User login
Some have heavier periods after COVID vaccine
Many women who got a COVID-19 vaccine have reported heavier bleeding during their periods since they had the shots.
A team of researchers investigated the trend and set out to find out who among the vaccinated were more likely to experience the menstruation changes.
The researchers were led by Katharine M.N. Lee, PhD, MS, of the division of public health sciences at Washington University in St. Louis. Their findings were published ahead of print in Science Advances.
The investigators analyzed more than 139,000 responses from an online survey from both currently and formerly menstruating women.
They found that, among people who have regular periods, about the same percentage had heavier bleeding after they got a COVID vaccine as had no change in bleeding after the vaccine (44% vs. 42%, respectively).
“A much smaller portion had lighter periods,” they write.
The phenomenon has been difficult to study because questions about changes in menstruation are not a standard part of vaccine trials.
Date of last period is often tracked in clinical trials to make sure a participant is not pregnant, but the questions about periods often stop there.
Additionally, periods are different for everyone and can be influenced by all sorts of environmental factors, so making associations regarding exposures is problematic.
No changes found to fertility
The authors emphasized that, generally, changes to menstrual bleeding are not uncommon nor dangerous. They also emphasized that the changes in bleeding don’t mean changes to fertility.
The uterine reproductive system is flexible when the body is under stress, they note.
“We know that running a marathon may influence hormone concentrations in the short term while not rendering that person infertile,” the authors write.
However, they acknowledge that investigating these reports is critical in building trust in medicine.
This report includes information that hasn’t been available through the clinical trial follow-up process.
For instance, the authors write, “To the best of our knowledge, our work is the first to examine breakthrough bleeding after vaccination in either pre- or postmenopausal people.”
Reports of changes to periods after vaccination started emerging in 2021. But without data, reports were largely dismissed, fueling criticism from those waging campaigns against COVID vaccines.
Dr. Lee and colleagues gathered data from those who responded to the online survey and detailed some trends.
People who were bleeding more heavily after vaccination were more likely to be older, Hispanic, had vaccine side effects of fever and fatigue, had been pregnant at some point, or had given birth.
People with regular periods who had endometriosis, prolonged bleeding during their periods, polycystic ovarian syndrome (PCOS) or fibroids were also more likely to have increased bleeding after a COVID vaccine.
Breakthrough bleeding
For people who don’t menstruate, but have not reached menopause, breakthrough bleeding happened more often in women who had been pregnant and/or had given birth.
Among respondents who were postmenopausal, breakthrough bleeding happened more often in younger people and/or those who are Hispanic.
More than a third of the respondents (39%) who use gender-affirming hormones that eliminate menstruation reported breakthrough bleeding after vaccination.
The majority of premenopausal people on long-acting, reversible contraception (71%) and the majority of postmenopausal respondents (66%) had breakthrough bleeding as well.
The authors note that you can’t compare the percentages who report these experiences in the survey with the incidence of those who would experience changes in menstrual bleeding in the general population.
The nature of the online survey means it may be naturally biased because the people who responded may be more often those who noted some change in their own menstrual experiences, particularly if that involved discomfort, pain, or fear.
Researchers also acknowledge that Black, Indigenous, Latinx, and other respondents of color are underrepresented in this research and that represents a limitation in the work.
Alison Edelman, MD, MPH, with the department of obstetrics and gynecology at Oregon Health & Science University in Portland, was not involved with Dr. Lee and associates’ study but has also studied the relationship between COVID vaccines and menstruation.
Her team’s study found that COVID vaccination is associated with a small change in time between periods but not length of periods.
She said about the work by Dr. Lee and colleagues, “This work really elevates the voices of the public and what they’re experiencing.”
The association makes sense, Dr. Edelman says, in that the reproductive system and the immune system talk to each other and inflammation in the immune system is going to be noticed by the system governing periods.
Lack of data on the relationship between exposures and menstruation didn’t start with COVID. “There has been a signal in the population before with other vaccines that’s been dismissed,” she said.
Tracking menstruation information in clinical trials can help physicians counsel women on what may be coming with any vaccine and alleviate fears and vaccine hesitancy, Dr. Edelman explained. It can also help vaccine developers know what to include in information about their product.
“When you are counseled about what to expect, it’s not as scary. That provides trust in the system,” she said. She likened it to original lack of data on whether COVID-19 vaccines would affect pregnancy.
“We have great science now that COVID vaccine does not affect fertility and [vaccine] does not impact pregnancy.”
Another important aspect of this paper is that it included subgroups not studied before regarding menstruation and breakthrough bleeding, such as those taking gender-affirming hormones, she added.
Menstruation has been often overlooked as important in clinical trial exposures but Dr. Edelman hopes this recent attention and question will escalate and prompt more research.
“I’m hoping with the immense outpouring from the public about how important this is, that future studies will look at this a little bit better,” she says.
She said when the National Institutes of Health opened up funding for trials on COVID-19 vaccines and menstruation, researchers got flooded with requests from women to share their stories.
“As a researcher – I’ve been doing research for over 20 years – that’s not something that usually happens. I would love to have that happen for every research project.”
The authors and Dr. Edelman declare that they have no competing interests. This research was supported in part by the University of Illinois Beckman Institute for Advanced Science and Technology, the University of Illinois Interdisciplinary Health Sciences Institute, the National Institutes of Health, the Foundation for Barnes-Jewish Hospital, and the Siteman Cancer Center.
Many women who got a COVID-19 vaccine have reported heavier bleeding during their periods since they had the shots.
A team of researchers investigated the trend and set out to find out who among the vaccinated were more likely to experience the menstruation changes.
The researchers were led by Katharine M.N. Lee, PhD, MS, of the division of public health sciences at Washington University in St. Louis. Their findings were published ahead of print in Science Advances.
The investigators analyzed more than 139,000 responses from an online survey from both currently and formerly menstruating women.
They found that, among people who have regular periods, about the same percentage had heavier bleeding after they got a COVID vaccine as had no change in bleeding after the vaccine (44% vs. 42%, respectively).
“A much smaller portion had lighter periods,” they write.
The phenomenon has been difficult to study because questions about changes in menstruation are not a standard part of vaccine trials.
Date of last period is often tracked in clinical trials to make sure a participant is not pregnant, but the questions about periods often stop there.
Additionally, periods are different for everyone and can be influenced by all sorts of environmental factors, so making associations regarding exposures is problematic.
No changes found to fertility
The authors emphasized that, generally, changes to menstrual bleeding are not uncommon nor dangerous. They also emphasized that the changes in bleeding don’t mean changes to fertility.
The uterine reproductive system is flexible when the body is under stress, they note.
“We know that running a marathon may influence hormone concentrations in the short term while not rendering that person infertile,” the authors write.
However, they acknowledge that investigating these reports is critical in building trust in medicine.
This report includes information that hasn’t been available through the clinical trial follow-up process.
For instance, the authors write, “To the best of our knowledge, our work is the first to examine breakthrough bleeding after vaccination in either pre- or postmenopausal people.”
Reports of changes to periods after vaccination started emerging in 2021. But without data, reports were largely dismissed, fueling criticism from those waging campaigns against COVID vaccines.
Dr. Lee and colleagues gathered data from those who responded to the online survey and detailed some trends.
People who were bleeding more heavily after vaccination were more likely to be older, Hispanic, had vaccine side effects of fever and fatigue, had been pregnant at some point, or had given birth.
People with regular periods who had endometriosis, prolonged bleeding during their periods, polycystic ovarian syndrome (PCOS) or fibroids were also more likely to have increased bleeding after a COVID vaccine.
Breakthrough bleeding
For people who don’t menstruate, but have not reached menopause, breakthrough bleeding happened more often in women who had been pregnant and/or had given birth.
Among respondents who were postmenopausal, breakthrough bleeding happened more often in younger people and/or those who are Hispanic.
More than a third of the respondents (39%) who use gender-affirming hormones that eliminate menstruation reported breakthrough bleeding after vaccination.
The majority of premenopausal people on long-acting, reversible contraception (71%) and the majority of postmenopausal respondents (66%) had breakthrough bleeding as well.
The authors note that you can’t compare the percentages who report these experiences in the survey with the incidence of those who would experience changes in menstrual bleeding in the general population.
The nature of the online survey means it may be naturally biased because the people who responded may be more often those who noted some change in their own menstrual experiences, particularly if that involved discomfort, pain, or fear.
Researchers also acknowledge that Black, Indigenous, Latinx, and other respondents of color are underrepresented in this research and that represents a limitation in the work.
Alison Edelman, MD, MPH, with the department of obstetrics and gynecology at Oregon Health & Science University in Portland, was not involved with Dr. Lee and associates’ study but has also studied the relationship between COVID vaccines and menstruation.
Her team’s study found that COVID vaccination is associated with a small change in time between periods but not length of periods.
She said about the work by Dr. Lee and colleagues, “This work really elevates the voices of the public and what they’re experiencing.”
The association makes sense, Dr. Edelman says, in that the reproductive system and the immune system talk to each other and inflammation in the immune system is going to be noticed by the system governing periods.
Lack of data on the relationship between exposures and menstruation didn’t start with COVID. “There has been a signal in the population before with other vaccines that’s been dismissed,” she said.
Tracking menstruation information in clinical trials can help physicians counsel women on what may be coming with any vaccine and alleviate fears and vaccine hesitancy, Dr. Edelman explained. It can also help vaccine developers know what to include in information about their product.
“When you are counseled about what to expect, it’s not as scary. That provides trust in the system,” she said. She likened it to original lack of data on whether COVID-19 vaccines would affect pregnancy.
“We have great science now that COVID vaccine does not affect fertility and [vaccine] does not impact pregnancy.”
Another important aspect of this paper is that it included subgroups not studied before regarding menstruation and breakthrough bleeding, such as those taking gender-affirming hormones, she added.
Menstruation has been often overlooked as important in clinical trial exposures but Dr. Edelman hopes this recent attention and question will escalate and prompt more research.
“I’m hoping with the immense outpouring from the public about how important this is, that future studies will look at this a little bit better,” she says.
She said when the National Institutes of Health opened up funding for trials on COVID-19 vaccines and menstruation, researchers got flooded with requests from women to share their stories.
“As a researcher – I’ve been doing research for over 20 years – that’s not something that usually happens. I would love to have that happen for every research project.”
The authors and Dr. Edelman declare that they have no competing interests. This research was supported in part by the University of Illinois Beckman Institute for Advanced Science and Technology, the University of Illinois Interdisciplinary Health Sciences Institute, the National Institutes of Health, the Foundation for Barnes-Jewish Hospital, and the Siteman Cancer Center.
Many women who got a COVID-19 vaccine have reported heavier bleeding during their periods since they had the shots.
A team of researchers investigated the trend and set out to find out who among the vaccinated were more likely to experience the menstruation changes.
The researchers were led by Katharine M.N. Lee, PhD, MS, of the division of public health sciences at Washington University in St. Louis. Their findings were published ahead of print in Science Advances.
The investigators analyzed more than 139,000 responses from an online survey from both currently and formerly menstruating women.
They found that, among people who have regular periods, about the same percentage had heavier bleeding after they got a COVID vaccine as had no change in bleeding after the vaccine (44% vs. 42%, respectively).
“A much smaller portion had lighter periods,” they write.
The phenomenon has been difficult to study because questions about changes in menstruation are not a standard part of vaccine trials.
Date of last period is often tracked in clinical trials to make sure a participant is not pregnant, but the questions about periods often stop there.
Additionally, periods are different for everyone and can be influenced by all sorts of environmental factors, so making associations regarding exposures is problematic.
No changes found to fertility
The authors emphasized that, generally, changes to menstrual bleeding are not uncommon nor dangerous. They also emphasized that the changes in bleeding don’t mean changes to fertility.
The uterine reproductive system is flexible when the body is under stress, they note.
“We know that running a marathon may influence hormone concentrations in the short term while not rendering that person infertile,” the authors write.
However, they acknowledge that investigating these reports is critical in building trust in medicine.
This report includes information that hasn’t been available through the clinical trial follow-up process.
For instance, the authors write, “To the best of our knowledge, our work is the first to examine breakthrough bleeding after vaccination in either pre- or postmenopausal people.”
Reports of changes to periods after vaccination started emerging in 2021. But without data, reports were largely dismissed, fueling criticism from those waging campaigns against COVID vaccines.
Dr. Lee and colleagues gathered data from those who responded to the online survey and detailed some trends.
People who were bleeding more heavily after vaccination were more likely to be older, Hispanic, had vaccine side effects of fever and fatigue, had been pregnant at some point, or had given birth.
People with regular periods who had endometriosis, prolonged bleeding during their periods, polycystic ovarian syndrome (PCOS) or fibroids were also more likely to have increased bleeding after a COVID vaccine.
Breakthrough bleeding
For people who don’t menstruate, but have not reached menopause, breakthrough bleeding happened more often in women who had been pregnant and/or had given birth.
Among respondents who were postmenopausal, breakthrough bleeding happened more often in younger people and/or those who are Hispanic.
More than a third of the respondents (39%) who use gender-affirming hormones that eliminate menstruation reported breakthrough bleeding after vaccination.
The majority of premenopausal people on long-acting, reversible contraception (71%) and the majority of postmenopausal respondents (66%) had breakthrough bleeding as well.
The authors note that you can’t compare the percentages who report these experiences in the survey with the incidence of those who would experience changes in menstrual bleeding in the general population.
The nature of the online survey means it may be naturally biased because the people who responded may be more often those who noted some change in their own menstrual experiences, particularly if that involved discomfort, pain, or fear.
Researchers also acknowledge that Black, Indigenous, Latinx, and other respondents of color are underrepresented in this research and that represents a limitation in the work.
Alison Edelman, MD, MPH, with the department of obstetrics and gynecology at Oregon Health & Science University in Portland, was not involved with Dr. Lee and associates’ study but has also studied the relationship between COVID vaccines and menstruation.
Her team’s study found that COVID vaccination is associated with a small change in time between periods but not length of periods.
She said about the work by Dr. Lee and colleagues, “This work really elevates the voices of the public and what they’re experiencing.”
The association makes sense, Dr. Edelman says, in that the reproductive system and the immune system talk to each other and inflammation in the immune system is going to be noticed by the system governing periods.
Lack of data on the relationship between exposures and menstruation didn’t start with COVID. “There has been a signal in the population before with other vaccines that’s been dismissed,” she said.
Tracking menstruation information in clinical trials can help physicians counsel women on what may be coming with any vaccine and alleviate fears and vaccine hesitancy, Dr. Edelman explained. It can also help vaccine developers know what to include in information about their product.
“When you are counseled about what to expect, it’s not as scary. That provides trust in the system,” she said. She likened it to original lack of data on whether COVID-19 vaccines would affect pregnancy.
“We have great science now that COVID vaccine does not affect fertility and [vaccine] does not impact pregnancy.”
Another important aspect of this paper is that it included subgroups not studied before regarding menstruation and breakthrough bleeding, such as those taking gender-affirming hormones, she added.
Menstruation has been often overlooked as important in clinical trial exposures but Dr. Edelman hopes this recent attention and question will escalate and prompt more research.
“I’m hoping with the immense outpouring from the public about how important this is, that future studies will look at this a little bit better,” she says.
She said when the National Institutes of Health opened up funding for trials on COVID-19 vaccines and menstruation, researchers got flooded with requests from women to share their stories.
“As a researcher – I’ve been doing research for over 20 years – that’s not something that usually happens. I would love to have that happen for every research project.”
The authors and Dr. Edelman declare that they have no competing interests. This research was supported in part by the University of Illinois Beckman Institute for Advanced Science and Technology, the University of Illinois Interdisciplinary Health Sciences Institute, the National Institutes of Health, the Foundation for Barnes-Jewish Hospital, and the Siteman Cancer Center.
FROM SCIENCE ADVANCES
BA.4 and BA.5 subvariants are more evasive of antibodies, but not of cellular immunity
The picture around the BA.4 and BA.5 subvariants of Omicron has been really confusing in that the pair is driving up cases but global COVID-19 deaths remain at their lowest level since the beginning of the pandemic. Explaining the two components of the immune response – antibodies versus cellular immune responses – can help us understand where we are in the pandemic and future booster options.
These two subvariants of Omicron, as of July 5, make up more than half of the COVID-19 strains in the United States and are expected to keep increasing. One of two reasons can lead to a variant or subvariant becoming dominant strain: increased transmissibility or evasion of antibodies.
Although BA.4 and BA.5 could be more transmissible than other subvariants of Omicron (which is already very transmissible), this has not yet been established in experiments showing increased affinity for the human receptor or in animal models. What we do know is that BA.4 and BA.5 seem to evade neutralizing antibodies conferred by the vaccines or even prior BA.1 infection (an earlier subvariant of Omicron), which could be the reason we are seeing so many reinfections now. Of note, BA.1 infection conferred antibodies that protected against subsequent BA.2 infection, so we did not see the same spike in cases in the United States with BA.2 (after a large BA.1 spike over the winter) earlier this spring.
Okay, so isn’t evasion of antibodies a bad thing? Of course it is but, luckily, our immune system is “redundant” and doesn›t just rely on antibodies to protect us from infection. In fact, antibodies (such as IgA, which is the mucosal antibody most prevalent in the nose and mouth, and IgG, which is the most prevalent antibody in the bloodstream) are our first line of COVID-19 defense in the nasal mucosa. Therefore, mild upper respiratory infections will be common as BA.4/BA.5 evade our nasal antibodies. Luckily, the rate of severe disease is remaining low throughout the world, probably because of the high amounts of cellular immunity to the virus. B and T cells are our protectors from severe disease.
For instance, two-dose vaccines are still conferring high rates of protection from severe disease with the BA.4 and BA.5 variants, with 87% protection against hospitalization per South Africa data. This is probably attributable to the fact that T-cell immunity from the vaccines remains protective across variants “from Alpha to Omicron,” as described by a recent and elegant paper.
Data from Qatar show that natural infection (even occurring up to 14 months ago) remains very protective (97.3%) against severe disease with the current circulating subvariants, including BA.4 and BA.5. Again, this is probably attributable to T cells which specifically amplify in response to a piece of the virus and help recruit cells to attack the pathogen directly.
The original BA.1 subvariant of Omicron has 26-32 mutations along its spike protein that differ from the “ancestral strain,” and BA.4 and BA.5 variants have a few more. Our T-cell response, even across a mutated spike protein, is so robust that we have not seen Omicron yet able to evade the many T cells (which we produce from the vaccines or infection) that descend upon the mutated virus to fight severe disease. Antibody-producing memory B cells, generated by the vaccines (or prior infection), have been shown to actually adapt their immune response to the variant to which they are exposed.
Therefore, the story of the BA.4 and BA.5 subvariants seems to remain about antibodies vs. cellular immunity. Our immunity in the United States is growing and is from both vaccination and natural infection, with 78.3% of the population having had at least one dose of the vaccine and at least 60% of adults (and 75% of children 0-18) having been exposed to the virus by February 2022, per the Centers for Disease Control and Prevention (with exposure probably much higher now in July 2022 after subsequent Omicron subvariants waves).
So, what about Omicron-specific boosters? A booster shot will just raise antibodies temporarily, but their effectiveness wanes several months later. Moreover, a booster shot against the ancestral strain is not very effective in neutralizing BA.4 and BA.5 (with a prior BA.1 Omicron infection being more effective than a booster). Luckily, Pfizer has promised a BA.4/BA.5-specific mRNA vaccine by October, and Moderna has promised a bivalent vaccine containing BA.4/BA.5 mRNA sequences around the same time. A vaccine that specifically increases antibodies against the most prevalent circulating strain should be important as a booster for those who are predisposed to severe breakthrough infections (for example, those with immunocompromise or older individuals with multiple comorbidities). Moreover, BA.4/BA.5–specific booster vaccines may help prevent mild infections for many individuals. Finally, any booster (or exposure) should diversify and broaden T-cell responses to the virus, and a booster shot will also expand the potency of B cells, making them better able to respond to the newest subvariants as we continue to live with COVID-19.
Monica Gandhi, MD, MPH, is an infectious diseases doctor, professor of medicine, and associate chief in the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco.
A version of this article first appeared on Medscape.com.
The picture around the BA.4 and BA.5 subvariants of Omicron has been really confusing in that the pair is driving up cases but global COVID-19 deaths remain at their lowest level since the beginning of the pandemic. Explaining the two components of the immune response – antibodies versus cellular immune responses – can help us understand where we are in the pandemic and future booster options.
These two subvariants of Omicron, as of July 5, make up more than half of the COVID-19 strains in the United States and are expected to keep increasing. One of two reasons can lead to a variant or subvariant becoming dominant strain: increased transmissibility or evasion of antibodies.
Although BA.4 and BA.5 could be more transmissible than other subvariants of Omicron (which is already very transmissible), this has not yet been established in experiments showing increased affinity for the human receptor or in animal models. What we do know is that BA.4 and BA.5 seem to evade neutralizing antibodies conferred by the vaccines or even prior BA.1 infection (an earlier subvariant of Omicron), which could be the reason we are seeing so many reinfections now. Of note, BA.1 infection conferred antibodies that protected against subsequent BA.2 infection, so we did not see the same spike in cases in the United States with BA.2 (after a large BA.1 spike over the winter) earlier this spring.
Okay, so isn’t evasion of antibodies a bad thing? Of course it is but, luckily, our immune system is “redundant” and doesn›t just rely on antibodies to protect us from infection. In fact, antibodies (such as IgA, which is the mucosal antibody most prevalent in the nose and mouth, and IgG, which is the most prevalent antibody in the bloodstream) are our first line of COVID-19 defense in the nasal mucosa. Therefore, mild upper respiratory infections will be common as BA.4/BA.5 evade our nasal antibodies. Luckily, the rate of severe disease is remaining low throughout the world, probably because of the high amounts of cellular immunity to the virus. B and T cells are our protectors from severe disease.
For instance, two-dose vaccines are still conferring high rates of protection from severe disease with the BA.4 and BA.5 variants, with 87% protection against hospitalization per South Africa data. This is probably attributable to the fact that T-cell immunity from the vaccines remains protective across variants “from Alpha to Omicron,” as described by a recent and elegant paper.
Data from Qatar show that natural infection (even occurring up to 14 months ago) remains very protective (97.3%) against severe disease with the current circulating subvariants, including BA.4 and BA.5. Again, this is probably attributable to T cells which specifically amplify in response to a piece of the virus and help recruit cells to attack the pathogen directly.
The original BA.1 subvariant of Omicron has 26-32 mutations along its spike protein that differ from the “ancestral strain,” and BA.4 and BA.5 variants have a few more. Our T-cell response, even across a mutated spike protein, is so robust that we have not seen Omicron yet able to evade the many T cells (which we produce from the vaccines or infection) that descend upon the mutated virus to fight severe disease. Antibody-producing memory B cells, generated by the vaccines (or prior infection), have been shown to actually adapt their immune response to the variant to which they are exposed.
Therefore, the story of the BA.4 and BA.5 subvariants seems to remain about antibodies vs. cellular immunity. Our immunity in the United States is growing and is from both vaccination and natural infection, with 78.3% of the population having had at least one dose of the vaccine and at least 60% of adults (and 75% of children 0-18) having been exposed to the virus by February 2022, per the Centers for Disease Control and Prevention (with exposure probably much higher now in July 2022 after subsequent Omicron subvariants waves).
So, what about Omicron-specific boosters? A booster shot will just raise antibodies temporarily, but their effectiveness wanes several months later. Moreover, a booster shot against the ancestral strain is not very effective in neutralizing BA.4 and BA.5 (with a prior BA.1 Omicron infection being more effective than a booster). Luckily, Pfizer has promised a BA.4/BA.5-specific mRNA vaccine by October, and Moderna has promised a bivalent vaccine containing BA.4/BA.5 mRNA sequences around the same time. A vaccine that specifically increases antibodies against the most prevalent circulating strain should be important as a booster for those who are predisposed to severe breakthrough infections (for example, those with immunocompromise or older individuals with multiple comorbidities). Moreover, BA.4/BA.5–specific booster vaccines may help prevent mild infections for many individuals. Finally, any booster (or exposure) should diversify and broaden T-cell responses to the virus, and a booster shot will also expand the potency of B cells, making them better able to respond to the newest subvariants as we continue to live with COVID-19.
Monica Gandhi, MD, MPH, is an infectious diseases doctor, professor of medicine, and associate chief in the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco.
A version of this article first appeared on Medscape.com.
