User login
In adults with prediabetes, vitamin D cuts diabetes risk
Results of the analysis, led by Anastassios G. Pittas, MD, MS, with the division of endocrinology, diabetes, and metabolism at Tufts Medical Center, in Boston, were published online in Annals of Internal Medicine (2023 Feb 7. doi: 10.7326/M22-3018).
All three eligible trials included in the analysis were randomized, double blinded, and placebo controlled. The three eligible trials tested three oral formulations of Vitamin D: cholecalciferol, 20,000 IU (500 mcg) weekly; cholecalciferol, 4,000 IU (100 mcg) daily; or eldecalcitol, 0.75 mcg daily, against placebos.
The authors of the new paper found that vitamin D reduced the risk for diabetes in people with prediabetes by a statistically significant 15% in adjusted analyses. The 3-year absolute risk reduction was 3.3%.
They found no difference in the rate ratios for adverse events (kidney stones, 1.17, 95% confidence interval, 0.69-1.99; hypercalcemia, 2.34; 95% CI, 0.83-6.66]; hypercalciuria, 1.65; 95% CI, 0.83-3.28]; death, 0.85; 95% CI, 0.31-2.36]) when study participants got vitamin D instead of placebo.
Differences from previous analyses
The relationship between vitamin D levels and risk for type 2 diabetes has been studied in previous trials and results have been mixed.
The authors note that two previous meta-analyses included trials “that had relatively short durations for assessment of diabetes risk (for example, ≤ 1 year), had high risk of bias (for example, open-label trials), or were not specifically designed and conducted for primary prevention of type 2 diabetes, potentially undermining the validity of the results.”
Each of the trials in this meta-analysis had a low risk of bias as determined by the Cochrane risk-of-bias tool, Dr. Pittas and colleagues said.
“The present study does not reach an opposite conclusion from the D2d study,” said Dr. Pittas, who coauthored that paper as well. “Rather, it confirms the results of the D2d study. In D2d and two other similar vitamin D and diabetes prevention trials (one in Norway and one in Japan), vitamin D reduced the rate of progression to diabetes in adults with prediabetes, but the observed differences were not statistically significant because the reported relative risk reductions (10%-13%) were smaller than each trial was powered to detect (25%-36%).”
“Individual participant data meta-analyses increase the statistical power to detect an effect. After combining data, we found that vitamin D reduced the risk of progression from prediabetes to diabetes by 15% and this result was statistically significant. So, the conclusion of the meta-analysis is essentially the same conclusion as in D2d and the other two trials. The difference is that the result is now statistically significant,” Dr. Pittas added.
Small reduction but large population
The authors acknowledged that the absolute risk reduction number is small, especially when compared with the risk reduction seen with intensive lifestyle changes (58%) and metformin (31%), as reported in an article published in the New England of Journal of Medicine (2002 Feb 7;346:393-403). But “extrapolating to the more than 374 million adults worldwide who have prediabetes suggests that inexpensive vitamin D supplementation could delay the development of diabetes in more than 10 million people,” they said.
As for how high vitamin D levels need to be, the authors write that their research indicates that the optimal level of vitamin D in the blood needed to reduce diabetes risk may be higher than an Institute of Medicine committee recommendation in 2011.
“The blood 25-hydroxy vitamin D level needed to optimally reduce diabetes risk may be near and possibly above the range of 125-150 nmol/L (50-60 ng/mL) that the 2011 Institute of Medicine Committee to Review Dietary Reference Intakes for Calcium and Vitamin D provided as the range corresponding to the tolerable upper intake level (UL) of 4,000 IU/d for vitamin D,” the authors of the new paper said.
Editorialists urge caution
In an accompanying editorial also published in the Annals of Internal Medicine, Malachi J. McKenna, MD, with the department of clinical chemistry, at St. Vincent’s University Hospital, and Mary A.T. Flynn, PhD, RD, with the Food Safety Authority of Ireland in Dublin, urge caution regarding vitamin D dosing.
They write that there are important distinctions between vitamin D supplements and vitamin D therapy, and the potential harms of high-dose vitamin D are still unclear.
“Vitamin D supplementation of 10 to 20 mcg (400 to 800 IU) daily can be applied safely at the population level to prevent skeletal and possibly nonskeletal disease. Very-high-dose vitamin D therapy might prevent type 2 diabetes in some patients but may also cause harm,” they note.
Dr. Pittas said in an interview that there have been some studies with high-dose vitamin D (up to 500,000 IU a year in one study) that reported an increased fall risk in older adults who had high fall risk. “However, these findings are not generalizable to other populations that are younger and at low or average fall risk, such as the prediabetes population to which the results of this meta-analysis apply,” he noted.
“The benefit-to-risk ratio for vitamin D depends on the target population and medical condition,” Dr. Pittas said. “The editorial refers to the NAM (National Academy of Medicine) vitamin D guidelines for the general, healthy population to promote bone health. The guidelines should not be extrapolated to specific populations, for example [patients with] prediabetes,” where the vitamin D benefit-to-risk ratio would be different from that in the general population.
Dr. Pittas and colleagues caution that the people studied in this meta-analysis were at high risk for type 2 diabetes, so these results do not apply to the general healthy population. The results also should not be extrapolated to people at average risk for any type of diabetes, they add.
Several physicians either declined to comment or did not respond to requests for comment on this research.
Dr. Pittas reports the National Institutes of Health and the American Diabetes Association made payments to his institution to conduct Vitamin D-related research. He is an unpaid cochair of the Endocrine Society’s Evaluation, Treatment and Prevention of Vitamin D Deficiency Clinical Practice Guideline team.
Coauthor Dr. Jorde reports grants from Novo Nordisk Foundation, North Norwegian Regional Health Authorities, and the Research Council of Norway.
Dr. Dawson-Hughes reports she is on the DSMB for AgNovos Healthcare. AgNovos is developing a bone implant to reduce hip fracture risk and she gets a stipend from the company. She reports Helsinn Therapeutics provided anamorelin and matching placebo for an NIH-funded clinical trial.
Dr. Trikalinos was supported by the D2d study. He is a technical methodological consultant to Latham and Watkins, who is retained by Pacira Pharmaceuticals.
Dr. Angellotti has been employed by Takeda and owns stock in the company.
The editorialists report no relevant financial relationships.
Results of the analysis, led by Anastassios G. Pittas, MD, MS, with the division of endocrinology, diabetes, and metabolism at Tufts Medical Center, in Boston, were published online in Annals of Internal Medicine (2023 Feb 7. doi: 10.7326/M22-3018).
All three eligible trials included in the analysis were randomized, double blinded, and placebo controlled. The three eligible trials tested three oral formulations of Vitamin D: cholecalciferol, 20,000 IU (500 mcg) weekly; cholecalciferol, 4,000 IU (100 mcg) daily; or eldecalcitol, 0.75 mcg daily, against placebos.
The authors of the new paper found that vitamin D reduced the risk for diabetes in people with prediabetes by a statistically significant 15% in adjusted analyses. The 3-year absolute risk reduction was 3.3%.
They found no difference in the rate ratios for adverse events (kidney stones, 1.17, 95% confidence interval, 0.69-1.99; hypercalcemia, 2.34; 95% CI, 0.83-6.66]; hypercalciuria, 1.65; 95% CI, 0.83-3.28]; death, 0.85; 95% CI, 0.31-2.36]) when study participants got vitamin D instead of placebo.
Differences from previous analyses
The relationship between vitamin D levels and risk for type 2 diabetes has been studied in previous trials and results have been mixed.
The authors note that two previous meta-analyses included trials “that had relatively short durations for assessment of diabetes risk (for example, ≤ 1 year), had high risk of bias (for example, open-label trials), or were not specifically designed and conducted for primary prevention of type 2 diabetes, potentially undermining the validity of the results.”
Each of the trials in this meta-analysis had a low risk of bias as determined by the Cochrane risk-of-bias tool, Dr. Pittas and colleagues said.
“The present study does not reach an opposite conclusion from the D2d study,” said Dr. Pittas, who coauthored that paper as well. “Rather, it confirms the results of the D2d study. In D2d and two other similar vitamin D and diabetes prevention trials (one in Norway and one in Japan), vitamin D reduced the rate of progression to diabetes in adults with prediabetes, but the observed differences were not statistically significant because the reported relative risk reductions (10%-13%) were smaller than each trial was powered to detect (25%-36%).”
“Individual participant data meta-analyses increase the statistical power to detect an effect. After combining data, we found that vitamin D reduced the risk of progression from prediabetes to diabetes by 15% and this result was statistically significant. So, the conclusion of the meta-analysis is essentially the same conclusion as in D2d and the other two trials. The difference is that the result is now statistically significant,” Dr. Pittas added.
Small reduction but large population
The authors acknowledged that the absolute risk reduction number is small, especially when compared with the risk reduction seen with intensive lifestyle changes (58%) and metformin (31%), as reported in an article published in the New England of Journal of Medicine (2002 Feb 7;346:393-403). But “extrapolating to the more than 374 million adults worldwide who have prediabetes suggests that inexpensive vitamin D supplementation could delay the development of diabetes in more than 10 million people,” they said.
As for how high vitamin D levels need to be, the authors write that their research indicates that the optimal level of vitamin D in the blood needed to reduce diabetes risk may be higher than an Institute of Medicine committee recommendation in 2011.
“The blood 25-hydroxy vitamin D level needed to optimally reduce diabetes risk may be near and possibly above the range of 125-150 nmol/L (50-60 ng/mL) that the 2011 Institute of Medicine Committee to Review Dietary Reference Intakes for Calcium and Vitamin D provided as the range corresponding to the tolerable upper intake level (UL) of 4,000 IU/d for vitamin D,” the authors of the new paper said.
Editorialists urge caution
In an accompanying editorial also published in the Annals of Internal Medicine, Malachi J. McKenna, MD, with the department of clinical chemistry, at St. Vincent’s University Hospital, and Mary A.T. Flynn, PhD, RD, with the Food Safety Authority of Ireland in Dublin, urge caution regarding vitamin D dosing.
They write that there are important distinctions between vitamin D supplements and vitamin D therapy, and the potential harms of high-dose vitamin D are still unclear.
“Vitamin D supplementation of 10 to 20 mcg (400 to 800 IU) daily can be applied safely at the population level to prevent skeletal and possibly nonskeletal disease. Very-high-dose vitamin D therapy might prevent type 2 diabetes in some patients but may also cause harm,” they note.
Dr. Pittas said in an interview that there have been some studies with high-dose vitamin D (up to 500,000 IU a year in one study) that reported an increased fall risk in older adults who had high fall risk. “However, these findings are not generalizable to other populations that are younger and at low or average fall risk, such as the prediabetes population to which the results of this meta-analysis apply,” he noted.
“The benefit-to-risk ratio for vitamin D depends on the target population and medical condition,” Dr. Pittas said. “The editorial refers to the NAM (National Academy of Medicine) vitamin D guidelines for the general, healthy population to promote bone health. The guidelines should not be extrapolated to specific populations, for example [patients with] prediabetes,” where the vitamin D benefit-to-risk ratio would be different from that in the general population.
Dr. Pittas and colleagues caution that the people studied in this meta-analysis were at high risk for type 2 diabetes, so these results do not apply to the general healthy population. The results also should not be extrapolated to people at average risk for any type of diabetes, they add.
Several physicians either declined to comment or did not respond to requests for comment on this research.
Dr. Pittas reports the National Institutes of Health and the American Diabetes Association made payments to his institution to conduct Vitamin D-related research. He is an unpaid cochair of the Endocrine Society’s Evaluation, Treatment and Prevention of Vitamin D Deficiency Clinical Practice Guideline team.
Coauthor Dr. Jorde reports grants from Novo Nordisk Foundation, North Norwegian Regional Health Authorities, and the Research Council of Norway.
Dr. Dawson-Hughes reports she is on the DSMB for AgNovos Healthcare. AgNovos is developing a bone implant to reduce hip fracture risk and she gets a stipend from the company. She reports Helsinn Therapeutics provided anamorelin and matching placebo for an NIH-funded clinical trial.
Dr. Trikalinos was supported by the D2d study. He is a technical methodological consultant to Latham and Watkins, who is retained by Pacira Pharmaceuticals.
Dr. Angellotti has been employed by Takeda and owns stock in the company.
The editorialists report no relevant financial relationships.
Results of the analysis, led by Anastassios G. Pittas, MD, MS, with the division of endocrinology, diabetes, and metabolism at Tufts Medical Center, in Boston, were published online in Annals of Internal Medicine (2023 Feb 7. doi: 10.7326/M22-3018).
All three eligible trials included in the analysis were randomized, double blinded, and placebo controlled. The three eligible trials tested three oral formulations of Vitamin D: cholecalciferol, 20,000 IU (500 mcg) weekly; cholecalciferol, 4,000 IU (100 mcg) daily; or eldecalcitol, 0.75 mcg daily, against placebos.
The authors of the new paper found that vitamin D reduced the risk for diabetes in people with prediabetes by a statistically significant 15% in adjusted analyses. The 3-year absolute risk reduction was 3.3%.
They found no difference in the rate ratios for adverse events (kidney stones, 1.17, 95% confidence interval, 0.69-1.99; hypercalcemia, 2.34; 95% CI, 0.83-6.66]; hypercalciuria, 1.65; 95% CI, 0.83-3.28]; death, 0.85; 95% CI, 0.31-2.36]) when study participants got vitamin D instead of placebo.
Differences from previous analyses
The relationship between vitamin D levels and risk for type 2 diabetes has been studied in previous trials and results have been mixed.
