User login
Screening for parasitic infections: One doctor’s experience
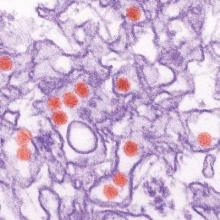
Soin, et al, reported an interesting case of strongyloidiasis in a refugee in their Photo Rounds article, “Rash, diarrhea, and eosinophilia” (J Fam Pract. 2015;64:655-658). They mentioned the importance of having a high degree of suspicion for parasitic infections among refugees. Indeed, health screenings for refugees are necessary and should include testing for parasitoses. However, there are several other issues to consider.
First, a single screening may not be effective. Thus, results should be verified with repeat screening tests. In my experience in Thailand, a single screening of migrants from nearby Indochinese countries failed to detect several infectious cases, including tuberculosis, malaria, and intestinal parasite infections. To optimize early detection and infection control, a repeated check-up system is needed. It should be noted, however, that a false-negative result for strongyloidiasis is not common from a stool examination or immunological test.1
Second, the mentioned symptoms of “rash, diarrhea, and eosinophilia” can be due to several etiologies and may have been caused by a completely separate illness. Or the findings might have been due to a forgotten condition, such as post-dengue infection illness.2
Finally, the existence of strongyloidiasis in the case presented by Soin, et al, could have been an incidental finding without a relationship to the exact pathology.
Viroj Wiwanitkit, MD
Bangkok, Thailand
1. Rodriguez EA, Abraham T, Williams FK. Severe strongyloidiasis with negative serology after corticosteroid treatment. Am J Case Rep. 2015;16:95-98.
2. Wiwanitkit V. Dengue fever: diagnosis and treatment. Expert Rev Anti Infect Ther. 2010;8:841-845.
Soin, et al, reported an interesting case of strongyloidiasis in a refugee in their Photo Rounds article, “Rash, diarrhea, and eosinophilia” (J Fam Pract. 2015;64:655-658). They mentioned the importance of having a high degree of suspicion for parasitic infections among refugees. Indeed, health screenings for refugees are necessary and should include testing for parasitoses. However, there are several other issues to consider.
First, a single screening may not be effective. Thus, results should be verified with repeat screening tests. In my experience in Thailand, a single screening of migrants from nearby Indochinese countries failed to detect several infectious cases, including tuberculosis, malaria, and intestinal parasite infections. To optimize early detection and infection control, a repeated check-up system is needed. It should be noted, however, that a false-negative result for strongyloidiasis is not common from a stool examination or immunological test.1
Second, the mentioned symptoms of “rash, diarrhea, and eosinophilia” can be due to several etiologies and may have been caused by a completely separate illness. Or the findings might have been due to a forgotten condition, such as post-dengue infection illness.2
Finally, the existence of strongyloidiasis in the case presented by Soin, et al, could have been an incidental finding without a relationship to the exact pathology.
Viroj Wiwanitkit, MD
Bangkok, Thailand
Soin, et al, reported an interesting case of strongyloidiasis in a refugee in their Photo Rounds article, “Rash, diarrhea, and eosinophilia” (J Fam Pract. 2015;64:655-658). They mentioned the importance of having a high degree of suspicion for parasitic infections among refugees. Indeed, health screenings for refugees are necessary and should include testing for parasitoses. However, there are several other issues to consider.
First, a single screening may not be effective. Thus, results should be verified with repeat screening tests. In my experience in Thailand, a single screening of migrants from nearby Indochinese countries failed to detect several infectious cases, including tuberculosis, malaria, and intestinal parasite infections. To optimize early detection and infection control, a repeated check-up system is needed. It should be noted, however, that a false-negative result for strongyloidiasis is not common from a stool examination or immunological test.1
Second, the mentioned symptoms of “rash, diarrhea, and eosinophilia” can be due to several etiologies and may have been caused by a completely separate illness. Or the findings might have been due to a forgotten condition, such as post-dengue infection illness.2
Finally, the existence of strongyloidiasis in the case presented by Soin, et al, could have been an incidental finding without a relationship to the exact pathology.
Viroj Wiwanitkit, MD
Bangkok, Thailand
1. Rodriguez EA, Abraham T, Williams FK. Severe strongyloidiasis with negative serology after corticosteroid treatment. Am J Case Rep. 2015;16:95-98.
2. Wiwanitkit V. Dengue fever: diagnosis and treatment. Expert Rev Anti Infect Ther. 2010;8:841-845.
1. Rodriguez EA, Abraham T, Williams FK. Severe strongyloidiasis with negative serology after corticosteroid treatment. Am J Case Rep. 2015;16:95-98.
2. Wiwanitkit V. Dengue fever: diagnosis and treatment. Expert Rev Anti Infect Ther. 2010;8:841-845.
An unconscious bias in this EHR study?
Like many physicians, I struggle with looking at my patients while they are talking and getting the stories that they tell me transcribed as accurately and completely as possible. After I read the article, “EHR use and patient satisfaction: What we learned” by Farber, et al, (J Fam Pract. 2015;64:687-696), I was struck by something.
Of the 126 patients chosen for the research, the educational level breakdown included 75% with at least some college education and 28% with postgraduate education. A study performed by the National Center for Veterans Analysis and Statistics published in 2015 has different statistics.1 Although a similar percentage had at least some college education, only 10.5% of the men and 12.4% of the women had postgraduate education.
In my practice, most of my patients who have worked with computers empathize with the amount of time that I spend looking at the screen. Those with less education are less agreeable. Since the patients were picked by their physicians to take part in the study, I wonder if there was an unconscious bias present.
Holly Leeds, MD
Auburn, Calif
1. National Center for Veterans Analysis and Statistics. Profile of Veterans: 2013. US Department of Veterans Affairs Web site. Available at: http://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2013.pdf. Accessed March 21, 2016.
Author's response:
Dr. Leeds brings up an interesting issue. It is possible that there is an unconscious bias on the part of physicians who participated in this study. Although the demographics are fairly similar to those that she cites, the veterans in our study were somewhat more educated.
If less well-educated subjects participated, this would make the data more impressive, in terms of less satisfaction with physicians who more readily focus their eyes on computer screens rather than on their patients. The fact that we did find this association is important for physicians who use EHR systems.
Neil J. Farber, MD, FACP
San Diego, Calif
Like many physicians, I struggle with looking at my patients while they are talking and getting the stories that they tell me transcribed as accurately and completely as possible. After I read the article, “EHR use and patient satisfaction: What we learned” by Farber, et al, (J Fam Pract. 2015;64:687-696), I was struck by something.
Of the 126 patients chosen for the research, the educational level breakdown included 75% with at least some college education and 28% with postgraduate education. A study performed by the National Center for Veterans Analysis and Statistics published in 2015 has different statistics.1 Although a similar percentage had at least some college education, only 10.5% of the men and 12.4% of the women had postgraduate education.
In my practice, most of my patients who have worked with computers empathize with the amount of time that I spend looking at the screen. Those with less education are less agreeable. Since the patients were picked by their physicians to take part in the study, I wonder if there was an unconscious bias present.
Holly Leeds, MD
Auburn, Calif
1. National Center for Veterans Analysis and Statistics. Profile of Veterans: 2013. US Department of Veterans Affairs Web site. Available at: http://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2013.pdf. Accessed March 21, 2016.
Author's response:
Dr. Leeds brings up an interesting issue. It is possible that there is an unconscious bias on the part of physicians who participated in this study. Although the demographics are fairly similar to those that she cites, the veterans in our study were somewhat more educated.
If less well-educated subjects participated, this would make the data more impressive, in terms of less satisfaction with physicians who more readily focus their eyes on computer screens rather than on their patients. The fact that we did find this association is important for physicians who use EHR systems.
Neil J. Farber, MD, FACP
San Diego, Calif
Like many physicians, I struggle with looking at my patients while they are talking and getting the stories that they tell me transcribed as accurately and completely as possible. After I read the article, “EHR use and patient satisfaction: What we learned” by Farber, et al, (J Fam Pract. 2015;64:687-696), I was struck by something.
Of the 126 patients chosen for the research, the educational level breakdown included 75% with at least some college education and 28% with postgraduate education. A study performed by the National Center for Veterans Analysis and Statistics published in 2015 has different statistics.1 Although a similar percentage had at least some college education, only 10.5% of the men and 12.4% of the women had postgraduate education.
In my practice, most of my patients who have worked with computers empathize with the amount of time that I spend looking at the screen. Those with less education are less agreeable. Since the patients were picked by their physicians to take part in the study, I wonder if there was an unconscious bias present.
Holly Leeds, MD
Auburn, Calif
1. National Center for Veterans Analysis and Statistics. Profile of Veterans: 2013. US Department of Veterans Affairs Web site. Available at: http://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2013.pdf. Accessed March 21, 2016.
Author's response:
Dr. Leeds brings up an interesting issue. It is possible that there is an unconscious bias on the part of physicians who participated in this study. Although the demographics are fairly similar to those that she cites, the veterans in our study were somewhat more educated.
If less well-educated subjects participated, this would make the data more impressive, in terms of less satisfaction with physicians who more readily focus their eyes on computer screens rather than on their patients. The fact that we did find this association is important for physicians who use EHR systems.
Neil J. Farber, MD, FACP
San Diego, Calif
Genetic and related laboratory tests in psychiatry: What mental health practitioners need to know
What has been the history of the development of laboratory tests in the field of psychiatry?
During my almost-40-year academic medical career, I have been interested in the development and incorporation of laboratory tests into psychiatry.1 This interest initially focused on therapeutic drug monitoring (TDM) and the genetics of drug responsiveness, with an emphasis on drug metabolism. In addition to TDM—which I have long believed is vastly underutilized in psychiatry—there have been many failed attempts to develop diagnostic tests, including tests to distinguish between what were postulated to be serotonergic and noradrenergic forms of major depression in the 1970s2,3 and the dexamethasone suppression test for melancholia in the 1980s.4 Recently, a 51-analyte immunoassay test was marketed by Rules-Based Medicine, Inc. (RBM), as an aid in the diagnosis of schizophrenia, but the test was found to suffer a high false-positive rate and was withdrawn from the market.5 Given this track record, caution is warranted when examining claims for new tests.
What types of tests are being developed?
Most tests in development are pharmacogenomic (PG)-based or immunoassay (IA)-based.
PG tests examine single nucleotide polymorphisms (SNP) in genes that code for pharmacokinetic mechanisms, primarily cytochrome P450 (CYP) enzymes responsible for drug metabolism and P-glycoprotein, responsible for drug transportation. The next most common type of test examines pharmacodynamic mechanisms, such as SNPs of specific receptor genes, including serotonin (or 5-hydroxytryptophan [5-HT] transporter [SET or 5-HTT]) or the 5-HT2A receptor.
The fact that CYP enzymes lead the list is not surprising: These enzymes and their role in the metabolism of specific drugs have been extensively studied since the late 1980s. Considerable data has been accumulated regarding variants of CYP enzymes, which convey clinically meaningful differences among individuals in terms of their ability to metabolize drug via these pathways. Individuals are commonly divided into 4 phenotypic categories: ultra-rapid, extensive (or normal), intermediate, and poor metabolizers. Based on these phenotypes, clinical consequences can be quantitated in terms of changes in drug concentration, concentration-dependent beneficial or adverse effects, and associated/recommended changes in dosing.
Research into the role of pharmacodynamic variants, however, is still in infancy and more difficult to measure in terms of assessing endpoints, with related limitations in clinical utility.
IA assays generally measure a variety of proteins, particularly those reflecting inflammatory processes (eg, various cytokines, such as interleukin-6).6 As with pharmacodynamic measures, research into the role of inflammatory biomarkers is in early stages. The clinical utility of associated tests is, therefore, less certain; witness the recent study5 I noted that revealed a high false-positive rate for the RBM schizophrenia panel in healthy controls. Nevertheless, considerable research is being conducted in all of these areas so that new developments might lend themselves to greater clinical utility.
(Note that PG biomarkers are trait measures, whereas IA biomarkers are state measures, so that complementary use of both types of tests might prove useful in diagnosis and clinical management. Although such integrative use of these 2 different types of tests generally is not done today.)
What does it take to market these tests?
At a minimum, offering these tests for sale requires that the laboratory be certified by the Centers for Medicare & Medicaid Services, according to the Clinical Laboratory Improvement Amendments (CLIA) standards (www.fda.gov/medicaldevices/deviceregulationandguidance/ivdregulatoryassistance/ucm124105.htm). CLIA-certified laboratories are required to demonstrate the analytical validity of tests that they offer—ie, the accuracy and reliability of the test in measuring a parameter of interest—but not the clinical validity or utility of those tests. The fact that a test in fact measures what it claims to be measuring in and of itself does not mean it has clinical validity or utility (see the discussion below).
Must the FDA approve laboratory tests?
No, but that situation might be changing.
Currently, only tests used in a setting considered high risk—eg, a test intended to detect or diagnose a malignancy or guide its treatment—requires formal FDA approval. The approval of such a test requires submission to the FDA of clinical data supporting its clinical validity and utility, in addition to evidence of analytic validity.
Even in such cases, the degree and quality of the clinical data required are generally not as high as would be required for approval of a drug. That distinction is understandable, given the type and quantity of data necessary for drug approval and the many years and billions of dollars it takes to accumulate such data. For most laboratory tests, providing the same level of data required to have a drug approved would be neither necessary nor feasible given the business model underlying most laboratories providing laboratory tests.
What do ‘clinical validity’ and ‘clinical utility’ mean?
These are higher evidence thresholds than is needed for analytic validity, although the latter is a necessary first step on the path to achieving these higher thresholds.
Clinical validity is the ability of a test to detect:
- a clinically meaningful measure, such as clinical response
- an adverse effect
- a biologically meaningful measure (eg, a drug level or a change in the electrocardiographic pattern).
Above the threshold of clinical validity is clinical utility, which is proof that the test can reliably be used to guide clinical management and thus meaningfully improve outcomes, such as guiding drug or dosage selection.
Is the use of PG testing recommended? If so, in what instances?
Specific types of PG testing is recommended by the FDA recommended. The FDA has been incorporating PG information into the labels of specific medications for several years; the agency has a Web site (www.fda.gov/drugs/scienceresearch/researchareas/pharmacogenetics/ucm083378.htm) that continuously updates this information. The involved drugs are in all therapeutic classes—from oncology to psychiatry.
More than 30 psychotropic drugs have PG information in their label; some of those drugs’ labels contain specific recommendations, such as obtaining PG information before selecting or starting a drug in a specific patient. An example is carbamazepine, for which the recommendation is to obtain HLA testing before starting the drug in patients of Han Chinese ancestry, because members of this large ethnic group are at greater risk of serious dermatologic adverse effects, including Stevens-Johnson syndrome.
In other instances, the recommendation is to do the testing before increasing beyond a specific dose. Examples of psychiatric drugs whose labels contain such PG information include pimozide and iloperidone as well as citalopram. In the FDA-approved label, guidance is provided that these drugs can be started without testing if prescribed at a reduced recommended starting dosage range, rather than the full starting dosage range. The guidance on these drugs further recommends testing for genetic CYP2D6 poor metabolizer (PM) status before dosing above that initial recommended, limited, starting dosage range.
The rationale for this guidance is to reduce the risk that (1) patients in question will achieve an excessively high plasma drug level that can cause significant prolongation of intracardiac conduction (eg, QTc prolongation) and thus (2) develop the potentially fatal arrhythmia torsades de pointes. Guidance is based on thorough QTc studies that were performed on each drug,7,8 which makes them examples of instances in which the test has clinical validity and utility as well as analytical validity.
To find PG labeling in the package insert for these drugs, visit: www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm.
What about data for other tests that are marketed and promoted by developers?
Sometimes, there are—literally—no data on available tests beyond the analytical validity of the test; other times, the amount and quality of clinical data are quite variable, ranging from results of ≥1 small retrospective studies without controls to results of prospective, randomized, controlled studies. Even among the latter, the developer may conduct and analyze their studies without oversight by an independent agency, such as the FDA.
This situation (1) raises concern that study results are not independent of the developer’s business interests and, as one might expect, (2) leads to controversy about whether the data are compelling—or not.9-12
What is a critical difference between PG test results and results of most laboratory tests?
PG tests are, as noted, trait rather than state characteristics. That means that the results do not change except for a phenomenon known as phenocoversion, discussed below. (Of course, advances in gene therapy might make it possible someday to change a person’s genetic makeup and for mitochondrial genes that is already possible.)
For this reason, PG test results should not get buried in the medical record, as might happen with, say, a patient’s serum potassium level at a given point in time. Instead, PG test results need to be carried forward continuously. Results also should be given to the patient as part of his (her) personal health record and to all other health care providers that the patient is seeing or will see in the future. Each health care provider who obtains PG test results should consider sending them to all current clinicians providing care for the patient at the same time as they are.
Is your functional status at a given moment the same as your genetic status?
No. There is a phenomenon known as phenoconversion in which a person’s current functional status may be different from what would be expected based on their genetic status.
CYP2D6 functional status is susceptible to phenoconversion as follows: Administering fluoxetine and paroxetine, for example, at 20 or 40 mg/d converts 66% and 95%, respectively, of patients who are CYP2D6 extensive (ie, normal) metabolizers into phenocopies of people who, genetically, lack the ability to metabolize drugs via CYP2D6 (ie, genotypic CYP2D6 PM). Based on a recent study of 900 participants in routine clinical care who were taking an antidepressant, 4% of the general U.S. population are genetically CYP2D6 PM; an additional 24% are phenotypically CYP2D6 PM because of concomitant administration of a CYP2D6 substantial inhibitor, such as bupropion, fluoxetine, paroxetine, or terbenafine.13
That is the reason a provider needs to know what drugs a patient is taking concomitantly—to consider the possibility of phenoconversion and, when necessary, to dose accordingly.
What does the future hold?
Development of tests for use in psychiatric practice is likely to grow substantially, for at least 2 reasons:
- There is a huge unmet need for clinically meaningful tests to aid in the provision of optimal patient care and, therefore, a tremendous business opportunity
- Knowledge in the biological basis of psychiatric disorders is growing exponentially; with that knowledge comes the ability to develop new tests.
