User login
Helping a patient buck the odds
I’m not going to get rich off Mike.
Of course, I’m not going to get rich off anyone, nor do I want to. I’m not here to rip anyone off.
Mike goes back with me, roughly 23 years.
He was born with cerebral palsy and refractory seizures. His birth mother gave him up quickly, and he was adopted by a couple who knew what they were getting into (to me that constitutes sainthood).
Over the years Mike has done his best to buck the odds. He’s tried to stay employed, in spite of his physical limitations, working variously as a janitor, grocery courtesy clerk, and store greeter. He tells me that he can still work and wants to, even with having to rely on public transportation.
By the time he came to me he’d been through several neurologists and even more failed epilepsy drugs. His brain MRI and EEGs showed multifocal seizures from numerous inoperable cortical heterotopias.
I dabbled with a few newer drugs at the time for him, without success. Finally, I reached for the neurological equivalent of unstable dynamite – Felbatol (felbamate).
As it often does, it worked. One of my attendings in training (you, Bob) told me it was the home-run drug. When nothing else worked, it might – but you had to handle it carefully.
Fortunately, after 23 years, that hasn’t happened. Mike’s labs have looked good. His seizures have dropped from several a week to a few per year.
Ten years ago Mike had to change insurance to one I don’t take, and had me forward his records to another neurologist. That office told him they don’t handle Felbatol. As did another. And another.
Mike, understandably, doesn’t want to change meds. This is the only drug that’s given him a decent quality of life, and let him have a job. That’s pretty important to him.
So, I see him for free now, once or twice a year. Sometimes he offers me a token payment of $5-$10, but I turn it down. He needs it more than I do, for bus fair to my office if nothing else.
I’m sure some would be critical of me, saying that I should be more open to new drugs and treatments. I am, believe me. But Mike can’t afford many of them, or the loss of work they’d entail if his seizures worsen. He doesn’t want to take that chance, and I don’t blame him.
Of course, none of us can see everyone for free. In fact, he’s the only one I do. I’m not greedy, but I also have to pay my rent, staff, and mortgage.
But taking money from Mike, who’s come up on the short end of the stick in so many ways, doesn’t seem right. I can’t do it, and really don’t want to.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’m not going to get rich off Mike.
Of course, I’m not going to get rich off anyone, nor do I want to. I’m not here to rip anyone off.
Mike goes back with me, roughly 23 years.
He was born with cerebral palsy and refractory seizures. His birth mother gave him up quickly, and he was adopted by a couple who knew what they were getting into (to me that constitutes sainthood).
Over the years Mike has done his best to buck the odds. He’s tried to stay employed, in spite of his physical limitations, working variously as a janitor, grocery courtesy clerk, and store greeter. He tells me that he can still work and wants to, even with having to rely on public transportation.
By the time he came to me he’d been through several neurologists and even more failed epilepsy drugs. His brain MRI and EEGs showed multifocal seizures from numerous inoperable cortical heterotopias.
I dabbled with a few newer drugs at the time for him, without success. Finally, I reached for the neurological equivalent of unstable dynamite – Felbatol (felbamate).
As it often does, it worked. One of my attendings in training (you, Bob) told me it was the home-run drug. When nothing else worked, it might – but you had to handle it carefully.
Fortunately, after 23 years, that hasn’t happened. Mike’s labs have looked good. His seizures have dropped from several a week to a few per year.
Ten years ago Mike had to change insurance to one I don’t take, and had me forward his records to another neurologist. That office told him they don’t handle Felbatol. As did another. And another.
Mike, understandably, doesn’t want to change meds. This is the only drug that’s given him a decent quality of life, and let him have a job. That’s pretty important to him.
So, I see him for free now, once or twice a year. Sometimes he offers me a token payment of $5-$10, but I turn it down. He needs it more than I do, for bus fair to my office if nothing else.
I’m sure some would be critical of me, saying that I should be more open to new drugs and treatments. I am, believe me. But Mike can’t afford many of them, or the loss of work they’d entail if his seizures worsen. He doesn’t want to take that chance, and I don’t blame him.
Of course, none of us can see everyone for free. In fact, he’s the only one I do. I’m not greedy, but I also have to pay my rent, staff, and mortgage.
But taking money from Mike, who’s come up on the short end of the stick in so many ways, doesn’t seem right. I can’t do it, and really don’t want to.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’m not going to get rich off Mike.
Of course, I’m not going to get rich off anyone, nor do I want to. I’m not here to rip anyone off.
Mike goes back with me, roughly 23 years.
He was born with cerebral palsy and refractory seizures. His birth mother gave him up quickly, and he was adopted by a couple who knew what they were getting into (to me that constitutes sainthood).
Over the years Mike has done his best to buck the odds. He’s tried to stay employed, in spite of his physical limitations, working variously as a janitor, grocery courtesy clerk, and store greeter. He tells me that he can still work and wants to, even with having to rely on public transportation.
By the time he came to me he’d been through several neurologists and even more failed epilepsy drugs. His brain MRI and EEGs showed multifocal seizures from numerous inoperable cortical heterotopias.
I dabbled with a few newer drugs at the time for him, without success. Finally, I reached for the neurological equivalent of unstable dynamite – Felbatol (felbamate).
As it often does, it worked. One of my attendings in training (you, Bob) told me it was the home-run drug. When nothing else worked, it might – but you had to handle it carefully.
Fortunately, after 23 years, that hasn’t happened. Mike’s labs have looked good. His seizures have dropped from several a week to a few per year.
Ten years ago Mike had to change insurance to one I don’t take, and had me forward his records to another neurologist. That office told him they don’t handle Felbatol. As did another. And another.
Mike, understandably, doesn’t want to change meds. This is the only drug that’s given him a decent quality of life, and let him have a job. That’s pretty important to him.
So, I see him for free now, once or twice a year. Sometimes he offers me a token payment of $5-$10, but I turn it down. He needs it more than I do, for bus fair to my office if nothing else.
I’m sure some would be critical of me, saying that I should be more open to new drugs and treatments. I am, believe me. But Mike can’t afford many of them, or the loss of work they’d entail if his seizures worsen. He doesn’t want to take that chance, and I don’t blame him.
Of course, none of us can see everyone for free. In fact, he’s the only one I do. I’m not greedy, but I also have to pay my rent, staff, and mortgage.
But taking money from Mike, who’s come up on the short end of the stick in so many ways, doesn’t seem right. I can’t do it, and really don’t want to.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Time to rebuild
A few months ago, after several months of considerable foot dragging, I wrote that I have accepted the American Academy of Pediatrics’ proclamation that we should begin to treat obesity as a disease.
While it may feel like we are just throwing in the towel, it sounds better if we admit that we may have reached the threshold beyond which total focus on prevention is not going to work.
I continue to be troubled by the lingering fear that, in declaring that obesity is a disease, we will suspend our current efforts at preventing the condition. Granted, most of these efforts at prevention have been woefully ineffective. However, I still believe that, much like ADHD, the rise in obesity in this country is a reflection of some serious flaws in our society. On the other hand, as an inveterate optimist I have not given up on the belief that we will find some yet-to-be-discovered changes in our societal fabric that will eventually turn the ship around.
With this somewhat contradictory combination of resignation and optimism in mind, I continue to seek out studies that hold some promise for prevention while we begin tinkering with the let’s-treat-it-like-a-disease approach.
I recently discovered a story about one such study from the Center for Economic and Social Research at the University of Southern California. Using data collected about adolescent dependents of military personnel, the researchers found that “exposure to a more advantageous built environment for more than 2 years was associated with lower probabilities of obesity.” Because more than half of these teenagers were living in housing that had been assigned by the military, the researchers could more easily control for a variety of factors some related to self-selection.
Interestingly, the data did not support associations between the adolescents’ diet, physical activity, or socioeconomic environments. The investigators noted that “more advantageous built environments were associated with lower consumption of unhealthy foods.” However, the study lacked the granularity to determine what segments of the built environment were most associated with the effect they were observing.
Like me, you may not be familiar with the term “built environment.” Turns out it is just exactly what we might expect – anything about the environment that is the result of human action – buildings, roadways, dams, neighborhoods – and what they do and don’t contain. For example, is the adolescent living in an environment that encourages walking or one that is overly motor vehicle–centric? Does his or her neighborhood have easily reachable grocery stores that offer a range of healthy foods or does the teenager live in a nutritional desert populated only by convenience stores? Is there ample space for outdoor physical activity?
The authors’ observation that the adolescents who benefited from living in advantageous environments had a lower consumption of unhealthy foods might suggest that access to a healthy diet might be a significant factor. For me, the take-home message is that in our search for preventive strategies we have barely scratched the surface. The observation that the associations these researchers were making was over a relatively short time span of 2 years should give us hope that if we think more broadly and creatively we may be to find solutions on a grand scale.
Over the last century we have built an environment that is clearly obesogenic. This paper offers a starting point from which we can learn which components of that environment are the most potent contributors to the obesity epidemic. Once we have that information the question remains: Can we find the political will to tear down and rebuilt?
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
A few months ago, after several months of considerable foot dragging, I wrote that I have accepted the American Academy of Pediatrics’ proclamation that we should begin to treat obesity as a disease.
While it may feel like we are just throwing in the towel, it sounds better if we admit that we may have reached the threshold beyond which total focus on prevention is not going to work.
I continue to be troubled by the lingering fear that, in declaring that obesity is a disease, we will suspend our current efforts at preventing the condition. Granted, most of these efforts at prevention have been woefully ineffective. However, I still believe that, much like ADHD, the rise in obesity in this country is a reflection of some serious flaws in our society. On the other hand, as an inveterate optimist I have not given up on the belief that we will find some yet-to-be-discovered changes in our societal fabric that will eventually turn the ship around.
With this somewhat contradictory combination of resignation and optimism in mind, I continue to seek out studies that hold some promise for prevention while we begin tinkering with the let’s-treat-it-like-a-disease approach.
I recently discovered a story about one such study from the Center for Economic and Social Research at the University of Southern California. Using data collected about adolescent dependents of military personnel, the researchers found that “exposure to a more advantageous built environment for more than 2 years was associated with lower probabilities of obesity.” Because more than half of these teenagers were living in housing that had been assigned by the military, the researchers could more easily control for a variety of factors some related to self-selection.
Interestingly, the data did not support associations between the adolescents’ diet, physical activity, or socioeconomic environments. The investigators noted that “more advantageous built environments were associated with lower consumption of unhealthy foods.” However, the study lacked the granularity to determine what segments of the built environment were most associated with the effect they were observing.
Like me, you may not be familiar with the term “built environment.” Turns out it is just exactly what we might expect – anything about the environment that is the result of human action – buildings, roadways, dams, neighborhoods – and what they do and don’t contain. For example, is the adolescent living in an environment that encourages walking or one that is overly motor vehicle–centric? Does his or her neighborhood have easily reachable grocery stores that offer a range of healthy foods or does the teenager live in a nutritional desert populated only by convenience stores? Is there ample space for outdoor physical activity?
The authors’ observation that the adolescents who benefited from living in advantageous environments had a lower consumption of unhealthy foods might suggest that access to a healthy diet might be a significant factor. For me, the take-home message is that in our search for preventive strategies we have barely scratched the surface. The observation that the associations these researchers were making was over a relatively short time span of 2 years should give us hope that if we think more broadly and creatively we may be to find solutions on a grand scale.
Over the last century we have built an environment that is clearly obesogenic. This paper offers a starting point from which we can learn which components of that environment are the most potent contributors to the obesity epidemic. Once we have that information the question remains: Can we find the political will to tear down and rebuilt?
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
A few months ago, after several months of considerable foot dragging, I wrote that I have accepted the American Academy of Pediatrics’ proclamation that we should begin to treat obesity as a disease.
While it may feel like we are just throwing in the towel, it sounds better if we admit that we may have reached the threshold beyond which total focus on prevention is not going to work.
I continue to be troubled by the lingering fear that, in declaring that obesity is a disease, we will suspend our current efforts at preventing the condition. Granted, most of these efforts at prevention have been woefully ineffective. However, I still believe that, much like ADHD, the rise in obesity in this country is a reflection of some serious flaws in our society. On the other hand, as an inveterate optimist I have not given up on the belief that we will find some yet-to-be-discovered changes in our societal fabric that will eventually turn the ship around.
With this somewhat contradictory combination of resignation and optimism in mind, I continue to seek out studies that hold some promise for prevention while we begin tinkering with the let’s-treat-it-like-a-disease approach.
I recently discovered a story about one such study from the Center for Economic and Social Research at the University of Southern California. Using data collected about adolescent dependents of military personnel, the researchers found that “exposure to a more advantageous built environment for more than 2 years was associated with lower probabilities of obesity.” Because more than half of these teenagers were living in housing that had been assigned by the military, the researchers could more easily control for a variety of factors some related to self-selection.
Interestingly, the data did not support associations between the adolescents’ diet, physical activity, or socioeconomic environments. The investigators noted that “more advantageous built environments were associated with lower consumption of unhealthy foods.” However, the study lacked the granularity to determine what segments of the built environment were most associated with the effect they were observing.
Like me, you may not be familiar with the term “built environment.” Turns out it is just exactly what we might expect – anything about the environment that is the result of human action – buildings, roadways, dams, neighborhoods – and what they do and don’t contain. For example, is the adolescent living in an environment that encourages walking or one that is overly motor vehicle–centric? Does his or her neighborhood have easily reachable grocery stores that offer a range of healthy foods or does the teenager live in a nutritional desert populated only by convenience stores? Is there ample space for outdoor physical activity?
The authors’ observation that the adolescents who benefited from living in advantageous environments had a lower consumption of unhealthy foods might suggest that access to a healthy diet might be a significant factor. For me, the take-home message is that in our search for preventive strategies we have barely scratched the surface. The observation that the associations these researchers were making was over a relatively short time span of 2 years should give us hope that if we think more broadly and creatively we may be to find solutions on a grand scale.
Over the last century we have built an environment that is clearly obesogenic. This paper offers a starting point from which we can learn which components of that environment are the most potent contributors to the obesity epidemic. Once we have that information the question remains: Can we find the political will to tear down and rebuilt?
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Family violence after COVID: Understanding coercive relationships
Despite the ability of some couples to pull together and manage through the COVID-19 pandemic, other couples and families failed to thrive. Increasing divorce rates have been noted nationwide with many disagreements being specifically about COVID.1
A review of over 1 million tweets, between April 12 and July 16, 2020, found an increase in calls to hotlines and increased reports of a variety of types of family violence. There were also more inquiries about social services for family violence, an increased presence from social movements, and more domestic violence-related news.2
The literature addressing family violence uses a variety of terms, so here are some definitions.
Domestic violence is defined as a pattern of behaviors used to gain or maintain power and control. Broadly speaking, domestic violence includes elder abuse, sibling abuse, child abuse, intimate partner abuse, parent abuse, and can also include people who don’t necessarily live together but who have an intimate relationship. Domestic violence centers use the Power and Control Wheel (see graphic) developed by the Domestic Abuse Intervention Project in Duluth, Minn., to describe how domestic violence occurs.
Intimate partner violence is more specific, referring to violence that happens between people in an ongoing or former intimate or romantic relationship, and is a subcategory of domestic violence.
Coercive control is the use of power for control and compliance. It is a dynamic and systematic process described in the top left corner of the Power and Control Wheel. Overt control occurs with the implication that “if you don’t follow the rules, I’ll kill you.” More subtle control is when obedience is forced through monopolizing resources, dictating preferred choices, microregulating a partner’s behavior, and deprivation of supports needed to exercise independent judgment.
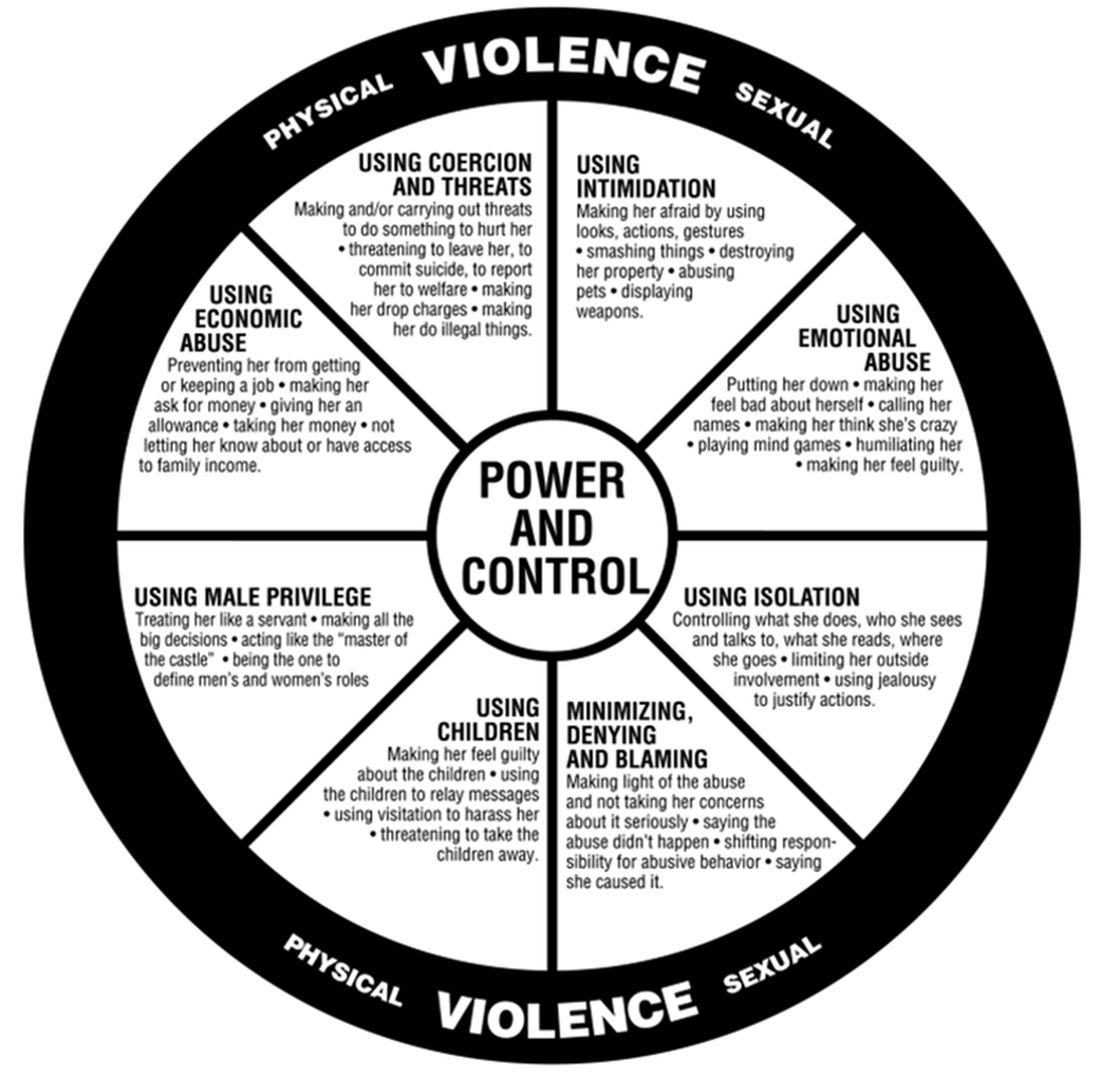
All interpersonal relationships have elements of persuasion and influence; however, the goal of coercive relationships is to maintain power and control. It is a dynamic of the relationship. Coercive control emphasizes the systematic, organized, multifaceted, and patterned nature of this interpersonal dynamic and can be considered to originate in the patriarchal dynamic where men control women.