The picture around the BA.4 and BA.5 subvariants of Omicron has been really confusing in that the pair is driving up cases but global COVID-19 deaths remain at their lowest level since the beginning of the pandemic. Explaining the two components of the immune response – antibodies versus cellular immune responses – can help us understand where we are in the pandemic and future booster options.
These two subvariants of Omicron, as of July 5, make up more than half of the COVID-19 strains in the United States and are expected to keep increasing. One of two reasons can lead to a variant or subvariant becoming dominant strain: increased transmissibility or evasion of antibodies.
Although BA.4 and BA.5 could be more transmissible than other subvariants of Omicron (which is already very transmissible), this has not yet been established in experiments showing increased affinity for the human receptor or in animal models. What we do know is that BA.4 and BA.5 seem to evade neutralizing antibodies conferred by the vaccines or even prior BA.1 infection (an earlier subvariant of Omicron), which could be the reason we are seeing so many reinfections now. Of note, BA.1 infection conferred antibodies that protected against subsequent BA.2 infection, so we did not see the same spike in cases in the United States with BA.2 (after a large BA.1 spike over the winter) earlier this spring.
Okay, so isn’t evasion of antibodies a bad thing? Of course it is but, luckily, our immune system is “redundant” and doesn›t just rely on antibodies to protect us from infection. In fact, antibodies (such as IgA, which is the mucosal antibody most prevalent in the nose and mouth, and IgG, which is the most prevalent antibody in the bloodstream) are our first line of COVID-19 defense in the nasal mucosa. Therefore, mild upper respiratory infections will be common as BA.4/BA.5 evade our nasal antibodies. Luckily, the rate of severe disease is remaining low throughout the world, probably because of the high amounts of cellular immunity to the virus. B and T cells are our protectors from severe disease.
For instance, two-dose vaccines are still conferring high rates of protection from severe disease with the BA.4 and BA.5 variants, with 87% protection against hospitalization per South Africa data. This is probably attributable to the fact that T-cell immunity from the vaccines remains protective across variants “from Alpha to Omicron,” as described by a recent and elegant paper.
Data from Qatar show that natural infection (even occurring up to 14 months ago) remains very protective (97.3%) against severe disease with the current circulating subvariants, including BA.4 and BA.5. Again, this is probably attributable to T cells which specifically amplify in response to a piece of the virus and help recruit cells to attack the pathogen directly.
The original BA.1 subvariant of Omicron has 26-32 mutations along its spike protein that differ from the “ancestral strain,” and BA.4 and BA.5 variants have a few more. Our T-cell response, even across a mutated spike protein, is so robust that we have not seen Omicron yet able to evade the many T cells (which we produce from the vaccines or infection) that descend upon the mutated virus to fight severe disease. Antibody-producing memory B cells, generated by the vaccines (or prior infection), have been shown to actually adapt their immune response to the variant to which they are exposed.
Therefore, the story of the BA.4 and BA.5 subvariants seems to remain about antibodies vs. cellular immunity. Our immunity in the United States is growing and is from both vaccination and natural infection, with 78.3% of the population having had at least one dose of the vaccine and at least 60% of adults (and 75% of children 0-18) having been exposed to the virus by February 2022, per the Centers for Disease Control and Prevention (with exposure probably much higher now in July 2022 after subsequent Omicron subvariants waves).
So, what about Omicron-specific boosters? A booster shot will just raise antibodies temporarily, but their effectiveness wanes several months later. Moreover, a booster shot against the ancestral strain is not very effective in neutralizing BA.4 and BA.5 (with a prior BA.1 Omicron infection being more effective than a booster). Luckily, Pfizer has promised a BA.4/BA.5-specific mRNA vaccine by October, and Moderna has promised a bivalent vaccine containing BA.4/BA.5 mRNA sequences around the same time. A vaccine that specifically increases antibodies against the most prevalent circulating strain should be important as a booster for those who are predisposed to severe breakthrough infections (for example, those with immunocompromise or older individuals with multiple comorbidities). Moreover, BA.4/BA.5–specific booster vaccines may help prevent mild infections for many individuals. Finally, any booster (or exposure) should diversify and broaden T-cell responses to the virus, and a booster shot will also expand the potency of B cells, making them better able to respond to the newest subvariants as we continue to live with COVID-19.
Monica Gandhi, MD, MPH, is an infectious diseases doctor, professor of medicine, and associate chief in the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco.
A version of this article first appeared on Medscape.com.
Acute hepatitis cases in children show declining trend; adenovirus, COVID-19 remain key leads
LONDON – Case numbers of acute hepatitis in children show “a declining trajectory,” and COVID-19 and adenovirus remain the most likely, but as yet unproven, causative agents, said experts in an update at the annual International Liver Congress sponsored by the European Association for the Study of the Liver.
Philippa Easterbrook, MD, medical expert at the World Health Organization Global HIV, Hepatitis, and STI Programme, shared the latest case numbers and working hypotheses of possible causative agents in the outbreak of acute hepatitis among children in Europe and beyond.
Global data across the five WHO regions show there were 244 cases in the past month, bringing the total to 894 probable cases reported since October 2021 from 33 countries.
“It’s important to remember that this includes new cases, as well as retrospectively identified cases,” Dr.Easterbrook said. “Over half (52%) are from the European region, while 262 cases (30% of the global total) are from the United Kingdom.”
Data from Europe and the United States show a declining trajectory of reports of new cases. “This is a positive development,” she said.
The second highest reporting region is the Americas, she said, with 368 cases total, 290 cases of which come from the United States, accounting for 35% of the global total.
“Together the United Kingdom and the United States make up 65% of the global total,” she said.
Dr. Easterbrook added that 17 of the 33 reporting countries had more than five cases. Most cases (75%) are in young children under 5 years of age.
Serious cases are relatively few, but 44 (5%) children have required liver transplantation. Data from the European region show that 30% have required intensive care at some point during their hospitalization. There have been 18 (2%) reported deaths.
Possible post-COVID phenomenon, adenovirus most commonly reported
Dr. Easterbrook acknowledged the emerging hypothesis of a post-COVID phenomenon.
“Is this a variant of the rare but recognized multisystem inflammatory syndrome condition in children that’s been reported, often 1-2 months after COVID, causing widespread organ damage?” But she pointed out that the reported COVID cases with hepatitis “don’t seem to fit these features.”
Adenovirus remains the most commonly detected virus in acute hepatitis in children, found in 53% of cases overall, she said. The adenovirus detection rate is higher in the United Kingdom, at 68%.
“There are quite high rates of detection, but they’re not in all cases. There does seem to be a high rate of detection in the younger age groups and in those who are developing severe disease, so perhaps there is some link to severity,” Dr. Easterbrook said.
The working hypotheses continue to favor adenovirus together with past or current SARS-CoV-2 infection, as proposed early in the outbreak, she said. “These either work independently or work together as cofactors in some way to result in hepatitis. And there has been some clear progress on this. WHO is bringing together the data from different countries on some of these working hypotheses.”
Dr. Easterbrook highlighted the importance of procuring global data, especially given that two countries are reporting the majority of cases and in high numbers. “It’s a mixed picture with different rates of adenovirus detection and of COVID,” she said. “We need good-quality data collected in a standardized way.” WHO is requesting that countries provide these data.
She also highlighted the need for good in-depth studies, citing the UK Health Security Agency as an example of this. “There’s only a few countries that have the capacity or the patient numbers to look at this in detail, for example, the U.K. and the UKHSA.”
She noted that the UKHSA had laid out a comprehensive, systematic set of further investigations. For example, a case-control study is trying to establish whether there is a difference in the rate of adenovirus detection in children with hepatitis compared with other hospitalized children at the same time. “This aims to really tease out whether adenovirus is a cause or just a bystander,” she said.
She added that there were also genetic studies investigating whether genes were predisposing some children to develop a more severe form of disease. Other studies are evaluating the immune response of the patients.
Dr. Easterbrook added that the WHO will soon launch a global survey asking whether the reports of acute hepatitis are greater than the expected background rate for cases of hepatitis of unknown etiology.
Acute hepatitis is not new, but high caseload is
Also speaking at the ILC special briefing was Maria Buti, MD, PhD, policy and public health chair for the European Association for the Study of the Liver, and chief of the internal medicine and hepatology department at Hospital General Universitari Valle Hebron in Barcelona.
Dr. Buti drew attention to the fact that severe acute hepatitis of unknown etiology in children is not new.
“We have cases of acute hepatitis that even needed liver transplantation some years ago, and every year in our clinics we see these type of patients,” Dr. Buti remarked. What is really new, she added, is the amount of cases, particularly in the United Kingdom.
Dr. Easterbrook and Dr. Buti have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LONDON – Case numbers of acute hepatitis in children show “a declining trajectory,” and COVID-19 and adenovirus remain the most likely, but as yet unproven, causative agents, said experts in an update at the annual International Liver Congress sponsored by the European Association for the Study of the Liver.
Philippa Easterbrook, MD, medical expert at the World Health Organization Global HIV, Hepatitis, and STI Programme, shared the latest case numbers and working hypotheses of possible causative agents in the outbreak of acute hepatitis among children in Europe and beyond.
Global data across the five WHO regions show there were 244 cases in the past month, bringing the total to 894 probable cases reported since October 2021 from 33 countries.
“It’s important to remember that this includes new cases, as well as retrospectively identified cases,” Dr.Easterbrook said. “Over half (52%) are from the European region, while 262 cases (30% of the global total) are from the United Kingdom.”
Data from Europe and the United States show a declining trajectory of reports of new cases. “This is a positive development,” she said.
The second highest reporting region is the Americas, she said, with 368 cases total, 290 cases of which come from the United States, accounting for 35% of the global total.
“Together the United Kingdom and the United States make up 65% of the global total,” she said.
Dr. Easterbrook added that 17 of the 33 reporting countries had more than five cases. Most cases (75%) are in young children under 5 years of age.
Serious cases are relatively few, but 44 (5%) children have required liver transplantation. Data from the European region show that 30% have required intensive care at some point during their hospitalization. There have been 18 (2%) reported deaths.
Possible post-COVID phenomenon, adenovirus most commonly reported
Dr. Easterbrook acknowledged the emerging hypothesis of a post-COVID phenomenon.
“Is this a variant of the rare but recognized multisystem inflammatory syndrome condition in children that’s been reported, often 1-2 months after COVID, causing widespread organ damage?” But she pointed out that the reported COVID cases with hepatitis “don’t seem to fit these features.”
Adenovirus remains the most commonly detected virus in acute hepatitis in children, found in 53% of cases overall, she said. The adenovirus detection rate is higher in the United Kingdom, at 68%.
“There are quite high rates of detection, but they’re not in all cases. There does seem to be a high rate of detection in the younger age groups and in those who are developing severe disease, so perhaps there is some link to severity,” Dr. Easterbrook said.
The working hypotheses continue to favor adenovirus together with past or current SARS-CoV-2 infection, as proposed early in the outbreak, she said. “These either work independently or work together as cofactors in some way to result in hepatitis. And there has been some clear progress on this. WHO is bringing together the data from different countries on some of these working hypotheses.”
Dr. Easterbrook highlighted the importance of procuring global data, especially given that two countries are reporting the majority of cases and in high numbers. “It’s a mixed picture with different rates of adenovirus detection and of COVID,” she said. “We need good-quality data collected in a standardized way.” WHO is requesting that countries provide these data.
She also highlighted the need for good in-depth studies, citing the UK Health Security Agency as an example of this. “There’s only a few countries that have the capacity or the patient numbers to look at this in detail, for example, the U.K. and the UKHSA.”
She noted that the UKHSA had laid out a comprehensive, systematic set of further investigations. For example, a case-control study is trying to establish whether there is a difference in the rate of adenovirus detection in children with hepatitis compared with other hospitalized children at the same time. “This aims to really tease out whether adenovirus is a cause or just a bystander,” she said.
She added that there were also genetic studies investigating whether genes were predisposing some children to develop a more severe form of disease. Other studies are evaluating the immune response of the patients.
Dr. Easterbrook added that the WHO will soon launch a global survey asking whether the reports of acute hepatitis are greater than the expected background rate for cases of hepatitis of unknown etiology.
Acute hepatitis is not new, but high caseload is
Also speaking at the ILC special briefing was Maria Buti, MD, PhD, policy and public health chair for the European Association for the Study of the Liver, and chief of the internal medicine and hepatology department at Hospital General Universitari Valle Hebron in Barcelona.
Dr. Buti drew attention to the fact that severe acute hepatitis of unknown etiology in children is not new.
“We have cases of acute hepatitis that even needed liver transplantation some years ago, and every year in our clinics we see these type of patients,” Dr. Buti remarked. What is really new, she added, is the amount of cases, particularly in the United Kingdom.
Dr. Easterbrook and Dr. Buti have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LONDON – Case numbers of acute hepatitis in children show “a declining trajectory,” and COVID-19 and adenovirus remain the most likely, but as yet unproven, causative agents, said experts in an update at the annual International Liver Congress sponsored by the European Association for the Study of the Liver.
Philippa Easterbrook, MD, medical expert at the World Health Organization Global HIV, Hepatitis, and STI Programme, shared the latest case numbers and working hypotheses of possible causative agents in the outbreak of acute hepatitis among children in Europe and beyond.
Global data across the five WHO regions show there were 244 cases in the past month, bringing the total to 894 probable cases reported since October 2021 from 33 countries.
“It’s important to remember that this includes new cases, as well as retrospectively identified cases,” Dr.Easterbrook said. “Over half (52%) are from the European region, while 262 cases (30% of the global total) are from the United Kingdom.”
Data from Europe and the United States show a declining trajectory of reports of new cases. “This is a positive development,” she said.
The second highest reporting region is the Americas, she said, with 368 cases total, 290 cases of which come from the United States, accounting for 35% of the global total.
“Together the United Kingdom and the United States make up 65% of the global total,” she said.
Dr. Easterbrook added that 17 of the 33 reporting countries had more than five cases. Most cases (75%) are in young children under 5 years of age.
Serious cases are relatively few, but 44 (5%) children have required liver transplantation. Data from the European region show that 30% have required intensive care at some point during their hospitalization. There have been 18 (2%) reported deaths.
Possible post-COVID phenomenon, adenovirus most commonly reported
Dr. Easterbrook acknowledged the emerging hypothesis of a post-COVID phenomenon.
“Is this a variant of the rare but recognized multisystem inflammatory syndrome condition in children that’s been reported, often 1-2 months after COVID, causing widespread organ damage?” But she pointed out that the reported COVID cases with hepatitis “don’t seem to fit these features.”
Adenovirus remains the most commonly detected virus in acute hepatitis in children, found in 53% of cases overall, she said. The adenovirus detection rate is higher in the United Kingdom, at 68%.
“There are quite high rates of detection, but they’re not in all cases. There does seem to be a high rate of detection in the younger age groups and in those who are developing severe disease, so perhaps there is some link to severity,” Dr. Easterbrook said.
The working hypotheses continue to favor adenovirus together with past or current SARS-CoV-2 infection, as proposed early in the outbreak, she said. “These either work independently or work together as cofactors in some way to result in hepatitis. And there has been some clear progress on this. WHO is bringing together the data from different countries on some of these working hypotheses.”
Dr. Easterbrook highlighted the importance of procuring global data, especially given that two countries are reporting the majority of cases and in high numbers. “It’s a mixed picture with different rates of adenovirus detection and of COVID,” she said. “We need good-quality data collected in a standardized way.” WHO is requesting that countries provide these data.
She also highlighted the need for good in-depth studies, citing the UK Health Security Agency as an example of this. “There’s only a few countries that have the capacity or the patient numbers to look at this in detail, for example, the U.K. and the UKHSA.”
She noted that the UKHSA had laid out a comprehensive, systematic set of further investigations. For example, a case-control study is trying to establish whether there is a difference in the rate of adenovirus detection in children with hepatitis compared with other hospitalized children at the same time. “This aims to really tease out whether adenovirus is a cause or just a bystander,” she said.
She added that there were also genetic studies investigating whether genes were predisposing some children to develop a more severe form of disease. Other studies are evaluating the immune response of the patients.
Dr. Easterbrook added that the WHO will soon launch a global survey asking whether the reports of acute hepatitis are greater than the expected background rate for cases of hepatitis of unknown etiology.
Acute hepatitis is not new, but high caseload is
Also speaking at the ILC special briefing was Maria Buti, MD, PhD, policy and public health chair for the European Association for the Study of the Liver, and chief of the internal medicine and hepatology department at Hospital General Universitari Valle Hebron in Barcelona.
Dr. Buti drew attention to the fact that severe acute hepatitis of unknown etiology in children is not new.
“We have cases of acute hepatitis that even needed liver transplantation some years ago, and every year in our clinics we see these type of patients,” Dr. Buti remarked. What is really new, she added, is the amount of cases, particularly in the United Kingdom.
Dr. Easterbrook and Dr. Buti have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ILC 2022
FDA, AMA prepare for potential COVID-19 shots for children younger than 6
Regulators and the nation’s largest physician organization took separate steps in recent days to prepare for expected authorization of use of COVID-19 vaccines in children younger than age 6.
The Food and Drug Administration on May 23 announced its Vaccines and Related Biological Products Advisory Committee will meet June 15 to discuss expanding the use of COVID vaccines from Pfizer and Moderna.
The panel will examine a request from Pfizer and its partner BioNTech for an emergency use authorization (EUA) of its vaccine to cover children ages 6 months through 4 years. The EUA expansion for the Moderna shot would cover children ages 6 months through 5 years, the FDA said.
Many parents and physicians have been urging regulators to clear COVID shots for young children, among whom rates of infection are high.
The American Medical Association in February announced an update of its Current Procedural Terminology (CPT) to prepare for an eventual FDA clearance of the Pfizer-BioNTech shot for children aged 6 months to younger than 5 years. On May 19, the association announced a new CPT update to prepare for FDA clearance for use of the Moderna COVID-19 vaccine for children 6 months through 5 years.
“Extending COVID-19 vaccination protection to approximately 18 million young children will significantly reduce their risk of COVID-19 infection, hospitalization, and death, and give their parents incredible peace of mind,” Gerald Harmon, MD, AMA’s president, said in a statement. “We strongly urge all parents to get their infants and toddlers vaccinated as soon as they are eligible for a COVID-19 vaccine.”
Both the Moderna and the Pfizer-BioNTech COVID vaccines would be given to these young children in low doses.
On May 23, Pfizer announced results from a phase 2/3 trial evaluating a series of three shots of its vaccine in children ages 6 months to younger than 5 years.
Vaccine efficacy, which was a secondary endpoint in this study, was 80.3% in this age group, Pfizer said. The analysis was based on 10 symptomatic cases of COVID-19. The trial’s protocol specifies a formal analysis will be performed when at least 21 cases have accrued from 7 days after the third dose. The company said it would share final data on the effectiveness of the vaccine once the results are available.
Moderna on April 28 issued a statement with details about testing of its vaccine in young children. Vaccine efficacy was estimated at about 51% for children aged 6 months to younger than 2 years and 37% for the children aged 2 years to younger than 6. Paul Burton, MD, Moderna’s chief medical officer, spoke about this rate during a May 1 appearance on CBS’ Face the Nation.
“What it means for parents, for caregivers, is that if they give the Moderna vaccine to these little kids, they would basically cut in half the risk of that child getting symptomatic COVID,” Dr. Burton said in the interview. “Now, the number, 50%, I know is often lower than we are used to seeing with our vaccine, but it’s because this study was conducted during a time of Omicron.”
The FDA’s vaccine advisory committee also will meet on June 14 discuss potential use under an EUA of Moderna’s COVID vaccine for children and teenagers aged 6-17 years. The Pfizer-BioNTech vaccine already is authorized under an EUA for people aged 5 years and older.
The FDA has to date granted both conditional clearances, or EUAs, and regular approvals for COVID vaccines.
EUAs are meant to be temporary, allowing for rapid introduction of medicines in response to public health crises such as the pandemic. The FDA also uses EUAs to provide initial clearances of additional indications for products, as would be the case with the authorizations Moderna and Pfizer-BioNTech are seeking for their COVID vaccines.
Companies that want to continue to sell EUA-cleared products or promote EUA-cleared indications beyond the time of the public health crisis must seek regular approvals.
The FDA cleared the Pfizer-BioNTech and Moderna COVID vaccines under EUAs in December 2020. The agency then granted a regular approval for the Pfizer-BioNTech vaccine for people ages 16 and older in August 2021 based on more robust data. Regular approval for the Moderna vaccine for people ages 18 and older followed in January 2022.
Varied reactions among parents
Attitudes in the United States about pediatric COVID vaccines are far from uniform.
The initial uptake has disappointed physicians and researchers, who have been urging wider use of the COVID vaccination among children and teens for whom the FDA already has granted a clearance. Many parents are hesitating to bring their children for the COVID vaccines, according to the Centers for Disease Control and Prevention. Only 35.4% of children ages 5-11 had received at least one dose of a COVID vaccine, CDC staff said during a meeting.
Yet many other parents are demanding this medicine for their young children, urging the FDA to move quickly to clear COVID shots.
A private Facebook group called “Protect Their Future: A Call to Action for COVID Vaccines in Kids <5” boasts about 6,200 members. Many parents and physicians have used Twitter in recent months to press for a speedy review of COVID vaccines for the youngest children, often using the hashtag #immunizeunder5s. A group called Protect Their Future, which uses @ImmunizeUnder5s as its Twitter handle, had 5,288 followers as of the afternoon of May 23.
A special panel of the House of Representatives, the Select Subcommittee on the Coronavirus Crisis, on May 23 joined those tweeting about the need to soon authorize COVID vaccines for very young children.
“Parents have been waiting many months for vaccines for their young children,” the subcommittee tweeted. “They deserve to hear from @US_FDA why this lengthy process has been in children’s best interests.”
A version of this article first appeared on Medscape.com.
Regulators and the nation’s largest physician organization took separate steps in recent days to prepare for expected authorization of use of COVID-19 vaccines in children younger than age 6.
The Food and Drug Administration on May 23 announced its Vaccines and Related Biological Products Advisory Committee will meet June 15 to discuss expanding the use of COVID vaccines from Pfizer and Moderna.
The panel will examine a request from Pfizer and its partner BioNTech for an emergency use authorization (EUA) of its vaccine to cover children ages 6 months through 4 years. The EUA expansion for the Moderna shot would cover children ages 6 months through 5 years, the FDA said.
Many parents and physicians have been urging regulators to clear COVID shots for young children, among whom rates of infection are high.
The American Medical Association in February announced an update of its Current Procedural Terminology (CPT) to prepare for an eventual FDA clearance of the Pfizer-BioNTech shot for children aged 6 months to younger than 5 years. On May 19, the association announced a new CPT update to prepare for FDA clearance for use of the Moderna COVID-19 vaccine for children 6 months through 5 years.
“Extending COVID-19 vaccination protection to approximately 18 million young children will significantly reduce their risk of COVID-19 infection, hospitalization, and death, and give their parents incredible peace of mind,” Gerald Harmon, MD, AMA’s president, said in a statement. “We strongly urge all parents to get their infants and toddlers vaccinated as soon as they are eligible for a COVID-19 vaccine.”
Both the Moderna and the Pfizer-BioNTech COVID vaccines would be given to these young children in low doses.
On May 23, Pfizer announced results from a phase 2/3 trial evaluating a series of three shots of its vaccine in children ages 6 months to younger than 5 years.
Vaccine efficacy, which was a secondary endpoint in this study, was 80.3% in this age group, Pfizer said. The analysis was based on 10 symptomatic cases of COVID-19. The trial’s protocol specifies a formal analysis will be performed when at least 21 cases have accrued from 7 days after the third dose. The company said it would share final data on the effectiveness of the vaccine once the results are available.
Moderna on April 28 issued a statement with details about testing of its vaccine in young children. Vaccine efficacy was estimated at about 51% for children aged 6 months to younger than 2 years and 37% for the children aged 2 years to younger than 6. Paul Burton, MD, Moderna’s chief medical officer, spoke about this rate during a May 1 appearance on CBS’ Face the Nation.
“What it means for parents, for caregivers, is that if they give the Moderna vaccine to these little kids, they would basically cut in half the risk of that child getting symptomatic COVID,” Dr. Burton said in the interview. “Now, the number, 50%, I know is often lower than we are used to seeing with our vaccine, but it’s because this study was conducted during a time of Omicron.”
The FDA’s vaccine advisory committee also will meet on June 14 discuss potential use under an EUA of Moderna’s COVID vaccine for children and teenagers aged 6-17 years. The Pfizer-BioNTech vaccine already is authorized under an EUA for people aged 5 years and older.
