User login
Lifestyle Medicine: Not Just for the Wealthy
Primary care clinicians understand that addressing lifestyle-related chronic disease health disparities in minority and lower-income communities is a significant opportunity to alleviate unnecessary suffering. Disparate health outcomes associated with underlying comorbidities during the COVID pandemic exposed the urgency of this problem.
When it comes to delivering evidence-based therapeutic lifestyle behavior interventions to these populations, however, there is a misconception that lifestyle medicine is only for the wealthy. Such a misconception needlessly widens the gap in health disparities because the truth is that everyone deserves access to lifestyle medicine. Fortunately, there are numerous successful examples of delivering these services to underresourced patients. We can all contribute to narrowing health inequities by sourcing increasingly abundant lifestyle medicine resources.
All patients’ lived experiences are unique, and there is a wide range of potential challenges to achieving lifestyle behavior change. Ignoring these obstacles is a disservice to patients and almost certainly results in treatment failure. Requirements to document SDOH have been a tremendous initial step.
The next step is to have conversations with every patient about the powerful outcomes of even small lifestyle changes. All too often, clinicians forgo conversations on lifestyle change with patients affected by adverse SDOH and assume that social obstacles automatically mean that patients are neither willing nor able to attempt behavior modification. Instead, it is an opportunity for clinicians, particularly those certified in lifestyle medicine, to meet patients where they are, work with them to identify solutions, and provide referrals to community-based organizations with resources to help.
Small Steps to Big Changes
Not all lifestyle behavior interventions need to be programmatic or time intensive. Clinicians can guide patients toward simple but specific actions that can make a difference in health outcomes over time. Small steps, like eating one can of beans or two bags of frozen leafy greens each week, are a good start toward adjusted eating patterns. The American College of Lifestyle Medicine offers a whole-food, plant-predominant meal guide to share with patients.
Individuals can increase their physical activity in their living rooms by doing sit-to-stands or balancing on one leg. Deep breathing and establishing a sleep routine are other lifestyle behavior changes without a price tag.
It is true that early adopters of lifestyle medicine often had difficulty practicing in underresourced communities. Those practitioners were forced to operate on a cash-pay basis, making access to care cost-prohibitive for many patients. However, board certification has been available since 2017, and lifestyle medicine is being integrated into medical schools and residency programs. Many such board-certified clinicians now work in large health systems and bill under the usual methods. There are also frameworks, such as the community-engaged lifestyle medicine model, showing how to treat patients affected by adverse SDOH effectively.
For example, patients at risk for malnutrition because of illnesses like chronic kidney disease, cancer, and heart failure receive medically tailored meals and access to a registered dietitian through a partnership between UC San Diego Health and Mama’s Kitchen. In Pennsylvania’s Lehigh Valley, where 1 in 10 of the approximately 700,000 residents face food insecurity, the Kellyn Foundation delivers fresh food through the Eat Real Food Mobile Market and offers whole-food, plant-predominant cooking classes, interactive elementary school programs focused on healthy lifestyle choices, and therapeutic lifestyle-change programs in community locations. Three months after launching new mobile market sites in Allentown, 1200 households were utilizing $15 weekly food vouchers through the program. Lifestyle medicine clinicians serve inner-city and rural areas in independent practices, large health systems, and community-based practice activities.
To improve access to lifestyle medicine in underresourced communities, more clinicians trained and certified in lifestyle medicine are needed. The Health Equity Achieved through Lifestyle Medicine Initiative supports a diverse lifestyle medicine workforce by offering scholarships to clinicians underrepresented in medicine and is working to train and certify at least one physician within each of the 1400 federally qualified health centers where clinicians are on the front lines of delivering care to the most underserved populations.
A meaningful first step for clinicians to address health disparities is to screen patients for and document SDOH. The American Academy of Family Physicians offers useful tools to screen patients, identify community-based resources, and help patients create action plans to overcome health risks and improve outcomes. In a promising trend to better support addressing SDOH in clinical care, the 2024 Medicare Physician Fee Schedule final rule included new codes to support this effort.
Not every patient will be ready or willing to begin a lifestyle medicine treatment plan. Still, all of them will be grateful for the opportunity to decide for themselves. If we are invested in narrowing health inequities, lifestyle medicine and behavior change must be a topic in clinical encounters with all our patients.
Dr. Collings, director of lifestyle medicine, Silicon Valley Medical Development, and past president, American College of Lifestyle Medicine, Mountain View, California, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Primary care clinicians understand that addressing lifestyle-related chronic disease health disparities in minority and lower-income communities is a significant opportunity to alleviate unnecessary suffering. Disparate health outcomes associated with underlying comorbidities during the COVID pandemic exposed the urgency of this problem.
When it comes to delivering evidence-based therapeutic lifestyle behavior interventions to these populations, however, there is a misconception that lifestyle medicine is only for the wealthy. Such a misconception needlessly widens the gap in health disparities because the truth is that everyone deserves access to lifestyle medicine. Fortunately, there are numerous successful examples of delivering these services to underresourced patients. We can all contribute to narrowing health inequities by sourcing increasingly abundant lifestyle medicine resources.
All patients’ lived experiences are unique, and there is a wide range of potential challenges to achieving lifestyle behavior change. Ignoring these obstacles is a disservice to patients and almost certainly results in treatment failure. Requirements to document SDOH have been a tremendous initial step.
The next step is to have conversations with every patient about the powerful outcomes of even small lifestyle changes. All too often, clinicians forgo conversations on lifestyle change with patients affected by adverse SDOH and assume that social obstacles automatically mean that patients are neither willing nor able to attempt behavior modification. Instead, it is an opportunity for clinicians, particularly those certified in lifestyle medicine, to meet patients where they are, work with them to identify solutions, and provide referrals to community-based organizations with resources to help.
Small Steps to Big Changes
Not all lifestyle behavior interventions need to be programmatic or time intensive. Clinicians can guide patients toward simple but specific actions that can make a difference in health outcomes over time. Small steps, like eating one can of beans or two bags of frozen leafy greens each week, are a good start toward adjusted eating patterns. The American College of Lifestyle Medicine offers a whole-food, plant-predominant meal guide to share with patients.
Individuals can increase their physical activity in their living rooms by doing sit-to-stands or balancing on one leg. Deep breathing and establishing a sleep routine are other lifestyle behavior changes without a price tag.
It is true that early adopters of lifestyle medicine often had difficulty practicing in underresourced communities. Those practitioners were forced to operate on a cash-pay basis, making access to care cost-prohibitive for many patients. However, board certification has been available since 2017, and lifestyle medicine is being integrated into medical schools and residency programs. Many such board-certified clinicians now work in large health systems and bill under the usual methods. There are also frameworks, such as the community-engaged lifestyle medicine model, showing how to treat patients affected by adverse SDOH effectively.
For example, patients at risk for malnutrition because of illnesses like chronic kidney disease, cancer, and heart failure receive medically tailored meals and access to a registered dietitian through a partnership between UC San Diego Health and Mama’s Kitchen. In Pennsylvania’s Lehigh Valley, where 1 in 10 of the approximately 700,000 residents face food insecurity, the Kellyn Foundation delivers fresh food through the Eat Real Food Mobile Market and offers whole-food, plant-predominant cooking classes, interactive elementary school programs focused on healthy lifestyle choices, and therapeutic lifestyle-change programs in community locations. Three months after launching new mobile market sites in Allentown, 1200 households were utilizing $15 weekly food vouchers through the program. Lifestyle medicine clinicians serve inner-city and rural areas in independent practices, large health systems, and community-based practice activities.
To improve access to lifestyle medicine in underresourced communities, more clinicians trained and certified in lifestyle medicine are needed. The Health Equity Achieved through Lifestyle Medicine Initiative supports a diverse lifestyle medicine workforce by offering scholarships to clinicians underrepresented in medicine and is working to train and certify at least one physician within each of the 1400 federally qualified health centers where clinicians are on the front lines of delivering care to the most underserved populations.
A meaningful first step for clinicians to address health disparities is to screen patients for and document SDOH. The American Academy of Family Physicians offers useful tools to screen patients, identify community-based resources, and help patients create action plans to overcome health risks and improve outcomes. In a promising trend to better support addressing SDOH in clinical care, the 2024 Medicare Physician Fee Schedule final rule included new codes to support this effort.
Not every patient will be ready or willing to begin a lifestyle medicine treatment plan. Still, all of them will be grateful for the opportunity to decide for themselves. If we are invested in narrowing health inequities, lifestyle medicine and behavior change must be a topic in clinical encounters with all our patients.
Dr. Collings, director of lifestyle medicine, Silicon Valley Medical Development, and past president, American College of Lifestyle Medicine, Mountain View, California, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Primary care clinicians understand that addressing lifestyle-related chronic disease health disparities in minority and lower-income communities is a significant opportunity to alleviate unnecessary suffering. Disparate health outcomes associated with underlying comorbidities during the COVID pandemic exposed the urgency of this problem.
When it comes to delivering evidence-based therapeutic lifestyle behavior interventions to these populations, however, there is a misconception that lifestyle medicine is only for the wealthy. Such a misconception needlessly widens the gap in health disparities because the truth is that everyone deserves access to lifestyle medicine. Fortunately, there are numerous successful examples of delivering these services to underresourced patients. We can all contribute to narrowing health inequities by sourcing increasingly abundant lifestyle medicine resources.
All patients’ lived experiences are unique, and there is a wide range of potential challenges to achieving lifestyle behavior change. Ignoring these obstacles is a disservice to patients and almost certainly results in treatment failure. Requirements to document SDOH have been a tremendous initial step.
The next step is to have conversations with every patient about the powerful outcomes of even small lifestyle changes. All too often, clinicians forgo conversations on lifestyle change with patients affected by adverse SDOH and assume that social obstacles automatically mean that patients are neither willing nor able to attempt behavior modification. Instead, it is an opportunity for clinicians, particularly those certified in lifestyle medicine, to meet patients where they are, work with them to identify solutions, and provide referrals to community-based organizations with resources to help.
Small Steps to Big Changes
Not all lifestyle behavior interventions need to be programmatic or time intensive. Clinicians can guide patients toward simple but specific actions that can make a difference in health outcomes over time. Small steps, like eating one can of beans or two bags of frozen leafy greens each week, are a good start toward adjusted eating patterns. The American College of Lifestyle Medicine offers a whole-food, plant-predominant meal guide to share with patients.
Individuals can increase their physical activity in their living rooms by doing sit-to-stands or balancing on one leg. Deep breathing and establishing a sleep routine are other lifestyle behavior changes without a price tag.
It is true that early adopters of lifestyle medicine often had difficulty practicing in underresourced communities. Those practitioners were forced to operate on a cash-pay basis, making access to care cost-prohibitive for many patients. However, board certification has been available since 2017, and lifestyle medicine is being integrated into medical schools and residency programs. Many such board-certified clinicians now work in large health systems and bill under the usual methods. There are also frameworks, such as the community-engaged lifestyle medicine model, showing how to treat patients affected by adverse SDOH effectively.
For example, patients at risk for malnutrition because of illnesses like chronic kidney disease, cancer, and heart failure receive medically tailored meals and access to a registered dietitian through a partnership between UC San Diego Health and Mama’s Kitchen. In Pennsylvania’s Lehigh Valley, where 1 in 10 of the approximately 700,000 residents face food insecurity, the Kellyn Foundation delivers fresh food through the Eat Real Food Mobile Market and offers whole-food, plant-predominant cooking classes, interactive elementary school programs focused on healthy lifestyle choices, and therapeutic lifestyle-change programs in community locations. Three months after launching new mobile market sites in Allentown, 1200 households were utilizing $15 weekly food vouchers through the program. Lifestyle medicine clinicians serve inner-city and rural areas in independent practices, large health systems, and community-based practice activities.
To improve access to lifestyle medicine in underresourced communities, more clinicians trained and certified in lifestyle medicine are needed. The Health Equity Achieved through Lifestyle Medicine Initiative supports a diverse lifestyle medicine workforce by offering scholarships to clinicians underrepresented in medicine and is working to train and certify at least one physician within each of the 1400 federally qualified health centers where clinicians are on the front lines of delivering care to the most underserved populations.
A meaningful first step for clinicians to address health disparities is to screen patients for and document SDOH. The American Academy of Family Physicians offers useful tools to screen patients, identify community-based resources, and help patients create action plans to overcome health risks and improve outcomes. In a promising trend to better support addressing SDOH in clinical care, the 2024 Medicare Physician Fee Schedule final rule included new codes to support this effort.
Not every patient will be ready or willing to begin a lifestyle medicine treatment plan. Still, all of them will be grateful for the opportunity to decide for themselves. If we are invested in narrowing health inequities, lifestyle medicine and behavior change must be a topic in clinical encounters with all our patients.
Dr. Collings, director of lifestyle medicine, Silicon Valley Medical Development, and past president, American College of Lifestyle Medicine, Mountain View, California, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Time-Restricted Eating Is Not a Metabolic Magic Bullet
This transcript has been edited for clarity.
One out of three American adults — about 100 million people in this country — have the metabolic syndrome. I’m showing you the official criteria here, but essentially this is a syndrome of insulin resistance and visceral adiposity that predisposes us to a host of chronic diseases such as diabetes, heart disease, and even dementia.
The metabolic syndrome is, fundamentally, a lifestyle disease. There is a direct line between our dietary habits and the wide availability of carbohydrate-rich, highly processed foods, and the rise in the syndrome in the population.
A saying I learned from one of my epidemiology teachers comes to mind: “Lifestyle diseases require lifestyle reinterventions.” But you know what? I’m not so sure anymore.
I’ve been around long enough to see multiple dietary fads come and go with varying efficacy. I grew up in the low-fat era, probably the most detrimental time to our national health as food manufacturers started replacing fats with carbohydrates, driving much of the problem we’re faced with today.
But I was also around for the Atkins diet and the low-carb craze — a healthier approach, all things being equal. And I’ve seen variants of these: the paleo diet (essentially a low-carb, high-protein diet based on minimally processed foods) and the Mediterranean diet, which sought to replace some percentage of fats with healthier fats.
And, of course, there is time-restricted eating.
Time-restricted eating, a variant of intermittent fasting, has the advantage of being very simple. No cookbooks, no recipes. Eat what you want — but limit it to certain hours in the day, ideally a window of less than 10 hours, such as 8 a.m. to 6 p.m.
When it comes to weight loss, the diets that work tend to work because they reduce calorie intake. I know, people will get angry about this, but thermodynamics is not just a good idea, it’s the law.
But weight loss is not the only reason we need to eat healthier. What we eat can impact our health in multiple ways; certain foods lead to more atherosclerosis, more inflammation, increased strain on the kidney and liver, and can affect our glucose homeostasis.
So I was really interested when I saw this article, “Time-Restricted Eating in Adults With Metabolic Syndrome,” appearing in Annals of Internal Medicine October 1, which examined the effect of time-restricted eating on the metabolic syndrome itself. Could this lifestyle intervention cure this lifestyle disease?
In the study, 108 individuals, all of whom had the metabolic syndrome but not full-blown diabetes, were randomized to usual care — basically, nutrition education — vs time-restricted eating. In that group, participants were instructed to reduce their window of eating by at least 4 hours to achieve an 8- to 10-hour eating window. The groups were followed for 3 months.
Now, before we get to the results, it’s important to remember that the success of a lifestyle intervention trial is quite dependent on how well people adhere to the lifestyle intervention. Time-restricted eating is not as easy as taking a pill once a day.
The researchers had participants log their consumption using a smartphone app to confirm whether they were adhering to that restricted eating window.
Broadly speaking, they did. At baseline, both groups had an eating window of about 14 hours a day — think 7 a.m. to 9 p.m. The intervention group reduced that to just under 10 hours, with 10% of days falling outside of the target window.
Lifestyle change achieved, the primary outcome was the change in hemoglobin A1c at 3 months. A1c integrates the serum glucose over time and is thus a good indicator of the success of the intervention in terms of insulin resistance. But the effect was, honestly, disappointing.
Technically, the time-restricted-eating group had a greater A1c change than the control group — by 0.1 percentage points. On average, they went from a baseline A1c of 5.87 to a 3-month A1c of 5.75.
Other metabolic syndrome markers were equally lackluster: no difference in fasting glucose, mean glucose, or fasting insulin.
There was some weight change. The control group, which got that dietary education, lost 1.5% of body weight over the 3 months. The time-restricted-eating group lost 3.3% — about 7 pounds, which is reasonable.
With that weight loss came statistically significant, albeit modest improvements in BMI, body fat percentage, and LDL cholesterol.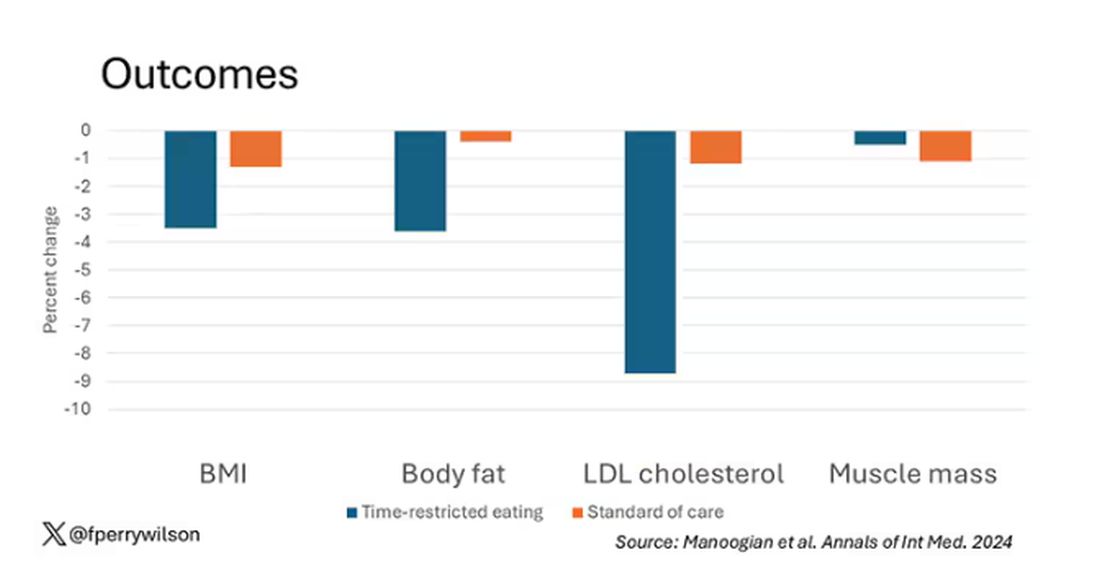
Of interest, despite the larger weight loss in the intermittent-fasting group, there was no difference in muscle mass loss, which is encouraging.