The authors note that two previous meta-analyses included trials “that had relatively short durations for assessment of diabetes risk (for example, ≤ 1 year), had high risk of bias (for example, open-label trials), or were not specifically designed and conducted for primary prevention of type 2 diabetes, potentially undermining the validity of the results.”
Each of the trials in this meta-analysis had a low risk of bias as determined by the Cochrane risk-of-bias tool, Dr. Pittas and colleagues said.
“The present study does not reach an opposite conclusion from the D2d study,” said Dr. Pittas, who coauthored that paper as well. “Rather, it confirms the results of the D2d study. In D2d and two other similar vitamin D and diabetes prevention trials (one in Norway and one in Japan), vitamin D reduced the rate of progression to diabetes in adults with prediabetes, but the observed differences were not statistically significant because the reported relative risk reductions (10%-13%) were smaller than each trial was powered to detect (25%-36%).”
“Individual participant data meta-analyses increase the statistical power to detect an effect. After combining data, we found that vitamin D reduced the risk of progression from prediabetes to diabetes by 15% and this result was statistically significant. So, the conclusion of the meta-analysis is essentially the same conclusion as in D2d and the other two trials. The difference is that the result is now statistically significant,” Dr. Pittas added.
Small reduction but large population
The authors acknowledged that the absolute risk reduction number is small, especially when compared with the risk reduction seen with intensive lifestyle changes (58%) and metformin (31%), as reported in an article published in the New England of Journal of Medicine (2002 Feb 7;346:393-403). But “extrapolating to the more than 374 million adults worldwide who have prediabetes suggests that inexpensive vitamin D supplementation could delay the development of diabetes in more than 10 million people,” they said.
As for how high vitamin D levels need to be, the authors write that their research indicates that the optimal level of vitamin D in the blood needed to reduce diabetes risk may be higher than an Institute of Medicine committee recommendation in 2011.
“The blood 25-hydroxy vitamin D level needed to optimally reduce diabetes risk may be near and possibly above the range of 125-150 nmol/L (50-60 ng/mL) that the 2011 Institute of Medicine Committee to Review Dietary Reference Intakes for Calcium and Vitamin D provided as the range corresponding to the tolerable upper intake level (UL) of 4,000 IU/d for vitamin D,” the authors of the new paper said.
Editorialists urge caution
In an accompanying editorial also published in the Annals of Internal Medicine, Malachi J. McKenna, MD, with the department of clinical chemistry, at St. Vincent’s University Hospital, and Mary A.T. Flynn, PhD, RD, with the Food Safety Authority of Ireland in Dublin, urge caution regarding vitamin D dosing.
They write that there are important distinctions between vitamin D supplements and vitamin D therapy, and the potential harms of high-dose vitamin D are still unclear.
“Vitamin D supplementation of 10 to 20 mcg (400 to 800 IU) daily can be applied safely at the population level to prevent skeletal and possibly nonskeletal disease. Very-high-dose vitamin D therapy might prevent type 2 diabetes in some patients but may also cause harm,” they note.
Dr. Pittas said in an interview that there have been some studies with high-dose vitamin D (up to 500,000 IU a year in one study) that reported an increased fall risk in older adults who had high fall risk. “However, these findings are not generalizable to other populations that are younger and at low or average fall risk, such as the prediabetes population to which the results of this meta-analysis apply,” he noted.
“The benefit-to-risk ratio for vitamin D depends on the target population and medical condition,” Dr. Pittas said. “The editorial refers to the NAM (National Academy of Medicine) vitamin D guidelines for the general, healthy population to promote bone health. The guidelines should not be extrapolated to specific populations, for example [patients with] prediabetes,” where the vitamin D benefit-to-risk ratio would be different from that in the general population.
Dr. Pittas and colleagues caution that the people studied in this meta-analysis were at high risk for type 2 diabetes, so these results do not apply to the general healthy population. The results also should not be extrapolated to people at average risk for any type of diabetes, they add.
Several physicians either declined to comment or did not respond to requests for comment on this research.
Dr. Pittas reports the National Institutes of Health and the American Diabetes Association made payments to his institution to conduct Vitamin D-related research. He is an unpaid cochair of the Endocrine Society’s Evaluation, Treatment and Prevention of Vitamin D Deficiency Clinical Practice Guideline team.
Coauthor Dr. Jorde reports grants from Novo Nordisk Foundation, North Norwegian Regional Health Authorities, and the Research Council of Norway.
Dr. Dawson-Hughes reports she is on the DSMB for AgNovos Healthcare. AgNovos is developing a bone implant to reduce hip fracture risk and she gets a stipend from the company. She reports Helsinn Therapeutics provided anamorelin and matching placebo for an NIH-funded clinical trial.
Dr. Trikalinos was supported by the D2d study. He is a technical methodological consultant to Latham and Watkins, who is retained by Pacira Pharmaceuticals.
Dr. Angellotti has been employed by Takeda and owns stock in the company.
The editorialists report no relevant financial relationships.
FROM ANNALS OF INTERNAL MEDICINE
FDA OKs sacituzumab govitecan for HR+ metastatic breast cancer
for patients with unresectable, locally advanced or metastatic hormone receptor (HR)–positive, HER2-negative breast cancer after endocrine-based therapy and at least two additional systemic therapies for metastatic disease.
Label expansion for the Trop-2–directed antibody-drug conjugate was based on the TROPICS-02 trial, which randomized 543 adults 1:1 to either sacituzumab govitecan 10 mg/kg IV on days 1 and 8 of a 21-day cycle or single agent chemotherapy, most often eribulin but also vinorelbine, gemcitabine, or capecitabine.
Median progression free survival was 5.5 months with sacituzumab govitecan versus 4 months with single agent chemotherapy (hazard ratio, 0.66; P = .0003). Median overall survival was 14.4 months in the sacituzumab govitecan group versus 11.2 months with chemotherapy (HR, 0.79), according to an FDA press release announcing the approval.
In a Gilead press release, Hope Rugo, MD, a breast cancer specialist at the University of California, San Francisco, and principal investigator for TROPICS-02, said the approval “is significant for the breast cancer community. We have had limited options to offer patients after endocrine-based therapy and chemotherapy, and to see a clinically meaningful survival benefit of more than 3 months with a quality-of-life benefit for these women is exceptional.”
The most common adverse events associated with sacituzumab govitecan in the trial, occurring in a quarter or more of participants, were decreased leukocyte count, decreased neutrophil count, decreased hemoglobin, decreased lymphocyte count, diarrhea, fatigue, nausea, alopecia, glucose elevation, constipation, and decreased albumin.
Labeling for the agent carries a boxedwarning of severe or life-threatening neutropenia and severe diarrhea.
The recommended dose is the trial dose: 10 mg/kg IV on days 1 and 8 of 21-day cycles until disease progression or unacceptable toxicity.
Sacituzumab govitecan was previously approved for unresectable, locally advanced or metastatic triple-negative breast cancer after two or more prior systemic therapies and locally advanced or metastatic urothelial cancer after platinum-based chemotherapy and either a PD-1 or PD-L1 inhibitor.
A version of this article first appeared on Medscape.com.
for patients with unresectable, locally advanced or metastatic hormone receptor (HR)–positive, HER2-negative breast cancer after endocrine-based therapy and at least two additional systemic therapies for metastatic disease.
Label expansion for the Trop-2–directed antibody-drug conjugate was based on the TROPICS-02 trial, which randomized 543 adults 1:1 to either sacituzumab govitecan 10 mg/kg IV on days 1 and 8 of a 21-day cycle or single agent chemotherapy, most often eribulin but also vinorelbine, gemcitabine, or capecitabine.
Median progression free survival was 5.5 months with sacituzumab govitecan versus 4 months with single agent chemotherapy (hazard ratio, 0.66; P = .0003). Median overall survival was 14.4 months in the sacituzumab govitecan group versus 11.2 months with chemotherapy (HR, 0.79), according to an FDA press release announcing the approval.
In a Gilead press release, Hope Rugo, MD, a breast cancer specialist at the University of California, San Francisco, and principal investigator for TROPICS-02, said the approval “is significant for the breast cancer community. We have had limited options to offer patients after endocrine-based therapy and chemotherapy, and to see a clinically meaningful survival benefit of more than 3 months with a quality-of-life benefit for these women is exceptional.”
The most common adverse events associated with sacituzumab govitecan in the trial, occurring in a quarter or more of participants, were decreased leukocyte count, decreased neutrophil count, decreased hemoglobin, decreased lymphocyte count, diarrhea, fatigue, nausea, alopecia, glucose elevation, constipation, and decreased albumin.
Labeling for the agent carries a boxedwarning of severe or life-threatening neutropenia and severe diarrhea.
The recommended dose is the trial dose: 10 mg/kg IV on days 1 and 8 of 21-day cycles until disease progression or unacceptable toxicity.
Sacituzumab govitecan was previously approved for unresectable, locally advanced or metastatic triple-negative breast cancer after two or more prior systemic therapies and locally advanced or metastatic urothelial cancer after platinum-based chemotherapy and either a PD-1 or PD-L1 inhibitor.
A version of this article first appeared on Medscape.com.
for patients with unresectable, locally advanced or metastatic hormone receptor (HR)–positive, HER2-negative breast cancer after endocrine-based therapy and at least two additional systemic therapies for metastatic disease.
Label expansion for the Trop-2–directed antibody-drug conjugate was based on the TROPICS-02 trial, which randomized 543 adults 1:1 to either sacituzumab govitecan 10 mg/kg IV on days 1 and 8 of a 21-day cycle or single agent chemotherapy, most often eribulin but also vinorelbine, gemcitabine, or capecitabine.
Median progression free survival was 5.5 months with sacituzumab govitecan versus 4 months with single agent chemotherapy (hazard ratio, 0.66; P = .0003). Median overall survival was 14.4 months in the sacituzumab govitecan group versus 11.2 months with chemotherapy (HR, 0.79), according to an FDA press release announcing the approval.
In a Gilead press release, Hope Rugo, MD, a breast cancer specialist at the University of California, San Francisco, and principal investigator for TROPICS-02, said the approval “is significant for the breast cancer community. We have had limited options to offer patients after endocrine-based therapy and chemotherapy, and to see a clinically meaningful survival benefit of more than 3 months with a quality-of-life benefit for these women is exceptional.”
The most common adverse events associated with sacituzumab govitecan in the trial, occurring in a quarter or more of participants, were decreased leukocyte count, decreased neutrophil count, decreased hemoglobin, decreased lymphocyte count, diarrhea, fatigue, nausea, alopecia, glucose elevation, constipation, and decreased albumin.
Labeling for the agent carries a boxedwarning of severe or life-threatening neutropenia and severe diarrhea.
The recommended dose is the trial dose: 10 mg/kg IV on days 1 and 8 of 21-day cycles until disease progression or unacceptable toxicity.
Sacituzumab govitecan was previously approved for unresectable, locally advanced or metastatic triple-negative breast cancer after two or more prior systemic therapies and locally advanced or metastatic urothelial cancer after platinum-based chemotherapy and either a PD-1 or PD-L1 inhibitor.
A version of this article first appeared on Medscape.com.
Despite limits, COVID vaccines protect CLL patients
These findings don’t reveal whether the T-cell boost actually provides extra protection against COVID-19. Still, the study suggests that patients with CLL should be vaccinated no matter which medications they’re taking, coauthor and hematologist/oncologist Clemens-Martin Wendtner, MD, of the Munich (Germany) Clinic, said in an interview.
“Do not defer or pause treatment,” said Dr. Wendtner, whose study was published in Blood Advances.
Patients with CLL appear to have among the weakest responses to the COVID-19 vaccine among people with various types of blood cancer. A meta-analysis published in 2022 found that seropositivity rates following vaccination were just 51% in patients with CLL, compared with 80%-90% in those with acute leukemia and 76%-80% of those with myeloma.
“Usually, the response rate to vaccination among the nonimmunocompromised would be 95%,” Dr. Wendtner said.
Research has also suggested that patients treated with B-cell pathway inhibitors and anti-CD20 antibodies are especially likely to have poorer responses to COVID-19 vaccines, no surprise considering that their job is to dampen the immune system. But there’s an unanswered question, according to Dr. Wendtner: Does “just measuring B-cell response tell us everything about the immune response?”
The new prospective, single-institution study aims to answer that question in patients who each received two types of vaccines. Researchers compared peripheral blood mononuclear cell transcriptional response with antibody and T-cell response rates in 15 patients with CLL/small lymphocytic lymphoma following vaccination with both the Pfizer-BioNTech and AstraZeneca vaccines.
The average antibody response was limited. “Overall, 7/15 of patients failed to mount a humoral response even after three-dose vaccination,” the researchers reported. All of the patients were “heavily pretreated” with CLL medications such as venetoclax, an anti-CD20 monoclonal antibody.
By contrast, the T-cell response was much stronger: 80% of patients (12/15) had a robust response, a number that grew to 90% (14/15) after a booster. This response is “almost ideal” considering that the response in a nonimmunocompromised person would be about 99%, Dr. Wendtner said.
The study also revealed that vaccine responses were weaker in patients who took a combination of a Bruton tyrosine kinase inhibitor and venetoclax within a year.
Four patients developed COVID-19 infections with the Omicron variant about 6 months after vaccination. All had mild symptoms. A lone patient had a history of COVID-19 infection prior to vaccination.
The researchers noted that the study had several limitations, including its small size, its reliance on a single institution, and the differences in treatments and vaccination protocols among the patient population.
