User login
Once-Weekly Insulin: A Game-Changer for Primary Care
Presented at the European Association for the Study of Diabetes (EASD) 2024 congress in Madrid, the QWINT-2 study established that. Study participants were, however, receiving noninsulin glucose-lowering agents, including glucagon-like peptide 1 (GLP-1) receptor agonists.
Slightly higher rates of mild to moderate hypoglycemia were noted with efsitora compared with degludec, but no significant differences in severe hypoglycemia were observed. Nor was there any difference in weight gain between groups, and adverse events were balanced between study arms.
This study positions insulin efsitora alongside once-weekly insulin icodec as a novel long-acting insulin therapy. In the ONWARDS 3 trial, icodec was noninferior to once-daily degludec, in terms of A1c reduction. It also had an adverse effect profile like that of efsitora with respect to hypoglycemia and weight change.
So, what are the implications of a once-weekly insulin for primary care?
“Game-changer” is an overused term, but from the perspective of primary care, it applies to once-weekly insulin.
I initiate basal insulin much less frequently these days, given the multitude of noninsulin options now available to me in primary care, particularly the GLP-1 receptor agonists and the dual GLP-1/glucose-dependent insulinotropic polypeptide receptor agonists. The American Diabetes Association/EASD 2022 consensus report also reminds me that GLP-1 receptor agonists should be considered in all individuals with T2D before insulin, unless they are contraindicated. GLP-1 receptor agonists are insulin-sparing agents with a lower injection burden and a lower risk for hypoglycemia. They also promote significant weight loss compared with basal insulin.
But progressive beta-cell decline and insulin deficiency are among the key pathophysiologic abnormalities in T2D. Eventually, many patients with T2D, despite lifestyle interventions and medication adherence, do require insulin.
Understandably, many of my patients have reservations about commencing insulin. Significant stigma about starting insulin persists, because others often perceive insulin use as a failure to manage T2D. Patients frequently fear injections, and many are worried about how insulin therapy, specifically the risk for hypoglycemia, will affect their daily activities such as driving.
Clinicians often experience therapeutic inertia, hesitating to escalate therapy to insulin because of a lack of confidence and competence, which often results from inadequate education. Lengthy referral-to-treatment waiting times are common in the United Kingdom, and there is concern about the workload implications associated with insulin initiation.
Workload is a particular concern for my community nursing colleagues, who must visit some of my more frail and functionally dependent patients daily to administer their insulin.
In addition, the delivery of high-quality diabetes care in nursing homes, particularly for patients requiring insulin, has been a perennial challenge in the UK, again because of a lack of confidence and competence due to an absence of education for nursing and ancillary staff.
Moreover, it is not appropriate to switch many of these frail patients to noninsulin therapies because of their insulinopenia, as well as the significant weight (and sometimes muscle) loss associated with GLP-1 receptor agonists. Also, sodium-glucose cotransporter 2 inhibitors are associated with a risk for volume depletion and diabetic ketoacidosis.
I believe that the availability of a once-weekly insulin will help overcome many of the above barriers.
From a patient’s viewpoint, simplification of insulin therapy with once-weekly insulin will substantially reduce the number of injections required (from 365 to 52 over 1 year). This change will improve compliance and concordance even in patients with injection anxiety. These results will hopefully translate into improved glycemic control and a lower risk for the complications of T2D. Real-world evidence for these outcomes is not yet available, however. Also, the reduced amount of insulin consumables that once-weekly dosing requires will also help improve the environmental footprint of insulin therapy.
From a clinician’s viewpoint, once-weekly insulin may seem less daunting and could reduce therapeutic inertia, thus facilitating earlier initiation of insulin therapy and reducing the risk for complications of T2D. Although education remains pivotal, this ease of dosing may be more acceptable to many clinicians because it has less of an effect on workload. This dosing could even save time because it requires less intensive follow-up than daily basal insulin does.
My community nurse colleagues were ecstatic when I mentioned that once-weekly basal insulin was on the horizon. This formulation could reduce the number of weekly home visits from 7 to just 1, thus freeing up considerable healthcare resources. And if once-weekly insulin is coupled with continuous glucose monitoring, then remote review of glucose data can further streamline and optimize the management of T2D in frail older patients. I am sure that my nursing-home colleagues will be equally enthusiastic about simplifying insulin regimens and monitoring.
Finally, an unanswered question is how I manage “sick days” for patients on weekly insulin dosing. Of course, the golden rule of never stopping insulin during intercurrent illness must be followed, but is any dose titration required for once-weekly insulin? I suspect not, but do I need to consider adding a once-daily basal insulin or rapid-acting insulin to mitigate the glucose counterregulatory hormone response during acute illness? Initially, I will be asking specialist diabetes teams for further advice on managing sick days.
In conclusion, once-weekly dosing of insulin is a game-changer for primary care and could finally be the driver to quash therapeutic inertia and address common patient barriers when escalation to insulin is required.
Dr. Fernando, general practitioner partner, North Berwick Health Centre, North Berwick, Scotland, disclosed ties with Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Dexcom, Daiichi Sankyo, Lilly, Menarini, Novartis, Novo Nordisk, Roche Diagnostics, Embecta, Roche Diabetes Care, and Sanofi.
A version of this article appeared on Medscape.com.
Presented at the European Association for the Study of Diabetes (EASD) 2024 congress in Madrid, the QWINT-2 study established that. Study participants were, however, receiving noninsulin glucose-lowering agents, including glucagon-like peptide 1 (GLP-1) receptor agonists.
Slightly higher rates of mild to moderate hypoglycemia were noted with efsitora compared with degludec, but no significant differences in severe hypoglycemia were observed. Nor was there any difference in weight gain between groups, and adverse events were balanced between study arms.
This study positions insulin efsitora alongside once-weekly insulin icodec as a novel long-acting insulin therapy. In the ONWARDS 3 trial, icodec was noninferior to once-daily degludec, in terms of A1c reduction. It also had an adverse effect profile like that of efsitora with respect to hypoglycemia and weight change.
So, what are the implications of a once-weekly insulin for primary care?
“Game-changer” is an overused term, but from the perspective of primary care, it applies to once-weekly insulin.
I initiate basal insulin much less frequently these days, given the multitude of noninsulin options now available to me in primary care, particularly the GLP-1 receptor agonists and the dual GLP-1/glucose-dependent insulinotropic polypeptide receptor agonists. The American Diabetes Association/EASD 2022 consensus report also reminds me that GLP-1 receptor agonists should be considered in all individuals with T2D before insulin, unless they are contraindicated. GLP-1 receptor agonists are insulin-sparing agents with a lower injection burden and a lower risk for hypoglycemia. They also promote significant weight loss compared with basal insulin.
But progressive beta-cell decline and insulin deficiency are among the key pathophysiologic abnormalities in T2D. Eventually, many patients with T2D, despite lifestyle interventions and medication adherence, do require insulin.
Understandably, many of my patients have reservations about commencing insulin. Significant stigma about starting insulin persists, because others often perceive insulin use as a failure to manage T2D. Patients frequently fear injections, and many are worried about how insulin therapy, specifically the risk for hypoglycemia, will affect their daily activities such as driving.
Clinicians often experience therapeutic inertia, hesitating to escalate therapy to insulin because of a lack of confidence and competence, which often results from inadequate education. Lengthy referral-to-treatment waiting times are common in the United Kingdom, and there is concern about the workload implications associated with insulin initiation.
Workload is a particular concern for my community nursing colleagues, who must visit some of my more frail and functionally dependent patients daily to administer their insulin.
In addition, the delivery of high-quality diabetes care in nursing homes, particularly for patients requiring insulin, has been a perennial challenge in the UK, again because of a lack of confidence and competence due to an absence of education for nursing and ancillary staff.
Moreover, it is not appropriate to switch many of these frail patients to noninsulin therapies because of their insulinopenia, as well as the significant weight (and sometimes muscle) loss associated with GLP-1 receptor agonists. Also, sodium-glucose cotransporter 2 inhibitors are associated with a risk for volume depletion and diabetic ketoacidosis.
I believe that the availability of a once-weekly insulin will help overcome many of the above barriers.
From a patient’s viewpoint, simplification of insulin therapy with once-weekly insulin will substantially reduce the number of injections required (from 365 to 52 over 1 year). This change will improve compliance and concordance even in patients with injection anxiety. These results will hopefully translate into improved glycemic control and a lower risk for the complications of T2D. Real-world evidence for these outcomes is not yet available, however. Also, the reduced amount of insulin consumables that once-weekly dosing requires will also help improve the environmental footprint of insulin therapy.
From a clinician’s viewpoint, once-weekly insulin may seem less daunting and could reduce therapeutic inertia, thus facilitating earlier initiation of insulin therapy and reducing the risk for complications of T2D. Although education remains pivotal, this ease of dosing may be more acceptable to many clinicians because it has less of an effect on workload. This dosing could even save time because it requires less intensive follow-up than daily basal insulin does.
My community nurse colleagues were ecstatic when I mentioned that once-weekly basal insulin was on the horizon. This formulation could reduce the number of weekly home visits from 7 to just 1, thus freeing up considerable healthcare resources. And if once-weekly insulin is coupled with continuous glucose monitoring, then remote review of glucose data can further streamline and optimize the management of T2D in frail older patients. I am sure that my nursing-home colleagues will be equally enthusiastic about simplifying insulin regimens and monitoring.
Finally, an unanswered question is how I manage “sick days” for patients on weekly insulin dosing. Of course, the golden rule of never stopping insulin during intercurrent illness must be followed, but is any dose titration required for once-weekly insulin? I suspect not, but do I need to consider adding a once-daily basal insulin or rapid-acting insulin to mitigate the glucose counterregulatory hormone response during acute illness? Initially, I will be asking specialist diabetes teams for further advice on managing sick days.
In conclusion, once-weekly dosing of insulin is a game-changer for primary care and could finally be the driver to quash therapeutic inertia and address common patient barriers when escalation to insulin is required.
Dr. Fernando, general practitioner partner, North Berwick Health Centre, North Berwick, Scotland, disclosed ties with Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Dexcom, Daiichi Sankyo, Lilly, Menarini, Novartis, Novo Nordisk, Roche Diagnostics, Embecta, Roche Diabetes Care, and Sanofi.
A version of this article appeared on Medscape.com.
Presented at the European Association for the Study of Diabetes (EASD) 2024 congress in Madrid, the QWINT-2 study established that. Study participants were, however, receiving noninsulin glucose-lowering agents, including glucagon-like peptide 1 (GLP-1) receptor agonists.
Slightly higher rates of mild to moderate hypoglycemia were noted with efsitora compared with degludec, but no significant differences in severe hypoglycemia were observed. Nor was there any difference in weight gain between groups, and adverse events were balanced between study arms.
This study positions insulin efsitora alongside once-weekly insulin icodec as a novel long-acting insulin therapy. In the ONWARDS 3 trial, icodec was noninferior to once-daily degludec, in terms of A1c reduction. It also had an adverse effect profile like that of efsitora with respect to hypoglycemia and weight change.
So, what are the implications of a once-weekly insulin for primary care?
“Game-changer” is an overused term, but from the perspective of primary care, it applies to once-weekly insulin.
I initiate basal insulin much less frequently these days, given the multitude of noninsulin options now available to me in primary care, particularly the GLP-1 receptor agonists and the dual GLP-1/glucose-dependent insulinotropic polypeptide receptor agonists. The American Diabetes Association/EASD 2022 consensus report also reminds me that GLP-1 receptor agonists should be considered in all individuals with T2D before insulin, unless they are contraindicated. GLP-1 receptor agonists are insulin-sparing agents with a lower injection burden and a lower risk for hypoglycemia. They also promote significant weight loss compared with basal insulin.
But progressive beta-cell decline and insulin deficiency are among the key pathophysiologic abnormalities in T2D. Eventually, many patients with T2D, despite lifestyle interventions and medication adherence, do require insulin.
Understandably, many of my patients have reservations about commencing insulin. Significant stigma about starting insulin persists, because others often perceive insulin use as a failure to manage T2D. Patients frequently fear injections, and many are worried about how insulin therapy, specifically the risk for hypoglycemia, will affect their daily activities such as driving.
Clinicians often experience therapeutic inertia, hesitating to escalate therapy to insulin because of a lack of confidence and competence, which often results from inadequate education. Lengthy referral-to-treatment waiting times are common in the United Kingdom, and there is concern about the workload implications associated with insulin initiation.
Workload is a particular concern for my community nursing colleagues, who must visit some of my more frail and functionally dependent patients daily to administer their insulin.
In addition, the delivery of high-quality diabetes care in nursing homes, particularly for patients requiring insulin, has been a perennial challenge in the UK, again because of a lack of confidence and competence due to an absence of education for nursing and ancillary staff.
Moreover, it is not appropriate to switch many of these frail patients to noninsulin therapies because of their insulinopenia, as well as the significant weight (and sometimes muscle) loss associated with GLP-1 receptor agonists. Also, sodium-glucose cotransporter 2 inhibitors are associated with a risk for volume depletion and diabetic ketoacidosis.
I believe that the availability of a once-weekly insulin will help overcome many of the above barriers.
From a patient’s viewpoint, simplification of insulin therapy with once-weekly insulin will substantially reduce the number of injections required (from 365 to 52 over 1 year). This change will improve compliance and concordance even in patients with injection anxiety. These results will hopefully translate into improved glycemic control and a lower risk for the complications of T2D. Real-world evidence for these outcomes is not yet available, however. Also, the reduced amount of insulin consumables that once-weekly dosing requires will also help improve the environmental footprint of insulin therapy.
From a clinician’s viewpoint, once-weekly insulin may seem less daunting and could reduce therapeutic inertia, thus facilitating earlier initiation of insulin therapy and reducing the risk for complications of T2D. Although education remains pivotal, this ease of dosing may be more acceptable to many clinicians because it has less of an effect on workload. This dosing could even save time because it requires less intensive follow-up than daily basal insulin does.
My community nurse colleagues were ecstatic when I mentioned that once-weekly basal insulin was on the horizon. This formulation could reduce the number of weekly home visits from 7 to just 1, thus freeing up considerable healthcare resources. And if once-weekly insulin is coupled with continuous glucose monitoring, then remote review of glucose data can further streamline and optimize the management of T2D in frail older patients. I am sure that my nursing-home colleagues will be equally enthusiastic about simplifying insulin regimens and monitoring.
Finally, an unanswered question is how I manage “sick days” for patients on weekly insulin dosing. Of course, the golden rule of never stopping insulin during intercurrent illness must be followed, but is any dose titration required for once-weekly insulin? I suspect not, but do I need to consider adding a once-daily basal insulin or rapid-acting insulin to mitigate the glucose counterregulatory hormone response during acute illness? Initially, I will be asking specialist diabetes teams for further advice on managing sick days.
In conclusion, once-weekly dosing of insulin is a game-changer for primary care and could finally be the driver to quash therapeutic inertia and address common patient barriers when escalation to insulin is required.
Dr. Fernando, general practitioner partner, North Berwick Health Centre, North Berwick, Scotland, disclosed ties with Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Dexcom, Daiichi Sankyo, Lilly, Menarini, Novartis, Novo Nordisk, Roche Diagnostics, Embecta, Roche Diabetes Care, and Sanofi.
A version of this article appeared on Medscape.com.
Abnormal TSH: Forget it or Fret it?
If you’re like most primary care clinicians, your email inbox is flooded with messages from patients with questions about lab results. A common query: Should I be worried about an abnormal value on a test of thyroid-stimulating hormone (TSH)?
For guidance, this news organization spoke with Angela Leung, MD, associate professor of medicine in the Division of Endocrinology, Diabetes & Metabolism at the UCLA David Geffen School of Medicine and an endocrinologist at UCLA and the VA Greater Los Angeles Healthcare System, and Karen Tsai, MD, assistant clinical professor of endocrinology at City of Hope Comprehensive Cancer Center in Duarte, California. The following interview has been edited for length and clarity.
Question: Why do you usually start by measuring TSH levels?
Dr. Leung: We need to measure the thyroid status in a way that integrates more information about the long-term thyroid status and not small changes in thyroid hormone levels. TSH is made by the pituitary gland in the brain, which integrates information about the signals of high and low levels from each of the different thyroid hormones.
Now we can measure the actual thyroid hormones — primarily we’re talking about T3 and T4 — but if we do that, we are relying on a single snapshot in the bloodstream at that moment. The levels might change throughout the day in response to ongoing metabolism and outside stresses. So we usually start by measuring the TSH level, which is a good representation of the compilation of all those things over the past 30 days or so.
Question: How do you describe a low TSH result to patients?
Dr. Leung: Whenever we encounter a low TSH level, we want to repeat the test because it is a dynamic test, and it can change in response to several factors. If it is indeed low, we’re thinking that perhaps there’s a little bit of extra thyroid hormone in the body. It can be either temporary or more chronic, but that higher amount of thyroid hormone is telling the pituitary gland in the brain to start making less. So TSH levels go low when we need less thyroid hormone.
Question: What are some of the reasons for a low TSH level?
Dr. Leung: One of the most common situations for a temporarily low TSH level I see is what we call nonthyroidal illness, like a common cold or just being under the weather. Other things that can artifactually lower the TSH level could be the use of steroids, such as prednisone for asthma or some sort of a rheumatologic condition. Also, the TSH level could be low if a person has been recently exposed to very high amounts of iodine, such as iodinated contrast needed for a CT scan.
If the TSH level remains persistently low, usually in the presence of high thyroid hormone (T3 and/or T4) levels, the most common reason for hyperthyroidism is Graves disease, in which there are autoantibodies — measurable in the blood — that can stimulate the thyroid gland in the neck to make extra thyroid hormone.
Question: And what does an elevated TSH level mean?
Dr. Leung: Again, we want to confirm that it is elevated. We need at least two tests to confirm a high TSH level. A persistently elevated TSH level is a signal there might be low thyroid hormone levels in the body, which could be transient or more longer lasting.
Question: What are some of the most common causes of an elevated TSH level?
Dr. Leung: If the TSH level is confirmed high and the thyroid hormone levels are low, the most common cause of hypothyroidism here in the United States is Hashimoto thyroiditis.
Globally, iodine deficiency is the most common reason for hypothyroidism and may be a problem in parts of the globe where there are endemically low iodine levels in soil, crops, and the food supply like not having enough iodized salt. The thyroid is reliant on having enough iodine as a micronutrient to make thyroid hormone. If it doesn’t, the thyroid really can’t make thyroid hormone. It’s important to also remember, though, that having too much iodine can result in hypo- or hyperthyroidism.
Dr. Tsai: I take a glance at their medication list. Some of the patients are on methimazole or levothyroxine, and those medications should be adjusted first to normalize the TSH level. Other medications like lithium and amiodarone can also cause elevated TSH levels. We are also seeing a lot of patients on cancer therapies, such as tyrosine kinase inhibitors or immunotherapy, that can cause an elevated TSH level.
Question: If the repeat TSH test shows that TSH levels are still elevated, what comes next in your workup?
Dr. Tsai: If there’s not a real clear-cut diagnosis, I’ll order the thyroid peroxidase antibody and the thyroglobulin antibody, although thyroid peroxidase antibody, which is indicative of autoimmune thyroid disease, alone is usually sufficient to make that diagnosis.
Question: Should clinicians follow thyroid antibodies over time?
Dr. Tsai: I usually don’t repeat the antibody tests. In those circumstances where patients who were diagnosed 50-60 years ago and perhaps it is unknown if they had the thyroid antibodies measured at the time and now they’re saying, “Do I actually have Hashimoto’s?” or “Do I really need to continue this for the rest of my life?” I do repeat antibody tests to help gauge if the patient’s levothyroxine can be stopped.
Question: How important is it to follow T4 or T3 levels?
Dr. Tsai: T4 and T3 levels can help differentiate overt thyroid dysfunction — where T3 and/or T4 levels will be abnormal — from subclinical thyroid dysfunction — where T3 and T4 levels would be normal. In general, although we do not fully appreciate the best metric to monitor hypo- or hyperthyroidism, because some patients with a normal TSH level still may have symptoms of thyroid dysfunction, these peripheral thyroid hormone levels are usually the most helpful at the time of initial diagnosis.
Question: What are your criteria for initiating treatment for hypothyroidism?
Dr. Tsai: If the TSH level > 10 mIU/L, I recommend levothyroxine hormone replacement. A lot of published data support clinical benefit in this group.
There is a gray area in those patients who have a TSH level higher than the upper limit of the reference range but less than 10. If the patient doesn’t have overt hypothyroid symptoms, I discuss the findings with the patient but don’t really feel eager to treat. I recommend checking the levels again in 6 months to see where that TSH goes, and if it worsens or becomes greater than 10 mIU/L, I then recommend levothyroxine hormone replacement.
It is also important to note that a TSH level of 5-7 may be an acceptable range for older patients, and they do not require levothyroxine.
The other category is patients whose TSH level is greater than the upper limit of the normal reference range but less than 10 and with overt hypothyroid symptoms such as fatigue, unintentional weight gain, constipation, or cold intolerance. In these patients, it is worthwhile to try a low dose of levothyroxine (25-50 mcg/d) and repeat TSH and free T4 tests in 6-8 weeks and see if the TSH level normalizes.
Dr. Leung: When you look at subclinical hypothyroidism, the situation of an isolated high TSH level in the setting of normal T4 levels, if the TSH level is mildly elevated in the 5-7 mIU/L range, there’s a 60% chance that it will normalize within 6 months.
Going back to Karen’s point, a lot of people are started and maintained on low doses of thyroid hormone forever and ever. A recent study on levothyroxine use found half of the prescriptions were unnecessary.
Question: In an era where many patients obtain much of their health information from TikTok, what’s your approach with patients with a normal TSH level who feel that they should have more testing or start treatment?
Dr. Tsai: Fatigue is one of the common referrals we get into our endocrinology practice, and everyone is convinced that their thyroid is the culprit. It is important to note, however, that fatigue can be due to different diseases such as anemia, depression, sleep disorders, or a recent viral illness.
TSH tests are readily available and cheap. I don’t mind ordering the lab test again if it helps give the patient some reassurance. I also find that patients are relieved once they hear from their endocrinologist that their thyroid is unlikely to be the cause of their fatigue.
Some other endocrine causes we may consider additionally working up include adrenal insufficiency, vitamin D deficiency, and diabetes. A comprehensive metabolic panel and complete blood count is part of my workup to rule out any gross electrolyte abnormalities or any new diagnosis of anemia, liver disease, or chronic kidney disease.
Question: What are your criteria for recommending that someone see an endocrinologist?
Dr. Tsai: Our primary care colleagues can do a workup and interpretation of thyroid function tests in most cases. In the situations where the thyroid function test results are discordant (ie, elevated TSH and elevated free T4 levels or low TSH and low free T4 levels) or difficult to interpret, it would be appropriate to refer the patient to an endocrinologist.
One of the common referrals that we do get from the community is a patient’s thyroid function tests going from hyperthyroid to hypothyroid without a clear explanation or the patient is suboptimally controlled with levothyroxine or methimazole. In those circumstances, it would be worthwhile to send to an endocrinologist try to discern an underlying cause or for optimization of medication.
Dr. Leung and Dr. Tsai had no financial disclosures.
A version of this article appeared on Medscape.com.
If you’re like most primary care clinicians, your email inbox is flooded with messages from patients with questions about lab results. A common query: Should I be worried about an abnormal value on a test of thyroid-stimulating hormone (TSH)?
For guidance, this news organization spoke with Angela Leung, MD, associate professor of medicine in the Division of Endocrinology, Diabetes & Metabolism at the UCLA David Geffen School of Medicine and an endocrinologist at UCLA and the VA Greater Los Angeles Healthcare System, and Karen Tsai, MD, assistant clinical professor of endocrinology at City of Hope Comprehensive Cancer Center in Duarte, California. The following interview has been edited for length and clarity.
Question: Why do you usually start by measuring TSH levels?
Dr. Leung: We need to measure the thyroid status in a way that integrates more information about the long-term thyroid status and not small changes in thyroid hormone levels. TSH is made by the pituitary gland in the brain, which integrates information about the signals of high and low levels from each of the different thyroid hormones.
Now we can measure the actual thyroid hormones — primarily we’re talking about T3 and T4 — but if we do that, we are relying on a single snapshot in the bloodstream at that moment. The levels might change throughout the day in response to ongoing metabolism and outside stresses. So we usually start by measuring the TSH level, which is a good representation of the compilation of all those things over the past 30 days or so.
Question: How do you describe a low TSH result to patients?
Dr. Leung: Whenever we encounter a low TSH level, we want to repeat the test because it is a dynamic test, and it can change in response to several factors. If it is indeed low, we’re thinking that perhaps there’s a little bit of extra thyroid hormone in the body. It can be either temporary or more chronic, but that higher amount of thyroid hormone is telling the pituitary gland in the brain to start making less. So TSH levels go low when we need less thyroid hormone.
Question: What are some of the reasons for a low TSH level?
Dr. Leung: One of the most common situations for a temporarily low TSH level I see is what we call nonthyroidal illness, like a common cold or just being under the weather. Other things that can artifactually lower the TSH level could be the use of steroids, such as prednisone for asthma or some sort of a rheumatologic condition. Also, the TSH level could be low if a person has been recently exposed to very high amounts of iodine, such as iodinated contrast needed for a CT scan.
If the TSH level remains persistently low, usually in the presence of high thyroid hormone (T3 and/or T4) levels, the most common reason for hyperthyroidism is Graves disease, in which there are autoantibodies — measurable in the blood — that can stimulate the thyroid gland in the neck to make extra thyroid hormone.
Question: And what does an elevated TSH level mean?
Dr. Leung: Again, we want to confirm that it is elevated. We need at least two tests to confirm a high TSH level. A persistently elevated TSH level is a signal there might be low thyroid hormone levels in the body, which could be transient or more longer lasting.
Question: What are some of the most common causes of an elevated TSH level?
Dr. Leung: If the TSH level is confirmed high and the thyroid hormone levels are low, the most common cause of hypothyroidism here in the United States is Hashimoto thyroiditis.
Globally, iodine deficiency is the most common reason for hypothyroidism and may be a problem in parts of the globe where there are endemically low iodine levels in soil, crops, and the food supply like not having enough iodized salt. The thyroid is reliant on having enough iodine as a micronutrient to make thyroid hormone. If it doesn’t, the thyroid really can’t make thyroid hormone. It’s important to also remember, though, that having too much iodine can result in hypo- or hyperthyroidism.
Dr. Tsai: I take a glance at their medication list. Some of the patients are on methimazole or levothyroxine, and those medications should be adjusted first to normalize the TSH level. Other medications like lithium and amiodarone can also cause elevated TSH levels. We are also seeing a lot of patients on cancer therapies, such as tyrosine kinase inhibitors or immunotherapy, that can cause an elevated TSH level.
Question: If the repeat TSH test shows that TSH levels are still elevated, what comes next in your workup?
Dr. Tsai: If there’s not a real clear-cut diagnosis, I’ll order the thyroid peroxidase antibody and the thyroglobulin antibody, although thyroid peroxidase antibody, which is indicative of autoimmune thyroid disease, alone is usually sufficient to make that diagnosis.
Question: Should clinicians follow thyroid antibodies over time?
Dr. Tsai: I usually don’t repeat the antibody tests. In those circumstances where patients who were diagnosed 50-60 years ago and perhaps it is unknown if they had the thyroid antibodies measured at the time and now they’re saying, “Do I actually have Hashimoto’s?” or “Do I really need to continue this for the rest of my life?” I do repeat antibody tests to help gauge if the patient’s levothyroxine can be stopped.
Question: How important is it to follow T4 or T3 levels?
Dr. Tsai: T4 and T3 levels can help differentiate overt thyroid dysfunction — where T3 and/or T4 levels will be abnormal — from subclinical thyroid dysfunction — where T3 and T4 levels would be normal. In general, although we do not fully appreciate the best metric to monitor hypo- or hyperthyroidism, because some patients with a normal TSH level still may have symptoms of thyroid dysfunction, these peripheral thyroid hormone levels are usually the most helpful at the time of initial diagnosis.
Question: What are your criteria for initiating treatment for hypothyroidism?
Dr. Tsai: If the TSH level > 10 mIU/L, I recommend levothyroxine hormone replacement. A lot of published data support clinical benefit in this group.
There is a gray area in those patients who have a TSH level higher than the upper limit of the reference range but less than 10. If the patient doesn’t have overt hypothyroid symptoms, I discuss the findings with the patient but don’t really feel eager to treat. I recommend checking the levels again in 6 months to see where that TSH goes, and if it worsens or becomes greater than 10 mIU/L, I then recommend levothyroxine hormone replacement.
It is also important to note that a TSH level of 5-7 may be an acceptable range for older patients, and they do not require levothyroxine.
The other category is patients whose TSH level is greater than the upper limit of the normal reference range but less than 10 and with overt hypothyroid symptoms such as fatigue, unintentional weight gain, constipation, or cold intolerance. In these patients, it is worthwhile to try a low dose of levothyroxine (25-50 mcg/d) and repeat TSH and free T4 tests in 6-8 weeks and see if the TSH level normalizes.
Dr. Leung: When you look at subclinical hypothyroidism, the situation of an isolated high TSH level in the setting of normal T4 levels, if the TSH level is mildly elevated in the 5-7 mIU/L range, there’s a 60% chance that it will normalize within 6 months.
