User login
Zulresso: Hope and lingering questions
The last decade has brought increasing awareness of the need to effectively screen for postpartum depression, with a majority of states across the country now having some sort of formal program by which women are screened for mood disorder during the postnatal period, typically with scales such as the Edinburgh Postnatal Depression Scale (EPDS).
In addition to effective screening is a pressing need for effective referral networks of clinicians who have both the expertise and time to manage the 10%-15% of women who have been identified and who suffer from postpartum psychiatric disorders – both postpartum mood and anxiety disorders. Several studies have suggested that only a small percentage of postpartum women who score with clinically significant level of depressive symptoms actually get to a clinician or, if they do get to a clinician, receive adequate treatment restoring their emotional well-being (J Clin Psychiatry. 2016 Sep;77[9]:1189-200).
Zulresso (brexanolone), a novel new antidepressant medication which recently received Food and Drug Administration approval for the treatment of postpartum depression, is a first-in-class molecule to get such approval. Zulresso is a neurosteroid, an analogue of allopregnanolone and a GABAA receptor–positive allosteric modulator, a primary inhibitory neurotransmitter in the brain.
There is every reason to believe that, as a class, this group of neurosteroid molecules are effective in treating depression in other populations aside from women with postpartum depression and hence may not be specific to the postpartum period. For example, recent presentations of preliminary data suggest other neurosteroids such as zuranolone (an oral medication also developed by Sage Therapeutics) is effective for both men and women who have major depression in addition to women suffering from postpartum depression.
Zulresso is approved through a Risk Evaluation and Mitigation Strategy–restricted program and, per that protocol, needs to be administered by a health care provider in a recognized health care setting intravenously over 2.5 days (60 hours). Because of concerns regarding increased sedation, continuous pulse oximetry is required, and this is outlined in a boxed warning in the prescribing information. Zulresso has been classified by the Drug Enforcement Administration (DEA) as a Schedule IV injection and is subject to the prescribing regulations for a controlled substance.
Since Zulresso’s approval, my colleagues and I at the Center for Women’s Mental Health have received numerous queries from patients and colleagues about our clinical impression of this new molecule with a different mechanism of action – a welcome addition to the antidepressant pharmacopeia. The question posed to us essentially is: Where does brexanolone fit into our algorithm for treating women who suffer from postpartum depression? And frequently, the follow-up query is: Because subjects in the clinical trials for this medication included women who had onset of depression either late in pregnancy or during the postpartum period, how specific is brexanolone with respect to being a targeted therapy for postpartum depression, compared with depression encountered in other clinical settings.
What clearly can be stated is that Zulresso has a rapid onset of action and was demonstrated across clinical trials to have sustained benefit up to 30 days after IV administration. The question is whether patients have sustained benefit after 30 days or if this is a medicine to be considered as a “bridge” to other treatment. Data answering that critical clinical question are unavailable at this time. From a clinical standpoint, do patients receive this treatment and get sent home on antidepressants, as we would for patients who receive ECT, often discharging them with prophylactic antidepressants to sustain the benefit of the treatment? Or do patients receive this new medicine with the clinician providing close follow-up, assuming a wait-and-see approach? Because data informing the answer to that question are not available, this decision will be made empirically, frequently factoring in the patient’s past clinical history where presumably more liberal use of antidepressant immediately after the administration of Zulresso will be pursued in those with histories of highly recurrent major depression.
So where might this new medicine fit into the treatment of postpartum depression of moderate severity, or modest to moderate severity? It should be kept in mind that for patients with mild to moderate postpartum depression, there are data supporting the efficacy of cognitive-behavioral therapy (CBT). CBT frequently is pursued with concurrent mobilization of substantial social support with good outcomes. In patients with more severe postpartum depression, there are data supporting the use of antidepressants, and in these patients as well, use of established support from the ever-growing network of community-based support groups and services can be particularly helpful. It is unlikely that Zulresso will be a first-line medication for the treatment of postpartum depression, but it may be particularly appropriate for patients with severe illness who have not responded to other interventions.
Other practical considerations regarding use of Zulresso include the requirement that the medicine be administered in hospitals that have established clinical infrastructure to accommodate this particular population of patients and where pharmacists and other relevant parties in hospitals have accepted the medicine into its drug formulary. While coverage by various insurance policies may vary, the cost of this new medication is substantial, between $24,000 and $34,000 per treatment, according to reports.
Where Zulresso fits into the pharmacopeia for treating postpartum depression may fall well beyond the issues of efficacy. Given all of the attention to this first-in-class medicine, Zulresso has reinforced the growing interest in the substantial prevalence and the morbidity associated with postpartum depression. It is hard to imagine Zulresso being used in cases of more mild to moderate depression, in which there is nonemergent opportunity to pursue options that do not require a new mom to absent herself from homelife with a newborn. However, in picking cases of severe new onset or recurrence of depression in postpartum women, the rapid onset of benefit that was noted within days could be an extraordinary relief and be the beginning of a road to wellness for some women.
Ultimately, the collaboration of patients with their doctors, the realities of cost, and the acceptability of use in various clinical settings will determine how Zulresso is incorporated into seeking treatment to mitigate the suffering associated with postpartum depression. We at the Center for Women’s Mental Health are interested in user experience with respect to this medicine and welcome comments from both patients and their doctors at [email protected].
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. This center was an investigator site for one of the studies supported by Sage Therapeutics, the manufacturer of Zulresso. Dr. Cohen is also the Edmund and Carroll Carpenter professor of psychiatry at Harvard Medical School, also in Boston. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
The last decade has brought increasing awareness of the need to effectively screen for postpartum depression, with a majority of states across the country now having some sort of formal program by which women are screened for mood disorder during the postnatal period, typically with scales such as the Edinburgh Postnatal Depression Scale (EPDS).
In addition to effective screening is a pressing need for effective referral networks of clinicians who have both the expertise and time to manage the 10%-15% of women who have been identified and who suffer from postpartum psychiatric disorders – both postpartum mood and anxiety disorders. Several studies have suggested that only a small percentage of postpartum women who score with clinically significant level of depressive symptoms actually get to a clinician or, if they do get to a clinician, receive adequate treatment restoring their emotional well-being (J Clin Psychiatry. 2016 Sep;77[9]:1189-200).
Zulresso (brexanolone), a novel new antidepressant medication which recently received Food and Drug Administration approval for the treatment of postpartum depression, is a first-in-class molecule to get such approval. Zulresso is a neurosteroid, an analogue of allopregnanolone and a GABAA receptor–positive allosteric modulator, a primary inhibitory neurotransmitter in the brain.
There is every reason to believe that, as a class, this group of neurosteroid molecules are effective in treating depression in other populations aside from women with postpartum depression and hence may not be specific to the postpartum period. For example, recent presentations of preliminary data suggest other neurosteroids such as zuranolone (an oral medication also developed by Sage Therapeutics) is effective for both men and women who have major depression in addition to women suffering from postpartum depression.
Zulresso is approved through a Risk Evaluation and Mitigation Strategy–restricted program and, per that protocol, needs to be administered by a health care provider in a recognized health care setting intravenously over 2.5 days (60 hours). Because of concerns regarding increased sedation, continuous pulse oximetry is required, and this is outlined in a boxed warning in the prescribing information. Zulresso has been classified by the Drug Enforcement Administration (DEA) as a Schedule IV injection and is subject to the prescribing regulations for a controlled substance.
Since Zulresso’s approval, my colleagues and I at the Center for Women’s Mental Health have received numerous queries from patients and colleagues about our clinical impression of this new molecule with a different mechanism of action – a welcome addition to the antidepressant pharmacopeia. The question posed to us essentially is: Where does brexanolone fit into our algorithm for treating women who suffer from postpartum depression? And frequently, the follow-up query is: Because subjects in the clinical trials for this medication included women who had onset of depression either late in pregnancy or during the postpartum period, how specific is brexanolone with respect to being a targeted therapy for postpartum depression, compared with depression encountered in other clinical settings.
What clearly can be stated is that Zulresso has a rapid onset of action and was demonstrated across clinical trials to have sustained benefit up to 30 days after IV administration. The question is whether patients have sustained benefit after 30 days or if this is a medicine to be considered as a “bridge” to other treatment. Data answering that critical clinical question are unavailable at this time. From a clinical standpoint, do patients receive this treatment and get sent home on antidepressants, as we would for patients who receive ECT, often discharging them with prophylactic antidepressants to sustain the benefit of the treatment? Or do patients receive this new medicine with the clinician providing close follow-up, assuming a wait-and-see approach? Because data informing the answer to that question are not available, this decision will be made empirically, frequently factoring in the patient’s past clinical history where presumably more liberal use of antidepressant immediately after the administration of Zulresso will be pursued in those with histories of highly recurrent major depression.
So where might this new medicine fit into the treatment of postpartum depression of moderate severity, or modest to moderate severity? It should be kept in mind that for patients with mild to moderate postpartum depression, there are data supporting the efficacy of cognitive-behavioral therapy (CBT). CBT frequently is pursued with concurrent mobilization of substantial social support with good outcomes. In patients with more severe postpartum depression, there are data supporting the use of antidepressants, and in these patients as well, use of established support from the ever-growing network of community-based support groups and services can be particularly helpful. It is unlikely that Zulresso will be a first-line medication for the treatment of postpartum depression, but it may be particularly appropriate for patients with severe illness who have not responded to other interventions.
Other practical considerations regarding use of Zulresso include the requirement that the medicine be administered in hospitals that have established clinical infrastructure to accommodate this particular population of patients and where pharmacists and other relevant parties in hospitals have accepted the medicine into its drug formulary. While coverage by various insurance policies may vary, the cost of this new medication is substantial, between $24,000 and $34,000 per treatment, according to reports.
Where Zulresso fits into the pharmacopeia for treating postpartum depression may fall well beyond the issues of efficacy. Given all of the attention to this first-in-class medicine, Zulresso has reinforced the growing interest in the substantial prevalence and the morbidity associated with postpartum depression. It is hard to imagine Zulresso being used in cases of more mild to moderate depression, in which there is nonemergent opportunity to pursue options that do not require a new mom to absent herself from homelife with a newborn. However, in picking cases of severe new onset or recurrence of depression in postpartum women, the rapid onset of benefit that was noted within days could be an extraordinary relief and be the beginning of a road to wellness for some women.
Ultimately, the collaboration of patients with their doctors, the realities of cost, and the acceptability of use in various clinical settings will determine how Zulresso is incorporated into seeking treatment to mitigate the suffering associated with postpartum depression. We at the Center for Women’s Mental Health are interested in user experience with respect to this medicine and welcome comments from both patients and their doctors at [email protected].
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. This center was an investigator site for one of the studies supported by Sage Therapeutics, the manufacturer of Zulresso. Dr. Cohen is also the Edmund and Carroll Carpenter professor of psychiatry at Harvard Medical School, also in Boston. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
The last decade has brought increasing awareness of the need to effectively screen for postpartum depression, with a majority of states across the country now having some sort of formal program by which women are screened for mood disorder during the postnatal period, typically with scales such as the Edinburgh Postnatal Depression Scale (EPDS).
In addition to effective screening is a pressing need for effective referral networks of clinicians who have both the expertise and time to manage the 10%-15% of women who have been identified and who suffer from postpartum psychiatric disorders – both postpartum mood and anxiety disorders. Several studies have suggested that only a small percentage of postpartum women who score with clinically significant level of depressive symptoms actually get to a clinician or, if they do get to a clinician, receive adequate treatment restoring their emotional well-being (J Clin Psychiatry. 2016 Sep;77[9]:1189-200).
Zulresso (brexanolone), a novel new antidepressant medication which recently received Food and Drug Administration approval for the treatment of postpartum depression, is a first-in-class molecule to get such approval. Zulresso is a neurosteroid, an analogue of allopregnanolone and a GABAA receptor–positive allosteric modulator, a primary inhibitory neurotransmitter in the brain.
There is every reason to believe that, as a class, this group of neurosteroid molecules are effective in treating depression in other populations aside from women with postpartum depression and hence may not be specific to the postpartum period. For example, recent presentations of preliminary data suggest other neurosteroids such as zuranolone (an oral medication also developed by Sage Therapeutics) is effective for both men and women who have major depression in addition to women suffering from postpartum depression.
Zulresso is approved through a Risk Evaluation and Mitigation Strategy–restricted program and, per that protocol, needs to be administered by a health care provider in a recognized health care setting intravenously over 2.5 days (60 hours). Because of concerns regarding increased sedation, continuous pulse oximetry is required, and this is outlined in a boxed warning in the prescribing information. Zulresso has been classified by the Drug Enforcement Administration (DEA) as a Schedule IV injection and is subject to the prescribing regulations for a controlled substance.
Since Zulresso’s approval, my colleagues and I at the Center for Women’s Mental Health have received numerous queries from patients and colleagues about our clinical impression of this new molecule with a different mechanism of action – a welcome addition to the antidepressant pharmacopeia. The question posed to us essentially is: Where does brexanolone fit into our algorithm for treating women who suffer from postpartum depression? And frequently, the follow-up query is: Because subjects in the clinical trials for this medication included women who had onset of depression either late in pregnancy or during the postpartum period, how specific is brexanolone with respect to being a targeted therapy for postpartum depression, compared with depression encountered in other clinical settings.
What clearly can be stated is that Zulresso has a rapid onset of action and was demonstrated across clinical trials to have sustained benefit up to 30 days after IV administration. The question is whether patients have sustained benefit after 30 days or if this is a medicine to be considered as a “bridge” to other treatment. Data answering that critical clinical question are unavailable at this time. From a clinical standpoint, do patients receive this treatment and get sent home on antidepressants, as we would for patients who receive ECT, often discharging them with prophylactic antidepressants to sustain the benefit of the treatment? Or do patients receive this new medicine with the clinician providing close follow-up, assuming a wait-and-see approach? Because data informing the answer to that question are not available, this decision will be made empirically, frequently factoring in the patient’s past clinical history where presumably more liberal use of antidepressant immediately after the administration of Zulresso will be pursued in those with histories of highly recurrent major depression.
So where might this new medicine fit into the treatment of postpartum depression of moderate severity, or modest to moderate severity? It should be kept in mind that for patients with mild to moderate postpartum depression, there are data supporting the efficacy of cognitive-behavioral therapy (CBT). CBT frequently is pursued with concurrent mobilization of substantial social support with good outcomes. In patients with more severe postpartum depression, there are data supporting the use of antidepressants, and in these patients as well, use of established support from the ever-growing network of community-based support groups and services can be particularly helpful. It is unlikely that Zulresso will be a first-line medication for the treatment of postpartum depression, but it may be particularly appropriate for patients with severe illness who have not responded to other interventions.
Other practical considerations regarding use of Zulresso include the requirement that the medicine be administered in hospitals that have established clinical infrastructure to accommodate this particular population of patients and where pharmacists and other relevant parties in hospitals have accepted the medicine into its drug formulary. While coverage by various insurance policies may vary, the cost of this new medication is substantial, between $24,000 and $34,000 per treatment, according to reports.
Where Zulresso fits into the pharmacopeia for treating postpartum depression may fall well beyond the issues of efficacy. Given all of the attention to this first-in-class medicine, Zulresso has reinforced the growing interest in the substantial prevalence and the morbidity associated with postpartum depression. It is hard to imagine Zulresso being used in cases of more mild to moderate depression, in which there is nonemergent opportunity to pursue options that do not require a new mom to absent herself from homelife with a newborn. However, in picking cases of severe new onset or recurrence of depression in postpartum women, the rapid onset of benefit that was noted within days could be an extraordinary relief and be the beginning of a road to wellness for some women.
Ultimately, the collaboration of patients with their doctors, the realities of cost, and the acceptability of use in various clinical settings will determine how Zulresso is incorporated into seeking treatment to mitigate the suffering associated with postpartum depression. We at the Center for Women’s Mental Health are interested in user experience with respect to this medicine and welcome comments from both patients and their doctors at [email protected].
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. This center was an investigator site for one of the studies supported by Sage Therapeutics, the manufacturer of Zulresso. Dr. Cohen is also the Edmund and Carroll Carpenter professor of psychiatry at Harvard Medical School, also in Boston. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Supporting our gender-diverse patients
CASE Patient has adverse effects from halted estrogen pills
JR twists her hands nervously as you step into the room. “They stopped my hormones,” she sighs as you pull up her lab results.
JR recently had been admitted to an inpatient cardiology unit for several days for a heart failure exacerbation. Her ankles are still swollen beneath her floral print skirt, but she is breathing much easier now. She is back at your primary care office, hoping to get clearance to restart her estrogen pills.
JR reports having mood swings and terrible nightmares while not taking her hormones, which she has been taking for more than 3 years. She hesitates before sharing, “One of the doctors kept asking me questions about my sex life that had nothing to do with my heart condition. I don’t want to go back there.”
Providing compassionate and comprehensive care to gender-nonconforming individuals is challenging for a multitude of reasons, from clinician ignorance to systemic discrimination. About 33% of transgender patients reported being harassed, denied care, or even being assaulted when seeking health care, while 23% reported avoiding going to the doctor altogether when sick or injured out of fear of discrimination.1
Unfortunately, now, further increases to barriers to care may be put in place. In late May of this year, the Department of Health and Human Services (HHS) proposed new regulations that would reverse previous regulations granted through section 1557 of the Affordable Care Act (ACA)—the Health Care Rights Law—which affirmed the rights of gender nonbinary persons to medical care. Among the proposed changes is the elimination of protections against discrimination in health care based on gender identity.2 The proposed regulation changes come on the heels of a federal court case, which seeks to declare that hospital systems may turn away patients based on gender identity.3
Unraveling rights afforded under the ACA
The Health Care Rights Law was passed under the ACA; it prohibits discrimination based on race, color, national origin, sex, age, and disability in health programs and activities receiving federal financial assistance. Multiple lower courts have supported that the rights of transgender individuals is included within these protections against discrimination on the basis of sex.4 These court rulings not only have ensured the ability of gender-diverse individuals to access care but also have enforced insurance coverage of therapies for gender dysphoria. It was only in 2014 that Medicaid began providing coverage for gender-affirming surgeries and eliminating language that such procedures were “experimental” or “cosmetic.” The 2016 passage of the ACA mandated that private insurance companies follow suit. Unfortunately, the recent proposed regulation changes to the Health Care Rights Law may spark a reversal from insurance companies as well. Such a setback would affect gender-diverse individuals’ hormone treatments as well as their ability to access a full spectrum of care within the health care system.
Continue to: ACOG urges nondiscriminatory practices...
ACOG urges nondiscriminatory practices
The proposed regulation changes to the Health Care Rights Law are from the Conscience and Religious Freedom Division of the HHS Office for Civil Rights, which was established in 2018 and has been advocating for the rights of health care providers to refuse to treat patients based on their own religious beliefs.5 We argue, however, that providing care to persons of varying backgrounds is not an assault on our individual liberties but rather a privilege as providers. As obstetrician-gynecologists, it may be easy to only consider cis-gendered women our responsibility. But our field also emphasizes individual empowerment above all else—we fight every day for our patients’ rights to contraception, fertility, pregnancy, parenthood, and sexual freedoms. Let us continue speaking up for the rights of all those who need gynecologic care, regardless of the pronouns they use.
“The American College of Obstetricians and Gynecologists urges health care providers to foster nondiscriminatory practices and policies to increase identification and to facilitate quality health care for transgender individuals, both in assisting with the transition if desired as well as providing long-term preventive health care.”6
We urge you to take action
- Reach out to your local representatives about protecting transgender health access
- Educate yourself on the unique needs of transgender individuals
- Read personal accounts
- Share your personal story
- Find referring providers near your practice
- 2015 US Transgender Survey. December 2016. https://www.transequality.org/sites/default/files/docs/USTS-Full-Report-FINAL.PDF. Accessed August 30, 2019.
- Musumeci M, Kates J, Dawson J, et al. HHS’ proposed changes to non-discrimination regulations under ACA section 1557. July 1, 2019. https://www.kff.org/disparities-policy/issue-brief/hhss-proposed-changes-to-non-discrimination-regulations-under-aca-section-1557/. Accessed August 30, 2019.
- Franciscan Alliance v. Burwell. ACLU website. https://www.aclu.org/cases/franciscan-alliance-v-burwell. Accessed August 30, 2019.
- Pear R. Trump plan would cut back health care protections for transgender people. April 21, 2018. https://www.nytimes.com/2018/04/21/us/politics/trump-transgender-health-care.html. Accessed August 30, 2019.
- U.S. Department of Health and Human Services. HHS announces new conscience and religious freedom division. January 18, 2018. https://www.hhs.gov/about/news/2018/01/18/hhs-ocr-announces-new-conscience-and-religious-freedom-division.html. Accessed August 30, 2019.
- American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women. Committee Opinion no. 512: health care for transgender individuals. Obstet Gynecol. 2011;118:1454–1458.
CASE Patient has adverse effects from halted estrogen pills
JR twists her hands nervously as you step into the room. “They stopped my hormones,” she sighs as you pull up her lab results.
JR recently had been admitted to an inpatient cardiology unit for several days for a heart failure exacerbation. Her ankles are still swollen beneath her floral print skirt, but she is breathing much easier now. She is back at your primary care office, hoping to get clearance to restart her estrogen pills.
JR reports having mood swings and terrible nightmares while not taking her hormones, which she has been taking for more than 3 years. She hesitates before sharing, “One of the doctors kept asking me questions about my sex life that had nothing to do with my heart condition. I don’t want to go back there.”
Providing compassionate and comprehensive care to gender-nonconforming individuals is challenging for a multitude of reasons, from clinician ignorance to systemic discrimination. About 33% of transgender patients reported being harassed, denied care, or even being assaulted when seeking health care, while 23% reported avoiding going to the doctor altogether when sick or injured out of fear of discrimination.1
Unfortunately, now, further increases to barriers to care may be put in place. In late May of this year, the Department of Health and Human Services (HHS) proposed new regulations that would reverse previous regulations granted through section 1557 of the Affordable Care Act (ACA)—the Health Care Rights Law—which affirmed the rights of gender nonbinary persons to medical care. Among the proposed changes is the elimination of protections against discrimination in health care based on gender identity.2 The proposed regulation changes come on the heels of a federal court case, which seeks to declare that hospital systems may turn away patients based on gender identity.3
Unraveling rights afforded under the ACA
The Health Care Rights Law was passed under the ACA; it prohibits discrimination based on race, color, national origin, sex, age, and disability in health programs and activities receiving federal financial assistance. Multiple lower courts have supported that the rights of transgender individuals is included within these protections against discrimination on the basis of sex.4 These court rulings not only have ensured the ability of gender-diverse individuals to access care but also have enforced insurance coverage of therapies for gender dysphoria. It was only in 2014 that Medicaid began providing coverage for gender-affirming surgeries and eliminating language that such procedures were “experimental” or “cosmetic.” The 2016 passage of the ACA mandated that private insurance companies follow suit. Unfortunately, the recent proposed regulation changes to the Health Care Rights Law may spark a reversal from insurance companies as well. Such a setback would affect gender-diverse individuals’ hormone treatments as well as their ability to access a full spectrum of care within the health care system.
Continue to: ACOG urges nondiscriminatory practices...
ACOG urges nondiscriminatory practices
The proposed regulation changes to the Health Care Rights Law are from the Conscience and Religious Freedom Division of the HHS Office for Civil Rights, which was established in 2018 and has been advocating for the rights of health care providers to refuse to treat patients based on their own religious beliefs.5 We argue, however, that providing care to persons of varying backgrounds is not an assault on our individual liberties but rather a privilege as providers. As obstetrician-gynecologists, it may be easy to only consider cis-gendered women our responsibility. But our field also emphasizes individual empowerment above all else—we fight every day for our patients’ rights to contraception, fertility, pregnancy, parenthood, and sexual freedoms. Let us continue speaking up for the rights of all those who need gynecologic care, regardless of the pronouns they use.
