatment for patients with MDD, no clear benefit exists for medication over psychological therapy, specifically evidence-based therapies (EBTs).11,12 The best studied and known EBTs are cognitive behavioral therapy for depression (CBT-D) and interpersonal therapy (IPT). Both are brief, targeted therapies with clear rules and expectations as opposed to more traditional long-term therapies, such as insight-oriented psychotherapy. These therapies generally involve weekly meetings with a therapist for about 12 weeks. They both require the patient to do homework during the therapy period; therefore, it is important for the patient to be a willing participant in these treatments to receive the maximum benefit. Many patients prefer not taking medication for depression, so psychotherapy is an excellent option. It is widely believed, although without clear evidence at this time, that the combination of medications and EBT offers improved outcomes. There are no contraindications to combining antidepressant medication and EBT.
Medications
Given the realities of practice settings and patient preferences, medication is often the most practical first-line treatment choice. The newer antidepressant medications are the most likely choices in the primary care setting for treating depression, because they offer effective treatment with less severe adverse effects (AEs) and are much safer than the early tricyclic antidepressant and monoamine oxidase inhibitor medications. There are numerous metaanalyses comparing the effectiveness of the commonly prescribed newer antidepressants that consistently show there is no absolutely best choice antidepressant. A number of studies have tried to identify predictors of response for a particular antidepressant, but these have not yielded clinically significant results. Additionally, there is little difference between an antidepressant’s rate of response and tolerability on a population scale.13 Therefore, it is the AE profile and alternate uses that usually drive the choice of which to use for a particular patient.
Before discussing antidepressant medications with patients, it is important to note the FDA-required black box warning for increased risk of suicidal ideation in young people aged ≤ 24 years. The data that led to this warning did not show an increased risk of suicide.14 In patients aged > 24 years, there is no difference in risk of suicidal ideation, and in patients aged > 65 years, the risk of suicidal ideation decreases with the start of antidepressant medication.1
SSRIs
Selective serotonin reuptake inhibitors (SSRIs) are a common first-line treatment for MDD. Of interest, all SSRIs share the same mechanism of action (MOA), so failure of a single agent does not preclude a trial of a second agent of the same class, because the chance of response is essentially the same as switching the patient to another class. However, after a second failure, there is less chance of response to another SSRI.5 These agents are used for depression, anxiety (long term not acute), posttraumatic stress disorder (PTSD), and obsessive-compulsive disorder. The AEs include nausea, headache, insomnia, dry mouth, and loss of emotion. Other than feeling emotionally blunted, most of these AEs are usually temporary and resolve in days. Sexual dysfunction is often the AE of SSRIs that draws primary concern from patients. The most frequently experienced sexual AE is delayed orgasm. A review of FDA package inserts showed rates from 7% (sertraline) to as high as 28% (high-dose paroxetine).16,17 Impotence or decreased libido is often more concerning for patients and occurs at 3% to 6%. A common concern for patients is weight gain, and SSRIs are considered weight neutral. There has been no demonstrated benefit from combining these medications or using them with selective serotonin norepinephrine reuptake inhibitors (SNRIs), which only exposes patients to increased risk of AEs.
Other Medications
Often used as first-line medications for MDD, SNRIs are also useful for MDD, anxiety (long term not acute), and PTSD. Duloxetine also has indications for diabetic peripheral neuropathy, fibromyalgia, and chronic musculoskeletal pain. The SNRIs share the same AEs as SSRIs; however, some patients show an increase in blood pressure with venlafaxine. There has been no demonstrated benefit from combining these medications or with SSRIs. These are also considered weight neutral.
Mirtazapine, bupropion, and trazodone are medications with MOAs that do not fit within the abovementioned categories and differ from one another as well. These carry FDA indications for MDD. Bupropion also has indications of smoking cessation and seasonal affective disorder for the extended-release form. The AEs are varied and unique to each medication. Mirtazapine may show a rapid improvement in mood within 2 weeks and very low risk of sexual AEs.18 Associated with some weight gain and sedation, mirtazapine is useful for patients who have sleep problems or who are experiencing weight loss. It is noted in practice to be more sedating at lower doses.
Bupropion is considered an activating medication that can lead to jitteriness and increased anxiety for some patients. Bupropion also is associated with some weight loss and will disrupt sleep if taken later in the day. Therefore, bupropion can be useful for overweight patients or those with significant energy problems. It has few sexual AEs.
Although not widely used as a primary agent for MDD, trazodone is commonly used at low doses to treat insomnia. Sedation and dry mouth are the primary AEs with some sexual AEs. These medications can be used alone, in combination with SSRIs or SNRIs, or with one another in what is commonly called rational pharmacology.
Patient Response
There is a significant portion of patients who are treated for MDD who will not have an adequate response to their first medication, somewhat analogous to patients who are treated for hypertension or diabetes. If patients are showing a response at 6 to 8 weeks but not remission, the medication trial should continue for 12 or more weeks, increasing the dose as tolerated. If the dose has been raised to the maximum and if there are still significant depressive symptoms, some combination or augmentation strategy should be tried. If patients show no improvement at 6 to 8 weeks, it is appropriate to discontinue the current treatment and trial another medication.
There are no clear guidelines for the best way to switch medications. Some practitioners cross taper; some discontinue one medication prior to starting another; and some abruptly stop one and start another (ie, changing between SSRIs, or from a SSRI to a SNRI). In all cases, some caution is recommended to avoid discontinuation syndromes, which often drive the rate of discontinuation of the medication: Many people have no problem; others are very sensitive to dosage changes.
If a patient has achieved remission with the medication, the next step is easy—continue the medication. It is generally accepted that 6 to 12 months of medication treatment after remission is best, to avoid relapse to another episode of MDD. If a patient desires to stop medication after that period, it is best to slowly titrate off over a month or more, as the patient tolerates, to avoid the discontinuation syndrome and lessen the risk of relapse. Remission rates for a single trial of medication are 35% to 45% and up to 65% with repeated medication changes.19 Therefore, 35% of patients are inadequately relieved of illness, referred to as treatment-resistant illness.
In the patient who has experienced some benefit but not remission after taking the dose to the highest approved or tolerated dose, the question is whether to switch from a medication that has shown some efficacy to another that may be better or to add another medication to augment the efficacy of the first treatment. There are many opinions regarding these decisions and about how to proceed.
Augmentation and Combination Treatments
Augmentation is the use of a nonantidepressant medication in addition to the antidepressant to improve the efficacy of the antidepressant medication. Combination treatments use 2 antidepressant medications to improve efficacy. Although there is clear evidence of benefit from a number of augmentation strategies, including some that are FDA approved, combination treatments have conflicting
published evidence of efficacy.20,21
The FDA-approved medications for augmenting antidepressant medications in treatment-resistant MDD are aripiprazole and quetiapine XR. Both are atypical antipsychotic medications with the intrinsic class risks associated with them. Only psychiatrists usually use them, because most systems require the approval of a specialist to use these agents. Of particular concern is the longterm risks of tardive dyskinesia, neuroleptic malignant syndrome, weight gain, metabolic syndrome, diabetes, and sudden death in geriatric patients with dementia that warranted a black box warning. Other well-established augmentation strategies used by psychiatrists include the usage of lithium or T3 hormone. Using these agents requires collaborative monitoring with the PCP to prevent potential renal, cardiac, and thyroid abnormalities.
Conclusions
Successful treatment of depression in medical settings can have a positive impact on medical conditions, such as potentially improving outcomes in the treatment of diabetes, cardiovascular problems, and pain. Active screening for depression followed by a safe and well-tolerated antidepressant may relieve symptoms in 6 to 8 weeks in about half of treated patients.
Tracking responses with one of the aforementioned scales is an excellent way to guide treatment decisions. Lack of tolerability or response should not discourage physicians from trying a different antidepressant. The successful treatment of depression requires patience but can make a big difference in the patient’s quality of life.
Click here to read the digital editition.
Although a common condition in the general population, major depressive disorder (MDD) is even more prevalent among patients receiving medical care. Its early recognition and successful treatment have significant implications in all fields of medicine, and the predominant burden of treatment falls on primary care providers (PCPs). The prevalence of MDD over 12 months is about 6.6%; lifetime risk is about 16.6%.1,2 About 50% of depressive episodes are rated severe or higher level. Of those having a depressive episode lasting 1 year, only 51% will seek medical help, and only 42% of these will receive adequate treatment.3 The illness is present across cultures and socioeconomic strata, although the actual rates vary. Women have about a 2-fold higher rate of MDD than do men.4
The illness can vary from mild to severe. Primary care providers usually provide treatment for patients who are at the mild-to-moderate end of the spectrum; more severely ill patients are likely to require consulting with mental health specialists. Patients who have 1 episode of MDD have a 50% chance of having a second episode during their lifetime. After a second episode, the risk of another episode rises to about 80%.5 After a third episode, it is presumed patients will continue to have subsequent episodes, because the risk rises to 90%. Among patients with MDD, about 35% will have a chronic, relapsing pattern of illness.6 Because of a relative shortage of specialty mental health providers in many areas served by federal practitioners, it is important to maximize the impact of a patient’s primary care team in treating this common and persistent illness.
Morbidity from MDD includes dysfunction in all spheres of life: Difficulties with work, home life, self-care, medication adherence, and mental health are all encountered. Although a minority of patients with depression will attempt or complete suicide, depression is a major predictor of suicide risk. However, it is a modifiable risk factor with successful treatment. Among the general population, the reported risk of suicide is about 11.5 per 100,000 per year.7 Among patients with psychiatric illness, the rate is higher.
Many patients treated through the federal system have been identified as having a risk of suicide higher than that of the general population. These populations include veterans (41% to 61% above the national average); Native Americans aged 18 to 24 years (about 2 to 3 times the national average); and active-duty military (18.7 per 100,000 per year, 50% above the national average).8-10
Diagnosis
Several subtypes of MDD and associated depressive disorders exist. The diagnosis requires a constellation of criteria for diagnosis. Using the full criteria to make an MDD diagnosis may seem excessive, but its use is critical to guide sound treatment decisions (ie, past presence of manic or hypomanic episode, which would signify bipolar disorder, or psychotic symptoms that would dictate a different path and a referral to a psychiatrist). For example, an antidepressant given to a patient with a history of hypomania or mania can trigger a full manic episode with all the potential morbidity that mania entails. The presence of psychotic symptoms leads down a different treatment path that requires antipsychotic medication.
A major depressive episode includes the sine qua non of depressed mood or anhedonia (lack of experiencing or seeking pleasurable activities) and 3 to 4 associated criteria, including poor energy (often noted as “I just can’t seem to get moving”), insomnia/hypersomnia (usually middle or late insomnia) with difficulty concentrating (often seen as problems making decisions), increased sense of guilt or worthlessness, psychomotor agitation or retardation (often noted by the patient’s spouse), significant weight loss or gain (5%), and thoughts of death/dying or suicidal ideation. These must be present most days over the previous 2 weeks.1 The time duration criteria are important because some patients may seem very distressed during a visit but do not meet the criteria for depression or have the chronic depressive symptoms of MDD. Symptoms not meeting the full criteria may likely be noted as unspecified depressive disorder or dysthymia (now termed persistent depressive disorder in Diagnostic and Statistical Manual of Mental Disorders, 5th Edition [DSM 5]).1 There are no FDA-approved treatments for these disorders; however, in cases requiring treatment, the standard of practice would be the same as for MDD.
The more severe types of MDD are usually not subtle and would likely require an immediate referral to a mental health professional. These include the presence of melancholic features, such as severe anhedonia, or loss of reactivity to normally pleasurable things, and at least 3 of the following: (1) distinct quality of depressed mood that is characterized as different from serious loss (ie, death of a loved one); (2) worse depression in the morning; (3) late insomnia (waking 2+ hours early); (4) marked psychomotor agitation or retardation; (5) significant anorexia or weight loss; and (6) excessive and inappropriate guilt; or the presence of psychotic features, such as delusions or hallucinations. There are other subtypes of MDD that can be found in DSM 5.
Treatment
Major depressive disorder can be viewed as analogous to common illnesses, such as hypertension or diabetes, which are treated by a PCP; screening for depression should be systematically included as part of primary care services. The clear goal of treatment for any illness is elimination or reduction of symptoms so they no longer cause any significant problem for the patient. In MDD remission is the complete resolution of depressive symptoms. Response is considered a 50% reduction of MDD symptom severity as rated on various depression scales (Table). Because no objective physical measurements exist for assessing a patient’s depression, using a rating scale is necessary to monitor the severity of depressive symptoms and a patient’s response to treatment.
There are many validated depression scales, both clinician and patient administered. A patient-administered scale can save time and provide needed information; however, the questions of a clinician-administered scale can help screen for depression and improve a clinician’s sensitivity to a patient’s depression even if the patient does not bring up the subject. These scales are available on the Internet and include instructions for proper use. Benefits of the scales include giving clinicians data to discuss with patients and helping patients track their progress. For example, patients do not always recall how they were doing before they started taking medications, so the scales can help them measure the improvement.
Psychotherapies
As first-line treatment for patients with MDD, no clear benefit exists for medication over psychological therapy, specifically evidence-based therapies (EBTs).11,12 The best studied and known EBTs are cognitive behavioral therapy for depression (CBT-D) and interpersonal therapy (IPT). Both are brief, targeted therapies with clear rules and expectations as opposed to more traditional long-term therapies, such as insight-oriented psychotherapy. These therapies generally involve weekly meetings with a therapist for about 12 weeks. They both require the patient to do homework during the therapy period; therefore, it is important for the patient to be a willing participant in these treatments to receive the maximum benefit. Many patients prefer not taking medication for depression, so psychotherapy is an excellent option. It is widely believed, although without clear evidence at this time, that the combination of medications and EBT offers improved outcomes. There are no contraindications to combining antidepressant medication and EBT.
Medications
Given the realities of practice settings and patient preferences, medication is often the most practical first-line treatment choice. The newer antidepressant medications are the most likely choices in the primary care setting for treating depression, because they offer effective treatment with less severe adverse effects (AEs) and are much safer than the early tricyclic antidepressant and monoamine oxidase inhibitor medications. There are numerous metaanalyses comparing the effectiveness of the commonly prescribed newer antidepressants that consistently show there is no absolutely best choice antidepressant. A number of studies have tried to identify predictors of response for a particular antidepressant, but these have not yielded clinically significant results. Additionally, there is little difference between an antidepressant’s rate of response and tolerability on a population scale.13 Therefore, it is the AE profile and alternate uses that usually drive the choice of which to use for a particular patient.
Before discussing antidepressant medications with patients, it is important to note the FDA-required black box warning for increased risk of suicidal ideation in young people aged ≤ 24 years. The data that led to this warning did not show an increased risk of suicide.14 In patients aged > 24 years, there is no difference in risk of suicidal ideation, and in patients aged > 65 years, the risk of suicidal ideation decreases with the start of antidepressant medication.1
SSRIs
Selective serotonin reuptake inhibitors (SSRIs) are a common first-line treatment for MDD. Of interest, all SSRIs share the same mechanism of action (MOA), so failure of a single agent does not preclude a trial of a second agent of the same class, because the chance of response is essentially the same as switching the patient to another class. However, after a second failure, there is less chance of response to another SSRI.5 These agents are used for depression, anxiety (long term not acute), posttraumatic stress disorder (PTSD), and obsessive-compulsive disorder. The AEs include nausea, headache, insomnia, dry mouth, and loss of emotion. Other than feeling emotionally blunted, most of these AEs are usually temporary and resolve in days. Sexual dysfunction is often the AE of SSRIs that draws primary concern from patients. The most frequently experienced sexual AE is delayed orgasm. A review of FDA package inserts showed rates from 7% (sertraline) to as high as 28% (high-dose paroxetine).16,17 Impotence or decreased libido is often more concerning for patients and occurs at 3% to 6%. A common concern for patients is weight gain, and SSRIs are considered weight neutral. There has been no demonstrated benefit from combining these medications or using them with selective serotonin norepinephrine reuptake inhibitors (SNRIs), which only exposes patients to increased risk of AEs.
Other Medications
Often used as first-line medications for MDD, SNRIs are also useful for MDD, anxiety (long term not acute), and PTSD. Duloxetine also has indications for diabetic peripheral neuropathy, fibromyalgia, and chronic musculoskeletal pain. The SNRIs share the same AEs as SSRIs; however, some patients show an increase in blood pressure with venlafaxine. There has been no demonstrated benefit from combining these medications or with SSRIs. These are also considered weight neutral.
Mirtazapine, bupropion, and trazodone are medications with MOAs that do not fit within the abovementioned categories and differ from one another as well. These carry FDA indications for MDD. Bupropion also has indications of smoking cessation and seasonal affective disorder for the extended-release form. The AEs are varied and unique to each medication. Mirtazapine may show a rapid improvement in mood within 2 weeks and very low risk of sexual AEs.18 Associated with some weight gain and sedation, mirtazapine is useful for patients who have sleep problems or who are experiencing weight loss. It is noted in practice to be more sedating at lower doses.
Bupropion is considered an activating medication that can lead to jitteriness and increased anxiety for some patients. Bupropion also is associated with some weight loss and will disrupt sleep if taken later in the day. Therefore, bupropion can be useful for overweight patients or those with significant energy problems. It has few sexual AEs.
Although not widely used as a primary agent for MDD, trazodone is commonly used at low doses to treat insomnia. Sedation and dry mouth are the primary AEs with some sexual AEs. These medications can be used alone, in combination with SSRIs or SNRIs, or with one another in what is commonly called rational pharmacology.
Patient Response
There is a significant portion of patients who are treated for MDD who will not have an adequate response to their first medication, somewhat analogous to patients who are treated for hypertension or diabetes. If patients are showing a response at 6 to 8 weeks but not remission, the medication trial should continue for 12 or more weeks, increasing the dose as tolerated. If the dose has been raised to the maximum and if there are still significant depressive symptoms, some combination or augmentation strategy should be tried. If patients show no improvement at 6 to 8 weeks, it is appropriate to discontinue the current treatment and trial another medication.
There are no clear guidelines for the best way to switch medications. Some practitioners cross taper; some discontinue one medication prior to starting another; and some abruptly stop one and start another (ie, changing between SSRIs, or from a SSRI to a SNRI). In all cases, some caution is recommended to avoid discontinuation syndromes, which often drive the rate of discontinuation of the medication: Many people have no problem; others are very sensitive to dosage changes.
If a patient has achieved remission with the medication, the next step is easy—continue the medication. It is generally accepted that 6 to 12 months of medication treatment after remission is best, to avoid relapse to another episode of MDD. If a patient desires to stop medication after that period, it is best to slowly titrate off over a month or more, as the patient tolerates, to avoid the discontinuation syndrome and lessen the risk of relapse. Remission rates for a single trial of medication are 35% to 45% and up to 65% with repeated medication changes.19 Therefore, 35% of patients are inadequately relieved of illness, referred to as treatment-resistant illness.
In the patient who has experienced some benefit but not remission after taking the dose to the highest approved or tolerated dose, the question is whether to switch from a medication that has shown some efficacy to another that may be better or to add another medication to augment the efficacy of the first treatment. There are many opinions regarding these decisions and about how to proceed.
Augmentation and Combination Treatments
Augmentation is the use of a nonantidepressant medication in addition to the antidepressant to improve the efficacy of the antidepressant medication. Combination treatments use 2 antidepressant medications to improve efficacy. Although there is clear evidence of benefit from a number of augmentation strategies, including some that are FDA approved, combination treatments have conflicting
published evidence of efficacy.20,21
The FDA-approved medications for augmenting antidepressant medications in treatment-resistant MDD are aripiprazole and quetiapine XR. Both are atypical antipsychotic medications with the intrinsic class risks associated with them. Only psychiatrists usually use them, because most systems require the approval of a specialist to use these agents. Of particular concern is the longterm risks of tardive dyskinesia, neuroleptic malignant syndrome, weight gain, metabolic syndrome, diabetes, and sudden death in geriatric patients with dementia that warranted a black box warning. Other well-established augmentation strategies used by psychiatrists include the usage of lithium or T3 hormone. Using these agents requires collaborative monitoring with the PCP to prevent potential renal, cardiac, and thyroid abnormalities.
Conclusions
Successful treatment of depression in medical settings can have a positive impact on medical conditions, such as potentially improving outcomes in the treatment of diabetes, cardiovascular problems, and pain. Active screening for depression followed by a safe and well-tolerated antidepressant may relieve symptoms in 6 to 8 weeks in about half of treated patients.
Tracking responses with one of the aforementioned scales is an excellent way to guide treatment decisions. Lack of tolerability or response should not discourage physicians from trying a different antidepressant. The successful treatment of depression requires patience but can make a big difference in the patient’s quality of life.
Click here to read the digital editition.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM5). 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
2. Kessler R, Berglund P, Demler O, Jin R, Merikangas K, Walters E. Lifetime prevalence and age-of-onset distributions of DSM-IV Disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
3. Kessler RC, Berglund P, Demler O, et al; National Comorbidity Survey Replication. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289(23):3095-3105.
4. Weissman MM, Bland RC, Canino GJ, et al. Cross-national epidemiology of major depressive and bipolar disorder. JAMA. 1996;276(4):293-299.
5. Burcusa SL, Iacono WG. Risk for recurrence in depression. Clin Psych Rev. 2007;279(8):959-985.
6. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. 3rd ed. Psychiatry Online Website. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published October 2010. Accessed February 17, 2016.
7. American Association of Suicidology. U.S.A. suicide: 2014 official final data. American Association of Suicidology Website. http://www.suicidology.org /Portals/14/docs/Resources/FactSheets/2014/2014datapgsv1b.pdf. Revised December 22, 2015. Accessed February 17, 2016.
8. Kang HK, Bullman TA, Smolenski DJ, Skopp NA, Gahm GA, Reger MA. Suicide risk among 1.3 million veterans who were on active duty during the Iraq and Afghanistan
wars. Ann Epidemiol. 2015;25(2):96-100.
9. Jiang C, Mitran A, Miniño A, Ni H. Racial and gender disparities in suicide among young adults aged 18-24: United States, 2009-2013. Centers for Disease Control and Prevention Website. http://www.cdc.gov/nchs/data/hestat/suicide/racial_and_gender_2009_2013.htm. Updated September 30, 2015. Accessed February 9, 2016.
10. Smolenski DJ, Reger MA, Bush NE, Skopp NA, Zhang Y, Campise RL. Department of Defense suicide event report 2013 annual report. National Center for Telehealth & Technology Website. http://t2health.dcoe.mil/sites/default/files/DoDSER-2013-Jan-13-2015-Final.pdf. Accessed February 9, 2016.
11. DeRubeis RJ, Hollon SD, Amsterdam JD, et al. Cognitive therapy vs medications in the treatment of moderate to severe depression. Arch Gen Psychiatry. 2005;62(4):409-416.
12. Elkin I, Shea MT, Watkins JT, et al. National Institute of Mental Health Treatment of Depression Collaborative Research Program. General effectiveness of treatments. Arch Gen Psychiatry. 1989;46(11):971-978.
13. Gartlehner G, Hansen RA, Reichenpfader U, et al. Drug class review: secondgeneration antidepressants final update 5 report. National Center for Biotechnology Information Website. http://www.ncbi.nlm.nih.gov/books/NBK54355/pdf/Bookshelf_NBK54355.pdf. Updated March 11, 2011. Accessed February 17, 2016.
14. Friedman RA. Antidepressants’ black-box warning—10 years later. N Engl J Med. 2014;371(18):1666-1668.
15. U.S. Food and Drug Administration. Antidepressant use in children, adolescents, and adults. Revisions to product labeling. U.S. Food and Drug Administration Website. http://www.fda.gov/downloads/Drugs/DrugSafety/InformationbyDrug-Class/UCM173233.pdf. Updated December 23, 2014. Accessed February 17, 2016.
16. Zoloft [package insert]. New York, NY: Pfizer; 2014.
17. Paxil [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2012.
18. Lavergne F, Berlin I, Gamma A, Stassen H, Angst J. Onset of improvement and response to mirtazapine in depression: a multicenter naturalistic study of 4771 patients. Neuropsychiatr Dis Treat. 2005;1(1):59-68.
19. Rush JA, Trivedi MH, Wisniewski SR, et al. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12): 1231-1242.
20. Rush AJ, Trivedi MH, Stewart JW, et al. Combining medications to enhance depression outcomes (CO-MED): acute and long-term outcomes of a single-blind randomized study. Am J Psychiatry. 2011;168(7):689-701.
21. Blier P, Ward HE, Tremblay P, Laberge L, Hébert C, Bergeron R. Combination of antidepressant medications from treatment initiation for major depressive disorder: a double-blind randomized study. Am J Psychiatry. 2010;167(3):281-288.
Note: Page numbers differ between the print issue and digital edition.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM5). 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
2. Kessler R, Berglund P, Demler O, Jin R, Merikangas K, Walters E. Lifetime prevalence and age-of-onset distributions of DSM-IV Disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
3. Kessler RC, Berglund P, Demler O, et al; National Comorbidity Survey Replication. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289(23):3095-3105.
4. Weissman MM, Bland RC, Canino GJ, et al. Cross-national epidemiology of major depressive and bipolar disorder. JAMA. 1996;276(4):293-299.
5. Burcusa SL, Iacono WG. Risk for recurrence in depression. Clin Psych Rev. 2007;279(8):959-985.
6. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. 3rd ed. Psychiatry Online Website. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published October 2010. Accessed February 17, 2016.
7. American Association of Suicidology. U.S.A. suicide: 2014 official final data. American Association of Suicidology Website. http://www.suicidology.org /Portals/14/docs/Resources/FactSheets/2014/2014datapgsv1b.pdf. Revised December 22, 2015. Accessed February 17, 2016.
8. Kang HK, Bullman TA, Smolenski DJ, Skopp NA, Gahm GA, Reger MA. Suicide risk among 1.3 million veterans who were on active duty during the Iraq and Afghanistan
wars. Ann Epidemiol. 2015;25(2):96-100.
9. Jiang C, Mitran A, Miniño A, Ni H. Racial and gender disparities in suicide among young adults aged 18-24: United States, 2009-2013. Centers for Disease Control and Prevention Website. http://www.cdc.gov/nchs/data/hestat/suicide/racial_and_gender_2009_2013.htm. Updated September 30, 2015. Accessed February 9, 2016.
10. Smolenski DJ, Reger MA, Bush NE, Skopp NA, Zhang Y, Campise RL. Department of Defense suicide event report 2013 annual report. National Center for Telehealth & Technology Website. http://t2health.dcoe.mil/sites/default/files/DoDSER-2013-Jan-13-2015-Final.pdf. Accessed February 9, 2016.
11. DeRubeis RJ, Hollon SD, Amsterdam JD, et al. Cognitive therapy vs medications in the treatment of moderate to severe depression. Arch Gen Psychiatry. 2005;62(4):409-416.
12. Elkin I, Shea MT, Watkins JT, et al. National Institute of Mental Health Treatment of Depression Collaborative Research Program. General effectiveness of treatments. Arch Gen Psychiatry. 1989;46(11):971-978.
13. Gartlehner G, Hansen RA, Reichenpfader U, et al. Drug class review: secondgeneration antidepressants final update 5 report. National Center for Biotechnology Information Website. http://www.ncbi.nlm.nih.gov/books/NBK54355/pdf/Bookshelf_NBK54355.pdf. Updated March 11, 2011. Accessed February 17, 2016.
14. Friedman RA. Antidepressants’ black-box warning—10 years later. N Engl J Med. 2014;371(18):1666-1668.
15. U.S. Food and Drug Administration. Antidepressant use in children, adolescents, and adults. Revisions to product labeling. U.S. Food and Drug Administration Website. http://www.fda.gov/downloads/Drugs/DrugSafety/InformationbyDrug-Class/UCM173233.pdf. Updated December 23, 2014. Accessed February 17, 2016.
16. Zoloft [package insert]. New York, NY: Pfizer; 2014.
17. Paxil [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2012.
18. Lavergne F, Berlin I, Gamma A, Stassen H, Angst J. Onset of improvement and response to mirtazapine in depression: a multicenter naturalistic study of 4771 patients. Neuropsychiatr Dis Treat. 2005;1(1):59-68.
19. Rush JA, Trivedi MH, Wisniewski SR, et al. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12): 1231-1242.
20. Rush AJ, Trivedi MH, Stewart JW, et al. Combining medications to enhance depression outcomes (CO-MED): acute and long-term outcomes of a single-blind randomized study. Am J Psychiatry. 2011;168(7):689-701.
21. Blier P, Ward HE, Tremblay P, Laberge L, Hébert C, Bergeron R. Combination of antidepressant medications from treatment initiation for major depressive disorder: a double-blind randomized study. Am J Psychiatry. 2010;167(3):281-288.
Note: Page numbers differ between the print issue and digital edition.
Impact of Psychotropic Medication Reviews on Prescribing Patterns
Due to the ever-expanding pool of psychopharmacologic agents available to treat mental health conditions, prescribers need to be vigilant about ensuring appropriate medication selection, evaluation, and monitoring. In response to this need, the Office of Mental Health Operations, Mental Health Services, and Pharmacy Benefits Management Services launched the Psychotropic Drug Safety Initiative (PDSI) to improve evidence-based psychotropic prescribing habits within the VA. Considering the large geriatric population in the VA, PDSI was particularly concerned with benzodiazepines and antipsychotic medications.
Background
The management of neuropsychiatric symptoms (NPS) for dementia is particularly burdensome to patients, caregivers, and prescribers. When nonpharmacologic interventions fail, patients are often prescribed antipsychotic medications to target these symptoms. The FDA has issued a black box warning regarding an increased risk of death associated with the use of both first- and second-generation antipsychotics for the treatment of dementia-related psychosis.1 Due to these risks, tapering or discontinuing these medications should be considered at regular intervals.
Recently, the Centers for Medicare & Medicaid Services (CMS) expanded its goal to reduce antipsychotic use in nursing facilities by 25% by the end of 2015 and 30% by the end of 2016.2 A review investigated the impact of withdrawal vs continuation of antipsychotic medications in the setting of Alzheimer dementia (AD) and found that antipsychotic medications could be withdrawn without detrimental effects on patient behaviors.3 However, the study also noted that those patients with severe NPS at baseline or who had a history of positive response to an antipsychotic might be at increased risk of relapse or have a shorter time to relapse when antipsychotic medications are withdrawn.
Benzodiazepines as a class may be used for many indications, including anxiety disorders, seizure disorders, sleep disorders, or muscle spasms. However, due to known risks of cognitive impairments, sedation, falls, or dependence and addiction, these agents are typically recommended for only short-term treatment. These risks are potentially amplified in a geriatric population, because elderly patients have an increased sensitivity to the effects of these agents and may have impaired hepatic or renal function, leading to accumulation.
Due to these risks, the American Geriatrics Society (AGS) recommends against the use of any benzodiazepines for the treatment of insomnia, agitation, or delirium. Additionally, the AGS recommends that use of these agents for the treatment of behavioral problems related to dementia be reserved for those who have failed nonpharmacologic options and are at risk to themselves or others.4
Growing evidence suggests that benzodiazepine use may increase the risk of developing AD. A recent casecontrol study compare records of 1,796 patients with an AD diagnosis to 7,184 patients with no cognitive deficits. This study found that patients with a history of benzodiazepine use had a 51% increase in risk for AD. Additionally, use of long-acting benzodiazepines, such as diazepam and clonazepam, was strongly associated with the development of AD.5 These data further illustrate the importance of minimizing the use of benzodiazepines.
To ensure proper use of these 2 classes of medications, clinical pharmacy specialists (CPSs) at the Lexington VAMC (LVAMC) in Kentucky began conducting psychotropic medication reviews (PMRs). Each PMR contained a brief summary of evidence-based recommendations (both pharmacologic and nonpharmacologic) and a clinical review of the patient’s medication history, including an evaluation of the appropriateness of current therapy and supportive documentation. Patients were candidates for PMR if they met one of the following criteria: (1) use of a benzodiazepine in a patient with dementia; (2) use of a benzodiazepine in a patient aged > 75 years; and (3) use of an antipsychotic in a patient with dementia. These criteria were selected based on guidance set forth by the PDSI. The figure provides a sample of the PMR template in the electronic medical record (EMR).
The purpose of this study was to evaluate the impact of a pharmacist-conducted PMR based on prescriber response to written recommendations. Additionally, this study characterized any observed differences in prescriber response based on discipline. The results of this study will be used to evaluate the efficacy of LVAMC’s current method of clinical pharmacy intervention and identify areas for process improvement. This study was reviewed and approved by the LVAMC institutional review board and research and development committee.
Methods
Patients were included in this study if they had a PMR note entered in the EMR between September 2014 and January 2015. Baseline demographic information collected included patient age and gender. One study author manually reviewed all PMR notes and collected the type of pharmacy intervention (characterized as recommendation for medication adjustment, patient education, both medication adjustment and patient education, or no recommendation), provider discipline, provider response to intervention, and any changes to medication therapy that occurred as a result of pharmacist intervention.
When patients were not cognitively able to receive education, family members or caretakers were educated. The primary outcome of this study was prescriber response to pharmacist recommendations, which was characterized as acknowledged, ignored, accepted, or declined with justification. In instances where both medication adjustment and patient education was recommended, the recommendation was considered to be accepted only if both components of the recommendation were addressed. The secondary outcome sought to identify any difference in provider response based on discipline.
Results
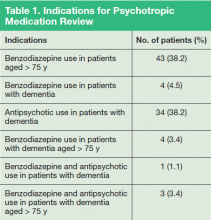
Eighty-nine patients were included in the study. Due to the nature of LVAMC, the patient population was fairly homogeneous, with an average patient age of 80 years (range 52-95 years); > 97% of patients were male. Fifty patients were noted to have prescriptions for benzodiazepines and were aged > 75 years, 11 patients had prescriptions for benzodiazepines and a diagnosis of dementia, and 38 patients had prescriptions for antipsychotics and a diagnosis of dementia. Several patients fell into more than one of the categories (Table 1).
Specific written recommendations were made for 69 (78%) patients, with 20 patients having appropriate documentation in the chart for therapy continuation. The most common documented reasons for continuation of therapy were (a) patient/caregiver educated and consent to continue therapy already documented; (b) recent dose reduction or discontinuation attempt failed; (c) recent successful dose reduction; or (d) documented risk to patient or others if medication were to be discontinued.
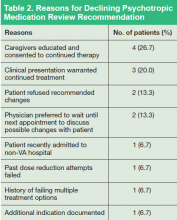
Most recommendations were for medication adjustments (n = 54; 78.3%). Six (8.7%) recommendations were for patient education only, and 9 (13%) recommendations were for both medication adjustment and patient education. Overall, 33 (48%) of recommendations were accepted, 21 (30%) were not acknowledged, and 15 (22%) were declined. The most common outcome of accepted recommendations was a dose reduction with full taper planned by provider. The most common reasons for declined recommendations were (a) caregivers were educated and consented to continued treatment; (b) clinical presentation warranted continued treatment; (c) patient refused recommended changes; and (d) prescriber preferred to wait until next appointment to discuss with patient (Table 2).
Forty-nine recommendations were made to prescribers in the Psychiatry Department, 17 to prescribers in the Home-Based Primary Care (HBPC) Department, 15 to prescribers in the Primary Care Department, and 8 to prescribers in the Neurology Department. Prescribers in the Primary Care Department accepted recommendations at the highest rate (n = 13; 69%), while Neurology Department prescribers (n = 2; 33%) accepted recommendations at the lowest rate.
Discussion
This study illustrates the impact of including a psychiatric CPS as part of the interdisciplinary care team. Through the implementation of a PMR process, CPSs were able to provide specific, unbiased recommendations for the safe use of medications. It was felt that CPSs might have a greater impact by offering patient-specific recommendations rather than providing general information about the risks of these medications, which many providers are aware of already. Because nearly half of all recommendations were accepted, the authors feel that the PMR is an effective way to deliver provider education and improve safe prescribing practices.
There will be times when the use of these agents in at-risk populations is justified and appropriately documented, as was the case for 20 patients in this study. The goals of this study were not only to improve the use of evidence-based medications, but also the process of documenting justification for the continued use of these agents. The 22% of recommendations that were declined with justification from the provider were considered successful, because the PMR note prompted the provider to document in a note clear justification for the use of the agent in question.
The majority of recommendations were made to prescribers in the Mental Health Department, which was expected given the 2 classes of medications evaluated in the study. However, primary care prescribers accepted recommendations at the highest rate. There are several possible explanations. First, mental health prescribers are more likely to have complex, treatment-resistant psychiatric patients than do other disciplines. Additionally, these prescribers have an increased level of familiarity and comfort with second-generation antipsychotics and benzodiazepines and may have been more confident in documenting justifications to continue therapy.
Neurologists were the least likely to accept PMR recommendations. Unlike other services, prescribers in the Neurology Department spend a significant amount of their time providing care to patients at a university hospital and, therefore, are not present on the VA campus on a daily basis. This location disparity can lead to less frequent contact between prescriber and CPSs and may impact the professional relationship between these departments. Also, both the Neurology Department and the home-based Primary Care Department did not have staff actively involved in the PDSI, which may have decreased prescriber familiarity with the goals and intentions of PDSI and therefore decreased provider responsiveness to PMR notes.
Sometimes PMR notes were entered in the EMR when the patient did not have an upcoming appointment with the prescriber. As a result, there were instances when recommendations could not be implemented due to time and workload constraints. Many providers acknowledged the importance of shared medical decision making and preferred to wait to make medication adjustments until patients could be seen in the clinic.
Psychotropic medication review is a continually developing process, and these results illustrate provider response to the initial 5 months of a new service. During the time frame, PMR notes had been entered for all veterans identified as using antipsychotics or benzodiazepines in the setting of dementia but for only a fraction of those identified as using benzodiazepines who were aged > 75 years. It is reasonable to expect that as prescribers become more familiar with the PMR process and its intentions, they may be more likely to acknowledge recommendations and to respond with the appropriate documentation.
Psychotropic medication reviews were initiated as part of a PGY-2 psychiatric pharmacy residency project, and as such, the impact on the CPS workflow was not evaluated. Although this study suggests that the use of PMR was effective in improving evidence-based prescribing, it does not evaluate whether this process is sustainable in the long-term for the CPS.
Conclusions
The results of this study illustrate the value of a psychiatric CPS. Through the implementation of a simple PMR service, CPSs were able to impact evidence-based prescribing and related documentation. With nearly 50% of the recommendations accepted, the authors believe that use of the PMR is an effective way to deliver provider education and improve safe prescribing practices. Further review of the PMR process will be needed to evaluate the impact and sustainability on CPS workflow.
Click here to read the digital edition.
1. U.S. Food and Drug Administration. Information for healthcare professionals: conventional antipsychotics, 2013. U.S. Food and Drug Administration Website. http://www. fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124830.htm. Updated August 15, 2013. Accessed February 8, 2016.
2. Sprague K. CMS sets new goals for reducing use of antipsychotic medications. Consult Pharm. 2015;30(2):3.
3. Declercq T, Petrovic M, Azermai M, et al. Withdrawal versus continuation of chronic antipsychotic drugs for behavioural and psychological symptoms in older people with dementia. Cochrane Database Syst Rev. 2013;3:CD007726.
4. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
5. Billioti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ. 2014;349:g5205
Note: Page numbers differ between the print issue and digital edition.
Due to the ever-expanding pool of psychopharmacologic agents available to treat mental health conditions, prescribers need to be vigilant about ensuring appropriate medication selection, evaluation, and monitoring. In response to this need, the Office of Mental Health Operations, Mental Health Services, and Pharmacy Benefits Management Services launched the Psychotropic Drug Safety Initiative (PDSI) to improve evidence-based psychotropic prescribing habits within the VA. Considering the large geriatric population in the VA, PDSI was particularly concerned with benzodiazepines and antipsychotic medications.
Background
The management of neuropsychiatric symptoms (NPS) for dementia is particularly burdensome to patients, caregivers, and prescribers. When nonpharmacologic interventions fail, patients are often prescribed antipsychotic medications to target these symptoms. The FDA has issued a black box warning regarding an increased risk of death associated with the use of both first- and second-generation antipsychotics for the treatment of dementia-related psychosis.1 Due to these risks, tapering or discontinuing these medications should be considered at regular intervals.
Recently, the Centers for Medicare & Medicaid Services (CMS) expanded its goal to reduce antipsychotic use in nursing facilities by 25% by the end of 2015 and 30% by the end of 2016.2 A review investigated the impact of withdrawal vs continuation of antipsychotic medications in the setting of Alzheimer dementia (AD) and found that antipsychotic medications could be withdrawn without detrimental effects on patient behaviors.3 However, the study also noted that those patients with severe NPS at baseline or who had a history of positive response to an antipsychotic might be at increased risk of relapse or have a shorter time to relapse when antipsychotic medications are withdrawn.
Benzodiazepines as a class may be used for many indications, including anxiety disorders, seizure disorders, sleep disorders, or muscle spasms. However, due to known risks of cognitive impairments, sedation, falls, or dependence and addiction, these agents are typically recommended for only short-term treatment. These risks are potentially amplified in a geriatric population, because elderly patients have an increased sensitivity to the effects of these agents and may have impaired hepatic or renal function, leading to accumulation.
Due to these risks, the American Geriatrics Society (AGS) recommends against the use of any benzodiazepines for the treatment of insomnia, agitation, or delirium. Additionally, the AGS recommends that use of these agents for the treatment of behavioral problems related to dementia be reserved for those who have failed nonpharmacologic options and are at risk to themselves or others.4
Growing evidence suggests that benzodiazepine use may increase the risk of developing AD. A recent casecontrol study compare records of 1,796 patients with an AD diagnosis to 7,184 patients with no cognitive deficits. This study found that patients with a history of benzodiazepine use had a 51% increase in risk for AD. Additionally, use of long-acting benzodiazepines, such as diazepam and clonazepam, was strongly associated with the development of AD.5 These data further illustrate the importance of minimizing the use of benzodiazepines.
To ensure proper use of these 2 classes of medications, clinical pharmacy specialists (CPSs) at the Lexington VAMC (LVAMC) in Kentucky began conducting psychotropic medication reviews (PMRs). Each PMR contained a brief summary of evidence-based recommendations (both pharmacologic and nonpharmacologic) and a clinical review of the patient’s medication history, including an evaluation of the appropriateness of current therapy and supportive documentation. Patients were candidates for PMR if they met one of the following criteria: (1) use of a benzodiazepine in a patient with dementia; (2) use of a benzodiazepine in a patient aged > 75 years; and (3) use of an antipsychotic in a patient with dementia. These criteria were selected based on guidance set forth by the PDSI. The figure provides a sample of the PMR template in the electronic medical record (EMR).
The purpose of this study was to evaluate the impact of a pharmacist-conducted PMR based on prescriber response to written recommendations. Additionally, this study characterized any observed differences in prescriber response based on discipline. The results of this study will be used to evaluate the efficacy of LVAMC’s current method of clinical pharmacy intervention and identify areas for process improvement. This study was reviewed and approved by the LVAMC institutional review board and research and development committee.
Methods
Patients were included in this study if they had a PMR note entered in the EMR between September 2014 and January 2015. Baseline demographic information collected included patient age and gender. One study author manually reviewed all PMR notes and collected the type of pharmacy intervention (characterized as recommendation for medication adjustment, patient education, both medication adjustment and patient education, or no recommendation), provider discipline, provider response to intervention, and any changes to medication therapy that occurred as a result of pharmacist intervention.
When patients were not cognitively able to receive education, family members or caretakers were educated. The primary outcome of this study was prescriber response to pharmacist recommendations, which was characterized as acknowledged, ignored, accepted, or declined with justification. In instances where both medication adjustment and patient education was recommended, the recommendation was considered to be accepted only if both components of the recommendation were addressed. The secondary outcome sought to identify any difference in provider response based on discipline.
Results
Eighty-nine patients were included in the study. Due to the nature of LVAMC, the patient population was fairly homogeneous, with an average patient age of 80 years (range 52-95 years); > 97% of patients were male. Fifty patients were noted to have prescriptions for benzodiazepines and were aged > 75 years, 11 patients had prescriptions for benzodiazepines and a diagnosis of dementia, and 38 patients had prescriptions for antipsychotics and a diagnosis of dementia. Several patients fell into more than one of the categories (Table 1).
Specific written recommendations were made for 69 (78%) patients, with 20 patients having appropriate documentation in the chart for therapy continuation. The most common documented reasons for continuation of therapy were (a) patient/caregiver educated and consent to continue therapy already documented; (b) recent dose reduction or discontinuation attempt failed; (c) recent successful dose reduction; or (d) documented risk to patient or others if medication were to be discontinued.
Most recommendations were for medication adjustments (n = 54; 78.3%). Six (8.7%) recommendations were for patient education only, and 9 (13%) recommendations were for both medication adjustment and patient education. Overall, 33 (48%) of recommendations were accepted, 21 (30%) were not acknowledged, and 15 (22%) were declined. The most common outcome of accepted recommendations was a dose reduction with full taper planned by provider. The most common reasons for declined recommendations were (a) caregivers were educated and consented to continued treatment; (b) clinical presentation warranted continued treatment; (c) patient refused recommended changes; and (d) prescriber preferred to wait until next appointment to discuss with patient (Table 2).
Forty-nine recommendations were made to prescribers in the Psychiatry Department, 17 to prescribers in the Home-Based Primary Care (HBPC) Department, 15 to prescribers in the Primary Care Department, and 8 to prescribers in the Neurology Department. Prescribers in the Primary Care Department accepted recommendations at the highest rate (n = 13; 69%), while Neurology Department prescribers (n = 2; 33%) accepted recommendations at the lowest rate.
Discussion
This study illustrates the impact of including a psychiatric CPS as part of the interdisciplinary care team. Through the implementation of a PMR process, CPSs were able to provide specific, unbiased recommendations for the safe use of medications. It was felt that CPSs might have a greater impact by offering patient-specific recommendations rather than providing general information about the risks of these medications, which many providers are aware of already. Because nearly half of all recommendations were accepted, the authors feel that the PMR is an effective way to deliver provider education and improve safe prescribing practices.
There will be times when the use of these agents in at-risk populations is justified and appropriately documented, as was the case for 20 patients in this study. The goals of this study were not only to improve the use of evidence-based medications, but also the process of documenting justification for the continued use of these agents. The 22% of recommendations that were declined with justification from the provider were considered successful, because the PMR note prompted the provider to document in a note clear justification for the use of the agent in question.
The majority of recommendations were made to prescribers in the Mental Health Department, which was expected given the 2 classes of medications evaluated in the study. However, primary care prescribers accepted recommendations at the highest rate. There are several possible explanations. First, mental health prescribers are more likely to have complex, treatment-resistant psychiatric patients than do other disciplines. Additionally, these prescribers have an increased level of familiarity and comfort with second-generation antipsychotics and benzodiazepines and may have been more confident in documenting justifications to continue therapy.
Neurologists were the least likely to accept PMR recommendations. Unlike other services, prescribers in the Neurology Department spend a significant amount of their time providing care to patients at a university hospital and, therefore, are not present on the VA campus on a daily basis. This location disparity can lead to less frequent contact between prescriber and CPSs and may impact the professional relationship between these departments. Also, both the Neurology Department and the home-based Primary Care Department did not have staff actively involved in the PDSI, which may have decreased prescriber familiarity with the goals and intentions of PDSI and therefore decreased provider responsiveness to PMR notes.
Sometimes PMR notes were entered in the EMR when the patient did not have an upcoming appointment with the prescriber. As a result, there were instances when recommendations could not be implemented due to time and workload constraints. Many providers acknowledged the importance of shared medical decision making and preferred to wait to make medication adjustments until patients could be seen in the clinic.
Psychotropic medication review is a continually developing process, and these results illustrate provider response to the initial 5 months of a new service. During the time frame, PMR notes had been entered for all veterans identified as using antipsychotics or benzodiazepines in the setting of dementia but for only a fraction of those identified as using benzodiazepines who were aged > 75 years. It is reasonable to expect that as prescribers become more familiar with the PMR process and its intentions, they may be more likely to acknowledge recommendations and to respond with the appropriate documentation.
Psychotropic medication reviews were initiated as part of a PGY-2 psychiatric pharmacy residency project, and as such, the impact on the CPS workflow was not evaluated. Although this study suggests that the use of PMR was effective in improving evidence-based prescribing, it does not evaluate whether this process is sustainable in the long-term for the CPS.
Conclusions
The results of this study illustrate the value of a psychiatric CPS. Through the implementation of a simple PMR service, CPSs were able to impact evidence-based prescribing and related documentation. With nearly 50% of the recommendations accepted, the authors believe that use of the PMR is an effective way to deliver provider education and improve safe prescribing practices. Further review of the PMR process will be needed to evaluate the impact and sustainability on CPS workflow.
Click here to read the digital edition.
Due to the ever-expanding pool of psychopharmacologic agents available to treat mental health conditions, prescribers need to be vigilant about ensuring appropriate medication selection, evaluation, and monitoring. In response to this need, the Office of Mental Health Operations, Mental Health Services, and Pharmacy Benefits Management Services launched the Psychotropic Drug Safety Initiative (PDSI) to improve evidence-based psychotropic prescribing habits within the VA. Considering the large geriatric population in the VA, PDSI was particularly concerned with benzodiazepines and antipsychotic medications.
Background
The management of neuropsychiatric symptoms (NPS) for dementia is particularly burdensome to patients, caregivers, and prescribers. When nonpharmacologic interventions fail, patients are often prescribed antipsychotic medications to target these symptoms. The FDA has issued a black box warning regarding an increased risk of death associated with the use of both first- and second-generation antipsychotics for the treatment of dementia-related psychosis.1 Due to these risks, tapering or discontinuing these medications should be considered at regular intervals.
Recently, the Centers for Medicare & Medicaid Services (CMS) expanded its goal to reduce antipsychotic use in nursing facilities by 25% by the end of 2015 and 30% by the end of 2016.2 A review investigated the impact of withdrawal vs continuation of antipsychotic medications in the setting of Alzheimer dementia (AD) and found that antipsychotic medications could be withdrawn without detrimental effects on patient behaviors.3 However, the study also noted that those patients with severe NPS at baseline or who had a history of positive response to an antipsychotic might be at increased risk of relapse or have a shorter time to relapse when antipsychotic medications are withdrawn.
Benzodiazepines as a class may be used for many indications, including anxiety disorders, seizure disorders, sleep disorders, or muscle spasms. However, due to known risks of cognitive impairments, sedation, falls, or dependence and addiction, these agents are typically recommended for only short-term treatment. These risks are potentially amplified in a geriatric population, because elderly patients have an increased sensitivity to the effects of these agents and may have impaired hepatic or renal function, leading to accumulation.
Due to these risks, the American Geriatrics Society (AGS) recommends against the use of any benzodiazepines for the treatment of insomnia, agitation, or delirium. Additionally, the AGS recommends that use of these agents for the treatment of behavioral problems related to dementia be reserved for those who have failed nonpharmacologic options and are at risk to themselves or others.4
Growing evidence suggests that benzodiazepine use may increase the risk of developing AD. A recent casecontrol study compare records of 1,796 patients with an AD diagnosis to 7,184 patients with no cognitive deficits. This study found that patients with a history of benzodiazepine use had a 51% increase in risk for AD. Additionally, use of long-acting benzodiazepines, such as diazepam and clonazepam, was strongly associated with the development of AD.5 These data further illustrate the importance of minimizing the use of benzodiazepines.
To ensure proper use of these 2 classes of medications, clinical pharmacy specialists (CPSs) at the Lexington VAMC (LVAMC) in Kentucky began conducting psychotropic medication reviews (PMRs). Each PMR contained a brief summary of evidence-based recommendations (both pharmacologic and nonpharmacologic) and a clinical review of the patient’s medication history, including an evaluation of the appropriateness of current therapy and supportive documentation. Patients were candidates for PMR if they met one of the following criteria: (1) use of a benzodiazepine in a patient with dementia; (2) use of a benzodiazepine in a patient aged > 75 years; and (3) use of an antipsychotic in a patient with dementia. These criteria were selected based on guidance set forth by the PDSI. The figure provides a sample of the PMR template in the electronic medical record (EMR).
The purpose of this study was to evaluate the impact of a pharmacist-conducted PMR based on prescriber response to written recommendations. Additionally, this study characterized any observed differences in prescriber response based on discipline. The results of this study will be used to evaluate the efficacy of LVAMC’s current method of clinical pharmacy intervention and identify areas for process improvement. This study was reviewed and approved by the LVAMC institutional review board and research and development committee.
Methods
Patients were included in this study if they had a PMR note entered in the EMR between September 2014 and January 2015. Baseline demographic information collected included patient age and gender. One study author manually reviewed all PMR notes and collected the type of pharmacy intervention (characterized as recommendation for medication adjustment, patient education, both medication adjustment and patient education, or no recommendation), provider discipline, provider response to intervention, and any changes to medication therapy that occurred as a result of pharmacist intervention.
When patients were not cognitively able to receive education, family members or caretakers were educated. The primary outcome of this study was prescriber response to pharmacist recommendations, which was characterized as acknowledged, ignored, accepted, or declined with justification. In instances where both medication adjustment and patient education was recommended, the recommendation was considered to be accepted only if both components of the recommendation were addressed. The secondary outcome sought to identify any difference in provider response based on discipline.
Results
Eighty-nine patients were included in the study. Due to the nature of LVAMC, the patient population was fairly homogeneous, with an average patient age of 80 years (range 52-95 years); > 97% of patients were male. Fifty patients were noted to have prescriptions for benzodiazepines and were aged > 75 years, 11 patients had prescriptions for benzodiazepines and a diagnosis of dementia, and 38 patients had prescriptions for antipsychotics and a diagnosis of dementia. Several patients fell into more than one of the categories (Table 1).
Specific written recommendations were made for 69 (78%) patients, with 20 patients having appropriate documentation in the chart for therapy continuation. The most common documented reasons for continuation of therapy were (a) patient/caregiver educated and consent to continue therapy already documented; (b) recent dose reduction or discontinuation attempt failed; (c) recent successful dose reduction; or (d) documented risk to patient or others if medication were to be discontinued.