Broadly speaking, the study showed that “a vaccine is not in vain” in patients with CLL, “although the doctor might not detect an antibody response,” Dr. Wendtner said. He added that mixing vaccine types should provide more protection. Start with a viral vector vaccine followed by an mRNA vaccine or vice versa, he suggested.
In an interview, infectious disease physician Joshua A. Hill, MD, from Fred Hutchinson Cancer Center, Seattle, who wasn’t involved with the study, said it makes “important and interesting observations to reinforce other studies with similar findings.”
Specifically, Dr. Hill said, “despite the absence of a robust antibody response some of these patients who are on active treatment, patients can still generate robust cellular immune responses in the form of T-cell immunity. Our understanding is that having T cell immunity will provide important additional protection for developing severe disease, although is less easily tested.”
As for the best vaccination strategies, Dr. Hill said “patients should get vaccinated as soon as they are eligible, according to standard guidelines. If patients have not yet started therapy, they should get their indicated vaccines before starting treatment whenever possible.”
The German study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases and the Bavarian State Ministry of Science and Art. Dr. Wendtner disclosed consultant fees from AstraZeneca and BioNTech, and another author disclosed consultant fees from AstraZeneca. The other authors reported no disclosures. Dr. Hill disclosed consultant fees from Moderna, Pfizer, and Gilead.
These findings don’t reveal whether the T-cell boost actually provides extra protection against COVID-19. Still, the study suggests that patients with CLL should be vaccinated no matter which medications they’re taking, coauthor and hematologist/oncologist Clemens-Martin Wendtner, MD, of the Munich (Germany) Clinic, said in an interview.
“Do not defer or pause treatment,” said Dr. Wendtner, whose study was published in Blood Advances.
Patients with CLL appear to have among the weakest responses to the COVID-19 vaccine among people with various types of blood cancer. A meta-analysis published in 2022 found that seropositivity rates following vaccination were just 51% in patients with CLL, compared with 80%-90% in those with acute leukemia and 76%-80% of those with myeloma.
“Usually, the response rate to vaccination among the nonimmunocompromised would be 95%,” Dr. Wendtner said.
Research has also suggested that patients treated with B-cell pathway inhibitors and anti-CD20 antibodies are especially likely to have poorer responses to COVID-19 vaccines, no surprise considering that their job is to dampen the immune system. But there’s an unanswered question, according to Dr. Wendtner: Does “just measuring B-cell response tell us everything about the immune response?”
The new prospective, single-institution study aims to answer that question in patients who each received two types of vaccines. Researchers compared peripheral blood mononuclear cell transcriptional response with antibody and T-cell response rates in 15 patients with CLL/small lymphocytic lymphoma following vaccination with both the Pfizer-BioNTech and AstraZeneca vaccines.
The average antibody response was limited. “Overall, 7/15 of patients failed to mount a humoral response even after three-dose vaccination,” the researchers reported. All of the patients were “heavily pretreated” with CLL medications such as venetoclax, an anti-CD20 monoclonal antibody.
By contrast, the T-cell response was much stronger: 80% of patients (12/15) had a robust response, a number that grew to 90% (14/15) after a booster. This response is “almost ideal” considering that the response in a nonimmunocompromised person would be about 99%, Dr. Wendtner said.
The study also revealed that vaccine responses were weaker in patients who took a combination of a Bruton tyrosine kinase inhibitor and venetoclax within a year.
Four patients developed COVID-19 infections with the Omicron variant about 6 months after vaccination. All had mild symptoms. A lone patient had a history of COVID-19 infection prior to vaccination.
The researchers noted that the study had several limitations, including its small size, its reliance on a single institution, and the differences in treatments and vaccination protocols among the patient population.
Broadly speaking, the study showed that “a vaccine is not in vain” in patients with CLL, “although the doctor might not detect an antibody response,” Dr. Wendtner said. He added that mixing vaccine types should provide more protection. Start with a viral vector vaccine followed by an mRNA vaccine or vice versa, he suggested.
In an interview, infectious disease physician Joshua A. Hill, MD, from Fred Hutchinson Cancer Center, Seattle, who wasn’t involved with the study, said it makes “important and interesting observations to reinforce other studies with similar findings.”
Specifically, Dr. Hill said, “despite the absence of a robust antibody response some of these patients who are on active treatment, patients can still generate robust cellular immune responses in the form of T-cell immunity. Our understanding is that having T cell immunity will provide important additional protection for developing severe disease, although is less easily tested.”
As for the best vaccination strategies, Dr. Hill said “patients should get vaccinated as soon as they are eligible, according to standard guidelines. If patients have not yet started therapy, they should get their indicated vaccines before starting treatment whenever possible.”
The German study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases and the Bavarian State Ministry of Science and Art. Dr. Wendtner disclosed consultant fees from AstraZeneca and BioNTech, and another author disclosed consultant fees from AstraZeneca. The other authors reported no disclosures. Dr. Hill disclosed consultant fees from Moderna, Pfizer, and Gilead.
These findings don’t reveal whether the T-cell boost actually provides extra protection against COVID-19. Still, the study suggests that patients with CLL should be vaccinated no matter which medications they’re taking, coauthor and hematologist/oncologist Clemens-Martin Wendtner, MD, of the Munich (Germany) Clinic, said in an interview.
“Do not defer or pause treatment,” said Dr. Wendtner, whose study was published in Blood Advances.
Patients with CLL appear to have among the weakest responses to the COVID-19 vaccine among people with various types of blood cancer. A meta-analysis published in 2022 found that seropositivity rates following vaccination were just 51% in patients with CLL, compared with 80%-90% in those with acute leukemia and 76%-80% of those with myeloma.
“Usually, the response rate to vaccination among the nonimmunocompromised would be 95%,” Dr. Wendtner said.
Research has also suggested that patients treated with B-cell pathway inhibitors and anti-CD20 antibodies are especially likely to have poorer responses to COVID-19 vaccines, no surprise considering that their job is to dampen the immune system. But there’s an unanswered question, according to Dr. Wendtner: Does “just measuring B-cell response tell us everything about the immune response?”
The new prospective, single-institution study aims to answer that question in patients who each received two types of vaccines. Researchers compared peripheral blood mononuclear cell transcriptional response with antibody and T-cell response rates in 15 patients with CLL/small lymphocytic lymphoma following vaccination with both the Pfizer-BioNTech and AstraZeneca vaccines.
The average antibody response was limited. “Overall, 7/15 of patients failed to mount a humoral response even after three-dose vaccination,” the researchers reported. All of the patients were “heavily pretreated” with CLL medications such as venetoclax, an anti-CD20 monoclonal antibody.
By contrast, the T-cell response was much stronger: 80% of patients (12/15) had a robust response, a number that grew to 90% (14/15) after a booster. This response is “almost ideal” considering that the response in a nonimmunocompromised person would be about 99%, Dr. Wendtner said.
The study also revealed that vaccine responses were weaker in patients who took a combination of a Bruton tyrosine kinase inhibitor and venetoclax within a year.
Four patients developed COVID-19 infections with the Omicron variant about 6 months after vaccination. All had mild symptoms. A lone patient had a history of COVID-19 infection prior to vaccination.
The researchers noted that the study had several limitations, including its small size, its reliance on a single institution, and the differences in treatments and vaccination protocols among the patient population.
Broadly speaking, the study showed that “a vaccine is not in vain” in patients with CLL, “although the doctor might not detect an antibody response,” Dr. Wendtner said. He added that mixing vaccine types should provide more protection. Start with a viral vector vaccine followed by an mRNA vaccine or vice versa, he suggested.
In an interview, infectious disease physician Joshua A. Hill, MD, from Fred Hutchinson Cancer Center, Seattle, who wasn’t involved with the study, said it makes “important and interesting observations to reinforce other studies with similar findings.”
Specifically, Dr. Hill said, “despite the absence of a robust antibody response some of these patients who are on active treatment, patients can still generate robust cellular immune responses in the form of T-cell immunity. Our understanding is that having T cell immunity will provide important additional protection for developing severe disease, although is less easily tested.”
As for the best vaccination strategies, Dr. Hill said “patients should get vaccinated as soon as they are eligible, according to standard guidelines. If patients have not yet started therapy, they should get their indicated vaccines before starting treatment whenever possible.”
The German study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases and the Bavarian State Ministry of Science and Art. Dr. Wendtner disclosed consultant fees from AstraZeneca and BioNTech, and another author disclosed consultant fees from AstraZeneca. The other authors reported no disclosures. Dr. Hill disclosed consultant fees from Moderna, Pfizer, and Gilead.
FROM BLOOD ADVANCES
First Humira biosimilar launches in U.S.
The first biosimilar for Humira, adalimumab-atto (Amjevita), is now available in the United States, according to an announcement on Jan. 31 by the manufacturer, Amgen. At least seven other U.S. Food and Drug Administration–approved Humira biosimilars are expected to become available later in 2023.
Amjevita was approved by the FDA in September 2016 for multiple inflammatory diseases, including rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis. The delayed launch was part of a global settlement with Humira’s manufacturer, AbbVie.
Humira (adalimumab) has been available since 2002 and is consistently one of the top-selling drugs in the United States. A single 40-mg Amjevita pen device will be available at two prices: a list price (wholesale acquisition cost) of $1,557.59, 55% below the current Humira list price, and a list price of $3,288.24, 5% below the current Humira list price, according to Amgen.
“Amgen’s goal is to provide broad access for patients by offering two options to health plans and pharmacy benefit managers,” the company said in the press release.
Patients are less likely to benefit from the more significant discount, said Marta Wosinska, PhD, a health care economist at the Brookings Institute in Washington, DC. It's expected that insurance companies will use the higher list price for Amjevita, she said, as this higher price will also likely have higher rebates. Rebates are payments to health insurance payers provided by drug manufacturers to promote use of an expensive drug. Some pharmacy benefit managers have already said that they plan to charge patients the same amount for Humira as its biosimilars, Dr. Wosinska said.
"For an existing patient, there's really no incentive for them to switch," she said in an interview.
So far only one insurance company, Kaiser Permanente, has plans to switch patients over to biosimilars, according to the health policy podcast Tradeoffs, and the insurer will stop covering Humira by the end of this year.
A version of this article first appeared on Medscape.com.
*This story was updated 2/1/2023.
The first biosimilar for Humira, adalimumab-atto (Amjevita), is now available in the United States, according to an announcement on Jan. 31 by the manufacturer, Amgen. At least seven other U.S. Food and Drug Administration–approved Humira biosimilars are expected to become available later in 2023.
Amjevita was approved by the FDA in September 2016 for multiple inflammatory diseases, including rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis. The delayed launch was part of a global settlement with Humira’s manufacturer, AbbVie.
Humira (adalimumab) has been available since 2002 and is consistently one of the top-selling drugs in the United States. A single 40-mg Amjevita pen device will be available at two prices: a list price (wholesale acquisition cost) of $1,557.59, 55% below the current Humira list price, and a list price of $3,288.24, 5% below the current Humira list price, according to Amgen.
“Amgen’s goal is to provide broad access for patients by offering two options to health plans and pharmacy benefit managers,” the company said in the press release.
Patients are less likely to benefit from the more significant discount, said Marta Wosinska, PhD, a health care economist at the Brookings Institute in Washington, DC. It's expected that insurance companies will use the higher list price for Amjevita, she said, as this higher price will also likely have higher rebates. Rebates are payments to health insurance payers provided by drug manufacturers to promote use of an expensive drug. Some pharmacy benefit managers have already said that they plan to charge patients the same amount for Humira as its biosimilars, Dr. Wosinska said.
"For an existing patient, there's really no incentive for them to switch," she said in an interview.
So far only one insurance company, Kaiser Permanente, has plans to switch patients over to biosimilars, according to the health policy podcast Tradeoffs, and the insurer will stop covering Humira by the end of this year.
A version of this article first appeared on Medscape.com.
*This story was updated 2/1/2023.
The first biosimilar for Humira, adalimumab-atto (Amjevita), is now available in the United States, according to an announcement on Jan. 31 by the manufacturer, Amgen. At least seven other U.S. Food and Drug Administration–approved Humira biosimilars are expected to become available later in 2023.
Amjevita was approved by the FDA in September 2016 for multiple inflammatory diseases, including rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis. The delayed launch was part of a global settlement with Humira’s manufacturer, AbbVie.
Humira (adalimumab) has been available since 2002 and is consistently one of the top-selling drugs in the United States. A single 40-mg Amjevita pen device will be available at two prices: a list price (wholesale acquisition cost) of $1,557.59, 55% below the current Humira list price, and a list price of $3,288.24, 5% below the current Humira list price, according to Amgen.
“Amgen’s goal is to provide broad access for patients by offering two options to health plans and pharmacy benefit managers,” the company said in the press release.
Patients are less likely to benefit from the more significant discount, said Marta Wosinska, PhD, a health care economist at the Brookings Institute in Washington, DC. It's expected that insurance companies will use the higher list price for Amjevita, she said, as this higher price will also likely have higher rebates. Rebates are payments to health insurance payers provided by drug manufacturers to promote use of an expensive drug. Some pharmacy benefit managers have already said that they plan to charge patients the same amount for Humira as its biosimilars, Dr. Wosinska said.
"For an existing patient, there's really no incentive for them to switch," she said in an interview.
So far only one insurance company, Kaiser Permanente, has plans to switch patients over to biosimilars, according to the health policy podcast Tradeoffs, and the insurer will stop covering Humira by the end of this year.
A version of this article first appeared on Medscape.com.
*This story was updated 2/1/2023.
