User login
Your Guide to COVID Vaccines for 2024-2025
The updated COVID vaccines for 2024-2025 are officially here, designed to target the latest variants and offer robust protection — but getting Americans to roll up their sleeves could prove harder than ever. With COVID cases on the decline, many people feel the urgency has passed.
As of December 2, the CDC reports that COVID test positivity remains low, rising slightly to 4.5% for the week ending November 23, compared with 4.2% the previous week. That’s a far cry from the early days of 2022, when positivity rates soared above 30%. Emergency room visits for COVID now make up just 0.5%, and deaths are down to 0.8% of total weekly fatalities, compared to 1% the previous week.
This steady improvement in the numbers may explain why a recent Pew Research Center survey revealed that 6 in 10 US adults have no plans to get the updated vaccine this year.
As of December 2, according to the CDC, just 19.7% of the US adult population and 9.4% of children had gotten the updated vaccine. The age group most likely? Adults ages 65 and older, with 41.6% getting the updated shot.
Despite the good news about declining cases, our pandemic history suggests a pre-holiday increase is likely. On November 20, the CDC warned it expects levels of both COVID and RSV (respiratory syncytial virus) to rise in the coming weeks — the familiar post-Thanksgiving, pre-Christmas, and Hanukkah increase.
Here’s what to know about the 2024-2025 vaccines — what’s available, how the updated versions are tested, how well each protects you, side effects and other safety information, the best time to get them, and where.
What’s Available?
Three updated vaccines, which work two different ways, are authorized or licensed by the FDA for the 2024-2025 season:
Novavax. A protein subunit vaccine, Novavax is authorized for emergency use by the FDA in people ages 12 and older. The vaccine makes a protein that mimics the SARS-CoV-2 virus’ version of the spike protein and combines it with an adjuvant or “booster” to stimulate a protective immune response. This year’s version targets the JN.1 variant.
Pfizer/BioNTech. Its Comirnaty is a fully licensed vaccine for people ages 12 and older. Its mechanism of action is by messenger RNA (mRNA). It works by instructing cells to produce viral proteins, triggering an immune response. Pfizer’s COVID vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
Moderna. Its Spikevax is a fully licensed vaccine for people ages 12 and older. It is also an mRNA vaccine. Moderna’s COVID-19 vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
How Effective Are They?
Before being approved for this year’s use, each company had to show its updated vaccine is effective against the currently circulating variants. For the 2 weeks ending November 23, KP.3.1.1 and XEC, from the Omicron lineage, made up the majority of cases, according to CDC data.
How do the vaccine makers know their updated vaccines are targeting the circulating variants? The companies use “pre-clinical” data, which means the updated versions have not yet been tested in people but in other ways, such as animal studies. But they do have to prove to the FDA that their updated vaccine can neutralize the circulating variants.
Companies continue to monitor their updated vaccines as new variants appear. Later in the season, there will be more specific information about how well each vaccine protects in people after tracking real-world data.
What About Side Effects?
The CDC lists comparable side effects for both mRNA and protein COVID vaccines, including pain and soreness from the needle, fatigue, headache, muscle pain joint pain, chills, fever, nausea, and vomiting.
Severe allergic reactions are rare, the CDC says, but cautions to be alert for low blood pressure, swelling of the lips, tongue, or throat, or difficulty breathing.
Which One Is Best?
“I consider the three currently available COVID vaccines — Pfizer, Moderna, and Novavax — interchangeable,’’ said Scott Roberts, MD, an infectious diseases specialist and assistant professor of medicine at Yale School of Medicine in New Haven, Connecticut. “There have not been head-to-head studies, and the initial vaccine studies for each were performed at different phases of the pandemic, so we do not have great data to guide which one is better than another.”
He does point out the different mechanisms of action, which may make a difference in people’s choice of vaccines. “So if someone has a reaction to one of them, they can switch to a different brand.”
Best Time to Get It?
“We have consistently seen COVID rates rise quite significantly in the winter season, especially around the holidays. So if anyone is on the fence and hasn’t gotten the updated vaccine yet, now is a great time to get it to maximize immunity for the holidays,” he said.
What’s next? In late October, the CDC recommended a second dose of the 2024-2025 vaccine 6 months after the first one for those age 65 and above and those 6 months old and older who are moderately or severely immunocompromised.
Now, while it’s tempting to think rates are down and will continue to drop steadily, Roberts reminds people that pandemic history suggests otherwise.
Coverage
Most people can get COVID-19 vaccines at no cost through their private health insurance, Medicaid, or Medicare. For the uninsured, there’s also the Vaccines for Children (VFC) program or access through state and local health departments and some health centers. Find details on the CDC website.
A version of this article first appeared on WebMD.
The updated COVID vaccines for 2024-2025 are officially here, designed to target the latest variants and offer robust protection — but getting Americans to roll up their sleeves could prove harder than ever. With COVID cases on the decline, many people feel the urgency has passed.
As of December 2, the CDC reports that COVID test positivity remains low, rising slightly to 4.5% for the week ending November 23, compared with 4.2% the previous week. That’s a far cry from the early days of 2022, when positivity rates soared above 30%. Emergency room visits for COVID now make up just 0.5%, and deaths are down to 0.8% of total weekly fatalities, compared to 1% the previous week.
This steady improvement in the numbers may explain why a recent Pew Research Center survey revealed that 6 in 10 US adults have no plans to get the updated vaccine this year.
As of December 2, according to the CDC, just 19.7% of the US adult population and 9.4% of children had gotten the updated vaccine. The age group most likely? Adults ages 65 and older, with 41.6% getting the updated shot.
Despite the good news about declining cases, our pandemic history suggests a pre-holiday increase is likely. On November 20, the CDC warned it expects levels of both COVID and RSV (respiratory syncytial virus) to rise in the coming weeks — the familiar post-Thanksgiving, pre-Christmas, and Hanukkah increase.
Here’s what to know about the 2024-2025 vaccines — what’s available, how the updated versions are tested, how well each protects you, side effects and other safety information, the best time to get them, and where.
What’s Available?
Three updated vaccines, which work two different ways, are authorized or licensed by the FDA for the 2024-2025 season:
Novavax. A protein subunit vaccine, Novavax is authorized for emergency use by the FDA in people ages 12 and older. The vaccine makes a protein that mimics the SARS-CoV-2 virus’ version of the spike protein and combines it with an adjuvant or “booster” to stimulate a protective immune response. This year’s version targets the JN.1 variant.
Pfizer/BioNTech. Its Comirnaty is a fully licensed vaccine for people ages 12 and older. Its mechanism of action is by messenger RNA (mRNA). It works by instructing cells to produce viral proteins, triggering an immune response. Pfizer’s COVID vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
Moderna. Its Spikevax is a fully licensed vaccine for people ages 12 and older. It is also an mRNA vaccine. Moderna’s COVID-19 vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
How Effective Are They?
Before being approved for this year’s use, each company had to show its updated vaccine is effective against the currently circulating variants. For the 2 weeks ending November 23, KP.3.1.1 and XEC, from the Omicron lineage, made up the majority of cases, according to CDC data.
How do the vaccine makers know their updated vaccines are targeting the circulating variants? The companies use “pre-clinical” data, which means the updated versions have not yet been tested in people but in other ways, such as animal studies. But they do have to prove to the FDA that their updated vaccine can neutralize the circulating variants.
Companies continue to monitor their updated vaccines as new variants appear. Later in the season, there will be more specific information about how well each vaccine protects in people after tracking real-world data.
What About Side Effects?
The CDC lists comparable side effects for both mRNA and protein COVID vaccines, including pain and soreness from the needle, fatigue, headache, muscle pain joint pain, chills, fever, nausea, and vomiting.
Severe allergic reactions are rare, the CDC says, but cautions to be alert for low blood pressure, swelling of the lips, tongue, or throat, or difficulty breathing.
Which One Is Best?
“I consider the three currently available COVID vaccines — Pfizer, Moderna, and Novavax — interchangeable,’’ said Scott Roberts, MD, an infectious diseases specialist and assistant professor of medicine at Yale School of Medicine in New Haven, Connecticut. “There have not been head-to-head studies, and the initial vaccine studies for each were performed at different phases of the pandemic, so we do not have great data to guide which one is better than another.”
He does point out the different mechanisms of action, which may make a difference in people’s choice of vaccines. “So if someone has a reaction to one of them, they can switch to a different brand.”
Best Time to Get It?
“We have consistently seen COVID rates rise quite significantly in the winter season, especially around the holidays. So if anyone is on the fence and hasn’t gotten the updated vaccine yet, now is a great time to get it to maximize immunity for the holidays,” he said.
What’s next? In late October, the CDC recommended a second dose of the 2024-2025 vaccine 6 months after the first one for those age 65 and above and those 6 months old and older who are moderately or severely immunocompromised.
Now, while it’s tempting to think rates are down and will continue to drop steadily, Roberts reminds people that pandemic history suggests otherwise.
Coverage
Most people can get COVID-19 vaccines at no cost through their private health insurance, Medicaid, or Medicare. For the uninsured, there’s also the Vaccines for Children (VFC) program or access through state and local health departments and some health centers. Find details on the CDC website.
A version of this article first appeared on WebMD.
The updated COVID vaccines for 2024-2025 are officially here, designed to target the latest variants and offer robust protection — but getting Americans to roll up their sleeves could prove harder than ever. With COVID cases on the decline, many people feel the urgency has passed.
As of December 2, the CDC reports that COVID test positivity remains low, rising slightly to 4.5% for the week ending November 23, compared with 4.2% the previous week. That’s a far cry from the early days of 2022, when positivity rates soared above 30%. Emergency room visits for COVID now make up just 0.5%, and deaths are down to 0.8% of total weekly fatalities, compared to 1% the previous week.
This steady improvement in the numbers may explain why a recent Pew Research Center survey revealed that 6 in 10 US adults have no plans to get the updated vaccine this year.
As of December 2, according to the CDC, just 19.7% of the US adult population and 9.4% of children had gotten the updated vaccine. The age group most likely? Adults ages 65 and older, with 41.6% getting the updated shot.
Despite the good news about declining cases, our pandemic history suggests a pre-holiday increase is likely. On November 20, the CDC warned it expects levels of both COVID and RSV (respiratory syncytial virus) to rise in the coming weeks — the familiar post-Thanksgiving, pre-Christmas, and Hanukkah increase.
Here’s what to know about the 2024-2025 vaccines — what’s available, how the updated versions are tested, how well each protects you, side effects and other safety information, the best time to get them, and where.
What’s Available?
Three updated vaccines, which work two different ways, are authorized or licensed by the FDA for the 2024-2025 season:
Novavax. A protein subunit vaccine, Novavax is authorized for emergency use by the FDA in people ages 12 and older. The vaccine makes a protein that mimics the SARS-CoV-2 virus’ version of the spike protein and combines it with an adjuvant or “booster” to stimulate a protective immune response. This year’s version targets the JN.1 variant.
Pfizer/BioNTech. Its Comirnaty is a fully licensed vaccine for people ages 12 and older. Its mechanism of action is by messenger RNA (mRNA). It works by instructing cells to produce viral proteins, triggering an immune response. Pfizer’s COVID vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
Moderna. Its Spikevax is a fully licensed vaccine for people ages 12 and older. It is also an mRNA vaccine. Moderna’s COVID-19 vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
How Effective Are They?
Before being approved for this year’s use, each company had to show its updated vaccine is effective against the currently circulating variants. For the 2 weeks ending November 23, KP.3.1.1 and XEC, from the Omicron lineage, made up the majority of cases, according to CDC data.
How do the vaccine makers know their updated vaccines are targeting the circulating variants? The companies use “pre-clinical” data, which means the updated versions have not yet been tested in people but in other ways, such as animal studies. But they do have to prove to the FDA that their updated vaccine can neutralize the circulating variants.
Companies continue to monitor their updated vaccines as new variants appear. Later in the season, there will be more specific information about how well each vaccine protects in people after tracking real-world data.
What About Side Effects?
The CDC lists comparable side effects for both mRNA and protein COVID vaccines, including pain and soreness from the needle, fatigue, headache, muscle pain joint pain, chills, fever, nausea, and vomiting.
Severe allergic reactions are rare, the CDC says, but cautions to be alert for low blood pressure, swelling of the lips, tongue, or throat, or difficulty breathing.
Which One Is Best?
“I consider the three currently available COVID vaccines — Pfizer, Moderna, and Novavax — interchangeable,’’ said Scott Roberts, MD, an infectious diseases specialist and assistant professor of medicine at Yale School of Medicine in New Haven, Connecticut. “There have not been head-to-head studies, and the initial vaccine studies for each were performed at different phases of the pandemic, so we do not have great data to guide which one is better than another.”
He does point out the different mechanisms of action, which may make a difference in people’s choice of vaccines. “So if someone has a reaction to one of them, they can switch to a different brand.”
Best Time to Get It?
“We have consistently seen COVID rates rise quite significantly in the winter season, especially around the holidays. So if anyone is on the fence and hasn’t gotten the updated vaccine yet, now is a great time to get it to maximize immunity for the holidays,” he said.
What’s next? In late October, the CDC recommended a second dose of the 2024-2025 vaccine 6 months after the first one for those age 65 and above and those 6 months old and older who are moderately or severely immunocompromised.
Now, while it’s tempting to think rates are down and will continue to drop steadily, Roberts reminds people that pandemic history suggests otherwise.
Coverage
Most people can get COVID-19 vaccines at no cost through their private health insurance, Medicaid, or Medicare. For the uninsured, there’s also the Vaccines for Children (VFC) program or access through state and local health departments and some health centers. Find details on the CDC website.
A version of this article first appeared on WebMD.
New Cancer Drugs: Do Patients Prefer Faster Access or Clinical Benefit?
When the Food and Drug Administration (FDA) grants cancer drugs accelerated approval, a key aim is to provide patients faster access to therapies that can benefit them.
The downside of a speedier approval timeline, however, is that it’s often not yet clear whether the new drugs will actually allow a patient to live longer or better. Information on overall survival and quality of life typically comes years later, after drugs undergo confirmatory trials, or sometimes not at all, if companies fail to conduct these trials.
During this waiting period, patients may be receiving a cancer drug that provides no real clinical benefit but comes with a host of toxicities.
In fact, the odds are about as good as a coin flip. For cancer drugs that have confirmatory trial data, more than half don’t ultimately provide an overall survival or quality of life benefit.
Inherent to the accelerated approval process is the assumption that patients are willing to accept this uncertainty in exchange for faster access.
But is that really the case?
The researchers asked about 870 adults with experience of cancer challenges — either their own cancer diagnosis or that of family or a close friend — whether they valued faster access or certainty that a drug really works.
In the study, participants imagined they had been diagnosed with cancer and could choose between two cancer drugs under investigation in clinical trials but with uncertain effectiveness, and a current standard treatment. Participants had to make a series of choices based on five scenarios.
The first two scenarios were based on the impact of the current standard treatment: A patient’s life expectancy on the standard treatment (6 months up to 3 years), and a patient’s physical health on the standard treatment (functional status restricted only during strenuous activities up to completely disabled).
The remaining three scenarios dealt with the two new drugs: The effect of the new drugs on a surrogate endpoint, progression-free survival (whether the drugs slowed tumor growth for an extra month or 5 additional months compared with the standard treatment), certainty that slowing tumor growth will improve survival (very low to high), and the wait time to access the drugs (immediately to as long as 2 years).
The researchers assessed the relative importance of survival benefit certainty vs wait time and how that balance shifted depending on the different scenarios.
Overall, the researchers found that, if there was no evidence linking the surrogate endpoint (progression-free survival) to overall survival, patients were willing to wait about 8 months for weak evidence of an overall survival benefit (ie, low certainty the drug will extend survival by 1-5 months), about 16 months for moderate certainty, and almost 22 months for high certainty.
Despite a willingness to wait for greater certainty, participants did value speed as well. Overall, respondents showed a strong preference against a 1-year delay in FDA approval time. People who were aged 55 years or more and were non-White individuals made less than $40,000 year as well as those with the lowest life expectancy on a current standard treatment were most sensitive to wait times while those with better functional status and longer life expectancies on a current treatment were less sensitive to longer wait times.
“Our results indicate that some patients (except those with the poorest prognoses) would find the additional time required to generate evidence on the survival benefit of new cancer drugs an acceptable tradeoff,” the study authors concluded.
Although people do place high value on timely access to new cancer drugs, especially if there are limited treatment options, many are willing to wait for greater certainty that a new drug provides an overall survival benefit, lead author Robin Forrest, MSc, with the Department of Health Policy, London School of Economics in England, said in an interview.
In the study, respondents also did not place significant value on whether the drug substantially slowed cancer growth. “In other words, substantial progression-free survival benefit of a drug did not compensate for lack of certainty about a drug’s benefit on survival in respondents’ drug choices,” the authors explained.
“In an effort to move quickly, we have accepted progression-free survival [as a surrogate endpoint],” Jyoti D. Patel, MD, oncologist with Northwestern Memorial Hospital, Chicago, Illinois, who wasn’t involved in the study. But a growing body of evidence indicates that progression-free survival is often a poor surrogate for overall survival. And what this study suggests is that “patients uniformly care about improvements in overall survival and the quality of that survival,” Patel said.
Bishal Gyawali, MD, PhD, was not surprised by the findings.
“I always thought this was the real-world scenario, but the problem is the voices of ordinary patients are not heard,” Gyawali, with Queen’s University, Kingston, Ontario, Canada, who also wasn’t involved in the study, said in an interview.
“What is heard is the loud noise of ‘we need access now, today, yesterday’ — ‘we don’t care if the drug doesn’t improve overall survival, we just need a drug, any drug’ — ‘we don’t care how much it costs, we need access today,’ ” Gyawali said. “Not saying this is wrong, but this is not the representation of all patients.”
However, the voices of patients who are more cautious and want evidence of benefit before accepting toxicities don’t make headlines, he added.
What this survey means from a policy perspective, said Gyawali, is that accelerated approvals that do not mandate survival endpoint in confirmatory trials are ignoring the need of many patients who prioritize certainty of benefit over speed of access.
The study was funded by the London School of Economics and Political Science Phelan United States Centre. Forrest had no relevant disclosures. Gyawali has received consulting fees from Vivio Health. Patel has various relationships with AbbVie, Anheart, AstraZeneca, Bristol-Myers Squibb, Guardant, Tempus, Sanofi, BluePrint, Takeda, and Gilead.
A version of this article first appeared on Medscape.com.
When the Food and Drug Administration (FDA) grants cancer drugs accelerated approval, a key aim is to provide patients faster access to therapies that can benefit them.
The downside of a speedier approval timeline, however, is that it’s often not yet clear whether the new drugs will actually allow a patient to live longer or better. Information on overall survival and quality of life typically comes years later, after drugs undergo confirmatory trials, or sometimes not at all, if companies fail to conduct these trials.
During this waiting period, patients may be receiving a cancer drug that provides no real clinical benefit but comes with a host of toxicities.
In fact, the odds are about as good as a coin flip. For cancer drugs that have confirmatory trial data, more than half don’t ultimately provide an overall survival or quality of life benefit.
Inherent to the accelerated approval process is the assumption that patients are willing to accept this uncertainty in exchange for faster access.
But is that really the case?
The researchers asked about 870 adults with experience of cancer challenges — either their own cancer diagnosis or that of family or a close friend — whether they valued faster access or certainty that a drug really works.
In the study, participants imagined they had been diagnosed with cancer and could choose between two cancer drugs under investigation in clinical trials but with uncertain effectiveness, and a current standard treatment. Participants had to make a series of choices based on five scenarios.
The first two scenarios were based on the impact of the current standard treatment: A patient’s life expectancy on the standard treatment (6 months up to 3 years), and a patient’s physical health on the standard treatment (functional status restricted only during strenuous activities up to completely disabled).
The remaining three scenarios dealt with the two new drugs: The effect of the new drugs on a surrogate endpoint, progression-free survival (whether the drugs slowed tumor growth for an extra month or 5 additional months compared with the standard treatment), certainty that slowing tumor growth will improve survival (very low to high), and the wait time to access the drugs (immediately to as long as 2 years).
The researchers assessed the relative importance of survival benefit certainty vs wait time and how that balance shifted depending on the different scenarios.
Overall, the researchers found that, if there was no evidence linking the surrogate endpoint (progression-free survival) to overall survival, patients were willing to wait about 8 months for weak evidence of an overall survival benefit (ie, low certainty the drug will extend survival by 1-5 months), about 16 months for moderate certainty, and almost 22 months for high certainty.
Despite a willingness to wait for greater certainty, participants did value speed as well. Overall, respondents showed a strong preference against a 1-year delay in FDA approval time. People who were aged 55 years or more and were non-White individuals made less than $40,000 year as well as those with the lowest life expectancy on a current standard treatment were most sensitive to wait times while those with better functional status and longer life expectancies on a current treatment were less sensitive to longer wait times.
“Our results indicate that some patients (except those with the poorest prognoses) would find the additional time required to generate evidence on the survival benefit of new cancer drugs an acceptable tradeoff,” the study authors concluded.
Although people do place high value on timely access to new cancer drugs, especially if there are limited treatment options, many are willing to wait for greater certainty that a new drug provides an overall survival benefit, lead author Robin Forrest, MSc, with the Department of Health Policy, London School of Economics in England, said in an interview.
In the study, respondents also did not place significant value on whether the drug substantially slowed cancer growth. “In other words, substantial progression-free survival benefit of a drug did not compensate for lack of certainty about a drug’s benefit on survival in respondents’ drug choices,” the authors explained.
“In an effort to move quickly, we have accepted progression-free survival [as a surrogate endpoint],” Jyoti D. Patel, MD, oncologist with Northwestern Memorial Hospital, Chicago, Illinois, who wasn’t involved in the study. But a growing body of evidence indicates that progression-free survival is often a poor surrogate for overall survival. And what this study suggests is that “patients uniformly care about improvements in overall survival and the quality of that survival,” Patel said.
Bishal Gyawali, MD, PhD, was not surprised by the findings.
“I always thought this was the real-world scenario, but the problem is the voices of ordinary patients are not heard,” Gyawali, with Queen’s University, Kingston, Ontario, Canada, who also wasn’t involved in the study, said in an interview.
“What is heard is the loud noise of ‘we need access now, today, yesterday’ — ‘we don’t care if the drug doesn’t improve overall survival, we just need a drug, any drug’ — ‘we don’t care how much it costs, we need access today,’ ” Gyawali said. “Not saying this is wrong, but this is not the representation of all patients.”
However, the voices of patients who are more cautious and want evidence of benefit before accepting toxicities don’t make headlines, he added.
What this survey means from a policy perspective, said Gyawali, is that accelerated approvals that do not mandate survival endpoint in confirmatory trials are ignoring the need of many patients who prioritize certainty of benefit over speed of access.
