User login
Local therapies show promise for metastatic lung cancer
“Don’t close the barn door after the horse is gone,” the old proverb goes. In other words, there’s no sense in trying to prevent something when it’s already too late.
In many ways and for many years, this saying has applied to providing local therapies to treat cancers that have metastasized to distant sites. I learned this lesson early on from my mentors and have relayed it to countless patients with advanced cancer over the past several decades.
But a growing body of evidence, alongside promising new therapies, highlights more and more exceptions to this long-held belief.
This concept was outlined decades ago for oligometastatic disease and has since been studied in greater depth, and is even being applied in practice. Local therapy for colorectal cancer with limited liver-only metastases is now established as a path to potentially excellent long-term survival. And prospective randomized trials of local therapies for oligometastatic lung cancer or prostate cancer have also demonstrated improvements in clinical outcomes that should lead us to strongly consider integrating local therapy for appropriately selected patients.
In addition, early retrospective studies have provided a proof of principle that patients with solitary brain or adrenal metastases from non–small cell lung cancer (NSCLC) can do exceptionally well and even remain disease-free for many years after definitive local therapy to the primary tumor and oligometastatic disease. For example, a recent press release on the LUNAR trial reported an improvement in overall survival with tumor-treating fields (TTFs), a local therapy, compared with docetaxel as second-line therapy for patients with advanced NSCLC.
That said, the selection process for who receives local therapy remains subjective. In practice, I see patients who fall well outside of conventional oligometastatic parameters but who are directed to local therapy, commonly when systemic therapy is considered futile or prohibitively toxic.
At the same time, however, I also see many patients who would be appropriate candidates for local therapy for oligometastatic disease for whom this strategy is not pursued, perhaps because some oncologists remain dubious about the value of local therapy in this setting. And although we await the full data from the LUNAR trial, I would expect TTFs to face challenges in broad adoption because it is a novel platform with cumbersome practical application, particularly outside of larger centers.
But beyond the potential for TTFs to change management of previously treated advanced NSCLC, I think the findings are more significant because they represent a step, perhaps even a quantum leap, in the role that local therapy could play in improving survival in a broad, unselected population with advanced disease. That is a far more meaningful prospect than conferring benefits in well-selected patients with a narrow subtype of lung cancer. It will be important to determine whether certain subgroups from the LUNAR trial are driving this overall survival benefit.
Local therapy may even have value in the advanced cancer setting beyond oligometastatic disease. That potential is being explored in the SABR-COMET-10 trial, which randomly assigned 159 patients with 4-10 metastatic lesions from various cancers to stereotactic ablative body radiation with standard systemic therapy or the latter alone. With overall survival as the primary endpoint, this study could further revise our understanding of the use of local therapy for treating patients whose cancer biology does not fit the definition of oligometastatic disease.
Does this evolving landscape mean that we were wrong to minimize the role of local therapy?
I don’t think so. The risk/benefit of local therapy today is predicated on two key factors that were absent a few decades ago. First, local therapies such as stereotactic ablative body radiation, minimally invasive surgery, and TTFs now offer disease control with far less attendant toxicity than conventional external beam radiation therapy or open surgery. Second, newer systemic therapies that include targeted therapies and immunotherapy confer remarkably greater disease control for far more patients than does conventional chemotherapy alone.
It is this combination of local therapy’s excellent therapeutic index applied against a background of far better systemic disease control that makes the interplay of local and systemic treatments a newly relevant, open question.
We have yet to see the details of several pivotal trials, but I feel that we should be prepared to question some of the historic dogma in our field to achieve better outcomes not just for selected, narrow subgroups but for a broader population with different types of metastatic cancer.
Dr. West is clinical associate professor, department of medical oncology, City of Hope Comprehensive Cancer Care, Duarte, Calif. He disclosed ties with Ariad/Takeda, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Genentech/Roche, Merck, Pfizer, and Spectrum. A version of this article originally appeared on Medscape.com.
“Don’t close the barn door after the horse is gone,” the old proverb goes. In other words, there’s no sense in trying to prevent something when it’s already too late.
In many ways and for many years, this saying has applied to providing local therapies to treat cancers that have metastasized to distant sites. I learned this lesson early on from my mentors and have relayed it to countless patients with advanced cancer over the past several decades.
But a growing body of evidence, alongside promising new therapies, highlights more and more exceptions to this long-held belief.
This concept was outlined decades ago for oligometastatic disease and has since been studied in greater depth, and is even being applied in practice. Local therapy for colorectal cancer with limited liver-only metastases is now established as a path to potentially excellent long-term survival. And prospective randomized trials of local therapies for oligometastatic lung cancer or prostate cancer have also demonstrated improvements in clinical outcomes that should lead us to strongly consider integrating local therapy for appropriately selected patients.
In addition, early retrospective studies have provided a proof of principle that patients with solitary brain or adrenal metastases from non–small cell lung cancer (NSCLC) can do exceptionally well and even remain disease-free for many years after definitive local therapy to the primary tumor and oligometastatic disease. For example, a recent press release on the LUNAR trial reported an improvement in overall survival with tumor-treating fields (TTFs), a local therapy, compared with docetaxel as second-line therapy for patients with advanced NSCLC.
That said, the selection process for who receives local therapy remains subjective. In practice, I see patients who fall well outside of conventional oligometastatic parameters but who are directed to local therapy, commonly when systemic therapy is considered futile or prohibitively toxic.
At the same time, however, I also see many patients who would be appropriate candidates for local therapy for oligometastatic disease for whom this strategy is not pursued, perhaps because some oncologists remain dubious about the value of local therapy in this setting. And although we await the full data from the LUNAR trial, I would expect TTFs to face challenges in broad adoption because it is a novel platform with cumbersome practical application, particularly outside of larger centers.
But beyond the potential for TTFs to change management of previously treated advanced NSCLC, I think the findings are more significant because they represent a step, perhaps even a quantum leap, in the role that local therapy could play in improving survival in a broad, unselected population with advanced disease. That is a far more meaningful prospect than conferring benefits in well-selected patients with a narrow subtype of lung cancer. It will be important to determine whether certain subgroups from the LUNAR trial are driving this overall survival benefit.
Local therapy may even have value in the advanced cancer setting beyond oligometastatic disease. That potential is being explored in the SABR-COMET-10 trial, which randomly assigned 159 patients with 4-10 metastatic lesions from various cancers to stereotactic ablative body radiation with standard systemic therapy or the latter alone. With overall survival as the primary endpoint, this study could further revise our understanding of the use of local therapy for treating patients whose cancer biology does not fit the definition of oligometastatic disease.
Does this evolving landscape mean that we were wrong to minimize the role of local therapy?
I don’t think so. The risk/benefit of local therapy today is predicated on two key factors that were absent a few decades ago. First, local therapies such as stereotactic ablative body radiation, minimally invasive surgery, and TTFs now offer disease control with far less attendant toxicity than conventional external beam radiation therapy or open surgery. Second, newer systemic therapies that include targeted therapies and immunotherapy confer remarkably greater disease control for far more patients than does conventional chemotherapy alone.
It is this combination of local therapy’s excellent therapeutic index applied against a background of far better systemic disease control that makes the interplay of local and systemic treatments a newly relevant, open question.
We have yet to see the details of several pivotal trials, but I feel that we should be prepared to question some of the historic dogma in our field to achieve better outcomes not just for selected, narrow subgroups but for a broader population with different types of metastatic cancer.
Dr. West is clinical associate professor, department of medical oncology, City of Hope Comprehensive Cancer Care, Duarte, Calif. He disclosed ties with Ariad/Takeda, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Genentech/Roche, Merck, Pfizer, and Spectrum. A version of this article originally appeared on Medscape.com.
“Don’t close the barn door after the horse is gone,” the old proverb goes. In other words, there’s no sense in trying to prevent something when it’s already too late.
In many ways and for many years, this saying has applied to providing local therapies to treat cancers that have metastasized to distant sites. I learned this lesson early on from my mentors and have relayed it to countless patients with advanced cancer over the past several decades.
But a growing body of evidence, alongside promising new therapies, highlights more and more exceptions to this long-held belief.
This concept was outlined decades ago for oligometastatic disease and has since been studied in greater depth, and is even being applied in practice. Local therapy for colorectal cancer with limited liver-only metastases is now established as a path to potentially excellent long-term survival. And prospective randomized trials of local therapies for oligometastatic lung cancer or prostate cancer have also demonstrated improvements in clinical outcomes that should lead us to strongly consider integrating local therapy for appropriately selected patients.
In addition, early retrospective studies have provided a proof of principle that patients with solitary brain or adrenal metastases from non–small cell lung cancer (NSCLC) can do exceptionally well and even remain disease-free for many years after definitive local therapy to the primary tumor and oligometastatic disease. For example, a recent press release on the LUNAR trial reported an improvement in overall survival with tumor-treating fields (TTFs), a local therapy, compared with docetaxel as second-line therapy for patients with advanced NSCLC.
That said, the selection process for who receives local therapy remains subjective. In practice, I see patients who fall well outside of conventional oligometastatic parameters but who are directed to local therapy, commonly when systemic therapy is considered futile or prohibitively toxic.
At the same time, however, I also see many patients who would be appropriate candidates for local therapy for oligometastatic disease for whom this strategy is not pursued, perhaps because some oncologists remain dubious about the value of local therapy in this setting. And although we await the full data from the LUNAR trial, I would expect TTFs to face challenges in broad adoption because it is a novel platform with cumbersome practical application, particularly outside of larger centers.
But beyond the potential for TTFs to change management of previously treated advanced NSCLC, I think the findings are more significant because they represent a step, perhaps even a quantum leap, in the role that local therapy could play in improving survival in a broad, unselected population with advanced disease. That is a far more meaningful prospect than conferring benefits in well-selected patients with a narrow subtype of lung cancer. It will be important to determine whether certain subgroups from the LUNAR trial are driving this overall survival benefit.
Local therapy may even have value in the advanced cancer setting beyond oligometastatic disease. That potential is being explored in the SABR-COMET-10 trial, which randomly assigned 159 patients with 4-10 metastatic lesions from various cancers to stereotactic ablative body radiation with standard systemic therapy or the latter alone. With overall survival as the primary endpoint, this study could further revise our understanding of the use of local therapy for treating patients whose cancer biology does not fit the definition of oligometastatic disease.
Does this evolving landscape mean that we were wrong to minimize the role of local therapy?
I don’t think so. The risk/benefit of local therapy today is predicated on two key factors that were absent a few decades ago. First, local therapies such as stereotactic ablative body radiation, minimally invasive surgery, and TTFs now offer disease control with far less attendant toxicity than conventional external beam radiation therapy or open surgery. Second, newer systemic therapies that include targeted therapies and immunotherapy confer remarkably greater disease control for far more patients than does conventional chemotherapy alone.
It is this combination of local therapy’s excellent therapeutic index applied against a background of far better systemic disease control that makes the interplay of local and systemic treatments a newly relevant, open question.
We have yet to see the details of several pivotal trials, but I feel that we should be prepared to question some of the historic dogma in our field to achieve better outcomes not just for selected, narrow subgroups but for a broader population with different types of metastatic cancer.
Dr. West is clinical associate professor, department of medical oncology, City of Hope Comprehensive Cancer Care, Duarte, Calif. He disclosed ties with Ariad/Takeda, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Genentech/Roche, Merck, Pfizer, and Spectrum. A version of this article originally appeared on Medscape.com.
Saying goodbye: How to transition teens to adult medical care
However, many clinicians feel insufficiently prepared to provide comprehensive transition services. This can result in the actual handoff or transfer into adult care being abrupt, incomplete, or outright unsuccessful. By following the recommended best practices of transitions, providers of pediatric care can ensure that this challenging goodbye prepares everyone for the next steps ahead.
Using a structured transition process
In 2011, a health care transition clinical report based on expert opinion and practice consensus and endorsed by the American Academy of Pediatrics, American Academy of Family Physicians, and American College of Physicians – Society of Internal Medicine was released. This report provided a decision-making algorithm for “practice-based implementation of transition for all youth beginning in early adolescence.”
The Got Transition organization, funded by the Maternal Child Health Bureau and Health Resources and Services Administration, provides web-based information and materials for health care providers and families to establish a smooth and successful transition. At the center of these recommendations are the Six Core Elements of Health Care Transition – the essential components of a structured transition process: 1) transition policy/guide; 2) tracking and monitoring; 3) readiness; 4) planning; 5) transfer of care, and 6) transition completion.
This transition process should start early in adolescence, preferably by age 12-14 years, to give adequate time to progress successfully through these elements and improve the likelihood of a smooth, final transfer into the care of an adult clinician.
Preparing your patients for transfer
Despite the availability of these recommendations, national surveys show that the overwhelming majority of adolescents with and without special health care needs report not receiving transition services. Lack of time, resources, interest, and patients being lost to care during adolescence all contribute to this deficit in care. Without transition preparation, the actual handoff or transfer to adult care can be difficult for adolescents, caregivers, and clinicians alike. Adolescents and caregivers may feel a sense of abandonment or have inadequate health knowledge/literacy, pediatric clinicians may fear that the patient is not ready for the expected independence, and adult clinicians face numerous challenges integrating these young patients into their practice.
A structured transition process can help the family and clinicians know what to expect during the transfer of care. Pediatric clinicians can gradually move from a pediatric model of care, in which the caregiver is the center of communication, to an adult model, putting the patient at the center. By encouraging the adolescent to be the direct communicator, the pediatric clinician can promote independence and assess health knowledge, allowing for education where gaps exist.
Assisting the patient in identifying and even meeting the adult clinician well ahead of the final transfer date can also make the process less daunting for the adolescent.
Adult clinicians should consider allowing more time for the first visit with a new young adult patient and welcome caregiver input early in the transfer process, particularly for patients with a chronic disease. By engaging patients and families in an intentional, gradual transition process with an expected outcome, all those involved will be more prepared for the final handoff.
Utilizing transition tools and engaging the adolescent
Numerous tools can assist in the preparation for transfer to adult care. These include transition summaries and emergency plans, which contain essential information such as current medical problems, allergies, medications, prior procedures and treatments, and sick day plans. Such tools can also be built into electronic medical records for easy modification and updating. They can be used as methods to engage and teach adolescents about their disease history and current regimen and can contain essential components for information handoff at the time of transfer to adult care. If the patient carries a rare diagnosis, or one that has historically been associated with lower survival to adulthood, these transfer documents can also include summary information about disease states and contact information for pediatric specialty clinicians.
Adolescent engagement in their health care during the time of transition can also be prompted through the use of patient portals within an electronic health record. Such portals put health information directly at the adolescent’s fingertips, provide them with an outlet for communication with their clinicians, and give reminders regarding health maintenance.
Completing the transfer: The final handoff
The best and most recommended means of relaying information at the time of transfer to adult care is a direct, verbal handoff between clinicians. This direct handoff has several goals:
(1) To ensure the patient has scheduled or attended the first appointment with the adult clinician
(2) To ensure record transfer has occurred successfully
(3) To answer any questions the receiving clinician may have about prior or ongoing care.
(4) To offer the adult clinician ongoing access to the pediatric clinician as an “expert” resource for additional questions.
By remaining available as a resource, the pediatric clinician can alleviate concerns for both the patient and caregiver as well as the receiving adult clinician.
As valuable as verbal handoffs can be, they are not always possible due to patients not having selected an adult clinician prior to leaving the pediatric clinician, an inability to reach the receiving clinician, and/or time limitations. Many of these barriers can be alleviated by early discussions of transitions of care as well as utilization of structured documentation tools as noted above.
It is also recommended that the pediatric clinician follows up with the patient and/or caregiver several months after the transfer is complete. This allows for the adolescent and/or the caregiver to reflect on the transition process and provide feedback to the pediatric clinicians and their practice for ongoing process improvement.
Reflection as a pediatrician
Ideally, all transition steps occur for the adolescent; in our opinion, a crucial component is to prepare the adolescent patient for the change from a pediatric to adult model of care, in which they are independent in their health communication and decision-making. By engaging adolescents to understand their health, how to maintain it, and when to seek care, we empower them to advocate for their own health as young adults. With appropriate health knowledge and literacy, adolescents are more likely to actively engage with their health care providers and make healthy lifestyle choices. So though saying goodbye may still be difficult, it can be done with the confidence that the patients will continue to get the care they need as they transition into adulthood.
Dr. Kim is assistant clinical professor, department of pediatrics, University of California, San Diego. Dr. Mennito is associate professor of pediatrics and internal medicine, Medical University of South Carolina, Charleston, S.C. Dr. Kim and Dr. Mennito have disclosed no relevant financial relationships. A version of this article originally appeared on Medscape.com.
