User login
Scams
It’s amazing how many phone calls I get from different agencies and groups:
The Drug Enforcement Administration – A car rented in your name was found with fentanyl in the trunk.
The Maricopa County Sheriff’s Department – There is a warrant for your arrest due to failure to show up for jury duty and/or as an expert witness.
Doctors Without Borders – We treated one of your patients while they were overseas and need payment for the supplies used.
The Arizona Medical Board – Your license has been suspended.
The Department of Health & Human Services – Your patient database has been posted on the dark web.
Of course, any of these problems can be fixed simply paying the caller a fee by credit card, Bitcoin, or purchasing gift cards and reading off the numbers to them.
Really.
As you’ve probably guessed, none of these calls are real, they’re just popular scams that have been circulating among doctors’ (and other) offices for the last several years. You may have gotten some of them yourself.
I’m sure the vast majority of us don’t fall for them, but the scammer on the other end doesn’t care.
And, realistically, that sucker could be any of us on a bad day. Timing is everything. If we’re frazzled by office events, or aware that the local medical board is looking into something, or have just been up all night at the hospital and are exhausted ... that’s when we’re most vulnerable, our razor’s edge is dull, our thought process slowed, and maybe at that moment we are just not as able to think clearly.
If I were younger I’d probably be more inclined to waste time messing around with them for the entertainment, trying to get them to give up on me after a while. But nowadays I have neither the time nor interest for that. In the rare cases that they make it past my secretary (which is pretty hard) I just hang up.
I’m not sure if it says more about us or them that this happens. I suppose doctors’ offices are the low-hanging fruit where they assume there’s money and (hopefully) someone who’s either gullible, not paying attention, or just not on top of things. As with any other business, if it weren’t profitable they wouldn’t do it. The best we can do is to make it as unprofitable as possible.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
It’s amazing how many phone calls I get from different agencies and groups:
The Drug Enforcement Administration – A car rented in your name was found with fentanyl in the trunk.
The Maricopa County Sheriff’s Department – There is a warrant for your arrest due to failure to show up for jury duty and/or as an expert witness.
Doctors Without Borders – We treated one of your patients while they were overseas and need payment for the supplies used.
The Arizona Medical Board – Your license has been suspended.
The Department of Health & Human Services – Your patient database has been posted on the dark web.
Of course, any of these problems can be fixed simply paying the caller a fee by credit card, Bitcoin, or purchasing gift cards and reading off the numbers to them.
Really.
As you’ve probably guessed, none of these calls are real, they’re just popular scams that have been circulating among doctors’ (and other) offices for the last several years. You may have gotten some of them yourself.
I’m sure the vast majority of us don’t fall for them, but the scammer on the other end doesn’t care.
And, realistically, that sucker could be any of us on a bad day. Timing is everything. If we’re frazzled by office events, or aware that the local medical board is looking into something, or have just been up all night at the hospital and are exhausted ... that’s when we’re most vulnerable, our razor’s edge is dull, our thought process slowed, and maybe at that moment we are just not as able to think clearly.
If I were younger I’d probably be more inclined to waste time messing around with them for the entertainment, trying to get them to give up on me after a while. But nowadays I have neither the time nor interest for that. In the rare cases that they make it past my secretary (which is pretty hard) I just hang up.
I’m not sure if it says more about us or them that this happens. I suppose doctors’ offices are the low-hanging fruit where they assume there’s money and (hopefully) someone who’s either gullible, not paying attention, or just not on top of things. As with any other business, if it weren’t profitable they wouldn’t do it. The best we can do is to make it as unprofitable as possible.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
It’s amazing how many phone calls I get from different agencies and groups:
The Drug Enforcement Administration – A car rented in your name was found with fentanyl in the trunk.
The Maricopa County Sheriff’s Department – There is a warrant for your arrest due to failure to show up for jury duty and/or as an expert witness.
Doctors Without Borders – We treated one of your patients while they were overseas and need payment for the supplies used.
The Arizona Medical Board – Your license has been suspended.
The Department of Health & Human Services – Your patient database has been posted on the dark web.
Of course, any of these problems can be fixed simply paying the caller a fee by credit card, Bitcoin, or purchasing gift cards and reading off the numbers to them.
Really.
As you’ve probably guessed, none of these calls are real, they’re just popular scams that have been circulating among doctors’ (and other) offices for the last several years. You may have gotten some of them yourself.
I’m sure the vast majority of us don’t fall for them, but the scammer on the other end doesn’t care.
And, realistically, that sucker could be any of us on a bad day. Timing is everything. If we’re frazzled by office events, or aware that the local medical board is looking into something, or have just been up all night at the hospital and are exhausted ... that’s when we’re most vulnerable, our razor’s edge is dull, our thought process slowed, and maybe at that moment we are just not as able to think clearly.
If I were younger I’d probably be more inclined to waste time messing around with them for the entertainment, trying to get them to give up on me after a while. But nowadays I have neither the time nor interest for that. In the rare cases that they make it past my secretary (which is pretty hard) I just hang up.
I’m not sure if it says more about us or them that this happens. I suppose doctors’ offices are the low-hanging fruit where they assume there’s money and (hopefully) someone who’s either gullible, not paying attention, or just not on top of things. As with any other business, if it weren’t profitable they wouldn’t do it. The best we can do is to make it as unprofitable as possible.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Must-read acute care medicine articles from 2022
When 2022 began, we started seeing some light at the end of the COVID-19 tunnel. Vaccines were widely available, and even with new variants of the virus still occasionally emerging, the rates of severe morbidity and mortality appeared to be decreasing.
Expectedly, journals appeared to start moving more toward mainstream topics and publications rather than what seemed like a major focus on COVID-19 publications. The resulting literature was fantastic.
Several of those topics were discussed in a prior Emergency Medicine Viewpoint from this news organization, and many more of the research advances of 2022 will be discussed in the near future. However, in this Viewpoint, I would like to present my annual review of my three “must-read” articles of the past year.
As in past years, I am choosing reviews of the literature rather than original research articles (which, all too often, become outdated or debunked within a few years). I choose these articles in the hopes that readers will not simply settle for my brief reviews of the key points but instead will feel compelled to download and read the entire articles. These publications address common conditions and quandaries we face in the daily practice of emergency medicine and are practice-changing.
Myocardial dysfunction after cardiac arrest: Tips and pitfalls
The management of post–cardiac arrest patients remains a hot topic in the resuscitation literature as we continue to understand that the immediate post-arrest period is critical to patient outcome.
Ortuno and colleagues reviewed the current literature on post-arrest care and wrote an outstanding summary of how to optimally care for these patients. More specifically, they focused on post-arrest patients who demonstrate continued shock, or “post–cardiac arrest myocardial dysfunction” (PCAMD).
They propose three mechanisms for the pathogenesis of PCAMD: ischemia reperfusion phenomenon, systemic inflammatory response, and increased catecholamine release
I will skip through the details of the pathophysiology that they describe in the article, but I certainly do recommend that everyone review their descriptions.
Management of these patients begins with a good hemodynamic assessment, which includes clinical markers of perfusion (blood pressure, capillary refill), ECG, and point-of-care ultrasound (POCUS). If the initial assessment reveals an obvious cause of the cardiac arrest (e.g., massive pulmonary embolism, myocardial infarction, pericardial tamponade), then the underlying cause should be treated expeditiously.
In the absence of an obvious treatable cause of the shock, the fluid status and cardiac function should be addressed with POCUS. If the patient is hypovolemic, intravenous fluids should be administered. If the fluid status is adequate, POCUS should be used to estimate the patient’s ventricular function. If the ventricle appears to be hyperdynamic with good contractility, shock should be treated with norepinephrine. On the other hand, if the ventricle is hypodynamic, dobutamine should be substituted for norepinephrine or, more often, added to norepinephrine.
The above represents a simplified summary of the critical points, but the authors do delve into further detail and also discuss some other options for therapies, including steroids, coronary revascularization, extracorporeal membrane oxygenation, and so on. The review is very thoughtful, thorough, and definitely worth a full read.
Top myths of diagnosis and management of infectious diseases in hospital medicine
Most, if not all of us in medicine, have heard the saying that 50% of what we learn in medical school (or residency) will turn out to be wrong. I certainly believe in this concept and consequently, like many of you, I enjoy reading about myths and misconceptions that we have been taught. With that in mind, I have to say that I love this article because it seems to have been written specifically to address what I was taught!
This author group, consisting mostly of clinical PharmDs who are experts in antibiotic use, provide us with an evidence-based discussion of myths and pitfalls in how antibiotics are often used in current clinical practice. The authors review their top 10 myths involving the use of antibiotics in treating infections in the hospital setting. A few of these relate more to the inpatient setting, but here are my favorite emergency department (ED)–related myths that they address:
- “Antibiotics do no harm.” The authors address the risk-benefit of antibiotics based on assumed vs. confirmed infections, including a brief discussion of adverse drug effects.
- “Antibiotic durations of 7, 14, or 21 days are typically necessary.” The authors address appropriate duration of antibiotic use and the fact that unnecessarily long durations of use can lead to resistance. They also provide reassurance that some infections can be treated with quite short durations of antibiotics.
- “If one drug is good, two (or more!) is better.” The use of multiple antibiotics, often with overlapping bacterial coverage, is rampant in medicine and further increases the risk for adverse drug effects and resistance.
- “Oral antibiotics are not as good as intravenous antibiotics for hospitalized patients.” This is definitely a myth that I learned. I recall being taught by many senior physicians that anyone sick enough for admission should be treated with intravenous antibiotics. As it turns out, absorption and effectiveness of most oral antibiotics is just as good as intravenous antibiotics, and the oral formulations are often safer.
- “A history of a penicillin allergy means the patient can never receive a beta-lactam antibiotic.” This is a myth that was debunked quite a few years ago, but it seems that many clinicians still need a reminder.
The authors included five more myths that are worth the read. This is an article that needs to be disseminated among all hospital clinicians.
Guidelines for low-risk, recurrent abdominal pain in the emergency department
The Society for Academic Emergency Medicine (SAEM) recently initiated a program focused on creating evidence-based approaches to challenging chief complaints and presentations in the emergency department (ED). In 2021, they published an approach to managing patients with recurrent, low-risk chest pain in the ED. This past year, they published their second guideline, focused on the management of patients with low-risk, recurrent abdominal pain in the ED.
Recurrent low-risk abdominal pain is a common and vexing presentation to EDs around the world, and there is little prior published guidance. Do all of these patients need repeat imaging? How do we manage their pain? Are there nonabdominal conditions that should be considered?
Broder and colleagues did a fantastic review of the current literature and, on behalf of SAEM, have provided a rational approach to optimal management of these patients. The four major questions they addressed, with brief summaries of their recommendations, are:
- Should adult ED patients with low-risk, recurrent and previously undifferentiated abdominal pain receive a repeat CT abdomen-pelvis (CTAP) after a negative CTAP within the past 12 months? This is a typical question that we all ponder when managing these patients. Unfortunately, the writing group found insufficient evidence to definitively identify populations in whom CTAP was recommended vs could be safely withheld. It is a bit disappointing that there is no definite answer to the question. On the other hand, it is reassuring to know that the world’s best evidence essentially says that it is perfectly appropriate to use your own good clinical judgment.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain with a negative CTAP receive additional imaging with abdominal ultrasound? In this case, the writing group found enough evidence, though low-level, to suggest against routine ultrasound in the absence of concern specifically for pelvic or hepatobiliary pathology. Like most tests, ultrasound is best used when there are specific concerns rather than being used in an undifferentiated fashion.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive screening for depression/anxiety? The writing group found enough evidence, though low-level again, to suggest that screening for depression and/or anxiety be performed during the ED evaluation. This could lead to successful therapy for the abdominal pain.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive nonopioid and/or nonpharmacologic analgesics? The writing group found little evidence to suggest for or against these analgesics, but they made a consensus recommendation suggesting an opioid-minimizing strategy for pain control.
Although the final recommendations of the writing group were not definitive or based on the strongest level of evidence, I find it helpful to have this guidance, nevertheless, on behalf of a major national organization. I also find it helpful to know that even with the best evidence available, optimal patient care will often boil down to physician experience and gestalt. I should also add that the overall article is chock-full of pearls and helpful information that will further inform the readers’ decisions, and so the full version is definitely worth the read.
In summary
There you have it – my three favorite practice-changing articles of 2022. Although I have tried to provide key points here, the full discussions of those key points in the published articles will provide a great deal more education than I can offer in this brief write-up, and so I strongly encourage everyone to read the full versions. Please be sure to include in the comments section your own pick for favorite or must-read articles from the past year.
Amal Mattu, MD, is a professor, vice chair of education, and codirector of the emergency cardiology fellowship in the department of emergency medicine at the University of Maryland, Baltimore. She reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
When 2022 began, we started seeing some light at the end of the COVID-19 tunnel. Vaccines were widely available, and even with new variants of the virus still occasionally emerging, the rates of severe morbidity and mortality appeared to be decreasing.
Expectedly, journals appeared to start moving more toward mainstream topics and publications rather than what seemed like a major focus on COVID-19 publications. The resulting literature was fantastic.
Several of those topics were discussed in a prior Emergency Medicine Viewpoint from this news organization, and many more of the research advances of 2022 will be discussed in the near future. However, in this Viewpoint, I would like to present my annual review of my three “must-read” articles of the past year.
As in past years, I am choosing reviews of the literature rather than original research articles (which, all too often, become outdated or debunked within a few years). I choose these articles in the hopes that readers will not simply settle for my brief reviews of the key points but instead will feel compelled to download and read the entire articles. These publications address common conditions and quandaries we face in the daily practice of emergency medicine and are practice-changing.
Myocardial dysfunction after cardiac arrest: Tips and pitfalls
The management of post–cardiac arrest patients remains a hot topic in the resuscitation literature as we continue to understand that the immediate post-arrest period is critical to patient outcome.
Ortuno and colleagues reviewed the current literature on post-arrest care and wrote an outstanding summary of how to optimally care for these patients. More specifically, they focused on post-arrest patients who demonstrate continued shock, or “post–cardiac arrest myocardial dysfunction” (PCAMD).
They propose three mechanisms for the pathogenesis of PCAMD: ischemia reperfusion phenomenon, systemic inflammatory response, and increased catecholamine release
I will skip through the details of the pathophysiology that they describe in the article, but I certainly do recommend that everyone review their descriptions.
Management of these patients begins with a good hemodynamic assessment, which includes clinical markers of perfusion (blood pressure, capillary refill), ECG, and point-of-care ultrasound (POCUS). If the initial assessment reveals an obvious cause of the cardiac arrest (e.g., massive pulmonary embolism, myocardial infarction, pericardial tamponade), then the underlying cause should be treated expeditiously.
In the absence of an obvious treatable cause of the shock, the fluid status and cardiac function should be addressed with POCUS. If the patient is hypovolemic, intravenous fluids should be administered. If the fluid status is adequate, POCUS should be used to estimate the patient’s ventricular function. If the ventricle appears to be hyperdynamic with good contractility, shock should be treated with norepinephrine. On the other hand, if the ventricle is hypodynamic, dobutamine should be substituted for norepinephrine or, more often, added to norepinephrine.
The above represents a simplified summary of the critical points, but the authors do delve into further detail and also discuss some other options for therapies, including steroids, coronary revascularization, extracorporeal membrane oxygenation, and so on. The review is very thoughtful, thorough, and definitely worth a full read.
Top myths of diagnosis and management of infectious diseases in hospital medicine
Most, if not all of us in medicine, have heard the saying that 50% of what we learn in medical school (or residency) will turn out to be wrong. I certainly believe in this concept and consequently, like many of you, I enjoy reading about myths and misconceptions that we have been taught. With that in mind, I have to say that I love this article because it seems to have been written specifically to address what I was taught!
This author group, consisting mostly of clinical PharmDs who are experts in antibiotic use, provide us with an evidence-based discussion of myths and pitfalls in how antibiotics are often used in current clinical practice. The authors review their top 10 myths involving the use of antibiotics in treating infections in the hospital setting. A few of these relate more to the inpatient setting, but here are my favorite emergency department (ED)–related myths that they address:
- “Antibiotics do no harm.” The authors address the risk-benefit of antibiotics based on assumed vs. confirmed infections, including a brief discussion of adverse drug effects.
- “Antibiotic durations of 7, 14, or 21 days are typically necessary.” The authors address appropriate duration of antibiotic use and the fact that unnecessarily long durations of use can lead to resistance. They also provide reassurance that some infections can be treated with quite short durations of antibiotics.
- “If one drug is good, two (or more!) is better.” The use of multiple antibiotics, often with overlapping bacterial coverage, is rampant in medicine and further increases the risk for adverse drug effects and resistance.
- “Oral antibiotics are not as good as intravenous antibiotics for hospitalized patients.” This is definitely a myth that I learned. I recall being taught by many senior physicians that anyone sick enough for admission should be treated with intravenous antibiotics. As it turns out, absorption and effectiveness of most oral antibiotics is just as good as intravenous antibiotics, and the oral formulations are often safer.
- “A history of a penicillin allergy means the patient can never receive a beta-lactam antibiotic.” This is a myth that was debunked quite a few years ago, but it seems that many clinicians still need a reminder.
The authors included five more myths that are worth the read. This is an article that needs to be disseminated among all hospital clinicians.
Guidelines for low-risk, recurrent abdominal pain in the emergency department
The Society for Academic Emergency Medicine (SAEM) recently initiated a program focused on creating evidence-based approaches to challenging chief complaints and presentations in the emergency department (ED). In 2021, they published an approach to managing patients with recurrent, low-risk chest pain in the ED. This past year, they published their second guideline, focused on the management of patients with low-risk, recurrent abdominal pain in the ED.
Recurrent low-risk abdominal pain is a common and vexing presentation to EDs around the world, and there is little prior published guidance. Do all of these patients need repeat imaging? How do we manage their pain? Are there nonabdominal conditions that should be considered?
Broder and colleagues did a fantastic review of the current literature and, on behalf of SAEM, have provided a rational approach to optimal management of these patients. The four major questions they addressed, with brief summaries of their recommendations, are:
- Should adult ED patients with low-risk, recurrent and previously undifferentiated abdominal pain receive a repeat CT abdomen-pelvis (CTAP) after a negative CTAP within the past 12 months? This is a typical question that we all ponder when managing these patients. Unfortunately, the writing group found insufficient evidence to definitively identify populations in whom CTAP was recommended vs could be safely withheld. It is a bit disappointing that there is no definite answer to the question. On the other hand, it is reassuring to know that the world’s best evidence essentially says that it is perfectly appropriate to use your own good clinical judgment.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain with a negative CTAP receive additional imaging with abdominal ultrasound? In this case, the writing group found enough evidence, though low-level, to suggest against routine ultrasound in the absence of concern specifically for pelvic or hepatobiliary pathology. Like most tests, ultrasound is best used when there are specific concerns rather than being used in an undifferentiated fashion.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive screening for depression/anxiety? The writing group found enough evidence, though low-level again, to suggest that screening for depression and/or anxiety be performed during the ED evaluation. This could lead to successful therapy for the abdominal pain.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive nonopioid and/or nonpharmacologic analgesics? The writing group found little evidence to suggest for or against these analgesics, but they made a consensus recommendation suggesting an opioid-minimizing strategy for pain control.
