User login
‘Impressive’ SOLO3 results should influence practice
In this edition of “Applying research to practice,” I highlight a study suggesting olaparib is helpful in patients BRCA mutations experiencing multiple relapses of ovarian cancer.
SOLO3 was the first phase 3 trial comparing the oral PARP inhibitor olaparib (OLA; 300 mg twice daily) with physician’s choice of intravenous single-agent chemotherapy (TPC) in relapsed high-grade serous or endometroid ovarian, fallopian tube, or primary peritoneal cancer (J Clin Oncol. 2020 Feb 19. doi: 10.1200/JCO.19.02745).
The trial involved 266 BRCA-mutated patients who had received two (approximately 50%) or more lines of platinum-based TPC. All patients were required to be completely platinum sensitive (progression beyond 12 months from last platinum exposure) or partially platinum sensitive (progression within 6-12 months).
Women were randomized to receive either OLA or nonplatinum TPC (pegylated liposomal doxorubicin, paclitaxel, gemcitabine, or topotecan). After an amendment to the study in 2017, the primary endpoint was objective response rate, determined by blinded independent central review, with a variety of secondary endpoints.
Among 223 patients with measurable disease, the objective response rate was 72.2% with OLA and 51.4% with TPC (odds ratio, 2.53; P = .002). Across all patients, the median progression-free survival was significantly better with OLA (13.4 months) than with TPC (9.2 months; P = .013). Overall survival data were immature.
The superiority of OLA for the primary endpoint was maintained in multiple subgroups of patients, including those who had received only two prior lines of therapy (OR, 3.44) and those who had three or more prior lines (OR, 2.21). Time to first subsequent therapy (HR, 0.48) and time to treatment discontinuation or death (HR, 0.17) were significantly longer for OLA than for TPC.
Adverse events were consistent with the established safety profiles of OLA and chemotherapy. The most common grade 3 or higher adverse events were anemia (21.3%) with OLA and neutropenia (15.8%) and hand-foot syndrome (11.8%) with TPC.
However, median treatment durations were substantially and consistently longer for OLA than for TPC, and there were fewer treatment discontinuations because of toxicity for OLA than for TPC. At the time of data cutoff, 43 patients in the OLA group and 1 patient in the TPC cohort remained on treatment.
How these results influence practice
The results of the SOLO3 trial are clear: Treatment with OLA is a reasonable alternative to nonplatinum-containing chemotherapy for women with BRCA mutations and platinum-sensitive ovarian cancer. OLA is a “chemotherapy-free” option for these patients in the second- and later-line settings.
Less clear are the following:
- How many patients with BRCA mutations will not have already received a PARP inhibitor in the frontline maintenance setting in the future? SOLO3 required modification in the accrual target and endpoint because of challenges in patient recruitment from the entry of PARP inhibitors into routine clinical practice.
- Would OLA be superior to a carboplatin doublet rather than a nonplatinum single agent in patients with two prior relapses of platinum-sensitive ovarian cancer? Standard practice would be for patients in the second-line setting to receive a platinum doublet.
- Is extending the platinum-free interval a worthwhile objective, or would some patients prefer a finite interval of a platinum doublet over an indefinite period of treatment with OLA?
All phase 3 clinical trials have limitations since they require years to complete and the applicability of the results are challenged by intercurrent advances in treatment options and diagnostic tests.
However, overall, the results of SOLO3 are impressive and should influence clinical practice for the subset of relapsed ovarian cancer patients who would have qualified to participate in it. OLA represents an important treatment advance for a group of patients who are trying to string together remission after remission, with limited negative impact on quality of life.
Dr. Lyss was an oncologist and researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
In this edition of “Applying research to practice,” I highlight a study suggesting olaparib is helpful in patients BRCA mutations experiencing multiple relapses of ovarian cancer.
SOLO3 was the first phase 3 trial comparing the oral PARP inhibitor olaparib (OLA; 300 mg twice daily) with physician’s choice of intravenous single-agent chemotherapy (TPC) in relapsed high-grade serous or endometroid ovarian, fallopian tube, or primary peritoneal cancer (J Clin Oncol. 2020 Feb 19. doi: 10.1200/JCO.19.02745).
The trial involved 266 BRCA-mutated patients who had received two (approximately 50%) or more lines of platinum-based TPC. All patients were required to be completely platinum sensitive (progression beyond 12 months from last platinum exposure) or partially platinum sensitive (progression within 6-12 months).
Women were randomized to receive either OLA or nonplatinum TPC (pegylated liposomal doxorubicin, paclitaxel, gemcitabine, or topotecan). After an amendment to the study in 2017, the primary endpoint was objective response rate, determined by blinded independent central review, with a variety of secondary endpoints.
Among 223 patients with measurable disease, the objective response rate was 72.2% with OLA and 51.4% with TPC (odds ratio, 2.53; P = .002). Across all patients, the median progression-free survival was significantly better with OLA (13.4 months) than with TPC (9.2 months; P = .013). Overall survival data were immature.
The superiority of OLA for the primary endpoint was maintained in multiple subgroups of patients, including those who had received only two prior lines of therapy (OR, 3.44) and those who had three or more prior lines (OR, 2.21). Time to first subsequent therapy (HR, 0.48) and time to treatment discontinuation or death (HR, 0.17) were significantly longer for OLA than for TPC.
Adverse events were consistent with the established safety profiles of OLA and chemotherapy. The most common grade 3 or higher adverse events were anemia (21.3%) with OLA and neutropenia (15.8%) and hand-foot syndrome (11.8%) with TPC.
However, median treatment durations were substantially and consistently longer for OLA than for TPC, and there were fewer treatment discontinuations because of toxicity for OLA than for TPC. At the time of data cutoff, 43 patients in the OLA group and 1 patient in the TPC cohort remained on treatment.
How these results influence practice
The results of the SOLO3 trial are clear: Treatment with OLA is a reasonable alternative to nonplatinum-containing chemotherapy for women with BRCA mutations and platinum-sensitive ovarian cancer. OLA is a “chemotherapy-free” option for these patients in the second- and later-line settings.
Less clear are the following:
- How many patients with BRCA mutations will not have already received a PARP inhibitor in the frontline maintenance setting in the future? SOLO3 required modification in the accrual target and endpoint because of challenges in patient recruitment from the entry of PARP inhibitors into routine clinical practice.
- Would OLA be superior to a carboplatin doublet rather than a nonplatinum single agent in patients with two prior relapses of platinum-sensitive ovarian cancer? Standard practice would be for patients in the second-line setting to receive a platinum doublet.
- Is extending the platinum-free interval a worthwhile objective, or would some patients prefer a finite interval of a platinum doublet over an indefinite period of treatment with OLA?
All phase 3 clinical trials have limitations since they require years to complete and the applicability of the results are challenged by intercurrent advances in treatment options and diagnostic tests.
However, overall, the results of SOLO3 are impressive and should influence clinical practice for the subset of relapsed ovarian cancer patients who would have qualified to participate in it. OLA represents an important treatment advance for a group of patients who are trying to string together remission after remission, with limited negative impact on quality of life.
Dr. Lyss was an oncologist and researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
In this edition of “Applying research to practice,” I highlight a study suggesting olaparib is helpful in patients BRCA mutations experiencing multiple relapses of ovarian cancer.
SOLO3 was the first phase 3 trial comparing the oral PARP inhibitor olaparib (OLA; 300 mg twice daily) with physician’s choice of intravenous single-agent chemotherapy (TPC) in relapsed high-grade serous or endometroid ovarian, fallopian tube, or primary peritoneal cancer (J Clin Oncol. 2020 Feb 19. doi: 10.1200/JCO.19.02745).
The trial involved 266 BRCA-mutated patients who had received two (approximately 50%) or more lines of platinum-based TPC. All patients were required to be completely platinum sensitive (progression beyond 12 months from last platinum exposure) or partially platinum sensitive (progression within 6-12 months).
Women were randomized to receive either OLA or nonplatinum TPC (pegylated liposomal doxorubicin, paclitaxel, gemcitabine, or topotecan). After an amendment to the study in 2017, the primary endpoint was objective response rate, determined by blinded independent central review, with a variety of secondary endpoints.
Among 223 patients with measurable disease, the objective response rate was 72.2% with OLA and 51.4% with TPC (odds ratio, 2.53; P = .002). Across all patients, the median progression-free survival was significantly better with OLA (13.4 months) than with TPC (9.2 months; P = .013). Overall survival data were immature.
The superiority of OLA for the primary endpoint was maintained in multiple subgroups of patients, including those who had received only two prior lines of therapy (OR, 3.44) and those who had three or more prior lines (OR, 2.21). Time to first subsequent therapy (HR, 0.48) and time to treatment discontinuation or death (HR, 0.17) were significantly longer for OLA than for TPC.
Adverse events were consistent with the established safety profiles of OLA and chemotherapy. The most common grade 3 or higher adverse events were anemia (21.3%) with OLA and neutropenia (15.8%) and hand-foot syndrome (11.8%) with TPC.
However, median treatment durations were substantially and consistently longer for OLA than for TPC, and there were fewer treatment discontinuations because of toxicity for OLA than for TPC. At the time of data cutoff, 43 patients in the OLA group and 1 patient in the TPC cohort remained on treatment.
How these results influence practice
The results of the SOLO3 trial are clear: Treatment with OLA is a reasonable alternative to nonplatinum-containing chemotherapy for women with BRCA mutations and platinum-sensitive ovarian cancer. OLA is a “chemotherapy-free” option for these patients in the second- and later-line settings.
Less clear are the following:
- How many patients with BRCA mutations will not have already received a PARP inhibitor in the frontline maintenance setting in the future? SOLO3 required modification in the accrual target and endpoint because of challenges in patient recruitment from the entry of PARP inhibitors into routine clinical practice.
- Would OLA be superior to a carboplatin doublet rather than a nonplatinum single agent in patients with two prior relapses of platinum-sensitive ovarian cancer? Standard practice would be for patients in the second-line setting to receive a platinum doublet.
- Is extending the platinum-free interval a worthwhile objective, or would some patients prefer a finite interval of a platinum doublet over an indefinite period of treatment with OLA?
All phase 3 clinical trials have limitations since they require years to complete and the applicability of the results are challenged by intercurrent advances in treatment options and diagnostic tests.
However, overall, the results of SOLO3 are impressive and should influence clinical practice for the subset of relapsed ovarian cancer patients who would have qualified to participate in it. OLA represents an important treatment advance for a group of patients who are trying to string together remission after remission, with limited negative impact on quality of life.
Dr. Lyss was an oncologist and researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
The power and promise of person-generated health data (Part II)
In Part I of our discussion we introduced the concept of person-generated health data (PGHD), defined as wellness and/or health-related data created, recorded, or gathered by individuals.
Such rich, longitudinal information is now being used in combination with traditional clinical information to predict, diagnose, and formulate treatment plans for diseases, as well as understand the safety and effectiveness of medical interventions.
Identifying a disease early
One novel example of digital technologies being used for early identification of disease was a promising 2019 study by Eli Lilly (in collaboration with Apple and Evidation Health) called the Lilly Exploratory Digital Assessment Study.
In this study, the feasibility of using PGHD for identifying physiological and behavioral signatures of cognitive impairment was examined for the purpose of seeking new methods to detect mild cognitive impairment (MCI) in a timely and cost-effective manner. The study enrolled 31 study participants with cognitive impairment and 82 without cognitive impairment. It used consumer-grade sensor technologies (the iPhone, Apple Watch, iPad, and Beddit sleep monitor) to continuously and unobtrusively collect data. Among the information the researchers collected were interaction with the phone keyboard, accelerometer data from the Apple Watch, volume of messages sent/received, and sleep cycles.1
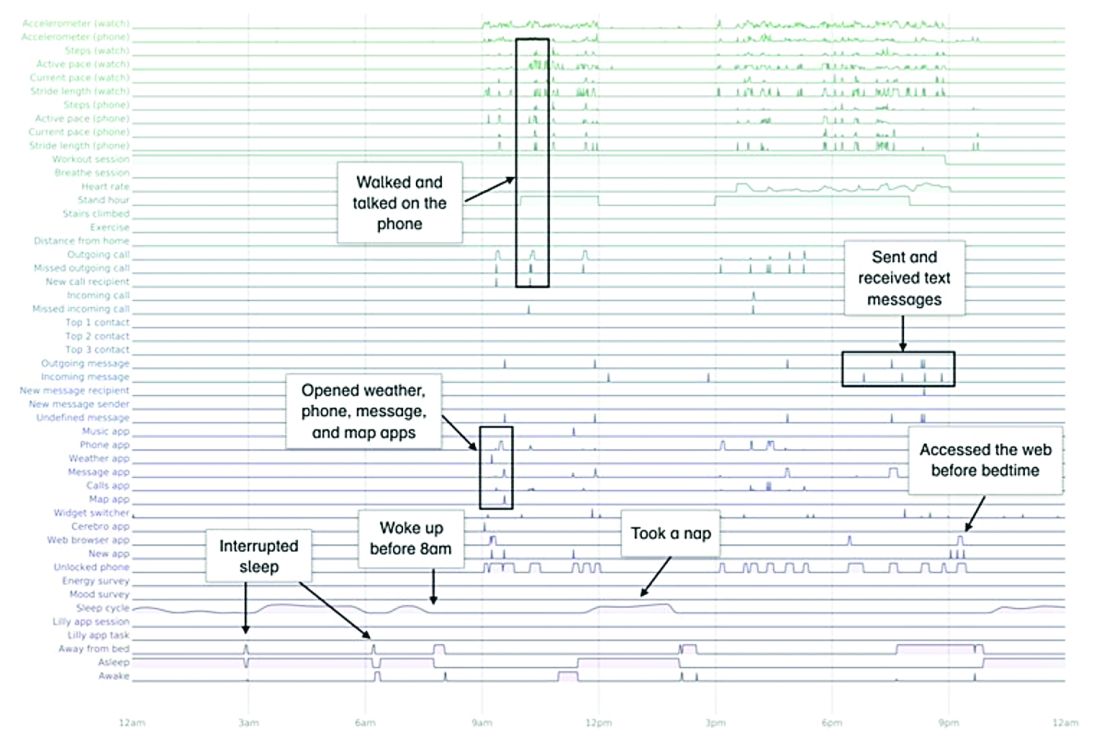
A total of 16 terabytes of data were collected over the course of 12 weeks. Data were organized into a behaviorgram (See Figure 1) that gives a holistic picture of a day in a patient’s life. A machine learning model was used to distinguish between behaviorgrams of symptomatic versus healthy controls, identifying typing speed, circadian rhythm shifts, and reliance on helper apps, among other things, as differentiating cognitively impaired from healthy controls. These behaviorgrams may someday serve as “fingerprints” of different diseases, with specific diseases displaying predictable patterns. In the near future, digital measures like the ones investigated in this study are likely to be used to help clinicians predict and diagnose disease, as well as to better understand disease progression and treatment response.
Leading to better health outcomes
The potential of PGHD to detect diseases early and lead to better health outcomes is being investigated in the Heartline study, a collaboration between Johnson & Johnson and Apple, which is supported by Evidation.2
This study aims to enroll 150,000 adults age 65 years and over to analyze the impact of Apple Watch–based early detection of irregular heart rhythms consistent with atrial fibrillation (AFib). The researchers’ hypothesis is that jointly detecting atrial fibrillation early and providing cardiovascular health programs to new AFib patients, will lead to patients being treated by a medical provider for AFib that otherwise would not have been detected. This, in turn, would lead to these AFib patients decreasing their risks of stroke and other serious cardiovascular events, including death, the study authors speculated.
Presenting new challenges
While PGHD has the potential to help people, it also presents new challenges. It is highly sensitive and personal – it can be as identifying as DNA.3
The vast amount of data that PGHD can collect from interaction with consumer wearable devices poses serious privacy risks if done improperly. To address those risks, companies like Evidation have built in protections. Evidation has an app, Achievement, that has enlisted a connected population of more than 3.5 million members who earn rewards for performing health-related actions, as tracked by wearables devices and apps. Through the Achievement app (See Figure 2.), members are provided opportunities to join research studies. As part of these studies, data collected from sensors and apps is used by permission of the member so that it is clear how their data are contributing to specific research questions or use cases.
This is a collaborative model of data collection built upon trust and permission and is substantially different than the collection of data from electronic health records (EHRs) – which is typically aggregated, deidentified, and commercialized, often without the patients’ knowledge or consent. Stringent protections, explicit permission, and transparency are absolutely imperative until privacy frameworks for data outside of HIPAA regulation catches up and protects patients from discrimination and unintended uses of their data.
Large connected cohorts can help advance our understanding of public health. In one study run on Achievement during the 2017-2018 flu season, a survey was sent to the Achievement population every week asking about symptoms of influenza-like illness and requesting permission to access historical data from their wearable around the influenza-like illness event.4 With the data, it was possible to analyze patterns of activity, sleep, and resting heart rate change around flu events. Resting heart rate, in particular, is shown to increase during fever and at the population level. In fact, through the use of PGHD, it is possible to use the fraction of people with resting heart rate above their usual baseline as a proxy to quantify the number of infected people in a region.5 This resting heart rate–informed flu surveillance method, if refined to increased accuracy, can work in near real time. This means it may be able detect influenza outbreaks days earlier than current epidemiological methods.
Health data generated by connected populations are in the early stages of development. It is clear that it will yield novel insights into health and disease. Only time will tell if it will be able to help clinicians and patients better predict, diagnose, and formulate treatment plans for disease.
Neil Skolnik, M.D. is a professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, and associate director of the Family Medicine Residency Program at Abington Jefferson Health. Luca Foschini PhD, is co-founder & chief data scientist at Evidation Health. Bray Patrick-Lake, MFS, is a patient thought leader and director of strategic partnerships at Evidation Health.
References
1. Chen R et al. Developing measures of cognitive impairment in the real world from consumer-grade multimodal sensor streams. KDD ’19. August 4–8, 2019 Aug 4-8.
2. The Heartline Study. https://www.heartline.com.
3. Foschini L. Privacy of Wearable and Sensors Data (or, the Lack Thereof?). Data Driven Investor, Medium. 2019.
4. Bradshaw B et al. Influenza surveillance using wearable mobile health devices. Online J Public Health Inform. 2019;11(1):e249.
5. Radin JM et al. Harnessing wearable device data to improve state-level real-time surveillance of influenza-like illness in the USA: a population-based study. Lancet Digital Health. 2020. doi: 10.1016/S2589-7500(19)30222-5.
In Part I of our discussion we introduced the concept of person-generated health data (PGHD), defined as wellness and/or health-related data created, recorded, or gathered by individuals.
Such rich, longitudinal information is now being used in combination with traditional clinical information to predict, diagnose, and formulate treatment plans for diseases, as well as understand the safety and effectiveness of medical interventions.
Identifying a disease early
One novel example of digital technologies being used for early identification of disease was a promising 2019 study by Eli Lilly (in collaboration with Apple and Evidation Health) called the Lilly Exploratory Digital Assessment Study.