Most professionals who work in this interdisciplinary area now refer to domestic violence as coercive control. Victimizers target women whom they sense they can control to get their own needs met. They are disinclined to invest in relationships with women who stress their own points of view, who do not readily accept blame when there is a disagreement, and who offer nurturing only when it is reciprocated.
In my office, if I think there are elements of coercion in a relationship, I bring out the Power and Control Wheel and the patient and I go over it. Good education is our responsibility. However, we all have met women who decide to stay in unhealthy relationships.
Assessing people who stay in coercive relationships
Fear
The most important first step is to assess safety. Are they afraid of increased violence if they challenge their partner? Restraining orders or other legal deterrents may not offer solace, as many women are clear that their spouse will come after them, if not tomorrow, then next week, or even next month. They are sure that they will not be safe.
In these cases, I go over safety steps with them so that if they decide to go, they will be prepared. I bring out the “safety box,” which includes the following action steps:
- Memorize important phone numbers of people to call in an emergency.
- If your children are old enough, teach them important phone numbers, including when to dial 911.
- If you can, open your own bank account.
- Stay in touch with friends. Get to know your neighbors. Don’t cut yourself off from people, even if you feel like you want to be alone.
- Rehearse your escape plan until you know it by heart.
- Leave a set of car keys, extra money, a change of clothes and copies of important documents with a trusted friend or relative: your own and your children’s birth certificates, children’s school and medical records, bank books, welfare identification, passport/green card, immigration papers, social security card, lease agreements or mortgage payment books, insurance papers, important addresses, and telephone numbers.
- Keep information about domestic violence in a safe place, where your abuser won’t find it, but where you can get it when you need to review it.
Some women may acknowledge that the risk of physical violence is not the determining factor in their decision to stay and have difficulty explaining why they choose to stay. I suggest that we then consider the following frames that have their origin in the study of the impact of trauma.
Shame
From this lens, abusive events are humiliating experiences, now represented as shame experiences. Humiliation and shame hide hostile feelings that the patient is not able to acknowledge.
“In shame, the self is the failure and others may reject or be critical of this exposed, flawed self.”3 Women will therefore remain attached to an abuser to avoid the exposure of their defective self.
Action steps: Empathic engagement and acknowledgment of shame and humiliation are key. For someone to overcome shame, they must face their sense of their defective self and have strategies to manage these feelings. The development of such strategies is the next step.
Trauma repetition and trauma bonding
Women subjected to domestic violence often respond with incapacitating traumatic syndromes. The concept of “trauma repetition” is suggested as a cause of vulnerability to repeated abuse, and “trauma bonding” is the term for the intense and tenacious bond that can form between abusers and victims.4
Trauma bonding implies that a sense of safety and closeness and secure attachment can only be reached through highly abusive engagement; anything else is experienced as “superficial, cold, or irrelevant.”5 Trauma bonding may have its origins in emotional neglect, according to self reports of 116 women.6Action steps: The literature on trauma is growing and many patients will benefit from good curated sources. Having a good list of books and website on hand is important. Discussion and exploration of the impact of trauma will be needed, and can be provided by someone who is available on a consistent and frequent basis. This work may be time consuming and difficult.
Some asides
1. Some psychiatrists proffer the explanation that these women who stay must be masochistic. The misogynistic concept of masochism still haunts the halls of psychiatry. It is usually offered as a way to dismiss these women’s concerns.
2. One of the obstacles to recognizing chronic mistreatment in relationships is that most abusive men simply “do not seem like abusers.” They have many good qualities, including times of kindness, warmth, and humor, especially in the initial period of a relationship. An abuser’s friends may think the world of him. He may have a successful work life and have no problems with drugs or alcohol. He may simply not fit anyone’s image of a cruel or intimidating person. So, when a woman feels her relationship spinning out of control, it may not occur to her that her partner is an abuser. Even if she does consider her partner to be overly controlling, others may question her perception.
3. Neutrality in family courts is systemic sexism/misogyny. When it comes to domestic violence, family courts tend to split the difference. Stephanie Brandt, MD, notes that The assumption that it is violence alone that matters has formed the basis of much clinical and legal confusion.7 As an analyst, she has gone against the grain of a favored neutrality and become active in the courts, noting the secondary victimization that occurs when a woman enters the legal system.
In summary, psychiatrists must reclaim our expertise in systemic dynamics and point out the role of systemic misogyny. Justices and other court officials need to be educated. Ideally, justice should be based on the equality of men and women in a society free of systemic misogyny. Unfortunately our society has not yet reached this position. In the meanwhile, we must think systemically about interpersonal dynamics. This is our lane. This should not be controversial.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at [email protected]. Dr. Heru would like to thank Dr. Stephanie Brandt for discussing this topic with her and supporting this work.
References
1. Ellyatt H. Arguing with your partner over Covid? You’re not alone, with the pandemic straining many relationships. 2022 Jan 21. https://www.cnbc.com/2022/01/21/covid-has-put-pressures-and-strains-on-relationships.html
2. Xue J et al. J Med Internet Res. 2020 Nov 6;22(11):e24361. doi: 10.2196/24361.
3. Dorahy MJ. J Trauma Dissociation. 2017 May-Jun;18(3):383-96. doi: 10.1080/15299732.2017.1295422.
4. Dutton DG and Painter SL. Victimology. 1981 Jan;6(1):139-55.
5. Sachs A. J Trauma Dissociation. 2017 May-Jun;18(3):319-39. doi: 10.1080/15299732.2017.1295400.
6. Krüger C and Fletcher L. J Trauma Dissociation. 2017 May-Jun;18(3):356-72. doi: 10.1080/15299732.2017.1295420.
7. Brandt S and Rudden M. Int J Appl Psychoanal Studies. 2020 Sept;17(3):215-31. doi: 10.1002/aps.1671.
Despite the ability of some couples to pull together and manage through the COVID-19 pandemic, other couples and families failed to thrive. Increasing divorce rates have been noted nationwide with many disagreements being specifically about COVID.1
A review of over 1 million tweets, between April 12 and July 16, 2020, found an increase in calls to hotlines and increased reports of a variety of types of family violence. There were also more inquiries about social services for family violence, an increased presence from social movements, and more domestic violence-related news.2
The literature addressing family violence uses a variety of terms, so here are some definitions.
Domestic violence is defined as a pattern of behaviors used to gain or maintain power and control. Broadly speaking, domestic violence includes elder abuse, sibling abuse, child abuse, intimate partner abuse, parent abuse, and can also include people who don’t necessarily live together but who have an intimate relationship. Domestic violence centers use the Power and Control Wheel (see graphic) developed by the Domestic Abuse Intervention Project in Duluth, Minn., to describe how domestic violence occurs.
Intimate partner violence is more specific, referring to violence that happens between people in an ongoing or former intimate or romantic relationship, and is a subcategory of domestic violence.
Coercive control is the use of power for control and compliance. It is a dynamic and systematic process described in the top left corner of the Power and Control Wheel. Overt control occurs with the implication that “if you don’t follow the rules, I’ll kill you.” More subtle control is when obedience is forced through monopolizing resources, dictating preferred choices, microregulating a partner’s behavior, and deprivation of supports needed to exercise independent judgment.

All interpersonal relationships have elements of persuasion and influence; however, the goal of coercive relationships is to maintain power and control. It is a dynamic of the relationship. Coercive control emphasizes the systematic, organized, multifaceted, and patterned nature of this interpersonal dynamic and can be considered to originate in the patriarchal dynamic where men control women.
Most professionals who work in this interdisciplinary area now refer to domestic violence as coercive control. Victimizers target women whom they sense they can control to get their own needs met. They are disinclined to invest in relationships with women who stress their own points of view, who do not readily accept blame when there is a disagreement, and who offer nurturing only when it is reciprocated.
In my office, if I think there are elements of coercion in a relationship, I bring out the Power and Control Wheel and the patient and I go over it. Good education is our responsibility. However, we all have met women who decide to stay in unhealthy relationships.
Assessing people who stay in coercive relationships
Fear
The most important first step is to assess safety. Are they afraid of increased violence if they challenge their partner? Restraining orders or other legal deterrents may not offer solace, as many women are clear that their spouse will come after them, if not tomorrow, then next week, or even next month. They are sure that they will not be safe.
In these cases, I go over safety steps with them so that if they decide to go, they will be prepared. I bring out the “safety box,” which includes the following action steps:
- Memorize important phone numbers of people to call in an emergency.
- If your children are old enough, teach them important phone numbers, including when to dial 911.
- If you can, open your own bank account.
- Stay in touch with friends. Get to know your neighbors. Don’t cut yourself off from people, even if you feel like you want to be alone.
- Rehearse your escape plan until you know it by heart.
- Leave a set of car keys, extra money, a change of clothes and copies of important documents with a trusted friend or relative: your own and your children’s birth certificates, children’s school and medical records, bank books, welfare identification, passport/green card, immigration papers, social security card, lease agreements or mortgage payment books, insurance papers, important addresses, and telephone numbers.
- Keep information about domestic violence in a safe place, where your abuser won’t find it, but where you can get it when you need to review it.
Some women may acknowledge that the risk of physical violence is not the determining factor in their decision to stay and have difficulty explaining why they choose to stay. I suggest that we then consider the following frames that have their origin in the study of the impact of trauma.
Shame
From this lens, abusive events are humiliating experiences, now represented as shame experiences. Humiliation and shame hide hostile feelings that the patient is not able to acknowledge.
“In shame, the self is the failure and others may reject or be critical of this exposed, flawed self.”3 Women will therefore remain attached to an abuser to avoid the exposure of their defective self.
Action steps: Empathic engagement and acknowledgment of shame and humiliation are key. For someone to overcome shame, they must face their sense of their defective self and have strategies to manage these feelings. The development of such strategies is the next step.
Trauma repetition and trauma bonding
Women subjected to domestic violence often respond with incapacitating traumatic syndromes. The concept of “trauma repetition” is suggested as a cause of vulnerability to repeated abuse, and “trauma bonding” is the term for the intense and tenacious bond that can form between abusers and victims.4
Trauma bonding implies that a sense of safety and closeness and secure attachment can only be reached through highly abusive engagement; anything else is experienced as “superficial, cold, or irrelevant.”5 Trauma bonding may have its origins in emotional neglect, according to self reports of 116 women.6Action steps: The literature on trauma is growing and many patients will benefit from good curated sources. Having a good list of books and website on hand is important. Discussion and exploration of the impact of trauma will be needed, and can be provided by someone who is available on a consistent and frequent basis. This work may be time consuming and difficult.
Some asides
1. Some psychiatrists proffer the explanation that these women who stay must be masochistic. The misogynistic concept of masochism still haunts the halls of psychiatry. It is usually offered as a way to dismiss these women’s concerns.
2. One of the obstacles to recognizing chronic mistreatment in relationships is that most abusive men simply “do not seem like abusers.” They have many good qualities, including times of kindness, warmth, and humor, especially in the initial period of a relationship. An abuser’s friends may think the world of him. He may have a successful work life and have no problems with drugs or alcohol. He may simply not fit anyone’s image of a cruel or intimidating person. So, when a woman feels her relationship spinning out of control, it may not occur to her that her partner is an abuser. Even if she does consider her partner to be overly controlling, others may question her perception.
3. Neutrality in family courts is systemic sexism/misogyny. When it comes to domestic violence, family courts tend to split the difference. Stephanie Brandt, MD, notes that The assumption that it is violence alone that matters has formed the basis of much clinical and legal confusion.7 As an analyst, she has gone against the grain of a favored neutrality and become active in the courts, noting the secondary victimization that occurs when a woman enters the legal system.
In summary, psychiatrists must reclaim our expertise in systemic dynamics and point out the role of systemic misogyny. Justices and other court officials need to be educated. Ideally, justice should be based on the equality of men and women in a society free of systemic misogyny. Unfortunately our society has not yet reached this position. In the meanwhile, we must think systemically about interpersonal dynamics. This is our lane. This should not be controversial.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at [email protected]. Dr. Heru would like to thank Dr. Stephanie Brandt for discussing this topic with her and supporting this work.
References
1. Ellyatt H. Arguing with your partner over Covid? You’re not alone, with the pandemic straining many relationships. 2022 Jan 21. https://www.cnbc.com/2022/01/21/covid-has-put-pressures-and-strains-on-relationships.html
2. Xue J et al. J Med Internet Res. 2020 Nov 6;22(11):e24361. doi: 10.2196/24361.
3. Dorahy MJ. J Trauma Dissociation. 2017 May-Jun;18(3):383-96. doi: 10.1080/15299732.2017.1295422.
4. Dutton DG and Painter SL. Victimology. 1981 Jan;6(1):139-55.
5. Sachs A. J Trauma Dissociation. 2017 May-Jun;18(3):319-39. doi: 10.1080/15299732.2017.1295400.
6. Krüger C and Fletcher L. J Trauma Dissociation. 2017 May-Jun;18(3):356-72. doi: 10.1080/15299732.2017.1295420.
7. Brandt S and Rudden M. Int J Appl Psychoanal Studies. 2020 Sept;17(3):215-31. doi: 10.1002/aps.1671.
Despite the ability of some couples to pull together and manage through the COVID-19 pandemic, other couples and families failed to thrive. Increasing divorce rates have been noted nationwide with many disagreements being specifically about COVID.1
A review of over 1 million tweets, between April 12 and July 16, 2020, found an increase in calls to hotlines and increased reports of a variety of types of family violence. There were also more inquiries about social services for family violence, an increased presence from social movements, and more domestic violence-related news.2
The literature addressing family violence uses a variety of terms, so here are some definitions.
Domestic violence is defined as a pattern of behaviors used to gain or maintain power and control. Broadly speaking, domestic violence includes elder abuse, sibling abuse, child abuse, intimate partner abuse, parent abuse, and can also include people who don’t necessarily live together but who have an intimate relationship. Domestic violence centers use the Power and Control Wheel (see graphic) developed by the Domestic Abuse Intervention Project in Duluth, Minn., to describe how domestic violence occurs.
Intimate partner violence is more specific, referring to violence that happens between people in an ongoing or former intimate or romantic relationship, and is a subcategory of domestic violence.
Coercive control is the use of power for control and compliance. It is a dynamic and systematic process described in the top left corner of the Power and Control Wheel. Overt control occurs with the implication that “if you don’t follow the rules, I’ll kill you.” More subtle control is when obedience is forced through monopolizing resources, dictating preferred choices, microregulating a partner’s behavior, and deprivation of supports needed to exercise independent judgment.

All interpersonal relationships have elements of persuasion and influence; however, the goal of coercive relationships is to maintain power and control. It is a dynamic of the relationship. Coercive control emphasizes the systematic, organized, multifaceted, and patterned nature of this interpersonal dynamic and can be considered to originate in the patriarchal dynamic where men control women.
Most professionals who work in this interdisciplinary area now refer to domestic violence as coercive control. Victimizers target women whom they sense they can control to get their own needs met. They are disinclined to invest in relationships with women who stress their own points of view, who do not readily accept blame when there is a disagreement, and who offer nurturing only when it is reciprocated.
In my office, if I think there are elements of coercion in a relationship, I bring out the Power and Control Wheel and the patient and I go over it. Good education is our responsibility. However, we all have met women who decide to stay in unhealthy relationships.
Assessing people who stay in coercive relationships
Fear
The most important first step is to assess safety. Are they afraid of increased violence if they challenge their partner? Restraining orders or other legal deterrents may not offer solace, as many women are clear that their spouse will come after them, if not tomorrow, then next week, or even next month. They are sure that they will not be safe.
In these cases, I go over safety steps with them so that if they decide to go, they will be prepared. I bring out the “safety box,” which includes the following action steps:
- Memorize important phone numbers of people to call in an emergency.
- If your children are old enough, teach them important phone numbers, including when to dial 911.
- If you can, open your own bank account.
- Stay in touch with friends. Get to know your neighbors. Don’t cut yourself off from people, even if you feel like you want to be alone.
- Rehearse your escape plan until you know it by heart.
- Leave a set of car keys, extra money, a change of clothes and copies of important documents with a trusted friend or relative: your own and your children’s birth certificates, children’s school and medical records, bank books, welfare identification, passport/green card, immigration papers, social security card, lease agreements or mortgage payment books, insurance papers, important addresses, and telephone numbers.
- Keep information about domestic violence in a safe place, where your abuser won’t find it, but where you can get it when you need to review it.
Some women may acknowledge that the risk of physical violence is not the determining factor in their decision to stay and have difficulty explaining why they choose to stay. I suggest that we then consider the following frames that have their origin in the study of the impact of trauma.