Although the final recommendations of the writing group were not definitive or based on the strongest level of evidence, I find it helpful to have this guidance, nevertheless, on behalf of a major national organization. I also find it helpful to know that even with the best evidence available, optimal patient care will often boil down to physician experience and gestalt. I should also add that the overall article is chock-full of pearls and helpful information that will further inform the readers’ decisions, and so the full version is definitely worth the read.
In summary
There you have it – my three favorite practice-changing articles of 2022. Although I have tried to provide key points here, the full discussions of those key points in the published articles will provide a great deal more education than I can offer in this brief write-up, and so I strongly encourage everyone to read the full versions. Please be sure to include in the comments section your own pick for favorite or must-read articles from the past year.
Amal Mattu, MD, is a professor, vice chair of education, and codirector of the emergency cardiology fellowship in the department of emergency medicine at the University of Maryland, Baltimore. She reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
When 2022 began, we started seeing some light at the end of the COVID-19 tunnel. Vaccines were widely available, and even with new variants of the virus still occasionally emerging, the rates of severe morbidity and mortality appeared to be decreasing.
Expectedly, journals appeared to start moving more toward mainstream topics and publications rather than what seemed like a major focus on COVID-19 publications. The resulting literature was fantastic.
Several of those topics were discussed in a prior Emergency Medicine Viewpoint from this news organization, and many more of the research advances of 2022 will be discussed in the near future. However, in this Viewpoint, I would like to present my annual review of my three “must-read” articles of the past year.
As in past years, I am choosing reviews of the literature rather than original research articles (which, all too often, become outdated or debunked within a few years). I choose these articles in the hopes that readers will not simply settle for my brief reviews of the key points but instead will feel compelled to download and read the entire articles. These publications address common conditions and quandaries we face in the daily practice of emergency medicine and are practice-changing.
Myocardial dysfunction after cardiac arrest: Tips and pitfalls
The management of post–cardiac arrest patients remains a hot topic in the resuscitation literature as we continue to understand that the immediate post-arrest period is critical to patient outcome.
Ortuno and colleagues reviewed the current literature on post-arrest care and wrote an outstanding summary of how to optimally care for these patients. More specifically, they focused on post-arrest patients who demonstrate continued shock, or “post–cardiac arrest myocardial dysfunction” (PCAMD).
They propose three mechanisms for the pathogenesis of PCAMD: ischemia reperfusion phenomenon, systemic inflammatory response, and increased catecholamine release
I will skip through the details of the pathophysiology that they describe in the article, but I certainly do recommend that everyone review their descriptions.
Management of these patients begins with a good hemodynamic assessment, which includes clinical markers of perfusion (blood pressure, capillary refill), ECG, and point-of-care ultrasound (POCUS). If the initial assessment reveals an obvious cause of the cardiac arrest (e.g., massive pulmonary embolism, myocardial infarction, pericardial tamponade), then the underlying cause should be treated expeditiously.
In the absence of an obvious treatable cause of the shock, the fluid status and cardiac function should be addressed with POCUS. If the patient is hypovolemic, intravenous fluids should be administered. If the fluid status is adequate, POCUS should be used to estimate the patient’s ventricular function. If the ventricle appears to be hyperdynamic with good contractility, shock should be treated with norepinephrine. On the other hand, if the ventricle is hypodynamic, dobutamine should be substituted for norepinephrine or, more often, added to norepinephrine.
The above represents a simplified summary of the critical points, but the authors do delve into further detail and also discuss some other options for therapies, including steroids, coronary revascularization, extracorporeal membrane oxygenation, and so on. The review is very thoughtful, thorough, and definitely worth a full read.
Top myths of diagnosis and management of infectious diseases in hospital medicine
Most, if not all of us in medicine, have heard the saying that 50% of what we learn in medical school (or residency) will turn out to be wrong. I certainly believe in this concept and consequently, like many of you, I enjoy reading about myths and misconceptions that we have been taught. With that in mind, I have to say that I love this article because it seems to have been written specifically to address what I was taught!
This author group, consisting mostly of clinical PharmDs who are experts in antibiotic use, provide us with an evidence-based discussion of myths and pitfalls in how antibiotics are often used in current clinical practice. The authors review their top 10 myths involving the use of antibiotics in treating infections in the hospital setting. A few of these relate more to the inpatient setting, but here are my favorite emergency department (ED)–related myths that they address:
- “Antibiotics do no harm.” The authors address the risk-benefit of antibiotics based on assumed vs. confirmed infections, including a brief discussion of adverse drug effects.
- “Antibiotic durations of 7, 14, or 21 days are typically necessary.” The authors address appropriate duration of antibiotic use and the fact that unnecessarily long durations of use can lead to resistance. They also provide reassurance that some infections can be treated with quite short durations of antibiotics.
- “If one drug is good, two (or more!) is better.” The use of multiple antibiotics, often with overlapping bacterial coverage, is rampant in medicine and further increases the risk for adverse drug effects and resistance.
- “Oral antibiotics are not as good as intravenous antibiotics for hospitalized patients.” This is definitely a myth that I learned. I recall being taught by many senior physicians that anyone sick enough for admission should be treated with intravenous antibiotics. As it turns out, absorption and effectiveness of most oral antibiotics is just as good as intravenous antibiotics, and the oral formulations are often safer.
- “A history of a penicillin allergy means the patient can never receive a beta-lactam antibiotic.” This is a myth that was debunked quite a few years ago, but it seems that many clinicians still need a reminder.
The authors included five more myths that are worth the read. This is an article that needs to be disseminated among all hospital clinicians.
Guidelines for low-risk, recurrent abdominal pain in the emergency department
The Society for Academic Emergency Medicine (SAEM) recently initiated a program focused on creating evidence-based approaches to challenging chief complaints and presentations in the emergency department (ED). In 2021, they published an approach to managing patients with recurrent, low-risk chest pain in the ED. This past year, they published their second guideline, focused on the management of patients with low-risk, recurrent abdominal pain in the ED.
Recurrent low-risk abdominal pain is a common and vexing presentation to EDs around the world, and there is little prior published guidance. Do all of these patients need repeat imaging? How do we manage their pain? Are there nonabdominal conditions that should be considered?
Broder and colleagues did a fantastic review of the current literature and, on behalf of SAEM, have provided a rational approach to optimal management of these patients. The four major questions they addressed, with brief summaries of their recommendations, are:
- Should adult ED patients with low-risk, recurrent and previously undifferentiated abdominal pain receive a repeat CT abdomen-pelvis (CTAP) after a negative CTAP within the past 12 months? This is a typical question that we all ponder when managing these patients. Unfortunately, the writing group found insufficient evidence to definitively identify populations in whom CTAP was recommended vs could be safely withheld. It is a bit disappointing that there is no definite answer to the question. On the other hand, it is reassuring to know that the world’s best evidence essentially says that it is perfectly appropriate to use your own good clinical judgment.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain with a negative CTAP receive additional imaging with abdominal ultrasound? In this case, the writing group found enough evidence, though low-level, to suggest against routine ultrasound in the absence of concern specifically for pelvic or hepatobiliary pathology. Like most tests, ultrasound is best used when there are specific concerns rather than being used in an undifferentiated fashion.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive screening for depression/anxiety? The writing group found enough evidence, though low-level again, to suggest that screening for depression and/or anxiety be performed during the ED evaluation. This could lead to successful therapy for the abdominal pain.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive nonopioid and/or nonpharmacologic analgesics? The writing group found little evidence to suggest for or against these analgesics, but they made a consensus recommendation suggesting an opioid-minimizing strategy for pain control.
Although the final recommendations of the writing group were not definitive or based on the strongest level of evidence, I find it helpful to have this guidance, nevertheless, on behalf of a major national organization. I also find it helpful to know that even with the best evidence available, optimal patient care will often boil down to physician experience and gestalt. I should also add that the overall article is chock-full of pearls and helpful information that will further inform the readers’ decisions, and so the full version is definitely worth the read.
In summary
There you have it – my three favorite practice-changing articles of 2022. Although I have tried to provide key points here, the full discussions of those key points in the published articles will provide a great deal more education than I can offer in this brief write-up, and so I strongly encourage everyone to read the full versions. Please be sure to include in the comments section your own pick for favorite or must-read articles from the past year.
Amal Mattu, MD, is a professor, vice chair of education, and codirector of the emergency cardiology fellowship in the department of emergency medicine at the University of Maryland, Baltimore. She reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The X-waiver is dead
In 2016, when Erin Schanning lost her brother Ethan to an overdose, she wanted to know what could have been done to have helped him. Ethan, who had struggled with opioids since getting a prescription for the drugs after a dental procedure in middle school, had tried dozens of treatments. But at the age of 30, he was gone.
“After my brother died, I started researching and was surprised to learn that there were many evidence-based ways to treat substance use disorder that he hadn’t had access to, even though he had doggedly pursued treatment,” Ms. Schanning told me in an interview. One of those treatments, buprenorphine, is one of the most effective tools that health care providers have to treat opioid use disorder. A partial opioid agonist, it reduces cravings and prevents overdose, decreasing mortality more effectively than almost any medication for any disease. Yet most providers have never prescribed it.
That may be about to change. The special license to prescribe the medication, commonly known as the “X-waiver,” was officially eliminated as part of the passage of the Mainstreaming Addiction Treatment (MAT) Act. Immediately, following the passage of the Act, any provider with a DEA license became eligible to prescribe buprenorphine to treat opioid use disorder, and limits on the number of patients they could treat were eliminated.
Previously, buprenorphine, which has a better safety profile than almost any other prescription opioid because of its ceiling effect on respiratory depression, nonetheless required providers to obtain a special license to prescribe it, and – prior to an executive order from the Biden administration – 8 to 24 hours of training to do so. This led to a misconception that buprenorphine was dangerous, and created barriers for treatment during the worst overdose crisis in our country’s history. More than 110,00 overdose deaths occurred in 2021, representing a 468% increase in the last 2 decades.
Along with the MAT Act, the Medication Access and Training Expansion Act was passed in the same spending bill, requiring all prescribers who obtain a DEA license to do 8 hours of training on the treatment of substance use disorders. According to the Act, addiction specialty societies will have a role in creating trainings. Medical schools and residencies will also be able to fulfill this requirement with a “comprehensive” curriculum that covers all approved medications for the treatment of substance use disorders.
The DEA has not yet confirmed what training will be accepted, according to the Chief Medical Officer of the Substance Abuse and Mental Health Services Administration, Neeraj Gandotra, MD, who spoke to me in an interview. However, it is required to do so by April 5, 2023. Dr. Gandotra also emphasized that state and local laws, as well as insurance requirements, remain in place, and may place other constraints on prescribing. According to the Act, this new rule will be in effect by June 2023.
As an addiction medicine specialist and longtime buprenorphine prescriber, I am excited about these changes but wary of lingering resistance among health care providers. Will providers who have chosen not to get an X-waiver now look for another reason to not treat patients with substance use disorders?
Ms. Schanning remains hopeful. “I’m incredibly optimistic that health care providers are going to learn about buprenorphine and prescribe it to patients, and that patients are going to start asking about this medication,” she told me. “Seven in 10 providers say that they do feel an obligation to treat their patients with [opioid use disorder], but the federal government has made it very difficult to do so.”
Now with the X-waiver gone, providers and patients may be able to push for a long overdue shift in how we treat and conceptualize substance use disorders, she noted.
“Health care providers need to recognize substance use disorder as a medical condition that deserves treatment, and to speak about it like a medical condition,” Ms. Schanning said, by, for instance, moving away from using words such as “abuse” and “clean” and, instead, talking about treatable substance use disorders that can improve with evidence-based care, such as buprenorphine and methadone. “We also need to share stories of success and hope with people,” she added. “Once you’ve seen how someone can be transformed by treatment, it’s really difficult to say that substance use disorder is a character flaw, or their fault.”
A patient-centered approach
Over the past decade of practicing medicine, I have experienced this transformation personally. In residency, I believed that people had to be ready for help, to stop using, to change. I failed to recognize that many of those same people were asking me for help, and I wasn’t offering what they needed. The person who had to change was me.
As I moved toward a patient-centered approach, lowering barriers to starting and remaining in treatment, and collaborating with teams that could meet people wherever they might be, addictions became the most rewarding part of my practice.
I have never had more people thank me spontaneously and deeply for the care I provide. Plus, I have never seen a more profound change in the students I work with than when they witness someone with a substance use disorder offered treatment that works.
The X-waiver was not the only barrier to care, and the overdose crisis is not slowing down. But maybe with a new tool widely accessible, more of us will be ready to help.
Dr. Poorman is board certified in internal medicine and addiction medicine, assistant professor of medicine, University of Illinois at Chicago, and provides primary care and addiction services in Chicago. Her views do not necessarily reflect the views of her employer. She has reported no relevant disclosures, and she serves on the editorial advisory board of Internal Medicine News.
In 2016, when Erin Schanning lost her brother Ethan to an overdose, she wanted to know what could have been done to have helped him. Ethan, who had struggled with opioids since getting a prescription for the drugs after a dental procedure in middle school, had tried dozens of treatments. But at the age of 30, he was gone.
“After my brother died, I started researching and was surprised to learn that there were many evidence-based ways to treat substance use disorder that he hadn’t had access to, even though he had doggedly pursued treatment,” Ms. Schanning told me in an interview. One of those treatments, buprenorphine, is one of the most effective tools that health care providers have to treat opioid use disorder. A partial opioid agonist, it reduces cravings and prevents overdose, decreasing mortality more effectively than almost any medication for any disease. Yet most providers have never prescribed it.
That may be about to change. The special license to prescribe the medication, commonly known as the “X-waiver,” was officially eliminated as part of the passage of the Mainstreaming Addiction Treatment (MAT) Act. Immediately, following the passage of the Act, any provider with a DEA license became eligible to prescribe buprenorphine to treat opioid use disorder, and limits on the number of patients they could treat were eliminated.
Previously, buprenorphine, which has a better safety profile than almost any other prescription opioid because of its ceiling effect on respiratory depression, nonetheless required providers to obtain a special license to prescribe it, and – prior to an executive order from the Biden administration – 8 to 24 hours of training to do so. This led to a misconception that buprenorphine was dangerous, and created barriers for treatment during the worst overdose crisis in our country’s history. More than 110,00 overdose deaths occurred in 2021, representing a 468% increase in the last 2 decades.
Along with the MAT Act, the Medication Access and Training Expansion Act was passed in the same spending bill, requiring all prescribers who obtain a DEA license to do 8 hours of training on the treatment of substance use disorders. According to the Act, addiction specialty societies will have a role in creating trainings. Medical schools and residencies will also be able to fulfill this requirement with a “comprehensive” curriculum that covers all approved medications for the treatment of substance use disorders.
The DEA has not yet confirmed what training will be accepted, according to the Chief Medical Officer of the Substance Abuse and Mental Health Services Administration, Neeraj Gandotra, MD, who spoke to me in an interview. However, it is required to do so by April 5, 2023. Dr. Gandotra also emphasized that state and local laws, as well as insurance requirements, remain in place, and may place other constraints on prescribing. According to the Act, this new rule will be in effect by June 2023.
As an addiction medicine specialist and longtime buprenorphine prescriber, I am excited about these changes but wary of lingering resistance among health care providers. Will providers who have chosen not to get an X-waiver now look for another reason to not treat patients with substance use disorders?
Ms. Schanning remains hopeful. “I’m incredibly optimistic that health care providers are going to learn about buprenorphine and prescribe it to patients, and that patients are going to start asking about this medication,” she told me. “Seven in 10 providers say that they do feel an obligation to treat their patients with [opioid use disorder], but the federal government has made it very difficult to do so.”
Now with the X-waiver gone, providers and patients may be able to push for a long overdue shift in how we treat and conceptualize substance use disorders, she noted.
“Health care providers need to recognize substance use disorder as a medical condition that deserves treatment, and to speak about it like a medical condition,” Ms. Schanning said, by, for instance, moving away from using words such as “abuse” and “clean” and, instead, talking about treatable substance use disorders that can improve with evidence-based care, such as buprenorphine and methadone. “We also need to share stories of success and hope with people,” she added. “Once you’ve seen how someone can be transformed by treatment, it’s really difficult to say that substance use disorder is a character flaw, or their fault.”
A patient-centered approach
Over the past decade of practicing medicine, I have experienced this transformation personally. In residency, I believed that people had to be ready for help, to stop using, to change. I failed to recognize that many of those same people were asking me for help, and I wasn’t offering what they needed. The person who had to change was me.
As I moved toward a patient-centered approach, lowering barriers to starting and remaining in treatment, and collaborating with teams that could meet people wherever they might be, addictions became the most rewarding part of my practice.
I have never had more people thank me spontaneously and deeply for the care I provide. Plus, I have never seen a more profound change in the students I work with than when they witness someone with a substance use disorder offered treatment that works.
The X-waiver was not the only barrier to care, and the overdose crisis is not slowing down. But maybe with a new tool widely accessible, more of us will be ready to help.
Dr. Poorman is board certified in internal medicine and addiction medicine, assistant professor of medicine, University of Illinois at Chicago, and provides primary care and addiction services in Chicago. Her views do not necessarily reflect the views of her employer. She has reported no relevant disclosures, and she serves on the editorial advisory board of Internal Medicine News.
In 2016, when Erin Schanning lost her brother Ethan to an overdose, she wanted to know what could have been done to have helped him. Ethan, who had struggled with opioids since getting a prescription for the drugs after a dental procedure in middle school, had tried dozens of treatments. But at the age of 30, he was gone.
“After my brother died, I started researching and was surprised to learn that there were many evidence-based ways to treat substance use disorder that he hadn’t had access to, even though he had doggedly pursued treatment,” Ms. Schanning told me in an interview. One of those treatments, buprenorphine, is one of the most effective tools that health care providers have to treat opioid use disorder. A partial opioid agonist, it reduces cravings and prevents overdose, decreasing mortality more effectively than almost any medication for any disease. Yet most providers have never prescribed it.
That may be about to change. The special license to prescribe the medication, commonly known as the “X-waiver,” was officially eliminated as part of the passage of the Mainstreaming Addiction Treatment (MAT) Act. Immediately, following the passage of the Act, any provider with a DEA license became eligible to prescribe buprenorphine to treat opioid use disorder, and limits on the number of patients they could treat were eliminated.
Previously, buprenorphine, which has a better safety profile than almost any other prescription opioid because of its ceiling effect on respiratory depression, nonetheless required providers to obtain a special license to prescribe it, and – prior to an executive order from the Biden administration – 8 to 24 hours of training to do so. This led to a misconception that buprenorphine was dangerous, and created barriers for treatment during the worst overdose crisis in our country’s history. More than 110,00 overdose deaths occurred in 2021, representing a 468% increase in the last 2 decades.
Along with the MAT Act, the Medication Access and Training Expansion Act was passed in the same spending bill, requiring all prescribers who obtain a DEA license to do 8 hours of training on the treatment of substance use disorders. According to the Act, addiction specialty societies will have a role in creating trainings. Medical schools and residencies will also be able to fulfill this requirement with a “comprehensive” curriculum that covers all approved medications for the treatment of substance use disorders.