In this study, the feasibility of using PGHD for identifying physiological and behavioral signatures of cognitive impairment was examined for the purpose of seeking new methods to detect mild cognitive impairment (MCI) in a timely and cost-effective manner. The study enrolled 31 study participants with cognitive impairment and 82 without cognitive impairment. It used consumer-grade sensor technologies (the iPhone, Apple Watch, iPad, and Beddit sleep monitor) to continuously and unobtrusively collect data. Among the information the researchers collected were interaction with the phone keyboard, accelerometer data from the Apple Watch, volume of messages sent/received, and sleep cycles.1

A total of 16 terabytes of data were collected over the course of 12 weeks. Data were organized into a behaviorgram (See Figure 1) that gives a holistic picture of a day in a patient’s life. A machine learning model was used to distinguish between behaviorgrams of symptomatic versus healthy controls, identifying typing speed, circadian rhythm shifts, and reliance on helper apps, among other things, as differentiating cognitively impaired from healthy controls. These behaviorgrams may someday serve as “fingerprints” of different diseases, with specific diseases displaying predictable patterns. In the near future, digital measures like the ones investigated in this study are likely to be used to help clinicians predict and diagnose disease, as well as to better understand disease progression and treatment response.
Leading to better health outcomes
The potential of PGHD to detect diseases early and lead to better health outcomes is being investigated in the Heartline study, a collaboration between Johnson & Johnson and Apple, which is supported by Evidation.2
This study aims to enroll 150,000 adults age 65 years and over to analyze the impact of Apple Watch–based early detection of irregular heart rhythms consistent with atrial fibrillation (AFib). The researchers’ hypothesis is that jointly detecting atrial fibrillation early and providing cardiovascular health programs to new AFib patients, will lead to patients being treated by a medical provider for AFib that otherwise would not have been detected. This, in turn, would lead to these AFib patients decreasing their risks of stroke and other serious cardiovascular events, including death, the study authors speculated.
Presenting new challenges
While PGHD has the potential to help people, it also presents new challenges. It is highly sensitive and personal – it can be as identifying as DNA.3
The vast amount of data that PGHD can collect from interaction with consumer wearable devices poses serious privacy risks if done improperly. To address those risks, companies like Evidation have built in protections. Evidation has an app, Achievement, that has enlisted a connected population of more than 3.5 million members who earn rewards for performing health-related actions, as tracked by wearables devices and apps. Through the Achievement app (See Figure 2.), members are provided opportunities to join research studies. As part of these studies, data collected from sensors and apps is used by permission of the member so that it is clear how their data are contributing to specific research questions or use cases.
This is a collaborative model of data collection built upon trust and permission and is substantially different than the collection of data from electronic health records (EHRs) – which is typically aggregated, deidentified, and commercialized, often without the patients’ knowledge or consent. Stringent protections, explicit permission, and transparency are absolutely imperative until privacy frameworks for data outside of HIPAA regulation catches up and protects patients from discrimination and unintended uses of their data.
Large connected cohorts can help advance our understanding of public health. In one study run on Achievement during the 2017-2018 flu season, a survey was sent to the Achievement population every week asking about symptoms of influenza-like illness and requesting permission to access historical data from their wearable around the influenza-like illness event.4 With the data, it was possible to analyze patterns of activity, sleep, and resting heart rate change around flu events. Resting heart rate, in particular, is shown to increase during fever and at the population level. In fact, through the use of PGHD, it is possible to use the fraction of people with resting heart rate above their usual baseline as a proxy to quantify the number of infected people in a region.5 This resting heart rate–informed flu surveillance method, if refined to increased accuracy, can work in near real time. This means it may be able detect influenza outbreaks days earlier than current epidemiological methods.
Health data generated by connected populations are in the early stages of development. It is clear that it will yield novel insights into health and disease. Only time will tell if it will be able to help clinicians and patients better predict, diagnose, and formulate treatment plans for disease.
Neil Skolnik, M.D. is a professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, and associate director of the Family Medicine Residency Program at Abington Jefferson Health. Luca Foschini PhD, is co-founder & chief data scientist at Evidation Health. Bray Patrick-Lake, MFS, is a patient thought leader and director of strategic partnerships at Evidation Health.
References
1. Chen R et al. Developing measures of cognitive impairment in the real world from consumer-grade multimodal sensor streams. KDD ’19. August 4–8, 2019 Aug 4-8.
2. The Heartline Study. https://www.heartline.com.
3. Foschini L. Privacy of Wearable and Sensors Data (or, the Lack Thereof?). Data Driven Investor, Medium. 2019.
4. Bradshaw B et al. Influenza surveillance using wearable mobile health devices. Online J Public Health Inform. 2019;11(1):e249.
5. Radin JM et al. Harnessing wearable device data to improve state-level real-time surveillance of influenza-like illness in the USA: a population-based study. Lancet Digital Health. 2020. doi: 10.1016/S2589-7500(19)30222-5.
In Part I of our discussion we introduced the concept of person-generated health data (PGHD), defined as wellness and/or health-related data created, recorded, or gathered by individuals.
Such rich, longitudinal information is now being used in combination with traditional clinical information to predict, diagnose, and formulate treatment plans for diseases, as well as understand the safety and effectiveness of medical interventions.
Identifying a disease early
One novel example of digital technologies being used for early identification of disease was a promising 2019 study by Eli Lilly (in collaboration with Apple and Evidation Health) called the Lilly Exploratory Digital Assessment Study.
In this study, the feasibility of using PGHD for identifying physiological and behavioral signatures of cognitive impairment was examined for the purpose of seeking new methods to detect mild cognitive impairment (MCI) in a timely and cost-effective manner. The study enrolled 31 study participants with cognitive impairment and 82 without cognitive impairment. It used consumer-grade sensor technologies (the iPhone, Apple Watch, iPad, and Beddit sleep monitor) to continuously and unobtrusively collect data. Among the information the researchers collected were interaction with the phone keyboard, accelerometer data from the Apple Watch, volume of messages sent/received, and sleep cycles.1

A total of 16 terabytes of data were collected over the course of 12 weeks. Data were organized into a behaviorgram (See Figure 1) that gives a holistic picture of a day in a patient’s life. A machine learning model was used to distinguish between behaviorgrams of symptomatic versus healthy controls, identifying typing speed, circadian rhythm shifts, and reliance on helper apps, among other things, as differentiating cognitively impaired from healthy controls. These behaviorgrams may someday serve as “fingerprints” of different diseases, with specific diseases displaying predictable patterns. In the near future, digital measures like the ones investigated in this study are likely to be used to help clinicians predict and diagnose disease, as well as to better understand disease progression and treatment response.
Leading to better health outcomes
The potential of PGHD to detect diseases early and lead to better health outcomes is being investigated in the Heartline study, a collaboration between Johnson & Johnson and Apple, which is supported by Evidation.2
This study aims to enroll 150,000 adults age 65 years and over to analyze the impact of Apple Watch–based early detection of irregular heart rhythms consistent with atrial fibrillation (AFib). The researchers’ hypothesis is that jointly detecting atrial fibrillation early and providing cardiovascular health programs to new AFib patients, will lead to patients being treated by a medical provider for AFib that otherwise would not have been detected. This, in turn, would lead to these AFib patients decreasing their risks of stroke and other serious cardiovascular events, including death, the study authors speculated.
Presenting new challenges
While PGHD has the potential to help people, it also presents new challenges. It is highly sensitive and personal – it can be as identifying as DNA.3
The vast amount of data that PGHD can collect from interaction with consumer wearable devices poses serious privacy risks if done improperly. To address those risks, companies like Evidation have built in protections. Evidation has an app, Achievement, that has enlisted a connected population of more than 3.5 million members who earn rewards for performing health-related actions, as tracked by wearables devices and apps. Through the Achievement app (See Figure 2.), members are provided opportunities to join research studies. As part of these studies, data collected from sensors and apps is used by permission of the member so that it is clear how their data are contributing to specific research questions or use cases.
This is a collaborative model of data collection built upon trust and permission and is substantially different than the collection of data from electronic health records (EHRs) – which is typically aggregated, deidentified, and commercialized, often without the patients’ knowledge or consent. Stringent protections, explicit permission, and transparency are absolutely imperative until privacy frameworks for data outside of HIPAA regulation catches up and protects patients from discrimination and unintended uses of their data.
Large connected cohorts can help advance our understanding of public health. In one study run on Achievement during the 2017-2018 flu season, a survey was sent to the Achievement population every week asking about symptoms of influenza-like illness and requesting permission to access historical data from their wearable around the influenza-like illness event.4 With the data, it was possible to analyze patterns of activity, sleep, and resting heart rate change around flu events. Resting heart rate, in particular, is shown to increase during fever and at the population level. In fact, through the use of PGHD, it is possible to use the fraction of people with resting heart rate above their usual baseline as a proxy to quantify the number of infected people in a region.5 This resting heart rate–informed flu surveillance method, if refined to increased accuracy, can work in near real time. This means it may be able detect influenza outbreaks days earlier than current epidemiological methods.
Health data generated by connected populations are in the early stages of development. It is clear that it will yield novel insights into health and disease. Only time will tell if it will be able to help clinicians and patients better predict, diagnose, and formulate treatment plans for disease.
Neil Skolnik, M.D. is a professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, and associate director of the Family Medicine Residency Program at Abington Jefferson Health. Luca Foschini PhD, is co-founder & chief data scientist at Evidation Health. Bray Patrick-Lake, MFS, is a patient thought leader and director of strategic partnerships at Evidation Health.
References
1. Chen R et al. Developing measures of cognitive impairment in the real world from consumer-grade multimodal sensor streams. KDD ’19. August 4–8, 2019 Aug 4-8.
2. The Heartline Study. https://www.heartline.com.
3. Foschini L. Privacy of Wearable and Sensors Data (or, the Lack Thereof?). Data Driven Investor, Medium. 2019.
4. Bradshaw B et al. Influenza surveillance using wearable mobile health devices. Online J Public Health Inform. 2019;11(1):e249.
5. Radin JM et al. Harnessing wearable device data to improve state-level real-time surveillance of influenza-like illness in the USA: a population-based study. Lancet Digital Health. 2020. doi: 10.1016/S2589-7500(19)30222-5.
Novel drugs approved in 2019
In 2019, the Food and Drug Administration approved 42 drugs, 6 of which will not be discussed here because of space limitations: recarbrio, a three-drug combination, containing imipenem, cilastatin, and relebactam; polatuzumab (Polivy) combined with bendamustine and a rituximab product; pretomanid combined with bedaquiline and linezolid; romosozumab (Evenity) for postmenopausal women; and alpelisib (Piqray) for postmenopausal women. In addition, darolutamide (Nubeqa) will not be included because it is indicated for the treatment of patients with prostate cancer. The remaining 36 agents are listed alphabetically below with the trade names in parentheses.
The molecular weights (if available), rounded to the nearest whole number, are shown in parentheses.
Air polymer-type a intrauterine foam (ExEm Foam), an ultrasound contrast agent, is indicated for sonohysterosalpingography to assess fallopian tube patency in women with known or suspected infertility. Animal studies have not been conducted, and the agent is contraindicated in pregnancy.
Afamelanotide implant (Scenesse) (1,647) is a melanocortin 1 receptor agonist that is indicated to increase pain-free light exposure in adult patients with a history of phototoxic reactions from erythropoietic protoporphyria. The drug caused no embryofetal toxicity in two species of rats. The molecular weight suggests that it will not cross the placenta, at least early in pregnancy.
Alpelisib (Piqray) (441) is a kinase inhibitor that is combined with fulvestrant for the treatment of advanced breast cancer in women and men. The molecular weight suggests that it can cross the human placenta. It is contraindicated in pregnancy because it can cause embryofetal toxicity.
Bremelanotide (Vyleesi) (1,025) is indicated for the treatment of premenopausal women with hypoactive sexual disorder. The drug caused fetal harm in dogs and mice. If a pregnant woman is exposed to the drug, health care providers are encouraged to call the VYLEESI Pregnancy Exposure Registry at 877-411-2510.
Brolucizumab (Beovu) (26,000) is a human vascular endothelial growth factor that is indicated for the treatment of neovascular age-related macular degeneration. In animals, it caused malformations, embryofetal resorption, and decreased fetal weight. Other adverse effects were follicular development, corpus luteum function, and fertility.
Caplacizumab (Cablivi) (28,000) is indicated for the treatment of adult patients with acquired thrombotic thrombocytopenia purpura, in combination with plasma exchange and immunosuppressive therapy. If used in pregnancy, there is a risk of hemorrhage in the mother and fetus. In guinea pigs given intramuscular doses of the drug, there was no evidence of adverse developmental outcomes.
Cefiderocol (Fetroja) (3,044) is an IV cephalosporin antibiotic indicated for the treatment of urinary tract infections, including pyelonephritis. The manufacturer states that it should be used in patients 18 years of age or older who have limited or no alternative treatment options. Consistent with other cephalosporins, no developmental adverse effects were observed in rats and mice.
Cenobamate (Xcopri) (268) is indicated for the treatment of partial-onset seizures in adults. In pregnant animals given the drug, there was increased embryo-fetal mortality, decreased fetal and offspring body weight, and neurobehavioral and reproductive impairment in offspring. If a pregnant woman receives this drug, encourage her to enroll in the North American Antiepileptic Drug Pregnancy Registry by calling the toll-free number 1-888-233-2334.
Crizanlizumab (Adakveo) (146,000) is indicated to reduce the frequency of vaso-occlusive crises in patients with sickle cell disease. In monkeys given doses slightly higher than those given to humans, there was increased fetal loss (abortions/stillbirths).
Entrectinib (Rozlytrek) (561) is a kinase inhibitor indicated for the treatment of cancer. The drug was teratogenic in rats. It is contraindicated in pregnancy because it can cause embryo-fetal toxicity.
Erdafitinib (Balversa) (447), a kinase inhibitor, is indicated for the treatment of locally advanced or metastatic urothelial carcinoma. In rats given doses during organogenesis with maternal exposures less than human exposures, the drug was teratogenic and caused embryofetal death. The manufacturer states that women of reproductive potential should use effective contraception during treatment and for 1 month after the last dose. The same advice was provided for male patients with female partners of reproductive potential. It is contraindicated in pregnancy because it can cause embryofetal toxicity.
Fedratinib (Inrebic) (616), a kinase inhibitor, is indicated for patients with intermediate-2 or high-risk primary or secondary myelofibrosis. The drug was teratogenic in rats when doses that were about 0.1 times the human exposure based on AUC (area under the curve) at the recommended daily dose during organogenesis. It is contraindicated in pregnancy because it can cause embryofetal toxicity.
Fluorodopa f18 (214) is a radioactive diagnostic agent. It is indicated for use in positron emission tomography to visualize dopaminergic nerve terminals in the striatum for evaluation of adult patients with suspected parkinsonian syndromes. The potential for adverse pregnant outcomes is based on the radiation dose and the gestational timing of exposure.
Givosiran sodium (Givlaari) (17,2460) is an aminolevulinate synthase 1-directed small interfering RNA given subcutaneously. It is indicated for the treatment of adults with acute hepatic porphyria. Doses less than 10 times the human dose in rats and rabbits produced maternal toxicity. In rats there was increased postimplantation loss, and in rats there was skeletal variation (incomplete ossification of pubes).
Golodirsen (Vyondys 53) (8,647) is indicated for the treatment of Duchenne muscular dystrophy given intravenously. There are no human or animal data available to assess the use of this drug during pregnancy.
Istradefylline (Nourianz) (384) is an adenosine receptor antagonist given orally. It is indicated as adjunctive treatment to levodopa/carbidopa in patients with Parkinson’s disease experiencing “off” episodes. In pregnant rats and rabbits, the drug was related to teratogenicity, embryo-fetal and offspring mortality, and growth deficits at clinically relevant exposures.
Lasmiditan (Reyvow) (436), a serotonin receptor agonist, is indicated for acute treatment of migraine with or without aura. In animals, the drug caused increased incidences of fetal defects, increased embryo-fetal and offspring mortality, and decreased fetal body weight at maternal exposures less than (rabbits) or greater than (rat) those observed clinically.
Lefamulin (Xenleta) (568) is an antibacterial agent available for oral and IV administration. They are indicated for the treatment of community-acquired bacterial pneumonia. The drug was teratogenic in rats at systemic exposures lower than those in humans, an increased incidence of post-implantation fetal loss and stillbirths, and decreased fetal body weights and ossification. There was also an apparent delay in sexual maturation in rats.
Luspatercept (Reblozyl) (76,000) is given subcutaneously for the treatment of anemia in patients with beta thalassemia who require regular red blood cell transfusions. In rats and rabbits, the drug cause increased embryo-fetal mortality, alteration to growth, and structural defects at exposures (based on AUC) that were about 13 times (rats) and 18 times (rabbits) the maximum recommended human dose.
Pexidartinib (Turalio) (454) is an oral kinase inhibitor that is indicated for the treatment of symptomatic tenosynovial giant cell tumor associated with severe morbidity or functional limitations and not amenable with surgery. In rats and rabbits, the drug caused malformations, increased post-implantation loss, and abortion at exposures nearly equal to the human exposure. It is contraindicated in pregnancy because it can cause embryo-fetal toxicity.
Pitolisant HCl (Wakix) (296) is an histamine-3 receptor antagonist/inverse agonist indicated for the treatment of excessive daytime sleepiness in patients with narcolepsy. The drug has caused maternal and embryofetal toxicity in rats and rabbits at doses greater than and equal to 13 times and greater than 4 times the maximum human dose, respectively. The manufacturer has a pregnancy exposure registry that patients can contact at 1-800-833-7460.
Prabotulinum toxin A (Jeuveau) (900,000) is an acetylcholine release inhibitor and a neuromuscular blocking agent indicated for the temporary improvement in the appearance of moderate to severe glabellar lines associated with corrugator and/or procerus muscle activity. The drug caused no adverse embryo-fetal in rats with doses up to 12 times the human dose.
Risankizumab-rzaa (Skyrizi) (molecular weight unknown), an interleukin-23 antagonist, is used for the treatment of moderate-to-severe plaque psoriasis. In pregnant monkeys, doses that were 20 times the maximum human dose increased fetal/infant loss.
Selinexor (Xpovio) (443) is an oral nuclear export inhibitor given in combination with dexamethasone for the treatment of relapsed or refractory myeloma. At doses lower than those used clinically, the drug caused structural abnormalities and alterations to growth in fetal rats.
Siponimod (Mayzent) (1,149) is an oral sphingosine 1-phosphate receptor modulator. It is indicated for the treatment of relapsing forms of multiple sclerosis. At low doses, the drug caused embryotoxicity and fetotoxicity in rats and rabbits including embryofetal deaths and abortions. The drug was teratogenic in both species.
Solriamfetol (Sunosi) (231) is an oral dopamine and norepinephrine reuptake inhibitor that is indicated to improve wakefulness in adult patients with excessive daytime sleepiness associated with narcolepsy or obstructive sleep apnea. The drug caused maternal and fetal toxicities in rats and rabbits and was teratogenic. The manufacturer has a pregnancy exposure registry to monitor pregnancy outcomes. Health care providers or patients can enroll in the program by calling 1-877-283-6220 or contacting the company.
Tafamidis meglumine (Vyndaqel) (503) and tafamidis (Vyndamax) (308) are indicated for the treatment of the cardiomyopathy of wild type or hereditary transthyretin-mediated amyloidosis to reduce cardiovascular mortality and cardiovascular-related hospitalization. In rabbits and rats, use of the drugs during pregnancy caused birth defects, embryo-fetal mortality, and fetal body weight reduction. Limited available data with Vyndaqel use in human pregnancy at a dose of 20 mg/day have not identified any drug-associated risks for major birth defects, miscarriage, or adverse maternal or fetal outcomes (see package insert).
Tenapanor (Ibsrela) (1,218) is indicated for the treatment of irritable bowel syndrome with constipation. The drug is minimally absorbed systemically, with plasma concentrations below the limit of quantification. No adverse maternal or fetal outcomes in rats or rabbits were observed. As reported by the manufacturer, in a small number of pregnant women, no drug-induced adverse maternal or fetal outcomes were identified.