Most recommendations were for medication adjustments (n = 54; 78.3%). Six (8.7%) recommendations were for patient education only, and 9 (13%) recommendations were for both medication adjustment and patient education. Overall, 33 (48%) of recommendations were accepted, 21 (30%) were not acknowledged, and 15 (22%) were declined. The most common outcome of accepted recommendations was a dose reduction with full taper planned by provider. The most common reasons for declined recommendations were (a) caregivers were educated and consented to continued treatment; (b) clinical presentation warranted continued treatment; (c) patient refused recommended changes; and (d) prescriber preferred to wait until next appointment to discuss with patient (Table 2).
Forty-nine recommendations were made to prescribers in the Psychiatry Department, 17 to prescribers in the Home-Based Primary Care (HBPC) Department, 15 to prescribers in the Primary Care Department, and 8 to prescribers in the Neurology Department. Prescribers in the Primary Care Department accepted recommendations at the highest rate (n = 13; 69%), while Neurology Department prescribers (n = 2; 33%) accepted recommendations at the lowest rate.
Discussion
This study illustrates the impact of including a psychiatric CPS as part of the interdisciplinary care team. Through the implementation of a PMR process, CPSs were able to provide specific, unbiased recommendations for the safe use of medications. It was felt that CPSs might have a greater impact by offering patient-specific recommendations rather than providing general information about the risks of these medications, which many providers are aware of already. Because nearly half of all recommendations were accepted, the authors feel that the PMR is an effective way to deliver provider education and improve safe prescribing practices.
There will be times when the use of these agents in at-risk populations is justified and appropriately documented, as was the case for 20 patients in this study. The goals of this study were not only to improve the use of evidence-based medications, but also the process of documenting justification for the continued use of these agents. The 22% of recommendations that were declined with justification from the provider were considered successful, because the PMR note prompted the provider to document in a note clear justification for the use of the agent in question.
The majority of recommendations were made to prescribers in the Mental Health Department, which was expected given the 2 classes of medications evaluated in the study. However, primary care prescribers accepted recommendations at the highest rate. There are several possible explanations. First, mental health prescribers are more likely to have complex, treatment-resistant psychiatric patients than do other disciplines. Additionally, these prescribers have an increased level of familiarity and comfort with second-generation antipsychotics and benzodiazepines and may have been more confident in documenting justifications to continue therapy.
Neurologists were the least likely to accept PMR recommendations. Unlike other services, prescribers in the Neurology Department spend a significant amount of their time providing care to patients at a university hospital and, therefore, are not present on the VA campus on a daily basis. This location disparity can lead to less frequent contact between prescriber and CPSs and may impact the professional relationship between these departments. Also, both the Neurology Department and the home-based Primary Care Department did not have staff actively involved in the PDSI, which may have decreased prescriber familiarity with the goals and intentions of PDSI and therefore decreased provider responsiveness to PMR notes.
Sometimes PMR notes were entered in the EMR when the patient did not have an upcoming appointment with the prescriber. As a result, there were instances when recommendations could not be implemented due to time and workload constraints. Many providers acknowledged the importance of shared medical decision making and preferred to wait to make medication adjustments until patients could be seen in the clinic.
Psychotropic medication review is a continually developing process, and these results illustrate provider response to the initial 5 months of a new service. During the time frame, PMR notes had been entered for all veterans identified as using antipsychotics or benzodiazepines in the setting of dementia but for only a fraction of those identified as using benzodiazepines who were aged > 75 years. It is reasonable to expect that as prescribers become more familiar with the PMR process and its intentions, they may be more likely to acknowledge recommendations and to respond with the appropriate documentation.
Psychotropic medication reviews were initiated as part of a PGY-2 psychiatric pharmacy residency project, and as such, the impact on the CPS workflow was not evaluated. Although this study suggests that the use of PMR was effective in improving evidence-based prescribing, it does not evaluate whether this process is sustainable in the long-term for the CPS.
Conclusions
The results of this study illustrate the value of a psychiatric CPS. Through the implementation of a simple PMR service, CPSs were able to impact evidence-based prescribing and related documentation. With nearly 50% of the recommendations accepted, the authors believe that use of the PMR is an effective way to deliver provider education and improve safe prescribing practices. Further review of the PMR process will be needed to evaluate the impact and sustainability on CPS workflow.
Click here to read the digital edition.
1. U.S. Food and Drug Administration. Information for healthcare professionals: conventional antipsychotics, 2013. U.S. Food and Drug Administration Website. http://www. fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124830.htm. Updated August 15, 2013. Accessed February 8, 2016.
2. Sprague K. CMS sets new goals for reducing use of antipsychotic medications. Consult Pharm. 2015;30(2):3.
3. Declercq T, Petrovic M, Azermai M, et al. Withdrawal versus continuation of chronic antipsychotic drugs for behavioural and psychological symptoms in older people with dementia. Cochrane Database Syst Rev. 2013;3:CD007726.
4. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
5. Billioti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ. 2014;349:g5205
Note: Page numbers differ between the print issue and digital edition.
1. U.S. Food and Drug Administration. Information for healthcare professionals: conventional antipsychotics, 2013. U.S. Food and Drug Administration Website. http://www. fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124830.htm. Updated August 15, 2013. Accessed February 8, 2016.
2. Sprague K. CMS sets new goals for reducing use of antipsychotic medications. Consult Pharm. 2015;30(2):3.
3. Declercq T, Petrovic M, Azermai M, et al. Withdrawal versus continuation of chronic antipsychotic drugs for behavioural and psychological symptoms in older people with dementia. Cochrane Database Syst Rev. 2013;3:CD007726.
4. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
5. Billioti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ. 2014;349:g5205
Note: Page numbers differ between the print issue and digital edition.
What to Do When Your Depressed Patient Develops Mania
This article has been adapted from an article originally published in Current Psychiatry (http://currentpsychiatry.com):Current Psychiatry. 2015;14(10):29-32,35-40,e6.
When a known depressed patient newly develops signs of mania or hypomania, a cascade of diagnostic and therapeutic questions ensues: Does the event “automatically” signify the presence of bipolar disorder (BD), or could manic symptoms be secondary to another underlying medical problem, a prescribed antidepressant or non-psychotropic medication, or illicit substances?
Even more questions confront the clinician: If mania symptoms are nothing more than an adverse drug reaction, will they go away by stopping the presumed offending agent? Or do symptoms always indicate the unmasking of a bipolar diathesis? Should anti-manic medication be prescribed immediately? If so, which one(s) and for how long? How extensive a medical or neurologic workup is indicated?
And how do you differentiate ambiguous hypomania symptoms (irritability, insomnia, agitation) from other phenomena, such as akathisia, anxiety, and overstimulation?
In this article, we present an overview of how to approach and answer these key questions, so that you can identify, comprehend, and manage manic symptoms that arise in the course of your patient’s treatment for depression (Box).
Does Disease Exist on a Unipolar-Bipolar Continuum.
There has been a resurgence of interest in Kraepelin’s original notion of mania and depression as falling along a continuum, rather than being distinct categories of pathology. True bipolar mania has its own identifiable epidemiology, familiality, and treatment, but symptomatic shades of gray often pose a formidable diagnostic and therapeutic challenge.
For example, the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) relaxed its definition of “mixed” episodes of BD to include subsyndromal mania features in unipolar depression. When a patient with unipolar depression develops a full, unequivocal manic episode, there usually isn’t much ambiguity or confusion about initial management: assure a safe environment, stop any antidepressants, rule out drug- or medically induced causes, and begin an acute anti-manic medication.
Next steps can, sometimes, be murkier:
- Formulate a definitive, overarching diagnosis
- Provide psycho-education
- Forecast return to work or school
- Discuss prognosis and likelihood of relapse
- Address necessary lifestyle modifications (eg, sleep hygiene, elimination of alcohol and illicit drug use)
- Determine whether indefinite maintenance pharmacotherapy is indicated—and, if so, with which medication(s).
A Diagnostic Formulation Isn’t Always Black and White
Ms. J, age 56, a medically healthy woman, has a 10-year history of depression and anxiety that has been treated effectively for most of that time with venlafaxine,
225 mg/d. The mother of 4 grown children, Ms. J has worked steadily for > 20 years as a flight attendant for an international airline.
Today, Ms. J is brought by ambulance from work to the emergency department in a paranoid and agitated state. The admission follows her having e-blasted airline corporate executives with a voluminous manifesto that she worked on around the clock the preceding week, in which she explained her bold ideas to revolutionize the airline industry, under her leadership.
Ms. J’s family history is unremarkable for psychiatric illness.
How Does One Approach A Case Such As Ms. J's?
Stark examples of classical mania, as depicted in this case vignette, are easy to recognize but not necessarily straightforward, nosologically. Consider the following not-so-straightforward elements of Ms. J’s case:
- A first-lifetime episode of mania or hypomania is rare after age 50
- Ms. J took a serotonin-norepinephrine reuptake inhibitor (SNRI) for many years without evidence of mood destabilization
- Years of repetitive chronobiological stress (including probable frequent time zone changes with likely sleep disruption) apparently did not trigger mood destabilization
- None of Ms. J’s 4 pregnancies led to postpartum mood episodes
- At least on the surface, there are no obvious features that point to likely causes of a secondary mania (eg, drug-induced, toxic, metabolic, or medical)
- Ms. J has no known family history of BD or any other mood disorder.
Approaching a case such as Ms. J’s must involve a systematic strategy that can best be broken into 2 segments: (1) a period of acute initial assessment and treatment and (2) later efforts focused on broader diagnostic evaluation and longer-term relapse prevention.
Initial Assessment and Treatment
Immediate assessment and management hinges on initial triage and forming a working diagnostic impression. Although full-blown mania usually is obvious (sometimes even without a formal interview), be alert to patients who might minimize or altogether disavow mania symptoms—often because of denial of illness, misidentification of symptoms, or impaired insight about changes in thinking, mood, or behavior.
Because florid mania, by definition, impairs psychosocial functioning, the context of an initial presentation often holds diagnostic relevance. Manic patients who display disruptive behaviors often are brought to treatment by a third party, whereas a less severely ill patient might be more inclined to seek treatment for herself (himself) when psychosis is absent and insight is less compromised or when the patient feels she (he) might be depressed.
It is not uncommon for a manic patient to report “depression” as the chief complaint or to omit elements related to psychomotor acceleration (such as racing thoughts or psychomotor agitation) in the description of symptoms. An accurate diagnosis often requires clinical probing and clarification of symptoms (eg, differentiating simple insomnia with consequent next-day fatigue from loss of the need for sleep with intact or even enhanced next-day energy) or discriminating racing thoughts from anxious ruminations that might be more intrusive than rapid.
Presentations of frank mania also can come to light as a consequence of symptoms, rather than as symptoms per se (eg, conflict in relationships, problems at work, financial reversals).
Particularly in patients who do not have a history of mania, avoid the temptation to begin or modify existing pharmacotherapy until you have performed a basic initial evaluation. Immediate considerations for initial assessment and management include the following:
Provide containment. Ensure a safe setting, level of care, and frequency of monitoring. Evaluate suicide risk (particularly when mixed features are present), and risk of withdrawal from any psychoactive substances.
Engage significant others. Close family members can provide essential history, particularly when a patient’s insight about her illness and need for treatment are impaired. Family members and significant others also often play important roles in helping to restrict access to finances, fostering medication adherence, preventing access to weapons in the home, and sharing information with providers about substance use or high-risk behavior.
Systematically assess for DSM-5 symptoms of mania and depression. DSM-5 modified criteria for mania/hypomania to necessitate increased energy, in addition to change in mood, to make a syndromal diagnosis. Useful during a clinical interview is the popular mnemonic DIGFAST to aid recognition of core mania symptoms:
- Distractibility
- Indiscretion/impulsivity
- Grandiosity
- Flight of ideas
- Activity increase
- Sleep deficit
- Talkativeness.
These symptoms should represent a departure from normal baseline characteristics; it often is helpful to ask a significant other or collateral historian how the
present symptoms differ from the patient’s usual state.
Assess for unstable medical conditions or toxicity states. When evaluating an acute change in mental status, toxicology screening is relatively standard and the absence of illicit substances should seldom, if ever, be taken for granted—especially because occult substance use can lead to identification of false-positive BD “cases.”1
Stop any antidepressant. During a manic episode, continuing antidepressant medication serves no purpose other than to contribute to or exacerbate mania symptoms. Nonetheless, observational studies demonstrate that approximately 15% of syndromally manic patients continue to receive an antidepressant, often when a clinician perceives more severe depression during mania, multiple prior depressive episodes,current anxiety, or rapid cycling.2
Importantly, antidepressants have been shown to harm, rather than alleviate, presentations that involve a mixed state, and have no demonstrated value in preventing post-manic depression.3 Mere elimination of an antidepressant might ease symptoms during a manic or mixed episode.4
In some cases, it might be advisable to taper, not abruptly stop, a short halflife serotonergic antidepressant, even in the setting of mania, to minimize the potential for aggravating autonomic dysregulation that can result from antidepressant discontinuation effects.
Begin anti-manic pharmacotherapy. Initiation of an anti-manic mood stabilizer, such as lithium and divalproex, has been standard in the treatment of acute mania.
In the 1990s, protocols for oral loading of divalproex (20 to 30 mg/kg/d) gained popularity for achieving more rapid symptom improvement than might occur with lithium. In the current era, atypical antipsychotics have all but replaced mood stabilizers as an initial intervention to contain mania symptoms quickly (and with less risk than firstgeneration antipsychotics for acute adverse motor effects from so-called rapid neuroleptization).
Because atypical antipsychotics often rapidly subdue mania, psychosis, and agitation, regardless of the underlying process, many practitioners might feel more comfortable initiating them than a mood stabilizer when the diagnosis is ambiguous or provisional, although their longer-term efficacy and safety, relative to traditional mood stabilizers, remains contested. Considerations for choosing from among feasible anti-manic pharmacotherapies are summarized in Table 1.
Normalize the sleep-wake cycle. Chronobiological and circadian variables, such as irregular sleep patterns, are thought to contribute to the pathophysiology of affective switch in BD. Behavioral and pharmacotherapeutic efforts to impose a normal sleep-wake schedule are considered fundamental to stabilizing acute mania.
Facilitate next steps after acute stabilization. For inpatients, this might involve step-down to a partial hospitalization or intensive outpatient program, alongside taking steps to ensure continued treatment adherence and minimize relapse.
What Medical and Neurologic Workup is Appropriate?
Not every first lifetime presentation of mania requires extensive medical and neurologic workup, particularly among patients who have a history of depression and those whose presentation neatly fits the demographic and clinical profile of newly emergent BD. Basic assessment should determine whether any new medication has been started that could plausibly contribute to abnormal mental status (Table 2).
Nevertheless, evaluation of almost all first presentations of mania should include:
- Urine toxicology screen
- Complete blood count
- Comprehensive metabolic panel
- Thyroid-stimulating hormone assay
- Serum vitamin B12 level assay
- Serum folic acid level assay
- Rapid plasma reagin test.
Clinical features that usually lead a clinician to pursue a more detailed medical and neurologic evaluation of first-episode mania include:
- Onset age > 40
- Absence of a family history of mood disorder
- Symptoms arising during a major medical illness
- Multiple medications
- Suspicion of a degenerative or hereditary neurologic disorder
- Altered state of consciousness
- Signs of cortical or diffuse subcortical dysfunction (eg, cognitive deficits, motor deficits, tremor)
- Abnormal vital signs.
Depending on the presentation, additional testing might include:
- Tests of HIV antibody, immune autoantibodies, and Lyme disease antibody
- Heavy metal screening (when suggested by environmental exposure)
- Lumbar puncture (eg, in a setting of manic delirium or suspected central nervous system infection or paraneoplastic syndrome)
- Neuroimaging (note: MRI provides better visualization than CT of white matter pathology and small vessel cerebrovascular disease) electroencephalography.
Making An Overarching Diagnosis: Is Mania Always Bipolar Disorder?
Mania is considered a manifestation of BD when symptoms cannot be attributed to another psychiatric condition, another underlying medical or neurologic condition, or a toxic-metabolic state (Tables 3 and 4). Classification of mania that occurs soon after antidepressant exposure in patients without a known history of BD continues to be the subject of debate, varying in its conceptualization across editions of DSM.
The National Institute of Mental Health (NIMH) Systematic Treatment Enhancement Program for Bipolar Disorder, or STEP-BD, observed a fairly low (approximately 10%) incidence of switch from depression to mania when an antidepressant is added to a mood stabilizer; the study authors concluded that much of what is presumed to be antidepressant-induced mania might simply be the natural course of illness.10
Notably, several reports suggest that antidepressants might pose a greater risk of mood destabilization in people with BD I than with either BD II or other suspected variants on the bipolar spectrum.
DSM-5 advises that a diagnosis of substance-induced mood disorder appropriately describes symptoms that spontaneously dissipate once an antidepressant has been discontinued, whereas a diagnosis of BD can be made when manic or hypomanic symptoms persist at a syndromal level after an antidepressant has been stopped and its physiological effects are no longer present. With respect to time course, the International Society of Bipolar Disorders proposes that, beyond 12 to 16 weeks after an antidepressant has been started or the dosage has been increased, it is unlikely that new-onset mania/hypomania can reasonably be attributed to “triggering” by an antidepressant (although antidepressants should be stopped when symptoms of mania emerge).11 Several clinical features have been linked in the literature with an increased susceptibility to BD after an initial depressive episode, including:
- Early (pre-adolescent) age at onset of first mood disorder episode6
- Family history of BD, highly recurrent depression, or psychosis12,13
- Psychosis when depressed.7,14
A number of other characteristics of depressive illness—including seasonal depression, atypical depressive features, suicidality, irritability, anxiety or substance use comorbidity, postpartum mood episodes, and brief recurrent depressive episodes—have been described in the literature as potential correlates of a bipolar diathesis; none have proved to be robust or pathognomonic of a BD diagnosis, as opposed to a unipolar diagnosis.
Data from the NIMH Collaborative Depression Study suggest that recurrent mania/hypomania after an antidepressant-associated polarity switch is greater when a family history of BD is present; other clinical variables might hold less predictive value.15 In addition, although some practitioners consider a history of nonresponse to trials of multiple antidepressants suggestive of an underlying bipolar process, polarity is only one of many variables that must be considered in the differential diagnosis of antidepressantresistant depression. Likewise, molecular genetic studies do not support a link between antidepressant nonresponse and the likelihood of a diagnosis of BD.16
Indefinite Pharmacotherapy for Bipolar Disorder
An important but nagging issue when diagnosing BD after a first manic (or hypomanic) episode is the implied need for indefinite pharmacotherapy to sustain remission and prevent relapse and recurrence.
The likelihood of subsequent depression or mania/hypomania remains high after an index manic/hypomanic episode, particularly for 6 to 8 months after recovery.8,17 Natural history data suggest that, during the year that follows a first lifetime mania, approximately 40% of patients experience a second manic episode.8 A second lifetime mania might be especially likely in patients whose index episode involved mood-congruent psychosis, low premorbid work functioning, and an initial manic episode, as opposed to a mixed episode or early age at onset.8,17
In the absence of randomized, placebo-controlled studies of maintenance pharmacotherapy after a first lifetime manic episode, clinical judgment often drives decisions about the duration of continuing pharmacotherapy after initial symptoms resolve. The Texas Medication Algorithm Project for BD advises that:
General practice at this time is lifetime medication following 2 manic episodes, or 1 episode if it was a severe episode and/or significant family history of bipolar or major depressive disorder is present. For a first episode of bipolar mania with no family history of bipolar or major depressive disorders, medication tapering and discontinuation may be considered after the continuation period is completed (usually 6 months in remission), depending on the severity of the first episode, surrounding factors, and prodromal history.18
Similarly, in the most recent (2004) Expert Consensus Guideline Series for the Treatment of Bipolar Disorder, 84% of practitioner−respondents favored indefinite mood stabilizer therapy after a second lifetime manic episode.19 No recommendation was made about the duration of maintenance pharmacotherapy after a first lifetime manic/hypomanic episode.
Avoid or Reintroduce an Antidepressant if Depression Recurs After a First Mania?
Controversies surrounding antidepressant use in BD are extensive; detailed discussion is beyond the scope of this review (Goldberg and Ghaemi provided a broader discussion of risks and benefits of antidepressants in BD).20 Although the main clinical concern regarding antidepressant use was, at one time, the potential to induce mania or accelerate the frequency of recurrent episodes, more recent, empirical studies suggest that the greater risk of using antidepressants for BD is lack of efficacy.10,21
If a careful longitudinal history and clinical evaluation reveal that an initial manic episode heralds the onset of BD, decisions about whether to avoid an antidepressant (as opposed to using other, more evidence-based interventions for bipolar depression) depend on a number of variables, including establishing whether the index episode was manic or hypomanic; ruling out current subthreshold mixed features; and clarifying how recently mania developed. Decisions about future antidepressant use (or avoidance) might be less clear if an index manic/hypomanic episode was brief and self-limited once the antidepressant was stopped.
Although some experts eschew antidepressant monotherapy after such occurrences, there is no body of literature to inform decisions about the safety or efficacy of undertaking a future antidepressant trial in such patients. That said, reasonable judgment probably includes several considerations:
- Re-exposure to the same antidepressant that was associated with an induction of mania is likely riskier than choosing a different antidepressant; in general, purely serotonergic antidepressants or bupropion are considered to pose less risk of mood destabilization than is seen with an SNRI or tricyclic antidepressant.
- After a manic episode, a subsequent antidepressant trial generally shouldn’t be attempted without concurrent anti-manic medication.
- Introducing any antidepressant is probably ill-advised in the recent (~2 months) aftermath of acute manic/hypomanic symptoms.22
- Patients and their significant other should be apprised of the risk of emerging symptoms of mania or hypomania, or mixed features, and should be familiar with key target symptoms to watch for. Prospective mood charting can be helpful.
- Patients should be monitored closely both for an exacerbation of depression and recurrence of mania/hypomania symptoms.
- Any antidepressant should be discontinued promptly at the first sign of psychomotor acceleration or the emergence of mixed features, as defined by DSM-5
Psychoeducation and Forecasting
Functional recovery from a manic episode can lag behind symptomatic recovery. Subsyndromal symptoms often persist after a full episode subsides.
Mania often is followed by a depressive episode, and questions inevitably arise about how to prevent and treat these episodes. Because the median duration of a manic episode is approximately 13 weeks, it is crucial for patients and their immediate family to recognize that recovery might be gradual, and that it will likely take time before she (he) can resume full-time responsibilities at work or school or in the home.23
Today, a patient who is hospitalized for severe acute mania (as Ms. J was, in the case vignette) seldom remains an inpatient long enough to achieve remission of symptoms; sometimes, she (he) might continue to manifest significant symptoms, even though decisions about the “medical necessity” of ongoing inpatient care tend to be governed mainly by issues of safety and imminent danger. (See this article at CurrentPsychiatry.com for a Table of considerations when making the transition from the acute phase to the continuation phase of treatment.20,24,25)
To minimize risk of relapse, psycho-education should include discussion of:
- Psychiatrically deleterious effects of alcohol and illicit drug use
- Suicide risk, including what to do in an emergency
- Protecting a regular sleep schedule and avoiding sleep deprivation
- The potential for poor medication adherence and management of side effects
- The role of adjunctive psychotherapy and effective stress management
- Familiarity with symptoms that serve as warning signs, and how to monitor their onset.
Click here to read the digital edition.
1. Goldberg JF, Garno JL, Callahan AM, et al. Overdiagnosis of bipolar disorder among substance use disorder in patients with mood instability. J Clin Psychiatry. 2008;69(11): 1751-1757.
2. Rosa AR, Cruz B, Franco C, et al. Why do clinicians maintain antidepressants in some patients with acute mania? Hints from the European Mania in Bipolar Longitudinal Evaluation of Medication (EMBLEM), a large naturalistic study. J Clin Psychiatry. 2010;71(8):1000-1006.
3. Goldberg JF, Perlis RH, Ghaemi SN, et al. Adjunctive antidepressant use and symptomatic recovery among bipolar depressed patients with concomitant manic symptoms: findings from the STEP-BD. Am J Psychiatry. 2007;164(9):1348-1355.
4. Bowers MB Jr, McKay BG, Mazure CM. Discontinuation of antidepressants in newly admitted psychotic patients. J Neuropsychiatr Clin Neurosci. 2003;15(2):227-230.
5. Perlis RH, Welge JA, Vornik LA, et al. Atypical antipsychotics in the treatment of mania: a meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry. 2006;67(4): 509-516.
6. Geller B, Zimmerman B, Williams M, et al. Bipolar disorder at prospective followup of adults who had prepubertal major depressive disorder. Am J Psychiatry. 2001;158(1): 125-127.
7. Goldberg JF, Harrow M, Whiteside JE. Risk for bipolar illness in patients initially hospitalized for unipolar depression. Am J Psychiatry. 2001;158(8):1265-1270.
8. Yatham LN, Kauer-Sant’Anna M, Bond DJ, et al. Course and outcome after the first manic episode in patients with bipolar disorder: prospective 12-month data from the Systematic Treatment Optimization Project for Early Mania project. Can J Psychiatry. 2009;54(2):105-112.
9. Chaudron LH, Pies RW. The relationship between postpartum psychosis and bipolar
disorder: a review. J Clin Psychiatry. 2003;64(11):1284-1292.
10. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
11. Tohen M, Frank E, Bowden CL, et al. The International Society for Bipolar Disorders (ISBD) Task Force report on the nomenclature of course and outcome in bipolar disorders. Bipolar Disord. 2009;11(15):453-473.
12. Schulze TG, Hedeker D, Zandi P, et al. What is familial about familial bipolar disorder? Resemblance among relatives across a broad spectrum of phenotypic characteristics. Arch Gen Psychiatry. 2006;63(12):1368-1376.
13. Song J, Bergen SE, Kuja-Halkola R, et al. Bipolar disorder and its relation to major psychiatric disorders: a family-based study in the Swedish population. Bipolar Disord. 2015;7(2):184-193.
14. Goes FS, Sadler B, Toolan J, et al. Psychotic features in bipolar and unipolar depression. Bipolar Disord. 2007;9(8):901-906.
15. Fiedorowicz JG, Endicott J, Solomon DA, et al. Course of illness following prospectively observed mania or hypomania in individuals presenting with unipolar depression. Bipolar Disord. 2007;14(6):664-671.
16. Tansey KE, Guipponi M, Domenici E, et al. Genetic susceptibility for bipolar disorder and response to antidepressants in major depressive disorder. Am J Med Genetics B Neuropsychiatr Genet. 2014;165B(1):77-83.
17. Tohen M, Zarate CA Jr, Hennen J, et al. The McLean-Harvard First-Episode Mania Study: prediction of recovery and first recurrence. Am J Psychiatry. 2003;160(12):2099-2107.
18. Suppes T, Dennehy EB, Swann AC, et al. Report of the Texas Consensus Conference Panel on medication treatment of bipolar disorder 2000. J Clin Psychiatry. 2002; 63(4):288-299.
19. Keck PE Jr, Perlis RH, Otto MW, et al. The Expert Consensus Guideline Series: treatment of bipolar disorder 2004. Postgrad Med Special Report. 2004:1-120.
20. Goldberg JF, Ghaemi SN. Benefits and limitations of antidepressants and traditional mood stabilizers for treatment of bipolar depression. Bipolar Disord. 2005; 7(suppl 5):3-12.
21. Sidor MM, MacQueen GM. Antidepressants for the acute treatment of bipolar
depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2): 156-167.
22. MacQueen GM, Trevor Young L, Marriott M, et al. Previous mood state predicts response and switch rates in patients with bipolar depression. Acta Psychiatr Scand. 2002;105(6):414-418.
23. Solomon DA, Leon AC, Coryell WH, et al. Longitudinal course of bipolar I disorder: duration of mood episodes. Arch Gen Psychiatry. 2010;67(4):339-347.
24. Tohen M, Chengappa KN, Suppes T, et al. Relapse prevention in bipolar I disorder: 18-month comparison of olanzapine plus mood stabiliser v. mood stabiliser alone. Br J Psychiatry. 2004;184:337-345.
25. Suppes T, Vieta E, Liu S, et al. Maintenance treatment for patients with bipolar I disorder: results from a North American study of quetiapine in combination with lithium or divalproex (trial 127). Am J Psychiatry. 2009;166(4): 476-488.
Note: Page numbers differ between the print issue and digital edition.
This article has been adapted from an article originally published in Current Psychiatry (http://currentpsychiatry.com):Current Psychiatry. 2015;14(10):29-32,35-40,e6.
When a known depressed patient newly develops signs of mania or hypomania, a cascade of diagnostic and therapeutic questions ensues: Does the event “automatically” signify the presence of bipolar disorder (BD), or could manic symptoms be secondary to another underlying medical problem, a prescribed antidepressant or non-psychotropic medication, or illicit substances?
Even more questions confront the clinician: If mania symptoms are nothing more than an adverse drug reaction, will they go away by stopping the presumed offending agent? Or do symptoms always indicate the unmasking of a bipolar diathesis? Should anti-manic medication be prescribed immediately? If so, which one(s) and for how long? How extensive a medical or neurologic workup is indicated?
And how do you differentiate ambiguous hypomania symptoms (irritability, insomnia, agitation) from other phenomena, such as akathisia, anxiety, and overstimulation?
In this article, we present an overview of how to approach and answer these key questions, so that you can identify, comprehend, and manage manic symptoms that arise in the course of your patient’s treatment for depression (Box).
Does Disease Exist on a Unipolar-Bipolar Continuum.
There has been a resurgence of interest in Kraepelin’s original notion of mania and depression as falling along a continuum, rather than being distinct categories of pathology. True bipolar mania has its own identifiable epidemiology, familiality, and treatment, but symptomatic shades of gray often pose a formidable diagnostic and therapeutic challenge.
For example, the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) relaxed its definition of “mixed” episodes of BD to include subsyndromal mania features in unipolar depression. When a patient with unipolar depression develops a full, unequivocal manic episode, there usually isn’t much ambiguity or confusion about initial management: assure a safe environment, stop any antidepressants, rule out drug- or medically induced causes, and begin an acute anti-manic medication.
Next steps can, sometimes, be murkier:
- Formulate a definitive, overarching diagnosis
- Provide psycho-education
- Forecast return to work or school
- Discuss prognosis and likelihood of relapse
- Address necessary lifestyle modifications (eg, sleep hygiene, elimination of alcohol and illicit drug use)
- Determine whether indefinite maintenance pharmacotherapy is indicated—and, if so, with which medication(s).
A Diagnostic Formulation Isn’t Always Black and White
Ms. J, age 56, a medically healthy woman, has a 10-year history of depression and anxiety that has been treated effectively for most of that time with venlafaxine,
225 mg/d. The mother of 4 grown children, Ms. J has worked steadily for > 20 years as a flight attendant for an international airline.
Today, Ms. J is brought by ambulance from work to the emergency department in a paranoid and agitated state. The admission follows her having e-blasted airline corporate executives with a voluminous manifesto that she worked on around the clock the preceding week, in which she explained her bold ideas to revolutionize the airline industry, under her leadership.
Ms. J’s family history is unremarkable for psychiatric illness.
How Does One Approach A Case Such As Ms. J's?
Stark examples of classical mania, as depicted in this case vignette, are easy to recognize but not necessarily straightforward, nosologically. Consider the following not-so-straightforward elements of Ms. J’s case:
- A first-lifetime episode of mania or hypomania is rare after age 50
- Ms. J took a serotonin-norepinephrine reuptake inhibitor (SNRI) for many years without evidence of mood destabilization
- Years of repetitive chronobiological stress (including probable frequent time zone changes with likely sleep disruption) apparently did not trigger mood destabilization
- None of Ms. J’s 4 pregnancies led to postpartum mood episodes
- At least on the surface, there are no obvious features that point to likely causes of a secondary mania (eg, drug-induced, toxic, metabolic, or medical)
- Ms. J has no known family history of BD or any other mood disorder.
Approaching a case such as Ms. J’s must involve a systematic strategy that can best be broken into 2 segments: (1) a period of acute initial assessment and treatment and (2) later efforts focused on broader diagnostic evaluation and longer-term relapse prevention.
Initial Assessment and Treatment
Immediate assessment and management hinges on initial triage and forming a working diagnostic impression. Although full-blown mania usually is obvious (sometimes even without a formal interview), be alert to patients who might minimize or altogether disavow mania symptoms—often because of denial of illness, misidentification of symptoms, or impaired insight about changes in thinking, mood, or behavior.
Because florid mania, by definition, impairs psychosocial functioning, the context of an initial presentation often holds diagnostic relevance. Manic patients who display disruptive behaviors often are brought to treatment by a third party, whereas a less severely ill patient might be more inclined to seek treatment for herself (himself) when psychosis is absent and insight is less compromised or when the patient feels she (he) might be depressed.
It is not uncommon for a manic patient to report “depression” as the chief complaint or to omit elements related to psychomotor acceleration (such as racing thoughts or psychomotor agitation) in the description of symptoms. An accurate diagnosis often requires clinical probing and clarification of symptoms (eg, differentiating simple insomnia with consequent next-day fatigue from loss of the need for sleep with intact or even enhanced next-day energy) or discriminating racing thoughts from anxious ruminations that might be more intrusive than rapid.
Presentations of frank mania also can come to light as a consequence of symptoms, rather than as symptoms per se (eg, conflict in relationships, problems at work, financial reversals).
Particularly in patients who do not have a history of mania, avoid the temptation to begin or modify existing pharmacotherapy until you have performed a basic initial evaluation. Immediate considerations for initial assessment and management include the following:
Provide containment. Ensure a safe setting, level of care, and frequency of monitoring. Evaluate suicide risk (particularly when mixed features are present), and risk of withdrawal from any psychoactive substances.
Engage significant others. Close family members can provide essential history, particularly when a patient’s insight about her illness and need for treatment are impaired. Family members and significant others also often play important roles in helping to restrict access to finances, fostering medication adherence, preventing access to weapons in the home, and sharing information with providers about substance use or high-risk behavior.
Systematically assess for DSM-5 symptoms of mania and depression. DSM-5 modified criteria for mania/hypomania to necessitate increased energy, in addition to change in mood, to make a syndromal diagnosis. Useful during a clinical interview is the popular mnemonic DIGFAST to aid recognition of core mania symptoms:
- Distractibility
- Indiscretion/impulsivity
- Grandiosity
- Flight of ideas
- Activity increase
- Sleep deficit
- Talkativeness.
These symptoms should represent a departure from normal baseline characteristics; it often is helpful to ask a significant other or collateral historian how the
present symptoms differ from the patient’s usual state.
Assess for unstable medical conditions or toxicity states. When evaluating an acute change in mental status, toxicology screening is relatively standard and the absence of illicit substances should seldom, if ever, be taken for granted—especially because occult substance use can lead to identification of false-positive BD “cases.”1
Stop any antidepressant. During a manic episode, continuing antidepressant medication serves no purpose other than to contribute to or exacerbate mania symptoms. Nonetheless, observational studies demonstrate that approximately 15% of syndromally manic patients continue to receive an antidepressant, often when a clinician perceives more severe depression during mania, multiple prior depressive episodes,current anxiety, or rapid cycling.2
Importantly, antidepressants have been shown to harm, rather than alleviate, presentations that involve a mixed state, and have no demonstrated value in preventing post-manic depression.3 Mere elimination of an antidepressant might ease symptoms during a manic or mixed episode.4
In some cases, it might be advisable to taper, not abruptly stop, a short halflife serotonergic antidepressant, even in the setting of mania, to minimize the potential for aggravating autonomic dysregulation that can result from antidepressant discontinuation effects.
Begin anti-manic pharmacotherapy. Initiation of an anti-manic mood stabilizer, such as lithium and divalproex, has been standard in the treatment of acute mania.
In the 1990s, protocols for oral loading of divalproex (20 to 30 mg/kg/d) gained popularity for achieving more rapid symptom improvement than might occur with lithium. In the current era, atypical antipsychotics have all but replaced mood stabilizers as an initial intervention to contain mania symptoms quickly (and with less risk than firstgeneration antipsychotics for acute adverse motor effects from so-called rapid neuroleptization).
Because atypical antipsychotics often rapidly subdue mania, psychosis, and agitation, regardless of the underlying process, many practitioners might feel more comfortable initiating them than a mood stabilizer when the diagnosis is ambiguous or provisional, although their longer-term efficacy and safety, relative to traditional mood stabilizers, remains contested. Considerations for choosing from among feasible anti-manic pharmacotherapies are summarized in Table 1.
Normalize the sleep-wake cycle. Chronobiological and circadian variables, such as irregular sleep patterns, are thought to contribute to the pathophysiology of affective switch in BD. Behavioral and pharmacotherapeutic efforts to impose a normal sleep-wake schedule are considered fundamental to stabilizing acute mania.
Facilitate next steps after acute stabilization. For inpatients, this might involve step-down to a partial hospitalization or intensive outpatient program, alongside taking steps to ensure continued treatment adherence and minimize relapse.
What Medical and Neurologic Workup is Appropriate?
Not every first lifetime presentation of mania requires extensive medical and neurologic workup, particularly among patients who have a history of depression and those whose presentation neatly fits the demographic and clinical profile of newly emergent BD. Basic assessment should determine whether any new medication has been started that could plausibly contribute to abnormal mental status (Table 2).
Nevertheless, evaluation of almost all first presentations of mania should include:
- Urine toxicology screen
- Complete blood count
- Comprehensive metabolic panel
- Thyroid-stimulating hormone assay
- Serum vitamin B12 level assay
- Serum folic acid level assay
- Rapid plasma reagin test.
Clinical features that usually lead a clinician to pursue a more detailed medical and neurologic evaluation of first-episode mania include:
- Onset age > 40
- Absence of a family history of mood disorder
- Symptoms arising during a major medical illness
- Multiple medications
- Suspicion of a degenerative or hereditary neurologic disorder
- Altered state of consciousness
- Signs of cortical or diffuse subcortical dysfunction (eg, cognitive deficits, motor deficits, tremor)
- Abnormal vital signs.
Depending on the presentation, additional testing might include:
- Tests of HIV antibody, immune autoantibodies, and Lyme disease antibody
- Heavy metal screening (when suggested by environmental exposure)
- Lumbar puncture (eg, in a setting of manic delirium or suspected central nervous system infection or paraneoplastic syndrome)
- Neuroimaging (note: MRI provides better visualization than CT of white matter pathology and small vessel cerebrovascular disease) electroencephalography.
Making An Overarching Diagnosis: Is Mania Always Bipolar Disorder?
Mania is considered a manifestation of BD when symptoms cannot be attributed to another psychiatric condition, another underlying medical or neurologic condition, or a toxic-metabolic state (Tables 3 and 4). Classification of mania that occurs soon after antidepressant exposure in patients without a known history of BD continues to be the subject of debate, varying in its conceptualization across editions of DSM.
The National Institute of Mental Health (NIMH) Systematic Treatment Enhancement Program for Bipolar Disorder, or STEP-BD, observed a fairly low (approximately 10%) incidence of switch from depression to mania when an antidepressant is added to a mood stabilizer; the study authors concluded that much of what is presumed to be antidepressant-induced mania might simply be the natural course of illness.10
Notably, several reports suggest that antidepressants might pose a greater risk of mood destabilization in people with BD I than with either BD II or other suspected variants on the bipolar spectrum.
DSM-5 advises that a diagnosis of substance-induced mood disorder appropriately describes symptoms that spontaneously dissipate once an antidepressant has been discontinued, whereas a diagnosis of BD can be made when manic or hypomanic symptoms persist at a syndromal level after an antidepressant has been stopped and its physiological effects are no longer present. With respect to time course, the International Society of Bipolar Disorders proposes that, beyond 12 to 16 weeks after an antidepressant has been started or the dosage has been increased, it is unlikely that new-onset mania/hypomania can reasonably be attributed to “triggering” by an antidepressant (although antidepressants should be stopped when symptoms of mania emerge).11 Several clinical features have been linked in the literature with an increased susceptibility to BD after an initial depressive episode, including:
- Early (pre-adolescent) age at onset of first mood disorder episode6
- Family history of BD, highly recurrent depression, or psychosis12,13
- Psychosis when depressed.7,14
A number of other characteristics of depressive illness—including seasonal depression, atypical depressive features, suicidality, irritability, anxiety or substance use comorbidity, postpartum mood episodes, and brief recurrent depressive episodes—have been described in the literature as potential correlates of a bipolar diathesis; none have proved to be robust or pathognomonic of a BD diagnosis, as opposed to a unipolar diagnosis.
Data from the NIMH Collaborative Depression Study suggest that recurrent mania/hypomania after an antidepressant-associated polarity switch is greater when a family history of BD is present; other clinical variables might hold less predictive value.15 In addition, although some practitioners consider a history of nonresponse to trials of multiple antidepressants suggestive of an underlying bipolar process, polarity is only one of many variables that must be considered in the differential diagnosis of antidepressantresistant depression. Likewise, molecular genetic studies do not support a link between antidepressant nonresponse and the likelihood of a diagnosis of BD.16
Indefinite Pharmacotherapy for Bipolar Disorder
An important but nagging issue when diagnosing BD after a first manic (or hypomanic) episode is the implied need for indefinite pharmacotherapy to sustain remission and prevent relapse and recurrence.
The likelihood of subsequent depression or mania/hypomania remains high after an index manic/hypomanic episode, particularly for 6 to 8 months after recovery.8,17 Natural history data suggest that, during the year that follows a first lifetime mania, approximately 40% of patients experience a second manic episode.8 A second lifetime mania might be especially likely in patients whose index episode involved mood-congruent psychosis, low premorbid work functioning, and an initial manic episode, as opposed to a mixed episode or early age at onset.8,17
In the absence of randomized, placebo-controlled studies of maintenance pharmacotherapy after a first lifetime manic episode, clinical judgment often drives decisions about the duration of continuing pharmacotherapy after initial symptoms resolve. The Texas Medication Algorithm Project for BD advises that:
General practice at this time is lifetime medication following 2 manic episodes, or 1 episode if it was a severe episode and/or significant family history of bipolar or major depressive disorder is present. For a first episode of bipolar mania with no family history of bipolar or major depressive disorders, medication tapering and discontinuation may be considered after the continuation period is completed (usually 6 months in remission), depending on the severity of the first episode, surrounding factors, and prodromal history.18
Similarly, in the most recent (2004) Expert Consensus Guideline Series for the Treatment of Bipolar Disorder, 84% of practitioner−respondents favored indefinite mood stabilizer therapy after a second lifetime manic episode.19 No recommendation was made about the duration of maintenance pharmacotherapy after a first lifetime manic/hypomanic episode.
Avoid or Reintroduce an Antidepressant if Depression Recurs After a First Mania?
Controversies surrounding antidepressant use in BD are extensive; detailed discussion is beyond the scope of this review (Goldberg and Ghaemi provided a broader discussion of risks and benefits of antidepressants in BD).20 Although the main clinical concern regarding antidepressant use was, at one time, the potential to induce mania or accelerate the frequency of recurrent episodes, more recent, empirical studies suggest that the greater risk of using antidepressants for BD is lack of efficacy.10,21
If a careful longitudinal history and clinical evaluation reveal that an initial manic episode heralds the onset of BD, decisions about whether to avoid an antidepressant (as opposed to using other, more evidence-based interventions for bipolar depression) depend on a number of variables, including establishing whether the index episode was manic or hypomanic; ruling out current subthreshold mixed features; and clarifying how recently mania developed. Decisions about future antidepressant use (or avoidance) might be less clear if an index manic/hypomanic episode was brief and self-limited once the antidepressant was stopped.
Although some experts eschew antidepressant monotherapy after such occurrences, there is no body of literature to inform decisions about the safety or efficacy of undertaking a future antidepressant trial in such patients. That said, reasonable judgment probably includes several considerations:
- Re-exposure to the same antidepressant that was associated with an induction of mania is likely riskier than choosing a different antidepressant; in general, purely serotonergic antidepressants or bupropion are considered to pose less risk of mood destabilization than is seen with an SNRI or tricyclic antidepressant.
- After a manic episode, a subsequent antidepressant trial generally shouldn’t be attempted without concurrent anti-manic medication.
- Introducing any antidepressant is probably ill-advised in the recent (~2 months) aftermath of acute manic/hypomanic symptoms.22
- Patients and their significant other should be apprised of the risk of emerging symptoms of mania or hypomania, or mixed features, and should be familiar with key target symptoms to watch for. Prospective mood charting can be helpful.
- Patients should be monitored closely both for an exacerbation of depression and recurrence of mania/hypomania symptoms.
- Any antidepressant should be discontinued promptly at the first sign of psychomotor acceleration or the emergence of mixed features, as defined by DSM-5
Psychoeducation and Forecasting
Functional recovery from a manic episode can lag behind symptomatic recovery. Subsyndromal symptoms often persist after a full episode subsides.
Mania often is followed by a depressive episode, and questions inevitably arise about how to prevent and treat these episodes. Because the median duration of a manic episode is approximately 13 weeks, it is crucial for patients and their immediate family to recognize that recovery might be gradual, and that it will likely take time before she (he) can resume full-time responsibilities at work or school or in the home.23
Today, a patient who is hospitalized for severe acute mania (as Ms. J was, in the case vignette) seldom remains an inpatient long enough to achieve remission of symptoms; sometimes, she (he) might continue to manifest significant symptoms, even though decisions about the “medical necessity” of ongoing inpatient care tend to be governed mainly by issues of safety and imminent danger. (See this article at CurrentPsychiatry.com for a Table of considerations when making the transition from the acute phase to the continuation phase of treatment.20,24,25)
To minimize risk of relapse, psycho-education should include discussion of:
- Psychiatrically deleterious effects of alcohol and illicit drug use
- Suicide risk, including what to do in an emergency
- Protecting a regular sleep schedule and avoiding sleep deprivation
- The potential for poor medication adherence and management of side effects
- The role of adjunctive psychotherapy and effective stress management
- Familiarity with symptoms that serve as warning signs, and how to monitor their onset.
Click here to read the digital edition.
This article has been adapted from an article originally published in Current Psychiatry (http://currentpsychiatry.com):Current Psychiatry. 2015;14(10):29-32,35-40,e6.
When a known depressed patient newly develops signs of mania or hypomania, a cascade of diagnostic and therapeutic questions ensues: Does the event “automatically” signify the presence of bipolar disorder (BD), or could manic symptoms be secondary to another underlying medical problem, a prescribed antidepressant or non-psychotropic medication, or illicit substances?
Even more questions confront the clinician: If mania symptoms are nothing more than an adverse drug reaction, will they go away by stopping the presumed offending agent? Or do symptoms always indicate the unmasking of a bipolar diathesis? Should anti-manic medication be prescribed immediately? If so, which one(s) and for how long? How extensive a medical or neurologic workup is indicated?
And how do you differentiate ambiguous hypomania symptoms (irritability, insomnia, agitation) from other phenomena, such as akathisia, anxiety, and overstimulation?
In this article, we present an overview of how to approach and answer these key questions, so that you can identify, comprehend, and manage manic symptoms that arise in the course of your patient’s treatment for depression (Box).
Does Disease Exist on a Unipolar-Bipolar Continuum.
There has been a resurgence of interest in Kraepelin’s original notion of mania and depression as falling along a continuum, rather than being distinct categories of pathology. True bipolar mania has its own identifiable epidemiology, familiality, and treatment, but symptomatic shades of gray often pose a formidable diagnostic and therapeutic challenge.
For example, the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) relaxed its definition of “mixed” episodes of BD to include subsyndromal mania features in unipolar depression. When a patient with unipolar depression develops a full, unequivocal manic episode, there usually isn’t much ambiguity or confusion about initial management: assure a safe environment, stop any antidepressants, rule out drug- or medically induced causes, and begin an acute anti-manic medication.
Next steps can, sometimes, be murkier:
- Formulate a definitive, overarching diagnosis
- Provide psycho-education
- Forecast return to work or school
- Discuss prognosis and likelihood of relapse
- Address necessary lifestyle modifications (eg, sleep hygiene, elimination of alcohol and illicit drug use)
- Determine whether indefinite maintenance pharmacotherapy is indicated—and, if so, with which medication(s).
A Diagnostic Formulation Isn’t Always Black and White
Ms. J, age 56, a medically healthy woman, has a 10-year history of depression and anxiety that has been treated effectively for most of that time with venlafaxine,
225 mg/d. The mother of 4 grown children, Ms. J has worked steadily for > 20 years as a flight attendant for an international airline.
Today, Ms. J is brought by ambulance from work to the emergency department in a paranoid and agitated state. The admission follows her having e-blasted airline corporate executives with a voluminous manifesto that she worked on around the clock the preceding week, in which she explained her bold ideas to revolutionize the airline industry, under her leadership.
Ms. J’s family history is unremarkable for psychiatric illness.
How Does One Approach A Case Such As Ms. J's?
Stark examples of classical mania, as depicted in this case vignette, are easy to recognize but not necessarily straightforward, nosologically. Consider the following not-so-straightforward elements of Ms. J’s case:
- A first-lifetime episode of mania or hypomania is rare after age 50
- Ms. J took a serotonin-norepinephrine reuptake inhibitor (SNRI) for many years without evidence of mood destabilization
- Years of repetitive chronobiological stress (including probable frequent time zone changes with likely sleep disruption) apparently did not trigger mood destabilization
- None of Ms. J’s 4 pregnancies led to postpartum mood episodes
- At least on the surface, there are no obvious features that point to likely causes of a secondary mania (eg, drug-induced, toxic, metabolic, or medical)
- Ms. J has no known family history of BD or any other mood disorder.
Approaching a case such as Ms. J’s must involve a systematic strategy that can best be broken into 2 segments: (1) a period of acute initial assessment and treatment and (2) later efforts focused on broader diagnostic evaluation and longer-term relapse prevention.
Initial Assessment and Treatment
Immediate assessment and management hinges on initial triage and forming a working diagnostic impression. Although full-blown mania usually is obvious (sometimes even without a formal interview), be alert to patients who might minimize or altogether disavow mania symptoms—often because of denial of illness, misidentification of symptoms, or impaired insight about changes in thinking, mood, or behavior.
Because florid mania, by definition, impairs psychosocial functioning, the context of an initial presentation often holds diagnostic relevance. Manic patients who display disruptive behaviors often are brought to treatment by a third party, whereas a less severely ill patient might be more inclined to seek treatment for herself (himself) when psychosis is absent and insight is less compromised or when the patient feels she (he) might be depressed.
It is not uncommon for a manic patient to report “depression” as the chief complaint or to omit elements related to psychomotor acceleration (such as racing thoughts or psychomotor agitation) in the description of symptoms. An accurate diagnosis often requires clinical probing and clarification of symptoms (eg, differentiating simple insomnia with consequent next-day fatigue from loss of the need for sleep with intact or even enhanced next-day energy) or discriminating racing thoughts from anxious ruminations that might be more intrusive than rapid.
Presentations of frank mania also can come to light as a consequence of symptoms, rather than as symptoms per se (eg, conflict in relationships, problems at work, financial reversals).
Particularly in patients who do not have a history of mania, avoid the temptation to begin or modify existing pharmacotherapy until you have performed a basic initial evaluation. Immediate considerations for initial assessment and management include the following:
Provide containment. Ensure a safe setting, level of care, and frequency of monitoring. Evaluate suicide risk (particularly when mixed features are present), and risk of withdrawal from any psychoactive substances.
Engage significant others. Close family members can provide essential history, particularly when a patient’s insight about her illness and need for treatment are impaired. Family members and significant others also often play important roles in helping to restrict access to finances, fostering medication adherence, preventing access to weapons in the home, and sharing information with providers about substance use or high-risk behavior.
Systematically assess for DSM-5 symptoms of mania and depression. DSM-5 modified criteria for mania/hypomania to necessitate increased energy, in addition to change in mood, to make a syndromal diagnosis. Useful during a clinical interview is the popular mnemonic DIGFAST to aid recognition of core mania symptoms:
- Distractibility
- Indiscretion/impulsivity
- Grandiosity
- Flight of ideas
- Activity increase
- Sleep deficit
- Talkativeness.
These symptoms should represent a departure from normal baseline characteristics; it often is helpful to ask a significant other or collateral historian how the
present symptoms differ from the patient’s usual state.
Assess for unstable medical conditions or toxicity states. When evaluating an acute change in mental status, toxicology screening is relatively standard and the absence of illicit substances should seldom, if ever, be taken for granted—especially because occult substance use can lead to identification of false-positive BD “cases.”1
Stop any antidepressant. During a manic episode, continuing antidepressant medication serves no purpose other than to contribute to or exacerbate mania symptoms. Nonetheless, observational studies demonstrate that approximately 15% of syndromally manic patients continue to receive an antidepressant, often when a clinician perceives more severe depression during mania, multiple prior depressive episodes,current anxiety, or rapid cycling.2
Importantly, antidepressants have been shown to harm, rather than alleviate, presentations that involve a mixed state, and have no demonstrated value in preventing post-manic depression.3 Mere elimination of an antidepressant might ease symptoms during a manic or mixed episode.4
In some cases, it might be advisable to taper, not abruptly stop, a short halflife serotonergic antidepressant, even in the setting of mania, to minimize the potential for aggravating autonomic dysregulation that can result from antidepressant discontinuation effects.
Begin anti-manic pharmacotherapy. Initiation of an anti-manic mood stabilizer, such as lithium and divalproex, has been standard in the treatment of acute mania.
In the 1990s, protocols for oral loading of divalproex (20 to 30 mg/kg/d) gained popularity for achieving more rapid symptom improvement than might occur with lithium. In the current era, atypical antipsychotics have all but replaced mood stabilizers as an initial intervention to contain mania symptoms quickly (and with less risk than firstgeneration antipsychotics for acute adverse motor effects from so-called rapid neuroleptization).
Because atypical antipsychotics often rapidly subdue mania, psychosis, and agitation, regardless of the underlying process, many practitioners might feel more comfortable initiating them than a mood stabilizer when the diagnosis is ambiguous or provisional, although their longer-term efficacy and safety, relative to traditional mood stabilizers, remains contested. Considerations for choosing from among feasible anti-manic pharmacotherapies are summarized in Table 1.
Normalize the sleep-wake cycle. Chronobiological and circadian variables, such as irregular sleep patterns, are thought to contribute to the pathophysiology of affective switch in BD. Behavioral and pharmacotherapeutic efforts to impose a normal sleep-wake schedule are considered fundamental to stabilizing acute mania.
