User login
More weight loss with surgery than new obesity meds: meta-analysis
SAN DIEGO – but glycemic control was similar after either treatment.
However, researchers have yet to directly compare bariatric surgery with new dual and even triple agonists that are in development.
The review by Shohinee Sarma, MD, MPH, and Patricia Palcu, MD, from the University of Toronto, was published in Obesity. Dr. Sarma also presented the findings virtually at the Obesity journal symposium at ObesityWeek® 2022.
Eric Ravussin, PhD, outgoing editor-in-chief of Obesity, explained to in an interview that this is one of five articles the editors chose from about 20 papers submitted for consideration for the symposium, and it was selected because it is a first review and meta-analysis of this direct comparison.
It showed that in “a straight head-to-head comparison, weight loss is larger by about 20 kg (44 lb) with bariatric surgery versus a GLP-1 agonist, but the improvement in glycemia (carbohydrate metabolism) was similar,” said Dr. Ravussin, from Pennington Biomedical Research Center, Louisiana State University, Baton Rouge.
Study limitations, which the authors also acknowledge, include that this was a small review of small studies: There were only six studies and 322 patients.
Moreover, the data are from 2007 to 2017, and newer weight-loss drugs are more potent.
Most studies in the review compared bariatric surgery with liraglutide, Dr. Ravussin noted, whereas, “we have now better GLP-1 agonists like semaglutide,” as well as drugs that are combinations of a GLP-1 agonist with another agonist or agonists.
“Tirzepatide, for example, which is a combination of a GLP-1 agonist and a [glucose-dependent insulinotropic polypeptide (GIP) agonist], is showing results that are very close to weight loss with bariatric surgery,” he observed.
There are quite a few other drugs in development, too, he continued, which are going to approach the weight loss obtained with bariatric surgery.
Novo Nordisk is coming out with a combination of an amylin analog (cagrilintide) and a GLP-1 agonist (semaglutide), he noted. “There are others coming in with GLP-1 and glucagon [dual agonists], and there is even a ... combo called triple G, which is a glucagon, GLP-1, and GIP [agonist].”
We now need a head-to-head comparison between bariatric surgery versus a combination drug like tirzepatide in a large population, he said.
“This is an exciting period,” Dr. Ravussin summarized, “because, 10 years ago, nobody thought that [results with] pharmacotherapy can approach bariatric surgery. Now we have other drugs that are still in development that are going to approach really close bariatric surgery.”
In an email to this news organization, Dr. Sarma noted that “due to the potent weight loss and glycemic benefits of GLP-1 agonists, patients who wish to avoid the risks of bariatric surgery may wish to discuss the option of medical therapy with their health professionals.”
“For next steps,” she said, “we need long-term studies comparing the weight-lowering, glycemic, and cardiovascular benefits of GLP-1 agonists in comparison to bariatric surgery for better counseling in obesity treatment.”
Three RCTs, three observational studies
The researchers searched the literature for randomized controlled trials (RCTs) and observational studies up to April 21, 2021, which directly compared absolute weight loss with a GLP-1 agonist – liraglutide, dulaglutide, semaglutide, exenatide, lixisenatide, and albiglutide (which are approved by the U.S. Food and Drug Administration or Health Canada) – versus any type of bariatric surgery including Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy, gastric banding, and biliopancreatic diversion.
The studies included patients aged 18 and older with a body mass index (BMI) greater than 25 kg/m2.
Secondary outcomes included change in BMI, and for patients with type 2 diabetes, change in A1c.
The researchers identified three RCTs and three observational studies, with diverse drugs and diverse types of bariatric surgery, which enrolled 13 to 134 patients, with follow-up from 6 months to 10 years.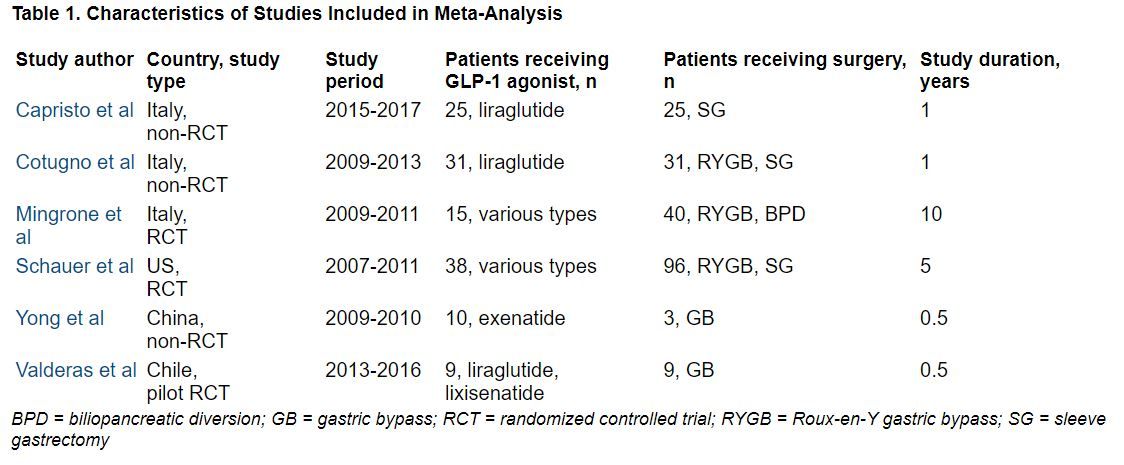
During follow-up, the overall mean weight loss was 22.7 kg greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 25.1 kg greater in the two non-RCTs with these data (Capristo et al. and Cotugno et al.).
The overall mean decrease in BMI was 8.2 kg/m2 greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 10.6 kg/m2 greater in the three non-RCTs with these data.
The overall mean decrease in A1c was 1.28% lower in the three RCTs with these data, and it was 0.9% lower in the one non-RCT with these data.
“In adults with obesity, bariatric surgery still confers the highest reductions in weight and BMI but confers similar effects in glycemic control when compared with GLP-1 agonists,” the researchers summarize.
Dr. Sarma received funding from the Clinical Investigator Program. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – but glycemic control was similar after either treatment.
However, researchers have yet to directly compare bariatric surgery with new dual and even triple agonists that are in development.
The review by Shohinee Sarma, MD, MPH, and Patricia Palcu, MD, from the University of Toronto, was published in Obesity. Dr. Sarma also presented the findings virtually at the Obesity journal symposium at ObesityWeek® 2022.
Eric Ravussin, PhD, outgoing editor-in-chief of Obesity, explained to in an interview that this is one of five articles the editors chose from about 20 papers submitted for consideration for the symposium, and it was selected because it is a first review and meta-analysis of this direct comparison.
It showed that in “a straight head-to-head comparison, weight loss is larger by about 20 kg (44 lb) with bariatric surgery versus a GLP-1 agonist, but the improvement in glycemia (carbohydrate metabolism) was similar,” said Dr. Ravussin, from Pennington Biomedical Research Center, Louisiana State University, Baton Rouge.
Study limitations, which the authors also acknowledge, include that this was a small review of small studies: There were only six studies and 322 patients.
Moreover, the data are from 2007 to 2017, and newer weight-loss drugs are more potent.
Most studies in the review compared bariatric surgery with liraglutide, Dr. Ravussin noted, whereas, “we have now better GLP-1 agonists like semaglutide,” as well as drugs that are combinations of a GLP-1 agonist with another agonist or agonists.
“Tirzepatide, for example, which is a combination of a GLP-1 agonist and a [glucose-dependent insulinotropic polypeptide (GIP) agonist], is showing results that are very close to weight loss with bariatric surgery,” he observed.
There are quite a few other drugs in development, too, he continued, which are going to approach the weight loss obtained with bariatric surgery.
Novo Nordisk is coming out with a combination of an amylin analog (cagrilintide) and a GLP-1 agonist (semaglutide), he noted. “There are others coming in with GLP-1 and glucagon [dual agonists], and there is even a ... combo called triple G, which is a glucagon, GLP-1, and GIP [agonist].”
We now need a head-to-head comparison between bariatric surgery versus a combination drug like tirzepatide in a large population, he said.
“This is an exciting period,” Dr. Ravussin summarized, “because, 10 years ago, nobody thought that [results with] pharmacotherapy can approach bariatric surgery. Now we have other drugs that are still in development that are going to approach really close bariatric surgery.”
In an email to this news organization, Dr. Sarma noted that “due to the potent weight loss and glycemic benefits of GLP-1 agonists, patients who wish to avoid the risks of bariatric surgery may wish to discuss the option of medical therapy with their health professionals.”
“For next steps,” she said, “we need long-term studies comparing the weight-lowering, glycemic, and cardiovascular benefits of GLP-1 agonists in comparison to bariatric surgery for better counseling in obesity treatment.”
Three RCTs, three observational studies
The researchers searched the literature for randomized controlled trials (RCTs) and observational studies up to April 21, 2021, which directly compared absolute weight loss with a GLP-1 agonist – liraglutide, dulaglutide, semaglutide, exenatide, lixisenatide, and albiglutide (which are approved by the U.S. Food and Drug Administration or Health Canada) – versus any type of bariatric surgery including Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy, gastric banding, and biliopancreatic diversion.
The studies included patients aged 18 and older with a body mass index (BMI) greater than 25 kg/m2.
Secondary outcomes included change in BMI, and for patients with type 2 diabetes, change in A1c.
The researchers identified three RCTs and three observational studies, with diverse drugs and diverse types of bariatric surgery, which enrolled 13 to 134 patients, with follow-up from 6 months to 10 years.
During follow-up, the overall mean weight loss was 22.7 kg greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 25.1 kg greater in the two non-RCTs with these data (Capristo et al. and Cotugno et al.).
The overall mean decrease in BMI was 8.2 kg/m2 greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 10.6 kg/m2 greater in the three non-RCTs with these data.
The overall mean decrease in A1c was 1.28% lower in the three RCTs with these data, and it was 0.9% lower in the one non-RCT with these data.
“In adults with obesity, bariatric surgery still confers the highest reductions in weight and BMI but confers similar effects in glycemic control when compared with GLP-1 agonists,” the researchers summarize.
Dr. Sarma received funding from the Clinical Investigator Program. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – but glycemic control was similar after either treatment.
However, researchers have yet to directly compare bariatric surgery with new dual and even triple agonists that are in development.
The review by Shohinee Sarma, MD, MPH, and Patricia Palcu, MD, from the University of Toronto, was published in Obesity. Dr. Sarma also presented the findings virtually at the Obesity journal symposium at ObesityWeek® 2022.
Eric Ravussin, PhD, outgoing editor-in-chief of Obesity, explained to in an interview that this is one of five articles the editors chose from about 20 papers submitted for consideration for the symposium, and it was selected because it is a first review and meta-analysis of this direct comparison.
It showed that in “a straight head-to-head comparison, weight loss is larger by about 20 kg (44 lb) with bariatric surgery versus a GLP-1 agonist, but the improvement in glycemia (carbohydrate metabolism) was similar,” said Dr. Ravussin, from Pennington Biomedical Research Center, Louisiana State University, Baton Rouge.
Study limitations, which the authors also acknowledge, include that this was a small review of small studies: There were only six studies and 322 patients.
Moreover, the data are from 2007 to 2017, and newer weight-loss drugs are more potent.
Most studies in the review compared bariatric surgery with liraglutide, Dr. Ravussin noted, whereas, “we have now better GLP-1 agonists like semaglutide,” as well as drugs that are combinations of a GLP-1 agonist with another agonist or agonists.
“Tirzepatide, for example, which is a combination of a GLP-1 agonist and a [glucose-dependent insulinotropic polypeptide (GIP) agonist], is showing results that are very close to weight loss with bariatric surgery,” he observed.
There are quite a few other drugs in development, too, he continued, which are going to approach the weight loss obtained with bariatric surgery.
Novo Nordisk is coming out with a combination of an amylin analog (cagrilintide) and a GLP-1 agonist (semaglutide), he noted. “There are others coming in with GLP-1 and glucagon [dual agonists], and there is even a ... combo called triple G, which is a glucagon, GLP-1, and GIP [agonist].”
We now need a head-to-head comparison between bariatric surgery versus a combination drug like tirzepatide in a large population, he said.
“This is an exciting period,” Dr. Ravussin summarized, “because, 10 years ago, nobody thought that [results with] pharmacotherapy can approach bariatric surgery. Now we have other drugs that are still in development that are going to approach really close bariatric surgery.”
In an email to this news organization, Dr. Sarma noted that “due to the potent weight loss and glycemic benefits of GLP-1 agonists, patients who wish to avoid the risks of bariatric surgery may wish to discuss the option of medical therapy with their health professionals.”
“For next steps,” she said, “we need long-term studies comparing the weight-lowering, glycemic, and cardiovascular benefits of GLP-1 agonists in comparison to bariatric surgery for better counseling in obesity treatment.”
Three RCTs, three observational studies
The researchers searched the literature for randomized controlled trials (RCTs) and observational studies up to April 21, 2021, which directly compared absolute weight loss with a GLP-1 agonist – liraglutide, dulaglutide, semaglutide, exenatide, lixisenatide, and albiglutide (which are approved by the U.S. Food and Drug Administration or Health Canada) – versus any type of bariatric surgery including Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy, gastric banding, and biliopancreatic diversion.
The studies included patients aged 18 and older with a body mass index (BMI) greater than 25 kg/m2.
Secondary outcomes included change in BMI, and for patients with type 2 diabetes, change in A1c.
The researchers identified three RCTs and three observational studies, with diverse drugs and diverse types of bariatric surgery, which enrolled 13 to 134 patients, with follow-up from 6 months to 10 years.
During follow-up, the overall mean weight loss was 22.7 kg greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 25.1 kg greater in the two non-RCTs with these data (Capristo et al. and Cotugno et al.).
The overall mean decrease in BMI was 8.2 kg/m2 greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 10.6 kg/m2 greater in the three non-RCTs with these data.
The overall mean decrease in A1c was 1.28% lower in the three RCTs with these data, and it was 0.9% lower in the one non-RCT with these data.
“In adults with obesity, bariatric surgery still confers the highest reductions in weight and BMI but confers similar effects in glycemic control when compared with GLP-1 agonists,” the researchers summarize.
Dr. Sarma received funding from the Clinical Investigator Program. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT OBESITYWEEK®
Bariatric surgery prompts visceral fat reduction, cardiac changes
Weight loss after bariatric surgery was linked with visceral fat reduction as well as reduced blood pressure, fasting glucose, and left ventricular remodeling, based an imaging study in 213 patients.
“We found that ventricular function measured by strain imaging improved in both the left and right sides of the heart, but function measured in the traditional method using endocardial motion [in other words, ejection fraction] actually worsened,” senior investigator Barry A. Borlaug, MD, said in an interview.
Although previous studies have shown positive effects of weight loss on the heart after bariatric surgery, most have been short term and have not specifically examined the effects of visceral fat reduction, wrote the investigators.
“We are in the middle of an increasing epidemic of obesity worldwide, but particularly in the United States, where it is currently projected that one in two adults will be obese by 2030,” added Dr. Borlaug of Mayo Clinic, Rochester, Minn. “Heart failure with preserved ejection fraction (HFpEF) is growing in tandem, and numerous recent studies have shown that obesity is one of the strongest risk factors for developing HFpEF, and that the severity of HFpEF is intimately linked to excess body fat. This suggests that therapies to reduce body fat could improve the cardiac abnormalities that cause HFpEF, which was our focus in this study,” he explained.
In the study, published in the Journal of the American College of Cardiology, the researchers reviewed echocardiography data from 213 obese patients before and more than 180 days after bariatric surgery. They also measured abdominal visceral adipose tissue (VAT) of 52 patients via computed tomography. The average age of the patients was 54 years, the average body mass index was 45 kg/m2, and 67% were women. Comorbidities included hypertension, diabetes, dyslipidemia, and obstructive sleep apnea.
The primary outcome was changes in cardiac structure and function.
