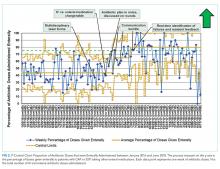
User login
Review of Radiologic Considerations in an Immunocompetent Patient With Primary Central Nervous System Lymphoma (FULL)
Central nervous system (CNS) lymphoma can be classified into 2 categories: primary CNS lymphoma (PCNSL), which includes disease limited to brain, eyes, spinal cord; and leptomeninges without coexisting or previous systemic lymphoma. Secondary CNS lymphoma (SCNSL) is essentially metastatic disease from a systemic primary site.1 The focus of this case presentation is PCNSL, with an emphasis on imaging characteristics and differential diagnosis.
The median age at diagnosis for PCNSL is 65 years, and the overall incidence has been decreasing since the mid-1990s, likely related to the increased use of highly-active antiretroviral therapy (HAART) in patients with AIDS.2,3 Although overall incidence has decreased, incidence in the elderly population has increased.4 Historically, PCNSL has been considered an AIDS-defining illness.5 These patients, among other immunocompromised patients, such as those on chronic immunosuppressive therapy, are at a higher risk for developing the malignancy.6
Clinical presentation varies because of the location of CNS involvement and may present with headache, mood or personality disturbances, or focal neurologic deficits. Seizures are less likely due to the tendency of PCNSL to spare gray matter. Initial workup generally includes a head computed tomography (CT) scan, as well as a contrast-enhanced magnetic resonance image (MRI), which may help direct clinicians to the appropriate diagnosis. However, there is significant overlap between the imaging characteristics of PCNSL and numerous other disease processes, including glioblastoma and demyelination. The imaging characteristics of PCNSL are considerably different depending on the patient’s immune status.7
This case illustrates a rare presentation of PCNSL in an immunocompetent patient whose MRI characteristics were seemingly more consistent with those seen in patients with immunodeficiency. The main differential diagnoses and key imaging characteristics, which may help obtain accurate diagnosis, will be discussed.
Case Presentation
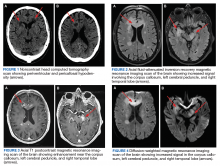
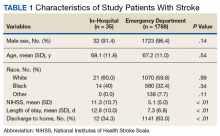
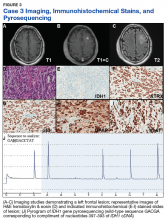
A 72-year-old male veteran presented with a 2-month history of subjective weakness in his upper and lower extremities progressing to multiple falls at home. He had no significant medical history other than a thymectomy at age 15 for an enlarged thymus, which per patient report, was benign. An initial laboratory test that included vitamin B12, folate, thyroid-stimulating hormone, complete blood cell count, and comprehensive metabolic panel, were unremarkable, with a white blood cell count of 8.5 K/uL. The initial neurologic evaluation did not show any focal neurologic deficits; however, during the initial hospital stay, the patient developed increasing lower extremity weakness on examination. A noncontrast CT head scan showed extensive nonspecific hypodensities within the periventricular white matter (Figure 1). A contrast-enhanced MRI showed enhancing lesions involving the corpus callosum, left cerebral peduncle, and right temporal lobe (Figures 2, 3, and 4). These lesions also exhibited significant restricted diffusion and a mild amount of surrounding vasogenic edema. The working diagnosis after the MRI included primary CNS lymphoma, multifocal glioblastoma, and tumefactive demyelinating disease. The patient was started on IV steroids and transferred for neurosurgical evaluation and biopsy at an outside hospital. The frontal lesion was biopsied, and the initial frozen section was consistent with lymphoma; a bone marrow biopsy was negative. The workup for immunodeficiency was unremarkable. Pathology revealed high-grade B-cell lymphoma, and the patient began a chemotherapy regimen.
Discussion
The workup of altered mental status, focal neurologic deficits, headaches, or other neurologic conditions often begins with a noncontrast CT scan. On CT, PCNSL generally appears isodense to hyperdense to gray matter, but appearance is variable. The often hyperdense appearance is attributable to the hypercellular nature of lymphoma. Many times, as in this case, CT may show only vague hypodensities, some of which may be associated with surrounding edema. This presentation is nonspecific and may be seen with advancing age due to changes of chronic microvascular ischemia as well as demyelination, other malignancies, and several other disease processes, both benign and malignant. After the initial CT scan, further workup requires evaluation with MRI. PCNSL exhibits restricted diffusion and variable signal intensity on T2-weighted imaging.
PCNSL is frequently centrally located within the periventricular white matter, often within the frontal lobe but can involve other lobes, the basal ganglia, brainstem, cerebellum, or less likely, the spinal canal.7 Contrary to primary CNS disease, secondary lymphoma within the CNS has been described classically as affecting a leptomeningeal (pia and arachnoid mater) distribution two-thirds of the time, with parenchymal involvement occurring in the other one-third of patients. A recent study by Malikova and colleagues found parenchymal involvement may be much more common than previously thought.1 Leptomeningeal spread of disease often involves the cranial nerves, subependymal regions, spinal cord, or spinal nerve roots. Dural involvement in primary or secondary lymphoma is rare.
PCNSL nearly always shows enhancement. Linear enhancement along perivascular spaces is highly characteristic of PCNSL. The typical appearance of PCNSL associated with immunodeficiency varies from that seen in an otherwise immunocompetent patient. Patients with immunodeficiency usually have multifocal involvement, central necrosis leading to a ring enhancement appearance, and have more propensity for spontaneous hemorrhage.7 Immunocompetent patients are less likely to present with multifocal disease and rarely show ring enhancement. Also, spontaneous hemorrhage is rare in immunocompetent patients. In our case, extensive multifocal involvement was present, whereas typically immunocompetent patients will present with a solitary homogeneously enhancing parenchymal mass.
The primary differential for PCNSL includes malignant glioma, tumefactive multiple sclerosis, metastatic disease, and in an immunocompromised patient, toxoplasmosis. The degree of associated vasogenic edema and mass effect is generally lower in PCNSL than that of malignant gliomas and metastasis. Also, PCNSL tends to spare the cerebral cortex.8
Classically, PCNSL, malignant gliomas, and demyelinating disease have been considered the main differential for lesions that cross midline and involve both cerebral hemispheres. Lymphoma generally exhibits more restricted diffusion than malignant gliomas and metastasis, attributable to the highly cellular nature of lymphoma.7 Tumefactive multiple sclerosis is associated with relatively minimal mass effect for lesion size and exhibits less restricted diffusion values when compared to high grade gliomas and PCNSL. One fairly specific finding for tumefactive demyelinating lesions is incomplete rim enhancement.9 Unfortunately, an MRI is not reliable in differentiating these entities, and biopsy is required for definitive diagnosis. Many advancing imaging modalities may help provide the correct diagnosis of PCNSL, including diffusion-weighted and apparent diffusion coefficient imaging, diffusion tensor imaging, MR spectroscopy and PET imaging.7
Conclusion
With the increasing use of HAART, the paradigm of PCNSL is shifting toward one predominantly affecting immunocompetent patients. PCNSL should be considered in any patient with multiple enhancing CNS lesions, regardless of immune status. Several key imaging characteristics may help differentiate PCNSL and other disease processes; however, at this time, biopsy is recommended for definitive diagnosis.
1. Malikova H, Burghardtova M, Koubska E, Mandys V, Kozak T, Weichet J. Secondary central nervous system lymphoma: spectrum of morphological MRI appearances. Neuropsychiatr Dis Treat. 2018;4:733-740.
2. Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro-Oncol. 2012;14(suppl 5):v1-v49.
3. Diamond C, Taylor TH, Aboumrad T, Anton-Culver H. Changes in acquired immunodeficiency syndrome-related non-Hodgkin lymphoma in the era of highly active antiretroviral therapy: incidence, presentation, treatment, and survival. Cancer. 2006;106(1):128-135.
4. O’Neill BP, Decker PA, Tieu C, Cerhan JR. The changing incidence of primary central nervous system lymphoma is driven primarily by the changing incidence in young and middle-aged men and differs from time trends in systemic diffuse large B-cell non-Hodgkins lymphoma. Am J Hematol. 2013;88(12):997-1000.
5. [no authors listed]. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(rr-17):1-19.
6. Maiuri F. Central nervous system lymphomas and immunodeficiency. Neurological Research. 1989;11(1):2-5.
7. Haldorsen IS, Espeland A, Larsson EM. Central nervous system lymphoma: characteristic findings on traditional and advanced imaging. AJNR Am J Neuroradiol. 2010;32(6):984-992.
8. Gómez Roselló E, Quiles Granado AM, Laguillo Sala G, Gutiérrez S. Primary central nervous system lymphoma in immunocompetent patients: spectrum of findings and differential characteristics. Radiología. 2018;60(4):280-289.
9. Mabray MC, Cohen BA, Villanueva-Meyer JE, et al. Performance of Apparent Diffusion Coefficient Values and Conventional MRI Features in Differentiating Tumefactive Demyelinating Lesions From Primary Brain Neoplasms. American Journal of Roentgenology. 2015;205(5):1075-1085.
Central nervous system (CNS) lymphoma can be classified into 2 categories: primary CNS lymphoma (PCNSL), which includes disease limited to brain, eyes, spinal cord; and leptomeninges without coexisting or previous systemic lymphoma. Secondary CNS lymphoma (SCNSL) is essentially metastatic disease from a systemic primary site.1 The focus of this case presentation is PCNSL, with an emphasis on imaging characteristics and differential diagnosis.
The median age at diagnosis for PCNSL is 65 years, and the overall incidence has been decreasing since the mid-1990s, likely related to the increased use of highly-active antiretroviral therapy (HAART) in patients with AIDS.2,3 Although overall incidence has decreased, incidence in the elderly population has increased.4 Historically, PCNSL has been considered an AIDS-defining illness.5 These patients, among other immunocompromised patients, such as those on chronic immunosuppressive therapy, are at a higher risk for developing the malignancy.6
Clinical presentation varies because of the location of CNS involvement and may present with headache, mood or personality disturbances, or focal neurologic deficits. Seizures are less likely due to the tendency of PCNSL to spare gray matter. Initial workup generally includes a head computed tomography (CT) scan, as well as a contrast-enhanced magnetic resonance image (MRI), which may help direct clinicians to the appropriate diagnosis. However, there is significant overlap between the imaging characteristics of PCNSL and numerous other disease processes, including glioblastoma and demyelination. The imaging characteristics of PCNSL are considerably different depending on the patient’s immune status.7
This case illustrates a rare presentation of PCNSL in an immunocompetent patient whose MRI characteristics were seemingly more consistent with those seen in patients with immunodeficiency. The main differential diagnoses and key imaging characteristics, which may help obtain accurate diagnosis, will be discussed.
Case Presentation
A 72-year-old male veteran presented with a 2-month history of subjective weakness in his upper and lower extremities progressing to multiple falls at home. He had no significant medical history other than a thymectomy at age 15 for an enlarged thymus, which per patient report, was benign. An initial laboratory test that included vitamin B12, folate, thyroid-stimulating hormone, complete blood cell count, and comprehensive metabolic panel, were unremarkable, with a white blood cell count of 8.5 K/uL. The initial neurologic evaluation did not show any focal neurologic deficits; however, during the initial hospital stay, the patient developed increasing lower extremity weakness on examination. A noncontrast CT head scan showed extensive nonspecific hypodensities within the periventricular white matter (Figure 1). A contrast-enhanced MRI showed enhancing lesions involving the corpus callosum, left cerebral peduncle, and right temporal lobe (Figures 2, 3, and 4). These lesions also exhibited significant restricted diffusion and a mild amount of surrounding vasogenic edema. The working diagnosis after the MRI included primary CNS lymphoma, multifocal glioblastoma, and tumefactive demyelinating disease. The patient was started on IV steroids and transferred for neurosurgical evaluation and biopsy at an outside hospital. The frontal lesion was biopsied, and the initial frozen section was consistent with lymphoma; a bone marrow biopsy was negative. The workup for immunodeficiency was unremarkable. Pathology revealed high-grade B-cell lymphoma, and the patient began a chemotherapy regimen.
Discussion
The workup of altered mental status, focal neurologic deficits, headaches, or other neurologic conditions often begins with a noncontrast CT scan. On CT, PCNSL generally appears isodense to hyperdense to gray matter, but appearance is variable. The often hyperdense appearance is attributable to the hypercellular nature of lymphoma. Many times, as in this case, CT may show only vague hypodensities, some of which may be associated with surrounding edema. This presentation is nonspecific and may be seen with advancing age due to changes of chronic microvascular ischemia as well as demyelination, other malignancies, and several other disease processes, both benign and malignant. After the initial CT scan, further workup requires evaluation with MRI. PCNSL exhibits restricted diffusion and variable signal intensity on T2-weighted imaging.
PCNSL is frequently centrally located within the periventricular white matter, often within the frontal lobe but can involve other lobes, the basal ganglia, brainstem, cerebellum, or less likely, the spinal canal.7 Contrary to primary CNS disease, secondary lymphoma within the CNS has been described classically as affecting a leptomeningeal (pia and arachnoid mater) distribution two-thirds of the time, with parenchymal involvement occurring in the other one-third of patients. A recent study by Malikova and colleagues found parenchymal involvement may be much more common than previously thought.1 Leptomeningeal spread of disease often involves the cranial nerves, subependymal regions, spinal cord, or spinal nerve roots. Dural involvement in primary or secondary lymphoma is rare.
PCNSL nearly always shows enhancement. Linear enhancement along perivascular spaces is highly characteristic of PCNSL. The typical appearance of PCNSL associated with immunodeficiency varies from that seen in an otherwise immunocompetent patient. Patients with immunodeficiency usually have multifocal involvement, central necrosis leading to a ring enhancement appearance, and have more propensity for spontaneous hemorrhage.7 Immunocompetent patients are less likely to present with multifocal disease and rarely show ring enhancement. Also, spontaneous hemorrhage is rare in immunocompetent patients. In our case, extensive multifocal involvement was present, whereas typically immunocompetent patients will present with a solitary homogeneously enhancing parenchymal mass.
The primary differential for PCNSL includes malignant glioma, tumefactive multiple sclerosis, metastatic disease, and in an immunocompromised patient, toxoplasmosis. The degree of associated vasogenic edema and mass effect is generally lower in PCNSL than that of malignant gliomas and metastasis. Also, PCNSL tends to spare the cerebral cortex.8
Classically, PCNSL, malignant gliomas, and demyelinating disease have been considered the main differential for lesions that cross midline and involve both cerebral hemispheres. Lymphoma generally exhibits more restricted diffusion than malignant gliomas and metastasis, attributable to the highly cellular nature of lymphoma.7 Tumefactive multiple sclerosis is associated with relatively minimal mass effect for lesion size and exhibits less restricted diffusion values when compared to high grade gliomas and PCNSL. One fairly specific finding for tumefactive demyelinating lesions is incomplete rim enhancement.9 Unfortunately, an MRI is not reliable in differentiating these entities, and biopsy is required for definitive diagnosis. Many advancing imaging modalities may help provide the correct diagnosis of PCNSL, including diffusion-weighted and apparent diffusion coefficient imaging, diffusion tensor imaging, MR spectroscopy and PET imaging.7
Conclusion
With the increasing use of HAART, the paradigm of PCNSL is shifting toward one predominantly affecting immunocompetent patients. PCNSL should be considered in any patient with multiple enhancing CNS lesions, regardless of immune status. Several key imaging characteristics may help differentiate PCNSL and other disease processes; however, at this time, biopsy is recommended for definitive diagnosis.
Central nervous system (CNS) lymphoma can be classified into 2 categories: primary CNS lymphoma (PCNSL), which includes disease limited to brain, eyes, spinal cord; and leptomeninges without coexisting or previous systemic lymphoma. Secondary CNS lymphoma (SCNSL) is essentially metastatic disease from a systemic primary site.1 The focus of this case presentation is PCNSL, with an emphasis on imaging characteristics and differential diagnosis.
The median age at diagnosis for PCNSL is 65 years, and the overall incidence has been decreasing since the mid-1990s, likely related to the increased use of highly-active antiretroviral therapy (HAART) in patients with AIDS.2,3 Although overall incidence has decreased, incidence in the elderly population has increased.4 Historically, PCNSL has been considered an AIDS-defining illness.5 These patients, among other immunocompromised patients, such as those on chronic immunosuppressive therapy, are at a higher risk for developing the malignancy.6
Clinical presentation varies because of the location of CNS involvement and may present with headache, mood or personality disturbances, or focal neurologic deficits. Seizures are less likely due to the tendency of PCNSL to spare gray matter. Initial workup generally includes a head computed tomography (CT) scan, as well as a contrast-enhanced magnetic resonance image (MRI), which may help direct clinicians to the appropriate diagnosis. However, there is significant overlap between the imaging characteristics of PCNSL and numerous other disease processes, including glioblastoma and demyelination. The imaging characteristics of PCNSL are considerably different depending on the patient’s immune status.7
This case illustrates a rare presentation of PCNSL in an immunocompetent patient whose MRI characteristics were seemingly more consistent with those seen in patients with immunodeficiency. The main differential diagnoses and key imaging characteristics, which may help obtain accurate diagnosis, will be discussed.
Case Presentation
A 72-year-old male veteran presented with a 2-month history of subjective weakness in his upper and lower extremities progressing to multiple falls at home. He had no significant medical history other than a thymectomy at age 15 for an enlarged thymus, which per patient report, was benign. An initial laboratory test that included vitamin B12, folate, thyroid-stimulating hormone, complete blood cell count, and comprehensive metabolic panel, were unremarkable, with a white blood cell count of 8.5 K/uL. The initial neurologic evaluation did not show any focal neurologic deficits; however, during the initial hospital stay, the patient developed increasing lower extremity weakness on examination. A noncontrast CT head scan showed extensive nonspecific hypodensities within the periventricular white matter (Figure 1). A contrast-enhanced MRI showed enhancing lesions involving the corpus callosum, left cerebral peduncle, and right temporal lobe (Figures 2, 3, and 4). These lesions also exhibited significant restricted diffusion and a mild amount of surrounding vasogenic edema. The working diagnosis after the MRI included primary CNS lymphoma, multifocal glioblastoma, and tumefactive demyelinating disease. The patient was started on IV steroids and transferred for neurosurgical evaluation and biopsy at an outside hospital. The frontal lesion was biopsied, and the initial frozen section was consistent with lymphoma; a bone marrow biopsy was negative. The workup for immunodeficiency was unremarkable. Pathology revealed high-grade B-cell lymphoma, and the patient began a chemotherapy regimen.
Discussion
The workup of altered mental status, focal neurologic deficits, headaches, or other neurologic conditions often begins with a noncontrast CT scan. On CT, PCNSL generally appears isodense to hyperdense to gray matter, but appearance is variable. The often hyperdense appearance is attributable to the hypercellular nature of lymphoma. Many times, as in this case, CT may show only vague hypodensities, some of which may be associated with surrounding edema. This presentation is nonspecific and may be seen with advancing age due to changes of chronic microvascular ischemia as well as demyelination, other malignancies, and several other disease processes, both benign and malignant. After the initial CT scan, further workup requires evaluation with MRI. PCNSL exhibits restricted diffusion and variable signal intensity on T2-weighted imaging.
PCNSL is frequently centrally located within the periventricular white matter, often within the frontal lobe but can involve other lobes, the basal ganglia, brainstem, cerebellum, or less likely, the spinal canal.7 Contrary to primary CNS disease, secondary lymphoma within the CNS has been described classically as affecting a leptomeningeal (pia and arachnoid mater) distribution two-thirds of the time, with parenchymal involvement occurring in the other one-third of patients. A recent study by Malikova and colleagues found parenchymal involvement may be much more common than previously thought.1 Leptomeningeal spread of disease often involves the cranial nerves, subependymal regions, spinal cord, or spinal nerve roots. Dural involvement in primary or secondary lymphoma is rare.
PCNSL nearly always shows enhancement. Linear enhancement along perivascular spaces is highly characteristic of PCNSL. The typical appearance of PCNSL associated with immunodeficiency varies from that seen in an otherwise immunocompetent patient. Patients with immunodeficiency usually have multifocal involvement, central necrosis leading to a ring enhancement appearance, and have more propensity for spontaneous hemorrhage.7 Immunocompetent patients are less likely to present with multifocal disease and rarely show ring enhancement. Also, spontaneous hemorrhage is rare in immunocompetent patients. In our case, extensive multifocal involvement was present, whereas typically immunocompetent patients will present with a solitary homogeneously enhancing parenchymal mass.
The primary differential for PCNSL includes malignant glioma, tumefactive multiple sclerosis, metastatic disease, and in an immunocompromised patient, toxoplasmosis. The degree of associated vasogenic edema and mass effect is generally lower in PCNSL than that of malignant gliomas and metastasis. Also, PCNSL tends to spare the cerebral cortex.8
Classically, PCNSL, malignant gliomas, and demyelinating disease have been considered the main differential for lesions that cross midline and involve both cerebral hemispheres. Lymphoma generally exhibits more restricted diffusion than malignant gliomas and metastasis, attributable to the highly cellular nature of lymphoma.7 Tumefactive multiple sclerosis is associated with relatively minimal mass effect for lesion size and exhibits less restricted diffusion values when compared to high grade gliomas and PCNSL. One fairly specific finding for tumefactive demyelinating lesions is incomplete rim enhancement.9 Unfortunately, an MRI is not reliable in differentiating these entities, and biopsy is required for definitive diagnosis. Many advancing imaging modalities may help provide the correct diagnosis of PCNSL, including diffusion-weighted and apparent diffusion coefficient imaging, diffusion tensor imaging, MR spectroscopy and PET imaging.7
Conclusion
With the increasing use of HAART, the paradigm of PCNSL is shifting toward one predominantly affecting immunocompetent patients. PCNSL should be considered in any patient with multiple enhancing CNS lesions, regardless of immune status. Several key imaging characteristics may help differentiate PCNSL and other disease processes; however, at this time, biopsy is recommended for definitive diagnosis.
1. Malikova H, Burghardtova M, Koubska E, Mandys V, Kozak T, Weichet J. Secondary central nervous system lymphoma: spectrum of morphological MRI appearances. Neuropsychiatr Dis Treat. 2018;4:733-740.
2. Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro-Oncol. 2012;14(suppl 5):v1-v49.
3. Diamond C, Taylor TH, Aboumrad T, Anton-Culver H. Changes in acquired immunodeficiency syndrome-related non-Hodgkin lymphoma in the era of highly active antiretroviral therapy: incidence, presentation, treatment, and survival. Cancer. 2006;106(1):128-135.
4. O’Neill BP, Decker PA, Tieu C, Cerhan JR. The changing incidence of primary central nervous system lymphoma is driven primarily by the changing incidence in young and middle-aged men and differs from time trends in systemic diffuse large B-cell non-Hodgkins lymphoma. Am J Hematol. 2013;88(12):997-1000.
5. [no authors listed]. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(rr-17):1-19.
6. Maiuri F. Central nervous system lymphomas and immunodeficiency. Neurological Research. 1989;11(1):2-5.
7. Haldorsen IS, Espeland A, Larsson EM. Central nervous system lymphoma: characteristic findings on traditional and advanced imaging. AJNR Am J Neuroradiol. 2010;32(6):984-992.
8. Gómez Roselló E, Quiles Granado AM, Laguillo Sala G, Gutiérrez S. Primary central nervous system lymphoma in immunocompetent patients: spectrum of findings and differential characteristics. Radiología. 2018;60(4):280-289.
9. Mabray MC, Cohen BA, Villanueva-Meyer JE, et al. Performance of Apparent Diffusion Coefficient Values and Conventional MRI Features in Differentiating Tumefactive Demyelinating Lesions From Primary Brain Neoplasms. American Journal of Roentgenology. 2015;205(5):1075-1085.
1. Malikova H, Burghardtova M, Koubska E, Mandys V, Kozak T, Weichet J. Secondary central nervous system lymphoma: spectrum of morphological MRI appearances. Neuropsychiatr Dis Treat. 2018;4:733-740.
2. Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro-Oncol. 2012;14(suppl 5):v1-v49.
3. Diamond C, Taylor TH, Aboumrad T, Anton-Culver H. Changes in acquired immunodeficiency syndrome-related non-Hodgkin lymphoma in the era of highly active antiretroviral therapy: incidence, presentation, treatment, and survival. Cancer. 2006;106(1):128-135.
4. O’Neill BP, Decker PA, Tieu C, Cerhan JR. The changing incidence of primary central nervous system lymphoma is driven primarily by the changing incidence in young and middle-aged men and differs from time trends in systemic diffuse large B-cell non-Hodgkins lymphoma. Am J Hematol. 2013;88(12):997-1000.
5. [no authors listed]. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(rr-17):1-19.
6. Maiuri F. Central nervous system lymphomas and immunodeficiency. Neurological Research. 1989;11(1):2-5.
7. Haldorsen IS, Espeland A, Larsson EM. Central nervous system lymphoma: characteristic findings on traditional and advanced imaging. AJNR Am J Neuroradiol. 2010;32(6):984-992.
8. Gómez Roselló E, Quiles Granado AM, Laguillo Sala G, Gutiérrez S. Primary central nervous system lymphoma in immunocompetent patients: spectrum of findings and differential characteristics. Radiología. 2018;60(4):280-289.
9. Mabray MC, Cohen BA, Villanueva-Meyer JE, et al. Performance of Apparent Diffusion Coefficient Values and Conventional MRI Features in Differentiating Tumefactive Demyelinating Lesions From Primary Brain Neoplasms. American Journal of Roentgenology. 2015;205(5):1075-1085.
Accuracy of Endoscopic Ultrasound in Staging of Early Rectal Cancer (FULL)
Endoscopic ultrasound can be highly accurate for the staging of neoplasms in early rectal cancer.
Colorectal cancer is the second most common cause of cancer death in the US, with one-third of all colorectal cancers occurring within the rectum. Each year, an estimated 40000 Americans are diagnosed with rectal cancer (RC).1,2 The prognosis and treatment of RC depends on both T and N stage at the time of diagnosis.3-5 According to the most recent National Comprehensive Cancer Network guidelines from May 2019, patients with T1 to T2N0 tumors should undergo transanal or transabdominal surgery upfront, whereas patients with T3 to T4N0 or any TN1 to 2 should start with neoadjuvant therapy for better locoregional control, followed by surgery.6 Therefore, the appropriate management of RC requires adequate staging.
Endoscopic ultrasound (EUS), magnetic resonance imaging (MRI), and computed tomography (CT) are the imaging techniques currently used to stage RC. In a meta-analysis of 90 articles published between 1985 and 2002 that compared the 3 radiologic modalities, Bipat and colleagues found that MRI and EUS had a similar sensitivity of 94%, whereas the specificity of EUS (86%) was significantly higher than that of MRI (69%) for muscularis propria invasion.7 CT was performed only in a limited number of trials because CT was considered inadequate to assess early T stage. For perirectal tissue invasion, the sensitivity of EUS was statistically higher than that of CT and MRI imaging: 90% compared with 79% and 82%, respectively. The specificity estimates for EUS, CT, and MRI were comparable: 75%, 78%, and 76%, respectively. The respective sensitivity and specificity of the 3 imaging modalities to evaluate lymph nodes were also comparable: EUS, 67% and 78%; CT, 55% and 74%; and MRI, 66% and 76%.
The role of EUS in the diagnosis and treatment of RC has long been validated.1,2-5 A meta-analysis of 42 studies involving 5039 patients found EUS to be highly accurate for differentiating various T stages.8 However, EUS cannot assess iliac and mesenteric lymph nodes or posterior tumor extension beyond endopelvic fascia in advanced RC. Notable heterogeneity was found among the studies in the meta-analyses with regard to the type of equipment used for staging, as well as the criteria used to assess the depth of penetration and nodal status. The recent introduction of phased-array coils and the development of T2-weighted fast spin sequences have improved the resolution of MRI. The MERCURY trial showed that extension of tumor to within 1 mm of the circumferential margin on high-resolution MRI correctly predicted margin involvement at the time of surgery in 92% of the patients.9 In the retrospective study by Balyasnikova and colleagues, MRI was found to correctly identify partial submucosal invasion and suitability for local excision in 89% of the cases.10
Therefore, both EUS and MRI are useful, more so than CT, in assessment of the depth of tumor invasion, nodal staging, and predicting the circumferential resection margin. The use of EUS, however, does not preclude the use of MRI, or vice versa. Rather, the 2 modalities can complement each other in staging and proper patient selection for treatment.11
Despite data supporting the value of EUS in staging RC, its use is limited by a high degree of operator dependence and a substantial learning curve,12-17 which may explain the low EUS accuracy observed in some reports.7,13,15 Given the presence of recognized alternatives such as MRI, we decided to reevaluate EUS accuracy for the staging of RC outside high-volume specialized centers and prospective clinical trials.
Methods
A retrospective chart review was performed that included all consecutive patients undergoing rectal ultrasound from January 2011 to August 2015 at the US Department of Veterans Affairs Medical Center (VAMC) in Memphis, Tennessee. Sixty-five patients with short-stocked or sessile lesions < 15 cm from anal margin staged T2N0M0 or lower by endorectal ultrasound (ERUS) were included. The patients with neoplasms staged in excess of T2 or N0 were excluded from the study because treatment protocol dictates immediate neoadjuvant treatment, the administration of which would affect subsequent histopathology.
For the 37 patients included in the final analysis, ERUS results were compared with surgical pathology to ascertain accuracy. The resections were performed endoscopically or surgically with a goal of obtaining clear margins. The choice of procedure depended on size, shape, location, and depth of invasion. All patients underwent clinical and endoscopic surveillance with flexible sigmoidoscopy/EUS every 3 to 6 months for the first 2 years. We used 2 different gold standards for surveillance depending on the type of procedure performed to remove the lesion. A pathology report was the gold standard used for patients who underwent surgery. In patients who underwent endoscopic resection, we used the lack of recurrent disease, determined by normal endoscopic and endoscopic ultrasound examination, to signify complete endoscopic resection and therefore adequate staging as an early neoplasm.
Results
From January 2011 to August 2015, 65 rectal ultrasounds were performed. All EUS procedures were performed by 1 physician (C Ruben Tombazzi). All patients had previous endoscopic evaluation and tissue diagnoses. Twenty-eight patients were excluded: 18 had T3 or N1 disease, 2 had T2N0 but refused surgery, 2 had anal cancer, 3 patients with suspected cancer had benign nonneoplastic disease (2 radiation proctitis, 1 normal rectal wall), and 3 underwent EUS for benign tumors (1 ganglioneuroma and 2 lipomas).
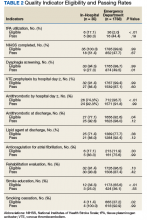
Thirty-seven patients were included in the study, 3 of whom were staged as T2N0 and 34 as T1N0 or lower by EUS. All patients were men ranging in age from 43 to 73 years (mean, 59 years). All 37 patients underwent endoscopic or surgical resection of their early rectal neoplasm. The final pathologic evaluation of the specimens demonstrated 14 carcinoid tumors, 11 adenocarcinomas, 6 tubular adenomas with high-grade dysplasia, and 6 benign adenomas. The preoperative EUS staging was confirmed for all patients, with 100% sensitivity, specificity, and accuracy. None of the patients who underwent endoscopic or surgical transanal resection had recurrence, determined by normal endoscopic and endoscopic ultrasound appearance, during a mean of 32.6 months surveillance.
Discussion
EUS has long been a recognized method for T and N staging of RC.1,3-5,7,8 Our data confirm that, in experienced hands, EUS is highly accurate in the staging of early rectal cancers.
The impact of EUS on the management of RC was demonstrated in a Mayo Clinic prospective blinded study.1 In that cohort of 80 consecutive patients who had previously had a CT for staging, EUS altered patient management in about 30% of cases. The most common change precipatated by EUS was the indication for additional neoadjuvant treatment.
However, the results have not been as encouraging when ERUS is performed outside of strict research protocol. A multicenter, prospective, country-wide quality assurance study from > 300 German hospitals was designed to assess the diagnostic accuracy of EUS in RC.13 Of 29206 patients, 7096 underwent surgery, without neoadjuvant treatment, and were included in the final analysis. The correspondence of tumor invasion with histopathology was 64.7%, with understaging of 18% and overstaging of 17.3%.13 These numbers were better in hospitals with greater experience performing ERUS: 73% accuracy in the centers with a case load of > 30 cases per year compared with 63.2% accuracy for the centers with < 10 cases a year. Marusch and colleagues had previously demonstrated an EUS accuracy of 63.3% in a study of 1463 patients with RC in Germany.14 Another study based out of the UK had similar findings. Ashraf and colleagues performed a database analyses from 20 UK centers and identified 165 patients with RC who underwent ERUS and endoscopic microsurgery.15 Compared with histopathology, EUS had 57.1% sensitivity, 73% specificity, and 42.9% accuracy for T1 cancers; EUS accuracy was 50% for T2 and 58% for T3 tumors. The authors concluded that the general accuracy of EUS in determining stage was around 50%, the statistical equivalent of flipping a coin.
The low accuracy of EUS observed by German and British multicenter studies13-15 was attributed to the difference that may exist in clinical trials at specialized centers compared with wider use of EUS in a community setting. As seen by our data, the Memphis VAMC is not a high-volume center for the treatment of RC. However, all our EUS procedures were performed and interpreted by a single operator (C. Ruben Tombazzi) with 18 years of EUS experience. We cannot conclude that no patient was overstaged, as patients receiving a stage of T3N0 or T > N0 received neoadjuvant treatment and were not included. However, we can conclude that no patient was understaged. All patients deemed to be T1 to T2N0 included in our study received accurate staging. Our results are consistent with the high accuracy of EUS reported from other centers with experience in diagnosis and treatment of RC.1,3-5,17,18
Although EUS is accurate in differentiating T1 from T2 tumors, it cannot reliably differentiate T1 from T0 lesions. In one study, 57.6% of adenomas and 30.7% of carcinomas in situ were staged as T1 on EUS, while almost half of T1 cancers were interpreted as T0.17 This drawback is a well-known limitation of EUS; although, the misinterpretation does not affect treatment, as both T0 and T1 lesions can be treated successfully by local excision alone, which was the algorithm used for our patients. The choice of the specific procedure for local excision was left to the clinicians and included transanal endoscopic or surgical resections. At a mean follow-up of 32.6 months, none of the 37 patients who underwent endoscopic or surgical transanal resection had evidence of recurrent disease.
A limitation of EUS, or any other imaging modality, is differentiating tumor invasion from peritumoral inflammation. The inflammation can render images of tumor borders ill-defined and irregular, which hinders precise staging. However, the accurate identification of tumors with deep involvement of the submucosa (T1sm3) is of importance, because these tumors are more advanced than the superficial and intermediate T1 lesions (T1sm1 and T1sm2, respectively).
Patients with RC whose lesions are considered T1sm3 are at higher risk of harboring lymph node metastases.18 Nascimbeni and colleagues had shown that the invasion into the lower third of the submucosa (sm3) was an independent risk factor for lower cancer-free survival among patients with T1 RC.19
Unlike rectal adenocarcinomas, the prognosis for carcinoid tumors correlates not only with the depth of invasion but also with the size of the tumor. The other adverse prognostic features include poor differentiation, high mitosis index, and lymphovascular invasion.20
EUS had been shown to be highly accurate in determining the precise carcinoid tumor size, depth of invasion, and lymph node metastases.20,21 In a study of 66 resected rectal carcinoid tumors by Ishii and colleagues, 57 lesions had a diameter of ≤ 10 mm and 9 lesions had a diameter of > 10 mm.21 All of the 57 carcinoid tumors with a diameter of ≤ 10 mm were confined to the submucosa. In contrast, 5 of the 9 lesions > 10 mm invaded the muscularis propria, 6 had a lymphovascular invasion, 4 were lymph node metastases, and 1 was a liver metastasis.
In our series, 4 of the 14 carcinoid tumors were > 10 mm but none were > 20 mm. None of the carcinoids with a diameter ≤ 10 mm invaded the muscularis propria. Of the 4 carcinoids > 10 mm, 1 was T2N0 and 3 were T1N0. All carcinoid tumors in our series were low grade and with low proliferation indexes, and all were treated successfully by local excision.
Conclusion
We believe our study shows that EUS can be highly accurate in staging rectal lesions, specifically lesions that are T1-T2N0, be they adenocarcinoma or carcinoid. Although we could not assess overstaging for lesions that were staged > T2 or > N0, we w
1. Harewood GC, Wiersema MJ, Nelson H, et al. A prospective, blinded assessment of the impact of preoperative staging on the management of rectal cancer. Gastroenterology. 2002;123(1):24-32.
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
3. Ahuja NK, Sauer BG, Wang AY, et al. Performance of endoscopic ultrasound in staging rectal adenocarcinoma appropriate for primary surgical resection. Clin Gastroenterol Hepatol. 2015;13:339-44.
4. Doornebosch PG, Bronkhorst PJ, Hop WC, Bode WA, Sing AK, de Graaf EJ. The role of endorectal ultrasound in therapeutic decision-making for local vs. transabdominal resection of rectal tumors. Dis Colon Rectum. 2008;51(1):38-42.
5. Santoro GA, Gizzi G, Pellegrini L, Battistella G, Di Falco G. The value of high-resolution three-dimensional endorectal ultrasonography in the management of submucosal invasive rectal tumors. Dis Colon Rectum. 2009;52(11):1837-1843.
6. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: rectal cancer, version 2.2019. https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Published May 15, 2019. Accessed July 19, 2019.
7. Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging—a meta-analysis. Radiology. 2004;232(3):773-783.
8. Puli SR, Bechtold ML, Reddy JB, Choudhary A, Antillon MR, Brugge WR. How good is endoscopic ultrasound in differentiating various T stages of rectal cancer? Meta-analysis and systematic review. Ann Surg Oncol. 2009;16(2):254-265.
9. MERCURY Study Group. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ. 2006;333(7572):779.
10. Balyasnikova S, Read J, Wotherspoon A, et al. Diagnostic accuracy of high-resolution MRI as a method to predict potentially safe endoscopic and surgical planes in patient with early rectal cancer. BMJ Open Gastroenterol. 2017;4(1):e000151.
11. Frasson M, Garcia-Granero E, Roda D, et al. Preoperative chemoradiation may not always be needed for patients with T3 and T2N+ rectal cancer. Cancer. 2011;117(14):3118-3125.
12. Rafaelsen SR, Sørensen T, Jakobsen A, Bisgaard C, Lindebjerg J. Transrectal ultrasonography and magnetic resonance imaging in the staging of rectal cancer. Effect of experience. Scand J Gastroenterol. 2008;43(4):440-446.
13. Marusch F, Ptok H, Sahm M, et al. Endorectal ultrasound in rectal carcinoma – do the literature results really correspond to the realities of routine clinical care? Endoscopy. 2011;43(5):425-431.
14. Marusch F, Koch A, Schmidt U, et al. Routine use of transrectal ultrasound in rectal carcinoma: results of a prospective multicenter study. Endoscopy. 2002;34(5):385-390.
15. Ashraf S, Hompes R, Slater A, et al; Association of Coloproctology of Great Britain and Ireland Transanal Endoscopic Microsurgery (TEM) Collaboration. A critical appraisal of endorectal ultrasound and transanal endoscopic microsurgery and decision-making in early rectal cancer. Colorectal Dis. 2012;14(7):821-826.
16. Harewood GC. Assessment of clinical impact of endoscopic ultrasound on rectal cancer. Am J Gastroenterol. 2004;99(4):623-627.
17. Zorcolo L, Fantola G, Cabras F, Marongiu L, D’Alia G, Casula G. Preoperative staging of patients with rectal tumors suitable for transanal endoscopic microsurgery (TEM): comparison of endorectal ultrasound and histopathologic findings. Surg Endosc. 2009;23(6):1384-1389.
18. Akasu T, Kondo H, Moriya Y, et al. Endoscopic ultrasonography and treatment of early stage rectal cancer. World J Surg. 2000;24(9):1061-1068.
19. Nascimbeni R, Nivatvongs S, Larson DR, Burgart LJ. Long-term survival after local excision for T1 carcinoma of the rectum. Dis Colon Rectum. 2004;47(11):1773-1779.
20. Park CH, Cheon JH, Kim JO, et al. Criteria for decision making after endoscopic resection of well-differentiated rectal carcinoids with regard to potential lymphatic spread. Endoscopy. 2011;43(9):790-795.
21. Ishii N, Horiki N, Itoh T, et al. Endoscopic submucosal dissection and preoperative assessment with endoscopic ultrasonography for the treatment of rectal carcinoid tumors. Surg Endosc. 2010;24(6):1413-1419.
Endoscopic ultrasound can be highly accurate for the staging of neoplasms in early rectal cancer.
Endoscopic ultrasound can be highly accurate for the staging of neoplasms in early rectal cancer.
Colorectal cancer is the second most common cause of cancer death in the US, with one-third of all colorectal cancers occurring within the rectum. Each year, an estimated 40000 Americans are diagnosed with rectal cancer (RC).1,2 The prognosis and treatment of RC depends on both T and N stage at the time of diagnosis.3-5 According to the most recent National Comprehensive Cancer Network guidelines from May 2019, patients with T1 to T2N0 tumors should undergo transanal or transabdominal surgery upfront, whereas patients with T3 to T4N0 or any TN1 to 2 should start with neoadjuvant therapy for better locoregional control, followed by surgery.6 Therefore, the appropriate management of RC requires adequate staging.
Endoscopic ultrasound (EUS), magnetic resonance imaging (MRI), and computed tomography (CT) are the imaging techniques currently used to stage RC. In a meta-analysis of 90 articles published between 1985 and 2002 that compared the 3 radiologic modalities, Bipat and colleagues found that MRI and EUS had a similar sensitivity of 94%, whereas the specificity of EUS (86%) was significantly higher than that of MRI (69%) for muscularis propria invasion.7 CT was performed only in a limited number of trials because CT was considered inadequate to assess early T stage. For perirectal tissue invasion, the sensitivity of EUS was statistically higher than that of CT and MRI imaging: 90% compared with 79% and 82%, respectively. The specificity estimates for EUS, CT, and MRI were comparable: 75%, 78%, and 76%, respectively. The respective sensitivity and specificity of the 3 imaging modalities to evaluate lymph nodes were also comparable: EUS, 67% and 78%; CT, 55% and 74%; and MRI, 66% and 76%.
The role of EUS in the diagnosis and treatment of RC has long been validated.1,2-5 A meta-analysis of 42 studies involving 5039 patients found EUS to be highly accurate for differentiating various T stages.8 However, EUS cannot assess iliac and mesenteric lymph nodes or posterior tumor extension beyond endopelvic fascia in advanced RC. Notable heterogeneity was found among the studies in the meta-analyses with regard to the type of equipment used for staging, as well as the criteria used to assess the depth of penetration and nodal status. The recent introduction of phased-array coils and the development of T2-weighted fast spin sequences have improved the resolution of MRI. The MERCURY trial showed that extension of tumor to within 1 mm of the circumferential margin on high-resolution MRI correctly predicted margin involvement at the time of surgery in 92% of the patients.9 In the retrospective study by Balyasnikova and colleagues, MRI was found to correctly identify partial submucosal invasion and suitability for local excision in 89% of the cases.10
Therefore, both EUS and MRI are useful, more so than CT, in assessment of the depth of tumor invasion, nodal staging, and predicting the circumferential resection margin. The use of EUS, however, does not preclude the use of MRI, or vice versa. Rather, the 2 modalities can complement each other in staging and proper patient selection for treatment.11
Despite data supporting the value of EUS in staging RC, its use is limited by a high degree of operator dependence and a substantial learning curve,12-17 which may explain the low EUS accuracy observed in some reports.7,13,15 Given the presence of recognized alternatives such as MRI, we decided to reevaluate EUS accuracy for the staging of RC outside high-volume specialized centers and prospective clinical trials.
Methods
A retrospective chart review was performed that included all consecutive patients undergoing rectal ultrasound from January 2011 to August 2015 at the US Department of Veterans Affairs Medical Center (VAMC) in Memphis, Tennessee. Sixty-five patients with short-stocked or sessile lesions < 15 cm from anal margin staged T2N0M0 or lower by endorectal ultrasound (ERUS) were included. The patients with neoplasms staged in excess of T2 or N0 were excluded from the study because treatment protocol dictates immediate neoadjuvant treatment, the administration of which would affect subsequent histopathology.
For the 37 patients included in the final analysis, ERUS results were compared with surgical pathology to ascertain accuracy. The resections were performed endoscopically or surgically with a goal of obtaining clear margins. The choice of procedure depended on size, shape, location, and depth of invasion. All patients underwent clinical and endoscopic surveillance with flexible sigmoidoscopy/EUS every 3 to 6 months for the first 2 years. We used 2 different gold standards for surveillance depending on the type of procedure performed to remove the lesion. A pathology report was the gold standard used for patients who underwent surgery. In patients who underwent endoscopic resection, we used the lack of recurrent disease, determined by normal endoscopic and endoscopic ultrasound examination, to signify complete endoscopic resection and therefore adequate staging as an early neoplasm.
Results
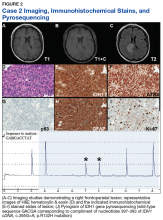
From January 2011 to August 2015, 65 rectal ultrasounds were performed. All EUS procedures were performed by 1 physician (C Ruben Tombazzi). All patients had previous endoscopic evaluation and tissue diagnoses. Twenty-eight patients were excluded: 18 had T3 or N1 disease, 2 had T2N0 but refused surgery, 2 had anal cancer, 3 patients with suspected cancer had benign nonneoplastic disease (2 radiation proctitis, 1 normal rectal wall), and 3 underwent EUS for benign tumors (1 ganglioneuroma and 2 lipomas).
Thirty-seven patients were included in the study, 3 of whom were staged as T2N0 and 34 as T1N0 or lower by EUS. All patients were men ranging in age from 43 to 73 years (mean, 59 years). All 37 patients underwent endoscopic or surgical resection of their early rectal neoplasm. The final pathologic evaluation of the specimens demonstrated 14 carcinoid tumors, 11 adenocarcinomas, 6 tubular adenomas with high-grade dysplasia, and 6 benign adenomas. The preoperative EUS staging was confirmed for all patients, with 100% sensitivity, specificity, and accuracy. None of the patients who underwent endoscopic or surgical transanal resection had recurrence, determined by normal endoscopic and endoscopic ultrasound appearance, during a mean of 32.6 months surveillance.
Discussion
EUS has long been a recognized method for T and N staging of RC.1,3-5,7,8 Our data confirm that, in experienced hands, EUS is highly accurate in the staging of early rectal cancers.
The impact of EUS on the management of RC was demonstrated in a Mayo Clinic prospective blinded study.1 In that cohort of 80 consecutive patients who had previously had a CT for staging, EUS altered patient management in about 30% of cases. The most common change precipatated by EUS was the indication for additional neoadjuvant treatment.
However, the results have not been as encouraging when ERUS is performed outside of strict research protocol. A multicenter, prospective, country-wide quality assurance study from > 300 German hospitals was designed to assess the diagnostic accuracy of EUS in RC.13 Of 29206 patients, 7096 underwent surgery, without neoadjuvant treatment, and were included in the final analysis. The correspondence of tumor invasion with histopathology was 64.7%, with understaging of 18% and overstaging of 17.3%.13 These numbers were better in hospitals with greater experience performing ERUS: 73% accuracy in the centers with a case load of > 30 cases per year compared with 63.2% accuracy for the centers with < 10 cases a year. Marusch and colleagues had previously demonstrated an EUS accuracy of 63.3% in a study of 1463 patients with RC in Germany.14 Another study based out of the UK had similar findings. Ashraf and colleagues performed a database analyses from 20 UK centers and identified 165 patients with RC who underwent ERUS and endoscopic microsurgery.15 Compared with histopathology, EUS had 57.1% sensitivity, 73% specificity, and 42.9% accuracy for T1 cancers; EUS accuracy was 50% for T2 and 58% for T3 tumors. The authors concluded that the general accuracy of EUS in determining stage was around 50%, the statistical equivalent of flipping a coin.
The low accuracy of EUS observed by German and British multicenter studies13-15 was attributed to the difference that may exist in clinical trials at specialized centers compared with wider use of EUS in a community setting. As seen by our data, the Memphis VAMC is not a high-volume center for the treatment of RC. However, all our EUS procedures were performed and interpreted by a single operator (C. Ruben Tombazzi) with 18 years of EUS experience. We cannot conclude that no patient was overstaged, as patients receiving a stage of T3N0 or T > N0 received neoadjuvant treatment and were not included. However, we can conclude that no patient was understaged. All patients deemed to be T1 to T2N0 included in our study received accurate staging. Our results are consistent with the high accuracy of EUS reported from other centers with experience in diagnosis and treatment of RC.1,3-5,17,18
Although EUS is accurate in differentiating T1 from T2 tumors, it cannot reliably differentiate T1 from T0 lesions. In one study, 57.6% of adenomas and 30.7% of carcinomas in situ were staged as T1 on EUS, while almost half of T1 cancers were interpreted as T0.17 This drawback is a well-known limitation of EUS; although, the misinterpretation does not affect treatment, as both T0 and T1 lesions can be treated successfully by local excision alone, which was the algorithm used for our patients. The choice of the specific procedure for local excision was left to the clinicians and included transanal endoscopic or surgical resections. At a mean follow-up of 32.6 months, none of the 37 patients who underwent endoscopic or surgical transanal resection had evidence of recurrent disease.
A limitation of EUS, or any other imaging modality, is differentiating tumor invasion from peritumoral inflammation. The inflammation can render images of tumor borders ill-defined and irregular, which hinders precise staging. However, the accurate identification of tumors with deep involvement of the submucosa (T1sm3) is of importance, because these tumors are more advanced than the superficial and intermediate T1 lesions (T1sm1 and T1sm2, respectively).
Patients with RC whose lesions are considered T1sm3 are at higher risk of harboring lymph node metastases.18 Nascimbeni and colleagues had shown that the invasion into the lower third of the submucosa (sm3) was an independent risk factor for lower cancer-free survival among patients with T1 RC.19
Unlike rectal adenocarcinomas, the prognosis for carcinoid tumors correlates not only with the depth of invasion but also with the size of the tumor. The other adverse prognostic features include poor differentiation, high mitosis index, and lymphovascular invasion.20
EUS had been shown to be highly accurate in determining the precise carcinoid tumor size, depth of invasion, and lymph node metastases.20,21 In a study of 66 resected rectal carcinoid tumors by Ishii and colleagues, 57 lesions had a diameter of ≤ 10 mm and 9 lesions had a diameter of > 10 mm.21 All of the 57 carcinoid tumors with a diameter of ≤ 10 mm were confined to the submucosa. In contrast, 5 of the 9 lesions > 10 mm invaded the muscularis propria, 6 had a lymphovascular invasion, 4 were lymph node metastases, and 1 was a liver metastasis.
In our series, 4 of the 14 carcinoid tumors were > 10 mm but none were > 20 mm. None of the carcinoids with a diameter ≤ 10 mm invaded the muscularis propria. Of the 4 carcinoids > 10 mm, 1 was T2N0 and 3 were T1N0. All carcinoid tumors in our series were low grade and with low proliferation indexes, and all were treated successfully by local excision.
Conclusion
We believe our study shows that EUS can be highly accurate in staging rectal lesions, specifically lesions that are T1-T2N0, be they adenocarcinoma or carcinoid. Although we could not assess overstaging for lesions that were staged > T2 or > N0, we w
Colorectal cancer is the second most common cause of cancer death in the US, with one-third of all colorectal cancers occurring within the rectum. Each year, an estimated 40000 Americans are diagnosed with rectal cancer (RC).1,2 The prognosis and treatment of RC depends on both T and N stage at the time of diagnosis.3-5 According to the most recent National Comprehensive Cancer Network guidelines from May 2019, patients with T1 to T2N0 tumors should undergo transanal or transabdominal surgery upfront, whereas patients with T3 to T4N0 or any TN1 to 2 should start with neoadjuvant therapy for better locoregional control, followed by surgery.6 Therefore, the appropriate management of RC requires adequate staging.
Endoscopic ultrasound (EUS), magnetic resonance imaging (MRI), and computed tomography (CT) are the imaging techniques currently used to stage RC. In a meta-analysis of 90 articles published between 1985 and 2002 that compared the 3 radiologic modalities, Bipat and colleagues found that MRI and EUS had a similar sensitivity of 94%, whereas the specificity of EUS (86%) was significantly higher than that of MRI (69%) for muscularis propria invasion.7 CT was performed only in a limited number of trials because CT was considered inadequate to assess early T stage. For perirectal tissue invasion, the sensitivity of EUS was statistically higher than that of CT and MRI imaging: 90% compared with 79% and 82%, respectively. The specificity estimates for EUS, CT, and MRI were comparable: 75%, 78%, and 76%, respectively. The respective sensitivity and specificity of the 3 imaging modalities to evaluate lymph nodes were also comparable: EUS, 67% and 78%; CT, 55% and 74%; and MRI, 66% and 76%.
The role of EUS in the diagnosis and treatment of RC has long been validated.1,2-5 A meta-analysis of 42 studies involving 5039 patients found EUS to be highly accurate for differentiating various T stages.8 However, EUS cannot assess iliac and mesenteric lymph nodes or posterior tumor extension beyond endopelvic fascia in advanced RC. Notable heterogeneity was found among the studies in the meta-analyses with regard to the type of equipment used for staging, as well as the criteria used to assess the depth of penetration and nodal status. The recent introduction of phased-array coils and the development of T2-weighted fast spin sequences have improved the resolution of MRI. The MERCURY trial showed that extension of tumor to within 1 mm of the circumferential margin on high-resolution MRI correctly predicted margin involvement at the time of surgery in 92% of the patients.9 In the retrospective study by Balyasnikova and colleagues, MRI was found to correctly identify partial submucosal invasion and suitability for local excision in 89% of the cases.10
Therefore, both EUS and MRI are useful, more so than CT, in assessment of the depth of tumor invasion, nodal staging, and predicting the circumferential resection margin. The use of EUS, however, does not preclude the use of MRI, or vice versa. Rather, the 2 modalities can complement each other in staging and proper patient selection for treatment.11
Despite data supporting the value of EUS in staging RC, its use is limited by a high degree of operator dependence and a substantial learning curve,12-17 which may explain the low EUS accuracy observed in some reports.7,13,15 Given the presence of recognized alternatives such as MRI, we decided to reevaluate EUS accuracy for the staging of RC outside high-volume specialized centers and prospective clinical trials.
Methods
A retrospective chart review was performed that included all consecutive patients undergoing rectal ultrasound from January 2011 to August 2015 at the US Department of Veterans Affairs Medical Center (VAMC) in Memphis, Tennessee. Sixty-five patients with short-stocked or sessile lesions < 15 cm from anal margin staged T2N0M0 or lower by endorectal ultrasound (ERUS) were included. The patients with neoplasms staged in excess of T2 or N0 were excluded from the study because treatment protocol dictates immediate neoadjuvant treatment, the administration of which would affect subsequent histopathology.
For the 37 patients included in the final analysis, ERUS results were compared with surgical pathology to ascertain accuracy. The resections were performed endoscopically or surgically with a goal of obtaining clear margins. The choice of procedure depended on size, shape, location, and depth of invasion. All patients underwent clinical and endoscopic surveillance with flexible sigmoidoscopy/EUS every 3 to 6 months for the first 2 years. We used 2 different gold standards for surveillance depending on the type of procedure performed to remove the lesion. A pathology report was the gold standard used for patients who underwent surgery. In patients who underwent endoscopic resection, we used the lack of recurrent disease, determined by normal endoscopic and endoscopic ultrasound examination, to signify complete endoscopic resection and therefore adequate staging as an early neoplasm.
Results
From January 2011 to August 2015, 65 rectal ultrasounds were performed. All EUS procedures were performed by 1 physician (C Ruben Tombazzi). All patients had previous endoscopic evaluation and tissue diagnoses. Twenty-eight patients were excluded: 18 had T3 or N1 disease, 2 had T2N0 but refused surgery, 2 had anal cancer, 3 patients with suspected cancer had benign nonneoplastic disease (2 radiation proctitis, 1 normal rectal wall), and 3 underwent EUS for benign tumors (1 ganglioneuroma and 2 lipomas).
Thirty-seven patients were included in the study, 3 of whom were staged as T2N0 and 34 as T1N0 or lower by EUS. All patients were men ranging in age from 43 to 73 years (mean, 59 years). All 37 patients underwent endoscopic or surgical resection of their early rectal neoplasm. The final pathologic evaluation of the specimens demonstrated 14 carcinoid tumors, 11 adenocarcinomas, 6 tubular adenomas with high-grade dysplasia, and 6 benign adenomas. The preoperative EUS staging was confirmed for all patients, with 100% sensitivity, specificity, and accuracy. None of the patients who underwent endoscopic or surgical transanal resection had recurrence, determined by normal endoscopic and endoscopic ultrasound appearance, during a mean of 32.6 months surveillance.
Discussion
EUS has long been a recognized method for T and N staging of RC.1,3-5,7,8 Our data confirm that, in experienced hands, EUS is highly accurate in the staging of early rectal cancers.
The impact of EUS on the management of RC was demonstrated in a Mayo Clinic prospective blinded study.1 In that cohort of 80 consecutive patients who had previously had a CT for staging, EUS altered patient management in about 30% of cases. The most common change precipatated by EUS was the indication for additional neoadjuvant treatment.
However, the results have not been as encouraging when ERUS is performed outside of strict research protocol. A multicenter, prospective, country-wide quality assurance study from > 300 German hospitals was designed to assess the diagnostic accuracy of EUS in RC.13 Of 29206 patients, 7096 underwent surgery, without neoadjuvant treatment, and were included in the final analysis. The correspondence of tumor invasion with histopathology was 64.7%, with understaging of 18% and overstaging of 17.3%.13 These numbers were better in hospitals with greater experience performing ERUS: 73% accuracy in the centers with a case load of > 30 cases per year compared with 63.2% accuracy for the centers with < 10 cases a year. Marusch and colleagues had previously demonstrated an EUS accuracy of 63.3% in a study of 1463 patients with RC in Germany.14 Another study based out of the UK had similar findings. Ashraf and colleagues performed a database analyses from 20 UK centers and identified 165 patients with RC who underwent ERUS and endoscopic microsurgery.15 Compared with histopathology, EUS had 57.1% sensitivity, 73% specificity, and 42.9% accuracy for T1 cancers; EUS accuracy was 50% for T2 and 58% for T3 tumors. The authors concluded that the general accuracy of EUS in determining stage was around 50%, the statistical equivalent of flipping a coin.
The low accuracy of EUS observed by German and British multicenter studies13-15 was attributed to the difference that may exist in clinical trials at specialized centers compared with wider use of EUS in a community setting. As seen by our data, the Memphis VAMC is not a high-volume center for the treatment of RC. However, all our EUS procedures were performed and interpreted by a single operator (C. Ruben Tombazzi) with 18 years of EUS experience. We cannot conclude that no patient was overstaged, as patients receiving a stage of T3N0 or T > N0 received neoadjuvant treatment and were not included. However, we can conclude that no patient was understaged. All patients deemed to be T1 to T2N0 included in our study received accurate staging. Our results are consistent with the high accuracy of EUS reported from other centers with experience in diagnosis and treatment of RC.1,3-5,17,18
Although EUS is accurate in differentiating T1 from T2 tumors, it cannot reliably differentiate T1 from T0 lesions. In one study, 57.6% of adenomas and 30.7% of carcinomas in situ were staged as T1 on EUS, while almost half of T1 cancers were interpreted as T0.17 This drawback is a well-known limitation of EUS; although, the misinterpretation does not affect treatment, as both T0 and T1 lesions can be treated successfully by local excision alone, which was the algorithm used for our patients. The choice of the specific procedure for local excision was left to the clinicians and included transanal endoscopic or surgical resections. At a mean follow-up of 32.6 months, none of the 37 patients who underwent endoscopic or surgical transanal resection had evidence of recurrent disease.
A limitation of EUS, or any other imaging modality, is differentiating tumor invasion from peritumoral inflammation. The inflammation can render images of tumor borders ill-defined and irregular, which hinders precise staging. However, the accurate identification of tumors with deep involvement of the submucosa (T1sm3) is of importance, because these tumors are more advanced than the superficial and intermediate T1 lesions (T1sm1 and T1sm2, respectively).
Patients with RC whose lesions are considered T1sm3 are at higher risk of harboring lymph node metastases.18 Nascimbeni and colleagues had shown that the invasion into the lower third of the submucosa (sm3) was an independent risk factor for lower cancer-free survival among patients with T1 RC.19
Unlike rectal adenocarcinomas, the prognosis for carcinoid tumors correlates not only with the depth of invasion but also with the size of the tumor. The other adverse prognostic features include poor differentiation, high mitosis index, and lymphovascular invasion.20
EUS had been shown to be highly accurate in determining the precise carcinoid tumor size, depth of invasion, and lymph node metastases.20,21 In a study of 66 resected rectal carcinoid tumors by Ishii and colleagues, 57 lesions had a diameter of ≤ 10 mm and 9 lesions had a diameter of > 10 mm.21 All of the 57 carcinoid tumors with a diameter of ≤ 10 mm were confined to the submucosa. In contrast, 5 of the 9 lesions > 10 mm invaded the muscularis propria, 6 had a lymphovascular invasion, 4 were lymph node metastases, and 1 was a liver metastasis.
In our series, 4 of the 14 carcinoid tumors were > 10 mm but none were > 20 mm. None of the carcinoids with a diameter ≤ 10 mm invaded the muscularis propria. Of the 4 carcinoids > 10 mm, 1 was T2N0 and 3 were T1N0. All carcinoid tumors in our series were low grade and with low proliferation indexes, and all were treated successfully by local excision.
Conclusion
We believe our study shows that EUS can be highly accurate in staging rectal lesions, specifically lesions that are T1-T2N0, be they adenocarcinoma or carcinoid. Although we could not assess overstaging for lesions that were staged > T2 or > N0, we w
1. Harewood GC, Wiersema MJ, Nelson H, et al. A prospective, blinded assessment of the impact of preoperative staging on the management of rectal cancer. Gastroenterology. 2002;123(1):24-32.
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
3. Ahuja NK, Sauer BG, Wang AY, et al. Performance of endoscopic ultrasound in staging rectal adenocarcinoma appropriate for primary surgical resection. Clin Gastroenterol Hepatol. 2015;13:339-44.
4. Doornebosch PG, Bronkhorst PJ, Hop WC, Bode WA, Sing AK, de Graaf EJ. The role of endorectal ultrasound in therapeutic decision-making for local vs. transabdominal resection of rectal tumors. Dis Colon Rectum. 2008;51(1):38-42.
5. Santoro GA, Gizzi G, Pellegrini L, Battistella G, Di Falco G. The value of high-resolution three-dimensional endorectal ultrasonography in the management of submucosal invasive rectal tumors. Dis Colon Rectum. 2009;52(11):1837-1843.
6. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: rectal cancer, version 2.2019. https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Published May 15, 2019. Accessed July 19, 2019.
7. Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging—a meta-analysis. Radiology. 2004;232(3):773-783.
8. Puli SR, Bechtold ML, Reddy JB, Choudhary A, Antillon MR, Brugge WR. How good is endoscopic ultrasound in differentiating various T stages of rectal cancer? Meta-analysis and systematic review. Ann Surg Oncol. 2009;16(2):254-265.
9. MERCURY Study Group. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ. 2006;333(7572):779.
10. Balyasnikova S, Read J, Wotherspoon A, et al. Diagnostic accuracy of high-resolution MRI as a method to predict potentially safe endoscopic and surgical planes in patient with early rectal cancer. BMJ Open Gastroenterol. 2017;4(1):e000151.
11. Frasson M, Garcia-Granero E, Roda D, et al. Preoperative chemoradiation may not always be needed for patients with T3 and T2N+ rectal cancer. Cancer. 2011;117(14):3118-3125.
12. Rafaelsen SR, Sørensen T, Jakobsen A, Bisgaard C, Lindebjerg J. Transrectal ultrasonography and magnetic resonance imaging in the staging of rectal cancer. Effect of experience. Scand J Gastroenterol. 2008;43(4):440-446.
13. Marusch F, Ptok H, Sahm M, et al. Endorectal ultrasound in rectal carcinoma – do the literature results really correspond to the realities of routine clinical care? Endoscopy. 2011;43(5):425-431.
14. Marusch F, Koch A, Schmidt U, et al. Routine use of transrectal ultrasound in rectal carcinoma: results of a prospective multicenter study. Endoscopy. 2002;34(5):385-390.
15. Ashraf S, Hompes R, Slater A, et al; Association of Coloproctology of Great Britain and Ireland Transanal Endoscopic Microsurgery (TEM) Collaboration. A critical appraisal of endorectal ultrasound and transanal endoscopic microsurgery and decision-making in early rectal cancer. Colorectal Dis. 2012;14(7):821-826.
16. Harewood GC. Assessment of clinical impact of endoscopic ultrasound on rectal cancer. Am J Gastroenterol. 2004;99(4):623-627.
17. Zorcolo L, Fantola G, Cabras F, Marongiu L, D’Alia G, Casula G. Preoperative staging of patients with rectal tumors suitable for transanal endoscopic microsurgery (TEM): comparison of endorectal ultrasound and histopathologic findings. Surg Endosc. 2009;23(6):1384-1389.
18. Akasu T, Kondo H, Moriya Y, et al. Endoscopic ultrasonography and treatment of early stage rectal cancer. World J Surg. 2000;24(9):1061-1068.
19. Nascimbeni R, Nivatvongs S, Larson DR, Burgart LJ. Long-term survival after local excision for T1 carcinoma of the rectum. Dis Colon Rectum. 2004;47(11):1773-1779.
20. Park CH, Cheon JH, Kim JO, et al. Criteria for decision making after endoscopic resection of well-differentiated rectal carcinoids with regard to potential lymphatic spread. Endoscopy. 2011;43(9):790-795.
21. Ishii N, Horiki N, Itoh T, et al. Endoscopic submucosal dissection and preoperative assessment with endoscopic ultrasonography for the treatment of rectal carcinoid tumors. Surg Endosc. 2010;24(6):1413-1419.
1. Harewood GC, Wiersema MJ, Nelson H, et al. A prospective, blinded assessment of the impact of preoperative staging on the management of rectal cancer. Gastroenterology. 2002;123(1):24-32.
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
3. Ahuja NK, Sauer BG, Wang AY, et al. Performance of endoscopic ultrasound in staging rectal adenocarcinoma appropriate for primary surgical resection. Clin Gastroenterol Hepatol. 2015;13:339-44.
4. Doornebosch PG, Bronkhorst PJ, Hop WC, Bode WA, Sing AK, de Graaf EJ. The role of endorectal ultrasound in therapeutic decision-making for local vs. transabdominal resection of rectal tumors. Dis Colon Rectum. 2008;51(1):38-42.
5. Santoro GA, Gizzi G, Pellegrini L, Battistella G, Di Falco G. The value of high-resolution three-dimensional endorectal ultrasonography in the management of submucosal invasive rectal tumors. Dis Colon Rectum. 2009;52(11):1837-1843.
6. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: rectal cancer, version 2.2019. https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Published May 15, 2019. Accessed July 19, 2019.
7. Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging—a meta-analysis. Radiology. 2004;232(3):773-783.
8. Puli SR, Bechtold ML, Reddy JB, Choudhary A, Antillon MR, Brugge WR. How good is endoscopic ultrasound in differentiating various T stages of rectal cancer? Meta-analysis and systematic review. Ann Surg Oncol. 2009;16(2):254-265.
9. MERCURY Study Group. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ. 2006;333(7572):779.
10. Balyasnikova S, Read J, Wotherspoon A, et al. Diagnostic accuracy of high-resolution MRI as a method to predict potentially safe endoscopic and surgical planes in patient with early rectal cancer. BMJ Open Gastroenterol. 2017;4(1):e000151.
11. Frasson M, Garcia-Granero E, Roda D, et al. Preoperative chemoradiation may not always be needed for patients with T3 and T2N+ rectal cancer. Cancer. 2011;117(14):3118-3125.
12. Rafaelsen SR, Sørensen T, Jakobsen A, Bisgaard C, Lindebjerg J. Transrectal ultrasonography and magnetic resonance imaging in the staging of rectal cancer. Effect of experience. Scand J Gastroenterol. 2008;43(4):440-446.
13. Marusch F, Ptok H, Sahm M, et al. Endorectal ultrasound in rectal carcinoma – do the literature results really correspond to the realities of routine clinical care? Endoscopy. 2011;43(5):425-431.
14. Marusch F, Koch A, Schmidt U, et al. Routine use of transrectal ultrasound in rectal carcinoma: results of a prospective multicenter study. Endoscopy. 2002;34(5):385-390.
15. Ashraf S, Hompes R, Slater A, et al; Association of Coloproctology of Great Britain and Ireland Transanal Endoscopic Microsurgery (TEM) Collaboration. A critical appraisal of endorectal ultrasound and transanal endoscopic microsurgery and decision-making in early rectal cancer. Colorectal Dis. 2012;14(7):821-826.
16. Harewood GC. Assessment of clinical impact of endoscopic ultrasound on rectal cancer. Am J Gastroenterol. 2004;99(4):623-627.
17. Zorcolo L, Fantola G, Cabras F, Marongiu L, D’Alia G, Casula G. Preoperative staging of patients with rectal tumors suitable for transanal endoscopic microsurgery (TEM): comparison of endorectal ultrasound and histopathologic findings. Surg Endosc. 2009;23(6):1384-1389.
18. Akasu T, Kondo H, Moriya Y, et al. Endoscopic ultrasonography and treatment of early stage rectal cancer. World J Surg. 2000;24(9):1061-1068.
19. Nascimbeni R, Nivatvongs S, Larson DR, Burgart LJ. Long-term survival after local excision for T1 carcinoma of the rectum. Dis Colon Rectum. 2004;47(11):1773-1779.
20. Park CH, Cheon JH, Kim JO, et al. Criteria for decision making after endoscopic resection of well-differentiated rectal carcinoids with regard to potential lymphatic spread. Endoscopy. 2011;43(9):790-795.
21. Ishii N, Horiki N, Itoh T, et al. Endoscopic submucosal dissection and preoperative assessment with endoscopic ultrasonography for the treatment of rectal carcinoid tumors. Surg Endosc. 2010;24(6):1413-1419.
Quality of Care for Veterans With In-Hospital Stroke
Stroke is a leading cause of death and long-term disability in the US.1 Quality improvement efforts for acute stroke care delivery have successfully led to increased rates of thrombolytic utilization.2 Increasing attention is now being paid to additional quality metrics for stroke care, including hospital management and initiation of appropriate secondary stroke prevention measures at discharge. Many organizations, including the Veterans Health Administration (VHA), use these measures to monitor health care quality and certify centers that are committed to excellence in stroke care.3-6 It is anticipated that collection, evaluation, and feedback from these data may lead to improvements in outcomes after stroke.7
Patients who experience onset of stroke symptoms while already admitted to a hospital may be uniquely suited for quality improvement strategies. In-hospital strokes (IHS) are not uncommon and have been associated with higher stroke severity and increased mortality compared with patients with stroke symptoms prior to arriving at the emergency department (ED).8-10 A potential reason for the higher observed mortality is that patients with IHS may have poorer access to acute stroke resources, such as stroke teams and neuroimaging, as well as increased rates of medical comorbidities.9,11,12 Furthermore, stroke management protocols are typically created based on ED resources, which may not be equivalent to resources available on inpatient settings.
Although many studies have examined clinical characteristics of patients with IHS, few studies have looked at the quality of stroke care for IHS. Information on stroke quality data is even more limited in VHA hospitals due to the small number of admitted patients with stroke.13 VHA released a directive on Acute Stroke Treatment (Directive 2011-03) in 2011 with a recent update in 2018, which aimed to implement quality improvement strategies for stroke care in VHA hospitals.14 Although focusing primarily on acute stroke care in the ED, this directive has led to increased awareness of areas for improvement, particularly among larger VHA hospitals. Prior to this directive, although national stroke guidelines were well-defined, more variability likely existed in stroke protocols and the manner in which stroke care was delivered across care settings. As efforts to measure and improve stroke care evolve, it is important to ensure that strategies used in ED settings also are implemented for patients already admitted to the hospital. This study seeks to define the quality of care in VHA hospitals between patients having an in-hospital ischemic stroke compared with those presenting to the ED.
Methods
As a secondary analysis, we examined stroke care quality data from an 11-site VHA stroke quality improvement study.15 Sites participating in this study were high stroke volume VHA hospitals from various geographic regions of the US. This study collected data on ICD-9 discharge diagnosis-defined ischemic stroke admissions between January 2009 and June 2012. Patient charts were reviewed by a group of central, trained abstractors who collected information on patient demographics, clinical history, and stroke characteristics. Stroke severity was defined using the National Institutes of Health Stroke Scale (NIHSS), assessed by standardized retrospective review of admission physical examination documentation.16 A multidisciplinary team defined 11 stroke quality indicators (QIs; the 8 Joint Commission indictors and 3 additional indicators: smoking cessation and dysphagia screening, and NIHSS assessment), and the chart abstractors’ data were used to evaluate eligibility and passing rates for each QI.
For our analysis, patients were stratified into 2 categories: patients admitted to the hospital for another diagnosis who developed an IHS, and patients presenting with stroke to the ED. We excluded patients transferred from other facilities. We then compared the demographic and clinical features of the 2 groups as well as eligibility and passing rates for each of the 11 QIs. Patients were recorded as eligible if they did not have any clinical contraindication to receiving the assessment or intervention measured by the quality metric. Passing rates were defined by the presence of clear documentation in the patient record that the quality metric was met or fulfilled. Comparisons were made using nonparametric Mann-Whitney U tests and chi-square tests. All tests were performed at α .05 level.
Results
A total of 1823 patients were included in this analysis: 35 IHS and 1788 ED strokes. The 2 groups did not differ with respect to age, race, or sex (Table 1). Patients with IHS had higher stroke severity (mean NIHSS 11.3 vs 5.1, P <.01) and longer length of stay than did ED patients with stroke (mean 12.8 vs 7.3 days, P < .01). Patients with IHS also were less likely to be discharged home when compared with ED patients with stroke (34.3% vs 63.8%, P < .01).
Table 2 summarizes our findings on eligibility and passing rates for the 11 QIs. For acute care metrics, we found that stroke severity documentation rates did not differ but were low for each patient group (51% vs 48%, P = .07). Patients with IHS were more likely to be eligible for IV tissue plasminogen activator (tPA; P < .01) although utilization rates did not differ. Only 2% of ED patients met eligibility criteria to receive tPA (36 of 1788), and among these patients only 16 actually received the drug. By comparison, 5 of 6 of eligible patients with IHS received tPA. Rates of dysphagia screening also were low for both groups, and patients with IHS were less likely to receive this screen prior to initiation of oral intake than were ED patients with stroke (27% vs 50%, P = .01).
Beyond the acute period, we found that patients with IHS were less likely than were ED patients with stroke to be eligible to receive antithrombotic therapy by 2 days after their initial stroke evaluation (74% vs 96%, P < .01), although treatment rates were similar between the 2 groups (P = .99). In patients with documented atrial fibrillation, initiation of anticoagulation therapy also did not differ (P = .99). The 2 groups were similar with respect to initiation of venous thromboembolism (VTE) prophylaxis (P = .596) and evaluation for rehabilitation needs (P = .42). Although rates of smoking cessation counseling and stroke education prior to discharge did not differ, overall rates of stroke education were very low for both groups (25% vs 36%, P = .55).
