User login
How ObGyns can best work with radiologists to optimize screening for patients with dense breasts
If your ObGyn practices are anything like ours, every time there is news coverage of a study regarding mammography or about efforts to pass a breast density inform law, your phone rings with patient calls. In fact, every density inform law enacted in the United States, except for in Illinois, directs patients to their referring provider—generally their ObGyn—to discuss the screening and risk implications of dense breast tissue.
The steady increased awareness of breast density means that we, as ObGyns and other primary care providers (PCPs), have additional responsibilities in managing the breast health of our patients. This includes guiding discussions with patients about what breast density means and whether supplemental screening beyond mammography might be beneficial.
As members of the Medical Advisory Board for DenseBreast-info.org (an online educational resource dedicated to providing breast density information to patients and health care professionals), we are aware of the growing body of evidence demonstrating improved detection of early breast cancer using supplemental screening in dense breasts. However, we know that there is confusion among clinicians about how and when to facilitate tailored screening for women with dense breasts or other breast cancer risk factors. Here we answer 6 questions focusing on how to navigate patient discussions around the topic and the best way to collaborate with radiologists to improve breast care for patients.
Play an active role
1. What role should ObGyns and PCPs play in women’s breast health?
Elizabeth Etkin-Kramer, MD: I am a firm believer that ObGyns and all women’s health providers should be able to assess their patients’ risk of breast cancer and explain the process for managing this risk with their patients. This explanation includes the clinical implications of breast density and when supplemental screening should be employed. It is also important for providers to know when to offer genetic testing and when a patient’s personal or family history indicates supplemental screening with breast magnetic resonance imaging (MRI).
DaCarla M. Albright, MD: I absolutely agree that PCPs, ObGyns, and family practitioners should spend the time to be educated about breast density and supplemental screening options. While the exact role providers play in managing patients’ breast health may vary depending on the practice type or location, the need for knowledge and comfort when talking with patients to help them make informed decisions is critical. Breast health and screening, including the importance of breast density, happen to be a particular interest of mine. I have participated in educational webinars, invited lectures, and breast cancer awareness media events on this topic in the past.
Continue to: Join forces with imaging centers...
Join forces with imaging centers
2. How can ObGyns and radiologists collaborate most effectively to use screening results to personalize breast care for patients?
Dr. Etkin-Kramer: It is important to have a close relationship with the radiologists that read our patients’ mammograms. We need to be able to easily contact the radiologist and quickly get clarification on a patient’s report or discuss next steps. Imaging centers should consider running outreach programs to educate their referring providers on how to risk assess, with this assessment inclusive of breast density. Dinner lectures or grand round meetings are effective to facilitate communication between the radiology community and the ObGyn community. Finally, as we all know, supplemental screening is often subject to copays and deductibles per insurance coverage. If advocacy groups, who are working to eliminate these types of costs, cannot get insurers to waive these payments, we need a less expensive self-pay option.
Dr. Albright: I definitely have and encourage an open line of communication between my practice and breast radiology, as well as our breast surgeons and cancer center to set up consultations as needed. We also invite our radiologists as guests to monthly practice meetings or grand rounds within our department to further improve access and open communication, as this environment is one in which greater provider education on density and adjunctive screening can be achieved.
Know when to refer a high-risk patient
3. Most ObGyns routinely collect family history and perform formal risk assessment. What do you need to know about referring patients to a high-risk program?
Dr. Etkin-Kramer: It is important as ObGyns to be knowledgeable about breast and ovarian cancer risk assessment and genetic testing for cancer susceptibility genes. Our patients expect that of us. I am comfortable doing risk assessment in my office, but I sometimes refer to other specialists in the community if the patient needs additional counseling. For risk assessment, I look at family and personal history, breast density, and other factors that might lead me to believe the patient might carry a hereditary cancer susceptibility gene, including Ashkenazi Jewish ancestry.1 When indicated, I check lifetime as well as short-term (5- to 10-year) risk, usually using Breast Cancer Surveillance Consortium (BCSC) or Tyrer-Cuzick/International Breast Cancer Intervention Study (IBIS) models, as these include breast density.
I discuss risk-reducing medications. The US Preventive Services Task Force recommends these agents if my patient’s 5-year risk of breast cancer is 1.67% or greater, and I strongly recommend chemoprevention when the patient’s 5-year BCSC risk exceeds 3%, provided likely benefits exceed risks.2,3 I discuss adding screening breast MRI if lifetime risk by Tyrer-Cuzick exceeds 20%. (Note that Gail and BCSC models are not recommended to be used to determine risk for purposes of supplemental screening with MRI as they do not consider paternal family history nor age of relatives at diagnosis.)
Dr. Albright: ObGyns should be able to ascertain a pertinent history and identify patients at risk for breast cancer based on their personal history, family history, and breast imaging/biopsy history, if relevant. We also need to improve our discussions of supplemental screening for patients who have heterogeneously dense or extremely dense breast tissue. I sense that some ObGyns may rely heavily on the radiologist to suggest supplemental screening, but patients actually look to ObGyns as their providers to have this knowledge and give them direction.
Since I practice at a large academic medical center, I have the opportunity to refer patients to our Breast Cancer Genetics Program because I may be limited on time for counseling in the office and do not want to miss salient details. With all of the information I have ascertained about the patient, I am able to determine and encourage appropriate screening and assure insurance coverage for adjunctive breast MRI when appropriate.
Continue to: Consider how you order patients’ screening to reduce barriers and cost...
Consider how you order patients’ screening to reduce barriers and cost
4. How would you suggest reducing barriers when referring patients for supplemental screening, such as MRI for high-risk women or ultrasound for those with dense breasts? Would you prefer it if such screening could be performed without additional script/referral? How does insurance coverage factor in?
Dr. Etkin-Kramer: I would love for a screening mammogram with possible ultrasound, on one script, to be the norm. One of the centers that I work with accepts a script written this way. Further, when a patient receives screening at a freestanding facility as opposed to a hospital, the fee for the supplemental screening may be lower because they do not add on a facility fee.
Dr. Albright: We have an order in our electronic health record that allows for screening mammography but adds on diagnostic mammography/bilateral ultrasonography, if indicated by imaging. I am mostly ordering that option now for all of my screening patients; rarely have I had issues with insurance accepting that script. As for when ordering an MRI, I always try to ensure that I have done the patient’s personal risk assessment and included that lifetime breast cancer risk on the order. If the risk is 20% or higher, I typically do not have any insurance coverage issues. If I am ordering MRI as supplemental screening, I typically order the “Fast MRI” protocol that our center offers. This order incurs a $299 out-of-pocket cost for the patient. Any patient with heterogeneously or extremely dense breasts on mammography should have this option, but it requires patient education, discussion with the provider, and an additional cost. I definitely think that insurers need to consider covering supplemental screening, since breast density is reportable in a majority of the US states and will soon be the national standard.
Pearls for guiding patients
5. How do you discuss breast density and the need for supplemental screening with your patients?
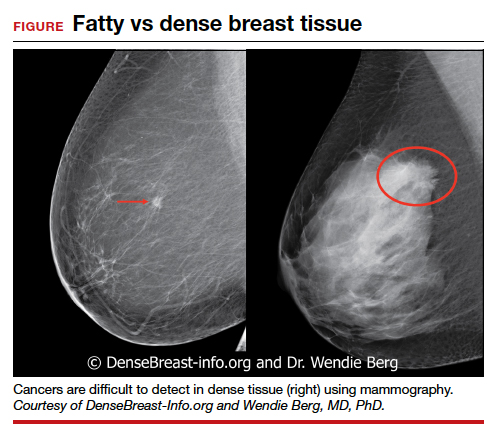
Dr. Etkin-Kramer: I strongly feel that my patients need to know when a screening test has limited ability to do its job. This is the case with dense breasts. Visuals help; when discussing breast density, I like the images supplied by DenseBreast-info.org (FIGURE). I explain the two implications of dense tissue:
- First, dense tissue makes it harder to visualize cancers in the breast—the denser the breasts, the less likely the radiologist can pick up a cancer, so mammographic sensitivity for extremely dense breasts can be as low as 25% to 50%.
- Second, high breast density adds to the risk of developing breast cancer. I explain that supplemental screening will pick up additional cancers in women with dense breasts. For example, breast ultrasound will pick up about 2-3/1000 additional breast cancers per year and MRI or molecular breast imaging (MBI) will pick up much more, perhaps 10/1000.
MRI is more invasive than an ultrasound and uses gadolinium, and MBI has more radiation. Supplemental screening is not endorsed by ACOG’s most recent Committee Opinion from 2017; 4 however, patients may choose to have it done. This is where shared-decision making is important.
I strongly recommend that all women’s health care providers complete the CME course on the DenseBreast-info.org website. “ Breast Density: Why It Matters ” is a certified educational program for referring physicians that helps health care professionals learn about breast density, its associated risks, and how best to guide patients regarding breast cancer screening.
Continue to: Dr. Albright...
Dr. Albright: When I discuss breast density, I make sure that patients understand that their mammogram determines the density of their breast tissue. I review that in the higher density categories (heterogeneously dense or extremely dense), there is a higher risk of missing cancer, and that these categories are also associated with a higher risk of breast cancer. I also discuss the potential need for supplemental screening, for which my institution primarily offers Fast MRI. However, we can offer breast ultrasonography instead as an option, especially for those concerned about gadolinium exposure. Our center offers either of these supplemental screenings at a cost of $299. I also review the lack of coverage for supplemental screening by some insurance carriers, as both providers and patients may need to advocate for insurer coverage of adjunct studies.
Educational resources
6. What reference materials, illustrations, or other tools do you use to educate your patients?
Dr. Etkin-Kramer: I frequently use handouts printed from the DenseBreast-info.org website, and there is now a brand new patient fact sheet that I have just started using. I also have an example of breast density categories from fatty replaced to extremely dense on my computer, and I am putting it on a new smart board.
Dr. Albright: The extensive resources available at DenseBreast-info.org can improve both patient and provider knowledge of these important issues, so I suggest patients visit that website, and I use many of the images and visuals to help explain breast density. I even use the materials from the website for educating my resident trainees on breast health and screening. ●
Nearly 16,000 children (up to age 19 years) face cancer-related treatment every year.1 For girls and young women, undergoing chest radiotherapy puts them at higher risk for secondary breast cancer. In fact, they have a 30% chance of developing such cancer by age 50—a risk that is similar to women with a BRCA1 mutation.2 Therefore, current recommendations for breast cancer screening among those who have undergone childhood chest radiation (≥20 Gy) are to begin annual mammography, with adjunct magnetic resonance imaging (MRI), at age 25 years (or 8 years after chest radiotherapy).3
To determine the benefits and risks of these recommendations, as well as of similar strategies, Yeh and colleagues performed simulation modeling using data from the Childhood Cancer Survivor Study and two CISNET (Cancer Intervention and Surveillance Modeling Network) models.4 For their study they targeted a cohort of female childhood cancer survivors having undergone chest radiotherapy and evaluated breast cancer screening with the following strategies:
- mammography plus MRI, starting at ages 25, 30, or 35 years and continuing to age 74
- MRI alone, starting at ages 25, 30, or 35 years and continuing to age 74.
They found that both strategies reduced the risk of breast cancer in the targeted cohort but that screening beginning at the earliest ages prevented most deaths. No screening at all was associated with a 10% to 11% lifetime risk of breast cancer, but mammography plus MRI beginning at age 25 reduced that risk by 56% to 71% depending on the model. Screening with MRI alone reduced mortality risk by 56% to 62%. When considering cost per quality adjusted life-year gained, the researchers found that screening beginning at age 30 to be the most cost-effective.4
Yeh and colleagues addressed concerns with mammography and radiation. Although they said the associated amount of radiation exposure is small, the use of mammography in women younger than age 30 is controversial—and not recommended by the American Cancer Society or the National Comprehensive Cancer Network.5,6
Bottom line. Yeh and colleagues conclude that MRI screening, with or without mammography, beginning between the ages of 25 and 30 should be emphasized in screening guidelines. They note the importance of insurance coverage for MRI in those at risk for breast cancer due to childhood radiation exposure.4
References
- National Cancer Institute. How common is cancer in children? https://www.cancer.gov/types/childhood-cancers/child-adolescentcancers-fact-sheet#how-common-is-cancer-in-children. Accessed September 25, 2020.
- Moskowitz CS, Chou JF, Wolden SL, et al. Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol. 2014;32:2217- 2223.
- Children’s Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. http:// www.survivorshipguidelines.org/pdf/2018/COG_LTFU_Guidelines_v5.pdf. Accessed September 25, 2020.
- Yeh JM, Lowry KP, Schechter CB, et al. Clinical benefits, harms, and cost-effectiveness of breast cancer screening for survivors of childhood cancer treated with chest radiation. Ann Intern Med. 2020;173:331-341.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Breast cancer screening and diagnosis version 1.2019. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed September 25, 2020.
- Bharucha PP, Chiu KE, Francois FM, et al. Genetic testing and screening recommendations for patients with hereditary breast cancer. RadioGraphics. 2020;40:913-936.
- Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol. 2011;29:2327-2333.
- Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol. 2015;22:3230-3235.
- American College of Obstetricians and Gynecologists. Committee opinion no. 625: management of women with dense breasts diagnosed by mammography [published correction appears in Obstet Gynecol. 2016;127:166]. Obstet Gynecol. 2015;125(3):750-751.
If your ObGyn practices are anything like ours, every time there is news coverage of a study regarding mammography or about efforts to pass a breast density inform law, your phone rings with patient calls. In fact, every density inform law enacted in the United States, except for in Illinois, directs patients to their referring provider—generally their ObGyn—to discuss the screening and risk implications of dense breast tissue.
The steady increased awareness of breast density means that we, as ObGyns and other primary care providers (PCPs), have additional responsibilities in managing the breast health of our patients. This includes guiding discussions with patients about what breast density means and whether supplemental screening beyond mammography might be beneficial.
As members of the Medical Advisory Board for DenseBreast-info.org (an online educational resource dedicated to providing breast density information to patients and health care professionals), we are aware of the growing body of evidence demonstrating improved detection of early breast cancer using supplemental screening in dense breasts. However, we know that there is confusion among clinicians about how and when to facilitate tailored screening for women with dense breasts or other breast cancer risk factors. Here we answer 6 questions focusing on how to navigate patient discussions around the topic and the best way to collaborate with radiologists to improve breast care for patients.
Play an active role
1. What role should ObGyns and PCPs play in women’s breast health?
Elizabeth Etkin-Kramer, MD: I am a firm believer that ObGyns and all women’s health providers should be able to assess their patients’ risk of breast cancer and explain the process for managing this risk with their patients. This explanation includes the clinical implications of breast density and when supplemental screening should be employed. It is also important for providers to know when to offer genetic testing and when a patient’s personal or family history indicates supplemental screening with breast magnetic resonance imaging (MRI).
DaCarla M. Albright, MD: I absolutely agree that PCPs, ObGyns, and family practitioners should spend the time to be educated about breast density and supplemental screening options. While the exact role providers play in managing patients’ breast health may vary depending on the practice type or location, the need for knowledge and comfort when talking with patients to help them make informed decisions is critical. Breast health and screening, including the importance of breast density, happen to be a particular interest of mine. I have participated in educational webinars, invited lectures, and breast cancer awareness media events on this topic in the past.
Continue to: Join forces with imaging centers...
Join forces with imaging centers
2. How can ObGyns and radiologists collaborate most effectively to use screening results to personalize breast care for patients?
Dr. Etkin-Kramer: It is important to have a close relationship with the radiologists that read our patients’ mammograms. We need to be able to easily contact the radiologist and quickly get clarification on a patient’s report or discuss next steps. Imaging centers should consider running outreach programs to educate their referring providers on how to risk assess, with this assessment inclusive of breast density. Dinner lectures or grand round meetings are effective to facilitate communication between the radiology community and the ObGyn community. Finally, as we all know, supplemental screening is often subject to copays and deductibles per insurance coverage. If advocacy groups, who are working to eliminate these types of costs, cannot get insurers to waive these payments, we need a less expensive self-pay option.
Dr. Albright: I definitely have and encourage an open line of communication between my practice and breast radiology, as well as our breast surgeons and cancer center to set up consultations as needed. We also invite our radiologists as guests to monthly practice meetings or grand rounds within our department to further improve access and open communication, as this environment is one in which greater provider education on density and adjunctive screening can be achieved.
Know when to refer a high-risk patient
3. Most ObGyns routinely collect family history and perform formal risk assessment. What do you need to know about referring patients to a high-risk program?
Dr. Etkin-Kramer: It is important as ObGyns to be knowledgeable about breast and ovarian cancer risk assessment and genetic testing for cancer susceptibility genes. Our patients expect that of us. I am comfortable doing risk assessment in my office, but I sometimes refer to other specialists in the community if the patient needs additional counseling. For risk assessment, I look at family and personal history, breast density, and other factors that might lead me to believe the patient might carry a hereditary cancer susceptibility gene, including Ashkenazi Jewish ancestry.1 When indicated, I check lifetime as well as short-term (5- to 10-year) risk, usually using Breast Cancer Surveillance Consortium (BCSC) or Tyrer-Cuzick/International Breast Cancer Intervention Study (IBIS) models, as these include breast density.
I discuss risk-reducing medications. The US Preventive Services Task Force recommends these agents if my patient’s 5-year risk of breast cancer is 1.67% or greater, and I strongly recommend chemoprevention when the patient’s 5-year BCSC risk exceeds 3%, provided likely benefits exceed risks.2,3 I discuss adding screening breast MRI if lifetime risk by Tyrer-Cuzick exceeds 20%. (Note that Gail and BCSC models are not recommended to be used to determine risk for purposes of supplemental screening with MRI as they do not consider paternal family history nor age of relatives at diagnosis.)
Dr. Albright: ObGyns should be able to ascertain a pertinent history and identify patients at risk for breast cancer based on their personal history, family history, and breast imaging/biopsy history, if relevant. We also need to improve our discussions of supplemental screening for patients who have heterogeneously dense or extremely dense breast tissue. I sense that some ObGyns may rely heavily on the radiologist to suggest supplemental screening, but patients actually look to ObGyns as their providers to have this knowledge and give them direction.
Since I practice at a large academic medical center, I have the opportunity to refer patients to our Breast Cancer Genetics Program because I may be limited on time for counseling in the office and do not want to miss salient details. With all of the information I have ascertained about the patient, I am able to determine and encourage appropriate screening and assure insurance coverage for adjunctive breast MRI when appropriate.
Continue to: Consider how you order patients’ screening to reduce barriers and cost...
Consider how you order patients’ screening to reduce barriers and cost
4. How would you suggest reducing barriers when referring patients for supplemental screening, such as MRI for high-risk women or ultrasound for those with dense breasts? Would you prefer it if such screening could be performed without additional script/referral? How does insurance coverage factor in?
Dr. Etkin-Kramer: I would love for a screening mammogram with possible ultrasound, on one script, to be the norm. One of the centers that I work with accepts a script written this way. Further, when a patient receives screening at a freestanding facility as opposed to a hospital, the fee for the supplemental screening may be lower because they do not add on a facility fee.
Dr. Albright: We have an order in our electronic health record that allows for screening mammography but adds on diagnostic mammography/bilateral ultrasonography, if indicated by imaging. I am mostly ordering that option now for all of my screening patients; rarely have I had issues with insurance accepting that script. As for when ordering an MRI, I always try to ensure that I have done the patient’s personal risk assessment and included that lifetime breast cancer risk on the order. If the risk is 20% or higher, I typically do not have any insurance coverage issues. If I am ordering MRI as supplemental screening, I typically order the “Fast MRI” protocol that our center offers. This order incurs a $299 out-of-pocket cost for the patient. Any patient with heterogeneously or extremely dense breasts on mammography should have this option, but it requires patient education, discussion with the provider, and an additional cost. I definitely think that insurers need to consider covering supplemental screening, since breast density is reportable in a majority of the US states and will soon be the national standard.
Pearls for guiding patients
5. How do you discuss breast density and the need for supplemental screening with your patients?
Dr. Etkin-Kramer: I strongly feel that my patients need to know when a screening test has limited ability to do its job. This is the case with dense breasts. Visuals help; when discussing breast density, I like the images supplied by DenseBreast-info.org (FIGURE). I explain the two implications of dense tissue:
- First, dense tissue makes it harder to visualize cancers in the breast—the denser the breasts, the less likely the radiologist can pick up a cancer, so mammographic sensitivity for extremely dense breasts can be as low as 25% to 50%.
- Second, high breast density adds to the risk of developing breast cancer. I explain that supplemental screening will pick up additional cancers in women with dense breasts. For example, breast ultrasound will pick up about 2-3/1000 additional breast cancers per year and MRI or molecular breast imaging (MBI) will pick up much more, perhaps 10/1000.

MRI is more invasive than an ultrasound and uses gadolinium, and MBI has more radiation. Supplemental screening is not endorsed by ACOG’s most recent Committee Opinion from 2017; 4 however, patients may choose to have it done. This is where shared-decision making is important.
I strongly recommend that all women’s health care providers complete the CME course on the DenseBreast-info.org website. “ Breast Density: Why It Matters ” is a certified educational program for referring physicians that helps health care professionals learn about breast density, its associated risks, and how best to guide patients regarding breast cancer screening.