Going back to Karen’s point, a lot of people are started and maintained on low doses of thyroid hormone forever and ever. A recent study on levothyroxine use found half of the prescriptions were unnecessary.
Question: In an era where many patients obtain much of their health information from TikTok, what’s your approach with patients with a normal TSH level who feel that they should have more testing or start treatment?
Dr. Tsai: Fatigue is one of the common referrals we get into our endocrinology practice, and everyone is convinced that their thyroid is the culprit. It is important to note, however, that fatigue can be due to different diseases such as anemia, depression, sleep disorders, or a recent viral illness.
TSH tests are readily available and cheap. I don’t mind ordering the lab test again if it helps give the patient some reassurance. I also find that patients are relieved once they hear from their endocrinologist that their thyroid is unlikely to be the cause of their fatigue.
Some other endocrine causes we may consider additionally working up include adrenal insufficiency, vitamin D deficiency, and diabetes. A comprehensive metabolic panel and complete blood count is part of my workup to rule out any gross electrolyte abnormalities or any new diagnosis of anemia, liver disease, or chronic kidney disease.
Question: What are your criteria for recommending that someone see an endocrinologist?
Dr. Tsai: Our primary care colleagues can do a workup and interpretation of thyroid function tests in most cases. In the situations where the thyroid function test results are discordant (ie, elevated TSH and elevated free T4 levels or low TSH and low free T4 levels) or difficult to interpret, it would be appropriate to refer the patient to an endocrinologist.
One of the common referrals that we do get from the community is a patient’s thyroid function tests going from hyperthyroid to hypothyroid without a clear explanation or the patient is suboptimally controlled with levothyroxine or methimazole. In those circumstances, it would be worthwhile to send to an endocrinologist try to discern an underlying cause or for optimization of medication.
Dr. Leung and Dr. Tsai had no financial disclosures.
A version of this article appeared on Medscape.com.
If you’re like most primary care clinicians, your email inbox is flooded with messages from patients with questions about lab results. A common query: Should I be worried about an abnormal value on a test of thyroid-stimulating hormone (TSH)?
For guidance, this news organization spoke with Angela Leung, MD, associate professor of medicine in the Division of Endocrinology, Diabetes & Metabolism at the UCLA David Geffen School of Medicine and an endocrinologist at UCLA and the VA Greater Los Angeles Healthcare System, and Karen Tsai, MD, assistant clinical professor of endocrinology at City of Hope Comprehensive Cancer Center in Duarte, California. The following interview has been edited for length and clarity.
Question: Why do you usually start by measuring TSH levels?
Dr. Leung: We need to measure the thyroid status in a way that integrates more information about the long-term thyroid status and not small changes in thyroid hormone levels. TSH is made by the pituitary gland in the brain, which integrates information about the signals of high and low levels from each of the different thyroid hormones.
Now we can measure the actual thyroid hormones — primarily we’re talking about T3 and T4 — but if we do that, we are relying on a single snapshot in the bloodstream at that moment. The levels might change throughout the day in response to ongoing metabolism and outside stresses. So we usually start by measuring the TSH level, which is a good representation of the compilation of all those things over the past 30 days or so.
Question: How do you describe a low TSH result to patients?
Dr. Leung: Whenever we encounter a low TSH level, we want to repeat the test because it is a dynamic test, and it can change in response to several factors. If it is indeed low, we’re thinking that perhaps there’s a little bit of extra thyroid hormone in the body. It can be either temporary or more chronic, but that higher amount of thyroid hormone is telling the pituitary gland in the brain to start making less. So TSH levels go low when we need less thyroid hormone.
Question: What are some of the reasons for a low TSH level?
Dr. Leung: One of the most common situations for a temporarily low TSH level I see is what we call nonthyroidal illness, like a common cold or just being under the weather. Other things that can artifactually lower the TSH level could be the use of steroids, such as prednisone for asthma or some sort of a rheumatologic condition. Also, the TSH level could be low if a person has been recently exposed to very high amounts of iodine, such as iodinated contrast needed for a CT scan.
If the TSH level remains persistently low, usually in the presence of high thyroid hormone (T3 and/or T4) levels, the most common reason for hyperthyroidism is Graves disease, in which there are autoantibodies — measurable in the blood — that can stimulate the thyroid gland in the neck to make extra thyroid hormone.
Question: And what does an elevated TSH level mean?
Dr. Leung: Again, we want to confirm that it is elevated. We need at least two tests to confirm a high TSH level. A persistently elevated TSH level is a signal there might be low thyroid hormone levels in the body, which could be transient or more longer lasting.
Question: What are some of the most common causes of an elevated TSH level?
Dr. Leung: If the TSH level is confirmed high and the thyroid hormone levels are low, the most common cause of hypothyroidism here in the United States is Hashimoto thyroiditis.
Globally, iodine deficiency is the most common reason for hypothyroidism and may be a problem in parts of the globe where there are endemically low iodine levels in soil, crops, and the food supply like not having enough iodized salt. The thyroid is reliant on having enough iodine as a micronutrient to make thyroid hormone. If it doesn’t, the thyroid really can’t make thyroid hormone. It’s important to also remember, though, that having too much iodine can result in hypo- or hyperthyroidism.
Dr. Tsai: I take a glance at their medication list. Some of the patients are on methimazole or levothyroxine, and those medications should be adjusted first to normalize the TSH level. Other medications like lithium and amiodarone can also cause elevated TSH levels. We are also seeing a lot of patients on cancer therapies, such as tyrosine kinase inhibitors or immunotherapy, that can cause an elevated TSH level.
Question: If the repeat TSH test shows that TSH levels are still elevated, what comes next in your workup?
Dr. Tsai: If there’s not a real clear-cut diagnosis, I’ll order the thyroid peroxidase antibody and the thyroglobulin antibody, although thyroid peroxidase antibody, which is indicative of autoimmune thyroid disease, alone is usually sufficient to make that diagnosis.
Question: Should clinicians follow thyroid antibodies over time?
Dr. Tsai: I usually don’t repeat the antibody tests. In those circumstances where patients who were diagnosed 50-60 years ago and perhaps it is unknown if they had the thyroid antibodies measured at the time and now they’re saying, “Do I actually have Hashimoto’s?” or “Do I really need to continue this for the rest of my life?” I do repeat antibody tests to help gauge if the patient’s levothyroxine can be stopped.
Question: How important is it to follow T4 or T3 levels?
Dr. Tsai: T4 and T3 levels can help differentiate overt thyroid dysfunction — where T3 and/or T4 levels will be abnormal — from subclinical thyroid dysfunction — where T3 and T4 levels would be normal. In general, although we do not fully appreciate the best metric to monitor hypo- or hyperthyroidism, because some patients with a normal TSH level still may have symptoms of thyroid dysfunction, these peripheral thyroid hormone levels are usually the most helpful at the time of initial diagnosis.
Question: What are your criteria for initiating treatment for hypothyroidism?
Dr. Tsai: If the TSH level > 10 mIU/L, I recommend levothyroxine hormone replacement. A lot of published data support clinical benefit in this group.
There is a gray area in those patients who have a TSH level higher than the upper limit of the reference range but less than 10. If the patient doesn’t have overt hypothyroid symptoms, I discuss the findings with the patient but don’t really feel eager to treat. I recommend checking the levels again in 6 months to see where that TSH goes, and if it worsens or becomes greater than 10 mIU/L, I then recommend levothyroxine hormone replacement.
It is also important to note that a TSH level of 5-7 may be an acceptable range for older patients, and they do not require levothyroxine.
The other category is patients whose TSH level is greater than the upper limit of the normal reference range but less than 10 and with overt hypothyroid symptoms such as fatigue, unintentional weight gain, constipation, or cold intolerance. In these patients, it is worthwhile to try a low dose of levothyroxine (25-50 mcg/d) and repeat TSH and free T4 tests in 6-8 weeks and see if the TSH level normalizes.
Dr. Leung: When you look at subclinical hypothyroidism, the situation of an isolated high TSH level in the setting of normal T4 levels, if the TSH level is mildly elevated in the 5-7 mIU/L range, there’s a 60% chance that it will normalize within 6 months.
Going back to Karen’s point, a lot of people are started and maintained on low doses of thyroid hormone forever and ever. A recent study on levothyroxine use found half of the prescriptions were unnecessary.
Question: In an era where many patients obtain much of their health information from TikTok, what’s your approach with patients with a normal TSH level who feel that they should have more testing or start treatment?
Dr. Tsai: Fatigue is one of the common referrals we get into our endocrinology practice, and everyone is convinced that their thyroid is the culprit. It is important to note, however, that fatigue can be due to different diseases such as anemia, depression, sleep disorders, or a recent viral illness.
TSH tests are readily available and cheap. I don’t mind ordering the lab test again if it helps give the patient some reassurance. I also find that patients are relieved once they hear from their endocrinologist that their thyroid is unlikely to be the cause of their fatigue.
Some other endocrine causes we may consider additionally working up include adrenal insufficiency, vitamin D deficiency, and diabetes. A comprehensive metabolic panel and complete blood count is part of my workup to rule out any gross electrolyte abnormalities or any new diagnosis of anemia, liver disease, or chronic kidney disease.
Question: What are your criteria for recommending that someone see an endocrinologist?
Dr. Tsai: Our primary care colleagues can do a workup and interpretation of thyroid function tests in most cases. In the situations where the thyroid function test results are discordant (ie, elevated TSH and elevated free T4 levels or low TSH and low free T4 levels) or difficult to interpret, it would be appropriate to refer the patient to an endocrinologist.
One of the common referrals that we do get from the community is a patient’s thyroid function tests going from hyperthyroid to hypothyroid without a clear explanation or the patient is suboptimally controlled with levothyroxine or methimazole. In those circumstances, it would be worthwhile to send to an endocrinologist try to discern an underlying cause or for optimization of medication.
Dr. Leung and Dr. Tsai had no financial disclosures.
A version of this article appeared on Medscape.com.
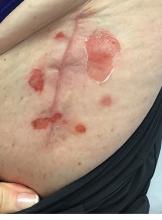
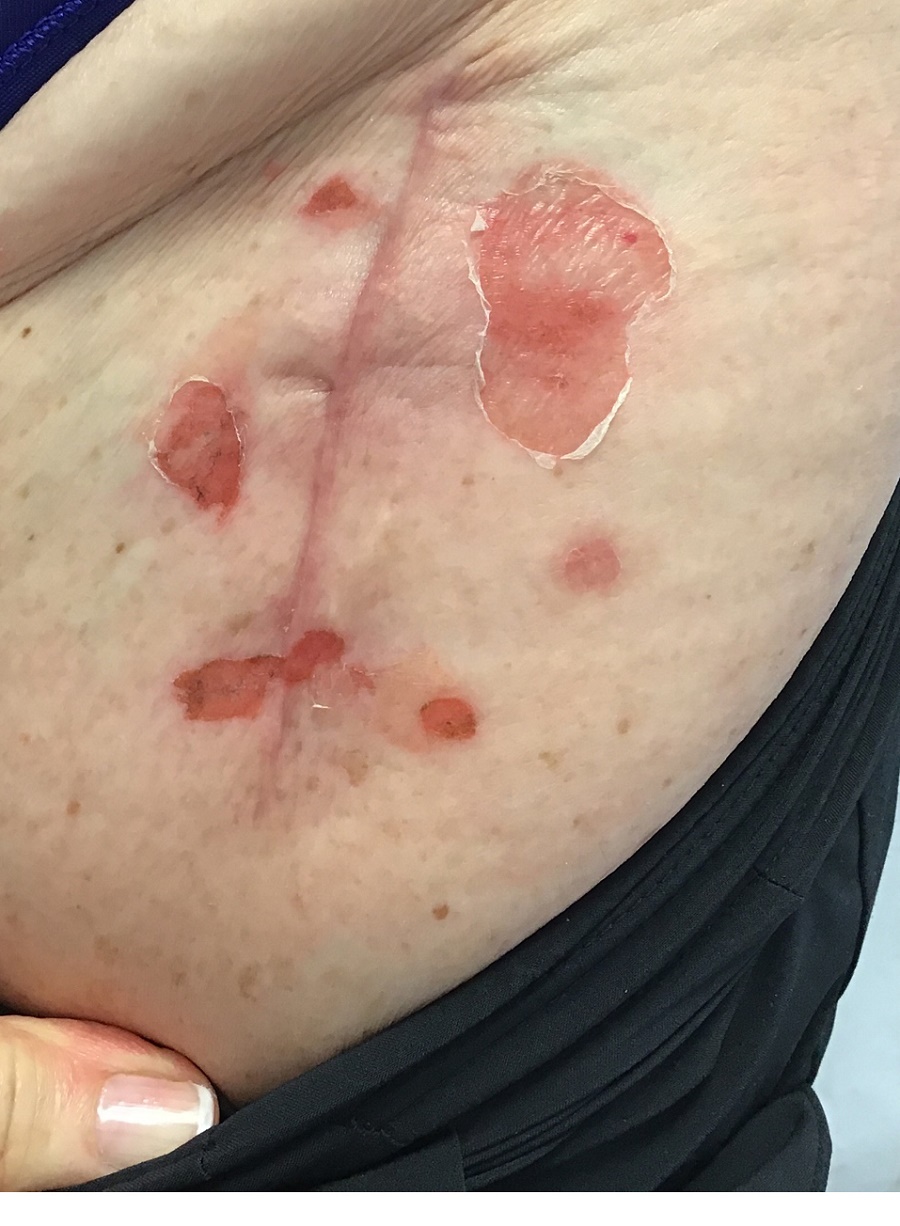
A 71-year-old White female developed erosions after hip replacement surgery 2 months prior to presentation
The patient had been diagnosed with pemphigus vulgaris (PV) 1 year prior to presentation with erosions on the axilla. Biopsy at that time revealed intraepithelial acantholytic blistering with areas of suprabasilar and subcorneal clefting. Direct immunofluorescence was positive for linear/granular IgG deposition throughout the epithelial cell surfaces, as well as linear/granular C3 deposits of the lower two thirds of the epithelial strata, consistent for pemphigus vulgaris.
There is likely a genetic predisposition. Medications that may induce pemphigus include penicillamine, nifedipine, or captopril.
Clinically, PV presents with flaccid blistering lesions that may be cutaneous and/or mucosal. Bullae can progress to erosions and crusting, which then heal with pigment alteration but not scarring. The most commonly affected sites are the mouth, intertriginous areas, face, and neck. Mucosal lesions can involve the lips, esophagus, conjunctiva, and genitals.
Biopsy for histology and direct immunofluorescence is important in distinguishing between PV and other blistering disorders. Up to 75% of patients with active disease also have a positive indirect immunofluorescence with circulating IgG.
There are numerous reports in the literature of PV occurring in previous surgical scars, and areas of friction or trauma. This so-called Koebner’s phenomenon is seen more commonly in several dermatologic conditions, such as psoriasis, lichen planus, verruca vulgaris, and vitiligo.
Treatment for PV is generally immunosuppressive. Systemic therapy usually begins with prednisone and then is transitioned to a steroid sparing agent such as mycophenolate mofetil. Other steroid sparing agents include azathioprine, methotrexate, cyclophosphamide, and intravenous immunoglobulin. Secondary infections are possible and should be treated. Topical therapies aimed at reducing pain, especially in mucosal lesions, can be beneficial.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cerottini JP et al. Eur J Dermatol. 2000 Oct-Nov;10(7):546-7.
Reichert-Penetrat S et al. Eur J Dermatol. 1998 Jan-Feb;8(1):60-2.
Saini P et al. Skinmed. 2020 Aug 1;18(4):252-253.
The patient had been diagnosed with pemphigus vulgaris (PV) 1 year prior to presentation with erosions on the axilla. Biopsy at that time revealed intraepithelial acantholytic blistering with areas of suprabasilar and subcorneal clefting. Direct immunofluorescence was positive for linear/granular IgG deposition throughout the epithelial cell surfaces, as well as linear/granular C3 deposits of the lower two thirds of the epithelial strata, consistent for pemphigus vulgaris.
There is likely a genetic predisposition. Medications that may induce pemphigus include penicillamine, nifedipine, or captopril.
Clinically, PV presents with flaccid blistering lesions that may be cutaneous and/or mucosal. Bullae can progress to erosions and crusting, which then heal with pigment alteration but not scarring. The most commonly affected sites are the mouth, intertriginous areas, face, and neck. Mucosal lesions can involve the lips, esophagus, conjunctiva, and genitals.
Biopsy for histology and direct immunofluorescence is important in distinguishing between PV and other blistering disorders. Up to 75% of patients with active disease also have a positive indirect immunofluorescence with circulating IgG.
There are numerous reports in the literature of PV occurring in previous surgical scars, and areas of friction or trauma. This so-called Koebner’s phenomenon is seen more commonly in several dermatologic conditions, such as psoriasis, lichen planus, verruca vulgaris, and vitiligo.
Treatment for PV is generally immunosuppressive. Systemic therapy usually begins with prednisone and then is transitioned to a steroid sparing agent such as mycophenolate mofetil. Other steroid sparing agents include azathioprine, methotrexate, cyclophosphamide, and intravenous immunoglobulin. Secondary infections are possible and should be treated. Topical therapies aimed at reducing pain, especially in mucosal lesions, can be beneficial.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cerottini JP et al. Eur J Dermatol. 2000 Oct-Nov;10(7):546-7.
Reichert-Penetrat S et al. Eur J Dermatol. 1998 Jan-Feb;8(1):60-2.
Saini P et al. Skinmed. 2020 Aug 1;18(4):252-253.
The patient had been diagnosed with pemphigus vulgaris (PV) 1 year prior to presentation with erosions on the axilla. Biopsy at that time revealed intraepithelial acantholytic blistering with areas of suprabasilar and subcorneal clefting. Direct immunofluorescence was positive for linear/granular IgG deposition throughout the epithelial cell surfaces, as well as linear/granular C3 deposits of the lower two thirds of the epithelial strata, consistent for pemphigus vulgaris.
There is likely a genetic predisposition. Medications that may induce pemphigus include penicillamine, nifedipine, or captopril.
Clinically, PV presents with flaccid blistering lesions that may be cutaneous and/or mucosal. Bullae can progress to erosions and crusting, which then heal with pigment alteration but not scarring. The most commonly affected sites are the mouth, intertriginous areas, face, and neck. Mucosal lesions can involve the lips, esophagus, conjunctiva, and genitals.
Biopsy for histology and direct immunofluorescence is important in distinguishing between PV and other blistering disorders. Up to 75% of patients with active disease also have a positive indirect immunofluorescence with circulating IgG.
There are numerous reports in the literature of PV occurring in previous surgical scars, and areas of friction or trauma. This so-called Koebner’s phenomenon is seen more commonly in several dermatologic conditions, such as psoriasis, lichen planus, verruca vulgaris, and vitiligo.
Treatment for PV is generally immunosuppressive. Systemic therapy usually begins with prednisone and then is transitioned to a steroid sparing agent such as mycophenolate mofetil. Other steroid sparing agents include azathioprine, methotrexate, cyclophosphamide, and intravenous immunoglobulin. Secondary infections are possible and should be treated. Topical therapies aimed at reducing pain, especially in mucosal lesions, can be beneficial.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cerottini JP et al. Eur J Dermatol. 2000 Oct-Nov;10(7):546-7.
Reichert-Penetrat S et al. Eur J Dermatol. 1998 Jan-Feb;8(1):60-2.
Saini P et al. Skinmed. 2020 Aug 1;18(4):252-253.
Treating Family: Ethicist Discusses Whether It’s Appropriate
This transcript has been edited for clarity.
There’s a very interesting story in the medical press. A few years ago, a plastic surgeon named Edmond Cabbabe was preparing to do a follow-up cosmetic procedure on his wife at Mercy Hospital South, which is a big hospital in the St. Louis, Missouri, area.
He put her on the operating schedule, and he had done that when he had performed the original operation on her. On the day of the surgery, he got a call from the hospital saying the procedure was canceled. They said that the hospital’s policy, maybe a new one, would not allow doctors to operate on family members.
This physician was a past president of the Missouri State Medical Association. I think he was also on the board or president of the American Medical Association (AMA) Foundation. This was a physician not only in a skilled area where he felt confident he could take care of his wife, but also someone who was prominent in medical politics and medical policy.
The AMA forever has had a policy that says don’t treat relatives. This physician basically said, I think that policy is too restrictive, too cautious, and it doesn’t make much sense to continue to say that you can’t treat family and friends.
By implication, he was saying, I know exactly what I’m doing in my field and I know exactly what I’m doing with her procedure. I should have a right to perform it. I think I do a great job and I’d be best for her.
If you look at medical boards, every once in a while in some state, someone is brought up on a charge of doing different things with family members and saying that they’re going to get censured. They don’t usually lose their license, but they get a reprimand or get told that is just not ethical to do.
I think, in the long run, the policy about not treating your family and friends makes sense. The problem is, as is well known from the social sciences and psychology, people get biased when they deal with those they care about, love, and hold close to them.
It’s hard for the doctor to be objective when dealing with people that they really like or love. It’s also difficult for patients because they may not want to bring up something or they are uncomfortable talking with a doctor who’s a family member or close friend. They may not want to complain. They may be a little bit embarrassed about things. It just adds an emotional edge, I think, that’s difficult.
All that said, do I know doctors who regularly prescribe, say, an ointment for something that’s itchy or some kind of a pill when allergy season breaks out? I do. Do I think they’re acting in a horribly unethical manner? I don’t.
You need some judgment here. There are absolutely minor things where objectivity, fear, and anxiety are not in play. You’re going to be able to prescribe the routine thing for the routine itch without worrying too much about whether it’s a stranger, a friend, or your daughter.
What sorts of things am I really talking about when I say that minor variability ought to be allowed? It’s one thing when someone has poison ivy and they’re going to need some kind of standard medicine to treat it. A very different area that’s much more dangerous, and one I would avoid, is in the mental health field, and for that matter, the pain field.
It’s tempting to say: “Oh, my relative is just having a bad time. I’ll give her a little bit of antidepressant medicine,” or “They seem to be having pain after an operation or something, and I’m going to give them a little bit of pain meds just to get them through.”
Those areas are flying red flags. It’s easy to abuse and easy for someone to become a user and manipulate a friend or a doctor who’s a relative into getting things that another doctor wouldn’t be giving. I think that’s the space where you’ve got to exercise extreme caution.
Time and again, when those people get called up in front of the boards for treating relatives, it’s in those spaces of mental health, anxiety, and pain control. Again, when you know that there’s a likelihood of abuse, I think that’s the place where the line has to hold. Don’t treat the relative. Don’t treat the friend.
At the end of the day, I wouldn’t change the AMA policy. I think we should keep it in place and morally try to discourage doctors from caring for those they’re close to or they have emotional ties to.
At the same time, as with all ethical situations, there has to be a little bit of wiggle room for those super-minor cases where it just makes sense to say: “You don’t have to go find somebody else to do this. I can prescribe this ointment or this minor thing for you. No one’s objectivity is going to be soured, and you’re not going to feel in any way at risk because I’m going to prescribe this for you.”
Common sense ought to prevail. The default position is don’t do it; however, maybe with a tiny bit of space for what’s minor, what’s routine, and what really does just save people some inconvenience, there I might just give a little.
Dr. Caplan, Director, Division of Medical Ethics, New York University Langone Medical Center, New York City, has disclosed relationships with Johnson & Johnson’s Panel for Compassionate Drug Use and Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
There’s a very interesting story in the medical press. A few years ago, a plastic surgeon named Edmond Cabbabe was preparing to do a follow-up cosmetic procedure on his wife at Mercy Hospital South, which is a big hospital in the St. Louis, Missouri, area.
He put her on the operating schedule, and he had done that when he had performed the original operation on her. On the day of the surgery, he got a call from the hospital saying the procedure was canceled. They said that the hospital’s policy, maybe a new one, would not allow doctors to operate on family members.
This physician was a past president of the Missouri State Medical Association. I think he was also on the board or president of the American Medical Association (AMA) Foundation. This was a physician not only in a skilled area where he felt confident he could take care of his wife, but also someone who was prominent in medical politics and medical policy.
The AMA forever has had a policy that says don’t treat relatives. This physician basically said, I think that policy is too restrictive, too cautious, and it doesn’t make much sense to continue to say that you can’t treat family and friends.
By implication, he was saying, I know exactly what I’m doing in my field and I know exactly what I’m doing with her procedure. I should have a right to perform it. I think I do a great job and I’d be best for her.
If you look at medical boards, every once in a while in some state, someone is brought up on a charge of doing different things with family members and saying that they’re going to get censured. They don’t usually lose their license, but they get a reprimand or get told that is just not ethical to do.
I think, in the long run, the policy about not treating your family and friends makes sense. The problem is, as is well known from the social sciences and psychology, people get biased when they deal with those they care about, love, and hold close to them.
It’s hard for the doctor to be objective when dealing with people that they really like or love. It’s also difficult for patients because they may not want to bring up something or they are uncomfortable talking with a doctor who’s a family member or close friend. They may not want to complain. They may be a little bit embarrassed about things. It just adds an emotional edge, I think, that’s difficult.
All that said, do I know doctors who regularly prescribe, say, an ointment for something that’s itchy or some kind of a pill when allergy season breaks out? I do. Do I think they’re acting in a horribly unethical manner? I don’t.
You need some judgment here. There are absolutely minor things where objectivity, fear, and anxiety are not in play. You’re going to be able to prescribe the routine thing for the routine itch without worrying too much about whether it’s a stranger, a friend, or your daughter.
What sorts of things am I really talking about when I say that minor variability ought to be allowed? It’s one thing when someone has poison ivy and they’re going to need some kind of standard medicine to treat it. A very different area that’s much more dangerous, and one I would avoid, is in the mental health field, and for that matter, the pain field.
It’s tempting to say: “Oh, my relative is just having a bad time. I’ll give her a little bit of antidepressant medicine,” or “They seem to be having pain after an operation or something, and I’m going to give them a little bit of pain meds just to get them through.”
Those areas are flying red flags. It’s easy to abuse and easy for someone to become a user and manipulate a friend or a doctor who’s a relative into getting things that another doctor wouldn’t be giving. I think that’s the space where you’ve got to exercise extreme caution.
Time and again, when those people get called up in front of the boards for treating relatives, it’s in those spaces of mental health, anxiety, and pain control. Again, when you know that there’s a likelihood of abuse, I think that’s the place where the line has to hold. Don’t treat the relative. Don’t treat the friend.
At the end of the day, I wouldn’t change the AMA policy. I think we should keep it in place and morally try to discourage doctors from caring for those they’re close to or they have emotional ties to.
At the same time, as with all ethical situations, there has to be a little bit of wiggle room for those super-minor cases where it just makes sense to say: “You don’t have to go find somebody else to do this. I can prescribe this ointment or this minor thing for you. No one’s objectivity is going to be soured, and you’re not going to feel in any way at risk because I’m going to prescribe this for you.”
Common sense ought to prevail. The default position is don’t do it; however, maybe with a tiny bit of space for what’s minor, what’s routine, and what really does just save people some inconvenience, there I might just give a little.
Dr. Caplan, Director, Division of Medical Ethics, New York University Langone Medical Center, New York City, has disclosed relationships with Johnson & Johnson’s Panel for Compassionate Drug Use and Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
There’s a very interesting story in the medical press. A few years ago, a plastic surgeon named Edmond Cabbabe was preparing to do a follow-up cosmetic procedure on his wife at Mercy Hospital South, which is a big hospital in the St. Louis, Missouri, area.
He put her on the operating schedule, and he had done that when he had performed the original operation on her. On the day of the surgery, he got a call from the hospital saying the procedure was canceled. They said that the hospital’s policy, maybe a new one, would not allow doctors to operate on family members.
This physician was a past president of the Missouri State Medical Association. I think he was also on the board or president of the American Medical Association (AMA) Foundation. This was a physician not only in a skilled area where he felt confident he could take care of his wife, but also someone who was prominent in medical politics and medical policy.
The AMA forever has had a policy that says don’t treat relatives. This physician basically said, I think that policy is too restrictive, too cautious, and it doesn’t make much sense to continue to say that you can’t treat family and friends.
By implication, he was saying, I know exactly what I’m doing in my field and I know exactly what I’m doing with her procedure. I should have a right to perform it. I think I do a great job and I’d be best for her.
If you look at medical boards, every once in a while in some state, someone is brought up on a charge of doing different things with family members and saying that they’re going to get censured. They don’t usually lose their license, but they get a reprimand or get told that is just not ethical to do.
I think, in the long run, the policy about not treating your family and friends makes sense. The problem is, as is well known from the social sciences and psychology, people get biased when they deal with those they care about, love, and hold close to them.
It’s hard for the doctor to be objective when dealing with people that they really like or love. It’s also difficult for patients because they may not want to bring up something or they are uncomfortable talking with a doctor who’s a family member or close friend. They may not want to complain. They may be a little bit embarrassed about things. It just adds an emotional edge, I think, that’s difficult.
All that said, do I know doctors who regularly prescribe, say, an ointment for something that’s itchy or some kind of a pill when allergy season breaks out? I do. Do I think they’re acting in a horribly unethical manner? I don’t.