However, many clinicians feel insufficiently prepared to provide comprehensive transition services. This can result in the actual handoff or transfer into adult care being abrupt, incomplete, or outright unsuccessful. By following the recommended best practices of transitions, providers of pediatric care can ensure that this challenging goodbye prepares everyone for the next steps ahead.
Using a structured transition process
In 2011, a health care transition clinical report based on expert opinion and practice consensus and endorsed by the American Academy of Pediatrics, American Academy of Family Physicians, and American College of Physicians – Society of Internal Medicine was released. This report provided a decision-making algorithm for “practice-based implementation of transition for all youth beginning in early adolescence.”
The Got Transition organization, funded by the Maternal Child Health Bureau and Health Resources and Services Administration, provides web-based information and materials for health care providers and families to establish a smooth and successful transition. At the center of these recommendations are the Six Core Elements of Health Care Transition – the essential components of a structured transition process: 1) transition policy/guide; 2) tracking and monitoring; 3) readiness; 4) planning; 5) transfer of care, and 6) transition completion.
This transition process should start early in adolescence, preferably by age 12-14 years, to give adequate time to progress successfully through these elements and improve the likelihood of a smooth, final transfer into the care of an adult clinician.
Preparing your patients for transfer
Despite the availability of these recommendations, national surveys show that the overwhelming majority of adolescents with and without special health care needs report not receiving transition services. Lack of time, resources, interest, and patients being lost to care during adolescence all contribute to this deficit in care. Without transition preparation, the actual handoff or transfer to adult care can be difficult for adolescents, caregivers, and clinicians alike. Adolescents and caregivers may feel a sense of abandonment or have inadequate health knowledge/literacy, pediatric clinicians may fear that the patient is not ready for the expected independence, and adult clinicians face numerous challenges integrating these young patients into their practice.
A structured transition process can help the family and clinicians know what to expect during the transfer of care. Pediatric clinicians can gradually move from a pediatric model of care, in which the caregiver is the center of communication, to an adult model, putting the patient at the center. By encouraging the adolescent to be the direct communicator, the pediatric clinician can promote independence and assess health knowledge, allowing for education where gaps exist.
Assisting the patient in identifying and even meeting the adult clinician well ahead of the final transfer date can also make the process less daunting for the adolescent.
Adult clinicians should consider allowing more time for the first visit with a new young adult patient and welcome caregiver input early in the transfer process, particularly for patients with a chronic disease. By engaging patients and families in an intentional, gradual transition process with an expected outcome, all those involved will be more prepared for the final handoff.
Utilizing transition tools and engaging the adolescent
Numerous tools can assist in the preparation for transfer to adult care. These include transition summaries and emergency plans, which contain essential information such as current medical problems, allergies, medications, prior procedures and treatments, and sick day plans. Such tools can also be built into electronic medical records for easy modification and updating. They can be used as methods to engage and teach adolescents about their disease history and current regimen and can contain essential components for information handoff at the time of transfer to adult care. If the patient carries a rare diagnosis, or one that has historically been associated with lower survival to adulthood, these transfer documents can also include summary information about disease states and contact information for pediatric specialty clinicians.
Adolescent engagement in their health care during the time of transition can also be prompted through the use of patient portals within an electronic health record. Such portals put health information directly at the adolescent’s fingertips, provide them with an outlet for communication with their clinicians, and give reminders regarding health maintenance.
Completing the transfer: The final handoff
The best and most recommended means of relaying information at the time of transfer to adult care is a direct, verbal handoff between clinicians. This direct handoff has several goals:
(1) To ensure the patient has scheduled or attended the first appointment with the adult clinician
(2) To ensure record transfer has occurred successfully
(3) To answer any questions the receiving clinician may have about prior or ongoing care.
(4) To offer the adult clinician ongoing access to the pediatric clinician as an “expert” resource for additional questions.
By remaining available as a resource, the pediatric clinician can alleviate concerns for both the patient and caregiver as well as the receiving adult clinician.
As valuable as verbal handoffs can be, they are not always possible due to patients not having selected an adult clinician prior to leaving the pediatric clinician, an inability to reach the receiving clinician, and/or time limitations. Many of these barriers can be alleviated by early discussions of transitions of care as well as utilization of structured documentation tools as noted above.
It is also recommended that the pediatric clinician follows up with the patient and/or caregiver several months after the transfer is complete. This allows for the adolescent and/or the caregiver to reflect on the transition process and provide feedback to the pediatric clinicians and their practice for ongoing process improvement.
Reflection as a pediatrician
Ideally, all transition steps occur for the adolescent; in our opinion, a crucial component is to prepare the adolescent patient for the change from a pediatric to adult model of care, in which they are independent in their health communication and decision-making. By engaging adolescents to understand their health, how to maintain it, and when to seek care, we empower them to advocate for their own health as young adults. With appropriate health knowledge and literacy, adolescents are more likely to actively engage with their health care providers and make healthy lifestyle choices. So though saying goodbye may still be difficult, it can be done with the confidence that the patients will continue to get the care they need as they transition into adulthood.
Dr. Kim is assistant clinical professor, department of pediatrics, University of California, San Diego. Dr. Mennito is associate professor of pediatrics and internal medicine, Medical University of South Carolina, Charleston, S.C. Dr. Kim and Dr. Mennito have disclosed no relevant financial relationships. A version of this article originally appeared on Medscape.com.
However, many clinicians feel insufficiently prepared to provide comprehensive transition services. This can result in the actual handoff or transfer into adult care being abrupt, incomplete, or outright unsuccessful. By following the recommended best practices of transitions, providers of pediatric care can ensure that this challenging goodbye prepares everyone for the next steps ahead.
Using a structured transition process
In 2011, a health care transition clinical report based on expert opinion and practice consensus and endorsed by the American Academy of Pediatrics, American Academy of Family Physicians, and American College of Physicians – Society of Internal Medicine was released. This report provided a decision-making algorithm for “practice-based implementation of transition for all youth beginning in early adolescence.”
The Got Transition organization, funded by the Maternal Child Health Bureau and Health Resources and Services Administration, provides web-based information and materials for health care providers and families to establish a smooth and successful transition. At the center of these recommendations are the Six Core Elements of Health Care Transition – the essential components of a structured transition process: 1) transition policy/guide; 2) tracking and monitoring; 3) readiness; 4) planning; 5) transfer of care, and 6) transition completion.
This transition process should start early in adolescence, preferably by age 12-14 years, to give adequate time to progress successfully through these elements and improve the likelihood of a smooth, final transfer into the care of an adult clinician.
Preparing your patients for transfer
Despite the availability of these recommendations, national surveys show that the overwhelming majority of adolescents with and without special health care needs report not receiving transition services. Lack of time, resources, interest, and patients being lost to care during adolescence all contribute to this deficit in care. Without transition preparation, the actual handoff or transfer to adult care can be difficult for adolescents, caregivers, and clinicians alike. Adolescents and caregivers may feel a sense of abandonment or have inadequate health knowledge/literacy, pediatric clinicians may fear that the patient is not ready for the expected independence, and adult clinicians face numerous challenges integrating these young patients into their practice.
A structured transition process can help the family and clinicians know what to expect during the transfer of care. Pediatric clinicians can gradually move from a pediatric model of care, in which the caregiver is the center of communication, to an adult model, putting the patient at the center. By encouraging the adolescent to be the direct communicator, the pediatric clinician can promote independence and assess health knowledge, allowing for education where gaps exist.
Assisting the patient in identifying and even meeting the adult clinician well ahead of the final transfer date can also make the process less daunting for the adolescent.
Adult clinicians should consider allowing more time for the first visit with a new young adult patient and welcome caregiver input early in the transfer process, particularly for patients with a chronic disease. By engaging patients and families in an intentional, gradual transition process with an expected outcome, all those involved will be more prepared for the final handoff.
Utilizing transition tools and engaging the adolescent
Numerous tools can assist in the preparation for transfer to adult care. These include transition summaries and emergency plans, which contain essential information such as current medical problems, allergies, medications, prior procedures and treatments, and sick day plans. Such tools can also be built into electronic medical records for easy modification and updating. They can be used as methods to engage and teach adolescents about their disease history and current regimen and can contain essential components for information handoff at the time of transfer to adult care. If the patient carries a rare diagnosis, or one that has historically been associated with lower survival to adulthood, these transfer documents can also include summary information about disease states and contact information for pediatric specialty clinicians.
Adolescent engagement in their health care during the time of transition can also be prompted through the use of patient portals within an electronic health record. Such portals put health information directly at the adolescent’s fingertips, provide them with an outlet for communication with their clinicians, and give reminders regarding health maintenance.
Completing the transfer: The final handoff
The best and most recommended means of relaying information at the time of transfer to adult care is a direct, verbal handoff between clinicians. This direct handoff has several goals:
(1) To ensure the patient has scheduled or attended the first appointment with the adult clinician
(2) To ensure record transfer has occurred successfully
(3) To answer any questions the receiving clinician may have about prior or ongoing care.
(4) To offer the adult clinician ongoing access to the pediatric clinician as an “expert” resource for additional questions.
By remaining available as a resource, the pediatric clinician can alleviate concerns for both the patient and caregiver as well as the receiving adult clinician.
As valuable as verbal handoffs can be, they are not always possible due to patients not having selected an adult clinician prior to leaving the pediatric clinician, an inability to reach the receiving clinician, and/or time limitations. Many of these barriers can be alleviated by early discussions of transitions of care as well as utilization of structured documentation tools as noted above.
It is also recommended that the pediatric clinician follows up with the patient and/or caregiver several months after the transfer is complete. This allows for the adolescent and/or the caregiver to reflect on the transition process and provide feedback to the pediatric clinicians and their practice for ongoing process improvement.
Reflection as a pediatrician
Ideally, all transition steps occur for the adolescent; in our opinion, a crucial component is to prepare the adolescent patient for the change from a pediatric to adult model of care, in which they are independent in their health communication and decision-making. By engaging adolescents to understand their health, how to maintain it, and when to seek care, we empower them to advocate for their own health as young adults. With appropriate health knowledge and literacy, adolescents are more likely to actively engage with their health care providers and make healthy lifestyle choices. So though saying goodbye may still be difficult, it can be done with the confidence that the patients will continue to get the care they need as they transition into adulthood.
Dr. Kim is assistant clinical professor, department of pediatrics, University of California, San Diego. Dr. Mennito is associate professor of pediatrics and internal medicine, Medical University of South Carolina, Charleston, S.C. Dr. Kim and Dr. Mennito have disclosed no relevant financial relationships. A version of this article originally appeared on Medscape.com.
Don’t keep your patients waiting
Recently, the results of a survey of consumers regarding their health care experiences were reported by Carta Healthcare. As you might expect, I’ve written about punctuality before, but this is such a ubiquitous problem that it bears repeating. Here are some suggestions:
Start on time. That seems obvious, but I’m always amazed at the number of doctors who admit to running late who also admit that they start late. If you’re in the hole before you even start, you can seldom dig yourself out. Sometimes an on-time start is the solution to the entire problem! If you doubt me, try it.
Book realistically. Everyone works at a different pace. Determine the number of patients you can comfortably see in an hour, and book only that number. If you want to see more patients, the solution is working longer hours or hiring physicians or physician extenders (or both), not overloading your schedule.
Time-stamp each chart. Pay attention to patient arrival times if your EHR records them, and step up your pace if you start to fall behind. If your EHR does not record arrival times or you are still using paper records, buy a time clock and have your receptionist time-stamp the “encounter form” that goes to the back with the patient. One glance at the stamp will tell you exactly how long that patient has been waiting.
Schedule all surgeries. If you haven’t scheduled the time necessary for a surgical procedure, don’t do it. It’s frequently tempting to “squeeze in” an excision, often because you feel guilty that the patient has already had to wait for you. But every unscheduled surgery puts you that much further behind. And hurrying through a procedure increases the risk of mistakes. Tell the patient that surgery requires extra time and it can’t be rushed, so you will have to schedule that time.
Work-ins come last, not first. Patients with urgent problems should be seen after scheduled patients. That may seem counterintuitive; receptionists often assume it’s better to squeeze them in early, while you’re still running on time. But doing that guarantees you will run late, and it isn’t fair to patients who have appointments and expect to be seen promptly.
Work-ins, on the other hand, expect a wait because they have no appointment. We tell them, “Our schedule is full today; but if you come at the end of hours, the doctor will see you. But you may have a wait.” Far from complaining, they invariably thank us for seeing them.
Seize the list. You know the list I mean. “Number 16: My right big toe itches. Number 17: I think I feel something on my back. Number 18: This weird chartreuse thing on my arm ...” One long list can leave an entire half-day schedule in shambles.
When a list is produced, the best option is to take it and read it yourself. Identify the most important two or three problems, and address them. For the rest, I will say, “This group of problems deserves a visit of its own, and we will schedule that visit.”
Ask if you can place the list (or a photocopy) in the patient’s chart. (It is, after all, important clinical information.) All of these problems are important to the patient and should be addressed – but on your schedule, not the patient’s.
Avoid interruptions. Especially phone calls. Unless it’s an emergency or an immediate family member, my receptionists say, “I’m sorry, the doctor is with patients. May I take a message?” Everyone – even other physicians – understands. But be sure to return those calls promptly.
Pharmaceutical reps should not be allowed to interrupt you, either. Have them make an appointment, just like everybody else.
There will be times, of course, when you run late. But these should be the exception rather than the rule. By streamlining your procedures and avoiding the pitfalls mentioned, you can give nearly every patient all the time he or she deserves without keeping the next patient waiting.
Incidentally, other common patient complaints in that survey were the following:
- Couldn’t schedule an appointment within a week.
- Spent too little time with me.
- Didn’t provide test results promptly.
- Didn’t respond to my phone calls promptly.
Now would be an excellent opportunity to identify and address any of those problems as well.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Recently, the results of a survey of consumers regarding their health care experiences were reported by Carta Healthcare. As you might expect, I’ve written about punctuality before, but this is such a ubiquitous problem that it bears repeating. Here are some suggestions:
Start on time. That seems obvious, but I’m always amazed at the number of doctors who admit to running late who also admit that they start late. If you’re in the hole before you even start, you can seldom dig yourself out. Sometimes an on-time start is the solution to the entire problem! If you doubt me, try it.
Book realistically. Everyone works at a different pace. Determine the number of patients you can comfortably see in an hour, and book only that number. If you want to see more patients, the solution is working longer hours or hiring physicians or physician extenders (or both), not overloading your schedule.
Time-stamp each chart. Pay attention to patient arrival times if your EHR records them, and step up your pace if you start to fall behind. If your EHR does not record arrival times or you are still using paper records, buy a time clock and have your receptionist time-stamp the “encounter form” that goes to the back with the patient. One glance at the stamp will tell you exactly how long that patient has been waiting.
Schedule all surgeries. If you haven’t scheduled the time necessary for a surgical procedure, don’t do it. It’s frequently tempting to “squeeze in” an excision, often because you feel guilty that the patient has already had to wait for you. But every unscheduled surgery puts you that much further behind. And hurrying through a procedure increases the risk of mistakes. Tell the patient that surgery requires extra time and it can’t be rushed, so you will have to schedule that time.
Work-ins come last, not first. Patients with urgent problems should be seen after scheduled patients. That may seem counterintuitive; receptionists often assume it’s better to squeeze them in early, while you’re still running on time. But doing that guarantees you will run late, and it isn’t fair to patients who have appointments and expect to be seen promptly.
Work-ins, on the other hand, expect a wait because they have no appointment. We tell them, “Our schedule is full today; but if you come at the end of hours, the doctor will see you. But you may have a wait.” Far from complaining, they invariably thank us for seeing them.
Seize the list. You know the list I mean. “Number 16: My right big toe itches. Number 17: I think I feel something on my back. Number 18: This weird chartreuse thing on my arm ...” One long list can leave an entire half-day schedule in shambles.
When a list is produced, the best option is to take it and read it yourself. Identify the most important two or three problems, and address them. For the rest, I will say, “This group of problems deserves a visit of its own, and we will schedule that visit.”
Ask if you can place the list (or a photocopy) in the patient’s chart. (It is, after all, important clinical information.) All of these problems are important to the patient and should be addressed – but on your schedule, not the patient’s.
Avoid interruptions. Especially phone calls. Unless it’s an emergency or an immediate family member, my receptionists say, “I’m sorry, the doctor is with patients. May I take a message?” Everyone – even other physicians – understands. But be sure to return those calls promptly.
Pharmaceutical reps should not be allowed to interrupt you, either. Have them make an appointment, just like everybody else.