The FDA has to date granted both conditional clearances, or EUAs, and regular approvals for COVID vaccines.
EUAs are meant to be temporary, allowing for rapid introduction of medicines in response to public health crises such as the pandemic. The FDA also uses EUAs to provide initial clearances of additional indications for products, as would be the case with the authorizations Moderna and Pfizer-BioNTech are seeking for their COVID vaccines.
Companies that want to continue to sell EUA-cleared products or promote EUA-cleared indications beyond the time of the public health crisis must seek regular approvals.
The FDA cleared the Pfizer-BioNTech and Moderna COVID vaccines under EUAs in December 2020. The agency then granted a regular approval for the Pfizer-BioNTech vaccine for people ages 16 and older in August 2021 based on more robust data. Regular approval for the Moderna vaccine for people ages 18 and older followed in January 2022.
Varied reactions among parents
Attitudes in the United States about pediatric COVID vaccines are far from uniform.
The initial uptake has disappointed physicians and researchers, who have been urging wider use of the COVID vaccination among children and teens for whom the FDA already has granted a clearance. Many parents are hesitating to bring their children for the COVID vaccines, according to the Centers for Disease Control and Prevention. Only 35.4% of children ages 5-11 had received at least one dose of a COVID vaccine, CDC staff said during a meeting.
Yet many other parents are demanding this medicine for their young children, urging the FDA to move quickly to clear COVID shots.
A private Facebook group called “Protect Their Future: A Call to Action for COVID Vaccines in Kids <5” boasts about 6,200 members. Many parents and physicians have used Twitter in recent months to press for a speedy review of COVID vaccines for the youngest children, often using the hashtag #immunizeunder5s. A group called Protect Their Future, which uses @ImmunizeUnder5s as its Twitter handle, had 5,288 followers as of the afternoon of May 23.
A special panel of the House of Representatives, the Select Subcommittee on the Coronavirus Crisis, on May 23 joined those tweeting about the need to soon authorize COVID vaccines for very young children.
“Parents have been waiting many months for vaccines for their young children,” the subcommittee tweeted. “They deserve to hear from @US_FDA why this lengthy process has been in children’s best interests.”
A version of this article first appeared on Medscape.com.
Regulators and the nation’s largest physician organization took separate steps in recent days to prepare for expected authorization of use of COVID-19 vaccines in children younger than age 6.
The Food and Drug Administration on May 23 announced its Vaccines and Related Biological Products Advisory Committee will meet June 15 to discuss expanding the use of COVID vaccines from Pfizer and Moderna.
The panel will examine a request from Pfizer and its partner BioNTech for an emergency use authorization (EUA) of its vaccine to cover children ages 6 months through 4 years. The EUA expansion for the Moderna shot would cover children ages 6 months through 5 years, the FDA said.
Many parents and physicians have been urging regulators to clear COVID shots for young children, among whom rates of infection are high.
The American Medical Association in February announced an update of its Current Procedural Terminology (CPT) to prepare for an eventual FDA clearance of the Pfizer-BioNTech shot for children aged 6 months to younger than 5 years. On May 19, the association announced a new CPT update to prepare for FDA clearance for use of the Moderna COVID-19 vaccine for children 6 months through 5 years.
“Extending COVID-19 vaccination protection to approximately 18 million young children will significantly reduce their risk of COVID-19 infection, hospitalization, and death, and give their parents incredible peace of mind,” Gerald Harmon, MD, AMA’s president, said in a statement. “We strongly urge all parents to get their infants and toddlers vaccinated as soon as they are eligible for a COVID-19 vaccine.”
Both the Moderna and the Pfizer-BioNTech COVID vaccines would be given to these young children in low doses.
On May 23, Pfizer announced results from a phase 2/3 trial evaluating a series of three shots of its vaccine in children ages 6 months to younger than 5 years.
Vaccine efficacy, which was a secondary endpoint in this study, was 80.3% in this age group, Pfizer said. The analysis was based on 10 symptomatic cases of COVID-19. The trial’s protocol specifies a formal analysis will be performed when at least 21 cases have accrued from 7 days after the third dose. The company said it would share final data on the effectiveness of the vaccine once the results are available.
Moderna on April 28 issued a statement with details about testing of its vaccine in young children. Vaccine efficacy was estimated at about 51% for children aged 6 months to younger than 2 years and 37% for the children aged 2 years to younger than 6. Paul Burton, MD, Moderna’s chief medical officer, spoke about this rate during a May 1 appearance on CBS’ Face the Nation.
“What it means for parents, for caregivers, is that if they give the Moderna vaccine to these little kids, they would basically cut in half the risk of that child getting symptomatic COVID,” Dr. Burton said in the interview. “Now, the number, 50%, I know is often lower than we are used to seeing with our vaccine, but it’s because this study was conducted during a time of Omicron.”
The FDA’s vaccine advisory committee also will meet on June 14 discuss potential use under an EUA of Moderna’s COVID vaccine for children and teenagers aged 6-17 years. The Pfizer-BioNTech vaccine already is authorized under an EUA for people aged 5 years and older.
The FDA has to date granted both conditional clearances, or EUAs, and regular approvals for COVID vaccines.
EUAs are meant to be temporary, allowing for rapid introduction of medicines in response to public health crises such as the pandemic. The FDA also uses EUAs to provide initial clearances of additional indications for products, as would be the case with the authorizations Moderna and Pfizer-BioNTech are seeking for their COVID vaccines.
Companies that want to continue to sell EUA-cleared products or promote EUA-cleared indications beyond the time of the public health crisis must seek regular approvals.
The FDA cleared the Pfizer-BioNTech and Moderna COVID vaccines under EUAs in December 2020. The agency then granted a regular approval for the Pfizer-BioNTech vaccine for people ages 16 and older in August 2021 based on more robust data. Regular approval for the Moderna vaccine for people ages 18 and older followed in January 2022.
Varied reactions among parents
Attitudes in the United States about pediatric COVID vaccines are far from uniform.
The initial uptake has disappointed physicians and researchers, who have been urging wider use of the COVID vaccination among children and teens for whom the FDA already has granted a clearance. Many parents are hesitating to bring their children for the COVID vaccines, according to the Centers for Disease Control and Prevention. Only 35.4% of children ages 5-11 had received at least one dose of a COVID vaccine, CDC staff said during a meeting.
Yet many other parents are demanding this medicine for their young children, urging the FDA to move quickly to clear COVID shots.
A private Facebook group called “Protect Their Future: A Call to Action for COVID Vaccines in Kids <5” boasts about 6,200 members. Many parents and physicians have used Twitter in recent months to press for a speedy review of COVID vaccines for the youngest children, often using the hashtag #immunizeunder5s. A group called Protect Their Future, which uses @ImmunizeUnder5s as its Twitter handle, had 5,288 followers as of the afternoon of May 23.
A special panel of the House of Representatives, the Select Subcommittee on the Coronavirus Crisis, on May 23 joined those tweeting about the need to soon authorize COVID vaccines for very young children.
“Parents have been waiting many months for vaccines for their young children,” the subcommittee tweeted. “They deserve to hear from @US_FDA why this lengthy process has been in children’s best interests.”
A version of this article first appeared on Medscape.com.
Direct-to-Consumer Teledermatology Growth: A Review and Outlook for the Future
In recent years, direct-to-consumer (DTC) teledermatology platforms have gained popularity as telehealth business models, allowing patients to directly initiate visits with physicians and purchase medications from single platforms. A shortage of dermatologists, improved technology, drug patent expirations, and rising health care costs accelerated the growth of DTC dermatology.1 During the COVID-19 pandemic, teledermatology adoption surged due to the need to provide care while social distancing and minimizing viral exposure. These needs prompted additional federal funding and loosened regulatory provisions.2 As the userbase of these companies has grown, so have their valuations.3 Although the DTC model has attracted the attention of patients and investors, its rise provokes many questions about patients acting as consumers in health care. Indeed, DTC telemedicine offers greater autonomy and convenience for patients, but it may impact the quality of care and the nature of physician-patient relationships, perhaps making them more transactional.
Evolution of DTC in Health Care
The DTC model emphasizes individual choice and accessible health care. Although the definition has evolved, the core idea is not new.4 Over decades, pharmaceutical companies have spent billions of dollars on DTC advertising, circumventing physicians by directly reaching patients with campaigns on prescription drugs and laboratory tests and shaping public definitions of diseases.5
The DTC model of care is fundamentally different from traditional care models in that it changes the roles of the patient and physician. Whereas early telehealth models required a health care provider to initiate teleconsultations with specialists, DTC telemedicine bypasses this step (eg, the patient can consult a dermatologist without needing a primary care provider’s input first). This care can then be provided by dermatologists with whom patients may or may not have pre-established relationships.4,6
Dermatology was an early adopter of DTC telemedicine. The shortage of dermatologists in the United States created demand for increasing accessibility to dermatologic care. Additionally, the visual nature of diagnosing dermatologic disease was ideal for platforms supporting image sharing.7 Early DTC providers were primarily individual companies offering teledermatology. However, many dermatologists can now offer DTC capabilities via companies such as Amwell and Teladoc Health.8
Over the last 2 decades, start-ups such as Warby Parker (eyeglasses) and Casper (mattresses) defined the DTC industry using borrowed supply chains, cohesive branding, heavy social media marketing, and web-only retail. Scalability, lack of competition, and abundant venture capital created competition across numerous markets.9 Health care capitalized on this DTC model, creating a $700 billion market for products ranging from hearing aids to over-the-counter medications.10
Borrowing from this DTC playbook, platforms were created to offer delivery of generic prescription drugs to patients’ doorsteps. However, unlike with other products bought online, a consumer cannot simply add prescription drugs to their shopping cart and check out. In all models of American medical practice, physicians still serve as gatekeepers, providing a safeguard for patients to ensure appropriate prescription and avoid negative consequences of unnecessary drug use. This new model effectively streamlines diagnosis, prescription, and drug delivery without the patient ever having to leave home. Combining the prescribing and selling of medications (2 tasks that traditionally have been separated) potentially creates financial conflicts of interest (COIs). Additionally, high utilization of health care, including more prescriptions and visits, does not necessarily equal high quality of care. The companies stand to benefit from extra care regardless of need, and thus these models must be scrutinized for any incentives driving unnecessary care and prescriptions.
Ultimately, DTC has evolved to encompass multiple definitions in health care (Table 1). Although all models provide health care, each offers a different modality of delivery. The primary service may be the sale of prescription drugs or simply telemedicine visits. This review primarily discusses DTC pharmaceutical telemedicine platforms that sell private-label drugs and also offer telemedicine services to streamline care. However, the history, risks, and benefits discussed may apply to all models.
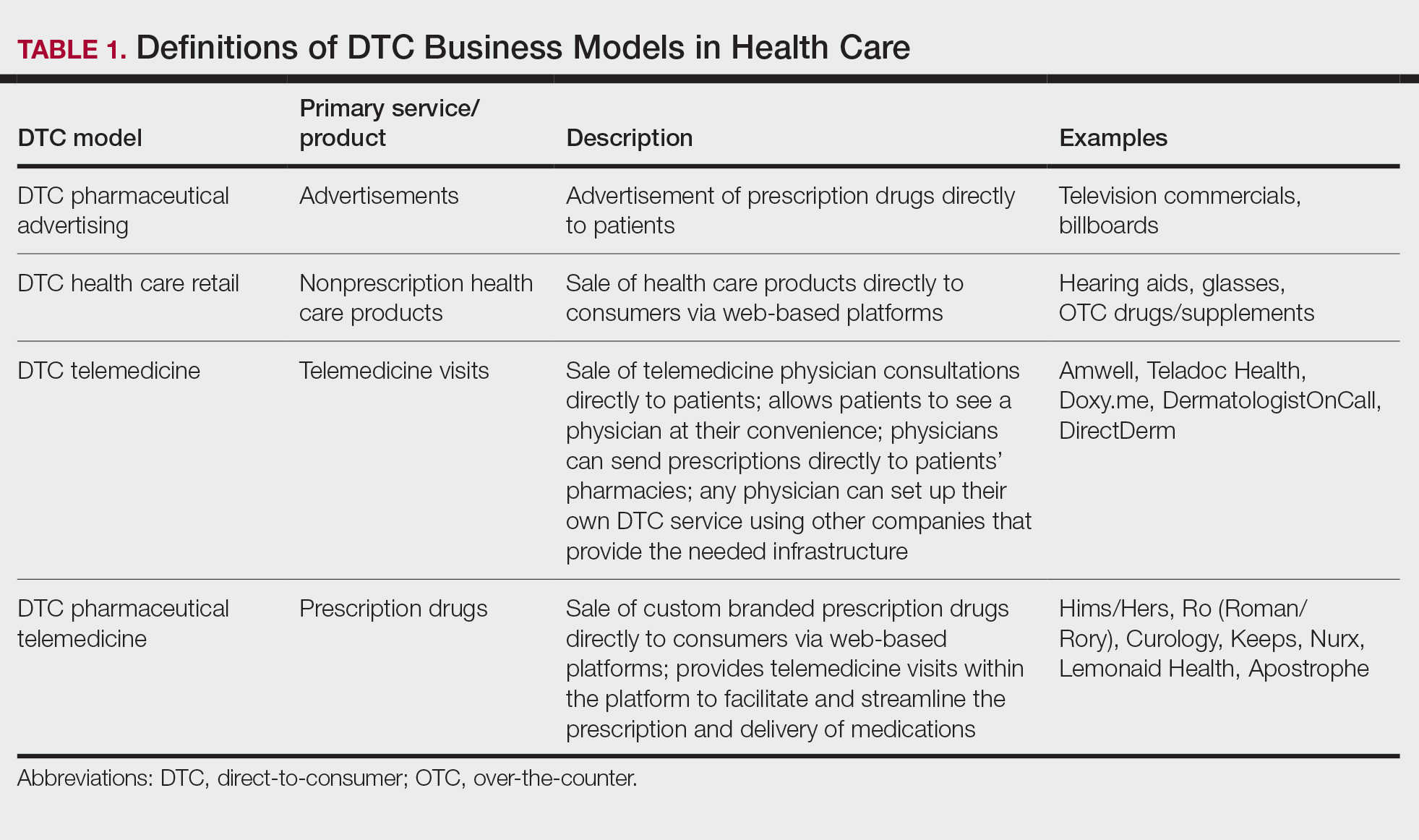
The DTC Landscape
Most DTC companies employ variations on a model with the same 3 main components: a triage questionnaire, telehealth services, and prescription/drug delivery (Figure). The triage questionnaire elicits a history of the patient’s presentation and medical history. Some companies may use artificial intelligence (AI) algorithms to tailor questions to patient needs. There are 2 modalities for patient-provider communication: synchronous and asynchronous. Synchronous communication entails real-time patient-physician conversations via audio only or video call. Asynchronous (or store-and-forward) communication refers to consultations provided via messaging or text-based modality, where a provider may respond to a patient within 24 hours.6 Direct-to-consumer platforms primarily use asynchronous visits (Table 2). However, some also use synchronous modalities if the provider deems it necessary or if state laws require it.
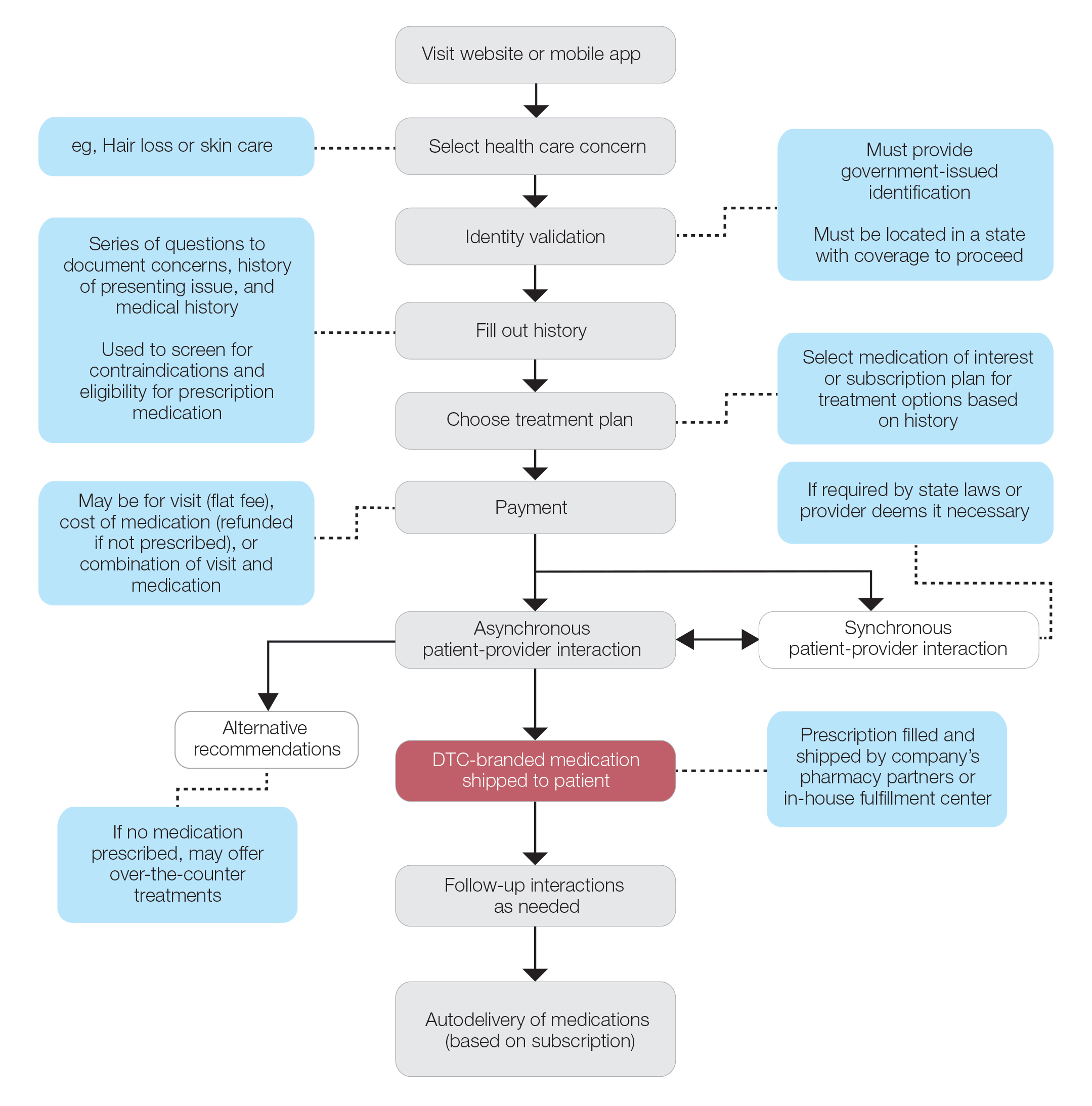
Once a provider has consulted with the patient, they can prescribe medication as needed. In certain cases, with adequate history, a prescription may be issued without a full physician visit. Furthermore, DTC companies require purchase of their custom-branded generic drugs. Prescriptions are fulfilled by the company’s pharmacy network and directly shipped to patients; few will allow patients to transfer a prescription to a pharmacy of their choice. Some platforms also sell supplements and over-the-counter medications.
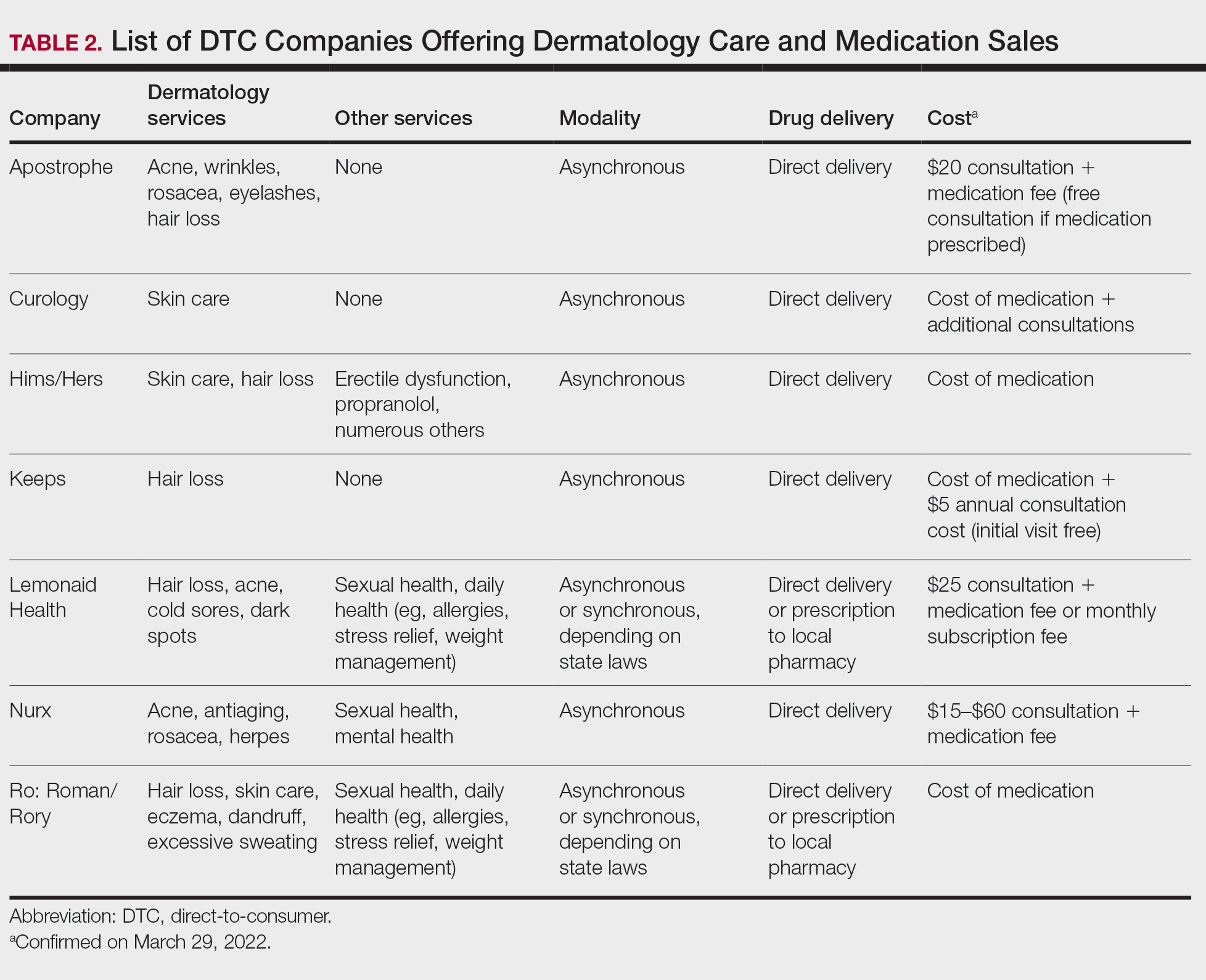
Payment models vary among these companies, and most do not accept insurance (Table 2). Select models may provide free consultations and only require payment for pharmaceuticals. Others charge for consultations but reallocate payment to the cost of medication if prescribed. Another model involves flat rates for consultations and additional charges for drugs but unlimited messaging with providers for the duration of the prescription. Moreover, patients can subscribe to monthly deliveries of their medications.
Foundation of DTC
Technological advances have enabled patients to receive remote treatment from a single platform offering video calls, AI, electronic medical record interoperability, and integration of drug supply chains. Even in its simplest form, AI is increasingly used, as it allows for programs and chatbots to screen and triage patients.11 Technology also has improved at targeted mass marketing through social media platforms and search engines (eg, companies can use age, interests, location, and other parameters to target individuals likely needing acne treatment).
Drug patent expirations are a key catalyst for the rise of DTC companies, creating an attractive business model with generic drugs as the core product. Since 2008, patents for medications treating chronic conditions, such as erectile dysfunction, have expired. These patent expirations are responsible for $198 billion in projected prescription sales between 2019 and 2024.1 Thus, it follows that DTC companies have seized this opportunity to act as middlemen, taking advantage of these generic medications’ lower costs to create platforms focused on personalization and accessibility.
