User login
Red wine’s potential benefits for cardiovascular health
In recent weeks, you may have noticed some familiar headlines about red wine and cardiovascular health. Why the sudden return of these stories? Because of an article recently published in the American Journal of Clinical Nutrition.
Funded in part by a grant from the São Paulo Research Foundation (FAPESP), the “Wine Flora Study” was carried out by prominent researchers from institutions in South America, Europe, and the United States: University of São Paulo; State University of Campinas, São Paulo; University of Brasília; University of Verona (Italy); Austrian Institute of Technology, Tulln; and Harvard Medical School, Boston. The team looked into the effects of red wine on gut flora and plasma levels of trimethylamine-N-oxide (TMAO). And what they found was quite interesting.
The study
Previous results have pointed to the beneficial effect that red wine has on the gut microbiome.
The Wine Flora Study involved 42 men (average age, 60 years) with documented coronary artery disease. The trial encompassed two 3-week interventions. In one, the participants consumed 250 mL of red wine per day; the red wine sample had an alcohol content (% v) of 12.75. The Brazilian Wine Institute produced and supplied the red wine: a 2014 Merlot bottled in August 2016 and customized for the study. The second intervention involved alcohol abstention.
Each intervention was preceded by a 2-week washout period. Because certain foods and drinks could interfere with the results, the participants were instructed not to consume alcoholic beverages, fermented foods (yogurt, kombucha, soy lecithin, kefir, sauerkraut, and other fermented vegetables), synthetic prebiotics (insulin, fructooligosaccharides), fiber, dairy, food polyphenols (grapes, grape juice, cranberries, strawberries), and probiotics.
At each intervention, the gut microbiota was analyzed via 16S ribosomal RNA highthroughput sequencing. This method makes it possible to identify bacterial species. The plasma metabolome of 20 randomly selected participants was evaluated by ultra–high-performance liquid chromatography with tandem mass spectrometry. In this method, liquid chromatography separates the compounds, and a mass spectrometer is used to analyze them.
One of the metabolites of interest was TMAO, which is produced from the trimethylamine released when gut bacteria process protein-rich foods. TMAO has been identified as playing a role in the development of atherosclerosis.
Results
with a difference in beta diversity and predominance of Parasutterella, Ruminococcaceae, several Bacteroides species, and Prevotella.
Plasma metabolomic analysis revealed significant changes in metabolites after red wine consumption, consistent with improved redox homeostasis, which is involved in the oxidative stress that promotes atherosclerosis.
Plasma TMAO, however, did not differ between red wine intervention and alcohol abstention.
Implications
The researchers concluded that modulation of the gut microbiota may contribute to the putative cardiovascular benefits of moderate red wine consumption. But, as they were careful to point out in the very title of the study, a red wine intervention does not modify plasma TMAO. They also mentioned that the 3-week period may have been too short for the findings to serve as the basis for promoting any meaningful modification. In addition, the team emphasized that these data remain hypothesisgenerating and pave the way for future research.
In an interview with FAPESP, the study’s corresponding author, Protásio Lemos da Luz, MD, PhD, warned about the risks associated with drinking too much alcohol (> 8.5 oz., or 250 mL, of wine daily).
It should be kept in mind that, in Brazil, people do not drink nearly as much wine as they do beer or liquor. Furthermore, the evidence that is available does not provide confirmation of the existence or the extent of the protective health effects associated with light or moderate alcohol intake.
This article was translated from the Medscape Portuguese edition. A version appeared on Medscape.com.
In recent weeks, you may have noticed some familiar headlines about red wine and cardiovascular health. Why the sudden return of these stories? Because of an article recently published in the American Journal of Clinical Nutrition.
Funded in part by a grant from the São Paulo Research Foundation (FAPESP), the “Wine Flora Study” was carried out by prominent researchers from institutions in South America, Europe, and the United States: University of São Paulo; State University of Campinas, São Paulo; University of Brasília; University of Verona (Italy); Austrian Institute of Technology, Tulln; and Harvard Medical School, Boston. The team looked into the effects of red wine on gut flora and plasma levels of trimethylamine-N-oxide (TMAO). And what they found was quite interesting.
The study
Previous results have pointed to the beneficial effect that red wine has on the gut microbiome.
The Wine Flora Study involved 42 men (average age, 60 years) with documented coronary artery disease. The trial encompassed two 3-week interventions. In one, the participants consumed 250 mL of red wine per day; the red wine sample had an alcohol content (% v) of 12.75. The Brazilian Wine Institute produced and supplied the red wine: a 2014 Merlot bottled in August 2016 and customized for the study. The second intervention involved alcohol abstention.
Each intervention was preceded by a 2-week washout period. Because certain foods and drinks could interfere with the results, the participants were instructed not to consume alcoholic beverages, fermented foods (yogurt, kombucha, soy lecithin, kefir, sauerkraut, and other fermented vegetables), synthetic prebiotics (insulin, fructooligosaccharides), fiber, dairy, food polyphenols (grapes, grape juice, cranberries, strawberries), and probiotics.
At each intervention, the gut microbiota was analyzed via 16S ribosomal RNA highthroughput sequencing. This method makes it possible to identify bacterial species. The plasma metabolome of 20 randomly selected participants was evaluated by ultra–high-performance liquid chromatography with tandem mass spectrometry. In this method, liquid chromatography separates the compounds, and a mass spectrometer is used to analyze them.
One of the metabolites of interest was TMAO, which is produced from the trimethylamine released when gut bacteria process protein-rich foods. TMAO has been identified as playing a role in the development of atherosclerosis.
Results
with a difference in beta diversity and predominance of Parasutterella, Ruminococcaceae, several Bacteroides species, and Prevotella.
Plasma metabolomic analysis revealed significant changes in metabolites after red wine consumption, consistent with improved redox homeostasis, which is involved in the oxidative stress that promotes atherosclerosis.
Plasma TMAO, however, did not differ between red wine intervention and alcohol abstention.
Implications
The researchers concluded that modulation of the gut microbiota may contribute to the putative cardiovascular benefits of moderate red wine consumption. But, as they were careful to point out in the very title of the study, a red wine intervention does not modify plasma TMAO. They also mentioned that the 3-week period may have been too short for the findings to serve as the basis for promoting any meaningful modification. In addition, the team emphasized that these data remain hypothesisgenerating and pave the way for future research.
In an interview with FAPESP, the study’s corresponding author, Protásio Lemos da Luz, MD, PhD, warned about the risks associated with drinking too much alcohol (> 8.5 oz., or 250 mL, of wine daily).
It should be kept in mind that, in Brazil, people do not drink nearly as much wine as they do beer or liquor. Furthermore, the evidence that is available does not provide confirmation of the existence or the extent of the protective health effects associated with light or moderate alcohol intake.
This article was translated from the Medscape Portuguese edition. A version appeared on Medscape.com.
In recent weeks, you may have noticed some familiar headlines about red wine and cardiovascular health. Why the sudden return of these stories? Because of an article recently published in the American Journal of Clinical Nutrition.
Funded in part by a grant from the São Paulo Research Foundation (FAPESP), the “Wine Flora Study” was carried out by prominent researchers from institutions in South America, Europe, and the United States: University of São Paulo; State University of Campinas, São Paulo; University of Brasília; University of Verona (Italy); Austrian Institute of Technology, Tulln; and Harvard Medical School, Boston. The team looked into the effects of red wine on gut flora and plasma levels of trimethylamine-N-oxide (TMAO). And what they found was quite interesting.
The study
Previous results have pointed to the beneficial effect that red wine has on the gut microbiome.
The Wine Flora Study involved 42 men (average age, 60 years) with documented coronary artery disease. The trial encompassed two 3-week interventions. In one, the participants consumed 250 mL of red wine per day; the red wine sample had an alcohol content (% v) of 12.75. The Brazilian Wine Institute produced and supplied the red wine: a 2014 Merlot bottled in August 2016 and customized for the study. The second intervention involved alcohol abstention.
Each intervention was preceded by a 2-week washout period. Because certain foods and drinks could interfere with the results, the participants were instructed not to consume alcoholic beverages, fermented foods (yogurt, kombucha, soy lecithin, kefir, sauerkraut, and other fermented vegetables), synthetic prebiotics (insulin, fructooligosaccharides), fiber, dairy, food polyphenols (grapes, grape juice, cranberries, strawberries), and probiotics.
At each intervention, the gut microbiota was analyzed via 16S ribosomal RNA highthroughput sequencing. This method makes it possible to identify bacterial species. The plasma metabolome of 20 randomly selected participants was evaluated by ultra–high-performance liquid chromatography with tandem mass spectrometry. In this method, liquid chromatography separates the compounds, and a mass spectrometer is used to analyze them.
One of the metabolites of interest was TMAO, which is produced from the trimethylamine released when gut bacteria process protein-rich foods. TMAO has been identified as playing a role in the development of atherosclerosis.
Results
with a difference in beta diversity and predominance of Parasutterella, Ruminococcaceae, several Bacteroides species, and Prevotella.
Plasma metabolomic analysis revealed significant changes in metabolites after red wine consumption, consistent with improved redox homeostasis, which is involved in the oxidative stress that promotes atherosclerosis.
Plasma TMAO, however, did not differ between red wine intervention and alcohol abstention.
Implications
The researchers concluded that modulation of the gut microbiota may contribute to the putative cardiovascular benefits of moderate red wine consumption. But, as they were careful to point out in the very title of the study, a red wine intervention does not modify plasma TMAO. They also mentioned that the 3-week period may have been too short for the findings to serve as the basis for promoting any meaningful modification. In addition, the team emphasized that these data remain hypothesisgenerating and pave the way for future research.
In an interview with FAPESP, the study’s corresponding author, Protásio Lemos da Luz, MD, PhD, warned about the risks associated with drinking too much alcohol (> 8.5 oz., or 250 mL, of wine daily).
It should be kept in mind that, in Brazil, people do not drink nearly as much wine as they do beer or liquor. Furthermore, the evidence that is available does not provide confirmation of the existence or the extent of the protective health effects associated with light or moderate alcohol intake.
This article was translated from the Medscape Portuguese edition. A version appeared on Medscape.com.
Alzheimer’s disease: What is ‘clinically meaningful’?
A recent report in Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association suggested that, at least for now, we need to lower the bar in Alzheimer’s disease drug trials.
Their point is that there’s no consensus on “clinically meaningful benefit.” Does it mean a complete cure for Alzheimer’s disease, with reversal of deficits? Or stopping disease progression where it is? Or just slowing things down enough that it means something to patients, family members, and caregivers?
The last one is, realistically, where we are now.
The problem with this is that many nonmedical people equate “treatment” with “cure,” which isn’t close to the truth for many diseases. In Alzheimer’s disease, it’s even trickier to figure out. There’s a disparity between imaging (which suggests something that should be quite effective) and clinical results (which aren’t nearly as impressive as the PET scans).
So when I prescribe any of the Alzheimer’s medications, I make it pretty clear to patients, and more importantly the patient’s family, what they can and can’t expect. This isn’t easy, because most will come back a month later, tell me their loved one is no better, and want to try something else. So I have to explain it again. These people aren’t stupid. They’re hopeful, and also facing an impossible question. “Better” is a lot easier to judge than “slowed progression.”
“Better” is a great word for migraines. Or seizures. Or Parkinson’s disease. These are condition where patients and families can tell us whether they’ve seen an improvement.
But with the current treatments for Alzheimer’s disease we’re asking patients and families “do you think you’ve gotten any worse than you would have if you hadn’t taken the drug at all?”
That’s an impossible question to answer, unless you’re following people with objective cognitive data over time and comparing them against a placebo group, which is how these drugs got here in the first place – we know they do that.
But to a family watching their loved ones go downhill, such reassurances aren’t what they want to hear.
Regrettably, it’s where things stand. While I want to strive for absolute success in these things, today it’s simply not possible. Maybe it never will be, though I hope it is.
But, for now, I agree that we need to reframe what we’re going to consider clinically meaningful. Sometimes you have to settle for a flight of stairs instead of an elevator, but still hope that you’ll get to the top. It just takes longer, and it’s better than not going anywhere at all.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
A recent report in Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association suggested that, at least for now, we need to lower the bar in Alzheimer’s disease drug trials.
Their point is that there’s no consensus on “clinically meaningful benefit.” Does it mean a complete cure for Alzheimer’s disease, with reversal of deficits? Or stopping disease progression where it is? Or just slowing things down enough that it means something to patients, family members, and caregivers?
The last one is, realistically, where we are now.
The problem with this is that many nonmedical people equate “treatment” with “cure,” which isn’t close to the truth for many diseases. In Alzheimer’s disease, it’s even trickier to figure out. There’s a disparity between imaging (which suggests something that should be quite effective) and clinical results (which aren’t nearly as impressive as the PET scans).
So when I prescribe any of the Alzheimer’s medications, I make it pretty clear to patients, and more importantly the patient’s family, what they can and can’t expect. This isn’t easy, because most will come back a month later, tell me their loved one is no better, and want to try something else. So I have to explain it again. These people aren’t stupid. They’re hopeful, and also facing an impossible question. “Better” is a lot easier to judge than “slowed progression.”
“Better” is a great word for migraines. Or seizures. Or Parkinson’s disease. These are condition where patients and families can tell us whether they’ve seen an improvement.
But with the current treatments for Alzheimer’s disease we’re asking patients and families “do you think you’ve gotten any worse than you would have if you hadn’t taken the drug at all?”
That’s an impossible question to answer, unless you’re following people with objective cognitive data over time and comparing them against a placebo group, which is how these drugs got here in the first place – we know they do that.
But to a family watching their loved ones go downhill, such reassurances aren’t what they want to hear.
Regrettably, it’s where things stand. While I want to strive for absolute success in these things, today it’s simply not possible. Maybe it never will be, though I hope it is.
But, for now, I agree that we need to reframe what we’re going to consider clinically meaningful. Sometimes you have to settle for a flight of stairs instead of an elevator, but still hope that you’ll get to the top. It just takes longer, and it’s better than not going anywhere at all.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
A recent report in Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association suggested that, at least for now, we need to lower the bar in Alzheimer’s disease drug trials.
Their point is that there’s no consensus on “clinically meaningful benefit.” Does it mean a complete cure for Alzheimer’s disease, with reversal of deficits? Or stopping disease progression where it is? Or just slowing things down enough that it means something to patients, family members, and caregivers?
The last one is, realistically, where we are now.
The problem with this is that many nonmedical people equate “treatment” with “cure,” which isn’t close to the truth for many diseases. In Alzheimer’s disease, it’s even trickier to figure out. There’s a disparity between imaging (which suggests something that should be quite effective) and clinical results (which aren’t nearly as impressive as the PET scans).
So when I prescribe any of the Alzheimer’s medications, I make it pretty clear to patients, and more importantly the patient’s family, what they can and can’t expect. This isn’t easy, because most will come back a month later, tell me their loved one is no better, and want to try something else. So I have to explain it again. These people aren’t stupid. They’re hopeful, and also facing an impossible question. “Better” is a lot easier to judge than “slowed progression.”
“Better” is a great word for migraines. Or seizures. Or Parkinson’s disease. These are condition where patients and families can tell us whether they’ve seen an improvement.
But with the current treatments for Alzheimer’s disease we’re asking patients and families “do you think you’ve gotten any worse than you would have if you hadn’t taken the drug at all?”