You need some judgment here. There are absolutely minor things where objectivity, fear, and anxiety are not in play. You’re going to be able to prescribe the routine thing for the routine itch without worrying too much about whether it’s a stranger, a friend, or your daughter.
What sorts of things am I really talking about when I say that minor variability ought to be allowed? It’s one thing when someone has poison ivy and they’re going to need some kind of standard medicine to treat it. A very different area that’s much more dangerous, and one I would avoid, is in the mental health field, and for that matter, the pain field.
It’s tempting to say: “Oh, my relative is just having a bad time. I’ll give her a little bit of antidepressant medicine,” or “They seem to be having pain after an operation or something, and I’m going to give them a little bit of pain meds just to get them through.”
Those areas are flying red flags. It’s easy to abuse and easy for someone to become a user and manipulate a friend or a doctor who’s a relative into getting things that another doctor wouldn’t be giving. I think that’s the space where you’ve got to exercise extreme caution.
Time and again, when those people get called up in front of the boards for treating relatives, it’s in those spaces of mental health, anxiety, and pain control. Again, when you know that there’s a likelihood of abuse, I think that’s the place where the line has to hold. Don’t treat the relative. Don’t treat the friend.
At the end of the day, I wouldn’t change the AMA policy. I think we should keep it in place and morally try to discourage doctors from caring for those they’re close to or they have emotional ties to.
At the same time, as with all ethical situations, there has to be a little bit of wiggle room for those super-minor cases where it just makes sense to say: “You don’t have to go find somebody else to do this. I can prescribe this ointment or this minor thing for you. No one’s objectivity is going to be soured, and you’re not going to feel in any way at risk because I’m going to prescribe this for you.”
Common sense ought to prevail. The default position is don’t do it; however, maybe with a tiny bit of space for what’s minor, what’s routine, and what really does just save people some inconvenience, there I might just give a little.
Dr. Caplan, Director, Division of Medical Ethics, New York University Langone Medical Center, New York City, has disclosed relationships with Johnson & Johnson’s Panel for Compassionate Drug Use and Medscape.
A version of this article first appeared on Medscape.com.
Harnessing Doxycycline for STI Prevention: A Vital Role for Primary Care Physicians
Primary care physicians frequently offer postexposure prophylaxis for various infections, including influenza, pertussis, tetanus, hepatitis, and Lyme disease, among others. However, the scope of postexposure prophylaxis in primary care is expanding, presenting an opportunity to further integrate it into patient care. As primary care providers, we have the unique advantage of being involved in both preventive care and immediate response, particularly in urgent care or triage scenarios. This dual role is crucial, as timely administration of postexposure prophylaxis can prevent infections from taking hold, especially following high-risk exposures.
Recently, the use of doxycycline as a form of postexposure prophylaxis for sexually transmitted infections (STIs) has gained attention. Traditionally, doxycycline has been used as preexposure or postexposure prophylaxis for conditions like malaria and Lyme disease but has not been widely employed for STI prevention until now. Doxycycline is a relatively common medication, generally safe with side effects that typically resolve upon discontinuation. Several open-label studies have shown that taking 200 mg of doxycycline within 72 hours of condomless sex significantly reduces the incidence of chlamydia, gonorrhea, and syphilis among gay, bisexual, and other men who have sex with men, as well as transgender women who have previously had a bacterial STI. However, these benefits have not been consistently observed among cisgender women and heterosexual men.
Given these findings, the Centers for Disease Control and Prevention now recommends that clinicians discuss the risks and benefits of doxycycline PEP (Doxy PEP) with gay, bisexual, and other men who have sex with men, as well as transgender women who have had a bacterial STI in the past 12 months. This discussion should be part of a shared decision-making process, advising the use of 200 mg of doxycycline within 72 hours of oral, vaginal, or anal sex, with the recommendation not to exceed 200 mg every 24 hours and to reassess the need for continued use every 3-6 months. Doxy PEP can be safely prescribed with preexposure prophylaxis for HIV (PrEP). Patients who receive PrEP may often be eligible for Doxy PEP, though the groups are not always the same.
The shared decision-making process is essential when considering Doxy PEP. While cost-effective and proven to reduce the risk of gonorrhea, chlamydia, and syphilis, its benefits vary among different populations. Moreover, some patients may experience side effects such as photosensitivity and gastrointestinal discomfort. Since the effectiveness of prophylaxis is closely tied to the timing of exposure and the patient’s current risk factors, it is important to regularly evaluate whether Doxy PEP remains beneficial. As there is not yet clear benefit to heterosexual men and cisgender women, opportunities still need to be explored for them.
Integrating Doxy PEP into a primary care practice can be done efficiently. A standing order protocol could be established for telehealth visits or nurse triage, allowing timely administration when patients report an exposure within 72 hours. It could also be incorporated into electronic medical records as part of a smart set for easy access to orders and as standard educational material in after-visit instructions. As this option is new, it is also important to discuss it with patients before they may need it so that they are aware should the need arise. While concerns about antibiotic resistance are valid, studies have not yet shown significant resistance issues related to Doxy PEP use, though ongoing monitoring is necessary.
You might wonder why primary care should prioritize this intervention. As the first point of contact, primary care providers are well-positioned to identify the need for prophylaxis, particularly since its effectiveness diminishes over time. Furthermore, the established, trusting relationships that primary care physicians often have with their patients create a nonjudgmental environment that encourages disclosure of potential exposures. This trust, combined with easier access to care, can make a significant difference in the timely provision of postexposure prophylaxis. By offering comprehensive, holistic care, including prophylaxis, primary care physicians can prevent infections and address conditions before they lead to serious complications. Therefore, family medicine physicians should consider incorporating Doxy PEP into their practices as a standard of care.
Dr. Wheat is vice chair of Diversity, Equity, and Inclusion, Department of Family and Community Medicine, and associate professor, Family and Community Medicine, at Northwestern University’s Feinberg School of Medicine, Chicago. She has no relevant financial disclosures.
References
Bachmann LH et al. CDC Clinical Guidelines on the Use of Doxycycline Postexposure Prophylaxis for Bacterial Sexually Transmitted Infection Prevention, United States, 2024. MMWR Recomm Rep 2024;73(No. RR-2):1-8.
Traeger MW et al. Potential Impact of Doxycycline Postexposure Prophylaxis Prescribing Strategies on Incidence of Bacterial Sexually Transmitted Infections. (Clin Infect Dis. 2023 Aug 18. doi: 10.1093/cid/ciad488).
Primary care physicians frequently offer postexposure prophylaxis for various infections, including influenza, pertussis, tetanus, hepatitis, and Lyme disease, among others. However, the scope of postexposure prophylaxis in primary care is expanding, presenting an opportunity to further integrate it into patient care. As primary care providers, we have the unique advantage of being involved in both preventive care and immediate response, particularly in urgent care or triage scenarios. This dual role is crucial, as timely administration of postexposure prophylaxis can prevent infections from taking hold, especially following high-risk exposures.
Recently, the use of doxycycline as a form of postexposure prophylaxis for sexually transmitted infections (STIs) has gained attention. Traditionally, doxycycline has been used as preexposure or postexposure prophylaxis for conditions like malaria and Lyme disease but has not been widely employed for STI prevention until now. Doxycycline is a relatively common medication, generally safe with side effects that typically resolve upon discontinuation. Several open-label studies have shown that taking 200 mg of doxycycline within 72 hours of condomless sex significantly reduces the incidence of chlamydia, gonorrhea, and syphilis among gay, bisexual, and other men who have sex with men, as well as transgender women who have previously had a bacterial STI. However, these benefits have not been consistently observed among cisgender women and heterosexual men.
Given these findings, the Centers for Disease Control and Prevention now recommends that clinicians discuss the risks and benefits of doxycycline PEP (Doxy PEP) with gay, bisexual, and other men who have sex with men, as well as transgender women who have had a bacterial STI in the past 12 months. This discussion should be part of a shared decision-making process, advising the use of 200 mg of doxycycline within 72 hours of oral, vaginal, or anal sex, with the recommendation not to exceed 200 mg every 24 hours and to reassess the need for continued use every 3-6 months. Doxy PEP can be safely prescribed with preexposure prophylaxis for HIV (PrEP). Patients who receive PrEP may often be eligible for Doxy PEP, though the groups are not always the same.
The shared decision-making process is essential when considering Doxy PEP. While cost-effective and proven to reduce the risk of gonorrhea, chlamydia, and syphilis, its benefits vary among different populations. Moreover, some patients may experience side effects such as photosensitivity and gastrointestinal discomfort. Since the effectiveness of prophylaxis is closely tied to the timing of exposure and the patient’s current risk factors, it is important to regularly evaluate whether Doxy PEP remains beneficial. As there is not yet clear benefit to heterosexual men and cisgender women, opportunities still need to be explored for them.
Integrating Doxy PEP into a primary care practice can be done efficiently. A standing order protocol could be established for telehealth visits or nurse triage, allowing timely administration when patients report an exposure within 72 hours. It could also be incorporated into electronic medical records as part of a smart set for easy access to orders and as standard educational material in after-visit instructions. As this option is new, it is also important to discuss it with patients before they may need it so that they are aware should the need arise. While concerns about antibiotic resistance are valid, studies have not yet shown significant resistance issues related to Doxy PEP use, though ongoing monitoring is necessary.
You might wonder why primary care should prioritize this intervention. As the first point of contact, primary care providers are well-positioned to identify the need for prophylaxis, particularly since its effectiveness diminishes over time. Furthermore, the established, trusting relationships that primary care physicians often have with their patients create a nonjudgmental environment that encourages disclosure of potential exposures. This trust, combined with easier access to care, can make a significant difference in the timely provision of postexposure prophylaxis. By offering comprehensive, holistic care, including prophylaxis, primary care physicians can prevent infections and address conditions before they lead to serious complications. Therefore, family medicine physicians should consider incorporating Doxy PEP into their practices as a standard of care.
Dr. Wheat is vice chair of Diversity, Equity, and Inclusion, Department of Family and Community Medicine, and associate professor, Family and Community Medicine, at Northwestern University’s Feinberg School of Medicine, Chicago. She has no relevant financial disclosures.
References
Bachmann LH et al. CDC Clinical Guidelines on the Use of Doxycycline Postexposure Prophylaxis for Bacterial Sexually Transmitted Infection Prevention, United States, 2024. MMWR Recomm Rep 2024;73(No. RR-2):1-8.
Traeger MW et al. Potential Impact of Doxycycline Postexposure Prophylaxis Prescribing Strategies on Incidence of Bacterial Sexually Transmitted Infections. (Clin Infect Dis. 2023 Aug 18. doi: 10.1093/cid/ciad488).
Primary care physicians frequently offer postexposure prophylaxis for various infections, including influenza, pertussis, tetanus, hepatitis, and Lyme disease, among others. However, the scope of postexposure prophylaxis in primary care is expanding, presenting an opportunity to further integrate it into patient care. As primary care providers, we have the unique advantage of being involved in both preventive care and immediate response, particularly in urgent care or triage scenarios. This dual role is crucial, as timely administration of postexposure prophylaxis can prevent infections from taking hold, especially following high-risk exposures.
Recently, the use of doxycycline as a form of postexposure prophylaxis for sexually transmitted infections (STIs) has gained attention. Traditionally, doxycycline has been used as preexposure or postexposure prophylaxis for conditions like malaria and Lyme disease but has not been widely employed for STI prevention until now. Doxycycline is a relatively common medication, generally safe with side effects that typically resolve upon discontinuation. Several open-label studies have shown that taking 200 mg of doxycycline within 72 hours of condomless sex significantly reduces the incidence of chlamydia, gonorrhea, and syphilis among gay, bisexual, and other men who have sex with men, as well as transgender women who have previously had a bacterial STI. However, these benefits have not been consistently observed among cisgender women and heterosexual men.
Given these findings, the Centers for Disease Control and Prevention now recommends that clinicians discuss the risks and benefits of doxycycline PEP (Doxy PEP) with gay, bisexual, and other men who have sex with men, as well as transgender women who have had a bacterial STI in the past 12 months. This discussion should be part of a shared decision-making process, advising the use of 200 mg of doxycycline within 72 hours of oral, vaginal, or anal sex, with the recommendation not to exceed 200 mg every 24 hours and to reassess the need for continued use every 3-6 months. Doxy PEP can be safely prescribed with preexposure prophylaxis for HIV (PrEP). Patients who receive PrEP may often be eligible for Doxy PEP, though the groups are not always the same.
The shared decision-making process is essential when considering Doxy PEP. While cost-effective and proven to reduce the risk of gonorrhea, chlamydia, and syphilis, its benefits vary among different populations. Moreover, some patients may experience side effects such as photosensitivity and gastrointestinal discomfort. Since the effectiveness of prophylaxis is closely tied to the timing of exposure and the patient’s current risk factors, it is important to regularly evaluate whether Doxy PEP remains beneficial. As there is not yet clear benefit to heterosexual men and cisgender women, opportunities still need to be explored for them.
Integrating Doxy PEP into a primary care practice can be done efficiently. A standing order protocol could be established for telehealth visits or nurse triage, allowing timely administration when patients report an exposure within 72 hours. It could also be incorporated into electronic medical records as part of a smart set for easy access to orders and as standard educational material in after-visit instructions. As this option is new, it is also important to discuss it with patients before they may need it so that they are aware should the need arise. While concerns about antibiotic resistance are valid, studies have not yet shown significant resistance issues related to Doxy PEP use, though ongoing monitoring is necessary.
You might wonder why primary care should prioritize this intervention. As the first point of contact, primary care providers are well-positioned to identify the need for prophylaxis, particularly since its effectiveness diminishes over time. Furthermore, the established, trusting relationships that primary care physicians often have with their patients create a nonjudgmental environment that encourages disclosure of potential exposures. This trust, combined with easier access to care, can make a significant difference in the timely provision of postexposure prophylaxis. By offering comprehensive, holistic care, including prophylaxis, primary care physicians can prevent infections and address conditions before they lead to serious complications. Therefore, family medicine physicians should consider incorporating Doxy PEP into their practices as a standard of care.
Dr. Wheat is vice chair of Diversity, Equity, and Inclusion, Department of Family and Community Medicine, and associate professor, Family and Community Medicine, at Northwestern University’s Feinberg School of Medicine, Chicago. She has no relevant financial disclosures.
References
Bachmann LH et al. CDC Clinical Guidelines on the Use of Doxycycline Postexposure Prophylaxis for Bacterial Sexually Transmitted Infection Prevention, United States, 2024. MMWR Recomm Rep 2024;73(No. RR-2):1-8.
Traeger MW et al. Potential Impact of Doxycycline Postexposure Prophylaxis Prescribing Strategies on Incidence of Bacterial Sexually Transmitted Infections. (Clin Infect Dis. 2023 Aug 18. doi: 10.1093/cid/ciad488).
Starting Mammography at Age 40 May Backfire Due to False Positives
Earlier this year, I wrote a Medscape commentary to explain my disagreement with the US Preventive Services Task Force (USPSTF)’s updated recommendation that all women at average risk for breast cancer start screening mammography at age 40. The bottom line is that when the evidence doesn’t change, the guidelines shouldn’t change. Since then, other screening experts have criticized the USPSTF guideline on similar grounds, and a national survey reported that nearly 4 out of 10 women in their 40s preferred to delay breast cancer screening after viewing a decision aid and a personalized breast cancer risk estimate.
The decision analysis performed for the USPSTF guideline estimated that compared with having mammography beginning at age 50, 1000 women who begin at age 40 experience 519 more false-positive results and 62 more benign breast biopsies. Another study suggested that anxiety and other psychosocial harms resulting from a false-positive test are similar between patients who require a biopsy vs additional imaging only. Of greater concern, women who have false-positive results are less likely to return for their next scheduled screening exam.
A recent analysis of 2005-2017 data from the US Breast Cancer Surveillance Consortium found that about 1 in 10 mammograms had a false-positive result. Sixty percent of these patients underwent immediate additional imaging, 27% were recalled for diagnostic imaging within the next few days to weeks, and 13% were advised to have a biopsy. While patients who had additional imaging at the same visit were only 1.9% less likely to return for screening mammography within 30 months compared with those with normal mammograms, women who were recalled for short-interval follow-up or recommended for biopsy were 15.9% and 10% less likely to return, respectively. For unclear reasons, women who identified as Asian or Hispanic had even lower rates of return screening after false-positive results.
These differences matter because women in their 40s, with the lowest incidence of breast cancer among those undergoing screening, have a lot of false positives. A patient who follows the USPSTF recommendation and starts screening at age 40 has a 42% chance of having at least one false positive with every-other-year screening, or a 61% chance with annual screening, by the time she turns 50. If some of these patients are so turned off by false positives that they don’t return for regular mammography in their 50s and 60s, when screening is the most likely to catch clinically significant cancers at treatable stages, then moving up the starting age may backfire and cause net harm.
The recently implemented FDA rule requiring mammography reports to include breast density could compound this problem. Because younger women are more likely to have dense breasts, more of them will probably decide to have supplemental imaging for cancer. I previously pointed out that we don’t know whether supplemental imaging with breast ultrasonography or MRI reduces cancer deaths, but we do know that it increases false-positive results.
I have personally cared for several patients who abandoned screening mammography for long stretches, or permanently, after having endured one or more benign biopsies prompted by a false-positive result. I vividly recall one woman in her 60s who was very reluctant to have screening tests in general, and mammography in particular, for that reason. After she had been my patient for a few years, I finally persuaded her to resume screening. We were both surprised when her first mammogram in more than a decade revealed an early-stage breast cancer. Fortunately, the tumor was successfully treated, but for her, an earlier false-positive result nearly ended up having critical health consequences.
Dr. Lin is associate director, Family Medicine Residency Program, Lancaster General Hospital, Lancaster, Pennsylvania. He blogs at Common Sense Family Doctor. He has no relevant financial relationships.
A version of this article appeared on Medscape.com.
Earlier this year, I wrote a Medscape commentary to explain my disagreement with the US Preventive Services Task Force (USPSTF)’s updated recommendation that all women at average risk for breast cancer start screening mammography at age 40. The bottom line is that when the evidence doesn’t change, the guidelines shouldn’t change. Since then, other screening experts have criticized the USPSTF guideline on similar grounds, and a national survey reported that nearly 4 out of 10 women in their 40s preferred to delay breast cancer screening after viewing a decision aid and a personalized breast cancer risk estimate.
The decision analysis performed for the USPSTF guideline estimated that compared with having mammography beginning at age 50, 1000 women who begin at age 40 experience 519 more false-positive results and 62 more benign breast biopsies. Another study suggested that anxiety and other psychosocial harms resulting from a false-positive test are similar between patients who require a biopsy vs additional imaging only. Of greater concern, women who have false-positive results are less likely to return for their next scheduled screening exam.
A recent analysis of 2005-2017 data from the US Breast Cancer Surveillance Consortium found that about 1 in 10 mammograms had a false-positive result. Sixty percent of these patients underwent immediate additional imaging, 27% were recalled for diagnostic imaging within the next few days to weeks, and 13% were advised to have a biopsy. While patients who had additional imaging at the same visit were only 1.9% less likely to return for screening mammography within 30 months compared with those with normal mammograms, women who were recalled for short-interval follow-up or recommended for biopsy were 15.9% and 10% less likely to return, respectively. For unclear reasons, women who identified as Asian or Hispanic had even lower rates of return screening after false-positive results.
These differences matter because women in their 40s, with the lowest incidence of breast cancer among those undergoing screening, have a lot of false positives. A patient who follows the USPSTF recommendation and starts screening at age 40 has a 42% chance of having at least one false positive with every-other-year screening, or a 61% chance with annual screening, by the time she turns 50. If some of these patients are so turned off by false positives that they don’t return for regular mammography in their 50s and 60s, when screening is the most likely to catch clinically significant cancers at treatable stages, then moving up the starting age may backfire and cause net harm.
The recently implemented FDA rule requiring mammography reports to include breast density could compound this problem. Because younger women are more likely to have dense breasts, more of them will probably decide to have supplemental imaging for cancer. I previously pointed out that we don’t know whether supplemental imaging with breast ultrasonography or MRI reduces cancer deaths, but we do know that it increases false-positive results.
I have personally cared for several patients who abandoned screening mammography for long stretches, or permanently, after having endured one or more benign biopsies prompted by a false-positive result. I vividly recall one woman in her 60s who was very reluctant to have screening tests in general, and mammography in particular, for that reason. After she had been my patient for a few years, I finally persuaded her to resume screening. We were both surprised when her first mammogram in more than a decade revealed an early-stage breast cancer. Fortunately, the tumor was successfully treated, but for her, an earlier false-positive result nearly ended up having critical health consequences.
Dr. Lin is associate director, Family Medicine Residency Program, Lancaster General Hospital, Lancaster, Pennsylvania. He blogs at Common Sense Family Doctor. He has no relevant financial relationships.
A version of this article appeared on Medscape.com.
Earlier this year, I wrote a Medscape commentary to explain my disagreement with the US Preventive Services Task Force (USPSTF)’s updated recommendation that all women at average risk for breast cancer start screening mammography at age 40. The bottom line is that when the evidence doesn’t change, the guidelines shouldn’t change. Since then, other screening experts have criticized the USPSTF guideline on similar grounds, and a national survey reported that nearly 4 out of 10 women in their 40s preferred to delay breast cancer screening after viewing a decision aid and a personalized breast cancer risk estimate.
The decision analysis performed for the USPSTF guideline estimated that compared with having mammography beginning at age 50, 1000 women who begin at age 40 experience 519 more false-positive results and 62 more benign breast biopsies. Another study suggested that anxiety and other psychosocial harms resulting from a false-positive test are similar between patients who require a biopsy vs additional imaging only. Of greater concern, women who have false-positive results are less likely to return for their next scheduled screening exam.
A recent analysis of 2005-2017 data from the US Breast Cancer Surveillance Consortium found that about 1 in 10 mammograms had a false-positive result. Sixty percent of these patients underwent immediate additional imaging, 27% were recalled for diagnostic imaging within the next few days to weeks, and 13% were advised to have a biopsy. While patients who had additional imaging at the same visit were only 1.9% less likely to return for screening mammography within 30 months compared with those with normal mammograms, women who were recalled for short-interval follow-up or recommended for biopsy were 15.9% and 10% less likely to return, respectively. For unclear reasons, women who identified as Asian or Hispanic had even lower rates of return screening after false-positive results.
These differences matter because women in their 40s, with the lowest incidence of breast cancer among those undergoing screening, have a lot of false positives. A patient who follows the USPSTF recommendation and starts screening at age 40 has a 42% chance of having at least one false positive with every-other-year screening, or a 61% chance with annual screening, by the time she turns 50. If some of these patients are so turned off by false positives that they don’t return for regular mammography in their 50s and 60s, when screening is the most likely to catch clinically significant cancers at treatable stages, then moving up the starting age may backfire and cause net harm.
The recently implemented FDA rule requiring mammography reports to include breast density could compound this problem. Because younger women are more likely to have dense breasts, more of them will probably decide to have supplemental imaging for cancer. I previously pointed out that we don’t know whether supplemental imaging with breast ultrasonography or MRI reduces cancer deaths, but we do know that it increases false-positive results.
I have personally cared for several patients who abandoned screening mammography for long stretches, or permanently, after having endured one or more benign biopsies prompted by a false-positive result. I vividly recall one woman in her 60s who was very reluctant to have screening tests in general, and mammography in particular, for that reason. After she had been my patient for a few years, I finally persuaded her to resume screening. We were both surprised when her first mammogram in more than a decade revealed an early-stage breast cancer. Fortunately, the tumor was successfully treated, but for her, an earlier false-positive result nearly ended up having critical health consequences.
Dr. Lin is associate director, Family Medicine Residency Program, Lancaster General Hospital, Lancaster, Pennsylvania. He blogs at Common Sense Family Doctor. He has no relevant financial relationships.
A version of this article appeared on Medscape.com.
Should There Be a Mandatory Retirement Age for Physicians?
This transcript has been edited for clarity.
I’d like to pose a question: When should doctors retire? When, as practicing physicians or surgeons, do we become too old to deliver competent service?
You will be amazed to hear, those of you who have listened to my videos before — and although it is a matter of public knowledge — that I’m 68. I know it’s impossible to imagine, due to this youthful appearance, visage, and so on, but I am. I’ve been a cancer doctor for 40 years; therefore, I need to think a little about retirement.
There are two elements of this for me. I’m a university professor, and in Oxford we did vote, as a democracy of scholars, to have a mandatory retirement age around 68. This is so that we can bring new blood forward so that we can create the space to promote new professors, to bring youngsters in to make new ideas, and to get rid of us fusty old lot.
The other argument would be, of course, that we are wise, we’re experienced, we are world-weary, and we’re successful — otherwise, we wouldn’t have lasted as academics as long. Nevertheless, we voted to do that.
It’s possible to have a discussion with the university to extend this, and for those of us who are clinical academics, I have an honorary appointment as a consultant cancer physician in the hospital and my university professorial appointment, too.
I can extend it probably until I’m about 70. It feels like a nice, round number at which to retire — somewhat arbitrarily, one would admit. But does that feel right?
In the United States, more than 25% of the physician workforce is over the age of 65. There are many studies showing that there is a 20% cognitive decline for most individuals between the ages of 45 and 65.
Are we as capable as an elderly workforce as once we were? Clearly, it’s hardly individualistic. It depends on each of our own health status, where we started from, and so on, but are there any general rules that we can apply? I think these are starting to creep in around the sense of revalidation.
In the United Kingdom, we have a General Medical Council (GMC). I need to have a license to practice from the GMC and a sense of fitness to practice. I have annual appraisals within the hospital system, in which I explore delivery of care, how I’m doing as a mentor, am I reaching the milestones I’ve set in terms of academic achievements, and so on.
This is a peer-to-peer process. We have senior physicians — people like myself — who act as appraisers to support our colleagues and to maintain that sense of fitness to practice. Every 5 years, I’m revalidated by the GMC. They take account of the annual appraisals and a report made by the senior physician within my hospital network who’s a so-called designated person.
These two elements come together with patient feedback, with 360-degree feedback from colleagues, and so on. This is quite a firmly regulated system that I think works. Our mandatory retirement age of 65 has gone. That was phased out by the government. In fact, our NHS is making an effort to retain older elders in the workforce.
They see the benefits of mentorship, experience, leadership, and networks. At a time when the majority of NHS are actively seeking to retire when 65, the NHS is trying to retain and pull back those of us who have been around for that wee bit longer and who still feel committed to doing it.
I’d be really interested to see what you think. There’s variation from country to country. I know that, in Australia, they’re talking about annual appraisals of doctors over the age of 70. I’d be very interested to hear what you think is likely to happen in the United States.
I think our system works pretty well, as long as you’re within the NHS and hospital system. If you wanted to still practice, but practice privately, you would still have to find somebody who’d be prepared to conduct appraisals and so on outside of the NHS. It’s an interesting area.
For myself, I still feel competent. Patients seem to like me. That’s an objective assessment by this 360-degree thing in which patients reflected very positively, indeed, in my approach to the delivery of the care and so on, as did colleagues. I’m still publishing, I go to meetings, I cheer things, bits and bobs. I’d say I’m a wee bit unusual in terms of still having a strong academic profile in doing stuff.
It’s an interesting question. Richard Doll, one of the world’s great epidemiologists who, of course, was the dominant discoverer of the link between smoking and lung cancer, was attending seminars, sitting in the front row, and coming into university 3 days a week at age 90, continuing to be contributory with his extraordinarily sharp intellect and vast, vast experience.
When I think of experience, all young cancer doctors are now immunologists. When I was a young doctor, I was a clinical pharmacologist. There are many lessons and tricks that I learned which I do need to pass on to the younger generation of today. What do you think? Should there be a mandatory retirement age? How do we best measure, assess, and revalidate elderly physicians and surgeons? How can we continue to contribute to those who choose to do so? For the time being, as always, thanks for listening.
Dr. Kerr is professor, Nuffield Department of Clinical Laboratory Science, University of Oxford, and professor of cancer medicine, Oxford Cancer Centre, Oxford, United Kingdom. He has disclosed ties with Celleron Therapeutics, Oxford Cancer Biomarkers (Board of Directors); Afrox (charity; Trustee); GlaxoSmithKline and Bayer HealthCare Pharmaceuticals (Consultant), Genomic Health; Merck Serono, and Roche.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I’d like to pose a question: When should doctors retire? When, as practicing physicians or surgeons, do we become too old to deliver competent service?
You will be amazed to hear, those of you who have listened to my videos before — and although it is a matter of public knowledge — that I’m 68. I know it’s impossible to imagine, due to this youthful appearance, visage, and so on, but I am. I’ve been a cancer doctor for 40 years; therefore, I need to think a little about retirement.
There are two elements of this for me. I’m a university professor, and in Oxford we did vote, as a democracy of scholars, to have a mandatory retirement age around 68. This is so that we can bring new blood forward so that we can create the space to promote new professors, to bring youngsters in to make new ideas, and to get rid of us fusty old lot.
The other argument would be, of course, that we are wise, we’re experienced, we are world-weary, and we’re successful — otherwise, we wouldn’t have lasted as academics as long. Nevertheless, we voted to do that.