Continue to: Dr. Albright...
Dr. Albright: When I discuss breast density, I make sure that patients understand that their mammogram determines the density of their breast tissue. I review that in the higher density categories (heterogeneously dense or extremely dense), there is a higher risk of missing cancer, and that these categories are also associated with a higher risk of breast cancer. I also discuss the potential need for supplemental screening, for which my institution primarily offers Fast MRI. However, we can offer breast ultrasonography instead as an option, especially for those concerned about gadolinium exposure. Our center offers either of these supplemental screenings at a cost of $299. I also review the lack of coverage for supplemental screening by some insurance carriers, as both providers and patients may need to advocate for insurer coverage of adjunct studies.
Educational resources
6. What reference materials, illustrations, or other tools do you use to educate your patients?
Dr. Etkin-Kramer: I frequently use handouts printed from the DenseBreast-info.org website, and there is now a brand new patient fact sheet that I have just started using. I also have an example of breast density categories from fatty replaced to extremely dense on my computer, and I am putting it on a new smart board.
Dr. Albright: The extensive resources available at DenseBreast-info.org can improve both patient and provider knowledge of these important issues, so I suggest patients visit that website, and I use many of the images and visuals to help explain breast density. I even use the materials from the website for educating my resident trainees on breast health and screening. ●
Nearly 16,000 children (up to age 19 years) face cancer-related treatment every year.1 For girls and young women, undergoing chest radiotherapy puts them at higher risk for secondary breast cancer. In fact, they have a 30% chance of developing such cancer by age 50—a risk that is similar to women with a BRCA1 mutation.2 Therefore, current recommendations for breast cancer screening among those who have undergone childhood chest radiation (≥20 Gy) are to begin annual mammography, with adjunct magnetic resonance imaging (MRI), at age 25 years (or 8 years after chest radiotherapy).3
To determine the benefits and risks of these recommendations, as well as of similar strategies, Yeh and colleagues performed simulation modeling using data from the Childhood Cancer Survivor Study and two CISNET (Cancer Intervention and Surveillance Modeling Network) models.4 For their study they targeted a cohort of female childhood cancer survivors having undergone chest radiotherapy and evaluated breast cancer screening with the following strategies:
- mammography plus MRI, starting at ages 25, 30, or 35 years and continuing to age 74
- MRI alone, starting at ages 25, 30, or 35 years and continuing to age 74.
They found that both strategies reduced the risk of breast cancer in the targeted cohort but that screening beginning at the earliest ages prevented most deaths. No screening at all was associated with a 10% to 11% lifetime risk of breast cancer, but mammography plus MRI beginning at age 25 reduced that risk by 56% to 71% depending on the model. Screening with MRI alone reduced mortality risk by 56% to 62%. When considering cost per quality adjusted life-year gained, the researchers found that screening beginning at age 30 to be the most cost-effective.4
Yeh and colleagues addressed concerns with mammography and radiation. Although they said the associated amount of radiation exposure is small, the use of mammography in women younger than age 30 is controversial—and not recommended by the American Cancer Society or the National Comprehensive Cancer Network.5,6
Bottom line. Yeh and colleagues conclude that MRI screening, with or without mammography, beginning between the ages of 25 and 30 should be emphasized in screening guidelines. They note the importance of insurance coverage for MRI in those at risk for breast cancer due to childhood radiation exposure.4
References
- National Cancer Institute. How common is cancer in children? https://www.cancer.gov/types/childhood-cancers/child-adolescentcancers-fact-sheet#how-common-is-cancer-in-children. Accessed September 25, 2020.
- Moskowitz CS, Chou JF, Wolden SL, et al. Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol. 2014;32:2217- 2223.
- Children’s Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. http:// www.survivorshipguidelines.org/pdf/2018/COG_LTFU_Guidelines_v5.pdf. Accessed September 25, 2020.
- Yeh JM, Lowry KP, Schechter CB, et al. Clinical benefits, harms, and cost-effectiveness of breast cancer screening for survivors of childhood cancer treated with chest radiation. Ann Intern Med. 2020;173:331-341.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Breast cancer screening and diagnosis version 1.2019. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed September 25, 2020.
If your ObGyn practices are anything like ours, every time there is news coverage of a study regarding mammography or about efforts to pass a breast density inform law, your phone rings with patient calls. In fact, every density inform law enacted in the United States, except for in Illinois, directs patients to their referring provider—generally their ObGyn—to discuss the screening and risk implications of dense breast tissue.
The steady increased awareness of breast density means that we, as ObGyns and other primary care providers (PCPs), have additional responsibilities in managing the breast health of our patients. This includes guiding discussions with patients about what breast density means and whether supplemental screening beyond mammography might be beneficial.
As members of the Medical Advisory Board for DenseBreast-info.org (an online educational resource dedicated to providing breast density information to patients and health care professionals), we are aware of the growing body of evidence demonstrating improved detection of early breast cancer using supplemental screening in dense breasts. However, we know that there is confusion among clinicians about how and when to facilitate tailored screening for women with dense breasts or other breast cancer risk factors. Here we answer 6 questions focusing on how to navigate patient discussions around the topic and the best way to collaborate with radiologists to improve breast care for patients.
Play an active role
1. What role should ObGyns and PCPs play in women’s breast health?
Elizabeth Etkin-Kramer, MD: I am a firm believer that ObGyns and all women’s health providers should be able to assess their patients’ risk of breast cancer and explain the process for managing this risk with their patients. This explanation includes the clinical implications of breast density and when supplemental screening should be employed. It is also important for providers to know when to offer genetic testing and when a patient’s personal or family history indicates supplemental screening with breast magnetic resonance imaging (MRI).
DaCarla M. Albright, MD: I absolutely agree that PCPs, ObGyns, and family practitioners should spend the time to be educated about breast density and supplemental screening options. While the exact role providers play in managing patients’ breast health may vary depending on the practice type or location, the need for knowledge and comfort when talking with patients to help them make informed decisions is critical. Breast health and screening, including the importance of breast density, happen to be a particular interest of mine. I have participated in educational webinars, invited lectures, and breast cancer awareness media events on this topic in the past.
Continue to: Join forces with imaging centers...
Join forces with imaging centers
2. How can ObGyns and radiologists collaborate most effectively to use screening results to personalize breast care for patients?
Dr. Etkin-Kramer: It is important to have a close relationship with the radiologists that read our patients’ mammograms. We need to be able to easily contact the radiologist and quickly get clarification on a patient’s report or discuss next steps. Imaging centers should consider running outreach programs to educate their referring providers on how to risk assess, with this assessment inclusive of breast density. Dinner lectures or grand round meetings are effective to facilitate communication between the radiology community and the ObGyn community. Finally, as we all know, supplemental screening is often subject to copays and deductibles per insurance coverage. If advocacy groups, who are working to eliminate these types of costs, cannot get insurers to waive these payments, we need a less expensive self-pay option.
Dr. Albright: I definitely have and encourage an open line of communication between my practice and breast radiology, as well as our breast surgeons and cancer center to set up consultations as needed. We also invite our radiologists as guests to monthly practice meetings or grand rounds within our department to further improve access and open communication, as this environment is one in which greater provider education on density and adjunctive screening can be achieved.
Know when to refer a high-risk patient
3. Most ObGyns routinely collect family history and perform formal risk assessment. What do you need to know about referring patients to a high-risk program?
Dr. Etkin-Kramer: It is important as ObGyns to be knowledgeable about breast and ovarian cancer risk assessment and genetic testing for cancer susceptibility genes. Our patients expect that of us. I am comfortable doing risk assessment in my office, but I sometimes refer to other specialists in the community if the patient needs additional counseling. For risk assessment, I look at family and personal history, breast density, and other factors that might lead me to believe the patient might carry a hereditary cancer susceptibility gene, including Ashkenazi Jewish ancestry.1 When indicated, I check lifetime as well as short-term (5- to 10-year) risk, usually using Breast Cancer Surveillance Consortium (BCSC) or Tyrer-Cuzick/International Breast Cancer Intervention Study (IBIS) models, as these include breast density.
I discuss risk-reducing medications. The US Preventive Services Task Force recommends these agents if my patient’s 5-year risk of breast cancer is 1.67% or greater, and I strongly recommend chemoprevention when the patient’s 5-year BCSC risk exceeds 3%, provided likely benefits exceed risks.2,3 I discuss adding screening breast MRI if lifetime risk by Tyrer-Cuzick exceeds 20%. (Note that Gail and BCSC models are not recommended to be used to determine risk for purposes of supplemental screening with MRI as they do not consider paternal family history nor age of relatives at diagnosis.)
Dr. Albright: ObGyns should be able to ascertain a pertinent history and identify patients at risk for breast cancer based on their personal history, family history, and breast imaging/biopsy history, if relevant. We also need to improve our discussions of supplemental screening for patients who have heterogeneously dense or extremely dense breast tissue. I sense that some ObGyns may rely heavily on the radiologist to suggest supplemental screening, but patients actually look to ObGyns as their providers to have this knowledge and give them direction.
Since I practice at a large academic medical center, I have the opportunity to refer patients to our Breast Cancer Genetics Program because I may be limited on time for counseling in the office and do not want to miss salient details. With all of the information I have ascertained about the patient, I am able to determine and encourage appropriate screening and assure insurance coverage for adjunctive breast MRI when appropriate.
Continue to: Consider how you order patients’ screening to reduce barriers and cost...
Consider how you order patients’ screening to reduce barriers and cost
4. How would you suggest reducing barriers when referring patients for supplemental screening, such as MRI for high-risk women or ultrasound for those with dense breasts? Would you prefer it if such screening could be performed without additional script/referral? How does insurance coverage factor in?
Dr. Etkin-Kramer: I would love for a screening mammogram with possible ultrasound, on one script, to be the norm. One of the centers that I work with accepts a script written this way. Further, when a patient receives screening at a freestanding facility as opposed to a hospital, the fee for the supplemental screening may be lower because they do not add on a facility fee.
Dr. Albright: We have an order in our electronic health record that allows for screening mammography but adds on diagnostic mammography/bilateral ultrasonography, if indicated by imaging. I am mostly ordering that option now for all of my screening patients; rarely have I had issues with insurance accepting that script. As for when ordering an MRI, I always try to ensure that I have done the patient’s personal risk assessment and included that lifetime breast cancer risk on the order. If the risk is 20% or higher, I typically do not have any insurance coverage issues. If I am ordering MRI as supplemental screening, I typically order the “Fast MRI” protocol that our center offers. This order incurs a $299 out-of-pocket cost for the patient. Any patient with heterogeneously or extremely dense breasts on mammography should have this option, but it requires patient education, discussion with the provider, and an additional cost. I definitely think that insurers need to consider covering supplemental screening, since breast density is reportable in a majority of the US states and will soon be the national standard.
Pearls for guiding patients
5. How do you discuss breast density and the need for supplemental screening with your patients?
Dr. Etkin-Kramer: I strongly feel that my patients need to know when a screening test has limited ability to do its job. This is the case with dense breasts. Visuals help; when discussing breast density, I like the images supplied by DenseBreast-info.org (FIGURE). I explain the two implications of dense tissue:
- First, dense tissue makes it harder to visualize cancers in the breast—the denser the breasts, the less likely the radiologist can pick up a cancer, so mammographic sensitivity for extremely dense breasts can be as low as 25% to 50%.
- Second, high breast density adds to the risk of developing breast cancer. I explain that supplemental screening will pick up additional cancers in women with dense breasts. For example, breast ultrasound will pick up about 2-3/1000 additional breast cancers per year and MRI or molecular breast imaging (MBI) will pick up much more, perhaps 10/1000.

MRI is more invasive than an ultrasound and uses gadolinium, and MBI has more radiation. Supplemental screening is not endorsed by ACOG’s most recent Committee Opinion from 2017; 4 however, patients may choose to have it done. This is where shared-decision making is important.
I strongly recommend that all women’s health care providers complete the CME course on the DenseBreast-info.org website. “ Breast Density: Why It Matters ” is a certified educational program for referring physicians that helps health care professionals learn about breast density, its associated risks, and how best to guide patients regarding breast cancer screening.
Continue to: Dr. Albright...
Dr. Albright: When I discuss breast density, I make sure that patients understand that their mammogram determines the density of their breast tissue. I review that in the higher density categories (heterogeneously dense or extremely dense), there is a higher risk of missing cancer, and that these categories are also associated with a higher risk of breast cancer. I also discuss the potential need for supplemental screening, for which my institution primarily offers Fast MRI. However, we can offer breast ultrasonography instead as an option, especially for those concerned about gadolinium exposure. Our center offers either of these supplemental screenings at a cost of $299. I also review the lack of coverage for supplemental screening by some insurance carriers, as both providers and patients may need to advocate for insurer coverage of adjunct studies.
Educational resources
6. What reference materials, illustrations, or other tools do you use to educate your patients?
Dr. Etkin-Kramer: I frequently use handouts printed from the DenseBreast-info.org website, and there is now a brand new patient fact sheet that I have just started using. I also have an example of breast density categories from fatty replaced to extremely dense on my computer, and I am putting it on a new smart board.
Dr. Albright: The extensive resources available at DenseBreast-info.org can improve both patient and provider knowledge of these important issues, so I suggest patients visit that website, and I use many of the images and visuals to help explain breast density. I even use the materials from the website for educating my resident trainees on breast health and screening. ●
Nearly 16,000 children (up to age 19 years) face cancer-related treatment every year.1 For girls and young women, undergoing chest radiotherapy puts them at higher risk for secondary breast cancer. In fact, they have a 30% chance of developing such cancer by age 50—a risk that is similar to women with a BRCA1 mutation.2 Therefore, current recommendations for breast cancer screening among those who have undergone childhood chest radiation (≥20 Gy) are to begin annual mammography, with adjunct magnetic resonance imaging (MRI), at age 25 years (or 8 years after chest radiotherapy).3
To determine the benefits and risks of these recommendations, as well as of similar strategies, Yeh and colleagues performed simulation modeling using data from the Childhood Cancer Survivor Study and two CISNET (Cancer Intervention and Surveillance Modeling Network) models.4 For their study they targeted a cohort of female childhood cancer survivors having undergone chest radiotherapy and evaluated breast cancer screening with the following strategies:
- mammography plus MRI, starting at ages 25, 30, or 35 years and continuing to age 74
- MRI alone, starting at ages 25, 30, or 35 years and continuing to age 74.
They found that both strategies reduced the risk of breast cancer in the targeted cohort but that screening beginning at the earliest ages prevented most deaths. No screening at all was associated with a 10% to 11% lifetime risk of breast cancer, but mammography plus MRI beginning at age 25 reduced that risk by 56% to 71% depending on the model. Screening with MRI alone reduced mortality risk by 56% to 62%. When considering cost per quality adjusted life-year gained, the researchers found that screening beginning at age 30 to be the most cost-effective.4
Yeh and colleagues addressed concerns with mammography and radiation. Although they said the associated amount of radiation exposure is small, the use of mammography in women younger than age 30 is controversial—and not recommended by the American Cancer Society or the National Comprehensive Cancer Network.5,6
Bottom line. Yeh and colleagues conclude that MRI screening, with or without mammography, beginning between the ages of 25 and 30 should be emphasized in screening guidelines. They note the importance of insurance coverage for MRI in those at risk for breast cancer due to childhood radiation exposure.4
References
- National Cancer Institute. How common is cancer in children? https://www.cancer.gov/types/childhood-cancers/child-adolescentcancers-fact-sheet#how-common-is-cancer-in-children. Accessed September 25, 2020.
- Moskowitz CS, Chou JF, Wolden SL, et al. Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol. 2014;32:2217- 2223.
- Children’s Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. http:// www.survivorshipguidelines.org/pdf/2018/COG_LTFU_Guidelines_v5.pdf. Accessed September 25, 2020.
- Yeh JM, Lowry KP, Schechter CB, et al. Clinical benefits, harms, and cost-effectiveness of breast cancer screening for survivors of childhood cancer treated with chest radiation. Ann Intern Med. 2020;173:331-341.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Breast cancer screening and diagnosis version 1.2019. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed September 25, 2020.
- Bharucha PP, Chiu KE, Francois FM, et al. Genetic testing and screening recommendations for patients with hereditary breast cancer. RadioGraphics. 2020;40:913-936.
- Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol. 2011;29:2327-2333.
- Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol. 2015;22:3230-3235.
- American College of Obstetricians and Gynecologists. Committee opinion no. 625: management of women with dense breasts diagnosed by mammography [published correction appears in Obstet Gynecol. 2016;127:166]. Obstet Gynecol. 2015;125(3):750-751.
- Bharucha PP, Chiu KE, Francois FM, et al. Genetic testing and screening recommendations for patients with hereditary breast cancer. RadioGraphics. 2020;40:913-936.
- Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol. 2011;29:2327-2333.
- Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol. 2015;22:3230-3235.
- American College of Obstetricians and Gynecologists. Committee opinion no. 625: management of women with dense breasts diagnosed by mammography [published correction appears in Obstet Gynecol. 2016;127:166]. Obstet Gynecol. 2015;125(3):750-751.
Restarting breast cancer screening after disruption not so simple
according to a modeling study reported at the 12th European Breast Cancer Conference.
Fallout of the pandemic has included reductions in cancer screening and diagnosis, said study investigator Lindy M. Kregting, a PhD student in the department of public health at Erasmus Medical Center, University Medical Center Rotterdam (the Netherlands).
In the Netherlands, new breast cancer diagnoses fell dramatically from historical levels starting in February. The number in April was less than half of that expected.
Ms. Kregting and colleagues used modeling to assess the impact of four strategies for restarting breast cancer screening in the Netherlands. The strategies differed regarding the population affected, the duration of the effects, and changes in stopping age. The usual situation, without any disruption, served as the comparator.
Results showed wide variation across strategies with respect to the increase in screening capacity needed during the latter half of this year – from 0% to 100% – and the excess breast cancer mortality occurring during 2020-2030 – from as many as 181 excess breast cancer deaths to as few as 14.
“The effects of the disruption are dependent on the chosen restart strategy,” Ms. Kregting summarized. “It would be preferred to immediately catch up because this minimizes the impact, but it also requires a very high capacity, so it may not always be possible. A proper alternative would be to increase the stopping age, so no screens are omitted, because this requires a rather normal capacity, and it will result in only small effects on incidence and mortality.”
As screening programs restart in some countries, there are still a lot of unknowns that could affect outcomes, including how many women will attend given that some may stay away out of fear, Ms. Kregting cautioned.
“We plan to do further model calculations when we know exactly what has happened. ... For now, we just assumed some reasonable disruption periods, and we assumed that capacity would be back to the original, before COVID-19, but I think we can say this is probably not the case,” she added.
Study details
Ms. Kregting and colleagues used Dutch breast cancer screening program parameters (biennial digital mammography for women aged 50-75 years) and a microsimulation screening analysis model to simulate four strategies for restarting breast cancer screening after a 6-month disruption:
- “Everyone delay,” a strategy in which all screening continues in the order planned with no change in the stopping age of 75 years (so that one in four women ultimately miss a screening during their lifetime)
- “First rounds no delay,” in which there is a delay in screening except for women having their first screening
- “Continue after stopping age,” in which there is a delay in screening but temporary increasing of the stopping age (to 76.5 years) to ensure all women get their final screen
- “Catch-up after stop,” in which capacity is increased to ensure full catch-up, with all delayed screens caught up in a 6-month period (the second half of 2020).
Results showed that 5,872 women would be screened in the latter half of 2020 if screening proceeded as usual without disruption. The necessary capacity was essentially the same with all of the restarting strategies, except for the catch-up-after-stop strategy, which would require a doubling of that number.
The temporal pattern of breast cancer incidence varied according to restart strategy early on, but incidence essentially returned to that expected with undisrupted screening by 2025 for all four strategies, with some small fluctuations thereafter.
The impact on breast cancer mortality differed considerably long term. It increased slightly and transiently above the expected level with the catch-up-after-stop strategy, but there were sizable, long-lasting increases with the other strategies, with excess deaths still seen in 2060 for the everyone-delay strategy.
In absolute terms, the excess number of breast cancer deaths during 2020-2030, compared with undisrupted screening, was 181 with the everyone-delay strategy, 155 with the first-rounds-no-delay strategy, 145 with the continue-after-stopping-age strategy, and just 14 with the catch-up-after-stop strategy. Ms. Kregting declined to provide numbers for other countries, given that the model is based on the Dutch population and screening program.
Results in context
“The unprecedented burden of COVID-19 on health systems worldwide has important implications for cancer care,” said invited discussant Alessandra Gennari, MD, PhD, of the University of Eastern Piedmont and Maggiore della Carità Hospital, both in Novara, Italy.
“There is a delay in diagnosis due to the fact that screening programs and diagnostic programs have been decreased or suspended in many Western countries where this is standard of care. Patients also are more reluctant to present to health care services, delaying their diagnosis,” Dr. Gennari said.
Findings of this new study add to those of similar studies undertaken in Italy (published in In Vivo) and the United Kingdom (published in The Lancet Oncology) showing the likely marked toll of the pandemic on cancer diagnosis and mortality, Dr. Gennari noted. Taken together, the findings underscore the urgent need for policy interventions to mitigate this impact.