Rising deductibles have led patients to consider cheaper out-of-pocket alternatives that are not covered by insurance.1 For example, insurers typically do not cover finasteride treatment for conditions deemed cosmetic, such as androgenetic alopecia.12 The low cost of generic drugs creates an attractive business model for patients and investors. According to GoodRx, the average retail price for a 30-day supply of brand-name finasteride (Propecia [Merck]) is $135.92, whereas generic finasteride is $75.24.13 Direct-to-consumer pharmaceutical companies offer a 30-day supply of generic finasteride ranging from $8.33 to $30.14 The average wholesale cost for retailers is an estimated $2.31 for 30 days.15 Although profit margins on generic medications may be lower, more affordable drugs increase the size of the total market. These prescriptions are available as subscription plans, resulting in recurring revenue.
Lax US pharmaceutical marketing regulations allow direct advertising to the general public.16 In 1997, the US Food and Drug Administration allowed DTC advertisements to replace summaries of serious and common adverse effects with short statements covering important risks or referrals to other sources for complete information. In 2015, the US Food and Drug Administration guidelines preventing encouragement of self-diagnosis and self-treatment were withdrawn.5 These changes enable DTC companies to launch large advertising campaigns and to accelerate customer acquisition, as the industry often describes it, with ease.
Rapid Growth and Implications
Increasing generic drug availability and improving telemedicine capabilities have the potential to reduce costs and barriers but also have the potential for financial gain. Venture capital funds have recognized this opportunity, reflected by millions of dollars of investments, and accelerated the growth of DTC health care start-ups. For example, Ro has raised $376 million from venture capital, valuing the company at $1.5 billion.3
Direct-to-consumer companies require a heavy focus on marketing campaigns for customer acquisition. Their aesthetically pleasing websites and aggressive campaigns target specific audiences based on demographics, digital use habits, and purchasing behavior.4 Some campaigns celebrate the ease of obtaining prescriptions.17 Companies have been effective in recruiting so-called millennial and Generation Z patients, known to search the internet for remedies prior to seeking physician consultations.18 Recognizing these needs, some platforms offer guides on diseases they treat, creating effective customer-acquisition funnels. Recruitment of these technology-friendly patients has proven effective, especially given the largely positive media coverage of DTC platforms––potentially serving as a surrogate for medical credibility for patients.18
Some DTC companies also market physically; skin care ads may be strategically placed in social media feeds, or even found near mirrors in public bathrooms.19 Marketing campaigns also involve disease awareness; such efforts serve to increase diagnoses and prescribed treatments while destigmatizing diseases. Although DTC companies argue this strategy empowers patients, these marketing habits have the potential to take advantage of uninformed patients. Campaigns could potentially medicalize normal experiences and expand disease definitions resulting in overdiagnosis, overtreatment, and wasted resources.5 For example, off-label propranolol use has been advertised to attract patients who might have “nerves that come creeping before an important presentation.”17 Disease awareness campaigns also may lead people to falsely believe unproven drug benefits.5 According to studies, DTC pharmaceutical advertisements are low in informational quality and result in increased patient visits and prescriptions despite cost-effective alternatives.5,20-22
Fragmentation of the health care system is another possible complication of DTC teledermatology. These companies operate as for-profit organizations separated from the rest of the health care system, raising concerns about care coordination.8 Vital health data may not be conveyed as patients move among different providers and pharmacies. One study found DTC teledermatology rarely offered to provide medical records or facilitate a referral to a local physician.23 Such a lack of communication is concerning, as medication errors are the leading cause of avoidable harm in health care.24
Direct-to-consumer care models also seemingly redefine the physician-patient relationship by turning patients into consumers. Patient interactions may seem transactional and streamlined toward sales. For these platforms, a visit often is set up as an evaluation of a patient’s suitability for a prescription, not necessarily for the best treatment modality for the problem. These companies primarily make money through the sale of prescription drugs, creating a potential COI that may undermine the patient-physician relationship. Although some companies have made it clear that medical care and pharmaceutical sales are provided by legally separate business entities and that they do not pay physicians on commission, a conflict may still exist given the financial importance of physicians prescribing medication to the success of the business.16
Even as DTC models advertise upon expanded access and choice, the companies largely prohibit patients from choosing their own pharmacy. Instead, they encourage patients to fill prescriptions with the company’s pharmacy network by claiming lower costs compared with competitors. One DTC company, Hims, is launching a prescription-fulfillment center to further consolidate their business.17,19,25 The inherent COI of issuing and fulfilling prescriptions raises concerns of patient harm.26 For example, when Dermatology.com launched as a DTC prescription skin medication shop backed by Bausch Health Companies Inc, its model included telemedicine consultation. Although consultations were provided by RxDefine, a third party, only Dermatology.com drugs were prescribed. Given the poor quality of care and obvious financial COI, an uproar in the dermatology community and advocacy by the American Academy of Dermatology led to the shutdown of Dermatology.com’s online prescription services.26
The quality of care among DTC telemedicine platforms has been equivocal. Some studies have reported equivalent care in person and online, while others have reported poor adherence to guidelines, overuse of antibiotics, and misdiagnosis.8,23 A vital portion of the DTC experience is the history questionnaire, which is geared to diagnosis and risk assessment.25 Resneck et al23 found diagnostic quality to be adequate for simple dermatologic clinical scenarios but poor for scenarios requiring more than basic histories. Although Ro has reported leveraging data from millions of interactions to ask the right questions and streamline visits, it is still unclear whether history questionnaires are adequate.17,27 Additionally, consultations may lack sufficient counseling on adverse effects, risks, or pregnancy warnings, as well as discussions on alternative treatments and preventative care.17,23 Finally, patients often are limited in their choice of dermatologist; the lack of a fully developed relationship increases concerns of follow-up and monitoring practices. Although some DTC platforms offer unlimited interactions with physicians for the duration of a prescription, it is unknown how often these services are utilized or how adequate the quality of these interactions is. This potential for lax follow-up is especially concerning for prescriptions that autorenew on a monthly basis and could result in unnecessary overtreatment.
Postpandemic and Future Outlook
The COVID-19 pandemic dramatically impacted the use of telemedicine. To minimize COVID-19 transmission, the Centers for Medicare & Medicaid Services and private payers expanded telehealth coverage and eliminated reimbursement and licensing barriers.28 A decade’s worth of regulatory changes and consumer adoption was accelerated to weeks, resulting in telemedicine companies reaching record-high visit numbers.29 McKinsey & Company estimated that telehealth visit numbers surged 50- to 175-fold compared with pre–COVID-19 numbers. Additionally, 76% of patients were interested in future telehealth use, and 64% of providers were more comfortable using telehealth than before the pandemic.30 For their part, US dermatologists reported an increase in telemedicine use from 14.1% to 96.9% since COVID-19.31
Exactly how much DTC pharmaceutical telemedicine companies are growing is unclear, but private investments may be an indication. A record $14.7 billion was invested in the digital health sector in the first half of 2021; the majority went to telehealth companies.30 Ro, which reported $230 million in revenue in 2020 and has served 6 million visits, raised $200 milllion in July 2020 and $500 million in March 2021.32 Although post–COVID-19 health care will certainly involve increased telemedicine, the extent remains unclear, as telehealth vendors saw decreased usage upon reopening of state economies. Ultimately, the postpandemic regulatory landscape is hard to predict.30
Although COVID-19 appears to have caused rapid growth for DTC platforms, it also may have spurred competition. Telemedicine providers have given independent dermatologists and health care systems the infrastructure to implement custom DTC services.33 Although systems do not directly sell prescription drugs, the target market is essentially the same: patients looking for instant virtual dermatologic care. Therefore, sustained telemedicine services offered by traditional practices and systems may prove detrimental to DTC companies. However, unlike most telemedicine services, DTC models are less affected by certain changes in regulation since they do not rely on insurance. If regulations are tightened and reimbursements for telehealth are not attractive for dermatologists, teledermatology services may see an overall decrease. If so, patients who appreciate teledermatology may shift to using DTC platforms, even if their insurance does not cover them. Still, a nationwide survey found 56% of respondents felt an established relationship with a physician prior to a telemedicine visit is important, which may create a barrier for DTC adoption.34
Conclusion
Direct-to-consumer teledermatology represents a growing for-profit model of health care that provides patients with seemingly affordable and convenient care. However, there is potential for overtreatment, misdiagnosis, and fragmentation of health care. It will be important to monitor and evaluate the quality of care that DTC teledermatology offers and advocate for appropriate regulations and oversight. Eventually, more patients will have medications prescribed and dermatologic care administered through DTC companies. Dermatologists will benefit from this knowledge of DTC models to properly counsel patients on the risks and benefits of their use.
- Vennare J. The DTC healthcare report. Fitt Insider. September 15, 2019. Accessed February 23, 2022. https://insider.fitt.co/direct-to-consumer-healthcare-startups/
- Kannampallil T, Ma J. Digital translucence: adapting telemedicine delivery post-COVID-19. Telemed J E Health. 2020;26:1120-1122.
- Farr C. Ro, a 3-year-old online health provider, just raised a new round that values it at $1.5 billion. CNBC. July 27, 2020. Accessed February 23, 2022. https://www.cnbc.com/2020/07/27/ro-raises-200-million-at-1point5-billion-valuation-250-million-sales.html
- Elliott T, Shih J. Direct to consumer telemedicine. Curr Allergy Asthma Rep. 2019;19:1.
- Schwartz LM, Woloshin S. Medical marketing in the United States, 1997-2016. JAMA. 2019;321:80-96.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Coates SJ, Kvedar J, Granstein RD. Teledermatology: from historical perspective to emerging techniques of the modern era. J Am Acad Dermatol. 2015;72:563-574.
- Rheuban KS, Krupinski EA, eds. Understanding Telehealth. McGraw-Hill Education; 2017.
- Schlesinger LA, Higgins M, Roseman S. Reinventing the direct-to-consumer business model. Harvard Business Review. March 31, 2020. Accessed February 23, 2022. https://hbr.org/2020/03/reinventing-the-direct-to-consumer-business-model
- Cohen AB, Mathews SC, Dorsey ER, et al. Direct-to-consumer digital health. Lancet Digit Health. 2020;2:E163-E165.
- 6 telehealth trends for 2020. Wolters Kluwer. Published January 27, 2021. Accessed February 23, 2022. https://www.wolterskluwer.com/en/expert-insights/6-telehealth-trends-for-2020
- Jadoo SA, Lipoff JB. Prescribing to save patients money: ethical considerations. J Am Acad Dermatol. 2018;78:826-828.
- Propecia. GoodRx. Accessed February 23, 2022. https://www.goodrx.com/propecia
- Lauer A. The truth about online hair-loss treatments like Roman and Hims, according to a dermatologist. InsideHook. January 13, 2020. Accessed February 23, 2022. https://www.insidehook.com/article/grooming/men-hair-loss-treatments-dermatologist-review
- Friedman Y. Drug price trends for NDC 16729-0089. DrugPatentWatch. Accessed February 23, 2022. https://www.drugpatentwatch.com/p/drug-price/ndc/index.php?query=16729-0089
- Curtis H, Milner J. Ethical concerns with online direct-to-consumer pharmaceutical companies. J Med Ethics. 2020;46:168-171.
- Jain T, Lu RJ, Mehrotra A. Prescriptions on demand: the growth of direct-to-consumer telemedicine companies. JAMA. 2019;322:925-926.
- Shahinyan RH, Amighi A, Carey AN, et al. Direct-to-consumer internet prescription platforms overlook crucial pathology found during traditional office evaluation of young men with erectile dysfunction. Urology. 2020;143:165-172.
- Ali M. Andrew Dudum—bold strategies that propelled Hims & Hers into unicorn status. Exit Strategy with Moiz Ali. Published April 2020. Accessed February 23, 2022. https://open.spotify.com/episode/6DtaJxwZDjvZSJI88DTf24?si=b3FHQiUIQY62YjfRHmnJBQ
- Klara K, Kim J, Ross JS. Direct-to-consumer broadcast advertisements for pharmaceuticals: off-label promotion and adherence to FDA guidelines. J Gen Intern Med. 2018;33:651-658.
- Sullivan HW, Aikin KJ, Poehlman J. Communicating risk information in direct-to-consumer prescription drug television ads: a content analysis. Health Commun. 2019;34:212-219.
- Applequist J, Ball JG. An updated analysis of direct-to-consumer television advertisements for prescription drugs. Ann Fam Med. 2018;16:211-216.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Patient safety. World Health Organization. Published September 13, 2019. Accessed February 1, 2022. https://www.who.int/news-room/fact-sheets/detail/patient-safety
- Bollmeier SG, Stevenson E, Finnegan P, et al. Direct to consumer telemedicine: is healthcare from home best? Mo Med. 2020;117:303-309.
26. Court E. Bausch yanked online prescribing after dermatologist backlash. Bloomberg.com. Published March 11, 2020. Accessed September 25, 2020. https://www.bloomberg.com/news/articles/2020-03-11/bausch-yanked-online-prescribing-after-dermatologist-backlash
27. Reitano Z. The future of healthcare: how Ro helps providers treat patients 2 minutes, 2 days, 2 weeks, and 2 years at a time. Medium. Published March 4, 2019. Accessed February 1, 2022. https://medium.com/ro-co/the-future-of-healthcare-how-ro-helps-providers-treat-patients-2-mins-2-days-2-weeks-and-2-10efc0679d7
28. Lee I, Kovarik C, Tejasvi T, et al. Telehealth: helping your patients and practice survive and thrive during the COVID-19 crisis with rapid quality implementation. J Am Acad Dermatol. 2020;82:1213-1214.
29. Pifer R. “Weeks where decades happen”: telehealth 6 months into COVID-19. Healthcare Dive. Published July 27, 2020. Accessed February 23, 2022. https://www.healthcaredive.com/news/telehealth-6-months-coronavirus/581447/
30. Bestsennyy O, Gilbert G, Harris A, et al. Telehealth: a quarter-trillion-dollar post-COVID-19 reality? McKinsey & Company. Updated July 9, 2021. Accessed February 23, 2022. https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality
31. Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
32. Jennings K. Digital health startup Ro raised $500 million at $5 billion valuation. Forbes. March 22, 2021. Accessed March 29, 2022. https://www.forbes.com/sites/katiejennings/2021/03/22/digital-health-startup-ro-raised-500-million-at-5-billion-valuation/?sh=695be0e462f5
33. Hollander JE, Carr BG. Virtually perfect? telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
34. Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
In recent years, direct-to-consumer (DTC) teledermatology platforms have gained popularity as telehealth business models, allowing patients to directly initiate visits with physicians and purchase medications from single platforms. A shortage of dermatologists, improved technology, drug patent expirations, and rising health care costs accelerated the growth of DTC dermatology.1 During the COVID-19 pandemic, teledermatology adoption surged due to the need to provide care while social distancing and minimizing viral exposure. These needs prompted additional federal funding and loosened regulatory provisions.2 As the userbase of these companies has grown, so have their valuations.3 Although the DTC model has attracted the attention of patients and investors, its rise provokes many questions about patients acting as consumers in health care. Indeed, DTC telemedicine offers greater autonomy and convenience for patients, but it may impact the quality of care and the nature of physician-patient relationships, perhaps making them more transactional.
Evolution of DTC in Health Care
The DTC model emphasizes individual choice and accessible health care. Although the definition has evolved, the core idea is not new.4 Over decades, pharmaceutical companies have spent billions of dollars on DTC advertising, circumventing physicians by directly reaching patients with campaigns on prescription drugs and laboratory tests and shaping public definitions of diseases.5
The DTC model of care is fundamentally different from traditional care models in that it changes the roles of the patient and physician. Whereas early telehealth models required a health care provider to initiate teleconsultations with specialists, DTC telemedicine bypasses this step (eg, the patient can consult a dermatologist without needing a primary care provider’s input first). This care can then be provided by dermatologists with whom patients may or may not have pre-established relationships.4,6
Dermatology was an early adopter of DTC telemedicine. The shortage of dermatologists in the United States created demand for increasing accessibility to dermatologic care. Additionally, the visual nature of diagnosing dermatologic disease was ideal for platforms supporting image sharing.7 Early DTC providers were primarily individual companies offering teledermatology. However, many dermatologists can now offer DTC capabilities via companies such as Amwell and Teladoc Health.8
Over the last 2 decades, start-ups such as Warby Parker (eyeglasses) and Casper (mattresses) defined the DTC industry using borrowed supply chains, cohesive branding, heavy social media marketing, and web-only retail. Scalability, lack of competition, and abundant venture capital created competition across numerous markets.9 Health care capitalized on this DTC model, creating a $700 billion market for products ranging from hearing aids to over-the-counter medications.10
Borrowing from this DTC playbook, platforms were created to offer delivery of generic prescription drugs to patients’ doorsteps. However, unlike with other products bought online, a consumer cannot simply add prescription drugs to their shopping cart and check out. In all models of American medical practice, physicians still serve as gatekeepers, providing a safeguard for patients to ensure appropriate prescription and avoid negative consequences of unnecessary drug use. This new model effectively streamlines diagnosis, prescription, and drug delivery without the patient ever having to leave home. Combining the prescribing and selling of medications (2 tasks that traditionally have been separated) potentially creates financial conflicts of interest (COIs). Additionally, high utilization of health care, including more prescriptions and visits, does not necessarily equal high quality of care. The companies stand to benefit from extra care regardless of need, and thus these models must be scrutinized for any incentives driving unnecessary care and prescriptions.
Ultimately, DTC has evolved to encompass multiple definitions in health care (Table 1). Although all models provide health care, each offers a different modality of delivery. The primary service may be the sale of prescription drugs or simply telemedicine visits. This review primarily discusses DTC pharmaceutical telemedicine platforms that sell private-label drugs and also offer telemedicine services to streamline care. However, the history, risks, and benefits discussed may apply to all models.

The DTC Landscape
Most DTC companies employ variations on a model with the same 3 main components: a triage questionnaire, telehealth services, and prescription/drug delivery (Figure). The triage questionnaire elicits a history of the patient’s presentation and medical history. Some companies may use artificial intelligence (AI) algorithms to tailor questions to patient needs. There are 2 modalities for patient-provider communication: synchronous and asynchronous. Synchronous communication entails real-time patient-physician conversations via audio only or video call. Asynchronous (or store-and-forward) communication refers to consultations provided via messaging or text-based modality, where a provider may respond to a patient within 24 hours.6 Direct-to-consumer platforms primarily use asynchronous visits (Table 2). However, some also use synchronous modalities if the provider deems it necessary or if state laws require it.

Once a provider has consulted with the patient, they can prescribe medication as needed. In certain cases, with adequate history, a prescription may be issued without a full physician visit. Furthermore, DTC companies require purchase of their custom-branded generic drugs. Prescriptions are fulfilled by the company’s pharmacy network and directly shipped to patients; few will allow patients to transfer a prescription to a pharmacy of their choice. Some platforms also sell supplements and over-the-counter medications.

Payment models vary among these companies, and most do not accept insurance (Table 2). Select models may provide free consultations and only require payment for pharmaceuticals. Others charge for consultations but reallocate payment to the cost of medication if prescribed. Another model involves flat rates for consultations and additional charges for drugs but unlimited messaging with providers for the duration of the prescription. Moreover, patients can subscribe to monthly deliveries of their medications.
Foundation of DTC
Technological advances have enabled patients to receive remote treatment from a single platform offering video calls, AI, electronic medical record interoperability, and integration of drug supply chains. Even in its simplest form, AI is increasingly used, as it allows for programs and chatbots to screen and triage patients.11 Technology also has improved at targeted mass marketing through social media platforms and search engines (eg, companies can use age, interests, location, and other parameters to target individuals likely needing acne treatment).
Drug patent expirations are a key catalyst for the rise of DTC companies, creating an attractive business model with generic drugs as the core product. Since 2008, patents for medications treating chronic conditions, such as erectile dysfunction, have expired. These patent expirations are responsible for $198 billion in projected prescription sales between 2019 and 2024.1 Thus, it follows that DTC companies have seized this opportunity to act as middlemen, taking advantage of these generic medications’ lower costs to create platforms focused on personalization and accessibility.
Rising deductibles have led patients to consider cheaper out-of-pocket alternatives that are not covered by insurance.1 For example, insurers typically do not cover finasteride treatment for conditions deemed cosmetic, such as androgenetic alopecia.12 The low cost of generic drugs creates an attractive business model for patients and investors. According to GoodRx, the average retail price for a 30-day supply of brand-name finasteride (Propecia [Merck]) is $135.92, whereas generic finasteride is $75.24.13 Direct-to-consumer pharmaceutical companies offer a 30-day supply of generic finasteride ranging from $8.33 to $30.14 The average wholesale cost for retailers is an estimated $2.31 for 30 days.15 Although profit margins on generic medications may be lower, more affordable drugs increase the size of the total market. These prescriptions are available as subscription plans, resulting in recurring revenue.
Lax US pharmaceutical marketing regulations allow direct advertising to the general public.16 In 1997, the US Food and Drug Administration allowed DTC advertisements to replace summaries of serious and common adverse effects with short statements covering important risks or referrals to other sources for complete information. In 2015, the US Food and Drug Administration guidelines preventing encouragement of self-diagnosis and self-treatment were withdrawn.5 These changes enable DTC companies to launch large advertising campaigns and to accelerate customer acquisition, as the industry often describes it, with ease.
Rapid Growth and Implications
Increasing generic drug availability and improving telemedicine capabilities have the potential to reduce costs and barriers but also have the potential for financial gain. Venture capital funds have recognized this opportunity, reflected by millions of dollars of investments, and accelerated the growth of DTC health care start-ups. For example, Ro has raised $376 million from venture capital, valuing the company at $1.5 billion.3
Direct-to-consumer companies require a heavy focus on marketing campaigns for customer acquisition. Their aesthetically pleasing websites and aggressive campaigns target specific audiences based on demographics, digital use habits, and purchasing behavior.4 Some campaigns celebrate the ease of obtaining prescriptions.17 Companies have been effective in recruiting so-called millennial and Generation Z patients, known to search the internet for remedies prior to seeking physician consultations.18 Recognizing these needs, some platforms offer guides on diseases they treat, creating effective customer-acquisition funnels. Recruitment of these technology-friendly patients has proven effective, especially given the largely positive media coverage of DTC platforms––potentially serving as a surrogate for medical credibility for patients.18
Some DTC companies also market physically; skin care ads may be strategically placed in social media feeds, or even found near mirrors in public bathrooms.19 Marketing campaigns also involve disease awareness; such efforts serve to increase diagnoses and prescribed treatments while destigmatizing diseases. Although DTC companies argue this strategy empowers patients, these marketing habits have the potential to take advantage of uninformed patients. Campaigns could potentially medicalize normal experiences and expand disease definitions resulting in overdiagnosis, overtreatment, and wasted resources.5 For example, off-label propranolol use has been advertised to attract patients who might have “nerves that come creeping before an important presentation.”17 Disease awareness campaigns also may lead people to falsely believe unproven drug benefits.5 According to studies, DTC pharmaceutical advertisements are low in informational quality and result in increased patient visits and prescriptions despite cost-effective alternatives.5,20-22
Fragmentation of the health care system is another possible complication of DTC teledermatology. These companies operate as for-profit organizations separated from the rest of the health care system, raising concerns about care coordination.8 Vital health data may not be conveyed as patients move among different providers and pharmacies. One study found DTC teledermatology rarely offered to provide medical records or facilitate a referral to a local physician.23 Such a lack of communication is concerning, as medication errors are the leading cause of avoidable harm in health care.24
Direct-to-consumer care models also seemingly redefine the physician-patient relationship by turning patients into consumers. Patient interactions may seem transactional and streamlined toward sales. For these platforms, a visit often is set up as an evaluation of a patient’s suitability for a prescription, not necessarily for the best treatment modality for the problem. These companies primarily make money through the sale of prescription drugs, creating a potential COI that may undermine the patient-physician relationship. Although some companies have made it clear that medical care and pharmaceutical sales are provided by legally separate business entities and that they do not pay physicians on commission, a conflict may still exist given the financial importance of physicians prescribing medication to the success of the business.16
Even as DTC models advertise upon expanded access and choice, the companies largely prohibit patients from choosing their own pharmacy. Instead, they encourage patients to fill prescriptions with the company’s pharmacy network by claiming lower costs compared with competitors. One DTC company, Hims, is launching a prescription-fulfillment center to further consolidate their business.17,19,25 The inherent COI of issuing and fulfilling prescriptions raises concerns of patient harm.26 For example, when Dermatology.com launched as a DTC prescription skin medication shop backed by Bausch Health Companies Inc, its model included telemedicine consultation. Although consultations were provided by RxDefine, a third party, only Dermatology.com drugs were prescribed. Given the poor quality of care and obvious financial COI, an uproar in the dermatology community and advocacy by the American Academy of Dermatology led to the shutdown of Dermatology.com’s online prescription services.26
The quality of care among DTC telemedicine platforms has been equivocal. Some studies have reported equivalent care in person and online, while others have reported poor adherence to guidelines, overuse of antibiotics, and misdiagnosis.8,23 A vital portion of the DTC experience is the history questionnaire, which is geared to diagnosis and risk assessment.25 Resneck et al23 found diagnostic quality to be adequate for simple dermatologic clinical scenarios but poor for scenarios requiring more than basic histories. Although Ro has reported leveraging data from millions of interactions to ask the right questions and streamline visits, it is still unclear whether history questionnaires are adequate.17,27 Additionally, consultations may lack sufficient counseling on adverse effects, risks, or pregnancy warnings, as well as discussions on alternative treatments and preventative care.17,23 Finally, patients often are limited in their choice of dermatologist; the lack of a fully developed relationship increases concerns of follow-up and monitoring practices. Although some DTC platforms offer unlimited interactions with physicians for the duration of a prescription, it is unknown how often these services are utilized or how adequate the quality of these interactions is. This potential for lax follow-up is especially concerning for prescriptions that autorenew on a monthly basis and could result in unnecessary overtreatment.