That’s an impossible question to answer, unless you’re following people with objective cognitive data over time and comparing them against a placebo group, which is how these drugs got here in the first place – we know they do that.
But to a family watching their loved ones go downhill, such reassurances aren’t what they want to hear.
Regrettably, it’s where things stand. While I want to strive for absolute success in these things, today it’s simply not possible. Maybe it never will be, though I hope it is.
But, for now, I agree that we need to reframe what we’re going to consider clinically meaningful. Sometimes you have to settle for a flight of stairs instead of an elevator, but still hope that you’ll get to the top. It just takes longer, and it’s better than not going anywhere at all.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Returning to normal after concussion
Last night I invested an hour and a half watching the first half of the Super Bowl ... because ... well, just because. As exciting as it might have been to watch, investing another 2 hours on the second half would have kept me up well past my bedtime. As I lay in bed with the thwack-thwack-thud of helmets hitting pads still reverberating in my ears, my thoughts drifted to the ever-shifting landscape of concussion management.
More than 2 decades ago, concussions were just beginning to exit the dark ages when loss of consciousness was the defining symptom or sign that most folks (and here I am including physicians) used to separate the run-of-the-mill stinger or bell-ringer from a “real” concussion.
The new era dawned with the appearance of clinics devoted to concussion management and the development of protocols that limited everything from physical exertion to reading and screen time. Schools were coaxed into subjecting their athletes to preparticipation testing sessions with the hope that creating a baseline cognitive assessment would somehow make the diagnosis and management of concussion feel more scientific. Many of the recommended management strategies were based on the intuitive but flawed notion of “brain rest.” If reading or bright lights aggravate patient’s symptoms, they should be avoided but otherwise resting the brain doesn’t seem to make sense.
Fortunately, there were, and hopefully will continue to be, clinicians willing to question hastily developed management protocols. One recent cohort study from Canada has found that, surprisingly, (to some experts), “early return to school was associated with a lower symptom burden” This association held true for both age groups the researches studied (8-12 years and 13-18 years). The authors conclude that delayed return to school “may be detrimental to recovery.” In this study, early return to school was defined as less than 3 days.
In another study, this one in the journal Pediatrics, the authors found that “the association of early screen time with postconcussion symptoms is not linear.” Their conclusion was that the best approach to clinical management of concussion should include a moderate amount of screen time.
After reading both of these studies I am heartened that we are now hearing voices suggesting a return to concussion management based on careful observation of the individual patient and common sense. A concussed brain is not a torn hamstring or a broken clavicle that under most circumstances will heal in a predictable amount of time. It is prudent to exclude the concussed patient from activities that carry a significant risk of reinjury until the symptoms have subsided. However, postconcussion symptoms are often vague and can be mistaken for or aggravated by a host of other conditions including learning disabilities, anxiety, and depression.
I hope that our experience with the COVID pandemic has taught us that removing children from school and their usual activities can have a serious negative effect on their emotional health and academic achievement. This seems to be particularly true for the young people who were already struggling to adjust to being a student. Getting out of the habit of going to school often intensifies the anxieties of an emotionally or academically challenged student. Each day away from the school atmosphere can compound the symptoms that may or may not have been triggered by the concussion.
The message here is clear that, whether we are talking about concussions or appendectomies or mononucleosis, the sooner we can return the child to something close to their old normal the more successful we will be in a helping them adjust to the new normal.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Last night I invested an hour and a half watching the first half of the Super Bowl ... because ... well, just because. As exciting as it might have been to watch, investing another 2 hours on the second half would have kept me up well past my bedtime. As I lay in bed with the thwack-thwack-thud of helmets hitting pads still reverberating in my ears, my thoughts drifted to the ever-shifting landscape of concussion management.
More than 2 decades ago, concussions were just beginning to exit the dark ages when loss of consciousness was the defining symptom or sign that most folks (and here I am including physicians) used to separate the run-of-the-mill stinger or bell-ringer from a “real” concussion.
The new era dawned with the appearance of clinics devoted to concussion management and the development of protocols that limited everything from physical exertion to reading and screen time. Schools were coaxed into subjecting their athletes to preparticipation testing sessions with the hope that creating a baseline cognitive assessment would somehow make the diagnosis and management of concussion feel more scientific. Many of the recommended management strategies were based on the intuitive but flawed notion of “brain rest.” If reading or bright lights aggravate patient’s symptoms, they should be avoided but otherwise resting the brain doesn’t seem to make sense.
Fortunately, there were, and hopefully will continue to be, clinicians willing to question hastily developed management protocols. One recent cohort study from Canada has found that, surprisingly, (to some experts), “early return to school was associated with a lower symptom burden” This association held true for both age groups the researches studied (8-12 years and 13-18 years). The authors conclude that delayed return to school “may be detrimental to recovery.” In this study, early return to school was defined as less than 3 days.
In another study, this one in the journal Pediatrics, the authors found that “the association of early screen time with postconcussion symptoms is not linear.” Their conclusion was that the best approach to clinical management of concussion should include a moderate amount of screen time.
After reading both of these studies I am heartened that we are now hearing voices suggesting a return to concussion management based on careful observation of the individual patient and common sense. A concussed brain is not a torn hamstring or a broken clavicle that under most circumstances will heal in a predictable amount of time. It is prudent to exclude the concussed patient from activities that carry a significant risk of reinjury until the symptoms have subsided. However, postconcussion symptoms are often vague and can be mistaken for or aggravated by a host of other conditions including learning disabilities, anxiety, and depression.
I hope that our experience with the COVID pandemic has taught us that removing children from school and their usual activities can have a serious negative effect on their emotional health and academic achievement. This seems to be particularly true for the young people who were already struggling to adjust to being a student. Getting out of the habit of going to school often intensifies the anxieties of an emotionally or academically challenged student. Each day away from the school atmosphere can compound the symptoms that may or may not have been triggered by the concussion.
The message here is clear that, whether we are talking about concussions or appendectomies or mononucleosis, the sooner we can return the child to something close to their old normal the more successful we will be in a helping them adjust to the new normal.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Last night I invested an hour and a half watching the first half of the Super Bowl ... because ... well, just because. As exciting as it might have been to watch, investing another 2 hours on the second half would have kept me up well past my bedtime. As I lay in bed with the thwack-thwack-thud of helmets hitting pads still reverberating in my ears, my thoughts drifted to the ever-shifting landscape of concussion management.
More than 2 decades ago, concussions were just beginning to exit the dark ages when loss of consciousness was the defining symptom or sign that most folks (and here I am including physicians) used to separate the run-of-the-mill stinger or bell-ringer from a “real” concussion.
The new era dawned with the appearance of clinics devoted to concussion management and the development of protocols that limited everything from physical exertion to reading and screen time. Schools were coaxed into subjecting their athletes to preparticipation testing sessions with the hope that creating a baseline cognitive assessment would somehow make the diagnosis and management of concussion feel more scientific. Many of the recommended management strategies were based on the intuitive but flawed notion of “brain rest.” If reading or bright lights aggravate patient’s symptoms, they should be avoided but otherwise resting the brain doesn’t seem to make sense.
Fortunately, there were, and hopefully will continue to be, clinicians willing to question hastily developed management protocols. One recent cohort study from Canada has found that, surprisingly, (to some experts), “early return to school was associated with a lower symptom burden” This association held true for both age groups the researches studied (8-12 years and 13-18 years). The authors conclude that delayed return to school “may be detrimental to recovery.” In this study, early return to school was defined as less than 3 days.
In another study, this one in the journal Pediatrics, the authors found that “the association of early screen time with postconcussion symptoms is not linear.” Their conclusion was that the best approach to clinical management of concussion should include a moderate amount of screen time.
After reading both of these studies I am heartened that we are now hearing voices suggesting a return to concussion management based on careful observation of the individual patient and common sense. A concussed brain is not a torn hamstring or a broken clavicle that under most circumstances will heal in a predictable amount of time. It is prudent to exclude the concussed patient from activities that carry a significant risk of reinjury until the symptoms have subsided. However, postconcussion symptoms are often vague and can be mistaken for or aggravated by a host of other conditions including learning disabilities, anxiety, and depression.
I hope that our experience with the COVID pandemic has taught us that removing children from school and their usual activities can have a serious negative effect on their emotional health and academic achievement. This seems to be particularly true for the young people who were already struggling to adjust to being a student. Getting out of the habit of going to school often intensifies the anxieties of an emotionally or academically challenged student. Each day away from the school atmosphere can compound the symptoms that may or may not have been triggered by the concussion.
The message here is clear that, whether we are talking about concussions or appendectomies or mononucleosis, the sooner we can return the child to something close to their old normal the more successful we will be in a helping them adjust to the new normal.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Toxic chemicals we consume without knowing it
Life expectancy is falling precipitously. Three-fourths of Americans are overweight or obese, half have diabetes or prediabetes, and a majority are metabolically unhealthy. Furthermore, the rates of allergic, inflammatory, and autoimmune diseases are rising at rates of 3%-9% per year in the West, far faster than the speed of genetic change in this population.
Of course, diet and lifestyle are major factors behind such trends, but a grossly underappreciated driver in what ails us is the role of environmental toxins and endocrine-disrupting chemicals. In years past, these factors have largely evaded the traditional Western medical establishment; however, mounting evidence now supports their significance in fertility, metabolic health, and cancer.
Although several industrial chemicals and toxins have been identified as carcinogens and have subsequently been regulated, many more remain persistent in the environment and continue to be freely used. It is therefore incumbent upon both the general public and clinicians to be knowledgeable about these exposures. Here, we review some of the most common exposures and the substantial health risks associated with them, along with some general guidance around best practices for how to minimize exposure.
Microplastics
“Microplastics” is a term used to describe small fragments or particles of plastic breakdown or microbeads from household or personal care products, measuring less than 5 mm in length.
Plastic waste is accumulating at alarming and devastating proportions – by 2050, it is estimated that by weight, there will be more plastic than fish in the oceans. That translates into hundreds of thousands of tons of microplastics and trillions of these particles in the seas. A recent study demonstrated that microplastics were present in the bloodstream in the majority of 22 otherwise healthy participants.
Since the 1950s, plastic exposure has been shown to promote tumorigenesis in animal studies, and in vitro studies have demonstrated the toxicity of microplastics at the cellular level. However, it is not well known whether the plastic itself is toxic or if it simply serves as a carrier for other environmental toxins to bioaccumulate.
According to Tasha Stoiber, a senior scientist at the Environmental Working Group, “Microplastics have been widely detected in fish and seafood, as well as other products like bottled water, beer, honey, and tap water.” The EWG states there are no formal advisories on fish consumption to avoid exposure to microplastics at the moment.
Pressure also is mounting for a ban on microbeads in personal care products.
Until such bans are put in place, it is advised to avoid single-use plastics, favor reusable tote bags for grocery shopping rather than plastic bags, and opt for loose leaf tea or paper tea bags rather than mesh-based alternatives.
Phthalates
Phthalates are chemicals used to make plastics soft and durable, as well as to bind fragrances. They are commonly found in household items such as vinyl (for example, flooring, shower curtains) and fragrances, air fresheners, and perfumes.
Phthalates are known hormone-disrupting chemicals, exposure to which has been associated with abnormal sexual and brain development in children, as well as lower levels of testosterone in men. Exposures are thought to occur via inhalation, ingestion, and skin contact; however, fasting studies demonstrate that a majority of exposure is probably food related.
To avoid phthalate exposures, recommendations include avoiding polyvinyl chloride plastics (particularly food containers, plastic wrap, and children’s toys), which are identifiable by the recycle code number 3, as well as air fresheners and fragranced products.
The EWG’s Skin Deep database provides an important resource on phthalate-free personal care products.
Despite pressure from consumer advocacy groups, the U.S. Food and Drug Administration has not yet banned phthalates in food packaging.
Bisphenol A (BPA)
BPA is a chemical additive used to make clear and hard polycarbonate plastics, as well as epoxy and thermal papers. BPA is one of the highest-volume chemicals, with roughly 6 billion pounds produced each year. BPA is traditionally found in many clear plastic bottles and sippy cups, as well as in the lining of canned foods.
Structurally, BPA acts as an estrogen mimetic and has been associated with cardiovascular disease, obesity, and male sexual dysfunction. Since 2012, BPA has been banned in sippy cups and baby bottles, but there is some debate as to whether its replacements (bisphenol S and bisphenol F) are any safer; they appear to have similar hormonal effects as BPA.
As with phthalates, the majority of ingestion is thought to be food related. BPA has been found in more than 90% of a representative study population in the United States.
Guidance advises avoiding polycarbonate plastics (identifiable with the recycling code number 7), as well as avoiding handling thermal papers such as tickets and receipts, if possible. Food and beverages should be stored in glass or stainless steel. If plastic must be used, opt for polycarbonate- and polyvinyl chloride–free plastics, and food and beverages should never be reheated in plastic containers or wrapping. Canned foods should ideally be avoided, particularly canned tunas and condensed soups. If canned products are bought, they should ideally be BPA free.
Dioxins and polychlorinated biphenyls (PCBs)
Dioxins are mainly the byproducts of industrial practices; they are released after incineration, trash burning, and fires. PCBs, which are somewhat structurally related to dioxins, were previously found in products such as flame retardants and coolants. Dioxins and PCBs are often grouped in the same category under the umbrella term “persistent organic pollutants” because they break down slowly and remain in the environment even after emissions have been curbed.
Tetrachlorodibenzodioxin, perhaps the best-known dioxin, is a known carcinogen. Dioxins also have been associated with a host of health implications in development, immunity, and reproductive and endocrine systems. Higher levels of PCB exposure have also been associated with an increased risk for mortality from cardiovascular disease.
Notably, dioxin emissions have been reduced by 90% since the 1980s, and the U.S. Environmental Protection Agency has banned the use of PCBs in industrial manufacturing since 1979. However, environmental dioxins and PCBs still enter the food chain and accumulate in fat.
The best ways to avoid exposures are through limiting meat, fish, and dairy consumption and trimming the skin and fat from meats. The level of dioxins and PCBs found in meat, eggs, fish, and dairy are approximately 5-10 times higher than they are in plant-based foods. Research has shown that farmed salmon is likely to be the most PCB-contaminated protein source in the U.S. diet; however, newer forms of land-based and sustainable aquaculture probably avoid this exposure.
Pesticides
The growth of modern monoculture agriculture in the United States over the past century has coincided with a dramatic surge in the use of industrial pesticides. In fact, over 90% of the U.S. population have pesticides in their urine and blood, regardless of where they live. Exposures are thought to be food related.
Approximately 1 billion pounds of pesticides are used annually in the United States, including nearly 300 million pounds of glyphosate, which has been identified as a probable carcinogen by European agencies. The EPA has not yet reached this conclusion, although the matter is currently being litigated.
A large European prospective cohort trial demonstrated a lower risk for cancer in those with a greater frequency of self-reported organic food consumption. In addition to cancer risk, relatively elevated blood levels of a pesticide known as beta-hexachlorocyclohexane (B-HCH) are associated with higher all-cause mortality. Also, exposure to DDE – a metabolite of DDT, a chlorinated pesticide heavily used in the 1940s-1960s that still persists in the environment today – has been shown to increase the risk for Alzheimer’s-type dementia as well as overall cognitive decline.