Similar to initiation of antithrombotic therapy in the hospital, we found lower rates of eligibility to receive antithrombotic therapy on discharge in the IHS group when compared with the ED group (77% vs 93%, P = .04). However, actual treatment initiation rates did not differ (P = .12). Use of lipid-lowering agents was similar for the 2 groups (P = .12).
Discussion
Our study found that veterans who develop an IHS received similar quality of care as did those presenting to the ED with stroke symptoms for many QIs, although there were some notable differences. We were pleased to find that overall rates of secondary stroke prevention initiation (antithrombotic and statin therapy), VTE prophylaxis, rehabilitation evaluations, and smoking cessation counseling were high for both groups, in keeping with evidence-based guidelines.17 This likely reflected the fact that these metrics typically involve care outside of the acute period and are less likely to be influenced by the location of initial stroke evaluation. Furthermore, efforts to improve smoking cessation and VTE prophylaxis are not exclusive to stroke care and have been the target of several nonstroke quality projects in the VHA. Many aspects of acute stroke care did differ, and present opportunities for quality improvement in the future.
In our sample, patients with IHS had higher IV thrombolytic eligibility, which has not typically been reported in other samples.10,11,18 In these studies, hospitalized patients have been reported to more often have contraindications to tPA, such as recent surgery or lack of stroke symptom recognition due to delirium or medication effects. Interestingly, patients presenting to VHA EDs had extremely low rates of tPA eligibility (2%), which is lower than many reported estimates of tPA eligibility outside of the VHA.19,20 This may be due to multiple influences, such as geographic barriers, patient perceptions about stroke symptoms, access to emergency medical services (EMS), EMS routing patterns, and social/cultural factors. Although not statistically significant due to small sample size, tPA use also was twice as high in the IHS group.
Given that a significant proportion of patients with IHS in the VHA system may be eligible for acute thrombolysis, our findings highlight the need for acute stroke protocols to ensure that patients with IHS receive the same rapid stroke assessment and access to thrombolytics as do patients evaluated in the ED. Further investigation is needed to determine whether there are unique features of patients with IHS in VHA hospitals, which may make them more eligible for IV thrombolysis.
Dysphagia is associated with increased risks for aspiration pneumonia in stroke patients.21 We found that patients with IHS were less likely to receive dysphagia screening compared with that of stroke patients admitted through the ED. This finding is consistent with the fact that care for patients with IHS is less frequently guided by specific stroke care protocols and algorithms that are more often used in EDs.8,11 Although attention to swallowing function may lead to improved outcomes in stroke, this can be easily overlooked in patients with IHS.22 However, low dysphagia screening also was found in patients admitted through the ED, suggesting that low screening rates cannot be solely explained by differences in where the initial stroke evaluation is occurring. These findings suggest a need for novel approaches to dysphagia screening in VHA stroke patients that can be universally implemented throughout the hospital.
Finally, we also found very low rates of stroke education prior to discharge for both groups. Given the risk of stroke recurrence and the overall poor level of public knowledge about stroke, providing patients with stroke with formal oral and written information on stroke is a critical component of secondary prevention.23,24 Educational tools, including those that are veteran specific, are now available for use in VHA hospitals and should be incorporated into quality improvement strategies for stroke care in VHA hospitals.
In 2012, the VHA Acute Stroke Treatment Directive was published in an effort to improve stroke care systemwide. Several of the metrics examined in this study are addressed in this directive. The data presented in this study is one of the only samples of stroke quality metrics within the VHA that largely predates the directive and can serve as a baseline comparator for future work examining stroke care after release of the directive. At present, although continuous internal reviews of quality data are ongoing, longitudinal description of stroke care quality since publication of the directive will help to inform future efforts to improve stroke care for veterans.
Limitations
Despite the strength of being a multicenter sampling of stroke care in high volume VHA hospitals, our study had several limitations. The IHS sample size was small, which limited our ability to evaluate differences between the groups, to evaluate generalizability, and account for estimation error.13 It is possible that differences existed between the groups that could not be observed in this sample due to small size (type II error) or that patient-specific characteristics not captured by these data could influence these metrics. Assessments of eligibility and passing were based on retrospective chart review and post hoc coding. Our sample assessed only patients who presented to larger VHA hospitals with higher stroke volumes, thus these findings may not be generalizable to smaller VHA hospitals with less systematized stroke care. This sample did not describe the specialty care services that were received by each patient, which may have influenced their stroke care. Finally, this study is an analysis of use of QIs in stroke care and did not examine how these indicators affect outcomes.
Conclusion
Despite reassuring findings for several inpatient ischemic stroke quality metrics, we found several differences in stroke care between patients with IHS compared with those presenting to the ED, emphasizing the need for standardized approaches to stroke care regardless of care setting. Although patients with IHS may be more likely to be eligible for tPA, these patients received dysphagia screening and less often than did ED patients with stroke. Ongoing quality initiatives should continue to place emphasis on improving all quality metrics (particularly dysphagia screening, stroke severity documentation, and stroke education) for patients with stroke at VHA hospitals across all care settings. Future work will be needed to examine how specific patient characteristics and revisions to stroke protocols may affect stroke quality metrics and outcomes between patients with IHS and those presenting to the ED.
Acknowledgments
The authors would like to thank Danielle Sager for her contributions to this project.
1. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28-e292.
2. Schwamm LH, Ali SF, Reeves MJ, et al. Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at Get With the Guidelines—Stroke hospitals. Circ Cardiovasc Qual Outcomes. 2013;6(5):543-549.
3. Reeves MJ, Parker C, Fonarow GC, Smith EE, Schwamm LH. Development of stroke performance measures: definitions, methods, and current measures. Stroke. 2010;41(7):1573-1578.
4. The Joint Commission. Certificate of distinction for primary stroke centers. https://www.jointcommission.org/certificate_of_distinction_for_primary_stroke_centers_/.Published April 30, 2012. Accessed July 9, 2019.
5. US Department of Veterans Affairs. Center highlight: acute ischemic stroke care for veterans. https://www.queri.research.va.gov/center_highlights/stroke.cfm. Updated February 20, 2014. Accessed July 16, 2019.
6. Chumbler NR, Jia H, Phipps MS, et al. Does inpatient quality of care differ by age among US veterans with ischemic stroke? J Stroke Cerebrovasc Dis. 2012;21(8):844-851.
7. Katzan IL, Spertus J, Bettger JP, et al; American Heart Association Stroke Council; Council on Quality of Care and Outcomes Research; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology. Risk adjustment of ischemic stroke outcomes for comparing hospital performance: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(3):918-944.
8. Cumbler E, Wald H, Bhatt DL, et al. Quality of care and outcomes for in-hospital ischemic stroke: findings from the National Get With the Guidelines—Stroke. Stroke. 2014;45(1):231-238.
9. Blacker DJ. In-hospital stroke. Lancet Neurol. 2003;2(12):741-746.
10. Farooq MU, Reeves MJ, Gargano J, Wehner S, Hickenbottom S, Majid A; Paul Coverdell National Acute Stroke Registry Michigan Prototype Investigators. In-hospital stroke in a statewide stroke registry. Cerebrovascular Dis. 2008;25(1-2):12-20.
11. Bhalla A, Smeeton N, Rudd AG, Heuschmann P, Wolfe CD. A comparison of characteristics and resource use between in-hospital and admitted patients with stroke. J Stroke Cerebrovasc Dis. 2010;19:(5)357-363.
12. Garcia-Santibanez R, Liang J, Walker A, Matos-Diaz I, Kahkeshani K, Boniece I. Comparison of stroke codes in the emergency room and inpatient setting. J Stroke Cerebrovasc Dis. 2015;24(8):1948-1950.
13. Arling G, Reeves M, Ross J, et al. Estimating and reporting on the quality of inpatient stroke care by Veterans Health Administration medical centers. Circ Cardiovasc Qual Outcomes. 2012;5(1):44-51.
14. US Department of Veterans Affairs. Treatment of Acute Ischemic Stroke (AIS). VHA Directive 2011-038. https://www.hsrd.research.va.gov/news/feature/stroke.cfm. Updated January 20, 2014. Accessed July 17, 2019.
15. Williams LS, Daggett V, Slaven J, et al. Abstract 18: Does quality improvement training add to audit and feedback for inpatient stroke care processes? [International Stroke Conference abstract 18] Stroke. 2014;45(suppl 1):A18.
16. Williams LS, Yilmaz EY, Lopez-Yunez AM. Retrospective assessment of initial stroke severity with the NIH Stroke Scale. Stroke. 2000;31(4):858-862.
17. Jauch EC, Saver JL, Adams HP Jr, et al; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947.
18. Park HJ, Cho HJ, Kim YD, et al. Comparison of the characteristics for in-hospital and out-of-hospital ischaemic strokes. Eur J Neurol. 2009;16(5):582-588.
19. Messé SR, Fonarow GC, Smith EE, et al. Use of tissue-type plasminogen activator before and after publication of the European Cooperative Acute Stroke Study III in Get With the Guidelines-Stroke. Circ Cardiovasc Qual Outcomes. 2012;5(3):321-326.
20. Allen NB, Kaltenbach L, Goldstein LB, et al. Regional variation in recommended treatments for ischemic stroke and TIA: Get With the Guidelines—Stroke 2003-2010. Stroke. 2012;43(7):1858-1864.
21. Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36(12):2756-2763.
22. Bravata DM, Wells CK, Lo AC, et al. Processes of care associated with acute stroke outcomes. Arch Intern Med. 2010;170(9):804-810.
23. Mosley I, Nicol M, Donnan G, Patrick I, Dewey H. Stroke symptoms and the decision to call for an ambulance. Stroke; a journal of cerebral circulation. 2007;38(2):361-366.
24. Jurkowski JM, Maniccia DM, Dennison BA, Samuels SJ, Spicer DA. Awareness of necessity to call 9-1-1 for stroke symptoms, upstate New York. Prev Chronic Dis. 2008;5(2):A41.
Stroke is a leading cause of death and long-term disability in the US.1 Quality improvement efforts for acute stroke care delivery have successfully led to increased rates of thrombolytic utilization.2 Increasing attention is now being paid to additional quality metrics for stroke care, including hospital management and initiation of appropriate secondary stroke prevention measures at discharge. Many organizations, including the Veterans Health Administration (VHA), use these measures to monitor health care quality and certify centers that are committed to excellence in stroke care.3-6 It is anticipated that collection, evaluation, and feedback from these data may lead to improvements in outcomes after stroke.7
Patients who experience onset of stroke symptoms while already admitted to a hospital may be uniquely suited for quality improvement strategies. In-hospital strokes (IHS) are not uncommon and have been associated with higher stroke severity and increased mortality compared with patients with stroke symptoms prior to arriving at the emergency department (ED).8-10 A potential reason for the higher observed mortality is that patients with IHS may have poorer access to acute stroke resources, such as stroke teams and neuroimaging, as well as increased rates of medical comorbidities.9,11,12 Furthermore, stroke management protocols are typically created based on ED resources, which may not be equivalent to resources available on inpatient settings.
Although many studies have examined clinical characteristics of patients with IHS, few studies have looked at the quality of stroke care for IHS. Information on stroke quality data is even more limited in VHA hospitals due to the small number of admitted patients with stroke.13 VHA released a directive on Acute Stroke Treatment (Directive 2011-03) in 2011 with a recent update in 2018, which aimed to implement quality improvement strategies for stroke care in VHA hospitals.14 Although focusing primarily on acute stroke care in the ED, this directive has led to increased awareness of areas for improvement, particularly among larger VHA hospitals. Prior to this directive, although national stroke guidelines were well-defined, more variability likely existed in stroke protocols and the manner in which stroke care was delivered across care settings. As efforts to measure and improve stroke care evolve, it is important to ensure that strategies used in ED settings also are implemented for patients already admitted to the hospital. This study seeks to define the quality of care in VHA hospitals between patients having an in-hospital ischemic stroke compared with those presenting to the ED.
Methods
As a secondary analysis, we examined stroke care quality data from an 11-site VHA stroke quality improvement study.15 Sites participating in this study were high stroke volume VHA hospitals from various geographic regions of the US. This study collected data on ICD-9 discharge diagnosis-defined ischemic stroke admissions between January 2009 and June 2012. Patient charts were reviewed by a group of central, trained abstractors who collected information on patient demographics, clinical history, and stroke characteristics. Stroke severity was defined using the National Institutes of Health Stroke Scale (NIHSS), assessed by standardized retrospective review of admission physical examination documentation.16 A multidisciplinary team defined 11 stroke quality indicators (QIs; the 8 Joint Commission indictors and 3 additional indicators: smoking cessation and dysphagia screening, and NIHSS assessment), and the chart abstractors’ data were used to evaluate eligibility and passing rates for each QI.
For our analysis, patients were stratified into 2 categories: patients admitted to the hospital for another diagnosis who developed an IHS, and patients presenting with stroke to the ED. We excluded patients transferred from other facilities. We then compared the demographic and clinical features of the 2 groups as well as eligibility and passing rates for each of the 11 QIs. Patients were recorded as eligible if they did not have any clinical contraindication to receiving the assessment or intervention measured by the quality metric. Passing rates were defined by the presence of clear documentation in the patient record that the quality metric was met or fulfilled. Comparisons were made using nonparametric Mann-Whitney U tests and chi-square tests. All tests were performed at α .05 level.
Results
A total of 1823 patients were included in this analysis: 35 IHS and 1788 ED strokes. The 2 groups did not differ with respect to age, race, or sex (Table 1). Patients with IHS had higher stroke severity (mean NIHSS 11.3 vs 5.1, P <.01) and longer length of stay than did ED patients with stroke (mean 12.8 vs 7.3 days, P < .01). Patients with IHS also were less likely to be discharged home when compared with ED patients with stroke (34.3% vs 63.8%, P < .01).
Table 2 summarizes our findings on eligibility and passing rates for the 11 QIs. For acute care metrics, we found that stroke severity documentation rates did not differ but were low for each patient group (51% vs 48%, P = .07). Patients with IHS were more likely to be eligible for IV tissue plasminogen activator (tPA; P < .01) although utilization rates did not differ. Only 2% of ED patients met eligibility criteria to receive tPA (36 of 1788), and among these patients only 16 actually received the drug. By comparison, 5 of 6 of eligible patients with IHS received tPA. Rates of dysphagia screening also were low for both groups, and patients with IHS were less likely to receive this screen prior to initiation of oral intake than were ED patients with stroke (27% vs 50%, P = .01).
Beyond the acute period, we found that patients with IHS were less likely than were ED patients with stroke to be eligible to receive antithrombotic therapy by 2 days after their initial stroke evaluation (74% vs 96%, P < .01), although treatment rates were similar between the 2 groups (P = .99). In patients with documented atrial fibrillation, initiation of anticoagulation therapy also did not differ (P = .99). The 2 groups were similar with respect to initiation of venous thromboembolism (VTE) prophylaxis (P = .596) and evaluation for rehabilitation needs (P = .42). Although rates of smoking cessation counseling and stroke education prior to discharge did not differ, overall rates of stroke education were very low for both groups (25% vs 36%, P = .55).
Similar to initiation of antithrombotic therapy in the hospital, we found lower rates of eligibility to receive antithrombotic therapy on discharge in the IHS group when compared with the ED group (77% vs 93%, P = .04). However, actual treatment initiation rates did not differ (P = .12). Use of lipid-lowering agents was similar for the 2 groups (P = .12).
Discussion
Our study found that veterans who develop an IHS received similar quality of care as did those presenting to the ED with stroke symptoms for many QIs, although there were some notable differences. We were pleased to find that overall rates of secondary stroke prevention initiation (antithrombotic and statin therapy), VTE prophylaxis, rehabilitation evaluations, and smoking cessation counseling were high for both groups, in keeping with evidence-based guidelines.17 This likely reflected the fact that these metrics typically involve care outside of the acute period and are less likely to be influenced by the location of initial stroke evaluation. Furthermore, efforts to improve smoking cessation and VTE prophylaxis are not exclusive to stroke care and have been the target of several nonstroke quality projects in the VHA. Many aspects of acute stroke care did differ, and present opportunities for quality improvement in the future.
In our sample, patients with IHS had higher IV thrombolytic eligibility, which has not typically been reported in other samples.10,11,18 In these studies, hospitalized patients have been reported to more often have contraindications to tPA, such as recent surgery or lack of stroke symptom recognition due to delirium or medication effects. Interestingly, patients presenting to VHA EDs had extremely low rates of tPA eligibility (2%), which is lower than many reported estimates of tPA eligibility outside of the VHA.19,20 This may be due to multiple influences, such as geographic barriers, patient perceptions about stroke symptoms, access to emergency medical services (EMS), EMS routing patterns, and social/cultural factors. Although not statistically significant due to small sample size, tPA use also was twice as high in the IHS group.
Given that a significant proportion of patients with IHS in the VHA system may be eligible for acute thrombolysis, our findings highlight the need for acute stroke protocols to ensure that patients with IHS receive the same rapid stroke assessment and access to thrombolytics as do patients evaluated in the ED. Further investigation is needed to determine whether there are unique features of patients with IHS in VHA hospitals, which may make them more eligible for IV thrombolysis.
Dysphagia is associated with increased risks for aspiration pneumonia in stroke patients.21 We found that patients with IHS were less likely to receive dysphagia screening compared with that of stroke patients admitted through the ED. This finding is consistent with the fact that care for patients with IHS is less frequently guided by specific stroke care protocols and algorithms that are more often used in EDs.8,11 Although attention to swallowing function may lead to improved outcomes in stroke, this can be easily overlooked in patients with IHS.22 However, low dysphagia screening also was found in patients admitted through the ED, suggesting that low screening rates cannot be solely explained by differences in where the initial stroke evaluation is occurring. These findings suggest a need for novel approaches to dysphagia screening in VHA stroke patients that can be universally implemented throughout the hospital.
Finally, we also found very low rates of stroke education prior to discharge for both groups. Given the risk of stroke recurrence and the overall poor level of public knowledge about stroke, providing patients with stroke with formal oral and written information on stroke is a critical component of secondary prevention.23,24 Educational tools, including those that are veteran specific, are now available for use in VHA hospitals and should be incorporated into quality improvement strategies for stroke care in VHA hospitals.
In 2012, the VHA Acute Stroke Treatment Directive was published in an effort to improve stroke care systemwide. Several of the metrics examined in this study are addressed in this directive. The data presented in this study is one of the only samples of stroke quality metrics within the VHA that largely predates the directive and can serve as a baseline comparator for future work examining stroke care after release of the directive. At present, although continuous internal reviews of quality data are ongoing, longitudinal description of stroke care quality since publication of the directive will help to inform future efforts to improve stroke care for veterans.
Limitations
Despite the strength of being a multicenter sampling of stroke care in high volume VHA hospitals, our study had several limitations. The IHS sample size was small, which limited our ability to evaluate differences between the groups, to evaluate generalizability, and account for estimation error.13 It is possible that differences existed between the groups that could not be observed in this sample due to small size (type II error) or that patient-specific characteristics not captured by these data could influence these metrics. Assessments of eligibility and passing were based on retrospective chart review and post hoc coding. Our sample assessed only patients who presented to larger VHA hospitals with higher stroke volumes, thus these findings may not be generalizable to smaller VHA hospitals with less systematized stroke care. This sample did not describe the specialty care services that were received by each patient, which may have influenced their stroke care. Finally, this study is an analysis of use of QIs in stroke care and did not examine how these indicators affect outcomes.
Conclusion
Despite reassuring findings for several inpatient ischemic stroke quality metrics, we found several differences in stroke care between patients with IHS compared with those presenting to the ED, emphasizing the need for standardized approaches to stroke care regardless of care setting. Although patients with IHS may be more likely to be eligible for tPA, these patients received dysphagia screening and less often than did ED patients with stroke. Ongoing quality initiatives should continue to place emphasis on improving all quality metrics (particularly dysphagia screening, stroke severity documentation, and stroke education) for patients with stroke at VHA hospitals across all care settings. Future work will be needed to examine how specific patient characteristics and revisions to stroke protocols may affect stroke quality metrics and outcomes between patients with IHS and those presenting to the ED.
Acknowledgments
The authors would like to thank Danielle Sager for her contributions to this project.
Stroke is a leading cause of death and long-term disability in the US.1 Quality improvement efforts for acute stroke care delivery have successfully led to increased rates of thrombolytic utilization.2 Increasing attention is now being paid to additional quality metrics for stroke care, including hospital management and initiation of appropriate secondary stroke prevention measures at discharge. Many organizations, including the Veterans Health Administration (VHA), use these measures to monitor health care quality and certify centers that are committed to excellence in stroke care.3-6 It is anticipated that collection, evaluation, and feedback from these data may lead to improvements in outcomes after stroke.7
Patients who experience onset of stroke symptoms while already admitted to a hospital may be uniquely suited for quality improvement strategies. In-hospital strokes (IHS) are not uncommon and have been associated with higher stroke severity and increased mortality compared with patients with stroke symptoms prior to arriving at the emergency department (ED).8-10 A potential reason for the higher observed mortality is that patients with IHS may have poorer access to acute stroke resources, such as stroke teams and neuroimaging, as well as increased rates of medical comorbidities.9,11,12 Furthermore, stroke management protocols are typically created based on ED resources, which may not be equivalent to resources available on inpatient settings.
Although many studies have examined clinical characteristics of patients with IHS, few studies have looked at the quality of stroke care for IHS. Information on stroke quality data is even more limited in VHA hospitals due to the small number of admitted patients with stroke.13 VHA released a directive on Acute Stroke Treatment (Directive 2011-03) in 2011 with a recent update in 2018, which aimed to implement quality improvement strategies for stroke care in VHA hospitals.14 Although focusing primarily on acute stroke care in the ED, this directive has led to increased awareness of areas for improvement, particularly among larger VHA hospitals. Prior to this directive, although national stroke guidelines were well-defined, more variability likely existed in stroke protocols and the manner in which stroke care was delivered across care settings. As efforts to measure and improve stroke care evolve, it is important to ensure that strategies used in ED settings also are implemented for patients already admitted to the hospital. This study seeks to define the quality of care in VHA hospitals between patients having an in-hospital ischemic stroke compared with those presenting to the ED.
Methods
As a secondary analysis, we examined stroke care quality data from an 11-site VHA stroke quality improvement study.15 Sites participating in this study were high stroke volume VHA hospitals from various geographic regions of the US. This study collected data on ICD-9 discharge diagnosis-defined ischemic stroke admissions between January 2009 and June 2012. Patient charts were reviewed by a group of central, trained abstractors who collected information on patient demographics, clinical history, and stroke characteristics. Stroke severity was defined using the National Institutes of Health Stroke Scale (NIHSS), assessed by standardized retrospective review of admission physical examination documentation.16 A multidisciplinary team defined 11 stroke quality indicators (QIs; the 8 Joint Commission indictors and 3 additional indicators: smoking cessation and dysphagia screening, and NIHSS assessment), and the chart abstractors’ data were used to evaluate eligibility and passing rates for each QI.
For our analysis, patients were stratified into 2 categories: patients admitted to the hospital for another diagnosis who developed an IHS, and patients presenting with stroke to the ED. We excluded patients transferred from other facilities. We then compared the demographic and clinical features of the 2 groups as well as eligibility and passing rates for each of the 11 QIs. Patients were recorded as eligible if they did not have any clinical contraindication to receiving the assessment or intervention measured by the quality metric. Passing rates were defined by the presence of clear documentation in the patient record that the quality metric was met or fulfilled. Comparisons were made using nonparametric Mann-Whitney U tests and chi-square tests. All tests were performed at α .05 level.
Results
A total of 1823 patients were included in this analysis: 35 IHS and 1788 ED strokes. The 2 groups did not differ with respect to age, race, or sex (Table 1). Patients with IHS had higher stroke severity (mean NIHSS 11.3 vs 5.1, P <.01) and longer length of stay than did ED patients with stroke (mean 12.8 vs 7.3 days, P < .01). Patients with IHS also were less likely to be discharged home when compared with ED patients with stroke (34.3% vs 63.8%, P < .01).
Table 2 summarizes our findings on eligibility and passing rates for the 11 QIs. For acute care metrics, we found that stroke severity documentation rates did not differ but were low for each patient group (51% vs 48%, P = .07). Patients with IHS were more likely to be eligible for IV tissue plasminogen activator (tPA; P < .01) although utilization rates did not differ. Only 2% of ED patients met eligibility criteria to receive tPA (36 of 1788), and among these patients only 16 actually received the drug. By comparison, 5 of 6 of eligible patients with IHS received tPA. Rates of dysphagia screening also were low for both groups, and patients with IHS were less likely to receive this screen prior to initiation of oral intake than were ED patients with stroke (27% vs 50%, P = .01).
Beyond the acute period, we found that patients with IHS were less likely than were ED patients with stroke to be eligible to receive antithrombotic therapy by 2 days after their initial stroke evaluation (74% vs 96%, P < .01), although treatment rates were similar between the 2 groups (P = .99). In patients with documented atrial fibrillation, initiation of anticoagulation therapy also did not differ (P = .99). The 2 groups were similar with respect to initiation of venous thromboembolism (VTE) prophylaxis (P = .596) and evaluation for rehabilitation needs (P = .42). Although rates of smoking cessation counseling and stroke education prior to discharge did not differ, overall rates of stroke education were very low for both groups (25% vs 36%, P = .55).
Similar to initiation of antithrombotic therapy in the hospital, we found lower rates of eligibility to receive antithrombotic therapy on discharge in the IHS group when compared with the ED group (77% vs 93%, P = .04). However, actual treatment initiation rates did not differ (P = .12). Use of lipid-lowering agents was similar for the 2 groups (P = .12).
Discussion
Our study found that veterans who develop an IHS received similar quality of care as did those presenting to the ED with stroke symptoms for many QIs, although there were some notable differences. We were pleased to find that overall rates of secondary stroke prevention initiation (antithrombotic and statin therapy), VTE prophylaxis, rehabilitation evaluations, and smoking cessation counseling were high for both groups, in keeping with evidence-based guidelines.17 This likely reflected the fact that these metrics typically involve care outside of the acute period and are less likely to be influenced by the location of initial stroke evaluation. Furthermore, efforts to improve smoking cessation and VTE prophylaxis are not exclusive to stroke care and have been the target of several nonstroke quality projects in the VHA. Many aspects of acute stroke care did differ, and present opportunities for quality improvement in the future.
In our sample, patients with IHS had higher IV thrombolytic eligibility, which has not typically been reported in other samples.10,11,18 In these studies, hospitalized patients have been reported to more often have contraindications to tPA, such as recent surgery or lack of stroke symptom recognition due to delirium or medication effects. Interestingly, patients presenting to VHA EDs had extremely low rates of tPA eligibility (2%), which is lower than many reported estimates of tPA eligibility outside of the VHA.19,20 This may be due to multiple influences, such as geographic barriers, patient perceptions about stroke symptoms, access to emergency medical services (EMS), EMS routing patterns, and social/cultural factors. Although not statistically significant due to small sample size, tPA use also was twice as high in the IHS group.
Given that a significant proportion of patients with IHS in the VHA system may be eligible for acute thrombolysis, our findings highlight the need for acute stroke protocols to ensure that patients with IHS receive the same rapid stroke assessment and access to thrombolytics as do patients evaluated in the ED. Further investigation is needed to determine whether there are unique features of patients with IHS in VHA hospitals, which may make them more eligible for IV thrombolysis.
Dysphagia is associated with increased risks for aspiration pneumonia in stroke patients.21 We found that patients with IHS were less likely to receive dysphagia screening compared with that of stroke patients admitted through the ED. This finding is consistent with the fact that care for patients with IHS is less frequently guided by specific stroke care protocols and algorithms that are more often used in EDs.8,11 Although attention to swallowing function may lead to improved outcomes in stroke, this can be easily overlooked in patients with IHS.22 However, low dysphagia screening also was found in patients admitted through the ED, suggesting that low screening rates cannot be solely explained by differences in where the initial stroke evaluation is occurring. These findings suggest a need for novel approaches to dysphagia screening in VHA stroke patients that can be universally implemented throughout the hospital.
Finally, we also found very low rates of stroke education prior to discharge for both groups. Given the risk of stroke recurrence and the overall poor level of public knowledge about stroke, providing patients with stroke with formal oral and written information on stroke is a critical component of secondary prevention.23,24 Educational tools, including those that are veteran specific, are now available for use in VHA hospitals and should be incorporated into quality improvement strategies for stroke care in VHA hospitals.
In 2012, the VHA Acute Stroke Treatment Directive was published in an effort to improve stroke care systemwide. Several of the metrics examined in this study are addressed in this directive. The data presented in this study is one of the only samples of stroke quality metrics within the VHA that largely predates the directive and can serve as a baseline comparator for future work examining stroke care after release of the directive. At present, although continuous internal reviews of quality data are ongoing, longitudinal description of stroke care quality since publication of the directive will help to inform future efforts to improve stroke care for veterans.
Limitations
Despite the strength of being a multicenter sampling of stroke care in high volume VHA hospitals, our study had several limitations. The IHS sample size was small, which limited our ability to evaluate differences between the groups, to evaluate generalizability, and account for estimation error.13 It is possible that differences existed between the groups that could not be observed in this sample due to small size (type II error) or that patient-specific characteristics not captured by these data could influence these metrics. Assessments of eligibility and passing were based on retrospective chart review and post hoc coding. Our sample assessed only patients who presented to larger VHA hospitals with higher stroke volumes, thus these findings may not be generalizable to smaller VHA hospitals with less systematized stroke care. This sample did not describe the specialty care services that were received by each patient, which may have influenced their stroke care. Finally, this study is an analysis of use of QIs in stroke care and did not examine how these indicators affect outcomes.
Conclusion
Despite reassuring findings for several inpatient ischemic stroke quality metrics, we found several differences in stroke care between patients with IHS compared with those presenting to the ED, emphasizing the need for standardized approaches to stroke care regardless of care setting. Although patients with IHS may be more likely to be eligible for tPA, these patients received dysphagia screening and less often than did ED patients with stroke. Ongoing quality initiatives should continue to place emphasis on improving all quality metrics (particularly dysphagia screening, stroke severity documentation, and stroke education) for patients with stroke at VHA hospitals across all care settings. Future work will be needed to examine how specific patient characteristics and revisions to stroke protocols may affect stroke quality metrics and outcomes between patients with IHS and those presenting to the ED.
Acknowledgments
The authors would like to thank Danielle Sager for her contributions to this project.
1. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28-e292.
2. Schwamm LH, Ali SF, Reeves MJ, et al. Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at Get With the Guidelines—Stroke hospitals. Circ Cardiovasc Qual Outcomes. 2013;6(5):543-549.
3. Reeves MJ, Parker C, Fonarow GC, Smith EE, Schwamm LH. Development of stroke performance measures: definitions, methods, and current measures. Stroke. 2010;41(7):1573-1578.
4. The Joint Commission. Certificate of distinction for primary stroke centers. https://www.jointcommission.org/certificate_of_distinction_for_primary_stroke_centers_/.Published April 30, 2012. Accessed July 9, 2019.
5. US Department of Veterans Affairs. Center highlight: acute ischemic stroke care for veterans. https://www.queri.research.va.gov/center_highlights/stroke.cfm. Updated February 20, 2014. Accessed July 16, 2019.
6. Chumbler NR, Jia H, Phipps MS, et al. Does inpatient quality of care differ by age among US veterans with ischemic stroke? J Stroke Cerebrovasc Dis. 2012;21(8):844-851.
7. Katzan IL, Spertus J, Bettger JP, et al; American Heart Association Stroke Council; Council on Quality of Care and Outcomes Research; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology. Risk adjustment of ischemic stroke outcomes for comparing hospital performance: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(3):918-944.
8. Cumbler E, Wald H, Bhatt DL, et al. Quality of care and outcomes for in-hospital ischemic stroke: findings from the National Get With the Guidelines—Stroke. Stroke. 2014;45(1):231-238.
9. Blacker DJ. In-hospital stroke. Lancet Neurol. 2003;2(12):741-746.
10. Farooq MU, Reeves MJ, Gargano J, Wehner S, Hickenbottom S, Majid A; Paul Coverdell National Acute Stroke Registry Michigan Prototype Investigators. In-hospital stroke in a statewide stroke registry. Cerebrovascular Dis. 2008;25(1-2):12-20.
11. Bhalla A, Smeeton N, Rudd AG, Heuschmann P, Wolfe CD. A comparison of characteristics and resource use between in-hospital and admitted patients with stroke. J Stroke Cerebrovasc Dis. 2010;19:(5)357-363.
12. Garcia-Santibanez R, Liang J, Walker A, Matos-Diaz I, Kahkeshani K, Boniece I. Comparison of stroke codes in the emergency room and inpatient setting. J Stroke Cerebrovasc Dis. 2015;24(8):1948-1950.
13. Arling G, Reeves M, Ross J, et al. Estimating and reporting on the quality of inpatient stroke care by Veterans Health Administration medical centers. Circ Cardiovasc Qual Outcomes. 2012;5(1):44-51.
14. US Department of Veterans Affairs. Treatment of Acute Ischemic Stroke (AIS). VHA Directive 2011-038. https://www.hsrd.research.va.gov/news/feature/stroke.cfm. Updated January 20, 2014. Accessed July 17, 2019.
15. Williams LS, Daggett V, Slaven J, et al. Abstract 18: Does quality improvement training add to audit and feedback for inpatient stroke care processes? [International Stroke Conference abstract 18] Stroke. 2014;45(suppl 1):A18.
16. Williams LS, Yilmaz EY, Lopez-Yunez AM. Retrospective assessment of initial stroke severity with the NIH Stroke Scale. Stroke. 2000;31(4):858-862.
17. Jauch EC, Saver JL, Adams HP Jr, et al; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947.
18. Park HJ, Cho HJ, Kim YD, et al. Comparison of the characteristics for in-hospital and out-of-hospital ischaemic strokes. Eur J Neurol. 2009;16(5):582-588.
19. Messé SR, Fonarow GC, Smith EE, et al. Use of tissue-type plasminogen activator before and after publication of the European Cooperative Acute Stroke Study III in Get With the Guidelines-Stroke. Circ Cardiovasc Qual Outcomes. 2012;5(3):321-326.
20. Allen NB, Kaltenbach L, Goldstein LB, et al. Regional variation in recommended treatments for ischemic stroke and TIA: Get With the Guidelines—Stroke 2003-2010. Stroke. 2012;43(7):1858-1864.
21. Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36(12):2756-2763.
22. Bravata DM, Wells CK, Lo AC, et al. Processes of care associated with acute stroke outcomes. Arch Intern Med. 2010;170(9):804-810.
23. Mosley I, Nicol M, Donnan G, Patrick I, Dewey H. Stroke symptoms and the decision to call for an ambulance. Stroke; a journal of cerebral circulation. 2007;38(2):361-366.
24. Jurkowski JM, Maniccia DM, Dennison BA, Samuels SJ, Spicer DA. Awareness of necessity to call 9-1-1 for stroke symptoms, upstate New York. Prev Chronic Dis. 2008;5(2):A41.
1. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28-e292.
2. Schwamm LH, Ali SF, Reeves MJ, et al. Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at Get With the Guidelines—Stroke hospitals. Circ Cardiovasc Qual Outcomes. 2013;6(5):543-549.
3. Reeves MJ, Parker C, Fonarow GC, Smith EE, Schwamm LH. Development of stroke performance measures: definitions, methods, and current measures. Stroke. 2010;41(7):1573-1578.
4. The Joint Commission. Certificate of distinction for primary stroke centers. https://www.jointcommission.org/certificate_of_distinction_for_primary_stroke_centers_/.Published April 30, 2012. Accessed July 9, 2019.
5. US Department of Veterans Affairs. Center highlight: acute ischemic stroke care for veterans. https://www.queri.research.va.gov/center_highlights/stroke.cfm. Updated February 20, 2014. Accessed July 16, 2019.
6. Chumbler NR, Jia H, Phipps MS, et al. Does inpatient quality of care differ by age among US veterans with ischemic stroke? J Stroke Cerebrovasc Dis. 2012;21(8):844-851.
7. Katzan IL, Spertus J, Bettger JP, et al; American Heart Association Stroke Council; Council on Quality of Care and Outcomes Research; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology. Risk adjustment of ischemic stroke outcomes for comparing hospital performance: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(3):918-944.
8. Cumbler E, Wald H, Bhatt DL, et al. Quality of care and outcomes for in-hospital ischemic stroke: findings from the National Get With the Guidelines—Stroke. Stroke. 2014;45(1):231-238.
9. Blacker DJ. In-hospital stroke. Lancet Neurol. 2003;2(12):741-746.
10. Farooq MU, Reeves MJ, Gargano J, Wehner S, Hickenbottom S, Majid A; Paul Coverdell National Acute Stroke Registry Michigan Prototype Investigators. In-hospital stroke in a statewide stroke registry. Cerebrovascular Dis. 2008;25(1-2):12-20.
11. Bhalla A, Smeeton N, Rudd AG, Heuschmann P, Wolfe CD. A comparison of characteristics and resource use between in-hospital and admitted patients with stroke. J Stroke Cerebrovasc Dis. 2010;19:(5)357-363.
12. Garcia-Santibanez R, Liang J, Walker A, Matos-Diaz I, Kahkeshani K, Boniece I. Comparison of stroke codes in the emergency room and inpatient setting. J Stroke Cerebrovasc Dis. 2015;24(8):1948-1950.
13. Arling G, Reeves M, Ross J, et al. Estimating and reporting on the quality of inpatient stroke care by Veterans Health Administration medical centers. Circ Cardiovasc Qual Outcomes. 2012;5(1):44-51.
14. US Department of Veterans Affairs. Treatment of Acute Ischemic Stroke (AIS). VHA Directive 2011-038. https://www.hsrd.research.va.gov/news/feature/stroke.cfm. Updated January 20, 2014. Accessed July 17, 2019.
15. Williams LS, Daggett V, Slaven J, et al. Abstract 18: Does quality improvement training add to audit and feedback for inpatient stroke care processes? [International Stroke Conference abstract 18] Stroke. 2014;45(suppl 1):A18.
16. Williams LS, Yilmaz EY, Lopez-Yunez AM. Retrospective assessment of initial stroke severity with the NIH Stroke Scale. Stroke. 2000;31(4):858-862.
17. Jauch EC, Saver JL, Adams HP Jr, et al; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947.
18. Park HJ, Cho HJ, Kim YD, et al. Comparison of the characteristics for in-hospital and out-of-hospital ischaemic strokes. Eur J Neurol. 2009;16(5):582-588.
19. Messé SR, Fonarow GC, Smith EE, et al. Use of tissue-type plasminogen activator before and after publication of the European Cooperative Acute Stroke Study III in Get With the Guidelines-Stroke. Circ Cardiovasc Qual Outcomes. 2012;5(3):321-326.
20. Allen NB, Kaltenbach L, Goldstein LB, et al. Regional variation in recommended treatments for ischemic stroke and TIA: Get With the Guidelines—Stroke 2003-2010. Stroke. 2012;43(7):1858-1864.
21. Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36(12):2756-2763.
22. Bravata DM, Wells CK, Lo AC, et al. Processes of care associated with acute stroke outcomes. Arch Intern Med. 2010;170(9):804-810.
23. Mosley I, Nicol M, Donnan G, Patrick I, Dewey H. Stroke symptoms and the decision to call for an ambulance. Stroke; a journal of cerebral circulation. 2007;38(2):361-366.
24. Jurkowski JM, Maniccia DM, Dennison BA, Samuels SJ, Spicer DA. Awareness of necessity to call 9-1-1 for stroke symptoms, upstate New York. Prev Chronic Dis. 2008;5(2):A41.
VHA Practice Guideline Recommendations for Diffuse Gliomas (FULL)
Over the past few decades, our understanding of the molecular underpinning of primary neoplasms of the central nervous system (CNS) has progressed substantially. Thanks in large part to this expansion in our knowledge base, the World Health Organization (WHO) has recently updated its classification of tumors of the CNS.1 One of the key elements of this update was the inclusion of molecular diagnostic criteria for the classification of infiltrating gliomas. While the previous classification system was based upon histologic subtypes of the tumor (astrocytoma, oligodendroglioma, and oligoastrocytoma), the revised classification system incorporates molecular testing to establish the genetic characteristics of the tumor to reach a final integrated diagnosis.
In this article, we present 3 cases to highlight some of these recent changes in the WHO diagnostic categories of primary CNS tumors and to illustrate the role of specific molecular tests in reaching a final integrated diagnosis. We then propose a clinical practice guideline for the Veterans Health Administration (VHA) that recommends use of molecular testing for veterans as part of the diagnostic workup of primary CNS neoplasms.
Purpose
In 2013 the VHA National Director of Pathology & Laboratory Medicine Services (P&LMS) chartered a national molecular genetics pathology workgroup (MGPW) that was charged with 4 specific tasks: (1) Provide recommendations about the effective use of molecular genetic testing for veterans; (2) Promote increased quality and availability of molecular testing within the VHA; (3) Encourage internal referral testing; and (4) Create an organizational structure and policies for molecular genetic testing and laboratory developed tests. The workgroup is currently composed of 4 subcommittees: genetic medicine, hematopathology, pharmacogenomics, and molecular oncology. The molecular oncology subcommittee is focused upon molecular genetic testing for solid tumors.
This article is intended to be the first of several publications from the molecular oncology subcommittee of the MGPW that address some of the aforementioned tasks. Similar to the recent publication from the hematopathology subcommittee of the MGPW, this article focuses on CNS neoplasms.2
Scope of Problem
The incidence of tumors of the CNS in the US population varies among age groups. It is the most common solid tumor in children aged < 14 years and represents a significant cause of mortality across all age groups.3 Of CNS tumors, diffuse gliomas comprise about 20% of the tumors and more than 70% of the primary malignant CNS tumors.3 Analysis of the VA Central Cancer Registry data from 2010 to 2014 identified 1,186 veterans (about 237 veterans per year) who were diagnosed with diffuse gliomas. (Lynch, Kulich, Colman, unpublished data, February 2018). While the majority (nearly 80%) of these cases were glioblastomas (GBMs), unfortunately a majority of these cases did not undergo molecular testing (Lynch, Kulich, Colman, unpublished data, February 2018).
Although this low rate of testing may be in part reflective of the period from which these data were gleaned (ie, prior to the WHO release of their updated the classification of tumors of the CNS), it is important to raise VA practitioners’ awareness of these recent changes to ensure that veterans receive the proper diagnosis and treatment for their disease. Thus, while the number of veterans diagnosed with diffuse gliomas within the VHA is relatively small in comparison to other malignancies, such as prostatic adenocarcinomas and lung carcinomas, the majority of diffuse gliomas do not seem to be receiving the molecular testing that would be necessary for (1) appropriate classification under the recently revised WHO recommendations; and (2) making important treatment decisions.
Case Presentations
Case 1. A veteran of the Gulf War presented with a 3-month history of possible narcoleptic events associated with a motor vehicle accident. Magnetic resonance imaging (MRI) revealed a large left frontal mass lesion with minimal surrounding edema without appreciable contrast enhancement (Figures 1A, 1B, and 1C).
Neither mitotic figures nor endothelial proliferation were identified. Immunohistochemical stains revealed a lack of R132H mutant IDH1 protein expression, a loss of nuclear staining for ATRX protein within a substantial number of cells, and a clonal pattern of p53 protein overexpression (Figures 1E, 1F, and 1G). The lesion demonstrated diffuse glial fibrillary acidic protein (GFAP) immunoreactivity and a low proliferation index (as determined by Ki-67 staining; estimated at less than 5%) (Figures 1H and 1I).
Based upon these results, an initial morphologic diagnosis of diffuse glioma was issued, and tissue was subjected to a variety of nucleic acid-based tests. While fluorescence in situ hybridization (FISH) studies were negative for 1p/19q codeletion, pyrosequencing analysis revealed the presence of a c.394C>T (R132C) mutation of the IDH1 gene (Figure 1J). The University of Pittsburgh Medical Center’s GlioSeq targeted next-generation sequence (NGS) analysis confirmed the presence of the c.394C > T mutation in IDH1 gene.4 Based upon this additional information, a final integrated morphologic and molecular diagnosis of diffuse astrocytoma, IDH-mutant was rendered.
Case 2. A Vietnam War veteran presented with a 6-week history of new onset falls with associated left lower extremity weakness. A MRI revealed a right frontoparietal mass lesion with surrounding edema without appreciable contrast enhancement (Figures 2A, 2B, and 2C).
Immunohistochemical stains revealed R132H mutant IDH1 protein expression, retention of nuclear staining for ATRX protein, the lack of a clonal pattern of p53 protein overexpression, diffuse GFAP immunoreactivity, and a proliferation index (as determined by Ki-67 staining) focally approaching 20% (Figures 2E, 2F, 2G, 2H and 2I).
Based upon these results, an initial morphologic diagnosis of diffuse (high grade) glioma was issued, and tissue was subjected to a variety of nucleic acid-based tests. The FISH studies were positive for 1p/19q codeletion, and pyrosequencing analysis confirmed the immunohistochemical findings of a c.395G>A (R132H) mutation of the IDH1 gene (Figure 2J). GlioSeq targeted NGS analysis confirmed the presence of the c.395G>A mutation in the IDH1 gene, a mutation in the telomerase reverse transcriptase (TERT) promoter, and possible decreased copy number of the CIC (chromosome 1p) and FUBP1 (chromosome 19q) genes.
A final integrated morphologic and molecular diagnosis of anaplastic oligodendroglioma, IDH-mutant and 1p/19q-codeleted was rendered based on the additional information. With this final diagnosis, methylation analysis of the MGMT gene promoter, which was performed for prognostic and predictive purposes, was identified in this case.5,6
Case 3. A veteran of the Vietnam War presented with a new onset seizure. A MRI revealed a focally contrast-enhancing mass with surrounding edema within the left frontal lobe (Figures 3A, 3B, and 3C).
Hematoxylin and eosin (H&E) stained sections following formalin fixation and paraffin embedding demonstrated similar findings (Figure 3D), and while mitotic figures were readily identified, areas of necrosis were not identified and endothelial proliferation was not a prominent feature. Immunohistochemical stains revealed no evidence of R132H mutant IDH1 protein expression, retention of nuclear staining for ATRX protein, a clonal pattern of p53 protein overexpression, patchy GFAP immunoreactivity, and a proliferation index (as determined by Ki-67 staining) focally approaching 50% (Figures 3E, 3F, 3G, 3H, and 3I).
Based upon these results, an initial morphologic diagnosis of diffuse (high grade) glioma was issued, and the tissue was subjected to a variety of nucleic acid-based tests. The FISH studies were negative for EGFR gene amplification and 1p/19q codeletion, although a gain of the long arm of chromosome 1 was detected. Pyrosequencing analysis for mutations in codon 132 of the IDH1 gene revealed no mutations (Figure 3J). GlioSeq targeted NGS analysis identified mutations within the NF1, TP53, and PIK3CA genes without evidence of mutations in the IDH1, IDH2, ATRX, H3F3A, or EGFR genes or the TERT promoter. Based upon this additional information, a final integrated morphologic and molecular diagnosis of GBM, IDH wild-type was issued. The MGMT gene promoter was negative for methylation, a finding that has prognostic and predictive impact with regard to treatment with temazolamide.7-9
New Diffuse Glioma Classification
Since the issuance of the previous edition of the WHO classification of CNS tumors in 2007, several sentinel discoveries have been made that have advanced our understanding of the underlying biology of primary CNS neoplasms. Since a detailed review of these findings is beyond the scope and purpose of this manuscript and salient reviews on the topic can be found elsewhere, we will focus on the molecular findings that have been incorporated into the recently revised WHO classification.10 The importance of providing such information for proper patient management is illustrated by the recent acknowledgement by the American Academy of Neurology that molecular testing of brain tumors is a specific area in which there is a need for quality improvement.11 Therefore, it is critical that these underlying molecular abnormalities are identified to allow for proper classification and treatment of diffuse gliomas in the veteran population.
As noted previously, based on VA cancer registry data, diffuse gliomas are the most commonly encountered primary CNS cancers in the veteran population. Several of the aforementioned seminal discoveries have been incorporated into the updated classification of diffuse gliomas. While the recently updated WHO classification allows for the assignment of “not otherwise specified (NOS)” diagnostic designation, this category must be limited to cases where there is insufficient data to allow for a more precise classification due to sample limitations and not simply due to a failure of VA pathology laboratories to pursue the appropriate diagnostic testing.
Figure 4 presents the recommended diagnostic workflow for the workup of diffuse gliomas. As illustrated in the above cases, a variety of different methodologies, including immunohistochemical, FISH, loss of heterozygosity analysis, traditional and NGS may be applied when elucidating the status of molecular events at critical diagnostic branch points.
Diagnostic Uses of Molecular Testing
While the case studies in this article demonstrate the use of ancillary testing and provide a suggested strategy for properly subclassifying diffuse gliomas, inherent in this strategy is the assumption that, based upon the initial clinical and pathologic information available, one can accurately categorize the lesion as a diffuse glioma. In reality, such a distinction is not always a straightforward endeavor. It is well recognized that a proportion of low-grade, typically radiologically circumscribed, CNS neoplasms, such as pilocytic astrocytomas and glioneuronal tumors, may infiltrate the surrounding brain parenchyma. In addition, many of these low-grade CNS neoplasms also may have growth patterns that are shared with diffuse gliomas, a diagnostic challenge that often can be further hampered by the inherent limitations involved in obtaining adequate samples for diagnosis from the CNS.
Although there are limitations and caveats, molecular diagnostic testing may be invaluable in properly classifying CNS tumors in such situations. The finding of mutations in the IDH1 or IDH2 genes has been shown to be very valuable in distinguishing low-grade diffuse glioma from both nonneoplastic and low-grade circumscribed neuroepithelial neoplasms that may exhibit growth patterns that can mimic those of diffuse gliomas.15-17 Conversely, finding abnormalities in the BRAF gene in a brain neoplasm that has a low-grade morphology suggests that the lesion may represent one of these low-grade lesions such as a pleomorphic xanthoastrocytoma, pilocytic astrocytoma, or mixed neuronal-glial tumor as opposed to a diffuse glioma.18,19
Depending upon the environment in which one practices, small biopsy specimens may be prevalent, and unfortunately, it is not uncommon to obtain a biopsy that exhibits a histologic growth pattern that is discordant from what one would predict based on the clinical context and imaging findings. Molecular testing may be useful in resolving discordances in such situations. If a biopsy of a ring-enhancing lesion demonstrates a diffuse glioma that doesn’t meet WHO grade IV criteria, applying methodologies that look for genetic features commonly encountered in high-grade astrocytomas may identify genetic abnormalities that suggest a more aggressive lesion than is indicated by the histologic findings. The presence of genetic abnormalities such as homozygous deletion of the CDKN2A gene, TERT promoter mutation, loss of heterozygosity of chromosome 10q and/or phosphatase and tensin homolog (PTEN) mutations, EGFR gene amplification or the presence of the EGFR variant III are a few findings that would suggest the aforementioned sample may represent an undersampling of a higher grade diffuse astrocytoma, which would be important information to convey to the treating clinicians.20-26
Testing In the VA
The goals of the MPWG include promoting increased quality and availability of genetic testing within the VHA as well as encouraging internal referral testing. An informal survey of the chiefs of VA Pathology and Laboratory Medicine Services was conducted in November of 2017 in an attempt to identify internal VA pathology laboratories currently conducting testing that may be of use in the workup of diffuse gliomas (Table 1).
The VA currently offers NGS panels for patients with advanced-stage malignancies under the auspices of the Precision Oncology Program, whose reports provide both (1) mutational analyses for genes such as TP53, ATRX, NF1, BRAF, PTEN, TERT IDH1, and IDH2 that may be useful in the proper classifying of high-grade diffuse gliomas; and (2) information regarding clinical trials for which the veteran may be eligible for based on their glioma’s mutational profile. Interested VA providers should visit tinyurl.com/precisiononcology/ for more information about this program. Finally, although internal testing within VA laboratories is recommended to allow for the development of more cost-effective testing, testing may be performed through many nationally contracted reference laboratories.
Conclusion
In light of the recent progress made in our understanding of the molecular events of gliomagenesis, the way we diagnose diffuse gliomas within the CNS has undergone a major paradigm shift. While histology still plays a critical role in the process, we believe that additional ancillary testing is a requirement for all diffuse gliomas diagnosed within VA pathology laboratories. In the context of recently encountered cases, we have provided a recommended workflow highlighting the testing that can be performed to allow for the proper diagnosis of our veterans with diffuse gliomas (Figure 4).
Unless limited by the amount of tissue available for such tests, ancillary testing must be performed on all diffuse gliomas diagnosed within the VA system to ensure proper diagnosis and treatment of our veterans with diffuse gliomas.
Acknowledgments
The authors thank Dr. Craig M. Horbinski (Feinberg School of Medicine, Northwestern University) and Dr. Geoffrey H. Murdoch (University of Pittsburgh) for their constructive criticism of the manuscript. We also thank the following individuals for past service as members of the molecular oncology subcommittee of the MGPW: Dr. George Ansstas (Washington University School of Medicine), Dr. Osssama Hemadeh (Bay Pines VA Health Care System), Dr. James Herman (VA Pittsburgh Healthcare System), and Dr. Ryan Phan (formerly of the VA Greater Los Angeles Healthcare System) as well as the members of the Veterans Administration pathology and laboratory medicine service molecular genetics pathology workgroup.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies.
Dr. Kulich is the Acting Chief of Pathology and Laboratory Medicine Service at VA Pittsburgh Healthcare System and member of the Division of Neuropathology at University of Pittsburgh Department of Pathology, Dr. Duvvuri is an Otolaryngologist at VA Pittsburgh Healthcare System, and Dr. Passero is the Section Chief of Hematology\Oncology at VA Pittsburgh Healthcare System in Pennsylvania. Dr. Becker is an Oncologist at VA-New York Harbor Healthcare System. Dr. Dacic is a Pathologist at University of Pittsburgh Department of Pathology in Pennsylvania. Dr. Ehsan is Chief of Pathology and Laboratory Medicine Services at the South Texas Veterans Healthcare System in San Antonio. Dr. Gutkin is the former Chief of Pathology and Laboratory Medicine Service at VA Pittsburgh Healthcare System. Dr. Hou is a Pathologist at St. Louis VA Medical Center in Missouri. Dr. Icardi is the VA National Director of Pathology and Laboratory Medicine Services. Dr. Lyle is a Pathologist at Bay Pine Health Care System in Florida. Dr. Lynch is an Investigator at VA Salt Lake Health Care System Informatics and Computing Infrastructure. Dr. Montgomery is an Oncologist at VA Puget Sound Health Care System, in Seattle, Washington. Dr. Przygodzki is the Director of Genomic Medicine Implementation and Associate Director of Genomic Medicine for the VA. Dr. Colman is a Neuro-Oncologist at George E. Wahlen VA Medical Center and the Director of Medical Neuro-Oncology at the Huntsman Cancer Institute, Salt Lake City, Utah.
Correspondence: Dr. Kulich ([email protected])
1. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803-820.
2. Wang-Rodriguez J, Yunes A, Phan R, et al. The challenges of precision medicine and new advances in molecular diagnostic testing in hematolymphoid malignancies: impact on the VHA. Fed Pract. 2017;34(suppl 5):S38-S49.
3. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19(suppl 5):v1-v88.
4. Nikiforova MN, Wald AI, Melan MA, et al. Targeted next-generation sequencing panel (GlioSeq) provides comprehensive genetic profiling of central nervous system tumors. Neuro Oncol. 2016;18(3)379-387.
5. Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90(19):1473-1479.
6. van den Bent MJ, Erdem-Eraslan L, Idbaih A, et al. MGMT-STP27 methylation status as predictive marker for response to PCV in anaplastic oligodendrogliomas and oligoastrocytomas. A report from EORTC study 26951. Clin Cancer Res. 2013;19(19):5513-5522.
7. Stupp R, Hegi ME, Mason WP, et al; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459-466.
8. Malmstrom A, Gronberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916-926.
9. van den Bent MJ, Kros JM. Predictive and prognostic markers in neuro-oncology. J Neuropathol Exp Neurol. 2007;66(12):1074-1081.
10. Chen R, Smith-Cohn M, Cohen AL, Colman H. Glioma subclassifications and their clinical significance. Neurotherapeutics. 2017;14(2):284-297.
11. Jordan JT, Sanders AE, Armstrong T, et al. Quality improvement in neurology: neuro-oncology quality measurement set. Neurology. 2018;90(14):652-658.
12. Chen L, Voronovich Z, Clark K, et al. Predicting the likelihood of an isocitrate dehydrogenase 1 or 2 mutation in diagnoses of infiltrative glioma. Neuro Oncol. 2014;16(11):1478-1483.
13. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997-1003.
14. Wick W, Platten M, Meisner C, et al; NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707-715.
15. Horbinski C, Kofler J, Kelly LM, Murdoch GH, Nikiforova MN. Diagnostic use of IDH1/2 mutation analysis in routine clinical testing of formalin-fixed, paraffin-embedded glioma tissues. J Neuropathol Exp Neurol. 2009;68(12):1319-1325.
16. Camelo-Piragua S, Jansen M, Ganguly A, Kim JC, Louis DN, Nutt CL. Mutant IDH1-specific immunohistochemistry distinguishes diffuse astrocytoma from astrocytosis. Acta Neuropathol. 2010;119(4):509-511.
17. Horbinski C, Kofler J, Yeaney G, et al. Isocitrate dehydrogenase 1 analysis differentiates gangliogliomas from infiltrative gliomas. Brain Pathol. 2011;21(5):564-574.
18. Berghoff AS, Preusser M. BRAF alterations in brain tumours: molecular pathology and therapeutic opportunities. Curr Opin Neurol. 2014;27(6):689-696.
19. Korshunov A, Meyer J, Capper D, et al. Combined molecular analysis of BRAF and IDH1 distinguishes pilocytic astrocytoma from diffuse astrocytoma. Acta Neuropathol. 2009;118(3):401-405.
20. Fuller CE, Schmidt RE, Roth KA, et al. Clinical utility of fluorescence in situ hybridization (FISH) in morphologically ambiguous gliomas with hybrid oligodendroglial/astrocytic features. J Neuropathol Exp Neurol. 2003;62(11):1118-1128.
21. Horbinski C. Practical molecular diagnostics in neuropathology: making a tough job a little easier. Semin Diagn Pathol. 2010;27(2):105-113.
22. Fuller GN, Bigner SH. Amplified cellular oncogenes in neoplasms of the human central nervous system. Mutat Res. 1992;276(3):299-306.
23. Brennan CW, Verhaak RG, McKenna A, et al; TCGA Research Network. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462-477.
24. Aldape K, Zadeh G, Mansouri S, Reifenberger G, von Deimling A. Glioblastoma: pathology, molecular mechanisms and markers. Acta Neuropathol. 2015;129(6):829-848.
25. Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110(15):6021-6026.
26. Nikiforova MN, Hamilton RL. Molecular diagnostics of gliomas. Arch Pathol Lab Med. 2011;135(5):558-568.
Over the past few decades, our understanding of the molecular underpinning of primary neoplasms of the central nervous system (CNS) has progressed substantially. Thanks in large part to this expansion in our knowledge base, the World Health Organization (WHO) has recently updated its classification of tumors of the CNS.1 One of the key elements of this update was the inclusion of molecular diagnostic criteria for the classification of infiltrating gliomas. While the previous classification system was based upon histologic subtypes of the tumor (astrocytoma, oligodendroglioma, and oligoastrocytoma), the revised classification system incorporates molecular testing to establish the genetic characteristics of the tumor to reach a final integrated diagnosis.
In this article, we present 3 cases to highlight some of these recent changes in the WHO diagnostic categories of primary CNS tumors and to illustrate the role of specific molecular tests in reaching a final integrated diagnosis. We then propose a clinical practice guideline for the Veterans Health Administration (VHA) that recommends use of molecular testing for veterans as part of the diagnostic workup of primary CNS neoplasms.
Purpose
In 2013 the VHA National Director of Pathology & Laboratory Medicine Services (P&LMS) chartered a national molecular genetics pathology workgroup (MGPW) that was charged with 4 specific tasks: (1) Provide recommendations about the effective use of molecular genetic testing for veterans; (2) Promote increased quality and availability of molecular testing within the VHA; (3) Encourage internal referral testing; and (4) Create an organizational structure and policies for molecular genetic testing and laboratory developed tests. The workgroup is currently composed of 4 subcommittees: genetic medicine, hematopathology, pharmacogenomics, and molecular oncology. The molecular oncology subcommittee is focused upon molecular genetic testing for solid tumors.
This article is intended to be the first of several publications from the molecular oncology subcommittee of the MGPW that address some of the aforementioned tasks. Similar to the recent publication from the hematopathology subcommittee of the MGPW, this article focuses on CNS neoplasms.2
Scope of Problem
The incidence of tumors of the CNS in the US population varies among age groups. It is the most common solid tumor in children aged < 14 years and represents a significant cause of mortality across all age groups.3 Of CNS tumors, diffuse gliomas comprise about 20% of the tumors and more than 70% of the primary malignant CNS tumors.3 Analysis of the VA Central Cancer Registry data from 2010 to 2014 identified 1,186 veterans (about 237 veterans per year) who were diagnosed with diffuse gliomas. (Lynch, Kulich, Colman, unpublished data, February 2018). While the majority (nearly 80%) of these cases were glioblastomas (GBMs), unfortunately a majority of these cases did not undergo molecular testing (Lynch, Kulich, Colman, unpublished data, February 2018).
Although this low rate of testing may be in part reflective of the period from which these data were gleaned (ie, prior to the WHO release of their updated the classification of tumors of the CNS), it is important to raise VA practitioners’ awareness of these recent changes to ensure that veterans receive the proper diagnosis and treatment for their disease. Thus, while the number of veterans diagnosed with diffuse gliomas within the VHA is relatively small in comparison to other malignancies, such as prostatic adenocarcinomas and lung carcinomas, the majority of diffuse gliomas do not seem to be receiving the molecular testing that would be necessary for (1) appropriate classification under the recently revised WHO recommendations; and (2) making important treatment decisions.
Case Presentations
Case 1. A veteran of the Gulf War presented with a 3-month history of possible narcoleptic events associated with a motor vehicle accident. Magnetic resonance imaging (MRI) revealed a large left frontal mass lesion with minimal surrounding edema without appreciable contrast enhancement (Figures 1A, 1B, and 1C).
Neither mitotic figures nor endothelial proliferation were identified. Immunohistochemical stains revealed a lack of R132H mutant IDH1 protein expression, a loss of nuclear staining for ATRX protein within a substantial number of cells, and a clonal pattern of p53 protein overexpression (Figures 1E, 1F, and 1G). The lesion demonstrated diffuse glial fibrillary acidic protein (GFAP) immunoreactivity and a low proliferation index (as determined by Ki-67 staining; estimated at less than 5%) (Figures 1H and 1I).
Based upon these results, an initial morphologic diagnosis of diffuse glioma was issued, and tissue was subjected to a variety of nucleic acid-based tests. While fluorescence in situ hybridization (FISH) studies were negative for 1p/19q codeletion, pyrosequencing analysis revealed the presence of a c.394C>T (R132C) mutation of the IDH1 gene (Figure 1J). The University of Pittsburgh Medical Center’s GlioSeq targeted next-generation sequence (NGS) analysis confirmed the presence of the c.394C > T mutation in IDH1 gene.4 Based upon this additional information, a final integrated morphologic and molecular diagnosis of diffuse astrocytoma, IDH-mutant was rendered.
Case 2. A Vietnam War veteran presented with a 6-week history of new onset falls with associated left lower extremity weakness. A MRI revealed a right frontoparietal mass lesion with surrounding edema without appreciable contrast enhancement (Figures 2A, 2B, and 2C).
Immunohistochemical stains revealed R132H mutant IDH1 protein expression, retention of nuclear staining for ATRX protein, the lack of a clonal pattern of p53 protein overexpression, diffuse GFAP immunoreactivity, and a proliferation index (as determined by Ki-67 staining) focally approaching 20% (Figures 2E, 2F, 2G, 2H and 2I).
Based upon these results, an initial morphologic diagnosis of diffuse (high grade) glioma was issued, and tissue was subjected to a variety of nucleic acid-based tests. The FISH studies were positive for 1p/19q codeletion, and pyrosequencing analysis confirmed the immunohistochemical findings of a c.395G>A (R132H) mutation of the IDH1 gene (Figure 2J). GlioSeq targeted NGS analysis confirmed the presence of the c.395G>A mutation in the IDH1 gene, a mutation in the telomerase reverse transcriptase (TERT) promoter, and possible decreased copy number of the CIC (chromosome 1p) and FUBP1 (chromosome 19q) genes.
A final integrated morphologic and molecular diagnosis of anaplastic oligodendroglioma, IDH-mutant and 1p/19q-codeleted was rendered based on the additional information. With this final diagnosis, methylation analysis of the MGMT gene promoter, which was performed for prognostic and predictive purposes, was identified in this case.5,6
Case 3. A veteran of the Vietnam War presented with a new onset seizure. A MRI revealed a focally contrast-enhancing mass with surrounding edema within the left frontal lobe (Figures 3A, 3B, and 3C).
Hematoxylin and eosin (H&E) stained sections following formalin fixation and paraffin embedding demonstrated similar findings (Figure 3D), and while mitotic figures were readily identified, areas of necrosis were not identified and endothelial proliferation was not a prominent feature. Immunohistochemical stains revealed no evidence of R132H mutant IDH1 protein expression, retention of nuclear staining for ATRX protein, a clonal pattern of p53 protein overexpression, patchy GFAP immunoreactivity, and a proliferation index (as determined by Ki-67 staining) focally approaching 50% (Figures 3E, 3F, 3G, 3H, and 3I).
Based upon these results, an initial morphologic diagnosis of diffuse (high grade) glioma was issued, and the tissue was subjected to a variety of nucleic acid-based tests. The FISH studies were negative for EGFR gene amplification and 1p/19q codeletion, although a gain of the long arm of chromosome 1 was detected. Pyrosequencing analysis for mutations in codon 132 of the IDH1 gene revealed no mutations (Figure 3J). GlioSeq targeted NGS analysis identified mutations within the NF1, TP53, and PIK3CA genes without evidence of mutations in the IDH1, IDH2, ATRX, H3F3A, or EGFR genes or the TERT promoter. Based upon this additional information, a final integrated morphologic and molecular diagnosis of GBM, IDH wild-type was issued. The MGMT gene promoter was negative for methylation, a finding that has prognostic and predictive impact with regard to treatment with temazolamide.7-9
New Diffuse Glioma Classification
Since the issuance of the previous edition of the WHO classification of CNS tumors in 2007, several sentinel discoveries have been made that have advanced our understanding of the underlying biology of primary CNS neoplasms. Since a detailed review of these findings is beyond the scope and purpose of this manuscript and salient reviews on the topic can be found elsewhere, we will focus on the molecular findings that have been incorporated into the recently revised WHO classification.10 The importance of providing such information for proper patient management is illustrated by the recent acknowledgement by the American Academy of Neurology that molecular testing of brain tumors is a specific area in which there is a need for quality improvement.11 Therefore, it is critical that these underlying molecular abnormalities are identified to allow for proper classification and treatment of diffuse gliomas in the veteran population.
As noted previously, based on VA cancer registry data, diffuse gliomas are the most commonly encountered primary CNS cancers in the veteran population. Several of the aforementioned seminal discoveries have been incorporated into the updated classification of diffuse gliomas. While the recently updated WHO classification allows for the assignment of “not otherwise specified (NOS)” diagnostic designation, this category must be limited to cases where there is insufficient data to allow for a more precise classification due to sample limitations and not simply due to a failure of VA pathology laboratories to pursue the appropriate diagnostic testing.
Figure 4 presents the recommended diagnostic workflow for the workup of diffuse gliomas. As illustrated in the above cases, a variety of different methodologies, including immunohistochemical, FISH, loss of heterozygosity analysis, traditional and NGS may be applied when elucidating the status of molecular events at critical diagnostic branch points.
Diagnostic Uses of Molecular Testing
While the case studies in this article demonstrate the use of ancillary testing and provide a suggested strategy for properly subclassifying diffuse gliomas, inherent in this strategy is the assumption that, based upon the initial clinical and pathologic information available, one can accurately categorize the lesion as a diffuse glioma. In reality, such a distinction is not always a straightforward endeavor. It is well recognized that a proportion of low-grade, typically radiologically circumscribed, CNS neoplasms, such as pilocytic astrocytomas and glioneuronal tumors, may infiltrate the surrounding brain parenchyma. In addition, many of these low-grade CNS neoplasms also may have growth patterns that are shared with diffuse gliomas, a diagnostic challenge that often can be further hampered by the inherent limitations involved in obtaining adequate samples for diagnosis from the CNS.
Although there are limitations and caveats, molecular diagnostic testing may be invaluable in properly classifying CNS tumors in such situations. The finding of mutations in the IDH1 or IDH2 genes has been shown to be very valuable in distinguishing low-grade diffuse glioma from both nonneoplastic and low-grade circumscribed neuroepithelial neoplasms that may exhibit growth patterns that can mimic those of diffuse gliomas.15-17 Conversely, finding abnormalities in the BRAF gene in a brain neoplasm that has a low-grade morphology suggests that the lesion may represent one of these low-grade lesions such as a pleomorphic xanthoastrocytoma, pilocytic astrocytoma, or mixed neuronal-glial tumor as opposed to a diffuse glioma.18,19
Depending upon the environment in which one practices, small biopsy specimens may be prevalent, and unfortunately, it is not uncommon to obtain a biopsy that exhibits a histologic growth pattern that is discordant from what one would predict based on the clinical context and imaging findings. Molecular testing may be useful in resolving discordances in such situations. If a biopsy of a ring-enhancing lesion demonstrates a diffuse glioma that doesn’t meet WHO grade IV criteria, applying methodologies that look for genetic features commonly encountered in high-grade astrocytomas may identify genetic abnormalities that suggest a more aggressive lesion than is indicated by the histologic findings. The presence of genetic abnormalities such as homozygous deletion of the CDKN2A gene, TERT promoter mutation, loss of heterozygosity of chromosome 10q and/or phosphatase and tensin homolog (PTEN) mutations, EGFR gene amplification or the presence of the EGFR variant III are a few findings that would suggest the aforementioned sample may represent an undersampling of a higher grade diffuse astrocytoma, which would be important information to convey to the treating clinicians.20-26
Testing In the VA
The goals of the MPWG include promoting increased quality and availability of genetic testing within the VHA as well as encouraging internal referral testing. An informal survey of the chiefs of VA Pathology and Laboratory Medicine Services was conducted in November of 2017 in an attempt to identify internal VA pathology laboratories currently conducting testing that may be of use in the workup of diffuse gliomas (Table 1).
The VA currently offers NGS panels for patients with advanced-stage malignancies under the auspices of the Precision Oncology Program, whose reports provide both (1) mutational analyses for genes such as TP53, ATRX, NF1, BRAF, PTEN, TERT IDH1, and IDH2 that may be useful in the proper classifying of high-grade diffuse gliomas; and (2) information regarding clinical trials for which the veteran may be eligible for based on their glioma’s mutational profile. Interested VA providers should visit tinyurl.com/precisiononcology/ for more information about this program. Finally, although internal testing within VA laboratories is recommended to allow for the development of more cost-effective testing, testing may be performed through many nationally contracted reference laboratories.
Conclusion
In light of the recent progress made in our understanding of the molecular events of gliomagenesis, the way we diagnose diffuse gliomas within the CNS has undergone a major paradigm shift. While histology still plays a critical role in the process, we believe that additional ancillary testing is a requirement for all diffuse gliomas diagnosed within VA pathology laboratories. In the context of recently encountered cases, we have provided a recommended workflow highlighting the testing that can be performed to allow for the proper diagnosis of our veterans with diffuse gliomas (Figure 4).
Unless limited by the amount of tissue available for such tests, ancillary testing must be performed on all diffuse gliomas diagnosed within the VA system to ensure proper diagnosis and treatment of our veterans with diffuse gliomas.