The study was funded by the London School of Economics and Political Science Phelan United States Centre. Forrest had no relevant disclosures. Gyawali has received consulting fees from Vivio Health. Patel has various relationships with AbbVie, Anheart, AstraZeneca, Bristol-Myers Squibb, Guardant, Tempus, Sanofi, BluePrint, Takeda, and Gilead.
A version of this article first appeared on Medscape.com.
When the Food and Drug Administration (FDA) grants cancer drugs accelerated approval, a key aim is to provide patients faster access to therapies that can benefit them.
The downside of a speedier approval timeline, however, is that it’s often not yet clear whether the new drugs will actually allow a patient to live longer or better. Information on overall survival and quality of life typically comes years later, after drugs undergo confirmatory trials, or sometimes not at all, if companies fail to conduct these trials.
During this waiting period, patients may be receiving a cancer drug that provides no real clinical benefit but comes with a host of toxicities.
In fact, the odds are about as good as a coin flip. For cancer drugs that have confirmatory trial data, more than half don’t ultimately provide an overall survival or quality of life benefit.
Inherent to the accelerated approval process is the assumption that patients are willing to accept this uncertainty in exchange for faster access.
But is that really the case?
The researchers asked about 870 adults with experience of cancer challenges — either their own cancer diagnosis or that of family or a close friend — whether they valued faster access or certainty that a drug really works.
In the study, participants imagined they had been diagnosed with cancer and could choose between two cancer drugs under investigation in clinical trials but with uncertain effectiveness, and a current standard treatment. Participants had to make a series of choices based on five scenarios.
The first two scenarios were based on the impact of the current standard treatment: A patient’s life expectancy on the standard treatment (6 months up to 3 years), and a patient’s physical health on the standard treatment (functional status restricted only during strenuous activities up to completely disabled).
The remaining three scenarios dealt with the two new drugs: The effect of the new drugs on a surrogate endpoint, progression-free survival (whether the drugs slowed tumor growth for an extra month or 5 additional months compared with the standard treatment), certainty that slowing tumor growth will improve survival (very low to high), and the wait time to access the drugs (immediately to as long as 2 years).
The researchers assessed the relative importance of survival benefit certainty vs wait time and how that balance shifted depending on the different scenarios.
Overall, the researchers found that, if there was no evidence linking the surrogate endpoint (progression-free survival) to overall survival, patients were willing to wait about 8 months for weak evidence of an overall survival benefit (ie, low certainty the drug will extend survival by 1-5 months), about 16 months for moderate certainty, and almost 22 months for high certainty.
Despite a willingness to wait for greater certainty, participants did value speed as well. Overall, respondents showed a strong preference against a 1-year delay in FDA approval time. People who were aged 55 years or more and were non-White individuals made less than $40,000 year as well as those with the lowest life expectancy on a current standard treatment were most sensitive to wait times while those with better functional status and longer life expectancies on a current treatment were less sensitive to longer wait times.
“Our results indicate that some patients (except those with the poorest prognoses) would find the additional time required to generate evidence on the survival benefit of new cancer drugs an acceptable tradeoff,” the study authors concluded.
Although people do place high value on timely access to new cancer drugs, especially if there are limited treatment options, many are willing to wait for greater certainty that a new drug provides an overall survival benefit, lead author Robin Forrest, MSc, with the Department of Health Policy, London School of Economics in England, said in an interview.
In the study, respondents also did not place significant value on whether the drug substantially slowed cancer growth. “In other words, substantial progression-free survival benefit of a drug did not compensate for lack of certainty about a drug’s benefit on survival in respondents’ drug choices,” the authors explained.
“In an effort to move quickly, we have accepted progression-free survival [as a surrogate endpoint],” Jyoti D. Patel, MD, oncologist with Northwestern Memorial Hospital, Chicago, Illinois, who wasn’t involved in the study. But a growing body of evidence indicates that progression-free survival is often a poor surrogate for overall survival. And what this study suggests is that “patients uniformly care about improvements in overall survival and the quality of that survival,” Patel said.
Bishal Gyawali, MD, PhD, was not surprised by the findings.
“I always thought this was the real-world scenario, but the problem is the voices of ordinary patients are not heard,” Gyawali, with Queen’s University, Kingston, Ontario, Canada, who also wasn’t involved in the study, said in an interview.
“What is heard is the loud noise of ‘we need access now, today, yesterday’ — ‘we don’t care if the drug doesn’t improve overall survival, we just need a drug, any drug’ — ‘we don’t care how much it costs, we need access today,’ ” Gyawali said. “Not saying this is wrong, but this is not the representation of all patients.”
However, the voices of patients who are more cautious and want evidence of benefit before accepting toxicities don’t make headlines, he added.
What this survey means from a policy perspective, said Gyawali, is that accelerated approvals that do not mandate survival endpoint in confirmatory trials are ignoring the need of many patients who prioritize certainty of benefit over speed of access.
The study was funded by the London School of Economics and Political Science Phelan United States Centre. Forrest had no relevant disclosures. Gyawali has received consulting fees from Vivio Health. Patel has various relationships with AbbVie, Anheart, AstraZeneca, Bristol-Myers Squibb, Guardant, Tempus, Sanofi, BluePrint, Takeda, and Gilead.
A version of this article first appeared on Medscape.com.
FROM THE LANCET ONCOLOGY
Clopidogrel Tops Aspirin Post-PCI, Even in High-Risk Cases
TOPLINE:
The beneficial effect of clopidogrel monotherapy over aspirin monotherapy in patients who underwent percutaneous coronary intervention (PCI) and remained event free for 6-18 months on dual antiplatelet therapy (DAPT) is consistent, regardless of bleeding risk or PCI complexity, according to a post hoc analysis of the HOST-EXAM trial.
METHODOLOGY:
- The HOST-EXAM Extended study conducted across 37 sites in South Korea included patients who underwent PCI with drug-eluting stents and remained free of clinical events for 6-18 months post-PCI, while receiving DAPT.
- This post hoc analysis of the HOST-EXAM Extended study compared the effectiveness of long-term daily clopidogrel (75 mg) with that of aspirin monotherapy (100 mg) after PCI, according to bleeding risk and procedural complexity in 3974 patients (mean age, 63 years; 75% men) who were followed for up to 5.9 years.
- High bleeding risk was reported in 866 patients, and 849 patients underwent complex PCI.
- Patients were classified into four distinct risk groups: No bleeding risk and noncomplex PCI, no bleeding risk and complex PCI, high bleeding risk and noncomplex PCI, and high bleeding risk and complex PCI.
- The co-primary endpoints were thrombotic composite events (cardiovascular death, nonfatal myocardial infarction, stroke, readmission due to acute coronary syndrome, and definite/probable stent thrombosis) and any bleeding event.
TAKEAWAY:
- Thrombotic composite events (hazard ratio [HR], 2.15; P < .001) and any bleeding event (HR, 3.64; P < .001) were more frequent in patients with a high bleeding risk than in those without.
- However, there was no difference in the risk for thrombotic composite events or any bleeding event by PCI complexity.
- The long-term benefits of clopidogrel monotherapy over aspirin monotherapy were seen in all patients, regardless of bleeding risks (P for interaction = .38 for thrombotic composite events and P for interaction = .20 for any bleeding event) or PCI complexity (P for interaction = .12 for thrombotic composite events and P for interaction = .62 for any bleeding event).
- The greatest risk reduction in thrombotic composite events with clopidogrel monotherapy occurred in patients with a high bleeding risk who underwent complex PCI (HR, 0.46; P = .03).
IN PRACTICE:
“[In this study], no significant interaction was found between treatment arms and risk groups, denoting that the beneficial impact of clopidogrel monotherapy was consistent regardless of HBR [high bleeding risk] or PCI complexity,” the authors wrote.
SOURCE:
This study was led by Jeehoon Kang, MD, Seoul National University College of Medicine and Seoul National University Hospital, Seoul, Republic of Korea. It was published online on November 27, 2024, in JAMA Cardiology.
LIMITATIONS:
As this study is a post hoc analysis, the findings should be considered primarily hypothesis generating. This study was conducted exclusively in an East Asian population and may not be generalizable to other ethnic groups. The definitions of high bleeding risk and complex PCI used in this analysis were not prespecified in the study protocol of the HOST-EXAM trial. Certain criteria defining high bleeding risk were not analyzed as they fell under the exclusion criteria of the HOST-EXAM trial or were not recorded in the study case report form.
DISCLOSURES:
This study was supported by grants from the Patient-Centered Clinical Research Coordinating Center and Seoul National University Hospital. One author reported receiving grants and personal fees from various pharmaceutical companies outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The beneficial effect of clopidogrel monotherapy over aspirin monotherapy in patients who underwent percutaneous coronary intervention (PCI) and remained event free for 6-18 months on dual antiplatelet therapy (DAPT) is consistent, regardless of bleeding risk or PCI complexity, according to a post hoc analysis of the HOST-EXAM trial.
METHODOLOGY:
- The HOST-EXAM Extended study conducted across 37 sites in South Korea included patients who underwent PCI with drug-eluting stents and remained free of clinical events for 6-18 months post-PCI, while receiving DAPT.
- This post hoc analysis of the HOST-EXAM Extended study compared the effectiveness of long-term daily clopidogrel (75 mg) with that of aspirin monotherapy (100 mg) after PCI, according to bleeding risk and procedural complexity in 3974 patients (mean age, 63 years; 75% men) who were followed for up to 5.9 years.
- High bleeding risk was reported in 866 patients, and 849 patients underwent complex PCI.
- Patients were classified into four distinct risk groups: No bleeding risk and noncomplex PCI, no bleeding risk and complex PCI, high bleeding risk and noncomplex PCI, and high bleeding risk and complex PCI.
- The co-primary endpoints were thrombotic composite events (cardiovascular death, nonfatal myocardial infarction, stroke, readmission due to acute coronary syndrome, and definite/probable stent thrombosis) and any bleeding event.
TAKEAWAY:
- Thrombotic composite events (hazard ratio [HR], 2.15; P < .001) and any bleeding event (HR, 3.64; P < .001) were more frequent in patients with a high bleeding risk than in those without.
- However, there was no difference in the risk for thrombotic composite events or any bleeding event by PCI complexity.
- The long-term benefits of clopidogrel monotherapy over aspirin monotherapy were seen in all patients, regardless of bleeding risks (P for interaction = .38 for thrombotic composite events and P for interaction = .20 for any bleeding event) or PCI complexity (P for interaction = .12 for thrombotic composite events and P for interaction = .62 for any bleeding event).
- The greatest risk reduction in thrombotic composite events with clopidogrel monotherapy occurred in patients with a high bleeding risk who underwent complex PCI (HR, 0.46; P = .03).
IN PRACTICE:
“[In this study], no significant interaction was found between treatment arms and risk groups, denoting that the beneficial impact of clopidogrel monotherapy was consistent regardless of HBR [high bleeding risk] or PCI complexity,” the authors wrote.
SOURCE:
This study was led by Jeehoon Kang, MD, Seoul National University College of Medicine and Seoul National University Hospital, Seoul, Republic of Korea. It was published online on November 27, 2024, in JAMA Cardiology.
LIMITATIONS:
As this study is a post hoc analysis, the findings should be considered primarily hypothesis generating. This study was conducted exclusively in an East Asian population and may not be generalizable to other ethnic groups. The definitions of high bleeding risk and complex PCI used in this analysis were not prespecified in the study protocol of the HOST-EXAM trial. Certain criteria defining high bleeding risk were not analyzed as they fell under the exclusion criteria of the HOST-EXAM trial or were not recorded in the study case report form.
DISCLOSURES:
This study was supported by grants from the Patient-Centered Clinical Research Coordinating Center and Seoul National University Hospital. One author reported receiving grants and personal fees from various pharmaceutical companies outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The beneficial effect of clopidogrel monotherapy over aspirin monotherapy in patients who underwent percutaneous coronary intervention (PCI) and remained event free for 6-18 months on dual antiplatelet therapy (DAPT) is consistent, regardless of bleeding risk or PCI complexity, according to a post hoc analysis of the HOST-EXAM trial.
METHODOLOGY:
- The HOST-EXAM Extended study conducted across 37 sites in South Korea included patients who underwent PCI with drug-eluting stents and remained free of clinical events for 6-18 months post-PCI, while receiving DAPT.
- This post hoc analysis of the HOST-EXAM Extended study compared the effectiveness of long-term daily clopidogrel (75 mg) with that of aspirin monotherapy (100 mg) after PCI, according to bleeding risk and procedural complexity in 3974 patients (mean age, 63 years; 75% men) who were followed for up to 5.9 years.
- High bleeding risk was reported in 866 patients, and 849 patients underwent complex PCI.
- Patients were classified into four distinct risk groups: No bleeding risk and noncomplex PCI, no bleeding risk and complex PCI, high bleeding risk and noncomplex PCI, and high bleeding risk and complex PCI.
- The co-primary endpoints were thrombotic composite events (cardiovascular death, nonfatal myocardial infarction, stroke, readmission due to acute coronary syndrome, and definite/probable stent thrombosis) and any bleeding event.
TAKEAWAY:
- Thrombotic composite events (hazard ratio [HR], 2.15; P < .001) and any bleeding event (HR, 3.64; P < .001) were more frequent in patients with a high bleeding risk than in those without.
- However, there was no difference in the risk for thrombotic composite events or any bleeding event by PCI complexity.
- The long-term benefits of clopidogrel monotherapy over aspirin monotherapy were seen in all patients, regardless of bleeding risks (P for interaction = .38 for thrombotic composite events and P for interaction = .20 for any bleeding event) or PCI complexity (P for interaction = .12 for thrombotic composite events and P for interaction = .62 for any bleeding event).
- The greatest risk reduction in thrombotic composite events with clopidogrel monotherapy occurred in patients with a high bleeding risk who underwent complex PCI (HR, 0.46; P = .03).
IN PRACTICE:
“[In this study], no significant interaction was found between treatment arms and risk groups, denoting that the beneficial impact of clopidogrel monotherapy was consistent regardless of HBR [high bleeding risk] or PCI complexity,” the authors wrote.
SOURCE:
This study was led by Jeehoon Kang, MD, Seoul National University College of Medicine and Seoul National University Hospital, Seoul, Republic of Korea. It was published online on November 27, 2024, in JAMA Cardiology.
LIMITATIONS:
As this study is a post hoc analysis, the findings should be considered primarily hypothesis generating. This study was conducted exclusively in an East Asian population and may not be generalizable to other ethnic groups. The definitions of high bleeding risk and complex PCI used in this analysis were not prespecified in the study protocol of the HOST-EXAM trial. Certain criteria defining high bleeding risk were not analyzed as they fell under the exclusion criteria of the HOST-EXAM trial or were not recorded in the study case report form.
DISCLOSURES:
This study was supported by grants from the Patient-Centered Clinical Research Coordinating Center and Seoul National University Hospital. One author reported receiving grants and personal fees from various pharmaceutical companies outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
CGM Use, GLP-1s, Drinking Water Key of 2025 ADA Standards
plus a strong endorsement for drinking water and much more.
The Standards of Care — 2025 were published December 9 as a supplement to Diabetes Care. The standards “incorporate the latest information from clinical trial data and knowledge of diabetes management into a comprehensive guidelines document that will assist physicians in managing patients with diabetes in their practices,” said Mandeep Bajaj, MBBS, ADA’s President, Medicine & Science.
In an interview, Bajaj highlighted some of the most important of the clinical updates in 2024, including the following:
- Consideration of the use of continuous glucose monitoring devices in adults with type 2 diabetes (T2D) who don’t use insulin. Medicare and many other payers currently only cover CGM for people who use insulin or are otherwise at risk for hypoglycemia. However, some CGMs are now available over the counter, Bajaj pointed out.
- Actions to be taken in the event of medication shortages. The ADA published guidance for this in the case of GLP-1 RAs on December 2. Essentially ADA advised substituting a different GLP-1 RA if possible. Nonapproved products aren’t recommended, but guidance is provided for people who choose to use them.
- Use of GLP-1 RAs for heart and kidney health. Recommendations were revised to explicitly advise on choice of pharmacotherapy for individuals with T2D, based on new data on those with established or high risk for atherosclerotic cardiovascular disease, heart failure with preserved ejection fraction, and chronic kidney disease.
- Treatment of MAFLD with moderate or advanced liver fibrosis. A new recommendation for use of a thyroid hormone receptor–beta agonist is based on trial data for resmetirom. Moreover, Bajaj noted, “we’ve adopted the new nomenclature, which was previously NAFLD and NASH, and now is MAFLD and MASH [metabolic-associated steatohepatitis].”
- Advice to continue weight management therapy beyond achieving weight loss goals. This is based on a large amount of evidence that “stopping these therapies are associated with weight regain and increased cardiovascular risk,” Bajaj said, adding that this recommendation was made in collaboration with the Obesity Society.
- Antibody-based screening for presymptomatic T1D in family members of people with T2D and others who may be at risk. “Individuals who test autoantibody positive should be provided with or referred for counseling about the risk of developing diabetes, diabetes symptoms, and [diabetic ketoacidosis] prevention and should be given consideration for referral to a specialized center for further evaluation and/or consideration of a clinical trial or approved therapy to potentially delay development of clinical diabetes,” the document says.
- Screen for psychosocial issues. People with diabetes should be screened for concerns including diabetes distress, depression, anxiety, fear of hypoglycemia, and disordered eating behaviors. “People on insulin or sulfonylureas may have fear of hypoglycemia, but diabetes distress can happen to anyone with diabetes,” Bajaj pointed out. Caregivers and family members should be screened as well, the document advises.
- Drink water, not soda. In the nutrition section, a new recommendation strongly advises drinking water instead of nutritive or nonnutritive sweetened beverages. “This is an important recommendation. So, when patients ask what’s the best thing to drink, our answer is drink water rather than Coca Cola or Diet Coke,” Bajaj said. But, what about people with diabetes who can’t quit their diet soda habit? “We’ve said that the nonnutritive sweetener is preferred over sugar sweetener, provided it’s in moderation and short term ... but the best is water.”
Bajaj has received grant support from ADA. He had no further disclosures.
A version of this article first appeared on Medscape.com.
plus a strong endorsement for drinking water and much more.
The Standards of Care — 2025 were published December 9 as a supplement to Diabetes Care. The standards “incorporate the latest information from clinical trial data and knowledge of diabetes management into a comprehensive guidelines document that will assist physicians in managing patients with diabetes in their practices,” said Mandeep Bajaj, MBBS, ADA’s President, Medicine & Science.
In an interview, Bajaj highlighted some of the most important of the clinical updates in 2024, including the following:
- Consideration of the use of continuous glucose monitoring devices in adults with type 2 diabetes (T2D) who don’t use insulin. Medicare and many other payers currently only cover CGM for people who use insulin or are otherwise at risk for hypoglycemia. However, some CGMs are now available over the counter, Bajaj pointed out.
- Actions to be taken in the event of medication shortages. The ADA published guidance for this in the case of GLP-1 RAs on December 2. Essentially ADA advised substituting a different GLP-1 RA if possible. Nonapproved products aren’t recommended, but guidance is provided for people who choose to use them.
- Use of GLP-1 RAs for heart and kidney health. Recommendations were revised to explicitly advise on choice of pharmacotherapy for individuals with T2D, based on new data on those with established or high risk for atherosclerotic cardiovascular disease, heart failure with preserved ejection fraction, and chronic kidney disease.
- Treatment of MAFLD with moderate or advanced liver fibrosis. A new recommendation for use of a thyroid hormone receptor–beta agonist is based on trial data for resmetirom. Moreover, Bajaj noted, “we’ve adopted the new nomenclature, which was previously NAFLD and NASH, and now is MAFLD and MASH [metabolic-associated steatohepatitis].”
- Advice to continue weight management therapy beyond achieving weight loss goals. This is based on a large amount of evidence that “stopping these therapies are associated with weight regain and increased cardiovascular risk,” Bajaj said, adding that this recommendation was made in collaboration with the Obesity Society.
- Antibody-based screening for presymptomatic T1D in family members of people with T2D and others who may be at risk. “Individuals who test autoantibody positive should be provided with or referred for counseling about the risk of developing diabetes, diabetes symptoms, and [diabetic ketoacidosis] prevention and should be given consideration for referral to a specialized center for further evaluation and/or consideration of a clinical trial or approved therapy to potentially delay development of clinical diabetes,” the document says.
- Screen for psychosocial issues. People with diabetes should be screened for concerns including diabetes distress, depression, anxiety, fear of hypoglycemia, and disordered eating behaviors. “People on insulin or sulfonylureas may have fear of hypoglycemia, but diabetes distress can happen to anyone with diabetes,” Bajaj pointed out. Caregivers and family members should be screened as well, the document advises.
- Drink water, not soda. In the nutrition section, a new recommendation strongly advises drinking water instead of nutritive or nonnutritive sweetened beverages. “This is an important recommendation. So, when patients ask what’s the best thing to drink, our answer is drink water rather than Coca Cola or Diet Coke,” Bajaj said. But, what about people with diabetes who can’t quit their diet soda habit? “We’ve said that the nonnutritive sweetener is preferred over sugar sweetener, provided it’s in moderation and short term ... but the best is water.”
Bajaj has received grant support from ADA. He had no further disclosures.
A version of this article first appeared on Medscape.com.
plus a strong endorsement for drinking water and much more.
The Standards of Care — 2025 were published December 9 as a supplement to Diabetes Care. The standards “incorporate the latest information from clinical trial data and knowledge of diabetes management into a comprehensive guidelines document that will assist physicians in managing patients with diabetes in their practices,” said Mandeep Bajaj, MBBS, ADA’s President, Medicine & Science.
In an interview, Bajaj highlighted some of the most important of the clinical updates in 2024, including the following:
- Consideration of the use of continuous glucose monitoring devices in adults with type 2 diabetes (T2D) who don’t use insulin. Medicare and many other payers currently only cover CGM for people who use insulin or are otherwise at risk for hypoglycemia. However, some CGMs are now available over the counter, Bajaj pointed out.
- Actions to be taken in the event of medication shortages. The ADA published guidance for this in the case of GLP-1 RAs on December 2. Essentially ADA advised substituting a different GLP-1 RA if possible. Nonapproved products aren’t recommended, but guidance is provided for people who choose to use them.
- Use of GLP-1 RAs for heart and kidney health. Recommendations were revised to explicitly advise on choice of pharmacotherapy for individuals with T2D, based on new data on those with established or high risk for atherosclerotic cardiovascular disease, heart failure with preserved ejection fraction, and chronic kidney disease.
- Treatment of MAFLD with moderate or advanced liver fibrosis. A new recommendation for use of a thyroid hormone receptor–beta agonist is based on trial data for resmetirom. Moreover, Bajaj noted, “we’ve adopted the new nomenclature, which was previously NAFLD and NASH, and now is MAFLD and MASH [metabolic-associated steatohepatitis].”
- Advice to continue weight management therapy beyond achieving weight loss goals. This is based on a large amount of evidence that “stopping these therapies are associated with weight regain and increased cardiovascular risk,” Bajaj said, adding that this recommendation was made in collaboration with the Obesity Society.