After a median follow-up of 5.3 years, patients overall averaged a 23% reduction in body weight and a 22% reduction in BMI. In the 52 patients with abdominal scans, the VAT area decreased by 30% overall. Changes in left ventricular mass were significantly correlated to changes in the VAT.
Epicardial adipose thickness decreased by 14% overall. Left and right ventricular longitudinal strains improved at follow-up, but left atrial strain deteriorated, the researchers noted.
Although the mechanism of action remains unclear, the results suggest that left ventricular remodeling was associated with visceral adiposity rather than subcutaneous fat, the researchers wrote.
They also found that right ventricular strain was negatively correlated with VAT, but not with body weight or BMI.
“These findings suggest that weight loss, particularly reduction in visceral adiposity, benefits [right ventricular] structure and function in a manner akin to that observed in the [left ventricle],” the researchers noted.
Some surprises and limitations
Dr. Borlaug said he found some, but not all, of the results surprising. “Earlier studies had shown evidence for benefit from weight loss on cardiac structure and function, but had been limited by smaller sample sizes, shorter durations of evaluation, and variable methods used,” he said in an interview.
The findings that strain imaging showed both left and right ventricular function improved while EF declined “shows some of the problems with using EF, as it is affected by chamber size and geometry. We have previously shown that patients with HFpEF display an increase in fat around the heart, and this affects cardiac function and interaction between the left and right sides of the heart, so we expected to see that this fat depot would be reduced, and this was indeed the case,” Dr. Borlaug added.
In the current study, “visceral fat was most strongly tied to the heart remodeling in obesity, and changes in visceral fat were most strongly tied to improvements in cardiac structure following weight loss,” Dr. Borlaug told this news organization. “This further supports this concept that excess visceral fat plays a key role in HFpEF, especially in the abdomen and around the heart,” he said.
However, “The biggest surprise was the discordant effects in the left atrium,” Dr. Borlaug said. “Left atrial remodeling and dysfunction play a crucial role in HFpEF as well, and we expected that this would improve following weight loss, but in fact we observed that left atrial function deteriorated, and other indicators of atrial myopathy worsened, including higher estimates of left atrial pressures and increased prevalence of atrial fibrillation,” he said.
This difference emphasizes that weight loss may not address all abnormalities that lead to HFpEF, although a key limitation of the current study was the lack of a control group of patients with the same degree of obesity and no weight-loss intervention, and the deterioration in left atrial function might have been even greater in the absence of weight loss, Dr. Borlaug added.
Larger numbers support effects
Previous research shows that structural heart changes associated with obesity can be reversed through weight loss, but the current study fills a gap by providing long-term data in a larger sample than previously studied, wrote Paul Heidenreich, MD, of Stanford (Calif.) University in an accompanying editorial).
“There has been uncertainty regarding the prolonged effect of weight loss on cardiac function; this study was larger than many prior studies and provided a longer follow-up,” Dr. Heidenreich said in an interview.
“One unusual finding was that, while weight loss led to left ventricle reverse remodeling (reduction in wall thickness), the same effect was not seen for the left atrium; the left atrial size continued to increase,” he said. “I would have expected the left atrial changes to mirror the changes in the left ventricle,” he noted.
The findings support the greater cardiac risk of visceral vs. subcutaneous adipose tissue, and although body mass index will retain prognostic value, measures of central obesity are more likely predictors of cardiac structural changes and events and should be reported in clinical studies, Dr. Heidenreich wrote.
However, “We need a better understanding of the factors that influence left atrial remodeling and reverse remodeling,” Dr. Heidenreich told this news organization. “While left ventricular compliance and pressure play a role, there are other factors that need to be elucidated,” he said.
Studies in progress may inform practice
The current data call for further study to test novel treatments to facilitate weight loss in patients with HFpEF and those at risk for HFpEF, and some of these studies with medicines are underway, Dr. Borlaug said in the interview.
“Until such studies are completed, we will not truly understand the effects of weight loss on the heart, but the present data certainly provide strong support that patients who have obesity and HFpEF or are at risk for HFpEF should try to lose weight through lifestyle interventions,” he said.
Whether the cardiac changes seen in the current study would be different with nonsurgical weight loss remains a key question because many obese patients are reluctant to undergo bariatric surgery, Dr. Borlaug said. “We cannot assess whether the effects would differ with nonsurgical weight loss, and this requires further study,” he added.
As for additional research, “Randomized, controlled trials of weight-loss interventions, with appropriate controls and comprehensive assessments of cardiac structure, function, and hemodynamics will be most informative,” said Dr. Borlaug. “Larger trials powered to evaluate cardiovascular outcomes such as heart failure hospitalization or cardiovascular death also are critically important to better understand the role of weight loss to treat and prevent HFpEF, the ultimate form of obesity-related heart disease,” he emphasized.
The study was supported in part by grants to lead author Dr. Hidemi Sorimachi of the Mayo Clinic from the Uehara Memorial Foundation, Japan, and to corresponding author Dr. Borlaug from the National Institutes of Health. Dr. Borlaug also disclosed previous grants from National Institutes of Health/National Heart, Lung, and Blood Institute, AstraZeneca, Corvia, Medtronic, GlaxoSmithKline, Mesoblast, Novartis, and Tenax Therapeutics; and consulting fees from Actelion, Amgen, Aria, Axon Therapies, Boehringer Ingelheim, Edwards Lifesciences, Eli Lilly, Imbria, Janssen, Merck, Novo Nordisk, and VADovations. Dr. Heidenreich had no financial disclosures.
Weight loss after bariatric surgery was linked with visceral fat reduction as well as reduced blood pressure, fasting glucose, and left ventricular remodeling, based an imaging study in 213 patients.
“We found that ventricular function measured by strain imaging improved in both the left and right sides of the heart, but function measured in the traditional method using endocardial motion [in other words, ejection fraction] actually worsened,” senior investigator Barry A. Borlaug, MD, said in an interview.
Although previous studies have shown positive effects of weight loss on the heart after bariatric surgery, most have been short term and have not specifically examined the effects of visceral fat reduction, wrote the investigators.
“We are in the middle of an increasing epidemic of obesity worldwide, but particularly in the United States, where it is currently projected that one in two adults will be obese by 2030,” added Dr. Borlaug of Mayo Clinic, Rochester, Minn. “Heart failure with preserved ejection fraction (HFpEF) is growing in tandem, and numerous recent studies have shown that obesity is one of the strongest risk factors for developing HFpEF, and that the severity of HFpEF is intimately linked to excess body fat. This suggests that therapies to reduce body fat could improve the cardiac abnormalities that cause HFpEF, which was our focus in this study,” he explained.
In the study, published in the Journal of the American College of Cardiology, the researchers reviewed echocardiography data from 213 obese patients before and more than 180 days after bariatric surgery. They also measured abdominal visceral adipose tissue (VAT) of 52 patients via computed tomography. The average age of the patients was 54 years, the average body mass index was 45 kg/m2, and 67% were women. Comorbidities included hypertension, diabetes, dyslipidemia, and obstructive sleep apnea.
The primary outcome was changes in cardiac structure and function.
After a median follow-up of 5.3 years, patients overall averaged a 23% reduction in body weight and a 22% reduction in BMI. In the 52 patients with abdominal scans, the VAT area decreased by 30% overall. Changes in left ventricular mass were significantly correlated to changes in the VAT.
Epicardial adipose thickness decreased by 14% overall. Left and right ventricular longitudinal strains improved at follow-up, but left atrial strain deteriorated, the researchers noted.
Although the mechanism of action remains unclear, the results suggest that left ventricular remodeling was associated with visceral adiposity rather than subcutaneous fat, the researchers wrote.
They also found that right ventricular strain was negatively correlated with VAT, but not with body weight or BMI.
“These findings suggest that weight loss, particularly reduction in visceral adiposity, benefits [right ventricular] structure and function in a manner akin to that observed in the [left ventricle],” the researchers noted.
Some surprises and limitations
Dr. Borlaug said he found some, but not all, of the results surprising. “Earlier studies had shown evidence for benefit from weight loss on cardiac structure and function, but had been limited by smaller sample sizes, shorter durations of evaluation, and variable methods used,” he said in an interview.
The findings that strain imaging showed both left and right ventricular function improved while EF declined “shows some of the problems with using EF, as it is affected by chamber size and geometry. We have previously shown that patients with HFpEF display an increase in fat around the heart, and this affects cardiac function and interaction between the left and right sides of the heart, so we expected to see that this fat depot would be reduced, and this was indeed the case,” Dr. Borlaug added.
In the current study, “visceral fat was most strongly tied to the heart remodeling in obesity, and changes in visceral fat were most strongly tied to improvements in cardiac structure following weight loss,” Dr. Borlaug told this news organization. “This further supports this concept that excess visceral fat plays a key role in HFpEF, especially in the abdomen and around the heart,” he said.
However, “The biggest surprise was the discordant effects in the left atrium,” Dr. Borlaug said. “Left atrial remodeling and dysfunction play a crucial role in HFpEF as well, and we expected that this would improve following weight loss, but in fact we observed that left atrial function deteriorated, and other indicators of atrial myopathy worsened, including higher estimates of left atrial pressures and increased prevalence of atrial fibrillation,” he said.
This difference emphasizes that weight loss may not address all abnormalities that lead to HFpEF, although a key limitation of the current study was the lack of a control group of patients with the same degree of obesity and no weight-loss intervention, and the deterioration in left atrial function might have been even greater in the absence of weight loss, Dr. Borlaug added.
Larger numbers support effects
Previous research shows that structural heart changes associated with obesity can be reversed through weight loss, but the current study fills a gap by providing long-term data in a larger sample than previously studied, wrote Paul Heidenreich, MD, of Stanford (Calif.) University in an accompanying editorial).
“There has been uncertainty regarding the prolonged effect of weight loss on cardiac function; this study was larger than many prior studies and provided a longer follow-up,” Dr. Heidenreich said in an interview.
“One unusual finding was that, while weight loss led to left ventricle reverse remodeling (reduction in wall thickness), the same effect was not seen for the left atrium; the left atrial size continued to increase,” he said. “I would have expected the left atrial changes to mirror the changes in the left ventricle,” he noted.
The findings support the greater cardiac risk of visceral vs. subcutaneous adipose tissue, and although body mass index will retain prognostic value, measures of central obesity are more likely predictors of cardiac structural changes and events and should be reported in clinical studies, Dr. Heidenreich wrote.
However, “We need a better understanding of the factors that influence left atrial remodeling and reverse remodeling,” Dr. Heidenreich told this news organization. “While left ventricular compliance and pressure play a role, there are other factors that need to be elucidated,” he said.
Studies in progress may inform practice
The current data call for further study to test novel treatments to facilitate weight loss in patients with HFpEF and those at risk for HFpEF, and some of these studies with medicines are underway, Dr. Borlaug said in the interview.
“Until such studies are completed, we will not truly understand the effects of weight loss on the heart, but the present data certainly provide strong support that patients who have obesity and HFpEF or are at risk for HFpEF should try to lose weight through lifestyle interventions,” he said.
Whether the cardiac changes seen in the current study would be different with nonsurgical weight loss remains a key question because many obese patients are reluctant to undergo bariatric surgery, Dr. Borlaug said. “We cannot assess whether the effects would differ with nonsurgical weight loss, and this requires further study,” he added.
As for additional research, “Randomized, controlled trials of weight-loss interventions, with appropriate controls and comprehensive assessments of cardiac structure, function, and hemodynamics will be most informative,” said Dr. Borlaug. “Larger trials powered to evaluate cardiovascular outcomes such as heart failure hospitalization or cardiovascular death also are critically important to better understand the role of weight loss to treat and prevent HFpEF, the ultimate form of obesity-related heart disease,” he emphasized.
The study was supported in part by grants to lead author Dr. Hidemi Sorimachi of the Mayo Clinic from the Uehara Memorial Foundation, Japan, and to corresponding author Dr. Borlaug from the National Institutes of Health. Dr. Borlaug also disclosed previous grants from National Institutes of Health/National Heart, Lung, and Blood Institute, AstraZeneca, Corvia, Medtronic, GlaxoSmithKline, Mesoblast, Novartis, and Tenax Therapeutics; and consulting fees from Actelion, Amgen, Aria, Axon Therapies, Boehringer Ingelheim, Edwards Lifesciences, Eli Lilly, Imbria, Janssen, Merck, Novo Nordisk, and VADovations. Dr. Heidenreich had no financial disclosures.
Weight loss after bariatric surgery was linked with visceral fat reduction as well as reduced blood pressure, fasting glucose, and left ventricular remodeling, based an imaging study in 213 patients.
“We found that ventricular function measured by strain imaging improved in both the left and right sides of the heart, but function measured in the traditional method using endocardial motion [in other words, ejection fraction] actually worsened,” senior investigator Barry A. Borlaug, MD, said in an interview.
Although previous studies have shown positive effects of weight loss on the heart after bariatric surgery, most have been short term and have not specifically examined the effects of visceral fat reduction, wrote the investigators.
“We are in the middle of an increasing epidemic of obesity worldwide, but particularly in the United States, where it is currently projected that one in two adults will be obese by 2030,” added Dr. Borlaug of Mayo Clinic, Rochester, Minn. “Heart failure with preserved ejection fraction (HFpEF) is growing in tandem, and numerous recent studies have shown that obesity is one of the strongest risk factors for developing HFpEF, and that the severity of HFpEF is intimately linked to excess body fat. This suggests that therapies to reduce body fat could improve the cardiac abnormalities that cause HFpEF, which was our focus in this study,” he explained.
In the study, published in the Journal of the American College of Cardiology, the researchers reviewed echocardiography data from 213 obese patients before and more than 180 days after bariatric surgery. They also measured abdominal visceral adipose tissue (VAT) of 52 patients via computed tomography. The average age of the patients was 54 years, the average body mass index was 45 kg/m2, and 67% were women. Comorbidities included hypertension, diabetes, dyslipidemia, and obstructive sleep apnea.
The primary outcome was changes in cardiac structure and function.
After a median follow-up of 5.3 years, patients overall averaged a 23% reduction in body weight and a 22% reduction in BMI. In the 52 patients with abdominal scans, the VAT area decreased by 30% overall. Changes in left ventricular mass were significantly correlated to changes in the VAT.
Epicardial adipose thickness decreased by 14% overall. Left and right ventricular longitudinal strains improved at follow-up, but left atrial strain deteriorated, the researchers noted.
Although the mechanism of action remains unclear, the results suggest that left ventricular remodeling was associated with visceral adiposity rather than subcutaneous fat, the researchers wrote.
They also found that right ventricular strain was negatively correlated with VAT, but not with body weight or BMI.
“These findings suggest that weight loss, particularly reduction in visceral adiposity, benefits [right ventricular] structure and function in a manner akin to that observed in the [left ventricle],” the researchers noted.
Some surprises and limitations
Dr. Borlaug said he found some, but not all, of the results surprising. “Earlier studies had shown evidence for benefit from weight loss on cardiac structure and function, but had been limited by smaller sample sizes, shorter durations of evaluation, and variable methods used,” he said in an interview.
The findings that strain imaging showed both left and right ventricular function improved while EF declined “shows some of the problems with using EF, as it is affected by chamber size and geometry. We have previously shown that patients with HFpEF display an increase in fat around the heart, and this affects cardiac function and interaction between the left and right sides of the heart, so we expected to see that this fat depot would be reduced, and this was indeed the case,” Dr. Borlaug added.
In the current study, “visceral fat was most strongly tied to the heart remodeling in obesity, and changes in visceral fat were most strongly tied to improvements in cardiac structure following weight loss,” Dr. Borlaug told this news organization. “This further supports this concept that excess visceral fat plays a key role in HFpEF, especially in the abdomen and around the heart,” he said.
However, “The biggest surprise was the discordant effects in the left atrium,” Dr. Borlaug said. “Left atrial remodeling and dysfunction play a crucial role in HFpEF as well, and we expected that this would improve following weight loss, but in fact we observed that left atrial function deteriorated, and other indicators of atrial myopathy worsened, including higher estimates of left atrial pressures and increased prevalence of atrial fibrillation,” he said.