Facilitate next steps after acute stabilization. For inpatients, this might involve step-down to a partial hospitalization or intensive outpatient program, alongside taking steps to ensure continued treatment adherence and minimize relapse.
What Medical and Neurologic Workup is Appropriate?
Not every first lifetime presentation of mania requires extensive medical and neurologic workup, particularly among patients who have a history of depression and those whose presentation neatly fits the demographic and clinical profile of newly emergent BD. Basic assessment should determine whether any new medication has been started that could plausibly contribute to abnormal mental status (Table 2).
Nevertheless, evaluation of almost all first presentations of mania should include:
- Urine toxicology screen
- Complete blood count
- Comprehensive metabolic panel
- Thyroid-stimulating hormone assay
- Serum vitamin B12 level assay
- Serum folic acid level assay
- Rapid plasma reagin test.
Clinical features that usually lead a clinician to pursue a more detailed medical and neurologic evaluation of first-episode mania include:
- Onset age > 40
- Absence of a family history of mood disorder
- Symptoms arising during a major medical illness
- Multiple medications
- Suspicion of a degenerative or hereditary neurologic disorder
- Altered state of consciousness
- Signs of cortical or diffuse subcortical dysfunction (eg, cognitive deficits, motor deficits, tremor)
- Abnormal vital signs.
Depending on the presentation, additional testing might include:
- Tests of HIV antibody, immune autoantibodies, and Lyme disease antibody
- Heavy metal screening (when suggested by environmental exposure)
- Lumbar puncture (eg, in a setting of manic delirium or suspected central nervous system infection or paraneoplastic syndrome)
- Neuroimaging (note: MRI provides better visualization than CT of white matter pathology and small vessel cerebrovascular disease) electroencephalography.
Making An Overarching Diagnosis: Is Mania Always Bipolar Disorder?
Mania is considered a manifestation of BD when symptoms cannot be attributed to another psychiatric condition, another underlying medical or neurologic condition, or a toxic-metabolic state (Tables 3 and 4). Classification of mania that occurs soon after antidepressant exposure in patients without a known history of BD continues to be the subject of debate, varying in its conceptualization across editions of DSM.
The National Institute of Mental Health (NIMH) Systematic Treatment Enhancement Program for Bipolar Disorder, or STEP-BD, observed a fairly low (approximately 10%) incidence of switch from depression to mania when an antidepressant is added to a mood stabilizer; the study authors concluded that much of what is presumed to be antidepressant-induced mania might simply be the natural course of illness.10
Notably, several reports suggest that antidepressants might pose a greater risk of mood destabilization in people with BD I than with either BD II or other suspected variants on the bipolar spectrum.
DSM-5 advises that a diagnosis of substance-induced mood disorder appropriately describes symptoms that spontaneously dissipate once an antidepressant has been discontinued, whereas a diagnosis of BD can be made when manic or hypomanic symptoms persist at a syndromal level after an antidepressant has been stopped and its physiological effects are no longer present. With respect to time course, the International Society of Bipolar Disorders proposes that, beyond 12 to 16 weeks after an antidepressant has been started or the dosage has been increased, it is unlikely that new-onset mania/hypomania can reasonably be attributed to “triggering” by an antidepressant (although antidepressants should be stopped when symptoms of mania emerge).11 Several clinical features have been linked in the literature with an increased susceptibility to BD after an initial depressive episode, including:
- Early (pre-adolescent) age at onset of first mood disorder episode6
- Family history of BD, highly recurrent depression, or psychosis12,13
- Psychosis when depressed.7,14
A number of other characteristics of depressive illness—including seasonal depression, atypical depressive features, suicidality, irritability, anxiety or substance use comorbidity, postpartum mood episodes, and brief recurrent depressive episodes—have been described in the literature as potential correlates of a bipolar diathesis; none have proved to be robust or pathognomonic of a BD diagnosis, as opposed to a unipolar diagnosis.
Data from the NIMH Collaborative Depression Study suggest that recurrent mania/hypomania after an antidepressant-associated polarity switch is greater when a family history of BD is present; other clinical variables might hold less predictive value.15 In addition, although some practitioners consider a history of nonresponse to trials of multiple antidepressants suggestive of an underlying bipolar process, polarity is only one of many variables that must be considered in the differential diagnosis of antidepressantresistant depression. Likewise, molecular genetic studies do not support a link between antidepressant nonresponse and the likelihood of a diagnosis of BD.16
Indefinite Pharmacotherapy for Bipolar Disorder
An important but nagging issue when diagnosing BD after a first manic (or hypomanic) episode is the implied need for indefinite pharmacotherapy to sustain remission and prevent relapse and recurrence.
The likelihood of subsequent depression or mania/hypomania remains high after an index manic/hypomanic episode, particularly for 6 to 8 months after recovery.8,17 Natural history data suggest that, during the year that follows a first lifetime mania, approximately 40% of patients experience a second manic episode.8 A second lifetime mania might be especially likely in patients whose index episode involved mood-congruent psychosis, low premorbid work functioning, and an initial manic episode, as opposed to a mixed episode or early age at onset.8,17
In the absence of randomized, placebo-controlled studies of maintenance pharmacotherapy after a first lifetime manic episode, clinical judgment often drives decisions about the duration of continuing pharmacotherapy after initial symptoms resolve. The Texas Medication Algorithm Project for BD advises that:
General practice at this time is lifetime medication following 2 manic episodes, or 1 episode if it was a severe episode and/or significant family history of bipolar or major depressive disorder is present. For a first episode of bipolar mania with no family history of bipolar or major depressive disorders, medication tapering and discontinuation may be considered after the continuation period is completed (usually 6 months in remission), depending on the severity of the first episode, surrounding factors, and prodromal history.18
Similarly, in the most recent (2004) Expert Consensus Guideline Series for the Treatment of Bipolar Disorder, 84% of practitioner−respondents favored indefinite mood stabilizer therapy after a second lifetime manic episode.19 No recommendation was made about the duration of maintenance pharmacotherapy after a first lifetime manic/hypomanic episode.
Avoid or Reintroduce an Antidepressant if Depression Recurs After a First Mania?
Controversies surrounding antidepressant use in BD are extensive; detailed discussion is beyond the scope of this review (Goldberg and Ghaemi provided a broader discussion of risks and benefits of antidepressants in BD).20 Although the main clinical concern regarding antidepressant use was, at one time, the potential to induce mania or accelerate the frequency of recurrent episodes, more recent, empirical studies suggest that the greater risk of using antidepressants for BD is lack of efficacy.10,21
If a careful longitudinal history and clinical evaluation reveal that an initial manic episode heralds the onset of BD, decisions about whether to avoid an antidepressant (as opposed to using other, more evidence-based interventions for bipolar depression) depend on a number of variables, including establishing whether the index episode was manic or hypomanic; ruling out current subthreshold mixed features; and clarifying how recently mania developed. Decisions about future antidepressant use (or avoidance) might be less clear if an index manic/hypomanic episode was brief and self-limited once the antidepressant was stopped.
Although some experts eschew antidepressant monotherapy after such occurrences, there is no body of literature to inform decisions about the safety or efficacy of undertaking a future antidepressant trial in such patients. That said, reasonable judgment probably includes several considerations:
- Re-exposure to the same antidepressant that was associated with an induction of mania is likely riskier than choosing a different antidepressant; in general, purely serotonergic antidepressants or bupropion are considered to pose less risk of mood destabilization than is seen with an SNRI or tricyclic antidepressant.
- After a manic episode, a subsequent antidepressant trial generally shouldn’t be attempted without concurrent anti-manic medication.
- Introducing any antidepressant is probably ill-advised in the recent (~2 months) aftermath of acute manic/hypomanic symptoms.22
- Patients and their significant other should be apprised of the risk of emerging symptoms of mania or hypomania, or mixed features, and should be familiar with key target symptoms to watch for. Prospective mood charting can be helpful.
- Patients should be monitored closely both for an exacerbation of depression and recurrence of mania/hypomania symptoms.
- Any antidepressant should be discontinued promptly at the first sign of psychomotor acceleration or the emergence of mixed features, as defined by DSM-5
Psychoeducation and Forecasting
Functional recovery from a manic episode can lag behind symptomatic recovery. Subsyndromal symptoms often persist after a full episode subsides.
Mania often is followed by a depressive episode, and questions inevitably arise about how to prevent and treat these episodes. Because the median duration of a manic episode is approximately 13 weeks, it is crucial for patients and their immediate family to recognize that recovery might be gradual, and that it will likely take time before she (he) can resume full-time responsibilities at work or school or in the home.23
Today, a patient who is hospitalized for severe acute mania (as Ms. J was, in the case vignette) seldom remains an inpatient long enough to achieve remission of symptoms; sometimes, she (he) might continue to manifest significant symptoms, even though decisions about the “medical necessity” of ongoing inpatient care tend to be governed mainly by issues of safety and imminent danger. (See this article at CurrentPsychiatry.com for a Table of considerations when making the transition from the acute phase to the continuation phase of treatment.20,24,25)
To minimize risk of relapse, psycho-education should include discussion of:
- Psychiatrically deleterious effects of alcohol and illicit drug use
- Suicide risk, including what to do in an emergency
- Protecting a regular sleep schedule and avoiding sleep deprivation
- The potential for poor medication adherence and management of side effects
- The role of adjunctive psychotherapy and effective stress management
- Familiarity with symptoms that serve as warning signs, and how to monitor their onset.
Click here to read the digital edition.
1. Goldberg JF, Garno JL, Callahan AM, et al. Overdiagnosis of bipolar disorder among substance use disorder in patients with mood instability. J Clin Psychiatry. 2008;69(11): 1751-1757.
2. Rosa AR, Cruz B, Franco C, et al. Why do clinicians maintain antidepressants in some patients with acute mania? Hints from the European Mania in Bipolar Longitudinal Evaluation of Medication (EMBLEM), a large naturalistic study. J Clin Psychiatry. 2010;71(8):1000-1006.
3. Goldberg JF, Perlis RH, Ghaemi SN, et al. Adjunctive antidepressant use and symptomatic recovery among bipolar depressed patients with concomitant manic symptoms: findings from the STEP-BD. Am J Psychiatry. 2007;164(9):1348-1355.
4. Bowers MB Jr, McKay BG, Mazure CM. Discontinuation of antidepressants in newly admitted psychotic patients. J Neuropsychiatr Clin Neurosci. 2003;15(2):227-230.
5. Perlis RH, Welge JA, Vornik LA, et al. Atypical antipsychotics in the treatment of mania: a meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry. 2006;67(4): 509-516.
6. Geller B, Zimmerman B, Williams M, et al. Bipolar disorder at prospective followup of adults who had prepubertal major depressive disorder. Am J Psychiatry. 2001;158(1): 125-127.
7. Goldberg JF, Harrow M, Whiteside JE. Risk for bipolar illness in patients initially hospitalized for unipolar depression. Am J Psychiatry. 2001;158(8):1265-1270.
8. Yatham LN, Kauer-Sant’Anna M, Bond DJ, et al. Course and outcome after the first manic episode in patients with bipolar disorder: prospective 12-month data from the Systematic Treatment Optimization Project for Early Mania project. Can J Psychiatry. 2009;54(2):105-112.
9. Chaudron LH, Pies RW. The relationship between postpartum psychosis and bipolar
disorder: a review. J Clin Psychiatry. 2003;64(11):1284-1292.
10. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
11. Tohen M, Frank E, Bowden CL, et al. The International Society for Bipolar Disorders (ISBD) Task Force report on the nomenclature of course and outcome in bipolar disorders. Bipolar Disord. 2009;11(15):453-473.
12. Schulze TG, Hedeker D, Zandi P, et al. What is familial about familial bipolar disorder? Resemblance among relatives across a broad spectrum of phenotypic characteristics. Arch Gen Psychiatry. 2006;63(12):1368-1376.
13. Song J, Bergen SE, Kuja-Halkola R, et al. Bipolar disorder and its relation to major psychiatric disorders: a family-based study in the Swedish population. Bipolar Disord. 2015;7(2):184-193.
14. Goes FS, Sadler B, Toolan J, et al. Psychotic features in bipolar and unipolar depression. Bipolar Disord. 2007;9(8):901-906.
15. Fiedorowicz JG, Endicott J, Solomon DA, et al. Course of illness following prospectively observed mania or hypomania in individuals presenting with unipolar depression. Bipolar Disord. 2007;14(6):664-671.
16. Tansey KE, Guipponi M, Domenici E, et al. Genetic susceptibility for bipolar disorder and response to antidepressants in major depressive disorder. Am J Med Genetics B Neuropsychiatr Genet. 2014;165B(1):77-83.
17. Tohen M, Zarate CA Jr, Hennen J, et al. The McLean-Harvard First-Episode Mania Study: prediction of recovery and first recurrence. Am J Psychiatry. 2003;160(12):2099-2107.
18. Suppes T, Dennehy EB, Swann AC, et al. Report of the Texas Consensus Conference Panel on medication treatment of bipolar disorder 2000. J Clin Psychiatry. 2002; 63(4):288-299.
19. Keck PE Jr, Perlis RH, Otto MW, et al. The Expert Consensus Guideline Series: treatment of bipolar disorder 2004. Postgrad Med Special Report. 2004:1-120.
20. Goldberg JF, Ghaemi SN. Benefits and limitations of antidepressants and traditional mood stabilizers for treatment of bipolar depression. Bipolar Disord. 2005; 7(suppl 5):3-12.
21. Sidor MM, MacQueen GM. Antidepressants for the acute treatment of bipolar
depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2): 156-167.
22. MacQueen GM, Trevor Young L, Marriott M, et al. Previous mood state predicts response and switch rates in patients with bipolar depression. Acta Psychiatr Scand. 2002;105(6):414-418.
23. Solomon DA, Leon AC, Coryell WH, et al. Longitudinal course of bipolar I disorder: duration of mood episodes. Arch Gen Psychiatry. 2010;67(4):339-347.
24. Tohen M, Chengappa KN, Suppes T, et al. Relapse prevention in bipolar I disorder: 18-month comparison of olanzapine plus mood stabiliser v. mood stabiliser alone. Br J Psychiatry. 2004;184:337-345.
25. Suppes T, Vieta E, Liu S, et al. Maintenance treatment for patients with bipolar I disorder: results from a North American study of quetiapine in combination with lithium or divalproex (trial 127). Am J Psychiatry. 2009;166(4): 476-488.
Note: Page numbers differ between the print issue and digital edition.
1. Goldberg JF, Garno JL, Callahan AM, et al. Overdiagnosis of bipolar disorder among substance use disorder in patients with mood instability. J Clin Psychiatry. 2008;69(11): 1751-1757.
2. Rosa AR, Cruz B, Franco C, et al. Why do clinicians maintain antidepressants in some patients with acute mania? Hints from the European Mania in Bipolar Longitudinal Evaluation of Medication (EMBLEM), a large naturalistic study. J Clin Psychiatry. 2010;71(8):1000-1006.
3. Goldberg JF, Perlis RH, Ghaemi SN, et al. Adjunctive antidepressant use and symptomatic recovery among bipolar depressed patients with concomitant manic symptoms: findings from the STEP-BD. Am J Psychiatry. 2007;164(9):1348-1355.
4. Bowers MB Jr, McKay BG, Mazure CM. Discontinuation of antidepressants in newly admitted psychotic patients. J Neuropsychiatr Clin Neurosci. 2003;15(2):227-230.
5. Perlis RH, Welge JA, Vornik LA, et al. Atypical antipsychotics in the treatment of mania: a meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry. 2006;67(4): 509-516.
6. Geller B, Zimmerman B, Williams M, et al. Bipolar disorder at prospective followup of adults who had prepubertal major depressive disorder. Am J Psychiatry. 2001;158(1): 125-127.
7. Goldberg JF, Harrow M, Whiteside JE. Risk for bipolar illness in patients initially hospitalized for unipolar depression. Am J Psychiatry. 2001;158(8):1265-1270.
8. Yatham LN, Kauer-Sant’Anna M, Bond DJ, et al. Course and outcome after the first manic episode in patients with bipolar disorder: prospective 12-month data from the Systematic Treatment Optimization Project for Early Mania project. Can J Psychiatry. 2009;54(2):105-112.
9. Chaudron LH, Pies RW. The relationship between postpartum psychosis and bipolar
disorder: a review. J Clin Psychiatry. 2003;64(11):1284-1292.
10. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
11. Tohen M, Frank E, Bowden CL, et al. The International Society for Bipolar Disorders (ISBD) Task Force report on the nomenclature of course and outcome in bipolar disorders. Bipolar Disord. 2009;11(15):453-473.
12. Schulze TG, Hedeker D, Zandi P, et al. What is familial about familial bipolar disorder? Resemblance among relatives across a broad spectrum of phenotypic characteristics. Arch Gen Psychiatry. 2006;63(12):1368-1376.
13. Song J, Bergen SE, Kuja-Halkola R, et al. Bipolar disorder and its relation to major psychiatric disorders: a family-based study in the Swedish population. Bipolar Disord. 2015;7(2):184-193.
14. Goes FS, Sadler B, Toolan J, et al. Psychotic features in bipolar and unipolar depression. Bipolar Disord. 2007;9(8):901-906.
15. Fiedorowicz JG, Endicott J, Solomon DA, et al. Course of illness following prospectively observed mania or hypomania in individuals presenting with unipolar depression. Bipolar Disord. 2007;14(6):664-671.
16. Tansey KE, Guipponi M, Domenici E, et al. Genetic susceptibility for bipolar disorder and response to antidepressants in major depressive disorder. Am J Med Genetics B Neuropsychiatr Genet. 2014;165B(1):77-83.
17. Tohen M, Zarate CA Jr, Hennen J, et al. The McLean-Harvard First-Episode Mania Study: prediction of recovery and first recurrence. Am J Psychiatry. 2003;160(12):2099-2107.
18. Suppes T, Dennehy EB, Swann AC, et al. Report of the Texas Consensus Conference Panel on medication treatment of bipolar disorder 2000. J Clin Psychiatry. 2002; 63(4):288-299.
19. Keck PE Jr, Perlis RH, Otto MW, et al. The Expert Consensus Guideline Series: treatment of bipolar disorder 2004. Postgrad Med Special Report. 2004:1-120.
20. Goldberg JF, Ghaemi SN. Benefits and limitations of antidepressants and traditional mood stabilizers for treatment of bipolar depression. Bipolar Disord. 2005; 7(suppl 5):3-12.
21. Sidor MM, MacQueen GM. Antidepressants for the acute treatment of bipolar
depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2): 156-167.
22. MacQueen GM, Trevor Young L, Marriott M, et al. Previous mood state predicts response and switch rates in patients with bipolar depression. Acta Psychiatr Scand. 2002;105(6):414-418.
23. Solomon DA, Leon AC, Coryell WH, et al. Longitudinal course of bipolar I disorder: duration of mood episodes. Arch Gen Psychiatry. 2010;67(4):339-347.
24. Tohen M, Chengappa KN, Suppes T, et al. Relapse prevention in bipolar I disorder: 18-month comparison of olanzapine plus mood stabiliser v. mood stabiliser alone. Br J Psychiatry. 2004;184:337-345.
25. Suppes T, Vieta E, Liu S, et al. Maintenance treatment for patients with bipolar I disorder: results from a North American study of quetiapine in combination with lithium or divalproex (trial 127). Am J Psychiatry. 2009;166(4): 476-488.
Note: Page numbers differ between the print issue and digital edition.
2016 Update on abnormal uterine bleeding
How abnormal uterine bleeding (AUB) is managed has a significant impact on health care. In the United States, almost one-third of all gynecologic visits are related to AUB, with estimated annual direct costs of up to $1.55 billion and indirect costs as high as $36 billion.1 Not surprisingly, office-based procedures for AUB are being emphasized. While in the short term it is more cost efficient to perform surgery in the office rather than in the operating room, questions have arisen regarding the long-term efficacy and durability of in-office procedures. Insurers are undoubtedly raising these questions as well.
Notably, some ObGyns are early adopters of office-based surgery while others tend to adopt in-office procedures more slowly. As the literature for such procedures for AUB matures to provide more data on efficacy and acceptability, we will have a greater evidence base for understanding which procedures are more appropriate for the office. And while practice shifts sometimes occur due to cost-containment initiatives, some shifts are patient driven. Studies that address these driving variables, as well as efficacy considerations, are helpful. As we counsel women about procedures for AUB, the relative advantages and disadvantages of available treatment settings likely will become a greater part of that discussion so that they can make an informed decision.
In this Update, we discuss the results of 3 studies that examined various procedures and settings for AUB management:
- outpatient vs inpatient polypectomy
- hysteroscopic morcellation of polyps and myomas in an office vs ambulatory surgical center
- comparative costs of endometrial ablation and hysterectomy.
Outpatient vs inpatient polypectomy: Similar success rates in the short term
Cooper NA, Clark TJ, Middleton L, et al; OPT Trial Collaborative Group. Outpatient versus inpatient uterine polyp treatment for abnormal uterine bleeding: randomised controlled non-inferiority study. BMJ. 2015;350:h1398. doi:10.1136/bmj.h1398.
A collaborative group in the United Kingdom studied the common problem of endometrial polyps. Their objective was to evaluate whether outpatient polypectomy was as effective and well accepted as polypectomy performed in the operating room (OR).
Patients with a hysteroscopically diagnosed polyp were randomly assigned to hysteroscopic polyp removal in either a hysteroscopy clinic or an OR; polyp removal was performed using miniature mechanical or electrosurgical instruments. The primary outcome was successful treatment, determined by the participants’ assessment of their bleeding at 6 months.
Overall, 73% of women (166 of 228) in the clinic group and 80% (168 of 211) in the OR group reported a successful response to surgery at 6 months, with treatment effects being maintained at 12 and 24 months. A “see and treat” approach—that is, treatment carried out at the same time as diagnosis—was possible in 72% of women (174 of 242).
Partial or failed polyp removal occurred in 46 of 242 women (19%) in the clinic group, mostly because of pain issues, and in 18 of 233 women (7%) in the OR group (relative risk, 2.5; 95% confidence interval, 1.5−4.1; P<.001). Four uterine perforations (2% of patients) occurred in the OR group.
Mean pain scores were higher in the clinic group, and treatment was unacceptable for 2% of the women in each group.
The results of this trial show that clinic polypectomy has some limitations, but the outpatient procedure was deemed noninferior to polypectomy performed in the OR for the successful alleviation of uterine bleeding associated with uterine polyps.
What this EVIDENCE means for practice
Office-based polypectomy allowed a “see and treat” model in 72% of cases. Office polypectomy had similar successful therapeutic responses as inpatient polypectomy; however, over a 2-year follow-up period, women treated in the office were twice as likely to undergo at least 1 further polyp removal and were 1.6 times more likely to have further gynecologic surgery.
In-office hysteroscopic morcellation of polyps and myomas improves health-related quality of life
Rubino RJ, Lukes AS. Twelve-month outcomes for patients undergoing hysteroscopic morcellation of uterine polyps and myomas in an office or ambulatory surgical center. J Minim Invasive Gynecol. 2015;22(2):285–290.
Is it feasible to morcellate fibroids, as well as polyps, in the clinic? Rubino and colleagues investigated this question in a randomized, prospective clinical trial. They examined the efficacy of hysteroscopic removal of polyps and myomas on health-related quality of life and symptom severity at 1-year postprocedure. Women aged 18 to 55 years, with hysteroscopic and saline-infusion sonogram–assessed polyps and/or type 0 or I myomas (1.5−3.0 cm), were enrolled from 9 US clinical sites. Some patient populations were excluded, such as women with a long narcotic abuse history, current intrauterine device (IUD), type II submucous myomas, and type I fundal myomas.
A total of 118 pathologies were removed in 74 patients. Forty-two women were treated in the office setting; 32 were treated in the OR setting. Among the 118 pathologies removed, 53 were removed in the office (28 myomas and 25 polyps), and 55 were removed in the OR (14 myomas and 41 polyps).
The percentage of patients who reported being satisfied or highly satisfied was higher in the OR cohort (96.5%) compared with the office cohort (83.3%), although this difference was not statistically significant (P = .06). The percentage of patients who had 100% of their pathology removed was significantly higher in those with polyps compared with patients with myomas (96.0% vs 63.6%, respectively; P<.01).
These findings indicate that there were several cases in which the majority of a myoma was removed but a small residual portion remained. This disparity was especially pronounced in the office setting, where 96% of polyps were completely removed, compared with 52% of fibroids. There was no statistically significant difference in health-related quality of life between patients with complete removal and those with residual pathology, and there was no difference in satisfaction rates between patients who were treated in the office and those treated in the OR.
What this EVIDENCE means for practice
In general, office-based hysteroscopic myomectomy and polypectomy using morcellation for small- to medium-size lesions was associated with low rates of adverse events, high physician acceptance, and significant durable health-related quality-of-life improvements for up to 12 months post‑ procedure. Partial removal of myomas did not seem to be a significant factor in patients’ perceived outcomes.
Endometrial ablation for AUB costs less, has fewer complications at 1 year than hysterectomy
Miller JD, Lenhart GM, Bonafede MM, Lukes AS, Laughlin-Tommaso SK. Cost-effectiveness of global endometrial ablation vs hysterectomy for treatment of abnormal uterine bleeding: US commercial and Medicaid payer perspectives. Popul Health Manag. 2015;18(5):373–382.
Endometrial ablation often is performed in the office for AUB management. Miller and colleagues suggested that cost-effectiveness modeling studies of endometrial ablation for AUB treatment from a US perspective are lacking. They therefore designed a study to model the cost-effectiveness of endometrial ablation versus hysterectomy for treatment of AUB from both commercial and Medicaid payer perspectives.
They developed a decision-tree, state-transition (semi-Markov) model to simulate 2 hypothetical patient cohorts of women with AUB: one treated with endometrial ablation and the other with hysterectomy. Twenty-one health states were included in the model of intervention with endometrial ablation or hysterectomy; these comprised postablation reintervention with secondary ablation, tranexamic acid, or a levonorgestrel-containing IUD due to AUB, use of adjunctive pharmacotherapy following ablation, and a small probability of death from hysterectomy or actuarial death from all other causes.
The 1-year direct costs of endometrial ablation were $7,352 and $6,306 in the commercial payer and Medicaid payer perspectives, respectively; these were about half the costs of hysterectomy. The cost differential between the 2 treatments narrowed over time but, even at 5 years, endometrial ablation costs were still one-third less than hysterectomy costs.
In the first year, 35.6% of patients who had a hysterectomy and only 17.1% of patients undergoing ablation had complications. Short-term results were similar under the Medicaid perspective. By 5 years intervention/reintervention, however, complications of endometrial ablation were higher than those for hysterectomy by about 1.6%.
Over a 5-year time frame, direct costs of endometrial ablation were lower than those of hysterectomy from both the commercial payer and Medicaid perspectives. In the commercial payer analysis, the indirect costs of endometrial ablation were also lower than for hysterectomy, with 38.5 workdays lost for endometrial ablation compared with 55.3 days lost for hysterectomy, resulting in indirect costs of $8,976 versus $13,087.
What this EVIDENCE means for practice
Costs and cost-effectiveness of endometrial ablation from a US perspective are understudied. This model estimates a financial advantage for endometrial ablation over hysterectomy from both the commercial payer and Medicaid payer perspectives. Over a variety of time frames, endometrial ablation may save costs while reducing treatment complications and lost workdays. From the patient perspective, this model suggests better quality of life in the short term after endometrial ablation. It will be interesting to see whether longer term impacts show this model to be predictive.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Reference
- Liu Z, Doan QV, Blumenthal P, Dubois RW. A systematic review evaluating health-related quality of life, work impairment, and healthcare costs and utilization in abnormal uterine bleeding. Value Health. 2007;10(3):183–194.
How abnormal uterine bleeding (AUB) is managed has a significant impact on health care. In the United States, almost one-third of all gynecologic visits are related to AUB, with estimated annual direct costs of up to $1.55 billion and indirect costs as high as $36 billion.1 Not surprisingly, office-based procedures for AUB are being emphasized. While in the short term it is more cost efficient to perform surgery in the office rather than in the operating room, questions have arisen regarding the long-term efficacy and durability of in-office procedures. Insurers are undoubtedly raising these questions as well.
Notably, some ObGyns are early adopters of office-based surgery while others tend to adopt in-office procedures more slowly. As the literature for such procedures for AUB matures to provide more data on efficacy and acceptability, we will have a greater evidence base for understanding which procedures are more appropriate for the office. And while practice shifts sometimes occur due to cost-containment initiatives, some shifts are patient driven. Studies that address these driving variables, as well as efficacy considerations, are helpful. As we counsel women about procedures for AUB, the relative advantages and disadvantages of available treatment settings likely will become a greater part of that discussion so that they can make an informed decision.
In this Update, we discuss the results of 3 studies that examined various procedures and settings for AUB management:
- outpatient vs inpatient polypectomy
- hysteroscopic morcellation of polyps and myomas in an office vs ambulatory surgical center
- comparative costs of endometrial ablation and hysterectomy.
Outpatient vs inpatient polypectomy: Similar success rates in the short term
Cooper NA, Clark TJ, Middleton L, et al; OPT Trial Collaborative Group. Outpatient versus inpatient uterine polyp treatment for abnormal uterine bleeding: randomised controlled non-inferiority study. BMJ. 2015;350:h1398. doi:10.1136/bmj.h1398.
A collaborative group in the United Kingdom studied the common problem of endometrial polyps. Their objective was to evaluate whether outpatient polypectomy was as effective and well accepted as polypectomy performed in the operating room (OR).
Patients with a hysteroscopically diagnosed polyp were randomly assigned to hysteroscopic polyp removal in either a hysteroscopy clinic or an OR; polyp removal was performed using miniature mechanical or electrosurgical instruments. The primary outcome was successful treatment, determined by the participants’ assessment of their bleeding at 6 months.
Overall, 73% of women (166 of 228) in the clinic group and 80% (168 of 211) in the OR group reported a successful response to surgery at 6 months, with treatment effects being maintained at 12 and 24 months. A “see and treat” approach—that is, treatment carried out at the same time as diagnosis—was possible in 72% of women (174 of 242).
Partial or failed polyp removal occurred in 46 of 242 women (19%) in the clinic group, mostly because of pain issues, and in 18 of 233 women (7%) in the OR group (relative risk, 2.5; 95% confidence interval, 1.5−4.1; P<.001). Four uterine perforations (2% of patients) occurred in the OR group.
Mean pain scores were higher in the clinic group, and treatment was unacceptable for 2% of the women in each group.
The results of this trial show that clinic polypectomy has some limitations, but the outpatient procedure was deemed noninferior to polypectomy performed in the OR for the successful alleviation of uterine bleeding associated with uterine polyps.
What this EVIDENCE means for practice
Office-based polypectomy allowed a “see and treat” model in 72% of cases. Office polypectomy had similar successful therapeutic responses as inpatient polypectomy; however, over a 2-year follow-up period, women treated in the office were twice as likely to undergo at least 1 further polyp removal and were 1.6 times more likely to have further gynecologic surgery.
In-office hysteroscopic morcellation of polyps and myomas improves health-related quality of life
Rubino RJ, Lukes AS. Twelve-month outcomes for patients undergoing hysteroscopic morcellation of uterine polyps and myomas in an office or ambulatory surgical center. J Minim Invasive Gynecol. 2015;22(2):285–290.
Is it feasible to morcellate fibroids, as well as polyps, in the clinic? Rubino and colleagues investigated this question in a randomized, prospective clinical trial. They examined the efficacy of hysteroscopic removal of polyps and myomas on health-related quality of life and symptom severity at 1-year postprocedure. Women aged 18 to 55 years, with hysteroscopic and saline-infusion sonogram–assessed polyps and/or type 0 or I myomas (1.5−3.0 cm), were enrolled from 9 US clinical sites. Some patient populations were excluded, such as women with a long narcotic abuse history, current intrauterine device (IUD), type II submucous myomas, and type I fundal myomas.
A total of 118 pathologies were removed in 74 patients. Forty-two women were treated in the office setting; 32 were treated in the OR setting. Among the 118 pathologies removed, 53 were removed in the office (28 myomas and 25 polyps), and 55 were removed in the OR (14 myomas and 41 polyps).
The percentage of patients who reported being satisfied or highly satisfied was higher in the OR cohort (96.5%) compared with the office cohort (83.3%), although this difference was not statistically significant (P = .06). The percentage of patients who had 100% of their pathology removed was significantly higher in those with polyps compared with patients with myomas (96.0% vs 63.6%, respectively; P<.01).
These findings indicate that there were several cases in which the majority of a myoma was removed but a small residual portion remained. This disparity was especially pronounced in the office setting, where 96% of polyps were completely removed, compared with 52% of fibroids. There was no statistically significant difference in health-related quality of life between patients with complete removal and those with residual pathology, and there was no difference in satisfaction rates between patients who were treated in the office and those treated in the OR.
What this EVIDENCE means for practice
In general, office-based hysteroscopic myomectomy and polypectomy using morcellation for small- to medium-size lesions was associated with low rates of adverse events, high physician acceptance, and significant durable health-related quality-of-life improvements for up to 12 months post‑ procedure. Partial removal of myomas did not seem to be a significant factor in patients’ perceived outcomes.
Endometrial ablation for AUB costs less, has fewer complications at 1 year than hysterectomy
Miller JD, Lenhart GM, Bonafede MM, Lukes AS, Laughlin-Tommaso SK. Cost-effectiveness of global endometrial ablation vs hysterectomy for treatment of abnormal uterine bleeding: US commercial and Medicaid payer perspectives. Popul Health Manag. 2015;18(5):373–382.
Endometrial ablation often is performed in the office for AUB management. Miller and colleagues suggested that cost-effectiveness modeling studies of endometrial ablation for AUB treatment from a US perspective are lacking. They therefore designed a study to model the cost-effectiveness of endometrial ablation versus hysterectomy for treatment of AUB from both commercial and Medicaid payer perspectives.
They developed a decision-tree, state-transition (semi-Markov) model to simulate 2 hypothetical patient cohorts of women with AUB: one treated with endometrial ablation and the other with hysterectomy. Twenty-one health states were included in the model of intervention with endometrial ablation or hysterectomy; these comprised postablation reintervention with secondary ablation, tranexamic acid, or a levonorgestrel-containing IUD due to AUB, use of adjunctive pharmacotherapy following ablation, and a small probability of death from hysterectomy or actuarial death from all other causes.
The 1-year direct costs of endometrial ablation were $7,352 and $6,306 in the commercial payer and Medicaid payer perspectives, respectively; these were about half the costs of hysterectomy. The cost differential between the 2 treatments narrowed over time but, even at 5 years, endometrial ablation costs were still one-third less than hysterectomy costs.
In the first year, 35.6% of patients who had a hysterectomy and only 17.1% of patients undergoing ablation had complications. Short-term results were similar under the Medicaid perspective. By 5 years intervention/reintervention, however, complications of endometrial ablation were higher than those for hysterectomy by about 1.6%.
Over a 5-year time frame, direct costs of endometrial ablation were lower than those of hysterectomy from both the commercial payer and Medicaid perspectives. In the commercial payer analysis, the indirect costs of endometrial ablation were also lower than for hysterectomy, with 38.5 workdays lost for endometrial ablation compared with 55.3 days lost for hysterectomy, resulting in indirect costs of $8,976 versus $13,087.
What this EVIDENCE means for practice
Costs and cost-effectiveness of endometrial ablation from a US perspective are understudied. This model estimates a financial advantage for endometrial ablation over hysterectomy from both the commercial payer and Medicaid payer perspectives. Over a variety of time frames, endometrial ablation may save costs while reducing treatment complications and lost workdays. From the patient perspective, this model suggests better quality of life in the short term after endometrial ablation. It will be interesting to see whether longer term impacts show this model to be predictive.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
How abnormal uterine bleeding (AUB) is managed has a significant impact on health care. In the United States, almost one-third of all gynecologic visits are related to AUB, with estimated annual direct costs of up to $1.55 billion and indirect costs as high as $36 billion.1 Not surprisingly, office-based procedures for AUB are being emphasized. While in the short term it is more cost efficient to perform surgery in the office rather than in the operating room, questions have arisen regarding the long-term efficacy and durability of in-office procedures. Insurers are undoubtedly raising these questions as well.
Notably, some ObGyns are early adopters of office-based surgery while others tend to adopt in-office procedures more slowly. As the literature for such procedures for AUB matures to provide more data on efficacy and acceptability, we will have a greater evidence base for understanding which procedures are more appropriate for the office. And while practice shifts sometimes occur due to cost-containment initiatives, some shifts are patient driven. Studies that address these driving variables, as well as efficacy considerations, are helpful. As we counsel women about procedures for AUB, the relative advantages and disadvantages of available treatment settings likely will become a greater part of that discussion so that they can make an informed decision.
In this Update, we discuss the results of 3 studies that examined various procedures and settings for AUB management:
- outpatient vs inpatient polypectomy
- hysteroscopic morcellation of polyps and myomas in an office vs ambulatory surgical center
- comparative costs of endometrial ablation and hysterectomy.
Outpatient vs inpatient polypectomy: Similar success rates in the short term
Cooper NA, Clark TJ, Middleton L, et al; OPT Trial Collaborative Group. Outpatient versus inpatient uterine polyp treatment for abnormal uterine bleeding: randomised controlled non-inferiority study. BMJ. 2015;350:h1398. doi:10.1136/bmj.h1398.
A collaborative group in the United Kingdom studied the common problem of endometrial polyps. Their objective was to evaluate whether outpatient polypectomy was as effective and well accepted as polypectomy performed in the operating room (OR).
Patients with a hysteroscopically diagnosed polyp were randomly assigned to hysteroscopic polyp removal in either a hysteroscopy clinic or an OR; polyp removal was performed using miniature mechanical or electrosurgical instruments. The primary outcome was successful treatment, determined by the participants’ assessment of their bleeding at 6 months.
Overall, 73% of women (166 of 228) in the clinic group and 80% (168 of 211) in the OR group reported a successful response to surgery at 6 months, with treatment effects being maintained at 12 and 24 months. A “see and treat” approach—that is, treatment carried out at the same time as diagnosis—was possible in 72% of women (174 of 242).
Partial or failed polyp removal occurred in 46 of 242 women (19%) in the clinic group, mostly because of pain issues, and in 18 of 233 women (7%) in the OR group (relative risk, 2.5; 95% confidence interval, 1.5−4.1; P<.001). Four uterine perforations (2% of patients) occurred in the OR group.
Mean pain scores were higher in the clinic group, and treatment was unacceptable for 2% of the women in each group.
The results of this trial show that clinic polypectomy has some limitations, but the outpatient procedure was deemed noninferior to polypectomy performed in the OR for the successful alleviation of uterine bleeding associated with uterine polyps.
What this EVIDENCE means for practice
Office-based polypectomy allowed a “see and treat” model in 72% of cases. Office polypectomy had similar successful therapeutic responses as inpatient polypectomy; however, over a 2-year follow-up period, women treated in the office were twice as likely to undergo at least 1 further polyp removal and were 1.6 times more likely to have further gynecologic surgery.
In-office hysteroscopic morcellation of polyps and myomas improves health-related quality of life
Rubino RJ, Lukes AS. Twelve-month outcomes for patients undergoing hysteroscopic morcellation of uterine polyps and myomas in an office or ambulatory surgical center. J Minim Invasive Gynecol. 2015;22(2):285–290.
Is it feasible to morcellate fibroids, as well as polyps, in the clinic? Rubino and colleagues investigated this question in a randomized, prospective clinical trial. They examined the efficacy of hysteroscopic removal of polyps and myomas on health-related quality of life and symptom severity at 1-year postprocedure. Women aged 18 to 55 years, with hysteroscopic and saline-infusion sonogram–assessed polyps and/or type 0 or I myomas (1.5−3.0 cm), were enrolled from 9 US clinical sites. Some patient populations were excluded, such as women with a long narcotic abuse history, current intrauterine device (IUD), type II submucous myomas, and type I fundal myomas.
A total of 118 pathologies were removed in 74 patients. Forty-two women were treated in the office setting; 32 were treated in the OR setting. Among the 118 pathologies removed, 53 were removed in the office (28 myomas and 25 polyps), and 55 were removed in the OR (14 myomas and 41 polyps).
The percentage of patients who reported being satisfied or highly satisfied was higher in the OR cohort (96.5%) compared with the office cohort (83.3%), although this difference was not statistically significant (P = .06). The percentage of patients who had 100% of their pathology removed was significantly higher in those with polyps compared with patients with myomas (96.0% vs 63.6%, respectively; P<.01).
These findings indicate that there were several cases in which the majority of a myoma was removed but a small residual portion remained. This disparity was especially pronounced in the office setting, where 96% of polyps were completely removed, compared with 52% of fibroids. There was no statistically significant difference in health-related quality of life between patients with complete removal and those with residual pathology, and there was no difference in satisfaction rates between patients who were treated in the office and those treated in the OR.
What this EVIDENCE means for practice
In general, office-based hysteroscopic myomectomy and polypectomy using morcellation for small- to medium-size lesions was associated with low rates of adverse events, high physician acceptance, and significant durable health-related quality-of-life improvements for up to 12 months post‑ procedure. Partial removal of myomas did not seem to be a significant factor in patients’ perceived outcomes.
Endometrial ablation for AUB costs less, has fewer complications at 1 year than hysterectomy
Miller JD, Lenhart GM, Bonafede MM, Lukes AS, Laughlin-Tommaso SK. Cost-effectiveness of global endometrial ablation vs hysterectomy for treatment of abnormal uterine bleeding: US commercial and Medicaid payer perspectives. Popul Health Manag. 2015;18(5):373–382.
Endometrial ablation often is performed in the office for AUB management. Miller and colleagues suggested that cost-effectiveness modeling studies of endometrial ablation for AUB treatment from a US perspective are lacking. They therefore designed a study to model the cost-effectiveness of endometrial ablation versus hysterectomy for treatment of AUB from both commercial and Medicaid payer perspectives.
They developed a decision-tree, state-transition (semi-Markov) model to simulate 2 hypothetical patient cohorts of women with AUB: one treated with endometrial ablation and the other with hysterectomy. Twenty-one health states were included in the model of intervention with endometrial ablation or hysterectomy; these comprised postablation reintervention with secondary ablation, tranexamic acid, or a levonorgestrel-containing IUD due to AUB, use of adjunctive pharmacotherapy following ablation, and a small probability of death from hysterectomy or actuarial death from all other causes.
The 1-year direct costs of endometrial ablation were $7,352 and $6,306 in the commercial payer and Medicaid payer perspectives, respectively; these were about half the costs of hysterectomy. The cost differential between the 2 treatments narrowed over time but, even at 5 years, endometrial ablation costs were still one-third less than hysterectomy costs.
In the first year, 35.6% of patients who had a hysterectomy and only 17.1% of patients undergoing ablation had complications. Short-term results were similar under the Medicaid perspective. By 5 years intervention/reintervention, however, complications of endometrial ablation were higher than those for hysterectomy by about 1.6%.
Over a 5-year time frame, direct costs of endometrial ablation were lower than those of hysterectomy from both the commercial payer and Medicaid perspectives. In the commercial payer analysis, the indirect costs of endometrial ablation were also lower than for hysterectomy, with 38.5 workdays lost for endometrial ablation compared with 55.3 days lost for hysterectomy, resulting in indirect costs of $8,976 versus $13,087.
What this EVIDENCE means for practice
Costs and cost-effectiveness of endometrial ablation from a US perspective are understudied. This model estimates a financial advantage for endometrial ablation over hysterectomy from both the commercial payer and Medicaid payer perspectives. Over a variety of time frames, endometrial ablation may save costs while reducing treatment complications and lost workdays. From the patient perspective, this model suggests better quality of life in the short term after endometrial ablation. It will be interesting to see whether longer term impacts show this model to be predictive.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Reference
- Liu Z, Doan QV, Blumenthal P, Dubois RW. A systematic review evaluating health-related quality of life, work impairment, and healthcare costs and utilization in abnormal uterine bleeding. Value Health. 2007;10(3):183–194.
Reference
- Liu Z, Doan QV, Blumenthal P, Dubois RW. A systematic review evaluating health-related quality of life, work impairment, and healthcare costs and utilization in abnormal uterine bleeding. Value Health. 2007;10(3):183–194.
IN THIS ARTICLE
- Polypectomy in the clinic vs OR
- In-office polyp, fibroid morcellation
- Cost-effectiveness of endometrial ablation
Transvaginal mesh for prolapse: Where are we in 2016?
TRANSVAGINAL MESH FOR POP: NEW AND
NOTEWORTHY RESOURCES
• Pelvic Floor Disorders Registry: Overview, registry resources, FAQs, more
• Vaginal mesh manufacturer closing due to lawsuit concerns, Wall Street Journal, 2/29/16
Approximately 300,000 surgeries for pelvic organ prolapse (POP) are performed annually in the United States. In 2006, the peak of synthetic mesh use for prolapse surgery, one-third of all prolapse operations involved some mesh use.1,2 The use of vaginal mesh has declined since the US Food and Drug Administration (FDA) issued warnings in 2008 and 2011.
Historically, the use of mesh for gynecologic surgery began in the 1970s, with abdominal POP repair.3 Transvaginal mesh use for POP surgeries became FDA-cleared in 2004. The first cleared mesh device was classified as class II (moderate risk).3 Subsequent mesh devices were given 510(k) clearance, which bypasses clinical trials and requires manufacturers only to show that their product is substantially equivalent to one already on the market.4 More than 40 companies began the manufacturing of mesh devices in the 10 years following the initial cleared device.3
Of course, much controversy has surrounded mesh use in recent years, with common adverse events reported, including severe pelvic pain, pain during intercourse, infection, bleeding, organ perforation, and problems from mesh eroding into surrounding tissues.3 The FDA very recently (in January 2016) reclassified this device from moderate risk to high risk (class III), after indicating in May 2014 that such action was necessary. (See “Timeline of FDA’s actions regarding surgical mesh for pelvic organ prolapse” on page 46.) This reclassification requires a premarket approval application to be filed for each device, with safety and efficacy demonstrated. There are approximately 5 companies currently manufacturing mesh for transvaginal POP repair.3
OBG Management recently sat down with Cheryl Iglesia, MD, director of the Section of Female Pelvic Medicine and Reconstructive Surgery at MedStar Washington Hospital Center and professor in the Departments of Obstetrics/Gynecology and Urology at Georgetown University School of Medicine in Washington, DC. Dr. Iglesia serves, from 2011 through 2017, as a member on the FDA Obstetrics and Gynecology Devices Panel, and she addressed lessons learned over the past decade on synthetic and biologic mesh at the Pelvic Anatomy and Gynecologic Surgery (PAGS) symposium in Las Vegas, Nevada, this past December.
In this Q&A article, she addresses the current state of transvaginal mesh use and how it relates to the innovation adaptation curve (otherwise known as the Hype Cycle), how new mesh types differ from older ones, and how the specialty can move into a future of POP surgery in which innovation and data will rule.
OBG Management: Where is transvaginal mesh use on the so-called “Hype Cycle,” or innovation adaptation curve?
Cheryl B. Iglesia, MD: The Hype Cycle was developed and branded by the Gartner company, an information technology advisory and research firm. This cycle refers to the graphical depictions of how a technology or application will evolve over time. After all, new technologies may make bold promises, and the hype may not translate to commercial viability. Each cycle drills down into the key phases of a technology’s life cycle: the trigger, peak of inflated expectations, trough of disillusionment, slope of enlightenment, and plateau of productivity.5
If we use the Hype Cycle to drill down the phases of transvaginal mesh’s life cycle, we begin in 2004 with the FDA clearance of the first vaginal mesh system (FIGURE).6 The height of its use (the “peak of inflated expectation”) was around 2006, when essentially one-third of all annual surgeries performed for prolapse repair used some type of mesh placed either abdominally or transvaginally.2
Subsequently, adverse events began being reported to the Manufacturer and User Facility Device Experience (MAUDE) database. In 2008, the FDA published its first notification of serious complications associated with transvaginal placement of surgical mesh, with more than 1,000 reports from 9 surgical mesh manufacturers.7 A second alert followed in 2011.8 By this time, we had reached our “trough of disillusionment.”
In 2016, we have reached the “plateau of productivity” on the innovation adaptation curve. During this phase on the Hype Cycle the criteria for assessing the technology’s viability are clearly defined. I say we are in this phase because now we have a way of completing more postmarket surveillance on mesh devices. We now can see what applying the technology is like in the real world, generalized across many different surgeons’ hands, and we have a way of performing comparative studies with native tissue.
OBG Management: How do the new types of mesh differ from those that have been removed from the market?
Dr. Iglesia: In January 2012, there were about 40 types of surgical mesh available from more than 30 manufacturers of transvaginal mesh. At that time, the FDA imposed 522 orders on these companies, requiring them to provide up to 3 years of postmarket data on the safety and effectiveness of their devices.9 Some companies ceased production, including Johnson and Johnson and CR Bard. Today, there are about a half-dozen mesh types on the market, and these are undergoing evaluation.
First-generation meshes were the size of a sheet of paper; now, meshes can fit on the palm of your hand. They also do not have the legs or the arms that are placed using trocars through the transobturator or ischioanal fossae, which can approach nearby nerves, arteries, or other vital structures. They are significantly lighter weight, and some have color to make the native tissue and mesh interface more apparent.
Mesh contraction,10 inflammation of the mesh involving surrounding soft tissue,11 and stress shearing at the mesh/soft tissue interface12 have been implicated as potential causes of pain with synthetic mesh. The most commonly available synthetic mesh today is type 1 polypropylene (macroporous monofilament), with a large pore size (usually greater than 75 microns).
Non−cross linked biologic grafts also are available currently, with several cross-linked grafts removed from the market by 2013 because their design was associated with graft stiffness and shrinkage, which had the potential to distort the pelvic anatomy.
Non−cross linked biologic grafts may be associated with fewer mesh-related complications compared with synthetic mesh, but there are limited data on their use in POP repair and there are many unanswered questions. The current concerns with biologics are their tensile properties, foreign body reactions, and documented autolysis. Modifications to them may affect their soft tissue reactivity, but outcomes depend on the technique used for implantation.
OBG Management: When do you consider vaginal mesh use for prolapse?
Dr. Iglesia: A recent Cochrane review shows that some data favor mesh for decreased recurrence, but there are trade-offs.13 I consider mesh use in the setting of recurrent prolapse, especially anterior, for advanced-stageprolapse, and under certain situations, including when there is a known collagen deficiency and there are contraindications to abdominal surgery. However, pelvic pain always is a concern, and surgeons should be extremely careful when choosing to use mesh in patients with known chronic pelvic pain.
The FDA recommends that clinicians treating patients with POP recognize that POP can be treated successfully without mesh and that this native tissue repair will avoid completely the risk of mesh-related complications (TABLE 1).14 Patients should be made aware of alternatives to vaginal mesh when deciding on surgical repair, including nonsurgical options, native tissue repair, and abdominally (laparoscopic, robotic, or open) placed sacrocolpopexy mesh.
OBG Management: How does the Pelvic Floor Disorders Registry solve issues that existed prior to the mesh controversy?
Dr. Iglesia: The Pelvic Floor Disorders Registry (PFDR), which can be accessed online (http://www.pfdr.org), is a private and public collaboration including many medical societies: the American Urogynecologic Society (AUGS), the American College of Obstetricians and Gynecologists, the American Urologic Association, the National Institutes of Health, the FDA, and industry. Its objectives are 3-fold15:
- to collect, store, and analyze clinical data related to POP treatment
- to establish common data elements and quality metrics
- to provide a framework for external stakeholders to conduct POP research (TABLE 2).
All involved PFDR partners, which also includes patient advocates, reached consensus on the outcomes that matter scientifically in terms of objective cure rates and complications as well as on subjective outcomes that matter most to patien
Quite frankly, subjective patient-reported outcomes probably trump any other outcome because, in general, patients are risk averse—which is to say that they are much more easily accepting of recurrence or failure than of a serious adverse event from a mesh-related complication. With the PFDR, we are able to capture not only that objective data but also the critically important patient-centered outcomes.16
With the PFDR, a patient who goes to surgeon B following a complication with surgeon A can still be followed. I look forward to the tracking capability within the registry and the many prospective comparative trials that can be conducted.
Unfortunately, differences between older and newer transvaginal mesh delivery systems will not be evaluated as part of the required 522 studies within the PFDR; however, I really look forward to seeing the data roll out on the second generation vaginal mesh kits compared to native tissue repai
The PFDR has 2 options for volunteer registry participation, the PFDR-Quality Improvement and PFDR-Research. I encourage specialists who are board-certified in Female Pelvic Medicine and Reconstructive Surgery to be involved in the quality improvement research. For this, physicians basically can track their own success and complication rates, including nonsurgical outcomes. This information could be helpful to achieving our ongoing goal of getting better at what we do surgically. If you are doing well, it will be very validating. Your patients will be happy, you will have good outcomes, and that probably will not be bad for marketing your practice.
There may be some opportunities to reach the health-related quality indicators that we need to meet right now as part of government-mandated initiatives. For many reasons, it is important for surgeons who are performing a high volume of POP surgeries per year to get involved in the PFDR. In fact, even if you are not performing surgery, you still can get involved with the nonsurgical pessary side. This also is important information for us to move forward with as a specialty as we seek to understand the natural history of POP.
The PFDR will serve many different purposes—one of the best of which is that we are going to be able to safely promote mesh technology for the most appropriate cases and not stifle innovation. The comparison groups, already built in to the registry, will allow for native tissue arms to be compared head to head with the currently available meshes. In addition, we will be able to see signals sooner if certain products or patient profiles, and even individual surgeon outcomes, are concerning.
Cheryl B. Iglesia, MD, was the Keynote Speaker at the Pelvic Anatomy and Gynecologic Surgery (PAGS) Symposium held in Las Vegas, Nevada, December 10–12, 2015. This article was developed from Dr. Iglesia's presentation titled "The How, Why and Where of Synthetic and Biologic Mesh: Lessons Learned."
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Urogynecologic surgical mesh: Update on the safety and effectiveness of transvaginal placement for pelvic organ prolapse. Washington DC: US Food and Drug Administration, Centers for Devices and Radiological Health; July 2011.