There will be times, of course, when you run late. But these should be the exception rather than the rule. By streamlining your procedures and avoiding the pitfalls mentioned, you can give nearly every patient all the time he or she deserves without keeping the next patient waiting.
Incidentally, other common patient complaints in that survey were the following:
- Couldn’t schedule an appointment within a week.
- Spent too little time with me.
- Didn’t provide test results promptly.
- Didn’t respond to my phone calls promptly.
Now would be an excellent opportunity to identify and address any of those problems as well.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Recently, the results of a survey of consumers regarding their health care experiences were reported by Carta Healthcare. As you might expect, I’ve written about punctuality before, but this is such a ubiquitous problem that it bears repeating. Here are some suggestions:
Start on time. That seems obvious, but I’m always amazed at the number of doctors who admit to running late who also admit that they start late. If you’re in the hole before you even start, you can seldom dig yourself out. Sometimes an on-time start is the solution to the entire problem! If you doubt me, try it.
Book realistically. Everyone works at a different pace. Determine the number of patients you can comfortably see in an hour, and book only that number. If you want to see more patients, the solution is working longer hours or hiring physicians or physician extenders (or both), not overloading your schedule.
Time-stamp each chart. Pay attention to patient arrival times if your EHR records them, and step up your pace if you start to fall behind. If your EHR does not record arrival times or you are still using paper records, buy a time clock and have your receptionist time-stamp the “encounter form” that goes to the back with the patient. One glance at the stamp will tell you exactly how long that patient has been waiting.
Schedule all surgeries. If you haven’t scheduled the time necessary for a surgical procedure, don’t do it. It’s frequently tempting to “squeeze in” an excision, often because you feel guilty that the patient has already had to wait for you. But every unscheduled surgery puts you that much further behind. And hurrying through a procedure increases the risk of mistakes. Tell the patient that surgery requires extra time and it can’t be rushed, so you will have to schedule that time.
Work-ins come last, not first. Patients with urgent problems should be seen after scheduled patients. That may seem counterintuitive; receptionists often assume it’s better to squeeze them in early, while you’re still running on time. But doing that guarantees you will run late, and it isn’t fair to patients who have appointments and expect to be seen promptly.
Work-ins, on the other hand, expect a wait because they have no appointment. We tell them, “Our schedule is full today; but if you come at the end of hours, the doctor will see you. But you may have a wait.” Far from complaining, they invariably thank us for seeing them.
Seize the list. You know the list I mean. “Number 16: My right big toe itches. Number 17: I think I feel something on my back. Number 18: This weird chartreuse thing on my arm ...” One long list can leave an entire half-day schedule in shambles.
When a list is produced, the best option is to take it and read it yourself. Identify the most important two or three problems, and address them. For the rest, I will say, “This group of problems deserves a visit of its own, and we will schedule that visit.”
Ask if you can place the list (or a photocopy) in the patient’s chart. (It is, after all, important clinical information.) All of these problems are important to the patient and should be addressed – but on your schedule, not the patient’s.
Avoid interruptions. Especially phone calls. Unless it’s an emergency or an immediate family member, my receptionists say, “I’m sorry, the doctor is with patients. May I take a message?” Everyone – even other physicians – understands. But be sure to return those calls promptly.
Pharmaceutical reps should not be allowed to interrupt you, either. Have them make an appointment, just like everybody else.
There will be times, of course, when you run late. But these should be the exception rather than the rule. By streamlining your procedures and avoiding the pitfalls mentioned, you can give nearly every patient all the time he or she deserves without keeping the next patient waiting.
Incidentally, other common patient complaints in that survey were the following:
- Couldn’t schedule an appointment within a week.
- Spent too little time with me.
- Didn’t provide test results promptly.
- Didn’t respond to my phone calls promptly.
Now would be an excellent opportunity to identify and address any of those problems as well.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Money can buy health but it may not be affordable
It is said that “Money cannot buy happiness.” But ask anyone who does not have it and you will find that money can buy security and freedom from many fears and aggravations. There are no guarantees, but the odds are better with resources than without them. Nations with higher per capita gross domestic product have longer life expectancies, have more freedom, and offer more opportunities for the favored subsets of their populations. The adage is that a rising tide lifts all boats.
It is also said that “Money cannot buy health.” That is also a half lie. Again, there is no guarantee that money can cure what ails you. But over the past 4 decades, modern medical care has added on average 3 years to the average American’s life expectancy. These gains in life expectancy came at a steep cost. The United States has been paying a luxurious, premium price for health care compared with similarly developed nations. When I started my career studying health economics 40 years ago, U.S. health care had increased from 6% of the gross domestic product, was passing 9%, and per my professors, appeared headed for 12% – at which point the predictions were that the sky would start falling. Costs continued to rise and for the past decade about 17% of the U.S. GDP has been going to health care before the pandemic blip.
U.S. life expectancy in the past 150 years nearly doubled. This was primarily due to public health measures rather than the small contributions of medical care for individuals. Life expectancy has mostly risen by reducing infant mortality and childhood mortality. The civil engineers who figured out how to get clean running water into cities and sewage out without the two intermingling have saved far more lives than all the doctors graduating from the medical schools. Better nutrition, better sanitation, vaccines, and, to a smaller extent, antibiotics, have all contributed worthy but lesser effects.
When it comes to individual behavior rather than public health measures, the biggest effect comes from advice physicians have been doling out for decades: Stop smoking, exercise, wear your seatbelt, and do not drink and drive. Over the past generation, medical technology added an incremental improvement to the life span (life expectancy once you attain age 60). This has occurred in cardiovascular health by treating high blood pressure, reducing stroke risk, and lowering hemoglobin A1c. Those measures added about 3 years to life span before the obesity epidemic, the opioid epidemic, and the COVID pandemic wiped out the gains. In round numbers, the price for those 3 extra years of life was about the earnings of working an extra 1-2 years in a lifetime. To share those benefits with everyone, the working stiff has to postpone retirement and work an extra 3 years.
Most recently, modern medicine has produced some very expensive therapies that can add months or years to the lives of people with certain diseases. These therapies may involve over $100,000 worth of marginally beneficial medication that statistically adds a few months to the lives of people with certain varieties of prostate, lung, and breast cancers. Similarly, treatments for chronic disease such as AIDS and cystic fibrosis may involve ongoing medication costs of $10,000 or $20,000 or more per year every year for a lifetime. There are even more extreme examples on the horizon. CAR T-cells and Aduhelm [aducanumab] have the potential to break the Medicare bank. Kaftrio [ivacaftor/tezacaftor/elexacaftor], for cystic fibrosis, is being offered only to wealthier countries.
Medical expenses are hard to analyze because the cited “cost” of many of these medications is like a manufacturer’s suggested retail price that few are actually paying. I am a highly educated consumer, and a physician, but various policies at insurance companies and at national chain pharmacies make it impossible for me to comparison shop for lower prices on my personal medicines. It is not just medications. Over the past 15 years, I have noticed that the “cost” charged for routine lab tests I get have also increased to be 300%-900% higher than what the lab ultimately gets paid by my insurance. It is a farce, and I am chagrined to have been a part of the medical system (managed care organizations, pharmacies, hospitals, and government) that concocted this Frankenstein’s monster of health care payments.
Communicating these issues to a polarized public will require leadership. It will also require better speech writing than the distorted story President Biden used to discuss insulin prices during the State of the Union address. The expensive insulin analogues in common use for diabetes are not the same insulin that was discovered a century ago. The newer medications do provide extra benefits. Assigning a fair value to those benefits is the real issue.
Dr. Powell is a retired pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
It is said that “Money cannot buy happiness.” But ask anyone who does not have it and you will find that money can buy security and freedom from many fears and aggravations. There are no guarantees, but the odds are better with resources than without them. Nations with higher per capita gross domestic product have longer life expectancies, have more freedom, and offer more opportunities for the favored subsets of their populations. The adage is that a rising tide lifts all boats.
It is also said that “Money cannot buy health.” That is also a half lie. Again, there is no guarantee that money can cure what ails you. But over the past 4 decades, modern medical care has added on average 3 years to the average American’s life expectancy. These gains in life expectancy came at a steep cost. The United States has been paying a luxurious, premium price for health care compared with similarly developed nations. When I started my career studying health economics 40 years ago, U.S. health care had increased from 6% of the gross domestic product, was passing 9%, and per my professors, appeared headed for 12% – at which point the predictions were that the sky would start falling. Costs continued to rise and for the past decade about 17% of the U.S. GDP has been going to health care before the pandemic blip.
U.S. life expectancy in the past 150 years nearly doubled. This was primarily due to public health measures rather than the small contributions of medical care for individuals. Life expectancy has mostly risen by reducing infant mortality and childhood mortality. The civil engineers who figured out how to get clean running water into cities and sewage out without the two intermingling have saved far more lives than all the doctors graduating from the medical schools. Better nutrition, better sanitation, vaccines, and, to a smaller extent, antibiotics, have all contributed worthy but lesser effects.
When it comes to individual behavior rather than public health measures, the biggest effect comes from advice physicians have been doling out for decades: Stop smoking, exercise, wear your seatbelt, and do not drink and drive. Over the past generation, medical technology added an incremental improvement to the life span (life expectancy once you attain age 60). This has occurred in cardiovascular health by treating high blood pressure, reducing stroke risk, and lowering hemoglobin A1c. Those measures added about 3 years to life span before the obesity epidemic, the opioid epidemic, and the COVID pandemic wiped out the gains. In round numbers, the price for those 3 extra years of life was about the earnings of working an extra 1-2 years in a lifetime. To share those benefits with everyone, the working stiff has to postpone retirement and work an extra 3 years.
Most recently, modern medicine has produced some very expensive therapies that can add months or years to the lives of people with certain diseases. These therapies may involve over $100,000 worth of marginally beneficial medication that statistically adds a few months to the lives of people with certain varieties of prostate, lung, and breast cancers. Similarly, treatments for chronic disease such as AIDS and cystic fibrosis may involve ongoing medication costs of $10,000 or $20,000 or more per year every year for a lifetime. There are even more extreme examples on the horizon. CAR T-cells and Aduhelm [aducanumab] have the potential to break the Medicare bank. Kaftrio [ivacaftor/tezacaftor/elexacaftor], for cystic fibrosis, is being offered only to wealthier countries.
Medical expenses are hard to analyze because the cited “cost” of many of these medications is like a manufacturer’s suggested retail price that few are actually paying. I am a highly educated consumer, and a physician, but various policies at insurance companies and at national chain pharmacies make it impossible for me to comparison shop for lower prices on my personal medicines. It is not just medications. Over the past 15 years, I have noticed that the “cost” charged for routine lab tests I get have also increased to be 300%-900% higher than what the lab ultimately gets paid by my insurance. It is a farce, and I am chagrined to have been a part of the medical system (managed care organizations, pharmacies, hospitals, and government) that concocted this Frankenstein’s monster of health care payments.
Communicating these issues to a polarized public will require leadership. It will also require better speech writing than the distorted story President Biden used to discuss insulin prices during the State of the Union address. The expensive insulin analogues in common use for diabetes are not the same insulin that was discovered a century ago. The newer medications do provide extra benefits. Assigning a fair value to those benefits is the real issue.
Dr. Powell is a retired pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
It is said that “Money cannot buy happiness.” But ask anyone who does not have it and you will find that money can buy security and freedom from many fears and aggravations. There are no guarantees, but the odds are better with resources than without them. Nations with higher per capita gross domestic product have longer life expectancies, have more freedom, and offer more opportunities for the favored subsets of their populations. The adage is that a rising tide lifts all boats.
It is also said that “Money cannot buy health.” That is also a half lie. Again, there is no guarantee that money can cure what ails you. But over the past 4 decades, modern medical care has added on average 3 years to the average American’s life expectancy. These gains in life expectancy came at a steep cost. The United States has been paying a luxurious, premium price for health care compared with similarly developed nations. When I started my career studying health economics 40 years ago, U.S. health care had increased from 6% of the gross domestic product, was passing 9%, and per my professors, appeared headed for 12% – at which point the predictions were that the sky would start falling. Costs continued to rise and for the past decade about 17% of the U.S. GDP has been going to health care before the pandemic blip.
U.S. life expectancy in the past 150 years nearly doubled. This was primarily due to public health measures rather than the small contributions of medical care for individuals. Life expectancy has mostly risen by reducing infant mortality and childhood mortality. The civil engineers who figured out how to get clean running water into cities and sewage out without the two intermingling have saved far more lives than all the doctors graduating from the medical schools. Better nutrition, better sanitation, vaccines, and, to a smaller extent, antibiotics, have all contributed worthy but lesser effects.
When it comes to individual behavior rather than public health measures, the biggest effect comes from advice physicians have been doling out for decades: Stop smoking, exercise, wear your seatbelt, and do not drink and drive. Over the past generation, medical technology added an incremental improvement to the life span (life expectancy once you attain age 60). This has occurred in cardiovascular health by treating high blood pressure, reducing stroke risk, and lowering hemoglobin A1c. Those measures added about 3 years to life span before the obesity epidemic, the opioid epidemic, and the COVID pandemic wiped out the gains. In round numbers, the price for those 3 extra years of life was about the earnings of working an extra 1-2 years in a lifetime. To share those benefits with everyone, the working stiff has to postpone retirement and work an extra 3 years.
Most recently, modern medicine has produced some very expensive therapies that can add months or years to the lives of people with certain diseases. These therapies may involve over $100,000 worth of marginally beneficial medication that statistically adds a few months to the lives of people with certain varieties of prostate, lung, and breast cancers. Similarly, treatments for chronic disease such as AIDS and cystic fibrosis may involve ongoing medication costs of $10,000 or $20,000 or more per year every year for a lifetime. There are even more extreme examples on the horizon. CAR T-cells and Aduhelm [aducanumab] have the potential to break the Medicare bank. Kaftrio [ivacaftor/tezacaftor/elexacaftor], for cystic fibrosis, is being offered only to wealthier countries.
Medical expenses are hard to analyze because the cited “cost” of many of these medications is like a manufacturer’s suggested retail price that few are actually paying. I am a highly educated consumer, and a physician, but various policies at insurance companies and at national chain pharmacies make it impossible for me to comparison shop for lower prices on my personal medicines. It is not just medications. Over the past 15 years, I have noticed that the “cost” charged for routine lab tests I get have also increased to be 300%-900% higher than what the lab ultimately gets paid by my insurance. It is a farce, and I am chagrined to have been a part of the medical system (managed care organizations, pharmacies, hospitals, and government) that concocted this Frankenstein’s monster of health care payments.
Communicating these issues to a polarized public will require leadership. It will also require better speech writing than the distorted story President Biden used to discuss insulin prices during the State of the Union address. The expensive insulin analogues in common use for diabetes are not the same insulin that was discovered a century ago. The newer medications do provide extra benefits. Assigning a fair value to those benefits is the real issue.
Dr. Powell is a retired pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
Lack of motivation to change can be deadly
For 15 years I rounded at Jefferson Medical College in Philadelphia as a psychiatric consultant with the chair of the department of otolaryngology, his residents, and medical students to see severely ill head and neck cancer patients.
Most of these patients were very depressed, dealing with the severe losses of disfigurement, with decreased self-esteem, and the functional losses of mastication, smell, hearing, and taste. Further exacerbating their depression were the functional limitations of social skills they experienced, with attendant alienation, decreased concentration, persistence, and pace – as well as decreased adaptive skills.
