User login
Neurosurgery Operating Room Efficiency During the COVID-19 Era
From the Department of Neurological Surgery, Vanderbilt University Medical Center, Nashville, TN (Stefan W. Koester, Puja Jagasia, and Drs. Liles, Dambrino IV, Feldman, and Chambless), and the Department of Anesthesiology, Vanderbilt University Medical Center, Nashville, TN (Drs. Mathews and Tiwari).
ABSTRACT
Background: The COVID-19 pandemic has had broad effects on surgical care, including operating room (OR) staffing, personal protective equipment (PPE) utilization, and newly implemented anti-infective measures. Our aim was to assess neurosurgery OR efficiency before the COVID-19 pandemic, during peak COVID-19, and during current times.
Methods: Institutional perioperative databases at a single, high-volume neurosurgical center were queried for operations performed from December 2019 until October 2021. March 12, 2020, the day that the state of Tennessee declared a state of emergency, was chosen as the onset of the COVID-19 pandemic. The 90-day periods before and after this day were used to define the pre-COVID-19, peak-COVID-19, and post-peak restrictions time periods for comparative analysis. Outcomes included delay in first-start and OR turnover time between neurosurgical cases. Preset threshold times were used in analyses to adjust for normal leniency in OR scheduling (15 minutes for first start and 90 minutes for turnover). Univariate analysis used Wilcoxon rank-sum test for continuous outcomes, while chi-square test and Fisher’s exact test were used for categorical comparisons. Significance was defined as P < .05.
Results: First-start time was analyzed in 426 pre-COVID-19, 357 peak-restrictions, and 2304 post-peak-restrictions cases. The unadjusted mean delay length was found to be significantly different between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes, 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004). The adjusted average delay length and proportion of cases delayed beyond the 15-minute threshold were not significantly different. The proportion of cases that started early, as well as significantly early past a 15-minute threshold, have not been impacted. There was no significant change in turnover time during peak restrictions relative to the pre-COVID-19 period (88 [100] minutes vs 85 [95] minutes), and turnover time has since remained unchanged (83 [87] minutes).
Conclusion: Our center was able to maintain OR efficiency before, during, and after peak restrictions even while instituting advanced infection-control strategies. While there were significant changes, delays were relatively small in magnitude.
Keywords: operating room timing, hospital efficiency, socioeconomics, pandemic.
The COVID-19 pandemic has led to major changes in patient care both from a surgical perspective and in regard to inpatient hospital course. Safety protocols nationwide have been implemented to protect both patients and providers. Some elements of surgical care have drastically changed, including operating room (OR) staffing, personal protective equipment (PPE) utilization, and increased sterilization measures. Furloughs, layoffs, and reassignments due to the focus on nonelective and COVID-19–related cases challenged OR staffing and efficiency. Operating room staff with COVID-19 exposures or COVID-19 infections also caused last-minute changes in staffing. All of these scenarios can cause issues due to actual understaffing or due to staff members being pushed into highly specialized areas, such as neurosurgery, in which they have very little experience. A further obstacle to OR efficiency included policy changes involving PPE utilization, sterilization measures, and supply chain shortages of necessary resources such as PPE.
Neurosurgery in particular has been susceptible to COVID-19–related system-wide changes given operator proximity to the patient’s respiratory passages, frequency of emergent cases, and varying anesthetic needs, as well as the high level of specialization needed to perform neurosurgical care. Previous studies have shown a change in the makeup of neurosurgical patients seeking care, as well as in the acuity of neurological consult of these patients.1 A study in orthopedic surgery by Andreata et al demonstrated worsened OR efficiency, with significantly increased first-start and turnover times.2 In the COVID-19 era, OR quality and safety are crucially important to both patients and providers. Providing this safe and effective care in an efficient manner is important for optimal neurosurgical management in the long term.3 Moreover, the financial burden of implementing new protocols and standards can be compounded by additional financial losses due to reduced OR efficiency.
Methods
To analyze the effect of COVID-19 on neurosurgical OR efficiency, institutional perioperative databases at a single high-volume center were queried for operations performed from December 2019 until October 2021. March 12, 2020, was chosen as the onset of COVID-19 for analytic purposes, as this was the date when the state of Tennessee declared a state of emergency. The 90-day periods before and after this date were used for comparative analysis for pre-COVID-19, peak COVID-19, and post-peak-restrictions time periods. The peak COVID-19 period was defined as the 90-day period following the initial onset of COVID-19 and the surge of cases. For comparison purposes, post-peak COVID-19 was defined as the months following the first peak until October 2021 (approximately 17 months). COVID-19 burden was determined using a COVID-19 single-institution census of confirmed cases by polymerase chain reaction (PCR) for which the average number of cases of COVID-19 during a given month was determined. This number is a scaled trend, and a true number of COVID-19 cases in our hospital was not reported.