“These interventions should focus on increasing routine diagnostic capacity, through which up to 40% of patients with cancer are diagnosed,” Dr. Gennari recommended. “Public health messaging is needed that accurately conveys the risk of severe illness from COVID-19 versus the risks of not seeking health care advice if patients are symptomatic. Finally, there is a need for provision of evidence-based data on which clinicians can adequately base their decision on how to manage the risks of cancer patients and the risks and benefits of procedures during the pandemic.”
The current study did not have any specific funding, and Ms. Kregting disclosed no conflicts of interest. Dr. Gennari disclosed relationships with Roche, Eisai, Lilly, AstraZeneca, Daiichi Sankyo, Merck, Novartis, and Pfizer.
SOURCE: Kregting L et al. EBCC-12 Virtual Conference, Abstract 24.
according to a modeling study reported at the 12th European Breast Cancer Conference.
Fallout of the pandemic has included reductions in cancer screening and diagnosis, said study investigator Lindy M. Kregting, a PhD student in the department of public health at Erasmus Medical Center, University Medical Center Rotterdam (the Netherlands).
In the Netherlands, new breast cancer diagnoses fell dramatically from historical levels starting in February. The number in April was less than half of that expected.
Ms. Kregting and colleagues used modeling to assess the impact of four strategies for restarting breast cancer screening in the Netherlands. The strategies differed regarding the population affected, the duration of the effects, and changes in stopping age. The usual situation, without any disruption, served as the comparator.
Results showed wide variation across strategies with respect to the increase in screening capacity needed during the latter half of this year – from 0% to 100% – and the excess breast cancer mortality occurring during 2020-2030 – from as many as 181 excess breast cancer deaths to as few as 14.
“The effects of the disruption are dependent on the chosen restart strategy,” Ms. Kregting summarized. “It would be preferred to immediately catch up because this minimizes the impact, but it also requires a very high capacity, so it may not always be possible. A proper alternative would be to increase the stopping age, so no screens are omitted, because this requires a rather normal capacity, and it will result in only small effects on incidence and mortality.”
As screening programs restart in some countries, there are still a lot of unknowns that could affect outcomes, including how many women will attend given that some may stay away out of fear, Ms. Kregting cautioned.
“We plan to do further model calculations when we know exactly what has happened. ... For now, we just assumed some reasonable disruption periods, and we assumed that capacity would be back to the original, before COVID-19, but I think we can say this is probably not the case,” she added.
Study details
Ms. Kregting and colleagues used Dutch breast cancer screening program parameters (biennial digital mammography for women aged 50-75 years) and a microsimulation screening analysis model to simulate four strategies for restarting breast cancer screening after a 6-month disruption:
- “Everyone delay,” a strategy in which all screening continues in the order planned with no change in the stopping age of 75 years (so that one in four women ultimately miss a screening during their lifetime)
- “First rounds no delay,” in which there is a delay in screening except for women having their first screening
- “Continue after stopping age,” in which there is a delay in screening but temporary increasing of the stopping age (to 76.5 years) to ensure all women get their final screen
- “Catch-up after stop,” in which capacity is increased to ensure full catch-up, with all delayed screens caught up in a 6-month period (the second half of 2020).
Results showed that 5,872 women would be screened in the latter half of 2020 if screening proceeded as usual without disruption. The necessary capacity was essentially the same with all of the restarting strategies, except for the catch-up-after-stop strategy, which would require a doubling of that number.
The temporal pattern of breast cancer incidence varied according to restart strategy early on, but incidence essentially returned to that expected with undisrupted screening by 2025 for all four strategies, with some small fluctuations thereafter.
The impact on breast cancer mortality differed considerably long term. It increased slightly and transiently above the expected level with the catch-up-after-stop strategy, but there were sizable, long-lasting increases with the other strategies, with excess deaths still seen in 2060 for the everyone-delay strategy.
In absolute terms, the excess number of breast cancer deaths during 2020-2030, compared with undisrupted screening, was 181 with the everyone-delay strategy, 155 with the first-rounds-no-delay strategy, 145 with the continue-after-stopping-age strategy, and just 14 with the catch-up-after-stop strategy. Ms. Kregting declined to provide numbers for other countries, given that the model is based on the Dutch population and screening program.
Results in context
“The unprecedented burden of COVID-19 on health systems worldwide has important implications for cancer care,” said invited discussant Alessandra Gennari, MD, PhD, of the University of Eastern Piedmont and Maggiore della Carità Hospital, both in Novara, Italy.
“There is a delay in diagnosis due to the fact that screening programs and diagnostic programs have been decreased or suspended in many Western countries where this is standard of care. Patients also are more reluctant to present to health care services, delaying their diagnosis,” Dr. Gennari said.
Findings of this new study add to those of similar studies undertaken in Italy (published in In Vivo) and the United Kingdom (published in The Lancet Oncology) showing the likely marked toll of the pandemic on cancer diagnosis and mortality, Dr. Gennari noted. Taken together, the findings underscore the urgent need for policy interventions to mitigate this impact.
“These interventions should focus on increasing routine diagnostic capacity, through which up to 40% of patients with cancer are diagnosed,” Dr. Gennari recommended. “Public health messaging is needed that accurately conveys the risk of severe illness from COVID-19 versus the risks of not seeking health care advice if patients are symptomatic. Finally, there is a need for provision of evidence-based data on which clinicians can adequately base their decision on how to manage the risks of cancer patients and the risks and benefits of procedures during the pandemic.”
The current study did not have any specific funding, and Ms. Kregting disclosed no conflicts of interest. Dr. Gennari disclosed relationships with Roche, Eisai, Lilly, AstraZeneca, Daiichi Sankyo, Merck, Novartis, and Pfizer.
SOURCE: Kregting L et al. EBCC-12 Virtual Conference, Abstract 24.
according to a modeling study reported at the 12th European Breast Cancer Conference.
Fallout of the pandemic has included reductions in cancer screening and diagnosis, said study investigator Lindy M. Kregting, a PhD student in the department of public health at Erasmus Medical Center, University Medical Center Rotterdam (the Netherlands).
In the Netherlands, new breast cancer diagnoses fell dramatically from historical levels starting in February. The number in April was less than half of that expected.
Ms. Kregting and colleagues used modeling to assess the impact of four strategies for restarting breast cancer screening in the Netherlands. The strategies differed regarding the population affected, the duration of the effects, and changes in stopping age. The usual situation, without any disruption, served as the comparator.
Results showed wide variation across strategies with respect to the increase in screening capacity needed during the latter half of this year – from 0% to 100% – and the excess breast cancer mortality occurring during 2020-2030 – from as many as 181 excess breast cancer deaths to as few as 14.
“The effects of the disruption are dependent on the chosen restart strategy,” Ms. Kregting summarized. “It would be preferred to immediately catch up because this minimizes the impact, but it also requires a very high capacity, so it may not always be possible. A proper alternative would be to increase the stopping age, so no screens are omitted, because this requires a rather normal capacity, and it will result in only small effects on incidence and mortality.”
As screening programs restart in some countries, there are still a lot of unknowns that could affect outcomes, including how many women will attend given that some may stay away out of fear, Ms. Kregting cautioned.
“We plan to do further model calculations when we know exactly what has happened. ... For now, we just assumed some reasonable disruption periods, and we assumed that capacity would be back to the original, before COVID-19, but I think we can say this is probably not the case,” she added.
Study details
Ms. Kregting and colleagues used Dutch breast cancer screening program parameters (biennial digital mammography for women aged 50-75 years) and a microsimulation screening analysis model to simulate four strategies for restarting breast cancer screening after a 6-month disruption:
- “Everyone delay,” a strategy in which all screening continues in the order planned with no change in the stopping age of 75 years (so that one in four women ultimately miss a screening during their lifetime)
- “First rounds no delay,” in which there is a delay in screening except for women having their first screening
- “Continue after stopping age,” in which there is a delay in screening but temporary increasing of the stopping age (to 76.5 years) to ensure all women get their final screen
- “Catch-up after stop,” in which capacity is increased to ensure full catch-up, with all delayed screens caught up in a 6-month period (the second half of 2020).
Results showed that 5,872 women would be screened in the latter half of 2020 if screening proceeded as usual without disruption. The necessary capacity was essentially the same with all of the restarting strategies, except for the catch-up-after-stop strategy, which would require a doubling of that number.
The temporal pattern of breast cancer incidence varied according to restart strategy early on, but incidence essentially returned to that expected with undisrupted screening by 2025 for all four strategies, with some small fluctuations thereafter.
The impact on breast cancer mortality differed considerably long term. It increased slightly and transiently above the expected level with the catch-up-after-stop strategy, but there were sizable, long-lasting increases with the other strategies, with excess deaths still seen in 2060 for the everyone-delay strategy.
In absolute terms, the excess number of breast cancer deaths during 2020-2030, compared with undisrupted screening, was 181 with the everyone-delay strategy, 155 with the first-rounds-no-delay strategy, 145 with the continue-after-stopping-age strategy, and just 14 with the catch-up-after-stop strategy. Ms. Kregting declined to provide numbers for other countries, given that the model is based on the Dutch population and screening program.
Results in context
“The unprecedented burden of COVID-19 on health systems worldwide has important implications for cancer care,” said invited discussant Alessandra Gennari, MD, PhD, of the University of Eastern Piedmont and Maggiore della Carità Hospital, both in Novara, Italy.
“There is a delay in diagnosis due to the fact that screening programs and diagnostic programs have been decreased or suspended in many Western countries where this is standard of care. Patients also are more reluctant to present to health care services, delaying their diagnosis,” Dr. Gennari said.
Findings of this new study add to those of similar studies undertaken in Italy (published in In Vivo) and the United Kingdom (published in The Lancet Oncology) showing the likely marked toll of the pandemic on cancer diagnosis and mortality, Dr. Gennari noted. Taken together, the findings underscore the urgent need for policy interventions to mitigate this impact.
“These interventions should focus on increasing routine diagnostic capacity, through which up to 40% of patients with cancer are diagnosed,” Dr. Gennari recommended. “Public health messaging is needed that accurately conveys the risk of severe illness from COVID-19 versus the risks of not seeking health care advice if patients are symptomatic. Finally, there is a need for provision of evidence-based data on which clinicians can adequately base their decision on how to manage the risks of cancer patients and the risks and benefits of procedures during the pandemic.”
The current study did not have any specific funding, and Ms. Kregting disclosed no conflicts of interest. Dr. Gennari disclosed relationships with Roche, Eisai, Lilly, AstraZeneca, Daiichi Sankyo, Merck, Novartis, and Pfizer.
SOURCE: Kregting L et al. EBCC-12 Virtual Conference, Abstract 24.
FROM EBCC-12 VIRTUAL CONFERENCE
Radiotherapy planning scans reveal breast cancer patients’ CVD risk
Radiotherapy planning scans may be a rich untapped source of information for estimating the risk of cardiovascular disease (CVD) in breast cancer patients, a large study suggests.
Researchers found that breast cancer patients with a coronary artery calcifications (CAC) score exceeding 400 had nearly four times the adjusted risk of fatal and nonfatal CVD events when compared with patients who had a CAC score of 0.
Patients with scores exceeding 400 also had more than eight times the risk of coronary heart disease events. The associations were especially strong in the subset of patients who received anthracycline-containing chemotherapy.
Helena Verkooijen, MD, PhD, of University Medical Center Utrecht (the Netherlands) presented these findings at the 12th European Breast Cancer Conference.
Dr. Verkooijen noted that, over the past 50 years, breast cancer has dramatically declined as a cause of death among breast cancer survivors, while CVD has continued to account for about 20% of the total deaths in this population.
CACs are sometimes incidentally seen in radiotherapy planning CT scans. “Right now, this information is not often used for patient stratification or informing patients about their cardiovascular risk, and this is a pity, because we know that it is an independent risk factor, and, often, the presence of calcifications can occur in the absence of other cardiovascular risk factors,” Dr. Verkooijen said.
Study details
Dr. Verkooijen and and colleagues from the Bragataston Study Group retrospectively studied 15,919 breast cancer patients who had radiotherapy planning CT scans during 2004-2016 at three Dutch institutions.
The researchers used an automated deep-learning algorithm (described in Radiology) to detect and quantify coronary calcium in planning CT scans and calculate CAC scores, classifying them into five categories.
The median follow-up was 51.6 months. Most women (70%) did not have any calcium detected in their coronary arteries (CAC score of 0), while 3% fell into the highest category (CAC score of >400).
The incidence of nonfatal and fatal CVD events increased with CAC score:
- 5.1% with a score of 0.
- 8.5% with a score of 1-10.
- 13.5% with a score of 11-100.
- 17.6% with a score of 101-400.
- 28.0% with a score greater than 400.
In analyses adjusted for age, laterality of radiation, and receipt of cardiotoxic agents – anthracyclines and trastuzumab – women with a score exceeding 400 had sharply elevated adjusted risks of CVD events (hazard ratio, 3.7), of coronary heart disease events specifically (HR, 8.2), and of death from any cause (HR, 2.8), when compared with peers who had a CAC score of 0.
On further scrutiny of CVD events, the pattern was similar regardless of whether radiation was left- or right-sided. However, the association was stronger among women who received anthracyclines as compared with counterparts who did not, with a nearly six-fold higher risk for those with highest versus lowest CAC scores.
When the women were surveyed, nearly 90% said they wanted to be informed about their CAC score and associated CVD risk, even in the absence of evidence-based risk reduction strategies.
Applying the results
“We believe that this is the first time that anyone has conducted a study on this topic on a scale like this, and we show that it is possible to relatively easily identify women at a very high risk of CVD,” Dr. Verkooijen said. “But what do we do with this information, because these scans are not made to answer this question. … This is information that we get that we haven’t really requested. I think we should only use this information when we have really shown that we can help patients reduce their risk of cardiovascular disease.”
To that end, Dr. Verkooijen and colleagues are planning additional research that will look at the potential benefit of referring high-risk patients for cardioprevention strategies and at the role of using the CAC score to personalize treatment strategies.
“This is an interesting and novel approach to predicting cardiac events for patients undergoing breast cancer treatment,” Meena S. Moran, MD, of Yale University in New Haven, Conn., commented in an interview.
The approach would likely be feasible in typical practice with widespread availability of the automated algorithm and might even alter treatment planning in real time, she said. “From the standpoint of radiation oncology, it would mean running the software to generate a CAC score, which would allow for modifications in decision-making during treatment planning, such as whether or not to include the internal mammary nodal chain in a patient who may be in the ‘gray zone’ for regional nodal radiation. For example, if a patient has a high CAC score, plus if they have received (or are receiving) cardiotoxic drugs, radiation oncologists can use that information as an additional factor to consider in the decision-making of whether or not to include the internal mammary chain, which inevitably can increase the dose delivered to the heart,” Dr. Moran elaborated.
Dr. Verkooijen’s study was supported by the Dutch Cancer Society, the European Commission, the Dutch Digestive Foundation, the Netherlands Organisation for Scientific Research, and Elekta. Dr. Verkooijen and Dr. Moran disclosed no conflicts of interest.
SOURCE: Gal R et al. EBCC-12 Virtual Congress, Abstract 7.
Radiotherapy planning scans may be a rich untapped source of information for estimating the risk of cardiovascular disease (CVD) in breast cancer patients, a large study suggests.
Researchers found that breast cancer patients with a coronary artery calcifications (CAC) score exceeding 400 had nearly four times the adjusted risk of fatal and nonfatal CVD events when compared with patients who had a CAC score of 0.
Patients with scores exceeding 400 also had more than eight times the risk of coronary heart disease events. The associations were especially strong in the subset of patients who received anthracycline-containing chemotherapy.
Helena Verkooijen, MD, PhD, of University Medical Center Utrecht (the Netherlands) presented these findings at the 12th European Breast Cancer Conference.
Dr. Verkooijen noted that, over the past 50 years, breast cancer has dramatically declined as a cause of death among breast cancer survivors, while CVD has continued to account for about 20% of the total deaths in this population.
CACs are sometimes incidentally seen in radiotherapy planning CT scans. “Right now, this information is not often used for patient stratification or informing patients about their cardiovascular risk, and this is a pity, because we know that it is an independent risk factor, and, often, the presence of calcifications can occur in the absence of other cardiovascular risk factors,” Dr. Verkooijen said.
Study details
Dr. Verkooijen and and colleagues from the Bragataston Study Group retrospectively studied 15,919 breast cancer patients who had radiotherapy planning CT scans during 2004-2016 at three Dutch institutions.
The researchers used an automated deep-learning algorithm (described in Radiology) to detect and quantify coronary calcium in planning CT scans and calculate CAC scores, classifying them into five categories.
The median follow-up was 51.6 months. Most women (70%) did not have any calcium detected in their coronary arteries (CAC score of 0), while 3% fell into the highest category (CAC score of >400).
The incidence of nonfatal and fatal CVD events increased with CAC score:
- 5.1% with a score of 0.
- 8.5% with a score of 1-10.
- 13.5% with a score of 11-100.
- 17.6% with a score of 101-400.
- 28.0% with a score greater than 400.
In analyses adjusted for age, laterality of radiation, and receipt of cardiotoxic agents – anthracyclines and trastuzumab – women with a score exceeding 400 had sharply elevated adjusted risks of CVD events (hazard ratio, 3.7), of coronary heart disease events specifically (HR, 8.2), and of death from any cause (HR, 2.8), when compared with peers who had a CAC score of 0.
On further scrutiny of CVD events, the pattern was similar regardless of whether radiation was left- or right-sided. However, the association was stronger among women who received anthracyclines as compared with counterparts who did not, with a nearly six-fold higher risk for those with highest versus lowest CAC scores.
When the women were surveyed, nearly 90% said they wanted to be informed about their CAC score and associated CVD risk, even in the absence of evidence-based risk reduction strategies.
Applying the results
“We believe that this is the first time that anyone has conducted a study on this topic on a scale like this, and we show that it is possible to relatively easily identify women at a very high risk of CVD,” Dr. Verkooijen said. “But what do we do with this information, because these scans are not made to answer this question. … This is information that we get that we haven’t really requested. I think we should only use this information when we have really shown that we can help patients reduce their risk of cardiovascular disease.”
To that end, Dr. Verkooijen and colleagues are planning additional research that will look at the potential benefit of referring high-risk patients for cardioprevention strategies and at the role of using the CAC score to personalize treatment strategies.
“This is an interesting and novel approach to predicting cardiac events for patients undergoing breast cancer treatment,” Meena S. Moran, MD, of Yale University in New Haven, Conn., commented in an interview.
The approach would likely be feasible in typical practice with widespread availability of the automated algorithm and might even alter treatment planning in real time, she said. “From the standpoint of radiation oncology, it would mean running the software to generate a CAC score, which would allow for modifications in decision-making during treatment planning, such as whether or not to include the internal mammary nodal chain in a patient who may be in the ‘gray zone’ for regional nodal radiation. For example, if a patient has a high CAC score, plus if they have received (or are receiving) cardiotoxic drugs, radiation oncologists can use that information as an additional factor to consider in the decision-making of whether or not to include the internal mammary chain, which inevitably can increase the dose delivered to the heart,” Dr. Moran elaborated.
Dr. Verkooijen’s study was supported by the Dutch Cancer Society, the European Commission, the Dutch Digestive Foundation, the Netherlands Organisation for Scientific Research, and Elekta. Dr. Verkooijen and Dr. Moran disclosed no conflicts of interest.
SOURCE: Gal R et al. EBCC-12 Virtual Congress, Abstract 7.
Radiotherapy planning scans may be a rich untapped source of information for estimating the risk of cardiovascular disease (CVD) in breast cancer patients, a large study suggests.
Researchers found that breast cancer patients with a coronary artery calcifications (CAC) score exceeding 400 had nearly four times the adjusted risk of fatal and nonfatal CVD events when compared with patients who had a CAC score of 0.
Patients with scores exceeding 400 also had more than eight times the risk of coronary heart disease events. The associations were especially strong in the subset of patients who received anthracycline-containing chemotherapy.
Helena Verkooijen, MD, PhD, of University Medical Center Utrecht (the Netherlands) presented these findings at the 12th European Breast Cancer Conference.
Dr. Verkooijen noted that, over the past 50 years, breast cancer has dramatically declined as a cause of death among breast cancer survivors, while CVD has continued to account for about 20% of the total deaths in this population.
CACs are sometimes incidentally seen in radiotherapy planning CT scans. “Right now, this information is not often used for patient stratification or informing patients about their cardiovascular risk, and this is a pity, because we know that it is an independent risk factor, and, often, the presence of calcifications can occur in the absence of other cardiovascular risk factors,” Dr. Verkooijen said.
Study details
Dr. Verkooijen and and colleagues from the Bragataston Study Group retrospectively studied 15,919 breast cancer patients who had radiotherapy planning CT scans during 2004-2016 at three Dutch institutions.
The researchers used an automated deep-learning algorithm (described in Radiology) to detect and quantify coronary calcium in planning CT scans and calculate CAC scores, classifying them into five categories.