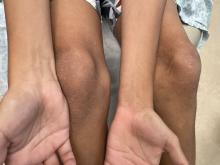
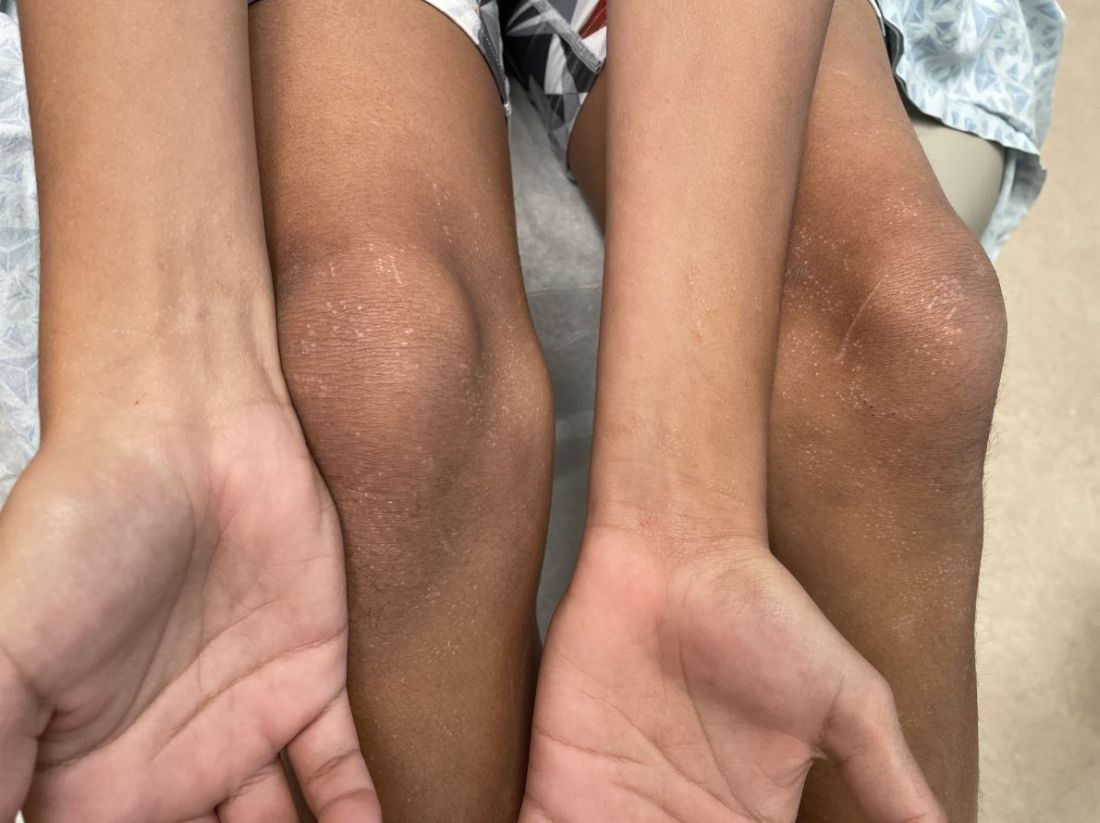
Many of these patients were interjecting a great deal of anger and were very anxious dealing with their disabling surgeries and nonideal recoveries. I witnessed patients dealing with horrific losses – of their tongues, their mandibles, and facial bones – that were chilling, even more horrific than the textbook pictures that I saw in medical school.
Many of these patients I followed with medication management and psychotherapy as outpatients after seeing them during their hospitalization. Throughout the medical literature a direct relationship has been shown between head and neck cancers and alcohol abuse, chewing tobacco, and smoking, and it became apparent that many of these patients were dealing with alcohol and tobacco issues before their cancers. I would have thought that having gone through these horrendous experiences would have been an incentive to stop abusing. To the contrary, after following these patients, I found the majority (about two-thirds) continued with their old habits, even with my interventions.
Susan A. Cohen, DMD, a dentist who has practiced for over 20 years, has also witnessed comparable outcomes, having seen and referred similar cancer patients to the appropriate medical specialists, and upon following these patients noticed that about the same percentage (two-thirds) continued their alcohol and tobacco habits. A common theme and defense mechanism of these patients was denial, and they would often say something like “I have a great doctor who can fix anything, and I don’t have to worry about my habits.” In using the primitive oral defense mechanism of denial, they had problems taking responsibility for their own actions and changing their habits.
Furthermore, Dr. Susan Cohen reveals that abusing tobacco causes severe periodontal problems, including the loss of teeth. She also notes that the same patients have exhibited decreased personal oral hygiene, which further aggravates periodontal disease, loss of dentition, and increases the likelihood of cancers of the mouth and esophagus. She discovered that the losses that occur cause patients to become more depressed and continue the vicious cycle of self-medication with alcohol and tobacco.
In conclusion, we both found that despite disfigurement and loss of function, these postsurgical patients – for the most part – continued their abusive habits.
Dr. Richard W. Cohen is a psychiatrist who has been in private practice for more than 40 years and is on the editorial advisory board for Clinical Psychiatry News. Dr. Susan A. Cohen has practiced dentistry for over 20 years. The Cohens, who are married, are based in Philadelphia.
For 15 years I rounded at Jefferson Medical College in Philadelphia as a psychiatric consultant with the chair of the department of otolaryngology, his residents, and medical students to see severely ill head and neck cancer patients.
Most of these patients were very depressed, dealing with the severe losses of disfigurement, with decreased self-esteem, and the functional losses of mastication, smell, hearing, and taste. Further exacerbating their depression were the functional limitations of social skills they experienced, with attendant alienation, decreased concentration, persistence, and pace – as well as decreased adaptive skills.
Many of these patients were interjecting a great deal of anger and were very anxious dealing with their disabling surgeries and nonideal recoveries. I witnessed patients dealing with horrific losses – of their tongues, their mandibles, and facial bones – that were chilling, even more horrific than the textbook pictures that I saw in medical school.
Many of these patients I followed with medication management and psychotherapy as outpatients after seeing them during their hospitalization. Throughout the medical literature a direct relationship has been shown between head and neck cancers and alcohol abuse, chewing tobacco, and smoking, and it became apparent that many of these patients were dealing with alcohol and tobacco issues before their cancers. I would have thought that having gone through these horrendous experiences would have been an incentive to stop abusing. To the contrary, after following these patients, I found the majority (about two-thirds) continued with their old habits, even with my interventions.
Susan A. Cohen, DMD, a dentist who has practiced for over 20 years, has also witnessed comparable outcomes, having seen and referred similar cancer patients to the appropriate medical specialists, and upon following these patients noticed that about the same percentage (two-thirds) continued their alcohol and tobacco habits. A common theme and defense mechanism of these patients was denial, and they would often say something like “I have a great doctor who can fix anything, and I don’t have to worry about my habits.” In using the primitive oral defense mechanism of denial, they had problems taking responsibility for their own actions and changing their habits.
Furthermore, Dr. Susan Cohen reveals that abusing tobacco causes severe periodontal problems, including the loss of teeth. She also notes that the same patients have exhibited decreased personal oral hygiene, which further aggravates periodontal disease, loss of dentition, and increases the likelihood of cancers of the mouth and esophagus. She discovered that the losses that occur cause patients to become more depressed and continue the vicious cycle of self-medication with alcohol and tobacco.
In conclusion, we both found that despite disfigurement and loss of function, these postsurgical patients – for the most part – continued their abusive habits.
Dr. Richard W. Cohen is a psychiatrist who has been in private practice for more than 40 years and is on the editorial advisory board for Clinical Psychiatry News. Dr. Susan A. Cohen has practiced dentistry for over 20 years. The Cohens, who are married, are based in Philadelphia.
For 15 years I rounded at Jefferson Medical College in Philadelphia as a psychiatric consultant with the chair of the department of otolaryngology, his residents, and medical students to see severely ill head and neck cancer patients.
Most of these patients were very depressed, dealing with the severe losses of disfigurement, with decreased self-esteem, and the functional losses of mastication, smell, hearing, and taste. Further exacerbating their depression were the functional limitations of social skills they experienced, with attendant alienation, decreased concentration, persistence, and pace – as well as decreased adaptive skills.
Many of these patients were interjecting a great deal of anger and were very anxious dealing with their disabling surgeries and nonideal recoveries. I witnessed patients dealing with horrific losses – of their tongues, their mandibles, and facial bones – that were chilling, even more horrific than the textbook pictures that I saw in medical school.
Many of these patients I followed with medication management and psychotherapy as outpatients after seeing them during their hospitalization. Throughout the medical literature a direct relationship has been shown between head and neck cancers and alcohol abuse, chewing tobacco, and smoking, and it became apparent that many of these patients were dealing with alcohol and tobacco issues before their cancers. I would have thought that having gone through these horrendous experiences would have been an incentive to stop abusing. To the contrary, after following these patients, I found the majority (about two-thirds) continued with their old habits, even with my interventions.
Susan A. Cohen, DMD, a dentist who has practiced for over 20 years, has also witnessed comparable outcomes, having seen and referred similar cancer patients to the appropriate medical specialists, and upon following these patients noticed that about the same percentage (two-thirds) continued their alcohol and tobacco habits. A common theme and defense mechanism of these patients was denial, and they would often say something like “I have a great doctor who can fix anything, and I don’t have to worry about my habits.” In using the primitive oral defense mechanism of denial, they had problems taking responsibility for their own actions and changing their habits.
Furthermore, Dr. Susan Cohen reveals that abusing tobacco causes severe periodontal problems, including the loss of teeth. She also notes that the same patients have exhibited decreased personal oral hygiene, which further aggravates periodontal disease, loss of dentition, and increases the likelihood of cancers of the mouth and esophagus. She discovered that the losses that occur cause patients to become more depressed and continue the vicious cycle of self-medication with alcohol and tobacco.
In conclusion, we both found that despite disfigurement and loss of function, these postsurgical patients – for the most part – continued their abusive habits.
Dr. Richard W. Cohen is a psychiatrist who has been in private practice for more than 40 years and is on the editorial advisory board for Clinical Psychiatry News. Dr. Susan A. Cohen has practiced dentistry for over 20 years. The Cohens, who are married, are based in Philadelphia.
Could ChatGPT write this column?
, but I am starting to think it is the real deal. Just how powerful is it? Well, ChatGPT might in fact be writing this column right now. It isn’t. No really, it’s me. But if not for the few cues (“super-buzzy”) that you’ll recognize as my writing voice, there might not be any way for you to know if I wrote this or not.
It’s perfectly OK if you’ve no clue what I’m talking about. ChatGPT is an AI chatbot that burst into public view just a couple months ago. Not your parent’s chatbot, this one is capable of answering questions in conversational language. It is jaw-droppingly good. Like Google, you can type in a question and it offers you answers. Rather than giving you a list of websites and a few Wikipedia blurbs, however, ChatGPT answers your question in human-like text. It can also create content on demand. For example, I asked it to write a Valentine poem to a dermatologist, and it gave me five stanzas starting with:
Oh gentle healer of skin so fair,
Not good enough to send to my wife. But not bad.
If you ask it again, it will create a whole new one for you. Amusing, yes? What if you asked ChatGPT to explain psoriasis, or any medical condition for that matter, to a patient? The replies are quite good. Some even better than what I’m currently using for my patients. It can also offer treatment recommendations, vacation advice, and plan, with recipes, a dinner party for six with one vegan and one gluten-free couple. If you are a programmer, it can write code. Ask it for a Wordpress plugin to add to your website and your eyes will widen as you see it magically appear before you. What if you find that you just don’t like your daughter’s new boyfriend? Yep, it will write the text or email for you to help with this discussion. I’ve saved that one.
I tried “What are treatments for bullous pemphigoid that has been refractory to topical steroid, oral prednisone, and oral tetracyclines?” It replied with five ideas, including the standard methotrexate and azathioprine but also IVIG, Rituxan, even other biologics. Write an op note? Appeal a denied prior authorization to a payer? Write a clinic note for a complete skin exam? Check, check, check. Are you starting to think it might be the real deal, too?
Before we sell the farm though, there are significant limitations. Despite how swotty ChatGPT seems, it is not smart. That is, “it” has no idea what “it” is saying. ChatGPT is an incredibly sophisticated algorithm that has learned the probability of what word comes next in a conversation. To do so, it read the Internet. Billions (trillions?) of words make it possible to predict what is the best answer to any question. But – it’s only as good as the Internet, so there’s that. My patient who used ChatGPT has dissecting cellulitis and asked what to do for scarring alopecia. Some of the answers were reasonable, but some, such as transplanting hairs into the scarred areas, would not likely be helpful. That is unless ChatGPT knows something I don’t.
Having wasted hours of time playing with this thing rather than writing my column, I asked ChatGPT to write an article about itself in the style of Christopher Hitchens. It was nothing like his incisive and eloquent prose, but it wrote 500 words in a few seconds ending with:
“The reality is that there is no substitute for human interaction and empathy in the field of dermatology. Dermatologists must be cautious in their adoption of ChatGPT and ensure that they are not sacrificing the quality of patient care in the pursuit of efficiency and convenience.”
I’m not sure I could have said it better myself.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
, but I am starting to think it is the real deal. Just how powerful is it? Well, ChatGPT might in fact be writing this column right now. It isn’t. No really, it’s me. But if not for the few cues (“super-buzzy”) that you’ll recognize as my writing voice, there might not be any way for you to know if I wrote this or not.
It’s perfectly OK if you’ve no clue what I’m talking about. ChatGPT is an AI chatbot that burst into public view just a couple months ago. Not your parent’s chatbot, this one is capable of answering questions in conversational language. It is jaw-droppingly good. Like Google, you can type in a question and it offers you answers. Rather than giving you a list of websites and a few Wikipedia blurbs, however, ChatGPT answers your question in human-like text. It can also create content on demand. For example, I asked it to write a Valentine poem to a dermatologist, and it gave me five stanzas starting with:
Oh gentle healer of skin so fair,
Not good enough to send to my wife. But not bad.
If you ask it again, it will create a whole new one for you. Amusing, yes? What if you asked ChatGPT to explain psoriasis, or any medical condition for that matter, to a patient? The replies are quite good. Some even better than what I’m currently using for my patients. It can also offer treatment recommendations, vacation advice, and plan, with recipes, a dinner party for six with one vegan and one gluten-free couple. If you are a programmer, it can write code. Ask it for a Wordpress plugin to add to your website and your eyes will widen as you see it magically appear before you. What if you find that you just don’t like your daughter’s new boyfriend? Yep, it will write the text or email for you to help with this discussion. I’ve saved that one.
I tried “What are treatments for bullous pemphigoid that has been refractory to topical steroid, oral prednisone, and oral tetracyclines?” It replied with five ideas, including the standard methotrexate and azathioprine but also IVIG, Rituxan, even other biologics. Write an op note? Appeal a denied prior authorization to a payer? Write a clinic note for a complete skin exam? Check, check, check. Are you starting to think it might be the real deal, too?
Before we sell the farm though, there are significant limitations. Despite how swotty ChatGPT seems, it is not smart. That is, “it” has no idea what “it” is saying. ChatGPT is an incredibly sophisticated algorithm that has learned the probability of what word comes next in a conversation. To do so, it read the Internet. Billions (trillions?) of words make it possible to predict what is the best answer to any question. But – it’s only as good as the Internet, so there’s that. My patient who used ChatGPT has dissecting cellulitis and asked what to do for scarring alopecia. Some of the answers were reasonable, but some, such as transplanting hairs into the scarred areas, would not likely be helpful. That is unless ChatGPT knows something I don’t.
Having wasted hours of time playing with this thing rather than writing my column, I asked ChatGPT to write an article about itself in the style of Christopher Hitchens. It was nothing like his incisive and eloquent prose, but it wrote 500 words in a few seconds ending with:
“The reality is that there is no substitute for human interaction and empathy in the field of dermatology. Dermatologists must be cautious in their adoption of ChatGPT and ensure that they are not sacrificing the quality of patient care in the pursuit of efficiency and convenience.”
I’m not sure I could have said it better myself.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
, but I am starting to think it is the real deal. Just how powerful is it? Well, ChatGPT might in fact be writing this column right now. It isn’t. No really, it’s me. But if not for the few cues (“super-buzzy”) that you’ll recognize as my writing voice, there might not be any way for you to know if I wrote this or not.
It’s perfectly OK if you’ve no clue what I’m talking about. ChatGPT is an AI chatbot that burst into public view just a couple months ago. Not your parent’s chatbot, this one is capable of answering questions in conversational language. It is jaw-droppingly good. Like Google, you can type in a question and it offers you answers. Rather than giving you a list of websites and a few Wikipedia blurbs, however, ChatGPT answers your question in human-like text. It can also create content on demand. For example, I asked it to write a Valentine poem to a dermatologist, and it gave me five stanzas starting with:
Oh gentle healer of skin so fair,
Not good enough to send to my wife. But not bad.
If you ask it again, it will create a whole new one for you. Amusing, yes? What if you asked ChatGPT to explain psoriasis, or any medical condition for that matter, to a patient? The replies are quite good. Some even better than what I’m currently using for my patients. It can also offer treatment recommendations, vacation advice, and plan, with recipes, a dinner party for six with one vegan and one gluten-free couple. If you are a programmer, it can write code. Ask it for a Wordpress plugin to add to your website and your eyes will widen as you see it magically appear before you. What if you find that you just don’t like your daughter’s new boyfriend? Yep, it will write the text or email for you to help with this discussion. I’ve saved that one.
I tried “What are treatments for bullous pemphigoid that has been refractory to topical steroid, oral prednisone, and oral tetracyclines?” It replied with five ideas, including the standard methotrexate and azathioprine but also IVIG, Rituxan, even other biologics. Write an op note? Appeal a denied prior authorization to a payer? Write a clinic note for a complete skin exam? Check, check, check. Are you starting to think it might be the real deal, too?