Shame
From this lens, abusive events are humiliating experiences, now represented as shame experiences. Humiliation and shame hide hostile feelings that the patient is not able to acknowledge.
“In shame, the self is the failure and others may reject or be critical of this exposed, flawed self.”3 Women will therefore remain attached to an abuser to avoid the exposure of their defective self.
Action steps: Empathic engagement and acknowledgment of shame and humiliation are key. For someone to overcome shame, they must face their sense of their defective self and have strategies to manage these feelings. The development of such strategies is the next step.
Trauma repetition and trauma bonding
Women subjected to domestic violence often respond with incapacitating traumatic syndromes. The concept of “trauma repetition” is suggested as a cause of vulnerability to repeated abuse, and “trauma bonding” is the term for the intense and tenacious bond that can form between abusers and victims.4
Trauma bonding implies that a sense of safety and closeness and secure attachment can only be reached through highly abusive engagement; anything else is experienced as “superficial, cold, or irrelevant.”5 Trauma bonding may have its origins in emotional neglect, according to self reports of 116 women.6Action steps: The literature on trauma is growing and many patients will benefit from good curated sources. Having a good list of books and website on hand is important. Discussion and exploration of the impact of trauma will be needed, and can be provided by someone who is available on a consistent and frequent basis. This work may be time consuming and difficult.
Some asides
1. Some psychiatrists proffer the explanation that these women who stay must be masochistic. The misogynistic concept of masochism still haunts the halls of psychiatry. It is usually offered as a way to dismiss these women’s concerns.
2. One of the obstacles to recognizing chronic mistreatment in relationships is that most abusive men simply “do not seem like abusers.” They have many good qualities, including times of kindness, warmth, and humor, especially in the initial period of a relationship. An abuser’s friends may think the world of him. He may have a successful work life and have no problems with drugs or alcohol. He may simply not fit anyone’s image of a cruel or intimidating person. So, when a woman feels her relationship spinning out of control, it may not occur to her that her partner is an abuser. Even if she does consider her partner to be overly controlling, others may question her perception.
3. Neutrality in family courts is systemic sexism/misogyny. When it comes to domestic violence, family courts tend to split the difference. Stephanie Brandt, MD, notes that The assumption that it is violence alone that matters has formed the basis of much clinical and legal confusion.7 As an analyst, she has gone against the grain of a favored neutrality and become active in the courts, noting the secondary victimization that occurs when a woman enters the legal system.
In summary, psychiatrists must reclaim our expertise in systemic dynamics and point out the role of systemic misogyny. Justices and other court officials need to be educated. Ideally, justice should be based on the equality of men and women in a society free of systemic misogyny. Unfortunately our society has not yet reached this position. In the meanwhile, we must think systemically about interpersonal dynamics. This is our lane. This should not be controversial.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at [email protected]. Dr. Heru would like to thank Dr. Stephanie Brandt for discussing this topic with her and supporting this work.
References
1. Ellyatt H. Arguing with your partner over Covid? You’re not alone, with the pandemic straining many relationships. 2022 Jan 21. https://www.cnbc.com/2022/01/21/covid-has-put-pressures-and-strains-on-relationships.html
2. Xue J et al. J Med Internet Res. 2020 Nov 6;22(11):e24361. doi: 10.2196/24361.
3. Dorahy MJ. J Trauma Dissociation. 2017 May-Jun;18(3):383-96. doi: 10.1080/15299732.2017.1295422.
4. Dutton DG and Painter SL. Victimology. 1981 Jan;6(1):139-55.
5. Sachs A. J Trauma Dissociation. 2017 May-Jun;18(3):319-39. doi: 10.1080/15299732.2017.1295400.
6. Krüger C and Fletcher L. J Trauma Dissociation. 2017 May-Jun;18(3):356-72. doi: 10.1080/15299732.2017.1295420.
7. Brandt S and Rudden M. Int J Appl Psychoanal Studies. 2020 Sept;17(3):215-31. doi: 10.1002/aps.1671.
Picking up the premotor symptoms of Parkinson’s
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. We had a great discussion on Parkinson’s Disease for Primary Care with Dr. Albert Hung. Paul, this was something that really made me nervous. I didn’t have a lot of comfort with it. But he taught us a lot of tips about how to recognize Parkinson’s.
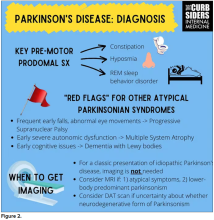
I hadn’t been as aware of the premotor symptoms: constipation, hyposmia (loss of sense of smell), and rapid eye movement sleep behavior disorder. If patients have those early on and they aren’t explained by other things (especially the REM sleep behavior disorder), you should really key in because those patients are at risk of developing Parkinson’s years down the line. Those symptoms could present first, which just kind of blew my mind.
What tips do you have about how to recognize Parkinson’s? Do you want to talk about the physical exam?
Paul N. Williams, MD: You know I love the physical exam stuff, so I’m happy to talk about that.
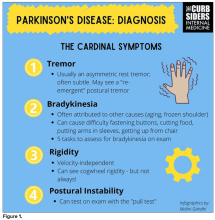
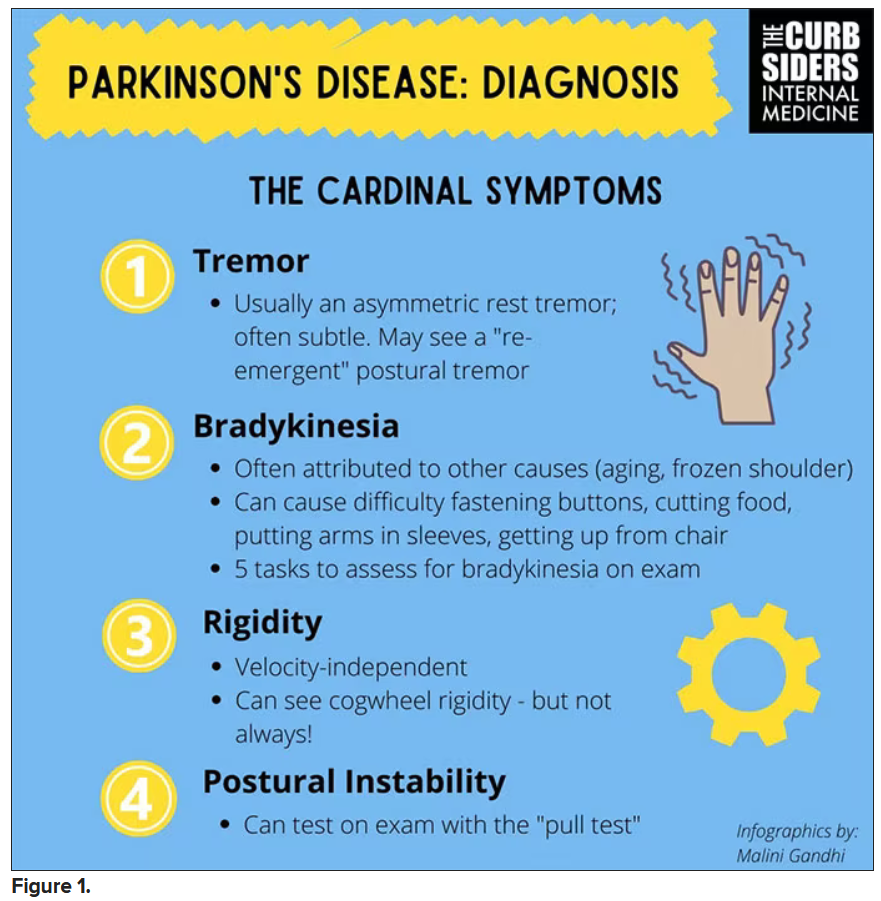
You were deeply upset that cogwheel rigidity was not pathognomonic for Parkinson’s, but you made the point – and our guest agreed – that asymmetry tends to be the key here. And I really appreciated the point about reemergent tremor. This is this idea of a resting tremor. If someone has more parkinsonian features, you might see an intention tremor with essential tremor. If they reach out, it might seem steady at first, but if they hold long enough, then the tremor may kind of reemerge. I thought that was a neat distinction.
And this idea of cogwheel rigidity is a combination of some of the cardinal features of Parkinson’s – it’s a little bit of tremor and a little bit of rigidity too. There’s a baseline increase in tone, and then the tremor is superimposed on top of that. When you’re feeling cogwheeling, that’s actually what you’re feeling on examination. Parkinson’s, with all of its physical exam findings has always fascinated me.
Dr. Watto: He also told us about some red flags.
With classic idiopathic parkinsonism, there’s asymmetric involvement of the tremor. So red flags include a symmetric tremor, which might be something other than idiopathic parkinsonism. He also mentioned that one of the reasons you may want to get imaging (which is not always necessary if someone has a classic presentation), is if you see lower body–predominant symptoms of parkinsonism. These patients have rigidity or slowness of movement in their legs, but their upper bodies are not affected. They don’t have masked facies or the tremor in their hands. You might get an MRI in that case because that could be presentation of vascular dementia or vascular disease in the brain or even normal pressure hydrocephalus, which is a treatable condition. That would be one reason to get imaging.
What if the patient was exposed to a drug like a dopamine antagonist? They will get better in a couple of days, right?
Dr. Williams: This was a really fascinating point because we typically think if a patient’s symptoms are related to a drug exposure – in this case, drug-induced parkinsonism – we can just stop the medication and the symptoms will disappear in a couple of days as the drug leaves the system. But as it turns out, it might take much longer. A mistake that Dr Hung often sees is that the clinician stops the possibly offending agent, but when they don’t see an immediate relief of symptoms, they assume the drug wasn’t causing them. You really have to give the patient a fair shot off the medication to experience recovery because those symptoms can last weeks or even months after the drug is discontinued.
Dr. Watto: Dr Hung looks at the patient’s problem list and asks whether is there any reason this patient might have been exposed to one of these medications?
We’re not going to get too much into specific Parkinson’s treatment, but I was glad to hear that exercise actually improves mobility and may even have some neuroprotective effects. He mentioned ongoing trials looking at that. We always love an excuse to tell patients that they should be moving around more and being physically active.
Dr. Williams: That was one of the more shocking things I learned, that exercise might actually be good for you. That will deeply inform my practice. Many of the treatments that we use for Parkinson’s only address symptoms. They don’t address progression or fix anything, but exercise can help with that.
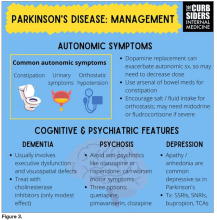
Dr. Watto: Paul, the last question I wanted to ask you is about our role in primary care. Patients with Parkinson’s have autonomic symptoms. They have neurocognitive symptoms. What is our role in that as primary care physicians?
Dr. Williams: Myriad symptoms can accompany Parkinson’s, and we have experience with most of them. We should all feel fairly comfortable dealing with constipation, which can be a very bothersome symptom. And we can use our full arsenal for symptoms such as depression, anxiety, and even apathy – the anhedonia, which apparently can be the predominant feature. We do have the tools to address these problems.
This might be a situation where we might reach for bupropion or a tricyclic antidepressant, which might not be your initial choice for a patient with a possibly annoying mood disorder. But for someone with Parkinson’s disease, this actually may be very helpful. We know how to manage a lot of the symptoms that come along with Parkinson’s that are not just the motor symptoms, and we should take ownership of those things.
Dr. Watto: You can hear the rest of this podcast here. This has been another episode of The Curbsiders bringing you a little knowledge food for your brain hole. Until next time, I’ve been Dr Matthew Frank Watto.
Dr. Williams: And I’m Dr Paul Nelson Williams.
Dr. Watto is a clinical assistant professor, department of medicine, at the University of Pennsylvania, Philadelphia. Dr. Williams is Associate Professor of Clinical Medicine, Department of General Internal Medicine, at Temple University, Philadelphia. Neither Dr. Watto nor Dr. Williams reported any relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. We had a great discussion on Parkinson’s Disease for Primary Care with Dr. Albert Hung. Paul, this was something that really made me nervous. I didn’t have a lot of comfort with it. But he taught us a lot of tips about how to recognize Parkinson’s.
I hadn’t been as aware of the premotor symptoms: constipation, hyposmia (loss of sense of smell), and rapid eye movement sleep behavior disorder. If patients have those early on and they aren’t explained by other things (especially the REM sleep behavior disorder), you should really key in because those patients are at risk of developing Parkinson’s years down the line. Those symptoms could present first, which just kind of blew my mind.
What tips do you have about how to recognize Parkinson’s? Do you want to talk about the physical exam?
Paul N. Williams, MD: You know I love the physical exam stuff, so I’m happy to talk about that.
You were deeply upset that cogwheel rigidity was not pathognomonic for Parkinson’s, but you made the point – and our guest agreed – that asymmetry tends to be the key here. And I really appreciated the point about reemergent tremor. This is this idea of a resting tremor. If someone has more parkinsonian features, you might see an intention tremor with essential tremor. If they reach out, it might seem steady at first, but if they hold long enough, then the tremor may kind of reemerge. I thought that was a neat distinction.
And this idea of cogwheel rigidity is a combination of some of the cardinal features of Parkinson’s – it’s a little bit of tremor and a little bit of rigidity too. There’s a baseline increase in tone, and then the tremor is superimposed on top of that. When you’re feeling cogwheeling, that’s actually what you’re feeling on examination. Parkinson’s, with all of its physical exam findings has always fascinated me.
Dr. Watto: He also told us about some red flags.
With classic idiopathic parkinsonism, there’s asymmetric involvement of the tremor. So red flags include a symmetric tremor, which might be something other than idiopathic parkinsonism. He also mentioned that one of the reasons you may want to get imaging (which is not always necessary if someone has a classic presentation), is if you see lower body–predominant symptoms of parkinsonism. These patients have rigidity or slowness of movement in their legs, but their upper bodies are not affected. They don’t have masked facies or the tremor in their hands. You might get an MRI in that case because that could be presentation of vascular dementia or vascular disease in the brain or even normal pressure hydrocephalus, which is a treatable condition. That would be one reason to get imaging.
What if the patient was exposed to a drug like a dopamine antagonist? They will get better in a couple of days, right?
Dr. Williams: This was a really fascinating point because we typically think if a patient’s symptoms are related to a drug exposure – in this case, drug-induced parkinsonism – we can just stop the medication and the symptoms will disappear in a couple of days as the drug leaves the system. But as it turns out, it might take much longer. A mistake that Dr Hung often sees is that the clinician stops the possibly offending agent, but when they don’t see an immediate relief of symptoms, they assume the drug wasn’t causing them. You really have to give the patient a fair shot off the medication to experience recovery because those symptoms can last weeks or even months after the drug is discontinued.
Dr. Watto: Dr Hung looks at the patient’s problem list and asks whether is there any reason this patient might have been exposed to one of these medications?
We’re not going to get too much into specific Parkinson’s treatment, but I was glad to hear that exercise actually improves mobility and may even have some neuroprotective effects. He mentioned ongoing trials looking at that. We always love an excuse to tell patients that they should be moving around more and being physically active.
Dr. Williams: That was one of the more shocking things I learned, that exercise might actually be good for you. That will deeply inform my practice. Many of the treatments that we use for Parkinson’s only address symptoms. They don’t address progression or fix anything, but exercise can help with that.
Dr. Watto: Paul, the last question I wanted to ask you is about our role in primary care. Patients with Parkinson’s have autonomic symptoms. They have neurocognitive symptoms. What is our role in that as primary care physicians?
Dr. Williams: Myriad symptoms can accompany Parkinson’s, and we have experience with most of them. We should all feel fairly comfortable dealing with constipation, which can be a very bothersome symptom. And we can use our full arsenal for symptoms such as depression, anxiety, and even apathy – the anhedonia, which apparently can be the predominant feature. We do have the tools to address these problems.
This might be a situation where we might reach for bupropion or a tricyclic antidepressant, which might not be your initial choice for a patient with a possibly annoying mood disorder. But for someone with Parkinson’s disease, this actually may be very helpful. We know how to manage a lot of the symptoms that come along with Parkinson’s that are not just the motor symptoms, and we should take ownership of those things.
Dr. Watto: You can hear the rest of this podcast here. This has been another episode of The Curbsiders bringing you a little knowledge food for your brain hole. Until next time, I’ve been Dr Matthew Frank Watto.
Dr. Williams: And I’m Dr Paul Nelson Williams.
Dr. Watto is a clinical assistant professor, department of medicine, at the University of Pennsylvania, Philadelphia. Dr. Williams is Associate Professor of Clinical Medicine, Department of General Internal Medicine, at Temple University, Philadelphia. Neither Dr. Watto nor Dr. Williams reported any relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. We had a great discussion on Parkinson’s Disease for Primary Care with Dr. Albert Hung. Paul, this was something that really made me nervous. I didn’t have a lot of comfort with it. But he taught us a lot of tips about how to recognize Parkinson’s.
I hadn’t been as aware of the premotor symptoms: constipation, hyposmia (loss of sense of smell), and rapid eye movement sleep behavior disorder. If patients have those early on and they aren’t explained by other things (especially the REM sleep behavior disorder), you should really key in because those patients are at risk of developing Parkinson’s years down the line. Those symptoms could present first, which just kind of blew my mind.
What tips do you have about how to recognize Parkinson’s? Do you want to talk about the physical exam?
Paul N. Williams, MD: You know I love the physical exam stuff, so I’m happy to talk about that.
You were deeply upset that cogwheel rigidity was not pathognomonic for Parkinson’s, but you made the point – and our guest agreed – that asymmetry tends to be the key here. And I really appreciated the point about reemergent tremor. This is this idea of a resting tremor. If someone has more parkinsonian features, you might see an intention tremor with essential tremor. If they reach out, it might seem steady at first, but if they hold long enough, then the tremor may kind of reemerge. I thought that was a neat distinction.
And this idea of cogwheel rigidity is a combination of some of the cardinal features of Parkinson’s – it’s a little bit of tremor and a little bit of rigidity too. There’s a baseline increase in tone, and then the tremor is superimposed on top of that. When you’re feeling cogwheeling, that’s actually what you’re feeling on examination. Parkinson’s, with all of its physical exam findings has always fascinated me.