Triclabendazole (Egaten) (360), an oral anthelmintic, is indicated for the treatment of fascioliasis. The drug was not teratogenic in mice and rabbits.
Trifarotene (Aklief) (460) cream is a retinoid that is indicated for the topical treatment of acne vulgaris. Animal data was related to oral retinoids and it not applicable to this agent. The manufacturer reported that available data from the use of the cream in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes.
Upadacitinib (Rinvoq) (389) is an oral Janus inhibitor. It is indicated for the treatment of moderate to severe active rheumatoid arthritis in patients who have had an inadequate response or intolerance to methotrexate. The drug caused increases in fetal malformations when given to rats and rabbits during organogenesis.
Voxelotor (Oxbryta) (337) is an oral hemoglobin S polymerization inhibitor indicated for the treatment of sickle cell disease. In rats and rabbits, there was no evidence of adverse developmental outcomes.
Zanubrutinib (Brukinsa) (472), an oral kinase inhibitor, is indicated for the treatment of mantle cell lymphoma. The drug caused embryofetal toxicity in pregnant rats, including malformations. It is contraindicated in pregnancy because it can cause embryo-fetal toxicity.
Breastfeeding
Brexanolone (Zulresso) (319) is indicated for the treatment of postpartum depression. It is given as a continuous IV infusion over 60 hours. The drug, at exposures close to those seen in humans, did not cause structural defects in rabbits and rats, but did cause fetal toxicity. Because patients are at risk of excessive sedation or sudden loss of consciousness when receiving the drug, it is only available through a restricted program called the ZULRESSO REMS. Health care providers are encouraged to register patients by calling the National Pregnancy Registry for Antidepressants at 844-405-6185. To obtain a list of health care facilities enrolled in the program call 844-472-4379.
Nearly all of the above drugs will cross into a woman’s colostrum during the first 48 hours post partum. These amounts should be very small, but not breastfeeding is the best choice.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at [email protected].
In 2019, the Food and Drug Administration approved 42 drugs, 6 of which will not be discussed here because of space limitations: recarbrio, a three-drug combination, containing imipenem, cilastatin, and relebactam; polatuzumab (Polivy) combined with bendamustine and a rituximab product; pretomanid combined with bedaquiline and linezolid; romosozumab (Evenity) for postmenopausal women; and alpelisib (Piqray) for postmenopausal women. In addition, darolutamide (Nubeqa) will not be included because it is indicated for the treatment of patients with prostate cancer. The remaining 36 agents are listed alphabetically below with the trade names in parentheses.
The molecular weights (if available), rounded to the nearest whole number, are shown in parentheses.
Air polymer-type a intrauterine foam (ExEm Foam), an ultrasound contrast agent, is indicated for sonohysterosalpingography to assess fallopian tube patency in women with known or suspected infertility. Animal studies have not been conducted, and the agent is contraindicated in pregnancy.
Afamelanotide implant (Scenesse) (1,647) is a melanocortin 1 receptor agonist that is indicated to increase pain-free light exposure in adult patients with a history of phototoxic reactions from erythropoietic protoporphyria. The drug caused no embryofetal toxicity in two species of rats. The molecular weight suggests that it will not cross the placenta, at least early in pregnancy.
Alpelisib (Piqray) (441) is a kinase inhibitor that is combined with fulvestrant for the treatment of advanced breast cancer in women and men. The molecular weight suggests that it can cross the human placenta. It is contraindicated in pregnancy because it can cause embryofetal toxicity.
Bremelanotide (Vyleesi) (1,025) is indicated for the treatment of premenopausal women with hypoactive sexual disorder. The drug caused fetal harm in dogs and mice. If a pregnant woman is exposed to the drug, health care providers are encouraged to call the VYLEESI Pregnancy Exposure Registry at 877-411-2510.
Brolucizumab (Beovu) (26,000) is a human vascular endothelial growth factor that is indicated for the treatment of neovascular age-related macular degeneration. In animals, it caused malformations, embryofetal resorption, and decreased fetal weight. Other adverse effects were follicular development, corpus luteum function, and fertility.
Caplacizumab (Cablivi) (28,000) is indicated for the treatment of adult patients with acquired thrombotic thrombocytopenia purpura, in combination with plasma exchange and immunosuppressive therapy. If used in pregnancy, there is a risk of hemorrhage in the mother and fetus. In guinea pigs given intramuscular doses of the drug, there was no evidence of adverse developmental outcomes.
Cefiderocol (Fetroja) (3,044) is an IV cephalosporin antibiotic indicated for the treatment of urinary tract infections, including pyelonephritis. The manufacturer states that it should be used in patients 18 years of age or older who have limited or no alternative treatment options. Consistent with other cephalosporins, no developmental adverse effects were observed in rats and mice.
Cenobamate (Xcopri) (268) is indicated for the treatment of partial-onset seizures in adults. In pregnant animals given the drug, there was increased embryo-fetal mortality, decreased fetal and offspring body weight, and neurobehavioral and reproductive impairment in offspring. If a pregnant woman receives this drug, encourage her to enroll in the North American Antiepileptic Drug Pregnancy Registry by calling the toll-free number 1-888-233-2334.
Crizanlizumab (Adakveo) (146,000) is indicated to reduce the frequency of vaso-occlusive crises in patients with sickle cell disease. In monkeys given doses slightly higher than those given to humans, there was increased fetal loss (abortions/stillbirths).
Entrectinib (Rozlytrek) (561) is a kinase inhibitor indicated for the treatment of cancer. The drug was teratogenic in rats. It is contraindicated in pregnancy because it can cause embryo-fetal toxicity.
Erdafitinib (Balversa) (447), a kinase inhibitor, is indicated for the treatment of locally advanced or metastatic urothelial carcinoma. In rats given doses during organogenesis with maternal exposures less than human exposures, the drug was teratogenic and caused embryofetal death. The manufacturer states that women of reproductive potential should use effective contraception during treatment and for 1 month after the last dose. The same advice was provided for male patients with female partners of reproductive potential. It is contraindicated in pregnancy because it can cause embryofetal toxicity.
Fedratinib (Inrebic) (616), a kinase inhibitor, is indicated for patients with intermediate-2 or high-risk primary or secondary myelofibrosis. The drug was teratogenic in rats when doses that were about 0.1 times the human exposure based on AUC (area under the curve) at the recommended daily dose during organogenesis. It is contraindicated in pregnancy because it can cause embryofetal toxicity.
Fluorodopa f18 (214) is a radioactive diagnostic agent. It is indicated for use in positron emission tomography to visualize dopaminergic nerve terminals in the striatum for evaluation of adult patients with suspected parkinsonian syndromes. The potential for adverse pregnant outcomes is based on the radiation dose and the gestational timing of exposure.
Givosiran sodium (Givlaari) (17,2460) is an aminolevulinate synthase 1-directed small interfering RNA given subcutaneously. It is indicated for the treatment of adults with acute hepatic porphyria. Doses less than 10 times the human dose in rats and rabbits produced maternal toxicity. In rats there was increased postimplantation loss, and in rats there was skeletal variation (incomplete ossification of pubes).
Golodirsen (Vyondys 53) (8,647) is indicated for the treatment of Duchenne muscular dystrophy given intravenously. There are no human or animal data available to assess the use of this drug during pregnancy.
Istradefylline (Nourianz) (384) is an adenosine receptor antagonist given orally. It is indicated as adjunctive treatment to levodopa/carbidopa in patients with Parkinson’s disease experiencing “off” episodes. In pregnant rats and rabbits, the drug was related to teratogenicity, embryo-fetal and offspring mortality, and growth deficits at clinically relevant exposures.
Lasmiditan (Reyvow) (436), a serotonin receptor agonist, is indicated for acute treatment of migraine with or without aura. In animals, the drug caused increased incidences of fetal defects, increased embryo-fetal and offspring mortality, and decreased fetal body weight at maternal exposures less than (rabbits) or greater than (rat) those observed clinically.
Lefamulin (Xenleta) (568) is an antibacterial agent available for oral and IV administration. They are indicated for the treatment of community-acquired bacterial pneumonia. The drug was teratogenic in rats at systemic exposures lower than those in humans, an increased incidence of post-implantation fetal loss and stillbirths, and decreased fetal body weights and ossification. There was also an apparent delay in sexual maturation in rats.
Luspatercept (Reblozyl) (76,000) is given subcutaneously for the treatment of anemia in patients with beta thalassemia who require regular red blood cell transfusions. In rats and rabbits, the drug cause increased embryo-fetal mortality, alteration to growth, and structural defects at exposures (based on AUC) that were about 13 times (rats) and 18 times (rabbits) the maximum recommended human dose.
Pexidartinib (Turalio) (454) is an oral kinase inhibitor that is indicated for the treatment of symptomatic tenosynovial giant cell tumor associated with severe morbidity or functional limitations and not amenable with surgery. In rats and rabbits, the drug caused malformations, increased post-implantation loss, and abortion at exposures nearly equal to the human exposure. It is contraindicated in pregnancy because it can cause embryo-fetal toxicity.
Pitolisant HCl (Wakix) (296) is an histamine-3 receptor antagonist/inverse agonist indicated for the treatment of excessive daytime sleepiness in patients with narcolepsy. The drug has caused maternal and embryofetal toxicity in rats and rabbits at doses greater than and equal to 13 times and greater than 4 times the maximum human dose, respectively. The manufacturer has a pregnancy exposure registry that patients can contact at 1-800-833-7460.
Prabotulinum toxin A (Jeuveau) (900,000) is an acetylcholine release inhibitor and a neuromuscular blocking agent indicated for the temporary improvement in the appearance of moderate to severe glabellar lines associated with corrugator and/or procerus muscle activity. The drug caused no adverse embryo-fetal in rats with doses up to 12 times the human dose.
Risankizumab-rzaa (Skyrizi) (molecular weight unknown), an interleukin-23 antagonist, is used for the treatment of moderate-to-severe plaque psoriasis. In pregnant monkeys, doses that were 20 times the maximum human dose increased fetal/infant loss.
Selinexor (Xpovio) (443) is an oral nuclear export inhibitor given in combination with dexamethasone for the treatment of relapsed or refractory myeloma. At doses lower than those used clinically, the drug caused structural abnormalities and alterations to growth in fetal rats.
Siponimod (Mayzent) (1,149) is an oral sphingosine 1-phosphate receptor modulator. It is indicated for the treatment of relapsing forms of multiple sclerosis. At low doses, the drug caused embryotoxicity and fetotoxicity in rats and rabbits including embryofetal deaths and abortions. The drug was teratogenic in both species.
Solriamfetol (Sunosi) (231) is an oral dopamine and norepinephrine reuptake inhibitor that is indicated to improve wakefulness in adult patients with excessive daytime sleepiness associated with narcolepsy or obstructive sleep apnea. The drug caused maternal and fetal toxicities in rats and rabbits and was teratogenic. The manufacturer has a pregnancy exposure registry to monitor pregnancy outcomes. Health care providers or patients can enroll in the program by calling 1-877-283-6220 or contacting the company.
Tafamidis meglumine (Vyndaqel) (503) and tafamidis (Vyndamax) (308) are indicated for the treatment of the cardiomyopathy of wild type or hereditary transthyretin-mediated amyloidosis to reduce cardiovascular mortality and cardiovascular-related hospitalization. In rabbits and rats, use of the drugs during pregnancy caused birth defects, embryo-fetal mortality, and fetal body weight reduction. Limited available data with Vyndaqel use in human pregnancy at a dose of 20 mg/day have not identified any drug-associated risks for major birth defects, miscarriage, or adverse maternal or fetal outcomes (see package insert).
Tenapanor (Ibsrela) (1,218) is indicated for the treatment of irritable bowel syndrome with constipation. The drug is minimally absorbed systemically, with plasma concentrations below the limit of quantification. No adverse maternal or fetal outcomes in rats or rabbits were observed. As reported by the manufacturer, in a small number of pregnant women, no drug-induced adverse maternal or fetal outcomes were identified.
Triclabendazole (Egaten) (360), an oral anthelmintic, is indicated for the treatment of fascioliasis. The drug was not teratogenic in mice and rabbits.
Trifarotene (Aklief) (460) cream is a retinoid that is indicated for the topical treatment of acne vulgaris. Animal data was related to oral retinoids and it not applicable to this agent. The manufacturer reported that available data from the use of the cream in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes.
Upadacitinib (Rinvoq) (389) is an oral Janus inhibitor. It is indicated for the treatment of moderate to severe active rheumatoid arthritis in patients who have had an inadequate response or intolerance to methotrexate. The drug caused increases in fetal malformations when given to rats and rabbits during organogenesis.
Voxelotor (Oxbryta) (337) is an oral hemoglobin S polymerization inhibitor indicated for the treatment of sickle cell disease. In rats and rabbits, there was no evidence of adverse developmental outcomes.
Zanubrutinib (Brukinsa) (472), an oral kinase inhibitor, is indicated for the treatment of mantle cell lymphoma. The drug caused embryofetal toxicity in pregnant rats, including malformations. It is contraindicated in pregnancy because it can cause embryo-fetal toxicity.
Breastfeeding
Brexanolone (Zulresso) (319) is indicated for the treatment of postpartum depression. It is given as a continuous IV infusion over 60 hours. The drug, at exposures close to those seen in humans, did not cause structural defects in rabbits and rats, but did cause fetal toxicity. Because patients are at risk of excessive sedation or sudden loss of consciousness when receiving the drug, it is only available through a restricted program called the ZULRESSO REMS. Health care providers are encouraged to register patients by calling the National Pregnancy Registry for Antidepressants at 844-405-6185. To obtain a list of health care facilities enrolled in the program call 844-472-4379.
Nearly all of the above drugs will cross into a woman’s colostrum during the first 48 hours post partum. These amounts should be very small, but not breastfeeding is the best choice.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at [email protected].
In 2019, the Food and Drug Administration approved 42 drugs, 6 of which will not be discussed here because of space limitations: recarbrio, a three-drug combination, containing imipenem, cilastatin, and relebactam; polatuzumab (Polivy) combined with bendamustine and a rituximab product; pretomanid combined with bedaquiline and linezolid; romosozumab (Evenity) for postmenopausal women; and alpelisib (Piqray) for postmenopausal women. In addition, darolutamide (Nubeqa) will not be included because it is indicated for the treatment of patients with prostate cancer. The remaining 36 agents are listed alphabetically below with the trade names in parentheses.
The molecular weights (if available), rounded to the nearest whole number, are shown in parentheses.
Air polymer-type a intrauterine foam (ExEm Foam), an ultrasound contrast agent, is indicated for sonohysterosalpingography to assess fallopian tube patency in women with known or suspected infertility. Animal studies have not been conducted, and the agent is contraindicated in pregnancy.
Afamelanotide implant (Scenesse) (1,647) is a melanocortin 1 receptor agonist that is indicated to increase pain-free light exposure in adult patients with a history of phototoxic reactions from erythropoietic protoporphyria. The drug caused no embryofetal toxicity in two species of rats. The molecular weight suggests that it will not cross the placenta, at least early in pregnancy.
Alpelisib (Piqray) (441) is a kinase inhibitor that is combined with fulvestrant for the treatment of advanced breast cancer in women and men. The molecular weight suggests that it can cross the human placenta. It is contraindicated in pregnancy because it can cause embryofetal toxicity.
Bremelanotide (Vyleesi) (1,025) is indicated for the treatment of premenopausal women with hypoactive sexual disorder. The drug caused fetal harm in dogs and mice. If a pregnant woman is exposed to the drug, health care providers are encouraged to call the VYLEESI Pregnancy Exposure Registry at 877-411-2510.
Brolucizumab (Beovu) (26,000) is a human vascular endothelial growth factor that is indicated for the treatment of neovascular age-related macular degeneration. In animals, it caused malformations, embryofetal resorption, and decreased fetal weight. Other adverse effects were follicular development, corpus luteum function, and fertility.
Caplacizumab (Cablivi) (28,000) is indicated for the treatment of adult patients with acquired thrombotic thrombocytopenia purpura, in combination with plasma exchange and immunosuppressive therapy. If used in pregnancy, there is a risk of hemorrhage in the mother and fetus. In guinea pigs given intramuscular doses of the drug, there was no evidence of adverse developmental outcomes.
Cefiderocol (Fetroja) (3,044) is an IV cephalosporin antibiotic indicated for the treatment of urinary tract infections, including pyelonephritis. The manufacturer states that it should be used in patients 18 years of age or older who have limited or no alternative treatment options. Consistent with other cephalosporins, no developmental adverse effects were observed in rats and mice.
Cenobamate (Xcopri) (268) is indicated for the treatment of partial-onset seizures in adults. In pregnant animals given the drug, there was increased embryo-fetal mortality, decreased fetal and offspring body weight, and neurobehavioral and reproductive impairment in offspring. If a pregnant woman receives this drug, encourage her to enroll in the North American Antiepileptic Drug Pregnancy Registry by calling the toll-free number 1-888-233-2334.
Crizanlizumab (Adakveo) (146,000) is indicated to reduce the frequency of vaso-occlusive crises in patients with sickle cell disease. In monkeys given doses slightly higher than those given to humans, there was increased fetal loss (abortions/stillbirths).
Entrectinib (Rozlytrek) (561) is a kinase inhibitor indicated for the treatment of cancer. The drug was teratogenic in rats. It is contraindicated in pregnancy because it can cause embryo-fetal toxicity.
Erdafitinib (Balversa) (447), a kinase inhibitor, is indicated for the treatment of locally advanced or metastatic urothelial carcinoma. In rats given doses during organogenesis with maternal exposures less than human exposures, the drug was teratogenic and caused embryofetal death. The manufacturer states that women of reproductive potential should use effective contraception during treatment and for 1 month after the last dose. The same advice was provided for male patients with female partners of reproductive potential. It is contraindicated in pregnancy because it can cause embryofetal toxicity.
Fedratinib (Inrebic) (616), a kinase inhibitor, is indicated for patients with intermediate-2 or high-risk primary or secondary myelofibrosis. The drug was teratogenic in rats when doses that were about 0.1 times the human exposure based on AUC (area under the curve) at the recommended daily dose during organogenesis. It is contraindicated in pregnancy because it can cause embryofetal toxicity.
Fluorodopa f18 (214) is a radioactive diagnostic agent. It is indicated for use in positron emission tomography to visualize dopaminergic nerve terminals in the striatum for evaluation of adult patients with suspected parkinsonian syndromes. The potential for adverse pregnant outcomes is based on the radiation dose and the gestational timing of exposure.
Givosiran sodium (Givlaari) (17,2460) is an aminolevulinate synthase 1-directed small interfering RNA given subcutaneously. It is indicated for the treatment of adults with acute hepatic porphyria. Doses less than 10 times the human dose in rats and rabbits produced maternal toxicity. In rats there was increased postimplantation loss, and in rats there was skeletal variation (incomplete ossification of pubes).
Golodirsen (Vyondys 53) (8,647) is indicated for the treatment of Duchenne muscular dystrophy given intravenously. There are no human or animal data available to assess the use of this drug during pregnancy.
Istradefylline (Nourianz) (384) is an adenosine receptor antagonist given orally. It is indicated as adjunctive treatment to levodopa/carbidopa in patients with Parkinson’s disease experiencing “off” episodes. In pregnant rats and rabbits, the drug was related to teratogenicity, embryo-fetal and offspring mortality, and growth deficits at clinically relevant exposures.