Before we sell the farm though, there are significant limitations. Despite how swotty ChatGPT seems, it is not smart. That is, “it” has no idea what “it” is saying. ChatGPT is an incredibly sophisticated algorithm that has learned the probability of what word comes next in a conversation. To do so, it read the Internet. Billions (trillions?) of words make it possible to predict what is the best answer to any question. But – it’s only as good as the Internet, so there’s that. My patient who used ChatGPT has dissecting cellulitis and asked what to do for scarring alopecia. Some of the answers were reasonable, but some, such as transplanting hairs into the scarred areas, would not likely be helpful. That is unless ChatGPT knows something I don’t.
Having wasted hours of time playing with this thing rather than writing my column, I asked ChatGPT to write an article about itself in the style of Christopher Hitchens. It was nothing like his incisive and eloquent prose, but it wrote 500 words in a few seconds ending with:
“The reality is that there is no substitute for human interaction and empathy in the field of dermatology. Dermatologists must be cautious in their adoption of ChatGPT and ensure that they are not sacrificing the quality of patient care in the pursuit of efficiency and convenience.”
I’m not sure I could have said it better myself.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Joint effort: CBD not just innocent bystander in weed
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
I visited a legal cannabis dispensary in Massachusetts a few years ago, mostly to see what the hype was about. There I was, knowing basically nothing about pot, as the gentle stoner behind the counter explained to me the differences between the various strains. Acapulco Gold is buoyant and energizing; Purple Kush is sleepy, relaxed, dissociative. Here’s a strain that makes you feel nostalgic; here’s one that helps you focus. It was as complicated and as oddly specific as a fancy wine tasting – and, I had a feeling, about as reliable.
It’s a plant, after all, and though delta-9-tetrahydrocannabinol (THC) is the chemical responsible for its euphoric effects, it is far from the only substance in there.
The second most important compound in cannabis is cannabidiol, and most people will tell you that CBD is the gentle yin to THC’s paranoiac yang. Hence your local ganja barista reminding you that, if you don›t want all those anxiety-inducing side effects of THC, grab a strain with a nice CBD balance.
But is it true? A new study appearing in JAMA Network Open suggests, in fact, that it’s quite the opposite. This study is from Austin Zamarripa and colleagues, who clearly sit at the researcher cool kids table.
Eighteen adults who had abstained from marijuana use for at least a month participated in this trial (which is way more fun than anything we do in my lab at Yale). In random order, separated by at least a week, they ate some special brownies.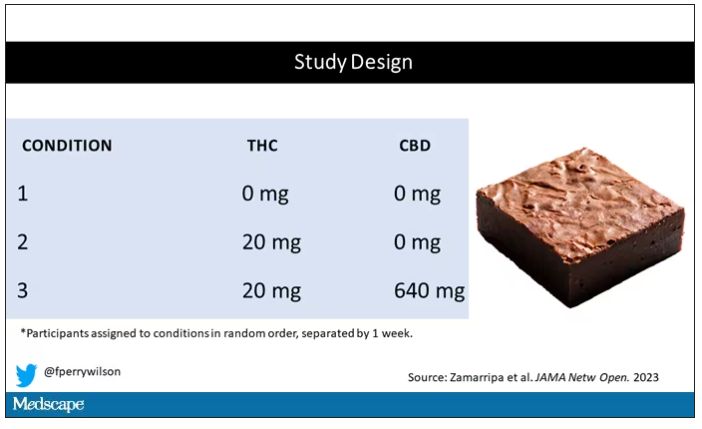
Condition one was a control brownie, condition two was a brownie containing 20 mg of THC, and condition three was a brownie containing 20 mg of THC and 640 mg of CBD. Participants were assigned each condition in random order, separated by at least a week.
A side note on doses for those of you who, like me, are not totally weed literate. A dose of 20 mg of THC is about a third of what you might find in a typical joint these days (though it’s about double the THC content of a joint in the ‘70s – I believe the technical term is “doobie”). And 640 mg of CBD is a decent dose, as 5 mg per kilogram is what some folks start with to achieve therapeutic effects.
Both THC and CBD interact with the cytochrome p450 system in the liver. This matters when you’re ingesting them instead of smoking them because you have first-pass metabolism to contend with. And, because of that p450 inhibition, it’s possible that CBD might actually increase the amount of THC that gets into your bloodstream from the brownie, or gummy, or pizza sauce, or whatever.
Let’s get to the results, starting with blood THC concentration. It’s not subtle. With CBD on board the THC concentration rises higher faster, with roughly double the area under the curve.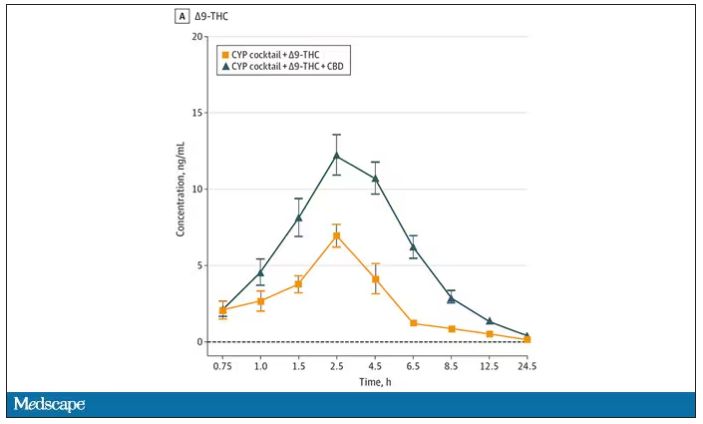
And, unsurprisingly, the subjective experience correlated with those higher levels. Individuals rated the “drug effect” higher with the combo. But, interestingly, the “pleasant” drug effect didn’t change much, while the unpleasant effects were substantially higher. No mitigation of THC anxiety here – quite the opposite. CBD made the anxiety worse.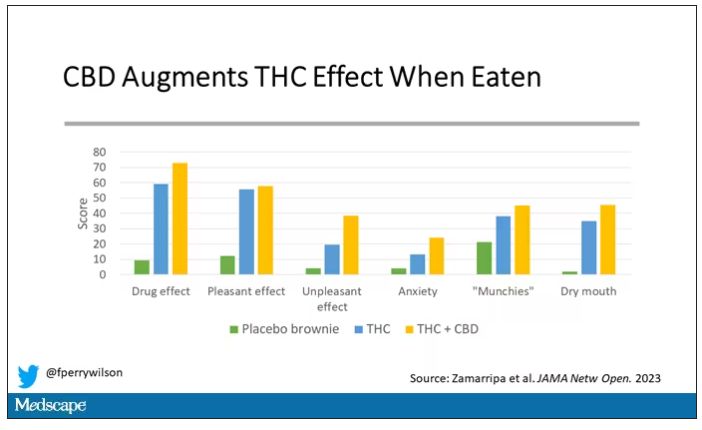
Cognitive effects were equally profound. Scores on a digit symbol substitution test and a paced serial addition task were all substantially worse when CBD was mixed with THC.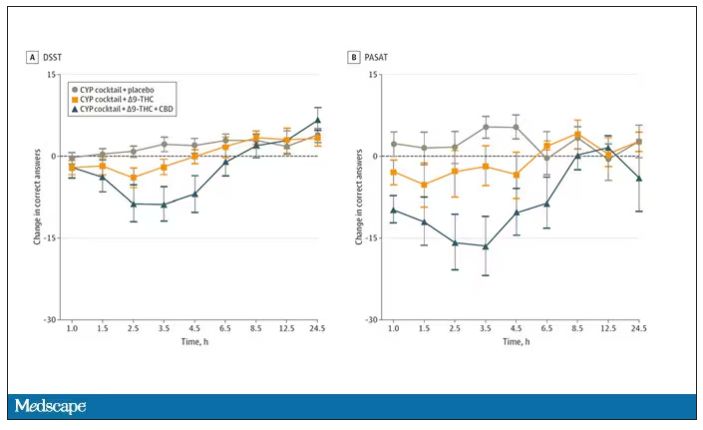
And for those of you who want some more objective measures, check out the heart rate. Despite the purported “calming” nature of CBD, heart rates were way higher when individuals were exposed to both chemicals.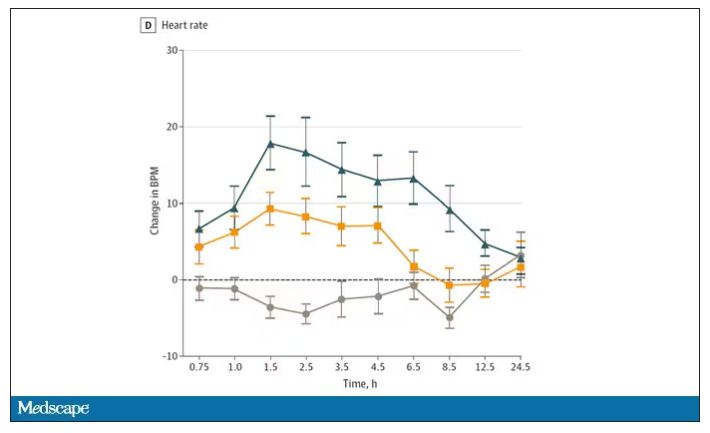
The picture here is quite clear, though the mechanism is not. At least when talking edibles, CBD enhances the effects of THC, and not necessarily for the better. It may be that CBD is competing with some of the proteins that metabolize THC, thus prolonging its effects. CBD may also directly inhibit those enzymes. But whatever the case, I think we can safely say the myth that CBD makes the effects of THC more mild or more tolerable is busted.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
I visited a legal cannabis dispensary in Massachusetts a few years ago, mostly to see what the hype was about. There I was, knowing basically nothing about pot, as the gentle stoner behind the counter explained to me the differences between the various strains. Acapulco Gold is buoyant and energizing; Purple Kush is sleepy, relaxed, dissociative. Here’s a strain that makes you feel nostalgic; here’s one that helps you focus. It was as complicated and as oddly specific as a fancy wine tasting – and, I had a feeling, about as reliable.
It’s a plant, after all, and though delta-9-tetrahydrocannabinol (THC) is the chemical responsible for its euphoric effects, it is far from the only substance in there.
The second most important compound in cannabis is cannabidiol, and most people will tell you that CBD is the gentle yin to THC’s paranoiac yang. Hence your local ganja barista reminding you that, if you don›t want all those anxiety-inducing side effects of THC, grab a strain with a nice CBD balance.
But is it true? A new study appearing in JAMA Network Open suggests, in fact, that it’s quite the opposite. This study is from Austin Zamarripa and colleagues, who clearly sit at the researcher cool kids table.
Eighteen adults who had abstained from marijuana use for at least a month participated in this trial (which is way more fun than anything we do in my lab at Yale). In random order, separated by at least a week, they ate some special brownies.
Condition one was a control brownie, condition two was a brownie containing 20 mg of THC, and condition three was a brownie containing 20 mg of THC and 640 mg of CBD. Participants were assigned each condition in random order, separated by at least a week.
A side note on doses for those of you who, like me, are not totally weed literate. A dose of 20 mg of THC is about a third of what you might find in a typical joint these days (though it’s about double the THC content of a joint in the ‘70s – I believe the technical term is “doobie”). And 640 mg of CBD is a decent dose, as 5 mg per kilogram is what some folks start with to achieve therapeutic effects.
Both THC and CBD interact with the cytochrome p450 system in the liver. This matters when you’re ingesting them instead of smoking them because you have first-pass metabolism to contend with. And, because of that p450 inhibition, it’s possible that CBD might actually increase the amount of THC that gets into your bloodstream from the brownie, or gummy, or pizza sauce, or whatever.
Let’s get to the results, starting with blood THC concentration. It’s not subtle. With CBD on board the THC concentration rises higher faster, with roughly double the area under the curve.
And, unsurprisingly, the subjective experience correlated with those higher levels. Individuals rated the “drug effect” higher with the combo. But, interestingly, the “pleasant” drug effect didn’t change much, while the unpleasant effects were substantially higher. No mitigation of THC anxiety here – quite the opposite. CBD made the anxiety worse.
Cognitive effects were equally profound. Scores on a digit symbol substitution test and a paced serial addition task were all substantially worse when CBD was mixed with THC.
And for those of you who want some more objective measures, check out the heart rate. Despite the purported “calming” nature of CBD, heart rates were way higher when individuals were exposed to both chemicals.
The picture here is quite clear, though the mechanism is not. At least when talking edibles, CBD enhances the effects of THC, and not necessarily for the better. It may be that CBD is competing with some of the proteins that metabolize THC, thus prolonging its effects. CBD may also directly inhibit those enzymes. But whatever the case, I think we can safely say the myth that CBD makes the effects of THC more mild or more tolerable is busted.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
I visited a legal cannabis dispensary in Massachusetts a few years ago, mostly to see what the hype was about. There I was, knowing basically nothing about pot, as the gentle stoner behind the counter explained to me the differences between the various strains. Acapulco Gold is buoyant and energizing; Purple Kush is sleepy, relaxed, dissociative. Here’s a strain that makes you feel nostalgic; here’s one that helps you focus. It was as complicated and as oddly specific as a fancy wine tasting – and, I had a feeling, about as reliable.
It’s a plant, after all, and though delta-9-tetrahydrocannabinol (THC) is the chemical responsible for its euphoric effects, it is far from the only substance in there.
The second most important compound in cannabis is cannabidiol, and most people will tell you that CBD is the gentle yin to THC’s paranoiac yang. Hence your local ganja barista reminding you that, if you don›t want all those anxiety-inducing side effects of THC, grab a strain with a nice CBD balance.
But is it true? A new study appearing in JAMA Network Open suggests, in fact, that it’s quite the opposite. This study is from Austin Zamarripa and colleagues, who clearly sit at the researcher cool kids table.
Eighteen adults who had abstained from marijuana use for at least a month participated in this trial (which is way more fun than anything we do in my lab at Yale). In random order, separated by at least a week, they ate some special brownies.
Condition one was a control brownie, condition two was a brownie containing 20 mg of THC, and condition three was a brownie containing 20 mg of THC and 640 mg of CBD. Participants were assigned each condition in random order, separated by at least a week.
A side note on doses for those of you who, like me, are not totally weed literate. A dose of 20 mg of THC is about a third of what you might find in a typical joint these days (though it’s about double the THC content of a joint in the ‘70s – I believe the technical term is “doobie”). And 640 mg of CBD is a decent dose, as 5 mg per kilogram is what some folks start with to achieve therapeutic effects.
Both THC and CBD interact with the cytochrome p450 system in the liver. This matters when you’re ingesting them instead of smoking them because you have first-pass metabolism to contend with. And, because of that p450 inhibition, it’s possible that CBD might actually increase the amount of THC that gets into your bloodstream from the brownie, or gummy, or pizza sauce, or whatever.
Let’s get to the results, starting with blood THC concentration. It’s not subtle. With CBD on board the THC concentration rises higher faster, with roughly double the area under the curve.
And, unsurprisingly, the subjective experience correlated with those higher levels. Individuals rated the “drug effect” higher with the combo. But, interestingly, the “pleasant” drug effect didn’t change much, while the unpleasant effects were substantially higher. No mitigation of THC anxiety here – quite the opposite. CBD made the anxiety worse.
Cognitive effects were equally profound. Scores on a digit symbol substitution test and a paced serial addition task were all substantially worse when CBD was mixed with THC.
And for those of you who want some more objective measures, check out the heart rate. Despite the purported “calming” nature of CBD, heart rates were way higher when individuals were exposed to both chemicals.
The picture here is quite clear, though the mechanism is not. At least when talking edibles, CBD enhances the effects of THC, and not necessarily for the better. It may be that CBD is competing with some of the proteins that metabolize THC, thus prolonging its effects. CBD may also directly inhibit those enzymes. But whatever the case, I think we can safely say the myth that CBD makes the effects of THC more mild or more tolerable is busted.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn.
A version of this article first appeared on Medscape.com.
The 5-year survival rate for pancreatic cancer is increasing
John Whyte, MD: Hello, I’m Dr. John Whyte, the Chief Medical Officer of WebMD. One of those cancers was pancreatic cancer, which historically has had a very low survival rate. What’s going on here? Are we doing better with diagnosis, treatment, a combination?
Joining me today is Dr. Lynn Matrisian. She is PanCAN’s chief science officer. Dr. Matrisian, thanks for joining me today. It’s great to see you.
Lynn Matrisian, PhD, MBA: Great to be here. Thank you.
Dr. Whyte: Well, tell me what your first reaction was when you saw the recent data from the American Cancer Society. What one word would you use?
Dr. Matrisian: Hopeful. I think hopeful in general that survival rates are increasing, not for all cancers, but for many cancers. We continue to make progress. Research is making a difference. And we’re making progress against cancer in general.