Neurosurgical and neuroendovascular cases were included in the analysis. Outcomes included delay in first-start and OR turnover time between neurosurgical cases, defined as the time from the patient leaving the room until the next patient entered the room. Preset threshold times were used in analyses to adjust for normal leniency in OR scheduling (15 minutes for first start and 90 minutes for turnover, which is a standard for our single-institution perioperative center). Statistical analyses, including data aggregation, were performed using R, version 4.0.1 (R Foundation for Statistical Computing). Patients’ demographic and clinical characteristics were analyzed using an independent 2-sample t-test for interval variables and a chi-square test for categorical variables. Significance was defined as P < .05.
Results
First-Start Time
First-start time was analyzed in 426 pre-COVID-19, 357 peak-COVID-19, and 2304 post-peak-COVID-19 cases. The unadjusted mean delay length was significantly different between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes, 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004) (Table 1). 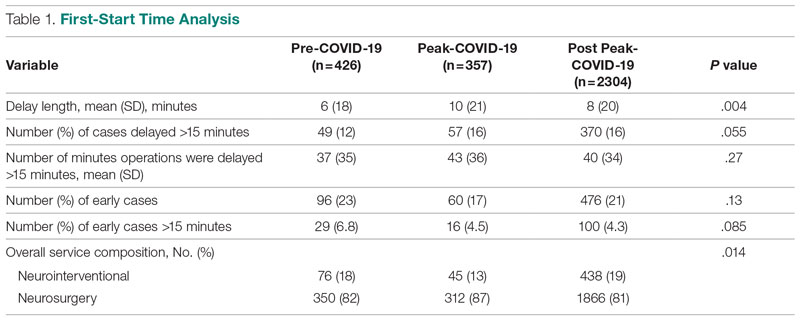
The adjusted average delay length and proportion of cases delayed beyond the 15-minute threshold were not significantly different, but they have been slightly higher since the onset of COVID-19. The proportion of cases that have started early, as well as significantly early past a 15-minute threshold, have also trended down since the onset of the COVID-19 pandemic, but this difference was again not significant. The temporal relationship of first-start delay, both unadjusted and adjusted, from December 2019 to October 2021 is shown in Figure 1. The trend of increasing delay is loosely associated with the COVID-19 burden experienced by our hospital. The start of COVID-19 as well as both COVID-19 peaks have been associated with increased delays in our hospital.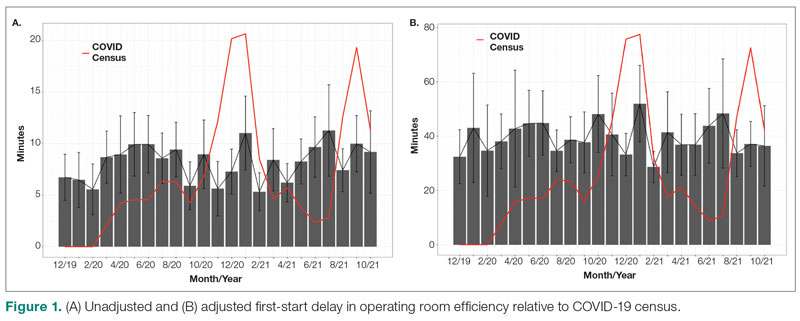
Turnover Time
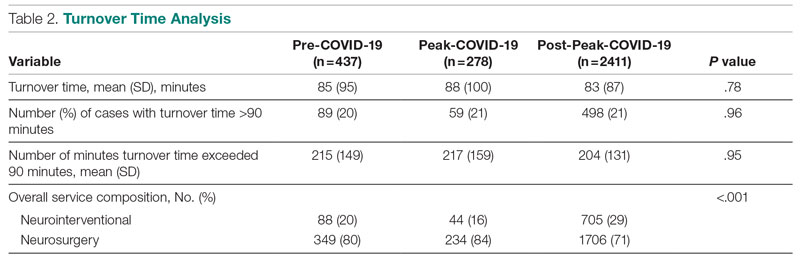
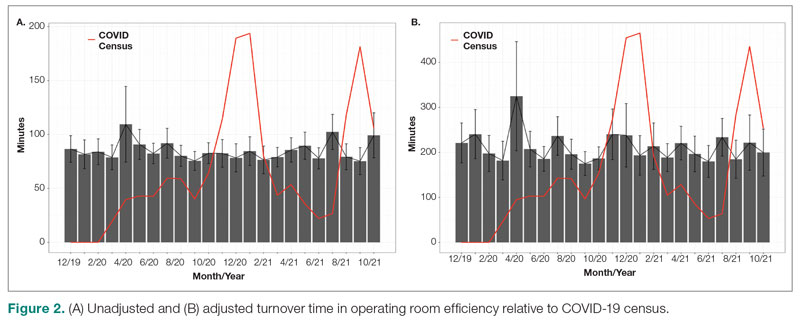
Turnover time was assessed in 437 pre-COVID-19, 278 peak-restrictions, and 2411 post-peak-restrictions cases. Turnover time during peak restrictions was not significantly different from pre-COVID-19 (88 [100] vs 85 [95]) and has since remained relatively unchanged (83 [87], P = .78). A similar trend held for comparisons of proportion of cases with turnover time past 90 minutes and average times past the 90-minute threshold (Table 2). The temporal relationship between COVID-19 burden and turnover time, both unadjusted and adjusted, from December 2019 to October 2021 is shown in Figure 2. Both figures demonstrate a slight initial increase in turnover time delay at the start of COVID-19, which stabilized with little variation thereafter.