This difference emphasizes that weight loss may not address all abnormalities that lead to HFpEF, although a key limitation of the current study was the lack of a control group of patients with the same degree of obesity and no weight-loss intervention, and the deterioration in left atrial function might have been even greater in the absence of weight loss, Dr. Borlaug added.
Larger numbers support effects
Previous research shows that structural heart changes associated with obesity can be reversed through weight loss, but the current study fills a gap by providing long-term data in a larger sample than previously studied, wrote Paul Heidenreich, MD, of Stanford (Calif.) University in an accompanying editorial).
“There has been uncertainty regarding the prolonged effect of weight loss on cardiac function; this study was larger than many prior studies and provided a longer follow-up,” Dr. Heidenreich said in an interview.
“One unusual finding was that, while weight loss led to left ventricle reverse remodeling (reduction in wall thickness), the same effect was not seen for the left atrium; the left atrial size continued to increase,” he said. “I would have expected the left atrial changes to mirror the changes in the left ventricle,” he noted.
The findings support the greater cardiac risk of visceral vs. subcutaneous adipose tissue, and although body mass index will retain prognostic value, measures of central obesity are more likely predictors of cardiac structural changes and events and should be reported in clinical studies, Dr. Heidenreich wrote.
However, “We need a better understanding of the factors that influence left atrial remodeling and reverse remodeling,” Dr. Heidenreich told this news organization. “While left ventricular compliance and pressure play a role, there are other factors that need to be elucidated,” he said.
Studies in progress may inform practice
The current data call for further study to test novel treatments to facilitate weight loss in patients with HFpEF and those at risk for HFpEF, and some of these studies with medicines are underway, Dr. Borlaug said in the interview.
“Until such studies are completed, we will not truly understand the effects of weight loss on the heart, but the present data certainly provide strong support that patients who have obesity and HFpEF or are at risk for HFpEF should try to lose weight through lifestyle interventions,” he said.
Whether the cardiac changes seen in the current study would be different with nonsurgical weight loss remains a key question because many obese patients are reluctant to undergo bariatric surgery, Dr. Borlaug said. “We cannot assess whether the effects would differ with nonsurgical weight loss, and this requires further study,” he added.
As for additional research, “Randomized, controlled trials of weight-loss interventions, with appropriate controls and comprehensive assessments of cardiac structure, function, and hemodynamics will be most informative,” said Dr. Borlaug. “Larger trials powered to evaluate cardiovascular outcomes such as heart failure hospitalization or cardiovascular death also are critically important to better understand the role of weight loss to treat and prevent HFpEF, the ultimate form of obesity-related heart disease,” he emphasized.
The study was supported in part by grants to lead author Dr. Hidemi Sorimachi of the Mayo Clinic from the Uehara Memorial Foundation, Japan, and to corresponding author Dr. Borlaug from the National Institutes of Health. Dr. Borlaug also disclosed previous grants from National Institutes of Health/National Heart, Lung, and Blood Institute, AstraZeneca, Corvia, Medtronic, GlaxoSmithKline, Mesoblast, Novartis, and Tenax Therapeutics; and consulting fees from Actelion, Amgen, Aria, Axon Therapies, Boehringer Ingelheim, Edwards Lifesciences, Eli Lilly, Imbria, Janssen, Merck, Novo Nordisk, and VADovations. Dr. Heidenreich had no financial disclosures.
FROM JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Bariatric surgery may up risk for epilepsy
Analyzing health records, investigators compared almost 17,000 patients who had undergone bariatric surgery with more than 620,000 individuals with obesity who had not undergone the surgery.
During a minimum 3-year follow-up period, the surgery group had a 45% higher risk of developing epilepsy than the nonsurgery group. Moreover, patients who had a stroke after their bariatric surgery were 14 times more likely to develop epilepsy than those who did not have a stroke.
“When considering having bariatric surgery, people should talk to their doctors about the benefits and risks,” senior investigator Jorge Burneo, MD, professor of neurology, biostatistics, and epidemiology and endowed chair in epilepsy at Western University, London, told this news organization.
“While there are many health benefits of weight loss, our findings suggest that epilepsy is a long-term risk of bariatric surgery for weight loss,” Dr. Burneo said.
The findings were published online in Neurology.
Unrecognized risk factor?
Bariatric surgery has become more common as global rates of obesity have increased. The surgery has been shown to reduce the risk for serious obesity-related conditions, the researchers note.
However, “in addition to the positive outcomes of bariatric surgery, several long-term neurological complications have also been identified,” they write.
One previous study reported increased epilepsy risk following gastric bypass. Those findings “suggest that bariatric surgery may be an unrecognized epilepsy risk factor; however, this possible association has not been thoroughly explored,” write the investigators.
Dr. Burneo said he conducted the study because he has seen patients with epilepsy in his clinic who were “without risk factors, with normal MRIs, who shared the history of having bariatric surgery before the development of epilepsy.”
The researchers’ primary objective was to “assess whether epilepsy risk is elevated following bariatric surgery for weight loss relative to a nonsurgical cohort of patients who are obese,” he noted.
The study used linked administrative health databases in Ontario, Canada. Patients were accrued from July 1, 2010, to Dec. 31, 2016, and were followed until Dec. 31, 2019. The analysis included 639,472 participants, 2.7% of whom had undergone bariatric surgery.
The “exposed” cohort consisted of all Ontario residents aged 18 years or older who had undergone bariatric surgery during the 6-year period (n = 16,958; 65.1% women; mean age, 47.4 years), while the “unexposed” cohort consisted of patients hospitalized with a diagnosis of obesity who had not undergone bariatric surgery (n = 622,514; 62.8% women; mean age, 47.6 years).
Patients with a history of seizures, epilepsy, epilepsy risk factors, prior brain surgery, psychiatric disorders, or drug or alcohol abuse/dependence were excluded from the analysis.
The researchers collected data on patients’ sociodemographic characteristics at the index date, as well as Charlson Comorbidity Index scores during the 2 years prior to index, and data regarding several specific comorbidities, such as diabetes mellitus, hypertension, sleep apnea, depression/anxiety, and cardiovascular factors.
The exposed and unexposed cohorts were followed for a median period of 5.8 and 5.9 person-years, respectively.
‘Unclear’ mechanisms
Before weighting, 0.4% of participants in the exposed cohort (n = 73) developed epilepsy, versus 0.2% of participants in the unexposed cohort (n = 1,260) by the end of the follow-up period.
In the weighted cohorts, there were 50.1 epilepsy diagnoses per 100,000 person-years, versus 34.1 per 100,000 person-years (rate difference, 16 per 100,000 person-years).
The multivariable analysis of the weighted cohort showed the hazard ratio for epilepsy cases that were associated with bariatric surgery was 1.45 (95% confidence interval, 1.35-1.56), after adjusting for sleep apnea and including stroke as a time-varying covariate.
Having a stroke during the follow-up period increased epilepsy 14-fold in the exposed cohort (HR, 14.03; 95% CI, 4.25-46.25).
The investigators note that they were unable to measure obesity status or body mass index throughout the study and that some obesity-related comorbidities “may affect epilepsy risk.”
In addition, Dr. Burneo reported that the study did not investigate potential causes and mechanisms of the association between bariatric surgery and epilepsy risk.
Hypotheses “include potential nutritional deficiencies, receipt of general anesthesia, or other unclear causes,” he said.
“Future research should investigate epilepsy as a potential long-term complication of bariatric surgery, exploring the possible effects of this procedure,” Dr. Burneo added.
Risk-benefit discussion
In a comment, Jacqueline French, MD, professor of neurology at NYU Grossman School of Medicine, and director of NYU’s Epilepsy Study Consortium, said she was “not 100% surprised by the findings” because she has seen in her clinical practice “a number of patients who developed epilepsy after bariatric surgery or had a history of bariatric surgery at the time they developed epilepsy.”
On the other hand, she has also seen patients who did not have a history of bariatric surgery and who developed epilepsy.
“I’m unable to tell if there is an association, although I’ve had it at the back of my head as a thought and wondered about it,” said Dr. French, who is also the chief medical and innovation officer at the Epilepsy Foundation. She was not involved with the study.
She noted that possible mechanisms underlying the association are that gastric bypass surgery leads to a “significant alteration” in nutrient absorption. Moreover, “we now know that the microbiome is associated with epilepsy” and that changes occur in the gut microbiome after bariatric surgery, Dr. French said.
There are two take-home messages for practicing clinicians, she added.
“Although the risk [of developing epilepsy] is very low, it should be presented as part of the risks and benefits to patients considering bariatric surgery,” she said.
“It’s equally important to follow up on the potential differences in these patients who go on to develop epilepsy following bariatric surgery,” said Dr. French. “Is there a certain metabolic profile or some nutrient previously absorbed that now is not absorbed that might predispose people to risk?”
This would be “enormously important to know because it might not just pertain to these people but to a whole other cohort of people who develop epilepsy,” Dr. French concluded.
The study was funded by the Ontario Ministry of Health and Ministry of Long-Term Care and by the Jack Cowin Endowed Chair in Epilepsy Research at Western University. Dr. Burneo holds the Jack Cowin Endowed Chair in Epilepsy Research at Western University. The other investigators and Dr. French have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Analyzing health records, investigators compared almost 17,000 patients who had undergone bariatric surgery with more than 620,000 individuals with obesity who had not undergone the surgery.
During a minimum 3-year follow-up period, the surgery group had a 45% higher risk of developing epilepsy than the nonsurgery group. Moreover, patients who had a stroke after their bariatric surgery were 14 times more likely to develop epilepsy than those who did not have a stroke.
“When considering having bariatric surgery, people should talk to their doctors about the benefits and risks,” senior investigator Jorge Burneo, MD, professor of neurology, biostatistics, and epidemiology and endowed chair in epilepsy at Western University, London, told this news organization.
“While there are many health benefits of weight loss, our findings suggest that epilepsy is a long-term risk of bariatric surgery for weight loss,” Dr. Burneo said.
The findings were published online in Neurology.
Unrecognized risk factor?
Bariatric surgery has become more common as global rates of obesity have increased. The surgery has been shown to reduce the risk for serious obesity-related conditions, the researchers note.
However, “in addition to the positive outcomes of bariatric surgery, several long-term neurological complications have also been identified,” they write.
One previous study reported increased epilepsy risk following gastric bypass. Those findings “suggest that bariatric surgery may be an unrecognized epilepsy risk factor; however, this possible association has not been thoroughly explored,” write the investigators.
Dr. Burneo said he conducted the study because he has seen patients with epilepsy in his clinic who were “without risk factors, with normal MRIs, who shared the history of having bariatric surgery before the development of epilepsy.”
The researchers’ primary objective was to “assess whether epilepsy risk is elevated following bariatric surgery for weight loss relative to a nonsurgical cohort of patients who are obese,” he noted.
The study used linked administrative health databases in Ontario, Canada. Patients were accrued from July 1, 2010, to Dec. 31, 2016, and were followed until Dec. 31, 2019. The analysis included 639,472 participants, 2.7% of whom had undergone bariatric surgery.
The “exposed” cohort consisted of all Ontario residents aged 18 years or older who had undergone bariatric surgery during the 6-year period (n = 16,958; 65.1% women; mean age, 47.4 years), while the “unexposed” cohort consisted of patients hospitalized with a diagnosis of obesity who had not undergone bariatric surgery (n = 622,514; 62.8% women; mean age, 47.6 years).
Patients with a history of seizures, epilepsy, epilepsy risk factors, prior brain surgery, psychiatric disorders, or drug or alcohol abuse/dependence were excluded from the analysis.
The researchers collected data on patients’ sociodemographic characteristics at the index date, as well as Charlson Comorbidity Index scores during the 2 years prior to index, and data regarding several specific comorbidities, such as diabetes mellitus, hypertension, sleep apnea, depression/anxiety, and cardiovascular factors.
The exposed and unexposed cohorts were followed for a median period of 5.8 and 5.9 person-years, respectively.
‘Unclear’ mechanisms
Before weighting, 0.4% of participants in the exposed cohort (n = 73) developed epilepsy, versus 0.2% of participants in the unexposed cohort (n = 1,260) by the end of the follow-up period.
In the weighted cohorts, there were 50.1 epilepsy diagnoses per 100,000 person-years, versus 34.1 per 100,000 person-years (rate difference, 16 per 100,000 person-years).
The multivariable analysis of the weighted cohort showed the hazard ratio for epilepsy cases that were associated with bariatric surgery was 1.45 (95% confidence interval, 1.35-1.56), after adjusting for sleep apnea and including stroke as a time-varying covariate.
Having a stroke during the follow-up period increased epilepsy 14-fold in the exposed cohort (HR, 14.03; 95% CI, 4.25-46.25).
The investigators note that they were unable to measure obesity status or body mass index throughout the study and that some obesity-related comorbidities “may affect epilepsy risk.”
In addition, Dr. Burneo reported that the study did not investigate potential causes and mechanisms of the association between bariatric surgery and epilepsy risk.
Hypotheses “include potential nutritional deficiencies, receipt of general anesthesia, or other unclear causes,” he said.
“Future research should investigate epilepsy as a potential long-term complication of bariatric surgery, exploring the possible effects of this procedure,” Dr. Burneo added.
Risk-benefit discussion
In a comment, Jacqueline French, MD, professor of neurology at NYU Grossman School of Medicine, and director of NYU’s Epilepsy Study Consortium, said she was “not 100% surprised by the findings” because she has seen in her clinical practice “a number of patients who developed epilepsy after bariatric surgery or had a history of bariatric surgery at the time they developed epilepsy.”
On the other hand, she has also seen patients who did not have a history of bariatric surgery and who developed epilepsy.
“I’m unable to tell if there is an association, although I’ve had it at the back of my head as a thought and wondered about it,” said Dr. French, who is also the chief medical and innovation officer at the Epilepsy Foundation. She was not involved with the study.
She noted that possible mechanisms underlying the association are that gastric bypass surgery leads to a “significant alteration” in nutrient absorption. Moreover, “we now know that the microbiome is associated with epilepsy” and that changes occur in the gut microbiome after bariatric surgery, Dr. French said.
There are two take-home messages for practicing clinicians, she added.
“Although the risk [of developing epilepsy] is very low, it should be presented as part of the risks and benefits to patients considering bariatric surgery,” she said.
“It’s equally important to follow up on the potential differences in these patients who go on to develop epilepsy following bariatric surgery,” said Dr. French. “Is there a certain metabolic profile or some nutrient previously absorbed that now is not absorbed that might predispose people to risk?”
This would be “enormously important to know because it might not just pertain to these people but to a whole other cohort of people who develop epilepsy,” Dr. French concluded.
The study was funded by the Ontario Ministry of Health and Ministry of Long-Term Care and by the Jack Cowin Endowed Chair in Epilepsy Research at Western University. Dr. Burneo holds the Jack Cowin Endowed Chair in Epilepsy Research at Western University. The other investigators and Dr. French have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Analyzing health records, investigators compared almost 17,000 patients who had undergone bariatric surgery with more than 620,000 individuals with obesity who had not undergone the surgery.
During a minimum 3-year follow-up period, the surgery group had a 45% higher risk of developing epilepsy than the nonsurgery group. Moreover, patients who had a stroke after their bariatric surgery were 14 times more likely to develop epilepsy than those who did not have a stroke.
“When considering having bariatric surgery, people should talk to their doctors about the benefits and risks,” senior investigator Jorge Burneo, MD, professor of neurology, biostatistics, and epidemiology and endowed chair in epilepsy at Western University, London, told this news organization.
“While there are many health benefits of weight loss, our findings suggest that epilepsy is a long-term risk of bariatric surgery for weight loss,” Dr. Burneo said.
The findings were published online in Neurology.
Unrecognized risk factor?
Bariatric surgery has become more common as global rates of obesity have increased. The surgery has been shown to reduce the risk for serious obesity-related conditions, the researchers note.