Lasmiditan (Reyvow) (436), a serotonin receptor agonist, is indicated for acute treatment of migraine with or without aura. In animals, the drug caused increased incidences of fetal defects, increased embryo-fetal and offspring mortality, and decreased fetal body weight at maternal exposures less than (rabbits) or greater than (rat) those observed clinically.
Lefamulin (Xenleta) (568) is an antibacterial agent available for oral and IV administration. They are indicated for the treatment of community-acquired bacterial pneumonia. The drug was teratogenic in rats at systemic exposures lower than those in humans, an increased incidence of post-implantation fetal loss and stillbirths, and decreased fetal body weights and ossification. There was also an apparent delay in sexual maturation in rats.
Luspatercept (Reblozyl) (76,000) is given subcutaneously for the treatment of anemia in patients with beta thalassemia who require regular red blood cell transfusions. In rats and rabbits, the drug cause increased embryo-fetal mortality, alteration to growth, and structural defects at exposures (based on AUC) that were about 13 times (rats) and 18 times (rabbits) the maximum recommended human dose.
Pexidartinib (Turalio) (454) is an oral kinase inhibitor that is indicated for the treatment of symptomatic tenosynovial giant cell tumor associated with severe morbidity or functional limitations and not amenable with surgery. In rats and rabbits, the drug caused malformations, increased post-implantation loss, and abortion at exposures nearly equal to the human exposure. It is contraindicated in pregnancy because it can cause embryo-fetal toxicity.
Pitolisant HCl (Wakix) (296) is an histamine-3 receptor antagonist/inverse agonist indicated for the treatment of excessive daytime sleepiness in patients with narcolepsy. The drug has caused maternal and embryofetal toxicity in rats and rabbits at doses greater than and equal to 13 times and greater than 4 times the maximum human dose, respectively. The manufacturer has a pregnancy exposure registry that patients can contact at 1-800-833-7460.
Prabotulinum toxin A (Jeuveau) (900,000) is an acetylcholine release inhibitor and a neuromuscular blocking agent indicated for the temporary improvement in the appearance of moderate to severe glabellar lines associated with corrugator and/or procerus muscle activity. The drug caused no adverse embryo-fetal in rats with doses up to 12 times the human dose.
Risankizumab-rzaa (Skyrizi) (molecular weight unknown), an interleukin-23 antagonist, is used for the treatment of moderate-to-severe plaque psoriasis. In pregnant monkeys, doses that were 20 times the maximum human dose increased fetal/infant loss.
Selinexor (Xpovio) (443) is an oral nuclear export inhibitor given in combination with dexamethasone for the treatment of relapsed or refractory myeloma. At doses lower than those used clinically, the drug caused structural abnormalities and alterations to growth in fetal rats.
Siponimod (Mayzent) (1,149) is an oral sphingosine 1-phosphate receptor modulator. It is indicated for the treatment of relapsing forms of multiple sclerosis. At low doses, the drug caused embryotoxicity and fetotoxicity in rats and rabbits including embryofetal deaths and abortions. The drug was teratogenic in both species.
Solriamfetol (Sunosi) (231) is an oral dopamine and norepinephrine reuptake inhibitor that is indicated to improve wakefulness in adult patients with excessive daytime sleepiness associated with narcolepsy or obstructive sleep apnea. The drug caused maternal and fetal toxicities in rats and rabbits and was teratogenic. The manufacturer has a pregnancy exposure registry to monitor pregnancy outcomes. Health care providers or patients can enroll in the program by calling 1-877-283-6220 or contacting the company.
Tafamidis meglumine (Vyndaqel) (503) and tafamidis (Vyndamax) (308) are indicated for the treatment of the cardiomyopathy of wild type or hereditary transthyretin-mediated amyloidosis to reduce cardiovascular mortality and cardiovascular-related hospitalization. In rabbits and rats, use of the drugs during pregnancy caused birth defects, embryo-fetal mortality, and fetal body weight reduction. Limited available data with Vyndaqel use in human pregnancy at a dose of 20 mg/day have not identified any drug-associated risks for major birth defects, miscarriage, or adverse maternal or fetal outcomes (see package insert).
Tenapanor (Ibsrela) (1,218) is indicated for the treatment of irritable bowel syndrome with constipation. The drug is minimally absorbed systemically, with plasma concentrations below the limit of quantification. No adverse maternal or fetal outcomes in rats or rabbits were observed. As reported by the manufacturer, in a small number of pregnant women, no drug-induced adverse maternal or fetal outcomes were identified.
Triclabendazole (Egaten) (360), an oral anthelmintic, is indicated for the treatment of fascioliasis. The drug was not teratogenic in mice and rabbits.
Trifarotene (Aklief) (460) cream is a retinoid that is indicated for the topical treatment of acne vulgaris. Animal data was related to oral retinoids and it not applicable to this agent. The manufacturer reported that available data from the use of the cream in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes.
Upadacitinib (Rinvoq) (389) is an oral Janus inhibitor. It is indicated for the treatment of moderate to severe active rheumatoid arthritis in patients who have had an inadequate response or intolerance to methotrexate. The drug caused increases in fetal malformations when given to rats and rabbits during organogenesis.
Voxelotor (Oxbryta) (337) is an oral hemoglobin S polymerization inhibitor indicated for the treatment of sickle cell disease. In rats and rabbits, there was no evidence of adverse developmental outcomes.
Zanubrutinib (Brukinsa) (472), an oral kinase inhibitor, is indicated for the treatment of mantle cell lymphoma. The drug caused embryofetal toxicity in pregnant rats, including malformations. It is contraindicated in pregnancy because it can cause embryo-fetal toxicity.
Breastfeeding
Brexanolone (Zulresso) (319) is indicated for the treatment of postpartum depression. It is given as a continuous IV infusion over 60 hours. The drug, at exposures close to those seen in humans, did not cause structural defects in rabbits and rats, but did cause fetal toxicity. Because patients are at risk of excessive sedation or sudden loss of consciousness when receiving the drug, it is only available through a restricted program called the ZULRESSO REMS. Health care providers are encouraged to register patients by calling the National Pregnancy Registry for Antidepressants at 844-405-6185. To obtain a list of health care facilities enrolled in the program call 844-472-4379.
Nearly all of the above drugs will cross into a woman’s colostrum during the first 48 hours post partum. These amounts should be very small, but not breastfeeding is the best choice.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at [email protected].
Coronavirus and Dermatology: A Resident’s Perspective
On January 30, 2020, the World Health Organization declared the outbreak of coronavirus disease 2019 (COVID-19) a public health emergency of international concern.1 Severe acute respiratory syndrome–associated coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19, is an enveloped, single-stranded RNA virus. It is the seventh known coronavirus to infect humans and third zoonotic Coronaviridae to cause fatal respiratory illness, along with SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV).2 There has been a rapid shift in the geographic center of the outbreak as well as the numbers of confirmed cases and deaths. Although the first cases in late 2019 and early 2020 were in China, by mid-March Italy became the center of the pandemic, with a steep increase in the number of cases in other European countries and the United States.3 Although COVID-19 does not have known dermatologic manifestations, it has the potential for wide-reaching impact on our field.
Strained Resources
In the United States, COVID-19 initially was associated with international travel but is now rapidly spreading throughout the community. I am currently a dermatology resident at New York-Presbyterian, Columbia campus, in New York, New York, a city that unfortunately finds itself underprepared to handle this unprecedented crisis. As of Monday, March 16, 2020, New York-Presbyterian made the decision to postpone all elective procedures, including Mohs micrographic surgery, to preserve hospital resources, including trained personnel, personal protective equipment, ventilators, and hospital beds. There have not been clear-cut guidelines regarding how to approach other dermatologic care for our patients, including routine clinic visits and inpatient dermatology consultations, leaving decisions up to individual departments and providers.
It would be prudent to learn from our colleagues in China who report steps that have been successful in preventing nosocomial spread of COVID-19 in the dermatologic setting. Tao et al4 described their protocols in both the outpatient and inpatient dermatologic setting, beginning with strict triage before patients can even enter a clinic building for their outpatient appointment. Those who screen positive are sent to a fever clinic for further evaluation, which may include a rapid computed tomography scan (using a machine that may perform 200 chest computed tomography scans per day) and SARS-CoV-2 polymerase chain reaction.5 For inpatient dermatologic consultation of patients with known COVID-19, telehealth and multidisciplinary meetings are first and second line, respectively, with bedside dermatologic consultation as a last resort.4 Chen et al6 described similarly strict triage protocols as well as physician use of full-body personal protective equipment during all patient encounters. These measures are taken in light of the well-documented phenomenon of asymptomatic carriage and transmission, as all patients entering their dermatologic clinics have screened negative for symptomatic SARS-CoV-2 infection.6
Conferences and Education
Coronavirus is impacting the education of millions of individuals worldwide, including that of dermatology residents. The Annual Meeting of the American Academy of Dermatology, which was scheduled to take place in March 2020, was canceled due to COVID-19.7 The American Board of Dermatology has released a statement indicating that for all dermatology residents, time spent in COVID-19–mandated quarantine will count as clinical education if residents are able to work with their program to complete independent structured academic activity during that time.8 We also must consider the possibility that dermatology residents are reassigned to work outside of our specialty, resulting in less time and experience caring for patients with dermatologic conditions. Dermatologists in other countries have been called upon to care for COVID-19 patients, even reported to be working in intensive care units in Italy.9 Virtual technologies may be used in novel ways to support dermatology resident education throughout this process.
Final Thoughts
As physicians, dermatologists are in the position to educate their patients regarding prevention strategies, especially given that the lay press disseminates confusing and inaccurate information. The World Health Organization provides specific guidance, focusing on handwashing, respiratory hygiene, social distancing, and encouraging symptomatic patients to seek remote care whenever possible.10 Many of our patients are at high risk of complications due to COVID-19, whether due to their age or because they are immunocompromised. As the situation unfolds, the impact on and role of dermatology in this crisis will continue to evolve.
- Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV)[news release]. Geneva, Switzerland: World Health Organization; January 30, 2020. https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-%282005%29-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-%282019-ncov%29. Accessed March 16, 2020.
- Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273.
- World Health Organization. Coronavirus disease 2019: Situation Report—58. March 18, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200318-sitrep-58-covid-19.pdf?sfvrsn=20876712_2. Accessed March 19, 2020.
- Tao J, Song Z, Yang L, et al. Emergency management for preventing and controlling nosocomial infection of 2019 novel coronavirus: implications for the dermatology department [published online March 5, 2020]. Br J Dermatol. doi:10.1111/bjd.19011.
- McNeil DG. Jr. Inside China’s all out war on the coronavirus. The New York Times. March 4, 2020. https://www.nytimes.com/2020/03/04/health/coronavirus-china-aylward.html?smid=tw-nytimes&smtyp=cur. Accessed March 19, 2020.
- Chen Y, Pradhan S, Xue S. What are we doing in the dermatology outpatient department amidst the raging of the 2019 novel coronavirus? J Am Acad Dermatol. 2020;82:1034.
- Hruza GJ. 2020 annual AAD meeting is canceled due to COVID-19 outbreak. American Academy of Dermatology website. https://www.aad.org/member/meetings/am2020/faqs/coronavirus. Accessed March 16, 2020.
- American Board of Dermatology. Impact of COVID-19 on dermatology resident education. March 6, 2020. https://www.abderm.org/2978.aspx. Accessed March 16, 2020.
- “It’s Like a War” [podcast]. The Daily. March 17, 2020. https://www.nytimes.com/2020/03/17/podcasts/the-daily/italy-coronavirus.html?action=click&module=audio-series-bar®ion=header&pgtype=Article. Accessed March 20, 2020.
- World Health Organization. Coronavirus disease (COVID-19) advice for public. March 18, 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Accessed March 19, 2020.
On January 30, 2020, the World Health Organization declared the outbreak of coronavirus disease 2019 (COVID-19) a public health emergency of international concern.1 Severe acute respiratory syndrome–associated coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19, is an enveloped, single-stranded RNA virus. It is the seventh known coronavirus to infect humans and third zoonotic Coronaviridae to cause fatal respiratory illness, along with SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV).2 There has been a rapid shift in the geographic center of the outbreak as well as the numbers of confirmed cases and deaths. Although the first cases in late 2019 and early 2020 were in China, by mid-March Italy became the center of the pandemic, with a steep increase in the number of cases in other European countries and the United States.3 Although COVID-19 does not have known dermatologic manifestations, it has the potential for wide-reaching impact on our field.
Strained Resources
In the United States, COVID-19 initially was associated with international travel but is now rapidly spreading throughout the community. I am currently a dermatology resident at New York-Presbyterian, Columbia campus, in New York, New York, a city that unfortunately finds itself underprepared to handle this unprecedented crisis. As of Monday, March 16, 2020, New York-Presbyterian made the decision to postpone all elective procedures, including Mohs micrographic surgery, to preserve hospital resources, including trained personnel, personal protective equipment, ventilators, and hospital beds. There have not been clear-cut guidelines regarding how to approach other dermatologic care for our patients, including routine clinic visits and inpatient dermatology consultations, leaving decisions up to individual departments and providers.
It would be prudent to learn from our colleagues in China who report steps that have been successful in preventing nosocomial spread of COVID-19 in the dermatologic setting. Tao et al4 described their protocols in both the outpatient and inpatient dermatologic setting, beginning with strict triage before patients can even enter a clinic building for their outpatient appointment. Those who screen positive are sent to a fever clinic for further evaluation, which may include a rapid computed tomography scan (using a machine that may perform 200 chest computed tomography scans per day) and SARS-CoV-2 polymerase chain reaction.5 For inpatient dermatologic consultation of patients with known COVID-19, telehealth and multidisciplinary meetings are first and second line, respectively, with bedside dermatologic consultation as a last resort.4 Chen et al6 described similarly strict triage protocols as well as physician use of full-body personal protective equipment during all patient encounters. These measures are taken in light of the well-documented phenomenon of asymptomatic carriage and transmission, as all patients entering their dermatologic clinics have screened negative for symptomatic SARS-CoV-2 infection.6
Conferences and Education
Coronavirus is impacting the education of millions of individuals worldwide, including that of dermatology residents. The Annual Meeting of the American Academy of Dermatology, which was scheduled to take place in March 2020, was canceled due to COVID-19.7 The American Board of Dermatology has released a statement indicating that for all dermatology residents, time spent in COVID-19–mandated quarantine will count as clinical education if residents are able to work with their program to complete independent structured academic activity during that time.8 We also must consider the possibility that dermatology residents are reassigned to work outside of our specialty, resulting in less time and experience caring for patients with dermatologic conditions. Dermatologists in other countries have been called upon to care for COVID-19 patients, even reported to be working in intensive care units in Italy.9 Virtual technologies may be used in novel ways to support dermatology resident education throughout this process.
Final Thoughts
As physicians, dermatologists are in the position to educate their patients regarding prevention strategies, especially given that the lay press disseminates confusing and inaccurate information. The World Health Organization provides specific guidance, focusing on handwashing, respiratory hygiene, social distancing, and encouraging symptomatic patients to seek remote care whenever possible.10 Many of our patients are at high risk of complications due to COVID-19, whether due to their age or because they are immunocompromised. As the situation unfolds, the impact on and role of dermatology in this crisis will continue to evolve.
On January 30, 2020, the World Health Organization declared the outbreak of coronavirus disease 2019 (COVID-19) a public health emergency of international concern.1 Severe acute respiratory syndrome–associated coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19, is an enveloped, single-stranded RNA virus. It is the seventh known coronavirus to infect humans and third zoonotic Coronaviridae to cause fatal respiratory illness, along with SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV).2 There has been a rapid shift in the geographic center of the outbreak as well as the numbers of confirmed cases and deaths. Although the first cases in late 2019 and early 2020 were in China, by mid-March Italy became the center of the pandemic, with a steep increase in the number of cases in other European countries and the United States.3 Although COVID-19 does not have known dermatologic manifestations, it has the potential for wide-reaching impact on our field.
Strained Resources
In the United States, COVID-19 initially was associated with international travel but is now rapidly spreading throughout the community. I am currently a dermatology resident at New York-Presbyterian, Columbia campus, in New York, New York, a city that unfortunately finds itself underprepared to handle this unprecedented crisis. As of Monday, March 16, 2020, New York-Presbyterian made the decision to postpone all elective procedures, including Mohs micrographic surgery, to preserve hospital resources, including trained personnel, personal protective equipment, ventilators, and hospital beds. There have not been clear-cut guidelines regarding how to approach other dermatologic care for our patients, including routine clinic visits and inpatient dermatology consultations, leaving decisions up to individual departments and providers.
It would be prudent to learn from our colleagues in China who report steps that have been successful in preventing nosocomial spread of COVID-19 in the dermatologic setting. Tao et al4 described their protocols in both the outpatient and inpatient dermatologic setting, beginning with strict triage before patients can even enter a clinic building for their outpatient appointment. Those who screen positive are sent to a fever clinic for further evaluation, which may include a rapid computed tomography scan (using a machine that may perform 200 chest computed tomography scans per day) and SARS-CoV-2 polymerase chain reaction.5 For inpatient dermatologic consultation of patients with known COVID-19, telehealth and multidisciplinary meetings are first and second line, respectively, with bedside dermatologic consultation as a last resort.4 Chen et al6 described similarly strict triage protocols as well as physician use of full-body personal protective equipment during all patient encounters. These measures are taken in light of the well-documented phenomenon of asymptomatic carriage and transmission, as all patients entering their dermatologic clinics have screened negative for symptomatic SARS-CoV-2 infection.6
Conferences and Education
Coronavirus is impacting the education of millions of individuals worldwide, including that of dermatology residents. The Annual Meeting of the American Academy of Dermatology, which was scheduled to take place in March 2020, was canceled due to COVID-19.7 The American Board of Dermatology has released a statement indicating that for all dermatology residents, time spent in COVID-19–mandated quarantine will count as clinical education if residents are able to work with their program to complete independent structured academic activity during that time.8 We also must consider the possibility that dermatology residents are reassigned to work outside of our specialty, resulting in less time and experience caring for patients with dermatologic conditions. Dermatologists in other countries have been called upon to care for COVID-19 patients, even reported to be working in intensive care units in Italy.9 Virtual technologies may be used in novel ways to support dermatology resident education throughout this process.
Final Thoughts
As physicians, dermatologists are in the position to educate their patients regarding prevention strategies, especially given that the lay press disseminates confusing and inaccurate information. The World Health Organization provides specific guidance, focusing on handwashing, respiratory hygiene, social distancing, and encouraging symptomatic patients to seek remote care whenever possible.10 Many of our patients are at high risk of complications due to COVID-19, whether due to their age or because they are immunocompromised. As the situation unfolds, the impact on and role of dermatology in this crisis will continue to evolve.
- Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV)[news release]. Geneva, Switzerland: World Health Organization; January 30, 2020. https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-%282005%29-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-%282019-ncov%29. Accessed March 16, 2020.
- Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273.
- World Health Organization. Coronavirus disease 2019: Situation Report—58. March 18, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200318-sitrep-58-covid-19.pdf?sfvrsn=20876712_2. Accessed March 19, 2020.
- Tao J, Song Z, Yang L, et al. Emergency management for preventing and controlling nosocomial infection of 2019 novel coronavirus: implications for the dermatology department [published online March 5, 2020]. Br J Dermatol. doi:10.1111/bjd.19011.
- McNeil DG. Jr. Inside China’s all out war on the coronavirus. The New York Times. March 4, 2020. https://www.nytimes.com/2020/03/04/health/coronavirus-china-aylward.html?smid=tw-nytimes&smtyp=cur. Accessed March 19, 2020.