Because these chlorinated pesticides are often fat soluble, they seem to accumulate in animal products. Therefore, people consuming a vegetarian diet have been found to have lower levels of B-HCH. This has led to the recommendation that consumers of produce should favor organic over conventional, if possible. Here too, the EWG provides an important resource to consumers in the form of shopper guides regarding pesticides in produce.
Per- and polyfluoroalkyl substances (PFAS)
PFAS are a group of fluorinated compounds discovered in the 1930s. Their chemical composition includes a durable carbon-fluoride bond, giving them a persistence within the environment that has led to their being referred to as “forever chemicals.”
PFAS have been detected in the blood of 98% of Americans, and in the rainwater of locations as far afield as Tibet and Antarctica. Even low levels of exposure have been associated with an increased risk for cancer, liver disease, low birth weight, and hormonal disruption.
The properties of PFAS also make them both durable at very high heat and water repellent. Notoriously, the chemical was used by 3M to make Scotchgard for carpets and fabrics and by Dupont to make Teflon for nonstick coating of pots and pans. Although perfluorooctanoic acid (PFOA) was removed from nonstick cookware in 2013, PFAS – a family of thousands of synthetic compounds – remain common in fast-food packaging, water- and stain-repellent clothing, firefighting foam, and personal care products. PFAS are released into the environment during the breakdown of these consumer and industrial products, as well as from dumping from waste facilities.
Alarmingly, the EWG notes that up to 200 million Americans may be exposed to PFAS in their drinking water. In March 2021, the EPA announced that they will be regulating PFAS in drinking water; however, the regulations have not been finalized. Currently, it is up to individual states to test for its presence in the water. The EWG has compiled a map of all known PFAS contamination sites.
To avoid or prevent exposures from PFAS, recommendations include filtering tap water with either reverse osmosis or activated carbon filters, as well as avoiding fast food and carry-out food, if possible, and consumer products labeled as “water resistant,” “stain-resistant,” and “nonstick.”
In a testament to how harmful these chemicals are, the EPA recently revised their lifetime health advisories for PFAS, such as PFOA, to 0.004 parts per trillion, which is more than 10,000 times smaller than the previous limit of 70 parts per trillion. The EPA also has proposed formally designating certain PFAS chemicals as “hazardous substances.”
Dr. Goel, clinical assistant professor of medicine at Weill Cornell Medicine, New York, has disclosed no relevant financial relationships. A version of this article originally appeared on Medscape.com.
Life expectancy is falling precipitously. Three-fourths of Americans are overweight or obese, half have diabetes or prediabetes, and a majority are metabolically unhealthy. Furthermore, the rates of allergic, inflammatory, and autoimmune diseases are rising at rates of 3%-9% per year in the West, far faster than the speed of genetic change in this population.
Of course, diet and lifestyle are major factors behind such trends, but a grossly underappreciated driver in what ails us is the role of environmental toxins and endocrine-disrupting chemicals. In years past, these factors have largely evaded the traditional Western medical establishment; however, mounting evidence now supports their significance in fertility, metabolic health, and cancer.
Although several industrial chemicals and toxins have been identified as carcinogens and have subsequently been regulated, many more remain persistent in the environment and continue to be freely used. It is therefore incumbent upon both the general public and clinicians to be knowledgeable about these exposures. Here, we review some of the most common exposures and the substantial health risks associated with them, along with some general guidance around best practices for how to minimize exposure.
Microplastics
“Microplastics” is a term used to describe small fragments or particles of plastic breakdown or microbeads from household or personal care products, measuring less than 5 mm in length.
Plastic waste is accumulating at alarming and devastating proportions – by 2050, it is estimated that by weight, there will be more plastic than fish in the oceans. That translates into hundreds of thousands of tons of microplastics and trillions of these particles in the seas. A recent study demonstrated that microplastics were present in the bloodstream in the majority of 22 otherwise healthy participants.
Since the 1950s, plastic exposure has been shown to promote tumorigenesis in animal studies, and in vitro studies have demonstrated the toxicity of microplastics at the cellular level. However, it is not well known whether the plastic itself is toxic or if it simply serves as a carrier for other environmental toxins to bioaccumulate.
According to Tasha Stoiber, a senior scientist at the Environmental Working Group, “Microplastics have been widely detected in fish and seafood, as well as other products like bottled water, beer, honey, and tap water.” The EWG states there are no formal advisories on fish consumption to avoid exposure to microplastics at the moment.
Pressure also is mounting for a ban on microbeads in personal care products.
Until such bans are put in place, it is advised to avoid single-use plastics, favor reusable tote bags for grocery shopping rather than plastic bags, and opt for loose leaf tea or paper tea bags rather than mesh-based alternatives.
Phthalates
Phthalates are chemicals used to make plastics soft and durable, as well as to bind fragrances. They are commonly found in household items such as vinyl (for example, flooring, shower curtains) and fragrances, air fresheners, and perfumes.
Phthalates are known hormone-disrupting chemicals, exposure to which has been associated with abnormal sexual and brain development in children, as well as lower levels of testosterone in men. Exposures are thought to occur via inhalation, ingestion, and skin contact; however, fasting studies demonstrate that a majority of exposure is probably food related.
To avoid phthalate exposures, recommendations include avoiding polyvinyl chloride plastics (particularly food containers, plastic wrap, and children’s toys), which are identifiable by the recycle code number 3, as well as air fresheners and fragranced products.
The EWG’s Skin Deep database provides an important resource on phthalate-free personal care products.
Despite pressure from consumer advocacy groups, the U.S. Food and Drug Administration has not yet banned phthalates in food packaging.
Bisphenol A (BPA)
BPA is a chemical additive used to make clear and hard polycarbonate plastics, as well as epoxy and thermal papers. BPA is one of the highest-volume chemicals, with roughly 6 billion pounds produced each year. BPA is traditionally found in many clear plastic bottles and sippy cups, as well as in the lining of canned foods.
Structurally, BPA acts as an estrogen mimetic and has been associated with cardiovascular disease, obesity, and male sexual dysfunction. Since 2012, BPA has been banned in sippy cups and baby bottles, but there is some debate as to whether its replacements (bisphenol S and bisphenol F) are any safer; they appear to have similar hormonal effects as BPA.
As with phthalates, the majority of ingestion is thought to be food related. BPA has been found in more than 90% of a representative study population in the United States.
Guidance advises avoiding polycarbonate plastics (identifiable with the recycling code number 7), as well as avoiding handling thermal papers such as tickets and receipts, if possible. Food and beverages should be stored in glass or stainless steel. If plastic must be used, opt for polycarbonate- and polyvinyl chloride–free plastics, and food and beverages should never be reheated in plastic containers or wrapping. Canned foods should ideally be avoided, particularly canned tunas and condensed soups. If canned products are bought, they should ideally be BPA free.
Dioxins and polychlorinated biphenyls (PCBs)
Dioxins are mainly the byproducts of industrial practices; they are released after incineration, trash burning, and fires. PCBs, which are somewhat structurally related to dioxins, were previously found in products such as flame retardants and coolants. Dioxins and PCBs are often grouped in the same category under the umbrella term “persistent organic pollutants” because they break down slowly and remain in the environment even after emissions have been curbed.
Tetrachlorodibenzodioxin, perhaps the best-known dioxin, is a known carcinogen. Dioxins also have been associated with a host of health implications in development, immunity, and reproductive and endocrine systems. Higher levels of PCB exposure have also been associated with an increased risk for mortality from cardiovascular disease.
Notably, dioxin emissions have been reduced by 90% since the 1980s, and the U.S. Environmental Protection Agency has banned the use of PCBs in industrial manufacturing since 1979. However, environmental dioxins and PCBs still enter the food chain and accumulate in fat.
The best ways to avoid exposures are through limiting meat, fish, and dairy consumption and trimming the skin and fat from meats. The level of dioxins and PCBs found in meat, eggs, fish, and dairy are approximately 5-10 times higher than they are in plant-based foods. Research has shown that farmed salmon is likely to be the most PCB-contaminated protein source in the U.S. diet; however, newer forms of land-based and sustainable aquaculture probably avoid this exposure.
Pesticides
The growth of modern monoculture agriculture in the United States over the past century has coincided with a dramatic surge in the use of industrial pesticides. In fact, over 90% of the U.S. population have pesticides in their urine and blood, regardless of where they live. Exposures are thought to be food related.
Approximately 1 billion pounds of pesticides are used annually in the United States, including nearly 300 million pounds of glyphosate, which has been identified as a probable carcinogen by European agencies. The EPA has not yet reached this conclusion, although the matter is currently being litigated.
A large European prospective cohort trial demonstrated a lower risk for cancer in those with a greater frequency of self-reported organic food consumption. In addition to cancer risk, relatively elevated blood levels of a pesticide known as beta-hexachlorocyclohexane (B-HCH) are associated with higher all-cause mortality. Also, exposure to DDE – a metabolite of DDT, a chlorinated pesticide heavily used in the 1940s-1960s that still persists in the environment today – has been shown to increase the risk for Alzheimer’s-type dementia as well as overall cognitive decline.
Because these chlorinated pesticides are often fat soluble, they seem to accumulate in animal products. Therefore, people consuming a vegetarian diet have been found to have lower levels of B-HCH. This has led to the recommendation that consumers of produce should favor organic over conventional, if possible. Here too, the EWG provides an important resource to consumers in the form of shopper guides regarding pesticides in produce.
Per- and polyfluoroalkyl substances (PFAS)
PFAS are a group of fluorinated compounds discovered in the 1930s. Their chemical composition includes a durable carbon-fluoride bond, giving them a persistence within the environment that has led to their being referred to as “forever chemicals.”
PFAS have been detected in the blood of 98% of Americans, and in the rainwater of locations as far afield as Tibet and Antarctica. Even low levels of exposure have been associated with an increased risk for cancer, liver disease, low birth weight, and hormonal disruption.
The properties of PFAS also make them both durable at very high heat and water repellent. Notoriously, the chemical was used by 3M to make Scotchgard for carpets and fabrics and by Dupont to make Teflon for nonstick coating of pots and pans. Although perfluorooctanoic acid (PFOA) was removed from nonstick cookware in 2013, PFAS – a family of thousands of synthetic compounds – remain common in fast-food packaging, water- and stain-repellent clothing, firefighting foam, and personal care products. PFAS are released into the environment during the breakdown of these consumer and industrial products, as well as from dumping from waste facilities.
Alarmingly, the EWG notes that up to 200 million Americans may be exposed to PFAS in their drinking water. In March 2021, the EPA announced that they will be regulating PFAS in drinking water; however, the regulations have not been finalized. Currently, it is up to individual states to test for its presence in the water. The EWG has compiled a map of all known PFAS contamination sites.
To avoid or prevent exposures from PFAS, recommendations include filtering tap water with either reverse osmosis or activated carbon filters, as well as avoiding fast food and carry-out food, if possible, and consumer products labeled as “water resistant,” “stain-resistant,” and “nonstick.”
In a testament to how harmful these chemicals are, the EPA recently revised their lifetime health advisories for PFAS, such as PFOA, to 0.004 parts per trillion, which is more than 10,000 times smaller than the previous limit of 70 parts per trillion. The EPA also has proposed formally designating certain PFAS chemicals as “hazardous substances.”
Dr. Goel, clinical assistant professor of medicine at Weill Cornell Medicine, New York, has disclosed no relevant financial relationships. A version of this article originally appeared on Medscape.com.
Life expectancy is falling precipitously. Three-fourths of Americans are overweight or obese, half have diabetes or prediabetes, and a majority are metabolically unhealthy. Furthermore, the rates of allergic, inflammatory, and autoimmune diseases are rising at rates of 3%-9% per year in the West, far faster than the speed of genetic change in this population.
Of course, diet and lifestyle are major factors behind such trends, but a grossly underappreciated driver in what ails us is the role of environmental toxins and endocrine-disrupting chemicals. In years past, these factors have largely evaded the traditional Western medical establishment; however, mounting evidence now supports their significance in fertility, metabolic health, and cancer.
Although several industrial chemicals and toxins have been identified as carcinogens and have subsequently been regulated, many more remain persistent in the environment and continue to be freely used. It is therefore incumbent upon both the general public and clinicians to be knowledgeable about these exposures. Here, we review some of the most common exposures and the substantial health risks associated with them, along with some general guidance around best practices for how to minimize exposure.
Microplastics
“Microplastics” is a term used to describe small fragments or particles of plastic breakdown or microbeads from household or personal care products, measuring less than 5 mm in length.
Plastic waste is accumulating at alarming and devastating proportions – by 2050, it is estimated that by weight, there will be more plastic than fish in the oceans. That translates into hundreds of thousands of tons of microplastics and trillions of these particles in the seas. A recent study demonstrated that microplastics were present in the bloodstream in the majority of 22 otherwise healthy participants.
Since the 1950s, plastic exposure has been shown to promote tumorigenesis in animal studies, and in vitro studies have demonstrated the toxicity of microplastics at the cellular level. However, it is not well known whether the plastic itself is toxic or if it simply serves as a carrier for other environmental toxins to bioaccumulate.
According to Tasha Stoiber, a senior scientist at the Environmental Working Group, “Microplastics have been widely detected in fish and seafood, as well as other products like bottled water, beer, honey, and tap water.” The EWG states there are no formal advisories on fish consumption to avoid exposure to microplastics at the moment.
Pressure also is mounting for a ban on microbeads in personal care products.
Until such bans are put in place, it is advised to avoid single-use plastics, favor reusable tote bags for grocery shopping rather than plastic bags, and opt for loose leaf tea or paper tea bags rather than mesh-based alternatives.
Phthalates
Phthalates are chemicals used to make plastics soft and durable, as well as to bind fragrances. They are commonly found in household items such as vinyl (for example, flooring, shower curtains) and fragrances, air fresheners, and perfumes.
Phthalates are known hormone-disrupting chemicals, exposure to which has been associated with abnormal sexual and brain development in children, as well as lower levels of testosterone in men. Exposures are thought to occur via inhalation, ingestion, and skin contact; however, fasting studies demonstrate that a majority of exposure is probably food related.
To avoid phthalate exposures, recommendations include avoiding polyvinyl chloride plastics (particularly food containers, plastic wrap, and children’s toys), which are identifiable by the recycle code number 3, as well as air fresheners and fragranced products.
The EWG’s Skin Deep database provides an important resource on phthalate-free personal care products.
Despite pressure from consumer advocacy groups, the U.S. Food and Drug Administration has not yet banned phthalates in food packaging.
Bisphenol A (BPA)
BPA is a chemical additive used to make clear and hard polycarbonate plastics, as well as epoxy and thermal papers. BPA is one of the highest-volume chemicals, with roughly 6 billion pounds produced each year. BPA is traditionally found in many clear plastic bottles and sippy cups, as well as in the lining of canned foods.
Structurally, BPA acts as an estrogen mimetic and has been associated with cardiovascular disease, obesity, and male sexual dysfunction. Since 2012, BPA has been banned in sippy cups and baby bottles, but there is some debate as to whether its replacements (bisphenol S and bisphenol F) are any safer; they appear to have similar hormonal effects as BPA.
As with phthalates, the majority of ingestion is thought to be food related. BPA has been found in more than 90% of a representative study population in the United States.
Guidance advises avoiding polycarbonate plastics (identifiable with the recycling code number 7), as well as avoiding handling thermal papers such as tickets and receipts, if possible. Food and beverages should be stored in glass or stainless steel. If plastic must be used, opt for polycarbonate- and polyvinyl chloride–free plastics, and food and beverages should never be reheated in plastic containers or wrapping. Canned foods should ideally be avoided, particularly canned tunas and condensed soups. If canned products are bought, they should ideally be BPA free.