However, “in addition to the positive outcomes of bariatric surgery, several long-term neurological complications have also been identified,” they write.
One previous study reported increased epilepsy risk following gastric bypass. Those findings “suggest that bariatric surgery may be an unrecognized epilepsy risk factor; however, this possible association has not been thoroughly explored,” write the investigators.
Dr. Burneo said he conducted the study because he has seen patients with epilepsy in his clinic who were “without risk factors, with normal MRIs, who shared the history of having bariatric surgery before the development of epilepsy.”
The researchers’ primary objective was to “assess whether epilepsy risk is elevated following bariatric surgery for weight loss relative to a nonsurgical cohort of patients who are obese,” he noted.
The study used linked administrative health databases in Ontario, Canada. Patients were accrued from July 1, 2010, to Dec. 31, 2016, and were followed until Dec. 31, 2019. The analysis included 639,472 participants, 2.7% of whom had undergone bariatric surgery.
The “exposed” cohort consisted of all Ontario residents aged 18 years or older who had undergone bariatric surgery during the 6-year period (n = 16,958; 65.1% women; mean age, 47.4 years), while the “unexposed” cohort consisted of patients hospitalized with a diagnosis of obesity who had not undergone bariatric surgery (n = 622,514; 62.8% women; mean age, 47.6 years).
Patients with a history of seizures, epilepsy, epilepsy risk factors, prior brain surgery, psychiatric disorders, or drug or alcohol abuse/dependence were excluded from the analysis.
The researchers collected data on patients’ sociodemographic characteristics at the index date, as well as Charlson Comorbidity Index scores during the 2 years prior to index, and data regarding several specific comorbidities, such as diabetes mellitus, hypertension, sleep apnea, depression/anxiety, and cardiovascular factors.
The exposed and unexposed cohorts were followed for a median period of 5.8 and 5.9 person-years, respectively.
‘Unclear’ mechanisms
Before weighting, 0.4% of participants in the exposed cohort (n = 73) developed epilepsy, versus 0.2% of participants in the unexposed cohort (n = 1,260) by the end of the follow-up period.
In the weighted cohorts, there were 50.1 epilepsy diagnoses per 100,000 person-years, versus 34.1 per 100,000 person-years (rate difference, 16 per 100,000 person-years).
The multivariable analysis of the weighted cohort showed the hazard ratio for epilepsy cases that were associated with bariatric surgery was 1.45 (95% confidence interval, 1.35-1.56), after adjusting for sleep apnea and including stroke as a time-varying covariate.
Having a stroke during the follow-up period increased epilepsy 14-fold in the exposed cohort (HR, 14.03; 95% CI, 4.25-46.25).
The investigators note that they were unable to measure obesity status or body mass index throughout the study and that some obesity-related comorbidities “may affect epilepsy risk.”
In addition, Dr. Burneo reported that the study did not investigate potential causes and mechanisms of the association between bariatric surgery and epilepsy risk.
Hypotheses “include potential nutritional deficiencies, receipt of general anesthesia, or other unclear causes,” he said.
“Future research should investigate epilepsy as a potential long-term complication of bariatric surgery, exploring the possible effects of this procedure,” Dr. Burneo added.
Risk-benefit discussion
In a comment, Jacqueline French, MD, professor of neurology at NYU Grossman School of Medicine, and director of NYU’s Epilepsy Study Consortium, said she was “not 100% surprised by the findings” because she has seen in her clinical practice “a number of patients who developed epilepsy after bariatric surgery or had a history of bariatric surgery at the time they developed epilepsy.”
On the other hand, she has also seen patients who did not have a history of bariatric surgery and who developed epilepsy.
“I’m unable to tell if there is an association, although I’ve had it at the back of my head as a thought and wondered about it,” said Dr. French, who is also the chief medical and innovation officer at the Epilepsy Foundation. She was not involved with the study.
She noted that possible mechanisms underlying the association are that gastric bypass surgery leads to a “significant alteration” in nutrient absorption. Moreover, “we now know that the microbiome is associated with epilepsy” and that changes occur in the gut microbiome after bariatric surgery, Dr. French said.
There are two take-home messages for practicing clinicians, she added.
“Although the risk [of developing epilepsy] is very low, it should be presented as part of the risks and benefits to patients considering bariatric surgery,” she said.
“It’s equally important to follow up on the potential differences in these patients who go on to develop epilepsy following bariatric surgery,” said Dr. French. “Is there a certain metabolic profile or some nutrient previously absorbed that now is not absorbed that might predispose people to risk?”
This would be “enormously important to know because it might not just pertain to these people but to a whole other cohort of people who develop epilepsy,” Dr. French concluded.
The study was funded by the Ontario Ministry of Health and Ministry of Long-Term Care and by the Jack Cowin Endowed Chair in Epilepsy Research at Western University. Dr. Burneo holds the Jack Cowin Endowed Chair in Epilepsy Research at Western University. The other investigators and Dr. French have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
New ESC guidelines for cutting CV risk in noncardiac surgery
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2022
Weight-loss surgery has a big effect on marriage
Kristal was only in her mid-30s when she decided to have surgery. Her doctor said it was too early. But the Oregon mom of three had found herself in the hospital twice for obesity-related lung complications before her 35th birthday. So she got the gastric sleeve.
And at first it seemed like the best decision for her and her family. She was losing weight – 100 pounds in 16 months – and so was her husband. The whole family was more active and seemed to have more energy. But then her husband’s weight began to creep back up.
While she joined a running group and signed up for half-marathons, her husband’s depression and drinking worsened. The healthier lifestyle they’d shared was now an unspoken wedge between them.
And the added attention Kristal was getting from men and women because of her thinner size only added to the tension. After 30 years together and 22 years of marriage, the high school sweethearts divorced in June 2021. Kristal’s weight loss wasn’t the only problem, but she and her ex-husband believe it was the beginning of the end.
An unexpected outcome?
New research from the University of Pittsburgh found that Kristal’s experience is a common one. The study looked at data from 1,441 bariatric surgery patients and found that never-married patients were over 50% more likely to get married, and married patients were more than twice as likely to get divorced, compared to the general U.S. population.
This U.S. data follows two Scandinavian studies from 2018 and 2020 that found similar relationship changes after bariatric surgery. But the postsurgery divorce rate in the United States was only about half that found in the Danish and Swedish studies, according to the new study published in Annals of Surgery.
It’s important to note that even with an increase in the divorce rate, most marriages in the study were unchanged, said epidemiologist and lead author Wendy King, PhD. In fact, 81% of couples were still married 5 years after surgery. But where the U.S. population has a divorce rate of 3.5%, bariatric patients in the study had an 8% divorce rate. Likewise, those who’d never been married before the surgery had a marriage rate of 18%, compared to 7% in the U.S. population.
Surgery certainly isn’t a death sentence for a patient’s love life. But the uptick in marriage and divorce suggests bariatric surgery significantly impacts how people engage in relationships.
“It makes sense,” said clinical psychologist Rachel Goldman, PhD, who specializes in health and wellness issues and bariatric surgery cases in New York City. “People are changing their lifestyle.” And those changes don’t start or stop the day of surgery, they begin as soon as someone decides to have surgery and continue as a lifelong process, she said.
For some patients, these healthy habits may offer a “new lease on life,” said Dr. King. According to the study, patients who had better physical health after surgery were more likely to get married.
But the continual lifestyle changes can dramatically impact the rituals of existing relationships, said Dr. Goldman. Maybe a couple loved to go out and enjoy an extravagant meal before surgery, or they had ice cream and watched a movie every Friday. The habit changes that come with bariatric surgery can require one partner to focus less on those rituals.
These sorts of changes may leave one or both people feeling like their partner is turning away from them, said Don Cole, DMin, a relationship therapist and clinical director at the Gottman Institute in Seattle, a think tank focused on the science of relationships. The person who had surgery may feel unsupported in the new journey if the partner keeps advocating for unhealthy habits, he said. And the person who didn’t have surgery may feel cast aside by the partner’s new health priorities.
Changes, even those that are positive and healthy, create a kind of crisis for relationships, Dr. Cole said. It’s not just bariatric surgery. Bringing a baby into the home, infertility treatments, and substance abuse recovery are all considered positive changes that are also predictors of relationship dissatisfaction and divorce, he said.
A couple could have a range of emotions after one partner gets bariatric surgery, Dr. Cole said. Unfortunately, “my experience as a therapist says they aren’t that good [at talking about it],” he said.
But bariatric surgery isn’t the only thing at play in these relationship changes, according to the study. Married patients had a much lower chance of separation or divorce (13%) than patients who were unmarried but living together (44%) by 5 years after surgery. Similarly, most people who were already separated either got divorced or resumed being married. It’s as if the surgery and lifestyle changes served as a catalyst for people who already had one foot out of (or in) the door, Dr. Goldman said.
A high sexual desire after surgery was also a predictor of divorce. In fact, there were more things before surgery that impacted divorce than surgery-related changes. It’s possible that many of these patients are “on the path toward change already,” Dr. King said. “Who knows how much the surgery had to do with it.”
Dr. Goldman recalled a patient who, before surgery, had very low self-worth. She wasn’t satisfied with her relationship but admitted to staying because she didn’t believe she could do any better than her current partner. After surgery, her perspective radically changed. She started to get healthier, invested in her education, and changed jobs. And when her partner refused to join her in making changes, she left. Maybe some of these patients “were already thinking about leaving but just didn’t have the confidence,” Dr. Goldman said.
Still, it’s critical that patients receive more counseling on how choosing to have bariatric surgery can impact their relationship before and after their weight loss procedure, Dr. King said. It should be the standard of care.
Currently, relationship-specific counseling isn’t required, Dr. Goldman said. Most programs do require a psychosocial evaluation before surgery, “but they are quite varied.” And even in programs where relationships are mentioned, there often isn’t a psychologist or licensed mental health professional on the team.
Since Dr. King’s previous research on substance abuse after bariatric surgery changed common practice in the field, Dr. Goldman said she hopes the new data will have a similar influence and relationship counseling will become the norm.
Dr. Cole actually had bariatric surgery. He recalled potential relationship issues were briefly mentioned. Someone at the clinic said if his marriage felt challenged, he should seek help from a professional, and that was it.
For Dr. Cole, there were unexpected negative feelings of shame and disappointment after surgery. He felt the extreme weight loss was all his colleagues could talk about and was very disappointed when there was no change in his chronic pain, a primary reason he had the procedure.
Fortunately, he could talk to his wife – also is a relationship therapist at Gottman – about the range of emotions. “One of the things that we know that creates a deep sense of trust is [when] I know my partner is there for me when I’m not well,” Dr. Cole said.
But these negative emotions can be the very things that feel most difficult to talk about or hear from a partner. It’s hard to share our own negative feelings and to hear someone else’s, Dr. Cole said.
He advises creating a new “ritual of connection: moments in time when you plan to turn toward one another.”
That could be a daily walk, where you intentionally talk about the surgery-related changes that both of you have had. Dr. Cole said to ask yourself, “Are we intentional about turning toward one another in those [challenging] moments?”
A version of this article first appeared on WebMD.com.
Kristal was only in her mid-30s when she decided to have surgery. Her doctor said it was too early. But the Oregon mom of three had found herself in the hospital twice for obesity-related lung complications before her 35th birthday. So she got the gastric sleeve.
And at first it seemed like the best decision for her and her family. She was losing weight – 100 pounds in 16 months – and so was her husband. The whole family was more active and seemed to have more energy. But then her husband’s weight began to creep back up.
While she joined a running group and signed up for half-marathons, her husband’s depression and drinking worsened. The healthier lifestyle they’d shared was now an unspoken wedge between them.
And the added attention Kristal was getting from men and women because of her thinner size only added to the tension. After 30 years together and 22 years of marriage, the high school sweethearts divorced in June 2021. Kristal’s weight loss wasn’t the only problem, but she and her ex-husband believe it was the beginning of the end.
An unexpected outcome?
New research from the University of Pittsburgh found that Kristal’s experience is a common one. The study looked at data from 1,441 bariatric surgery patients and found that never-married patients were over 50% more likely to get married, and married patients were more than twice as likely to get divorced, compared to the general U.S. population.
This U.S. data follows two Scandinavian studies from 2018 and 2020 that found similar relationship changes after bariatric surgery. But the postsurgery divorce rate in the United States was only about half that found in the Danish and Swedish studies, according to the new study published in Annals of Surgery.
It’s important to note that even with an increase in the divorce rate, most marriages in the study were unchanged, said epidemiologist and lead author Wendy King, PhD. In fact, 81% of couples were still married 5 years after surgery. But where the U.S. population has a divorce rate of 3.5%, bariatric patients in the study had an 8% divorce rate. Likewise, those who’d never been married before the surgery had a marriage rate of 18%, compared to 7% in the U.S. population.
Surgery certainly isn’t a death sentence for a patient’s love life. But the uptick in marriage and divorce suggests bariatric surgery significantly impacts how people engage in relationships.
“It makes sense,” said clinical psychologist Rachel Goldman, PhD, who specializes in health and wellness issues and bariatric surgery cases in New York City. “People are changing their lifestyle.” And those changes don’t start or stop the day of surgery, they begin as soon as someone decides to have surgery and continue as a lifelong process, she said.
For some patients, these healthy habits may offer a “new lease on life,” said Dr. King. According to the study, patients who had better physical health after surgery were more likely to get married.
But the continual lifestyle changes can dramatically impact the rituals of existing relationships, said Dr. Goldman. Maybe a couple loved to go out and enjoy an extravagant meal before surgery, or they had ice cream and watched a movie every Friday. The habit changes that come with bariatric surgery can require one partner to focus less on those rituals.
These sorts of changes may leave one or both people feeling like their partner is turning away from them, said Don Cole, DMin, a relationship therapist and clinical director at the Gottman Institute in Seattle, a think tank focused on the science of relationships. The person who had surgery may feel unsupported in the new journey if the partner keeps advocating for unhealthy habits, he said. And the person who didn’t have surgery may feel cast aside by the partner’s new health priorities.
Changes, even those that are positive and healthy, create a kind of crisis for relationships, Dr. Cole said. It’s not just bariatric surgery. Bringing a baby into the home, infertility treatments, and substance abuse recovery are all considered positive changes that are also predictors of relationship dissatisfaction and divorce, he said.
A couple could have a range of emotions after one partner gets bariatric surgery, Dr. Cole said. Unfortunately, “my experience as a therapist says they aren’t that good [at talking about it],” he said.
But bariatric surgery isn’t the only thing at play in these relationship changes, according to the study. Married patients had a much lower chance of separation or divorce (13%) than patients who were unmarried but living together (44%) by 5 years after surgery. Similarly, most people who were already separated either got divorced or resumed being married. It’s as if the surgery and lifestyle changes served as a catalyst for people who already had one foot out of (or in) the door, Dr. Goldman said.
A high sexual desire after surgery was also a predictor of divorce. In fact, there were more things before surgery that impacted divorce than surgery-related changes. It’s possible that many of these patients are “on the path toward change already,” Dr. King said. “Who knows how much the surgery had to do with it.”
Dr. Goldman recalled a patient who, before surgery, had very low self-worth. She wasn’t satisfied with her relationship but admitted to staying because she didn’t believe she could do any better than her current partner. After surgery, her perspective radically changed. She started to get healthier, invested in her education, and changed jobs. And when her partner refused to join her in making changes, she left. Maybe some of these patients “were already thinking about leaving but just didn’t have the confidence,” Dr. Goldman said.
Still, it’s critical that patients receive more counseling on how choosing to have bariatric surgery can impact their relationship before and after their weight loss procedure, Dr. King said. It should be the standard of care.
Currently, relationship-specific counseling isn’t required, Dr. Goldman said. Most programs do require a psychosocial evaluation before surgery, “but they are quite varied.” And even in programs where relationships are mentioned, there often isn’t a psychologist or licensed mental health professional on the team.