The DEA has not yet confirmed what training will be accepted, according to the Chief Medical Officer of the Substance Abuse and Mental Health Services Administration, Neeraj Gandotra, MD, who spoke to me in an interview. However, it is required to do so by April 5, 2023. Dr. Gandotra also emphasized that state and local laws, as well as insurance requirements, remain in place, and may place other constraints on prescribing. According to the Act, this new rule will be in effect by June 2023.
As an addiction medicine specialist and longtime buprenorphine prescriber, I am excited about these changes but wary of lingering resistance among health care providers. Will providers who have chosen not to get an X-waiver now look for another reason to not treat patients with substance use disorders?
Ms. Schanning remains hopeful. “I’m incredibly optimistic that health care providers are going to learn about buprenorphine and prescribe it to patients, and that patients are going to start asking about this medication,” she told me. “Seven in 10 providers say that they do feel an obligation to treat their patients with [opioid use disorder], but the federal government has made it very difficult to do so.”
Now with the X-waiver gone, providers and patients may be able to push for a long overdue shift in how we treat and conceptualize substance use disorders, she noted.
“Health care providers need to recognize substance use disorder as a medical condition that deserves treatment, and to speak about it like a medical condition,” Ms. Schanning said, by, for instance, moving away from using words such as “abuse” and “clean” and, instead, talking about treatable substance use disorders that can improve with evidence-based care, such as buprenorphine and methadone. “We also need to share stories of success and hope with people,” she added. “Once you’ve seen how someone can be transformed by treatment, it’s really difficult to say that substance use disorder is a character flaw, or their fault.”
A patient-centered approach
Over the past decade of practicing medicine, I have experienced this transformation personally. In residency, I believed that people had to be ready for help, to stop using, to change. I failed to recognize that many of those same people were asking me for help, and I wasn’t offering what they needed. The person who had to change was me.
As I moved toward a patient-centered approach, lowering barriers to starting and remaining in treatment, and collaborating with teams that could meet people wherever they might be, addictions became the most rewarding part of my practice.
I have never had more people thank me spontaneously and deeply for the care I provide. Plus, I have never seen a more profound change in the students I work with than when they witness someone with a substance use disorder offered treatment that works.
The X-waiver was not the only barrier to care, and the overdose crisis is not slowing down. But maybe with a new tool widely accessible, more of us will be ready to help.
Dr. Poorman is board certified in internal medicine and addiction medicine, assistant professor of medicine, University of Illinois at Chicago, and provides primary care and addiction services in Chicago. Her views do not necessarily reflect the views of her employer. She has reported no relevant disclosures, and she serves on the editorial advisory board of Internal Medicine News.
The five biggest changes in the 2023 adult vaccine schedules
This transcript has been edited for clarity.
Hello. I’m Dr Sandra Fryhofer. Welcome to Medicine Matters.
It’s a new year, which means a new ACIP adult immunization schedule – a valuable resource collating ACIP’s most up-to-date vaccination recommendations.
Here are this year’s five most important changes:
- COVID vaccines now front and center
- New emphasis on polio vaccination
- Inclusion of some nonvaccine products (such as monoclonal antibody products)
- Pharmacists group has approved the schedule for the first time
- New shared clinical decision-making option for pneumococcal vaccines
The schedule’s organization remains the same. It still has four sections:
- Table 1: vaccinations by age
- Table 2: vaccinations by medical condition and other indications
- The Notes section (alphabetically ordered by vaccine type)
- Appendix listing of vaccine-specific contraindications and precautions
But what’s unique this year is that some of the abbreviations have historical implications. The first change is no big surprise in light of what we’ve gone through in the past few years. COVID vaccines are listed first on the cover page by brand name for those authorized and by company name for those still under US emergency use authorization. They’re also listed first on the graphics and in the notes.
COVID and mRNA and protein-based vaccines have now been assigned official abbreviations based on vaccine platform and valency.
- 1vCOV-mRNA: Comirnaty/Pfizer-BioNTech and Spikevax Moderna COVID-19 vaccines
- 2vCOV-mRNA: Pfizer-BioNTech and Moderna bivalent COVID-19 vaccines
- 1vCOV-aPS: Novavax COVID-19 vaccine
Also remarkable is the absence of COVID viral vector vaccines on the list. However, the viral vector COVID vaccine (which has been available but is not preferred) does have a CDC website link in the Notes section.
A sad but necessary inclusion was triggered by recent polio cases in New York. Polio was believed to be eradicated, and we thought adults no longer needed to be vaccinated against polio. In the new schedule, the polio vaccine is listed on the cover page but is not included in the tables. Current polio vaccination recommendations are now in the Notes section.
Also of historical significance and something that may set a precedent is the inclusion of nonvaccine products. The value of COVID preexposure prophylaxis with products including monoclonal antibodies (such as Evusheld) for people who are moderately or severely immunocompromised is mentioned in the Notes section.
For the first time ever, the schedule has been approved by the American Pharmacists Association, which validates pharmacists as established partners in vaccine administration.
Color-code key
One aspect of the schedule that has not changed is the color-code key:
- Yellow: Recommended if the patient meets the age requirement
- Purple: Indicated for those with additional risk factors or another indication
- Blue: Recommended based on shared clinical decision-making
- Orange: Precaution
- Red: Contraindicated or not recommended; the vaccine should not be administered. Overlays on the red more precisely clarify whether a vaccine is really contraindicated or just not recommended. An asterisk on red means vaccinate after pregnancy if indicated.
- Gray: No recommendation or not applicable
Vaccinations by age
Table 1 lists recommended vaccinations by age. There is one major change. COVID vaccines are on the first row of the graphic, with the need for both a primary series and boosters emphasized on the overlay. The notes have hyperlinks to the most up-to-date COVID vaccination recommendations.
Pneumococcal vaccination. Pneumococcal vaccination is routinely recommended starting at age 65. Current recommendations for those not previously vaccinated have not changed since last year. But on Table 1, the bottom half of the row for those 65 or older is now blue (and that’s new). This new color blue means shared clinical decision-making and applies to people who were previously considered fully vaccinated with the now extinct combination of PCV13 and PPSV23. These patients now have the option of getting a dose of PCV20 five years after completing their PCV13-PPSV23 combo series. This option is blue because the decision is up to you and your patient.
Check the notes for more pneumococcal vaccination details. For example, for those partially vaccinated using lower valency vaccines, there’s an option of substituting PCV20 for PPSV23 to broaden and increase durability of protection.
The pneumococcal vaccination recommendation options are complicated. A new pneumococcal vaccination app can help.
Hepatitis B. For adults under age 60, the color code for the hepatitis B vaccine is yellow, meaning it’s indicated for all. For older patients, the color code is purple. If a patient who is age 60 or older wants the hepatitis B vaccine, they can have it even in the absence of additional risk indications.
Vaccinations by medical condition or other indications
Other than a few minor word changes on the overlay, the only thing that’s new is the COVID vaccine row.
This table is helpful for matching vaccine recommendations with specific medical conditions, including pregnancy, immunocompromise, HIV (with specifics according to CD4 count), asplenia, complement deficiencies, heart disease, lung disease, alcoholism, chronic liver disease, diabetes, health care personnel, and men who have sex with men.
Use this table to dot the i’s and cross the t’s when it comes to vaccination recommendations. For example, take a look at the pregnancy column. Live virus vaccines, including LAIV, MMR, and varicella, are contraindicated and color-coded red. MMR and varicella also have an asterisk, meaning vaccinate after pregnancy if indicated. HPV vaccines are not live virus vaccines, but the overlay says they are not recommended during pregnancy. The asterisk indicates that you can vaccinate after pregnancy.
Vaccine notes
The notes are in alphabetical order, and their organization (routine, special situations, and shared clinical decision-making when indicated) has not changed. They are concise and succinct, but sometimes they’re not enough. That’s why vaccine-specific links to more complete recommendations are so convenient.
Notes for hepatitis B contain nuances on specific dosing for vaccinating patients on dialysis, as well as a reminder that newer hepatitis C vaccines such as Heplisav and PreHevbrio are not recommended during pregnancy due to lack of safety data.
For influenza, everyone 6 months or older still needs yearly flu vaccination with an age- and health-appropriate flu vaccine. But for those aged 65 or older, the notes specify the three vaccine versions now preferred: high-dose, recombinant, or adjuvanted versions. However, if these aren’t available, it’s better to get any flu vaccine than to go without.
Under meningococcal vaccines, the notes for MenACWY and MenB are combined. For MenB, trade names Bexsero and Trumenba are specified because the products are not interchangeable. Booster intervals for those still at risk are different for each vaccine type: every 5 years for MenACWY boosters, and every 2-3 years for boosts of MenB.
The recent polio cases in New York have put polio vaccination in the spotlight. ACIP has now reinstated its Polio Vaccine Work Group. The new schedule lists polio vaccines on the cover page. Current recommendations have been added to the notes section. Routine vaccination for adults is not necessary, at least for now. However, those at increased risk for exposure to polio fall in the special-situation category. For those at increased risk who have completed a polio vaccine series, a single lifetime IPV booster can be given. For those at increased risk who have not completed their polio vaccine series, now would be the time to finish the series.
Appendix
The final step in using the new schedule is checking the appendix and its list of vaccine-specific contraindications and precautions.
I hope this review of the new ACIP adult immunization schedule has been helpful. For Medicine Matters, I’m Dr. Sandra Fryhofer.
Dr. Fryhofer is clinical associate professor of medicine, Emory University, Atlanta. She reported numerous conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hello. I’m Dr Sandra Fryhofer. Welcome to Medicine Matters.
It’s a new year, which means a new ACIP adult immunization schedule – a valuable resource collating ACIP’s most up-to-date vaccination recommendations.
Here are this year’s five most important changes:
- COVID vaccines now front and center
- New emphasis on polio vaccination
- Inclusion of some nonvaccine products (such as monoclonal antibody products)
- Pharmacists group has approved the schedule for the first time
- New shared clinical decision-making option for pneumococcal vaccines
The schedule’s organization remains the same. It still has four sections:
- Table 1: vaccinations by age
- Table 2: vaccinations by medical condition and other indications
- The Notes section (alphabetically ordered by vaccine type)
- Appendix listing of vaccine-specific contraindications and precautions
But what’s unique this year is that some of the abbreviations have historical implications. The first change is no big surprise in light of what we’ve gone through in the past few years. COVID vaccines are listed first on the cover page by brand name for those authorized and by company name for those still under US emergency use authorization. They’re also listed first on the graphics and in the notes.
COVID and mRNA and protein-based vaccines have now been assigned official abbreviations based on vaccine platform and valency.
- 1vCOV-mRNA: Comirnaty/Pfizer-BioNTech and Spikevax Moderna COVID-19 vaccines
- 2vCOV-mRNA: Pfizer-BioNTech and Moderna bivalent COVID-19 vaccines
- 1vCOV-aPS: Novavax COVID-19 vaccine
Also remarkable is the absence of COVID viral vector vaccines on the list. However, the viral vector COVID vaccine (which has been available but is not preferred) does have a CDC website link in the Notes section.
A sad but necessary inclusion was triggered by recent polio cases in New York. Polio was believed to be eradicated, and we thought adults no longer needed to be vaccinated against polio. In the new schedule, the polio vaccine is listed on the cover page but is not included in the tables. Current polio vaccination recommendations are now in the Notes section.
Also of historical significance and something that may set a precedent is the inclusion of nonvaccine products. The value of COVID preexposure prophylaxis with products including monoclonal antibodies (such as Evusheld) for people who are moderately or severely immunocompromised is mentioned in the Notes section.
For the first time ever, the schedule has been approved by the American Pharmacists Association, which validates pharmacists as established partners in vaccine administration.
Color-code key
One aspect of the schedule that has not changed is the color-code key:
- Yellow: Recommended if the patient meets the age requirement
- Purple: Indicated for those with additional risk factors or another indication
- Blue: Recommended based on shared clinical decision-making
- Orange: Precaution
- Red: Contraindicated or not recommended; the vaccine should not be administered. Overlays on the red more precisely clarify whether a vaccine is really contraindicated or just not recommended. An asterisk on red means vaccinate after pregnancy if indicated.
- Gray: No recommendation or not applicable
Vaccinations by age
Table 1 lists recommended vaccinations by age. There is one major change. COVID vaccines are on the first row of the graphic, with the need for both a primary series and boosters emphasized on the overlay. The notes have hyperlinks to the most up-to-date COVID vaccination recommendations.
Pneumococcal vaccination. Pneumococcal vaccination is routinely recommended starting at age 65. Current recommendations for those not previously vaccinated have not changed since last year. But on Table 1, the bottom half of the row for those 65 or older is now blue (and that’s new). This new color blue means shared clinical decision-making and applies to people who were previously considered fully vaccinated with the now extinct combination of PCV13 and PPSV23. These patients now have the option of getting a dose of PCV20 five years after completing their PCV13-PPSV23 combo series. This option is blue because the decision is up to you and your patient.
Check the notes for more pneumococcal vaccination details. For example, for those partially vaccinated using lower valency vaccines, there’s an option of substituting PCV20 for PPSV23 to broaden and increase durability of protection.
The pneumococcal vaccination recommendation options are complicated. A new pneumococcal vaccination app can help.
Hepatitis B. For adults under age 60, the color code for the hepatitis B vaccine is yellow, meaning it’s indicated for all. For older patients, the color code is purple. If a patient who is age 60 or older wants the hepatitis B vaccine, they can have it even in the absence of additional risk indications.
Vaccinations by medical condition or other indications
Other than a few minor word changes on the overlay, the only thing that’s new is the COVID vaccine row.
This table is helpful for matching vaccine recommendations with specific medical conditions, including pregnancy, immunocompromise, HIV (with specifics according to CD4 count), asplenia, complement deficiencies, heart disease, lung disease, alcoholism, chronic liver disease, diabetes, health care personnel, and men who have sex with men.
Use this table to dot the i’s and cross the t’s when it comes to vaccination recommendations. For example, take a look at the pregnancy column. Live virus vaccines, including LAIV, MMR, and varicella, are contraindicated and color-coded red. MMR and varicella also have an asterisk, meaning vaccinate after pregnancy if indicated. HPV vaccines are not live virus vaccines, but the overlay says they are not recommended during pregnancy. The asterisk indicates that you can vaccinate after pregnancy.
Vaccine notes
The notes are in alphabetical order, and their organization (routine, special situations, and shared clinical decision-making when indicated) has not changed. They are concise and succinct, but sometimes they’re not enough. That’s why vaccine-specific links to more complete recommendations are so convenient.
Notes for hepatitis B contain nuances on specific dosing for vaccinating patients on dialysis, as well as a reminder that newer hepatitis C vaccines such as Heplisav and PreHevbrio are not recommended during pregnancy due to lack of safety data.
For influenza, everyone 6 months or older still needs yearly flu vaccination with an age- and health-appropriate flu vaccine. But for those aged 65 or older, the notes specify the three vaccine versions now preferred: high-dose, recombinant, or adjuvanted versions. However, if these aren’t available, it’s better to get any flu vaccine than to go without.
Under meningococcal vaccines, the notes for MenACWY and MenB are combined. For MenB, trade names Bexsero and Trumenba are specified because the products are not interchangeable. Booster intervals for those still at risk are different for each vaccine type: every 5 years for MenACWY boosters, and every 2-3 years for boosts of MenB.
The recent polio cases in New York have put polio vaccination in the spotlight. ACIP has now reinstated its Polio Vaccine Work Group. The new schedule lists polio vaccines on the cover page. Current recommendations have been added to the notes section. Routine vaccination for adults is not necessary, at least for now. However, those at increased risk for exposure to polio fall in the special-situation category. For those at increased risk who have completed a polio vaccine series, a single lifetime IPV booster can be given. For those at increased risk who have not completed their polio vaccine series, now would be the time to finish the series.
Appendix
The final step in using the new schedule is checking the appendix and its list of vaccine-specific contraindications and precautions.
I hope this review of the new ACIP adult immunization schedule has been helpful. For Medicine Matters, I’m Dr. Sandra Fryhofer.
Dr. Fryhofer is clinical associate professor of medicine, Emory University, Atlanta. She reported numerous conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hello. I’m Dr Sandra Fryhofer. Welcome to Medicine Matters.
It’s a new year, which means a new ACIP adult immunization schedule – a valuable resource collating ACIP’s most up-to-date vaccination recommendations.
Here are this year’s five most important changes:
- COVID vaccines now front and center
- New emphasis on polio vaccination
- Inclusion of some nonvaccine products (such as monoclonal antibody products)
- Pharmacists group has approved the schedule for the first time
- New shared clinical decision-making option for pneumococcal vaccines
The schedule’s organization remains the same. It still has four sections:
- Table 1: vaccinations by age
- Table 2: vaccinations by medical condition and other indications
- The Notes section (alphabetically ordered by vaccine type)
- Appendix listing of vaccine-specific contraindications and precautions
But what’s unique this year is that some of the abbreviations have historical implications. The first change is no big surprise in light of what we’ve gone through in the past few years. COVID vaccines are listed first on the cover page by brand name for those authorized and by company name for those still under US emergency use authorization. They’re also listed first on the graphics and in the notes.
COVID and mRNA and protein-based vaccines have now been assigned official abbreviations based on vaccine platform and valency.
- 1vCOV-mRNA: Comirnaty/Pfizer-BioNTech and Spikevax Moderna COVID-19 vaccines
- 2vCOV-mRNA: Pfizer-BioNTech and Moderna bivalent COVID-19 vaccines
- 1vCOV-aPS: Novavax COVID-19 vaccine
Also remarkable is the absence of COVID viral vector vaccines on the list. However, the viral vector COVID vaccine (which has been available but is not preferred) does have a CDC website link in the Notes section.
A sad but necessary inclusion was triggered by recent polio cases in New York. Polio was believed to be eradicated, and we thought adults no longer needed to be vaccinated against polio. In the new schedule, the polio vaccine is listed on the cover page but is not included in the tables. Current polio vaccination recommendations are now in the Notes section.
Also of historical significance and something that may set a precedent is the inclusion of nonvaccine products. The value of COVID preexposure prophylaxis with products including monoclonal antibodies (such as Evusheld) for people who are moderately or severely immunocompromised is mentioned in the Notes section.
For the first time ever, the schedule has been approved by the American Pharmacists Association, which validates pharmacists as established partners in vaccine administration.
Color-code key
One aspect of the schedule that has not changed is the color-code key:
- Yellow: Recommended if the patient meets the age requirement
- Purple: Indicated for those with additional risk factors or another indication
- Blue: Recommended based on shared clinical decision-making
- Orange: Precaution
- Red: Contraindicated or not recommended; the vaccine should not be administered. Overlays on the red more precisely clarify whether a vaccine is really contraindicated or just not recommended. An asterisk on red means vaccinate after pregnancy if indicated.