Dioxins and polychlorinated biphenyls (PCBs)
Dioxins are mainly the byproducts of industrial practices; they are released after incineration, trash burning, and fires. PCBs, which are somewhat structurally related to dioxins, were previously found in products such as flame retardants and coolants. Dioxins and PCBs are often grouped in the same category under the umbrella term “persistent organic pollutants” because they break down slowly and remain in the environment even after emissions have been curbed.
Tetrachlorodibenzodioxin, perhaps the best-known dioxin, is a known carcinogen. Dioxins also have been associated with a host of health implications in development, immunity, and reproductive and endocrine systems. Higher levels of PCB exposure have also been associated with an increased risk for mortality from cardiovascular disease.
Notably, dioxin emissions have been reduced by 90% since the 1980s, and the U.S. Environmental Protection Agency has banned the use of PCBs in industrial manufacturing since 1979. However, environmental dioxins and PCBs still enter the food chain and accumulate in fat.
The best ways to avoid exposures are through limiting meat, fish, and dairy consumption and trimming the skin and fat from meats. The level of dioxins and PCBs found in meat, eggs, fish, and dairy are approximately 5-10 times higher than they are in plant-based foods. Research has shown that farmed salmon is likely to be the most PCB-contaminated protein source in the U.S. diet; however, newer forms of land-based and sustainable aquaculture probably avoid this exposure.
Pesticides
The growth of modern monoculture agriculture in the United States over the past century has coincided with a dramatic surge in the use of industrial pesticides. In fact, over 90% of the U.S. population have pesticides in their urine and blood, regardless of where they live. Exposures are thought to be food related.
Approximately 1 billion pounds of pesticides are used annually in the United States, including nearly 300 million pounds of glyphosate, which has been identified as a probable carcinogen by European agencies. The EPA has not yet reached this conclusion, although the matter is currently being litigated.
A large European prospective cohort trial demonstrated a lower risk for cancer in those with a greater frequency of self-reported organic food consumption. In addition to cancer risk, relatively elevated blood levels of a pesticide known as beta-hexachlorocyclohexane (B-HCH) are associated with higher all-cause mortality. Also, exposure to DDE – a metabolite of DDT, a chlorinated pesticide heavily used in the 1940s-1960s that still persists in the environment today – has been shown to increase the risk for Alzheimer’s-type dementia as well as overall cognitive decline.
Because these chlorinated pesticides are often fat soluble, they seem to accumulate in animal products. Therefore, people consuming a vegetarian diet have been found to have lower levels of B-HCH. This has led to the recommendation that consumers of produce should favor organic over conventional, if possible. Here too, the EWG provides an important resource to consumers in the form of shopper guides regarding pesticides in produce.
Per- and polyfluoroalkyl substances (PFAS)
PFAS are a group of fluorinated compounds discovered in the 1930s. Their chemical composition includes a durable carbon-fluoride bond, giving them a persistence within the environment that has led to their being referred to as “forever chemicals.”
PFAS have been detected in the blood of 98% of Americans, and in the rainwater of locations as far afield as Tibet and Antarctica. Even low levels of exposure have been associated with an increased risk for cancer, liver disease, low birth weight, and hormonal disruption.
The properties of PFAS also make them both durable at very high heat and water repellent. Notoriously, the chemical was used by 3M to make Scotchgard for carpets and fabrics and by Dupont to make Teflon for nonstick coating of pots and pans. Although perfluorooctanoic acid (PFOA) was removed from nonstick cookware in 2013, PFAS – a family of thousands of synthetic compounds – remain common in fast-food packaging, water- and stain-repellent clothing, firefighting foam, and personal care products. PFAS are released into the environment during the breakdown of these consumer and industrial products, as well as from dumping from waste facilities.
Alarmingly, the EWG notes that up to 200 million Americans may be exposed to PFAS in their drinking water. In March 2021, the EPA announced that they will be regulating PFAS in drinking water; however, the regulations have not been finalized. Currently, it is up to individual states to test for its presence in the water. The EWG has compiled a map of all known PFAS contamination sites.
To avoid or prevent exposures from PFAS, recommendations include filtering tap water with either reverse osmosis or activated carbon filters, as well as avoiding fast food and carry-out food, if possible, and consumer products labeled as “water resistant,” “stain-resistant,” and “nonstick.”
In a testament to how harmful these chemicals are, the EPA recently revised their lifetime health advisories for PFAS, such as PFOA, to 0.004 parts per trillion, which is more than 10,000 times smaller than the previous limit of 70 parts per trillion. The EPA also has proposed formally designating certain PFAS chemicals as “hazardous substances.”
Dr. Goel, clinical assistant professor of medicine at Weill Cornell Medicine, New York, has disclosed no relevant financial relationships. A version of this article originally appeared on Medscape.com.
Physicians and clinicians should be required to get flu shots: Ethicist
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the Division of Medical Ethics at New York University’s Grossman School of Medicine, where I’m the director.
I’ve long believed that every health care institution – nursing homes, hospitals, clinics, home care, hospice – should require flu shots for all doctors and all nurses because it is the easiest, cheapest, and most ethical way to protect the workforce, who you need to be in there when flu outbreaks take place, and to protect patients against getting the flu when they come into hospital settings and get exposed to health care workers who may have the flu already but don’t know it.
In a recent poll, I was happy to see that the majority of physicians surveyed agreed with me: 65% said they supported mandatory flu vaccination in hospitals and only 23% said they did not. I think flu vaccination is something that has already been shown to be useful and important, not only in stopping people from getting the flu but also in making sure that they don’t get as sick when they get the flu.
Just like COVID-19 vaccination, it doesn’t always prevent somebody from getting infected, but if you get it, it keeps you from winding up sick at home, or worse – from dying and winding up in the morgue. Flu kills many, many people every year. We don’t want that to happen. A flu vaccine will really help prevent deaths, help prevent the number of symptoms that somebody gets, and will get people back to work. The benefits are pretty clear.
Does the flu vaccine work equally well every year? It does not. Some years, the strains that are picked for the vaccine don’t match the ones that circulate, and we don’t get as much protection as we hoped for. I think the safety side is so strong that it’s worth making the investment and the effort to promote mandatory flu vaccination.
Can you opt out on religious grounds? Well, some hospitals permit that at New York University. You have to go before a committee and make a case that your exemption on religious grounds is based on an authentic set of beliefs that are deeply held, and not just something you thought up the day before flu vaccine requirements went into effect.
There may be room for some exemptions – obviously, for health reasons. If people think that the flu vaccine is dangerous to them and can get a physician to agree and sign off that they are not appropriate to vaccinate, okay.
On the other hand, if you’re working with an especially vulnerable population – newborns, people who are immunosuppressed – then I think you’ve got to be vaccinated and you shouldn’t be working around people who are at huge risk of getting the flu if you refuse to be vaccinated or, for that matter, can’t be vaccinated.
Would I extend these mandates? Yes, I would. I’d extend them to COVID-19 vaccination and to measles vaccination. I think physicians and nurses should be good role models. They should get vaccinated. We know that the best available evidence says that vaccination for infectious disease is safe. It is really the best thing we can do to combat a variety of diseases such as the flu and COVID-19.
It seems to me that, in addition, the data that are out there in terms of risks from flu and COVID-19 – deaths in places like nursing homes – are overwhelming about the importance of trying to get staff vaccinated so they don’t bring flu into an institutionalized population. This is similar for prison health and many other settings where people are kept close together and staff may move from place to place, rotating from institution to institution, spreading infectious disease.
I’m going to go with the poll. Let’s keep pushing for health care workers to do the right thing and to be good role models. Let’s get everybody a flu vaccination. Let’s extend it to a COVID-19 vaccination and its boosters.
Let’s try to show the nation that health care is going to be guided by good science, a duty to one’s own health, and a duty to one’s patients. It shouldn’t be political. It should be based on what works best for the interests of health care providers and those they care for.
I’m Art Caplan at the New York University Grossman School of Medicine. Thanks for watching.
Dr. Caplan has disclosed the following relevant financial relationships: Served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position). Serves as a contributing author and advisor for Medscape. A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the Division of Medical Ethics at New York University’s Grossman School of Medicine, where I’m the director.
I’ve long believed that every health care institution – nursing homes, hospitals, clinics, home care, hospice – should require flu shots for all doctors and all nurses because it is the easiest, cheapest, and most ethical way to protect the workforce, who you need to be in there when flu outbreaks take place, and to protect patients against getting the flu when they come into hospital settings and get exposed to health care workers who may have the flu already but don’t know it.
In a recent poll, I was happy to see that the majority of physicians surveyed agreed with me: 65% said they supported mandatory flu vaccination in hospitals and only 23% said they did not. I think flu vaccination is something that has already been shown to be useful and important, not only in stopping people from getting the flu but also in making sure that they don’t get as sick when they get the flu.
Just like COVID-19 vaccination, it doesn’t always prevent somebody from getting infected, but if you get it, it keeps you from winding up sick at home, or worse – from dying and winding up in the morgue. Flu kills many, many people every year. We don’t want that to happen. A flu vaccine will really help prevent deaths, help prevent the number of symptoms that somebody gets, and will get people back to work. The benefits are pretty clear.
Does the flu vaccine work equally well every year? It does not. Some years, the strains that are picked for the vaccine don’t match the ones that circulate, and we don’t get as much protection as we hoped for. I think the safety side is so strong that it’s worth making the investment and the effort to promote mandatory flu vaccination.
Can you opt out on religious grounds? Well, some hospitals permit that at New York University. You have to go before a committee and make a case that your exemption on religious grounds is based on an authentic set of beliefs that are deeply held, and not just something you thought up the day before flu vaccine requirements went into effect.
There may be room for some exemptions – obviously, for health reasons. If people think that the flu vaccine is dangerous to them and can get a physician to agree and sign off that they are not appropriate to vaccinate, okay.
On the other hand, if you’re working with an especially vulnerable population – newborns, people who are immunosuppressed – then I think you’ve got to be vaccinated and you shouldn’t be working around people who are at huge risk of getting the flu if you refuse to be vaccinated or, for that matter, can’t be vaccinated.
Would I extend these mandates? Yes, I would. I’d extend them to COVID-19 vaccination and to measles vaccination. I think physicians and nurses should be good role models. They should get vaccinated. We know that the best available evidence says that vaccination for infectious disease is safe. It is really the best thing we can do to combat a variety of diseases such as the flu and COVID-19.
It seems to me that, in addition, the data that are out there in terms of risks from flu and COVID-19 – deaths in places like nursing homes – are overwhelming about the importance of trying to get staff vaccinated so they don’t bring flu into an institutionalized population. This is similar for prison health and many other settings where people are kept close together and staff may move from place to place, rotating from institution to institution, spreading infectious disease.
I’m going to go with the poll. Let’s keep pushing for health care workers to do the right thing and to be good role models. Let’s get everybody a flu vaccination. Let’s extend it to a COVID-19 vaccination and its boosters.
Let’s try to show the nation that health care is going to be guided by good science, a duty to one’s own health, and a duty to one’s patients. It shouldn’t be political. It should be based on what works best for the interests of health care providers and those they care for.
I’m Art Caplan at the New York University Grossman School of Medicine. Thanks for watching.
Dr. Caplan has disclosed the following relevant financial relationships: Served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position). Serves as a contributing author and advisor for Medscape. A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the Division of Medical Ethics at New York University’s Grossman School of Medicine, where I’m the director.
I’ve long believed that every health care institution – nursing homes, hospitals, clinics, home care, hospice – should require flu shots for all doctors and all nurses because it is the easiest, cheapest, and most ethical way to protect the workforce, who you need to be in there when flu outbreaks take place, and to protect patients against getting the flu when they come into hospital settings and get exposed to health care workers who may have the flu already but don’t know it.
In a recent poll, I was happy to see that the majority of physicians surveyed agreed with me: 65% said they supported mandatory flu vaccination in hospitals and only 23% said they did not. I think flu vaccination is something that has already been shown to be useful and important, not only in stopping people from getting the flu but also in making sure that they don’t get as sick when they get the flu.
Just like COVID-19 vaccination, it doesn’t always prevent somebody from getting infected, but if you get it, it keeps you from winding up sick at home, or worse – from dying and winding up in the morgue. Flu kills many, many people every year. We don’t want that to happen. A flu vaccine will really help prevent deaths, help prevent the number of symptoms that somebody gets, and will get people back to work. The benefits are pretty clear.
Does the flu vaccine work equally well every year? It does not. Some years, the strains that are picked for the vaccine don’t match the ones that circulate, and we don’t get as much protection as we hoped for. I think the safety side is so strong that it’s worth making the investment and the effort to promote mandatory flu vaccination.
Can you opt out on religious grounds? Well, some hospitals permit that at New York University. You have to go before a committee and make a case that your exemption on religious grounds is based on an authentic set of beliefs that are deeply held, and not just something you thought up the day before flu vaccine requirements went into effect.
There may be room for some exemptions – obviously, for health reasons. If people think that the flu vaccine is dangerous to them and can get a physician to agree and sign off that they are not appropriate to vaccinate, okay.
On the other hand, if you’re working with an especially vulnerable population – newborns, people who are immunosuppressed – then I think you’ve got to be vaccinated and you shouldn’t be working around people who are at huge risk of getting the flu if you refuse to be vaccinated or, for that matter, can’t be vaccinated.
Would I extend these mandates? Yes, I would. I’d extend them to COVID-19 vaccination and to measles vaccination. I think physicians and nurses should be good role models. They should get vaccinated. We know that the best available evidence says that vaccination for infectious disease is safe. It is really the best thing we can do to combat a variety of diseases such as the flu and COVID-19.
It seems to me that, in addition, the data that are out there in terms of risks from flu and COVID-19 – deaths in places like nursing homes – are overwhelming about the importance of trying to get staff vaccinated so they don’t bring flu into an institutionalized population. This is similar for prison health and many other settings where people are kept close together and staff may move from place to place, rotating from institution to institution, spreading infectious disease.
I’m going to go with the poll. Let’s keep pushing for health care workers to do the right thing and to be good role models. Let’s get everybody a flu vaccination. Let’s extend it to a COVID-19 vaccination and its boosters.
Let’s try to show the nation that health care is going to be guided by good science, a duty to one’s own health, and a duty to one’s patients. It shouldn’t be political. It should be based on what works best for the interests of health care providers and those they care for.
I’m Art Caplan at the New York University Grossman School of Medicine. Thanks for watching.
Dr. Caplan has disclosed the following relevant financial relationships: Served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position). Serves as a contributing author and advisor for Medscape. A version of this article originally appeared on Medscape.com.
Postop RT: Meaningful survival improvement in N2 lung cancer
This transcript has been edited for clarity.
I’m Mark Kris from Memorial Sloan Kettering, speaking today about a topic that’s become quite controversial, which is
Data from clinical trials and data from a SEER study showed approximately 7% improvement in overall survival in patients with N2 disease who received PORT. There has been a very clear demonstration of an improved local control rate in every trial that’s ever looked at PORT.
However, there was a randomized trial, the Lung ART trial, where patients were randomized to get PORT or not. PORT was delivered in a way that is not routinely used now. In that trial, the benefit of PORT was found in terms of local control, almost doubling control within the mediastinum.