- Antibody-based screening for presymptomatic T1D in family members of people with T2D and others who may be at risk. “Individuals who test autoantibody positive should be provided with or referred for counseling about the risk of developing diabetes, diabetes symptoms, and [diabetic ketoacidosis] prevention and should be given consideration for referral to a specialized center for further evaluation and/or consideration of a clinical trial or approved therapy to potentially delay development of clinical diabetes,” the document says.
- Screen for psychosocial issues. People with diabetes should be screened for concerns including diabetes distress, depression, anxiety, fear of hypoglycemia, and disordered eating behaviors. “People on insulin or sulfonylureas may have fear of hypoglycemia, but diabetes distress can happen to anyone with diabetes,” Bajaj pointed out. Caregivers and family members should be screened as well, the document advises.
- Drink water, not soda. In the nutrition section, a new recommendation strongly advises drinking water instead of nutritive or nonnutritive sweetened beverages. “This is an important recommendation. So, when patients ask what’s the best thing to drink, our answer is drink water rather than Coca Cola or Diet Coke,” Bajaj said. But, what about people with diabetes who can’t quit their diet soda habit? “We’ve said that the nonnutritive sweetener is preferred over sugar sweetener, provided it’s in moderation and short term ... but the best is water.”
Bajaj has received grant support from ADA. He had no further disclosures.
A version of this article first appeared on Medscape.com.
Freezing the Pain: A New Way to Treat Rib Fractures
This transcript has been edited for clarity.
Robert D. Glatter, MD: Hi. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Joining me today to discuss a novel way to treat pain related to conditions such as rib fractures and burns is Dr. Sergey Motov, an emergency physician with expertise in pain management and research director in the Department of Emergency Medicine at Maimonides Medical Center in Brooklyn, New York.
Also joining me is Dr. Gary Schwartz, vice chair of pain and anesthesiology at Maimonides Medical Center. Dr. Schwartz is board certified in anesthesiology and interventional pain management.
Welcome, Sergey and Gary.
Sergey M. Motov, MD: Thank you, Robert.
Gary S. Schwartz, MD: Thank you, Robert.
Traditional Approaches to Pain Relief
Glatter: It’s a pleasure to have you both. Sergey, we were chatting earlier this week and you had mentioned a novel approach to treating a common condition we encounter in the emergency department — rib fractures.
As we all know, they’re very painful and can lead to pulmonary complications, including atelectasis, pneumonia due to splinting and lack of proper pain management, along with the use of incentive spirometry.
Sergey and Gary, can you describe traditional approaches to alleviating the pain associated with rib fractures? What do we typically use? Then we’ll get to some novel treatments that we’re here to discuss.
Motov: I’m going to use the emergency medicine approach to rib fractures. As you pointed out, pain relief is of utmost importance.
With the advent and acquiring of the amazing technique of interventional pain management, physicians, for the most part, are very astute about providing nerve blocks to alleviate pain, at least in immediate need. I’m talking about the relatively short term, 1-5 hours, in the emergency department.
Primarily, we focus on fascial plane blocks such as serratus anterior plane block. Traditionally, ED physicians don’t use much of the intercostal blocks. At times, we can direct the spinal block to cover the lateral aspect of the chest wall.
As part of the multimodal approach, we can use NSAIDs. If there’s a contraindication, we can use opioids. There are some data to support consideration of using topical formularies such as a lidocaine patch, but they are somewhat conflicting.
The question becomes what you’re going to send a patient home with. Again, traditional teaching is either opioids, immediate release with a short course, plus or minus NSAIDs, plus or minus acetaminophen.
The issue with rib fractures is that, while we can manage immediate and super-acute pain presentation in the ED and then discharge up to 24-72 hours, what happens afterwards is very challenging. Acute intercostal neuralgia related to traumatic rib fractures is semi-manageable, but if it’s inappropriately treated, it has a great tendency to transform into chronic intercostal neuralgia. It contributes a great deal of disability and morbidity.
Several years ago, I came across an entity called cryoneurolysis (cryo ─ cold temperature; neurolysis ─ freezing the nerve). I’m excited to be here today because Gary is the one who’s pioneering and championing this technique in our institution.
Cryoneurolysis: Mechanisms of Action and Benefits
Glatter: Gary, what do you see as the main role for this procedure at this time?
Schwartz: As Sergey alluded to, the traditional approach of opiates has side effects (ie, constipation, addiction, and tolerance). Unfortunately, many of these rib fractures occur in older patients. They come in anticoagulated, so they can’t have NSAIDs.
Sergey and his team in the ER have been pioneers in giving short-acting local anesthetic blocks that could last anywhere from 12 to 24 hours. There are long-acting local anesthetics that we can get out to 72 hours.
Unfortunately, these rib fractures and the pain associated with them, in addition to the intercostal neuralgia, could take weeks to heal. That’s where cryoneurolysis comes in. We’re all used to ice or cold temperature. For example, if your child gets an ear piercing, they put some ice on their earlobe beforehand, it numbs it up, and they don’t feel pain. It allows them to get their ears pierced without pain, but it’s short-acting.
What we have now are handheld devices with tips about as long as a pen, 3.5 inches, that allow you to go down precisely to these intercostal nerves that innervate the ribs and give a cold lesion that freezes these nerves.
The benefit of it is it’s not permanent like cryoablation, like we’ve seen for tumor necrosis, which destroys outside tissues. It’s really a small lesion, about 16 mm x 8 mm, which is enough to engulf the nerve and pretty much stun it.
It causes axonotmesis, but the epineurium, the endoneurium, and the perineurium — the inner workings of the nerve — stay intact, so it regrows. It just destroys the myelin sheath and the axon.
Glatter: You’re creating a scarring effect; is that what you’re saying? In other words, you’re doing a cold-temperature freeze and stunning the nerve. My question is, does it regrow? Is this a permanent type of injury?
Schwartz: With Wallerian degeneration, nerves do regrow after injuries.
Unfortunately, as you two probably see in the ER for big traumas, where the nerve is transected, those unfortunately do not grow back. This is considered a grade 2 lesion, so the Wallerian degeneration recurs. The nerves grow, depending on the literature you look at, about 0.5-2 mm per day.
This intervention gives us at least 3 months of relief for the patient, which is in the time frame where the rib fracture will heal, hopefully with no damage to the nerve from the fracture, and they go on living their life without having to take opiates or having to stop their anticoagulation.
Because prior to this, when I was a pain fellow, we used to put epidurals in many of these patients. The problem with that is patients can’t go home, and if they’re anticoagulated, you can’t place it because of the risk of a spinal hematoma.
Potential Use in Ventilation Weaning
Glatter: This is something we encounter daily, and certainly for those patients who have more numerous rib fractures or flail chest, this could be even more devastating, as well as for those who get intubated.
Do you see any role, in terms of ventilator weaning, in using this technique specifically in the ICU setting?
Schwartz: That’s an interesting concept. I’m not so sure about ventilator weaning, but we’ve used this in the hospital for rib fractures from traumas where patients had such severe fractures and had to go to the operating room for rib plating, and did necessitate an epidural. We’ve used this to discontinue their epidural and transition them to get the patient home.
I think that is part of the care, not only in the ER but in the hospital as well. We need to treat the patients, but we also have to have a transition plan to get them out of the hospital. Not that we don’t want to treat our patients, but we have to have a plan to get them home. I’m guessing that might be an interesting stage of research in the future if it does help with weaning from a ventilator.
Glatter: There are some studies out there suggesting that there can be some utility in terms of ventilator weaning using this technique. The ability of this to change how we manage pain is just incredible.
Sergey, do you feel that this is something that you could implement in your ED with your patients in the near future?
Motov: Definitely. I have personally been a very big proponent of it. I’m the theoreticist because I’ve covered a great deal of literature, and now having Gary and his team doing this in our institution, it’s a shame not to capitalize on it. I’m slowly moving toward figuring out the way of collaborative effort to have Gary and his team help my team and our colleagues, bring him on board, and maybe broaden the integration for pain management.
I believe, as Gary emphasized, that geriatric traumatic pain injuries are critically important due to the presence of comorbidities, potential drug interactions, and the challenges of managing these factors effectively.
There is one thing I want to bring up, and Gary, please support me on it. The procedure itself is fascinating because it provides long-term pain relief and reduces morbidity. I wouldn’t say mortality, just reduced morbidity. However, we need to be very conscious of the fact that this blockade, this ice-ball freezing of the nerve, can be detrimental to motor nerves. If your whole goal or idea of faster recovery after postoperative knee or hip replacements, or any traumatic lower- or upper-extremity surgery, includes blockade of motor nerves, it’s not going to be beneficial.
I believe the primary therapeutic application of this technology lies in targeting sensory nerves. For instance, intercostal nerves could be a focus in cases of rib fractures. Additionally, this approach shows promise for treating burns, particularly in the lower and upper extremities. Specifically, targeting nerves such as the lateral femoral cutaneous nerve or the anterior femoral cutaneous nerve could effectively neutralize pain and provide significant relief for weeks, if not months.
Based on additional predilection to what particular indications would be, maybe occipital headache with cervicalgia, occipital nerve block — it’s a sensory block — can benefit from it. Slowly but surely, there’s a slew of painful syndromes for which cryoneurolysis might have a great deal of use in the emergency department.
Cryoneurolysis for Other Pain Syndromes
Glatter: Gary, I’ll let you expand upon additional uses that you see. You did mention one on our chat earlier this week, which was postmastectomy pain syndrome with the intercostal brachial nerve. That’s a very compelling area of interest, certainly for the number of women that go through mastectomies or lumpectomies and that have axillary dissection or nerve injury.
Schwartz: Post-mastectomy is one way you could use this device and technology to attack painful syndromes, such as postmastectomy syndrome. Mastectomies are one of the most common surgeries performed in the United States, but I believe it’s a top three for post-op chronic pain, which we don’t normally think of.
There was a great study by a team in San Diego where they did intercostal brachial and intercostal nerve blocks on multiple nerves, and they decreased pain up to 3 months after the surgery and decreased opiates.
As Sergey alluded to, it’s approved for any peripheral nerve in the body. We’ve used it in our pain office for occipital neuralgia, postherpetic neuralgia, chronic rib pain after fractures, and surgery. Some of the most common uses are for superficial, sensory, genicular nerves, the lateral femoral cutaneous nerve, the anterior femoral cutaneous nerve, and the infrapatellar branch of the saphenous.
You could numb the skin preoperatively before a painful surgery, such as a total knee replacement — or as we like to call it, a total knee arthroplasty — to reduce opiates, improve function, and decrease length of stay. You could attack any sensory nerve.
We’ve utilized that already in our private practice. We’re trying to transition into the hospital to have everyone who gets a knee arthroplasty have this technology to decrease opiates, improve function, and recover faster.
This is quite interesting and motivating for me because when I first started, we had a femoral catheter to block the motor femoral nerve or an epidural. Patients were in the hospital for 3-5 days with the CPM [continuous passive motion] machine, which is like a medieval torture device that you might see in Mad Max — where you’re kind of moving the patient’s knee back and forth after surgery, and they were miserable, taking patient-controlled analgesia and high-dose opiates. Now, we’re freezing these nerves beforehand, doing our nerve blocks in the operating room with long-acting local anesthetic, and patients are going home the same day with minimal or even no opiates sometimes.
Implications for Patient Mobility and DVT Risk
Glatter: You’re getting up to 3 months of relief in that setting, doing it as you described?
Schwartz: Yes, up to 3 months of relief, which is huge, because most patients recovering from a knee arthroplasty, at about the 6- to 8-week mark, have improved range of motion, they have their 110° flexion, they have their extension, and they’re getting back to their normal life.
You cover the whole postoperative rehab, where patients don’t have to get recurring refills, they can participate in physical therapy. As you both know, part of the recovery process is to be able to interact with family and friends without being sleepy, angry, and in pain all day, so they can get back to their normal function.
Glatter: In terms of this procedure, would there be any increase in deep vein thrombosis (DVT) in relation to this, by chance?
Schwartz: Actually, there’s less of a risk of DVT because patients have less pain, so they can get up and move faster. Some of my surgical colleagues who have implemented this in their practice have gotten away from using the stronger anticoagulation like Xarelto (rivaroxaban) or Coumadin (warfarin), and they just give them baby aspirin postoperatively because their patients are going home the same day and walking. It’s probably safer for patients. There’s no research out there yet to show that, but we all know that the more you move and the more you’re not lying around, the lower the risk of having a DVT or a blood clot.
There are studies showing that there’s no damage to blood vessels, other than if you stick it with the needle, because the nitrogen gas in this that allows the ice ball to form does not get injected into the body. It’s all resorbed in the machine. The only thing the body sees is this ice ball, which would melt if you hit a blood vessel because we should be 98 °F and the ice ball is -88 °F. There’s no gas injected into the body either, so there’s no risk of a gas embolism.
Training and Implementation
Glatter: I was going to ask you about air emboli, and you perfectly led right into that.
In terms of training requirements, currently, what do you envision as a way we can train residents and fellows to do this? Is this currently something being considered in curriculum?
Schwartz: We are going to train our residents first. I’m training the attendings. Before you use this technology, you should have a basic understanding of ultrasound, how to use the device, the different settings, and what the risks are for each procedure you’re doing.
Let’s say, as Sergey alluded to, with an intercostal nerve block, you could have a pneumothorax. You have to be able to identify the rib, where the nerve should lie, the innermost intercostal muscle you could see on the newer ultrasounds, and where the pleura lies. People should start with just basic ultrasound training and then advance to a typical intercostal nerve block.
Once you master that, the procedure with the device is not much different than an intercostal nerve block, except you have a handheld device and the needle is just as long as a pen, 3.5 inches.
If you could do a nerve block with a spinal needle, you could do the procedure. Once people have the technical ultrasound skills, then they can advance to needle-based procedures, and once you have that training, you could use this procedure safely and efficaciously.
Glatter: Sergey, do you see this as requiring quite a bit of time and training in your program?
Motov: I mentioned earlier, before we started, that with the advent of ultrasound-guided nerve blocks, the vast majority of physicians are becoming very comfortable and fairly effective with maneuvering a needle and the ultrasound probe. The learning curve is essentially the same. The only difference is, as Gary pointed out, some of the nerves could be new to ED folks, but the technique, the understanding, the visualization, and the knowledge of anatomy are essentially the same.
As he pointed out, if you can use it with a spinal needle and local anesthetic, the procedure becomes exactly the same. It’s a slightly different drug and a different needle, and instead of local anesthetic, you’re using a gas at cold temperatures, and that’s pretty much it.
Glatter: Are there any other barriers to adoption in terms of cost, the device itself, or the companies that manufacture these handheld devices?
Schwartz: There’s always cost associated with the new device, needles, and the gas. Thankfully, they’re covered by Medicare, Medicaid, and most commercial insurances in the current framework, which I think is important. I think Congress is seeing the benefits of opiate sparing that Sergey helped lead in the ED.
At AABP Integrative Pain Care and Wellness and Maimonides, we’re doing this intraoperatively as well. I think the government is seeing that. There was a NOPAIN Act passed in 2023 that, starting January 1, 2025, will allow certain approved companies, devices, and medications to have to be repaid by CMS, Centers for Medicare & Medicaid Services, in the hospital setting and in the outpatient departments. In the inpatient surgical stays, we could have less opiates. I think that’s important. It is reimbursed now. Obviously, there is a cost associated.
The other benefit of this procedure and these techniques is, as Sergey alluded to, it’s done under ultrasound. The way we all learn procedures, whether it be central lines or chest tubes, is the blind technique. There is no good way to practice. In my interventional pain practice, many of our original techniques were done under fluoroscopy, and we don’t want to get extra radiation during practice.
The benefit of ultrasound and the advent of handheld ultrasound devices is that we can practice scanning and techniques on ourselves and on colleagues, without the fear of radiation. Other than the fact that we need to shower after the surgical lube is on from the scanning gel, you could practice your techniques in a safe way without harming a patient or yourself.
Future Directions in Pain Management Techniques
Glatter: Absolutely. Do you see any role for possibly stellate ganglion blocks, which are a bit riskier and have greater depth?
Schwartz: People are looking at different studies because, again, it’s a needle-based technology. We do many stellate ganglion blocks. I have not done it for this procedure yet, but that’s the next step of what I try. Under ultrasound, we could see the longus colli muscle and we could see the carotid artery. Obviously, we don’t see the ganglion per se, but anatomically, we know where it lies. You could drop a couple of lesions on there and give a theoretic prolonged sympathetic block, which might help with symptoms of complex regional pain syndrome.
I know there are some studies that have looked at stellate ganglion blocks for long-COVID symptoms. Unfortunately, it looks like we’re back in another wave right now. I think that’s the next step of the technology.
Glatter: Getting back to the emergency department, burns are something we see commonly — such painful conditions. This is something that could really provide significant relief, especially with burns that involve the chest wall, not just extremity burns.
Motov: I agree with you. Burns would be a very good indication to utilize this technique. Just listening to you and Gary, another thing that pops into my head, which may have actually some science behind it, would be any traumatic amputations done in a civilian environment or even in the military in a combat situation.
A person who has either an upper or lower extremity that is partially or completely severed or amputated, and the pain — God knows how bad it is — if not properly treated, it is going to be a very long recovery. That’s, I believe, another percutaneous condition where cryoneurolysis will be very beneficial to freeze those nerves, allowing patients to recover through rehab acute care, acute phases, rehabilitation, and move on with their lives.
Glatter: In the setting of a painful distal radius fracture, a femur fracture, and things of that nature, Gary, do you see this as a modality in conjunction with emergency medicine colleagues as being something that’s going to really become an important part of our armamentarium?
Schwartz: I do think it’s going to become more important in the future, as there are more studies to show what nerves you could block with cryoneurolysis in the longer term. I think you might see people start using these for fractures, especially for fractures that are not operable at the time or if a patient needs to be optimized prior to surgery.
As Sergey alluded to, it’s optimal in burns. People have been looking for relief of stump pain or postamputation pain. There’s a big researcher in Canada who’s been looking at pain with spasticity for people with cerebral palsy and poststroke issues, where they can’t move and they have pain moving an extremity after these conditions. We’re at just the tip of the iceberg as to where people are going to use this hand-held technology in the future.
Glatter: We use long-acting nerve blocks for hip fractures already in the emergency department. Why not employ this technique, which would have longer effects and limit opiate use?
Schwartz: It might even help a certain subset of the population, at least in Brooklyn, where we have a large elderly population. I believe it’s one of the oldest boroughs in the country, and definitely in New York.
There are some people that go on to surgery just because they might be bedbound, but it’s the pain that is dictating their surgical procedure, not that they’re ever going to walk again.
It’s maybe the next step to look for. If you could block this nerve for 3 months or longer, they’re still going to be bedbound, but maybe you could avoid a surgical procedure that carries its own morbidity and mortality, which I see a big interest in in the future.
Glatter: Absolutely. The idea behind treating spasticity is very important from an occupational therapy standpoint — eating, activities of daily living — just the basics.
Getting someone’s fingers released, being able to move their legs again, and getting them out of contracture states, I think, has a huge role.
Schwartz: Not only for the patient but also for the caregivers. For many of these patients, if they’re contracted fully and the pain from the spasticity is preventing their caregivers from moving them, it’s difficult to put on a shirt, pants, and so on.
One other point I’d like to make is that it’s reproducible. It’s not one-and-done. If the pain comes back from any of these conditions, you could treat again with another cryoneurolysis treatment. The current literature to date shows that it’s just as effective time and time again. I’ve seen clinically that you can repeat this procedure, whereas some of our other procedures that we do in medicine are not as reproducible, which is important for some of these chronic conditions.
Glatter: You had mentioned reimbursement earlier. Currently, this procedure is reimbursed under Medicare, Medicaid, and third-party payers, I assume?
Schwartz: Not all, but many commercial insurers. Yes for Medicare.
Final Takeaways
Glatter: Reimbursement has to be really universal because if this is shown to be more effective and limits opiate use, then there’s no question in my mind that this is such a groundbreaking procedure.
I’ll let you both give a few pearls for our audience to summarize our discussion.
Motov: I’d say it’s somewhat long overdue that this technique and pain-relieving modality should enter the emergency department, with the auspices and the beautiful collaborative effort between emergency department folks and interventional anesthesiologists, pain management specialists, collaborative training, and a collaborative goal of improving patients’ pain throughout the entire journey during the healthcare system.
That would be my only pearl. Just reach out to your colleagues within your respective institutions who you believe have aptitude, knowledge, and expertise. Reach out, get trained, and start passing down the knowledge to your faculty, and by virtue of extension, to your fellow residents and colleagues.
Schwartz: He took the words right out of my mouth. Communication and collaboration are the two most important things. There’s a shortage of physicians in this country. We can only each do so much, so we should each utilize and implement this technology to affect and help as many patients as possible.
We can decrease the amount of opiates, help our patients, help our family members in our community live with decreased pain, improve their function, and just get back to their lives and keep pushing the envelope of what’s the next step in treatment.
Again, like we went from giving opiates for this and that’s it — maybe an epidural, maybe a 5- to 6-hour intercostal nerve block — to fascial plane blocks like Sergey said, to more advanced procedures, to now we can give months of relief.
I think the communication, the collaboration, and the camaraderie among our different specialties are important to push the envelope to help our patients.
Glatter: That’s so well put. I completely agree.
I want to thank both of you for a very lively discussion. It was very informative. Your expertise is greatly appreciated and will certainly benefit our audience. Thank you both again.
Dr. Glatter is an assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. Dr. Motov is professor of emergency medicine and director of research in the Department of Emergency Medicine at Maimonides Medical Center in Brooklyn, New York. Dr. Schwartz is co-owner and primary clinic director at AABP Integrative Pain Care in Brooklyn, New York. Schwartz currently serves as the co-director of AABP Integrative Pain Care and Wellness and the vice chair of pain and anesthesiology for Maimonides Medical Center. Dr. Schwartz reported conflicts of interest with Pacira Biosciences and Dorsal Health; neither Dr. Glatter nor Dr. Motov reported relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Robert D. Glatter, MD: Hi. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Joining me today to discuss a novel way to treat pain related to conditions such as rib fractures and burns is Dr. Sergey Motov, an emergency physician with expertise in pain management and research director in the Department of Emergency Medicine at Maimonides Medical Center in Brooklyn, New York.
Also joining me is Dr. Gary Schwartz, vice chair of pain and anesthesiology at Maimonides Medical Center. Dr. Schwartz is board certified in anesthesiology and interventional pain management.
Welcome, Sergey and Gary.
Sergey M. Motov, MD: Thank you, Robert.
Gary S. Schwartz, MD: Thank you, Robert.
Traditional Approaches to Pain Relief
Glatter: It’s a pleasure to have you both. Sergey, we were chatting earlier this week and you had mentioned a novel approach to treating a common condition we encounter in the emergency department — rib fractures.