Taken together, we can say that, yes, it seems like time-restricted eating can help people lose some weight. This is essentially due to the fact that people eat fewer calories when they do time-restricted eating, as you can see here.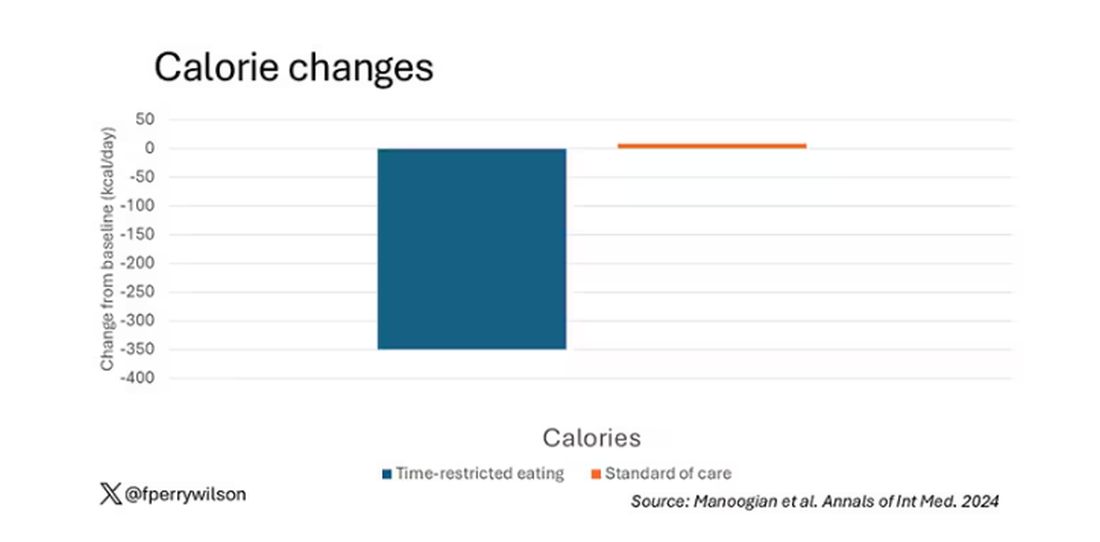
But, in the end, this trial examined whether this relatively straightforward lifestyle intervention would move the needle in terms of metabolic syndrome, and the data are not very compelling for that.
This graph shows how many of those five factors for metabolic syndrome the individuals in this trial had from the start to the end. You see that, over the 3 months, seven people in the time-restricted-eating group moved from having three criteria to two or one — being “cured” of metabolic syndrome, if you will. Nine people in the standard group were cured by that definition. Remember, they had to have at least three to have the syndrome and thus be eligible for the trial. 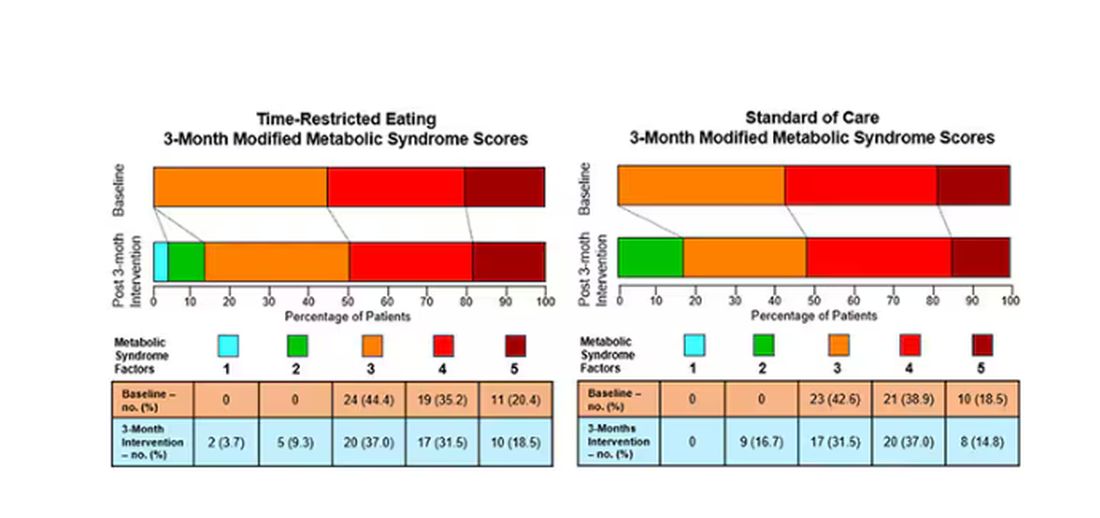
So If it just leads to weight loss by forcing people to consume less calories, then we need to acknowledge that we probably have better methods to achieve this same end. Ten years ago, I would have said that lifestyle change is the only way to end the epidemic of the metabolic syndrome in this country. Today, well, we live in a world of GLP-1 weight loss drugs. It is simply a different world now. Yes, they are expensive. Yes, they have side effects. But we need to evaluate them against the comparison. And so far, lifestyle changes alone are really no comparison.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
One out of three American adults — about 100 million people in this country — have the metabolic syndrome. I’m showing you the official criteria here, but essentially this is a syndrome of insulin resistance and visceral adiposity that predisposes us to a host of chronic diseases such as diabetes, heart disease, and even dementia.
The metabolic syndrome is, fundamentally, a lifestyle disease. There is a direct line between our dietary habits and the wide availability of carbohydrate-rich, highly processed foods, and the rise in the syndrome in the population.
A saying I learned from one of my epidemiology teachers comes to mind: “Lifestyle diseases require lifestyle reinterventions.” But you know what? I’m not so sure anymore.
I’ve been around long enough to see multiple dietary fads come and go with varying efficacy. I grew up in the low-fat era, probably the most detrimental time to our national health as food manufacturers started replacing fats with carbohydrates, driving much of the problem we’re faced with today.
But I was also around for the Atkins diet and the low-carb craze — a healthier approach, all things being equal. And I’ve seen variants of these: the paleo diet (essentially a low-carb, high-protein diet based on minimally processed foods) and the Mediterranean diet, which sought to replace some percentage of fats with healthier fats.
And, of course, there is time-restricted eating.
Time-restricted eating, a variant of intermittent fasting, has the advantage of being very simple. No cookbooks, no recipes. Eat what you want — but limit it to certain hours in the day, ideally a window of less than 10 hours, such as 8 a.m. to 6 p.m.
When it comes to weight loss, the diets that work tend to work because they reduce calorie intake. I know, people will get angry about this, but thermodynamics is not just a good idea, it’s the law.
But weight loss is not the only reason we need to eat healthier. What we eat can impact our health in multiple ways; certain foods lead to more atherosclerosis, more inflammation, increased strain on the kidney and liver, and can affect our glucose homeostasis.
So I was really interested when I saw this article, “Time-Restricted Eating in Adults With Metabolic Syndrome,” appearing in Annals of Internal Medicine October 1, which examined the effect of time-restricted eating on the metabolic syndrome itself. Could this lifestyle intervention cure this lifestyle disease?
In the study, 108 individuals, all of whom had the metabolic syndrome but not full-blown diabetes, were randomized to usual care — basically, nutrition education — vs time-restricted eating. In that group, participants were instructed to reduce their window of eating by at least 4 hours to achieve an 8- to 10-hour eating window. The groups were followed for 3 months.
Now, before we get to the results, it’s important to remember that the success of a lifestyle intervention trial is quite dependent on how well people adhere to the lifestyle intervention. Time-restricted eating is not as easy as taking a pill once a day.
The researchers had participants log their consumption using a smartphone app to confirm whether they were adhering to that restricted eating window.
Broadly speaking, they did. At baseline, both groups had an eating window of about 14 hours a day — think 7 a.m. to 9 p.m. The intervention group reduced that to just under 10 hours, with 10% of days falling outside of the target window.
Lifestyle change achieved, the primary outcome was the change in hemoglobin A1c at 3 months. A1c integrates the serum glucose over time and is thus a good indicator of the success of the intervention in terms of insulin resistance. But the effect was, honestly, disappointing.
Technically, the time-restricted-eating group had a greater A1c change than the control group — by 0.1 percentage points. On average, they went from a baseline A1c of 5.87 to a 3-month A1c of 5.75.
Other metabolic syndrome markers were equally lackluster: no difference in fasting glucose, mean glucose, or fasting insulin.
There was some weight change. The control group, which got that dietary education, lost 1.5% of body weight over the 3 months. The time-restricted-eating group lost 3.3% — about 7 pounds, which is reasonable.
With that weight loss came statistically significant, albeit modest improvements in BMI, body fat percentage, and LDL cholesterol.
Of interest, despite the larger weight loss in the intermittent-fasting group, there was no difference in muscle mass loss, which is encouraging.
Taken together, we can say that, yes, it seems like time-restricted eating can help people lose some weight. This is essentially due to the fact that people eat fewer calories when they do time-restricted eating, as you can see here.
But, in the end, this trial examined whether this relatively straightforward lifestyle intervention would move the needle in terms of metabolic syndrome, and the data are not very compelling for that.
This graph shows how many of those five factors for metabolic syndrome the individuals in this trial had from the start to the end. You see that, over the 3 months, seven people in the time-restricted-eating group moved from having three criteria to two or one — being “cured” of metabolic syndrome, if you will. Nine people in the standard group were cured by that definition. Remember, they had to have at least three to have the syndrome and thus be eligible for the trial. 
So If it just leads to weight loss by forcing people to consume less calories, then we need to acknowledge that we probably have better methods to achieve this same end. Ten years ago, I would have said that lifestyle change is the only way to end the epidemic of the metabolic syndrome in this country. Today, well, we live in a world of GLP-1 weight loss drugs. It is simply a different world now. Yes, they are expensive. Yes, they have side effects. But we need to evaluate them against the comparison. And so far, lifestyle changes alone are really no comparison.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
One out of three American adults — about 100 million people in this country — have the metabolic syndrome. I’m showing you the official criteria here, but essentially this is a syndrome of insulin resistance and visceral adiposity that predisposes us to a host of chronic diseases such as diabetes, heart disease, and even dementia.
The metabolic syndrome is, fundamentally, a lifestyle disease. There is a direct line between our dietary habits and the wide availability of carbohydrate-rich, highly processed foods, and the rise in the syndrome in the population.
A saying I learned from one of my epidemiology teachers comes to mind: “Lifestyle diseases require lifestyle reinterventions.” But you know what? I’m not so sure anymore.
I’ve been around long enough to see multiple dietary fads come and go with varying efficacy. I grew up in the low-fat era, probably the most detrimental time to our national health as food manufacturers started replacing fats with carbohydrates, driving much of the problem we’re faced with today.
But I was also around for the Atkins diet and the low-carb craze — a healthier approach, all things being equal. And I’ve seen variants of these: the paleo diet (essentially a low-carb, high-protein diet based on minimally processed foods) and the Mediterranean diet, which sought to replace some percentage of fats with healthier fats.
And, of course, there is time-restricted eating.
Time-restricted eating, a variant of intermittent fasting, has the advantage of being very simple. No cookbooks, no recipes. Eat what you want — but limit it to certain hours in the day, ideally a window of less than 10 hours, such as 8 a.m. to 6 p.m.
When it comes to weight loss, the diets that work tend to work because they reduce calorie intake. I know, people will get angry about this, but thermodynamics is not just a good idea, it’s the law.
But weight loss is not the only reason we need to eat healthier. What we eat can impact our health in multiple ways; certain foods lead to more atherosclerosis, more inflammation, increased strain on the kidney and liver, and can affect our glucose homeostasis.
So I was really interested when I saw this article, “Time-Restricted Eating in Adults With Metabolic Syndrome,” appearing in Annals of Internal Medicine October 1, which examined the effect of time-restricted eating on the metabolic syndrome itself. Could this lifestyle intervention cure this lifestyle disease?
In the study, 108 individuals, all of whom had the metabolic syndrome but not full-blown diabetes, were randomized to usual care — basically, nutrition education — vs time-restricted eating. In that group, participants were instructed to reduce their window of eating by at least 4 hours to achieve an 8- to 10-hour eating window. The groups were followed for 3 months.
Now, before we get to the results, it’s important to remember that the success of a lifestyle intervention trial is quite dependent on how well people adhere to the lifestyle intervention. Time-restricted eating is not as easy as taking a pill once a day.
The researchers had participants log their consumption using a smartphone app to confirm whether they were adhering to that restricted eating window.
Broadly speaking, they did. At baseline, both groups had an eating window of about 14 hours a day — think 7 a.m. to 9 p.m. The intervention group reduced that to just under 10 hours, with 10% of days falling outside of the target window.
Lifestyle change achieved, the primary outcome was the change in hemoglobin A1c at 3 months. A1c integrates the serum glucose over time and is thus a good indicator of the success of the intervention in terms of insulin resistance. But the effect was, honestly, disappointing.
Technically, the time-restricted-eating group had a greater A1c change than the control group — by 0.1 percentage points. On average, they went from a baseline A1c of 5.87 to a 3-month A1c of 5.75.
Other metabolic syndrome markers were equally lackluster: no difference in fasting glucose, mean glucose, or fasting insulin.
There was some weight change. The control group, which got that dietary education, lost 1.5% of body weight over the 3 months. The time-restricted-eating group lost 3.3% — about 7 pounds, which is reasonable.
With that weight loss came statistically significant, albeit modest improvements in BMI, body fat percentage, and LDL cholesterol.
Of interest, despite the larger weight loss in the intermittent-fasting group, there was no difference in muscle mass loss, which is encouraging.
Taken together, we can say that, yes, it seems like time-restricted eating can help people lose some weight. This is essentially due to the fact that people eat fewer calories when they do time-restricted eating, as you can see here.
But, in the end, this trial examined whether this relatively straightforward lifestyle intervention would move the needle in terms of metabolic syndrome, and the data are not very compelling for that.
This graph shows how many of those five factors for metabolic syndrome the individuals in this trial had from the start to the end. You see that, over the 3 months, seven people in the time-restricted-eating group moved from having three criteria to two or one — being “cured” of metabolic syndrome, if you will. Nine people in the standard group were cured by that definition. Remember, they had to have at least three to have the syndrome and thus be eligible for the trial. 
So If it just leads to weight loss by forcing people to consume less calories, then we need to acknowledge that we probably have better methods to achieve this same end. Ten years ago, I would have said that lifestyle change is the only way to end the epidemic of the metabolic syndrome in this country. Today, well, we live in a world of GLP-1 weight loss drugs. It is simply a different world now. Yes, they are expensive. Yes, they have side effects. But we need to evaluate them against the comparison. And so far, lifestyle changes alone are really no comparison.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Public Health, Not Politics, Should Drive Mask Policies, Says Ethicist
This transcript has been edited for clarity.
I recently saw a ban that has me very worried, concerned, and strongly in opposition.
Basically, the standard kind of medical mask would be captured, although I think their aim in doing this was to try to discourage people at political protests from being able to wear masks and hide their identity. They’re basically trying to discourage that. This is particularly triggered by, I think, protests about the invasion of Israel, the war that resulted in Gaza, and the demonstrations that have gone on around the country, with many people masked.
There may be issues about what is acceptable to wear when you go to a demonstration. I don’t claim to know about the civil rights of that.
In a time at which COVID-19 is flourishing, really on the rebound, expanding fast, and still causing 600 deaths a week; the flu season is going to be upon us soon enough; and there are also concerns about the possibility of avian flu jumping into the human population, it is absolutely the wrong time to single out those who are trying to mask for health reasons.
Basically, there are two strong reasons. One, there are people out there who wear a medical mask or mask for a medical reason because they have an underlying disease. They may have had a transplant or they may feel they’re immunocompromised for some reason. They worry that, if they don’t wear a mask, they’re going to get an infection from something like COVID-19 or flu, which could really be super-dangerous for them.
The other reason people mask is to protect their family members. They may have someone who’s immunocompromised in the family, or they’re doing it kindly and altruistically to protect the rest of us and to stop viruses from circulating.
These bans are not taking into account public health. They’re being brought forward in the midst of political heat about demonstrations and political issues. I think they should be opposed. I do not think they should be enacted.
I think the medical rights of people with disabilities and immunologic disorders, and those who want to mask to prevent getting sick at a time at which infectious diseases are still circulating and killing people, ought to take priority. Public health, in this case, should drive our policies about masks.
Dr. Caplan, director, Division of Medical Ethics, New York University Langone Medical Center, New York, NY, served on Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position), and is a contributing author and adviser for Medscape.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I recently saw a ban that has me very worried, concerned, and strongly in opposition.
Basically, the standard kind of medical mask would be captured, although I think their aim in doing this was to try to discourage people at political protests from being able to wear masks and hide their identity. They’re basically trying to discourage that. This is particularly triggered by, I think, protests about the invasion of Israel, the war that resulted in Gaza, and the demonstrations that have gone on around the country, with many people masked.
There may be issues about what is acceptable to wear when you go to a demonstration. I don’t claim to know about the civil rights of that.
In a time at which COVID-19 is flourishing, really on the rebound, expanding fast, and still causing 600 deaths a week; the flu season is going to be upon us soon enough; and there are also concerns about the possibility of avian flu jumping into the human population, it is absolutely the wrong time to single out those who are trying to mask for health reasons.
Basically, there are two strong reasons. One, there are people out there who wear a medical mask or mask for a medical reason because they have an underlying disease. They may have had a transplant or they may feel they’re immunocompromised for some reason. They worry that, if they don’t wear a mask, they’re going to get an infection from something like COVID-19 or flu, which could really be super-dangerous for them.
The other reason people mask is to protect their family members. They may have someone who’s immunocompromised in the family, or they’re doing it kindly and altruistically to protect the rest of us and to stop viruses from circulating.
These bans are not taking into account public health. They’re being brought forward in the midst of political heat about demonstrations and political issues. I think they should be opposed. I do not think they should be enacted.
I think the medical rights of people with disabilities and immunologic disorders, and those who want to mask to prevent getting sick at a time at which infectious diseases are still circulating and killing people, ought to take priority. Public health, in this case, should drive our policies about masks.
Dr. Caplan, director, Division of Medical Ethics, New York University Langone Medical Center, New York, NY, served on Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position), and is a contributing author and adviser for Medscape.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I recently saw a ban that has me very worried, concerned, and strongly in opposition.
Basically, the standard kind of medical mask would be captured, although I think their aim in doing this was to try to discourage people at political protests from being able to wear masks and hide their identity. They’re basically trying to discourage that. This is particularly triggered by, I think, protests about the invasion of Israel, the war that resulted in Gaza, and the demonstrations that have gone on around the country, with many people masked.
There may be issues about what is acceptable to wear when you go to a demonstration. I don’t claim to know about the civil rights of that.
In a time at which COVID-19 is flourishing, really on the rebound, expanding fast, and still causing 600 deaths a week; the flu season is going to be upon us soon enough; and there are also concerns about the possibility of avian flu jumping into the human population, it is absolutely the wrong time to single out those who are trying to mask for health reasons.
Basically, there are two strong reasons. One, there are people out there who wear a medical mask or mask for a medical reason because they have an underlying disease. They may have had a transplant or they may feel they’re immunocompromised for some reason. They worry that, if they don’t wear a mask, they’re going to get an infection from something like COVID-19 or flu, which could really be super-dangerous for them.
The other reason people mask is to protect their family members. They may have someone who’s immunocompromised in the family, or they’re doing it kindly and altruistically to protect the rest of us and to stop viruses from circulating.
These bans are not taking into account public health. They’re being brought forward in the midst of political heat about demonstrations and political issues. I think they should be opposed. I do not think they should be enacted.