Dr. Whyte: You’re passionate, as our viewers know, about pancreatic cancer. And that’s been one of the hardest cancers to treat, and one of the lowest survival rates. But there’s some encouraging news that we saw, didn’t we?
Dr. Matrisian: Yes. So the 5-year survival rate for pancreatic cancer went up a whole percentage. It’s at 12% now. And what’s really good is it was at 11% last year. It was at 10% the year before. So that’s 2 years in a row that we’ve had an increase in the 5-year survival rate for pancreatic cancer. So we’re hopeful that’s a trajectory that we can really capitalize on is how fast we’re making progress in this disease.
Dr. Whyte: I want to put it into context, Lynn. Because some people might be thinking, 1%? Like you’re excited about 1%? That doesn’t seem that much. But correct me if I’m wrong. A one percentage point increase means 641 more loved ones will enjoy life’s moments, as you put it, 5 years after their diagnosis that otherwise wouldn’t have. What does that practically mean to viewers?
Dr. Matrisian: That means that more than 600 people in the United States will hug a loved one 5 years after that diagnosis of pancreatic cancer. It is a very deadly disease. But we’re going to, by continuing to make progress, it gives those moments to those people. And it means that we’re making progress against the disease in general.
Dr. Whyte: So even 1%, and 1% each year, does have value.
Dr. Matrisian: It has a lot of value.
Dr. Whyte: What’s driving this improvement? Is it better screening? And we’re not so great still in screening a pancreatic cancer. Is it the innovation in cancer treatments? What do you think is accounting for what we hope is this trajectory of increases in 5-year survival?
Dr. Matrisian: Right, so the nice thing the reason that we like looking at 5-year survival rates is because it takes into account all of those things. And we have actually made progress in all of those things. So by looking at those that are diagnosed with pancreatic cancer in general as a whole, and looking at their survival, we are looking at better treatments. People who are getting pancreatic cancer later are living longer as a result of better treatments.
But it’s not just that. It’s also, if you’re diagnosed earlier, your 5-year survival rate is higher. More people who are diagnosed early live to five years than those that are diagnosed later. So within that statistic, there are more people who are diagnosed earlier. And those people also live longer. So it takes into account all of those things, which is why we really like to look at that five-year survival rate for a disease like pancreatic cancer.
Dr. Whyte: Where are we on screening? Because we always want to catch people early. That gives them that greatest chance of survival. Have we made much improvements there? And if we have, what are they?
Dr. Matrisian: Well we have made improvements there are more people that are now diagnosed with localized disease than there were 20 years ago. So that is increasing. And we’re still doing it really by being aware of the symptoms right now. Being aware that kind of chronic indigestion, lower back pain that won’t go away, these are signs and symptoms. And especially things like jaundice ...
Dr. Whyte: That yellow color that they might see.
Dr. Matrisian: Yes, that yellow colors in your eye, that’s a really important symptom that would certainly send people to the doctor in order to look at this. So some of it is being more aware and finding the disease earlier. But what we’re really hoping for is some sort of blood test or some sort of other way of looking through medical records and identifying those people that need to go and be checked.
Dr. Whyte: Now we chatted about that almost two years ago. So tell me the progress that we’ve made. How are we doing?
Dr. Matrisian: Yeah, well there’s a number of companies now that have blood tests that are available. They still need more work. They still need more studies to really understand how good they are at finding pancreatic cancer early. But we didn’t have them a couple of years ago. And so it’s really a very exciting time in the field, that there’s companies that were taking advantage of research for many years and actually turning it into a commercial product that is available for people to check.
Dr. Whyte: And then what about treatments? More treatment options today than there were just a few years ago, but still a lot of progress to be made. So when we talk about even 12% 5-year survival, we’d love to see it much more. And you talk about, I don’t want to misquote, so correct me if I’m wrong. Your goal is 20%. Five-year survival by 2030. That’s not too far. So, Lynn, how are we going to get there?
Dr. Matrisian: Okay, well this is our mission. And that’s exactly our goal, 20% by 2030. So we’ve got some work to do. And we are working at both fronts. You’re right, we need better treatments. And so we’ve set up a clinical trial platform where we can look at a lot of different treatments much more efficiently, much faster, kind of taking advantage of an infrastructure to do that. And that’s called Precision Promise. And we’re excited about that as a way to get new treatments for advanced pancreatic cancer.
And then we’re also working on the early detection end. We think an important symptom of pancreatic cancer that isn’t often recognized is new onset diabetes, sudden diabetes in those over 50 where that person did not have diabetes before. So it’s new, looks like type 2 diabetes, but it’s actually caused by pancreatic cancer.
And so we have an initiative, The Early Detection Initiative, that is taking advantage of that. And seeing if we image people right away based on that symptom, can we find pancreatic cancer early? So we think it’s important to look both at trying to diagnose it earlier, as well as trying to treat it better for advanced disease.
Dr. Whyte: Yeah. You know, at WebMD we’re always trying to empower people with better information so they can also become advocates for their health. You’re an expert in advocacy on pancreatic cancer. So what’s your advice to listeners as to how they become good advocates for themselves or advocates in general for loved ones who have pancreatic cancer?
Dr. Matrisian: Yeah. Yeah. Well certainly, knowledge is power. And so the real thing to do is to call the Pancreatic Cancer Action Network. This is what we do. We stay up on the most current information. We have very experienced case managers who can help navigate the complexities of pancreatic cancer at every stage of the journey.
Or if you have questions about pancreatic cancer, call PanCAN. Go to PanCAN.org and give us a call. Because it’s really that knowledge, knowing what it is that you need to get more knowledge about, how to advocate for yourself is very important in a disease, in any disease, but in particular a disease like pancreatic cancer.
Dr. Whyte: And I don’t want to dismiss the progress that we’ve made, that you’ve just referenced in terms of the increased survival. But there’s still a long way to go. We need a lot more dollars for research. We need a lot more clinical trials to take place. What’s your message to a viewer who’s been diagnosed with pancreatic cancer or a loved one? What’s your message, Lynn, today for them?
Dr. Matrisian: Well, first, get as much knowledge as you can. Call PanCAN, and let us help you help your loved one. But then help us. Let’s do research. Let’s do more research. Let’s understand this disease better so we can make those kinds of progress in both treatment and early detection.
And PanCAN works very hard at understanding the disease and setting up research programs that are going to make a difference, that are going to get us to that aggressive goal of 20% survival by 2030. So there is a lot of things that can be done, raise awareness to your friends and neighbors about the disease, lots of things that will help this whole field.
Dr. Whyte: What’s your feeling on second opinions? Given that this can be a difficult cancer to treat, given that there’s emerging therapies that are always developing, when you have a diagnosis of pancreatic cancer, is it important to consider getting a second opinion?
Dr. Matrisian: Yes. Yes, it is. And our case managers will help with that process. We do think it’s important.
Dr. Whyte: Because sometimes, Lynn, people just want to get started, right? Get it out of me. Get treatment. And sometimes getting a second opinion, doing some genomic testing can take time. So what’s your response to that?
Dr. Matrisian: Yeah. Yeah. Well we say, your care team is very important. Who is on your care team, and it may take a little time to find the right people on your care team. But that is an incredibly important step. Sometimes it’s not just one person. Sometimes you need more than one doctor, more than one nurse, more than one type of specialty to help you deal with this. And taking the time to do that is incredibly important.
Yes, you need to – you do need to act. But act smart. And do it with knowledge. Do it really understanding what your options are, and advocate for yourself.
Dr. Whyte: And surround yourself as you reference with that right care team for you, because that’s the most important thing when you have any type of cancer diagnosis. Dr. Lynn Matrisian, I want to thank you for taking time today.
Dr. Matrisian: Thank you so much, John.
A version of this article first appeared on Medscape.com.
John Whyte, MD: Hello, I’m Dr. John Whyte, the Chief Medical Officer of WebMD. One of those cancers was pancreatic cancer, which historically has had a very low survival rate. What’s going on here? Are we doing better with diagnosis, treatment, a combination?
Joining me today is Dr. Lynn Matrisian. She is PanCAN’s chief science officer. Dr. Matrisian, thanks for joining me today. It’s great to see you.
Lynn Matrisian, PhD, MBA: Great to be here. Thank you.
Dr. Whyte: Well, tell me what your first reaction was when you saw the recent data from the American Cancer Society. What one word would you use?
Dr. Matrisian: Hopeful. I think hopeful in general that survival rates are increasing, not for all cancers, but for many cancers. We continue to make progress. Research is making a difference. And we’re making progress against cancer in general.
Dr. Whyte: You’re passionate, as our viewers know, about pancreatic cancer. And that’s been one of the hardest cancers to treat, and one of the lowest survival rates. But there’s some encouraging news that we saw, didn’t we?
Dr. Matrisian: Yes. So the 5-year survival rate for pancreatic cancer went up a whole percentage. It’s at 12% now. And what’s really good is it was at 11% last year. It was at 10% the year before. So that’s 2 years in a row that we’ve had an increase in the 5-year survival rate for pancreatic cancer. So we’re hopeful that’s a trajectory that we can really capitalize on is how fast we’re making progress in this disease.
Dr. Whyte: I want to put it into context, Lynn. Because some people might be thinking, 1%? Like you’re excited about 1%? That doesn’t seem that much. But correct me if I’m wrong. A one percentage point increase means 641 more loved ones will enjoy life’s moments, as you put it, 5 years after their diagnosis that otherwise wouldn’t have. What does that practically mean to viewers?
Dr. Matrisian: That means that more than 600 people in the United States will hug a loved one 5 years after that diagnosis of pancreatic cancer. It is a very deadly disease. But we’re going to, by continuing to make progress, it gives those moments to those people. And it means that we’re making progress against the disease in general.
Dr. Whyte: So even 1%, and 1% each year, does have value.
Dr. Matrisian: It has a lot of value.
Dr. Whyte: What’s driving this improvement? Is it better screening? And we’re not so great still in screening a pancreatic cancer. Is it the innovation in cancer treatments? What do you think is accounting for what we hope is this trajectory of increases in 5-year survival?
Dr. Matrisian: Right, so the nice thing the reason that we like looking at 5-year survival rates is because it takes into account all of those things. And we have actually made progress in all of those things. So by looking at those that are diagnosed with pancreatic cancer in general as a whole, and looking at their survival, we are looking at better treatments. People who are getting pancreatic cancer later are living longer as a result of better treatments.
But it’s not just that. It’s also, if you’re diagnosed earlier, your 5-year survival rate is higher. More people who are diagnosed early live to five years than those that are diagnosed later. So within that statistic, there are more people who are diagnosed earlier. And those people also live longer. So it takes into account all of those things, which is why we really like to look at that five-year survival rate for a disease like pancreatic cancer.
Dr. Whyte: Where are we on screening? Because we always want to catch people early. That gives them that greatest chance of survival. Have we made much improvements there? And if we have, what are they?
Dr. Matrisian: Well we have made improvements there are more people that are now diagnosed with localized disease than there were 20 years ago. So that is increasing. And we’re still doing it really by being aware of the symptoms right now. Being aware that kind of chronic indigestion, lower back pain that won’t go away, these are signs and symptoms. And especially things like jaundice ...
Dr. Whyte: That yellow color that they might see.
Dr. Matrisian: Yes, that yellow colors in your eye, that’s a really important symptom that would certainly send people to the doctor in order to look at this. So some of it is being more aware and finding the disease earlier. But what we’re really hoping for is some sort of blood test or some sort of other way of looking through medical records and identifying those people that need to go and be checked.
Dr. Whyte: Now we chatted about that almost two years ago. So tell me the progress that we’ve made. How are we doing?
Dr. Matrisian: Yeah, well there’s a number of companies now that have blood tests that are available. They still need more work. They still need more studies to really understand how good they are at finding pancreatic cancer early. But we didn’t have them a couple of years ago. And so it’s really a very exciting time in the field, that there’s companies that were taking advantage of research for many years and actually turning it into a commercial product that is available for people to check.
Dr. Whyte: And then what about treatments? More treatment options today than there were just a few years ago, but still a lot of progress to be made. So when we talk about even 12% 5-year survival, we’d love to see it much more. And you talk about, I don’t want to misquote, so correct me if I’m wrong. Your goal is 20%. Five-year survival by 2030. That’s not too far. So, Lynn, how are we going to get there?
Dr. Matrisian: Okay, well this is our mission. And that’s exactly our goal, 20% by 2030. So we’ve got some work to do. And we are working at both fronts. You’re right, we need better treatments. And so we’ve set up a clinical trial platform where we can look at a lot of different treatments much more efficiently, much faster, kind of taking advantage of an infrastructure to do that. And that’s called Precision Promise. And we’re excited about that as a way to get new treatments for advanced pancreatic cancer.
And then we’re also working on the early detection end. We think an important symptom of pancreatic cancer that isn’t often recognized is new onset diabetes, sudden diabetes in those over 50 where that person did not have diabetes before. So it’s new, looks like type 2 diabetes, but it’s actually caused by pancreatic cancer.
And so we have an initiative, The Early Detection Initiative, that is taking advantage of that. And seeing if we image people right away based on that symptom, can we find pancreatic cancer early? So we think it’s important to look both at trying to diagnose it earlier, as well as trying to treat it better for advanced disease.
Dr. Whyte: Yeah. You know, at WebMD we’re always trying to empower people with better information so they can also become advocates for their health. You’re an expert in advocacy on pancreatic cancer. So what’s your advice to listeners as to how they become good advocates for themselves or advocates in general for loved ones who have pancreatic cancer?
Dr. Matrisian: Yeah. Yeah. Well certainly, knowledge is power. And so the real thing to do is to call the Pancreatic Cancer Action Network. This is what we do. We stay up on the most current information. We have very experienced case managers who can help navigate the complexities of pancreatic cancer at every stage of the journey.
Or if you have questions about pancreatic cancer, call PanCAN. Go to PanCAN.org and give us a call. Because it’s really that knowledge, knowing what it is that you need to get more knowledge about, how to advocate for yourself is very important in a disease, in any disease, but in particular a disease like pancreatic cancer.
Dr. Whyte: And I don’t want to dismiss the progress that we’ve made, that you’ve just referenced in terms of the increased survival. But there’s still a long way to go. We need a lot more dollars for research. We need a lot more clinical trials to take place. What’s your message to a viewer who’s been diagnosed with pancreatic cancer or a loved one? What’s your message, Lynn, today for them?
Dr. Matrisian: Well, first, get as much knowledge as you can. Call PanCAN, and let us help you help your loved one. But then help us. Let’s do research. Let’s do more research. Let’s understand this disease better so we can make those kinds of progress in both treatment and early detection.
And PanCAN works very hard at understanding the disease and setting up research programs that are going to make a difference, that are going to get us to that aggressive goal of 20% survival by 2030. So there is a lot of things that can be done, raise awareness to your friends and neighbors about the disease, lots of things that will help this whole field.
Dr. Whyte: What’s your feeling on second opinions? Given that this can be a difficult cancer to treat, given that there’s emerging therapies that are always developing, when you have a diagnosis of pancreatic cancer, is it important to consider getting a second opinion?
Dr. Matrisian: Yes. Yes, it is. And our case managers will help with that process. We do think it’s important.
Dr. Whyte: Because sometimes, Lynn, people just want to get started, right? Get it out of me. Get treatment. And sometimes getting a second opinion, doing some genomic testing can take time. So what’s your response to that?
Dr. Matrisian: Yeah. Yeah. Well we say, your care team is very important. Who is on your care team, and it may take a little time to find the right people on your care team. But that is an incredibly important step. Sometimes it’s not just one person. Sometimes you need more than one doctor, more than one nurse, more than one type of specialty to help you deal with this. And taking the time to do that is incredibly important.
Yes, you need to – you do need to act. But act smart. And do it with knowledge. Do it really understanding what your options are, and advocate for yourself.
Dr. Whyte: And surround yourself as you reference with that right care team for you, because that’s the most important thing when you have any type of cancer diagnosis. Dr. Lynn Matrisian, I want to thank you for taking time today.
Dr. Matrisian: Thank you so much, John.
A version of this article first appeared on Medscape.com.
John Whyte, MD: Hello, I’m Dr. John Whyte, the Chief Medical Officer of WebMD. One of those cancers was pancreatic cancer, which historically has had a very low survival rate. What’s going on here? Are we doing better with diagnosis, treatment, a combination?
Joining me today is Dr. Lynn Matrisian. She is PanCAN’s chief science officer. Dr. Matrisian, thanks for joining me today. It’s great to see you.
Lynn Matrisian, PhD, MBA: Great to be here. Thank you.
Dr. Whyte: Well, tell me what your first reaction was when you saw the recent data from the American Cancer Society. What one word would you use?