As we all know, they’re very painful and can lead to pulmonary complications, including atelectasis, pneumonia due to splinting and lack of proper pain management, along with the use of incentive spirometry.
Sergey and Gary, can you describe traditional approaches to alleviating the pain associated with rib fractures? What do we typically use? Then we’ll get to some novel treatments that we’re here to discuss.
Motov: I’m going to use the emergency medicine approach to rib fractures. As you pointed out, pain relief is of utmost importance.
With the advent and acquiring of the amazing technique of interventional pain management, physicians, for the most part, are very astute about providing nerve blocks to alleviate pain, at least in immediate need. I’m talking about the relatively short term, 1-5 hours, in the emergency department.
Primarily, we focus on fascial plane blocks such as serratus anterior plane block. Traditionally, ED physicians don’t use much of the intercostal blocks. At times, we can direct the spinal block to cover the lateral aspect of the chest wall.
As part of the multimodal approach, we can use NSAIDs. If there’s a contraindication, we can use opioids. There are some data to support consideration of using topical formularies such as a lidocaine patch, but they are somewhat conflicting.
The question becomes what you’re going to send a patient home with. Again, traditional teaching is either opioids, immediate release with a short course, plus or minus NSAIDs, plus or minus acetaminophen.
The issue with rib fractures is that, while we can manage immediate and super-acute pain presentation in the ED and then discharge up to 24-72 hours, what happens afterwards is very challenging. Acute intercostal neuralgia related to traumatic rib fractures is semi-manageable, but if it’s inappropriately treated, it has a great tendency to transform into chronic intercostal neuralgia. It contributes a great deal of disability and morbidity.
Several years ago, I came across an entity called cryoneurolysis (cryo ─ cold temperature; neurolysis ─ freezing the nerve). I’m excited to be here today because Gary is the one who’s pioneering and championing this technique in our institution.
Cryoneurolysis: Mechanisms of Action and Benefits
Glatter: Gary, what do you see as the main role for this procedure at this time?
Schwartz: As Sergey alluded to, the traditional approach of opiates has side effects (ie, constipation, addiction, and tolerance). Unfortunately, many of these rib fractures occur in older patients. They come in anticoagulated, so they can’t have NSAIDs.
Sergey and his team in the ER have been pioneers in giving short-acting local anesthetic blocks that could last anywhere from 12 to 24 hours. There are long-acting local anesthetics that we can get out to 72 hours.
Unfortunately, these rib fractures and the pain associated with them, in addition to the intercostal neuralgia, could take weeks to heal. That’s where cryoneurolysis comes in. We’re all used to ice or cold temperature. For example, if your child gets an ear piercing, they put some ice on their earlobe beforehand, it numbs it up, and they don’t feel pain. It allows them to get their ears pierced without pain, but it’s short-acting.
What we have now are handheld devices with tips about as long as a pen, 3.5 inches, that allow you to go down precisely to these intercostal nerves that innervate the ribs and give a cold lesion that freezes these nerves.
The benefit of it is it’s not permanent like cryoablation, like we’ve seen for tumor necrosis, which destroys outside tissues. It’s really a small lesion, about 16 mm x 8 mm, which is enough to engulf the nerve and pretty much stun it.
It causes axonotmesis, but the epineurium, the endoneurium, and the perineurium — the inner workings of the nerve — stay intact, so it regrows. It just destroys the myelin sheath and the axon.
Glatter: You’re creating a scarring effect; is that what you’re saying? In other words, you’re doing a cold-temperature freeze and stunning the nerve. My question is, does it regrow? Is this a permanent type of injury?
Schwartz: With Wallerian degeneration, nerves do regrow after injuries.
Unfortunately, as you two probably see in the ER for big traumas, where the nerve is transected, those unfortunately do not grow back. This is considered a grade 2 lesion, so the Wallerian degeneration recurs. The nerves grow, depending on the literature you look at, about 0.5-2 mm per day.
This intervention gives us at least 3 months of relief for the patient, which is in the time frame where the rib fracture will heal, hopefully with no damage to the nerve from the fracture, and they go on living their life without having to take opiates or having to stop their anticoagulation.
Because prior to this, when I was a pain fellow, we used to put epidurals in many of these patients. The problem with that is patients can’t go home, and if they’re anticoagulated, you can’t place it because of the risk of a spinal hematoma.
Potential Use in Ventilation Weaning
Glatter: This is something we encounter daily, and certainly for those patients who have more numerous rib fractures or flail chest, this could be even more devastating, as well as for those who get intubated.
Do you see any role, in terms of ventilator weaning, in using this technique specifically in the ICU setting?
Schwartz: That’s an interesting concept. I’m not so sure about ventilator weaning, but we’ve used this in the hospital for rib fractures from traumas where patients had such severe fractures and had to go to the operating room for rib plating, and did necessitate an epidural. We’ve used this to discontinue their epidural and transition them to get the patient home.
I think that is part of the care, not only in the ER but in the hospital as well. We need to treat the patients, but we also have to have a transition plan to get them out of the hospital. Not that we don’t want to treat our patients, but we have to have a plan to get them home. I’m guessing that might be an interesting stage of research in the future if it does help with weaning from a ventilator.
Glatter: There are some studies out there suggesting that there can be some utility in terms of ventilator weaning using this technique. The ability of this to change how we manage pain is just incredible.
Sergey, do you feel that this is something that you could implement in your ED with your patients in the near future?
Motov: Definitely. I have personally been a very big proponent of it. I’m the theoreticist because I’ve covered a great deal of literature, and now having Gary and his team doing this in our institution, it’s a shame not to capitalize on it. I’m slowly moving toward figuring out the way of collaborative effort to have Gary and his team help my team and our colleagues, bring him on board, and maybe broaden the integration for pain management.
I believe, as Gary emphasized, that geriatric traumatic pain injuries are critically important due to the presence of comorbidities, potential drug interactions, and the challenges of managing these factors effectively.
There is one thing I want to bring up, and Gary, please support me on it. The procedure itself is fascinating because it provides long-term pain relief and reduces morbidity. I wouldn’t say mortality, just reduced morbidity. However, we need to be very conscious of the fact that this blockade, this ice-ball freezing of the nerve, can be detrimental to motor nerves. If your whole goal or idea of faster recovery after postoperative knee or hip replacements, or any traumatic lower- or upper-extremity surgery, includes blockade of motor nerves, it’s not going to be beneficial.
I believe the primary therapeutic application of this technology lies in targeting sensory nerves. For instance, intercostal nerves could be a focus in cases of rib fractures. Additionally, this approach shows promise for treating burns, particularly in the lower and upper extremities. Specifically, targeting nerves such as the lateral femoral cutaneous nerve or the anterior femoral cutaneous nerve could effectively neutralize pain and provide significant relief for weeks, if not months.
Based on additional predilection to what particular indications would be, maybe occipital headache with cervicalgia, occipital nerve block — it’s a sensory block — can benefit from it. Slowly but surely, there’s a slew of painful syndromes for which cryoneurolysis might have a great deal of use in the emergency department.
Cryoneurolysis for Other Pain Syndromes
Glatter: Gary, I’ll let you expand upon additional uses that you see. You did mention one on our chat earlier this week, which was postmastectomy pain syndrome with the intercostal brachial nerve. That’s a very compelling area of interest, certainly for the number of women that go through mastectomies or lumpectomies and that have axillary dissection or nerve injury.
Schwartz: Post-mastectomy is one way you could use this device and technology to attack painful syndromes, such as postmastectomy syndrome. Mastectomies are one of the most common surgeries performed in the United States, but I believe it’s a top three for post-op chronic pain, which we don’t normally think of.
There was a great study by a team in San Diego where they did intercostal brachial and intercostal nerve blocks on multiple nerves, and they decreased pain up to 3 months after the surgery and decreased opiates.
As Sergey alluded to, it’s approved for any peripheral nerve in the body. We’ve used it in our pain office for occipital neuralgia, postherpetic neuralgia, chronic rib pain after fractures, and surgery. Some of the most common uses are for superficial, sensory, genicular nerves, the lateral femoral cutaneous nerve, the anterior femoral cutaneous nerve, and the infrapatellar branch of the saphenous.
You could numb the skin preoperatively before a painful surgery, such as a total knee replacement — or as we like to call it, a total knee arthroplasty — to reduce opiates, improve function, and decrease length of stay. You could attack any sensory nerve.
We’ve utilized that already in our private practice. We’re trying to transition into the hospital to have everyone who gets a knee arthroplasty have this technology to decrease opiates, improve function, and recover faster.
This is quite interesting and motivating for me because when I first started, we had a femoral catheter to block the motor femoral nerve or an epidural. Patients were in the hospital for 3-5 days with the CPM [continuous passive motion] machine, which is like a medieval torture device that you might see in Mad Max — where you’re kind of moving the patient’s knee back and forth after surgery, and they were miserable, taking patient-controlled analgesia and high-dose opiates. Now, we’re freezing these nerves beforehand, doing our nerve blocks in the operating room with long-acting local anesthetic, and patients are going home the same day with minimal or even no opiates sometimes.
Implications for Patient Mobility and DVT Risk
Glatter: You’re getting up to 3 months of relief in that setting, doing it as you described?
Schwartz: Yes, up to 3 months of relief, which is huge, because most patients recovering from a knee arthroplasty, at about the 6- to 8-week mark, have improved range of motion, they have their 110° flexion, they have their extension, and they’re getting back to their normal life.
You cover the whole postoperative rehab, where patients don’t have to get recurring refills, they can participate in physical therapy. As you both know, part of the recovery process is to be able to interact with family and friends without being sleepy, angry, and in pain all day, so they can get back to their normal function.
Glatter: In terms of this procedure, would there be any increase in deep vein thrombosis (DVT) in relation to this, by chance?
Schwartz: Actually, there’s less of a risk of DVT because patients have less pain, so they can get up and move faster. Some of my surgical colleagues who have implemented this in their practice have gotten away from using the stronger anticoagulation like Xarelto (rivaroxaban) or Coumadin (warfarin), and they just give them baby aspirin postoperatively because their patients are going home the same day and walking. It’s probably safer for patients. There’s no research out there yet to show that, but we all know that the more you move and the more you’re not lying around, the lower the risk of having a DVT or a blood clot.
There are studies showing that there’s no damage to blood vessels, other than if you stick it with the needle, because the nitrogen gas in this that allows the ice ball to form does not get injected into the body. It’s all resorbed in the machine. The only thing the body sees is this ice ball, which would melt if you hit a blood vessel because we should be 98 °F and the ice ball is -88 °F. There’s no gas injected into the body either, so there’s no risk of a gas embolism.
Training and Implementation
Glatter: I was going to ask you about air emboli, and you perfectly led right into that.
In terms of training requirements, currently, what do you envision as a way we can train residents and fellows to do this? Is this currently something being considered in curriculum?
Schwartz: We are going to train our residents first. I’m training the attendings. Before you use this technology, you should have a basic understanding of ultrasound, how to use the device, the different settings, and what the risks are for each procedure you’re doing.
Let’s say, as Sergey alluded to, with an intercostal nerve block, you could have a pneumothorax. You have to be able to identify the rib, where the nerve should lie, the innermost intercostal muscle you could see on the newer ultrasounds, and where the pleura lies. People should start with just basic ultrasound training and then advance to a typical intercostal nerve block.
Once you master that, the procedure with the device is not much different than an intercostal nerve block, except you have a handheld device and the needle is just as long as a pen, 3.5 inches.
If you could do a nerve block with a spinal needle, you could do the procedure. Once people have the technical ultrasound skills, then they can advance to needle-based procedures, and once you have that training, you could use this procedure safely and efficaciously.
Glatter: Sergey, do you see this as requiring quite a bit of time and training in your program?
Motov: I mentioned earlier, before we started, that with the advent of ultrasound-guided nerve blocks, the vast majority of physicians are becoming very comfortable and fairly effective with maneuvering a needle and the ultrasound probe. The learning curve is essentially the same. The only difference is, as Gary pointed out, some of the nerves could be new to ED folks, but the technique, the understanding, the visualization, and the knowledge of anatomy are essentially the same.
As he pointed out, if you can use it with a spinal needle and local anesthetic, the procedure becomes exactly the same. It’s a slightly different drug and a different needle, and instead of local anesthetic, you’re using a gas at cold temperatures, and that’s pretty much it.
Glatter: Are there any other barriers to adoption in terms of cost, the device itself, or the companies that manufacture these handheld devices?
Schwartz: There’s always cost associated with the new device, needles, and the gas. Thankfully, they’re covered by Medicare, Medicaid, and most commercial insurances in the current framework, which I think is important. I think Congress is seeing the benefits of opiate sparing that Sergey helped lead in the ED.
At AABP Integrative Pain Care and Wellness and Maimonides, we’re doing this intraoperatively as well. I think the government is seeing that. There was a NOPAIN Act passed in 2023 that, starting January 1, 2025, will allow certain approved companies, devices, and medications to have to be repaid by CMS, Centers for Medicare & Medicaid Services, in the hospital setting and in the outpatient departments. In the inpatient surgical stays, we could have less opiates. I think that’s important. It is reimbursed now. Obviously, there is a cost associated.
The other benefit of this procedure and these techniques is, as Sergey alluded to, it’s done under ultrasound. The way we all learn procedures, whether it be central lines or chest tubes, is the blind technique. There is no good way to practice. In my interventional pain practice, many of our original techniques were done under fluoroscopy, and we don’t want to get extra radiation during practice.
The benefit of ultrasound and the advent of handheld ultrasound devices is that we can practice scanning and techniques on ourselves and on colleagues, without the fear of radiation. Other than the fact that we need to shower after the surgical lube is on from the scanning gel, you could practice your techniques in a safe way without harming a patient or yourself.
Future Directions in Pain Management Techniques
Glatter: Absolutely. Do you see any role for possibly stellate ganglion blocks, which are a bit riskier and have greater depth?
Schwartz: People are looking at different studies because, again, it’s a needle-based technology. We do many stellate ganglion blocks. I have not done it for this procedure yet, but that’s the next step of what I try. Under ultrasound, we could see the longus colli muscle and we could see the carotid artery. Obviously, we don’t see the ganglion per se, but anatomically, we know where it lies. You could drop a couple of lesions on there and give a theoretic prolonged sympathetic block, which might help with symptoms of complex regional pain syndrome.
I know there are some studies that have looked at stellate ganglion blocks for long-COVID symptoms. Unfortunately, it looks like we’re back in another wave right now. I think that’s the next step of the technology.
Glatter: Getting back to the emergency department, burns are something we see commonly — such painful conditions. This is something that could really provide significant relief, especially with burns that involve the chest wall, not just extremity burns.
Motov: I agree with you. Burns would be a very good indication to utilize this technique. Just listening to you and Gary, another thing that pops into my head, which may have actually some science behind it, would be any traumatic amputations done in a civilian environment or even in the military in a combat situation.
A person who has either an upper or lower extremity that is partially or completely severed or amputated, and the pain — God knows how bad it is — if not properly treated, it is going to be a very long recovery. That’s, I believe, another percutaneous condition where cryoneurolysis will be very beneficial to freeze those nerves, allowing patients to recover through rehab acute care, acute phases, rehabilitation, and move on with their lives.
Glatter: In the setting of a painful distal radius fracture, a femur fracture, and things of that nature, Gary, do you see this as a modality in conjunction with emergency medicine colleagues as being something that’s going to really become an important part of our armamentarium?
Schwartz: I do think it’s going to become more important in the future, as there are more studies to show what nerves you could block with cryoneurolysis in the longer term. I think you might see people start using these for fractures, especially for fractures that are not operable at the time or if a patient needs to be optimized prior to surgery.
As Sergey alluded to, it’s optimal in burns. People have been looking for relief of stump pain or postamputation pain. There’s a big researcher in Canada who’s been looking at pain with spasticity for people with cerebral palsy and poststroke issues, where they can’t move and they have pain moving an extremity after these conditions. We’re at just the tip of the iceberg as to where people are going to use this hand-held technology in the future.
Glatter: We use long-acting nerve blocks for hip fractures already in the emergency department. Why not employ this technique, which would have longer effects and limit opiate use?
Schwartz: It might even help a certain subset of the population, at least in Brooklyn, where we have a large elderly population. I believe it’s one of the oldest boroughs in the country, and definitely in New York.
There are some people that go on to surgery just because they might be bedbound, but it’s the pain that is dictating their surgical procedure, not that they’re ever going to walk again.
It’s maybe the next step to look for. If you could block this nerve for 3 months or longer, they’re still going to be bedbound, but maybe you could avoid a surgical procedure that carries its own morbidity and mortality, which I see a big interest in in the future.
Glatter: Absolutely. The idea behind treating spasticity is very important from an occupational therapy standpoint — eating, activities of daily living — just the basics.
Getting someone’s fingers released, being able to move their legs again, and getting them out of contracture states, I think, has a huge role.
Schwartz: Not only for the patient but also for the caregivers. For many of these patients, if they’re contracted fully and the pain from the spasticity is preventing their caregivers from moving them, it’s difficult to put on a shirt, pants, and so on.
One other point I’d like to make is that it’s reproducible. It’s not one-and-done. If the pain comes back from any of these conditions, you could treat again with another cryoneurolysis treatment. The current literature to date shows that it’s just as effective time and time again. I’ve seen clinically that you can repeat this procedure, whereas some of our other procedures that we do in medicine are not as reproducible, which is important for some of these chronic conditions.
Glatter: You had mentioned reimbursement earlier. Currently, this procedure is reimbursed under Medicare, Medicaid, and third-party payers, I assume?
Schwartz: Not all, but many commercial insurers. Yes for Medicare.
Final Takeaways
Glatter: Reimbursement has to be really universal because if this is shown to be more effective and limits opiate use, then there’s no question in my mind that this is such a groundbreaking procedure.
I’ll let you both give a few pearls for our audience to summarize our discussion.
Motov: I’d say it’s somewhat long overdue that this technique and pain-relieving modality should enter the emergency department, with the auspices and the beautiful collaborative effort between emergency department folks and interventional anesthesiologists, pain management specialists, collaborative training, and a collaborative goal of improving patients’ pain throughout the entire journey during the healthcare system.
That would be my only pearl. Just reach out to your colleagues within your respective institutions who you believe have aptitude, knowledge, and expertise. Reach out, get trained, and start passing down the knowledge to your faculty, and by virtue of extension, to your fellow residents and colleagues.
Schwartz: He took the words right out of my mouth. Communication and collaboration are the two most important things. There’s a shortage of physicians in this country. We can only each do so much, so we should each utilize and implement this technology to affect and help as many patients as possible.
We can decrease the amount of opiates, help our patients, help our family members in our community live with decreased pain, improve their function, and just get back to their lives and keep pushing the envelope of what’s the next step in treatment.
Again, like we went from giving opiates for this and that’s it — maybe an epidural, maybe a 5- to 6-hour intercostal nerve block — to fascial plane blocks like Sergey said, to more advanced procedures, to now we can give months of relief.
I think the communication, the collaboration, and the camaraderie among our different specialties are important to push the envelope to help our patients.
Glatter: That’s so well put. I completely agree.
I want to thank both of you for a very lively discussion. It was very informative. Your expertise is greatly appreciated and will certainly benefit our audience. Thank you both again.
Dr. Glatter is an assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. Dr. Motov is professor of emergency medicine and director of research in the Department of Emergency Medicine at Maimonides Medical Center in Brooklyn, New York. Dr. Schwartz is co-owner and primary clinic director at AABP Integrative Pain Care in Brooklyn, New York. Schwartz currently serves as the co-director of AABP Integrative Pain Care and Wellness and the vice chair of pain and anesthesiology for Maimonides Medical Center. Dr. Schwartz reported conflicts of interest with Pacira Biosciences and Dorsal Health; neither Dr. Glatter nor Dr. Motov reported relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Robert D. Glatter, MD: Hi. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Joining me today to discuss a novel way to treat pain related to conditions such as rib fractures and burns is Dr. Sergey Motov, an emergency physician with expertise in pain management and research director in the Department of Emergency Medicine at Maimonides Medical Center in Brooklyn, New York.
Also joining me is Dr. Gary Schwartz, vice chair of pain and anesthesiology at Maimonides Medical Center. Dr. Schwartz is board certified in anesthesiology and interventional pain management.
Welcome, Sergey and Gary.
Sergey M. Motov, MD: Thank you, Robert.
Gary S. Schwartz, MD: Thank you, Robert.
Traditional Approaches to Pain Relief
Glatter: It’s a pleasure to have you both. Sergey, we were chatting earlier this week and you had mentioned a novel approach to treating a common condition we encounter in the emergency department — rib fractures.
As we all know, they’re very painful and can lead to pulmonary complications, including atelectasis, pneumonia due to splinting and lack of proper pain management, along with the use of incentive spirometry.
Sergey and Gary, can you describe traditional approaches to alleviating the pain associated with rib fractures? What do we typically use? Then we’ll get to some novel treatments that we’re here to discuss.
Motov: I’m going to use the emergency medicine approach to rib fractures. As you pointed out, pain relief is of utmost importance.
With the advent and acquiring of the amazing technique of interventional pain management, physicians, for the most part, are very astute about providing nerve blocks to alleviate pain, at least in immediate need. I’m talking about the relatively short term, 1-5 hours, in the emergency department.
Primarily, we focus on fascial plane blocks such as serratus anterior plane block. Traditionally, ED physicians don’t use much of the intercostal blocks. At times, we can direct the spinal block to cover the lateral aspect of the chest wall.
As part of the multimodal approach, we can use NSAIDs. If there’s a contraindication, we can use opioids. There are some data to support consideration of using topical formularies such as a lidocaine patch, but they are somewhat conflicting.
The question becomes what you’re going to send a patient home with. Again, traditional teaching is either opioids, immediate release with a short course, plus or minus NSAIDs, plus or minus acetaminophen.
The issue with rib fractures is that, while we can manage immediate and super-acute pain presentation in the ED and then discharge up to 24-72 hours, what happens afterwards is very challenging. Acute intercostal neuralgia related to traumatic rib fractures is semi-manageable, but if it’s inappropriately treated, it has a great tendency to transform into chronic intercostal neuralgia. It contributes a great deal of disability and morbidity.
Several years ago, I came across an entity called cryoneurolysis (cryo ─ cold temperature; neurolysis ─ freezing the nerve). I’m excited to be here today because Gary is the one who’s pioneering and championing this technique in our institution.
Cryoneurolysis: Mechanisms of Action and Benefits
Glatter: Gary, what do you see as the main role for this procedure at this time?
Schwartz: As Sergey alluded to, the traditional approach of opiates has side effects (ie, constipation, addiction, and tolerance). Unfortunately, many of these rib fractures occur in older patients. They come in anticoagulated, so they can’t have NSAIDs.