Since Dr. King’s previous research on substance abuse after bariatric surgery changed common practice in the field, Dr. Goldman said she hopes the new data will have a similar influence and relationship counseling will become the norm.
Dr. Cole actually had bariatric surgery. He recalled potential relationship issues were briefly mentioned. Someone at the clinic said if his marriage felt challenged, he should seek help from a professional, and that was it.
For Dr. Cole, there were unexpected negative feelings of shame and disappointment after surgery. He felt the extreme weight loss was all his colleagues could talk about and was very disappointed when there was no change in his chronic pain, a primary reason he had the procedure.
Fortunately, he could talk to his wife – also is a relationship therapist at Gottman – about the range of emotions. “One of the things that we know that creates a deep sense of trust is [when] I know my partner is there for me when I’m not well,” Dr. Cole said.
But these negative emotions can be the very things that feel most difficult to talk about or hear from a partner. It’s hard to share our own negative feelings and to hear someone else’s, Dr. Cole said.
He advises creating a new “ritual of connection: moments in time when you plan to turn toward one another.”
That could be a daily walk, where you intentionally talk about the surgery-related changes that both of you have had. Dr. Cole said to ask yourself, “Are we intentional about turning toward one another in those [challenging] moments?”
A version of this article first appeared on WebMD.com.
Kristal was only in her mid-30s when she decided to have surgery. Her doctor said it was too early. But the Oregon mom of three had found herself in the hospital twice for obesity-related lung complications before her 35th birthday. So she got the gastric sleeve.
And at first it seemed like the best decision for her and her family. She was losing weight – 100 pounds in 16 months – and so was her husband. The whole family was more active and seemed to have more energy. But then her husband’s weight began to creep back up.
While she joined a running group and signed up for half-marathons, her husband’s depression and drinking worsened. The healthier lifestyle they’d shared was now an unspoken wedge between them.
And the added attention Kristal was getting from men and women because of her thinner size only added to the tension. After 30 years together and 22 years of marriage, the high school sweethearts divorced in June 2021. Kristal’s weight loss wasn’t the only problem, but she and her ex-husband believe it was the beginning of the end.
An unexpected outcome?
New research from the University of Pittsburgh found that Kristal’s experience is a common one. The study looked at data from 1,441 bariatric surgery patients and found that never-married patients were over 50% more likely to get married, and married patients were more than twice as likely to get divorced, compared to the general U.S. population.
This U.S. data follows two Scandinavian studies from 2018 and 2020 that found similar relationship changes after bariatric surgery. But the postsurgery divorce rate in the United States was only about half that found in the Danish and Swedish studies, according to the new study published in Annals of Surgery.
It’s important to note that even with an increase in the divorce rate, most marriages in the study were unchanged, said epidemiologist and lead author Wendy King, PhD. In fact, 81% of couples were still married 5 years after surgery. But where the U.S. population has a divorce rate of 3.5%, bariatric patients in the study had an 8% divorce rate. Likewise, those who’d never been married before the surgery had a marriage rate of 18%, compared to 7% in the U.S. population.
Surgery certainly isn’t a death sentence for a patient’s love life. But the uptick in marriage and divorce suggests bariatric surgery significantly impacts how people engage in relationships.
“It makes sense,” said clinical psychologist Rachel Goldman, PhD, who specializes in health and wellness issues and bariatric surgery cases in New York City. “People are changing their lifestyle.” And those changes don’t start or stop the day of surgery, they begin as soon as someone decides to have surgery and continue as a lifelong process, she said.
For some patients, these healthy habits may offer a “new lease on life,” said Dr. King. According to the study, patients who had better physical health after surgery were more likely to get married.
But the continual lifestyle changes can dramatically impact the rituals of existing relationships, said Dr. Goldman. Maybe a couple loved to go out and enjoy an extravagant meal before surgery, or they had ice cream and watched a movie every Friday. The habit changes that come with bariatric surgery can require one partner to focus less on those rituals.
These sorts of changes may leave one or both people feeling like their partner is turning away from them, said Don Cole, DMin, a relationship therapist and clinical director at the Gottman Institute in Seattle, a think tank focused on the science of relationships. The person who had surgery may feel unsupported in the new journey if the partner keeps advocating for unhealthy habits, he said. And the person who didn’t have surgery may feel cast aside by the partner’s new health priorities.
Changes, even those that are positive and healthy, create a kind of crisis for relationships, Dr. Cole said. It’s not just bariatric surgery. Bringing a baby into the home, infertility treatments, and substance abuse recovery are all considered positive changes that are also predictors of relationship dissatisfaction and divorce, he said.
A couple could have a range of emotions after one partner gets bariatric surgery, Dr. Cole said. Unfortunately, “my experience as a therapist says they aren’t that good [at talking about it],” he said.
But bariatric surgery isn’t the only thing at play in these relationship changes, according to the study. Married patients had a much lower chance of separation or divorce (13%) than patients who were unmarried but living together (44%) by 5 years after surgery. Similarly, most people who were already separated either got divorced or resumed being married. It’s as if the surgery and lifestyle changes served as a catalyst for people who already had one foot out of (or in) the door, Dr. Goldman said.
A high sexual desire after surgery was also a predictor of divorce. In fact, there were more things before surgery that impacted divorce than surgery-related changes. It’s possible that many of these patients are “on the path toward change already,” Dr. King said. “Who knows how much the surgery had to do with it.”
Dr. Goldman recalled a patient who, before surgery, had very low self-worth. She wasn’t satisfied with her relationship but admitted to staying because she didn’t believe she could do any better than her current partner. After surgery, her perspective radically changed. She started to get healthier, invested in her education, and changed jobs. And when her partner refused to join her in making changes, she left. Maybe some of these patients “were already thinking about leaving but just didn’t have the confidence,” Dr. Goldman said.
Still, it’s critical that patients receive more counseling on how choosing to have bariatric surgery can impact their relationship before and after their weight loss procedure, Dr. King said. It should be the standard of care.
Currently, relationship-specific counseling isn’t required, Dr. Goldman said. Most programs do require a psychosocial evaluation before surgery, “but they are quite varied.” And even in programs where relationships are mentioned, there often isn’t a psychologist or licensed mental health professional on the team.
Since Dr. King’s previous research on substance abuse after bariatric surgery changed common practice in the field, Dr. Goldman said she hopes the new data will have a similar influence and relationship counseling will become the norm.
Dr. Cole actually had bariatric surgery. He recalled potential relationship issues were briefly mentioned. Someone at the clinic said if his marriage felt challenged, he should seek help from a professional, and that was it.
For Dr. Cole, there were unexpected negative feelings of shame and disappointment after surgery. He felt the extreme weight loss was all his colleagues could talk about and was very disappointed when there was no change in his chronic pain, a primary reason he had the procedure.
Fortunately, he could talk to his wife – also is a relationship therapist at Gottman – about the range of emotions. “One of the things that we know that creates a deep sense of trust is [when] I know my partner is there for me when I’m not well,” Dr. Cole said.
But these negative emotions can be the very things that feel most difficult to talk about or hear from a partner. It’s hard to share our own negative feelings and to hear someone else’s, Dr. Cole said.
He advises creating a new “ritual of connection: moments in time when you plan to turn toward one another.”
That could be a daily walk, where you intentionally talk about the surgery-related changes that both of you have had. Dr. Cole said to ask yourself, “Are we intentional about turning toward one another in those [challenging] moments?”
A version of this article first appeared on WebMD.com.
FROM ANNALS OF SURGERY
FDA clears endoscopic devices for sleeve gastroplasty, bariatric revision
The Food and Drug Administration has cleared for marketing the first devices indicated for endoscopic sleeve gastroplasty (ESG) and endoscopic bariatric revision, according to the manufacturer.
The Apollo ESG, Apollo ESG Sx, Apollo Revise, and Apollo Revise Sx systems made by Apollo Endosurgery were reviewed through the de novo premarket review pathway, a regulatory pathway for low- to moderate-risk devices of a new type.
“The Apollo ESG and Apollo Revise systems offer a compelling mix of effectiveness, safety, durability, and convenience for treatment of patients with obesity,” Chas McKhann, president and CEO of the company, said in a news release.
“The authorization of these new endoscopic systems represents a major step forward in addressing the global obesity epidemic,” Mr. McKhann added.
The Apollo ESG and Apollo ESG Sx systems are intended for use by trained gastroenterologists or surgeons to facilitate weight loss in adults with obesity who have failed to lose weight or maintain weight loss through more conservative measures, the company says.
The Apollo Revise and Apollo Revise Sx systems allow gastroenterologists or surgeons to perform transoral outlet reduction (TORe) as a revision to a previous bariatric procedure.
Studies have shown that 10 years after bariatric surgery, patients have regained an average of 20%-30% of weight they initially lost. Bariatric revision procedures are the fastest growing segment of the bariatric surgery market.
TORe is an endoscopic procedure performed to revise a previous gastric bypass and like ESG, can be performed as a same-day procedure without incisions or scars.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has cleared for marketing the first devices indicated for endoscopic sleeve gastroplasty (ESG) and endoscopic bariatric revision, according to the manufacturer.
The Apollo ESG, Apollo ESG Sx, Apollo Revise, and Apollo Revise Sx systems made by Apollo Endosurgery were reviewed through the de novo premarket review pathway, a regulatory pathway for low- to moderate-risk devices of a new type.
“The Apollo ESG and Apollo Revise systems offer a compelling mix of effectiveness, safety, durability, and convenience for treatment of patients with obesity,” Chas McKhann, president and CEO of the company, said in a news release.
“The authorization of these new endoscopic systems represents a major step forward in addressing the global obesity epidemic,” Mr. McKhann added.
The Apollo ESG and Apollo ESG Sx systems are intended for use by trained gastroenterologists or surgeons to facilitate weight loss in adults with obesity who have failed to lose weight or maintain weight loss through more conservative measures, the company says.
The Apollo Revise and Apollo Revise Sx systems allow gastroenterologists or surgeons to perform transoral outlet reduction (TORe) as a revision to a previous bariatric procedure.
Studies have shown that 10 years after bariatric surgery, patients have regained an average of 20%-30% of weight they initially lost. Bariatric revision procedures are the fastest growing segment of the bariatric surgery market.
TORe is an endoscopic procedure performed to revise a previous gastric bypass and like ESG, can be performed as a same-day procedure without incisions or scars.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has cleared for marketing the first devices indicated for endoscopic sleeve gastroplasty (ESG) and endoscopic bariatric revision, according to the manufacturer.
The Apollo ESG, Apollo ESG Sx, Apollo Revise, and Apollo Revise Sx systems made by Apollo Endosurgery were reviewed through the de novo premarket review pathway, a regulatory pathway for low- to moderate-risk devices of a new type.
“The Apollo ESG and Apollo Revise systems offer a compelling mix of effectiveness, safety, durability, and convenience for treatment of patients with obesity,” Chas McKhann, president and CEO of the company, said in a news release.
“The authorization of these new endoscopic systems represents a major step forward in addressing the global obesity epidemic,” Mr. McKhann added.
The Apollo ESG and Apollo ESG Sx systems are intended for use by trained gastroenterologists or surgeons to facilitate weight loss in adults with obesity who have failed to lose weight or maintain weight loss through more conservative measures, the company says.
The Apollo Revise and Apollo Revise Sx systems allow gastroenterologists or surgeons to perform transoral outlet reduction (TORe) as a revision to a previous bariatric procedure.
Studies have shown that 10 years after bariatric surgery, patients have regained an average of 20%-30% of weight they initially lost. Bariatric revision procedures are the fastest growing segment of the bariatric surgery market.
TORe is an endoscopic procedure performed to revise a previous gastric bypass and like ESG, can be performed as a same-day procedure without incisions or scars.
A version of this article first appeared on Medscape.com.
Pediatric obesity treatment options: Beyond lifestyle modification
Pediatric obesity is a serious problem, not only in the United States but worldwide. Unfortunately, the ongoing COVID-19 pandemic has worsened the epidemic of childhood obesity. Solutions for treating the millions of children and adolescents with obesity are desperately needed because prevention efforts over the past several decades have not been sufficient in slowing the steady rise in obesity prevalence.
Lifestyle modification, including dietary changes, increases in activity, and behavioral modification, are the cornerstone of any obesity treatment, but they alone are not powerful enough to treat obesity by itself in the vast majority of children and adolescents. This is because obesity is not a lifestyle choice; rather, it is a disease, and a disease that has a tremendous amount of biology driving individuals toward weight gain and the propensity toward weight regain if weight is lost.
Fortunately, the tools to treat the underlying biology driving obesity are becoming safer, more effective, and more widely used every year. The two most effective biology-based treatments for pediatric obesity are antiobesity medications and bariatric surgery. These two treatments, when accompanied by lifestyle modification, have the potential to reduce not only body weight but also treat many other risk factors, such as prediabetes, diabetes, high blood pressure, poor cholesterol, liver disease, and sleep apnea, as well as others.
Rise in antiobesity medications
Antiobesity medications are developing at a rapid pace. Seven medications have been approved by the Food and Drug Administration for adults, and three medications (phentermine, orlistat, and liraglutide) are now approved for children and adolescents.
The number of antiobesity medications for use in children and adolescents is expected to expand to five, with semaglutide and phentermine-topiramate (Qsymia) both completing trials in adolescents in 2022. Each of these medications works by treating the biology that drives weight gain, whether it is decreasing impulsivity, reducing hunger and appetite hormone pathways, or improving energy regulation pathways. Weight loss at 1 year for currently FDA-approved medications in adolescents ranges from 3% to 6% on average, depending on the medications. The newer medications already FDA approved in adults that will soon, hopefully, be available in pediatrics result in 10%-16% weight loss on average.
A common parent and patient question regarding antiobesity medications is: “If I start an antiobesity medication, how long will I need to be on it?” The simple answer is: “Probably for the rest of your life.”
This can be a shock to hear, but obesity treatment is very similar to that of hypertension or diabetes. Using high blood pressure as an example: If a patient has high blood pressure (for example, 160/90 mm Hg), they will be prescribed a medication to treat it. Once blood pressure comes down to near-normal levels (for example, 120/80 mm Hg), a dose will be maintained, not removed, because that is the biological mediator keeping the blood pressure low. Removal of the medication would result in blood pressure going back to homeostasis (160/90 mm Hg in our example) in a short period of time).
The same can be said for obesity. For example, if a 16-year-old girl is prescribed liraglutide, a glucagonlike peptide–1 receptor agonist, and loses 10% of her body weight at 1 year, that is great success. Why would we remove the medication that is treating the underlying biology causing successful weight loss?
In short, we would not want to do that. Even if our example patient only maintained that 10% initial weight loss, that would be very successful, just like someone maintaining their low blood pressure. As medications begin to develop at a rapid pace and become more available to pediatric patients, the messaging and conversation around anti-obesity medications must continue to focus on obesity being a biological disease and not a behavior for treatment to be effective and not stigmatized.
Bariatric surgery most effective treatment for pediatric obesity
Currently, the most effective treatment for pediatric obesity is bariatric surgery. The two most commonly used surgical procedures today are the sleeve gastrectomy and gastric bypass. Sleeve gastrectomy works by removing 75%-85% of the stomach and creating a new stomach, called a “sleeve.” Gastric bypass works by separating the stomach into two parts and connecting one part of the new stomach into the intestine.
Both surgeries are very effective at treating obesity in adolescents, with an average weight loss of 30%-35%. Surgery is not just a restrictive means of controlling body weight; it also changes key hormones for appetite and satiety that signal the brain. In fact, many of the same biological signals that are changed by surgery are the same signals being targeted by antiobesity medications. Long-term outcome of bariatric surgery in adolescents, provided by Teen-LABS, show it to be safe and maybe even more effective than in adults for treating diabetes and hypertension, with similar weight loss.
Does treatment outweigh the potential risks?