Postpandemic and Future Outlook
The COVID-19 pandemic dramatically impacted the use of telemedicine. To minimize COVID-19 transmission, the Centers for Medicare & Medicaid Services and private payers expanded telehealth coverage and eliminated reimbursement and licensing barriers.28 A decade’s worth of regulatory changes and consumer adoption was accelerated to weeks, resulting in telemedicine companies reaching record-high visit numbers.29 McKinsey & Company estimated that telehealth visit numbers surged 50- to 175-fold compared with pre–COVID-19 numbers. Additionally, 76% of patients were interested in future telehealth use, and 64% of providers were more comfortable using telehealth than before the pandemic.30 For their part, US dermatologists reported an increase in telemedicine use from 14.1% to 96.9% since COVID-19.31
Exactly how much DTC pharmaceutical telemedicine companies are growing is unclear, but private investments may be an indication. A record $14.7 billion was invested in the digital health sector in the first half of 2021; the majority went to telehealth companies.30 Ro, which reported $230 million in revenue in 2020 and has served 6 million visits, raised $200 milllion in July 2020 and $500 million in March 2021.32 Although post–COVID-19 health care will certainly involve increased telemedicine, the extent remains unclear, as telehealth vendors saw decreased usage upon reopening of state economies. Ultimately, the postpandemic regulatory landscape is hard to predict.30
Although COVID-19 appears to have caused rapid growth for DTC platforms, it also may have spurred competition. Telemedicine providers have given independent dermatologists and health care systems the infrastructure to implement custom DTC services.33 Although systems do not directly sell prescription drugs, the target market is essentially the same: patients looking for instant virtual dermatologic care. Therefore, sustained telemedicine services offered by traditional practices and systems may prove detrimental to DTC companies. However, unlike most telemedicine services, DTC models are less affected by certain changes in regulation since they do not rely on insurance. If regulations are tightened and reimbursements for telehealth are not attractive for dermatologists, teledermatology services may see an overall decrease. If so, patients who appreciate teledermatology may shift to using DTC platforms, even if their insurance does not cover them. Still, a nationwide survey found 56% of respondents felt an established relationship with a physician prior to a telemedicine visit is important, which may create a barrier for DTC adoption.34
Conclusion
Direct-to-consumer teledermatology represents a growing for-profit model of health care that provides patients with seemingly affordable and convenient care. However, there is potential for overtreatment, misdiagnosis, and fragmentation of health care. It will be important to monitor and evaluate the quality of care that DTC teledermatology offers and advocate for appropriate regulations and oversight. Eventually, more patients will have medications prescribed and dermatologic care administered through DTC companies. Dermatologists will benefit from this knowledge of DTC models to properly counsel patients on the risks and benefits of their use.
In recent years, direct-to-consumer (DTC) teledermatology platforms have gained popularity as telehealth business models, allowing patients to directly initiate visits with physicians and purchase medications from single platforms. A shortage of dermatologists, improved technology, drug patent expirations, and rising health care costs accelerated the growth of DTC dermatology.1 During the COVID-19 pandemic, teledermatology adoption surged due to the need to provide care while social distancing and minimizing viral exposure. These needs prompted additional federal funding and loosened regulatory provisions.2 As the userbase of these companies has grown, so have their valuations.3 Although the DTC model has attracted the attention of patients and investors, its rise provokes many questions about patients acting as consumers in health care. Indeed, DTC telemedicine offers greater autonomy and convenience for patients, but it may impact the quality of care and the nature of physician-patient relationships, perhaps making them more transactional.
Evolution of DTC in Health Care
The DTC model emphasizes individual choice and accessible health care. Although the definition has evolved, the core idea is not new.4 Over decades, pharmaceutical companies have spent billions of dollars on DTC advertising, circumventing physicians by directly reaching patients with campaigns on prescription drugs and laboratory tests and shaping public definitions of diseases.5
The DTC model of care is fundamentally different from traditional care models in that it changes the roles of the patient and physician. Whereas early telehealth models required a health care provider to initiate teleconsultations with specialists, DTC telemedicine bypasses this step (eg, the patient can consult a dermatologist without needing a primary care provider’s input first). This care can then be provided by dermatologists with whom patients may or may not have pre-established relationships.4,6
Dermatology was an early adopter of DTC telemedicine. The shortage of dermatologists in the United States created demand for increasing accessibility to dermatologic care. Additionally, the visual nature of diagnosing dermatologic disease was ideal for platforms supporting image sharing.7 Early DTC providers were primarily individual companies offering teledermatology. However, many dermatologists can now offer DTC capabilities via companies such as Amwell and Teladoc Health.8
Over the last 2 decades, start-ups such as Warby Parker (eyeglasses) and Casper (mattresses) defined the DTC industry using borrowed supply chains, cohesive branding, heavy social media marketing, and web-only retail. Scalability, lack of competition, and abundant venture capital created competition across numerous markets.9 Health care capitalized on this DTC model, creating a $700 billion market for products ranging from hearing aids to over-the-counter medications.10
Borrowing from this DTC playbook, platforms were created to offer delivery of generic prescription drugs to patients’ doorsteps. However, unlike with other products bought online, a consumer cannot simply add prescription drugs to their shopping cart and check out. In all models of American medical practice, physicians still serve as gatekeepers, providing a safeguard for patients to ensure appropriate prescription and avoid negative consequences of unnecessary drug use. This new model effectively streamlines diagnosis, prescription, and drug delivery without the patient ever having to leave home. Combining the prescribing and selling of medications (2 tasks that traditionally have been separated) potentially creates financial conflicts of interest (COIs). Additionally, high utilization of health care, including more prescriptions and visits, does not necessarily equal high quality of care. The companies stand to benefit from extra care regardless of need, and thus these models must be scrutinized for any incentives driving unnecessary care and prescriptions.
Ultimately, DTC has evolved to encompass multiple definitions in health care (Table 1). Although all models provide health care, each offers a different modality of delivery. The primary service may be the sale of prescription drugs or simply telemedicine visits. This review primarily discusses DTC pharmaceutical telemedicine platforms that sell private-label drugs and also offer telemedicine services to streamline care. However, the history, risks, and benefits discussed may apply to all models.

The DTC Landscape
Most DTC companies employ variations on a model with the same 3 main components: a triage questionnaire, telehealth services, and prescription/drug delivery (Figure). The triage questionnaire elicits a history of the patient’s presentation and medical history. Some companies may use artificial intelligence (AI) algorithms to tailor questions to patient needs. There are 2 modalities for patient-provider communication: synchronous and asynchronous. Synchronous communication entails real-time patient-physician conversations via audio only or video call. Asynchronous (or store-and-forward) communication refers to consultations provided via messaging or text-based modality, where a provider may respond to a patient within 24 hours.6 Direct-to-consumer platforms primarily use asynchronous visits (Table 2). However, some also use synchronous modalities if the provider deems it necessary or if state laws require it.

Once a provider has consulted with the patient, they can prescribe medication as needed. In certain cases, with adequate history, a prescription may be issued without a full physician visit. Furthermore, DTC companies require purchase of their custom-branded generic drugs. Prescriptions are fulfilled by the company’s pharmacy network and directly shipped to patients; few will allow patients to transfer a prescription to a pharmacy of their choice. Some platforms also sell supplements and over-the-counter medications.

Payment models vary among these companies, and most do not accept insurance (Table 2). Select models may provide free consultations and only require payment for pharmaceuticals. Others charge for consultations but reallocate payment to the cost of medication if prescribed. Another model involves flat rates for consultations and additional charges for drugs but unlimited messaging with providers for the duration of the prescription. Moreover, patients can subscribe to monthly deliveries of their medications.
Foundation of DTC
Technological advances have enabled patients to receive remote treatment from a single platform offering video calls, AI, electronic medical record interoperability, and integration of drug supply chains. Even in its simplest form, AI is increasingly used, as it allows for programs and chatbots to screen and triage patients.11 Technology also has improved at targeted mass marketing through social media platforms and search engines (eg, companies can use age, interests, location, and other parameters to target individuals likely needing acne treatment).
Drug patent expirations are a key catalyst for the rise of DTC companies, creating an attractive business model with generic drugs as the core product. Since 2008, patents for medications treating chronic conditions, such as erectile dysfunction, have expired. These patent expirations are responsible for $198 billion in projected prescription sales between 2019 and 2024.1 Thus, it follows that DTC companies have seized this opportunity to act as middlemen, taking advantage of these generic medications’ lower costs to create platforms focused on personalization and accessibility.
Rising deductibles have led patients to consider cheaper out-of-pocket alternatives that are not covered by insurance.1 For example, insurers typically do not cover finasteride treatment for conditions deemed cosmetic, such as androgenetic alopecia.12 The low cost of generic drugs creates an attractive business model for patients and investors. According to GoodRx, the average retail price for a 30-day supply of brand-name finasteride (Propecia [Merck]) is $135.92, whereas generic finasteride is $75.24.13 Direct-to-consumer pharmaceutical companies offer a 30-day supply of generic finasteride ranging from $8.33 to $30.14 The average wholesale cost for retailers is an estimated $2.31 for 30 days.15 Although profit margins on generic medications may be lower, more affordable drugs increase the size of the total market. These prescriptions are available as subscription plans, resulting in recurring revenue.
Lax US pharmaceutical marketing regulations allow direct advertising to the general public.16 In 1997, the US Food and Drug Administration allowed DTC advertisements to replace summaries of serious and common adverse effects with short statements covering important risks or referrals to other sources for complete information. In 2015, the US Food and Drug Administration guidelines preventing encouragement of self-diagnosis and self-treatment were withdrawn.5 These changes enable DTC companies to launch large advertising campaigns and to accelerate customer acquisition, as the industry often describes it, with ease.
Rapid Growth and Implications
Increasing generic drug availability and improving telemedicine capabilities have the potential to reduce costs and barriers but also have the potential for financial gain. Venture capital funds have recognized this opportunity, reflected by millions of dollars of investments, and accelerated the growth of DTC health care start-ups. For example, Ro has raised $376 million from venture capital, valuing the company at $1.5 billion.3
Direct-to-consumer companies require a heavy focus on marketing campaigns for customer acquisition. Their aesthetically pleasing websites and aggressive campaigns target specific audiences based on demographics, digital use habits, and purchasing behavior.4 Some campaigns celebrate the ease of obtaining prescriptions.17 Companies have been effective in recruiting so-called millennial and Generation Z patients, known to search the internet for remedies prior to seeking physician consultations.18 Recognizing these needs, some platforms offer guides on diseases they treat, creating effective customer-acquisition funnels. Recruitment of these technology-friendly patients has proven effective, especially given the largely positive media coverage of DTC platforms––potentially serving as a surrogate for medical credibility for patients.18
Some DTC companies also market physically; skin care ads may be strategically placed in social media feeds, or even found near mirrors in public bathrooms.19 Marketing campaigns also involve disease awareness; such efforts serve to increase diagnoses and prescribed treatments while destigmatizing diseases. Although DTC companies argue this strategy empowers patients, these marketing habits have the potential to take advantage of uninformed patients. Campaigns could potentially medicalize normal experiences and expand disease definitions resulting in overdiagnosis, overtreatment, and wasted resources.5 For example, off-label propranolol use has been advertised to attract patients who might have “nerves that come creeping before an important presentation.”17 Disease awareness campaigns also may lead people to falsely believe unproven drug benefits.5 According to studies, DTC pharmaceutical advertisements are low in informational quality and result in increased patient visits and prescriptions despite cost-effective alternatives.5,20-22
Fragmentation of the health care system is another possible complication of DTC teledermatology. These companies operate as for-profit organizations separated from the rest of the health care system, raising concerns about care coordination.8 Vital health data may not be conveyed as patients move among different providers and pharmacies. One study found DTC teledermatology rarely offered to provide medical records or facilitate a referral to a local physician.23 Such a lack of communication is concerning, as medication errors are the leading cause of avoidable harm in health care.24
Direct-to-consumer care models also seemingly redefine the physician-patient relationship by turning patients into consumers. Patient interactions may seem transactional and streamlined toward sales. For these platforms, a visit often is set up as an evaluation of a patient’s suitability for a prescription, not necessarily for the best treatment modality for the problem. These companies primarily make money through the sale of prescription drugs, creating a potential COI that may undermine the patient-physician relationship. Although some companies have made it clear that medical care and pharmaceutical sales are provided by legally separate business entities and that they do not pay physicians on commission, a conflict may still exist given the financial importance of physicians prescribing medication to the success of the business.16
Even as DTC models advertise upon expanded access and choice, the companies largely prohibit patients from choosing their own pharmacy. Instead, they encourage patients to fill prescriptions with the company’s pharmacy network by claiming lower costs compared with competitors. One DTC company, Hims, is launching a prescription-fulfillment center to further consolidate their business.17,19,25 The inherent COI of issuing and fulfilling prescriptions raises concerns of patient harm.26 For example, when Dermatology.com launched as a DTC prescription skin medication shop backed by Bausch Health Companies Inc, its model included telemedicine consultation. Although consultations were provided by RxDefine, a third party, only Dermatology.com drugs were prescribed. Given the poor quality of care and obvious financial COI, an uproar in the dermatology community and advocacy by the American Academy of Dermatology led to the shutdown of Dermatology.com’s online prescription services.26
The quality of care among DTC telemedicine platforms has been equivocal. Some studies have reported equivalent care in person and online, while others have reported poor adherence to guidelines, overuse of antibiotics, and misdiagnosis.8,23 A vital portion of the DTC experience is the history questionnaire, which is geared to diagnosis and risk assessment.25 Resneck et al23 found diagnostic quality to be adequate for simple dermatologic clinical scenarios but poor for scenarios requiring more than basic histories. Although Ro has reported leveraging data from millions of interactions to ask the right questions and streamline visits, it is still unclear whether history questionnaires are adequate.17,27 Additionally, consultations may lack sufficient counseling on adverse effects, risks, or pregnancy warnings, as well as discussions on alternative treatments and preventative care.17,23 Finally, patients often are limited in their choice of dermatologist; the lack of a fully developed relationship increases concerns of follow-up and monitoring practices. Although some DTC platforms offer unlimited interactions with physicians for the duration of a prescription, it is unknown how often these services are utilized or how adequate the quality of these interactions is. This potential for lax follow-up is especially concerning for prescriptions that autorenew on a monthly basis and could result in unnecessary overtreatment.
Postpandemic and Future Outlook
The COVID-19 pandemic dramatically impacted the use of telemedicine. To minimize COVID-19 transmission, the Centers for Medicare & Medicaid Services and private payers expanded telehealth coverage and eliminated reimbursement and licensing barriers.28 A decade’s worth of regulatory changes and consumer adoption was accelerated to weeks, resulting in telemedicine companies reaching record-high visit numbers.29 McKinsey & Company estimated that telehealth visit numbers surged 50- to 175-fold compared with pre–COVID-19 numbers. Additionally, 76% of patients were interested in future telehealth use, and 64% of providers were more comfortable using telehealth than before the pandemic.30 For their part, US dermatologists reported an increase in telemedicine use from 14.1% to 96.9% since COVID-19.31
Exactly how much DTC pharmaceutical telemedicine companies are growing is unclear, but private investments may be an indication. A record $14.7 billion was invested in the digital health sector in the first half of 2021; the majority went to telehealth companies.30 Ro, which reported $230 million in revenue in 2020 and has served 6 million visits, raised $200 milllion in July 2020 and $500 million in March 2021.32 Although post–COVID-19 health care will certainly involve increased telemedicine, the extent remains unclear, as telehealth vendors saw decreased usage upon reopening of state economies. Ultimately, the postpandemic regulatory landscape is hard to predict.30
Although COVID-19 appears to have caused rapid growth for DTC platforms, it also may have spurred competition. Telemedicine providers have given independent dermatologists and health care systems the infrastructure to implement custom DTC services.33 Although systems do not directly sell prescription drugs, the target market is essentially the same: patients looking for instant virtual dermatologic care. Therefore, sustained telemedicine services offered by traditional practices and systems may prove detrimental to DTC companies. However, unlike most telemedicine services, DTC models are less affected by certain changes in regulation since they do not rely on insurance. If regulations are tightened and reimbursements for telehealth are not attractive for dermatologists, teledermatology services may see an overall decrease. If so, patients who appreciate teledermatology may shift to using DTC platforms, even if their insurance does not cover them. Still, a nationwide survey found 56% of respondents felt an established relationship with a physician prior to a telemedicine visit is important, which may create a barrier for DTC adoption.34
Conclusion
Direct-to-consumer teledermatology represents a growing for-profit model of health care that provides patients with seemingly affordable and convenient care. However, there is potential for overtreatment, misdiagnosis, and fragmentation of health care. It will be important to monitor and evaluate the quality of care that DTC teledermatology offers and advocate for appropriate regulations and oversight. Eventually, more patients will have medications prescribed and dermatologic care administered through DTC companies. Dermatologists will benefit from this knowledge of DTC models to properly counsel patients on the risks and benefits of their use.
- Vennare J. The DTC healthcare report. Fitt Insider. September 15, 2019. Accessed February 23, 2022. https://insider.fitt.co/direct-to-consumer-healthcare-startups/
- Kannampallil T, Ma J. Digital translucence: adapting telemedicine delivery post-COVID-19. Telemed J E Health. 2020;26:1120-1122.
- Farr C. Ro, a 3-year-old online health provider, just raised a new round that values it at $1.5 billion. CNBC. July 27, 2020. Accessed February 23, 2022. https://www.cnbc.com/2020/07/27/ro-raises-200-million-at-1point5-billion-valuation-250-million-sales.html
- Elliott T, Shih J. Direct to consumer telemedicine. Curr Allergy Asthma Rep. 2019;19:1.
- Schwartz LM, Woloshin S. Medical marketing in the United States, 1997-2016. JAMA. 2019;321:80-96.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Coates SJ, Kvedar J, Granstein RD. Teledermatology: from historical perspective to emerging techniques of the modern era. J Am Acad Dermatol. 2015;72:563-574.
- Rheuban KS, Krupinski EA, eds. Understanding Telehealth. McGraw-Hill Education; 2017.
- Schlesinger LA, Higgins M, Roseman S. Reinventing the direct-to-consumer business model. Harvard Business Review. March 31, 2020. Accessed February 23, 2022. https://hbr.org/2020/03/reinventing-the-direct-to-consumer-business-model
- Cohen AB, Mathews SC, Dorsey ER, et al. Direct-to-consumer digital health. Lancet Digit Health. 2020;2:E163-E165.
- 6 telehealth trends for 2020. Wolters Kluwer. Published January 27, 2021. Accessed February 23, 2022. https://www.wolterskluwer.com/en/expert-insights/6-telehealth-trends-for-2020
- Jadoo SA, Lipoff JB. Prescribing to save patients money: ethical considerations. J Am Acad Dermatol. 2018;78:826-828.
- Propecia. GoodRx. Accessed February 23, 2022. https://www.goodrx.com/propecia
- Lauer A. The truth about online hair-loss treatments like Roman and Hims, according to a dermatologist. InsideHook. January 13, 2020. Accessed February 23, 2022. https://www.insidehook.com/article/grooming/men-hair-loss-treatments-dermatologist-review
- Friedman Y. Drug price trends for NDC 16729-0089. DrugPatentWatch. Accessed February 23, 2022. https://www.drugpatentwatch.com/p/drug-price/ndc/index.php?query=16729-0089
- Curtis H, Milner J. Ethical concerns with online direct-to-consumer pharmaceutical companies. J Med Ethics. 2020;46:168-171.
- Jain T, Lu RJ, Mehrotra A. Prescriptions on demand: the growth of direct-to-consumer telemedicine companies. JAMA. 2019;322:925-926.
- Shahinyan RH, Amighi A, Carey AN, et al. Direct-to-consumer internet prescription platforms overlook crucial pathology found during traditional office evaluation of young men with erectile dysfunction. Urology. 2020;143:165-172.
- Ali M. Andrew Dudum—bold strategies that propelled Hims & Hers into unicorn status. Exit Strategy with Moiz Ali. Published April 2020. Accessed February 23, 2022. https://open.spotify.com/episode/6DtaJxwZDjvZSJI88DTf24?si=b3FHQiUIQY62YjfRHmnJBQ
- Klara K, Kim J, Ross JS. Direct-to-consumer broadcast advertisements for pharmaceuticals: off-label promotion and adherence to FDA guidelines. J Gen Intern Med. 2018;33:651-658.
- Sullivan HW, Aikin KJ, Poehlman J. Communicating risk information in direct-to-consumer prescription drug television ads: a content analysis. Health Commun. 2019;34:212-219.
- Applequist J, Ball JG. An updated analysis of direct-to-consumer television advertisements for prescription drugs. Ann Fam Med. 2018;16:211-216.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Patient safety. World Health Organization. Published September 13, 2019. Accessed February 1, 2022. https://www.who.int/news-room/fact-sheets/detail/patient-safety
- Bollmeier SG, Stevenson E, Finnegan P, et al. Direct to consumer telemedicine: is healthcare from home best? Mo Med. 2020;117:303-309.
26. Court E. Bausch yanked online prescribing after dermatologist backlash. Bloomberg.com. Published March 11, 2020. Accessed September 25, 2020. https://www.bloomberg.com/news/articles/2020-03-11/bausch-yanked-online-prescribing-after-dermatologist-backlash
27. Reitano Z. The future of healthcare: how Ro helps providers treat patients 2 minutes, 2 days, 2 weeks, and 2 years at a time. Medium. Published March 4, 2019. Accessed February 1, 2022. https://medium.com/ro-co/the-future-of-healthcare-how-ro-helps-providers-treat-patients-2-mins-2-days-2-weeks-and-2-10efc0679d7
28. Lee I, Kovarik C, Tejasvi T, et al. Telehealth: helping your patients and practice survive and thrive during the COVID-19 crisis with rapid quality implementation. J Am Acad Dermatol. 2020;82:1213-1214.
29. Pifer R. “Weeks where decades happen”: telehealth 6 months into COVID-19. Healthcare Dive. Published July 27, 2020. Accessed February 23, 2022. https://www.healthcaredive.com/news/telehealth-6-months-coronavirus/581447/
30. Bestsennyy O, Gilbert G, Harris A, et al. Telehealth: a quarter-trillion-dollar post-COVID-19 reality? McKinsey & Company. Updated July 9, 2021. Accessed February 23, 2022. https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality
31. Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
32. Jennings K. Digital health startup Ro raised $500 million at $5 billion valuation. Forbes. March 22, 2021. Accessed March 29, 2022. https://www.forbes.com/sites/katiejennings/2021/03/22/digital-health-startup-ro-raised-500-million-at-5-billion-valuation/?sh=695be0e462f5
33. Hollander JE, Carr BG. Virtually perfect? telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
34. Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
- Vennare J. The DTC healthcare report. Fitt Insider. September 15, 2019. Accessed February 23, 2022. https://insider.fitt.co/direct-to-consumer-healthcare-startups/
- Kannampallil T, Ma J. Digital translucence: adapting telemedicine delivery post-COVID-19. Telemed J E Health. 2020;26:1120-1122.
- Farr C. Ro, a 3-year-old online health provider, just raised a new round that values it at $1.5 billion. CNBC. July 27, 2020. Accessed February 23, 2022. https://www.cnbc.com/2020/07/27/ro-raises-200-million-at-1point5-billion-valuation-250-million-sales.html
- Elliott T, Shih J. Direct to consumer telemedicine. Curr Allergy Asthma Rep. 2019;19:1.