Sergey and his team in the ER have been pioneers in giving short-acting local anesthetic blocks that could last anywhere from 12 to 24 hours. There are long-acting local anesthetics that we can get out to 72 hours.
Unfortunately, these rib fractures and the pain associated with them, in addition to the intercostal neuralgia, could take weeks to heal. That’s where cryoneurolysis comes in. We’re all used to ice or cold temperature. For example, if your child gets an ear piercing, they put some ice on their earlobe beforehand, it numbs it up, and they don’t feel pain. It allows them to get their ears pierced without pain, but it’s short-acting.
What we have now are handheld devices with tips about as long as a pen, 3.5 inches, that allow you to go down precisely to these intercostal nerves that innervate the ribs and give a cold lesion that freezes these nerves.
The benefit of it is it’s not permanent like cryoablation, like we’ve seen for tumor necrosis, which destroys outside tissues. It’s really a small lesion, about 16 mm x 8 mm, which is enough to engulf the nerve and pretty much stun it.
It causes axonotmesis, but the epineurium, the endoneurium, and the perineurium — the inner workings of the nerve — stay intact, so it regrows. It just destroys the myelin sheath and the axon.
Glatter: You’re creating a scarring effect; is that what you’re saying? In other words, you’re doing a cold-temperature freeze and stunning the nerve. My question is, does it regrow? Is this a permanent type of injury?
Schwartz: With Wallerian degeneration, nerves do regrow after injuries.
Unfortunately, as you two probably see in the ER for big traumas, where the nerve is transected, those unfortunately do not grow back. This is considered a grade 2 lesion, so the Wallerian degeneration recurs. The nerves grow, depending on the literature you look at, about 0.5-2 mm per day.
This intervention gives us at least 3 months of relief for the patient, which is in the time frame where the rib fracture will heal, hopefully with no damage to the nerve from the fracture, and they go on living their life without having to take opiates or having to stop their anticoagulation.
Because prior to this, when I was a pain fellow, we used to put epidurals in many of these patients. The problem with that is patients can’t go home, and if they’re anticoagulated, you can’t place it because of the risk of a spinal hematoma.
Potential Use in Ventilation Weaning
Glatter: This is something we encounter daily, and certainly for those patients who have more numerous rib fractures or flail chest, this could be even more devastating, as well as for those who get intubated.
Do you see any role, in terms of ventilator weaning, in using this technique specifically in the ICU setting?
Schwartz: That’s an interesting concept. I’m not so sure about ventilator weaning, but we’ve used this in the hospital for rib fractures from traumas where patients had such severe fractures and had to go to the operating room for rib plating, and did necessitate an epidural. We’ve used this to discontinue their epidural and transition them to get the patient home.
I think that is part of the care, not only in the ER but in the hospital as well. We need to treat the patients, but we also have to have a transition plan to get them out of the hospital. Not that we don’t want to treat our patients, but we have to have a plan to get them home. I’m guessing that might be an interesting stage of research in the future if it does help with weaning from a ventilator.
Glatter: There are some studies out there suggesting that there can be some utility in terms of ventilator weaning using this technique. The ability of this to change how we manage pain is just incredible.
Sergey, do you feel that this is something that you could implement in your ED with your patients in the near future?
Motov: Definitely. I have personally been a very big proponent of it. I’m the theoreticist because I’ve covered a great deal of literature, and now having Gary and his team doing this in our institution, it’s a shame not to capitalize on it. I’m slowly moving toward figuring out the way of collaborative effort to have Gary and his team help my team and our colleagues, bring him on board, and maybe broaden the integration for pain management.
I believe, as Gary emphasized, that geriatric traumatic pain injuries are critically important due to the presence of comorbidities, potential drug interactions, and the challenges of managing these factors effectively.
There is one thing I want to bring up, and Gary, please support me on it. The procedure itself is fascinating because it provides long-term pain relief and reduces morbidity. I wouldn’t say mortality, just reduced morbidity. However, we need to be very conscious of the fact that this blockade, this ice-ball freezing of the nerve, can be detrimental to motor nerves. If your whole goal or idea of faster recovery after postoperative knee or hip replacements, or any traumatic lower- or upper-extremity surgery, includes blockade of motor nerves, it’s not going to be beneficial.
I believe the primary therapeutic application of this technology lies in targeting sensory nerves. For instance, intercostal nerves could be a focus in cases of rib fractures. Additionally, this approach shows promise for treating burns, particularly in the lower and upper extremities. Specifically, targeting nerves such as the lateral femoral cutaneous nerve or the anterior femoral cutaneous nerve could effectively neutralize pain and provide significant relief for weeks, if not months.
Based on additional predilection to what particular indications would be, maybe occipital headache with cervicalgia, occipital nerve block — it’s a sensory block — can benefit from it. Slowly but surely, there’s a slew of painful syndromes for which cryoneurolysis might have a great deal of use in the emergency department.
Cryoneurolysis for Other Pain Syndromes
Glatter: Gary, I’ll let you expand upon additional uses that you see. You did mention one on our chat earlier this week, which was postmastectomy pain syndrome with the intercostal brachial nerve. That’s a very compelling area of interest, certainly for the number of women that go through mastectomies or lumpectomies and that have axillary dissection or nerve injury.
Schwartz: Post-mastectomy is one way you could use this device and technology to attack painful syndromes, such as postmastectomy syndrome. Mastectomies are one of the most common surgeries performed in the United States, but I believe it’s a top three for post-op chronic pain, which we don’t normally think of.
There was a great study by a team in San Diego where they did intercostal brachial and intercostal nerve blocks on multiple nerves, and they decreased pain up to 3 months after the surgery and decreased opiates.
As Sergey alluded to, it’s approved for any peripheral nerve in the body. We’ve used it in our pain office for occipital neuralgia, postherpetic neuralgia, chronic rib pain after fractures, and surgery. Some of the most common uses are for superficial, sensory, genicular nerves, the lateral femoral cutaneous nerve, the anterior femoral cutaneous nerve, and the infrapatellar branch of the saphenous.
You could numb the skin preoperatively before a painful surgery, such as a total knee replacement — or as we like to call it, a total knee arthroplasty — to reduce opiates, improve function, and decrease length of stay. You could attack any sensory nerve.
We’ve utilized that already in our private practice. We’re trying to transition into the hospital to have everyone who gets a knee arthroplasty have this technology to decrease opiates, improve function, and recover faster.
This is quite interesting and motivating for me because when I first started, we had a femoral catheter to block the motor femoral nerve or an epidural. Patients were in the hospital for 3-5 days with the CPM [continuous passive motion] machine, which is like a medieval torture device that you might see in Mad Max — where you’re kind of moving the patient’s knee back and forth after surgery, and they were miserable, taking patient-controlled analgesia and high-dose opiates. Now, we’re freezing these nerves beforehand, doing our nerve blocks in the operating room with long-acting local anesthetic, and patients are going home the same day with minimal or even no opiates sometimes.
Implications for Patient Mobility and DVT Risk
Glatter: You’re getting up to 3 months of relief in that setting, doing it as you described?
Schwartz: Yes, up to 3 months of relief, which is huge, because most patients recovering from a knee arthroplasty, at about the 6- to 8-week mark, have improved range of motion, they have their 110° flexion, they have their extension, and they’re getting back to their normal life.
You cover the whole postoperative rehab, where patients don’t have to get recurring refills, they can participate in physical therapy. As you both know, part of the recovery process is to be able to interact with family and friends without being sleepy, angry, and in pain all day, so they can get back to their normal function.
Glatter: In terms of this procedure, would there be any increase in deep vein thrombosis (DVT) in relation to this, by chance?
Schwartz: Actually, there’s less of a risk of DVT because patients have less pain, so they can get up and move faster. Some of my surgical colleagues who have implemented this in their practice have gotten away from using the stronger anticoagulation like Xarelto (rivaroxaban) or Coumadin (warfarin), and they just give them baby aspirin postoperatively because their patients are going home the same day and walking. It’s probably safer for patients. There’s no research out there yet to show that, but we all know that the more you move and the more you’re not lying around, the lower the risk of having a DVT or a blood clot.
There are studies showing that there’s no damage to blood vessels, other than if you stick it with the needle, because the nitrogen gas in this that allows the ice ball to form does not get injected into the body. It’s all resorbed in the machine. The only thing the body sees is this ice ball, which would melt if you hit a blood vessel because we should be 98 °F and the ice ball is -88 °F. There’s no gas injected into the body either, so there’s no risk of a gas embolism.
Training and Implementation
Glatter: I was going to ask you about air emboli, and you perfectly led right into that.
In terms of training requirements, currently, what do you envision as a way we can train residents and fellows to do this? Is this currently something being considered in curriculum?
Schwartz: We are going to train our residents first. I’m training the attendings. Before you use this technology, you should have a basic understanding of ultrasound, how to use the device, the different settings, and what the risks are for each procedure you’re doing.
Let’s say, as Sergey alluded to, with an intercostal nerve block, you could have a pneumothorax. You have to be able to identify the rib, where the nerve should lie, the innermost intercostal muscle you could see on the newer ultrasounds, and where the pleura lies. People should start with just basic ultrasound training and then advance to a typical intercostal nerve block.
Once you master that, the procedure with the device is not much different than an intercostal nerve block, except you have a handheld device and the needle is just as long as a pen, 3.5 inches.
If you could do a nerve block with a spinal needle, you could do the procedure. Once people have the technical ultrasound skills, then they can advance to needle-based procedures, and once you have that training, you could use this procedure safely and efficaciously.
Glatter: Sergey, do you see this as requiring quite a bit of time and training in your program?
Motov: I mentioned earlier, before we started, that with the advent of ultrasound-guided nerve blocks, the vast majority of physicians are becoming very comfortable and fairly effective with maneuvering a needle and the ultrasound probe. The learning curve is essentially the same. The only difference is, as Gary pointed out, some of the nerves could be new to ED folks, but the technique, the understanding, the visualization, and the knowledge of anatomy are essentially the same.
As he pointed out, if you can use it with a spinal needle and local anesthetic, the procedure becomes exactly the same. It’s a slightly different drug and a different needle, and instead of local anesthetic, you’re using a gas at cold temperatures, and that’s pretty much it.
Glatter: Are there any other barriers to adoption in terms of cost, the device itself, or the companies that manufacture these handheld devices?
Schwartz: There’s always cost associated with the new device, needles, and the gas. Thankfully, they’re covered by Medicare, Medicaid, and most commercial insurances in the current framework, which I think is important. I think Congress is seeing the benefits of opiate sparing that Sergey helped lead in the ED.
At AABP Integrative Pain Care and Wellness and Maimonides, we’re doing this intraoperatively as well. I think the government is seeing that. There was a NOPAIN Act passed in 2023 that, starting January 1, 2025, will allow certain approved companies, devices, and medications to have to be repaid by CMS, Centers for Medicare & Medicaid Services, in the hospital setting and in the outpatient departments. In the inpatient surgical stays, we could have less opiates. I think that’s important. It is reimbursed now. Obviously, there is a cost associated.
The other benefit of this procedure and these techniques is, as Sergey alluded to, it’s done under ultrasound. The way we all learn procedures, whether it be central lines or chest tubes, is the blind technique. There is no good way to practice. In my interventional pain practice, many of our original techniques were done under fluoroscopy, and we don’t want to get extra radiation during practice.
The benefit of ultrasound and the advent of handheld ultrasound devices is that we can practice scanning and techniques on ourselves and on colleagues, without the fear of radiation. Other than the fact that we need to shower after the surgical lube is on from the scanning gel, you could practice your techniques in a safe way without harming a patient or yourself.
Future Directions in Pain Management Techniques
Glatter: Absolutely. Do you see any role for possibly stellate ganglion blocks, which are a bit riskier and have greater depth?
Schwartz: People are looking at different studies because, again, it’s a needle-based technology. We do many stellate ganglion blocks. I have not done it for this procedure yet, but that’s the next step of what I try. Under ultrasound, we could see the longus colli muscle and we could see the carotid artery. Obviously, we don’t see the ganglion per se, but anatomically, we know where it lies. You could drop a couple of lesions on there and give a theoretic prolonged sympathetic block, which might help with symptoms of complex regional pain syndrome.
I know there are some studies that have looked at stellate ganglion blocks for long-COVID symptoms. Unfortunately, it looks like we’re back in another wave right now. I think that’s the next step of the technology.
Glatter: Getting back to the emergency department, burns are something we see commonly — such painful conditions. This is something that could really provide significant relief, especially with burns that involve the chest wall, not just extremity burns.
Motov: I agree with you. Burns would be a very good indication to utilize this technique. Just listening to you and Gary, another thing that pops into my head, which may have actually some science behind it, would be any traumatic amputations done in a civilian environment or even in the military in a combat situation.
A person who has either an upper or lower extremity that is partially or completely severed or amputated, and the pain — God knows how bad it is — if not properly treated, it is going to be a very long recovery. That’s, I believe, another percutaneous condition where cryoneurolysis will be very beneficial to freeze those nerves, allowing patients to recover through rehab acute care, acute phases, rehabilitation, and move on with their lives.
Glatter: In the setting of a painful distal radius fracture, a femur fracture, and things of that nature, Gary, do you see this as a modality in conjunction with emergency medicine colleagues as being something that’s going to really become an important part of our armamentarium?
Schwartz: I do think it’s going to become more important in the future, as there are more studies to show what nerves you could block with cryoneurolysis in the longer term. I think you might see people start using these for fractures, especially for fractures that are not operable at the time or if a patient needs to be optimized prior to surgery.
As Sergey alluded to, it’s optimal in burns. People have been looking for relief of stump pain or postamputation pain. There’s a big researcher in Canada who’s been looking at pain with spasticity for people with cerebral palsy and poststroke issues, where they can’t move and they have pain moving an extremity after these conditions. We’re at just the tip of the iceberg as to where people are going to use this hand-held technology in the future.
Glatter: We use long-acting nerve blocks for hip fractures already in the emergency department. Why not employ this technique, which would have longer effects and limit opiate use?
Schwartz: It might even help a certain subset of the population, at least in Brooklyn, where we have a large elderly population. I believe it’s one of the oldest boroughs in the country, and definitely in New York.
There are some people that go on to surgery just because they might be bedbound, but it’s the pain that is dictating their surgical procedure, not that they’re ever going to walk again.
It’s maybe the next step to look for. If you could block this nerve for 3 months or longer, they’re still going to be bedbound, but maybe you could avoid a surgical procedure that carries its own morbidity and mortality, which I see a big interest in in the future.
Glatter: Absolutely. The idea behind treating spasticity is very important from an occupational therapy standpoint — eating, activities of daily living — just the basics.
Getting someone’s fingers released, being able to move their legs again, and getting them out of contracture states, I think, has a huge role.
Schwartz: Not only for the patient but also for the caregivers. For many of these patients, if they’re contracted fully and the pain from the spasticity is preventing their caregivers from moving them, it’s difficult to put on a shirt, pants, and so on.
One other point I’d like to make is that it’s reproducible. It’s not one-and-done. If the pain comes back from any of these conditions, you could treat again with another cryoneurolysis treatment. The current literature to date shows that it’s just as effective time and time again. I’ve seen clinically that you can repeat this procedure, whereas some of our other procedures that we do in medicine are not as reproducible, which is important for some of these chronic conditions.
Glatter: You had mentioned reimbursement earlier. Currently, this procedure is reimbursed under Medicare, Medicaid, and third-party payers, I assume?
Schwartz: Not all, but many commercial insurers. Yes for Medicare.
Final Takeaways
Glatter: Reimbursement has to be really universal because if this is shown to be more effective and limits opiate use, then there’s no question in my mind that this is such a groundbreaking procedure.
I’ll let you both give a few pearls for our audience to summarize our discussion.
Motov: I’d say it’s somewhat long overdue that this technique and pain-relieving modality should enter the emergency department, with the auspices and the beautiful collaborative effort between emergency department folks and interventional anesthesiologists, pain management specialists, collaborative training, and a collaborative goal of improving patients’ pain throughout the entire journey during the healthcare system.
That would be my only pearl. Just reach out to your colleagues within your respective institutions who you believe have aptitude, knowledge, and expertise. Reach out, get trained, and start passing down the knowledge to your faculty, and by virtue of extension, to your fellow residents and colleagues.
Schwartz: He took the words right out of my mouth. Communication and collaboration are the two most important things. There’s a shortage of physicians in this country. We can only each do so much, so we should each utilize and implement this technology to affect and help as many patients as possible.
We can decrease the amount of opiates, help our patients, help our family members in our community live with decreased pain, improve their function, and just get back to their lives and keep pushing the envelope of what’s the next step in treatment.
Again, like we went from giving opiates for this and that’s it — maybe an epidural, maybe a 5- to 6-hour intercostal nerve block — to fascial plane blocks like Sergey said, to more advanced procedures, to now we can give months of relief.
I think the communication, the collaboration, and the camaraderie among our different specialties are important to push the envelope to help our patients.
Glatter: That’s so well put. I completely agree.
I want to thank both of you for a very lively discussion. It was very informative. Your expertise is greatly appreciated and will certainly benefit our audience. Thank you both again.
Dr. Glatter is an assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. Dr. Motov is professor of emergency medicine and director of research in the Department of Emergency Medicine at Maimonides Medical Center in Brooklyn, New York. Dr. Schwartz is co-owner and primary clinic director at AABP Integrative Pain Care in Brooklyn, New York. Schwartz currently serves as the co-director of AABP Integrative Pain Care and Wellness and the vice chair of pain and anesthesiology for Maimonides Medical Center. Dr. Schwartz reported conflicts of interest with Pacira Biosciences and Dorsal Health; neither Dr. Glatter nor Dr. Motov reported relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Skin Stress Biomarker May Predict Nerve Damage in Early T2D
TOPLINE:
Increased cutaneous carbonyl stress is linked to slower nerve conduction in patients with metabolically well-controlled, recent-onset type 2 diabetes (T2D) and can predict the development of neuropathic deficits over 5 years.
METHODOLOGY:
- Accumulation of advanced glycation end products (AGEs), which results from endogenous carbonyl stress, may be a potential target for preventing and treating the diabetic sensorimotor polyneuropathy (DSPN) that is a common complication of T2D.
- Researchers investigated novel cutaneous biomarkers for the development and progression of DSPN in 160 individuals with recent-onset T2D (diagnosed within 12 months or less) and 144 individuals with normal glucose tolerance, all recruited consecutively from the German Diabetes Study baseline cohort.
- Peripheral nerve function was assessed through nerve conduction studies, quantitative sensory testing, and clinical neuropathy scores.
- Skin biopsies were used to analyze intraepidermal nerve fiber density, endothelial integrity, cutaneous oxidative stress markers, and cutaneous carbonyl stress markers, including AGE autofluorescence and argpyrimidine area.
- Skin autofluorescence was measured noninvasively using an AGE reader device.
- A subgroup of 80 patients with T2D were reassessed after 5 years to evaluate the progression of neurophysiological deficits.
TAKEAWAY:
- Patients with recent-onset T2D had greater AGE autofluorescence and argpyrimidine area (P ≤ .05 for both) and lower nerve fiber density (P ≤ .05) than individuals with normal glucose tolerance.
- In patients with T2D, AGE autofluorescence was inversely associated with nerve conduction (P = .0002, P = .002, and P = .001 for peroneal motor, median motor, and sural sensory nerve conduction velocity, respectively) and positively associated with AGE reader measurements (P < .05); no such associations were observed in those with normal glucose tolerance.
- In the prospective T2D cohort, associations were noted between cutaneous markers for AGEs and endothelial cells at baseline and changes in nerve function indices over a 5-year period.
IN PRACTICE:
“Prospective analyses revealed some predictive value of cutaneous AGEs and lower endothelial integrity for declining nerve function, supporting the role of carbonyl stress in the development and progression of DSPN, representing a potential therapeutic target,” the authors wrote.
SOURCE:
The study was led by Gidon J. Bönhof, Department of Endocrinology and Diabetology, Medical Faculty, University Hospital Düsseldorf, Heinrich Heine University Düsseldorf, Düsseldorf, Germany. It was published online in Diabetes Care.
LIMITATIONS:
The observational design of the study limited the ability to draw causal conclusions. The groups were not matched for age or body mass index. Various mechanisms related to DSPN were analyzed; however, specific pathways of AGEs were not studied in detail. The relatively low number of individuals with clinically manifested DSPN limited the exploration of different stages of the condition.
DISCLOSURES:
The study was supported by a German Center for Diabetes Research grant. The German Diabetes Study was supported by the German Diabetes Center funded by the German Federal Ministry of Health (Berlin), the Ministry of Innovation, Science, Research and Technology of North Rhine-Westphalia (Düsseldorf, Germany), and grants from the German Federal Ministry of Education and Research to the German Center for Diabetes Research e.V. No relevant conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Increased cutaneous carbonyl stress is linked to slower nerve conduction in patients with metabolically well-controlled, recent-onset type 2 diabetes (T2D) and can predict the development of neuropathic deficits over 5 years.
METHODOLOGY:
- Accumulation of advanced glycation end products (AGEs), which results from endogenous carbonyl stress, may be a potential target for preventing and treating the diabetic sensorimotor polyneuropathy (DSPN) that is a common complication of T2D.
- Researchers investigated novel cutaneous biomarkers for the development and progression of DSPN in 160 individuals with recent-onset T2D (diagnosed within 12 months or less) and 144 individuals with normal glucose tolerance, all recruited consecutively from the German Diabetes Study baseline cohort.
- Peripheral nerve function was assessed through nerve conduction studies, quantitative sensory testing, and clinical neuropathy scores.
- Skin biopsies were used to analyze intraepidermal nerve fiber density, endothelial integrity, cutaneous oxidative stress markers, and cutaneous carbonyl stress markers, including AGE autofluorescence and argpyrimidine area.
- Skin autofluorescence was measured noninvasively using an AGE reader device.
- A subgroup of 80 patients with T2D were reassessed after 5 years to evaluate the progression of neurophysiological deficits.
TAKEAWAY:
- Patients with recent-onset T2D had greater AGE autofluorescence and argpyrimidine area (P ≤ .05 for both) and lower nerve fiber density (P ≤ .05) than individuals with normal glucose tolerance.
- In patients with T2D, AGE autofluorescence was inversely associated with nerve conduction (P = .0002, P = .002, and P = .001 for peroneal motor, median motor, and sural sensory nerve conduction velocity, respectively) and positively associated with AGE reader measurements (P < .05); no such associations were observed in those with normal glucose tolerance.
- In the prospective T2D cohort, associations were noted between cutaneous markers for AGEs and endothelial cells at baseline and changes in nerve function indices over a 5-year period.
IN PRACTICE:
“Prospective analyses revealed some predictive value of cutaneous AGEs and lower endothelial integrity for declining nerve function, supporting the role of carbonyl stress in the development and progression of DSPN, representing a potential therapeutic target,” the authors wrote.