“The American College of Obstetricians and Gynecologists urges health care providers to foster nondiscriminatory practices and policies to increase identification and to facilitate quality health care for transgender individuals, both in assisting with the transition if desired as well as providing long-term preventive health care.”6
We urge you to take action
- Reach out to your local representatives about protecting transgender health access
- Educate yourself on the unique needs of transgender individuals
- Read personal accounts
- Share your personal story
- Find referring providers near your practice
CASE Patient has adverse effects from halted estrogen pills
JR twists her hands nervously as you step into the room. “They stopped my hormones,” she sighs as you pull up her lab results.
JR recently had been admitted to an inpatient cardiology unit for several days for a heart failure exacerbation. Her ankles are still swollen beneath her floral print skirt, but she is breathing much easier now. She is back at your primary care office, hoping to get clearance to restart her estrogen pills.
JR reports having mood swings and terrible nightmares while not taking her hormones, which she has been taking for more than 3 years. She hesitates before sharing, “One of the doctors kept asking me questions about my sex life that had nothing to do with my heart condition. I don’t want to go back there.”
Providing compassionate and comprehensive care to gender-nonconforming individuals is challenging for a multitude of reasons, from clinician ignorance to systemic discrimination. About 33% of transgender patients reported being harassed, denied care, or even being assaulted when seeking health care, while 23% reported avoiding going to the doctor altogether when sick or injured out of fear of discrimination.1
Unfortunately, now, further increases to barriers to care may be put in place. In late May of this year, the Department of Health and Human Services (HHS) proposed new regulations that would reverse previous regulations granted through section 1557 of the Affordable Care Act (ACA)—the Health Care Rights Law—which affirmed the rights of gender nonbinary persons to medical care. Among the proposed changes is the elimination of protections against discrimination in health care based on gender identity.2 The proposed regulation changes come on the heels of a federal court case, which seeks to declare that hospital systems may turn away patients based on gender identity.3
Unraveling rights afforded under the ACA
The Health Care Rights Law was passed under the ACA; it prohibits discrimination based on race, color, national origin, sex, age, and disability in health programs and activities receiving federal financial assistance. Multiple lower courts have supported that the rights of transgender individuals is included within these protections against discrimination on the basis of sex.4 These court rulings not only have ensured the ability of gender-diverse individuals to access care but also have enforced insurance coverage of therapies for gender dysphoria. It was only in 2014 that Medicaid began providing coverage for gender-affirming surgeries and eliminating language that such procedures were “experimental” or “cosmetic.” The 2016 passage of the ACA mandated that private insurance companies follow suit. Unfortunately, the recent proposed regulation changes to the Health Care Rights Law may spark a reversal from insurance companies as well. Such a setback would affect gender-diverse individuals’ hormone treatments as well as their ability to access a full spectrum of care within the health care system.
Continue to: ACOG urges nondiscriminatory practices...
ACOG urges nondiscriminatory practices
The proposed regulation changes to the Health Care Rights Law are from the Conscience and Religious Freedom Division of the HHS Office for Civil Rights, which was established in 2018 and has been advocating for the rights of health care providers to refuse to treat patients based on their own religious beliefs.5 We argue, however, that providing care to persons of varying backgrounds is not an assault on our individual liberties but rather a privilege as providers. As obstetrician-gynecologists, it may be easy to only consider cis-gendered women our responsibility. But our field also emphasizes individual empowerment above all else—we fight every day for our patients’ rights to contraception, fertility, pregnancy, parenthood, and sexual freedoms. Let us continue speaking up for the rights of all those who need gynecologic care, regardless of the pronouns they use.
“The American College of Obstetricians and Gynecologists urges health care providers to foster nondiscriminatory practices and policies to increase identification and to facilitate quality health care for transgender individuals, both in assisting with the transition if desired as well as providing long-term preventive health care.”6
We urge you to take action
- Reach out to your local representatives about protecting transgender health access
- Educate yourself on the unique needs of transgender individuals
- Read personal accounts
- Share your personal story
- Find referring providers near your practice
- 2015 US Transgender Survey. December 2016. https://www.transequality.org/sites/default/files/docs/USTS-Full-Report-FINAL.PDF. Accessed August 30, 2019.
- Musumeci M, Kates J, Dawson J, et al. HHS’ proposed changes to non-discrimination regulations under ACA section 1557. July 1, 2019. https://www.kff.org/disparities-policy/issue-brief/hhss-proposed-changes-to-non-discrimination-regulations-under-aca-section-1557/. Accessed August 30, 2019.
- Franciscan Alliance v. Burwell. ACLU website. https://www.aclu.org/cases/franciscan-alliance-v-burwell. Accessed August 30, 2019.
- Pear R. Trump plan would cut back health care protections for transgender people. April 21, 2018. https://www.nytimes.com/2018/04/21/us/politics/trump-transgender-health-care.html. Accessed August 30, 2019.
- U.S. Department of Health and Human Services. HHS announces new conscience and religious freedom division. January 18, 2018. https://www.hhs.gov/about/news/2018/01/18/hhs-ocr-announces-new-conscience-and-religious-freedom-division.html. Accessed August 30, 2019.
- American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women. Committee Opinion no. 512: health care for transgender individuals. Obstet Gynecol. 2011;118:1454–1458.
- 2015 US Transgender Survey. December 2016. https://www.transequality.org/sites/default/files/docs/USTS-Full-Report-FINAL.PDF. Accessed August 30, 2019.
- Musumeci M, Kates J, Dawson J, et al. HHS’ proposed changes to non-discrimination regulations under ACA section 1557. July 1, 2019. https://www.kff.org/disparities-policy/issue-brief/hhss-proposed-changes-to-non-discrimination-regulations-under-aca-section-1557/. Accessed August 30, 2019.
- Franciscan Alliance v. Burwell. ACLU website. https://www.aclu.org/cases/franciscan-alliance-v-burwell. Accessed August 30, 2019.
- Pear R. Trump plan would cut back health care protections for transgender people. April 21, 2018. https://www.nytimes.com/2018/04/21/us/politics/trump-transgender-health-care.html. Accessed August 30, 2019.
- U.S. Department of Health and Human Services. HHS announces new conscience and religious freedom division. January 18, 2018. https://www.hhs.gov/about/news/2018/01/18/hhs-ocr-announces-new-conscience-and-religious-freedom-division.html. Accessed August 30, 2019.
- American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women. Committee Opinion no. 512: health care for transgender individuals. Obstet Gynecol. 2011;118:1454–1458.
Medical Cannabis: Not just a passing fad
In this issue of JFP, Weinstein and Worster provide a wealth of information about prescribing marijuana. Medical marijuana (Cannabis) is now legal in the majority of states, so it’s likely that some of your patients are using marijuana for symptom relief. For those physicians who elect to prescribe marijuana, reading this review will help you avoid harming patients while maximizing potential benefits.
I say “potential benefits” because the research evidence to support benefit for most conditions and symptoms is weak at best. In addition to the JAMA meta-analysis cited by Weinstein and Worster,1 several meta-analyses and systematic reviews published since January 2018 reach similar conclusions.2-4
Marijuana can provide significant relief from chemotherapy-induced nausea and vomiting, and it is effective in reducing intractable seizures in 2 rare pediatric seizure disorders. There may be some benefit for treatment of spasticity, and there may be some therapeutic value for relief of neuropathic pain, although the evidence is not strong. Interestingly, there is some preliminary evidence that cannabis can improve gastrointestinal symptoms in patients with Crohn's disease and ulcerative colitis.5,6
Why do people use marijuana as medicine? A meta-analysis found that pain (64%), anxiety (50%), and depression/mood (34%) were common reasons.7 People use marijuana for a plethora of other conditions and symptoms, which is reflected in the long list of “approved” conditions in most state medical marijuana laws. The problem I have with prescribing cannabis for non-neuropathic pain, anxiety, and depression is that there is no good randomized trial evidence of its effectiveness beyond a placebo effect (which is probably quite strong considering the psychotropic effects of marijuana). And, as Weinstein and Worster point out, there is evidence of increased mental health symptoms in chronic marijuana users.
Regardless of the scientific evidence, use of cannabis for symptom relief is unlikely to be a passing fad. Surveys show that about 70% of users believe they receive benefit from it.8 Therefore, it behooves us to be prepared to discuss the pros and cons of cannabis use with our patients—even if we decide not to prescribe it. Warn patients with anxiety and depression that it is unlikely to be effective and may make matters worse.
There is intense interest in medical marijuana and better research will likely change the way we use cannabis for medical purposes in the future. So, for now, our best approach is to stay informed as the research unfolds.
1. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456-2473.
2. Allan GM, Finley CR, Ton J, et al. Systematic review of systematic reviews for medical cannabinoids: pain, nausea and vomiting, spasticity, and harms. Can Fam Physician. 2018;64:E78-E94.
3. Abrams DI. The therapeutic effects of cannabis and cannabinoids: an update from the National Academies of Sciences, Engineering and Medicine report. Eur J Intern Med. 2018;49:7-11.
4. Mücke M, Phillips T, Radbruch L, et al. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2018;3:CD012182.
5. Kafil TS, Nguyen TM, MacDonald JK, et al. Cannabis for the treatment of Crohn’s disease. Cochrane Database Syst Rev. 2018;11:CD012853.
6. Kafil TS, Nguyen TM, MacDonald JK, et al. Cannabis for the treatment of ulcerative colitis. Cochrane Database Syst Rev. 2018;11:CD012954.
7. Kosiba JD, Maisto SA, Ditre JW. Patient-reported use of medical cannabis for pain, anxiety, and depression symptoms: systematic review and meta-analysis. Soc Sci Med. 2019;233:181-192.
8. Park JY, Wu LT. Prevalence, reasons, perceived effects, and correlates of medical marijuana use: a review. Drug Alcohol Depend. 2017;177:1–13. Epub 2017 May 16.
In this issue of JFP, Weinstein and Worster provide a wealth of information about prescribing marijuana. Medical marijuana (Cannabis) is now legal in the majority of states, so it’s likely that some of your patients are using marijuana for symptom relief. For those physicians who elect to prescribe marijuana, reading this review will help you avoid harming patients while maximizing potential benefits.
I say “potential benefits” because the research evidence to support benefit for most conditions and symptoms is weak at best. In addition to the JAMA meta-analysis cited by Weinstein and Worster,1 several meta-analyses and systematic reviews published since January 2018 reach similar conclusions.2-4
Marijuana can provide significant relief from chemotherapy-induced nausea and vomiting, and it is effective in reducing intractable seizures in 2 rare pediatric seizure disorders. There may be some benefit for treatment of spasticity, and there may be some therapeutic value for relief of neuropathic pain, although the evidence is not strong. Interestingly, there is some preliminary evidence that cannabis can improve gastrointestinal symptoms in patients with Crohn's disease and ulcerative colitis.5,6
Why do people use marijuana as medicine? A meta-analysis found that pain (64%), anxiety (50%), and depression/mood (34%) were common reasons.7 People use marijuana for a plethora of other conditions and symptoms, which is reflected in the long list of “approved” conditions in most state medical marijuana laws. The problem I have with prescribing cannabis for non-neuropathic pain, anxiety, and depression is that there is no good randomized trial evidence of its effectiveness beyond a placebo effect (which is probably quite strong considering the psychotropic effects of marijuana). And, as Weinstein and Worster point out, there is evidence of increased mental health symptoms in chronic marijuana users.
Regardless of the scientific evidence, use of cannabis for symptom relief is unlikely to be a passing fad. Surveys show that about 70% of users believe they receive benefit from it.8 Therefore, it behooves us to be prepared to discuss the pros and cons of cannabis use with our patients—even if we decide not to prescribe it. Warn patients with anxiety and depression that it is unlikely to be effective and may make matters worse.
There is intense interest in medical marijuana and better research will likely change the way we use cannabis for medical purposes in the future. So, for now, our best approach is to stay informed as the research unfolds.
In this issue of JFP, Weinstein and Worster provide a wealth of information about prescribing marijuana. Medical marijuana (Cannabis) is now legal in the majority of states, so it’s likely that some of your patients are using marijuana for symptom relief. For those physicians who elect to prescribe marijuana, reading this review will help you avoid harming patients while maximizing potential benefits.
I say “potential benefits” because the research evidence to support benefit for most conditions and symptoms is weak at best. In addition to the JAMA meta-analysis cited by Weinstein and Worster,1 several meta-analyses and systematic reviews published since January 2018 reach similar conclusions.2-4
Marijuana can provide significant relief from chemotherapy-induced nausea and vomiting, and it is effective in reducing intractable seizures in 2 rare pediatric seizure disorders. There may be some benefit for treatment of spasticity, and there may be some therapeutic value for relief of neuropathic pain, although the evidence is not strong. Interestingly, there is some preliminary evidence that cannabis can improve gastrointestinal symptoms in patients with Crohn's disease and ulcerative colitis.5,6
Why do people use marijuana as medicine? A meta-analysis found that pain (64%), anxiety (50%), and depression/mood (34%) were common reasons.7 People use marijuana for a plethora of other conditions and symptoms, which is reflected in the long list of “approved” conditions in most state medical marijuana laws. The problem I have with prescribing cannabis for non-neuropathic pain, anxiety, and depression is that there is no good randomized trial evidence of its effectiveness beyond a placebo effect (which is probably quite strong considering the psychotropic effects of marijuana). And, as Weinstein and Worster point out, there is evidence of increased mental health symptoms in chronic marijuana users.
Regardless of the scientific evidence, use of cannabis for symptom relief is unlikely to be a passing fad. Surveys show that about 70% of users believe they receive benefit from it.8 Therefore, it behooves us to be prepared to discuss the pros and cons of cannabis use with our patients—even if we decide not to prescribe it. Warn patients with anxiety and depression that it is unlikely to be effective and may make matters worse.
There is intense interest in medical marijuana and better research will likely change the way we use cannabis for medical purposes in the future. So, for now, our best approach is to stay informed as the research unfolds.
1. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456-2473.
2. Allan GM, Finley CR, Ton J, et al. Systematic review of systematic reviews for medical cannabinoids: pain, nausea and vomiting, spasticity, and harms. Can Fam Physician. 2018;64:E78-E94.
3. Abrams DI. The therapeutic effects of cannabis and cannabinoids: an update from the National Academies of Sciences, Engineering and Medicine report. Eur J Intern Med. 2018;49:7-11.
4. Mücke M, Phillips T, Radbruch L, et al. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2018;3:CD012182.
5. Kafil TS, Nguyen TM, MacDonald JK, et al. Cannabis for the treatment of Crohn’s disease. Cochrane Database Syst Rev. 2018;11:CD012853.
6. Kafil TS, Nguyen TM, MacDonald JK, et al. Cannabis for the treatment of ulcerative colitis. Cochrane Database Syst Rev. 2018;11:CD012954.
7. Kosiba JD, Maisto SA, Ditre JW. Patient-reported use of medical cannabis for pain, anxiety, and depression symptoms: systematic review and meta-analysis. Soc Sci Med. 2019;233:181-192.
8. Park JY, Wu LT. Prevalence, reasons, perceived effects, and correlates of medical marijuana use: a review. Drug Alcohol Depend. 2017;177:1–13. Epub 2017 May 16.
1. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456-2473.
2. Allan GM, Finley CR, Ton J, et al. Systematic review of systematic reviews for medical cannabinoids: pain, nausea and vomiting, spasticity, and harms. Can Fam Physician. 2018;64:E78-E94.
3. Abrams DI. The therapeutic effects of cannabis and cannabinoids: an update from the National Academies of Sciences, Engineering and Medicine report. Eur J Intern Med. 2018;49:7-11.
4. Mücke M, Phillips T, Radbruch L, et al. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2018;3:CD012182.
5. Kafil TS, Nguyen TM, MacDonald JK, et al. Cannabis for the treatment of Crohn’s disease. Cochrane Database Syst Rev. 2018;11:CD012853.
6. Kafil TS, Nguyen TM, MacDonald JK, et al. Cannabis for the treatment of ulcerative colitis. Cochrane Database Syst Rev. 2018;11:CD012954.
7. Kosiba JD, Maisto SA, Ditre JW. Patient-reported use of medical cannabis for pain, anxiety, and depression symptoms: systematic review and meta-analysis. Soc Sci Med. 2019;233:181-192.
8. Park JY, Wu LT. Prevalence, reasons, perceived effects, and correlates of medical marijuana use: a review. Drug Alcohol Depend. 2017;177:1–13. Epub 2017 May 16.
Women with epilepsy: 5 clinical pearls for contraception and preconception counseling
In 2015, 1.2% of the US population was estimated to have active epilepsy.1 For neurologists, key goals in the treatment of epilepsy include: controlling seizures, minimizing adverse effects of antiepileptic drugs (AEDs) and optimizing quality of life. For obstetrician-gynecologists, women with epilepsy (WWE) have unique contraceptive, preconception, and obstetric needs that require highly specialized approaches to care. Here, I highlight 5 care points that are important to keep in mind when counseling WWE.
1. Enzyme-inducing AEDs reduce the effectiveness of estrogen-progestin and some progestin contraceptives.
AEDs can induce hepatic enzymes that accelerate steroid hormone metabolism, producing clinically important reductions in bioavailable steroid hormone concentration (TABLE 1). According to Lexicomp, AEDs that are inducers of hepatic enzymes that metabolize steroid hormones include: carbamazepine (Tegretol), eslicarbazepine (Aptiom), felbamate (Felbatol), oxcarbazepine (Trileptal), perampanel (Fycompa), phenobarbital, phenytoin (Dilantin), primidone (Mysoline), rufinamide (Banzel), and topiramate (Topamax) (at dosages >200 mg daily). According to Lexicomp, the following AEDs do not cause clinically significant changes in hepatic enzymes that metabolize steroid hormones: acetazolamide (Diamox), clonazepam (Klonopin), ethosuximide (Zarontin), gabapentin (Neurontin), lacosamide (Vimpat), levetiracetam (Keppra), pregabalin (Lyrica), tiagabine (Gabitril), vigabatrin (Vigadrone), and zonisamide (Zonegran).2,3 In addition, lamotrigine (Lamictal) and valproate (Depakote) do not significantly influence the metabolism of contraceptive steroids,4,5 but contraceptive steroids significantly influence their metabolism (TABLE 2).
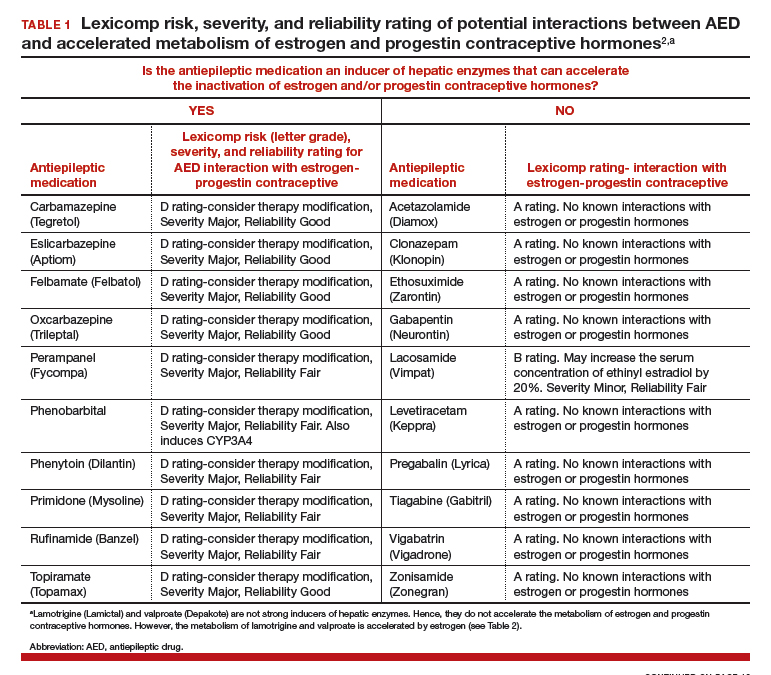

For WWE taking an AED that accelerates steroid hormone metabolism, estrogen-progestin contraceptive failure is common. In a survey of 111 WWE taking both an oral contraceptive and an AED, 27 reported becoming pregnant while taking the oral contraceptive.6 Carbamazepine, a strong inducer of hepatic enzymes, was the most frequently used AED in this sample.
Many studies report that carbamazepine accelerates the metabolisms of estrogen and progestins and reduces contraceptive efficacy. For example, in one study 20 healthy women were administered an ethinyl estradiol (20 µg)-levonorgestrel (100 µg) contraceptive, and randomly assigned to either receive carbamazepine 600 mg daily or a placebo pill.7 In this study, based on serum progesterone measurements, 5 of 10 women in the carbamazepine group ovulated, compared with 1 of 10 women in the placebo group. Women taking carbamazepine had integrated serum ethinyl estradiol and levonorgestrel concentrations approximately 45% lower than women taking placebo.7 Other studies also report that carbamazepine accelerates steroid hormone metabolism and reduces the circulating concentration of ethinyl estradiol, norethindrone, and levonorgestrel by about 50%.5,8
WWE taking an AED that induces hepatic enzymes should be counseled to use a copper or levonorgestrel (LNG) intrauterine device (IUD) or depot medroxyprogesterone acetate (DMPA) for contraception.9 WWE taking AEDs that do not induce hepatic enzymes can be offered the full array of contraceptive options, as outlined in Table 1. Occasionally, a WWE taking an AED that is an inducer of hepatic enzymes may strongly prefer to use an estrogen-progestin contraceptive and decline the preferred option of using an IUD or DMPA. If an estrogen-progestin contraceptive is to be prescribed, safeguards to reduce the risk of pregnancy include:
- prescribe a contraceptive with ≥35 µg of ethinyl estradiol
- prescribe a contraceptive with the highest dose of progestin with a long half-life (drospirenone, desogestrel, levonorgestrel)
- consider continuous hormonal contraception rather than 4 or 7 days off hormones and
- recommend use of a barrier contraceptive in addition to the hormonal contraceptive.
The effectiveness of levonorgestrel emergency contraception may also be reduced in WWE taking an enzyme-inducing AED. In these cases, some experts recommend a regimen of two doses of levonorgestrel 1.5 mg, separated by 12 hours.10 The effectiveness of progestin subdermal contraceptives may be reduced in women taking phenytoin. In one study of 9 WWE using a progestin subdermal implant, phenytoin reduced the circulating levonorgestrel level by approximately 40%.11
Continue to: 2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives...
2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives.