Acknowledgments
The authors thank Dr. Craig M. Horbinski (Feinberg School of Medicine, Northwestern University) and Dr. Geoffrey H. Murdoch (University of Pittsburgh) for their constructive criticism of the manuscript. We also thank the following individuals for past service as members of the molecular oncology subcommittee of the MGPW: Dr. George Ansstas (Washington University School of Medicine), Dr. Osssama Hemadeh (Bay Pines VA Health Care System), Dr. James Herman (VA Pittsburgh Healthcare System), and Dr. Ryan Phan (formerly of the VA Greater Los Angeles Healthcare System) as well as the members of the Veterans Administration pathology and laboratory medicine service molecular genetics pathology workgroup.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies.
Dr. Kulich is the Acting Chief of Pathology and Laboratory Medicine Service at VA Pittsburgh Healthcare System and member of the Division of Neuropathology at University of Pittsburgh Department of Pathology, Dr. Duvvuri is an Otolaryngologist at VA Pittsburgh Healthcare System, and Dr. Passero is the Section Chief of Hematology\Oncology at VA Pittsburgh Healthcare System in Pennsylvania. Dr. Becker is an Oncologist at VA-New York Harbor Healthcare System. Dr. Dacic is a Pathologist at University of Pittsburgh Department of Pathology in Pennsylvania. Dr. Ehsan is Chief of Pathology and Laboratory Medicine Services at the South Texas Veterans Healthcare System in San Antonio. Dr. Gutkin is the former Chief of Pathology and Laboratory Medicine Service at VA Pittsburgh Healthcare System. Dr. Hou is a Pathologist at St. Louis VA Medical Center in Missouri. Dr. Icardi is the VA National Director of Pathology and Laboratory Medicine Services. Dr. Lyle is a Pathologist at Bay Pine Health Care System in Florida. Dr. Lynch is an Investigator at VA Salt Lake Health Care System Informatics and Computing Infrastructure. Dr. Montgomery is an Oncologist at VA Puget Sound Health Care System, in Seattle, Washington. Dr. Przygodzki is the Director of Genomic Medicine Implementation and Associate Director of Genomic Medicine for the VA. Dr. Colman is a Neuro-Oncologist at George E. Wahlen VA Medical Center and the Director of Medical Neuro-Oncology at the Huntsman Cancer Institute, Salt Lake City, Utah.
Correspondence: Dr. Kulich ([email protected])
Over the past few decades, our understanding of the molecular underpinning of primary neoplasms of the central nervous system (CNS) has progressed substantially. Thanks in large part to this expansion in our knowledge base, the World Health Organization (WHO) has recently updated its classification of tumors of the CNS.1 One of the key elements of this update was the inclusion of molecular diagnostic criteria for the classification of infiltrating gliomas. While the previous classification system was based upon histologic subtypes of the tumor (astrocytoma, oligodendroglioma, and oligoastrocytoma), the revised classification system incorporates molecular testing to establish the genetic characteristics of the tumor to reach a final integrated diagnosis.
In this article, we present 3 cases to highlight some of these recent changes in the WHO diagnostic categories of primary CNS tumors and to illustrate the role of specific molecular tests in reaching a final integrated diagnosis. We then propose a clinical practice guideline for the Veterans Health Administration (VHA) that recommends use of molecular testing for veterans as part of the diagnostic workup of primary CNS neoplasms.
Purpose
In 2013 the VHA National Director of Pathology & Laboratory Medicine Services (P&LMS) chartered a national molecular genetics pathology workgroup (MGPW) that was charged with 4 specific tasks: (1) Provide recommendations about the effective use of molecular genetic testing for veterans; (2) Promote increased quality and availability of molecular testing within the VHA; (3) Encourage internal referral testing; and (4) Create an organizational structure and policies for molecular genetic testing and laboratory developed tests. The workgroup is currently composed of 4 subcommittees: genetic medicine, hematopathology, pharmacogenomics, and molecular oncology. The molecular oncology subcommittee is focused upon molecular genetic testing for solid tumors.
This article is intended to be the first of several publications from the molecular oncology subcommittee of the MGPW that address some of the aforementioned tasks. Similar to the recent publication from the hematopathology subcommittee of the MGPW, this article focuses on CNS neoplasms.2
Scope of Problem
The incidence of tumors of the CNS in the US population varies among age groups. It is the most common solid tumor in children aged < 14 years and represents a significant cause of mortality across all age groups.3 Of CNS tumors, diffuse gliomas comprise about 20% of the tumors and more than 70% of the primary malignant CNS tumors.3 Analysis of the VA Central Cancer Registry data from 2010 to 2014 identified 1,186 veterans (about 237 veterans per year) who were diagnosed with diffuse gliomas. (Lynch, Kulich, Colman, unpublished data, February 2018). While the majority (nearly 80%) of these cases were glioblastomas (GBMs), unfortunately a majority of these cases did not undergo molecular testing (Lynch, Kulich, Colman, unpublished data, February 2018).
Although this low rate of testing may be in part reflective of the period from which these data were gleaned (ie, prior to the WHO release of their updated the classification of tumors of the CNS), it is important to raise VA practitioners’ awareness of these recent changes to ensure that veterans receive the proper diagnosis and treatment for their disease. Thus, while the number of veterans diagnosed with diffuse gliomas within the VHA is relatively small in comparison to other malignancies, such as prostatic adenocarcinomas and lung carcinomas, the majority of diffuse gliomas do not seem to be receiving the molecular testing that would be necessary for (1) appropriate classification under the recently revised WHO recommendations; and (2) making important treatment decisions.
Case Presentations
Case 1. A veteran of the Gulf War presented with a 3-month history of possible narcoleptic events associated with a motor vehicle accident. Magnetic resonance imaging (MRI) revealed a large left frontal mass lesion with minimal surrounding edema without appreciable contrast enhancement (Figures 1A, 1B, and 1C).
Neither mitotic figures nor endothelial proliferation were identified. Immunohistochemical stains revealed a lack of R132H mutant IDH1 protein expression, a loss of nuclear staining for ATRX protein within a substantial number of cells, and a clonal pattern of p53 protein overexpression (Figures 1E, 1F, and 1G). The lesion demonstrated diffuse glial fibrillary acidic protein (GFAP) immunoreactivity and a low proliferation index (as determined by Ki-67 staining; estimated at less than 5%) (Figures 1H and 1I).
Based upon these results, an initial morphologic diagnosis of diffuse glioma was issued, and tissue was subjected to a variety of nucleic acid-based tests. While fluorescence in situ hybridization (FISH) studies were negative for 1p/19q codeletion, pyrosequencing analysis revealed the presence of a c.394C>T (R132C) mutation of the IDH1 gene (Figure 1J). The University of Pittsburgh Medical Center’s GlioSeq targeted next-generation sequence (NGS) analysis confirmed the presence of the c.394C > T mutation in IDH1 gene.4 Based upon this additional information, a final integrated morphologic and molecular diagnosis of diffuse astrocytoma, IDH-mutant was rendered.
Case 2. A Vietnam War veteran presented with a 6-week history of new onset falls with associated left lower extremity weakness. A MRI revealed a right frontoparietal mass lesion with surrounding edema without appreciable contrast enhancement (Figures 2A, 2B, and 2C).
Immunohistochemical stains revealed R132H mutant IDH1 protein expression, retention of nuclear staining for ATRX protein, the lack of a clonal pattern of p53 protein overexpression, diffuse GFAP immunoreactivity, and a proliferation index (as determined by Ki-67 staining) focally approaching 20% (Figures 2E, 2F, 2G, 2H and 2I).
Based upon these results, an initial morphologic diagnosis of diffuse (high grade) glioma was issued, and tissue was subjected to a variety of nucleic acid-based tests. The FISH studies were positive for 1p/19q codeletion, and pyrosequencing analysis confirmed the immunohistochemical findings of a c.395G>A (R132H) mutation of the IDH1 gene (Figure 2J). GlioSeq targeted NGS analysis confirmed the presence of the c.395G>A mutation in the IDH1 gene, a mutation in the telomerase reverse transcriptase (TERT) promoter, and possible decreased copy number of the CIC (chromosome 1p) and FUBP1 (chromosome 19q) genes.
A final integrated morphologic and molecular diagnosis of anaplastic oligodendroglioma, IDH-mutant and 1p/19q-codeleted was rendered based on the additional information. With this final diagnosis, methylation analysis of the MGMT gene promoter, which was performed for prognostic and predictive purposes, was identified in this case.5,6
Case 3. A veteran of the Vietnam War presented with a new onset seizure. A MRI revealed a focally contrast-enhancing mass with surrounding edema within the left frontal lobe (Figures 3A, 3B, and 3C).
Hematoxylin and eosin (H&E) stained sections following formalin fixation and paraffin embedding demonstrated similar findings (Figure 3D), and while mitotic figures were readily identified, areas of necrosis were not identified and endothelial proliferation was not a prominent feature. Immunohistochemical stains revealed no evidence of R132H mutant IDH1 protein expression, retention of nuclear staining for ATRX protein, a clonal pattern of p53 protein overexpression, patchy GFAP immunoreactivity, and a proliferation index (as determined by Ki-67 staining) focally approaching 50% (Figures 3E, 3F, 3G, 3H, and 3I).
Based upon these results, an initial morphologic diagnosis of diffuse (high grade) glioma was issued, and the tissue was subjected to a variety of nucleic acid-based tests. The FISH studies were negative for EGFR gene amplification and 1p/19q codeletion, although a gain of the long arm of chromosome 1 was detected. Pyrosequencing analysis for mutations in codon 132 of the IDH1 gene revealed no mutations (Figure 3J). GlioSeq targeted NGS analysis identified mutations within the NF1, TP53, and PIK3CA genes without evidence of mutations in the IDH1, IDH2, ATRX, H3F3A, or EGFR genes or the TERT promoter. Based upon this additional information, a final integrated morphologic and molecular diagnosis of GBM, IDH wild-type was issued. The MGMT gene promoter was negative for methylation, a finding that has prognostic and predictive impact with regard to treatment with temazolamide.7-9
New Diffuse Glioma Classification
Since the issuance of the previous edition of the WHO classification of CNS tumors in 2007, several sentinel discoveries have been made that have advanced our understanding of the underlying biology of primary CNS neoplasms. Since a detailed review of these findings is beyond the scope and purpose of this manuscript and salient reviews on the topic can be found elsewhere, we will focus on the molecular findings that have been incorporated into the recently revised WHO classification.10 The importance of providing such information for proper patient management is illustrated by the recent acknowledgement by the American Academy of Neurology that molecular testing of brain tumors is a specific area in which there is a need for quality improvement.11 Therefore, it is critical that these underlying molecular abnormalities are identified to allow for proper classification and treatment of diffuse gliomas in the veteran population.
As noted previously, based on VA cancer registry data, diffuse gliomas are the most commonly encountered primary CNS cancers in the veteran population. Several of the aforementioned seminal discoveries have been incorporated into the updated classification of diffuse gliomas. While the recently updated WHO classification allows for the assignment of “not otherwise specified (NOS)” diagnostic designation, this category must be limited to cases where there is insufficient data to allow for a more precise classification due to sample limitations and not simply due to a failure of VA pathology laboratories to pursue the appropriate diagnostic testing.
Figure 4 presents the recommended diagnostic workflow for the workup of diffuse gliomas. As illustrated in the above cases, a variety of different methodologies, including immunohistochemical, FISH, loss of heterozygosity analysis, traditional and NGS may be applied when elucidating the status of molecular events at critical diagnostic branch points.
Diagnostic Uses of Molecular Testing
While the case studies in this article demonstrate the use of ancillary testing and provide a suggested strategy for properly subclassifying diffuse gliomas, inherent in this strategy is the assumption that, based upon the initial clinical and pathologic information available, one can accurately categorize the lesion as a diffuse glioma. In reality, such a distinction is not always a straightforward endeavor. It is well recognized that a proportion of low-grade, typically radiologically circumscribed, CNS neoplasms, such as pilocytic astrocytomas and glioneuronal tumors, may infiltrate the surrounding brain parenchyma. In addition, many of these low-grade CNS neoplasms also may have growth patterns that are shared with diffuse gliomas, a diagnostic challenge that often can be further hampered by the inherent limitations involved in obtaining adequate samples for diagnosis from the CNS.
Although there are limitations and caveats, molecular diagnostic testing may be invaluable in properly classifying CNS tumors in such situations. The finding of mutations in the IDH1 or IDH2 genes has been shown to be very valuable in distinguishing low-grade diffuse glioma from both nonneoplastic and low-grade circumscribed neuroepithelial neoplasms that may exhibit growth patterns that can mimic those of diffuse gliomas.15-17 Conversely, finding abnormalities in the BRAF gene in a brain neoplasm that has a low-grade morphology suggests that the lesion may represent one of these low-grade lesions such as a pleomorphic xanthoastrocytoma, pilocytic astrocytoma, or mixed neuronal-glial tumor as opposed to a diffuse glioma.18,19
Depending upon the environment in which one practices, small biopsy specimens may be prevalent, and unfortunately, it is not uncommon to obtain a biopsy that exhibits a histologic growth pattern that is discordant from what one would predict based on the clinical context and imaging findings. Molecular testing may be useful in resolving discordances in such situations. If a biopsy of a ring-enhancing lesion demonstrates a diffuse glioma that doesn’t meet WHO grade IV criteria, applying methodologies that look for genetic features commonly encountered in high-grade astrocytomas may identify genetic abnormalities that suggest a more aggressive lesion than is indicated by the histologic findings. The presence of genetic abnormalities such as homozygous deletion of the CDKN2A gene, TERT promoter mutation, loss of heterozygosity of chromosome 10q and/or phosphatase and tensin homolog (PTEN) mutations, EGFR gene amplification or the presence of the EGFR variant III are a few findings that would suggest the aforementioned sample may represent an undersampling of a higher grade diffuse astrocytoma, which would be important information to convey to the treating clinicians.20-26
Testing In the VA
The goals of the MPWG include promoting increased quality and availability of genetic testing within the VHA as well as encouraging internal referral testing. An informal survey of the chiefs of VA Pathology and Laboratory Medicine Services was conducted in November of 2017 in an attempt to identify internal VA pathology laboratories currently conducting testing that may be of use in the workup of diffuse gliomas (Table 1).
The VA currently offers NGS panels for patients with advanced-stage malignancies under the auspices of the Precision Oncology Program, whose reports provide both (1) mutational analyses for genes such as TP53, ATRX, NF1, BRAF, PTEN, TERT IDH1, and IDH2 that may be useful in the proper classifying of high-grade diffuse gliomas; and (2) information regarding clinical trials for which the veteran may be eligible for based on their glioma’s mutational profile. Interested VA providers should visit tinyurl.com/precisiononcology/ for more information about this program. Finally, although internal testing within VA laboratories is recommended to allow for the development of more cost-effective testing, testing may be performed through many nationally contracted reference laboratories.
Conclusion
In light of the recent progress made in our understanding of the molecular events of gliomagenesis, the way we diagnose diffuse gliomas within the CNS has undergone a major paradigm shift. While histology still plays a critical role in the process, we believe that additional ancillary testing is a requirement for all diffuse gliomas diagnosed within VA pathology laboratories. In the context of recently encountered cases, we have provided a recommended workflow highlighting the testing that can be performed to allow for the proper diagnosis of our veterans with diffuse gliomas (Figure 4).
Unless limited by the amount of tissue available for such tests, ancillary testing must be performed on all diffuse gliomas diagnosed within the VA system to ensure proper diagnosis and treatment of our veterans with diffuse gliomas.