SOURCE:
The study was led by Gidon J. Bönhof, Department of Endocrinology and Diabetology, Medical Faculty, University Hospital Düsseldorf, Heinrich Heine University Düsseldorf, Düsseldorf, Germany. It was published online in Diabetes Care.
LIMITATIONS:
The observational design of the study limited the ability to draw causal conclusions. The groups were not matched for age or body mass index. Various mechanisms related to DSPN were analyzed; however, specific pathways of AGEs were not studied in detail. The relatively low number of individuals with clinically manifested DSPN limited the exploration of different stages of the condition.
DISCLOSURES:
The study was supported by a German Center for Diabetes Research grant. The German Diabetes Study was supported by the German Diabetes Center funded by the German Federal Ministry of Health (Berlin), the Ministry of Innovation, Science, Research and Technology of North Rhine-Westphalia (Düsseldorf, Germany), and grants from the German Federal Ministry of Education and Research to the German Center for Diabetes Research e.V. No relevant conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Increased cutaneous carbonyl stress is linked to slower nerve conduction in patients with metabolically well-controlled, recent-onset type 2 diabetes (T2D) and can predict the development of neuropathic deficits over 5 years.
METHODOLOGY:
- Accumulation of advanced glycation end products (AGEs), which results from endogenous carbonyl stress, may be a potential target for preventing and treating the diabetic sensorimotor polyneuropathy (DSPN) that is a common complication of T2D.
- Researchers investigated novel cutaneous biomarkers for the development and progression of DSPN in 160 individuals with recent-onset T2D (diagnosed within 12 months or less) and 144 individuals with normal glucose tolerance, all recruited consecutively from the German Diabetes Study baseline cohort.
- Peripheral nerve function was assessed through nerve conduction studies, quantitative sensory testing, and clinical neuropathy scores.
- Skin biopsies were used to analyze intraepidermal nerve fiber density, endothelial integrity, cutaneous oxidative stress markers, and cutaneous carbonyl stress markers, including AGE autofluorescence and argpyrimidine area.
- Skin autofluorescence was measured noninvasively using an AGE reader device.
- A subgroup of 80 patients with T2D were reassessed after 5 years to evaluate the progression of neurophysiological deficits.
TAKEAWAY:
- Patients with recent-onset T2D had greater AGE autofluorescence and argpyrimidine area (P ≤ .05 for both) and lower nerve fiber density (P ≤ .05) than individuals with normal glucose tolerance.
- In patients with T2D, AGE autofluorescence was inversely associated with nerve conduction (P = .0002, P = .002, and P = .001 for peroneal motor, median motor, and sural sensory nerve conduction velocity, respectively) and positively associated with AGE reader measurements (P < .05); no such associations were observed in those with normal glucose tolerance.
- In the prospective T2D cohort, associations were noted between cutaneous markers for AGEs and endothelial cells at baseline and changes in nerve function indices over a 5-year period.
IN PRACTICE:
“Prospective analyses revealed some predictive value of cutaneous AGEs and lower endothelial integrity for declining nerve function, supporting the role of carbonyl stress in the development and progression of DSPN, representing a potential therapeutic target,” the authors wrote.
SOURCE:
The study was led by Gidon J. Bönhof, Department of Endocrinology and Diabetology, Medical Faculty, University Hospital Düsseldorf, Heinrich Heine University Düsseldorf, Düsseldorf, Germany. It was published online in Diabetes Care.
LIMITATIONS:
The observational design of the study limited the ability to draw causal conclusions. The groups were not matched for age or body mass index. Various mechanisms related to DSPN were analyzed; however, specific pathways of AGEs were not studied in detail. The relatively low number of individuals with clinically manifested DSPN limited the exploration of different stages of the condition.
DISCLOSURES:
The study was supported by a German Center for Diabetes Research grant. The German Diabetes Study was supported by the German Diabetes Center funded by the German Federal Ministry of Health (Berlin), the Ministry of Innovation, Science, Research and Technology of North Rhine-Westphalia (Düsseldorf, Germany), and grants from the German Federal Ministry of Education and Research to the German Center for Diabetes Research e.V. No relevant conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Congress and VA Aim to Improve Health Care Access for Rural Veterans
Veterans living in rural areas are often too far away from health care institutions to easily travel to their appointments. Even if they can drive, the cost of gas and other related travel expenses may be too much for some. Telehealth was meant to help relieve that problem, but poor internet access can mitigate its convenience and accessibility for those patients. Two proposals offer solutions.
In February, Sens. Jon Ossoff (D-GA), Susan Collins (R-ME), and John Thune (R-SD) introduced the Rural Veterans Transportation to Care Act, a bill that would expand eligibility to the US Department of Veterans Affairs (VA) Highly Rural Transportation Grants, a program currently only available to counties with < 7 people per square mile.
“As I’ve sat down with veterans in rural areas across Georgia, one of their key concerns is lack of transportation,” Sen. Ossoff said. “That’s why I’m introducing this bipartisan bill to ensure veterans have more access to transportation services that can bring them to VA clinics and medical centers to get the care they need.”
Amanda Flener and her husband, John, a veteran wounded while serving in Iraq, were driving as long as 3 hours from Fitzgerald, Georgia (population 8900) to attend his medical appointments. In the last 2 years, Flener told the Daily Yonder she had put nearly 72,000 miles on her vehicle. Following hurricane Helene, she said, "We had been driving 30 miles just to get gas to power our generator … and we were fortunate to be able to do that.”
Telehealth appointments can help fill coverage gaps, Flener said. But even while paying for the most expensive internet plan available in her county, the signal isn't always strong enough. Telehealth care is "progress, for sure," Flener said. "So, we pay for the best Wi-Fi we can get in our area, but it isn't always reliable enough to take the video calls from the VA."
As a result, veterans and their caregivers could benefit not only from the bipartisan transportation proposal, but also from a decision announced in November. The VA is proposing to eliminate copayments for all VA telehealth services and establish a grant program to fund designated VA telehealth access points in non-VA facilities, with a focus on rural and medically underserved communities.
The program, called Accessing Telehealth through Local Area Stations (ATLAS), would provide funding to organizations — including nonprofits and private businesses — to offer veterans comfortable, private spaces equipped with high-speed internet access and the technology to remotely meet with VA clinicians. Grants would also provide designated funding to train on-site personnel to support the program.
These proposed changes would advance the VA’s and the Biden-Harris Administration’s ongoing efforts to lower costs and expand access to care for veterans. They also could make a life-changing difference for the 2.7 million rural veterans enrolled in VA health care.
According to a 2024 RAND study, just under half of military and veteran caregivers live in a county without a VA facility, and nearly half live in a primary care physician shortage area. For military/veteran caregivers in particular, the survey found, reduced access to support related to the more complicated care some patients require, greater distances to reach opportunities (eg, retail, economic, or social), and even differences in Wi-Fi/broadband internet access may create “unique needs.” The survey found that 24% of rural military/veteran caregivers did not have reliable broadband internet.
“Waiving copays for telehealth services and launching this grant program are both major steps forward in ensuring veterans can access health care where and when they need it,” said VA Secretary Denis McDonough. “VA is the best and most affordable care in America for veterans — with these steps, we can make it easier for veterans to access their earned VA health care.”
The rulemaking can be viewed in the Federal Register under public inspection, and is open for comment. The VA anticipates a notice of funding opportunity for this grant program following publication of the final rule.
Veterans living in rural areas are often too far away from health care institutions to easily travel to their appointments. Even if they can drive, the cost of gas and other related travel expenses may be too much for some. Telehealth was meant to help relieve that problem, but poor internet access can mitigate its convenience and accessibility for those patients. Two proposals offer solutions.
In February, Sens. Jon Ossoff (D-GA), Susan Collins (R-ME), and John Thune (R-SD) introduced the Rural Veterans Transportation to Care Act, a bill that would expand eligibility to the US Department of Veterans Affairs (VA) Highly Rural Transportation Grants, a program currently only available to counties with < 7 people per square mile.
“As I’ve sat down with veterans in rural areas across Georgia, one of their key concerns is lack of transportation,” Sen. Ossoff said. “That’s why I’m introducing this bipartisan bill to ensure veterans have more access to transportation services that can bring them to VA clinics and medical centers to get the care they need.”
Amanda Flener and her husband, John, a veteran wounded while serving in Iraq, were driving as long as 3 hours from Fitzgerald, Georgia (population 8900) to attend his medical appointments. In the last 2 years, Flener told the Daily Yonder she had put nearly 72,000 miles on her vehicle. Following hurricane Helene, she said, "We had been driving 30 miles just to get gas to power our generator … and we were fortunate to be able to do that.”
Telehealth appointments can help fill coverage gaps, Flener said. But even while paying for the most expensive internet plan available in her county, the signal isn't always strong enough. Telehealth care is "progress, for sure," Flener said. "So, we pay for the best Wi-Fi we can get in our area, but it isn't always reliable enough to take the video calls from the VA."
As a result, veterans and their caregivers could benefit not only from the bipartisan transportation proposal, but also from a decision announced in November. The VA is proposing to eliminate copayments for all VA telehealth services and establish a grant program to fund designated VA telehealth access points in non-VA facilities, with a focus on rural and medically underserved communities.
The program, called Accessing Telehealth through Local Area Stations (ATLAS), would provide funding to organizations — including nonprofits and private businesses — to offer veterans comfortable, private spaces equipped with high-speed internet access and the technology to remotely meet with VA clinicians. Grants would also provide designated funding to train on-site personnel to support the program.
These proposed changes would advance the VA’s and the Biden-Harris Administration’s ongoing efforts to lower costs and expand access to care for veterans. They also could make a life-changing difference for the 2.7 million rural veterans enrolled in VA health care.
According to a 2024 RAND study, just under half of military and veteran caregivers live in a county without a VA facility, and nearly half live in a primary care physician shortage area. For military/veteran caregivers in particular, the survey found, reduced access to support related to the more complicated care some patients require, greater distances to reach opportunities (eg, retail, economic, or social), and even differences in Wi-Fi/broadband internet access may create “unique needs.” The survey found that 24% of rural military/veteran caregivers did not have reliable broadband internet.
“Waiving copays for telehealth services and launching this grant program are both major steps forward in ensuring veterans can access health care where and when they need it,” said VA Secretary Denis McDonough. “VA is the best and most affordable care in America for veterans — with these steps, we can make it easier for veterans to access their earned VA health care.”
The rulemaking can be viewed in the Federal Register under public inspection, and is open for comment. The VA anticipates a notice of funding opportunity for this grant program following publication of the final rule.
Veterans living in rural areas are often too far away from health care institutions to easily travel to their appointments. Even if they can drive, the cost of gas and other related travel expenses may be too much for some. Telehealth was meant to help relieve that problem, but poor internet access can mitigate its convenience and accessibility for those patients. Two proposals offer solutions.
In February, Sens. Jon Ossoff (D-GA), Susan Collins (R-ME), and John Thune (R-SD) introduced the Rural Veterans Transportation to Care Act, a bill that would expand eligibility to the US Department of Veterans Affairs (VA) Highly Rural Transportation Grants, a program currently only available to counties with < 7 people per square mile.
“As I’ve sat down with veterans in rural areas across Georgia, one of their key concerns is lack of transportation,” Sen. Ossoff said. “That’s why I’m introducing this bipartisan bill to ensure veterans have more access to transportation services that can bring them to VA clinics and medical centers to get the care they need.”
Amanda Flener and her husband, John, a veteran wounded while serving in Iraq, were driving as long as 3 hours from Fitzgerald, Georgia (population 8900) to attend his medical appointments. In the last 2 years, Flener told the Daily Yonder she had put nearly 72,000 miles on her vehicle. Following hurricane Helene, she said, "We had been driving 30 miles just to get gas to power our generator … and we were fortunate to be able to do that.”
Telehealth appointments can help fill coverage gaps, Flener said. But even while paying for the most expensive internet plan available in her county, the signal isn't always strong enough. Telehealth care is "progress, for sure," Flener said. "So, we pay for the best Wi-Fi we can get in our area, but it isn't always reliable enough to take the video calls from the VA."
As a result, veterans and their caregivers could benefit not only from the bipartisan transportation proposal, but also from a decision announced in November. The VA is proposing to eliminate copayments for all VA telehealth services and establish a grant program to fund designated VA telehealth access points in non-VA facilities, with a focus on rural and medically underserved communities.
The program, called Accessing Telehealth through Local Area Stations (ATLAS), would provide funding to organizations — including nonprofits and private businesses — to offer veterans comfortable, private spaces equipped with high-speed internet access and the technology to remotely meet with VA clinicians. Grants would also provide designated funding to train on-site personnel to support the program.
These proposed changes would advance the VA’s and the Biden-Harris Administration’s ongoing efforts to lower costs and expand access to care for veterans. They also could make a life-changing difference for the 2.7 million rural veterans enrolled in VA health care.
According to a 2024 RAND study, just under half of military and veteran caregivers live in a county without a VA facility, and nearly half live in a primary care physician shortage area. For military/veteran caregivers in particular, the survey found, reduced access to support related to the more complicated care some patients require, greater distances to reach opportunities (eg, retail, economic, or social), and even differences in Wi-Fi/broadband internet access may create “unique needs.” The survey found that 24% of rural military/veteran caregivers did not have reliable broadband internet.
“Waiving copays for telehealth services and launching this grant program are both major steps forward in ensuring veterans can access health care where and when they need it,” said VA Secretary Denis McDonough. “VA is the best and most affordable care in America for veterans — with these steps, we can make it easier for veterans to access their earned VA health care.”
The rulemaking can be viewed in the Federal Register under public inspection, and is open for comment. The VA anticipates a notice of funding opportunity for this grant program following publication of the final rule.
The Cause of All That Stress: Tonsillectomy?
This transcript has been edited for clarity.
You know those times in your life when you’re just feeling ... stressed? You’re on the edge; you have no chill; everything just sort of gets to you. If you can step away from the anxiety for a moment, you might ask yourself where it’s all coming from. Is it really the stuff in your inbox at work or is it money issues at home? Is it something with your relationship, or maybe it’s your sleep quality or your diet? One thing you probably won’t blame for those acute stress reactions is the tonsillectomy you had as a kid. But according to new research, maybe you should.
Tonsillectomy and adenoidectomy are among the most common surgical procedures young people in the United States undergo, with about 300,000 cases a year, according to recent numbers. That’s down a bit from numbers a decade or so ago, but suffice it to say, a good chunk of the population is walking around right now without their tonsils.
The data supporting tonsillectomy have never been great. The two big indications for the surgery are recurrent sore throat — data show that tonsillectomy reduces this by about 0.7 sore throats per year— and obstructive sleep apnea (OSA). The data for improvement of OSA are a bit better than the data for sore throats.
Also, tonsillectomy is a relatively quick, relatively well-reimbursed surgery with indications that are — let’s be honest — somewhat subjective, and so variation is high. One study found that in a single Vermont town, nearly 60% of the population had had their tonsils removed by the time they turned 18. A few towns over, the rate was 20%.
A few factors have led to the decline of tonsillectomy in recent years. Reimbursement rates have gone down a bit. Additionally, better data collection and statistical analysis have shown that the benefits of the procedure are relatively modest.
And then there is a body of medical literature that at first struck me as surprising and almost bizarre: data linking tonsillectomy to subsequent physical and psychiatric disorders.
I teach a course on interpretation of the medical literature, and one of the first things I teach my students is to check their gut when they see the conclusion of a study.
Basically, even before you read the data, have a sense in your own mind if the hypothesis seems reasonable. If a paper is going to conclude that smoking leads to increased risk for bone cancer, I’d say that seems like a reasonable thing to study. If a paper purports to show a link between eating poultry and bone cancer, I’m going to be reading it with quite a bit more skepticism.
The technical term for that process is assessing “biologic plausibility.” If we’re talking tonsils, we have to ask ourselves: Is it plausible that removing someone’s tonsils when they are young should lead to major problems in the future?
At first blush, it didn’t seem very plausible to me.
But the truth is, there are quite a few studies out there demonstrating links like this: links between tonsillectomy and irritable bowel syndrome; links between tonsillectomy and cancer; links between tonsillectomy and depression.
And this week, appearing in JAMA Network Open, is a study linking tonsillectomy with stress disorders.
Researchers leveraged Sweden’s health database, which contains longitudinal data on basically every person who has lived in Sweden since 1981. This database let them know who had a tonsillectomy or adenoidectomy, and when, and what happened to them later in life.
I think the best way to present these data is to show you what they found, and then challenge that finding, and then show you what they did in anticipation of the challenges we would have to their findings. It’s a pretty thorough study.
So, topline results here. The researchers first identified 83,957 individuals who had their tonsils removed. They matched each of them with 10 controls who did not have their tonsils removed but were the same age and sex.
Over around 30 years of follow-up, those people who had their tonsils removed were 43% more likely to develop a stress-related disorder. Among the specific disorders, the risk for PTSD was substantially higher: 55% higher in the tonsillectomy group.
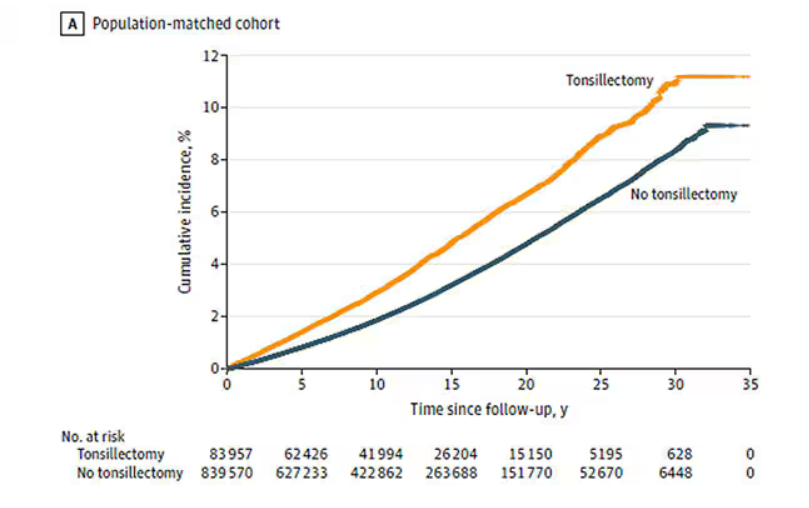
That’s pretty surprising, but I bet you already want to push back against this. Sure, the control group was the same age and sex, but other factors might be different between the two groups. You’d be right to think so. People who got their tonsils out were more likely to have parents with a history of stress-related disorders and who had lower educational attainment. But the primary results were adjusted for those factors.
There’s more to a family than parental educational attainment, of course. To account for household factors that might be harder to measure, the researchers created a second control group, this one comprising the siblings of people who had their tonsils removed but who hadn’t themselves had their tonsils removed.
The relationship between tonsillectomy and stress disorders in this population was not quite as robust but still present: a 34% increase in any stress disorder and a 41% increase in the risk for PTSD.
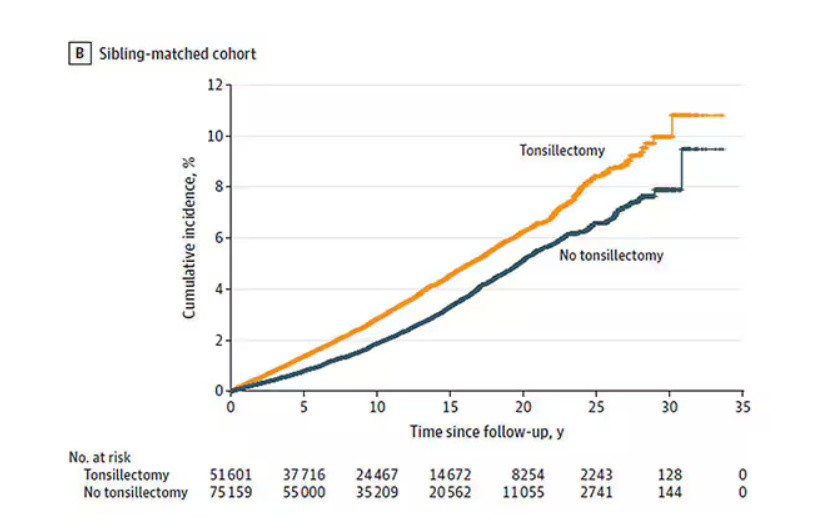
Maybe kids who get their tonsils out are just followed more closely thereafter, so doctors might notice a stress disorder and document it in the medical record; whereas with other kids it might go unnoticed. This is known as ascertainment bias. The researchers addressed this in a sensitivity analysis where they excluded new diagnoses of stress disorders that occurred in the first 3 years after tonsillectomy. The results were largely unchanged.
So how do we explain these data? We observe a correlation between tonsillectomy in youth and stress disorders in later life. But correlation is not causation. One possibility, perhaps even the most likely possibility, is that tonsillectomy is a marker of some other problem. Maybe these kids are more prone to infections and are therefore more likely to need their tonsils removed. Then, after a lifetime of more infections than average, their stress responses are higher. Or maybe kids with a higher BMI are more likely to have their tonsils removed due to sleep apnea concerns, and it’s that elevated BMI that leads to higher stress in later life.
Or maybe this is causal. Maybe there actually is biological plausibility here. The authors suggest that removal of tonsils might lead to broader changes in the immune system; after all, tonsillar tissue is on the front line of our defense against pathogens that might enter our bodies through our mouths or noses. Immunologic changes lead to greater inflammation over time, and there is decent evidence to link chronic inflammation to a variety of physical and psychological disorders.
In support of this, the authors show that the kids with tonsillectomy were more likely to be hospitalized for an infectious disease in the future as well, in magnitudes similar to the increased risk for stress. But they don’t actually show that the relationship between tonsillectomy and stress is mediated by that increased risk for infectious disease.
In the end, I find these data really intriguing. Before I dug into the literature, it seemed highly unlikely that removal of these small lumps of tissue would have much of an effect on anything. Now I’m not so sure. A few things can be removed from the human body without any consequences, but it can be hard to know exactly what those consequences are.
That said, given the rather marginal benefits of tonsillectomy and the growing number of studies expanding on the risks, I expect that we’ll see the rates of the surgery decline even further in the future.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Connecticut. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
You know those times in your life when you’re just feeling ... stressed? You’re on the edge; you have no chill; everything just sort of gets to you. If you can step away from the anxiety for a moment, you might ask yourself where it’s all coming from. Is it really the stuff in your inbox at work or is it money issues at home? Is it something with your relationship, or maybe it’s your sleep quality or your diet? One thing you probably won’t blame for those acute stress reactions is the tonsillectomy you had as a kid. But according to new research, maybe you should.
Tonsillectomy and adenoidectomy are among the most common surgical procedures young people in the United States undergo, with about 300,000 cases a year, according to recent numbers. That’s down a bit from numbers a decade or so ago, but suffice it to say, a good chunk of the population is walking around right now without their tonsils.
The data supporting tonsillectomy have never been great. The two big indications for the surgery are recurrent sore throat — data show that tonsillectomy reduces this by about 0.7 sore throats per year— and obstructive sleep apnea (OSA). The data for improvement of OSA are a bit better than the data for sore throats.