A recent example comes from a research group that devised a test that could predict suicidality.14 Time will tell whether this test or a derivative of it enters practice. Nevertheless, it is a harbinger of the likely dramatic changes in the landscape of clinical medicine particularly as it applies to psychiatry.
Given these developments, the syndromic diagnoses in DSM-5 will in the future likely be replaced by a new diagnostic schema that breaks down existing heterogenous syndromic diagnoses into pathophysiologically and etiologically meaningful entities using insights gained from genetic and biomarker data as well as functional brain imaging. Theoretically, those insights will lead to new modalities of treatment, including somatic treatments that target novel mechanisms of action, coupled to more effective psychosocial therapies—with both therapies guided by diagnostic tests to monitor response to specific treatment interventions.
During this transition from the past to the future, answers to the questions I’ve posed here about laboratory testing in psychiatry will, I hope, help the practitioner understand, evaluate, and incorporate these changes readily into practice.
1. Preskorn SH, Biggs JT. Use of tricyclic antidepressant blood levels. N Engl J Med. 1978;298(3):166.
2. Schildkraut JJ. Biogenic amines and affective disorders. Annu Rev Med. 1974;25(0):333-348.
3. Maas JW. Biogenic amines and depression. Biochemical and pharmacological separation of two types of depression. Arch Gen Psychiatry. 1975;32(11):1357-1361.
4. Carroll BJ, Feinberg M, Greden JF, et al. A specific laboratory test for the diagnosis of melancholia. Standardization, validation, and clinical utility. Arch Gen Psychiatry. 1981;38(1):15-22.
5. Wehler C, Preskorn S. High false-positive rate of a putative biomarker test to aid in the diagnosis of schizophrenia. J Clin Psychiatry. In press.
6. Savitz J, Preskorn S, Teague TK, et al. Minocycline and aspirin in the treatment of bipolar depression: a protocol for a proof-of-concept, randomised, double-blind, placebo-controlled, 2x2 clinical trial. BMJ Open. 2012;2(1):e000643. doi: 10.1136/bmjopen-2011-000643.
7. Rogers HL, Bhattaram A, Zineh I, et al. CYP2D6 genotype information to guide pimozide treatment in adult and pediatric patients: basis for the U.S. Food and Drug Administration’s new dosing recommendations. J Clin Psychiatry. 2012;73(9):1187-1190.
8. Potkin S, Preskorn S, Hochfeld M, et al. A thorough QTc study of 3 doses of iloperidone including metabolic inhibition via CYP2D6 and/or CYP3A4 inhibition and a comparison to quetiapine and ziprasidone. J Clin Psychopharmacol. 2013;33(1):3-10.
9. Howland RH. Pharmacogenetic testing in psychiatry: not (quite) ready for primetime. J Psychosoc Nurs Ment Health Serv. 2014;52(11):13-16.
10. Rosenblat JD, Lee Y, McIntyre RS. Does pharmacogenomics testing improve clinical outcomes for major depressive disorder? A systematic review of clinical trials and cost-effectiveness studies. J Clin Psychiatry. In press.
11. Nassan M, Nicholson WT, Elliott MA, et al. Pharmacokinetic pharmacogenetic prescribing guidelines for antidepressants: a template for psychiatric precision medicine. Mayo Clin Proc. In press.
12. Altar CA, Carhart JM, Allen JD, et al. Clinical validity: combinatorial pharmacogenomics predicts antidepressant responses and healthcare utilizations better than single gene phenotypes. Pharmacogenomics J. 2015;15(5):443-451.
13. Preskorn S, Kane C, Lobello K, et al. Cytochrome P450 2D6 phenoconversion is common in patients being treated for depression: implications for personalized medicine. J Clin Psychiatry. 2013;74(6):614-621.
14. Niculescu AB, Levey DF, Phalen PL, et al. Understanding and predicting suicidality using a combined genomic and clinical risk assessment approach. Mol Psychiatry. 2015;20(11):1266-1285.
What has been the history of the development of laboratory tests in the field of psychiatry?
During my almost-40-year academic medical career, I have been interested in the development and incorporation of laboratory tests into psychiatry.1 This interest initially focused on therapeutic drug monitoring (TDM) and the genetics of drug responsiveness, with an emphasis on drug metabolism. In addition to TDM—which I have long believed is vastly underutilized in psychiatry—there have been many failed attempts to develop diagnostic tests, including tests to distinguish between what were postulated to be serotonergic and noradrenergic forms of major depression in the 1970s2,3 and the dexamethasone suppression test for melancholia in the 1980s.4 Recently, a 51-analyte immunoassay test was marketed by Rules-Based Medicine, Inc. (RBM), as an aid in the diagnosis of schizophrenia, but the test was found to suffer a high false-positive rate and was withdrawn from the market.5 Given this track record, caution is warranted when examining claims for new tests.
What types of tests are being developed?
Most tests in development are pharmacogenomic (PG)-based or immunoassay (IA)-based.
PG tests examine single nucleotide polymorphisms (SNP) in genes that code for pharmacokinetic mechanisms, primarily cytochrome P450 (CYP) enzymes responsible for drug metabolism and P-glycoprotein, responsible for drug transportation. The next most common type of test examines pharmacodynamic mechanisms, such as SNPs of specific receptor genes, including serotonin (or 5-hydroxytryptophan [5-HT] transporter [SET or 5-HTT]) or the 5-HT2A receptor.
The fact that CYP enzymes lead the list is not surprising: These enzymes and their role in the metabolism of specific drugs have been extensively studied since the late 1980s. Considerable data has been accumulated regarding variants of CYP enzymes, which convey clinically meaningful differences among individuals in terms of their ability to metabolize drug via these pathways. Individuals are commonly divided into 4 phenotypic categories: ultra-rapid, extensive (or normal), intermediate, and poor metabolizers. Based on these phenotypes, clinical consequences can be quantitated in terms of changes in drug concentration, concentration-dependent beneficial or adverse effects, and associated/recommended changes in dosing.
Research into the role of pharmacodynamic variants, however, is still in infancy and more difficult to measure in terms of assessing endpoints, with related limitations in clinical utility.
IA assays generally measure a variety of proteins, particularly those reflecting inflammatory processes (eg, various cytokines, such as interleukin-6).6 As with pharmacodynamic measures, research into the role of inflammatory biomarkers is in early stages. The clinical utility of associated tests is, therefore, less certain; witness the recent study5 I noted that revealed a high false-positive rate for the RBM schizophrenia panel in healthy controls. Nevertheless, considerable research is being conducted in all of these areas so that new developments might lend themselves to greater clinical utility.
(Note that PG biomarkers are trait measures, whereas IA biomarkers are state measures, so that complementary use of both types of tests might prove useful in diagnosis and clinical management. Although such integrative use of these 2 different types of tests generally is not done today.)
What does it take to market these tests?
At a minimum, offering these tests for sale requires that the laboratory be certified by the Centers for Medicare & Medicaid Services, according to the Clinical Laboratory Improvement Amendments (CLIA) standards (www.fda.gov/medicaldevices/deviceregulationandguidance/ivdregulatoryassistance/ucm124105.htm). CLIA-certified laboratories are required to demonstrate the analytical validity of tests that they offer—ie, the accuracy and reliability of the test in measuring a parameter of interest—but not the clinical validity or utility of those tests. The fact that a test in fact measures what it claims to be measuring in and of itself does not mean it has clinical validity or utility (see the discussion below).
Must the FDA approve laboratory tests?
No, but that situation might be changing.
Currently, only tests used in a setting considered high risk—eg, a test intended to detect or diagnose a malignancy or guide its treatment—requires formal FDA approval. The approval of such a test requires submission to the FDA of clinical data supporting its clinical validity and utility, in addition to evidence of analytic validity.
Even in such cases, the degree and quality of the clinical data required are generally not as high as would be required for approval of a drug. That distinction is understandable, given the type and quantity of data necessary for drug approval and the many years and billions of dollars it takes to accumulate such data. For most laboratory tests, providing the same level of data required to have a drug approved would be neither necessary nor feasible given the business model underlying most laboratories providing laboratory tests.
What do ‘clinical validity’ and ‘clinical utility’ mean?
These are higher evidence thresholds than is needed for analytic validity, although the latter is a necessary first step on the path to achieving these higher thresholds.
Clinical validity is the ability of a test to detect:
- a clinically meaningful measure, such as clinical response
- an adverse effect
- a biologically meaningful measure (eg, a drug level or a change in the electrocardiographic pattern).
Above the threshold of clinical validity is clinical utility, which is proof that the test can reliably be used to guide clinical management and thus meaningfully improve outcomes, such as guiding drug or dosage selection.
Is the use of PG testing recommended? If so, in what instances?
Specific types of PG testing is recommended by the FDA recommended. The FDA has been incorporating PG information into the labels of specific medications for several years; the agency has a Web site (www.fda.gov/drugs/scienceresearch/researchareas/pharmacogenetics/ucm083378.htm) that continuously updates this information. The involved drugs are in all therapeutic classes—from oncology to psychiatry.
More than 30 psychotropic drugs have PG information in their label; some of those drugs’ labels contain specific recommendations, such as obtaining PG information before selecting or starting a drug in a specific patient. An example is carbamazepine, for which the recommendation is to obtain HLA testing before starting the drug in patients of Han Chinese ancestry, because members of this large ethnic group are at greater risk of serious dermatologic adverse effects, including Stevens-Johnson syndrome.
In other instances, the recommendation is to do the testing before increasing beyond a specific dose. Examples of psychiatric drugs whose labels contain such PG information include pimozide and iloperidone as well as citalopram. In the FDA-approved label, guidance is provided that these drugs can be started without testing if prescribed at a reduced recommended starting dosage range, rather than the full starting dosage range. The guidance on these drugs further recommends testing for genetic CYP2D6 poor metabolizer (PM) status before dosing above that initial recommended, limited, starting dosage range.
The rationale for this guidance is to reduce the risk that (1) patients in question will achieve an excessively high plasma drug level that can cause significant prolongation of intracardiac conduction (eg, QTc prolongation) and thus (2) develop the potentially fatal arrhythmia torsades de pointes. Guidance is based on thorough QTc studies that were performed on each drug,7,8 which makes them examples of instances in which the test has clinical validity and utility as well as analytical validity.
To find PG labeling in the package insert for these drugs, visit: www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm.
What about data for other tests that are marketed and promoted by developers?
Sometimes, there are—literally—no data on available tests beyond the analytical validity of the test; other times, the amount and quality of clinical data are quite variable, ranging from results of ≥1 small retrospective studies without controls to results of prospective, randomized, controlled studies. Even among the latter, the developer may conduct and analyze their studies without oversight by an independent agency, such as the FDA.
This situation (1) raises concern that study results are not independent of the developer’s business interests and, as one might expect, (2) leads to controversy about whether the data are compelling—or not.9-12
What is a critical difference between PG test results and results of most laboratory tests?
PG tests are, as noted, trait rather than state characteristics. That means that the results do not change except for a phenomenon known as phenocoversion, discussed below. (Of course, advances in gene therapy might make it possible someday to change a person’s genetic makeup and for mitochondrial genes that is already possible.)
For this reason, PG test results should not get buried in the medical record, as might happen with, say, a patient’s serum potassium level at a given point in time. Instead, PG test results need to be carried forward continuously. Results also should be given to the patient as part of his (her) personal health record and to all other health care providers that the patient is seeing or will see in the future. Each health care provider who obtains PG test results should consider sending them to all current clinicians providing care for the patient at the same time as they are.
Is your functional status at a given moment the same as your genetic status?
No. There is a phenomenon known as phenoconversion in which a person’s current functional status may be different from what would be expected based on their genetic status.
CYP2D6 functional status is susceptible to phenoconversion as follows: Administering fluoxetine and paroxetine, for example, at 20 or 40 mg/d converts 66% and 95%, respectively, of patients who are CYP2D6 extensive (ie, normal) metabolizers into phenocopies of people who, genetically, lack the ability to metabolize drugs via CYP2D6 (ie, genotypic CYP2D6 PM). Based on a recent study of 900 participants in routine clinical care who were taking an antidepressant, 4% of the general U.S. population are genetically CYP2D6 PM; an additional 24% are phenotypically CYP2D6 PM because of concomitant administration of a CYP2D6 substantial inhibitor, such as bupropion, fluoxetine, paroxetine, or terbenafine.13
That is the reason a provider needs to know what drugs a patient is taking concomitantly—to consider the possibility of phenoconversion and, when necessary, to dose accordingly.
What does the future hold?
Development of tests for use in psychiatric practice is likely to grow substantially, for at least 2 reasons:
- There is a huge unmet need for clinically meaningful tests to aid in the provision of optimal patient care and, therefore, a tremendous business opportunity
- Knowledge in the biological basis of psychiatric disorders is growing exponentially; with that knowledge comes the ability to develop new tests.
A recent example comes from a research group that devised a test that could predict suicidality.14 Time will tell whether this test or a derivative of it enters practice. Nevertheless, it is a harbinger of the likely dramatic changes in the landscape of clinical medicine particularly as it applies to psychiatry.
Given these developments, the syndromic diagnoses in DSM-5 will in the future likely be replaced by a new diagnostic schema that breaks down existing heterogenous syndromic diagnoses into pathophysiologically and etiologically meaningful entities using insights gained from genetic and biomarker data as well as functional brain imaging. Theoretically, those insights will lead to new modalities of treatment, including somatic treatments that target novel mechanisms of action, coupled to more effective psychosocial therapies—with both therapies guided by diagnostic tests to monitor response to specific treatment interventions.
During this transition from the past to the future, answers to the questions I’ve posed here about laboratory testing in psychiatry will, I hope, help the practitioner understand, evaluate, and incorporate these changes readily into practice.
What has been the history of the development of laboratory tests in the field of psychiatry?
During my almost-40-year academic medical career, I have been interested in the development and incorporation of laboratory tests into psychiatry.1 This interest initially focused on therapeutic drug monitoring (TDM) and the genetics of drug responsiveness, with an emphasis on drug metabolism. In addition to TDM—which I have long believed is vastly underutilized in psychiatry—there have been many failed attempts to develop diagnostic tests, including tests to distinguish between what were postulated to be serotonergic and noradrenergic forms of major depression in the 1970s2,3 and the dexamethasone suppression test for melancholia in the 1980s.4 Recently, a 51-analyte immunoassay test was marketed by Rules-Based Medicine, Inc. (RBM), as an aid in the diagnosis of schizophrenia, but the test was found to suffer a high false-positive rate and was withdrawn from the market.5 Given this track record, caution is warranted when examining claims for new tests.
What types of tests are being developed?
Most tests in development are pharmacogenomic (PG)-based or immunoassay (IA)-based.
PG tests examine single nucleotide polymorphisms (SNP) in genes that code for pharmacokinetic mechanisms, primarily cytochrome P450 (CYP) enzymes responsible for drug metabolism and P-glycoprotein, responsible for drug transportation. The next most common type of test examines pharmacodynamic mechanisms, such as SNPs of specific receptor genes, including serotonin (or 5-hydroxytryptophan [5-HT] transporter [SET or 5-HTT]) or the 5-HT2A receptor.
The fact that CYP enzymes lead the list is not surprising: These enzymes and their role in the metabolism of specific drugs have been extensively studied since the late 1980s. Considerable data has been accumulated regarding variants of CYP enzymes, which convey clinically meaningful differences among individuals in terms of their ability to metabolize drug via these pathways. Individuals are commonly divided into 4 phenotypic categories: ultra-rapid, extensive (or normal), intermediate, and poor metabolizers. Based on these phenotypes, clinical consequences can be quantitated in terms of changes in drug concentration, concentration-dependent beneficial or adverse effects, and associated/recommended changes in dosing.
Research into the role of pharmacodynamic variants, however, is still in infancy and more difficult to measure in terms of assessing endpoints, with related limitations in clinical utility.
IA assays generally measure a variety of proteins, particularly those reflecting inflammatory processes (eg, various cytokines, such as interleukin-6).6 As with pharmacodynamic measures, research into the role of inflammatory biomarkers is in early stages. The clinical utility of associated tests is, therefore, less certain; witness the recent study5 I noted that revealed a high false-positive rate for the RBM schizophrenia panel in healthy controls. Nevertheless, considerable research is being conducted in all of these areas so that new developments might lend themselves to greater clinical utility.
(Note that PG biomarkers are trait measures, whereas IA biomarkers are state measures, so that complementary use of both types of tests might prove useful in diagnosis and clinical management. Although such integrative use of these 2 different types of tests generally is not done today.)
What does it take to market these tests?
At a minimum, offering these tests for sale requires that the laboratory be certified by the Centers for Medicare & Medicaid Services, according to the Clinical Laboratory Improvement Amendments (CLIA) standards (www.fda.gov/medicaldevices/deviceregulationandguidance/ivdregulatoryassistance/ucm124105.htm). CLIA-certified laboratories are required to demonstrate the analytical validity of tests that they offer—ie, the accuracy and reliability of the test in measuring a parameter of interest—but not the clinical validity or utility of those tests. The fact that a test in fact measures what it claims to be measuring in and of itself does not mean it has clinical validity or utility (see the discussion below).
Must the FDA approve laboratory tests?
No, but that situation might be changing.
Currently, only tests used in a setting considered high risk—eg, a test intended to detect or diagnose a malignancy or guide its treatment—requires formal FDA approval. The approval of such a test requires submission to the FDA of clinical data supporting its clinical validity and utility, in addition to evidence of analytic validity.
Even in such cases, the degree and quality of the clinical data required are generally not as high as would be required for approval of a drug. That distinction is understandable, given the type and quantity of data necessary for drug approval and the many years and billions of dollars it takes to accumulate such data. For most laboratory tests, providing the same level of data required to have a drug approved would be neither necessary nor feasible given the business model underlying most laboratories providing laboratory tests.
What do ‘clinical validity’ and ‘clinical utility’ mean?
These are higher evidence thresholds than is needed for analytic validity, although the latter is a necessary first step on the path to achieving these higher thresholds.
Clinical validity is the ability of a test to detect:
- a clinically meaningful measure, such as clinical response
- an adverse effect
- a biologically meaningful measure (eg, a drug level or a change in the electrocardiographic pattern).