Acknowledgments
The authors thank Dr. Craig M. Horbinski (Feinberg School of Medicine, Northwestern University) and Dr. Geoffrey H. Murdoch (University of Pittsburgh) for their constructive criticism of the manuscript. We also thank the following individuals for past service as members of the molecular oncology subcommittee of the MGPW: Dr. George Ansstas (Washington University School of Medicine), Dr. Osssama Hemadeh (Bay Pines VA Health Care System), Dr. James Herman (VA Pittsburgh Healthcare System), and Dr. Ryan Phan (formerly of the VA Greater Los Angeles Healthcare System) as well as the members of the Veterans Administration pathology and laboratory medicine service molecular genetics pathology workgroup.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies.
Dr. Kulich is the Acting Chief of Pathology and Laboratory Medicine Service at VA Pittsburgh Healthcare System and member of the Division of Neuropathology at University of Pittsburgh Department of Pathology, Dr. Duvvuri is an Otolaryngologist at VA Pittsburgh Healthcare System, and Dr. Passero is the Section Chief of Hematology\Oncology at VA Pittsburgh Healthcare System in Pennsylvania. Dr. Becker is an Oncologist at VA-New York Harbor Healthcare System. Dr. Dacic is a Pathologist at University of Pittsburgh Department of Pathology in Pennsylvania. Dr. Ehsan is Chief of Pathology and Laboratory Medicine Services at the South Texas Veterans Healthcare System in San Antonio. Dr. Gutkin is the former Chief of Pathology and Laboratory Medicine Service at VA Pittsburgh Healthcare System. Dr. Hou is a Pathologist at St. Louis VA Medical Center in Missouri. Dr. Icardi is the VA National Director of Pathology and Laboratory Medicine Services. Dr. Lyle is a Pathologist at Bay Pine Health Care System in Florida. Dr. Lynch is an Investigator at VA Salt Lake Health Care System Informatics and Computing Infrastructure. Dr. Montgomery is an Oncologist at VA Puget Sound Health Care System, in Seattle, Washington. Dr. Przygodzki is the Director of Genomic Medicine Implementation and Associate Director of Genomic Medicine for the VA. Dr. Colman is a Neuro-Oncologist at George E. Wahlen VA Medical Center and the Director of Medical Neuro-Oncology at the Huntsman Cancer Institute, Salt Lake City, Utah.
Correspondence: Dr. Kulich ([email protected])
1. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803-820.
2. Wang-Rodriguez J, Yunes A, Phan R, et al. The challenges of precision medicine and new advances in molecular diagnostic testing in hematolymphoid malignancies: impact on the VHA. Fed Pract. 2017;34(suppl 5):S38-S49.
3. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19(suppl 5):v1-v88.
4. Nikiforova MN, Wald AI, Melan MA, et al. Targeted next-generation sequencing panel (GlioSeq) provides comprehensive genetic profiling of central nervous system tumors. Neuro Oncol. 2016;18(3)379-387.
5. Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90(19):1473-1479.
6. van den Bent MJ, Erdem-Eraslan L, Idbaih A, et al. MGMT-STP27 methylation status as predictive marker for response to PCV in anaplastic oligodendrogliomas and oligoastrocytomas. A report from EORTC study 26951. Clin Cancer Res. 2013;19(19):5513-5522.
7. Stupp R, Hegi ME, Mason WP, et al; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459-466.
8. Malmstrom A, Gronberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916-926.
9. van den Bent MJ, Kros JM. Predictive and prognostic markers in neuro-oncology. J Neuropathol Exp Neurol. 2007;66(12):1074-1081.
10. Chen R, Smith-Cohn M, Cohen AL, Colman H. Glioma subclassifications and their clinical significance. Neurotherapeutics. 2017;14(2):284-297.
11. Jordan JT, Sanders AE, Armstrong T, et al. Quality improvement in neurology: neuro-oncology quality measurement set. Neurology. 2018;90(14):652-658.
12. Chen L, Voronovich Z, Clark K, et al. Predicting the likelihood of an isocitrate dehydrogenase 1 or 2 mutation in diagnoses of infiltrative glioma. Neuro Oncol. 2014;16(11):1478-1483.
13. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997-1003.
14. Wick W, Platten M, Meisner C, et al; NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707-715.
15. Horbinski C, Kofler J, Kelly LM, Murdoch GH, Nikiforova MN. Diagnostic use of IDH1/2 mutation analysis in routine clinical testing of formalin-fixed, paraffin-embedded glioma tissues. J Neuropathol Exp Neurol. 2009;68(12):1319-1325.
16. Camelo-Piragua S, Jansen M, Ganguly A, Kim JC, Louis DN, Nutt CL. Mutant IDH1-specific immunohistochemistry distinguishes diffuse astrocytoma from astrocytosis. Acta Neuropathol. 2010;119(4):509-511.
17. Horbinski C, Kofler J, Yeaney G, et al. Isocitrate dehydrogenase 1 analysis differentiates gangliogliomas from infiltrative gliomas. Brain Pathol. 2011;21(5):564-574.
18. Berghoff AS, Preusser M. BRAF alterations in brain tumours: molecular pathology and therapeutic opportunities. Curr Opin Neurol. 2014;27(6):689-696.
19. Korshunov A, Meyer J, Capper D, et al. Combined molecular analysis of BRAF and IDH1 distinguishes pilocytic astrocytoma from diffuse astrocytoma. Acta Neuropathol. 2009;118(3):401-405.
20. Fuller CE, Schmidt RE, Roth KA, et al. Clinical utility of fluorescence in situ hybridization (FISH) in morphologically ambiguous gliomas with hybrid oligodendroglial/astrocytic features. J Neuropathol Exp Neurol. 2003;62(11):1118-1128.
21. Horbinski C. Practical molecular diagnostics in neuropathology: making a tough job a little easier. Semin Diagn Pathol. 2010;27(2):105-113.
22. Fuller GN, Bigner SH. Amplified cellular oncogenes in neoplasms of the human central nervous system. Mutat Res. 1992;276(3):299-306.
23. Brennan CW, Verhaak RG, McKenna A, et al; TCGA Research Network. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462-477.
24. Aldape K, Zadeh G, Mansouri S, Reifenberger G, von Deimling A. Glioblastoma: pathology, molecular mechanisms and markers. Acta Neuropathol. 2015;129(6):829-848.
25. Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110(15):6021-6026.
26. Nikiforova MN, Hamilton RL. Molecular diagnostics of gliomas. Arch Pathol Lab Med. 2011;135(5):558-568.
1. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803-820.
2. Wang-Rodriguez J, Yunes A, Phan R, et al. The challenges of precision medicine and new advances in molecular diagnostic testing in hematolymphoid malignancies: impact on the VHA. Fed Pract. 2017;34(suppl 5):S38-S49.
3. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19(suppl 5):v1-v88.
4. Nikiforova MN, Wald AI, Melan MA, et al. Targeted next-generation sequencing panel (GlioSeq) provides comprehensive genetic profiling of central nervous system tumors. Neuro Oncol. 2016;18(3)379-387.
5. Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90(19):1473-1479.
6. van den Bent MJ, Erdem-Eraslan L, Idbaih A, et al. MGMT-STP27 methylation status as predictive marker for response to PCV in anaplastic oligodendrogliomas and oligoastrocytomas. A report from EORTC study 26951. Clin Cancer Res. 2013;19(19):5513-5522.
7. Stupp R, Hegi ME, Mason WP, et al; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459-466.
8. Malmstrom A, Gronberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916-926.
9. van den Bent MJ, Kros JM. Predictive and prognostic markers in neuro-oncology. J Neuropathol Exp Neurol. 2007;66(12):1074-1081.
10. Chen R, Smith-Cohn M, Cohen AL, Colman H. Glioma subclassifications and their clinical significance. Neurotherapeutics. 2017;14(2):284-297.
11. Jordan JT, Sanders AE, Armstrong T, et al. Quality improvement in neurology: neuro-oncology quality measurement set. Neurology. 2018;90(14):652-658.
12. Chen L, Voronovich Z, Clark K, et al. Predicting the likelihood of an isocitrate dehydrogenase 1 or 2 mutation in diagnoses of infiltrative glioma. Neuro Oncol. 2014;16(11):1478-1483.
13. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997-1003.
14. Wick W, Platten M, Meisner C, et al; NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707-715.
15. Horbinski C, Kofler J, Kelly LM, Murdoch GH, Nikiforova MN. Diagnostic use of IDH1/2 mutation analysis in routine clinical testing of formalin-fixed, paraffin-embedded glioma tissues. J Neuropathol Exp Neurol. 2009;68(12):1319-1325.
16. Camelo-Piragua S, Jansen M, Ganguly A, Kim JC, Louis DN, Nutt CL. Mutant IDH1-specific immunohistochemistry distinguishes diffuse astrocytoma from astrocytosis. Acta Neuropathol. 2010;119(4):509-511.
17. Horbinski C, Kofler J, Yeaney G, et al. Isocitrate dehydrogenase 1 analysis differentiates gangliogliomas from infiltrative gliomas. Brain Pathol. 2011;21(5):564-574.
18. Berghoff AS, Preusser M. BRAF alterations in brain tumours: molecular pathology and therapeutic opportunities. Curr Opin Neurol. 2014;27(6):689-696.
19. Korshunov A, Meyer J, Capper D, et al. Combined molecular analysis of BRAF and IDH1 distinguishes pilocytic astrocytoma from diffuse astrocytoma. Acta Neuropathol. 2009;118(3):401-405.
20. Fuller CE, Schmidt RE, Roth KA, et al. Clinical utility of fluorescence in situ hybridization (FISH) in morphologically ambiguous gliomas with hybrid oligodendroglial/astrocytic features. J Neuropathol Exp Neurol. 2003;62(11):1118-1128.
21. Horbinski C. Practical molecular diagnostics in neuropathology: making a tough job a little easier. Semin Diagn Pathol. 2010;27(2):105-113.
22. Fuller GN, Bigner SH. Amplified cellular oncogenes in neoplasms of the human central nervous system. Mutat Res. 1992;276(3):299-306.
23. Brennan CW, Verhaak RG, McKenna A, et al; TCGA Research Network. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462-477.
24. Aldape K, Zadeh G, Mansouri S, Reifenberger G, von Deimling A. Glioblastoma: pathology, molecular mechanisms and markers. Acta Neuropathol. 2015;129(6):829-848.
25. Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110(15):6021-6026.
26. Nikiforova MN, Hamilton RL. Molecular diagnostics of gliomas. Arch Pathol Lab Med. 2011;135(5):558-568.
Association of Nausea and Length of Stay with Carbohydrate Loading Prior to Total Joint Arthroplasty
From Stony Brook Medical Center, Stony Brook, NY (Dr. Blum), and NYU Winthrop Medical Center,
Abstract
- Background: Enhanced Recovery After Surgery (ERAS) is a multimodal, standardized approach to the surgical patient that incorporates evidenced-based interventions designed to achieve rapid recovery after surgery by minimizing the patient’s stress response. One aspect of ERAS, carbohydrate loading, has been shown in multiple randomized controlled trials to result in postoperative benefits in patients undergoing colorectal surgery, but there appears to be insufficient data to make definitive recommendations for or against carbohydrate loading in joint replacement patients.
- Objective: To evaluate postoperative nausea and length of stay (LOS) after a preoperative carbohydrate loading protocol was initiated for patients undergoing total joint replacement.
- Design: Retrospective chart review.
- Setting and participants: 100 patients who underwent either total knee or hip arthroplasty at Winthrop University Hospital, Mineola, NY, in the past 4 years and either had (n = 50) or had not received preoperative carbohydrate supplements (n = 50).
- Methods: Using the total joint database, the medical record was reviewed for the patient’s demographics, LOS, documentation of postoperative nausea, and number of doses of antiemetic medication given to the patient.
- Results: The mean LOS for the carbohydrate-loading group and non-carbohydrate group was 1.9 days and 2.6 days. respectively, a difference of 0.70 days (P < 0.0001). The carbohydrate-loaded group received a total of 13 doses of antiemetic medications and the non-carbohydrate group received 21 doses. The average number of antiemetic doses given to a patient postoperatively was 0.26 for the carbohydrate-loaded group and 0.42 for the non-carbohydrate-loaded group. The difference was 0.16 doses (P < 0.7815).
- Conclusion: The implementation of carbohydrate loading decreased LOS for joint replacement patients by approximately 1 day. Additionally, there was a trend towards decreased antiemetic use and fewer documented cases of postoperative nausea after carbohydrate loading.
Keywords: carbohydrate loading, ERAS, joint arthroplasty, length of stay, nausea.
Enhanced Recovery After Surgery (ERAS) is a multimodal, standardized approach to the surgical patient that incorporates evidenced-based interventions designed to achieve rapid recovery after surgery by minimizing the patient’s stress response.1-4 The ERAS protocols have been shown to reduce complications, decrease length of stay (LOS), and improve patient outcomes.3-7 The program was originally designed to facilitate recovery after colorectal operative procedures by maintaining preoperative organ function and reducing the postoperative stress response. This was done through a coordinated program of preoperative counseling, optimizing nutritional status, standardizing analgesic regimens, and early mobilization.3
The principles of an ERAS program with standardized pre- and postoperative protocols appear ideally suited for the total joint arthroplasty patient.1,3-5 Prior studies have demonstrated ERAS to be effective in facilitating decreased LOS, with no apparent increase in readmission rates or complications for both colorectal and joint arthroplasty patients.1-7 The protocols have also been shown to be cost-effective, with decreased incidence of postoperative complications, including thromboembolic disease and infections.3,4,6
An important tenet of ERAS protocols is optimizing the nutritional status of the patient prior to surgery.6 This includes avoidance of preoperative fasting in conjunction with carbohydrate loading. ERAS protocols instruct the patient to ingest a carbohydrate-rich beverage 2 hours prior to surgery. The concept of allowing a patient to eat prior to surgery is based on the preference for the patient to present for surgery in an anabolic rather than a catabolic state.2,3,11 Patients in an anabolic state undergo less postoperative protein and nitrogen losses, which appears to facilitate wound healing.2,6,11
There have been multiple randomized controlled trials demonstrating the postoperative benefits of carbohydrate loading prior to colorectal surgery.2,6
Another potential benefit of preoperative carbohydrate loading is a decrease in postoperative nausea.1,5,12-14 A decrease in nausea in theory would allow for earlier mobilization with physical therapy and potentially a shorter LOS. Hence, the goal of this study was to examine the impact of preoperative carbohydrate loading on postoperative nausea directly, as well as on LOS, at a single institution in the setting of an ERAS protocol.
Methods
We retrospectively reviewed the records of 100 patients who underwent total hip or total knee replacement between 2014 and 2018 at NYU Winthrop University Hospital, Mineola, NY. Fifty patients had received preoperative carbohydrate supplements and 50 patients had not. The remainder of the total joint protocol was identical for the 2 groups.
Protocol
All patients attended preoperative educational classes. For patients receiving carbohydrate loading, written and oral instructions were given for the patient to drink Ensure Clear followed by 8 ounces of water before going to bed the night before surgery. They were also instructed to drink the Ensure Pre-Surgery Drink 2 hours prior to their operative procedure. Patients with diabetes were instructed to drink the Ensure Glucerna Clear drink the night before surgery. No carbohydrate drink was given on the day of surgery until a finger-stick glucose level was performed upon arrival at the hospital. Spinal anesthesia was utilized in all patients, with adductor canal block supplementation for patients undergoing total knee replacement. Orders were written to have physical therapy evaluate the patients in the PACU to facilitate ambulation. Pre- and postoperative pain protocols were identical for the 2 groups.
Data Collection
A chart review was performed using the patients’ medical record numbers from the joint replacement database at our institution. Exemption was obtained for the project from our institution’s Institutional Review Board (IRB).
Analysis
Descriptive statistics (mean, standard deviation, and median for continuous variables; frequencies and percentages for categorical variables) were calculated separately by group. The 2 groups were compared using the chi-square test or Fisher’s exact test, as deemed appropriate, for categorical variables, the 2-sample t-test for age, and the Mann-Whitney test for LOS and number of antiemetic doses given. A result was considered statistically significant at the P < 0.05 level of significance. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
The carbohydrate-loading group (n = 50) and the non-carbohydrate-loading group (n = 50) were comparable for age, gender, type of arthroplasty, episodes of vomiting, diabetes, and nerve block (Table). 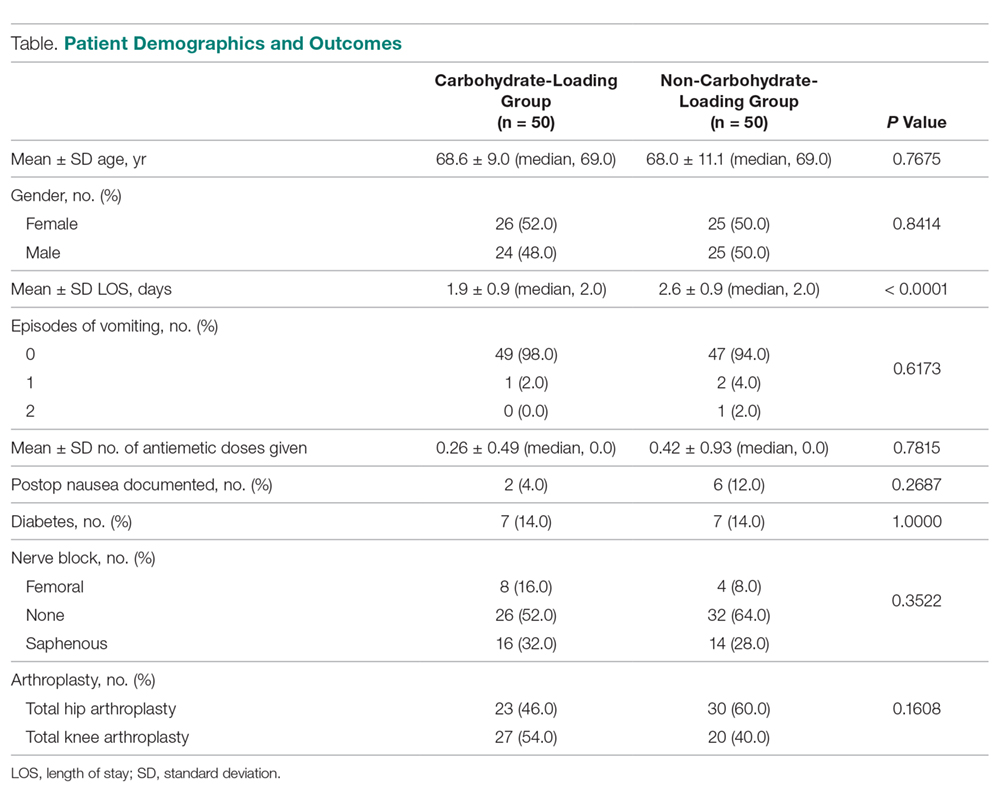
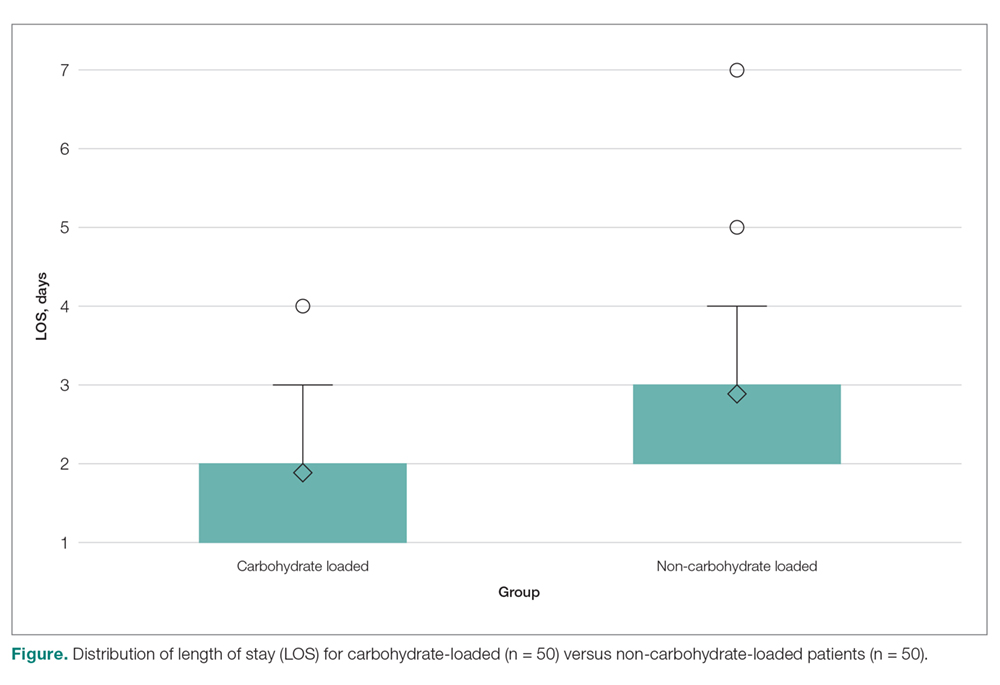
Discussion
In this study we explored whether carbohydrate loading prior to total joint replacement influenced postoperative nausea and LOS in a single institution. The 2 groups appeared similar in terms of demographics as well as the types of surgical procedures performed. After initiation of the carbohydrate-loading protocol, LOS decreased by approximately 1 day. There was also a trend toward decreased usage of antiemetics in the carbohydrate-loaded group, although the final values were not statistically significant. There were also fewer documented cases of postoperative nausea in the carbohydrate-loaded group.
The failure to find a statistical difference in postoperative antiemetic usage between carbohydrate-loaded and non-carbohydrate-loaded patients may be due to incomplete documentation (ie, not all patients who were nauseous having their symptoms documented in the chart). Due to the small number of antiemetic doses given to each patient, we may have lacked the necessary numbers to visualize the difference between the groups. We were unable to perform a post-hoc power calculation with our current data. Additionally, the decrease seen in LOS may not have been due solely to carbohydrate loading, since the data were collected over multiple years during implementation of the ERAS protocol. There is a possibility that the ERAS protocol, which is multimodal, was better implemented as time progressed, adding a confounding variable to our data. Despite these limitations, however, we were able to demonstrate a decreased LOS for patients who underwent total joint replacement with the initiation of a preoperative carbohydrate-loading ERAS protocol. Furthermore, there was a trend toward decreased documented postoperative nausea and decreased antiemetic use in the group that avoided fasting and received carbohydrate supplements.
This decrease in LOS by almost 1 day is consistent with multiple prior studies that demonstrated a similar decrease when implementing an ERAS protocol.3-5,7 The trend towards lower antiemetic use and less postoperative nausea in the carbohydrate-loading ERAS protocol gives merit to further research on this topic, with the goal of finding an optimal preoperative practice that allows patients to experience rapid mobilization, minimal postoperative nausea, and faster recovery overall.
Conclusion
Corresponding author: Christopher L. Blum, MD, Stony Brook Medical Center, Stony Brook, NY; [email protected].
Financial disclosures: None.
1. Proudfoot S, Bennett B, Duff S, Palmer J. Implementation and effects of Enhanced Recovery After Surgery for hip and knee replacements and fractured neck of femur in New Zealand orthopaedic services. N Z Med J. 2017;130:77-90.
2. Geltzeiler CB, Rotramel A, Wilson C, et al. Prospective study of colorectal enhanced recovery after surgery in a community hospital. JAMA Surg. 2014;149:955-961.
3. Soffin EM, YaDeau JT. Enhanced recovery after surgery for primary hip and knee arthroplasty: a review of the evidence. Br J Anaesth. 2016;117(suppl 3):iii62-iii72.
4. Stowers MD, Manuopangai L, Hill AG, et al. Enhanced Recovery After Surgery in elective hip and knee arthroplasty reduces length of hospital stay. ANZ J Surg. 2016;86:475-479.
5. Gwynne-Jones DP, Martin G, Crane C. Enhanced Recovery After Surgery for hip and knee replacements. Orthop Nurs. 2017;36:203-210.
6. Semerjian A, Milbar N, Kates M, et al. Hospital charges and length of stay following radical cystectomy in the enhanced recovery after surgery era. Urology. 2018;111:86-91.
7. Stambough JB, Nunley RM, Curry MC, et al. Rapid recovery protocols for primary total hip arthroplasty can safely reduce length of stay without increasing readmissions. J Arthroplasty. 2015;30:521-526.
8. Ljungqvist O, Soreide E. Preoperative fasting. Br J Surg. 2003;90:400-406.
9. Riis J, Lomholt B, Haxholdt O, et al. Immediate and long-term mental recovery from general versus epidural anesthesia in elderly patients. Acta Anaesthesiol Scand. 1983;27:44-49.
10. Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183:630-641.
11. Svanfeldt M, Thorell A, Hausel J, Soop M, et al. Randomized clinical trial of the effect of preoperative oral carbohydrate treatment on postoperative whole-body protein and glucose kinetics. Br J Surg. 2007;94:1342-1350.
12. Halaszynski TM, Juda R, Silverman DG. Optimizing postoperative outcomes with efficient preoperative assessment and management. Crit Care Med. 2004;32(4 suppl):S76-S86.
13. Aronsson A, Al-Ani NA, Brismar K, Hedstrom M. A carbohydrate-rich drink shortly before surgery affected IGF-I bioavailability after a total hip replacement. A double-blind placebo controlled study on 29 patients. Aging Clin Exp Res. 2009;21:97-101.
14. Bilku DK, Dennison AR, Hall TC, Metcalfe MS, Garcea G. Role of preoperative carbohydrate loading: a systematic review. Ann R Coll Surg Engl. 2014;96:15-22.
From Stony Brook Medical Center, Stony Brook, NY (Dr. Blum), and NYU Winthrop Medical Center,
Abstract
- Background: Enhanced Recovery After Surgery (ERAS) is a multimodal, standardized approach to the surgical patient that incorporates evidenced-based interventions designed to achieve rapid recovery after surgery by minimizing the patient’s stress response. One aspect of ERAS, carbohydrate loading, has been shown in multiple randomized controlled trials to result in postoperative benefits in patients undergoing colorectal surgery, but there appears to be insufficient data to make definitive recommendations for or against carbohydrate loading in joint replacement patients.
- Objective: To evaluate postoperative nausea and length of stay (LOS) after a preoperative carbohydrate loading protocol was initiated for patients undergoing total joint replacement.
- Design: Retrospective chart review.
- Setting and participants: 100 patients who underwent either total knee or hip arthroplasty at Winthrop University Hospital, Mineola, NY, in the past 4 years and either had (n = 50) or had not received preoperative carbohydrate supplements (n = 50).
- Methods: Using the total joint database, the medical record was reviewed for the patient’s demographics, LOS, documentation of postoperative nausea, and number of doses of antiemetic medication given to the patient.
- Results: The mean LOS for the carbohydrate-loading group and non-carbohydrate group was 1.9 days and 2.6 days. respectively, a difference of 0.70 days (P < 0.0001). The carbohydrate-loaded group received a total of 13 doses of antiemetic medications and the non-carbohydrate group received 21 doses. The average number of antiemetic doses given to a patient postoperatively was 0.26 for the carbohydrate-loaded group and 0.42 for the non-carbohydrate-loaded group. The difference was 0.16 doses (P < 0.7815).
- Conclusion: The implementation of carbohydrate loading decreased LOS for joint replacement patients by approximately 1 day. Additionally, there was a trend towards decreased antiemetic use and fewer documented cases of postoperative nausea after carbohydrate loading.
Keywords: carbohydrate loading, ERAS, joint arthroplasty, length of stay, nausea.
Enhanced Recovery After Surgery (ERAS) is a multimodal, standardized approach to the surgical patient that incorporates evidenced-based interventions designed to achieve rapid recovery after surgery by minimizing the patient’s stress response.1-4 The ERAS protocols have been shown to reduce complications, decrease length of stay (LOS), and improve patient outcomes.3-7 The program was originally designed to facilitate recovery after colorectal operative procedures by maintaining preoperative organ function and reducing the postoperative stress response. This was done through a coordinated program of preoperative counseling, optimizing nutritional status, standardizing analgesic regimens, and early mobilization.3
The principles of an ERAS program with standardized pre- and postoperative protocols appear ideally suited for the total joint arthroplasty patient.1,3-5 Prior studies have demonstrated ERAS to be effective in facilitating decreased LOS, with no apparent increase in readmission rates or complications for both colorectal and joint arthroplasty patients.1-7 The protocols have also been shown to be cost-effective, with decreased incidence of postoperative complications, including thromboembolic disease and infections.3,4,6
An important tenet of ERAS protocols is optimizing the nutritional status of the patient prior to surgery.6 This includes avoidance of preoperative fasting in conjunction with carbohydrate loading. ERAS protocols instruct the patient to ingest a carbohydrate-rich beverage 2 hours prior to surgery. The concept of allowing a patient to eat prior to surgery is based on the preference for the patient to present for surgery in an anabolic rather than a catabolic state.2,3,11 Patients in an anabolic state undergo less postoperative protein and nitrogen losses, which appears to facilitate wound healing.2,6,11
There have been multiple randomized controlled trials demonstrating the postoperative benefits of carbohydrate loading prior to colorectal surgery.2,6
Another potential benefit of preoperative carbohydrate loading is a decrease in postoperative nausea.1,5,12-14 A decrease in nausea in theory would allow for earlier mobilization with physical therapy and potentially a shorter LOS. Hence, the goal of this study was to examine the impact of preoperative carbohydrate loading on postoperative nausea directly, as well as on LOS, at a single institution in the setting of an ERAS protocol.
Methods
We retrospectively reviewed the records of 100 patients who underwent total hip or total knee replacement between 2014 and 2018 at NYU Winthrop University Hospital, Mineola, NY. Fifty patients had received preoperative carbohydrate supplements and 50 patients had not. The remainder of the total joint protocol was identical for the 2 groups.
Protocol
All patients attended preoperative educational classes. For patients receiving carbohydrate loading, written and oral instructions were given for the patient to drink Ensure Clear followed by 8 ounces of water before going to bed the night before surgery. They were also instructed to drink the Ensure Pre-Surgery Drink 2 hours prior to their operative procedure. Patients with diabetes were instructed to drink the Ensure Glucerna Clear drink the night before surgery. No carbohydrate drink was given on the day of surgery until a finger-stick glucose level was performed upon arrival at the hospital. Spinal anesthesia was utilized in all patients, with adductor canal block supplementation for patients undergoing total knee replacement. Orders were written to have physical therapy evaluate the patients in the PACU to facilitate ambulation. Pre- and postoperative pain protocols were identical for the 2 groups.
Data Collection
A chart review was performed using the patients’ medical record numbers from the joint replacement database at our institution. Exemption was obtained for the project from our institution’s Institutional Review Board (IRB).
Analysis
Descriptive statistics (mean, standard deviation, and median for continuous variables; frequencies and percentages for categorical variables) were calculated separately by group. The 2 groups were compared using the chi-square test or Fisher’s exact test, as deemed appropriate, for categorical variables, the 2-sample t-test for age, and the Mann-Whitney test for LOS and number of antiemetic doses given. A result was considered statistically significant at the P < 0.05 level of significance. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
The carbohydrate-loading group (n = 50) and the non-carbohydrate-loading group (n = 50) were comparable for age, gender, type of arthroplasty, episodes of vomiting, diabetes, and nerve block (Table). 