I think the medical rights of people with disabilities and immunologic disorders, and those who want to mask to prevent getting sick at a time at which infectious diseases are still circulating and killing people, ought to take priority. Public health, in this case, should drive our policies about masks.
Dr. Caplan, director, Division of Medical Ethics, New York University Langone Medical Center, New York, NY, served on Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position), and is a contributing author and adviser for Medscape.
A version of this article appeared on Medscape.com.
Considerations for the Use of Biologics in Pregnancy
Biologics have revolutionized dermatologic treatment, offering substantial relief from chronic and debilitating skin conditions such as psoriasis,
Biologics for Cutaneous Conditions
Biologics—tumor necrosis factor (TNF) α inhibitors; IL-17, IL-23, IL-12, and IL-36 inhibitors; and agents such as omalizumab and dupilumab—have shown remarkable efficacy in controlling severe or recalcitrant dermatologic conditions and typically are more effective than traditional systemic therapies.1 For instance, randomized clinical trials (RCTs) and real-world data have shown that patients with psoriasis can achieve considerable skin clearance with biologics, greatly enhancing QOL.2 Adalimumab and secukinumab, which have been approved for use in moderate to severe cases of hidradenitis suppurativa, reduce the frequency of painful nodules and abscesses, thereby decreasing pain and improving QOL. Dupilumab, an IL-4/13 receptor antagonist, has revolutionized the treatment of AD by drastically reducing itch and skin lesions and improving QOL.3 For chronic urticaria, the anti-IgE antibody omalizumab has effectively reduced the incidence of hives and itching, providing pronounced symptom relief when traditional antihistamines fail.4 Use of rituximab, an anti-CD20 monoclonal antibody, has led to remission in severe cases of pemphigus vulgaris and bullous pemphigoid.5
Impact of Untreated Cutaneous Conditions in Pregnancy
When treating patients who are pregnant, dermatologists must consider the health of both the expectant mother and the developing fetus. This dual focus complicates decision-making, particularly with the use of biologics. Untreated cutaneous conditions can profoundly impact a pregnant patient’s health and QOL as well as lead to pregnancy complications affecting the fetus, such as preterm birth or low birth weight. In some studies, moderate to severe psoriasis has been associated with increased risk for complications during pregnancy, including preeclampsia and intrauterine growth restriction.6 Although specific data on hidradenitis suppurativa are lacking, the highly inflammatory nature of the condition suggests similar adverse effects on pregnancy.7 Atopic dermatitis can be exacerbated during pregnancy due to a shift in the immune system to become more allergic dominant.8 Generalized pustular psoriasis manifests with widespread pustules, fever, and systemic inflammation, posing serious risks to both the mother and the fetus if left untreated9; in such a life-threatening scenario, the use of potent treatments such as spesolimab, an IL-36 receptor antagonist, may be warranted. Therefore, managing these conditions effectively is crucial not only for the mother’s health but also for fetal well-being.
Which Biologics Can Dermatologists Safely Prescribe?
Despite the benefits, many dermatologists are hesitant to prescribe biologics to pregnant patients due to the lack of understanding and definitive safety data.10,11 Although there are no RCTs that involve pregnant patients, current evidence suggests that several biologics are not teratogenic and do not cause fetal malformations. Extensive postexposure data support the safety of TNF-α inhibitors during pregnancy.12 Research has shown that children exposed to these agents in utero have normal development, infection rates, and vaccination outcomes comparable to nonexposed children. For example, a systematic review and meta-analysis found no significant increase in the risk for major congenital malformations, spontaneous abortions, or preterm births among patients exposed to anti–TNF-α agents during pregnancy.2 The Organization of Teratology Information Specialists Autoimmune Diseases in Pregnancy Project has provided valuable real-world data indicating that the use of TNF-α inhibitors in pregnancy, particularly during the first trimester, does not substantially elevate the risk for adverse outcomes.13 These findings have been corroborated by several other registry studies and RCTs, providing a robust safety profile for these agents during pregnancy.14
Similarly, postexposure data on IL-17 and IL-12/23 inhibitors indicate a favorable safety profile, though the sample sizes are smaller than those for anti–TNF-α agents.12,14 Studies of drugs such as secukinumab (IL-17 inhibitor), guselkumab (IL-23 inhibitor), or ustekinumab (IL-12/23 inhibitor) have shown no association with teratogenic effects or increased risk for miscarriage.14 However, agents such as spesolimab (IL-36 inhibitor) are relatively new, and ongoing studies are expected to provide more comprehensive safety data.15 Similarly, omalizumab and dupilumab have not been associated with increased risk for fetal malformations or adverse pregnancy outcomes. Omalizumab, indicated for chronic urticaria, has a good safety profile in pregnancy, with no significant increase in adverse outcomes reported in studies and registries.16 Dupilumab, used for AD, has demonstrated safety in pregnancy, with ongoing studies continuing to monitor outcomes.17
Conversely, rituximab (an anti-CD20 antibody for autoimmune bullous diseases) has shown evidence of adverse pregnancy outcomes, including fetal harm.18 Its use generally is discouraged unless deemed absolutely necessary, and no safer alternatives are available. Rituximab can cross the placenta, especially in the second and third trimesters, and has been associated with B-cell depletion in the fetus, leading to potential immunosuppression and increased risk for infections.5
Although the data on the safety of biologics in pregnancy are largely reassuring, it is essential to recognize that potential risks have not been ruled out entirely. There are extensive safety data for anti–TNF-α inhibitors, which provides a level of confidence; although newer agents such as IL-17 and IL-23 inhibitors have shown promising early results, further research is required to solidify their safety profiles during pregnancy.
Dermatologists must balance the risks and benefits of using biologics in pregnant patients. This decision-making process involves careful consideration of the severity of the mother’s condition, the potential risks to the fetus, and the availability of alternative treatments. For many severe dermatologic conditions, the benefits of biologics in controlling disease activity and improving QOL may outweigh the potential risks, especially when other treatments have failed or are not suitable.
Final Thoughts
The increasing use of biologics in dermatology has undoubtedly improved the management of severe skin conditions, substantially enhancing patients’ QOL. As more data become available and clinical guidelines evolve, health care providers will be better equipped to make informed decisions about the use of biologics, particularly in pregnant patients. Collaborative efforts between dermatologists, obstetricians, and researchers will help refine treatment guidelines and ensure that pregnant patients with severe dermatologic conditions receive the best possible care.
For now, although the current evidence supports the safety of many biologics during pregnancy,10,11 individualized care and informed decision-making remain paramount. Careful management and adherence to current guidelines make it possible to navigate the complexities of treating severe dermatologic conditions in pregnant patients, ensuring the best outcomes for both mother and child.
- Sehgal VN, Pandhi D, Khurana A. Biologics in dermatology: an integrated review. Indian J Dermatol. 2014; 59:425-441. doi:10.4103/0019-5154.139859
- Mahadevan U, Wolf DC, Dubinsky M, et al. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:286-292. doi:10.1016/j.cgh.2012.11.011
- Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-2348.
- Saini SS, Bindslev-Jensen C, Maurer M, et al. Efficacy and safety of omalizumab in patients with chronic idiopathic/spontaneous urticaria who remain symptomatic on H1 antihistamines: a randomized, placebo-controlled study. J Invest Dermatol. 2015;135:67-75. doi:10.1038/jid.2014.306
- Mariette X, Forger F, Abraham B, et al. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis. 2018;77:228-233. doi:10.1136/annrheumdis-2017-212196
- Yang Y-W, Chen C-S, Chen Y-H, et al. Psoriasis and pregnancy outcomes: a nationwide population-based study. J Am Acad Dermatol. 2011;64:71-77.
- Zouboulis CC, Del Marmol V, Mrowietz U, et al. Hidradenitis suppurativa/acne inversa: criteria for diagnosis, severity assessment, classification and disease evaluation. Dermatology. 2015;231:184-190.
- Balakirski G, Novak N. Atopic dermatitis and pregnancy. J Allergy Clin Immunol. 2022;149:1185-1194. doi:10.1016/j.jaci.2022.01.010
- Bachelez H, Choon S-E, Marrakchi S, et al. Inhibition of the interleukin-36 pathway for the treatment of generalized pustular psoriasis. N Engl J Med. 2019;380:981-983.
- McMullan P, Yaghi M, Truong TM, et al. Safety of dermatologic medications in pregnancy and lactation: an update—part I: pregnancy. J Am Acad Dermatol. Published online January 25, 2024. doi:10.1016/j.jaad.2023.10.072
- Yaghi M, McMullan P, Truong TM, et al. Safety of dermatologic medications in pregnancy and lactation: an update—part II: lactation. J Am Acad Dermatol. Published online January 25, 2024. doi:10.1016/j.jaad.2023.10.071
- Owczarek W, Walecka I, Lesiak A, et al. The use of biological drugs in psoriasis patients prior to pregnancy, during pregnancy and lactation: a review of current clinical guidelines. Postepy Dermatol Alergol. 2020;37:821-830. doi:10.5114/ada.2020.102089
- Organization of Teratology Information Services (OTIS) Autoimmune Diseases in Pregnancy Project. ClinicalTrials.gov identifier: NCT00116272. Updated October 6, 2023. Accessed August 29, 2024. https://clinicaltrials.gov/study/NCT00116272
- Sanchez-Garcia V, Hernandez-Quiles R, de-Miguel-Balsa E, et al. Exposure to biologic therapy before and during pregnancy in patients with psoriasis: systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2023;37:1971-1990. doi:10.1111/jdv.19238
- Silverberg JI, Boguniewicz M, Hanifin J, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis is efficacious regardless of age of disease onset: a post hoc analysis of two phase 3 clinical trials. Dermatol Ther (Heidelb). 2022;12:2731-2746. doi:10.1007/s13555-022-00822-x
- Levi-Schaffer F, Mankuta D. Omalizumab safety in pregnancy. J Allergy Clin Immunol. 2020;145:481-483. doi:10.1016/j.jaci.2019.11.018
- Thaci D, Simpson EL, Beck LA, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2016;387:40-52.
- Chakravarty EF, Murray ER, Kelman A, et al. Pregnancy outcomes after maternal exposure to rituximab. Blood. 2011;117:1499-1506. doi:10.1182/blood-2010-07-295444
Biologics have revolutionized dermatologic treatment, offering substantial relief from chronic and debilitating skin conditions such as psoriasis,
Biologics for Cutaneous Conditions
Biologics—tumor necrosis factor (TNF) α inhibitors; IL-17, IL-23, IL-12, and IL-36 inhibitors; and agents such as omalizumab and dupilumab—have shown remarkable efficacy in controlling severe or recalcitrant dermatologic conditions and typically are more effective than traditional systemic therapies.1 For instance, randomized clinical trials (RCTs) and real-world data have shown that patients with psoriasis can achieve considerable skin clearance with biologics, greatly enhancing QOL.2 Adalimumab and secukinumab, which have been approved for use in moderate to severe cases of hidradenitis suppurativa, reduce the frequency of painful nodules and abscesses, thereby decreasing pain and improving QOL. Dupilumab, an IL-4/13 receptor antagonist, has revolutionized the treatment of AD by drastically reducing itch and skin lesions and improving QOL.3 For chronic urticaria, the anti-IgE antibody omalizumab has effectively reduced the incidence of hives and itching, providing pronounced symptom relief when traditional antihistamines fail.4 Use of rituximab, an anti-CD20 monoclonal antibody, has led to remission in severe cases of pemphigus vulgaris and bullous pemphigoid.5
Impact of Untreated Cutaneous Conditions in Pregnancy
When treating patients who are pregnant, dermatologists must consider the health of both the expectant mother and the developing fetus. This dual focus complicates decision-making, particularly with the use of biologics. Untreated cutaneous conditions can profoundly impact a pregnant patient’s health and QOL as well as lead to pregnancy complications affecting the fetus, such as preterm birth or low birth weight. In some studies, moderate to severe psoriasis has been associated with increased risk for complications during pregnancy, including preeclampsia and intrauterine growth restriction.6 Although specific data on hidradenitis suppurativa are lacking, the highly inflammatory nature of the condition suggests similar adverse effects on pregnancy.7 Atopic dermatitis can be exacerbated during pregnancy due to a shift in the immune system to become more allergic dominant.8 Generalized pustular psoriasis manifests with widespread pustules, fever, and systemic inflammation, posing serious risks to both the mother and the fetus if left untreated9; in such a life-threatening scenario, the use of potent treatments such as spesolimab, an IL-36 receptor antagonist, may be warranted. Therefore, managing these conditions effectively is crucial not only for the mother’s health but also for fetal well-being.
Which Biologics Can Dermatologists Safely Prescribe?
Despite the benefits, many dermatologists are hesitant to prescribe biologics to pregnant patients due to the lack of understanding and definitive safety data.10,11 Although there are no RCTs that involve pregnant patients, current evidence suggests that several biologics are not teratogenic and do not cause fetal malformations. Extensive postexposure data support the safety of TNF-α inhibitors during pregnancy.12 Research has shown that children exposed to these agents in utero have normal development, infection rates, and vaccination outcomes comparable to nonexposed children. For example, a systematic review and meta-analysis found no significant increase in the risk for major congenital malformations, spontaneous abortions, or preterm births among patients exposed to anti–TNF-α agents during pregnancy.2 The Organization of Teratology Information Specialists Autoimmune Diseases in Pregnancy Project has provided valuable real-world data indicating that the use of TNF-α inhibitors in pregnancy, particularly during the first trimester, does not substantially elevate the risk for adverse outcomes.13 These findings have been corroborated by several other registry studies and RCTs, providing a robust safety profile for these agents during pregnancy.14
Similarly, postexposure data on IL-17 and IL-12/23 inhibitors indicate a favorable safety profile, though the sample sizes are smaller than those for anti–TNF-α agents.12,14 Studies of drugs such as secukinumab (IL-17 inhibitor), guselkumab (IL-23 inhibitor), or ustekinumab (IL-12/23 inhibitor) have shown no association with teratogenic effects or increased risk for miscarriage.14 However, agents such as spesolimab (IL-36 inhibitor) are relatively new, and ongoing studies are expected to provide more comprehensive safety data.15 Similarly, omalizumab and dupilumab have not been associated with increased risk for fetal malformations or adverse pregnancy outcomes. Omalizumab, indicated for chronic urticaria, has a good safety profile in pregnancy, with no significant increase in adverse outcomes reported in studies and registries.16 Dupilumab, used for AD, has demonstrated safety in pregnancy, with ongoing studies continuing to monitor outcomes.17
Conversely, rituximab (an anti-CD20 antibody for autoimmune bullous diseases) has shown evidence of adverse pregnancy outcomes, including fetal harm.18 Its use generally is discouraged unless deemed absolutely necessary, and no safer alternatives are available. Rituximab can cross the placenta, especially in the second and third trimesters, and has been associated with B-cell depletion in the fetus, leading to potential immunosuppression and increased risk for infections.5
Although the data on the safety of biologics in pregnancy are largely reassuring, it is essential to recognize that potential risks have not been ruled out entirely. There are extensive safety data for anti–TNF-α inhibitors, which provides a level of confidence; although newer agents such as IL-17 and IL-23 inhibitors have shown promising early results, further research is required to solidify their safety profiles during pregnancy.
Dermatologists must balance the risks and benefits of using biologics in pregnant patients. This decision-making process involves careful consideration of the severity of the mother’s condition, the potential risks to the fetus, and the availability of alternative treatments. For many severe dermatologic conditions, the benefits of biologics in controlling disease activity and improving QOL may outweigh the potential risks, especially when other treatments have failed or are not suitable.
Final Thoughts
The increasing use of biologics in dermatology has undoubtedly improved the management of severe skin conditions, substantially enhancing patients’ QOL. As more data become available and clinical guidelines evolve, health care providers will be better equipped to make informed decisions about the use of biologics, particularly in pregnant patients. Collaborative efforts between dermatologists, obstetricians, and researchers will help refine treatment guidelines and ensure that pregnant patients with severe dermatologic conditions receive the best possible care.
For now, although the current evidence supports the safety of many biologics during pregnancy,10,11 individualized care and informed decision-making remain paramount. Careful management and adherence to current guidelines make it possible to navigate the complexities of treating severe dermatologic conditions in pregnant patients, ensuring the best outcomes for both mother and child.
Biologics have revolutionized dermatologic treatment, offering substantial relief from chronic and debilitating skin conditions such as psoriasis,
Biologics for Cutaneous Conditions
Biologics—tumor necrosis factor (TNF) α inhibitors; IL-17, IL-23, IL-12, and IL-36 inhibitors; and agents such as omalizumab and dupilumab—have shown remarkable efficacy in controlling severe or recalcitrant dermatologic conditions and typically are more effective than traditional systemic therapies.1 For instance, randomized clinical trials (RCTs) and real-world data have shown that patients with psoriasis can achieve considerable skin clearance with biologics, greatly enhancing QOL.2 Adalimumab and secukinumab, which have been approved for use in moderate to severe cases of hidradenitis suppurativa, reduce the frequency of painful nodules and abscesses, thereby decreasing pain and improving QOL. Dupilumab, an IL-4/13 receptor antagonist, has revolutionized the treatment of AD by drastically reducing itch and skin lesions and improving QOL.3 For chronic urticaria, the anti-IgE antibody omalizumab has effectively reduced the incidence of hives and itching, providing pronounced symptom relief when traditional antihistamines fail.4 Use of rituximab, an anti-CD20 monoclonal antibody, has led to remission in severe cases of pemphigus vulgaris and bullous pemphigoid.5
Impact of Untreated Cutaneous Conditions in Pregnancy
When treating patients who are pregnant, dermatologists must consider the health of both the expectant mother and the developing fetus. This dual focus complicates decision-making, particularly with the use of biologics. Untreated cutaneous conditions can profoundly impact a pregnant patient’s health and QOL as well as lead to pregnancy complications affecting the fetus, such as preterm birth or low birth weight. In some studies, moderate to severe psoriasis has been associated with increased risk for complications during pregnancy, including preeclampsia and intrauterine growth restriction.6 Although specific data on hidradenitis suppurativa are lacking, the highly inflammatory nature of the condition suggests similar adverse effects on pregnancy.7 Atopic dermatitis can be exacerbated during pregnancy due to a shift in the immune system to become more allergic dominant.8 Generalized pustular psoriasis manifests with widespread pustules, fever, and systemic inflammation, posing serious risks to both the mother and the fetus if left untreated9; in such a life-threatening scenario, the use of potent treatments such as spesolimab, an IL-36 receptor antagonist, may be warranted. Therefore, managing these conditions effectively is crucial not only for the mother’s health but also for fetal well-being.
Which Biologics Can Dermatologists Safely Prescribe?