Above the threshold of clinical validity is clinical utility, which is proof that the test can reliably be used to guide clinical management and thus meaningfully improve outcomes, such as guiding drug or dosage selection.
Is the use of PG testing recommended? If so, in what instances?
Specific types of PG testing is recommended by the FDA recommended. The FDA has been incorporating PG information into the labels of specific medications for several years; the agency has a Web site (www.fda.gov/drugs/scienceresearch/researchareas/pharmacogenetics/ucm083378.htm) that continuously updates this information. The involved drugs are in all therapeutic classes—from oncology to psychiatry.
More than 30 psychotropic drugs have PG information in their label; some of those drugs’ labels contain specific recommendations, such as obtaining PG information before selecting or starting a drug in a specific patient. An example is carbamazepine, for which the recommendation is to obtain HLA testing before starting the drug in patients of Han Chinese ancestry, because members of this large ethnic group are at greater risk of serious dermatologic adverse effects, including Stevens-Johnson syndrome.
In other instances, the recommendation is to do the testing before increasing beyond a specific dose. Examples of psychiatric drugs whose labels contain such PG information include pimozide and iloperidone as well as citalopram. In the FDA-approved label, guidance is provided that these drugs can be started without testing if prescribed at a reduced recommended starting dosage range, rather than the full starting dosage range. The guidance on these drugs further recommends testing for genetic CYP2D6 poor metabolizer (PM) status before dosing above that initial recommended, limited, starting dosage range.
The rationale for this guidance is to reduce the risk that (1) patients in question will achieve an excessively high plasma drug level that can cause significant prolongation of intracardiac conduction (eg, QTc prolongation) and thus (2) develop the potentially fatal arrhythmia torsades de pointes. Guidance is based on thorough QTc studies that were performed on each drug,7,8 which makes them examples of instances in which the test has clinical validity and utility as well as analytical validity.
To find PG labeling in the package insert for these drugs, visit: www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm.
What about data for other tests that are marketed and promoted by developers?
Sometimes, there are—literally—no data on available tests beyond the analytical validity of the test; other times, the amount and quality of clinical data are quite variable, ranging from results of ≥1 small retrospective studies without controls to results of prospective, randomized, controlled studies. Even among the latter, the developer may conduct and analyze their studies without oversight by an independent agency, such as the FDA.
This situation (1) raises concern that study results are not independent of the developer’s business interests and, as one might expect, (2) leads to controversy about whether the data are compelling—or not.9-12
What is a critical difference between PG test results and results of most laboratory tests?
PG tests are, as noted, trait rather than state characteristics. That means that the results do not change except for a phenomenon known as phenocoversion, discussed below. (Of course, advances in gene therapy might make it possible someday to change a person’s genetic makeup and for mitochondrial genes that is already possible.)
For this reason, PG test results should not get buried in the medical record, as might happen with, say, a patient’s serum potassium level at a given point in time. Instead, PG test results need to be carried forward continuously. Results also should be given to the patient as part of his (her) personal health record and to all other health care providers that the patient is seeing or will see in the future. Each health care provider who obtains PG test results should consider sending them to all current clinicians providing care for the patient at the same time as they are.
Is your functional status at a given moment the same as your genetic status?
No. There is a phenomenon known as phenoconversion in which a person’s current functional status may be different from what would be expected based on their genetic status.
CYP2D6 functional status is susceptible to phenoconversion as follows: Administering fluoxetine and paroxetine, for example, at 20 or 40 mg/d converts 66% and 95%, respectively, of patients who are CYP2D6 extensive (ie, normal) metabolizers into phenocopies of people who, genetically, lack the ability to metabolize drugs via CYP2D6 (ie, genotypic CYP2D6 PM). Based on a recent study of 900 participants in routine clinical care who were taking an antidepressant, 4% of the general U.S. population are genetically CYP2D6 PM; an additional 24% are phenotypically CYP2D6 PM because of concomitant administration of a CYP2D6 substantial inhibitor, such as bupropion, fluoxetine, paroxetine, or terbenafine.13
That is the reason a provider needs to know what drugs a patient is taking concomitantly—to consider the possibility of phenoconversion and, when necessary, to dose accordingly.
What does the future hold?
Development of tests for use in psychiatric practice is likely to grow substantially, for at least 2 reasons:
- There is a huge unmet need for clinically meaningful tests to aid in the provision of optimal patient care and, therefore, a tremendous business opportunity
- Knowledge in the biological basis of psychiatric disorders is growing exponentially; with that knowledge comes the ability to develop new tests.
A recent example comes from a research group that devised a test that could predict suicidality.14 Time will tell whether this test or a derivative of it enters practice. Nevertheless, it is a harbinger of the likely dramatic changes in the landscape of clinical medicine particularly as it applies to psychiatry.
Given these developments, the syndromic diagnoses in DSM-5 will in the future likely be replaced by a new diagnostic schema that breaks down existing heterogenous syndromic diagnoses into pathophysiologically and etiologically meaningful entities using insights gained from genetic and biomarker data as well as functional brain imaging. Theoretically, those insights will lead to new modalities of treatment, including somatic treatments that target novel mechanisms of action, coupled to more effective psychosocial therapies—with both therapies guided by diagnostic tests to monitor response to specific treatment interventions.
During this transition from the past to the future, answers to the questions I’ve posed here about laboratory testing in psychiatry will, I hope, help the practitioner understand, evaluate, and incorporate these changes readily into practice.
1. Preskorn SH, Biggs JT. Use of tricyclic antidepressant blood levels. N Engl J Med. 1978;298(3):166.
2. Schildkraut JJ. Biogenic amines and affective disorders. Annu Rev Med. 1974;25(0):333-348.
3. Maas JW. Biogenic amines and depression. Biochemical and pharmacological separation of two types of depression. Arch Gen Psychiatry. 1975;32(11):1357-1361.
4. Carroll BJ, Feinberg M, Greden JF, et al. A specific laboratory test for the diagnosis of melancholia. Standardization, validation, and clinical utility. Arch Gen Psychiatry. 1981;38(1):15-22.
5. Wehler C, Preskorn S. High false-positive rate of a putative biomarker test to aid in the diagnosis of schizophrenia. J Clin Psychiatry. In press.
6. Savitz J, Preskorn S, Teague TK, et al. Minocycline and aspirin in the treatment of bipolar depression: a protocol for a proof-of-concept, randomised, double-blind, placebo-controlled, 2x2 clinical trial. BMJ Open. 2012;2(1):e000643. doi: 10.1136/bmjopen-2011-000643.
7. Rogers HL, Bhattaram A, Zineh I, et al. CYP2D6 genotype information to guide pimozide treatment in adult and pediatric patients: basis for the U.S. Food and Drug Administration’s new dosing recommendations. J Clin Psychiatry. 2012;73(9):1187-1190.
8. Potkin S, Preskorn S, Hochfeld M, et al. A thorough QTc study of 3 doses of iloperidone including metabolic inhibition via CYP2D6 and/or CYP3A4 inhibition and a comparison to quetiapine and ziprasidone. J Clin Psychopharmacol. 2013;33(1):3-10.
9. Howland RH. Pharmacogenetic testing in psychiatry: not (quite) ready for primetime. J Psychosoc Nurs Ment Health Serv. 2014;52(11):13-16.
10. Rosenblat JD, Lee Y, McIntyre RS. Does pharmacogenomics testing improve clinical outcomes for major depressive disorder? A systematic review of clinical trials and cost-effectiveness studies. J Clin Psychiatry. In press.
11. Nassan M, Nicholson WT, Elliott MA, et al. Pharmacokinetic pharmacogenetic prescribing guidelines for antidepressants: a template for psychiatric precision medicine. Mayo Clin Proc. In press.
12. Altar CA, Carhart JM, Allen JD, et al. Clinical validity: combinatorial pharmacogenomics predicts antidepressant responses and healthcare utilizations better than single gene phenotypes. Pharmacogenomics J. 2015;15(5):443-451.
13. Preskorn S, Kane C, Lobello K, et al. Cytochrome P450 2D6 phenoconversion is common in patients being treated for depression: implications for personalized medicine. J Clin Psychiatry. 2013;74(6):614-621.
14. Niculescu AB, Levey DF, Phalen PL, et al. Understanding and predicting suicidality using a combined genomic and clinical risk assessment approach. Mol Psychiatry. 2015;20(11):1266-1285.
1. Preskorn SH, Biggs JT. Use of tricyclic antidepressant blood levels. N Engl J Med. 1978;298(3):166.
2. Schildkraut JJ. Biogenic amines and affective disorders. Annu Rev Med. 1974;25(0):333-348.
3. Maas JW. Biogenic amines and depression. Biochemical and pharmacological separation of two types of depression. Arch Gen Psychiatry. 1975;32(11):1357-1361.
4. Carroll BJ, Feinberg M, Greden JF, et al. A specific laboratory test for the diagnosis of melancholia. Standardization, validation, and clinical utility. Arch Gen Psychiatry. 1981;38(1):15-22.
5. Wehler C, Preskorn S. High false-positive rate of a putative biomarker test to aid in the diagnosis of schizophrenia. J Clin Psychiatry. In press.
6. Savitz J, Preskorn S, Teague TK, et al. Minocycline and aspirin in the treatment of bipolar depression: a protocol for a proof-of-concept, randomised, double-blind, placebo-controlled, 2x2 clinical trial. BMJ Open. 2012;2(1):e000643. doi: 10.1136/bmjopen-2011-000643.
7. Rogers HL, Bhattaram A, Zineh I, et al. CYP2D6 genotype information to guide pimozide treatment in adult and pediatric patients: basis for the U.S. Food and Drug Administration’s new dosing recommendations. J Clin Psychiatry. 2012;73(9):1187-1190.
8. Potkin S, Preskorn S, Hochfeld M, et al. A thorough QTc study of 3 doses of iloperidone including metabolic inhibition via CYP2D6 and/or CYP3A4 inhibition and a comparison to quetiapine and ziprasidone. J Clin Psychopharmacol. 2013;33(1):3-10.
9. Howland RH. Pharmacogenetic testing in psychiatry: not (quite) ready for primetime. J Psychosoc Nurs Ment Health Serv. 2014;52(11):13-16.
10. Rosenblat JD, Lee Y, McIntyre RS. Does pharmacogenomics testing improve clinical outcomes for major depressive disorder? A systematic review of clinical trials and cost-effectiveness studies. J Clin Psychiatry. In press.
11. Nassan M, Nicholson WT, Elliott MA, et al. Pharmacokinetic pharmacogenetic prescribing guidelines for antidepressants: a template for psychiatric precision medicine. Mayo Clin Proc. In press.
12. Altar CA, Carhart JM, Allen JD, et al. Clinical validity: combinatorial pharmacogenomics predicts antidepressant responses and healthcare utilizations better than single gene phenotypes. Pharmacogenomics J. 2015;15(5):443-451.
13. Preskorn S, Kane C, Lobello K, et al. Cytochrome P450 2D6 phenoconversion is common in patients being treated for depression: implications for personalized medicine. J Clin Psychiatry. 2013;74(6):614-621.
14. Niculescu AB, Levey DF, Phalen PL, et al. Understanding and predicting suicidality using a combined genomic and clinical risk assessment approach. Mol Psychiatry. 2015;20(11):1266-1285.
The Death of Altruism, or, Can I Get a Preceptor, Please?
If you ask most physicians, PAs, and NPs why they decided to pursue a career in health care, the clichéd and somewhat unwavering response usually includes some variation of “I want to help people.” Although this view is extolled and encouraged, today’s complicated (and at times competitive) practice of medicine has a way of robbing us of that altruism.
What prompted these musings? The other day, the PA program at our university received a letter from a physician group that, for years, has provided us with quality preceptors for our students. The gist of the letter was to inform us that they could no longer take our PA students, because the time spent precepting resulted in reduced productivity and the compensation offered was not enough to offset that loss.
Now, don’t get me wrong, I understand. In today’s “corporate” mentality, productivity makes or breaks a practice. But we have also seen the number of PAs, NPs, and physicians—including alumni of our program—who offer themselves as preceptors dwindle.
Why is this happening, and what can we do about it? With more than 200 PA and 350 NP programs (and counting), the pressure to find and maintain clinical rotation sites and preceptors for thousands of PA and NP students will continue to increase.
We might first consider critical aspects of our “contract” with society—the one that defines our professionalism. Once we take that oath (whether the Hippocratic or another developed by and for our professions), we vow to put patient welfare first. In return for your service to patients, your contribution to the public good, and your assurance of competence and a high level of morality, integrity, accountability, transparency, and objective advice … you will be conferred very significant privileges as a PA or an NP. Those privileges include trust, self-regulation, participation in public policy, funding for what we value in both patient care and medical research, and many personal rewards.
However, there is one other stipulation: The social contract calls for altruistic service. But altruism specifies that one’s deeds benefit the recipient and not necessarily one’s self. The word altruism is derived from Latin through French and refers to “the good of others as the end of moral action.” Auguste Comte, a French philosopher, coined the term. He believed that individuals had a moral obligation to reject self-interest and live for others.1
I am not sure that is possible in today’s society. Can you “altruistically” relinquish your personal interests, your autonomy, and the primary reason for your existence—life, liberty, and the pursuit of happiness? My answer is maybe not. On the other hand, selfishness becomes a virtue when your own happiness is tied to service.
But enough philosophizing. What does this mean for us? A preceptor is one “who teaches, counsels, inspires, serves as a role model, and supports the growth and development of an individual (the novice) for a fixed and limited amount of time, with the specific purpose of socializing the novice into a new role.”2 A preceptor fills three primary roles: nurturer, educator, and mentor. He or she guides and enhances the learning experience of students by providing ideas, information, resources, and feedback.
I would argue that altruism is as important in giving back to our professions as is quality patient care. Think back to your student days (which may be a ways back for some of us). Remember anxiously entering the clinic or hospital on your first day and nervously approaching the front desk to ask for your assigned preceptor, who would play a key role in your transition?
All new professionals acclimate themselves with guidance from preceptors. We all have favorite preceptors and cherished experiences. Our preceptors were dedicated to our success and gladly gave of their time for the betterment of the profession. I am fearful that today’s preceptors do not have the same dedication to students. But please, tell me I am wrong!
Continue for multifactorial barriers >>
In fairness, I do think there are multifactorial barriers facing today’s preceptors. Discussion of this issue with colleagues produced the following reasons for the changes we’ve seen:
Preceptor skill level. As educators, we may have failed in our efforts to teach our preceptors how to mentor or precept. Some may not be confident enough in their own skills to be able to precept—or perhaps they are just indifferent.
Time and resources. Time and reimbursement are factors in almost every aspect of health care nowadays. It’s just not possible to say “yes” as often as we used to; the pressure to see patients and maintain or bolster the bottom line is immense. It may be that younger generations of clinicians would be willing to precept, if not for the difficulties associated with reimbursement and lack of time.
Income is now often based on production: the more you see, the more you are paid. With the complexities of ICD-10, the documentation process is much more complex. And then there’s the need to document everything thoroughly in the hopes of allaying litigation somewhere down the road. And so on … What it comes down to is that if the preceptor is unable to see the required number of patients because of time spent precepting a student, many administrators will not support the concept of “giving back.”
Financial compensation for precepting. Paying preceptors is now the norm rather than the exception. The time and effort spent is certainly worth compensation, but with decreasing education dollars, the overall quality of PA and NP education could suffer. Tuition is already high and, whether we like it or not, the cost of paying preceptors will eventually roll over into increased fees and/or tuition to the student.
There is also the issue of who gets the compensation—the institution or the preceptor—which causes some angst among preceptors. Some clinical departments or institutions are paid a lump sum to allow for released time for the provider; others allow the provider to be compensated directly.3 One group I am aware of decided to split the compensation evenly, as they felt this would be the most equitable solution.
Lastly, the amount of compensation varies. This can create a competitive atmosphere as programs “bid” against one another to secure precepting services.
Student preparation. Taking on students is a sacrifice, and the preceptor rightfully should expect them to be ready and willing to learn. At times, there is lack of preparation on the part of the student to be ready to dive in and see patients and not take all day!
But a bigger issue, I think, is that we don’t seem to instill in the new generations of professionals the responsibility to give back to our professions. So many barriers to our professions have been mitigated—we have recognition, reimbursement, and prescriptive authority, as well as the respect of our colleagues—that today’s students don’t have the “fights to fight” that we did. I think that contributes to the decreased dedication (beyond that to the patients, that is). Maybe too, we viewed our choice of profession as a calling—a vocation in which you have to give back to those who come after you—whereas many people today see it as just a job.
Practice is too specialized. More and more PAs and NPs practice in specialties (and some in subspecialties). This means they may not be equipped to offer the generalist or basic specialty clinical education required by the program.
So, yes, the decline of altruism may be a sign of our changing times. But nonetheless, we need to prepare our NP and PA students to step into real-world roles and provide quality patient care. We must identify solutions to this problem, because we cannot simply “drop” precepting or clinical rotations. So what can we do?
Many PA and NP programs have worked hard to develop relationships with preceptors and institutions that support the process. Here are some ways to foster those relationships and bring back the altruism needed to precept in today’s society:
• Seek nonfinancial incentives for preceptors, such as Category 1 CE/CME credit, discounts for conference attendance, opportunities for academic advancement (eg, adjunct clinical professor status), and access to resources via the university library.
• Advocate for a state Preceptor Tax Incentive Program (such as the one in the state of Georgia).
• Reduce the (often copious) amount of paperwork that educational institutions require of preceptors.
We also need to ensure that our students are adequately prepared. In the past decade, students enter our programs with high expectations of what they will get out of a clinical rotation and a preceptor. Some may lack a touch of humility or a servant’s mind, both of which are important to their success.
An NP colleague recently decided to take a “student vacation” for six months, as her opinion was that many of the students she had precepted were not well prepared, either academically or in work/life experience. Her assessment of increasing class sizes was that some students are there “just to fill the program up” and make it profitable. To my colleague’s way of thinking, there is a disservice being done to students who are on rotations and not ready for them. We need to make sure our students are prepared to face the challenges of clinical rotations.