Estrogens, but not progestins, are known to reduce the serum concentration of lamotrigine by about 50%.12,13 This is a clinically significant pharmacologic interaction. Consequently, when a cyclic estrogen-progestin contraceptive is prescribed to a woman taking lamotrigine, oscillation in lamotrigine serum concentration can occur. When the woman is taking estrogen-containing pills, lamotrigine levels decrease, which increases the risk of seizure. When the woman is not taking the estrogen-containing pills, lamotrigine levels increase, possibly causing such adverse effects as nausea and vomiting. If a woman taking lamotrigine insists on using an estrogen-progestin contraceptive, the medication should be prescribed in a continuous regimen and the neurologist alerted so that they can increase the dose of lamotrigine and intensify their monitoring of lamotrigine levels. Lamotrigine does not change the metabolism of ethinyl estradiol and has minimal impact on the metabolism of levonorgestrel.4
3. Estrogen-progestin contraceptives require valproate dosage adjustment.
A few studies report that estrogen-progestin contraceptives accelerate the metabolism of valproate and reduce circulating valproate concentration,14,15 as noted in Table 2.In one study, estrogen-progestin contraceptive was associated with 18% and 29% decreases in total and unbound valproate concentrations, respectively.14 Valproate may induce polycystic ovary syndrome in women.16 Therefore, it is common that valproate and an estrogen-progestin contraceptive are co-prescribed. In these situations, the neurologist should be alerted prior to prescribing an estrogen-progestin contraceptive to WWE taking valproate so that dosage adjustment may occur, if indicated. Valproate does not appear to change the metabolism of ethinyl estradiol or levonorgestrel.5
4. Preconception counseling: Before conception consider using an AED with low teratogenicity.
Valproate is a potent teratogen, and consideration should be given to discontinuing valproate prior to conception. In a study of 1,788 pregnancies exposed to valproate, the risk of a major congenital malformation was 10% for valproate monotherapy, 11.3% for valproate combined with lamotrigine, and 11.7% for valproate combined with another AED, but not lamotrigine.17 At a valproate dose of ≥1,500 mg daily, the risk of major malformation was 24% for valproate monotherapy, 31% for valproate plus lamotrigine, and 19% for valproate plus another AED, but not lamotrigine.17 Valproate is reported to be associated with the following major congenital malformations: spina bifida, ventricular and atrial septal defects, pulmonary valve atresia, hypoplastic left heart syndrome, cleft palate, anorectal atresia, and hypospadias.18
In a study of 7,555 pregnancies in women using a single AED, the risk of major congenital anomalies varied greatly among the AEDs, including: valproate (10.3%), phenobarbital (6.5%), phenytoin (6.4%), carbamazepine (5.5%), topiramate (3.9%), oxcarbazepine (3.0%), lamotrigine (2.9%), and levetiracetam (2.8%).19 For WWE considering pregnancy, many experts recommend use of lamotrigine, levetiracetam, or oxcarbazepine to minimize the risk of fetal anomalies.
Continue to: 5. Folic acid...
5. Folic acid: Although the optimal dose for WWE taking an AED and planning to become pregnant is unknown, a high dose is reasonable.
The American College of Obstetricians and Gynecologists (ACOG) recommends that women planning pregnancy take 0.4 mg of folic acid daily, starting at least 1 month before pregnancy and continuing through at least the 12th week of gestation.20 ACOG also recommends that women at high risk of a neural tube defect should take 4 mg of folic acid daily. WWE taking a teratogenic AED are known to be at increased risk for fetal malformations, including neural tube defects. Should these women take 4 mg of folic acid daily? ACOG notes that, for women taking valproate, the benefit of high-dose folic acid (4 mg daily) has not been definitively proven,21 and guidelines from the American Academy of Neurology do not recommend high-dose folic acid for women receiving AEDs.22 Hence, ACOG does not recommend that WWE taking an AED take high-dose folic acid.
By contrast, the Royal College of Obstetricians and Gynecologists (RCOG) recommends that all WWE planning a pregnancy take folic acid 5 mg daily, initiated 3 months before conception and continued through the first trimester of pregnancy.23 The RCOG notes that among WWE taking an AED, intelligence quotient is greater in children whose mothers took folic acid during pregnancy.24 Given the potential benefit of folic acid on long-term outcomes and the known safety of folic acid, it is reasonable to recommend high-dose folic acid for WWE.
Final takeaways
Surveys consistently report that WWE have a low-level of awareness about the interaction between AEDs and hormonal contraceptives and the teratogenicity of AEDs. For example, in a survey of 2,000 WWE, 45% who were taking an enzyme-inducing AED and an estrogen-progestin oral contraceptive reported that they had not been warned about the potential interaction between the medications.25 Surprisingly, surveys of neurologists and obstetrician-gynecologists also report that there is a low level of awareness about the interaction between AEDs and hormonal contraceptives.26 When providing contraceptive counseling for WWE, prioritize the use of a copper or levonorgestrel IUD. When providing preconception counseling for WWE, educate the patient about the high teratogenicity of valproate and the lower risk of malformations associated with the use of lamotrigine, levetiracetam, and oxcarbazepine.
For most women with epilepsy, maintaining a valid driver's license is important for completion of daily life tasks. Most states require that a patient with seizures be seizure-free for 6 to 12 months to operate a motor vehicle. Estrogen-containing hormonal contraceptives can reduce the concentration of some AEDs, such as lamotrigine. Hence, it is important that the patient be aware of this interaction and that the primary neurologist be alerted if an estrogen-containing contraceptive is prescribed to a woman taking lamotrigine or valproate. Specific state laws related to epilepsy and driving are available at the Epilepsy Foundation website (https://www.epilepsy.com/driving-laws).
- Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy - United States 2015. MMWR Morb Mortal Wkly Rep. 2017;66:821-825.
- Lexicomp. https://www.wolterskluwercdi.com/lexicomp-online/. Accessed August 16, 2019.
- Reimers A, Brodtkorb E, Sabers A. Interactions between hormonal contraception and antiepileptic drugs: clinical and mechanistic considerations. Seizure. 2015;28:66-70.
- Sidhu J, Job S, Singh S, et al. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61:191-199.
- Crawford P, Chadwick D, Cleland P, et al. The lack of effect of sodium valproate on the pharmacokinetics of oral contraceptive steroids. Contraception. 1986;33:23-29.
- Fairgrieve SD, Jackson M, Jonas P, et al. Population-based, prospective study of the care of women with epilepsy in pregnancy. BMJ. 2000;321:674-675.
- Davis AR, Westhoff CL, Stanczyk FZ. Carbamazepine coadministration with an oral contraceptive: effects on steroid pharmacokinetics, ovulation, and bleeding. Epilepsia. 2011;52:243-247.
- Doose DR, Wang SS, Padmanabhan M, et al. Effect of topiramate or carbamazepine on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in healthy obese and nonobese female subjects. Epilepsia. 2003;44:540-549.
- Vieira CS, Pack A, Roberts K, et al. A pilot study of levonorgestrel concentrations and bleeding patterns in women with epilepsy using a levonorgestrel IUD and treated with antiepileptic drugs. Contraception. 2019;99:251-255.
- O'Brien MD, Guillebaud J. Contraception for women with epilepsy. Epilepsia. 2006;47:1419-1422.
- Haukkamaa M. Contraception by Norplant subdermal capsules is not reliable in epileptic patients on anticonvulsant treatment. Contraception. 1986;33:559-565.
- Sabers A, Buchholt JM, Uldall P, et al. Lamotrigine plasma levels reduced by oral contraceptives. Epilepsy Res. 2001;47:151-154.
- Reimers A, Helde G, Brodtkorb E. Ethinyl estradiol, not progestogens, reduces lamotrigine serum concentrations. Epilepsia. 2005;46:1414-1417.
- Galimberti CA, Mazzucchelli I, Arbasino C, et al. Increased apparent oral clearance of valproic acid during intake of combined contraceptive steroids in women with epilepsy. Epilepsia. 2006;47:1569-1572.
- Herzog AG, Farina EL, Blum AS. Serum valproate levels with oral contraceptive use. Epilepsia. 2005;46:970-971.
- Morrell MJ, Hayes FJ, Sluss PM, et al. Hyperandrogenism, ovulatory dysfunction, and polycystic ovary syndrome with valproate versus lamotrigine. Ann Neurol. 2008;64:200-211.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Dose-dependent teratogenicity of valproate in mono- and polytherapy: an observational study. Neurology. 2015;85:866-872.
- Blotière PO, Raguideau F, Weill A, et al. Risks of 23 specific malformations associated with prenatal exposure to 10 antiepileptic drugs. Neurology. 2019;93:e167-e180.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17:530-538.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 187: neural tube defects. Obstet Gynecol. 2017;130:e279-e290.
- Ban L, Fleming KM, Doyle P, et al. Congenital anomalies in children of mothers taking antiepileptic drugs with and without periconceptional high dose folic acid use: a population-based cohort study. PLoS One. 2015;10:e0131130.
- Harden CL, Pennell PB, Koppel BS, et al; American Academy of Neurology and American Epilepsy Society. Practice parameter update: management issues for women with epilepsy--focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breastfeeding: report of the Quality Standards Subcommittee and Therapeutics and technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73:142-149.
- Royal College of Obstetricians and Gynecologists. Epilepsy in pregnancy. Green-top Guideline No. 68; June 2016. https://www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg68_epilepsy.pdf. Accessed August 16, 2019.
- Meador KJ, Baker GA, Browning N, et al; NEAD Study Group. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12:244-252.
- Crawford P, Hudson S. Understanding the information needs of women with epilepsy at different life stages: results of the 'Ideal World' survey. Seizure. 2003;12:502-507.
- Krauss GL, Brandt J, Campbell M, et al. Antiepileptic medication and oral contraceptive interactions: a national survey of neurologists and obstetricians. Neurology. 1996;46:1534-1539.
In 2015, 1.2% of the US population was estimated to have active epilepsy.1 For neurologists, key goals in the treatment of epilepsy include: controlling seizures, minimizing adverse effects of antiepileptic drugs (AEDs) and optimizing quality of life. For obstetrician-gynecologists, women with epilepsy (WWE) have unique contraceptive, preconception, and obstetric needs that require highly specialized approaches to care. Here, I highlight 5 care points that are important to keep in mind when counseling WWE.
1. Enzyme-inducing AEDs reduce the effectiveness of estrogen-progestin and some progestin contraceptives.
AEDs can induce hepatic enzymes that accelerate steroid hormone metabolism, producing clinically important reductions in bioavailable steroid hormone concentration (TABLE 1). According to Lexicomp, AEDs that are inducers of hepatic enzymes that metabolize steroid hormones include: carbamazepine (Tegretol), eslicarbazepine (Aptiom), felbamate (Felbatol), oxcarbazepine (Trileptal), perampanel (Fycompa), phenobarbital, phenytoin (Dilantin), primidone (Mysoline), rufinamide (Banzel), and topiramate (Topamax) (at dosages >200 mg daily). According to Lexicomp, the following AEDs do not cause clinically significant changes in hepatic enzymes that metabolize steroid hormones: acetazolamide (Diamox), clonazepam (Klonopin), ethosuximide (Zarontin), gabapentin (Neurontin), lacosamide (Vimpat), levetiracetam (Keppra), pregabalin (Lyrica), tiagabine (Gabitril), vigabatrin (Vigadrone), and zonisamide (Zonegran).2,3 In addition, lamotrigine (Lamictal) and valproate (Depakote) do not significantly influence the metabolism of contraceptive steroids,4,5 but contraceptive steroids significantly influence their metabolism (TABLE 2).


For WWE taking an AED that accelerates steroid hormone metabolism, estrogen-progestin contraceptive failure is common. In a survey of 111 WWE taking both an oral contraceptive and an AED, 27 reported becoming pregnant while taking the oral contraceptive.6 Carbamazepine, a strong inducer of hepatic enzymes, was the most frequently used AED in this sample.
Many studies report that carbamazepine accelerates the metabolisms of estrogen and progestins and reduces contraceptive efficacy. For example, in one study 20 healthy women were administered an ethinyl estradiol (20 µg)-levonorgestrel (100 µg) contraceptive, and randomly assigned to either receive carbamazepine 600 mg daily or a placebo pill.7 In this study, based on serum progesterone measurements, 5 of 10 women in the carbamazepine group ovulated, compared with 1 of 10 women in the placebo group. Women taking carbamazepine had integrated serum ethinyl estradiol and levonorgestrel concentrations approximately 45% lower than women taking placebo.7 Other studies also report that carbamazepine accelerates steroid hormone metabolism and reduces the circulating concentration of ethinyl estradiol, norethindrone, and levonorgestrel by about 50%.5,8
WWE taking an AED that induces hepatic enzymes should be counseled to use a copper or levonorgestrel (LNG) intrauterine device (IUD) or depot medroxyprogesterone acetate (DMPA) for contraception.9 WWE taking AEDs that do not induce hepatic enzymes can be offered the full array of contraceptive options, as outlined in Table 1. Occasionally, a WWE taking an AED that is an inducer of hepatic enzymes may strongly prefer to use an estrogen-progestin contraceptive and decline the preferred option of using an IUD or DMPA. If an estrogen-progestin contraceptive is to be prescribed, safeguards to reduce the risk of pregnancy include:
- prescribe a contraceptive with ≥35 µg of ethinyl estradiol
- prescribe a contraceptive with the highest dose of progestin with a long half-life (drospirenone, desogestrel, levonorgestrel)
- consider continuous hormonal contraception rather than 4 or 7 days off hormones and
- recommend use of a barrier contraceptive in addition to the hormonal contraceptive.
The effectiveness of levonorgestrel emergency contraception may also be reduced in WWE taking an enzyme-inducing AED. In these cases, some experts recommend a regimen of two doses of levonorgestrel 1.5 mg, separated by 12 hours.10 The effectiveness of progestin subdermal contraceptives may be reduced in women taking phenytoin. In one study of 9 WWE using a progestin subdermal implant, phenytoin reduced the circulating levonorgestrel level by approximately 40%.11
Continue to: 2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives...
2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives.
Estrogens, but not progestins, are known to reduce the serum concentration of lamotrigine by about 50%.12,13 This is a clinically significant pharmacologic interaction. Consequently, when a cyclic estrogen-progestin contraceptive is prescribed to a woman taking lamotrigine, oscillation in lamotrigine serum concentration can occur. When the woman is taking estrogen-containing pills, lamotrigine levels decrease, which increases the risk of seizure. When the woman is not taking the estrogen-containing pills, lamotrigine levels increase, possibly causing such adverse effects as nausea and vomiting. If a woman taking lamotrigine insists on using an estrogen-progestin contraceptive, the medication should be prescribed in a continuous regimen and the neurologist alerted so that they can increase the dose of lamotrigine and intensify their monitoring of lamotrigine levels. Lamotrigine does not change the metabolism of ethinyl estradiol and has minimal impact on the metabolism of levonorgestrel.4
3. Estrogen-progestin contraceptives require valproate dosage adjustment.
A few studies report that estrogen-progestin contraceptives accelerate the metabolism of valproate and reduce circulating valproate concentration,14,15 as noted in Table 2.In one study, estrogen-progestin contraceptive was associated with 18% and 29% decreases in total and unbound valproate concentrations, respectively.14 Valproate may induce polycystic ovary syndrome in women.16 Therefore, it is common that valproate and an estrogen-progestin contraceptive are co-prescribed. In these situations, the neurologist should be alerted prior to prescribing an estrogen-progestin contraceptive to WWE taking valproate so that dosage adjustment may occur, if indicated. Valproate does not appear to change the metabolism of ethinyl estradiol or levonorgestrel.5
4. Preconception counseling: Before conception consider using an AED with low teratogenicity.
Valproate is a potent teratogen, and consideration should be given to discontinuing valproate prior to conception. In a study of 1,788 pregnancies exposed to valproate, the risk of a major congenital malformation was 10% for valproate monotherapy, 11.3% for valproate combined with lamotrigine, and 11.7% for valproate combined with another AED, but not lamotrigine.17 At a valproate dose of ≥1,500 mg daily, the risk of major malformation was 24% for valproate monotherapy, 31% for valproate plus lamotrigine, and 19% for valproate plus another AED, but not lamotrigine.17 Valproate is reported to be associated with the following major congenital malformations: spina bifida, ventricular and atrial septal defects, pulmonary valve atresia, hypoplastic left heart syndrome, cleft palate, anorectal atresia, and hypospadias.18
In a study of 7,555 pregnancies in women using a single AED, the risk of major congenital anomalies varied greatly among the AEDs, including: valproate (10.3%), phenobarbital (6.5%), phenytoin (6.4%), carbamazepine (5.5%), topiramate (3.9%), oxcarbazepine (3.0%), lamotrigine (2.9%), and levetiracetam (2.8%).19 For WWE considering pregnancy, many experts recommend use of lamotrigine, levetiracetam, or oxcarbazepine to minimize the risk of fetal anomalies.
Continue to: 5. Folic acid...
5. Folic acid: Although the optimal dose for WWE taking an AED and planning to become pregnant is unknown, a high dose is reasonable.
The American College of Obstetricians and Gynecologists (ACOG) recommends that women planning pregnancy take 0.4 mg of folic acid daily, starting at least 1 month before pregnancy and continuing through at least the 12th week of gestation.20 ACOG also recommends that women at high risk of a neural tube defect should take 4 mg of folic acid daily. WWE taking a teratogenic AED are known to be at increased risk for fetal malformations, including neural tube defects. Should these women take 4 mg of folic acid daily? ACOG notes that, for women taking valproate, the benefit of high-dose folic acid (4 mg daily) has not been definitively proven,21 and guidelines from the American Academy of Neurology do not recommend high-dose folic acid for women receiving AEDs.22 Hence, ACOG does not recommend that WWE taking an AED take high-dose folic acid.
By contrast, the Royal College of Obstetricians and Gynecologists (RCOG) recommends that all WWE planning a pregnancy take folic acid 5 mg daily, initiated 3 months before conception and continued through the first trimester of pregnancy.23 The RCOG notes that among WWE taking an AED, intelligence quotient is greater in children whose mothers took folic acid during pregnancy.24 Given the potential benefit of folic acid on long-term outcomes and the known safety of folic acid, it is reasonable to recommend high-dose folic acid for WWE.
Final takeaways
Surveys consistently report that WWE have a low-level of awareness about the interaction between AEDs and hormonal contraceptives and the teratogenicity of AEDs. For example, in a survey of 2,000 WWE, 45% who were taking an enzyme-inducing AED and an estrogen-progestin oral contraceptive reported that they had not been warned about the potential interaction between the medications.25 Surprisingly, surveys of neurologists and obstetrician-gynecologists also report that there is a low level of awareness about the interaction between AEDs and hormonal contraceptives.26 When providing contraceptive counseling for WWE, prioritize the use of a copper or levonorgestrel IUD. When providing preconception counseling for WWE, educate the patient about the high teratogenicity of valproate and the lower risk of malformations associated with the use of lamotrigine, levetiracetam, and oxcarbazepine.
For most women with epilepsy, maintaining a valid driver's license is important for completion of daily life tasks. Most states require that a patient with seizures be seizure-free for 6 to 12 months to operate a motor vehicle. Estrogen-containing hormonal contraceptives can reduce the concentration of some AEDs, such as lamotrigine. Hence, it is important that the patient be aware of this interaction and that the primary neurologist be alerted if an estrogen-containing contraceptive is prescribed to a woman taking lamotrigine or valproate. Specific state laws related to epilepsy and driving are available at the Epilepsy Foundation website (https://www.epilepsy.com/driving-laws).
In 2015, 1.2% of the US population was estimated to have active epilepsy.1 For neurologists, key goals in the treatment of epilepsy include: controlling seizures, minimizing adverse effects of antiepileptic drugs (AEDs) and optimizing quality of life. For obstetrician-gynecologists, women with epilepsy (WWE) have unique contraceptive, preconception, and obstetric needs that require highly specialized approaches to care. Here, I highlight 5 care points that are important to keep in mind when counseling WWE.
1. Enzyme-inducing AEDs reduce the effectiveness of estrogen-progestin and some progestin contraceptives.
AEDs can induce hepatic enzymes that accelerate steroid hormone metabolism, producing clinically important reductions in bioavailable steroid hormone concentration (TABLE 1). According to Lexicomp, AEDs that are inducers of hepatic enzymes that metabolize steroid hormones include: carbamazepine (Tegretol), eslicarbazepine (Aptiom), felbamate (Felbatol), oxcarbazepine (Trileptal), perampanel (Fycompa), phenobarbital, phenytoin (Dilantin), primidone (Mysoline), rufinamide (Banzel), and topiramate (Topamax) (at dosages >200 mg daily). According to Lexicomp, the following AEDs do not cause clinically significant changes in hepatic enzymes that metabolize steroid hormones: acetazolamide (Diamox), clonazepam (Klonopin), ethosuximide (Zarontin), gabapentin (Neurontin), lacosamide (Vimpat), levetiracetam (Keppra), pregabalin (Lyrica), tiagabine (Gabitril), vigabatrin (Vigadrone), and zonisamide (Zonegran).2,3 In addition, lamotrigine (Lamictal) and valproate (Depakote) do not significantly influence the metabolism of contraceptive steroids,4,5 but contraceptive steroids significantly influence their metabolism (TABLE 2).


For WWE taking an AED that accelerates steroid hormone metabolism, estrogen-progestin contraceptive failure is common. In a survey of 111 WWE taking both an oral contraceptive and an AED, 27 reported becoming pregnant while taking the oral contraceptive.6 Carbamazepine, a strong inducer of hepatic enzymes, was the most frequently used AED in this sample.
Many studies report that carbamazepine accelerates the metabolisms of estrogen and progestins and reduces contraceptive efficacy. For example, in one study 20 healthy women were administered an ethinyl estradiol (20 µg)-levonorgestrel (100 µg) contraceptive, and randomly assigned to either receive carbamazepine 600 mg daily or a placebo pill.7 In this study, based on serum progesterone measurements, 5 of 10 women in the carbamazepine group ovulated, compared with 1 of 10 women in the placebo group. Women taking carbamazepine had integrated serum ethinyl estradiol and levonorgestrel concentrations approximately 45% lower than women taking placebo.7 Other studies also report that carbamazepine accelerates steroid hormone metabolism and reduces the circulating concentration of ethinyl estradiol, norethindrone, and levonorgestrel by about 50%.5,8
WWE taking an AED that induces hepatic enzymes should be counseled to use a copper or levonorgestrel (LNG) intrauterine device (IUD) or depot medroxyprogesterone acetate (DMPA) for contraception.9 WWE taking AEDs that do not induce hepatic enzymes can be offered the full array of contraceptive options, as outlined in Table 1. Occasionally, a WWE taking an AED that is an inducer of hepatic enzymes may strongly prefer to use an estrogen-progestin contraceptive and decline the preferred option of using an IUD or DMPA. If an estrogen-progestin contraceptive is to be prescribed, safeguards to reduce the risk of pregnancy include:
- prescribe a contraceptive with ≥35 µg of ethinyl estradiol
- prescribe a contraceptive with the highest dose of progestin with a long half-life (drospirenone, desogestrel, levonorgestrel)
- consider continuous hormonal contraception rather than 4 or 7 days off hormones and
- recommend use of a barrier contraceptive in addition to the hormonal contraceptive.
The effectiveness of levonorgestrel emergency contraception may also be reduced in WWE taking an enzyme-inducing AED. In these cases, some experts recommend a regimen of two doses of levonorgestrel 1.5 mg, separated by 12 hours.10 The effectiveness of progestin subdermal contraceptives may be reduced in women taking phenytoin. In one study of 9 WWE using a progestin subdermal implant, phenytoin reduced the circulating levonorgestrel level by approximately 40%.11
Continue to: 2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives...
2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives.