The difference in overall survival was less than 12%. Again, I’m not surprised to see that because the improvement in overall survival is probably somewhere between 5% and 10%. They also found an excess of deaths, probably due to cardiac causes from the radiation in the radiation arm.
However, the trial used a type of radiation not used at this point – it used conformal, but now we would use 3D. And its ability at the time of the trial to estimate and lower cardiac risk was not what it is today. Owing to the design of the trial, it was not a significant difference and has largely been interpreted as saying that the PORT doesn’t work.
First, let’s please go to the guidelines. I’m going to the ASCO guidelines, which say that patients with mediastinal disease should not routinely get PORT, but they should be routinely referred to a radiation oncologist for consideration of PORT. I don’t think anything that’s been published so far changes that.
I think each case needs to be individualized and requires the specialty care of a radiation oncologist to weigh the pros and cons of PORT. It also depends upon the treatment plan. Can the heart be spared? Are there radiation techniques available that would eliminate or lessen heart exposure, such as using protons? The point is that PORT is still needed.
When we look at the trials of patients receiving adjuvant therapy – and I’m looking particularly at the ADAURA trial where patients received adjuvant osimertinib – the greatest number of failures now is in the chest. We have to look for good ways to cut down on failure in the chest. Unfortunately, failure in the chest means ultimately failure and lack of cure, and we have to do a better job at that. I think PORT can play a role there.
Please, when you have patients with N2 disease, after the completion of systemic therapies, think about the use of PORT and get the advice of a radiation oncologist to meet with the patient, review their clinical situation, and assess whether or not PORT could be useful for that patient.
That is following the NCCN guidelines, which were not changed on the basis of the Lung ART paper. I think we owe it to our patients to make sure that those who could benefit from this additional therapy receive it.
I’ll put it to you that radiation delivered in the most innovative way – taking very careful account of the effects on the heart – can improve local control. There’s no question about that. I think PORT has the ability to improve survival by a small amount – probably less than 12%, which I will agree the Lung ART trial showed – but still an important amount for patients with this condition.
Mark G. Kris, MD, is chief of the thoracic oncology service and the William and Joy Ruane Chair in Thoracic Oncology at Memorial Sloan Kettering Cancer Center in New York City. He reported conflicts of interest with Arial Pharmaceuticals, Pfizer, PUMA, and Roche/Genentech. A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m Mark Kris from Memorial Sloan Kettering, speaking today about a topic that’s become quite controversial, which is
Data from clinical trials and data from a SEER study showed approximately 7% improvement in overall survival in patients with N2 disease who received PORT. There has been a very clear demonstration of an improved local control rate in every trial that’s ever looked at PORT.
However, there was a randomized trial, the Lung ART trial, where patients were randomized to get PORT or not. PORT was delivered in a way that is not routinely used now. In that trial, the benefit of PORT was found in terms of local control, almost doubling control within the mediastinum.
The difference in overall survival was less than 12%. Again, I’m not surprised to see that because the improvement in overall survival is probably somewhere between 5% and 10%. They also found an excess of deaths, probably due to cardiac causes from the radiation in the radiation arm.
However, the trial used a type of radiation not used at this point – it used conformal, but now we would use 3D. And its ability at the time of the trial to estimate and lower cardiac risk was not what it is today. Owing to the design of the trial, it was not a significant difference and has largely been interpreted as saying that the PORT doesn’t work.
First, let’s please go to the guidelines. I’m going to the ASCO guidelines, which say that patients with mediastinal disease should not routinely get PORT, but they should be routinely referred to a radiation oncologist for consideration of PORT. I don’t think anything that’s been published so far changes that.
I think each case needs to be individualized and requires the specialty care of a radiation oncologist to weigh the pros and cons of PORT. It also depends upon the treatment plan. Can the heart be spared? Are there radiation techniques available that would eliminate or lessen heart exposure, such as using protons? The point is that PORT is still needed.
When we look at the trials of patients receiving adjuvant therapy – and I’m looking particularly at the ADAURA trial where patients received adjuvant osimertinib – the greatest number of failures now is in the chest. We have to look for good ways to cut down on failure in the chest. Unfortunately, failure in the chest means ultimately failure and lack of cure, and we have to do a better job at that. I think PORT can play a role there.
Please, when you have patients with N2 disease, after the completion of systemic therapies, think about the use of PORT and get the advice of a radiation oncologist to meet with the patient, review their clinical situation, and assess whether or not PORT could be useful for that patient.
That is following the NCCN guidelines, which were not changed on the basis of the Lung ART paper. I think we owe it to our patients to make sure that those who could benefit from this additional therapy receive it.
I’ll put it to you that radiation delivered in the most innovative way – taking very careful account of the effects on the heart – can improve local control. There’s no question about that. I think PORT has the ability to improve survival by a small amount – probably less than 12%, which I will agree the Lung ART trial showed – but still an important amount for patients with this condition.
Mark G. Kris, MD, is chief of the thoracic oncology service and the William and Joy Ruane Chair in Thoracic Oncology at Memorial Sloan Kettering Cancer Center in New York City. He reported conflicts of interest with Arial Pharmaceuticals, Pfizer, PUMA, and Roche/Genentech. A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m Mark Kris from Memorial Sloan Kettering, speaking today about a topic that’s become quite controversial, which is
Data from clinical trials and data from a SEER study showed approximately 7% improvement in overall survival in patients with N2 disease who received PORT. There has been a very clear demonstration of an improved local control rate in every trial that’s ever looked at PORT.
However, there was a randomized trial, the Lung ART trial, where patients were randomized to get PORT or not. PORT was delivered in a way that is not routinely used now. In that trial, the benefit of PORT was found in terms of local control, almost doubling control within the mediastinum.
The difference in overall survival was less than 12%. Again, I’m not surprised to see that because the improvement in overall survival is probably somewhere between 5% and 10%. They also found an excess of deaths, probably due to cardiac causes from the radiation in the radiation arm.
However, the trial used a type of radiation not used at this point – it used conformal, but now we would use 3D. And its ability at the time of the trial to estimate and lower cardiac risk was not what it is today. Owing to the design of the trial, it was not a significant difference and has largely been interpreted as saying that the PORT doesn’t work.
First, let’s please go to the guidelines. I’m going to the ASCO guidelines, which say that patients with mediastinal disease should not routinely get PORT, but they should be routinely referred to a radiation oncologist for consideration of PORT. I don’t think anything that’s been published so far changes that.
I think each case needs to be individualized and requires the specialty care of a radiation oncologist to weigh the pros and cons of PORT. It also depends upon the treatment plan. Can the heart be spared? Are there radiation techniques available that would eliminate or lessen heart exposure, such as using protons? The point is that PORT is still needed.
When we look at the trials of patients receiving adjuvant therapy – and I’m looking particularly at the ADAURA trial where patients received adjuvant osimertinib – the greatest number of failures now is in the chest. We have to look for good ways to cut down on failure in the chest. Unfortunately, failure in the chest means ultimately failure and lack of cure, and we have to do a better job at that. I think PORT can play a role there.
Please, when you have patients with N2 disease, after the completion of systemic therapies, think about the use of PORT and get the advice of a radiation oncologist to meet with the patient, review their clinical situation, and assess whether or not PORT could be useful for that patient.
That is following the NCCN guidelines, which were not changed on the basis of the Lung ART paper. I think we owe it to our patients to make sure that those who could benefit from this additional therapy receive it.
I’ll put it to you that radiation delivered in the most innovative way – taking very careful account of the effects on the heart – can improve local control. There’s no question about that. I think PORT has the ability to improve survival by a small amount – probably less than 12%, which I will agree the Lung ART trial showed – but still an important amount for patients with this condition.
Mark G. Kris, MD, is chief of the thoracic oncology service and the William and Joy Ruane Chair in Thoracic Oncology at Memorial Sloan Kettering Cancer Center in New York City. He reported conflicts of interest with Arial Pharmaceuticals, Pfizer, PUMA, and Roche/Genentech. A version of this article first appeared on Medscape.com.
The way I see it
I’ve worn glasses since I was 8, when a routine school vision test showed I was nearsighted. Except for an ill-fated 3-month attempt at contact lenses when I was 16, glasses have been just another part of my daily routine.
The last time I got new ones was in 2018, and my vision always seemed “off” after that. I took them back to the store a few times and was told I’d adjust to them and that things would be fine, So after a few weeks of doggedly wearing them I adjusted to them. I still felt like something was slightly off, but then I was busy, and then came the pandemic, and then my eye doctor retired and I had to find a new one ... so going to get my glasses prescription rechecked kept getting pushed back.
As so many of us do over time, I’ve gotten used to taking my glasses off to read things up close, like a book, or to do a detailed jigsaw puzzle. This has gotten worse over time, and so finally I made an appointment with a new eye doctor.
I handed him my previous prescription. He did a reading off the lenses, looked at the prescription again, gave me a perplexed look, and started the usual eye exam, asking me to read different lines as he switched lenses around. This went on for 10-15 minutes.
“The right lens wasn’t made correctly,” he told me. “You’ve been working off your left eye for the last 5 years.”
He returned my glasses and I put them on. He covered my left eye and showed me how, without realizing it, I was tilting my head back to bring distant items into focus on the right – the opposite of what I should be doing – and with both eyes would adjust my position to use the left eye.
The next morning, while working at my desk, I realized for the first time that I had my head turned slightly right to bring the left eye a tad closer to the screen. In a job where we’re trained to look for such minutiae in patients I’d missed it on myself. A friend even suggested I submit my story as a case report – “An unusual cause of a head-tilt in a middle-aged male” – to a journal.
It’s an interesting commentary on how adaptable the brain is at handling vision changes. It was several hundred million years ago when the brain figured out how to invert images that were seen upside down, and it continues to find ways to compensate for field cuts, cranial nerve palsies, and other lesions. Including flawed spectacles.
When my new eyeglasses arrive, my brain will have to readjust. This time, though, I’m curious and will try to pay better attention to my own reactions. If I can.
so we don’t notice an issue.
Amazing stuff if you think about it.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’ve worn glasses since I was 8, when a routine school vision test showed I was nearsighted. Except for an ill-fated 3-month attempt at contact lenses when I was 16, glasses have been just another part of my daily routine.
The last time I got new ones was in 2018, and my vision always seemed “off” after that. I took them back to the store a few times and was told I’d adjust to them and that things would be fine, So after a few weeks of doggedly wearing them I adjusted to them. I still felt like something was slightly off, but then I was busy, and then came the pandemic, and then my eye doctor retired and I had to find a new one ... so going to get my glasses prescription rechecked kept getting pushed back.
As so many of us do over time, I’ve gotten used to taking my glasses off to read things up close, like a book, or to do a detailed jigsaw puzzle. This has gotten worse over time, and so finally I made an appointment with a new eye doctor.
I handed him my previous prescription. He did a reading off the lenses, looked at the prescription again, gave me a perplexed look, and started the usual eye exam, asking me to read different lines as he switched lenses around. This went on for 10-15 minutes.
“The right lens wasn’t made correctly,” he told me. “You’ve been working off your left eye for the last 5 years.”
He returned my glasses and I put them on. He covered my left eye and showed me how, without realizing it, I was tilting my head back to bring distant items into focus on the right – the opposite of what I should be doing – and with both eyes would adjust my position to use the left eye.
The next morning, while working at my desk, I realized for the first time that I had my head turned slightly right to bring the left eye a tad closer to the screen. In a job where we’re trained to look for such minutiae in patients I’d missed it on myself. A friend even suggested I submit my story as a case report – “An unusual cause of a head-tilt in a middle-aged male” – to a journal.
It’s an interesting commentary on how adaptable the brain is at handling vision changes. It was several hundred million years ago when the brain figured out how to invert images that were seen upside down, and it continues to find ways to compensate for field cuts, cranial nerve palsies, and other lesions. Including flawed spectacles.
When my new eyeglasses arrive, my brain will have to readjust. This time, though, I’m curious and will try to pay better attention to my own reactions. If I can.
so we don’t notice an issue.
Amazing stuff if you think about it.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’ve worn glasses since I was 8, when a routine school vision test showed I was nearsighted. Except for an ill-fated 3-month attempt at contact lenses when I was 16, glasses have been just another part of my daily routine.
The last time I got new ones was in 2018, and my vision always seemed “off” after that. I took them back to the store a few times and was told I’d adjust to them and that things would be fine, So after a few weeks of doggedly wearing them I adjusted to them. I still felt like something was slightly off, but then I was busy, and then came the pandemic, and then my eye doctor retired and I had to find a new one ... so going to get my glasses prescription rechecked kept getting pushed back.
As so many of us do over time, I’ve gotten used to taking my glasses off to read things up close, like a book, or to do a detailed jigsaw puzzle. This has gotten worse over time, and so finally I made an appointment with a new eye doctor.
I handed him my previous prescription. He did a reading off the lenses, looked at the prescription again, gave me a perplexed look, and started the usual eye exam, asking me to read different lines as he switched lenses around. This went on for 10-15 minutes.
“The right lens wasn’t made correctly,” he told me. “You’ve been working off your left eye for the last 5 years.”
He returned my glasses and I put them on. He covered my left eye and showed me how, without realizing it, I was tilting my head back to bring distant items into focus on the right – the opposite of what I should be doing – and with both eyes would adjust my position to use the left eye.
The next morning, while working at my desk, I realized for the first time that I had my head turned slightly right to bring the left eye a tad closer to the screen. In a job where we’re trained to look for such minutiae in patients I’d missed it on myself. A friend even suggested I submit my story as a case report – “An unusual cause of a head-tilt in a middle-aged male” – to a journal.
It’s an interesting commentary on how adaptable the brain is at handling vision changes. It was several hundred million years ago when the brain figured out how to invert images that were seen upside down, and it continues to find ways to compensate for field cuts, cranial nerve palsies, and other lesions. Including flawed spectacles.
When my new eyeglasses arrive, my brain will have to readjust. This time, though, I’m curious and will try to pay better attention to my own reactions. If I can.
so we don’t notice an issue.
Amazing stuff if you think about it.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Is there still a role for tubal surgery in the modern world of IVF?
According to the Centers for Disease Control and Preventions, in 2019 2.1% of all infants born in the United States were conceived by assisted reproductive technology (ART). Now 45 years old, ART, namely in vitro fertilization (IVF), is offered in nearly 500 clinics in the United States, contributing to over 300,000 treatment cycles per year.
A tubal factor is responsible for 30% of female infertility and may involve proximal and/or distal tubal occlusion, irrespective of pelvic adhesions.1 Before the advent of IVF, the sole approach to the treatment of a tubal factor had been surgery. Given its success and minimal invasiveness, IVF is increasingly being offered to circumvent a tubal factor for infertility. This month we examine the utility of surgical treatment of tubal factor infertility. The options for fertility with a history of bilateral tubal ligation was covered in a prior Reproductive Rounds column.
Tubal disease and pelvic adhesions prevent the normal transport of the oocyte and sperm through the fallopian tube. The primary etiology of tubal factor infertility is pelvic inflammatory disease, mainly caused by chlamydia or gonorrhea. Other conditions that may interfere with tubal transport include severe endometriosis, adhesions from previous surgery, or nontubal infection (for example, appendicitis, inflammatory bowel disease), pelvic tuberculosis, and salpingitis isthmica nodosa (that is, diverticulosis of the fallopian tube).