We also need to encourage a renewed sense of altruism in our students, so that they feel compelled to precept when they are alumni. So how do we enhance our preparation of future clinicians through precepting? I would love to hear from you about barriers you have encountered—and preferably, some viable solutions! You can reach me at [email protected].
References
1. Altruism: ethics. Encyclopaedia Britannica. www.britannica.com/topic/altruism-ethics. Accessed March 10, 2016.
2. Morrow KL. Preceptorships in Nursing Staff Development. Rockville, MD: Aspen Systems Corp; 1984.
3. Physician Assistant Education Association. Payment of clinical sites and preceptors in PA education [issue brief]. http://paeaonline.org/wp-content/uploads/2015/09/PaymentClinicalSites-PreceptorsPAEducation.pdf. Accessed March 10, 2016.
If you ask most physicians, PAs, and NPs why they decided to pursue a career in health care, the clichéd and somewhat unwavering response usually includes some variation of “I want to help people.” Although this view is extolled and encouraged, today’s complicated (and at times competitive) practice of medicine has a way of robbing us of that altruism.
What prompted these musings? The other day, the PA program at our university received a letter from a physician group that, for years, has provided us with quality preceptors for our students. The gist of the letter was to inform us that they could no longer take our PA students, because the time spent precepting resulted in reduced productivity and the compensation offered was not enough to offset that loss.
Now, don’t get me wrong, I understand. In today’s “corporate” mentality, productivity makes or breaks a practice. But we have also seen the number of PAs, NPs, and physicians—including alumni of our program—who offer themselves as preceptors dwindle.
Why is this happening, and what can we do about it? With more than 200 PA and 350 NP programs (and counting), the pressure to find and maintain clinical rotation sites and preceptors for thousands of PA and NP students will continue to increase.
We might first consider critical aspects of our “contract” with society—the one that defines our professionalism. Once we take that oath (whether the Hippocratic or another developed by and for our professions), we vow to put patient welfare first. In return for your service to patients, your contribution to the public good, and your assurance of competence and a high level of morality, integrity, accountability, transparency, and objective advice … you will be conferred very significant privileges as a PA or an NP. Those privileges include trust, self-regulation, participation in public policy, funding for what we value in both patient care and medical research, and many personal rewards.
However, there is one other stipulation: The social contract calls for altruistic service. But altruism specifies that one’s deeds benefit the recipient and not necessarily one’s self. The word altruism is derived from Latin through French and refers to “the good of others as the end of moral action.” Auguste Comte, a French philosopher, coined the term. He believed that individuals had a moral obligation to reject self-interest and live for others.1
I am not sure that is possible in today’s society. Can you “altruistically” relinquish your personal interests, your autonomy, and the primary reason for your existence—life, liberty, and the pursuit of happiness? My answer is maybe not. On the other hand, selfishness becomes a virtue when your own happiness is tied to service.
But enough philosophizing. What does this mean for us? A preceptor is one “who teaches, counsels, inspires, serves as a role model, and supports the growth and development of an individual (the novice) for a fixed and limited amount of time, with the specific purpose of socializing the novice into a new role.”2 A preceptor fills three primary roles: nurturer, educator, and mentor. He or she guides and enhances the learning experience of students by providing ideas, information, resources, and feedback.
I would argue that altruism is as important in giving back to our professions as is quality patient care. Think back to your student days (which may be a ways back for some of us). Remember anxiously entering the clinic or hospital on your first day and nervously approaching the front desk to ask for your assigned preceptor, who would play a key role in your transition?
All new professionals acclimate themselves with guidance from preceptors. We all have favorite preceptors and cherished experiences. Our preceptors were dedicated to our success and gladly gave of their time for the betterment of the profession. I am fearful that today’s preceptors do not have the same dedication to students. But please, tell me I am wrong!
Continue for multifactorial barriers >>
In fairness, I do think there are multifactorial barriers facing today’s preceptors. Discussion of this issue with colleagues produced the following reasons for the changes we’ve seen:
Preceptor skill level. As educators, we may have failed in our efforts to teach our preceptors how to mentor or precept. Some may not be confident enough in their own skills to be able to precept—or perhaps they are just indifferent.
Time and resources. Time and reimbursement are factors in almost every aspect of health care nowadays. It’s just not possible to say “yes” as often as we used to; the pressure to see patients and maintain or bolster the bottom line is immense. It may be that younger generations of clinicians would be willing to precept, if not for the difficulties associated with reimbursement and lack of time.
Income is now often based on production: the more you see, the more you are paid. With the complexities of ICD-10, the documentation process is much more complex. And then there’s the need to document everything thoroughly in the hopes of allaying litigation somewhere down the road. And so on … What it comes down to is that if the preceptor is unable to see the required number of patients because of time spent precepting a student, many administrators will not support the concept of “giving back.”
Financial compensation for precepting. Paying preceptors is now the norm rather than the exception. The time and effort spent is certainly worth compensation, but with decreasing education dollars, the overall quality of PA and NP education could suffer. Tuition is already high and, whether we like it or not, the cost of paying preceptors will eventually roll over into increased fees and/or tuition to the student.
There is also the issue of who gets the compensation—the institution or the preceptor—which causes some angst among preceptors. Some clinical departments or institutions are paid a lump sum to allow for released time for the provider; others allow the provider to be compensated directly.3 One group I am aware of decided to split the compensation evenly, as they felt this would be the most equitable solution.
Lastly, the amount of compensation varies. This can create a competitive atmosphere as programs “bid” against one another to secure precepting services.
Student preparation. Taking on students is a sacrifice, and the preceptor rightfully should expect them to be ready and willing to learn. At times, there is lack of preparation on the part of the student to be ready to dive in and see patients and not take all day!
But a bigger issue, I think, is that we don’t seem to instill in the new generations of professionals the responsibility to give back to our professions. So many barriers to our professions have been mitigated—we have recognition, reimbursement, and prescriptive authority, as well as the respect of our colleagues—that today’s students don’t have the “fights to fight” that we did. I think that contributes to the decreased dedication (beyond that to the patients, that is). Maybe too, we viewed our choice of profession as a calling—a vocation in which you have to give back to those who come after you—whereas many people today see it as just a job.
Practice is too specialized. More and more PAs and NPs practice in specialties (and some in subspecialties). This means they may not be equipped to offer the generalist or basic specialty clinical education required by the program.
So, yes, the decline of altruism may be a sign of our changing times. But nonetheless, we need to prepare our NP and PA students to step into real-world roles and provide quality patient care. We must identify solutions to this problem, because we cannot simply “drop” precepting or clinical rotations. So what can we do?
Many PA and NP programs have worked hard to develop relationships with preceptors and institutions that support the process. Here are some ways to foster those relationships and bring back the altruism needed to precept in today’s society:
• Seek nonfinancial incentives for preceptors, such as Category 1 CE/CME credit, discounts for conference attendance, opportunities for academic advancement (eg, adjunct clinical professor status), and access to resources via the university library.
• Advocate for a state Preceptor Tax Incentive Program (such as the one in the state of Georgia).
• Reduce the (often copious) amount of paperwork that educational institutions require of preceptors.
We also need to ensure that our students are adequately prepared. In the past decade, students enter our programs with high expectations of what they will get out of a clinical rotation and a preceptor. Some may lack a touch of humility or a servant’s mind, both of which are important to their success.
An NP colleague recently decided to take a “student vacation” for six months, as her opinion was that many of the students she had precepted were not well prepared, either academically or in work/life experience. Her assessment of increasing class sizes was that some students are there “just to fill the program up” and make it profitable. To my colleague’s way of thinking, there is a disservice being done to students who are on rotations and not ready for them. We need to make sure our students are prepared to face the challenges of clinical rotations.
We also need to encourage a renewed sense of altruism in our students, so that they feel compelled to precept when they are alumni. So how do we enhance our preparation of future clinicians through precepting? I would love to hear from you about barriers you have encountered—and preferably, some viable solutions! You can reach me at [email protected].
References
1. Altruism: ethics. Encyclopaedia Britannica. www.britannica.com/topic/altruism-ethics. Accessed March 10, 2016.
2. Morrow KL. Preceptorships in Nursing Staff Development. Rockville, MD: Aspen Systems Corp; 1984.
3. Physician Assistant Education Association. Payment of clinical sites and preceptors in PA education [issue brief]. http://paeaonline.org/wp-content/uploads/2015/09/PaymentClinicalSites-PreceptorsPAEducation.pdf. Accessed March 10, 2016.
If you ask most physicians, PAs, and NPs why they decided to pursue a career in health care, the clichéd and somewhat unwavering response usually includes some variation of “I want to help people.” Although this view is extolled and encouraged, today’s complicated (and at times competitive) practice of medicine has a way of robbing us of that altruism.
What prompted these musings? The other day, the PA program at our university received a letter from a physician group that, for years, has provided us with quality preceptors for our students. The gist of the letter was to inform us that they could no longer take our PA students, because the time spent precepting resulted in reduced productivity and the compensation offered was not enough to offset that loss.
Now, don’t get me wrong, I understand. In today’s “corporate” mentality, productivity makes or breaks a practice. But we have also seen the number of PAs, NPs, and physicians—including alumni of our program—who offer themselves as preceptors dwindle.
Why is this happening, and what can we do about it? With more than 200 PA and 350 NP programs (and counting), the pressure to find and maintain clinical rotation sites and preceptors for thousands of PA and NP students will continue to increase.
We might first consider critical aspects of our “contract” with society—the one that defines our professionalism. Once we take that oath (whether the Hippocratic or another developed by and for our professions), we vow to put patient welfare first. In return for your service to patients, your contribution to the public good, and your assurance of competence and a high level of morality, integrity, accountability, transparency, and objective advice … you will be conferred very significant privileges as a PA or an NP. Those privileges include trust, self-regulation, participation in public policy, funding for what we value in both patient care and medical research, and many personal rewards.
However, there is one other stipulation: The social contract calls for altruistic service. But altruism specifies that one’s deeds benefit the recipient and not necessarily one’s self. The word altruism is derived from Latin through French and refers to “the good of others as the end of moral action.” Auguste Comte, a French philosopher, coined the term. He believed that individuals had a moral obligation to reject self-interest and live for others.1
I am not sure that is possible in today’s society. Can you “altruistically” relinquish your personal interests, your autonomy, and the primary reason for your existence—life, liberty, and the pursuit of happiness? My answer is maybe not. On the other hand, selfishness becomes a virtue when your own happiness is tied to service.
But enough philosophizing. What does this mean for us? A preceptor is one “who teaches, counsels, inspires, serves as a role model, and supports the growth and development of an individual (the novice) for a fixed and limited amount of time, with the specific purpose of socializing the novice into a new role.”2 A preceptor fills three primary roles: nurturer, educator, and mentor. He or she guides and enhances the learning experience of students by providing ideas, information, resources, and feedback.
I would argue that altruism is as important in giving back to our professions as is quality patient care. Think back to your student days (which may be a ways back for some of us). Remember anxiously entering the clinic or hospital on your first day and nervously approaching the front desk to ask for your assigned preceptor, who would play a key role in your transition?
All new professionals acclimate themselves with guidance from preceptors. We all have favorite preceptors and cherished experiences. Our preceptors were dedicated to our success and gladly gave of their time for the betterment of the profession. I am fearful that today’s preceptors do not have the same dedication to students. But please, tell me I am wrong!
Continue for multifactorial barriers >>
In fairness, I do think there are multifactorial barriers facing today’s preceptors. Discussion of this issue with colleagues produced the following reasons for the changes we’ve seen:
Preceptor skill level. As educators, we may have failed in our efforts to teach our preceptors how to mentor or precept. Some may not be confident enough in their own skills to be able to precept—or perhaps they are just indifferent.
Time and resources. Time and reimbursement are factors in almost every aspect of health care nowadays. It’s just not possible to say “yes” as often as we used to; the pressure to see patients and maintain or bolster the bottom line is immense. It may be that younger generations of clinicians would be willing to precept, if not for the difficulties associated with reimbursement and lack of time.
Income is now often based on production: the more you see, the more you are paid. With the complexities of ICD-10, the documentation process is much more complex. And then there’s the need to document everything thoroughly in the hopes of allaying litigation somewhere down the road. And so on … What it comes down to is that if the preceptor is unable to see the required number of patients because of time spent precepting a student, many administrators will not support the concept of “giving back.”
Financial compensation for precepting. Paying preceptors is now the norm rather than the exception. The time and effort spent is certainly worth compensation, but with decreasing education dollars, the overall quality of PA and NP education could suffer. Tuition is already high and, whether we like it or not, the cost of paying preceptors will eventually roll over into increased fees and/or tuition to the student.
There is also the issue of who gets the compensation—the institution or the preceptor—which causes some angst among preceptors. Some clinical departments or institutions are paid a lump sum to allow for released time for the provider; others allow the provider to be compensated directly.3 One group I am aware of decided to split the compensation evenly, as they felt this would be the most equitable solution.
Lastly, the amount of compensation varies. This can create a competitive atmosphere as programs “bid” against one another to secure precepting services.
Student preparation. Taking on students is a sacrifice, and the preceptor rightfully should expect them to be ready and willing to learn. At times, there is lack of preparation on the part of the student to be ready to dive in and see patients and not take all day!
But a bigger issue, I think, is that we don’t seem to instill in the new generations of professionals the responsibility to give back to our professions. So many barriers to our professions have been mitigated—we have recognition, reimbursement, and prescriptive authority, as well as the respect of our colleagues—that today’s students don’t have the “fights to fight” that we did. I think that contributes to the decreased dedication (beyond that to the patients, that is). Maybe too, we viewed our choice of profession as a calling—a vocation in which you have to give back to those who come after you—whereas many people today see it as just a job.
Practice is too specialized. More and more PAs and NPs practice in specialties (and some in subspecialties). This means they may not be equipped to offer the generalist or basic specialty clinical education required by the program.
So, yes, the decline of altruism may be a sign of our changing times. But nonetheless, we need to prepare our NP and PA students to step into real-world roles and provide quality patient care. We must identify solutions to this problem, because we cannot simply “drop” precepting or clinical rotations. So what can we do?
Many PA and NP programs have worked hard to develop relationships with preceptors and institutions that support the process. Here are some ways to foster those relationships and bring back the altruism needed to precept in today’s society:
• Seek nonfinancial incentives for preceptors, such as Category 1 CE/CME credit, discounts for conference attendance, opportunities for academic advancement (eg, adjunct clinical professor status), and access to resources via the university library.
• Advocate for a state Preceptor Tax Incentive Program (such as the one in the state of Georgia).
• Reduce the (often copious) amount of paperwork that educational institutions require of preceptors.
We also need to ensure that our students are adequately prepared. In the past decade, students enter our programs with high expectations of what they will get out of a clinical rotation and a preceptor. Some may lack a touch of humility or a servant’s mind, both of which are important to their success.
An NP colleague recently decided to take a “student vacation” for six months, as her opinion was that many of the students she had precepted were not well prepared, either academically or in work/life experience. Her assessment of increasing class sizes was that some students are there “just to fill the program up” and make it profitable. To my colleague’s way of thinking, there is a disservice being done to students who are on rotations and not ready for them. We need to make sure our students are prepared to face the challenges of clinical rotations.
We also need to encourage a renewed sense of altruism in our students, so that they feel compelled to precept when they are alumni. So how do we enhance our preparation of future clinicians through precepting? I would love to hear from you about barriers you have encountered—and preferably, some viable solutions! You can reach me at [email protected].
References
1. Altruism: ethics. Encyclopaedia Britannica. www.britannica.com/topic/altruism-ethics. Accessed March 10, 2016.
2. Morrow KL. Preceptorships in Nursing Staff Development. Rockville, MD: Aspen Systems Corp; 1984.
3. Physician Assistant Education Association. Payment of clinical sites and preceptors in PA education [issue brief]. http://paeaonline.org/wp-content/uploads/2015/09/PaymentClinicalSites-PreceptorsPAEducation.pdf. Accessed March 10, 2016.
Randomized corporate clinical trials
The randomized clinical trial (RCT) has been the bulwark of the development of effective and safe medical and surgical therapy. Developed over the last half-century, they have proved the benefit and safety of new drugs and procedures and provided guidance to physicians in choosing therapeutic choices for their patients. They have replaced intuitive care that had largely directed medical care for centuries with a degree of science in making therapeutic decisions.
Although less than perfect, the RCT has been the reason for the remarkable success that we have achieved in decreasing the mortality of heart disease. This success has been driven largely by scientific altruism modified to some degree by the financial benefits of both the medical profession and the pharmaceutical industry.
As corporate hospital and third-party payers expand their role in the pattern of health care, there is the potential to make changes in how care is rendered beyond the choice of medical or surgical therapy. The expansion of care regionally and nationally provides tempting targets to modify the quality and efficacy on a large scale. It also provides potential cost-saving targets and generates corporate profits. Decisions at the corporate or administrative area in the past have been initiated on the basis of competitive costs and on intuitive decision making. The size of the market, however, provides an opportunity to test a variety of administrative plans that can lead to cost saving. These initiatives, well-meaning in their genesis, may result in patient participation in “studies” in which the patient may have a limited knowledge and benefit and uncertain risks.
RCTs demand informed consent by patients and unbiased temporal oversight by safety boards when searching for answers to questions based on contemporary knowledge. At present, there is no specific role for the patient’s voluntary and informed participation should a corporate representative initiate a “study” to answer a corporate question. Before we embark on “research” in patient care, it is imperative that we consider the principles established by RCTs. I propose two scenarios that may provide illustrative insight:
1. A nurse administrator wishes to explore the question of the cost savings achieved on a general medical ward by changing the current nurse staffing of eight professional nurses to a staffing with two professional nurses and six nursing assistants. The administrator will use personnel costs as the endpoint of the study and consider a variety of secondary issues such as the duration of time to respond to a call, sacral erythema, and wrong-dose administration, or other quality measurements.
2. The administrator of the catheterization laboratory decides to compare two catheters in the laboratory driven again by the costs of the catheters. The primary endpoint of the study will be cost savings. Secondary measurements will be time in the catheterization laboratory and postprocedure bleeding.