Estrogens, but not progestins, are known to reduce the serum concentration of lamotrigine by about 50%.12,13 This is a clinically significant pharmacologic interaction. Consequently, when a cyclic estrogen-progestin contraceptive is prescribed to a woman taking lamotrigine, oscillation in lamotrigine serum concentration can occur. When the woman is taking estrogen-containing pills, lamotrigine levels decrease, which increases the risk of seizure. When the woman is not taking the estrogen-containing pills, lamotrigine levels increase, possibly causing such adverse effects as nausea and vomiting. If a woman taking lamotrigine insists on using an estrogen-progestin contraceptive, the medication should be prescribed in a continuous regimen and the neurologist alerted so that they can increase the dose of lamotrigine and intensify their monitoring of lamotrigine levels. Lamotrigine does not change the metabolism of ethinyl estradiol and has minimal impact on the metabolism of levonorgestrel.4
3. Estrogen-progestin contraceptives require valproate dosage adjustment.
A few studies report that estrogen-progestin contraceptives accelerate the metabolism of valproate and reduce circulating valproate concentration,14,15 as noted in Table 2.In one study, estrogen-progestin contraceptive was associated with 18% and 29% decreases in total and unbound valproate concentrations, respectively.14 Valproate may induce polycystic ovary syndrome in women.16 Therefore, it is common that valproate and an estrogen-progestin contraceptive are co-prescribed. In these situations, the neurologist should be alerted prior to prescribing an estrogen-progestin contraceptive to WWE taking valproate so that dosage adjustment may occur, if indicated. Valproate does not appear to change the metabolism of ethinyl estradiol or levonorgestrel.5
4. Preconception counseling: Before conception consider using an AED with low teratogenicity.
Valproate is a potent teratogen, and consideration should be given to discontinuing valproate prior to conception. In a study of 1,788 pregnancies exposed to valproate, the risk of a major congenital malformation was 10% for valproate monotherapy, 11.3% for valproate combined with lamotrigine, and 11.7% for valproate combined with another AED, but not lamotrigine.17 At a valproate dose of ≥1,500 mg daily, the risk of major malformation was 24% for valproate monotherapy, 31% for valproate plus lamotrigine, and 19% for valproate plus another AED, but not lamotrigine.17 Valproate is reported to be associated with the following major congenital malformations: spina bifida, ventricular and atrial septal defects, pulmonary valve atresia, hypoplastic left heart syndrome, cleft palate, anorectal atresia, and hypospadias.18
In a study of 7,555 pregnancies in women using a single AED, the risk of major congenital anomalies varied greatly among the AEDs, including: valproate (10.3%), phenobarbital (6.5%), phenytoin (6.4%), carbamazepine (5.5%), topiramate (3.9%), oxcarbazepine (3.0%), lamotrigine (2.9%), and levetiracetam (2.8%).19 For WWE considering pregnancy, many experts recommend use of lamotrigine, levetiracetam, or oxcarbazepine to minimize the risk of fetal anomalies.
Continue to: 5. Folic acid...
5. Folic acid: Although the optimal dose for WWE taking an AED and planning to become pregnant is unknown, a high dose is reasonable.
The American College of Obstetricians and Gynecologists (ACOG) recommends that women planning pregnancy take 0.4 mg of folic acid daily, starting at least 1 month before pregnancy and continuing through at least the 12th week of gestation.20 ACOG also recommends that women at high risk of a neural tube defect should take 4 mg of folic acid daily. WWE taking a teratogenic AED are known to be at increased risk for fetal malformations, including neural tube defects. Should these women take 4 mg of folic acid daily? ACOG notes that, for women taking valproate, the benefit of high-dose folic acid (4 mg daily) has not been definitively proven,21 and guidelines from the American Academy of Neurology do not recommend high-dose folic acid for women receiving AEDs.22 Hence, ACOG does not recommend that WWE taking an AED take high-dose folic acid.
By contrast, the Royal College of Obstetricians and Gynecologists (RCOG) recommends that all WWE planning a pregnancy take folic acid 5 mg daily, initiated 3 months before conception and continued through the first trimester of pregnancy.23 The RCOG notes that among WWE taking an AED, intelligence quotient is greater in children whose mothers took folic acid during pregnancy.24 Given the potential benefit of folic acid on long-term outcomes and the known safety of folic acid, it is reasonable to recommend high-dose folic acid for WWE.
Final takeaways
Surveys consistently report that WWE have a low-level of awareness about the interaction between AEDs and hormonal contraceptives and the teratogenicity of AEDs. For example, in a survey of 2,000 WWE, 45% who were taking an enzyme-inducing AED and an estrogen-progestin oral contraceptive reported that they had not been warned about the potential interaction between the medications.25 Surprisingly, surveys of neurologists and obstetrician-gynecologists also report that there is a low level of awareness about the interaction between AEDs and hormonal contraceptives.26 When providing contraceptive counseling for WWE, prioritize the use of a copper or levonorgestrel IUD. When providing preconception counseling for WWE, educate the patient about the high teratogenicity of valproate and the lower risk of malformations associated with the use of lamotrigine, levetiracetam, and oxcarbazepine.
For most women with epilepsy, maintaining a valid driver's license is important for completion of daily life tasks. Most states require that a patient with seizures be seizure-free for 6 to 12 months to operate a motor vehicle. Estrogen-containing hormonal contraceptives can reduce the concentration of some AEDs, such as lamotrigine. Hence, it is important that the patient be aware of this interaction and that the primary neurologist be alerted if an estrogen-containing contraceptive is prescribed to a woman taking lamotrigine or valproate. Specific state laws related to epilepsy and driving are available at the Epilepsy Foundation website (https://www.epilepsy.com/driving-laws).
- Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy - United States 2015. MMWR Morb Mortal Wkly Rep. 2017;66:821-825.
- Lexicomp. https://www.wolterskluwercdi.com/lexicomp-online/. Accessed August 16, 2019.
- Reimers A, Brodtkorb E, Sabers A. Interactions between hormonal contraception and antiepileptic drugs: clinical and mechanistic considerations. Seizure. 2015;28:66-70.
- Sidhu J, Job S, Singh S, et al. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61:191-199.
- Crawford P, Chadwick D, Cleland P, et al. The lack of effect of sodium valproate on the pharmacokinetics of oral contraceptive steroids. Contraception. 1986;33:23-29.
- Fairgrieve SD, Jackson M, Jonas P, et al. Population-based, prospective study of the care of women with epilepsy in pregnancy. BMJ. 2000;321:674-675.
- Davis AR, Westhoff CL, Stanczyk FZ. Carbamazepine coadministration with an oral contraceptive: effects on steroid pharmacokinetics, ovulation, and bleeding. Epilepsia. 2011;52:243-247.
- Doose DR, Wang SS, Padmanabhan M, et al. Effect of topiramate or carbamazepine on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in healthy obese and nonobese female subjects. Epilepsia. 2003;44:540-549.
- Vieira CS, Pack A, Roberts K, et al. A pilot study of levonorgestrel concentrations and bleeding patterns in women with epilepsy using a levonorgestrel IUD and treated with antiepileptic drugs. Contraception. 2019;99:251-255.
- O'Brien MD, Guillebaud J. Contraception for women with epilepsy. Epilepsia. 2006;47:1419-1422.
- Haukkamaa M. Contraception by Norplant subdermal capsules is not reliable in epileptic patients on anticonvulsant treatment. Contraception. 1986;33:559-565.
- Sabers A, Buchholt JM, Uldall P, et al. Lamotrigine plasma levels reduced by oral contraceptives. Epilepsy Res. 2001;47:151-154.
- Reimers A, Helde G, Brodtkorb E. Ethinyl estradiol, not progestogens, reduces lamotrigine serum concentrations. Epilepsia. 2005;46:1414-1417.
- Galimberti CA, Mazzucchelli I, Arbasino C, et al. Increased apparent oral clearance of valproic acid during intake of combined contraceptive steroids in women with epilepsy. Epilepsia. 2006;47:1569-1572.
- Herzog AG, Farina EL, Blum AS. Serum valproate levels with oral contraceptive use. Epilepsia. 2005;46:970-971.
- Morrell MJ, Hayes FJ, Sluss PM, et al. Hyperandrogenism, ovulatory dysfunction, and polycystic ovary syndrome with valproate versus lamotrigine. Ann Neurol. 2008;64:200-211.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Dose-dependent teratogenicity of valproate in mono- and polytherapy: an observational study. Neurology. 2015;85:866-872.
- Blotière PO, Raguideau F, Weill A, et al. Risks of 23 specific malformations associated with prenatal exposure to 10 antiepileptic drugs. Neurology. 2019;93:e167-e180.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17:530-538.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 187: neural tube defects. Obstet Gynecol. 2017;130:e279-e290.
- Ban L, Fleming KM, Doyle P, et al. Congenital anomalies in children of mothers taking antiepileptic drugs with and without periconceptional high dose folic acid use: a population-based cohort study. PLoS One. 2015;10:e0131130.
- Harden CL, Pennell PB, Koppel BS, et al; American Academy of Neurology and American Epilepsy Society. Practice parameter update: management issues for women with epilepsy--focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breastfeeding: report of the Quality Standards Subcommittee and Therapeutics and technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73:142-149.
- Royal College of Obstetricians and Gynecologists. Epilepsy in pregnancy. Green-top Guideline No. 68; June 2016. https://www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg68_epilepsy.pdf. Accessed August 16, 2019.
- Meador KJ, Baker GA, Browning N, et al; NEAD Study Group. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12:244-252.
- Crawford P, Hudson S. Understanding the information needs of women with epilepsy at different life stages: results of the 'Ideal World' survey. Seizure. 2003;12:502-507.
- Krauss GL, Brandt J, Campbell M, et al. Antiepileptic medication and oral contraceptive interactions: a national survey of neurologists and obstetricians. Neurology. 1996;46:1534-1539.
- Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy - United States 2015. MMWR Morb Mortal Wkly Rep. 2017;66:821-825.
- Lexicomp. https://www.wolterskluwercdi.com/lexicomp-online/. Accessed August 16, 2019.
- Reimers A, Brodtkorb E, Sabers A. Interactions between hormonal contraception and antiepileptic drugs: clinical and mechanistic considerations. Seizure. 2015;28:66-70.
- Sidhu J, Job S, Singh S, et al. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61:191-199.
- Crawford P, Chadwick D, Cleland P, et al. The lack of effect of sodium valproate on the pharmacokinetics of oral contraceptive steroids. Contraception. 1986;33:23-29.
- Fairgrieve SD, Jackson M, Jonas P, et al. Population-based, prospective study of the care of women with epilepsy in pregnancy. BMJ. 2000;321:674-675.
- Davis AR, Westhoff CL, Stanczyk FZ. Carbamazepine coadministration with an oral contraceptive: effects on steroid pharmacokinetics, ovulation, and bleeding. Epilepsia. 2011;52:243-247.
- Doose DR, Wang SS, Padmanabhan M, et al. Effect of topiramate or carbamazepine on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in healthy obese and nonobese female subjects. Epilepsia. 2003;44:540-549.
- Vieira CS, Pack A, Roberts K, et al. A pilot study of levonorgestrel concentrations and bleeding patterns in women with epilepsy using a levonorgestrel IUD and treated with antiepileptic drugs. Contraception. 2019;99:251-255.
- O'Brien MD, Guillebaud J. Contraception for women with epilepsy. Epilepsia. 2006;47:1419-1422.
- Haukkamaa M. Contraception by Norplant subdermal capsules is not reliable in epileptic patients on anticonvulsant treatment. Contraception. 1986;33:559-565.
- Sabers A, Buchholt JM, Uldall P, et al. Lamotrigine plasma levels reduced by oral contraceptives. Epilepsy Res. 2001;47:151-154.
- Reimers A, Helde G, Brodtkorb E. Ethinyl estradiol, not progestogens, reduces lamotrigine serum concentrations. Epilepsia. 2005;46:1414-1417.
- Galimberti CA, Mazzucchelli I, Arbasino C, et al. Increased apparent oral clearance of valproic acid during intake of combined contraceptive steroids in women with epilepsy. Epilepsia. 2006;47:1569-1572.
- Herzog AG, Farina EL, Blum AS. Serum valproate levels with oral contraceptive use. Epilepsia. 2005;46:970-971.
- Morrell MJ, Hayes FJ, Sluss PM, et al. Hyperandrogenism, ovulatory dysfunction, and polycystic ovary syndrome with valproate versus lamotrigine. Ann Neurol. 2008;64:200-211.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Dose-dependent teratogenicity of valproate in mono- and polytherapy: an observational study. Neurology. 2015;85:866-872.
- Blotière PO, Raguideau F, Weill A, et al. Risks of 23 specific malformations associated with prenatal exposure to 10 antiepileptic drugs. Neurology. 2019;93:e167-e180.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17:530-538.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 187: neural tube defects. Obstet Gynecol. 2017;130:e279-e290.
- Ban L, Fleming KM, Doyle P, et al. Congenital anomalies in children of mothers taking antiepileptic drugs with and without periconceptional high dose folic acid use: a population-based cohort study. PLoS One. 2015;10:e0131130.
- Harden CL, Pennell PB, Koppel BS, et al; American Academy of Neurology and American Epilepsy Society. Practice parameter update: management issues for women with epilepsy--focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breastfeeding: report of the Quality Standards Subcommittee and Therapeutics and technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73:142-149.
- Royal College of Obstetricians and Gynecologists. Epilepsy in pregnancy. Green-top Guideline No. 68; June 2016. https://www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg68_epilepsy.pdf. Accessed August 16, 2019.
- Meador KJ, Baker GA, Browning N, et al; NEAD Study Group. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12:244-252.
- Crawford P, Hudson S. Understanding the information needs of women with epilepsy at different life stages: results of the 'Ideal World' survey. Seizure. 2003;12:502-507.
- Krauss GL, Brandt J, Campbell M, et al. Antiepileptic medication and oral contraceptive interactions: a national survey of neurologists and obstetricians. Neurology. 1996;46:1534-1539.
Part 1: Finding Your Inner Leader
Is it my imagination, or has there been a lot of discussion on leadership lately? In the past 3 years, all the meetings I have attended had at least 1 presentation on leadership or the traits of leaders. Sometimes—even in the oddest places—I have come across an article with “leadership” in the title. In fact, a serendipitous discovery of 2 publications is what inspired me to write this.
I spotted the first one in a reading basket when I was on vacation: It was an interview with Benjamin Zander, the conductor of the Boston Philharmonic Orchestra and the Boston Philharmonic Youth Orchestra.1 I was especially interested to read this because when I was in graduate school, Benjamin (the father of one of my classmates) visited our campus to give a presentation on talent and self-confidence. I can still hear him conducting us to emphatically believe in ourselves and others. This was echoed in his interview: “Never doubt the capacity of the people you lead to accomplish whatever you dream for them.” What a powerful concept: Believe in the possibilities of someone!
The second was an American Legion Auxiliary column, which emphasized that “leadership is not a title, but a responsibility.”2 This struck a chord with me because some prominent leaders don’t appear to ascribe to that assessment (although they should!).
After digesting these articles, I started thinking: What, exactly, is leadership? What do we even mean by leadership? How do we measure it? Is it measurable? How do we know when we see or experience good leadership? Can one learn to become a leader by simply reading a “how-to” article?
I think I can answer these questions with 2 principles that have guided me through my career as an NP: (1) Make use of another leader’s expertise to guide you, and (2) Continue to grow amid any setbacks.
For example, my transition from a full-time clinician to a Health Policy Coordinator or “policy wonk” did not have a distinct trajectory. Although my core set of clinical skills were essential, I knew early on that I had to expand by adapting specific organizational skills that would enable me to grow in my new role. But how was I to prioritize which skills to improve? More than a simple trial-and-error approach was required; I needed guidance. Fortunately, my new boss was willing to share her experience and the lessons she learned on the job. Key among them was to recognize the skills I already had—communicating and coordinating—and to develop those skills to be more effective in my new position.
Later in my career, I worked with colleagues to pursue legislation for NP prescriptive authority in Massachusetts. The political arena of Commonwealth’s health care laws was especially pivotal in changing how I saw setbacks. These weren’t to be accepted as a failure but as a challenge to figure out how to better succeed the next time. For several years, I was told “No” before we finally got a bill passed. But each round of my testimony was an opportunity to educate lawmakers and the public on the valuable role of NPs and the quality of care we provide. I try to share this story with new NPs as a good example of why they should persist through adversity.
Continue to: Over the next 3 weeks...
Over the next 3 weeks, join us on Thursdays as we continue to discuss what it means to be a leader—from the pitfalls to the victories. For those who are leaders or who work for one, please share your thoughts, experiences, and lessons learned. Maybe you can give a shoutout to someone who was a positive influence!
1. Labarre P. Leadership—Ben Zander. Fast Company website. www.fastcompany.com/35825/leadership-ben-zander. Published November 30, 1998. Accessed August 27, 2019.
2. Volunteer beyond the ALA: serve on boards, committees. American Legion Auxiliary. November 2018:29.
Is it my imagination, or has there been a lot of discussion on leadership lately? In the past 3 years, all the meetings I have attended had at least 1 presentation on leadership or the traits of leaders. Sometimes—even in the oddest places—I have come across an article with “leadership” in the title. In fact, a serendipitous discovery of 2 publications is what inspired me to write this.
I spotted the first one in a reading basket when I was on vacation: It was an interview with Benjamin Zander, the conductor of the Boston Philharmonic Orchestra and the Boston Philharmonic Youth Orchestra.1 I was especially interested to read this because when I was in graduate school, Benjamin (the father of one of my classmates) visited our campus to give a presentation on talent and self-confidence. I can still hear him conducting us to emphatically believe in ourselves and others. This was echoed in his interview: “Never doubt the capacity of the people you lead to accomplish whatever you dream for them.” What a powerful concept: Believe in the possibilities of someone!
The second was an American Legion Auxiliary column, which emphasized that “leadership is not a title, but a responsibility.”2 This struck a chord with me because some prominent leaders don’t appear to ascribe to that assessment (although they should!).
After digesting these articles, I started thinking: What, exactly, is leadership? What do we even mean by leadership? How do we measure it? Is it measurable? How do we know when we see or experience good leadership? Can one learn to become a leader by simply reading a “how-to” article?
I think I can answer these questions with 2 principles that have guided me through my career as an NP: (1) Make use of another leader’s expertise to guide you, and (2) Continue to grow amid any setbacks.
For example, my transition from a full-time clinician to a Health Policy Coordinator or “policy wonk” did not have a distinct trajectory. Although my core set of clinical skills were essential, I knew early on that I had to expand by adapting specific organizational skills that would enable me to grow in my new role. But how was I to prioritize which skills to improve? More than a simple trial-and-error approach was required; I needed guidance. Fortunately, my new boss was willing to share her experience and the lessons she learned on the job. Key among them was to recognize the skills I already had—communicating and coordinating—and to develop those skills to be more effective in my new position.
Later in my career, I worked with colleagues to pursue legislation for NP prescriptive authority in Massachusetts. The political arena of Commonwealth’s health care laws was especially pivotal in changing how I saw setbacks. These weren’t to be accepted as a failure but as a challenge to figure out how to better succeed the next time. For several years, I was told “No” before we finally got a bill passed. But each round of my testimony was an opportunity to educate lawmakers and the public on the valuable role of NPs and the quality of care we provide. I try to share this story with new NPs as a good example of why they should persist through adversity.
Continue to: Over the next 3 weeks...
Over the next 3 weeks, join us on Thursdays as we continue to discuss what it means to be a leader—from the pitfalls to the victories. For those who are leaders or who work for one, please share your thoughts, experiences, and lessons learned. Maybe you can give a shoutout to someone who was a positive influence!
Is it my imagination, or has there been a lot of discussion on leadership lately? In the past 3 years, all the meetings I have attended had at least 1 presentation on leadership or the traits of leaders. Sometimes—even in the oddest places—I have come across an article with “leadership” in the title. In fact, a serendipitous discovery of 2 publications is what inspired me to write this.
I spotted the first one in a reading basket when I was on vacation: It was an interview with Benjamin Zander, the conductor of the Boston Philharmonic Orchestra and the Boston Philharmonic Youth Orchestra.1 I was especially interested to read this because when I was in graduate school, Benjamin (the father of one of my classmates) visited our campus to give a presentation on talent and self-confidence. I can still hear him conducting us to emphatically believe in ourselves and others. This was echoed in his interview: “Never doubt the capacity of the people you lead to accomplish whatever you dream for them.” What a powerful concept: Believe in the possibilities of someone!
The second was an American Legion Auxiliary column, which emphasized that “leadership is not a title, but a responsibility.”2 This struck a chord with me because some prominent leaders don’t appear to ascribe to that assessment (although they should!).
After digesting these articles, I started thinking: What, exactly, is leadership? What do we even mean by leadership? How do we measure it? Is it measurable? How do we know when we see or experience good leadership? Can one learn to become a leader by simply reading a “how-to” article?
I think I can answer these questions with 2 principles that have guided me through my career as an NP: (1) Make use of another leader’s expertise to guide you, and (2) Continue to grow amid any setbacks.
For example, my transition from a full-time clinician to a Health Policy Coordinator or “policy wonk” did not have a distinct trajectory. Although my core set of clinical skills were essential, I knew early on that I had to expand by adapting specific organizational skills that would enable me to grow in my new role. But how was I to prioritize which skills to improve? More than a simple trial-and-error approach was required; I needed guidance. Fortunately, my new boss was willing to share her experience and the lessons she learned on the job. Key among them was to recognize the skills I already had—communicating and coordinating—and to develop those skills to be more effective in my new position.
Later in my career, I worked with colleagues to pursue legislation for NP prescriptive authority in Massachusetts. The political arena of Commonwealth’s health care laws was especially pivotal in changing how I saw setbacks. These weren’t to be accepted as a failure but as a challenge to figure out how to better succeed the next time. For several years, I was told “No” before we finally got a bill passed. But each round of my testimony was an opportunity to educate lawmakers and the public on the valuable role of NPs and the quality of care we provide. I try to share this story with new NPs as a good example of why they should persist through adversity.
Continue to: Over the next 3 weeks...
Over the next 3 weeks, join us on Thursdays as we continue to discuss what it means to be a leader—from the pitfalls to the victories. For those who are leaders or who work for one, please share your thoughts, experiences, and lessons learned. Maybe you can give a shoutout to someone who was a positive influence!
1. Labarre P. Leadership—Ben Zander. Fast Company website. www.fastcompany.com/35825/leadership-ben-zander. Published November 30, 1998. Accessed August 27, 2019.
2. Volunteer beyond the ALA: serve on boards, committees. American Legion Auxiliary. November 2018:29.
1. Labarre P. Leadership—Ben Zander. Fast Company website. www.fastcompany.com/35825/leadership-ben-zander. Published November 30, 1998. Accessed August 27, 2019.
2. Volunteer beyond the ALA: serve on boards, committees. American Legion Auxiliary. November 2018:29.
ID Blog: The story of syphilis, part I
Rise of a global scourge
The Great Pox, the French Disease, Cupid’s Disease – syphilis has had many names throughout history.
Why should we care about the history of syphilis? Surely syphilis has reached the status of a nonentity disease – in-and-out of the doctor’s office with a course of antibiotics and farewell to the problem. And on the surface, that is certainly true. For now. In the developed world. For those with access to reasonable health care.
But that is all the shiny surface of modern medical triumph. Despite successes in prevention throughout the late 20th and early 21st century, syphilis is making comeback. A growing reservoir of syphilis, often untreated, lies hidden by the invisibility of poorer nations and increasingly in the lower economic strata of the developed world. And the danger is increased by the rise of antibiotic-resistant strains of the disease.
Over the last decade, the European Union and several other high-income countries observed an increasing syphilis trend, according to a recent report by the European Centre for Disease Prevention and Control. And in the United States, the Centers for Disease Control and Prevention has expressed concern over “the rising tide of syphilis” and a “devastating surge in congenital syphilis.” Many reasons have been suggested for this resurgence of syphilis, including the prevalence of unprotected sex and the overall increase in multiple sexual partners in the sexually active population. This trend has been ascribed to a reduced fear of acquiring HIV from condomless sex because of the rise of antiretroviral therapies, which make HIV infection no longer a death sentence for those who have access to and can afford the drugs.