Dr. Matrisian: Hopeful. I think hopeful in general that survival rates are increasing, not for all cancers, but for many cancers. We continue to make progress. Research is making a difference. And we’re making progress against cancer in general.
Dr. Whyte: You’re passionate, as our viewers know, about pancreatic cancer. And that’s been one of the hardest cancers to treat, and one of the lowest survival rates. But there’s some encouraging news that we saw, didn’t we?
Dr. Matrisian: Yes. So the 5-year survival rate for pancreatic cancer went up a whole percentage. It’s at 12% now. And what’s really good is it was at 11% last year. It was at 10% the year before. So that’s 2 years in a row that we’ve had an increase in the 5-year survival rate for pancreatic cancer. So we’re hopeful that’s a trajectory that we can really capitalize on is how fast we’re making progress in this disease.
Dr. Whyte: I want to put it into context, Lynn. Because some people might be thinking, 1%? Like you’re excited about 1%? That doesn’t seem that much. But correct me if I’m wrong. A one percentage point increase means 641 more loved ones will enjoy life’s moments, as you put it, 5 years after their diagnosis that otherwise wouldn’t have. What does that practically mean to viewers?
Dr. Matrisian: That means that more than 600 people in the United States will hug a loved one 5 years after that diagnosis of pancreatic cancer. It is a very deadly disease. But we’re going to, by continuing to make progress, it gives those moments to those people. And it means that we’re making progress against the disease in general.
Dr. Whyte: So even 1%, and 1% each year, does have value.
Dr. Matrisian: It has a lot of value.
Dr. Whyte: What’s driving this improvement? Is it better screening? And we’re not so great still in screening a pancreatic cancer. Is it the innovation in cancer treatments? What do you think is accounting for what we hope is this trajectory of increases in 5-year survival?
Dr. Matrisian: Right, so the nice thing the reason that we like looking at 5-year survival rates is because it takes into account all of those things. And we have actually made progress in all of those things. So by looking at those that are diagnosed with pancreatic cancer in general as a whole, and looking at their survival, we are looking at better treatments. People who are getting pancreatic cancer later are living longer as a result of better treatments.
But it’s not just that. It’s also, if you’re diagnosed earlier, your 5-year survival rate is higher. More people who are diagnosed early live to five years than those that are diagnosed later. So within that statistic, there are more people who are diagnosed earlier. And those people also live longer. So it takes into account all of those things, which is why we really like to look at that five-year survival rate for a disease like pancreatic cancer.
Dr. Whyte: Where are we on screening? Because we always want to catch people early. That gives them that greatest chance of survival. Have we made much improvements there? And if we have, what are they?
Dr. Matrisian: Well we have made improvements there are more people that are now diagnosed with localized disease than there were 20 years ago. So that is increasing. And we’re still doing it really by being aware of the symptoms right now. Being aware that kind of chronic indigestion, lower back pain that won’t go away, these are signs and symptoms. And especially things like jaundice ...
Dr. Whyte: That yellow color that they might see.
Dr. Matrisian: Yes, that yellow colors in your eye, that’s a really important symptom that would certainly send people to the doctor in order to look at this. So some of it is being more aware and finding the disease earlier. But what we’re really hoping for is some sort of blood test or some sort of other way of looking through medical records and identifying those people that need to go and be checked.
Dr. Whyte: Now we chatted about that almost two years ago. So tell me the progress that we’ve made. How are we doing?
Dr. Matrisian: Yeah, well there’s a number of companies now that have blood tests that are available. They still need more work. They still need more studies to really understand how good they are at finding pancreatic cancer early. But we didn’t have them a couple of years ago. And so it’s really a very exciting time in the field, that there’s companies that were taking advantage of research for many years and actually turning it into a commercial product that is available for people to check.
Dr. Whyte: And then what about treatments? More treatment options today than there were just a few years ago, but still a lot of progress to be made. So when we talk about even 12% 5-year survival, we’d love to see it much more. And you talk about, I don’t want to misquote, so correct me if I’m wrong. Your goal is 20%. Five-year survival by 2030. That’s not too far. So, Lynn, how are we going to get there?
Dr. Matrisian: Okay, well this is our mission. And that’s exactly our goal, 20% by 2030. So we’ve got some work to do. And we are working at both fronts. You’re right, we need better treatments. And so we’ve set up a clinical trial platform where we can look at a lot of different treatments much more efficiently, much faster, kind of taking advantage of an infrastructure to do that. And that’s called Precision Promise. And we’re excited about that as a way to get new treatments for advanced pancreatic cancer.
And then we’re also working on the early detection end. We think an important symptom of pancreatic cancer that isn’t often recognized is new onset diabetes, sudden diabetes in those over 50 where that person did not have diabetes before. So it’s new, looks like type 2 diabetes, but it’s actually caused by pancreatic cancer.
And so we have an initiative, The Early Detection Initiative, that is taking advantage of that. And seeing if we image people right away based on that symptom, can we find pancreatic cancer early? So we think it’s important to look both at trying to diagnose it earlier, as well as trying to treat it better for advanced disease.
Dr. Whyte: Yeah. You know, at WebMD we’re always trying to empower people with better information so they can also become advocates for their health. You’re an expert in advocacy on pancreatic cancer. So what’s your advice to listeners as to how they become good advocates for themselves or advocates in general for loved ones who have pancreatic cancer?
Dr. Matrisian: Yeah. Yeah. Well certainly, knowledge is power. And so the real thing to do is to call the Pancreatic Cancer Action Network. This is what we do. We stay up on the most current information. We have very experienced case managers who can help navigate the complexities of pancreatic cancer at every stage of the journey.
Or if you have questions about pancreatic cancer, call PanCAN. Go to PanCAN.org and give us a call. Because it’s really that knowledge, knowing what it is that you need to get more knowledge about, how to advocate for yourself is very important in a disease, in any disease, but in particular a disease like pancreatic cancer.
Dr. Whyte: And I don’t want to dismiss the progress that we’ve made, that you’ve just referenced in terms of the increased survival. But there’s still a long way to go. We need a lot more dollars for research. We need a lot more clinical trials to take place. What’s your message to a viewer who’s been diagnosed with pancreatic cancer or a loved one? What’s your message, Lynn, today for them?
Dr. Matrisian: Well, first, get as much knowledge as you can. Call PanCAN, and let us help you help your loved one. But then help us. Let’s do research. Let’s do more research. Let’s understand this disease better so we can make those kinds of progress in both treatment and early detection.
And PanCAN works very hard at understanding the disease and setting up research programs that are going to make a difference, that are going to get us to that aggressive goal of 20% survival by 2030. So there is a lot of things that can be done, raise awareness to your friends and neighbors about the disease, lots of things that will help this whole field.
Dr. Whyte: What’s your feeling on second opinions? Given that this can be a difficult cancer to treat, given that there’s emerging therapies that are always developing, when you have a diagnosis of pancreatic cancer, is it important to consider getting a second opinion?
Dr. Matrisian: Yes. Yes, it is. And our case managers will help with that process. We do think it’s important.
Dr. Whyte: Because sometimes, Lynn, people just want to get started, right? Get it out of me. Get treatment. And sometimes getting a second opinion, doing some genomic testing can take time. So what’s your response to that?
Dr. Matrisian: Yeah. Yeah. Well we say, your care team is very important. Who is on your care team, and it may take a little time to find the right people on your care team. But that is an incredibly important step. Sometimes it’s not just one person. Sometimes you need more than one doctor, more than one nurse, more than one type of specialty to help you deal with this. And taking the time to do that is incredibly important.
Yes, you need to – you do need to act. But act smart. And do it with knowledge. Do it really understanding what your options are, and advocate for yourself.
Dr. Whyte: And surround yourself as you reference with that right care team for you, because that’s the most important thing when you have any type of cancer diagnosis. Dr. Lynn Matrisian, I want to thank you for taking time today.
Dr. Matrisian: Thank you so much, John.
A version of this article first appeared on Medscape.com.
Forced hospitalization for mental illness not a permanent solution
I met Eleanor when I was writing a book on involuntary psychiatric treatment. She was very ill when she presented to an emergency department in Northern California. She was looking for help and would have signed herself in, but after waiting 8 hours with no food or medical attention, she walked out and went to another hospital.
At this point, she was agitated and distressed and began screaming uncontrollably. The physician in the second ED did not offer her the option of signing in, and she was placed on a 72-hour hold and subsequently held in the hospital for 3 weeks after a judge committed her.
Like so many issues, involuntary psychiatric care is highly polarized. Some groups favor legislation to make involuntary treatment easier, while patient advocacy and civil rights groups vehemently oppose such legislation.
We don’t hear from these combatants as much as we hear from those who trumpet their views on abortion or gun control, yet this battlefield exists. It is not surprising that when New York City Mayor Eric Adams announced a plan to hospitalize homeless people with mental illnesses – involuntarily if necessary, and at the discretion of the police – people were outraged.
New York City is not the only place using this strategy to address the problem of mental illness and homelessness; California has enacted similar legislation, and every major city has homeless citizens.
Eleanor was not homeless, and fortunately, she recovered and returned to her family. However, she remained distressed and traumatized by her hospitalization for years. “It sticks with you,” she told me. “I would rather die than go in again.”
I wish I could tell you that Eleanor is unique in saying that she would rather die than go to a hospital unit for treatment, but it is not an uncommon sentiment for patients. Some people who are charged with crimes and end up in the judicial system will opt to go to jail rather than to a psychiatric hospital. It is also not easy to access outpatient psychiatric treatment.
Barriers to care
Many psychiatrists don’t participate with insurance networks, and publicly funded clinics may have long waiting lists, so illnesses escalate until there is a crisis and hospitalization is necessary. For many, stigma and fear of potential professional repercussions are significant barriers to care.
What are the issues that legislation attempts to address? The first is the standard for hospitalizing individuals against their will. In some states, the patient must be dangerous, while in others there is a lower standard of “gravely disabled,” and finally there are those that promote a standard of a “need for treatment.”
The second is related to medicating people against their will, a process that can be rightly perceived as an assault if the patient refuses to take oral medications and must be held down for injections. Next, the use of outpatient civil commitment – legally requiring people to get treatment if they are not in the hospital – has been increasingly invoked as a way to prevent mass murders and random violence against strangers.
All but four states have some legislation for outpatient commitment, euphemistically called Assisted Outpatient Treatment (AOT), yet these laws are difficult to enforce and expensive to enact. They are also not fully effective.
In New York City, Kendra’s Law has not eliminated subway violence by people with psychiatric disturbances, and the shooter who killed 32 people and wounded 17 others at Virginia Tech in 2007 had previously been ordered by a judge to go to outpatient treatment, but he simply never showed up for his appointment.
Finally, the battle includes the right of patients to refuse to have their psychiatric information released to their caretakers under the Health Insurance Portability and Accountability Act of 1996 – a measure that many families believe would help them to get loved ones to take medications and go to appointments.
The concern about how to negotiate the needs of society and the civil rights of people with psychiatric disorders has been with us for centuries. There is a strong antipsychiatry movement that asserts that psychotropic medications are ineffective or harmful and refers to patients as “psychiatric survivors.” We value the right to medical autonomy, and when there is controversy over the validity of a treatment, there is even more controversy over forcing it upon people.
Psychiatric medications are very effective and benefit many people, but they don’t help everyone, and some people experience side effects. Also, we can’t deny that involuntary care can go wrong; the conservatorship of Britney Spears for 13 years is a very public example.
Multiple stakeholders
Many have a stake in how this plays out. There are the patients, who may be suffering and unable to recognize that they are ill, who may have valid reasons for not wanting the treatments, and who ideally should have the right to refuse care.
There are the families who watch their loved ones suffer, deteriorate, and miss the opportunities that life has to offer; who do not want their children to be homeless or incarcerated; and who may be at risk from violent behavior.
There are the mental health professionals who want to do what’s in the best interest of their patients while following legal and ethical mandates, who worry about being sued for tragic outcomes, and who can’t meet the current demand for services.
There is the taxpayer who foots the bill for disability payments, lost productivity, and institutionalization. There is our society that worries that people with psychiatric disorders will commit random acts of violence.
Finally, there are the insurers, who want to pay for as little care as possible and throw up constant hurdles in the treatment process. We must acknowledge that resources used for involuntary treatment are diverted away from those who want care.
Eleanor had many advantages that unhoused people don’t have: a supportive family, health insurance, and the financial means to pay a psychiatrist who respected her wishes to wean off her medications. She returned to a comfortable home and to personal and occupational success.
It is tragic that we have people living on the streets because of a psychiatric disorder, addiction, poverty, or some combination of these. No one should be unhoused. If the rationale of hospitalization is to decrease violence, I am not hopeful. The Epidemiologic Catchment Area study shows that people with psychiatric disorders are responsible for only 4% of all violence.
The logistics of determining which people living on the streets have psychiatric disorders, transporting them safely to medical facilities, and then finding the resources to provide for compassionate and thoughtful care in meaningful and sustained ways are very challenging.
If we don’t want people living on the streets, we need to create supports, including infrastructure to facilitate housing, access to mental health care, and addiction treatment before we resort to involuntary hospitalization.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore. She has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
I met Eleanor when I was writing a book on involuntary psychiatric treatment. She was very ill when she presented to an emergency department in Northern California. She was looking for help and would have signed herself in, but after waiting 8 hours with no food or medical attention, she walked out and went to another hospital.
At this point, she was agitated and distressed and began screaming uncontrollably. The physician in the second ED did not offer her the option of signing in, and she was placed on a 72-hour hold and subsequently held in the hospital for 3 weeks after a judge committed her.
Like so many issues, involuntary psychiatric care is highly polarized. Some groups favor legislation to make involuntary treatment easier, while patient advocacy and civil rights groups vehemently oppose such legislation.
We don’t hear from these combatants as much as we hear from those who trumpet their views on abortion or gun control, yet this battlefield exists. It is not surprising that when New York City Mayor Eric Adams announced a plan to hospitalize homeless people with mental illnesses – involuntarily if necessary, and at the discretion of the police – people were outraged.
New York City is not the only place using this strategy to address the problem of mental illness and homelessness; California has enacted similar legislation, and every major city has homeless citizens.
Eleanor was not homeless, and fortunately, she recovered and returned to her family. However, she remained distressed and traumatized by her hospitalization for years. “It sticks with you,” she told me. “I would rather die than go in again.”
I wish I could tell you that Eleanor is unique in saying that she would rather die than go to a hospital unit for treatment, but it is not an uncommon sentiment for patients. Some people who are charged with crimes and end up in the judicial system will opt to go to jail rather than to a psychiatric hospital. It is also not easy to access outpatient psychiatric treatment.
Barriers to care
Many psychiatrists don’t participate with insurance networks, and publicly funded clinics may have long waiting lists, so illnesses escalate until there is a crisis and hospitalization is necessary. For many, stigma and fear of potential professional repercussions are significant barriers to care.
What are the issues that legislation attempts to address? The first is the standard for hospitalizing individuals against their will. In some states, the patient must be dangerous, while in others there is a lower standard of “gravely disabled,” and finally there are those that promote a standard of a “need for treatment.”
The second is related to medicating people against their will, a process that can be rightly perceived as an assault if the patient refuses to take oral medications and must be held down for injections. Next, the use of outpatient civil commitment – legally requiring people to get treatment if they are not in the hospital – has been increasingly invoked as a way to prevent mass murders and random violence against strangers.
All but four states have some legislation for outpatient commitment, euphemistically called Assisted Outpatient Treatment (AOT), yet these laws are difficult to enforce and expensive to enact. They are also not fully effective.
In New York City, Kendra’s Law has not eliminated subway violence by people with psychiatric disturbances, and the shooter who killed 32 people and wounded 17 others at Virginia Tech in 2007 had previously been ordered by a judge to go to outpatient treatment, but he simply never showed up for his appointment.
Finally, the battle includes the right of patients to refuse to have their psychiatric information released to their caretakers under the Health Insurance Portability and Accountability Act of 1996 – a measure that many families believe would help them to get loved ones to take medications and go to appointments.
The concern about how to negotiate the needs of society and the civil rights of people with psychiatric disorders has been with us for centuries. There is a strong antipsychiatry movement that asserts that psychotropic medications are ineffective or harmful and refers to patients as “psychiatric survivors.” We value the right to medical autonomy, and when there is controversy over the validity of a treatment, there is even more controversy over forcing it upon people.
Psychiatric medications are very effective and benefit many people, but they don’t help everyone, and some people experience side effects. Also, we can’t deny that involuntary care can go wrong; the conservatorship of Britney Spears for 13 years is a very public example.