Dr. Watto: He also told us about some red flags.
With classic idiopathic parkinsonism, there’s asymmetric involvement of the tremor. So red flags include a symmetric tremor, which might be something other than idiopathic parkinsonism. He also mentioned that one of the reasons you may want to get imaging (which is not always necessary if someone has a classic presentation), is if you see lower body–predominant symptoms of parkinsonism. These patients have rigidity or slowness of movement in their legs, but their upper bodies are not affected. They don’t have masked facies or the tremor in their hands. You might get an MRI in that case because that could be presentation of vascular dementia or vascular disease in the brain or even normal pressure hydrocephalus, which is a treatable condition. That would be one reason to get imaging.
What if the patient was exposed to a drug like a dopamine antagonist? They will get better in a couple of days, right?
Dr. Williams: This was a really fascinating point because we typically think if a patient’s symptoms are related to a drug exposure – in this case, drug-induced parkinsonism – we can just stop the medication and the symptoms will disappear in a couple of days as the drug leaves the system. But as it turns out, it might take much longer. A mistake that Dr Hung often sees is that the clinician stops the possibly offending agent, but when they don’t see an immediate relief of symptoms, they assume the drug wasn’t causing them. You really have to give the patient a fair shot off the medication to experience recovery because those symptoms can last weeks or even months after the drug is discontinued.
Dr. Watto: Dr Hung looks at the patient’s problem list and asks whether is there any reason this patient might have been exposed to one of these medications?
We’re not going to get too much into specific Parkinson’s treatment, but I was glad to hear that exercise actually improves mobility and may even have some neuroprotective effects. He mentioned ongoing trials looking at that. We always love an excuse to tell patients that they should be moving around more and being physically active.
Dr. Williams: That was one of the more shocking things I learned, that exercise might actually be good for you. That will deeply inform my practice. Many of the treatments that we use for Parkinson’s only address symptoms. They don’t address progression or fix anything, but exercise can help with that.
Dr. Watto: Paul, the last question I wanted to ask you is about our role in primary care. Patients with Parkinson’s have autonomic symptoms. They have neurocognitive symptoms. What is our role in that as primary care physicians?
Dr. Williams: Myriad symptoms can accompany Parkinson’s, and we have experience with most of them. We should all feel fairly comfortable dealing with constipation, which can be a very bothersome symptom. And we can use our full arsenal for symptoms such as depression, anxiety, and even apathy – the anhedonia, which apparently can be the predominant feature. We do have the tools to address these problems.
This might be a situation where we might reach for bupropion or a tricyclic antidepressant, which might not be your initial choice for a patient with a possibly annoying mood disorder. But for someone with Parkinson’s disease, this actually may be very helpful. We know how to manage a lot of the symptoms that come along with Parkinson’s that are not just the motor symptoms, and we should take ownership of those things.
Dr. Watto: You can hear the rest of this podcast here. This has been another episode of The Curbsiders bringing you a little knowledge food for your brain hole. Until next time, I’ve been Dr Matthew Frank Watto.
Dr. Williams: And I’m Dr Paul Nelson Williams.
Dr. Watto is a clinical assistant professor, department of medicine, at the University of Pennsylvania, Philadelphia. Dr. Williams is Associate Professor of Clinical Medicine, Department of General Internal Medicine, at Temple University, Philadelphia. Neither Dr. Watto nor Dr. Williams reported any relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Parkinson’s disease: What’s trauma got to do with it?
This transcript has been edited for clarity.
Kathrin LaFaver, MD: Hello. I’m happy to talk today to Dr. Indu Subramanian, clinical professor at University of California, Los Angeles, and director of the Parkinson’s Disease Research, Education and Clinical Center in Los Angeles. I am a neurologist in Saratoga Springs, New York, and we will be talking today about Indu’s new paper on childhood trauma and Parkinson’s disease. Welcome and thanks for taking the time.
Indu Subramanian, MD: Thank you so much for letting us highlight this important topic.
Dr. LaFaver: There are many papers published every month on Parkinson’s disease, but this topic stands out because it’s not a thing that has been commonly looked at. What gave you the idea to study this?
Neurology behind other specialties
Dr. Subramanian: Kathrin, you and I have been looking at things that can inform us about our patients – the person who’s standing in front of us when they come in and we’re giving them this diagnosis. I think that so much of what we’ve done [in the past] is a cookie cutter approach to giving everybody the standard treatment. [We’ve been assuming that] It doesn’t matter if they’re a man or woman. It doesn’t matter if they’re a veteran. It doesn’t matter if they may be from a minoritized population.
We’ve also been interested in approaches that are outside the box, right? We have this integrative medicine and lifestyle medicine background. I’ve been going to those meetings and really been struck by the mounting evidence on the importance of things like early adverse childhood events (ACEs), what zip code you live in, what your pollution index is, and how these things can affect people through their life and their health.
I think that it is high time neurologists pay attention to this. There’s been mounting evidence throughout many disease states, various types of cancers, and mental health. Cardiology is much more advanced, but we haven’t had much data in neurology. In fact, when we went to write this paper, there were just one or two papers that were looking at multiple sclerosis or general neurologic issues, but really nothing in Parkinson’s disease.
We know that Parkinson’s disease is not only a motor disease that affects mental health, but that it also affects nonmotor issues. Childhood adversity may affect how people progress or how quickly they may get a disease, and we were interested in how it may manifest in a disease like Parkinson’s disease.
That was the framework going to meetings. As we wrote this paper and were in various editing stages, there was a beautiful paper that came out by Nadine Burke Harris and team that really was a call to action for neurologists and caring about trauma.
Dr. LaFaver: I couldn’t agree more. It’s really an underrecognized issue. With my own background, being very interested in functional movement disorders, psychosomatic disorders, and so on, it becomes much more evident how common a trauma background is, not only for people we were traditionally asking about.
Why don’t you summarize your findings for us?
Adverse childhood events
Dr. Subramanian: This is a web-based survey, so obviously, these are patient self-reports of their disease. We have a large cohort of people that we’ve been following over 7 years. I’m looking at modifiable variables and what really impacts Parkinson’s disease. Some of our previous papers have looked at diet, exercise, and loneliness. This is the same cohort.
We ended up putting the ACEs questionnaire, which is 10 questions looking at whether you were exposed to certain things in your household below the age of 18. This is a relatively standard questionnaire that’s administered one time, and you get a score out of 10. This is something that has been pushed, at least in the state of California, as something that we should be checking more in all people coming in.
We introduced the survey, and we didn’t force everyone to take it. Unfortunately, there was 20% or so of our patients who chose not to answer these questions. One has to ask, who are those people that didn’t answer the questions? Are they the ones that may have had trauma and these questions were triggering? It was a gap. We didn’t add extra questions to explore why people didn’t answer those questions.
We have to also put this in context. We have a patient population that’s largely quite affluent, who are able to access web-based surveys through their computer, and largely Caucasian; there are not many minoritized populations in our cohort. We want to do better with that. We actually were able to gather a decent number of women. We represent women quite well in our survey. I think that’s because of this online approach and some of the things that we’re studying.
In our survey, we broke it down into people who had no ACEs, one to three ACEs, or four or more ACEs. This is a standard way to break down ACEs so that we’re able to categorize what to do with these patient populations.
What we saw – and it’s preliminary evidence – is that people who had higher ACE scores seemed to have more symptom severity when we controlled for things like years since diagnosis, age, and gender. They also seem to have a worse quality of life. There was some indication that there were more nonmotor issues in those populations, as you might expect, such as anxiety, depression, and things that presumably ACEs can affect separately.
There are some confounders, but I think we really want to use this as the first piece of evidence to hopefully pave the way for caring about trauma in Parkinson’s disease moving forward.
Dr. LaFaver: Thank you so much for that summary. You already mentioned the main methodology you used.
What is the next step for you? How do you see these findings informing our clinical care? Do you have suggestions for all of the neurologists listening in this regard?
PD not yet considered ACE-related
Dr. Subramanian: Dr. Burke Harris was the former surgeon general in California. She’s a woman of color and a brilliant speaker, and she had worked in inner cities, I think in San Francisco, with pediatric populations, seeing these effects of adversity in that time frame.
You see this population at risk, and then you’re following this cohort, which we knew from the Kaiser cohort determines earlier morbidity and mortality across a number of disease states. We’re seeing things like more heart attacks, more diabetes, and all kinds of things in these populations. This is not new news; we just have not been focusing on this.
In her paper, this call to action, they had talked about some ACE-related conditions that currently do not include Parkinson’s disease. There are three ACE-related neurologic conditions that people should be aware of. One is in the headache/pain universe. Another is in the stroke universe, and that’s understandable, given cardiovascular risk factors . Then the third is in this dementia risk category. I think Parkinson’s disease, as we know, can be associated with dementia. A large percentage of our patients get dementia, but we don’t have Parkinson’s disease called out in this framework.
What people are talking about is if you have no ACEs or are in this middle category of one to three ACEs and you don’t have an ACE-related diagnosis – which Parkinson’s disease is not currently – we just give some basic counseling about the importance of lifestyle. I think we would love to see that anyway. They’re talking about things like exercise, diet, sleep, social connection, getting out in nature, things like that, so just general counseling on the importance of that.
Then if you’re in this higher-risk category, and so with these ACE-related neurologic conditions, including dementia, headache, and stroke, if you had this middle range of one to three ACEs, they’re getting additional resources. Some of them may be referred for social work help or mental health support and things like that.
I’d really love to see that happening in Parkinson’s disease, because I think we have so many needs in our population. I’m always hoping to advocate for more mental health needs that are scarce and resources in the social support realm because I believe that social connection and social support is a huge buffer for this trauma.
ACEs are just one type of trauma. I take care of veterans in the Veterans [Affairs Department]. We have some information now coming out about posttraumatic stress disorder, predisposing to certain things in Parkinson’s disease, possibly head injury, and things like that. I think we have populations at risk that we can hopefully screen at intake, and I’m really pushing for that.
Maybe it’s not the neurologist that does this intake. It might be someone else on the team that can spend some time doing these questionnaires and understand if your patient has a high ACE score. Unless you ask, many patients don’t necessarily come forward to talk about this. I really am pushing for trying to screen and trying to advocate for more research in this area so that we can classify Parkinson’s disease as an ACE-related condition and thus give more resources from the mental health world, and also the social support world, to our patients.
Dr. LaFaver: Thank you. There are many important points, and I think it’s a very important thing to recognize that it may not be only trauma in childhood but also throughout life, as you said, and might really influence nonmotor symptoms of Parkinson’s disease in particular, including anxiety and pain, which are often difficult to treat.
I think there’s much more to do in research, advocacy, and education. We’re going to educate patients about this, and also educate other neurologists and providers. I think you mentioned that trauma-informed care is getting its spotlight in primary care and other specialties. I think we have catching up to do in neurology, and I think this is a really important work toward that goal.
Thank you so much for your work and for taking the time to share your thoughts. I hope to talk to you again soon.
Dr. Subramanian: Thank you so much, Kathrin.
Dr. LaFaver has disclosed no relevant financial relationships. Dr. Subramanian disclosed ties with Acorda Therapeutics.
A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Kathrin LaFaver, MD: Hello. I’m happy to talk today to Dr. Indu Subramanian, clinical professor at University of California, Los Angeles, and director of the Parkinson’s Disease Research, Education and Clinical Center in Los Angeles. I am a neurologist in Saratoga Springs, New York, and we will be talking today about Indu’s new paper on childhood trauma and Parkinson’s disease. Welcome and thanks for taking the time.
Indu Subramanian, MD: Thank you so much for letting us highlight this important topic.
Dr. LaFaver: There are many papers published every month on Parkinson’s disease, but this topic stands out because it’s not a thing that has been commonly looked at. What gave you the idea to study this?
Neurology behind other specialties
Dr. Subramanian: Kathrin, you and I have been looking at things that can inform us about our patients – the person who’s standing in front of us when they come in and we’re giving them this diagnosis. I think that so much of what we’ve done [in the past] is a cookie cutter approach to giving everybody the standard treatment. [We’ve been assuming that] It doesn’t matter if they’re a man or woman. It doesn’t matter if they’re a veteran. It doesn’t matter if they may be from a minoritized population.
We’ve also been interested in approaches that are outside the box, right? We have this integrative medicine and lifestyle medicine background. I’ve been going to those meetings and really been struck by the mounting evidence on the importance of things like early adverse childhood events (ACEs), what zip code you live in, what your pollution index is, and how these things can affect people through their life and their health.
I think that it is high time neurologists pay attention to this. There’s been mounting evidence throughout many disease states, various types of cancers, and mental health. Cardiology is much more advanced, but we haven’t had much data in neurology. In fact, when we went to write this paper, there were just one or two papers that were looking at multiple sclerosis or general neurologic issues, but really nothing in Parkinson’s disease.
We know that Parkinson’s disease is not only a motor disease that affects mental health, but that it also affects nonmotor issues. Childhood adversity may affect how people progress or how quickly they may get a disease, and we were interested in how it may manifest in a disease like Parkinson’s disease.
That was the framework going to meetings. As we wrote this paper and were in various editing stages, there was a beautiful paper that came out by Nadine Burke Harris and team that really was a call to action for neurologists and caring about trauma.
Dr. LaFaver: I couldn’t agree more. It’s really an underrecognized issue. With my own background, being very interested in functional movement disorders, psychosomatic disorders, and so on, it becomes much more evident how common a trauma background is, not only for people we were traditionally asking about.
Why don’t you summarize your findings for us?
Adverse childhood events
Dr. Subramanian: This is a web-based survey, so obviously, these are patient self-reports of their disease. We have a large cohort of people that we’ve been following over 7 years. I’m looking at modifiable variables and what really impacts Parkinson’s disease. Some of our previous papers have looked at diet, exercise, and loneliness. This is the same cohort.
We ended up putting the ACEs questionnaire, which is 10 questions looking at whether you were exposed to certain things in your household below the age of 18. This is a relatively standard questionnaire that’s administered one time, and you get a score out of 10. This is something that has been pushed, at least in the state of California, as something that we should be checking more in all people coming in.
We introduced the survey, and we didn’t force everyone to take it. Unfortunately, there was 20% or so of our patients who chose not to answer these questions. One has to ask, who are those people that didn’t answer the questions? Are they the ones that may have had trauma and these questions were triggering? It was a gap. We didn’t add extra questions to explore why people didn’t answer those questions.
We have to also put this in context. We have a patient population that’s largely quite affluent, who are able to access web-based surveys through their computer, and largely Caucasian; there are not many minoritized populations in our cohort. We want to do better with that. We actually were able to gather a decent number of women. We represent women quite well in our survey. I think that’s because of this online approach and some of the things that we’re studying.
In our survey, we broke it down into people who had no ACEs, one to three ACEs, or four or more ACEs. This is a standard way to break down ACEs so that we’re able to categorize what to do with these patient populations.
What we saw – and it’s preliminary evidence – is that people who had higher ACE scores seemed to have more symptom severity when we controlled for things like years since diagnosis, age, and gender. They also seem to have a worse quality of life. There was some indication that there were more nonmotor issues in those populations, as you might expect, such as anxiety, depression, and things that presumably ACEs can affect separately.
There are some confounders, but I think we really want to use this as the first piece of evidence to hopefully pave the way for caring about trauma in Parkinson’s disease moving forward.
Dr. LaFaver: Thank you so much for that summary. You already mentioned the main methodology you used.
What is the next step for you? How do you see these findings informing our clinical care? Do you have suggestions for all of the neurologists listening in this regard?
PD not yet considered ACE-related
Dr. Subramanian: Dr. Burke Harris was the former surgeon general in California. She’s a woman of color and a brilliant speaker, and she had worked in inner cities, I think in San Francisco, with pediatric populations, seeing these effects of adversity in that time frame.
You see this population at risk, and then you’re following this cohort, which we knew from the Kaiser cohort determines earlier morbidity and mortality across a number of disease states. We’re seeing things like more heart attacks, more diabetes, and all kinds of things in these populations. This is not new news; we just have not been focusing on this.
In her paper, this call to action, they had talked about some ACE-related conditions that currently do not include Parkinson’s disease. There are three ACE-related neurologic conditions that people should be aware of. One is in the headache/pain universe. Another is in the stroke universe, and that’s understandable, given cardiovascular risk factors . Then the third is in this dementia risk category. I think Parkinson’s disease, as we know, can be associated with dementia. A large percentage of our patients get dementia, but we don’t have Parkinson’s disease called out in this framework.
What people are talking about is if you have no ACEs or are in this middle category of one to three ACEs and you don’t have an ACE-related diagnosis – which Parkinson’s disease is not currently – we just give some basic counseling about the importance of lifestyle. I think we would love to see that anyway. They’re talking about things like exercise, diet, sleep, social connection, getting out in nature, things like that, so just general counseling on the importance of that.
Then if you’re in this higher-risk category, and so with these ACE-related neurologic conditions, including dementia, headache, and stroke, if you had this middle range of one to three ACEs, they’re getting additional resources. Some of them may be referred for social work help or mental health support and things like that.
I’d really love to see that happening in Parkinson’s disease, because I think we have so many needs in our population. I’m always hoping to advocate for more mental health needs that are scarce and resources in the social support realm because I believe that social connection and social support is a huge buffer for this trauma.
ACEs are just one type of trauma. I take care of veterans in the Veterans [Affairs Department]. We have some information now coming out about posttraumatic stress disorder, predisposing to certain things in Parkinson’s disease, possibly head injury, and things like that. I think we have populations at risk that we can hopefully screen at intake, and I’m really pushing for that.
Maybe it’s not the neurologist that does this intake. It might be someone else on the team that can spend some time doing these questionnaires and understand if your patient has a high ACE score. Unless you ask, many patients don’t necessarily come forward to talk about this. I really am pushing for trying to screen and trying to advocate for more research in this area so that we can classify Parkinson’s disease as an ACE-related condition and thus give more resources from the mental health world, and also the social support world, to our patients.
Dr. LaFaver: Thank you. There are many important points, and I think it’s a very important thing to recognize that it may not be only trauma in childhood but also throughout life, as you said, and might really influence nonmotor symptoms of Parkinson’s disease in particular, including anxiety and pain, which are often difficult to treat.