Despite the benefits, many dermatologists are hesitant to prescribe biologics to pregnant patients due to the lack of understanding and definitive safety data.10,11 Although there are no RCTs that involve pregnant patients, current evidence suggests that several biologics are not teratogenic and do not cause fetal malformations. Extensive postexposure data support the safety of TNF-α inhibitors during pregnancy.12 Research has shown that children exposed to these agents in utero have normal development, infection rates, and vaccination outcomes comparable to nonexposed children. For example, a systematic review and meta-analysis found no significant increase in the risk for major congenital malformations, spontaneous abortions, or preterm births among patients exposed to anti–TNF-α agents during pregnancy.2 The Organization of Teratology Information Specialists Autoimmune Diseases in Pregnancy Project has provided valuable real-world data indicating that the use of TNF-α inhibitors in pregnancy, particularly during the first trimester, does not substantially elevate the risk for adverse outcomes.13 These findings have been corroborated by several other registry studies and RCTs, providing a robust safety profile for these agents during pregnancy.14
Similarly, postexposure data on IL-17 and IL-12/23 inhibitors indicate a favorable safety profile, though the sample sizes are smaller than those for anti–TNF-α agents.12,14 Studies of drugs such as secukinumab (IL-17 inhibitor), guselkumab (IL-23 inhibitor), or ustekinumab (IL-12/23 inhibitor) have shown no association with teratogenic effects or increased risk for miscarriage.14 However, agents such as spesolimab (IL-36 inhibitor) are relatively new, and ongoing studies are expected to provide more comprehensive safety data.15 Similarly, omalizumab and dupilumab have not been associated with increased risk for fetal malformations or adverse pregnancy outcomes. Omalizumab, indicated for chronic urticaria, has a good safety profile in pregnancy, with no significant increase in adverse outcomes reported in studies and registries.16 Dupilumab, used for AD, has demonstrated safety in pregnancy, with ongoing studies continuing to monitor outcomes.17
Conversely, rituximab (an anti-CD20 antibody for autoimmune bullous diseases) has shown evidence of adverse pregnancy outcomes, including fetal harm.18 Its use generally is discouraged unless deemed absolutely necessary, and no safer alternatives are available. Rituximab can cross the placenta, especially in the second and third trimesters, and has been associated with B-cell depletion in the fetus, leading to potential immunosuppression and increased risk for infections.5
Although the data on the safety of biologics in pregnancy are largely reassuring, it is essential to recognize that potential risks have not been ruled out entirely. There are extensive safety data for anti–TNF-α inhibitors, which provides a level of confidence; although newer agents such as IL-17 and IL-23 inhibitors have shown promising early results, further research is required to solidify their safety profiles during pregnancy.
Dermatologists must balance the risks and benefits of using biologics in pregnant patients. This decision-making process involves careful consideration of the severity of the mother’s condition, the potential risks to the fetus, and the availability of alternative treatments. For many severe dermatologic conditions, the benefits of biologics in controlling disease activity and improving QOL may outweigh the potential risks, especially when other treatments have failed or are not suitable.
Final Thoughts
The increasing use of biologics in dermatology has undoubtedly improved the management of severe skin conditions, substantially enhancing patients’ QOL. As more data become available and clinical guidelines evolve, health care providers will be better equipped to make informed decisions about the use of biologics, particularly in pregnant patients. Collaborative efforts between dermatologists, obstetricians, and researchers will help refine treatment guidelines and ensure that pregnant patients with severe dermatologic conditions receive the best possible care.
For now, although the current evidence supports the safety of many biologics during pregnancy,10,11 individualized care and informed decision-making remain paramount. Careful management and adherence to current guidelines make it possible to navigate the complexities of treating severe dermatologic conditions in pregnant patients, ensuring the best outcomes for both mother and child.
- Sehgal VN, Pandhi D, Khurana A. Biologics in dermatology: an integrated review. Indian J Dermatol. 2014; 59:425-441. doi:10.4103/0019-5154.139859
- Mahadevan U, Wolf DC, Dubinsky M, et al. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:286-292. doi:10.1016/j.cgh.2012.11.011
- Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-2348.
- Saini SS, Bindslev-Jensen C, Maurer M, et al. Efficacy and safety of omalizumab in patients with chronic idiopathic/spontaneous urticaria who remain symptomatic on H1 antihistamines: a randomized, placebo-controlled study. J Invest Dermatol. 2015;135:67-75. doi:10.1038/jid.2014.306
- Mariette X, Forger F, Abraham B, et al. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis. 2018;77:228-233. doi:10.1136/annrheumdis-2017-212196
- Yang Y-W, Chen C-S, Chen Y-H, et al. Psoriasis and pregnancy outcomes: a nationwide population-based study. J Am Acad Dermatol. 2011;64:71-77.
- Zouboulis CC, Del Marmol V, Mrowietz U, et al. Hidradenitis suppurativa/acne inversa: criteria for diagnosis, severity assessment, classification and disease evaluation. Dermatology. 2015;231:184-190.
- Balakirski G, Novak N. Atopic dermatitis and pregnancy. J Allergy Clin Immunol. 2022;149:1185-1194. doi:10.1016/j.jaci.2022.01.010
- Bachelez H, Choon S-E, Marrakchi S, et al. Inhibition of the interleukin-36 pathway for the treatment of generalized pustular psoriasis. N Engl J Med. 2019;380:981-983.
- McMullan P, Yaghi M, Truong TM, et al. Safety of dermatologic medications in pregnancy and lactation: an update—part I: pregnancy. J Am Acad Dermatol. Published online January 25, 2024. doi:10.1016/j.jaad.2023.10.072
- Yaghi M, McMullan P, Truong TM, et al. Safety of dermatologic medications in pregnancy and lactation: an update—part II: lactation. J Am Acad Dermatol. Published online January 25, 2024. doi:10.1016/j.jaad.2023.10.071
- Owczarek W, Walecka I, Lesiak A, et al. The use of biological drugs in psoriasis patients prior to pregnancy, during pregnancy and lactation: a review of current clinical guidelines. Postepy Dermatol Alergol. 2020;37:821-830. doi:10.5114/ada.2020.102089
- Organization of Teratology Information Services (OTIS) Autoimmune Diseases in Pregnancy Project. ClinicalTrials.gov identifier: NCT00116272. Updated October 6, 2023. Accessed August 29, 2024. https://clinicaltrials.gov/study/NCT00116272
- Sanchez-Garcia V, Hernandez-Quiles R, de-Miguel-Balsa E, et al. Exposure to biologic therapy before and during pregnancy in patients with psoriasis: systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2023;37:1971-1990. doi:10.1111/jdv.19238
- Silverberg JI, Boguniewicz M, Hanifin J, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis is efficacious regardless of age of disease onset: a post hoc analysis of two phase 3 clinical trials. Dermatol Ther (Heidelb). 2022;12:2731-2746. doi:10.1007/s13555-022-00822-x
- Levi-Schaffer F, Mankuta D. Omalizumab safety in pregnancy. J Allergy Clin Immunol. 2020;145:481-483. doi:10.1016/j.jaci.2019.11.018
- Thaci D, Simpson EL, Beck LA, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2016;387:40-52.
- Chakravarty EF, Murray ER, Kelman A, et al. Pregnancy outcomes after maternal exposure to rituximab. Blood. 2011;117:1499-1506. doi:10.1182/blood-2010-07-295444
- Sehgal VN, Pandhi D, Khurana A. Biologics in dermatology: an integrated review. Indian J Dermatol. 2014; 59:425-441. doi:10.4103/0019-5154.139859
- Mahadevan U, Wolf DC, Dubinsky M, et al. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:286-292. doi:10.1016/j.cgh.2012.11.011
- Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-2348.
- Saini SS, Bindslev-Jensen C, Maurer M, et al. Efficacy and safety of omalizumab in patients with chronic idiopathic/spontaneous urticaria who remain symptomatic on H1 antihistamines: a randomized, placebo-controlled study. J Invest Dermatol. 2015;135:67-75. doi:10.1038/jid.2014.306
- Mariette X, Forger F, Abraham B, et al. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis. 2018;77:228-233. doi:10.1136/annrheumdis-2017-212196
- Yang Y-W, Chen C-S, Chen Y-H, et al. Psoriasis and pregnancy outcomes: a nationwide population-based study. J Am Acad Dermatol. 2011;64:71-77.
- Zouboulis CC, Del Marmol V, Mrowietz U, et al. Hidradenitis suppurativa/acne inversa: criteria for diagnosis, severity assessment, classification and disease evaluation. Dermatology. 2015;231:184-190.
- Balakirski G, Novak N. Atopic dermatitis and pregnancy. J Allergy Clin Immunol. 2022;149:1185-1194. doi:10.1016/j.jaci.2022.01.010
- Bachelez H, Choon S-E, Marrakchi S, et al. Inhibition of the interleukin-36 pathway for the treatment of generalized pustular psoriasis. N Engl J Med. 2019;380:981-983.
- McMullan P, Yaghi M, Truong TM, et al. Safety of dermatologic medications in pregnancy and lactation: an update—part I: pregnancy. J Am Acad Dermatol. Published online January 25, 2024. doi:10.1016/j.jaad.2023.10.072
- Yaghi M, McMullan P, Truong TM, et al. Safety of dermatologic medications in pregnancy and lactation: an update—part II: lactation. J Am Acad Dermatol. Published online January 25, 2024. doi:10.1016/j.jaad.2023.10.071
- Owczarek W, Walecka I, Lesiak A, et al. The use of biological drugs in psoriasis patients prior to pregnancy, during pregnancy and lactation: a review of current clinical guidelines. Postepy Dermatol Alergol. 2020;37:821-830. doi:10.5114/ada.2020.102089
- Organization of Teratology Information Services (OTIS) Autoimmune Diseases in Pregnancy Project. ClinicalTrials.gov identifier: NCT00116272. Updated October 6, 2023. Accessed August 29, 2024. https://clinicaltrials.gov/study/NCT00116272
- Sanchez-Garcia V, Hernandez-Quiles R, de-Miguel-Balsa E, et al. Exposure to biologic therapy before and during pregnancy in patients with psoriasis: systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2023;37:1971-1990. doi:10.1111/jdv.19238
- Silverberg JI, Boguniewicz M, Hanifin J, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis is efficacious regardless of age of disease onset: a post hoc analysis of two phase 3 clinical trials. Dermatol Ther (Heidelb). 2022;12:2731-2746. doi:10.1007/s13555-022-00822-x
- Levi-Schaffer F, Mankuta D. Omalizumab safety in pregnancy. J Allergy Clin Immunol. 2020;145:481-483. doi:10.1016/j.jaci.2019.11.018
- Thaci D, Simpson EL, Beck LA, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2016;387:40-52.
- Chakravarty EF, Murray ER, Kelman A, et al. Pregnancy outcomes after maternal exposure to rituximab. Blood. 2011;117:1499-1506. doi:10.1182/blood-2010-07-295444
Alzheimer’s and Comorbidities: Implications for Patient Care
Alzheimer’s disease (AD), the most common cause of dementia, is the fifth leading cause of death in the United States. An estimated 6.9 million Americans aged 65 years or older have AD. Comorbid conditions in AD may exacerbate the progression of dementia and negatively affect overall health.
Although the exact mechanisms remain unclear, systemic inflammation is thought to play a significant role in the development of many common comorbidities associated with AD. Among the most frequently observed comorbid conditions are hypertension, diabetes, and depression. The presence of these comorbidities affects the treatment and management of AD, underscoring the need to understand the mechanisms of their interrelationship and develop effective management strategies.
Hypertension
Hypertension is a well-established risk factor for numerous health conditions, including AD. A comprehensive review of five meta-analyses and 52 primary studies revealed that elevated systolic blood pressure (SBP) correlates with an 11 % increased risk of developing AD, raising the question of whether early intervention and control of blood pressure would mitigate the risk for AD later in life.
Findings from the Northern Manhattan Study suggest that although elevated SBP contributes to cognitive decline in older patients, the use of antihypertensive medications can neutralize the effects of high SBP on certain cognitive functions. Furthermore, a systematic review and meta-analysis comprising 12 trials (92,135 participants) demonstrated a significant reduction in the risk for dementia and cognitive impairment with antihypertensive treatment.
Notably, a retrospective cohort study involving 69,081 participants treated with beta-blockers for hypertension found that beta-blockers with high blood-brain barrier permeability were associated with a reduced risk for AD compared with those with low blood-brain barrier permeability. Additionally, a secondary analysis of the SPRINT trial found antihypertensive medications that stimulate vs inhibit type 2 and 4 angiotensin II receptors were associated with a lower incidence of cognitive impairment. Although further clinical trials are necessary to directly assess specific medications, these findings emphasize the potential of antihypertensive treatment as a strategic approach to reduce the risk for AD.
Type 2 Diabetes
The connection between AD and type 2 diabetes is such that AD is sometimes referred to as “type 3 diabetes.” Both diseases share some of the same underlying pathophysiologic mechanisms, particularly the development of insulin resistance and oxidative stress. A prospective cohort study of 10,095 participants showed that diabetes was significantly associated with a higher risk of developing dementia; this risk is even greater in patients who develop diabetes at an earlier age.
In an interview with this news organization, Alvaro Pascual-Leone, MD, PhD, a professor of neurology at Harvard Medical School, Boston, said, “In addition to being a comorbidity factor, diabetes appears to be a predisposing risk factor for AD.” This is supported by a comprehensive literature review showing an increased progression from mild cognitive impairment (MCI) to dementia in patients with diabetes, prediabetes, or metabolic syndrome, with a pooled odds ratio for dementia progression in individuals with diabetes of 1.53.
Owing to the overlapping pathophysiologic mechanisms in AD and diabetes, treating one condition may have beneficial effects on the other. A systematic umbrella review and meta-analysis that included 10 meta-analyses across nine classes of diabetes drugs found a protective effect against dementia with the use of metformin, thiazolidinediones (including pioglitazone), glucagon-like peptide 1 receptor agonists, and sodium-glucose cotransporter 2 inhibitors. Moreover, a cohort study of 12,220 patients who discontinued metformin early (ie, stopped using metformin without a prior history of abnormal kidney function) and 29,126 patients considered routine users found an increased risk for dementia in the early terminator group. Although further research is warranted, the concurrent treatment of AD and diabetes with antidiabetic agents holds considerable promise.
Depression and Anxiety
Anxiety and depression are significant risk factors for AD, and conversely, AD increases the likelihood of developing these psychiatric conditions. A systematic review of 14,760 studies showed dysthymia often emerges during the early stages of AD as an emotional response to cognitive decline.
Data from the Australian Imaging Biomarkers and Lifestyle study showed a markedly elevated risk for AD and MCI among individuals with preexisting anxiety or depression. This study also found that age, sex, and marital status are important determinants, with men and single individuals with depression being particularly susceptible to developing AD. Conversely, a cohort study of 129,410 AD patients with AD, 390,088 patients with all-cause dementia, and 3,900,880 age-matched controls without a history of depression showed a cumulative incidence of depression of 13% in the AD group vs 3% in the control group, suggesting a heightened risk for depression following an AD diagnosis.
These findings underscore the importance of targeted screening and assessment for patients with anxiety and depression who may be at risk for AD or those diagnosed with AD who are at risk for subsequent depression and anxiety. Although antidepressants are effective in treating depression in general, their efficacy in AD-related depression is of variable quality, probably owing to differing pathophysiologic mechanisms of the disease. Further research is necessary to explore both pharmacologic and nonpharmacologic interventions for treating depression in AD patients. Some studies have found that cognitive behavioral-therapy can be effective in improving depression in patients with AD.
Sleep Disorders
Research has shown a strong correlation between AD and sleep disorders, particularly obstructive sleep apnea, insomnia, and circadian rhythm disruptions. Additionally, studies suggest that insomnia and sleep deprivation contribute to increased amyloid beta production and tau pathology, hallmark features of AD. A scoping review of 70 studies proposed that this relationship is mediated by the glymphatic system (glial-dependent waste clearance pathway in the central nervous system), and that sleep deprivation disrupts its function, leading to protein accumulation and subsequent neurologic symptoms of AD. Another study showed that sleep deprivation triggers glial cell activation, initiating an inflammatory cascade that accelerates AD progression.
Given that the gold standard treatment for obstructive sleep apnea is continuous positive airway pressure (CPAP), it has been hypothesized that CPAP could also alleviate AD symptoms owing to shared pathophysiologic mechanisms of these conditions. A large systemic review found that CPAP use improved AD symptoms in patients with mild AD or MCI, though other sleep interventions, such as cognitive-behavioral therapy and melatonin supplementation, have yielded mixed outcomes. However, most studies in this area are small in scale, and there remains a paucity of research on treating sleep disorders in AD patients, indicating a need for further investigation.
Musculoskeletal Disorders
Although no direct causative link has been established, research indicates an association between osteoarthritis (OA) and dementia, likely because of similar pathophysiologic mechanisms, including systemic inflammation. Longitudinal analyses of data from the Alzheimer’s Disease Neuroimaging Initiative study found cognitively normal older individuals with OA experience more rapid declines in hippocampal volumes compared to those without OA, suggesting that OA may elevate the risk of cognitive impairment. Current treatments for OA, such as nonsteroidal anti-inflammatory drugs, glucocorticoids, and disease-modifying OA drugs, might also help alleviate AD symptoms related to inflammation, though the research in this area is limited.
AD has also been linked to osteoporosis. In a longitudinal follow-up study involving 78,994 patients with osteoporosis and 78,994 controls, AD developed in 5856 patients with osteoporosis compared with 3761 patients in the control group. These findings represent a 1.27-fold higher incidence of AD in patients with osteoporosis than in the control group, suggesting that osteoporosis might be a risk factor for AD.
Additionally, research has identified a relationship between AD and increased fracture risk and decreased bone mineral density, with AD patients exhibiting a significantly higher likelihood of bone fractures compared with those without AD. “Falls and fractures, aside from the risk they pose in all geriatric patients, in individuals with cognitive impairment — whether due to AD or another cause — have higher risk to cause delirium and that can result in greater morbidity and mortality and a lasting increase in cognitive disability,” stated Dr. Pascual-Leone. Current recommendations emphasize exercise and fall prevention strategies to reduce fracture risk in patients with AD, but there is a lack of comprehensive research on the safety and efficacy of osteoporosis medications in this population.
Implications for Clinical Practice
The intricate interplay between AD and its comorbidities highlights the need for a comprehensive and integrated approach to patient care. The overlapping pathophysiologic mechanisms suggest that these comorbidities can contribute to the evolution and progression of AD. Likewise, AD can exacerbate comorbid conditions. As such, a holistic assessment strategy that prioritizes early detection and management of comorbid conditions to mitigate their impact on AD progression would be beneficial. Dr. Pascual-Leone added, “The presence of any of these comorbidities suggests a need to screen for MCI earlier than might otherwise be indicated or as part of the treatment for the comorbid condition. In many cases, patients can make lifestyle modifications that improve not only the comorbid condition but also reduce its effect on dementia.” In doing so, healthcare providers can help improve patient outcomes and enhance the overall quality of life for individuals living with AD.
Alissa Hershberger, Professor of Nursing, University of Central Missouri, Lee’s Summit, Missouri, has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Alzheimer’s disease (AD), the most common cause of dementia, is the fifth leading cause of death in the United States. An estimated 6.9 million Americans aged 65 years or older have AD. Comorbid conditions in AD may exacerbate the progression of dementia and negatively affect overall health.