Men who have sex with men are the most affected population cited, which may in part be related to the trend in unprotected sex that has accompanied the decreasing fear of HIV. But in some countries, syphilis rates among heterosexual populations are on the increase as well. Even more troubling were the increases in syphilis diagnosed among pregnant women that were reported in high-income settings outside of the European Union, which led to increases in congenital syphilis infections.
According to a 2018 update on the global epidemiology of syphilis, each year an estimated 6 million new cases are diagnosed in people aged 15-49 years, with more than 300,000 fetal and neonatal deaths attributed to the disease. An additional 215,000 infants are at increased risk of early death because of prenatal infection.
For syphilis is indeed a nasty disease. But a remarkable one as well. It presents an almost textbook example of disease evolution and adaptation writ large. It is also a disease with equally remarkable properties – acute, systemic, latent, eruptive, and congenital in its various manifestations. As Sir William Osler, one of the brightest lights of medical education of his time, said in 1897: “I often tell my students that it [syphilis] is the only disease which they require to know thoroughly. Know syphilis in all its manifestations and relations, and all other things clinical will be added unto you.”
Syphilis is caused by the spirochete Treponema pallidum subspecies pallidum and is generally acquired by sexual contact. Congenital syphilis infection occurs by transplacental transmission.
In its modern manifestation, the disease evolves through several stages – primary, secondary, and tertiary. Primary, noncongenital infection is characterized by a lesion. This chancre, as it is called, occurs at the original site of infection, typically between 10 days and 3 months after exposure. The chancre usually appears on the genitals, but given the variety of sexual behaviors, chancres can also occur on the rectum, tongue, pharynx, breast, and so on. The myth of only choosing “a clean partner,” one without visible lesions, is misleading because vaginal and rectal lesions may not be easy to spot yet still remain profoundly infectious.
The secondary stage of an untreated infection occurs 2-3 months after the onset of chancre, and results in multisystem involvement as the spirochetes spread through the bloodstream. Symptoms include skin rash (involving the palms and the soles of the feet) and potentially a variety of other dermatologic manifestations. Fever and swollen lymph nodes may also be present before the disease moves into a latent stage, in which no clinical symptoms are evident. Following this, tertiary syphilis can occur 10-30 years after the initial infection in about 30% of the untreated population, resulting in neurosyphilis, cardiovascular syphilis, or late benign syphilis. Disease progression in tertiary syphilis can lead to dementia, disfigurement, and death.
Sounds bad, doesn’t it? But what we’ve just recounted is the relatively benign disease that modern syphilis has become. Syphilis began as a sweeping, lethal epidemic in the late 15th century spreading dread across the world from the Americas to Europe and then to Asia at a speed equal to the fastest sailing ships of the era.
Syphilis first appeared in Naples in its epidemic form in 1495. Recent anthropological and historical consensus has suggested that syphilis, as we know it today, like tobacco, potatoes, and maize is a product of the Americas that was brought to the Old World by the intrepid exploits of one Christopher Columbus in 1493. Just as the Spanish inadvertently brought smallpox to devastate the population of the New World, Christopher Columbus appears to have brought epidemic syphilis to the Old World in an ironic twist of fate.
Ruy Diaz de Isla, one of two Spanish physicians present when Christopher Columbus returned from his first voyage to America, wrote in a manuscript that Pinzon de Palos, the pilot of Columbus, and also other members of the crew already suffered from symptoms of what was likely syphilis on their return from the New World
Although there has been some controversy regarding the origin of the syphilis epidemic, a recent molecular study using a large collection of pathogenic Treponema strains indicated that venereal syphilis arose relatively recently in human history, and that the closest related syphilis-causing strains were found in South America, providing support for the Columbian theory of syphilis’s origin.
Syphilis flamed across Europe like wildfire, lit by a series of small wars that started shortly after Columbus’s return. Soldiers throughout history have indulged themselves in activities well primed for the spread of venereal disease, and the doughty warriors of the late 15th century were no exception. And throughout the next 500-plus years, syphilis and war rode across the world in tandem, like the white and red horsemen of the Apocalypse.
In its initial launch, syphilis had the help of Charles VIII, the King of France, who had invaded Italy in early 1495 with an army of more than 30,000 mercenaries recruited from across Europe. His forces conquered Naples, which was primarily defended by Spanish mercenaries.
When Charles VIII broke up his army, “mercenaries, infected with a mysterious, serious disease, returned to their native lands or moved elsewhere to wage war, spreading the disease across Europe.” The “Great Pox” initially struck Italy, France, Germany, and Switzerland in 1495; then Holland and Greece in the following year, reaching England and Scotland by 1497; and then Hungary, Poland, and the Scandinavian countries by 1500.
As this period was the Age of Exploration, French, Dutch, and English sailors soon carried syphilis across the rest of an unsuspecting world, with the disease reaching India in 1498 before moving also to Africa and then throughout the rest of Asia in the early 16th century.
And yet, one of the most remarkable parts of the story is the rapid transformation of syphilis from a deadly virulent epidemic to a (comparatively) benign endemic status. Which will be the subject of my next posting.

Mark Lesney is the managing editor of MDedge.com/IDPractioner . He has a PhD in Plant Virology and a PhD in the History of Science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor at the Georgetown University School of Medicine, Department of Biochemistry and Molecular & Cellular Biology, Washington, DC.
Rise of a global scourge
Rise of a global scourge
The Great Pox, the French Disease, Cupid’s Disease – syphilis has had many names throughout history.
Why should we care about the history of syphilis? Surely syphilis has reached the status of a nonentity disease – in-and-out of the doctor’s office with a course of antibiotics and farewell to the problem. And on the surface, that is certainly true. For now. In the developed world. For those with access to reasonable health care.
But that is all the shiny surface of modern medical triumph. Despite successes in prevention throughout the late 20th and early 21st century, syphilis is making comeback. A growing reservoir of syphilis, often untreated, lies hidden by the invisibility of poorer nations and increasingly in the lower economic strata of the developed world. And the danger is increased by the rise of antibiotic-resistant strains of the disease.
Over the last decade, the European Union and several other high-income countries observed an increasing syphilis trend, according to a recent report by the European Centre for Disease Prevention and Control. And in the United States, the Centers for Disease Control and Prevention has expressed concern over “the rising tide of syphilis” and a “devastating surge in congenital syphilis.” Many reasons have been suggested for this resurgence of syphilis, including the prevalence of unprotected sex and the overall increase in multiple sexual partners in the sexually active population. This trend has been ascribed to a reduced fear of acquiring HIV from condomless sex because of the rise of antiretroviral therapies, which make HIV infection no longer a death sentence for those who have access to and can afford the drugs.
Men who have sex with men are the most affected population cited, which may in part be related to the trend in unprotected sex that has accompanied the decreasing fear of HIV. But in some countries, syphilis rates among heterosexual populations are on the increase as well. Even more troubling were the increases in syphilis diagnosed among pregnant women that were reported in high-income settings outside of the European Union, which led to increases in congenital syphilis infections.
According to a 2018 update on the global epidemiology of syphilis, each year an estimated 6 million new cases are diagnosed in people aged 15-49 years, with more than 300,000 fetal and neonatal deaths attributed to the disease. An additional 215,000 infants are at increased risk of early death because of prenatal infection.
For syphilis is indeed a nasty disease. But a remarkable one as well. It presents an almost textbook example of disease evolution and adaptation writ large. It is also a disease with equally remarkable properties – acute, systemic, latent, eruptive, and congenital in its various manifestations. As Sir William Osler, one of the brightest lights of medical education of his time, said in 1897: “I often tell my students that it [syphilis] is the only disease which they require to know thoroughly. Know syphilis in all its manifestations and relations, and all other things clinical will be added unto you.”
Syphilis is caused by the spirochete Treponema pallidum subspecies pallidum and is generally acquired by sexual contact. Congenital syphilis infection occurs by transplacental transmission.
In its modern manifestation, the disease evolves through several stages – primary, secondary, and tertiary. Primary, noncongenital infection is characterized by a lesion. This chancre, as it is called, occurs at the original site of infection, typically between 10 days and 3 months after exposure. The chancre usually appears on the genitals, but given the variety of sexual behaviors, chancres can also occur on the rectum, tongue, pharynx, breast, and so on. The myth of only choosing “a clean partner,” one without visible lesions, is misleading because vaginal and rectal lesions may not be easy to spot yet still remain profoundly infectious.
The secondary stage of an untreated infection occurs 2-3 months after the onset of chancre, and results in multisystem involvement as the spirochetes spread through the bloodstream. Symptoms include skin rash (involving the palms and the soles of the feet) and potentially a variety of other dermatologic manifestations. Fever and swollen lymph nodes may also be present before the disease moves into a latent stage, in which no clinical symptoms are evident. Following this, tertiary syphilis can occur 10-30 years after the initial infection in about 30% of the untreated population, resulting in neurosyphilis, cardiovascular syphilis, or late benign syphilis. Disease progression in tertiary syphilis can lead to dementia, disfigurement, and death.
Sounds bad, doesn’t it? But what we’ve just recounted is the relatively benign disease that modern syphilis has become. Syphilis began as a sweeping, lethal epidemic in the late 15th century spreading dread across the world from the Americas to Europe and then to Asia at a speed equal to the fastest sailing ships of the era.
Syphilis first appeared in Naples in its epidemic form in 1495. Recent anthropological and historical consensus has suggested that syphilis, as we know it today, like tobacco, potatoes, and maize is a product of the Americas that was brought to the Old World by the intrepid exploits of one Christopher Columbus in 1493. Just as the Spanish inadvertently brought smallpox to devastate the population of the New World, Christopher Columbus appears to have brought epidemic syphilis to the Old World in an ironic twist of fate.
Ruy Diaz de Isla, one of two Spanish physicians present when Christopher Columbus returned from his first voyage to America, wrote in a manuscript that Pinzon de Palos, the pilot of Columbus, and also other members of the crew already suffered from symptoms of what was likely syphilis on their return from the New World
Although there has been some controversy regarding the origin of the syphilis epidemic, a recent molecular study using a large collection of pathogenic Treponema strains indicated that venereal syphilis arose relatively recently in human history, and that the closest related syphilis-causing strains were found in South America, providing support for the Columbian theory of syphilis’s origin.
Syphilis flamed across Europe like wildfire, lit by a series of small wars that started shortly after Columbus’s return. Soldiers throughout history have indulged themselves in activities well primed for the spread of venereal disease, and the doughty warriors of the late 15th century were no exception. And throughout the next 500-plus years, syphilis and war rode across the world in tandem, like the white and red horsemen of the Apocalypse.
In its initial launch, syphilis had the help of Charles VIII, the King of France, who had invaded Italy in early 1495 with an army of more than 30,000 mercenaries recruited from across Europe. His forces conquered Naples, which was primarily defended by Spanish mercenaries.
When Charles VIII broke up his army, “mercenaries, infected with a mysterious, serious disease, returned to their native lands or moved elsewhere to wage war, spreading the disease across Europe.” The “Great Pox” initially struck Italy, France, Germany, and Switzerland in 1495; then Holland and Greece in the following year, reaching England and Scotland by 1497; and then Hungary, Poland, and the Scandinavian countries by 1500.
As this period was the Age of Exploration, French, Dutch, and English sailors soon carried syphilis across the rest of an unsuspecting world, with the disease reaching India in 1498 before moving also to Africa and then throughout the rest of Asia in the early 16th century.
And yet, one of the most remarkable parts of the story is the rapid transformation of syphilis from a deadly virulent epidemic to a (comparatively) benign endemic status. Which will be the subject of my next posting.

Mark Lesney is the managing editor of MDedge.com/IDPractioner . He has a PhD in Plant Virology and a PhD in the History of Science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor at the Georgetown University School of Medicine, Department of Biochemistry and Molecular & Cellular Biology, Washington, DC.
The Great Pox, the French Disease, Cupid’s Disease – syphilis has had many names throughout history.
Why should we care about the history of syphilis? Surely syphilis has reached the status of a nonentity disease – in-and-out of the doctor’s office with a course of antibiotics and farewell to the problem. And on the surface, that is certainly true. For now. In the developed world. For those with access to reasonable health care.
But that is all the shiny surface of modern medical triumph. Despite successes in prevention throughout the late 20th and early 21st century, syphilis is making comeback. A growing reservoir of syphilis, often untreated, lies hidden by the invisibility of poorer nations and increasingly in the lower economic strata of the developed world. And the danger is increased by the rise of antibiotic-resistant strains of the disease.
Over the last decade, the European Union and several other high-income countries observed an increasing syphilis trend, according to a recent report by the European Centre for Disease Prevention and Control. And in the United States, the Centers for Disease Control and Prevention has expressed concern over “the rising tide of syphilis” and a “devastating surge in congenital syphilis.” Many reasons have been suggested for this resurgence of syphilis, including the prevalence of unprotected sex and the overall increase in multiple sexual partners in the sexually active population. This trend has been ascribed to a reduced fear of acquiring HIV from condomless sex because of the rise of antiretroviral therapies, which make HIV infection no longer a death sentence for those who have access to and can afford the drugs.
Men who have sex with men are the most affected population cited, which may in part be related to the trend in unprotected sex that has accompanied the decreasing fear of HIV. But in some countries, syphilis rates among heterosexual populations are on the increase as well. Even more troubling were the increases in syphilis diagnosed among pregnant women that were reported in high-income settings outside of the European Union, which led to increases in congenital syphilis infections.
According to a 2018 update on the global epidemiology of syphilis, each year an estimated 6 million new cases are diagnosed in people aged 15-49 years, with more than 300,000 fetal and neonatal deaths attributed to the disease. An additional 215,000 infants are at increased risk of early death because of prenatal infection.
For syphilis is indeed a nasty disease. But a remarkable one as well. It presents an almost textbook example of disease evolution and adaptation writ large. It is also a disease with equally remarkable properties – acute, systemic, latent, eruptive, and congenital in its various manifestations. As Sir William Osler, one of the brightest lights of medical education of his time, said in 1897: “I often tell my students that it [syphilis] is the only disease which they require to know thoroughly. Know syphilis in all its manifestations and relations, and all other things clinical will be added unto you.”
Syphilis is caused by the spirochete Treponema pallidum subspecies pallidum and is generally acquired by sexual contact. Congenital syphilis infection occurs by transplacental transmission.
In its modern manifestation, the disease evolves through several stages – primary, secondary, and tertiary. Primary, noncongenital infection is characterized by a lesion. This chancre, as it is called, occurs at the original site of infection, typically between 10 days and 3 months after exposure. The chancre usually appears on the genitals, but given the variety of sexual behaviors, chancres can also occur on the rectum, tongue, pharynx, breast, and so on. The myth of only choosing “a clean partner,” one without visible lesions, is misleading because vaginal and rectal lesions may not be easy to spot yet still remain profoundly infectious.
The secondary stage of an untreated infection occurs 2-3 months after the onset of chancre, and results in multisystem involvement as the spirochetes spread through the bloodstream. Symptoms include skin rash (involving the palms and the soles of the feet) and potentially a variety of other dermatologic manifestations. Fever and swollen lymph nodes may also be present before the disease moves into a latent stage, in which no clinical symptoms are evident. Following this, tertiary syphilis can occur 10-30 years after the initial infection in about 30% of the untreated population, resulting in neurosyphilis, cardiovascular syphilis, or late benign syphilis. Disease progression in tertiary syphilis can lead to dementia, disfigurement, and death.
Sounds bad, doesn’t it? But what we’ve just recounted is the relatively benign disease that modern syphilis has become. Syphilis began as a sweeping, lethal epidemic in the late 15th century spreading dread across the world from the Americas to Europe and then to Asia at a speed equal to the fastest sailing ships of the era.
Syphilis first appeared in Naples in its epidemic form in 1495. Recent anthropological and historical consensus has suggested that syphilis, as we know it today, like tobacco, potatoes, and maize is a product of the Americas that was brought to the Old World by the intrepid exploits of one Christopher Columbus in 1493. Just as the Spanish inadvertently brought smallpox to devastate the population of the New World, Christopher Columbus appears to have brought epidemic syphilis to the Old World in an ironic twist of fate.
Ruy Diaz de Isla, one of two Spanish physicians present when Christopher Columbus returned from his first voyage to America, wrote in a manuscript that Pinzon de Palos, the pilot of Columbus, and also other members of the crew already suffered from symptoms of what was likely syphilis on their return from the New World
Although there has been some controversy regarding the origin of the syphilis epidemic, a recent molecular study using a large collection of pathogenic Treponema strains indicated that venereal syphilis arose relatively recently in human history, and that the closest related syphilis-causing strains were found in South America, providing support for the Columbian theory of syphilis’s origin.
Syphilis flamed across Europe like wildfire, lit by a series of small wars that started shortly after Columbus’s return. Soldiers throughout history have indulged themselves in activities well primed for the spread of venereal disease, and the doughty warriors of the late 15th century were no exception. And throughout the next 500-plus years, syphilis and war rode across the world in tandem, like the white and red horsemen of the Apocalypse.
In its initial launch, syphilis had the help of Charles VIII, the King of France, who had invaded Italy in early 1495 with an army of more than 30,000 mercenaries recruited from across Europe. His forces conquered Naples, which was primarily defended by Spanish mercenaries.
When Charles VIII broke up his army, “mercenaries, infected with a mysterious, serious disease, returned to their native lands or moved elsewhere to wage war, spreading the disease across Europe.” The “Great Pox” initially struck Italy, France, Germany, and Switzerland in 1495; then Holland and Greece in the following year, reaching England and Scotland by 1497; and then Hungary, Poland, and the Scandinavian countries by 1500.
As this period was the Age of Exploration, French, Dutch, and English sailors soon carried syphilis across the rest of an unsuspecting world, with the disease reaching India in 1498 before moving also to Africa and then throughout the rest of Asia in the early 16th century.
And yet, one of the most remarkable parts of the story is the rapid transformation of syphilis from a deadly virulent epidemic to a (comparatively) benign endemic status. Which will be the subject of my next posting.

Mark Lesney is the managing editor of MDedge.com/IDPractioner . He has a PhD in Plant Virology and a PhD in the History of Science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor at the Georgetown University School of Medicine, Department of Biochemistry and Molecular & Cellular Biology, Washington, DC.
Revolutionizing Atopic Dermatitis
Impressive progress has been made in recent years in the management and treatment of atopic dermatitis (AD) and its comorbidities; however, there is a major need for state-of-the-art, evidence-based, multidisciplinary education for AD management. To address this need, the first Revolutionizing Atopic Dermatitis (RAD) Conference was held in April 2019 in Chicago, Illinois, featuring cutting-edge research presented by globally recognized experts in dermatology, allergy and immunology, sleep medicine, ophthalmology, and nursing care. The following is a recap of the latest topics in AD research presented at the conference.
Diagnosis and Assessment of AD: Jonathan I. Silverberg, MD, PhD, MPH
Although diagnosis of AD typically is straightforward in children, it can be challenging in adults, even for expert clinicians. These challenges stem from the different lesional distribution and morphology of AD in adults vs children.1,2 Additionally, the conditions included in the differential diagnosis of AD (eg, allergic contact dermatitis, cutaneous T-cell lymphoma, psoriasis) are far more common in adults than in children. Formal diagnostic criteria can be useful to improve the diagnosis of AD in clinical practice.3 It is important to note that flexural lesions and early disease onset are diagnostic criteria in AD; nevertheless, neither are essential nor sufficient on their own to make the diagnosis.
Patch Testing: Jacob P. Thyssen, MD, PhD, DmSci, and Noreen Heer Nicol, PhD, RN, FNP, NEA-BC
Patch testing can be used in AD patients to rule out contact dermatitis as an alternative or comorbid diagnosis.4-6 Because contact dermatitis can mimic AD, patch testing is recommended for all patients with adolescent and adult-onset AD.5 Additionally, refractory cases of AD across all ages, especially prior to initiation of systemic therapy, warrant patch testing. The unique challenges of patch testing in AD patients were reviewed.
Patient Panel
Atopic dermatitis can be a considerable disease burden on both patients and society in general. At the 2019 RAD Conference, a panel of patients bravely shared their AD journeys. Their eye-opening stories highlighted opportunities for improving real-world assessment and management of AD. Some key takeaways included the importance of adequately assessing the symptom burden of AD and not merely relying on visual inspection of the skin. The need for long-term treatment approaches beyond quick fixes with steroids also was discussed.
Pathogenesis of AD: Mark Boguniewicz, MD
There have been many advances in our understanding of the complex pathogenesis of AD,7-11 which is characterized by an altered skin barrier and immune dysregulation. Filaggrin deficiency in the skin has structural and biophysical consequences. A subset of patients with AD has filaggrin loss-of-function genetic polymorphisms inherited in an autosomal-semidominant pattern; however, many other genetic polymorphisms have been identified that affect different components of the skin architecture and immune system. Many cytokine pathways have been found to be upregulated in AD lesions, including IL-13, IL-4, IL-31, and IL-5 in acute and chronic lesions, and IFN-γ and other helper T cell (TH1) cytokines in chronic lesions. IL-4 and IL-13 (TH2 cytokines) have been shown to decrease epidermal expression of filaggrin and lead to lipid abnormalities in the skin of patients with AD. Even normal-appearing, nonlesional skin has substantial immune activation and barrier abnormalities in patients with moderate to severe AD. Activation of different immune pathways may contribute to the heterogeneous clinical presentation of AD. There also is an increasingly recognized role of superantigen-producing Staphylococcus aureus and decreased microbial diversity in AD.
Therapies for AD
The advances in our understanding of AD pathophysiology have led to the development of 2 recently approved therapeutic agents.7-10 Crisaborole ointment 2% is a topical phosphodiesterase 4 inhibitor that was approved by the US Food and Drug Administration in 2016 for treatment of mild to moderate AD. Treatment with crisaborole ointment 2% demonstrated improvement in lesion severity, itch, and quality of life in children and adults with AD. Dupilumab, an injectable biologic therapy that inhibits IL-4 and IL-13 signaling, was approved by the US Food and Drug Administration in 2017 for adults and in 2019 for adolescents aged 12 to 17 years with moderate to severe AD. The expert panel of speakers at the 2019 RAD Conference discussed many practical clinical pearls regarding patient education, optimization of both short- and long-term efficacy, and prevention and management of treatment-related adverse events. The discussion included evidence-based guidelines for bathing practices and topical therapy in AD, as well as practical pearls for patient and provider education in AD, reviewed by Dr. Nicol. Evidence-based guidelines for use of phototherapy and systemic and biologic therapy for AD also were highlighted by Dr. Silverberg.
After decades of limited therapeutic options, there is a large therapeutic pipeline of topical, oral, and biologic agents in development for the treatment AD.7-9 Dr. Boguniewicz reviewed the state-of-the-art treatments that are the furthest advanced in development. Many of these agents may be approved within the next couple of years and look promising in terms of their potential to improve the care of patients with AD.
Comorbidities of AD
The impact of AD is not just skin deep. Atopic dermatitis is associated with myriad comorbid health conditions.12-16 Dr. Boguniewicz reviewed the relationship between AD and atopic comorbidities, including asthma, hay fever, and food allergies, which are common across all AD patients. In addition, a subset of children with AD demonstrated the atopic march, in which AD first appears early in life followed by the development of other atopic comorbidities in later childhood or adulthood. In particular, children with filaggrin null mutations were found to be at increased risk of early-onset, severe, persistent AD with asthma and allergic sensitization.17 More recently, eosinophilic esophagitis was demonstrated to be a late-onset comorbidity of the atopic march.18 The allergy guidelines for which patients are appropriate candidates for food and/or aeroallergen testing were discussed,19 and it was emphasized that patients with AD should not routinely receive this testing.