I think there’s much more to do in research, advocacy, and education. We’re going to educate patients about this, and also educate other neurologists and providers. I think you mentioned that trauma-informed care is getting its spotlight in primary care and other specialties. I think we have catching up to do in neurology, and I think this is a really important work toward that goal.
Thank you so much for your work and for taking the time to share your thoughts. I hope to talk to you again soon.
Dr. Subramanian: Thank you so much, Kathrin.
Dr. LaFaver has disclosed no relevant financial relationships. Dr. Subramanian disclosed ties with Acorda Therapeutics.
A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Kathrin LaFaver, MD: Hello. I’m happy to talk today to Dr. Indu Subramanian, clinical professor at University of California, Los Angeles, and director of the Parkinson’s Disease Research, Education and Clinical Center in Los Angeles. I am a neurologist in Saratoga Springs, New York, and we will be talking today about Indu’s new paper on childhood trauma and Parkinson’s disease. Welcome and thanks for taking the time.
Indu Subramanian, MD: Thank you so much for letting us highlight this important topic.
Dr. LaFaver: There are many papers published every month on Parkinson’s disease, but this topic stands out because it’s not a thing that has been commonly looked at. What gave you the idea to study this?
Neurology behind other specialties
Dr. Subramanian: Kathrin, you and I have been looking at things that can inform us about our patients – the person who’s standing in front of us when they come in and we’re giving them this diagnosis. I think that so much of what we’ve done [in the past] is a cookie cutter approach to giving everybody the standard treatment. [We’ve been assuming that] It doesn’t matter if they’re a man or woman. It doesn’t matter if they’re a veteran. It doesn’t matter if they may be from a minoritized population.
We’ve also been interested in approaches that are outside the box, right? We have this integrative medicine and lifestyle medicine background. I’ve been going to those meetings and really been struck by the mounting evidence on the importance of things like early adverse childhood events (ACEs), what zip code you live in, what your pollution index is, and how these things can affect people through their life and their health.
I think that it is high time neurologists pay attention to this. There’s been mounting evidence throughout many disease states, various types of cancers, and mental health. Cardiology is much more advanced, but we haven’t had much data in neurology. In fact, when we went to write this paper, there were just one or two papers that were looking at multiple sclerosis or general neurologic issues, but really nothing in Parkinson’s disease.
We know that Parkinson’s disease is not only a motor disease that affects mental health, but that it also affects nonmotor issues. Childhood adversity may affect how people progress or how quickly they may get a disease, and we were interested in how it may manifest in a disease like Parkinson’s disease.
That was the framework going to meetings. As we wrote this paper and were in various editing stages, there was a beautiful paper that came out by Nadine Burke Harris and team that really was a call to action for neurologists and caring about trauma.
Dr. LaFaver: I couldn’t agree more. It’s really an underrecognized issue. With my own background, being very interested in functional movement disorders, psychosomatic disorders, and so on, it becomes much more evident how common a trauma background is, not only for people we were traditionally asking about.
Why don’t you summarize your findings for us?
Adverse childhood events
Dr. Subramanian: This is a web-based survey, so obviously, these are patient self-reports of their disease. We have a large cohort of people that we’ve been following over 7 years. I’m looking at modifiable variables and what really impacts Parkinson’s disease. Some of our previous papers have looked at diet, exercise, and loneliness. This is the same cohort.
We ended up putting the ACEs questionnaire, which is 10 questions looking at whether you were exposed to certain things in your household below the age of 18. This is a relatively standard questionnaire that’s administered one time, and you get a score out of 10. This is something that has been pushed, at least in the state of California, as something that we should be checking more in all people coming in.
We introduced the survey, and we didn’t force everyone to take it. Unfortunately, there was 20% or so of our patients who chose not to answer these questions. One has to ask, who are those people that didn’t answer the questions? Are they the ones that may have had trauma and these questions were triggering? It was a gap. We didn’t add extra questions to explore why people didn’t answer those questions.
We have to also put this in context. We have a patient population that’s largely quite affluent, who are able to access web-based surveys through their computer, and largely Caucasian; there are not many minoritized populations in our cohort. We want to do better with that. We actually were able to gather a decent number of women. We represent women quite well in our survey. I think that’s because of this online approach and some of the things that we’re studying.
In our survey, we broke it down into people who had no ACEs, one to three ACEs, or four or more ACEs. This is a standard way to break down ACEs so that we’re able to categorize what to do with these patient populations.
What we saw – and it’s preliminary evidence – is that people who had higher ACE scores seemed to have more symptom severity when we controlled for things like years since diagnosis, age, and gender. They also seem to have a worse quality of life. There was some indication that there were more nonmotor issues in those populations, as you might expect, such as anxiety, depression, and things that presumably ACEs can affect separately.
There are some confounders, but I think we really want to use this as the first piece of evidence to hopefully pave the way for caring about trauma in Parkinson’s disease moving forward.
Dr. LaFaver: Thank you so much for that summary. You already mentioned the main methodology you used.
What is the next step for you? How do you see these findings informing our clinical care? Do you have suggestions for all of the neurologists listening in this regard?
PD not yet considered ACE-related
Dr. Subramanian: Dr. Burke Harris was the former surgeon general in California. She’s a woman of color and a brilliant speaker, and she had worked in inner cities, I think in San Francisco, with pediatric populations, seeing these effects of adversity in that time frame.
You see this population at risk, and then you’re following this cohort, which we knew from the Kaiser cohort determines earlier morbidity and mortality across a number of disease states. We’re seeing things like more heart attacks, more diabetes, and all kinds of things in these populations. This is not new news; we just have not been focusing on this.
In her paper, this call to action, they had talked about some ACE-related conditions that currently do not include Parkinson’s disease. There are three ACE-related neurologic conditions that people should be aware of. One is in the headache/pain universe. Another is in the stroke universe, and that’s understandable, given cardiovascular risk factors . Then the third is in this dementia risk category. I think Parkinson’s disease, as we know, can be associated with dementia. A large percentage of our patients get dementia, but we don’t have Parkinson’s disease called out in this framework.
What people are talking about is if you have no ACEs or are in this middle category of one to three ACEs and you don’t have an ACE-related diagnosis – which Parkinson’s disease is not currently – we just give some basic counseling about the importance of lifestyle. I think we would love to see that anyway. They’re talking about things like exercise, diet, sleep, social connection, getting out in nature, things like that, so just general counseling on the importance of that.
Then if you’re in this higher-risk category, and so with these ACE-related neurologic conditions, including dementia, headache, and stroke, if you had this middle range of one to three ACEs, they’re getting additional resources. Some of them may be referred for social work help or mental health support and things like that.
I’d really love to see that happening in Parkinson’s disease, because I think we have so many needs in our population. I’m always hoping to advocate for more mental health needs that are scarce and resources in the social support realm because I believe that social connection and social support is a huge buffer for this trauma.
ACEs are just one type of trauma. I take care of veterans in the Veterans [Affairs Department]. We have some information now coming out about posttraumatic stress disorder, predisposing to certain things in Parkinson’s disease, possibly head injury, and things like that. I think we have populations at risk that we can hopefully screen at intake, and I’m really pushing for that.
Maybe it’s not the neurologist that does this intake. It might be someone else on the team that can spend some time doing these questionnaires and understand if your patient has a high ACE score. Unless you ask, many patients don’t necessarily come forward to talk about this. I really am pushing for trying to screen and trying to advocate for more research in this area so that we can classify Parkinson’s disease as an ACE-related condition and thus give more resources from the mental health world, and also the social support world, to our patients.
Dr. LaFaver: Thank you. There are many important points, and I think it’s a very important thing to recognize that it may not be only trauma in childhood but also throughout life, as you said, and might really influence nonmotor symptoms of Parkinson’s disease in particular, including anxiety and pain, which are often difficult to treat.
I think there’s much more to do in research, advocacy, and education. We’re going to educate patients about this, and also educate other neurologists and providers. I think you mentioned that trauma-informed care is getting its spotlight in primary care and other specialties. I think we have catching up to do in neurology, and I think this is a really important work toward that goal.
Thank you so much for your work and for taking the time to share your thoughts. I hope to talk to you again soon.
Dr. Subramanian: Thank you so much, Kathrin.
Dr. LaFaver has disclosed no relevant financial relationships. Dr. Subramanian disclosed ties with Acorda Therapeutics.
A version of this article originally appeared on Medscape.com.
The sacrifice of orthodoxy: Maintaining collegiality in psychiatry
Psychiatrists practice in a wide array of ways. We approach our work and our patients with beliefs and preconceptions that develop over time. Our training has significant influence, though our own personalities and biases also affect our understanding.
Psychiatrists have philosophical lenses through which they see patients. We can reflect and see some standard archetypes. We are familiar with the reductionistic pharmacologist, the somatic treatment specialist, the psychodynamic ‘guru,’ and the medicolegally paralyzed practitioner. It is without judgment that we lay these out, for our very point is that we have these constituent parts within our own clinical identities. The intensity with which we subscribe to these clinical sensibilities could contribute to a biased orthodoxy.
Orthodoxy can be defined as an accepted theory that stems from an authoritative entity. This is a well-known phenomenon that continues to be visible. For example, one can quickly peruse psychodynamic literature to find one school of thought criticizing another. It is not without some confrontation and even interpersonal rifts that the lineage of psychoanalytic theory has evolved. This has always been of interest to us. A core facet of psychoanalysis is empathy, truly knowing the inner state of a different person. And yet, the very bastions of this clinical sensibility frequently resort to veiled attacks on those in their field who have opposing views. It then begs the question: If even enlightened institutions fail at a nonjudgmental approach toward their colleagues, what hope is there for the rest of us clinicians, mired in the thick of day-to-day clinical practice?
It is our contention that the odds are against us. Even the aforementioned critique of psychoanalytic orthodoxy is just another example of how we humans organize our experience. Even as we write an article in argument against unbridled critique, we find it difficult to do so without engaging in it. For to criticize another is to help shore up our own personal identities. This is especially the case when clinicians deal with issues that we feel strongly about. The human psyche has a need to organize its experience, as “our experience of ourselves is fundamental to how we operate in the world. Our subjective experience is the phenomenology of all that one might be aware of.”1
In this vein, we would like to cite attribution theory. This is a view of human behavior within social psychology. The Austrian psychologist Fritz Heider, PhD, investigated “the domain of social interactions, wondering how people perceive each other in interaction and especially how they make sense of each other’s behavior.”2 Attribution theory suggests that as humans organize our social interactions, we may make two basic assumptions. One is that our own behavior is highly affected by an environment that is beyond our control. The second is that when judging the behavior of others, we are more likely to attribute it to internal traits that they have. A classic example is automobile traffic. When we see someone driving erratically, we are more likely to blame them for being an inherently bad driver. However, if attention is called to our own driving, we are more likely to cite external factors such as rush hour, a bad driver around us, or a faulty vehicle.
We would like to reference one last model of human behavior. It has become customary within the field of neuroscience to view the brain as a predictive organ: “Theories of prediction in perception, action, and learning suggest that the brain serves to reduce the discrepancies between expectation and actual experience, i.e., by reducing the prediction error.”3 Perception itself has recently been described as a controlled hallucination, where the brain makes predictions of what it thinks it is about to see based on past experiences. Visual stimulus ultimately takes time to enter our eyes and be processed in the brain – “predictions would need to preactivate neural representations that would typically be driven by sensory input, before the actual arrival of that input.”4 It thus seems to be an inherent method of the brain to anticipate visual and even social events to help human beings sustain themselves.
Having spoken of a psychoanalytic conceptualization of self-organization, the theory of attribution, and research into social neuroscience, we turn our attention back to the central question that this article would like to address.
When we find ourselves busy in rote clinical practice, we believe the likelihood of intercollegiate mentalization is low; our ability to relate to our peers becomes strained. We ultimately do not practice in a vacuum. Psychiatrists, even those in a solo private practice, are ultimately part of a community of providers who, more or less, follow some emergent ‘standard of care.’ This can be a vague concept; but one that takes on a concrete form in the minds of certain clinicians and certainly in the setting of a medicolegal court. Yet, the psychiatrists that we know all have very stereotyped ways of practice. And at the heart of it, we all think that we are right.
We can use polypharmacy as an example. Imagine that you have a new patient intake, who tells you that they are transferring care from another psychiatrist. They inform you of their medication regimen. This patient presents on eight or more psychotropics. Many of us may have a visceral reaction at this point and, following the aforementioned attribution theory, we may ask ourselves what ‘quack’ of a doctor would do this. Yet some among us would think that a very competent psychopharmacologist was daring enough to use the full armamentarium of psychopharmacology to help this patient, who must be treatment refractory.
When speaking with such a patient, we would be quick to reflect on our own parsimonious use of medications. We would tell ourselves that we are responsible providers and would be quick to recommend discontinuation of medications. This would help us feel better about ourselves, and would of course assuage the ever-present medicolegal ‘big brother’ in our minds. It is through this very process that we affirm our self-identities. For if this patient’s previous physician was a bad psychiatrist, then we are a good psychiatrist. It is through this process that our clinical selves find confirmation.
We do not mean to reduce the complexities of human behavior to quick stereotypes. However, it is our belief that when confronted with clinical or philosophical disputes with our colleagues, the basic rules of human behavior will attempt to dissolve and override efforts at mentalization, collegiality, or interpersonal sensitivity. For to accept a clinical practice view that is different from ours would be akin to giving up the essence of our clinical identities. It could be compared to the fragmentation process of a vulnerable psyche when confronted with a reality that is at odds with preconceived notions and experiences.
While we may be able to appreciate the nuances and sensibilities of another provider, we believe it would be particularly difficult for most of us to actually attempt to practice in a fashion that is not congruent with our own organizers of experience. Whether or not our practice style is ‘perfect,’ it has worked for us. Social neuroscience and our understanding of the organization of the self would predict that we would hold onto our way of practice with all the mind’s defenses. Externalization, denial, and projection could all be called into action in this battle against existential fragmentation.
Do we seek to portray a clinical world where there is no hope for genuine modeling of clinical sensibilities to other psychiatrists? That is not our intention. Yet it seems that many of the theoretical frameworks that we subscribe to argue against this possibility. We would be hypocritical if we did not here state that our own theoretical frameworks are yet other examples of “organizers of experience.” Attribution theory, intersubjectivity, and social neuroscience are simply our ways of organizing the chaos of perceptions, ideas, and intricacies of human behavior.
If we accept that psychiatrists, like all human beings, are trapped in a subjective experience, then we can be more playful and flexible when interacting with our colleagues. We do not have to be as defensive of our practices and accusatory of others. If we practice daily according to some orthodoxy, then we color our experiences of the patient and of our colleagues’ ways of practice. We automatically start off on the wrong foot. And yet, to give up this orthodoxy would, by definition, be disorganizing and fragmenting to us. For as Nietzsche said, “truth is an illusion without which a certain species could not survive.”5
Dr. Khalafian practices full time as a general outpatient psychiatrist. He trained at the University of California, San Diego, for his psychiatric residency and currently works as a telepsychiatrist, serving an outpatient clinic population in northern California. Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Badre and Dr. Khalafian have no conflicts of interest.
References
1. Buirski P and Haglund P. Making sense together: The intersubjective approach to psychotherapy. Northvale, NJ: Jason Aronson; 2001.
2. Malle BF. Attribution theories: How people make sense of behavior. In Chadee D (ed.), Theories in social psychology. pp. 72-95. Wiley-Blackwell; 2011.
3. Brown EC and Brune M. The role of prediction in social neuroscience. Front Hum Neurosci. 2012 May 24;6:147. doi: 10.3389/fnhum.2012.00147.
4. Blom T et al. Predictions drive neural representations of visual events ahead of incoming sensory information. Proc Natl Acad Sci USA. 2020 Mar 31;117(13):7510-7515. doi: 10.1073/pnas.1917777117.
5. Yalom I. The Gift of Therapy. Harper Perennial; 2002.
Psychiatrists practice in a wide array of ways. We approach our work and our patients with beliefs and preconceptions that develop over time. Our training has significant influence, though our own personalities and biases also affect our understanding.
Psychiatrists have philosophical lenses through which they see patients. We can reflect and see some standard archetypes. We are familiar with the reductionistic pharmacologist, the somatic treatment specialist, the psychodynamic ‘guru,’ and the medicolegally paralyzed practitioner. It is without judgment that we lay these out, for our very point is that we have these constituent parts within our own clinical identities. The intensity with which we subscribe to these clinical sensibilities could contribute to a biased orthodoxy.
Orthodoxy can be defined as an accepted theory that stems from an authoritative entity. This is a well-known phenomenon that continues to be visible. For example, one can quickly peruse psychodynamic literature to find one school of thought criticizing another. It is not without some confrontation and even interpersonal rifts that the lineage of psychoanalytic theory has evolved. This has always been of interest to us. A core facet of psychoanalysis is empathy, truly knowing the inner state of a different person. And yet, the very bastions of this clinical sensibility frequently resort to veiled attacks on those in their field who have opposing views. It then begs the question: If even enlightened institutions fail at a nonjudgmental approach toward their colleagues, what hope is there for the rest of us clinicians, mired in the thick of day-to-day clinical practice?
It is our contention that the odds are against us. Even the aforementioned critique of psychoanalytic orthodoxy is just another example of how we humans organize our experience. Even as we write an article in argument against unbridled critique, we find it difficult to do so without engaging in it. For to criticize another is to help shore up our own personal identities. This is especially the case when clinicians deal with issues that we feel strongly about. The human psyche has a need to organize its experience, as “our experience of ourselves is fundamental to how we operate in the world. Our subjective experience is the phenomenology of all that one might be aware of.”1
In this vein, we would like to cite attribution theory. This is a view of human behavior within social psychology. The Austrian psychologist Fritz Heider, PhD, investigated “the domain of social interactions, wondering how people perceive each other in interaction and especially how they make sense of each other’s behavior.”2 Attribution theory suggests that as humans organize our social interactions, we may make two basic assumptions. One is that our own behavior is highly affected by an environment that is beyond our control. The second is that when judging the behavior of others, we are more likely to attribute it to internal traits that they have. A classic example is automobile traffic. When we see someone driving erratically, we are more likely to blame them for being an inherently bad driver. However, if attention is called to our own driving, we are more likely to cite external factors such as rush hour, a bad driver around us, or a faulty vehicle.