The median follow-up was 51.6 months. Most women (70%) did not have any calcium detected in their coronary arteries (CAC score of 0), while 3% fell into the highest category (CAC score of >400).
The incidence of nonfatal and fatal CVD events increased with CAC score:
- 5.1% with a score of 0.
- 8.5% with a score of 1-10.
- 13.5% with a score of 11-100.
- 17.6% with a score of 101-400.
- 28.0% with a score greater than 400.
In analyses adjusted for age, laterality of radiation, and receipt of cardiotoxic agents – anthracyclines and trastuzumab – women with a score exceeding 400 had sharply elevated adjusted risks of CVD events (hazard ratio, 3.7), of coronary heart disease events specifically (HR, 8.2), and of death from any cause (HR, 2.8), when compared with peers who had a CAC score of 0.
On further scrutiny of CVD events, the pattern was similar regardless of whether radiation was left- or right-sided. However, the association was stronger among women who received anthracyclines as compared with counterparts who did not, with a nearly six-fold higher risk for those with highest versus lowest CAC scores.
When the women were surveyed, nearly 90% said they wanted to be informed about their CAC score and associated CVD risk, even in the absence of evidence-based risk reduction strategies.
Applying the results
“We believe that this is the first time that anyone has conducted a study on this topic on a scale like this, and we show that it is possible to relatively easily identify women at a very high risk of CVD,” Dr. Verkooijen said. “But what do we do with this information, because these scans are not made to answer this question. … This is information that we get that we haven’t really requested. I think we should only use this information when we have really shown that we can help patients reduce their risk of cardiovascular disease.”
To that end, Dr. Verkooijen and colleagues are planning additional research that will look at the potential benefit of referring high-risk patients for cardioprevention strategies and at the role of using the CAC score to personalize treatment strategies.
“This is an interesting and novel approach to predicting cardiac events for patients undergoing breast cancer treatment,” Meena S. Moran, MD, of Yale University in New Haven, Conn., commented in an interview.
The approach would likely be feasible in typical practice with widespread availability of the automated algorithm and might even alter treatment planning in real time, she said. “From the standpoint of radiation oncology, it would mean running the software to generate a CAC score, which would allow for modifications in decision-making during treatment planning, such as whether or not to include the internal mammary nodal chain in a patient who may be in the ‘gray zone’ for regional nodal radiation. For example, if a patient has a high CAC score, plus if they have received (or are receiving) cardiotoxic drugs, radiation oncologists can use that information as an additional factor to consider in the decision-making of whether or not to include the internal mammary chain, which inevitably can increase the dose delivered to the heart,” Dr. Moran elaborated.
Dr. Verkooijen’s study was supported by the Dutch Cancer Society, the European Commission, the Dutch Digestive Foundation, the Netherlands Organisation for Scientific Research, and Elekta. Dr. Verkooijen and Dr. Moran disclosed no conflicts of interest.
SOURCE: Gal R et al. EBCC-12 Virtual Congress, Abstract 7.
FROM EBCC-12 VIRTUAL CONFERENCE
Breast cancer screening complexities
Breast cancer in women remains one of the most common types of cancer in the United States, affecting about one in eight women1 over the course of their lifetime. Despite its pervasiveness, the 5-year survival rate for women with breast cancer remains high, estimated at around 90%2 based on data from 2010-2016, in large part because of early detection and treatment through screening. However, many organizations disagree on when to start and how often to screen women at average risk.
Important to discussions about breast cancer screening is the trend that many women delay childbirth until their 30s and 40s. In 2018 the birth rate increased for women ages 35-44, and the mean age of first birth increased from the prior year across all racial and ethnic groups.3 Therefore, ob.gyns. may need to consider that their patients not only may have increased risk of developing breast cancer based on age alone – women aged 35-44 have four times greater risk of disease than women aged 20-342 – but that the pregnancy itself may further exacerbate risk in older women. A 2019 pooled analysis found that women who were older at first birth had a greater chance of developing breast cancer compared with women with no children.4
In addition, ob.gyns. should consider that their patients may have received a breast cancer diagnosis prior to initiation or completion of their family plans or that their patients are cancer survivors – in 2013-2017, breast cancer was the most common form of cancer in adolescents and young adults.5 Thus, practitioners should be prepared to discuss not only options for fertility preservation but the evidence regarding cancer recurrence after pregnancy.
We have invited Dr. Katherine Tkaczuk, professor of medicine at the University of Maryland School of Medicine* and director of the breast evaluation and treatment program at the Marlene and Stewart Greenebaum Comprehensive Cancer Center, to discuss the vital role of screening in the shared decision-making process of breast cancer prevention.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore,* as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
Correction, 1/8/21: *An earlier version of this article misstated the university affiliations for Dr. Tkaczuk and Dr. Reece.
References
1. U.S. Breast Cancer Statistics. breastcancer.org.
2. “Cancer Stat Facts: Female Breast Cancer,” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
3. Martin JA et al. “Births: Final Data for 2018.” National Vital Statistics Reports. 2019 Nov 27;68(13):1-46.
4. Nichols HB et al. Ann Intern Med. 2019 Jan;170(1):22-30.
5. “Cancer Stat Facts: Cancer Among Adolescents and Young Adults (AYAs) (Ages 15-39),” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
Breast cancer in women remains one of the most common types of cancer in the United States, affecting about one in eight women1 over the course of their lifetime. Despite its pervasiveness, the 5-year survival rate for women with breast cancer remains high, estimated at around 90%2 based on data from 2010-2016, in large part because of early detection and treatment through screening. However, many organizations disagree on when to start and how often to screen women at average risk.
Important to discussions about breast cancer screening is the trend that many women delay childbirth until their 30s and 40s. In 2018 the birth rate increased for women ages 35-44, and the mean age of first birth increased from the prior year across all racial and ethnic groups.3 Therefore, ob.gyns. may need to consider that their patients not only may have increased risk of developing breast cancer based on age alone – women aged 35-44 have four times greater risk of disease than women aged 20-342 – but that the pregnancy itself may further exacerbate risk in older women. A 2019 pooled analysis found that women who were older at first birth had a greater chance of developing breast cancer compared with women with no children.4
In addition, ob.gyns. should consider that their patients may have received a breast cancer diagnosis prior to initiation or completion of their family plans or that their patients are cancer survivors – in 2013-2017, breast cancer was the most common form of cancer in adolescents and young adults.5 Thus, practitioners should be prepared to discuss not only options for fertility preservation but the evidence regarding cancer recurrence after pregnancy.
We have invited Dr. Katherine Tkaczuk, professor of medicine at the University of Maryland School of Medicine* and director of the breast evaluation and treatment program at the Marlene and Stewart Greenebaum Comprehensive Cancer Center, to discuss the vital role of screening in the shared decision-making process of breast cancer prevention.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore,* as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
Correction, 1/8/21: *An earlier version of this article misstated the university affiliations for Dr. Tkaczuk and Dr. Reece.
References
1. U.S. Breast Cancer Statistics. breastcancer.org.
2. “Cancer Stat Facts: Female Breast Cancer,” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
3. Martin JA et al. “Births: Final Data for 2018.” National Vital Statistics Reports. 2019 Nov 27;68(13):1-46.
4. Nichols HB et al. Ann Intern Med. 2019 Jan;170(1):22-30.
5. “Cancer Stat Facts: Cancer Among Adolescents and Young Adults (AYAs) (Ages 15-39),” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
Breast cancer in women remains one of the most common types of cancer in the United States, affecting about one in eight women1 over the course of their lifetime. Despite its pervasiveness, the 5-year survival rate for women with breast cancer remains high, estimated at around 90%2 based on data from 2010-2016, in large part because of early detection and treatment through screening. However, many organizations disagree on when to start and how often to screen women at average risk.
Important to discussions about breast cancer screening is the trend that many women delay childbirth until their 30s and 40s. In 2018 the birth rate increased for women ages 35-44, and the mean age of first birth increased from the prior year across all racial and ethnic groups.3 Therefore, ob.gyns. may need to consider that their patients not only may have increased risk of developing breast cancer based on age alone – women aged 35-44 have four times greater risk of disease than women aged 20-342 – but that the pregnancy itself may further exacerbate risk in older women. A 2019 pooled analysis found that women who were older at first birth had a greater chance of developing breast cancer compared with women with no children.4
In addition, ob.gyns. should consider that their patients may have received a breast cancer diagnosis prior to initiation or completion of their family plans or that their patients are cancer survivors – in 2013-2017, breast cancer was the most common form of cancer in adolescents and young adults.5 Thus, practitioners should be prepared to discuss not only options for fertility preservation but the evidence regarding cancer recurrence after pregnancy.
We have invited Dr. Katherine Tkaczuk, professor of medicine at the University of Maryland School of Medicine* and director of the breast evaluation and treatment program at the Marlene and Stewart Greenebaum Comprehensive Cancer Center, to discuss the vital role of screening in the shared decision-making process of breast cancer prevention.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore,* as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
Correction, 1/8/21: *An earlier version of this article misstated the university affiliations for Dr. Tkaczuk and Dr. Reece.
References
1. U.S. Breast Cancer Statistics. breastcancer.org.
2. “Cancer Stat Facts: Female Breast Cancer,” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
3. Martin JA et al. “Births: Final Data for 2018.” National Vital Statistics Reports. 2019 Nov 27;68(13):1-46.
4. Nichols HB et al. Ann Intern Med. 2019 Jan;170(1):22-30.
5. “Cancer Stat Facts: Cancer Among Adolescents and Young Adults (AYAs) (Ages 15-39),” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
An oncologist’s view on screening mammography
Screening mammography has contributed to the lowering of mortality from breast cancer by facilitating earlier diagnosis and a lower stage at diagnosis. With more effective treatment options for women who are diagnosed with lower-stage breast cancer, the current 5-year survival rate has risen to 90% – significantly higher than the 5-year survival rate of 75% in 1975.1
Women who are at much higher risk for developing breast cancer – mainly because of family history, certain genetic mutations, or a history of radiation therapy to the chest – will benefit the most from earlier and more frequent screening mammography as well as enhanced screening with non-x-ray methods of breast imaging. It is important that ob.gyns. help to identify these women.
However, the majority of women who are screened with mammography are at “average risk,” with a lifetime risk for developing breast cancer of 12.9%, based on 2015-2017 data from the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results Program (SEER).1 The median age at diagnosis of breast cancer in the U.S. is 62 years,1 and advancing age is the most important risk factor for these women.
A 20% relative risk reduction in breast cancer mortality with screening mammography has been demonstrated both in systematic reviews of randomized and observational studies2 and in a meta-analysis of 11 randomized trials comparing screening and no screening.3 Even though the majority of randomized trials were done in the age of film mammography, experts believe that we still see at least a 20% reduction today.
Among average-risk women, those aged 50-74 with a life expectancy of at least 10 years will benefit the most from regular screening. According to the 2016 screening guideline of the United States Preventive Services Task Force (USPSTF), relative risk reductions in breast cancer mortality from mammography screening, by age group, are 0.88 (confidence interval, 0.73-1.003) for ages 39-49; 0.86 (CI, 0.68-0.97) for ages 50-59; 0.67 (CI, 0.55-0.91) for ages 60-69; and 0.80 (CI, 0.51 to 1.28) for ages 70-74.2
For women aged 40-49 years, most of the guidelines in the United States recommend individualized screening every 1 or 2 years – screening that is guided by shared decision-making that takes into account each woman’s values regarding relative harms and benefits. This is because their risk of developing breast cancer is relatively low while the risk of false-positive results can be higher.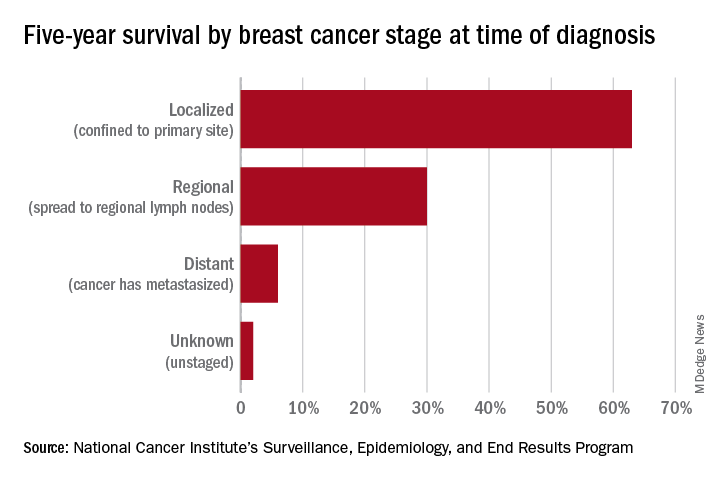
A few exceptions include guidelines by the National Comprehensive Cancer Network (NCCN) and the American College of Radiology, which recommend annual screening mammography starting at age 40 years for all average-risk women. In our program, we adhere to these latter recommendations and advise annual digital 3-D mammograms starting at age 40 and continuing until age 74, or longer if the woman is otherwise healthy with a life expectancy greater than 10 years.
Screening and overdiagnosis
Overdiagnosis – the diagnosis of cancers that may not actually cause mortality or may not even have become apparent without screening – is a concern for all women undergoing routine screening for breast cancer. There is significant uncertainty about its frequency, however.
Research cited by the USPSTF suggests that as many as one in five women diagnosed with breast cancer over approximately 10 years will be overdiagnosed. Other modeling studies have estimated one in eight overdiagnoses, for women aged 50-75 years specifically. By the more conservative estimate, according to the USPSTF, one breast cancer death will be prevented for every 2-3 cases of unnecessary treatment.2
Ductal carcinoma in situ is confined to the mammary ductal-lobular system and lacks the classic characteristics of cancer. Technically, it should not metastasize. But we do not know with certainty which cases of DCIS will or will not progress to invasive cancer. Therefore these women often are offered surgical approaches mirroring invasive cancer treatments (lumpectomy with radiation or even mastectomy in some cases), while for some, such treatments may be unnecessary.
Screening younger women (40-49)
Shared decision-making is always important for breast cancer screening, but in our program we routinely recommend annual screening in average-risk women starting at age 40 for several reasons. For one, younger women may present with more aggressive types of breast cancer such as triple-negative breast cancer. These are much less common than hormone-receptor positive breast cancers – they represent 15%-20% of all breast cancers – but they are faster growing and may develop in the interim if women are screened less often (at 2-year intervals).
In addition, finding an invasive breast cancer early is almost always beneficial. Earlier diagnosis (lower stage at diagnosis) is associated with increased breast cancer-specific and overall survival, as well as less-aggressive treatment approaches.
As a medical oncologist who treats women with breast cancer, I see these benefits firsthand. With earlier diagnosis, we are more likely to offer less aggressive surgical approaches such as partial mastectomy (lumpectomy) and sentinel lymph node biopsy as opposed to total mastectomy with axillary lymph node dissection, the latter of which is more likely to be associated with lymphedema and which can lead to postmastectomy chest wall pain syndromes.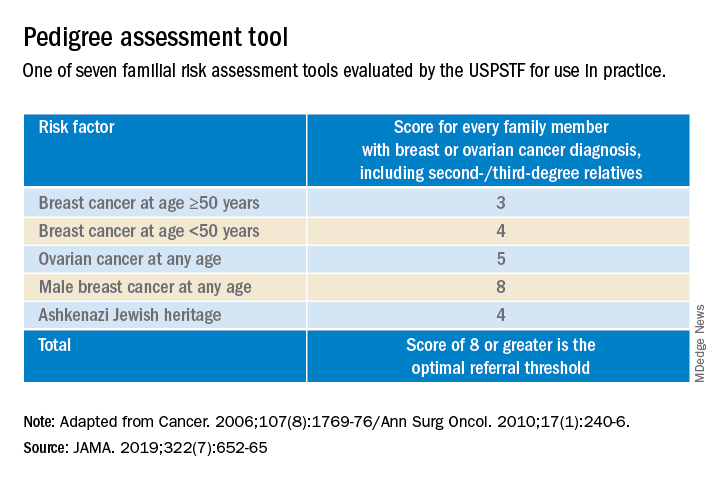
We also are able to use less aggressive radiation therapy approaches such as partial breast radiation, and less aggressive breast cancer–specific systemic treatments for women with a lower stage of breast cancer at diagnosis. In some cases, adjuvant or neoadjuvant chemotherapy may not be needed – and when it is necessary, shorter courses of chemotherapy or targeted chemotherapeutic regimens may be offered. This means lower systemic toxicities, both early and late, such as less cytopenias, risk of infections, mucositis, hair loss, cardiotoxicity, secondary malignancies/leukemia, and peripheral sensory neuropathy.
It is important to note that Black women in the United States have the highest death rate from breast cancer – 27.3 per 100,000 per year, versus 19.6 per 100,000 per year for White women1 – and that younger Black women appear to have a higher risk of developing triple-negative breast cancer, a more aggressive type of breast cancer. The higher breast cancer mortality in Black women is likely multifactorial and may be attributed partly to disparities in health care and partly to tumor biology. The case for annual screening in this population thus seems especially strong.
Screening modalities
Digital 3-D mammography, or digital breast tomosynthesis (DBT), is widely considered to be a more sensitive screening tool than conventional digital mammography alone. The NCCN recommends DBT for women with an average risk of developing breast cancer starting at age 40,4,5 and the USPSTF, while offering no recommendation on DBT as a primary screening method (“insufficient evidence”), says that DBT appears to increase cancer detection rates.2 So, I do routinely recommend it.
DBT may be especially beneficial for women with dense breast tissue (determined mammographically), who are most often premenopausal women – particularly non-Hispanic White women. Dense breast tissue itself can contribute to an increased risk of breast cancer – an approximately 20% higher relative risk in an average-risk woman with heterogeneously dense breast tissue, and an approximately 100% higher relative risk in a woman with extremely dense breasts6 – but unfortunately it affects the sensitivity and specificity of screening mammography.
I do not recommend routine supplemental screening with other methods (breast ultrasonography or MRI) for women at average risk of breast cancer who have dense breasts. MRI with gadolinium contrast is recommended as an adjunct to mammography for women who have a lifetime risk of developing breast cancer of more than 20%-25% (e.g., women with known BRCA1/2 mutations or radiation to breast tissue), and can be done annually at the same time as the screening mammogram is done. Some clinicians and patients prefer to alternate these two tests – one every 6 months.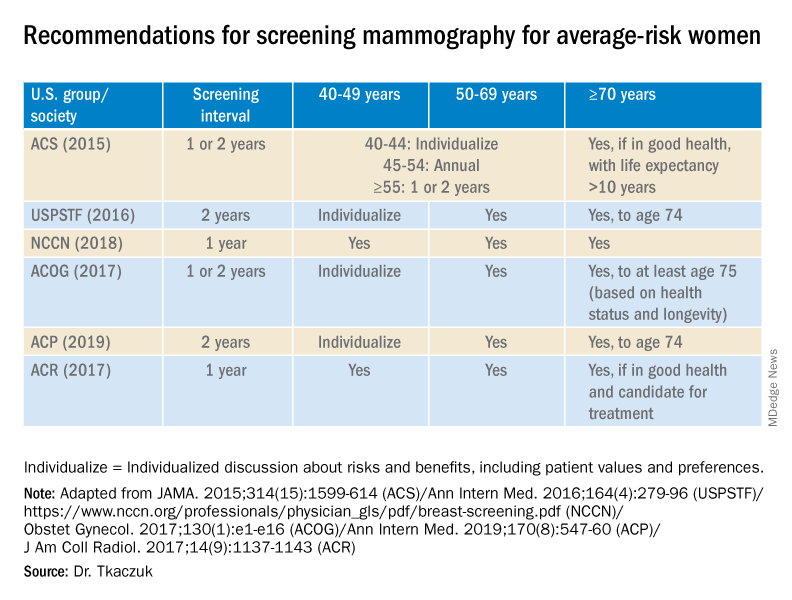
Screening breast MRI is more sensitive but less specific than mammography; combining the two screening modalities leads to overall increased sensitivity and specificity in high-risk populations.
Risk assessment
Identifying higher-risk women who need to be sent to a genetic counselor is critically important. The USPSTF recommends that women who have family members with breast, ovarian, tubal or peritoneal cancer, or who have an ancestry associated with BRCA1/2 gene mutations, be assessed with a brief familial risk assessment tool such as the Pedigree Assessment Tool. This and other validated tools have been evaluated by the USPSTF and can be used to guide referrals to genetic counseling for more definitive risk assessment.7
These tools are different from general breast cancer risk assessment models, such as the NCI’s Breast Cancer Risk Assessment Tool,8 which are designed to calculate the 5-year and lifetime risk of developing invasive breast cancer for an average-risk woman but not to identify BRCA-related cancer risk. (The NCI’s tool is based on the Gail model, which has been widely used over the years.)
The general risk assessment models use a women’s personal medical and reproductive history as well as the history of breast cancer among her first-degree relatives to estimate her risk.
Dr. Tkaczuk reported that she has no disclosures.
References
1. “Cancer Stat Facts: Female Breast Cancer.” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
2. Siu AL et al. Ann Intern Med. 2016 Feb 16. doi: 10.7326/M15-2886.
3. Independent UK Panel on Breast Cancer Screening. Lancet. 2012 Nov 17;380(9855):1778-86.
4. NCCN guidelines for Detection, Prevention, & Risk Reduction: Breast Cancer Screening and Diagnosis. National Comprehensive Cancer Network.