Although the exact mechanisms remain unclear, systemic inflammation is thought to play a significant role in the development of many common comorbidities associated with AD. Among the most frequently observed comorbid conditions are hypertension, diabetes, and depression. The presence of these comorbidities affects the treatment and management of AD, underscoring the need to understand the mechanisms of their interrelationship and develop effective management strategies.
Hypertension
Hypertension is a well-established risk factor for numerous health conditions, including AD. A comprehensive review of five meta-analyses and 52 primary studies revealed that elevated systolic blood pressure (SBP) correlates with an 11 % increased risk of developing AD, raising the question of whether early intervention and control of blood pressure would mitigate the risk for AD later in life.
Findings from the Northern Manhattan Study suggest that although elevated SBP contributes to cognitive decline in older patients, the use of antihypertensive medications can neutralize the effects of high SBP on certain cognitive functions. Furthermore, a systematic review and meta-analysis comprising 12 trials (92,135 participants) demonstrated a significant reduction in the risk for dementia and cognitive impairment with antihypertensive treatment.
Notably, a retrospective cohort study involving 69,081 participants treated with beta-blockers for hypertension found that beta-blockers with high blood-brain barrier permeability were associated with a reduced risk for AD compared with those with low blood-brain barrier permeability. Additionally, a secondary analysis of the SPRINT trial found antihypertensive medications that stimulate vs inhibit type 2 and 4 angiotensin II receptors were associated with a lower incidence of cognitive impairment. Although further clinical trials are necessary to directly assess specific medications, these findings emphasize the potential of antihypertensive treatment as a strategic approach to reduce the risk for AD.
Type 2 Diabetes
The connection between AD and type 2 diabetes is such that AD is sometimes referred to as “type 3 diabetes.” Both diseases share some of the same underlying pathophysiologic mechanisms, particularly the development of insulin resistance and oxidative stress. A prospective cohort study of 10,095 participants showed that diabetes was significantly associated with a higher risk of developing dementia; this risk is even greater in patients who develop diabetes at an earlier age.
In an interview with this news organization, Alvaro Pascual-Leone, MD, PhD, a professor of neurology at Harvard Medical School, Boston, said, “In addition to being a comorbidity factor, diabetes appears to be a predisposing risk factor for AD.” This is supported by a comprehensive literature review showing an increased progression from mild cognitive impairment (MCI) to dementia in patients with diabetes, prediabetes, or metabolic syndrome, with a pooled odds ratio for dementia progression in individuals with diabetes of 1.53.
Owing to the overlapping pathophysiologic mechanisms in AD and diabetes, treating one condition may have beneficial effects on the other. A systematic umbrella review and meta-analysis that included 10 meta-analyses across nine classes of diabetes drugs found a protective effect against dementia with the use of metformin, thiazolidinediones (including pioglitazone), glucagon-like peptide 1 receptor agonists, and sodium-glucose cotransporter 2 inhibitors. Moreover, a cohort study of 12,220 patients who discontinued metformin early (ie, stopped using metformin without a prior history of abnormal kidney function) and 29,126 patients considered routine users found an increased risk for dementia in the early terminator group. Although further research is warranted, the concurrent treatment of AD and diabetes with antidiabetic agents holds considerable promise.
Depression and Anxiety
Anxiety and depression are significant risk factors for AD, and conversely, AD increases the likelihood of developing these psychiatric conditions. A systematic review of 14,760 studies showed dysthymia often emerges during the early stages of AD as an emotional response to cognitive decline.
Data from the Australian Imaging Biomarkers and Lifestyle study showed a markedly elevated risk for AD and MCI among individuals with preexisting anxiety or depression. This study also found that age, sex, and marital status are important determinants, with men and single individuals with depression being particularly susceptible to developing AD. Conversely, a cohort study of 129,410 AD patients with AD, 390,088 patients with all-cause dementia, and 3,900,880 age-matched controls without a history of depression showed a cumulative incidence of depression of 13% in the AD group vs 3% in the control group, suggesting a heightened risk for depression following an AD diagnosis.
These findings underscore the importance of targeted screening and assessment for patients with anxiety and depression who may be at risk for AD or those diagnosed with AD who are at risk for subsequent depression and anxiety. Although antidepressants are effective in treating depression in general, their efficacy in AD-related depression is of variable quality, probably owing to differing pathophysiologic mechanisms of the disease. Further research is necessary to explore both pharmacologic and nonpharmacologic interventions for treating depression in AD patients. Some studies have found that cognitive behavioral-therapy can be effective in improving depression in patients with AD.
Sleep Disorders
Research has shown a strong correlation between AD and sleep disorders, particularly obstructive sleep apnea, insomnia, and circadian rhythm disruptions. Additionally, studies suggest that insomnia and sleep deprivation contribute to increased amyloid beta production and tau pathology, hallmark features of AD. A scoping review of 70 studies proposed that this relationship is mediated by the glymphatic system (glial-dependent waste clearance pathway in the central nervous system), and that sleep deprivation disrupts its function, leading to protein accumulation and subsequent neurologic symptoms of AD. Another study showed that sleep deprivation triggers glial cell activation, initiating an inflammatory cascade that accelerates AD progression.
Given that the gold standard treatment for obstructive sleep apnea is continuous positive airway pressure (CPAP), it has been hypothesized that CPAP could also alleviate AD symptoms owing to shared pathophysiologic mechanisms of these conditions. A large systemic review found that CPAP use improved AD symptoms in patients with mild AD or MCI, though other sleep interventions, such as cognitive-behavioral therapy and melatonin supplementation, have yielded mixed outcomes. However, most studies in this area are small in scale, and there remains a paucity of research on treating sleep disorders in AD patients, indicating a need for further investigation.
Musculoskeletal Disorders
Although no direct causative link has been established, research indicates an association between osteoarthritis (OA) and dementia, likely because of similar pathophysiologic mechanisms, including systemic inflammation. Longitudinal analyses of data from the Alzheimer’s Disease Neuroimaging Initiative study found cognitively normal older individuals with OA experience more rapid declines in hippocampal volumes compared to those without OA, suggesting that OA may elevate the risk of cognitive impairment. Current treatments for OA, such as nonsteroidal anti-inflammatory drugs, glucocorticoids, and disease-modifying OA drugs, might also help alleviate AD symptoms related to inflammation, though the research in this area is limited.
AD has also been linked to osteoporosis. In a longitudinal follow-up study involving 78,994 patients with osteoporosis and 78,994 controls, AD developed in 5856 patients with osteoporosis compared with 3761 patients in the control group. These findings represent a 1.27-fold higher incidence of AD in patients with osteoporosis than in the control group, suggesting that osteoporosis might be a risk factor for AD.
Additionally, research has identified a relationship between AD and increased fracture risk and decreased bone mineral density, with AD patients exhibiting a significantly higher likelihood of bone fractures compared with those without AD. “Falls and fractures, aside from the risk they pose in all geriatric patients, in individuals with cognitive impairment — whether due to AD or another cause — have higher risk to cause delirium and that can result in greater morbidity and mortality and a lasting increase in cognitive disability,” stated Dr. Pascual-Leone. Current recommendations emphasize exercise and fall prevention strategies to reduce fracture risk in patients with AD, but there is a lack of comprehensive research on the safety and efficacy of osteoporosis medications in this population.
Implications for Clinical Practice
The intricate interplay between AD and its comorbidities highlights the need for a comprehensive and integrated approach to patient care. The overlapping pathophysiologic mechanisms suggest that these comorbidities can contribute to the evolution and progression of AD. Likewise, AD can exacerbate comorbid conditions. As such, a holistic assessment strategy that prioritizes early detection and management of comorbid conditions to mitigate their impact on AD progression would be beneficial. Dr. Pascual-Leone added, “The presence of any of these comorbidities suggests a need to screen for MCI earlier than might otherwise be indicated or as part of the treatment for the comorbid condition. In many cases, patients can make lifestyle modifications that improve not only the comorbid condition but also reduce its effect on dementia.” In doing so, healthcare providers can help improve patient outcomes and enhance the overall quality of life for individuals living with AD.
Alissa Hershberger, Professor of Nursing, University of Central Missouri, Lee’s Summit, Missouri, has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Alzheimer’s disease (AD), the most common cause of dementia, is the fifth leading cause of death in the United States. An estimated 6.9 million Americans aged 65 years or older have AD. Comorbid conditions in AD may exacerbate the progression of dementia and negatively affect overall health.
Although the exact mechanisms remain unclear, systemic inflammation is thought to play a significant role in the development of many common comorbidities associated with AD. Among the most frequently observed comorbid conditions are hypertension, diabetes, and depression. The presence of these comorbidities affects the treatment and management of AD, underscoring the need to understand the mechanisms of their interrelationship and develop effective management strategies.
Hypertension
Hypertension is a well-established risk factor for numerous health conditions, including AD. A comprehensive review of five meta-analyses and 52 primary studies revealed that elevated systolic blood pressure (SBP) correlates with an 11 % increased risk of developing AD, raising the question of whether early intervention and control of blood pressure would mitigate the risk for AD later in life.
Findings from the Northern Manhattan Study suggest that although elevated SBP contributes to cognitive decline in older patients, the use of antihypertensive medications can neutralize the effects of high SBP on certain cognitive functions. Furthermore, a systematic review and meta-analysis comprising 12 trials (92,135 participants) demonstrated a significant reduction in the risk for dementia and cognitive impairment with antihypertensive treatment.
Notably, a retrospective cohort study involving 69,081 participants treated with beta-blockers for hypertension found that beta-blockers with high blood-brain barrier permeability were associated with a reduced risk for AD compared with those with low blood-brain barrier permeability. Additionally, a secondary analysis of the SPRINT trial found antihypertensive medications that stimulate vs inhibit type 2 and 4 angiotensin II receptors were associated with a lower incidence of cognitive impairment. Although further clinical trials are necessary to directly assess specific medications, these findings emphasize the potential of antihypertensive treatment as a strategic approach to reduce the risk for AD.
Type 2 Diabetes
The connection between AD and type 2 diabetes is such that AD is sometimes referred to as “type 3 diabetes.” Both diseases share some of the same underlying pathophysiologic mechanisms, particularly the development of insulin resistance and oxidative stress. A prospective cohort study of 10,095 participants showed that diabetes was significantly associated with a higher risk of developing dementia; this risk is even greater in patients who develop diabetes at an earlier age.
In an interview with this news organization, Alvaro Pascual-Leone, MD, PhD, a professor of neurology at Harvard Medical School, Boston, said, “In addition to being a comorbidity factor, diabetes appears to be a predisposing risk factor for AD.” This is supported by a comprehensive literature review showing an increased progression from mild cognitive impairment (MCI) to dementia in patients with diabetes, prediabetes, or metabolic syndrome, with a pooled odds ratio for dementia progression in individuals with diabetes of 1.53.
Owing to the overlapping pathophysiologic mechanisms in AD and diabetes, treating one condition may have beneficial effects on the other. A systematic umbrella review and meta-analysis that included 10 meta-analyses across nine classes of diabetes drugs found a protective effect against dementia with the use of metformin, thiazolidinediones (including pioglitazone), glucagon-like peptide 1 receptor agonists, and sodium-glucose cotransporter 2 inhibitors. Moreover, a cohort study of 12,220 patients who discontinued metformin early (ie, stopped using metformin without a prior history of abnormal kidney function) and 29,126 patients considered routine users found an increased risk for dementia in the early terminator group. Although further research is warranted, the concurrent treatment of AD and diabetes with antidiabetic agents holds considerable promise.
Depression and Anxiety
Anxiety and depression are significant risk factors for AD, and conversely, AD increases the likelihood of developing these psychiatric conditions. A systematic review of 14,760 studies showed dysthymia often emerges during the early stages of AD as an emotional response to cognitive decline.
Data from the Australian Imaging Biomarkers and Lifestyle study showed a markedly elevated risk for AD and MCI among individuals with preexisting anxiety or depression. This study also found that age, sex, and marital status are important determinants, with men and single individuals with depression being particularly susceptible to developing AD. Conversely, a cohort study of 129,410 AD patients with AD, 390,088 patients with all-cause dementia, and 3,900,880 age-matched controls without a history of depression showed a cumulative incidence of depression of 13% in the AD group vs 3% in the control group, suggesting a heightened risk for depression following an AD diagnosis.
These findings underscore the importance of targeted screening and assessment for patients with anxiety and depression who may be at risk for AD or those diagnosed with AD who are at risk for subsequent depression and anxiety. Although antidepressants are effective in treating depression in general, their efficacy in AD-related depression is of variable quality, probably owing to differing pathophysiologic mechanisms of the disease. Further research is necessary to explore both pharmacologic and nonpharmacologic interventions for treating depression in AD patients. Some studies have found that cognitive behavioral-therapy can be effective in improving depression in patients with AD.
Sleep Disorders
Research has shown a strong correlation between AD and sleep disorders, particularly obstructive sleep apnea, insomnia, and circadian rhythm disruptions. Additionally, studies suggest that insomnia and sleep deprivation contribute to increased amyloid beta production and tau pathology, hallmark features of AD. A scoping review of 70 studies proposed that this relationship is mediated by the glymphatic system (glial-dependent waste clearance pathway in the central nervous system), and that sleep deprivation disrupts its function, leading to protein accumulation and subsequent neurologic symptoms of AD. Another study showed that sleep deprivation triggers glial cell activation, initiating an inflammatory cascade that accelerates AD progression.
Given that the gold standard treatment for obstructive sleep apnea is continuous positive airway pressure (CPAP), it has been hypothesized that CPAP could also alleviate AD symptoms owing to shared pathophysiologic mechanisms of these conditions. A large systemic review found that CPAP use improved AD symptoms in patients with mild AD or MCI, though other sleep interventions, such as cognitive-behavioral therapy and melatonin supplementation, have yielded mixed outcomes. However, most studies in this area are small in scale, and there remains a paucity of research on treating sleep disorders in AD patients, indicating a need for further investigation.
Musculoskeletal Disorders
Although no direct causative link has been established, research indicates an association between osteoarthritis (OA) and dementia, likely because of similar pathophysiologic mechanisms, including systemic inflammation. Longitudinal analyses of data from the Alzheimer’s Disease Neuroimaging Initiative study found cognitively normal older individuals with OA experience more rapid declines in hippocampal volumes compared to those without OA, suggesting that OA may elevate the risk of cognitive impairment. Current treatments for OA, such as nonsteroidal anti-inflammatory drugs, glucocorticoids, and disease-modifying OA drugs, might also help alleviate AD symptoms related to inflammation, though the research in this area is limited.
AD has also been linked to osteoporosis. In a longitudinal follow-up study involving 78,994 patients with osteoporosis and 78,994 controls, AD developed in 5856 patients with osteoporosis compared with 3761 patients in the control group. These findings represent a 1.27-fold higher incidence of AD in patients with osteoporosis than in the control group, suggesting that osteoporosis might be a risk factor for AD.
Additionally, research has identified a relationship between AD and increased fracture risk and decreased bone mineral density, with AD patients exhibiting a significantly higher likelihood of bone fractures compared with those without AD. “Falls and fractures, aside from the risk they pose in all geriatric patients, in individuals with cognitive impairment — whether due to AD or another cause — have higher risk to cause delirium and that can result in greater morbidity and mortality and a lasting increase in cognitive disability,” stated Dr. Pascual-Leone. Current recommendations emphasize exercise and fall prevention strategies to reduce fracture risk in patients with AD, but there is a lack of comprehensive research on the safety and efficacy of osteoporosis medications in this population.
Implications for Clinical Practice
The intricate interplay between AD and its comorbidities highlights the need for a comprehensive and integrated approach to patient care. The overlapping pathophysiologic mechanisms suggest that these comorbidities can contribute to the evolution and progression of AD. Likewise, AD can exacerbate comorbid conditions. As such, a holistic assessment strategy that prioritizes early detection and management of comorbid conditions to mitigate their impact on AD progression would be beneficial. Dr. Pascual-Leone added, “The presence of any of these comorbidities suggests a need to screen for MCI earlier than might otherwise be indicated or as part of the treatment for the comorbid condition. In many cases, patients can make lifestyle modifications that improve not only the comorbid condition but also reduce its effect on dementia.” In doing so, healthcare providers can help improve patient outcomes and enhance the overall quality of life for individuals living with AD.
Alissa Hershberger, Professor of Nursing, University of Central Missouri, Lee’s Summit, Missouri, has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Following the Light
Percutaneous endoscopic gastrostomy (PEG) was first introduced in the early 1980s by surgeons Michael Gauderer and Jeffrey Ponsky as a less-invasive alternative to surgical gastrostomy via open laparotomy. The concept was born after the pair observed that the light from an endoscope in an infant undergoing endoscopy caused the abdominal wall to glow in the darkened operating room.
In fact, PEG was among the first procedures that defined minimally invasive surgery, a concept that has now revolutionized the surgical field. Since that time, PEG has evolved as a preferred method for patients needing long-term nutritional support for various indications. By 2001, approximately 216,000 PEGs were placed annually in the United States. While the volume of PEG procedures has declined in recent years at some institutions as practice patterns have shifted toward interventional radiology–placed gastrostomy tubes, evaluation of patients for PEG insertion, removal, or management of PEG complications remains a core area of gastroenterology practice.
Among the most important roles of the gastroenterologist in considering potential PEG candidates is to determine whether an appropriate indication exists, a decision that requires a detailed understanding of a patient’s overall clinical condition, goals of care, values, and preferences. a common GI consultation that deserves thoughtful consideration and demands effective communication among members of the multidisciplinary team and with patients.
Also in our October issue, we highlight a recently published large multicohort study from Gastroenterology elucidating clinical, serologic, and genetic factors associated with extraintestinal manifestations in IBD. We also review key updates to colonoscopy quality indicators, including modifications to existing indicators such as ADR and the addition of two new “priority indicators” — rate of inadequate bowel prep and sessile serrated lesion detection rate.
In this month’s Member Spotlight, Dr. Stephanie Pointer of Digestive & Liver Health Specialists in Nashville, Tennessee, shares the many ways in which she has given back to her community through music and mentoring while leading a thriving GI practice. We hope you enjoy this, and all the coverage included in our October issue.
Megan A. Adams, MD, JD, MSc
Editor in Chief
Percutaneous endoscopic gastrostomy (PEG) was first introduced in the early 1980s by surgeons Michael Gauderer and Jeffrey Ponsky as a less-invasive alternative to surgical gastrostomy via open laparotomy. The concept was born after the pair observed that the light from an endoscope in an infant undergoing endoscopy caused the abdominal wall to glow in the darkened operating room.
In fact, PEG was among the first procedures that defined minimally invasive surgery, a concept that has now revolutionized the surgical field. Since that time, PEG has evolved as a preferred method for patients needing long-term nutritional support for various indications. By 2001, approximately 216,000 PEGs were placed annually in the United States. While the volume of PEG procedures has declined in recent years at some institutions as practice patterns have shifted toward interventional radiology–placed gastrostomy tubes, evaluation of patients for PEG insertion, removal, or management of PEG complications remains a core area of gastroenterology practice.