We would like to reference one last model of human behavior. It has become customary within the field of neuroscience to view the brain as a predictive organ: “Theories of prediction in perception, action, and learning suggest that the brain serves to reduce the discrepancies between expectation and actual experience, i.e., by reducing the prediction error.”3 Perception itself has recently been described as a controlled hallucination, where the brain makes predictions of what it thinks it is about to see based on past experiences. Visual stimulus ultimately takes time to enter our eyes and be processed in the brain – “predictions would need to preactivate neural representations that would typically be driven by sensory input, before the actual arrival of that input.”4 It thus seems to be an inherent method of the brain to anticipate visual and even social events to help human beings sustain themselves.
Having spoken of a psychoanalytic conceptualization of self-organization, the theory of attribution, and research into social neuroscience, we turn our attention back to the central question that this article would like to address.
When we find ourselves busy in rote clinical practice, we believe the likelihood of intercollegiate mentalization is low; our ability to relate to our peers becomes strained. We ultimately do not practice in a vacuum. Psychiatrists, even those in a solo private practice, are ultimately part of a community of providers who, more or less, follow some emergent ‘standard of care.’ This can be a vague concept; but one that takes on a concrete form in the minds of certain clinicians and certainly in the setting of a medicolegal court. Yet, the psychiatrists that we know all have very stereotyped ways of practice. And at the heart of it, we all think that we are right.
We can use polypharmacy as an example. Imagine that you have a new patient intake, who tells you that they are transferring care from another psychiatrist. They inform you of their medication regimen. This patient presents on eight or more psychotropics. Many of us may have a visceral reaction at this point and, following the aforementioned attribution theory, we may ask ourselves what ‘quack’ of a doctor would do this. Yet some among us would think that a very competent psychopharmacologist was daring enough to use the full armamentarium of psychopharmacology to help this patient, who must be treatment refractory.
When speaking with such a patient, we would be quick to reflect on our own parsimonious use of medications. We would tell ourselves that we are responsible providers and would be quick to recommend discontinuation of medications. This would help us feel better about ourselves, and would of course assuage the ever-present medicolegal ‘big brother’ in our minds. It is through this very process that we affirm our self-identities. For if this patient’s previous physician was a bad psychiatrist, then we are a good psychiatrist. It is through this process that our clinical selves find confirmation.
We do not mean to reduce the complexities of human behavior to quick stereotypes. However, it is our belief that when confronted with clinical or philosophical disputes with our colleagues, the basic rules of human behavior will attempt to dissolve and override efforts at mentalization, collegiality, or interpersonal sensitivity. For to accept a clinical practice view that is different from ours would be akin to giving up the essence of our clinical identities. It could be compared to the fragmentation process of a vulnerable psyche when confronted with a reality that is at odds with preconceived notions and experiences.
While we may be able to appreciate the nuances and sensibilities of another provider, we believe it would be particularly difficult for most of us to actually attempt to practice in a fashion that is not congruent with our own organizers of experience. Whether or not our practice style is ‘perfect,’ it has worked for us. Social neuroscience and our understanding of the organization of the self would predict that we would hold onto our way of practice with all the mind’s defenses. Externalization, denial, and projection could all be called into action in this battle against existential fragmentation.
Do we seek to portray a clinical world where there is no hope for genuine modeling of clinical sensibilities to other psychiatrists? That is not our intention. Yet it seems that many of the theoretical frameworks that we subscribe to argue against this possibility. We would be hypocritical if we did not here state that our own theoretical frameworks are yet other examples of “organizers of experience.” Attribution theory, intersubjectivity, and social neuroscience are simply our ways of organizing the chaos of perceptions, ideas, and intricacies of human behavior.
If we accept that psychiatrists, like all human beings, are trapped in a subjective experience, then we can be more playful and flexible when interacting with our colleagues. We do not have to be as defensive of our practices and accusatory of others. If we practice daily according to some orthodoxy, then we color our experiences of the patient and of our colleagues’ ways of practice. We automatically start off on the wrong foot. And yet, to give up this orthodoxy would, by definition, be disorganizing and fragmenting to us. For as Nietzsche said, “truth is an illusion without which a certain species could not survive.”5
Dr. Khalafian practices full time as a general outpatient psychiatrist. He trained at the University of California, San Diego, for his psychiatric residency and currently works as a telepsychiatrist, serving an outpatient clinic population in northern California. Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Badre and Dr. Khalafian have no conflicts of interest.
References
1. Buirski P and Haglund P. Making sense together: The intersubjective approach to psychotherapy. Northvale, NJ: Jason Aronson; 2001.
2. Malle BF. Attribution theories: How people make sense of behavior. In Chadee D (ed.), Theories in social psychology. pp. 72-95. Wiley-Blackwell; 2011.
3. Brown EC and Brune M. The role of prediction in social neuroscience. Front Hum Neurosci. 2012 May 24;6:147. doi: 10.3389/fnhum.2012.00147.
4. Blom T et al. Predictions drive neural representations of visual events ahead of incoming sensory information. Proc Natl Acad Sci USA. 2020 Mar 31;117(13):7510-7515. doi: 10.1073/pnas.1917777117.
5. Yalom I. The Gift of Therapy. Harper Perennial; 2002.
Psychiatrists practice in a wide array of ways. We approach our work and our patients with beliefs and preconceptions that develop over time. Our training has significant influence, though our own personalities and biases also affect our understanding.
Psychiatrists have philosophical lenses through which they see patients. We can reflect and see some standard archetypes. We are familiar with the reductionistic pharmacologist, the somatic treatment specialist, the psychodynamic ‘guru,’ and the medicolegally paralyzed practitioner. It is without judgment that we lay these out, for our very point is that we have these constituent parts within our own clinical identities. The intensity with which we subscribe to these clinical sensibilities could contribute to a biased orthodoxy.
Orthodoxy can be defined as an accepted theory that stems from an authoritative entity. This is a well-known phenomenon that continues to be visible. For example, one can quickly peruse psychodynamic literature to find one school of thought criticizing another. It is not without some confrontation and even interpersonal rifts that the lineage of psychoanalytic theory has evolved. This has always been of interest to us. A core facet of psychoanalysis is empathy, truly knowing the inner state of a different person. And yet, the very bastions of this clinical sensibility frequently resort to veiled attacks on those in their field who have opposing views. It then begs the question: If even enlightened institutions fail at a nonjudgmental approach toward their colleagues, what hope is there for the rest of us clinicians, mired in the thick of day-to-day clinical practice?
It is our contention that the odds are against us. Even the aforementioned critique of psychoanalytic orthodoxy is just another example of how we humans organize our experience. Even as we write an article in argument against unbridled critique, we find it difficult to do so without engaging in it. For to criticize another is to help shore up our own personal identities. This is especially the case when clinicians deal with issues that we feel strongly about. The human psyche has a need to organize its experience, as “our experience of ourselves is fundamental to how we operate in the world. Our subjective experience is the phenomenology of all that one might be aware of.”1
In this vein, we would like to cite attribution theory. This is a view of human behavior within social psychology. The Austrian psychologist Fritz Heider, PhD, investigated “the domain of social interactions, wondering how people perceive each other in interaction and especially how they make sense of each other’s behavior.”2 Attribution theory suggests that as humans organize our social interactions, we may make two basic assumptions. One is that our own behavior is highly affected by an environment that is beyond our control. The second is that when judging the behavior of others, we are more likely to attribute it to internal traits that they have. A classic example is automobile traffic. When we see someone driving erratically, we are more likely to blame them for being an inherently bad driver. However, if attention is called to our own driving, we are more likely to cite external factors such as rush hour, a bad driver around us, or a faulty vehicle.
We would like to reference one last model of human behavior. It has become customary within the field of neuroscience to view the brain as a predictive organ: “Theories of prediction in perception, action, and learning suggest that the brain serves to reduce the discrepancies between expectation and actual experience, i.e., by reducing the prediction error.”3 Perception itself has recently been described as a controlled hallucination, where the brain makes predictions of what it thinks it is about to see based on past experiences. Visual stimulus ultimately takes time to enter our eyes and be processed in the brain – “predictions would need to preactivate neural representations that would typically be driven by sensory input, before the actual arrival of that input.”4 It thus seems to be an inherent method of the brain to anticipate visual and even social events to help human beings sustain themselves.
Having spoken of a psychoanalytic conceptualization of self-organization, the theory of attribution, and research into social neuroscience, we turn our attention back to the central question that this article would like to address.
When we find ourselves busy in rote clinical practice, we believe the likelihood of intercollegiate mentalization is low; our ability to relate to our peers becomes strained. We ultimately do not practice in a vacuum. Psychiatrists, even those in a solo private practice, are ultimately part of a community of providers who, more or less, follow some emergent ‘standard of care.’ This can be a vague concept; but one that takes on a concrete form in the minds of certain clinicians and certainly in the setting of a medicolegal court. Yet, the psychiatrists that we know all have very stereotyped ways of practice. And at the heart of it, we all think that we are right.
We can use polypharmacy as an example. Imagine that you have a new patient intake, who tells you that they are transferring care from another psychiatrist. They inform you of their medication regimen. This patient presents on eight or more psychotropics. Many of us may have a visceral reaction at this point and, following the aforementioned attribution theory, we may ask ourselves what ‘quack’ of a doctor would do this. Yet some among us would think that a very competent psychopharmacologist was daring enough to use the full armamentarium of psychopharmacology to help this patient, who must be treatment refractory.
When speaking with such a patient, we would be quick to reflect on our own parsimonious use of medications. We would tell ourselves that we are responsible providers and would be quick to recommend discontinuation of medications. This would help us feel better about ourselves, and would of course assuage the ever-present medicolegal ‘big brother’ in our minds. It is through this very process that we affirm our self-identities. For if this patient’s previous physician was a bad psychiatrist, then we are a good psychiatrist. It is through this process that our clinical selves find confirmation.
We do not mean to reduce the complexities of human behavior to quick stereotypes. However, it is our belief that when confronted with clinical or philosophical disputes with our colleagues, the basic rules of human behavior will attempt to dissolve and override efforts at mentalization, collegiality, or interpersonal sensitivity. For to accept a clinical practice view that is different from ours would be akin to giving up the essence of our clinical identities. It could be compared to the fragmentation process of a vulnerable psyche when confronted with a reality that is at odds with preconceived notions and experiences.
While we may be able to appreciate the nuances and sensibilities of another provider, we believe it would be particularly difficult for most of us to actually attempt to practice in a fashion that is not congruent with our own organizers of experience. Whether or not our practice style is ‘perfect,’ it has worked for us. Social neuroscience and our understanding of the organization of the self would predict that we would hold onto our way of practice with all the mind’s defenses. Externalization, denial, and projection could all be called into action in this battle against existential fragmentation.
Do we seek to portray a clinical world where there is no hope for genuine modeling of clinical sensibilities to other psychiatrists? That is not our intention. Yet it seems that many of the theoretical frameworks that we subscribe to argue against this possibility. We would be hypocritical if we did not here state that our own theoretical frameworks are yet other examples of “organizers of experience.” Attribution theory, intersubjectivity, and social neuroscience are simply our ways of organizing the chaos of perceptions, ideas, and intricacies of human behavior.
If we accept that psychiatrists, like all human beings, are trapped in a subjective experience, then we can be more playful and flexible when interacting with our colleagues. We do not have to be as defensive of our practices and accusatory of others. If we practice daily according to some orthodoxy, then we color our experiences of the patient and of our colleagues’ ways of practice. We automatically start off on the wrong foot. And yet, to give up this orthodoxy would, by definition, be disorganizing and fragmenting to us. For as Nietzsche said, “truth is an illusion without which a certain species could not survive.”5
Dr. Khalafian practices full time as a general outpatient psychiatrist. He trained at the University of California, San Diego, for his psychiatric residency and currently works as a telepsychiatrist, serving an outpatient clinic population in northern California. Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Badre and Dr. Khalafian have no conflicts of interest.
References
1. Buirski P and Haglund P. Making sense together: The intersubjective approach to psychotherapy. Northvale, NJ: Jason Aronson; 2001.
2. Malle BF. Attribution theories: How people make sense of behavior. In Chadee D (ed.), Theories in social psychology. pp. 72-95. Wiley-Blackwell; 2011.
3. Brown EC and Brune M. The role of prediction in social neuroscience. Front Hum Neurosci. 2012 May 24;6:147. doi: 10.3389/fnhum.2012.00147.
4. Blom T et al. Predictions drive neural representations of visual events ahead of incoming sensory information. Proc Natl Acad Sci USA. 2020 Mar 31;117(13):7510-7515. doi: 10.1073/pnas.1917777117.
5. Yalom I. The Gift of Therapy. Harper Perennial; 2002.
TNT: You need it, but guidelines won’t give it to you
Hi, everyone. I’m Dr Kenny Lin. I am a family physician and associate director of the Lancaster (Pa.) General Hospital Family Medicine Residency, and I blog at Common Sense Family Doctor.
For most of my career, I have precepted residents in primary care clinics. Generally, 1st years are scheduled to see a patient every 45 minutes on average, then every 30 minutes after the first 6 months. By the 3rd year, residents are scheduled to see a patient every 15 minutes to approximate the frequency at which patients are routinely seen in practice. Adult health maintenance visits are typically allotted two slots, for a total of 30 minutes.
The gradually increased pace of seeing patients is a challenge for many residents. It requires them to not only perform more focused medical histories and physical examinations but also to address a select handful of issues in patients who may have a long list of health concerns or preventive care needs. Prioritizing tasks into those that are necessary to do today vs. those that can be deferred to a follow-up visit is an essential skill that is equal parts art and science. In a previous commentary, I wrote about a research group’s efforts to create visual decision aids to generate individualized estimates of life expectancy gains from various preventive services.
Of course, it’s uncommon to have the luxury of focusing exclusively on preventive care in older adults, most of whom have one or more chronic conditions. Obesity, diabetes, hypertension, hypothyroidism, chronic obstructive pulmonary disease, coronary artery disease, and chronic kidney disease each has its own set of management guidelines. According to a recent estimate, following all guideline recommendations for chronic diseases plus those for preventive and acute care would require a primary care physician with a nationally representative panel of adult patients to work an impossible 27-hour day. That’s another good reason for me to continue seeing children in practice!
In a commentary in The BMJ, Dr Minna Johansson and colleagues argued that guideline panels should explicitly consider the estimated clinician time needed to improve the desired outcome for one person in the targeted population, a metric that they call “time needed to treat” (TNT). For example, to implement a National Institute for Health and Care Excellence (NICE) guideline for U.K. general practitioners (GPs) about providing advice to physically inactive adults would require 3 hours for one more person to increase their self-reported physical activity. For a patient panel of 2000 adults, the absolute TNT would be 167 hours per GP, representing 15% of yearly total face time with all patients, which seems clearly excessive.
In fact, the U.S. Preventive Services Task Force does occasionally consider the “opportunity costs” of recommending preventive services. When they first reviewed screening for chronic obstructive pulmonary disease (COPD) in 2008, the USPSTF reasoned that the minimal benefit of screening hundreds of patients to prevent a single COPD exacerbation was at least offset by the time and resources it would take to perform spirometry on every adult with a smoking history, a conclusion that it reaffirmed last year. In contrast to NICE, the USPSTF recommends selectively counseling adults without cardiovascular risk factors to promote a healthy diet and physical activity rather than counseling every single person.
Other US guideline groups would do well to adopt the advice of Johansson and colleagues to consider TNT. Last year, the Women’s Preventive Services Initiative (WPSI) recommended counseling every woman aged 40-60 years with normal or overweight body mass index “to maintain weight or limit weight gain to prevent obesity.” Though preventing obesity is a laudable goal, I’d prefer to counsel those who are obese and suffering from obesity-related conditions and spend my 15 or 30 minutes with others doing something more valuable, like listening to the patient. As Dr. Johansson and colleagues wrote in their commentary, “Healthcare policies also need to account for the time clinicians should spend listening in silence, noticing carefully, and cocreating sensible plans of care with patients.”
Having served on several guideline panels in the past, I believe that thoughtfully developed evidence-based guidelines can help family physicians care for patients. But guidelines will never make up all of medicine, particularly primary care, where following too many well-intended recommendations can sometimes get in the way of being a good doctor.
A version of this article first appeared on Medscape.com.
Hi, everyone. I’m Dr Kenny Lin. I am a family physician and associate director of the Lancaster (Pa.) General Hospital Family Medicine Residency, and I blog at Common Sense Family Doctor.
For most of my career, I have precepted residents in primary care clinics. Generally, 1st years are scheduled to see a patient every 45 minutes on average, then every 30 minutes after the first 6 months. By the 3rd year, residents are scheduled to see a patient every 15 minutes to approximate the frequency at which patients are routinely seen in practice. Adult health maintenance visits are typically allotted two slots, for a total of 30 minutes.
The gradually increased pace of seeing patients is a challenge for many residents. It requires them to not only perform more focused medical histories and physical examinations but also to address a select handful of issues in patients who may have a long list of health concerns or preventive care needs. Prioritizing tasks into those that are necessary to do today vs. those that can be deferred to a follow-up visit is an essential skill that is equal parts art and science. In a previous commentary, I wrote about a research group’s efforts to create visual decision aids to generate individualized estimates of life expectancy gains from various preventive services.
Of course, it’s uncommon to have the luxury of focusing exclusively on preventive care in older adults, most of whom have one or more chronic conditions. Obesity, diabetes, hypertension, hypothyroidism, chronic obstructive pulmonary disease, coronary artery disease, and chronic kidney disease each has its own set of management guidelines. According to a recent estimate, following all guideline recommendations for chronic diseases plus those for preventive and acute care would require a primary care physician with a nationally representative panel of adult patients to work an impossible 27-hour day. That’s another good reason for me to continue seeing children in practice!