Multiple stakeholders
Many have a stake in how this plays out. There are the patients, who may be suffering and unable to recognize that they are ill, who may have valid reasons for not wanting the treatments, and who ideally should have the right to refuse care.
There are the families who watch their loved ones suffer, deteriorate, and miss the opportunities that life has to offer; who do not want their children to be homeless or incarcerated; and who may be at risk from violent behavior.
There are the mental health professionals who want to do what’s in the best interest of their patients while following legal and ethical mandates, who worry about being sued for tragic outcomes, and who can’t meet the current demand for services.
There is the taxpayer who foots the bill for disability payments, lost productivity, and institutionalization. There is our society that worries that people with psychiatric disorders will commit random acts of violence.
Finally, there are the insurers, who want to pay for as little care as possible and throw up constant hurdles in the treatment process. We must acknowledge that resources used for involuntary treatment are diverted away from those who want care.
Eleanor had many advantages that unhoused people don’t have: a supportive family, health insurance, and the financial means to pay a psychiatrist who respected her wishes to wean off her medications. She returned to a comfortable home and to personal and occupational success.
It is tragic that we have people living on the streets because of a psychiatric disorder, addiction, poverty, or some combination of these. No one should be unhoused. If the rationale of hospitalization is to decrease violence, I am not hopeful. The Epidemiologic Catchment Area study shows that people with psychiatric disorders are responsible for only 4% of all violence.
The logistics of determining which people living on the streets have psychiatric disorders, transporting them safely to medical facilities, and then finding the resources to provide for compassionate and thoughtful care in meaningful and sustained ways are very challenging.
If we don’t want people living on the streets, we need to create supports, including infrastructure to facilitate housing, access to mental health care, and addiction treatment before we resort to involuntary hospitalization.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore. She has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
I met Eleanor when I was writing a book on involuntary psychiatric treatment. She was very ill when she presented to an emergency department in Northern California. She was looking for help and would have signed herself in, but after waiting 8 hours with no food or medical attention, she walked out and went to another hospital.
At this point, she was agitated and distressed and began screaming uncontrollably. The physician in the second ED did not offer her the option of signing in, and she was placed on a 72-hour hold and subsequently held in the hospital for 3 weeks after a judge committed her.
Like so many issues, involuntary psychiatric care is highly polarized. Some groups favor legislation to make involuntary treatment easier, while patient advocacy and civil rights groups vehemently oppose such legislation.
We don’t hear from these combatants as much as we hear from those who trumpet their views on abortion or gun control, yet this battlefield exists. It is not surprising that when New York City Mayor Eric Adams announced a plan to hospitalize homeless people with mental illnesses – involuntarily if necessary, and at the discretion of the police – people were outraged.
New York City is not the only place using this strategy to address the problem of mental illness and homelessness; California has enacted similar legislation, and every major city has homeless citizens.
Eleanor was not homeless, and fortunately, she recovered and returned to her family. However, she remained distressed and traumatized by her hospitalization for years. “It sticks with you,” she told me. “I would rather die than go in again.”
I wish I could tell you that Eleanor is unique in saying that she would rather die than go to a hospital unit for treatment, but it is not an uncommon sentiment for patients. Some people who are charged with crimes and end up in the judicial system will opt to go to jail rather than to a psychiatric hospital. It is also not easy to access outpatient psychiatric treatment.
Barriers to care
Many psychiatrists don’t participate with insurance networks, and publicly funded clinics may have long waiting lists, so illnesses escalate until there is a crisis and hospitalization is necessary. For many, stigma and fear of potential professional repercussions are significant barriers to care.
What are the issues that legislation attempts to address? The first is the standard for hospitalizing individuals against their will. In some states, the patient must be dangerous, while in others there is a lower standard of “gravely disabled,” and finally there are those that promote a standard of a “need for treatment.”
The second is related to medicating people against their will, a process that can be rightly perceived as an assault if the patient refuses to take oral medications and must be held down for injections. Next, the use of outpatient civil commitment – legally requiring people to get treatment if they are not in the hospital – has been increasingly invoked as a way to prevent mass murders and random violence against strangers.
All but four states have some legislation for outpatient commitment, euphemistically called Assisted Outpatient Treatment (AOT), yet these laws are difficult to enforce and expensive to enact. They are also not fully effective.
In New York City, Kendra’s Law has not eliminated subway violence by people with psychiatric disturbances, and the shooter who killed 32 people and wounded 17 others at Virginia Tech in 2007 had previously been ordered by a judge to go to outpatient treatment, but he simply never showed up for his appointment.
Finally, the battle includes the right of patients to refuse to have their psychiatric information released to their caretakers under the Health Insurance Portability and Accountability Act of 1996 – a measure that many families believe would help them to get loved ones to take medications and go to appointments.
The concern about how to negotiate the needs of society and the civil rights of people with psychiatric disorders has been with us for centuries. There is a strong antipsychiatry movement that asserts that psychotropic medications are ineffective or harmful and refers to patients as “psychiatric survivors.” We value the right to medical autonomy, and when there is controversy over the validity of a treatment, there is even more controversy over forcing it upon people.
Psychiatric medications are very effective and benefit many people, but they don’t help everyone, and some people experience side effects. Also, we can’t deny that involuntary care can go wrong; the conservatorship of Britney Spears for 13 years is a very public example.
Multiple stakeholders
Many have a stake in how this plays out. There are the patients, who may be suffering and unable to recognize that they are ill, who may have valid reasons for not wanting the treatments, and who ideally should have the right to refuse care.
There are the families who watch their loved ones suffer, deteriorate, and miss the opportunities that life has to offer; who do not want their children to be homeless or incarcerated; and who may be at risk from violent behavior.
There are the mental health professionals who want to do what’s in the best interest of their patients while following legal and ethical mandates, who worry about being sued for tragic outcomes, and who can’t meet the current demand for services.
There is the taxpayer who foots the bill for disability payments, lost productivity, and institutionalization. There is our society that worries that people with psychiatric disorders will commit random acts of violence.
Finally, there are the insurers, who want to pay for as little care as possible and throw up constant hurdles in the treatment process. We must acknowledge that resources used for involuntary treatment are diverted away from those who want care.
Eleanor had many advantages that unhoused people don’t have: a supportive family, health insurance, and the financial means to pay a psychiatrist who respected her wishes to wean off her medications. She returned to a comfortable home and to personal and occupational success.
It is tragic that we have people living on the streets because of a psychiatric disorder, addiction, poverty, or some combination of these. No one should be unhoused. If the rationale of hospitalization is to decrease violence, I am not hopeful. The Epidemiologic Catchment Area study shows that people with psychiatric disorders are responsible for only 4% of all violence.
The logistics of determining which people living on the streets have psychiatric disorders, transporting them safely to medical facilities, and then finding the resources to provide for compassionate and thoughtful care in meaningful and sustained ways are very challenging.
If we don’t want people living on the streets, we need to create supports, including infrastructure to facilitate housing, access to mental health care, and addiction treatment before we resort to involuntary hospitalization.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore. She has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
An 11-year-old boy presents with small itchy bumps on the wrists, face, arms, and legs
The patient was diagnosed with lichen nitidus, given the characteristic clinical presentation.
Lichen nitidus is a rare chronic inflammatory condition of the skin that most commonly presents in children and young adults and does not seem to be restricted to any sex or race. The classic lesions are described as asymptomatic to slightly pruritic, small (1 mm), skin-colored to hypopigmented flat-topped papules.
Koebner phenomenon is usually seen in which the skin lesions appear in areas of traumatized healthy skin. The extremities, abdomen, chest, and penis are common locations for the lesions to occur. Rarely, the oral mucosa or nails can be involved. It has been described in patients with a diagnosis of Crohn’s disease, Niemann-Pick disease, Down syndrome, and HIV. The rare, generalized purpuric variant has been reported in a few cases associated with interferon and ribavirin treatment for hepatitis C infection and nivolumab treatment for cancer. The pathophysiology of lichen nitidus is unknown.
Lichen nitidus can occur in the presence of other skin conditions like lichen planus, atopic dermatitis, vitiligo, erythema nodosum, and lichen spinulosus. Histopathologic characteristics of lichen nitidus are described as a “ball and claw” of epidermal rete around a lymphohistiocytic infiltrate. Parakeratosis overlying epidermal atrophy and focal basal liquefaction degeneration is also seen.
The differential diagnosis of lichen nitidus includes flat warts, which can present as clusters of small flat-topped papules that can show a pseudo-Koebner phenomenon (where the virus is seeded in traumatized skin). The morphological difference between the condition is that lichen nitidus lesions are usually monomorphic, compared with flat warts, which usually present with different sizes and shapes.
Patients with a history of allergic contact dermatitis may present with a generalized monomorphic eruption of skin-colored papules (known as ID reaction) that can sometimes be very similar to lichen nitidus. Allergic contact dermatitis tends to respond fairly quickly to topical or systemic corticosteroids, unlike lichen nitidus. There are a few reports that consider lichen nitidus to be a variant of lichen planus, although they have different histopathologic findings. Lichen planus lesions are described as polygonal, pruritic, purple to pink papules most commonly seen on the wrists, lower back, and ankles. Lichen planus can be seen in patients with hepatitis C and may also occur secondary to medication.
Milia are small keratin cysts on the skin that are commonly seen in babies as primary milia and can be seen in older children secondary to trauma (commonly on the eyelids) or medications. Given their size and monomorphic appearance, they can sometimes be confused with lichen nitidus.
Lichen nitidus is often asymptomatic and the lesions resolve within a few months to years. Topical corticosteroids can be helpful to alleviate the symptoms in patients who present with pruritus. In more persistent and generalized cases, phototherapy, systemic corticosteroids, acitretin, isotretinoin, or cyclosporine can be considered.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Chu J and Lam JM. CMAJ. 2014 Dec 9;186(18):E688.
Lestringant G et al. Dermatology 1996;192:171-3.
Peterson JA et al. Proc (Bayl Univ Med Cent). 2021 Aug 25;35(1):70-2.
Schwartz C and Goodman MB. “Lichen nitidus,” in StatPearls. Treasure Island, Fla.: StatPearls Publishing, 2022.
The patient was diagnosed with lichen nitidus, given the characteristic clinical presentation.
Lichen nitidus is a rare chronic inflammatory condition of the skin that most commonly presents in children and young adults and does not seem to be restricted to any sex or race. The classic lesions are described as asymptomatic to slightly pruritic, small (1 mm), skin-colored to hypopigmented flat-topped papules.
Koebner phenomenon is usually seen in which the skin lesions appear in areas of traumatized healthy skin. The extremities, abdomen, chest, and penis are common locations for the lesions to occur. Rarely, the oral mucosa or nails can be involved. It has been described in patients with a diagnosis of Crohn’s disease, Niemann-Pick disease, Down syndrome, and HIV. The rare, generalized purpuric variant has been reported in a few cases associated with interferon and ribavirin treatment for hepatitis C infection and nivolumab treatment for cancer. The pathophysiology of lichen nitidus is unknown.
Lichen nitidus can occur in the presence of other skin conditions like lichen planus, atopic dermatitis, vitiligo, erythema nodosum, and lichen spinulosus. Histopathologic characteristics of lichen nitidus are described as a “ball and claw” of epidermal rete around a lymphohistiocytic infiltrate. Parakeratosis overlying epidermal atrophy and focal basal liquefaction degeneration is also seen.
The differential diagnosis of lichen nitidus includes flat warts, which can present as clusters of small flat-topped papules that can show a pseudo-Koebner phenomenon (where the virus is seeded in traumatized skin). The morphological difference between the condition is that lichen nitidus lesions are usually monomorphic, compared with flat warts, which usually present with different sizes and shapes.
Patients with a history of allergic contact dermatitis may present with a generalized monomorphic eruption of skin-colored papules (known as ID reaction) that can sometimes be very similar to lichen nitidus. Allergic contact dermatitis tends to respond fairly quickly to topical or systemic corticosteroids, unlike lichen nitidus. There are a few reports that consider lichen nitidus to be a variant of lichen planus, although they have different histopathologic findings. Lichen planus lesions are described as polygonal, pruritic, purple to pink papules most commonly seen on the wrists, lower back, and ankles. Lichen planus can be seen in patients with hepatitis C and may also occur secondary to medication.
Milia are small keratin cysts on the skin that are commonly seen in babies as primary milia and can be seen in older children secondary to trauma (commonly on the eyelids) or medications. Given their size and monomorphic appearance, they can sometimes be confused with lichen nitidus.
Lichen nitidus is often asymptomatic and the lesions resolve within a few months to years. Topical corticosteroids can be helpful to alleviate the symptoms in patients who present with pruritus. In more persistent and generalized cases, phototherapy, systemic corticosteroids, acitretin, isotretinoin, or cyclosporine can be considered.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Chu J and Lam JM. CMAJ. 2014 Dec 9;186(18):E688.
Lestringant G et al. Dermatology 1996;192:171-3.
Peterson JA et al. Proc (Bayl Univ Med Cent). 2021 Aug 25;35(1):70-2.
Schwartz C and Goodman MB. “Lichen nitidus,” in StatPearls. Treasure Island, Fla.: StatPearls Publishing, 2022.
The patient was diagnosed with lichen nitidus, given the characteristic clinical presentation.
Lichen nitidus is a rare chronic inflammatory condition of the skin that most commonly presents in children and young adults and does not seem to be restricted to any sex or race. The classic lesions are described as asymptomatic to slightly pruritic, small (1 mm), skin-colored to hypopigmented flat-topped papules.
Koebner phenomenon is usually seen in which the skin lesions appear in areas of traumatized healthy skin. The extremities, abdomen, chest, and penis are common locations for the lesions to occur. Rarely, the oral mucosa or nails can be involved. It has been described in patients with a diagnosis of Crohn’s disease, Niemann-Pick disease, Down syndrome, and HIV. The rare, generalized purpuric variant has been reported in a few cases associated with interferon and ribavirin treatment for hepatitis C infection and nivolumab treatment for cancer. The pathophysiology of lichen nitidus is unknown.
Lichen nitidus can occur in the presence of other skin conditions like lichen planus, atopic dermatitis, vitiligo, erythema nodosum, and lichen spinulosus. Histopathologic characteristics of lichen nitidus are described as a “ball and claw” of epidermal rete around a lymphohistiocytic infiltrate. Parakeratosis overlying epidermal atrophy and focal basal liquefaction degeneration is also seen.
The differential diagnosis of lichen nitidus includes flat warts, which can present as clusters of small flat-topped papules that can show a pseudo-Koebner phenomenon (where the virus is seeded in traumatized skin). The morphological difference between the condition is that lichen nitidus lesions are usually monomorphic, compared with flat warts, which usually present with different sizes and shapes.
Patients with a history of allergic contact dermatitis may present with a generalized monomorphic eruption of skin-colored papules (known as ID reaction) that can sometimes be very similar to lichen nitidus. Allergic contact dermatitis tends to respond fairly quickly to topical or systemic corticosteroids, unlike lichen nitidus. There are a few reports that consider lichen nitidus to be a variant of lichen planus, although they have different histopathologic findings. Lichen planus lesions are described as polygonal, pruritic, purple to pink papules most commonly seen on the wrists, lower back, and ankles. Lichen planus can be seen in patients with hepatitis C and may also occur secondary to medication.
Milia are small keratin cysts on the skin that are commonly seen in babies as primary milia and can be seen in older children secondary to trauma (commonly on the eyelids) or medications. Given their size and monomorphic appearance, they can sometimes be confused with lichen nitidus.
Lichen nitidus is often asymptomatic and the lesions resolve within a few months to years. Topical corticosteroids can be helpful to alleviate the symptoms in patients who present with pruritus. In more persistent and generalized cases, phototherapy, systemic corticosteroids, acitretin, isotretinoin, or cyclosporine can be considered.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Chu J and Lam JM. CMAJ. 2014 Dec 9;186(18):E688.
Lestringant G et al. Dermatology 1996;192:171-3.
Peterson JA et al. Proc (Bayl Univ Med Cent). 2021 Aug 25;35(1):70-2.
Schwartz C and Goodman MB. “Lichen nitidus,” in StatPearls. Treasure Island, Fla.: StatPearls Publishing, 2022.
An 11-year-old male with a prior history of atopic dermatitis as a young child, presents with 6 months of slightly itchy, small bumps on the wrists, face, arms, and legs. Has been treated with fluocinolone oil and hydrocortisone 2.5% for a month with no change in the lesions. Besides the use of topical corticosteroids, he has not been taking any other medications.
On physical examination he has multiple skin-colored, flat-topped papules that coalesce into plaques on the arms, legs, chest, and back (Photo 1). Koebner phenomenon was also seen on the knees and arms. There were no lesions in the mouth or on the nails.