Discussion
In this study we explored whether carbohydrate loading prior to total joint replacement influenced postoperative nausea and LOS in a single institution. The 2 groups appeared similar in terms of demographics as well as the types of surgical procedures performed. After initiation of the carbohydrate-loading protocol, LOS decreased by approximately 1 day. There was also a trend toward decreased usage of antiemetics in the carbohydrate-loaded group, although the final values were not statistically significant. There were also fewer documented cases of postoperative nausea in the carbohydrate-loaded group.
The failure to find a statistical difference in postoperative antiemetic usage between carbohydrate-loaded and non-carbohydrate-loaded patients may be due to incomplete documentation (ie, not all patients who were nauseous having their symptoms documented in the chart). Due to the small number of antiemetic doses given to each patient, we may have lacked the necessary numbers to visualize the difference between the groups. We were unable to perform a post-hoc power calculation with our current data. Additionally, the decrease seen in LOS may not have been due solely to carbohydrate loading, since the data were collected over multiple years during implementation of the ERAS protocol. There is a possibility that the ERAS protocol, which is multimodal, was better implemented as time progressed, adding a confounding variable to our data. Despite these limitations, however, we were able to demonstrate a decreased LOS for patients who underwent total joint replacement with the initiation of a preoperative carbohydrate-loading ERAS protocol. Furthermore, there was a trend toward decreased documented postoperative nausea and decreased antiemetic use in the group that avoided fasting and received carbohydrate supplements.
This decrease in LOS by almost 1 day is consistent with multiple prior studies that demonstrated a similar decrease when implementing an ERAS protocol.3-5,7 The trend towards lower antiemetic use and less postoperative nausea in the carbohydrate-loading ERAS protocol gives merit to further research on this topic, with the goal of finding an optimal preoperative practice that allows patients to experience rapid mobilization, minimal postoperative nausea, and faster recovery overall.
Conclusion
Corresponding author: Christopher L. Blum, MD, Stony Brook Medical Center, Stony Brook, NY; [email protected].
Financial disclosures: None.
From Stony Brook Medical Center, Stony Brook, NY (Dr. Blum), and NYU Winthrop Medical Center,
Abstract
- Background: Enhanced Recovery After Surgery (ERAS) is a multimodal, standardized approach to the surgical patient that incorporates evidenced-based interventions designed to achieve rapid recovery after surgery by minimizing the patient’s stress response. One aspect of ERAS, carbohydrate loading, has been shown in multiple randomized controlled trials to result in postoperative benefits in patients undergoing colorectal surgery, but there appears to be insufficient data to make definitive recommendations for or against carbohydrate loading in joint replacement patients.
- Objective: To evaluate postoperative nausea and length of stay (LOS) after a preoperative carbohydrate loading protocol was initiated for patients undergoing total joint replacement.
- Design: Retrospective chart review.
- Setting and participants: 100 patients who underwent either total knee or hip arthroplasty at Winthrop University Hospital, Mineola, NY, in the past 4 years and either had (n = 50) or had not received preoperative carbohydrate supplements (n = 50).
- Methods: Using the total joint database, the medical record was reviewed for the patient’s demographics, LOS, documentation of postoperative nausea, and number of doses of antiemetic medication given to the patient.
- Results: The mean LOS for the carbohydrate-loading group and non-carbohydrate group was 1.9 days and 2.6 days. respectively, a difference of 0.70 days (P < 0.0001). The carbohydrate-loaded group received a total of 13 doses of antiemetic medications and the non-carbohydrate group received 21 doses. The average number of antiemetic doses given to a patient postoperatively was 0.26 for the carbohydrate-loaded group and 0.42 for the non-carbohydrate-loaded group. The difference was 0.16 doses (P < 0.7815).
- Conclusion: The implementation of carbohydrate loading decreased LOS for joint replacement patients by approximately 1 day. Additionally, there was a trend towards decreased antiemetic use and fewer documented cases of postoperative nausea after carbohydrate loading.
Keywords: carbohydrate loading, ERAS, joint arthroplasty, length of stay, nausea.
Enhanced Recovery After Surgery (ERAS) is a multimodal, standardized approach to the surgical patient that incorporates evidenced-based interventions designed to achieve rapid recovery after surgery by minimizing the patient’s stress response.1-4 The ERAS protocols have been shown to reduce complications, decrease length of stay (LOS), and improve patient outcomes.3-7 The program was originally designed to facilitate recovery after colorectal operative procedures by maintaining preoperative organ function and reducing the postoperative stress response. This was done through a coordinated program of preoperative counseling, optimizing nutritional status, standardizing analgesic regimens, and early mobilization.3
The principles of an ERAS program with standardized pre- and postoperative protocols appear ideally suited for the total joint arthroplasty patient.1,3-5 Prior studies have demonstrated ERAS to be effective in facilitating decreased LOS, with no apparent increase in readmission rates or complications for both colorectal and joint arthroplasty patients.1-7 The protocols have also been shown to be cost-effective, with decreased incidence of postoperative complications, including thromboembolic disease and infections.3,4,6
An important tenet of ERAS protocols is optimizing the nutritional status of the patient prior to surgery.6 This includes avoidance of preoperative fasting in conjunction with carbohydrate loading. ERAS protocols instruct the patient to ingest a carbohydrate-rich beverage 2 hours prior to surgery. The concept of allowing a patient to eat prior to surgery is based on the preference for the patient to present for surgery in an anabolic rather than a catabolic state.2,3,11 Patients in an anabolic state undergo less postoperative protein and nitrogen losses, which appears to facilitate wound healing.2,6,11
There have been multiple randomized controlled trials demonstrating the postoperative benefits of carbohydrate loading prior to colorectal surgery.2,6
Another potential benefit of preoperative carbohydrate loading is a decrease in postoperative nausea.1,5,12-14 A decrease in nausea in theory would allow for earlier mobilization with physical therapy and potentially a shorter LOS. Hence, the goal of this study was to examine the impact of preoperative carbohydrate loading on postoperative nausea directly, as well as on LOS, at a single institution in the setting of an ERAS protocol.
Methods
We retrospectively reviewed the records of 100 patients who underwent total hip or total knee replacement between 2014 and 2018 at NYU Winthrop University Hospital, Mineola, NY. Fifty patients had received preoperative carbohydrate supplements and 50 patients had not. The remainder of the total joint protocol was identical for the 2 groups.
Protocol
All patients attended preoperative educational classes. For patients receiving carbohydrate loading, written and oral instructions were given for the patient to drink Ensure Clear followed by 8 ounces of water before going to bed the night before surgery. They were also instructed to drink the Ensure Pre-Surgery Drink 2 hours prior to their operative procedure. Patients with diabetes were instructed to drink the Ensure Glucerna Clear drink the night before surgery. No carbohydrate drink was given on the day of surgery until a finger-stick glucose level was performed upon arrival at the hospital. Spinal anesthesia was utilized in all patients, with adductor canal block supplementation for patients undergoing total knee replacement. Orders were written to have physical therapy evaluate the patients in the PACU to facilitate ambulation. Pre- and postoperative pain protocols were identical for the 2 groups.
Data Collection
A chart review was performed using the patients’ medical record numbers from the joint replacement database at our institution. Exemption was obtained for the project from our institution’s Institutional Review Board (IRB).
Analysis
Descriptive statistics (mean, standard deviation, and median for continuous variables; frequencies and percentages for categorical variables) were calculated separately by group. The 2 groups were compared using the chi-square test or Fisher’s exact test, as deemed appropriate, for categorical variables, the 2-sample t-test for age, and the Mann-Whitney test for LOS and number of antiemetic doses given. A result was considered statistically significant at the P < 0.05 level of significance. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
The carbohydrate-loading group (n = 50) and the non-carbohydrate-loading group (n = 50) were comparable for age, gender, type of arthroplasty, episodes of vomiting, diabetes, and nerve block (Table). 

Discussion
In this study we explored whether carbohydrate loading prior to total joint replacement influenced postoperative nausea and LOS in a single institution. The 2 groups appeared similar in terms of demographics as well as the types of surgical procedures performed. After initiation of the carbohydrate-loading protocol, LOS decreased by approximately 1 day. There was also a trend toward decreased usage of antiemetics in the carbohydrate-loaded group, although the final values were not statistically significant. There were also fewer documented cases of postoperative nausea in the carbohydrate-loaded group.
The failure to find a statistical difference in postoperative antiemetic usage between carbohydrate-loaded and non-carbohydrate-loaded patients may be due to incomplete documentation (ie, not all patients who were nauseous having their symptoms documented in the chart). Due to the small number of antiemetic doses given to each patient, we may have lacked the necessary numbers to visualize the difference between the groups. We were unable to perform a post-hoc power calculation with our current data. Additionally, the decrease seen in LOS may not have been due solely to carbohydrate loading, since the data were collected over multiple years during implementation of the ERAS protocol. There is a possibility that the ERAS protocol, which is multimodal, was better implemented as time progressed, adding a confounding variable to our data. Despite these limitations, however, we were able to demonstrate a decreased LOS for patients who underwent total joint replacement with the initiation of a preoperative carbohydrate-loading ERAS protocol. Furthermore, there was a trend toward decreased documented postoperative nausea and decreased antiemetic use in the group that avoided fasting and received carbohydrate supplements.
This decrease in LOS by almost 1 day is consistent with multiple prior studies that demonstrated a similar decrease when implementing an ERAS protocol.3-5,7 The trend towards lower antiemetic use and less postoperative nausea in the carbohydrate-loading ERAS protocol gives merit to further research on this topic, with the goal of finding an optimal preoperative practice that allows patients to experience rapid mobilization, minimal postoperative nausea, and faster recovery overall.
Conclusion
Corresponding author: Christopher L. Blum, MD, Stony Brook Medical Center, Stony Brook, NY; [email protected].
Financial disclosures: None.
1. Proudfoot S, Bennett B, Duff S, Palmer J. Implementation and effects of Enhanced Recovery After Surgery for hip and knee replacements and fractured neck of femur in New Zealand orthopaedic services. N Z Med J. 2017;130:77-90.
2. Geltzeiler CB, Rotramel A, Wilson C, et al. Prospective study of colorectal enhanced recovery after surgery in a community hospital. JAMA Surg. 2014;149:955-961.
3. Soffin EM, YaDeau JT. Enhanced recovery after surgery for primary hip and knee arthroplasty: a review of the evidence. Br J Anaesth. 2016;117(suppl 3):iii62-iii72.
4. Stowers MD, Manuopangai L, Hill AG, et al. Enhanced Recovery After Surgery in elective hip and knee arthroplasty reduces length of hospital stay. ANZ J Surg. 2016;86:475-479.
5. Gwynne-Jones DP, Martin G, Crane C. Enhanced Recovery After Surgery for hip and knee replacements. Orthop Nurs. 2017;36:203-210.
6. Semerjian A, Milbar N, Kates M, et al. Hospital charges and length of stay following radical cystectomy in the enhanced recovery after surgery era. Urology. 2018;111:86-91.
7. Stambough JB, Nunley RM, Curry MC, et al. Rapid recovery protocols for primary total hip arthroplasty can safely reduce length of stay without increasing readmissions. J Arthroplasty. 2015;30:521-526.
8. Ljungqvist O, Soreide E. Preoperative fasting. Br J Surg. 2003;90:400-406.
9. Riis J, Lomholt B, Haxholdt O, et al. Immediate and long-term mental recovery from general versus epidural anesthesia in elderly patients. Acta Anaesthesiol Scand. 1983;27:44-49.
10. Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183:630-641.
11. Svanfeldt M, Thorell A, Hausel J, Soop M, et al. Randomized clinical trial of the effect of preoperative oral carbohydrate treatment on postoperative whole-body protein and glucose kinetics. Br J Surg. 2007;94:1342-1350.
12. Halaszynski TM, Juda R, Silverman DG. Optimizing postoperative outcomes with efficient preoperative assessment and management. Crit Care Med. 2004;32(4 suppl):S76-S86.
13. Aronsson A, Al-Ani NA, Brismar K, Hedstrom M. A carbohydrate-rich drink shortly before surgery affected IGF-I bioavailability after a total hip replacement. A double-blind placebo controlled study on 29 patients. Aging Clin Exp Res. 2009;21:97-101.
14. Bilku DK, Dennison AR, Hall TC, Metcalfe MS, Garcea G. Role of preoperative carbohydrate loading: a systematic review. Ann R Coll Surg Engl. 2014;96:15-22.
1. Proudfoot S, Bennett B, Duff S, Palmer J. Implementation and effects of Enhanced Recovery After Surgery for hip and knee replacements and fractured neck of femur in New Zealand orthopaedic services. N Z Med J. 2017;130:77-90.
2. Geltzeiler CB, Rotramel A, Wilson C, et al. Prospective study of colorectal enhanced recovery after surgery in a community hospital. JAMA Surg. 2014;149:955-961.
3. Soffin EM, YaDeau JT. Enhanced recovery after surgery for primary hip and knee arthroplasty: a review of the evidence. Br J Anaesth. 2016;117(suppl 3):iii62-iii72.
4. Stowers MD, Manuopangai L, Hill AG, et al. Enhanced Recovery After Surgery in elective hip and knee arthroplasty reduces length of hospital stay. ANZ J Surg. 2016;86:475-479.
5. Gwynne-Jones DP, Martin G, Crane C. Enhanced Recovery After Surgery for hip and knee replacements. Orthop Nurs. 2017;36:203-210.
6. Semerjian A, Milbar N, Kates M, et al. Hospital charges and length of stay following radical cystectomy in the enhanced recovery after surgery era. Urology. 2018;111:86-91.
7. Stambough JB, Nunley RM, Curry MC, et al. Rapid recovery protocols for primary total hip arthroplasty can safely reduce length of stay without increasing readmissions. J Arthroplasty. 2015;30:521-526.
8. Ljungqvist O, Soreide E. Preoperative fasting. Br J Surg. 2003;90:400-406.
9. Riis J, Lomholt B, Haxholdt O, et al. Immediate and long-term mental recovery from general versus epidural anesthesia in elderly patients. Acta Anaesthesiol Scand. 1983;27:44-49.
10. Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183:630-641.
11. Svanfeldt M, Thorell A, Hausel J, Soop M, et al. Randomized clinical trial of the effect of preoperative oral carbohydrate treatment on postoperative whole-body protein and glucose kinetics. Br J Surg. 2007;94:1342-1350.
12. Halaszynski TM, Juda R, Silverman DG. Optimizing postoperative outcomes with efficient preoperative assessment and management. Crit Care Med. 2004;32(4 suppl):S76-S86.
13. Aronsson A, Al-Ani NA, Brismar K, Hedstrom M. A carbohydrate-rich drink shortly before surgery affected IGF-I bioavailability after a total hip replacement. A double-blind placebo controlled study on 29 patients. Aging Clin Exp Res. 2009;21:97-101.
14. Bilku DK, Dennison AR, Hall TC, Metcalfe MS, Garcea G. Role of preoperative carbohydrate loading: a systematic review. Ann R Coll Surg Engl. 2014;96:15-22.
The benefits of a standardized approach to opioid prescribing
ABSTRACT
Purpose The “opioid epidemic” in the United States has received increasing attention over the past few years. Most drug overdose deaths involve an opioid, and prescription opioid deaths have quadrupled since 1999. We sought to improve patient safety and adhere to clinical guidelines by standardizing opioid prescribing in our practice.
Methods We implemented a standardized approach to opioid prescribing based on Arizona Department of Health Services guidelines. All of our providers received instruction on Arizona’s Controlled Substance Prescription Monitoring Program (AZCSPMP) database and were encouraged to use it online. Our goal was for patients to have quarterly office visits, complete random urine drug screens, and sign a controlled substance agreement (CSA). The CSA acknowledged their understanding of the risks and benefits of opioid therapy as well as our updated prescribing policies.
Results Three-hundred fifty-eight of our practice’s patients were receiving chronic opioid therapy. All providers enrolled in AZCSPMP and used it for patient care. We increased rates of signed CSAs from 4.5% to 43.6%, and urine drug screening from 0.8% to 20.1%. For 325 patients remaining in the practice after our interventions, a postintervention chart review demonstrated a statistically significant discontinuation of opioid therapy (71/325, 21.8%; 95% confidence interval, 17.4%-26.7%).
Conclusion Implementation of a standardized opioid prescribing process resulted in discontinuation of therapy for some patients. Rates increased for signed CSAs and completed random urine drug screening. Future process interventions may improve patient and provider adherence. All primary care physicians should examine their prescribing processes to enhance the safety of opioid therapy.
[polldaddy:10370177]
The US opioid epidemic has received increased attention both nationally and at the state level over the past 2 years. This attention is warranted given the significant societal burden of opioid misuse, abuse, and overdose. Most drug overdose deaths (> 6/10) involve an opioid.1 Deaths from prescription opioids have quadrupled since 1999 in the United States.2 Arizona, the state in which we practice, ranked sixth highest in the nation for drug overdose deaths and had the fifth highest opioid prescribing rate in 2011.3 In response to the growing epidemic, the Centers for Disease Control and Prevention (CDC) released guidelines in 2016 for prescribing and monitoring opioids for chronic pain.4
Chronic nonterminal pain (CNTP) remains a significant cause of human suffering and is more prevalent in the United States than cancer, diabetes, and heart disease combined.5 The increased use of opioids since 1999 to ease CNTP has not reduced Americans’ reports of pain overall.6,7 Given the growing opioid epidemic and disease burden of CNTP, we embarked on a quality improvement (QI) project to safely prescribe and refill opioid medications in the Department of Family Medicine at the Mayo Clinic Arizona.
METHODS
This project received an exemption from internal review board evaluation as a QI intervention. We used a team-based approach to address standardization of opioid prescribing and monitoring within our practice. The team included physicians (MD/DO), nurses (LPN/RN), and allied health staff (MA), operations and administrative personnel, and information technology (IT) support. We did not involve patients in the initial design of our project. With future quality efforts in this area, we plan to involve patients in design processes.
Continue to: We began by identifying...
We began by identifying the scope of the problem, establishing criteria to search the electronic medical record (EMR) and identify appropriate patients. Chronic pain is often defined as pain lasting more than 3 months. Chronic opioid therapy (COT) has been defined as opioid use lasting longer than 3 months.8 Working with our IT colleagues, we defined COT patients as those with 3 or more prescriptions for opioids in the past year or those who received ≥ 30 pills a month (ie, patients who received 180 pills with 2 prescriptions written for the year). This definition gave us the ability to query our EMR to determine which patients were on COT, and we prepared lists of patients by primary care provider (FIGURE). Providers reviewed the lists to ensure these individuals were in fact on COT for CNTP. The number of patients identified after EMR query and provider review was 358, comprising 2.6% of 14,000 empaneled patients.
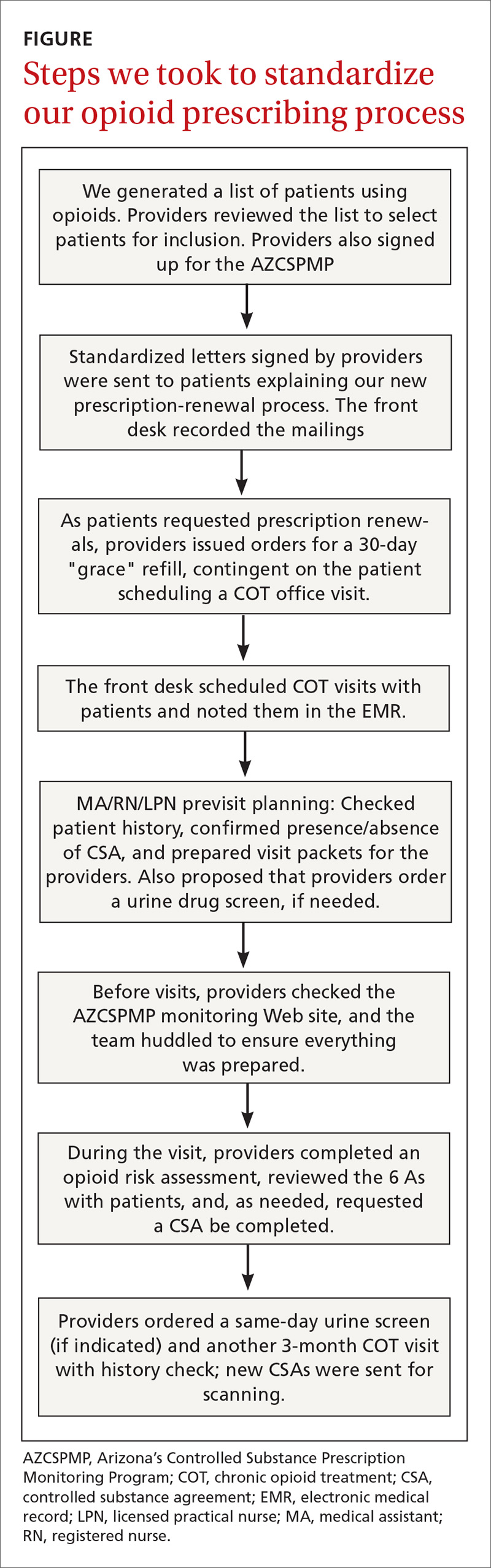
We based our interventions on the Arizona Department of Health Services 2014 opioid prescribing guidelines.3 The Arizona guidelines used existing national and state opioid prescribing guidelines along with clinical practice guidelines. Our study began prior to the 2016 CDC guidelines, so they were not used in this study. Our practice guidelines recommended that all 23 of our providers (MDs, DOs, and NPs) sign up for Arizona’s Controlled Substance Prescription Monitoring Program (AZCSPMP). We asked each patient to sign a controlled substance agreement (CSA), acknowledging their awareness of our proposed processes and the discussion of opioid therapy. Patients were expected to have face-to-face visits with providers at least quarterly and to complete a random urine drug screen at least annually. Patients were not incentivized to complete the process. We placed reminder calls for appointments just as we do for regular appointments.
Providers were asked to complete the Opioid Risk Tool9 with the patient at the initial visit, discuss the risks, benefits, and alternatives of long-term use of opioid medication, and review the 6 As (analgesia, activity, aberrant drug related behavior, adverse effects, affect, and adjunctive treatments). On the day before each patient visit, providers were reminded by a note in the EMR schedule to check AZCSPMP. Initial appointment times would be 30 minutes and follow-up appointments would be scheduled for 15 minutes if only addressing COT.
The QI project was introduced at an all-staff meeting in October 2015 that included providers, allied health staff, front desk personnel, and administrative staff, with the goal of beginning our COT process in November. We mailed letters to COT patients describing our new guidelines and asking them to call to schedule an appointment. If patients on COT came into the office for an alternate appointment and had not yet been seen for a COT visit, providers were encouraged to complete the COT process at that time.
We created a standard order set in the EMR for initial and follow-up visits and for the urine drug screen. We also added an interactive form to the EMR allowing providers to electronically complete the Opioid Risk Tool, and to confirm CSA completion and AZCSPMP review. We developed a database that would query the EMR for patient office visit frequency, CSA completion, and urine drug screen collection. We also placed paper copies of forms in exam rooms with a laminated instruction sheet reviewing the process steps and the 6 As.
Continue to: Soft rollout was...
Soft rollout was November 1, 2015, to assist in working through the process before full rollout. We asked providers to complete the full process on at least 1 patient during this period. This run-through would help ensure that allied health staff who room the patients would have the CSA and Opioid Risk Tool already in the chart before the visit. Full rollout was January 2, 2016. Every 2 to 4 weeks after the full rollout, regular email reminders were sent to providers about the project process and allowed for any feedback about issues that arose.
We provided regular updates and discussed the process at department meetings monthly. Quarterly data were reviewed and discussed for the first year of implementation. Providers and staff completed a chart review for each COT patient at project completion, to determine whether opioids had been decreased (in dosage) or discontinued, a nonopioid medicine had been initiated to augment pain control, or whether patients had died or left the practice.
Statistical analysis
We summarized binary data as counts and proportions and compared them using the chi square test. We summarized discrete data by their mean and standard deviation. To analyze binary variables measured repeatedly in time, we used the logistic generalized estimating equation (GEE) with an autoregressive (AR-1) correlation structure. We computed 95% confidence intervals (CIs)for odds ratios using the empirical or “sandwich” standard error estimates. For discrete variables representing counts, we used the negative binomial regression model.
For count data, a Poisson model is typically used; in our case the variance was considerably larger than the mean, exceeding the Poisson-model requirement that they not be significantly different if not exactly the same. This implies that the data are “over dispersed” or more variable than a Poisson model is thought to be able to model accurately. We therefore used a negative binomial model, which is regarded as the better model in this situation. The 95% CIs for the estimate resulting from the negative binomial regression model were computed using the profile-likelihood.10 All GEEs were clustered on patients (n = 358). We used SAS version 9.3 (Cary, NC) for all analyses.

RESULTS
All providers enrolled for AZCSPMP. CSA completion increased from 16 (4.5%) at baseline to 156 (43.6%) after intervention (P < .001). Patients completed a urine drug screen more frequently as well, from 3 (0.8%) to 72 (20.1%) (P < .001) (TABLES 1 and 2). No statistically significant change was noted in the frequency of office visits.