It’s possible to have a discussion with the university to extend this, and for those of us who are clinical academics, I have an honorary appointment as a consultant cancer physician in the hospital and my university professorial appointment, too.
I can extend it probably until I’m about 70. It feels like a nice, round number at which to retire — somewhat arbitrarily, one would admit. But does that feel right?
In the United States, more than 25% of the physician workforce is over the age of 65. There are many studies showing that there is a 20% cognitive decline for most individuals between the ages of 45 and 65.
Are we as capable as an elderly workforce as once we were? Clearly, it’s hardly individualistic. It depends on each of our own health status, where we started from, and so on, but are there any general rules that we can apply? I think these are starting to creep in around the sense of revalidation.
In the United Kingdom, we have a General Medical Council (GMC). I need to have a license to practice from the GMC and a sense of fitness to practice. I have annual appraisals within the hospital system, in which I explore delivery of care, how I’m doing as a mentor, am I reaching the milestones I’ve set in terms of academic achievements, and so on.
This is a peer-to-peer process. We have senior physicians — people like myself — who act as appraisers to support our colleagues and to maintain that sense of fitness to practice. Every 5 years, I’m revalidated by the GMC. They take account of the annual appraisals and a report made by the senior physician within my hospital network who’s a so-called designated person.
These two elements come together with patient feedback, with 360-degree feedback from colleagues, and so on. This is quite a firmly regulated system that I think works. Our mandatory retirement age of 65 has gone. That was phased out by the government. In fact, our NHS is making an effort to retain older elders in the workforce.
They see the benefits of mentorship, experience, leadership, and networks. At a time when the majority of NHS are actively seeking to retire when 65, the NHS is trying to retain and pull back those of us who have been around for that wee bit longer and who still feel committed to doing it.
I’d be really interested to see what you think. There’s variation from country to country. I know that, in Australia, they’re talking about annual appraisals of doctors over the age of 70. I’d be very interested to hear what you think is likely to happen in the United States.
I think our system works pretty well, as long as you’re within the NHS and hospital system. If you wanted to still practice, but practice privately, you would still have to find somebody who’d be prepared to conduct appraisals and so on outside of the NHS. It’s an interesting area.
For myself, I still feel competent. Patients seem to like me. That’s an objective assessment by this 360-degree thing in which patients reflected very positively, indeed, in my approach to the delivery of the care and so on, as did colleagues. I’m still publishing, I go to meetings, I cheer things, bits and bobs. I’d say I’m a wee bit unusual in terms of still having a strong academic profile in doing stuff.
It’s an interesting question. Richard Doll, one of the world’s great epidemiologists who, of course, was the dominant discoverer of the link between smoking and lung cancer, was attending seminars, sitting in the front row, and coming into university 3 days a week at age 90, continuing to be contributory with his extraordinarily sharp intellect and vast, vast experience.
When I think of experience, all young cancer doctors are now immunologists. When I was a young doctor, I was a clinical pharmacologist. There are many lessons and tricks that I learned which I do need to pass on to the younger generation of today. What do you think? Should there be a mandatory retirement age? How do we best measure, assess, and revalidate elderly physicians and surgeons? How can we continue to contribute to those who choose to do so? For the time being, as always, thanks for listening.
Dr. Kerr is professor, Nuffield Department of Clinical Laboratory Science, University of Oxford, and professor of cancer medicine, Oxford Cancer Centre, Oxford, United Kingdom. He has disclosed ties with Celleron Therapeutics, Oxford Cancer Biomarkers (Board of Directors); Afrox (charity; Trustee); GlaxoSmithKline and Bayer HealthCare Pharmaceuticals (Consultant), Genomic Health; Merck Serono, and Roche.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I’d like to pose a question: When should doctors retire? When, as practicing physicians or surgeons, do we become too old to deliver competent service?
You will be amazed to hear, those of you who have listened to my videos before — and although it is a matter of public knowledge — that I’m 68. I know it’s impossible to imagine, due to this youthful appearance, visage, and so on, but I am. I’ve been a cancer doctor for 40 years; therefore, I need to think a little about retirement.
There are two elements of this for me. I’m a university professor, and in Oxford we did vote, as a democracy of scholars, to have a mandatory retirement age around 68. This is so that we can bring new blood forward so that we can create the space to promote new professors, to bring youngsters in to make new ideas, and to get rid of us fusty old lot.
The other argument would be, of course, that we are wise, we’re experienced, we are world-weary, and we’re successful — otherwise, we wouldn’t have lasted as academics as long. Nevertheless, we voted to do that.
It’s possible to have a discussion with the university to extend this, and for those of us who are clinical academics, I have an honorary appointment as a consultant cancer physician in the hospital and my university professorial appointment, too.
I can extend it probably until I’m about 70. It feels like a nice, round number at which to retire — somewhat arbitrarily, one would admit. But does that feel right?
In the United States, more than 25% of the physician workforce is over the age of 65. There are many studies showing that there is a 20% cognitive decline for most individuals between the ages of 45 and 65.
Are we as capable as an elderly workforce as once we were? Clearly, it’s hardly individualistic. It depends on each of our own health status, where we started from, and so on, but are there any general rules that we can apply? I think these are starting to creep in around the sense of revalidation.
In the United Kingdom, we have a General Medical Council (GMC). I need to have a license to practice from the GMC and a sense of fitness to practice. I have annual appraisals within the hospital system, in which I explore delivery of care, how I’m doing as a mentor, am I reaching the milestones I’ve set in terms of academic achievements, and so on.
This is a peer-to-peer process. We have senior physicians — people like myself — who act as appraisers to support our colleagues and to maintain that sense of fitness to practice. Every 5 years, I’m revalidated by the GMC. They take account of the annual appraisals and a report made by the senior physician within my hospital network who’s a so-called designated person.
These two elements come together with patient feedback, with 360-degree feedback from colleagues, and so on. This is quite a firmly regulated system that I think works. Our mandatory retirement age of 65 has gone. That was phased out by the government. In fact, our NHS is making an effort to retain older elders in the workforce.
They see the benefits of mentorship, experience, leadership, and networks. At a time when the majority of NHS are actively seeking to retire when 65, the NHS is trying to retain and pull back those of us who have been around for that wee bit longer and who still feel committed to doing it.
I’d be really interested to see what you think. There’s variation from country to country. I know that, in Australia, they’re talking about annual appraisals of doctors over the age of 70. I’d be very interested to hear what you think is likely to happen in the United States.
I think our system works pretty well, as long as you’re within the NHS and hospital system. If you wanted to still practice, but practice privately, you would still have to find somebody who’d be prepared to conduct appraisals and so on outside of the NHS. It’s an interesting area.
For myself, I still feel competent. Patients seem to like me. That’s an objective assessment by this 360-degree thing in which patients reflected very positively, indeed, in my approach to the delivery of the care and so on, as did colleagues. I’m still publishing, I go to meetings, I cheer things, bits and bobs. I’d say I’m a wee bit unusual in terms of still having a strong academic profile in doing stuff.
It’s an interesting question. Richard Doll, one of the world’s great epidemiologists who, of course, was the dominant discoverer of the link between smoking and lung cancer, was attending seminars, sitting in the front row, and coming into university 3 days a week at age 90, continuing to be contributory with his extraordinarily sharp intellect and vast, vast experience.
When I think of experience, all young cancer doctors are now immunologists. When I was a young doctor, I was a clinical pharmacologist. There are many lessons and tricks that I learned which I do need to pass on to the younger generation of today. What do you think? Should there be a mandatory retirement age? How do we best measure, assess, and revalidate elderly physicians and surgeons? How can we continue to contribute to those who choose to do so? For the time being, as always, thanks for listening.
Dr. Kerr is professor, Nuffield Department of Clinical Laboratory Science, University of Oxford, and professor of cancer medicine, Oxford Cancer Centre, Oxford, United Kingdom. He has disclosed ties with Celleron Therapeutics, Oxford Cancer Biomarkers (Board of Directors); Afrox (charity; Trustee); GlaxoSmithKline and Bayer HealthCare Pharmaceuticals (Consultant), Genomic Health; Merck Serono, and Roche.
A version of this article appeared on Medscape.com.
Hidden in Plain Sight: The Growing Epidemic of Ultraprocessed Food Addiction
Yet, even as this evidence mounted, these food items have become increasingly prominent in diets globally.
Now, recent studies are unlocking why cutting back on ultraprocessed foods can be so challenging. In their ability to fuel intense cravings, loss of control, and even withdrawal symptoms, ultraprocessed foods appear as capable of triggering addiction as traditional culprits like tobacco and alcohol.
This has driven efforts to better understand the addictive nature of these foods and identify strategies for combating it.
The Key Role of the Food Industry
Some foods are more likely to trigger addictions than others. For instance, in our studies, participants frequently mention chocolate, pizza, French fries, potato chips, and soda as some of the most addictive foods. What these foods all share is an ability to deliver high doses of refined carbohydrates, fat, or salt at levels exceeding those found in natural foods (eg, fruits, vegetables, beans).
Furthermore, ultraprocessed foods are industrially mass-produced in a process that relies on the heavy use of flavor enhancers and additives, as well as preservatives and packaging that make them shelf-stable. This has flooded our food supply with cheap, accessible, hyperrewarding foods that our brains are not well equipped to resist.
To add to these already substantial effects, the food industry often employs strategies reminiscent of Big Tobacco. They engineer foods to hit our “bliss points,” maximizing craving and fostering brand loyalty from a young age. This product engineering, coupled with aggressive marketing, makes these foods both attractive and seemingly ubiquitous.
How Many People Are Affected?
Addiction to ultraprocessed food is more common than you might think. According to the Yale Food Addiction Scale — a tool that uses the same criteria for diagnosing substance use disorders to assess ultraprocessed food addiction (UPFA) — about 14% of adults and 12% of children show clinically significant signs of addiction to such foods. This is quite similar to addiction rates among adults for legal substances like alcohol and tobacco.
Research has shown that behaviors and brain mechanisms contributing to addictive disorders, such as cravings and impulsivity, also apply to UPFA.
Many more people outside of those who meet the criteria for UPFA are influenced by their addictive properties. Picture a teenager craving a sugary drink after school, a child needing the morning cereal fix, or adults reaching for candy and fast food; these scenarios illustrate how addictive ultraprocessed foods permeate our daily lives.
From a public health standpoint, this comes at a significant cost. Even experiencing one or two symptoms of UPFA, such as intense cravings or a feeling of loss of control over intake, can lead to consuming too many calories, sugar, fat, and sodium in a way that puts health at risk.
Clinical Implications
Numerous studies have found that individuals who exhibit UPFA have more severe mental and physical health challenges. For example, UPFA is associated with higher rates of diet-related diseases (like type 2 diabetes), greater overall mental health issues, and generally poorer outcomes in weight loss treatments.
Despite the growing understanding of UPFA’s relevance in clinical settings, research is still limited on how to best treat, manage, or prevent it. Most of the existing work has focused on investigating whether UPFA is indeed a real condition, with efforts to create clinical guidelines only just beginning.
Of note, UPFA isn’t officially recognized as a diagnosis — yet. If it were, it could spark much more research into how to handle it clinically.
There is some debate about whether we really need this new diagnosis, given that eating disorders are already recognized. However, the statistics tell a different story: Around 14% of people might have UPFA compared with about 1% for binge-type eating disorders. This suggests that many individuals with problematic eating habits are currently flying under the radar with our existing diagnostic categories.
What’s even more concerning is that these individuals often suffer significant problems and exhibit distinct brain differences, even if they do not neatly fit into an existing eating disorder diagnosis. Officially recognizing UPFA could open up new avenues for support and lead to better treatments aimed at reducing compulsive eating patterns.
Treatment Options
Treatment options for UPFA are still being explored. Initial evidence suggests that medications used for treating substance addiction, such as naltrexone and bupropion, might help with highly processed food addiction as well. Newer drugs, like glucagon-like peptide-1 receptor agonists, which appear to curb food cravings and manage addictive behaviors, also look promising.
Psychosocial approaches can also be used to address UPFA. Strategies include:
- Helping individuals become more aware of their triggers for addictive patterns of intake. This often involves identifying certain types of food (eg, potato chips, candy), specific places or times of day (eg, sitting on the couch at night while watching TV), and particular emotional states (eg, anger, loneliness, boredom, sadness). Increasing awareness of personal triggers can help people minimize their exposure to these and develop coping strategies when they do arise.
- Many people use ultraprocessed foods to cope with challenging emotions. Helping individuals develop healthier strategies to regulate their emotions can be key. This may include seeking out social support, journaling, going for a walk, or practicing mindfulness.
- UPFA can be associated with erratic and inconsistent eating patterns. Stabilizing eating habits by consuming regular meals composed of more minimally processed foods (eg, vegetables, fruits, high-quality protein, beans) can help heal the body and reduce vulnerability to ultraprocessed food triggers.
- Many people with UPFA have other existing mental health conditions, including mood disorders, anxiety, substance use disorders, or trauma-related disorders. Addressing these co-occurring mental health conditions can help reduce reliance on ultraprocessed foods.
Public-policy interventions may also help safeguard vulnerable populations from developing UPFA. For instance, support exists for policies to protect children from cigarette marketing and to put clear addiction warning labels on cigarette packages. A similar approach could be applied to reduce the harms associated with ultraprocessed foods, particularly for children.
Combating this growing problem requires treating ultraprocessed foods like other addictive substances. By identifying the threat posed by these common food items, we can not only help patients with UPFA, but also potentially stave off the development of several diet-related conditions.
Dr. Gearhardt, professor of psychology, University of Michigan, Ann Arbor, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Yet, even as this evidence mounted, these food items have become increasingly prominent in diets globally.
Now, recent studies are unlocking why cutting back on ultraprocessed foods can be so challenging. In their ability to fuel intense cravings, loss of control, and even withdrawal symptoms, ultraprocessed foods appear as capable of triggering addiction as traditional culprits like tobacco and alcohol.
This has driven efforts to better understand the addictive nature of these foods and identify strategies for combating it.
The Key Role of the Food Industry
Some foods are more likely to trigger addictions than others. For instance, in our studies, participants frequently mention chocolate, pizza, French fries, potato chips, and soda as some of the most addictive foods. What these foods all share is an ability to deliver high doses of refined carbohydrates, fat, or salt at levels exceeding those found in natural foods (eg, fruits, vegetables, beans).
Furthermore, ultraprocessed foods are industrially mass-produced in a process that relies on the heavy use of flavor enhancers and additives, as well as preservatives and packaging that make them shelf-stable. This has flooded our food supply with cheap, accessible, hyperrewarding foods that our brains are not well equipped to resist.
To add to these already substantial effects, the food industry often employs strategies reminiscent of Big Tobacco. They engineer foods to hit our “bliss points,” maximizing craving and fostering brand loyalty from a young age. This product engineering, coupled with aggressive marketing, makes these foods both attractive and seemingly ubiquitous.
How Many People Are Affected?
Addiction to ultraprocessed food is more common than you might think. According to the Yale Food Addiction Scale — a tool that uses the same criteria for diagnosing substance use disorders to assess ultraprocessed food addiction (UPFA) — about 14% of adults and 12% of children show clinically significant signs of addiction to such foods. This is quite similar to addiction rates among adults for legal substances like alcohol and tobacco.
Research has shown that behaviors and brain mechanisms contributing to addictive disorders, such as cravings and impulsivity, also apply to UPFA.
Many more people outside of those who meet the criteria for UPFA are influenced by their addictive properties. Picture a teenager craving a sugary drink after school, a child needing the morning cereal fix, or adults reaching for candy and fast food; these scenarios illustrate how addictive ultraprocessed foods permeate our daily lives.
From a public health standpoint, this comes at a significant cost. Even experiencing one or two symptoms of UPFA, such as intense cravings or a feeling of loss of control over intake, can lead to consuming too many calories, sugar, fat, and sodium in a way that puts health at risk.
Clinical Implications
Numerous studies have found that individuals who exhibit UPFA have more severe mental and physical health challenges. For example, UPFA is associated with higher rates of diet-related diseases (like type 2 diabetes), greater overall mental health issues, and generally poorer outcomes in weight loss treatments.
Despite the growing understanding of UPFA’s relevance in clinical settings, research is still limited on how to best treat, manage, or prevent it. Most of the existing work has focused on investigating whether UPFA is indeed a real condition, with efforts to create clinical guidelines only just beginning.
Of note, UPFA isn’t officially recognized as a diagnosis — yet. If it were, it could spark much more research into how to handle it clinically.
There is some debate about whether we really need this new diagnosis, given that eating disorders are already recognized. However, the statistics tell a different story: Around 14% of people might have UPFA compared with about 1% for binge-type eating disorders. This suggests that many individuals with problematic eating habits are currently flying under the radar with our existing diagnostic categories.
What’s even more concerning is that these individuals often suffer significant problems and exhibit distinct brain differences, even if they do not neatly fit into an existing eating disorder diagnosis. Officially recognizing UPFA could open up new avenues for support and lead to better treatments aimed at reducing compulsive eating patterns.
Treatment Options
Treatment options for UPFA are still being explored. Initial evidence suggests that medications used for treating substance addiction, such as naltrexone and bupropion, might help with highly processed food addiction as well. Newer drugs, like glucagon-like peptide-1 receptor agonists, which appear to curb food cravings and manage addictive behaviors, also look promising.
Psychosocial approaches can also be used to address UPFA. Strategies include:
- Helping individuals become more aware of their triggers for addictive patterns of intake. This often involves identifying certain types of food (eg, potato chips, candy), specific places or times of day (eg, sitting on the couch at night while watching TV), and particular emotional states (eg, anger, loneliness, boredom, sadness). Increasing awareness of personal triggers can help people minimize their exposure to these and develop coping strategies when they do arise.
- Many people use ultraprocessed foods to cope with challenging emotions. Helping individuals develop healthier strategies to regulate their emotions can be key. This may include seeking out social support, journaling, going for a walk, or practicing mindfulness.
- UPFA can be associated with erratic and inconsistent eating patterns. Stabilizing eating habits by consuming regular meals composed of more minimally processed foods (eg, vegetables, fruits, high-quality protein, beans) can help heal the body and reduce vulnerability to ultraprocessed food triggers.
- Many people with UPFA have other existing mental health conditions, including mood disorders, anxiety, substance use disorders, or trauma-related disorders. Addressing these co-occurring mental health conditions can help reduce reliance on ultraprocessed foods.
Public-policy interventions may also help safeguard vulnerable populations from developing UPFA. For instance, support exists for policies to protect children from cigarette marketing and to put clear addiction warning labels on cigarette packages. A similar approach could be applied to reduce the harms associated with ultraprocessed foods, particularly for children.
Combating this growing problem requires treating ultraprocessed foods like other addictive substances. By identifying the threat posed by these common food items, we can not only help patients with UPFA, but also potentially stave off the development of several diet-related conditions.
Dr. Gearhardt, professor of psychology, University of Michigan, Ann Arbor, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Yet, even as this evidence mounted, these food items have become increasingly prominent in diets globally.
Now, recent studies are unlocking why cutting back on ultraprocessed foods can be so challenging. In their ability to fuel intense cravings, loss of control, and even withdrawal symptoms, ultraprocessed foods appear as capable of triggering addiction as traditional culprits like tobacco and alcohol.
This has driven efforts to better understand the addictive nature of these foods and identify strategies for combating it.
The Key Role of the Food Industry
Some foods are more likely to trigger addictions than others. For instance, in our studies, participants frequently mention chocolate, pizza, French fries, potato chips, and soda as some of the most addictive foods. What these foods all share is an ability to deliver high doses of refined carbohydrates, fat, or salt at levels exceeding those found in natural foods (eg, fruits, vegetables, beans).
Furthermore, ultraprocessed foods are industrially mass-produced in a process that relies on the heavy use of flavor enhancers and additives, as well as preservatives and packaging that make them shelf-stable. This has flooded our food supply with cheap, accessible, hyperrewarding foods that our brains are not well equipped to resist.
To add to these already substantial effects, the food industry often employs strategies reminiscent of Big Tobacco. They engineer foods to hit our “bliss points,” maximizing craving and fostering brand loyalty from a young age. This product engineering, coupled with aggressive marketing, makes these foods both attractive and seemingly ubiquitous.
How Many People Are Affected?
Addiction to ultraprocessed food is more common than you might think. According to the Yale Food Addiction Scale — a tool that uses the same criteria for diagnosing substance use disorders to assess ultraprocessed food addiction (UPFA) — about 14% of adults and 12% of children show clinically significant signs of addiction to such foods. This is quite similar to addiction rates among adults for legal substances like alcohol and tobacco.
Research has shown that behaviors and brain mechanisms contributing to addictive disorders, such as cravings and impulsivity, also apply to UPFA.
Many more people outside of those who meet the criteria for UPFA are influenced by their addictive properties. Picture a teenager craving a sugary drink after school, a child needing the morning cereal fix, or adults reaching for candy and fast food; these scenarios illustrate how addictive ultraprocessed foods permeate our daily lives.
From a public health standpoint, this comes at a significant cost. Even experiencing one or two symptoms of UPFA, such as intense cravings or a feeling of loss of control over intake, can lead to consuming too many calories, sugar, fat, and sodium in a way that puts health at risk.
Clinical Implications
Numerous studies have found that individuals who exhibit UPFA have more severe mental and physical health challenges. For example, UPFA is associated with higher rates of diet-related diseases (like type 2 diabetes), greater overall mental health issues, and generally poorer outcomes in weight loss treatments.
Despite the growing understanding of UPFA’s relevance in clinical settings, research is still limited on how to best treat, manage, or prevent it. Most of the existing work has focused on investigating whether UPFA is indeed a real condition, with efforts to create clinical guidelines only just beginning.
Of note, UPFA isn’t officially recognized as a diagnosis — yet. If it were, it could spark much more research into how to handle it clinically.
There is some debate about whether we really need this new diagnosis, given that eating disorders are already recognized. However, the statistics tell a different story: Around 14% of people might have UPFA compared with about 1% for binge-type eating disorders. This suggests that many individuals with problematic eating habits are currently flying under the radar with our existing diagnostic categories.
What’s even more concerning is that these individuals often suffer significant problems and exhibit distinct brain differences, even if they do not neatly fit into an existing eating disorder diagnosis. Officially recognizing UPFA could open up new avenues for support and lead to better treatments aimed at reducing compulsive eating patterns.
Treatment Options
Treatment options for UPFA are still being explored. Initial evidence suggests that medications used for treating substance addiction, such as naltrexone and bupropion, might help with highly processed food addiction as well. Newer drugs, like glucagon-like peptide-1 receptor agonists, which appear to curb food cravings and manage addictive behaviors, also look promising.
Psychosocial approaches can also be used to address UPFA. Strategies include:
- Helping individuals become more aware of their triggers for addictive patterns of intake. This often involves identifying certain types of food (eg, potato chips, candy), specific places or times of day (eg, sitting on the couch at night while watching TV), and particular emotional states (eg, anger, loneliness, boredom, sadness). Increasing awareness of personal triggers can help people minimize their exposure to these and develop coping strategies when they do arise.
- Many people use ultraprocessed foods to cope with challenging emotions. Helping individuals develop healthier strategies to regulate their emotions can be key. This may include seeking out social support, journaling, going for a walk, or practicing mindfulness.
- UPFA can be associated with erratic and inconsistent eating patterns. Stabilizing eating habits by consuming regular meals composed of more minimally processed foods (eg, vegetables, fruits, high-quality protein, beans) can help heal the body and reduce vulnerability to ultraprocessed food triggers.
- Many people with UPFA have other existing mental health conditions, including mood disorders, anxiety, substance use disorders, or trauma-related disorders. Addressing these co-occurring mental health conditions can help reduce reliance on ultraprocessed foods.
Public-policy interventions may also help safeguard vulnerable populations from developing UPFA. For instance, support exists for policies to protect children from cigarette marketing and to put clear addiction warning labels on cigarette packages. A similar approach could be applied to reduce the harms associated with ultraprocessed foods, particularly for children.
Combating this growing problem requires treating ultraprocessed foods like other addictive substances. By identifying the threat posed by these common food items, we can not only help patients with UPFA, but also potentially stave off the development of several diet-related conditions.
Dr. Gearhardt, professor of psychology, University of Michigan, Ann Arbor, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Coffee’s ‘Sweet Spot’: Daily Consumption and Cardiometabolic Risk
Each and every day, 1 billion people on this planet ingest a particular psychoactive substance. This chemical has fairly profound physiologic effects. It increases levels of nitric oxide in the blood, leads to vasodilation, and, of course, makes you feel more awake. The substance comes in many forms but almost always in a liquid medium. Do you have it yet? That’s right. The substance is caffeine, quite possibly the healthiest recreational drug that has ever been discovered.
This might be my New England upbringing speaking, but when it comes to lifestyle and health, one of the rules I’ve internalized is that things that are pleasurable are generally bad for you. I know, I know — some of you love to exercise. Some of you love doing crosswords. But you know what I mean. I’m talking French fries, smoked meats, drugs, smoking, alcohol, binge-watching Firefly. You’d be suspicious if a study came out suggesting that eating ice cream in bed reduces your risk for heart attack, and so would I. So I’m always on the lookout for those unicorns of lifestyle factors, those rare things that you want to do and are also good for you.
So far, the data are strong for three things: sleeping, (safe) sexual activity, and coffee. You’ll have to stay tuned for articles about the first two. Today, we’re brewing up some deeper insights about the power of java.
I was inspired to write this article because of a paper, “Habitual Coffee, Tea, and Caffeine Consumption, Circulating Metabolites, and the Risk of Cardiometabolic Multimorbidity,” appearing September 17 in The Journal of Clinical Endocrinology and Metabolism (JCEM).
This is not the first study to suggest that coffee intake may be beneficial. A 2013 meta-analysis summarized the results of 36 studies with more than a million participants and found a U-shaped relationship between coffee intake and cardiovascular risk. The sweet spot was at three to five cups a day; people drinking that much coffee had about a 15% reduced risk for cardiovascular disease compared with nondrinkers.
But here’s the thing. Coffee contains caffeine, but it is much more than that. It is a heady brew of various chemicals and compounds, phenols, and chlorogenic acids. And, of course, you can get caffeine from stuff that isn’t coffee — natural things like tea — and decidedly unnatural things like energy drinks. How do you figure out where the benefit really lies?
The JCEM study leveraged the impressive UK Biobank dataset to figure this out. The Biobank recruited more than half a million people from the UK between 2006 and 2010 and collected a wealth of data from each of them: surveys, blood samples, biometrics, medical imaging — the works. And then they followed what would happen to those people medically over time. It’s a pretty amazing resource.
But for the purposes of this study, what you need to know is that just under 200,000 of those participants met the key criteria for this study: being free from cardiovascular disease at baseline; having completed a detailed survey about their coffee, tea, and other caffeinated beverage intake; and having adequate follow-up. A subset of that number, just under 100,000, had metabolomic data — which is where this study really gets interesting.
We’ll dive into the metabolome in a moment, but first let’s just talk about the main finding, the relationship between coffee, tea, or caffeine and cardiovascular disease. But to do that, we need to acknowledge that people who drink a lot of coffee are different from people who don’t, and it might be those differences, not the coffee itself, that are beneficial.
What were those differences? People who drank more coffee tended to be a bit older, were less likely to be female, and were slightly more likely to engage in physical activity. They ate less processed meat but also fewer vegetables. Some of those factors, like being female, are generally protective against cardiovascular disease; but some, like age, are definitely not. The authors adjusted for these and multiple other factors, including alcohol intake, BMI, kidney function, and many others to try to disentangle the effect of being the type of person who drinks a lot of coffee from the drinking a lot of coffee itself.