- Gray: No recommendation or not applicable
Vaccinations by age
Table 1 lists recommended vaccinations by age. There is one major change. COVID vaccines are on the first row of the graphic, with the need for both a primary series and boosters emphasized on the overlay. The notes have hyperlinks to the most up-to-date COVID vaccination recommendations.
Pneumococcal vaccination. Pneumococcal vaccination is routinely recommended starting at age 65. Current recommendations for those not previously vaccinated have not changed since last year. But on Table 1, the bottom half of the row for those 65 or older is now blue (and that’s new). This new color blue means shared clinical decision-making and applies to people who were previously considered fully vaccinated with the now extinct combination of PCV13 and PPSV23. These patients now have the option of getting a dose of PCV20 five years after completing their PCV13-PPSV23 combo series. This option is blue because the decision is up to you and your patient.
Check the notes for more pneumococcal vaccination details. For example, for those partially vaccinated using lower valency vaccines, there’s an option of substituting PCV20 for PPSV23 to broaden and increase durability of protection.
The pneumococcal vaccination recommendation options are complicated. A new pneumococcal vaccination app can help.
Hepatitis B. For adults under age 60, the color code for the hepatitis B vaccine is yellow, meaning it’s indicated for all. For older patients, the color code is purple. If a patient who is age 60 or older wants the hepatitis B vaccine, they can have it even in the absence of additional risk indications.
Vaccinations by medical condition or other indications
Other than a few minor word changes on the overlay, the only thing that’s new is the COVID vaccine row.
This table is helpful for matching vaccine recommendations with specific medical conditions, including pregnancy, immunocompromise, HIV (with specifics according to CD4 count), asplenia, complement deficiencies, heart disease, lung disease, alcoholism, chronic liver disease, diabetes, health care personnel, and men who have sex with men.
Use this table to dot the i’s and cross the t’s when it comes to vaccination recommendations. For example, take a look at the pregnancy column. Live virus vaccines, including LAIV, MMR, and varicella, are contraindicated and color-coded red. MMR and varicella also have an asterisk, meaning vaccinate after pregnancy if indicated. HPV vaccines are not live virus vaccines, but the overlay says they are not recommended during pregnancy. The asterisk indicates that you can vaccinate after pregnancy.
Vaccine notes
The notes are in alphabetical order, and their organization (routine, special situations, and shared clinical decision-making when indicated) has not changed. They are concise and succinct, but sometimes they’re not enough. That’s why vaccine-specific links to more complete recommendations are so convenient.
Notes for hepatitis B contain nuances on specific dosing for vaccinating patients on dialysis, as well as a reminder that newer hepatitis C vaccines such as Heplisav and PreHevbrio are not recommended during pregnancy due to lack of safety data.
For influenza, everyone 6 months or older still needs yearly flu vaccination with an age- and health-appropriate flu vaccine. But for those aged 65 or older, the notes specify the three vaccine versions now preferred: high-dose, recombinant, or adjuvanted versions. However, if these aren’t available, it’s better to get any flu vaccine than to go without.
Under meningococcal vaccines, the notes for MenACWY and MenB are combined. For MenB, trade names Bexsero and Trumenba are specified because the products are not interchangeable. Booster intervals for those still at risk are different for each vaccine type: every 5 years for MenACWY boosters, and every 2-3 years for boosts of MenB.
The recent polio cases in New York have put polio vaccination in the spotlight. ACIP has now reinstated its Polio Vaccine Work Group. The new schedule lists polio vaccines on the cover page. Current recommendations have been added to the notes section. Routine vaccination for adults is not necessary, at least for now. However, those at increased risk for exposure to polio fall in the special-situation category. For those at increased risk who have completed a polio vaccine series, a single lifetime IPV booster can be given. For those at increased risk who have not completed their polio vaccine series, now would be the time to finish the series.
Appendix
The final step in using the new schedule is checking the appendix and its list of vaccine-specific contraindications and precautions.
I hope this review of the new ACIP adult immunization schedule has been helpful. For Medicine Matters, I’m Dr. Sandra Fryhofer.
Dr. Fryhofer is clinical associate professor of medicine, Emory University, Atlanta. She reported numerous conflicts of interest.
A version of this article first appeared on Medscape.com.
Finding catatonia requires knowing what to look for
Catatonia is a psychomotor syndrome identified by its clinical phenotype. Unlike common psychiatric syndromes such as major depression that are characterized by self-report of symptoms, catatonia is identified chiefly by empirically evaluated signs on clinical evaluation. Its signs are recognized through observation, physical examination, or elicitation by clinical maneuvers or the presentation of stimuli. However, catatonia is often overlooked even though its clinical signs are often visibly apparent, including to the casual observer.
Why is catatonia underdiagnosed? A key modifiable factor appears to be a prevalent misunderstanding over what catatonia looks like.1 We have sought to address this in a few ways.
First identified was the need for comprehensive educational resources on how to assess for and recognize catatonia. Using the Bush-Francis Catatonia Rating Scale – the most widely used scale for catatonia in both research and clinical settings and the most cited publication in the catatonia literature – our team developed the BFCRS Training Manual and Coding Guide.2,3 This manual expands on the definitions of each BFCRS item based on how it was originally operationalized by the scale’s authors. Subsequently, we created a comprehensive set of educational resources including videos illustrating how to assess for catatonia, a video for each of the 23 items on the BFCRS, and self-assessment tools. All resources are freely available online at https://bfcrs.urmc.edu.4
Through this project it became apparent that there are many discrepancies across the field regarding the phenotype of catatonia. Specifically, a recent review inspired by this project set about to characterize the scope of distinctions across diagnostic systems and rating scales.5 For instance, each diagnostic system and rating scale includes a unique set of signs, approaches diagnostic thresholds differently, and often operationalizes clinical features in ways that lead either to criterion overlap (for example, combativeness would be scored both as combativeness and agitation on ICD-11) or contradictions with other systems or scales (for example, varied definitions of waxy flexibility). In the face of so many inconsistencies, what is a clinician to do? What follows is a discussion of how to apply the insights from this recent review in clinical and research settings.
Starting with DSM-5-TR and ICD-11 – the current editions of the two leading diagnostic systems – one might ask: How do they compare?6,7 Overall, these two systems are broadly aligned in terms of the catatonic syndrome. Both systems identify individual clinical signs (as opposed to symptom complexes). Both require three features as a diagnostic threshold. Most of the same clinical signs are included in both systems, and the definitions of individual items are largely equivalent. Additionally, both systems allow for diagnosis of catatonia in association with psychiatric and medical conditions and include a category for unspecified catatonia.
Despite these core agreements, though, there are several important distinctions. First, whereas all 12 signs included in DSM-5-TR count toward an ICD-11 catatonia diagnosis, the opposite cannot be said. ICD-11 includes several features that are not in DSM-5-TR: rigidity, verbigeration, withdrawal, staring, ambitendency, impulsivity, and combativeness. Next, autonomic abnormality, which signifies the most severe type of catatonia called malignant catatonia, is included as a potential comorbidity in ICD-11 but not mentioned in DSM-5-TR. Third, ICD-11 includes a separate diagnosis for substance-induced catatonia, whereas this condition would be diagnosed as unspecified catatonia in DSM-5-TR.
There are also elements missing from both systems. The most notable of these is that neither system specifies the period over which findings must be present for diagnosis. By clinical convention, the practical definition of 24 hours is appropriate in most instances. The clinical features identified during direct evaluation are usually sufficient for diagnosis, but additional signs observed or documented over the prior 24 hours should be incorporated as part of the clinical evaluation. Another distinction is how to handle clinical features before and after lorazepam challenge. As noted in the BFCRS Training Manual, it would be appropriate to compare “state assessments” (that is, restricted to features identified only during direct, in-person assessment) from before and after lorazepam administration to document improvement.4
Whereas DSM-5-TR and ICD-11 are broadly in agreement, comparing these systems with catatonia rating scales reveals many sources of potential confusion, but also concrete guidance on operationalizing individual items.5 How exactly should each of catatonia’s clinical signs be defined? Descriptions differ, and thresholds of duration and frequency vary considerably across scales. As a result, clinicians who use different scales and then convert these results to diagnostic criteria are liable to come to different clinical conclusions. For instance, both echophenomena and negativism must be elicited more than five times to be scored per Northoff,8 but even a single convincing instance of either would be scored on the BFCRS as “occasional.”2
Such discrepancies are important because, whereas the psychometric properties of several catatonia scales have been documented, there are no analogous studies on the DSM-5-TR and ICD-11 criteria. Therefore, it is essential for clinicians and researchers to document how diagnostic criteria have been operationalized. The most practical and evidence-based way to do this is to use a clinically validated scale and convert these to diagnostic criteria, yet in doing so a few modifications will be necessary.
Of the available clinical scales, the BFCRS is best positioned for clinical use. The BFCRS has been validated clinically and has good reliability, detailed item definitions and audiovisual examples available. In addition, it is the only scale with a published semistructured evaluation (see initial paper and Training Manual), which takes about 5 minutes.2,4 In terms of utility, all 12 signs included by DSM-5-TR are among the first 14 items on the BFCRS, which constitutes a standalone tool known as the Bush-Francis Catatonia Screening Instrument (BFCSI, see Table).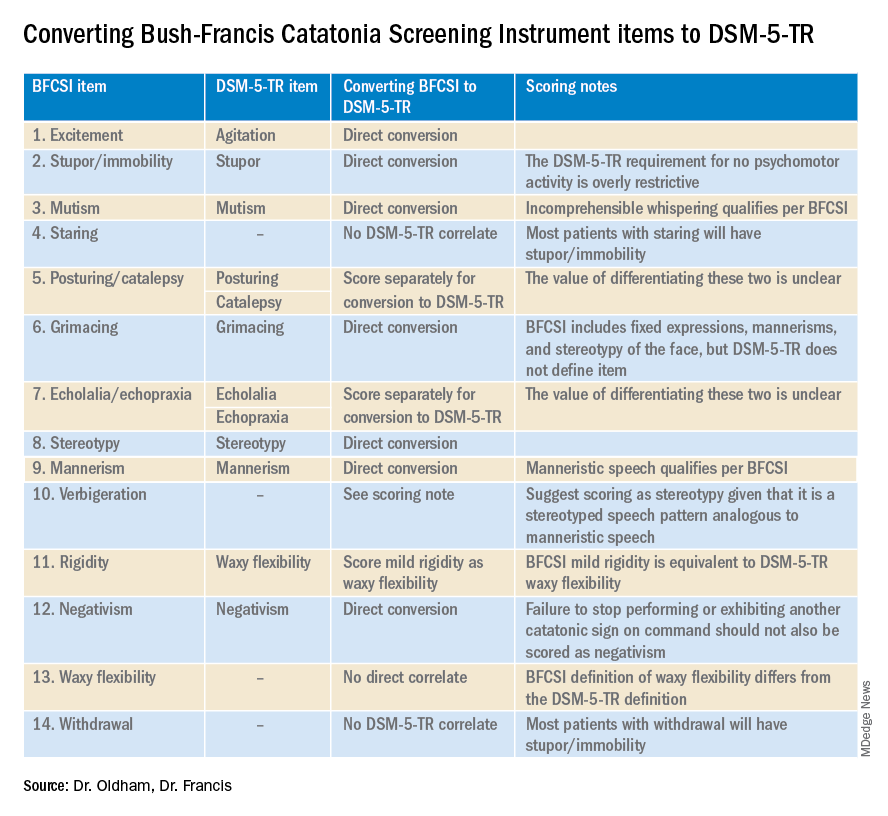
Many fundamental questions remain about catatonia,but the importance of a shared understanding of its clinical features is clear.9 Catatonia should be on the differential whenever a patient exhibits a markedly altered level of activity or grossly abnormal behavior, especially when inappropriate to context. We encourage readers to familiarize themselves with the phenotype of catatonia through online educational resources4 because the optimal care of patients with catatonia requires – at a minimum – that we know what we’re looking for.
Dr. Oldham is assistant professor of psychiatry at the University of Rochester (N.Y.) Medical Center. Dr. Francis is professor of psychiatry at Penn State University, Hershey. The authors declare no relevant conflicts of interest. Funding for the educational project hosted at https://bfcrs.urmc.edu was provided by the department of psychiatry at the University of Rochester Medical Center. Dr. Oldham is currently supported by a K23 career development award from the National Institute on Aging (AG072383). The educational resources referenced in this piece could not have been created were it not for the intellectual and thespian collaboration of Joshua R. Wortzel, MD, who is currently a fellow in child and adolescent psychiatry at Brown University, Providence, R.I. The authors are also indebted to Hochang B. Lee, MD, for his gracious support of this project.
References
1. Wortzel JR et al. J Clin Psychiatry. 2021 Aug 17;82(5):21m14025. doi: 10.4088/JCP.21m14025.
2. Bush G et al. Acta Psychiatr Scand. 1996 Feb;93(2):129-36. doi: 10.1111/j.1600-0447.1996.tb09814.x.
3. Weleff J et al. J Acad Consult Liaison Psychiatry. 2023 Jan-Feb;64(1):13-27. doi:10.1016/j.jaclp.2022.07.002.
4. Oldham MA et al. Bush-Francis Catatonia Rating Scale Assessment Resources. University of Rochester Medical Center, Department of Psychiatry. https://bfcrs.urmc.edu.
5. Oldham MA. Schizophr Res. 2022 Aug 19;S0920-9964(22)00294-8. doi: 10.1016/j.schres.2022.08.002.
6. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5-TR. Washington, D.C.: American Psychiatric Association Publishing, 2022.
7. World Health Organization. ICD-11 for Mortality and Morbidity Stastistics. 2022. https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/486722075.
8. Northoff G et al. Mov Disord. May 1999;14(3):404-16. doi: 10.1002/1531-8257(199905)14:3<404::AID-MDS1004>3.0.CO;2-5.
9. Walther S et al. The Lancet Psychiatry. 2019 Jul;6(7):610-9. doi: 10.1016/S2215-0366(18)30474-7.
Catatonia is a psychomotor syndrome identified by its clinical phenotype. Unlike common psychiatric syndromes such as major depression that are characterized by self-report of symptoms, catatonia is identified chiefly by empirically evaluated signs on clinical evaluation. Its signs are recognized through observation, physical examination, or elicitation by clinical maneuvers or the presentation of stimuli. However, catatonia is often overlooked even though its clinical signs are often visibly apparent, including to the casual observer.
Why is catatonia underdiagnosed? A key modifiable factor appears to be a prevalent misunderstanding over what catatonia looks like.1 We have sought to address this in a few ways.
First identified was the need for comprehensive educational resources on how to assess for and recognize catatonia. Using the Bush-Francis Catatonia Rating Scale – the most widely used scale for catatonia in both research and clinical settings and the most cited publication in the catatonia literature – our team developed the BFCRS Training Manual and Coding Guide.2,3 This manual expands on the definitions of each BFCRS item based on how it was originally operationalized by the scale’s authors. Subsequently, we created a comprehensive set of educational resources including videos illustrating how to assess for catatonia, a video for each of the 23 items on the BFCRS, and self-assessment tools. All resources are freely available online at https://bfcrs.urmc.edu.4
Through this project it became apparent that there are many discrepancies across the field regarding the phenotype of catatonia. Specifically, a recent review inspired by this project set about to characterize the scope of distinctions across diagnostic systems and rating scales.5 For instance, each diagnostic system and rating scale includes a unique set of signs, approaches diagnostic thresholds differently, and often operationalizes clinical features in ways that lead either to criterion overlap (for example, combativeness would be scored both as combativeness and agitation on ICD-11) or contradictions with other systems or scales (for example, varied definitions of waxy flexibility). In the face of so many inconsistencies, what is a clinician to do? What follows is a discussion of how to apply the insights from this recent review in clinical and research settings.
Starting with DSM-5-TR and ICD-11 – the current editions of the two leading diagnostic systems – one might ask: How do they compare?6,7 Overall, these two systems are broadly aligned in terms of the catatonic syndrome. Both systems identify individual clinical signs (as opposed to symptom complexes). Both require three features as a diagnostic threshold. Most of the same clinical signs are included in both systems, and the definitions of individual items are largely equivalent. Additionally, both systems allow for diagnosis of catatonia in association with psychiatric and medical conditions and include a category for unspecified catatonia.
Despite these core agreements, though, there are several important distinctions. First, whereas all 12 signs included in DSM-5-TR count toward an ICD-11 catatonia diagnosis, the opposite cannot be said. ICD-11 includes several features that are not in DSM-5-TR: rigidity, verbigeration, withdrawal, staring, ambitendency, impulsivity, and combativeness. Next, autonomic abnormality, which signifies the most severe type of catatonia called malignant catatonia, is included as a potential comorbidity in ICD-11 but not mentioned in DSM-5-TR. Third, ICD-11 includes a separate diagnosis for substance-induced catatonia, whereas this condition would be diagnosed as unspecified catatonia in DSM-5-TR.
There are also elements missing from both systems. The most notable of these is that neither system specifies the period over which findings must be present for diagnosis. By clinical convention, the practical definition of 24 hours is appropriate in most instances. The clinical features identified during direct evaluation are usually sufficient for diagnosis, but additional signs observed or documented over the prior 24 hours should be incorporated as part of the clinical evaluation. Another distinction is how to handle clinical features before and after lorazepam challenge. As noted in the BFCRS Training Manual, it would be appropriate to compare “state assessments” (that is, restricted to features identified only during direct, in-person assessment) from before and after lorazepam administration to document improvement.4
Whereas DSM-5-TR and ICD-11 are broadly in agreement, comparing these systems with catatonia rating scales reveals many sources of potential confusion, but also concrete guidance on operationalizing individual items.5 How exactly should each of catatonia’s clinical signs be defined? Descriptions differ, and thresholds of duration and frequency vary considerably across scales. As a result, clinicians who use different scales and then convert these results to diagnostic criteria are liable to come to different clinical conclusions. For instance, both echophenomena and negativism must be elicited more than five times to be scored per Northoff,8 but even a single convincing instance of either would be scored on the BFCRS as “occasional.”2
Such discrepancies are important because, whereas the psychometric properties of several catatonia scales have been documented, there are no analogous studies on the DSM-5-TR and ICD-11 criteria. Therefore, it is essential for clinicians and researchers to document how diagnostic criteria have been operationalized. The most practical and evidence-based way to do this is to use a clinically validated scale and convert these to diagnostic criteria, yet in doing so a few modifications will be necessary.
Of the available clinical scales, the BFCRS is best positioned for clinical use. The BFCRS has been validated clinically and has good reliability, detailed item definitions and audiovisual examples available. In addition, it is the only scale with a published semistructured evaluation (see initial paper and Training Manual), which takes about 5 minutes.2,4 In terms of utility, all 12 signs included by DSM-5-TR are among the first 14 items on the BFCRS, which constitutes a standalone tool known as the Bush-Francis Catatonia Screening Instrument (BFCSI, see Table).
Many fundamental questions remain about catatonia,but the importance of a shared understanding of its clinical features is clear.9 Catatonia should be on the differential whenever a patient exhibits a markedly altered level of activity or grossly abnormal behavior, especially when inappropriate to context. We encourage readers to familiarize themselves with the phenotype of catatonia through online educational resources4 because the optimal care of patients with catatonia requires – at a minimum – that we know what we’re looking for.