Continue to: We excluded 33 patients...
We excluded 33 patients from the post-intervention chart review (TABLE 3). Twenty-seven had left the practice and 6 had died, leaving 325 patients included in the post-intervention chart review.
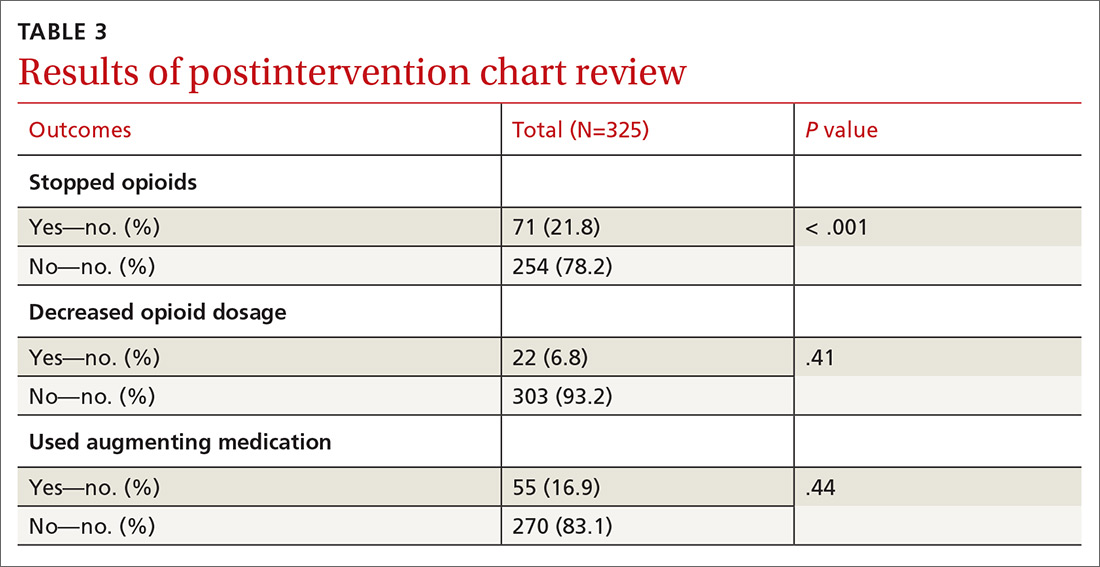
There was a statistically significant association between patients who discontinued opioids and those who neglected to sign a CSA (P < .001) (TABLE 4). We tested for associations between office visit frequency and process step completion. There was a nonsignificant trend between increased frequency of office visits and opioid dose reduction. Patients who stopped opioids had fewer office visits (TABLE 5), while patients who had initiated a medication to augment pain relief had more frequent office visits (TABLE 6).
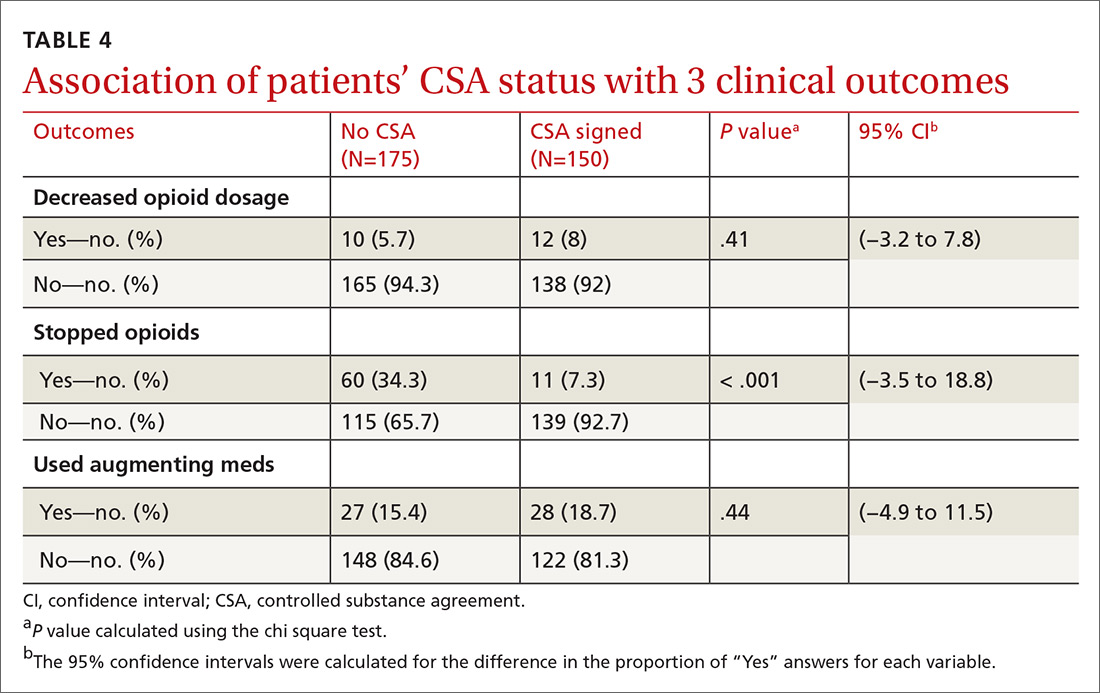
DISCUSSION
Our interventions to improve the quality of our COT processes were moderately successful. We achieved statistically significant increases in our rates of CSA completion and in urine drug screening. However, these increases were not as clinically impactful as we had hoped. Improvements in both patient and provider adherence are needed. We plan to engage allied health staff more fully to assist with adherence and thereby improve quality. This study was not intended to obtain patient-oriented outcomes, such as decreased pain and improved function. The study was designed to improve patient safety and to standardize a process for prescribing and monitoring patients on COT. In the future we plan to look at patient outcomes and expand our focus to patients on high-dose opioids and those on combination therapy with benzodiazepines.
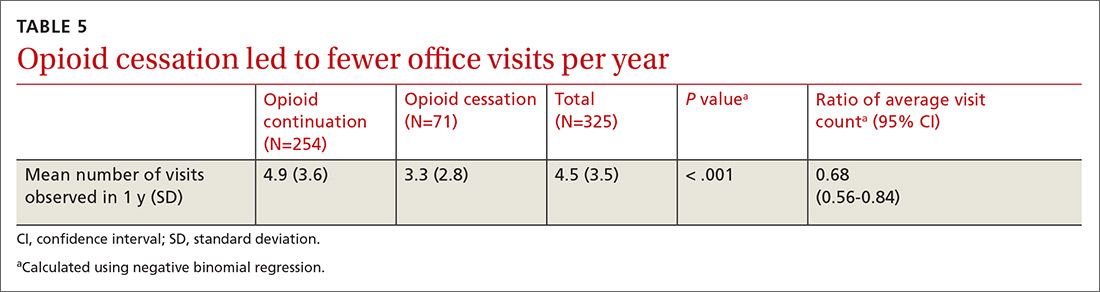
We believe the most impactful process steps were our letters sent to COT patients describing our updated, standardized prescribing process, and the ensuing provider-patient discussion to review the risks, benefits, and alternatives to opioid therapy. This frank discussion of treatment options resulted in more than 1 in 5 patients electing to discontinue COT.
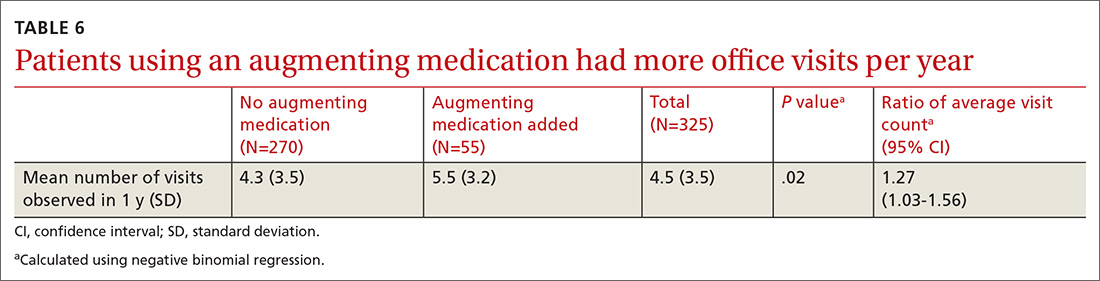
There was an association between opioid discontinuation and patients not signing the CSA. This may have been due to patients deciding to discontinue opioids at the initiation review with providers after they received their letter. Therefore, signing the agreement was no longer necessary.
Continue to: We noted that some patients...
We noted that some patients elected to begin a new, nonopioid medication intended to augment their pain relief. However, they did not decrease their use of opioid medicines. We did not collect pain rating scale scores to determine whether the addition of augmenting medicines provided a reduction in pain perception.
Close monitoring of COT patients with frequent office visits may have had an impact on their care. We noted an association between more frequent visits and initiation of pain augmentation medicines. There was also a nonsignificant trend between office visit frequency and dose reduction. These are topics we may re-examine in our practice over time. There was no change in office visit frequency with our intervention, likely a result of these patients having frequent office visits for multiple comorbid medical conditions at baseline.
Evidence of similar benefits in primary care practices that standardized their opioid prescribing guidelines for patients on COT11 illustrates the importance of such a process for ensuring patient safety and decreasing opioid dosage and use.
Limitations to our project are that we did not measure functional changes and quality-of-life scores for patients. We also did not note the opioid dosages for individuals who chose to stop using opioids.
Looking forward. Based on our experience, patient notification with discussion of COT risks, benefits, and alternatives, as well as implementation of a process to monitor COT, appear to be related to patients’ decisions to discontinue COT. Our new standard process did show QI in the process steps but remained suboptimal to our expectations of clinical impact. More frequent office visits may impact patient decisions to reduce opioid dose and to add an augmenting pain medication. We plan to increase the involvement and responsibilities of our allied health staff in our processes to improve rates of adherence and the overall quality of how we manage patients on chronic opioid therapy.
CORRESPONDENCE
David Patchett, DO, Mayo Clinic, 13400 East Shea Blvd, Scottsdale, AZ 85259; [email protected]
1. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths — United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1445-1452.
2. CDC. Opioid data analysis and resources. https://www.cdc.gov/drugoverdose/data/analysis.html. Published December 19, 2018. Accessed May 27, 2019.
3. Arizona Department of Health Services. Arizona opioid prescribing guidelines. https://www.azdhs.gov/documents/audiences/clinicians/clinical-guidelines-recommendations/prescribing-guidelines/az-opiod-prescribing-guidelines.pdf. Published November 2014. Accessed May 27, 2019.
4. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
5. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
6. Chang H, Daubresse M, Kruszewski S, et al. Prevalence and treatment of pain in EDs in the United States, 2000 to 2010. Am J Emerg Med. 2014;32:421-431.
7. Daubresse M, Chang H, Yu Y, et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000 - 2010. Med Care. 2013;51:870-878.
8. Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162:276-286.
9. Webster LR, Webster RM. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432-442.
10. Hilbe JM. Negative Binomial Regression. Cambridge, United Kingdom: Cambridge University Press; 2013.
11. Liebschutz JM, Xuan Z, Shanahan CW, et al. Improving adherence to long-term opioid therapy guidelines to reduce opioid misuse in primary care: a cluster-randomized clinical trial. JAMA Intern Med. 2017;177:1265-1272.
ABSTRACT
Purpose The “opioid epidemic” in the United States has received increasing attention over the past few years. Most drug overdose deaths involve an opioid, and prescription opioid deaths have quadrupled since 1999. We sought to improve patient safety and adhere to clinical guidelines by standardizing opioid prescribing in our practice.
Methods We implemented a standardized approach to opioid prescribing based on Arizona Department of Health Services guidelines. All of our providers received instruction on Arizona’s Controlled Substance Prescription Monitoring Program (AZCSPMP) database and were encouraged to use it online. Our goal was for patients to have quarterly office visits, complete random urine drug screens, and sign a controlled substance agreement (CSA). The CSA acknowledged their understanding of the risks and benefits of opioid therapy as well as our updated prescribing policies.
Results Three-hundred fifty-eight of our practice’s patients were receiving chronic opioid therapy. All providers enrolled in AZCSPMP and used it for patient care. We increased rates of signed CSAs from 4.5% to 43.6%, and urine drug screening from 0.8% to 20.1%. For 325 patients remaining in the practice after our interventions, a postintervention chart review demonstrated a statistically significant discontinuation of opioid therapy (71/325, 21.8%; 95% confidence interval, 17.4%-26.7%).
Conclusion Implementation of a standardized opioid prescribing process resulted in discontinuation of therapy for some patients. Rates increased for signed CSAs and completed random urine drug screening. Future process interventions may improve patient and provider adherence. All primary care physicians should examine their prescribing processes to enhance the safety of opioid therapy.
[polldaddy:10370177]
The US opioid epidemic has received increased attention both nationally and at the state level over the past 2 years. This attention is warranted given the significant societal burden of opioid misuse, abuse, and overdose. Most drug overdose deaths (> 6/10) involve an opioid.1 Deaths from prescription opioids have quadrupled since 1999 in the United States.2 Arizona, the state in which we practice, ranked sixth highest in the nation for drug overdose deaths and had the fifth highest opioid prescribing rate in 2011.3 In response to the growing epidemic, the Centers for Disease Control and Prevention (CDC) released guidelines in 2016 for prescribing and monitoring opioids for chronic pain.4
Chronic nonterminal pain (CNTP) remains a significant cause of human suffering and is more prevalent in the United States than cancer, diabetes, and heart disease combined.5 The increased use of opioids since 1999 to ease CNTP has not reduced Americans’ reports of pain overall.6,7 Given the growing opioid epidemic and disease burden of CNTP, we embarked on a quality improvement (QI) project to safely prescribe and refill opioid medications in the Department of Family Medicine at the Mayo Clinic Arizona.
METHODS
This project received an exemption from internal review board evaluation as a QI intervention. We used a team-based approach to address standardization of opioid prescribing and monitoring within our practice. The team included physicians (MD/DO), nurses (LPN/RN), and allied health staff (MA), operations and administrative personnel, and information technology (IT) support. We did not involve patients in the initial design of our project. With future quality efforts in this area, we plan to involve patients in design processes.
Continue to: We began by identifying...
We began by identifying the scope of the problem, establishing criteria to search the electronic medical record (EMR) and identify appropriate patients. Chronic pain is often defined as pain lasting more than 3 months. Chronic opioid therapy (COT) has been defined as opioid use lasting longer than 3 months.8 Working with our IT colleagues, we defined COT patients as those with 3 or more prescriptions for opioids in the past year or those who received ≥ 30 pills a month (ie, patients who received 180 pills with 2 prescriptions written for the year). This definition gave us the ability to query our EMR to determine which patients were on COT, and we prepared lists of patients by primary care provider (FIGURE). Providers reviewed the lists to ensure these individuals were in fact on COT for CNTP. The number of patients identified after EMR query and provider review was 358, comprising 2.6% of 14,000 empaneled patients.

We based our interventions on the Arizona Department of Health Services 2014 opioid prescribing guidelines.3 The Arizona guidelines used existing national and state opioid prescribing guidelines along with clinical practice guidelines. Our study began prior to the 2016 CDC guidelines, so they were not used in this study. Our practice guidelines recommended that all 23 of our providers (MDs, DOs, and NPs) sign up for Arizona’s Controlled Substance Prescription Monitoring Program (AZCSPMP). We asked each patient to sign a controlled substance agreement (CSA), acknowledging their awareness of our proposed processes and the discussion of opioid therapy. Patients were expected to have face-to-face visits with providers at least quarterly and to complete a random urine drug screen at least annually. Patients were not incentivized to complete the process. We placed reminder calls for appointments just as we do for regular appointments.
Providers were asked to complete the Opioid Risk Tool9 with the patient at the initial visit, discuss the risks, benefits, and alternatives of long-term use of opioid medication, and review the 6 As (analgesia, activity, aberrant drug related behavior, adverse effects, affect, and adjunctive treatments). On the day before each patient visit, providers were reminded by a note in the EMR schedule to check AZCSPMP. Initial appointment times would be 30 minutes and follow-up appointments would be scheduled for 15 minutes if only addressing COT.
The QI project was introduced at an all-staff meeting in October 2015 that included providers, allied health staff, front desk personnel, and administrative staff, with the goal of beginning our COT process in November. We mailed letters to COT patients describing our new guidelines and asking them to call to schedule an appointment. If patients on COT came into the office for an alternate appointment and had not yet been seen for a COT visit, providers were encouraged to complete the COT process at that time.
We created a standard order set in the EMR for initial and follow-up visits and for the urine drug screen. We also added an interactive form to the EMR allowing providers to electronically complete the Opioid Risk Tool, and to confirm CSA completion and AZCSPMP review. We developed a database that would query the EMR for patient office visit frequency, CSA completion, and urine drug screen collection. We also placed paper copies of forms in exam rooms with a laminated instruction sheet reviewing the process steps and the 6 As.
Continue to: Soft rollout was...
Soft rollout was November 1, 2015, to assist in working through the process before full rollout. We asked providers to complete the full process on at least 1 patient during this period. This run-through would help ensure that allied health staff who room the patients would have the CSA and Opioid Risk Tool already in the chart before the visit. Full rollout was January 2, 2016. Every 2 to 4 weeks after the full rollout, regular email reminders were sent to providers about the project process and allowed for any feedback about issues that arose.
We provided regular updates and discussed the process at department meetings monthly. Quarterly data were reviewed and discussed for the first year of implementation. Providers and staff completed a chart review for each COT patient at project completion, to determine whether opioids had been decreased (in dosage) or discontinued, a nonopioid medicine had been initiated to augment pain control, or whether patients had died or left the practice.
Statistical analysis
We summarized binary data as counts and proportions and compared them using the chi square test. We summarized discrete data by their mean and standard deviation. To analyze binary variables measured repeatedly in time, we used the logistic generalized estimating equation (GEE) with an autoregressive (AR-1) correlation structure. We computed 95% confidence intervals (CIs)for odds ratios using the empirical or “sandwich” standard error estimates. For discrete variables representing counts, we used the negative binomial regression model.
For count data, a Poisson model is typically used; in our case the variance was considerably larger than the mean, exceeding the Poisson-model requirement that they not be significantly different if not exactly the same. This implies that the data are “over dispersed” or more variable than a Poisson model is thought to be able to model accurately. We therefore used a negative binomial model, which is regarded as the better model in this situation. The 95% CIs for the estimate resulting from the negative binomial regression model were computed using the profile-likelihood.10 All GEEs were clustered on patients (n = 358). We used SAS version 9.3 (Cary, NC) for all analyses.

RESULTS
All providers enrolled for AZCSPMP. CSA completion increased from 16 (4.5%) at baseline to 156 (43.6%) after intervention (P < .001). Patients completed a urine drug screen more frequently as well, from 3 (0.8%) to 72 (20.1%) (P < .001) (TABLES 1 and 2). No statistically significant change was noted in the frequency of office visits.

Continue to: We excluded 33 patients...
We excluded 33 patients from the post-intervention chart review (TABLE 3). Twenty-seven had left the practice and 6 had died, leaving 325 patients included in the post-intervention chart review.

There was a statistically significant association between patients who discontinued opioids and those who neglected to sign a CSA (P < .001) (TABLE 4). We tested for associations between office visit frequency and process step completion. There was a nonsignificant trend between increased frequency of office visits and opioid dose reduction. Patients who stopped opioids had fewer office visits (TABLE 5), while patients who had initiated a medication to augment pain relief had more frequent office visits (TABLE 6).

DISCUSSION
Our interventions to improve the quality of our COT processes were moderately successful. We achieved statistically significant increases in our rates of CSA completion and in urine drug screening. However, these increases were not as clinically impactful as we had hoped. Improvements in both patient and provider adherence are needed. We plan to engage allied health staff more fully to assist with adherence and thereby improve quality. This study was not intended to obtain patient-oriented outcomes, such as decreased pain and improved function. The study was designed to improve patient safety and to standardize a process for prescribing and monitoring patients on COT. In the future we plan to look at patient outcomes and expand our focus to patients on high-dose opioids and those on combination therapy with benzodiazepines.

We believe the most impactful process steps were our letters sent to COT patients describing our updated, standardized prescribing process, and the ensuing provider-patient discussion to review the risks, benefits, and alternatives to opioid therapy. This frank discussion of treatment options resulted in more than 1 in 5 patients electing to discontinue COT.

There was an association between opioid discontinuation and patients not signing the CSA. This may have been due to patients deciding to discontinue opioids at the initiation review with providers after they received their letter. Therefore, signing the agreement was no longer necessary.
Continue to: We noted that some patients...
We noted that some patients elected to begin a new, nonopioid medication intended to augment their pain relief. However, they did not decrease their use of opioid medicines. We did not collect pain rating scale scores to determine whether the addition of augmenting medicines provided a reduction in pain perception.
Close monitoring of COT patients with frequent office visits may have had an impact on their care. We noted an association between more frequent visits and initiation of pain augmentation medicines. There was also a nonsignificant trend between office visit frequency and dose reduction. These are topics we may re-examine in our practice over time. There was no change in office visit frequency with our intervention, likely a result of these patients having frequent office visits for multiple comorbid medical conditions at baseline.
Evidence of similar benefits in primary care practices that standardized their opioid prescribing guidelines for patients on COT11 illustrates the importance of such a process for ensuring patient safety and decreasing opioid dosage and use.
Limitations to our project are that we did not measure functional changes and quality-of-life scores for patients. We also did not note the opioid dosages for individuals who chose to stop using opioids.
Looking forward. Based on our experience, patient notification with discussion of COT risks, benefits, and alternatives, as well as implementation of a process to monitor COT, appear to be related to patients’ decisions to discontinue COT. Our new standard process did show QI in the process steps but remained suboptimal to our expectations of clinical impact. More frequent office visits may impact patient decisions to reduce opioid dose and to add an augmenting pain medication. We plan to increase the involvement and responsibilities of our allied health staff in our processes to improve rates of adherence and the overall quality of how we manage patients on chronic opioid therapy.
CORRESPONDENCE
David Patchett, DO, Mayo Clinic, 13400 East Shea Blvd, Scottsdale, AZ 85259; [email protected]
ABSTRACT
Purpose The “opioid epidemic” in the United States has received increasing attention over the past few years. Most drug overdose deaths involve an opioid, and prescription opioid deaths have quadrupled since 1999. We sought to improve patient safety and adhere to clinical guidelines by standardizing opioid prescribing in our practice.
Methods We implemented a standardized approach to opioid prescribing based on Arizona Department of Health Services guidelines. All of our providers received instruction on Arizona’s Controlled Substance Prescription Monitoring Program (AZCSPMP) database and were encouraged to use it online. Our goal was for patients to have quarterly office visits, complete random urine drug screens, and sign a controlled substance agreement (CSA). The CSA acknowledged their understanding of the risks and benefits of opioid therapy as well as our updated prescribing policies.
Results Three-hundred fifty-eight of our practice’s patients were receiving chronic opioid therapy. All providers enrolled in AZCSPMP and used it for patient care. We increased rates of signed CSAs from 4.5% to 43.6%, and urine drug screening from 0.8% to 20.1%. For 325 patients remaining in the practice after our interventions, a postintervention chart review demonstrated a statistically significant discontinuation of opioid therapy (71/325, 21.8%; 95% confidence interval, 17.4%-26.7%).
Conclusion Implementation of a standardized opioid prescribing process resulted in discontinuation of therapy for some patients. Rates increased for signed CSAs and completed random urine drug screening. Future process interventions may improve patient and provider adherence. All primary care physicians should examine their prescribing processes to enhance the safety of opioid therapy.
[polldaddy:10370177]
The US opioid epidemic has received increased attention both nationally and at the state level over the past 2 years. This attention is warranted given the significant societal burden of opioid misuse, abuse, and overdose. Most drug overdose deaths (> 6/10) involve an opioid.1 Deaths from prescription opioids have quadrupled since 1999 in the United States.2 Arizona, the state in which we practice, ranked sixth highest in the nation for drug overdose deaths and had the fifth highest opioid prescribing rate in 2011.3 In response to the growing epidemic, the Centers for Disease Control and Prevention (CDC) released guidelines in 2016 for prescribing and monitoring opioids for chronic pain.4
Chronic nonterminal pain (CNTP) remains a significant cause of human suffering and is more prevalent in the United States than cancer, diabetes, and heart disease combined.5 The increased use of opioids since 1999 to ease CNTP has not reduced Americans’ reports of pain overall.6,7 Given the growing opioid epidemic and disease burden of CNTP, we embarked on a quality improvement (QI) project to safely prescribe and refill opioid medications in the Department of Family Medicine at the Mayo Clinic Arizona.
METHODS
This project received an exemption from internal review board evaluation as a QI intervention. We used a team-based approach to address standardization of opioid prescribing and monitoring within our practice. The team included physicians (MD/DO), nurses (LPN/RN), and allied health staff (MA), operations and administrative personnel, and information technology (IT) support. We did not involve patients in the initial design of our project. With future quality efforts in this area, we plan to involve patients in design processes.
Continue to: We began by identifying...
We began by identifying the scope of the problem, establishing criteria to search the electronic medical record (EMR) and identify appropriate patients. Chronic pain is often defined as pain lasting more than 3 months. Chronic opioid therapy (COT) has been defined as opioid use lasting longer than 3 months.8 Working with our IT colleagues, we defined COT patients as those with 3 or more prescriptions for opioids in the past year or those who received ≥ 30 pills a month (ie, patients who received 180 pills with 2 prescriptions written for the year). This definition gave us the ability to query our EMR to determine which patients were on COT, and we prepared lists of patients by primary care provider (FIGURE). Providers reviewed the lists to ensure these individuals were in fact on COT for CNTP. The number of patients identified after EMR query and provider review was 358, comprising 2.6% of 14,000 empaneled patients.

We based our interventions on the Arizona Department of Health Services 2014 opioid prescribing guidelines.3 The Arizona guidelines used existing national and state opioid prescribing guidelines along with clinical practice guidelines. Our study began prior to the 2016 CDC guidelines, so they were not used in this study. Our practice guidelines recommended that all 23 of our providers (MDs, DOs, and NPs) sign up for Arizona’s Controlled Substance Prescription Monitoring Program (AZCSPMP). We asked each patient to sign a controlled substance agreement (CSA), acknowledging their awareness of our proposed processes and the discussion of opioid therapy. Patients were expected to have face-to-face visits with providers at least quarterly and to complete a random urine drug screen at least annually. Patients were not incentivized to complete the process. We placed reminder calls for appointments just as we do for regular appointments.
Providers were asked to complete the Opioid Risk Tool9 with the patient at the initial visit, discuss the risks, benefits, and alternatives of long-term use of opioid medication, and review the 6 As (analgesia, activity, aberrant drug related behavior, adverse effects, affect, and adjunctive treatments). On the day before each patient visit, providers were reminded by a note in the EMR schedule to check AZCSPMP. Initial appointment times would be 30 minutes and follow-up appointments would be scheduled for 15 minutes if only addressing COT.
The QI project was introduced at an all-staff meeting in October 2015 that included providers, allied health staff, front desk personnel, and administrative staff, with the goal of beginning our COT process in November. We mailed letters to COT patients describing our new guidelines and asking them to call to schedule an appointment. If patients on COT came into the office for an alternate appointment and had not yet been seen for a COT visit, providers were encouraged to complete the COT process at that time.
We created a standard order set in the EMR for initial and follow-up visits and for the urine drug screen. We also added an interactive form to the EMR allowing providers to electronically complete the Opioid Risk Tool, and to confirm CSA completion and AZCSPMP review. We developed a database that would query the EMR for patient office visit frequency, CSA completion, and urine drug screen collection. We also placed paper copies of forms in exam rooms with a laminated instruction sheet reviewing the process steps and the 6 As.
Continue to: Soft rollout was...
Soft rollout was November 1, 2015, to assist in working through the process before full rollout. We asked providers to complete the full process on at least 1 patient during this period. This run-through would help ensure that allied health staff who room the patients would have the CSA and Opioid Risk Tool already in the chart before the visit. Full rollout was January 2, 2016. Every 2 to 4 weeks after the full rollout, regular email reminders were sent to providers about the project process and allowed for any feedback about issues that arose.
We provided regular updates and discussed the process at department meetings monthly. Quarterly data were reviewed and discussed for the first year of implementation. Providers and staff completed a chart review for each COT patient at project completion, to determine whether opioids had been decreased (in dosage) or discontinued, a nonopioid medicine had been initiated to augment pain control, or whether patients had died or left the practice.
Statistical analysis
We summarized binary data as counts and proportions and compared them using the chi square test. We summarized discrete data by their mean and standard deviation. To analyze binary variables measured repeatedly in time, we used the logistic generalized estimating equation (GEE) with an autoregressive (AR-1) correlation structure. We computed 95% confidence intervals (CIs)for odds ratios using the empirical or “sandwich” standard error estimates. For discrete variables representing counts, we used the negative binomial regression model.
For count data, a Poisson model is typically used; in our case the variance was considerably larger than the mean, exceeding the Poisson-model requirement that they not be significantly different if not exactly the same. This implies that the data are “over dispersed” or more variable than a Poisson model is thought to be able to model accurately. We therefore used a negative binomial model, which is regarded as the better model in this situation. The 95% CIs for the estimate resulting from the negative binomial regression model were computed using the profile-likelihood.10 All GEEs were clustered on patients (n = 358). We used SAS version 9.3 (Cary, NC) for all analyses.

RESULTS
All providers enrolled for AZCSPMP. CSA completion increased from 16 (4.5%) at baseline to 156 (43.6%) after intervention (P < .001). Patients completed a urine drug screen more frequently as well, from 3 (0.8%) to 72 (20.1%) (P < .001) (TABLES 1 and 2). No statistically significant change was noted in the frequency of office visits.

Continue to: We excluded 33 patients...
We excluded 33 patients from the post-intervention chart review (TABLE 3). Twenty-seven had left the practice and 6 had died, leaving 325 patients included in the post-intervention chart review.

There was a statistically significant association between patients who discontinued opioids and those who neglected to sign a CSA (P < .001) (TABLE 4). We tested for associations between office visit frequency and process step completion. There was a nonsignificant trend between increased frequency of office visits and opioid dose reduction. Patients who stopped opioids had fewer office visits (TABLE 5), while patients who had initiated a medication to augment pain relief had more frequent office visits (TABLE 6).

DISCUSSION
Our interventions to improve the quality of our COT processes were moderately successful. We achieved statistically significant increases in our rates of CSA completion and in urine drug screening. However, these increases were not as clinically impactful as we had hoped. Improvements in both patient and provider adherence are needed. We plan to engage allied health staff more fully to assist with adherence and thereby improve quality. This study was not intended to obtain patient-oriented outcomes, such as decreased pain and improved function. The study was designed to improve patient safety and to standardize a process for prescribing and monitoring patients on COT. In the future we plan to look at patient outcomes and expand our focus to patients on high-dose opioids and those on combination therapy with benzodiazepines.

We believe the most impactful process steps were our letters sent to COT patients describing our updated, standardized prescribing process, and the ensuing provider-patient discussion to review the risks, benefits, and alternatives to opioid therapy. This frank discussion of treatment options resulted in more than 1 in 5 patients electing to discontinue COT.