These are the results of the fully adjusted model. Compared with nonconsumers, you can see that people in the higher range of coffee, tea, or just caffeine intake have almost a 40% reduction in cardiovascular disease in follow-up.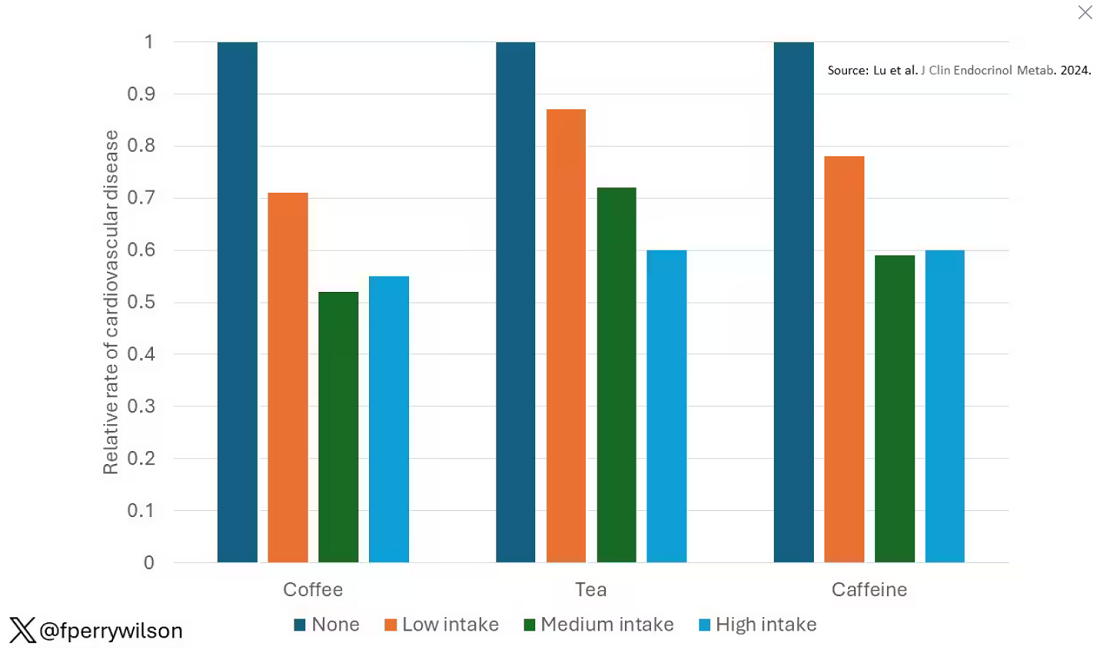
Looking at the benefit across the spectrum of intake, you again see that U-shaped curve, suggesting that a sweet spot for daily consumption can be found around 3 cups of coffee or tea (or 250 mg of caffeine). A standard energy drink contains about 120 mg of caffeine. 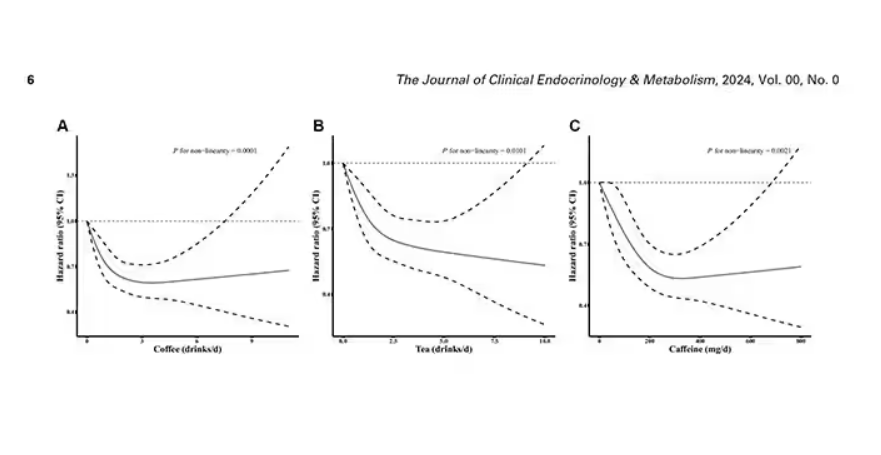
But if this is true, it would be good to know why. To figure that out, the authors turned to the metabolome. The idea here is that your body is constantly breaking stuff down, taking all these proteins and chemicals and compounds that we ingest and turning them into metabolites. Using advanced measurement techniques, researchers can measure hundreds or even thousands of metabolites from a single blood sample. They provide information, obviously, about the food you eat and the drinks you drink, but what is really intriguing is that some metabolites are associated with better health and some with worse
In this study, researchers measured 168 individual metabolites. Eighty of them, nearly half, were significantly altered in people who drank more coffee.
This figure summarizes the findings, and yes, this is way too complicated. 
But here’s how to interpret it. The inner ring shows you how certain metabolites are associated with cardiovascular disease. The outer rings show you how those metabolites are associated with coffee, tea, or caffeine. The interesting part is that the sections of the ring (outer rings and inner rings) are very different colors.
Like here.
What you see here is a fairly profound effect that coffee, tea, or caffeine intake has on metabolites of VLDL — bad cholesterol. The beverages lower it, and, of course, higher levels lead to cardiovascular disease. This means that this is a potential causal pathway from coffee intake to heart protection.
And that’s not the only one.
You see a similar relationship for saturated fatty acids. Higher levels lead to cardiovascular disease, and coffee intake lowers levels. The reverse works too: Lower levels of histidine (an amino acid) increase cardiovascular risk, and coffee seems to raise those levels.
Is this all too good to be true? It’s hard to say. The data on coffee’s benefits have been remarkably consistent. Still, I wouldn’t be a good doctor if I didn’t mention that clearly there is a difference between a cup of black coffee and a venti caramel Frappuccino.
Nevertheless, coffee remains firmly in my holy trinity of enjoyable things that are, for whatever reason, still good for you. So, when you’re having that second, or third, or maybe fourth cup of the day, you can take that to heart.
Dr. Wilson, associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Each and every day, 1 billion people on this planet ingest a particular psychoactive substance. This chemical has fairly profound physiologic effects. It increases levels of nitric oxide in the blood, leads to vasodilation, and, of course, makes you feel more awake. The substance comes in many forms but almost always in a liquid medium. Do you have it yet? That’s right. The substance is caffeine, quite possibly the healthiest recreational drug that has ever been discovered.
This might be my New England upbringing speaking, but when it comes to lifestyle and health, one of the rules I’ve internalized is that things that are pleasurable are generally bad for you. I know, I know — some of you love to exercise. Some of you love doing crosswords. But you know what I mean. I’m talking French fries, smoked meats, drugs, smoking, alcohol, binge-watching Firefly. You’d be suspicious if a study came out suggesting that eating ice cream in bed reduces your risk for heart attack, and so would I. So I’m always on the lookout for those unicorns of lifestyle factors, those rare things that you want to do and are also good for you.
So far, the data are strong for three things: sleeping, (safe) sexual activity, and coffee. You’ll have to stay tuned for articles about the first two. Today, we’re brewing up some deeper insights about the power of java.
I was inspired to write this article because of a paper, “Habitual Coffee, Tea, and Caffeine Consumption, Circulating Metabolites, and the Risk of Cardiometabolic Multimorbidity,” appearing September 17 in The Journal of Clinical Endocrinology and Metabolism (JCEM).
This is not the first study to suggest that coffee intake may be beneficial. A 2013 meta-analysis summarized the results of 36 studies with more than a million participants and found a U-shaped relationship between coffee intake and cardiovascular risk. The sweet spot was at three to five cups a day; people drinking that much coffee had about a 15% reduced risk for cardiovascular disease compared with nondrinkers.
But here’s the thing. Coffee contains caffeine, but it is much more than that. It is a heady brew of various chemicals and compounds, phenols, and chlorogenic acids. And, of course, you can get caffeine from stuff that isn’t coffee — natural things like tea — and decidedly unnatural things like energy drinks. How do you figure out where the benefit really lies?
The JCEM study leveraged the impressive UK Biobank dataset to figure this out. The Biobank recruited more than half a million people from the UK between 2006 and 2010 and collected a wealth of data from each of them: surveys, blood samples, biometrics, medical imaging — the works. And then they followed what would happen to those people medically over time. It’s a pretty amazing resource.
But for the purposes of this study, what you need to know is that just under 200,000 of those participants met the key criteria for this study: being free from cardiovascular disease at baseline; having completed a detailed survey about their coffee, tea, and other caffeinated beverage intake; and having adequate follow-up. A subset of that number, just under 100,000, had metabolomic data — which is where this study really gets interesting.
We’ll dive into the metabolome in a moment, but first let’s just talk about the main finding, the relationship between coffee, tea, or caffeine and cardiovascular disease. But to do that, we need to acknowledge that people who drink a lot of coffee are different from people who don’t, and it might be those differences, not the coffee itself, that are beneficial.
What were those differences? People who drank more coffee tended to be a bit older, were less likely to be female, and were slightly more likely to engage in physical activity. They ate less processed meat but also fewer vegetables. Some of those factors, like being female, are generally protective against cardiovascular disease; but some, like age, are definitely not. The authors adjusted for these and multiple other factors, including alcohol intake, BMI, kidney function, and many others to try to disentangle the effect of being the type of person who drinks a lot of coffee from the drinking a lot of coffee itself.
These are the results of the fully adjusted model. Compared with nonconsumers, you can see that people in the higher range of coffee, tea, or just caffeine intake have almost a 40% reduction in cardiovascular disease in follow-up.
Looking at the benefit across the spectrum of intake, you again see that U-shaped curve, suggesting that a sweet spot for daily consumption can be found around 3 cups of coffee or tea (or 250 mg of caffeine). A standard energy drink contains about 120 mg of caffeine. 
But if this is true, it would be good to know why. To figure that out, the authors turned to the metabolome. The idea here is that your body is constantly breaking stuff down, taking all these proteins and chemicals and compounds that we ingest and turning them into metabolites. Using advanced measurement techniques, researchers can measure hundreds or even thousands of metabolites from a single blood sample. They provide information, obviously, about the food you eat and the drinks you drink, but what is really intriguing is that some metabolites are associated with better health and some with worse
In this study, researchers measured 168 individual metabolites. Eighty of them, nearly half, were significantly altered in people who drank more coffee.
This figure summarizes the findings, and yes, this is way too complicated. 
But here’s how to interpret it. The inner ring shows you how certain metabolites are associated with cardiovascular disease. The outer rings show you how those metabolites are associated with coffee, tea, or caffeine. The interesting part is that the sections of the ring (outer rings and inner rings) are very different colors.
Like here.
What you see here is a fairly profound effect that coffee, tea, or caffeine intake has on metabolites of VLDL — bad cholesterol. The beverages lower it, and, of course, higher levels lead to cardiovascular disease. This means that this is a potential causal pathway from coffee intake to heart protection.
And that’s not the only one.
You see a similar relationship for saturated fatty acids. Higher levels lead to cardiovascular disease, and coffee intake lowers levels. The reverse works too: Lower levels of histidine (an amino acid) increase cardiovascular risk, and coffee seems to raise those levels.
Is this all too good to be true? It’s hard to say. The data on coffee’s benefits have been remarkably consistent. Still, I wouldn’t be a good doctor if I didn’t mention that clearly there is a difference between a cup of black coffee and a venti caramel Frappuccino.
Nevertheless, coffee remains firmly in my holy trinity of enjoyable things that are, for whatever reason, still good for you. So, when you’re having that second, or third, or maybe fourth cup of the day, you can take that to heart.
Dr. Wilson, associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Each and every day, 1 billion people on this planet ingest a particular psychoactive substance. This chemical has fairly profound physiologic effects. It increases levels of nitric oxide in the blood, leads to vasodilation, and, of course, makes you feel more awake. The substance comes in many forms but almost always in a liquid medium. Do you have it yet? That’s right. The substance is caffeine, quite possibly the healthiest recreational drug that has ever been discovered.
This might be my New England upbringing speaking, but when it comes to lifestyle and health, one of the rules I’ve internalized is that things that are pleasurable are generally bad for you. I know, I know — some of you love to exercise. Some of you love doing crosswords. But you know what I mean. I’m talking French fries, smoked meats, drugs, smoking, alcohol, binge-watching Firefly. You’d be suspicious if a study came out suggesting that eating ice cream in bed reduces your risk for heart attack, and so would I. So I’m always on the lookout for those unicorns of lifestyle factors, those rare things that you want to do and are also good for you.
So far, the data are strong for three things: sleeping, (safe) sexual activity, and coffee. You’ll have to stay tuned for articles about the first two. Today, we’re brewing up some deeper insights about the power of java.
I was inspired to write this article because of a paper, “Habitual Coffee, Tea, and Caffeine Consumption, Circulating Metabolites, and the Risk of Cardiometabolic Multimorbidity,” appearing September 17 in The Journal of Clinical Endocrinology and Metabolism (JCEM).
This is not the first study to suggest that coffee intake may be beneficial. A 2013 meta-analysis summarized the results of 36 studies with more than a million participants and found a U-shaped relationship between coffee intake and cardiovascular risk. The sweet spot was at three to five cups a day; people drinking that much coffee had about a 15% reduced risk for cardiovascular disease compared with nondrinkers.
But here’s the thing. Coffee contains caffeine, but it is much more than that. It is a heady brew of various chemicals and compounds, phenols, and chlorogenic acids. And, of course, you can get caffeine from stuff that isn’t coffee — natural things like tea — and decidedly unnatural things like energy drinks. How do you figure out where the benefit really lies?
The JCEM study leveraged the impressive UK Biobank dataset to figure this out. The Biobank recruited more than half a million people from the UK between 2006 and 2010 and collected a wealth of data from each of them: surveys, blood samples, biometrics, medical imaging — the works. And then they followed what would happen to those people medically over time. It’s a pretty amazing resource.
But for the purposes of this study, what you need to know is that just under 200,000 of those participants met the key criteria for this study: being free from cardiovascular disease at baseline; having completed a detailed survey about their coffee, tea, and other caffeinated beverage intake; and having adequate follow-up. A subset of that number, just under 100,000, had metabolomic data — which is where this study really gets interesting.
We’ll dive into the metabolome in a moment, but first let’s just talk about the main finding, the relationship between coffee, tea, or caffeine and cardiovascular disease. But to do that, we need to acknowledge that people who drink a lot of coffee are different from people who don’t, and it might be those differences, not the coffee itself, that are beneficial.
What were those differences? People who drank more coffee tended to be a bit older, were less likely to be female, and were slightly more likely to engage in physical activity. They ate less processed meat but also fewer vegetables. Some of those factors, like being female, are generally protective against cardiovascular disease; but some, like age, are definitely not. The authors adjusted for these and multiple other factors, including alcohol intake, BMI, kidney function, and many others to try to disentangle the effect of being the type of person who drinks a lot of coffee from the drinking a lot of coffee itself.
These are the results of the fully adjusted model. Compared with nonconsumers, you can see that people in the higher range of coffee, tea, or just caffeine intake have almost a 40% reduction in cardiovascular disease in follow-up.
Looking at the benefit across the spectrum of intake, you again see that U-shaped curve, suggesting that a sweet spot for daily consumption can be found around 3 cups of coffee or tea (or 250 mg of caffeine). A standard energy drink contains about 120 mg of caffeine. 
But if this is true, it would be good to know why. To figure that out, the authors turned to the metabolome. The idea here is that your body is constantly breaking stuff down, taking all these proteins and chemicals and compounds that we ingest and turning them into metabolites. Using advanced measurement techniques, researchers can measure hundreds or even thousands of metabolites from a single blood sample. They provide information, obviously, about the food you eat and the drinks you drink, but what is really intriguing is that some metabolites are associated with better health and some with worse
In this study, researchers measured 168 individual metabolites. Eighty of them, nearly half, were significantly altered in people who drank more coffee.
This figure summarizes the findings, and yes, this is way too complicated. 
But here’s how to interpret it. The inner ring shows you how certain metabolites are associated with cardiovascular disease. The outer rings show you how those metabolites are associated with coffee, tea, or caffeine. The interesting part is that the sections of the ring (outer rings and inner rings) are very different colors.
Like here.
What you see here is a fairly profound effect that coffee, tea, or caffeine intake has on metabolites of VLDL — bad cholesterol. The beverages lower it, and, of course, higher levels lead to cardiovascular disease. This means that this is a potential causal pathway from coffee intake to heart protection.
And that’s not the only one.
You see a similar relationship for saturated fatty acids. Higher levels lead to cardiovascular disease, and coffee intake lowers levels. The reverse works too: Lower levels of histidine (an amino acid) increase cardiovascular risk, and coffee seems to raise those levels.
Is this all too good to be true? It’s hard to say. The data on coffee’s benefits have been remarkably consistent. Still, I wouldn’t be a good doctor if I didn’t mention that clearly there is a difference between a cup of black coffee and a venti caramel Frappuccino.
Nevertheless, coffee remains firmly in my holy trinity of enjoyable things that are, for whatever reason, still good for you. So, when you’re having that second, or third, or maybe fourth cup of the day, you can take that to heart.
Dr. Wilson, associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Impact of Expanded Eligibility for Veterans With Other Than Honorable Discharges on Treatment Courts and VA Mental Health Care
In April 2022, the US Department of Veterans Affairs (VA) revised its behavioral health care eligibility policies to provide comprehensive mental and behavioral health care to former service members who received an Other Than Honorable (OTH) discharge characterization upon separation from military service.1 This policy shift represents a marked expansion in eligibility practices (Table 1 includes amended eligibility criteria).
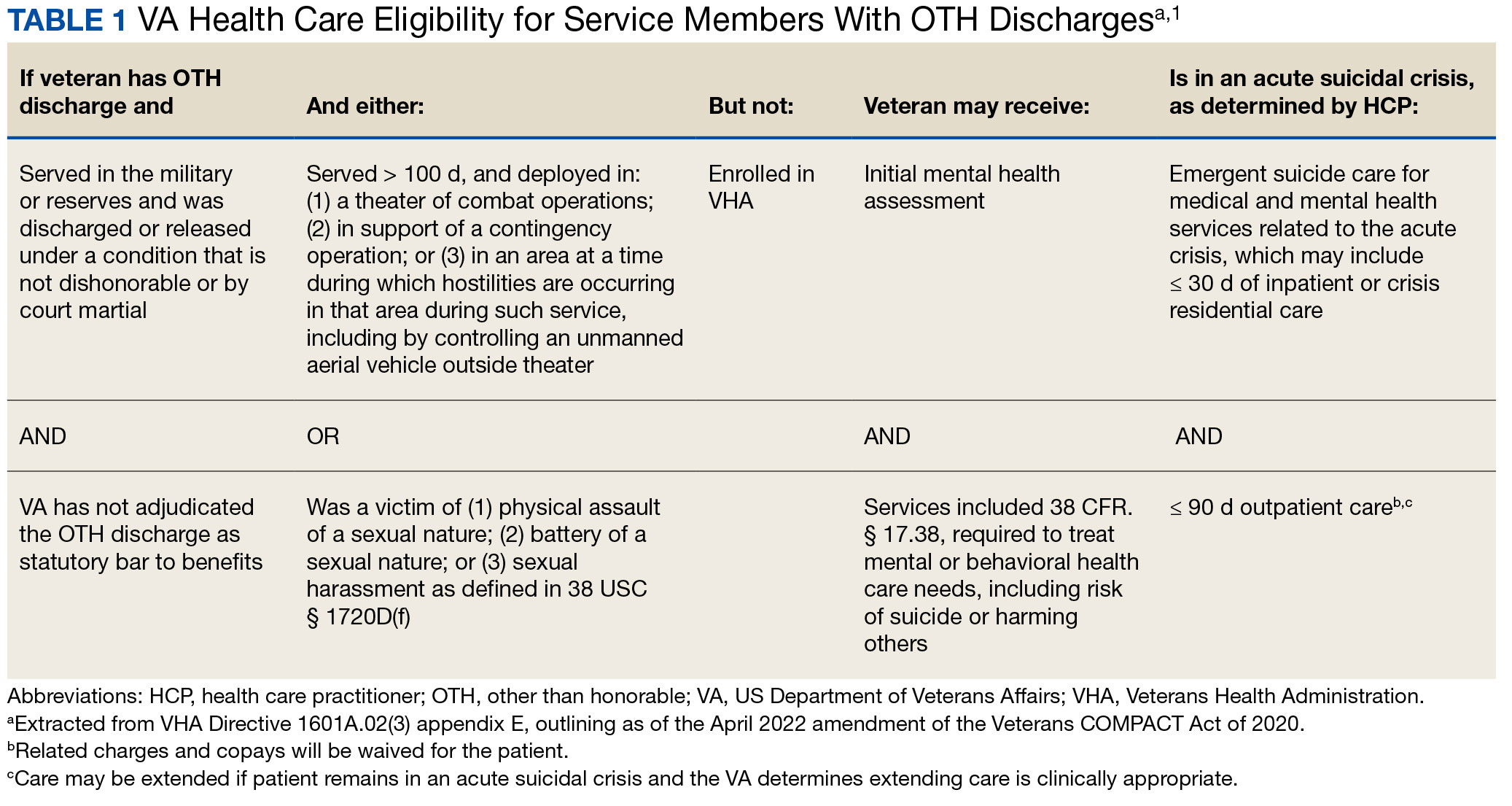
Since June 2017, eligibility policies allowed veterans with OTH discharges to receive “emergent mental health services” needed to stabilize acute mental health crises related to military service (eg, acute escalations in suicide risk).2,3 Previously, veterans with OTH discharges were largely ineligible for VA-based health care; these individuals were only able to access Veterans Health Administration (VHA) mental and behavioral health care through limited channels of eligibility (eg, for treatment of military sexual trauma or psychosis or other mental illness within 2 years of discharge).4,5 The impetus for expansions in eligibility stemmed from VA efforts to reduce the suicide rate among veterans.6-8 Implications of such expansion extend beyond suicide prevention efforts, with notable promised effects on the care of veterans with criminal-legal involvement. This article highlights potential effects of recent eligibility expansions on veterans with criminal-legal involvement and makes specific recommendations for agencies and organizations serving these veterans.
OTHER THAN HONORABLE DISCHARGE
The US Department of Defense delineates 6 discharge characterizations provided to service members upon separation from military service: honorable, general under honorable conditions, OTH, bad conduct, dishonorable, and uncharacterized. Honorable discharge characterizations are considered to reflect general concordance between service member behavior and military standards; general discharge characterizations reflect some disparity between the service member’s behavior and military standards; OTH, bad conduct, and dishonorable discharge characterizations reflect serious disparities between the service member’s behavior and military standards; and uncharacterized discharge characterizations are given when other discharge characterizations are deemed inappropriate.9,10 OTH discharge characterizations are typically issued under instances of misconduct, fraudulent entry, security reasons, or in lieu of trial by court martial.9,10
Recent research suggests that about 85% of service members receive an honorable discharge characterization upon separation from military service, 8% receive general, 6% receive OTH, and 1% receive bad conduct or dishonorable discharges.11 In 2017, the VA estimated there were > 500,000 prior service members with OTH discharge characterizations, which has grown over time (1.9% during the Korean Conflict, 2.5% during the Vietnam War Era, 3.9% during the Cold War, 4.8% in the Persian Gulf War, and 5.8% in the post-9/11 era).7,11
The OTH discharge characterization is 1 of 3 less than honorable discharges informally referred to as bad papers (ie, OTH, bad conduct, or dishonorable). Former service members receiving these discharge characterizations face significant social stigma and structural discrimination upon military discharge, including significant hurdles to employment and educational pursuits as well as notable social alienation.12 Due to their discharge characterization, some are viewed as less deserving of the veteran title, and until recently, many did not qualify for the complex legal definition of veteran as established by the Congress.11,13 Veterans with OTH discharge characterizations have also historically been excluded from services (eg, VHA care),3 benefits (eg, disability compensation),14 and protections (eg, Uniformed Services Employment and Reemployment Rights Act)15 offered to veterans with honorable or general discharge characterizations. However, eligibility policies have gradually expanded, providing veterans with OTH discharges with access to VHA-based mental and behavioral health services and VA supportive housing assistance.1,3,16
Perhaps due to their historical exclusion from VA services, there is limited research available on the behavioral health and associated needs of veterans with OTH discharges. Some scholars argue that historical exclusions have exacerbated underlying difficulties faced by this population, thereby contributing to stark health and social disparities across discharge types.14,15,17 Studies with large veteran samples, for example, reflect notable demographic and behavioral health differences across discharge types. Compared to routinely discharged veterans, veterans with OTH discharges are significantly more likely to be younger, have lower income, use substances, have a history of criminal-legal involvement, and have mental and physical health difficulties.18,19
Substantial evidence also suggests a historical racial bias, with service members of color being disproportionately more likely to receive an OTH discharge.12 Similarly, across all branches of military service, Black service members are significantly more likely to face general or special court martial in military justice proceedings when compared with White service members.20 Service members from gender and sexual minorities are also disproportionately impacted by the OTH designation. Historically, many have been discharged with bad papers due to discriminatory policies, such as Don’t Ask Don’t Tell, which discriminated on the basis of sexual orientation between December 1993 and September 2011, and Directive-type Memorandum-19-004, which banned transgender persons from military service between April 2019 and January 2021.21,22
There is also significant mental health bias in the provision of OTH discharges, such that OTH characterizations are disproportionately represented among individuals with mental health disorders.18-20 Veterans discharged from military service due to behavioral misconduct are significantly more likely to meet diagnostic criteria for various behavioral health conditions and to experience homelessness, criminal-legal involvement, and suicidal ideation and behavior compared with routinely-discharged veterans.23-28
Consistent with their comparatively higher rates of criminal-legal involvement relative to routinely discharged veterans, veterans with OTH discharges are disproportionately represented in criminal justice settings. While veterans with OTH discharges represent only 6% of discharging service members and 2.5% of community-based veterans, they represent 10% of incarcerated veterans.11,18,23,29 Preliminary research suggests veterans with OTH discharges may be at higher risk for lifetime incarceration, though the association between OTH discharge and frequency of lifetime arrests remains unclear.18,30
VETERANS TREATMENT COURTS
Given the overrepresentation of veterans with OTH discharges in criminal-legal settings, consideration for this subset of the veteran population and its unique needs is commonplace among problem-solving courts that service veterans. First conceptualized in 2004, Veterans Treatment Courts (VTCs) are specialized problem-solving courts that divert veterans away from traditional judicial court and penal systems and into community-based supervision and treatment (most commonly behavioral health services).31-34 Although each VTC program is unique in structure, policies, and procedures, most VTCs can be characterized by certain key elements, including voluntary participation, plea requirements, delayed sentencing (often including reduced or dismissed charges), integration of military culture into court proceedings, a rehabilitative vs adversarial approach to decreasing risk of future criminal behavior, mandated treatment and supervision during participation, and use of veteran mentors to provide peer support.32-35 Eligibility requirements vary; however, many restrict participation to veterans with honorable discharge types and charges for nonviolent offenses.32,33,35-37
VTCs connect veterans within the criminal-legal system to needed behavioral health, community, and social services.31-33,37 VTC participants are commonly connected to case management, behavioral health care, therapeutic journaling programs, and vocational rehabilitation.38,39 Accordingly, the most common difficulties faced by veterans participating in these courts include substance use, mental health, family issues, anger management and/or aggressive behavior, and homelessness.36,39 There is limited research on the effectiveness of VTCs. Evidence on their overall effectiveness is largely mixed, though some studies suggest VTC graduates tend to have lower recidivism rates than offenders more broadly or persons who terminate VTC programs prior to completion.40,41 Other studies suggest that VTC participants are more likely to have jail sanctions, new arrests, and new incarcerations relative to nontreatment court participants.42 Notably, experimental designs (considered the gold standard in assessing effectiveness) to date have not been applied to evaluate the effectiveness of VTCs; as such, the effectiveness of these programs remains an area in need of continued empirical investigation.