Among the most important roles of the gastroenterologist in considering potential PEG candidates is to determine whether an appropriate indication exists, a decision that requires a detailed understanding of a patient’s overall clinical condition, goals of care, values, and preferences. a common GI consultation that deserves thoughtful consideration and demands effective communication among members of the multidisciplinary team and with patients.
Also in our October issue, we highlight a recently published large multicohort study from Gastroenterology elucidating clinical, serologic, and genetic factors associated with extraintestinal manifestations in IBD. We also review key updates to colonoscopy quality indicators, including modifications to existing indicators such as ADR and the addition of two new “priority indicators” — rate of inadequate bowel prep and sessile serrated lesion detection rate.
In this month’s Member Spotlight, Dr. Stephanie Pointer of Digestive & Liver Health Specialists in Nashville, Tennessee, shares the many ways in which she has given back to her community through music and mentoring while leading a thriving GI practice. We hope you enjoy this, and all the coverage included in our October issue.
Megan A. Adams, MD, JD, MSc
Editor in Chief
Percutaneous endoscopic gastrostomy (PEG) was first introduced in the early 1980s by surgeons Michael Gauderer and Jeffrey Ponsky as a less-invasive alternative to surgical gastrostomy via open laparotomy. The concept was born after the pair observed that the light from an endoscope in an infant undergoing endoscopy caused the abdominal wall to glow in the darkened operating room.
In fact, PEG was among the first procedures that defined minimally invasive surgery, a concept that has now revolutionized the surgical field. Since that time, PEG has evolved as a preferred method for patients needing long-term nutritional support for various indications. By 2001, approximately 216,000 PEGs were placed annually in the United States. While the volume of PEG procedures has declined in recent years at some institutions as practice patterns have shifted toward interventional radiology–placed gastrostomy tubes, evaluation of patients for PEG insertion, removal, or management of PEG complications remains a core area of gastroenterology practice.
Among the most important roles of the gastroenterologist in considering potential PEG candidates is to determine whether an appropriate indication exists, a decision that requires a detailed understanding of a patient’s overall clinical condition, goals of care, values, and preferences. a common GI consultation that deserves thoughtful consideration and demands effective communication among members of the multidisciplinary team and with patients.
Also in our October issue, we highlight a recently published large multicohort study from Gastroenterology elucidating clinical, serologic, and genetic factors associated with extraintestinal manifestations in IBD. We also review key updates to colonoscopy quality indicators, including modifications to existing indicators such as ADR and the addition of two new “priority indicators” — rate of inadequate bowel prep and sessile serrated lesion detection rate.
In this month’s Member Spotlight, Dr. Stephanie Pointer of Digestive & Liver Health Specialists in Nashville, Tennessee, shares the many ways in which she has given back to her community through music and mentoring while leading a thriving GI practice. We hope you enjoy this, and all the coverage included in our October issue.
Megan A. Adams, MD, JD, MSc
Editor in Chief
Guidance for Practicing Primary Care: World Health Organization’s Updated Influenza Guidelines for 2024
As primary care physicians, we are often the first ones patients see when they become infected with influenza. According to Centers for Disease Control and Prevention statistics, approximately 5%-20% of the US population will be infected with influenza every year. Additionally, more than 200,000 of these patients will be hospitalized because of complications related to influenza.
Earlier in September, the World Health Organization (WHO) issued its latest clinical practice guidelines for influenza for the 2024-2025 season. This is a 213-page document aimed at healthcare providers who treat patients infected with influenza. It includes treatment for those with severe and nonsevere influenza infections, those in both the outpatient and hospitalized setting, as well as medication prophylaxis for those exposed to the virus. Additionally, it defines risk estimates for those who are at risk of being hospitalized or dying. In contrast, previous updates focused on management of severe influenza or those at risk of severe influenza.
These guidelines cover recommendations regarding all the antiviral medications for treating influenza used around the world. For the purpose of this article, we will focus on those most commonly used in the United States.
A newer medication discussed was baloxavir. It is recommended to be used for patients with nonsevere influenza who are at high risk for progression to severe disease. The advice is to not use it for those with little risk of progression to severe disease. Oseltamivir is recommended for those with severe infection.
The guidelines recommend against using antibiotics for those who have a low likelihood of having a bacterial coinfection. As primary care doctors, we often prescribe medications to help with symptoms. These guidelines recommend against the use of corticosteroids and antibiotics but did advise that NSAIDs could be used for symptom relief.
One of the important parts of these guidelines is prevention in patients who have been exposed but are asymptomatic. They recommend baloxavir or oseltamivir but only for those patients who are at high risk of being hospitalized if they were to become infected. Any of the antivirals can be used for patients who are exposed to the novel influenza A, which is associated with a higher mortality rate. Caution when prescribing antivirals is recommended in immunocompromised patients because there is more drug resistance seen in these patients.
These updates also discuss the use of different influenza tests. In the outpatient setting, primary doctors don’t have time for test results that may take 2 days to come back. Only rapid tests make the sense in the primary care setting. Additionally, in the age of COVID, it is important to make an accurate diagnosis so we should be testing patients. There is resistance seen with the antivirals we prescribe for influenza so prescribing them empirically without a confirmed diagnosis of influenza may be doing more harm than good.
One gap in these recommendations is vaccination. This topic was not covered at all. It would be helpful to have a strategy in place to prevent infection in populations rather than focusing just on exposed individuals. A discussion of when and who and to vaccinate would be helpful. Research into the effectiveness of vaccines is key and more accurate development of a season’s influenza vaccine would be beneficial. Currently, there is much vaccine misinformation being spread around. Education and information regarding influenza vaccines, especially coming from WHO, is crucial.
Another failure of these recommendations is that the guidelines apply only to those who present within a few days of becoming symptomatic. As family doctors, we know many of our patients self-treat or consult Google. They often don’t come for medical care until they’ve been sick for a week or longer. There are no guidelines for these patients.
In general, these guidelines are comprehensive and do a great job discussing the current medications available. However, more is needed to increase vaccination rates. Patients need to know that if they may be sick with influenza, they need to seek medical care as soon as possible. We, as family doctors, need to do a better job of risk-stratifying our patients and prescribing prophylactic medication when suitable. Every infection we prevent aids in the health of our community and the global population at large.
Dr. Girgis practices family medicine in South River, New Jersey, and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, New Jersey. She has no relevant conflicts of interest.
As primary care physicians, we are often the first ones patients see when they become infected with influenza. According to Centers for Disease Control and Prevention statistics, approximately 5%-20% of the US population will be infected with influenza every year. Additionally, more than 200,000 of these patients will be hospitalized because of complications related to influenza.
Earlier in September, the World Health Organization (WHO) issued its latest clinical practice guidelines for influenza for the 2024-2025 season. This is a 213-page document aimed at healthcare providers who treat patients infected with influenza. It includes treatment for those with severe and nonsevere influenza infections, those in both the outpatient and hospitalized setting, as well as medication prophylaxis for those exposed to the virus. Additionally, it defines risk estimates for those who are at risk of being hospitalized or dying. In contrast, previous updates focused on management of severe influenza or those at risk of severe influenza.
These guidelines cover recommendations regarding all the antiviral medications for treating influenza used around the world. For the purpose of this article, we will focus on those most commonly used in the United States.
A newer medication discussed was baloxavir. It is recommended to be used for patients with nonsevere influenza who are at high risk for progression to severe disease. The advice is to not use it for those with little risk of progression to severe disease. Oseltamivir is recommended for those with severe infection.
The guidelines recommend against using antibiotics for those who have a low likelihood of having a bacterial coinfection. As primary care doctors, we often prescribe medications to help with symptoms. These guidelines recommend against the use of corticosteroids and antibiotics but did advise that NSAIDs could be used for symptom relief.
One of the important parts of these guidelines is prevention in patients who have been exposed but are asymptomatic. They recommend baloxavir or oseltamivir but only for those patients who are at high risk of being hospitalized if they were to become infected. Any of the antivirals can be used for patients who are exposed to the novel influenza A, which is associated with a higher mortality rate. Caution when prescribing antivirals is recommended in immunocompromised patients because there is more drug resistance seen in these patients.
These updates also discuss the use of different influenza tests. In the outpatient setting, primary doctors don’t have time for test results that may take 2 days to come back. Only rapid tests make the sense in the primary care setting. Additionally, in the age of COVID, it is important to make an accurate diagnosis so we should be testing patients. There is resistance seen with the antivirals we prescribe for influenza so prescribing them empirically without a confirmed diagnosis of influenza may be doing more harm than good.
One gap in these recommendations is vaccination. This topic was not covered at all. It would be helpful to have a strategy in place to prevent infection in populations rather than focusing just on exposed individuals. A discussion of when and who and to vaccinate would be helpful. Research into the effectiveness of vaccines is key and more accurate development of a season’s influenza vaccine would be beneficial. Currently, there is much vaccine misinformation being spread around. Education and information regarding influenza vaccines, especially coming from WHO, is crucial.
Another failure of these recommendations is that the guidelines apply only to those who present within a few days of becoming symptomatic. As family doctors, we know many of our patients self-treat or consult Google. They often don’t come for medical care until they’ve been sick for a week or longer. There are no guidelines for these patients.
In general, these guidelines are comprehensive and do a great job discussing the current medications available. However, more is needed to increase vaccination rates. Patients need to know that if they may be sick with influenza, they need to seek medical care as soon as possible. We, as family doctors, need to do a better job of risk-stratifying our patients and prescribing prophylactic medication when suitable. Every infection we prevent aids in the health of our community and the global population at large.
Dr. Girgis practices family medicine in South River, New Jersey, and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, New Jersey. She has no relevant conflicts of interest.
As primary care physicians, we are often the first ones patients see when they become infected with influenza. According to Centers for Disease Control and Prevention statistics, approximately 5%-20% of the US population will be infected with influenza every year. Additionally, more than 200,000 of these patients will be hospitalized because of complications related to influenza.
Earlier in September, the World Health Organization (WHO) issued its latest clinical practice guidelines for influenza for the 2024-2025 season. This is a 213-page document aimed at healthcare providers who treat patients infected with influenza. It includes treatment for those with severe and nonsevere influenza infections, those in both the outpatient and hospitalized setting, as well as medication prophylaxis for those exposed to the virus. Additionally, it defines risk estimates for those who are at risk of being hospitalized or dying. In contrast, previous updates focused on management of severe influenza or those at risk of severe influenza.
These guidelines cover recommendations regarding all the antiviral medications for treating influenza used around the world. For the purpose of this article, we will focus on those most commonly used in the United States.
A newer medication discussed was baloxavir. It is recommended to be used for patients with nonsevere influenza who are at high risk for progression to severe disease. The advice is to not use it for those with little risk of progression to severe disease. Oseltamivir is recommended for those with severe infection.
The guidelines recommend against using antibiotics for those who have a low likelihood of having a bacterial coinfection. As primary care doctors, we often prescribe medications to help with symptoms. These guidelines recommend against the use of corticosteroids and antibiotics but did advise that NSAIDs could be used for symptom relief.
One of the important parts of these guidelines is prevention in patients who have been exposed but are asymptomatic. They recommend baloxavir or oseltamivir but only for those patients who are at high risk of being hospitalized if they were to become infected. Any of the antivirals can be used for patients who are exposed to the novel influenza A, which is associated with a higher mortality rate. Caution when prescribing antivirals is recommended in immunocompromised patients because there is more drug resistance seen in these patients.
These updates also discuss the use of different influenza tests. In the outpatient setting, primary doctors don’t have time for test results that may take 2 days to come back. Only rapid tests make the sense in the primary care setting. Additionally, in the age of COVID, it is important to make an accurate diagnosis so we should be testing patients. There is resistance seen with the antivirals we prescribe for influenza so prescribing them empirically without a confirmed diagnosis of influenza may be doing more harm than good.
One gap in these recommendations is vaccination. This topic was not covered at all. It would be helpful to have a strategy in place to prevent infection in populations rather than focusing just on exposed individuals. A discussion of when and who and to vaccinate would be helpful. Research into the effectiveness of vaccines is key and more accurate development of a season’s influenza vaccine would be beneficial. Currently, there is much vaccine misinformation being spread around. Education and information regarding influenza vaccines, especially coming from WHO, is crucial.
Another failure of these recommendations is that the guidelines apply only to those who present within a few days of becoming symptomatic. As family doctors, we know many of our patients self-treat or consult Google. They often don’t come for medical care until they’ve been sick for a week or longer. There are no guidelines for these patients.
In general, these guidelines are comprehensive and do a great job discussing the current medications available. However, more is needed to increase vaccination rates. Patients need to know that if they may be sick with influenza, they need to seek medical care as soon as possible. We, as family doctors, need to do a better job of risk-stratifying our patients and prescribing prophylactic medication when suitable. Every infection we prevent aids in the health of our community and the global population at large.
Dr. Girgis practices family medicine in South River, New Jersey, and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, New Jersey. She has no relevant conflicts of interest.
Five Essential Nutrients for Patients on GLP-1s
Fatigue, nausea, acid reflux, muscle loss, and the dreaded “Ozempic face” are side effects from using glucagon-like peptide 1 (GLP-1) receptor agonists (RAs) such as semaglutide or the dual glucose-dependent insulinotropic polypeptide (GIP)/GLP-1 RA tirzepatide to control blood sugar and promote weight loss.
But what I’ve learned from working with hundreds of patients on these medications, and others, is that most (if not all) of these side effects can be minimized by ensuring proper nutrition.
Setting patients up for success requires dietary education and counseling, along with regular monitoring to determine any nutritional deficiencies. Although adequate intake of all the macro and micronutrients is obviously important,
Protein
My patients are probably sick of hearing me talk about protein, but without the constant reinforcement, many of them wouldn’t consume enough of this macronutrient to maintain their baseline lean body mass. The recommended dietary allowance (RDA) for protein (0.8 g/kg bodyweight) doesn’t cut it, especially for older, obese patients, who need closer to 1.0-1.2 g/kg bodyweight to maintain their muscle mass. For example, for a 250-lb patient, I would recommend 114-136 g protein per day. This is equivalent to roughly 15 oz of cooked animal protein. It’s important to note, though, that individuals with kidney disease must limit their protein intake to 0.6-0.8 g/kg bodyweight per day, to avoid overtaxing their kidneys. In this situation, the benefit of increased protein intake does not outweigh the risk of harming the kidneys.
It’s often challenging for patients with suppressed appetites to even think about eating a large hunk of meat or fish, let alone consume it. Plus, eating more than 3-4 oz of protein in one meal can make some patients extremely uncomfortable, owing to the medication’s effect on gastric emptying. This means that daily protein intake must be spread out over multiple mini-meals.
For patients who need more than 100 g of protein per day, protein powders and premade protein shakes can provide 20-30 g protein to fill in the gaps. Although I always try to promote food first, protein supplements have been game changers for my patients, especially those who find solid food less appealing on the medication, or those who avoid animal protein.
Clinicians should have their patients monitor changes in their lean body mass using a dual-energy x-ray absorptiometry scan or a bioelectrical impedance scale; this can be a helpful tool in assessing whether protein intake is sufficient.
Fiber
Even my most knowledgeable and compliant patients will experience some constipation. Generally speaking, when you eat less, you will have fewer bowel movements. Combine that with delayed gastric emptying and reduced fiber intake, and you have a perfect storm. Many patients are simply not able to get in the recommended 25-35 g fiber per day through food, because fibrous foods are filling. If they are prioritizing the protein in their meal, they will not have enough room for all the vegetables on their plate.
To ensure that patients are getting sufficient fiber, clinicians should push consumption of certain vegetables and fruits, such as carrots, broccoli, Brussels sprouts, raspberries, blackberries, and apples, as well as beans and legumes. (Salads are great, but greens like spinach are not as fibrous as one might think.) If the fruit and veggie intake isn’t up to par, a fiber supplement such as psyllium husk can provide an effective boost.
Vitamin B12
Use of these medications is associated with a reduction in vitamin B12 levels, in part because delayed gastric emptying may affect B12 absorption. Low dietary intake of B12 while on the medications can also be to blame, though. The best food sources are animal proteins, so if possible, patients should prioritize having fish, lean meat, eggs, and dairy daily.
Vegetarians and vegans, who are at an increased risk for deficiency, can incorporate nutritional yeast, an excellent source of vitamin B12, into their daily routine. It is beneficial for patients to get blood work periodically to check on B12 status, because insufficient B12 can contribute to the fatigue patients experience while on the medication.
Calcium
Individuals should have calcium on their radar, because weight loss is associated with a decrease in bone mineral density. Adequate intake of the mineral is crucial for optimal bone health, particularly among postmenopausal women and those who are at risk of developing osteoporosis. The RDA for calcium is 1000-1200 mg/d, which an estimated 50% of obese individuals do not take in.
Although dairy products are well-known for being rich in calcium, there are other great sources. Dark green leafy vegetables, such as cooked collard greens and spinach, provide nearly 300 mg per cup. Tofu and sardines are also calcium powerhouses. Despite the plethora of calcium-rich foods, however, some patients may need a calcium supplement.
Vitamin D
Vitamin D deficiency or insufficiency is common among individuals with obesity, so even before these patients start the medications, supplementation may be warranted. The vitamin’s role in promoting calcium absorption, as well as in bone remodeling, make adequate intake essential for patients experiencing significant weight loss.
Clinicians should emphasize regular consumption of fatty fish, such as salmon, as well as eggs, mushrooms, and vitamin D–fortified milks. But unfortunately, that’s where the list of vitamin D–rich foods ends, so taking a vitamin D supplement will be necessary for many patients.
Regularly monitoring patients on GLP-1 RAs through blood work to check vitamin levels and body composition analysis can be helpful in assessing nutritional status while losing weight. Clinicians can also encourage their patients to work with a registered dietitian who is familiar with these medications, so they can develop optimal eating habits throughout their health journey.
Ms. Hanks, a registered dietitian in New York City, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Fatigue, nausea, acid reflux, muscle loss, and the dreaded “Ozempic face” are side effects from using glucagon-like peptide 1 (GLP-1) receptor agonists (RAs) such as semaglutide or the dual glucose-dependent insulinotropic polypeptide (GIP)/GLP-1 RA tirzepatide to control blood sugar and promote weight loss.
But what I’ve learned from working with hundreds of patients on these medications, and others, is that most (if not all) of these side effects can be minimized by ensuring proper nutrition.
Setting patients up for success requires dietary education and counseling, along with regular monitoring to determine any nutritional deficiencies. Although adequate intake of all the macro and micronutrients is obviously important,
Protein
My patients are probably sick of hearing me talk about protein, but without the constant reinforcement, many of them wouldn’t consume enough of this macronutrient to maintain their baseline lean body mass. The recommended dietary allowance (RDA) for protein (0.8 g/kg bodyweight) doesn’t cut it, especially for older, obese patients, who need closer to 1.0-1.2 g/kg bodyweight to maintain their muscle mass. For example, for a 250-lb patient, I would recommend 114-136 g protein per day. This is equivalent to roughly 15 oz of cooked animal protein. It’s important to note, though, that individuals with kidney disease must limit their protein intake to 0.6-0.8 g/kg bodyweight per day, to avoid overtaxing their kidneys. In this situation, the benefit of increased protein intake does not outweigh the risk of harming the kidneys.