5. NCCN guidelines for Detection, Prevention, & Risk Reduction: Breast Cancer Risk Reduction. National Comprehensive Cancer Network.
6. Ziv E et al. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2090-5.
7. USPSTF. JAMA. 2019;322(7):652-65.
8. The Breast Cancer Risk Assessment Tool. National Cancer Institute.
Screening mammography has contributed to the lowering of mortality from breast cancer by facilitating earlier diagnosis and a lower stage at diagnosis. With more effective treatment options for women who are diagnosed with lower-stage breast cancer, the current 5-year survival rate has risen to 90% – significantly higher than the 5-year survival rate of 75% in 1975.1
Women who are at much higher risk for developing breast cancer – mainly because of family history, certain genetic mutations, or a history of radiation therapy to the chest – will benefit the most from earlier and more frequent screening mammography as well as enhanced screening with non-x-ray methods of breast imaging. It is important that ob.gyns. help to identify these women.
However, the majority of women who are screened with mammography are at “average risk,” with a lifetime risk for developing breast cancer of 12.9%, based on 2015-2017 data from the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results Program (SEER).1 The median age at diagnosis of breast cancer in the U.S. is 62 years,1 and advancing age is the most important risk factor for these women.
A 20% relative risk reduction in breast cancer mortality with screening mammography has been demonstrated both in systematic reviews of randomized and observational studies2 and in a meta-analysis of 11 randomized trials comparing screening and no screening.3 Even though the majority of randomized trials were done in the age of film mammography, experts believe that we still see at least a 20% reduction today.
Among average-risk women, those aged 50-74 with a life expectancy of at least 10 years will benefit the most from regular screening. According to the 2016 screening guideline of the United States Preventive Services Task Force (USPSTF), relative risk reductions in breast cancer mortality from mammography screening, by age group, are 0.88 (confidence interval, 0.73-1.003) for ages 39-49; 0.86 (CI, 0.68-0.97) for ages 50-59; 0.67 (CI, 0.55-0.91) for ages 60-69; and 0.80 (CI, 0.51 to 1.28) for ages 70-74.2
For women aged 40-49 years, most of the guidelines in the United States recommend individualized screening every 1 or 2 years – screening that is guided by shared decision-making that takes into account each woman’s values regarding relative harms and benefits. This is because their risk of developing breast cancer is relatively low while the risk of false-positive results can be higher.
A few exceptions include guidelines by the National Comprehensive Cancer Network (NCCN) and the American College of Radiology, which recommend annual screening mammography starting at age 40 years for all average-risk women. In our program, we adhere to these latter recommendations and advise annual digital 3-D mammograms starting at age 40 and continuing until age 74, or longer if the woman is otherwise healthy with a life expectancy greater than 10 years.
Screening and overdiagnosis
Overdiagnosis – the diagnosis of cancers that may not actually cause mortality or may not even have become apparent without screening – is a concern for all women undergoing routine screening for breast cancer. There is significant uncertainty about its frequency, however.
Research cited by the USPSTF suggests that as many as one in five women diagnosed with breast cancer over approximately 10 years will be overdiagnosed. Other modeling studies have estimated one in eight overdiagnoses, for women aged 50-75 years specifically. By the more conservative estimate, according to the USPSTF, one breast cancer death will be prevented for every 2-3 cases of unnecessary treatment.2
Ductal carcinoma in situ is confined to the mammary ductal-lobular system and lacks the classic characteristics of cancer. Technically, it should not metastasize. But we do not know with certainty which cases of DCIS will or will not progress to invasive cancer. Therefore these women often are offered surgical approaches mirroring invasive cancer treatments (lumpectomy with radiation or even mastectomy in some cases), while for some, such treatments may be unnecessary.
Screening younger women (40-49)
Shared decision-making is always important for breast cancer screening, but in our program we routinely recommend annual screening in average-risk women starting at age 40 for several reasons. For one, younger women may present with more aggressive types of breast cancer such as triple-negative breast cancer. These are much less common than hormone-receptor positive breast cancers – they represent 15%-20% of all breast cancers – but they are faster growing and may develop in the interim if women are screened less often (at 2-year intervals).
In addition, finding an invasive breast cancer early is almost always beneficial. Earlier diagnosis (lower stage at diagnosis) is associated with increased breast cancer-specific and overall survival, as well as less-aggressive treatment approaches.
As a medical oncologist who treats women with breast cancer, I see these benefits firsthand. With earlier diagnosis, we are more likely to offer less aggressive surgical approaches such as partial mastectomy (lumpectomy) and sentinel lymph node biopsy as opposed to total mastectomy with axillary lymph node dissection, the latter of which is more likely to be associated with lymphedema and which can lead to postmastectomy chest wall pain syndromes.
We also are able to use less aggressive radiation therapy approaches such as partial breast radiation, and less aggressive breast cancer–specific systemic treatments for women with a lower stage of breast cancer at diagnosis. In some cases, adjuvant or neoadjuvant chemotherapy may not be needed – and when it is necessary, shorter courses of chemotherapy or targeted chemotherapeutic regimens may be offered. This means lower systemic toxicities, both early and late, such as less cytopenias, risk of infections, mucositis, hair loss, cardiotoxicity, secondary malignancies/leukemia, and peripheral sensory neuropathy.
It is important to note that Black women in the United States have the highest death rate from breast cancer – 27.3 per 100,000 per year, versus 19.6 per 100,000 per year for White women1 – and that younger Black women appear to have a higher risk of developing triple-negative breast cancer, a more aggressive type of breast cancer. The higher breast cancer mortality in Black women is likely multifactorial and may be attributed partly to disparities in health care and partly to tumor biology. The case for annual screening in this population thus seems especially strong.
Screening modalities
Digital 3-D mammography, or digital breast tomosynthesis (DBT), is widely considered to be a more sensitive screening tool than conventional digital mammography alone. The NCCN recommends DBT for women with an average risk of developing breast cancer starting at age 40,4,5 and the USPSTF, while offering no recommendation on DBT as a primary screening method (“insufficient evidence”), says that DBT appears to increase cancer detection rates.2 So, I do routinely recommend it.
DBT may be especially beneficial for women with dense breast tissue (determined mammographically), who are most often premenopausal women – particularly non-Hispanic White women. Dense breast tissue itself can contribute to an increased risk of breast cancer – an approximately 20% higher relative risk in an average-risk woman with heterogeneously dense breast tissue, and an approximately 100% higher relative risk in a woman with extremely dense breasts6 – but unfortunately it affects the sensitivity and specificity of screening mammography.
I do not recommend routine supplemental screening with other methods (breast ultrasonography or MRI) for women at average risk of breast cancer who have dense breasts. MRI with gadolinium contrast is recommended as an adjunct to mammography for women who have a lifetime risk of developing breast cancer of more than 20%-25% (e.g., women with known BRCA1/2 mutations or radiation to breast tissue), and can be done annually at the same time as the screening mammogram is done. Some clinicians and patients prefer to alternate these two tests – one every 6 months.
Screening breast MRI is more sensitive but less specific than mammography; combining the two screening modalities leads to overall increased sensitivity and specificity in high-risk populations.
Risk assessment
Identifying higher-risk women who need to be sent to a genetic counselor is critically important. The USPSTF recommends that women who have family members with breast, ovarian, tubal or peritoneal cancer, or who have an ancestry associated with BRCA1/2 gene mutations, be assessed with a brief familial risk assessment tool such as the Pedigree Assessment Tool. This and other validated tools have been evaluated by the USPSTF and can be used to guide referrals to genetic counseling for more definitive risk assessment.7
These tools are different from general breast cancer risk assessment models, such as the NCI’s Breast Cancer Risk Assessment Tool,8 which are designed to calculate the 5-year and lifetime risk of developing invasive breast cancer for an average-risk woman but not to identify BRCA-related cancer risk. (The NCI’s tool is based on the Gail model, which has been widely used over the years.)
The general risk assessment models use a women’s personal medical and reproductive history as well as the history of breast cancer among her first-degree relatives to estimate her risk.
Dr. Tkaczuk reported that she has no disclosures.
References
1. “Cancer Stat Facts: Female Breast Cancer.” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
2. Siu AL et al. Ann Intern Med. 2016 Feb 16. doi: 10.7326/M15-2886.
3. Independent UK Panel on Breast Cancer Screening. Lancet. 2012 Nov 17;380(9855):1778-86.
4. NCCN guidelines for Detection, Prevention, & Risk Reduction: Breast Cancer Screening and Diagnosis. National Comprehensive Cancer Network.
5. NCCN guidelines for Detection, Prevention, & Risk Reduction: Breast Cancer Risk Reduction. National Comprehensive Cancer Network.
6. Ziv E et al. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2090-5.
7. USPSTF. JAMA. 2019;322(7):652-65.
8. The Breast Cancer Risk Assessment Tool. National Cancer Institute.
Screening mammography has contributed to the lowering of mortality from breast cancer by facilitating earlier diagnosis and a lower stage at diagnosis. With more effective treatment options for women who are diagnosed with lower-stage breast cancer, the current 5-year survival rate has risen to 90% – significantly higher than the 5-year survival rate of 75% in 1975.1
Women who are at much higher risk for developing breast cancer – mainly because of family history, certain genetic mutations, or a history of radiation therapy to the chest – will benefit the most from earlier and more frequent screening mammography as well as enhanced screening with non-x-ray methods of breast imaging. It is important that ob.gyns. help to identify these women.
However, the majority of women who are screened with mammography are at “average risk,” with a lifetime risk for developing breast cancer of 12.9%, based on 2015-2017 data from the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results Program (SEER).1 The median age at diagnosis of breast cancer in the U.S. is 62 years,1 and advancing age is the most important risk factor for these women.
A 20% relative risk reduction in breast cancer mortality with screening mammography has been demonstrated both in systematic reviews of randomized and observational studies2 and in a meta-analysis of 11 randomized trials comparing screening and no screening.3 Even though the majority of randomized trials were done in the age of film mammography, experts believe that we still see at least a 20% reduction today.
Among average-risk women, those aged 50-74 with a life expectancy of at least 10 years will benefit the most from regular screening. According to the 2016 screening guideline of the United States Preventive Services Task Force (USPSTF), relative risk reductions in breast cancer mortality from mammography screening, by age group, are 0.88 (confidence interval, 0.73-1.003) for ages 39-49; 0.86 (CI, 0.68-0.97) for ages 50-59; 0.67 (CI, 0.55-0.91) for ages 60-69; and 0.80 (CI, 0.51 to 1.28) for ages 70-74.2
For women aged 40-49 years, most of the guidelines in the United States recommend individualized screening every 1 or 2 years – screening that is guided by shared decision-making that takes into account each woman’s values regarding relative harms and benefits. This is because their risk of developing breast cancer is relatively low while the risk of false-positive results can be higher.
A few exceptions include guidelines by the National Comprehensive Cancer Network (NCCN) and the American College of Radiology, which recommend annual screening mammography starting at age 40 years for all average-risk women. In our program, we adhere to these latter recommendations and advise annual digital 3-D mammograms starting at age 40 and continuing until age 74, or longer if the woman is otherwise healthy with a life expectancy greater than 10 years.
Screening and overdiagnosis
Overdiagnosis – the diagnosis of cancers that may not actually cause mortality or may not even have become apparent without screening – is a concern for all women undergoing routine screening for breast cancer. There is significant uncertainty about its frequency, however.
Research cited by the USPSTF suggests that as many as one in five women diagnosed with breast cancer over approximately 10 years will be overdiagnosed. Other modeling studies have estimated one in eight overdiagnoses, for women aged 50-75 years specifically. By the more conservative estimate, according to the USPSTF, one breast cancer death will be prevented for every 2-3 cases of unnecessary treatment.2
Ductal carcinoma in situ is confined to the mammary ductal-lobular system and lacks the classic characteristics of cancer. Technically, it should not metastasize. But we do not know with certainty which cases of DCIS will or will not progress to invasive cancer. Therefore these women often are offered surgical approaches mirroring invasive cancer treatments (lumpectomy with radiation or even mastectomy in some cases), while for some, such treatments may be unnecessary.
Screening younger women (40-49)
Shared decision-making is always important for breast cancer screening, but in our program we routinely recommend annual screening in average-risk women starting at age 40 for several reasons. For one, younger women may present with more aggressive types of breast cancer such as triple-negative breast cancer. These are much less common than hormone-receptor positive breast cancers – they represent 15%-20% of all breast cancers – but they are faster growing and may develop in the interim if women are screened less often (at 2-year intervals).
In addition, finding an invasive breast cancer early is almost always beneficial. Earlier diagnosis (lower stage at diagnosis) is associated with increased breast cancer-specific and overall survival, as well as less-aggressive treatment approaches.
As a medical oncologist who treats women with breast cancer, I see these benefits firsthand. With earlier diagnosis, we are more likely to offer less aggressive surgical approaches such as partial mastectomy (lumpectomy) and sentinel lymph node biopsy as opposed to total mastectomy with axillary lymph node dissection, the latter of which is more likely to be associated with lymphedema and which can lead to postmastectomy chest wall pain syndromes.
We also are able to use less aggressive radiation therapy approaches such as partial breast radiation, and less aggressive breast cancer–specific systemic treatments for women with a lower stage of breast cancer at diagnosis. In some cases, adjuvant or neoadjuvant chemotherapy may not be needed – and when it is necessary, shorter courses of chemotherapy or targeted chemotherapeutic regimens may be offered. This means lower systemic toxicities, both early and late, such as less cytopenias, risk of infections, mucositis, hair loss, cardiotoxicity, secondary malignancies/leukemia, and peripheral sensory neuropathy.
It is important to note that Black women in the United States have the highest death rate from breast cancer – 27.3 per 100,000 per year, versus 19.6 per 100,000 per year for White women1 – and that younger Black women appear to have a higher risk of developing triple-negative breast cancer, a more aggressive type of breast cancer. The higher breast cancer mortality in Black women is likely multifactorial and may be attributed partly to disparities in health care and partly to tumor biology. The case for annual screening in this population thus seems especially strong.
Screening modalities
Digital 3-D mammography, or digital breast tomosynthesis (DBT), is widely considered to be a more sensitive screening tool than conventional digital mammography alone. The NCCN recommends DBT for women with an average risk of developing breast cancer starting at age 40,4,5 and the USPSTF, while offering no recommendation on DBT as a primary screening method (“insufficient evidence”), says that DBT appears to increase cancer detection rates.2 So, I do routinely recommend it.
DBT may be especially beneficial for women with dense breast tissue (determined mammographically), who are most often premenopausal women – particularly non-Hispanic White women. Dense breast tissue itself can contribute to an increased risk of breast cancer – an approximately 20% higher relative risk in an average-risk woman with heterogeneously dense breast tissue, and an approximately 100% higher relative risk in a woman with extremely dense breasts6 – but unfortunately it affects the sensitivity and specificity of screening mammography.
I do not recommend routine supplemental screening with other methods (breast ultrasonography or MRI) for women at average risk of breast cancer who have dense breasts. MRI with gadolinium contrast is recommended as an adjunct to mammography for women who have a lifetime risk of developing breast cancer of more than 20%-25% (e.g., women with known BRCA1/2 mutations or radiation to breast tissue), and can be done annually at the same time as the screening mammogram is done. Some clinicians and patients prefer to alternate these two tests – one every 6 months.
Screening breast MRI is more sensitive but less specific than mammography; combining the two screening modalities leads to overall increased sensitivity and specificity in high-risk populations.
Risk assessment
Identifying higher-risk women who need to be sent to a genetic counselor is critically important. The USPSTF recommends that women who have family members with breast, ovarian, tubal or peritoneal cancer, or who have an ancestry associated with BRCA1/2 gene mutations, be assessed with a brief familial risk assessment tool such as the Pedigree Assessment Tool. This and other validated tools have been evaluated by the USPSTF and can be used to guide referrals to genetic counseling for more definitive risk assessment.7
These tools are different from general breast cancer risk assessment models, such as the NCI’s Breast Cancer Risk Assessment Tool,8 which are designed to calculate the 5-year and lifetime risk of developing invasive breast cancer for an average-risk woman but not to identify BRCA-related cancer risk. (The NCI’s tool is based on the Gail model, which has been widely used over the years.)
The general risk assessment models use a women’s personal medical and reproductive history as well as the history of breast cancer among her first-degree relatives to estimate her risk.
Dr. Tkaczuk reported that she has no disclosures.
References
1. “Cancer Stat Facts: Female Breast Cancer.” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
2. Siu AL et al. Ann Intern Med. 2016 Feb 16. doi: 10.7326/M15-2886.
3. Independent UK Panel on Breast Cancer Screening. Lancet. 2012 Nov 17;380(9855):1778-86.
4. NCCN guidelines for Detection, Prevention, & Risk Reduction: Breast Cancer Screening and Diagnosis. National Comprehensive Cancer Network.
5. NCCN guidelines for Detection, Prevention, & Risk Reduction: Breast Cancer Risk Reduction. National Comprehensive Cancer Network.
6. Ziv E et al. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2090-5.
7. USPSTF. JAMA. 2019;322(7):652-65.
8. The Breast Cancer Risk Assessment Tool. National Cancer Institute.
The scope of under- and overtreatment in older adults with cancer
Because of physiological changes with aging and differences in cancer biology, caring for older adults (OAs) with cancer requires careful assessment and planning.
Clark Dumontier, MD, of Brigham and Women’s Hospital in Boston, and colleagues sought to define the meaning of the terms “undertreatment” and “overtreatment” for OAs with cancer in a scoping literature review published in the Journal of Clinical Oncology.
Though OAs are typically defined as adults aged 65 years and older, in this review, the authors defined OAs as patients aged 60 years and older.
The authors theorized that a scoping review of papers about this patient population could provide clues about limitations in the oncology literature and guidance about patient management and future research. Despite comprising the majority of cancer patients, OAs are underrepresented in clinical trials.
About scoping reviews
Scoping reviews are used to identify existing evidence in a field, clarify concepts or definitions in the literature, survey how research on a topic is conducted, and identify knowledge gaps. In addition, scoping reviews summarize available evidence without answering a discrete research question.
Industry standards for scoping reviews have been established by the Johanna Briggs Institute and Preferred Reporting Items for Systematic Reviews and Meta-analyses extension for scoping reviews. According to these standards, scoping reviews should:
- Establish eligibility criteria with a rationale for each criterion clearly explained
- Search multiple databases in multiple languages
- Include “gray literature,” defined as studies that are unpublished or difficult to locate
- Have several independent reviewers screen titles and abstracts
- Ask multiple independent reviewers to review full text articles
- Present results with charts or diagrams that align with the review’s objective
- Graphically depict the decision process for including/excluding sources
- Identify implications for further research.
In their review, Dr. DuMontier and colleagues fulfilled many of the aforementioned criteria. The team searched three English-language databases for titles and abstracts that included the terms undertreatment and/or overtreatment, and were related to OAs with cancer, inclusive of all types of articles, cancer types, and treatments.
Definitions of undertreatment and overtreatment were extracted, and categories underlying these definitions were derived. Within a random subset of articles, two coauthors independently determined final categories of definitions and independently assigned those categories.
Findings and implications
To define OA, Dr. DuMontier and colleagues used a cutoff of 60 years or older. Articles mentioning undertreatment (n = 236), overtreatment (n = 71), or both (n = 51) met criteria for inclusion (n = 256), but only 14 articles (5.5%) explicitly provided formal definitions.
For most of the reviewed articles, the authors judged definitions from the surrounding context. In a random subset of 50 articles, there was a high level of agreement (87.1%; κ = 0.81) between two coauthors in independently assigning categories of definitions.
Undertreatment was applied to therapy that was less than recommended (148 articles; 62.7%) or less than recommended with worse outcomes (88 articles; 37.3%).
Overtreatment most commonly denoted intensive treatment of an OA in whom harms outweighed the benefits of treatment (38 articles; 53.5%) or intensive treatment of a cancer not expected to affect the OA during the patient’s remaining life (33 articles; 46.5%).
Overall, the authors found that undertreatment and overtreatment of OAs with cancer are imprecisely defined concepts. Formal geriatric assessment was recommended in just over half of articles, and only 26.2% recommended formal assessments of age-related vulnerabilities for management. The authors proposed definitions that accounted for both oncologic factors and geriatric domains.
Care of individual patients and clinical research
National Comprehensive Cancer Network (NCCN) guidelines for OAs with cancer recommend initial consideration of overall life expectancy. If a patient is a candidate for cancer treatment on that basis, the next recommended assessment is that of the patient’s capacity to understand the relevant information, appreciate the underlying values and overall medical situation, reason through decisions, and communicate a choice that is consistent with the patient’s articulated goals.
In the pretreatment evaluation of OAs in whom there are no concerns about tolerance to antineoplastic therapy, NCCN guidelines suggest geriatric screening with standardized tools and, if abnormal, comprehensive geriatric screening. The guidelines recommend considering alternative treatment options if nonmodifiable abnormalities are identified.