Angioedema risk jumps when switching HF meds
New renin-angiotensin-system (RAS) inhibitor therapy using sacubitril-valsartan (Entresto) is no more likely to cause angioedema than starting out with an ACE inhibitor or angiotensin receptor blocker (ARB).
But the risk climbs when such patients start on an ACE inhibitor or ARB and then switch to sacubitril-valsartan, compared with those prescribed the newer drug, the only available angiotensin receptor-neprilysin inhibitor (ARNI), in the first place.
Those findings and others from a large database analysis, by researchers at the Food and Drug Administration and Harvard Medical School, may clarify and help alleviate a residual safety concern about the ARNI – that it might promote angioedema – that persists after the drug’s major HF trials.
The angioedema risk increased the most right after the switch to the ARNI from one of the older RAS inhibitors. For example, the overall risk doubled for patients who started with an ARB then switched to sacubitril-valsartan, compared with those who started on the newer drug. But it went up about 2.5 times during the first 14 days after the switch.
A similar pattern emerged for ACE inhibitors, but the increased angioedema risk reached significance only within 2 weeks of the switch from an ACE inhibitor to sacubitril-valsartan compared to starting on the latter.
The analysis, based on data from the FDA’s Sentinel adverse event reporting system, was published in the Journal of the American College of Cardiology.
A rare complication, but ...
Angioedema was rare overall in the study, with an unadjusted rate of about 6.75 per 1,000 person-years for users of ACE inhibitors, less than half that rate for ARB users, and only one-fifth that rate for sacubitril-valsartan recipients.
But even a rare complication can be a worry for drugs as widely used as RAS inhibitors. And it’s not unusual for patients cautiously started on an ACE inhibitor or ARB to be switched to sacubitril-valsartan, which is only recently a core guideline–recommended therapy for HF with reduced ejection fraction.
Such patients transitioning to the ARNI, the current study suggests, should probably be watched closely for signs of angioedema for 2 weeks but especially during the first few days. Indeed, the study’s event curves show most of the extra risk “popping up” right after the switch to sacubitril-valsartan, lead author Efe Eworuke, PhD, told this news organization.
The ARNI’s labeling, which states the drug should follow ACE inhibitors only after 36-hour washout period, “has done justice to this issue,” she said. But “whether clinicians are adhering to that, we can’t tell.”
Potentially, patients who miss the 36-hour washout between ACE inhibitors or ARBs and sacubitril-valsartan may account for the excess angioedema risk seen in the analysis, said Dr. Eworuke, with the FDA’s Center for Drug Evaluation and Research, Silver Spring, Md.
But the analysis doesn’t nail down the window of excess risk to only 36 hours. It suggests that patients switching to the ARNI – even those pausing for 36 hours in between drugs – should probably be monitored “2 weeks or longer,” she said. “They could still have angioedema after the washout period.”
Indeed, the “timing of the switch may be critical,” according to an editorial accompanying the report. “Perhaps a longer initial exposure period of ACE inhibitor or ARB,” beyond 2 weeks, “should be considered before switching to an ARNI,” contended Robert L. Page II, PharmD, MSPH, University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences, Aurora.
Moreover, he wrote, the study suggests that “initiation of an ARNI de novo may be safer compared with trialing an ACE inhibitor or ARB then switching to an ARNI,” and “should be a consideration when beginning guideline-directed medical therapy for patients with HF.”
New RAS inhibition with ARNI ‘protective’
Compared with ARNI “new users” who had not received any RAS inhibitor in the prior 6 months, patients in the study who switched from an ACE inhibitor to ARNI (41,548 matched pairs) showed a hazard ratio (HR) for angioedema of 1.62 (95% confidence interval [CI], 0.91-2.89), that is, only a “trend,” the report states.
But that trend became significant when the analysis considered only angioedema cases in the first 14 days after the drug switch: HR, 1.98 (95% CI, 1.11-3.53).
Those switching from an ARB to ARNI, compared with ARNI new users (37,893 matched pairs), showed a significant HR for angioedema of 2.03 (95% CI, 1.16-3.54). The effect was more pronounced when considering only angioedema arising in the first 2 weeks: HR, 2.45 (95% CI, 1.36-4.43).
Compared with new use of ACE inhibitors, new ARNI use (41,998 matched pairs) was “protective,” the report states, with an HR for angioedema of 0.18 (95% CI, 0.11-0.29). So was a switch from ACE inhibitors to the ARNI (69,639 matched pairs), with an HR of 0.31 (95% CI, 0.23-0.43).
But compared with starting with an ARB, ARNI new use (43,755 matched pairs) had a null effect on angioedema risk, HR, 0.59 (95% CI, 0.35-1.01); as did switching from an ARB to ARNI (49,137 matched pairs), HR, 0.85 (95% CI, 0.58-1.26).
The analysis has limitations, Dr. Eworuke acknowledged. The comparator groups probably differed in unknown ways given the limits of propensity matching, for example, and because the FDA’s Sentinel system data can reflect only cases that are reported, the study probably underestimates the true prevalence of angioedema.
For example, a patient may see a clinician for a milder case that resolves without a significant intervention, she noted. But “those types of angioedema would not have been captured by our study.”
Dr. Eworuke disclosed that her comments reflect her views and are not those of the Food and Drug Administration; she and the other authors, as well as editorialist Dr. Page, report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New renin-angiotensin-system (RAS) inhibitor therapy using sacubitril-valsartan (Entresto) is no more likely to cause angioedema than starting out with an ACE inhibitor or angiotensin receptor blocker (ARB).
But the risk climbs when such patients start on an ACE inhibitor or ARB and then switch to sacubitril-valsartan, compared with those prescribed the newer drug, the only available angiotensin receptor-neprilysin inhibitor (ARNI), in the first place.
Those findings and others from a large database analysis, by researchers at the Food and Drug Administration and Harvard Medical School, may clarify and help alleviate a residual safety concern about the ARNI – that it might promote angioedema – that persists after the drug’s major HF trials.
The angioedema risk increased the most right after the switch to the ARNI from one of the older RAS inhibitors. For example, the overall risk doubled for patients who started with an ARB then switched to sacubitril-valsartan, compared with those who started on the newer drug. But it went up about 2.5 times during the first 14 days after the switch.
A similar pattern emerged for ACE inhibitors, but the increased angioedema risk reached significance only within 2 weeks of the switch from an ACE inhibitor to sacubitril-valsartan compared to starting on the latter.
The analysis, based on data from the FDA’s Sentinel adverse event reporting system, was published in the Journal of the American College of Cardiology.
A rare complication, but ...
Angioedema was rare overall in the study, with an unadjusted rate of about 6.75 per 1,000 person-years for users of ACE inhibitors, less than half that rate for ARB users, and only one-fifth that rate for sacubitril-valsartan recipients.
But even a rare complication can be a worry for drugs as widely used as RAS inhibitors. And it’s not unusual for patients cautiously started on an ACE inhibitor or ARB to be switched to sacubitril-valsartan, which is only recently a core guideline–recommended therapy for HF with reduced ejection fraction.
Such patients transitioning to the ARNI, the current study suggests, should probably be watched closely for signs of angioedema for 2 weeks but especially during the first few days. Indeed, the study’s event curves show most of the extra risk “popping up” right after the switch to sacubitril-valsartan, lead author Efe Eworuke, PhD, told this news organization.
The ARNI’s labeling, which states the drug should follow ACE inhibitors only after 36-hour washout period, “has done justice to this issue,” she said. But “whether clinicians are adhering to that, we can’t tell.”
Potentially, patients who miss the 36-hour washout between ACE inhibitors or ARBs and sacubitril-valsartan may account for the excess angioedema risk seen in the analysis, said Dr. Eworuke, with the FDA’s Center for Drug Evaluation and Research, Silver Spring, Md.
But the analysis doesn’t nail down the window of excess risk to only 36 hours. It suggests that patients switching to the ARNI – even those pausing for 36 hours in between drugs – should probably be monitored “2 weeks or longer,” she said. “They could still have angioedema after the washout period.”
Indeed, the “timing of the switch may be critical,” according to an editorial accompanying the report. “Perhaps a longer initial exposure period of ACE inhibitor or ARB,” beyond 2 weeks, “should be considered before switching to an ARNI,” contended Robert L. Page II, PharmD, MSPH, University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences, Aurora.
Moreover, he wrote, the study suggests that “initiation of an ARNI de novo may be safer compared with trialing an ACE inhibitor or ARB then switching to an ARNI,” and “should be a consideration when beginning guideline-directed medical therapy for patients with HF.”
New RAS inhibition with ARNI ‘protective’
Compared with ARNI “new users” who had not received any RAS inhibitor in the prior 6 months, patients in the study who switched from an ACE inhibitor to ARNI (41,548 matched pairs) showed a hazard ratio (HR) for angioedema of 1.62 (95% confidence interval [CI], 0.91-2.89), that is, only a “trend,” the report states.
But that trend became significant when the analysis considered only angioedema cases in the first 14 days after the drug switch: HR, 1.98 (95% CI, 1.11-3.53).
Those switching from an ARB to ARNI, compared with ARNI new users (37,893 matched pairs), showed a significant HR for angioedema of 2.03 (95% CI, 1.16-3.54). The effect was more pronounced when considering only angioedema arising in the first 2 weeks: HR, 2.45 (95% CI, 1.36-4.43).
Compared with new use of ACE inhibitors, new ARNI use (41,998 matched pairs) was “protective,” the report states, with an HR for angioedema of 0.18 (95% CI, 0.11-0.29). So was a switch from ACE inhibitors to the ARNI (69,639 matched pairs), with an HR of 0.31 (95% CI, 0.23-0.43).
But compared with starting with an ARB, ARNI new use (43,755 matched pairs) had a null effect on angioedema risk, HR, 0.59 (95% CI, 0.35-1.01); as did switching from an ARB to ARNI (49,137 matched pairs), HR, 0.85 (95% CI, 0.58-1.26).
The analysis has limitations, Dr. Eworuke acknowledged. The comparator groups probably differed in unknown ways given the limits of propensity matching, for example, and because the FDA’s Sentinel system data can reflect only cases that are reported, the study probably underestimates the true prevalence of angioedema.
For example, a patient may see a clinician for a milder case that resolves without a significant intervention, she noted. But “those types of angioedema would not have been captured by our study.”
Dr. Eworuke disclosed that her comments reflect her views and are not those of the Food and Drug Administration; she and the other authors, as well as editorialist Dr. Page, report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New renin-angiotensin-system (RAS) inhibitor therapy using sacubitril-valsartan (Entresto) is no more likely to cause angioedema than starting out with an ACE inhibitor or angiotensin receptor blocker (ARB).
But the risk climbs when such patients start on an ACE inhibitor or ARB and then switch to sacubitril-valsartan, compared with those prescribed the newer drug, the only available angiotensin receptor-neprilysin inhibitor (ARNI), in the first place.
Those findings and others from a large database analysis, by researchers at the Food and Drug Administration and Harvard Medical School, may clarify and help alleviate a residual safety concern about the ARNI – that it might promote angioedema – that persists after the drug’s major HF trials.
The angioedema risk increased the most right after the switch to the ARNI from one of the older RAS inhibitors. For example, the overall risk doubled for patients who started with an ARB then switched to sacubitril-valsartan, compared with those who started on the newer drug. But it went up about 2.5 times during the first 14 days after the switch.
A similar pattern emerged for ACE inhibitors, but the increased angioedema risk reached significance only within 2 weeks of the switch from an ACE inhibitor to sacubitril-valsartan compared to starting on the latter.
The analysis, based on data from the FDA’s Sentinel adverse event reporting system, was published in the Journal of the American College of Cardiology.
A rare complication, but ...
Angioedema was rare overall in the study, with an unadjusted rate of about 6.75 per 1,000 person-years for users of ACE inhibitors, less than half that rate for ARB users, and only one-fifth that rate for sacubitril-valsartan recipients.
But even a rare complication can be a worry for drugs as widely used as RAS inhibitors. And it’s not unusual for patients cautiously started on an ACE inhibitor or ARB to be switched to sacubitril-valsartan, which is only recently a core guideline–recommended therapy for HF with reduced ejection fraction.
Such patients transitioning to the ARNI, the current study suggests, should probably be watched closely for signs of angioedema for 2 weeks but especially during the first few days. Indeed, the study’s event curves show most of the extra risk “popping up” right after the switch to sacubitril-valsartan, lead author Efe Eworuke, PhD, told this news organization.
The ARNI’s labeling, which states the drug should follow ACE inhibitors only after 36-hour washout period, “has done justice to this issue,” she said. But “whether clinicians are adhering to that, we can’t tell.”
Potentially, patients who miss the 36-hour washout between ACE inhibitors or ARBs and sacubitril-valsartan may account for the excess angioedema risk seen in the analysis, said Dr. Eworuke, with the FDA’s Center for Drug Evaluation and Research, Silver Spring, Md.
But the analysis doesn’t nail down the window of excess risk to only 36 hours. It suggests that patients switching to the ARNI – even those pausing for 36 hours in between drugs – should probably be monitored “2 weeks or longer,” she said. “They could still have angioedema after the washout period.”
Indeed, the “timing of the switch may be critical,” according to an editorial accompanying the report. “Perhaps a longer initial exposure period of ACE inhibitor or ARB,” beyond 2 weeks, “should be considered before switching to an ARNI,” contended Robert L. Page II, PharmD, MSPH, University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences, Aurora.
Moreover, he wrote, the study suggests that “initiation of an ARNI de novo may be safer compared with trialing an ACE inhibitor or ARB then switching to an ARNI,” and “should be a consideration when beginning guideline-directed medical therapy for patients with HF.”