Atopic dermatitis is associated with many other comorbidities, including sleep disturbances. Phyllis C. Zee, MD, PhD, provided a brilliant review of circadian regulation of physiology and the immune system. Sleep is one of the most important determinants of patients’ health and well-being. Atopic dermatitis is associated with disturbances of sleep and circadian rhythms. Sleep disturbances are gaining recognition as an important end point to assess for improvement in clinical practice and trials.
Patients with AD have long been recognized to have increased ophthalmic comorbidities, including allergic conjunctivitis, atopic keratoconjunctivitis, and cataracts. More recently, conjunctivitis has emerged as an important adverse event with dupilumab treatment.20 Jeanine Baqai, MD, reviewed the various ophthalmic comorbidities and shared numerous clinical signs of ophthalmic comorbidities that dermatologists can assess with the naked eye (no slit-lamp examination needed). Pearls to manage dupilumab-related conjunctivitis shared by Dr. Baqai and the speaker panel included elimination of eye rubbing, cold compresses, avoidance of exacerbating factors, artificial tears, and timely referral to an ophthalmologist. Medications discussed were mast cell stabilizers, antihistamines, and corticosteroids and calcineurin inhibitors.
Final Thoughts
There has been an explosion of new research that has increased our understanding of all aspects of AD, and the standard of care is truly being revolutionized. Clinicians should stay tuned to a wealth of new evidence-based recommendations coming down the pike.
- Vakharia PP, Silverberg JI. Adult-onset atopic dermatitis: characteristics and management [published online May 28, 2019]. Am J Clin Dermatol. doi:10.1007/s40257-019-00453-7.
- Silverberg JI. Adult-onset atopic dermatitis. J Allergy Clin Immunol Pract. 2019;7:28-33.
- Hanifin J, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol (Stockh). 1980;92(suppl):44-47.
- Hamann CR, Hamann D, Egeberg A, et al. Association between atopic dermatitis and contact sensitization: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:70-78.
- Owen JL, Vakharia PP, Silverberg JI. The role and diagnosis of allergic contact dermatitis in patients with atopic dermatitis. Am J Clin Dermatol. 2018;19:293-302.
- Rastogi S, Patel KR, Singam V, et al. Allergic contact dermatitis to personal care products and topical medications in adults with atopic dermatitis [published online July 25, 2018]. J Am Acad Dermatol. 2018;79:1028-1033.e6.
- Vakharia PP, Silverberg JI. New and emerging therapies for paediatric atopic dermatitis. Lancet Child Adolesc Health. 2019;3:343-353.
- Vakharia PP, Silverberg JI. New therapies for atopic dermatitis: additional treatment classes [published online December 14, 2017]. J Am Acad Dermatol. 2018;78(3 suppl 1):S76-S83.
- Silverberg JI. Atopic dermatitis treatment: current state of the art and emerging therapies. Allergy Asthma Proc. 2017;38:243-249.
- Vakharia PP, Silverberg JI. Monoclonal antibodies for atopic dermatitis: progress and potential. BioDrugs. 2017;31:409-422.
- Silverberg NB, Silverberg JI. Inside out or outside in: does atopic dermatitis disrupt barrier function or does disruption of barrier function trigger atopic dermatitis? Cutis. 2015;96:359-361.
- Silverberg JI. Comorbidities and the impact of atopic dermatitis. Ann Allergy Asthma Immunol. 2019;123:144-151.
- Brunner PM, Silverberg JI, Guttman-Yassky E, et al. Increasing comorbidities suggest that atopic dermatitis is a systemic disorder. J Invest Dermatol. 2017;137:18-25.
- Silverberg J, Garg N, Silverberg NB. New developments in comorbidities of atopic dermatitis. Cutis. 2014;93:222-224.
- Silverberg JI. Selected comorbidities of atopic dermatitis: atopy, neuropsychiatric, and musculoskeletal disorders. Clin Dermatol. 2017;35:360-366.
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Association of atopic dermatitis with allergic, autoimmune, and cardiovascular comorbidities in US adults. Ann Allergy Asthma Immunol. 2018;121:604-612.e603.
- Henderson J, Northstone K, Lee SP, et al. The burden of disease associated with filaggrin mutations: a population-based, longitudinal birth cohort study. J Allergy Clin Immunol. 2008;121:872-877.e879.
- Hill DA, Grundmeier RW, Ramos M, et al. Eosinophilic esophagitis is a late manifestation of the allergic march. J Allergy Clin Immunol. 2018;6:1528-1533.
- Boyce JA, Assa’ad A, Burks AW, et al; NIAID-Sponsored Expert Panel. Guidelines for th
e diagnosis and management of food allergy in the United States. J Allergy Clin Immunol. 2010;126:1105-1118. - Akinlade B, Guttman-Yassky E, de Bruin-Weller M, et al. Conjunctivitis in dupilumab clinical trials [published online March 9, 2019]. Br J Dermatol. doi:10.1111/bjd.17869.
Impressive progress has been made in recent years in the management and treatment of atopic dermatitis (AD) and its comorbidities; however, there is a major need for state-of-the-art, evidence-based, multidisciplinary education for AD management. To address this need, the first Revolutionizing Atopic Dermatitis (RAD) Conference was held in April 2019 in Chicago, Illinois, featuring cutting-edge research presented by globally recognized experts in dermatology, allergy and immunology, sleep medicine, ophthalmology, and nursing care. The following is a recap of the latest topics in AD research presented at the conference.
Diagnosis and Assessment of AD: Jonathan I. Silverberg, MD, PhD, MPH
Although diagnosis of AD typically is straightforward in children, it can be challenging in adults, even for expert clinicians. These challenges stem from the different lesional distribution and morphology of AD in adults vs children.1,2 Additionally, the conditions included in the differential diagnosis of AD (eg, allergic contact dermatitis, cutaneous T-cell lymphoma, psoriasis) are far more common in adults than in children. Formal diagnostic criteria can be useful to improve the diagnosis of AD in clinical practice.3 It is important to note that flexural lesions and early disease onset are diagnostic criteria in AD; nevertheless, neither are essential nor sufficient on their own to make the diagnosis.
Patch Testing: Jacob P. Thyssen, MD, PhD, DmSci, and Noreen Heer Nicol, PhD, RN, FNP, NEA-BC
Patch testing can be used in AD patients to rule out contact dermatitis as an alternative or comorbid diagnosis.4-6 Because contact dermatitis can mimic AD, patch testing is recommended for all patients with adolescent and adult-onset AD.5 Additionally, refractory cases of AD across all ages, especially prior to initiation of systemic therapy, warrant patch testing. The unique challenges of patch testing in AD patients were reviewed.
Patient Panel
Atopic dermatitis can be a considerable disease burden on both patients and society in general. At the 2019 RAD Conference, a panel of patients bravely shared their AD journeys. Their eye-opening stories highlighted opportunities for improving real-world assessment and management of AD. Some key takeaways included the importance of adequately assessing the symptom burden of AD and not merely relying on visual inspection of the skin. The need for long-term treatment approaches beyond quick fixes with steroids also was discussed.
Pathogenesis of AD: Mark Boguniewicz, MD
There have been many advances in our understanding of the complex pathogenesis of AD,7-11 which is characterized by an altered skin barrier and immune dysregulation. Filaggrin deficiency in the skin has structural and biophysical consequences. A subset of patients with AD has filaggrin loss-of-function genetic polymorphisms inherited in an autosomal-semidominant pattern; however, many other genetic polymorphisms have been identified that affect different components of the skin architecture and immune system. Many cytokine pathways have been found to be upregulated in AD lesions, including IL-13, IL-4, IL-31, and IL-5 in acute and chronic lesions, and IFN-γ and other helper T cell (TH1) cytokines in chronic lesions. IL-4 and IL-13 (TH2 cytokines) have been shown to decrease epidermal expression of filaggrin and lead to lipid abnormalities in the skin of patients with AD. Even normal-appearing, nonlesional skin has substantial immune activation and barrier abnormalities in patients with moderate to severe AD. Activation of different immune pathways may contribute to the heterogeneous clinical presentation of AD. There also is an increasingly recognized role of superantigen-producing Staphylococcus aureus and decreased microbial diversity in AD.
Therapies for AD
The advances in our understanding of AD pathophysiology have led to the development of 2 recently approved therapeutic agents.7-10 Crisaborole ointment 2% is a topical phosphodiesterase 4 inhibitor that was approved by the US Food and Drug Administration in 2016 for treatment of mild to moderate AD. Treatment with crisaborole ointment 2% demonstrated improvement in lesion severity, itch, and quality of life in children and adults with AD. Dupilumab, an injectable biologic therapy that inhibits IL-4 and IL-13 signaling, was approved by the US Food and Drug Administration in 2017 for adults and in 2019 for adolescents aged 12 to 17 years with moderate to severe AD. The expert panel of speakers at the 2019 RAD Conference discussed many practical clinical pearls regarding patient education, optimization of both short- and long-term efficacy, and prevention and management of treatment-related adverse events. The discussion included evidence-based guidelines for bathing practices and topical therapy in AD, as well as practical pearls for patient and provider education in AD, reviewed by Dr. Nicol. Evidence-based guidelines for use of phototherapy and systemic and biologic therapy for AD also were highlighted by Dr. Silverberg.
After decades of limited therapeutic options, there is a large therapeutic pipeline of topical, oral, and biologic agents in development for the treatment AD.7-9 Dr. Boguniewicz reviewed the state-of-the-art treatments that are the furthest advanced in development. Many of these agents may be approved within the next couple of years and look promising in terms of their potential to improve the care of patients with AD.
Comorbidities of AD
The impact of AD is not just skin deep. Atopic dermatitis is associated with myriad comorbid health conditions.12-16 Dr. Boguniewicz reviewed the relationship between AD and atopic comorbidities, including asthma, hay fever, and food allergies, which are common across all AD patients. In addition, a subset of children with AD demonstrated the atopic march, in which AD first appears early in life followed by the development of other atopic comorbidities in later childhood or adulthood. In particular, children with filaggrin null mutations were found to be at increased risk of early-onset, severe, persistent AD with asthma and allergic sensitization.17 More recently, eosinophilic esophagitis was demonstrated to be a late-onset comorbidity of the atopic march.18 The allergy guidelines for which patients are appropriate candidates for food and/or aeroallergen testing were discussed,19 and it was emphasized that patients with AD should not routinely receive this testing.
Atopic dermatitis is associated with many other comorbidities, including sleep disturbances. Phyllis C. Zee, MD, PhD, provided a brilliant review of circadian regulation of physiology and the immune system. Sleep is one of the most important determinants of patients’ health and well-being. Atopic dermatitis is associated with disturbances of sleep and circadian rhythms. Sleep disturbances are gaining recognition as an important end point to assess for improvement in clinical practice and trials.
Patients with AD have long been recognized to have increased ophthalmic comorbidities, including allergic conjunctivitis, atopic keratoconjunctivitis, and cataracts. More recently, conjunctivitis has emerged as an important adverse event with dupilumab treatment.20 Jeanine Baqai, MD, reviewed the various ophthalmic comorbidities and shared numerous clinical signs of ophthalmic comorbidities that dermatologists can assess with the naked eye (no slit-lamp examination needed). Pearls to manage dupilumab-related conjunctivitis shared by Dr. Baqai and the speaker panel included elimination of eye rubbing, cold compresses, avoidance of exacerbating factors, artificial tears, and timely referral to an ophthalmologist. Medications discussed were mast cell stabilizers, antihistamines, and corticosteroids and calcineurin inhibitors.
Final Thoughts
There has been an explosion of new research that has increased our understanding of all aspects of AD, and the standard of care is truly being revolutionized. Clinicians should stay tuned to a wealth of new evidence-based recommendations coming down the pike.
Impressive progress has been made in recent years in the management and treatment of atopic dermatitis (AD) and its comorbidities; however, there is a major need for state-of-the-art, evidence-based, multidisciplinary education for AD management. To address this need, the first Revolutionizing Atopic Dermatitis (RAD) Conference was held in April 2019 in Chicago, Illinois, featuring cutting-edge research presented by globally recognized experts in dermatology, allergy and immunology, sleep medicine, ophthalmology, and nursing care. The following is a recap of the latest topics in AD research presented at the conference.
Diagnosis and Assessment of AD: Jonathan I. Silverberg, MD, PhD, MPH
Although diagnosis of AD typically is straightforward in children, it can be challenging in adults, even for expert clinicians. These challenges stem from the different lesional distribution and morphology of AD in adults vs children.1,2 Additionally, the conditions included in the differential diagnosis of AD (eg, allergic contact dermatitis, cutaneous T-cell lymphoma, psoriasis) are far more common in adults than in children. Formal diagnostic criteria can be useful to improve the diagnosis of AD in clinical practice.3 It is important to note that flexural lesions and early disease onset are diagnostic criteria in AD; nevertheless, neither are essential nor sufficient on their own to make the diagnosis.
Patch Testing: Jacob P. Thyssen, MD, PhD, DmSci, and Noreen Heer Nicol, PhD, RN, FNP, NEA-BC
Patch testing can be used in AD patients to rule out contact dermatitis as an alternative or comorbid diagnosis.4-6 Because contact dermatitis can mimic AD, patch testing is recommended for all patients with adolescent and adult-onset AD.5 Additionally, refractory cases of AD across all ages, especially prior to initiation of systemic therapy, warrant patch testing. The unique challenges of patch testing in AD patients were reviewed.
Patient Panel
Atopic dermatitis can be a considerable disease burden on both patients and society in general. At the 2019 RAD Conference, a panel of patients bravely shared their AD journeys. Their eye-opening stories highlighted opportunities for improving real-world assessment and management of AD. Some key takeaways included the importance of adequately assessing the symptom burden of AD and not merely relying on visual inspection of the skin. The need for long-term treatment approaches beyond quick fixes with steroids also was discussed.
Pathogenesis of AD: Mark Boguniewicz, MD
There have been many advances in our understanding of the complex pathogenesis of AD,7-11 which is characterized by an altered skin barrier and immune dysregulation. Filaggrin deficiency in the skin has structural and biophysical consequences. A subset of patients with AD has filaggrin loss-of-function genetic polymorphisms inherited in an autosomal-semidominant pattern; however, many other genetic polymorphisms have been identified that affect different components of the skin architecture and immune system. Many cytokine pathways have been found to be upregulated in AD lesions, including IL-13, IL-4, IL-31, and IL-5 in acute and chronic lesions, and IFN-γ and other helper T cell (TH1) cytokines in chronic lesions. IL-4 and IL-13 (TH2 cytokines) have been shown to decrease epidermal expression of filaggrin and lead to lipid abnormalities in the skin of patients with AD. Even normal-appearing, nonlesional skin has substantial immune activation and barrier abnormalities in patients with moderate to severe AD. Activation of different immune pathways may contribute to the heterogeneous clinical presentation of AD. There also is an increasingly recognized role of superantigen-producing Staphylococcus aureus and decreased microbial diversity in AD.
Therapies for AD
The advances in our understanding of AD pathophysiology have led to the development of 2 recently approved therapeutic agents.7-10 Crisaborole ointment 2% is a topical phosphodiesterase 4 inhibitor that was approved by the US Food and Drug Administration in 2016 for treatment of mild to moderate AD. Treatment with crisaborole ointment 2% demonstrated improvement in lesion severity, itch, and quality of life in children and adults with AD. Dupilumab, an injectable biologic therapy that inhibits IL-4 and IL-13 signaling, was approved by the US Food and Drug Administration in 2017 for adults and in 2019 for adolescents aged 12 to 17 years with moderate to severe AD. The expert panel of speakers at the 2019 RAD Conference discussed many practical clinical pearls regarding patient education, optimization of both short- and long-term efficacy, and prevention and management of treatment-related adverse events. The discussion included evidence-based guidelines for bathing practices and topical therapy in AD, as well as practical pearls for patient and provider education in AD, reviewed by Dr. Nicol. Evidence-based guidelines for use of phototherapy and systemic and biologic therapy for AD also were highlighted by Dr. Silverberg.
After decades of limited therapeutic options, there is a large therapeutic pipeline of topical, oral, and biologic agents in development for the treatment AD.7-9 Dr. Boguniewicz reviewed the state-of-the-art treatments that are the furthest advanced in development. Many of these agents may be approved within the next couple of years and look promising in terms of their potential to improve the care of patients with AD.
Comorbidities of AD
The impact of AD is not just skin deep. Atopic dermatitis is associated with myriad comorbid health conditions.12-16 Dr. Boguniewicz reviewed the relationship between AD and atopic comorbidities, including asthma, hay fever, and food allergies, which are common across all AD patients. In addition, a subset of children with AD demonstrated the atopic march, in which AD first appears early in life followed by the development of other atopic comorbidities in later childhood or adulthood. In particular, children with filaggrin null mutations were found to be at increased risk of early-onset, severe, persistent AD with asthma and allergic sensitization.17 More recently, eosinophilic esophagitis was demonstrated to be a late-onset comorbidity of the atopic march.18 The allergy guidelines for which patients are appropriate candidates for food and/or aeroallergen testing were discussed,19 and it was emphasized that patients with AD should not routinely receive this testing.
Atopic dermatitis is associated with many other comorbidities, including sleep disturbances. Phyllis C. Zee, MD, PhD, provided a brilliant review of circadian regulation of physiology and the immune system. Sleep is one of the most important determinants of patients’ health and well-being. Atopic dermatitis is associated with disturbances of sleep and circadian rhythms. Sleep disturbances are gaining recognition as an important end point to assess for improvement in clinical practice and trials.
Patients with AD have long been recognized to have increased ophthalmic comorbidities, including allergic conjunctivitis, atopic keratoconjunctivitis, and cataracts. More recently, conjunctivitis has emerged as an important adverse event with dupilumab treatment.20 Jeanine Baqai, MD, reviewed the various ophthalmic comorbidities and shared numerous clinical signs of ophthalmic comorbidities that dermatologists can assess with the naked eye (no slit-lamp examination needed). Pearls to manage dupilumab-related conjunctivitis shared by Dr. Baqai and the speaker panel included elimination of eye rubbing, cold compresses, avoidance of exacerbating factors, artificial tears, and timely referral to an ophthalmologist. Medications discussed were mast cell stabilizers, antihistamines, and corticosteroids and calcineurin inhibitors.
Final Thoughts
There has been an explosion of new research that has increased our understanding of all aspects of AD, and the standard of care is truly being revolutionized. Clinicians should stay tuned to a wealth of new evidence-based recommendations coming down the pike.
- Vakharia PP, Silverberg JI. Adult-onset atopic dermatitis: characteristics and management [published online May 28, 2019]. Am J Clin Dermatol. doi:10.1007/s40257-019-00453-7.
- Silverberg JI. Adult-onset atopic dermatitis. J Allergy Clin Immunol Pract. 2019;7:28-33.
- Hanifin J, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol (Stockh). 1980;92(suppl):44-47.
- Hamann CR, Hamann D, Egeberg A, et al. Association between atopic dermatitis and contact sensitization: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:70-78.
- Owen JL, Vakharia PP, Silverberg JI. The role and diagnosis of allergic contact dermatitis in patients with atopic dermatitis. Am J Clin Dermatol. 2018;19:293-302.
- Rastogi S, Patel KR, Singam V, et al. Allergic contact dermatitis to personal care products and topical medications in adults with atopic dermatitis [published online July 25, 2018]. J Am Acad Dermatol. 2018;79:1028-1033.e6.
- Vakharia PP, Silverberg JI. New and emerging therapies for paediatric atopic dermatitis. Lancet Child Adolesc Health. 2019;3:343-353.
- Vakharia PP, Silverberg JI. New therapies for atopic dermatitis: additional treatment classes [published online December 14, 2017]. J Am Acad Dermatol. 2018;78(3 suppl 1):S76-S83.
- Silverberg JI. Atopic dermatitis treatment: current state of the art and emerging therapies. Allergy Asthma Proc. 2017;38:243-249.
- Vakharia PP, Silverberg JI. Monoclonal antibodies for atopic dermatitis: progress and potential. BioDrugs. 2017;31:409-422.
- Silverberg NB, Silverberg JI. Inside out or outside in: does atopic dermatitis disrupt barrier function or does disruption of barrier function trigger atopic dermatitis? Cutis. 2015;96:359-361.
- Silverberg JI. Comorbidities and the impact of atopic dermatitis. Ann Allergy Asthma Immunol. 2019;123:144-151.
- Brunner PM, Silverberg JI, Guttman-Yassky E, et al. Increasing comorbidities suggest that atopic dermatitis is a systemic disorder. J Invest Dermatol. 2017;137:18-25.
- Silverberg J, Garg N, Silverberg NB. New developments in comorbidities of atopic dermatitis. Cutis. 2014;93:222-224.
- Silverberg JI. Selected comorbidities of atopic dermatitis: atopy, neuropsychiatric, and musculoskeletal disorders. Clin Dermatol. 2017;35:360-366.
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Association of atopic dermatitis with allergic, autoimmune, and cardiovascular comorbidities in US adults. Ann Allergy Asthma Immunol. 2018;121:604-612.e603.
- Henderson J, Northstone K, Lee SP, et al. The burden of disease associated with filaggrin mutations: a population-based, longitudinal birth cohort study. J Allergy Clin Immunol. 2008;121:872-877.e879.
- Hill DA, Grundmeier RW, Ramos M, et al. Eosinophilic esophagitis is a late manifestation of the allergic march. J Allergy Clin Immunol. 2018;6:1528-1533.
- Boyce JA, Assa’ad A, Burks AW, et al; NIAID-Sponsored Expert Panel. Guidelines for th
e diagnosis and management of food allergy in the United States. J Allergy Clin Immunol. 2010;126:1105-1118. - Akinlade B, Guttman-Yassky E, de Bruin-Weller M, et al. Conjunctivitis in dupilumab clinical trials [published online March 9, 2019]. Br J Dermatol. doi:10.1111/bjd.17869.
- Vakharia PP, Silverberg JI. Adult-onset atopic dermatitis: characteristics and management [published online May 28, 2019]. Am J Clin Dermatol. doi:10.1007/s40257-019-00453-7.
- Silverberg JI. Adult-onset atopic dermatitis. J Allergy Clin Immunol Pract. 2019;7:28-33.
- Hanifin J, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol (Stockh). 1980;92(suppl):44-47.
- Hamann CR, Hamann D, Egeberg A, et al. Association between atopic dermatitis and contact sensitization: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:70-78.
- Owen JL, Vakharia PP, Silverberg JI. The role and diagnosis of allergic contact dermatitis in patients with atopic dermatitis. Am J Clin Dermatol. 2018;19:293-302.
- Rastogi S, Patel KR, Singam V, et al. Allergic contact dermatitis to personal care products and topical medications in adults with atopic dermatitis [published online July 25, 2018]. J Am Acad Dermatol. 2018;79:1028-1033.e6.
- Vakharia PP, Silverberg JI. New and emerging therapies for paediatric atopic dermatitis. Lancet Child Adolesc Health. 2019;3:343-353.
- Vakharia PP, Silverberg JI. New therapies for atopic dermatitis: additional treatment classes [published online December 14, 2017]. J Am Acad Dermatol. 2018;78(3 suppl 1):S76-S83.
- Silverberg JI. Atopic dermatitis treatment: current state of the art and emerging therapies. Allergy Asthma Proc. 2017;38:243-249.
- Vakharia PP, Silverberg JI. Monoclonal antibodies for atopic dermatitis: progress and potential. BioDrugs. 2017;31:409-422.