It’s often challenging for patients with suppressed appetites to even think about eating a large hunk of meat or fish, let alone consume it. Plus, eating more than 3-4 oz of protein in one meal can make some patients extremely uncomfortable, owing to the medication’s effect on gastric emptying. This means that daily protein intake must be spread out over multiple mini-meals.
For patients who need more than 100 g of protein per day, protein powders and premade protein shakes can provide 20-30 g protein to fill in the gaps. Although I always try to promote food first, protein supplements have been game changers for my patients, especially those who find solid food less appealing on the medication, or those who avoid animal protein.
Clinicians should have their patients monitor changes in their lean body mass using a dual-energy x-ray absorptiometry scan or a bioelectrical impedance scale; this can be a helpful tool in assessing whether protein intake is sufficient.
Fiber
Even my most knowledgeable and compliant patients will experience some constipation. Generally speaking, when you eat less, you will have fewer bowel movements. Combine that with delayed gastric emptying and reduced fiber intake, and you have a perfect storm. Many patients are simply not able to get in the recommended 25-35 g fiber per day through food, because fibrous foods are filling. If they are prioritizing the protein in their meal, they will not have enough room for all the vegetables on their plate.
To ensure that patients are getting sufficient fiber, clinicians should push consumption of certain vegetables and fruits, such as carrots, broccoli, Brussels sprouts, raspberries, blackberries, and apples, as well as beans and legumes. (Salads are great, but greens like spinach are not as fibrous as one might think.) If the fruit and veggie intake isn’t up to par, a fiber supplement such as psyllium husk can provide an effective boost.
Vitamin B12
Use of these medications is associated with a reduction in vitamin B12 levels, in part because delayed gastric emptying may affect B12 absorption. Low dietary intake of B12 while on the medications can also be to blame, though. The best food sources are animal proteins, so if possible, patients should prioritize having fish, lean meat, eggs, and dairy daily.
Vegetarians and vegans, who are at an increased risk for deficiency, can incorporate nutritional yeast, an excellent source of vitamin B12, into their daily routine. It is beneficial for patients to get blood work periodically to check on B12 status, because insufficient B12 can contribute to the fatigue patients experience while on the medication.
Calcium
Individuals should have calcium on their radar, because weight loss is associated with a decrease in bone mineral density. Adequate intake of the mineral is crucial for optimal bone health, particularly among postmenopausal women and those who are at risk of developing osteoporosis. The RDA for calcium is 1000-1200 mg/d, which an estimated 50% of obese individuals do not take in.
Although dairy products are well-known for being rich in calcium, there are other great sources. Dark green leafy vegetables, such as cooked collard greens and spinach, provide nearly 300 mg per cup. Tofu and sardines are also calcium powerhouses. Despite the plethora of calcium-rich foods, however, some patients may need a calcium supplement.
Vitamin D
Vitamin D deficiency or insufficiency is common among individuals with obesity, so even before these patients start the medications, supplementation may be warranted. The vitamin’s role in promoting calcium absorption, as well as in bone remodeling, make adequate intake essential for patients experiencing significant weight loss.
Clinicians should emphasize regular consumption of fatty fish, such as salmon, as well as eggs, mushrooms, and vitamin D–fortified milks. But unfortunately, that’s where the list of vitamin D–rich foods ends, so taking a vitamin D supplement will be necessary for many patients.
Regularly monitoring patients on GLP-1 RAs through blood work to check vitamin levels and body composition analysis can be helpful in assessing nutritional status while losing weight. Clinicians can also encourage their patients to work with a registered dietitian who is familiar with these medications, so they can develop optimal eating habits throughout their health journey.
Ms. Hanks, a registered dietitian in New York City, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Fatigue, nausea, acid reflux, muscle loss, and the dreaded “Ozempic face” are side effects from using glucagon-like peptide 1 (GLP-1) receptor agonists (RAs) such as semaglutide or the dual glucose-dependent insulinotropic polypeptide (GIP)/GLP-1 RA tirzepatide to control blood sugar and promote weight loss.
But what I’ve learned from working with hundreds of patients on these medications, and others, is that most (if not all) of these side effects can be minimized by ensuring proper nutrition.
Setting patients up for success requires dietary education and counseling, along with regular monitoring to determine any nutritional deficiencies. Although adequate intake of all the macro and micronutrients is obviously important,
Protein
My patients are probably sick of hearing me talk about protein, but without the constant reinforcement, many of them wouldn’t consume enough of this macronutrient to maintain their baseline lean body mass. The recommended dietary allowance (RDA) for protein (0.8 g/kg bodyweight) doesn’t cut it, especially for older, obese patients, who need closer to 1.0-1.2 g/kg bodyweight to maintain their muscle mass. For example, for a 250-lb patient, I would recommend 114-136 g protein per day. This is equivalent to roughly 15 oz of cooked animal protein. It’s important to note, though, that individuals with kidney disease must limit their protein intake to 0.6-0.8 g/kg bodyweight per day, to avoid overtaxing their kidneys. In this situation, the benefit of increased protein intake does not outweigh the risk of harming the kidneys.
It’s often challenging for patients with suppressed appetites to even think about eating a large hunk of meat or fish, let alone consume it. Plus, eating more than 3-4 oz of protein in one meal can make some patients extremely uncomfortable, owing to the medication’s effect on gastric emptying. This means that daily protein intake must be spread out over multiple mini-meals.
For patients who need more than 100 g of protein per day, protein powders and premade protein shakes can provide 20-30 g protein to fill in the gaps. Although I always try to promote food first, protein supplements have been game changers for my patients, especially those who find solid food less appealing on the medication, or those who avoid animal protein.
Clinicians should have their patients monitor changes in their lean body mass using a dual-energy x-ray absorptiometry scan or a bioelectrical impedance scale; this can be a helpful tool in assessing whether protein intake is sufficient.
Fiber
Even my most knowledgeable and compliant patients will experience some constipation. Generally speaking, when you eat less, you will have fewer bowel movements. Combine that with delayed gastric emptying and reduced fiber intake, and you have a perfect storm. Many patients are simply not able to get in the recommended 25-35 g fiber per day through food, because fibrous foods are filling. If they are prioritizing the protein in their meal, they will not have enough room for all the vegetables on their plate.
To ensure that patients are getting sufficient fiber, clinicians should push consumption of certain vegetables and fruits, such as carrots, broccoli, Brussels sprouts, raspberries, blackberries, and apples, as well as beans and legumes. (Salads are great, but greens like spinach are not as fibrous as one might think.) If the fruit and veggie intake isn’t up to par, a fiber supplement such as psyllium husk can provide an effective boost.
Vitamin B12
Use of these medications is associated with a reduction in vitamin B12 levels, in part because delayed gastric emptying may affect B12 absorption. Low dietary intake of B12 while on the medications can also be to blame, though. The best food sources are animal proteins, so if possible, patients should prioritize having fish, lean meat, eggs, and dairy daily.
Vegetarians and vegans, who are at an increased risk for deficiency, can incorporate nutritional yeast, an excellent source of vitamin B12, into their daily routine. It is beneficial for patients to get blood work periodically to check on B12 status, because insufficient B12 can contribute to the fatigue patients experience while on the medication.
Calcium
Individuals should have calcium on their radar, because weight loss is associated with a decrease in bone mineral density. Adequate intake of the mineral is crucial for optimal bone health, particularly among postmenopausal women and those who are at risk of developing osteoporosis. The RDA for calcium is 1000-1200 mg/d, which an estimated 50% of obese individuals do not take in.
Although dairy products are well-known for being rich in calcium, there are other great sources. Dark green leafy vegetables, such as cooked collard greens and spinach, provide nearly 300 mg per cup. Tofu and sardines are also calcium powerhouses. Despite the plethora of calcium-rich foods, however, some patients may need a calcium supplement.
Vitamin D
Vitamin D deficiency or insufficiency is common among individuals with obesity, so even before these patients start the medications, supplementation may be warranted. The vitamin’s role in promoting calcium absorption, as well as in bone remodeling, make adequate intake essential for patients experiencing significant weight loss.
Clinicians should emphasize regular consumption of fatty fish, such as salmon, as well as eggs, mushrooms, and vitamin D–fortified milks. But unfortunately, that’s where the list of vitamin D–rich foods ends, so taking a vitamin D supplement will be necessary for many patients.
Regularly monitoring patients on GLP-1 RAs through blood work to check vitamin levels and body composition analysis can be helpful in assessing nutritional status while losing weight. Clinicians can also encourage their patients to work with a registered dietitian who is familiar with these medications, so they can develop optimal eating habits throughout their health journey.
Ms. Hanks, a registered dietitian in New York City, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Doing the Best They Can
Our dermatology department is composed of 25 doctors spread across 4 offices. It can be difficult to sustain cohesion so we have a few rituals to help hold us together. One is the morning huddle. This is a stand-up meeting lasting 3-5 minutes at 8:42 a.m. (just before the 8:45 a.m. patients). Led by our staff, huddle is a quick review of the priorities, issues, and celebrations across our department. While enthusiastically celebrating a staff member’s promotion one morning, a patient swung open the exam door and shouted, “What’s going on out here?! I’m sitting here waiting!” before slamming the door closed again. “Well, that was unnecessary,” our morning lead interjected as she went to reprimand him.
His behavior was easily recognizable to any doctor with children. It was an emotional outburst we call a tantrum. Although a graphic of tantrums by age would show a steep curve that drops precipitously after 4-years-old (please God, I hope), it persists throughout life. Even adults have tantrums. After? When I broke my pinky toe saving the family from flaming tornadoes a few weeks ago (I ran into the sofa), I flung the ice bag across the room in frustration. “You’ve a right to be mad,” my wife said returning the ice to where I was elevating my foot. She was spot on, it is understandable that I would be angry. It will be weeks before I can run again. And also my toe was broken. Both things were true.
“Two things are true” is a technique for managing tantrums in toddlers. I first learned of it from Dr. Becky Kennedy, a clinical psychologist specializing in family therapy. She has a popular podcast called “Good Inside” based on her book of the same name. Her approach is to use positive psychology with an emphasis on connecting with children to not only shape behavior, but also to help them learn to manage their emotions. I read her book to level up dad skills and realized many of her principles are applicable to various types of relationships. Instead of viewing behaviors as an end, she instead recommends using them as an opportunity to probe for understanding. Assume they are doing the best they can. When my 4-year-old obstinately refused to go to bed despite the usual colored night lights and bedtime rituals, it seemed she was being a typical tantrum-y toddler. The more I insisted — lights-out! the more she resisted. It wasn’t until I asked why that I learned she was worried that the trash truck was going to come overnight. What seemed like just a behavioral problem, time for bed, was actually an opportunity for her to be seen and for us to connect.
I was finishing up with a patient last week when my medical assistant interrupted to advise my next patient was leaving. I walked out to see her storm into the corridor heading for the exit. “I am sorry, you must be quite frustrated having to wait for me.” “Yes, you don’t respect my time,” she said loudly enough for everyone pretending to not notice. I coaxed her back into the room and sat down. After apologizing for her wait and explaining it was because an urgent patient had been added to my schedule, she calmed down and allowed me to continue. At her previous visit, I had biopsied a firm dermal papule on her upper abdomen that turned out to be metastatic breast cancer. She was treated years ago and believed she was in complete remission. Now she was alone, terrified, and wanted her full appointment with me. Because I was running late, she assumed I wouldn’t have the time for her. It was an opportunity for me to connect with her and help her feel safe. I would have missed that opportunity if I had labeled her as just another angry “Karen” brassly asserting herself.
Dr. Kennedy talks a lot in her book about taking the “Most generous interpretation” of whatever behavioral issue arises. Take the time to validate what they are feeling and empathize as best as we can. Acknowledge that it’s normal to be angry and also these are the truths we have to work with. Two truths commonly appear in these emotional episodes. One, the immutable facts, for example, insurance doesn’t cover that drug, and two, your right to be frustrated by that. Above all, remember you, the doctor, are good inside as is your discourteous patient, disaffected staff member or sometimes mendacious teenager. “All good decisions start with feeling secure and nothing feels more secure than being recognized for the good people we are,” says Dr. Kennedy. True I believe even if we sometimes slam the door.
Dr. Benabio is chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X. Write to him at [email protected].
Our dermatology department is composed of 25 doctors spread across 4 offices. It can be difficult to sustain cohesion so we have a few rituals to help hold us together. One is the morning huddle. This is a stand-up meeting lasting 3-5 minutes at 8:42 a.m. (just before the 8:45 a.m. patients). Led by our staff, huddle is a quick review of the priorities, issues, and celebrations across our department. While enthusiastically celebrating a staff member’s promotion one morning, a patient swung open the exam door and shouted, “What’s going on out here?! I’m sitting here waiting!” before slamming the door closed again. “Well, that was unnecessary,” our morning lead interjected as she went to reprimand him.
His behavior was easily recognizable to any doctor with children. It was an emotional outburst we call a tantrum. Although a graphic of tantrums by age would show a steep curve that drops precipitously after 4-years-old (please God, I hope), it persists throughout life. Even adults have tantrums. After? When I broke my pinky toe saving the family from flaming tornadoes a few weeks ago (I ran into the sofa), I flung the ice bag across the room in frustration. “You’ve a right to be mad,” my wife said returning the ice to where I was elevating my foot. She was spot on, it is understandable that I would be angry. It will be weeks before I can run again. And also my toe was broken. Both things were true.
“Two things are true” is a technique for managing tantrums in toddlers. I first learned of it from Dr. Becky Kennedy, a clinical psychologist specializing in family therapy. She has a popular podcast called “Good Inside” based on her book of the same name. Her approach is to use positive psychology with an emphasis on connecting with children to not only shape behavior, but also to help them learn to manage their emotions. I read her book to level up dad skills and realized many of her principles are applicable to various types of relationships. Instead of viewing behaviors as an end, she instead recommends using them as an opportunity to probe for understanding. Assume they are doing the best they can. When my 4-year-old obstinately refused to go to bed despite the usual colored night lights and bedtime rituals, it seemed she was being a typical tantrum-y toddler. The more I insisted — lights-out! the more she resisted. It wasn’t until I asked why that I learned she was worried that the trash truck was going to come overnight. What seemed like just a behavioral problem, time for bed, was actually an opportunity for her to be seen and for us to connect.
I was finishing up with a patient last week when my medical assistant interrupted to advise my next patient was leaving. I walked out to see her storm into the corridor heading for the exit. “I am sorry, you must be quite frustrated having to wait for me.” “Yes, you don’t respect my time,” she said loudly enough for everyone pretending to not notice. I coaxed her back into the room and sat down. After apologizing for her wait and explaining it was because an urgent patient had been added to my schedule, she calmed down and allowed me to continue. At her previous visit, I had biopsied a firm dermal papule on her upper abdomen that turned out to be metastatic breast cancer. She was treated years ago and believed she was in complete remission. Now she was alone, terrified, and wanted her full appointment with me. Because I was running late, she assumed I wouldn’t have the time for her. It was an opportunity for me to connect with her and help her feel safe. I would have missed that opportunity if I had labeled her as just another angry “Karen” brassly asserting herself.
Dr. Kennedy talks a lot in her book about taking the “Most generous interpretation” of whatever behavioral issue arises. Take the time to validate what they are feeling and empathize as best as we can. Acknowledge that it’s normal to be angry and also these are the truths we have to work with. Two truths commonly appear in these emotional episodes. One, the immutable facts, for example, insurance doesn’t cover that drug, and two, your right to be frustrated by that. Above all, remember you, the doctor, are good inside as is your discourteous patient, disaffected staff member or sometimes mendacious teenager. “All good decisions start with feeling secure and nothing feels more secure than being recognized for the good people we are,” says Dr. Kennedy. True I believe even if we sometimes slam the door.
Dr. Benabio is chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X. Write to him at [email protected].
Our dermatology department is composed of 25 doctors spread across 4 offices. It can be difficult to sustain cohesion so we have a few rituals to help hold us together. One is the morning huddle. This is a stand-up meeting lasting 3-5 minutes at 8:42 a.m. (just before the 8:45 a.m. patients). Led by our staff, huddle is a quick review of the priorities, issues, and celebrations across our department. While enthusiastically celebrating a staff member’s promotion one morning, a patient swung open the exam door and shouted, “What’s going on out here?! I’m sitting here waiting!” before slamming the door closed again. “Well, that was unnecessary,” our morning lead interjected as she went to reprimand him.
His behavior was easily recognizable to any doctor with children. It was an emotional outburst we call a tantrum. Although a graphic of tantrums by age would show a steep curve that drops precipitously after 4-years-old (please God, I hope), it persists throughout life. Even adults have tantrums. After? When I broke my pinky toe saving the family from flaming tornadoes a few weeks ago (I ran into the sofa), I flung the ice bag across the room in frustration. “You’ve a right to be mad,” my wife said returning the ice to where I was elevating my foot. She was spot on, it is understandable that I would be angry. It will be weeks before I can run again. And also my toe was broken. Both things were true.
“Two things are true” is a technique for managing tantrums in toddlers. I first learned of it from Dr. Becky Kennedy, a clinical psychologist specializing in family therapy. She has a popular podcast called “Good Inside” based on her book of the same name. Her approach is to use positive psychology with an emphasis on connecting with children to not only shape behavior, but also to help them learn to manage their emotions. I read her book to level up dad skills and realized many of her principles are applicable to various types of relationships. Instead of viewing behaviors as an end, she instead recommends using them as an opportunity to probe for understanding. Assume they are doing the best they can. When my 4-year-old obstinately refused to go to bed despite the usual colored night lights and bedtime rituals, it seemed she was being a typical tantrum-y toddler. The more I insisted — lights-out! the more she resisted. It wasn’t until I asked why that I learned she was worried that the trash truck was going to come overnight. What seemed like just a behavioral problem, time for bed, was actually an opportunity for her to be seen and for us to connect.
I was finishing up with a patient last week when my medical assistant interrupted to advise my next patient was leaving. I walked out to see her storm into the corridor heading for the exit. “I am sorry, you must be quite frustrated having to wait for me.” “Yes, you don’t respect my time,” she said loudly enough for everyone pretending to not notice. I coaxed her back into the room and sat down. After apologizing for her wait and explaining it was because an urgent patient had been added to my schedule, she calmed down and allowed me to continue. At her previous visit, I had biopsied a firm dermal papule on her upper abdomen that turned out to be metastatic breast cancer. She was treated years ago and believed she was in complete remission. Now she was alone, terrified, and wanted her full appointment with me. Because I was running late, she assumed I wouldn’t have the time for her. It was an opportunity for me to connect with her and help her feel safe. I would have missed that opportunity if I had labeled her as just another angry “Karen” brassly asserting herself.
Dr. Kennedy talks a lot in her book about taking the “Most generous interpretation” of whatever behavioral issue arises. Take the time to validate what they are feeling and empathize as best as we can. Acknowledge that it’s normal to be angry and also these are the truths we have to work with. Two truths commonly appear in these emotional episodes. One, the immutable facts, for example, insurance doesn’t cover that drug, and two, your right to be frustrated by that. Above all, remember you, the doctor, are good inside as is your discourteous patient, disaffected staff member or sometimes mendacious teenager. “All good decisions start with feeling secure and nothing feels more secure than being recognized for the good people we are,” says Dr. Kennedy. True I believe even if we sometimes slam the door.