New RAS inhibition with ARNI ‘protective’
Compared with ARNI “new users” who had not received any RAS inhibitor in the prior 6 months, patients in the study who switched from an ACE inhibitor to ARNI (41,548 matched pairs) showed a hazard ratio (HR) for angioedema of 1.62 (95% confidence interval [CI], 0.91-2.89), that is, only a “trend,” the report states.
But that trend became significant when the analysis considered only angioedema cases in the first 14 days after the drug switch: HR, 1.98 (95% CI, 1.11-3.53).
Those switching from an ARB to ARNI, compared with ARNI new users (37,893 matched pairs), showed a significant HR for angioedema of 2.03 (95% CI, 1.16-3.54). The effect was more pronounced when considering only angioedema arising in the first 2 weeks: HR, 2.45 (95% CI, 1.36-4.43).
Compared with new use of ACE inhibitors, new ARNI use (41,998 matched pairs) was “protective,” the report states, with an HR for angioedema of 0.18 (95% CI, 0.11-0.29). So was a switch from ACE inhibitors to the ARNI (69,639 matched pairs), with an HR of 0.31 (95% CI, 0.23-0.43).
But compared with starting with an ARB, ARNI new use (43,755 matched pairs) had a null effect on angioedema risk, HR, 0.59 (95% CI, 0.35-1.01); as did switching from an ARB to ARNI (49,137 matched pairs), HR, 0.85 (95% CI, 0.58-1.26).
The analysis has limitations, Dr. Eworuke acknowledged. The comparator groups probably differed in unknown ways given the limits of propensity matching, for example, and because the FDA’s Sentinel system data can reflect only cases that are reported, the study probably underestimates the true prevalence of angioedema.
For example, a patient may see a clinician for a milder case that resolves without a significant intervention, she noted. But “those types of angioedema would not have been captured by our study.”
Dr. Eworuke disclosed that her comments reflect her views and are not those of the Food and Drug Administration; she and the other authors, as well as editorialist Dr. Page, report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
FDA approves pirtobrutinib for r/r mantle cell lymphoma
Pirtobrutinib is the first and only noncovalent Bruton’s tyrosine kinase inhibitor approved for use in this MCL setting, manufacturer Eli Lilly noted in a press release.
“The approval of Jaypirca represents an important advance for patients with relapsed or refractory MCL, who currently have limited options and historically have had a poor prognosis following discontinuation of treatment with a covalent Bruton’s tyrosine kinase inhibitor,” senior author Michael Wang, MD, University of Texas MD Anderson Cancer Center, Houston, said in the release.
The approval was based on efficacy demonstrated in the open-label, single-arm, phase 1/2 BRUIN trial – a multicenter study assessing 200 mg once-daily oral pirtobrutinib monotherapy in 120 patients with MCL who had previously received a Bruton’s tyrosine kinase inhibitor, most often ibrutinib (Imbruvica, 67%) acalabrutinib (Calquence, 30%) and zanubrutinib (Brukinsa, 8%). Pirtobrutinib was continued until disease progression or unacceptable toxicity.
Study participants had a median of three prior lines of therapy, and 83% discontinued their last Bruton’s tyrosine kinase inhibitor because of refractory or progressive disease.
The overall response rate in pirtobrutinib-treated patients was 50% with a complete response rate of 13%. Estimated median duration of response was 8.3 months, and the estimated duration of response at 6 months occurred in nearly two-thirds of patients.
Adverse reactions that occurred in at least 15% of patients included fatigue, musculoskeletal pain, diarrhea, edema, dyspnea, pneumonia, and bruising. Grade 3 or 4 laboratory abnormalities occurring in at least 10% of patients included decreased neutrophil counts, lymphocyte counts, and platelet counts.
Prescribing information for pirtobrutinib includes warnings and precautions for infections, hemorrhage, cytopenias, atrial fibrillation and flutter, and second primary malignancies, noted the FDA, which granted priority review, fast track designation, and orphan drug designation for the application submitted by Eli Lilly.
“Jaypirca can reestablish Bruton’s tyrosine kinase inhibition in MCL patients previously treated with a covalent Bruton’s tyrosine kinase inhibitor (ibrutinib, acalabrutinib, or zanubrutinib) and extend the benefit of targeting the Bruton’s tyrosine kinase pathway,” according to Eli Lilly’s release.
Dr. Wang added that the agent “has the potential to meaningfully impact the treatment paradigm for relapsed and refractory MCL patients.”
Meghan Gutierrez, CEO at the Lymphoma Research Foundation, also noted that “the approval of Jaypirca brings a new treatment option and, along with that, new hope for people with relapsed or refractory MCL.”
The drug is expected to be available in the United States in the coming weeks, and the confirmatory phase 3 BRUIN trial is currently enrolling patients, Eli Lilly announced. The company also indicated the list price would be $21,000 for a 30-day supply of the 200-mg dose.
Serious adverse events believed to be associated with the use of pirtobrutinib or any medicine or device should be reported to the FDA’s MedWatch Reporting System or by calling 1-800-FDA-1088.
A version of this article first appeared on Medscape.com.
Pirtobrutinib is the first and only noncovalent Bruton’s tyrosine kinase inhibitor approved for use in this MCL setting, manufacturer Eli Lilly noted in a press release.
“The approval of Jaypirca represents an important advance for patients with relapsed or refractory MCL, who currently have limited options and historically have had a poor prognosis following discontinuation of treatment with a covalent Bruton’s tyrosine kinase inhibitor,” senior author Michael Wang, MD, University of Texas MD Anderson Cancer Center, Houston, said in the release.
The approval was based on efficacy demonstrated in the open-label, single-arm, phase 1/2 BRUIN trial – a multicenter study assessing 200 mg once-daily oral pirtobrutinib monotherapy in 120 patients with MCL who had previously received a Bruton’s tyrosine kinase inhibitor, most often ibrutinib (Imbruvica, 67%) acalabrutinib (Calquence, 30%) and zanubrutinib (Brukinsa, 8%). Pirtobrutinib was continued until disease progression or unacceptable toxicity.
Study participants had a median of three prior lines of therapy, and 83% discontinued their last Bruton’s tyrosine kinase inhibitor because of refractory or progressive disease.
The overall response rate in pirtobrutinib-treated patients was 50% with a complete response rate of 13%. Estimated median duration of response was 8.3 months, and the estimated duration of response at 6 months occurred in nearly two-thirds of patients.
Adverse reactions that occurred in at least 15% of patients included fatigue, musculoskeletal pain, diarrhea, edema, dyspnea, pneumonia, and bruising. Grade 3 or 4 laboratory abnormalities occurring in at least 10% of patients included decreased neutrophil counts, lymphocyte counts, and platelet counts.
Prescribing information for pirtobrutinib includes warnings and precautions for infections, hemorrhage, cytopenias, atrial fibrillation and flutter, and second primary malignancies, noted the FDA, which granted priority review, fast track designation, and orphan drug designation for the application submitted by Eli Lilly.
“Jaypirca can reestablish Bruton’s tyrosine kinase inhibition in MCL patients previously treated with a covalent Bruton’s tyrosine kinase inhibitor (ibrutinib, acalabrutinib, or zanubrutinib) and extend the benefit of targeting the Bruton’s tyrosine kinase pathway,” according to Eli Lilly’s release.
Dr. Wang added that the agent “has the potential to meaningfully impact the treatment paradigm for relapsed and refractory MCL patients.”
Meghan Gutierrez, CEO at the Lymphoma Research Foundation, also noted that “the approval of Jaypirca brings a new treatment option and, along with that, new hope for people with relapsed or refractory MCL.”
The drug is expected to be available in the United States in the coming weeks, and the confirmatory phase 3 BRUIN trial is currently enrolling patients, Eli Lilly announced. The company also indicated the list price would be $21,000 for a 30-day supply of the 200-mg dose.
Serious adverse events believed to be associated with the use of pirtobrutinib or any medicine or device should be reported to the FDA’s MedWatch Reporting System or by calling 1-800-FDA-1088.
A version of this article first appeared on Medscape.com.
Pirtobrutinib is the first and only noncovalent Bruton’s tyrosine kinase inhibitor approved for use in this MCL setting, manufacturer Eli Lilly noted in a press release.
“The approval of Jaypirca represents an important advance for patients with relapsed or refractory MCL, who currently have limited options and historically have had a poor prognosis following discontinuation of treatment with a covalent Bruton’s tyrosine kinase inhibitor,” senior author Michael Wang, MD, University of Texas MD Anderson Cancer Center, Houston, said in the release.
The approval was based on efficacy demonstrated in the open-label, single-arm, phase 1/2 BRUIN trial – a multicenter study assessing 200 mg once-daily oral pirtobrutinib monotherapy in 120 patients with MCL who had previously received a Bruton’s tyrosine kinase inhibitor, most often ibrutinib (Imbruvica, 67%) acalabrutinib (Calquence, 30%) and zanubrutinib (Brukinsa, 8%). Pirtobrutinib was continued until disease progression or unacceptable toxicity.
Study participants had a median of three prior lines of therapy, and 83% discontinued their last Bruton’s tyrosine kinase inhibitor because of refractory or progressive disease.
The overall response rate in pirtobrutinib-treated patients was 50% with a complete response rate of 13%. Estimated median duration of response was 8.3 months, and the estimated duration of response at 6 months occurred in nearly two-thirds of patients.
Adverse reactions that occurred in at least 15% of patients included fatigue, musculoskeletal pain, diarrhea, edema, dyspnea, pneumonia, and bruising. Grade 3 or 4 laboratory abnormalities occurring in at least 10% of patients included decreased neutrophil counts, lymphocyte counts, and platelet counts.
Prescribing information for pirtobrutinib includes warnings and precautions for infections, hemorrhage, cytopenias, atrial fibrillation and flutter, and second primary malignancies, noted the FDA, which granted priority review, fast track designation, and orphan drug designation for the application submitted by Eli Lilly.
“Jaypirca can reestablish Bruton’s tyrosine kinase inhibition in MCL patients previously treated with a covalent Bruton’s tyrosine kinase inhibitor (ibrutinib, acalabrutinib, or zanubrutinib) and extend the benefit of targeting the Bruton’s tyrosine kinase pathway,” according to Eli Lilly’s release.
Dr. Wang added that the agent “has the potential to meaningfully impact the treatment paradigm for relapsed and refractory MCL patients.”
Meghan Gutierrez, CEO at the Lymphoma Research Foundation, also noted that “the approval of Jaypirca brings a new treatment option and, along with that, new hope for people with relapsed or refractory MCL.”
The drug is expected to be available in the United States in the coming weeks, and the confirmatory phase 3 BRUIN trial is currently enrolling patients, Eli Lilly announced. The company also indicated the list price would be $21,000 for a 30-day supply of the 200-mg dose.
Serious adverse events believed to be associated with the use of pirtobrutinib or any medicine or device should be reported to the FDA’s MedWatch Reporting System or by calling 1-800-FDA-1088.
A version of this article first appeared on Medscape.com.
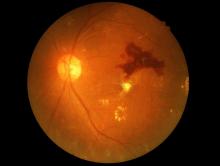
Eye check important before starting semaglutide for diabetes
A small potential increased risk of retinopathy worsening at 1 year with injected semaglutide (Ozempic, Novo Nordisk), a glucagon-like peptide 1 (GLP-1) agonist approved for type 2 diabetes, doesn’t outweigh the drug’s cardiovascular benefits but does highlight the need for baseline ophthalmologic evaluation before initiating treatment and ongoing retinal monitoring, researchers say.
That conclusion was based on data from a meta-analysis of the seven major cardiovascular outcomes trials of GLP-1 agonists currently on the market.
The findings were recently published in Diabetes & Metabolic Syndrome: Clinical Research & Reviews, by Stewart G. Albert, MD, and colleagues.
Concerns about retinopathy worsening with the GLP-1 agonist drug class first arose from the SUSTAIN-6 cardiovascular outcomes trial for injectable semaglutide, although a subsequent analysis of data from that trial appeared to suggest the problem is likely due to rapid glucose-lowering in already vulnerable patients rather than a drug-specific effect. This effect had been previously reported, most notably in the landmark Diabetes Control and Complications Trial.
In this new meta-analysis, “we showed that with improvements in A1c there were correlations with decreases in the rate of cardiovascular events but increases in the rate of retinopathy,” Dr. Albert, of St. Louis University, told this news organization.
“As a class of drugs, we did not find an increased rate of retinopathy. The effect of GLP-1 agonists on retinopathy did not appear to be due to an immediate direct toxic effect of the drug. The worsening of the rate of retinopathy was seen with semaglutide after 1 year of therapy and when there was a decrease in A1c of 1%,” he explained.
He noted that because the increased risk was seen primarily among those who already had retinopathy at baseline, “it would seem prudent to know the level of retinopathy either before or plan for close ophthalmologic monitoring around the time of drug initiation ... We routinely evaluate patients with known type 2 diabetes mellitus at yearly intervals for retinopathy. From our data, we saw worsening at 1 year of drug exposure, but we do not know the exact time when the changes occurred during that year.”
The Ozempic label advises that “patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy” but doesn’t specifically mention baseline assessment at the time of drug initiation.
No increase in retinopathy risk for GLP-1 agonist class overall
The seven trials in the meta-analysis comprised 56,004 participants, with baseline retinopathy prevalence ranging from 9% to 31%.