- Silverberg NB, Silverberg JI. Inside out or outside in: does atopic dermatitis disrupt barrier function or does disruption of barrier function trigger atopic dermatitis? Cutis. 2015;96:359-361.
- Silverberg JI. Comorbidities and the impact of atopic dermatitis. Ann Allergy Asthma Immunol. 2019;123:144-151.
- Brunner PM, Silverberg JI, Guttman-Yassky E, et al. Increasing comorbidities suggest that atopic dermatitis is a systemic disorder. J Invest Dermatol. 2017;137:18-25.
- Silverberg J, Garg N, Silverberg NB. New developments in comorbidities of atopic dermatitis. Cutis. 2014;93:222-224.
- Silverberg JI. Selected comorbidities of atopic dermatitis: atopy, neuropsychiatric, and musculoskeletal disorders. Clin Dermatol. 2017;35:360-366.
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Association of atopic dermatitis with allergic, autoimmune, and cardiovascular comorbidities in US adults. Ann Allergy Asthma Immunol. 2018;121:604-612.e603.
- Henderson J, Northstone K, Lee SP, et al. The burden of disease associated with filaggrin mutations: a population-based, longitudinal birth cohort study. J Allergy Clin Immunol. 2008;121:872-877.e879.
- Hill DA, Grundmeier RW, Ramos M, et al. Eosinophilic esophagitis is a late manifestation of the allergic march. J Allergy Clin Immunol. 2018;6:1528-1533.
- Boyce JA, Assa’ad A, Burks AW, et al; NIAID-Sponsored Expert Panel. Guidelines for th
e diagnosis and management of food allergy in the United States. J Allergy Clin Immunol. 2010;126:1105-1118. - Akinlade B, Guttman-Yassky E, de Bruin-Weller M, et al. Conjunctivitis in dupilumab clinical trials [published online March 9, 2019]. Br J Dermatol. doi:10.1111/bjd.17869.
Reframing Clinician Distress: Moral Injury Not Burnout
*This version has been corrected. In the original version the first sentence incorrectly referred to moral injury instead of burnout.
For more than a decade, the term burnout has been used to describe clinician distress.1,2 Although some clinicians in federal health care systems may be protected from some of the drivers of burnout, other federal practitioners suffer from rule-driven health care practices and distant, top-down administration. The demand for health care is expanding, driven by the aging of the US population.3 Massive information technology investments, which promised efficiency for health care providers,4 have instead delivered a triple blow: They have diverted capital resources that might have been used to hire additional caregivers,5 diverted the time and attention of those already engaged in patient care,6 and done little to improve patient outcomes.7 Reimbursements are falling, and the only way for health systems to maintain their revenue is to increase the number of patients each clinician sees per day.8 As the resources of time and attention shrink, and as spending continues with no improvement in patient outcomes, clinician distress is on the rise.9 It will be important to understand exactly what the drivers of the problem are for federal clinicians so that solutions can be appropriately targeted. The first step in addressing the epidemic of physician distress is using the most accurate terminology to describe it.
Freudenberger defined burnout in 1975 as a constellation of symptoms—malaise, fatigue, frustration, cynicism, and inefficacy—that arise from “making excessive demands on energy, strength, or resources” in the workplace.10 The term was borrowed from other fields and applied to health care in the hopes of readily transferring the solutions that had worked in other industries to address a growing crisis among physicians. Unfortunately, the crisis in health care has proven resistant to solutions that have worked elsewhere, and many clinicians have resisted being characterized as burned out, citing a subtle, elusive disconnect between what they have experienced and what burnout encapsulates.
In July 2018, the conversation about clinician distress shifted with an article we wrote in STAT that described the moral injury of health care.11 The concept of moral injury was first described in service members who returned from the Vietnam War with symptoms that loosely fit a diagnosis of posttraumatic stress disorder (PTSD), but which did not respond to standard PTSD treatment and contained symptoms outside the PTSD constellation.12 On closer assessment, what these service members were experiencing had a different driver. Whereas those with PTSD experienced a real and imminent threat to their mortality and had come back deeply concerned for their individual, physical safety, those with this different presentation experienced repeated insults to their morality and had returned questioning whether they were still, at their core, moral beings. They had been forced, in some way, to act contrary to what their beliefs dictated was right by killing civilians on orders from their superiors, for example. This was a different category of psychological injury that required different treatment.
Moral injury occurs when we perpetrate, bear witness to, or fail to prevent an act that transgresses our deeply held moral beliefs. In the health care context, that deeply held moral belief is the oath each of us took when embarking on our paths as health care providers: Put the needs of patients first. That oath is the lynchpin of our working lives and our guiding principle when searching for the right course of action. But as clinicians, we are increasingly forced to consider the demands of other stakeholders—the electronic medical record (EMR), the insurers, the hospital, the health care system, even our own financial security—before the needs of our patients. Every time we are forced to make a decision that contravenes our patients’ best interests, we feel a sting of moral injustice. Over time, these repetitive insults amass into moral injury.
The difference between burnout and moral injury is important because using different terminology reframes the problem and the solutions. Burnout suggests that the problem resides within the individual, who is in some way deficient. It implies that the individual lacks the resources or resilience to withstand the work environment. Since the problem is in the individual, the solutions to burnout must be in the individual, too, and therefore, it is the individual’s responsibility to find and implement them. Many of the solutions to physician distress posited to date revolve around this conception; hence, the focus on yoga, mindfulness, wellness retreats, and meditation.13 While there is nothing inherently wrong with any of those practices, it is absurd to believe that yoga will solve the problems of treating a cancer patient with a declined preauthorization for chemotherapy, having no time to discuss a complex diagnosis, or relying on a computer system that places metrics ahead of communication. These problems are not the result of some failing on the part of the individual clinician.
Moral injury, on the other hand, describes the challenge of simultaneously knowing what care patients need but being unable to provide it due to constraints that are beyond our control. Moral injury is the consequence of the ever-present double binds in health care: Do we take care of our patient, the hospital, the insurer, the EMR, the health care system, or our productivity metrics first? There should be only 1 answer to that question, but the current business framework of medicine pressures us to serve all these masters at once. Moral injury locates the source of distress in a broken system, not a broken individual, and allows us to direct solutions at the causes of distress. And in the end, addressing the drivers of moral injury on a large scale may be the most effective preventive treatment for its cumulative effects among health care providers.
The long-term solutions to moral injury demand changes in the business framework of health care. The solutions reside not in promoting mindfulness or resilience among individual physicians, but in creating a health care environment that finally acknowledges the value of the time clinicians and patients spend together developing the trust, understanding, and compassion that accompany a true relationship. The long-term solutions to moral injury include a health care system that prioritizes healing over profit and that trusts its clinicians to always put their patients’ best interests first.
Treating moral injury will not be simple. It cannot happen quickly, and it will not happen without widespread clinician engagement. Change can begin when clinicians identify the double binds they face every day and convey those challenges to their administrators. If administrators and clinicians are willing to work together to resolve these double binds, health care will improve for everyone.
The following are our recommendations for how you can bring change both locally and on a broader scale.
Bring together the 2 sides of the health care house: administrators and clinicians. Invite administrators to join you on rounds, in clinic, or in the operating room. Ask them to follow you during a night of call or to spend an overnight shift with you in the emergency department. The majority of people, including health care administrators, have had only glancing encounters with the medical system. They see their primary care doctor, have regular screening procedures, and maybe get treated for a routine illness or injury. None of those encounters expose them to the depth of challenge in the system.
It takes exposure over a longer duration, or with greater intensity, to appreciate the tensions and double binds that patients and clinicians face regularly.14,15 Whether or not the administrators accept your invitation, you must also ask to see the challenges from their side. Block out an afternoon, a day, or a week to follow them and learn where they struggle in their work. Only when we understand the other party’s perspective can we truly begin to empathize and communicate meaningfully. That profound understanding is the place where commonality and compromises are found.
Make clinician satisfaction a financial priority. Although care team well-being is now part of the quadruple aim (patient experience, population health, reducing costs, and provider experience), organizations must be held accountable to ensure it is a priority. If we choose to link patient satisfaction with clinician compensation, why not link clinician satisfaction with executive compensation?
Make sure every physician leader has and uses the cell phone number of his or her legislators. Hospitals and big pharma have nearly bottomless lobbying budgets, which makes competing with them for lawmakers’ attention a formidable prospect. Despite this, physician leaders (ie, chief wellness officer, department chairperson, medical society president, etc) have a responsibility to communicate with legislators about the needs of patients (their constituents) and what role our legislators can play in fulfilling those needs. We must understand how policy, regulation, and legislation work, and we need to find seats at every table where the decisions that impact clinical care are made. The first step is opening lines of communication with those who have the power to enact large-scale change.
Reestablish a sense of community among clinicians. Too often clinicians are pitted against one another as resources shrink. Doctors compete with each other for referrals, advanced practitioners and nurses compete with doctors, and everyone feels overstressed. What we tend to forget is that we are all working toward the same goal: To give patients the best care possible. It’s time to view each other with the presumption of charity and to have each other’s backs. Uniting for support, camaraderie, mentorship, and activism is a necessary step in making change.
1 . West CP, Dyrbye LN, Sloan JA, Shanafelt TD. Single item measures of emotional exhaustion and depersonalization are useful for assessing burnout in medical professionals. J Gen Intern Med. 2009;24(12):1318-1321.
2. Shanafelt TD, Noseworthy JH. Executive leadership and physician well-being: nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. 2017;92(1):129-146.
3. Institute of Medicine (US) National Cancer Policy Forum. Ensuring Quality Cancer Care through the Oncology Workforce: Sustaining Care in the 21st Century: Workshop Summary. Washington, DC: National Academies Press; 2009.
4. Menachemi N, Collum TH. Benefits and drawbacks of electronic health record systems. Risk Manag Healthc Policy. 2011;4:47-55.
5. Palabindala V, Pamarthy A, Jonnalagadda NR. Adoption of electronic health records and barriers. J Community Hosp Intern Med Perspect. 2016;6(5):32643.
6. Zeng X. The impacts of electronic health record implementation on the health care workforce. N C Med J. 2016;77(2):112-114.
7. Squires D. U.S. health care from a global perspective: spending, use of services, prices, and health in 13 countries. https://www.commonwealthfund.org/publications/issue-briefs/2015/oct/us-health-care-global-perspective. Published October 8, 2015. Accessed August 19, 2019.
8. Fifer R. Health care economics: the real source of reimbursement problems. https://www.asha.org/Articles/Health-Care-Economics-The-Real-Source-of-Reimbursement-Problems/. Published July 2016. Accessed August 19, 2019.
9. Jha AK, Iliff AR, Chaoui AA, Defossez S, Bombaugh MC, Miller YR. A crisis in health care: a call to action on physician burnout. http://www.massmed.org/News-and-Publications/MMS-News-Releases/Physician-Burnout-Report-2018/. Published March 28, 2019. Accessed August 19, 2019.
10. Freudenberger HJ. The staff burn-out syndrome in alternative institutions. Psychother Theory Res Pract. 1975;12(1):73-82.
11. Dean W, Talbot S. Physicians aren’t “burning out.” They’re suffering from moral injury. STAT . July 26, 2018. https://www.statnews.com/2018/07/26/physicians-not-burning-out-they-are-suffering-moral-injury/. Accessed August 19, 2019.
12. Shay J. Moral injury. Psychoanal Psych. 2014;31(2):182-191.
13. Sinsky C, Shanafelt TD, Murphy ML, et al. Creating the organizational foundation for joy in medicine: organizational changes lead to physician satisfaction. https://edhub.ama-assn.org/steps-forward/module/2702510. Published September 7, 2017. Accessed August 19, 2019.
14. Golshan Ma. When a cancer surgeon becomes a cancer patient. https://elemental.medium.com/when-a-cancer-surgeon-becomes-a-cancer-patient-3b9d984066da. Published June 25, 2019. Accessed August 19, 2019.
15. Joseph S, Japa S. We were inspired to become primary care physicians. Now we’re reconsidering a field in crisis. STAT . June 20, 2019. https://www.statnews.com/2019/06/20/primary-care-field-crisis/. Accessed August 19, 2019.
*This version has been corrected. In the original version the first sentence incorrectly referred to moral injury instead of burnout.
For more than a decade, the term burnout has been used to describe clinician distress.1,2 Although some clinicians in federal health care systems may be protected from some of the drivers of burnout, other federal practitioners suffer from rule-driven health care practices and distant, top-down administration. The demand for health care is expanding, driven by the aging of the US population.3 Massive information technology investments, which promised efficiency for health care providers,4 have instead delivered a triple blow: They have diverted capital resources that might have been used to hire additional caregivers,5 diverted the time and attention of those already engaged in patient care,6 and done little to improve patient outcomes.7 Reimbursements are falling, and the only way for health systems to maintain their revenue is to increase the number of patients each clinician sees per day.8 As the resources of time and attention shrink, and as spending continues with no improvement in patient outcomes, clinician distress is on the rise.9 It will be important to understand exactly what the drivers of the problem are for federal clinicians so that solutions can be appropriately targeted. The first step in addressing the epidemic of physician distress is using the most accurate terminology to describe it.
Freudenberger defined burnout in 1975 as a constellation of symptoms—malaise, fatigue, frustration, cynicism, and inefficacy—that arise from “making excessive demands on energy, strength, or resources” in the workplace.10 The term was borrowed from other fields and applied to health care in the hopes of readily transferring the solutions that had worked in other industries to address a growing crisis among physicians. Unfortunately, the crisis in health care has proven resistant to solutions that have worked elsewhere, and many clinicians have resisted being characterized as burned out, citing a subtle, elusive disconnect between what they have experienced and what burnout encapsulates.
In July 2018, the conversation about clinician distress shifted with an article we wrote in STAT that described the moral injury of health care.11 The concept of moral injury was first described in service members who returned from the Vietnam War with symptoms that loosely fit a diagnosis of posttraumatic stress disorder (PTSD), but which did not respond to standard PTSD treatment and contained symptoms outside the PTSD constellation.12 On closer assessment, what these service members were experiencing had a different driver. Whereas those with PTSD experienced a real and imminent threat to their mortality and had come back deeply concerned for their individual, physical safety, those with this different presentation experienced repeated insults to their morality and had returned questioning whether they were still, at their core, moral beings. They had been forced, in some way, to act contrary to what their beliefs dictated was right by killing civilians on orders from their superiors, for example. This was a different category of psychological injury that required different treatment.
Moral injury occurs when we perpetrate, bear witness to, or fail to prevent an act that transgresses our deeply held moral beliefs. In the health care context, that deeply held moral belief is the oath each of us took when embarking on our paths as health care providers: Put the needs of patients first. That oath is the lynchpin of our working lives and our guiding principle when searching for the right course of action. But as clinicians, we are increasingly forced to consider the demands of other stakeholders—the electronic medical record (EMR), the insurers, the hospital, the health care system, even our own financial security—before the needs of our patients. Every time we are forced to make a decision that contravenes our patients’ best interests, we feel a sting of moral injustice. Over time, these repetitive insults amass into moral injury.
The difference between burnout and moral injury is important because using different terminology reframes the problem and the solutions. Burnout suggests that the problem resides within the individual, who is in some way deficient. It implies that the individual lacks the resources or resilience to withstand the work environment. Since the problem is in the individual, the solutions to burnout must be in the individual, too, and therefore, it is the individual’s responsibility to find and implement them. Many of the solutions to physician distress posited to date revolve around this conception; hence, the focus on yoga, mindfulness, wellness retreats, and meditation.13 While there is nothing inherently wrong with any of those practices, it is absurd to believe that yoga will solve the problems of treating a cancer patient with a declined preauthorization for chemotherapy, having no time to discuss a complex diagnosis, or relying on a computer system that places metrics ahead of communication. These problems are not the result of some failing on the part of the individual clinician.
Moral injury, on the other hand, describes the challenge of simultaneously knowing what care patients need but being unable to provide it due to constraints that are beyond our control. Moral injury is the consequence of the ever-present double binds in health care: Do we take care of our patient, the hospital, the insurer, the EMR, the health care system, or our productivity metrics first? There should be only 1 answer to that question, but the current business framework of medicine pressures us to serve all these masters at once. Moral injury locates the source of distress in a broken system, not a broken individual, and allows us to direct solutions at the causes of distress. And in the end, addressing the drivers of moral injury on a large scale may be the most effective preventive treatment for its cumulative effects among health care providers.
The long-term solutions to moral injury demand changes in the business framework of health care. The solutions reside not in promoting mindfulness or resilience among individual physicians, but in creating a health care environment that finally acknowledges the value of the time clinicians and patients spend together developing the trust, understanding, and compassion that accompany a true relationship. The long-term solutions to moral injury include a health care system that prioritizes healing over profit and that trusts its clinicians to always put their patients’ best interests first.
Treating moral injury will not be simple. It cannot happen quickly, and it will not happen without widespread clinician engagement. Change can begin when clinicians identify the double binds they face every day and convey those challenges to their administrators. If administrators and clinicians are willing to work together to resolve these double binds, health care will improve for everyone.
The following are our recommendations for how you can bring change both locally and on a broader scale.
Bring together the 2 sides of the health care house: administrators and clinicians. Invite administrators to join you on rounds, in clinic, or in the operating room. Ask them to follow you during a night of call or to spend an overnight shift with you in the emergency department. The majority of people, including health care administrators, have had only glancing encounters with the medical system. They see their primary care doctor, have regular screening procedures, and maybe get treated for a routine illness or injury. None of those encounters expose them to the depth of challenge in the system.
It takes exposure over a longer duration, or with greater intensity, to appreciate the tensions and double binds that patients and clinicians face regularly.14,15 Whether or not the administrators accept your invitation, you must also ask to see the challenges from their side. Block out an afternoon, a day, or a week to follow them and learn where they struggle in their work. Only when we understand the other party’s perspective can we truly begin to empathize and communicate meaningfully. That profound understanding is the place where commonality and compromises are found.
Make clinician satisfaction a financial priority. Although care team well-being is now part of the quadruple aim (patient experience, population health, reducing costs, and provider experience), organizations must be held accountable to ensure it is a priority. If we choose to link patient satisfaction with clinician compensation, why not link clinician satisfaction with executive compensation?
Make sure every physician leader has and uses the cell phone number of his or her legislators. Hospitals and big pharma have nearly bottomless lobbying budgets, which makes competing with them for lawmakers’ attention a formidable prospect. Despite this, physician leaders (ie, chief wellness officer, department chairperson, medical society president, etc) have a responsibility to communicate with legislators about the needs of patients (their constituents) and what role our legislators can play in fulfilling those needs. We must understand how policy, regulation, and legislation work, and we need to find seats at every table where the decisions that impact clinical care are made. The first step is opening lines of communication with those who have the power to enact large-scale change.
Reestablish a sense of community among clinicians. Too often clinicians are pitted against one another as resources shrink. Doctors compete with each other for referrals, advanced practitioners and nurses compete with doctors, and everyone feels overstressed. What we tend to forget is that we are all working toward the same goal: To give patients the best care possible. It’s time to view each other with the presumption of charity and to have each other’s backs. Uniting for support, camaraderie, mentorship, and activism is a necessary step in making change.
*This version has been corrected. In the original version the first sentence incorrectly referred to moral injury instead of burnout.
For more than a decade, the term burnout has been used to describe clinician distress.1,2 Although some clinicians in federal health care systems may be protected from some of the drivers of burnout, other federal practitioners suffer from rule-driven health care practices and distant, top-down administration. The demand for health care is expanding, driven by the aging of the US population.3 Massive information technology investments, which promised efficiency for health care providers,4 have instead delivered a triple blow: They have diverted capital resources that might have been used to hire additional caregivers,5 diverted the time and attention of those already engaged in patient care,6 and done little to improve patient outcomes.7 Reimbursements are falling, and the only way for health systems to maintain their revenue is to increase the number of patients each clinician sees per day.8 As the resources of time and attention shrink, and as spending continues with no improvement in patient outcomes, clinician distress is on the rise.9 It will be important to understand exactly what the drivers of the problem are for federal clinicians so that solutions can be appropriately targeted. The first step in addressing the epidemic of physician distress is using the most accurate terminology to describe it.
Freudenberger defined burnout in 1975 as a constellation of symptoms—malaise, fatigue, frustration, cynicism, and inefficacy—that arise from “making excessive demands on energy, strength, or resources” in the workplace.10 The term was borrowed from other fields and applied to health care in the hopes of readily transferring the solutions that had worked in other industries to address a growing crisis among physicians. Unfortunately, the crisis in health care has proven resistant to solutions that have worked elsewhere, and many clinicians have resisted being characterized as burned out, citing a subtle, elusive disconnect between what they have experienced and what burnout encapsulates.
In July 2018, the conversation about clinician distress shifted with an article we wrote in STAT that described the moral injury of health care.11 The concept of moral injury was first described in service members who returned from the Vietnam War with symptoms that loosely fit a diagnosis of posttraumatic stress disorder (PTSD), but which did not respond to standard PTSD treatment and contained symptoms outside the PTSD constellation.12 On closer assessment, what these service members were experiencing had a different driver. Whereas those with PTSD experienced a real and imminent threat to their mortality and had come back deeply concerned for their individual, physical safety, those with this different presentation experienced repeated insults to their morality and had returned questioning whether they were still, at their core, moral beings. They had been forced, in some way, to act contrary to what their beliefs dictated was right by killing civilians on orders from their superiors, for example. This was a different category of psychological injury that required different treatment.
Moral injury occurs when we perpetrate, bear witness to, or fail to prevent an act that transgresses our deeply held moral beliefs. In the health care context, that deeply held moral belief is the oath each of us took when embarking on our paths as health care providers: Put the needs of patients first. That oath is the lynchpin of our working lives and our guiding principle when searching for the right course of action. But as clinicians, we are increasingly forced to consider the demands of other stakeholders—the electronic medical record (EMR), the insurers, the hospital, the health care system, even our own financial security—before the needs of our patients. Every time we are forced to make a decision that contravenes our patients’ best interests, we feel a sting of moral injustice. Over time, these repetitive insults amass into moral injury.
The difference between burnout and moral injury is important because using different terminology reframes the problem and the solutions. Burnout suggests that the problem resides within the individual, who is in some way deficient. It implies that the individual lacks the resources or resilience to withstand the work environment. Since the problem is in the individual, the solutions to burnout must be in the individual, too, and therefore, it is the individual’s responsibility to find and implement them. Many of the solutions to physician distress posited to date revolve around this conception; hence, the focus on yoga, mindfulness, wellness retreats, and meditation.13 While there is nothing inherently wrong with any of those practices, it is absurd to believe that yoga will solve the problems of treating a cancer patient with a declined preauthorization for chemotherapy, having no time to discuss a complex diagnosis, or relying on a computer system that places metrics ahead of communication. These problems are not the result of some failing on the part of the individual clinician.
Moral injury, on the other hand, describes the challenge of simultaneously knowing what care patients need but being unable to provide it due to constraints that are beyond our control. Moral injury is the consequence of the ever-present double binds in health care: Do we take care of our patient, the hospital, the insurer, the EMR, the health care system, or our productivity metrics first? There should be only 1 answer to that question, but the current business framework of medicine pressures us to serve all these masters at once. Moral injury locates the source of distress in a broken system, not a broken individual, and allows us to direct solutions at the causes of distress. And in the end, addressing the drivers of moral injury on a large scale may be the most effective preventive treatment for its cumulative effects among health care providers.