Although obesity surgery and antiobesity medications are more successful at treating obesity in children and adolescents than lifestyle medications, they do have some risks. Surgery, depending on the type of surgery, can cause nutritional deficiencies, reduce body mineral density, and is a life-changing medical procedure. Antiobesity medications, depending on the type, can cause nausea and vomiting and increase heart rate – and because they are relatively new, we do not fully understand the long-term impact of continued use past 1 year.
However, an important question to ask is: “Do the risks of obesity surgery and antiobesity medications outweigh the risk of having lifelong obesity?” The answer to me and many of my colleagues is: “Yes!” Although there are risks associated with the two best treatments for pediatric obesity, those risks under proper supervision of a medical professional far outweigh the risks of not properly treating obesity and allowing it to persist and get worse over many years to come. Obesity is a disease deeply rooted in biology, and we must use biology-based treatments to tackle this problem in children and adolescents, who deserve the best care and treatments possible.
Dr. Ryder is assistant professor of pediatrics and associate director of research, Center for Pediatric Obesity Medicine, at the University of Minnesota, Minneapolis. She reported receiving donations for clinical trials from Boehringer Ingelheim. A version of this article first appeared on Medscape.com.
Pediatric obesity is a serious problem, not only in the United States but worldwide. Unfortunately, the ongoing COVID-19 pandemic has worsened the epidemic of childhood obesity. Solutions for treating the millions of children and adolescents with obesity are desperately needed because prevention efforts over the past several decades have not been sufficient in slowing the steady rise in obesity prevalence.
Lifestyle modification, including dietary changes, increases in activity, and behavioral modification, are the cornerstone of any obesity treatment, but they alone are not powerful enough to treat obesity by itself in the vast majority of children and adolescents. This is because obesity is not a lifestyle choice; rather, it is a disease, and a disease that has a tremendous amount of biology driving individuals toward weight gain and the propensity toward weight regain if weight is lost.
Fortunately, the tools to treat the underlying biology driving obesity are becoming safer, more effective, and more widely used every year. The two most effective biology-based treatments for pediatric obesity are antiobesity medications and bariatric surgery. These two treatments, when accompanied by lifestyle modification, have the potential to reduce not only body weight but also treat many other risk factors, such as prediabetes, diabetes, high blood pressure, poor cholesterol, liver disease, and sleep apnea, as well as others.
Rise in antiobesity medications
Antiobesity medications are developing at a rapid pace. Seven medications have been approved by the Food and Drug Administration for adults, and three medications (phentermine, orlistat, and liraglutide) are now approved for children and adolescents.
The number of antiobesity medications for use in children and adolescents is expected to expand to five, with semaglutide and phentermine-topiramate (Qsymia) both completing trials in adolescents in 2022. Each of these medications works by treating the biology that drives weight gain, whether it is decreasing impulsivity, reducing hunger and appetite hormone pathways, or improving energy regulation pathways. Weight loss at 1 year for currently FDA-approved medications in adolescents ranges from 3% to 6% on average, depending on the medications. The newer medications already FDA approved in adults that will soon, hopefully, be available in pediatrics result in 10%-16% weight loss on average.
A common parent and patient question regarding antiobesity medications is: “If I start an antiobesity medication, how long will I need to be on it?” The simple answer is: “Probably for the rest of your life.”
This can be a shock to hear, but obesity treatment is very similar to that of hypertension or diabetes. Using high blood pressure as an example: If a patient has high blood pressure (for example, 160/90 mm Hg), they will be prescribed a medication to treat it. Once blood pressure comes down to near-normal levels (for example, 120/80 mm Hg), a dose will be maintained, not removed, because that is the biological mediator keeping the blood pressure low. Removal of the medication would result in blood pressure going back to homeostasis (160/90 mm Hg in our example) in a short period of time).
The same can be said for obesity. For example, if a 16-year-old girl is prescribed liraglutide, a glucagonlike peptide–1 receptor agonist, and loses 10% of her body weight at 1 year, that is great success. Why would we remove the medication that is treating the underlying biology causing successful weight loss?
In short, we would not want to do that. Even if our example patient only maintained that 10% initial weight loss, that would be very successful, just like someone maintaining their low blood pressure. As medications begin to develop at a rapid pace and become more available to pediatric patients, the messaging and conversation around anti-obesity medications must continue to focus on obesity being a biological disease and not a behavior for treatment to be effective and not stigmatized.
Bariatric surgery most effective treatment for pediatric obesity
Currently, the most effective treatment for pediatric obesity is bariatric surgery. The two most commonly used surgical procedures today are the sleeve gastrectomy and gastric bypass. Sleeve gastrectomy works by removing 75%-85% of the stomach and creating a new stomach, called a “sleeve.” Gastric bypass works by separating the stomach into two parts and connecting one part of the new stomach into the intestine.
Both surgeries are very effective at treating obesity in adolescents, with an average weight loss of 30%-35%. Surgery is not just a restrictive means of controlling body weight; it also changes key hormones for appetite and satiety that signal the brain. In fact, many of the same biological signals that are changed by surgery are the same signals being targeted by antiobesity medications. Long-term outcome of bariatric surgery in adolescents, provided by Teen-LABS, show it to be safe and maybe even more effective than in adults for treating diabetes and hypertension, with similar weight loss.
Does treatment outweigh the potential risks?
Although obesity surgery and antiobesity medications are more successful at treating obesity in children and adolescents than lifestyle medications, they do have some risks. Surgery, depending on the type of surgery, can cause nutritional deficiencies, reduce body mineral density, and is a life-changing medical procedure. Antiobesity medications, depending on the type, can cause nausea and vomiting and increase heart rate – and because they are relatively new, we do not fully understand the long-term impact of continued use past 1 year.
However, an important question to ask is: “Do the risks of obesity surgery and antiobesity medications outweigh the risk of having lifelong obesity?” The answer to me and many of my colleagues is: “Yes!” Although there are risks associated with the two best treatments for pediatric obesity, those risks under proper supervision of a medical professional far outweigh the risks of not properly treating obesity and allowing it to persist and get worse over many years to come. Obesity is a disease deeply rooted in biology, and we must use biology-based treatments to tackle this problem in children and adolescents, who deserve the best care and treatments possible.
Dr. Ryder is assistant professor of pediatrics and associate director of research, Center for Pediatric Obesity Medicine, at the University of Minnesota, Minneapolis. She reported receiving donations for clinical trials from Boehringer Ingelheim. A version of this article first appeared on Medscape.com.
Pediatric obesity is a serious problem, not only in the United States but worldwide. Unfortunately, the ongoing COVID-19 pandemic has worsened the epidemic of childhood obesity. Solutions for treating the millions of children and adolescents with obesity are desperately needed because prevention efforts over the past several decades have not been sufficient in slowing the steady rise in obesity prevalence.
Lifestyle modification, including dietary changes, increases in activity, and behavioral modification, are the cornerstone of any obesity treatment, but they alone are not powerful enough to treat obesity by itself in the vast majority of children and adolescents. This is because obesity is not a lifestyle choice; rather, it is a disease, and a disease that has a tremendous amount of biology driving individuals toward weight gain and the propensity toward weight regain if weight is lost.
Fortunately, the tools to treat the underlying biology driving obesity are becoming safer, more effective, and more widely used every year. The two most effective biology-based treatments for pediatric obesity are antiobesity medications and bariatric surgery. These two treatments, when accompanied by lifestyle modification, have the potential to reduce not only body weight but also treat many other risk factors, such as prediabetes, diabetes, high blood pressure, poor cholesterol, liver disease, and sleep apnea, as well as others.
Rise in antiobesity medications
Antiobesity medications are developing at a rapid pace. Seven medications have been approved by the Food and Drug Administration for adults, and three medications (phentermine, orlistat, and liraglutide) are now approved for children and adolescents.
The number of antiobesity medications for use in children and adolescents is expected to expand to five, with semaglutide and phentermine-topiramate (Qsymia) both completing trials in adolescents in 2022. Each of these medications works by treating the biology that drives weight gain, whether it is decreasing impulsivity, reducing hunger and appetite hormone pathways, or improving energy regulation pathways. Weight loss at 1 year for currently FDA-approved medications in adolescents ranges from 3% to 6% on average, depending on the medications. The newer medications already FDA approved in adults that will soon, hopefully, be available in pediatrics result in 10%-16% weight loss on average.
A common parent and patient question regarding antiobesity medications is: “If I start an antiobesity medication, how long will I need to be on it?” The simple answer is: “Probably for the rest of your life.”
This can be a shock to hear, but obesity treatment is very similar to that of hypertension or diabetes. Using high blood pressure as an example: If a patient has high blood pressure (for example, 160/90 mm Hg), they will be prescribed a medication to treat it. Once blood pressure comes down to near-normal levels (for example, 120/80 mm Hg), a dose will be maintained, not removed, because that is the biological mediator keeping the blood pressure low. Removal of the medication would result in blood pressure going back to homeostasis (160/90 mm Hg in our example) in a short period of time).
The same can be said for obesity. For example, if a 16-year-old girl is prescribed liraglutide, a glucagonlike peptide–1 receptor agonist, and loses 10% of her body weight at 1 year, that is great success. Why would we remove the medication that is treating the underlying biology causing successful weight loss?
In short, we would not want to do that. Even if our example patient only maintained that 10% initial weight loss, that would be very successful, just like someone maintaining their low blood pressure. As medications begin to develop at a rapid pace and become more available to pediatric patients, the messaging and conversation around anti-obesity medications must continue to focus on obesity being a biological disease and not a behavior for treatment to be effective and not stigmatized.
Bariatric surgery most effective treatment for pediatric obesity
Currently, the most effective treatment for pediatric obesity is bariatric surgery. The two most commonly used surgical procedures today are the sleeve gastrectomy and gastric bypass. Sleeve gastrectomy works by removing 75%-85% of the stomach and creating a new stomach, called a “sleeve.” Gastric bypass works by separating the stomach into two parts and connecting one part of the new stomach into the intestine.
Both surgeries are very effective at treating obesity in adolescents, with an average weight loss of 30%-35%. Surgery is not just a restrictive means of controlling body weight; it also changes key hormones for appetite and satiety that signal the brain. In fact, many of the same biological signals that are changed by surgery are the same signals being targeted by antiobesity medications. Long-term outcome of bariatric surgery in adolescents, provided by Teen-LABS, show it to be safe and maybe even more effective than in adults for treating diabetes and hypertension, with similar weight loss.
Does treatment outweigh the potential risks?
Although obesity surgery and antiobesity medications are more successful at treating obesity in children and adolescents than lifestyle medications, they do have some risks. Surgery, depending on the type of surgery, can cause nutritional deficiencies, reduce body mineral density, and is a life-changing medical procedure. Antiobesity medications, depending on the type, can cause nausea and vomiting and increase heart rate – and because they are relatively new, we do not fully understand the long-term impact of continued use past 1 year.
However, an important question to ask is: “Do the risks of obesity surgery and antiobesity medications outweigh the risk of having lifelong obesity?” The answer to me and many of my colleagues is: “Yes!” Although there are risks associated with the two best treatments for pediatric obesity, those risks under proper supervision of a medical professional far outweigh the risks of not properly treating obesity and allowing it to persist and get worse over many years to come. Obesity is a disease deeply rooted in biology, and we must use biology-based treatments to tackle this problem in children and adolescents, who deserve the best care and treatments possible.
Dr. Ryder is assistant professor of pediatrics and associate director of research, Center for Pediatric Obesity Medicine, at the University of Minnesota, Minneapolis. She reported receiving donations for clinical trials from Boehringer Ingelheim. A version of this article first appeared on Medscape.com.
More reflux after sleeve gastrectomy vs. gastric bypass at 10 years
Sleeve gastrectomy (SG) and Roux-en-Y gastric bypass (RYGB) each led to good and sustainable weight loss 10 years later, although reflux was more prevalent after SG, according to the Sleeve vs. Bypass (SLEEVEPASS) randomized clinical trial.
At 10 years, there were no statistically significant between-procedure differences in type 2 diabetes remission, dyslipidemia, or obstructive sleep apnea, but hypertension remission was greater with RYGB.
However, importantly, the cumulative incidence of Barrett’s esophagus was similar after both procedures (4%) and markedly lower than reported in previous trials (14%-17%).
To their knowledge, this is the largest randomized controlled trial with the longest follow-up comparing these two laparoscopic bariatric surgeries, Paulina Salminen, MD, PhD, and colleagues write in their study published online in JAMA Surgery.
They aimed to clarify the “controversial issues” of long-term gastroesophageal reflux disease (GERD) symptoms, endoscopic esophagitis, and Barrett’s esophagus after SG vs. RYGB.
The findings showed that “there was no difference in the prevalence of Barrett’s esophagus, contrary to previous reports of alarming rates of Barrett’s [esophagus] after sleeve gastrectomy,” Dr. Salminen from Turku (Finland) University Hospital, told this news organization in an email.
“However, our results also show that esophagitis and GERD symptoms are significantly more prevalent after sleeve [gastrectomy], and GERD is an important factor to be considered in the preoperative assessment of bariatric surgery and procedure choice,” she said.
The takeaway is that “we have two good procedures providing good and sustainable 10-year results for both weight loss and remission of comorbidities” for severe obesity, a major health risk, Dr. Salminen summarized.
10-year data analysis
Long-term outcomes from randomized clinical trials of laparoscopic SG vs. RYGB are limited, and recent studies have shown a high incidence of worsening of de novo GERD, esophagitis, and Barrett’s esophagus, after laparoscopic SG, Dr. Salminen and colleagues write.
To investigate, they analyzed 10-year data from SLEEVEPASS, which had randomized 240 adult patients with severe obesity to either SG or RYGB at three hospitals in Finland during 2008-2010.
At baseline, 121 patients were randomized to SG and 119 to RYGB. They had a mean age of 48 years, a mean body mass index of 45.9 kg/m2, and 70% were women.
Two patients never had the surgery, and at 10 years, 10 patients had died of causes unrelated to bariatric surgery.
At 10 years, 193 of the 288 remaining patients (85%) completed the follow-up for weight loss and other comorbidity outcomes, and 176 of 228 (77%) underwent gastroscopy.
The primary study endpoint of the trial was percent excess weight loss (%EWL). At 10 years, the median %EWL was 43.5% after SG vs. 50.7% after RYGB, with a wide range for both procedures (roughly 2%-110% excess weight loss). Mean estimate %EWL was not equivalent, with it being 8.4% in favor of RYGB.
After SG and RYGB, there were no statistically significant differences in type 2 diabetes remission (26% and 33%, respectively), dyslipidemia (19% and 35%, respectively), or obstructive sleep apnea (16% and 31%, respectively).
Hypertension remission was superior after RYGB (8% vs. 24%; P = .04).
Esophagitis was more prevalent after SG (31% vs. 7%; P < .001).
‘Very important study’
“This is a very important study, the first to report 10-year results of a randomized controlled trial comparing the two most frequently used bariatric operations, SG and RYGB,” Beat Peter Müller, MD, MBA, and Adrian Billeter, MD, PhD, who were not involved with this research, told this news organization in an email.
“The results will have a major impact on the future of bariatric surgery,” according to Dr. Müller and Dr. Billeter, from Heidelberg (Germany) University.
The most relevant findings are the GERD outcomes, they said. Because of the high rate of upper endoscopies at 10 years (73%), the study allowed a good assessment of this.
“While this study confirms that SG is a GERD-prone procedure, it clearly demonstrates that GERD after SG does not induce severe esophagitis and Barrett’s esophagus,” they said.
Most importantly, the rate of Barrett’s esophagus, the precursor lesion of adenocarcinomas of the esophago-gastric junction is similar (4%) after both operations and there was no dysplasia in either group, they stressed.
“The main problem after SG remains new-onset GERD, for which still no predictive parameter exists,” according to Dr. Müller and Dr. Billeter.
“The take home message … is that GERD after SG is generally mild and the risk of Barrett’s esophagus is equally higher after SG and RYGB,” they said. “Therefore, all patients after any bariatric operations should undergo regular upper endoscopies.”