Proximal tubal occlusion
During a hysterosalpingogram (HSG), transient uterine cornual spasm can result if a woman experiences significant uterine cramping, thereby resulting in a false-positive diagnosis of proximal tubal occlusion. When a repeat HSG is gently performed with slow instillation of contrast, uterine cramping is less likely, and the tubal patency rate is 60%. PTO may also result from plugs of mucus and amorphous debris, but this is not true occlusion.2 In cases with unilateral PTO, controlled ovarian hyperstimulation with intrauterine insemination has resulted in pregnancy rates similar to those in patients with unexplained infertility.3
Reconstructive surgery for bilateral PTO has limited effectiveness and the risk of subsequent ectopic pregnancy is as high as 20%.4 A more successful option is fluoroscopic tubal catheterization (FTC), an outpatient procedure performed in a radiology or infertility center. FTC uses a coaxial catheter system where the outer catheter is guided through the tubal ostium and an inner catheter is atraumatically advanced to overcome the blockage. This procedure is 85% successful for tubal patency with 50% of patients conceiving in the first 12 months; one-third of time the tubes reocclude. After the reestablishment of patency with FTC, the chance of achieving a live birth is 22% and the risk of ectopic pregnancy is 4%.5
Treatment of distal tubal occlusion – the hydrosalpinx
Surgery for treating tubal factor infertility is most successful in women with distal tubal obstruction (DTO), often caused by a hydrosalpinx. Fimbrioplasty is the lysis of fimbrial adhesions or dilatation of fimbrial strictures; the tube is patent, but there are adhesive bands that surround the terminal end with preserved tubal rugae. Gentle introduction of an alligator laparoscopic forceps into the tubal ostium followed by opening and withdrawal of the forceps helps to stretch the tube and release minor degrees of fimbrial agglutination.6
A hydrosalpinx is diagnosed by DTO with dilation and intraluminal fluid accumulation along with the reduction/loss of endothelial cilia. Left untreated, a hydrosalpinx can lead to a 50% reduction in IVF pregnancy rates.7 Tube-sparing treatment involves neosalpingostomy to create a new tubal opening. A nonsurgical approach, ultrasound-guided aspiration of hydrosalpinges, has not been shown to significantly increase the rate of clinical pregnancy. Efficacy for improving fertility is generally poor, but depends upon tubal wall thickness, ampullary dilation, presence of mucosal folds, percentage of ciliated cells in the fimbrial end, and peritubal adhesions.8
Evidence supports that laparoscopic salpingectomy in women with hydrosalpinges improves the outcomes of IVF treatment, compared with no surgical intervention.9 The improvement in pregnancy and live birth rates likely stems from the elimination of the retrograde flow of embryotoxic fluid that disrupts implantation. Endometrial receptivity markers (endometrial cell adhesion molecules, integrins, and HOXA10) have been shown to be reduced in the presence of hydrosalpinx.10 A small, randomized trial demonstrated that bipolar diathermy prior to IVF improved pregnancy outcomes.11 PTO was not more effective than salpingectomy. Conceptions, without IVF, have been reported following salpingectomy for unilateral hydrosalpinx.12
In a series including 434 patients with DTO who underwent laparoscopic fimbrioplasty (enlargement of the ostium) or neosalpingostomy (creation of a new ostium) by a single surgeon, 5-year actuarial delivery rates decreased as the severity of tubal occlusion increased; the ectopic rate was stable at approximately 15%.13 A prospective study reported that the relative increase in the pregnancy rate after salpingectomy was greatest in women with a large hydrosalpinx visible on ultrasound.14
Because of the possible risks of decreased ovarian reserve secondary to interruption of ovarian blood supply, salpingectomy should be done with minimal thermal injury and very close to the fallopian tube.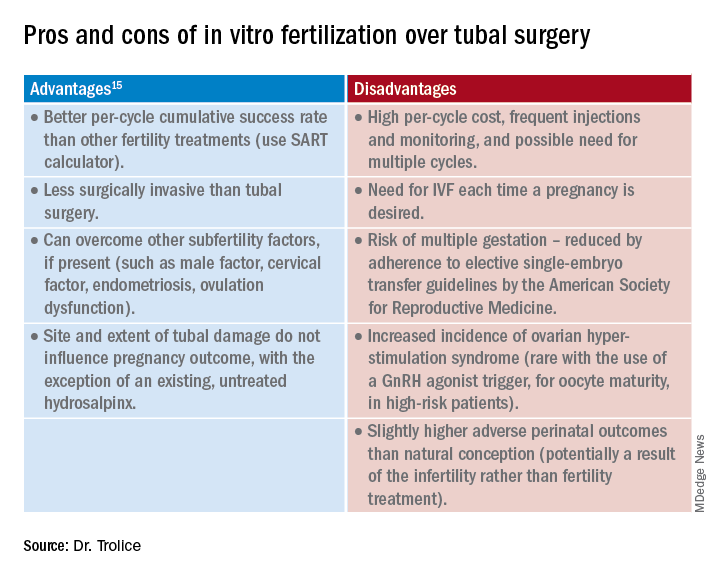
Summary
Surgery may be considered for young women with mild distal tubal disease as one surgical procedure can lead to several pregnancies whereas IVF must be performed each time pregnancy is desired. IVF is more likely than surgery to be successful in women with bilateral hydrosalpinx, in those with pelvic adhesions, in older reproductive aged women, and for both proximal and distal tubal occlusion.15 An online prediction calculator from the Society for Assisted Reproductive Technology (SART) can be helpful in counseling patients on personalized expectations for IVF pregnancy outcomes.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Ambildhuke K et al. Cureus. 2022;1:14(11):e30990.
2. Fatemeh Z et al. Br J Radiol. 2021 Jun 1;94(1122):20201386.
3. Farhi J et al. Fertil Steril. 2007 Aug;88(2):396.
4. Honoré GM et al. Fertil Steril. 1999;71(5):785.
5. De Silva PM et al. Hum Reprod. 2017;32(4):836.
6. Namnoum A and Murphy A. “Diagnostic and Operative Laparoscopy,” in Te Linde’s Operative Gynecology, 8th ed. Philadelphia: Lippincott-Raven, 1997, pp. 389.
7. Camus E et al.Hum Reprod. 1999;14(5):1243.
8. Marana R et al. Hum Reprod. 1999;14(12):2991-5.
9. Johnson N et al. Cochrane Database Syst Rev. 2010 Jan 20;2010(1):CD002125.
10. Savaris RF et al. Fertil Steril. 2006 Jan;85(1):188.
11. Kontoravdis A et al. Fertil Steril. 2006;86(6):1642.
12. Sagoskin AW et al. Hum Reprod. 2003;18(12):2634.
13. Audebert A et al. Fertil Steril. 2014;102(4):1203.
14. Bildirici I et al. Hum Reprod. 2001;16(11):2422.
15. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2012;97(3):539.
According to the Centers for Disease Control and Preventions, in 2019 2.1% of all infants born in the United States were conceived by assisted reproductive technology (ART). Now 45 years old, ART, namely in vitro fertilization (IVF), is offered in nearly 500 clinics in the United States, contributing to over 300,000 treatment cycles per year.
A tubal factor is responsible for 30% of female infertility and may involve proximal and/or distal tubal occlusion, irrespective of pelvic adhesions.1 Before the advent of IVF, the sole approach to the treatment of a tubal factor had been surgery. Given its success and minimal invasiveness, IVF is increasingly being offered to circumvent a tubal factor for infertility. This month we examine the utility of surgical treatment of tubal factor infertility. The options for fertility with a history of bilateral tubal ligation was covered in a prior Reproductive Rounds column.
Tubal disease and pelvic adhesions prevent the normal transport of the oocyte and sperm through the fallopian tube. The primary etiology of tubal factor infertility is pelvic inflammatory disease, mainly caused by chlamydia or gonorrhea. Other conditions that may interfere with tubal transport include severe endometriosis, adhesions from previous surgery, or nontubal infection (for example, appendicitis, inflammatory bowel disease), pelvic tuberculosis, and salpingitis isthmica nodosa (that is, diverticulosis of the fallopian tube).
Proximal tubal occlusion
During a hysterosalpingogram (HSG), transient uterine cornual spasm can result if a woman experiences significant uterine cramping, thereby resulting in a false-positive diagnosis of proximal tubal occlusion. When a repeat HSG is gently performed with slow instillation of contrast, uterine cramping is less likely, and the tubal patency rate is 60%. PTO may also result from plugs of mucus and amorphous debris, but this is not true occlusion.2 In cases with unilateral PTO, controlled ovarian hyperstimulation with intrauterine insemination has resulted in pregnancy rates similar to those in patients with unexplained infertility.3
Reconstructive surgery for bilateral PTO has limited effectiveness and the risk of subsequent ectopic pregnancy is as high as 20%.4 A more successful option is fluoroscopic tubal catheterization (FTC), an outpatient procedure performed in a radiology or infertility center. FTC uses a coaxial catheter system where the outer catheter is guided through the tubal ostium and an inner catheter is atraumatically advanced to overcome the blockage. This procedure is 85% successful for tubal patency with 50% of patients conceiving in the first 12 months; one-third of time the tubes reocclude. After the reestablishment of patency with FTC, the chance of achieving a live birth is 22% and the risk of ectopic pregnancy is 4%.5
Treatment of distal tubal occlusion – the hydrosalpinx
Surgery for treating tubal factor infertility is most successful in women with distal tubal obstruction (DTO), often caused by a hydrosalpinx. Fimbrioplasty is the lysis of fimbrial adhesions or dilatation of fimbrial strictures; the tube is patent, but there are adhesive bands that surround the terminal end with preserved tubal rugae. Gentle introduction of an alligator laparoscopic forceps into the tubal ostium followed by opening and withdrawal of the forceps helps to stretch the tube and release minor degrees of fimbrial agglutination.6
A hydrosalpinx is diagnosed by DTO with dilation and intraluminal fluid accumulation along with the reduction/loss of endothelial cilia. Left untreated, a hydrosalpinx can lead to a 50% reduction in IVF pregnancy rates.7 Tube-sparing treatment involves neosalpingostomy to create a new tubal opening. A nonsurgical approach, ultrasound-guided aspiration of hydrosalpinges, has not been shown to significantly increase the rate of clinical pregnancy. Efficacy for improving fertility is generally poor, but depends upon tubal wall thickness, ampullary dilation, presence of mucosal folds, percentage of ciliated cells in the fimbrial end, and peritubal adhesions.8
Evidence supports that laparoscopic salpingectomy in women with hydrosalpinges improves the outcomes of IVF treatment, compared with no surgical intervention.9 The improvement in pregnancy and live birth rates likely stems from the elimination of the retrograde flow of embryotoxic fluid that disrupts implantation. Endometrial receptivity markers (endometrial cell adhesion molecules, integrins, and HOXA10) have been shown to be reduced in the presence of hydrosalpinx.10 A small, randomized trial demonstrated that bipolar diathermy prior to IVF improved pregnancy outcomes.11 PTO was not more effective than salpingectomy. Conceptions, without IVF, have been reported following salpingectomy for unilateral hydrosalpinx.12
In a series including 434 patients with DTO who underwent laparoscopic fimbrioplasty (enlargement of the ostium) or neosalpingostomy (creation of a new ostium) by a single surgeon, 5-year actuarial delivery rates decreased as the severity of tubal occlusion increased; the ectopic rate was stable at approximately 15%.13 A prospective study reported that the relative increase in the pregnancy rate after salpingectomy was greatest in women with a large hydrosalpinx visible on ultrasound.14
Because of the possible risks of decreased ovarian reserve secondary to interruption of ovarian blood supply, salpingectomy should be done with minimal thermal injury and very close to the fallopian tube.
Summary
Surgery may be considered for young women with mild distal tubal disease as one surgical procedure can lead to several pregnancies whereas IVF must be performed each time pregnancy is desired. IVF is more likely than surgery to be successful in women with bilateral hydrosalpinx, in those with pelvic adhesions, in older reproductive aged women, and for both proximal and distal tubal occlusion.15 An online prediction calculator from the Society for Assisted Reproductive Technology (SART) can be helpful in counseling patients on personalized expectations for IVF pregnancy outcomes.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Ambildhuke K et al. Cureus. 2022;1:14(11):e30990.
2. Fatemeh Z et al. Br J Radiol. 2021 Jun 1;94(1122):20201386.
3. Farhi J et al. Fertil Steril. 2007 Aug;88(2):396.
4. Honoré GM et al. Fertil Steril. 1999;71(5):785.
5. De Silva PM et al. Hum Reprod. 2017;32(4):836.
6. Namnoum A and Murphy A. “Diagnostic and Operative Laparoscopy,” in Te Linde’s Operative Gynecology, 8th ed. Philadelphia: Lippincott-Raven, 1997, pp. 389.
7. Camus E et al.Hum Reprod. 1999;14(5):1243.
8. Marana R et al. Hum Reprod. 1999;14(12):2991-5.
9. Johnson N et al. Cochrane Database Syst Rev. 2010 Jan 20;2010(1):CD002125.
10. Savaris RF et al. Fertil Steril. 2006 Jan;85(1):188.
11. Kontoravdis A et al. Fertil Steril. 2006;86(6):1642.
12. Sagoskin AW et al. Hum Reprod. 2003;18(12):2634.
13. Audebert A et al. Fertil Steril. 2014;102(4):1203.
14. Bildirici I et al. Hum Reprod. 2001;16(11):2422.
15. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2012;97(3):539.
According to the Centers for Disease Control and Preventions, in 2019 2.1% of all infants born in the United States were conceived by assisted reproductive technology (ART). Now 45 years old, ART, namely in vitro fertilization (IVF), is offered in nearly 500 clinics in the United States, contributing to over 300,000 treatment cycles per year.
A tubal factor is responsible for 30% of female infertility and may involve proximal and/or distal tubal occlusion, irrespective of pelvic adhesions.1 Before the advent of IVF, the sole approach to the treatment of a tubal factor had been surgery. Given its success and minimal invasiveness, IVF is increasingly being offered to circumvent a tubal factor for infertility. This month we examine the utility of surgical treatment of tubal factor infertility. The options for fertility with a history of bilateral tubal ligation was covered in a prior Reproductive Rounds column.
Tubal disease and pelvic adhesions prevent the normal transport of the oocyte and sperm through the fallopian tube. The primary etiology of tubal factor infertility is pelvic inflammatory disease, mainly caused by chlamydia or gonorrhea. Other conditions that may interfere with tubal transport include severe endometriosis, adhesions from previous surgery, or nontubal infection (for example, appendicitis, inflammatory bowel disease), pelvic tuberculosis, and salpingitis isthmica nodosa (that is, diverticulosis of the fallopian tube).