Dr. Oldham is assistant professor of psychiatry at the University of Rochester (N.Y.) Medical Center. Dr. Francis is professor of psychiatry at Penn State University, Hershey. The authors declare no relevant conflicts of interest. Funding for the educational project hosted at https://bfcrs.urmc.edu was provided by the department of psychiatry at the University of Rochester Medical Center. Dr. Oldham is currently supported by a K23 career development award from the National Institute on Aging (AG072383). The educational resources referenced in this piece could not have been created were it not for the intellectual and thespian collaboration of Joshua R. Wortzel, MD, who is currently a fellow in child and adolescent psychiatry at Brown University, Providence, R.I. The authors are also indebted to Hochang B. Lee, MD, for his gracious support of this project.
References
1. Wortzel JR et al. J Clin Psychiatry. 2021 Aug 17;82(5):21m14025. doi: 10.4088/JCP.21m14025.
2. Bush G et al. Acta Psychiatr Scand. 1996 Feb;93(2):129-36. doi: 10.1111/j.1600-0447.1996.tb09814.x.
3. Weleff J et al. J Acad Consult Liaison Psychiatry. 2023 Jan-Feb;64(1):13-27. doi:10.1016/j.jaclp.2022.07.002.
4. Oldham MA et al. Bush-Francis Catatonia Rating Scale Assessment Resources. University of Rochester Medical Center, Department of Psychiatry. https://bfcrs.urmc.edu.
5. Oldham MA. Schizophr Res. 2022 Aug 19;S0920-9964(22)00294-8. doi: 10.1016/j.schres.2022.08.002.
6. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5-TR. Washington, D.C.: American Psychiatric Association Publishing, 2022.
7. World Health Organization. ICD-11 for Mortality and Morbidity Stastistics. 2022. https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/486722075.
8. Northoff G et al. Mov Disord. May 1999;14(3):404-16. doi: 10.1002/1531-8257(199905)14:3<404::AID-MDS1004>3.0.CO;2-5.
9. Walther S et al. The Lancet Psychiatry. 2019 Jul;6(7):610-9. doi: 10.1016/S2215-0366(18)30474-7.
Catatonia is a psychomotor syndrome identified by its clinical phenotype. Unlike common psychiatric syndromes such as major depression that are characterized by self-report of symptoms, catatonia is identified chiefly by empirically evaluated signs on clinical evaluation. Its signs are recognized through observation, physical examination, or elicitation by clinical maneuvers or the presentation of stimuli. However, catatonia is often overlooked even though its clinical signs are often visibly apparent, including to the casual observer.
Why is catatonia underdiagnosed? A key modifiable factor appears to be a prevalent misunderstanding over what catatonia looks like.1 We have sought to address this in a few ways.
First identified was the need for comprehensive educational resources on how to assess for and recognize catatonia. Using the Bush-Francis Catatonia Rating Scale – the most widely used scale for catatonia in both research and clinical settings and the most cited publication in the catatonia literature – our team developed the BFCRS Training Manual and Coding Guide.2,3 This manual expands on the definitions of each BFCRS item based on how it was originally operationalized by the scale’s authors. Subsequently, we created a comprehensive set of educational resources including videos illustrating how to assess for catatonia, a video for each of the 23 items on the BFCRS, and self-assessment tools. All resources are freely available online at https://bfcrs.urmc.edu.4
Through this project it became apparent that there are many discrepancies across the field regarding the phenotype of catatonia. Specifically, a recent review inspired by this project set about to characterize the scope of distinctions across diagnostic systems and rating scales.5 For instance, each diagnostic system and rating scale includes a unique set of signs, approaches diagnostic thresholds differently, and often operationalizes clinical features in ways that lead either to criterion overlap (for example, combativeness would be scored both as combativeness and agitation on ICD-11) or contradictions with other systems or scales (for example, varied definitions of waxy flexibility). In the face of so many inconsistencies, what is a clinician to do? What follows is a discussion of how to apply the insights from this recent review in clinical and research settings.
Starting with DSM-5-TR and ICD-11 – the current editions of the two leading diagnostic systems – one might ask: How do they compare?6,7 Overall, these two systems are broadly aligned in terms of the catatonic syndrome. Both systems identify individual clinical signs (as opposed to symptom complexes). Both require three features as a diagnostic threshold. Most of the same clinical signs are included in both systems, and the definitions of individual items are largely equivalent. Additionally, both systems allow for diagnosis of catatonia in association with psychiatric and medical conditions and include a category for unspecified catatonia.
Despite these core agreements, though, there are several important distinctions. First, whereas all 12 signs included in DSM-5-TR count toward an ICD-11 catatonia diagnosis, the opposite cannot be said. ICD-11 includes several features that are not in DSM-5-TR: rigidity, verbigeration, withdrawal, staring, ambitendency, impulsivity, and combativeness. Next, autonomic abnormality, which signifies the most severe type of catatonia called malignant catatonia, is included as a potential comorbidity in ICD-11 but not mentioned in DSM-5-TR. Third, ICD-11 includes a separate diagnosis for substance-induced catatonia, whereas this condition would be diagnosed as unspecified catatonia in DSM-5-TR.
There are also elements missing from both systems. The most notable of these is that neither system specifies the period over which findings must be present for diagnosis. By clinical convention, the practical definition of 24 hours is appropriate in most instances. The clinical features identified during direct evaluation are usually sufficient for diagnosis, but additional signs observed or documented over the prior 24 hours should be incorporated as part of the clinical evaluation. Another distinction is how to handle clinical features before and after lorazepam challenge. As noted in the BFCRS Training Manual, it would be appropriate to compare “state assessments” (that is, restricted to features identified only during direct, in-person assessment) from before and after lorazepam administration to document improvement.4
Whereas DSM-5-TR and ICD-11 are broadly in agreement, comparing these systems with catatonia rating scales reveals many sources of potential confusion, but also concrete guidance on operationalizing individual items.5 How exactly should each of catatonia’s clinical signs be defined? Descriptions differ, and thresholds of duration and frequency vary considerably across scales. As a result, clinicians who use different scales and then convert these results to diagnostic criteria are liable to come to different clinical conclusions. For instance, both echophenomena and negativism must be elicited more than five times to be scored per Northoff,8 but even a single convincing instance of either would be scored on the BFCRS as “occasional.”2
Such discrepancies are important because, whereas the psychometric properties of several catatonia scales have been documented, there are no analogous studies on the DSM-5-TR and ICD-11 criteria. Therefore, it is essential for clinicians and researchers to document how diagnostic criteria have been operationalized. The most practical and evidence-based way to do this is to use a clinically validated scale and convert these to diagnostic criteria, yet in doing so a few modifications will be necessary.
Of the available clinical scales, the BFCRS is best positioned for clinical use. The BFCRS has been validated clinically and has good reliability, detailed item definitions and audiovisual examples available. In addition, it is the only scale with a published semistructured evaluation (see initial paper and Training Manual), which takes about 5 minutes.2,4 In terms of utility, all 12 signs included by DSM-5-TR are among the first 14 items on the BFCRS, which constitutes a standalone tool known as the Bush-Francis Catatonia Screening Instrument (BFCSI, see Table).
Many fundamental questions remain about catatonia,but the importance of a shared understanding of its clinical features is clear.9 Catatonia should be on the differential whenever a patient exhibits a markedly altered level of activity or grossly abnormal behavior, especially when inappropriate to context. We encourage readers to familiarize themselves with the phenotype of catatonia through online educational resources4 because the optimal care of patients with catatonia requires – at a minimum – that we know what we’re looking for.
Dr. Oldham is assistant professor of psychiatry at the University of Rochester (N.Y.) Medical Center. Dr. Francis is professor of psychiatry at Penn State University, Hershey. The authors declare no relevant conflicts of interest. Funding for the educational project hosted at https://bfcrs.urmc.edu was provided by the department of psychiatry at the University of Rochester Medical Center. Dr. Oldham is currently supported by a K23 career development award from the National Institute on Aging (AG072383). The educational resources referenced in this piece could not have been created were it not for the intellectual and thespian collaboration of Joshua R. Wortzel, MD, who is currently a fellow in child and adolescent psychiatry at Brown University, Providence, R.I. The authors are also indebted to Hochang B. Lee, MD, for his gracious support of this project.
References
1. Wortzel JR et al. J Clin Psychiatry. 2021 Aug 17;82(5):21m14025. doi: 10.4088/JCP.21m14025.
2. Bush G et al. Acta Psychiatr Scand. 1996 Feb;93(2):129-36. doi: 10.1111/j.1600-0447.1996.tb09814.x.
3. Weleff J et al. J Acad Consult Liaison Psychiatry. 2023 Jan-Feb;64(1):13-27. doi:10.1016/j.jaclp.2022.07.002.
4. Oldham MA et al. Bush-Francis Catatonia Rating Scale Assessment Resources. University of Rochester Medical Center, Department of Psychiatry. https://bfcrs.urmc.edu.
5. Oldham MA. Schizophr Res. 2022 Aug 19;S0920-9964(22)00294-8. doi: 10.1016/j.schres.2022.08.002.
6. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5-TR. Washington, D.C.: American Psychiatric Association Publishing, 2022.
7. World Health Organization. ICD-11 for Mortality and Morbidity Stastistics. 2022. https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/486722075.
8. Northoff G et al. Mov Disord. May 1999;14(3):404-16. doi: 10.1002/1531-8257(199905)14:3<404::AID-MDS1004>3.0.CO;2-5.
9. Walther S et al. The Lancet Psychiatry. 2019 Jul;6(7):610-9. doi: 10.1016/S2215-0366(18)30474-7.
AAP vs. AED on obesity treatment: Is there a middle ground?
While there is little controversy that both obesity and eating disorders represent important public health concerns, each deserving of clinical attention, how best to address one without worsening the other has been the crux of the discussion.
Sparking the dispute was a recent publication from the American Academy of Pediatrics that outlines the scope of the obesity problem and makes specific recommendations for assessment and treatment.1 The ambitious 100-page document, with 801 citations, puts new emphasis on the medical and psychological costs associated with obesity and advocates that pediatric primary care clinicians be more assertive in its treatment. While the guidelines certainly don’t urge the use of medications or surgery options as first-line treatment, the new recommendations do put them on the table as options.
In response, the Academy of Eating Disorders issued a public statement outlining several concerns regarding these guidelines that centered around a lack of a detailed plan to screen and address eating disorders; concerns that pediatricians don’t have the level of training and “skills” to conduct these conversations with patients and families with enough sensitivity; and worries about the premature use of antiobesity medications and surgeries in this population.2
It is fair to say that the critique was sharply worded, invoking physicians’ Hippocratic oath, criticizing their training, and suggesting that the guidelines could be biased by pharmaceutical industry influence (of note, the authors of the guidelines reported no ties to any pharmaceutical company). The AED urged that the guidelines be “revised” after consultation with other groups, including them.
Not unexpectedly, this response, especially coming from a group whose leadership and members are primarily nonphysicians, triggered its own sharp rebukes, including a recent commentary that counter-accused some of the eating disorder clinicians of being more concerned with their pet diets than actual health improvements.3
After everyone takes some deep breaths, it’s worth looking to see if there is some middle ground to explore here. The AAP document, to my reading, shows some important acknowledgments of the stigma associated with being overweight, even coming from pediatricians themselves. One passage reads, “Pediatricians and other PHCPs [primary health care providers] have been – and remain – a source of weight bias. They first need to uncover and address their own attitudes regarding children with obesity. Understanding weight stigma and bias, and learning how to reduce it in the clinical setting, sets the stage for productive discussions and improved relationships between families and pediatricians or other PHCPs.”
The guidelines also include some suggestions for how to talk to youth and families about obesity in less stigmatizing ways and offer a fairly lengthy summary of motivational interviewing techniques as they might apply to obesity discussions and lifestyle change. There is also a section on the interface between obesity and eating disorders with suggestions for further reading on their assessment and management.4
Indeed, research has looked specifically at how to minimize the triggering of eating disorders when addressing weight problems, a concern that has been raised by pediatricians themselves as documented in a qualitative study that also invoked the “do no harm” principle.5 One study asked more than 2,000 teens about how various conversations about weight affected their behavior.6 A main finding from that study was that conversations that focused on healthy eating rather than weight per se were less likely to be associated with unhealthy weight control behaviors. This message was emphasized in a publication that came from the AAP itself; it addresses the interaction between eating disorders and obesity.7 Strangely, however, the suggestion to try to minimize the focus on weight in discussions with patients isn’t well emphasized in the publication.
Overall, though, the AAP guidelines offer a well-informed and balanced approach to helping overweight youth. Pediatricians and other pediatric primary care clinicians are frequently called upon to engage in extremely sensitive and difficult discussions with patients and families on a wide variety of topics and most do so quite skillfully, especially when given the proper time and tools. While it is an area in which many of us, including mental health professionals, could do better, it’s no surprise that the AED’s disparaging of pediatricians’ communication competence came off as insulting. Similarly, productive dialogue would be likely enhanced if both sides avoided unfounded speculation about bias and motive and worked from a good faith perspective that all of us are engaged in this important discussion because of a desire to improve the lives of kids.
From my reading, it is quite a stretch to conclude that this document is urging a hasty and financially driven descent into GLP-1 analogues and bariatric surgery. That said, this wouldn’t be the first time a professional organization issues detailed, thoughtful, and nuanced care guidelines only to have them “condensed” within the practical confines of a busy office practice. Leaders would do well to remember that there remains much work to do to empower clinicians to be able to follow these guidelines as intended.
Dr. Rettew is a child and adolescent psychiatrist with Lane County Behavioral Health in Eugene, Ore., and Oregon Health & Science University, Portland. His latest book is “Parenting Made Complicated: What Science Really Knows About the Greatest Debates of Early Childhood.”
References
1. Hampl SE et al. Pediatrics. 2023;151(2):e2022060640.
2. Academy of Eating Disorders. Jan. 26, 2023. Accessed February 2, 2023. Available at The Academy for Eating Disorders Releases a Statement on the Recent American Academy of Pediatrics Clinical Practice Guideline for Weight-Related Care: First, Do No Harm (newswise.com).
3. Freedhoff Y. MDedge Pediatrics 2023. Available at https://www.mdedge.com/pediatrics/article/260894/obesity/weight-bias-affects-views-kids-obesity-recommendations?channel=52.
4. Hornberger LL, Lane MA et al. Pediatrics. 2021;147(1):e202004027989.
5. Loth KA, Lebow J et al. Global Pediatric Health. 2021;8:1-9.
6. Berge JM et al. JAMA Pediatrics. 2013;167(8):746-53.
7. Golden NH et al. Pediatrics. 2016;138(3):e20161649.
While there is little controversy that both obesity and eating disorders represent important public health concerns, each deserving of clinical attention, how best to address one without worsening the other has been the crux of the discussion.
Sparking the dispute was a recent publication from the American Academy of Pediatrics that outlines the scope of the obesity problem and makes specific recommendations for assessment and treatment.1 The ambitious 100-page document, with 801 citations, puts new emphasis on the medical and psychological costs associated with obesity and advocates that pediatric primary care clinicians be more assertive in its treatment. While the guidelines certainly don’t urge the use of medications or surgery options as first-line treatment, the new recommendations do put them on the table as options.
In response, the Academy of Eating Disorders issued a public statement outlining several concerns regarding these guidelines that centered around a lack of a detailed plan to screen and address eating disorders; concerns that pediatricians don’t have the level of training and “skills” to conduct these conversations with patients and families with enough sensitivity; and worries about the premature use of antiobesity medications and surgeries in this population.2
It is fair to say that the critique was sharply worded, invoking physicians’ Hippocratic oath, criticizing their training, and suggesting that the guidelines could be biased by pharmaceutical industry influence (of note, the authors of the guidelines reported no ties to any pharmaceutical company). The AED urged that the guidelines be “revised” after consultation with other groups, including them.
Not unexpectedly, this response, especially coming from a group whose leadership and members are primarily nonphysicians, triggered its own sharp rebukes, including a recent commentary that counter-accused some of the eating disorder clinicians of being more concerned with their pet diets than actual health improvements.3
After everyone takes some deep breaths, it’s worth looking to see if there is some middle ground to explore here. The AAP document, to my reading, shows some important acknowledgments of the stigma associated with being overweight, even coming from pediatricians themselves. One passage reads, “Pediatricians and other PHCPs [primary health care providers] have been – and remain – a source of weight bias. They first need to uncover and address their own attitudes regarding children with obesity. Understanding weight stigma and bias, and learning how to reduce it in the clinical setting, sets the stage for productive discussions and improved relationships between families and pediatricians or other PHCPs.”
The guidelines also include some suggestions for how to talk to youth and families about obesity in less stigmatizing ways and offer a fairly lengthy summary of motivational interviewing techniques as they might apply to obesity discussions and lifestyle change. There is also a section on the interface between obesity and eating disorders with suggestions for further reading on their assessment and management.4
Indeed, research has looked specifically at how to minimize the triggering of eating disorders when addressing weight problems, a concern that has been raised by pediatricians themselves as documented in a qualitative study that also invoked the “do no harm” principle.5 One study asked more than 2,000 teens about how various conversations about weight affected their behavior.6 A main finding from that study was that conversations that focused on healthy eating rather than weight per se were less likely to be associated with unhealthy weight control behaviors. This message was emphasized in a publication that came from the AAP itself; it addresses the interaction between eating disorders and obesity.7 Strangely, however, the suggestion to try to minimize the focus on weight in discussions with patients isn’t well emphasized in the publication.
Overall, though, the AAP guidelines offer a well-informed and balanced approach to helping overweight youth. Pediatricians and other pediatric primary care clinicians are frequently called upon to engage in extremely sensitive and difficult discussions with patients and families on a wide variety of topics and most do so quite skillfully, especially when given the proper time and tools. While it is an area in which many of us, including mental health professionals, could do better, it’s no surprise that the AED’s disparaging of pediatricians’ communication competence came off as insulting. Similarly, productive dialogue would be likely enhanced if both sides avoided unfounded speculation about bias and motive and worked from a good faith perspective that all of us are engaged in this important discussion because of a desire to improve the lives of kids.
From my reading, it is quite a stretch to conclude that this document is urging a hasty and financially driven descent into GLP-1 analogues and bariatric surgery. That said, this wouldn’t be the first time a professional organization issues detailed, thoughtful, and nuanced care guidelines only to have them “condensed” within the practical confines of a busy office practice. Leaders would do well to remember that there remains much work to do to empower clinicians to be able to follow these guidelines as intended.
Dr. Rettew is a child and adolescent psychiatrist with Lane County Behavioral Health in Eugene, Ore., and Oregon Health & Science University, Portland. His latest book is “Parenting Made Complicated: What Science Really Knows About the Greatest Debates of Early Childhood.”
References
1. Hampl SE et al. Pediatrics. 2023;151(2):e2022060640.
2. Academy of Eating Disorders. Jan. 26, 2023. Accessed February 2, 2023. Available at The Academy for Eating Disorders Releases a Statement on the Recent American Academy of Pediatrics Clinical Practice Guideline for Weight-Related Care: First, Do No Harm (newswise.com).