However, “RYGB still leads to an increase in proton-pump inhibitor use, despite RYGB being one of the most effective antireflux procedures,” they said. “This finding needs further investigation.”
Furthermore, “a 4% Barrett esophagus rate 10 years after RYGB is troublesome, and the reasons should be investigated,” they added.
“Another relevant finding is that after 10 years, RYGB has a statistically better weight loss, which reaches the primary endpoint of the SLEEVEPASS trial for the first time,” they noted, yet the clinical relevance of this is not clear, since there was no difference in resolution of comorbidities, except for hypertension.
Gyanprakash A. Ketwaroo, MD, of Baylor College of Medicine, Houston, who was not involved with this research, agreed that “the study shows durable and good weight loss for either type of laparoscopic surgery with important metabolic effects and confirms the long-term benefits of weight-loss surgery.”
“What is somewhat new is the lower levels of Barrett’s esophagus after sleeve gastrectomy compared with several earlier studies,” he told this news organization in an email.
“This is somewhat incongruent with the relatively high incidence of postsleeve esophagitis noted in the study, which is an accepted risk factor for Barrett’s esophagus,” he continued. “Thus, I believe concern will still remain about GERD-related complications, including Barrett’s [esophagus], after sleeve gastrectomy.”
“This paper highlights the need for larger prospective studies, especially those that include diverse, older populations with multiple risk factors for Barrett’s esophagus,” Dr. Ketwaroo said.
Looking ahead
Using a large data set, such as that from SLEEVEPASS and possibly with data from the SM-BOSS trial and the BariSurg trial, with machine learning and other sophisticated analyses might identify parameters that could be used to choose the best operation for an individual patient, Dr. Salminen speculated.
“I think what we have learned from these long-term follow-up results is that GERD assessment should be a part of the preoperative assessment, and for patients who have preoperative GERD symptoms and GERD-related endoscopic findings (e.g., hiatal hernia), gastric bypass would be a more optimal procedure choice, if there are no contraindications for it,” she said.
Patient discussions should also cover “long-term symptoms, for example, abdominal pain after RYGB,” she added.
“I am looking forward to our future 20-year follow-up results,” Dr. Salminen said, “which will shed more light on this topic of postoperative [endoscopic] surveillance.
In the meantime, “preoperative gastroscopy is necessary and beneficial, at least when considering sleeve gastrectomy,” she said.
The SLEEVEPASS trial was supported by the Mary and Georg C. Ehrnrooth Foundation, the Government Research Foundation (in a grant awarded to Turku University Hospital), the Orion Research Foundation, the Paulo Foundation, and the Gastroenterological Research Foundation. Dr. Salminen reported receiving grants from the Government Research Foundation awarded to Turku University Hospital and the Mary and Georg C. Ehrnrooth Foundation. Another coauthor received grants from the Orion Research Foundation, the Paulo Foundation, and the Gastroenterological Research Foundation during the study. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
Sleeve gastrectomy (SG) and Roux-en-Y gastric bypass (RYGB) each led to good and sustainable weight loss 10 years later, although reflux was more prevalent after SG, according to the Sleeve vs. Bypass (SLEEVEPASS) randomized clinical trial.
At 10 years, there were no statistically significant between-procedure differences in type 2 diabetes remission, dyslipidemia, or obstructive sleep apnea, but hypertension remission was greater with RYGB.
However, importantly, the cumulative incidence of Barrett’s esophagus was similar after both procedures (4%) and markedly lower than reported in previous trials (14%-17%).
To their knowledge, this is the largest randomized controlled trial with the longest follow-up comparing these two laparoscopic bariatric surgeries, Paulina Salminen, MD, PhD, and colleagues write in their study published online in JAMA Surgery.
They aimed to clarify the “controversial issues” of long-term gastroesophageal reflux disease (GERD) symptoms, endoscopic esophagitis, and Barrett’s esophagus after SG vs. RYGB.
The findings showed that “there was no difference in the prevalence of Barrett’s esophagus, contrary to previous reports of alarming rates of Barrett’s [esophagus] after sleeve gastrectomy,” Dr. Salminen from Turku (Finland) University Hospital, told this news organization in an email.
“However, our results also show that esophagitis and GERD symptoms are significantly more prevalent after sleeve [gastrectomy], and GERD is an important factor to be considered in the preoperative assessment of bariatric surgery and procedure choice,” she said.
The takeaway is that “we have two good procedures providing good and sustainable 10-year results for both weight loss and remission of comorbidities” for severe obesity, a major health risk, Dr. Salminen summarized.
10-year data analysis
Long-term outcomes from randomized clinical trials of laparoscopic SG vs. RYGB are limited, and recent studies have shown a high incidence of worsening of de novo GERD, esophagitis, and Barrett’s esophagus, after laparoscopic SG, Dr. Salminen and colleagues write.
To investigate, they analyzed 10-year data from SLEEVEPASS, which had randomized 240 adult patients with severe obesity to either SG or RYGB at three hospitals in Finland during 2008-2010.
At baseline, 121 patients were randomized to SG and 119 to RYGB. They had a mean age of 48 years, a mean body mass index of 45.9 kg/m2, and 70% were women.
Two patients never had the surgery, and at 10 years, 10 patients had died of causes unrelated to bariatric surgery.
At 10 years, 193 of the 288 remaining patients (85%) completed the follow-up for weight loss and other comorbidity outcomes, and 176 of 228 (77%) underwent gastroscopy.
The primary study endpoint of the trial was percent excess weight loss (%EWL). At 10 years, the median %EWL was 43.5% after SG vs. 50.7% after RYGB, with a wide range for both procedures (roughly 2%-110% excess weight loss). Mean estimate %EWL was not equivalent, with it being 8.4% in favor of RYGB.
After SG and RYGB, there were no statistically significant differences in type 2 diabetes remission (26% and 33%, respectively), dyslipidemia (19% and 35%, respectively), or obstructive sleep apnea (16% and 31%, respectively).
Hypertension remission was superior after RYGB (8% vs. 24%; P = .04).
Esophagitis was more prevalent after SG (31% vs. 7%; P < .001).
‘Very important study’
“This is a very important study, the first to report 10-year results of a randomized controlled trial comparing the two most frequently used bariatric operations, SG and RYGB,” Beat Peter Müller, MD, MBA, and Adrian Billeter, MD, PhD, who were not involved with this research, told this news organization in an email.
“The results will have a major impact on the future of bariatric surgery,” according to Dr. Müller and Dr. Billeter, from Heidelberg (Germany) University.
The most relevant findings are the GERD outcomes, they said. Because of the high rate of upper endoscopies at 10 years (73%), the study allowed a good assessment of this.
“While this study confirms that SG is a GERD-prone procedure, it clearly demonstrates that GERD after SG does not induce severe esophagitis and Barrett’s esophagus,” they said.
Most importantly, the rate of Barrett’s esophagus, the precursor lesion of adenocarcinomas of the esophago-gastric junction is similar (4%) after both operations and there was no dysplasia in either group, they stressed.
“The main problem after SG remains new-onset GERD, for which still no predictive parameter exists,” according to Dr. Müller and Dr. Billeter.
“The take home message … is that GERD after SG is generally mild and the risk of Barrett’s esophagus is equally higher after SG and RYGB,” they said. “Therefore, all patients after any bariatric operations should undergo regular upper endoscopies.”
However, “RYGB still leads to an increase in proton-pump inhibitor use, despite RYGB being one of the most effective antireflux procedures,” they said. “This finding needs further investigation.”
Furthermore, “a 4% Barrett esophagus rate 10 years after RYGB is troublesome, and the reasons should be investigated,” they added.
“Another relevant finding is that after 10 years, RYGB has a statistically better weight loss, which reaches the primary endpoint of the SLEEVEPASS trial for the first time,” they noted, yet the clinical relevance of this is not clear, since there was no difference in resolution of comorbidities, except for hypertension.
Gyanprakash A. Ketwaroo, MD, of Baylor College of Medicine, Houston, who was not involved with this research, agreed that “the study shows durable and good weight loss for either type of laparoscopic surgery with important metabolic effects and confirms the long-term benefits of weight-loss surgery.”
“What is somewhat new is the lower levels of Barrett’s esophagus after sleeve gastrectomy compared with several earlier studies,” he told this news organization in an email.
“This is somewhat incongruent with the relatively high incidence of postsleeve esophagitis noted in the study, which is an accepted risk factor for Barrett’s esophagus,” he continued. “Thus, I believe concern will still remain about GERD-related complications, including Barrett’s [esophagus], after sleeve gastrectomy.”
“This paper highlights the need for larger prospective studies, especially those that include diverse, older populations with multiple risk factors for Barrett’s esophagus,” Dr. Ketwaroo said.
Looking ahead
Using a large data set, such as that from SLEEVEPASS and possibly with data from the SM-BOSS trial and the BariSurg trial, with machine learning and other sophisticated analyses might identify parameters that could be used to choose the best operation for an individual patient, Dr. Salminen speculated.
“I think what we have learned from these long-term follow-up results is that GERD assessment should be a part of the preoperative assessment, and for patients who have preoperative GERD symptoms and GERD-related endoscopic findings (e.g., hiatal hernia), gastric bypass would be a more optimal procedure choice, if there are no contraindications for it,” she said.
Patient discussions should also cover “long-term symptoms, for example, abdominal pain after RYGB,” she added.
“I am looking forward to our future 20-year follow-up results,” Dr. Salminen said, “which will shed more light on this topic of postoperative [endoscopic] surveillance.
In the meantime, “preoperative gastroscopy is necessary and beneficial, at least when considering sleeve gastrectomy,” she said.
The SLEEVEPASS trial was supported by the Mary and Georg C. Ehrnrooth Foundation, the Government Research Foundation (in a grant awarded to Turku University Hospital), the Orion Research Foundation, the Paulo Foundation, and the Gastroenterological Research Foundation. Dr. Salminen reported receiving grants from the Government Research Foundation awarded to Turku University Hospital and the Mary and Georg C. Ehrnrooth Foundation. Another coauthor received grants from the Orion Research Foundation, the Paulo Foundation, and the Gastroenterological Research Foundation during the study. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
Sleeve gastrectomy (SG) and Roux-en-Y gastric bypass (RYGB) each led to good and sustainable weight loss 10 years later, although reflux was more prevalent after SG, according to the Sleeve vs. Bypass (SLEEVEPASS) randomized clinical trial.
At 10 years, there were no statistically significant between-procedure differences in type 2 diabetes remission, dyslipidemia, or obstructive sleep apnea, but hypertension remission was greater with RYGB.
However, importantly, the cumulative incidence of Barrett’s esophagus was similar after both procedures (4%) and markedly lower than reported in previous trials (14%-17%).
To their knowledge, this is the largest randomized controlled trial with the longest follow-up comparing these two laparoscopic bariatric surgeries, Paulina Salminen, MD, PhD, and colleagues write in their study published online in JAMA Surgery.
They aimed to clarify the “controversial issues” of long-term gastroesophageal reflux disease (GERD) symptoms, endoscopic esophagitis, and Barrett’s esophagus after SG vs. RYGB.
The findings showed that “there was no difference in the prevalence of Barrett’s esophagus, contrary to previous reports of alarming rates of Barrett’s [esophagus] after sleeve gastrectomy,” Dr. Salminen from Turku (Finland) University Hospital, told this news organization in an email.
“However, our results also show that esophagitis and GERD symptoms are significantly more prevalent after sleeve [gastrectomy], and GERD is an important factor to be considered in the preoperative assessment of bariatric surgery and procedure choice,” she said.
The takeaway is that “we have two good procedures providing good and sustainable 10-year results for both weight loss and remission of comorbidities” for severe obesity, a major health risk, Dr. Salminen summarized.
10-year data analysis
Long-term outcomes from randomized clinical trials of laparoscopic SG vs. RYGB are limited, and recent studies have shown a high incidence of worsening of de novo GERD, esophagitis, and Barrett’s esophagus, after laparoscopic SG, Dr. Salminen and colleagues write.
To investigate, they analyzed 10-year data from SLEEVEPASS, which had randomized 240 adult patients with severe obesity to either SG or RYGB at three hospitals in Finland during 2008-2010.
At baseline, 121 patients were randomized to SG and 119 to RYGB. They had a mean age of 48 years, a mean body mass index of 45.9 kg/m2, and 70% were women.
Two patients never had the surgery, and at 10 years, 10 patients had died of causes unrelated to bariatric surgery.
At 10 years, 193 of the 288 remaining patients (85%) completed the follow-up for weight loss and other comorbidity outcomes, and 176 of 228 (77%) underwent gastroscopy.
The primary study endpoint of the trial was percent excess weight loss (%EWL). At 10 years, the median %EWL was 43.5% after SG vs. 50.7% after RYGB, with a wide range for both procedures (roughly 2%-110% excess weight loss). Mean estimate %EWL was not equivalent, with it being 8.4% in favor of RYGB.
After SG and RYGB, there were no statistically significant differences in type 2 diabetes remission (26% and 33%, respectively), dyslipidemia (19% and 35%, respectively), or obstructive sleep apnea (16% and 31%, respectively).
Hypertension remission was superior after RYGB (8% vs. 24%; P = .04).
Esophagitis was more prevalent after SG (31% vs. 7%; P < .001).
‘Very important study’
“This is a very important study, the first to report 10-year results of a randomized controlled trial comparing the two most frequently used bariatric operations, SG and RYGB,” Beat Peter Müller, MD, MBA, and Adrian Billeter, MD, PhD, who were not involved with this research, told this news organization in an email.
“The results will have a major impact on the future of bariatric surgery,” according to Dr. Müller and Dr. Billeter, from Heidelberg (Germany) University.
The most relevant findings are the GERD outcomes, they said. Because of the high rate of upper endoscopies at 10 years (73%), the study allowed a good assessment of this.
“While this study confirms that SG is a GERD-prone procedure, it clearly demonstrates that GERD after SG does not induce severe esophagitis and Barrett’s esophagus,” they said.
Most importantly, the rate of Barrett’s esophagus, the precursor lesion of adenocarcinomas of the esophago-gastric junction is similar (4%) after both operations and there was no dysplasia in either group, they stressed.
“The main problem after SG remains new-onset GERD, for which still no predictive parameter exists,” according to Dr. Müller and Dr. Billeter.
“The take home message … is that GERD after SG is generally mild and the risk of Barrett’s esophagus is equally higher after SG and RYGB,” they said. “Therefore, all patients after any bariatric operations should undergo regular upper endoscopies.”
However, “RYGB still leads to an increase in proton-pump inhibitor use, despite RYGB being one of the most effective antireflux procedures,” they said. “This finding needs further investigation.”
Furthermore, “a 4% Barrett esophagus rate 10 years after RYGB is troublesome, and the reasons should be investigated,” they added.
“Another relevant finding is that after 10 years, RYGB has a statistically better weight loss, which reaches the primary endpoint of the SLEEVEPASS trial for the first time,” they noted, yet the clinical relevance of this is not clear, since there was no difference in resolution of comorbidities, except for hypertension.
Gyanprakash A. Ketwaroo, MD, of Baylor College of Medicine, Houston, who was not involved with this research, agreed that “the study shows durable and good weight loss for either type of laparoscopic surgery with important metabolic effects and confirms the long-term benefits of weight-loss surgery.”
“What is somewhat new is the lower levels of Barrett’s esophagus after sleeve gastrectomy compared with several earlier studies,” he told this news organization in an email.
“This is somewhat incongruent with the relatively high incidence of postsleeve esophagitis noted in the study, which is an accepted risk factor for Barrett’s esophagus,” he continued. “Thus, I believe concern will still remain about GERD-related complications, including Barrett’s [esophagus], after sleeve gastrectomy.”
“This paper highlights the need for larger prospective studies, especially those that include diverse, older populations with multiple risk factors for Barrett’s esophagus,” Dr. Ketwaroo said.