- Rogo-Gupta L, Rodriguez LV, Litwin MS, et al. Trends in surgical mesh use for pelvic organ prolapse from 2000 to 2010. Obstet Gynecol. 2012;120(5):1105–1115.
- FDA strengthens requirements for surgical mesh for the transvaginal repair of pelvic organ prolapse to address safety risks. US Food and Drug Administration website. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm479732.htm. Updated January 6, 2016. Accessed February 12, 2016.
- Premarket notification 510(k). US Food and Drug Administration website. http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/HowtoMarketYourDevice/PremarketSubmissions/PremarketNotification510k/default.htm. Updated September, 16, 2016. Accessed February 11, 2016.
- Gartner Hype Cycle. Gartner website. http://www.gartner.com/technology/research/methodologies/hype-cycle.jsp. Accessed February 12, 2016.
- Barber MD. Thirty-third American Urogynecologic Society Annual Meeting Presidential Address: the end of the beginning. Female Pelvic Med Reconstr Surg. 2013;19(1):2−7.
- FDA public health notification: Serious complications associated with transvaginal placement of surgical mesh in repair of pelvic organ prolapse and stress urinary incontinence. US Food and Drug Administration website. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm061976.htm. Updated August 6, 2015. Accessed February 12, 2016.
- Update on serious complications associated with transvaginal placement of surgical mesh for pelvic organ prolapse: FDA safety communication. US Food and Drug Administration website. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm262435.htm. Updated October 6, 2014. Accessed February 12, 2016.
- Hughes C. FDA issues 522 orders for postmarket surveillance studies: urogynecologic surgical mesh implants. American Urogynecologic Society website. . Published January 5, 2012. Accessed February 12, 2016.
- Feiner B, Maher C. Vaginal mesh contraction: definition, clinical presentation, and management. Obstet Gynecol. 2010;115(2 pt 1):325–330.
- Ozkan N, Kayaoglu HA, Ersoy OF, Celik A, Kurt GS, Arabaci E. Effects of two different meshes used in hernia repair on nerve transport. J Am Coll Surg. 2008;207(5):670–675.
- Clemons JL, Weinstein M, Guess MK, et al; AUGS Research Committee. Impact of the 2011 FDA transvaginal mesh safety update on AUGS members’ use of synthetic mesh and biologic grafts in pelvic reconstructive surgery. Female Pelvic Med Reconstr Surg. 2013;19(4):191–198.
- Maher C, Feiner B, Baessler K, Haya N, Marjoribanks J. Transvaginal mesh or grafts compared with native tissue repair for vaginal prolapse. Cocrane Database Syst Rev. 2016;2;CD012079.
- Information for Health Care Providers for POP. US Food and Drug Administration website. http://www.fda.gov/medicaldevices/productsandmedicalprocedures/implantsandprosthetics/urogynsurgicalmesh/ucm345204.htm. Updated January 4, 2016. Accessed February 13, 2016.
- Bradley CS, Visco AG, Weber LeBrun EE, Barber MD. The Pelvic Floor Disorders Registry: purpose and development [published online ahead of print January 30, 2016]. Female Pelvic Med Reconstr Surg.
- FDA strengthens requirements for surgical mesh for the transvaginal repair of pelvic organ prolapse to address safety risks. US Food and Drug Administration website. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncementsFeiner B, Maher C. Vaginal mesh contraction: definition, clinical presentation, and management.
TRANSVAGINAL MESH FOR POP: NEW AND
NOTEWORTHY RESOURCES
• Pelvic Floor Disorders Registry: Overview, registry resources, FAQs, more
• Vaginal mesh manufacturer closing due to lawsuit concerns, Wall Street Journal, 2/29/16
Approximately 300,000 surgeries for pelvic organ prolapse (POP) are performed annually in the United States. In 2006, the peak of synthetic mesh use for prolapse surgery, one-third of all prolapse operations involved some mesh use.1,2 The use of vaginal mesh has declined since the US Food and Drug Administration (FDA) issued warnings in 2008 and 2011.
Historically, the use of mesh for gynecologic surgery began in the 1970s, with abdominal POP repair.3 Transvaginal mesh use for POP surgeries became FDA-cleared in 2004. The first cleared mesh device was classified as class II (moderate risk).3 Subsequent mesh devices were given 510(k) clearance, which bypasses clinical trials and requires manufacturers only to show that their product is substantially equivalent to one already on the market.4 More than 40 companies began the manufacturing of mesh devices in the 10 years following the initial cleared device.3
Of course, much controversy has surrounded mesh use in recent years, with common adverse events reported, including severe pelvic pain, pain during intercourse, infection, bleeding, organ perforation, and problems from mesh eroding into surrounding tissues.3 The FDA very recently (in January 2016) reclassified this device from moderate risk to high risk (class III), after indicating in May 2014 that such action was necessary. (See “Timeline of FDA’s actions regarding surgical mesh for pelvic organ prolapse” on page 46.) This reclassification requires a premarket approval application to be filed for each device, with safety and efficacy demonstrated. There are approximately 5 companies currently manufacturing mesh for transvaginal POP repair.3
OBG Management recently sat down with Cheryl Iglesia, MD, director of the Section of Female Pelvic Medicine and Reconstructive Surgery at MedStar Washington Hospital Center and professor in the Departments of Obstetrics/Gynecology and Urology at Georgetown University School of Medicine in Washington, DC. Dr. Iglesia serves, from 2011 through 2017, as a member on the FDA Obstetrics and Gynecology Devices Panel, and she addressed lessons learned over the past decade on synthetic and biologic mesh at the Pelvic Anatomy and Gynecologic Surgery (PAGS) symposium in Las Vegas, Nevada, this past December.
In this Q&A article, she addresses the current state of transvaginal mesh use and how it relates to the innovation adaptation curve (otherwise known as the Hype Cycle), how new mesh types differ from older ones, and how the specialty can move into a future of POP surgery in which innovation and data will rule.
OBG Management: Where is transvaginal mesh use on the so-called “Hype Cycle,” or innovation adaptation curve?
Cheryl B. Iglesia, MD: The Hype Cycle was developed and branded by the Gartner company, an information technology advisory and research firm. This cycle refers to the graphical depictions of how a technology or application will evolve over time. After all, new technologies may make bold promises, and the hype may not translate to commercial viability. Each cycle drills down into the key phases of a technology’s life cycle: the trigger, peak of inflated expectations, trough of disillusionment, slope of enlightenment, and plateau of productivity.5
If we use the Hype Cycle to drill down the phases of transvaginal mesh’s life cycle, we begin in 2004 with the FDA clearance of the first vaginal mesh system (FIGURE).6 The height of its use (the “peak of inflated expectation”) was around 2006, when essentially one-third of all annual surgeries performed for prolapse repair used some type of mesh placed either abdominally or transvaginally.2
Subsequently, adverse events began being reported to the Manufacturer and User Facility Device Experience (MAUDE) database. In 2008, the FDA published its first notification of serious complications associated with transvaginal placement of surgical mesh, with more than 1,000 reports from 9 surgical mesh manufacturers.7 A second alert followed in 2011.8 By this time, we had reached our “trough of disillusionment.”
In 2016, we have reached the “plateau of productivity” on the innovation adaptation curve. During this phase on the Hype Cycle the criteria for assessing the technology’s viability are clearly defined. I say we are in this phase because now we have a way of completing more postmarket surveillance on mesh devices. We now can see what applying the technology is like in the real world, generalized across many different surgeons’ hands, and we have a way of performing comparative studies with native tissue.
OBG Management: How do the new types of mesh differ from those that have been removed from the market?
Dr. Iglesia: In January 2012, there were about 40 types of surgical mesh available from more than 30 manufacturers of transvaginal mesh. At that time, the FDA imposed 522 orders on these companies, requiring them to provide up to 3 years of postmarket data on the safety and effectiveness of their devices.9 Some companies ceased production, including Johnson and Johnson and CR Bard. Today, there are about a half-dozen mesh types on the market, and these are undergoing evaluation.
First-generation meshes were the size of a sheet of paper; now, meshes can fit on the palm of your hand. They also do not have the legs or the arms that are placed using trocars through the transobturator or ischioanal fossae, which can approach nearby nerves, arteries, or other vital structures. They are significantly lighter weight, and some have color to make the native tissue and mesh interface more apparent.
Mesh contraction,10 inflammation of the mesh involving surrounding soft tissue,11 and stress shearing at the mesh/soft tissue interface12 have been implicated as potential causes of pain with synthetic mesh. The most commonly available synthetic mesh today is type 1 polypropylene (macroporous monofilament), with a large pore size (usually greater than 75 microns).
Non−cross linked biologic grafts also are available currently, with several cross-linked grafts removed from the market by 2013 because their design was associated with graft stiffness and shrinkage, which had the potential to distort the pelvic anatomy.
Non−cross linked biologic grafts may be associated with fewer mesh-related complications compared with synthetic mesh, but there are limited data on their use in POP repair and there are many unanswered questions. The current concerns with biologics are their tensile properties, foreign body reactions, and documented autolysis. Modifications to them may affect their soft tissue reactivity, but outcomes depend on the technique used for implantation.
OBG Management: When do you consider vaginal mesh use for prolapse?
Dr. Iglesia: A recent Cochrane review shows that some data favor mesh for decreased recurrence, but there are trade-offs.13 I consider mesh use in the setting of recurrent prolapse, especially anterior, for advanced-stageprolapse, and under certain situations, including when there is a known collagen deficiency and there are contraindications to abdominal surgery. However, pelvic pain always is a concern, and surgeons should be extremely careful when choosing to use mesh in patients with known chronic pelvic pain.
The FDA recommends that clinicians treating patients with POP recognize that POP can be treated successfully without mesh and that this native tissue repair will avoid completely the risk of mesh-related complications (TABLE 1).14 Patients should be made aware of alternatives to vaginal mesh when deciding on surgical repair, including nonsurgical options, native tissue repair, and abdominally (laparoscopic, robotic, or open) placed sacrocolpopexy mesh.
OBG Management: How does the Pelvic Floor Disorders Registry solve issues that existed prior to the mesh controversy?
Dr. Iglesia: The Pelvic Floor Disorders Registry (PFDR), which can be accessed online (http://www.pfdr.org), is a private and public collaboration including many medical societies: the American Urogynecologic Society (AUGS), the American College of Obstetricians and Gynecologists, the American Urologic Association, the National Institutes of Health, the FDA, and industry. Its objectives are 3-fold15:
- to collect, store, and analyze clinical data related to POP treatment
- to establish common data elements and quality metrics
- to provide a framework for external stakeholders to conduct POP research (TABLE 2).
All involved PFDR partners, which also includes patient advocates, reached consensus on the outcomes that matter scientifically in terms of objective cure rates and complications as well as on subjective outcomes that matter most to patien
Quite frankly, subjective patient-reported outcomes probably trump any other outcome because, in general, patients are risk averse—which is to say that they are much more easily accepting of recurrence or failure than of a serious adverse event from a mesh-related complication. With the PFDR, we are able to capture not only that objective data but also the critically important patient-centered outcomes.16
With the PFDR, a patient who goes to surgeon B following a complication with surgeon A can still be followed. I look forward to the tracking capability within the registry and the many prospective comparative trials that can be conducted.
Unfortunately, differences between older and newer transvaginal mesh delivery systems will not be evaluated as part of the required 522 studies within the PFDR; however, I really look forward to seeing the data roll out on the second generation vaginal mesh kits compared to native tissue repai
The PFDR has 2 options for volunteer registry participation, the PFDR-Quality Improvement and PFDR-Research. I encourage specialists who are board-certified in Female Pelvic Medicine and Reconstructive Surgery to be involved in the quality improvement research. For this, physicians basically can track their own success and complication rates, including nonsurgical outcomes. This information could be helpful to achieving our ongoing goal of getting better at what we do surgically. If you are doing well, it will be very validating. Your patients will be happy, you will have good outcomes, and that probably will not be bad for marketing your practice.
There may be some opportunities to reach the health-related quality indicators that we need to meet right now as part of government-mandated initiatives. For many reasons, it is important for surgeons who are performing a high volume of POP surgeries per year to get involved in the PFDR. In fact, even if you are not performing surgery, you still can get involved with the nonsurgical pessary side. This also is important information for us to move forward with as a specialty as we seek to understand the natural history of POP.
The PFDR will serve many different purposes—one of the best of which is that we are going to be able to safely promote mesh technology for the most appropriate cases and not stifle innovation. The comparison groups, already built in to the registry, will allow for native tissue arms to be compared head to head with the currently available meshes. In addition, we will be able to see signals sooner if certain products or patient profiles, and even individual surgeon outcomes, are concerning.
Cheryl B. Iglesia, MD, was the Keynote Speaker at the Pelvic Anatomy and Gynecologic Surgery (PAGS) Symposium held in Las Vegas, Nevada, December 10–12, 2015. This article was developed from Dr. Iglesia's presentation titled "The How, Why and Where of Synthetic and Biologic Mesh: Lessons Learned."
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
TRANSVAGINAL MESH FOR POP: NEW AND
NOTEWORTHY RESOURCES
• Pelvic Floor Disorders Registry: Overview, registry resources, FAQs, more
• Vaginal mesh manufacturer closing due to lawsuit concerns, Wall Street Journal, 2/29/16
Approximately 300,000 surgeries for pelvic organ prolapse (POP) are performed annually in the United States. In 2006, the peak of synthetic mesh use for prolapse surgery, one-third of all prolapse operations involved some mesh use.1,2 The use of vaginal mesh has declined since the US Food and Drug Administration (FDA) issued warnings in 2008 and 2011.
Historically, the use of mesh for gynecologic surgery began in the 1970s, with abdominal POP repair.3 Transvaginal mesh use for POP surgeries became FDA-cleared in 2004. The first cleared mesh device was classified as class II (moderate risk).3 Subsequent mesh devices were given 510(k) clearance, which bypasses clinical trials and requires manufacturers only to show that their product is substantially equivalent to one already on the market.4 More than 40 companies began the manufacturing of mesh devices in the 10 years following the initial cleared device.3
Of course, much controversy has surrounded mesh use in recent years, with common adverse events reported, including severe pelvic pain, pain during intercourse, infection, bleeding, organ perforation, and problems from mesh eroding into surrounding tissues.3 The FDA very recently (in January 2016) reclassified this device from moderate risk to high risk (class III), after indicating in May 2014 that such action was necessary. (See “Timeline of FDA’s actions regarding surgical mesh for pelvic organ prolapse” on page 46.) This reclassification requires a premarket approval application to be filed for each device, with safety and efficacy demonstrated. There are approximately 5 companies currently manufacturing mesh for transvaginal POP repair.3
OBG Management recently sat down with Cheryl Iglesia, MD, director of the Section of Female Pelvic Medicine and Reconstructive Surgery at MedStar Washington Hospital Center and professor in the Departments of Obstetrics/Gynecology and Urology at Georgetown University School of Medicine in Washington, DC. Dr. Iglesia serves, from 2011 through 2017, as a member on the FDA Obstetrics and Gynecology Devices Panel, and she addressed lessons learned over the past decade on synthetic and biologic mesh at the Pelvic Anatomy and Gynecologic Surgery (PAGS) symposium in Las Vegas, Nevada, this past December.
In this Q&A article, she addresses the current state of transvaginal mesh use and how it relates to the innovation adaptation curve (otherwise known as the Hype Cycle), how new mesh types differ from older ones, and how the specialty can move into a future of POP surgery in which innovation and data will rule.
OBG Management: Where is transvaginal mesh use on the so-called “Hype Cycle,” or innovation adaptation curve?
Cheryl B. Iglesia, MD: The Hype Cycle was developed and branded by the Gartner company, an information technology advisory and research firm. This cycle refers to the graphical depictions of how a technology or application will evolve over time. After all, new technologies may make bold promises, and the hype may not translate to commercial viability. Each cycle drills down into the key phases of a technology’s life cycle: the trigger, peak of inflated expectations, trough of disillusionment, slope of enlightenment, and plateau of productivity.5
If we use the Hype Cycle to drill down the phases of transvaginal mesh’s life cycle, we begin in 2004 with the FDA clearance of the first vaginal mesh system (FIGURE).6 The height of its use (the “peak of inflated expectation”) was around 2006, when essentially one-third of all annual surgeries performed for prolapse repair used some type of mesh placed either abdominally or transvaginally.2
Subsequently, adverse events began being reported to the Manufacturer and User Facility Device Experience (MAUDE) database. In 2008, the FDA published its first notification of serious complications associated with transvaginal placement of surgical mesh, with more than 1,000 reports from 9 surgical mesh manufacturers.7 A second alert followed in 2011.8 By this time, we had reached our “trough of disillusionment.”
In 2016, we have reached the “plateau of productivity” on the innovation adaptation curve. During this phase on the Hype Cycle the criteria for assessing the technology’s viability are clearly defined. I say we are in this phase because now we have a way of completing more postmarket surveillance on mesh devices. We now can see what applying the technology is like in the real world, generalized across many different surgeons’ hands, and we have a way of performing comparative studies with native tissue.
OBG Management: How do the new types of mesh differ from those that have been removed from the market?
Dr. Iglesia: In January 2012, there were about 40 types of surgical mesh available from more than 30 manufacturers of transvaginal mesh. At that time, the FDA imposed 522 orders on these companies, requiring them to provide up to 3 years of postmarket data on the safety and effectiveness of their devices.9 Some companies ceased production, including Johnson and Johnson and CR Bard. Today, there are about a half-dozen mesh types on the market, and these are undergoing evaluation.
First-generation meshes were the size of a sheet of paper; now, meshes can fit on the palm of your hand. They also do not have the legs or the arms that are placed using trocars through the transobturator or ischioanal fossae, which can approach nearby nerves, arteries, or other vital structures. They are significantly lighter weight, and some have color to make the native tissue and mesh interface more apparent.
Mesh contraction,10 inflammation of the mesh involving surrounding soft tissue,11 and stress shearing at the mesh/soft tissue interface12 have been implicated as potential causes of pain with synthetic mesh. The most commonly available synthetic mesh today is type 1 polypropylene (macroporous monofilament), with a large pore size (usually greater than 75 microns).
Non−cross linked biologic grafts also are available currently, with several cross-linked grafts removed from the market by 2013 because their design was associated with graft stiffness and shrinkage, which had the potential to distort the pelvic anatomy.
Non−cross linked biologic grafts may be associated with fewer mesh-related complications compared with synthetic mesh, but there are limited data on their use in POP repair and there are many unanswered questions. The current concerns with biologics are their tensile properties, foreign body reactions, and documented autolysis. Modifications to them may affect their soft tissue reactivity, but outcomes depend on the technique used for implantation.
OBG Management: When do you consider vaginal mesh use for prolapse?
Dr. Iglesia: A recent Cochrane review shows that some data favor mesh for decreased recurrence, but there are trade-offs.13 I consider mesh use in the setting of recurrent prolapse, especially anterior, for advanced-stageprolapse, and under certain situations, including when there is a known collagen deficiency and there are contraindications to abdominal surgery. However, pelvic pain always is a concern, and surgeons should be extremely careful when choosing to use mesh in patients with known chronic pelvic pain.
The FDA recommends that clinicians treating patients with POP recognize that POP can be treated successfully without mesh and that this native tissue repair will avoid completely the risk of mesh-related complications (TABLE 1).14 Patients should be made aware of alternatives to vaginal mesh when deciding on surgical repair, including nonsurgical options, native tissue repair, and abdominally (laparoscopic, robotic, or open) placed sacrocolpopexy mesh.
OBG Management: How does the Pelvic Floor Disorders Registry solve issues that existed prior to the mesh controversy?
Dr. Iglesia: The Pelvic Floor Disorders Registry (PFDR), which can be accessed online (http://www.pfdr.org), is a private and public collaboration including many medical societies: the American Urogynecologic Society (AUGS), the American College of Obstetricians and Gynecologists, the American Urologic Association, the National Institutes of Health, the FDA, and industry. Its objectives are 3-fold15:
- to collect, store, and analyze clinical data related to POP treatment
- to establish common data elements and quality metrics
- to provide a framework for external stakeholders to conduct POP research (TABLE 2).
All involved PFDR partners, which also includes patient advocates, reached consensus on the outcomes that matter scientifically in terms of objective cure rates and complications as well as on subjective outcomes that matter most to patien
Quite frankly, subjective patient-reported outcomes probably trump any other outcome because, in general, patients are risk averse—which is to say that they are much more easily accepting of recurrence or failure than of a serious adverse event from a mesh-related complication. With the PFDR, we are able to capture not only that objective data but also the critically important patient-centered outcomes.16
With the PFDR, a patient who goes to surgeon B following a complication with surgeon A can still be followed. I look forward to the tracking capability within the registry and the many prospective comparative trials that can be conducted.
Unfortunately, differences between older and newer transvaginal mesh delivery systems will not be evaluated as part of the required 522 studies within the PFDR; however, I really look forward to seeing the data roll out on the second generation vaginal mesh kits compared to native tissue repai
The PFDR has 2 options for volunteer registry participation, the PFDR-Quality Improvement and PFDR-Research. I encourage specialists who are board-certified in Female Pelvic Medicine and Reconstructive Surgery to be involved in the quality improvement research. For this, physicians basically can track their own success and complication rates, including nonsurgical outcomes. This information could be helpful to achieving our ongoing goal of getting better at what we do surgically. If you are doing well, it will be very validating. Your patients will be happy, you will have good outcomes, and that probably will not be bad for marketing your practice.
There may be some opportunities to reach the health-related quality indicators that we need to meet right now as part of government-mandated initiatives. For many reasons, it is important for surgeons who are performing a high volume of POP surgeries per year to get involved in the PFDR. In fact, even if you are not performing surgery, you still can get involved with the nonsurgical pessary side. This also is important information for us to move forward with as a specialty as we seek to understand the natural history of POP.
The PFDR will serve many different purposes—one of the best of which is that we are going to be able to safely promote mesh technology for the most appropriate cases and not stifle innovation. The comparison groups, already built in to the registry, will allow for native tissue arms to be compared head to head with the currently available meshes. In addition, we will be able to see signals sooner if certain products or patient profiles, and even individual surgeon outcomes, are concerning.
Cheryl B. Iglesia, MD, was the Keynote Speaker at the Pelvic Anatomy and Gynecologic Surgery (PAGS) Symposium held in Las Vegas, Nevada, December 10–12, 2015. This article was developed from Dr. Iglesia's presentation titled "The How, Why and Where of Synthetic and Biologic Mesh: Lessons Learned."
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Urogynecologic surgical mesh: Update on the safety and effectiveness of transvaginal placement for pelvic organ prolapse. Washington DC: US Food and Drug Administration, Centers for Devices and Radiological Health; July 2011.
- Rogo-Gupta L, Rodriguez LV, Litwin MS, et al. Trends in surgical mesh use for pelvic organ prolapse from 2000 to 2010. Obstet Gynecol. 2012;120(5):1105–1115.
- FDA strengthens requirements for surgical mesh for the transvaginal repair of pelvic organ prolapse to address safety risks. US Food and Drug Administration website. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm479732.htm. Updated January 6, 2016. Accessed February 12, 2016.
- Premarket notification 510(k). US Food and Drug Administration website. http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/HowtoMarketYourDevice/PremarketSubmissions/PremarketNotification510k/default.htm. Updated September, 16, 2016. Accessed February 11, 2016.
- Gartner Hype Cycle. Gartner website. http://www.gartner.com/technology/research/methodologies/hype-cycle.jsp. Accessed February 12, 2016.
- Barber MD. Thirty-third American Urogynecologic Society Annual Meeting Presidential Address: the end of the beginning. Female Pelvic Med Reconstr Surg. 2013;19(1):2−7.
- FDA public health notification: Serious complications associated with transvaginal placement of surgical mesh in repair of pelvic organ prolapse and stress urinary incontinence. US Food and Drug Administration website. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm061976.htm. Updated August 6, 2015. Accessed February 12, 2016.
- Update on serious complications associated with transvaginal placement of surgical mesh for pelvic organ prolapse: FDA safety communication. US Food and Drug Administration website. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm262435.htm. Updated October 6, 2014. Accessed February 12, 2016.
- Hughes C. FDA issues 522 orders for postmarket surveillance studies: urogynecologic surgical mesh implants. American Urogynecologic Society website. . Published January 5, 2012. Accessed February 12, 2016.
- Feiner B, Maher C. Vaginal mesh contraction: definition, clinical presentation, and management. Obstet Gynecol. 2010;115(2 pt 1):325–330.
- Ozkan N, Kayaoglu HA, Ersoy OF, Celik A, Kurt GS, Arabaci E. Effects of two different meshes used in hernia repair on nerve transport. J Am Coll Surg. 2008;207(5):670–675.
- Clemons JL, Weinstein M, Guess MK, et al; AUGS Research Committee. Impact of the 2011 FDA transvaginal mesh safety update on AUGS members’ use of synthetic mesh and biologic grafts in pelvic reconstructive surgery. Female Pelvic Med Reconstr Surg. 2013;19(4):191–198.
- Maher C, Feiner B, Baessler K, Haya N, Marjoribanks J. Transvaginal mesh or grafts compared with native tissue repair for vaginal prolapse. Cocrane Database Syst Rev. 2016;2;CD012079.
- Information for Health Care Providers for POP. US Food and Drug Administration website. http://www.fda.gov/medicaldevices/productsandmedicalprocedures/implantsandprosthetics/urogynsurgicalmesh/ucm345204.htm. Updated January 4, 2016. Accessed February 13, 2016.
- Bradley CS, Visco AG, Weber LeBrun EE, Barber MD. The Pelvic Floor Disorders Registry: purpose and development [published online ahead of print January 30, 2016]. Female Pelvic Med Reconstr Surg.
- FDA strengthens requirements for surgical mesh for the transvaginal repair of pelvic organ prolapse to address safety risks. US Food and Drug Administration website. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncementsFeiner B, Maher C. Vaginal mesh contraction: definition, clinical presentation, and management.
- Urogynecologic surgical mesh: Update on the safety and effectiveness of transvaginal placement for pelvic organ prolapse. Washington DC: US Food and Drug Administration, Centers for Devices and Radiological Health; July 2011.
- Rogo-Gupta L, Rodriguez LV, Litwin MS, et al. Trends in surgical mesh use for pelvic organ prolapse from 2000 to 2010. Obstet Gynecol. 2012;120(5):1105–1115.
- FDA strengthens requirements for surgical mesh for the transvaginal repair of pelvic organ prolapse to address safety risks. US Food and Drug Administration website. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm479732.htm. Updated January 6, 2016. Accessed February 12, 2016.
- Premarket notification 510(k). US Food and Drug Administration website. http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/HowtoMarketYourDevice/PremarketSubmissions/PremarketNotification510k/default.htm. Updated September, 16, 2016. Accessed February 11, 2016.
- Gartner Hype Cycle. Gartner website. http://www.gartner.com/technology/research/methodologies/hype-cycle.jsp. Accessed February 12, 2016.
- Barber MD. Thirty-third American Urogynecologic Society Annual Meeting Presidential Address: the end of the beginning. Female Pelvic Med Reconstr Surg. 2013;19(1):2−7.
- FDA public health notification: Serious complications associated with transvaginal placement of surgical mesh in repair of pelvic organ prolapse and stress urinary incontinence. US Food and Drug Administration website. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm061976.htm. Updated August 6, 2015. Accessed February 12, 2016.
- Update on serious complications associated with transvaginal placement of surgical mesh for pelvic organ prolapse: FDA safety communication. US Food and Drug Administration website. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm262435.htm. Updated October 6, 2014. Accessed February 12, 2016.
- Hughes C. FDA issues 522 orders for postmarket surveillance studies: urogynecologic surgical mesh implants. American Urogynecologic Society website. . Published January 5, 2012. Accessed February 12, 2016.
- Feiner B, Maher C. Vaginal mesh contraction: definition, clinical presentation, and management. Obstet Gynecol. 2010;115(2 pt 1):325–330.
- Ozkan N, Kayaoglu HA, Ersoy OF, Celik A, Kurt GS, Arabaci E. Effects of two different meshes used in hernia repair on nerve transport. J Am Coll Surg. 2008;207(5):670–675.
- Clemons JL, Weinstein M, Guess MK, et al; AUGS Research Committee. Impact of the 2011 FDA transvaginal mesh safety update on AUGS members’ use of synthetic mesh and biologic grafts in pelvic reconstructive surgery. Female Pelvic Med Reconstr Surg. 2013;19(4):191–198.
- Maher C, Feiner B, Baessler K, Haya N, Marjoribanks J. Transvaginal mesh or grafts compared with native tissue repair for vaginal prolapse. Cocrane Database Syst Rev. 2016;2;CD012079.
- Information for Health Care Providers for POP. US Food and Drug Administration website. http://www.fda.gov/medicaldevices/productsandmedicalprocedures/implantsandprosthetics/urogynsurgicalmesh/ucm345204.htm. Updated January 4, 2016. Accessed February 13, 2016.
- Bradley CS, Visco AG, Weber LeBrun EE, Barber MD. The Pelvic Floor Disorders Registry: purpose and development [published online ahead of print January 30, 2016]. Female Pelvic Med Reconstr Surg.
- FDA strengthens requirements for surgical mesh for the transvaginal repair of pelvic organ prolapse to address safety risks. US Food and Drug Administration website. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncementsFeiner B, Maher C. Vaginal mesh contraction: definition, clinical presentation, and management.
In this Article
- Transvaginal mesh and the “Hype Cycle”
- Newer vs older mesh types
- Solutions offered by the Pelvic Floor Disorders Registry
Cheryl B. Iglesia, MD, was the Keynote Speaker at the Pelvic Anatomy and Gynecologic Surgery (PAGS) Symposium held in Las Vegas, Nevada, December 10–12, 2015. This article was developed from Dr. Iglesia's presentation titled "The How, Why and Where of Synthetic and Biologic Mesh: Lessons Learned."
A Call for More Autopsies
In the past 50 years, the number of autopsies at most U.S. hospitals has dropped drastically. The decline is due in part to a “widespread perception” that new imaging techniques and laboratory tests have improved diagnostic accuracy to the extent that an autopsy is considered obsolete, say researchers from Temple University Hospital in Philadelphia, Pennsylvania. But the researchers claim that autopsies are still relevant and “a valuable tool to evaluate diagnostic accuracy.”
According to the researchers, autopsy studies continue to show that the proportions of clinical misdiagnosis have remained largely unchanged. They also found that autopsies performed over 10 years of 821 adults showed 8% had clinically undiagnosed malignancies—similar to the numbers found in studies done 10 years earlier. Out of 66 cases, 26 revealed undiagnosed malignancies directly related to the primary cause of death. In 16 autopsies, there was no clinical suspicion of malignancy, but the primary cause of death (such as acute bronchopneumonia and gastrointestinal perforation) was directly related to an undiagnosed neoplasm. In 10 cases, there was clinical suspicion of malignancy based on history, radiologic studies, and laboratory tests but without definite tissue diagnosis.
The researchers note that some studies have suggested a link between short hospital stays and missed diagnoses. But in the current study, length of stay had no bearing on a patient’s having an unsuspected malignancy. “Ironically,” the cases with no clinical suspicion involved longer hospital stays. In at least 5 cases the hospital stay was for more than 5 days. Moreover, CT scans of the thorax/abdomen raised no suspicion of cancer.
The researchers say their findings make a “strong case for a vigorous hospital autopsy program,” and for using autopsy data to improve performance in clinical, radiologic, and laboratory services. Their study “reiterates the value of the hospital autopsy as an auditing tool for diagnostic accuracy.”
Source: Parajuli S, Aneja A, Mukherjee A. Hum Pathol. 2016; 48:32-36.
doi: 10.1016/j.humpath.2015.09.040.
In the past 50 years, the number of autopsies at most U.S. hospitals has dropped drastically. The decline is due in part to a “widespread perception” that new imaging techniques and laboratory tests have improved diagnostic accuracy to the extent that an autopsy is considered obsolete, say researchers from Temple University Hospital in Philadelphia, Pennsylvania. But the researchers claim that autopsies are still relevant and “a valuable tool to evaluate diagnostic accuracy.”
According to the researchers, autopsy studies continue to show that the proportions of clinical misdiagnosis have remained largely unchanged. They also found that autopsies performed over 10 years of 821 adults showed 8% had clinically undiagnosed malignancies—similar to the numbers found in studies done 10 years earlier. Out of 66 cases, 26 revealed undiagnosed malignancies directly related to the primary cause of death. In 16 autopsies, there was no clinical suspicion of malignancy, but the primary cause of death (such as acute bronchopneumonia and gastrointestinal perforation) was directly related to an undiagnosed neoplasm. In 10 cases, there was clinical suspicion of malignancy based on history, radiologic studies, and laboratory tests but without definite tissue diagnosis.
The researchers note that some studies have suggested a link between short hospital stays and missed diagnoses. But in the current study, length of stay had no bearing on a patient’s having an unsuspected malignancy. “Ironically,” the cases with no clinical suspicion involved longer hospital stays. In at least 5 cases the hospital stay was for more than 5 days. Moreover, CT scans of the thorax/abdomen raised no suspicion of cancer.
The researchers say their findings make a “strong case for a vigorous hospital autopsy program,” and for using autopsy data to improve performance in clinical, radiologic, and laboratory services. Their study “reiterates the value of the hospital autopsy as an auditing tool for diagnostic accuracy.”
Source: Parajuli S, Aneja A, Mukherjee A. Hum Pathol. 2016; 48:32-36.
doi: 10.1016/j.humpath.2015.09.040.
In the past 50 years, the number of autopsies at most U.S. hospitals has dropped drastically. The decline is due in part to a “widespread perception” that new imaging techniques and laboratory tests have improved diagnostic accuracy to the extent that an autopsy is considered obsolete, say researchers from Temple University Hospital in Philadelphia, Pennsylvania. But the researchers claim that autopsies are still relevant and “a valuable tool to evaluate diagnostic accuracy.”
According to the researchers, autopsy studies continue to show that the proportions of clinical misdiagnosis have remained largely unchanged. They also found that autopsies performed over 10 years of 821 adults showed 8% had clinically undiagnosed malignancies—similar to the numbers found in studies done 10 years earlier. Out of 66 cases, 26 revealed undiagnosed malignancies directly related to the primary cause of death. In 16 autopsies, there was no clinical suspicion of malignancy, but the primary cause of death (such as acute bronchopneumonia and gastrointestinal perforation) was directly related to an undiagnosed neoplasm. In 10 cases, there was clinical suspicion of malignancy based on history, radiologic studies, and laboratory tests but without definite tissue diagnosis.
The researchers note that some studies have suggested a link between short hospital stays and missed diagnoses. But in the current study, length of stay had no bearing on a patient’s having an unsuspected malignancy. “Ironically,” the cases with no clinical suspicion involved longer hospital stays. In at least 5 cases the hospital stay was for more than 5 days. Moreover, CT scans of the thorax/abdomen raised no suspicion of cancer.
The researchers say their findings make a “strong case for a vigorous hospital autopsy program,” and for using autopsy data to improve performance in clinical, radiologic, and laboratory services. Their study “reiterates the value of the hospital autopsy as an auditing tool for diagnostic accuracy.”
Source: Parajuli S, Aneja A, Mukherjee A. Hum Pathol. 2016; 48:32-36.
doi: 10.1016/j.humpath.2015.09.040.
Zika virus: Counseling considerations for this emerging perinatal threat
Zika virus infection in the news
- CDC: Zika virus disease cases by US state or territory, updated periodically
- CDC: Q&As for ObGyns on pregnant women and Zika virus, 2/9/16
- CDC: Zika virus infection among US pregnant travelers, 2/26/16
- CDC: Interim guidelines for health care providers caring for infants and children with possible Zika virus infection, 2/19/16
- SMFM statement: Ultrasound screening for fetal microcephaly following Zika virus exposure, 2/16/16
- FDA approves first Zika diagnostic test for commercial use. Newsweek, 2/26/16
- NIH accelerates timeline for human trials of Zika vaccine. The Washington Post, 2/17/16
- Patient resource: Zika virus and pregnancy fact sheet from MotherToBaby.org
- Zika virus article collection from New England Journal of Medicine
- Zika infection diagnosed in 18 pregnant US women who traveled to Zika-affected areas
- FDA grants emergency approval to new 3-in-1 lab test for Zika
- ACOG Practice Advisory: Updated interim guidance for care of women of reproductive age during a Zika virus outbreak, 3/31/16
- MMWR: Patterns in Zika virus testing and infection, 4/22/16
- What insect repellents are safe during pregnancy? 5/19/16
- Zika virus and complications: Q&A from WHO, 5/31/16
- WHO strengthens guidelines to prevent sexual transmission of Zika virus, 5/31/16
- Ultrasound screening for fetal microcephaly following Zika virus exposure (from AJOG), 6/1/16
- CDC: Interim guidance for interpretation of Zika virus antibody test results, 6/3/16
- First Zika vaccine to begin testing in human trials, The Washington Post, 6/20/16
- NIH launches the Zika in Infants and Pregnancy (ZIP) international study, 6/21/16
CASE 1: Pregnant traveler asks: Should I be tested for Zika virus?
A 28-year-old Hispanic woman (G3P2) at 15 weeks’ gestation visits your office for a routine prenatal care appointment. She reports having returned from a 3-week holiday in Brazil 2 days ago, and she is concerned about having experienced fever, malaise, arthralgias, and a disseminated erythematous rash. She has since heard about the Zika virus and asks you if she and her baby are in danger and whether she should be tested for the disease.
What should you tell this patient?
The Zika virus is an RNA Flavivirus, transmitted primarily by the Aedes aegypti mosquito.1 This virus is closely related to the organisms that cause dengue fever, yellow fever, chikungunya infection, and West Nile infection. By feeding on infected prey, mosquitoes can transmit the virus to humans through bites. They breed near pools of stagnant water, can survive both indoors and outdoors, and prefer to be near people. These mosquitoes bite mostly during daylight hours, so it is essential that people use insect repellent throughout the day while in endemic areas.2 These mosquitoes live only in tropical regions; however, the Aedes albopictus mosquito, also known as the Asian tiger mosquito, lives in temperate regions and can transmit the Zika virus as well3 (FIGURE 1).
| FIGURE 1 Aedes aegypti and Aedes albopictus mosquitoes | ||
 |  | |
Aedes aegypti (left) and Aedes albopictus (right) mosquitoes. Aedes mosquitoes are the main transmission vector for the Zika virus. | ||
|
The Zika virus was first discovered in 1947 when it was isolated from a rhesus monkey in Uganda. It subsequently spread to Southeast Asia and eventually caused major outbreaks in the Yap Islands of Micronesia (2007)4 and French Polynesia (2013).5 In 2015, local transmission of the Zika virus infection was noted in Brazil, and, most recently, a pandemic of Zika virus infection has occurred throughout South America, Central America, and the Caribbean islands. To date, local mosquito-borne virus transmission has not occurred in the continental United States, although at least 82 cases acquired during travel to infected areas have been reported.6
Additionally, there have been rare cases involving spread of this virus from infected blood transfusions and through sexual contact.7 In February 2016, the first case of locally acquired Zika virus infection was reported in Texas following sexual transmission of the disease.8
Clinical manifestations of Zika virus infection
Eighty percent of patients infected with Zika virus remain asymptomatic. The illness is short-lived, occurring 2 to 12 days following the mosquito bite, and infected individuals usually do not require hospitalization or experience serious morbidity. When symptoms are present, they typically include low-grade fever (37.8° to 38.5°C), maculopapular rash, arthralgias of the hands and feet, and nonpurulent conjunctivitis. Patients also may experience headache, retro-orbital pain, myalgia, and, rarely, abdominal pain, nausea, vomiting, diarrhea, ulcerations of mucous membranes, and pruritus.9 Guillain-Barré syndrome has been reported in association with Zika virus infection10; however, a definitive cause-effect relationship has not been proven.
If a pregnant woman is infected with the Zika virus, perinatal transmission can occur, either through uteroplacental transmission or vertically from mother to child at the time of delivery. Zika virus RNA has been detected in blood, amniotic fluid, semen, saliva, cerebrospinal fluid, urine, and breast milk. Although the virus has been shown to be present in breast milk, there has been no evidence of viral replication in milk or reported transmission in breastfed infants.11 Pregnant women are not known to have increased susceptibility to Zika virus infection when compared with the general population, and there is no evidence to suggest pregnant women will have a more serious illness if infected.
The Zika virus has been strongly associated with congenital microcephaly and fetal loss among women infected during pregnancy.12 Following the recent large outbreak in Brazil, an alarmingly high number of Brazilian newborns with microcephaly have been observed. The total now exceeds 4,000. Because of these ominous findings, fetuses and neonates born to women with a history of infection should be evaluated for adverse effects of congenital infection.
Management strategies for Zika virus exposure during pregnancy
The incidence of Zika virus infection during pregnancy remains unknown. However, a pregnant woman may be infected in any trimester, and maternal-fetal transmission of the virus can occur throughout pregnancy. If a patient is pregnant and has travelled to areas of Zika virus transmission, or has had unprotected sexual contact with a partner who has had exposure, she should be carefully screened with a detailed review of systems and ultrasonography to evaluate for fetal microcephaly or intracranial calcifications. The US Centers for Disease Control and Prevention (CDC) initially recommended that, if a patient exhibited 2 or more symptoms consistent with Zika virus infection within 2 weeks of exposure or if sonographic evidence revealed fetal microcephaly or intracranial calcifications, she should be tested for Zika virus infection.11
More recently, the CDC issued new guidelines recommending that even asymptomatic women with exposure have serologic testing for infection and that all exposed women undergo serial ultrasound assessments.13 The CDC also recommends offering retesting in the mid second trimester for women who were exposed very early in gestation.
The best diagnostic test for infection is reverse transcriptase-polymerase chain reaction (RT-PCR), and, ideally, it should be completed within 4 days of symptom onset. Beyond 4 days after symptom onset, testing for Zika virus immunoglobulin M (IgM)-specific antibody and neutralizing antibody should be performed in addition to the RT-PCR test. At times, interpretation of antibody testing can be problematic because cross-reaction with related arboviruses is common. Moreover, Zika viremia decreases rapidly over time; therefore, if serum is collected even 5 to 7 days after symptom onset, a negative test does not definitively exclude infection (TABLE 1).
In the United States, local health departments should be contacted to facilitate testing, as the tests described above are not currently commercially available. If the local health department is unable to perform this testing, clinicians should contact the CDC’s Division of Vector-Borne Diseases (telephone: 1-970-221-6400) or visit their website (http://www.cdc.gov/ncezid/dvbd/specimensub/arboviral-shipping.html) for detailed instructions on specimen submission.
Testing is not indicated for women without a history of travel to areas where Zika virus infection is endemic or without a history of unprotected sexual contact with someone who has been exposed to the infection.
Following the delivery of a live infant to an infected or exposed mother, detailed histopathologic evaluation of the placenta and umbilical cord should be performed. Frozen sections of placental and cord tissue should be tested for Zika virus RNA, and cord serum should be tested for Zika and dengue virus IgM and neutralizing antibodies. In cases of fetal loss in the setting of relevant travel history or exposure (particularly maternal symptoms or sonographic evidence of microcephaly), RT-PCR testing and immunohistochemistry should be completed on fetal tissues, umbilical cord, and placenta.2
Treatment is supportive
At present, there is no vaccine for the Zika virus, and no hyperimmune globulin or anti‑ viral chemotherapy is available. Treatment is therefore supportive. Patients should be encouraged to rest and maintain hydration. The preferred antipyretic and analgesic is acetaminophen (650 mg orally every 6 hours or 1,000 mg orally every 8 hours). Aspirin should be avoided until dengue infection has been ruled out because of the related risk of bleeding with hemorrhagic fever. Nonsteroidal anti-inflammatory drugs should be avoided in the second half of pregnancy because of their effect on fetal renal blood flow (oligohydramnios) and stricture of the ductus arteriosus.
CASE 1 Continued
Given this patient’s recent travel, exposure to mosquito-borne illness, and clinical manifestations of malaise, rash, and joint pain, you proceed with serologic testing. The RT-PCR test is positive for Zika virus.
What should be the next step in the management of this patient?
Prenatal diagnosis and fetal surveillance
The recent epidemic of microcephaly and poor pregnancy outcomes reported in Brazil has been alarming and demonstrates an almost 20-fold increase in incidence of this condition between 2014–2015.14 Careful surveillance is needed for this birth defect and other poor pregnancy outcomes in association with the Zika virus. To date, a direct causal relationship between Zika virus infection and microcephaly has not been unequivocally established15; however; these microcephaly cases have yet to be attributed to any other cause (FIGURE 2)
| FIGURE 2 Microcephaly: associated with Zika virus infection in pregnancy |
 |
Illustration depicts a child with congenital microcephaly (left) and one with head circumference within the mean SD (right). |
Following the outbreak in Brazil, a task force and registry were established to investigate microcephaly and other birth defects associated with Zika virus infection. In one small investigation, 35 cases of microcephaly were reported, and 71% of the infants were seriously affected (head circumference >3 SD below the mean). Fifty percent of babies had at least one neurologic abnormality, and, of the 27 patients who had neuroimaging studies, all had distinct abnormalities, including widespread brain calcifications and cell migration abnormalities, such as lissencephaly, pachgyria, and ventriculomegaly due to cortical atrophy.16
In addition to microcephaly, fetal ultrasound monitoring has revealed focal brain abnormalities, such as asymmetric cerebral hemispheres, ventriculomegaly, displacement of the midline, failure to visualize the corpus callosum, failure of thalamic development, and the presence of intraocular and brain calcifications.17
In collaboration with the CDC, the American College of Obstetricians and Gynecologists and the Society for Maternal Fetal-Medicine have developed guidelines to monitor fetal growth in women with laboratory evidence of Zika virus infection.18 Recommendations include having a detailed anatomy ultrasound and serial growth sonograms every 3 to 4 weeks, along with referral to a maternal-fetal medicine or infectious disease specialist.
If the pregnancy is beyond 15 weeks’ gestational age, an amniocentesis should be performed in symptomatic patients and in those with abnormal ultrasound findings. Amniotic fluid should be tested for Zika virus with RT-PCR (FIGURE 3).12 The sensitivity and specificity of amniotic fluid RT-PCR in detecting congenital infection, as well as the predictive value of a fetal anomaly, remain unknown at this time. For this reason, a patient must be counseled carefully regarding the benefits of confirming intrauterine infection versus the slight risks of premature rupture of membranes, infection, and pregnancy loss related to amniocentesis.
Once diagnosed, microcephaly cannot be “fixed.” However, pregnancy termination is an option that some parents may choose once they are aware of the diagnosis and prognosis of microcephaly. Moreover, even for parents who would not choose abortion, there may be considerable value in being prepared for the care of a severely disabled child. Microcephaly has many possible causes, Zika virus infection being just one. Others include genetic syndromes and other congenital infections, such as cytomegalovirus (CMV) infection and toxoplasmosis. Amniocentesis therefore may help the clinician sort through these causes. For both CMV infection and toxoplasmosis, certain antenatal treatments may be helpful in lessening the severity of fetal injury.
CASE 2 Pregnant patient has travel plans
A 34-year-old woman (G1P0) presents to you for her first prenatal visit. She mentions she plans to take a cruise through the Eastern Caribbean in 2 weeks. Following the history and physical examination, what should you tell this patient?
Perinatal counseling: Limiting exposure is best
As mentioned, there is currently no treatment, prophylactic medication, or vaccination for Zika virus infection. Because of the virus’s significant associations with adverse pregnancy outcomes, birth defects, and fetal loss, the CDC has issued a travel advisory urging pregnant women to avoid travel to areas when Zika virus infection is prevalent. Currently, Zika virus outbreaks are occurring throughout South and Central America, the Pacific Islands, and Africa, and the infection is expected to spread, mainly due to international air travel. If travel to these areas is inevitable, women should take rigorous precautions to avoid exposure to mosquito bites and infection (TABLE 2).
If a woman was infected with laboratory-confirmed Zika virus infection in a prior pregnancy, she should not be at risk for congenital infection during her next pregnancy. This is mainly because the period of viremia is short-lived and lasts approximately 5 to 7 days.2
Further, based on documented sexual transmission of the virus, pregnant women should abstain from sexual activity or should consistently and correctly use condoms with partners who have Zika virus infection or exposure to the virus until further evidence is available.
Stay informed
Zika virus infection is now pandemic; it has evolved from an isolated disease of the tropics to one that is sweeping the Western hemisphere. It is being reported daily in new locations around the world. Given the unsettling association of Zika virus infection with birth defects, careful obstetric surveillance of exposed or symptomatic patients is imperative. Clinicians must carefully screen patients with potential risk of exposure and be prepared to offer appropriate perinatal counseling and diagnostic testing during pregnancy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Dyer O. Zika virus spreads across Americas as concerns mount over birth defects. BMJ. 2015;351:h6983.
- Centers for Disease Control and Prevention. Zika virus. Atlanta, GA: US Dept of Health and Human Services; 2015. http://www.cdc.gov/zika/index.html. Accessed February 12, 2016.
- Bogoch II, Brady OJ, Kraemer MU, et al. Anticipating the international spread of Zika virus from Brazil. Lancet. 2016;387(10016):335–336.
- Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536–2543.
- Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill. 2014;19(13):pii:20751.
- Centers for Disease Control and Prevention. Zika virus disease in the United States, 2015–2016. http://www.cdc.gov/zika/geo/united-states.html. Accessed February 12, 2016.
- Foy BD, Kobylinski KC, Chilson Foy JL, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17(5):880–882.
- Dallas County Health and Human Services. DCHHS reports first Zika virus case in Dallas County acquired through sexual transmission. http://www.dallascounty.org/department/hhs /press/documents/PR2-2-16DCHHSReportsFirstCaseofZikaVirusThroughSexualTransmission.pdf. Accessed February 3, 2016.
- Ministry of Health, Manuatu Hauora. Zika virus. http://www.health.govt.nz/our-work/diseases-and-conditions/zika -virus. Accessed January 13, 2016.
- Oehler E, Watrin L, Larre P, et al. Zika virus infection complicated by Guillain-Barre syndrome—case report, French Polynesia, December 2013. Euro Surveill. 2014;19:4–6.
- Centers for Disease Control and Prevention. Zika virus: transmission. http://www.cdc.gov/zika/transmission/index.html. Accessed January 20, 2016.
- Petersen EE, Staples JE, Meaney-Delamn, D et al. Interim guidelines for pregnant women during a Zika virus outbreak—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30–33.
- Oduyebo T, Petersen EE, Rasmussen SA, et al. Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(5):122–127.
- Pan American Health Organization, World Health Organization. Epidemiological alert: neurological syndrome, congenital malformations, and Zika virus infection. Implications for public health in the Americas. December 1,2015. http://www.paho.org/hq/index.php?option=com_doc man&task=doc_view&Itemid=270&gid=32405&lang=en. Accessed January 13, 2016.
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus epidemic in the Americas: potential associations with microcephaly and Guillain-Barré syndrome. December 10, 2015. http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association -with-microcephaly-rapid-risk-assessment.pdf. Accessed January 13, 2016.
- Schuler-Faccini L, Ribeiro EM, Feitosa IM, et al; Brazilian Medical Genetics Society—Zika Embryopathy Task Force. Possible association between Zika virus infection and microcephaly—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(3):59–62.
- Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol. 2016;47(1):6–7.
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus epidemic in the Americas: potential associations with microcephaly and Guillain-Barré syndrome. December 10, 2015. http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association.
Zika virus infection in the news
- CDC: Zika virus disease cases by US state or territory, updated periodically
- CDC: Q&As for ObGyns on pregnant women and Zika virus, 2/9/16
- CDC: Zika virus infection among US pregnant travelers, 2/26/16
- CDC: Interim guidelines for health care providers caring for infants and children with possible Zika virus infection, 2/19/16
- SMFM statement: Ultrasound screening for fetal microcephaly following Zika virus exposure, 2/16/16
- FDA approves first Zika diagnostic test for commercial use. Newsweek, 2/26/16
- NIH accelerates timeline for human trials of Zika vaccine. The Washington Post, 2/17/16
- Patient resource: Zika virus and pregnancy fact sheet from MotherToBaby.org
- Zika virus article collection from New England Journal of Medicine
- Zika infection diagnosed in 18 pregnant US women who traveled to Zika-affected areas
- FDA grants emergency approval to new 3-in-1 lab test for Zika
- ACOG Practice Advisory: Updated interim guidance for care of women of reproductive age during a Zika virus outbreak, 3/31/16
- MMWR: Patterns in Zika virus testing and infection, 4/22/16
- What insect repellents are safe during pregnancy? 5/19/16
- Zika virus and complications: Q&A from WHO, 5/31/16
- WHO strengthens guidelines to prevent sexual transmission of Zika virus, 5/31/16
- Ultrasound screening for fetal microcephaly following Zika virus exposure (from AJOG), 6/1/16
- CDC: Interim guidance for interpretation of Zika virus antibody test results, 6/3/16
- First Zika vaccine to begin testing in human trials, The Washington Post, 6/20/16
- NIH launches the Zika in Infants and Pregnancy (ZIP) international study, 6/21/16
CASE 1: Pregnant traveler asks: Should I be tested for Zika virus?
A 28-year-old Hispanic woman (G3P2) at 15 weeks’ gestation visits your office for a routine prenatal care appointment. She reports having returned from a 3-week holiday in Brazil 2 days ago, and she is concerned about having experienced fever, malaise, arthralgias, and a disseminated erythematous rash. She has since heard about the Zika virus and asks you if she and her baby are in danger and whether she should be tested for the disease.
What should you tell this patient?