Dr. Benabio is chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X. Write to him at [email protected].
The Sexual Revolution Has Been Great — For Men
During the month of September, Sexual Health Awareness Month, it may help to notice something: Men and their doctors have significantly more options to help with sexual function than do women and their clinicians. Moreover, .
Not convinced? Let’s take a quick tour.
The New Sexual Revolution and the Growing Anger
Around the time of the release of the book and movie Fifty Shades of Grey, Newsweek put the cultural sensation on its cover.
I bought the magazine at the airport and, while waiting for my plane, showed the story to a woman sitting next to me. “What do you think — is this the new ‘sexual revolution’?” I asked her.
She glanced at the cover and answered as accurately as if she had written the article: “In the ’60s, it became okay for women to have sex; now, it’s okay for women to demand good sex.”
I would add to that: Women are demanding good sex, and they want to define for themselves what “good” means.
That social revolution rages, still.
You would think that the demand would bring a corresponding response in clinical medicine. You would be wrong. Although efforts in some sectors are heroic, overall, the results are lagging the forward movement of women wanting better sex.
The Lag in Sexual Education
To examine the progression of the education of physicians regarding the treatment of female sexual dysfunction (FSD), Codispoti and colleagues examined the curricula of seven medical schools in and around Chicago. They found the following: Only one institution identified all anatomic components of the clitoris — one! Four of the seven discussed the physiology of the female orgasm. Only three of the seven highlighted the prevalence and epidemiology of FSD or the treatments for FSD. Only one of the seven explained how to do a genitourinary physical exam specific to assessing FSD.
When assessing obstetrics and gynecology clinical materials, sexual pleasure, arousal, and libido were not included anywhere in the curricula.
I have been teaching physicians about the therapies I developed (over 5000 clinicians in 50-plus countries over the past 14 years). During those sessions, I often stop the class and ask, “Who in here was taught how to retract the foreskin and examine the penis for phimosis?”
All hands will go up.
Then I will ask, “Who in here was taught in medical school how to retract the clitoral hood and examine the clitoris for phimosis?”
Not once has anyone raised a hand.
The Sex Remedies Gap
When I first published research offering support for using platelet-rich plasma to improve sexual function in women, women had not one drug approved by the US Food and Drug Administration (FDA) for the treatment of sexual dysfunction — none. Men had over 20. Today, men have a growing number of FDA-approved drugs for erectile dysfunction, including the “fils”; women have three.
Women have access to only one FDA-approved medication that primarily affects the genitalia: prasterone. This drug is indicated only for the treatment of pain in postmenopausal women. It does not directly enhance desire or improve orgasms. Said another way, although the incidence of sexual dysfunction is higher in premenopausal women than in other groups, they do not have a single approved medication designed to improve the function of their genitalia.
The other two of the three available drugs — flibanserin and bremelanotide — primarily affect the brain and could accurately be called psychoactive agents. They are available only for premenopausal women to improve desire. Flibanserin resulted in one extra sexual encounter per month on average, and patients are advised to avoid alcohol while using the drug. The other can make you vomit.
I do think all three of these treatments can be of great help to some women. I am not advising their disappearance. But in contrast to what is available to men, they are woefully inadequate.
Historical Perspective
In 1980, the medical establishment believed “most instances of acquired impotence are psychogenic.” Then, with the accidental discovery of the benefits of phosphodiesterase type 5 inhibitors, we realized that most cases of male sexual dysfunction involve the vasculature of the genitalia, not the neuroses of the brain. Yet, our two FDA-approved drugs for women with sexual dysfunction are designed to affect the brain. Women have nothing but off-label therapies to improve the function of the genitalia.
Despite the fact research supports the use of testosterone in women for both libido and orgasm, and despite the fact millions of women are treated with testosterone off-label for the benefit of sexual function, the only widely used FDA-approved class of drugs for women that affects testosterone — birth control pills, by blocking pituitary hormone production (the way they prevent pregnancy) — lowers the production of testosterone.
One might wonder, considering our expanded understanding of the endocrinology of both men and women, at the irony of why it is acceptable to lower the testosterone level of an adolescent girl knowingly, as if her development did not require the hormone (such would never be acceptable in an adolescent male unless sexual transitioning were the goal); yet, we are fearful of giving testosterone to grown women who can no longer make it.
Premenopausal Women: An Orphan Population
The concept of “orphan populations” can partially explain the gap in available therapies between men and women.
Women of childbearing age are risky to study; so, with testosterone, for example, it is safer and cheaper for pharmaceutical companies to prove the benefits for men and ride the profits from the off-label use for women. I don’t mean to condemn the manufacturers of testosterone, only to point out the phenomenon of why up to 30% of the prescriptions written by a primary care physician are off-label; off-label use is common among cardiologists (46%); up to 90% of children in the hospital receive at least one off-label drug; and approval of drugs for premenopausal women is more expensive than approval of drugs for men.
What Can Be Done?
The regrettable situation does not reflect evil intent on the part of regulators, educators, or physicians. But the gap between what women want and what medical education and the pharmaceutical-regulatory complex are providing is intolerably wide.
First, I would recommend a standard, required curriculum for the study of female sexual anatomy and function be established and widely adopted by medical schools. The reproductive system contains different components and a different purpose from the orgasm system, with modest overlap. Both systems should be taught in every medical school.
Second, physicians should be required to undergo a course in understanding their own sexuality. Research demonstrates doctors will avoid conversations about sex, and it seems to me this could be secondary to being uncomfortable with their own sexuality. After all, to talk with a patient about sex, you cannot be fearful of where the conversation may lead.
Third, the FDA might reconsider the requirements for the approval of drugs for FSD. Currently, to approve a drug for men, an objective finding — ie, an erection — can be sufficient. However, a higher bar, “satisfaction,” which is subjective, must be obtained with women.
Regenerative therapies have proved helpful but are not yet widely adopted; more grant money for the study of regenerative therapies would be a good start here.
Finally, by the definition of FSD, a woman must be psychologically distressed. The idea of sex is not pleasure alone. Sexual function affects family relationships, emotional health, confidence, even sleep, as well as the emotional well-being of the children who live in the house. Saying women are wonderfully and mysteriously made may be poetic, but it is not an excuse for not learning more and closing the gaps.
Dr. Runels is medical director of the Cellular Medicine Association, Fairhope, Alabama. He reported conflicts of interest with the Cellular Medicine Association, Runels Research Institute, Institute for Lichen Sclerosus, and Vulvar Health. A version of this article first appeared on Medscape.com.
During the month of September, Sexual Health Awareness Month, it may help to notice something: Men and their doctors have significantly more options to help with sexual function than do women and their clinicians. Moreover, .
Not convinced? Let’s take a quick tour.
The New Sexual Revolution and the Growing Anger
Around the time of the release of the book and movie Fifty Shades of Grey, Newsweek put the cultural sensation on its cover.
I bought the magazine at the airport and, while waiting for my plane, showed the story to a woman sitting next to me. “What do you think — is this the new ‘sexual revolution’?” I asked her.
She glanced at the cover and answered as accurately as if she had written the article: “In the ’60s, it became okay for women to have sex; now, it’s okay for women to demand good sex.”
I would add to that: Women are demanding good sex, and they want to define for themselves what “good” means.
That social revolution rages, still.
You would think that the demand would bring a corresponding response in clinical medicine. You would be wrong. Although efforts in some sectors are heroic, overall, the results are lagging the forward movement of women wanting better sex.
The Lag in Sexual Education
To examine the progression of the education of physicians regarding the treatment of female sexual dysfunction (FSD), Codispoti and colleagues examined the curricula of seven medical schools in and around Chicago. They found the following: Only one institution identified all anatomic components of the clitoris — one! Four of the seven discussed the physiology of the female orgasm. Only three of the seven highlighted the prevalence and epidemiology of FSD or the treatments for FSD. Only one of the seven explained how to do a genitourinary physical exam specific to assessing FSD.
When assessing obstetrics and gynecology clinical materials, sexual pleasure, arousal, and libido were not included anywhere in the curricula.
I have been teaching physicians about the therapies I developed (over 5000 clinicians in 50-plus countries over the past 14 years). During those sessions, I often stop the class and ask, “Who in here was taught how to retract the foreskin and examine the penis for phimosis?”
All hands will go up.
Then I will ask, “Who in here was taught in medical school how to retract the clitoral hood and examine the clitoris for phimosis?”
Not once has anyone raised a hand.
The Sex Remedies Gap
When I first published research offering support for using platelet-rich plasma to improve sexual function in women, women had not one drug approved by the US Food and Drug Administration (FDA) for the treatment of sexual dysfunction — none. Men had over 20. Today, men have a growing number of FDA-approved drugs for erectile dysfunction, including the “fils”; women have three.
Women have access to only one FDA-approved medication that primarily affects the genitalia: prasterone. This drug is indicated only for the treatment of pain in postmenopausal women. It does not directly enhance desire or improve orgasms. Said another way, although the incidence of sexual dysfunction is higher in premenopausal women than in other groups, they do not have a single approved medication designed to improve the function of their genitalia.
The other two of the three available drugs — flibanserin and bremelanotide — primarily affect the brain and could accurately be called psychoactive agents. They are available only for premenopausal women to improve desire. Flibanserin resulted in one extra sexual encounter per month on average, and patients are advised to avoid alcohol while using the drug. The other can make you vomit.
I do think all three of these treatments can be of great help to some women. I am not advising their disappearance. But in contrast to what is available to men, they are woefully inadequate.
Historical Perspective
In 1980, the medical establishment believed “most instances of acquired impotence are psychogenic.” Then, with the accidental discovery of the benefits of phosphodiesterase type 5 inhibitors, we realized that most cases of male sexual dysfunction involve the vasculature of the genitalia, not the neuroses of the brain. Yet, our two FDA-approved drugs for women with sexual dysfunction are designed to affect the brain. Women have nothing but off-label therapies to improve the function of the genitalia.
Despite the fact research supports the use of testosterone in women for both libido and orgasm, and despite the fact millions of women are treated with testosterone off-label for the benefit of sexual function, the only widely used FDA-approved class of drugs for women that affects testosterone — birth control pills, by blocking pituitary hormone production (the way they prevent pregnancy) — lowers the production of testosterone.
One might wonder, considering our expanded understanding of the endocrinology of both men and women, at the irony of why it is acceptable to lower the testosterone level of an adolescent girl knowingly, as if her development did not require the hormone (such would never be acceptable in an adolescent male unless sexual transitioning were the goal); yet, we are fearful of giving testosterone to grown women who can no longer make it.
Premenopausal Women: An Orphan Population
The concept of “orphan populations” can partially explain the gap in available therapies between men and women.
Women of childbearing age are risky to study; so, with testosterone, for example, it is safer and cheaper for pharmaceutical companies to prove the benefits for men and ride the profits from the off-label use for women. I don’t mean to condemn the manufacturers of testosterone, only to point out the phenomenon of why up to 30% of the prescriptions written by a primary care physician are off-label; off-label use is common among cardiologists (46%); up to 90% of children in the hospital receive at least one off-label drug; and approval of drugs for premenopausal women is more expensive than approval of drugs for men.
What Can Be Done?
The regrettable situation does not reflect evil intent on the part of regulators, educators, or physicians. But the gap between what women want and what medical education and the pharmaceutical-regulatory complex are providing is intolerably wide.
First, I would recommend a standard, required curriculum for the study of female sexual anatomy and function be established and widely adopted by medical schools. The reproductive system contains different components and a different purpose from the orgasm system, with modest overlap. Both systems should be taught in every medical school.
Second, physicians should be required to undergo a course in understanding their own sexuality. Research demonstrates doctors will avoid conversations about sex, and it seems to me this could be secondary to being uncomfortable with their own sexuality. After all, to talk with a patient about sex, you cannot be fearful of where the conversation may lead.
Third, the FDA might reconsider the requirements for the approval of drugs for FSD. Currently, to approve a drug for men, an objective finding — ie, an erection — can be sufficient. However, a higher bar, “satisfaction,” which is subjective, must be obtained with women.
Regenerative therapies have proved helpful but are not yet widely adopted; more grant money for the study of regenerative therapies would be a good start here.
Finally, by the definition of FSD, a woman must be psychologically distressed. The idea of sex is not pleasure alone. Sexual function affects family relationships, emotional health, confidence, even sleep, as well as the emotional well-being of the children who live in the house. Saying women are wonderfully and mysteriously made may be poetic, but it is not an excuse for not learning more and closing the gaps.
Dr. Runels is medical director of the Cellular Medicine Association, Fairhope, Alabama. He reported conflicts of interest with the Cellular Medicine Association, Runels Research Institute, Institute for Lichen Sclerosus, and Vulvar Health. A version of this article first appeared on Medscape.com.
During the month of September, Sexual Health Awareness Month, it may help to notice something: Men and their doctors have significantly more options to help with sexual function than do women and their clinicians. Moreover, .
Not convinced? Let’s take a quick tour.
The New Sexual Revolution and the Growing Anger
Around the time of the release of the book and movie Fifty Shades of Grey, Newsweek put the cultural sensation on its cover.
I bought the magazine at the airport and, while waiting for my plane, showed the story to a woman sitting next to me. “What do you think — is this the new ‘sexual revolution’?” I asked her.
She glanced at the cover and answered as accurately as if she had written the article: “In the ’60s, it became okay for women to have sex; now, it’s okay for women to demand good sex.”
I would add to that: Women are demanding good sex, and they want to define for themselves what “good” means.
That social revolution rages, still.
You would think that the demand would bring a corresponding response in clinical medicine. You would be wrong. Although efforts in some sectors are heroic, overall, the results are lagging the forward movement of women wanting better sex.
The Lag in Sexual Education
To examine the progression of the education of physicians regarding the treatment of female sexual dysfunction (FSD), Codispoti and colleagues examined the curricula of seven medical schools in and around Chicago. They found the following: Only one institution identified all anatomic components of the clitoris — one! Four of the seven discussed the physiology of the female orgasm. Only three of the seven highlighted the prevalence and epidemiology of FSD or the treatments for FSD. Only one of the seven explained how to do a genitourinary physical exam specific to assessing FSD.
When assessing obstetrics and gynecology clinical materials, sexual pleasure, arousal, and libido were not included anywhere in the curricula.
I have been teaching physicians about the therapies I developed (over 5000 clinicians in 50-plus countries over the past 14 years). During those sessions, I often stop the class and ask, “Who in here was taught how to retract the foreskin and examine the penis for phimosis?”
All hands will go up.
Then I will ask, “Who in here was taught in medical school how to retract the clitoral hood and examine the clitoris for phimosis?”
Not once has anyone raised a hand.
The Sex Remedies Gap
When I first published research offering support for using platelet-rich plasma to improve sexual function in women, women had not one drug approved by the US Food and Drug Administration (FDA) for the treatment of sexual dysfunction — none. Men had over 20. Today, men have a growing number of FDA-approved drugs for erectile dysfunction, including the “fils”; women have three.
Women have access to only one FDA-approved medication that primarily affects the genitalia: prasterone. This drug is indicated only for the treatment of pain in postmenopausal women. It does not directly enhance desire or improve orgasms. Said another way, although the incidence of sexual dysfunction is higher in premenopausal women than in other groups, they do not have a single approved medication designed to improve the function of their genitalia.
The other two of the three available drugs — flibanserin and bremelanotide — primarily affect the brain and could accurately be called psychoactive agents. They are available only for premenopausal women to improve desire. Flibanserin resulted in one extra sexual encounter per month on average, and patients are advised to avoid alcohol while using the drug. The other can make you vomit.
I do think all three of these treatments can be of great help to some women. I am not advising their disappearance. But in contrast to what is available to men, they are woefully inadequate.
Historical Perspective
In 1980, the medical establishment believed “most instances of acquired impotence are psychogenic.” Then, with the accidental discovery of the benefits of phosphodiesterase type 5 inhibitors, we realized that most cases of male sexual dysfunction involve the vasculature of the genitalia, not the neuroses of the brain. Yet, our two FDA-approved drugs for women with sexual dysfunction are designed to affect the brain. Women have nothing but off-label therapies to improve the function of the genitalia.
Despite the fact research supports the use of testosterone in women for both libido and orgasm, and despite the fact millions of women are treated with testosterone off-label for the benefit of sexual function, the only widely used FDA-approved class of drugs for women that affects testosterone — birth control pills, by blocking pituitary hormone production (the way they prevent pregnancy) — lowers the production of testosterone.
One might wonder, considering our expanded understanding of the endocrinology of both men and women, at the irony of why it is acceptable to lower the testosterone level of an adolescent girl knowingly, as if her development did not require the hormone (such would never be acceptable in an adolescent male unless sexual transitioning were the goal); yet, we are fearful of giving testosterone to grown women who can no longer make it.
Premenopausal Women: An Orphan Population
The concept of “orphan populations” can partially explain the gap in available therapies between men and women.
Women of childbearing age are risky to study; so, with testosterone, for example, it is safer and cheaper for pharmaceutical companies to prove the benefits for men and ride the profits from the off-label use for women. I don’t mean to condemn the manufacturers of testosterone, only to point out the phenomenon of why up to 30% of the prescriptions written by a primary care physician are off-label; off-label use is common among cardiologists (46%); up to 90% of children in the hospital receive at least one off-label drug; and approval of drugs for premenopausal women is more expensive than approval of drugs for men.
What Can Be Done?
The regrettable situation does not reflect evil intent on the part of regulators, educators, or physicians. But the gap between what women want and what medical education and the pharmaceutical-regulatory complex are providing is intolerably wide.
First, I would recommend a standard, required curriculum for the study of female sexual anatomy and function be established and widely adopted by medical schools. The reproductive system contains different components and a different purpose from the orgasm system, with modest overlap. Both systems should be taught in every medical school.
Second, physicians should be required to undergo a course in understanding their own sexuality. Research demonstrates doctors will avoid conversations about sex, and it seems to me this could be secondary to being uncomfortable with their own sexuality. After all, to talk with a patient about sex, you cannot be fearful of where the conversation may lead.
Third, the FDA might reconsider the requirements for the approval of drugs for FSD. Currently, to approve a drug for men, an objective finding — ie, an erection — can be sufficient. However, a higher bar, “satisfaction,” which is subjective, must be obtained with women.
Regenerative therapies have proved helpful but are not yet widely adopted; more grant money for the study of regenerative therapies would be a good start here.
Finally, by the definition of FSD, a woman must be psychologically distressed. The idea of sex is not pleasure alone. Sexual function affects family relationships, emotional health, confidence, even sleep, as well as the emotional well-being of the children who live in the house. Saying women are wonderfully and mysteriously made may be poetic, but it is not an excuse for not learning more and closing the gaps.
Dr. Runels is medical director of the Cellular Medicine Association, Fairhope, Alabama. He reported conflicts of interest with the Cellular Medicine Association, Runels Research Institute, Institute for Lichen Sclerosus, and Vulvar Health. A version of this article first appeared on Medscape.com.