In both cases, the patient may or not have been informed about the research project in which they have been enrolled. In addition, there is no temporal assessment of the occurrence of adverse events that could occur during the progress of the “study” to ensure its safety. Many of the safeguards that have been developed around the RCT need to be incorporated into the design of these investigations.
This, of course, is all hypothetical, but on the other hand, it is not beyond the realm of possibility; as we move from “local care” to a system driven by anonymous personnel, it is important to obtain the consent of the patient and to establish procedures to ensure their safety.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
The randomized clinical trial (RCT) has been the bulwark of the development of effective and safe medical and surgical therapy. Developed over the last half-century, they have proved the benefit and safety of new drugs and procedures and provided guidance to physicians in choosing therapeutic choices for their patients. They have replaced intuitive care that had largely directed medical care for centuries with a degree of science in making therapeutic decisions.
Although less than perfect, the RCT has been the reason for the remarkable success that we have achieved in decreasing the mortality of heart disease. This success has been driven largely by scientific altruism modified to some degree by the financial benefits of both the medical profession and the pharmaceutical industry.
As corporate hospital and third-party payers expand their role in the pattern of health care, there is the potential to make changes in how care is rendered beyond the choice of medical or surgical therapy. The expansion of care regionally and nationally provides tempting targets to modify the quality and efficacy on a large scale. It also provides potential cost-saving targets and generates corporate profits. Decisions at the corporate or administrative area in the past have been initiated on the basis of competitive costs and on intuitive decision making. The size of the market, however, provides an opportunity to test a variety of administrative plans that can lead to cost saving. These initiatives, well-meaning in their genesis, may result in patient participation in “studies” in which the patient may have a limited knowledge and benefit and uncertain risks.
RCTs demand informed consent by patients and unbiased temporal oversight by safety boards when searching for answers to questions based on contemporary knowledge. At present, there is no specific role for the patient’s voluntary and informed participation should a corporate representative initiate a “study” to answer a corporate question. Before we embark on “research” in patient care, it is imperative that we consider the principles established by RCTs. I propose two scenarios that may provide illustrative insight:
1. A nurse administrator wishes to explore the question of the cost savings achieved on a general medical ward by changing the current nurse staffing of eight professional nurses to a staffing with two professional nurses and six nursing assistants. The administrator will use personnel costs as the endpoint of the study and consider a variety of secondary issues such as the duration of time to respond to a call, sacral erythema, and wrong-dose administration, or other quality measurements.
2. The administrator of the catheterization laboratory decides to compare two catheters in the laboratory driven again by the costs of the catheters. The primary endpoint of the study will be cost savings. Secondary measurements will be time in the catheterization laboratory and postprocedure bleeding.
In both cases, the patient may or not have been informed about the research project in which they have been enrolled. In addition, there is no temporal assessment of the occurrence of adverse events that could occur during the progress of the “study” to ensure its safety. Many of the safeguards that have been developed around the RCT need to be incorporated into the design of these investigations.
This, of course, is all hypothetical, but on the other hand, it is not beyond the realm of possibility; as we move from “local care” to a system driven by anonymous personnel, it is important to obtain the consent of the patient and to establish procedures to ensure their safety.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
The randomized clinical trial (RCT) has been the bulwark of the development of effective and safe medical and surgical therapy. Developed over the last half-century, they have proved the benefit and safety of new drugs and procedures and provided guidance to physicians in choosing therapeutic choices for their patients. They have replaced intuitive care that had largely directed medical care for centuries with a degree of science in making therapeutic decisions.
Although less than perfect, the RCT has been the reason for the remarkable success that we have achieved in decreasing the mortality of heart disease. This success has been driven largely by scientific altruism modified to some degree by the financial benefits of both the medical profession and the pharmaceutical industry.
As corporate hospital and third-party payers expand their role in the pattern of health care, there is the potential to make changes in how care is rendered beyond the choice of medical or surgical therapy. The expansion of care regionally and nationally provides tempting targets to modify the quality and efficacy on a large scale. It also provides potential cost-saving targets and generates corporate profits. Decisions at the corporate or administrative area in the past have been initiated on the basis of competitive costs and on intuitive decision making. The size of the market, however, provides an opportunity to test a variety of administrative plans that can lead to cost saving. These initiatives, well-meaning in their genesis, may result in patient participation in “studies” in which the patient may have a limited knowledge and benefit and uncertain risks.
RCTs demand informed consent by patients and unbiased temporal oversight by safety boards when searching for answers to questions based on contemporary knowledge. At present, there is no specific role for the patient’s voluntary and informed participation should a corporate representative initiate a “study” to answer a corporate question. Before we embark on “research” in patient care, it is imperative that we consider the principles established by RCTs. I propose two scenarios that may provide illustrative insight:
1. A nurse administrator wishes to explore the question of the cost savings achieved on a general medical ward by changing the current nurse staffing of eight professional nurses to a staffing with two professional nurses and six nursing assistants. The administrator will use personnel costs as the endpoint of the study and consider a variety of secondary issues such as the duration of time to respond to a call, sacral erythema, and wrong-dose administration, or other quality measurements.
2. The administrator of the catheterization laboratory decides to compare two catheters in the laboratory driven again by the costs of the catheters. The primary endpoint of the study will be cost savings. Secondary measurements will be time in the catheterization laboratory and postprocedure bleeding.
In both cases, the patient may or not have been informed about the research project in which they have been enrolled. In addition, there is no temporal assessment of the occurrence of adverse events that could occur during the progress of the “study” to ensure its safety. Many of the safeguards that have been developed around the RCT need to be incorporated into the design of these investigations.
This, of course, is all hypothetical, but on the other hand, it is not beyond the realm of possibility; as we move from “local care” to a system driven by anonymous personnel, it is important to obtain the consent of the patient and to establish procedures to ensure their safety.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
Considering learning disorders
Introduction
Often, it can be difficult for providers to fully understand why children may be presenting with behavioral problems that appear to be occurring principally in specific environments. Parents may bring their children for an evaluation based upon reports they are receiving from teachers and other school personnel who may be observing more prominent oppositionality, social struggles, troubles following instructions, and frustration intolerance than the parents are experiencing in the home. Additionally, youth may begin to display school refusal and voice strong negative feelings about school. Although at face value, these problems may indicate potential diagnoses like oppositional defiant disorder and attention-deficit/hyperactivity disorder, one also should consider other underlying “non–mental health” issues that could greatly influence school success and present with a range of emotional and behavioral struggles.
Case Summary
Brynn is an animated, social, and strong-willed 4 year-old girl who experienced delays in her receptive and expressive language that prompted her engagement in early intervention around 18 months of age. She and her family continued to receive developmental services over the next few years and, at the age of 3 years, because of ongoing speech and language challenges, she was enrolled in a preschool individualized education program. In her program, Brynn participates in an array of specialized instruction, but her educators comment that she is not making expected academic progress and has troubles “holding onto information,” focusing, and participating meaningfully with peers in a consistent manner.
At times, Brynn can be impulsive, aggressive, and volatile – behavioral traits that mom denies are occurring in the home. A diagnosis of an autism spectrum disorder has been ruled out, and Brynn’s hearing and vision are tested to be normal. Brynn’s mother feels confused by the reports she’s getting from school; “I want Brynn to be happy,” she shares. “I needed special help to read in high school, and I worry about her future.”
Discussion
Learning disorders (or learning disabilities, using educational terminology) are defined as neurologically rooted problems that affect academic achievement via the receiving, processing, or communication of information. They occur in 1 in 10 children (Pediatrics. 2007 Feb;119 Suppl 1:S77-83) and can present with specific problems in reading, writing, and mathematics while having considerable influence on related aptitudes in language, social ability, self-help, and motor functioning. Dyslexia – a developmental reading disorder – is the most common type of learning disorder (LD). Although it’s not clear what causes learning disorders, there are several factors that are thought to play a role in their development, including hereditary factors and problems with pregnancy and birth. Having developmental delays also can place children at risk for having later learning problems that may not be identifiable until a child enters a more structured learning environment. At clinical visits during the preschool years, particularly with a child who may have had earlier developmental concerns, the pediatrician should inquire about a range of “warning signs” that may indicate a need for additional screening and evaluation for specific learning issues.
The National Center for Learning Disabilities offers a range of practical tips for pediatricians who may want to further explore parental and teacher concerns by asking questions related to literacy, writing, language, and social-emotional skills, attention, and gross and fine motor movements in a developmentally informed manner. Further exploring Brynn’s mother’s comments in the context of her daughter not progressing in school revealed a history of difficulty retaining new words, troubles learning colors and shapes, challenges remembering rules, and particular difficulties engaging in group play with other 4-year-olds – all potential signals for a learning disorder. This alerted educators that she may indeed be struggling with issues beyond that of an enduring speech-language delay.
With the suspicion that Brynn was presenting with signs and symptoms suggestive of a learning disorder, her family was educated about the Individuals with Disabilities Education Act, and it was recommended she receive a comprehensive psychoeducational evaluation to further assess her intellectual profile, academic achievement, social functioning, and performance in the classroom using standardized tools. These tools, among other objectives, can help the child’s team offer a more definitive LD diagnosis while informing the potential development of special education supports and assessing for an intellectual disability. The DSM 5 indicates that LD diagnostic criteria are to be met based upon a clinical synthesis of history, school reports, and psychoeducational assessment.
It’s been long established that children with learning problems frequently have co-occurring emotional and behavioral troubles (Arch Dis Child. 1974 Apr; 49[4]:249-56), many of which also should be considered as differential diagnoses in a child with school problems – as such, a complicated interplay of learning disorders and internalizing and externalizing conditions is often appreciated in school-age children with academic difficulties. At times, learning disorders can lead to emotional distress and could also be misdiagnosed as primary emotional and behavioral challenges. Specifically, children with learning problems are at risk for struggling with low self-esteem, loneliness, and anxiety, which also can be associated with mood disorders, school dropout, victimization, and engaging in substance use.
In Brynn’s case, the results of the Teacher Report Forms and Child Behavior Checklists were reviewed, revealing some evidence that she was experiencing clinical-level problems with her attention, but a discrepancy was noted between the teacher and parent report (the teachers endorsing more clinically significant symptoms). Although co-occurring attention-deficit/hyperactivity disorder (ADHD) is not uncommon in children with a learning disorder (Pediatrics. 2011 Mar;127[3]:462-70), we did not feel Brynn met criteria for this. We elected not to provide an ADHD diagnosis but are mindful that her attentional concerns should be closely monitored over time; a diagnosis may be more relevant in the future, perhaps influencing Brynn’s eligibility for services and treatment planning. Furthermore, comorbidity with ADHD is predictive of worse mental health outcomes, compared with learning disabilities presenting without ADHD.
Clinical Pearl
Pediatricians should consider the possibility of a child having a learning disorder in youth who display risk factors (family history of learning concerns, atypical development, prematurity, etc.) and have problems at school. Such problems may be presenting with emotional and behavioral symptoms that could mask a child’s primary learning impairments. Learning disorders also frequently co-occur with psychiatric conditions, but engaging children in effective interventions (school-based supports) could potentially attenuate the risk for the development of mental health problems. Also, promoting emotional wellness and fostering self-worth may improve the academic performance of children with learning disorders.
Dr. Dickerson, a child and adolescent psychiatrist, is an assistant professor of psychiatry at the University of Vermont, Burlington. He is the director of the university’s autism diagnostic clinic. Dr. Dickerson said he had no relevant financial disclosures. Contact Dr. Dickerson at [email protected].
Introduction
Often, it can be difficult for providers to fully understand why children may be presenting with behavioral problems that appear to be occurring principally in specific environments. Parents may bring their children for an evaluation based upon reports they are receiving from teachers and other school personnel who may be observing more prominent oppositionality, social struggles, troubles following instructions, and frustration intolerance than the parents are experiencing in the home. Additionally, youth may begin to display school refusal and voice strong negative feelings about school. Although at face value, these problems may indicate potential diagnoses like oppositional defiant disorder and attention-deficit/hyperactivity disorder, one also should consider other underlying “non–mental health” issues that could greatly influence school success and present with a range of emotional and behavioral struggles.
Case Summary
Brynn is an animated, social, and strong-willed 4 year-old girl who experienced delays in her receptive and expressive language that prompted her engagement in early intervention around 18 months of age. She and her family continued to receive developmental services over the next few years and, at the age of 3 years, because of ongoing speech and language challenges, she was enrolled in a preschool individualized education program. In her program, Brynn participates in an array of specialized instruction, but her educators comment that she is not making expected academic progress and has troubles “holding onto information,” focusing, and participating meaningfully with peers in a consistent manner.
At times, Brynn can be impulsive, aggressive, and volatile – behavioral traits that mom denies are occurring in the home. A diagnosis of an autism spectrum disorder has been ruled out, and Brynn’s hearing and vision are tested to be normal. Brynn’s mother feels confused by the reports she’s getting from school; “I want Brynn to be happy,” she shares. “I needed special help to read in high school, and I worry about her future.”
Discussion
Learning disorders (or learning disabilities, using educational terminology) are defined as neurologically rooted problems that affect academic achievement via the receiving, processing, or communication of information. They occur in 1 in 10 children (Pediatrics. 2007 Feb;119 Suppl 1:S77-83) and can present with specific problems in reading, writing, and mathematics while having considerable influence on related aptitudes in language, social ability, self-help, and motor functioning. Dyslexia – a developmental reading disorder – is the most common type of learning disorder (LD). Although it’s not clear what causes learning disorders, there are several factors that are thought to play a role in their development, including hereditary factors and problems with pregnancy and birth. Having developmental delays also can place children at risk for having later learning problems that may not be identifiable until a child enters a more structured learning environment. At clinical visits during the preschool years, particularly with a child who may have had earlier developmental concerns, the pediatrician should inquire about a range of “warning signs” that may indicate a need for additional screening and evaluation for specific learning issues.
The National Center for Learning Disabilities offers a range of practical tips for pediatricians who may want to further explore parental and teacher concerns by asking questions related to literacy, writing, language, and social-emotional skills, attention, and gross and fine motor movements in a developmentally informed manner. Further exploring Brynn’s mother’s comments in the context of her daughter not progressing in school revealed a history of difficulty retaining new words, troubles learning colors and shapes, challenges remembering rules, and particular difficulties engaging in group play with other 4-year-olds – all potential signals for a learning disorder. This alerted educators that she may indeed be struggling with issues beyond that of an enduring speech-language delay.
With the suspicion that Brynn was presenting with signs and symptoms suggestive of a learning disorder, her family was educated about the Individuals with Disabilities Education Act, and it was recommended she receive a comprehensive psychoeducational evaluation to further assess her intellectual profile, academic achievement, social functioning, and performance in the classroom using standardized tools. These tools, among other objectives, can help the child’s team offer a more definitive LD diagnosis while informing the potential development of special education supports and assessing for an intellectual disability. The DSM 5 indicates that LD diagnostic criteria are to be met based upon a clinical synthesis of history, school reports, and psychoeducational assessment.
It’s been long established that children with learning problems frequently have co-occurring emotional and behavioral troubles (Arch Dis Child. 1974 Apr; 49[4]:249-56), many of which also should be considered as differential diagnoses in a child with school problems – as such, a complicated interplay of learning disorders and internalizing and externalizing conditions is often appreciated in school-age children with academic difficulties. At times, learning disorders can lead to emotional distress and could also be misdiagnosed as primary emotional and behavioral challenges. Specifically, children with learning problems are at risk for struggling with low self-esteem, loneliness, and anxiety, which also can be associated with mood disorders, school dropout, victimization, and engaging in substance use.
In Brynn’s case, the results of the Teacher Report Forms and Child Behavior Checklists were reviewed, revealing some evidence that she was experiencing clinical-level problems with her attention, but a discrepancy was noted between the teacher and parent report (the teachers endorsing more clinically significant symptoms). Although co-occurring attention-deficit/hyperactivity disorder (ADHD) is not uncommon in children with a learning disorder (Pediatrics. 2011 Mar;127[3]:462-70), we did not feel Brynn met criteria for this. We elected not to provide an ADHD diagnosis but are mindful that her attentional concerns should be closely monitored over time; a diagnosis may be more relevant in the future, perhaps influencing Brynn’s eligibility for services and treatment planning. Furthermore, comorbidity with ADHD is predictive of worse mental health outcomes, compared with learning disabilities presenting without ADHD.
Clinical Pearl
Pediatricians should consider the possibility of a child having a learning disorder in youth who display risk factors (family history of learning concerns, atypical development, prematurity, etc.) and have problems at school. Such problems may be presenting with emotional and behavioral symptoms that could mask a child’s primary learning impairments. Learning disorders also frequently co-occur with psychiatric conditions, but engaging children in effective interventions (school-based supports) could potentially attenuate the risk for the development of mental health problems. Also, promoting emotional wellness and fostering self-worth may improve the academic performance of children with learning disorders.
Dr. Dickerson, a child and adolescent psychiatrist, is an assistant professor of psychiatry at the University of Vermont, Burlington. He is the director of the university’s autism diagnostic clinic. Dr. Dickerson said he had no relevant financial disclosures. Contact Dr. Dickerson at [email protected].
Introduction
Often, it can be difficult for providers to fully understand why children may be presenting with behavioral problems that appear to be occurring principally in specific environments. Parents may bring their children for an evaluation based upon reports they are receiving from teachers and other school personnel who may be observing more prominent oppositionality, social struggles, troubles following instructions, and frustration intolerance than the parents are experiencing in the home. Additionally, youth may begin to display school refusal and voice strong negative feelings about school. Although at face value, these problems may indicate potential diagnoses like oppositional defiant disorder and attention-deficit/hyperactivity disorder, one also should consider other underlying “non–mental health” issues that could greatly influence school success and present with a range of emotional and behavioral struggles.
Case Summary
Brynn is an animated, social, and strong-willed 4 year-old girl who experienced delays in her receptive and expressive language that prompted her engagement in early intervention around 18 months of age. She and her family continued to receive developmental services over the next few years and, at the age of 3 years, because of ongoing speech and language challenges, she was enrolled in a preschool individualized education program. In her program, Brynn participates in an array of specialized instruction, but her educators comment that she is not making expected academic progress and has troubles “holding onto information,” focusing, and participating meaningfully with peers in a consistent manner.