The Zika virus is an RNA Flavivirus, transmitted primarily by the Aedes aegypti mosquito.1 This virus is closely related to the organisms that cause dengue fever, yellow fever, chikungunya infection, and West Nile infection. By feeding on infected prey, mosquitoes can transmit the virus to humans through bites. They breed near pools of stagnant water, can survive both indoors and outdoors, and prefer to be near people. These mosquitoes bite mostly during daylight hours, so it is essential that people use insect repellent throughout the day while in endemic areas.2 These mosquitoes live only in tropical regions; however, the Aedes albopictus mosquito, also known as the Asian tiger mosquito, lives in temperate regions and can transmit the Zika virus as well3 (FIGURE 1).
| FIGURE 1 Aedes aegypti and Aedes albopictus mosquitoes | ||
 |  | |
Aedes aegypti (left) and Aedes albopictus (right) mosquitoes. Aedes mosquitoes are the main transmission vector for the Zika virus. | ||
|
The Zika virus was first discovered in 1947 when it was isolated from a rhesus monkey in Uganda. It subsequently spread to Southeast Asia and eventually caused major outbreaks in the Yap Islands of Micronesia (2007)4 and French Polynesia (2013).5 In 2015, local transmission of the Zika virus infection was noted in Brazil, and, most recently, a pandemic of Zika virus infection has occurred throughout South America, Central America, and the Caribbean islands. To date, local mosquito-borne virus transmission has not occurred in the continental United States, although at least 82 cases acquired during travel to infected areas have been reported.6
Additionally, there have been rare cases involving spread of this virus from infected blood transfusions and through sexual contact.7 In February 2016, the first case of locally acquired Zika virus infection was reported in Texas following sexual transmission of the disease.8
Clinical manifestations of Zika virus infection
Eighty percent of patients infected with Zika virus remain asymptomatic. The illness is short-lived, occurring 2 to 12 days following the mosquito bite, and infected individuals usually do not require hospitalization or experience serious morbidity. When symptoms are present, they typically include low-grade fever (37.8° to 38.5°C), maculopapular rash, arthralgias of the hands and feet, and nonpurulent conjunctivitis. Patients also may experience headache, retro-orbital pain, myalgia, and, rarely, abdominal pain, nausea, vomiting, diarrhea, ulcerations of mucous membranes, and pruritus.9 Guillain-Barré syndrome has been reported in association with Zika virus infection10; however, a definitive cause-effect relationship has not been proven.
If a pregnant woman is infected with the Zika virus, perinatal transmission can occur, either through uteroplacental transmission or vertically from mother to child at the time of delivery. Zika virus RNA has been detected in blood, amniotic fluid, semen, saliva, cerebrospinal fluid, urine, and breast milk. Although the virus has been shown to be present in breast milk, there has been no evidence of viral replication in milk or reported transmission in breastfed infants.11 Pregnant women are not known to have increased susceptibility to Zika virus infection when compared with the general population, and there is no evidence to suggest pregnant women will have a more serious illness if infected.
The Zika virus has been strongly associated with congenital microcephaly and fetal loss among women infected during pregnancy.12 Following the recent large outbreak in Brazil, an alarmingly high number of Brazilian newborns with microcephaly have been observed. The total now exceeds 4,000. Because of these ominous findings, fetuses and neonates born to women with a history of infection should be evaluated for adverse effects of congenital infection.
Management strategies for Zika virus exposure during pregnancy
The incidence of Zika virus infection during pregnancy remains unknown. However, a pregnant woman may be infected in any trimester, and maternal-fetal transmission of the virus can occur throughout pregnancy. If a patient is pregnant and has travelled to areas of Zika virus transmission, or has had unprotected sexual contact with a partner who has had exposure, she should be carefully screened with a detailed review of systems and ultrasonography to evaluate for fetal microcephaly or intracranial calcifications. The US Centers for Disease Control and Prevention (CDC) initially recommended that, if a patient exhibited 2 or more symptoms consistent with Zika virus infection within 2 weeks of exposure or if sonographic evidence revealed fetal microcephaly or intracranial calcifications, she should be tested for Zika virus infection.11
More recently, the CDC issued new guidelines recommending that even asymptomatic women with exposure have serologic testing for infection and that all exposed women undergo serial ultrasound assessments.13 The CDC also recommends offering retesting in the mid second trimester for women who were exposed very early in gestation.
The best diagnostic test for infection is reverse transcriptase-polymerase chain reaction (RT-PCR), and, ideally, it should be completed within 4 days of symptom onset. Beyond 4 days after symptom onset, testing for Zika virus immunoglobulin M (IgM)-specific antibody and neutralizing antibody should be performed in addition to the RT-PCR test. At times, interpretation of antibody testing can be problematic because cross-reaction with related arboviruses is common. Moreover, Zika viremia decreases rapidly over time; therefore, if serum is collected even 5 to 7 days after symptom onset, a negative test does not definitively exclude infection (TABLE 1).
In the United States, local health departments should be contacted to facilitate testing, as the tests described above are not currently commercially available. If the local health department is unable to perform this testing, clinicians should contact the CDC’s Division of Vector-Borne Diseases (telephone: 1-970-221-6400) or visit their website (http://www.cdc.gov/ncezid/dvbd/specimensub/arboviral-shipping.html) for detailed instructions on specimen submission.
Testing is not indicated for women without a history of travel to areas where Zika virus infection is endemic or without a history of unprotected sexual contact with someone who has been exposed to the infection.
Following the delivery of a live infant to an infected or exposed mother, detailed histopathologic evaluation of the placenta and umbilical cord should be performed. Frozen sections of placental and cord tissue should be tested for Zika virus RNA, and cord serum should be tested for Zika and dengue virus IgM and neutralizing antibodies. In cases of fetal loss in the setting of relevant travel history or exposure (particularly maternal symptoms or sonographic evidence of microcephaly), RT-PCR testing and immunohistochemistry should be completed on fetal tissues, umbilical cord, and placenta.2
Treatment is supportive
At present, there is no vaccine for the Zika virus, and no hyperimmune globulin or anti‑ viral chemotherapy is available. Treatment is therefore supportive. Patients should be encouraged to rest and maintain hydration. The preferred antipyretic and analgesic is acetaminophen (650 mg orally every 6 hours or 1,000 mg orally every 8 hours). Aspirin should be avoided until dengue infection has been ruled out because of the related risk of bleeding with hemorrhagic fever. Nonsteroidal anti-inflammatory drugs should be avoided in the second half of pregnancy because of their effect on fetal renal blood flow (oligohydramnios) and stricture of the ductus arteriosus.
CASE 1 Continued
Given this patient’s recent travel, exposure to mosquito-borne illness, and clinical manifestations of malaise, rash, and joint pain, you proceed with serologic testing. The RT-PCR test is positive for Zika virus.
What should be the next step in the management of this patient?
Prenatal diagnosis and fetal surveillance
The recent epidemic of microcephaly and poor pregnancy outcomes reported in Brazil has been alarming and demonstrates an almost 20-fold increase in incidence of this condition between 2014–2015.14 Careful surveillance is needed for this birth defect and other poor pregnancy outcomes in association with the Zika virus. To date, a direct causal relationship between Zika virus infection and microcephaly has not been unequivocally established15; however; these microcephaly cases have yet to be attributed to any other cause (FIGURE 2)
| FIGURE 2 Microcephaly: associated with Zika virus infection in pregnancy |
 |
Illustration depicts a child with congenital microcephaly (left) and one with head circumference within the mean SD (right). |
Following the outbreak in Brazil, a task force and registry were established to investigate microcephaly and other birth defects associated with Zika virus infection. In one small investigation, 35 cases of microcephaly were reported, and 71% of the infants were seriously affected (head circumference >3 SD below the mean). Fifty percent of babies had at least one neurologic abnormality, and, of the 27 patients who had neuroimaging studies, all had distinct abnormalities, including widespread brain calcifications and cell migration abnormalities, such as lissencephaly, pachgyria, and ventriculomegaly due to cortical atrophy.16
In addition to microcephaly, fetal ultrasound monitoring has revealed focal brain abnormalities, such as asymmetric cerebral hemispheres, ventriculomegaly, displacement of the midline, failure to visualize the corpus callosum, failure of thalamic development, and the presence of intraocular and brain calcifications.17
In collaboration with the CDC, the American College of Obstetricians and Gynecologists and the Society for Maternal Fetal-Medicine have developed guidelines to monitor fetal growth in women with laboratory evidence of Zika virus infection.18 Recommendations include having a detailed anatomy ultrasound and serial growth sonograms every 3 to 4 weeks, along with referral to a maternal-fetal medicine or infectious disease specialist.
If the pregnancy is beyond 15 weeks’ gestational age, an amniocentesis should be performed in symptomatic patients and in those with abnormal ultrasound findings. Amniotic fluid should be tested for Zika virus with RT-PCR (FIGURE 3).12 The sensitivity and specificity of amniotic fluid RT-PCR in detecting congenital infection, as well as the predictive value of a fetal anomaly, remain unknown at this time. For this reason, a patient must be counseled carefully regarding the benefits of confirming intrauterine infection versus the slight risks of premature rupture of membranes, infection, and pregnancy loss related to amniocentesis.
Once diagnosed, microcephaly cannot be “fixed.” However, pregnancy termination is an option that some parents may choose once they are aware of the diagnosis and prognosis of microcephaly. Moreover, even for parents who would not choose abortion, there may be considerable value in being prepared for the care of a severely disabled child. Microcephaly has many possible causes, Zika virus infection being just one. Others include genetic syndromes and other congenital infections, such as cytomegalovirus (CMV) infection and toxoplasmosis. Amniocentesis therefore may help the clinician sort through these causes. For both CMV infection and toxoplasmosis, certain antenatal treatments may be helpful in lessening the severity of fetal injury.
CASE 2 Pregnant patient has travel plans
A 34-year-old woman (G1P0) presents to you for her first prenatal visit. She mentions she plans to take a cruise through the Eastern Caribbean in 2 weeks. Following the history and physical examination, what should you tell this patient?
Perinatal counseling: Limiting exposure is best
As mentioned, there is currently no treatment, prophylactic medication, or vaccination for Zika virus infection. Because of the virus’s significant associations with adverse pregnancy outcomes, birth defects, and fetal loss, the CDC has issued a travel advisory urging pregnant women to avoid travel to areas when Zika virus infection is prevalent. Currently, Zika virus outbreaks are occurring throughout South and Central America, the Pacific Islands, and Africa, and the infection is expected to spread, mainly due to international air travel. If travel to these areas is inevitable, women should take rigorous precautions to avoid exposure to mosquito bites and infection (TABLE 2).
If a woman was infected with laboratory-confirmed Zika virus infection in a prior pregnancy, she should not be at risk for congenital infection during her next pregnancy. This is mainly because the period of viremia is short-lived and lasts approximately 5 to 7 days.2
Further, based on documented sexual transmission of the virus, pregnant women should abstain from sexual activity or should consistently and correctly use condoms with partners who have Zika virus infection or exposure to the virus until further evidence is available.
Stay informed
Zika virus infection is now pandemic; it has evolved from an isolated disease of the tropics to one that is sweeping the Western hemisphere. It is being reported daily in new locations around the world. Given the unsettling association of Zika virus infection with birth defects, careful obstetric surveillance of exposed or symptomatic patients is imperative. Clinicians must carefully screen patients with potential risk of exposure and be prepared to offer appropriate perinatal counseling and diagnostic testing during pregnancy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Zika virus infection in the news
- CDC: Zika virus disease cases by US state or territory, updated periodically
- CDC: Q&As for ObGyns on pregnant women and Zika virus, 2/9/16
- CDC: Zika virus infection among US pregnant travelers, 2/26/16
- CDC: Interim guidelines for health care providers caring for infants and children with possible Zika virus infection, 2/19/16
- SMFM statement: Ultrasound screening for fetal microcephaly following Zika virus exposure, 2/16/16
- FDA approves first Zika diagnostic test for commercial use. Newsweek, 2/26/16
- NIH accelerates timeline for human trials of Zika vaccine. The Washington Post, 2/17/16
- Patient resource: Zika virus and pregnancy fact sheet from MotherToBaby.org
- Zika virus article collection from New England Journal of Medicine
- Zika infection diagnosed in 18 pregnant US women who traveled to Zika-affected areas
- FDA grants emergency approval to new 3-in-1 lab test for Zika
- ACOG Practice Advisory: Updated interim guidance for care of women of reproductive age during a Zika virus outbreak, 3/31/16
- MMWR: Patterns in Zika virus testing and infection, 4/22/16
- What insect repellents are safe during pregnancy? 5/19/16
- Zika virus and complications: Q&A from WHO, 5/31/16
- WHO strengthens guidelines to prevent sexual transmission of Zika virus, 5/31/16
- Ultrasound screening for fetal microcephaly following Zika virus exposure (from AJOG), 6/1/16
- CDC: Interim guidance for interpretation of Zika virus antibody test results, 6/3/16
- First Zika vaccine to begin testing in human trials, The Washington Post, 6/20/16
- NIH launches the Zika in Infants and Pregnancy (ZIP) international study, 6/21/16
CASE 1: Pregnant traveler asks: Should I be tested for Zika virus?
A 28-year-old Hispanic woman (G3P2) at 15 weeks’ gestation visits your office for a routine prenatal care appointment. She reports having returned from a 3-week holiday in Brazil 2 days ago, and she is concerned about having experienced fever, malaise, arthralgias, and a disseminated erythematous rash. She has since heard about the Zika virus and asks you if she and her baby are in danger and whether she should be tested for the disease.
What should you tell this patient?
The Zika virus is an RNA Flavivirus, transmitted primarily by the Aedes aegypti mosquito.1 This virus is closely related to the organisms that cause dengue fever, yellow fever, chikungunya infection, and West Nile infection. By feeding on infected prey, mosquitoes can transmit the virus to humans through bites. They breed near pools of stagnant water, can survive both indoors and outdoors, and prefer to be near people. These mosquitoes bite mostly during daylight hours, so it is essential that people use insect repellent throughout the day while in endemic areas.2 These mosquitoes live only in tropical regions; however, the Aedes albopictus mosquito, also known as the Asian tiger mosquito, lives in temperate regions and can transmit the Zika virus as well3 (FIGURE 1).
| FIGURE 1 Aedes aegypti and Aedes albopictus mosquitoes | ||
 |  | |
Aedes aegypti (left) and Aedes albopictus (right) mosquitoes. Aedes mosquitoes are the main transmission vector for the Zika virus. | ||
|
The Zika virus was first discovered in 1947 when it was isolated from a rhesus monkey in Uganda. It subsequently spread to Southeast Asia and eventually caused major outbreaks in the Yap Islands of Micronesia (2007)4 and French Polynesia (2013).5 In 2015, local transmission of the Zika virus infection was noted in Brazil, and, most recently, a pandemic of Zika virus infection has occurred throughout South America, Central America, and the Caribbean islands. To date, local mosquito-borne virus transmission has not occurred in the continental United States, although at least 82 cases acquired during travel to infected areas have been reported.6
Additionally, there have been rare cases involving spread of this virus from infected blood transfusions and through sexual contact.7 In February 2016, the first case of locally acquired Zika virus infection was reported in Texas following sexual transmission of the disease.8
Clinical manifestations of Zika virus infection
Eighty percent of patients infected with Zika virus remain asymptomatic. The illness is short-lived, occurring 2 to 12 days following the mosquito bite, and infected individuals usually do not require hospitalization or experience serious morbidity. When symptoms are present, they typically include low-grade fever (37.8° to 38.5°C), maculopapular rash, arthralgias of the hands and feet, and nonpurulent conjunctivitis. Patients also may experience headache, retro-orbital pain, myalgia, and, rarely, abdominal pain, nausea, vomiting, diarrhea, ulcerations of mucous membranes, and pruritus.9 Guillain-Barré syndrome has been reported in association with Zika virus infection10; however, a definitive cause-effect relationship has not been proven.
If a pregnant woman is infected with the Zika virus, perinatal transmission can occur, either through uteroplacental transmission or vertically from mother to child at the time of delivery. Zika virus RNA has been detected in blood, amniotic fluid, semen, saliva, cerebrospinal fluid, urine, and breast milk. Although the virus has been shown to be present in breast milk, there has been no evidence of viral replication in milk or reported transmission in breastfed infants.11 Pregnant women are not known to have increased susceptibility to Zika virus infection when compared with the general population, and there is no evidence to suggest pregnant women will have a more serious illness if infected.
The Zika virus has been strongly associated with congenital microcephaly and fetal loss among women infected during pregnancy.12 Following the recent large outbreak in Brazil, an alarmingly high number of Brazilian newborns with microcephaly have been observed. The total now exceeds 4,000. Because of these ominous findings, fetuses and neonates born to women with a history of infection should be evaluated for adverse effects of congenital infection.
Management strategies for Zika virus exposure during pregnancy
The incidence of Zika virus infection during pregnancy remains unknown. However, a pregnant woman may be infected in any trimester, and maternal-fetal transmission of the virus can occur throughout pregnancy. If a patient is pregnant and has travelled to areas of Zika virus transmission, or has had unprotected sexual contact with a partner who has had exposure, she should be carefully screened with a detailed review of systems and ultrasonography to evaluate for fetal microcephaly or intracranial calcifications. The US Centers for Disease Control and Prevention (CDC) initially recommended that, if a patient exhibited 2 or more symptoms consistent with Zika virus infection within 2 weeks of exposure or if sonographic evidence revealed fetal microcephaly or intracranial calcifications, she should be tested for Zika virus infection.11
More recently, the CDC issued new guidelines recommending that even asymptomatic women with exposure have serologic testing for infection and that all exposed women undergo serial ultrasound assessments.13 The CDC also recommends offering retesting in the mid second trimester for women who were exposed very early in gestation.
The best diagnostic test for infection is reverse transcriptase-polymerase chain reaction (RT-PCR), and, ideally, it should be completed within 4 days of symptom onset. Beyond 4 days after symptom onset, testing for Zika virus immunoglobulin M (IgM)-specific antibody and neutralizing antibody should be performed in addition to the RT-PCR test. At times, interpretation of antibody testing can be problematic because cross-reaction with related arboviruses is common. Moreover, Zika viremia decreases rapidly over time; therefore, if serum is collected even 5 to 7 days after symptom onset, a negative test does not definitively exclude infection (TABLE 1).
In the United States, local health departments should be contacted to facilitate testing, as the tests described above are not currently commercially available. If the local health department is unable to perform this testing, clinicians should contact the CDC’s Division of Vector-Borne Diseases (telephone: 1-970-221-6400) or visit their website (http://www.cdc.gov/ncezid/dvbd/specimensub/arboviral-shipping.html) for detailed instructions on specimen submission.
Testing is not indicated for women without a history of travel to areas where Zika virus infection is endemic or without a history of unprotected sexual contact with someone who has been exposed to the infection.
Following the delivery of a live infant to an infected or exposed mother, detailed histopathologic evaluation of the placenta and umbilical cord should be performed. Frozen sections of placental and cord tissue should be tested for Zika virus RNA, and cord serum should be tested for Zika and dengue virus IgM and neutralizing antibodies. In cases of fetal loss in the setting of relevant travel history or exposure (particularly maternal symptoms or sonographic evidence of microcephaly), RT-PCR testing and immunohistochemistry should be completed on fetal tissues, umbilical cord, and placenta.2
Treatment is supportive
At present, there is no vaccine for the Zika virus, and no hyperimmune globulin or anti‑ viral chemotherapy is available. Treatment is therefore supportive. Patients should be encouraged to rest and maintain hydration. The preferred antipyretic and analgesic is acetaminophen (650 mg orally every 6 hours or 1,000 mg orally every 8 hours). Aspirin should be avoided until dengue infection has been ruled out because of the related risk of bleeding with hemorrhagic fever. Nonsteroidal anti-inflammatory drugs should be avoided in the second half of pregnancy because of their effect on fetal renal blood flow (oligohydramnios) and stricture of the ductus arteriosus.
CASE 1 Continued
Given this patient’s recent travel, exposure to mosquito-borne illness, and clinical manifestations of malaise, rash, and joint pain, you proceed with serologic testing. The RT-PCR test is positive for Zika virus.
What should be the next step in the management of this patient?
Prenatal diagnosis and fetal surveillance
The recent epidemic of microcephaly and poor pregnancy outcomes reported in Brazil has been alarming and demonstrates an almost 20-fold increase in incidence of this condition between 2014–2015.14 Careful surveillance is needed for this birth defect and other poor pregnancy outcomes in association with the Zika virus. To date, a direct causal relationship between Zika virus infection and microcephaly has not been unequivocally established15; however; these microcephaly cases have yet to be attributed to any other cause (FIGURE 2)
| FIGURE 2 Microcephaly: associated with Zika virus infection in pregnancy |
 |
Illustration depicts a child with congenital microcephaly (left) and one with head circumference within the mean SD (right). |
Following the outbreak in Brazil, a task force and registry were established to investigate microcephaly and other birth defects associated with Zika virus infection. In one small investigation, 35 cases of microcephaly were reported, and 71% of the infants were seriously affected (head circumference >3 SD below the mean). Fifty percent of babies had at least one neurologic abnormality, and, of the 27 patients who had neuroimaging studies, all had distinct abnormalities, including widespread brain calcifications and cell migration abnormalities, such as lissencephaly, pachgyria, and ventriculomegaly due to cortical atrophy.16
In addition to microcephaly, fetal ultrasound monitoring has revealed focal brain abnormalities, such as asymmetric cerebral hemispheres, ventriculomegaly, displacement of the midline, failure to visualize the corpus callosum, failure of thalamic development, and the presence of intraocular and brain calcifications.17
In collaboration with the CDC, the American College of Obstetricians and Gynecologists and the Society for Maternal Fetal-Medicine have developed guidelines to monitor fetal growth in women with laboratory evidence of Zika virus infection.18 Recommendations include having a detailed anatomy ultrasound and serial growth sonograms every 3 to 4 weeks, along with referral to a maternal-fetal medicine or infectious disease specialist.
If the pregnancy is beyond 15 weeks’ gestational age, an amniocentesis should be performed in symptomatic patients and in those with abnormal ultrasound findings. Amniotic fluid should be tested for Zika virus with RT-PCR (FIGURE 3).12 The sensitivity and specificity of amniotic fluid RT-PCR in detecting congenital infection, as well as the predictive value of a fetal anomaly, remain unknown at this time. For this reason, a patient must be counseled carefully regarding the benefits of confirming intrauterine infection versus the slight risks of premature rupture of membranes, infection, and pregnancy loss related to amniocentesis.
Once diagnosed, microcephaly cannot be “fixed.” However, pregnancy termination is an option that some parents may choose once they are aware of the diagnosis and prognosis of microcephaly. Moreover, even for parents who would not choose abortion, there may be considerable value in being prepared for the care of a severely disabled child. Microcephaly has many possible causes, Zika virus infection being just one. Others include genetic syndromes and other congenital infections, such as cytomegalovirus (CMV) infection and toxoplasmosis. Amniocentesis therefore may help the clinician sort through these causes. For both CMV infection and toxoplasmosis, certain antenatal treatments may be helpful in lessening the severity of fetal injury.
CASE 2 Pregnant patient has travel plans
A 34-year-old woman (G1P0) presents to you for her first prenatal visit. She mentions she plans to take a cruise through the Eastern Caribbean in 2 weeks. Following the history and physical examination, what should you tell this patient?
Perinatal counseling: Limiting exposure is best
As mentioned, there is currently no treatment, prophylactic medication, or vaccination for Zika virus infection. Because of the virus’s significant associations with adverse pregnancy outcomes, birth defects, and fetal loss, the CDC has issued a travel advisory urging pregnant women to avoid travel to areas when Zika virus infection is prevalent. Currently, Zika virus outbreaks are occurring throughout South and Central America, the Pacific Islands, and Africa, and the infection is expected to spread, mainly due to international air travel. If travel to these areas is inevitable, women should take rigorous precautions to avoid exposure to mosquito bites and infection (TABLE 2).
If a woman was infected with laboratory-confirmed Zika virus infection in a prior pregnancy, she should not be at risk for congenital infection during her next pregnancy. This is mainly because the period of viremia is short-lived and lasts approximately 5 to 7 days.2
Further, based on documented sexual transmission of the virus, pregnant women should abstain from sexual activity or should consistently and correctly use condoms with partners who have Zika virus infection or exposure to the virus until further evidence is available.
Stay informed
Zika virus infection is now pandemic; it has evolved from an isolated disease of the tropics to one that is sweeping the Western hemisphere. It is being reported daily in new locations around the world. Given the unsettling association of Zika virus infection with birth defects, careful obstetric surveillance of exposed or symptomatic patients is imperative. Clinicians must carefully screen patients with potential risk of exposure and be prepared to offer appropriate perinatal counseling and diagnostic testing during pregnancy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Dyer O. Zika virus spreads across Americas as concerns mount over birth defects. BMJ. 2015;351:h6983.
- Centers for Disease Control and Prevention. Zika virus. Atlanta, GA: US Dept of Health and Human Services; 2015. http://www.cdc.gov/zika/index.html. Accessed February 12, 2016.
- Bogoch II, Brady OJ, Kraemer MU, et al. Anticipating the international spread of Zika virus from Brazil. Lancet. 2016;387(10016):335–336.
- Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536–2543.
- Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill. 2014;19(13):pii:20751.
- Centers for Disease Control and Prevention. Zika virus disease in the United States, 2015–2016. http://www.cdc.gov/zika/geo/united-states.html. Accessed February 12, 2016.
- Foy BD, Kobylinski KC, Chilson Foy JL, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17(5):880–882.
- Dallas County Health and Human Services. DCHHS reports first Zika virus case in Dallas County acquired through sexual transmission. http://www.dallascounty.org/department/hhs /press/documents/PR2-2-16DCHHSReportsFirstCaseofZikaVirusThroughSexualTransmission.pdf. Accessed February 3, 2016.
- Ministry of Health, Manuatu Hauora. Zika virus. http://www.health.govt.nz/our-work/diseases-and-conditions/zika -virus. Accessed January 13, 2016.
- Oehler E, Watrin L, Larre P, et al. Zika virus infection complicated by Guillain-Barre syndrome—case report, French Polynesia, December 2013. Euro Surveill. 2014;19:4–6.
- Centers for Disease Control and Prevention. Zika virus: transmission. http://www.cdc.gov/zika/transmission/index.html. Accessed January 20, 2016.
- Petersen EE, Staples JE, Meaney-Delamn, D et al. Interim guidelines for pregnant women during a Zika virus outbreak—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30–33.
- Oduyebo T, Petersen EE, Rasmussen SA, et al. Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(5):122–127.
- Pan American Health Organization, World Health Organization. Epidemiological alert: neurological syndrome, congenital malformations, and Zika virus infection. Implications for public health in the Americas. December 1,2015. http://www.paho.org/hq/index.php?option=com_doc man&task=doc_view&Itemid=270&gid=32405&lang=en. Accessed January 13, 2016.
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus epidemic in the Americas: potential associations with microcephaly and Guillain-Barré syndrome. December 10, 2015. http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association -with-microcephaly-rapid-risk-assessment.pdf. Accessed January 13, 2016.
- Schuler-Faccini L, Ribeiro EM, Feitosa IM, et al; Brazilian Medical Genetics Society—Zika Embryopathy Task Force. Possible association between Zika virus infection and microcephaly—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(3):59–62.
- Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol. 2016;47(1):6–7.
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus epidemic in the Americas: potential associations with microcephaly and Guillain-Barré syndrome. December 10, 2015. http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association.
- Dyer O. Zika virus spreads across Americas as concerns mount over birth defects. BMJ. 2015;351:h6983.
- Centers for Disease Control and Prevention. Zika virus. Atlanta, GA: US Dept of Health and Human Services; 2015. http://www.cdc.gov/zika/index.html. Accessed February 12, 2016.
- Bogoch II, Brady OJ, Kraemer MU, et al. Anticipating the international spread of Zika virus from Brazil. Lancet. 2016;387(10016):335–336.
- Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536–2543.
- Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill. 2014;19(13):pii:20751.
- Centers for Disease Control and Prevention. Zika virus disease in the United States, 2015–2016. http://www.cdc.gov/zika/geo/united-states.html. Accessed February 12, 2016.
- Foy BD, Kobylinski KC, Chilson Foy JL, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17(5):880–882.
- Dallas County Health and Human Services. DCHHS reports first Zika virus case in Dallas County acquired through sexual transmission. http://www.dallascounty.org/department/hhs /press/documents/PR2-2-16DCHHSReportsFirstCaseofZikaVirusThroughSexualTransmission.pdf. Accessed February 3, 2016.
- Ministry of Health, Manuatu Hauora. Zika virus. http://www.health.govt.nz/our-work/diseases-and-conditions/zika -virus. Accessed January 13, 2016.
- Oehler E, Watrin L, Larre P, et al. Zika virus infection complicated by Guillain-Barre syndrome—case report, French Polynesia, December 2013. Euro Surveill. 2014;19:4–6.
- Centers for Disease Control and Prevention. Zika virus: transmission. http://www.cdc.gov/zika/transmission/index.html. Accessed January 20, 2016.
- Petersen EE, Staples JE, Meaney-Delamn, D et al. Interim guidelines for pregnant women during a Zika virus outbreak—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30–33.
- Oduyebo T, Petersen EE, Rasmussen SA, et al. Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(5):122–127.
- Pan American Health Organization, World Health Organization. Epidemiological alert: neurological syndrome, congenital malformations, and Zika virus infection. Implications for public health in the Americas. December 1,2015. http://www.paho.org/hq/index.php?option=com_doc man&task=doc_view&Itemid=270&gid=32405&lang=en. Accessed January 13, 2016.
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus epidemic in the Americas: potential associations with microcephaly and Guillain-Barré syndrome. December 10, 2015. http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association -with-microcephaly-rapid-risk-assessment.pdf. Accessed January 13, 2016.
- Schuler-Faccini L, Ribeiro EM, Feitosa IM, et al; Brazilian Medical Genetics Society—Zika Embryopathy Task Force. Possible association between Zika virus infection and microcephaly—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(3):59–62.
- Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol. 2016;47(1):6–7.
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus epidemic in the Americas: potential associations with microcephaly and Guillain-Barré syndrome. December 10, 2015. http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association.
In this Article
- Management strategies for pregnant patients with Zika virus exposure
- Fetal surveillance
- Perinatal counseling on exposure prevention
- Algorithm for evaluation and management
Cardiorenal Syndrome Type 1: Renal Dysfunction in Acute Decompensated Heart Failure
From the Cardiovascular Division, Department of Internal Medicine, University of Minnesota, Minneapolis, MN.
Abstract
- Objective: To present a review of cardiorenal syndrome type 1 (CRS1).
- Methods: Review of the literature.
- Results: Acute kidney injury occurs in approximately one-third of patients with acute decompensated heart failure (ADHF) and the resultant condition was named CRS1. A growing body of literature shows CRS1 patients are at high risk for poor outcomes, and thus there is an urgent need to understand the pathophysiology and subsequently develop effective treatments. In this review we discuss prevalence, proposed pathophysiology including hemodynamic and nonhemodynamic factors, prognosticating variables, data for different treatment strategies, and ongoing clinical trials and highlight questions and problems physicians will face moving forward with this common and challenging condition.
- Conclusion: Further research is needed to understand the pathophysiology of this complex clinical entity and to develop effective treatments.
Acute decompensated heart failure (ADHF) is an epidemic facing physicians throughout the world. In the United States alone, ADHF accounts for over 1 million hospitalizations annually, with costs in 2012 reaching $30.7 billion [1]. Despite the advances in chronic heart failure management, ADHF continues to be associated with poor outcomes as exemplified by 30-day readmission rates of over 20% and in-hospital mortality rates of 5% to 6%, both of which have not significantly improved over the past 20 years [2,3]. One of the strongest predictors of adverse outcomes in ADHF is renal dysfunction. An analysis from the Acute Decompensated Heart Failure National Registry (ADHERE) revealed the combination of renal dysfunction (creatinine > 2.75 mg/dL and blood urea nitrogen (BUN) > 43 mg/dL) and hypotension (systolic blood pressure (SBP) < 115 mm Hg) upon admission was associated with an in-hospital mortality of > 20% [4]. The Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) registry documented a 16.3% in-hospital mortality when patients had a SBP < 100 mm Hg and creatinine > 2.0 mg/dL at admission [5].
The presence of acute kidney injury in the setting of ADHF is a very common occurrence and was termed cardiorenal syndrome type 1 (CRS1) [6]. The prevalence of CRS1 in single-centered studies ranged from 32% to 40% of all ADHF admissions [7,8]. If this estimate holds true throughout the United States, there would be 320,000 to 400,000 hospitalizations for CRS1 annually, highlighting the magnitude of this problem. Moreover, with the number of patients with heart failure expected to continue to rise, CRS1 will only become more prevalent in the future. In this review we discuss the prevalence, proposed pathophysiology including hemodynamic and nonhemodynamic factors, prognosticating variables, data for different treatment strategies, ongoing clinical trials, and highlight questions and problems physicians will face moving forward in this common and challenging condition.
Pathogenesis of CRS1
Hemodynamic Effects
The early hypothesis for renal dysfunction in ADHF centered on hemodynamics, as reduced cardiac output was believed to decrease renal perfusion. However, analysis of invasive hemodynamics from patients with ADHF suggested that central venous pressure (CVP) was actually a better predictor of the development of CRS1 than cardiac output. In a single-center study conducted at the Cleveland Clinic, hemodynamics from 145 patients with ADHF were evaluated and surprisingly baseline cardiac index was greater in the patients with CRS1 than patients without renal dysfunction (2.0 ± 0.8 L/min/m2 vs 1.8 ± 0.4 L/min/m2; P = 0.008). However, baseline CVP was higher in the CRS1 group (18 ± 7 mm Hg vs 12 ± 6 mm Hg; P = 0.001), and there was a heightened risk of developing CRS1 as CVP increased. In fact, 75% of the patients with a CVP of > 24 mm Hg developed renal impairment [9]. In a retrospective study of the Evaluation Study of Congestive Heart Failure and Pulmonary Arterial Catheter Effectiveness (ESCAPE) trial, the only hemodynamic parameter that correlated with baseline creatinine was CVP. However, no invasive measures predicted worsening renal function during hospitalization [10]. Finally, an experiment that used isolated canine kidneys showed increased venous pressure acutely reduced urine production. Interestingly, this relationship was dependent on arterial pressure; as arterial flow decreased smaller increases in CVP were needed to reduce urine output [11]. Together, these data suggest increased CVP plays an important role in CRS1, but imply hemodynamics alone may not fully explain the pathophysiology of CRS1.
Inflammation
As information about how hemodynamics incompletely predict renal dysfunction in ADHF became available, alternative hypotheses were investigated to gain a deeper understanding of the pathophysiology underlying CRS1. A pathological role of inflammation in CRS1 has gained attention due to recent publications. First of all, serum levels of the pro-inflammatory cytokines TNF-a and IL-6 were elevated in patients with CRS1 when compared to health controls [12]. Interestingly, Virzi et al showed that the median value of IL-6 was 5 times higher in CRS1 patients when compared to ADHF patients without renal dysfunction [13]. The negative consequences of elevated serum cytokines were demonstrated when incubation of a human cell line of monocytes with serum from CRS1 patients induced apoptosis in 81% of cells compared to just 11% of cells with control serum [12]. It is possible that cytokine-induced apoptosis could occur in other cell types in different organs in patients with CRS1, which may contribute to both cardiac and renal dysfunction. Finally, analysis from a rat model of CRS1 revealed macrophage infiltration into the kidneys and increased numbers of activated monocytes in the peripheral blood. Interestingly, monocyte/macrophage depletion using liposome clodronate prevented chronic renal dysfunction in the rat model [14]. In summary, these data suggest inflammation contributes to CRS1 pathophysiology, but more experimental data is needed to determine if there is a causal relationship.
Oxidative Stress
Very recently, oxidative stress was proposed to play a role in CRS1. Virzi et al analyzed serum levels of markers of oxidative stress and compared ADHF patients without renal impairment to CRS1 patients. The markers of oxidative stress, which included myeloperoxidase, nitric oxide, copper/zinc superoxide dismutase, and endogenous peroxidase, were all significantly higher in CRS1 patients [13]. While provocative, the tissues responsible for the generation of these molecules and the subsequent effects have not yet been fully elucidated.
Prognostication
Severity of Acute Kidney Injury
Initial publications did not document a strong link between kidney injury and poor outcomes in ADHF. Firstly, Ather et al performed a single-centered study that investigated how change in renal function defined by change in creatinine, estimated GFR, and BUN affected outcomes one year post admission for ADHF. Kidney injury defined by a change in creatinine or in estimated GFR was not associated with increased risk of mortality, but a change in BUN was associated with increased mortality in a univariate analysis [15]. Testani et al retrospectively analyzed patients from the ESCAPE trial and found worsening renal function defined by a ≥ 20% reduction in estimated GFR was not significantly associated with 180-day mortality, but there was a trend towards higher mortality (hazard ration 1.4; P = 0.11) [16]. Importantly, neither of 2 these studies assessed how severity of AKI impacted outcomes, which may have contributed to the weak relationships observed.
Diuretic Responsiveness
Voors et al performed a retrospective analysis of diuretic responsiveness in 1161 patients from the Relaxin in Acute Heart Failure (RELAX-AHF) trial. Diuretic responsiveness was defined as weight change (kg) per diuretic dose (IV furosemide and PO furosemide) over 5 days and then patients were separated into tertiles. The lowest tertile group had an approximate 20% incidence of 60-day combined end-point of death, heart failure or renal failure readmission compared to less than 10% incidence in the middle and upper tertiles. Interestingly, when the effects of worsening renal function (WRF), defined as creatinine change of ≥ 0.3 mg/dL, were examined in patients stratified by diuretic response, WRF did not offer additional prognostic information [19].
Finally, Valenete et al analyzed diuretic response in 1745 patients from the PROTECT trial (Placebo-Controlled Randomized Study of the Selective A1-Adenosine Receptor Antagonist Rolofylline for Patients Hospitalized with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function). Diuretic response was calculated using the weight change per 40 mg of furosemide, and as diuretic response declined there was increasing risk of 60-day rehospitalization and 180-day mortality rates. In fact, the lowest quintile responders had a 25% mortality rate at 180 days [20].
Emerging Biomarkers
Urine Neutrophil Gelatinase-Associated Lipocalin
Because previous studies showed urinary levels of NGAL was an earlier and more reliable marker of renal dysfunction than creatinine in AKI [21], it was studied as a possible biomarker for the development of CRS1 in ADHF. A single-centered study quantified levels of urine NGAL in 100 patients admitted with heart failure and then tracked the rates of acute kidney injury. Urine NGAL was elevated in patients that developed AKI and a cut-off value 12 ng/mL had a sensitivity of 79% and specificity of 67% for predicting CRS1 [22]. While promising, further studies are needed to better define the role of NGAL in CRS1.
Cystatin C
Cystatin C is a ubiquitously expressed cysteine protease that has a constant production rate and is freely filtered by the glomerulus without being secreted into the tubules, and has effectively prognosticated outcomes in ADHF [23]. Lassus et al showed an adjusted hazard ratio of 3.2 (2.0–5.3) for 12-month mortality when cystatin C levels were elevated. Moreover, patients with the highest tertitle of NT-proBNP and cystatin C had a 48.7% 1-year mortality. Interestingly, patients with an elevated cystatin C but normal creatinine had a 40.6% 1-year mortality compared to 12.6% for those with normal cystatin C and creatinine [24]. Furthermore, Arimoto et al showed elevated cystatin C predicted death or rehospitalization in a small cohort of ADHF patients in Japan [25]. Also, Naruse et al showed cystatin C was a better predictor of cardiac death than estimated GFR by the Modification of Diet in Renal Disease Study (MDRD) equation [26]. Finally, Manzano-Fernandez et al showed the highest tertile of cystatin C was a significant independent risk factor for 2-year death or rehospitalization while creatinine and MDRD estimates of GFR were not [27]. In agreement with Lassus et al, elevations in either 2 or 3 of cystatin C, troponin, and NT-proBNP predicted death or rehospitalization when compared to those with normal levels of these 3 markers [27]. In conclusion, cystatin C either alone or in combination with other biomarkers identifies high-risk patients.
Kidney Injury Molecule 1
Kidney injury molecule 1 (KIM-1) is a type-1 cell membrane glycoprotein expressed in regenerating proximal tubular cells but not under normal conditions [28]. Although associated with increased risk of hospitalization and mortality in chronic heart failure [29,30], elevated levels of urinary KIM-1 did not predict mortality in ADHF [31]. Further studies are needed to elucidate the utility of KIM-1 in CRS1.
Treatment Approaches
Diuretics
Loop diuretics are the main treatment for decongestion of patients with CRS1. To date, no clinical trial has compared the different loop diuretics (furosemide, bumetanide, torsemide, or ethacrynic acid) to each other, so there is no clear choice of which loop diuretic is the best. However, dosing scheme was investigated in the Dose Optimization Strategies Evaluation (DOSE) trial. In this trial, 308 patients were randomized in a 1:1:1:1 design in which patients were placed in groups with low-dose (equivalent to oral dose) or high-dose (2.5 times oral dose) intermittent parental therapy or alternatively low-dose or high-dose continuous drip therapy. There were no differences in dyspnea, fluid changes, change in creatinine, hospital length stay, or rehospitalization and death rates when the intermittent and drip approaches were compared. However, the high-dose arm had decreased dyspnea, increased volume removal, but there were more occurrences of AKIs when compared to the low-dose arm [32].
In clinical practice, if loop diuretic treatment does not result in the desired urine output, a second-site diuretic may be added to potentiate diuresis. Unfortunately, there is little data on this common clinical practice and thus the optimal choice of second site agent (chlorthiazide or metolazone) is unknown. Frequently, the deciding factor is based upon cost or concern that oral absorption of metolazone will be ineffective. However, Moranville et al recently performed a retrospective assessment comparing chlorthiazide (22 patients) to metolazone (33 patients) in ADHF patients with renal dysfunction defined by a creatinine clearance of 15–50 mL/min. There was a nonsignificant trend towards increased urine output in the metolazone group, no differences in the rates of adverse events, and the chlorthiazide group actually had a longer hospital stay [33]. While potentially promising results, the retrospective nature of the study made it difficult to determine if the differences were due to treatment approach or dissimilarities of patient illness. Nonetheless, physicians must remain vigilant when implementing the second-site diuretic approach because it can lead to marked diuretic response leading to metabolic derangements including hypokalemia, hyponatremia, hypomagnesaemia, and metabolic alkalosis.
Inotropes
The use of inotropic agents such as dobutamine or milrinone can be used to augment cardiac function when there is a known low-output state for better renal perfusion in CRS1. Unfortunately, there is little objective data available about the utility of this widely implemented approach. The Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of a Chronic Heart Failure (OPTIME-HF) trial did not show improved renal function with milrinone treatment [34]. The use of levosimendan, a cardiac calcium sensitizer that increases contractility not currently approved in the United States, was compared to dobutamine in the Survival of Patients With Acute Heart Failure in Need of Intravenous Inotropic Support (SURVIVE) trial, and there were no differences in rates of renal failure when the 2 groups were compared [35]. Nonetheless, if cardiac output is severely compromised, inotropes can be used for CRS1 treatment, but they should be used cautiously due the increased risks of lethal arrhythmias.
Dopamine
Use of low-dose dopamine to stimulate D1 and D2 receptors as a way to increase renal blood flow and promote increased glomerular filtration and urine production was extensively studied in ADHF. A small trial showed use of low dose dopamine had renal protective effects in a total of 20 patients [36]. However, when larger trials were conducted, such beneficial results were not consistently observed. The Dopamine in Acute Decompensated Heart Failure (DAD-HF I) trial compared low-dose furosemide plus low-dose dopamine (5 µg/kg/min) to high-dose furosemide alone in 60 patients. There were no differences in total diuresis, hospital stay, and 60-day mortality or rehospitalization rates, but there was a reduction in the renal dysfunction at the 24-hour time point in the dopamine-treated arm (6.7% versus 30%) [37]. The Dopamine in Acute Decompensated Heart Failure II trial randomized 161 ADHF patients to high-dose furosemide, low-dose furosemide and lose dose dopamine (5 µg/kg/min), or low-dose furosemide and assessed dyspnea, worsening renal function, length of stay, 60-day and one-year all-cause mortality and hospitalization for heart failure. Dopamine treatment did not improve any of the outcomes measured [38]. Finally, the most recent trial to examine the effects of dopamine was the Renal Optimization Strategies Evaluation (ROSE) trial. In this trial, there were 360 patients with ADHF randomized to nesiritide or dopamine versus placebo in a 2:1 design. When comparing dopamine (111 patients) treatment to placebo (115 patients), there were no differences in urine output, renal function as determined by cystatin C levels, or symptomatic improvements. However, there was more tachycardia in the dopamine group [39]. Currently, there is not strong evidence supporting routine use of dopamine in CRS1.
Nesiritide
Use of nesiritide, recombinant brain natriuretic peptide, was also investigated as a way to enhance urine production through the natriuretic effects of the peptide. The first attempt to explore this hypothesis was the B-Type Natriuretic Peptide in Cardiorenal Decompensation Syndrome (BNP-CARDS) trial. BNP-CARDS showed a 48-hour infusion of nesiritide (39 patients) or placebo (36 patients) in patients with ADHF and renal dysfunction (estimated GFR between 15–60 mL/min) did not reduce the incidence of worsening renal function as defined by a rise in serum creatinine by 20% [40]. A similar approach was implemented in the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND-HF) trial which examined over 7000 patients with ADHF. 3496 patients were treated with nesiritide and 3511 patients were treated with placebo for 24 hours and up to 7 days. Nesiritide treatment did not alter dyspnea at 6 and 24 hours, improve renal function as determined by creatinine change, or alter the combined end-point of rehospitalization or death 30 at days [41]. The ROSE trial examined the effects of nesiritide (117 patients) versus placebo (115 patients) for urine production, change in renal function as defined by change in cystatin C, and decongestion (urinary sodium excretion, weight change, and change in NT-proBNP) at 72 hours. Nesiritide did not alter any of the outcomes investigated [39]. Finally, a single-centered study conducted at the Mayo Clinic examined the effects of nesiritide (37 patients) or placebo (35 patients) with ADHF and pre-existing renal dysfunction (estimated GFR between 20 and 60 mL/min). These investigators found nesiritide treatment resulted in less renal dysfunction as measured by creatinine and BUN, but no changes in diuretic responsiveness, duration of hospitalization, or rehospitalization rates. Nesiritide did reduce serum endothelin levels, but had no effect on ANP, NT-pro BNP, renin, angiotensin II, or aldosterone [42]. In summary, nesiritide does not appear to have significant renal protective effects in ADHF.
Adenosine A1 Receptor Antagonists
The use of adenosine receptor antagonists to prevent adenosine-mediated vasoconstriction of renal vasculature in ADHF has also been examined. The first study conducted was a small double-blind randomized-controlled trial that investigated the effects of rolofylline, an adenosine A-1 antagonist, in patients with ADHF and an estimated creatinine clearance of 20-80 mL/min. The study had 27 patients in the placebo arm, 29 patients that received 2.5 mg of rolofylline, 31 patients received 15 mg of rolofylline, 30 patients received 30 mg of rolofylline, and 29 patients received 60 mg of rolofylline, all of which was daily for up to 3 days. Rolofylline treatment increased urine output on day 1 and improved renal function on day 2 [43]. These positive results led to the Placebo-Controlled Randomized Study of Selective Adenosine A1 Receptor Antagonist Rolofylline for Patients with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function (PROTECT) Trial. PROTECT assessed the effects of rolofylline (1356) or placebo (677) in patients with ADHF and an estimated creatinine clearance between 20 and 80 mL/min. There were no significant differences in renal function out to 14 days, but rolofylline led to more weight loss than placebo [44,45]. In a subgroup analysis of patients with severe baseline renal dysfunction (creatinine clearance of less than 30 mL/min), rolofylline reduced the combined 60-day end-point of hospitalization due to cardiovascular or renal cause and death [45]. Finally, the Effects of KW-3902 Injectable Emulsion on Heart Failure Signs and Symptoms, Diuresis, Renal Function, and Clinical Outcomes in Subjects Hospitalized with Worsening Renal Function and Heart Failure Requiring Intravenous Therapy (REACH-UP) trial probed the effects of rolofylline (36 patients) or placebo (40 patients) in patients with ADHF and renal impairment (creatinine clearance of 20-60 mL/min). Rolofylline treatment did not alter renal function, but there was a nonsignificant trend towards reduction in 60-day combined end-point of hospitalization due to renal or cardiovascular causes or death [46]. In summary, the use of rolofylline has not been conclusively associated with improved outcomes in CRS1.
Vasopressin Antagonists
The use of vasopressin antagonists to induce aquaphoresis and combat hyponatremia was studied in ADHF. Vasopressin antagonists were first investigated in the Acute and Chronic Therapeutic Impact of a Vasopressin Antagonist (ACTIV) trial. ACTIV involved 3 doses of tolvaptan (78 patients received 30 mg, 84 patients received 60 mg, and 77 patients received 90 mg) versus placebo (80 patients), and tolvaptan increased urine production and decreased body weight compared to placebo without compromising renal function. A post-hoc analysis of patients with renal dysfunction (BUN > 29 mg/dL) and severe volume overload revealed a survival benefit at 60 days [47]. The Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVERST) trial compared placebo (2061 patients) versus 30 mg/day of tolvaptan (2072 patients) within 48 hours after admission in an identical 2-trial design. Tolvaptan increased weight loss and reduced dyspnea acutely but did not alter all-cause mortality or cardiovascular or heart failure hospitalization rates out to 24 months post index hospitalization [48,49]. These data suggest vasopressin antagonists may potentiate diuresis acutely but likely do not improve long-term outcomes.
Corticosteroids
The use of corticosteroids in ADHF has been controversial as there were initial concerns that corticosteroids would increase fluid retention. However, corticosteroids augmented diuretic response and improved renal function in 13 ADHF patients who had inadequate response to sequential nephron blockage [50]. Furthermore, Zhang et al showed that prednisone treatment in 35 patients admitted with ADHF increased urinary volume, reduced dyspnea, reduced uric acid, and improved renal function [51]. These promising results led to the Cardiac Outcome Prevention Effectiveness of Glucocorticoids in Acute Decompensated Heart Failure (COPE-ADHF) trial. In this single-centered study, 102 patients with ADHF were randomized to either placebo [51] or corticosteroids [51] and the outcomes recorded included urinary volume, change in creatinine, and cardiovascular death at 30 days. Use of corticosteroids improved renal function, increased urine output, and reduced mortality (3/51 in corticosteroid group versus 10/51 in the placebo group) [52]. The mechanisms underlying the improvements with corticosteroids were not determined, but were hypothesized to be facilitation of natriuretic peptides or dilation of renal vasculature through activation of nitric oxide pathway or dopaminergic system.
Serelaxin
Serelaxin is a recombinantly expressed human relaxin-2, a peptide hormone present during pregnancy which facilitates physiological cardiovascular and renal adaptations [53–55], which showed potential benefits in CRS1. Analysis of the RELAX-AHF trial revealed serelaxin reduced incidence of worsening renal function at day 2 of treatment as defined by changes in serum creatinine, cystatin C, and BUN. Importantly, worsening renal function defined by cystatin C changes was associated with increased 180-day mortality in this analysis [56]. The mechanisms by which serelaxin prevented renal dysfunction are currently unknown as serelaxin treatment did not improve diuretic efficiency [19].
Ultrafiltration
Another treatment choice in CRS1 is mechanical removal of salt and water via ultrafiltration. Ultrafiltration showed early promise in Ultrafiltration Versus Intravenous Diuretics for Patients Hospitalized for Acute Decompensated Heart Failure trial (UNLOAD) trial. In this study, 200 patients with ADHF were randomized to either ultrafiltration or medical management with loop diuretics. Use of ultrafiltration increased volume removal without any differences in renal function and reduced rehospitalization rates at 90 days [57].
However, when ultrafiltration was employed specifically in CRS1 patients in the Cardiorenal Rescue Study in Acute Decompensated Heart Failure trial (CARESS-HF), UF was not superior to medical treatment. There were 188 patients studied in CARESS-HF, and in the ultrafiltration arm there was increased risk of renal dysfunction, no differences in volume removal, and no change in rehospitalization rates at 90 days [58]. When trying to reconcile UNLOAD and CARESS-HF, the medical treatment arm in CARESS-HF was much more standardized and aggressive and UNLOAD was earlier implementation of ultrafiltration, which may have explained the differences. Interestingly, ultrafiltration was hypothesized to be advantageous over diuretic therapy through reduced activation of the renin-angiotensin-aldosterone system, but analysis of the patients from CARESS-HF showed higher levels of plasma renin activity and no difference in aldosterone levels in ultrafiltration patients [59].
Two meta-analyses have examined the use of ultrafiltration versus medical management in ADHF and both showed ultrafiltration was more effective in volume removal than medical therapy but did not improve rehospitalization or mortality rates [60,61]. This fact combined with the risks of vascular access placement and bleeding from anticoagulation limits to routine use of ultrafiltration in CRS1.
Continuous Renal Replacement Therapy
Once renal function deteriorates to the point that renal replacement therapy is needed for both volume removal and solute clearance in CRS1, continuous renal replacement therapy (CRRT) may be implemented. Unfortunately, there are few available data for this group of advanced CRS1 patients to guide physicians. There was a single-centered study conducted in Egypt that randomized 40 ADHF patients to either IV furosemide or CRRT. The patients treated with CRRT had greater weight loss and decreased length of stay in the ICU, but there were no differences in dialysis dependence rates or 30-day mortality [62]. Two single-centered studies reported outcomes associated with advanced CRS1 requiring CRRT. In a study conducted at the Cleveland Clinic, 63 patients with CRS1 were treated with ultrafiltration, of which 37 were converted to CRRT due to worsening renal function. Of the 37 patients treated with CRRT, 16 died in the hospital and 4 were discharged with hospice care [63]. In another retrospective study performed at the University of Alabama-Birmingham, use of rescue CRRT in advanced CRS1 was examined in 37 patients. 23 patients died during hospitalization and 2 were discharged to hospice care [64]. Combination of the Cleveland Clinic and University of Alabama-Birmingham studies revealed patients requiring CRRT in the setting of advanced CRS1 had an in-hospital mortality or palliative discharge rate of 60.8% (45/74). Clearly, this population needs further investigation to prevent such poor outcomes.