Referral to a geriatric clinical specialist, use of the Cancer and Aging Research Group’s Chemo Toxicity Calculator, and calculation of Chemotherapy Risk Assessment Scale for High-Age Patients score are specifically suggested if high-risk procedures (such as chemotherapy, radiation, or complex surgery, which most oncologists would consider to be “another day in the office”) are contemplated.
The American Society of Clinical Oncology (ASCO) guidelines for geriatric oncology are similarly detailed and endorse similar evaluations and management.
Employing disease-centric and geriatric domains
Dr. DuMontier and colleagues noted that, for OAs with comorbidity or psychosocial challenges, surrogate survival endpoints are unrelated to quality of life (QOL) outcomes. Nonetheless, QOL is valued by OAs at least as much as survival improvement.
Through no fault of their own, the authors’ conclusion that undertreatment and overtreatment are imperfectly defined concepts has a certain neutrality to it. However, the terms undertreatment and overtreatment are commonly used to signify that inappropriate treatment decisions were made. Therefore, the terms are inherently negative and pejorative.
As with most emotionally charged issues in oncology, it is ideal for professionals in our field to take charge when deficiencies exist. ASCO, NCCN, and the authors of this scoping review have provided a conceptual basis for doing so.
An integrated oncologist-geriatrician approach was shown to be effective in the randomized INTEGERATE trial, showing improved QOL, reduced hospital admissions, and reduced early treatment discontinuation from adverse events (ASCO 2020, Abstract 12011).
Therefore, those clinicians who have not formally, systematically, and routinely supplemented the traditional disease-centric endpoints with patient-centered criteria need to do so.
Similarly, a retrospective study published in JAMA Network Open demonstrated that geriatric and surgical comanagement of OAs with cancer was associated with significantly lower 90-day postoperative mortality and receipt of more supportive care services (physical therapy, occupational therapy, speech and swallow rehabilitation, and nutrition services), in comparison with management from the surgical service only.
These clinical and administrative changes will not only enhance patient management but also facilitate the clinical trials required to clarify optimal treatment intensity. As that occurs, we will be able to apply as much precision to the care of OAs with cancer as we do in other areas of cancer treatment.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Dumontier C et al. J Clin Oncol. 2020 Aug 1;38(22):2558-2569.
Because of physiological changes with aging and differences in cancer biology, caring for older adults (OAs) with cancer requires careful assessment and planning.
Clark Dumontier, MD, of Brigham and Women’s Hospital in Boston, and colleagues sought to define the meaning of the terms “undertreatment” and “overtreatment” for OAs with cancer in a scoping literature review published in the Journal of Clinical Oncology.
Though OAs are typically defined as adults aged 65 years and older, in this review, the authors defined OAs as patients aged 60 years and older.
The authors theorized that a scoping review of papers about this patient population could provide clues about limitations in the oncology literature and guidance about patient management and future research. Despite comprising the majority of cancer patients, OAs are underrepresented in clinical trials.
About scoping reviews
Scoping reviews are used to identify existing evidence in a field, clarify concepts or definitions in the literature, survey how research on a topic is conducted, and identify knowledge gaps. In addition, scoping reviews summarize available evidence without answering a discrete research question.
Industry standards for scoping reviews have been established by the Johanna Briggs Institute and Preferred Reporting Items for Systematic Reviews and Meta-analyses extension for scoping reviews. According to these standards, scoping reviews should:
- Establish eligibility criteria with a rationale for each criterion clearly explained
- Search multiple databases in multiple languages
- Include “gray literature,” defined as studies that are unpublished or difficult to locate
- Have several independent reviewers screen titles and abstracts
- Ask multiple independent reviewers to review full text articles
- Present results with charts or diagrams that align with the review’s objective
- Graphically depict the decision process for including/excluding sources
- Identify implications for further research.
In their review, Dr. DuMontier and colleagues fulfilled many of the aforementioned criteria. The team searched three English-language databases for titles and abstracts that included the terms undertreatment and/or overtreatment, and were related to OAs with cancer, inclusive of all types of articles, cancer types, and treatments.
Definitions of undertreatment and overtreatment were extracted, and categories underlying these definitions were derived. Within a random subset of articles, two coauthors independently determined final categories of definitions and independently assigned those categories.
Findings and implications
To define OA, Dr. DuMontier and colleagues used a cutoff of 60 years or older. Articles mentioning undertreatment (n = 236), overtreatment (n = 71), or both (n = 51) met criteria for inclusion (n = 256), but only 14 articles (5.5%) explicitly provided formal definitions.
For most of the reviewed articles, the authors judged definitions from the surrounding context. In a random subset of 50 articles, there was a high level of agreement (87.1%; κ = 0.81) between two coauthors in independently assigning categories of definitions.
Undertreatment was applied to therapy that was less than recommended (148 articles; 62.7%) or less than recommended with worse outcomes (88 articles; 37.3%).
Overtreatment most commonly denoted intensive treatment of an OA in whom harms outweighed the benefits of treatment (38 articles; 53.5%) or intensive treatment of a cancer not expected to affect the OA during the patient’s remaining life (33 articles; 46.5%).
Overall, the authors found that undertreatment and overtreatment of OAs with cancer are imprecisely defined concepts. Formal geriatric assessment was recommended in just over half of articles, and only 26.2% recommended formal assessments of age-related vulnerabilities for management. The authors proposed definitions that accounted for both oncologic factors and geriatric domains.
Care of individual patients and clinical research
National Comprehensive Cancer Network (NCCN) guidelines for OAs with cancer recommend initial consideration of overall life expectancy. If a patient is a candidate for cancer treatment on that basis, the next recommended assessment is that of the patient’s capacity to understand the relevant information, appreciate the underlying values and overall medical situation, reason through decisions, and communicate a choice that is consistent with the patient’s articulated goals.
In the pretreatment evaluation of OAs in whom there are no concerns about tolerance to antineoplastic therapy, NCCN guidelines suggest geriatric screening with standardized tools and, if abnormal, comprehensive geriatric screening. The guidelines recommend considering alternative treatment options if nonmodifiable abnormalities are identified.
Referral to a geriatric clinical specialist, use of the Cancer and Aging Research Group’s Chemo Toxicity Calculator, and calculation of Chemotherapy Risk Assessment Scale for High-Age Patients score are specifically suggested if high-risk procedures (such as chemotherapy, radiation, or complex surgery, which most oncologists would consider to be “another day in the office”) are contemplated.
The American Society of Clinical Oncology (ASCO) guidelines for geriatric oncology are similarly detailed and endorse similar evaluations and management.
Employing disease-centric and geriatric domains
Dr. DuMontier and colleagues noted that, for OAs with comorbidity or psychosocial challenges, surrogate survival endpoints are unrelated to quality of life (QOL) outcomes. Nonetheless, QOL is valued by OAs at least as much as survival improvement.
Through no fault of their own, the authors’ conclusion that undertreatment and overtreatment are imperfectly defined concepts has a certain neutrality to it. However, the terms undertreatment and overtreatment are commonly used to signify that inappropriate treatment decisions were made. Therefore, the terms are inherently negative and pejorative.
As with most emotionally charged issues in oncology, it is ideal for professionals in our field to take charge when deficiencies exist. ASCO, NCCN, and the authors of this scoping review have provided a conceptual basis for doing so.
An integrated oncologist-geriatrician approach was shown to be effective in the randomized INTEGERATE trial, showing improved QOL, reduced hospital admissions, and reduced early treatment discontinuation from adverse events (ASCO 2020, Abstract 12011).
Therefore, those clinicians who have not formally, systematically, and routinely supplemented the traditional disease-centric endpoints with patient-centered criteria need to do so.
Similarly, a retrospective study published in JAMA Network Open demonstrated that geriatric and surgical comanagement of OAs with cancer was associated with significantly lower 90-day postoperative mortality and receipt of more supportive care services (physical therapy, occupational therapy, speech and swallow rehabilitation, and nutrition services), in comparison with management from the surgical service only.
These clinical and administrative changes will not only enhance patient management but also facilitate the clinical trials required to clarify optimal treatment intensity. As that occurs, we will be able to apply as much precision to the care of OAs with cancer as we do in other areas of cancer treatment.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Dumontier C et al. J Clin Oncol. 2020 Aug 1;38(22):2558-2569.
Because of physiological changes with aging and differences in cancer biology, caring for older adults (OAs) with cancer requires careful assessment and planning.
Clark Dumontier, MD, of Brigham and Women’s Hospital in Boston, and colleagues sought to define the meaning of the terms “undertreatment” and “overtreatment” for OAs with cancer in a scoping literature review published in the Journal of Clinical Oncology.
Though OAs are typically defined as adults aged 65 years and older, in this review, the authors defined OAs as patients aged 60 years and older.
The authors theorized that a scoping review of papers about this patient population could provide clues about limitations in the oncology literature and guidance about patient management and future research. Despite comprising the majority of cancer patients, OAs are underrepresented in clinical trials.
About scoping reviews
Scoping reviews are used to identify existing evidence in a field, clarify concepts or definitions in the literature, survey how research on a topic is conducted, and identify knowledge gaps. In addition, scoping reviews summarize available evidence without answering a discrete research question.
Industry standards for scoping reviews have been established by the Johanna Briggs Institute and Preferred Reporting Items for Systematic Reviews and Meta-analyses extension for scoping reviews. According to these standards, scoping reviews should:
- Establish eligibility criteria with a rationale for each criterion clearly explained
- Search multiple databases in multiple languages
- Include “gray literature,” defined as studies that are unpublished or difficult to locate
- Have several independent reviewers screen titles and abstracts
- Ask multiple independent reviewers to review full text articles
- Present results with charts or diagrams that align with the review’s objective
- Graphically depict the decision process for including/excluding sources
- Identify implications for further research.
In their review, Dr. DuMontier and colleagues fulfilled many of the aforementioned criteria. The team searched three English-language databases for titles and abstracts that included the terms undertreatment and/or overtreatment, and were related to OAs with cancer, inclusive of all types of articles, cancer types, and treatments.
Definitions of undertreatment and overtreatment were extracted, and categories underlying these definitions were derived. Within a random subset of articles, two coauthors independently determined final categories of definitions and independently assigned those categories.
Findings and implications
To define OA, Dr. DuMontier and colleagues used a cutoff of 60 years or older. Articles mentioning undertreatment (n = 236), overtreatment (n = 71), or both (n = 51) met criteria for inclusion (n = 256), but only 14 articles (5.5%) explicitly provided formal definitions.
For most of the reviewed articles, the authors judged definitions from the surrounding context. In a random subset of 50 articles, there was a high level of agreement (87.1%; κ = 0.81) between two coauthors in independently assigning categories of definitions.
Undertreatment was applied to therapy that was less than recommended (148 articles; 62.7%) or less than recommended with worse outcomes (88 articles; 37.3%).
Overtreatment most commonly denoted intensive treatment of an OA in whom harms outweighed the benefits of treatment (38 articles; 53.5%) or intensive treatment of a cancer not expected to affect the OA during the patient’s remaining life (33 articles; 46.5%).
Overall, the authors found that undertreatment and overtreatment of OAs with cancer are imprecisely defined concepts. Formal geriatric assessment was recommended in just over half of articles, and only 26.2% recommended formal assessments of age-related vulnerabilities for management. The authors proposed definitions that accounted for both oncologic factors and geriatric domains.
Care of individual patients and clinical research
National Comprehensive Cancer Network (NCCN) guidelines for OAs with cancer recommend initial consideration of overall life expectancy. If a patient is a candidate for cancer treatment on that basis, the next recommended assessment is that of the patient’s capacity to understand the relevant information, appreciate the underlying values and overall medical situation, reason through decisions, and communicate a choice that is consistent with the patient’s articulated goals.
In the pretreatment evaluation of OAs in whom there are no concerns about tolerance to antineoplastic therapy, NCCN guidelines suggest geriatric screening with standardized tools and, if abnormal, comprehensive geriatric screening. The guidelines recommend considering alternative treatment options if nonmodifiable abnormalities are identified.
Referral to a geriatric clinical specialist, use of the Cancer and Aging Research Group’s Chemo Toxicity Calculator, and calculation of Chemotherapy Risk Assessment Scale for High-Age Patients score are specifically suggested if high-risk procedures (such as chemotherapy, radiation, or complex surgery, which most oncologists would consider to be “another day in the office”) are contemplated.
The American Society of Clinical Oncology (ASCO) guidelines for geriatric oncology are similarly detailed and endorse similar evaluations and management.
Employing disease-centric and geriatric domains
Dr. DuMontier and colleagues noted that, for OAs with comorbidity or psychosocial challenges, surrogate survival endpoints are unrelated to quality of life (QOL) outcomes. Nonetheless, QOL is valued by OAs at least as much as survival improvement.
Through no fault of their own, the authors’ conclusion that undertreatment and overtreatment are imperfectly defined concepts has a certain neutrality to it. However, the terms undertreatment and overtreatment are commonly used to signify that inappropriate treatment decisions were made. Therefore, the terms are inherently negative and pejorative.
As with most emotionally charged issues in oncology, it is ideal for professionals in our field to take charge when deficiencies exist. ASCO, NCCN, and the authors of this scoping review have provided a conceptual basis for doing so.
An integrated oncologist-geriatrician approach was shown to be effective in the randomized INTEGERATE trial, showing improved QOL, reduced hospital admissions, and reduced early treatment discontinuation from adverse events (ASCO 2020, Abstract 12011).
Therefore, those clinicians who have not formally, systematically, and routinely supplemented the traditional disease-centric endpoints with patient-centered criteria need to do so.
Similarly, a retrospective study published in JAMA Network Open demonstrated that geriatric and surgical comanagement of OAs with cancer was associated with significantly lower 90-day postoperative mortality and receipt of more supportive care services (physical therapy, occupational therapy, speech and swallow rehabilitation, and nutrition services), in comparison with management from the surgical service only.
These clinical and administrative changes will not only enhance patient management but also facilitate the clinical trials required to clarify optimal treatment intensity. As that occurs, we will be able to apply as much precision to the care of OAs with cancer as we do in other areas of cancer treatment.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Dumontier C et al. J Clin Oncol. 2020 Aug 1;38(22):2558-2569.
Cancer disparities: One of the most pressing public health issues
“The burden of cancer is not shouldered equally by all segments of the U.S. population,” the AACR adds. “The adverse differences in cancer burden that exist among certain population groups are one of the most pressing public health challenges that we face in the United States.”
AACR president Antoni Ribas, MD, PhD, gave some examples of these disparities at a September 16 Congressional briefing that focused on the inaugural AACR Cancer Disparities Progress Report 2020.
He noted that:
- Black men have more than double the rate of death from prostate cancer compared with men of other racial and ethnic groups.
- Hispanic children are 24% more likely to develop leukemia than non-Hispanic children.
- Non-Hispanic Black children and adolescents with cancer are more than 50% more likely to die from the cancer than non-Hispanic white children and adolescents with cancer.
- Women of low socioeconomic status with early stage ovarian cancer are 50% less likely to receive recommended care than are women of high socioeconomic status.
- In addition to racial and ethnic minority groups, other populations that bear a disproportionate burden when it comes to cancer include individuals lacking adequate health insurance coverage, immigrants, those with disabilities, residents in rural areas, and members of the lesbian, gay, bisexual, and transgender communities.
“It is absolutely unacceptable that advances in cancer care and treatment are not benefiting everyone equally,” Ribas commented.
Making progress against cancer
Progress being made against cancer was highlighted in another publication, the annual AACR Cancer Progress Report 2020.
U.S. cancer deaths declined by 29% between 1991 and 2017, translating to nearly 3 million cancer deaths avoided, the report notes. In addition, 5-year survival rates for all cancers combined increased from 49% in the mid-1970s to 70% for patients diagnosed from 2010-2016.
Between August 2019 and July 31 of this year, the U.S. Food and Drug Administration approved 20 new anticancer drugs for various cancer types and 15 new indications for previously approved cancer drugs, marking the highest number of approvals in one 12-month period since AACR started producing these reports 10 years ago.
A continuing reduction in the cigarette smoking rate among US adults, which is now below 14%, is contributing greatly to declines in lung cancer rates, which have largely driven the improvements in cancer survival, the AACR noted.
This report also notes that progress has been made toward reducing cancer disparities. Overall disparities in cancer death rates among racial and ethnic groups are less pronounced now than they have been in the past two decades. For example, the overall cancer death rate for African American patients was 33% higher than for White patients in 1990 but just 14% higher in 2016.
However, both reports agree that more must be done to reduce cancer disparities even further.
They highlight initiatives that are underway, including:
- The draft guidance issued by the FDA to promote diversification of clinical trial populations.
- The National Institutes of Health’s (NIH’s) Continuing Umbrella of Research Experiences (CURE) program supporting underrepresented students and scientists along their academic and research career pathway.
- The Centers for Disease Control and Prevention’s Racial and Ethnic Approaches to Community Health (REACH) program, a grant-making program focused on encouraging preventive behaviors in underserved communities.
- The NIH’s All of Us program, which is gathering information from the genomes of 1 million healthy individuals with a focus on recruitment from historically underrepresented populations.
Ribas also announced that AACR has established a task force to focus on racial inequalities in cancer research.
Eliminating disparities would save money, argued John D. Carpten, PhD, from the University of Southern California, Los Angeles, who chaired the steering committee that developed the AACR Cancer Disparities Progress Report.
Carpten noted research showing that eliminating disparities for racial and ethnic minorities between 2003 and 2006 would have reduced health care costs by more than $1 trillion in the United States. This underscores the potentially far-reaching impact of efforts to eliminate disparities, he said.
“Without a doubt, socioeconomics and inequities in access to quality care represent major factors influencing cancer health disparities, and these disparities will persist until we address these issues” he said.
Both progress reports culminate in a call to action, largely focused on the need for “unwavering, bipartisan support from Congress, in the form of robust and sustained annual increases in funding for the NIH, NCI [National Cancer Institute], and FDA,” which is vital for accelerating the pace of progress.
The challenge is now compounded by the ongoing COVID-19 pandemic: Both progress reports note that racial and ethnic minorities, including African Americans, are not only affected disproportionately by cancer, but also by COVID-19, further highlighting the “stark inequities in health care.”
Ribas further called for action from national leadership and the scientific community.
“During this unprecedented time in our nation’s history, there is also a need for our nation’s leaders to take on a much bigger role in confronting and combating the structural and systemic racism that contributes to health disparities,” he said. The “pervasive racism and social injustices” that have contributed to disparities in both COVID-19 and cancer underscore the need for “the scientific community to step up and partner with Congress to assess and address this issue within the research community.”
This article first appeared on Medscape.com.
“The burden of cancer is not shouldered equally by all segments of the U.S. population,” the AACR adds. “The adverse differences in cancer burden that exist among certain population groups are one of the most pressing public health challenges that we face in the United States.”
AACR president Antoni Ribas, MD, PhD, gave some examples of these disparities at a September 16 Congressional briefing that focused on the inaugural AACR Cancer Disparities Progress Report 2020.
He noted that:
- Black men have more than double the rate of death from prostate cancer compared with men of other racial and ethnic groups.
- Hispanic children are 24% more likely to develop leukemia than non-Hispanic children.
- Non-Hispanic Black children and adolescents with cancer are more than 50% more likely to die from the cancer than non-Hispanic white children and adolescents with cancer.
- Women of low socioeconomic status with early stage ovarian cancer are 50% less likely to receive recommended care than are women of high socioeconomic status.
- In addition to racial and ethnic minority groups, other populations that bear a disproportionate burden when it comes to cancer include individuals lacking adequate health insurance coverage, immigrants, those with disabilities, residents in rural areas, and members of the lesbian, gay, bisexual, and transgender communities.
“It is absolutely unacceptable that advances in cancer care and treatment are not benefiting everyone equally,” Ribas commented.
Making progress against cancer
Progress being made against cancer was highlighted in another publication, the annual AACR Cancer Progress Report 2020.
U.S. cancer deaths declined by 29% between 1991 and 2017, translating to nearly 3 million cancer deaths avoided, the report notes. In addition, 5-year survival rates for all cancers combined increased from 49% in the mid-1970s to 70% for patients diagnosed from 2010-2016.
Between August 2019 and July 31 of this year, the U.S. Food and Drug Administration approved 20 new anticancer drugs for various cancer types and 15 new indications for previously approved cancer drugs, marking the highest number of approvals in one 12-month period since AACR started producing these reports 10 years ago.
A continuing reduction in the cigarette smoking rate among US adults, which is now below 14%, is contributing greatly to declines in lung cancer rates, which have largely driven the improvements in cancer survival, the AACR noted.
This report also notes that progress has been made toward reducing cancer disparities. Overall disparities in cancer death rates among racial and ethnic groups are less pronounced now than they have been in the past two decades. For example, the overall cancer death rate for African American patients was 33% higher than for White patients in 1990 but just 14% higher in 2016.
However, both reports agree that more must be done to reduce cancer disparities even further.
They highlight initiatives that are underway, including:
- The draft guidance issued by the FDA to promote diversification of clinical trial populations.
- The National Institutes of Health’s (NIH’s) Continuing Umbrella of Research Experiences (CURE) program supporting underrepresented students and scientists along their academic and research career pathway.