- Schwartz LM, Woloshin S. Medical marketing in the United States, 1997-2016. JAMA. 2019;321:80-96.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Coates SJ, Kvedar J, Granstein RD. Teledermatology: from historical perspective to emerging techniques of the modern era. J Am Acad Dermatol. 2015;72:563-574.
- Rheuban KS, Krupinski EA, eds. Understanding Telehealth. McGraw-Hill Education; 2017.
- Schlesinger LA, Higgins M, Roseman S. Reinventing the direct-to-consumer business model. Harvard Business Review. March 31, 2020. Accessed February 23, 2022. https://hbr.org/2020/03/reinventing-the-direct-to-consumer-business-model
- Cohen AB, Mathews SC, Dorsey ER, et al. Direct-to-consumer digital health. Lancet Digit Health. 2020;2:E163-E165.
- 6 telehealth trends for 2020. Wolters Kluwer. Published January 27, 2021. Accessed February 23, 2022. https://www.wolterskluwer.com/en/expert-insights/6-telehealth-trends-for-2020
- Jadoo SA, Lipoff JB. Prescribing to save patients money: ethical considerations. J Am Acad Dermatol. 2018;78:826-828.
- Propecia. GoodRx. Accessed February 23, 2022. https://www.goodrx.com/propecia
- Lauer A. The truth about online hair-loss treatments like Roman and Hims, according to a dermatologist. InsideHook. January 13, 2020. Accessed February 23, 2022. https://www.insidehook.com/article/grooming/men-hair-loss-treatments-dermatologist-review
- Friedman Y. Drug price trends for NDC 16729-0089. DrugPatentWatch. Accessed February 23, 2022. https://www.drugpatentwatch.com/p/drug-price/ndc/index.php?query=16729-0089
- Curtis H, Milner J. Ethical concerns with online direct-to-consumer pharmaceutical companies. J Med Ethics. 2020;46:168-171.
- Jain T, Lu RJ, Mehrotra A. Prescriptions on demand: the growth of direct-to-consumer telemedicine companies. JAMA. 2019;322:925-926.
- Shahinyan RH, Amighi A, Carey AN, et al. Direct-to-consumer internet prescription platforms overlook crucial pathology found during traditional office evaluation of young men with erectile dysfunction. Urology. 2020;143:165-172.
- Ali M. Andrew Dudum—bold strategies that propelled Hims & Hers into unicorn status. Exit Strategy with Moiz Ali. Published April 2020. Accessed February 23, 2022. https://open.spotify.com/episode/6DtaJxwZDjvZSJI88DTf24?si=b3FHQiUIQY62YjfRHmnJBQ
- Klara K, Kim J, Ross JS. Direct-to-consumer broadcast advertisements for pharmaceuticals: off-label promotion and adherence to FDA guidelines. J Gen Intern Med. 2018;33:651-658.
- Sullivan HW, Aikin KJ, Poehlman J. Communicating risk information in direct-to-consumer prescription drug television ads: a content analysis. Health Commun. 2019;34:212-219.
- Applequist J, Ball JG. An updated analysis of direct-to-consumer television advertisements for prescription drugs. Ann Fam Med. 2018;16:211-216.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Patient safety. World Health Organization. Published September 13, 2019. Accessed February 1, 2022. https://www.who.int/news-room/fact-sheets/detail/patient-safety
- Bollmeier SG, Stevenson E, Finnegan P, et al. Direct to consumer telemedicine: is healthcare from home best? Mo Med. 2020;117:303-309.
26. Court E. Bausch yanked online prescribing after dermatologist backlash. Bloomberg.com. Published March 11, 2020. Accessed September 25, 2020. https://www.bloomberg.com/news/articles/2020-03-11/bausch-yanked-online-prescribing-after-dermatologist-backlash
27. Reitano Z. The future of healthcare: how Ro helps providers treat patients 2 minutes, 2 days, 2 weeks, and 2 years at a time. Medium. Published March 4, 2019. Accessed February 1, 2022. https://medium.com/ro-co/the-future-of-healthcare-how-ro-helps-providers-treat-patients-2-mins-2-days-2-weeks-and-2-10efc0679d7
28. Lee I, Kovarik C, Tejasvi T, et al. Telehealth: helping your patients and practice survive and thrive during the COVID-19 crisis with rapid quality implementation. J Am Acad Dermatol. 2020;82:1213-1214.
29. Pifer R. “Weeks where decades happen”: telehealth 6 months into COVID-19. Healthcare Dive. Published July 27, 2020. Accessed February 23, 2022. https://www.healthcaredive.com/news/telehealth-6-months-coronavirus/581447/
30. Bestsennyy O, Gilbert G, Harris A, et al. Telehealth: a quarter-trillion-dollar post-COVID-19 reality? McKinsey & Company. Updated July 9, 2021. Accessed February 23, 2022. https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality
31. Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
32. Jennings K. Digital health startup Ro raised $500 million at $5 billion valuation. Forbes. March 22, 2021. Accessed March 29, 2022. https://www.forbes.com/sites/katiejennings/2021/03/22/digital-health-startup-ro-raised-500-million-at-5-billion-valuation/?sh=695be0e462f5
33. Hollander JE, Carr BG. Virtually perfect? telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
34. Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
Practice Points
- Direct-to-consumer (DTC) teledermatology platforms are for-profit companies that provide telemedicine visits and sell prescription drugs directly to patients.
- Although they are growing in popularity, DTC teledermatology platforms may lead to overdiagnosis, overtreatment, and fragmentation of health care. Knowledge of teledermatology will be vital to counsel patients on the risks and benefits of these platforms.
‘Overwhelming’ need to study COVID vaccine–associated tinnitus
It’s now known that tinnitus may be an unexpected side effect of SARS-CoV-2 vaccination, and there is an urgent need to understand the precise mechanisms and best treatment for vaccine-associated tinnitus, researchers say.
As of mid-September 2021, 12,247 cases of tinnitus, or ringing in the ears, following COVID-19 vaccination had been reported to the Vaccine Adverse Event Reporting System of the U.S. Centers for Disease Control and Prevention.
“Despite several cases of tinnitus being reported following SARS-CoV-2 vaccination, the precise pathophysiology is still not clear,” write Syed Hassan Ahmed, 3rd-year MBBS student, Dow University of Health Sciences, Karachi, Pakistan, and coauthors.
The researchers review what is known and unknown about SARS-CoV-2 vaccine-associated tinnitus in an article published online Feb. 11 in Annals of Medicine and Surgery.
Molecular mimicry?
The researchers say cross-reactivity between anti-spike SARS-CoV-2 antibodies and otologic antigens is one possibility, based on the mechanisms behind other COVID-19 vaccine–induced disorders and the phenomenon of molecular mimicry.
“The heptapeptide resemblance between coronavirus spike glycoprotein and numerous human proteins further supports molecular mimicry as a potential mechanism behind such vaccine-induced disorders,” they write.
Anti-spike antibodies may react with antigens anywhere along the auditory pathway and fuel an inflammatory reaction, they point out.
“Therefore, understanding the phenomenon of cross-reactivity and molecular mimicry may be helpful in postulating potential treatment behind not only tinnitus but also the rare events of vaccination associated hearing loss and other otologic manifestations,” the authors say.
Genetic predispositions and associated conditions may also play a significant role in determining whether an individual develops vaccine-induced tinnitus.
Stress and anxiety following COVID vaccination may also play a role, inasmuch as anxiety-related adverse events following vaccination have been reported. Vaccine-related anxiety as a potential cause of tinnitus developing after vaccination needs to be explored, they write.
Jury out on best management
How best to manage COVID vaccine-associated tinnitus also remains unclear, but it starts with a well-established diagnosis, the authors say.
A well-focused and detailed history and examination are essential, with particular emphasis placed on preexisting health conditions, specifically, autoimmune diseases, such as Hashimoto thyroiditis; otologic conditions, such as sensorineural hearing loss; glaucoma; and psychological well-being. According to the review, patients often present with a history of one or more of these disorders.
“However, any such association has not yet been established and requires further investigation to be concluded as potential risk factors for vaccine-induced tinnitus,” they caution.
Routine cranial nerve examination, otoscopy, Weber test, and Rinne test, which are used for tinnitus diagnosis in general, may be helpful for confirmation of vaccine-associated tinnitus.
Owing to the significant association between tinnitus and hearing impairment, audiology should also performed, the authors say.
Although treatments for non–vaccine-induced tinnitus vary significantly, corticosteroids are the top treatment choice for SARS-CoV-2 vaccine-induced tinnitus reported in the literature.
Trials of other drug and nondrug interventions that may uniquely help with vaccine-associated tinnitus are urgently needed, the authors say.
Summing up, the reviewers say, “Although the incidence of COVID-19 vaccine-associated tinnitus is rare, there is an overwhelming need to discern the precise pathophysiology and clinical management as a better understanding of adverse events may help in encountering vaccine hesitancy and hence fostering the COVID-19 global vaccination program.
“Despite the incidence of adverse events, the benefits of the SARS-CoV-2 vaccine in reducing hospitalization and deaths continue to outweigh the rare ramifications,” they conclude.
The research had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It’s now known that tinnitus may be an unexpected side effect of SARS-CoV-2 vaccination, and there is an urgent need to understand the precise mechanisms and best treatment for vaccine-associated tinnitus, researchers say.
As of mid-September 2021, 12,247 cases of tinnitus, or ringing in the ears, following COVID-19 vaccination had been reported to the Vaccine Adverse Event Reporting System of the U.S. Centers for Disease Control and Prevention.
“Despite several cases of tinnitus being reported following SARS-CoV-2 vaccination, the precise pathophysiology is still not clear,” write Syed Hassan Ahmed, 3rd-year MBBS student, Dow University of Health Sciences, Karachi, Pakistan, and coauthors.
The researchers review what is known and unknown about SARS-CoV-2 vaccine-associated tinnitus in an article published online Feb. 11 in Annals of Medicine and Surgery.
Molecular mimicry?
The researchers say cross-reactivity between anti-spike SARS-CoV-2 antibodies and otologic antigens is one possibility, based on the mechanisms behind other COVID-19 vaccine–induced disorders and the phenomenon of molecular mimicry.
“The heptapeptide resemblance between coronavirus spike glycoprotein and numerous human proteins further supports molecular mimicry as a potential mechanism behind such vaccine-induced disorders,” they write.
Anti-spike antibodies may react with antigens anywhere along the auditory pathway and fuel an inflammatory reaction, they point out.
“Therefore, understanding the phenomenon of cross-reactivity and molecular mimicry may be helpful in postulating potential treatment behind not only tinnitus but also the rare events of vaccination associated hearing loss and other otologic manifestations,” the authors say.
Genetic predispositions and associated conditions may also play a significant role in determining whether an individual develops vaccine-induced tinnitus.
Stress and anxiety following COVID vaccination may also play a role, inasmuch as anxiety-related adverse events following vaccination have been reported. Vaccine-related anxiety as a potential cause of tinnitus developing after vaccination needs to be explored, they write.
Jury out on best management
How best to manage COVID vaccine-associated tinnitus also remains unclear, but it starts with a well-established diagnosis, the authors say.
A well-focused and detailed history and examination are essential, with particular emphasis placed on preexisting health conditions, specifically, autoimmune diseases, such as Hashimoto thyroiditis; otologic conditions, such as sensorineural hearing loss; glaucoma; and psychological well-being. According to the review, patients often present with a history of one or more of these disorders.
“However, any such association has not yet been established and requires further investigation to be concluded as potential risk factors for vaccine-induced tinnitus,” they caution.
Routine cranial nerve examination, otoscopy, Weber test, and Rinne test, which are used for tinnitus diagnosis in general, may be helpful for confirmation of vaccine-associated tinnitus.
Owing to the significant association between tinnitus and hearing impairment, audiology should also performed, the authors say.
Although treatments for non–vaccine-induced tinnitus vary significantly, corticosteroids are the top treatment choice for SARS-CoV-2 vaccine-induced tinnitus reported in the literature.
Trials of other drug and nondrug interventions that may uniquely help with vaccine-associated tinnitus are urgently needed, the authors say.
Summing up, the reviewers say, “Although the incidence of COVID-19 vaccine-associated tinnitus is rare, there is an overwhelming need to discern the precise pathophysiology and clinical management as a better understanding of adverse events may help in encountering vaccine hesitancy and hence fostering the COVID-19 global vaccination program.
“Despite the incidence of adverse events, the benefits of the SARS-CoV-2 vaccine in reducing hospitalization and deaths continue to outweigh the rare ramifications,” they conclude.
The research had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It’s now known that tinnitus may be an unexpected side effect of SARS-CoV-2 vaccination, and there is an urgent need to understand the precise mechanisms and best treatment for vaccine-associated tinnitus, researchers say.
As of mid-September 2021, 12,247 cases of tinnitus, or ringing in the ears, following COVID-19 vaccination had been reported to the Vaccine Adverse Event Reporting System of the U.S. Centers for Disease Control and Prevention.
“Despite several cases of tinnitus being reported following SARS-CoV-2 vaccination, the precise pathophysiology is still not clear,” write Syed Hassan Ahmed, 3rd-year MBBS student, Dow University of Health Sciences, Karachi, Pakistan, and coauthors.
The researchers review what is known and unknown about SARS-CoV-2 vaccine-associated tinnitus in an article published online Feb. 11 in Annals of Medicine and Surgery.
Molecular mimicry?
The researchers say cross-reactivity between anti-spike SARS-CoV-2 antibodies and otologic antigens is one possibility, based on the mechanisms behind other COVID-19 vaccine–induced disorders and the phenomenon of molecular mimicry.
“The heptapeptide resemblance between coronavirus spike glycoprotein and numerous human proteins further supports molecular mimicry as a potential mechanism behind such vaccine-induced disorders,” they write.
Anti-spike antibodies may react with antigens anywhere along the auditory pathway and fuel an inflammatory reaction, they point out.
“Therefore, understanding the phenomenon of cross-reactivity and molecular mimicry may be helpful in postulating potential treatment behind not only tinnitus but also the rare events of vaccination associated hearing loss and other otologic manifestations,” the authors say.
Genetic predispositions and associated conditions may also play a significant role in determining whether an individual develops vaccine-induced tinnitus.
Stress and anxiety following COVID vaccination may also play a role, inasmuch as anxiety-related adverse events following vaccination have been reported. Vaccine-related anxiety as a potential cause of tinnitus developing after vaccination needs to be explored, they write.
Jury out on best management
How best to manage COVID vaccine-associated tinnitus also remains unclear, but it starts with a well-established diagnosis, the authors say.
A well-focused and detailed history and examination are essential, with particular emphasis placed on preexisting health conditions, specifically, autoimmune diseases, such as Hashimoto thyroiditis; otologic conditions, such as sensorineural hearing loss; glaucoma; and psychological well-being. According to the review, patients often present with a history of one or more of these disorders.
“However, any such association has not yet been established and requires further investigation to be concluded as potential risk factors for vaccine-induced tinnitus,” they caution.
Routine cranial nerve examination, otoscopy, Weber test, and Rinne test, which are used for tinnitus diagnosis in general, may be helpful for confirmation of vaccine-associated tinnitus.
Owing to the significant association between tinnitus and hearing impairment, audiology should also performed, the authors say.
Although treatments for non–vaccine-induced tinnitus vary significantly, corticosteroids are the top treatment choice for SARS-CoV-2 vaccine-induced tinnitus reported in the literature.
Trials of other drug and nondrug interventions that may uniquely help with vaccine-associated tinnitus are urgently needed, the authors say.
Summing up, the reviewers say, “Although the incidence of COVID-19 vaccine-associated tinnitus is rare, there is an overwhelming need to discern the precise pathophysiology and clinical management as a better understanding of adverse events may help in encountering vaccine hesitancy and hence fostering the COVID-19 global vaccination program.
“Despite the incidence of adverse events, the benefits of the SARS-CoV-2 vaccine in reducing hospitalization and deaths continue to outweigh the rare ramifications,” they conclude.
The research had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF MEDICINE AND SURGERY
COVID-19 vaccine does not affect in vitro fertilization outcomes
Getting a COVID-19 mRNA vaccine did not affect pregnancy rates for women trying to conceive with in vitro fertilization or ovarian response to treatment, findings of a new study indicate.
The study was led by Sarit Avraham, MD, with the IVF unit, department of obstetrics and gynecology, Shamir Medical Center in Tzrifi, Israel. The findings were published online in Fertility and Sterility in a preproof version.
“Women should be vaccinated for COVID-19 prior to attempting to conceive via IVF treatments, given the higher risk of severe illness in pregnant women,” the authors wrote.
Doubts arose from “the theoretical concept of the supposed similarity between the SARS-CoV-2 spike protein and the syncytin protein that is speculated to take part in the fertilization process and the formation of the placenta,” the authors wrote.
Some then assumed that the COVID vaccine might kick off an immune response that could affect implantation and pregnancy. But this study and others before it found otherwise.
Researchers included 200 vaccinated women trying to conceive with IVF treatments in the retrospective study, and compared them with 200 unvaccinated patients of similar age (average age in both groups, 36 years) who were not previously infected with COVID-19. All the women were undergoing IVF from January to April 2021 and all the vaccinated women completed two doses of the BNT162b2 (Pfizer/BioNTech) vaccine at least 2 weeks before ovarian stimulation.
Researchers compared the average number of oocytes retrieved and clinical pregnancy rates between the two groups.
No difference between groups
Two hundred patients underwent oocyte retrieval 14-68 days after receiving a COVID shot; there was no significant difference by vaccination status in the number retrieved per cycle (10.63 in the vaccinated group vs. 10.72 in the unvaccinated group; P = .93).
There was also no difference in the clinical pregnancy rates after fresh embryo transfers. The rate among 128 vaccinated patients was 32.8% versus 33.1% in the 133 unvaccinated patients (P = .96), with 42 and 44 clinical pregnancies, respectively.
A total of 113 patients (66 in the study group and 47 in the controls) underwent freeze-all cycles to preserve fertility and fertilization rates were similar between vaccinated and unvaccinated (55.43% vaccinated vs. 54.29% unvaccinated; P = .73). The average number of cryopreserved embryos was 3.59 (vaccinated) versus 3.28 (unvaccinated) (P = .80).
In a subanalysis of outcomes by age, researchers found vaccination status had no effect on number of oocytes or pregnancy rates in the 39-and-older group. That’s important because it shows the vaccine did not affect outcomes even in a population with reduced ovarian reserves, the authors wrote.
The authors noted one of the study’s limitations is that it didn’t include information about vaccination or past infection status of the male partners.
Question should be put to rest
Sarah Cross, MD, a maternal-fetal medicine specialist at the University of Minnesota, Minneapolis, said the study is the biggest she’s seen that concludes COVID vaccinations are safe and highly encouraged for women before trying to conceive, but other smaller studies have come to the same conclusion.
She pointed to research including a study from 2021 with similar findings that concluded: “Physicians and public health personnel can counsel women of reproductive age that neither previous illness with COVID-19 nor antibodies produced from vaccination to COVID-19 will cause sterility.”
She said she thinks the question of whether COVID shots are safe with IVF has been answered and the results of the latest study add proof to counter misinformation around the issue.
“The COVID-19 vaccine does not affect fertility,” she said. “I don’t know how many more [studies] we need.”
The harm is in not getting vaccinated, she said. Pregnancy significantly increases a woman’s chance of getting severe COVID, the need for hospitalization, mechanical ventilation, and risk of death.
“I personally have never had a hospitalized patient who’s been vaccinated,” Dr. Cross said. “The worst thing for the fetus is to have a critically ill mother.”
Dr. Cross, whose high-risk patients include those seeking counseling before IVF, added: “I would counsel all of them that they should absolutely get vaccinated prior to pregnancy, when they’re pregnant, whenever it is, as soon as they possibly can.”
The study authors and Dr. Cross report no relevant financial relationships.
Getting a COVID-19 mRNA vaccine did not affect pregnancy rates for women trying to conceive with in vitro fertilization or ovarian response to treatment, findings of a new study indicate.
The study was led by Sarit Avraham, MD, with the IVF unit, department of obstetrics and gynecology, Shamir Medical Center in Tzrifi, Israel. The findings were published online in Fertility and Sterility in a preproof version.
“Women should be vaccinated for COVID-19 prior to attempting to conceive via IVF treatments, given the higher risk of severe illness in pregnant women,” the authors wrote.
Doubts arose from “the theoretical concept of the supposed similarity between the SARS-CoV-2 spike protein and the syncytin protein that is speculated to take part in the fertilization process and the formation of the placenta,” the authors wrote.
Some then assumed that the COVID vaccine might kick off an immune response that could affect implantation and pregnancy. But this study and others before it found otherwise.
Researchers included 200 vaccinated women trying to conceive with IVF treatments in the retrospective study, and compared them with 200 unvaccinated patients of similar age (average age in both groups, 36 years) who were not previously infected with COVID-19. All the women were undergoing IVF from January to April 2021 and all the vaccinated women completed two doses of the BNT162b2 (Pfizer/BioNTech) vaccine at least 2 weeks before ovarian stimulation.
Researchers compared the average number of oocytes retrieved and clinical pregnancy rates between the two groups.
No difference between groups
Two hundred patients underwent oocyte retrieval 14-68 days after receiving a COVID shot; there was no significant difference by vaccination status in the number retrieved per cycle (10.63 in the vaccinated group vs. 10.72 in the unvaccinated group; P = .93).
There was also no difference in the clinical pregnancy rates after fresh embryo transfers. The rate among 128 vaccinated patients was 32.8% versus 33.1% in the 133 unvaccinated patients (P = .96), with 42 and 44 clinical pregnancies, respectively.
A total of 113 patients (66 in the study group and 47 in the controls) underwent freeze-all cycles to preserve fertility and fertilization rates were similar between vaccinated and unvaccinated (55.43% vaccinated vs. 54.29% unvaccinated; P = .73). The average number of cryopreserved embryos was 3.59 (vaccinated) versus 3.28 (unvaccinated) (P = .80).
In a subanalysis of outcomes by age, researchers found vaccination status had no effect on number of oocytes or pregnancy rates in the 39-and-older group. That’s important because it shows the vaccine did not affect outcomes even in a population with reduced ovarian reserves, the authors wrote.
The authors noted one of the study’s limitations is that it didn’t include information about vaccination or past infection status of the male partners.
Question should be put to rest
Sarah Cross, MD, a maternal-fetal medicine specialist at the University of Minnesota, Minneapolis, said the study is the biggest she’s seen that concludes COVID vaccinations are safe and highly encouraged for women before trying to conceive, but other smaller studies have come to the same conclusion.
She pointed to research including a study from 2021 with similar findings that concluded: “Physicians and public health personnel can counsel women of reproductive age that neither previous illness with COVID-19 nor antibodies produced from vaccination to COVID-19 will cause sterility.”
She said she thinks the question of whether COVID shots are safe with IVF has been answered and the results of the latest study add proof to counter misinformation around the issue.
“The COVID-19 vaccine does not affect fertility,” she said. “I don’t know how many more [studies] we need.”
The harm is in not getting vaccinated, she said. Pregnancy significantly increases a woman’s chance of getting severe COVID, the need for hospitalization, mechanical ventilation, and risk of death.
“I personally have never had a hospitalized patient who’s been vaccinated,” Dr. Cross said. “The worst thing for the fetus is to have a critically ill mother.”
Dr. Cross, whose high-risk patients include those seeking counseling before IVF, added: “I would counsel all of them that they should absolutely get vaccinated prior to pregnancy, when they’re pregnant, whenever it is, as soon as they possibly can.”
The study authors and Dr. Cross report no relevant financial relationships.
Getting a COVID-19 mRNA vaccine did not affect pregnancy rates for women trying to conceive with in vitro fertilization or ovarian response to treatment, findings of a new study indicate.
The study was led by Sarit Avraham, MD, with the IVF unit, department of obstetrics and gynecology, Shamir Medical Center in Tzrifi, Israel. The findings were published online in Fertility and Sterility in a preproof version.
“Women should be vaccinated for COVID-19 prior to attempting to conceive via IVF treatments, given the higher risk of severe illness in pregnant women,” the authors wrote.
Doubts arose from “the theoretical concept of the supposed similarity between the SARS-CoV-2 spike protein and the syncytin protein that is speculated to take part in the fertilization process and the formation of the placenta,” the authors wrote.
Some then assumed that the COVID vaccine might kick off an immune response that could affect implantation and pregnancy. But this study and others before it found otherwise.