Like all problem-solving courts, VTCs occasionally struggle to connect participating defendants with appropriate care, particularly when encountering structural barriers (eg, insurance, transportation) and/or complex behavioral health needs (eg, personality disorders).34,43 As suicide rates among veterans experiencing criminal-legal involvement surge (about 150 per 100,000 in 2021, a 10% increase from 2020 to 2021 compared to about 40 per 100,000 and a 1.8% increase among other veterans), efficiency of adequate care coordination is vital.44 Many VTCs rely on VTC-VA partnerships and collaborations to navigate these difficulties and facilitate connection of participating veterans to needed services.32-34,45 For example, within the VHA, Veterans Justice Outreach (VJO) and Health Care for Re-Entry Veterans (HCRV) specialists assist and bridge the gap between the criminal-legal system (including, but not limited to VTCs) and VA services by engaging veterans involved in the criminal-legal system and connecting them to needed VA-based services (Table 2). Generally, VJO specialists support veterans involved with the front end of the criminallegal system (eg, arrest, pretrial incarceration, or participation in VTCs), while HCRV specialists tend to support veterans transitioning back into the community after a period of incarceration.46,47 Specific to VTCs, VJO specialists typically serve as liaisons between the courts and VA, coordinating VA services for defendants to fulfill their terms of VTC participation.46
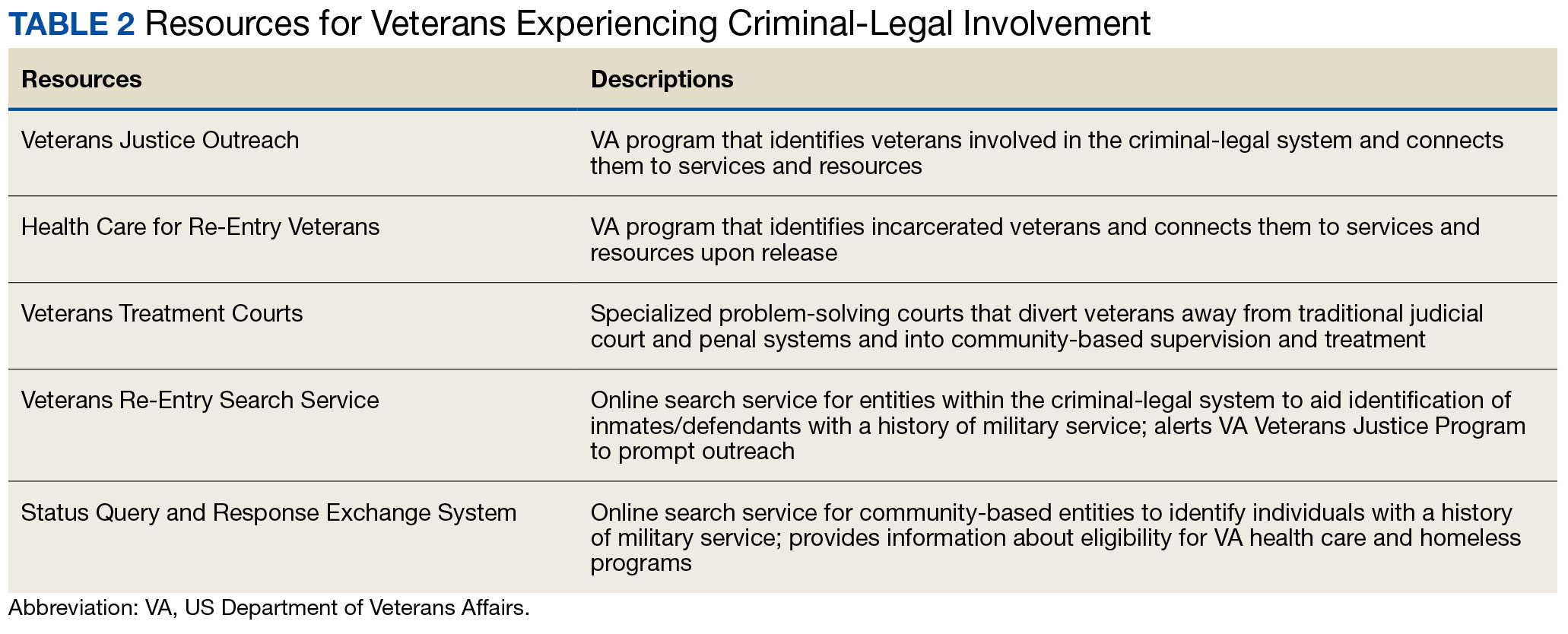
The historical exclusion of veterans with OTH discharge characterizations from VA-based services has restricted many from accessing VTC programs.32 Of 17 VTC programs active in Pennsylvania in 2014, only 5 accepted veterans with OTstayH discharges, and 3 required application to and eligibility for VA benefits.33 Similarly, in national surveys of VTC programs, about 1 in 3 report excluding veterans deemed ineligible for VA services.35,36 When veterans with OTH discharges have accessed VTC programs, they have historically relied on non-VA, community-based programming to fulfill treatment mandates, which may be less suited to addressing the unique needs of veterans.48
Veterans who utilize VTCs receive several benefits, namely peer support and mentorship, acceptance into a veteran-centric space, and connection with specially trained staff capable of supporting the veteran through applications for a range of VA benefits (eg, service connection, housing support).31-33,37 Given the disparate prevalence of OTH discharge characterizations among service members from racial, sexual, and gender minorities and among service members with mental health disorders, exclusion of veterans with OTH discharges from VTCs solely based on the type of discharge likely contributes to structural inequities among these already underserved groups by restricting access to these potential benefits. Such structural inequity stands in direct conflict with VTC best practice standards, which admonish programs to adjust eligibility requirements to facilitate access to treatment court programs for historically marginalized groups.49
ELIGIBILITY EXPANSIONS
Given the overrepresentation of veterans with OTH discharge characterizations within the criminal-legal system and historical barriers of these veterans to access needed mental and behavioral health care, expansions in VA eligibility policies could have immense implications for VTCs. First, these expansions could mitigate common barriers to connecting VTC-participating veterans with OTH discharges with needed behavioral health care by allowing these veterans to access established, VA-based services and programming. Expansion may also allow VTCs to serve as a key intercept point for identifying and engaging veterans with OTH discharges who may be unaware of their eligibility for VA-based behavioral health care.
Access to VA health care services is a major resource for VTC participants and a common requirement.32 Eligibility expansion should ease access barriers veterans with OTH discharges commonly face. By providing a potential source of treatment, expansions may also support OTH eligibility practices within VTCs, particularly practices that require participants to be eligible for VA health care.33,35,36 Some VTCs may continue to determine eligibility on the basis of discharge status and remain inaccessible to veterans with OTH discharge characterizations without program-level policy changes.32,36,37
Communicating changes in eligibility policies relevant to veterans with OTH discharges may be a challenge, because many of these individuals have no established channels of communication with the VA. Because veterans with OTH discharges are at increased risk for legal system involvement, VTCs may serve as a unique point of contact to help facilitate communication.18 For example, upon referral to a VTC, veterans with OTH discharges can be identified, VA health care eligibility can be verified, and veterans can connect to VA staff to facilitate enrollment in VA services and care.
VJO specialists are in a favorable position to serve a critical role in utilizing VTCs as a potential intercept point for engaging veterans with OTH discharge characterizations. As outlined in the STRONG Veterans Act of 2022, VJOs are mandated to “spread awareness and understanding of veteran eligibility for the [VJO] Program, including the eligibility of veterans who were discharged from service in the Armed Forces under conditions other than honorable.”50 The Act further requires VJOs to be annually trained in communicating eligibility changes as they arise. Accordingly, VJOs receive ongoing training in a wide variety of critical outreach topics, including changes in eligibility; while VJOs cannot make eligibility determinations, they are tasked with enrolling all veterans involved in the criminal-legal system with whom they interact into VHA services, whether through typical or special eligibility criteria (M. Stimmel, PhD, National Training Director for Veteran Justice Programming, oral communication, July 14, 2023). VJOs therefore routinely serve in this capacity of facilitating VA enrollment of veterans with OTH discharge characterizations.
Recommendations to Veteran-Servicing Judicial Programs
Considering these potential implications, professionals routinely interacting with veterans involved in the criminal-legal system should become familiarized with recent changes in VA eligibility policies. Such familiarization would support the identification of veterans previously considered ineligible for care; provision of education to these veterans regarding their new eligibility; and referral to appropriate VA-based behavioral health care options. Although conceptually simple, executing such an educational campaign may prove logistically difficult. Given their historical exclusion from VA services, veterans with OTH discharge characterizations are unlikely to seek VA-based services in times of need, instead relying on a broad swath of civilian community-based organizations and resources. Usual approaches to advertising VHA health care policy changes (eg, by notifying VA employees and/or departments providing corresponding services or by circulating information to veteran-focused mailing lists and organizations) likely would prove insufficient. Educational campaigns to disseminate information about recent OTH eligibility changes should instead consider partnering with traditionally civilian, communitybased organizations and institutions, such as state bar associations, legal aid networks, case management services, nonveteran treatment court programs (eg, drug courts, or domestic violence courts), or probation/ parole programs. Because national surveys suggest generally low military cultural competence among civilian populations, providing concurrent support in developing foundational veteran cultural competencies (eg, how to phrase questions about military service history, or understanding discharge characterizations) may be necessary to ensure effective identification and engagement of veteran clients.48
Programs that serve veterans with criminal-legal involvement should also consider potential relevance of recent OTH eligibility changes to program operations. VTC program staff and key partners (eg, judges, case managers, district attorneys, or defense attorneys), should revisit policies and procedures surrounding the engagement of veterans with OTH discharges within VTC programs and strategies for connecting these veterans with needed services. VTC programs that have historically excluded veterans with OTH discharges due to associated difficulties in locating and connecting with needed services should consider expanding eligibility policies considering recent shifts in VA behavioral health care eligibility.33,35,36 Within the VHA, VJO specialists can play a critical role in supporting these VTC eligibility and cultural shifts. Some evidence suggests a large proportion of VTC referrals are facilitated by VJO specialists and that many such referrals are identified when veterans involved with the criminal-legal system seek VA support and/or services.33 Given the historical exclusion of veterans with OTH discharges from VA care, strategies used by VJO specialists to identify, connect, and engage veterans with OTH discharges with VTCs and other services may be beneficial.
Even with knowledge of VA eligibility changes and considerations of these changes on local operations, many forensic settings and programs struggle to identify veterans. These difficulties are likely amplified among veterans with OTH discharge characterizations, who may be hesitant to self-disclose their military service history due to fear of stigma and/or views of OTH discharge characterizations as undeserving of the veteran title.12 The VA offers 2 tools to aid in identification of veterans for these settings: the Veterans Re-Entry Search Service (VRSS) and Status Query and Response Exchange System (SQUARES). For VRSS, correctional facilities, courts, and other criminal justice entities upload a simple spreadsheet that contains basic identifying information of inmates or defendants in their system. VRSS returns information about which inmates or defendants have a history of military service and alerts VA Veterans Justice Programs staff so they can conduct outreach. A pilot study conducted by the California Department of Corrections and Rehabilitation found that 2.7% of its inmate population self-identified as veterans, while VRSS identified 7.7% of inmates with a history of military service. This difference represented about 5000 previously unidentified veterans.51 Similarly, community entities that partner with the VA, such as law enforcement or homeless service programs, can be approved to become a SQUARES user and submit identifying information of individuals with whom they interact directly into the SQUARES search engine. SQUARES then directly returns information about the individual’s veteran status and eligibility for VA health care and homeless programs.
Other Eligibility Limitations
VTCs and other professionals looking to refer veterans with OTH discharge characterizations to VA-based behavioral health care should be aware of potential limitations in eligibility and access. Specifically, although veterans with OTH discharges are now broadly eligible for VA-based behavioral health care and homeless programs, they remain ineligible for other forms of health care, including primary care and nonbehavioral specialty care.1 Research has found a strong comorbidity between behavioral and nonbehavioral health concerns, particularly within historically marginalized demographic groups.52-55 Because these historically marginalized groups are often overrepresented among persons with criminal-legal involvement, veterans with OTH discharges, and VTC participants, such comorbidities require consideration by services or programming designed to support veterans with criminal-legal involvement.12,56-58 Connection with VA-based health care will therefore continue to fall short of addressing all health care needs of veterans with OTH discharges and effective case management will require considerable treatment coordination between VA behavioral health care practitioners (HCPs) and community-based HCPs (eg, primary care professionals or nonbehavioral HCPs).
Implications for VA Mental Health Care
Recent eligibility expansions will also have inevitable consequences for VA mental health care systems. For many years, these systems have been overburdened by high caseloads and clinician burnout.59,60 Given the generally elevated rates of mental health and substance use concerns among veterans with OTH discharge characterizations, expansions hold the potential to further burden caseloads with clinically complex, high-risk, high-need clients. Nevertheless, these expansions are also structured in a way that forces existing systems to absorb the responsibilities of providing necessary care to these veterans. To mitigate detrimental effects of eligibility expansions on the broader VA mental health system, clinicians should be explicitly trained in identifying veterans with OTH discharge characterizations and the implications of discharge status on broader health care eligibility. Treatment of veterans with OTH discharges may also benefit from close coordination between mental health professionals and behavioral health care coordinators to ensure appropriate coordination of care between VA- and non–VA-based HCPs.
CONCLUSIONS
Recent changes to VA eligibility policies now allow comprehensive mental and behavioral health care services to be provided to veterans with OTH discharges.1 Compared to routinely discharged veterans, veterans with OTH discharges are more likely to be persons of color, sexual or gender minorities, and experiencing mental health-related difficulties. Given the disproportionate mental health burden often faced by veterans with OTH discharges and relative overrepresentation of these veterans in judicial and correctional systems, these changes have considerable implications for programs and services designed to support veterans with criminallegal involvement. Professionals within these systems, particularly VTC programs, are therefore encouraged to familiarize themselves with recent changes in VA eligibility policies and to revisit strategies, policies, and procedures surrounding the engagement and enrollment of veterans with OTH discharge characterizations. Doing so may ensure veterans with OTH discharges are effectively connected to needed behavioral health care services.
- US Department of Veterans, Veterans Health Administration. VHA Directive 1601A.02(6): Eligibility Determination. Updated March 6, 2024. Accessed August 8, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8908
- Mental and behavioral health care for certain former members of the Armed Forces. 38 USC §1720I (2018). Accessed August 5, 2024. https://uscode.house.gov/view.xhtml?req=(title:38%20section:1720I%20edition:prelim
- US Department of Veterans, Veterans Health Administration. VHA Directive 1601A.02, Eligibility Determination. June 7, 2017.
- US Department of Veterans, Veterans Health Administration. VHA Directive 1115(1), Military Sexual Trauma (MST) Program. May 8, 2018. Accessed August 5, 2024. https:// www.va.gov/vhapublications/viewpublication.asp?pub_ID=6402
- US Department of Veterans Affairs. Tentative Eligibility Determinations; Presumptive Eligibility for Psychosis and Other Mental Illness. 38 CFR §17.109. May 14, 2013. Accessed August 5, 2024. https://www.federalregister.gov/documents/2013/05/14/2013-11410/tentative-eligibility-determinations-presumptive-eligibility-for-psychosis-and-other-mental-illness
- US Department of Veterans Affairs, VA Office of Mental Health and Suicide Prevention. National strategy for preventing veteran suicide 2018-2028. Published September 2018. Accessed August 5, 2024. https://www.mentalhealth.va.gov/suicide_prevention/docs/Office-of-Mental-Health-and-Suicide-Prevention-National-Strategy-for-Preventing-Veterans-Suicide.pdf
- US Department of Veterans Affairs. VA secretary announces intention to expand mental health care to former service members with other-than-honorable discharges and in crisis. Press Release. March 8, 2017. Accessed August 5, 2024. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=2867
- Smith C. Dramatic increase in mental health services to other-than-honorable discharge veterans. VA News. February 23, 2022. Accessed August 5, 2024. https://news.va.gov/100460/dramatic-increase-in-mental-health-services-to-other-than-honorable-discharge-veterans/
- US Department of Defense. DoD Instruction 1332.14. Enlisted administrative separations. Updated August 1, 2024. Accessed August 5, 2024. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/133214p.pdf
- US Department of Defense. DoD Instruction 1332.30. Commissioned officer administrative separations. Updated September 9, 2021. Accessed August 5, 2024. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/133230p.pdf
- OUTVETS, Legal Services Center of Harvard Law School, Veterans Legal Services. Turned away: how the VA unlawfully denies healthcare to veterans with bad paper discharges. 2020. Accessed August 5, 2024. https://legalservicescenter.org/wp-content/uploads/Turn-Away-Report.pdf
- McClean H. Discharged and discarded: the collateral consequences of a less-than-honorable military discharge. Columbia Law Rev. 2021;121(7):2203-2268.
- Veterans Benefits, General Provisions, Definitions. 38 USC §101(2) (1958). Accessed August 5, 2024. https://uscode.house.gov/view.xhtml?req=granuleid:USC-prelim-title38-section101&num=0&edition=prelim
- Bedford JR. Other than honorable discharges: unfair and unjust life sentences of decreased earning capacity. U Penn J Law Pub Affairs. 2021;6(4):687.
- Karin ML. Other than honorable discrimination. Case Western Reserve Law Rev. 2016;67(1):135-191. https://scholarlycommons.law.case.edu/caselrev/vol67/iss1/9
- Veteran HOUSE Act of 2020, HR 2398, 116th Cong, (2020). Accessed August 5, 2024. https://www.congress.gov/bill/116th-congress/house-bill/2398
- Scapardine D. Leaving other than honorable soldiers behind: how the Departments of Defense and Veterans Affairs inadvertently created a health and social crisis. Md Law Rev. 2017;76(4):1133-1165.
- Elbogen EB, Wagner HR, Brancu M, et al. Psychosocial risk factors and other than honorable military discharge: providing healthcare to previously ineligible veterans. Mil Med. 2018;183(9-10):e532-e538. doi:10.1093/milmed/usx128
- Tsai J, Rosenheck RA. Characteristics and health needs of veterans with other-than-honorable discharges: expanding eligibility in the Veterans Health Administration. Mil Med. 2018;183(5-6):e153-e157. doi:10.1093/milmed/usx110
- Christensen DM, Tsilker Y. Racial disparities in military justice: findings of substantial and persistent racial disparities within the United States military justice system. Accessed August 5, 2024. https://www.protectourdefenders.com/wp-content/uploads/2017/05/Report_20.pdf
- Don’t Ask Don’t Tell, 10 USC §654 (1993) (Repealed 2010). Accessed August 5, 2024. http://www.gpo.gov/fdsys/pkg/USCODE-2010-title10/pdf/USCODE-2010-title10-subtitleA-partII-chap37-sec654.pdf
- Palm Center. The making of a ban: how DTM-19-004 works to push transgender people out of military service. 2019. March 20, 2019. Accessed August 5, 2024. https://www.palmcenter.org/wp-content/uploads/2019/04/The-Making-of-a-Ban.pdf
- Edwards ER, Greene AL, Epshteyn G, Gromatsky M, Kinney AR, Holliday R. Mental health of incarcerated veterans and civilians: latent class analysis of the 2016 Survey of Prison Inmates. Crim Justice Behav. 2022;49(12):1800- 1821. doi:10.1177/00938548221121142
- Brignone E, Fargo JD, Blais RK, Carter ME, Samore MH, Gundlapalli AV. Non-routine discharge from military service: mental illness, substance use disorders, and suicidality. Am J Prev Med. 2017;52(5):557-565. doi:10.1016/j.amepre.2016.11.015
- Gamache G, Rosenheck R, Tessler R. Military discharge status of homeless veterans with mental illness. Mil Med. 2000;165(11):803-808. doi:10.1093/milmed/165.11.803
- Gundlapalli AV, Fargo JD, Metraux S, et al. Military Misconduct and Homelessness Among US Veterans Separated From Active Duty, 2001-2012. JAMA. 2015;314(8):832-834. doi:10.1001/jama.2015.8207
- Brooks Holliday S, Pedersen ER. The association between discharge status, mental health, and substance misuse among young adult veterans. Psychiatry Res. 2017;256:428-434. doi:10.1016/j.psychres.2017.07.011
- Williamson RB. DOD Health: Actions Needed to Ensure Post-Traumatic Stress Disorder and Traumatic Brain Injury are Considered in Misconduct Separations. US Government Accountability Office; 2017. Accessed August 5, 2024. https://apps.dtic.mil/sti/pdfs/AD1168610.pdf
- Maruschak LM, Bronson J, Alper M. Indicators of mental health problems reported by prisoners: survey of prison inmates. US Department of Justice Bureau of Justice Statistics. June 2021. Accessed August 5, 2024. https://bjs.ojp.gov/sites/g/files/xyckuh236/files/media/document/imhprpspi16st.pdf
- Brooke E, Gau J. Military service and lifetime arrests: examining the effects of the total military experience on arrests in a sample of prison inmates. Crim Justice Policy Rev. 2018;29(1):24-44. doi:10.1177/0887403415619007
- Russell RT. Veterans treatment court: a proactive approach. N Engl J Crim Civ Confin. 2009;35:357-372.
- Cartwright T. To care for him who shall have borne the battle: the recent development of Veterans Treatment Courts in America. Stanford Law Pol Rev. 2011;22:295-316.
- Douds AS, Ahlin EM, Howard D, Stigerwalt S. Varieties of veterans’ courts: a statewide assessment of veterans’ treatment court components. Crim Justice Policy Rev. 2017;28:740-769. doi:10.1177/0887403415620633
- Rowen J. Worthy of justice: a veterans treatment court in practice. Law Policy. 2020;42(1):78-100. doi:10.1111/lapo.12142
- Timko C, Flatley B, Tjemsland A, et al. A longitudinal examination of veterans treatment courts’ characteristics and eligibility criteria. Justice Res Policy. 2016;17(2):123-136.
- Baldwin JM. Executive summary: national survey of veterans treatment courts. SSRN. Preprint posted online June 5, 2013. Accessed August 5, 2024. doi:10.2139/ssrn.2274138
- Renz T. Veterans treatment court: a hand up rather than lock up. Richmond Public Interest Law Rev. 2013;17(3):697-705. https://scholarship.richmond.edu/pilr/vol17/iss3/6
- Knudsen KJ, Wingenfeld S. A specialized treatment court for veterans with trauma exposure: implications for the field. Community Ment Health J. 2016;52(2):127-135. doi:10.1007/s10597-015-9845-9
- McCall JD, Tsai J, Gordon AJ. Veterans treatment court research: participant characteristics, outcomes, and gaps in the literature. J Offender Rehabil. 2018;57:384-401. doi:10.1080/10509674.2018.1510864
- Smith JS. The Anchorage, Alaska veterans court and recidivism: July 6, 2004 – December 31, 2010. Alsk Law Rev. 2012;29(1):93-111.
- Hartley RD, Baldwin JM. Waging war on recidivism among justice-involved veterans: an impact evaluation of a large urban veterans treatment court. Crim Justice Policy Rev. 2019;30(1):52-78. doi:10.1177/0887403416650490
- Tsai J, Flatley B, Kasprow WJ, Clark S, Finlay A. Diversion of veterans with criminal justice involvement to treatment courts: participant characteristics and outcomes. Psychiatr Serv. 2017;68(4):375-383. doi:10.1176/appi.ps.201600233
- Edwards ER, Sissoko DR, Abrams D, Samost D, La Gamma S, Geraci J. Connecting mental health court participants with services: process, challenges, and recommendations. Psychol Public Policy Law. 2020;26(4):463-475. doi:10.1037/law0000236
- US Department of Veterans Affairs, VA Office of Mental Health and Suicide Prevention. 2023 National Veteran Suicide Prevention Annual Report. US Department of Veterans Affairs; November 2023. Accessed August 5, 2024. https://www.mentalhealth.va.gov/docs/data-sheets/2023/2023-National-Veteran-Suicide-Prevention-Annual-Report-FINAL-508.pdf
- Finlay AK, Clark S, Blue-Howells J, et al. Logic model of the Department of Veterans Affairs’ role in veterans treatment courts. Drug Court Rev. 2019;2:45-62.
- Finlay AK, Smelson D, Sawh L, et al. U.S. Department of Veterans Affairs veterans justice outreach program: connecting justice-involved veterans with mental health and substance use disorder treatment. Crim Justice Policy Rev. 2016;27(2):10.1177/0887403414562601. doi:10.1177/0887403414562601
- Finlay AK, Stimmel M, Blue-Howells J, et al. Use of Veterans Health Administration mental health and substance use disorder treatment after exiting prison: the health care for reentry veterans program. Adm Policy Ment Health. 2017;44(2):177-187. doi:10.1007/s10488-015-0708-z
- Meyer EG, Writer BW, Brim W. The Importance of Military Cultural Competence. Curr Psychiatry Rep. 2016;18(3):26. doi:10.1007/s11920-016-0662-9
- National Association of Drug Court Professionals. Adult Drug Court Best Practice Standards Volume I. National Association of Drug Court Professionals; 2013. Accessed August 5, 2024. https://allrise.org/publications/standards/
- STRONG Veterans Act of 2022, HR 6411, 117th Cong (2022). https://www.congress.gov/bill/117th-congress/house-bill/6411/text
- Pelletier D, Clark S, Davis L. Veterans reentry search service (VRSS) and the SQUARES application. Presented at: National Association of Drug Court Professionals Conference; August 15-18, 2021; National Harbor, Maryland.
- Scott KM, Lim C, Al-Hamzawi A, et al. Association of Mental Disorders With Subsequent Chronic Physical Conditions: World Mental Health Surveys From 17 Countries. JAMA Psychiatry. 2016;73(2):150-158. doi:10.1001/jamapsychiatry.2015.2688
- Ahmed N, Conway CA. Medical and mental health comorbidities among minority racial/ethnic groups in the United States. J Soc Beh Health Sci. 2020;14(1):153-168. doi:10.5590/JSBHS.2020.14.1.11
- Hanna B, Desai R, Parekh T, Guirguis E, Kumar G, Sachdeva R. Psychiatric disorders in the U.S. transgender population. Ann Epidemiol. 2019;39:1-7.e1. doi:10.1016/j.annepidem.2019.09.009
- Watkins DC, Assari S, Johnson-Lawrence V. Race and ethnic group differences in comorbid major depressive disorder, generalized anxiety disorder, and chronic medical conditions. J Racial Ethn Health Disparities. 2015;2(3):385- 394. doi:10.1007/s40615-015-0085-z
- Baldwin J. Whom do they serve? National examination of veterans treatment court participants and their challenges. Crim Justice Policy Rev. 2017;28(6):515-554. doi:10.1177/0887403415606184
- Beatty LG, Snell TL. Profile of prison inmates, 2016. US Department of Justice Bureau of Justice Statistics. December 2021. Accessed August 5, 2024. https://bjs.ojp.gov/content/pub/pdf/ppi16.pdf
- Al-Rousan T, Rubenstein L, Sieleni B, Deol H, Wallace RB. Inside the nation’s largest mental health institution: a prevalence study in a state prison system. BMC Public Health. 2017;17(1):342. doi:10.1186/s12889-017-4257-0
- Rosen CS, Kaplan AN, Nelson DB, et al. Implementation context and burnout among Department of Veterans Affairs psychotherapists prior to and during the COVID-19 pandemic. J Affect Disord. 2023;320:517-524. doi:10.1016/j.jad.2022.09.141
- Tsai J, Jones N, Klee A, Deegan D. Job burnout among mental health staff at a veterans affairs psychosocial rehabilitation center. Community Ment Health J. 2020;56(2):294- 297. doi:10.1007/s10597-019-00487-5
In April 2022, the US Department of Veterans Affairs (VA) revised its behavioral health care eligibility policies to provide comprehensive mental and behavioral health care to former service members who received an Other Than Honorable (OTH) discharge characterization upon separation from military service.1 This policy shift represents a marked expansion in eligibility practices (Table 1 includes amended eligibility criteria).

Since June 2017, eligibility policies allowed veterans with OTH discharges to receive “emergent mental health services” needed to stabilize acute mental health crises related to military service (eg, acute escalations in suicide risk).2,3 Previously, veterans with OTH discharges were largely ineligible for VA-based health care; these individuals were only able to access Veterans Health Administration (VHA) mental and behavioral health care through limited channels of eligibility (eg, for treatment of military sexual trauma or psychosis or other mental illness within 2 years of discharge).4,5 The impetus for expansions in eligibility stemmed from VA efforts to reduce the suicide rate among veterans.6-8 Implications of such expansion extend beyond suicide prevention efforts, with notable promised effects on the care of veterans with criminal-legal involvement. This article highlights potential effects of recent eligibility expansions on veterans with criminal-legal involvement and makes specific recommendations for agencies and organizations serving these veterans.
OTHER THAN HONORABLE DISCHARGE
The US Department of Defense delineates 6 discharge characterizations provided to service members upon separation from military service: honorable, general under honorable conditions, OTH, bad conduct, dishonorable, and uncharacterized. Honorable discharge characterizations are considered to reflect general concordance between service member behavior and military standards; general discharge characterizations reflect some disparity between the service member’s behavior and military standards; OTH, bad conduct, and dishonorable discharge characterizations reflect serious disparities between the service member’s behavior and military standards; and uncharacterized discharge characterizations are given when other discharge characterizations are deemed inappropriate.9,10 OTH discharge characterizations are typically issued under instances of misconduct, fraudulent entry, security reasons, or in lieu of trial by court martial.9,10
Recent research suggests that about 85% of service members receive an honorable discharge characterization upon separation from military service, 8% receive general, 6% receive OTH, and 1% receive bad conduct or dishonorable discharges.11 In 2017, the VA estimated there were > 500,000 prior service members with OTH discharge characterizations, which has grown over time (1.9% during the Korean Conflict, 2.5% during the Vietnam War Era, 3.9% during the Cold War, 4.8% in the Persian Gulf War, and 5.8% in the post-9/11 era).7,11
The OTH discharge characterization is 1 of 3 less than honorable discharges informally referred to as bad papers (ie, OTH, bad conduct, or dishonorable). Former service members receiving these discharge characterizations face significant social stigma and structural discrimination upon military discharge, including significant hurdles to employment and educational pursuits as well as notable social alienation.12 Due to their discharge characterization, some are viewed as less deserving of the veteran title, and until recently, many did not qualify for the complex legal definition of veteran as established by the Congress.11,13 Veterans with OTH discharge characterizations have also historically been excluded from services (eg, VHA care),3 benefits (eg, disability compensation),14 and protections (eg, Uniformed Services Employment and Reemployment Rights Act)15 offered to veterans with honorable or general discharge characterizations. However, eligibility policies have gradually expanded, providing veterans with OTH discharges with access to VHA-based mental and behavioral health services and VA supportive housing assistance.1,3,16
Perhaps due to their historical exclusion from VA services, there is limited research available on the behavioral health and associated needs of veterans with OTH discharges. Some scholars argue that historical exclusions have exacerbated underlying difficulties faced by this population, thereby contributing to stark health and social disparities across discharge types.14,15,17 Studies with large veteran samples, for example, reflect notable demographic and behavioral health differences across discharge types. Compared to routinely discharged veterans, veterans with OTH discharges are significantly more likely to be younger, have lower income, use substances, have a history of criminal-legal involvement, and have mental and physical health difficulties.18,19
Substantial evidence also suggests a historical racial bias, with service members of color being disproportionately more likely to receive an OTH discharge.12 Similarly, across all branches of military service, Black service members are significantly more likely to face general or special court martial in military justice proceedings when compared with White service members.20 Service members from gender and sexual minorities are also disproportionately impacted by the OTH designation. Historically, many have been discharged with bad papers due to discriminatory policies, such as Don’t Ask Don’t Tell, which discriminated on the basis of sexual orientation between December 1993 and September 2011, and Directive-type Memorandum-19-004, which banned transgender persons from military service between April 2019 and January 2021.21,22
There is also significant mental health bias in the provision of OTH discharges, such that OTH characterizations are disproportionately represented among individuals with mental health disorders.18-20 Veterans discharged from military service due to behavioral misconduct are significantly more likely to meet diagnostic criteria for various behavioral health conditions and to experience homelessness, criminal-legal involvement, and suicidal ideation and behavior compared with routinely-discharged veterans.23-28
Consistent with their comparatively higher rates of criminal-legal involvement relative to routinely discharged veterans, veterans with OTH discharges are disproportionately represented in criminal justice settings. While veterans with OTH discharges represent only 6% of discharging service members and 2.5% of community-based veterans, they represent 10% of incarcerated veterans.11,18,23,29 Preliminary research suggests veterans with OTH discharges may be at higher risk for lifetime incarceration, though the association between OTH discharge and frequency of lifetime arrests remains unclear.18,30
VETERANS TREATMENT COURTS
Given the overrepresentation of veterans with OTH discharges in criminal-legal settings, consideration for this subset of the veteran population and its unique needs is commonplace among problem-solving courts that service veterans. First conceptualized in 2004, Veterans Treatment Courts (VTCs) are specialized problem-solving courts that divert veterans away from traditional judicial court and penal systems and into community-based supervision and treatment (most commonly behavioral health services).31-34 Although each VTC program is unique in structure, policies, and procedures, most VTCs can be characterized by certain key elements, including voluntary participation, plea requirements, delayed sentencing (often including reduced or dismissed charges), integration of military culture into court proceedings, a rehabilitative vs adversarial approach to decreasing risk of future criminal behavior, mandated treatment and supervision during participation, and use of veteran mentors to provide peer support.32-35 Eligibility requirements vary; however, many restrict participation to veterans with honorable discharge types and charges for nonviolent offenses.32,33,35-37
VTCs connect veterans within the criminal-legal system to needed behavioral health, community, and social services.31-33,37 VTC participants are commonly connected to case management, behavioral health care, therapeutic journaling programs, and vocational rehabilitation.38,39 Accordingly, the most common difficulties faced by veterans participating in these courts include substance use, mental health, family issues, anger management and/or aggressive behavior, and homelessness.36,39 There is limited research on the effectiveness of VTCs. Evidence on their overall effectiveness is largely mixed, though some studies suggest VTC graduates tend to have lower recidivism rates than offenders more broadly or persons who terminate VTC programs prior to completion.40,41 Other studies suggest that VTC participants are more likely to have jail sanctions, new arrests, and new incarcerations relative to nontreatment court participants.42 Notably, experimental designs (considered the gold standard in assessing effectiveness) to date have not been applied to evaluate the effectiveness of VTCs; as such, the effectiveness of these programs remains an area in need of continued empirical investigation.