Future Treatment Options
Ongoing and Unreported Clinical Trials
Unfortunately, none of the current treatments for CRS1 have definitive improvements in outcomes, but there are several ongoing clinical trials which will hopefully identify novel treatment strategies. First of all, the Acetazolamide and Spironolactone to Increase Natriuresis in Congestive Heart Failure (Diuresis-CHF) trial is being conducted in Belgium. This study will examine the effects of acetazolamide with low dose diuretic versus high dose diuretics in one aim and the effects of upfront spironolactone in another. The outcomes analyzed will include total natriuresis, potassium homeostasis, NT-proBNP changes, change in renal function, peak serum levels of renin and aldosterone, weight change, urine volume, and change in edema (NCT01973335). The Protocolized Diuretic Strategy in Cardiorenal Failure (ProDius) trial is being performed at the University of Pittsburgh, and will determine the effects of a protocolized diuretic approach to target 3-5 liters of urine production a day versus standard therapy and will track the change in body weight, length of hospitalization, reshospitalization rates, mortality rates, venous compliance of internal jugular vein, clinical decongestion, change in renal function, and urine output (NCT01921829). The Levosimendan versus Dobutamine for Renal Function in Heart Failure (ELDOR) study is ongoing in Sweden and will probe the acute effects of levosimendan and dobutamine on renal perfusion. The endpoints will include changes in renal blood flow, GFR, renal vascular resistance, central hemodynamics, renal oxygen extraction and consumptions, and filtration fraction (NCT02133105). Finally, the Safety and Efficacy of Low Dose Hypertonic Saline and High Dose Furosemide for Congestive Heart Failure (REaCH) trial probed the effects of combination of hypertonic saline and furosemide versus furosemide in patients with ADHF and renal impairment defined by a GFR<60 mL/min. The outcomes were change in renal function, diuretic response, length of hospital stay, readmission rates, weight loss, BNP levels, and included a cost analysis. The study was completed but results are not currently available (NCT01028170)
Should Inflammation Be Targeted in CRS1?
Although proposed to play a role in the pathophysiology of CRS1, inflammation has not been explicitly targeted as a treatment for CRS1. One possible way to combat inflammation could be inhibition of the IL-6 pathway, which is support by preclinical work as previous studies showed IL-6 knockout mice were resistant to HgCl2-induced renal injury and death [65] and IL-6 has negative inotropic effects in both isolated cardiomyocytes [66] and intact animals [67]. Thus, IL-6 antagonism may improve both cardiac and renal function, an ideal scenario for CRS1 patients. The availability of tocilizumab, an FDA-approved humanized antibody to the IL-6 receptor, may allow for investigation of this hypothesis in the future. Although not examined in the COPE-ADHF trial, an alternative explanation for the improvements associated with corticosteroids treatment were the anti-inflammatory effects. If this were true, corticosteroids would represent a relatively cheap treatment option for CRS1 patients, but more studies need to be conducted before this approach is widely implemented. Finally, use of cytokine profiling may be used to enrich a population of CRS1 patients that could be investigated in future clinical trials using anti-inflammatory medications.
Unanswered Questions Moving Forward
Severity of AKI and Treatment Effects
An important unknown that warrants further investigation is if the severity of AKI should dictate treatment choice in CRS1. As discussed above, increasing severity of AKI resulted in elevated risk of adverse events, but it remains unknown whether different treatments offer benefits for more or less severe renal impairment. Perhaps, future studies aimed at defining outcomes from different treatment strategies stratified by severity of renal dysfunction may reveal which patients benefit from the various treatment options for CRS1.
How Do We Best Define Renal Dysfunction in CRS1?
Currently, there is no accepted definition of renal dysfunction in CRS1. As discussed above, using the AKIN, KDIGO, or RIFLE scoring systems or diuretic responsiveness effectively differentiated outcomes in patients with CRS1. However, an agreed-upon definition would likely benefit the field going forward so this population could be systematically investigated in future studies.
Conclusion
In summary, CRS1 is a common clinical entity associated with poor patient outcomes. A complex pathophysiology marked by reduced cardiac output, increased central venous pressure, inflammation, and oxidative stress underlies the disease process. Unfortunately, no current treatment approach shows consistent improvements in outcomes, highlighting the urgent need for further research to reduce the burden that CRS1 imposes.
Corresponding author: Kurt W. Prins, MD, PhD, MMC 580 Mayo, 420 Delaware St SE, Minneapolis, MN 55455, [email protected].
Funding/support: Dr. Prins is funded by NIH F32 grant HL129554 and Dr. Thenappen is funded by AHA Scientist Development Grant 15SDG25560048.
1. Mozaffarian D, Benjamin EJ, Go AS,et al. Heart disease and stroke statistics--2015 update: A report from the american heart association. Circulation 2015;131:e29–322.
2. Gheorghiade M, Vaduganathan M, Fonarow GC, Bonow RO. Rehospitalization for heart failure: problems and perspectives. J Am Coll Cardiol 2013;61:391–403.
3. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the medicare fee-for-service program. N Engl J Med 2009;360:1418–28.
4. Fonarow GC, Adams KF Jr, Abraham WT, et al and ADHERE Scientific Advisory Committee, Study Group, and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: Classification and regression tree analysis. JAMA 2005;293:572–80.
5. Abraham WT, Fonarow GC, Albert NM, et al. Predictors of in-hospital mortality in patients hospitalized for heart failure: Insights from the organized program to initiate lifesaving treatment in hospitalized patients with heart failure (OPTIMIZE-HF). J Am Coll Cardiol 2008;52:347–56.
6. Ronco C, Haapio M, House AA, et al. Cardiorenal syndrome. J Am Coll Cardiol 2008:52:1527–39.
7. Roy AK, Mc Gorrian C, Treacy C, et al. A comparison of traditional and novel definitions (RIFLE, AKIN, and KDIGO) of acute kidney injury for the prediction of outcomes in acute decompensated heart failure. Cardiorenal Med 2013;3:26–37.
8. Li Z, Cai L, Liang X, et al. Identification and predicting short-term prognosis of early cardiorenal syndrome type 1: KDIGO is superior to RIFLE or AKIN. PLoS One 2014;9:e114369.
9. Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009:53:589–96.
10. Nohria A, Hasselblad V, Stebbins A, et al. Cardiorenal interactions: Insights from the ESCAPE trial. J Am Coll Cardiol 2008:51:1268–74.
11. Winton FR. The influence of venous pressure on the isolated mammalian kidney. J Physiol 1931;72:49–61.
12. Virzi GM, Torregrossa R, Cruz DN, et al. Cardiorenal syndrome type 1 may be immunologically mediated: A pilot evaluation of monocyte apoptosis. Cardiorenal Med 2012;2:33–42.
13. Virzi GM, Clementi A, de Cal M, et al. Oxidative stress: Dual pathway induction in cardiorenal syndrome type 1 pathogenesis. Oxid Med Cell Longev 2015;391790.
14. Cho E, Kim M, Ko YS, et al. Role of inflammation in the pathogenesis of cardiorenal syndrome in a rat myocardial infarction model. Nephrol Dial Transplant 2013;28:2766–78.
15. Ather S, Bavishi C, McCauley MD, et al. Worsening renal function is not associated with response to treatment in acute heart failure. Int J Cardiol 2013;167:1912–7.
16. Testani JM, McCauley BD, Kimmel SE, Shannon RP. Characteristics of patients with improvement or worsening in renal function during treatment of acute decompensated heart failure. Am J Cardiol 2010;106:1763–69.
17. Hata N, Yokoyama S, Shinada T, et al. Acute kidney injury and outcomes in acute decompensated heart failure: Evaluation of the RIFLE criteria in an acutely ill heart failure population. Eur J Heart Fail 2010;12:32–7.
18. Testani JM, Brisco MA, Turner JM, et al. Loop diuretic efficiency: A metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ Heart Fail 2014;7:261–70.
19. Voors AA, Davison BA, Teerlink JR, et al. Diuretic response in patients with acute decompensated heart failure: Characteristics and clinical outcome--an analysis from RELAX-AHF. Eur J Heart Fail 2014;16:1230–40.
20. Valente MA, Voors AA, Damman K, et al. Diuretic response in acute heart failure: Clinical characteristics and prognostic significance. Eur Heart J 2014;35:1284–93.
21. Devarajan P. Neutrophil gelatinase-associated lipocalin: A troponin-like biomarker for human acute kidney injury. Nephrology (Carlton) 2010;15:419–28.
22. Soyler C, Tanriover MD, Ascioglu S, et al. Urine neutrophil gelatinase-associated lipocalin levels predict acute kidney injury in acute decompensated heart failure patients. Ren Fail 2015;5.
23. Brisco MA,Testani JM. Novel renal biomarkers to assess cardiorenal syndrome. Curr Heart Fail Rep 2014;11;485–99.
24. Lassus J, Harjola VP, Sund R, et al. and FINN-AKVA Study group. Prognostic value of cystatin C in acute heart failure in relation to other markers of renal function and NT-proBNP. Eur Heart J 2007;28:1841–7.
25. Arimoto T, Takeishi Y, Niizeki T, et al. Cystatin C, a novel measure of renal function, is an independent predictor of cardiac events in patients with heart failure. J Card Fail 2005;11:595–601.
26. Naruse H, Ishii J, Kawai T, et al. Cystatin C in acute heart failure without advanced renal impairment. Am J Med 2009;122:566–73.
27. Manzano-Fernandez S, Boronat-Garcia M, Albaladejo-Oton MD, et al. Complementary prognostic value of cystatin C, N-terminal pro-B-type natriuretic peptide and cardiac troponin T in patients with acute heart failure. Am J Cardiol 2009;103:1753–9.
28. Bonventre JV, Yang L. Kidney injury molecule-1. Curr Opin Crit Care 2010;16:556–61.
29. Damman K, Van Veldhuisen DJ, Navis G, et al. Tubular damage in chronic systolic heart failure is associated with reduced survival independent of glomerular filtration rate. Heart 2010;96:1297–302.
30. Jungbauer CG, Birner C, Jung B, et al. Kidney injury molecule-1 and N-acetyl-beta-D-glucosaminidase in chronic heart failure: Possible biomarkers of cardiorenal syndrome. Eur J Heart Fail 2011;13:1104–10.
31. Verbrugge FH, Dupont M, Shao Z, et al. Novel urinary biomarkers in detecting acute kidney injury, persistent renal impairment, and all-cause mortality following decongestive therapy in acute decompensated heart failure. J Card Fail 2013;19:621–8.
32. Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011;364:797–805.
33. Moranville MP, Choi S, Hogg J, et al. Comparison of metolazone versus chlorothiazide in acute decompensated heart failure with diuretic resistance. Cardiovasc Ther 2015;33;42–9.
34. Cuffe MS, Califf RM, Adams KF Jr, et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: A randomized controlled trial. JAMA 2002;287:1541–7.
35. Mebazaa A, Nieminen MS, Packer M, et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: The SURVIVE randomized trial. JAMA 2007;297:1883–91.
36. Varriale P, Mossavi A. The benefit of low-dose dopamine during vigorous diuresis for congestive heart failure associated with renal insufficiency: Does it protect renal function? Clin Cardiol 1997;20:627–30.
37. Giamouzis G, Butler J, Starling RC, et al. Impact of dopamine infusion on renal function in hospitalized heart failure patients: Results of the dopamine in acute decompensated heart failure (DAD-HF) trial. J Card Fail 2010;16:922–30.
38. Triposkiadis FK, Butler J, Karayannis G, et al. Efficacy and safety of high dose versus low dose furosemide with or without dopamine infusion: The dopamine in acute decompensated heart failure II (DAD-HF II) trial. Int J Cardiol 2014;172:115–21.
39. Chen HH, Anstrom KJ, Givertz MM, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: The ROSE acute heart failure randomized trial. JAMA 2013;310:2533–43.
40. Witteles RM, Kao D, Christopherson D, et al. Impact of nesiritide on renal function in patients with acute decompensated heart failure and pre-existing renal dysfunction a randomized, double-blind, placebo-controlled clinical trial. J Am Coll Cardiol 2007;50:1835–40.
41 O'Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med 2011;365:32–43.
42. Owan TE, Chen HH, Frantz RP, et al. The effects of nesiritide on renal function and diuretic responsiveness in acutely decompensated heart failure patients with renal dysfunction. J Card Fail 2008;14:267–75.
43. Givertz MM, Massie BM, Fields TK, et al and CKI-201 and CKI-202 Investigators. The effects of KW-3902, an adenosine A1-receptor antagonist,on diuresis and renal function in patients with acute decompensated heart failure and renal impairment or diuretic resistance. J Am Coll Cardiol 2007;50:1551–60.
44. Massie BM, O'Connor CM, Metra M, et al. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N Engl J Med 2010;363:1419–28.
45. Voors AA, Dittrich HC, Massie BM, et al. Effects of the adenosine A1 receptor antagonist rolofylline on renal function in patients with acute heart failure and renal dysfunction: Results from PROTECT (placebo-controlled randomized study of the selective adenosine A1 receptor antagonist rolofylline for patients hospitalized with acute decompensated heart failure and volume overload to assess treatment effect on congestion and renal function). J Am Coll Cardiol 2011;57:1899–907.
46. Gottlieb SS, Givertz MM, Metra M, et al. The effects of adenosine A(1) receptor antagonism in patients with acute decompensated heart failure and worsening renal function: The REACH UP study. J Card Fail 2010;16:714–9.
47. Gheorghiade M, Gattis WA, O'Connor CM, et al. Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: A randomized controlled trial. JAMA 2004;291:1963–71.
48. Gheorghiade M, Konstam MA, Burnett JC Jr, et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: The EVEREST clinical status trials. JAMA 2007;297:1332–43.
49. Konstam MA, Gheorghiade M, Burnett JC Jr, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: The EVEREST outcome trial. JAMA 2007;297:1319–31.
50. Liu C, Liu G, Zhou C, et al. Potent diuretic effects of prednisone in heart failure patients with refractory diuretic resistance. Can J Cardiol 2007;23:865–8.
51. Zhang H, Liu C, Ji Z, et al. Prednisone adding to usual care treatment for refractory decompensated congestive heart failure. Int Heart J 2008;49:587–95.
52. Liu C, Liu K and COPE-ADHF Study Group. Cardiac outcome prevention effectiveness of glucocorticoids in acute decompensated heart failure: COPE-ADHF study. J Cardiovasc Pharmacol 2014;63:333–8.
53. Teichman SL, Unemori E, Teerlink JR, et al. Relaxin: Review of biology and potential role in treating heart failure. Curr Heart Fail Rep 2010;7:75–82.
54. Conrad KP, Shroff SG. Effects of relaxin on arterial dilation, remodeling, and mechanical properties. Curr Hypertens Rep 2011;13:409–20.
55. Du XJ, Bathgate RA, Samuel CS, et al. Cardiovascular effects of relaxin: From basic science to clinical therapy. Nat Rev Cardiol 2010;7:48–58.
56. Metra M, Cotter G, Davison BA, et al. Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the relaxin in acute heart failure (RELAX-AHF) development program: Correlation with outcomes. J Am Coll Cardiol 2013;61:196-206.
57. Costanzo MR, Guglin ME, Saltzberg MT, et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol 2007;49:675–83.
58. Bart BA, Goldsmith SR, Lee KL, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012;367:2296–304.
59. Mentz RJ, Stevens SR, DeVore AD, et al. Decongestion strategies and renin-angiotensin-aldosterone system activation in acute heart failure. JACC Heart Fail 2015;3:97–107.
60. Ebrahim B, Sindhura K, Okoroh J, et al. Meta-analysis of ultrafiltration versus diuretics treatment option for overload volume reduction in patients with acute decompensated heart failure. Arq Bras Cardiol 2015;104:417–25.
61. Kwong JS, Yu CM. Ultrafiltration for acute decompensated heart failure: A systematic review and meta-analysis of randomized controlled trials. Int J Cardiol 2014;172:395–402.
62. Badawy SS, Fahmy A. Efficacy and cardiovascular tolerability of continuous veno-venous hemodiafiltration in acute decompensated heart failure: A randomized comparative study. J Crit Care 2012;27:106.e7-106.13.
63. Patarroyo M, Wehbe E, Hanna M, et al. Cardiorenal outcomes after slow continuous ultrafiltration therapy in refractory patients with advanced decompensated heart failure. J Am Coll Cardiol 2012;60:1906–12.
64. Prins KW, Wille KM, Tallaj JA, Tolwani AJ. Assessing continuous renal replacement therapy as a rescue strategy in cardiorenal syndrome 1. Clin Kidney J 2015;8:87–92.
65. Nechemia-Arbely Y, Barkan D, Pizov G, et al. IL-6/IL-6R axis plays a critical role in acute kidney injury. J Am Soc Nephrol 2008;19:1106–15.
66. Pathan N, Franklin JL, Eleftherohorinou H, et al. Myocardial depressant effects of interleukin 6 in meningococcal sepsis are regulated by p38 mitogen-activated protein kinase. Crit Care Med 2011;39:1692–711.
67. Janssen SP, Gayan-Ramirez G, Van den Bergh A, et al. Interleukin-6 causes myocardial failure and skeletal muscle atrophy in rats. Circulation 2005;111:996–1005.
68. Bellomo R, Ronco C, Kellum JA and Acute Dialysis Quality Initiative workgroup. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: The second international consensus conference of the acute dialysis quality initiative (ADQI) group. Crit Care 2004;8:R204-12.
69. Mehta RL, Kellum JA, Shah SV, et al and Acute Kidney Injury Network. Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31.
70. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guidelines for acute kidney injury. Kidney Inter Suppl 2012;2:19–36.
From the Cardiovascular Division, Department of Internal Medicine, University of Minnesota, Minneapolis, MN.
Abstract
- Objective: To present a review of cardiorenal syndrome type 1 (CRS1).
- Methods: Review of the literature.
- Results: Acute kidney injury occurs in approximately one-third of patients with acute decompensated heart failure (ADHF) and the resultant condition was named CRS1. A growing body of literature shows CRS1 patients are at high risk for poor outcomes, and thus there is an urgent need to understand the pathophysiology and subsequently develop effective treatments. In this review we discuss prevalence, proposed pathophysiology including hemodynamic and nonhemodynamic factors, prognosticating variables, data for different treatment strategies, and ongoing clinical trials and highlight questions and problems physicians will face moving forward with this common and challenging condition.
- Conclusion: Further research is needed to understand the pathophysiology of this complex clinical entity and to develop effective treatments.
Acute decompensated heart failure (ADHF) is an epidemic facing physicians throughout the world. In the United States alone, ADHF accounts for over 1 million hospitalizations annually, with costs in 2012 reaching $30.7 billion [1]. Despite the advances in chronic heart failure management, ADHF continues to be associated with poor outcomes as exemplified by 30-day readmission rates of over 20% and in-hospital mortality rates of 5% to 6%, both of which have not significantly improved over the past 20 years [2,3]. One of the strongest predictors of adverse outcomes in ADHF is renal dysfunction. An analysis from the Acute Decompensated Heart Failure National Registry (ADHERE) revealed the combination of renal dysfunction (creatinine > 2.75 mg/dL and blood urea nitrogen (BUN) > 43 mg/dL) and hypotension (systolic blood pressure (SBP) < 115 mm Hg) upon admission was associated with an in-hospital mortality of > 20% [4]. The Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) registry documented a 16.3% in-hospital mortality when patients had a SBP < 100 mm Hg and creatinine > 2.0 mg/dL at admission [5].
The presence of acute kidney injury in the setting of ADHF is a very common occurrence and was termed cardiorenal syndrome type 1 (CRS1) [6]. The prevalence of CRS1 in single-centered studies ranged from 32% to 40% of all ADHF admissions [7,8]. If this estimate holds true throughout the United States, there would be 320,000 to 400,000 hospitalizations for CRS1 annually, highlighting the magnitude of this problem. Moreover, with the number of patients with heart failure expected to continue to rise, CRS1 will only become more prevalent in the future. In this review we discuss the prevalence, proposed pathophysiology including hemodynamic and nonhemodynamic factors, prognosticating variables, data for different treatment strategies, ongoing clinical trials, and highlight questions and problems physicians will face moving forward in this common and challenging condition.
Pathogenesis of CRS1
Hemodynamic Effects
The early hypothesis for renal dysfunction in ADHF centered on hemodynamics, as reduced cardiac output was believed to decrease renal perfusion. However, analysis of invasive hemodynamics from patients with ADHF suggested that central venous pressure (CVP) was actually a better predictor of the development of CRS1 than cardiac output. In a single-center study conducted at the Cleveland Clinic, hemodynamics from 145 patients with ADHF were evaluated and surprisingly baseline cardiac index was greater in the patients with CRS1 than patients without renal dysfunction (2.0 ± 0.8 L/min/m2 vs 1.8 ± 0.4 L/min/m2; P = 0.008). However, baseline CVP was higher in the CRS1 group (18 ± 7 mm Hg vs 12 ± 6 mm Hg; P = 0.001), and there was a heightened risk of developing CRS1 as CVP increased. In fact, 75% of the patients with a CVP of > 24 mm Hg developed renal impairment [9]. In a retrospective study of the Evaluation Study of Congestive Heart Failure and Pulmonary Arterial Catheter Effectiveness (ESCAPE) trial, the only hemodynamic parameter that correlated with baseline creatinine was CVP. However, no invasive measures predicted worsening renal function during hospitalization [10]. Finally, an experiment that used isolated canine kidneys showed increased venous pressure acutely reduced urine production. Interestingly, this relationship was dependent on arterial pressure; as arterial flow decreased smaller increases in CVP were needed to reduce urine output [11]. Together, these data suggest increased CVP plays an important role in CRS1, but imply hemodynamics alone may not fully explain the pathophysiology of CRS1.
Inflammation
As information about how hemodynamics incompletely predict renal dysfunction in ADHF became available, alternative hypotheses were investigated to gain a deeper understanding of the pathophysiology underlying CRS1. A pathological role of inflammation in CRS1 has gained attention due to recent publications. First of all, serum levels of the pro-inflammatory cytokines TNF-a and IL-6 were elevated in patients with CRS1 when compared to health controls [12]. Interestingly, Virzi et al showed that the median value of IL-6 was 5 times higher in CRS1 patients when compared to ADHF patients without renal dysfunction [13]. The negative consequences of elevated serum cytokines were demonstrated when incubation of a human cell line of monocytes with serum from CRS1 patients induced apoptosis in 81% of cells compared to just 11% of cells with control serum [12]. It is possible that cytokine-induced apoptosis could occur in other cell types in different organs in patients with CRS1, which may contribute to both cardiac and renal dysfunction. Finally, analysis from a rat model of CRS1 revealed macrophage infiltration into the kidneys and increased numbers of activated monocytes in the peripheral blood. Interestingly, monocyte/macrophage depletion using liposome clodronate prevented chronic renal dysfunction in the rat model [14]. In summary, these data suggest inflammation contributes to CRS1 pathophysiology, but more experimental data is needed to determine if there is a causal relationship.
Oxidative Stress
Very recently, oxidative stress was proposed to play a role in CRS1. Virzi et al analyzed serum levels of markers of oxidative stress and compared ADHF patients without renal impairment to CRS1 patients. The markers of oxidative stress, which included myeloperoxidase, nitric oxide, copper/zinc superoxide dismutase, and endogenous peroxidase, were all significantly higher in CRS1 patients [13]. While provocative, the tissues responsible for the generation of these molecules and the subsequent effects have not yet been fully elucidated.
Prognostication
Severity of Acute Kidney Injury
Initial publications did not document a strong link between kidney injury and poor outcomes in ADHF. Firstly, Ather et al performed a single-centered study that investigated how change in renal function defined by change in creatinine, estimated GFR, and BUN affected outcomes one year post admission for ADHF. Kidney injury defined by a change in creatinine or in estimated GFR was not associated with increased risk of mortality, but a change in BUN was associated with increased mortality in a univariate analysis [15]. Testani et al retrospectively analyzed patients from the ESCAPE trial and found worsening renal function defined by a ≥ 20% reduction in estimated GFR was not significantly associated with 180-day mortality, but there was a trend towards higher mortality (hazard ration 1.4; P = 0.11) [16]. Importantly, neither of 2 these studies assessed how severity of AKI impacted outcomes, which may have contributed to the weak relationships observed.
Diuretic Responsiveness
Voors et al performed a retrospective analysis of diuretic responsiveness in 1161 patients from the Relaxin in Acute Heart Failure (RELAX-AHF) trial. Diuretic responsiveness was defined as weight change (kg) per diuretic dose (IV furosemide and PO furosemide) over 5 days and then patients were separated into tertiles. The lowest tertile group had an approximate 20% incidence of 60-day combined end-point of death, heart failure or renal failure readmission compared to less than 10% incidence in the middle and upper tertiles. Interestingly, when the effects of worsening renal function (WRF), defined as creatinine change of ≥ 0.3 mg/dL, were examined in patients stratified by diuretic response, WRF did not offer additional prognostic information [19].
Finally, Valenete et al analyzed diuretic response in 1745 patients from the PROTECT trial (Placebo-Controlled Randomized Study of the Selective A1-Adenosine Receptor Antagonist Rolofylline for Patients Hospitalized with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function). Diuretic response was calculated using the weight change per 40 mg of furosemide, and as diuretic response declined there was increasing risk of 60-day rehospitalization and 180-day mortality rates. In fact, the lowest quintile responders had a 25% mortality rate at 180 days [20].
Emerging Biomarkers
Urine Neutrophil Gelatinase-Associated Lipocalin
Because previous studies showed urinary levels of NGAL was an earlier and more reliable marker of renal dysfunction than creatinine in AKI [21], it was studied as a possible biomarker for the development of CRS1 in ADHF. A single-centered study quantified levels of urine NGAL in 100 patients admitted with heart failure and then tracked the rates of acute kidney injury. Urine NGAL was elevated in patients that developed AKI and a cut-off value 12 ng/mL had a sensitivity of 79% and specificity of 67% for predicting CRS1 [22]. While promising, further studies are needed to better define the role of NGAL in CRS1.
Cystatin C
Cystatin C is a ubiquitously expressed cysteine protease that has a constant production rate and is freely filtered by the glomerulus without being secreted into the tubules, and has effectively prognosticated outcomes in ADHF [23]. Lassus et al showed an adjusted hazard ratio of 3.2 (2.0–5.3) for 12-month mortality when cystatin C levels were elevated. Moreover, patients with the highest tertitle of NT-proBNP and cystatin C had a 48.7% 1-year mortality. Interestingly, patients with an elevated cystatin C but normal creatinine had a 40.6% 1-year mortality compared to 12.6% for those with normal cystatin C and creatinine [24]. Furthermore, Arimoto et al showed elevated cystatin C predicted death or rehospitalization in a small cohort of ADHF patients in Japan [25]. Also, Naruse et al showed cystatin C was a better predictor of cardiac death than estimated GFR by the Modification of Diet in Renal Disease Study (MDRD) equation [26]. Finally, Manzano-Fernandez et al showed the highest tertile of cystatin C was a significant independent risk factor for 2-year death or rehospitalization while creatinine and MDRD estimates of GFR were not [27]. In agreement with Lassus et al, elevations in either 2 or 3 of cystatin C, troponin, and NT-proBNP predicted death or rehospitalization when compared to those with normal levels of these 3 markers [27]. In conclusion, cystatin C either alone or in combination with other biomarkers identifies high-risk patients.
Kidney Injury Molecule 1
Kidney injury molecule 1 (KIM-1) is a type-1 cell membrane glycoprotein expressed in regenerating proximal tubular cells but not under normal conditions [28]. Although associated with increased risk of hospitalization and mortality in chronic heart failure [29,30], elevated levels of urinary KIM-1 did not predict mortality in ADHF [31]. Further studies are needed to elucidate the utility of KIM-1 in CRS1.
Treatment Approaches
Diuretics
Loop diuretics are the main treatment for decongestion of patients with CRS1. To date, no clinical trial has compared the different loop diuretics (furosemide, bumetanide, torsemide, or ethacrynic acid) to each other, so there is no clear choice of which loop diuretic is the best. However, dosing scheme was investigated in the Dose Optimization Strategies Evaluation (DOSE) trial. In this trial, 308 patients were randomized in a 1:1:1:1 design in which patients were placed in groups with low-dose (equivalent to oral dose) or high-dose (2.5 times oral dose) intermittent parental therapy or alternatively low-dose or high-dose continuous drip therapy. There were no differences in dyspnea, fluid changes, change in creatinine, hospital length stay, or rehospitalization and death rates when the intermittent and drip approaches were compared. However, the high-dose arm had decreased dyspnea, increased volume removal, but there were more occurrences of AKIs when compared to the low-dose arm [32].
In clinical practice, if loop diuretic treatment does not result in the desired urine output, a second-site diuretic may be added to potentiate diuresis. Unfortunately, there is little data on this common clinical practice and thus the optimal choice of second site agent (chlorthiazide or metolazone) is unknown. Frequently, the deciding factor is based upon cost or concern that oral absorption of metolazone will be ineffective. However, Moranville et al recently performed a retrospective assessment comparing chlorthiazide (22 patients) to metolazone (33 patients) in ADHF patients with renal dysfunction defined by a creatinine clearance of 15–50 mL/min. There was a nonsignificant trend towards increased urine output in the metolazone group, no differences in the rates of adverse events, and the chlorthiazide group actually had a longer hospital stay [33]. While potentially promising results, the retrospective nature of the study made it difficult to determine if the differences were due to treatment approach or dissimilarities of patient illness. Nonetheless, physicians must remain vigilant when implementing the second-site diuretic approach because it can lead to marked diuretic response leading to metabolic derangements including hypokalemia, hyponatremia, hypomagnesaemia, and metabolic alkalosis.
Inotropes
The use of inotropic agents such as dobutamine or milrinone can be used to augment cardiac function when there is a known low-output state for better renal perfusion in CRS1. Unfortunately, there is little objective data available about the utility of this widely implemented approach. The Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of a Chronic Heart Failure (OPTIME-HF) trial did not show improved renal function with milrinone treatment [34]. The use of levosimendan, a cardiac calcium sensitizer that increases contractility not currently approved in the United States, was compared to dobutamine in the Survival of Patients With Acute Heart Failure in Need of Intravenous Inotropic Support (SURVIVE) trial, and there were no differences in rates of renal failure when the 2 groups were compared [35]. Nonetheless, if cardiac output is severely compromised, inotropes can be used for CRS1 treatment, but they should be used cautiously due the increased risks of lethal arrhythmias.
Dopamine
Use of low-dose dopamine to stimulate D1 and D2 receptors as a way to increase renal blood flow and promote increased glomerular filtration and urine production was extensively studied in ADHF. A small trial showed use of low dose dopamine had renal protective effects in a total of 20 patients [36]. However, when larger trials were conducted, such beneficial results were not consistently observed. The Dopamine in Acute Decompensated Heart Failure (DAD-HF I) trial compared low-dose furosemide plus low-dose dopamine (5 µg/kg/min) to high-dose furosemide alone in 60 patients. There were no differences in total diuresis, hospital stay, and 60-day mortality or rehospitalization rates, but there was a reduction in the renal dysfunction at the 24-hour time point in the dopamine-treated arm (6.7% versus 30%) [37]. The Dopamine in Acute Decompensated Heart Failure II trial randomized 161 ADHF patients to high-dose furosemide, low-dose furosemide and lose dose dopamine (5 µg/kg/min), or low-dose furosemide and assessed dyspnea, worsening renal function, length of stay, 60-day and one-year all-cause mortality and hospitalization for heart failure. Dopamine treatment did not improve any of the outcomes measured [38]. Finally, the most recent trial to examine the effects of dopamine was the Renal Optimization Strategies Evaluation (ROSE) trial. In this trial, there were 360 patients with ADHF randomized to nesiritide or dopamine versus placebo in a 2:1 design. When comparing dopamine (111 patients) treatment to placebo (115 patients), there were no differences in urine output, renal function as determined by cystatin C levels, or symptomatic improvements. However, there was more tachycardia in the dopamine group [39]. Currently, there is not strong evidence supporting routine use of dopamine in CRS1.
Nesiritide
Use of nesiritide, recombinant brain natriuretic peptide, was also investigated as a way to enhance urine production through the natriuretic effects of the peptide. The first attempt to explore this hypothesis was the B-Type Natriuretic Peptide in Cardiorenal Decompensation Syndrome (BNP-CARDS) trial. BNP-CARDS showed a 48-hour infusion of nesiritide (39 patients) or placebo (36 patients) in patients with ADHF and renal dysfunction (estimated GFR between 15–60 mL/min) did not reduce the incidence of worsening renal function as defined by a rise in serum creatinine by 20% [40]. A similar approach was implemented in the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND-HF) trial which examined over 7000 patients with ADHF. 3496 patients were treated with nesiritide and 3511 patients were treated with placebo for 24 hours and up to 7 days. Nesiritide treatment did not alter dyspnea at 6 and 24 hours, improve renal function as determined by creatinine change, or alter the combined end-point of rehospitalization or death 30 at days [41]. The ROSE trial examined the effects of nesiritide (117 patients) versus placebo (115 patients) for urine production, change in renal function as defined by change in cystatin C, and decongestion (urinary sodium excretion, weight change, and change in NT-proBNP) at 72 hours. Nesiritide did not alter any of the outcomes investigated [39]. Finally, a single-centered study conducted at the Mayo Clinic examined the effects of nesiritide (37 patients) or placebo (35 patients) with ADHF and pre-existing renal dysfunction (estimated GFR between 20 and 60 mL/min). These investigators found nesiritide treatment resulted in less renal dysfunction as measured by creatinine and BUN, but no changes in diuretic responsiveness, duration of hospitalization, or rehospitalization rates. Nesiritide did reduce serum endothelin levels, but had no effect on ANP, NT-pro BNP, renin, angiotensin II, or aldosterone [42]. In summary, nesiritide does not appear to have significant renal protective effects in ADHF.
Adenosine A1 Receptor Antagonists
The use of adenosine receptor antagonists to prevent adenosine-mediated vasoconstriction of renal vasculature in ADHF has also been examined. The first study conducted was a small double-blind randomized-controlled trial that investigated the effects of rolofylline, an adenosine A-1 antagonist, in patients with ADHF and an estimated creatinine clearance of 20-80 mL/min. The study had 27 patients in the placebo arm, 29 patients that received 2.5 mg of rolofylline, 31 patients received 15 mg of rolofylline, 30 patients received 30 mg of rolofylline, and 29 patients received 60 mg of rolofylline, all of which was daily for up to 3 days. Rolofylline treatment increased urine output on day 1 and improved renal function on day 2 [43]. These positive results led to the Placebo-Controlled Randomized Study of Selective Adenosine A1 Receptor Antagonist Rolofylline for Patients with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function (PROTECT) Trial. PROTECT assessed the effects of rolofylline (1356) or placebo (677) in patients with ADHF and an estimated creatinine clearance between 20 and 80 mL/min. There were no significant differences in renal function out to 14 days, but rolofylline led to more weight loss than placebo [44,45]. In a subgroup analysis of patients with severe baseline renal dysfunction (creatinine clearance of less than 30 mL/min), rolofylline reduced the combined 60-day end-point of hospitalization due to cardiovascular or renal cause and death [45]. Finally, the Effects of KW-3902 Injectable Emulsion on Heart Failure Signs and Symptoms, Diuresis, Renal Function, and Clinical Outcomes in Subjects Hospitalized with Worsening Renal Function and Heart Failure Requiring Intravenous Therapy (REACH-UP) trial probed the effects of rolofylline (36 patients) or placebo (40 patients) in patients with ADHF and renal impairment (creatinine clearance of 20-60 mL/min). Rolofylline treatment did not alter renal function, but there was a nonsignificant trend towards reduction in 60-day combined end-point of hospitalization due to renal or cardiovascular causes or death [46]. In summary, the use of rolofylline has not been conclusively associated with improved outcomes in CRS1.
Vasopressin Antagonists
The use of vasopressin antagonists to induce aquaphoresis and combat hyponatremia was studied in ADHF. Vasopressin antagonists were first investigated in the Acute and Chronic Therapeutic Impact of a Vasopressin Antagonist (ACTIV) trial. ACTIV involved 3 doses of tolvaptan (78 patients received 30 mg, 84 patients received 60 mg, and 77 patients received 90 mg) versus placebo (80 patients), and tolvaptan increased urine production and decreased body weight compared to placebo without compromising renal function. A post-hoc analysis of patients with renal dysfunction (BUN > 29 mg/dL) and severe volume overload revealed a survival benefit at 60 days [47]. The Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVERST) trial compared placebo (2061 patients) versus 30 mg/day of tolvaptan (2072 patients) within 48 hours after admission in an identical 2-trial design. Tolvaptan increased weight loss and reduced dyspnea acutely but did not alter all-cause mortality or cardiovascular or heart failure hospitalization rates out to 24 months post index hospitalization [48,49]. These data suggest vasopressin antagonists may potentiate diuresis acutely but likely do not improve long-term outcomes.
Corticosteroids
The use of corticosteroids in ADHF has been controversial as there were initial concerns that corticosteroids would increase fluid retention. However, corticosteroids augmented diuretic response and improved renal function in 13 ADHF patients who had inadequate response to sequential nephron blockage [50]. Furthermore, Zhang et al showed that prednisone treatment in 35 patients admitted with ADHF increased urinary volume, reduced dyspnea, reduced uric acid, and improved renal function [51]. These promising results led to the Cardiac Outcome Prevention Effectiveness of Glucocorticoids in Acute Decompensated Heart Failure (COPE-ADHF) trial. In this single-centered study, 102 patients with ADHF were randomized to either placebo [51] or corticosteroids [51] and the outcomes recorded included urinary volume, change in creatinine, and cardiovascular death at 30 days. Use of corticosteroids improved renal function, increased urine output, and reduced mortality (3/51 in corticosteroid group versus 10/51 in the placebo group) [52]. The mechanisms underlying the improvements with corticosteroids were not determined, but were hypothesized to be facilitation of natriuretic peptides or dilation of renal vasculature through activation of nitric oxide pathway or dopaminergic system.
Serelaxin
Serelaxin is a recombinantly expressed human relaxin-2, a peptide hormone present during pregnancy which facilitates physiological cardiovascular and renal adaptations [53–55], which showed potential benefits in CRS1. Analysis of the RELAX-AHF trial revealed serelaxin reduced incidence of worsening renal function at day 2 of treatment as defined by changes in serum creatinine, cystatin C, and BUN. Importantly, worsening renal function defined by cystatin C changes was associated with increased 180-day mortality in this analysis [56]. The mechanisms by which serelaxin prevented renal dysfunction are currently unknown as serelaxin treatment did not improve diuretic efficiency [19].
Ultrafiltration
Another treatment choice in CRS1 is mechanical removal of salt and water via ultrafiltration. Ultrafiltration showed early promise in Ultrafiltration Versus Intravenous Diuretics for Patients Hospitalized for Acute Decompensated Heart Failure trial (UNLOAD) trial. In this study, 200 patients with ADHF were randomized to either ultrafiltration or medical management with loop diuretics. Use of ultrafiltration increased volume removal without any differences in renal function and reduced rehospitalization rates at 90 days [57].
However, when ultrafiltration was employed specifically in CRS1 patients in the Cardiorenal Rescue Study in Acute Decompensated Heart Failure trial (CARESS-HF), UF was not superior to medical treatment. There were 188 patients studied in CARESS-HF, and in the ultrafiltration arm there was increased risk of renal dysfunction, no differences in volume removal, and no change in rehospitalization rates at 90 days [58]. When trying to reconcile UNLOAD and CARESS-HF, the medical treatment arm in CARESS-HF was much more standardized and aggressive and UNLOAD was earlier implementation of ultrafiltration, which may have explained the differences. Interestingly, ultrafiltration was hypothesized to be advantageous over diuretic therapy through reduced activation of the renin-angiotensin-aldosterone system, but analysis of the patients from CARESS-HF showed higher levels of plasma renin activity and no difference in aldosterone levels in ultrafiltration patients [59].
Two meta-analyses have examined the use of ultrafiltration versus medical management in ADHF and both showed ultrafiltration was more effective in volume removal than medical therapy but did not improve rehospitalization or mortality rates [60,61]. This fact combined with the risks of vascular access placement and bleeding from anticoagulation limits to routine use of ultrafiltration in CRS1.
Continuous Renal Replacement Therapy
Once renal function deteriorates to the point that renal replacement therapy is needed for both volume removal and solute clearance in CRS1, continuous renal replacement therapy (CRRT) may be implemented. Unfortunately, there are few available data for this group of advanced CRS1 patients to guide physicians. There was a single-centered study conducted in Egypt that randomized 40 ADHF patients to either IV furosemide or CRRT. The patients treated with CRRT had greater weight loss and decreased length of stay in the ICU, but there were no differences in dialysis dependence rates or 30-day mortality [62]. Two single-centered studies reported outcomes associated with advanced CRS1 requiring CRRT. In a study conducted at the Cleveland Clinic, 63 patients with CRS1 were treated with ultrafiltration, of which 37 were converted to CRRT due to worsening renal function. Of the 37 patients treated with CRRT, 16 died in the hospital and 4 were discharged with hospice care [63]. In another retrospective study performed at the University of Alabama-Birmingham, use of rescue CRRT in advanced CRS1 was examined in 37 patients. 23 patients died during hospitalization and 2 were discharged to hospice care [64]. Combination of the Cleveland Clinic and University of Alabama-Birmingham studies revealed patients requiring CRRT in the setting of advanced CRS1 had an in-hospital mortality or palliative discharge rate of 60.8% (45/74). Clearly, this population needs further investigation to prevent such poor outcomes.
Future Treatment Options
Ongoing and Unreported Clinical Trials
Unfortunately, none of the current treatments for CRS1 have definitive improvements in outcomes, but there are several ongoing clinical trials which will hopefully identify novel treatment strategies. First of all, the Acetazolamide and Spironolactone to Increase Natriuresis in Congestive Heart Failure (Diuresis-CHF) trial is being conducted in Belgium. This study will examine the effects of acetazolamide with low dose diuretic versus high dose diuretics in one aim and the effects of upfront spironolactone in another. The outcomes analyzed will include total natriuresis, potassium homeostasis, NT-proBNP changes, change in renal function, peak serum levels of renin and aldosterone, weight change, urine volume, and change in edema (NCT01973335). The Protocolized Diuretic Strategy in Cardiorenal Failure (ProDius) trial is being performed at the University of Pittsburgh, and will determine the effects of a protocolized diuretic approach to target 3-5 liters of urine production a day versus standard therapy and will track the change in body weight, length of hospitalization, reshospitalization rates, mortality rates, venous compliance of internal jugular vein, clinical decongestion, change in renal function, and urine output (NCT01921829). The Levosimendan versus Dobutamine for Renal Function in Heart Failure (ELDOR) study is ongoing in Sweden and will probe the acute effects of levosimendan and dobutamine on renal perfusion. The endpoints will include changes in renal blood flow, GFR, renal vascular resistance, central hemodynamics, renal oxygen extraction and consumptions, and filtration fraction (NCT02133105). Finally, the Safety and Efficacy of Low Dose Hypertonic Saline and High Dose Furosemide for Congestive Heart Failure (REaCH) trial probed the effects of combination of hypertonic saline and furosemide versus furosemide in patients with ADHF and renal impairment defined by a GFR<60 mL/min. The outcomes were change in renal function, diuretic response, length of hospital stay, readmission rates, weight loss, BNP levels, and included a cost analysis. The study was completed but results are not currently available (NCT01028170)
Should Inflammation Be Targeted in CRS1?
Although proposed to play a role in the pathophysiology of CRS1, inflammation has not been explicitly targeted as a treatment for CRS1. One possible way to combat inflammation could be inhibition of the IL-6 pathway, which is support by preclinical work as previous studies showed IL-6 knockout mice were resistant to HgCl2-induced renal injury and death [65] and IL-6 has negative inotropic effects in both isolated cardiomyocytes [66] and intact animals [67]. Thus, IL-6 antagonism may improve both cardiac and renal function, an ideal scenario for CRS1 patients. The availability of tocilizumab, an FDA-approved humanized antibody to the IL-6 receptor, may allow for investigation of this hypothesis in the future. Although not examined in the COPE-ADHF trial, an alternative explanation for the improvements associated with corticosteroids treatment were the anti-inflammatory effects. If this were true, corticosteroids would represent a relatively cheap treatment option for CRS1 patients, but more studies need to be conducted before this approach is widely implemented. Finally, use of cytokine profiling may be used to enrich a population of CRS1 patients that could be investigated in future clinical trials using anti-inflammatory medications.
Unanswered Questions Moving Forward
Severity of AKI and Treatment Effects
An important unknown that warrants further investigation is if the severity of AKI should dictate treatment choice in CRS1. As discussed above, increasing severity of AKI resulted in elevated risk of adverse events, but it remains unknown whether different treatments offer benefits for more or less severe renal impairment. Perhaps, future studies aimed at defining outcomes from different treatment strategies stratified by severity of renal dysfunction may reveal which patients benefit from the various treatment options for CRS1.
How Do We Best Define Renal Dysfunction in CRS1?
Currently, there is no accepted definition of renal dysfunction in CRS1. As discussed above, using the AKIN, KDIGO, or RIFLE scoring systems or diuretic responsiveness effectively differentiated outcomes in patients with CRS1. However, an agreed-upon definition would likely benefit the field going forward so this population could be systematically investigated in future studies.
Conclusion
In summary, CRS1 is a common clinical entity associated with poor patient outcomes. A complex pathophysiology marked by reduced cardiac output, increased central venous pressure, inflammation, and oxidative stress underlies the disease process. Unfortunately, no current treatment approach shows consistent improvements in outcomes, highlighting the urgent need for further research to reduce the burden that CRS1 imposes.
Corresponding author: Kurt W. Prins, MD, PhD, MMC 580 Mayo, 420 Delaware St SE, Minneapolis, MN 55455, [email protected].
Funding/support: Dr. Prins is funded by NIH F32 grant HL129554 and Dr. Thenappen is funded by AHA Scientist Development Grant 15SDG25560048.
From the Cardiovascular Division, Department of Internal Medicine, University of Minnesota, Minneapolis, MN.
Abstract
- Objective: To present a review of cardiorenal syndrome type 1 (CRS1).
- Methods: Review of the literature.
- Results: Acute kidney injury occurs in approximately one-third of patients with acute decompensated heart failure (ADHF) and the resultant condition was named CRS1. A growing body of literature shows CRS1 patients are at high risk for poor outcomes, and thus there is an urgent need to understand the pathophysiology and subsequently develop effective treatments. In this review we discuss prevalence, proposed pathophysiology including hemodynamic and nonhemodynamic factors, prognosticating variables, data for different treatment strategies, and ongoing clinical trials and highlight questions and problems physicians will face moving forward with this common and challenging condition.
- Conclusion: Further research is needed to understand the pathophysiology of this complex clinical entity and to develop effective treatments.
Acute decompensated heart failure (ADHF) is an epidemic facing physicians throughout the world. In the United States alone, ADHF accounts for over 1 million hospitalizations annually, with costs in 2012 reaching $30.7 billion [1]. Despite the advances in chronic heart failure management, ADHF continues to be associated with poor outcomes as exemplified by 30-day readmission rates of over 20% and in-hospital mortality rates of 5% to 6%, both of which have not significantly improved over the past 20 years [2,3]. One of the strongest predictors of adverse outcomes in ADHF is renal dysfunction. An analysis from the Acute Decompensated Heart Failure National Registry (ADHERE) revealed the combination of renal dysfunction (creatinine > 2.75 mg/dL and blood urea nitrogen (BUN) > 43 mg/dL) and hypotension (systolic blood pressure (SBP) < 115 mm Hg) upon admission was associated with an in-hospital mortality of > 20% [4]. The Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) registry documented a 16.3% in-hospital mortality when patients had a SBP < 100 mm Hg and creatinine > 2.0 mg/dL at admission [5].
The presence of acute kidney injury in the setting of ADHF is a very common occurrence and was termed cardiorenal syndrome type 1 (CRS1) [6]. The prevalence of CRS1 in single-centered studies ranged from 32% to 40% of all ADHF admissions [7,8]. If this estimate holds true throughout the United States, there would be 320,000 to 400,000 hospitalizations for CRS1 annually, highlighting the magnitude of this problem. Moreover, with the number of patients with heart failure expected to continue to rise, CRS1 will only become more prevalent in the future. In this review we discuss the prevalence, proposed pathophysiology including hemodynamic and nonhemodynamic factors, prognosticating variables, data for different treatment strategies, ongoing clinical trials, and highlight questions and problems physicians will face moving forward in this common and challenging condition.
Pathogenesis of CRS1
Hemodynamic Effects
The early hypothesis for renal dysfunction in ADHF centered on hemodynamics, as reduced cardiac output was believed to decrease renal perfusion. However, analysis of invasive hemodynamics from patients with ADHF suggested that central venous pressure (CVP) was actually a better predictor of the development of CRS1 than cardiac output. In a single-center study conducted at the Cleveland Clinic, hemodynamics from 145 patients with ADHF were evaluated and surprisingly baseline cardiac index was greater in the patients with CRS1 than patients without renal dysfunction (2.0 ± 0.8 L/min/m2 vs 1.8 ± 0.4 L/min/m2; P = 0.008). However, baseline CVP was higher in the CRS1 group (18 ± 7 mm Hg vs 12 ± 6 mm Hg; P = 0.001), and there was a heightened risk of developing CRS1 as CVP increased. In fact, 75% of the patients with a CVP of > 24 mm Hg developed renal impairment [9]. In a retrospective study of the Evaluation Study of Congestive Heart Failure and Pulmonary Arterial Catheter Effectiveness (ESCAPE) trial, the only hemodynamic parameter that correlated with baseline creatinine was CVP. However, no invasive measures predicted worsening renal function during hospitalization [10]. Finally, an experiment that used isolated canine kidneys showed increased venous pressure acutely reduced urine production. Interestingly, this relationship was dependent on arterial pressure; as arterial flow decreased smaller increases in CVP were needed to reduce urine output [11]. Together, these data suggest increased CVP plays an important role in CRS1, but imply hemodynamics alone may not fully explain the pathophysiology of CRS1.
Inflammation
As information about how hemodynamics incompletely predict renal dysfunction in ADHF became available, alternative hypotheses were investigated to gain a deeper understanding of the pathophysiology underlying CRS1. A pathological role of inflammation in CRS1 has gained attention due to recent publications. First of all, serum levels of the pro-inflammatory cytokines TNF-a and IL-6 were elevated in patients with CRS1 when compared to health controls [12]. Interestingly, Virzi et al showed that the median value of IL-6 was 5 times higher in CRS1 patients when compared to ADHF patients without renal dysfunction [13]. The negative consequences of elevated serum cytokines were demonstrated when incubation of a human cell line of monocytes with serum from CRS1 patients induced apoptosis in 81% of cells compared to just 11% of cells with control serum [12]. It is possible that cytokine-induced apoptosis could occur in other cell types in different organs in patients with CRS1, which may contribute to both cardiac and renal dysfunction. Finally, analysis from a rat model of CRS1 revealed macrophage infiltration into the kidneys and increased numbers of activated monocytes in the peripheral blood. Interestingly, monocyte/macrophage depletion using liposome clodronate prevented chronic renal dysfunction in the rat model [14]. In summary, these data suggest inflammation contributes to CRS1 pathophysiology, but more experimental data is needed to determine if there is a causal relationship.
Oxidative Stress
Very recently, oxidative stress was proposed to play a role in CRS1. Virzi et al analyzed serum levels of markers of oxidative stress and compared ADHF patients without renal impairment to CRS1 patients. The markers of oxidative stress, which included myeloperoxidase, nitric oxide, copper/zinc superoxide dismutase, and endogenous peroxidase, were all significantly higher in CRS1 patients [13]. While provocative, the tissues responsible for the generation of these molecules and the subsequent effects have not yet been fully elucidated.
Prognostication
Severity of Acute Kidney Injury
Initial publications did not document a strong link between kidney injury and poor outcomes in ADHF. Firstly, Ather et al performed a single-centered study that investigated how change in renal function defined by change in creatinine, estimated GFR, and BUN affected outcomes one year post admission for ADHF. Kidney injury defined by a change in creatinine or in estimated GFR was not associated with increased risk of mortality, but a change in BUN was associated with increased mortality in a univariate analysis [15]. Testani et al retrospectively analyzed patients from the ESCAPE trial and found worsening renal function defined by a ≥ 20% reduction in estimated GFR was not significantly associated with 180-day mortality, but there was a trend towards higher mortality (hazard ration 1.4; P = 0.11) [16]. Importantly, neither of 2 these studies assessed how severity of AKI impacted outcomes, which may have contributed to the weak relationships observed.
Diuretic Responsiveness
Voors et al performed a retrospective analysis of diuretic responsiveness in 1161 patients from the Relaxin in Acute Heart Failure (RELAX-AHF) trial. Diuretic responsiveness was defined as weight change (kg) per diuretic dose (IV furosemide and PO furosemide) over 5 days and then patients were separated into tertiles. The lowest tertile group had an approximate 20% incidence of 60-day combined end-point of death, heart failure or renal failure readmission compared to less than 10% incidence in the middle and upper tertiles. Interestingly, when the effects of worsening renal function (WRF), defined as creatinine change of ≥ 0.3 mg/dL, were examined in patients stratified by diuretic response, WRF did not offer additional prognostic information [19].
Finally, Valenete et al analyzed diuretic response in 1745 patients from the PROTECT trial (Placebo-Controlled Randomized Study of the Selective A1-Adenosine Receptor Antagonist Rolofylline for Patients Hospitalized with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function). Diuretic response was calculated using the weight change per 40 mg of furosemide, and as diuretic response declined there was increasing risk of 60-day rehospitalization and 180-day mortality rates. In fact, the lowest quintile responders had a 25% mortality rate at 180 days [20].
Emerging Biomarkers
Urine Neutrophil Gelatinase-Associated Lipocalin
Because previous studies showed urinary levels of NGAL was an earlier and more reliable marker of renal dysfunction than creatinine in AKI [21], it was studied as a possible biomarker for the development of CRS1 in ADHF. A single-centered study quantified levels of urine NGAL in 100 patients admitted with heart failure and then tracked the rates of acute kidney injury. Urine NGAL was elevated in patients that developed AKI and a cut-off value 12 ng/mL had a sensitivity of 79% and specificity of 67% for predicting CRS1 [22]. While promising, further studies are needed to better define the role of NGAL in CRS1.