Also, tonsillectomy is a relatively quick, relatively well-reimbursed surgery with indications that are — let’s be honest — somewhat subjective, and so variation is high. One study found that in a single Vermont town, nearly 60% of the population had had their tonsils removed by the time they turned 18. A few towns over, the rate was 20%.
A few factors have led to the decline of tonsillectomy in recent years. Reimbursement rates have gone down a bit. Additionally, better data collection and statistical analysis have shown that the benefits of the procedure are relatively modest.
And then there is a body of medical literature that at first struck me as surprising and almost bizarre: data linking tonsillectomy to subsequent physical and psychiatric disorders.
I teach a course on interpretation of the medical literature, and one of the first things I teach my students is to check their gut when they see the conclusion of a study.
Basically, even before you read the data, have a sense in your own mind if the hypothesis seems reasonable. If a paper is going to conclude that smoking leads to increased risk for bone cancer, I’d say that seems like a reasonable thing to study. If a paper purports to show a link between eating poultry and bone cancer, I’m going to be reading it with quite a bit more skepticism.
The technical term for that process is assessing “biologic plausibility.” If we’re talking tonsils, we have to ask ourselves: Is it plausible that removing someone’s tonsils when they are young should lead to major problems in the future?
At first blush, it didn’t seem very plausible to me.
But the truth is, there are quite a few studies out there demonstrating links like this: links between tonsillectomy and irritable bowel syndrome; links between tonsillectomy and cancer; links between tonsillectomy and depression.
And this week, appearing in JAMA Network Open, is a study linking tonsillectomy with stress disorders.
Researchers leveraged Sweden’s health database, which contains longitudinal data on basically every person who has lived in Sweden since 1981. This database let them know who had a tonsillectomy or adenoidectomy, and when, and what happened to them later in life.
I think the best way to present these data is to show you what they found, and then challenge that finding, and then show you what they did in anticipation of the challenges we would have to their findings. It’s a pretty thorough study.
So, topline results here. The researchers first identified 83,957 individuals who had their tonsils removed. They matched each of them with 10 controls who did not have their tonsils removed but were the same age and sex.
Over around 30 years of follow-up, those people who had their tonsils removed were 43% more likely to develop a stress-related disorder. Among the specific disorders, the risk for PTSD was substantially higher: 55% higher in the tonsillectomy group.

That’s pretty surprising, but I bet you already want to push back against this. Sure, the control group was the same age and sex, but other factors might be different between the two groups. You’d be right to think so. People who got their tonsils out were more likely to have parents with a history of stress-related disorders and who had lower educational attainment. But the primary results were adjusted for those factors.
There’s more to a family than parental educational attainment, of course. To account for household factors that might be harder to measure, the researchers created a second control group, this one comprising the siblings of people who had their tonsils removed but who hadn’t themselves had their tonsils removed.
The relationship between tonsillectomy and stress disorders in this population was not quite as robust but still present: a 34% increase in any stress disorder and a 41% increase in the risk for PTSD.

Maybe kids who get their tonsils out are just followed more closely thereafter, so doctors might notice a stress disorder and document it in the medical record; whereas with other kids it might go unnoticed. This is known as ascertainment bias. The researchers addressed this in a sensitivity analysis where they excluded new diagnoses of stress disorders that occurred in the first 3 years after tonsillectomy. The results were largely unchanged.
So how do we explain these data? We observe a correlation between tonsillectomy in youth and stress disorders in later life. But correlation is not causation. One possibility, perhaps even the most likely possibility, is that tonsillectomy is a marker of some other problem. Maybe these kids are more prone to infections and are therefore more likely to need their tonsils removed. Then, after a lifetime of more infections than average, their stress responses are higher. Or maybe kids with a higher BMI are more likely to have their tonsils removed due to sleep apnea concerns, and it’s that elevated BMI that leads to higher stress in later life.
Or maybe this is causal. Maybe there actually is biological plausibility here. The authors suggest that removal of tonsils might lead to broader changes in the immune system; after all, tonsillar tissue is on the front line of our defense against pathogens that might enter our bodies through our mouths or noses. Immunologic changes lead to greater inflammation over time, and there is decent evidence to link chronic inflammation to a variety of physical and psychological disorders.
In support of this, the authors show that the kids with tonsillectomy were more likely to be hospitalized for an infectious disease in the future as well, in magnitudes similar to the increased risk for stress. But they don’t actually show that the relationship between tonsillectomy and stress is mediated by that increased risk for infectious disease.
In the end, I find these data really intriguing. Before I dug into the literature, it seemed highly unlikely that removal of these small lumps of tissue would have much of an effect on anything. Now I’m not so sure. A few things can be removed from the human body without any consequences, but it can be hard to know exactly what those consequences are.
That said, given the rather marginal benefits of tonsillectomy and the growing number of studies expanding on the risks, I expect that we’ll see the rates of the surgery decline even further in the future.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Connecticut. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
You know those times in your life when you’re just feeling ... stressed? You’re on the edge; you have no chill; everything just sort of gets to you. If you can step away from the anxiety for a moment, you might ask yourself where it’s all coming from. Is it really the stuff in your inbox at work or is it money issues at home? Is it something with your relationship, or maybe it’s your sleep quality or your diet? One thing you probably won’t blame for those acute stress reactions is the tonsillectomy you had as a kid. But according to new research, maybe you should.
Tonsillectomy and adenoidectomy are among the most common surgical procedures young people in the United States undergo, with about 300,000 cases a year, according to recent numbers. That’s down a bit from numbers a decade or so ago, but suffice it to say, a good chunk of the population is walking around right now without their tonsils.
The data supporting tonsillectomy have never been great. The two big indications for the surgery are recurrent sore throat — data show that tonsillectomy reduces this by about 0.7 sore throats per year— and obstructive sleep apnea (OSA). The data for improvement of OSA are a bit better than the data for sore throats.
Also, tonsillectomy is a relatively quick, relatively well-reimbursed surgery with indications that are — let’s be honest — somewhat subjective, and so variation is high. One study found that in a single Vermont town, nearly 60% of the population had had their tonsils removed by the time they turned 18. A few towns over, the rate was 20%.
A few factors have led to the decline of tonsillectomy in recent years. Reimbursement rates have gone down a bit. Additionally, better data collection and statistical analysis have shown that the benefits of the procedure are relatively modest.
And then there is a body of medical literature that at first struck me as surprising and almost bizarre: data linking tonsillectomy to subsequent physical and psychiatric disorders.
I teach a course on interpretation of the medical literature, and one of the first things I teach my students is to check their gut when they see the conclusion of a study.
Basically, even before you read the data, have a sense in your own mind if the hypothesis seems reasonable. If a paper is going to conclude that smoking leads to increased risk for bone cancer, I’d say that seems like a reasonable thing to study. If a paper purports to show a link between eating poultry and bone cancer, I’m going to be reading it with quite a bit more skepticism.
The technical term for that process is assessing “biologic plausibility.” If we’re talking tonsils, we have to ask ourselves: Is it plausible that removing someone’s tonsils when they are young should lead to major problems in the future?
At first blush, it didn’t seem very plausible to me.
But the truth is, there are quite a few studies out there demonstrating links like this: links between tonsillectomy and irritable bowel syndrome; links between tonsillectomy and cancer; links between tonsillectomy and depression.
And this week, appearing in JAMA Network Open, is a study linking tonsillectomy with stress disorders.
Researchers leveraged Sweden’s health database, which contains longitudinal data on basically every person who has lived in Sweden since 1981. This database let them know who had a tonsillectomy or adenoidectomy, and when, and what happened to them later in life.
I think the best way to present these data is to show you what they found, and then challenge that finding, and then show you what they did in anticipation of the challenges we would have to their findings. It’s a pretty thorough study.
So, topline results here. The researchers first identified 83,957 individuals who had their tonsils removed. They matched each of them with 10 controls who did not have their tonsils removed but were the same age and sex.
Over around 30 years of follow-up, those people who had their tonsils removed were 43% more likely to develop a stress-related disorder. Among the specific disorders, the risk for PTSD was substantially higher: 55% higher in the tonsillectomy group.

That’s pretty surprising, but I bet you already want to push back against this. Sure, the control group was the same age and sex, but other factors might be different between the two groups. You’d be right to think so. People who got their tonsils out were more likely to have parents with a history of stress-related disorders and who had lower educational attainment. But the primary results were adjusted for those factors.
There’s more to a family than parental educational attainment, of course. To account for household factors that might be harder to measure, the researchers created a second control group, this one comprising the siblings of people who had their tonsils removed but who hadn’t themselves had their tonsils removed.
The relationship between tonsillectomy and stress disorders in this population was not quite as robust but still present: a 34% increase in any stress disorder and a 41% increase in the risk for PTSD.

Maybe kids who get their tonsils out are just followed more closely thereafter, so doctors might notice a stress disorder and document it in the medical record; whereas with other kids it might go unnoticed. This is known as ascertainment bias. The researchers addressed this in a sensitivity analysis where they excluded new diagnoses of stress disorders that occurred in the first 3 years after tonsillectomy. The results were largely unchanged.
So how do we explain these data? We observe a correlation between tonsillectomy in youth and stress disorders in later life. But correlation is not causation. One possibility, perhaps even the most likely possibility, is that tonsillectomy is a marker of some other problem. Maybe these kids are more prone to infections and are therefore more likely to need their tonsils removed. Then, after a lifetime of more infections than average, their stress responses are higher. Or maybe kids with a higher BMI are more likely to have their tonsils removed due to sleep apnea concerns, and it’s that elevated BMI that leads to higher stress in later life.
Or maybe this is causal. Maybe there actually is biological plausibility here. The authors suggest that removal of tonsils might lead to broader changes in the immune system; after all, tonsillar tissue is on the front line of our defense against pathogens that might enter our bodies through our mouths or noses. Immunologic changes lead to greater inflammation over time, and there is decent evidence to link chronic inflammation to a variety of physical and psychological disorders.
In support of this, the authors show that the kids with tonsillectomy were more likely to be hospitalized for an infectious disease in the future as well, in magnitudes similar to the increased risk for stress. But they don’t actually show that the relationship between tonsillectomy and stress is mediated by that increased risk for infectious disease.
In the end, I find these data really intriguing. Before I dug into the literature, it seemed highly unlikely that removal of these small lumps of tissue would have much of an effect on anything. Now I’m not so sure. A few things can be removed from the human body without any consequences, but it can be hard to know exactly what those consequences are.
That said, given the rather marginal benefits of tonsillectomy and the growing number of studies expanding on the risks, I expect that we’ll see the rates of the surgery decline even further in the future.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Connecticut. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Is Vitamin E Beneficial for Bone Health?
Vitamin E may be best known for boosting skin and eye health as well as immune function. In recent years, researchers have explored the potential benefits of vitamin E on bone loss, especially in women with menopause-related osteoporosis. While data are beginning to roll in from these studies, evidence supporting a positive impact of vitamin E on osteoporosis and hip fracture risk in perimenopausal women remains elusive.
For osteoporosis, the rationale for using vitamin E is based on its antioxidant activity, which can scavenge potentially damaging free radicals. Researchers have asked whether vitamin E can help maintain the integrity of bone matrix and stimulate bone formation while minimizing bone resorption, particularly in trabecular (spongy) bone, the bone compartment preferentially affected in perimenopausal bone loss.
Vitamin E mostly consists of two isomers: alpha-tocopherol and gamma-tocopherol. Alpha-tocopherol has higher antioxidant activity and is found in nuts, seeds, vegetable oils, green leafy vegetables, fortified cereals, and vitamin E supplements. Gamma-tocopherol is known for its superior anti-inflammatory properties and accounts for about 70% of the total vitamin E intake in a typical American diet, largely sourced from soybean and other vegetable oils.
Benefits and Risks in Bone Loss Studies
Perimenopausal bone loss is caused, to a great extent, by the decrease in sex hormones. Studies of vitamin E in ovariectomized rats have yielded mixed results. This animal model lacks sex hormones and has similar bone changes to those of postmenopausal women. Some animal studies have suggested a positive effect of vitamin E on bone while others have reported no effect.
Studies in humans also have produced conflicting reports of positive, neutral, and negative associations of vitamin E with bone health. For example, the Women’s Health Initiative examined the relationship between vitamin and mineral antioxidants and bone health in postmenopausal women and found no significant association between antioxidants and bone mineral density.
Another study examining data from children and adolescents enrolled in the National Health and Nutrition Examination Survey (NHANES) database found an inverse association between alpha-tocopherol and lumbar spine bone density, suggesting a deleterious effect on bone. Inverse associations also have been reported in certain studies of postmenopausal women.
High doses of alpha-tocopherol have been linked to a risk for impaired bone health through a variety of mechanisms, such as interference with vitamin K metabolism; competitive binding for alpha-tocopherol transfer protein, inhibiting the entry of beneficial vitamin E isomers, including gamma-tocopherol; and pro-oxidant effects that harm bone. Thus, postmenopausal women taking vitamin E supplements primarily as high doses of alpha-tocopherol might be hindering their bone health.
Data for gamma-tocopherol are more promising. Some studies hypothesize that gamma-tocopherol might uncouple bone turnover, leading to increased bone formation without affecting bone resorption. Further, a randomized controlled study of mixed tocopherols (rather than alpha-tocopherol) vs placebo reported a protective effect of this preparation on bone outcomes by suppressing bone resorption. This raises the importance of considering the specific forms of vitamin E when evaluating its role in bone health.
Limitations of Current Studies
Researchers acknowledge several limitations in studies to date. For example, there are very few randomized controlled trials assessing the impact of vitamin E on bone health. Most studies are cross-sectional or observational, even when longitudinal. Cross-sectional and observational designs prevent us from establishing a causal relationship between vitamin E and bone endpoints.
Such designs also run the risk of additional confounders that may affect associations between vitamin E and bone, or the lack thereof. These could include both known and unknown confounders. Of note, gamma-tocopherol intake data were not available for certain NHANES studies.
Further, people often consume multiple nutrients and supplements, complicating the identification of specific nutrient-disease associations. Most human studies estimate tocopherol intake by dietary questionnaires or measure serum tocopherol levels, which reflect short-term dietary intake, while bone mineral density is probably influenced by long-term dietary patterns.
Too Soon to Prescribe Vitamin E for Bone Health
Some nutrition experts advocate for vitamin E supplements containing mixed tocopherols, specifically suggesting a ratio of 50-100 IU of gamma-tocopherol per 400 IU of D-alpha-tocopherol. Additional research is essential to confirm and further clarify the role of gamma-tocopherol in bone formation and resorption. In fact, it is also important to explore the influence of other compounds in the vitamin E family on skeletal health.
Until more data are available, we would recommend following the Institute of Medicine’s guidelines for the recommended daily allowance (RDA) of vitamin E. This is age dependent, ranging from 4 to 11 mg/d between the ages of 0 and 13 years, and 15 mg/d thereafter.
Overall, evidence of vitamin E’s impact on osteoporosis and hip fracture risk in perimenopausal women remains inconclusive. Although some observational and interventional studies suggest potential benefits, more interventional studies, particularly randomized controlled trials, are necessary to explore the risks and benefits of vitamin E supplementation and serum vitamin E levels on bone density and fracture risk more thoroughly.
Dr. Pani, Assistant Professor, Department of Internal Medicine, UVA School of Medicine; Medical Director, Department of General Medicine, Same Day Care Clinic, both in Charlottesville, has disclosed no relevant financial relationships. Dr. Misra, Professor, Chair, Physician-in-Chief, Department of Pediatrics, University of Virginia, and UVA Health Children’s, Charlottesville, has disclosed being a key opinion leader for Lumos Pharma.
A version of this article appeared on Medscape.com.
Vitamin E may be best known for boosting skin and eye health as well as immune function. In recent years, researchers have explored the potential benefits of vitamin E on bone loss, especially in women with menopause-related osteoporosis. While data are beginning to roll in from these studies, evidence supporting a positive impact of vitamin E on osteoporosis and hip fracture risk in perimenopausal women remains elusive.
For osteoporosis, the rationale for using vitamin E is based on its antioxidant activity, which can scavenge potentially damaging free radicals. Researchers have asked whether vitamin E can help maintain the integrity of bone matrix and stimulate bone formation while minimizing bone resorption, particularly in trabecular (spongy) bone, the bone compartment preferentially affected in perimenopausal bone loss.
Vitamin E mostly consists of two isomers: alpha-tocopherol and gamma-tocopherol. Alpha-tocopherol has higher antioxidant activity and is found in nuts, seeds, vegetable oils, green leafy vegetables, fortified cereals, and vitamin E supplements. Gamma-tocopherol is known for its superior anti-inflammatory properties and accounts for about 70% of the total vitamin E intake in a typical American diet, largely sourced from soybean and other vegetable oils.
Benefits and Risks in Bone Loss Studies
Perimenopausal bone loss is caused, to a great extent, by the decrease in sex hormones. Studies of vitamin E in ovariectomized rats have yielded mixed results. This animal model lacks sex hormones and has similar bone changes to those of postmenopausal women. Some animal studies have suggested a positive effect of vitamin E on bone while others have reported no effect.
Studies in humans also have produced conflicting reports of positive, neutral, and negative associations of vitamin E with bone health. For example, the Women’s Health Initiative examined the relationship between vitamin and mineral antioxidants and bone health in postmenopausal women and found no significant association between antioxidants and bone mineral density.
Another study examining data from children and adolescents enrolled in the National Health and Nutrition Examination Survey (NHANES) database found an inverse association between alpha-tocopherol and lumbar spine bone density, suggesting a deleterious effect on bone. Inverse associations also have been reported in certain studies of postmenopausal women.
High doses of alpha-tocopherol have been linked to a risk for impaired bone health through a variety of mechanisms, such as interference with vitamin K metabolism; competitive binding for alpha-tocopherol transfer protein, inhibiting the entry of beneficial vitamin E isomers, including gamma-tocopherol; and pro-oxidant effects that harm bone. Thus, postmenopausal women taking vitamin E supplements primarily as high doses of alpha-tocopherol might be hindering their bone health.
Data for gamma-tocopherol are more promising. Some studies hypothesize that gamma-tocopherol might uncouple bone turnover, leading to increased bone formation without affecting bone resorption. Further, a randomized controlled study of mixed tocopherols (rather than alpha-tocopherol) vs placebo reported a protective effect of this preparation on bone outcomes by suppressing bone resorption. This raises the importance of considering the specific forms of vitamin E when evaluating its role in bone health.
Limitations of Current Studies
Researchers acknowledge several limitations in studies to date. For example, there are very few randomized controlled trials assessing the impact of vitamin E on bone health. Most studies are cross-sectional or observational, even when longitudinal. Cross-sectional and observational designs prevent us from establishing a causal relationship between vitamin E and bone endpoints.
Such designs also run the risk of additional confounders that may affect associations between vitamin E and bone, or the lack thereof. These could include both known and unknown confounders. Of note, gamma-tocopherol intake data were not available for certain NHANES studies.
Further, people often consume multiple nutrients and supplements, complicating the identification of specific nutrient-disease associations. Most human studies estimate tocopherol intake by dietary questionnaires or measure serum tocopherol levels, which reflect short-term dietary intake, while bone mineral density is probably influenced by long-term dietary patterns.
Too Soon to Prescribe Vitamin E for Bone Health
Some nutrition experts advocate for vitamin E supplements containing mixed tocopherols, specifically suggesting a ratio of 50-100 IU of gamma-tocopherol per 400 IU of D-alpha-tocopherol. Additional research is essential to confirm and further clarify the role of gamma-tocopherol in bone formation and resorption. In fact, it is also important to explore the influence of other compounds in the vitamin E family on skeletal health.
Until more data are available, we would recommend following the Institute of Medicine’s guidelines for the recommended daily allowance (RDA) of vitamin E. This is age dependent, ranging from 4 to 11 mg/d between the ages of 0 and 13 years, and 15 mg/d thereafter.
Overall, evidence of vitamin E’s impact on osteoporosis and hip fracture risk in perimenopausal women remains inconclusive. Although some observational and interventional studies suggest potential benefits, more interventional studies, particularly randomized controlled trials, are necessary to explore the risks and benefits of vitamin E supplementation and serum vitamin E levels on bone density and fracture risk more thoroughly.
Dr. Pani, Assistant Professor, Department of Internal Medicine, UVA School of Medicine; Medical Director, Department of General Medicine, Same Day Care Clinic, both in Charlottesville, has disclosed no relevant financial relationships. Dr. Misra, Professor, Chair, Physician-in-Chief, Department of Pediatrics, University of Virginia, and UVA Health Children’s, Charlottesville, has disclosed being a key opinion leader for Lumos Pharma.
A version of this article appeared on Medscape.com.
Vitamin E may be best known for boosting skin and eye health as well as immune function. In recent years, researchers have explored the potential benefits of vitamin E on bone loss, especially in women with menopause-related osteoporosis. While data are beginning to roll in from these studies, evidence supporting a positive impact of vitamin E on osteoporosis and hip fracture risk in perimenopausal women remains elusive.
For osteoporosis, the rationale for using vitamin E is based on its antioxidant activity, which can scavenge potentially damaging free radicals. Researchers have asked whether vitamin E can help maintain the integrity of bone matrix and stimulate bone formation while minimizing bone resorption, particularly in trabecular (spongy) bone, the bone compartment preferentially affected in perimenopausal bone loss.
Vitamin E mostly consists of two isomers: alpha-tocopherol and gamma-tocopherol. Alpha-tocopherol has higher antioxidant activity and is found in nuts, seeds, vegetable oils, green leafy vegetables, fortified cereals, and vitamin E supplements. Gamma-tocopherol is known for its superior anti-inflammatory properties and accounts for about 70% of the total vitamin E intake in a typical American diet, largely sourced from soybean and other vegetable oils.
Benefits and Risks in Bone Loss Studies
Perimenopausal bone loss is caused, to a great extent, by the decrease in sex hormones. Studies of vitamin E in ovariectomized rats have yielded mixed results. This animal model lacks sex hormones and has similar bone changes to those of postmenopausal women. Some animal studies have suggested a positive effect of vitamin E on bone while others have reported no effect.
Studies in humans also have produced conflicting reports of positive, neutral, and negative associations of vitamin E with bone health. For example, the Women’s Health Initiative examined the relationship between vitamin and mineral antioxidants and bone health in postmenopausal women and found no significant association between antioxidants and bone mineral density.
Another study examining data from children and adolescents enrolled in the National Health and Nutrition Examination Survey (NHANES) database found an inverse association between alpha-tocopherol and lumbar spine bone density, suggesting a deleterious effect on bone. Inverse associations also have been reported in certain studies of postmenopausal women.