- The Centers for Disease Control and Prevention’s Racial and Ethnic Approaches to Community Health (REACH) program, a grant-making program focused on encouraging preventive behaviors in underserved communities.
- The NIH’s All of Us program, which is gathering information from the genomes of 1 million healthy individuals with a focus on recruitment from historically underrepresented populations.
Ribas also announced that AACR has established a task force to focus on racial inequalities in cancer research.
Eliminating disparities would save money, argued John D. Carpten, PhD, from the University of Southern California, Los Angeles, who chaired the steering committee that developed the AACR Cancer Disparities Progress Report.
Carpten noted research showing that eliminating disparities for racial and ethnic minorities between 2003 and 2006 would have reduced health care costs by more than $1 trillion in the United States. This underscores the potentially far-reaching impact of efforts to eliminate disparities, he said.
“Without a doubt, socioeconomics and inequities in access to quality care represent major factors influencing cancer health disparities, and these disparities will persist until we address these issues” he said.
Both progress reports culminate in a call to action, largely focused on the need for “unwavering, bipartisan support from Congress, in the form of robust and sustained annual increases in funding for the NIH, NCI [National Cancer Institute], and FDA,” which is vital for accelerating the pace of progress.
The challenge is now compounded by the ongoing COVID-19 pandemic: Both progress reports note that racial and ethnic minorities, including African Americans, are not only affected disproportionately by cancer, but also by COVID-19, further highlighting the “stark inequities in health care.”
Ribas further called for action from national leadership and the scientific community.
“During this unprecedented time in our nation’s history, there is also a need for our nation’s leaders to take on a much bigger role in confronting and combating the structural and systemic racism that contributes to health disparities,” he said. The “pervasive racism and social injustices” that have contributed to disparities in both COVID-19 and cancer underscore the need for “the scientific community to step up and partner with Congress to assess and address this issue within the research community.”
This article first appeared on Medscape.com.
“The burden of cancer is not shouldered equally by all segments of the U.S. population,” the AACR adds. “The adverse differences in cancer burden that exist among certain population groups are one of the most pressing public health challenges that we face in the United States.”
AACR president Antoni Ribas, MD, PhD, gave some examples of these disparities at a September 16 Congressional briefing that focused on the inaugural AACR Cancer Disparities Progress Report 2020.
He noted that:
- Black men have more than double the rate of death from prostate cancer compared with men of other racial and ethnic groups.
- Hispanic children are 24% more likely to develop leukemia than non-Hispanic children.
- Non-Hispanic Black children and adolescents with cancer are more than 50% more likely to die from the cancer than non-Hispanic white children and adolescents with cancer.
- Women of low socioeconomic status with early stage ovarian cancer are 50% less likely to receive recommended care than are women of high socioeconomic status.
- In addition to racial and ethnic minority groups, other populations that bear a disproportionate burden when it comes to cancer include individuals lacking adequate health insurance coverage, immigrants, those with disabilities, residents in rural areas, and members of the lesbian, gay, bisexual, and transgender communities.
“It is absolutely unacceptable that advances in cancer care and treatment are not benefiting everyone equally,” Ribas commented.
Making progress against cancer
Progress being made against cancer was highlighted in another publication, the annual AACR Cancer Progress Report 2020.
U.S. cancer deaths declined by 29% between 1991 and 2017, translating to nearly 3 million cancer deaths avoided, the report notes. In addition, 5-year survival rates for all cancers combined increased from 49% in the mid-1970s to 70% for patients diagnosed from 2010-2016.
Between August 2019 and July 31 of this year, the U.S. Food and Drug Administration approved 20 new anticancer drugs for various cancer types and 15 new indications for previously approved cancer drugs, marking the highest number of approvals in one 12-month period since AACR started producing these reports 10 years ago.
A continuing reduction in the cigarette smoking rate among US adults, which is now below 14%, is contributing greatly to declines in lung cancer rates, which have largely driven the improvements in cancer survival, the AACR noted.
This report also notes that progress has been made toward reducing cancer disparities. Overall disparities in cancer death rates among racial and ethnic groups are less pronounced now than they have been in the past two decades. For example, the overall cancer death rate for African American patients was 33% higher than for White patients in 1990 but just 14% higher in 2016.
However, both reports agree that more must be done to reduce cancer disparities even further.
They highlight initiatives that are underway, including:
- The draft guidance issued by the FDA to promote diversification of clinical trial populations.
- The National Institutes of Health’s (NIH’s) Continuing Umbrella of Research Experiences (CURE) program supporting underrepresented students and scientists along their academic and research career pathway.
- The Centers for Disease Control and Prevention’s Racial and Ethnic Approaches to Community Health (REACH) program, a grant-making program focused on encouraging preventive behaviors in underserved communities.
- The NIH’s All of Us program, which is gathering information from the genomes of 1 million healthy individuals with a focus on recruitment from historically underrepresented populations.
Ribas also announced that AACR has established a task force to focus on racial inequalities in cancer research.
Eliminating disparities would save money, argued John D. Carpten, PhD, from the University of Southern California, Los Angeles, who chaired the steering committee that developed the AACR Cancer Disparities Progress Report.
Carpten noted research showing that eliminating disparities for racial and ethnic minorities between 2003 and 2006 would have reduced health care costs by more than $1 trillion in the United States. This underscores the potentially far-reaching impact of efforts to eliminate disparities, he said.
“Without a doubt, socioeconomics and inequities in access to quality care represent major factors influencing cancer health disparities, and these disparities will persist until we address these issues” he said.
Both progress reports culminate in a call to action, largely focused on the need for “unwavering, bipartisan support from Congress, in the form of robust and sustained annual increases in funding for the NIH, NCI [National Cancer Institute], and FDA,” which is vital for accelerating the pace of progress.
The challenge is now compounded by the ongoing COVID-19 pandemic: Both progress reports note that racial and ethnic minorities, including African Americans, are not only affected disproportionately by cancer, but also by COVID-19, further highlighting the “stark inequities in health care.”
Ribas further called for action from national leadership and the scientific community.
“During this unprecedented time in our nation’s history, there is also a need for our nation’s leaders to take on a much bigger role in confronting and combating the structural and systemic racism that contributes to health disparities,” he said. The “pervasive racism and social injustices” that have contributed to disparities in both COVID-19 and cancer underscore the need for “the scientific community to step up and partner with Congress to assess and address this issue within the research community.”
This article first appeared on Medscape.com.
Divergent findings with paclitaxel and nab-paclitaxel in TNBC
The trials, IMpassion130 and IMpassion131, both enrolled patients with metastatic or unresectable, locally advanced TNBC.
In IMpassion131, adding atezolizumab to paclitaxel did not improve progression-free survival (PFS) or overall survival (OS), regardless of programmed death–ligand 1 (PD-L1) expression.
In IMpassion130, adding atezolizumab to nab-paclitaxel did not improve OS in the intention-to-treat (ITT) population but did provide a “clinically meaningful” improvement in OS among PD-L1-positive patients, according to investigators.
IMpassion130 and IMpassion131 were presented during the same session at the European Society for Medical Oncology (ESMO) Virtual Congress 2020.
Potential reasons for the different outcomes in the two studies require further exploration, according to David Miles, MD, of Mount Vernon Cancer Centre in Northwood, England, who presented the findings from IMpassion131.
ESMO discussant Lisa A. Carey, MD, of the University of North Carolina at Chapel Hill, posited three possible explanations for the divergent findings. The steroids necessary with paclitaxel dosing may have had a negative effect on immune checkpoint inhibitor activity, differences in study populations may have played a role, or the divergent findings could be caused by chance.
Steroid use in IMpassion131 could have played a negative role because of its lympholytic activity, but other indications with steroid use have not demonstrated attenuated benefits, said Leisha A. Emens, MD, PhD, of the University of Pittsburgh Medical Center, who presented the findings from IMpassion130 at ESMO 2020.
“If I were a patient, based on the data to date, I would want nab-paclitaxel with atezolizumab,” Dr. Emens said.
Trial details
Both trials are phase 3, double-blind, placebo-controlled studies of women with metastatic or unresectable locally advanced TNBC who had received no prior therapy for advanced TNBC.
IMpassion130 included 451 patients randomized to atezolizumab plus nab-paclitaxel and 451 randomized to placebo plus nab-paclitaxel. Patients received nab-paclitaxel at a starting dose of 100 mg/m2 via IV infusion on days 1, 8, and 15 of each 28-day cycle for at least six cycles.
In both studies, patients received atezolizumab at 840 mg on days 1 and 15 of a 28-day cycle in their active treatment arms.
IMpassion131 included 651 patients randomized 2:1 to atezolizumab plus paclitaxel (n = 431) or placebo plus paclitaxel (n = 220). Patients received paclitaxel at 90 mg/m2 on days 1, 8, and 15 every 28 days until disease progression or unacceptable toxicity.
Baseline characteristics were well balanced between the treatment arms in both studies. Less than half of patients – 45% in IMpassion131 and 41% in IMpassion130 – were PD-L1 positive.
Results of IMpassion131
The primary endpoint in IMpassion131 was PFS, and there was no significant difference in PFS between the treatment arms.
“The primary objective of IMpassion131 was not met,” Dr. Miles said. “[The] addition of atezolizumab to paclitaxel did not significantly improve PFS in patients with PD-L1-positive metastatic triple-negative breast cancer.”
In the PD-L1-positive population, the median PFS was 5.7 months in the placebo arm and 6.0 months in the atezolizumab arm (stratified hazard ratio, 0.82, P = .20).
In the ITT population, the median PFS was 5.6 months in the control arm and 5.7 months in the atezolizumab arm (HR, 0.86).
In subgroup analyses, Dr. Miles noted, “There was no clue about adverse or beneficial effects in any subgroup.”
The updated OS analysis demonstrated no benefit with atezolizumab in the ITT population or the PD-L1-positive population. In fact, there was a trend toward better OS for the control group in the latter analysis.
In the PD-L1-positive population, the median OS was 28.3 months in the control arm and 22.1 months in the atezolizumab arm (HR, 1.12). The 2-year OS rates were 51% and 49%, respectively.
In the ITT population, the median OS was 22.8 months in the control arm and 19.2 months in the atezolizumab arm (HR, 1.11). The 2-year OS rates were 45% and 42%, respectively.
The safety profile of the atezolizumab-paclitaxel combination was consistent with known side effects of the individual drugs, Dr. Miles said. There were four fatal treatment-related adverse events in the atezolizumab arm.
Results of IMpassion130
Presenting the final OS analysis from IMpassion130, Dr. Emens noted that the study’s findings have led to recommendations for atezolizumab plus nab-paclitaxel as first-line treatment of PD-L1-positive TNBC in international guidelines.
The median OS in the ITT population was 18.7 months in the placebo arm and 21.0 months in the atezolizumab arm (stratified HR, 0.87, P = .077). The 3-year OS rates were 25% and 28%, respectively.
The median OS in the PD-L1-positive population was 17.9 months in the placebo arm and 25.4 months in the atezolizumab arm (HR, 0.67). The 3-year OS rates were 22% and 36%, respectively.
A P value is not available for the between-arm OS comparison in the PD-L1-positive population. OS was not formally tested in this group because the OS boundary for statistical significance was not crossed in the ITT population. However, Dr. Emens said there was a “clinically meaningful” OS benefit observed with atezolizumab in the PD-L1-positive patients.
Treatment withdrawals caused by adverse events were more common in the atezolizumab arm (19% vs. 8%). The most common of these was neuropathy, Dr. Emens said. However, she noted that atezolizumab-related adverse events were generally low grade and easily managed.
“These results support a positive benefit-risk profile for atezolizumab plus nab-paclitaxel as first-line therapy in patients with PD-L1-positive metastatic triple-negative breast cancer,” Dr. Emens concluded.
Both studies were funded by F. Hoffman–La Roche. Dr. Miles, Dr. Emens, and Dr. Carey disclosed financial relationships with Roche and other companies.
SOURCES: Miles D et al. ESMO 2020, Abstract LBA15; Emens LA et al. ESMO 2020, Abstract LBA16.
The trials, IMpassion130 and IMpassion131, both enrolled patients with metastatic or unresectable, locally advanced TNBC.
In IMpassion131, adding atezolizumab to paclitaxel did not improve progression-free survival (PFS) or overall survival (OS), regardless of programmed death–ligand 1 (PD-L1) expression.
In IMpassion130, adding atezolizumab to nab-paclitaxel did not improve OS in the intention-to-treat (ITT) population but did provide a “clinically meaningful” improvement in OS among PD-L1-positive patients, according to investigators.
IMpassion130 and IMpassion131 were presented during the same session at the European Society for Medical Oncology (ESMO) Virtual Congress 2020.
Potential reasons for the different outcomes in the two studies require further exploration, according to David Miles, MD, of Mount Vernon Cancer Centre in Northwood, England, who presented the findings from IMpassion131.
ESMO discussant Lisa A. Carey, MD, of the University of North Carolina at Chapel Hill, posited three possible explanations for the divergent findings. The steroids necessary with paclitaxel dosing may have had a negative effect on immune checkpoint inhibitor activity, differences in study populations may have played a role, or the divergent findings could be caused by chance.
Steroid use in IMpassion131 could have played a negative role because of its lympholytic activity, but other indications with steroid use have not demonstrated attenuated benefits, said Leisha A. Emens, MD, PhD, of the University of Pittsburgh Medical Center, who presented the findings from IMpassion130 at ESMO 2020.
“If I were a patient, based on the data to date, I would want nab-paclitaxel with atezolizumab,” Dr. Emens said.
Trial details
Both trials are phase 3, double-blind, placebo-controlled studies of women with metastatic or unresectable locally advanced TNBC who had received no prior therapy for advanced TNBC.
IMpassion130 included 451 patients randomized to atezolizumab plus nab-paclitaxel and 451 randomized to placebo plus nab-paclitaxel. Patients received nab-paclitaxel at a starting dose of 100 mg/m2 via IV infusion on days 1, 8, and 15 of each 28-day cycle for at least six cycles.
In both studies, patients received atezolizumab at 840 mg on days 1 and 15 of a 28-day cycle in their active treatment arms.
IMpassion131 included 651 patients randomized 2:1 to atezolizumab plus paclitaxel (n = 431) or placebo plus paclitaxel (n = 220). Patients received paclitaxel at 90 mg/m2 on days 1, 8, and 15 every 28 days until disease progression or unacceptable toxicity.
Baseline characteristics were well balanced between the treatment arms in both studies. Less than half of patients – 45% in IMpassion131 and 41% in IMpassion130 – were PD-L1 positive.
Results of IMpassion131
The primary endpoint in IMpassion131 was PFS, and there was no significant difference in PFS between the treatment arms.
“The primary objective of IMpassion131 was not met,” Dr. Miles said. “[The] addition of atezolizumab to paclitaxel did not significantly improve PFS in patients with PD-L1-positive metastatic triple-negative breast cancer.”
In the PD-L1-positive population, the median PFS was 5.7 months in the placebo arm and 6.0 months in the atezolizumab arm (stratified hazard ratio, 0.82, P = .20).
In the ITT population, the median PFS was 5.6 months in the control arm and 5.7 months in the atezolizumab arm (HR, 0.86).
In subgroup analyses, Dr. Miles noted, “There was no clue about adverse or beneficial effects in any subgroup.”
The updated OS analysis demonstrated no benefit with atezolizumab in the ITT population or the PD-L1-positive population. In fact, there was a trend toward better OS for the control group in the latter analysis.
In the PD-L1-positive population, the median OS was 28.3 months in the control arm and 22.1 months in the atezolizumab arm (HR, 1.12). The 2-year OS rates were 51% and 49%, respectively.
In the ITT population, the median OS was 22.8 months in the control arm and 19.2 months in the atezolizumab arm (HR, 1.11). The 2-year OS rates were 45% and 42%, respectively.
The safety profile of the atezolizumab-paclitaxel combination was consistent with known side effects of the individual drugs, Dr. Miles said. There were four fatal treatment-related adverse events in the atezolizumab arm.
Results of IMpassion130
Presenting the final OS analysis from IMpassion130, Dr. Emens noted that the study’s findings have led to recommendations for atezolizumab plus nab-paclitaxel as first-line treatment of PD-L1-positive TNBC in international guidelines.
The median OS in the ITT population was 18.7 months in the placebo arm and 21.0 months in the atezolizumab arm (stratified HR, 0.87, P = .077). The 3-year OS rates were 25% and 28%, respectively.
The median OS in the PD-L1-positive population was 17.9 months in the placebo arm and 25.4 months in the atezolizumab arm (HR, 0.67). The 3-year OS rates were 22% and 36%, respectively.
A P value is not available for the between-arm OS comparison in the PD-L1-positive population. OS was not formally tested in this group because the OS boundary for statistical significance was not crossed in the ITT population. However, Dr. Emens said there was a “clinically meaningful” OS benefit observed with atezolizumab in the PD-L1-positive patients.
Treatment withdrawals caused by adverse events were more common in the atezolizumab arm (19% vs. 8%). The most common of these was neuropathy, Dr. Emens said. However, she noted that atezolizumab-related adverse events were generally low grade and easily managed.
“These results support a positive benefit-risk profile for atezolizumab plus nab-paclitaxel as first-line therapy in patients with PD-L1-positive metastatic triple-negative breast cancer,” Dr. Emens concluded.
Both studies were funded by F. Hoffman–La Roche. Dr. Miles, Dr. Emens, and Dr. Carey disclosed financial relationships with Roche and other companies.
SOURCES: Miles D et al. ESMO 2020, Abstract LBA15; Emens LA et al. ESMO 2020, Abstract LBA16.
The trials, IMpassion130 and IMpassion131, both enrolled patients with metastatic or unresectable, locally advanced TNBC.
In IMpassion131, adding atezolizumab to paclitaxel did not improve progression-free survival (PFS) or overall survival (OS), regardless of programmed death–ligand 1 (PD-L1) expression.
In IMpassion130, adding atezolizumab to nab-paclitaxel did not improve OS in the intention-to-treat (ITT) population but did provide a “clinically meaningful” improvement in OS among PD-L1-positive patients, according to investigators.
IMpassion130 and IMpassion131 were presented during the same session at the European Society for Medical Oncology (ESMO) Virtual Congress 2020.
Potential reasons for the different outcomes in the two studies require further exploration, according to David Miles, MD, of Mount Vernon Cancer Centre in Northwood, England, who presented the findings from IMpassion131.
ESMO discussant Lisa A. Carey, MD, of the University of North Carolina at Chapel Hill, posited three possible explanations for the divergent findings. The steroids necessary with paclitaxel dosing may have had a negative effect on immune checkpoint inhibitor activity, differences in study populations may have played a role, or the divergent findings could be caused by chance.
Steroid use in IMpassion131 could have played a negative role because of its lympholytic activity, but other indications with steroid use have not demonstrated attenuated benefits, said Leisha A. Emens, MD, PhD, of the University of Pittsburgh Medical Center, who presented the findings from IMpassion130 at ESMO 2020.
“If I were a patient, based on the data to date, I would want nab-paclitaxel with atezolizumab,” Dr. Emens said.
Trial details
Both trials are phase 3, double-blind, placebo-controlled studies of women with metastatic or unresectable locally advanced TNBC who had received no prior therapy for advanced TNBC.
IMpassion130 included 451 patients randomized to atezolizumab plus nab-paclitaxel and 451 randomized to placebo plus nab-paclitaxel. Patients received nab-paclitaxel at a starting dose of 100 mg/m2 via IV infusion on days 1, 8, and 15 of each 28-day cycle for at least six cycles.
In both studies, patients received atezolizumab at 840 mg on days 1 and 15 of a 28-day cycle in their active treatment arms.
IMpassion131 included 651 patients randomized 2:1 to atezolizumab plus paclitaxel (n = 431) or placebo plus paclitaxel (n = 220). Patients received paclitaxel at 90 mg/m2 on days 1, 8, and 15 every 28 days until disease progression or unacceptable toxicity.
Baseline characteristics were well balanced between the treatment arms in both studies. Less than half of patients – 45% in IMpassion131 and 41% in IMpassion130 – were PD-L1 positive.
Results of IMpassion131
The primary endpoint in IMpassion131 was PFS, and there was no significant difference in PFS between the treatment arms.
“The primary objective of IMpassion131 was not met,” Dr. Miles said. “[The] addition of atezolizumab to paclitaxel did not significantly improve PFS in patients with PD-L1-positive metastatic triple-negative breast cancer.”
In the PD-L1-positive population, the median PFS was 5.7 months in the placebo arm and 6.0 months in the atezolizumab arm (stratified hazard ratio, 0.82, P = .20).
In the ITT population, the median PFS was 5.6 months in the control arm and 5.7 months in the atezolizumab arm (HR, 0.86).
In subgroup analyses, Dr. Miles noted, “There was no clue about adverse or beneficial effects in any subgroup.”
The updated OS analysis demonstrated no benefit with atezolizumab in the ITT population or the PD-L1-positive population. In fact, there was a trend toward better OS for the control group in the latter analysis.
In the PD-L1-positive population, the median OS was 28.3 months in the control arm and 22.1 months in the atezolizumab arm (HR, 1.12). The 2-year OS rates were 51% and 49%, respectively.