Cystatin C
Cystatin C is a ubiquitously expressed cysteine protease that has a constant production rate and is freely filtered by the glomerulus without being secreted into the tubules, and has effectively prognosticated outcomes in ADHF [23]. Lassus et al showed an adjusted hazard ratio of 3.2 (2.0–5.3) for 12-month mortality when cystatin C levels were elevated. Moreover, patients with the highest tertitle of NT-proBNP and cystatin C had a 48.7% 1-year mortality. Interestingly, patients with an elevated cystatin C but normal creatinine had a 40.6% 1-year mortality compared to 12.6% for those with normal cystatin C and creatinine [24]. Furthermore, Arimoto et al showed elevated cystatin C predicted death or rehospitalization in a small cohort of ADHF patients in Japan [25]. Also, Naruse et al showed cystatin C was a better predictor of cardiac death than estimated GFR by the Modification of Diet in Renal Disease Study (MDRD) equation [26]. Finally, Manzano-Fernandez et al showed the highest tertile of cystatin C was a significant independent risk factor for 2-year death or rehospitalization while creatinine and MDRD estimates of GFR were not [27]. In agreement with Lassus et al, elevations in either 2 or 3 of cystatin C, troponin, and NT-proBNP predicted death or rehospitalization when compared to those with normal levels of these 3 markers [27]. In conclusion, cystatin C either alone or in combination with other biomarkers identifies high-risk patients.
Kidney Injury Molecule 1
Kidney injury molecule 1 (KIM-1) is a type-1 cell membrane glycoprotein expressed in regenerating proximal tubular cells but not under normal conditions [28]. Although associated with increased risk of hospitalization and mortality in chronic heart failure [29,30], elevated levels of urinary KIM-1 did not predict mortality in ADHF [31]. Further studies are needed to elucidate the utility of KIM-1 in CRS1.
Treatment Approaches
Diuretics
Loop diuretics are the main treatment for decongestion of patients with CRS1. To date, no clinical trial has compared the different loop diuretics (furosemide, bumetanide, torsemide, or ethacrynic acid) to each other, so there is no clear choice of which loop diuretic is the best. However, dosing scheme was investigated in the Dose Optimization Strategies Evaluation (DOSE) trial. In this trial, 308 patients were randomized in a 1:1:1:1 design in which patients were placed in groups with low-dose (equivalent to oral dose) or high-dose (2.5 times oral dose) intermittent parental therapy or alternatively low-dose or high-dose continuous drip therapy. There were no differences in dyspnea, fluid changes, change in creatinine, hospital length stay, or rehospitalization and death rates when the intermittent and drip approaches were compared. However, the high-dose arm had decreased dyspnea, increased volume removal, but there were more occurrences of AKIs when compared to the low-dose arm [32].
In clinical practice, if loop diuretic treatment does not result in the desired urine output, a second-site diuretic may be added to potentiate diuresis. Unfortunately, there is little data on this common clinical practice and thus the optimal choice of second site agent (chlorthiazide or metolazone) is unknown. Frequently, the deciding factor is based upon cost or concern that oral absorption of metolazone will be ineffective. However, Moranville et al recently performed a retrospective assessment comparing chlorthiazide (22 patients) to metolazone (33 patients) in ADHF patients with renal dysfunction defined by a creatinine clearance of 15–50 mL/min. There was a nonsignificant trend towards increased urine output in the metolazone group, no differences in the rates of adverse events, and the chlorthiazide group actually had a longer hospital stay [33]. While potentially promising results, the retrospective nature of the study made it difficult to determine if the differences were due to treatment approach or dissimilarities of patient illness. Nonetheless, physicians must remain vigilant when implementing the second-site diuretic approach because it can lead to marked diuretic response leading to metabolic derangements including hypokalemia, hyponatremia, hypomagnesaemia, and metabolic alkalosis.
Inotropes
The use of inotropic agents such as dobutamine or milrinone can be used to augment cardiac function when there is a known low-output state for better renal perfusion in CRS1. Unfortunately, there is little objective data available about the utility of this widely implemented approach. The Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of a Chronic Heart Failure (OPTIME-HF) trial did not show improved renal function with milrinone treatment [34]. The use of levosimendan, a cardiac calcium sensitizer that increases contractility not currently approved in the United States, was compared to dobutamine in the Survival of Patients With Acute Heart Failure in Need of Intravenous Inotropic Support (SURVIVE) trial, and there were no differences in rates of renal failure when the 2 groups were compared [35]. Nonetheless, if cardiac output is severely compromised, inotropes can be used for CRS1 treatment, but they should be used cautiously due the increased risks of lethal arrhythmias.
Dopamine
Use of low-dose dopamine to stimulate D1 and D2 receptors as a way to increase renal blood flow and promote increased glomerular filtration and urine production was extensively studied in ADHF. A small trial showed use of low dose dopamine had renal protective effects in a total of 20 patients [36]. However, when larger trials were conducted, such beneficial results were not consistently observed. The Dopamine in Acute Decompensated Heart Failure (DAD-HF I) trial compared low-dose furosemide plus low-dose dopamine (5 µg/kg/min) to high-dose furosemide alone in 60 patients. There were no differences in total diuresis, hospital stay, and 60-day mortality or rehospitalization rates, but there was a reduction in the renal dysfunction at the 24-hour time point in the dopamine-treated arm (6.7% versus 30%) [37]. The Dopamine in Acute Decompensated Heart Failure II trial randomized 161 ADHF patients to high-dose furosemide, low-dose furosemide and lose dose dopamine (5 µg/kg/min), or low-dose furosemide and assessed dyspnea, worsening renal function, length of stay, 60-day and one-year all-cause mortality and hospitalization for heart failure. Dopamine treatment did not improve any of the outcomes measured [38]. Finally, the most recent trial to examine the effects of dopamine was the Renal Optimization Strategies Evaluation (ROSE) trial. In this trial, there were 360 patients with ADHF randomized to nesiritide or dopamine versus placebo in a 2:1 design. When comparing dopamine (111 patients) treatment to placebo (115 patients), there were no differences in urine output, renal function as determined by cystatin C levels, or symptomatic improvements. However, there was more tachycardia in the dopamine group [39]. Currently, there is not strong evidence supporting routine use of dopamine in CRS1.
Nesiritide
Use of nesiritide, recombinant brain natriuretic peptide, was also investigated as a way to enhance urine production through the natriuretic effects of the peptide. The first attempt to explore this hypothesis was the B-Type Natriuretic Peptide in Cardiorenal Decompensation Syndrome (BNP-CARDS) trial. BNP-CARDS showed a 48-hour infusion of nesiritide (39 patients) or placebo (36 patients) in patients with ADHF and renal dysfunction (estimated GFR between 15–60 mL/min) did not reduce the incidence of worsening renal function as defined by a rise in serum creatinine by 20% [40]. A similar approach was implemented in the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND-HF) trial which examined over 7000 patients with ADHF. 3496 patients were treated with nesiritide and 3511 patients were treated with placebo for 24 hours and up to 7 days. Nesiritide treatment did not alter dyspnea at 6 and 24 hours, improve renal function as determined by creatinine change, or alter the combined end-point of rehospitalization or death 30 at days [41]. The ROSE trial examined the effects of nesiritide (117 patients) versus placebo (115 patients) for urine production, change in renal function as defined by change in cystatin C, and decongestion (urinary sodium excretion, weight change, and change in NT-proBNP) at 72 hours. Nesiritide did not alter any of the outcomes investigated [39]. Finally, a single-centered study conducted at the Mayo Clinic examined the effects of nesiritide (37 patients) or placebo (35 patients) with ADHF and pre-existing renal dysfunction (estimated GFR between 20 and 60 mL/min). These investigators found nesiritide treatment resulted in less renal dysfunction as measured by creatinine and BUN, but no changes in diuretic responsiveness, duration of hospitalization, or rehospitalization rates. Nesiritide did reduce serum endothelin levels, but had no effect on ANP, NT-pro BNP, renin, angiotensin II, or aldosterone [42]. In summary, nesiritide does not appear to have significant renal protective effects in ADHF.
Adenosine A1 Receptor Antagonists
The use of adenosine receptor antagonists to prevent adenosine-mediated vasoconstriction of renal vasculature in ADHF has also been examined. The first study conducted was a small double-blind randomized-controlled trial that investigated the effects of rolofylline, an adenosine A-1 antagonist, in patients with ADHF and an estimated creatinine clearance of 20-80 mL/min. The study had 27 patients in the placebo arm, 29 patients that received 2.5 mg of rolofylline, 31 patients received 15 mg of rolofylline, 30 patients received 30 mg of rolofylline, and 29 patients received 60 mg of rolofylline, all of which was daily for up to 3 days. Rolofylline treatment increased urine output on day 1 and improved renal function on day 2 [43]. These positive results led to the Placebo-Controlled Randomized Study of Selective Adenosine A1 Receptor Antagonist Rolofylline for Patients with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function (PROTECT) Trial. PROTECT assessed the effects of rolofylline (1356) or placebo (677) in patients with ADHF and an estimated creatinine clearance between 20 and 80 mL/min. There were no significant differences in renal function out to 14 days, but rolofylline led to more weight loss than placebo [44,45]. In a subgroup analysis of patients with severe baseline renal dysfunction (creatinine clearance of less than 30 mL/min), rolofylline reduced the combined 60-day end-point of hospitalization due to cardiovascular or renal cause and death [45]. Finally, the Effects of KW-3902 Injectable Emulsion on Heart Failure Signs and Symptoms, Diuresis, Renal Function, and Clinical Outcomes in Subjects Hospitalized with Worsening Renal Function and Heart Failure Requiring Intravenous Therapy (REACH-UP) trial probed the effects of rolofylline (36 patients) or placebo (40 patients) in patients with ADHF and renal impairment (creatinine clearance of 20-60 mL/min). Rolofylline treatment did not alter renal function, but there was a nonsignificant trend towards reduction in 60-day combined end-point of hospitalization due to renal or cardiovascular causes or death [46]. In summary, the use of rolofylline has not been conclusively associated with improved outcomes in CRS1.
Vasopressin Antagonists
The use of vasopressin antagonists to induce aquaphoresis and combat hyponatremia was studied in ADHF. Vasopressin antagonists were first investigated in the Acute and Chronic Therapeutic Impact of a Vasopressin Antagonist (ACTIV) trial. ACTIV involved 3 doses of tolvaptan (78 patients received 30 mg, 84 patients received 60 mg, and 77 patients received 90 mg) versus placebo (80 patients), and tolvaptan increased urine production and decreased body weight compared to placebo without compromising renal function. A post-hoc analysis of patients with renal dysfunction (BUN > 29 mg/dL) and severe volume overload revealed a survival benefit at 60 days [47]. The Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVERST) trial compared placebo (2061 patients) versus 30 mg/day of tolvaptan (2072 patients) within 48 hours after admission in an identical 2-trial design. Tolvaptan increased weight loss and reduced dyspnea acutely but did not alter all-cause mortality or cardiovascular or heart failure hospitalization rates out to 24 months post index hospitalization [48,49]. These data suggest vasopressin antagonists may potentiate diuresis acutely but likely do not improve long-term outcomes.
Corticosteroids
The use of corticosteroids in ADHF has been controversial as there were initial concerns that corticosteroids would increase fluid retention. However, corticosteroids augmented diuretic response and improved renal function in 13 ADHF patients who had inadequate response to sequential nephron blockage [50]. Furthermore, Zhang et al showed that prednisone treatment in 35 patients admitted with ADHF increased urinary volume, reduced dyspnea, reduced uric acid, and improved renal function [51]. These promising results led to the Cardiac Outcome Prevention Effectiveness of Glucocorticoids in Acute Decompensated Heart Failure (COPE-ADHF) trial. In this single-centered study, 102 patients with ADHF were randomized to either placebo [51] or corticosteroids [51] and the outcomes recorded included urinary volume, change in creatinine, and cardiovascular death at 30 days. Use of corticosteroids improved renal function, increased urine output, and reduced mortality (3/51 in corticosteroid group versus 10/51 in the placebo group) [52]. The mechanisms underlying the improvements with corticosteroids were not determined, but were hypothesized to be facilitation of natriuretic peptides or dilation of renal vasculature through activation of nitric oxide pathway or dopaminergic system.
Serelaxin
Serelaxin is a recombinantly expressed human relaxin-2, a peptide hormone present during pregnancy which facilitates physiological cardiovascular and renal adaptations [53–55], which showed potential benefits in CRS1. Analysis of the RELAX-AHF trial revealed serelaxin reduced incidence of worsening renal function at day 2 of treatment as defined by changes in serum creatinine, cystatin C, and BUN. Importantly, worsening renal function defined by cystatin C changes was associated with increased 180-day mortality in this analysis [56]. The mechanisms by which serelaxin prevented renal dysfunction are currently unknown as serelaxin treatment did not improve diuretic efficiency [19].
Ultrafiltration
Another treatment choice in CRS1 is mechanical removal of salt and water via ultrafiltration. Ultrafiltration showed early promise in Ultrafiltration Versus Intravenous Diuretics for Patients Hospitalized for Acute Decompensated Heart Failure trial (UNLOAD) trial. In this study, 200 patients with ADHF were randomized to either ultrafiltration or medical management with loop diuretics. Use of ultrafiltration increased volume removal without any differences in renal function and reduced rehospitalization rates at 90 days [57].
However, when ultrafiltration was employed specifically in CRS1 patients in the Cardiorenal Rescue Study in Acute Decompensated Heart Failure trial (CARESS-HF), UF was not superior to medical treatment. There were 188 patients studied in CARESS-HF, and in the ultrafiltration arm there was increased risk of renal dysfunction, no differences in volume removal, and no change in rehospitalization rates at 90 days [58]. When trying to reconcile UNLOAD and CARESS-HF, the medical treatment arm in CARESS-HF was much more standardized and aggressive and UNLOAD was earlier implementation of ultrafiltration, which may have explained the differences. Interestingly, ultrafiltration was hypothesized to be advantageous over diuretic therapy through reduced activation of the renin-angiotensin-aldosterone system, but analysis of the patients from CARESS-HF showed higher levels of plasma renin activity and no difference in aldosterone levels in ultrafiltration patients [59].
Two meta-analyses have examined the use of ultrafiltration versus medical management in ADHF and both showed ultrafiltration was more effective in volume removal than medical therapy but did not improve rehospitalization or mortality rates [60,61]. This fact combined with the risks of vascular access placement and bleeding from anticoagulation limits to routine use of ultrafiltration in CRS1.
Continuous Renal Replacement Therapy
Once renal function deteriorates to the point that renal replacement therapy is needed for both volume removal and solute clearance in CRS1, continuous renal replacement therapy (CRRT) may be implemented. Unfortunately, there are few available data for this group of advanced CRS1 patients to guide physicians. There was a single-centered study conducted in Egypt that randomized 40 ADHF patients to either IV furosemide or CRRT. The patients treated with CRRT had greater weight loss and decreased length of stay in the ICU, but there were no differences in dialysis dependence rates or 30-day mortality [62]. Two single-centered studies reported outcomes associated with advanced CRS1 requiring CRRT. In a study conducted at the Cleveland Clinic, 63 patients with CRS1 were treated with ultrafiltration, of which 37 were converted to CRRT due to worsening renal function. Of the 37 patients treated with CRRT, 16 died in the hospital and 4 were discharged with hospice care [63]. In another retrospective study performed at the University of Alabama-Birmingham, use of rescue CRRT in advanced CRS1 was examined in 37 patients. 23 patients died during hospitalization and 2 were discharged to hospice care [64]. Combination of the Cleveland Clinic and University of Alabama-Birmingham studies revealed patients requiring CRRT in the setting of advanced CRS1 had an in-hospital mortality or palliative discharge rate of 60.8% (45/74). Clearly, this population needs further investigation to prevent such poor outcomes.
Future Treatment Options
Ongoing and Unreported Clinical Trials
Unfortunately, none of the current treatments for CRS1 have definitive improvements in outcomes, but there are several ongoing clinical trials which will hopefully identify novel treatment strategies. First of all, the Acetazolamide and Spironolactone to Increase Natriuresis in Congestive Heart Failure (Diuresis-CHF) trial is being conducted in Belgium. This study will examine the effects of acetazolamide with low dose diuretic versus high dose diuretics in one aim and the effects of upfront spironolactone in another. The outcomes analyzed will include total natriuresis, potassium homeostasis, NT-proBNP changes, change in renal function, peak serum levels of renin and aldosterone, weight change, urine volume, and change in edema (NCT01973335). The Protocolized Diuretic Strategy in Cardiorenal Failure (ProDius) trial is being performed at the University of Pittsburgh, and will determine the effects of a protocolized diuretic approach to target 3-5 liters of urine production a day versus standard therapy and will track the change in body weight, length of hospitalization, reshospitalization rates, mortality rates, venous compliance of internal jugular vein, clinical decongestion, change in renal function, and urine output (NCT01921829). The Levosimendan versus Dobutamine for Renal Function in Heart Failure (ELDOR) study is ongoing in Sweden and will probe the acute effects of levosimendan and dobutamine on renal perfusion. The endpoints will include changes in renal blood flow, GFR, renal vascular resistance, central hemodynamics, renal oxygen extraction and consumptions, and filtration fraction (NCT02133105). Finally, the Safety and Efficacy of Low Dose Hypertonic Saline and High Dose Furosemide for Congestive Heart Failure (REaCH) trial probed the effects of combination of hypertonic saline and furosemide versus furosemide in patients with ADHF and renal impairment defined by a GFR<60 mL/min. The outcomes were change in renal function, diuretic response, length of hospital stay, readmission rates, weight loss, BNP levels, and included a cost analysis. The study was completed but results are not currently available (NCT01028170)
Should Inflammation Be Targeted in CRS1?
Although proposed to play a role in the pathophysiology of CRS1, inflammation has not been explicitly targeted as a treatment for CRS1. One possible way to combat inflammation could be inhibition of the IL-6 pathway, which is support by preclinical work as previous studies showed IL-6 knockout mice were resistant to HgCl2-induced renal injury and death [65] and IL-6 has negative inotropic effects in both isolated cardiomyocytes [66] and intact animals [67]. Thus, IL-6 antagonism may improve both cardiac and renal function, an ideal scenario for CRS1 patients. The availability of tocilizumab, an FDA-approved humanized antibody to the IL-6 receptor, may allow for investigation of this hypothesis in the future. Although not examined in the COPE-ADHF trial, an alternative explanation for the improvements associated with corticosteroids treatment were the anti-inflammatory effects. If this were true, corticosteroids would represent a relatively cheap treatment option for CRS1 patients, but more studies need to be conducted before this approach is widely implemented. Finally, use of cytokine profiling may be used to enrich a population of CRS1 patients that could be investigated in future clinical trials using anti-inflammatory medications.
Unanswered Questions Moving Forward
Severity of AKI and Treatment Effects
An important unknown that warrants further investigation is if the severity of AKI should dictate treatment choice in CRS1. As discussed above, increasing severity of AKI resulted in elevated risk of adverse events, but it remains unknown whether different treatments offer benefits for more or less severe renal impairment. Perhaps, future studies aimed at defining outcomes from different treatment strategies stratified by severity of renal dysfunction may reveal which patients benefit from the various treatment options for CRS1.
How Do We Best Define Renal Dysfunction in CRS1?
Currently, there is no accepted definition of renal dysfunction in CRS1. As discussed above, using the AKIN, KDIGO, or RIFLE scoring systems or diuretic responsiveness effectively differentiated outcomes in patients with CRS1. However, an agreed-upon definition would likely benefit the field going forward so this population could be systematically investigated in future studies.
Conclusion
In summary, CRS1 is a common clinical entity associated with poor patient outcomes. A complex pathophysiology marked by reduced cardiac output, increased central venous pressure, inflammation, and oxidative stress underlies the disease process. Unfortunately, no current treatment approach shows consistent improvements in outcomes, highlighting the urgent need for further research to reduce the burden that CRS1 imposes.
Corresponding author: Kurt W. Prins, MD, PhD, MMC 580 Mayo, 420 Delaware St SE, Minneapolis, MN 55455, [email protected].
Funding/support: Dr. Prins is funded by NIH F32 grant HL129554 and Dr. Thenappen is funded by AHA Scientist Development Grant 15SDG25560048.
1. Mozaffarian D, Benjamin EJ, Go AS,et al. Heart disease and stroke statistics--2015 update: A report from the american heart association. Circulation 2015;131:e29–322.
2. Gheorghiade M, Vaduganathan M, Fonarow GC, Bonow RO. Rehospitalization for heart failure: problems and perspectives. J Am Coll Cardiol 2013;61:391–403.
3. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the medicare fee-for-service program. N Engl J Med 2009;360:1418–28.
4. Fonarow GC, Adams KF Jr, Abraham WT, et al and ADHERE Scientific Advisory Committee, Study Group, and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: Classification and regression tree analysis. JAMA 2005;293:572–80.
5. Abraham WT, Fonarow GC, Albert NM, et al. Predictors of in-hospital mortality in patients hospitalized for heart failure: Insights from the organized program to initiate lifesaving treatment in hospitalized patients with heart failure (OPTIMIZE-HF). J Am Coll Cardiol 2008;52:347–56.
6. Ronco C, Haapio M, House AA, et al. Cardiorenal syndrome. J Am Coll Cardiol 2008:52:1527–39.
7. Roy AK, Mc Gorrian C, Treacy C, et al. A comparison of traditional and novel definitions (RIFLE, AKIN, and KDIGO) of acute kidney injury for the prediction of outcomes in acute decompensated heart failure. Cardiorenal Med 2013;3:26–37.
8. Li Z, Cai L, Liang X, et al. Identification and predicting short-term prognosis of early cardiorenal syndrome type 1: KDIGO is superior to RIFLE or AKIN. PLoS One 2014;9:e114369.
9. Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009:53:589–96.
10. Nohria A, Hasselblad V, Stebbins A, et al. Cardiorenal interactions: Insights from the ESCAPE trial. J Am Coll Cardiol 2008:51:1268–74.
11. Winton FR. The influence of venous pressure on the isolated mammalian kidney. J Physiol 1931;72:49–61.
12. Virzi GM, Torregrossa R, Cruz DN, et al. Cardiorenal syndrome type 1 may be immunologically mediated: A pilot evaluation of monocyte apoptosis. Cardiorenal Med 2012;2:33–42.
13. Virzi GM, Clementi A, de Cal M, et al. Oxidative stress: Dual pathway induction in cardiorenal syndrome type 1 pathogenesis. Oxid Med Cell Longev 2015;391790.
14. Cho E, Kim M, Ko YS, et al. Role of inflammation in the pathogenesis of cardiorenal syndrome in a rat myocardial infarction model. Nephrol Dial Transplant 2013;28:2766–78.
15. Ather S, Bavishi C, McCauley MD, et al. Worsening renal function is not associated with response to treatment in acute heart failure. Int J Cardiol 2013;167:1912–7.
16. Testani JM, McCauley BD, Kimmel SE, Shannon RP. Characteristics of patients with improvement or worsening in renal function during treatment of acute decompensated heart failure. Am J Cardiol 2010;106:1763–69.
17. Hata N, Yokoyama S, Shinada T, et al. Acute kidney injury and outcomes in acute decompensated heart failure: Evaluation of the RIFLE criteria in an acutely ill heart failure population. Eur J Heart Fail 2010;12:32–7.
18. Testani JM, Brisco MA, Turner JM, et al. Loop diuretic efficiency: A metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ Heart Fail 2014;7:261–70.
19. Voors AA, Davison BA, Teerlink JR, et al. Diuretic response in patients with acute decompensated heart failure: Characteristics and clinical outcome--an analysis from RELAX-AHF. Eur J Heart Fail 2014;16:1230–40.
20. Valente MA, Voors AA, Damman K, et al. Diuretic response in acute heart failure: Clinical characteristics and prognostic significance. Eur Heart J 2014;35:1284–93.
21. Devarajan P. Neutrophil gelatinase-associated lipocalin: A troponin-like biomarker for human acute kidney injury. Nephrology (Carlton) 2010;15:419–28.
22. Soyler C, Tanriover MD, Ascioglu S, et al. Urine neutrophil gelatinase-associated lipocalin levels predict acute kidney injury in acute decompensated heart failure patients. Ren Fail 2015;5.
23. Brisco MA,Testani JM. Novel renal biomarkers to assess cardiorenal syndrome. Curr Heart Fail Rep 2014;11;485–99.
24. Lassus J, Harjola VP, Sund R, et al. and FINN-AKVA Study group. Prognostic value of cystatin C in acute heart failure in relation to other markers of renal function and NT-proBNP. Eur Heart J 2007;28:1841–7.
25. Arimoto T, Takeishi Y, Niizeki T, et al. Cystatin C, a novel measure of renal function, is an independent predictor of cardiac events in patients with heart failure. J Card Fail 2005;11:595–601.
26. Naruse H, Ishii J, Kawai T, et al. Cystatin C in acute heart failure without advanced renal impairment. Am J Med 2009;122:566–73.
27. Manzano-Fernandez S, Boronat-Garcia M, Albaladejo-Oton MD, et al. Complementary prognostic value of cystatin C, N-terminal pro-B-type natriuretic peptide and cardiac troponin T in patients with acute heart failure. Am J Cardiol 2009;103:1753–9.
28. Bonventre JV, Yang L. Kidney injury molecule-1. Curr Opin Crit Care 2010;16:556–61.
29. Damman K, Van Veldhuisen DJ, Navis G, et al. Tubular damage in chronic systolic heart failure is associated with reduced survival independent of glomerular filtration rate. Heart 2010;96:1297–302.
30. Jungbauer CG, Birner C, Jung B, et al. Kidney injury molecule-1 and N-acetyl-beta-D-glucosaminidase in chronic heart failure: Possible biomarkers of cardiorenal syndrome. Eur J Heart Fail 2011;13:1104–10.
31. Verbrugge FH, Dupont M, Shao Z, et al. Novel urinary biomarkers in detecting acute kidney injury, persistent renal impairment, and all-cause mortality following decongestive therapy in acute decompensated heart failure. J Card Fail 2013;19:621–8.
32. Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011;364:797–805.
33. Moranville MP, Choi S, Hogg J, et al. Comparison of metolazone versus chlorothiazide in acute decompensated heart failure with diuretic resistance. Cardiovasc Ther 2015;33;42–9.
34. Cuffe MS, Califf RM, Adams KF Jr, et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: A randomized controlled trial. JAMA 2002;287:1541–7.
35. Mebazaa A, Nieminen MS, Packer M, et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: The SURVIVE randomized trial. JAMA 2007;297:1883–91.
36. Varriale P, Mossavi A. The benefit of low-dose dopamine during vigorous diuresis for congestive heart failure associated with renal insufficiency: Does it protect renal function? Clin Cardiol 1997;20:627–30.
37. Giamouzis G, Butler J, Starling RC, et al. Impact of dopamine infusion on renal function in hospitalized heart failure patients: Results of the dopamine in acute decompensated heart failure (DAD-HF) trial. J Card Fail 2010;16:922–30.
38. Triposkiadis FK, Butler J, Karayannis G, et al. Efficacy and safety of high dose versus low dose furosemide with or without dopamine infusion: The dopamine in acute decompensated heart failure II (DAD-HF II) trial. Int J Cardiol 2014;172:115–21.
39. Chen HH, Anstrom KJ, Givertz MM, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: The ROSE acute heart failure randomized trial. JAMA 2013;310:2533–43.
40. Witteles RM, Kao D, Christopherson D, et al. Impact of nesiritide on renal function in patients with acute decompensated heart failure and pre-existing renal dysfunction a randomized, double-blind, placebo-controlled clinical trial. J Am Coll Cardiol 2007;50:1835–40.
41 O'Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med 2011;365:32–43.
42. Owan TE, Chen HH, Frantz RP, et al. The effects of nesiritide on renal function and diuretic responsiveness in acutely decompensated heart failure patients with renal dysfunction. J Card Fail 2008;14:267–75.
43. Givertz MM, Massie BM, Fields TK, et al and CKI-201 and CKI-202 Investigators. The effects of KW-3902, an adenosine A1-receptor antagonist,on diuresis and renal function in patients with acute decompensated heart failure and renal impairment or diuretic resistance. J Am Coll Cardiol 2007;50:1551–60.
44. Massie BM, O'Connor CM, Metra M, et al. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N Engl J Med 2010;363:1419–28.
45. Voors AA, Dittrich HC, Massie BM, et al. Effects of the adenosine A1 receptor antagonist rolofylline on renal function in patients with acute heart failure and renal dysfunction: Results from PROTECT (placebo-controlled randomized study of the selective adenosine A1 receptor antagonist rolofylline for patients hospitalized with acute decompensated heart failure and volume overload to assess treatment effect on congestion and renal function). J Am Coll Cardiol 2011;57:1899–907.
46. Gottlieb SS, Givertz MM, Metra M, et al. The effects of adenosine A(1) receptor antagonism in patients with acute decompensated heart failure and worsening renal function: The REACH UP study. J Card Fail 2010;16:714–9.
47. Gheorghiade M, Gattis WA, O'Connor CM, et al. Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: A randomized controlled trial. JAMA 2004;291:1963–71.
48. Gheorghiade M, Konstam MA, Burnett JC Jr, et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: The EVEREST clinical status trials. JAMA 2007;297:1332–43.
49. Konstam MA, Gheorghiade M, Burnett JC Jr, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: The EVEREST outcome trial. JAMA 2007;297:1319–31.
50. Liu C, Liu G, Zhou C, et al. Potent diuretic effects of prednisone in heart failure patients with refractory diuretic resistance. Can J Cardiol 2007;23:865–8.
51. Zhang H, Liu C, Ji Z, et al. Prednisone adding to usual care treatment for refractory decompensated congestive heart failure. Int Heart J 2008;49:587–95.
52. Liu C, Liu K and COPE-ADHF Study Group. Cardiac outcome prevention effectiveness of glucocorticoids in acute decompensated heart failure: COPE-ADHF study. J Cardiovasc Pharmacol 2014;63:333–8.
53. Teichman SL, Unemori E, Teerlink JR, et al. Relaxin: Review of biology and potential role in treating heart failure. Curr Heart Fail Rep 2010;7:75–82.
54. Conrad KP, Shroff SG. Effects of relaxin on arterial dilation, remodeling, and mechanical properties. Curr Hypertens Rep 2011;13:409–20.
55. Du XJ, Bathgate RA, Samuel CS, et al. Cardiovascular effects of relaxin: From basic science to clinical therapy. Nat Rev Cardiol 2010;7:48–58.
56. Metra M, Cotter G, Davison BA, et al. Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the relaxin in acute heart failure (RELAX-AHF) development program: Correlation with outcomes. J Am Coll Cardiol 2013;61:196-206.
57. Costanzo MR, Guglin ME, Saltzberg MT, et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol 2007;49:675–83.
58. Bart BA, Goldsmith SR, Lee KL, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012;367:2296–304.
59. Mentz RJ, Stevens SR, DeVore AD, et al. Decongestion strategies and renin-angiotensin-aldosterone system activation in acute heart failure. JACC Heart Fail 2015;3:97–107.
60. Ebrahim B, Sindhura K, Okoroh J, et al. Meta-analysis of ultrafiltration versus diuretics treatment option for overload volume reduction in patients with acute decompensated heart failure. Arq Bras Cardiol 2015;104:417–25.
61. Kwong JS, Yu CM. Ultrafiltration for acute decompensated heart failure: A systematic review and meta-analysis of randomized controlled trials. Int J Cardiol 2014;172:395–402.
62. Badawy SS, Fahmy A. Efficacy and cardiovascular tolerability of continuous veno-venous hemodiafiltration in acute decompensated heart failure: A randomized comparative study. J Crit Care 2012;27:106.e7-106.13.
63. Patarroyo M, Wehbe E, Hanna M, et al. Cardiorenal outcomes after slow continuous ultrafiltration therapy in refractory patients with advanced decompensated heart failure. J Am Coll Cardiol 2012;60:1906–12.
64. Prins KW, Wille KM, Tallaj JA, Tolwani AJ. Assessing continuous renal replacement therapy as a rescue strategy in cardiorenal syndrome 1. Clin Kidney J 2015;8:87–92.
65. Nechemia-Arbely Y, Barkan D, Pizov G, et al. IL-6/IL-6R axis plays a critical role in acute kidney injury. J Am Soc Nephrol 2008;19:1106–15.
66. Pathan N, Franklin JL, Eleftherohorinou H, et al. Myocardial depressant effects of interleukin 6 in meningococcal sepsis are regulated by p38 mitogen-activated protein kinase. Crit Care Med 2011;39:1692–711.
67. Janssen SP, Gayan-Ramirez G, Van den Bergh A, et al. Interleukin-6 causes myocardial failure and skeletal muscle atrophy in rats. Circulation 2005;111:996–1005.
68. Bellomo R, Ronco C, Kellum JA and Acute Dialysis Quality Initiative workgroup. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: The second international consensus conference of the acute dialysis quality initiative (ADQI) group. Crit Care 2004;8:R204-12.
69. Mehta RL, Kellum JA, Shah SV, et al and Acute Kidney Injury Network. Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31.
70. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guidelines for acute kidney injury. Kidney Inter Suppl 2012;2:19–36.
1. Mozaffarian D, Benjamin EJ, Go AS,et al. Heart disease and stroke statistics--2015 update: A report from the american heart association. Circulation 2015;131:e29–322.
2. Gheorghiade M, Vaduganathan M, Fonarow GC, Bonow RO. Rehospitalization for heart failure: problems and perspectives. J Am Coll Cardiol 2013;61:391–403.
3. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the medicare fee-for-service program. N Engl J Med 2009;360:1418–28.
4. Fonarow GC, Adams KF Jr, Abraham WT, et al and ADHERE Scientific Advisory Committee, Study Group, and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: Classification and regression tree analysis. JAMA 2005;293:572–80.
5. Abraham WT, Fonarow GC, Albert NM, et al. Predictors of in-hospital mortality in patients hospitalized for heart failure: Insights from the organized program to initiate lifesaving treatment in hospitalized patients with heart failure (OPTIMIZE-HF). J Am Coll Cardiol 2008;52:347–56.
6. Ronco C, Haapio M, House AA, et al. Cardiorenal syndrome. J Am Coll Cardiol 2008:52:1527–39.
7. Roy AK, Mc Gorrian C, Treacy C, et al. A comparison of traditional and novel definitions (RIFLE, AKIN, and KDIGO) of acute kidney injury for the prediction of outcomes in acute decompensated heart failure. Cardiorenal Med 2013;3:26–37.
8. Li Z, Cai L, Liang X, et al. Identification and predicting short-term prognosis of early cardiorenal syndrome type 1: KDIGO is superior to RIFLE or AKIN. PLoS One 2014;9:e114369.
9. Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009:53:589–96.
10. Nohria A, Hasselblad V, Stebbins A, et al. Cardiorenal interactions: Insights from the ESCAPE trial. J Am Coll Cardiol 2008:51:1268–74.
11. Winton FR. The influence of venous pressure on the isolated mammalian kidney. J Physiol 1931;72:49–61.
12. Virzi GM, Torregrossa R, Cruz DN, et al. Cardiorenal syndrome type 1 may be immunologically mediated: A pilot evaluation of monocyte apoptosis. Cardiorenal Med 2012;2:33–42.
13. Virzi GM, Clementi A, de Cal M, et al. Oxidative stress: Dual pathway induction in cardiorenal syndrome type 1 pathogenesis. Oxid Med Cell Longev 2015;391790.
14. Cho E, Kim M, Ko YS, et al. Role of inflammation in the pathogenesis of cardiorenal syndrome in a rat myocardial infarction model. Nephrol Dial Transplant 2013;28:2766–78.
15. Ather S, Bavishi C, McCauley MD, et al. Worsening renal function is not associated with response to treatment in acute heart failure. Int J Cardiol 2013;167:1912–7.
16. Testani JM, McCauley BD, Kimmel SE, Shannon RP. Characteristics of patients with improvement or worsening in renal function during treatment of acute decompensated heart failure. Am J Cardiol 2010;106:1763–69.
17. Hata N, Yokoyama S, Shinada T, et al. Acute kidney injury and outcomes in acute decompensated heart failure: Evaluation of the RIFLE criteria in an acutely ill heart failure population. Eur J Heart Fail 2010;12:32–7.
18. Testani JM, Brisco MA, Turner JM, et al. Loop diuretic efficiency: A metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ Heart Fail 2014;7:261–70.
19. Voors AA, Davison BA, Teerlink JR, et al. Diuretic response in patients with acute decompensated heart failure: Characteristics and clinical outcome--an analysis from RELAX-AHF. Eur J Heart Fail 2014;16:1230–40.
20. Valente MA, Voors AA, Damman K, et al. Diuretic response in acute heart failure: Clinical characteristics and prognostic significance. Eur Heart J 2014;35:1284–93.
21. Devarajan P. Neutrophil gelatinase-associated lipocalin: A troponin-like biomarker for human acute kidney injury. Nephrology (Carlton) 2010;15:419–28.
22. Soyler C, Tanriover MD, Ascioglu S, et al. Urine neutrophil gelatinase-associated lipocalin levels predict acute kidney injury in acute decompensated heart failure patients. Ren Fail 2015;5.
23. Brisco MA,Testani JM. Novel renal biomarkers to assess cardiorenal syndrome. Curr Heart Fail Rep 2014;11;485–99.
24. Lassus J, Harjola VP, Sund R, et al. and FINN-AKVA Study group. Prognostic value of cystatin C in acute heart failure in relation to other markers of renal function and NT-proBNP. Eur Heart J 2007;28:1841–7.
25. Arimoto T, Takeishi Y, Niizeki T, et al. Cystatin C, a novel measure of renal function, is an independent predictor of cardiac events in patients with heart failure. J Card Fail 2005;11:595–601.
26. Naruse H, Ishii J, Kawai T, et al. Cystatin C in acute heart failure without advanced renal impairment. Am J Med 2009;122:566–73.
27. Manzano-Fernandez S, Boronat-Garcia M, Albaladejo-Oton MD, et al. Complementary prognostic value of cystatin C, N-terminal pro-B-type natriuretic peptide and cardiac troponin T in patients with acute heart failure. Am J Cardiol 2009;103:1753–9.
28. Bonventre JV, Yang L. Kidney injury molecule-1. Curr Opin Crit Care 2010;16:556–61.
29. Damman K, Van Veldhuisen DJ, Navis G, et al. Tubular damage in chronic systolic heart failure is associated with reduced survival independent of glomerular filtration rate. Heart 2010;96:1297–302.
30. Jungbauer CG, Birner C, Jung B, et al. Kidney injury molecule-1 and N-acetyl-beta-D-glucosaminidase in chronic heart failure: Possible biomarkers of cardiorenal syndrome. Eur J Heart Fail 2011;13:1104–10.
31. Verbrugge FH, Dupont M, Shao Z, et al. Novel urinary biomarkers in detecting acute kidney injury, persistent renal impairment, and all-cause mortality following decongestive therapy in acute decompensated heart failure. J Card Fail 2013;19:621–8.
32. Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011;364:797–805.
33. Moranville MP, Choi S, Hogg J, et al. Comparison of metolazone versus chlorothiazide in acute decompensated heart failure with diuretic resistance. Cardiovasc Ther 2015;33;42–9.
34. Cuffe MS, Califf RM, Adams KF Jr, et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: A randomized controlled trial. JAMA 2002;287:1541–7.
35. Mebazaa A, Nieminen MS, Packer M, et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: The SURVIVE randomized trial. JAMA 2007;297:1883–91.
36. Varriale P, Mossavi A. The benefit of low-dose dopamine during vigorous diuresis for congestive heart failure associated with renal insufficiency: Does it protect renal function? Clin Cardiol 1997;20:627–30.
37. Giamouzis G, Butler J, Starling RC, et al. Impact of dopamine infusion on renal function in hospitalized heart failure patients: Results of the dopamine in acute decompensated heart failure (DAD-HF) trial. J Card Fail 2010;16:922–30.
38. Triposkiadis FK, Butler J, Karayannis G, et al. Efficacy and safety of high dose versus low dose furosemide with or without dopamine infusion: The dopamine in acute decompensated heart failure II (DAD-HF II) trial. Int J Cardiol 2014;172:115–21.
39. Chen HH, Anstrom KJ, Givertz MM, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: The ROSE acute heart failure randomized trial. JAMA 2013;310:2533–43.
40. Witteles RM, Kao D, Christopherson D, et al. Impact of nesiritide on renal function in patients with acute decompensated heart failure and pre-existing renal dysfunction a randomized, double-blind, placebo-controlled clinical trial. J Am Coll Cardiol 2007;50:1835–40.
41 O'Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med 2011;365:32–43.
42. Owan TE, Chen HH, Frantz RP, et al. The effects of nesiritide on renal function and diuretic responsiveness in acutely decompensated heart failure patients with renal dysfunction. J Card Fail 2008;14:267–75.
43. Givertz MM, Massie BM, Fields TK, et al and CKI-201 and CKI-202 Investigators. The effects of KW-3902, an adenosine A1-receptor antagonist,on diuresis and renal function in patients with acute decompensated heart failure and renal impairment or diuretic resistance. J Am Coll Cardiol 2007;50:1551–60.
44. Massie BM, O'Connor CM, Metra M, et al. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N Engl J Med 2010;363:1419–28.
45. Voors AA, Dittrich HC, Massie BM, et al. Effects of the adenosine A1 receptor antagonist rolofylline on renal function in patients with acute heart failure and renal dysfunction: Results from PROTECT (placebo-controlled randomized study of the selective adenosine A1 receptor antagonist rolofylline for patients hospitalized with acute decompensated heart failure and volume overload to assess treatment effect on congestion and renal function). J Am Coll Cardiol 2011;57:1899–907.
46. Gottlieb SS, Givertz MM, Metra M, et al. The effects of adenosine A(1) receptor antagonism in patients with acute decompensated heart failure and worsening renal function: The REACH UP study. J Card Fail 2010;16:714–9.
47. Gheorghiade M, Gattis WA, O'Connor CM, et al. Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: A randomized controlled trial. JAMA 2004;291:1963–71.
48. Gheorghiade M, Konstam MA, Burnett JC Jr, et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: The EVEREST clinical status trials. JAMA 2007;297:1332–43.
49. Konstam MA, Gheorghiade M, Burnett JC Jr, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: The EVEREST outcome trial. JAMA 2007;297:1319–31.
50. Liu C, Liu G, Zhou C, et al. Potent diuretic effects of prednisone in heart failure patients with refractory diuretic resistance. Can J Cardiol 2007;23:865–8.
51. Zhang H, Liu C, Ji Z, et al. Prednisone adding to usual care treatment for refractory decompensated congestive heart failure. Int Heart J 2008;49:587–95.
52. Liu C, Liu K and COPE-ADHF Study Group. Cardiac outcome prevention effectiveness of glucocorticoids in acute decompensated heart failure: COPE-ADHF study. J Cardiovasc Pharmacol 2014;63:333–8.
53. Teichman SL, Unemori E, Teerlink JR, et al. Relaxin: Review of biology and potential role in treating heart failure. Curr Heart Fail Rep 2010;7:75–82.
54. Conrad KP, Shroff SG. Effects of relaxin on arterial dilation, remodeling, and mechanical properties. Curr Hypertens Rep 2011;13:409–20.
55. Du XJ, Bathgate RA, Samuel CS, et al. Cardiovascular effects of relaxin: From basic science to clinical therapy. Nat Rev Cardiol 2010;7:48–58.
56. Metra M, Cotter G, Davison BA, et al. Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the relaxin in acute heart failure (RELAX-AHF) development program: Correlation with outcomes. J Am Coll Cardiol 2013;61:196-206.
57. Costanzo MR, Guglin ME, Saltzberg MT, et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol 2007;49:675–83.
58. Bart BA, Goldsmith SR, Lee KL, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012;367:2296–304.
59. Mentz RJ, Stevens SR, DeVore AD, et al. Decongestion strategies and renin-angiotensin-aldosterone system activation in acute heart failure. JACC Heart Fail 2015;3:97–107.
60. Ebrahim B, Sindhura K, Okoroh J, et al. Meta-analysis of ultrafiltration versus diuretics treatment option for overload volume reduction in patients with acute decompensated heart failure. Arq Bras Cardiol 2015;104:417–25.
61. Kwong JS, Yu CM. Ultrafiltration for acute decompensated heart failure: A systematic review and meta-analysis of randomized controlled trials. Int J Cardiol 2014;172:395–402.
62. Badawy SS, Fahmy A. Efficacy and cardiovascular tolerability of continuous veno-venous hemodiafiltration in acute decompensated heart failure: A randomized comparative study. J Crit Care 2012;27:106.e7-106.13.
63. Patarroyo M, Wehbe E, Hanna M, et al. Cardiorenal outcomes after slow continuous ultrafiltration therapy in refractory patients with advanced decompensated heart failure. J Am Coll Cardiol 2012;60:1906–12.
64. Prins KW, Wille KM, Tallaj JA, Tolwani AJ. Assessing continuous renal replacement therapy as a rescue strategy in cardiorenal syndrome 1. Clin Kidney J 2015;8:87–92.
65. Nechemia-Arbely Y, Barkan D, Pizov G, et al. IL-6/IL-6R axis plays a critical role in acute kidney injury. J Am Soc Nephrol 2008;19:1106–15.
66. Pathan N, Franklin JL, Eleftherohorinou H, et al. Myocardial depressant effects of interleukin 6 in meningococcal sepsis are regulated by p38 mitogen-activated protein kinase. Crit Care Med 2011;39:1692–711.
67. Janssen SP, Gayan-Ramirez G, Van den Bergh A, et al. Interleukin-6 causes myocardial failure and skeletal muscle atrophy in rats. Circulation 2005;111:996–1005.
68. Bellomo R, Ronco C, Kellum JA and Acute Dialysis Quality Initiative workgroup. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: The second international consensus conference of the acute dialysis quality initiative (ADQI) group. Crit Care 2004;8:R204-12.
69. Mehta RL, Kellum JA, Shah SV, et al and Acute Kidney Injury Network. Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31.
70. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guidelines for acute kidney injury. Kidney Inter Suppl 2012;2:19–36.
Tracking a Tumor
Is there a universal cancer fingerprint? Researchers at the National Institutes of Health believe they may have found a potential common biomarker for 5 different tumor types. The clue is a “methylation signature”—evidence of a chemical modification of DNA. Methylation controls the expression of genes, and higher amounts of DNA methylation reduce a gene’s activity, like a dimmer switch on a light fixture.
In an earlier study using DNA taken from solid tumors, the researchers found a methylation signature in 15 tumor types in 13 different organs around the gene called ZNF154. In the new study, the researchers uncovered methylation in colon, lung, breast, stomach, and endometrial cancers. All the tumor types and subtypes consistently produced the same methylation mark around ZNF154.
Researchers developed a computer program that looked at methylation marks in the DNA of people with and without cancer and were able to predict a threshold for detecting tumor DNA. Because tumors often shed DNA into the bloodstream, the researchers were able to calculate the proportions of circulating tumor DNA. The researchers hope their results lead to a blood test that can diagnose cancers at early stages.
Currently, blood tests are specific to a known tumor type. Clinicians must first find the tumor and then sequence a sample from it before they can track the tumor-specific mutations in the blood. By contrast, a method derived from the methylation signatures would mean no prior knowledge of the cancer was required. The tests would be less intrusive than that of other screening methods and could be used to follow high-risk patients or monitor the activity of a tumor during treatment.
Source:
National Institutes of Health. NIH researchers identify striking genomic signature shared by five types of cancer [news release]. National Institutes of Health Website. http://www.nih.gov/news-events/news-releases/nih-researchers-identify-striking-genomic-signature-shared-five-types-cancer. Published February 5, 2016. Accessed February 29, 2016.
Is there a universal cancer fingerprint? Researchers at the National Institutes of Health believe they may have found a potential common biomarker for 5 different tumor types. The clue is a “methylation signature”—evidence of a chemical modification of DNA. Methylation controls the expression of genes, and higher amounts of DNA methylation reduce a gene’s activity, like a dimmer switch on a light fixture.
In an earlier study using DNA taken from solid tumors, the researchers found a methylation signature in 15 tumor types in 13 different organs around the gene called ZNF154. In the new study, the researchers uncovered methylation in colon, lung, breast, stomach, and endometrial cancers. All the tumor types and subtypes consistently produced the same methylation mark around ZNF154.
Researchers developed a computer program that looked at methylation marks in the DNA of people with and without cancer and were able to predict a threshold for detecting tumor DNA. Because tumors often shed DNA into the bloodstream, the researchers were able to calculate the proportions of circulating tumor DNA. The researchers hope their results lead to a blood test that can diagnose cancers at early stages.
Currently, blood tests are specific to a known tumor type. Clinicians must first find the tumor and then sequence a sample from it before they can track the tumor-specific mutations in the blood. By contrast, a method derived from the methylation signatures would mean no prior knowledge of the cancer was required. The tests would be less intrusive than that of other screening methods and could be used to follow high-risk patients or monitor the activity of a tumor during treatment.
Source:
National Institutes of Health. NIH researchers identify striking genomic signature shared by five types of cancer [news release]. National Institutes of Health Website. http://www.nih.gov/news-events/news-releases/nih-researchers-identify-striking-genomic-signature-shared-five-types-cancer. Published February 5, 2016. Accessed February 29, 2016.
Is there a universal cancer fingerprint? Researchers at the National Institutes of Health believe they may have found a potential common biomarker for 5 different tumor types. The clue is a “methylation signature”—evidence of a chemical modification of DNA. Methylation controls the expression of genes, and higher amounts of DNA methylation reduce a gene’s activity, like a dimmer switch on a light fixture.
In an earlier study using DNA taken from solid tumors, the researchers found a methylation signature in 15 tumor types in 13 different organs around the gene called ZNF154. In the new study, the researchers uncovered methylation in colon, lung, breast, stomach, and endometrial cancers. All the tumor types and subtypes consistently produced the same methylation mark around ZNF154.
Researchers developed a computer program that looked at methylation marks in the DNA of people with and without cancer and were able to predict a threshold for detecting tumor DNA. Because tumors often shed DNA into the bloodstream, the researchers were able to calculate the proportions of circulating tumor DNA. The researchers hope their results lead to a blood test that can diagnose cancers at early stages.
Currently, blood tests are specific to a known tumor type. Clinicians must first find the tumor and then sequence a sample from it before they can track the tumor-specific mutations in the blood. By contrast, a method derived from the methylation signatures would mean no prior knowledge of the cancer was required. The tests would be less intrusive than that of other screening methods and could be used to follow high-risk patients or monitor the activity of a tumor during treatment.
Source:
National Institutes of Health. NIH researchers identify striking genomic signature shared by five types of cancer [news release]. National Institutes of Health Website. http://www.nih.gov/news-events/news-releases/nih-researchers-identify-striking-genomic-signature-shared-five-types-cancer. Published February 5, 2016. Accessed February 29, 2016.
Current Management of Nephrolithiasis
Case
A 39-year-old woman presented to the ED with a chief complaint of intermittent right flank pain that radiated into her groin area. She stated the pain had begun suddenly, 4 hours prior to arrival, and was accompanied by nausea and vomiting. The patient said that she had taken acetaminophen for the pain, but had received no relief. Regarding history, according to the patient, her last menstrual period ended 2 days earlier. She denied any urinary symptoms, diarrhea, or constipation. She had no history of abdominal surgery and was currently not on any medications.
The patient’s vital signs at presentation were: temperature 98.7°F; blood pressure, 130/90 mm Hg; heart rate, 110 beats/minute; and respiratory rate, 18 breaths/minute. Oxygen saturation was 98% on room air. On physical examination, she appeared to be in mild distress, pacing around the room. There was moderate right costovertebral tenderness on percussion; the abdomen was soft and nontender.
Incidence
As ED visits for nephrolithiasis are increasing, so too are the health-care costs associated with this condition. Between 1992 and 2009, emergent-care presentations for nephrolithiasis rose from 178 to 340 visits per 100,000 individuals.1 Approximately 1 in 11 people in the United States will be affected by nephrolithiasis during their lifetime.2 Estimated health-care costs associated with these complaints were roughly $2 billion in 2000—an increase of 50% since 1994.2
Evaluation and Diagnosis
Laboratory Evaluation
Urinalysis is one of the initial studies for patients with suspected nephrolithiasis. Although hematuria is a classic finding associated with renal calculi, its sensitivity on microscopic analysis is around 84%. Therefore, the absence of hematuria does not exclude renal colic in the differential diagnosis.3
In addition to detecting hematuria, urinalysis can also reveal an underlying infection. One study by Abrahamian et al4 found that roughly 8% of patients presenting with acute nephrolithiasis had a urinary tract infection (UTI)—many without any clinical findings of infection. The presence of pyuria, however, has only moderate accuracy in identifying UTIs in patients with kidney stones.4 If an infected stone cannot be excluded clinically, computed tomography (CT) is indicated.
Mild leukocytosis (ie, <15,000 cells/mcL) is another common finding in patients with acute renal colic.5 A leukocyte count >15,000 cells/mcL is suspicious for infection or other pathology. A blood-chemistry panel to evaluate renal function is appropriate as a baseline—particularly for patients in whom treatment with a nonsteroidal anti-inflammatory (NSAID) drug is anticipated.
With the ability to visualize renal calculi (Figure 1), the use of noncontrast CT has become a standard initial imaging modality in assessing patients with renal colic. Between 1992 and 2009, the use of CT to evaluate patients presenting with flank pain for suspected renal colic more than tripled from 21% to 71%.6 An analysis performed by the American College of National Radiology Data Registry7 shows the mean radiation dose given by institutions for renal colic CT is unnecessarily high, and that few institutions follow CT-stone protocols aimed at minimizing radiation exposure while still maintaining proper diagnostic accuracy. A typical CT of the abdomen and pelvis is equivalent to over 100 two-view chest X-rays.8 Though controversial, data from a white paper by the American College of Radiology suggest that the ionizing radiation exposure from just one CT for renal colic causes an increase in lifetime cancer risk.9
Despite the increase in CT imaging to evaluate patients presenting to the ED with nephrolithiasis/flank pain, the proportion of patients diagnosed with a kidney stone remained the same between 2000 and 2008, with no significant change in outcomes.10-12 Moreover, the use of CT as an initial imaging modality in patients presenting with flank pain—but with no sign of infection—is unlikely to reveal important alternative findings.13
Regarding the sensitivity of CT in detecting nephrolithiasis, one study demonstrates a sensitivity of 100% and a specificity of 94% for noncontrast CT.14 Controversy, however, still exists regarding the necessity and utility of CT in diagnosing nephrolithiasis,15 and CT is one of the top 10 tests included in the American College of Emergency Physicians (ACEP) 2014 Choosing Wisely campaign. In this campaign, ACEP recommended emergency physicians (EPs) avoid abdominal and pelvic CT in otherwise healthy patients younger than age 50 years who present with symptoms consistent with uncomplicated renal colic and who have a known history of nephrolithiasis or ureterolithiasis.15 The ACEP also noted that CTs in this context do not often change treatment decisions and are associated with unnecessary radiation exposure and cost.15
While keeping the aforementioned recommendations in mind, if an EP intends to refer a renal colic patient to a urologist a CT scan is necessary either in the ED or as an outpatient. In all cases (except perhaps in patients in whom there is a history of renal stones), the urologist will need this study to determine the size and location of the stone in order to provide recommendations for management.