High doses of alpha-tocopherol have been linked to a risk for impaired bone health through a variety of mechanisms, such as interference with vitamin K metabolism; competitive binding for alpha-tocopherol transfer protein, inhibiting the entry of beneficial vitamin E isomers, including gamma-tocopherol; and pro-oxidant effects that harm bone. Thus, postmenopausal women taking vitamin E supplements primarily as high doses of alpha-tocopherol might be hindering their bone health.
Data for gamma-tocopherol are more promising. Some studies hypothesize that gamma-tocopherol might uncouple bone turnover, leading to increased bone formation without affecting bone resorption. Further, a randomized controlled study of mixed tocopherols (rather than alpha-tocopherol) vs placebo reported a protective effect of this preparation on bone outcomes by suppressing bone resorption. This raises the importance of considering the specific forms of vitamin E when evaluating its role in bone health.
Limitations of Current Studies
Researchers acknowledge several limitations in studies to date. For example, there are very few randomized controlled trials assessing the impact of vitamin E on bone health. Most studies are cross-sectional or observational, even when longitudinal. Cross-sectional and observational designs prevent us from establishing a causal relationship between vitamin E and bone endpoints.
Such designs also run the risk of additional confounders that may affect associations between vitamin E and bone, or the lack thereof. These could include both known and unknown confounders. Of note, gamma-tocopherol intake data were not available for certain NHANES studies.
Further, people often consume multiple nutrients and supplements, complicating the identification of specific nutrient-disease associations. Most human studies estimate tocopherol intake by dietary questionnaires or measure serum tocopherol levels, which reflect short-term dietary intake, while bone mineral density is probably influenced by long-term dietary patterns.
Too Soon to Prescribe Vitamin E for Bone Health
Some nutrition experts advocate for vitamin E supplements containing mixed tocopherols, specifically suggesting a ratio of 50-100 IU of gamma-tocopherol per 400 IU of D-alpha-tocopherol. Additional research is essential to confirm and further clarify the role of gamma-tocopherol in bone formation and resorption. In fact, it is also important to explore the influence of other compounds in the vitamin E family on skeletal health.
Until more data are available, we would recommend following the Institute of Medicine’s guidelines for the recommended daily allowance (RDA) of vitamin E. This is age dependent, ranging from 4 to 11 mg/d between the ages of 0 and 13 years, and 15 mg/d thereafter.
Overall, evidence of vitamin E’s impact on osteoporosis and hip fracture risk in perimenopausal women remains inconclusive. Although some observational and interventional studies suggest potential benefits, more interventional studies, particularly randomized controlled trials, are necessary to explore the risks and benefits of vitamin E supplementation and serum vitamin E levels on bone density and fracture risk more thoroughly.
Dr. Pani, Assistant Professor, Department of Internal Medicine, UVA School of Medicine; Medical Director, Department of General Medicine, Same Day Care Clinic, both in Charlottesville, has disclosed no relevant financial relationships. Dr. Misra, Professor, Chair, Physician-in-Chief, Department of Pediatrics, University of Virginia, and UVA Health Children’s, Charlottesville, has disclosed being a key opinion leader for Lumos Pharma.
A version of this article appeared on Medscape.com.
More Biologics May Be Breaking Through for COPD
New biologic drugs for chronic obstructive pulmonary disease (COPD) are finally here, said Stephen Rennard, MD, in a presentation in a session on new drugs at the 2024 GOLD International COPD Conference.
The therapeutic goals of biologics remain the same as with other treatments for COPD, namely restoration of normal inflammatory response and alteration of disease progression, as well as restoration of lost structure and function and improvement of systemic effects, Rennard said in his presentation. Most studies of new and up-and-coming drugs have improvement in acute exacerbation of COPD as the primary outcome.
The Biology Behind the Biologics
T2 inflammation is “an inflammatory cascade led by IL [interleukin]-4, IL-13, and IL-5,” Mona Bafadhel, MD, chair of Respiratory Medicine at King’s College London in England, said in her presentation during the session.
Bafadhel, who served as one of the investigators on the BOREAS and NOTUS studies, explained some of the science behind the development of the new biologics.
Eosinophils are powerful regulators of immune response and inflammation by stimulating T-cell production and affecting other immune cell types, she noted.
In the context of COPD and drug development, high blood eosinophil counts have been associated with increased COPD-related exacerbations, Bafadhel said. She cited data from a Dutch study of more than 7000 patients with COPD (with and without clinical diagnoses), in which absolute eosinophil counts ≥ 3.3% were associated with increased risk for severe exacerbations of 32% and 84% across all patients with COPD and clinical COPD, respectively.
Understanding the mechanisms of the eosinophil in COPD is important for research and development, Bafadhel said. Along with standardizing measurement of T2 inflammatory markers (IL-4, IL-13, and IL-5), more research is needed to fully understand the role of eosinophils in immunoregulation and repair.
Fitting the Biologic to the Patient
Several recent studies of up-and-coming biologics have focused on subsets of COPD patients, said Dave Singh, MD, professor of clinical pharmacology and respiratory medicine at The University of Manchester in England, in his presentation at the meeting. In September 2024, the Food and Drug Administration approved dupilumab as the first biologic treatment for patients with uncontrolled COPD and type 2 inflammation on the basis of eosinophil counts. Singh cited data from the BOREAS and NOTUS studies in which dupilumab significantly reduced exacerbations and improved lung function in these patients, compared with a placebo.
Mepolizumab, a biologic approved for asthma, is not currently approved for COPD, but data from a 2017 study showed a trend toward reduced exacerbations, compared with placebo, in a subset of patients with high blood eosinophil counts, Singh said.
In addition, a recent unpublished phase 3 study (MATINEE) showed a reduction in the annualized rate of exacerbations, compared with placebo, on the basis of up to 2 years’ follow-up.
Singh also highlighted data from a phase 2a study of astegolimab, a biologic drug that focuses on the IL-33 receptor, in which COPD exacerbation rates were not significantly different between treatment and placebo groups. However, astegolimab has shown safety and efficacy in adults with severe asthma and is under development in phase 3 trials for COPD.
Tezepelumab, which was approved by the FDA in 2021 as an add-on therapy for severe asthma in patients aged 12 years or older, is also in development as a therapy for COPD exacerbations, Singh said.
In a study presented at the 2024 American Thoracic Society annual meeting, Singh and colleagues found that tezepelumab at a subcutaneous dose of 420 mg every 4 weeks reduced the annualized rate of moderate or severe COPD exacerbations compared with placebo based on data from approximately 300 patients, although the difference was not statistically significant.
Itepekimab, another biologic, showed promise in a phase 2a genetic association study involving current and former smokers with moderate to severe COPD, Singh said.
In that study, published in 2022 in The Lancet Respiratory Medicine, itepekimab failed to meet the primary endpoint in the overall study population of reduced annualized rate of moderate to severe exacerbations; however, a subgroup analysis of former smokers showed a significant (42%) reduction in exacerbations, Singh said in his presentation. Two phase 3 clinical studies (AERIFY-1/2) are ongoing to confirm the safety and efficacy of itepekimab in former smokers with COPD.
Takeaways and Next Steps
“These therapies provide the first new classes of medications approved for COPD in nearly 20 years,” said David M. Mannino, MD, of the University of Kentucky, Lexington, in an interview. “Dupilumab will be available to a subset of patients who are poorly controlled and have evidence of high eosinophils in their blood and is only used once every 2 weeks,” added Mannino, who has served as a consultant to companies developing COPD drugs.
Both dupilumab and ensifentrine, a phosphodiesterase (PDE) 3 and PDE4 inhibitor also recently approved for maintenance treatment of COPD, have been shown in clinical trials to reduce exacerbations and improve symptoms, said Mannino. Both offer additional options for patients who continue to have symptoms and exacerbations in spite of their current therapy.
Some barriers to the use of biologics in practice include the high cost. “Access and overcoming insurance-related issues such as preauthorization and high copays will be a challenge,” he said. Also, because dupilumab is an injectable drug, some patient training will be required.
Newer biologic therapies in development are also injectables, but some studies are examining longer time intervals as long as every 6 months, which could be a major advancement for some patients. The newer therapies in development are similar to dupilumab in that they will be injected therapies. Some in development are looking at longer time intervals as long as every 6 months, which may be a major advancement for some patients. “All of these therapies, however, are currently targeting more advanced or serious disease,” he said.
Looking ahead, more therapies are needed for the treatment of early COPD, as well as therapies that can be administered to a large number of patients at a reasonable cost, Mannino added.
Rennard disclosed serving as a consultant for Verona Pharma, Sanofi, Beyond Air, RS BioTherapeutics, RespirAI, and Roche, as well as speaker fees from Sanofi and temporary ownership interest while employed by AstraZeneca. Rennard is also the founder of Great Plains Biometrix. Bafadhel disclosed funding from the National Institute for Health Research (NIHR), grants from Asthma + Lung UK, Horizon Europe, NIHR, and AstraZeneca to her institution, and honoraria from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, and Pfizer. Singh disclosed relationships including speaking sponsorships, honoraria, and advisory board memberships for Adovate, Aerogen, Almirall, Apogee, Arrowhead, AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, Cipla, Connect Biopharm, Covis, CSL Behring, DevPro Biopharm, Elpen, Empirico, EpiEndo, Genentech, Generate Biomedicines, GlaxoSmithKline, Glenmark, Kamada, Kinaset Therapeutics, Kymera, Menarini, MicroA, OM Pharma, Orion, Pieris Pharmaceuticals, Pulmatrix, Revolo, Roivant Sciences, Sanofi, Synairgen, Tetherex, Teva, Theravance Biopharma, Upstream, and Verona Pharma. Mannino disclosed serving as a consultant to multiple companies currently developing COPD therapies (AstraZeneca, GlaxoSmithKline, Roche, Regeneron, Sanofi, Genentech, Amgen, and Chiesi).
A version of this article appeared on Medscape.com.
New biologic drugs for chronic obstructive pulmonary disease (COPD) are finally here, said Stephen Rennard, MD, in a presentation in a session on new drugs at the 2024 GOLD International COPD Conference.
The therapeutic goals of biologics remain the same as with other treatments for COPD, namely restoration of normal inflammatory response and alteration of disease progression, as well as restoration of lost structure and function and improvement of systemic effects, Rennard said in his presentation. Most studies of new and up-and-coming drugs have improvement in acute exacerbation of COPD as the primary outcome.
The Biology Behind the Biologics
T2 inflammation is “an inflammatory cascade led by IL [interleukin]-4, IL-13, and IL-5,” Mona Bafadhel, MD, chair of Respiratory Medicine at King’s College London in England, said in her presentation during the session.
Bafadhel, who served as one of the investigators on the BOREAS and NOTUS studies, explained some of the science behind the development of the new biologics.
Eosinophils are powerful regulators of immune response and inflammation by stimulating T-cell production and affecting other immune cell types, she noted.
In the context of COPD and drug development, high blood eosinophil counts have been associated with increased COPD-related exacerbations, Bafadhel said. She cited data from a Dutch study of more than 7000 patients with COPD (with and without clinical diagnoses), in which absolute eosinophil counts ≥ 3.3% were associated with increased risk for severe exacerbations of 32% and 84% across all patients with COPD and clinical COPD, respectively.
Understanding the mechanisms of the eosinophil in COPD is important for research and development, Bafadhel said. Along with standardizing measurement of T2 inflammatory markers (IL-4, IL-13, and IL-5), more research is needed to fully understand the role of eosinophils in immunoregulation and repair.
Fitting the Biologic to the Patient
Several recent studies of up-and-coming biologics have focused on subsets of COPD patients, said Dave Singh, MD, professor of clinical pharmacology and respiratory medicine at The University of Manchester in England, in his presentation at the meeting. In September 2024, the Food and Drug Administration approved dupilumab as the first biologic treatment for patients with uncontrolled COPD and type 2 inflammation on the basis of eosinophil counts. Singh cited data from the BOREAS and NOTUS studies in which dupilumab significantly reduced exacerbations and improved lung function in these patients, compared with a placebo.
Mepolizumab, a biologic approved for asthma, is not currently approved for COPD, but data from a 2017 study showed a trend toward reduced exacerbations, compared with placebo, in a subset of patients with high blood eosinophil counts, Singh said.
In addition, a recent unpublished phase 3 study (MATINEE) showed a reduction in the annualized rate of exacerbations, compared with placebo, on the basis of up to 2 years’ follow-up.
Singh also highlighted data from a phase 2a study of astegolimab, a biologic drug that focuses on the IL-33 receptor, in which COPD exacerbation rates were not significantly different between treatment and placebo groups. However, astegolimab has shown safety and efficacy in adults with severe asthma and is under development in phase 3 trials for COPD.
Tezepelumab, which was approved by the FDA in 2021 as an add-on therapy for severe asthma in patients aged 12 years or older, is also in development as a therapy for COPD exacerbations, Singh said.
In a study presented at the 2024 American Thoracic Society annual meeting, Singh and colleagues found that tezepelumab at a subcutaneous dose of 420 mg every 4 weeks reduced the annualized rate of moderate or severe COPD exacerbations compared with placebo based on data from approximately 300 patients, although the difference was not statistically significant.
Itepekimab, another biologic, showed promise in a phase 2a genetic association study involving current and former smokers with moderate to severe COPD, Singh said.
In that study, published in 2022 in The Lancet Respiratory Medicine, itepekimab failed to meet the primary endpoint in the overall study population of reduced annualized rate of moderate to severe exacerbations; however, a subgroup analysis of former smokers showed a significant (42%) reduction in exacerbations, Singh said in his presentation. Two phase 3 clinical studies (AERIFY-1/2) are ongoing to confirm the safety and efficacy of itepekimab in former smokers with COPD.
Takeaways and Next Steps
“These therapies provide the first new classes of medications approved for COPD in nearly 20 years,” said David M. Mannino, MD, of the University of Kentucky, Lexington, in an interview. “Dupilumab will be available to a subset of patients who are poorly controlled and have evidence of high eosinophils in their blood and is only used once every 2 weeks,” added Mannino, who has served as a consultant to companies developing COPD drugs.
Both dupilumab and ensifentrine, a phosphodiesterase (PDE) 3 and PDE4 inhibitor also recently approved for maintenance treatment of COPD, have been shown in clinical trials to reduce exacerbations and improve symptoms, said Mannino. Both offer additional options for patients who continue to have symptoms and exacerbations in spite of their current therapy.
Some barriers to the use of biologics in practice include the high cost. “Access and overcoming insurance-related issues such as preauthorization and high copays will be a challenge,” he said. Also, because dupilumab is an injectable drug, some patient training will be required.
Newer biologic therapies in development are also injectables, but some studies are examining longer time intervals as long as every 6 months, which could be a major advancement for some patients. The newer therapies in development are similar to dupilumab in that they will be injected therapies. Some in development are looking at longer time intervals as long as every 6 months, which may be a major advancement for some patients. “All of these therapies, however, are currently targeting more advanced or serious disease,” he said.
Looking ahead, more therapies are needed for the treatment of early COPD, as well as therapies that can be administered to a large number of patients at a reasonable cost, Mannino added.
Rennard disclosed serving as a consultant for Verona Pharma, Sanofi, Beyond Air, RS BioTherapeutics, RespirAI, and Roche, as well as speaker fees from Sanofi and temporary ownership interest while employed by AstraZeneca. Rennard is also the founder of Great Plains Biometrix. Bafadhel disclosed funding from the National Institute for Health Research (NIHR), grants from Asthma + Lung UK, Horizon Europe, NIHR, and AstraZeneca to her institution, and honoraria from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, and Pfizer. Singh disclosed relationships including speaking sponsorships, honoraria, and advisory board memberships for Adovate, Aerogen, Almirall, Apogee, Arrowhead, AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, Cipla, Connect Biopharm, Covis, CSL Behring, DevPro Biopharm, Elpen, Empirico, EpiEndo, Genentech, Generate Biomedicines, GlaxoSmithKline, Glenmark, Kamada, Kinaset Therapeutics, Kymera, Menarini, MicroA, OM Pharma, Orion, Pieris Pharmaceuticals, Pulmatrix, Revolo, Roivant Sciences, Sanofi, Synairgen, Tetherex, Teva, Theravance Biopharma, Upstream, and Verona Pharma. Mannino disclosed serving as a consultant to multiple companies currently developing COPD therapies (AstraZeneca, GlaxoSmithKline, Roche, Regeneron, Sanofi, Genentech, Amgen, and Chiesi).
A version of this article appeared on Medscape.com.
New biologic drugs for chronic obstructive pulmonary disease (COPD) are finally here, said Stephen Rennard, MD, in a presentation in a session on new drugs at the 2024 GOLD International COPD Conference.
The therapeutic goals of biologics remain the same as with other treatments for COPD, namely restoration of normal inflammatory response and alteration of disease progression, as well as restoration of lost structure and function and improvement of systemic effects, Rennard said in his presentation. Most studies of new and up-and-coming drugs have improvement in acute exacerbation of COPD as the primary outcome.
The Biology Behind the Biologics
T2 inflammation is “an inflammatory cascade led by IL [interleukin]-4, IL-13, and IL-5,” Mona Bafadhel, MD, chair of Respiratory Medicine at King’s College London in England, said in her presentation during the session.
Bafadhel, who served as one of the investigators on the BOREAS and NOTUS studies, explained some of the science behind the development of the new biologics.
Eosinophils are powerful regulators of immune response and inflammation by stimulating T-cell production and affecting other immune cell types, she noted.
In the context of COPD and drug development, high blood eosinophil counts have been associated with increased COPD-related exacerbations, Bafadhel said. She cited data from a Dutch study of more than 7000 patients with COPD (with and without clinical diagnoses), in which absolute eosinophil counts ≥ 3.3% were associated with increased risk for severe exacerbations of 32% and 84% across all patients with COPD and clinical COPD, respectively.
Understanding the mechanisms of the eosinophil in COPD is important for research and development, Bafadhel said. Along with standardizing measurement of T2 inflammatory markers (IL-4, IL-13, and IL-5), more research is needed to fully understand the role of eosinophils in immunoregulation and repair.
Fitting the Biologic to the Patient
Several recent studies of up-and-coming biologics have focused on subsets of COPD patients, said Dave Singh, MD, professor of clinical pharmacology and respiratory medicine at The University of Manchester in England, in his presentation at the meeting. In September 2024, the Food and Drug Administration approved dupilumab as the first biologic treatment for patients with uncontrolled COPD and type 2 inflammation on the basis of eosinophil counts. Singh cited data from the BOREAS and NOTUS studies in which dupilumab significantly reduced exacerbations and improved lung function in these patients, compared with a placebo.
Mepolizumab, a biologic approved for asthma, is not currently approved for COPD, but data from a 2017 study showed a trend toward reduced exacerbations, compared with placebo, in a subset of patients with high blood eosinophil counts, Singh said.
In addition, a recent unpublished phase 3 study (MATINEE) showed a reduction in the annualized rate of exacerbations, compared with placebo, on the basis of up to 2 years’ follow-up.
Singh also highlighted data from a phase 2a study of astegolimab, a biologic drug that focuses on the IL-33 receptor, in which COPD exacerbation rates were not significantly different between treatment and placebo groups. However, astegolimab has shown safety and efficacy in adults with severe asthma and is under development in phase 3 trials for COPD.
Tezepelumab, which was approved by the FDA in 2021 as an add-on therapy for severe asthma in patients aged 12 years or older, is also in development as a therapy for COPD exacerbations, Singh said.
In a study presented at the 2024 American Thoracic Society annual meeting, Singh and colleagues found that tezepelumab at a subcutaneous dose of 420 mg every 4 weeks reduced the annualized rate of moderate or severe COPD exacerbations compared with placebo based on data from approximately 300 patients, although the difference was not statistically significant.
Itepekimab, another biologic, showed promise in a phase 2a genetic association study involving current and former smokers with moderate to severe COPD, Singh said.
In that study, published in 2022 in The Lancet Respiratory Medicine, itepekimab failed to meet the primary endpoint in the overall study population of reduced annualized rate of moderate to severe exacerbations; however, a subgroup analysis of former smokers showed a significant (42%) reduction in exacerbations, Singh said in his presentation. Two phase 3 clinical studies (AERIFY-1/2) are ongoing to confirm the safety and efficacy of itepekimab in former smokers with COPD.
Takeaways and Next Steps
“These therapies provide the first new classes of medications approved for COPD in nearly 20 years,” said David M. Mannino, MD, of the University of Kentucky, Lexington, in an interview. “Dupilumab will be available to a subset of patients who are poorly controlled and have evidence of high eosinophils in their blood and is only used once every 2 weeks,” added Mannino, who has served as a consultant to companies developing COPD drugs.
Both dupilumab and ensifentrine, a phosphodiesterase (PDE) 3 and PDE4 inhibitor also recently approved for maintenance treatment of COPD, have been shown in clinical trials to reduce exacerbations and improve symptoms, said Mannino. Both offer additional options for patients who continue to have symptoms and exacerbations in spite of their current therapy.
Some barriers to the use of biologics in practice include the high cost. “Access and overcoming insurance-related issues such as preauthorization and high copays will be a challenge,” he said. Also, because dupilumab is an injectable drug, some patient training will be required.
Newer biologic therapies in development are also injectables, but some studies are examining longer time intervals as long as every 6 months, which could be a major advancement for some patients. The newer therapies in development are similar to dupilumab in that they will be injected therapies. Some in development are looking at longer time intervals as long as every 6 months, which may be a major advancement for some patients. “All of these therapies, however, are currently targeting more advanced or serious disease,” he said.
Looking ahead, more therapies are needed for the treatment of early COPD, as well as therapies that can be administered to a large number of patients at a reasonable cost, Mannino added.
Rennard disclosed serving as a consultant for Verona Pharma, Sanofi, Beyond Air, RS BioTherapeutics, RespirAI, and Roche, as well as speaker fees from Sanofi and temporary ownership interest while employed by AstraZeneca. Rennard is also the founder of Great Plains Biometrix. Bafadhel disclosed funding from the National Institute for Health Research (NIHR), grants from Asthma + Lung UK, Horizon Europe, NIHR, and AstraZeneca to her institution, and honoraria from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, and Pfizer. Singh disclosed relationships including speaking sponsorships, honoraria, and advisory board memberships for Adovate, Aerogen, Almirall, Apogee, Arrowhead, AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, Cipla, Connect Biopharm, Covis, CSL Behring, DevPro Biopharm, Elpen, Empirico, EpiEndo, Genentech, Generate Biomedicines, GlaxoSmithKline, Glenmark, Kamada, Kinaset Therapeutics, Kymera, Menarini, MicroA, OM Pharma, Orion, Pieris Pharmaceuticals, Pulmatrix, Revolo, Roivant Sciences, Sanofi, Synairgen, Tetherex, Teva, Theravance Biopharma, Upstream, and Verona Pharma. Mannino disclosed serving as a consultant to multiple companies currently developing COPD therapies (AstraZeneca, GlaxoSmithKline, Roche, Regeneron, Sanofi, Genentech, Amgen, and Chiesi).
A version of this article appeared on Medscape.com.