In a commentary in The BMJ, Dr Minna Johansson and colleagues argued that guideline panels should explicitly consider the estimated clinician time needed to improve the desired outcome for one person in the targeted population, a metric that they call “time needed to treat” (TNT). For example, to implement a National Institute for Health and Care Excellence (NICE) guideline for U.K. general practitioners (GPs) about providing advice to physically inactive adults would require 3 hours for one more person to increase their self-reported physical activity. For a patient panel of 2000 adults, the absolute TNT would be 167 hours per GP, representing 15% of yearly total face time with all patients, which seems clearly excessive.
In fact, the U.S. Preventive Services Task Force does occasionally consider the “opportunity costs” of recommending preventive services. When they first reviewed screening for chronic obstructive pulmonary disease (COPD) in 2008, the USPSTF reasoned that the minimal benefit of screening hundreds of patients to prevent a single COPD exacerbation was at least offset by the time and resources it would take to perform spirometry on every adult with a smoking history, a conclusion that it reaffirmed last year. In contrast to NICE, the USPSTF recommends selectively counseling adults without cardiovascular risk factors to promote a healthy diet and physical activity rather than counseling every single person.
Other US guideline groups would do well to adopt the advice of Johansson and colleagues to consider TNT. Last year, the Women’s Preventive Services Initiative (WPSI) recommended counseling every woman aged 40-60 years with normal or overweight body mass index “to maintain weight or limit weight gain to prevent obesity.” Though preventing obesity is a laudable goal, I’d prefer to counsel those who are obese and suffering from obesity-related conditions and spend my 15 or 30 minutes with others doing something more valuable, like listening to the patient. As Dr. Johansson and colleagues wrote in their commentary, “Healthcare policies also need to account for the time clinicians should spend listening in silence, noticing carefully, and cocreating sensible plans of care with patients.”
Having served on several guideline panels in the past, I believe that thoughtfully developed evidence-based guidelines can help family physicians care for patients. But guidelines will never make up all of medicine, particularly primary care, where following too many well-intended recommendations can sometimes get in the way of being a good doctor.
A version of this article first appeared on Medscape.com.
Hi, everyone. I’m Dr Kenny Lin. I am a family physician and associate director of the Lancaster (Pa.) General Hospital Family Medicine Residency, and I blog at Common Sense Family Doctor.
For most of my career, I have precepted residents in primary care clinics. Generally, 1st years are scheduled to see a patient every 45 minutes on average, then every 30 minutes after the first 6 months. By the 3rd year, residents are scheduled to see a patient every 15 minutes to approximate the frequency at which patients are routinely seen in practice. Adult health maintenance visits are typically allotted two slots, for a total of 30 minutes.
The gradually increased pace of seeing patients is a challenge for many residents. It requires them to not only perform more focused medical histories and physical examinations but also to address a select handful of issues in patients who may have a long list of health concerns or preventive care needs. Prioritizing tasks into those that are necessary to do today vs. those that can be deferred to a follow-up visit is an essential skill that is equal parts art and science. In a previous commentary, I wrote about a research group’s efforts to create visual decision aids to generate individualized estimates of life expectancy gains from various preventive services.
Of course, it’s uncommon to have the luxury of focusing exclusively on preventive care in older adults, most of whom have one or more chronic conditions. Obesity, diabetes, hypertension, hypothyroidism, chronic obstructive pulmonary disease, coronary artery disease, and chronic kidney disease each has its own set of management guidelines. According to a recent estimate, following all guideline recommendations for chronic diseases plus those for preventive and acute care would require a primary care physician with a nationally representative panel of adult patients to work an impossible 27-hour day. That’s another good reason for me to continue seeing children in practice!
In a commentary in The BMJ, Dr Minna Johansson and colleagues argued that guideline panels should explicitly consider the estimated clinician time needed to improve the desired outcome for one person in the targeted population, a metric that they call “time needed to treat” (TNT). For example, to implement a National Institute for Health and Care Excellence (NICE) guideline for U.K. general practitioners (GPs) about providing advice to physically inactive adults would require 3 hours for one more person to increase their self-reported physical activity. For a patient panel of 2000 adults, the absolute TNT would be 167 hours per GP, representing 15% of yearly total face time with all patients, which seems clearly excessive.
In fact, the U.S. Preventive Services Task Force does occasionally consider the “opportunity costs” of recommending preventive services. When they first reviewed screening for chronic obstructive pulmonary disease (COPD) in 2008, the USPSTF reasoned that the minimal benefit of screening hundreds of patients to prevent a single COPD exacerbation was at least offset by the time and resources it would take to perform spirometry on every adult with a smoking history, a conclusion that it reaffirmed last year. In contrast to NICE, the USPSTF recommends selectively counseling adults without cardiovascular risk factors to promote a healthy diet and physical activity rather than counseling every single person.
Other US guideline groups would do well to adopt the advice of Johansson and colleagues to consider TNT. Last year, the Women’s Preventive Services Initiative (WPSI) recommended counseling every woman aged 40-60 years with normal or overweight body mass index “to maintain weight or limit weight gain to prevent obesity.” Though preventing obesity is a laudable goal, I’d prefer to counsel those who are obese and suffering from obesity-related conditions and spend my 15 or 30 minutes with others doing something more valuable, like listening to the patient. As Dr. Johansson and colleagues wrote in their commentary, “Healthcare policies also need to account for the time clinicians should spend listening in silence, noticing carefully, and cocreating sensible plans of care with patients.”
Having served on several guideline panels in the past, I believe that thoughtfully developed evidence-based guidelines can help family physicians care for patients. But guidelines will never make up all of medicine, particularly primary care, where following too many well-intended recommendations can sometimes get in the way of being a good doctor.
A version of this article first appeared on Medscape.com.
The end of the telemedicine era?
I started taking care of Jim, a 68-year-old man with metastatic renal cell carcinoma back in the fall of 2018. Jim lived far from our clinic in the rural western Sierra Mountains and had a hard time getting to Santa Monica, but needed ongoing pain and symptom management, as well as follow-up visits with oncology and discussions with our teams about preparing for the end of life.
Luckily for Jim, the Centers for Medicare & Medicaid Services had relaxed the rules around telehealth because of the public health emergency, and we were easily able to provide telemedicine visits throughout the pandemic ensuring that Jim retained access to the care team that had managed his cancer for several years at that point. This would not have been possible without the use of telemedicine – at least not without great effort and expense by Jim to make frequent trips to our Santa Monica clinic.
So, you can imagine my apprehension when I received an email the other day from our billing department, informing billing providers like myself that “telehealth visits are still covered through the end of the year.” While this initially seemed like reassuring news, it immediately begged the question – what happens at the end of the year? What will care look like for patients like Jim who live at a significant distance from their providers?
The end of the COVID-19 public health emergency on May 11 has prompted states to reevaluate the future of telehealth for Medicaid and Medicare recipients. Most states plan to make some telehealth services permanent, particularly in rural areas. While other telehealth services have been extended through Dec. 31, 2024, under the Consolidated Appropriations Act of 2023.
But still, We can now see very ill patients in their own homes without imposing an undue burden on them to come in for yet another office visit. Prior to the public health emergency, our embedded palliative care program would see patients only when they were in the oncology clinic so as to not burden them with having to travel to yet another clinic. This made our palliative providers less efficient since patients were being seen by multiple providers in the same space, which led to some time spent waiting around. It also frequently tied up our clinic exam rooms for long periods of time, delaying care for patients sitting in the waiting room.
Telehealth changed that virtually overnight. With the widespread availability of smartphones and tablets, patients could stay at home and speak more comfortably in their own surroundings – especially about the difficult topics we tend to dig into in palliative care – such as fears, suffering, grief, loss, legacy, regret, trauma, gratitude, dying – without the impersonal, aseptic environment of a clinic. We could visit with their family/caregivers, kids, and their pets. We could tour their living space and see how they were managing from a functional standpoint. We could get to know aspects of our patients’ lives that we’d never have seen in the clinic that could help us understand their goals and values better and help care for them more fully.
The benefit to the institution was also measurable. We could see our patients faster – the time from referral to consult dropped dramatically because patients could be scheduled for next-day virtual visits instead of having to wait for them to come back to an oncology visit. We could do quick symptom-focused visits that prior to telehealth would have been conducted by phone without the ability to perform at the very least an observational physical exam of the patient, which is important when prescribing medications to medically frail populations.
If telemedicine goes, how will it affect outpatient palliative care?
If that goes away, I do not know what will happen to outpatient palliative care. I can tell you we will be much less efficient in terms of when we see patients. There will probably be a higher clinic burden to patients, as well as higher financial toxicity to patients (Parking in the structure attached to my office building is $22 per day). And, what about the uncaptured costs associated with transportation for those whose illness prevents them from driving themselves? This can range from Uber costs to the time cost for a patient’s family member to take off work and arrange for childcare in order to drive the patient to a clinic for a visit.
In February, I received emails from the Drug Enforcement Agency suggesting that they, too, may roll back providers’ ability to prescribe controlled substances to patients who are mainly receiving telehealth services. While I understand and fully support the need to curb inappropriate overprescribing of controlled medications, I am concerned about the unintended consequences to cancer patients who live at a remote distance from their oncologists and palliative care providers. I remain hopeful that DEA will consider a carveout exception for those patients who have cancer, are receiving palliative care services, or are deemed to be at the end of life, much like the chronic opioid guidelines developed by the Centers for Disease Control and Prevention have done.
Telemedicine in essential care
Back to Jim. Using telehealth and electronic prescribing, our oncology and palliative care programs were able to keep Jim comfortable and at home through the end of his life. He did not have to travel 3 hours each way to get care. He did not have to spend money on parking and gas, and his daughter did not have to take days off work and arrange for a babysitter in order to drive him to our clinic. We partnered with a local pharmacy that was willing to special order medications for Jim when his pain became worse and he required a long-acting opioid. We partnered with a local home health company that kept a close eye on Jim and let us know when he seemed to be declining further, prompting discussions about transitioning to hospice.
I’m proud of the fact that our group helped Jim stay in comfortable surroundings and out of the clinic and hospital over the last 6 months of his life, but that would never have happened without the safe and thoughtful use of telehealth by our team.
Ironically, because of a public health emergency, we were able to provide efficient and high-quality palliative care at the right time, to the right person, in the right place, satisfying CMS goals to provide better care for patients and whole populations at lower costs.
Ms. D’Ambruoso is a hospice and palliative care nurse practitioner for UCLA Health Cancer Care, Santa Monica, Calif.
I started taking care of Jim, a 68-year-old man with metastatic renal cell carcinoma back in the fall of 2018. Jim lived far from our clinic in the rural western Sierra Mountains and had a hard time getting to Santa Monica, but needed ongoing pain and symptom management, as well as follow-up visits with oncology and discussions with our teams about preparing for the end of life.
Luckily for Jim, the Centers for Medicare & Medicaid Services had relaxed the rules around telehealth because of the public health emergency, and we were easily able to provide telemedicine visits throughout the pandemic ensuring that Jim retained access to the care team that had managed his cancer for several years at that point. This would not have been possible without the use of telemedicine – at least not without great effort and expense by Jim to make frequent trips to our Santa Monica clinic.
So, you can imagine my apprehension when I received an email the other day from our billing department, informing billing providers like myself that “telehealth visits are still covered through the end of the year.” While this initially seemed like reassuring news, it immediately begged the question – what happens at the end of the year? What will care look like for patients like Jim who live at a significant distance from their providers?
The end of the COVID-19 public health emergency on May 11 has prompted states to reevaluate the future of telehealth for Medicaid and Medicare recipients. Most states plan to make some telehealth services permanent, particularly in rural areas. While other telehealth services have been extended through Dec. 31, 2024, under the Consolidated Appropriations Act of 2023.
But still, We can now see very ill patients in their own homes without imposing an undue burden on them to come in for yet another office visit. Prior to the public health emergency, our embedded palliative care program would see patients only when they were in the oncology clinic so as to not burden them with having to travel to yet another clinic. This made our palliative providers less efficient since patients were being seen by multiple providers in the same space, which led to some time spent waiting around. It also frequently tied up our clinic exam rooms for long periods of time, delaying care for patients sitting in the waiting room.
Telehealth changed that virtually overnight. With the widespread availability of smartphones and tablets, patients could stay at home and speak more comfortably in their own surroundings – especially about the difficult topics we tend to dig into in palliative care – such as fears, suffering, grief, loss, legacy, regret, trauma, gratitude, dying – without the impersonal, aseptic environment of a clinic. We could visit with their family/caregivers, kids, and their pets. We could tour their living space and see how they were managing from a functional standpoint. We could get to know aspects of our patients’ lives that we’d never have seen in the clinic that could help us understand their goals and values better and help care for them more fully.
The benefit to the institution was also measurable. We could see our patients faster – the time from referral to consult dropped dramatically because patients could be scheduled for next-day virtual visits instead of having to wait for them to come back to an oncology visit. We could do quick symptom-focused visits that prior to telehealth would have been conducted by phone without the ability to perform at the very least an observational physical exam of the patient, which is important when prescribing medications to medically frail populations.
If telemedicine goes, how will it affect outpatient palliative care?
If that goes away, I do not know what will happen to outpatient palliative care. I can tell you we will be much less efficient in terms of when we see patients. There will probably be a higher clinic burden to patients, as well as higher financial toxicity to patients (Parking in the structure attached to my office building is $22 per day). And, what about the uncaptured costs associated with transportation for those whose illness prevents them from driving themselves? This can range from Uber costs to the time cost for a patient’s family member to take off work and arrange for childcare in order to drive the patient to a clinic for a visit.
In February, I received emails from the Drug Enforcement Agency suggesting that they, too, may roll back providers’ ability to prescribe controlled substances to patients who are mainly receiving telehealth services. While I understand and fully support the need to curb inappropriate overprescribing of controlled medications, I am concerned about the unintended consequences to cancer patients who live at a remote distance from their oncologists and palliative care providers. I remain hopeful that DEA will consider a carveout exception for those patients who have cancer, are receiving palliative care services, or are deemed to be at the end of life, much like the chronic opioid guidelines developed by the Centers for Disease Control and Prevention have done.
Telemedicine in essential care
Back to Jim. Using telehealth and electronic prescribing, our oncology and palliative care programs were able to keep Jim comfortable and at home through the end of his life. He did not have to travel 3 hours each way to get care. He did not have to spend money on parking and gas, and his daughter did not have to take days off work and arrange for a babysitter in order to drive him to our clinic. We partnered with a local pharmacy that was willing to special order medications for Jim when his pain became worse and he required a long-acting opioid. We partnered with a local home health company that kept a close eye on Jim and let us know when he seemed to be declining further, prompting discussions about transitioning to hospice.
I’m proud of the fact that our group helped Jim stay in comfortable surroundings and out of the clinic and hospital over the last 6 months of his life, but that would never have happened without the safe and thoughtful use of telehealth by our team.
Ironically, because of a public health emergency, we were able to provide efficient and high-quality palliative care at the right time, to the right person, in the right place, satisfying CMS goals to provide better care for patients and whole populations at lower costs.
Ms. D’Ambruoso is a hospice and palliative care nurse practitioner for UCLA Health Cancer Care, Santa Monica, Calif.
I started taking care of Jim, a 68-year-old man with metastatic renal cell carcinoma back in the fall of 2018. Jim lived far from our clinic in the rural western Sierra Mountains and had a hard time getting to Santa Monica, but needed ongoing pain and symptom management, as well as follow-up visits with oncology and discussions with our teams about preparing for the end of life.
Luckily for Jim, the Centers for Medicare & Medicaid Services had relaxed the rules around telehealth because of the public health emergency, and we were easily able to provide telemedicine visits throughout the pandemic ensuring that Jim retained access to the care team that had managed his cancer for several years at that point. This would not have been possible without the use of telemedicine – at least not without great effort and expense by Jim to make frequent trips to our Santa Monica clinic.
So, you can imagine my apprehension when I received an email the other day from our billing department, informing billing providers like myself that “telehealth visits are still covered through the end of the year.” While this initially seemed like reassuring news, it immediately begged the question – what happens at the end of the year? What will care look like for patients like Jim who live at a significant distance from their providers?
The end of the COVID-19 public health emergency on May 11 has prompted states to reevaluate the future of telehealth for Medicaid and Medicare recipients. Most states plan to make some telehealth services permanent, particularly in rural areas. While other telehealth services have been extended through Dec. 31, 2024, under the Consolidated Appropriations Act of 2023.
But still, We can now see very ill patients in their own homes without imposing an undue burden on them to come in for yet another office visit. Prior to the public health emergency, our embedded palliative care program would see patients only when they were in the oncology clinic so as to not burden them with having to travel to yet another clinic. This made our palliative providers less efficient since patients were being seen by multiple providers in the same space, which led to some time spent waiting around. It also frequently tied up our clinic exam rooms for long periods of time, delaying care for patients sitting in the waiting room.
Telehealth changed that virtually overnight. With the widespread availability of smartphones and tablets, patients could stay at home and speak more comfortably in their own surroundings – especially about the difficult topics we tend to dig into in palliative care – such as fears, suffering, grief, loss, legacy, regret, trauma, gratitude, dying – without the impersonal, aseptic environment of a clinic. We could visit with their family/caregivers, kids, and their pets. We could tour their living space and see how they were managing from a functional standpoint. We could get to know aspects of our patients’ lives that we’d never have seen in the clinic that could help us understand their goals and values better and help care for them more fully.
The benefit to the institution was also measurable. We could see our patients faster – the time from referral to consult dropped dramatically because patients could be scheduled for next-day virtual visits instead of having to wait for them to come back to an oncology visit. We could do quick symptom-focused visits that prior to telehealth would have been conducted by phone without the ability to perform at the very least an observational physical exam of the patient, which is important when prescribing medications to medically frail populations.
If telemedicine goes, how will it affect outpatient palliative care?
If that goes away, I do not know what will happen to outpatient palliative care. I can tell you we will be much less efficient in terms of when we see patients. There will probably be a higher clinic burden to patients, as well as higher financial toxicity to patients (Parking in the structure attached to my office building is $22 per day). And, what about the uncaptured costs associated with transportation for those whose illness prevents them from driving themselves? This can range from Uber costs to the time cost for a patient’s family member to take off work and arrange for childcare in order to drive the patient to a clinic for a visit.