For the GLP-1 agonist class overall, there was no significant increase in the relative rate (RR) of retinopathy (RR, 1.09; P = .36), while there were significant reductions in relative rates of major adverse cardiac events, overall deaths, and cardiovascular deaths (all P < .001 or P = .001).
The increased retinopathy risk was seen only in the subcutaneous semaglutide group (RR, 1.73; P = .02).
The overall number needed to harm was 1,000 and the number to treat was 77. For semaglutide, those values were 77 and 43, respectively.
There was a significant correlation between a decrease in major adverse cardiac events and a decrease in A1c (P = .014), while for retinopathy, the risk increased with improved A1c (P = .076).
Semaglutide subanalysis finds increased retinopathy worsening
Dr. Albert and colleagues conducted a separate subanalysis of 11 studies of semaglutide that enrolled 11,894 patients, of which 6 studies (n = 5,610) were of oral semaglutide (Rybelsus) and 5 studies were of subcutaneous semaglutide (Ozempic; n = 6,284).
In the subanalysis, there was an overall increase in relative rates of new or worsening retinopathy (RR, 1.218; P = .049).
The change in relative rate of retinopathy was predominantly found for subcutaneous semaglutide given for longer than 1 year (RR, 1.559; P = .022) and decreases in A1c of more than 1.0% (RR, 1.590; P = .016). No such differences were seen with oral semaglutide.
A further evaluation of the data without the SUSTAIN 6 trial showed no effect on retinopathy but the analysis lacked power.
Dr. Albert told this news organization: “We did not find an immediate toxic effect of any drug. However, we cannot rule out that there was a cumulative effect of the dose over longer times.”
No disclosures were given.
A version of this article first appeared on Medscape.com.
A small potential increased risk of retinopathy worsening at 1 year with injected semaglutide (Ozempic, Novo Nordisk), a glucagon-like peptide 1 (GLP-1) agonist approved for type 2 diabetes, doesn’t outweigh the drug’s cardiovascular benefits but does highlight the need for baseline ophthalmologic evaluation before initiating treatment and ongoing retinal monitoring, researchers say.
That conclusion was based on data from a meta-analysis of the seven major cardiovascular outcomes trials of GLP-1 agonists currently on the market.
The findings were recently published in Diabetes & Metabolic Syndrome: Clinical Research & Reviews, by Stewart G. Albert, MD, and colleagues.
Concerns about retinopathy worsening with the GLP-1 agonist drug class first arose from the SUSTAIN-6 cardiovascular outcomes trial for injectable semaglutide, although a subsequent analysis of data from that trial appeared to suggest the problem is likely due to rapid glucose-lowering in already vulnerable patients rather than a drug-specific effect. This effect had been previously reported, most notably in the landmark Diabetes Control and Complications Trial.
In this new meta-analysis, “we showed that with improvements in A1c there were correlations with decreases in the rate of cardiovascular events but increases in the rate of retinopathy,” Dr. Albert, of St. Louis University, told this news organization.
“As a class of drugs, we did not find an increased rate of retinopathy. The effect of GLP-1 agonists on retinopathy did not appear to be due to an immediate direct toxic effect of the drug. The worsening of the rate of retinopathy was seen with semaglutide after 1 year of therapy and when there was a decrease in A1c of 1%,” he explained.
He noted that because the increased risk was seen primarily among those who already had retinopathy at baseline, “it would seem prudent to know the level of retinopathy either before or plan for close ophthalmologic monitoring around the time of drug initiation ... We routinely evaluate patients with known type 2 diabetes mellitus at yearly intervals for retinopathy. From our data, we saw worsening at 1 year of drug exposure, but we do not know the exact time when the changes occurred during that year.”
The Ozempic label advises that “patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy” but doesn’t specifically mention baseline assessment at the time of drug initiation.
No increase in retinopathy risk for GLP-1 agonist class overall
The seven trials in the meta-analysis comprised 56,004 participants, with baseline retinopathy prevalence ranging from 9% to 31%.
For the GLP-1 agonist class overall, there was no significant increase in the relative rate (RR) of retinopathy (RR, 1.09; P = .36), while there were significant reductions in relative rates of major adverse cardiac events, overall deaths, and cardiovascular deaths (all P < .001 or P = .001).
The increased retinopathy risk was seen only in the subcutaneous semaglutide group (RR, 1.73; P = .02).
The overall number needed to harm was 1,000 and the number to treat was 77. For semaglutide, those values were 77 and 43, respectively.
There was a significant correlation between a decrease in major adverse cardiac events and a decrease in A1c (P = .014), while for retinopathy, the risk increased with improved A1c (P = .076).
Semaglutide subanalysis finds increased retinopathy worsening
Dr. Albert and colleagues conducted a separate subanalysis of 11 studies of semaglutide that enrolled 11,894 patients, of which 6 studies (n = 5,610) were of oral semaglutide (Rybelsus) and 5 studies were of subcutaneous semaglutide (Ozempic; n = 6,284).
In the subanalysis, there was an overall increase in relative rates of new or worsening retinopathy (RR, 1.218; P = .049).
The change in relative rate of retinopathy was predominantly found for subcutaneous semaglutide given for longer than 1 year (RR, 1.559; P = .022) and decreases in A1c of more than 1.0% (RR, 1.590; P = .016). No such differences were seen with oral semaglutide.
A further evaluation of the data without the SUSTAIN 6 trial showed no effect on retinopathy but the analysis lacked power.
Dr. Albert told this news organization: “We did not find an immediate toxic effect of any drug. However, we cannot rule out that there was a cumulative effect of the dose over longer times.”
No disclosures were given.
A version of this article first appeared on Medscape.com.
A small potential increased risk of retinopathy worsening at 1 year with injected semaglutide (Ozempic, Novo Nordisk), a glucagon-like peptide 1 (GLP-1) agonist approved for type 2 diabetes, doesn’t outweigh the drug’s cardiovascular benefits but does highlight the need for baseline ophthalmologic evaluation before initiating treatment and ongoing retinal monitoring, researchers say.
That conclusion was based on data from a meta-analysis of the seven major cardiovascular outcomes trials of GLP-1 agonists currently on the market.
The findings were recently published in Diabetes & Metabolic Syndrome: Clinical Research & Reviews, by Stewart G. Albert, MD, and colleagues.
Concerns about retinopathy worsening with the GLP-1 agonist drug class first arose from the SUSTAIN-6 cardiovascular outcomes trial for injectable semaglutide, although a subsequent analysis of data from that trial appeared to suggest the problem is likely due to rapid glucose-lowering in already vulnerable patients rather than a drug-specific effect. This effect had been previously reported, most notably in the landmark Diabetes Control and Complications Trial.
In this new meta-analysis, “we showed that with improvements in A1c there were correlations with decreases in the rate of cardiovascular events but increases in the rate of retinopathy,” Dr. Albert, of St. Louis University, told this news organization.
“As a class of drugs, we did not find an increased rate of retinopathy. The effect of GLP-1 agonists on retinopathy did not appear to be due to an immediate direct toxic effect of the drug. The worsening of the rate of retinopathy was seen with semaglutide after 1 year of therapy and when there was a decrease in A1c of 1%,” he explained.
He noted that because the increased risk was seen primarily among those who already had retinopathy at baseline, “it would seem prudent to know the level of retinopathy either before or plan for close ophthalmologic monitoring around the time of drug initiation ... We routinely evaluate patients with known type 2 diabetes mellitus at yearly intervals for retinopathy. From our data, we saw worsening at 1 year of drug exposure, but we do not know the exact time when the changes occurred during that year.”
The Ozempic label advises that “patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy” but doesn’t specifically mention baseline assessment at the time of drug initiation.
No increase in retinopathy risk for GLP-1 agonist class overall
The seven trials in the meta-analysis comprised 56,004 participants, with baseline retinopathy prevalence ranging from 9% to 31%.
For the GLP-1 agonist class overall, there was no significant increase in the relative rate (RR) of retinopathy (RR, 1.09; P = .36), while there were significant reductions in relative rates of major adverse cardiac events, overall deaths, and cardiovascular deaths (all P < .001 or P = .001).
The increased retinopathy risk was seen only in the subcutaneous semaglutide group (RR, 1.73; P = .02).
The overall number needed to harm was 1,000 and the number to treat was 77. For semaglutide, those values were 77 and 43, respectively.
There was a significant correlation between a decrease in major adverse cardiac events and a decrease in A1c (P = .014), while for retinopathy, the risk increased with improved A1c (P = .076).
Semaglutide subanalysis finds increased retinopathy worsening
Dr. Albert and colleagues conducted a separate subanalysis of 11 studies of semaglutide that enrolled 11,894 patients, of which 6 studies (n = 5,610) were of oral semaglutide (Rybelsus) and 5 studies were of subcutaneous semaglutide (Ozempic; n = 6,284).
In the subanalysis, there was an overall increase in relative rates of new or worsening retinopathy (RR, 1.218; P = .049).
The change in relative rate of retinopathy was predominantly found for subcutaneous semaglutide given for longer than 1 year (RR, 1.559; P = .022) and decreases in A1c of more than 1.0% (RR, 1.590; P = .016). No such differences were seen with oral semaglutide.
A further evaluation of the data without the SUSTAIN 6 trial showed no effect on retinopathy but the analysis lacked power.
Dr. Albert told this news organization: “We did not find an immediate toxic effect of any drug. However, we cannot rule out that there was a cumulative effect of the dose over longer times.”
No disclosures were given.
A version of this article first appeared on Medscape.com.
FROM DIABETES & METABOLIC SYNDROME: CLINICAL RESEARCH & REVIEWS
PCSK9 inhibitors for severe COVID? Pilot trial signals of benefit
PCSK9 inhibitors may best be known for their powerful LDL-lowering effects but are less appreciated as anti-inflammatory agents with potential beyond cardiovascular health.
In a small pilot trial, for example, patients hospitalized with severe COVID-19 who received a single injection of PCSK9 inhibitor became less sick and more likely to survive than those given a placebo. Their 30-day risk of death or intubation fell significantly, as did their levels of the inflammatory cytokine interleukin 6 (IL-6).
Indeed, survival gains in the PCSK9-inhibitor group were greatest among patients with higher baseline concentrations of IL-6. Although the trial wasn’t powered for clinical outcomes, it suggests the drugs’ efficacy in COVID-19 tracks with intensity of inflammation, proposes a report published in the Journal of the American College of Cardiology.
Therefore, “PCSK9 inhibition may represent a novel therapeutic pathway in addition to currently recommended therapeutic approaches for severe COVID-19,” conclude the authors, led by Eliano P. Navarese, MD, PhD, Nicolaus Copernicus University, Bydgoszcz, Poland.
PCSK9 inhibitors as anti-inflammatories
Although the study was small and only hypothesis-generating, the fact that outcomes for actively treated patients were proportional to baseline IL-6 levels “strongly suggests that PCSK9 inhibition can directly modulate inflammation in COVID-19,” argues an editorial accompanying the report.
and likely sheds light on “mechanisms through which PCSK9 inhibition dually modulates lipoprotein metabolism and inflammation,” write Sascha N. Goonewardena, MD, University of Michigan, Ann Arbor, and Robert S. Rosenson, MD, Icahn School of Medicine at Mount Sinai, New York.
The results are consistent with prior evidence that the drugs are anti-inflammatory at least partly because of their interference with inflammatory pathways triggered by PCSK9 and mediated by IL-6, as described by Dr. Navarese and colleagues.
Indeed, they write, PCSK9 inhibitors may improve COVID outcomes mostly through mechanisms unrelated to LDL-receptor expression, “including direct inhibition of PCSK9-triggered inflammation.”
If true, the authors observe, it might explain “why the positive findings of the present study have not been consistently observed in trials involving other lipid-lowering agents, such as statins.” Those drugs are well-known to decrease levels of the inflammatory biomarker C-reactive protein.
In patients with stable coronary disease, in whom inflammation is typically tracked by measuring CRP, “the PCSK9 inhibitors have not been shown to have an anti-inflammatory effect,” Dr. Rosenson further explained.
But the current study’s patients with acute, severe COVID-19, a “profound inflammatory insult” with upregulation of IL-6, were “a good population” for evaluating the drugs’ potential anti-inflammatory effects, Dr. Rosenson said in an interview. The results “are quite enticing but require corroboration in a larger trial.”
A single injection
The IMPACT-SIRIO 5 trial entered 60 adults hospitalized with severe COVID-19 and elevated IL-6 at four centers in Poland. Patients with other known active infections were excluded.
They were randomly assigned double-blind to receive a 140 mg injection of evolocumab (Repatha) or placebo. The 2 groups were similar with respect to demographics, body-mass index, time since symptom onset, and treatments for managing COVID-19 and its complications.
Rates of death or need for intubation at 30 days, the primary endpoint, were 23.3% in the PCSK9-inhibitor group and 53.3% for controls, a risk difference of 30% (95% confidence interval –53.4% to –6.6%). The median durations of oxygen therapy were significantly different at 13 days and 20 days, respectively, the report states.
Serum IL-6 levels fell further over 30 days in the PCSK9-inhibitor group (–56% vs. –21% among controls). A drop by more than 90% was seen in 60% of patients in the PCSK9-inhibitor group and in 27% of controls.
The average hospital stay was shorter for those getting the PCSK9 inhibitor, compared with placebo, 16 days versus 22 days, and their 30-day mortality was numerically lower, 16% versus 33.3%.
Patients’ baseline IL-6 levels above the median, the report states, had a lower mortality on the PCSK9 inhibitor versus placebo (risk difference –37.5%; 95% CI –68.2% to –6.70%).