Ultrasound
Clinical Decision Score
Moore et al,17 authors of the Size, Topography, Location, Obstruction, Number of stones, and Evaluation (STONE) scoring system, developed a classification system for patients with suspected nephrolithiasis. This system places patients into low-, moderate-, and high-score groups, with corresponding probabilities of ureteral stone based on symptoms and epidemiological classifications.
The intent of the STONE system is to accurately predict, based on classification, the likelihood of a patient having a simple ureteral stone versus a more significant, complicated stone and to help guide which, if any, imaging studies are indicated. For example, a lower STONE score would help guide the decision to defer advanced imaging studies that would be unlikely to reveal an alternate serious diagnosis. Likewise, an individual with a high STONE score could potentially receive ultrasonography, reduced-dose CT, or no further imaging.
The STONE score performs fairly well and appears to be superior to physician gestalt, with an area under the receiver operating characteristic curve (AUC) of .78 compared to .68 with physician gestalt. This system, however, is not always accurate in its classification and has been shown to have 87% specificity at the high end to rule in stone and 96% sensitivity rate at the low end to rule out a stone. Of course, when using a clinical decision rule to rule in or rule out a stone, a tool with a very high specificity is preferred. Although the STONE scoring system does show promise, further studies are needed before it can be applied clinically.17
Treatment
Analgesia
By inhibiting prostaglandin synthesis, NSAIDs reduce inflammation and ureteral muscular hyperactivity.18 A recent Cochrane review of over 50 studies concluded that NSAIDs were effective in relieving acute renal colic pain.19 A systematic review by Holdgate and Pollock20 shows that patients treated with NSAIDs achieve greater reductions in pain scores and are less likely to require additional analgesia in the short term compared to patients treated with opioids. Although opioid medications are effective in relieving pain associated with nephrolithiasis, this class of drugs can exacerbate the nausea often associated with this condition. This same study also showed that patients who were prescribed NSAIDs following an ED visit for renal colic required less medication for pain control, experienced less nausea, and had greater improvements in their pain.20
Nevertheless, the utility of opiates as an adjunct therapy should not be overlooked. For example, in patients with renal colic, numerous studies show treatment with a combination of an NSAID and opiate provides superior pain relief compared to either treatment modality in isolation.21 Opioid analgesia may be indicated in patients in whom NSAIDs are not recommended or contraindicated (eg, elderly patients, patients with renal disease). While NSAIDs address the underlying pathophysiology associated with renal colic, they are sometimes not the best treatment option. Depending on the situation, treatment with an opioid should instead be considered.
Intravenous Fluid Therapy
A 2012 Cochrane Review of randomized control trials (RCT) on intravenous (IV) fluid therapy hydration/diuretic use concluded that there was “no reliable evidence in the literature to support the use of diuretics and high-volume fluid therapy for people with acute ureteric colic.” The review, however, did note that further investigation is warranted for a definitive answer.22 Another study by Springhart et al23 showed no difference in pain or stone expulsion between large-volume (2 L IV fluids over 2 hours) and small-volume fluid administration (20 mL/h). Regarding administration, the use of IV fluids in renal colic is no different than the usual indications for fluid therapy in the ED and should be restricted to patients with signs of dehydration or kidney injury.
Many patients with renal colic will have decreased oral intake from the pain and nausea associated with the stone and may be vomiting. Under these circumstances, it is reasonable to rehydrate the patients, even though large-volume hydration with the intent of aiding stone expulsion or improving pain has not been shown efficacious. Conversely, in addition to the perceived benefit of rehydrating patients, a small amount of fluid hydration may improve the visualization of hydronephrosis on ultrasound.24
Medical Expulsive Therapy
For many years, clinicians have considered the use of tamsulosin, an α1-receptor blocker, as well as nifedipine, a calcium channel blocker, in treating renal colic due to the theoretical benefit of reducing ureteral smooth muscle spasm/constriction thus expediting stone passage. Over the years, dozens of studies showed positive benefit in the use of medical expulsive therapy (MET). A 2014 Cochrane Review demonstrated that patients treated with α1-blockers experienced a higher stone-free rate and shorter time to stone expulsion, and concluded that α1-blockers should be offered as one of the primary treatment modalities in MET.25 This review, however, has been criticized for using a number of studies with very small patient samples, non-peer-reviewed abstracts, and low-quality study designs.26
More recently, in April 2015, Lancet published a large RCT from 24 hospitals in the United Kingdom, comparing placebo versus 400 mcg tamsulosin and 30 mg nifedipine. The authors concluded that “tamsulosin 400 mcg and nifedipine 30 mg are not effective at decreasing the need for further treatment to achieve stone clearance in 4 weeks for patients with expectantly managed ureteric colic.”27 Another large double-blind, placebo-controlled, randomized, multicenter trial by Furyk et al28 in July 2015 went a step further and evaluated distal stones, which have historically caused complications requiring intervention. They concluded that there was “no benefit overall of 0.4 mg of tamsulosin daily for patients with distal ureteric calculi less than or equal to 10 mm in terms of spontaneous passage, time to stone passage, pain, or analgesia requirements. In the subgroup with large stones (5 to 10 mm), tamsulosin did increase passage and should be considered.”28 Based on these recent studies, the use of tamsulosin in patients with stones larger than 5 mm—but not those with smaller stones—appears to be an appropriate treatment option.
Patient Disposition
The American Urological Association cited indications for urgent/emergent urological interventions necessitating the need for inpatient admission and further workup.29 Patients who do not fall into any of the categories outlined in the Table may be seen on an outpatient basis. These patients may be treated symptomatically until they can follow up with a urologist, who will determine expectant management versus intervention.
Prognosis
The majority of stones <5mm will pass spontaneously.30 Larger stones may still pass spontaneously but are more likely to require lithotripsy or other urologic intervention; therefore, patients with stones >5 mm should be referred to urology services.30
Recurrence
Patients with a first-time kidney stone have a 30% to 50% chance of disease recurrence within 5 years,31 and a 60% to 80% chance of recurrence during their lifetime.32 Those with a family history of nephrolithiasis are likely to develop an earlier onset of stones as well as experience more frequent recurrent episodes.33 Patients with recurrent disease should undergo outpatient risk stratification, including stone-composition analysis and assessment for modifiable risk factors.
Case Conclusion
The patient’s urinalysis demonstrated microscopic hematuria; blood urea nitrogen and creatinine levels were within normal limits. As the patient was tachycardic and appeared mildly dehydrated, an IV infusion of 1 L normal saline was initiated, along with ketorolac and ondansetron for symptomatic relief. A POC ultrasound of the right kidney revealed mild-to-moderate hydronephrosis; the left kidney appeared sonographically normal. Since this patient had no history of nephrolithiasis, a nonenhanced CT of the abdomen was obtained, which revealed moderate, right-sided hydronephrosis and a 3-mm distal ureteral stone. Once the patient’s symptoms were controlled, she was discharged home with a prescription for ibuprofen for symptomatic relief and instructions to follow up with her PCP.
Conclusion
The evaluation and treatment of nephrolithiasis is important due to its increasing prevalence, as well as implications on costs to the health-care system and to patients themselves. The workup and treatment of nephrolithiasis has been and continues to be the subject of much controversy. Until very recently, treatment recommendations were founded on physiological theories more so than robust research. In an era where improved imaging technology is becoming more readily available in the ED, EPs should weigh the pros and cons of its utilization for common ED complaints such as nephrolithiasis.
Dr Parsa is an assistant professor in the department of emergency medicine, Texas Tech University Health Sciences Center, El Paso. Dr Khafi is a resident in the department of emergency medicine, Texas Tech University Health Sciences Center, El Paso.
- Scales CD Jr, Smith AC, Hanley JM, Saigal CS; Urologic Diseases in America Project. Prevalence of kidney stones in the United States. Eur Urol. 2012;62(1):160-165.
- Pearle MS, Calhoun EA, Curhan GC; Urologic Diseases of America Project: urolithiasis. J Urol. 2005;173(3):848-857.
- Luchs JS, Katz DS, Lane MJ et al. Utility of hematuria testing in patients with suspected renal colic: correlation with unenhanced helical CT results. Urology. 2002;59(6):839-842.
- Abrahamian FM, Krishnadasan A, Mower WR, Moran GJ, Talan DA. Association of pyuria and clinical characteristics with the presence of urinary tract infection among patients with acute nephrolithiasis. Ann Emerg Med. 2013;62(5):526-533.
- Yilmaz S, Pekdemir M, Aksu NM, Koyuncu N, Cinar O, Akpinar E. A multicenter case–control study of diagnostic tests for urinary tract infection in the presence of urolithiasis. Urol Res. 2011;40(1):61-65. doi:10.1007/s00240-011-0402-x.
- Fwu CW, Eggers PW, Kimmel PL, Kusek JW, Kirkali Z. Emergency department visits, use of imaging, and drugs for urolithiasis have increased in the United States. Kidney Int. 2013;83(3):479-486. doi:10.1038/ki.2012.419.
- Lukasiewicz A, Bhargavan-Chatfield M, Coombs L, et al. Radiation dose index of renal colic protocol CT studies in the United States: a report from the American College of Radiology National Radiology Data Registry. Radiology. 2014;271(2):445-451. doi:10.1148/radiol.14131601.
- Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 248(1):254-263.
- Amis ES Jr, Butler PF, Applegate KE, et al; American College of Radiology. American College of Radiology white paper on radiation dose in medicine. J Am Coll Radiol. 2007;4(5):272-284.
- Hyams ES, Korley FK, Pham JC, Matlaga BR. Trends in imaging use during the emergency department evaluation of flank pain. J Urol. 2011;186(6):2270-2274. doi:10.1016/j.juro.2011.07.079.
- Ripollés T, Agramunt M, Errando J, Martínez MJ, Coronel B, Morales M. Suspected ureteral colic: plain film and sonography vs unenhanced helical CT. A prospective study in 66 patients. Eur Radiol. 2004;14(1):129-36. doi:10.1007/s00330-003-1924-1926.
- Westphalen AC, Hsia RY, Maselli JH, Wang R, Gonzales R. Radiological imaging of patients with suspected urinary tract stones: national trends, diagnoses, and predictors. Acad Emerg Med. 2011;18(7):699-707. doi:10.1111/j.1553-2712.2011.01103.x.
- Moore CL, Daniels B, Singh D, Luty S, Molinaro A. Prevalence and clinical importance of alternative causes of symptoms using a renal colic computed tomography protocol in patients with flank or back pain and absence of pyuria. Acad Emerg Med. 2013;20(5):470-478. doi:10.1111/acem.12127.
- Chen MY, Zagoria RJ. Can noncontrast helical computed tomography replace intravenous urography for evaluation of patients with acute urinary tract colic? J Emerg Med. 1999;17(2):299-303.
- American College of Emergency Physicians. Five things physicians and patients should question. Choosing Wisely Web site. 2013;10:1-5. Available at: http://www.choosingwisely.org/societies/american-college-of-emergency-physicians/. Accessed February 10, 2016.
- Smith-Bindman R, Aubin C, Bailitz J, et al. Ultrasonography versus computed tomography for suspected nephrolithiasis. N Engl J Med. 2014;371(12):1100-1110. doi:10.1056/nejmoa1404446.
- Moore CL, Bomann S, Daniels B, et al. Derivation and validation of a clinical prediction rule for uncomplicated ureteral stone—the STONE score: retrospective and prospective observational cohort studies. BMJ. 2014;348:g2191. doi:10.1136/bmj.g2191.
- Cole RS, Fry CH, Shuttleworth KE. The action of the prostaglandins on isolated human ureteric smooth muscle. Br J Urol. 1988;61(1):19-26.
- Afshar K, Jafari S, Marks AJ, Eftekhari R, McNeily AE. Nonsteroidal anti-inflammatory drugs (NSAIDs) and non-opioids for acute renal colic. Cochrane Database Syst Rev. 2015;6:CD006027. doi:10.1002/14651858.CD006027.pub2.
- Holdgate A, Pollock T. Systematic review of the relative efficacy of non-steroidal anti-inflammatory drugs and opioids in the treatment of acute renal colic. BMJ. 2004;328(7453):1401. doi:10.1136/bmj.38119.581991.55.
- Safdar B, Degutis LC, Landry K, Vedere SR, Moscovitz HC, D’Onofrio G. Intravenous morphine plus ketorolac is superior to either drug alone for treatment of acute renal colic. Ann Emerg Med. 2006;48(2):173-181, 181.e1. doi:10.1016/j.annemergmed.2006.03.013.
- Worster AS, Bhanich Supapol W. Fluids and diuretics for acute ureteric colic. Cochrane Database Syst Rev. 2012;15;2:CD004926. doi:10.1002/14651858.CD004926.pub3.
- Springhart WP, Marguet CG, Sur RL, et al. Forced versus minimal intravenous hydration in the management of acute renal colic: a randomized trial. J. Endourol. 2006;20(10):713-716. doi:10.1089/end.2006.20.713.
- Morse JW, Hill R, Greissinger WP, Patterson JW, Melanson SW, Heller MB. Rapid oral hydration results in hydronephrosis as demonstrated by bedside ultrasound. Ann Emerg Med. 1999;34(2):134-140. doi:10.1016/s0196-0644(99)70221-0.
- Campschroer T, Zhu Y, Duijvesz D, Grobbee DE, Lock MT. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014;4:CD008509. doi:10.1002/14651858.CD008509.pub2.
- Radecki R. Sadly inadequate Cochrane review of renal colic. Emergency Medicine Literature of Note. 2014. Available at: http://www.emlitofnote.com/2014/04/sadly-inadequate-cochrane-review-of.html. Accessed February 10, 2016.
- Pickard R, Starr K, MacLennan G, et al. Medical expulsive therapy in adults with ureteric colic: a multicentre, randomised, placebo-controlled trial. Lancet. 2015;386(9991):341-349. doi:10.1016/S0140-6736(15)60933-3.
- Furyk JS, Chu K, Banks C, et al. Distal ureteric stones and tamsulosin: a double-blind, placebo-controlled, randomized, multicenter trial. Ann Emerg Med. 2016;67(1):86-95.e2. doi:10.1016/j.annemergmed.2015.06.001.
- Kidney stones. American Urological Association Web site. 2016. Available at: https://www.auanet.org/education/kidney-stones.cfm. Accessed February 10, 2016.
- Miller OF, Kane CJ. Time to stone passage for observed ureteral calculi: a guide for patient education. J Urol. 1999;162(3 Pt 1):688-690.
- Lipkin ME, Preminger GM. Demystifying the medical management of nephrolithiasis. Rev Urol. 2011;13(1):34-38.
- Morton AR, Iliescu EA, Wilson JW. Nephrology: 1. Investigation and treatment of recurrent kidney stones. CMAJ. 2002;166(2):213-218.
- Koyuncu HH, Yencilek F, Eryildirim B, Sarica K. Family history in stone disease: how important is it for the onset of the disease and the incidence of recurrence? Urol Res. 2010;38(2):105-109. doi:10.1007/s00240-009-0249-6.
Case
A 39-year-old woman presented to the ED with a chief complaint of intermittent right flank pain that radiated into her groin area. She stated the pain had begun suddenly, 4 hours prior to arrival, and was accompanied by nausea and vomiting. The patient said that she had taken acetaminophen for the pain, but had received no relief. Regarding history, according to the patient, her last menstrual period ended 2 days earlier. She denied any urinary symptoms, diarrhea, or constipation. She had no history of abdominal surgery and was currently not on any medications.
The patient’s vital signs at presentation were: temperature 98.7°F; blood pressure, 130/90 mm Hg; heart rate, 110 beats/minute; and respiratory rate, 18 breaths/minute. Oxygen saturation was 98% on room air. On physical examination, she appeared to be in mild distress, pacing around the room. There was moderate right costovertebral tenderness on percussion; the abdomen was soft and nontender.
Incidence
As ED visits for nephrolithiasis are increasing, so too are the health-care costs associated with this condition. Between 1992 and 2009, emergent-care presentations for nephrolithiasis rose from 178 to 340 visits per 100,000 individuals.1 Approximately 1 in 11 people in the United States will be affected by nephrolithiasis during their lifetime.2 Estimated health-care costs associated with these complaints were roughly $2 billion in 2000—an increase of 50% since 1994.2
Evaluation and Diagnosis
Laboratory Evaluation
Urinalysis is one of the initial studies for patients with suspected nephrolithiasis. Although hematuria is a classic finding associated with renal calculi, its sensitivity on microscopic analysis is around 84%. Therefore, the absence of hematuria does not exclude renal colic in the differential diagnosis.3
In addition to detecting hematuria, urinalysis can also reveal an underlying infection. One study by Abrahamian et al4 found that roughly 8% of patients presenting with acute nephrolithiasis had a urinary tract infection (UTI)—many without any clinical findings of infection. The presence of pyuria, however, has only moderate accuracy in identifying UTIs in patients with kidney stones.4 If an infected stone cannot be excluded clinically, computed tomography (CT) is indicated.
Mild leukocytosis (ie, <15,000 cells/mcL) is another common finding in patients with acute renal colic.5 A leukocyte count >15,000 cells/mcL is suspicious for infection or other pathology. A blood-chemistry panel to evaluate renal function is appropriate as a baseline—particularly for patients in whom treatment with a nonsteroidal anti-inflammatory (NSAID) drug is anticipated.
With the ability to visualize renal calculi (Figure 1), the use of noncontrast CT has become a standard initial imaging modality in assessing patients with renal colic. Between 1992 and 2009, the use of CT to evaluate patients presenting with flank pain for suspected renal colic more than tripled from 21% to 71%.6 An analysis performed by the American College of National Radiology Data Registry7 shows the mean radiation dose given by institutions for renal colic CT is unnecessarily high, and that few institutions follow CT-stone protocols aimed at minimizing radiation exposure while still maintaining proper diagnostic accuracy. A typical CT of the abdomen and pelvis is equivalent to over 100 two-view chest X-rays.8 Though controversial, data from a white paper by the American College of Radiology suggest that the ionizing radiation exposure from just one CT for renal colic causes an increase in lifetime cancer risk.9
Despite the increase in CT imaging to evaluate patients presenting to the ED with nephrolithiasis/flank pain, the proportion of patients diagnosed with a kidney stone remained the same between 2000 and 2008, with no significant change in outcomes.10-12 Moreover, the use of CT as an initial imaging modality in patients presenting with flank pain—but with no sign of infection—is unlikely to reveal important alternative findings.13
Regarding the sensitivity of CT in detecting nephrolithiasis, one study demonstrates a sensitivity of 100% and a specificity of 94% for noncontrast CT.14 Controversy, however, still exists regarding the necessity and utility of CT in diagnosing nephrolithiasis,15 and CT is one of the top 10 tests included in the American College of Emergency Physicians (ACEP) 2014 Choosing Wisely campaign. In this campaign, ACEP recommended emergency physicians (EPs) avoid abdominal and pelvic CT in otherwise healthy patients younger than age 50 years who present with symptoms consistent with uncomplicated renal colic and who have a known history of nephrolithiasis or ureterolithiasis.15 The ACEP also noted that CTs in this context do not often change treatment decisions and are associated with unnecessary radiation exposure and cost.15
While keeping the aforementioned recommendations in mind, if an EP intends to refer a renal colic patient to a urologist a CT scan is necessary either in the ED or as an outpatient. In all cases (except perhaps in patients in whom there is a history of renal stones), the urologist will need this study to determine the size and location of the stone in order to provide recommendations for management.
Ultrasound
Clinical Decision Score
Moore et al,17 authors of the Size, Topography, Location, Obstruction, Number of stones, and Evaluation (STONE) scoring system, developed a classification system for patients with suspected nephrolithiasis. This system places patients into low-, moderate-, and high-score groups, with corresponding probabilities of ureteral stone based on symptoms and epidemiological classifications.
The intent of the STONE system is to accurately predict, based on classification, the likelihood of a patient having a simple ureteral stone versus a more significant, complicated stone and to help guide which, if any, imaging studies are indicated. For example, a lower STONE score would help guide the decision to defer advanced imaging studies that would be unlikely to reveal an alternate serious diagnosis. Likewise, an individual with a high STONE score could potentially receive ultrasonography, reduced-dose CT, or no further imaging.
The STONE score performs fairly well and appears to be superior to physician gestalt, with an area under the receiver operating characteristic curve (AUC) of .78 compared to .68 with physician gestalt. This system, however, is not always accurate in its classification and has been shown to have 87% specificity at the high end to rule in stone and 96% sensitivity rate at the low end to rule out a stone. Of course, when using a clinical decision rule to rule in or rule out a stone, a tool with a very high specificity is preferred. Although the STONE scoring system does show promise, further studies are needed before it can be applied clinically.17
Treatment
Analgesia
By inhibiting prostaglandin synthesis, NSAIDs reduce inflammation and ureteral muscular hyperactivity.18 A recent Cochrane review of over 50 studies concluded that NSAIDs were effective in relieving acute renal colic pain.19 A systematic review by Holdgate and Pollock20 shows that patients treated with NSAIDs achieve greater reductions in pain scores and are less likely to require additional analgesia in the short term compared to patients treated with opioids. Although opioid medications are effective in relieving pain associated with nephrolithiasis, this class of drugs can exacerbate the nausea often associated with this condition. This same study also showed that patients who were prescribed NSAIDs following an ED visit for renal colic required less medication for pain control, experienced less nausea, and had greater improvements in their pain.20
Nevertheless, the utility of opiates as an adjunct therapy should not be overlooked. For example, in patients with renal colic, numerous studies show treatment with a combination of an NSAID and opiate provides superior pain relief compared to either treatment modality in isolation.21 Opioid analgesia may be indicated in patients in whom NSAIDs are not recommended or contraindicated (eg, elderly patients, patients with renal disease). While NSAIDs address the underlying pathophysiology associated with renal colic, they are sometimes not the best treatment option. Depending on the situation, treatment with an opioid should instead be considered.
Intravenous Fluid Therapy
A 2012 Cochrane Review of randomized control trials (RCT) on intravenous (IV) fluid therapy hydration/diuretic use concluded that there was “no reliable evidence in the literature to support the use of diuretics and high-volume fluid therapy for people with acute ureteric colic.” The review, however, did note that further investigation is warranted for a definitive answer.22 Another study by Springhart et al23 showed no difference in pain or stone expulsion between large-volume (2 L IV fluids over 2 hours) and small-volume fluid administration (20 mL/h). Regarding administration, the use of IV fluids in renal colic is no different than the usual indications for fluid therapy in the ED and should be restricted to patients with signs of dehydration or kidney injury.
Many patients with renal colic will have decreased oral intake from the pain and nausea associated with the stone and may be vomiting. Under these circumstances, it is reasonable to rehydrate the patients, even though large-volume hydration with the intent of aiding stone expulsion or improving pain has not been shown efficacious. Conversely, in addition to the perceived benefit of rehydrating patients, a small amount of fluid hydration may improve the visualization of hydronephrosis on ultrasound.24
Medical Expulsive Therapy
For many years, clinicians have considered the use of tamsulosin, an α1-receptor blocker, as well as nifedipine, a calcium channel blocker, in treating renal colic due to the theoretical benefit of reducing ureteral smooth muscle spasm/constriction thus expediting stone passage. Over the years, dozens of studies showed positive benefit in the use of medical expulsive therapy (MET). A 2014 Cochrane Review demonstrated that patients treated with α1-blockers experienced a higher stone-free rate and shorter time to stone expulsion, and concluded that α1-blockers should be offered as one of the primary treatment modalities in MET.25 This review, however, has been criticized for using a number of studies with very small patient samples, non-peer-reviewed abstracts, and low-quality study designs.26
More recently, in April 2015, Lancet published a large RCT from 24 hospitals in the United Kingdom, comparing placebo versus 400 mcg tamsulosin and 30 mg nifedipine. The authors concluded that “tamsulosin 400 mcg and nifedipine 30 mg are not effective at decreasing the need for further treatment to achieve stone clearance in 4 weeks for patients with expectantly managed ureteric colic.”27 Another large double-blind, placebo-controlled, randomized, multicenter trial by Furyk et al28 in July 2015 went a step further and evaluated distal stones, which have historically caused complications requiring intervention. They concluded that there was “no benefit overall of 0.4 mg of tamsulosin daily for patients with distal ureteric calculi less than or equal to 10 mm in terms of spontaneous passage, time to stone passage, pain, or analgesia requirements. In the subgroup with large stones (5 to 10 mm), tamsulosin did increase passage and should be considered.”28 Based on these recent studies, the use of tamsulosin in patients with stones larger than 5 mm—but not those with smaller stones—appears to be an appropriate treatment option.
Patient Disposition
The American Urological Association cited indications for urgent/emergent urological interventions necessitating the need for inpatient admission and further workup.29 Patients who do not fall into any of the categories outlined in the Table may be seen on an outpatient basis. These patients may be treated symptomatically until they can follow up with a urologist, who will determine expectant management versus intervention.
Prognosis
The majority of stones <5mm will pass spontaneously.30 Larger stones may still pass spontaneously but are more likely to require lithotripsy or other urologic intervention; therefore, patients with stones >5 mm should be referred to urology services.30
Recurrence
Patients with a first-time kidney stone have a 30% to 50% chance of disease recurrence within 5 years,31 and a 60% to 80% chance of recurrence during their lifetime.32 Those with a family history of nephrolithiasis are likely to develop an earlier onset of stones as well as experience more frequent recurrent episodes.33 Patients with recurrent disease should undergo outpatient risk stratification, including stone-composition analysis and assessment for modifiable risk factors.
Case Conclusion
The patient’s urinalysis demonstrated microscopic hematuria; blood urea nitrogen and creatinine levels were within normal limits. As the patient was tachycardic and appeared mildly dehydrated, an IV infusion of 1 L normal saline was initiated, along with ketorolac and ondansetron for symptomatic relief. A POC ultrasound of the right kidney revealed mild-to-moderate hydronephrosis; the left kidney appeared sonographically normal. Since this patient had no history of nephrolithiasis, a nonenhanced CT of the abdomen was obtained, which revealed moderate, right-sided hydronephrosis and a 3-mm distal ureteral stone. Once the patient’s symptoms were controlled, she was discharged home with a prescription for ibuprofen for symptomatic relief and instructions to follow up with her PCP.
Conclusion
The evaluation and treatment of nephrolithiasis is important due to its increasing prevalence, as well as implications on costs to the health-care system and to patients themselves. The workup and treatment of nephrolithiasis has been and continues to be the subject of much controversy. Until very recently, treatment recommendations were founded on physiological theories more so than robust research. In an era where improved imaging technology is becoming more readily available in the ED, EPs should weigh the pros and cons of its utilization for common ED complaints such as nephrolithiasis.
Dr Parsa is an assistant professor in the department of emergency medicine, Texas Tech University Health Sciences Center, El Paso. Dr Khafi is a resident in the department of emergency medicine, Texas Tech University Health Sciences Center, El Paso.
Case
A 39-year-old woman presented to the ED with a chief complaint of intermittent right flank pain that radiated into her groin area. She stated the pain had begun suddenly, 4 hours prior to arrival, and was accompanied by nausea and vomiting. The patient said that she had taken acetaminophen for the pain, but had received no relief. Regarding history, according to the patient, her last menstrual period ended 2 days earlier. She denied any urinary symptoms, diarrhea, or constipation. She had no history of abdominal surgery and was currently not on any medications.
The patient’s vital signs at presentation were: temperature 98.7°F; blood pressure, 130/90 mm Hg; heart rate, 110 beats/minute; and respiratory rate, 18 breaths/minute. Oxygen saturation was 98% on room air. On physical examination, she appeared to be in mild distress, pacing around the room. There was moderate right costovertebral tenderness on percussion; the abdomen was soft and nontender.
Incidence
As ED visits for nephrolithiasis are increasing, so too are the health-care costs associated with this condition. Between 1992 and 2009, emergent-care presentations for nephrolithiasis rose from 178 to 340 visits per 100,000 individuals.1 Approximately 1 in 11 people in the United States will be affected by nephrolithiasis during their lifetime.2 Estimated health-care costs associated with these complaints were roughly $2 billion in 2000—an increase of 50% since 1994.2
Evaluation and Diagnosis
Laboratory Evaluation
Urinalysis is one of the initial studies for patients with suspected nephrolithiasis. Although hematuria is a classic finding associated with renal calculi, its sensitivity on microscopic analysis is around 84%. Therefore, the absence of hematuria does not exclude renal colic in the differential diagnosis.3
In addition to detecting hematuria, urinalysis can also reveal an underlying infection. One study by Abrahamian et al4 found that roughly 8% of patients presenting with acute nephrolithiasis had a urinary tract infection (UTI)—many without any clinical findings of infection. The presence of pyuria, however, has only moderate accuracy in identifying UTIs in patients with kidney stones.4 If an infected stone cannot be excluded clinically, computed tomography (CT) is indicated.
Mild leukocytosis (ie, <15,000 cells/mcL) is another common finding in patients with acute renal colic.5 A leukocyte count >15,000 cells/mcL is suspicious for infection or other pathology. A blood-chemistry panel to evaluate renal function is appropriate as a baseline—particularly for patients in whom treatment with a nonsteroidal anti-inflammatory (NSAID) drug is anticipated.
With the ability to visualize renal calculi (Figure 1), the use of noncontrast CT has become a standard initial imaging modality in assessing patients with renal colic. Between 1992 and 2009, the use of CT to evaluate patients presenting with flank pain for suspected renal colic more than tripled from 21% to 71%.6 An analysis performed by the American College of National Radiology Data Registry7 shows the mean radiation dose given by institutions for renal colic CT is unnecessarily high, and that few institutions follow CT-stone protocols aimed at minimizing radiation exposure while still maintaining proper diagnostic accuracy. A typical CT of the abdomen and pelvis is equivalent to over 100 two-view chest X-rays.8 Though controversial, data from a white paper by the American College of Radiology suggest that the ionizing radiation exposure from just one CT for renal colic causes an increase in lifetime cancer risk.9
Despite the increase in CT imaging to evaluate patients presenting to the ED with nephrolithiasis/flank pain, the proportion of patients diagnosed with a kidney stone remained the same between 2000 and 2008, with no significant change in outcomes.10-12 Moreover, the use of CT as an initial imaging modality in patients presenting with flank pain—but with no sign of infection—is unlikely to reveal important alternative findings.13
Regarding the sensitivity of CT in detecting nephrolithiasis, one study demonstrates a sensitivity of 100% and a specificity of 94% for noncontrast CT.14 Controversy, however, still exists regarding the necessity and utility of CT in diagnosing nephrolithiasis,15 and CT is one of the top 10 tests included in the American College of Emergency Physicians (ACEP) 2014 Choosing Wisely campaign. In this campaign, ACEP recommended emergency physicians (EPs) avoid abdominal and pelvic CT in otherwise healthy patients younger than age 50 years who present with symptoms consistent with uncomplicated renal colic and who have a known history of nephrolithiasis or ureterolithiasis.15 The ACEP also noted that CTs in this context do not often change treatment decisions and are associated with unnecessary radiation exposure and cost.15
While keeping the aforementioned recommendations in mind, if an EP intends to refer a renal colic patient to a urologist a CT scan is necessary either in the ED or as an outpatient. In all cases (except perhaps in patients in whom there is a history of renal stones), the urologist will need this study to determine the size and location of the stone in order to provide recommendations for management.
Ultrasound
Clinical Decision Score
Moore et al,17 authors of the Size, Topography, Location, Obstruction, Number of stones, and Evaluation (STONE) scoring system, developed a classification system for patients with suspected nephrolithiasis. This system places patients into low-, moderate-, and high-score groups, with corresponding probabilities of ureteral stone based on symptoms and epidemiological classifications.
The intent of the STONE system is to accurately predict, based on classification, the likelihood of a patient having a simple ureteral stone versus a more significant, complicated stone and to help guide which, if any, imaging studies are indicated. For example, a lower STONE score would help guide the decision to defer advanced imaging studies that would be unlikely to reveal an alternate serious diagnosis. Likewise, an individual with a high STONE score could potentially receive ultrasonography, reduced-dose CT, or no further imaging.
The STONE score performs fairly well and appears to be superior to physician gestalt, with an area under the receiver operating characteristic curve (AUC) of .78 compared to .68 with physician gestalt. This system, however, is not always accurate in its classification and has been shown to have 87% specificity at the high end to rule in stone and 96% sensitivity rate at the low end to rule out a stone. Of course, when using a clinical decision rule to rule in or rule out a stone, a tool with a very high specificity is preferred. Although the STONE scoring system does show promise, further studies are needed before it can be applied clinically.17
Treatment
Analgesia
By inhibiting prostaglandin synthesis, NSAIDs reduce inflammation and ureteral muscular hyperactivity.18 A recent Cochrane review of over 50 studies concluded that NSAIDs were effective in relieving acute renal colic pain.19 A systematic review by Holdgate and Pollock20 shows that patients treated with NSAIDs achieve greater reductions in pain scores and are less likely to require additional analgesia in the short term compared to patients treated with opioids. Although opioid medications are effective in relieving pain associated with nephrolithiasis, this class of drugs can exacerbate the nausea often associated with this condition. This same study also showed that patients who were prescribed NSAIDs following an ED visit for renal colic required less medication for pain control, experienced less nausea, and had greater improvements in their pain.20
Nevertheless, the utility of opiates as an adjunct therapy should not be overlooked. For example, in patients with renal colic, numerous studies show treatment with a combination of an NSAID and opiate provides superior pain relief compared to either treatment modality in isolation.21 Opioid analgesia may be indicated in patients in whom NSAIDs are not recommended or contraindicated (eg, elderly patients, patients with renal disease). While NSAIDs address the underlying pathophysiology associated with renal colic, they are sometimes not the best treatment option. Depending on the situation, treatment with an opioid should instead be considered.
Intravenous Fluid Therapy
A 2012 Cochrane Review of randomized control trials (RCT) on intravenous (IV) fluid therapy hydration/diuretic use concluded that there was “no reliable evidence in the literature to support the use of diuretics and high-volume fluid therapy for people with acute ureteric colic.” The review, however, did note that further investigation is warranted for a definitive answer.22 Another study by Springhart et al23 showed no difference in pain or stone expulsion between large-volume (2 L IV fluids over 2 hours) and small-volume fluid administration (20 mL/h). Regarding administration, the use of IV fluids in renal colic is no different than the usual indications for fluid therapy in the ED and should be restricted to patients with signs of dehydration or kidney injury.
Many patients with renal colic will have decreased oral intake from the pain and nausea associated with the stone and may be vomiting. Under these circumstances, it is reasonable to rehydrate the patients, even though large-volume hydration with the intent of aiding stone expulsion or improving pain has not been shown efficacious. Conversely, in addition to the perceived benefit of rehydrating patients, a small amount of fluid hydration may improve the visualization of hydronephrosis on ultrasound.24
Medical Expulsive Therapy
For many years, clinicians have considered the use of tamsulosin, an α1-receptor blocker, as well as nifedipine, a calcium channel blocker, in treating renal colic due to the theoretical benefit of reducing ureteral smooth muscle spasm/constriction thus expediting stone passage. Over the years, dozens of studies showed positive benefit in the use of medical expulsive therapy (MET). A 2014 Cochrane Review demonstrated that patients treated with α1-blockers experienced a higher stone-free rate and shorter time to stone expulsion, and concluded that α1-blockers should be offered as one of the primary treatment modalities in MET.25 This review, however, has been criticized for using a number of studies with very small patient samples, non-peer-reviewed abstracts, and low-quality study designs.26
More recently, in April 2015, Lancet published a large RCT from 24 hospitals in the United Kingdom, comparing placebo versus 400 mcg tamsulosin and 30 mg nifedipine. The authors concluded that “tamsulosin 400 mcg and nifedipine 30 mg are not effective at decreasing the need for further treatment to achieve stone clearance in 4 weeks for patients with expectantly managed ureteric colic.”27 Another large double-blind, placebo-controlled, randomized, multicenter trial by Furyk et al28 in July 2015 went a step further and evaluated distal stones, which have historically caused complications requiring intervention. They concluded that there was “no benefit overall of 0.4 mg of tamsulosin daily for patients with distal ureteric calculi less than or equal to 10 mm in terms of spontaneous passage, time to stone passage, pain, or analgesia requirements. In the subgroup with large stones (5 to 10 mm), tamsulosin did increase passage and should be considered.”28 Based on these recent studies, the use of tamsulosin in patients with stones larger than 5 mm—but not those with smaller stones—appears to be an appropriate treatment option.
Patient Disposition
The American Urological Association cited indications for urgent/emergent urological interventions necessitating the need for inpatient admission and further workup.29 Patients who do not fall into any of the categories outlined in the Table may be seen on an outpatient basis. These patients may be treated symptomatically until they can follow up with a urologist, who will determine expectant management versus intervention.
Prognosis
The majority of stones <5mm will pass spontaneously.30 Larger stones may still pass spontaneously but are more likely to require lithotripsy or other urologic intervention; therefore, patients with stones >5 mm should be referred to urology services.30
Recurrence
Patients with a first-time kidney stone have a 30% to 50% chance of disease recurrence within 5 years,31 and a 60% to 80% chance of recurrence during their lifetime.32 Those with a family history of nephrolithiasis are likely to develop an earlier onset of stones as well as experience more frequent recurrent episodes.33 Patients with recurrent disease should undergo outpatient risk stratification, including stone-composition analysis and assessment for modifiable risk factors.
Case Conclusion
The patient’s urinalysis demonstrated microscopic hematuria; blood urea nitrogen and creatinine levels were within normal limits. As the patient was tachycardic and appeared mildly dehydrated, an IV infusion of 1 L normal saline was initiated, along with ketorolac and ondansetron for symptomatic relief. A POC ultrasound of the right kidney revealed mild-to-moderate hydronephrosis; the left kidney appeared sonographically normal. Since this patient had no history of nephrolithiasis, a nonenhanced CT of the abdomen was obtained, which revealed moderate, right-sided hydronephrosis and a 3-mm distal ureteral stone. Once the patient’s symptoms were controlled, she was discharged home with a prescription for ibuprofen for symptomatic relief and instructions to follow up with her PCP.
Conclusion
The evaluation and treatment of nephrolithiasis is important due to its increasing prevalence, as well as implications on costs to the health-care system and to patients themselves. The workup and treatment of nephrolithiasis has been and continues to be the subject of much controversy. Until very recently, treatment recommendations were founded on physiological theories more so than robust research. In an era where improved imaging technology is becoming more readily available in the ED, EPs should weigh the pros and cons of its utilization for common ED complaints such as nephrolithiasis.
Dr Parsa is an assistant professor in the department of emergency medicine, Texas Tech University Health Sciences Center, El Paso. Dr Khafi is a resident in the department of emergency medicine, Texas Tech University Health Sciences Center, El Paso.
- Scales CD Jr, Smith AC, Hanley JM, Saigal CS; Urologic Diseases in America Project. Prevalence of kidney stones in the United States. Eur Urol. 2012;62(1):160-165.
- Pearle MS, Calhoun EA, Curhan GC; Urologic Diseases of America Project: urolithiasis. J Urol. 2005;173(3):848-857.
- Luchs JS, Katz DS, Lane MJ et al. Utility of hematuria testing in patients with suspected renal colic: correlation with unenhanced helical CT results. Urology. 2002;59(6):839-842.
- Abrahamian FM, Krishnadasan A, Mower WR, Moran GJ, Talan DA. Association of pyuria and clinical characteristics with the presence of urinary tract infection among patients with acute nephrolithiasis. Ann Emerg Med. 2013;62(5):526-533.
- Yilmaz S, Pekdemir M, Aksu NM, Koyuncu N, Cinar O, Akpinar E. A multicenter case–control study of diagnostic tests for urinary tract infection in the presence of urolithiasis. Urol Res. 2011;40(1):61-65. doi:10.1007/s00240-011-0402-x.
- Fwu CW, Eggers PW, Kimmel PL, Kusek JW, Kirkali Z. Emergency department visits, use of imaging, and drugs for urolithiasis have increased in the United States. Kidney Int. 2013;83(3):479-486. doi:10.1038/ki.2012.419.
- Lukasiewicz A, Bhargavan-Chatfield M, Coombs L, et al. Radiation dose index of renal colic protocol CT studies in the United States: a report from the American College of Radiology National Radiology Data Registry. Radiology. 2014;271(2):445-451. doi:10.1148/radiol.14131601.
- Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 248(1):254-263.
- Amis ES Jr, Butler PF, Applegate KE, et al; American College of Radiology. American College of Radiology white paper on radiation dose in medicine. J Am Coll Radiol. 2007;4(5):272-284.
- Hyams ES, Korley FK, Pham JC, Matlaga BR. Trends in imaging use during the emergency department evaluation of flank pain. J Urol. 2011;186(6):2270-2274. doi:10.1016/j.juro.2011.07.079.
- Ripollés T, Agramunt M, Errando J, Martínez MJ, Coronel B, Morales M. Suspected ureteral colic: plain film and sonography vs unenhanced helical CT. A prospective study in 66 patients. Eur Radiol. 2004;14(1):129-36. doi:10.1007/s00330-003-1924-1926.
- Westphalen AC, Hsia RY, Maselli JH, Wang R, Gonzales R. Radiological imaging of patients with suspected urinary tract stones: national trends, diagnoses, and predictors. Acad Emerg Med. 2011;18(7):699-707. doi:10.1111/j.1553-2712.2011.01103.x.
- Moore CL, Daniels B, Singh D, Luty S, Molinaro A. Prevalence and clinical importance of alternative causes of symptoms using a renal colic computed tomography protocol in patients with flank or back pain and absence of pyuria. Acad Emerg Med. 2013;20(5):470-478. doi:10.1111/acem.12127.
- Chen MY, Zagoria RJ. Can noncontrast helical computed tomography replace intravenous urography for evaluation of patients with acute urinary tract colic? J Emerg Med. 1999;17(2):299-303.
- American College of Emergency Physicians. Five things physicians and patients should question. Choosing Wisely Web site. 2013;10:1-5. Available at: http://www.choosingwisely.org/societies/american-college-of-emergency-physicians/. Accessed February 10, 2016.
- Smith-Bindman R, Aubin C, Bailitz J, et al. Ultrasonography versus computed tomography for suspected nephrolithiasis. N Engl J Med. 2014;371(12):1100-1110. doi:10.1056/nejmoa1404446.
- Moore CL, Bomann S, Daniels B, et al. Derivation and validation of a clinical prediction rule for uncomplicated ureteral stone—the STONE score: retrospective and prospective observational cohort studies. BMJ. 2014;348:g2191. doi:10.1136/bmj.g2191.
- Cole RS, Fry CH, Shuttleworth KE. The action of the prostaglandins on isolated human ureteric smooth muscle. Br J Urol. 1988;61(1):19-26.
- Afshar K, Jafari S, Marks AJ, Eftekhari R, McNeily AE. Nonsteroidal anti-inflammatory drugs (NSAIDs) and non-opioids for acute renal colic. Cochrane Database Syst Rev. 2015;6:CD006027. doi:10.1002/14651858.CD006027.pub2.
- Holdgate A, Pollock T. Systematic review of the relative efficacy of non-steroidal anti-inflammatory drugs and opioids in the treatment of acute renal colic. BMJ. 2004;328(7453):1401. doi:10.1136/bmj.38119.581991.55.
- Safdar B, Degutis LC, Landry K, Vedere SR, Moscovitz HC, D’Onofrio G. Intravenous morphine plus ketorolac is superior to either drug alone for treatment of acute renal colic. Ann Emerg Med. 2006;48(2):173-181, 181.e1. doi:10.1016/j.annemergmed.2006.03.013.
- Worster AS, Bhanich Supapol W. Fluids and diuretics for acute ureteric colic. Cochrane Database Syst Rev. 2012;15;2:CD004926. doi:10.1002/14651858.CD004926.pub3.
- Springhart WP, Marguet CG, Sur RL, et al. Forced versus minimal intravenous hydration in the management of acute renal colic: a randomized trial. J. Endourol. 2006;20(10):713-716. doi:10.1089/end.2006.20.713.
- Morse JW, Hill R, Greissinger WP, Patterson JW, Melanson SW, Heller MB. Rapid oral hydration results in hydronephrosis as demonstrated by bedside ultrasound. Ann Emerg Med. 1999;34(2):134-140. doi:10.1016/s0196-0644(99)70221-0.
- Campschroer T, Zhu Y, Duijvesz D, Grobbee DE, Lock MT. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014;4:CD008509. doi:10.1002/14651858.CD008509.pub2.
- Radecki R. Sadly inadequate Cochrane review of renal colic. Emergency Medicine Literature of Note. 2014. Available at: http://www.emlitofnote.com/2014/04/sadly-inadequate-cochrane-review-of.html. Accessed February 10, 2016.
- Pickard R, Starr K, MacLennan G, et al. Medical expulsive therapy in adults with ureteric colic: a multicentre, randomised, placebo-controlled trial. Lancet. 2015;386(9991):341-349. doi:10.1016/S0140-6736(15)60933-3.
- Furyk JS, Chu K, Banks C, et al. Distal ureteric stones and tamsulosin: a double-blind, placebo-controlled, randomized, multicenter trial. Ann Emerg Med. 2016;67(1):86-95.e2. doi:10.1016/j.annemergmed.2015.06.001.
- Kidney stones. American Urological Association Web site. 2016. Available at: https://www.auanet.org/education/kidney-stones.cfm. Accessed February 10, 2016.
- Miller OF, Kane CJ. Time to stone passage for observed ureteral calculi: a guide for patient education. J Urol. 1999;162(3 Pt 1):688-690.
- Lipkin ME, Preminger GM. Demystifying the medical management of nephrolithiasis. Rev Urol. 2011;13(1):34-38.
- Morton AR, Iliescu EA, Wilson JW. Nephrology: 1. Investigation and treatment of recurrent kidney stones. CMAJ. 2002;166(2):213-218.
- Koyuncu HH, Yencilek F, Eryildirim B, Sarica K. Family history in stone disease: how important is it for the onset of the disease and the incidence of recurrence? Urol Res. 2010;38(2):105-109. doi:10.1007/s00240-009-0249-6.
- Scales CD Jr, Smith AC, Hanley JM, Saigal CS; Urologic Diseases in America Project. Prevalence of kidney stones in the United States. Eur Urol. 2012;62(1):160-165.
- Pearle MS, Calhoun EA, Curhan GC; Urologic Diseases of America Project: urolithiasis. J Urol. 2005;173(3):848-857.
- Luchs JS, Katz DS, Lane MJ et al. Utility of hematuria testing in patients with suspected renal colic: correlation with unenhanced helical CT results. Urology. 2002;59(6):839-842.
- Abrahamian FM, Krishnadasan A, Mower WR, Moran GJ, Talan DA. Association of pyuria and clinical characteristics with the presence of urinary tract infection among patients with acute nephrolithiasis. Ann Emerg Med. 2013;62(5):526-533.
- Yilmaz S, Pekdemir M, Aksu NM, Koyuncu N, Cinar O, Akpinar E. A multicenter case–control study of diagnostic tests for urinary tract infection in the presence of urolithiasis. Urol Res. 2011;40(1):61-65. doi:10.1007/s00240-011-0402-x.
- Fwu CW, Eggers PW, Kimmel PL, Kusek JW, Kirkali Z. Emergency department visits, use of imaging, and drugs for urolithiasis have increased in the United States. Kidney Int. 2013;83(3):479-486. doi:10.1038/ki.2012.419.
- Lukasiewicz A, Bhargavan-Chatfield M, Coombs L, et al. Radiation dose index of renal colic protocol CT studies in the United States: a report from the American College of Radiology National Radiology Data Registry. Radiology. 2014;271(2):445-451. doi:10.1148/radiol.14131601.
- Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 248(1):254-263.
- Amis ES Jr, Butler PF, Applegate KE, et al; American College of Radiology. American College of Radiology white paper on radiation dose in medicine. J Am Coll Radiol. 2007;4(5):272-284.
- Hyams ES, Korley FK, Pham JC, Matlaga BR. Trends in imaging use during the emergency department evaluation of flank pain. J Urol. 2011;186(6):2270-2274. doi:10.1016/j.juro.2011.07.079.
- Ripollés T, Agramunt M, Errando J, Martínez MJ, Coronel B, Morales M. Suspected ureteral colic: plain film and sonography vs unenhanced helical CT. A prospective study in 66 patients. Eur Radiol. 2004;14(1):129-36. doi:10.1007/s00330-003-1924-1926.
- Westphalen AC, Hsia RY, Maselli JH, Wang R, Gonzales R. Radiological imaging of patients with suspected urinary tract stones: national trends, diagnoses, and predictors. Acad Emerg Med. 2011;18(7):699-707. doi:10.1111/j.1553-2712.2011.01103.x.
- Moore CL, Daniels B, Singh D, Luty S, Molinaro A. Prevalence and clinical importance of alternative causes of symptoms using a renal colic computed tomography protocol in patients with flank or back pain and absence of pyuria. Acad Emerg Med. 2013;20(5):470-478. doi:10.1111/acem.12127.
- Chen MY, Zagoria RJ. Can noncontrast helical computed tomography replace intravenous urography for evaluation of patients with acute urinary tract colic? J Emerg Med. 1999;17(2):299-303.
- American College of Emergency Physicians. Five things physicians and patients should question. Choosing Wisely Web site. 2013;10:1-5. Available at: http://www.choosingwisely.org/societies/american-college-of-emergency-physicians/. Accessed February 10, 2016.
- Smith-Bindman R, Aubin C, Bailitz J, et al. Ultrasonography versus computed tomography for suspected nephrolithiasis. N Engl J Med. 2014;371(12):1100-1110. doi:10.1056/nejmoa1404446.
- Moore CL, Bomann S, Daniels B, et al. Derivation and validation of a clinical prediction rule for uncomplicated ureteral stone—the STONE score: retrospective and prospective observational cohort studies. BMJ. 2014;348:g2191. doi:10.1136/bmj.g2191.
- Cole RS, Fry CH, Shuttleworth KE. The action of the prostaglandins on isolated human ureteric smooth muscle. Br J Urol. 1988;61(1):19-26.
- Afshar K, Jafari S, Marks AJ, Eftekhari R, McNeily AE. Nonsteroidal anti-inflammatory drugs (NSAIDs) and non-opioids for acute renal colic. Cochrane Database Syst Rev. 2015;6:CD006027. doi:10.1002/14651858.CD006027.pub2.
- Holdgate A, Pollock T. Systematic review of the relative efficacy of non-steroidal anti-inflammatory drugs and opioids in the treatment of acute renal colic. BMJ. 2004;328(7453):1401. doi:10.1136/bmj.38119.581991.55.
- Safdar B, Degutis LC, Landry K, Vedere SR, Moscovitz HC, D’Onofrio G. Intravenous morphine plus ketorolac is superior to either drug alone for treatment of acute renal colic. Ann Emerg Med. 2006;48(2):173-181, 181.e1. doi:10.1016/j.annemergmed.2006.03.013.
- Worster AS, Bhanich Supapol W. Fluids and diuretics for acute ureteric colic. Cochrane Database Syst Rev. 2012;15;2:CD004926. doi:10.1002/14651858.CD004926.pub3.
- Springhart WP, Marguet CG, Sur RL, et al. Forced versus minimal intravenous hydration in the management of acute renal colic: a randomized trial. J. Endourol. 2006;20(10):713-716. doi:10.1089/end.2006.20.713.
- Morse JW, Hill R, Greissinger WP, Patterson JW, Melanson SW, Heller MB. Rapid oral hydration results in hydronephrosis as demonstrated by bedside ultrasound. Ann Emerg Med. 1999;34(2):134-140. doi:10.1016/s0196-0644(99)70221-0.
- Campschroer T, Zhu Y, Duijvesz D, Grobbee DE, Lock MT. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014;4:CD008509. doi:10.1002/14651858.CD008509.pub2.
- Radecki R. Sadly inadequate Cochrane review of renal colic. Emergency Medicine Literature of Note. 2014. Available at: http://www.emlitofnote.com/2014/04/sadly-inadequate-cochrane-review-of.html. Accessed February 10, 2016.
- Pickard R, Starr K, MacLennan G, et al. Medical expulsive therapy in adults with ureteric colic: a multicentre, randomised, placebo-controlled trial. Lancet. 2015;386(9991):341-349. doi:10.1016/S0140-6736(15)60933-3.
- Furyk JS, Chu K, Banks C, et al. Distal ureteric stones and tamsulosin: a double-blind, placebo-controlled, randomized, multicenter trial. Ann Emerg Med. 2016;67(1):86-95.e2. doi:10.1016/j.annemergmed.2015.06.001.
- Kidney stones. American Urological Association Web site. 2016. Available at: https://www.auanet.org/education/kidney-stones.cfm. Accessed February 10, 2016.
- Miller OF, Kane CJ. Time to stone passage for observed ureteral calculi: a guide for patient education. J Urol. 1999;162(3 Pt 1):688-690.
- Lipkin ME, Preminger GM. Demystifying the medical management of nephrolithiasis. Rev Urol. 2011;13(1):34-38.
- Morton AR, Iliescu EA, Wilson JW. Nephrology: 1. Investigation and treatment of recurrent kidney stones. CMAJ. 2002;166(2):213-218.
- Koyuncu HH, Yencilek F, Eryildirim B, Sarica K. Family history in stone disease: how important is it for the onset of the disease and the incidence of recurrence? Urol Res. 2010;38(2):105-109. doi:10.1007/s00240-009-0249-6.