Researchers included 200 vaccinated women trying to conceive with IVF treatments in the retrospective study, and compared them with 200 unvaccinated patients of similar age (average age in both groups, 36 years) who were not previously infected with COVID-19. All the women were undergoing IVF from January to April 2021 and all the vaccinated women completed two doses of the BNT162b2 (Pfizer/BioNTech) vaccine at least 2 weeks before ovarian stimulation.
Researchers compared the average number of oocytes retrieved and clinical pregnancy rates between the two groups.
No difference between groups
Two hundred patients underwent oocyte retrieval 14-68 days after receiving a COVID shot; there was no significant difference by vaccination status in the number retrieved per cycle (10.63 in the vaccinated group vs. 10.72 in the unvaccinated group; P = .93).
There was also no difference in the clinical pregnancy rates after fresh embryo transfers. The rate among 128 vaccinated patients was 32.8% versus 33.1% in the 133 unvaccinated patients (P = .96), with 42 and 44 clinical pregnancies, respectively.
A total of 113 patients (66 in the study group and 47 in the controls) underwent freeze-all cycles to preserve fertility and fertilization rates were similar between vaccinated and unvaccinated (55.43% vaccinated vs. 54.29% unvaccinated; P = .73). The average number of cryopreserved embryos was 3.59 (vaccinated) versus 3.28 (unvaccinated) (P = .80).
In a subanalysis of outcomes by age, researchers found vaccination status had no effect on number of oocytes or pregnancy rates in the 39-and-older group. That’s important because it shows the vaccine did not affect outcomes even in a population with reduced ovarian reserves, the authors wrote.
The authors noted one of the study’s limitations is that it didn’t include information about vaccination or past infection status of the male partners.
Question should be put to rest
Sarah Cross, MD, a maternal-fetal medicine specialist at the University of Minnesota, Minneapolis, said the study is the biggest she’s seen that concludes COVID vaccinations are safe and highly encouraged for women before trying to conceive, but other smaller studies have come to the same conclusion.
She pointed to research including a study from 2021 with similar findings that concluded: “Physicians and public health personnel can counsel women of reproductive age that neither previous illness with COVID-19 nor antibodies produced from vaccination to COVID-19 will cause sterility.”
She said she thinks the question of whether COVID shots are safe with IVF has been answered and the results of the latest study add proof to counter misinformation around the issue.
“The COVID-19 vaccine does not affect fertility,” she said. “I don’t know how many more [studies] we need.”
The harm is in not getting vaccinated, she said. Pregnancy significantly increases a woman’s chance of getting severe COVID, the need for hospitalization, mechanical ventilation, and risk of death.
“I personally have never had a hospitalized patient who’s been vaccinated,” Dr. Cross said. “The worst thing for the fetus is to have a critically ill mother.”
Dr. Cross, whose high-risk patients include those seeking counseling before IVF, added: “I would counsel all of them that they should absolutely get vaccinated prior to pregnancy, when they’re pregnant, whenever it is, as soon as they possibly can.”
The study authors and Dr. Cross report no relevant financial relationships.
FROM FERTILITY AND STERILITY
Enough is enough: the pandemic and loss of female oncologists
Imagine this: As a young girl, you decide you want to become a doctor when you grow up. You spend countless hours studying, researching, and volunteering to eventually make it into medical school. Four years later, you graduate top of your class and match into your first-choice residency program. You are so proud of yourself!
During your last year of residency, a pandemic takes the entire world by storm. You persevere through your last 14 months of residency that included additional time in the ICU, not seeing your colleagues, and interviewing for your new job all from your own living room. After all of this, you finally get to start doing what you have been waiting to do for the past decade: train with the brilliant minds in hematology and oncology.
All of a sudden, You start to question: If these incredible women have decided that the sacrifice this career requires is too much, then (1) How will I survive? and (2) Did I make a huge mistake in my career decision? Spoiler alert: This girl is me.
The World Health Organization defines burnout as a “syndrome conceptualized as resulting from chronic workplace stress that has not been successfully managed. It is characterized by energy depletion or exhaustion, increased mental distance from one’s job, and reduced professional efficacy.”
We know that 33% of oncologists are feeling burned out right now, according to the Medscape National Physician Burnout & Suicide Report 2021. Of the 51% of female physicians that are burned out, work-life balance has been identified as the biggest workplace concern to them. Research has shown that hours per week devoted to direct patient care is the dominant predictor of burnout for practicing oncologists. But in academic oncology, that is followed by grant deadlines, manuscript rejections, and the constant reminders that you are a new face in oncology, a specialty that was previously male-dominated.
In less than a year, we have had several key female oncologists leave our cancer center. While some made the decision to retire early, two of them chose to pivot their careers and leave clinical medicine to assist with drug development and clinical trials. Although this is extremely important work for cancer care, I was shocked to hear that these amazing and successful clinicians were choosing to remove all direct patient care from their practice, when for many of them, patient care was what motivated them to pursue medicine in the first place. They were loved by their patients, respected as researchers, and well known as educators within the division.
One shared that she no longer felt like she could be a good mother, wife, or daughter with what was currently being demanded of her to have a successful academic career. In hearing this news, I was saddened to have to say goodbye to a mentor of mine and immediately started second-guessing my career choice. I felt that my goal of having an impactful career and prosperous home life was not only unattainable but potentially unrealistic.
While we know that female physicians already experience a greater degree of burnout, the pandemic has only added fuel to the fire. This is especially true in cancer care. It has been estimated that new cancer diagnosis have decreased by as much as 23% since the beginning of the pandemic. This delay in diagnosis will lead to patients presenting with more advanced disease, busier clinic schedules, and worsened clinical outcomes for years to come. With no end in sight, I worry what this will mean for women currently in oncology, in addition to those in training or deciding if they should pursue this as a career.
Extrapolating evidence from prior epidemics, physicians are at increased risk for burnout due to immediate and long-term effects from this pandemic. We need to act now to not only continue addressing previously existing individual and organizational causes of burnout but also develop strategies to provide support for the COVID-19–specific impacts on oncologists’ well-being. An editorial published by the American Society of Clinical Oncology provides helpful suggestions on how to do this.
A recent cross-sectional survey found that 22% of academic female oncologists were likely or very likely to pursue a career outside of academia in the next 5 years. Losing these women would be detrimental to the field. This would mean a significant number of patients losing their long-term oncologists with whom they have years of care, trainees losing their professional and research mentors to guide and help mold them into successful independent practitioners and researchers, and arguably most important, little girls losing role models to show them that regardless of their gender, they can become an oncologist.Dr. Poterala is a current hematology and oncology fellow at the University of Wisconsin Carbone Cancer Center, Madison. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Imagine this: As a young girl, you decide you want to become a doctor when you grow up. You spend countless hours studying, researching, and volunteering to eventually make it into medical school. Four years later, you graduate top of your class and match into your first-choice residency program. You are so proud of yourself!
During your last year of residency, a pandemic takes the entire world by storm. You persevere through your last 14 months of residency that included additional time in the ICU, not seeing your colleagues, and interviewing for your new job all from your own living room. After all of this, you finally get to start doing what you have been waiting to do for the past decade: train with the brilliant minds in hematology and oncology.
All of a sudden, You start to question: If these incredible women have decided that the sacrifice this career requires is too much, then (1) How will I survive? and (2) Did I make a huge mistake in my career decision? Spoiler alert: This girl is me.
The World Health Organization defines burnout as a “syndrome conceptualized as resulting from chronic workplace stress that has not been successfully managed. It is characterized by energy depletion or exhaustion, increased mental distance from one’s job, and reduced professional efficacy.”
We know that 33% of oncologists are feeling burned out right now, according to the Medscape National Physician Burnout & Suicide Report 2021. Of the 51% of female physicians that are burned out, work-life balance has been identified as the biggest workplace concern to them. Research has shown that hours per week devoted to direct patient care is the dominant predictor of burnout for practicing oncologists. But in academic oncology, that is followed by grant deadlines, manuscript rejections, and the constant reminders that you are a new face in oncology, a specialty that was previously male-dominated.
In less than a year, we have had several key female oncologists leave our cancer center. While some made the decision to retire early, two of them chose to pivot their careers and leave clinical medicine to assist with drug development and clinical trials. Although this is extremely important work for cancer care, I was shocked to hear that these amazing and successful clinicians were choosing to remove all direct patient care from their practice, when for many of them, patient care was what motivated them to pursue medicine in the first place. They were loved by their patients, respected as researchers, and well known as educators within the division.
One shared that she no longer felt like she could be a good mother, wife, or daughter with what was currently being demanded of her to have a successful academic career. In hearing this news, I was saddened to have to say goodbye to a mentor of mine and immediately started second-guessing my career choice. I felt that my goal of having an impactful career and prosperous home life was not only unattainable but potentially unrealistic.
While we know that female physicians already experience a greater degree of burnout, the pandemic has only added fuel to the fire. This is especially true in cancer care. It has been estimated that new cancer diagnosis have decreased by as much as 23% since the beginning of the pandemic. This delay in diagnosis will lead to patients presenting with more advanced disease, busier clinic schedules, and worsened clinical outcomes for years to come. With no end in sight, I worry what this will mean for women currently in oncology, in addition to those in training or deciding if they should pursue this as a career.
Extrapolating evidence from prior epidemics, physicians are at increased risk for burnout due to immediate and long-term effects from this pandemic. We need to act now to not only continue addressing previously existing individual and organizational causes of burnout but also develop strategies to provide support for the COVID-19–specific impacts on oncologists’ well-being. An editorial published by the American Society of Clinical Oncology provides helpful suggestions on how to do this.
A recent cross-sectional survey found that 22% of academic female oncologists were likely or very likely to pursue a career outside of academia in the next 5 years. Losing these women would be detrimental to the field. This would mean a significant number of patients losing their long-term oncologists with whom they have years of care, trainees losing their professional and research mentors to guide and help mold them into successful independent practitioners and researchers, and arguably most important, little girls losing role models to show them that regardless of their gender, they can become an oncologist.Dr. Poterala is a current hematology and oncology fellow at the University of Wisconsin Carbone Cancer Center, Madison. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Imagine this: As a young girl, you decide you want to become a doctor when you grow up. You spend countless hours studying, researching, and volunteering to eventually make it into medical school. Four years later, you graduate top of your class and match into your first-choice residency program. You are so proud of yourself!
During your last year of residency, a pandemic takes the entire world by storm. You persevere through your last 14 months of residency that included additional time in the ICU, not seeing your colleagues, and interviewing for your new job all from your own living room. After all of this, you finally get to start doing what you have been waiting to do for the past decade: train with the brilliant minds in hematology and oncology.
All of a sudden, You start to question: If these incredible women have decided that the sacrifice this career requires is too much, then (1) How will I survive? and (2) Did I make a huge mistake in my career decision? Spoiler alert: This girl is me.
The World Health Organization defines burnout as a “syndrome conceptualized as resulting from chronic workplace stress that has not been successfully managed. It is characterized by energy depletion or exhaustion, increased mental distance from one’s job, and reduced professional efficacy.”
We know that 33% of oncologists are feeling burned out right now, according to the Medscape National Physician Burnout & Suicide Report 2021. Of the 51% of female physicians that are burned out, work-life balance has been identified as the biggest workplace concern to them. Research has shown that hours per week devoted to direct patient care is the dominant predictor of burnout for practicing oncologists. But in academic oncology, that is followed by grant deadlines, manuscript rejections, and the constant reminders that you are a new face in oncology, a specialty that was previously male-dominated.
In less than a year, we have had several key female oncologists leave our cancer center. While some made the decision to retire early, two of them chose to pivot their careers and leave clinical medicine to assist with drug development and clinical trials. Although this is extremely important work for cancer care, I was shocked to hear that these amazing and successful clinicians were choosing to remove all direct patient care from their practice, when for many of them, patient care was what motivated them to pursue medicine in the first place. They were loved by their patients, respected as researchers, and well known as educators within the division.
One shared that she no longer felt like she could be a good mother, wife, or daughter with what was currently being demanded of her to have a successful academic career. In hearing this news, I was saddened to have to say goodbye to a mentor of mine and immediately started second-guessing my career choice. I felt that my goal of having an impactful career and prosperous home life was not only unattainable but potentially unrealistic.
While we know that female physicians already experience a greater degree of burnout, the pandemic has only added fuel to the fire. This is especially true in cancer care. It has been estimated that new cancer diagnosis have decreased by as much as 23% since the beginning of the pandemic. This delay in diagnosis will lead to patients presenting with more advanced disease, busier clinic schedules, and worsened clinical outcomes for years to come. With no end in sight, I worry what this will mean for women currently in oncology, in addition to those in training or deciding if they should pursue this as a career.
Extrapolating evidence from prior epidemics, physicians are at increased risk for burnout due to immediate and long-term effects from this pandemic. We need to act now to not only continue addressing previously existing individual and organizational causes of burnout but also develop strategies to provide support for the COVID-19–specific impacts on oncologists’ well-being. An editorial published by the American Society of Clinical Oncology provides helpful suggestions on how to do this.
A recent cross-sectional survey found that 22% of academic female oncologists were likely or very likely to pursue a career outside of academia in the next 5 years. Losing these women would be detrimental to the field. This would mean a significant number of patients losing their long-term oncologists with whom they have years of care, trainees losing their professional and research mentors to guide and help mold them into successful independent practitioners and researchers, and arguably most important, little girls losing role models to show them that regardless of their gender, they can become an oncologist.Dr. Poterala is a current hematology and oncology fellow at the University of Wisconsin Carbone Cancer Center, Madison. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
At-home geriatric assessment offers cost-effective alternative to hospital
The comprehensive geriatric assessment (CGA) is an established strategy for guiding care of older adults in a hospital setting, but its use in other settings has not been well studied, Surya Singh, PhD, of the University of Oxford (England), and colleagues wrote in their paper published in Age and Ageing. Hospital at home is active treatment by health care professionals in the patient’s home for a condition that otherwise would require acute hospital inpatient care, for a limited time period.
Interest in providing health care in the home as an alternative to hospitalization is on the rise as a way to improve patient outcomes and reduce costs, but actual cost-effectiveness data on HAH interventions are limited, the authors said. “Wide scale implementation of such services has also been constrained by the practical difficulties of designing and delivering services that cut across primary and secondary care, might involve social care and require different workforce and funding arrangements.”
In this study, the researchers conducted a cost-effectiveness analysis alongside a randomized trial of an admission avoidance CGA hospital at home (CGAHAH) service as an alternative to hospital admission. They identified individuals aged 65 years and older who were living in the community but being considered for an unplanned hospital admission in the United Kingdom. A total of 700 individuals were randomized to CGAHAH and 355 to hospital care using a 2:1 ratio. Patients were assessed at baseline in the community or in an acute care setting before being transferred to CGAHAH service. These services included access to social workers, home care, district nursing, community rehabilitation, community mental health services and acute hospital services, such as diagnostic tests and transfer to hospital. The core workforce usually included consultant geriatricians, junior doctors, nurse practitioners, health care assistants or support workers, physiotherapists, occupational therapists, and community pharmacists. There were at least daily virtual ward rounds
Comparison between HAH and in-hospital groups
Patients in the CGAHAH group had a mean of 7.17 days of care, and those in hospital had a mean of 4.92 hospital days. At 6 months’ follow-up, the mean number of care days was 9.47 in the CGAHAH group and 10.58 in the hospital group, which was a nonsignificant difference.
“For complete cases, we found that allocation to CGAHAH resulted in 3 fewer days in hospital, a difference that was reduced to 1 day at 6 months follow-up,” the researchers wrote.
Overall, after adjusting for baseline variables, the health and social care costs after 6 months were less for CGAHAH than admission to hospital. The average cost differences between the two were approximately $3,000 or 2,265 pounds. The cost difference remained and increased to a mean difference of 2,840 pounds in favor of HAH after adding informal care/societal costs.
In addition, patients randomized to CGAHAH were less likely to have been admitted to long-term residential care at 6 months follow-up, compared with the hospital group; the mean days in residential care at 6 months were 3.43 and 6.14, respectively.
Both groups showed an approximate 15% decrease in measures of quality of life from baseline to 6 months, and no differences were noted in quality-adjusted survival between the groups.
Pandemic ‘has accelerated interest’ in HAH
“Health systems around the world are exploring alternatives to hospital admission, such as hospital at home, to act as a buffer to the increasing demand for hospital care,” corresponding author Sasha Shepperd, MSc, DPhil, said in an interview. “This is partly due to a growing older population with increased health needs, but also an emphasis on providing health care that limits a decline in capacity for the older population. Inevitably, the COVID-19 pandemic has accelerated interest in hospital at home to create additional acute health care capacity.”
The take home-message supports the home service option. “If you can access a hospital-at-home service, consider this as an option for older people who would otherwise be admitted to hospital and are eligible for hospital at home care. However, is important that the provision of hospital at home is adequately resourced, and that families and caregivers are supported,” she said.
“Barriers include delivering a different type of service that requires easy access to hospital services, including admission if required; a trained workforce to provide multidisciplinary care in a patient’s home; and ensuring a good fit with existing health and social care services,” Dr. Shepperd said.
Future research areas include the demands placed on caregivers from hospital-at-home services, and how the provision of hospital at home impacts hospital and community services, she added.
Findings support use of HAH
The data from the current study support the use of a hospital at home concept, especially in the geriatric age population, for acute health conditions that could be managed at home rather than acutely in a hospital-based environment,” Noel Deep, MD, emphasized in an interview.
Dr. Deep, who is a general internist in group practice in Antigo, Wisc., said he was not surprised by the study findings.
“I am a big proponent of the hospital at home approach to taking care of patients who can be safely and appropriately managed in the familiarity and comfort of their own home environment with help from physicians, nurses, and other home health care services,” he said. “It is a valuable option for appropriately screened and selected patients to be provided this approach to management of their acute health care situations.”
Primary care physicians should explore using HAH when faced with the decision of admitting an elderly individual to the hospital for management of an acute worsening of a chronic medical condition or a reversible acute illness, said Dr. Deep, who serves on the editorial advisory board of Internal Medicine News.
The current study reinforces previous studies and data showing the benefits of managing acute health problems of elderly individuals in their home environment. These benefits include “an opportunity to free up the emergency rooms and hospitals for providing care to those individuals who truly would be best served by being admitted to the hospital,” Dr. Deep explained. Home care for the elderly “would also lead to decreased utilization of the personal protective equipment and limit exposure of the vulnerable elderly individuals to the coronavirus. Primary care physicians should always explore this possibility of providing care to the patients in their homes if it is a viable option.
“While our practice environment [in the United States] is slightly different than that referenced in this article, many, if not almost all, of our primary care physicians provide care to the geriatric age population and provide assessment and management which would be comparable to this comprehensive geriatric assessment that is discussed in the article,” and many primary care physicians have seen similar results in outcomes that the study shows, said Dr. Deep. The available research and expert opinions are quite similar and agree upon the positive outcomes in terms of providing the CGAHAH approach.
Study is important but raises questions
The study is important because patient-centered, effective care should be the goal of any health system, William Golden, MD, of the University of Arkansas for Medical Sciences, Little Rock, said in an interview.
Dr. Golden also noted that the study raised a number of questions. How each patient entered the treatment protocol was not clear. “Similarly, it is not clear whether admission criteria and resource costs in England cross to the United States experience.”
“Having close follow up of patients at home as opposed to an ‘observation status’ could be a nice innovation, but more details are needed to consider implementation in a specific community setting,” he emphasized.
As for the clinical value of the study for primary care, “primary care professionals should welcome well-staffed alternatives to inpatient care for select patient presentations,” said Dr. Golden, who is also a member of the editorial advisory board of Internal Medicine News.
The current study does not identify the conditions that were treated at home and the logistics of delivering such services, which limits comparison with what experts have seen in practice in terms of outcomes using the CGAHAH, he said. “Interested practitioners would benefit from literature detailing the staffing and decision support tools that form the core framework of this innovation.”
Limitations and strengths of study, according to authors
The study findings were limited by several factors including the calculation of CGAHAH based on service budgets, rather than from collecting information on the actual resources used; potential errors in patients’ estimation of their informal care; and lack of data on a differential impact of CGAHAH for underserved communities, the researchers noted.
However, the results were strengthened by the large study population and randomized design, and support the value of CGAHAH, which addresses the need for management of multiple long-term conditions and the potential decline in functional and cognitive ability in older adults, they said. Providing CGAHAH as an alternative to admission to hospital for older people, with a focus on multidimensional assessment, is one option that might reduce reliance on hospitalization and residential care and at a lower cost.
The study was supported by the National Institute for Health Research, and several coauthors received individual grants from the NIHR, with no other financial conflicts to disclose. Dr. Golden and Dr. Deep had no financial conflicts to disclose.
The comprehensive geriatric assessment (CGA) is an established strategy for guiding care of older adults in a hospital setting, but its use in other settings has not been well studied, Surya Singh, PhD, of the University of Oxford (England), and colleagues wrote in their paper published in Age and Ageing. Hospital at home is active treatment by health care professionals in the patient’s home for a condition that otherwise would require acute hospital inpatient care, for a limited time period.
Interest in providing health care in the home as an alternative to hospitalization is on the rise as a way to improve patient outcomes and reduce costs, but actual cost-effectiveness data on HAH interventions are limited, the authors said. “Wide scale implementation of such services has also been constrained by the practical difficulties of designing and delivering services that cut across primary and secondary care, might involve social care and require different workforce and funding arrangements.”
In this study, the researchers conducted a cost-effectiveness analysis alongside a randomized trial of an admission avoidance CGA hospital at home (CGAHAH) service as an alternative to hospital admission. They identified individuals aged 65 years and older who were living in the community but being considered for an unplanned hospital admission in the United Kingdom. A total of 700 individuals were randomized to CGAHAH and 355 to hospital care using a 2:1 ratio. Patients were assessed at baseline in the community or in an acute care setting before being transferred to CGAHAH service. These services included access to social workers, home care, district nursing, community rehabilitation, community mental health services and acute hospital services, such as diagnostic tests and transfer to hospital. The core workforce usually included consultant geriatricians, junior doctors, nurse practitioners, health care assistants or support workers, physiotherapists, occupational therapists, and community pharmacists. There were at least daily virtual ward rounds
Comparison between HAH and in-hospital groups
Patients in the CGAHAH group had a mean of 7.17 days of care, and those in hospital had a mean of 4.92 hospital days. At 6 months’ follow-up, the mean number of care days was 9.47 in the CGAHAH group and 10.58 in the hospital group, which was a nonsignificant difference.
“For complete cases, we found that allocation to CGAHAH resulted in 3 fewer days in hospital, a difference that was reduced to 1 day at 6 months follow-up,” the researchers wrote.
Overall, after adjusting for baseline variables, the health and social care costs after 6 months were less for CGAHAH than admission to hospital. The average cost differences between the two were approximately $3,000 or 2,265 pounds. The cost difference remained and increased to a mean difference of 2,840 pounds in favor of HAH after adding informal care/societal costs.
In addition, patients randomized to CGAHAH were less likely to have been admitted to long-term residential care at 6 months follow-up, compared with the hospital group; the mean days in residential care at 6 months were 3.43 and 6.14, respectively.
Both groups showed an approximate 15% decrease in measures of quality of life from baseline to 6 months, and no differences were noted in quality-adjusted survival between the groups.
Pandemic ‘has accelerated interest’ in HAH
“Health systems around the world are exploring alternatives to hospital admission, such as hospital at home, to act as a buffer to the increasing demand for hospital care,” corresponding author Sasha Shepperd, MSc, DPhil, said in an interview. “This is partly due to a growing older population with increased health needs, but also an emphasis on providing health care that limits a decline in capacity for the older population. Inevitably, the COVID-19 pandemic has accelerated interest in hospital at home to create additional acute health care capacity.”
The take home-message supports the home service option. “If you can access a hospital-at-home service, consider this as an option for older people who would otherwise be admitted to hospital and are eligible for hospital at home care. However, is important that the provision of hospital at home is adequately resourced, and that families and caregivers are supported,” she said.
“Barriers include delivering a different type of service that requires easy access to hospital services, including admission if required; a trained workforce to provide multidisciplinary care in a patient’s home; and ensuring a good fit with existing health and social care services,” Dr. Shepperd said.
Future research areas include the demands placed on caregivers from hospital-at-home services, and how the provision of hospital at home impacts hospital and community services, she added.
Findings support use of HAH
The data from the current study support the use of a hospital at home concept, especially in the geriatric age population, for acute health conditions that could be managed at home rather than acutely in a hospital-based environment,” Noel Deep, MD, emphasized in an interview.