There was an association between opioid discontinuation and patients not signing the CSA. This may have been due to patients deciding to discontinue opioids at the initiation review with providers after they received their letter. Therefore, signing the agreement was no longer necessary.
Continue to: We noted that some patients...
We noted that some patients elected to begin a new, nonopioid medication intended to augment their pain relief. However, they did not decrease their use of opioid medicines. We did not collect pain rating scale scores to determine whether the addition of augmenting medicines provided a reduction in pain perception.
Close monitoring of COT patients with frequent office visits may have had an impact on their care. We noted an association between more frequent visits and initiation of pain augmentation medicines. There was also a nonsignificant trend between office visit frequency and dose reduction. These are topics we may re-examine in our practice over time. There was no change in office visit frequency with our intervention, likely a result of these patients having frequent office visits for multiple comorbid medical conditions at baseline.
Evidence of similar benefits in primary care practices that standardized their opioid prescribing guidelines for patients on COT11 illustrates the importance of such a process for ensuring patient safety and decreasing opioid dosage and use.
Limitations to our project are that we did not measure functional changes and quality-of-life scores for patients. We also did not note the opioid dosages for individuals who chose to stop using opioids.
Looking forward. Based on our experience, patient notification with discussion of COT risks, benefits, and alternatives, as well as implementation of a process to monitor COT, appear to be related to patients’ decisions to discontinue COT. Our new standard process did show QI in the process steps but remained suboptimal to our expectations of clinical impact. More frequent office visits may impact patient decisions to reduce opioid dose and to add an augmenting pain medication. We plan to increase the involvement and responsibilities of our allied health staff in our processes to improve rates of adherence and the overall quality of how we manage patients on chronic opioid therapy.
CORRESPONDENCE
David Patchett, DO, Mayo Clinic, 13400 East Shea Blvd, Scottsdale, AZ 85259; [email protected]
1. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths — United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1445-1452.
2. CDC. Opioid data analysis and resources. https://www.cdc.gov/drugoverdose/data/analysis.html. Published December 19, 2018. Accessed May 27, 2019.
3. Arizona Department of Health Services. Arizona opioid prescribing guidelines. https://www.azdhs.gov/documents/audiences/clinicians/clinical-guidelines-recommendations/prescribing-guidelines/az-opiod-prescribing-guidelines.pdf. Published November 2014. Accessed May 27, 2019.
4. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
5. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
6. Chang H, Daubresse M, Kruszewski S, et al. Prevalence and treatment of pain in EDs in the United States, 2000 to 2010. Am J Emerg Med. 2014;32:421-431.
7. Daubresse M, Chang H, Yu Y, et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000 - 2010. Med Care. 2013;51:870-878.
8. Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162:276-286.
9. Webster LR, Webster RM. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432-442.
10. Hilbe JM. Negative Binomial Regression. Cambridge, United Kingdom: Cambridge University Press; 2013.
11. Liebschutz JM, Xuan Z, Shanahan CW, et al. Improving adherence to long-term opioid therapy guidelines to reduce opioid misuse in primary care: a cluster-randomized clinical trial. JAMA Intern Med. 2017;177:1265-1272.
1. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths — United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1445-1452.
2. CDC. Opioid data analysis and resources. https://www.cdc.gov/drugoverdose/data/analysis.html. Published December 19, 2018. Accessed May 27, 2019.
3. Arizona Department of Health Services. Arizona opioid prescribing guidelines. https://www.azdhs.gov/documents/audiences/clinicians/clinical-guidelines-recommendations/prescribing-guidelines/az-opiod-prescribing-guidelines.pdf. Published November 2014. Accessed May 27, 2019.
4. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
5. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
6. Chang H, Daubresse M, Kruszewski S, et al. Prevalence and treatment of pain in EDs in the United States, 2000 to 2010. Am J Emerg Med. 2014;32:421-431.
7. Daubresse M, Chang H, Yu Y, et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000 - 2010. Med Care. 2013;51:870-878.
8. Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162:276-286.
9. Webster LR, Webster RM. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432-442.
10. Hilbe JM. Negative Binomial Regression. Cambridge, United Kingdom: Cambridge University Press; 2013.
11. Liebschutz JM, Xuan Z, Shanahan CW, et al. Improving adherence to long-term opioid therapy guidelines to reduce opioid misuse in primary care: a cluster-randomized clinical trial. JAMA Intern Med. 2017;177:1265-1272.
Discharge Medical Complexity, Change in Medical Complexity and Pediatric 30-day Readmission
Hospitalizations are disruptive, stressful, and costly for patients and families.1-5 Hospital readmissions subject families to the additional morbidity inherent to hospitalization and place patients at additional risk of hospital-acquired conditions or other harm.6-9 In pediatrics, hospital readmissions are common for specific conditions;10 with rates varying across institutions;10,11 and as many as one-third of unplanned pediatric readmissions are potentially preventable.12
Reducing pediatric readmissions requires a deeper understanding of the mechanisms through which readmissions occur. Medical complexity—specifically chronic conditions and use of medical technology—is associated with increased risk of readmission.13,14 Polypharmacy at discharge has also been associated with readmission.15,16 However, prior studies on polypharmacy and readmission risk examined the count of total medications and did not consider the nuances of scheduled versus as-needed medications, or the frequency of doses. These nuances may be critical to caregivers as discharge medical complexity can be overwhelming, even in diagnoses which are not traditionally considered complex.17 Finally, of potentially greater importance than medical complexity at discharge is a change in medical complexity during a hospitalization—for example, new diagnoses or new technologies that require additional education in hospital and management at home.
We sought to further understand the relationship between discharge medical complexity and readmission risk with regards to polypharmacy and home healthcare referrals at discharge. Specifically, we hypothesized that a change in medical complexity during an admission—ie, a new chronic diagnosis or new technology—would be a more prominent risk factor for readmission than discharge complexity alone. We examined these factors in the context of length of stay (LOS) since this is a marker of in-hospital severity of illness and a potentially modifiable function of time allowed for in-hospital teaching and discharge preparation.
METHODS
We conducted a retrospective, case-control study of pediatric hospitalizations at one tertiary care children’s hospital. Children <18 years were eligible for inclusion. Normal birth hospitalizations were excluded. We randomly selected one hospitalization from each child as the index visit. We identified cases, hospitalizations at C.S. Mott Children’s Hospital between 2008 and 2012 with a subsequent unplanned 30-day readmission,18 and matched them one to one with hospitalizations at the same hospital during the same period without subsequent readmission. We matched cases to controls based on the month of admission to account for seasonality of certain illnesses. We also matched on distance and direction from the hospital to the patient’s home to account for the potential to have readmissions to other institutions. We utilized both distance and direction recognizing that a family living 30 miles in one direction would be closer to an urban area with access to more facilities, as opposed to 30 miles in another direction in a rural area without additional access. We subsequently performed medical record review to abstract relevant covariates.
Primary Predictors
Medical Complexity Models (Models 1 and 2):
We evaluated three attributes of discharge medical complexity abstracted by medical record review—discharge medications, technology assistance (ie, tracheostomy, cerebral spinal fluid ventricular shunt, enteral feeding tube, central line), and the need for home healthcare after discharge. We counted discharge medications based on the number of medications listed on the discharge summary separated into scheduled or as needed.19 We also considered the number of scheduled doses to be administered in a 24-hour period (see Appendix methods for more information on counting discharge medications). For assistance by technology, we considered the presence of tracheostomy, cerebral spinal fluid ventricular shunt, enteral feeding tube, and central lines. While we describe these technologies separately, for multivariable analyses we considered the presence of any of the four types of technology.
Change in Medical Complexity Models (Models 3 and 4)
We examined two aspects of change in medical complexity—the presence of a new complex chronic condition (CCC)20 diagnosed during the hospitalization, and a new reliance on medical technology. The presence of new CCC was determined by comparing discharge diagnoses to past medical history abstracted by medical record review. A new CCC was defined as any complex chronic condition that was captured in the discharge diagnoses but was not evident in the past medical history. By definition, all CCCs coded during birth hospitalization (eg, at discharge from the neonatal intensive care unit) were assigned to “new” CCC. We calculated a kappa statistic to determine interrater reliability in determining the designation of new CCC. A sensitivity analysis examining these birth CCCs was also performed comparing no new CCC, new CCC, and new CCC after birth hospitalization. The methods appendix provides additional information on considering new CCCs. New technology, abstracted from chart review, was defined as technology placed during hospitalization that remained in place at discharge. If a child with existing technology had additional technology placed during the hospitalization (eg, a new tracheostomy in a child with a previously placed enteral feeding tube), the encounter was considered as having new technology placed.
Covariates
We created different sets of multivariable models to account for patient/hospitalization characteristics.
Statistical Analysis
A review of 600 cases and 600 controls yields 89% power to detect statistical significance for covariates with an odds ratio of 1.25 (β = 0.22) if the candidate covariate has low to moderate correlation with other covariates (<0.3). If a candidate covariate has a moderate correlation with other covariates (0.6), we have 89% power to detect an odds ratio of 1.35 (β = 0.30).21 We calculated odds of 30-days unplanned readmission using conditional logistic regression to account for matched case-control design. All the analyses were performed using STATA 13 (Stata Corp., College Station, Texas).
RESULTS
Of the 41,422 eligible index hospitalizations during the study period, 9.4% resulted in a 30-day unplanned readmission. After randomly selecting one hospitalization per child, there were 781 eligible cases. We subsequent matched all but one eligible case to a control. We randomly selected encounters for medical record review, reviewing a total of 1,212 encounters. After excluding pairs with incomplete records, we included 595 cases and 595 controls in this analysis (Figure). Patient/hospitalization characteristics are displayed in Table 1. The most frequent primary discharge diagnoses are displayed in Appendix Table 1.
Models of Medical Complexity at Discharge
Polypharmacy after discharge was common for both readmitted and nonreadmitted patients. Children who experienced unplanned readmission in 30 days were discharged with a median of four different scheduled medications (interquartile range [IQR] 2,7) which translated into a median of six (IQR 3,12) scheduled doses in a 24-hour period. In comparison, children without an unplanned readmission had a median of two different scheduled medications (IQR 1,3) with a median of three (IQR 0,7) scheduled doses in a 24-hour period. Medical technology was more common in case children (42%) than in control children (14%). Central lines and enteral tubes were the most common forms of medical technology in both cases and controls. Home health referral was common in both cases (44%) and controls (23%; Table 1).
In Model 1 (adjusting only for patient characteristics; Table 3), being discharged on two or more scheduled medications was associated with higher odds of readmission compared to being discharged without medications, with additional medications associated with even higher odds of readmission. Children with any technology had higher odds of readmission than children without medical technology. Likewise, home healthcare visits after discharge were associated with elevated odds of readmission in multivariable analyses without LOS. However, after adding LOS to the model (Model 2), home healthcare visits were no longer significantly associated with readmission.
Change in Medical Complexity Models
The adjudication of new CCCs had good reliability (Κ = 0.72). New CCCs occurred in 18% and new technologies occurred in 17% of cases. Comparatively, new CCCs occurred in 10% and new technologies in 7% of hospitalizations in control children (Table 1). In bivariate analyses, both aspects of change in medical complexity were associated with higher odds of readmission (Table 2). In multivariate analysis with patient characteristics (Model 3; Table 3), all aspects of change in complexity were associated with elevated odds of readmission. A new CCC was associated with higher odds of readmission (adjusted OR (AOR) 1.75, 95% CI: 1.11-2.75) as was new technology during admission (AOR 1.84, 95%CI: 1.09-3.10). Furthermore, the odds of readmission for medical complexity variables (polypharmacy and home healthcare need) remained largely unchanged when adding the change in medical complexity variables (ie, comparing Model 1 and Model 3). However, when accounting for LOS (Model 4), neither the acquisition of a new CCC nor the addition of new technology was associated with readmission. The most common form of new technology was central line followed by nonsurgically placed enteral tube (Appendix Table 2). Finally, in sensitivity analyses (results not detailed), separating new CCC acquired at birth and new CCCs in nonbirth hospitalizations, compared to hospitalizations with no new CCC, yielded similar results as the primary analyses.
DISCUSSION
We examined multiple attributes of polypharmacy—the number of scheduled medications, number of as-needed medications, and number of scheduled doses per 24 hours. Interestingly, only the scheduled medications (count of medication and number of doses) were associated with elevated readmission risk. As-needed medications have heterogeneity in the level of importance from critical (eg, seizure rescue) to discretionary (eg, antipyretics, creams). The burden of managing these types of medications may still be high (ie, parents must decide when to administer a critical medication); however, this burden does not translate into increased readmission risk in this population.
Not surprisingly, greater medical complexity—as defined by higher numbers of scheduled discharge medications and technology assistance—is associated with 30-day readmission risk. Our analyses do not allow us to determine how much of the increased risk is due to additional care burden and risks of polypharmacy versus the inherent increase in complexity and severity of illness for which polypharmacy is a marker. Tailoring discharge regimens to the realities of daily life, with the goal of “minimally disruptive medicine”22,23 (eg, integrating manageable discharge medication routines into school and work schedules), is not a common feature of pediatric discharge planning. For adult patients with complex medical conditions, tailoring medication regimens in a minimally disruptive way is known to improve outcomes.24 Similarly, adopting minimally disruptive techniques to integrate the polypharmacy inherent in discharge could potentially mitigate some of the readmission risks for children and adolescents.
Contrary to our hypothesis, new technologies and new diagnoses did not confer additional readmission risk when accounting for LOS and patient characteristics. One potential explanation is varying risks conveyed by different types of new technologies placed during hospitalization. Central lines, the most common form of new technology, is associated with higher odds of reutilization in unadjusted analyses. However, the second most common form of new technology, nonsurgically placed enteral feeding tube, was not. Further analyses of the differential effects of new technology should be further examined in larger datasets. Additionally, the lack of additional readmission risk from new technology may relate to additional teaching and support provided to families of patients undergoing unfamiliar procedures offsets the risks inherent of greater complexity. If so, it may be that the more intensive teaching and postdischarge support provided to families with new technology or a new diagnosis could be replicated through refresher teaching during hospitalizations, when a patient’s state of health is status quo for the family (ie, the child was admitted and discharged with the same technology and diagnoses). This notion is supported by prior work that demonstrated successful readmission reduction interventions for children with chronic conditions often rely on enhanced education or coaching.25,26
We elected to present models both with and without LOS as a confounder because it is a potentially modifiable attribute of hospitalization. Change in medical complexity aspects were significantly associated with readmission in multivariable models without LOS. However, with the addition of LOS, they were no longer significant. Thus, the readmission risk of new complexity is accounted for by the readmission risk inherent in a longer LOS. This finding prompts additional questions that merit further study: is it that LOS is a general marker for heightened complexity, or is it that a longer LOS can modify readmission risk through additional in-hospital care and time for enhanced education?
Our study has several strengths. We were able to discern true complexity at the time of discharge through medical record review. For example, if a child had a peripherally inserted central catheter placed during hospitalization, it cannot be ascertained through administrative data without medical record review if the technology was removed or in place at discharge. Likewise, medical record review allows for identification of medical technology which is not surgically implanted (eg, nasogastric feeding tubes). Given the “fog” families report as part of their in-hospital experience and its threats to education and postdischarge contingency planning,17 we felt it important to evaluate medical technology regardless of whether or not it was surgically placed. Additionally, the more detailed and nuanced understanding gained of polypharmacy burden can better inform both risk prediction models and interventions to improve the transition from hospital to home.
This study
CONCLUSION
Medical complexity at discharge is associated with pediatric readmission risk. Contrary to our hypothesis, the addition of new technologies and new CCC diagnoses are not associated with pediatric readmission, after accounting for patient and hospitalization factors including LOS. The dynamics of LOS as a risk factor for readmission for children with medical complexity are likely multifaceted and merit further investigation in a multi-institutional study.
Disclosures
The authors report no potential conflicts of interest.
Funding
This work was supported by a grant from the Agency for Healthcare Research and Quality (1K08HS204735-01A1) and a grant from the Blue Cross Blue Shield of Michigan Foundation.
1. Diaz-Caneja A, Gledhill J, Weaver T, Nadel S, Garralda E. A child’s admission to hospital: a qualitative study examining the experiences of parents. Intensive Care Med. 2005;31(9):1248-1254. https://doi.org/10.1007/s00134-005-2728-8.
2. Lapillonne A, Regnault A, Gournay V, et al. Impact on parents of bronchiolitis hospitalization of full-term, preterm and congenital heart disease infants. BMC Pediatrics. 2012;12:171. https://doi.org/10.1186/1471-2431-12-171.
3. Leader S, Jacobson P, Marcin J, Vardis R, Sorrentino M, Murray D. A method for identifying the financial burden of hospitalized infants on families. Value Health. 2002;5(1):55-59. https://doi.org/10.1046/j.1524-4733.2002.51076.x.
4. Leidy NK, Margolis MK, Marcin JP, et al. The impact of severe respiratory syncytial virus on the child, caregiver, and family during hospitalization and recovery. Pediatrics. 2005;115(6):1536-1546. https://doi.org/10.1542/peds.2004-1149.
5. Rennick JE, Johnston CC, Dougherty G, Platt R, Ritchie JA. Children’s psychological responses after critical illness and exposure to invasive technology. J Dev Behav Pediatr. 2002;23(3):133-144. PubMed
6. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. https://doi.org/10.1056/NEJM199102073240604.
7. Kohn LT, Corrigan J, Donaldson MS. To err is human: building a safer health system. Washington DC: National Academy Press; 2000.
8. Landrigan CP, Parry GJ, Bones CB, Hackbarth AD, Goldmann DA, Sharek PJ. Temporal trends in rates of patient harm resulting from medical care. N Engl J Med. 2010;363(22):2124-2134. https://doi.org/10.1056/NEJMsa1004404.
9. Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of healthcare-associated infections. N Engl J Med. 2014;370(13):1198-1208. https://doi.org/10.1056/NEJMoa1306801.
10. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351.
11. Bardach NS, Vittinghoff E, Asteria-Penaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. https://doi.org/10.1542/peds.2012-3527.
12. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-day hospital readmissions at a children’s hospital. Pediatrics. 2016;138(2):pii: e20154182. https://doi.org/10.1542/peds.2015-4182.
13. Bucholz EM, Gay JC, Hall M, Harris M, Berry JG. Timing and causes of common pediatric readmissions. J Pediatr. 2018;200:240-248. https://doi.org/10.1016/j.jpeds.2018.04.044.
14. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. https://doi.org/10.1001/jama.2011.122.
15. Winer JC, Aragona E, Fields AI, Stockwell DC. Comparison of clinical risk factors among pediatric patients with single admission, multiple admissions (without any 7-day readmissions), and 7-day readmission. Hosp Pediatr. 2016;6(3):119-125. https://doi.org/10.1542/hpeds.2015-0110.
16. Brittan MS, Martin S, Anderson L, Moss A, Torok MR. An electronic health record tool designed to improve pediatric hospital discharge has low predictive utility for readmissions. J Hosp Med. 2018;13(11):779-782. https://doi.org/10.12788/jhm.3043.
17. Solan LG, Beck AF, Brunswick SA, et al. The family perspective on hospital to home transitions: a qualitative study. Pediatrics. 2015;136(6):e1539-e1549. https://doi.org/10.1542/peds.2015-2098.
18. Auger KA, Mueller EL, Weinberg SH, et al. A validated method for identifying unplanned pediatric readmission. J Pediatr. 2016;170:105-112. https://doi.org/10.1016/j.jpeds.2015.11.051.
19. Auger KA, Shah SS, Davis MD, Brady PW. Counting the Ways to Count Medications: The Challenges of Defining Pediatric Polypharmacy. J Hosp Med. 2019;14(8):506-507. https://doi.org/10.12788/jhm.3213.
20. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatrics. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199.
21. Hsieh FY. Sample size tables for logistic regression. Stat Med. 1989;8(7):795-802. https://doi.org/10.1002/sim.4780080704.
22. May C, Montori VM, Mair FS. We need minimally disruptive medicine. BMJ. 2009;339:b2803. https://doi.org/10.1136/bmj.b2803.
23. Leppin AL, Montori VM, Gionfriddo MR. Minimally disruptive medicine: a pragmatically comprehensive model for delivering care to patients with multiple chronic conditions. Healthcare (Basel). 2015;3(1):50-63. https://doi.org/10.3390/healthcare3010050.
24. Serrano V, Spencer-Bonilla G, Boehmer KR, Montori VM. Minimally disruptive medicine for patients with diabetes. Curr Diab Rep. 2017;17(11):104. https://doi.org/10.1007/s11892-017-0935-7.
25. Auger KA, Kenyon CC, Feudtner C, Davis MM. Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2013;9(4):251-260. https://doi.org/10.1002/jhm.2134.
26. Coller RJ, Klitzner TS, Lerner CF, et al. Complex care hospital use and postdischarge coaching: a randomized controlled trial. Pediatrics. 2018;142(2):pii: e20174278. https://doi.org/10.1542/peds.2017-4278.
27. Hain PD, Gay JC, Berutti TW, Whitney GM, Wang W, Saville BR. Preventability of early readmissions at a children’s hospital. Pediatrics. 2013;131(1):e171-e181. https://doi.org/10.1542/peds.2012-0820.
28. Auger KA, Teufel RJ, 2nd, Harris JM, 2nd, et al. Children’s hospital characteristics and readmission metrics. Pediatrics. 2017;139(2). https://doi.org/10.1542/peds.2016-1720.
29. Gay JC, Agrawal R, Auger KA, et al. Rates and impact of potentially preventable readmissions at children’s hospitals. J Pediatr. 2015;166(3):613-619 e615. https://doi.org/10.1016/j.jpeds.2014.10.052.
Hospitalizations are disruptive, stressful, and costly for patients and families.1-5 Hospital readmissions subject families to the additional morbidity inherent to hospitalization and place patients at additional risk of hospital-acquired conditions or other harm.6-9 In pediatrics, hospital readmissions are common for specific conditions;10 with rates varying across institutions;10,11 and as many as one-third of unplanned pediatric readmissions are potentially preventable.12
Reducing pediatric readmissions requires a deeper understanding of the mechanisms through which readmissions occur. Medical complexity—specifically chronic conditions and use of medical technology—is associated with increased risk of readmission.13,14 Polypharmacy at discharge has also been associated with readmission.15,16 However, prior studies on polypharmacy and readmission risk examined the count of total medications and did not consider the nuances of scheduled versus as-needed medications, or the frequency of doses. These nuances may be critical to caregivers as discharge medical complexity can be overwhelming, even in diagnoses which are not traditionally considered complex.17 Finally, of potentially greater importance than medical complexity at discharge is a change in medical complexity during a hospitalization—for example, new diagnoses or new technologies that require additional education in hospital and management at home.
We sought to further understand the relationship between discharge medical complexity and readmission risk with regards to polypharmacy and home healthcare referrals at discharge. Specifically, we hypothesized that a change in medical complexity during an admission—ie, a new chronic diagnosis or new technology—would be a more prominent risk factor for readmission than discharge complexity alone. We examined these factors in the context of length of stay (LOS) since this is a marker of in-hospital severity of illness and a potentially modifiable function of time allowed for in-hospital teaching and discharge preparation.
METHODS
We conducted a retrospective, case-control study of pediatric hospitalizations at one tertiary care children’s hospital. Children <18 years were eligible for inclusion. Normal birth hospitalizations were excluded. We randomly selected one hospitalization from each child as the index visit. We identified cases, hospitalizations at C.S. Mott Children’s Hospital between 2008 and 2012 with a subsequent unplanned 30-day readmission,18 and matched them one to one with hospitalizations at the same hospital during the same period without subsequent readmission. We matched cases to controls based on the month of admission to account for seasonality of certain illnesses. We also matched on distance and direction from the hospital to the patient’s home to account for the potential to have readmissions to other institutions. We utilized both distance and direction recognizing that a family living 30 miles in one direction would be closer to an urban area with access to more facilities, as opposed to 30 miles in another direction in a rural area without additional access. We subsequently performed medical record review to abstract relevant covariates.
Primary Predictors
Medical Complexity Models (Models 1 and 2):
We evaluated three attributes of discharge medical complexity abstracted by medical record review—discharge medications, technology assistance (ie, tracheostomy, cerebral spinal fluid ventricular shunt, enteral feeding tube, central line), and the need for home healthcare after discharge. We counted discharge medications based on the number of medications listed on the discharge summary separated into scheduled or as needed.19 We also considered the number of scheduled doses to be administered in a 24-hour period (see Appendix methods for more information on counting discharge medications). For assistance by technology, we considered the presence of tracheostomy, cerebral spinal fluid ventricular shunt, enteral feeding tube, and central lines. While we describe these technologies separately, for multivariable analyses we considered the presence of any of the four types of technology.
Change in Medical Complexity Models (Models 3 and 4)
We examined two aspects of change in medical complexity—the presence of a new complex chronic condition (CCC)20 diagnosed during the hospitalization, and a new reliance on medical technology. The presence of new CCC was determined by comparing discharge diagnoses to past medical history abstracted by medical record review. A new CCC was defined as any complex chronic condition that was captured in the discharge diagnoses but was not evident in the past medical history. By definition, all CCCs coded during birth hospitalization (eg, at discharge from the neonatal intensive care unit) were assigned to “new” CCC. We calculated a kappa statistic to determine interrater reliability in determining the designation of new CCC. A sensitivity analysis examining these birth CCCs was also performed comparing no new CCC, new CCC, and new CCC after birth hospitalization. The methods appendix provides additional information on considering new CCCs. New technology, abstracted from chart review, was defined as technology placed during hospitalization that remained in place at discharge. If a child with existing technology had additional technology placed during the hospitalization (eg, a new tracheostomy in a child with a previously placed enteral feeding tube), the encounter was considered as having new technology placed.
Covariates
We created different sets of multivariable models to account for patient/hospitalization characteristics.
Statistical Analysis
A review of 600 cases and 600 controls yields 89% power to detect statistical significance for covariates with an odds ratio of 1.25 (β = 0.22) if the candidate covariate has low to moderate correlation with other covariates (<0.3). If a candidate covariate has a moderate correlation with other covariates (0.6), we have 89% power to detect an odds ratio of 1.35 (β = 0.30).21 We calculated odds of 30-days unplanned readmission using conditional logistic regression to account for matched case-control design. All the analyses were performed using STATA 13 (Stata Corp., College Station, Texas).
RESULTS
Of the 41,422 eligible index hospitalizations during the study period, 9.4% resulted in a 30-day unplanned readmission. After randomly selecting one hospitalization per child, there were 781 eligible cases. We subsequent matched all but one eligible case to a control. We randomly selected encounters for medical record review, reviewing a total of 1,212 encounters. After excluding pairs with incomplete records, we included 595 cases and 595 controls in this analysis (Figure). Patient/hospitalization characteristics are displayed in Table 1. The most frequent primary discharge diagnoses are displayed in Appendix Table 1.
Models of Medical Complexity at Discharge
Polypharmacy after discharge was common for both readmitted and nonreadmitted patients. Children who experienced unplanned readmission in 30 days were discharged with a median of four different scheduled medications (interquartile range [IQR] 2,7) which translated into a median of six (IQR 3,12) scheduled doses in a 24-hour period. In comparison, children without an unplanned readmission had a median of two different scheduled medications (IQR 1,3) with a median of three (IQR 0,7) scheduled doses in a 24-hour period. Medical technology was more common in case children (42%) than in control children (14%). Central lines and enteral tubes were the most common forms of medical technology in both cases and controls. Home health referral was common in both cases (44%) and controls (23%; Table 1).
In Model 1 (adjusting only for patient characteristics; Table 3), being discharged on two or more scheduled medications was associated with higher odds of readmission compared to being discharged without medications, with additional medications associated with even higher odds of readmission. Children with any technology had higher odds of readmission than children without medical technology. Likewise, home healthcare visits after discharge were associated with elevated odds of readmission in multivariable analyses without LOS. However, after adding LOS to the model (Model 2), home healthcare visits were no longer significantly associated with readmission.
Change in Medical Complexity Models
The adjudication of new CCCs had good reliability (Κ = 0.72). New CCCs occurred in 18% and new technologies occurred in 17% of cases. Comparatively, new CCCs occurred in 10% and new technologies in 7% of hospitalizations in control children (Table 1). In bivariate analyses, both aspects of change in medical complexity were associated with higher odds of readmission (Table 2). In multivariate analysis with patient characteristics (Model 3; Table 3), all aspects of change in complexity were associated with elevated odds of readmission. A new CCC was associated with higher odds of readmission (adjusted OR (AOR) 1.75, 95% CI: 1.11-2.75) as was new technology during admission (AOR 1.84, 95%CI: 1.09-3.10). Furthermore, the odds of readmission for medical complexity variables (polypharmacy and home healthcare need) remained largely unchanged when adding the change in medical complexity variables (ie, comparing Model 1 and Model 3). However, when accounting for LOS (Model 4), neither the acquisition of a new CCC nor the addition of new technology was associated with readmission. The most common form of new technology was central line followed by nonsurgically placed enteral tube (Appendix Table 2). Finally, in sensitivity analyses (results not detailed), separating new CCC acquired at birth and new CCCs in nonbirth hospitalizations, compared to hospitalizations with no new CCC, yielded similar results as the primary analyses.
DISCUSSION
We examined multiple attributes of polypharmacy—the number of scheduled medications, number of as-needed medications, and number of scheduled doses per 24 hours. Interestingly, only the scheduled medications (count of medication and number of doses) were associated with elevated readmission risk. As-needed medications have heterogeneity in the level of importance from critical (eg, seizure rescue) to discretionary (eg, antipyretics, creams). The burden of managing these types of medications may still be high (ie, parents must decide when to administer a critical medication); however, this burden does not translate into increased readmission risk in this population.
Not surprisingly, greater medical complexity—as defined by higher numbers of scheduled discharge medications and technology assistance—is associated with 30-day readmission risk. Our analyses do not allow us to determine how much of the increased risk is due to additional care burden and risks of polypharmacy versus the inherent increase in complexity and severity of illness for which polypharmacy is a marker. Tailoring discharge regimens to the realities of daily life, with the goal of “minimally disruptive medicine”22,23 (eg, integrating manageable discharge medication routines into school and work schedules), is not a common feature of pediatric discharge planning. For adult patients with complex medical conditions, tailoring medication regimens in a minimally disruptive way is known to improve outcomes.24 Similarly, adopting minimally disruptive techniques to integrate the polypharmacy inherent in discharge could potentially mitigate some of the readmission risks for children and adolescents.
Contrary to our hypothesis, new technologies and new diagnoses did not confer additional readmission risk when accounting for LOS and patient characteristics. One potential explanation is varying risks conveyed by different types of new technologies placed during hospitalization. Central lines, the most common form of new technology, is associated with higher odds of reutilization in unadjusted analyses. However, the second most common form of new technology, nonsurgically placed enteral feeding tube, was not. Further analyses of the differential effects of new technology should be further examined in larger datasets. Additionally, the lack of additional readmission risk from new technology may relate to additional teaching and support provided to families of patients undergoing unfamiliar procedures offsets the risks inherent of greater complexity. If so, it may be that the more intensive teaching and postdischarge support provided to families with new technology or a new diagnosis could be replicated through refresher teaching during hospitalizations, when a patient’s state of health is status quo for the family (ie, the child was admitted and discharged with the same technology and diagnoses). This notion is supported by prior work that demonstrated successful readmission reduction interventions for children with chronic conditions often rely on enhanced education or coaching.25,26
We elected to present models both with and without LOS as a confounder because it is a potentially modifiable attribute of hospitalization. Change in medical complexity aspects were significantly associated with readmission in multivariable models without LOS. However, with the addition of LOS, they were no longer significant. Thus, the readmission risk of new complexity is accounted for by the readmission risk inherent in a longer LOS. This finding prompts additional questions that merit further study: is it that LOS is a general marker for heightened complexity, or is it that a longer LOS can modify readmission risk through additional in-hospital care and time for enhanced education?
Our study has several strengths. We were able to discern true complexity at the time of discharge through medical record review. For example, if a child had a peripherally inserted central catheter placed during hospitalization, it cannot be ascertained through administrative data without medical record review if the technology was removed or in place at discharge. Likewise, medical record review allows for identification of medical technology which is not surgically implanted (eg, nasogastric feeding tubes). Given the “fog” families report as part of their in-hospital experience and its threats to education and postdischarge contingency planning,17 we felt it important to evaluate medical technology regardless of whether or not it was surgically placed. Additionally, the more detailed and nuanced understanding gained of polypharmacy burden can better inform both risk prediction models and interventions to improve the transition from hospital to home.
This study
CONCLUSION
Medical complexity at discharge is associated with pediatric readmission risk. Contrary to our hypothesis, the addition of new technologies and new CCC diagnoses are not associated with pediatric readmission, after accounting for patient and hospitalization factors including LOS. The dynamics of LOS as a risk factor for readmission for children with medical complexity are likely multifaceted and merit further investigation in a multi-institutional study.
Disclosures
The authors report no potential conflicts of interest.
Funding
This work was supported by a grant from the Agency for Healthcare Research and Quality (1K08HS204735-01A1) and a grant from the Blue Cross Blue Shield of Michigan Foundation.
Hospitalizations are disruptive, stressful, and costly for patients and families.1-5 Hospital readmissions subject families to the additional morbidity inherent to hospitalization and place patients at additional risk of hospital-acquired conditions or other harm.6-9 In pediatrics, hospital readmissions are common for specific conditions;10 with rates varying across institutions;10,11 and as many as one-third of unplanned pediatric readmissions are potentially preventable.12
Reducing pediatric readmissions requires a deeper understanding of the mechanisms through which readmissions occur. Medical complexity—specifically chronic conditions and use of medical technology—is associated with increased risk of readmission.13,14 Polypharmacy at discharge has also been associated with readmission.15,16 However, prior studies on polypharmacy and readmission risk examined the count of total medications and did not consider the nuances of scheduled versus as-needed medications, or the frequency of doses. These nuances may be critical to caregivers as discharge medical complexity can be overwhelming, even in diagnoses which are not traditionally considered complex.17 Finally, of potentially greater importance than medical complexity at discharge is a change in medical complexity during a hospitalization—for example, new diagnoses or new technologies that require additional education in hospital and management at home.
We sought to further understand the relationship between discharge medical complexity and readmission risk with regards to polypharmacy and home healthcare referrals at discharge. Specifically, we hypothesized that a change in medical complexity during an admission—ie, a new chronic diagnosis or new technology—would be a more prominent risk factor for readmission than discharge complexity alone. We examined these factors in the context of length of stay (LOS) since this is a marker of in-hospital severity of illness and a potentially modifiable function of time allowed for in-hospital teaching and discharge preparation.
METHODS
We conducted a retrospective, case-control study of pediatric hospitalizations at one tertiary care children’s hospital. Children <18 years were eligible for inclusion. Normal birth hospitalizations were excluded. We randomly selected one hospitalization from each child as the index visit. We identified cases, hospitalizations at C.S. Mott Children’s Hospital between 2008 and 2012 with a subsequent unplanned 30-day readmission,18 and matched them one to one with hospitalizations at the same hospital during the same period without subsequent readmission. We matched cases to controls based on the month of admission to account for seasonality of certain illnesses. We also matched on distance and direction from the hospital to the patient’s home to account for the potential to have readmissions to other institutions. We utilized both distance and direction recognizing that a family living 30 miles in one direction would be closer to an urban area with access to more facilities, as opposed to 30 miles in another direction in a rural area without additional access. We subsequently performed medical record review to abstract relevant covariates.
Primary Predictors
Medical Complexity Models (Models 1 and 2):
We evaluated three attributes of discharge medical complexity abstracted by medical record review—discharge medications, technology assistance (ie, tracheostomy, cerebral spinal fluid ventricular shunt, enteral feeding tube, central line), and the need for home healthcare after discharge. We counted discharge medications based on the number of medications listed on the discharge summary separated into scheduled or as needed.19 We also considered the number of scheduled doses to be administered in a 24-hour period (see Appendix methods for more information on counting discharge medications). For assistance by technology, we considered the presence of tracheostomy, cerebral spinal fluid ventricular shunt, enteral feeding tube, and central lines. While we describe these technologies separately, for multivariable analyses we considered the presence of any of the four types of technology.
Change in Medical Complexity Models (Models 3 and 4)
We examined two aspects of change in medical complexity—the presence of a new complex chronic condition (CCC)20 diagnosed during the hospitalization, and a new reliance on medical technology. The presence of new CCC was determined by comparing discharge diagnoses to past medical history abstracted by medical record review. A new CCC was defined as any complex chronic condition that was captured in the discharge diagnoses but was not evident in the past medical history. By definition, all CCCs coded during birth hospitalization (eg, at discharge from the neonatal intensive care unit) were assigned to “new” CCC. We calculated a kappa statistic to determine interrater reliability in determining the designation of new CCC. A sensitivity analysis examining these birth CCCs was also performed comparing no new CCC, new CCC, and new CCC after birth hospitalization. The methods appendix provides additional information on considering new CCCs. New technology, abstracted from chart review, was defined as technology placed during hospitalization that remained in place at discharge. If a child with existing technology had additional technology placed during the hospitalization (eg, a new tracheostomy in a child with a previously placed enteral feeding tube), the encounter was considered as having new technology placed.
Covariates
We created different sets of multivariable models to account for patient/hospitalization characteristics.
Statistical Analysis
A review of 600 cases and 600 controls yields 89% power to detect statistical significance for covariates with an odds ratio of 1.25 (β = 0.22) if the candidate covariate has low to moderate correlation with other covariates (<0.3). If a candidate covariate has a moderate correlation with other covariates (0.6), we have 89% power to detect an odds ratio of 1.35 (β = 0.30).21 We calculated odds of 30-days unplanned readmission using conditional logistic regression to account for matched case-control design. All the analyses were performed using STATA 13 (Stata Corp., College Station, Texas).
RESULTS
Of the 41,422 eligible index hospitalizations during the study period, 9.4% resulted in a 30-day unplanned readmission. After randomly selecting one hospitalization per child, there were 781 eligible cases. We subsequent matched all but one eligible case to a control. We randomly selected encounters for medical record review, reviewing a total of 1,212 encounters. After excluding pairs with incomplete records, we included 595 cases and 595 controls in this analysis (Figure). Patient/hospitalization characteristics are displayed in Table 1. The most frequent primary discharge diagnoses are displayed in Appendix Table 1.
Models of Medical Complexity at Discharge
Polypharmacy after discharge was common for both readmitted and nonreadmitted patients. Children who experienced unplanned readmission in 30 days were discharged with a median of four different scheduled medications (interquartile range [IQR] 2,7) which translated into a median of six (IQR 3,12) scheduled doses in a 24-hour period. In comparison, children without an unplanned readmission had a median of two different scheduled medications (IQR 1,3) with a median of three (IQR 0,7) scheduled doses in a 24-hour period. Medical technology was more common in case children (42%) than in control children (14%). Central lines and enteral tubes were the most common forms of medical technology in both cases and controls. Home health referral was common in both cases (44%) and controls (23%; Table 1).
In Model 1 (adjusting only for patient characteristics; Table 3), being discharged on two or more scheduled medications was associated with higher odds of readmission compared to being discharged without medications, with additional medications associated with even higher odds of readmission. Children with any technology had higher odds of readmission than children without medical technology. Likewise, home healthcare visits after discharge were associated with elevated odds of readmission in multivariable analyses without LOS. However, after adding LOS to the model (Model 2), home healthcare visits were no longer significantly associated with readmission.
Change in Medical Complexity Models
The adjudication of new CCCs had good reliability (Κ = 0.72). New CCCs occurred in 18% and new technologies occurred in 17% of cases. Comparatively, new CCCs occurred in 10% and new technologies in 7% of hospitalizations in control children (Table 1). In bivariate analyses, both aspects of change in medical complexity were associated with higher odds of readmission (Table 2). In multivariate analysis with patient characteristics (Model 3; Table 3), all aspects of change in complexity were associated with elevated odds of readmission. A new CCC was associated with higher odds of readmission (adjusted OR (AOR) 1.75, 95% CI: 1.11-2.75) as was new technology during admission (AOR 1.84, 95%CI: 1.09-3.10). Furthermore, the odds of readmission for medical complexity variables (polypharmacy and home healthcare need) remained largely unchanged when adding the change in medical complexity variables (ie, comparing Model 1 and Model 3). However, when accounting for LOS (Model 4), neither the acquisition of a new CCC nor the addition of new technology was associated with readmission. The most common form of new technology was central line followed by nonsurgically placed enteral tube (Appendix Table 2). Finally, in sensitivity analyses (results not detailed), separating new CCC acquired at birth and new CCCs in nonbirth hospitalizations, compared to hospitalizations with no new CCC, yielded similar results as the primary analyses.
DISCUSSION
We examined multiple attributes of polypharmacy—the number of scheduled medications, number of as-needed medications, and number of scheduled doses per 24 hours. Interestingly, only the scheduled medications (count of medication and number of doses) were associated with elevated readmission risk. As-needed medications have heterogeneity in the level of importance from critical (eg, seizure rescue) to discretionary (eg, antipyretics, creams). The burden of managing these types of medications may still be high (ie, parents must decide when to administer a critical medication); however, this burden does not translate into increased readmission risk in this population.
Not surprisingly, greater medical complexity—as defined by higher numbers of scheduled discharge medications and technology assistance—is associated with 30-day readmission risk. Our analyses do not allow us to determine how much of the increased risk is due to additional care burden and risks of polypharmacy versus the inherent increase in complexity and severity of illness for which polypharmacy is a marker. Tailoring discharge regimens to the realities of daily life, with the goal of “minimally disruptive medicine”22,23 (eg, integrating manageable discharge medication routines into school and work schedules), is not a common feature of pediatric discharge planning. For adult patients with complex medical conditions, tailoring medication regimens in a minimally disruptive way is known to improve outcomes.24 Similarly, adopting minimally disruptive techniques to integrate the polypharmacy inherent in discharge could potentially mitigate some of the readmission risks for children and adolescents.
Contrary to our hypothesis, new technologies and new diagnoses did not confer additional readmission risk when accounting for LOS and patient characteristics. One potential explanation is varying risks conveyed by different types of new technologies placed during hospitalization. Central lines, the most common form of new technology, is associated with higher odds of reutilization in unadjusted analyses. However, the second most common form of new technology, nonsurgically placed enteral feeding tube, was not. Further analyses of the differential effects of new technology should be further examined in larger datasets. Additionally, the lack of additional readmission risk from new technology may relate to additional teaching and support provided to families of patients undergoing unfamiliar procedures offsets the risks inherent of greater complexity. If so, it may be that the more intensive teaching and postdischarge support provided to families with new technology or a new diagnosis could be replicated through refresher teaching during hospitalizations, when a patient’s state of health is status quo for the family (ie, the child was admitted and discharged with the same technology and diagnoses). This notion is supported by prior work that demonstrated successful readmission reduction interventions for children with chronic conditions often rely on enhanced education or coaching.25,26
We elected to present models both with and without LOS as a confounder because it is a potentially modifiable attribute of hospitalization. Change in medical complexity aspects were significantly associated with readmission in multivariable models without LOS. However, with the addition of LOS, they were no longer significant. Thus, the readmission risk of new complexity is accounted for by the readmission risk inherent in a longer LOS. This finding prompts additional questions that merit further study: is it that LOS is a general marker for heightened complexity, or is it that a longer LOS can modify readmission risk through additional in-hospital care and time for enhanced education?
Our study has several strengths. We were able to discern true complexity at the time of discharge through medical record review. For example, if a child had a peripherally inserted central catheter placed during hospitalization, it cannot be ascertained through administrative data without medical record review if the technology was removed or in place at discharge. Likewise, medical record review allows for identification of medical technology which is not surgically implanted (eg, nasogastric feeding tubes). Given the “fog” families report as part of their in-hospital experience and its threats to education and postdischarge contingency planning,17 we felt it important to evaluate medical technology regardless of whether or not it was surgically placed. Additionally, the more detailed and nuanced understanding gained of polypharmacy burden can better inform both risk prediction models and interventions to improve the transition from hospital to home.
This study
CONCLUSION
Medical complexity at discharge is associated with pediatric readmission risk. Contrary to our hypothesis, the addition of new technologies and new CCC diagnoses are not associated with pediatric readmission, after accounting for patient and hospitalization factors including LOS. The dynamics of LOS as a risk factor for readmission for children with medical complexity are likely multifaceted and merit further investigation in a multi-institutional study.
Disclosures
The authors report no potential conflicts of interest.
Funding
This work was supported by a grant from the Agency for Healthcare Research and Quality (1K08HS204735-01A1) and a grant from the Blue Cross Blue Shield of Michigan Foundation.
1. Diaz-Caneja A, Gledhill J, Weaver T, Nadel S, Garralda E. A child’s admission to hospital: a qualitative study examining the experiences of parents. Intensive Care Med. 2005;31(9):1248-1254. https://doi.org/10.1007/s00134-005-2728-8.
2. Lapillonne A, Regnault A, Gournay V, et al. Impact on parents of bronchiolitis hospitalization of full-term, preterm and congenital heart disease infants. BMC Pediatrics. 2012;12:171. https://doi.org/10.1186/1471-2431-12-171.
3. Leader S, Jacobson P, Marcin J, Vardis R, Sorrentino M, Murray D. A method for identifying the financial burden of hospitalized infants on families. Value Health. 2002;5(1):55-59. https://doi.org/10.1046/j.1524-4733.2002.51076.x.
4. Leidy NK, Margolis MK, Marcin JP, et al. The impact of severe respiratory syncytial virus on the child, caregiver, and family during hospitalization and recovery. Pediatrics. 2005;115(6):1536-1546. https://doi.org/10.1542/peds.2004-1149.
5. Rennick JE, Johnston CC, Dougherty G, Platt R, Ritchie JA. Children’s psychological responses after critical illness and exposure to invasive technology. J Dev Behav Pediatr. 2002;23(3):133-144. PubMed
6. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. https://doi.org/10.1056/NEJM199102073240604.
7. Kohn LT, Corrigan J, Donaldson MS. To err is human: building a safer health system. Washington DC: National Academy Press; 2000.
8. Landrigan CP, Parry GJ, Bones CB, Hackbarth AD, Goldmann DA, Sharek PJ. Temporal trends in rates of patient harm resulting from medical care. N Engl J Med. 2010;363(22):2124-2134. https://doi.org/10.1056/NEJMsa1004404.
9. Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of healthcare-associated infections. N Engl J Med. 2014;370(13):1198-1208. https://doi.org/10.1056/NEJMoa1306801.
10. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351.
11. Bardach NS, Vittinghoff E, Asteria-Penaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. https://doi.org/10.1542/peds.2012-3527.
12. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-day hospital readmissions at a children’s hospital. Pediatrics. 2016;138(2):pii: e20154182. https://doi.org/10.1542/peds.2015-4182.
13. Bucholz EM, Gay JC, Hall M, Harris M, Berry JG. Timing and causes of common pediatric readmissions. J Pediatr. 2018;200:240-248. https://doi.org/10.1016/j.jpeds.2018.04.044.
14. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. https://doi.org/10.1001/jama.2011.122.
15. Winer JC, Aragona E, Fields AI, Stockwell DC. Comparison of clinical risk factors among pediatric patients with single admission, multiple admissions (without any 7-day readmissions), and 7-day readmission. Hosp Pediatr. 2016;6(3):119-125. https://doi.org/10.1542/hpeds.2015-0110.
16. Brittan MS, Martin S, Anderson L, Moss A, Torok MR. An electronic health record tool designed to improve pediatric hospital discharge has low predictive utility for readmissions. J Hosp Med. 2018;13(11):779-782. https://doi.org/10.12788/jhm.3043.
17. Solan LG, Beck AF, Brunswick SA, et al. The family perspective on hospital to home transitions: a qualitative study. Pediatrics. 2015;136(6):e1539-e1549. https://doi.org/10.1542/peds.2015-2098.
18. Auger KA, Mueller EL, Weinberg SH, et al. A validated method for identifying unplanned pediatric readmission. J Pediatr. 2016;170:105-112. https://doi.org/10.1016/j.jpeds.2015.11.051.
19. Auger KA, Shah SS, Davis MD, Brady PW. Counting the Ways to Count Medications: The Challenges of Defining Pediatric Polypharmacy. J Hosp Med. 2019;14(8):506-507. https://doi.org/10.12788/jhm.3213.
20. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatrics. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199.
21. Hsieh FY. Sample size tables for logistic regression. Stat Med. 1989;8(7):795-802. https://doi.org/10.1002/sim.4780080704.
22. May C, Montori VM, Mair FS. We need minimally disruptive medicine. BMJ. 2009;339:b2803. https://doi.org/10.1136/bmj.b2803.
23. Leppin AL, Montori VM, Gionfriddo MR. Minimally disruptive medicine: a pragmatically comprehensive model for delivering care to patients with multiple chronic conditions. Healthcare (Basel). 2015;3(1):50-63. https://doi.org/10.3390/healthcare3010050.
24. Serrano V, Spencer-Bonilla G, Boehmer KR, Montori VM. Minimally disruptive medicine for patients with diabetes. Curr Diab Rep. 2017;17(11):104. https://doi.org/10.1007/s11892-017-0935-7.
25. Auger KA, Kenyon CC, Feudtner C, Davis MM. Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2013;9(4):251-260. https://doi.org/10.1002/jhm.2134.
26. Coller RJ, Klitzner TS, Lerner CF, et al. Complex care hospital use and postdischarge coaching: a randomized controlled trial. Pediatrics. 2018;142(2):pii: e20174278. https://doi.org/10.1542/peds.2017-4278.
27. Hain PD, Gay JC, Berutti TW, Whitney GM, Wang W, Saville BR. Preventability of early readmissions at a children’s hospital. Pediatrics. 2013;131(1):e171-e181. https://doi.org/10.1542/peds.2012-0820.
28. Auger KA, Teufel RJ, 2nd, Harris JM, 2nd, et al. Children’s hospital characteristics and readmission metrics. Pediatrics. 2017;139(2). https://doi.org/10.1542/peds.2016-1720.
29. Gay JC, Agrawal R, Auger KA, et al. Rates and impact of potentially preventable readmissions at children’s hospitals. J Pediatr. 2015;166(3):613-619 e615. https://doi.org/10.1016/j.jpeds.2014.10.052.
1. Diaz-Caneja A, Gledhill J, Weaver T, Nadel S, Garralda E. A child’s admission to hospital: a qualitative study examining the experiences of parents. Intensive Care Med. 2005;31(9):1248-1254. https://doi.org/10.1007/s00134-005-2728-8.
2. Lapillonne A, Regnault A, Gournay V, et al. Impact on parents of bronchiolitis hospitalization of full-term, preterm and congenital heart disease infants. BMC Pediatrics. 2012;12:171. https://doi.org/10.1186/1471-2431-12-171.
3. Leader S, Jacobson P, Marcin J, Vardis R, Sorrentino M, Murray D. A method for identifying the financial burden of hospitalized infants on families. Value Health. 2002;5(1):55-59. https://doi.org/10.1046/j.1524-4733.2002.51076.x.
4. Leidy NK, Margolis MK, Marcin JP, et al. The impact of severe respiratory syncytial virus on the child, caregiver, and family during hospitalization and recovery. Pediatrics. 2005;115(6):1536-1546. https://doi.org/10.1542/peds.2004-1149.
5. Rennick JE, Johnston CC, Dougherty G, Platt R, Ritchie JA. Children’s psychological responses after critical illness and exposure to invasive technology. J Dev Behav Pediatr. 2002;23(3):133-144. PubMed
6. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. https://doi.org/10.1056/NEJM199102073240604.
7. Kohn LT, Corrigan J, Donaldson MS. To err is human: building a safer health system. Washington DC: National Academy Press; 2000.
8. Landrigan CP, Parry GJ, Bones CB, Hackbarth AD, Goldmann DA, Sharek PJ. Temporal trends in rates of patient harm resulting from medical care. N Engl J Med. 2010;363(22):2124-2134. https://doi.org/10.1056/NEJMsa1004404.
9. Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of healthcare-associated infections. N Engl J Med. 2014;370(13):1198-1208. https://doi.org/10.1056/NEJMoa1306801.
10. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351.
11. Bardach NS, Vittinghoff E, Asteria-Penaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. https://doi.org/10.1542/peds.2012-3527.
12. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-day hospital readmissions at a children’s hospital. Pediatrics. 2016;138(2):pii: e20154182. https://doi.org/10.1542/peds.2015-4182.
13. Bucholz EM, Gay JC, Hall M, Harris M, Berry JG. Timing and causes of common pediatric readmissions. J Pediatr. 2018;200:240-248. https://doi.org/10.1016/j.jpeds.2018.04.044.
14. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. https://doi.org/10.1001/jama.2011.122.
15. Winer JC, Aragona E, Fields AI, Stockwell DC. Comparison of clinical risk factors among pediatric patients with single admission, multiple admissions (without any 7-day readmissions), and 7-day readmission. Hosp Pediatr. 2016;6(3):119-125. https://doi.org/10.1542/hpeds.2015-0110.
16. Brittan MS, Martin S, Anderson L, Moss A, Torok MR. An electronic health record tool designed to improve pediatric hospital discharge has low predictive utility for readmissions. J Hosp Med. 2018;13(11):779-782. https://doi.org/10.12788/jhm.3043.
17. Solan LG, Beck AF, Brunswick SA, et al. The family perspective on hospital to home transitions: a qualitative study. Pediatrics. 2015;136(6):e1539-e1549. https://doi.org/10.1542/peds.2015-2098.
18. Auger KA, Mueller EL, Weinberg SH, et al. A validated method for identifying unplanned pediatric readmission. J Pediatr. 2016;170:105-112. https://doi.org/10.1016/j.jpeds.2015.11.051.
19. Auger KA, Shah SS, Davis MD, Brady PW. Counting the Ways to Count Medications: The Challenges of Defining Pediatric Polypharmacy. J Hosp Med. 2019;14(8):506-507. https://doi.org/10.12788/jhm.3213.
20. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatrics. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199.
21. Hsieh FY. Sample size tables for logistic regression. Stat Med. 1989;8(7):795-802. https://doi.org/10.1002/sim.4780080704.
22. May C, Montori VM, Mair FS. We need minimally disruptive medicine. BMJ. 2009;339:b2803. https://doi.org/10.1136/bmj.b2803.
23. Leppin AL, Montori VM, Gionfriddo MR. Minimally disruptive medicine: a pragmatically comprehensive model for delivering care to patients with multiple chronic conditions. Healthcare (Basel). 2015;3(1):50-63. https://doi.org/10.3390/healthcare3010050.
24. Serrano V, Spencer-Bonilla G, Boehmer KR, Montori VM. Minimally disruptive medicine for patients with diabetes. Curr Diab Rep. 2017;17(11):104. https://doi.org/10.1007/s11892-017-0935-7.
25. Auger KA, Kenyon CC, Feudtner C, Davis MM. Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2013;9(4):251-260. https://doi.org/10.1002/jhm.2134.
26. Coller RJ, Klitzner TS, Lerner CF, et al. Complex care hospital use and postdischarge coaching: a randomized controlled trial. Pediatrics. 2018;142(2):pii: e20174278. https://doi.org/10.1542/peds.2017-4278.
27. Hain PD, Gay JC, Berutti TW, Whitney GM, Wang W, Saville BR. Preventability of early readmissions at a children’s hospital. Pediatrics. 2013;131(1):e171-e181. https://doi.org/10.1542/peds.2012-0820.
28. Auger KA, Teufel RJ, 2nd, Harris JM, 2nd, et al. Children’s hospital characteristics and readmission metrics. Pediatrics. 2017;139(2). https://doi.org/10.1542/peds.2016-1720.
29. Gay JC, Agrawal R, Auger KA, et al. Rates and impact of potentially preventable readmissions at children’s hospitals. J Pediatr. 2015;166(3):613-619 e615. https://doi.org/10.1016/j.jpeds.2014.10.052.
© 2019 Society of Hospital Medicine
Opioid Utilization and Perception of Pain Control in Hospitalized Patients: A Cross-Sectional Study of 11 Sites in 8 Countries
Since 2000, the United States has seen a marked increase in opioid prescribing1-3 and opioid-related complications, including overdoses, hospitalizations, and deaths.2,4,5 A study from 2015 showed that more than one-third of the US civilian noninstitutionalized population reported receiving an opioid prescription in the prior year, with 12.5% reporting misuse, and, of those, 16.7% reported a prescription use disorder.6 While there has been a slight decrease in opioid prescriptions in the US since 2012, rates of opioid prescribing in 2015 were three times higher than in 1999 and approximately four times higher than in Europe in 2015.3,7
Pain is commonly reported by hospitalized patients,8,9 and opioids are often a mainstay of treatment;9,10 however, treatment with opioids can have a number of adverse outcomes.2,10,11 Short-term exposure to opioids can lead to long-term use,12-16 and patients on opioids are at an increased risk for subsequent hospitalization and longer inpatient lengths of stay.5
Physician prescribing practices for opioids and patient expectations for pain control vary as a function of geographic region and culture,10,12,17,18 and pain is influenced by the cultural context in which it occurs.17,19-22 Treatment of pain may also be affected by limited access to or restrictions on selected medications, as well as by cultural biases.23 Whether these variations in the treatment of pain are reflected in patients’ satisfaction with pain control is uncertain.
We sought to compare the inpatient analgesic prescribing practices and patients’ perceptions of pain control for medical patients in four teaching hospitals in the US and in seven teaching hospitals in seven other countries.
METHODS
Study Design
We utilized a cross-sectional, observational design. The study was approved by the Institutional Review Boards at all participating sites.
Setting
The study was conducted at 11 academic hospitals in eight countries from October 8, 2013 to August 31, 2015. Sites in the US included Denver Health in Denver, Colorado; the University of Colorado Hospital in Aurora, Colorado; Hennepin Healthcare in Minneapolis, Minnesota; and Legacy Health in Portland, Oregon. Sites outside the US included McMaster University in Hamilton, Ontario, Canada; Hospital de la Santa Creu i Sant Pau, Universitat Autonòma de Barcelona in Barcelona, Spain; the University of Study of Milan and the University Ospedale “Luigi Sacco” in Milan, Italy, the National Taiwan University Hospital, in Taipei, Taiwan, the University of Ulsan College of Medicine, Asan Medical Center, in Seoul, Korea, the Imperial College, Chelsea and Westminster Hospital, in London, United Kingdom and Dunedin Hospital, Dunedin, New Zealand.
Inclusion and Exclusion Criteria
We included patients 18-89 years of age (20-89 in Taiwan because patients under 20 years of age in this country are a restricted group with respect to participating in research), admitted to an internal medicine service from the Emergency Department or Urgent Care clinic with an acute illness for a minimum of 24 hours (with time zero defined as the time care was initiated in the Emergency Department or Urgent Care Clinic), who reported pain at some time during the first 24-36 hours of their hospitalization and who provided informed consent. In the US, “admission” included both observation and inpatient status. We limited the patient population to those admitted via emergency departments and urgent care clinics in order to enroll similar patient populations across sites.
Scheduled admissions, patients transferred from an outside facility, patients admitted directly from a clinic, and those receiving care in intensive care units were excluded. We also excluded patients who were incarcerated, pregnant, those who received major surgery within the previous 14 days, those with a known diagnosis of active cancer, and those who were receiving palliative or hospice care. Patients receiving care from an investigator in the study at the time of enrollment were not eligible due to the potential conflict of interest.
Patient Screening
Primary teams were contacted to determine if any patients on their service might meet the criteria for inclusion in the study on preselected study days chosen on the basis of the research team’s availability. Identified patients were then screened to establish if they met the eligibility criteria. Patients were asked directly if they had experienced pain during their preadmission evaluation or during their hospitalization.
Data Collection
All patients were hospitalized at the time they gave consent and when data were collected. Data were collected via interviews with patients, as well as through chart review. We recorded patients’ age, gender, race, admitting diagnosis(es), length of stay, psychiatric illness, illicit drug use, whether they reported receiving opioid analgesics at the time of hospitalization, whether they were prescribed opioids and/or nonopioid analgesics during their hospitalization, the median and maximum doses of opioids prescribed and dispensed, and whether they were discharged on opioids. The question of illicit drug use was asked of all patients with the exception of those hospitalized in South Korea due to potential legal implications.
Opioid prescribing and receipt of opioids was recorded based upon current provider orders and medication administration records, respectively. Perception of and satisfaction with pain control was assessed with the American Pain Society Patient Outcome Questionnaire–Modified (APS-POQ-Modified).24,25 Versions of this survey have been validated in English as well as in other languages and cultures.26-28 Because hospitalization practices could differ across hospitals and in different countries, we compared patients’ severity of illness by using Charlson comorbidity scores. Consent forms and the APS-POQ were translated into each country’s primary language according to established processes.29 The survey was filled out by having site investigators read questions aloud and by use of a large-font visual analog scale to aid patients’ verbal responses.
Data were collected and managed using a secure, web-based application electronic data capture tool (Research Electronic Data Capture [REDCap], Nashville, Tennessee), hosted at Denver Health.30
Study Size
Preliminary data from the internal medicine units at our institution suggested that 40% of patients without cancer received opioid analgesics during their hospitalization. Assuming 90% power to detect an absolute difference in the proportion of inpatient medical patients who are receiving opioid analgesics during their hospital stay of 17%, a two-sided type 1 error rate of 0.05, six hospitals in the US, and nine hospitals from all other countries, we calculated an initial sample size of 150 patients per site. This sample size was considered feasible for enrollment in a busy inpatient clinical setting. Study end points were to either reach the goal number of patients (150 per site) or the predetermined study end date, whichever came first.
Data Analysis
We generated means with standard deviations (SDs) and medians with interquartile ranges (IQRs) for normally and nonnormally distributed continuous variables, respectively, and frequencies for categorical variables. We used linear mixed modeling for the analysis of continuous variables. For binary outcomes, our data were fitted to a generalized linear mixed model with logit as the link function and a binary distribution. For ordinal variables, specifically patient-reported satisfaction with pain control and the opinion statements, the data were fitted to a generalized linear mixed model with a cumulative logit link and a multinomial distribution. Hospital was included as a random effect in all models to account for patients cared for in the same hospital.
Country of origin, dichotomized as US or non-US, was the independent variable of interest for all models. An interaction term for exposure to opioids prior to admission and country was entered into all models to explore whether differences in the effect of country existed for patients who reported taking opioids prior to admission and those who did not.
The models for the frequency with which analgesics were given, doses of opioids given during hospitalization and at discharge, patient-reported pain score, and patient-reported satisfaction with pain control were adjusted for (1) age, (2) gender, (3) Charlson Comorbidity Index, (4) length of stay, (5) history of illicit drug use, (6) history of psychiatric illness, (7) daily dose in morphine milligram equivalents (MME) for opioids prior to admission, (8) average pain score, and (9) hospital. The patient-reported satisfaction with pain control model was also adjusted for whether or not opioids were given to the patient during their hospitalization. P < .05 was considered to indicate significance. All analyses were performed using SAS Enterprise Guide 7.1 (SAS Institute, Inc., Cary, North Carolina). We reported data on medications that were prescribed and dispensed (as opposed to just prescribed and not necessarily given). Opioids prescribed at discharge represented the total possible opioids that could be given based upon the order/prescription (eg, oxycodone 5 mg every 6 hours as needed for pain would be counted as 20 mg/24 hours maximum possible dose followed by conversion to MME).
Missing Data
When there were missing data, a query was sent to sites to verify if the data were retrievable. If retrievable, the data were then entered. Data were missing in 5% and 2% of patients who did or did not report taking an opioid prior to admission, respectively. If a variable was included in a specific statistical test, then subjects with missing data were excluded from that analysis (ie, complete case analysis).
RESULTS
We approached 1,309 eligible patients, of which 981 provided informed consent, for a response rate of 75%; 503 from the US and 478 patients from other countries (Figure). In unadjusted analyses, we found no significant differences between US and non-US patients in age (mean age 51, SD 15 vs 59, SD 19; P = .30), race, ethnicity, or Charlson comorbidity index scores (median 2, IQR 1-3 vs 3, IQR 1-4; P = .45). US patients had shorter lengths of stay (median 3 days, IQR 2-4 vs 6 days, IQR 3-11; P = .04), a more frequent history of illicit drug use (33% vs 6%; P = .003), a higher frequency of psychiatric illness (27% vs 8%; P < .0001), and more were receiving opioid analgesics prior to admission (38% vs 17%; P = .007) than those hospitalized in other countries (Table 1, Appendix 1). The primary admitting diagnoses for all patients in the study are listed in Appendix 2. Opioid prescribing practices across the individual sites are shown in Appendix 3.
Patients Taking Opioids Prior to Admission
After adjusting for relevant covariates, we found that more patients in the US were given opioids during their hospitalization and in higher doses than patients from other countries and more were prescribed opioids at discharge. Fewer patients in the US were dispensed nonopioid analgesics during their hospitalization than patients from other countries, but this difference was not significant (Table 2). Appendix 4 shows the types of nonopioid pain medications prescribed in the US and other countries.
After adjustment for relevant covariates, US patients reported greater pain severity at the time they completed their pain surveys. We found no significant difference in satisfaction with pain control between patients from the US and other countries in the models, regardless of whether we included average pain score or opioid receipt during hospitalization in the model (Table 3).
In unadjusted analyses, compared with patients hospitalized in other countries, more patients in the US stated that they would like a stronger dose of analgesic if they were still in pain, though the difference was nonsignificant, and US patients were more likely to agree with the statement that people become addicted to pain medication easily and less likely to agree with the statement that it is easier to endure pain than deal with the side effects of pain medications (Table 3).
Patients Not Taking Opioids Prior to Admission
After adjusting for relevant covariates, we found no significant difference in the proportion of US patients provided with nonopioid pain medications during their hospitalization compared with patients in other countries, but a greater percentage of US patients were given opioids during their hospitalization and at discharge and in higher doses (Table 2).
After adjusting for relevant covariates, US patients reported greater pain severity at the time they completed their pain surveys and greater pain severity in the 24-36 hours prior to completing the survey than patients from other countries, but we found no difference in patient satisfaction with pain control (Table 3). After we included the average pain score and whether or not opioids were given to the patient during their hospitalization in this model, patients in the US were more likely to report a higher level of satisfaction with pain control than patients in all other countries (P = .001).
In unadjusted analyses, compared with patients hospitalized in other countries, those in the US were less likely to agree with the statement that good patients avoid talking about pain (Table 3).
Patient Satisfaction and Opioid Receipt
Among patients cared for in the US, after controlling for the average pain score, we did not find a significant association between receiving opioids while in the hospital and satisfaction with pain control for patients who either did or did not endorse taking opioids prior to admission (P = .38 and P = .24, respectively). Among patients cared for in all other countries, after controlling for the average pain score, we found a significant association between receiving opioids while in the hospital and a lower level of satisfaction with pain control for patients who reported taking opioids prior to admission (P = .02) but not for patients who did not report taking opioids prior to admission (P = .08).
DISCUSSION
Compared with patients hospitalized in other countries, a greater percentage of those hospitalized in the US were prescribed opioid analgesics both during hospitalization and at the time of discharge, even after adjustment for pain severity. In addition, patients hospitalized in the US reported greater pain severity at the time they completed their pain surveys and in the 24 to 36 hours prior to completing the survey than patients from other countries. In this sample, satisfaction, beliefs, and expectations about pain control differed between patients in the US and other sites. Our study also suggests that opioid receipt did not lead to improved patient satisfaction with pain control.
The frequency with which we observed opioid analgesics being prescribed during hospitalization in US hospitals (79%) was higher than the 51% of patients who received opioids reported by Herzig and colleagues.10 Patients in our study had a higher prevalence of illicit drug abuse and psychiatric illness, and our study only included patients who reported pain at some point during their hospitalization. We also studied prescribing practices through analysis of provider orders and medication administration records at the time the patient was hospitalized.
While we observed that physicians in the US more frequently prescribed opioid analgesics during hospitalizations than physicians working in other countries, we also observed that patients in the US reported higher levels of pain during their hospitalization. After adjusting for a number of variables, including pain severity, however, we still found that opioids were more commonly prescribed during hospitalizations by physicians working in the US sites studied than by physicians in the non-US sites.
Opioid prescribing practices varied across the sites sampled in our study. While the US sites, Taiwan, and Korea tended to be heavier utilizers of opioids during hospitalization, there were notable differences in discharge prescribing of opioids, with the US sites more commonly prescribing opioids and higher MME for patients who did not report taking opioids prior to their hospitalization (Appendix 3). A sensitivity analysis was conducted excluding South Korea from modeling, given that patients there were not asked about illicit opioid use. There were no important changes in the magnitude or direction of the results.
Our study supports previous studies indicating that there are cultural and societal differences when it comes to the experience of pain and the expectations around pain control.17,20-22,31 Much of the focus on reducing opioid utilization has been on provider practices32 and on prescription drug monitoring programs.33 Our findings suggest that another area of focus that may be important in mitigating the opioid epidemic is patient expectations of pain control.
Our study has a number of strengths. First, we included 11 hospitals from eight different countries. Second, we believe this is the first study to assess opioid prescribing and dispensing practices during hospitalization as well as at the time of discharge. Third, patient perceptions of pain control were assessed in conjunction with analgesic prescribing and were assessed during hospitalization. Fourth, we had high response rates for patient participation in our study. Fifth, we found much larger differences in opioid prescribing than anticipated, and thus, while we did not achieve the sample size originally planned for either the number of hospitals or patients enrolled per hospital, we were sufficiently powered. This is likely secondary to the fact that the population we studied was one that specifically reported pain, resulting in the larger differences seen.
Our study also had a number of limitations. First, the prescribing practices in countries other than the US are represented by only one hospital per country and, in some countries, by limited numbers of patients. While we studied four sites in the US, we did not have a site in the Northeast, a region previously shown to have lower prescribing rates.10 Additionally, patient samples for the US sites compared with the sites in other countries varied considerably with respect to ethnicity. While some studies in US patients have shown that opioid prescribing may vary based on race/ethnicity,34 we are uncertain as to how this might impact a study that crosses multiple countries. We also had a low number of patients receiving opioids prior to hospitalization for several of the non-US countries, which reduced the power to detect differences in this subgroup. Previous research has shown that there are wide variations in prescribing practices even within countries;10,12,18 therefore, caution should be taken when generalizing our findings. Second, we assessed analgesic prescribing patterns and pain control during the first 24 to 36 hours of hospitalization and did not consider hospital days beyond this timeframe with the exception of noting what medications were prescribed at discharge. We chose this methodology in an attempt to eliminate as many differences that might exist in the duration of hospitalization across many countries. Third, investigators in the study administered the survey, and respondents may have been affected by social desirability bias in how the survey questions were answered. Because investigators were not a part of the care team of any study patients, we believe this to be unlikely. Fourth, our study was conducted from October 8, 2013 to August 31, 2015 and the opioid epidemic is dynamic. Accordingly, our data may not reflect current opioid prescribing practices or patients’ current beliefs regarding pain control. Fifth, we did not collect demographic data on the patients who did not participate and could not look for systematic differences between participants and nonparticipants. Sixth, we relied on patients to self-report whether they were taking opioids prior to hospitalization or using illicit drugs. Seventh, we found comorbid mental health conditions to be more frequent in the US population studied. Previous work has shown regional variation in mental health conditions,35,36 which could have affected our findings. To account for this, our models included psychiatric illness.
CONCLUSIONS
Our data suggest that physicians in the US may prescribe opioids more frequently during patients’ hospitalizations and at discharge than their colleagues in other countries. We also found that patient satisfaction, beliefs, and expectations about pain control differed between patients in the US and other sites. Although the small number of hospitals included in our sample coupled with the small sample size in some of the non-US countries limits the generalizability of our findings, the data suggest that reducing the opioid epidemic in the US may require addressing patients’ expectations regarding pain control in addition to providers’ inpatient analgesic prescribing patterns.
Disclosures
The authors report no conflicts of interest.
Funding
The authors report no funding source for this work.
1. Pletcher MJ, Kertesz SG, Kohn MA, Gonzales R. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA. 2008;299(1):70-78. https://doi.org/10.1001/jama.2007.64.
2. Herzig SJ. Growing concerns regarding long-term opioid use: the hospitalization hazard. J Hosp Med. 2015;10(7):469-470. https://doi.org/10.1002/jhm.2369.
3. Guy GP Jr, Zhang K, Bohm MK, et al. Vital Signs: changes in opioid prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(26):697-704. https://doi.org/10.15585/mmwr.mm6626a4.
4. Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981-1985. https://doi.org/10.1056/NEJMp1011512.
5. Liang Y, Turner BJ. National cohort study of opioid analgesic dose and risk of future hospitalization. J Hosp Med. 2015;10(7):425-431. https://doi.org/10.1002/jhm.2350.
6. Han B, Compton WM, Blanco C, et al. Prescription opioid use, misuse, and use disorders in U.S. Adults: 2015 national survey on drug use and health. Ann Intern Med. 2017;167(5):293-301. https://doi.org/10.7326/M17-0865.
7. Schuchat A, Houry D, Guy GP, Jr. New data on opioid use and prescribing in the United States. JAMA. 2017;318(5):425-426. https://doi.org/10.1001/jama.2017.8913.
8. Sawyer J, Haslam L, Robinson S, Daines P, Stilos K. Pain prevalence study in a large Canadian teaching hospital. Pain Manag Nurs. 2008;9(3):104-112. https://doi.org/10.1016/j.pmn.2008.02.001.
9. Gupta A, Daigle S, Mojica J, Hurley RW. Patient perception of pain care in hospitals in the United States. J Pain Res. 2009;2:157-164. https://doi.org/10.2147/JPR.S7903.
10. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. https://doi.org/10.1002/jhm.2102.
11. Kanjanarat P, Winterstein AG, Johns TE, et al. Nature of preventable adverse drug events in hospitals: a literature review. Am J Health Syst Pharm. 2003;60(17):1750-1759. https://doi.org/10.1093/ajhp/60.17.1750.
12. Jena AB, Goldman D, Karaca-Mandic P. Hospital prescribing of opioids to medicare beneficiaries. JAMA Intern Med. 2016;176(7):990-997. https://doi.org/10.1001/jamainternmed.2016.2737.
13. Hooten WM, St Sauver JL, McGree ME, Jacobson DJ, Warner DO. Incidence and risk factors for progression From short-term to episodic or long-term opioid prescribing: A population-based study. Mayo Clin Proc. 2015;90(7):850-856. https://doi.org/10.1016/j.mayocp.2015.04.012.
14. Alam A, Gomes T, Zheng H, et al. Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med. 2012;172(5):425-430. https://doi.org/10.1001/archinternmed.2011.1827.
15. Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med. 2017;376(7):663-673. https://doi.org/10.1056/NEJMsa1610524.
16. Calcaterra SL, Scarbro S, Hull ML, et al. Prediction of future chronic opioid use Among hospitalized patients. J Gen Intern Med. 2018;33(6):898-905. https://doi.org/10.1007/s11606-018-4335-8.
17. Callister LC. Cultural influences on pain perceptions and behaviors. Home Health Care Manag Pract. 2003;15(3):207-211. https://doi.org/10.1177/1084822302250687.
18. Paulozzi LJ, Mack KA, Hockenberry JM. Vital signs: Variation among states in prescribing of opioid pain relievers and benzodiazepines--United States, 2012. J Saf Res. 2014;63(26):563-568. https://doi.org/10.1016/j.jsr.2014.09.001.
19. Callister LC, Khalaf I, Semenic S, Kartchner R, Vehvilainen-Julkunen K. The pain of childbirth: perceptions of culturally diverse women. Pain Manag Nurs. 2003;4(4):145-154. https://doi.org/10.1016/S1524-9042(03)00028-6.
20. Moore R, Brødsgaard I, Mao TK, Miller ML, Dworkin SF. Perceived need for local anesthesia in tooth drilling among Anglo-Americans, Chinese, and Scandinavians. Anesth Prog. 1998;45(1):22-28.
21. Kankkunen PM, Vehviläinen-Julkunen KM, Pietilä AM, et al. A tale of two countries: comparison of the perceptions of analgesics among Finnish and American parents. Pain Manag Nurs. 2008;9(3):113-119. https://doi.org/10.1016/j.pmn.2007.12.003.
22. Hanoch Y, Katsikopoulos KV, Gummerum M, Brass EP. American and German students’ knowledge, perceptions, and behaviors with respect to over-the-counter pain relievers. Health Psychol. 2007;26(6):802-806. https://doi.org/10.1037/0278-6133.26.6.802.
23. Manjiani D, Paul DB, Kunnumpurath S, Kaye AD, Vadivelu N. Availability and utilization of opioids for pain management: global issues. Ochsner J. 2014;14(2):208-215.
24. Quality improvement guidelines for the treatment of acute pain and cancer pain. JAMA. 1995;274(23):1874-1880.
25. McNeill JA, Sherwood GD, Starck PL, Thompson CJ. Assessing clinical outcomes: patient satisfaction with pain management. J Pain Symptom Manag. 1998;16(1):29-40. https://doi.org/10.1016/S0885-3924(98)00034-7.
26. Ferrari R, Novello C, Catania G, Visentin M. Patients’ satisfaction with pain management: the Italian version of the Patient Outcome Questionnaire of the American Pain Society. Recenti Prog Med. 2010;101(7–8):283-288.
27. Malouf J, Andión O, Torrubia R, Cañellas M, Baños JE. A survey of perceptions with pain management in Spanish inpatients. J Pain Symptom Manag. 2006;32(4):361-371. https://doi.org/10.1016/j.jpainsymman.2006.05.006.
28. Gordon DB, Polomano RC, Pellino TA, et al. Revised American Pain Society Patient Outcome Questionnaire (APS-POQ-R) for quality improvement of pain management in hospitalized adults: preliminary psychometric evaluation. J Pain. 2010;11(11):1172-1186. https://doi.org/10.1016/j.jpain.2010.02.012.
29. Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976). 2000;25(24):3186-3191. https://doi.org/10.1097/00007632-200012150-00014.
30. Harris PA, Taylor R, Thielke R, et al. Research Electronic Data Capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010.
31. Duman F. After surgery in Germany, I wanted Vicodin, not herbal tea. New York Times. January 27, 2018. https://www.nytimes.com/2018/01/27/opinion/sunday/surgery-germany-vicodin.html. Accessed November 6, 2018.
32. Beaudoin FL, Banerjee GN, Mello MJ. State-level and system-level opioid prescribing policies: the impact on provider practices and overdose deaths, a systematic review. J Opioid Manag. 2016;12(2):109-118. https://doi.org/10.5055/jom.2016.0322. 33. Bao Y, Wen K, Johnson P, et al. Assessing the impact of state policies for prescription drug monitoring programs on high-risk opioid prescriptions. Health Aff (Millwood). 2018;37(10):1596-1604. https://doi.org/10.1377/hlthaff.2018.0512.
33. Bao Y, Wen K, Johnson P, et al. Assessing the impact of state policies for prescription drug monitoring programs on high-risk opioid prescriptions. Health Aff (Millwood). 2018;37(10):1596-1604. https://doi.org/10.1377/hlthaff.2018.0512.
34. Friedman J, Kim D, Schneberk T, et al. Assessment of racial/ethnic and income disparities in the prescription of opioids and other controlled medications in California. JAMA Intern Med. 2019. https://doi.org/10.1001/jamainternmed.2018.6721.
35. Steel Z, Marnane C, Iranpour C, et al. The global prevalence of common mental disorders: a systematic review and meta-analysis 1980-2013. Int J Epidemiol. 2014;43(2):476-493. https://doi.org/10.1093/ije/dyu038.
36. Simon GE, Goldberg DP, Von Korff M, Ustün TB. Understanding cross-national differences in depression prevalence. Psychol Med. 2002;32(4):585-594. https://doi.org/10.1017/S0033291702005457.
Since 2000, the United States has seen a marked increase in opioid prescribing1-3 and opioid-related complications, including overdoses, hospitalizations, and deaths.2,4,5 A study from 2015 showed that more than one-third of the US civilian noninstitutionalized population reported receiving an opioid prescription in the prior year, with 12.5% reporting misuse, and, of those, 16.7% reported a prescription use disorder.6 While there has been a slight decrease in opioid prescriptions in the US since 2012, rates of opioid prescribing in 2015 were three times higher than in 1999 and approximately four times higher than in Europe in 2015.3,7
Pain is commonly reported by hospitalized patients,8,9 and opioids are often a mainstay of treatment;9,10 however, treatment with opioids can have a number of adverse outcomes.2,10,11 Short-term exposure to opioids can lead to long-term use,12-16 and patients on opioids are at an increased risk for subsequent hospitalization and longer inpatient lengths of stay.5
Physician prescribing practices for opioids and patient expectations for pain control vary as a function of geographic region and culture,10,12,17,18 and pain is influenced by the cultural context in which it occurs.17,19-22 Treatment of pain may also be affected by limited access to or restrictions on selected medications, as well as by cultural biases.23 Whether these variations in the treatment of pain are reflected in patients’ satisfaction with pain control is uncertain.
We sought to compare the inpatient analgesic prescribing practices and patients’ perceptions of pain control for medical patients in four teaching hospitals in the US and in seven teaching hospitals in seven other countries.
METHODS
Study Design
We utilized a cross-sectional, observational design. The study was approved by the Institutional Review Boards at all participating sites.
Setting
The study was conducted at 11 academic hospitals in eight countries from October 8, 2013 to August 31, 2015. Sites in the US included Denver Health in Denver, Colorado; the University of Colorado Hospital in Aurora, Colorado; Hennepin Healthcare in Minneapolis, Minnesota; and Legacy Health in Portland, Oregon. Sites outside the US included McMaster University in Hamilton, Ontario, Canada; Hospital de la Santa Creu i Sant Pau, Universitat Autonòma de Barcelona in Barcelona, Spain; the University of Study of Milan and the University Ospedale “Luigi Sacco” in Milan, Italy, the National Taiwan University Hospital, in Taipei, Taiwan, the University of Ulsan College of Medicine, Asan Medical Center, in Seoul, Korea, the Imperial College, Chelsea and Westminster Hospital, in London, United Kingdom and Dunedin Hospital, Dunedin, New Zealand.
Inclusion and Exclusion Criteria
We included patients 18-89 years of age (20-89 in Taiwan because patients under 20 years of age in this country are a restricted group with respect to participating in research), admitted to an internal medicine service from the Emergency Department or Urgent Care clinic with an acute illness for a minimum of 24 hours (with time zero defined as the time care was initiated in the Emergency Department or Urgent Care Clinic), who reported pain at some time during the first 24-36 hours of their hospitalization and who provided informed consent. In the US, “admission” included both observation and inpatient status. We limited the patient population to those admitted via emergency departments and urgent care clinics in order to enroll similar patient populations across sites.
Scheduled admissions, patients transferred from an outside facility, patients admitted directly from a clinic, and those receiving care in intensive care units were excluded. We also excluded patients who were incarcerated, pregnant, those who received major surgery within the previous 14 days, those with a known diagnosis of active cancer, and those who were receiving palliative or hospice care. Patients receiving care from an investigator in the study at the time of enrollment were not eligible due to the potential conflict of interest.
Patient Screening
Primary teams were contacted to determine if any patients on their service might meet the criteria for inclusion in the study on preselected study days chosen on the basis of the research team’s availability. Identified patients were then screened to establish if they met the eligibility criteria. Patients were asked directly if they had experienced pain during their preadmission evaluation or during their hospitalization.
Data Collection
All patients were hospitalized at the time they gave consent and when data were collected. Data were collected via interviews with patients, as well as through chart review. We recorded patients’ age, gender, race, admitting diagnosis(es), length of stay, psychiatric illness, illicit drug use, whether they reported receiving opioid analgesics at the time of hospitalization, whether they were prescribed opioids and/or nonopioid analgesics during their hospitalization, the median and maximum doses of opioids prescribed and dispensed, and whether they were discharged on opioids. The question of illicit drug use was asked of all patients with the exception of those hospitalized in South Korea due to potential legal implications.
Opioid prescribing and receipt of opioids was recorded based upon current provider orders and medication administration records, respectively. Perception of and satisfaction with pain control was assessed with the American Pain Society Patient Outcome Questionnaire–Modified (APS-POQ-Modified).24,25 Versions of this survey have been validated in English as well as in other languages and cultures.26-28 Because hospitalization practices could differ across hospitals and in different countries, we compared patients’ severity of illness by using Charlson comorbidity scores. Consent forms and the APS-POQ were translated into each country’s primary language according to established processes.29 The survey was filled out by having site investigators read questions aloud and by use of a large-font visual analog scale to aid patients’ verbal responses.
Data were collected and managed using a secure, web-based application electronic data capture tool (Research Electronic Data Capture [REDCap], Nashville, Tennessee), hosted at Denver Health.30
Study Size
Preliminary data from the internal medicine units at our institution suggested that 40% of patients without cancer received opioid analgesics during their hospitalization. Assuming 90% power to detect an absolute difference in the proportion of inpatient medical patients who are receiving opioid analgesics during their hospital stay of 17%, a two-sided type 1 error rate of 0.05, six hospitals in the US, and nine hospitals from all other countries, we calculated an initial sample size of 150 patients per site. This sample size was considered feasible for enrollment in a busy inpatient clinical setting. Study end points were to either reach the goal number of patients (150 per site) or the predetermined study end date, whichever came first.
Data Analysis
We generated means with standard deviations (SDs) and medians with interquartile ranges (IQRs) for normally and nonnormally distributed continuous variables, respectively, and frequencies for categorical variables. We used linear mixed modeling for the analysis of continuous variables. For binary outcomes, our data were fitted to a generalized linear mixed model with logit as the link function and a binary distribution. For ordinal variables, specifically patient-reported satisfaction with pain control and the opinion statements, the data were fitted to a generalized linear mixed model with a cumulative logit link and a multinomial distribution. Hospital was included as a random effect in all models to account for patients cared for in the same hospital.
Country of origin, dichotomized as US or non-US, was the independent variable of interest for all models. An interaction term for exposure to opioids prior to admission and country was entered into all models to explore whether differences in the effect of country existed for patients who reported taking opioids prior to admission and those who did not.
The models for the frequency with which analgesics were given, doses of opioids given during hospitalization and at discharge, patient-reported pain score, and patient-reported satisfaction with pain control were adjusted for (1) age, (2) gender, (3) Charlson Comorbidity Index, (4) length of stay, (5) history of illicit drug use, (6) history of psychiatric illness, (7) daily dose in morphine milligram equivalents (MME) for opioids prior to admission, (8) average pain score, and (9) hospital. The patient-reported satisfaction with pain control model was also adjusted for whether or not opioids were given to the patient during their hospitalization. P < .05 was considered to indicate significance. All analyses were performed using SAS Enterprise Guide 7.1 (SAS Institute, Inc., Cary, North Carolina). We reported data on medications that were prescribed and dispensed (as opposed to just prescribed and not necessarily given). Opioids prescribed at discharge represented the total possible opioids that could be given based upon the order/prescription (eg, oxycodone 5 mg every 6 hours as needed for pain would be counted as 20 mg/24 hours maximum possible dose followed by conversion to MME).
Missing Data
When there were missing data, a query was sent to sites to verify if the data were retrievable. If retrievable, the data were then entered. Data were missing in 5% and 2% of patients who did or did not report taking an opioid prior to admission, respectively. If a variable was included in a specific statistical test, then subjects with missing data were excluded from that analysis (ie, complete case analysis).
RESULTS
We approached 1,309 eligible patients, of which 981 provided informed consent, for a response rate of 75%; 503 from the US and 478 patients from other countries (Figure). In unadjusted analyses, we found no significant differences between US and non-US patients in age (mean age 51, SD 15 vs 59, SD 19; P = .30), race, ethnicity, or Charlson comorbidity index scores (median 2, IQR 1-3 vs 3, IQR 1-4; P = .45). US patients had shorter lengths of stay (median 3 days, IQR 2-4 vs 6 days, IQR 3-11; P = .04), a more frequent history of illicit drug use (33% vs 6%; P = .003), a higher frequency of psychiatric illness (27% vs 8%; P < .0001), and more were receiving opioid analgesics prior to admission (38% vs 17%; P = .007) than those hospitalized in other countries (Table 1, Appendix 1). The primary admitting diagnoses for all patients in the study are listed in Appendix 2. Opioid prescribing practices across the individual sites are shown in Appendix 3.
Patients Taking Opioids Prior to Admission
After adjusting for relevant covariates, we found that more patients in the US were given opioids during their hospitalization and in higher doses than patients from other countries and more were prescribed opioids at discharge. Fewer patients in the US were dispensed nonopioid analgesics during their hospitalization than patients from other countries, but this difference was not significant (Table 2). Appendix 4 shows the types of nonopioid pain medications prescribed in the US and other countries.
After adjustment for relevant covariates, US patients reported greater pain severity at the time they completed their pain surveys. We found no significant difference in satisfaction with pain control between patients from the US and other countries in the models, regardless of whether we included average pain score or opioid receipt during hospitalization in the model (Table 3).
In unadjusted analyses, compared with patients hospitalized in other countries, more patients in the US stated that they would like a stronger dose of analgesic if they were still in pain, though the difference was nonsignificant, and US patients were more likely to agree with the statement that people become addicted to pain medication easily and less likely to agree with the statement that it is easier to endure pain than deal with the side effects of pain medications (Table 3).
Patients Not Taking Opioids Prior to Admission
After adjusting for relevant covariates, we found no significant difference in the proportion of US patients provided with nonopioid pain medications during their hospitalization compared with patients in other countries, but a greater percentage of US patients were given opioids during their hospitalization and at discharge and in higher doses (Table 2).
After adjusting for relevant covariates, US patients reported greater pain severity at the time they completed their pain surveys and greater pain severity in the 24-36 hours prior to completing the survey than patients from other countries, but we found no difference in patient satisfaction with pain control (Table 3). After we included the average pain score and whether or not opioids were given to the patient during their hospitalization in this model, patients in the US were more likely to report a higher level of satisfaction with pain control than patients in all other countries (P = .001).
In unadjusted analyses, compared with patients hospitalized in other countries, those in the US were less likely to agree with the statement that good patients avoid talking about pain (Table 3).
Patient Satisfaction and Opioid Receipt
Among patients cared for in the US, after controlling for the average pain score, we did not find a significant association between receiving opioids while in the hospital and satisfaction with pain control for patients who either did or did not endorse taking opioids prior to admission (P = .38 and P = .24, respectively). Among patients cared for in all other countries, after controlling for the average pain score, we found a significant association between receiving opioids while in the hospital and a lower level of satisfaction with pain control for patients who reported taking opioids prior to admission (P = .02) but not for patients who did not report taking opioids prior to admission (P = .08).
DISCUSSION
Compared with patients hospitalized in other countries, a greater percentage of those hospitalized in the US were prescribed opioid analgesics both during hospitalization and at the time of discharge, even after adjustment for pain severity. In addition, patients hospitalized in the US reported greater pain severity at the time they completed their pain surveys and in the 24 to 36 hours prior to completing the survey than patients from other countries. In this sample, satisfaction, beliefs, and expectations about pain control differed between patients in the US and other sites. Our study also suggests that opioid receipt did not lead to improved patient satisfaction with pain control.
The frequency with which we observed opioid analgesics being prescribed during hospitalization in US hospitals (79%) was higher than the 51% of patients who received opioids reported by Herzig and colleagues.10 Patients in our study had a higher prevalence of illicit drug abuse and psychiatric illness, and our study only included patients who reported pain at some point during their hospitalization. We also studied prescribing practices through analysis of provider orders and medication administration records at the time the patient was hospitalized.
While we observed that physicians in the US more frequently prescribed opioid analgesics during hospitalizations than physicians working in other countries, we also observed that patients in the US reported higher levels of pain during their hospitalization. After adjusting for a number of variables, including pain severity, however, we still found that opioids were more commonly prescribed during hospitalizations by physicians working in the US sites studied than by physicians in the non-US sites.
Opioid prescribing practices varied across the sites sampled in our study. While the US sites, Taiwan, and Korea tended to be heavier utilizers of opioids during hospitalization, there were notable differences in discharge prescribing of opioids, with the US sites more commonly prescribing opioids and higher MME for patients who did not report taking opioids prior to their hospitalization (Appendix 3). A sensitivity analysis was conducted excluding South Korea from modeling, given that patients there were not asked about illicit opioid use. There were no important changes in the magnitude or direction of the results.
Our study supports previous studies indicating that there are cultural and societal differences when it comes to the experience of pain and the expectations around pain control.17,20-22,31 Much of the focus on reducing opioid utilization has been on provider practices32 and on prescription drug monitoring programs.33 Our findings suggest that another area of focus that may be important in mitigating the opioid epidemic is patient expectations of pain control.
Our study has a number of strengths. First, we included 11 hospitals from eight different countries. Second, we believe this is the first study to assess opioid prescribing and dispensing practices during hospitalization as well as at the time of discharge. Third, patient perceptions of pain control were assessed in conjunction with analgesic prescribing and were assessed during hospitalization. Fourth, we had high response rates for patient participation in our study. Fifth, we found much larger differences in opioid prescribing than anticipated, and thus, while we did not achieve the sample size originally planned for either the number of hospitals or patients enrolled per hospital, we were sufficiently powered. This is likely secondary to the fact that the population we studied was one that specifically reported pain, resulting in the larger differences seen.
Our study also had a number of limitations. First, the prescribing practices in countries other than the US are represented by only one hospital per country and, in some countries, by limited numbers of patients. While we studied four sites in the US, we did not have a site in the Northeast, a region previously shown to have lower prescribing rates.10 Additionally, patient samples for the US sites compared with the sites in other countries varied considerably with respect to ethnicity. While some studies in US patients have shown that opioid prescribing may vary based on race/ethnicity,34 we are uncertain as to how this might impact a study that crosses multiple countries. We also had a low number of patients receiving opioids prior to hospitalization for several of the non-US countries, which reduced the power to detect differences in this subgroup. Previous research has shown that there are wide variations in prescribing practices even within countries;10,12,18 therefore, caution should be taken when generalizing our findings. Second, we assessed analgesic prescribing patterns and pain control during the first 24 to 36 hours of hospitalization and did not consider hospital days beyond this timeframe with the exception of noting what medications were prescribed at discharge. We chose this methodology in an attempt to eliminate as many differences that might exist in the duration of hospitalization across many countries. Third, investigators in the study administered the survey, and respondents may have been affected by social desirability bias in how the survey questions were answered. Because investigators were not a part of the care team of any study patients, we believe this to be unlikely. Fourth, our study was conducted from October 8, 2013 to August 31, 2015 and the opioid epidemic is dynamic. Accordingly, our data may not reflect current opioid prescribing practices or patients’ current beliefs regarding pain control. Fifth, we did not collect demographic data on the patients who did not participate and could not look for systematic differences between participants and nonparticipants. Sixth, we relied on patients to self-report whether they were taking opioids prior to hospitalization or using illicit drugs. Seventh, we found comorbid mental health conditions to be more frequent in the US population studied. Previous work has shown regional variation in mental health conditions,35,36 which could have affected our findings. To account for this, our models included psychiatric illness.
CONCLUSIONS
Our data suggest that physicians in the US may prescribe opioids more frequently during patients’ hospitalizations and at discharge than their colleagues in other countries. We also found that patient satisfaction, beliefs, and expectations about pain control differed between patients in the US and other sites. Although the small number of hospitals included in our sample coupled with the small sample size in some of the non-US countries limits the generalizability of our findings, the data suggest that reducing the opioid epidemic in the US may require addressing patients’ expectations regarding pain control in addition to providers’ inpatient analgesic prescribing patterns.
Disclosures
The authors report no conflicts of interest.
Funding
The authors report no funding source for this work.
Since 2000, the United States has seen a marked increase in opioid prescribing1-3 and opioid-related complications, including overdoses, hospitalizations, and deaths.2,4,5 A study from 2015 showed that more than one-third of the US civilian noninstitutionalized population reported receiving an opioid prescription in the prior year, with 12.5% reporting misuse, and, of those, 16.7% reported a prescription use disorder.6 While there has been a slight decrease in opioid prescriptions in the US since 2012, rates of opioid prescribing in 2015 were three times higher than in 1999 and approximately four times higher than in Europe in 2015.3,7