- Chen Y, Pradhan S, Xue S. What are we doing in the dermatology outpatient department amidst the raging of the 2019 novel coronavirus? J Am Acad Dermatol. 2020;82:1034.
- Hruza GJ. 2020 annual AAD meeting is canceled due to COVID-19 outbreak. American Academy of Dermatology website. https://www.aad.org/member/meetings/am2020/faqs/coronavirus. Accessed March 16, 2020.
- American Board of Dermatology. Impact of COVID-19 on dermatology resident education. March 6, 2020. https://www.abderm.org/2978.aspx. Accessed March 16, 2020.
- “It’s Like a War” [podcast]. The Daily. March 17, 2020. https://www.nytimes.com/2020/03/17/podcasts/the-daily/italy-coronavirus.html?action=click&module=audio-series-bar®ion=header&pgtype=Article. Accessed March 20, 2020.
- World Health Organization. Coronavirus disease (COVID-19) advice for public. March 18, 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Accessed March 19, 2020.
- Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV)[news release]. Geneva, Switzerland: World Health Organization; January 30, 2020. https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-%282005%29-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-%282019-ncov%29. Accessed March 16, 2020.
- Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273.
- World Health Organization. Coronavirus disease 2019: Situation Report—58. March 18, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200318-sitrep-58-covid-19.pdf?sfvrsn=20876712_2. Accessed March 19, 2020.
- Tao J, Song Z, Yang L, et al. Emergency management for preventing and controlling nosocomial infection of 2019 novel coronavirus: implications for the dermatology department [published online March 5, 2020]. Br J Dermatol. doi:10.1111/bjd.19011.
- McNeil DG. Jr. Inside China’s all out war on the coronavirus. The New York Times. March 4, 2020. https://www.nytimes.com/2020/03/04/health/coronavirus-china-aylward.html?smid=tw-nytimes&smtyp=cur. Accessed March 19, 2020.
- Chen Y, Pradhan S, Xue S. What are we doing in the dermatology outpatient department amidst the raging of the 2019 novel coronavirus? J Am Acad Dermatol. 2020;82:1034.
- Hruza GJ. 2020 annual AAD meeting is canceled due to COVID-19 outbreak. American Academy of Dermatology website. https://www.aad.org/member/meetings/am2020/faqs/coronavirus. Accessed March 16, 2020.
- American Board of Dermatology. Impact of COVID-19 on dermatology resident education. March 6, 2020. https://www.abderm.org/2978.aspx. Accessed March 16, 2020.
- “It’s Like a War” [podcast]. The Daily. March 17, 2020. https://www.nytimes.com/2020/03/17/podcasts/the-daily/italy-coronavirus.html?action=click&module=audio-series-bar®ion=header&pgtype=Article. Accessed March 20, 2020.
- World Health Organization. Coronavirus disease (COVID-19) advice for public. March 18, 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Accessed March 19, 2020.
Perspective from the heartland: Cancer care and research during a public health crisis
I have no knowledge of, or experience with, managing a cancer patient during a pandemic. However, from the published and otherwise shared experience of others, we should not allow ourselves to underestimate the voracity of the coronavirus pandemic on our patients, communities, and health care systems.
Data from China suggest cancer patients infected with SARS-CoV-2 face a 3.5 times higher risk of mechanical ventilation, intensive care unit admission, or death, compared with infected patients without cancer (Lancet Oncol 2020;21:335-7).
Health care workers in Seattle have also shared their experiences battling coronavirus infections in cancer patients (J Natl Compr Canc Netw. 2020 Mar 20. doi: 10.6004/jnccn.2020.7560). Masumi Ueda, MD, of Seattle Cancer Care Alliance, and colleagues reviewed their decisions in multiple domains over a 7-week period, during which the state of Washington went from a single case of SARS-CoV-2 infection to nearly 650 cases and 40 deaths.
Making tough treatment decisions
Dr. Ueda and colleagues contrasted their customary resource-rich, innovation-oriented, cancer-combatting environment with their current circumstance, in which they must prioritize treatment for patients for whom the risk-reward balance has tilted substantially toward “risk.”
The authors noted that their most difficult decisions were those regarding delay of cancer treatment. They suggested that plans for potentially curative adjuvant therapy should likely proceed, but, for patients with metastatic disease, the equation is more nuanced.
In some cases, treatment should be delayed or interrupted with recognition of how that could result in worsening performance status and admission for symptom palliation, further stressing inpatient resources.
The authors suggested scenarios for prioritizing cancer surgery. For example, several months of systemic therapy (ideally, low-risk systemic therapy such as hormone therapy for breast or prostate cancer) and surgical delay may be worthwhile, without compromising patient care.
Patients with aggressive hematologic malignancy requiring urgent systemic treatment (potentially stem cell transplantation and cellular immunotherapies) should be treated promptly. However, even in those cases, opportunities should be sought to lessen immunosuppression and transition care as quickly as possible to the outpatient clinic, according to guidelines from the American Society of Transplantation and Cellular Therapy.
See one, do one, teach one
Rendering patient care during a pandemic would be unique for me. However, I, like all physicians, am familiar with feelings of inadequacy at times of professional challenge. On countless occasions, I have started my day or walked into a patient’s room wondering whether I will have the fortitude, knowledge, creativity, or help I need to get through that day or make that patient “better” by any definition of that word.
We all know the formula: “Work hard. Make evidence-based, personalized decisions for those who have entrusted their care to us. Learn from those encounters. Teach from our knowledge and experience – that is, ‘See one, do one, teach one.’ ”
The Seattle oncologists are living the lives of first responders and deserve our admiration for putting pen to paper so we can learn from their considerable, relevant experience.
Similar admiration is due to Giuseppe Curigliano, MD, of the European Institute of Oncology in Milan. In the ASCO Daily News, Dr. Curigliano described an epidemic that, within 3 weeks, overloaded the health care system across northern Italy.
Hospitalization was needed for over 60% of infected patients, and nearly 15% of those patients needed intensive care unit services for respiratory distress. The Italians centralized oncology care in specialized hubs, with spokes of institutions working in parallel to provide cancer-specific care in a COVID-free environment.
To build upon cancer-specific information from Italy and other areas hard-hit by COVID-19, more than 30 cancer centers have joined together to form the COVID-19 and Cancer Consortium. The consortium’s website hosts a survey designed to “capture details related to cancer patients presumed to have COVID-19.”
Calculating deaths and long-term consequences for cancer care delivery
It is proper that the authors from China, Italy, and Seattle did not focus attention on the case fatality rate from the COVID-19 pandemic among cancer patients. To say the least, it would be complicated to tally the direct mortality – either overall or in clinically important subsets of patients, including country-specific cohorts.
What we know from published reports is that, in Italy, cancer patients account for about 20% of deaths from coronavirus. In China, the case-fatality rate for patients with cancer was 5.6% (JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648).
However, we know nothing about the indirect death toll from malignancy (without coronavirus infection) that was untreated or managed less than optimally because of personnel and physical resources that were diverted to COVID-19–associated cases.
Similarly, we cannot begin to estimate indirect consequences of the pandemic to oncology practices, such as accelerated burnout and posttraumatic stress disorder, as well as the long-range effects of economic turmoil on patients, health care workers, and provider organizations.
What happens to cancer trials?
From China, Italy, and Seattle, thus far, there is little information about how the pandemic will affect the vital clinical research endeavor. The Seattle physicians did say they plan to enroll patients on clinical trials only when the trial offers a high chance of benefiting the patient over standard therapy alone.
Fortunately, the National Institutes of Health and Food and Drug Administration have released guidance documents related to clinical trials.
The National Cancer Institute (NCI) has also released guidance documents (March 13 guidance; March 23 guidance) for patients on clinical trials supported by the NCI Cancer Therapy Evaluation Program (CTEP) and the NCI Community Oncology Research Program (NCORP).
CTEP and NCORP are making reasonable accommodations to suspend monitoring visits and audits, allow tele–follow-up visits for patients, and permit local physicians to provide care for patients on study. In addition, with appropriate procedural adherence and documentation, CTEP and NCORP will allow oral investigational medicines to be mailed directly to patients’ homes.
Planned NCI National Clinical Trials Network meetings will be conducted via remote access webinars, conference calls, and similar technology. These adjustments – and probably many more to come – are geared toward facilitating ongoing care to proceed safely and with minimal risk for patients currently receiving investigational therapies and for the sites and investigators engaged in those studies.
Each of us has probably faced a personal “defining professional moment,” when we had to utilize every skill in our arsenal and examine the motivations that led us to a career in oncology. However, it is clear from the forgoing clinical and research processes and guidelines that the COVID-19 pandemic is such a defining professional moment for each of us, in every community we serve.
Critical junctures like this cause more rapid behavior change and innovation than the slow-moving pace that characterizes our idealized preferences. As oncologists who embrace new data and behavioral change, we stand to learn processes that will facilitate more perfected systems of care than the one that preceded this unprecedented crisis, promote more efficient sharing of high-quality information, and improve the outcome for our future patients.
Dr. Lyss was an oncologist and researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
I have no knowledge of, or experience with, managing a cancer patient during a pandemic. However, from the published and otherwise shared experience of others, we should not allow ourselves to underestimate the voracity of the coronavirus pandemic on our patients, communities, and health care systems.
Data from China suggest cancer patients infected with SARS-CoV-2 face a 3.5 times higher risk of mechanical ventilation, intensive care unit admission, or death, compared with infected patients without cancer (Lancet Oncol 2020;21:335-7).
Health care workers in Seattle have also shared their experiences battling coronavirus infections in cancer patients (J Natl Compr Canc Netw. 2020 Mar 20. doi: 10.6004/jnccn.2020.7560). Masumi Ueda, MD, of Seattle Cancer Care Alliance, and colleagues reviewed their decisions in multiple domains over a 7-week period, during which the state of Washington went from a single case of SARS-CoV-2 infection to nearly 650 cases and 40 deaths.
Making tough treatment decisions
Dr. Ueda and colleagues contrasted their customary resource-rich, innovation-oriented, cancer-combatting environment with their current circumstance, in which they must prioritize treatment for patients for whom the risk-reward balance has tilted substantially toward “risk.”
The authors noted that their most difficult decisions were those regarding delay of cancer treatment. They suggested that plans for potentially curative adjuvant therapy should likely proceed, but, for patients with metastatic disease, the equation is more nuanced.
In some cases, treatment should be delayed or interrupted with recognition of how that could result in worsening performance status and admission for symptom palliation, further stressing inpatient resources.
The authors suggested scenarios for prioritizing cancer surgery. For example, several months of systemic therapy (ideally, low-risk systemic therapy such as hormone therapy for breast or prostate cancer) and surgical delay may be worthwhile, without compromising patient care.
Patients with aggressive hematologic malignancy requiring urgent systemic treatment (potentially stem cell transplantation and cellular immunotherapies) should be treated promptly. However, even in those cases, opportunities should be sought to lessen immunosuppression and transition care as quickly as possible to the outpatient clinic, according to guidelines from the American Society of Transplantation and Cellular Therapy.
See one, do one, teach one
Rendering patient care during a pandemic would be unique for me. However, I, like all physicians, am familiar with feelings of inadequacy at times of professional challenge. On countless occasions, I have started my day or walked into a patient’s room wondering whether I will have the fortitude, knowledge, creativity, or help I need to get through that day or make that patient “better” by any definition of that word.
We all know the formula: “Work hard. Make evidence-based, personalized decisions for those who have entrusted their care to us. Learn from those encounters. Teach from our knowledge and experience – that is, ‘See one, do one, teach one.’ ”
The Seattle oncologists are living the lives of first responders and deserve our admiration for putting pen to paper so we can learn from their considerable, relevant experience.
Similar admiration is due to Giuseppe Curigliano, MD, of the European Institute of Oncology in Milan. In the ASCO Daily News, Dr. Curigliano described an epidemic that, within 3 weeks, overloaded the health care system across northern Italy.
Hospitalization was needed for over 60% of infected patients, and nearly 15% of those patients needed intensive care unit services for respiratory distress. The Italians centralized oncology care in specialized hubs, with spokes of institutions working in parallel to provide cancer-specific care in a COVID-free environment.
To build upon cancer-specific information from Italy and other areas hard-hit by COVID-19, more than 30 cancer centers have joined together to form the COVID-19 and Cancer Consortium. The consortium’s website hosts a survey designed to “capture details related to cancer patients presumed to have COVID-19.”
Calculating deaths and long-term consequences for cancer care delivery
It is proper that the authors from China, Italy, and Seattle did not focus attention on the case fatality rate from the COVID-19 pandemic among cancer patients. To say the least, it would be complicated to tally the direct mortality – either overall or in clinically important subsets of patients, including country-specific cohorts.
What we know from published reports is that, in Italy, cancer patients account for about 20% of deaths from coronavirus. In China, the case-fatality rate for patients with cancer was 5.6% (JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648).
However, we know nothing about the indirect death toll from malignancy (without coronavirus infection) that was untreated or managed less than optimally because of personnel and physical resources that were diverted to COVID-19–associated cases.
Similarly, we cannot begin to estimate indirect consequences of the pandemic to oncology practices, such as accelerated burnout and posttraumatic stress disorder, as well as the long-range effects of economic turmoil on patients, health care workers, and provider organizations.
What happens to cancer trials?
From China, Italy, and Seattle, thus far, there is little information about how the pandemic will affect the vital clinical research endeavor. The Seattle physicians did say they plan to enroll patients on clinical trials only when the trial offers a high chance of benefiting the patient over standard therapy alone.
Fortunately, the National Institutes of Health and Food and Drug Administration have released guidance documents related to clinical trials.
The National Cancer Institute (NCI) has also released guidance documents (March 13 guidance; March 23 guidance) for patients on clinical trials supported by the NCI Cancer Therapy Evaluation Program (CTEP) and the NCI Community Oncology Research Program (NCORP).
CTEP and NCORP are making reasonable accommodations to suspend monitoring visits and audits, allow tele–follow-up visits for patients, and permit local physicians to provide care for patients on study. In addition, with appropriate procedural adherence and documentation, CTEP and NCORP will allow oral investigational medicines to be mailed directly to patients’ homes.
Planned NCI National Clinical Trials Network meetings will be conducted via remote access webinars, conference calls, and similar technology. These adjustments – and probably many more to come – are geared toward facilitating ongoing care to proceed safely and with minimal risk for patients currently receiving investigational therapies and for the sites and investigators engaged in those studies.
Each of us has probably faced a personal “defining professional moment,” when we had to utilize every skill in our arsenal and examine the motivations that led us to a career in oncology. However, it is clear from the forgoing clinical and research processes and guidelines that the COVID-19 pandemic is such a defining professional moment for each of us, in every community we serve.
Critical junctures like this cause more rapid behavior change and innovation than the slow-moving pace that characterizes our idealized preferences. As oncologists who embrace new data and behavioral change, we stand to learn processes that will facilitate more perfected systems of care than the one that preceded this unprecedented crisis, promote more efficient sharing of high-quality information, and improve the outcome for our future patients.
Dr. Lyss was an oncologist and researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
I have no knowledge of, or experience with, managing a cancer patient during a pandemic. However, from the published and otherwise shared experience of others, we should not allow ourselves to underestimate the voracity of the coronavirus pandemic on our patients, communities, and health care systems.
Data from China suggest cancer patients infected with SARS-CoV-2 face a 3.5 times higher risk of mechanical ventilation, intensive care unit admission, or death, compared with infected patients without cancer (Lancet Oncol 2020;21:335-7).
Health care workers in Seattle have also shared their experiences battling coronavirus infections in cancer patients (J Natl Compr Canc Netw. 2020 Mar 20. doi: 10.6004/jnccn.2020.7560). Masumi Ueda, MD, of Seattle Cancer Care Alliance, and colleagues reviewed their decisions in multiple domains over a 7-week period, during which the state of Washington went from a single case of SARS-CoV-2 infection to nearly 650 cases and 40 deaths.
Making tough treatment decisions
Dr. Ueda and colleagues contrasted their customary resource-rich, innovation-oriented, cancer-combatting environment with their current circumstance, in which they must prioritize treatment for patients for whom the risk-reward balance has tilted substantially toward “risk.”
The authors noted that their most difficult decisions were those regarding delay of cancer treatment. They suggested that plans for potentially curative adjuvant therapy should likely proceed, but, for patients with metastatic disease, the equation is more nuanced.
In some cases, treatment should be delayed or interrupted with recognition of how that could result in worsening performance status and admission for symptom palliation, further stressing inpatient resources.
The authors suggested scenarios for prioritizing cancer surgery. For example, several months of systemic therapy (ideally, low-risk systemic therapy such as hormone therapy for breast or prostate cancer) and surgical delay may be worthwhile, without compromising patient care.
Patients with aggressive hematologic malignancy requiring urgent systemic treatment (potentially stem cell transplantation and cellular immunotherapies) should be treated promptly. However, even in those cases, opportunities should be sought to lessen immunosuppression and transition care as quickly as possible to the outpatient clinic, according to guidelines from the American Society of Transplantation and Cellular Therapy.
See one, do one, teach one
Rendering patient care during a pandemic would be unique for me. However, I, like all physicians, am familiar with feelings of inadequacy at times of professional challenge. On countless occasions, I have started my day or walked into a patient’s room wondering whether I will have the fortitude, knowledge, creativity, or help I need to get through that day or make that patient “better” by any definition of that word.
We all know the formula: “Work hard. Make evidence-based, personalized decisions for those who have entrusted their care to us. Learn from those encounters. Teach from our knowledge and experience – that is, ‘See one, do one, teach one.’ ”
The Seattle oncologists are living the lives of first responders and deserve our admiration for putting pen to paper so we can learn from their considerable, relevant experience.
Similar admiration is due to Giuseppe Curigliano, MD, of the European Institute of Oncology in Milan. In the ASCO Daily News, Dr. Curigliano described an epidemic that, within 3 weeks, overloaded the health care system across northern Italy.
Hospitalization was needed for over 60% of infected patients, and nearly 15% of those patients needed intensive care unit services for respiratory distress. The Italians centralized oncology care in specialized hubs, with spokes of institutions working in parallel to provide cancer-specific care in a COVID-free environment.
To build upon cancer-specific information from Italy and other areas hard-hit by COVID-19, more than 30 cancer centers have joined together to form the COVID-19 and Cancer Consortium. The consortium’s website hosts a survey designed to “capture details related to cancer patients presumed to have COVID-19.”
Calculating deaths and long-term consequences for cancer care delivery
It is proper that the authors from China, Italy, and Seattle did not focus attention on the case fatality rate from the COVID-19 pandemic among cancer patients. To say the least, it would be complicated to tally the direct mortality – either overall or in clinically important subsets of patients, including country-specific cohorts.
What we know from published reports is that, in Italy, cancer patients account for about 20% of deaths from coronavirus. In China, the case-fatality rate for patients with cancer was 5.6% (JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648).
However, we know nothing about the indirect death toll from malignancy (without coronavirus infection) that was untreated or managed less than optimally because of personnel and physical resources that were diverted to COVID-19–associated cases.
Similarly, we cannot begin to estimate indirect consequences of the pandemic to oncology practices, such as accelerated burnout and posttraumatic stress disorder, as well as the long-range effects of economic turmoil on patients, health care workers, and provider organizations.
What happens to cancer trials?
From China, Italy, and Seattle, thus far, there is little information about how the pandemic will affect the vital clinical research endeavor. The Seattle physicians did say they plan to enroll patients on clinical trials only when the trial offers a high chance of benefiting the patient over standard therapy alone.