The long-term solutions to moral injury demand changes in the business framework of health care. The solutions reside not in promoting mindfulness or resilience among individual physicians, but in creating a health care environment that finally acknowledges the value of the time clinicians and patients spend together developing the trust, understanding, and compassion that accompany a true relationship. The long-term solutions to moral injury include a health care system that prioritizes healing over profit and that trusts its clinicians to always put their patients’ best interests first.
Treating moral injury will not be simple. It cannot happen quickly, and it will not happen without widespread clinician engagement. Change can begin when clinicians identify the double binds they face every day and convey those challenges to their administrators. If administrators and clinicians are willing to work together to resolve these double binds, health care will improve for everyone.
The following are our recommendations for how you can bring change both locally and on a broader scale.
Bring together the 2 sides of the health care house: administrators and clinicians. Invite administrators to join you on rounds, in clinic, or in the operating room. Ask them to follow you during a night of call or to spend an overnight shift with you in the emergency department. The majority of people, including health care administrators, have had only glancing encounters with the medical system. They see their primary care doctor, have regular screening procedures, and maybe get treated for a routine illness or injury. None of those encounters expose them to the depth of challenge in the system.
It takes exposure over a longer duration, or with greater intensity, to appreciate the tensions and double binds that patients and clinicians face regularly.14,15 Whether or not the administrators accept your invitation, you must also ask to see the challenges from their side. Block out an afternoon, a day, or a week to follow them and learn where they struggle in their work. Only when we understand the other party’s perspective can we truly begin to empathize and communicate meaningfully. That profound understanding is the place where commonality and compromises are found.
Make clinician satisfaction a financial priority. Although care team well-being is now part of the quadruple aim (patient experience, population health, reducing costs, and provider experience), organizations must be held accountable to ensure it is a priority. If we choose to link patient satisfaction with clinician compensation, why not link clinician satisfaction with executive compensation?
Make sure every physician leader has and uses the cell phone number of his or her legislators. Hospitals and big pharma have nearly bottomless lobbying budgets, which makes competing with them for lawmakers’ attention a formidable prospect. Despite this, physician leaders (ie, chief wellness officer, department chairperson, medical society president, etc) have a responsibility to communicate with legislators about the needs of patients (their constituents) and what role our legislators can play in fulfilling those needs. We must understand how policy, regulation, and legislation work, and we need to find seats at every table where the decisions that impact clinical care are made. The first step is opening lines of communication with those who have the power to enact large-scale change.
Reestablish a sense of community among clinicians. Too often clinicians are pitted against one another as resources shrink. Doctors compete with each other for referrals, advanced practitioners and nurses compete with doctors, and everyone feels overstressed. What we tend to forget is that we are all working toward the same goal: To give patients the best care possible. It’s time to view each other with the presumption of charity and to have each other’s backs. Uniting for support, camaraderie, mentorship, and activism is a necessary step in making change.
1 . West CP, Dyrbye LN, Sloan JA, Shanafelt TD. Single item measures of emotional exhaustion and depersonalization are useful for assessing burnout in medical professionals. J Gen Intern Med. 2009;24(12):1318-1321.
2. Shanafelt TD, Noseworthy JH. Executive leadership and physician well-being: nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. 2017;92(1):129-146.
3. Institute of Medicine (US) National Cancer Policy Forum. Ensuring Quality Cancer Care through the Oncology Workforce: Sustaining Care in the 21st Century: Workshop Summary. Washington, DC: National Academies Press; 2009.
4. Menachemi N, Collum TH. Benefits and drawbacks of electronic health record systems. Risk Manag Healthc Policy. 2011;4:47-55.
5. Palabindala V, Pamarthy A, Jonnalagadda NR. Adoption of electronic health records and barriers. J Community Hosp Intern Med Perspect. 2016;6(5):32643.
6. Zeng X. The impacts of electronic health record implementation on the health care workforce. N C Med J. 2016;77(2):112-114.
7. Squires D. U.S. health care from a global perspective: spending, use of services, prices, and health in 13 countries. https://www.commonwealthfund.org/publications/issue-briefs/2015/oct/us-health-care-global-perspective. Published October 8, 2015. Accessed August 19, 2019.
8. Fifer R. Health care economics: the real source of reimbursement problems. https://www.asha.org/Articles/Health-Care-Economics-The-Real-Source-of-Reimbursement-Problems/. Published July 2016. Accessed August 19, 2019.
9. Jha AK, Iliff AR, Chaoui AA, Defossez S, Bombaugh MC, Miller YR. A crisis in health care: a call to action on physician burnout. http://www.massmed.org/News-and-Publications/MMS-News-Releases/Physician-Burnout-Report-2018/. Published March 28, 2019. Accessed August 19, 2019.
10. Freudenberger HJ. The staff burn-out syndrome in alternative institutions. Psychother Theory Res Pract. 1975;12(1):73-82.
11. Dean W, Talbot S. Physicians aren’t “burning out.” They’re suffering from moral injury. STAT . July 26, 2018. https://www.statnews.com/2018/07/26/physicians-not-burning-out-they-are-suffering-moral-injury/. Accessed August 19, 2019.
12. Shay J. Moral injury. Psychoanal Psych. 2014;31(2):182-191.
13. Sinsky C, Shanafelt TD, Murphy ML, et al. Creating the organizational foundation for joy in medicine: organizational changes lead to physician satisfaction. https://edhub.ama-assn.org/steps-forward/module/2702510. Published September 7, 2017. Accessed August 19, 2019.
14. Golshan Ma. When a cancer surgeon becomes a cancer patient. https://elemental.medium.com/when-a-cancer-surgeon-becomes-a-cancer-patient-3b9d984066da. Published June 25, 2019. Accessed August 19, 2019.
15. Joseph S, Japa S. We were inspired to become primary care physicians. Now we’re reconsidering a field in crisis. STAT . June 20, 2019. https://www.statnews.com/2019/06/20/primary-care-field-crisis/. Accessed August 19, 2019.
1 . West CP, Dyrbye LN, Sloan JA, Shanafelt TD. Single item measures of emotional exhaustion and depersonalization are useful for assessing burnout in medical professionals. J Gen Intern Med. 2009;24(12):1318-1321.
2. Shanafelt TD, Noseworthy JH. Executive leadership and physician well-being: nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. 2017;92(1):129-146.
3. Institute of Medicine (US) National Cancer Policy Forum. Ensuring Quality Cancer Care through the Oncology Workforce: Sustaining Care in the 21st Century: Workshop Summary. Washington, DC: National Academies Press; 2009.
4. Menachemi N, Collum TH. Benefits and drawbacks of electronic health record systems. Risk Manag Healthc Policy. 2011;4:47-55.
5. Palabindala V, Pamarthy A, Jonnalagadda NR. Adoption of electronic health records and barriers. J Community Hosp Intern Med Perspect. 2016;6(5):32643.
6. Zeng X. The impacts of electronic health record implementation on the health care workforce. N C Med J. 2016;77(2):112-114.
7. Squires D. U.S. health care from a global perspective: spending, use of services, prices, and health in 13 countries. https://www.commonwealthfund.org/publications/issue-briefs/2015/oct/us-health-care-global-perspective. Published October 8, 2015. Accessed August 19, 2019.
8. Fifer R. Health care economics: the real source of reimbursement problems. https://www.asha.org/Articles/Health-Care-Economics-The-Real-Source-of-Reimbursement-Problems/. Published July 2016. Accessed August 19, 2019.
9. Jha AK, Iliff AR, Chaoui AA, Defossez S, Bombaugh MC, Miller YR. A crisis in health care: a call to action on physician burnout. http://www.massmed.org/News-and-Publications/MMS-News-Releases/Physician-Burnout-Report-2018/. Published March 28, 2019. Accessed August 19, 2019.
10. Freudenberger HJ. The staff burn-out syndrome in alternative institutions. Psychother Theory Res Pract. 1975;12(1):73-82.
11. Dean W, Talbot S. Physicians aren’t “burning out.” They’re suffering from moral injury. STAT . July 26, 2018. https://www.statnews.com/2018/07/26/physicians-not-burning-out-they-are-suffering-moral-injury/. Accessed August 19, 2019.
12. Shay J. Moral injury. Psychoanal Psych. 2014;31(2):182-191.
13. Sinsky C, Shanafelt TD, Murphy ML, et al. Creating the organizational foundation for joy in medicine: organizational changes lead to physician satisfaction. https://edhub.ama-assn.org/steps-forward/module/2702510. Published September 7, 2017. Accessed August 19, 2019.
14. Golshan Ma. When a cancer surgeon becomes a cancer patient. https://elemental.medium.com/when-a-cancer-surgeon-becomes-a-cancer-patient-3b9d984066da. Published June 25, 2019. Accessed August 19, 2019.
15. Joseph S, Japa S. We were inspired to become primary care physicians. Now we’re reconsidering a field in crisis. STAT . June 20, 2019. https://www.statnews.com/2019/06/20/primary-care-field-crisis/. Accessed August 19, 2019.
Our EHRs have a drug problem
The “opioid epidemic” has become, perhaps, the most talked-about health crisis of the 21st century. It is a pervasive topic of discussion in the health literature and beyond, written about on the front pages of national newspapers and even mentioned in presidential state-of-the-union addresses.
As practicing physicians, we are all too familiar with the ills of chronic opioid use and have dealt with the implications of the crisis long before the issue attracted the public’s attention. In many ways, we have felt alone in bearing the burdens of caring for patients on chronic controlled substances. Until this point it has been our sacred duty to determine which patients are truly in need of those medications, and which are merely dependent on or – even worse – abusing them.
Health care providers have been largely blamed for the creation of this crisis, but we are not alone. Responsibility must also be shared by the pharmaceutical industry, health insurers, and even the government. Marketing practices, inadequate coverage of pain-relieving procedures and rehabilitation, and poorly-conceived drug policies have created an environment where it has been far too difficult to provide appropriate care for patients with chronic pain. As a result, patients who may have had an alternative to opioids were still started on these medications, and we – their physicians – have been left alone to manage the outcome.
Recently, however, health policy and public awareness have signaled a dramatic shift in the management of long-term pain medication. Significant legislation has been enacted on national, state, and local levels, and parties who are perceived to be responsible for the crisis are being held to task. For example, in August a landmark legal case was decided in an Oklahoma district court. Johnson & Johnson Pharmaceuticals was found guilty of promoting drug addiction through false and misleading marketing and was thus ordered to pay $572 million to the state to fund drug rehabilitation programs. This is likely a harbinger of many more such decisions to come, and the industry as a whole is bracing for the worst.
Physician prescribing practices are also being carefully scrutinized by the DEA, and a significant number of new “checks and balances” have been put in place to address dependence and addiction concerns. Unfortunately, as with all sweeping reform programs, there are good – and not-so-good – aspects to these changes. In many ways, the new tools at our disposal are a powerful way of mitigating drug dependence and diversion while protecting the sanctity of our “prescription pads.” Yet, as with so many other government mandates, we are burdened with the onus of complying with the new mandates for each and every opioid prescription, while our EHRs provide little help. This means more “clicks” for us, which can feel quite burdensome. It doesn’t need to be this way. Below are two straightforward things that can and should occur in order for providers to feel unburdened and to fully embrace the changes.
PDMP integration
One of the major ways of controlling prescription opioid abuse is through effective monitoring. Forty-nine of the 50 U.S. states have developed Prescription Drug Monitoring Programs (PDMPs), with Missouri being the only holdout (due to the politics of individual privacy concerns and conflation with gun control legislation). Most – though not all – of the states with a PDMP also mandate that physicians query a database prior to prescribing controlled substances. While noble and helpful in principle, querying a PDMP can be cumbersome, and the process is rarely integrated into the EHR workflow. Instead, physicians typically need to login to a separate website and manually transpose patient data to search the database. While most states have offered to subsidize PDMP integration with electronic records, EHR vendors have been very slow to develop the capability, leaving most physicians with no choice but to continue the aforementioned workflow. That is, if they comply at all; many well-meaning physicians have told us that they find themselves too harried to use the PDMP consistently. This reduces the value of these databases and places the physicians at significant risk. In some states, failure to query the database can lead to loss of a doctor’s medical license. It is high time that EHR vendors step up and integrate with every state’s prescription drug database.
Electronic prescribing of controlled substances
The other major milestone in prescription opioid management is the electronic prescribing of controlled substances (EPCS). This received national priority when the SUPPORT for Patients and Communities Act was signed into federal law in October of 2018. Included in this act is a requirement that, by January of 2021, all controlled substance prescriptions covered under Medicare Part D be sent electronically. Taking this as inspiration, many states and private companies have adopted more aggressive policies, choosing to implement electronic prescription requirements prior to the 2021 deadline. In Pennsylvania, where we practice, an EPCS requirement goes into effect in October of this year (2019). National pharmacy chains have also taken a more proactive approach. Walmart, for example, has decided that it will require EPCS nationwide in all of its stores beginning in January of 2020.
Essentially physicians have no choice – if they plan to continue to prescribe controlled substances, they will need to begin doing so electronically. Unfortunately, this may not be a straightforward process. While most EHRs offer some sort of EPCS solution, it is typically far from user friendly. Setting up EPCS can be costly and incredibly time consuming, and the procedure of actually submitting controlled prescriptions can be onerous and add many extra clicks. If vendors are serious about assisting in solving the opioid crisis, they need to make streamlining the steps of EPCS a high priority.
A prescription for success
As with so many other topics we’ve written about, we face an ever-increasing burden to provide quality patient care while complying with cumbersome and often unfunded external mandates. In the case of the opioid crisis, we believe we can do better. Our prescription for success? Streamlined workflow, smarter EHRs, and fewer clicks. There is no question that physicians and patients will benefit from effective implementation of the new tools at our disposal, but we need EHR vendors to step up and help carry the load.
Dr. Notte is a family physician and associate chief medical information officer for Abington (Pa.) Jefferson Health. Follow him on Twitter @doctornotte. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
The “opioid epidemic” has become, perhaps, the most talked-about health crisis of the 21st century. It is a pervasive topic of discussion in the health literature and beyond, written about on the front pages of national newspapers and even mentioned in presidential state-of-the-union addresses.
As practicing physicians, we are all too familiar with the ills of chronic opioid use and have dealt with the implications of the crisis long before the issue attracted the public’s attention. In many ways, we have felt alone in bearing the burdens of caring for patients on chronic controlled substances. Until this point it has been our sacred duty to determine which patients are truly in need of those medications, and which are merely dependent on or – even worse – abusing them.
Health care providers have been largely blamed for the creation of this crisis, but we are not alone. Responsibility must also be shared by the pharmaceutical industry, health insurers, and even the government. Marketing practices, inadequate coverage of pain-relieving procedures and rehabilitation, and poorly-conceived drug policies have created an environment where it has been far too difficult to provide appropriate care for patients with chronic pain. As a result, patients who may have had an alternative to opioids were still started on these medications, and we – their physicians – have been left alone to manage the outcome.
Recently, however, health policy and public awareness have signaled a dramatic shift in the management of long-term pain medication. Significant legislation has been enacted on national, state, and local levels, and parties who are perceived to be responsible for the crisis are being held to task. For example, in August a landmark legal case was decided in an Oklahoma district court. Johnson & Johnson Pharmaceuticals was found guilty of promoting drug addiction through false and misleading marketing and was thus ordered to pay $572 million to the state to fund drug rehabilitation programs. This is likely a harbinger of many more such decisions to come, and the industry as a whole is bracing for the worst.
Physician prescribing practices are also being carefully scrutinized by the DEA, and a significant number of new “checks and balances” have been put in place to address dependence and addiction concerns. Unfortunately, as with all sweeping reform programs, there are good – and not-so-good – aspects to these changes. In many ways, the new tools at our disposal are a powerful way of mitigating drug dependence and diversion while protecting the sanctity of our “prescription pads.” Yet, as with so many other government mandates, we are burdened with the onus of complying with the new mandates for each and every opioid prescription, while our EHRs provide little help. This means more “clicks” for us, which can feel quite burdensome. It doesn’t need to be this way. Below are two straightforward things that can and should occur in order for providers to feel unburdened and to fully embrace the changes.
PDMP integration
One of the major ways of controlling prescription opioid abuse is through effective monitoring. Forty-nine of the 50 U.S. states have developed Prescription Drug Monitoring Programs (PDMPs), with Missouri being the only holdout (due to the politics of individual privacy concerns and conflation with gun control legislation). Most – though not all – of the states with a PDMP also mandate that physicians query a database prior to prescribing controlled substances. While noble and helpful in principle, querying a PDMP can be cumbersome, and the process is rarely integrated into the EHR workflow. Instead, physicians typically need to login to a separate website and manually transpose patient data to search the database. While most states have offered to subsidize PDMP integration with electronic records, EHR vendors have been very slow to develop the capability, leaving most physicians with no choice but to continue the aforementioned workflow. That is, if they comply at all; many well-meaning physicians have told us that they find themselves too harried to use the PDMP consistently. This reduces the value of these databases and places the physicians at significant risk. In some states, failure to query the database can lead to loss of a doctor’s medical license. It is high time that EHR vendors step up and integrate with every state’s prescription drug database.
Electronic prescribing of controlled substances
The other major milestone in prescription opioid management is the electronic prescribing of controlled substances (EPCS). This received national priority when the SUPPORT for Patients and Communities Act was signed into federal law in October of 2018. Included in this act is a requirement that, by January of 2021, all controlled substance prescriptions covered under Medicare Part D be sent electronically. Taking this as inspiration, many states and private companies have adopted more aggressive policies, choosing to implement electronic prescription requirements prior to the 2021 deadline. In Pennsylvania, where we practice, an EPCS requirement goes into effect in October of this year (2019). National pharmacy chains have also taken a more proactive approach. Walmart, for example, has decided that it will require EPCS nationwide in all of its stores beginning in January of 2020.
Essentially physicians have no choice – if they plan to continue to prescribe controlled substances, they will need to begin doing so electronically. Unfortunately, this may not be a straightforward process. While most EHRs offer some sort of EPCS solution, it is typically far from user friendly. Setting up EPCS can be costly and incredibly time consuming, and the procedure of actually submitting controlled prescriptions can be onerous and add many extra clicks. If vendors are serious about assisting in solving the opioid crisis, they need to make streamlining the steps of EPCS a high priority.
A prescription for success
As with so many other topics we’ve written about, we face an ever-increasing burden to provide quality patient care while complying with cumbersome and often unfunded external mandates. In the case of the opioid crisis, we believe we can do better. Our prescription for success? Streamlined workflow, smarter EHRs, and fewer clicks. There is no question that physicians and patients will benefit from effective implementation of the new tools at our disposal, but we need EHR vendors to step up and help carry the load.
Dr. Notte is a family physician and associate chief medical information officer for Abington (Pa.) Jefferson Health. Follow him on Twitter @doctornotte. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
The “opioid epidemic” has become, perhaps, the most talked-about health crisis of the 21st century. It is a pervasive topic of discussion in the health literature and beyond, written about on the front pages of national newspapers and even mentioned in presidential state-of-the-union addresses.
As practicing physicians, we are all too familiar with the ills of chronic opioid use and have dealt with the implications of the crisis long before the issue attracted the public’s attention. In many ways, we have felt alone in bearing the burdens of caring for patients on chronic controlled substances. Until this point it has been our sacred duty to determine which patients are truly in need of those medications, and which are merely dependent on or – even worse – abusing them.
Health care providers have been largely blamed for the creation of this crisis, but we are not alone. Responsibility must also be shared by the pharmaceutical industry, health insurers, and even the government. Marketing practices, inadequate coverage of pain-relieving procedures and rehabilitation, and poorly-conceived drug policies have created an environment where it has been far too difficult to provide appropriate care for patients with chronic pain. As a result, patients who may have had an alternative to opioids were still started on these medications, and we – their physicians – have been left alone to manage the outcome.
Recently, however, health policy and public awareness have signaled a dramatic shift in the management of long-term pain medication. Significant legislation has been enacted on national, state, and local levels, and parties who are perceived to be responsible for the crisis are being held to task. For example, in August a landmark legal case was decided in an Oklahoma district court. Johnson & Johnson Pharmaceuticals was found guilty of promoting drug addiction through false and misleading marketing and was thus ordered to pay $572 million to the state to fund drug rehabilitation programs. This is likely a harbinger of many more such decisions to come, and the industry as a whole is bracing for the worst.
Physician prescribing practices are also being carefully scrutinized by the DEA, and a significant number of new “checks and balances” have been put in place to address dependence and addiction concerns. Unfortunately, as with all sweeping reform programs, there are good – and not-so-good – aspects to these changes. In many ways, the new tools at our disposal are a powerful way of mitigating drug dependence and diversion while protecting the sanctity of our “prescription pads.” Yet, as with so many other government mandates, we are burdened with the onus of complying with the new mandates for each and every opioid prescription, while our EHRs provide little help. This means more “clicks” for us, which can feel quite burdensome. It doesn’t need to be this way. Below are two straightforward things that can and should occur in order for providers to feel unburdened and to fully embrace the changes.
PDMP integration
One of the major ways of controlling prescription opioid abuse is through effective monitoring. Forty-nine of the 50 U.S. states have developed Prescription Drug Monitoring Programs (PDMPs), with Missouri being the only holdout (due to the politics of individual privacy concerns and conflation with gun control legislation). Most – though not all – of the states with a PDMP also mandate that physicians query a database prior to prescribing controlled substances. While noble and helpful in principle, querying a PDMP can be cumbersome, and the process is rarely integrated into the EHR workflow. Instead, physicians typically need to login to a separate website and manually transpose patient data to search the database. While most states have offered to subsidize PDMP integration with electronic records, EHR vendors have been very slow to develop the capability, leaving most physicians with no choice but to continue the aforementioned workflow. That is, if they comply at all; many well-meaning physicians have told us that they find themselves too harried to use the PDMP consistently. This reduces the value of these databases and places the physicians at significant risk. In some states, failure to query the database can lead to loss of a doctor’s medical license. It is high time that EHR vendors step up and integrate with every state’s prescription drug database.
Electronic prescribing of controlled substances
The other major milestone in prescription opioid management is the electronic prescribing of controlled substances (EPCS). This received national priority when the SUPPORT for Patients and Communities Act was signed into federal law in October of 2018. Included in this act is a requirement that, by January of 2021, all controlled substance prescriptions covered under Medicare Part D be sent electronically. Taking this as inspiration, many states and private companies have adopted more aggressive policies, choosing to implement electronic prescription requirements prior to the 2021 deadline. In Pennsylvania, where we practice, an EPCS requirement goes into effect in October of this year (2019). National pharmacy chains have also taken a more proactive approach. Walmart, for example, has decided that it will require EPCS nationwide in all of its stores beginning in January of 2020.
Essentially physicians have no choice – if they plan to continue to prescribe controlled substances, they will need to begin doing so electronically. Unfortunately, this may not be a straightforward process. While most EHRs offer some sort of EPCS solution, it is typically far from user friendly. Setting up EPCS can be costly and incredibly time consuming, and the procedure of actually submitting controlled prescriptions can be onerous and add many extra clicks. If vendors are serious about assisting in solving the opioid crisis, they need to make streamlining the steps of EPCS a high priority.
A prescription for success
As with so many other topics we’ve written about, we face an ever-increasing burden to provide quality patient care while complying with cumbersome and often unfunded external mandates. In the case of the opioid crisis, we believe we can do better. Our prescription for success? Streamlined workflow, smarter EHRs, and fewer clicks. There is no question that physicians and patients will benefit from effective implementation of the new tools at our disposal, but we need EHR vendors to step up and help carry the load.
Dr. Notte is a family physician and associate chief medical information officer for Abington (Pa.) Jefferson Health. Follow him on Twitter @doctornotte. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.