A larger trial to corroborate these results would potentially enter similar patients hospitalized with COVID-19 with reproducible evidence of an ongoing cytokine storm, such as elevated levels of IL-6, who would be assigned to either a PCSK9 inhibitor or placebo, Dr. Rosenson proposed.
Although the current primary endpoint that combines mortality and intubation was “reasonable” for a small pilot trial, he said, if the researchers embark on a larger study, “they’ll want to look at those events separately.”
Dr. Navarese discloses receiving speaker and consultancy fees from Amgen, Sanofi-Regeneron, Bayer; and grants from Abbott. Disclosures for the other authors are in the report. Rosenson discloses receiving research funding to his institution from Amgen, Arrowhead, Eli Lilly, Novartis, and Regeneron; consulting fees from Amgen, Arrowhead, CRISPR Therapeutics, Eli Lilly, Lipigon, Novartis, Precision Biosciences, Regeneron, Ultragenyx, and Verve; speaking fees from Amgen, Kowa, and Regeneron; and royalties from Wolters Kluwer; and owning stock in MediMergent. Dr. Goonewardena reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
PCSK9 inhibitors may best be known for their powerful LDL-lowering effects but are less appreciated as anti-inflammatory agents with potential beyond cardiovascular health.
In a small pilot trial, for example, patients hospitalized with severe COVID-19 who received a single injection of PCSK9 inhibitor became less sick and more likely to survive than those given a placebo. Their 30-day risk of death or intubation fell significantly, as did their levels of the inflammatory cytokine interleukin 6 (IL-6).
Indeed, survival gains in the PCSK9-inhibitor group were greatest among patients with higher baseline concentrations of IL-6. Although the trial wasn’t powered for clinical outcomes, it suggests the drugs’ efficacy in COVID-19 tracks with intensity of inflammation, proposes a report published in the Journal of the American College of Cardiology.
Therefore, “PCSK9 inhibition may represent a novel therapeutic pathway in addition to currently recommended therapeutic approaches for severe COVID-19,” conclude the authors, led by Eliano P. Navarese, MD, PhD, Nicolaus Copernicus University, Bydgoszcz, Poland.
PCSK9 inhibitors as anti-inflammatories
Although the study was small and only hypothesis-generating, the fact that outcomes for actively treated patients were proportional to baseline IL-6 levels “strongly suggests that PCSK9 inhibition can directly modulate inflammation in COVID-19,” argues an editorial accompanying the report.
and likely sheds light on “mechanisms through which PCSK9 inhibition dually modulates lipoprotein metabolism and inflammation,” write Sascha N. Goonewardena, MD, University of Michigan, Ann Arbor, and Robert S. Rosenson, MD, Icahn School of Medicine at Mount Sinai, New York.
The results are consistent with prior evidence that the drugs are anti-inflammatory at least partly because of their interference with inflammatory pathways triggered by PCSK9 and mediated by IL-6, as described by Dr. Navarese and colleagues.
Indeed, they write, PCSK9 inhibitors may improve COVID outcomes mostly through mechanisms unrelated to LDL-receptor expression, “including direct inhibition of PCSK9-triggered inflammation.”
If true, the authors observe, it might explain “why the positive findings of the present study have not been consistently observed in trials involving other lipid-lowering agents, such as statins.” Those drugs are well-known to decrease levels of the inflammatory biomarker C-reactive protein.
In patients with stable coronary disease, in whom inflammation is typically tracked by measuring CRP, “the PCSK9 inhibitors have not been shown to have an anti-inflammatory effect,” Dr. Rosenson further explained.
But the current study’s patients with acute, severe COVID-19, a “profound inflammatory insult” with upregulation of IL-6, were “a good population” for evaluating the drugs’ potential anti-inflammatory effects, Dr. Rosenson said in an interview. The results “are quite enticing but require corroboration in a larger trial.”
A single injection
The IMPACT-SIRIO 5 trial entered 60 adults hospitalized with severe COVID-19 and elevated IL-6 at four centers in Poland. Patients with other known active infections were excluded.
They were randomly assigned double-blind to receive a 140 mg injection of evolocumab (Repatha) or placebo. The 2 groups were similar with respect to demographics, body-mass index, time since symptom onset, and treatments for managing COVID-19 and its complications.
Rates of death or need for intubation at 30 days, the primary endpoint, were 23.3% in the PCSK9-inhibitor group and 53.3% for controls, a risk difference of 30% (95% confidence interval –53.4% to –6.6%). The median durations of oxygen therapy were significantly different at 13 days and 20 days, respectively, the report states.
Serum IL-6 levels fell further over 30 days in the PCSK9-inhibitor group (–56% vs. –21% among controls). A drop by more than 90% was seen in 60% of patients in the PCSK9-inhibitor group and in 27% of controls.
The average hospital stay was shorter for those getting the PCSK9 inhibitor, compared with placebo, 16 days versus 22 days, and their 30-day mortality was numerically lower, 16% versus 33.3%.
Patients’ baseline IL-6 levels above the median, the report states, had a lower mortality on the PCSK9 inhibitor versus placebo (risk difference –37.5%; 95% CI –68.2% to –6.70%).
A larger trial to corroborate these results would potentially enter similar patients hospitalized with COVID-19 with reproducible evidence of an ongoing cytokine storm, such as elevated levels of IL-6, who would be assigned to either a PCSK9 inhibitor or placebo, Dr. Rosenson proposed.
Although the current primary endpoint that combines mortality and intubation was “reasonable” for a small pilot trial, he said, if the researchers embark on a larger study, “they’ll want to look at those events separately.”
Dr. Navarese discloses receiving speaker and consultancy fees from Amgen, Sanofi-Regeneron, Bayer; and grants from Abbott. Disclosures for the other authors are in the report. Rosenson discloses receiving research funding to his institution from Amgen, Arrowhead, Eli Lilly, Novartis, and Regeneron; consulting fees from Amgen, Arrowhead, CRISPR Therapeutics, Eli Lilly, Lipigon, Novartis, Precision Biosciences, Regeneron, Ultragenyx, and Verve; speaking fees from Amgen, Kowa, and Regeneron; and royalties from Wolters Kluwer; and owning stock in MediMergent. Dr. Goonewardena reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
PCSK9 inhibitors may best be known for their powerful LDL-lowering effects but are less appreciated as anti-inflammatory agents with potential beyond cardiovascular health.
In a small pilot trial, for example, patients hospitalized with severe COVID-19 who received a single injection of PCSK9 inhibitor became less sick and more likely to survive than those given a placebo. Their 30-day risk of death or intubation fell significantly, as did their levels of the inflammatory cytokine interleukin 6 (IL-6).
Indeed, survival gains in the PCSK9-inhibitor group were greatest among patients with higher baseline concentrations of IL-6. Although the trial wasn’t powered for clinical outcomes, it suggests the drugs’ efficacy in COVID-19 tracks with intensity of inflammation, proposes a report published in the Journal of the American College of Cardiology.
Therefore, “PCSK9 inhibition may represent a novel therapeutic pathway in addition to currently recommended therapeutic approaches for severe COVID-19,” conclude the authors, led by Eliano P. Navarese, MD, PhD, Nicolaus Copernicus University, Bydgoszcz, Poland.
PCSK9 inhibitors as anti-inflammatories
Although the study was small and only hypothesis-generating, the fact that outcomes for actively treated patients were proportional to baseline IL-6 levels “strongly suggests that PCSK9 inhibition can directly modulate inflammation in COVID-19,” argues an editorial accompanying the report.
and likely sheds light on “mechanisms through which PCSK9 inhibition dually modulates lipoprotein metabolism and inflammation,” write Sascha N. Goonewardena, MD, University of Michigan, Ann Arbor, and Robert S. Rosenson, MD, Icahn School of Medicine at Mount Sinai, New York.
The results are consistent with prior evidence that the drugs are anti-inflammatory at least partly because of their interference with inflammatory pathways triggered by PCSK9 and mediated by IL-6, as described by Dr. Navarese and colleagues.
Indeed, they write, PCSK9 inhibitors may improve COVID outcomes mostly through mechanisms unrelated to LDL-receptor expression, “including direct inhibition of PCSK9-triggered inflammation.”
If true, the authors observe, it might explain “why the positive findings of the present study have not been consistently observed in trials involving other lipid-lowering agents, such as statins.” Those drugs are well-known to decrease levels of the inflammatory biomarker C-reactive protein.
In patients with stable coronary disease, in whom inflammation is typically tracked by measuring CRP, “the PCSK9 inhibitors have not been shown to have an anti-inflammatory effect,” Dr. Rosenson further explained.
But the current study’s patients with acute, severe COVID-19, a “profound inflammatory insult” with upregulation of IL-6, were “a good population” for evaluating the drugs’ potential anti-inflammatory effects, Dr. Rosenson said in an interview. The results “are quite enticing but require corroboration in a larger trial.”
A single injection
The IMPACT-SIRIO 5 trial entered 60 adults hospitalized with severe COVID-19 and elevated IL-6 at four centers in Poland. Patients with other known active infections were excluded.
They were randomly assigned double-blind to receive a 140 mg injection of evolocumab (Repatha) or placebo. The 2 groups were similar with respect to demographics, body-mass index, time since symptom onset, and treatments for managing COVID-19 and its complications.
Rates of death or need for intubation at 30 days, the primary endpoint, were 23.3% in the PCSK9-inhibitor group and 53.3% for controls, a risk difference of 30% (95% confidence interval –53.4% to –6.6%). The median durations of oxygen therapy were significantly different at 13 days and 20 days, respectively, the report states.
Serum IL-6 levels fell further over 30 days in the PCSK9-inhibitor group (–56% vs. –21% among controls). A drop by more than 90% was seen in 60% of patients in the PCSK9-inhibitor group and in 27% of controls.
The average hospital stay was shorter for those getting the PCSK9 inhibitor, compared with placebo, 16 days versus 22 days, and their 30-day mortality was numerically lower, 16% versus 33.3%.
Patients’ baseline IL-6 levels above the median, the report states, had a lower mortality on the PCSK9 inhibitor versus placebo (risk difference –37.5%; 95% CI –68.2% to –6.70%).
A larger trial to corroborate these results would potentially enter similar patients hospitalized with COVID-19 with reproducible evidence of an ongoing cytokine storm, such as elevated levels of IL-6, who would be assigned to either a PCSK9 inhibitor or placebo, Dr. Rosenson proposed.
Although the current primary endpoint that combines mortality and intubation was “reasonable” for a small pilot trial, he said, if the researchers embark on a larger study, “they’ll want to look at those events separately.”
Dr. Navarese discloses receiving speaker and consultancy fees from Amgen, Sanofi-Regeneron, Bayer; and grants from Abbott. Disclosures for the other authors are in the report. Rosenson discloses receiving research funding to his institution from Amgen, Arrowhead, Eli Lilly, Novartis, and Regeneron; consulting fees from Amgen, Arrowhead, CRISPR Therapeutics, Eli Lilly, Lipigon, Novartis, Precision Biosciences, Regeneron, Ultragenyx, and Verve; speaking fees from Amgen, Kowa, and Regeneron; and royalties from Wolters Kluwer; and owning stock in MediMergent. Dr. Goonewardena reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA approves new type 2 diabetes drug bexagliflozin
The U.S. Food and Drug Administration has approved bexagliflozin (Brenzavvy, TheracosBio) for the treatment of adults with type 2 diabetes.
The once-daily 20-mg oral sodium-glucose cotransporter 2 (SGLT2) inhibitor is indicated as an adjunct to diet and exercise to improve glycemic control for those with type 2 diabetes, but not type 1 diabetes. It can be used in adults with an estimated glomerular filtration rate (eGFR) > 30 mL/min per 1.73 m2.
Approval was based on results from 23 clinical trials with more than 5,000 participants, including more than 300 patients with stage 3 kidney disease (eGFR < 60 and > 30 mL/min per 1.73 m2).
In the phase 3 studies, bexagliflozin significantly reduced hemoglobin A1c and fasting blood glucose at 24 weeks as monotherapy or as add-on to metformin and other glucose-lowering drugs and combinations. It also produced modest reductions in body weight and systolic blood pressure.
In the phase 3 Bexagliflozin Efficacy and Safety Trial (BEST) cardiovascular outcomes trial, the drug met its efficacy and safety objectives in patients at high cardiovascular risk. Noninferiority was demonstrated for the composite outcome of cardiovascular death, myocardial infarction, stroke, or unstable angina.
“As a class of drugs, SGLT2 inhibitors have shown tremendous benefit in treating adults with type 2 diabetes,” said Mason Freeman, MD, director of the Translational Research Center at Massachusetts General Hospital, Boston, in a press release from TheracosBio.
“Being involved in all of the clinical trials for Brenzavvy, I am greatly impressed with the efficacy of the drug in reducing blood glucose levels and I believe it is an important addition to the SGLT2 inhibitor class of drugs.”
As with other SGLT2 inhibitors, adverse events seen in the trials include ketoacidosis, lower limb amputation, volume depletion, urosepsis, pyelonephritis, Fournier’s gangrene, genital mycotic infections, and hypoglycemia when used with insulin or insulin secretagogues.
Bexagliflozin joins an already crowded field of SGLT2 inhibitors, some of which have been approved for additional cardiovascular and kidney indications.
Of interest, bexagliflozin was approved by the FDA for diabetes in cats in December 2022, as the first oral new animal drug to improve glycemic control in otherwise healthy cats with diabetes not previously treated with insulin.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved bexagliflozin (Brenzavvy, TheracosBio) for the treatment of adults with type 2 diabetes.