Pain is commonly reported by hospitalized patients,8,9 and opioids are often a mainstay of treatment;9,10 however, treatment with opioids can have a number of adverse outcomes.2,10,11 Short-term exposure to opioids can lead to long-term use,12-16 and patients on opioids are at an increased risk for subsequent hospitalization and longer inpatient lengths of stay.5
Physician prescribing practices for opioids and patient expectations for pain control vary as a function of geographic region and culture,10,12,17,18 and pain is influenced by the cultural context in which it occurs.17,19-22 Treatment of pain may also be affected by limited access to or restrictions on selected medications, as well as by cultural biases.23 Whether these variations in the treatment of pain are reflected in patients’ satisfaction with pain control is uncertain.
We sought to compare the inpatient analgesic prescribing practices and patients’ perceptions of pain control for medical patients in four teaching hospitals in the US and in seven teaching hospitals in seven other countries.
METHODS
Study Design
We utilized a cross-sectional, observational design. The study was approved by the Institutional Review Boards at all participating sites.
Setting
The study was conducted at 11 academic hospitals in eight countries from October 8, 2013 to August 31, 2015. Sites in the US included Denver Health in Denver, Colorado; the University of Colorado Hospital in Aurora, Colorado; Hennepin Healthcare in Minneapolis, Minnesota; and Legacy Health in Portland, Oregon. Sites outside the US included McMaster University in Hamilton, Ontario, Canada; Hospital de la Santa Creu i Sant Pau, Universitat Autonòma de Barcelona in Barcelona, Spain; the University of Study of Milan and the University Ospedale “Luigi Sacco” in Milan, Italy, the National Taiwan University Hospital, in Taipei, Taiwan, the University of Ulsan College of Medicine, Asan Medical Center, in Seoul, Korea, the Imperial College, Chelsea and Westminster Hospital, in London, United Kingdom and Dunedin Hospital, Dunedin, New Zealand.
Inclusion and Exclusion Criteria
We included patients 18-89 years of age (20-89 in Taiwan because patients under 20 years of age in this country are a restricted group with respect to participating in research), admitted to an internal medicine service from the Emergency Department or Urgent Care clinic with an acute illness for a minimum of 24 hours (with time zero defined as the time care was initiated in the Emergency Department or Urgent Care Clinic), who reported pain at some time during the first 24-36 hours of their hospitalization and who provided informed consent. In the US, “admission” included both observation and inpatient status. We limited the patient population to those admitted via emergency departments and urgent care clinics in order to enroll similar patient populations across sites.
Scheduled admissions, patients transferred from an outside facility, patients admitted directly from a clinic, and those receiving care in intensive care units were excluded. We also excluded patients who were incarcerated, pregnant, those who received major surgery within the previous 14 days, those with a known diagnosis of active cancer, and those who were receiving palliative or hospice care. Patients receiving care from an investigator in the study at the time of enrollment were not eligible due to the potential conflict of interest.
Patient Screening
Primary teams were contacted to determine if any patients on their service might meet the criteria for inclusion in the study on preselected study days chosen on the basis of the research team’s availability. Identified patients were then screened to establish if they met the eligibility criteria. Patients were asked directly if they had experienced pain during their preadmission evaluation or during their hospitalization.
Data Collection
All patients were hospitalized at the time they gave consent and when data were collected. Data were collected via interviews with patients, as well as through chart review. We recorded patients’ age, gender, race, admitting diagnosis(es), length of stay, psychiatric illness, illicit drug use, whether they reported receiving opioid analgesics at the time of hospitalization, whether they were prescribed opioids and/or nonopioid analgesics during their hospitalization, the median and maximum doses of opioids prescribed and dispensed, and whether they were discharged on opioids. The question of illicit drug use was asked of all patients with the exception of those hospitalized in South Korea due to potential legal implications.
Opioid prescribing and receipt of opioids was recorded based upon current provider orders and medication administration records, respectively. Perception of and satisfaction with pain control was assessed with the American Pain Society Patient Outcome Questionnaire–Modified (APS-POQ-Modified).24,25 Versions of this survey have been validated in English as well as in other languages and cultures.26-28 Because hospitalization practices could differ across hospitals and in different countries, we compared patients’ severity of illness by using Charlson comorbidity scores. Consent forms and the APS-POQ were translated into each country’s primary language according to established processes.29 The survey was filled out by having site investigators read questions aloud and by use of a large-font visual analog scale to aid patients’ verbal responses.
Data were collected and managed using a secure, web-based application electronic data capture tool (Research Electronic Data Capture [REDCap], Nashville, Tennessee), hosted at Denver Health.30
Study Size
Preliminary data from the internal medicine units at our institution suggested that 40% of patients without cancer received opioid analgesics during their hospitalization. Assuming 90% power to detect an absolute difference in the proportion of inpatient medical patients who are receiving opioid analgesics during their hospital stay of 17%, a two-sided type 1 error rate of 0.05, six hospitals in the US, and nine hospitals from all other countries, we calculated an initial sample size of 150 patients per site. This sample size was considered feasible for enrollment in a busy inpatient clinical setting. Study end points were to either reach the goal number of patients (150 per site) or the predetermined study end date, whichever came first.
Data Analysis
We generated means with standard deviations (SDs) and medians with interquartile ranges (IQRs) for normally and nonnormally distributed continuous variables, respectively, and frequencies for categorical variables. We used linear mixed modeling for the analysis of continuous variables. For binary outcomes, our data were fitted to a generalized linear mixed model with logit as the link function and a binary distribution. For ordinal variables, specifically patient-reported satisfaction with pain control and the opinion statements, the data were fitted to a generalized linear mixed model with a cumulative logit link and a multinomial distribution. Hospital was included as a random effect in all models to account for patients cared for in the same hospital.
Country of origin, dichotomized as US or non-US, was the independent variable of interest for all models. An interaction term for exposure to opioids prior to admission and country was entered into all models to explore whether differences in the effect of country existed for patients who reported taking opioids prior to admission and those who did not.
The models for the frequency with which analgesics were given, doses of opioids given during hospitalization and at discharge, patient-reported pain score, and patient-reported satisfaction with pain control were adjusted for (1) age, (2) gender, (3) Charlson Comorbidity Index, (4) length of stay, (5) history of illicit drug use, (6) history of psychiatric illness, (7) daily dose in morphine milligram equivalents (MME) for opioids prior to admission, (8) average pain score, and (9) hospital. The patient-reported satisfaction with pain control model was also adjusted for whether or not opioids were given to the patient during their hospitalization. P < .05 was considered to indicate significance. All analyses were performed using SAS Enterprise Guide 7.1 (SAS Institute, Inc., Cary, North Carolina). We reported data on medications that were prescribed and dispensed (as opposed to just prescribed and not necessarily given). Opioids prescribed at discharge represented the total possible opioids that could be given based upon the order/prescription (eg, oxycodone 5 mg every 6 hours as needed for pain would be counted as 20 mg/24 hours maximum possible dose followed by conversion to MME).
Missing Data
When there were missing data, a query was sent to sites to verify if the data were retrievable. If retrievable, the data were then entered. Data were missing in 5% and 2% of patients who did or did not report taking an opioid prior to admission, respectively. If a variable was included in a specific statistical test, then subjects with missing data were excluded from that analysis (ie, complete case analysis).
RESULTS
We approached 1,309 eligible patients, of which 981 provided informed consent, for a response rate of 75%; 503 from the US and 478 patients from other countries (Figure). In unadjusted analyses, we found no significant differences between US and non-US patients in age (mean age 51, SD 15 vs 59, SD 19; P = .30), race, ethnicity, or Charlson comorbidity index scores (median 2, IQR 1-3 vs 3, IQR 1-4; P = .45). US patients had shorter lengths of stay (median 3 days, IQR 2-4 vs 6 days, IQR 3-11; P = .04), a more frequent history of illicit drug use (33% vs 6%; P = .003), a higher frequency of psychiatric illness (27% vs 8%; P < .0001), and more were receiving opioid analgesics prior to admission (38% vs 17%; P = .007) than those hospitalized in other countries (Table 1, Appendix 1). The primary admitting diagnoses for all patients in the study are listed in Appendix 2. Opioid prescribing practices across the individual sites are shown in Appendix 3.
Patients Taking Opioids Prior to Admission
After adjusting for relevant covariates, we found that more patients in the US were given opioids during their hospitalization and in higher doses than patients from other countries and more were prescribed opioids at discharge. Fewer patients in the US were dispensed nonopioid analgesics during their hospitalization than patients from other countries, but this difference was not significant (Table 2). Appendix 4 shows the types of nonopioid pain medications prescribed in the US and other countries.
After adjustment for relevant covariates, US patients reported greater pain severity at the time they completed their pain surveys. We found no significant difference in satisfaction with pain control between patients from the US and other countries in the models, regardless of whether we included average pain score or opioid receipt during hospitalization in the model (Table 3).
In unadjusted analyses, compared with patients hospitalized in other countries, more patients in the US stated that they would like a stronger dose of analgesic if they were still in pain, though the difference was nonsignificant, and US patients were more likely to agree with the statement that people become addicted to pain medication easily and less likely to agree with the statement that it is easier to endure pain than deal with the side effects of pain medications (Table 3).
Patients Not Taking Opioids Prior to Admission
After adjusting for relevant covariates, we found no significant difference in the proportion of US patients provided with nonopioid pain medications during their hospitalization compared with patients in other countries, but a greater percentage of US patients were given opioids during their hospitalization and at discharge and in higher doses (Table 2).
After adjusting for relevant covariates, US patients reported greater pain severity at the time they completed their pain surveys and greater pain severity in the 24-36 hours prior to completing the survey than patients from other countries, but we found no difference in patient satisfaction with pain control (Table 3). After we included the average pain score and whether or not opioids were given to the patient during their hospitalization in this model, patients in the US were more likely to report a higher level of satisfaction with pain control than patients in all other countries (P = .001).
In unadjusted analyses, compared with patients hospitalized in other countries, those in the US were less likely to agree with the statement that good patients avoid talking about pain (Table 3).
Patient Satisfaction and Opioid Receipt
Among patients cared for in the US, after controlling for the average pain score, we did not find a significant association between receiving opioids while in the hospital and satisfaction with pain control for patients who either did or did not endorse taking opioids prior to admission (P = .38 and P = .24, respectively). Among patients cared for in all other countries, after controlling for the average pain score, we found a significant association between receiving opioids while in the hospital and a lower level of satisfaction with pain control for patients who reported taking opioids prior to admission (P = .02) but not for patients who did not report taking opioids prior to admission (P = .08).
DISCUSSION
Compared with patients hospitalized in other countries, a greater percentage of those hospitalized in the US were prescribed opioid analgesics both during hospitalization and at the time of discharge, even after adjustment for pain severity. In addition, patients hospitalized in the US reported greater pain severity at the time they completed their pain surveys and in the 24 to 36 hours prior to completing the survey than patients from other countries. In this sample, satisfaction, beliefs, and expectations about pain control differed between patients in the US and other sites. Our study also suggests that opioid receipt did not lead to improved patient satisfaction with pain control.
The frequency with which we observed opioid analgesics being prescribed during hospitalization in US hospitals (79%) was higher than the 51% of patients who received opioids reported by Herzig and colleagues.10 Patients in our study had a higher prevalence of illicit drug abuse and psychiatric illness, and our study only included patients who reported pain at some point during their hospitalization. We also studied prescribing practices through analysis of provider orders and medication administration records at the time the patient was hospitalized.
While we observed that physicians in the US more frequently prescribed opioid analgesics during hospitalizations than physicians working in other countries, we also observed that patients in the US reported higher levels of pain during their hospitalization. After adjusting for a number of variables, including pain severity, however, we still found that opioids were more commonly prescribed during hospitalizations by physicians working in the US sites studied than by physicians in the non-US sites.
Opioid prescribing practices varied across the sites sampled in our study. While the US sites, Taiwan, and Korea tended to be heavier utilizers of opioids during hospitalization, there were notable differences in discharge prescribing of opioids, with the US sites more commonly prescribing opioids and higher MME for patients who did not report taking opioids prior to their hospitalization (Appendix 3). A sensitivity analysis was conducted excluding South Korea from modeling, given that patients there were not asked about illicit opioid use. There were no important changes in the magnitude or direction of the results.
Our study supports previous studies indicating that there are cultural and societal differences when it comes to the experience of pain and the expectations around pain control.17,20-22,31 Much of the focus on reducing opioid utilization has been on provider practices32 and on prescription drug monitoring programs.33 Our findings suggest that another area of focus that may be important in mitigating the opioid epidemic is patient expectations of pain control.
Our study has a number of strengths. First, we included 11 hospitals from eight different countries. Second, we believe this is the first study to assess opioid prescribing and dispensing practices during hospitalization as well as at the time of discharge. Third, patient perceptions of pain control were assessed in conjunction with analgesic prescribing and were assessed during hospitalization. Fourth, we had high response rates for patient participation in our study. Fifth, we found much larger differences in opioid prescribing than anticipated, and thus, while we did not achieve the sample size originally planned for either the number of hospitals or patients enrolled per hospital, we were sufficiently powered. This is likely secondary to the fact that the population we studied was one that specifically reported pain, resulting in the larger differences seen.
Our study also had a number of limitations. First, the prescribing practices in countries other than the US are represented by only one hospital per country and, in some countries, by limited numbers of patients. While we studied four sites in the US, we did not have a site in the Northeast, a region previously shown to have lower prescribing rates.10 Additionally, patient samples for the US sites compared with the sites in other countries varied considerably with respect to ethnicity. While some studies in US patients have shown that opioid prescribing may vary based on race/ethnicity,34 we are uncertain as to how this might impact a study that crosses multiple countries. We also had a low number of patients receiving opioids prior to hospitalization for several of the non-US countries, which reduced the power to detect differences in this subgroup. Previous research has shown that there are wide variations in prescribing practices even within countries;10,12,18 therefore, caution should be taken when generalizing our findings. Second, we assessed analgesic prescribing patterns and pain control during the first 24 to 36 hours of hospitalization and did not consider hospital days beyond this timeframe with the exception of noting what medications were prescribed at discharge. We chose this methodology in an attempt to eliminate as many differences that might exist in the duration of hospitalization across many countries. Third, investigators in the study administered the survey, and respondents may have been affected by social desirability bias in how the survey questions were answered. Because investigators were not a part of the care team of any study patients, we believe this to be unlikely. Fourth, our study was conducted from October 8, 2013 to August 31, 2015 and the opioid epidemic is dynamic. Accordingly, our data may not reflect current opioid prescribing practices or patients’ current beliefs regarding pain control. Fifth, we did not collect demographic data on the patients who did not participate and could not look for systematic differences between participants and nonparticipants. Sixth, we relied on patients to self-report whether they were taking opioids prior to hospitalization or using illicit drugs. Seventh, we found comorbid mental health conditions to be more frequent in the US population studied. Previous work has shown regional variation in mental health conditions,35,36 which could have affected our findings. To account for this, our models included psychiatric illness.
CONCLUSIONS
Our data suggest that physicians in the US may prescribe opioids more frequently during patients’ hospitalizations and at discharge than their colleagues in other countries. We also found that patient satisfaction, beliefs, and expectations about pain control differed between patients in the US and other sites. Although the small number of hospitals included in our sample coupled with the small sample size in some of the non-US countries limits the generalizability of our findings, the data suggest that reducing the opioid epidemic in the US may require addressing patients’ expectations regarding pain control in addition to providers’ inpatient analgesic prescribing patterns.
Disclosures
The authors report no conflicts of interest.
Funding
The authors report no funding source for this work.
1. Pletcher MJ, Kertesz SG, Kohn MA, Gonzales R. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA. 2008;299(1):70-78. https://doi.org/10.1001/jama.2007.64.
2. Herzig SJ. Growing concerns regarding long-term opioid use: the hospitalization hazard. J Hosp Med. 2015;10(7):469-470. https://doi.org/10.1002/jhm.2369.
3. Guy GP Jr, Zhang K, Bohm MK, et al. Vital Signs: changes in opioid prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(26):697-704. https://doi.org/10.15585/mmwr.mm6626a4.
4. Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981-1985. https://doi.org/10.1056/NEJMp1011512.
5. Liang Y, Turner BJ. National cohort study of opioid analgesic dose and risk of future hospitalization. J Hosp Med. 2015;10(7):425-431. https://doi.org/10.1002/jhm.2350.
6. Han B, Compton WM, Blanco C, et al. Prescription opioid use, misuse, and use disorders in U.S. Adults: 2015 national survey on drug use and health. Ann Intern Med. 2017;167(5):293-301. https://doi.org/10.7326/M17-0865.
7. Schuchat A, Houry D, Guy GP, Jr. New data on opioid use and prescribing in the United States. JAMA. 2017;318(5):425-426. https://doi.org/10.1001/jama.2017.8913.
8. Sawyer J, Haslam L, Robinson S, Daines P, Stilos K. Pain prevalence study in a large Canadian teaching hospital. Pain Manag Nurs. 2008;9(3):104-112. https://doi.org/10.1016/j.pmn.2008.02.001.
9. Gupta A, Daigle S, Mojica J, Hurley RW. Patient perception of pain care in hospitals in the United States. J Pain Res. 2009;2:157-164. https://doi.org/10.2147/JPR.S7903.
10. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. https://doi.org/10.1002/jhm.2102.
11. Kanjanarat P, Winterstein AG, Johns TE, et al. Nature of preventable adverse drug events in hospitals: a literature review. Am J Health Syst Pharm. 2003;60(17):1750-1759. https://doi.org/10.1093/ajhp/60.17.1750.
12. Jena AB, Goldman D, Karaca-Mandic P. Hospital prescribing of opioids to medicare beneficiaries. JAMA Intern Med. 2016;176(7):990-997. https://doi.org/10.1001/jamainternmed.2016.2737.
13. Hooten WM, St Sauver JL, McGree ME, Jacobson DJ, Warner DO. Incidence and risk factors for progression From short-term to episodic or long-term opioid prescribing: A population-based study. Mayo Clin Proc. 2015;90(7):850-856. https://doi.org/10.1016/j.mayocp.2015.04.012.
14. Alam A, Gomes T, Zheng H, et al. Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med. 2012;172(5):425-430. https://doi.org/10.1001/archinternmed.2011.1827.
15. Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med. 2017;376(7):663-673. https://doi.org/10.1056/NEJMsa1610524.
16. Calcaterra SL, Scarbro S, Hull ML, et al. Prediction of future chronic opioid use Among hospitalized patients. J Gen Intern Med. 2018;33(6):898-905. https://doi.org/10.1007/s11606-018-4335-8.
17. Callister LC. Cultural influences on pain perceptions and behaviors. Home Health Care Manag Pract. 2003;15(3):207-211. https://doi.org/10.1177/1084822302250687.
18. Paulozzi LJ, Mack KA, Hockenberry JM. Vital signs: Variation among states in prescribing of opioid pain relievers and benzodiazepines--United States, 2012. J Saf Res. 2014;63(26):563-568. https://doi.org/10.1016/j.jsr.2014.09.001.
19. Callister LC, Khalaf I, Semenic S, Kartchner R, Vehvilainen-Julkunen K. The pain of childbirth: perceptions of culturally diverse women. Pain Manag Nurs. 2003;4(4):145-154. https://doi.org/10.1016/S1524-9042(03)00028-6.
20. Moore R, Brødsgaard I, Mao TK, Miller ML, Dworkin SF. Perceived need for local anesthesia in tooth drilling among Anglo-Americans, Chinese, and Scandinavians. Anesth Prog. 1998;45(1):22-28.
21. Kankkunen PM, Vehviläinen-Julkunen KM, Pietilä AM, et al. A tale of two countries: comparison of the perceptions of analgesics among Finnish and American parents. Pain Manag Nurs. 2008;9(3):113-119. https://doi.org/10.1016/j.pmn.2007.12.003.
22. Hanoch Y, Katsikopoulos KV, Gummerum M, Brass EP. American and German students’ knowledge, perceptions, and behaviors with respect to over-the-counter pain relievers. Health Psychol. 2007;26(6):802-806. https://doi.org/10.1037/0278-6133.26.6.802.
23. Manjiani D, Paul DB, Kunnumpurath S, Kaye AD, Vadivelu N. Availability and utilization of opioids for pain management: global issues. Ochsner J. 2014;14(2):208-215.
24. Quality improvement guidelines for the treatment of acute pain and cancer pain. JAMA. 1995;274(23):1874-1880.
25. McNeill JA, Sherwood GD, Starck PL, Thompson CJ. Assessing clinical outcomes: patient satisfaction with pain management. J Pain Symptom Manag. 1998;16(1):29-40. https://doi.org/10.1016/S0885-3924(98)00034-7.
26. Ferrari R, Novello C, Catania G, Visentin M. Patients’ satisfaction with pain management: the Italian version of the Patient Outcome Questionnaire of the American Pain Society. Recenti Prog Med. 2010;101(7–8):283-288.
27. Malouf J, Andión O, Torrubia R, Cañellas M, Baños JE. A survey of perceptions with pain management in Spanish inpatients. J Pain Symptom Manag. 2006;32(4):361-371. https://doi.org/10.1016/j.jpainsymman.2006.05.006.
28. Gordon DB, Polomano RC, Pellino TA, et al. Revised American Pain Society Patient Outcome Questionnaire (APS-POQ-R) for quality improvement of pain management in hospitalized adults: preliminary psychometric evaluation. J Pain. 2010;11(11):1172-1186. https://doi.org/10.1016/j.jpain.2010.02.012.
29. Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976). 2000;25(24):3186-3191. https://doi.org/10.1097/00007632-200012150-00014.
30. Harris PA, Taylor R, Thielke R, et al. Research Electronic Data Capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010.
31. Duman F. After surgery in Germany, I wanted Vicodin, not herbal tea. New York Times. January 27, 2018. https://www.nytimes.com/2018/01/27/opinion/sunday/surgery-germany-vicodin.html. Accessed November 6, 2018.
32. Beaudoin FL, Banerjee GN, Mello MJ. State-level and system-level opioid prescribing policies: the impact on provider practices and overdose deaths, a systematic review. J Opioid Manag. 2016;12(2):109-118. https://doi.org/10.5055/jom.2016.0322. 33. Bao Y, Wen K, Johnson P, et al. Assessing the impact of state policies for prescription drug monitoring programs on high-risk opioid prescriptions. Health Aff (Millwood). 2018;37(10):1596-1604. https://doi.org/10.1377/hlthaff.2018.0512.
33. Bao Y, Wen K, Johnson P, et al. Assessing the impact of state policies for prescription drug monitoring programs on high-risk opioid prescriptions. Health Aff (Millwood). 2018;37(10):1596-1604. https://doi.org/10.1377/hlthaff.2018.0512.
34. Friedman J, Kim D, Schneberk T, et al. Assessment of racial/ethnic and income disparities in the prescription of opioids and other controlled medications in California. JAMA Intern Med. 2019. https://doi.org/10.1001/jamainternmed.2018.6721.
35. Steel Z, Marnane C, Iranpour C, et al. The global prevalence of common mental disorders: a systematic review and meta-analysis 1980-2013. Int J Epidemiol. 2014;43(2):476-493. https://doi.org/10.1093/ije/dyu038.
36. Simon GE, Goldberg DP, Von Korff M, Ustün TB. Understanding cross-national differences in depression prevalence. Psychol Med. 2002;32(4):585-594. https://doi.org/10.1017/S0033291702005457.
1. Pletcher MJ, Kertesz SG, Kohn MA, Gonzales R. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA. 2008;299(1):70-78. https://doi.org/10.1001/jama.2007.64.
2. Herzig SJ. Growing concerns regarding long-term opioid use: the hospitalization hazard. J Hosp Med. 2015;10(7):469-470. https://doi.org/10.1002/jhm.2369.
3. Guy GP Jr, Zhang K, Bohm MK, et al. Vital Signs: changes in opioid prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(26):697-704. https://doi.org/10.15585/mmwr.mm6626a4.
4. Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981-1985. https://doi.org/10.1056/NEJMp1011512.
5. Liang Y, Turner BJ. National cohort study of opioid analgesic dose and risk of future hospitalization. J Hosp Med. 2015;10(7):425-431. https://doi.org/10.1002/jhm.2350.
6. Han B, Compton WM, Blanco C, et al. Prescription opioid use, misuse, and use disorders in U.S. Adults: 2015 national survey on drug use and health. Ann Intern Med. 2017;167(5):293-301. https://doi.org/10.7326/M17-0865.
7. Schuchat A, Houry D, Guy GP, Jr. New data on opioid use and prescribing in the United States. JAMA. 2017;318(5):425-426. https://doi.org/10.1001/jama.2017.8913.
8. Sawyer J, Haslam L, Robinson S, Daines P, Stilos K. Pain prevalence study in a large Canadian teaching hospital. Pain Manag Nurs. 2008;9(3):104-112. https://doi.org/10.1016/j.pmn.2008.02.001.
9. Gupta A, Daigle S, Mojica J, Hurley RW. Patient perception of pain care in hospitals in the United States. J Pain Res. 2009;2:157-164. https://doi.org/10.2147/JPR.S7903.
10. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. https://doi.org/10.1002/jhm.2102.
11. Kanjanarat P, Winterstein AG, Johns TE, et al. Nature of preventable adverse drug events in hospitals: a literature review. Am J Health Syst Pharm. 2003;60(17):1750-1759. https://doi.org/10.1093/ajhp/60.17.1750.
12. Jena AB, Goldman D, Karaca-Mandic P. Hospital prescribing of opioids to medicare beneficiaries. JAMA Intern Med. 2016;176(7):990-997. https://doi.org/10.1001/jamainternmed.2016.2737.
13. Hooten WM, St Sauver JL, McGree ME, Jacobson DJ, Warner DO. Incidence and risk factors for progression From short-term to episodic or long-term opioid prescribing: A population-based study. Mayo Clin Proc. 2015;90(7):850-856. https://doi.org/10.1016/j.mayocp.2015.04.012.
14. Alam A, Gomes T, Zheng H, et al. Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med. 2012;172(5):425-430. https://doi.org/10.1001/archinternmed.2011.1827.
15. Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med. 2017;376(7):663-673. https://doi.org/10.1056/NEJMsa1610524.
16. Calcaterra SL, Scarbro S, Hull ML, et al. Prediction of future chronic opioid use Among hospitalized patients. J Gen Intern Med. 2018;33(6):898-905. https://doi.org/10.1007/s11606-018-4335-8.
17. Callister LC. Cultural influences on pain perceptions and behaviors. Home Health Care Manag Pract. 2003;15(3):207-211. https://doi.org/10.1177/1084822302250687.
18. Paulozzi LJ, Mack KA, Hockenberry JM. Vital signs: Variation among states in prescribing of opioid pain relievers and benzodiazepines--United States, 2012. J Saf Res. 2014;63(26):563-568. https://doi.org/10.1016/j.jsr.2014.09.001.
19. Callister LC, Khalaf I, Semenic S, Kartchner R, Vehvilainen-Julkunen K. The pain of childbirth: perceptions of culturally diverse women. Pain Manag Nurs. 2003;4(4):145-154. https://doi.org/10.1016/S1524-9042(03)00028-6.
20. Moore R, Brødsgaard I, Mao TK, Miller ML, Dworkin SF. Perceived need for local anesthesia in tooth drilling among Anglo-Americans, Chinese, and Scandinavians. Anesth Prog. 1998;45(1):22-28.
21. Kankkunen PM, Vehviläinen-Julkunen KM, Pietilä AM, et al. A tale of two countries: comparison of the perceptions of analgesics among Finnish and American parents. Pain Manag Nurs. 2008;9(3):113-119. https://doi.org/10.1016/j.pmn.2007.12.003.
22. Hanoch Y, Katsikopoulos KV, Gummerum M, Brass EP. American and German students’ knowledge, perceptions, and behaviors with respect to over-the-counter pain relievers. Health Psychol. 2007;26(6):802-806. https://doi.org/10.1037/0278-6133.26.6.802.
23. Manjiani D, Paul DB, Kunnumpurath S, Kaye AD, Vadivelu N. Availability and utilization of opioids for pain management: global issues. Ochsner J. 2014;14(2):208-215.
24. Quality improvement guidelines for the treatment of acute pain and cancer pain. JAMA. 1995;274(23):1874-1880.
25. McNeill JA, Sherwood GD, Starck PL, Thompson CJ. Assessing clinical outcomes: patient satisfaction with pain management. J Pain Symptom Manag. 1998;16(1):29-40. https://doi.org/10.1016/S0885-3924(98)00034-7.
26. Ferrari R, Novello C, Catania G, Visentin M. Patients’ satisfaction with pain management: the Italian version of the Patient Outcome Questionnaire of the American Pain Society. Recenti Prog Med. 2010;101(7–8):283-288.
27. Malouf J, Andión O, Torrubia R, Cañellas M, Baños JE. A survey of perceptions with pain management in Spanish inpatients. J Pain Symptom Manag. 2006;32(4):361-371. https://doi.org/10.1016/j.jpainsymman.2006.05.006.
28. Gordon DB, Polomano RC, Pellino TA, et al. Revised American Pain Society Patient Outcome Questionnaire (APS-POQ-R) for quality improvement of pain management in hospitalized adults: preliminary psychometric evaluation. J Pain. 2010;11(11):1172-1186. https://doi.org/10.1016/j.jpain.2010.02.012.
29. Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976). 2000;25(24):3186-3191. https://doi.org/10.1097/00007632-200012150-00014.
30. Harris PA, Taylor R, Thielke R, et al. Research Electronic Data Capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010.
31. Duman F. After surgery in Germany, I wanted Vicodin, not herbal tea. New York Times. January 27, 2018. https://www.nytimes.com/2018/01/27/opinion/sunday/surgery-germany-vicodin.html. Accessed November 6, 2018.
32. Beaudoin FL, Banerjee GN, Mello MJ. State-level and system-level opioid prescribing policies: the impact on provider practices and overdose deaths, a systematic review. J Opioid Manag. 2016;12(2):109-118. https://doi.org/10.5055/jom.2016.0322. 33. Bao Y, Wen K, Johnson P, et al. Assessing the impact of state policies for prescription drug monitoring programs on high-risk opioid prescriptions. Health Aff (Millwood). 2018;37(10):1596-1604. https://doi.org/10.1377/hlthaff.2018.0512.
33. Bao Y, Wen K, Johnson P, et al. Assessing the impact of state policies for prescription drug monitoring programs on high-risk opioid prescriptions. Health Aff (Millwood). 2018;37(10):1596-1604. https://doi.org/10.1377/hlthaff.2018.0512.
34. Friedman J, Kim D, Schneberk T, et al. Assessment of racial/ethnic and income disparities in the prescription of opioids and other controlled medications in California. JAMA Intern Med. 2019. https://doi.org/10.1001/jamainternmed.2018.6721.
35. Steel Z, Marnane C, Iranpour C, et al. The global prevalence of common mental disorders: a systematic review and meta-analysis 1980-2013. Int J Epidemiol. 2014;43(2):476-493. https://doi.org/10.1093/ije/dyu038.
36. Simon GE, Goldberg DP, Von Korff M, Ustün TB. Understanding cross-national differences in depression prevalence. Psychol Med. 2002;32(4):585-594. https://doi.org/10.1017/S0033291702005457.
© 2019 Society of Hospital Medicine
Improving the Transition of Intravenous to Enteral Antibiotics in Pediatric Patients with Pneumonia or Skin and Soft Tissue Infections
Intravenous (IV) antibiotics are commonly used in hospitalized pediatric patients to treat bacterial infections. Antimicrobial stewardship guidelines published by the Infectious Diseases Society of America (IDSA) recommend institutions develop a systematic plan to convert from IV to enteral antibiotics, as early transition may reduce healthcare costs, decrease length of stay (LOS), and avoid prolonged IV access complications1 such as extravasation, thrombosis, and catheter-associated infections.2-5
Pediatric patients with community-acquired pneumonia (CAP) and mild skin and soft tissue infections (SSTI) may not require IV antibiotics, even if the patient is hospitalized.6 Although national guidelines for pediatric CAP and SSTI recommend IV antibiotics for hospitalized patients, these guidelines state that mild infections may be treated with enteral antibiotics and emphasize discontinuation of IV antibiotics when the patient meets discharge criteria.7,8 Furthermore, several enteral antibiotics used for the treatment of CAP and SSTI, such as cephalexin and clindamycin,9 have excellent bioavailability (>90%) or can achieve sufficient concentrations to attain the pharmacodynamic target (ie, amoxicillin and trimethoprim–sulfamethoxazole).10,11 Nonetheless, the guidelines do not explicitly outline criteria regarding the transition from IV to enteral antibiotics.7,8
At our institution, patients admitted to Hospital Medicine (HM) often remained on IV antibiotics until discharge. Data review revealed that antibiotic treatment of CAP and SSTI posed the greatest opportunity for early conversion to enteral therapy based on the high frequency of admissions and the ability of commonly used enteral antibiotics to attain pharmacodynamic targets. We sought to change practice culture by decoupling transition to enteral antibiotics from discharge and use administration of other enteral medications as an objective indicator for transition. Our aim was to increase the proportion of enterally administered antibiotic doses for HM patients aged >60 days admitted with uncomplicated CAP or SSTI from 44% to 75% in eight months.
METHODS
Context
Cincinnati Children’s Hospital Medical Center (CCHMC) is a large, urban, academic hospital. The HM division has 45 attendings and admits >8,000 general pediatric patients annually. The five HM teams at the main campus consist of attendings, fellows, residents, and medical students. One HM team serves as the resident quality improvement (QI) team where residents collaborate in a longitudinal study under the guidance of QI-trained coaches. The focus of this QI initiative was determined by resident consensus and aligned with a high-value care curriculum.12
To identify the target patient population, we investigated IV antimicrobials frequently used in HM patients. Ampicillin and clindamycin are commonly used IV antibiotics, most frequently corresponding with the diagnoses of CAP and SSTI, respectively, accounting for half of all antibiotic use on the HM service. Amoxicillin, the enteral equivalent of ampicillin, can achieve sufficient concentrations to attain the pharmacodynamic target at infection sites, and clindamycin has high bioavailability, making them ideal options for early transition. Our institution’s robust antimicrobial stewardship program has published local guidelines on using amoxicillin as the enteral antibiotic of choice for uncomplicated CAP, but it does not provide guidance on the timing of transition for either CAP or SSTI; the clinical team makes this decision.
HM attendings were surveyed to determine the criteria used to transition from IV to enteral antibiotics for patients with CAP or SSTI. The survey illustrated practice variability with providers using differing clinical criteria to signal the timing of transition. Additionally, only 49% of respondents (n = 37) rated themselves as “very comfortable” with residents making autonomous decisions to transition to enteral antibiotics. We chose to use the administration of other enteral medications, instead of discharge readiness, as an objective indicator of a patient’s readiness to transition to enteral antibiotics, given the low-risk patient population and the ability of the enteral antibiotics commonly used for CAP and SSTI to achieve pharmacodynamic targets.
The study population included patients aged >60 days admitted to HM with CAP or SSTI treated with any antibiotic. We excluded patients with potential complications or significant progression of their disease process, including patients with parapneumonic effusions or chest tubes, patients who underwent bronchoscopy, and patients with osteomyelitis, septic arthritis, or preseptal or orbital cellulitis. Past medical history and clinical status on admission were not used to exclude patients.
Interventions
Our multidisciplinary team, formed in January 2017, included HM attendings, HM fellows, pediatric residents, a critical care attending, a pharmacy resident, and an antimicrobial stewardship pharmacist. Under the guidance of QI coaches, the residents on the HM QI team developed and tested all interventions on their team and then determined which interventions would spread to the other four teams. The nursing director of our primary HM unit disseminated project updates to bedside nurses. A simplified failure mode and effects analysis identified areas for improvement and potential interventions. Interventions focused on the following key drivers (Figure 1): increased prescriber awareness of medication charge, standardization of conversion from IV to enteral antibiotics, clear definition of the patients ready for transition, ongoing evaluation of the antimicrobial plan, timely recognition by prescribers of patients ready for transition, culture shift regarding the appropriate administration route in the inpatient setting, and transparency of data. The team implemented sequential Plan-Do-Study-Act (PDSA) cycles13 to test the interventions.
Charge Table
To improve knowledge about the increased charge for commonly used IV medications compared with enteral formulations, a table comparing relative charges was shared during monthly resident morning conferences and at an HM faculty meeting. The table included charge comparisons between ampicillin and amoxicillin and IV and enteral clindamycin.
Standardized Language in Electronic Health Record (EHR) Antibiotic Plan on Rounds
Standardized language to document antibiotic transition plans was added to admission and progress note templates in the EHR. The standard template prompted residents to (1) define clinical transition criteria, (2) discuss attending comfort with transition overnight (based on survey results), and (3) document patient preference of solid or liquid dosage forms. Plans were reviewed and updated daily. We hypothesized that since residents use the information in the daily progress notes, including assessments and plans, to present on rounds, inclusion of the transition criteria in the note would prompt transition plan discussions.
Communication Bundle
To promote early transition to enteral antibiotics, we standardized the discussion about antibiotic transition between residents and attendings. During a weekly preexisting meeting, the resident QI team reviewed preferences for transitions with the new service attending. By identifying attending preferences early, residents were able to proactively transition patients who met the criteria (eg, antibiotic transition in the evening instead of waiting until morning rounds). This discussion also provided an opportunity to engage service attendings in the QI efforts, which were also shared at HM faculty meetings quarterly.
Recognizing that in times of high census, discussion of patient plans may be abbreviated during rounds, residents were asked to identify all patients on IV antibiotics while reviewing patient medication orders prior to rounds. As part of an existing daily prerounds huddle to discuss rounding logistics, residents listed all patients on IV antibiotics and discussed which patients were ready for transition. If patients could not be transitioned immediately, the team identified the transition criteria.
At preexisting evening huddles between overnight shift HM residents and the evening HM attending, residents identified patients who were prescribed IV antibiotics and discussed readiness for enteral transition. If a patient could be transitioned overnight, enteral antibiotic orders were placed. Overnight residents were also encouraged to review the transition criteria with families upon admission.
Real-time Identification of Failures and Feedback
For two weeks, the EHR was queried daily to identify patients admitted for uncomplicated CAP and SSTI who were on antibiotics as well as other enteral medications. A failure was defined as an IV antibiotic dose given to a patient who was administered any enteral medication. Residents on the QI team approached residents on other HM teams whenever patients were identified as a failed transition to learn about failure reasons.
Study of the Interventions
Data for HM patients who met the inclusion criteria were collected weekly from January 2016 through June 2018 via EHR query. We initially searched for diagnoses that fit under the disease categories of pneumonia and SSTI in the EHR, which generated a list of International Classification of Disease-9 and -10 Diagnosis codes (Appendix Figure 1). The query identified patients based on these codes and reported whether the identified patients took a dose of any enteral medication, excluding nystatin, sildenafil, tacrolimus, and mouthwashes, which are commonly continued during NPO status due to no need for absorption or limited parenteral options. It also reported the ordered route of administration for the queried antibiotics (Appendix Figure 1).
The 2016 calendar year established our baseline to account for seasonal variability. Data were reported weekly and reviewed to evaluate the impact of PDSA cycles and inform new interventions.
Measures
Our process measure was the total number of enteral antibiotic doses divided by all antibiotic doses in patients receiving any enteral medication. We reasoned that if patients were well enough to take medications enterally, they could be given an enteral antibiotic that is highly bioavailable or readily achieves concentrations that attain pharmacodynamic targets. This practice change was a culture shift, decoupling the switch to enteral antibiotics from discharge readiness. Our EHR query reported only the antibiotic doses given to patients who took an enteral medication on the day of antibiotic administration and excluded patients who received only IV medications.
Outcome measures included antimicrobial costs per patient encounter using average wholesale prices, which were reported in our EHR query, and LOS. To ensure that transitions of IV to enteral antibiotics were not negatively impacting patient outcomes, patient readmissions within seven days served as a balancing measure.
Analysis
An annotated statistical process control p-chart tracked the impact of interventions on the proportion of antibiotic doses that were enterally administered during hospitalization. An x-bar and an s-chart tracked the impact of interventions on antimicrobial costs per patient encounter and on LOS. A p-chart and an encounters-between g-chart were used to evaluate the impact of our interventions on readmissions. Control chart rules for identifying special cause were used for center line shifts.14
Ethical Considerations
This study was part of a larger study of the residency high-value care curriculum,12 which was deemed exempt by the CCHMC IRB.
RESULTS
The baseline data collected included 372 patients and the postintervention period in 2017 included 326 patients (Table). Approximately two-thirds of patients had a diagnosis of CAP.
The percentage of antibiotic doses given enterally increased from 44% to 80% within eight months (Figure 2). When studying the impact of interventions, residents on the HM QI team found that the standard EHR template added to daily notes did not consistently prompt residents to discuss antibiotic plans and thus was abandoned. Initial improvement coincided with standardizing discussions between residents and attendings regarding transitions. Furthermore, discussion of all patients on IV antibiotics during the prerounds huddle allowed for reliable, daily communication about antibiotic plans and was subsequently spread to and adopted by all HM teams. The percentage of enterally administered antibiotic doses increased to >75% after the evening huddle, which involved all HM teams, and real-time identification of failures on all HM teams with provider feedback. Despite variability when the total number of antibiotic doses prescribed per week was low (<10), we demonstrated sustainability for 11 months (Figure 2), during which the prerounds and evening huddle discussions were continued and an updated control chart was shown monthly to residents during their educational conferences.
Residents on the QI team spoke directly with other HM residents when there were missed opportunities for transition. Based on these discussions and intermittent chart reviews, common reasons for failure to transition in patients with CAP included admission for failed outpatient enteral treatment, recent evaluation by critical care physicians for possible transfer to the intensive care unit, and difficulty weaning oxygen. For patients with SSTI, hand abscesses requiring drainage by surgery and treatment failure with other antibiotics constituted many of the IV antibiotic doses given to patients on enteral medications.
Antimicrobial costs per patient encounter decreased by 70% over one year; the shift in costs coincided with the second shift in our process measure (Appendix Figure 2A). Based on an estimate of 350 patients admitted per year for uncomplicated CAP or SSTI, this translates to an annual cost savings of approximately $29,000. The standard deviation of costs per patient encounter decreased by 84% (Appendix Figure 2B), suggesting a decrease in the variability of prescribing practices.
The average LOS in our patient population prior to intervention was 2.1 days and did not change (Appendix Figure 2C), but the standard deviation decreased by >50% (Appendix Figure 2D). There was no shift in the mean seven-day readmission rate or the number of encounters between readmissions (2.6% and 26, respectively; Appendix Figure 3). In addition, the hospital billing department did not identify an increase in insurance denials related to the route of antibiotic administration.
DISCUSSION
Summary
Using improvement science, we promoted earlier transition to enteral antibiotics for children hospitalized with uncomplicated CAP and SSTI by linking the decision for transition to the ability to take other enteral medications, rather than to discharge readiness. We increased the percentage of enterally administered antibiotic doses in this patient population from 44% to 80% in eight months. Although we did not observe a decrease in LOS as previously noted in a cost analysis study comparing pediatric patients with CAP treated with oral antibiotics versus those treated with IV antibiotics,15 we did find a decrease in LOS variability and in antimicrobial costs to our patients. These cost savings did not include potential savings from nursing or pharmacy labor. In addition, we noted a decrease in the variability in antibiotic prescribing practice, which demonstrates provider ability and willingness to couple antibiotic route transition to an objective characteristic (administration of other enteral medications).
A strength of our study was that residents, the most frequent prescribers of antibiotics on our HM service, were highly involved in the QI initiative, including defining the SMART aim, identifying key drivers, developing interventions, and completing sequential PDSA cycles. Under the guidance of QI-trained coaches, residents developed feasible interventions and assessed their success in real time. Consistent with other studies,16,17 resident buy-in and involvement led to the success of our improvement study.
Interpretation
Despite emerging evidence regarding the timing of transition to enteral antibiotics, several factors impeded early transition at our institution, including physician culture, variable practice habits, and hospital workflow. Evidence supports the use of enteral antibiotics in immunocompetent children hospitalized for uncomplicated CAP who do not have chronic lung disease, are not in shock, and have oxygen saturations >85%.6 Although existing literature suggests that in pediatric patients admitted for SSTIs not involving the eye or bone, IV antibiotics may be transitioned when clinical improvement, evidenced by a reduction in fever or erythema, is noted,6 enteral antibiotics that achieve appropriate concentrations to attain pharmacodynamic targets should have the same efficacy as that of IV antibiotics.9 Using the criterion of administration of any medication enterally to identify a patient’s readiness to transition, we were able to overcome practice variation among providers who may have differing opinions of what constitutes clinical improvement. Of note, new evidence is emerging on predictors of enteral antibiotic treatment failure in patients with CAP and SSTI to guide transition timing, but these studies have largely focused on the adult population or were performed in the outpatient and emergency department (ED) settings.18,19 Regardless, the stable number of encounters between readmissions in our patient population likely indicates that treatment failure in these patients was rare.
Rising healthcare costs have led to concerns around sustainability of the healthcare system;20,21 tackling overuse in clinical practice, as in our study, is one mitigation strategy. Several studies have used QI methods to facilitate the provision of high-value care through the decrease of continuous monitor overuse and extraneous ordering of electrolytes.22,23 Our QI study adds to the high-value care literature by safely decreasing the use of IV antibiotics. One retrospective study demonstrated that a one-day decrease in the use of IV antibiotics in pneumonia resulted in decreased costs without an increase in readmissions, similar to our findings.24 In adults, QI initiatives aimed at improving early transition of antibiotics utilized electronic trigger tools.25,26 Fischer et al. used active orders for scheduled enteral medications or an enteral diet as indication that a patient’s IV medications could be converted to enteral form.26
Our work is not without limitations. The list of ICD-9 and -10 codes used to query the EHR did not capture all diagnoses that would be considered as uncomplicated CAP or SSTI. However, we included an extensive list of diagnoses to ensure that the majority of patients meeting our inclusion criteria were captured. Our process measure did not account for patients on IV antibiotics who were not administered other enteral medications but tolerating an enteral diet. These patients were not identified in our EHR query and were not included in our process measure as a failure. However, in latter interventions, residents identified all patients on IV antibiotics, so that patients not identified by our EHR query benefited from our work. Furthermore, this QI study was conducted at a single institution and several interventions took advantage of preexisting structured huddles and a resident QI curriculum, which may not exist at other institutions. Our study does highlight that engaging frontline providers, such as residents, to review antibiotic orders consistently and question the appropriateness of the administration route is key to making incremental changes in prescribing practices.
CONCLUSIONS
Through a partnership between HM and Pharmacy and with substantial resident involvement, we improved the transition of IV antibiotics in patients with CAP or SSTI by increasing the percentage of enterally administered antibiotic doses and reducing antimicrobial costs and variability in antibiotic prescribing practices. This work illustrates how reducing overuse of IV antibiotics promotes high-value care and aligns with initiatives to prevent avoidable harm.27 Our work highlights that standardized discussions about medication orders to create consensus around enteral antibiotic transitions, real-time feedback, and challenging the status quo can influence practice habits and effect change.
Next steps include testing automated methods to notify providers of opportunities for transition from IV to enteral antibiotics through embedded clinical decision support, a method similar to the electronic trigger tools used in adult QI studies.25,26 Since our prerounds huddle includes identifying all patients on IV antibiotics, studying the transition to enteral antibiotics and its effect on prescribing practices in other diagnoses (ie, urinary tract infection and osteomyelitis) may contribute to spreading these efforts. Partnering with our ED colleagues may be an important next step, as several patients admitted to HM on IV antibiotics are given their first dose in the ED.
Acknowledgments
The authors would like to thank the faculty of the James M. Anderson Center for Health Systems Excellence Intermediate Improvement Science Series for their guidance in the planning of this project. The authors would also like to thank Ms. Ursula Bradshaw and Mr. Michael Ponti-Zins for obtaining the hospital data on length of stay and readmissions. The authors acknowledge Dr. Philip Hagedorn for his assistance with the software that queries the electronic health record and Dr. Laura Brower and Dr. Joanna Thomson for their assistance with statistical analysis. The authors are grateful to all the residents and coaches on the QI Hospital Medicine team who contributed ideas on study design and interventions.
1. Dellit TH, Owens RC, McGowan JE, Jr, et al. Infectious diseases society of America and the society for healthcare epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159-177. https://doi.org/10.1086/510393.
2. Shah SS, Srivastava R, Wu S, et al. Intravenous Versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6). https://doi.org/10.1542/peds.2016-1692.
3. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822.
4. Jumani K, Advani S, Reich NG, Gosey L, Milstone AM. Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429-435.https://doi.org/10.1001/jamapediatrics.2013.775.
5. Zaoutis T, Localio AR, Leckerman K, et al. Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics. 2009;123(2):636-642. https://doi.org/10.1542/peds.2008-0596.
6. McMullan BJ, Andresen D, Blyth CC, et al. Antibiotic duration and timing of the switch from intravenous to oral route for bacterial infections in children: systematic review and guidelines. Lancet Infect Dis. 2016;16(8):e139-e152. https://doi.org/10.1016/S1473-3099(16)30024-X.
7. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-e76. https://doi.org/10.1093/cid/cir531.
8. Stevens DL, Bisno AL, Chambers HF, et al. Executive summary: practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America. Clin Infect Dis. 2014;59(2):147-159. https://doi.org/10.1093/cid/ciu444.
9. MacGregor RR, Graziani AL. Oral administration of antibiotics: a rational alternative to the parenteral route. Clin Infect Dis. 1997;24(3):457-467. https://doi.org/10.1093/clinids/24.3.457.
10. Downes KJ, Hahn A, Wiles J, Courter JD, Vinks AA. Dose optimisation of antibiotics in children: application of pharmacokinetics/pharmacodynamics in paediatrics. Int J Antimicrob Agents. 2014;43(3):223-230. https://doi.org/10.1016/j.ijantimicag.2013.11.006.
11. Autmizguine J, Melloni C, Hornik CP, et al. Population pharmacokinetics of trimethoprim-sulfamethoxazole in infants and children. Antimicrob Agents Chemother. 2018;62(1):e01813-e01817. https://doi.org/10.1128/AAC.01813-17.
12. Dewan M, Herrmann LE, Tchou MJ, et al. Development and evaluation of high-value pediatrics: a high-value care pediatric resident curriculum. Hosp Pediatr. 2018;8(12):785-792. https://doi.org/10.1542/hpeds.2018-0115
13. Langley GJ, Moen RD, Nolan KM, Nolan TW, Norman CL, Provost LP. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. New Jersey, US: John Wiley & Sons; 2009.
14. Benneyan JC. Use and interpretation of statistical quality control charts. Int J Qual Health Care. 1998;10(1):69-73. https://doi.org/10.1093/intqhc/10.1.69.
15. Lorgelly PK, Atkinson M, Lakhanpaul M, et al. Oral versus i.v. antibiotics for community-acquired pneumonia in children: a cost-minimisation analysis. Eur Respir J. 2010;35(4):858-864. https://doi.org/10.1183/09031936.00087209.
16. Vidyarthi AR, Green AL, Rosenbluth G, Baron RB. Engaging residents and fellows to improve institution-wide quality: the first six years of a novel financial incentive program. Acad Med. 2014;89(3):460-468. https://doi.org/10.1097/ACM.0000000000000159.
17. Stinnett-Donnelly JM, Stevens PG, Hood VL. Developing a high value care programme from the bottom up: a programme of faculty-resident improvement projects targeting harmful or unnecessary care. BMJ Qual Saf. 2016;25(11):901-908. https://doi.org/10.1136/bmjqs-2015-004546.
18. Peterson D, McLeod S, Woolfrey K, McRae A. Predictors of failure of empiric outpatient antibiotic therapy in emergency department patients with uncomplicated cellulitis. Acad Emerg Med. 2014;21(5):526-531. https://doi.org/10.1111/acem.12371.
19. Yadav K, Suh KN, Eagles D, et al. Predictors of oral antibiotic treatment failure for non-purulent skin and soft tissue infections in the emergency department. Acad Emerg Med. 2018;20(S1):S24-S25. https://doi.org/10.1017/cem.2018.114.
20. Organisation for Economic Co-operation and Development. Healthcare costs unsustainable in advanced economies without reform. http://www.oecd.org/health/healthcarecostsunsustainableinadvancedeconomieswithoutreform.htm. Accessed June 28, 2018; 2015.
21. Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012;307(14):1513-1516. https://doi.org/10.1001/jama.2012.362.
22. Schondelmeyer AC, Simmons JM, Statile AM, et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135(4):e1044-e1051. https://doi.org/10.1542/peds.2014-2295.
23. Tchou MJ, Tang Girdwood S, Wormser B, et al. Reducing electrolyte testing in hospitalized children by using quality improvement methods. Pediatrics. 2018;141(5). https://doi.org/10.1542/peds.2017-3187.
24. Christensen EW, Spaulding AB, Pomputius WF, Grapentine SP. Effects of hospital practice patterns for antibiotic administration for pneumonia on hospital lengths of stay and costs. J Pediatr Infect Dis Soc. 2019;8(2):115-121. https://doi.org/10.1093/jpids/piy003.
25. Berrevoets MAH, Pot JHLW, Houterman AE, et al. An electronic trigger tool to optimise intravenous to oral antibiotic switch: a controlled, interrupted time series study. Antimicrob Resist Infect Control. 2017;6:81. https://doi.org/10.1186/s13756-017-0239-3.
26. Fischer MA, Solomon DH, Teich JM, Avorn J. Conversion from intravenous to oral medications: assessment of a computerized intervention for hospitalized patients. Arch Intern Med. 2003;163(21):2585-2589. https://doi.org/10.1001/archinte.163.21.2585.
27. Schroeder AR, Harris SJ, Newman TB. Safely doing less: a missing component of the patient safety dialogue. Pediatrics. 2011;128(6):e1596-e1597. https://doi.org/10.1542/peds.2011-2726.
Intravenous (IV) antibiotics are commonly used in hospitalized pediatric patients to treat bacterial infections. Antimicrobial stewardship guidelines published by the Infectious Diseases Society of America (IDSA) recommend institutions develop a systematic plan to convert from IV to enteral antibiotics, as early transition may reduce healthcare costs, decrease length of stay (LOS), and avoid prolonged IV access complications1 such as extravasation, thrombosis, and catheter-associated infections.2-5
Pediatric patients with community-acquired pneumonia (CAP) and mild skin and soft tissue infections (SSTI) may not require IV antibiotics, even if the patient is hospitalized.6 Although national guidelines for pediatric CAP and SSTI recommend IV antibiotics for hospitalized patients, these guidelines state that mild infections may be treated with enteral antibiotics and emphasize discontinuation of IV antibiotics when the patient meets discharge criteria.7,8 Furthermore, several enteral antibiotics used for the treatment of CAP and SSTI, such as cephalexin and clindamycin,9 have excellent bioavailability (>90%) or can achieve sufficient concentrations to attain the pharmacodynamic target (ie, amoxicillin and trimethoprim–sulfamethoxazole).10,11 Nonetheless, the guidelines do not explicitly outline criteria regarding the transition from IV to enteral antibiotics.7,8
At our institution, patients admitted to Hospital Medicine (HM) often remained on IV antibiotics until discharge. Data review revealed that antibiotic treatment of CAP and SSTI posed the greatest opportunity for early conversion to enteral therapy based on the high frequency of admissions and the ability of commonly used enteral antibiotics to attain pharmacodynamic targets. We sought to change practice culture by decoupling transition to enteral antibiotics from discharge and use administration of other enteral medications as an objective indicator for transition. Our aim was to increase the proportion of enterally administered antibiotic doses for HM patients aged >60 days admitted with uncomplicated CAP or SSTI from 44% to 75% in eight months.
METHODS
Context
Cincinnati Children’s Hospital Medical Center (CCHMC) is a large, urban, academic hospital. The HM division has 45 attendings and admits >8,000 general pediatric patients annually. The five HM teams at the main campus consist of attendings, fellows, residents, and medical students. One HM team serves as the resident quality improvement (QI) team where residents collaborate in a longitudinal study under the guidance of QI-trained coaches. The focus of this QI initiative was determined by resident consensus and aligned with a high-value care curriculum.12
To identify the target patient population, we investigated IV antimicrobials frequently used in HM patients. Ampicillin and clindamycin are commonly used IV antibiotics, most frequently corresponding with the diagnoses of CAP and SSTI, respectively, accounting for half of all antibiotic use on the HM service. Amoxicillin, the enteral equivalent of ampicillin, can achieve sufficient concentrations to attain the pharmacodynamic target at infection sites, and clindamycin has high bioavailability, making them ideal options for early transition. Our institution’s robust antimicrobial stewardship program has published local guidelines on using amoxicillin as the enteral antibiotic of choice for uncomplicated CAP, but it does not provide guidance on the timing of transition for either CAP or SSTI; the clinical team makes this decision.
HM attendings were surveyed to determine the criteria used to transition from IV to enteral antibiotics for patients with CAP or SSTI. The survey illustrated practice variability with providers using differing clinical criteria to signal the timing of transition. Additionally, only 49% of respondents (n = 37) rated themselves as “very comfortable” with residents making autonomous decisions to transition to enteral antibiotics. We chose to use the administration of other enteral medications, instead of discharge readiness, as an objective indicator of a patient’s readiness to transition to enteral antibiotics, given the low-risk patient population and the ability of the enteral antibiotics commonly used for CAP and SSTI to achieve pharmacodynamic targets.
The study population included patients aged >60 days admitted to HM with CAP or SSTI treated with any antibiotic. We excluded patients with potential complications or significant progression of their disease process, including patients with parapneumonic effusions or chest tubes, patients who underwent bronchoscopy, and patients with osteomyelitis, septic arthritis, or preseptal or orbital cellulitis. Past medical history and clinical status on admission were not used to exclude patients.
Interventions
Our multidisciplinary team, formed in January 2017, included HM attendings, HM fellows, pediatric residents, a critical care attending, a pharmacy resident, and an antimicrobial stewardship pharmacist. Under the guidance of QI coaches, the residents on the HM QI team developed and tested all interventions on their team and then determined which interventions would spread to the other four teams. The nursing director of our primary HM unit disseminated project updates to bedside nurses. A simplified failure mode and effects analysis identified areas for improvement and potential interventions. Interventions focused on the following key drivers (Figure 1): increased prescriber awareness of medication charge, standardization of conversion from IV to enteral antibiotics, clear definition of the patients ready for transition, ongoing evaluation of the antimicrobial plan, timely recognition by prescribers of patients ready for transition, culture shift regarding the appropriate administration route in the inpatient setting, and transparency of data. The team implemented sequential Plan-Do-Study-Act (PDSA) cycles13 to test the interventions.
Charge Table
To improve knowledge about the increased charge for commonly used IV medications compared with enteral formulations, a table comparing relative charges was shared during monthly resident morning conferences and at an HM faculty meeting. The table included charge comparisons between ampicillin and amoxicillin and IV and enteral clindamycin.
Standardized Language in Electronic Health Record (EHR) Antibiotic Plan on Rounds
Standardized language to document antibiotic transition plans was added to admission and progress note templates in the EHR. The standard template prompted residents to (1) define clinical transition criteria, (2) discuss attending comfort with transition overnight (based on survey results), and (3) document patient preference of solid or liquid dosage forms. Plans were reviewed and updated daily. We hypothesized that since residents use the information in the daily progress notes, including assessments and plans, to present on rounds, inclusion of the transition criteria in the note would prompt transition plan discussions.
Communication Bundle
To promote early transition to enteral antibiotics, we standardized the discussion about antibiotic transition between residents and attendings. During a weekly preexisting meeting, the resident QI team reviewed preferences for transitions with the new service attending. By identifying attending preferences early, residents were able to proactively transition patients who met the criteria (eg, antibiotic transition in the evening instead of waiting until morning rounds). This discussion also provided an opportunity to engage service attendings in the QI efforts, which were also shared at HM faculty meetings quarterly.
Recognizing that in times of high census, discussion of patient plans may be abbreviated during rounds, residents were asked to identify all patients on IV antibiotics while reviewing patient medication orders prior to rounds. As part of an existing daily prerounds huddle to discuss rounding logistics, residents listed all patients on IV antibiotics and discussed which patients were ready for transition. If patients could not be transitioned immediately, the team identified the transition criteria.
At preexisting evening huddles between overnight shift HM residents and the evening HM attending, residents identified patients who were prescribed IV antibiotics and discussed readiness for enteral transition. If a patient could be transitioned overnight, enteral antibiotic orders were placed. Overnight residents were also encouraged to review the transition criteria with families upon admission.
Real-time Identification of Failures and Feedback
For two weeks, the EHR was queried daily to identify patients admitted for uncomplicated CAP and SSTI who were on antibiotics as well as other enteral medications. A failure was defined as an IV antibiotic dose given to a patient who was administered any enteral medication. Residents on the QI team approached residents on other HM teams whenever patients were identified as a failed transition to learn about failure reasons.
Study of the Interventions
Data for HM patients who met the inclusion criteria were collected weekly from January 2016 through June 2018 via EHR query. We initially searched for diagnoses that fit under the disease categories of pneumonia and SSTI in the EHR, which generated a list of International Classification of Disease-9 and -10 Diagnosis codes (Appendix Figure 1). The query identified patients based on these codes and reported whether the identified patients took a dose of any enteral medication, excluding nystatin, sildenafil, tacrolimus, and mouthwashes, which are commonly continued during NPO status due to no need for absorption or limited parenteral options. It also reported the ordered route of administration for the queried antibiotics (Appendix Figure 1).
The 2016 calendar year established our baseline to account for seasonal variability. Data were reported weekly and reviewed to evaluate the impact of PDSA cycles and inform new interventions.
Measures
Our process measure was the total number of enteral antibiotic doses divided by all antibiotic doses in patients receiving any enteral medication. We reasoned that if patients were well enough to take medications enterally, they could be given an enteral antibiotic that is highly bioavailable or readily achieves concentrations that attain pharmacodynamic targets. This practice change was a culture shift, decoupling the switch to enteral antibiotics from discharge readiness. Our EHR query reported only the antibiotic doses given to patients who took an enteral medication on the day of antibiotic administration and excluded patients who received only IV medications.
Outcome measures included antimicrobial costs per patient encounter using average wholesale prices, which were reported in our EHR query, and LOS. To ensure that transitions of IV to enteral antibiotics were not negatively impacting patient outcomes, patient readmissions within seven days served as a balancing measure.
Analysis
An annotated statistical process control p-chart tracked the impact of interventions on the proportion of antibiotic doses that were enterally administered during hospitalization. An x-bar and an s-chart tracked the impact of interventions on antimicrobial costs per patient encounter and on LOS. A p-chart and an encounters-between g-chart were used to evaluate the impact of our interventions on readmissions. Control chart rules for identifying special cause were used for center line shifts.14
Ethical Considerations
This study was part of a larger study of the residency high-value care curriculum,12 which was deemed exempt by the CCHMC IRB.
RESULTS
The baseline data collected included 372 patients and the postintervention period in 2017 included 326 patients (Table). Approximately two-thirds of patients had a diagnosis of CAP.
The percentage of antibiotic doses given enterally increased from 44% to 80% within eight months (Figure 2). When studying the impact of interventions, residents on the HM QI team found that the standard EHR template added to daily notes did not consistently prompt residents to discuss antibiotic plans and thus was abandoned. Initial improvement coincided with standardizing discussions between residents and attendings regarding transitions. Furthermore, discussion of all patients on IV antibiotics during the prerounds huddle allowed for reliable, daily communication about antibiotic plans and was subsequently spread to and adopted by all HM teams. The percentage of enterally administered antibiotic doses increased to >75% after the evening huddle, which involved all HM teams, and real-time identification of failures on all HM teams with provider feedback. Despite variability when the total number of antibiotic doses prescribed per week was low (<10), we demonstrated sustainability for 11 months (Figure 2), during which the prerounds and evening huddle discussions were continued and an updated control chart was shown monthly to residents during their educational conferences.
Residents on the QI team spoke directly with other HM residents when there were missed opportunities for transition. Based on these discussions and intermittent chart reviews, common reasons for failure to transition in patients with CAP included admission for failed outpatient enteral treatment, recent evaluation by critical care physicians for possible transfer to the intensive care unit, and difficulty weaning oxygen. For patients with SSTI, hand abscesses requiring drainage by surgery and treatment failure with other antibiotics constituted many of the IV antibiotic doses given to patients on enteral medications.
Antimicrobial costs per patient encounter decreased by 70% over one year; the shift in costs coincided with the second shift in our process measure (Appendix Figure 2A). Based on an estimate of 350 patients admitted per year for uncomplicated CAP or SSTI, this translates to an annual cost savings of approximately $29,000. The standard deviation of costs per patient encounter decreased by 84% (Appendix Figure 2B), suggesting a decrease in the variability of prescribing practices.
The average LOS in our patient population prior to intervention was 2.1 days and did not change (Appendix Figure 2C), but the standard deviation decreased by >50% (Appendix Figure 2D). There was no shift in the mean seven-day readmission rate or the number of encounters between readmissions (2.6% and 26, respectively; Appendix Figure 3). In addition, the hospital billing department did not identify an increase in insurance denials related to the route of antibiotic administration.
DISCUSSION
Summary
Using improvement science, we promoted earlier transition to enteral antibiotics for children hospitalized with uncomplicated CAP and SSTI by linking the decision for transition to the ability to take other enteral medications, rather than to discharge readiness. We increased the percentage of enterally administered antibiotic doses in this patient population from 44% to 80% in eight months. Although we did not observe a decrease in LOS as previously noted in a cost analysis study comparing pediatric patients with CAP treated with oral antibiotics versus those treated with IV antibiotics,15 we did find a decrease in LOS variability and in antimicrobial costs to our patients. These cost savings did not include potential savings from nursing or pharmacy labor. In addition, we noted a decrease in the variability in antibiotic prescribing practice, which demonstrates provider ability and willingness to couple antibiotic route transition to an objective characteristic (administration of other enteral medications).
A strength of our study was that residents, the most frequent prescribers of antibiotics on our HM service, were highly involved in the QI initiative, including defining the SMART aim, identifying key drivers, developing interventions, and completing sequential PDSA cycles. Under the guidance of QI-trained coaches, residents developed feasible interventions and assessed their success in real time. Consistent with other studies,16,17 resident buy-in and involvement led to the success of our improvement study.
Interpretation
Despite emerging evidence regarding the timing of transition to enteral antibiotics, several factors impeded early transition at our institution, including physician culture, variable practice habits, and hospital workflow. Evidence supports the use of enteral antibiotics in immunocompetent children hospitalized for uncomplicated CAP who do not have chronic lung disease, are not in shock, and have oxygen saturations >85%.6 Although existing literature suggests that in pediatric patients admitted for SSTIs not involving the eye or bone, IV antibiotics may be transitioned when clinical improvement, evidenced by a reduction in fever or erythema, is noted,6 enteral antibiotics that achieve appropriate concentrations to attain pharmacodynamic targets should have the same efficacy as that of IV antibiotics.9 Using the criterion of administration of any medication enterally to identify a patient’s readiness to transition, we were able to overcome practice variation among providers who may have differing opinions of what constitutes clinical improvement. Of note, new evidence is emerging on predictors of enteral antibiotic treatment failure in patients with CAP and SSTI to guide transition timing, but these studies have largely focused on the adult population or were performed in the outpatient and emergency department (ED) settings.18,19 Regardless, the stable number of encounters between readmissions in our patient population likely indicates that treatment failure in these patients was rare.
Rising healthcare costs have led to concerns around sustainability of the healthcare system;20,21 tackling overuse in clinical practice, as in our study, is one mitigation strategy. Several studies have used QI methods to facilitate the provision of high-value care through the decrease of continuous monitor overuse and extraneous ordering of electrolytes.22,23 Our QI study adds to the high-value care literature by safely decreasing the use of IV antibiotics. One retrospective study demonstrated that a one-day decrease in the use of IV antibiotics in pneumonia resulted in decreased costs without an increase in readmissions, similar to our findings.24 In adults, QI initiatives aimed at improving early transition of antibiotics utilized electronic trigger tools.25,26 Fischer et al. used active orders for scheduled enteral medications or an enteral diet as indication that a patient’s IV medications could be converted to enteral form.26
Our work is not without limitations. The list of ICD-9 and -10 codes used to query the EHR did not capture all diagnoses that would be considered as uncomplicated CAP or SSTI. However, we included an extensive list of diagnoses to ensure that the majority of patients meeting our inclusion criteria were captured. Our process measure did not account for patients on IV antibiotics who were not administered other enteral medications but tolerating an enteral diet. These patients were not identified in our EHR query and were not included in our process measure as a failure. However, in latter interventions, residents identified all patients on IV antibiotics, so that patients not identified by our EHR query benefited from our work. Furthermore, this QI study was conducted at a single institution and several interventions took advantage of preexisting structured huddles and a resident QI curriculum, which may not exist at other institutions. Our study does highlight that engaging frontline providers, such as residents, to review antibiotic orders consistently and question the appropriateness of the administration route is key to making incremental changes in prescribing practices.
CONCLUSIONS
Through a partnership between HM and Pharmacy and with substantial resident involvement, we improved the transition of IV antibiotics in patients with CAP or SSTI by increasing the percentage of enterally administered antibiotic doses and reducing antimicrobial costs and variability in antibiotic prescribing practices. This work illustrates how reducing overuse of IV antibiotics promotes high-value care and aligns with initiatives to prevent avoidable harm.27 Our work highlights that standardized discussions about medication orders to create consensus around enteral antibiotic transitions, real-time feedback, and challenging the status quo can influence practice habits and effect change.
Next steps include testing automated methods to notify providers of opportunities for transition from IV to enteral antibiotics through embedded clinical decision support, a method similar to the electronic trigger tools used in adult QI studies.25,26 Since our prerounds huddle includes identifying all patients on IV antibiotics, studying the transition to enteral antibiotics and its effect on prescribing practices in other diagnoses (ie, urinary tract infection and osteomyelitis) may contribute to spreading these efforts. Partnering with our ED colleagues may be an important next step, as several patients admitted to HM on IV antibiotics are given their first dose in the ED.
Acknowledgments
The authors would like to thank the faculty of the James M. Anderson Center for Health Systems Excellence Intermediate Improvement Science Series for their guidance in the planning of this project. The authors would also like to thank Ms. Ursula Bradshaw and Mr. Michael Ponti-Zins for obtaining the hospital data on length of stay and readmissions. The authors acknowledge Dr. Philip Hagedorn for his assistance with the software that queries the electronic health record and Dr. Laura Brower and Dr. Joanna Thomson for their assistance with statistical analysis. The authors are grateful to all the residents and coaches on the QI Hospital Medicine team who contributed ideas on study design and interventions.
Intravenous (IV) antibiotics are commonly used in hospitalized pediatric patients to treat bacterial infections. Antimicrobial stewardship guidelines published by the Infectious Diseases Society of America (IDSA) recommend institutions develop a systematic plan to convert from IV to enteral antibiotics, as early transition may reduce healthcare costs, decrease length of stay (LOS), and avoid prolonged IV access complications1 such as extravasation, thrombosis, and catheter-associated infections.2-5
Pediatric patients with community-acquired pneumonia (CAP) and mild skin and soft tissue infections (SSTI) may not require IV antibiotics, even if the patient is hospitalized.6 Although national guidelines for pediatric CAP and SSTI recommend IV antibiotics for hospitalized patients, these guidelines state that mild infections may be treated with enteral antibiotics and emphasize discontinuation of IV antibiotics when the patient meets discharge criteria.7,8 Furthermore, several enteral antibiotics used for the treatment of CAP and SSTI, such as cephalexin and clindamycin,9 have excellent bioavailability (>90%) or can achieve sufficient concentrations to attain the pharmacodynamic target (ie, amoxicillin and trimethoprim–sulfamethoxazole).10,11 Nonetheless, the guidelines do not explicitly outline criteria regarding the transition from IV to enteral antibiotics.7,8
At our institution, patients admitted to Hospital Medicine (HM) often remained on IV antibiotics until discharge. Data review revealed that antibiotic treatment of CAP and SSTI posed the greatest opportunity for early conversion to enteral therapy based on the high frequency of admissions and the ability of commonly used enteral antibiotics to attain pharmacodynamic targets. We sought to change practice culture by decoupling transition to enteral antibiotics from discharge and use administration of other enteral medications as an objective indicator for transition. Our aim was to increase the proportion of enterally administered antibiotic doses for HM patients aged >60 days admitted with uncomplicated CAP or SSTI from 44% to 75% in eight months.
METHODS
Context
Cincinnati Children’s Hospital Medical Center (CCHMC) is a large, urban, academic hospital. The HM division has 45 attendings and admits >8,000 general pediatric patients annually. The five HM teams at the main campus consist of attendings, fellows, residents, and medical students. One HM team serves as the resident quality improvement (QI) team where residents collaborate in a longitudinal study under the guidance of QI-trained coaches. The focus of this QI initiative was determined by resident consensus and aligned with a high-value care curriculum.12
To identify the target patient population, we investigated IV antimicrobials frequently used in HM patients. Ampicillin and clindamycin are commonly used IV antibiotics, most frequently corresponding with the diagnoses of CAP and SSTI, respectively, accounting for half of all antibiotic use on the HM service. Amoxicillin, the enteral equivalent of ampicillin, can achieve sufficient concentrations to attain the pharmacodynamic target at infection sites, and clindamycin has high bioavailability, making them ideal options for early transition. Our institution’s robust antimicrobial stewardship program has published local guidelines on using amoxicillin as the enteral antibiotic of choice for uncomplicated CAP, but it does not provide guidance on the timing of transition for either CAP or SSTI; the clinical team makes this decision.
HM attendings were surveyed to determine the criteria used to transition from IV to enteral antibiotics for patients with CAP or SSTI. The survey illustrated practice variability with providers using differing clinical criteria to signal the timing of transition. Additionally, only 49% of respondents (n = 37) rated themselves as “very comfortable” with residents making autonomous decisions to transition to enteral antibiotics. We chose to use the administration of other enteral medications, instead of discharge readiness, as an objective indicator of a patient’s readiness to transition to enteral antibiotics, given the low-risk patient population and the ability of the enteral antibiotics commonly used for CAP and SSTI to achieve pharmacodynamic targets.
The study population included patients aged >60 days admitted to HM with CAP or SSTI treated with any antibiotic. We excluded patients with potential complications or significant progression of their disease process, including patients with parapneumonic effusions or chest tubes, patients who underwent bronchoscopy, and patients with osteomyelitis, septic arthritis, or preseptal or orbital cellulitis. Past medical history and clinical status on admission were not used to exclude patients.
Interventions
Our multidisciplinary team, formed in January 2017, included HM attendings, HM fellows, pediatric residents, a critical care attending, a pharmacy resident, and an antimicrobial stewardship pharmacist. Under the guidance of QI coaches, the residents on the HM QI team developed and tested all interventions on their team and then determined which interventions would spread to the other four teams. The nursing director of our primary HM unit disseminated project updates to bedside nurses. A simplified failure mode and effects analysis identified areas for improvement and potential interventions. Interventions focused on the following key drivers (Figure 1): increased prescriber awareness of medication charge, standardization of conversion from IV to enteral antibiotics, clear definition of the patients ready for transition, ongoing evaluation of the antimicrobial plan, timely recognition by prescribers of patients ready for transition, culture shift regarding the appropriate administration route in the inpatient setting, and transparency of data. The team implemented sequential Plan-Do-Study-Act (PDSA) cycles13 to test the interventions.
Charge Table
To improve knowledge about the increased charge for commonly used IV medications compared with enteral formulations, a table comparing relative charges was shared during monthly resident morning conferences and at an HM faculty meeting. The table included charge comparisons between ampicillin and amoxicillin and IV and enteral clindamycin.
Standardized Language in Electronic Health Record (EHR) Antibiotic Plan on Rounds
Standardized language to document antibiotic transition plans was added to admission and progress note templates in the EHR. The standard template prompted residents to (1) define clinical transition criteria, (2) discuss attending comfort with transition overnight (based on survey results), and (3) document patient preference of solid or liquid dosage forms. Plans were reviewed and updated daily. We hypothesized that since residents use the information in the daily progress notes, including assessments and plans, to present on rounds, inclusion of the transition criteria in the note would prompt transition plan discussions.
Communication Bundle
To promote early transition to enteral antibiotics, we standardized the discussion about antibiotic transition between residents and attendings. During a weekly preexisting meeting, the resident QI team reviewed preferences for transitions with the new service attending. By identifying attending preferences early, residents were able to proactively transition patients who met the criteria (eg, antibiotic transition in the evening instead of waiting until morning rounds). This discussion also provided an opportunity to engage service attendings in the QI efforts, which were also shared at HM faculty meetings quarterly.
Recognizing that in times of high census, discussion of patient plans may be abbreviated during rounds, residents were asked to identify all patients on IV antibiotics while reviewing patient medication orders prior to rounds. As part of an existing daily prerounds huddle to discuss rounding logistics, residents listed all patients on IV antibiotics and discussed which patients were ready for transition. If patients could not be transitioned immediately, the team identified the transition criteria.
At preexisting evening huddles between overnight shift HM residents and the evening HM attending, residents identified patients who were prescribed IV antibiotics and discussed readiness for enteral transition. If a patient could be transitioned overnight, enteral antibiotic orders were placed. Overnight residents were also encouraged to review the transition criteria with families upon admission.
Real-time Identification of Failures and Feedback
For two weeks, the EHR was queried daily to identify patients admitted for uncomplicated CAP and SSTI who were on antibiotics as well as other enteral medications. A failure was defined as an IV antibiotic dose given to a patient who was administered any enteral medication. Residents on the QI team approached residents on other HM teams whenever patients were identified as a failed transition to learn about failure reasons.
Study of the Interventions
Data for HM patients who met the inclusion criteria were collected weekly from January 2016 through June 2018 via EHR query. We initially searched for diagnoses that fit under the disease categories of pneumonia and SSTI in the EHR, which generated a list of International Classification of Disease-9 and -10 Diagnosis codes (Appendix Figure 1). The query identified patients based on these codes and reported whether the identified patients took a dose of any enteral medication, excluding nystatin, sildenafil, tacrolimus, and mouthwashes, which are commonly continued during NPO status due to no need for absorption or limited parenteral options. It also reported the ordered route of administration for the queried antibiotics (Appendix Figure 1).
The 2016 calendar year established our baseline to account for seasonal variability. Data were reported weekly and reviewed to evaluate the impact of PDSA cycles and inform new interventions.
Measures
Our process measure was the total number of enteral antibiotic doses divided by all antibiotic doses in patients receiving any enteral medication. We reasoned that if patients were well enough to take medications enterally, they could be given an enteral antibiotic that is highly bioavailable or readily achieves concentrations that attain pharmacodynamic targets. This practice change was a culture shift, decoupling the switch to enteral antibiotics from discharge readiness. Our EHR query reported only the antibiotic doses given to patients who took an enteral medication on the day of antibiotic administration and excluded patients who received only IV medications.
Outcome measures included antimicrobial costs per patient encounter using average wholesale prices, which were reported in our EHR query, and LOS. To ensure that transitions of IV to enteral antibiotics were not negatively impacting patient outcomes, patient readmissions within seven days served as a balancing measure.
Analysis
An annotated statistical process control p-chart tracked the impact of interventions on the proportion of antibiotic doses that were enterally administered during hospitalization. An x-bar and an s-chart tracked the impact of interventions on antimicrobial costs per patient encounter and on LOS. A p-chart and an encounters-between g-chart were used to evaluate the impact of our interventions on readmissions. Control chart rules for identifying special cause were used for center line shifts.14
Ethical Considerations
This study was part of a larger study of the residency high-value care curriculum,12 which was deemed exempt by the CCHMC IRB.
RESULTS
The baseline data collected included 372 patients and the postintervention period in 2017 included 326 patients (Table). Approximately two-thirds of patients had a diagnosis of CAP.
The percentage of antibiotic doses given enterally increased from 44% to 80% within eight months (Figure 2). When studying the impact of interventions, residents on the HM QI team found that the standard EHR template added to daily notes did not consistently prompt residents to discuss antibiotic plans and thus was abandoned. Initial improvement coincided with standardizing discussions between residents and attendings regarding transitions. Furthermore, discussion of all patients on IV antibiotics during the prerounds huddle allowed for reliable, daily communication about antibiotic plans and was subsequently spread to and adopted by all HM teams. The percentage of enterally administered antibiotic doses increased to >75% after the evening huddle, which involved all HM teams, and real-time identification of failures on all HM teams with provider feedback. Despite variability when the total number of antibiotic doses prescribed per week was low (<10), we demonstrated sustainability for 11 months (Figure 2), during which the prerounds and evening huddle discussions were continued and an updated control chart was shown monthly to residents during their educational conferences.
Residents on the QI team spoke directly with other HM residents when there were missed opportunities for transition. Based on these discussions and intermittent chart reviews, common reasons for failure to transition in patients with CAP included admission for failed outpatient enteral treatment, recent evaluation by critical care physicians for possible transfer to the intensive care unit, and difficulty weaning oxygen. For patients with SSTI, hand abscesses requiring drainage by surgery and treatment failure with other antibiotics constituted many of the IV antibiotic doses given to patients on enteral medications.
Antimicrobial costs per patient encounter decreased by 70% over one year; the shift in costs coincided with the second shift in our process measure (Appendix Figure 2A). Based on an estimate of 350 patients admitted per year for uncomplicated CAP or SSTI, this translates to an annual cost savings of approximately $29,000. The standard deviation of costs per patient encounter decreased by 84% (Appendix Figure 2B), suggesting a decrease in the variability of prescribing practices.
The average LOS in our patient population prior to intervention was 2.1 days and did not change (Appendix Figure 2C), but the standard deviation decreased by >50% (Appendix Figure 2D). There was no shift in the mean seven-day readmission rate or the number of encounters between readmissions (2.6% and 26, respectively; Appendix Figure 3). In addition, the hospital billing department did not identify an increase in insurance denials related to the route of antibiotic administration.
DISCUSSION
Summary
Using improvement science, we promoted earlier transition to enteral antibiotics for children hospitalized with uncomplicated CAP and SSTI by linking the decision for transition to the ability to take other enteral medications, rather than to discharge readiness. We increased the percentage of enterally administered antibiotic doses in this patient population from 44% to 80% in eight months. Although we did not observe a decrease in LOS as previously noted in a cost analysis study comparing pediatric patients with CAP treated with oral antibiotics versus those treated with IV antibiotics,15 we did find a decrease in LOS variability and in antimicrobial costs to our patients. These cost savings did not include potential savings from nursing or pharmacy labor. In addition, we noted a decrease in the variability in antibiotic prescribing practice, which demonstrates provider ability and willingness to couple antibiotic route transition to an objective characteristic (administration of other enteral medications).
A strength of our study was that residents, the most frequent prescribers of antibiotics on our HM service, were highly involved in the QI initiative, including defining the SMART aim, identifying key drivers, developing interventions, and completing sequential PDSA cycles. Under the guidance of QI-trained coaches, residents developed feasible interventions and assessed their success in real time. Consistent with other studies,16,17 resident buy-in and involvement led to the success of our improvement study.
Interpretation
Despite emerging evidence regarding the timing of transition to enteral antibiotics, several factors impeded early transition at our institution, including physician culture, variable practice habits, and hospital workflow. Evidence supports the use of enteral antibiotics in immunocompetent children hospitalized for uncomplicated CAP who do not have chronic lung disease, are not in shock, and have oxygen saturations >85%.6 Although existing literature suggests that in pediatric patients admitted for SSTIs not involving the eye or bone, IV antibiotics may be transitioned when clinical improvement, evidenced by a reduction in fever or erythema, is noted,6 enteral antibiotics that achieve appropriate concentrations to attain pharmacodynamic targets should have the same efficacy as that of IV antibiotics.9 Using the criterion of administration of any medication enterally to identify a patient’s readiness to transition, we were able to overcome practice variation among providers who may have differing opinions of what constitutes clinical improvement. Of note, new evidence is emerging on predictors of enteral antibiotic treatment failure in patients with CAP and SSTI to guide transition timing, but these studies have largely focused on the adult population or were performed in the outpatient and emergency department (ED) settings.18,19 Regardless, the stable number of encounters between readmissions in our patient population likely indicates that treatment failure in these patients was rare.
Rising healthcare costs have led to concerns around sustainability of the healthcare system;20,21 tackling overuse in clinical practice, as in our study, is one mitigation strategy. Several studies have used QI methods to facilitate the provision of high-value care through the decrease of continuous monitor overuse and extraneous ordering of electrolytes.22,23 Our QI study adds to the high-value care literature by safely decreasing the use of IV antibiotics. One retrospective study demonstrated that a one-day decrease in the use of IV antibiotics in pneumonia resulted in decreased costs without an increase in readmissions, similar to our findings.24 In adults, QI initiatives aimed at improving early transition of antibiotics utilized electronic trigger tools.25,26 Fischer et al. used active orders for scheduled enteral medications or an enteral diet as indication that a patient’s IV medications could be converted to enteral form.26
Our work is not without limitations. The list of ICD-9 and -10 codes used to query the EHR did not capture all diagnoses that would be considered as uncomplicated CAP or SSTI. However, we included an extensive list of diagnoses to ensure that the majority of patients meeting our inclusion criteria were captured. Our process measure did not account for patients on IV antibiotics who were not administered other enteral medications but tolerating an enteral diet. These patients were not identified in our EHR query and were not included in our process measure as a failure. However, in latter interventions, residents identified all patients on IV antibiotics, so that patients not identified by our EHR query benefited from our work. Furthermore, this QI study was conducted at a single institution and several interventions took advantage of preexisting structured huddles and a resident QI curriculum, which may not exist at other institutions. Our study does highlight that engaging frontline providers, such as residents, to review antibiotic orders consistently and question the appropriateness of the administration route is key to making incremental changes in prescribing practices.
CONCLUSIONS
Through a partnership between HM and Pharmacy and with substantial resident involvement, we improved the transition of IV antibiotics in patients with CAP or SSTI by increasing the percentage of enterally administered antibiotic doses and reducing antimicrobial costs and variability in antibiotic prescribing practices. This work illustrates how reducing overuse of IV antibiotics promotes high-value care and aligns with initiatives to prevent avoidable harm.27 Our work highlights that standardized discussions about medication orders to create consensus around enteral antibiotic transitions, real-time feedback, and challenging the status quo can influence practice habits and effect change.
Next steps include testing automated methods to notify providers of opportunities for transition from IV to enteral antibiotics through embedded clinical decision support, a method similar to the electronic trigger tools used in adult QI studies.25,26 Since our prerounds huddle includes identifying all patients on IV antibiotics, studying the transition to enteral antibiotics and its effect on prescribing practices in other diagnoses (ie, urinary tract infection and osteomyelitis) may contribute to spreading these efforts. Partnering with our ED colleagues may be an important next step, as several patients admitted to HM on IV antibiotics are given their first dose in the ED.
Acknowledgments
The authors would like to thank the faculty of the James M. Anderson Center for Health Systems Excellence Intermediate Improvement Science Series for their guidance in the planning of this project. The authors would also like to thank Ms. Ursula Bradshaw and Mr. Michael Ponti-Zins for obtaining the hospital data on length of stay and readmissions. The authors acknowledge Dr. Philip Hagedorn for his assistance with the software that queries the electronic health record and Dr. Laura Brower and Dr. Joanna Thomson for their assistance with statistical analysis. The authors are grateful to all the residents and coaches on the QI Hospital Medicine team who contributed ideas on study design and interventions.
1. Dellit TH, Owens RC, McGowan JE, Jr, et al. Infectious diseases society of America and the society for healthcare epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159-177. https://doi.org/10.1086/510393.
2. Shah SS, Srivastava R, Wu S, et al. Intravenous Versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6). https://doi.org/10.1542/peds.2016-1692.
3. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822.
4. Jumani K, Advani S, Reich NG, Gosey L, Milstone AM. Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429-435.https://doi.org/10.1001/jamapediatrics.2013.775.
5. Zaoutis T, Localio AR, Leckerman K, et al. Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics. 2009;123(2):636-642. https://doi.org/10.1542/peds.2008-0596.
6. McMullan BJ, Andresen D, Blyth CC, et al. Antibiotic duration and timing of the switch from intravenous to oral route for bacterial infections in children: systematic review and guidelines. Lancet Infect Dis. 2016;16(8):e139-e152. https://doi.org/10.1016/S1473-3099(16)30024-X.
7. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-e76. https://doi.org/10.1093/cid/cir531.
8. Stevens DL, Bisno AL, Chambers HF, et al. Executive summary: practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America. Clin Infect Dis. 2014;59(2):147-159. https://doi.org/10.1093/cid/ciu444.
9. MacGregor RR, Graziani AL. Oral administration of antibiotics: a rational alternative to the parenteral route. Clin Infect Dis. 1997;24(3):457-467. https://doi.org/10.1093/clinids/24.3.457.
10. Downes KJ, Hahn A, Wiles J, Courter JD, Vinks AA. Dose optimisation of antibiotics in children: application of pharmacokinetics/pharmacodynamics in paediatrics. Int J Antimicrob Agents. 2014;43(3):223-230. https://doi.org/10.1016/j.ijantimicag.2013.11.006.
11. Autmizguine J, Melloni C, Hornik CP, et al. Population pharmacokinetics of trimethoprim-sulfamethoxazole in infants and children. Antimicrob Agents Chemother. 2018;62(1):e01813-e01817. https://doi.org/10.1128/AAC.01813-17.
12. Dewan M, Herrmann LE, Tchou MJ, et al. Development and evaluation of high-value pediatrics: a high-value care pediatric resident curriculum. Hosp Pediatr. 2018;8(12):785-792. https://doi.org/10.1542/hpeds.2018-0115
13. Langley GJ, Moen RD, Nolan KM, Nolan TW, Norman CL, Provost LP. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. New Jersey, US: John Wiley & Sons; 2009.
14. Benneyan JC. Use and interpretation of statistical quality control charts. Int J Qual Health Care. 1998;10(1):69-73. https://doi.org/10.1093/intqhc/10.1.69.
15. Lorgelly PK, Atkinson M, Lakhanpaul M, et al. Oral versus i.v. antibiotics for community-acquired pneumonia in children: a cost-minimisation analysis. Eur Respir J. 2010;35(4):858-864. https://doi.org/10.1183/09031936.00087209.
16. Vidyarthi AR, Green AL, Rosenbluth G, Baron RB. Engaging residents and fellows to improve institution-wide quality: the first six years of a novel financial incentive program. Acad Med. 2014;89(3):460-468. https://doi.org/10.1097/ACM.0000000000000159.
17. Stinnett-Donnelly JM, Stevens PG, Hood VL. Developing a high value care programme from the bottom up: a programme of faculty-resident improvement projects targeting harmful or unnecessary care. BMJ Qual Saf. 2016;25(11):901-908. https://doi.org/10.1136/bmjqs-2015-004546.
18. Peterson D, McLeod S, Woolfrey K, McRae A. Predictors of failure of empiric outpatient antibiotic therapy in emergency department patients with uncomplicated cellulitis. Acad Emerg Med. 2014;21(5):526-531. https://doi.org/10.1111/acem.12371.
19. Yadav K, Suh KN, Eagles D, et al. Predictors of oral antibiotic treatment failure for non-purulent skin and soft tissue infections in the emergency department. Acad Emerg Med. 2018;20(S1):S24-S25. https://doi.org/10.1017/cem.2018.114.
20. Organisation for Economic Co-operation and Development. Healthcare costs unsustainable in advanced economies without reform. http://www.oecd.org/health/healthcarecostsunsustainableinadvancedeconomieswithoutreform.htm. Accessed June 28, 2018; 2015.
21. Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012;307(14):1513-1516. https://doi.org/10.1001/jama.2012.362.
22. Schondelmeyer AC, Simmons JM, Statile AM, et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135(4):e1044-e1051. https://doi.org/10.1542/peds.2014-2295.
23. Tchou MJ, Tang Girdwood S, Wormser B, et al. Reducing electrolyte testing in hospitalized children by using quality improvement methods. Pediatrics. 2018;141(5). https://doi.org/10.1542/peds.2017-3187.
24. Christensen EW, Spaulding AB, Pomputius WF, Grapentine SP. Effects of hospital practice patterns for antibiotic administration for pneumonia on hospital lengths of stay and costs. J Pediatr Infect Dis Soc. 2019;8(2):115-121. https://doi.org/10.1093/jpids/piy003.
25. Berrevoets MAH, Pot JHLW, Houterman AE, et al. An electronic trigger tool to optimise intravenous to oral antibiotic switch: a controlled, interrupted time series study. Antimicrob Resist Infect Control. 2017;6:81. https://doi.org/10.1186/s13756-017-0239-3.
26. Fischer MA, Solomon DH, Teich JM, Avorn J. Conversion from intravenous to oral medications: assessment of a computerized intervention for hospitalized patients. Arch Intern Med. 2003;163(21):2585-2589. https://doi.org/10.1001/archinte.163.21.2585.
27. Schroeder AR, Harris SJ, Newman TB. Safely doing less: a missing component of the patient safety dialogue. Pediatrics. 2011;128(6):e1596-e1597. https://doi.org/10.1542/peds.2011-2726.
1. Dellit TH, Owens RC, McGowan JE, Jr, et al. Infectious diseases society of America and the society for healthcare epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159-177. https://doi.org/10.1086/510393.
2. Shah SS, Srivastava R, Wu S, et al. Intravenous Versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6). https://doi.org/10.1542/peds.2016-1692.
3. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822.
4. Jumani K, Advani S, Reich NG, Gosey L, Milstone AM. Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429-435.https://doi.org/10.1001/jamapediatrics.2013.775.
5. Zaoutis T, Localio AR, Leckerman K, et al. Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics. 2009;123(2):636-642. https://doi.org/10.1542/peds.2008-0596.
6. McMullan BJ, Andresen D, Blyth CC, et al. Antibiotic duration and timing of the switch from intravenous to oral route for bacterial infections in children: systematic review and guidelines. Lancet Infect Dis. 2016;16(8):e139-e152. https://doi.org/10.1016/S1473-3099(16)30024-X.
7. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-e76. https://doi.org/10.1093/cid/cir531.
8. Stevens DL, Bisno AL, Chambers HF, et al. Executive summary: practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America. Clin Infect Dis. 2014;59(2):147-159. https://doi.org/10.1093/cid/ciu444.
9. MacGregor RR, Graziani AL. Oral administration of antibiotics: a rational alternative to the parenteral route. Clin Infect Dis. 1997;24(3):457-467. https://doi.org/10.1093/clinids/24.3.457.
10. Downes KJ, Hahn A, Wiles J, Courter JD, Vinks AA. Dose optimisation of antibiotics in children: application of pharmacokinetics/pharmacodynamics in paediatrics. Int J Antimicrob Agents. 2014;43(3):223-230. https://doi.org/10.1016/j.ijantimicag.2013.11.006.
11. Autmizguine J, Melloni C, Hornik CP, et al. Population pharmacokinetics of trimethoprim-sulfamethoxazole in infants and children. Antimicrob Agents Chemother. 2018;62(1):e01813-e01817. https://doi.org/10.1128/AAC.01813-17.
12. Dewan M, Herrmann LE, Tchou MJ, et al. Development and evaluation of high-value pediatrics: a high-value care pediatric resident curriculum. Hosp Pediatr. 2018;8(12):785-792. https://doi.org/10.1542/hpeds.2018-0115
13. Langley GJ, Moen RD, Nolan KM, Nolan TW, Norman CL, Provost LP. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. New Jersey, US: John Wiley & Sons; 2009.
14. Benneyan JC. Use and interpretation of statistical quality control charts. Int J Qual Health Care. 1998;10(1):69-73. https://doi.org/10.1093/intqhc/10.1.69.
15. Lorgelly PK, Atkinson M, Lakhanpaul M, et al. Oral versus i.v. antibiotics for community-acquired pneumonia in children: a cost-minimisation analysis. Eur Respir J. 2010;35(4):858-864. https://doi.org/10.1183/09031936.00087209.
16. Vidyarthi AR, Green AL, Rosenbluth G, Baron RB. Engaging residents and fellows to improve institution-wide quality: the first six years of a novel financial incentive program. Acad Med. 2014;89(3):460-468. https://doi.org/10.1097/ACM.0000000000000159.
17. Stinnett-Donnelly JM, Stevens PG, Hood VL. Developing a high value care programme from the bottom up: a programme of faculty-resident improvement projects targeting harmful or unnecessary care. BMJ Qual Saf. 2016;25(11):901-908. https://doi.org/10.1136/bmjqs-2015-004546.
18. Peterson D, McLeod S, Woolfrey K, McRae A. Predictors of failure of empiric outpatient antibiotic therapy in emergency department patients with uncomplicated cellulitis. Acad Emerg Med. 2014;21(5):526-531. https://doi.org/10.1111/acem.12371.
19. Yadav K, Suh KN, Eagles D, et al. Predictors of oral antibiotic treatment failure for non-purulent skin and soft tissue infections in the emergency department. Acad Emerg Med. 2018;20(S1):S24-S25. https://doi.org/10.1017/cem.2018.114.
20. Organisation for Economic Co-operation and Development. Healthcare costs unsustainable in advanced economies without reform. http://www.oecd.org/health/healthcarecostsunsustainableinadvancedeconomieswithoutreform.htm. Accessed June 28, 2018; 2015.
21. Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012;307(14):1513-1516. https://doi.org/10.1001/jama.2012.362.
22. Schondelmeyer AC, Simmons JM, Statile AM, et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135(4):e1044-e1051. https://doi.org/10.1542/peds.2014-2295.
23. Tchou MJ, Tang Girdwood S, Wormser B, et al. Reducing electrolyte testing in hospitalized children by using quality improvement methods. Pediatrics. 2018;141(5). https://doi.org/10.1542/peds.2017-3187.
24. Christensen EW, Spaulding AB, Pomputius WF, Grapentine SP. Effects of hospital practice patterns for antibiotic administration for pneumonia on hospital lengths of stay and costs. J Pediatr Infect Dis Soc. 2019;8(2):115-121. https://doi.org/10.1093/jpids/piy003.
25. Berrevoets MAH, Pot JHLW, Houterman AE, et al. An electronic trigger tool to optimise intravenous to oral antibiotic switch: a controlled, interrupted time series study. Antimicrob Resist Infect Control. 2017;6:81. https://doi.org/10.1186/s13756-017-0239-3.
26. Fischer MA, Solomon DH, Teich JM, Avorn J. Conversion from intravenous to oral medications: assessment of a computerized intervention for hospitalized patients. Arch Intern Med. 2003;163(21):2585-2589. https://doi.org/10.1001/archinte.163.21.2585.
27. Schroeder AR, Harris SJ, Newman TB. Safely doing less: a missing component of the patient safety dialogue. Pediatrics. 2011;128(6):e1596-e1597. https://doi.org/10.1542/peds.2011-2726.
© 2019 Society of Hospital Medicine