In February, I received emails from the Drug Enforcement Agency suggesting that they, too, may roll back providers’ ability to prescribe controlled substances to patients who are mainly receiving telehealth services. While I understand and fully support the need to curb inappropriate overprescribing of controlled medications, I am concerned about the unintended consequences to cancer patients who live at a remote distance from their oncologists and palliative care providers. I remain hopeful that DEA will consider a carveout exception for those patients who have cancer, are receiving palliative care services, or are deemed to be at the end of life, much like the chronic opioid guidelines developed by the Centers for Disease Control and Prevention have done.
Telemedicine in essential care
Back to Jim. Using telehealth and electronic prescribing, our oncology and palliative care programs were able to keep Jim comfortable and at home through the end of his life. He did not have to travel 3 hours each way to get care. He did not have to spend money on parking and gas, and his daughter did not have to take days off work and arrange for a babysitter in order to drive him to our clinic. We partnered with a local pharmacy that was willing to special order medications for Jim when his pain became worse and he required a long-acting opioid. We partnered with a local home health company that kept a close eye on Jim and let us know when he seemed to be declining further, prompting discussions about transitioning to hospice.
I’m proud of the fact that our group helped Jim stay in comfortable surroundings and out of the clinic and hospital over the last 6 months of his life, but that would never have happened without the safe and thoughtful use of telehealth by our team.
Ironically, because of a public health emergency, we were able to provide efficient and high-quality palliative care at the right time, to the right person, in the right place, satisfying CMS goals to provide better care for patients and whole populations at lower costs.
Ms. D’Ambruoso is a hospice and palliative care nurse practitioner for UCLA Health Cancer Care, Santa Monica, Calif.
Take time to relax and enjoy the ride
This past weekend was one of my least-favorite parts of the annual cycle: I shut off and drained my hot tub.
I’ve always loved sitting in hot tubs, as far back as I can remember. Growing up on family vacations I preferred them to the pool. So when I was grown up and could afford one, I got it for my house.
I spend my winter weekend afternoons relaxing in it with a can of beer, some bottles of iced tea, and a pile of journals or a book. I put instrumental jazz on my phone and spend a few pleasant hours there, catching up on my reading.
But, as the Phoenix weather swings back to summer temps, it’s time to turn it off until next November.
It’s interesting the ways we mark the passage of time in our lives. The traditional standards are New Year’s, major holidays, and birthdays. Some may mark it by their favorite sports seasons starting.
In medicine we may mark it by patient ages, or a drug that we thought just came to market now going generic, or realizing our state or DEA license is up for renewal.
It doesn’t really matter how you mark the time – it’s going to happen whether you do or don’t. The person you see in the mirror is the same one there since you were tall enough to see over the bathroom countertop. Isn’t it just the ones around us who change?
As Phoenix moves back to a summer footing, and as someone who’s been through 56 of them, it’s hard not to think about it. Summer vacations growing up, summer classes in college, summer elective rotations in medical school. Now I work year-round and watch the same cycle play out with my kids in college.
You often hear the phrase “a hundred years from now it won’t make a difference.” Probably true. In 2123 the time I spent relaxing in my hot tub won’t mean anything, or be remembered by anyone.
But I’m not sitting in it to think about that. I’m in it because I have what I have now, and none of us will ever have that again. And part of that, to me, is enjoying some time in the hot tub.
Because That may not matter in one hundred years, but it matters to me today. And that’s what’s really important.
To all of us.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
This past weekend was one of my least-favorite parts of the annual cycle: I shut off and drained my hot tub.
I’ve always loved sitting in hot tubs, as far back as I can remember. Growing up on family vacations I preferred them to the pool. So when I was grown up and could afford one, I got it for my house.
I spend my winter weekend afternoons relaxing in it with a can of beer, some bottles of iced tea, and a pile of journals or a book. I put instrumental jazz on my phone and spend a few pleasant hours there, catching up on my reading.
But, as the Phoenix weather swings back to summer temps, it’s time to turn it off until next November.
It’s interesting the ways we mark the passage of time in our lives. The traditional standards are New Year’s, major holidays, and birthdays. Some may mark it by their favorite sports seasons starting.
In medicine we may mark it by patient ages, or a drug that we thought just came to market now going generic, or realizing our state or DEA license is up for renewal.
It doesn’t really matter how you mark the time – it’s going to happen whether you do or don’t. The person you see in the mirror is the same one there since you were tall enough to see over the bathroom countertop. Isn’t it just the ones around us who change?
As Phoenix moves back to a summer footing, and as someone who’s been through 56 of them, it’s hard not to think about it. Summer vacations growing up, summer classes in college, summer elective rotations in medical school. Now I work year-round and watch the same cycle play out with my kids in college.
You often hear the phrase “a hundred years from now it won’t make a difference.” Probably true. In 2123 the time I spent relaxing in my hot tub won’t mean anything, or be remembered by anyone.
But I’m not sitting in it to think about that. I’m in it because I have what I have now, and none of us will ever have that again. And part of that, to me, is enjoying some time in the hot tub.
Because That may not matter in one hundred years, but it matters to me today. And that’s what’s really important.
To all of us.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
This past weekend was one of my least-favorite parts of the annual cycle: I shut off and drained my hot tub.
I’ve always loved sitting in hot tubs, as far back as I can remember. Growing up on family vacations I preferred them to the pool. So when I was grown up and could afford one, I got it for my house.
I spend my winter weekend afternoons relaxing in it with a can of beer, some bottles of iced tea, and a pile of journals or a book. I put instrumental jazz on my phone and spend a few pleasant hours there, catching up on my reading.
But, as the Phoenix weather swings back to summer temps, it’s time to turn it off until next November.
It’s interesting the ways we mark the passage of time in our lives. The traditional standards are New Year’s, major holidays, and birthdays. Some may mark it by their favorite sports seasons starting.
In medicine we may mark it by patient ages, or a drug that we thought just came to market now going generic, or realizing our state or DEA license is up for renewal.
It doesn’t really matter how you mark the time – it’s going to happen whether you do or don’t. The person you see in the mirror is the same one there since you were tall enough to see over the bathroom countertop. Isn’t it just the ones around us who change?
As Phoenix moves back to a summer footing, and as someone who’s been through 56 of them, it’s hard not to think about it. Summer vacations growing up, summer classes in college, summer elective rotations in medical school. Now I work year-round and watch the same cycle play out with my kids in college.
You often hear the phrase “a hundred years from now it won’t make a difference.” Probably true. In 2123 the time I spent relaxing in my hot tub won’t mean anything, or be remembered by anyone.
But I’m not sitting in it to think about that. I’m in it because I have what I have now, and none of us will ever have that again. And part of that, to me, is enjoying some time in the hot tub.
Because That may not matter in one hundred years, but it matters to me today. And that’s what’s really important.
To all of us.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Are parents infecting their children with contagious negativity?
A couple of weeks ago I stumbled across a report of a Pew Research Center’s survey titled “Parenting in America today” (Pew Research Center. Jan. 24, 2023), which found that 40% of parents in the United States with children younger than 18 are “extremely or very worried” that at some point their children might struggle with anxiety or depression. Thirty-six percent replied that they were “somewhat” worried. This total of more than 75% represents a significant change from the 2015 Pew Center survey in which only 54% of parents were “somewhat” worried about their children’s mental health.
Prompted by these findings I began work on a column in which I planned to encourage pediatricians to think more like family physicians when we were working with children who were experiencing serious mental health problems. My primary message was going to be that we should turn more of our attention to the mental health of the anxious parents who must endure the often long and frustrating path toward effective psychiatric care for their children. This might come in the form of some simple suggestions about nonpharmacologic self-help strategies. Or, it could mean encouraging parents to seek psychiatric care or counseling for themselves as they wait for help for their child.
However, as I began that column, my thoughts kept drifting toward a broader consideration of the relationship between parents and pediatric mental health. If mental health of children is causing their parents to be anxious and depressed isn’t it just as likely that this is a bidirectional connection? This was not exactly an “aha” moment for me because it is a relationship I have considered for sometime. However, it is a concept that I have come to realize is receiving far too little attention.
There are exceptions. For example, a recent opinion piece in the New York Times by David French, “What if Kids Are Sad and Stressed Because Their Parents Are?” (March 19, 2023) echoes many of my concerns. Drawing on his experiences traveling around college campuses, Mr. French observes, “Just as parents are upset about their children’s anxiety and depression, children are anxious about their parent’s mental health.”
He notes that an August 2022 NBC News poll found that 58% of registered voters feel this country’s best days are behind it and joins me in imagining that this negative mind set is filtering down to the pediatric population. He acknowledges that there are other likely contributors to teen unhappiness including the ubiquity of smart phones, the secularization of society, and the media’s focus on the political divide. However, Mr. French wonders if the parenting style that results in childhood experiences that are dominated by adult supervision and protection may also be playing a large role.
In his conclusion, Mr. French asks us to consider “How much fear and anxiety should we import to our lives and homes?” as we adults search for an answer.
As I continued to drill down for other possible solutions, I encountered an avenue of psychological research that suggests that instead of, or in addition to, filtering out the anxiety-generating deluge of information, we begin to give some thought to how our beliefs may be coloring our perception of reality.
Jeremy D.W. Clifton, PhD, a psychologist at the University of Pennsylvania Positive Psychology Center has done extensive research on the relationship between our basic beliefs about the world (known as primal beliefs or simply primals in psychologist lingo) and how we interpret reality. For example, one of your primal beliefs may be that the world is a dangerous place. I, on the other hand, may see the world as a stimulating environment offering me endless opportunities to explore. I may see the world as an abundant resource limited only by my creativity. You, however, see it as a barren wasteland.
Dr. Clifton’s research has shown that our primals (at least those of adults) are relatively immutable through one’s lifetime and “do not appear to be the consequence of our experiences.” For example, living in a ZIP code with a high crime rate does not predict that you will see the world as a dangerous place. Nor does being affluent guarantee that an adult sees the world rich with opportunities.
It is unclear exactly when and by what process we develop our primal beliefs, but it is safe to say our parents probably play a large role. Exactly to what degree the tsunami of bad news we are allowing to inundate our children’s lives plays a role is unclear. However, it is reasonable to assume that news about climate change, school shootings, and the pandemic must be a contributor.
According to Dr. Clifton, there is some evidence that certain mind exercises, when applied diligently, can occasionally modify the primal beliefs of an individual who sees the world as dangerous and barren. Until such strategies become more readily accessible, the best we can do is acknowledge that our children are like canaries in a coal mine full of negative perceptions, then do our best to clear the air.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
A couple of weeks ago I stumbled across a report of a Pew Research Center’s survey titled “Parenting in America today” (Pew Research Center. Jan. 24, 2023), which found that 40% of parents in the United States with children younger than 18 are “extremely or very worried” that at some point their children might struggle with anxiety or depression. Thirty-six percent replied that they were “somewhat” worried. This total of more than 75% represents a significant change from the 2015 Pew Center survey in which only 54% of parents were “somewhat” worried about their children’s mental health.
Prompted by these findings I began work on a column in which I planned to encourage pediatricians to think more like family physicians when we were working with children who were experiencing serious mental health problems. My primary message was going to be that we should turn more of our attention to the mental health of the anxious parents who must endure the often long and frustrating path toward effective psychiatric care for their children. This might come in the form of some simple suggestions about nonpharmacologic self-help strategies. Or, it could mean encouraging parents to seek psychiatric care or counseling for themselves as they wait for help for their child.
However, as I began that column, my thoughts kept drifting toward a broader consideration of the relationship between parents and pediatric mental health. If mental health of children is causing their parents to be anxious and depressed isn’t it just as likely that this is a bidirectional connection? This was not exactly an “aha” moment for me because it is a relationship I have considered for sometime. However, it is a concept that I have come to realize is receiving far too little attention.
There are exceptions. For example, a recent opinion piece in the New York Times by David French, “What if Kids Are Sad and Stressed Because Their Parents Are?” (March 19, 2023) echoes many of my concerns. Drawing on his experiences traveling around college campuses, Mr. French observes, “Just as parents are upset about their children’s anxiety and depression, children are anxious about their parent’s mental health.”
He notes that an August 2022 NBC News poll found that 58% of registered voters feel this country’s best days are behind it and joins me in imagining that this negative mind set is filtering down to the pediatric population. He acknowledges that there are other likely contributors to teen unhappiness including the ubiquity of smart phones, the secularization of society, and the media’s focus on the political divide. However, Mr. French wonders if the parenting style that results in childhood experiences that are dominated by adult supervision and protection may also be playing a large role.
In his conclusion, Mr. French asks us to consider “How much fear and anxiety should we import to our lives and homes?” as we adults search for an answer.
As I continued to drill down for other possible solutions, I encountered an avenue of psychological research that suggests that instead of, or in addition to, filtering out the anxiety-generating deluge of information, we begin to give some thought to how our beliefs may be coloring our perception of reality.
Jeremy D.W. Clifton, PhD, a psychologist at the University of Pennsylvania Positive Psychology Center has done extensive research on the relationship between our basic beliefs about the world (known as primal beliefs or simply primals in psychologist lingo) and how we interpret reality. For example, one of your primal beliefs may be that the world is a dangerous place. I, on the other hand, may see the world as a stimulating environment offering me endless opportunities to explore. I may see the world as an abundant resource limited only by my creativity. You, however, see it as a barren wasteland.
Dr. Clifton’s research has shown that our primals (at least those of adults) are relatively immutable through one’s lifetime and “do not appear to be the consequence of our experiences.” For example, living in a ZIP code with a high crime rate does not predict that you will see the world as a dangerous place. Nor does being affluent guarantee that an adult sees the world rich with opportunities.
It is unclear exactly when and by what process we develop our primal beliefs, but it is safe to say our parents probably play a large role. Exactly to what degree the tsunami of bad news we are allowing to inundate our children’s lives plays a role is unclear. However, it is reasonable to assume that news about climate change, school shootings, and the pandemic must be a contributor.
According to Dr. Clifton, there is some evidence that certain mind exercises, when applied diligently, can occasionally modify the primal beliefs of an individual who sees the world as dangerous and barren. Until such strategies become more readily accessible, the best we can do is acknowledge that our children are like canaries in a coal mine full of negative perceptions, then do our best to clear the air.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
A couple of weeks ago I stumbled across a report of a Pew Research Center’s survey titled “Parenting in America today” (Pew Research Center. Jan. 24, 2023), which found that 40% of parents in the United States with children younger than 18 are “extremely or very worried” that at some point their children might struggle with anxiety or depression. Thirty-six percent replied that they were “somewhat” worried. This total of more than 75% represents a significant change from the 2015 Pew Center survey in which only 54% of parents were “somewhat” worried about their children’s mental health.
Prompted by these findings I began work on a column in which I planned to encourage pediatricians to think more like family physicians when we were working with children who were experiencing serious mental health problems. My primary message was going to be that we should turn more of our attention to the mental health of the anxious parents who must endure the often long and frustrating path toward effective psychiatric care for their children. This might come in the form of some simple suggestions about nonpharmacologic self-help strategies. Or, it could mean encouraging parents to seek psychiatric care or counseling for themselves as they wait for help for their child.
However, as I began that column, my thoughts kept drifting toward a broader consideration of the relationship between parents and pediatric mental health. If mental health of children is causing their parents to be anxious and depressed isn’t it just as likely that this is a bidirectional connection? This was not exactly an “aha” moment for me because it is a relationship I have considered for sometime. However, it is a concept that I have come to realize is receiving far too little attention.
There are exceptions. For example, a recent opinion piece in the New York Times by David French, “What if Kids Are Sad and Stressed Because Their Parents Are?” (March 19, 2023) echoes many of my concerns. Drawing on his experiences traveling around college campuses, Mr. French observes, “Just as parents are upset about their children’s anxiety and depression, children are anxious about their parent’s mental health.”
He notes that an August 2022 NBC News poll found that 58% of registered voters feel this country’s best days are behind it and joins me in imagining that this negative mind set is filtering down to the pediatric population. He acknowledges that there are other likely contributors to teen unhappiness including the ubiquity of smart phones, the secularization of society, and the media’s focus on the political divide. However, Mr. French wonders if the parenting style that results in childhood experiences that are dominated by adult supervision and protection may also be playing a large role.
In his conclusion, Mr. French asks us to consider “How much fear and anxiety should we import to our lives and homes?” as we adults search for an answer.
As I continued to drill down for other possible solutions, I encountered an avenue of psychological research that suggests that instead of, or in addition to, filtering out the anxiety-generating deluge of information, we begin to give some thought to how our beliefs may be coloring our perception of reality.
Jeremy D.W. Clifton, PhD, a psychologist at the University of Pennsylvania Positive Psychology Center has done extensive research on the relationship between our basic beliefs about the world (known as primal beliefs or simply primals in psychologist lingo) and how we interpret reality. For example, one of your primal beliefs may be that the world is a dangerous place. I, on the other hand, may see the world as a stimulating environment offering me endless opportunities to explore. I may see the world as an abundant resource limited only by my creativity. You, however, see it as a barren wasteland.
Dr. Clifton’s research has shown that our primals (at least those of adults) are relatively immutable through one’s lifetime and “do not appear to be the consequence of our experiences.” For example, living in a ZIP code with a high crime rate does not predict that you will see the world as a dangerous place. Nor does being affluent guarantee that an adult sees the world rich with opportunities.
It is unclear exactly when and by what process we develop our primal beliefs, but it is safe to say our parents probably play a large role. Exactly to what degree the tsunami of bad news we are allowing to inundate our children’s lives plays a role is unclear. However, it is reasonable to assume that news about climate change, school shootings, and the pandemic must be a contributor.
According to Dr. Clifton, there is some evidence that certain mind exercises, when applied diligently, can occasionally modify the primal beliefs of an individual who sees the world as dangerous and barren. Until such strategies become more readily accessible, the best we can do is acknowledge that our children are like canaries in a coal mine full of negative perceptions, then do our best to clear the air.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].