At times, Brynn can be impulsive, aggressive, and volatile – behavioral traits that mom denies are occurring in the home. A diagnosis of an autism spectrum disorder has been ruled out, and Brynn’s hearing and vision are tested to be normal. Brynn’s mother feels confused by the reports she’s getting from school; “I want Brynn to be happy,” she shares. “I needed special help to read in high school, and I worry about her future.”
Discussion
Learning disorders (or learning disabilities, using educational terminology) are defined as neurologically rooted problems that affect academic achievement via the receiving, processing, or communication of information. They occur in 1 in 10 children (Pediatrics. 2007 Feb;119 Suppl 1:S77-83) and can present with specific problems in reading, writing, and mathematics while having considerable influence on related aptitudes in language, social ability, self-help, and motor functioning. Dyslexia – a developmental reading disorder – is the most common type of learning disorder (LD). Although it’s not clear what causes learning disorders, there are several factors that are thought to play a role in their development, including hereditary factors and problems with pregnancy and birth. Having developmental delays also can place children at risk for having later learning problems that may not be identifiable until a child enters a more structured learning environment. At clinical visits during the preschool years, particularly with a child who may have had earlier developmental concerns, the pediatrician should inquire about a range of “warning signs” that may indicate a need for additional screening and evaluation for specific learning issues.
The National Center for Learning Disabilities offers a range of practical tips for pediatricians who may want to further explore parental and teacher concerns by asking questions related to literacy, writing, language, and social-emotional skills, attention, and gross and fine motor movements in a developmentally informed manner. Further exploring Brynn’s mother’s comments in the context of her daughter not progressing in school revealed a history of difficulty retaining new words, troubles learning colors and shapes, challenges remembering rules, and particular difficulties engaging in group play with other 4-year-olds – all potential signals for a learning disorder. This alerted educators that she may indeed be struggling with issues beyond that of an enduring speech-language delay.
With the suspicion that Brynn was presenting with signs and symptoms suggestive of a learning disorder, her family was educated about the Individuals with Disabilities Education Act, and it was recommended she receive a comprehensive psychoeducational evaluation to further assess her intellectual profile, academic achievement, social functioning, and performance in the classroom using standardized tools. These tools, among other objectives, can help the child’s team offer a more definitive LD diagnosis while informing the potential development of special education supports and assessing for an intellectual disability. The DSM 5 indicates that LD diagnostic criteria are to be met based upon a clinical synthesis of history, school reports, and psychoeducational assessment.
It’s been long established that children with learning problems frequently have co-occurring emotional and behavioral troubles (Arch Dis Child. 1974 Apr; 49[4]:249-56), many of which also should be considered as differential diagnoses in a child with school problems – as such, a complicated interplay of learning disorders and internalizing and externalizing conditions is often appreciated in school-age children with academic difficulties. At times, learning disorders can lead to emotional distress and could also be misdiagnosed as primary emotional and behavioral challenges. Specifically, children with learning problems are at risk for struggling with low self-esteem, loneliness, and anxiety, which also can be associated with mood disorders, school dropout, victimization, and engaging in substance use.
In Brynn’s case, the results of the Teacher Report Forms and Child Behavior Checklists were reviewed, revealing some evidence that she was experiencing clinical-level problems with her attention, but a discrepancy was noted between the teacher and parent report (the teachers endorsing more clinically significant symptoms). Although co-occurring attention-deficit/hyperactivity disorder (ADHD) is not uncommon in children with a learning disorder (Pediatrics. 2011 Mar;127[3]:462-70), we did not feel Brynn met criteria for this. We elected not to provide an ADHD diagnosis but are mindful that her attentional concerns should be closely monitored over time; a diagnosis may be more relevant in the future, perhaps influencing Brynn’s eligibility for services and treatment planning. Furthermore, comorbidity with ADHD is predictive of worse mental health outcomes, compared with learning disabilities presenting without ADHD.
Clinical Pearl
Pediatricians should consider the possibility of a child having a learning disorder in youth who display risk factors (family history of learning concerns, atypical development, prematurity, etc.) and have problems at school. Such problems may be presenting with emotional and behavioral symptoms that could mask a child’s primary learning impairments. Learning disorders also frequently co-occur with psychiatric conditions, but engaging children in effective interventions (school-based supports) could potentially attenuate the risk for the development of mental health problems. Also, promoting emotional wellness and fostering self-worth may improve the academic performance of children with learning disorders.
Dr. Dickerson, a child and adolescent psychiatrist, is an assistant professor of psychiatry at the University of Vermont, Burlington. He is the director of the university’s autism diagnostic clinic. Dr. Dickerson said he had no relevant financial disclosures. Contact Dr. Dickerson at [email protected].
Does sharing genetic risk change behavior?
In the era of individualized (or precision) medicine, we are presented with a unique opportunity to peer into the genetic “maps” of our patients. Through this window, we can envision the self-evident present or predict a possible future.
For the front-line provider, knowing that we could someday have a large amount of these data to deal with can be overwhelming. We may be loath to think that, amongst all the other daily battles we wage with current disease states, we may now need to understand and explain risk for future disease states.
But would we be more likely to use these data if we thought that they would change patient behavior? Maybe.
So does it?
Gareth Hollands, Ph.D., of the University of Cambridge, England, and his colleagues conducted a brilliantly timed and welcome systematic review of the literature assessing the impact of communicating DNA-based disease risk estimates on risk-reducing health behaviors and motivation to engage in such behaviors (BMJ. 2016 Mar 15;352:i1102).
Eighteen studies were found reporting on seven behavioral outcomes, including smoking cessation (six studies, n = 2,663), diet (seven studies, n = 1,784), and physical activity (six studies, n = 1,704). The smoking studies related genetic risk for lung or esophageal cancer; the diet studies related risk for diabetes, obesity, cardiovascular disease, hypertension, hyperlipidemia, and Alzheimer’s disease; and the physical activity studies related risks similar to the diet studies.
No evidence was found that communicating DNA-based risk increased smoking cessation or led to positive changes in diet or physical activity. Nor did the investigators find any effects on motivation to change behavior. Although this information is not motivating to patients, no evidence was found suggesting that it is demotivating, either.
If neither behavior nor motivation is modified by DNA-based risk assessment, what is it good for? As the authors pointed out, this information can be used for clinical risk stratification and for refining screening and treatment procedures.
It’s important to note that this puts the responsibility for the required action in response to DNA data in the hands of medical providers – sadly reminding us that the list of ways to motivate patients to change behavior remains frustratingly short.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article.
In the era of individualized (or precision) medicine, we are presented with a unique opportunity to peer into the genetic “maps” of our patients. Through this window, we can envision the self-evident present or predict a possible future.
For the front-line provider, knowing that we could someday have a large amount of these data to deal with can be overwhelming. We may be loath to think that, amongst all the other daily battles we wage with current disease states, we may now need to understand and explain risk for future disease states.
But would we be more likely to use these data if we thought that they would change patient behavior? Maybe.
So does it?
Gareth Hollands, Ph.D., of the University of Cambridge, England, and his colleagues conducted a brilliantly timed and welcome systematic review of the literature assessing the impact of communicating DNA-based disease risk estimates on risk-reducing health behaviors and motivation to engage in such behaviors (BMJ. 2016 Mar 15;352:i1102).
Eighteen studies were found reporting on seven behavioral outcomes, including smoking cessation (six studies, n = 2,663), diet (seven studies, n = 1,784), and physical activity (six studies, n = 1,704). The smoking studies related genetic risk for lung or esophageal cancer; the diet studies related risk for diabetes, obesity, cardiovascular disease, hypertension, hyperlipidemia, and Alzheimer’s disease; and the physical activity studies related risks similar to the diet studies.
No evidence was found that communicating DNA-based risk increased smoking cessation or led to positive changes in diet or physical activity. Nor did the investigators find any effects on motivation to change behavior. Although this information is not motivating to patients, no evidence was found suggesting that it is demotivating, either.
If neither behavior nor motivation is modified by DNA-based risk assessment, what is it good for? As the authors pointed out, this information can be used for clinical risk stratification and for refining screening and treatment procedures.
It’s important to note that this puts the responsibility for the required action in response to DNA data in the hands of medical providers – sadly reminding us that the list of ways to motivate patients to change behavior remains frustratingly short.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article.
In the era of individualized (or precision) medicine, we are presented with a unique opportunity to peer into the genetic “maps” of our patients. Through this window, we can envision the self-evident present or predict a possible future.
For the front-line provider, knowing that we could someday have a large amount of these data to deal with can be overwhelming. We may be loath to think that, amongst all the other daily battles we wage with current disease states, we may now need to understand and explain risk for future disease states.
But would we be more likely to use these data if we thought that they would change patient behavior? Maybe.
So does it?
Gareth Hollands, Ph.D., of the University of Cambridge, England, and his colleagues conducted a brilliantly timed and welcome systematic review of the literature assessing the impact of communicating DNA-based disease risk estimates on risk-reducing health behaviors and motivation to engage in such behaviors (BMJ. 2016 Mar 15;352:i1102).
Eighteen studies were found reporting on seven behavioral outcomes, including smoking cessation (six studies, n = 2,663), diet (seven studies, n = 1,784), and physical activity (six studies, n = 1,704). The smoking studies related genetic risk for lung or esophageal cancer; the diet studies related risk for diabetes, obesity, cardiovascular disease, hypertension, hyperlipidemia, and Alzheimer’s disease; and the physical activity studies related risks similar to the diet studies.
No evidence was found that communicating DNA-based risk increased smoking cessation or led to positive changes in diet or physical activity. Nor did the investigators find any effects on motivation to change behavior. Although this information is not motivating to patients, no evidence was found suggesting that it is demotivating, either.
If neither behavior nor motivation is modified by DNA-based risk assessment, what is it good for? As the authors pointed out, this information can be used for clinical risk stratification and for refining screening and treatment procedures.
It’s important to note that this puts the responsibility for the required action in response to DNA data in the hands of medical providers – sadly reminding us that the list of ways to motivate patients to change behavior remains frustratingly short.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article.
Perinatal depression screening: New recommendations and challenges
It was almost a year ago that the American College of Obstetricians and Gynecologists came out unequivocally in favor of universal screening for perinatal depression.
In the revised policy statement from ACOG’s Committee on Obstetric Practice, the college recommended that physicians screen women for depression and anxiety symptoms at least once during the perinatal period using a standard, validated tool. ACOG also noted that screening must be coupled with appropriate follow-up and that clinical staff must be prepared to start therapy or refer patients to treatment (Obstet. Gynecol. 2015;125:1268-71).
This move toward routine screening was intuitive given the prevalence of perinatal mood and anxiety disorders.
Fast forward to January 2016 and the U.S. Preventive Services Task Force final recommendation calling for screening all adults for depression, including the at-risk populations of pregnant and postpartum women. Much like the ACOG guidelines, the USPSTF recommendations call for adequate systems to ensure treatment and follow-up (JAMA. 2016 Jan. 26;315[4]:380-7).
These recommendations, although timely, derive from relatively sparse data on the actual effectiveness of perinatal screening. Although the move toward screening is welcome and simply commonsense, it is concerning that there has been very little systematic study of the effectiveness of screening for such a prevalent and impactful illness. At the end of the day, the question remains: Will screening for perinatal depression in obstetric and possibly pediatric settings lead to improved outcomes for patients and families?
We’re screening, but will it make a difference?
As more U.S. states, along with other countries around the world, have begun routine screening of women in the perinatal period, it’s become clear that screening itself is easy to do. What has yet to be adequately demonstrated is how screening moves us toward getting women into treatment and ultimately toward getting women well.
New Jersey and Illinois are good examples of states that should be applauded for recognizing early on how important it is to identify women with perinatal depression. But even in these early-adopter states, the actual implementation of referral systems has been lacking.
Here in Massachusetts, we have a state-funded program designed to teach local women’s health providers – including ob.gyns. – about diagnosing perinatal depression. The MCPAP (Massachusetts Child Psychiatry Access Project) for Moms program also offers resources for consultation and referral. The program is fairly new, so it’s still unclear whether ob.gyns. and primary care physicians will accept the role of de facto mental health treaters, as well as whether the women who are identified through screening will go on to recover acutely and, more importantly, over the long term.
These experiences among the states highlight how great a challenge it is to go from screening to positive health outcomes for women.
Downstream difficulties
A lack of evidence isn’t the only problem. A recent editorial in the Lancet raised the concern that the currently available screening tests are not suitable for clinical practice. The suggestion read to some like heresy.
The Edinburgh Postnatal Depression Scale, which is the most commonly used screening instrument, has a positive predictive value of detecting major depressive disorder of 47%-64%, according to the editorial, making it prone to delivering false positives (doi: 10.1016/S0140-6736[16]00265-8).
“This situation is potentially dangerous,” the Lancet editorial noted, since results of qualitative studies “suggest that women are extremely concerned about depression screening, about the stigma associated with a diagnosis of depression, and that a positive result might lead to an automatic social service referral, and potentially removal of their baby.”
A recent article, published in the New York Times, raises an additional concern about what a depression diagnosis could mean for insurability. The article highlights the experience of a woman whose diagnosis of postpartum depression is creating difficulties for her in getting life insurance. The point is underscored that it is perfectly legal for life and disability insurers to charge more to patients with a diagnosis of mental illness or to deny coverage outright.
No going back
The whole issue of perinatal depression screening has opened a Pandora’s box, and that is a good thing. The conversation is long overdue in America. It is time for greater national awareness and focus on a disease that is as prevalent as perinatal depression and as disabling for women and their families.
The focus up to this point has been on perinatal depression screening, but we’re about to see a shift toward building the community infrastructure that will be critical for managing patients, including those women who have previously been marginalized and have had very poor access to care.
Widespread screening and treatment will also require a level of cooperation between advocacy groups and providers who are multidisciplinary in their approach, taking advantage of both pharmacologic and nonpharmacologic approaches. A model of crossdisciplinary collaboration will include, for example, providers from psychiatrists to therapists to doulas to social workers to clinicians who focus on mother-infant interaction. It is a long list and models for such collaboration are somewhat lacking.
One good example of a pilot effort for such a collaboration is the Massachusetts Postpartum Depression Commission, which includes a full spectrum of participants from doulas, social workers, and perinatal psychiatrists to lay people. The partnerships and the networking that’s going on across disciplines is absolutely new and is going to be essential if we’re going to manage an issue as large as the treatment of perinatal depression.
The enhanced awareness of the need to screen for, identify, and treat postpartum depression will also lead to better tools with greater specificity, perhaps using new technologies for better identification and treatment, everything from telemedicine to smartphone applications.
There will certainly be growing pains as we gather evidence, refine our screening instruments, and build referral systems, but I don’t see this as a reason not to identify illness in this vulnerable population. Rather, it is a charge to the field that there is work to be done.
Dr. Cohen is the director of the Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications.
It was almost a year ago that the American College of Obstetricians and Gynecologists came out unequivocally in favor of universal screening for perinatal depression.
In the revised policy statement from ACOG’s Committee on Obstetric Practice, the college recommended that physicians screen women for depression and anxiety symptoms at least once during the perinatal period using a standard, validated tool. ACOG also noted that screening must be coupled with appropriate follow-up and that clinical staff must be prepared to start therapy or refer patients to treatment (Obstet. Gynecol. 2015;125:1268-71).
This move toward routine screening was intuitive given the prevalence of perinatal mood and anxiety disorders.
Fast forward to January 2016 and the U.S. Preventive Services Task Force final recommendation calling for screening all adults for depression, including the at-risk populations of pregnant and postpartum women. Much like the ACOG guidelines, the USPSTF recommendations call for adequate systems to ensure treatment and follow-up (JAMA. 2016 Jan. 26;315[4]:380-7).
These recommendations, although timely, derive from relatively sparse data on the actual effectiveness of perinatal screening. Although the move toward screening is welcome and simply commonsense, it is concerning that there has been very little systematic study of the effectiveness of screening for such a prevalent and impactful illness. At the end of the day, the question remains: Will screening for perinatal depression in obstetric and possibly pediatric settings lead to improved outcomes for patients and families?
We’re screening, but will it make a difference?
As more U.S. states, along with other countries around the world, have begun routine screening of women in the perinatal period, it’s become clear that screening itself is easy to do. What has yet to be adequately demonstrated is how screening moves us toward getting women into treatment and ultimately toward getting women well.
New Jersey and Illinois are good examples of states that should be applauded for recognizing early on how important it is to identify women with perinatal depression. But even in these early-adopter states, the actual implementation of referral systems has been lacking.
Here in Massachusetts, we have a state-funded program designed to teach local women’s health providers – including ob.gyns. – about diagnosing perinatal depression. The MCPAP (Massachusetts Child Psychiatry Access Project) for Moms program also offers resources for consultation and referral. The program is fairly new, so it’s still unclear whether ob.gyns. and primary care physicians will accept the role of de facto mental health treaters, as well as whether the women who are identified through screening will go on to recover acutely and, more importantly, over the long term.
These experiences among the states highlight how great a challenge it is to go from screening to positive health outcomes for women.
Downstream difficulties
A lack of evidence isn’t the only problem. A recent editorial in the Lancet raised the concern that the currently available screening tests are not suitable for clinical practice. The suggestion read to some like heresy.
The Edinburgh Postnatal Depression Scale, which is the most commonly used screening instrument, has a positive predictive value of detecting major depressive disorder of 47%-64%, according to the editorial, making it prone to delivering false positives (doi: 10.1016/S0140-6736[16]00265-8).
“This situation is potentially dangerous,” the Lancet editorial noted, since results of qualitative studies “suggest that women are extremely concerned about depression screening, about the stigma associated with a diagnosis of depression, and that a positive result might lead to an automatic social service referral, and potentially removal of their baby.”
A recent article, published in the New York Times, raises an additional concern about what a depression diagnosis could mean for insurability. The article highlights the experience of a woman whose diagnosis of postpartum depression is creating difficulties for her in getting life insurance. The point is underscored that it is perfectly legal for life and disability insurers to charge more to patients with a diagnosis of mental illness or to deny coverage outright.