Fortunately, the National Institutes of Health and Food and Drug Administration have released guidance documents related to clinical trials.
The National Cancer Institute (NCI) has also released guidance documents (March 13 guidance; March 23 guidance) for patients on clinical trials supported by the NCI Cancer Therapy Evaluation Program (CTEP) and the NCI Community Oncology Research Program (NCORP).
CTEP and NCORP are making reasonable accommodations to suspend monitoring visits and audits, allow tele–follow-up visits for patients, and permit local physicians to provide care for patients on study. In addition, with appropriate procedural adherence and documentation, CTEP and NCORP will allow oral investigational medicines to be mailed directly to patients’ homes.
Planned NCI National Clinical Trials Network meetings will be conducted via remote access webinars, conference calls, and similar technology. These adjustments – and probably many more to come – are geared toward facilitating ongoing care to proceed safely and with minimal risk for patients currently receiving investigational therapies and for the sites and investigators engaged in those studies.
Each of us has probably faced a personal “defining professional moment,” when we had to utilize every skill in our arsenal and examine the motivations that led us to a career in oncology. However, it is clear from the forgoing clinical and research processes and guidelines that the COVID-19 pandemic is such a defining professional moment for each of us, in every community we serve.
Critical junctures like this cause more rapid behavior change and innovation than the slow-moving pace that characterizes our idealized preferences. As oncologists who embrace new data and behavioral change, we stand to learn processes that will facilitate more perfected systems of care than the one that preceded this unprecedented crisis, promote more efficient sharing of high-quality information, and improve the outcome for our future patients.
Dr. Lyss was an oncologist and researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Psychiatrists deemed ‘essential’ in time of COVID-19
New American Psychiatric Association poll shows depth of anxiety
The coronavirus pandemic weighs heavily on psychiatric patients with conditions such as anxiety, depression and PTSD. Meanwhile, a national poll released March 25 by the American Psychiatric Association shows that almost half of all Americans are anxious about contracting COVID-19 and 40% are anxious about becoming seriously ill or dying from the virus. In light of stressors on patients and nonpatients alike, mental health professionals have a key role in helping to alleviate suffering tied to the public health crisis, according to psychiatrists from across the country.
“There’s so much we can do to help people put order on this chaos,” said Shaili Jain, MD, section chief of outpatient mental health with the Veterans Affairs Palo Alto (Calif.) Health Care System, in an interview. “We are essential workers in this time.”
Dr. Jain, who specializes in treating PTSD, said those patients are especially vulnerable to the stress and disruptions spawned by the pandemic. “When you go to the grocery store and there’s no food, that can be triggering for people who survived situations with a feeling of calamity or panic,” she said. “People are reporting worsening of nightmares and spontaneous panic attacks after having been stable with symptoms for many months. These are the kinds of stories that are starting to filter through.”
To make things even more difficult, she said, shelter-in-place orders are preventing patients from taking advantage of healthy coping strategies, such as working out at the gym or going to support groups. “We have an invaluable role to play in trying to prevent long-term consequences by going into problem-solving modes with patients.” Dr. Jain offered several tips that might help patients who are suffering:
- Use technology to stay in touch with support communities and boost self-care. “How can you be flexible with FaceTime, Skype, or phone even if you might not be able to have that face-to-face time? What are you doing to double down on your efforts at self-care – listening to music, reading, daily meditation, or walks? Double down on what you can do to prevent anxiety and stress levels from building up.”
- Take breaks from the news, which can contribute to hypervigilance and disrupted sleep. “I’m seeing that people are going down these rabbit holes of having the news or social media on 24/7,” Dr. Jain said. “You have to stay informed. But you need to pick trusted news sources and have chunks of time that are free of coronavirus coverage.” Understand that life is going to be difficult for a while. “We’re doing a lot of reassurance and education,” she said, “helping people to know and accept that the next few days, weeks, and months are going to be stressful.”
Dr. Jain cautioned colleagues, however, that “there will be a tsunami” of mental illness when the coronavirus crisis lifts. She is especially concerned about patient populations that are socioeconomically disadvantaged already and how their lives with be affected by lost wages, unemployment, and business failures. “Medical professionals will see the consequences of this in the days and weeks and months after the pandemic has settled,” she predicted.
The APA poll shows that, early in the crisis, more than 60% of people are anxious about family and loved ones contracting COVID-19.
Maintaining ‘reflective space’ essential
At the Austen Riggs Center, a psychiatric residential treatment facility in Stockbridge, Mass., staff and patients are adjusting to new rules that aim to prevent transmission of the novel coronavirus. “Social distancing requirements are having a huge impact,” said Eric M. Plakun, MD, medical director and CEO of Austen Riggs, in an interview. “You can’t have groups in the same way; you can’t have families come in for a family meeting; you can’t have quite the same the freedom to come and go. A lot of management issues are being addressed, but it is crucial also to maintain the ‘reflective space’ essential to do the kind of clinical work we do.” One approach, he said, is virtual meetings with colleagues that address on-the-job management issues, but also leave a space for how staff members are feeling.
“It’s easy to get into crisis-response mode,” he said, “where you’re always managing but never leave a space to talk about vulnerability, helplessness, and fear.”
As the facility’s staff adjusts by embracing teleconference technology and adapting group meetings to the 6-feet-apart rule,
Dr. Plakun said he said, noting that patients have approached staff members to say they want to collaborate about changes. “That’s a credible offer we intend to accept.”
Still, communicating with patients as a whole about the coronavirus can be difficult. As Dr. Plakun noted, it’s now impossible to bring 75 people together into one room for a meeting. “If you have four to five smaller meetings, how do you maintain some congruence in the information that’s presented?”
Dr. Plakun suggested that colleagues find time to engage in the familiar, such as face-to-face clinical work. “That’s been the most reassuring and rewarding part of my day since it feels almost like normal,” he said.
Stocking up on medications
Jessica “Jessi” Gold, MD, MS, an assistant professor at Washington University in St. Louis, often treats college students. Asian students started to worry early in the pandemic, she said in an interview.
“At the beginning, there were a lot of concerns about the public’s view: ‘Did this come from China? Is it China’s fault?’ A lot of our students felt that if they coughed, and they were a white person, they’d be OK. But if they were Asian, everyone would wonder why they were in class and not at home. That got worse over time: the fear about – and anxiety from – stoking racism.”
Later, as classes began to be canceled, Dr. Gold started to see the psychological effects of disruption and uncertainty about the future. “This can lead people to feel like what they knew before is just not there anymore. This can obviously cause anxiety but also has the potential to cause depression.” Patients also might slip into overuse of alcohol and drugs, or they might engage in other kinds of harmful behavior. Eating disorders, for example, “are ways to have control when other things aren’t in control,” she said.
Dr. Gold pointed to research into the mental health after effects of quarantines, such as those imposed during the SARS outbreak. A review of 24 studies published this year found that most “reported negative psychological effects, including post-traumatic stress symptoms, confusion, and anger. Stressors included longer quarantine duration, infection fears, frustration, boredom, inadequate supplies, inadequate information, financial loss, and stigma. Some researchers have suggested long-lasting effects” (Lancet. 2020;395:912-20).
Dr. Gold is urging patients to recall the warning signs that alerted them to psychological downturns in the past: “Try to remember what those warning signs are and pay attention to whether you see them.” And, Dr. Gold said, she asks patients to think about what has helped them get better.
In some cases, she said, patients are already preparing themselves for experiencing mental distress by stocking up on medications. “Some people have a bottle of 10-20 pills that they only use in emergencies and keep as a kind of security blanket,” she said, and she’s seen some of them ask for refills. It seems they’ve either taken the pills recently or want to stash them just in case. This makes sense, since their anxiety is higher, she said.
Dr. Gold cautioned that psychiatrists need to be careful to not overextend themselves when they’re not treating patients. “It is easy to be therapist to friends, family, and colleagues,” she said, “but we need to take care of ourselves, too.”
Dr. Jain is author of “The Unspeakable Mind: Stories of Trauma and Healing From the Frontlines of PTSD Science” (New York: Harper, 2019). She has no other disclosures. Dr. Plakun and Dr. Gold reported no relevant disclosures.
New American Psychiatric Association poll shows depth of anxiety
New American Psychiatric Association poll shows depth of anxiety
The coronavirus pandemic weighs heavily on psychiatric patients with conditions such as anxiety, depression and PTSD. Meanwhile, a national poll released March 25 by the American Psychiatric Association shows that almost half of all Americans are anxious about contracting COVID-19 and 40% are anxious about becoming seriously ill or dying from the virus. In light of stressors on patients and nonpatients alike, mental health professionals have a key role in helping to alleviate suffering tied to the public health crisis, according to psychiatrists from across the country.
“There’s so much we can do to help people put order on this chaos,” said Shaili Jain, MD, section chief of outpatient mental health with the Veterans Affairs Palo Alto (Calif.) Health Care System, in an interview. “We are essential workers in this time.”
Dr. Jain, who specializes in treating PTSD, said those patients are especially vulnerable to the stress and disruptions spawned by the pandemic. “When you go to the grocery store and there’s no food, that can be triggering for people who survived situations with a feeling of calamity or panic,” she said. “People are reporting worsening of nightmares and spontaneous panic attacks after having been stable with symptoms for many months. These are the kinds of stories that are starting to filter through.”
To make things even more difficult, she said, shelter-in-place orders are preventing patients from taking advantage of healthy coping strategies, such as working out at the gym or going to support groups. “We have an invaluable role to play in trying to prevent long-term consequences by going into problem-solving modes with patients.” Dr. Jain offered several tips that might help patients who are suffering:
- Use technology to stay in touch with support communities and boost self-care. “How can you be flexible with FaceTime, Skype, or phone even if you might not be able to have that face-to-face time? What are you doing to double down on your efforts at self-care – listening to music, reading, daily meditation, or walks? Double down on what you can do to prevent anxiety and stress levels from building up.”
- Take breaks from the news, which can contribute to hypervigilance and disrupted sleep. “I’m seeing that people are going down these rabbit holes of having the news or social media on 24/7,” Dr. Jain said. “You have to stay informed. But you need to pick trusted news sources and have chunks of time that are free of coronavirus coverage.” Understand that life is going to be difficult for a while. “We’re doing a lot of reassurance and education,” she said, “helping people to know and accept that the next few days, weeks, and months are going to be stressful.”
Dr. Jain cautioned colleagues, however, that “there will be a tsunami” of mental illness when the coronavirus crisis lifts. She is especially concerned about patient populations that are socioeconomically disadvantaged already and how their lives with be affected by lost wages, unemployment, and business failures. “Medical professionals will see the consequences of this in the days and weeks and months after the pandemic has settled,” she predicted.
The APA poll shows that, early in the crisis, more than 60% of people are anxious about family and loved ones contracting COVID-19.
Maintaining ‘reflective space’ essential
At the Austen Riggs Center, a psychiatric residential treatment facility in Stockbridge, Mass., staff and patients are adjusting to new rules that aim to prevent transmission of the novel coronavirus. “Social distancing requirements are having a huge impact,” said Eric M. Plakun, MD, medical director and CEO of Austen Riggs, in an interview. “You can’t have groups in the same way; you can’t have families come in for a family meeting; you can’t have quite the same the freedom to come and go. A lot of management issues are being addressed, but it is crucial also to maintain the ‘reflective space’ essential to do the kind of clinical work we do.” One approach, he said, is virtual meetings with colleagues that address on-the-job management issues, but also leave a space for how staff members are feeling.
“It’s easy to get into crisis-response mode,” he said, “where you’re always managing but never leave a space to talk about vulnerability, helplessness, and fear.”
As the facility’s staff adjusts by embracing teleconference technology and adapting group meetings to the 6-feet-apart rule,
Dr. Plakun said he said, noting that patients have approached staff members to say they want to collaborate about changes. “That’s a credible offer we intend to accept.”
Still, communicating with patients as a whole about the coronavirus can be difficult. As Dr. Plakun noted, it’s now impossible to bring 75 people together into one room for a meeting. “If you have four to five smaller meetings, how do you maintain some congruence in the information that’s presented?”
Dr. Plakun suggested that colleagues find time to engage in the familiar, such as face-to-face clinical work. “That’s been the most reassuring and rewarding part of my day since it feels almost like normal,” he said.
Stocking up on medications
Jessica “Jessi” Gold, MD, MS, an assistant professor at Washington University in St. Louis, often treats college students. Asian students started to worry early in the pandemic, she said in an interview.
“At the beginning, there were a lot of concerns about the public’s view: ‘Did this come from China? Is it China’s fault?’ A lot of our students felt that if they coughed, and they were a white person, they’d be OK. But if they were Asian, everyone would wonder why they were in class and not at home. That got worse over time: the fear about – and anxiety from – stoking racism.”
Later, as classes began to be canceled, Dr. Gold started to see the psychological effects of disruption and uncertainty about the future. “This can lead people to feel like what they knew before is just not there anymore. This can obviously cause anxiety but also has the potential to cause depression.” Patients also might slip into overuse of alcohol and drugs, or they might engage in other kinds of harmful behavior. Eating disorders, for example, “are ways to have control when other things aren’t in control,” she said.
Dr. Gold pointed to research into the mental health after effects of quarantines, such as those imposed during the SARS outbreak. A review of 24 studies published this year found that most “reported negative psychological effects, including post-traumatic stress symptoms, confusion, and anger. Stressors included longer quarantine duration, infection fears, frustration, boredom, inadequate supplies, inadequate information, financial loss, and stigma. Some researchers have suggested long-lasting effects” (Lancet. 2020;395:912-20).
Dr. Gold is urging patients to recall the warning signs that alerted them to psychological downturns in the past: “Try to remember what those warning signs are and pay attention to whether you see them.” And, Dr. Gold said, she asks patients to think about what has helped them get better.
In some cases, she said, patients are already preparing themselves for experiencing mental distress by stocking up on medications. “Some people have a bottle of 10-20 pills that they only use in emergencies and keep as a kind of security blanket,” she said, and she’s seen some of them ask for refills. It seems they’ve either taken the pills recently or want to stash them just in case. This makes sense, since their anxiety is higher, she said.
Dr. Gold cautioned that psychiatrists need to be careful to not overextend themselves when they’re not treating patients. “It is easy to be therapist to friends, family, and colleagues,” she said, “but we need to take care of ourselves, too.”
Dr. Jain is author of “The Unspeakable Mind: Stories of Trauma and Healing From the Frontlines of PTSD Science” (New York: Harper, 2019). She has no other disclosures. Dr. Plakun and Dr. Gold reported no relevant disclosures.
The coronavirus pandemic weighs heavily on psychiatric patients with conditions such as anxiety, depression and PTSD. Meanwhile, a national poll released March 25 by the American Psychiatric Association shows that almost half of all Americans are anxious about contracting COVID-19 and 40% are anxious about becoming seriously ill or dying from the virus. In light of stressors on patients and nonpatients alike, mental health professionals have a key role in helping to alleviate suffering tied to the public health crisis, according to psychiatrists from across the country.
“There’s so much we can do to help people put order on this chaos,” said Shaili Jain, MD, section chief of outpatient mental health with the Veterans Affairs Palo Alto (Calif.) Health Care System, in an interview. “We are essential workers in this time.”
Dr. Jain, who specializes in treating PTSD, said those patients are especially vulnerable to the stress and disruptions spawned by the pandemic. “When you go to the grocery store and there’s no food, that can be triggering for people who survived situations with a feeling of calamity or panic,” she said. “People are reporting worsening of nightmares and spontaneous panic attacks after having been stable with symptoms for many months. These are the kinds of stories that are starting to filter through.”
To make things even more difficult, she said, shelter-in-place orders are preventing patients from taking advantage of healthy coping strategies, such as working out at the gym or going to support groups. “We have an invaluable role to play in trying to prevent long-term consequences by going into problem-solving modes with patients.” Dr. Jain offered several tips that might help patients who are suffering:
- Use technology to stay in touch with support communities and boost self-care. “How can you be flexible with FaceTime, Skype, or phone even if you might not be able to have that face-to-face time? What are you doing to double down on your efforts at self-care – listening to music, reading, daily meditation, or walks? Double down on what you can do to prevent anxiety and stress levels from building up.”
- Take breaks from the news, which can contribute to hypervigilance and disrupted sleep. “I’m seeing that people are going down these rabbit holes of having the news or social media on 24/7,” Dr. Jain said. “You have to stay informed. But you need to pick trusted news sources and have chunks of time that are free of coronavirus coverage.” Understand that life is going to be difficult for a while. “We’re doing a lot of reassurance and education,” she said, “helping people to know and accept that the next few days, weeks, and months are going to be stressful.”
Dr. Jain cautioned colleagues, however, that “there will be a tsunami” of mental illness when the coronavirus crisis lifts. She is especially concerned about patient populations that are socioeconomically disadvantaged already and how their lives with be affected by lost wages, unemployment, and business failures. “Medical professionals will see the consequences of this in the days and weeks and months after the pandemic has settled,” she predicted.
The APA poll shows that, early in the crisis, more than 60% of people are anxious about family and loved ones contracting COVID-19.
Maintaining ‘reflective space’ essential
At the Austen Riggs Center, a psychiatric residential treatment facility in Stockbridge, Mass., staff and patients are adjusting to new rules that aim to prevent transmission of the novel coronavirus. “Social distancing requirements are having a huge impact,” said Eric M. Plakun, MD, medical director and CEO of Austen Riggs, in an interview. “You can’t have groups in the same way; you can’t have families come in for a family meeting; you can’t have quite the same the freedom to come and go. A lot of management issues are being addressed, but it is crucial also to maintain the ‘reflective space’ essential to do the kind of clinical work we do.” One approach, he said, is virtual meetings with colleagues that address on-the-job management issues, but also leave a space for how staff members are feeling.
“It’s easy to get into crisis-response mode,” he said, “where you’re always managing but never leave a space to talk about vulnerability, helplessness, and fear.”
As the facility’s staff adjusts by embracing teleconference technology and adapting group meetings to the 6-feet-apart rule,
Dr. Plakun said he said, noting that patients have approached staff members to say they want to collaborate about changes. “That’s a credible offer we intend to accept.”
Still, communicating with patients as a whole about the coronavirus can be difficult. As Dr. Plakun noted, it’s now impossible to bring 75 people together into one room for a meeting. “If you have four to five smaller meetings, how do you maintain some congruence in the information that’s presented?”
Dr. Plakun suggested that colleagues find time to engage in the familiar, such as face-to-face clinical work. “That’s been the most reassuring and rewarding part of my day since it feels almost like normal,” he said.
Stocking up on medications
Jessica “Jessi” Gold, MD, MS, an assistant professor at Washington University in St. Louis, often treats college students. Asian students started to worry early in the pandemic, she said in an interview.
“At the beginning, there were a lot of concerns about the public’s view: ‘Did this come from China? Is it China’s fault?’ A lot of our students felt that if they coughed, and they were a white person, they’d be OK. But if they were Asian, everyone would wonder why they were in class and not at home. That got worse over time: the fear about – and anxiety from – stoking racism.”
Later, as classes began to be canceled, Dr. Gold started to see the psychological effects of disruption and uncertainty about the future. “This can lead people to feel like what they knew before is just not there anymore. This can obviously cause anxiety but also has the potential to cause depression.” Patients also might slip into overuse of alcohol and drugs, or they might engage in other kinds of harmful behavior. Eating disorders, for example, “are ways to have control when other things aren’t in control,” she said.