3. Freedhoff Y. MDedge Pediatrics 2023. Available at https://www.mdedge.com/pediatrics/article/260894/obesity/weight-bias-affects-views-kids-obesity-recommendations?channel=52.
4. Hornberger LL, Lane MA et al. Pediatrics. 2021;147(1):e202004027989.
5. Loth KA, Lebow J et al. Global Pediatric Health. 2021;8:1-9.
6. Berge JM et al. JAMA Pediatrics. 2013;167(8):746-53.
7. Golden NH et al. Pediatrics. 2016;138(3):e20161649.
While there is little controversy that both obesity and eating disorders represent important public health concerns, each deserving of clinical attention, how best to address one without worsening the other has been the crux of the discussion.
Sparking the dispute was a recent publication from the American Academy of Pediatrics that outlines the scope of the obesity problem and makes specific recommendations for assessment and treatment.1 The ambitious 100-page document, with 801 citations, puts new emphasis on the medical and psychological costs associated with obesity and advocates that pediatric primary care clinicians be more assertive in its treatment. While the guidelines certainly don’t urge the use of medications or surgery options as first-line treatment, the new recommendations do put them on the table as options.
In response, the Academy of Eating Disorders issued a public statement outlining several concerns regarding these guidelines that centered around a lack of a detailed plan to screen and address eating disorders; concerns that pediatricians don’t have the level of training and “skills” to conduct these conversations with patients and families with enough sensitivity; and worries about the premature use of antiobesity medications and surgeries in this population.2
It is fair to say that the critique was sharply worded, invoking physicians’ Hippocratic oath, criticizing their training, and suggesting that the guidelines could be biased by pharmaceutical industry influence (of note, the authors of the guidelines reported no ties to any pharmaceutical company). The AED urged that the guidelines be “revised” after consultation with other groups, including them.
Not unexpectedly, this response, especially coming from a group whose leadership and members are primarily nonphysicians, triggered its own sharp rebukes, including a recent commentary that counter-accused some of the eating disorder clinicians of being more concerned with their pet diets than actual health improvements.3
After everyone takes some deep breaths, it’s worth looking to see if there is some middle ground to explore here. The AAP document, to my reading, shows some important acknowledgments of the stigma associated with being overweight, even coming from pediatricians themselves. One passage reads, “Pediatricians and other PHCPs [primary health care providers] have been – and remain – a source of weight bias. They first need to uncover and address their own attitudes regarding children with obesity. Understanding weight stigma and bias, and learning how to reduce it in the clinical setting, sets the stage for productive discussions and improved relationships between families and pediatricians or other PHCPs.”
The guidelines also include some suggestions for how to talk to youth and families about obesity in less stigmatizing ways and offer a fairly lengthy summary of motivational interviewing techniques as they might apply to obesity discussions and lifestyle change. There is also a section on the interface between obesity and eating disorders with suggestions for further reading on their assessment and management.4
Indeed, research has looked specifically at how to minimize the triggering of eating disorders when addressing weight problems, a concern that has been raised by pediatricians themselves as documented in a qualitative study that also invoked the “do no harm” principle.5 One study asked more than 2,000 teens about how various conversations about weight affected their behavior.6 A main finding from that study was that conversations that focused on healthy eating rather than weight per se were less likely to be associated with unhealthy weight control behaviors. This message was emphasized in a publication that came from the AAP itself; it addresses the interaction between eating disorders and obesity.7 Strangely, however, the suggestion to try to minimize the focus on weight in discussions with patients isn’t well emphasized in the publication.
Overall, though, the AAP guidelines offer a well-informed and balanced approach to helping overweight youth. Pediatricians and other pediatric primary care clinicians are frequently called upon to engage in extremely sensitive and difficult discussions with patients and families on a wide variety of topics and most do so quite skillfully, especially when given the proper time and tools. While it is an area in which many of us, including mental health professionals, could do better, it’s no surprise that the AED’s disparaging of pediatricians’ communication competence came off as insulting. Similarly, productive dialogue would be likely enhanced if both sides avoided unfounded speculation about bias and motive and worked from a good faith perspective that all of us are engaged in this important discussion because of a desire to improve the lives of kids.
From my reading, it is quite a stretch to conclude that this document is urging a hasty and financially driven descent into GLP-1 analogues and bariatric surgery. That said, this wouldn’t be the first time a professional organization issues detailed, thoughtful, and nuanced care guidelines only to have them “condensed” within the practical confines of a busy office practice. Leaders would do well to remember that there remains much work to do to empower clinicians to be able to follow these guidelines as intended.
Dr. Rettew is a child and adolescent psychiatrist with Lane County Behavioral Health in Eugene, Ore., and Oregon Health & Science University, Portland. His latest book is “Parenting Made Complicated: What Science Really Knows About the Greatest Debates of Early Childhood.”
References
1. Hampl SE et al. Pediatrics. 2023;151(2):e2022060640.
2. Academy of Eating Disorders. Jan. 26, 2023. Accessed February 2, 2023. Available at The Academy for Eating Disorders Releases a Statement on the Recent American Academy of Pediatrics Clinical Practice Guideline for Weight-Related Care: First, Do No Harm (newswise.com).
3. Freedhoff Y. MDedge Pediatrics 2023. Available at https://www.mdedge.com/pediatrics/article/260894/obesity/weight-bias-affects-views-kids-obesity-recommendations?channel=52.
4. Hornberger LL, Lane MA et al. Pediatrics. 2021;147(1):e202004027989.
5. Loth KA, Lebow J et al. Global Pediatric Health. 2021;8:1-9.
6. Berge JM et al. JAMA Pediatrics. 2013;167(8):746-53.
7. Golden NH et al. Pediatrics. 2016;138(3):e20161649.
A doctor intervenes in a fiery car crash
Emergencies happen anywhere, anytime, and sometimes physicians find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a Medscape series telling these stories.
I was coming off a 48-hour shift plus a day of doing outpatient sedation at Sparrow Hospital in Lansing. It was December and Michigan-cold.
I drove on the side of the road where I wasn’t really supposed to and got closer. An SUV had crashed into one of the big concrete structures under the bridge. I saw people running around but wasn’t able to spot EMS or any health care workers. From where I was, I could identify four kids who had already been extricated and one adult still in the driver’s seat. I estimated the kids’ ages were around 7, 5, 3, and an infant who was a few months old. I left my car and went to help.
I was able to peg the ages correctly because I’m a pediatric critical care physician. As a specialty, we’re not commonly known. We oversee patient care in intensive care units, except the patients are children. Part of the job is that we’re experts at triaging. We recognize what’s life-threatening and less so.
The kids were with some adults who kept them warm with blankets. I examined each of them. The infant was asleep but arousable and acting like a normal baby. The 3-year-old boy was vomiting and appeared very fatigued. The 5-year-old boy had a forehead laceration and was in and out of consciousness. The 7-year-old girl was screaming because of different injuries.
While all of the children were concerning to me, I identified one in particular: the 5-year-old boy. It was obvious he needed serious medical attention and fast. So, I kept that little guy in mind. The others had sustained significant injuries, but my best guess was they could get to a hospital and be stabilized.
That said, I’m a trauma instructor, and one of the things I always tell trainees is: Trauma is a black box. On the outside, it may seem like a patient doesn’t have a lot of injuries. But underneath, there might be something worse, like a brain injury. Or the chest might have taken a blunt impact affecting the heart. There may be internal bleeding somewhere in the belly. It’s really hard to tease out what exactly is going on without equipment and testing.
I didn’t even have a pulse oximeter or heart rate monitor. I pretty much just went by the appearance of the child: pulse, heart rate, awareness, things like that.
After the kids, I moved to look at the man in the car. The front end had already caught fire. I could see the driver – the kids’ father, I guessed – unconscious and hunched over. I was wondering, Why hasn’t this guy been extricated?
I approached the car on the front passenger side. And then I just had this feeling. I knew I needed to step back. Immediately.
I did. And a few seconds later, the whole car exploded in flames.
I believe God is in control of everything. I tried to get to that man. But the scene was unsafe. Later I learned that several people, including a young nurse at the scene, had tried to get to him as well.
When EMS came, I identified myself. Obviously, these people do very, very important work. But they may be more used to the 60-year-old heart attack, the 25-year-old gunshot wound, the occasional ill child. I thought that four kids – each with possible critical poly-traumatic injuries – posed a challenge to anyone.
I told them, “This is what I do on a daily basis, and this is the kid I’m worried about the most. The other kids are definitely worrisome, but I would prioritize getting this kid to the hospital first. Can I ride with you?” They agreed.
We got that boy and his older sister into the first ambulance (she was in a lot of pain, the result of a femur fracture). The two other kids rode in the second ambulance. The hospital where I had just left was 10 minutes away. I called the other pediatric critical care doctor there, my partner. He thought I was calling for a routine issue – no such luck. I said, “I’m with four kids who are level-1 traumas in two ambulances and I’m heading to the hospital right now, ETA 10 minutes.”
En route, I thought the little boy might lose consciousness at any moment. He needed a breathing tube, and I debated whether it should be done in the ambulance vs. waiting until we got to the emergency room. Based on my judgment and his vital signs, I elected to wait to have it done it in a more controlled environment. Had I felt like he was in immediate need of an airway, I would’ve attempted it. But those are the tough calls that you must make.
My partner had alerted the trauma and emergency medicine teams at the hospital. By the time we arrived, my partner was down in the ER with the trauma team and ER staff. Everyone was ready. Then it was like divide and conquer. He attended to one of the kids. The ER team and I were with the little guy I was really worried about. We had his breathing tube in within minutes. The trauma team attended to the other two.
All the kids were stabilized and then admitted to the pediatric intensive care unit. I’m happy to say that all of them did well in the end. Even the little guy I was worried about the most.
I must say this incident gave me perspective on what EMS goes through. The field medicine we do in the United States is still in its infancy in a lot of ways. One of the things I would love to see in the future is a mobile ICU. After a critical illness hits, sometimes you only have seconds, minutes, maybe hours if you’re lucky. The earlier you can get patients the treatment they need, the better the outcomes.
I like taking care of critically ill children and their families. It fits my personality. And it’s a wonderful cause. But you have to be ready for tragic cases like this one. Yes, the children came out alive, but the accident claimed a life in a horrible way. And there was nothing I could do about it.
Critical care takes an emotional, psychological, and physical toll. It’s a roller coaster: Some kids do well; some kids don’t do well. All I can do is hold myself accountable. I keep my emotions in check, whether the outcome is positive or negative. And I do my best.
Mohamed Hani Farhat, MD, is a pediatric critical care physician at the University of Michigan C.S. Mott Children’s Hospital in Ann Arbor and Sparrow Hospital in Lansing, Mich. Are you a physician with a dramatic medical story outside the clinic? Medscape would love to consider your story for Is There a Doctor in the House? Please email your contact information and a short summary of your story to [email protected] . A version of this article appeared on Medscape.com.
Emergencies happen anywhere, anytime, and sometimes physicians find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a Medscape series telling these stories.
I was coming off a 48-hour shift plus a day of doing outpatient sedation at Sparrow Hospital in Lansing. It was December and Michigan-cold.
I drove on the side of the road where I wasn’t really supposed to and got closer. An SUV had crashed into one of the big concrete structures under the bridge. I saw people running around but wasn’t able to spot EMS or any health care workers. From where I was, I could identify four kids who had already been extricated and one adult still in the driver’s seat. I estimated the kids’ ages were around 7, 5, 3, and an infant who was a few months old. I left my car and went to help.
I was able to peg the ages correctly because I’m a pediatric critical care physician. As a specialty, we’re not commonly known. We oversee patient care in intensive care units, except the patients are children. Part of the job is that we’re experts at triaging. We recognize what’s life-threatening and less so.
The kids were with some adults who kept them warm with blankets. I examined each of them. The infant was asleep but arousable and acting like a normal baby. The 3-year-old boy was vomiting and appeared very fatigued. The 5-year-old boy had a forehead laceration and was in and out of consciousness. The 7-year-old girl was screaming because of different injuries.
While all of the children were concerning to me, I identified one in particular: the 5-year-old boy. It was obvious he needed serious medical attention and fast. So, I kept that little guy in mind. The others had sustained significant injuries, but my best guess was they could get to a hospital and be stabilized.
That said, I’m a trauma instructor, and one of the things I always tell trainees is: Trauma is a black box. On the outside, it may seem like a patient doesn’t have a lot of injuries. But underneath, there might be something worse, like a brain injury. Or the chest might have taken a blunt impact affecting the heart. There may be internal bleeding somewhere in the belly. It’s really hard to tease out what exactly is going on without equipment and testing.
I didn’t even have a pulse oximeter or heart rate monitor. I pretty much just went by the appearance of the child: pulse, heart rate, awareness, things like that.
After the kids, I moved to look at the man in the car. The front end had already caught fire. I could see the driver – the kids’ father, I guessed – unconscious and hunched over. I was wondering, Why hasn’t this guy been extricated?
I approached the car on the front passenger side. And then I just had this feeling. I knew I needed to step back. Immediately.
I did. And a few seconds later, the whole car exploded in flames.
I believe God is in control of everything. I tried to get to that man. But the scene was unsafe. Later I learned that several people, including a young nurse at the scene, had tried to get to him as well.
When EMS came, I identified myself. Obviously, these people do very, very important work. But they may be more used to the 60-year-old heart attack, the 25-year-old gunshot wound, the occasional ill child. I thought that four kids – each with possible critical poly-traumatic injuries – posed a challenge to anyone.
I told them, “This is what I do on a daily basis, and this is the kid I’m worried about the most. The other kids are definitely worrisome, but I would prioritize getting this kid to the hospital first. Can I ride with you?” They agreed.
We got that boy and his older sister into the first ambulance (she was in a lot of pain, the result of a femur fracture). The two other kids rode in the second ambulance. The hospital where I had just left was 10 minutes away. I called the other pediatric critical care doctor there, my partner. He thought I was calling for a routine issue – no such luck. I said, “I’m with four kids who are level-1 traumas in two ambulances and I’m heading to the hospital right now, ETA 10 minutes.”
En route, I thought the little boy might lose consciousness at any moment. He needed a breathing tube, and I debated whether it should be done in the ambulance vs. waiting until we got to the emergency room. Based on my judgment and his vital signs, I elected to wait to have it done it in a more controlled environment. Had I felt like he was in immediate need of an airway, I would’ve attempted it. But those are the tough calls that you must make.
My partner had alerted the trauma and emergency medicine teams at the hospital. By the time we arrived, my partner was down in the ER with the trauma team and ER staff. Everyone was ready. Then it was like divide and conquer. He attended to one of the kids. The ER team and I were with the little guy I was really worried about. We had his breathing tube in within minutes. The trauma team attended to the other two.
All the kids were stabilized and then admitted to the pediatric intensive care unit. I’m happy to say that all of them did well in the end. Even the little guy I was worried about the most.
I must say this incident gave me perspective on what EMS goes through. The field medicine we do in the United States is still in its infancy in a lot of ways. One of the things I would love to see in the future is a mobile ICU. After a critical illness hits, sometimes you only have seconds, minutes, maybe hours if you’re lucky. The earlier you can get patients the treatment they need, the better the outcomes.
I like taking care of critically ill children and their families. It fits my personality. And it’s a wonderful cause. But you have to be ready for tragic cases like this one. Yes, the children came out alive, but the accident claimed a life in a horrible way. And there was nothing I could do about it.
Critical care takes an emotional, psychological, and physical toll. It’s a roller coaster: Some kids do well; some kids don’t do well. All I can do is hold myself accountable. I keep my emotions in check, whether the outcome is positive or negative. And I do my best.
Mohamed Hani Farhat, MD, is a pediatric critical care physician at the University of Michigan C.S. Mott Children’s Hospital in Ann Arbor and Sparrow Hospital in Lansing, Mich. Are you a physician with a dramatic medical story outside the clinic? Medscape would love to consider your story for Is There a Doctor in the House? Please email your contact information and a short summary of your story to [email protected] . A version of this article appeared on Medscape.com.
Emergencies happen anywhere, anytime, and sometimes physicians find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a Medscape series telling these stories.
I was coming off a 48-hour shift plus a day of doing outpatient sedation at Sparrow Hospital in Lansing. It was December and Michigan-cold.
I drove on the side of the road where I wasn’t really supposed to and got closer. An SUV had crashed into one of the big concrete structures under the bridge. I saw people running around but wasn’t able to spot EMS or any health care workers. From where I was, I could identify four kids who had already been extricated and one adult still in the driver’s seat. I estimated the kids’ ages were around 7, 5, 3, and an infant who was a few months old. I left my car and went to help.
I was able to peg the ages correctly because I’m a pediatric critical care physician. As a specialty, we’re not commonly known. We oversee patient care in intensive care units, except the patients are children. Part of the job is that we’re experts at triaging. We recognize what’s life-threatening and less so.
The kids were with some adults who kept them warm with blankets. I examined each of them. The infant was asleep but arousable and acting like a normal baby. The 3-year-old boy was vomiting and appeared very fatigued. The 5-year-old boy had a forehead laceration and was in and out of consciousness. The 7-year-old girl was screaming because of different injuries.
While all of the children were concerning to me, I identified one in particular: the 5-year-old boy. It was obvious he needed serious medical attention and fast. So, I kept that little guy in mind. The others had sustained significant injuries, but my best guess was they could get to a hospital and be stabilized.
That said, I’m a trauma instructor, and one of the things I always tell trainees is: Trauma is a black box. On the outside, it may seem like a patient doesn’t have a lot of injuries. But underneath, there might be something worse, like a brain injury. Or the chest might have taken a blunt impact affecting the heart. There may be internal bleeding somewhere in the belly. It’s really hard to tease out what exactly is going on without equipment and testing.
I didn’t even have a pulse oximeter or heart rate monitor. I pretty much just went by the appearance of the child: pulse, heart rate, awareness, things like that.
After the kids, I moved to look at the man in the car. The front end had already caught fire. I could see the driver – the kids’ father, I guessed – unconscious and hunched over. I was wondering, Why hasn’t this guy been extricated?
I approached the car on the front passenger side. And then I just had this feeling. I knew I needed to step back. Immediately.
I did. And a few seconds later, the whole car exploded in flames.
I believe God is in control of everything. I tried to get to that man. But the scene was unsafe. Later I learned that several people, including a young nurse at the scene, had tried to get to him as well.
When EMS came, I identified myself. Obviously, these people do very, very important work. But they may be more used to the 60-year-old heart attack, the 25-year-old gunshot wound, the occasional ill child. I thought that four kids – each with possible critical poly-traumatic injuries – posed a challenge to anyone.
I told them, “This is what I do on a daily basis, and this is the kid I’m worried about the most. The other kids are definitely worrisome, but I would prioritize getting this kid to the hospital first. Can I ride with you?” They agreed.
We got that boy and his older sister into the first ambulance (she was in a lot of pain, the result of a femur fracture). The two other kids rode in the second ambulance. The hospital where I had just left was 10 minutes away. I called the other pediatric critical care doctor there, my partner. He thought I was calling for a routine issue – no such luck. I said, “I’m with four kids who are level-1 traumas in two ambulances and I’m heading to the hospital right now, ETA 10 minutes.”