Dr. Deep, who is a general internist in group practice in Antigo, Wisc., said he was not surprised by the study findings.
“I am a big proponent of the hospital at home approach to taking care of patients who can be safely and appropriately managed in the familiarity and comfort of their own home environment with help from physicians, nurses, and other home health care services,” he said. “It is a valuable option for appropriately screened and selected patients to be provided this approach to management of their acute health care situations.”
Primary care physicians should explore using HAH when faced with the decision of admitting an elderly individual to the hospital for management of an acute worsening of a chronic medical condition or a reversible acute illness, said Dr. Deep, who serves on the editorial advisory board of Internal Medicine News.
The current study reinforces previous studies and data showing the benefits of managing acute health problems of elderly individuals in their home environment. These benefits include “an opportunity to free up the emergency rooms and hospitals for providing care to those individuals who truly would be best served by being admitted to the hospital,” Dr. Deep explained. Home care for the elderly “would also lead to decreased utilization of the personal protective equipment and limit exposure of the vulnerable elderly individuals to the coronavirus. Primary care physicians should always explore this possibility of providing care to the patients in their homes if it is a viable option.
“While our practice environment [in the United States] is slightly different than that referenced in this article, many, if not almost all, of our primary care physicians provide care to the geriatric age population and provide assessment and management which would be comparable to this comprehensive geriatric assessment that is discussed in the article,” and many primary care physicians have seen similar results in outcomes that the study shows, said Dr. Deep. The available research and expert opinions are quite similar and agree upon the positive outcomes in terms of providing the CGAHAH approach.
Study is important but raises questions
The study is important because patient-centered, effective care should be the goal of any health system, William Golden, MD, of the University of Arkansas for Medical Sciences, Little Rock, said in an interview.
Dr. Golden also noted that the study raised a number of questions. How each patient entered the treatment protocol was not clear. “Similarly, it is not clear whether admission criteria and resource costs in England cross to the United States experience.”
“Having close follow up of patients at home as opposed to an ‘observation status’ could be a nice innovation, but more details are needed to consider implementation in a specific community setting,” he emphasized.
As for the clinical value of the study for primary care, “primary care professionals should welcome well-staffed alternatives to inpatient care for select patient presentations,” said Dr. Golden, who is also a member of the editorial advisory board of Internal Medicine News.
The current study does not identify the conditions that were treated at home and the logistics of delivering such services, which limits comparison with what experts have seen in practice in terms of outcomes using the CGAHAH, he said. “Interested practitioners would benefit from literature detailing the staffing and decision support tools that form the core framework of this innovation.”
Limitations and strengths of study, according to authors
The study findings were limited by several factors including the calculation of CGAHAH based on service budgets, rather than from collecting information on the actual resources used; potential errors in patients’ estimation of their informal care; and lack of data on a differential impact of CGAHAH for underserved communities, the researchers noted.
However, the results were strengthened by the large study population and randomized design, and support the value of CGAHAH, which addresses the need for management of multiple long-term conditions and the potential decline in functional and cognitive ability in older adults, they said. Providing CGAHAH as an alternative to admission to hospital for older people, with a focus on multidimensional assessment, is one option that might reduce reliance on hospitalization and residential care and at a lower cost.
The study was supported by the National Institute for Health Research, and several coauthors received individual grants from the NIHR, with no other financial conflicts to disclose. Dr. Golden and Dr. Deep had no financial conflicts to disclose.
The comprehensive geriatric assessment (CGA) is an established strategy for guiding care of older adults in a hospital setting, but its use in other settings has not been well studied, Surya Singh, PhD, of the University of Oxford (England), and colleagues wrote in their paper published in Age and Ageing. Hospital at home is active treatment by health care professionals in the patient’s home for a condition that otherwise would require acute hospital inpatient care, for a limited time period.
Interest in providing health care in the home as an alternative to hospitalization is on the rise as a way to improve patient outcomes and reduce costs, but actual cost-effectiveness data on HAH interventions are limited, the authors said. “Wide scale implementation of such services has also been constrained by the practical difficulties of designing and delivering services that cut across primary and secondary care, might involve social care and require different workforce and funding arrangements.”
In this study, the researchers conducted a cost-effectiveness analysis alongside a randomized trial of an admission avoidance CGA hospital at home (CGAHAH) service as an alternative to hospital admission. They identified individuals aged 65 years and older who were living in the community but being considered for an unplanned hospital admission in the United Kingdom. A total of 700 individuals were randomized to CGAHAH and 355 to hospital care using a 2:1 ratio. Patients were assessed at baseline in the community or in an acute care setting before being transferred to CGAHAH service. These services included access to social workers, home care, district nursing, community rehabilitation, community mental health services and acute hospital services, such as diagnostic tests and transfer to hospital. The core workforce usually included consultant geriatricians, junior doctors, nurse practitioners, health care assistants or support workers, physiotherapists, occupational therapists, and community pharmacists. There were at least daily virtual ward rounds
Comparison between HAH and in-hospital groups
Patients in the CGAHAH group had a mean of 7.17 days of care, and those in hospital had a mean of 4.92 hospital days. At 6 months’ follow-up, the mean number of care days was 9.47 in the CGAHAH group and 10.58 in the hospital group, which was a nonsignificant difference.
“For complete cases, we found that allocation to CGAHAH resulted in 3 fewer days in hospital, a difference that was reduced to 1 day at 6 months follow-up,” the researchers wrote.
Overall, after adjusting for baseline variables, the health and social care costs after 6 months were less for CGAHAH than admission to hospital. The average cost differences between the two were approximately $3,000 or 2,265 pounds. The cost difference remained and increased to a mean difference of 2,840 pounds in favor of HAH after adding informal care/societal costs.
In addition, patients randomized to CGAHAH were less likely to have been admitted to long-term residential care at 6 months follow-up, compared with the hospital group; the mean days in residential care at 6 months were 3.43 and 6.14, respectively.
Both groups showed an approximate 15% decrease in measures of quality of life from baseline to 6 months, and no differences were noted in quality-adjusted survival between the groups.
Pandemic ‘has accelerated interest’ in HAH
“Health systems around the world are exploring alternatives to hospital admission, such as hospital at home, to act as a buffer to the increasing demand for hospital care,” corresponding author Sasha Shepperd, MSc, DPhil, said in an interview. “This is partly due to a growing older population with increased health needs, but also an emphasis on providing health care that limits a decline in capacity for the older population. Inevitably, the COVID-19 pandemic has accelerated interest in hospital at home to create additional acute health care capacity.”
The take home-message supports the home service option. “If you can access a hospital-at-home service, consider this as an option for older people who would otherwise be admitted to hospital and are eligible for hospital at home care. However, is important that the provision of hospital at home is adequately resourced, and that families and caregivers are supported,” she said.
“Barriers include delivering a different type of service that requires easy access to hospital services, including admission if required; a trained workforce to provide multidisciplinary care in a patient’s home; and ensuring a good fit with existing health and social care services,” Dr. Shepperd said.
Future research areas include the demands placed on caregivers from hospital-at-home services, and how the provision of hospital at home impacts hospital and community services, she added.
Findings support use of HAH
The data from the current study support the use of a hospital at home concept, especially in the geriatric age population, for acute health conditions that could be managed at home rather than acutely in a hospital-based environment,” Noel Deep, MD, emphasized in an interview.
Dr. Deep, who is a general internist in group practice in Antigo, Wisc., said he was not surprised by the study findings.
“I am a big proponent of the hospital at home approach to taking care of patients who can be safely and appropriately managed in the familiarity and comfort of their own home environment with help from physicians, nurses, and other home health care services,” he said. “It is a valuable option for appropriately screened and selected patients to be provided this approach to management of their acute health care situations.”
Primary care physicians should explore using HAH when faced with the decision of admitting an elderly individual to the hospital for management of an acute worsening of a chronic medical condition or a reversible acute illness, said Dr. Deep, who serves on the editorial advisory board of Internal Medicine News.
The current study reinforces previous studies and data showing the benefits of managing acute health problems of elderly individuals in their home environment. These benefits include “an opportunity to free up the emergency rooms and hospitals for providing care to those individuals who truly would be best served by being admitted to the hospital,” Dr. Deep explained. Home care for the elderly “would also lead to decreased utilization of the personal protective equipment and limit exposure of the vulnerable elderly individuals to the coronavirus. Primary care physicians should always explore this possibility of providing care to the patients in their homes if it is a viable option.
“While our practice environment [in the United States] is slightly different than that referenced in this article, many, if not almost all, of our primary care physicians provide care to the geriatric age population and provide assessment and management which would be comparable to this comprehensive geriatric assessment that is discussed in the article,” and many primary care physicians have seen similar results in outcomes that the study shows, said Dr. Deep. The available research and expert opinions are quite similar and agree upon the positive outcomes in terms of providing the CGAHAH approach.
Study is important but raises questions
The study is important because patient-centered, effective care should be the goal of any health system, William Golden, MD, of the University of Arkansas for Medical Sciences, Little Rock, said in an interview.
Dr. Golden also noted that the study raised a number of questions. How each patient entered the treatment protocol was not clear. “Similarly, it is not clear whether admission criteria and resource costs in England cross to the United States experience.”
“Having close follow up of patients at home as opposed to an ‘observation status’ could be a nice innovation, but more details are needed to consider implementation in a specific community setting,” he emphasized.
As for the clinical value of the study for primary care, “primary care professionals should welcome well-staffed alternatives to inpatient care for select patient presentations,” said Dr. Golden, who is also a member of the editorial advisory board of Internal Medicine News.
The current study does not identify the conditions that were treated at home and the logistics of delivering such services, which limits comparison with what experts have seen in practice in terms of outcomes using the CGAHAH, he said. “Interested practitioners would benefit from literature detailing the staffing and decision support tools that form the core framework of this innovation.”
Limitations and strengths of study, according to authors
The study findings were limited by several factors including the calculation of CGAHAH based on service budgets, rather than from collecting information on the actual resources used; potential errors in patients’ estimation of their informal care; and lack of data on a differential impact of CGAHAH for underserved communities, the researchers noted.
However, the results were strengthened by the large study population and randomized design, and support the value of CGAHAH, which addresses the need for management of multiple long-term conditions and the potential decline in functional and cognitive ability in older adults, they said. Providing CGAHAH as an alternative to admission to hospital for older people, with a focus on multidimensional assessment, is one option that might reduce reliance on hospitalization and residential care and at a lower cost.
The study was supported by the National Institute for Health Research, and several coauthors received individual grants from the NIHR, with no other financial conflicts to disclose. Dr. Golden and Dr. Deep had no financial conflicts to disclose.
FROM AGE AND AGEING
Confusing messages on COVID taking a psychological toll
The Centers for Disease Control and Prevention’s decision to shorten the length of isolation time for asymptomatic Americans with COVID-19, regardless of their vaccination status, to 5 days from 10 days is confusing. I hope the agency reconsiders this decision.
After all, one of the CDC’s key messages during this pandemic has been that even people with asymptomatic COVID who have been vaccinated and boosted can transmit the disease. So it seems to me that the Dec. 27, 2021, recommendation about shortening the isolation time for COVID-19–positive people, like the agency’s earlier guidance encouraging people who are vaccinated to stop wearing masks while in indoor settings, runs contrary to good public health principles.
As an expert in human behavior, I am worried about the impact of these confusing messages on the psyche of people in general, as well as on our patients.
Mental health impact
Soon after the United States went on lockdown in March 2020, I wrote about the likelihood of a pandemic of PTSD, anxiety, and depression that would occur in the wake of rising COVID-19 rates. Well, it happened.
Many people have felt a sense of existential despair, depression, and anxiety. As we head into year No. 3 of disruption of our daily lives – and face the loss of more than 825,000 Americans to COVID – we continue to navigate this uncertainty. And now we must deal with Omicron, a variant that is so highly transmissible that it is apparently able to, in some cases, evade two-dose regimens of mRNA vaccines, boosters, and immunity from past infections, according to a report from Imperial College London. Yet, we are being told by some that Omicron might be less severe, compared with other variants. I worry that this assessment is misleading. In that same report, the Imperial College said it “found no evidence” that Omicron is less virulent than Delta, based on the risk of hospitalization and symptom status.
Meanwhile, animal studies suggest that the Omicron variant might lead to less lung damage than previous variants. A preprint article that is being considered for publication by a Nature Portfolio journal suggests that hamsters and mice infected with the Omicron variant do not have as much lung damage as those infected with other variants. More data need to come in for us to get a true understanding of Omicron’s virulence and transmissibility. We should keep an eye on Israel, which is launching a clinical trial of a second booster, or fourth mRNA shot.
As clinicians, we should give our patients and other people with whom we come in contact a sense of hope. In addition to urging people to get boosters, let’s tell them to err on the side of safety when it comes to this pandemic. That means encouraging them to remain isolated for longer than 5 days – until they test negative for COVID. It also means encouraging patients to wear high-quality face masks while inside public spaces – even in the absence of mandates. I have found it heartbreaking to watch televised broadcasts of sporting events held at some stadiums across the country where masks are not being worn. This absence of face coverings is counterintuitive at a time when some Broadway shows are closing. Even the great Radio City Rockettes shut down their holiday shows early in December 2021 because of COVID.
And, as I’ve argued before, we must not give up on unvaccinated people. I have had success in changing the minds of a few patients and some acquaintances with gentle, respectful prodding and vaccine education.
I would also like to see public health principles implemented in our schools and colleges. To protect the health of our children and young adults, we must continue to be nimble – which means school districts should implement layered prevention strategies, as the CDC recommends. This includes not only encouraging eligible staff members and students to get vaccinated, but requiring face masks inside school facilities, maintaining a physical distance of at least 3 feet, “screening testing, ventilation, handwashing, and staying home when sick.”
Furthermore, in deciding whether schools should remain open or be closed after positive COVID cases are discovered, officials should look at the vaccine demographics of that particular school. For example, if 15% of students are vaccinated in one school and 70% are vaccinated in another, the judgment would be different. Of course, it’s clearly best for schools to remain open, but perhaps closing them temporarily – perhaps for a week or 10 days – should be on the table if infection rates reach a certain level.
Now that we know more and have the benefit of getting more than 200 million Americans fully vaccinated, we can be far more selective about closings and openings. An important part of our strategy must be to communicate honestly with the public about which measures are best for safety. As a key tenet of cognitive-behavioral therapy tells us, “all-or-nothing” thinking is not productive. That should also be the case with our approach to managing COVID-19.
We don’t know the future of the pandemic. Yes, it will end, and possibly COVID will become endemic – like the flu. However, in the meantime, in addition to promoting vaccinations and boosters, we must rigorously encourage our patients to follow public health standards of masking, social distancing, and closing down businesses – and schools – temporarily.
This pandemic has taken a horrendous mental health toll on all of us – especially our patients and frontline health care workers. I’ve spoken with numerous people who were anxious, depressed, and showed signs of PTSD in early 2020; after they got vaccinated, COVID spread diminished, and as public health protocols began to lift, so did their spirits. Clearly for some, the benefit of psychiatric/psychological care centering on the pandemic has proven invaluable. In some ways, the pandemic has brought to the surface the importance of mental health care and removed some of the stigma from mental illness. And that’s a good thing.
Dr. London is a practicing psychiatrist who has been a newspaper columnist for 35 years, specializing in writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
The Centers for Disease Control and Prevention’s decision to shorten the length of isolation time for asymptomatic Americans with COVID-19, regardless of their vaccination status, to 5 days from 10 days is confusing. I hope the agency reconsiders this decision.
After all, one of the CDC’s key messages during this pandemic has been that even people with asymptomatic COVID who have been vaccinated and boosted can transmit the disease. So it seems to me that the Dec. 27, 2021, recommendation about shortening the isolation time for COVID-19–positive people, like the agency’s earlier guidance encouraging people who are vaccinated to stop wearing masks while in indoor settings, runs contrary to good public health principles.
As an expert in human behavior, I am worried about the impact of these confusing messages on the psyche of people in general, as well as on our patients.
Mental health impact
Soon after the United States went on lockdown in March 2020, I wrote about the likelihood of a pandemic of PTSD, anxiety, and depression that would occur in the wake of rising COVID-19 rates. Well, it happened.
Many people have felt a sense of existential despair, depression, and anxiety. As we head into year No. 3 of disruption of our daily lives – and face the loss of more than 825,000 Americans to COVID – we continue to navigate this uncertainty. And now we must deal with Omicron, a variant that is so highly transmissible that it is apparently able to, in some cases, evade two-dose regimens of mRNA vaccines, boosters, and immunity from past infections, according to a report from Imperial College London. Yet, we are being told by some that Omicron might be less severe, compared with other variants. I worry that this assessment is misleading. In that same report, the Imperial College said it “found no evidence” that Omicron is less virulent than Delta, based on the risk of hospitalization and symptom status.
Meanwhile, animal studies suggest that the Omicron variant might lead to less lung damage than previous variants. A preprint article that is being considered for publication by a Nature Portfolio journal suggests that hamsters and mice infected with the Omicron variant do not have as much lung damage as those infected with other variants. More data need to come in for us to get a true understanding of Omicron’s virulence and transmissibility. We should keep an eye on Israel, which is launching a clinical trial of a second booster, or fourth mRNA shot.
As clinicians, we should give our patients and other people with whom we come in contact a sense of hope. In addition to urging people to get boosters, let’s tell them to err on the side of safety when it comes to this pandemic. That means encouraging them to remain isolated for longer than 5 days – until they test negative for COVID. It also means encouraging patients to wear high-quality face masks while inside public spaces – even in the absence of mandates. I have found it heartbreaking to watch televised broadcasts of sporting events held at some stadiums across the country where masks are not being worn. This absence of face coverings is counterintuitive at a time when some Broadway shows are closing. Even the great Radio City Rockettes shut down their holiday shows early in December 2021 because of COVID.
And, as I’ve argued before, we must not give up on unvaccinated people. I have had success in changing the minds of a few patients and some acquaintances with gentle, respectful prodding and vaccine education.
I would also like to see public health principles implemented in our schools and colleges. To protect the health of our children and young adults, we must continue to be nimble – which means school districts should implement layered prevention strategies, as the CDC recommends. This includes not only encouraging eligible staff members and students to get vaccinated, but requiring face masks inside school facilities, maintaining a physical distance of at least 3 feet, “screening testing, ventilation, handwashing, and staying home when sick.”
Furthermore, in deciding whether schools should remain open or be closed after positive COVID cases are discovered, officials should look at the vaccine demographics of that particular school. For example, if 15% of students are vaccinated in one school and 70% are vaccinated in another, the judgment would be different. Of course, it’s clearly best for schools to remain open, but perhaps closing them temporarily – perhaps for a week or 10 days – should be on the table if infection rates reach a certain level.
Now that we know more and have the benefit of getting more than 200 million Americans fully vaccinated, we can be far more selective about closings and openings. An important part of our strategy must be to communicate honestly with the public about which measures are best for safety. As a key tenet of cognitive-behavioral therapy tells us, “all-or-nothing” thinking is not productive. That should also be the case with our approach to managing COVID-19.
We don’t know the future of the pandemic. Yes, it will end, and possibly COVID will become endemic – like the flu. However, in the meantime, in addition to promoting vaccinations and boosters, we must rigorously encourage our patients to follow public health standards of masking, social distancing, and closing down businesses – and schools – temporarily.
This pandemic has taken a horrendous mental health toll on all of us – especially our patients and frontline health care workers. I’ve spoken with numerous people who were anxious, depressed, and showed signs of PTSD in early 2020; after they got vaccinated, COVID spread diminished, and as public health protocols began to lift, so did their spirits. Clearly for some, the benefit of psychiatric/psychological care centering on the pandemic has proven invaluable. In some ways, the pandemic has brought to the surface the importance of mental health care and removed some of the stigma from mental illness. And that’s a good thing.
Dr. London is a practicing psychiatrist who has been a newspaper columnist for 35 years, specializing in writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
The Centers for Disease Control and Prevention’s decision to shorten the length of isolation time for asymptomatic Americans with COVID-19, regardless of their vaccination status, to 5 days from 10 days is confusing. I hope the agency reconsiders this decision.
After all, one of the CDC’s key messages during this pandemic has been that even people with asymptomatic COVID who have been vaccinated and boosted can transmit the disease. So it seems to me that the Dec. 27, 2021, recommendation about shortening the isolation time for COVID-19–positive people, like the agency’s earlier guidance encouraging people who are vaccinated to stop wearing masks while in indoor settings, runs contrary to good public health principles.
As an expert in human behavior, I am worried about the impact of these confusing messages on the psyche of people in general, as well as on our patients.
Mental health impact
Soon after the United States went on lockdown in March 2020, I wrote about the likelihood of a pandemic of PTSD, anxiety, and depression that would occur in the wake of rising COVID-19 rates. Well, it happened.
Many people have felt a sense of existential despair, depression, and anxiety. As we head into year No. 3 of disruption of our daily lives – and face the loss of more than 825,000 Americans to COVID – we continue to navigate this uncertainty. And now we must deal with Omicron, a variant that is so highly transmissible that it is apparently able to, in some cases, evade two-dose regimens of mRNA vaccines, boosters, and immunity from past infections, according to a report from Imperial College London. Yet, we are being told by some that Omicron might be less severe, compared with other variants. I worry that this assessment is misleading. In that same report, the Imperial College said it “found no evidence” that Omicron is less virulent than Delta, based on the risk of hospitalization and symptom status.
Meanwhile, animal studies suggest that the Omicron variant might lead to less lung damage than previous variants. A preprint article that is being considered for publication by a Nature Portfolio journal suggests that hamsters and mice infected with the Omicron variant do not have as much lung damage as those infected with other variants. More data need to come in for us to get a true understanding of Omicron’s virulence and transmissibility. We should keep an eye on Israel, which is launching a clinical trial of a second booster, or fourth mRNA shot.
As clinicians, we should give our patients and other people with whom we come in contact a sense of hope. In addition to urging people to get boosters, let’s tell them to err on the side of safety when it comes to this pandemic. That means encouraging them to remain isolated for longer than 5 days – until they test negative for COVID. It also means encouraging patients to wear high-quality face masks while inside public spaces – even in the absence of mandates. I have found it heartbreaking to watch televised broadcasts of sporting events held at some stadiums across the country where masks are not being worn. This absence of face coverings is counterintuitive at a time when some Broadway shows are closing. Even the great Radio City Rockettes shut down their holiday shows early in December 2021 because of COVID.
And, as I’ve argued before, we must not give up on unvaccinated people. I have had success in changing the minds of a few patients and some acquaintances with gentle, respectful prodding and vaccine education.
I would also like to see public health principles implemented in our schools and colleges. To protect the health of our children and young adults, we must continue to be nimble – which means school districts should implement layered prevention strategies, as the CDC recommends. This includes not only encouraging eligible staff members and students to get vaccinated, but requiring face masks inside school facilities, maintaining a physical distance of at least 3 feet, “screening testing, ventilation, handwashing, and staying home when sick.”
Furthermore, in deciding whether schools should remain open or be closed after positive COVID cases are discovered, officials should look at the vaccine demographics of that particular school. For example, if 15% of students are vaccinated in one school and 70% are vaccinated in another, the judgment would be different. Of course, it’s clearly best for schools to remain open, but perhaps closing them temporarily – perhaps for a week or 10 days – should be on the table if infection rates reach a certain level.
Now that we know more and have the benefit of getting more than 200 million Americans fully vaccinated, we can be far more selective about closings and openings. An important part of our strategy must be to communicate honestly with the public about which measures are best for safety. As a key tenet of cognitive-behavioral therapy tells us, “all-or-nothing” thinking is not productive. That should also be the case with our approach to managing COVID-19.
We don’t know the future of the pandemic. Yes, it will end, and possibly COVID will become endemic – like the flu. However, in the meantime, in addition to promoting vaccinations and boosters, we must rigorously encourage our patients to follow public health standards of masking, social distancing, and closing down businesses – and schools – temporarily.
This pandemic has taken a horrendous mental health toll on all of us – especially our patients and frontline health care workers. I’ve spoken with numerous people who were anxious, depressed, and showed signs of PTSD in early 2020; after they got vaccinated, COVID spread diminished, and as public health protocols began to lift, so did their spirits. Clearly for some, the benefit of psychiatric/psychological care centering on the pandemic has proven invaluable. In some ways, the pandemic has brought to the surface the importance of mental health care and removed some of the stigma from mental illness. And that’s a good thing.
Dr. London is a practicing psychiatrist who has been a newspaper columnist for 35 years, specializing in writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.