Like all problem-solving courts, VTCs occasionally struggle to connect participating defendants with appropriate care, particularly when encountering structural barriers (eg, insurance, transportation) and/or complex behavioral health needs (eg, personality disorders).34,43 As suicide rates among veterans experiencing criminal-legal involvement surge (about 150 per 100,000 in 2021, a 10% increase from 2020 to 2021 compared to about 40 per 100,000 and a 1.8% increase among other veterans), efficiency of adequate care coordination is vital.44 Many VTCs rely on VTC-VA partnerships and collaborations to navigate these difficulties and facilitate connection of participating veterans to needed services.32-34,45 For example, within the VHA, Veterans Justice Outreach (VJO) and Health Care for Re-Entry Veterans (HCRV) specialists assist and bridge the gap between the criminal-legal system (including, but not limited to VTCs) and VA services by engaging veterans involved in the criminal-legal system and connecting them to needed VA-based services (Table 2). Generally, VJO specialists support veterans involved with the front end of the criminallegal system (eg, arrest, pretrial incarceration, or participation in VTCs), while HCRV specialists tend to support veterans transitioning back into the community after a period of incarceration.46,47 Specific to VTCs, VJO specialists typically serve as liaisons between the courts and VA, coordinating VA services for defendants to fulfill their terms of VTC participation.46

The historical exclusion of veterans with OTH discharge characterizations from VA-based services has restricted many from accessing VTC programs.32 Of 17 VTC programs active in Pennsylvania in 2014, only 5 accepted veterans with OTstayH discharges, and 3 required application to and eligibility for VA benefits.33 Similarly, in national surveys of VTC programs, about 1 in 3 report excluding veterans deemed ineligible for VA services.35,36 When veterans with OTH discharges have accessed VTC programs, they have historically relied on non-VA, community-based programming to fulfill treatment mandates, which may be less suited to addressing the unique needs of veterans.48
Veterans who utilize VTCs receive several benefits, namely peer support and mentorship, acceptance into a veteran-centric space, and connection with specially trained staff capable of supporting the veteran through applications for a range of VA benefits (eg, service connection, housing support).31-33,37 Given the disparate prevalence of OTH discharge characterizations among service members from racial, sexual, and gender minorities and among service members with mental health disorders, exclusion of veterans with OTH discharges from VTCs solely based on the type of discharge likely contributes to structural inequities among these already underserved groups by restricting access to these potential benefits. Such structural inequity stands in direct conflict with VTC best practice standards, which admonish programs to adjust eligibility requirements to facilitate access to treatment court programs for historically marginalized groups.49
ELIGIBILITY EXPANSIONS
Given the overrepresentation of veterans with OTH discharge characterizations within the criminal-legal system and historical barriers of these veterans to access needed mental and behavioral health care, expansions in VA eligibility policies could have immense implications for VTCs. First, these expansions could mitigate common barriers to connecting VTC-participating veterans with OTH discharges with needed behavioral health care by allowing these veterans to access established, VA-based services and programming. Expansion may also allow VTCs to serve as a key intercept point for identifying and engaging veterans with OTH discharges who may be unaware of their eligibility for VA-based behavioral health care.
Access to VA health care services is a major resource for VTC participants and a common requirement.32 Eligibility expansion should ease access barriers veterans with OTH discharges commonly face. By providing a potential source of treatment, expansions may also support OTH eligibility practices within VTCs, particularly practices that require participants to be eligible for VA health care.33,35,36 Some VTCs may continue to determine eligibility on the basis of discharge status and remain inaccessible to veterans with OTH discharge characterizations without program-level policy changes.32,36,37
Communicating changes in eligibility policies relevant to veterans with OTH discharges may be a challenge, because many of these individuals have no established channels of communication with the VA. Because veterans with OTH discharges are at increased risk for legal system involvement, VTCs may serve as a unique point of contact to help facilitate communication.18 For example, upon referral to a VTC, veterans with OTH discharges can be identified, VA health care eligibility can be verified, and veterans can connect to VA staff to facilitate enrollment in VA services and care.
VJO specialists are in a favorable position to serve a critical role in utilizing VTCs as a potential intercept point for engaging veterans with OTH discharge characterizations. As outlined in the STRONG Veterans Act of 2022, VJOs are mandated to “spread awareness and understanding of veteran eligibility for the [VJO] Program, including the eligibility of veterans who were discharged from service in the Armed Forces under conditions other than honorable.”50 The Act further requires VJOs to be annually trained in communicating eligibility changes as they arise. Accordingly, VJOs receive ongoing training in a wide variety of critical outreach topics, including changes in eligibility; while VJOs cannot make eligibility determinations, they are tasked with enrolling all veterans involved in the criminal-legal system with whom they interact into VHA services, whether through typical or special eligibility criteria (M. Stimmel, PhD, National Training Director for Veteran Justice Programming, oral communication, July 14, 2023). VJOs therefore routinely serve in this capacity of facilitating VA enrollment of veterans with OTH discharge characterizations.
Recommendations to Veteran-Servicing Judicial Programs
Considering these potential implications, professionals routinely interacting with veterans involved in the criminal-legal system should become familiarized with recent changes in VA eligibility policies. Such familiarization would support the identification of veterans previously considered ineligible for care; provision of education to these veterans regarding their new eligibility; and referral to appropriate VA-based behavioral health care options. Although conceptually simple, executing such an educational campaign may prove logistically difficult. Given their historical exclusion from VA services, veterans with OTH discharge characterizations are unlikely to seek VA-based services in times of need, instead relying on a broad swath of civilian community-based organizations and resources. Usual approaches to advertising VHA health care policy changes (eg, by notifying VA employees and/or departments providing corresponding services or by circulating information to veteran-focused mailing lists and organizations) likely would prove insufficient. Educational campaigns to disseminate information about recent OTH eligibility changes should instead consider partnering with traditionally civilian, communitybased organizations and institutions, such as state bar associations, legal aid networks, case management services, nonveteran treatment court programs (eg, drug courts, or domestic violence courts), or probation/ parole programs. Because national surveys suggest generally low military cultural competence among civilian populations, providing concurrent support in developing foundational veteran cultural competencies (eg, how to phrase questions about military service history, or understanding discharge characterizations) may be necessary to ensure effective identification and engagement of veteran clients.48
Programs that serve veterans with criminal-legal involvement should also consider potential relevance of recent OTH eligibility changes to program operations. VTC program staff and key partners (eg, judges, case managers, district attorneys, or defense attorneys), should revisit policies and procedures surrounding the engagement of veterans with OTH discharges within VTC programs and strategies for connecting these veterans with needed services. VTC programs that have historically excluded veterans with OTH discharges due to associated difficulties in locating and connecting with needed services should consider expanding eligibility policies considering recent shifts in VA behavioral health care eligibility.33,35,36 Within the VHA, VJO specialists can play a critical role in supporting these VTC eligibility and cultural shifts. Some evidence suggests a large proportion of VTC referrals are facilitated by VJO specialists and that many such referrals are identified when veterans involved with the criminal-legal system seek VA support and/or services.33 Given the historical exclusion of veterans with OTH discharges from VA care, strategies used by VJO specialists to identify, connect, and engage veterans with OTH discharges with VTCs and other services may be beneficial.
Even with knowledge of VA eligibility changes and considerations of these changes on local operations, many forensic settings and programs struggle to identify veterans. These difficulties are likely amplified among veterans with OTH discharge characterizations, who may be hesitant to self-disclose their military service history due to fear of stigma and/or views of OTH discharge characterizations as undeserving of the veteran title.12 The VA offers 2 tools to aid in identification of veterans for these settings: the Veterans Re-Entry Search Service (VRSS) and Status Query and Response Exchange System (SQUARES). For VRSS, correctional facilities, courts, and other criminal justice entities upload a simple spreadsheet that contains basic identifying information of inmates or defendants in their system. VRSS returns information about which inmates or defendants have a history of military service and alerts VA Veterans Justice Programs staff so they can conduct outreach. A pilot study conducted by the California Department of Corrections and Rehabilitation found that 2.7% of its inmate population self-identified as veterans, while VRSS identified 7.7% of inmates with a history of military service. This difference represented about 5000 previously unidentified veterans.51 Similarly, community entities that partner with the VA, such as law enforcement or homeless service programs, can be approved to become a SQUARES user and submit identifying information of individuals with whom they interact directly into the SQUARES search engine. SQUARES then directly returns information about the individual’s veteran status and eligibility for VA health care and homeless programs.
Other Eligibility Limitations
VTCs and other professionals looking to refer veterans with OTH discharge characterizations to VA-based behavioral health care should be aware of potential limitations in eligibility and access. Specifically, although veterans with OTH discharges are now broadly eligible for VA-based behavioral health care and homeless programs, they remain ineligible for other forms of health care, including primary care and nonbehavioral specialty care.1 Research has found a strong comorbidity between behavioral and nonbehavioral health concerns, particularly within historically marginalized demographic groups.52-55 Because these historically marginalized groups are often overrepresented among persons with criminal-legal involvement, veterans with OTH discharges, and VTC participants, such comorbidities require consideration by services or programming designed to support veterans with criminal-legal involvement.12,56-58 Connection with VA-based health care will therefore continue to fall short of addressing all health care needs of veterans with OTH discharges and effective case management will require considerable treatment coordination between VA behavioral health care practitioners (HCPs) and community-based HCPs (eg, primary care professionals or nonbehavioral HCPs).
Implications for VA Mental Health Care
Recent eligibility expansions will also have inevitable consequences for VA mental health care systems. For many years, these systems have been overburdened by high caseloads and clinician burnout.59,60 Given the generally elevated rates of mental health and substance use concerns among veterans with OTH discharge characterizations, expansions hold the potential to further burden caseloads with clinically complex, high-risk, high-need clients. Nevertheless, these expansions are also structured in a way that forces existing systems to absorb the responsibilities of providing necessary care to these veterans. To mitigate detrimental effects of eligibility expansions on the broader VA mental health system, clinicians should be explicitly trained in identifying veterans with OTH discharge characterizations and the implications of discharge status on broader health care eligibility. Treatment of veterans with OTH discharges may also benefit from close coordination between mental health professionals and behavioral health care coordinators to ensure appropriate coordination of care between VA- and non–VA-based HCPs.
CONCLUSIONS
Recent changes to VA eligibility policies now allow comprehensive mental and behavioral health care services to be provided to veterans with OTH discharges.1 Compared to routinely discharged veterans, veterans with OTH discharges are more likely to be persons of color, sexual or gender minorities, and experiencing mental health-related difficulties. Given the disproportionate mental health burden often faced by veterans with OTH discharges and relative overrepresentation of these veterans in judicial and correctional systems, these changes have considerable implications for programs and services designed to support veterans with criminallegal involvement. Professionals within these systems, particularly VTC programs, are therefore encouraged to familiarize themselves with recent changes in VA eligibility policies and to revisit strategies, policies, and procedures surrounding the engagement and enrollment of veterans with OTH discharge characterizations. Doing so may ensure veterans with OTH discharges are effectively connected to needed behavioral health care services.
In April 2022, the US Department of Veterans Affairs (VA) revised its behavioral health care eligibility policies to provide comprehensive mental and behavioral health care to former service members who received an Other Than Honorable (OTH) discharge characterization upon separation from military service.1 This policy shift represents a marked expansion in eligibility practices (Table 1 includes amended eligibility criteria).

Since June 2017, eligibility policies allowed veterans with OTH discharges to receive “emergent mental health services” needed to stabilize acute mental health crises related to military service (eg, acute escalations in suicide risk).2,3 Previously, veterans with OTH discharges were largely ineligible for VA-based health care; these individuals were only able to access Veterans Health Administration (VHA) mental and behavioral health care through limited channels of eligibility (eg, for treatment of military sexual trauma or psychosis or other mental illness within 2 years of discharge).4,5 The impetus for expansions in eligibility stemmed from VA efforts to reduce the suicide rate among veterans.6-8 Implications of such expansion extend beyond suicide prevention efforts, with notable promised effects on the care of veterans with criminal-legal involvement. This article highlights potential effects of recent eligibility expansions on veterans with criminal-legal involvement and makes specific recommendations for agencies and organizations serving these veterans.
OTHER THAN HONORABLE DISCHARGE
The US Department of Defense delineates 6 discharge characterizations provided to service members upon separation from military service: honorable, general under honorable conditions, OTH, bad conduct, dishonorable, and uncharacterized. Honorable discharge characterizations are considered to reflect general concordance between service member behavior and military standards; general discharge characterizations reflect some disparity between the service member’s behavior and military standards; OTH, bad conduct, and dishonorable discharge characterizations reflect serious disparities between the service member’s behavior and military standards; and uncharacterized discharge characterizations are given when other discharge characterizations are deemed inappropriate.9,10 OTH discharge characterizations are typically issued under instances of misconduct, fraudulent entry, security reasons, or in lieu of trial by court martial.9,10
Recent research suggests that about 85% of service members receive an honorable discharge characterization upon separation from military service, 8% receive general, 6% receive OTH, and 1% receive bad conduct or dishonorable discharges.11 In 2017, the VA estimated there were > 500,000 prior service members with OTH discharge characterizations, which has grown over time (1.9% during the Korean Conflict, 2.5% during the Vietnam War Era, 3.9% during the Cold War, 4.8% in the Persian Gulf War, and 5.8% in the post-9/11 era).7,11
The OTH discharge characterization is 1 of 3 less than honorable discharges informally referred to as bad papers (ie, OTH, bad conduct, or dishonorable). Former service members receiving these discharge characterizations face significant social stigma and structural discrimination upon military discharge, including significant hurdles to employment and educational pursuits as well as notable social alienation.12 Due to their discharge characterization, some are viewed as less deserving of the veteran title, and until recently, many did not qualify for the complex legal definition of veteran as established by the Congress.11,13 Veterans with OTH discharge characterizations have also historically been excluded from services (eg, VHA care),3 benefits (eg, disability compensation),14 and protections (eg, Uniformed Services Employment and Reemployment Rights Act)15 offered to veterans with honorable or general discharge characterizations. However, eligibility policies have gradually expanded, providing veterans with OTH discharges with access to VHA-based mental and behavioral health services and VA supportive housing assistance.1,3,16
Perhaps due to their historical exclusion from VA services, there is limited research available on the behavioral health and associated needs of veterans with OTH discharges. Some scholars argue that historical exclusions have exacerbated underlying difficulties faced by this population, thereby contributing to stark health and social disparities across discharge types.14,15,17 Studies with large veteran samples, for example, reflect notable demographic and behavioral health differences across discharge types. Compared to routinely discharged veterans, veterans with OTH discharges are significantly more likely to be younger, have lower income, use substances, have a history of criminal-legal involvement, and have mental and physical health difficulties.18,19
Substantial evidence also suggests a historical racial bias, with service members of color being disproportionately more likely to receive an OTH discharge.12 Similarly, across all branches of military service, Black service members are significantly more likely to face general or special court martial in military justice proceedings when compared with White service members.20 Service members from gender and sexual minorities are also disproportionately impacted by the OTH designation. Historically, many have been discharged with bad papers due to discriminatory policies, such as Don’t Ask Don’t Tell, which discriminated on the basis of sexual orientation between December 1993 and September 2011, and Directive-type Memorandum-19-004, which banned transgender persons from military service between April 2019 and January 2021.21,22
There is also significant mental health bias in the provision of OTH discharges, such that OTH characterizations are disproportionately represented among individuals with mental health disorders.18-20 Veterans discharged from military service due to behavioral misconduct are significantly more likely to meet diagnostic criteria for various behavioral health conditions and to experience homelessness, criminal-legal involvement, and suicidal ideation and behavior compared with routinely-discharged veterans.23-28
Consistent with their comparatively higher rates of criminal-legal involvement relative to routinely discharged veterans, veterans with OTH discharges are disproportionately represented in criminal justice settings. While veterans with OTH discharges represent only 6% of discharging service members and 2.5% of community-based veterans, they represent 10% of incarcerated veterans.11,18,23,29 Preliminary research suggests veterans with OTH discharges may be at higher risk for lifetime incarceration, though the association between OTH discharge and frequency of lifetime arrests remains unclear.18,30
VETERANS TREATMENT COURTS
Given the overrepresentation of veterans with OTH discharges in criminal-legal settings, consideration for this subset of the veteran population and its unique needs is commonplace among problem-solving courts that service veterans. First conceptualized in 2004, Veterans Treatment Courts (VTCs) are specialized problem-solving courts that divert veterans away from traditional judicial court and penal systems and into community-based supervision and treatment (most commonly behavioral health services).31-34 Although each VTC program is unique in structure, policies, and procedures, most VTCs can be characterized by certain key elements, including voluntary participation, plea requirements, delayed sentencing (often including reduced or dismissed charges), integration of military culture into court proceedings, a rehabilitative vs adversarial approach to decreasing risk of future criminal behavior, mandated treatment and supervision during participation, and use of veteran mentors to provide peer support.32-35 Eligibility requirements vary; however, many restrict participation to veterans with honorable discharge types and charges for nonviolent offenses.32,33,35-37
VTCs connect veterans within the criminal-legal system to needed behavioral health, community, and social services.31-33,37 VTC participants are commonly connected to case management, behavioral health care, therapeutic journaling programs, and vocational rehabilitation.38,39 Accordingly, the most common difficulties faced by veterans participating in these courts include substance use, mental health, family issues, anger management and/or aggressive behavior, and homelessness.36,39 There is limited research on the effectiveness of VTCs. Evidence on their overall effectiveness is largely mixed, though some studies suggest VTC graduates tend to have lower recidivism rates than offenders more broadly or persons who terminate VTC programs prior to completion.40,41 Other studies suggest that VTC participants are more likely to have jail sanctions, new arrests, and new incarcerations relative to nontreatment court participants.42 Notably, experimental designs (considered the gold standard in assessing effectiveness) to date have not been applied to evaluate the effectiveness of VTCs; as such, the effectiveness of these programs remains an area in need of continued empirical investigation.
Like all problem-solving courts, VTCs occasionally struggle to connect participating defendants with appropriate care, particularly when encountering structural barriers (eg, insurance, transportation) and/or complex behavioral health needs (eg, personality disorders).34,43 As suicide rates among veterans experiencing criminal-legal involvement surge (about 150 per 100,000 in 2021, a 10% increase from 2020 to 2021 compared to about 40 per 100,000 and a 1.8% increase among other veterans), efficiency of adequate care coordination is vital.44 Many VTCs rely on VTC-VA partnerships and collaborations to navigate these difficulties and facilitate connection of participating veterans to needed services.32-34,45 For example, within the VHA, Veterans Justice Outreach (VJO) and Health Care for Re-Entry Veterans (HCRV) specialists assist and bridge the gap between the criminal-legal system (including, but not limited to VTCs) and VA services by engaging veterans involved in the criminal-legal system and connecting them to needed VA-based services (Table 2). Generally, VJO specialists support veterans involved with the front end of the criminallegal system (eg, arrest, pretrial incarceration, or participation in VTCs), while HCRV specialists tend to support veterans transitioning back into the community after a period of incarceration.46,47 Specific to VTCs, VJO specialists typically serve as liaisons between the courts and VA, coordinating VA services for defendants to fulfill their terms of VTC participation.46

The historical exclusion of veterans with OTH discharge characterizations from VA-based services has restricted many from accessing VTC programs.32 Of 17 VTC programs active in Pennsylvania in 2014, only 5 accepted veterans with OTstayH discharges, and 3 required application to and eligibility for VA benefits.33 Similarly, in national surveys of VTC programs, about 1 in 3 report excluding veterans deemed ineligible for VA services.35,36 When veterans with OTH discharges have accessed VTC programs, they have historically relied on non-VA, community-based programming to fulfill treatment mandates, which may be less suited to addressing the unique needs of veterans.48
Veterans who utilize VTCs receive several benefits, namely peer support and mentorship, acceptance into a veteran-centric space, and connection with specially trained staff capable of supporting the veteran through applications for a range of VA benefits (eg, service connection, housing support).31-33,37 Given the disparate prevalence of OTH discharge characterizations among service members from racial, sexual, and gender minorities and among service members with mental health disorders, exclusion of veterans with OTH discharges from VTCs solely based on the type of discharge likely contributes to structural inequities among these already underserved groups by restricting access to these potential benefits. Such structural inequity stands in direct conflict with VTC best practice standards, which admonish programs to adjust eligibility requirements to facilitate access to treatment court programs for historically marginalized groups.49
ELIGIBILITY EXPANSIONS
Given the overrepresentation of veterans with OTH discharge characterizations within the criminal-legal system and historical barriers of these veterans to access needed mental and behavioral health care, expansions in VA eligibility policies could have immense implications for VTCs. First, these expansions could mitigate common barriers to connecting VTC-participating veterans with OTH discharges with needed behavioral health care by allowing these veterans to access established, VA-based services and programming. Expansion may also allow VTCs to serve as a key intercept point for identifying and engaging veterans with OTH discharges who may be unaware of their eligibility for VA-based behavioral health care.
Access to VA health care services is a major resource for VTC participants and a common requirement.32 Eligibility expansion should ease access barriers veterans with OTH discharges commonly face. By providing a potential source of treatment, expansions may also support OTH eligibility practices within VTCs, particularly practices that require participants to be eligible for VA health care.33,35,36 Some VTCs may continue to determine eligibility on the basis of discharge status and remain inaccessible to veterans with OTH discharge characterizations without program-level policy changes.32,36,37
Communicating changes in eligibility policies relevant to veterans with OTH discharges may be a challenge, because many of these individuals have no established channels of communication with the VA. Because veterans with OTH discharges are at increased risk for legal system involvement, VTCs may serve as a unique point of contact to help facilitate communication.18 For example, upon referral to a VTC, veterans with OTH discharges can be identified, VA health care eligibility can be verified, and veterans can connect to VA staff to facilitate enrollment in VA services and care.
VJO specialists are in a favorable position to serve a critical role in utilizing VTCs as a potential intercept point for engaging veterans with OTH discharge characterizations. As outlined in the STRONG Veterans Act of 2022, VJOs are mandated to “spread awareness and understanding of veteran eligibility for the [VJO] Program, including the eligibility of veterans who were discharged from service in the Armed Forces under conditions other than honorable.”50 The Act further requires VJOs to be annually trained in communicating eligibility changes as they arise. Accordingly, VJOs receive ongoing training in a wide variety of critical outreach topics, including changes in eligibility; while VJOs cannot make eligibility determinations, they are tasked with enrolling all veterans involved in the criminal-legal system with whom they interact into VHA services, whether through typical or special eligibility criteria (M. Stimmel, PhD, National Training Director for Veteran Justice Programming, oral communication, July 14, 2023). VJOs therefore routinely serve in this capacity of facilitating VA enrollment of veterans with OTH discharge characterizations.
Recommendations to Veteran-Servicing Judicial Programs
Considering these potential implications, professionals routinely interacting with veterans involved in the criminal-legal system should become familiarized with recent changes in VA eligibility policies. Such familiarization would support the identification of veterans previously considered ineligible for care; provision of education to these veterans regarding their new eligibility; and referral to appropriate VA-based behavioral health care options. Although conceptually simple, executing such an educational campaign may prove logistically difficult. Given their historical exclusion from VA services, veterans with OTH discharge characterizations are unlikely to seek VA-based services in times of need, instead relying on a broad swath of civilian community-based organizations and resources. Usual approaches to advertising VHA health care policy changes (eg, by notifying VA employees and/or departments providing corresponding services or by circulating information to veteran-focused mailing lists and organizations) likely would prove insufficient. Educational campaigns to disseminate information about recent OTH eligibility changes should instead consider partnering with traditionally civilian, communitybased organizations and institutions, such as state bar associations, legal aid networks, case management services, nonveteran treatment court programs (eg, drug courts, or domestic violence courts), or probation/ parole programs. Because national surveys suggest generally low military cultural competence among civilian populations, providing concurrent support in developing foundational veteran cultural competencies (eg, how to phrase questions about military service history, or understanding discharge characterizations) may be necessary to ensure effective identification and engagement of veteran clients.48
Programs that serve veterans with criminal-legal involvement should also consider potential relevance of recent OTH eligibility changes to program operations. VTC program staff and key partners (eg, judges, case managers, district attorneys, or defense attorneys), should revisit policies and procedures surrounding the engagement of veterans with OTH discharges within VTC programs and strategies for connecting these veterans with needed services. VTC programs that have historically excluded veterans with OTH discharges due to associated difficulties in locating and connecting with needed services should consider expanding eligibility policies considering recent shifts in VA behavioral health care eligibility.33,35,36 Within the VHA, VJO specialists can play a critical role in supporting these VTC eligibility and cultural shifts. Some evidence suggests a large proportion of VTC referrals are facilitated by VJO specialists and that many such referrals are identified when veterans involved with the criminal-legal system seek VA support and/or services.33 Given the historical exclusion of veterans with OTH discharges from VA care, strategies used by VJO specialists to identify, connect, and engage veterans with OTH discharges with VTCs and other services may be beneficial.
Even with knowledge of VA eligibility changes and considerations of these changes on local operations, many forensic settings and programs struggle to identify veterans. These difficulties are likely amplified among veterans with OTH discharge characterizations, who may be hesitant to self-disclose their military service history due to fear of stigma and/or views of OTH discharge characterizations as undeserving of the veteran title.12 The VA offers 2 tools to aid in identification of veterans for these settings: the Veterans Re-Entry Search Service (VRSS) and Status Query and Response Exchange System (SQUARES). For VRSS, correctional facilities, courts, and other criminal justice entities upload a simple spreadsheet that contains basic identifying information of inmates or defendants in their system. VRSS returns information about which inmates or defendants have a history of military service and alerts VA Veterans Justice Programs staff so they can conduct outreach. A pilot study conducted by the California Department of Corrections and Rehabilitation found that 2.7% of its inmate population self-identified as veterans, while VRSS identified 7.7% of inmates with a history of military service. This difference represented about 5000 previously unidentified veterans.51 Similarly, community entities that partner with the VA, such as law enforcement or homeless service programs, can be approved to become a SQUARES user and submit identifying information of individuals with whom they interact directly into the SQUARES search engine. SQUARES then directly returns information about the individual’s veteran status and eligibility for VA health care and homeless programs.
Other Eligibility Limitations
VTCs and other professionals looking to refer veterans with OTH discharge characterizations to VA-based behavioral health care should be aware of potential limitations in eligibility and access. Specifically, although veterans with OTH discharges are now broadly eligible for VA-based behavioral health care and homeless programs, they remain ineligible for other forms of health care, including primary care and nonbehavioral specialty care.1 Research has found a strong comorbidity between behavioral and nonbehavioral health concerns, particularly within historically marginalized demographic groups.52-55 Because these historically marginalized groups are often overrepresented among persons with criminal-legal involvement, veterans with OTH discharges, and VTC participants, such comorbidities require consideration by services or programming designed to support veterans with criminal-legal involvement.12,56-58 Connection with VA-based health care will therefore continue to fall short of addressing all health care needs of veterans with OTH discharges and effective case management will require considerable treatment coordination between VA behavioral health care practitioners (HCPs) and community-based HCPs (eg, primary care professionals or nonbehavioral HCPs).