In the ITT population, the median OS was 22.8 months in the control arm and 19.2 months in the atezolizumab arm (HR, 1.11). The 2-year OS rates were 45% and 42%, respectively.
The safety profile of the atezolizumab-paclitaxel combination was consistent with known side effects of the individual drugs, Dr. Miles said. There were four fatal treatment-related adverse events in the atezolizumab arm.
Results of IMpassion130
Presenting the final OS analysis from IMpassion130, Dr. Emens noted that the study’s findings have led to recommendations for atezolizumab plus nab-paclitaxel as first-line treatment of PD-L1-positive TNBC in international guidelines.
The median OS in the ITT population was 18.7 months in the placebo arm and 21.0 months in the atezolizumab arm (stratified HR, 0.87, P = .077). The 3-year OS rates were 25% and 28%, respectively.
The median OS in the PD-L1-positive population was 17.9 months in the placebo arm and 25.4 months in the atezolizumab arm (HR, 0.67). The 3-year OS rates were 22% and 36%, respectively.
A P value is not available for the between-arm OS comparison in the PD-L1-positive population. OS was not formally tested in this group because the OS boundary for statistical significance was not crossed in the ITT population. However, Dr. Emens said there was a “clinically meaningful” OS benefit observed with atezolizumab in the PD-L1-positive patients.
Treatment withdrawals caused by adverse events were more common in the atezolizumab arm (19% vs. 8%). The most common of these was neuropathy, Dr. Emens said. However, she noted that atezolizumab-related adverse events were generally low grade and easily managed.
“These results support a positive benefit-risk profile for atezolizumab plus nab-paclitaxel as first-line therapy in patients with PD-L1-positive metastatic triple-negative breast cancer,” Dr. Emens concluded.
Both studies were funded by F. Hoffman–La Roche. Dr. Miles, Dr. Emens, and Dr. Carey disclosed financial relationships with Roche and other companies.
SOURCES: Miles D et al. ESMO 2020, Abstract LBA15; Emens LA et al. ESMO 2020, Abstract LBA16.
FROM ESMO 2020
Global stomach cancer deaths decline as colorectal cancer deaths stagnate, rise
The data suggest fewer people are dying from stomach cancer, but in some countries, the risk of colorectal cancer death is increasing or declining much more slowly than other causes of premature death.
As for other cancers, in more than half of the countries analyzed, the risk of liver and prostate cancer death is on the rise in men, and the risk of lung cancer death is on the rise in women.
The global decrease in the risk of stomach cancer death may be explained by the fact that stomach cancer’s main cause is Helicobacter pylori infection, which correlates with general food hygiene, the study’s corresponding author Majid Ezzati, PhD, professor of global environmental health at Imperial College London, said in an interview.
“Factors such as more widespread electrification and refrigeration tend to drive the rates down,” he explained.
Dr. Ezzati and colleagues detailed their findings in the second edition of the NCD Countdown 2030 report, recently published in The Lancet.
The report revolves around the Sustainable Development Goal (SDG) target 3.4, which is to reduce premature deaths from NCDs by one-third between 2015 and 2030. The causes of death include cancer, cardiovascular disease, chronic respiratory disease, and diabetes, which are collectively known as NCD4. “Premature” deaths are defined as deaths in people aged 30-70 years.
SDG target 3.4 is still attainable, according to Dr. Ezzati and colleagues. However, their report showed that many countries are falling short of this goal.
The findings come from an analysis of 2016 World Health Organization global estimate data on age-, sex-, and cause-specific mortality for 176 countries and territories with at least 200,000 inhabitants. Mathematical modeling was used to assess the number of approaches countries used to accelerate declines in mortality.
Results of the analysis
“Trends in the risk of death from 2010 to 2016 varied considerably among NCD4 causes of death,” Dr. Ezzati and colleagues wrote.
Stomach cancer, ischemic and hemorrhagic stroke, ischemic heart disease, and chronic respiratory diseases had the fastest rates of decline among risks of premature death.
In fact, stomach cancer was the fastest declining cause of death in 45 countries (25.6%) among men and in 40 countries (22.7%) among women.
On the other hand, the risk of premature death from colorectal, liver, breast, prostate, and other cancers declined more slowly than the risk of premature death from other NCDs.
The risk of death from colorectal, liver, and prostate cancers in men and lung cancer in women rose in more than 50% of the countries surveyed.
“The median annual rate of change in the probability of dying prematurely from various causes ranged from +0.2% per year for lung cancer to –2.5% per year for hemorrhagic stroke in women, and from +0.5% per year for colorectal cancer to –1.8% per year for hemorrhagic stroke in men,” the investigators summarized.
Explaining the GI cancer results
“There are dramatic differences between the upper and lower GI tract, both in terms of anatomy/embryologic origin but also in terms of exposures,” observed Mark Lewis, MD, medical director of the gastrointestinal oncology program at Intermountain Healthcare in Salt Lake City, in an interview.
H. pylori infection, family history, and diet factor into stomach cancer risk, Dr. Lewis said.
While family history isn’t modifiable, “we are much better now at identifying and eradicating the potentially carcinogenic H. pylori bacterium. In terms of diet, the advent of modern refrigeration has made the prevalence of heavily salted/preserved foods decline,” he added.
A 14-day course of treatment (with a proton pump inhibitor and antibiotics) can eliminate H. pylori, Dr. Lewis continued. “The prophylactic effect against gastric cancer is massive, cutting risk by roughly half,” he said.
At least in the United States, colorectal cancer rates have declined in people 50 years and older, but rates have risen sharply in younger age groups, increasing by 2% annually in the last decade, according to statistics in CA: A Cancer Journal for Clinicians.
“One prevailing theory is prior antibiotic prescriptions [even in childhood] might perturb the microbiome of the lower GI tract and predispose to cancer,” Dr. Lewis said, pointing to a recent study in the British Journal of Cancer that identified an association between repeated antibiotic use and colorectal cancer.
Reducing NCD deaths
Dr. Ezzati and colleagues said six high-income countries – Denmark, Luxembourg, New Zealand, Norway, Singapore, and South Korea – are likely to meet SDG target 3.4 if they maintain or exceed average rates of decline seen during 2010-2016. Seventeen countries are on track to reach the target for women, and 15 countries are on track for men.
High-income countries in Asia-Pacific, western Europe, Australasia, and Canada have seen the lowest NCD4 mortality risk, whereas low- and middle-income countries in sub-Saharan Africa and men in central Asia and eastern Europe have seen the highest risk.
“To move forward, we must learn from those countries that are doing well and replicate their strategies to NCD prevention and healthcare,” Dr. Ezzati said in a statement. “Our analysis shows that every country still has options to achieve SDG target 3.4, but they need to address multiple diseases and have strong health systems.”
Increasing access to effective cancer screening and diagnosing and treating cancers earlier could help reduce long-term health consequences and premature deaths from cancer, according to Dr. Ezzati and colleagues. Screening would help even the playing field on cancer diagnosis and survival rates between higher-income countries and low- and middle-income countries.
“This approach will allow earlier diagnosis during precancerous or early stages of disease, followed by treatment of those cancers with effective treatment,” the authors stated.
Tobacco and alcohol interventions and increasing access to quality primary care would also help tamp down on NCD-related deaths.
The authors acknowledged that low-income countries, which may be struggling with other health crises such as COVID-19 and Ebola, may find it a challenge to stage such interventions.
“COVID-19 has exposed how a failure to invest in effective public health to prevent NCDs and provide health care for people living with NCDs can come back to bite us,” said Katie Dain, CEO of the NCD Alliance.
“The good news is that all countries can still meet the 2030 targets, with sound policies and smart investments. NCD prevention and treatment can no longer be seen a ‘nice to have.’ It must be considered as part of pandemic preparedness,” she added.
COVID-19 should serve as an impetus for governments to invest in healthier lifestyle and diet habits and curb alcohol and tobacco use, according to an editorial in The Lancet related to the analysis.
The current report updates 2018’s first NCD Countdown Report, which linked NCD4 conditions to approximately 32 million or 80% of NCD deaths. Unlike the recent report, 2018’s data didn’t focus on specific diseases.
The current report was funded by Research England. Dr. Ezzati received a charitable grant from the AstraZeneca Young Health Programme and personal fees from Prudential and Scor, outside of this report. None of the other authors reported competing interests. Dr. Lewis has no relevant disclosures except that he is a commentator for Medscape, which is owned by the same parent company as MDedge.
SOURCE: Bennett JE et al. Lancet. 2020 Sep 3. doi: 10.1016/S0140-6736(20)31761-X.
The data suggest fewer people are dying from stomach cancer, but in some countries, the risk of colorectal cancer death is increasing or declining much more slowly than other causes of premature death.
As for other cancers, in more than half of the countries analyzed, the risk of liver and prostate cancer death is on the rise in men, and the risk of lung cancer death is on the rise in women.
The global decrease in the risk of stomach cancer death may be explained by the fact that stomach cancer’s main cause is Helicobacter pylori infection, which correlates with general food hygiene, the study’s corresponding author Majid Ezzati, PhD, professor of global environmental health at Imperial College London, said in an interview.
“Factors such as more widespread electrification and refrigeration tend to drive the rates down,” he explained.
Dr. Ezzati and colleagues detailed their findings in the second edition of the NCD Countdown 2030 report, recently published in The Lancet.
The report revolves around the Sustainable Development Goal (SDG) target 3.4, which is to reduce premature deaths from NCDs by one-third between 2015 and 2030. The causes of death include cancer, cardiovascular disease, chronic respiratory disease, and diabetes, which are collectively known as NCD4. “Premature” deaths are defined as deaths in people aged 30-70 years.
SDG target 3.4 is still attainable, according to Dr. Ezzati and colleagues. However, their report showed that many countries are falling short of this goal.
The findings come from an analysis of 2016 World Health Organization global estimate data on age-, sex-, and cause-specific mortality for 176 countries and territories with at least 200,000 inhabitants. Mathematical modeling was used to assess the number of approaches countries used to accelerate declines in mortality.
Results of the analysis
“Trends in the risk of death from 2010 to 2016 varied considerably among NCD4 causes of death,” Dr. Ezzati and colleagues wrote.
Stomach cancer, ischemic and hemorrhagic stroke, ischemic heart disease, and chronic respiratory diseases had the fastest rates of decline among risks of premature death.
In fact, stomach cancer was the fastest declining cause of death in 45 countries (25.6%) among men and in 40 countries (22.7%) among women.
On the other hand, the risk of premature death from colorectal, liver, breast, prostate, and other cancers declined more slowly than the risk of premature death from other NCDs.
The risk of death from colorectal, liver, and prostate cancers in men and lung cancer in women rose in more than 50% of the countries surveyed.
“The median annual rate of change in the probability of dying prematurely from various causes ranged from +0.2% per year for lung cancer to –2.5% per year for hemorrhagic stroke in women, and from +0.5% per year for colorectal cancer to –1.8% per year for hemorrhagic stroke in men,” the investigators summarized.
Explaining the GI cancer results
“There are dramatic differences between the upper and lower GI tract, both in terms of anatomy/embryologic origin but also in terms of exposures,” observed Mark Lewis, MD, medical director of the gastrointestinal oncology program at Intermountain Healthcare in Salt Lake City, in an interview.
H. pylori infection, family history, and diet factor into stomach cancer risk, Dr. Lewis said.
While family history isn’t modifiable, “we are much better now at identifying and eradicating the potentially carcinogenic H. pylori bacterium. In terms of diet, the advent of modern refrigeration has made the prevalence of heavily salted/preserved foods decline,” he added.
A 14-day course of treatment (with a proton pump inhibitor and antibiotics) can eliminate H. pylori, Dr. Lewis continued. “The prophylactic effect against gastric cancer is massive, cutting risk by roughly half,” he said.
At least in the United States, colorectal cancer rates have declined in people 50 years and older, but rates have risen sharply in younger age groups, increasing by 2% annually in the last decade, according to statistics in CA: A Cancer Journal for Clinicians.
“One prevailing theory is prior antibiotic prescriptions [even in childhood] might perturb the microbiome of the lower GI tract and predispose to cancer,” Dr. Lewis said, pointing to a recent study in the British Journal of Cancer that identified an association between repeated antibiotic use and colorectal cancer.
Reducing NCD deaths
Dr. Ezzati and colleagues said six high-income countries – Denmark, Luxembourg, New Zealand, Norway, Singapore, and South Korea – are likely to meet SDG target 3.4 if they maintain or exceed average rates of decline seen during 2010-2016. Seventeen countries are on track to reach the target for women, and 15 countries are on track for men.
High-income countries in Asia-Pacific, western Europe, Australasia, and Canada have seen the lowest NCD4 mortality risk, whereas low- and middle-income countries in sub-Saharan Africa and men in central Asia and eastern Europe have seen the highest risk.
“To move forward, we must learn from those countries that are doing well and replicate their strategies to NCD prevention and healthcare,” Dr. Ezzati said in a statement. “Our analysis shows that every country still has options to achieve SDG target 3.4, but they need to address multiple diseases and have strong health systems.”
Increasing access to effective cancer screening and diagnosing and treating cancers earlier could help reduce long-term health consequences and premature deaths from cancer, according to Dr. Ezzati and colleagues. Screening would help even the playing field on cancer diagnosis and survival rates between higher-income countries and low- and middle-income countries.
“This approach will allow earlier diagnosis during precancerous or early stages of disease, followed by treatment of those cancers with effective treatment,” the authors stated.
Tobacco and alcohol interventions and increasing access to quality primary care would also help tamp down on NCD-related deaths.
The authors acknowledged that low-income countries, which may be struggling with other health crises such as COVID-19 and Ebola, may find it a challenge to stage such interventions.
“COVID-19 has exposed how a failure to invest in effective public health to prevent NCDs and provide health care for people living with NCDs can come back to bite us,” said Katie Dain, CEO of the NCD Alliance.
“The good news is that all countries can still meet the 2030 targets, with sound policies and smart investments. NCD prevention and treatment can no longer be seen a ‘nice to have.’ It must be considered as part of pandemic preparedness,” she added.
COVID-19 should serve as an impetus for governments to invest in healthier lifestyle and diet habits and curb alcohol and tobacco use, according to an editorial in The Lancet related to the analysis.
The current report updates 2018’s first NCD Countdown Report, which linked NCD4 conditions to approximately 32 million or 80% of NCD deaths. Unlike the recent report, 2018’s data didn’t focus on specific diseases.
The current report was funded by Research England. Dr. Ezzati received a charitable grant from the AstraZeneca Young Health Programme and personal fees from Prudential and Scor, outside of this report. None of the other authors reported competing interests. Dr. Lewis has no relevant disclosures except that he is a commentator for Medscape, which is owned by the same parent company as MDedge.
SOURCE: Bennett JE et al. Lancet. 2020 Sep 3. doi: 10.1016/S0140-6736(20)31761-X.
The data suggest fewer people are dying from stomach cancer, but in some countries, the risk of colorectal cancer death is increasing or declining much more slowly than other causes of premature death.
As for other cancers, in more than half of the countries analyzed, the risk of liver and prostate cancer death is on the rise in men, and the risk of lung cancer death is on the rise in women.
The global decrease in the risk of stomach cancer death may be explained by the fact that stomach cancer’s main cause is Helicobacter pylori infection, which correlates with general food hygiene, the study’s corresponding author Majid Ezzati, PhD, professor of global environmental health at Imperial College London, said in an interview.
“Factors such as more widespread electrification and refrigeration tend to drive the rates down,” he explained.
Dr. Ezzati and colleagues detailed their findings in the second edition of the NCD Countdown 2030 report, recently published in The Lancet.
The report revolves around the Sustainable Development Goal (SDG) target 3.4, which is to reduce premature deaths from NCDs by one-third between 2015 and 2030. The causes of death include cancer, cardiovascular disease, chronic respiratory disease, and diabetes, which are collectively known as NCD4. “Premature” deaths are defined as deaths in people aged 30-70 years.
SDG target 3.4 is still attainable, according to Dr. Ezzati and colleagues. However, their report showed that many countries are falling short of this goal.
The findings come from an analysis of 2016 World Health Organization global estimate data on age-, sex-, and cause-specific mortality for 176 countries and territories with at least 200,000 inhabitants. Mathematical modeling was used to assess the number of approaches countries used to accelerate declines in mortality.
Results of the analysis
“Trends in the risk of death from 2010 to 2016 varied considerably among NCD4 causes of death,” Dr. Ezzati and colleagues wrote.
Stomach cancer, ischemic and hemorrhagic stroke, ischemic heart disease, and chronic respiratory diseases had the fastest rates of decline among risks of premature death.
In fact, stomach cancer was the fastest declining cause of death in 45 countries (25.6%) among men and in 40 countries (22.7%) among women.
On the other hand, the risk of premature death from colorectal, liver, breast, prostate, and other cancers declined more slowly than the risk of premature death from other NCDs.
The risk of death from colorectal, liver, and prostate cancers in men and lung cancer in women rose in more than 50% of the countries surveyed.
“The median annual rate of change in the probability of dying prematurely from various causes ranged from +0.2% per year for lung cancer to –2.5% per year for hemorrhagic stroke in women, and from +0.5% per year for colorectal cancer to –1.8% per year for hemorrhagic stroke in men,” the investigators summarized.
Explaining the GI cancer results
“There are dramatic differences between the upper and lower GI tract, both in terms of anatomy/embryologic origin but also in terms of exposures,” observed Mark Lewis, MD, medical director of the gastrointestinal oncology program at Intermountain Healthcare in Salt Lake City, in an interview.
H. pylori infection, family history, and diet factor into stomach cancer risk, Dr. Lewis said.
While family history isn’t modifiable, “we are much better now at identifying and eradicating the potentially carcinogenic H. pylori bacterium. In terms of diet, the advent of modern refrigeration has made the prevalence of heavily salted/preserved foods decline,” he added.
A 14-day course of treatment (with a proton pump inhibitor and antibiotics) can eliminate H. pylori, Dr. Lewis continued. “The prophylactic effect against gastric cancer is massive, cutting risk by roughly half,” he said.
At least in the United States, colorectal cancer rates have declined in people 50 years and older, but rates have risen sharply in younger age groups, increasing by 2% annually in the last decade, according to statistics in CA: A Cancer Journal for Clinicians.
“One prevailing theory is prior antibiotic prescriptions [even in childhood] might perturb the microbiome of the lower GI tract and predispose to cancer,” Dr. Lewis said, pointing to a recent study in the British Journal of Cancer that identified an association between repeated antibiotic use and colorectal cancer.
Reducing NCD deaths
Dr. Ezzati and colleagues said six high-income countries – Denmark, Luxembourg, New Zealand, Norway, Singapore, and South Korea – are likely to meet SDG target 3.4 if they maintain or exceed average rates of decline seen during 2010-2016. Seventeen countries are on track to reach the target for women, and 15 countries are on track for men.
High-income countries in Asia-Pacific, western Europe, Australasia, and Canada have seen the lowest NCD4 mortality risk, whereas low- and middle-income countries in sub-Saharan Africa and men in central Asia and eastern Europe have seen the highest risk.
“To move forward, we must learn from those countries that are doing well and replicate their strategies to NCD prevention and healthcare,” Dr. Ezzati said in a statement. “Our analysis shows that every country still has options to achieve SDG target 3.4, but they need to address multiple diseases and have strong health systems.”
Increasing access to effective cancer screening and diagnosing and treating cancers earlier could help reduce long-term health consequences and premature deaths from cancer, according to Dr. Ezzati and colleagues. Screening would help even the playing field on cancer diagnosis and survival rates between higher-income countries and low- and middle-income countries.
“This approach will allow earlier diagnosis during precancerous or early stages of disease, followed by treatment of those cancers with effective treatment,” the authors stated.
Tobacco and alcohol interventions and increasing access to quality primary care would also help tamp down on NCD-related deaths.
The authors acknowledged that low-income countries, which may be struggling with other health crises such as COVID-19 and Ebola, may find it a challenge to stage such interventions.
“COVID-19 has exposed how a failure to invest in effective public health to prevent NCDs and provide health care for people living with NCDs can come back to bite us,” said Katie Dain, CEO of the NCD Alliance.
“The good news is that all countries can still meet the 2030 targets, with sound policies and smart investments. NCD prevention and treatment can no longer be seen a ‘nice to have.’ It must be considered as part of pandemic preparedness,” she added.
COVID-19 should serve as an impetus for governments to invest in healthier lifestyle and diet habits and curb alcohol and tobacco use, according to an editorial in The Lancet related to the analysis.
The current report updates 2018’s first NCD Countdown Report, which linked NCD4 conditions to approximately 32 million or 80% of NCD deaths. Unlike the recent report, 2018’s data didn’t focus on specific diseases.
The current report was funded by Research England. Dr. Ezzati received a charitable grant from the AstraZeneca Young Health Programme and personal fees from Prudential and Scor, outside of this report. None of the other authors reported competing interests. Dr. Lewis has no relevant disclosures except that he is a commentator for Medscape, which is owned by the same parent company as MDedge.
SOURCE: Bennett JE et al. Lancet. 2020 Sep 3. doi: 10.1016/S0140-6736(20)31761-X.
FROM THE LANCET