No going back
The whole issue of perinatal depression screening has opened a Pandora’s box, and that is a good thing. The conversation is long overdue in America. It is time for greater national awareness and focus on a disease that is as prevalent as perinatal depression and as disabling for women and their families.
The focus up to this point has been on perinatal depression screening, but we’re about to see a shift toward building the community infrastructure that will be critical for managing patients, including those women who have previously been marginalized and have had very poor access to care.
Widespread screening and treatment will also require a level of cooperation between advocacy groups and providers who are multidisciplinary in their approach, taking advantage of both pharmacologic and nonpharmacologic approaches. A model of crossdisciplinary collaboration will include, for example, providers from psychiatrists to therapists to doulas to social workers to clinicians who focus on mother-infant interaction. It is a long list and models for such collaboration are somewhat lacking.
One good example of a pilot effort for such a collaboration is the Massachusetts Postpartum Depression Commission, which includes a full spectrum of participants from doulas, social workers, and perinatal psychiatrists to lay people. The partnerships and the networking that’s going on across disciplines is absolutely new and is going to be essential if we’re going to manage an issue as large as the treatment of perinatal depression.
The enhanced awareness of the need to screen for, identify, and treat postpartum depression will also lead to better tools with greater specificity, perhaps using new technologies for better identification and treatment, everything from telemedicine to smartphone applications.
There will certainly be growing pains as we gather evidence, refine our screening instruments, and build referral systems, but I don’t see this as a reason not to identify illness in this vulnerable population. Rather, it is a charge to the field that there is work to be done.
Dr. Cohen is the director of the Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications.
It was almost a year ago that the American College of Obstetricians and Gynecologists came out unequivocally in favor of universal screening for perinatal depression.
In the revised policy statement from ACOG’s Committee on Obstetric Practice, the college recommended that physicians screen women for depression and anxiety symptoms at least once during the perinatal period using a standard, validated tool. ACOG also noted that screening must be coupled with appropriate follow-up and that clinical staff must be prepared to start therapy or refer patients to treatment (Obstet. Gynecol. 2015;125:1268-71).
This move toward routine screening was intuitive given the prevalence of perinatal mood and anxiety disorders.
Fast forward to January 2016 and the U.S. Preventive Services Task Force final recommendation calling for screening all adults for depression, including the at-risk populations of pregnant and postpartum women. Much like the ACOG guidelines, the USPSTF recommendations call for adequate systems to ensure treatment and follow-up (JAMA. 2016 Jan. 26;315[4]:380-7).
These recommendations, although timely, derive from relatively sparse data on the actual effectiveness of perinatal screening. Although the move toward screening is welcome and simply commonsense, it is concerning that there has been very little systematic study of the effectiveness of screening for such a prevalent and impactful illness. At the end of the day, the question remains: Will screening for perinatal depression in obstetric and possibly pediatric settings lead to improved outcomes for patients and families?
We’re screening, but will it make a difference?
As more U.S. states, along with other countries around the world, have begun routine screening of women in the perinatal period, it’s become clear that screening itself is easy to do. What has yet to be adequately demonstrated is how screening moves us toward getting women into treatment and ultimately toward getting women well.
New Jersey and Illinois are good examples of states that should be applauded for recognizing early on how important it is to identify women with perinatal depression. But even in these early-adopter states, the actual implementation of referral systems has been lacking.
Here in Massachusetts, we have a state-funded program designed to teach local women’s health providers – including ob.gyns. – about diagnosing perinatal depression. The MCPAP (Massachusetts Child Psychiatry Access Project) for Moms program also offers resources for consultation and referral. The program is fairly new, so it’s still unclear whether ob.gyns. and primary care physicians will accept the role of de facto mental health treaters, as well as whether the women who are identified through screening will go on to recover acutely and, more importantly, over the long term.
These experiences among the states highlight how great a challenge it is to go from screening to positive health outcomes for women.
Downstream difficulties
A lack of evidence isn’t the only problem. A recent editorial in the Lancet raised the concern that the currently available screening tests are not suitable for clinical practice. The suggestion read to some like heresy.
The Edinburgh Postnatal Depression Scale, which is the most commonly used screening instrument, has a positive predictive value of detecting major depressive disorder of 47%-64%, according to the editorial, making it prone to delivering false positives (doi: 10.1016/S0140-6736[16]00265-8).
“This situation is potentially dangerous,” the Lancet editorial noted, since results of qualitative studies “suggest that women are extremely concerned about depression screening, about the stigma associated with a diagnosis of depression, and that a positive result might lead to an automatic social service referral, and potentially removal of their baby.”
A recent article, published in the New York Times, raises an additional concern about what a depression diagnosis could mean for insurability. The article highlights the experience of a woman whose diagnosis of postpartum depression is creating difficulties for her in getting life insurance. The point is underscored that it is perfectly legal for life and disability insurers to charge more to patients with a diagnosis of mental illness or to deny coverage outright.
No going back
The whole issue of perinatal depression screening has opened a Pandora’s box, and that is a good thing. The conversation is long overdue in America. It is time for greater national awareness and focus on a disease that is as prevalent as perinatal depression and as disabling for women and their families.
The focus up to this point has been on perinatal depression screening, but we’re about to see a shift toward building the community infrastructure that will be critical for managing patients, including those women who have previously been marginalized and have had very poor access to care.
Widespread screening and treatment will also require a level of cooperation between advocacy groups and providers who are multidisciplinary in their approach, taking advantage of both pharmacologic and nonpharmacologic approaches. A model of crossdisciplinary collaboration will include, for example, providers from psychiatrists to therapists to doulas to social workers to clinicians who focus on mother-infant interaction. It is a long list and models for such collaboration are somewhat lacking.
One good example of a pilot effort for such a collaboration is the Massachusetts Postpartum Depression Commission, which includes a full spectrum of participants from doulas, social workers, and perinatal psychiatrists to lay people. The partnerships and the networking that’s going on across disciplines is absolutely new and is going to be essential if we’re going to manage an issue as large as the treatment of perinatal depression.
The enhanced awareness of the need to screen for, identify, and treat postpartum depression will also lead to better tools with greater specificity, perhaps using new technologies for better identification and treatment, everything from telemedicine to smartphone applications.
There will certainly be growing pains as we gather evidence, refine our screening instruments, and build referral systems, but I don’t see this as a reason not to identify illness in this vulnerable population. Rather, it is a charge to the field that there is work to be done.
Dr. Cohen is the director of the Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications.
Zika virus: More questions than answers?
With spring break in full swing and summer vacations right around the corner, pediatricians are increasingly fielding questions from families about Zika virus.
“There are a lot of resources available online, but they’re constantly being updated, and it’s difficult to stay current,” a friend and fellow pediatrician confided. “It seems like there’s new information every day, but still as many questions as answers.”
A quick PubMed search validated her concern: More than 200 articles have been published about Zika virus since the beginning of the year. The Centers for Disease Control and Prevention and the World Health Organization post new information to their Zika websites regularly, if not daily, and the WHO has released a Zika app for clinicians. Understanding that the busy pediatrician may not always have time to peruse these authoritative references during the course of a day in the office, I’ve compiled some common questions and answers.
“Is Zika really as serious as the media portrays it?” asked the mother of two children as she contemplated Caribbean vacation plans. In truth, most healthy people infected with Zika virus never develop symptoms. Illness, when it occurs, is most often mild and includes low-grade fever, headache, arthralgia, myalgia, nonpurulent conjunctivitis, and a maculopapular rash. Unlike dengue, another Flavivirus carried by Aedes mosquitoes, Zika does not cause hemorrhagic fever, and death appears to be rare.
An understanding of Zika infection and neurologic complications is a work in progress. A 20-fold increase in the incidence of Guillain-Barré (GBS) cases was noted in French Polynesia during a 2013-2014 outbreak of Zika virus.
In a case-control study involving 42 patients hospitalized with GBS, 98% had anti–Zika virus IgM or IgG, and all had neutralizing antibodies against Zika virus, compared with 56% of 98 control patients (P less than .0001 ) (Lancet. 2016 Feb 29. doi: 10.1016/S0140-6736(16)00562-6).
To date, 10 countries or territories have reported GBS cases with confirmed Zika virus infection. According to the World Health Organization, “Zika virus is highly likely to be a cause of the elevated incidence of GBS in countries and territories in the Western Pacific and Americas,” but further research is needed. Zika has recently been associated with other neurologic disorders, including myelitis, and the full spectrum of disease is likely not yet known.
Most Zika virus infections are transmitted from the bite of an Aedes mosquito. What we know about Zika transmission among humans continues to evolve. Viremia can persist for 14 or more days after the onset of symptoms, during which time blood is a potential source of infection. Two possible cases of transfusion-related viral transmission are under investigation in Brazil, and during the French Polynesia outbreak, 3% of samples from asymptomatic blood donors contained detectable Zika RNA. The U.S. Food and Drug Administration has recommended that individuals who have lived in or traveled to an area with active Zika virus transmission defer blood donation for 4 weeks after departure from the area .
Zika virus also has been detected in the urine and saliva of infected individuals, but these fluids have not been linked to transmission. Sexual transmission from infected men to their partners is well documented, but the period of risk remains undefined. The virus can persist in the semen long after viremia clears, and in one individual, Zika virus was detected in the semen 62 days after symptom onset.
Maternal-fetal transmission can occur as early as the first trimester and as late as at the time of delivery. Zika virus has been recovered from both amniotic fluid and placentas. The consequences of maternal-fetal transmission are less certain. Coincident with an epidemic of Zika in Brazil, that country has observed a marked increase in the incidence of microcephaly. Between Oct. 22, 2015, and March 12, 2016, 6,480 cases of microcephaly and/or central nervous system malformation were reported in Brazil, contrasting sharply with the average of 163 cases reported annually from 2001 to 2014. Zika virus has been linked to 863 cases of microcephaly investigated thus far. Proving causality takes time, but the World Health Organization says the link between microcephaly and Zika infection is “strongly suspected.”
Because of the association between Zika virus and birth defects, including abnormal brain development, eye abnormalities, and hearing deficits, the CDC currently recommends that pregnant women not travel to areas with Zika transmission, while men who have lived in or traveled to an area with Zika and who have a pregnant partner should either use condoms or not have sex for the duration of the pregnancy.
The good news for nonpregnant women who contract Zika infection is that the infection is not thought to pose any risk to future pregnancies. Currently, there is no evidence that a fetus conceived after maternal viremia has resolved would be at risk for infection. Still, many unanswered questions remain about Zika infection during pregnancy. For example, it’s currently unknown how often infection is transmitted from an infected mother to her fetus, or if infection is more severe at a particular point in gestation.
Although Zika virus has been isolated from breast milk, no infections have been linked to breastfeeding, and mothers are encouraged to continue to nurse, even in areas with widespread transmission. Infection with Zika at the time of birth or later in childhood has not been linked to microcephaly. Beyond that, the long-term health outcomes of infants and children with Zika virus infection are unknown.
“How far north do you think the virus will spread?” one mom asked me. “Do I need to be worried?”
For public health officials, that’s the sixty-four thousand dollar question. To date, there have been no cases acquired as a result of a mosquito bite in the United States, but the edge of the outbreak continues to creep north. Local transmission of the virus was reported in Cuba on March 14.
As of March 16, 2016, 258 travel-associated Zika virus cases have been diagnosed in the United States, including 18 in pregnant women. Six of these were sexually transmitted. Theoretically, “onward transmission” from one of these cases could occur if the right kind of mosquito bites an infected person during the period of active viremia and then bites someone else, transferring a tiny amount of the virus-contaminated blood.
According to CDC experts, “Texas, Florida, and Hawaii are likely to be the U.S. states with the highest risk of experiencing local transmission of Zika virus by mosquitoes.” Although this estimate is based on prior experience with similar viruses, the principal vector of Zika, Aedes aegypti, has been identified as far west as California and in a number of states across the South, including my home state of Kentucky. Aedes albopictus mosquitoes also have been proven competent vectors for Zika virus transmission and are more widely distributed throughout the continental United States.
In a thoughtful review published in JAMA Pediatrics, “What Pediatricians and Other Clinicians Should Know About Zika Virus,” Dr. Mark W. Kline and Dr. Gordon E. Schutze noted that up to two-thirds of the U.S. population live in an area where Aedes mosquitoes are present at least part of the year (JAMA Pediatr. 2016 Feb 18. doi: 10.1001/jamapediatrics.2016.0429). Fortunately, transmission of dengue and chikungunya, two other viruses carried by the same insect, is still very uncommon. Public health experts are urging individuals with Zika virus infection to avoid mosquito bites during the first week of illness, to protect others.
We should start now counseling our patients and families to avoid mosquito bites at home and abroad. Besides Zika virus, mosquitoes transmit several pathogens in the United States each year, including West Nile virus, LaCrosse encephalitis virus, St. Louis encephalitis virus, and dengue.
Any collections of standing water should be eliminated, as these can be mosquito breeding grounds. These include flower pots, buckets, barrels, and discarded tires. The water in bird baths and pet dishes should be changed at least weekly, and children’s wading pools should be drained and stored on their side after use.
To the extent practical, exposed skin should be covered with long-sleeved shirts, long pants, and socks when individuals are in areas with mosquito activity. To enhance protection, clothing can be treated with permethrin, or pretreated clothing can be worn. An FDA-registered insect repellent should be applied to exposed skin, especially during hours of highest mosquito activity. Zika-carrying mosquitoes bite during the day, or dawn to dusk. Effective repellents include DEET, picaridin, IR3535, and oil of lemon eucalyptus, although families should read labels carefully as instructions for use vary, as does the recommended time period of reapplication. Combination sunscreen/insect repellent products are not recommended as repellent usually does not need to be reapplied as often as sunscreen. Parents also should be reminded not to use oil of lemon eucalyptus–containing products on children under 3 years of age.
“We’re going to get a lot more questions as the weather turns warmer,” said a colleague of mine. “I’m just waiting for the first call about a child who develops fever and a rash after a mosquito bite. Parents will wonder if it could be Zika.”
It is going to be an interesting summer. Stay tuned.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She had no relevant financial disclosures.
With spring break in full swing and summer vacations right around the corner, pediatricians are increasingly fielding questions from families about Zika virus.
“There are a lot of resources available online, but they’re constantly being updated, and it’s difficult to stay current,” a friend and fellow pediatrician confided. “It seems like there’s new information every day, but still as many questions as answers.”
A quick PubMed search validated her concern: More than 200 articles have been published about Zika virus since the beginning of the year. The Centers for Disease Control and Prevention and the World Health Organization post new information to their Zika websites regularly, if not daily, and the WHO has released a Zika app for clinicians. Understanding that the busy pediatrician may not always have time to peruse these authoritative references during the course of a day in the office, I’ve compiled some common questions and answers.
“Is Zika really as serious as the media portrays it?” asked the mother of two children as she contemplated Caribbean vacation plans. In truth, most healthy people infected with Zika virus never develop symptoms. Illness, when it occurs, is most often mild and includes low-grade fever, headache, arthralgia, myalgia, nonpurulent conjunctivitis, and a maculopapular rash. Unlike dengue, another Flavivirus carried by Aedes mosquitoes, Zika does not cause hemorrhagic fever, and death appears to be rare.
An understanding of Zika infection and neurologic complications is a work in progress. A 20-fold increase in the incidence of Guillain-Barré (GBS) cases was noted in French Polynesia during a 2013-2014 outbreak of Zika virus.
In a case-control study involving 42 patients hospitalized with GBS, 98% had anti–Zika virus IgM or IgG, and all had neutralizing antibodies against Zika virus, compared with 56% of 98 control patients (P less than .0001 ) (Lancet. 2016 Feb 29. doi: 10.1016/S0140-6736(16)00562-6).
To date, 10 countries or territories have reported GBS cases with confirmed Zika virus infection. According to the World Health Organization, “Zika virus is highly likely to be a cause of the elevated incidence of GBS in countries and territories in the Western Pacific and Americas,” but further research is needed. Zika has recently been associated with other neurologic disorders, including myelitis, and the full spectrum of disease is likely not yet known.
Most Zika virus infections are transmitted from the bite of an Aedes mosquito. What we know about Zika transmission among humans continues to evolve. Viremia can persist for 14 or more days after the onset of symptoms, during which time blood is a potential source of infection. Two possible cases of transfusion-related viral transmission are under investigation in Brazil, and during the French Polynesia outbreak, 3% of samples from asymptomatic blood donors contained detectable Zika RNA. The U.S. Food and Drug Administration has recommended that individuals who have lived in or traveled to an area with active Zika virus transmission defer blood donation for 4 weeks after departure from the area .
Zika virus also has been detected in the urine and saliva of infected individuals, but these fluids have not been linked to transmission. Sexual transmission from infected men to their partners is well documented, but the period of risk remains undefined. The virus can persist in the semen long after viremia clears, and in one individual, Zika virus was detected in the semen 62 days after symptom onset.
Maternal-fetal transmission can occur as early as the first trimester and as late as at the time of delivery. Zika virus has been recovered from both amniotic fluid and placentas. The consequences of maternal-fetal transmission are less certain. Coincident with an epidemic of Zika in Brazil, that country has observed a marked increase in the incidence of microcephaly. Between Oct. 22, 2015, and March 12, 2016, 6,480 cases of microcephaly and/or central nervous system malformation were reported in Brazil, contrasting sharply with the average of 163 cases reported annually from 2001 to 2014. Zika virus has been linked to 863 cases of microcephaly investigated thus far. Proving causality takes time, but the World Health Organization says the link between microcephaly and Zika infection is “strongly suspected.”
Because of the association between Zika virus and birth defects, including abnormal brain development, eye abnormalities, and hearing deficits, the CDC currently recommends that pregnant women not travel to areas with Zika transmission, while men who have lived in or traveled to an area with Zika and who have a pregnant partner should either use condoms or not have sex for the duration of the pregnancy.
The good news for nonpregnant women who contract Zika infection is that the infection is not thought to pose any risk to future pregnancies. Currently, there is no evidence that a fetus conceived after maternal viremia has resolved would be at risk for infection. Still, many unanswered questions remain about Zika infection during pregnancy. For example, it’s currently unknown how often infection is transmitted from an infected mother to her fetus, or if infection is more severe at a particular point in gestation.