Implications for VA Mental Health Care
Recent eligibility expansions will also have inevitable consequences for VA mental health care systems. For many years, these systems have been overburdened by high caseloads and clinician burnout.59,60 Given the generally elevated rates of mental health and substance use concerns among veterans with OTH discharge characterizations, expansions hold the potential to further burden caseloads with clinically complex, high-risk, high-need clients. Nevertheless, these expansions are also structured in a way that forces existing systems to absorb the responsibilities of providing necessary care to these veterans. To mitigate detrimental effects of eligibility expansions on the broader VA mental health system, clinicians should be explicitly trained in identifying veterans with OTH discharge characterizations and the implications of discharge status on broader health care eligibility. Treatment of veterans with OTH discharges may also benefit from close coordination between mental health professionals and behavioral health care coordinators to ensure appropriate coordination of care between VA- and non–VA-based HCPs.
CONCLUSIONS
Recent changes to VA eligibility policies now allow comprehensive mental and behavioral health care services to be provided to veterans with OTH discharges.1 Compared to routinely discharged veterans, veterans with OTH discharges are more likely to be persons of color, sexual or gender minorities, and experiencing mental health-related difficulties. Given the disproportionate mental health burden often faced by veterans with OTH discharges and relative overrepresentation of these veterans in judicial and correctional systems, these changes have considerable implications for programs and services designed to support veterans with criminallegal involvement. Professionals within these systems, particularly VTC programs, are therefore encouraged to familiarize themselves with recent changes in VA eligibility policies and to revisit strategies, policies, and procedures surrounding the engagement and enrollment of veterans with OTH discharge characterizations. Doing so may ensure veterans with OTH discharges are effectively connected to needed behavioral health care services.
- US Department of Veterans, Veterans Health Administration. VHA Directive 1601A.02(6): Eligibility Determination. Updated March 6, 2024. Accessed August 8, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8908
- Mental and behavioral health care for certain former members of the Armed Forces. 38 USC §1720I (2018). Accessed August 5, 2024. https://uscode.house.gov/view.xhtml?req=(title:38%20section:1720I%20edition:prelim
- US Department of Veterans, Veterans Health Administration. VHA Directive 1601A.02, Eligibility Determination. June 7, 2017.
- US Department of Veterans, Veterans Health Administration. VHA Directive 1115(1), Military Sexual Trauma (MST) Program. May 8, 2018. Accessed August 5, 2024. https:// www.va.gov/vhapublications/viewpublication.asp?pub_ID=6402
- US Department of Veterans Affairs. Tentative Eligibility Determinations; Presumptive Eligibility for Psychosis and Other Mental Illness. 38 CFR §17.109. May 14, 2013. Accessed August 5, 2024. https://www.federalregister.gov/documents/2013/05/14/2013-11410/tentative-eligibility-determinations-presumptive-eligibility-for-psychosis-and-other-mental-illness
- US Department of Veterans Affairs, VA Office of Mental Health and Suicide Prevention. National strategy for preventing veteran suicide 2018-2028. Published September 2018. Accessed August 5, 2024. https://www.mentalhealth.va.gov/suicide_prevention/docs/Office-of-Mental-Health-and-Suicide-Prevention-National-Strategy-for-Preventing-Veterans-Suicide.pdf
- US Department of Veterans Affairs. VA secretary announces intention to expand mental health care to former service members with other-than-honorable discharges and in crisis. Press Release. March 8, 2017. Accessed August 5, 2024. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=2867
- Smith C. Dramatic increase in mental health services to other-than-honorable discharge veterans. VA News. February 23, 2022. Accessed August 5, 2024. https://news.va.gov/100460/dramatic-increase-in-mental-health-services-to-other-than-honorable-discharge-veterans/
- US Department of Defense. DoD Instruction 1332.14. Enlisted administrative separations. Updated August 1, 2024. Accessed August 5, 2024. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/133214p.pdf
- US Department of Defense. DoD Instruction 1332.30. Commissioned officer administrative separations. Updated September 9, 2021. Accessed August 5, 2024. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/133230p.pdf
- OUTVETS, Legal Services Center of Harvard Law School, Veterans Legal Services. Turned away: how the VA unlawfully denies healthcare to veterans with bad paper discharges. 2020. Accessed August 5, 2024. https://legalservicescenter.org/wp-content/uploads/Turn-Away-Report.pdf
- McClean H. Discharged and discarded: the collateral consequences of a less-than-honorable military discharge. Columbia Law Rev. 2021;121(7):2203-2268.
- Veterans Benefits, General Provisions, Definitions. 38 USC §101(2) (1958). Accessed August 5, 2024. https://uscode.house.gov/view.xhtml?req=granuleid:USC-prelim-title38-section101&num=0&edition=prelim
- Bedford JR. Other than honorable discharges: unfair and unjust life sentences of decreased earning capacity. U Penn J Law Pub Affairs. 2021;6(4):687.
- Karin ML. Other than honorable discrimination. Case Western Reserve Law Rev. 2016;67(1):135-191. https://scholarlycommons.law.case.edu/caselrev/vol67/iss1/9
- Veteran HOUSE Act of 2020, HR 2398, 116th Cong, (2020). Accessed August 5, 2024. https://www.congress.gov/bill/116th-congress/house-bill/2398
- Scapardine D. Leaving other than honorable soldiers behind: how the Departments of Defense and Veterans Affairs inadvertently created a health and social crisis. Md Law Rev. 2017;76(4):1133-1165.
- Elbogen EB, Wagner HR, Brancu M, et al. Psychosocial risk factors and other than honorable military discharge: providing healthcare to previously ineligible veterans. Mil Med. 2018;183(9-10):e532-e538. doi:10.1093/milmed/usx128
- Tsai J, Rosenheck RA. Characteristics and health needs of veterans with other-than-honorable discharges: expanding eligibility in the Veterans Health Administration. Mil Med. 2018;183(5-6):e153-e157. doi:10.1093/milmed/usx110
- Christensen DM, Tsilker Y. Racial disparities in military justice: findings of substantial and persistent racial disparities within the United States military justice system. Accessed August 5, 2024. https://www.protectourdefenders.com/wp-content/uploads/2017/05/Report_20.pdf
- Don’t Ask Don’t Tell, 10 USC §654 (1993) (Repealed 2010). Accessed August 5, 2024. http://www.gpo.gov/fdsys/pkg/USCODE-2010-title10/pdf/USCODE-2010-title10-subtitleA-partII-chap37-sec654.pdf
- Palm Center. The making of a ban: how DTM-19-004 works to push transgender people out of military service. 2019. March 20, 2019. Accessed August 5, 2024. https://www.palmcenter.org/wp-content/uploads/2019/04/The-Making-of-a-Ban.pdf
- Edwards ER, Greene AL, Epshteyn G, Gromatsky M, Kinney AR, Holliday R. Mental health of incarcerated veterans and civilians: latent class analysis of the 2016 Survey of Prison Inmates. Crim Justice Behav. 2022;49(12):1800- 1821. doi:10.1177/00938548221121142
- Brignone E, Fargo JD, Blais RK, Carter ME, Samore MH, Gundlapalli AV. Non-routine discharge from military service: mental illness, substance use disorders, and suicidality. Am J Prev Med. 2017;52(5):557-565. doi:10.1016/j.amepre.2016.11.015
- Gamache G, Rosenheck R, Tessler R. Military discharge status of homeless veterans with mental illness. Mil Med. 2000;165(11):803-808. doi:10.1093/milmed/165.11.803
- Gundlapalli AV, Fargo JD, Metraux S, et al. Military Misconduct and Homelessness Among US Veterans Separated From Active Duty, 2001-2012. JAMA. 2015;314(8):832-834. doi:10.1001/jama.2015.8207
- Brooks Holliday S, Pedersen ER. The association between discharge status, mental health, and substance misuse among young adult veterans. Psychiatry Res. 2017;256:428-434. doi:10.1016/j.psychres.2017.07.011
- Williamson RB. DOD Health: Actions Needed to Ensure Post-Traumatic Stress Disorder and Traumatic Brain Injury are Considered in Misconduct Separations. US Government Accountability Office; 2017. Accessed August 5, 2024. https://apps.dtic.mil/sti/pdfs/AD1168610.pdf
- Maruschak LM, Bronson J, Alper M. Indicators of mental health problems reported by prisoners: survey of prison inmates. US Department of Justice Bureau of Justice Statistics. June 2021. Accessed August 5, 2024. https://bjs.ojp.gov/sites/g/files/xyckuh236/files/media/document/imhprpspi16st.pdf
- Brooke E, Gau J. Military service and lifetime arrests: examining the effects of the total military experience on arrests in a sample of prison inmates. Crim Justice Policy Rev. 2018;29(1):24-44. doi:10.1177/0887403415619007
- Russell RT. Veterans treatment court: a proactive approach. N Engl J Crim Civ Confin. 2009;35:357-372.
- Cartwright T. To care for him who shall have borne the battle: the recent development of Veterans Treatment Courts in America. Stanford Law Pol Rev. 2011;22:295-316.
- Douds AS, Ahlin EM, Howard D, Stigerwalt S. Varieties of veterans’ courts: a statewide assessment of veterans’ treatment court components. Crim Justice Policy Rev. 2017;28:740-769. doi:10.1177/0887403415620633
- Rowen J. Worthy of justice: a veterans treatment court in practice. Law Policy. 2020;42(1):78-100. doi:10.1111/lapo.12142
- Timko C, Flatley B, Tjemsland A, et al. A longitudinal examination of veterans treatment courts’ characteristics and eligibility criteria. Justice Res Policy. 2016;17(2):123-136.
- Baldwin JM. Executive summary: national survey of veterans treatment courts. SSRN. Preprint posted online June 5, 2013. Accessed August 5, 2024. doi:10.2139/ssrn.2274138
- Renz T. Veterans treatment court: a hand up rather than lock up. Richmond Public Interest Law Rev. 2013;17(3):697-705. https://scholarship.richmond.edu/pilr/vol17/iss3/6
- Knudsen KJ, Wingenfeld S. A specialized treatment court for veterans with trauma exposure: implications for the field. Community Ment Health J. 2016;52(2):127-135. doi:10.1007/s10597-015-9845-9
- McCall JD, Tsai J, Gordon AJ. Veterans treatment court research: participant characteristics, outcomes, and gaps in the literature. J Offender Rehabil. 2018;57:384-401. doi:10.1080/10509674.2018.1510864
- Smith JS. The Anchorage, Alaska veterans court and recidivism: July 6, 2004 – December 31, 2010. Alsk Law Rev. 2012;29(1):93-111.
- Hartley RD, Baldwin JM. Waging war on recidivism among justice-involved veterans: an impact evaluation of a large urban veterans treatment court. Crim Justice Policy Rev. 2019;30(1):52-78. doi:10.1177/0887403416650490
- Tsai J, Flatley B, Kasprow WJ, Clark S, Finlay A. Diversion of veterans with criminal justice involvement to treatment courts: participant characteristics and outcomes. Psychiatr Serv. 2017;68(4):375-383. doi:10.1176/appi.ps.201600233
- Edwards ER, Sissoko DR, Abrams D, Samost D, La Gamma S, Geraci J. Connecting mental health court participants with services: process, challenges, and recommendations. Psychol Public Policy Law. 2020;26(4):463-475. doi:10.1037/law0000236
- US Department of Veterans Affairs, VA Office of Mental Health and Suicide Prevention. 2023 National Veteran Suicide Prevention Annual Report. US Department of Veterans Affairs; November 2023. Accessed August 5, 2024. https://www.mentalhealth.va.gov/docs/data-sheets/2023/2023-National-Veteran-Suicide-Prevention-Annual-Report-FINAL-508.pdf
- Finlay AK, Clark S, Blue-Howells J, et al. Logic model of the Department of Veterans Affairs’ role in veterans treatment courts. Drug Court Rev. 2019;2:45-62.
- Finlay AK, Smelson D, Sawh L, et al. U.S. Department of Veterans Affairs veterans justice outreach program: connecting justice-involved veterans with mental health and substance use disorder treatment. Crim Justice Policy Rev. 2016;27(2):10.1177/0887403414562601. doi:10.1177/0887403414562601
- Finlay AK, Stimmel M, Blue-Howells J, et al. Use of Veterans Health Administration mental health and substance use disorder treatment after exiting prison: the health care for reentry veterans program. Adm Policy Ment Health. 2017;44(2):177-187. doi:10.1007/s10488-015-0708-z
- Meyer EG, Writer BW, Brim W. The Importance of Military Cultural Competence. Curr Psychiatry Rep. 2016;18(3):26. doi:10.1007/s11920-016-0662-9
- National Association of Drug Court Professionals. Adult Drug Court Best Practice Standards Volume I. National Association of Drug Court Professionals; 2013. Accessed August 5, 2024. https://allrise.org/publications/standards/
- STRONG Veterans Act of 2022, HR 6411, 117th Cong (2022). https://www.congress.gov/bill/117th-congress/house-bill/6411/text
- Pelletier D, Clark S, Davis L. Veterans reentry search service (VRSS) and the SQUARES application. Presented at: National Association of Drug Court Professionals Conference; August 15-18, 2021; National Harbor, Maryland.
- Scott KM, Lim C, Al-Hamzawi A, et al. Association of Mental Disorders With Subsequent Chronic Physical Conditions: World Mental Health Surveys From 17 Countries. JAMA Psychiatry. 2016;73(2):150-158. doi:10.1001/jamapsychiatry.2015.2688
- Ahmed N, Conway CA. Medical and mental health comorbidities among minority racial/ethnic groups in the United States. J Soc Beh Health Sci. 2020;14(1):153-168. doi:10.5590/JSBHS.2020.14.1.11
- Hanna B, Desai R, Parekh T, Guirguis E, Kumar G, Sachdeva R. Psychiatric disorders in the U.S. transgender population. Ann Epidemiol. 2019;39:1-7.e1. doi:10.1016/j.annepidem.2019.09.009
- Watkins DC, Assari S, Johnson-Lawrence V. Race and ethnic group differences in comorbid major depressive disorder, generalized anxiety disorder, and chronic medical conditions. J Racial Ethn Health Disparities. 2015;2(3):385- 394. doi:10.1007/s40615-015-0085-z
- Baldwin J. Whom do they serve? National examination of veterans treatment court participants and their challenges. Crim Justice Policy Rev. 2017;28(6):515-554. doi:10.1177/0887403415606184
- Beatty LG, Snell TL. Profile of prison inmates, 2016. US Department of Justice Bureau of Justice Statistics. December 2021. Accessed August 5, 2024. https://bjs.ojp.gov/content/pub/pdf/ppi16.pdf
- Al-Rousan T, Rubenstein L, Sieleni B, Deol H, Wallace RB. Inside the nation’s largest mental health institution: a prevalence study in a state prison system. BMC Public Health. 2017;17(1):342. doi:10.1186/s12889-017-4257-0
- Rosen CS, Kaplan AN, Nelson DB, et al. Implementation context and burnout among Department of Veterans Affairs psychotherapists prior to and during the COVID-19 pandemic. J Affect Disord. 2023;320:517-524. doi:10.1016/j.jad.2022.09.141
- Tsai J, Jones N, Klee A, Deegan D. Job burnout among mental health staff at a veterans affairs psychosocial rehabilitation center. Community Ment Health J. 2020;56(2):294- 297. doi:10.1007/s10597-019-00487-5
- US Department of Veterans, Veterans Health Administration. VHA Directive 1601A.02(6): Eligibility Determination. Updated March 6, 2024. Accessed August 8, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8908
- Mental and behavioral health care for certain former members of the Armed Forces. 38 USC §1720I (2018). Accessed August 5, 2024. https://uscode.house.gov/view.xhtml?req=(title:38%20section:1720I%20edition:prelim
- US Department of Veterans, Veterans Health Administration. VHA Directive 1601A.02, Eligibility Determination. June 7, 2017.
- US Department of Veterans, Veterans Health Administration. VHA Directive 1115(1), Military Sexual Trauma (MST) Program. May 8, 2018. Accessed August 5, 2024. https:// www.va.gov/vhapublications/viewpublication.asp?pub_ID=6402
- US Department of Veterans Affairs. Tentative Eligibility Determinations; Presumptive Eligibility for Psychosis and Other Mental Illness. 38 CFR §17.109. May 14, 2013. Accessed August 5, 2024. https://www.federalregister.gov/documents/2013/05/14/2013-11410/tentative-eligibility-determinations-presumptive-eligibility-for-psychosis-and-other-mental-illness
- US Department of Veterans Affairs, VA Office of Mental Health and Suicide Prevention. National strategy for preventing veteran suicide 2018-2028. Published September 2018. Accessed August 5, 2024. https://www.mentalhealth.va.gov/suicide_prevention/docs/Office-of-Mental-Health-and-Suicide-Prevention-National-Strategy-for-Preventing-Veterans-Suicide.pdf
- US Department of Veterans Affairs. VA secretary announces intention to expand mental health care to former service members with other-than-honorable discharges and in crisis. Press Release. March 8, 2017. Accessed August 5, 2024. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=2867
- Smith C. Dramatic increase in mental health services to other-than-honorable discharge veterans. VA News. February 23, 2022. Accessed August 5, 2024. https://news.va.gov/100460/dramatic-increase-in-mental-health-services-to-other-than-honorable-discharge-veterans/
- US Department of Defense. DoD Instruction 1332.14. Enlisted administrative separations. Updated August 1, 2024. Accessed August 5, 2024. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/133214p.pdf
- US Department of Defense. DoD Instruction 1332.30. Commissioned officer administrative separations. Updated September 9, 2021. Accessed August 5, 2024. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/133230p.pdf
- OUTVETS, Legal Services Center of Harvard Law School, Veterans Legal Services. Turned away: how the VA unlawfully denies healthcare to veterans with bad paper discharges. 2020. Accessed August 5, 2024. https://legalservicescenter.org/wp-content/uploads/Turn-Away-Report.pdf
- McClean H. Discharged and discarded: the collateral consequences of a less-than-honorable military discharge. Columbia Law Rev. 2021;121(7):2203-2268.
- Veterans Benefits, General Provisions, Definitions. 38 USC §101(2) (1958). Accessed August 5, 2024. https://uscode.house.gov/view.xhtml?req=granuleid:USC-prelim-title38-section101&num=0&edition=prelim
- Bedford JR. Other than honorable discharges: unfair and unjust life sentences of decreased earning capacity. U Penn J Law Pub Affairs. 2021;6(4):687.
- Karin ML. Other than honorable discrimination. Case Western Reserve Law Rev. 2016;67(1):135-191. https://scholarlycommons.law.case.edu/caselrev/vol67/iss1/9
- Veteran HOUSE Act of 2020, HR 2398, 116th Cong, (2020). Accessed August 5, 2024. https://www.congress.gov/bill/116th-congress/house-bill/2398
- Scapardine D. Leaving other than honorable soldiers behind: how the Departments of Defense and Veterans Affairs inadvertently created a health and social crisis. Md Law Rev. 2017;76(4):1133-1165.
- Elbogen EB, Wagner HR, Brancu M, et al. Psychosocial risk factors and other than honorable military discharge: providing healthcare to previously ineligible veterans. Mil Med. 2018;183(9-10):e532-e538. doi:10.1093/milmed/usx128
- Tsai J, Rosenheck RA. Characteristics and health needs of veterans with other-than-honorable discharges: expanding eligibility in the Veterans Health Administration. Mil Med. 2018;183(5-6):e153-e157. doi:10.1093/milmed/usx110
- Christensen DM, Tsilker Y. Racial disparities in military justice: findings of substantial and persistent racial disparities within the United States military justice system. Accessed August 5, 2024. https://www.protectourdefenders.com/wp-content/uploads/2017/05/Report_20.pdf
- Don’t Ask Don’t Tell, 10 USC §654 (1993) (Repealed 2010). Accessed August 5, 2024. http://www.gpo.gov/fdsys/pkg/USCODE-2010-title10/pdf/USCODE-2010-title10-subtitleA-partII-chap37-sec654.pdf
- Palm Center. The making of a ban: how DTM-19-004 works to push transgender people out of military service. 2019. March 20, 2019. Accessed August 5, 2024. https://www.palmcenter.org/wp-content/uploads/2019/04/The-Making-of-a-Ban.pdf
- Edwards ER, Greene AL, Epshteyn G, Gromatsky M, Kinney AR, Holliday R. Mental health of incarcerated veterans and civilians: latent class analysis of the 2016 Survey of Prison Inmates. Crim Justice Behav. 2022;49(12):1800- 1821. doi:10.1177/00938548221121142
- Brignone E, Fargo JD, Blais RK, Carter ME, Samore MH, Gundlapalli AV. Non-routine discharge from military service: mental illness, substance use disorders, and suicidality. Am J Prev Med. 2017;52(5):557-565. doi:10.1016/j.amepre.2016.11.015
- Gamache G, Rosenheck R, Tessler R. Military discharge status of homeless veterans with mental illness. Mil Med. 2000;165(11):803-808. doi:10.1093/milmed/165.11.803
- Gundlapalli AV, Fargo JD, Metraux S, et al. Military Misconduct and Homelessness Among US Veterans Separated From Active Duty, 2001-2012. JAMA. 2015;314(8):832-834. doi:10.1001/jama.2015.8207
- Brooks Holliday S, Pedersen ER. The association between discharge status, mental health, and substance misuse among young adult veterans. Psychiatry Res. 2017;256:428-434. doi:10.1016/j.psychres.2017.07.011
- Williamson RB. DOD Health: Actions Needed to Ensure Post-Traumatic Stress Disorder and Traumatic Brain Injury are Considered in Misconduct Separations. US Government Accountability Office; 2017. Accessed August 5, 2024. https://apps.dtic.mil/sti/pdfs/AD1168610.pdf
- Maruschak LM, Bronson J, Alper M. Indicators of mental health problems reported by prisoners: survey of prison inmates. US Department of Justice Bureau of Justice Statistics. June 2021. Accessed August 5, 2024. https://bjs.ojp.gov/sites/g/files/xyckuh236/files/media/document/imhprpspi16st.pdf
- Brooke E, Gau J. Military service and lifetime arrests: examining the effects of the total military experience on arrests in a sample of prison inmates. Crim Justice Policy Rev. 2018;29(1):24-44. doi:10.1177/0887403415619007
- Russell RT. Veterans treatment court: a proactive approach. N Engl J Crim Civ Confin. 2009;35:357-372.
- Cartwright T. To care for him who shall have borne the battle: the recent development of Veterans Treatment Courts in America. Stanford Law Pol Rev. 2011;22:295-316.
- Douds AS, Ahlin EM, Howard D, Stigerwalt S. Varieties of veterans’ courts: a statewide assessment of veterans’ treatment court components. Crim Justice Policy Rev. 2017;28:740-769. doi:10.1177/0887403415620633
- Rowen J. Worthy of justice: a veterans treatment court in practice. Law Policy. 2020;42(1):78-100. doi:10.1111/lapo.12142
- Timko C, Flatley B, Tjemsland A, et al. A longitudinal examination of veterans treatment courts’ characteristics and eligibility criteria. Justice Res Policy. 2016;17(2):123-136.
- Baldwin JM. Executive summary: national survey of veterans treatment courts. SSRN. Preprint posted online June 5, 2013. Accessed August 5, 2024. doi:10.2139/ssrn.2274138
- Renz T. Veterans treatment court: a hand up rather than lock up. Richmond Public Interest Law Rev. 2013;17(3):697-705. https://scholarship.richmond.edu/pilr/vol17/iss3/6
- Knudsen KJ, Wingenfeld S. A specialized treatment court for veterans with trauma exposure: implications for the field. Community Ment Health J. 2016;52(2):127-135. doi:10.1007/s10597-015-9845-9
- McCall JD, Tsai J, Gordon AJ. Veterans treatment court research: participant characteristics, outcomes, and gaps in the literature. J Offender Rehabil. 2018;57:384-401. doi:10.1080/10509674.2018.1510864
- Smith JS. The Anchorage, Alaska veterans court and recidivism: July 6, 2004 – December 31, 2010. Alsk Law Rev. 2012;29(1):93-111.
- Hartley RD, Baldwin JM. Waging war on recidivism among justice-involved veterans: an impact evaluation of a large urban veterans treatment court. Crim Justice Policy Rev. 2019;30(1):52-78. doi:10.1177/0887403416650490
- Tsai J, Flatley B, Kasprow WJ, Clark S, Finlay A. Diversion of veterans with criminal justice involvement to treatment courts: participant characteristics and outcomes. Psychiatr Serv. 2017;68(4):375-383. doi:10.1176/appi.ps.201600233
- Edwards ER, Sissoko DR, Abrams D, Samost D, La Gamma S, Geraci J. Connecting mental health court participants with services: process, challenges, and recommendations. Psychol Public Policy Law. 2020;26(4):463-475. doi:10.1037/law0000236
- US Department of Veterans Affairs, VA Office of Mental Health and Suicide Prevention. 2023 National Veteran Suicide Prevention Annual Report. US Department of Veterans Affairs; November 2023. Accessed August 5, 2024. https://www.mentalhealth.va.gov/docs/data-sheets/2023/2023-National-Veteran-Suicide-Prevention-Annual-Report-FINAL-508.pdf
- Finlay AK, Clark S, Blue-Howells J, et al. Logic model of the Department of Veterans Affairs’ role in veterans treatment courts. Drug Court Rev. 2019;2:45-62.
- Finlay AK, Smelson D, Sawh L, et al. U.S. Department of Veterans Affairs veterans justice outreach program: connecting justice-involved veterans with mental health and substance use disorder treatment. Crim Justice Policy Rev. 2016;27(2):10.1177/0887403414562601. doi:10.1177/0887403414562601
- Finlay AK, Stimmel M, Blue-Howells J, et al. Use of Veterans Health Administration mental health and substance use disorder treatment after exiting prison: the health care for reentry veterans program. Adm Policy Ment Health. 2017;44(2):177-187. doi:10.1007/s10488-015-0708-z
- Meyer EG, Writer BW, Brim W. The Importance of Military Cultural Competence. Curr Psychiatry Rep. 2016;18(3):26. doi:10.1007/s11920-016-0662-9
- National Association of Drug Court Professionals. Adult Drug Court Best Practice Standards Volume I. National Association of Drug Court Professionals; 2013. Accessed August 5, 2024. https://allrise.org/publications/standards/
- STRONG Veterans Act of 2022, HR 6411, 117th Cong (2022). https://www.congress.gov/bill/117th-congress/house-bill/6411/text
- Pelletier D, Clark S, Davis L. Veterans reentry search service (VRSS) and the SQUARES application. Presented at: National Association of Drug Court Professionals Conference; August 15-18, 2021; National Harbor, Maryland.
- Scott KM, Lim C, Al-Hamzawi A, et al. Association of Mental Disorders With Subsequent Chronic Physical Conditions: World Mental Health Surveys From 17 Countries. JAMA Psychiatry. 2016;73(2):150-158. doi:10.1001/jamapsychiatry.2015.2688
- Ahmed N, Conway CA. Medical and mental health comorbidities among minority racial/ethnic groups in the United States. J Soc Beh Health Sci. 2020;14(1):153-168. doi:10.5590/JSBHS.2020.14.1.11
- Hanna B, Desai R, Parekh T, Guirguis E, Kumar G, Sachdeva R. Psychiatric disorders in the U.S. transgender population. Ann Epidemiol. 2019;39:1-7.e1. doi:10.1016/j.annepidem.2019.09.009
- Watkins DC, Assari S, Johnson-Lawrence V. Race and ethnic group differences in comorbid major depressive disorder, generalized anxiety disorder, and chronic medical conditions. J Racial Ethn Health Disparities. 2015;2(3):385- 394. doi:10.1007/s40615-015-0085-z
- Baldwin J. Whom do they serve? National examination of veterans treatment court participants and their challenges. Crim Justice Policy Rev. 2017;28(6):515-554. doi:10.1177/0887403415606184
- Beatty LG, Snell TL. Profile of prison inmates, 2016. US Department of Justice Bureau of Justice Statistics. December 2021. Accessed August 5, 2024. https://bjs.ojp.gov/content/pub/pdf/ppi16.pdf
- Al-Rousan T, Rubenstein L, Sieleni B, Deol H, Wallace RB. Inside the nation’s largest mental health institution: a prevalence study in a state prison system. BMC Public Health. 2017;17(1):342. doi:10.1186/s12889-017-4257-0
- Rosen CS, Kaplan AN, Nelson DB, et al. Implementation context and burnout among Department of Veterans Affairs psychotherapists prior to and during the COVID-19 pandemic. J Affect Disord. 2023;320:517-524. doi:10.1016/j.jad.2022.09.141
- Tsai J, Jones N, Klee A, Deegan D. Job burnout among mental health staff at a veterans affairs psychosocial rehabilitation center. Community Ment Health J. 2020;56(2):294- 297. doi:10.1007/s10597-019-00487-5