En route, I thought the little boy might lose consciousness at any moment. He needed a breathing tube, and I debated whether it should be done in the ambulance vs. waiting until we got to the emergency room. Based on my judgment and his vital signs, I elected to wait to have it done it in a more controlled environment. Had I felt like he was in immediate need of an airway, I would’ve attempted it. But those are the tough calls that you must make.
My partner had alerted the trauma and emergency medicine teams at the hospital. By the time we arrived, my partner was down in the ER with the trauma team and ER staff. Everyone was ready. Then it was like divide and conquer. He attended to one of the kids. The ER team and I were with the little guy I was really worried about. We had his breathing tube in within minutes. The trauma team attended to the other two.
All the kids were stabilized and then admitted to the pediatric intensive care unit. I’m happy to say that all of them did well in the end. Even the little guy I was worried about the most.
I must say this incident gave me perspective on what EMS goes through. The field medicine we do in the United States is still in its infancy in a lot of ways. One of the things I would love to see in the future is a mobile ICU. After a critical illness hits, sometimes you only have seconds, minutes, maybe hours if you’re lucky. The earlier you can get patients the treatment they need, the better the outcomes.
I like taking care of critically ill children and their families. It fits my personality. And it’s a wonderful cause. But you have to be ready for tragic cases like this one. Yes, the children came out alive, but the accident claimed a life in a horrible way. And there was nothing I could do about it.
Critical care takes an emotional, psychological, and physical toll. It’s a roller coaster: Some kids do well; some kids don’t do well. All I can do is hold myself accountable. I keep my emotions in check, whether the outcome is positive or negative. And I do my best.
Mohamed Hani Farhat, MD, is a pediatric critical care physician at the University of Michigan C.S. Mott Children’s Hospital in Ann Arbor and Sparrow Hospital in Lansing, Mich. Are you a physician with a dramatic medical story outside the clinic? Medscape would love to consider your story for Is There a Doctor in the House? Please email your contact information and a short summary of your story to [email protected] . A version of this article appeared on Medscape.com.
Young children quickly outgrow the need for ear tubes
About half a million children between the ages of 1 and 3 years old have ear tube surgery in the United States every year at an annual cost exceeding $2 billion. It is the most common childhood surgery performed with anesthesia. It is a surgery commonly performed on children in most other high- and middle-income countries.
My group recently published a paper on the timing and necessity of tympanostomy tubes for recurrent otitis media in young children. The primary objective was to quantitatively examine recurrent acute otitis media (AOM) incidence with respect to age of occurrence, the influence of daycare attendance, and other risk factors in individual children. We introduced the concept of a “window of susceptibility” to AOM as new terminology referring to a child who has two or more closely spaced AOM occurrences during a window of time. We sought to know what to expect and how to advise the parent when a child presents with closely spaced AOMs.
A secondary objective was to develop models to predict the risk and timing of AOM recurrences based on the natural history of disease in young children who do not get tympanostomy tubes. Prediction models were developed to assist clinicians in understanding and explaining to parents the benefit of tympanostomy tubes based on the child’s age and number of AOMs.
The children were all from a primary care pediatric practice in Rochester, N.Y., which comprised a typical mixed demographic of largely middle-class, health care–insured families that was broadly representative of the racial/ethnic diversity in the community. The sample included both wealthy families and those living below the poverty line. The diagnosis of AOM was made based on the American Academy of Pediatrics guidance in which a presumed middle ear effusion and a full or bulging tympanic membrane were required. Almost all episodes (> 85%) of clinically diagnosed AOM cases were confirmed by culture of middle ear fluid collected by tympanocentesis to ensure diagnostic accuracy.
286 children who had ear infections were studied. We found that 80% of ear infections occurred during a very narrow window of susceptibility – age 6-21 months. About 72% of children had a window of susceptibility to ear infections that lasted 5 months or less; 97% of children had a window of susceptibility that lasted 10 months or less.
From this result, we observed that about 90% of children have a window of time lasting about 10 months when they get repeated ear infections. By the time a child gets three ear infections in 6 months (a period of time recommended by the AAP and American Academy of Otolaryngology–Head and Neck Surgery when ear tubes might be considered) and then a referral for ear tubes is made and the child gets an appointment with the ear, nose, and throat doctor, and surgery is scheduled, the ear infections were going to stop anyway.
In other words, millions of children worldwide have been getting ear tubes and physicians and parents saw that the ear infections stopped. So they concluded the ear tubes stopped the infections. We found the infections were going to stop anyway even if the child did not receive ear tubes because their susceptibility to ear infections is over by the time the surgery is performed. The child outgrew ear infections.
An exception was children in daycare at an early age. Our study found that children in daycare who are around 6 months old and start getting ear infections at that age are likely destined to have three or more ear infections in the first year of life. If children are going to be in daycare, perhaps those who need them should receive ear tubes early. Analysis of other demographic and risk factor covariates – sex, race/ethnicity, breastfeeding, siblings in the home, smoking in the home, atopy, and family history of otitis media – were not significantly associated with the number of AOMs in the child population we studied.
We developed a prediction model for doctors, so they could input a child’s age, number of ear infections, and daycare attendance and receive back an estimate of the number of likely future ear infections for that child. With that knowledge, physicians and parents can make more informed decisions.
Our message to clinicians and parents is to reconsider the necessity and timing of ear tube surgery for children with recurrent ear infections because the future is not predicted by the past. Children having several ear infections in a short time does not predict that they will have a similar number of ear infections in the future.
The study was supported by the National Institutes of Health awarded to Rochester Regional Health. Dr. Pichichero was principal investigator for the award.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
About half a million children between the ages of 1 and 3 years old have ear tube surgery in the United States every year at an annual cost exceeding $2 billion. It is the most common childhood surgery performed with anesthesia. It is a surgery commonly performed on children in most other high- and middle-income countries.
My group recently published a paper on the timing and necessity of tympanostomy tubes for recurrent otitis media in young children. The primary objective was to quantitatively examine recurrent acute otitis media (AOM) incidence with respect to age of occurrence, the influence of daycare attendance, and other risk factors in individual children. We introduced the concept of a “window of susceptibility” to AOM as new terminology referring to a child who has two or more closely spaced AOM occurrences during a window of time. We sought to know what to expect and how to advise the parent when a child presents with closely spaced AOMs.
A secondary objective was to develop models to predict the risk and timing of AOM recurrences based on the natural history of disease in young children who do not get tympanostomy tubes. Prediction models were developed to assist clinicians in understanding and explaining to parents the benefit of tympanostomy tubes based on the child’s age and number of AOMs.
The children were all from a primary care pediatric practice in Rochester, N.Y., which comprised a typical mixed demographic of largely middle-class, health care–insured families that was broadly representative of the racial/ethnic diversity in the community. The sample included both wealthy families and those living below the poverty line. The diagnosis of AOM was made based on the American Academy of Pediatrics guidance in which a presumed middle ear effusion and a full or bulging tympanic membrane were required. Almost all episodes (> 85%) of clinically diagnosed AOM cases were confirmed by culture of middle ear fluid collected by tympanocentesis to ensure diagnostic accuracy.
286 children who had ear infections were studied. We found that 80% of ear infections occurred during a very narrow window of susceptibility – age 6-21 months. About 72% of children had a window of susceptibility to ear infections that lasted 5 months or less; 97% of children had a window of susceptibility that lasted 10 months or less.
From this result, we observed that about 90% of children have a window of time lasting about 10 months when they get repeated ear infections. By the time a child gets three ear infections in 6 months (a period of time recommended by the AAP and American Academy of Otolaryngology–Head and Neck Surgery when ear tubes might be considered) and then a referral for ear tubes is made and the child gets an appointment with the ear, nose, and throat doctor, and surgery is scheduled, the ear infections were going to stop anyway.
In other words, millions of children worldwide have been getting ear tubes and physicians and parents saw that the ear infections stopped. So they concluded the ear tubes stopped the infections. We found the infections were going to stop anyway even if the child did not receive ear tubes because their susceptibility to ear infections is over by the time the surgery is performed. The child outgrew ear infections.
An exception was children in daycare at an early age. Our study found that children in daycare who are around 6 months old and start getting ear infections at that age are likely destined to have three or more ear infections in the first year of life. If children are going to be in daycare, perhaps those who need them should receive ear tubes early. Analysis of other demographic and risk factor covariates – sex, race/ethnicity, breastfeeding, siblings in the home, smoking in the home, atopy, and family history of otitis media – were not significantly associated with the number of AOMs in the child population we studied.
We developed a prediction model for doctors, so they could input a child’s age, number of ear infections, and daycare attendance and receive back an estimate of the number of likely future ear infections for that child. With that knowledge, physicians and parents can make more informed decisions.
Our message to clinicians and parents is to reconsider the necessity and timing of ear tube surgery for children with recurrent ear infections because the future is not predicted by the past. Children having several ear infections in a short time does not predict that they will have a similar number of ear infections in the future.
The study was supported by the National Institutes of Health awarded to Rochester Regional Health. Dr. Pichichero was principal investigator for the award.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
About half a million children between the ages of 1 and 3 years old have ear tube surgery in the United States every year at an annual cost exceeding $2 billion. It is the most common childhood surgery performed with anesthesia. It is a surgery commonly performed on children in most other high- and middle-income countries.
My group recently published a paper on the timing and necessity of tympanostomy tubes for recurrent otitis media in young children. The primary objective was to quantitatively examine recurrent acute otitis media (AOM) incidence with respect to age of occurrence, the influence of daycare attendance, and other risk factors in individual children. We introduced the concept of a “window of susceptibility” to AOM as new terminology referring to a child who has two or more closely spaced AOM occurrences during a window of time. We sought to know what to expect and how to advise the parent when a child presents with closely spaced AOMs.
A secondary objective was to develop models to predict the risk and timing of AOM recurrences based on the natural history of disease in young children who do not get tympanostomy tubes. Prediction models were developed to assist clinicians in understanding and explaining to parents the benefit of tympanostomy tubes based on the child’s age and number of AOMs.
The children were all from a primary care pediatric practice in Rochester, N.Y., which comprised a typical mixed demographic of largely middle-class, health care–insured families that was broadly representative of the racial/ethnic diversity in the community. The sample included both wealthy families and those living below the poverty line. The diagnosis of AOM was made based on the American Academy of Pediatrics guidance in which a presumed middle ear effusion and a full or bulging tympanic membrane were required. Almost all episodes (> 85%) of clinically diagnosed AOM cases were confirmed by culture of middle ear fluid collected by tympanocentesis to ensure diagnostic accuracy.
286 children who had ear infections were studied. We found that 80% of ear infections occurred during a very narrow window of susceptibility – age 6-21 months. About 72% of children had a window of susceptibility to ear infections that lasted 5 months or less; 97% of children had a window of susceptibility that lasted 10 months or less.
From this result, we observed that about 90% of children have a window of time lasting about 10 months when they get repeated ear infections. By the time a child gets three ear infections in 6 months (a period of time recommended by the AAP and American Academy of Otolaryngology–Head and Neck Surgery when ear tubes might be considered) and then a referral for ear tubes is made and the child gets an appointment with the ear, nose, and throat doctor, and surgery is scheduled, the ear infections were going to stop anyway.
In other words, millions of children worldwide have been getting ear tubes and physicians and parents saw that the ear infections stopped. So they concluded the ear tubes stopped the infections. We found the infections were going to stop anyway even if the child did not receive ear tubes because their susceptibility to ear infections is over by the time the surgery is performed. The child outgrew ear infections.
An exception was children in daycare at an early age. Our study found that children in daycare who are around 6 months old and start getting ear infections at that age are likely destined to have three or more ear infections in the first year of life. If children are going to be in daycare, perhaps those who need them should receive ear tubes early. Analysis of other demographic and risk factor covariates – sex, race/ethnicity, breastfeeding, siblings in the home, smoking in the home, atopy, and family history of otitis media – were not significantly associated with the number of AOMs in the child population we studied.
We developed a prediction model for doctors, so they could input a child’s age, number of ear infections, and daycare attendance and receive back an estimate of the number of likely future ear infections for that child. With that knowledge, physicians and parents can make more informed decisions.
Our message to clinicians and parents is to reconsider the necessity and timing of ear tube surgery for children with recurrent ear infections because the future is not predicted by the past. Children having several ear infections in a short time does not predict that they will have a similar number of ear infections in the future.
The study was supported by the National Institutes of Health awarded to Rochester Regional Health. Dr. Pichichero was principal investigator for the award.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
Three wild technologies about to change health care
When I was a child, I watched syndicated episodes of the original “Star Trek.” I was dazzled by the space travel, sure, but also the medical technology.
A handheld “tricorder” detected diseases, while an intramuscular injector (“hypospray”) could treat them. Sickbay “biobeds” came with real-time health monitors that looked futuristic at the time but seem primitive today.
Such visions inspired a lot of us kids to pursue science. Little did we know the real-life advances many of us would see in our lifetimes.
Artificial intelligence helping to spot disease, robots performing surgery, even video calls between doctor and patient – all these once sounded fantastical but now happen in clinical care.
Now, in the 23rd year of the 21st century, you might not believe wht we’ll be capable of next. Three especially wild examples are moving closer to clinical reality.
Human hibernation
Captain America, Han Solo, and “Star Trek” villain Khan – all were preserved at low temperatures and then revived, waking up alive and well months, decades, or centuries later. These are fictional examples, to be sure, but the science they’re rooted in is real.
(In one extreme case, a climber survived after almost 9 hours of efforts to revive him.)
Useful for a space traveler? Maybe not. But it’s potentially huge for someone with life-threatening injuries from a car accident or a gunshot wound.
That’s the thinking behind a breakthrough procedure that came after decades of research on pigs and dogs, now in a clinical trial. The idea: A person with massive blood loss whose heart has stopped is injected with an ice-cold fluid, cooling them from the inside, down to about 50° F.
Doctors already induce more modest hypothermia to protect the brain and other organs after cardiac arrest and during surgery on the aortic arch (the main artery carrying blood from the heart).
But this experimental procedure – called emergency preservation and resuscitation (EPR) – goes far beyond that, dramatically “decreasing the body’s need for oxygen and blood flow,” says Samuel Tisherman, MD, a trauma surgeon at the University of Maryland Medical Center and the trial’s lead researcher. This puts the patient in a state of suspended animation that “could buy time for surgeons to stop the bleeding and save more of these patients.”
The technique has been done on at least six patients, though none were reported to survive. The trial is expected to include 20 people by the time it wraps up in December, according to the listing on the U.S. clinical trials database. Though given the strict requirements for candidates (emergency trauma victims who are not likely to survive), one can’t exactly rely on a set schedule.
Still, the technology is promising. Someday we may even use it to keep patients in suspended animation for months or years, experts predict, helping astronauts through decades-long spaceflights, or stalling death in sick patients awaiting a cure.
Artificial womb
Another sci-fi classic: growing human babies outside the womb. Think the fetus fields from “The Matrix,” or the frozen embryos in “Alien: Covenant.”
In 1923, British biologist J.B.S. Haldane coined a term for that – ectogenesis. He predicted that 70% of pregnancies would take place, from fertilization to birth, in artificial wombs by 2074. That many seems unlikely, but the timeline is on track.
Developing an embryo outside the womb is already routine in in vitro fertilization. And technology enables preterm babies to survive through much of the second half of gestation. Normal human pregnancy is 40 weeks, and the youngest preterm baby ever to survive was 21 weeks and 1 day old, just a few days younger than a smattering of others who lived.
The biggest obstacle for babies younger than that is lung viability. Mechanical ventilation can damage the lungs and lead to a chronic (sometimes fatal) lung disease known as bronchopulmonary dysplasia. Avoiding this would mean figuring out a way to maintain fetal circulation – the intricate system that delivers oxygenated blood from the placenta to the fetus via the umbilical cord. Researchers at Children’s Hospital of Philadelphia have done this using a fetal lamb.
The key to their invention is a substitute placenta: an oxygenator connected to the lamb’s umbilical cord. Tubes inserted through the umbilical vein and arteries carry oxygenated blood from the “placenta” to the fetus, and deoxygenated blood back out. The lamb resides in an artificial, fluid-filled amniotic sac until its lungs and other organs are developed.
Fertility treatment could benefit, too. “An artificial womb may substitute in situations in which a gestational carrier – surrogate – is indicated,” says Paula Amato, MD, a professor of obstetrics and gynecology at Oregon Health and Science University, Portland. (Dr. Amato is not involved in the CHOP research.) For example: when the mother is missing a uterus or can’t carry a pregnancy safely.
No date is set for clinical trials yet. But according to the research, the main difference between human and lamb may come down to size. A lamb’s umbilical vessels are larger, so feeding in a tube is easier. With today’s advances in miniaturizing surgical methods, that seems like a challenge scientists can overcome.
Messenger RNA therapeutics
Back to “Star Trek.” The hypospray injector’s contents could cure just about any disease, even one newly discovered on a strange planet. That’s not unlike messenger RNA (mRNA) technology, a breakthrough that enabled scientists to quickly develop some of the first COVID-19 vaccines.
But vaccines are just the beginning of what this technology can do.
A whole field of immunotherapy is emerging that uses mRNA to deliver instructions to produce chimeric antigen receptor–modified immune cells (CAR-modified immune cells). These cells are engineered to target diseased cells and tissues, like cancer cells and harmful fibroblasts (scar tissue) that promote fibrosis in, for example, the heart and lungs.
The field is bursting with rodent research, and clinical trials have started for treating some advanced-stage malignancies.
Actual clinical use may be years away, but if all goes well, these medicines could help treat or even cure the core medical problems facing humanity. We’re talking cancer, heart disease, neurodegenerative disease – transforming one therapy into another by simply changing the mRNA’s “nucleotide sequence,” the blueprint containing instructions telling it what to do, and what disease to attack.
As this technology matures, we may start to feel as if we’re really on “Star Trek,” where Dr. Leonard “Bones” McCoy pulls out the same device to treat just about every disease or injury.
A version of this article first appeared on WebMD.com.
When I was a child, I watched syndicated episodes of the original “Star Trek.” I was dazzled by the space travel, sure, but also the medical technology.
A handheld “tricorder” detected diseases, while an intramuscular injector (“hypospray”) could treat them. Sickbay “biobeds” came with real-time health monitors that looked futuristic at the time but seem primitive today.
Such visions inspired a lot of us kids to pursue science. Little did we know the real-life advances many of us would see in our lifetimes.
Artificial intelligence helping to spot disease, robots performing surgery, even video calls between doctor and patient – all these once sounded fantastical but now happen in clinical care.
Now, in the 23rd year of the 21st century, you might not believe wht we’ll be capable of next. Three especially wild examples are moving closer to clinical reality.
Human hibernation
Captain America, Han Solo, and “Star Trek” villain Khan – all were preserved at low temperatures and then revived, waking up alive and well months, decades, or centuries later. These are fictional examples, to be sure, but the science they’re rooted in is real.
(In one extreme case, a climber survived after almost 9 hours of efforts to revive him.)