Proximal tubal occlusion
During a hysterosalpingogram (HSG), transient uterine cornual spasm can result if a woman experiences significant uterine cramping, thereby resulting in a false-positive diagnosis of proximal tubal occlusion. When a repeat HSG is gently performed with slow instillation of contrast, uterine cramping is less likely, and the tubal patency rate is 60%. PTO may also result from plugs of mucus and amorphous debris, but this is not true occlusion.2 In cases with unilateral PTO, controlled ovarian hyperstimulation with intrauterine insemination has resulted in pregnancy rates similar to those in patients with unexplained infertility.3
Reconstructive surgery for bilateral PTO has limited effectiveness and the risk of subsequent ectopic pregnancy is as high as 20%.4 A more successful option is fluoroscopic tubal catheterization (FTC), an outpatient procedure performed in a radiology or infertility center. FTC uses a coaxial catheter system where the outer catheter is guided through the tubal ostium and an inner catheter is atraumatically advanced to overcome the blockage. This procedure is 85% successful for tubal patency with 50% of patients conceiving in the first 12 months; one-third of time the tubes reocclude. After the reestablishment of patency with FTC, the chance of achieving a live birth is 22% and the risk of ectopic pregnancy is 4%.5
Treatment of distal tubal occlusion – the hydrosalpinx
Surgery for treating tubal factor infertility is most successful in women with distal tubal obstruction (DTO), often caused by a hydrosalpinx. Fimbrioplasty is the lysis of fimbrial adhesions or dilatation of fimbrial strictures; the tube is patent, but there are adhesive bands that surround the terminal end with preserved tubal rugae. Gentle introduction of an alligator laparoscopic forceps into the tubal ostium followed by opening and withdrawal of the forceps helps to stretch the tube and release minor degrees of fimbrial agglutination.6
A hydrosalpinx is diagnosed by DTO with dilation and intraluminal fluid accumulation along with the reduction/loss of endothelial cilia. Left untreated, a hydrosalpinx can lead to a 50% reduction in IVF pregnancy rates.7 Tube-sparing treatment involves neosalpingostomy to create a new tubal opening. A nonsurgical approach, ultrasound-guided aspiration of hydrosalpinges, has not been shown to significantly increase the rate of clinical pregnancy. Efficacy for improving fertility is generally poor, but depends upon tubal wall thickness, ampullary dilation, presence of mucosal folds, percentage of ciliated cells in the fimbrial end, and peritubal adhesions.8
Evidence supports that laparoscopic salpingectomy in women with hydrosalpinges improves the outcomes of IVF treatment, compared with no surgical intervention.9 The improvement in pregnancy and live birth rates likely stems from the elimination of the retrograde flow of embryotoxic fluid that disrupts implantation. Endometrial receptivity markers (endometrial cell adhesion molecules, integrins, and HOXA10) have been shown to be reduced in the presence of hydrosalpinx.10 A small, randomized trial demonstrated that bipolar diathermy prior to IVF improved pregnancy outcomes.11 PTO was not more effective than salpingectomy. Conceptions, without IVF, have been reported following salpingectomy for unilateral hydrosalpinx.12
In a series including 434 patients with DTO who underwent laparoscopic fimbrioplasty (enlargement of the ostium) or neosalpingostomy (creation of a new ostium) by a single surgeon, 5-year actuarial delivery rates decreased as the severity of tubal occlusion increased; the ectopic rate was stable at approximately 15%.13 A prospective study reported that the relative increase in the pregnancy rate after salpingectomy was greatest in women with a large hydrosalpinx visible on ultrasound.14
Because of the possible risks of decreased ovarian reserve secondary to interruption of ovarian blood supply, salpingectomy should be done with minimal thermal injury and very close to the fallopian tube.
Summary
Surgery may be considered for young women with mild distal tubal disease as one surgical procedure can lead to several pregnancies whereas IVF must be performed each time pregnancy is desired. IVF is more likely than surgery to be successful in women with bilateral hydrosalpinx, in those with pelvic adhesions, in older reproductive aged women, and for both proximal and distal tubal occlusion.15 An online prediction calculator from the Society for Assisted Reproductive Technology (SART) can be helpful in counseling patients on personalized expectations for IVF pregnancy outcomes.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Ambildhuke K et al. Cureus. 2022;1:14(11):e30990.
2. Fatemeh Z et al. Br J Radiol. 2021 Jun 1;94(1122):20201386.
3. Farhi J et al. Fertil Steril. 2007 Aug;88(2):396.
4. Honoré GM et al. Fertil Steril. 1999;71(5):785.
5. De Silva PM et al. Hum Reprod. 2017;32(4):836.
6. Namnoum A and Murphy A. “Diagnostic and Operative Laparoscopy,” in Te Linde’s Operative Gynecology, 8th ed. Philadelphia: Lippincott-Raven, 1997, pp. 389.
7. Camus E et al.Hum Reprod. 1999;14(5):1243.
8. Marana R et al. Hum Reprod. 1999;14(12):2991-5.
9. Johnson N et al. Cochrane Database Syst Rev. 2010 Jan 20;2010(1):CD002125.
10. Savaris RF et al. Fertil Steril. 2006 Jan;85(1):188.
11. Kontoravdis A et al. Fertil Steril. 2006;86(6):1642.
12. Sagoskin AW et al. Hum Reprod. 2003;18(12):2634.
13. Audebert A et al. Fertil Steril. 2014;102(4):1203.
14. Bildirici I et al. Hum Reprod. 2001;16(11):2422.
15. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2012;97(3):539.
What’s new in brain health?
This transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener from the medical faculty of the University of Duisburg-Essen in Germany.
Treatment of tension-type headache
I would like to start with headache. You are all aware that we have several new studies regarding the prevention of migraine, but very few studies involving nondrug treatments for tension-type headache.
A working group in Göttingen, Germany, conducted a study in people with frequent episodic and chronic tension-type headache. The first of the four randomized groups received traditional Chinese acupuncture for 3 months. The second group received physical therapy and exercise for 1 hour per week for 12 weeks. The third group received a combination of acupuncture and exercise. The last was a control group that received only standard care.
The outcome parameters of tension-type headache were evaluated after 6 months and again after 12 months. Previously, these same researchers published that the intensity but not the frequency of tension-type headache was reduced by active therapy.
In Cephalalgia, they published the outcome for the endpoints of depression, anxiety, and quality of life. Acupuncture, exercise, and the combination of the two improved depression, anxiety, and quality of life. This shows that nonmedical treatment is effective in people with frequent episodic and chronic tension-type headache.
Headache after COVID-19
The next study was published in Headache and discusses headache after COVID-19. In this review of published studies, more than 50% of people with COVID-19 develop headache. It is more frequent in young patients and people with preexisting primary headaches, such as migraine and tension-type headache. Prognosis is usually good, but some patients develop new, daily persistent headache, which is a major problem because treatment is unclear. We desperately need studies investigating how to treat this new, daily persistent headache after COVID-19.
SSRIs during COVID-19 infection
The next study also focuses on COVID-19. We have conflicting results from several studies suggesting that selective serotonin reuptake inhibitors might be effective in people with mild COVID-19 infection. This hypothesis was tested in a study in Brazil and was published in JAMA, The study included 1,288 outpatients with mild COVID-19 who either received 50 mg of fluvoxamine twice daily for 10 days or placebo. There was no benefit of the treatment for any outcome.
Preventing dementia with antihypertensive treatment
The next study was published in the European Heart Journal and addresses the question of whether effective antihypertensive treatment in elderly persons can prevent dementia. This is a meta-analysis of five placebo-controlled trials with more than 28,000 patients. The meta-analysis clearly shows that treating hypertension in elderly patients does prevent dementia. The benefit is higher if the blood pressure is lowered by a larger amount which also stays true for elderly patients. There is no negative impact of lowering blood pressure in this population.
Antiplatelet therapy
The next study was published in Stroke and reexamines whether resumption of antiplatelet therapy should be early or late in people who had an intracerebral hemorrhage while on antiplatelet therapy. In the Taiwanese Health Registry, this was studied in 1,584 patients. The researchers divided participants into groups based on whether antiplatelet therapy was resumed within 30 days or after 30 days. In 1 year, the rate of recurrent intracerebral hemorrhage was 3.2%. There was no difference whether antiplatelet therapy was resumed early or late.
Regular exercise in Parkinson’s disease
The final study is a review of nonmedical therapy. This meta-analysis of 19 randomized trials looked at the benefit of regular exercise in patients with Parkinson’s disease and depression. The analysis clearly showed that rigorous and moderate exercise improved depression in patients with Parkinson’s disease. This is very important because exercise improves not only the symptoms of Parkinson’s disease but also comorbid depression while presenting no serious adverse events or side effects.
Dr. Diener is a professor in the department of neurology at Stroke Center–Headache Center, University Duisburg-Essen, Germany. He disclosed ties with Abbott, Addex Pharma, Alder, Allergan, Almirall, Amgen, Autonomic Technology, AstraZeneca, Bayer Vital, Berlin Chemie, Bristol-Myers Squibb, Boehringer Ingelheim, Chordate, CoAxia, Corimmun, Covidien, Coherex, CoLucid, Daiichi Sankyo, D-Pharm, Electrocore, Fresenius, GlaxoSmithKline, Grunenthal, Janssen-Cilag, Labrys Biologics Lilly, La Roche, Lundbeck, 3M Medica, MSD, Medtronic, Menarini, MindFrame, Minster, Neuroscore, Neurobiological Technologies, Novartis, Novo Nordisk, Johnson & Johnson, Knoll, Paion, Parke-Davis, Pierre Fabre, Pfizer Inc, Schaper and Brummer, Sanofi-Aventis, Schering-Plough, Servier, Solvay, St. Jude, Talecris, Thrombogenics, WebMD Global, Weber and Weber, Wyeth, and Yamanouchi. Dr. Diener has served as editor of Aktuelle Neurologie, Arzneimitteltherapie, Kopfschmerz News, Stroke News, and the Treatment Guidelines of the German Neurological Society; as co-editor of Cephalalgia; and on the editorial board of The Lancet Neurology, Stroke, European Neurology, and Cerebrovascular Disorders. The department of neurology in Essen is supported by the German Research Council, the German Ministry of Education and Research, European Union, National Institutes of Health, Bertelsmann Foundation, and Heinz Nixdorf Foundation. Dr. Diener has no ownership interest and does not own stocks in any pharmaceutical company. A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener from the medical faculty of the University of Duisburg-Essen in Germany.
Treatment of tension-type headache
I would like to start with headache. You are all aware that we have several new studies regarding the prevention of migraine, but very few studies involving nondrug treatments for tension-type headache.
A working group in Göttingen, Germany, conducted a study in people with frequent episodic and chronic tension-type headache. The first of the four randomized groups received traditional Chinese acupuncture for 3 months. The second group received physical therapy and exercise for 1 hour per week for 12 weeks. The third group received a combination of acupuncture and exercise. The last was a control group that received only standard care.
The outcome parameters of tension-type headache were evaluated after 6 months and again after 12 months. Previously, these same researchers published that the intensity but not the frequency of tension-type headache was reduced by active therapy.
In Cephalalgia, they published the outcome for the endpoints of depression, anxiety, and quality of life. Acupuncture, exercise, and the combination of the two improved depression, anxiety, and quality of life. This shows that nonmedical treatment is effective in people with frequent episodic and chronic tension-type headache.
Headache after COVID-19
The next study was published in Headache and discusses headache after COVID-19. In this review of published studies, more than 50% of people with COVID-19 develop headache. It is more frequent in young patients and people with preexisting primary headaches, such as migraine and tension-type headache. Prognosis is usually good, but some patients develop new, daily persistent headache, which is a major problem because treatment is unclear. We desperately need studies investigating how to treat this new, daily persistent headache after COVID-19.
SSRIs during COVID-19 infection
The next study also focuses on COVID-19. We have conflicting results from several studies suggesting that selective serotonin reuptake inhibitors might be effective in people with mild COVID-19 infection. This hypothesis was tested in a study in Brazil and was published in JAMA, The study included 1,288 outpatients with mild COVID-19 who either received 50 mg of fluvoxamine twice daily for 10 days or placebo. There was no benefit of the treatment for any outcome.
Preventing dementia with antihypertensive treatment
The next study was published in the European Heart Journal and addresses the question of whether effective antihypertensive treatment in elderly persons can prevent dementia. This is a meta-analysis of five placebo-controlled trials with more than 28,000 patients. The meta-analysis clearly shows that treating hypertension in elderly patients does prevent dementia. The benefit is higher if the blood pressure is lowered by a larger amount which also stays true for elderly patients. There is no negative impact of lowering blood pressure in this population.
Antiplatelet therapy
The next study was published in Stroke and reexamines whether resumption of antiplatelet therapy should be early or late in people who had an intracerebral hemorrhage while on antiplatelet therapy. In the Taiwanese Health Registry, this was studied in 1,584 patients. The researchers divided participants into groups based on whether antiplatelet therapy was resumed within 30 days or after 30 days. In 1 year, the rate of recurrent intracerebral hemorrhage was 3.2%. There was no difference whether antiplatelet therapy was resumed early or late.
Regular exercise in Parkinson’s disease
The final study is a review of nonmedical therapy. This meta-analysis of 19 randomized trials looked at the benefit of regular exercise in patients with Parkinson’s disease and depression. The analysis clearly showed that rigorous and moderate exercise improved depression in patients with Parkinson’s disease. This is very important because exercise improves not only the symptoms of Parkinson’s disease but also comorbid depression while presenting no serious adverse events or side effects.
Dr. Diener is a professor in the department of neurology at Stroke Center–Headache Center, University Duisburg-Essen, Germany. He disclosed ties with Abbott, Addex Pharma, Alder, Allergan, Almirall, Amgen, Autonomic Technology, AstraZeneca, Bayer Vital, Berlin Chemie, Bristol-Myers Squibb, Boehringer Ingelheim, Chordate, CoAxia, Corimmun, Covidien, Coherex, CoLucid, Daiichi Sankyo, D-Pharm, Electrocore, Fresenius, GlaxoSmithKline, Grunenthal, Janssen-Cilag, Labrys Biologics Lilly, La Roche, Lundbeck, 3M Medica, MSD, Medtronic, Menarini, MindFrame, Minster, Neuroscore, Neurobiological Technologies, Novartis, Novo Nordisk, Johnson & Johnson, Knoll, Paion, Parke-Davis, Pierre Fabre, Pfizer Inc, Schaper and Brummer, Sanofi-Aventis, Schering-Plough, Servier, Solvay, St. Jude, Talecris, Thrombogenics, WebMD Global, Weber and Weber, Wyeth, and Yamanouchi. Dr. Diener has served as editor of Aktuelle Neurologie, Arzneimitteltherapie, Kopfschmerz News, Stroke News, and the Treatment Guidelines of the German Neurological Society; as co-editor of Cephalalgia; and on the editorial board of The Lancet Neurology, Stroke, European Neurology, and Cerebrovascular Disorders. The department of neurology in Essen is supported by the German Research Council, the German Ministry of Education and Research, European Union, National Institutes of Health, Bertelsmann Foundation, and Heinz Nixdorf Foundation. Dr. Diener has no ownership interest and does not own stocks in any pharmaceutical company. A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener from the medical faculty of the University of Duisburg-Essen in Germany.
Treatment of tension-type headache
I would like to start with headache. You are all aware that we have several new studies regarding the prevention of migraine, but very few studies involving nondrug treatments for tension-type headache.
A working group in Göttingen, Germany, conducted a study in people with frequent episodic and chronic tension-type headache. The first of the four randomized groups received traditional Chinese acupuncture for 3 months. The second group received physical therapy and exercise for 1 hour per week for 12 weeks. The third group received a combination of acupuncture and exercise. The last was a control group that received only standard care.
The outcome parameters of tension-type headache were evaluated after 6 months and again after 12 months. Previously, these same researchers published that the intensity but not the frequency of tension-type headache was reduced by active therapy.