Looking ahead
Using a large data set, such as that from SLEEVEPASS and possibly with data from the SM-BOSS trial and the BariSurg trial, with machine learning and other sophisticated analyses might identify parameters that could be used to choose the best operation for an individual patient, Dr. Salminen speculated.
“I think what we have learned from these long-term follow-up results is that GERD assessment should be a part of the preoperative assessment, and for patients who have preoperative GERD symptoms and GERD-related endoscopic findings (e.g., hiatal hernia), gastric bypass would be a more optimal procedure choice, if there are no contraindications for it,” she said.
Patient discussions should also cover “long-term symptoms, for example, abdominal pain after RYGB,” she added.
“I am looking forward to our future 20-year follow-up results,” Dr. Salminen said, “which will shed more light on this topic of postoperative [endoscopic] surveillance.
In the meantime, “preoperative gastroscopy is necessary and beneficial, at least when considering sleeve gastrectomy,” she said.
The SLEEVEPASS trial was supported by the Mary and Georg C. Ehrnrooth Foundation, the Government Research Foundation (in a grant awarded to Turku University Hospital), the Orion Research Foundation, the Paulo Foundation, and the Gastroenterological Research Foundation. Dr. Salminen reported receiving grants from the Government Research Foundation awarded to Turku University Hospital and the Mary and Georg C. Ehrnrooth Foundation. Another coauthor received grants from the Orion Research Foundation, the Paulo Foundation, and the Gastroenterological Research Foundation during the study. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
FROM JAMA SURGERY
Add AFib to noncardiac surgery risk evaluation: New support
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Avexitide promising for hypoglycemia after weight-loss surgery
Avexitide (Eiger Biopharmaceuticals), a first-in-class glucagonlike peptide (GLP)–1 receptor blocker, significantly reduced hypoglycemia in patients with refractory postbariatric hypoglycemia, new research finds.
Postbariatric hypoglycemia is a complication of bariatric surgery that is estimated to occur in about 29%-34% of people who undergo Roux-en-Y gastric bypass and in 11%-23% of those who undergo vertical sleeve gastrectomy. It typically manifests about 1-3 hours after meals and can lead to severe neuroglycopenic symptoms including blurred vision, confusion, drowsiness, and incoordination.
In addition, more than one-third (37%) with the condition have hypoglycemic unawareness. This can lead to seizures in about 59%, loss of consciousness and hospitalization in 50%, motor vehicle accidents, and even death. More than 90% with the condition consider themselves disabled, and 41% report being unable to work.
There are no currently approved medical treatments for postbariatric hypoglycemia. The standard of care is medical nutrition therapy involving a low-carbohydrate diet with carb restriction and small, frequent mixed meals. If this doesn’t work, off-label stepped pharmacotherapy has been tried, including acarbose (Precose), octreotide (Sandostatin), and diazoxide (Proglycem).
But “these are limited by efficacy and tolerability,” said Marilyn Tan, MD, who presented the findings from the phase 2 trial of avexitide at the annual meeting of the Endocrine Society.
In very severe cases, gastrostomy tubes or bypass reversal are options but those lead to weight regain and incomplete efficacy. “Safe, effective, and targeted therapies are needed urgently for postbariatric hypoglycemia,” said Dr. Tan, of the department of endocrinology at Stanford (Calif.) University.
The pathophysiology isn’t fully understood, but there appears to be an exaggerated GLP-1 response that leads to abnormal insulin secretion and symptomatic hyperinsulinemic hypoglycemia. Avexitide (formerly exendin 9-39), blocks the GLP-1 receptor and mitigates the excessive GLP-1 response, she explained.
Asked to comment, session moderator Michelle Van Name, MD, told this news organization, “This is a problem and it’s important for us to understand more about it and to identify different treatment options so these patients can continue to live their full, healthy lives post bariatric surgery.”
And, avexitide also holds potential for treating congenital hyperinsulinism, “which is a very challenging disease to treat in babies,” noted Dr. Van Name, a pediatric endocrinologist at Yale University, New Haven, Conn.
Drug reduced all levels of hypoglycemia, across surgery types
The study enrolled 14 women and 2 men with severe refractory postbariatric surgery hypoglycemia despite medical nutrition therapy. A majority (9) had undergone Roux-en-Y gastric bypass, 4 had vertical sleeve gastrectomy, 2 gastrectomy, and 1 had Nissen fundoplication. Seven patients (43.7%) had experienced loss of consciousness from hyperinsulinemic hypoglycemia. None had diabetes.
They were randomly assigned to either subcutaneous 45 mg of avexitide twice daily or 90 mg once daily for 14 days each, with a 2-day washout period followed by a switch to the other dose.
Both doses resulted in significant reductions in hypoglycemia as measured by self–blood glucose monitoring. The once-daily dose reduced level 1 hypoglycemia (glucose < 70 mg/dL) by 67.5% and it reduced level 2 (< 54 mg/dL) by 53.3% (P = .0043).
Even greater reductions were seen in severe hypoglycemia (that is, altered mental status/requiring assistance) – by 67.5% for the twice-daily dose (P = .0003) and by 66.1% with the once-daily dose (P = .0003).
“This is consistent with what we’ve seen in prior avexitide trials,” Dr. Tan noted.
More hypoglycemic events were captured using blinded continuous glucose monitoring (CGM), since it picked up episodes of which the patient was unaware. There were significant reductions in percentage time spent in level 1 and level 2 hypoglycemia, as well as in absolute number of hypoglycemic events over 14 days.
Here, the effect was greater with the once-daily 90 mg dose, with reductions of up to 65% in time spent and number of events, but results for the twice-daily dose were also significant, Dr. Tan said.
The drug was effective across all surgical subtypes. Patients who underwent vertical sleeve gastrectomy/gastrectomy had greater rates of hypoglycemia at baseline and “robust responses to avexitide subcutaneous injections. This supports the critical role of GLP-1,” Dr. Tan said.
There were no severe adverse events. The most commonly reported adverse events were diarrhea, headache, bloating, and injection-site reaction/bruising. All were mild and self-limited and resolved without treatment. No participants withdrew from the study.
Eiger Biopharmaceuticals is currently working with the U.S. Food and Drug Administration and the European Medicines Agency to design a phase 3 trial.
The study is an investor-initiated trial with funding from Eiger Biopharmaceuticals. Dr. Tan receives research support from Eiger Biopharmaceuticals as a site investigator. Dr. Van Name is an investigator for a trial sponsored by Provention Bio.
A version of this article first appeared on Medscape.com.
Avexitide (Eiger Biopharmaceuticals), a first-in-class glucagonlike peptide (GLP)–1 receptor blocker, significantly reduced hypoglycemia in patients with refractory postbariatric hypoglycemia, new research finds.
Postbariatric hypoglycemia is a complication of bariatric surgery that is estimated to occur in about 29%-34% of people who undergo Roux-en-Y gastric bypass and in 11%-23% of those who undergo vertical sleeve gastrectomy. It typically manifests about 1-3 hours after meals and can lead to severe neuroglycopenic symptoms including blurred vision, confusion, drowsiness, and incoordination.
In addition, more than one-third (37%) with the condition have hypoglycemic unawareness. This can lead to seizures in about 59%, loss of consciousness and hospitalization in 50%, motor vehicle accidents, and even death. More than 90% with the condition consider themselves disabled, and 41% report being unable to work.
There are no currently approved medical treatments for postbariatric hypoglycemia. The standard of care is medical nutrition therapy involving a low-carbohydrate diet with carb restriction and small, frequent mixed meals. If this doesn’t work, off-label stepped pharmacotherapy has been tried, including acarbose (Precose), octreotide (Sandostatin), and diazoxide (Proglycem).
But “these are limited by efficacy and tolerability,” said Marilyn Tan, MD, who presented the findings from the phase 2 trial of avexitide at the annual meeting of the Endocrine Society.
In very severe cases, gastrostomy tubes or bypass reversal are options but those lead to weight regain and incomplete efficacy. “Safe, effective, and targeted therapies are needed urgently for postbariatric hypoglycemia,” said Dr. Tan, of the department of endocrinology at Stanford (Calif.) University.
The pathophysiology isn’t fully understood, but there appears to be an exaggerated GLP-1 response that leads to abnormal insulin secretion and symptomatic hyperinsulinemic hypoglycemia. Avexitide (formerly exendin 9-39), blocks the GLP-1 receptor and mitigates the excessive GLP-1 response, she explained.
Asked to comment, session moderator Michelle Van Name, MD, told this news organization, “This is a problem and it’s important for us to understand more about it and to identify different treatment options so these patients can continue to live their full, healthy lives post bariatric surgery.”
And, avexitide also holds potential for treating congenital hyperinsulinism, “which is a very challenging disease to treat in babies,” noted Dr. Van Name, a pediatric endocrinologist at Yale University, New Haven, Conn.
Drug reduced all levels of hypoglycemia, across surgery types
The study enrolled 14 women and 2 men with severe refractory postbariatric surgery hypoglycemia despite medical nutrition therapy. A majority (9) had undergone Roux-en-Y gastric bypass, 4 had vertical sleeve gastrectomy, 2 gastrectomy, and 1 had Nissen fundoplication. Seven patients (43.7%) had experienced loss of consciousness from hyperinsulinemic hypoglycemia. None had diabetes.
They were randomly assigned to either subcutaneous 45 mg of avexitide twice daily or 90 mg once daily for 14 days each, with a 2-day washout period followed by a switch to the other dose.
Both doses resulted in significant reductions in hypoglycemia as measured by self–blood glucose monitoring. The once-daily dose reduced level 1 hypoglycemia (glucose < 70 mg/dL) by 67.5% and it reduced level 2 (< 54 mg/dL) by 53.3% (P = .0043).
Even greater reductions were seen in severe hypoglycemia (that is, altered mental status/requiring assistance) – by 67.5% for the twice-daily dose (P = .0003) and by 66.1% with the once-daily dose (P = .0003).
“This is consistent with what we’ve seen in prior avexitide trials,” Dr. Tan noted.
More hypoglycemic events were captured using blinded continuous glucose monitoring (CGM), since it picked up episodes of which the patient was unaware. There were significant reductions in percentage time spent in level 1 and level 2 hypoglycemia, as well as in absolute number of hypoglycemic events over 14 days.
Here, the effect was greater with the once-daily 90 mg dose, with reductions of up to 65% in time spent and number of events, but results for the twice-daily dose were also significant, Dr. Tan said.
The drug was effective across all surgical subtypes. Patients who underwent vertical sleeve gastrectomy/gastrectomy had greater rates of hypoglycemia at baseline and “robust responses to avexitide subcutaneous injections. This supports the critical role of GLP-1,” Dr. Tan said.
There were no severe adverse events. The most commonly reported adverse events were diarrhea, headache, bloating, and injection-site reaction/bruising. All were mild and self-limited and resolved without treatment. No participants withdrew from the study.
Eiger Biopharmaceuticals is currently working with the U.S. Food and Drug Administration and the European Medicines Agency to design a phase 3 trial.
The study is an investor-initiated trial with funding from Eiger Biopharmaceuticals. Dr. Tan receives research support from Eiger Biopharmaceuticals as a site investigator. Dr. Van Name is an investigator for a trial sponsored by Provention Bio.
A version of this article first appeared on Medscape.com.
Avexitide (Eiger Biopharmaceuticals), a first-in-class glucagonlike peptide (GLP)–1 receptor blocker, significantly reduced hypoglycemia in patients with refractory postbariatric hypoglycemia, new research finds.
Postbariatric hypoglycemia is a complication of bariatric surgery that is estimated to occur in about 29%-34% of people who undergo Roux-en-Y gastric bypass and in 11%-23% of those who undergo vertical sleeve gastrectomy. It typically manifests about 1-3 hours after meals and can lead to severe neuroglycopenic symptoms including blurred vision, confusion, drowsiness, and incoordination.
In addition, more than one-third (37%) with the condition have hypoglycemic unawareness. This can lead to seizures in about 59%, loss of consciousness and hospitalization in 50%, motor vehicle accidents, and even death. More than 90% with the condition consider themselves disabled, and 41% report being unable to work.
There are no currently approved medical treatments for postbariatric hypoglycemia. The standard of care is medical nutrition therapy involving a low-carbohydrate diet with carb restriction and small, frequent mixed meals. If this doesn’t work, off-label stepped pharmacotherapy has been tried, including acarbose (Precose), octreotide (Sandostatin), and diazoxide (Proglycem).
But “these are limited by efficacy and tolerability,” said Marilyn Tan, MD, who presented the findings from the phase 2 trial of avexitide at the annual meeting of the Endocrine Society.
In very severe cases, gastrostomy tubes or bypass reversal are options but those lead to weight regain and incomplete efficacy. “Safe, effective, and targeted therapies are needed urgently for postbariatric hypoglycemia,” said Dr. Tan, of the department of endocrinology at Stanford (Calif.) University.
The pathophysiology isn’t fully understood, but there appears to be an exaggerated GLP-1 response that leads to abnormal insulin secretion and symptomatic hyperinsulinemic hypoglycemia. Avexitide (formerly exendin 9-39), blocks the GLP-1 receptor and mitigates the excessive GLP-1 response, she explained.
Asked to comment, session moderator Michelle Van Name, MD, told this news organization, “This is a problem and it’s important for us to understand more about it and to identify different treatment options so these patients can continue to live their full, healthy lives post bariatric surgery.”
And, avexitide also holds potential for treating congenital hyperinsulinism, “which is a very challenging disease to treat in babies,” noted Dr. Van Name, a pediatric endocrinologist at Yale University, New Haven, Conn.
Drug reduced all levels of hypoglycemia, across surgery types
The study enrolled 14 women and 2 men with severe refractory postbariatric surgery hypoglycemia despite medical nutrition therapy. A majority (9) had undergone Roux-en-Y gastric bypass, 4 had vertical sleeve gastrectomy, 2 gastrectomy, and 1 had Nissen fundoplication. Seven patients (43.7%) had experienced loss of consciousness from hyperinsulinemic hypoglycemia. None had diabetes.
They were randomly assigned to either subcutaneous 45 mg of avexitide twice daily or 90 mg once daily for 14 days each, with a 2-day washout period followed by a switch to the other dose.
Both doses resulted in significant reductions in hypoglycemia as measured by self–blood glucose monitoring. The once-daily dose reduced level 1 hypoglycemia (glucose < 70 mg/dL) by 67.5% and it reduced level 2 (< 54 mg/dL) by 53.3% (P = .0043).
Even greater reductions were seen in severe hypoglycemia (that is, altered mental status/requiring assistance) – by 67.5% for the twice-daily dose (P = .0003) and by 66.1% with the once-daily dose (P = .0003).
“This is consistent with what we’ve seen in prior avexitide trials,” Dr. Tan noted.
More hypoglycemic events were captured using blinded continuous glucose monitoring (CGM), since it picked up episodes of which the patient was unaware. There were significant reductions in percentage time spent in level 1 and level 2 hypoglycemia, as well as in absolute number of hypoglycemic events over 14 days.
Here, the effect was greater with the once-daily 90 mg dose, with reductions of up to 65% in time spent and number of events, but results for the twice-daily dose were also significant, Dr. Tan said.
The drug was effective across all surgical subtypes. Patients who underwent vertical sleeve gastrectomy/gastrectomy had greater rates of hypoglycemia at baseline and “robust responses to avexitide subcutaneous injections. This supports the critical role of GLP-1,” Dr. Tan said.
There were no severe adverse events. The most commonly reported adverse events were diarrhea, headache, bloating, and injection-site reaction/bruising. All were mild and self-limited and resolved without treatment. No participants withdrew from the study.
Eiger Biopharmaceuticals is currently working with the U.S. Food and Drug Administration and the European Medicines Agency to design a phase 3 trial.
The study is an investor-initiated trial with funding from Eiger Biopharmaceuticals. Dr. Tan receives research support from Eiger Biopharmaceuticals as a site investigator. Dr. Van Name is an investigator for a trial sponsored by Provention Bio.
A version of this article first appeared on Medscape.com.
FROM ENDO 2022