Dr. Gold pointed to research into the mental health after effects of quarantines, such as those imposed during the SARS outbreak. A review of 24 studies published this year found that most “reported negative psychological effects, including post-traumatic stress symptoms, confusion, and anger. Stressors included longer quarantine duration, infection fears, frustration, boredom, inadequate supplies, inadequate information, financial loss, and stigma. Some researchers have suggested long-lasting effects” (Lancet. 2020;395:912-20).
Dr. Gold is urging patients to recall the warning signs that alerted them to psychological downturns in the past: “Try to remember what those warning signs are and pay attention to whether you see them.” And, Dr. Gold said, she asks patients to think about what has helped them get better.
In some cases, she said, patients are already preparing themselves for experiencing mental distress by stocking up on medications. “Some people have a bottle of 10-20 pills that they only use in emergencies and keep as a kind of security blanket,” she said, and she’s seen some of them ask for refills. It seems they’ve either taken the pills recently or want to stash them just in case. This makes sense, since their anxiety is higher, she said.
Dr. Gold cautioned that psychiatrists need to be careful to not overextend themselves when they’re not treating patients. “It is easy to be therapist to friends, family, and colleagues,” she said, “but we need to take care of ourselves, too.”
Dr. Jain is author of “The Unspeakable Mind: Stories of Trauma and Healing From the Frontlines of PTSD Science” (New York: Harper, 2019). She has no other disclosures. Dr. Plakun and Dr. Gold reported no relevant disclosures.
Is COVID-19 leading to a mental illness pandemic?
People living through this crisis are experiencing trauma
We are in the midst of an epidemic and possibly pandemic of anxiety and distress. The worry that folks have about themselves, families, finances, and work is overwhelming for millions.
I speak with people who report periods of racing thoughts jumping back in time and thinking of roads not taken. They also talk about their thoughts jumping forward with life plans of what they’ll do to change their lives in the future – if they survive COVID-19.
that is well-controlled with care (and even without care). Those people are suffering even more. Meanwhile, people with obsessive-compulsive disorder that had been under control appear to have worsened with the added stress.
Social distancing has disrupted our everyday routines. For many, there is no work, no spending time with people we care about, no going to movies or shows, no doing discretionary shopping, no going to school. Parents with children at home report frustration about balancing working from home with completing home-schooling packets. Physicians on the front lines of this unprecedented time report not having the proper protective equipment and worrying about the possibility of exposing their families to SARS-CoV-2.
We hear stories about the illness and even deaths of some young and middle-aged people with no underlying conditions, not to mention the loss of older adults. People are bursting into tears, and becoming easily frustrated and angry. Add in nightmares, ongoing anxiety states, insomnia, and decreased concentration.
We are seeing news reports of people stocking up on guns and ammunition and a case of one taking – and dying from – nonpharmaceutical grade chloroquine in an effort to prevent COVID-19.
I spoke with Juliana Tseng, PsyD, a clinical psychologist based in New York, and she said that the hype, half-truths, and false information from some outlets in the popular media are making things worse. Dr. Tseng added that the lack of coordination among local, state, and federal governments also is increasing fear and alienation.
As I see this period in time, my first thoughts are that we are witnessing a national epidemic of trauma. Specifically, what we have here is a clinical picture of PTSD.
PTSD is defined clearly as a traumatic disorder with a real or perceived fracture with life. Isolation (which we are creating as a way to “flatten the curve” or slow the spread of COVID-19), although that strategy is in our best personal and public health interests, is both painful and stressful. Frustration, flashbacks of past life experiences plus flashbacks of being ill are reported in people I’ve spoken with. Avoidance, even though it is planned in this instance, is part of the PTSD complex.
What can we as mental health professionals do to help alleviate this suffering?
First, of course, we must listen to the scientific experts and the data – and tell people to do the same. Most experts will say that COVID-19 is a mild or moderate illness for the vast majority of people. We also must encourage people to observe precautions outlined by the Centers for Disease Control and Prevention, such as distancing from people, hand washing, and avoiding those who are ill. Explain to people that, currently, there is no vaccine to prevent COVID-19. Treatment is mainly supportive, and some medication trials are being explored. However, we can empower people by helping them to develop skills aimed at increasing the ability to relax and focus on more positive aspects of life to break the chain of the stress and tension of anxiety as well as control the PTSD.
For more than 40 years, I have helped people master relaxation techniques and guided imagery. When taught properly, people are able to use these techniques on their own.
To begin, I teach people how to relax, using a simple three-point method:
- Get comfortable in a nice chair, and slowly count from one to three. At the count of one, do one thing: “roll your eyes up to the top of your head.”
- At the count of two, do two things, “close your lids on your eyes and take a deep breath.”
- At three, exhale slowly, relax your eyes, and concentrate on a restful feeling of floating.
- Do this for about 30 seconds to a minute.
- Count backward, from three to two to one and open your eyes.
The person will notice how nice and restful they will feel.
After that exercise, get the person to move to the graduate level and go beyond just relaxation. In the following exercise, people can go into a relaxed state by imagining a movie screen. Tell the person to do two things:
1. Look at the imagined movie screen and project on it any pleasant scene you wish; this is your screen. You will feel yourself becoming more and more relaxed. The person can do this one, two, three or whatever times a day. The exercise can last 1 minute or 5.
2. Incorporate the 1, 2, 3 relaxation described earlier, allowing yourself to float into this restful state and go to your movie screen. Now, on the screen, imagine a thick line down the center, and on the left side, project your worries and anxieties and fears. The idea is to see but not experience them. Then shift to the ride side of the screen, and again, visualize any pleasant scene you wish. Again, do this for 1 minute or 5 minutes, whatever works.
You will notice that the pleasant scene on the right will overcome the anxiety scene on the left, in that pleasantness, in most instances, overcomes anxiety. For many, these techniques have proved very useful – whether the problem is anxiety or fear – or both. In my experience, these techniques are a good beginning for controlling PTSD and successfully treating it.
We are in the midst of what could be the biggest public health crisis that America has faced since the 1918 pandemic, also known as the Spanish flu. The lockdowns, quarantines, and the myriad of other disruptions can lead to alienation. In fact, it would be strange for us not to experience strong emotions under these extreme conditions. Life will get better! In the meantime, let’s encourage people to hope, pray, and use relaxation techniques and guided imagery approaches to help control anxiety, worry, stress, and issues related to PTSD. These approaches can give our minds and bodies periods of relaxation and recovery, and ultimately, they can calm our minds.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
People living through this crisis are experiencing trauma
People living through this crisis are experiencing trauma
We are in the midst of an epidemic and possibly pandemic of anxiety and distress. The worry that folks have about themselves, families, finances, and work is overwhelming for millions.
I speak with people who report periods of racing thoughts jumping back in time and thinking of roads not taken. They also talk about their thoughts jumping forward with life plans of what they’ll do to change their lives in the future – if they survive COVID-19.
that is well-controlled with care (and even without care). Those people are suffering even more. Meanwhile, people with obsessive-compulsive disorder that had been under control appear to have worsened with the added stress.
Social distancing has disrupted our everyday routines. For many, there is no work, no spending time with people we care about, no going to movies or shows, no doing discretionary shopping, no going to school. Parents with children at home report frustration about balancing working from home with completing home-schooling packets. Physicians on the front lines of this unprecedented time report not having the proper protective equipment and worrying about the possibility of exposing their families to SARS-CoV-2.
We hear stories about the illness and even deaths of some young and middle-aged people with no underlying conditions, not to mention the loss of older adults. People are bursting into tears, and becoming easily frustrated and angry. Add in nightmares, ongoing anxiety states, insomnia, and decreased concentration.
We are seeing news reports of people stocking up on guns and ammunition and a case of one taking – and dying from – nonpharmaceutical grade chloroquine in an effort to prevent COVID-19.
I spoke with Juliana Tseng, PsyD, a clinical psychologist based in New York, and she said that the hype, half-truths, and false information from some outlets in the popular media are making things worse. Dr. Tseng added that the lack of coordination among local, state, and federal governments also is increasing fear and alienation.
As I see this period in time, my first thoughts are that we are witnessing a national epidemic of trauma. Specifically, what we have here is a clinical picture of PTSD.
PTSD is defined clearly as a traumatic disorder with a real or perceived fracture with life. Isolation (which we are creating as a way to “flatten the curve” or slow the spread of COVID-19), although that strategy is in our best personal and public health interests, is both painful and stressful. Frustration, flashbacks of past life experiences plus flashbacks of being ill are reported in people I’ve spoken with. Avoidance, even though it is planned in this instance, is part of the PTSD complex.
What can we as mental health professionals do to help alleviate this suffering?
First, of course, we must listen to the scientific experts and the data – and tell people to do the same. Most experts will say that COVID-19 is a mild or moderate illness for the vast majority of people. We also must encourage people to observe precautions outlined by the Centers for Disease Control and Prevention, such as distancing from people, hand washing, and avoiding those who are ill. Explain to people that, currently, there is no vaccine to prevent COVID-19. Treatment is mainly supportive, and some medication trials are being explored. However, we can empower people by helping them to develop skills aimed at increasing the ability to relax and focus on more positive aspects of life to break the chain of the stress and tension of anxiety as well as control the PTSD.
For more than 40 years, I have helped people master relaxation techniques and guided imagery. When taught properly, people are able to use these techniques on their own.
To begin, I teach people how to relax, using a simple three-point method:
- Get comfortable in a nice chair, and slowly count from one to three. At the count of one, do one thing: “roll your eyes up to the top of your head.”
- At the count of two, do two things, “close your lids on your eyes and take a deep breath.”
- At three, exhale slowly, relax your eyes, and concentrate on a restful feeling of floating.
- Do this for about 30 seconds to a minute.
- Count backward, from three to two to one and open your eyes.
The person will notice how nice and restful they will feel.
After that exercise, get the person to move to the graduate level and go beyond just relaxation. In the following exercise, people can go into a relaxed state by imagining a movie screen. Tell the person to do two things:
1. Look at the imagined movie screen and project on it any pleasant scene you wish; this is your screen. You will feel yourself becoming more and more relaxed. The person can do this one, two, three or whatever times a day. The exercise can last 1 minute or 5.
2. Incorporate the 1, 2, 3 relaxation described earlier, allowing yourself to float into this restful state and go to your movie screen. Now, on the screen, imagine a thick line down the center, and on the left side, project your worries and anxieties and fears. The idea is to see but not experience them. Then shift to the ride side of the screen, and again, visualize any pleasant scene you wish. Again, do this for 1 minute or 5 minutes, whatever works.
You will notice that the pleasant scene on the right will overcome the anxiety scene on the left, in that pleasantness, in most instances, overcomes anxiety. For many, these techniques have proved very useful – whether the problem is anxiety or fear – or both. In my experience, these techniques are a good beginning for controlling PTSD and successfully treating it.
We are in the midst of what could be the biggest public health crisis that America has faced since the 1918 pandemic, also known as the Spanish flu. The lockdowns, quarantines, and the myriad of other disruptions can lead to alienation. In fact, it would be strange for us not to experience strong emotions under these extreme conditions. Life will get better! In the meantime, let’s encourage people to hope, pray, and use relaxation techniques and guided imagery approaches to help control anxiety, worry, stress, and issues related to PTSD. These approaches can give our minds and bodies periods of relaxation and recovery, and ultimately, they can calm our minds.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
We are in the midst of an epidemic and possibly pandemic of anxiety and distress. The worry that folks have about themselves, families, finances, and work is overwhelming for millions.
I speak with people who report periods of racing thoughts jumping back in time and thinking of roads not taken. They also talk about their thoughts jumping forward with life plans of what they’ll do to change their lives in the future – if they survive COVID-19.
that is well-controlled with care (and even without care). Those people are suffering even more. Meanwhile, people with obsessive-compulsive disorder that had been under control appear to have worsened with the added stress.
Social distancing has disrupted our everyday routines. For many, there is no work, no spending time with people we care about, no going to movies or shows, no doing discretionary shopping, no going to school. Parents with children at home report frustration about balancing working from home with completing home-schooling packets. Physicians on the front lines of this unprecedented time report not having the proper protective equipment and worrying about the possibility of exposing their families to SARS-CoV-2.
We hear stories about the illness and even deaths of some young and middle-aged people with no underlying conditions, not to mention the loss of older adults. People are bursting into tears, and becoming easily frustrated and angry. Add in nightmares, ongoing anxiety states, insomnia, and decreased concentration.
We are seeing news reports of people stocking up on guns and ammunition and a case of one taking – and dying from – nonpharmaceutical grade chloroquine in an effort to prevent COVID-19.
I spoke with Juliana Tseng, PsyD, a clinical psychologist based in New York, and she said that the hype, half-truths, and false information from some outlets in the popular media are making things worse. Dr. Tseng added that the lack of coordination among local, state, and federal governments also is increasing fear and alienation.
As I see this period in time, my first thoughts are that we are witnessing a national epidemic of trauma. Specifically, what we have here is a clinical picture of PTSD.
PTSD is defined clearly as a traumatic disorder with a real or perceived fracture with life. Isolation (which we are creating as a way to “flatten the curve” or slow the spread of COVID-19), although that strategy is in our best personal and public health interests, is both painful and stressful. Frustration, flashbacks of past life experiences plus flashbacks of being ill are reported in people I’ve spoken with. Avoidance, even though it is planned in this instance, is part of the PTSD complex.
What can we as mental health professionals do to help alleviate this suffering?
First, of course, we must listen to the scientific experts and the data – and tell people to do the same. Most experts will say that COVID-19 is a mild or moderate illness for the vast majority of people. We also must encourage people to observe precautions outlined by the Centers for Disease Control and Prevention, such as distancing from people, hand washing, and avoiding those who are ill. Explain to people that, currently, there is no vaccine to prevent COVID-19. Treatment is mainly supportive, and some medication trials are being explored. However, we can empower people by helping them to develop skills aimed at increasing the ability to relax and focus on more positive aspects of life to break the chain of the stress and tension of anxiety as well as control the PTSD.
For more than 40 years, I have helped people master relaxation techniques and guided imagery. When taught properly, people are able to use these techniques on their own.
To begin, I teach people how to relax, using a simple three-point method:
- Get comfortable in a nice chair, and slowly count from one to three. At the count of one, do one thing: “roll your eyes up to the top of your head.”
- At the count of two, do two things, “close your lids on your eyes and take a deep breath.”
- At three, exhale slowly, relax your eyes, and concentrate on a restful feeling of floating.
- Do this for about 30 seconds to a minute.
- Count backward, from three to two to one and open your eyes.
The person will notice how nice and restful they will feel.
After that exercise, get the person to move to the graduate level and go beyond just relaxation. In the following exercise, people can go into a relaxed state by imagining a movie screen. Tell the person to do two things:
1. Look at the imagined movie screen and project on it any pleasant scene you wish; this is your screen. You will feel yourself becoming more and more relaxed. The person can do this one, two, three or whatever times a day. The exercise can last 1 minute or 5.
2. Incorporate the 1, 2, 3 relaxation described earlier, allowing yourself to float into this restful state and go to your movie screen. Now, on the screen, imagine a thick line down the center, and on the left side, project your worries and anxieties and fears. The idea is to see but not experience them. Then shift to the ride side of the screen, and again, visualize any pleasant scene you wish. Again, do this for 1 minute or 5 minutes, whatever works.
You will notice that the pleasant scene on the right will overcome the anxiety scene on the left, in that pleasantness, in most instances, overcomes anxiety. For many, these techniques have proved very useful – whether the problem is anxiety or fear – or both. In my experience, these techniques are a good beginning for controlling PTSD and successfully treating it.
We are in the midst of what could be the biggest public health crisis that America has faced since the 1918 pandemic, also known as the Spanish flu. The lockdowns, quarantines, and the myriad of other disruptions can lead to alienation. In fact, it would be strange for us not to experience strong emotions under these extreme conditions. Life will get better! In the meantime, let’s encourage people to hope, pray, and use relaxation techniques and guided imagery approaches to help control anxiety, worry, stress, and issues related to PTSD. These approaches can give our minds and bodies periods of relaxation and recovery, and ultimately, they can calm our minds.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
Dr. Douglas Paauw reflects on practicing in the COVID-19 world
As we are all facing uncertainties in caring for our patients amid the COVID-19 pandemic, I practice at the University of Washington, Seattle, in an area that initially had the highest prevalence of COVID-19 cases in the United States.
I have never felt better about being a part of the medical profession because of the altruism, compassion, and deep caring I have seen displayed by my colleagues, our nurses, our staff, and our students. I am proud to have worked with all of them while trying to figure out how to practice in this environment.
These times are really difficult and challenging as we face new problems every day. Last week, we had to send our students home, and we switched to phone and telehealth visits to keep our patients and staff safer.
I have had some unanticipated electronic messages from patients during this time. Two of my patients with major medical problems and very dependent on their medications were stranded internationally and running out of medications. I had the family of an incarcerated patient contact me for a letter because that patient was moved to a part of a jail where all patients with upper respiratory infection symptoms were being housed. My patient has severe immunosuppression, and they were requesting an exception for him.
Another of my patients, who has sarcoidosis and is immunosuppressed, informed me that her daughter who lives with her was diagnosed with COVID-19. After 3 days, this patient told me she had become febrile and short of breath. I instructed her patient to go to a hospital, where she was also diagnosed with COVID-19 and was admitted. This patient was discharged within 24 hours, because the utilization review department did not feel she should be in the hospital.
The lack of beds is forcing physicians to frequently make tough decisions like the one made for this patient. This unfortunate reality raises the question of: “How do you manage a patient you are worried about from his or her home?”
In this particular case, I sent my patient an oxygen saturation monitor. We touched base frequently, and I felt okay as long as her saturations on room air were above 90%. So far, she has done okay.
More recently, I received a message from a patient recently diagnosed with Mycobacterium avium complex. I learned that this patient and her disabled husband’s caregiver refused to continue to provide care to them, because my patient had a cough, which began 2 months prior. In this case, a COVID-19 test was done for the explicit purpose of getting the caregiver to return to work.
So how do we face this?
Burnout had been high before this difficult time. But now physicians are being called to care for more and sicker patients without the necessary personal protective gear. Our physicians have demonstrated strength and commitment to patients in their response to this challenge, but they need help from others, including regulators.
I think a first step that needs to be taken is to decrease the volume of documentation physicians are required to make in this time where we are forced to triage to what is most important and drop what isn’t. How is spending so much time documenting instead of seeing the high volumes of patients who need to be seen a good thing? Documentation to the level that Medicare has required isn’t going to work. In fact, it has never been a good thing and is a big driver of burnout.
Our health care system was broken and badly injured before this crisis, and I think now might be a time when positive changes for the future occur. In fact, COVID-19 has resulted in some temporary changes in medicine that I would like to see outlast this outbreak. The telehealth option is now available, for example, and this kind of care is covered much more broadly by Medicare under the 1135 waiver – this has been needed for years. Being able to conduct regular clinic visits via telehealth without the marked restrictions that were previously in place is a big advance. It is currently in place for this emergency only, but this is the time to start pushing hard to make sure this option will be permanent.
I invite you to help me fight for long-term change. Write a letter to the editor of your local newspaper or blog, share your thoughts on social media, and tweet. (I suggest using #documentationordoctors or, although a bit long, #excessivedocumentationcostslives.) This is an unprecedented time in modern medicine. Traumatic times are when the greatest changes occur. Let’s hope for the better.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He frequently contributes Pearl of the Month and Myth of the Month columns to MDedge, and he serves on the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact Dr. Paauw at [email protected].
As we are all facing uncertainties in caring for our patients amid the COVID-19 pandemic, I practice at the University of Washington, Seattle, in an area that initially had the highest prevalence of COVID-19 cases in the United States.