In Cephalalgia, they published the outcome for the endpoints of depression, anxiety, and quality of life. Acupuncture, exercise, and the combination of the two improved depression, anxiety, and quality of life. This shows that nonmedical treatment is effective in people with frequent episodic and chronic tension-type headache.
Headache after COVID-19
The next study was published in Headache and discusses headache after COVID-19. In this review of published studies, more than 50% of people with COVID-19 develop headache. It is more frequent in young patients and people with preexisting primary headaches, such as migraine and tension-type headache. Prognosis is usually good, but some patients develop new, daily persistent headache, which is a major problem because treatment is unclear. We desperately need studies investigating how to treat this new, daily persistent headache after COVID-19.
SSRIs during COVID-19 infection
The next study also focuses on COVID-19. We have conflicting results from several studies suggesting that selective serotonin reuptake inhibitors might be effective in people with mild COVID-19 infection. This hypothesis was tested in a study in Brazil and was published in JAMA, The study included 1,288 outpatients with mild COVID-19 who either received 50 mg of fluvoxamine twice daily for 10 days or placebo. There was no benefit of the treatment for any outcome.
Preventing dementia with antihypertensive treatment
The next study was published in the European Heart Journal and addresses the question of whether effective antihypertensive treatment in elderly persons can prevent dementia. This is a meta-analysis of five placebo-controlled trials with more than 28,000 patients. The meta-analysis clearly shows that treating hypertension in elderly patients does prevent dementia. The benefit is higher if the blood pressure is lowered by a larger amount which also stays true for elderly patients. There is no negative impact of lowering blood pressure in this population.
Antiplatelet therapy
The next study was published in Stroke and reexamines whether resumption of antiplatelet therapy should be early or late in people who had an intracerebral hemorrhage while on antiplatelet therapy. In the Taiwanese Health Registry, this was studied in 1,584 patients. The researchers divided participants into groups based on whether antiplatelet therapy was resumed within 30 days or after 30 days. In 1 year, the rate of recurrent intracerebral hemorrhage was 3.2%. There was no difference whether antiplatelet therapy was resumed early or late.
Regular exercise in Parkinson’s disease
The final study is a review of nonmedical therapy. This meta-analysis of 19 randomized trials looked at the benefit of regular exercise in patients with Parkinson’s disease and depression. The analysis clearly showed that rigorous and moderate exercise improved depression in patients with Parkinson’s disease. This is very important because exercise improves not only the symptoms of Parkinson’s disease but also comorbid depression while presenting no serious adverse events or side effects.
Dr. Diener is a professor in the department of neurology at Stroke Center–Headache Center, University Duisburg-Essen, Germany. He disclosed ties with Abbott, Addex Pharma, Alder, Allergan, Almirall, Amgen, Autonomic Technology, AstraZeneca, Bayer Vital, Berlin Chemie, Bristol-Myers Squibb, Boehringer Ingelheim, Chordate, CoAxia, Corimmun, Covidien, Coherex, CoLucid, Daiichi Sankyo, D-Pharm, Electrocore, Fresenius, GlaxoSmithKline, Grunenthal, Janssen-Cilag, Labrys Biologics Lilly, La Roche, Lundbeck, 3M Medica, MSD, Medtronic, Menarini, MindFrame, Minster, Neuroscore, Neurobiological Technologies, Novartis, Novo Nordisk, Johnson & Johnson, Knoll, Paion, Parke-Davis, Pierre Fabre, Pfizer Inc, Schaper and Brummer, Sanofi-Aventis, Schering-Plough, Servier, Solvay, St. Jude, Talecris, Thrombogenics, WebMD Global, Weber and Weber, Wyeth, and Yamanouchi. Dr. Diener has served as editor of Aktuelle Neurologie, Arzneimitteltherapie, Kopfschmerz News, Stroke News, and the Treatment Guidelines of the German Neurological Society; as co-editor of Cephalalgia; and on the editorial board of The Lancet Neurology, Stroke, European Neurology, and Cerebrovascular Disorders. The department of neurology in Essen is supported by the German Research Council, the German Ministry of Education and Research, European Union, National Institutes of Health, Bertelsmann Foundation, and Heinz Nixdorf Foundation. Dr. Diener has no ownership interest and does not own stocks in any pharmaceutical company. A version of this article originally appeared on Medscape.com.
A doctor must go to extremes to save a choking victim
Some time ago I was invited to join a bipartisan congressional task force on valley fever, also known as coccidioidomycosis. A large and diverse crowd attended the task force’s first meeting in Bakersfield, Calif. – a meeting for everyone: the medical profession, the public, it even included veterinarians.
The whole thing was a resounding success. Francis Collins was there, the just-retired director of the NIH. Tom Frieden, then-director of the Centers for Disease Control and Prevention was there, as were several congresspeople and also my college roommate, a retired Navy medical corps captain. I was enjoying it.
Afterward, we had a banquet dinner at a restaurant in downtown Bakersfield. One of the people there was a woman I knew well – her husband was a physician friend. The restaurant served steak and salmon, and this woman made the mistake of ordering the steak.
Not long after the entrees were served, I heard a commotion at the table just behind me. I turned around and saw that woman in distress. A piece of steak had wedged in her trachea and she couldn’t breathe.
Almost immediately, the chef showed up. I don’t know how he got there. The chef at this restaurant was a big guy. I mean, probably 6 feet, 5 inches tall and 275 pounds. He tried the Heimlich maneuver. It didn’t work.
At that point, I jumped up. I thought, “Well, maybe I know how to do this better than him.” Probably not, actually. I tried and couldn’t make it work either. So I knew we were going to have to do something.
Paul Krogstad, my friend and research partner who is a pediatric infectious disease physician, stepped up and tried to put his finger in her throat and dig it out. He couldn’t get it. The patient had lost consciousness.
So, I’m thinking, okay, there’s really only one choice. You have to get an airway surgically.
I said, “We have to put her down on the floor.” And then I said, “Knife!”
I was looking at the steak knives on the table and they weren’t to my liking for doing a procedure. My college roommate – the retired Navy man – whipped out this very good pocketknife.
I had never done this in my life.
While I was making the incision, somebody gave Paul a ballpoint pen and he broke it into pieces to make a tracheostomy tube. Once I’d made the little incision, I put the tube in. She wasn’t breathing, but she still had a pulse.
I leaned forward and blew into the tube and inflated her lungs. I could see her lungs balloon up. It was a nice feeling, because I knew I was clearly in the right place.
I can’t quite explain it, but while I was doing this, I was enormously calm and totally focused. I knew there was a crowd of people around me, all looking at me, but I wasn’t conscious of that.
It was really just the four of us: Paul and Tom and me and our patient. Those were the only people that I was really cognizant of. Paul and Tom were not panic stricken at all. I remember somebody shouting, “We have to start CPR!” and Frieden said, “No. We don’t.”
Moments later, she woke up, sat up, coughed, and shot the piece of steak across the room.
She was breathing on her own, but we still taped that tube into place. Somebody had already summoned an ambulance; they were there not very long after we completed this procedure. I got in the ambulance with her and we rode over to the emergency room at Mercy Truxtun.
She was stable and doing okay. I sat with her until a thoracic surgeon showed up. He checked out the situation and decided we didn’t need that tube and took it out. I didn’t want to take that out until I had a surgeon there who could do a formal tracheostomy.
They kept her in the hospital for 3 or 4 days. Now, this woman had always had difficulties swallowing, so steak may not have been the best choice. She still had trouble swallowing afterward but recovered.
I’ve known her and her husband a long time, so it was certainly rewarding to be able to provide this service. Years later, though, when her husband died, I spoke at his funeral. When she was speaking to the gathering, she said, “And oh, by the way, Royce, thanks for saving my life.”
That surprised me. I didn’t think we were going to go there.
I’d never tried to practice medicine “at the roadside” before. But that’s part of the career.
Royce Johnson, MD, is the chief of the division of infectious disease among other leadership positions at Kern Medical in Bakersfield, Calif., and the medical director of the Valley Fever Institute.
A version of this article first appeared on Medscape.com.
Some time ago I was invited to join a bipartisan congressional task force on valley fever, also known as coccidioidomycosis. A large and diverse crowd attended the task force’s first meeting in Bakersfield, Calif. – a meeting for everyone: the medical profession, the public, it even included veterinarians.
The whole thing was a resounding success. Francis Collins was there, the just-retired director of the NIH. Tom Frieden, then-director of the Centers for Disease Control and Prevention was there, as were several congresspeople and also my college roommate, a retired Navy medical corps captain. I was enjoying it.
Afterward, we had a banquet dinner at a restaurant in downtown Bakersfield. One of the people there was a woman I knew well – her husband was a physician friend. The restaurant served steak and salmon, and this woman made the mistake of ordering the steak.
Not long after the entrees were served, I heard a commotion at the table just behind me. I turned around and saw that woman in distress. A piece of steak had wedged in her trachea and she couldn’t breathe.
Almost immediately, the chef showed up. I don’t know how he got there. The chef at this restaurant was a big guy. I mean, probably 6 feet, 5 inches tall and 275 pounds. He tried the Heimlich maneuver. It didn’t work.
At that point, I jumped up. I thought, “Well, maybe I know how to do this better than him.” Probably not, actually. I tried and couldn’t make it work either. So I knew we were going to have to do something.
Paul Krogstad, my friend and research partner who is a pediatric infectious disease physician, stepped up and tried to put his finger in her throat and dig it out. He couldn’t get it. The patient had lost consciousness.
So, I’m thinking, okay, there’s really only one choice. You have to get an airway surgically.
I said, “We have to put her down on the floor.” And then I said, “Knife!”
I was looking at the steak knives on the table and they weren’t to my liking for doing a procedure. My college roommate – the retired Navy man – whipped out this very good pocketknife.
I had never done this in my life.
While I was making the incision, somebody gave Paul a ballpoint pen and he broke it into pieces to make a tracheostomy tube. Once I’d made the little incision, I put the tube in. She wasn’t breathing, but she still had a pulse.
I leaned forward and blew into the tube and inflated her lungs. I could see her lungs balloon up. It was a nice feeling, because I knew I was clearly in the right place.
I can’t quite explain it, but while I was doing this, I was enormously calm and totally focused. I knew there was a crowd of people around me, all looking at me, but I wasn’t conscious of that.
It was really just the four of us: Paul and Tom and me and our patient. Those were the only people that I was really cognizant of. Paul and Tom were not panic stricken at all. I remember somebody shouting, “We have to start CPR!” and Frieden said, “No. We don’t.”
Moments later, she woke up, sat up, coughed, and shot the piece of steak across the room.
She was breathing on her own, but we still taped that tube into place. Somebody had already summoned an ambulance; they were there not very long after we completed this procedure. I got in the ambulance with her and we rode over to the emergency room at Mercy Truxtun.
She was stable and doing okay. I sat with her until a thoracic surgeon showed up. He checked out the situation and decided we didn’t need that tube and took it out. I didn’t want to take that out until I had a surgeon there who could do a formal tracheostomy.
They kept her in the hospital for 3 or 4 days. Now, this woman had always had difficulties swallowing, so steak may not have been the best choice. She still had trouble swallowing afterward but recovered.
I’ve known her and her husband a long time, so it was certainly rewarding to be able to provide this service. Years later, though, when her husband died, I spoke at his funeral. When she was speaking to the gathering, she said, “And oh, by the way, Royce, thanks for saving my life.”
That surprised me. I didn’t think we were going to go there.
I’d never tried to practice medicine “at the roadside” before. But that’s part of the career.
Royce Johnson, MD, is the chief of the division of infectious disease among other leadership positions at Kern Medical in Bakersfield, Calif., and the medical director of the Valley Fever Institute.
A version of this article first appeared on Medscape.com.
Some time ago I was invited to join a bipartisan congressional task force on valley fever, also known as coccidioidomycosis. A large and diverse crowd attended the task force’s first meeting in Bakersfield, Calif. – a meeting for everyone: the medical profession, the public, it even included veterinarians.
The whole thing was a resounding success. Francis Collins was there, the just-retired director of the NIH. Tom Frieden, then-director of the Centers for Disease Control and Prevention was there, as were several congresspeople and also my college roommate, a retired Navy medical corps captain. I was enjoying it.
Afterward, we had a banquet dinner at a restaurant in downtown Bakersfield. One of the people there was a woman I knew well – her husband was a physician friend. The restaurant served steak and salmon, and this woman made the mistake of ordering the steak.
Not long after the entrees were served, I heard a commotion at the table just behind me. I turned around and saw that woman in distress. A piece of steak had wedged in her trachea and she couldn’t breathe.
Almost immediately, the chef showed up. I don’t know how he got there. The chef at this restaurant was a big guy. I mean, probably 6 feet, 5 inches tall and 275 pounds. He tried the Heimlich maneuver. It didn’t work.
At that point, I jumped up. I thought, “Well, maybe I know how to do this better than him.” Probably not, actually. I tried and couldn’t make it work either. So I knew we were going to have to do something.
Paul Krogstad, my friend and research partner who is a pediatric infectious disease physician, stepped up and tried to put his finger in her throat and dig it out. He couldn’t get it. The patient had lost consciousness.
So, I’m thinking, okay, there’s really only one choice. You have to get an airway surgically.
I said, “We have to put her down on the floor.” And then I said, “Knife!”
I was looking at the steak knives on the table and they weren’t to my liking for doing a procedure. My college roommate – the retired Navy man – whipped out this very good pocketknife.
I had never done this in my life.
While I was making the incision, somebody gave Paul a ballpoint pen and he broke it into pieces to make a tracheostomy tube. Once I’d made the little incision, I put the tube in. She wasn’t breathing, but she still had a pulse.
I leaned forward and blew into the tube and inflated her lungs. I could see her lungs balloon up. It was a nice feeling, because I knew I was clearly in the right place.
I can’t quite explain it, but while I was doing this, I was enormously calm and totally focused. I knew there was a crowd of people around me, all looking at me, but I wasn’t conscious of that.
It was really just the four of us: Paul and Tom and me and our patient. Those were the only people that I was really cognizant of. Paul and Tom were not panic stricken at all. I remember somebody shouting, “We have to start CPR!” and Frieden said, “No. We don’t.”
Moments later, she woke up, sat up, coughed, and shot the piece of steak across the room.
She was breathing on her own, but we still taped that tube into place. Somebody had already summoned an ambulance; they were there not very long after we completed this procedure. I got in the ambulance with her and we rode over to the emergency room at Mercy Truxtun.
She was stable and doing okay. I sat with her until a thoracic surgeon showed up. He checked out the situation and decided we didn’t need that tube and took it out. I didn’t want to take that out until I had a surgeon there who could do a formal tracheostomy.
They kept her in the hospital for 3 or 4 days. Now, this woman had always had difficulties swallowing, so steak may not have been the best choice. She still had trouble swallowing afterward but recovered.
I’ve known her and her husband a long time, so it was certainly rewarding to be able to provide this service. Years later, though, when her husband died, I spoke at his funeral. When she was speaking to the gathering, she said, “And oh, by the way, Royce, thanks for saving my life.”
That surprised me. I didn’t think we were going to go there.
I’d never tried to practice medicine “at the roadside” before. But that’s part of the career.
Royce Johnson, MD, is the chief of the division of infectious disease among other leadership positions at Kern Medical in Bakersfield, Calif., and the medical director of the Valley Fever Institute.
A version of this article first appeared on Medscape.com.