The once-daily 20-mg oral sodium-glucose cotransporter 2 (SGLT2) inhibitor is indicated as an adjunct to diet and exercise to improve glycemic control for those with type 2 diabetes, but not type 1 diabetes. It can be used in adults with an estimated glomerular filtration rate (eGFR) > 30 mL/min per 1.73 m2.
Approval was based on results from 23 clinical trials with more than 5,000 participants, including more than 300 patients with stage 3 kidney disease (eGFR < 60 and > 30 mL/min per 1.73 m2).
In the phase 3 studies, bexagliflozin significantly reduced hemoglobin A1c and fasting blood glucose at 24 weeks as monotherapy or as add-on to metformin and other glucose-lowering drugs and combinations. It also produced modest reductions in body weight and systolic blood pressure.
In the phase 3 Bexagliflozin Efficacy and Safety Trial (BEST) cardiovascular outcomes trial, the drug met its efficacy and safety objectives in patients at high cardiovascular risk. Noninferiority was demonstrated for the composite outcome of cardiovascular death, myocardial infarction, stroke, or unstable angina.
“As a class of drugs, SGLT2 inhibitors have shown tremendous benefit in treating adults with type 2 diabetes,” said Mason Freeman, MD, director of the Translational Research Center at Massachusetts General Hospital, Boston, in a press release from TheracosBio.
“Being involved in all of the clinical trials for Brenzavvy, I am greatly impressed with the efficacy of the drug in reducing blood glucose levels and I believe it is an important addition to the SGLT2 inhibitor class of drugs.”
As with other SGLT2 inhibitors, adverse events seen in the trials include ketoacidosis, lower limb amputation, volume depletion, urosepsis, pyelonephritis, Fournier’s gangrene, genital mycotic infections, and hypoglycemia when used with insulin or insulin secretagogues.
Bexagliflozin joins an already crowded field of SGLT2 inhibitors, some of which have been approved for additional cardiovascular and kidney indications.
Of interest, bexagliflozin was approved by the FDA for diabetes in cats in December 2022, as the first oral new animal drug to improve glycemic control in otherwise healthy cats with diabetes not previously treated with insulin.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved bexagliflozin (Brenzavvy, TheracosBio) for the treatment of adults with type 2 diabetes.
The once-daily 20-mg oral sodium-glucose cotransporter 2 (SGLT2) inhibitor is indicated as an adjunct to diet and exercise to improve glycemic control for those with type 2 diabetes, but not type 1 diabetes. It can be used in adults with an estimated glomerular filtration rate (eGFR) > 30 mL/min per 1.73 m2.
Approval was based on results from 23 clinical trials with more than 5,000 participants, including more than 300 patients with stage 3 kidney disease (eGFR < 60 and > 30 mL/min per 1.73 m2).
In the phase 3 studies, bexagliflozin significantly reduced hemoglobin A1c and fasting blood glucose at 24 weeks as monotherapy or as add-on to metformin and other glucose-lowering drugs and combinations. It also produced modest reductions in body weight and systolic blood pressure.
In the phase 3 Bexagliflozin Efficacy and Safety Trial (BEST) cardiovascular outcomes trial, the drug met its efficacy and safety objectives in patients at high cardiovascular risk. Noninferiority was demonstrated for the composite outcome of cardiovascular death, myocardial infarction, stroke, or unstable angina.
“As a class of drugs, SGLT2 inhibitors have shown tremendous benefit in treating adults with type 2 diabetes,” said Mason Freeman, MD, director of the Translational Research Center at Massachusetts General Hospital, Boston, in a press release from TheracosBio.
“Being involved in all of the clinical trials for Brenzavvy, I am greatly impressed with the efficacy of the drug in reducing blood glucose levels and I believe it is an important addition to the SGLT2 inhibitor class of drugs.”
As with other SGLT2 inhibitors, adverse events seen in the trials include ketoacidosis, lower limb amputation, volume depletion, urosepsis, pyelonephritis, Fournier’s gangrene, genital mycotic infections, and hypoglycemia when used with insulin or insulin secretagogues.
Bexagliflozin joins an already crowded field of SGLT2 inhibitors, some of which have been approved for additional cardiovascular and kidney indications.
Of interest, bexagliflozin was approved by the FDA for diabetes in cats in December 2022, as the first oral new animal drug to improve glycemic control in otherwise healthy cats with diabetes not previously treated with insulin.
A version of this article first appeared on Medscape.com.
Adding venetoclax improves ibrutinib outcomes in CLL
Investigators led by Philip Thompson, MD, a hematologist/oncologist at the center, explained that CLL patients receiving ibrutinib, a Bruton’s kinase inhibitor, “rarely achieve complete remission with undetectable measurable residual disease,” so they stay on the costly treatment indefinitely or until disease progression or accumulating adverse events force a switch to venetoclax.
Using the two agents together, instead of consecutively, may allow strong responders to stop treatment altogether and suboptimal responders to have longer remissions, they said.
“We would not advocate prolonged Bruton’s kinase inhibitor use prior to starting venetoclax in treatment-naive patients, as the safety and efficacy of commencing venetoclax after a 3-month ibrutinib monotherapy phase has been repeatedly demonstrated,” the team said.
However, the investigators noted that their “study was not intended to directly answer the question of whether combination therapy is superior to the current paradigm of sequential monotherapy.” Randomized trials are looking into the matter. The study was published recently as a preprint on ResearchSquare.com and has not been peer reviewed.
Complete remission in over half
The 45 adult subjects had one or more high-risk features for CLL progression and had received at least 1 year of ibrutinib at 140-420 mg once daily, depending on tolerance. They had bone marrow detectable disease at study entry but did not meet criteria for progression. Median duration of ibrutinib at baseline was 32 months, and about half the subjects were on it as their initial therapy.
Venetoclax, a BCL2 inhibitor with a completely different mechanisms of action, was added to ibrutinib for up to 2 years, escalated up to a target dose of 400 mg once daily.
On intention-to-treat analysis, venetoclax add-on improved ibrutinib response to complete remission in 55% of patients; complete remission was defined as less than 1 CLL cell per 10,000 leukocytes in bone marrow on two consecutive occasions 6 months apart.
The rate of undetectable bone marrow disease was 57% after 1 year of combined treatment and 71% after venetoclax completion, at which point 23 patients with undetectable disease stopped ibrutinib along with venetoclax.
Five patients had disease progression at a median of 41 months after venetoclax initiation, one during combined therapy, three during ibrutinib maintenance afterward, and one with Richter transformation after complete remission and discontinuation of all treatment. No patient had died from CLL.
“There has so far been no significant difference noted in” time to residual disease re-emergence, the team said, based on whether or not patients continued ibrutinib after venetoclax add-on.
There was no significant difference in the rate of bone marrow clearance according to the presence or absence of TP53 abnormalities, complex karyotypes, or prior treatment status.
The most common grade 3/4 adverse event was neutropenia in 20% of patients. Nine patients developed nonmelanoma skin cancer during the trial; six were diagnosed with other solid tumors; three came down with grade 3 infections, and two developed myelodysplastic syndrome, both with a prior history of chemotherapy.
No one stopped venetoclax because of toxicity, but about a third of subjects required dose reductions, most often because of neutropenia.
The study was funded by AbbVie, which is commercializing venetoclax along with Genentech. Investigators disclosed ties to both companies and many others. Dr. Thompson disclosed ties to AbbVie, Pharmacyclics, Lilly, Adaptive Biotechnologies, Janssen, Beigene, and Genentech.
Investigators led by Philip Thompson, MD, a hematologist/oncologist at the center, explained that CLL patients receiving ibrutinib, a Bruton’s kinase inhibitor, “rarely achieve complete remission with undetectable measurable residual disease,” so they stay on the costly treatment indefinitely or until disease progression or accumulating adverse events force a switch to venetoclax.
Using the two agents together, instead of consecutively, may allow strong responders to stop treatment altogether and suboptimal responders to have longer remissions, they said.
“We would not advocate prolonged Bruton’s kinase inhibitor use prior to starting venetoclax in treatment-naive patients, as the safety and efficacy of commencing venetoclax after a 3-month ibrutinib monotherapy phase has been repeatedly demonstrated,” the team said.
However, the investigators noted that their “study was not intended to directly answer the question of whether combination therapy is superior to the current paradigm of sequential monotherapy.” Randomized trials are looking into the matter. The study was published recently as a preprint on ResearchSquare.com and has not been peer reviewed.
Complete remission in over half
The 45 adult subjects had one or more high-risk features for CLL progression and had received at least 1 year of ibrutinib at 140-420 mg once daily, depending on tolerance. They had bone marrow detectable disease at study entry but did not meet criteria for progression. Median duration of ibrutinib at baseline was 32 months, and about half the subjects were on it as their initial therapy.
Venetoclax, a BCL2 inhibitor with a completely different mechanisms of action, was added to ibrutinib for up to 2 years, escalated up to a target dose of 400 mg once daily.
On intention-to-treat analysis, venetoclax add-on improved ibrutinib response to complete remission in 55% of patients; complete remission was defined as less than 1 CLL cell per 10,000 leukocytes in bone marrow on two consecutive occasions 6 months apart.
The rate of undetectable bone marrow disease was 57% after 1 year of combined treatment and 71% after venetoclax completion, at which point 23 patients with undetectable disease stopped ibrutinib along with venetoclax.
Five patients had disease progression at a median of 41 months after venetoclax initiation, one during combined therapy, three during ibrutinib maintenance afterward, and one with Richter transformation after complete remission and discontinuation of all treatment. No patient had died from CLL.
“There has so far been no significant difference noted in” time to residual disease re-emergence, the team said, based on whether or not patients continued ibrutinib after venetoclax add-on.
There was no significant difference in the rate of bone marrow clearance according to the presence or absence of TP53 abnormalities, complex karyotypes, or prior treatment status.
The most common grade 3/4 adverse event was neutropenia in 20% of patients. Nine patients developed nonmelanoma skin cancer during the trial; six were diagnosed with other solid tumors; three came down with grade 3 infections, and two developed myelodysplastic syndrome, both with a prior history of chemotherapy.
No one stopped venetoclax because of toxicity, but about a third of subjects required dose reductions, most often because of neutropenia.
The study was funded by AbbVie, which is commercializing venetoclax along with Genentech. Investigators disclosed ties to both companies and many others. Dr. Thompson disclosed ties to AbbVie, Pharmacyclics, Lilly, Adaptive Biotechnologies, Janssen, Beigene, and Genentech.
Investigators led by Philip Thompson, MD, a hematologist/oncologist at the center, explained that CLL patients receiving ibrutinib, a Bruton’s kinase inhibitor, “rarely achieve complete remission with undetectable measurable residual disease,” so they stay on the costly treatment indefinitely or until disease progression or accumulating adverse events force a switch to venetoclax.
Using the two agents together, instead of consecutively, may allow strong responders to stop treatment altogether and suboptimal responders to have longer remissions, they said.
“We would not advocate prolonged Bruton’s kinase inhibitor use prior to starting venetoclax in treatment-naive patients, as the safety and efficacy of commencing venetoclax after a 3-month ibrutinib monotherapy phase has been repeatedly demonstrated,” the team said.
However, the investigators noted that their “study was not intended to directly answer the question of whether combination therapy is superior to the current paradigm of sequential monotherapy.” Randomized trials are looking into the matter. The study was published recently as a preprint on ResearchSquare.com and has not been peer reviewed.
Complete remission in over half
The 45 adult subjects had one or more high-risk features for CLL progression and had received at least 1 year of ibrutinib at 140-420 mg once daily, depending on tolerance. They had bone marrow detectable disease at study entry but did not meet criteria for progression. Median duration of ibrutinib at baseline was 32 months, and about half the subjects were on it as their initial therapy.
Venetoclax, a BCL2 inhibitor with a completely different mechanisms of action, was added to ibrutinib for up to 2 years, escalated up to a target dose of 400 mg once daily.
On intention-to-treat analysis, venetoclax add-on improved ibrutinib response to complete remission in 55% of patients; complete remission was defined as less than 1 CLL cell per 10,000 leukocytes in bone marrow on two consecutive occasions 6 months apart.
The rate of undetectable bone marrow disease was 57% after 1 year of combined treatment and 71% after venetoclax completion, at which point 23 patients with undetectable disease stopped ibrutinib along with venetoclax.
Five patients had disease progression at a median of 41 months after venetoclax initiation, one during combined therapy, three during ibrutinib maintenance afterward, and one with Richter transformation after complete remission and discontinuation of all treatment. No patient had died from CLL.
“There has so far been no significant difference noted in” time to residual disease re-emergence, the team said, based on whether or not patients continued ibrutinib after venetoclax add-on.
There was no significant difference in the rate of bone marrow clearance according to the presence or absence of TP53 abnormalities, complex karyotypes, or prior treatment status.
The most common grade 3/4 adverse event was neutropenia in 20% of patients. Nine patients developed nonmelanoma skin cancer during the trial; six were diagnosed with other solid tumors; three came down with grade 3 infections, and two developed myelodysplastic syndrome, both with a prior history of chemotherapy.
No one stopped venetoclax because of toxicity, but about a third of subjects required dose reductions, most often because of neutropenia.
The study was funded by AbbVie, which is commercializing venetoclax along with Genentech. Investigators disclosed ties to both companies and many others. Dr. Thompson disclosed ties to AbbVie, Pharmacyclics, Lilly, Adaptive Biotechnologies, Janssen, Beigene, and Genentech.
FROM RESEARCHSQUARE