I have never felt better about being a part of the medical profession because of the altruism, compassion, and deep caring I have seen displayed by my colleagues, our nurses, our staff, and our students. I am proud to have worked with all of them while trying to figure out how to practice in this environment.
These times are really difficult and challenging as we face new problems every day. Last week, we had to send our students home, and we switched to phone and telehealth visits to keep our patients and staff safer.
I have had some unanticipated electronic messages from patients during this time. Two of my patients with major medical problems and very dependent on their medications were stranded internationally and running out of medications. I had the family of an incarcerated patient contact me for a letter because that patient was moved to a part of a jail where all patients with upper respiratory infection symptoms were being housed. My patient has severe immunosuppression, and they were requesting an exception for him.
Another of my patients, who has sarcoidosis and is immunosuppressed, informed me that her daughter who lives with her was diagnosed with COVID-19. After 3 days, this patient told me she had become febrile and short of breath. I instructed her patient to go to a hospital, where she was also diagnosed with COVID-19 and was admitted. This patient was discharged within 24 hours, because the utilization review department did not feel she should be in the hospital.
The lack of beds is forcing physicians to frequently make tough decisions like the one made for this patient. This unfortunate reality raises the question of: “How do you manage a patient you are worried about from his or her home?”
In this particular case, I sent my patient an oxygen saturation monitor. We touched base frequently, and I felt okay as long as her saturations on room air were above 90%. So far, she has done okay.
More recently, I received a message from a patient recently diagnosed with Mycobacterium avium complex. I learned that this patient and her disabled husband’s caregiver refused to continue to provide care to them, because my patient had a cough, which began 2 months prior. In this case, a COVID-19 test was done for the explicit purpose of getting the caregiver to return to work.
So how do we face this?
Burnout had been high before this difficult time. But now physicians are being called to care for more and sicker patients without the necessary personal protective gear. Our physicians have demonstrated strength and commitment to patients in their response to this challenge, but they need help from others, including regulators.
I think a first step that needs to be taken is to decrease the volume of documentation physicians are required to make in this time where we are forced to triage to what is most important and drop what isn’t. How is spending so much time documenting instead of seeing the high volumes of patients who need to be seen a good thing? Documentation to the level that Medicare has required isn’t going to work. In fact, it has never been a good thing and is a big driver of burnout.
Our health care system was broken and badly injured before this crisis, and I think now might be a time when positive changes for the future occur. In fact, COVID-19 has resulted in some temporary changes in medicine that I would like to see outlast this outbreak. The telehealth option is now available, for example, and this kind of care is covered much more broadly by Medicare under the 1135 waiver – this has been needed for years. Being able to conduct regular clinic visits via telehealth without the marked restrictions that were previously in place is a big advance. It is currently in place for this emergency only, but this is the time to start pushing hard to make sure this option will be permanent.
I invite you to help me fight for long-term change. Write a letter to the editor of your local newspaper or blog, share your thoughts on social media, and tweet. (I suggest using #documentationordoctors or, although a bit long, #excessivedocumentationcostslives.) This is an unprecedented time in modern medicine. Traumatic times are when the greatest changes occur. Let’s hope for the better.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He frequently contributes Pearl of the Month and Myth of the Month columns to MDedge, and he serves on the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact Dr. Paauw at [email protected].
As we are all facing uncertainties in caring for our patients amid the COVID-19 pandemic, I practice at the University of Washington, Seattle, in an area that initially had the highest prevalence of COVID-19 cases in the United States.
I have never felt better about being a part of the medical profession because of the altruism, compassion, and deep caring I have seen displayed by my colleagues, our nurses, our staff, and our students. I am proud to have worked with all of them while trying to figure out how to practice in this environment.
These times are really difficult and challenging as we face new problems every day. Last week, we had to send our students home, and we switched to phone and telehealth visits to keep our patients and staff safer.
I have had some unanticipated electronic messages from patients during this time. Two of my patients with major medical problems and very dependent on their medications were stranded internationally and running out of medications. I had the family of an incarcerated patient contact me for a letter because that patient was moved to a part of a jail where all patients with upper respiratory infection symptoms were being housed. My patient has severe immunosuppression, and they were requesting an exception for him.
Another of my patients, who has sarcoidosis and is immunosuppressed, informed me that her daughter who lives with her was diagnosed with COVID-19. After 3 days, this patient told me she had become febrile and short of breath. I instructed her patient to go to a hospital, where she was also diagnosed with COVID-19 and was admitted. This patient was discharged within 24 hours, because the utilization review department did not feel she should be in the hospital.
The lack of beds is forcing physicians to frequently make tough decisions like the one made for this patient. This unfortunate reality raises the question of: “How do you manage a patient you are worried about from his or her home?”
In this particular case, I sent my patient an oxygen saturation monitor. We touched base frequently, and I felt okay as long as her saturations on room air were above 90%. So far, she has done okay.
More recently, I received a message from a patient recently diagnosed with Mycobacterium avium complex. I learned that this patient and her disabled husband’s caregiver refused to continue to provide care to them, because my patient had a cough, which began 2 months prior. In this case, a COVID-19 test was done for the explicit purpose of getting the caregiver to return to work.
So how do we face this?
Burnout had been high before this difficult time. But now physicians are being called to care for more and sicker patients without the necessary personal protective gear. Our physicians have demonstrated strength and commitment to patients in their response to this challenge, but they need help from others, including regulators.
I think a first step that needs to be taken is to decrease the volume of documentation physicians are required to make in this time where we are forced to triage to what is most important and drop what isn’t. How is spending so much time documenting instead of seeing the high volumes of patients who need to be seen a good thing? Documentation to the level that Medicare has required isn’t going to work. In fact, it has never been a good thing and is a big driver of burnout.
Our health care system was broken and badly injured before this crisis, and I think now might be a time when positive changes for the future occur. In fact, COVID-19 has resulted in some temporary changes in medicine that I would like to see outlast this outbreak. The telehealth option is now available, for example, and this kind of care is covered much more broadly by Medicare under the 1135 waiver – this has been needed for years. Being able to conduct regular clinic visits via telehealth without the marked restrictions that were previously in place is a big advance. It is currently in place for this emergency only, but this is the time to start pushing hard to make sure this option will be permanent.
I invite you to help me fight for long-term change. Write a letter to the editor of your local newspaper or blog, share your thoughts on social media, and tweet. (I suggest using #documentationordoctors or, although a bit long, #excessivedocumentationcostslives.) This is an unprecedented time in modern medicine. Traumatic times are when the greatest changes occur. Let’s hope for the better.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He frequently contributes Pearl of the Month and Myth of the Month columns to MDedge, and he serves on the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact Dr. Paauw at [email protected].
CVH in pregnant women: Ample room for improvement
Cardiovascular disease is both common and chronic, and it remains the leading cause of death in women. Because it is a life-long condition, cardiovascular disease must be managed over the entire lifespan. In recognition of the important role of obstetricians and gynecologists in monitoring women’s health, the American Heart Association/American College of Obstetricians and Gynecologists 2018 guidelines1 promoted the use of “Life’s Simple 7”2 for assessing cardiovascular health (CVH) in women.
These seven metrics include diet, physical activity, smoking status, body mass index (BMI), blood pressure, total cholesterol, and fasting blood glucose levels. They have been shown to predict positive health outcomes in nonpregnant adults. However, until now, CVH had not been assessed in pregnant women.
Perak et al. recently performed the first cross-sectional study of the prevalence of CVH metrics in pregnant women using the AHA definition.3 Using data from the National Health and Nutrition Examination Surveys (NHANES), they used the Life’s Simple 7 metrics to assess CVH in 1,117 pregnant and 8,200 nonpregnant women in the United States aged 20-44 years. Each of the Life’s Simple 7 metrics was scored 0, 1, or 2 points, corresponding to a rating of poor, intermediate, or ideal, respectively. Thus, the total CVH score ranged from 0-14 points, with total scores of 0-7 indicating low CVH, 8-11 indicating moderate CVH, and 12-14 indicating high CVH.
which was even worse than in nonpregnant women, of whom only 13% were scored as having ideal CVH. Ideal scores were observed for 0.1% of pregnant women for diet, 27% for physical activity, 39% for cholesterol levels, 51% for BMI, 78% for smoking, 90% for blood pressure, and 92% for fasting blood glucose. Physical activity and cholesterol levels appeared to be the major drivers of the lower CVH scores in pregnant women.
Although further studies are warranted to determine the relevance of CVH during pregnancy to outcomes for both mother and offspring, the study by Perak et al. is an important step toward the development of pregnancy-specific guidelines and definitions for CVH metrics. These are stated goals of the AHA/ACOG that will help promote CVH in women across their lifespans, but which have not been possible due to scant data.
Emerging data suggest that cumulative lifetime exposure is a significant factor in cardiovascular disease outcomes; therefore, earlier intervention would have a more significant impact. Just as gestational diabetes is a predictor of future type 2 diabetes, CVH earlier in a woman’s life predicts cardiovascular disease later in life.4-7 The best data in this regard come from genetic and other studies of hyperlipidemia, which suggest that lowering lipid levels before symptoms develop may prevent cardiovascular disease. In contrast, treatment of patients with clinically manifest disease neither offers a cure nor prevents the occurrence of most cardiovascular events.
It is a particularly salient point in this regard that there currently are no guidelines on treatment of hypercholesterolemia during pregnancy. Notably, the study by Perak et al. suggested that cholesterol levels may have a significant impact on CVH in pregnant women. There also is emerging data supporting the importance of controlling blood pressure across the lifespan,7,8 including during pregnancy.9
For many women, their ob.gyn. is their primary care physician, and pregnancy is often the first time that a woman will have a substantial interaction with the health care system. The AHA/ACOG advisory panel described pregnancy as a “physiological stress test” for women that offers the opportunity to identify those at increased risk of cardiovascular disease.1
As pregnancy is a time when women particularly are motivated to improve their health,10 it also presents a valuable opportunity for physicians, including ob.gyns., to make a lifelong impact on the CVH of their patients through early identification, education, and intervention.
Dr. Charles Hong is the Melvin Sharoky, MD, Professor of Medicine and director of cardiovascular research in the department of medicine at the University of Maryland School of Medicine. Dr. E. Albert Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Neither physician had any relevant financial disclosures. Contact him at [email protected].
References
1. Circulation. 2018;137:e843–e852.
2. Circulation. 2010 Jan 20;121(4):586–613.
3. J Am Heart Assoc. 2020 Feb 17;9:e015123.
4. J Am Coll Cardiol. 2018 Sep 4;72(10):1141-56.
5. N Engl J Med. 2016 Dec 1;375:2144-53.
6. Nat Rev Cardiol. 2011 Nov 1;8(12):721-5.
7. J Am Coll Cardiol. 2019 Jul 23;74(3):330-41.
8. Circulation. 2020 Mar 2:141:725-7.
9. Circulation. 2013 Feb 12;127(6):681-90.
10. Nutrients. 2018 Aug 8. doi: 10.3390/nu10081032.
Cardiovascular disease is both common and chronic, and it remains the leading cause of death in women. Because it is a life-long condition, cardiovascular disease must be managed over the entire lifespan. In recognition of the important role of obstetricians and gynecologists in monitoring women’s health, the American Heart Association/American College of Obstetricians and Gynecologists 2018 guidelines1 promoted the use of “Life’s Simple 7”2 for assessing cardiovascular health (CVH) in women.
These seven metrics include diet, physical activity, smoking status, body mass index (BMI), blood pressure, total cholesterol, and fasting blood glucose levels. They have been shown to predict positive health outcomes in nonpregnant adults. However, until now, CVH had not been assessed in pregnant women.
Perak et al. recently performed the first cross-sectional study of the prevalence of CVH metrics in pregnant women using the AHA definition.3 Using data from the National Health and Nutrition Examination Surveys (NHANES), they used the Life’s Simple 7 metrics to assess CVH in 1,117 pregnant and 8,200 nonpregnant women in the United States aged 20-44 years. Each of the Life’s Simple 7 metrics was scored 0, 1, or 2 points, corresponding to a rating of poor, intermediate, or ideal, respectively. Thus, the total CVH score ranged from 0-14 points, with total scores of 0-7 indicating low CVH, 8-11 indicating moderate CVH, and 12-14 indicating high CVH.
which was even worse than in nonpregnant women, of whom only 13% were scored as having ideal CVH. Ideal scores were observed for 0.1% of pregnant women for diet, 27% for physical activity, 39% for cholesterol levels, 51% for BMI, 78% for smoking, 90% for blood pressure, and 92% for fasting blood glucose. Physical activity and cholesterol levels appeared to be the major drivers of the lower CVH scores in pregnant women.
Although further studies are warranted to determine the relevance of CVH during pregnancy to outcomes for both mother and offspring, the study by Perak et al. is an important step toward the development of pregnancy-specific guidelines and definitions for CVH metrics. These are stated goals of the AHA/ACOG that will help promote CVH in women across their lifespans, but which have not been possible due to scant data.
Emerging data suggest that cumulative lifetime exposure is a significant factor in cardiovascular disease outcomes; therefore, earlier intervention would have a more significant impact. Just as gestational diabetes is a predictor of future type 2 diabetes, CVH earlier in a woman’s life predicts cardiovascular disease later in life.4-7 The best data in this regard come from genetic and other studies of hyperlipidemia, which suggest that lowering lipid levels before symptoms develop may prevent cardiovascular disease. In contrast, treatment of patients with clinically manifest disease neither offers a cure nor prevents the occurrence of most cardiovascular events.
It is a particularly salient point in this regard that there currently are no guidelines on treatment of hypercholesterolemia during pregnancy. Notably, the study by Perak et al. suggested that cholesterol levels may have a significant impact on CVH in pregnant women. There also is emerging data supporting the importance of controlling blood pressure across the lifespan,7,8 including during pregnancy.9
For many women, their ob.gyn. is their primary care physician, and pregnancy is often the first time that a woman will have a substantial interaction with the health care system. The AHA/ACOG advisory panel described pregnancy as a “physiological stress test” for women that offers the opportunity to identify those at increased risk of cardiovascular disease.1
As pregnancy is a time when women particularly are motivated to improve their health,10 it also presents a valuable opportunity for physicians, including ob.gyns., to make a lifelong impact on the CVH of their patients through early identification, education, and intervention.
Dr. Charles Hong is the Melvin Sharoky, MD, Professor of Medicine and director of cardiovascular research in the department of medicine at the University of Maryland School of Medicine. Dr. E. Albert Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Neither physician had any relevant financial disclosures. Contact him at [email protected].
References
1. Circulation. 2018;137:e843–e852.
2. Circulation. 2010 Jan 20;121(4):586–613.
3. J Am Heart Assoc. 2020 Feb 17;9:e015123.
4. J Am Coll Cardiol. 2018 Sep 4;72(10):1141-56.
5. N Engl J Med. 2016 Dec 1;375:2144-53.
6. Nat Rev Cardiol. 2011 Nov 1;8(12):721-5.
7. J Am Coll Cardiol. 2019 Jul 23;74(3):330-41.
8. Circulation. 2020 Mar 2:141:725-7.
9. Circulation. 2013 Feb 12;127(6):681-90.
10. Nutrients. 2018 Aug 8. doi: 10.3390/nu10081032.
Cardiovascular disease is both common and chronic, and it remains the leading cause of death in women. Because it is a life-long condition, cardiovascular disease must be managed over the entire lifespan. In recognition of the important role of obstetricians and gynecologists in monitoring women’s health, the American Heart Association/American College of Obstetricians and Gynecologists 2018 guidelines1 promoted the use of “Life’s Simple 7”2 for assessing cardiovascular health (CVH) in women.
These seven metrics include diet, physical activity, smoking status, body mass index (BMI), blood pressure, total cholesterol, and fasting blood glucose levels. They have been shown to predict positive health outcomes in nonpregnant adults. However, until now, CVH had not been assessed in pregnant women.
Perak et al. recently performed the first cross-sectional study of the prevalence of CVH metrics in pregnant women using the AHA definition.3 Using data from the National Health and Nutrition Examination Surveys (NHANES), they used the Life’s Simple 7 metrics to assess CVH in 1,117 pregnant and 8,200 nonpregnant women in the United States aged 20-44 years. Each of the Life’s Simple 7 metrics was scored 0, 1, or 2 points, corresponding to a rating of poor, intermediate, or ideal, respectively. Thus, the total CVH score ranged from 0-14 points, with total scores of 0-7 indicating low CVH, 8-11 indicating moderate CVH, and 12-14 indicating high CVH.
which was even worse than in nonpregnant women, of whom only 13% were scored as having ideal CVH. Ideal scores were observed for 0.1% of pregnant women for diet, 27% for physical activity, 39% for cholesterol levels, 51% for BMI, 78% for smoking, 90% for blood pressure, and 92% for fasting blood glucose. Physical activity and cholesterol levels appeared to be the major drivers of the lower CVH scores in pregnant women.
Although further studies are warranted to determine the relevance of CVH during pregnancy to outcomes for both mother and offspring, the study by Perak et al. is an important step toward the development of pregnancy-specific guidelines and definitions for CVH metrics. These are stated goals of the AHA/ACOG that will help promote CVH in women across their lifespans, but which have not been possible due to scant data.
Emerging data suggest that cumulative lifetime exposure is a significant factor in cardiovascular disease outcomes; therefore, earlier intervention would have a more significant impact. Just as gestational diabetes is a predictor of future type 2 diabetes, CVH earlier in a woman’s life predicts cardiovascular disease later in life.4-7 The best data in this regard come from genetic and other studies of hyperlipidemia, which suggest that lowering lipid levels before symptoms develop may prevent cardiovascular disease. In contrast, treatment of patients with clinically manifest disease neither offers a cure nor prevents the occurrence of most cardiovascular events.
It is a particularly salient point in this regard that there currently are no guidelines on treatment of hypercholesterolemia during pregnancy. Notably, the study by Perak et al. suggested that cholesterol levels may have a significant impact on CVH in pregnant women. There also is emerging data supporting the importance of controlling blood pressure across the lifespan,7,8 including during pregnancy.9
For many women, their ob.gyn. is their primary care physician, and pregnancy is often the first time that a woman will have a substantial interaction with the health care system. The AHA/ACOG advisory panel described pregnancy as a “physiological stress test” for women that offers the opportunity to identify those at increased risk of cardiovascular disease.1
As pregnancy is a time when women particularly are motivated to improve their health,10 it also presents a valuable opportunity for physicians, including ob.gyns., to make a lifelong impact on the CVH of their patients through early identification, education, and intervention.
Dr. Charles Hong is the Melvin Sharoky, MD, Professor of Medicine and director of cardiovascular research in the department of medicine at the University of Maryland School of Medicine. Dr. E. Albert Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Neither physician had any relevant financial disclosures. Contact him at [email protected].
References
1. Circulation. 2018;137:e843–e852.
2. Circulation. 2010 Jan 20;121(4):586–613.
3. J Am Heart Assoc. 2020 Feb 17;9:e015123.
4. J Am Coll Cardiol. 2018 Sep 4;72(10):1141-56.
5. N Engl J Med. 2016 Dec 1;375:2144-53.
6. Nat Rev Cardiol. 2011 Nov 1;8(12):721-5.
7. J Am Coll Cardiol. 2019 Jul 23;74(3):330-41.
8. Circulation. 2020 Mar 2:141:725-7.
9. Circulation. 2013 Feb 12;127(6):681-90.
10. Nutrients. 2018 Aug 8. doi: 10.3390/nu10081032.