Although Zika virus has been isolated from breast milk, no infections have been linked to breastfeeding, and mothers are encouraged to continue to nurse, even in areas with widespread transmission. Infection with Zika at the time of birth or later in childhood has not been linked to microcephaly. Beyond that, the long-term health outcomes of infants and children with Zika virus infection are unknown.
“How far north do you think the virus will spread?” one mom asked me. “Do I need to be worried?”
For public health officials, that’s the sixty-four thousand dollar question. To date, there have been no cases acquired as a result of a mosquito bite in the United States, but the edge of the outbreak continues to creep north. Local transmission of the virus was reported in Cuba on March 14.
As of March 16, 2016, 258 travel-associated Zika virus cases have been diagnosed in the United States, including 18 in pregnant women. Six of these were sexually transmitted. Theoretically, “onward transmission” from one of these cases could occur if the right kind of mosquito bites an infected person during the period of active viremia and then bites someone else, transferring a tiny amount of the virus-contaminated blood.
According to CDC experts, “Texas, Florida, and Hawaii are likely to be the U.S. states with the highest risk of experiencing local transmission of Zika virus by mosquitoes.” Although this estimate is based on prior experience with similar viruses, the principal vector of Zika, Aedes aegypti, has been identified as far west as California and in a number of states across the South, including my home state of Kentucky. Aedes albopictus mosquitoes also have been proven competent vectors for Zika virus transmission and are more widely distributed throughout the continental United States.
In a thoughtful review published in JAMA Pediatrics, “What Pediatricians and Other Clinicians Should Know About Zika Virus,” Dr. Mark W. Kline and Dr. Gordon E. Schutze noted that up to two-thirds of the U.S. population live in an area where Aedes mosquitoes are present at least part of the year (JAMA Pediatr. 2016 Feb 18. doi: 10.1001/jamapediatrics.2016.0429). Fortunately, transmission of dengue and chikungunya, two other viruses carried by the same insect, is still very uncommon. Public health experts are urging individuals with Zika virus infection to avoid mosquito bites during the first week of illness, to protect others.
We should start now counseling our patients and families to avoid mosquito bites at home and abroad. Besides Zika virus, mosquitoes transmit several pathogens in the United States each year, including West Nile virus, LaCrosse encephalitis virus, St. Louis encephalitis virus, and dengue.
Any collections of standing water should be eliminated, as these can be mosquito breeding grounds. These include flower pots, buckets, barrels, and discarded tires. The water in bird baths and pet dishes should be changed at least weekly, and children’s wading pools should be drained and stored on their side after use.
To the extent practical, exposed skin should be covered with long-sleeved shirts, long pants, and socks when individuals are in areas with mosquito activity. To enhance protection, clothing can be treated with permethrin, or pretreated clothing can be worn. An FDA-registered insect repellent should be applied to exposed skin, especially during hours of highest mosquito activity. Zika-carrying mosquitoes bite during the day, or dawn to dusk. Effective repellents include DEET, picaridin, IR3535, and oil of lemon eucalyptus, although families should read labels carefully as instructions for use vary, as does the recommended time period of reapplication. Combination sunscreen/insect repellent products are not recommended as repellent usually does not need to be reapplied as often as sunscreen. Parents also should be reminded not to use oil of lemon eucalyptus–containing products on children under 3 years of age.
“We’re going to get a lot more questions as the weather turns warmer,” said a colleague of mine. “I’m just waiting for the first call about a child who develops fever and a rash after a mosquito bite. Parents will wonder if it could be Zika.”
It is going to be an interesting summer. Stay tuned.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She had no relevant financial disclosures.
With spring break in full swing and summer vacations right around the corner, pediatricians are increasingly fielding questions from families about Zika virus.
“There are a lot of resources available online, but they’re constantly being updated, and it’s difficult to stay current,” a friend and fellow pediatrician confided. “It seems like there’s new information every day, but still as many questions as answers.”
A quick PubMed search validated her concern: More than 200 articles have been published about Zika virus since the beginning of the year. The Centers for Disease Control and Prevention and the World Health Organization post new information to their Zika websites regularly, if not daily, and the WHO has released a Zika app for clinicians. Understanding that the busy pediatrician may not always have time to peruse these authoritative references during the course of a day in the office, I’ve compiled some common questions and answers.
“Is Zika really as serious as the media portrays it?” asked the mother of two children as she contemplated Caribbean vacation plans. In truth, most healthy people infected with Zika virus never develop symptoms. Illness, when it occurs, is most often mild and includes low-grade fever, headache, arthralgia, myalgia, nonpurulent conjunctivitis, and a maculopapular rash. Unlike dengue, another Flavivirus carried by Aedes mosquitoes, Zika does not cause hemorrhagic fever, and death appears to be rare.
An understanding of Zika infection and neurologic complications is a work in progress. A 20-fold increase in the incidence of Guillain-Barré (GBS) cases was noted in French Polynesia during a 2013-2014 outbreak of Zika virus.
In a case-control study involving 42 patients hospitalized with GBS, 98% had anti–Zika virus IgM or IgG, and all had neutralizing antibodies against Zika virus, compared with 56% of 98 control patients (P less than .0001 ) (Lancet. 2016 Feb 29. doi: 10.1016/S0140-6736(16)00562-6).
To date, 10 countries or territories have reported GBS cases with confirmed Zika virus infection. According to the World Health Organization, “Zika virus is highly likely to be a cause of the elevated incidence of GBS in countries and territories in the Western Pacific and Americas,” but further research is needed. Zika has recently been associated with other neurologic disorders, including myelitis, and the full spectrum of disease is likely not yet known.
Most Zika virus infections are transmitted from the bite of an Aedes mosquito. What we know about Zika transmission among humans continues to evolve. Viremia can persist for 14 or more days after the onset of symptoms, during which time blood is a potential source of infection. Two possible cases of transfusion-related viral transmission are under investigation in Brazil, and during the French Polynesia outbreak, 3% of samples from asymptomatic blood donors contained detectable Zika RNA. The U.S. Food and Drug Administration has recommended that individuals who have lived in or traveled to an area with active Zika virus transmission defer blood donation for 4 weeks after departure from the area .
Zika virus also has been detected in the urine and saliva of infected individuals, but these fluids have not been linked to transmission. Sexual transmission from infected men to their partners is well documented, but the period of risk remains undefined. The virus can persist in the semen long after viremia clears, and in one individual, Zika virus was detected in the semen 62 days after symptom onset.
Maternal-fetal transmission can occur as early as the first trimester and as late as at the time of delivery. Zika virus has been recovered from both amniotic fluid and placentas. The consequences of maternal-fetal transmission are less certain. Coincident with an epidemic of Zika in Brazil, that country has observed a marked increase in the incidence of microcephaly. Between Oct. 22, 2015, and March 12, 2016, 6,480 cases of microcephaly and/or central nervous system malformation were reported in Brazil, contrasting sharply with the average of 163 cases reported annually from 2001 to 2014. Zika virus has been linked to 863 cases of microcephaly investigated thus far. Proving causality takes time, but the World Health Organization says the link between microcephaly and Zika infection is “strongly suspected.”
Because of the association between Zika virus and birth defects, including abnormal brain development, eye abnormalities, and hearing deficits, the CDC currently recommends that pregnant women not travel to areas with Zika transmission, while men who have lived in or traveled to an area with Zika and who have a pregnant partner should either use condoms or not have sex for the duration of the pregnancy.
The good news for nonpregnant women who contract Zika infection is that the infection is not thought to pose any risk to future pregnancies. Currently, there is no evidence that a fetus conceived after maternal viremia has resolved would be at risk for infection. Still, many unanswered questions remain about Zika infection during pregnancy. For example, it’s currently unknown how often infection is transmitted from an infected mother to her fetus, or if infection is more severe at a particular point in gestation.
Although Zika virus has been isolated from breast milk, no infections have been linked to breastfeeding, and mothers are encouraged to continue to nurse, even in areas with widespread transmission. Infection with Zika at the time of birth or later in childhood has not been linked to microcephaly. Beyond that, the long-term health outcomes of infants and children with Zika virus infection are unknown.
“How far north do you think the virus will spread?” one mom asked me. “Do I need to be worried?”
For public health officials, that’s the sixty-four thousand dollar question. To date, there have been no cases acquired as a result of a mosquito bite in the United States, but the edge of the outbreak continues to creep north. Local transmission of the virus was reported in Cuba on March 14.
As of March 16, 2016, 258 travel-associated Zika virus cases have been diagnosed in the United States, including 18 in pregnant women. Six of these were sexually transmitted. Theoretically, “onward transmission” from one of these cases could occur if the right kind of mosquito bites an infected person during the period of active viremia and then bites someone else, transferring a tiny amount of the virus-contaminated blood.
According to CDC experts, “Texas, Florida, and Hawaii are likely to be the U.S. states with the highest risk of experiencing local transmission of Zika virus by mosquitoes.” Although this estimate is based on prior experience with similar viruses, the principal vector of Zika, Aedes aegypti, has been identified as far west as California and in a number of states across the South, including my home state of Kentucky. Aedes albopictus mosquitoes also have been proven competent vectors for Zika virus transmission and are more widely distributed throughout the continental United States.
In a thoughtful review published in JAMA Pediatrics, “What Pediatricians and Other Clinicians Should Know About Zika Virus,” Dr. Mark W. Kline and Dr. Gordon E. Schutze noted that up to two-thirds of the U.S. population live in an area where Aedes mosquitoes are present at least part of the year (JAMA Pediatr. 2016 Feb 18. doi: 10.1001/jamapediatrics.2016.0429). Fortunately, transmission of dengue and chikungunya, two other viruses carried by the same insect, is still very uncommon. Public health experts are urging individuals with Zika virus infection to avoid mosquito bites during the first week of illness, to protect others.
We should start now counseling our patients and families to avoid mosquito bites at home and abroad. Besides Zika virus, mosquitoes transmit several pathogens in the United States each year, including West Nile virus, LaCrosse encephalitis virus, St. Louis encephalitis virus, and dengue.
Any collections of standing water should be eliminated, as these can be mosquito breeding grounds. These include flower pots, buckets, barrels, and discarded tires. The water in bird baths and pet dishes should be changed at least weekly, and children’s wading pools should be drained and stored on their side after use.
To the extent practical, exposed skin should be covered with long-sleeved shirts, long pants, and socks when individuals are in areas with mosquito activity. To enhance protection, clothing can be treated with permethrin, or pretreated clothing can be worn. An FDA-registered insect repellent should be applied to exposed skin, especially during hours of highest mosquito activity. Zika-carrying mosquitoes bite during the day, or dawn to dusk. Effective repellents include DEET, picaridin, IR3535, and oil of lemon eucalyptus, although families should read labels carefully as instructions for use vary, as does the recommended time period of reapplication. Combination sunscreen/insect repellent products are not recommended as repellent usually does not need to be reapplied as often as sunscreen. Parents also should be reminded not to use oil of lemon eucalyptus–containing products on children under 3 years of age.
“We’re going to get a lot more questions as the weather turns warmer,” said a colleague of mine. “I’m just waiting for the first call about a child who develops fever and a rash after a mosquito bite. Parents will wonder if it could be Zika.”
It is going to be an interesting summer. Stay tuned.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She had no relevant financial disclosures.
Finding the right path for pain control
There has been a lot of publicity surrounding the increasing use of prescription painkillers and subsequent increase in deaths. In 2014, there were close to 19,000 deaths related to opioid painkiller overdose. State and federal governments have reacted with various initiatives, from changing the scheduling of Vicodin, to initiating prescription drug–monitoring programs, to limiting the number of pills dispensed. There are loud voices on either side of this debate, to be sure, but perhaps none so aggravating as the aggravated patient.
I did not start my practice with any prescribing “policy,” as I thought such policies were arbitrary. I am a physician, after all, so why wouldn’t I prescribe a narcotic if necessary? I also trained at a time when pain was considered “the fifth vital sign,” and we were taught to treat it aggressively.
But after a while you learn that trust in patients can be misplaced. You never forget the first nice lady whose urine drug screen comes back negative when you expected it to show the narcotic that you were prescribing her. You never forget the person who calls on a weekend claiming to be a patient of the practice and turns out not to be. And when your colleague gets her DEA number stolen and her signature forged, you finally learn that humanity is imperfect. What’s more, in your transition from young naive doctor to elder statesman, you learn that the push to treat pain so aggressively was achieved, in large part, by lobbying from the pharmaceutical industry.
Some patients of course can have a legitimate need for narcotics and truly derive benefit from them. In such patients, it is our practice to have the patient sign a pain “contract.” Such contracts are nonbinding, but give the narcotic prescription the gravitas that it deserves, underscoring the sacrosanct nature not just of the prescription but also of the physician-patient relationship. They specify the strength of the prescription, the number of pills dispensed, the pharmacy at which the prescription is to be filled, and the physician’s prerogative to do random drug screens.
The more vexing problem for rheumatologists involves patients with central sensitization and chronic pain. These patients seem predisposed to requiring escalating doses of narcotics, and they often have risk factors for developing narcotic abuse disorders. In addition, there is no evidence that chronic narcotic use provides any long-term benefit. But it is a rare chronic pain patient who is willing to accept that narcotics are not the answer to his or her problems.
One way to manage this is for the patients to not be given narcotics in the first place. That probably requires lobbying of a different kind: educating primary care providers and emergency department physicians on how to recognize chronic pain or central sensitization syndromes and disseminating the literature showing that narcotics have no long-term benefit in such cases.
Dr. Chan practices rheumatology in Pawtucket, R.I.
There has been a lot of publicity surrounding the increasing use of prescription painkillers and subsequent increase in deaths. In 2014, there were close to 19,000 deaths related to opioid painkiller overdose. State and federal governments have reacted with various initiatives, from changing the scheduling of Vicodin, to initiating prescription drug–monitoring programs, to limiting the number of pills dispensed. There are loud voices on either side of this debate, to be sure, but perhaps none so aggravating as the aggravated patient.
I did not start my practice with any prescribing “policy,” as I thought such policies were arbitrary. I am a physician, after all, so why wouldn’t I prescribe a narcotic if necessary? I also trained at a time when pain was considered “the fifth vital sign,” and we were taught to treat it aggressively.
But after a while you learn that trust in patients can be misplaced. You never forget the first nice lady whose urine drug screen comes back negative when you expected it to show the narcotic that you were prescribing her. You never forget the person who calls on a weekend claiming to be a patient of the practice and turns out not to be. And when your colleague gets her DEA number stolen and her signature forged, you finally learn that humanity is imperfect. What’s more, in your transition from young naive doctor to elder statesman, you learn that the push to treat pain so aggressively was achieved, in large part, by lobbying from the pharmaceutical industry.
Some patients of course can have a legitimate need for narcotics and truly derive benefit from them. In such patients, it is our practice to have the patient sign a pain “contract.” Such contracts are nonbinding, but give the narcotic prescription the gravitas that it deserves, underscoring the sacrosanct nature not just of the prescription but also of the physician-patient relationship. They specify the strength of the prescription, the number of pills dispensed, the pharmacy at which the prescription is to be filled, and the physician’s prerogative to do random drug screens.
The more vexing problem for rheumatologists involves patients with central sensitization and chronic pain. These patients seem predisposed to requiring escalating doses of narcotics, and they often have risk factors for developing narcotic abuse disorders. In addition, there is no evidence that chronic narcotic use provides any long-term benefit. But it is a rare chronic pain patient who is willing to accept that narcotics are not the answer to his or her problems.
One way to manage this is for the patients to not be given narcotics in the first place. That probably requires lobbying of a different kind: educating primary care providers and emergency department physicians on how to recognize chronic pain or central sensitization syndromes and disseminating the literature showing that narcotics have no long-term benefit in such cases.
Dr. Chan practices rheumatology in Pawtucket, R.I.
There has been a lot of publicity surrounding the increasing use of prescription painkillers and subsequent increase in deaths. In 2014, there were close to 19,000 deaths related to opioid painkiller overdose. State and federal governments have reacted with various initiatives, from changing the scheduling of Vicodin, to initiating prescription drug–monitoring programs, to limiting the number of pills dispensed. There are loud voices on either side of this debate, to be sure, but perhaps none so aggravating as the aggravated patient.
I did not start my practice with any prescribing “policy,” as I thought such policies were arbitrary. I am a physician, after all, so why wouldn’t I prescribe a narcotic if necessary? I also trained at a time when pain was considered “the fifth vital sign,” and we were taught to treat it aggressively.
But after a while you learn that trust in patients can be misplaced. You never forget the first nice lady whose urine drug screen comes back negative when you expected it to show the narcotic that you were prescribing her. You never forget the person who calls on a weekend claiming to be a patient of the practice and turns out not to be. And when your colleague gets her DEA number stolen and her signature forged, you finally learn that humanity is imperfect. What’s more, in your transition from young naive doctor to elder statesman, you learn that the push to treat pain so aggressively was achieved, in large part, by lobbying from the pharmaceutical industry.
Some patients of course can have a legitimate need for narcotics and truly derive benefit from them. In such patients, it is our practice to have the patient sign a pain “contract.” Such contracts are nonbinding, but give the narcotic prescription the gravitas that it deserves, underscoring the sacrosanct nature not just of the prescription but also of the physician-patient relationship. They specify the strength of the prescription, the number of pills dispensed, the pharmacy at which the prescription is to be filled, and the physician’s prerogative to do random drug screens.
The more vexing problem for rheumatologists involves patients with central sensitization and chronic pain. These patients seem predisposed to requiring escalating doses of narcotics, and they often have risk factors for developing narcotic abuse disorders. In addition, there is no evidence that chronic narcotic use provides any long-term benefit. But it is a rare chronic pain patient who is willing to accept that narcotics are not the answer to his or her problems.
One way to manage this is for the patients to not be given narcotics in the first place. That probably requires lobbying of a different kind: educating primary care providers and emergency department physicians on how to recognize chronic pain or central sensitization syndromes and disseminating the literature showing that narcotics have no long-term benefit in such cases.
Dr. Chan practices rheumatology in Pawtucket, R.I.