Useful for a space traveler? Maybe not. But it’s potentially huge for someone with life-threatening injuries from a car accident or a gunshot wound.
That’s the thinking behind a breakthrough procedure that came after decades of research on pigs and dogs, now in a clinical trial. The idea: A person with massive blood loss whose heart has stopped is injected with an ice-cold fluid, cooling them from the inside, down to about 50° F.
Doctors already induce more modest hypothermia to protect the brain and other organs after cardiac arrest and during surgery on the aortic arch (the main artery carrying blood from the heart).
But this experimental procedure – called emergency preservation and resuscitation (EPR) – goes far beyond that, dramatically “decreasing the body’s need for oxygen and blood flow,” says Samuel Tisherman, MD, a trauma surgeon at the University of Maryland Medical Center and the trial’s lead researcher. This puts the patient in a state of suspended animation that “could buy time for surgeons to stop the bleeding and save more of these patients.”
The technique has been done on at least six patients, though none were reported to survive. The trial is expected to include 20 people by the time it wraps up in December, according to the listing on the U.S. clinical trials database. Though given the strict requirements for candidates (emergency trauma victims who are not likely to survive), one can’t exactly rely on a set schedule.
Still, the technology is promising. Someday we may even use it to keep patients in suspended animation for months or years, experts predict, helping astronauts through decades-long spaceflights, or stalling death in sick patients awaiting a cure.
Artificial womb
Another sci-fi classic: growing human babies outside the womb. Think the fetus fields from “The Matrix,” or the frozen embryos in “Alien: Covenant.”
In 1923, British biologist J.B.S. Haldane coined a term for that – ectogenesis. He predicted that 70% of pregnancies would take place, from fertilization to birth, in artificial wombs by 2074. That many seems unlikely, but the timeline is on track.
Developing an embryo outside the womb is already routine in in vitro fertilization. And technology enables preterm babies to survive through much of the second half of gestation. Normal human pregnancy is 40 weeks, and the youngest preterm baby ever to survive was 21 weeks and 1 day old, just a few days younger than a smattering of others who lived.
The biggest obstacle for babies younger than that is lung viability. Mechanical ventilation can damage the lungs and lead to a chronic (sometimes fatal) lung disease known as bronchopulmonary dysplasia. Avoiding this would mean figuring out a way to maintain fetal circulation – the intricate system that delivers oxygenated blood from the placenta to the fetus via the umbilical cord. Researchers at Children’s Hospital of Philadelphia have done this using a fetal lamb.
The key to their invention is a substitute placenta: an oxygenator connected to the lamb’s umbilical cord. Tubes inserted through the umbilical vein and arteries carry oxygenated blood from the “placenta” to the fetus, and deoxygenated blood back out. The lamb resides in an artificial, fluid-filled amniotic sac until its lungs and other organs are developed.
Fertility treatment could benefit, too. “An artificial womb may substitute in situations in which a gestational carrier – surrogate – is indicated,” says Paula Amato, MD, a professor of obstetrics and gynecology at Oregon Health and Science University, Portland. (Dr. Amato is not involved in the CHOP research.) For example: when the mother is missing a uterus or can’t carry a pregnancy safely.
No date is set for clinical trials yet. But according to the research, the main difference between human and lamb may come down to size. A lamb’s umbilical vessels are larger, so feeding in a tube is easier. With today’s advances in miniaturizing surgical methods, that seems like a challenge scientists can overcome.
Messenger RNA therapeutics
Back to “Star Trek.” The hypospray injector’s contents could cure just about any disease, even one newly discovered on a strange planet. That’s not unlike messenger RNA (mRNA) technology, a breakthrough that enabled scientists to quickly develop some of the first COVID-19 vaccines.
But vaccines are just the beginning of what this technology can do.
A whole field of immunotherapy is emerging that uses mRNA to deliver instructions to produce chimeric antigen receptor–modified immune cells (CAR-modified immune cells). These cells are engineered to target diseased cells and tissues, like cancer cells and harmful fibroblasts (scar tissue) that promote fibrosis in, for example, the heart and lungs.
The field is bursting with rodent research, and clinical trials have started for treating some advanced-stage malignancies.
Actual clinical use may be years away, but if all goes well, these medicines could help treat or even cure the core medical problems facing humanity. We’re talking cancer, heart disease, neurodegenerative disease – transforming one therapy into another by simply changing the mRNA’s “nucleotide sequence,” the blueprint containing instructions telling it what to do, and what disease to attack.
As this technology matures, we may start to feel as if we’re really on “Star Trek,” where Dr. Leonard “Bones” McCoy pulls out the same device to treat just about every disease or injury.
A version of this article first appeared on WebMD.com.
When I was a child, I watched syndicated episodes of the original “Star Trek.” I was dazzled by the space travel, sure, but also the medical technology.
A handheld “tricorder” detected diseases, while an intramuscular injector (“hypospray”) could treat them. Sickbay “biobeds” came with real-time health monitors that looked futuristic at the time but seem primitive today.
Such visions inspired a lot of us kids to pursue science. Little did we know the real-life advances many of us would see in our lifetimes.
Artificial intelligence helping to spot disease, robots performing surgery, even video calls between doctor and patient – all these once sounded fantastical but now happen in clinical care.
Now, in the 23rd year of the 21st century, you might not believe wht we’ll be capable of next. Three especially wild examples are moving closer to clinical reality.
Human hibernation
Captain America, Han Solo, and “Star Trek” villain Khan – all were preserved at low temperatures and then revived, waking up alive and well months, decades, or centuries later. These are fictional examples, to be sure, but the science they’re rooted in is real.
(In one extreme case, a climber survived after almost 9 hours of efforts to revive him.)
Useful for a space traveler? Maybe not. But it’s potentially huge for someone with life-threatening injuries from a car accident or a gunshot wound.
That’s the thinking behind a breakthrough procedure that came after decades of research on pigs and dogs, now in a clinical trial. The idea: A person with massive blood loss whose heart has stopped is injected with an ice-cold fluid, cooling them from the inside, down to about 50° F.
Doctors already induce more modest hypothermia to protect the brain and other organs after cardiac arrest and during surgery on the aortic arch (the main artery carrying blood from the heart).
But this experimental procedure – called emergency preservation and resuscitation (EPR) – goes far beyond that, dramatically “decreasing the body’s need for oxygen and blood flow,” says Samuel Tisherman, MD, a trauma surgeon at the University of Maryland Medical Center and the trial’s lead researcher. This puts the patient in a state of suspended animation that “could buy time for surgeons to stop the bleeding and save more of these patients.”
The technique has been done on at least six patients, though none were reported to survive. The trial is expected to include 20 people by the time it wraps up in December, according to the listing on the U.S. clinical trials database. Though given the strict requirements for candidates (emergency trauma victims who are not likely to survive), one can’t exactly rely on a set schedule.
Still, the technology is promising. Someday we may even use it to keep patients in suspended animation for months or years, experts predict, helping astronauts through decades-long spaceflights, or stalling death in sick patients awaiting a cure.
Artificial womb
Another sci-fi classic: growing human babies outside the womb. Think the fetus fields from “The Matrix,” or the frozen embryos in “Alien: Covenant.”
In 1923, British biologist J.B.S. Haldane coined a term for that – ectogenesis. He predicted that 70% of pregnancies would take place, from fertilization to birth, in artificial wombs by 2074. That many seems unlikely, but the timeline is on track.
Developing an embryo outside the womb is already routine in in vitro fertilization. And technology enables preterm babies to survive through much of the second half of gestation. Normal human pregnancy is 40 weeks, and the youngest preterm baby ever to survive was 21 weeks and 1 day old, just a few days younger than a smattering of others who lived.
The biggest obstacle for babies younger than that is lung viability. Mechanical ventilation can damage the lungs and lead to a chronic (sometimes fatal) lung disease known as bronchopulmonary dysplasia. Avoiding this would mean figuring out a way to maintain fetal circulation – the intricate system that delivers oxygenated blood from the placenta to the fetus via the umbilical cord. Researchers at Children’s Hospital of Philadelphia have done this using a fetal lamb.
The key to their invention is a substitute placenta: an oxygenator connected to the lamb’s umbilical cord. Tubes inserted through the umbilical vein and arteries carry oxygenated blood from the “placenta” to the fetus, and deoxygenated blood back out. The lamb resides in an artificial, fluid-filled amniotic sac until its lungs and other organs are developed.
Fertility treatment could benefit, too. “An artificial womb may substitute in situations in which a gestational carrier – surrogate – is indicated,” says Paula Amato, MD, a professor of obstetrics and gynecology at Oregon Health and Science University, Portland. (Dr. Amato is not involved in the CHOP research.) For example: when the mother is missing a uterus or can’t carry a pregnancy safely.
No date is set for clinical trials yet. But according to the research, the main difference between human and lamb may come down to size. A lamb’s umbilical vessels are larger, so feeding in a tube is easier. With today’s advances in miniaturizing surgical methods, that seems like a challenge scientists can overcome.
Messenger RNA therapeutics
Back to “Star Trek.” The hypospray injector’s contents could cure just about any disease, even one newly discovered on a strange planet. That’s not unlike messenger RNA (mRNA) technology, a breakthrough that enabled scientists to quickly develop some of the first COVID-19 vaccines.
But vaccines are just the beginning of what this technology can do.
A whole field of immunotherapy is emerging that uses mRNA to deliver instructions to produce chimeric antigen receptor–modified immune cells (CAR-modified immune cells). These cells are engineered to target diseased cells and tissues, like cancer cells and harmful fibroblasts (scar tissue) that promote fibrosis in, for example, the heart and lungs.
The field is bursting with rodent research, and clinical trials have started for treating some advanced-stage malignancies.
Actual clinical use may be years away, but if all goes well, these medicines could help treat or even cure the core medical problems facing humanity. We’re talking cancer, heart disease, neurodegenerative disease – transforming one therapy into another by simply changing the mRNA’s “nucleotide sequence,” the blueprint containing instructions telling it what to do, and what disease to attack.
As this technology matures, we may start to feel as if we’re really on “Star Trek,” where Dr. Leonard “Bones” McCoy pulls out the same device to treat just about every disease or injury.
A version of this article first appeared on WebMD.com.
A new (old) drug joins the COVID fray, and guess what? It works
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.

At this point, with the monoclonals found to be essentially useless, we are left with remdesivir with its modest efficacy and Paxlovid, which, for some reason, people don’t seem to be taking.
Part of the reason the monoclonals have failed lately is because of their specificity; they are homogeneous antibodies targeted toward a very specific epitope that may change from variant to variant. We need a broader therapeutic, one that has activity across all variants — maybe even one that has activity against all viruses? We’ve got one. Interferon.
The first mention of interferon as a potential COVID therapy was at the very start of the pandemic, so I’m sort of surprised that the first large, randomized trial is only being reported now in the New England Journal of Medicine.
Before we dig into the results, let’s talk mechanism. This is a trial of interferon-lambda, also known as interleukin-29.
The lambda interferons were only discovered in 2003. They differ from the more familiar interferons only in their cellular receptors; the downstream effects seem quite similar. As opposed to the cellular receptors for interferon alfa, which are widely expressed, the receptors for lambda are restricted to epithelial tissues. This makes it a good choice as a COVID treatment, since the virus also preferentially targets those epithelial cells.
In this study, 1,951 participants from Brazil and Canada, but mostly Brazil, with new COVID infections who were not yet hospitalized were randomized to receive 180 mcg of interferon lambda or placebo.
This was a relatively current COVID trial, as you can see from the participant characteristics. The majority had been vaccinated, and nearly half of the infections were during the Omicron phase of the pandemic.
If you just want to cut to the chase, interferon worked.
The primary outcome – hospitalization or a prolonged emergency room visit for COVID – was 50% lower in the interferon group.
Key secondary outcomes, including death from COVID, were lower in the interferon group as well. These effects persisted across most of the subgroups I was looking out for.
Interferon seemed to help those who were already vaccinated and those who were unvaccinated. There’s a hint that it works better within the first few days of symptoms, which isn’t surprising; we’ve seen this for many of the therapeutics, including Paxlovid. Time is of the essence. Encouragingly, the effect was a bit more pronounced among those infected with Omicron.
Of course, if you have any experience with interferon, you know that the side effects can be pretty rough. In the bad old days when we treated hepatitis C infection with interferon, patients would get their injections on Friday in anticipation of being essentially out of commission with flu-like symptoms through the weekend. But we don’t see much evidence of adverse events in this trial, maybe due to the greater specificity of interferon lambda.
Putting it all together, the state of play for interferons in COVID may be changing. To date, the FDA has not recommended the use of interferon alfa or -beta for COVID-19, citing some data that they are ineffective or even harmful in hospitalized patients with COVID. Interferon lambda is not FDA approved and thus not even available in the United States. But the reason it has not been approved is that there has not been a large, well-conducted interferon lambda trial. Now there is. Will this study be enough to prompt an emergency use authorization? The elephant in the room, of course, is Paxlovid, which at this point has a longer safety track record and, importantly, is oral. I’d love to see a head-to-head trial. Short of that, I tend to be in favor of having more options on the table.
Dr. Perry Wilson is associate professor, department of medicine, and director, Clinical and Translational Research Accelerator, at Yale University, New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.

At this point, with the monoclonals found to be essentially useless, we are left with remdesivir with its modest efficacy and Paxlovid, which, for some reason, people don’t seem to be taking.
Part of the reason the monoclonals have failed lately is because of their specificity; they are homogeneous antibodies targeted toward a very specific epitope that may change from variant to variant. We need a broader therapeutic, one that has activity across all variants — maybe even one that has activity against all viruses? We’ve got one. Interferon.
The first mention of interferon as a potential COVID therapy was at the very start of the pandemic, so I’m sort of surprised that the first large, randomized trial is only being reported now in the New England Journal of Medicine.
Before we dig into the results, let’s talk mechanism. This is a trial of interferon-lambda, also known as interleukin-29.
The lambda interferons were only discovered in 2003. They differ from the more familiar interferons only in their cellular receptors; the downstream effects seem quite similar. As opposed to the cellular receptors for interferon alfa, which are widely expressed, the receptors for lambda are restricted to epithelial tissues. This makes it a good choice as a COVID treatment, since the virus also preferentially targets those epithelial cells.
In this study, 1,951 participants from Brazil and Canada, but mostly Brazil, with new COVID infections who were not yet hospitalized were randomized to receive 180 mcg of interferon lambda or placebo.
This was a relatively current COVID trial, as you can see from the participant characteristics. The majority had been vaccinated, and nearly half of the infections were during the Omicron phase of the pandemic.
If you just want to cut to the chase, interferon worked.
The primary outcome – hospitalization or a prolonged emergency room visit for COVID – was 50% lower in the interferon group.
Key secondary outcomes, including death from COVID, were lower in the interferon group as well. These effects persisted across most of the subgroups I was looking out for.
Interferon seemed to help those who were already vaccinated and those who were unvaccinated. There’s a hint that it works better within the first few days of symptoms, which isn’t surprising; we’ve seen this for many of the therapeutics, including Paxlovid. Time is of the essence. Encouragingly, the effect was a bit more pronounced among those infected with Omicron.
Of course, if you have any experience with interferon, you know that the side effects can be pretty rough. In the bad old days when we treated hepatitis C infection with interferon, patients would get their injections on Friday in anticipation of being essentially out of commission with flu-like symptoms through the weekend. But we don’t see much evidence of adverse events in this trial, maybe due to the greater specificity of interferon lambda.
Putting it all together, the state of play for interferons in COVID may be changing. To date, the FDA has not recommended the use of interferon alfa or -beta for COVID-19, citing some data that they are ineffective or even harmful in hospitalized patients with COVID. Interferon lambda is not FDA approved and thus not even available in the United States. But the reason it has not been approved is that there has not been a large, well-conducted interferon lambda trial. Now there is. Will this study be enough to prompt an emergency use authorization? The elephant in the room, of course, is Paxlovid, which at this point has a longer safety track record and, importantly, is oral. I’d love to see a head-to-head trial. Short of that, I tend to be in favor of having more options on the table.
Dr. Perry Wilson is associate professor, department of medicine, and director, Clinical and Translational Research Accelerator, at Yale University, New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.

At this point, with the monoclonals found to be essentially useless, we are left with remdesivir with its modest efficacy and Paxlovid, which, for some reason, people don’t seem to be taking.
Part of the reason the monoclonals have failed lately is because of their specificity; they are homogeneous antibodies targeted toward a very specific epitope that may change from variant to variant. We need a broader therapeutic, one that has activity across all variants — maybe even one that has activity against all viruses? We’ve got one. Interferon.
The first mention of interferon as a potential COVID therapy was at the very start of the pandemic, so I’m sort of surprised that the first large, randomized trial is only being reported now in the New England Journal of Medicine.
Before we dig into the results, let’s talk mechanism. This is a trial of interferon-lambda, also known as interleukin-29.
The lambda interferons were only discovered in 2003. They differ from the more familiar interferons only in their cellular receptors; the downstream effects seem quite similar. As opposed to the cellular receptors for interferon alfa, which are widely expressed, the receptors for lambda are restricted to epithelial tissues. This makes it a good choice as a COVID treatment, since the virus also preferentially targets those epithelial cells.
In this study, 1,951 participants from Brazil and Canada, but mostly Brazil, with new COVID infections who were not yet hospitalized were randomized to receive 180 mcg of interferon lambda or placebo.
This was a relatively current COVID trial, as you can see from the participant characteristics. The majority had been vaccinated, and nearly half of the infections were during the Omicron phase of the pandemic.
If you just want to cut to the chase, interferon worked.
The primary outcome – hospitalization or a prolonged emergency room visit for COVID – was 50% lower in the interferon group.
Key secondary outcomes, including death from COVID, were lower in the interferon group as well. These effects persisted across most of the subgroups I was looking out for.
Interferon seemed to help those who were already vaccinated and those who were unvaccinated. There’s a hint that it works better within the first few days of symptoms, which isn’t surprising; we’ve seen this for many of the therapeutics, including Paxlovid. Time is of the essence. Encouragingly, the effect was a bit more pronounced among those infected with Omicron.
Of course, if you have any experience with interferon, you know that the side effects can be pretty rough. In the bad old days when we treated hepatitis C infection with interferon, patients would get their injections on Friday in anticipation of being essentially out of commission with flu-like symptoms through the weekend. But we don’t see much evidence of adverse events in this trial, maybe due to the greater specificity of interferon lambda.
Putting it all together, the state of play for interferons in COVID may be changing. To date, the FDA has not recommended the use of interferon alfa or -beta for COVID-19, citing some data that they are ineffective or even harmful in hospitalized patients with COVID. Interferon lambda is not FDA approved and thus not even available in the United States. But the reason it has not been approved is that there has not been a large, well-conducted interferon lambda trial. Now there is. Will this study be enough to prompt an emergency use authorization? The elephant in the room, of course, is Paxlovid, which at this point has a longer safety track record and, importantly, is oral. I’d love to see a head-to-head trial. Short of that, I tend to be in favor of having more options on the table.
Dr. Perry Wilson is associate professor, department of medicine, and director, Clinical and Translational Research Accelerator, at Yale University, New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.