Discussion
We analyzed the OR efficiency metrics of first-start and turnover time during the 90-day period before COVID-19 (pre-COVID-19), the 90 days following Tennessee declaring a state of emergency (peak COVID-19), and the time following this period (post-COVID-19) for all neurosurgical and neuroendovascular cases at Vanderbilt University Medical Center (VUMC). We found a significant difference in unadjusted mean delay length in first-start time between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes for pre-COVID-19, peak-COVID-19, and post-COVID-19: 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004). No significant increase in turnover time between cases was found between these 3 time periods. Based on metrics from first-start delay and turnover time, our center was able to maintain OR efficiency before, during, and after peak COVID-19.
After the Centers for Disease Control and Prevention released guidelines recommending deferring elective procedures to conserve beds and PPE, VUMC made the decision to suspend all elective surgical procedures from March 18 to April 24, 2020. Prior research conducted during the COVID-19 pandemic has demonstrated more than 400 types of surgical procedures with negatively impacted outcomes when compared to surgical outcomes from the same time frame in 2018 and 2019.4 For more than 20 of these types of procedures, there was a significant association between procedure delay and adverse patient outcomes.4 Testing protocols for patients prior to surgery varied throughout the pandemic based on vaccination status and type of procedure. Before vaccines became widely available, all patients were required to obtain a PCR SARS-CoV-2 test within 48 to 72 hours of the scheduled procedure. If the patient’s procedure was urgent and testing was not feasible, the patient was treated as a SARS-CoV-2–positive patient, which required all health care workers involved in the case to wear gowns, gloves, surgical masks, and eye protection. Testing patients preoperatively likely helped to maintain OR efficiency since not all patients received test results prior to the scheduled procedure, leading to cancellations of cases and therefore more staff available for fewer cases.
After vaccines became widely available to the public, testing requirements for patients preoperatively were relaxed, and only patients who were not fully vaccinated or severely immunocompromised were required to test prior to procedures. However, approximately 40% of the population in Tennessee was fully vaccinated in 2021, which reflects the patient population of VUMC.5 This means that many patients who received care at VUMC were still tested prior to procedures.
Adopting adequate safety protocols was found to be key for OR efficiency during the COVID-19 pandemic since performing surgery increased the risk of infection for each health care worker in the OR.6 VUMC protocols identified procedures that required enhanced safety measures to prevent infection of health care workers and avoid staffing shortages, which would decrease OR efficiency. Protocols mandated that only anesthesia team members were allowed to be in the OR during intubation and extubation of patients, which could be one factor leading to increased delays and decreased efficiency for some institutions. Methods for neurosurgeons to decrease risk of infection in the OR include postponing all nonurgent cases, reappraising the necessity for general anesthesia and endotracheal intubation, considering alternative surgical approaches that avoid the respiratory tract, and limiting the use of aerosol-generating instruments.7,8 VUMC’s success in implementing these protocols likely explains why our center was able to maintain OR efficiency throughout the COVID-19 pandemic.
A study conducted by Andreata et al showed a significantly increased mean first-case delay and a nonsignificant increased turnover time in orthopedic surgeries in Northern Italy when comparing surgeries performed during the COVID-19 pandemic to those performed prior to COVID-19.2 Other studies have indicated a similar trend in decreased OR efficiency during COVID-19 in other areas around the world.9,10 These findings are not consistent with our own findings for neurosurgical and neuroendovascular surgeries at VUMC, and any change at our institution was relatively immaterial. Factors that threatened to change OR efficiency—but did not result in meaningful changes in our institutional experience—include delays due to pending COVID-19 test results, safety procedures such as PPE donning, and planning difficulties to ensure the existence of teams with non-overlapping providers in the case of a surgeon being infected.2,11-13
Globally, many surgery centers halted all elective surgeries during the initial COVID-19 spike to prevent a PPE shortage and mitigate risk of infection of patients and health care workers.8,12,14 However, there is no centralized definition of which neurosurgical procedures are elective, so that decision was made on a surgeon or center level, which could lead to variability in efficiency trends.14 One study on neurosurgical procedures during COVID-19 found a 30% decline in all cases and a 23% decline in emergent procedures, showing that the decrease in volume was not only due to cancellation of elective procedures.15 This decrease in elective and emergent surgeries created a backlog of surgeries as well as a loss in health care revenue, and caused many patients to go without adequate health care.10 Looking forward, it is imperative that surgical centers study trends in OR efficiency from COVID-19 and learn how to better maintain OR efficiency during future pandemic conditions to prevent a backlog of cases, loss of health care revenue, and decreased health care access.
Limitations
Our data are from a single center and therefore may not be representative of experiences of other hospitals due to different populations and different impacts from COVID-19. However, given our center’s high volume and diverse patient population, we believe our analysis highlights important trends in neurosurgery practice. Notably, data for patient and OR timing are digitally generated and are entered manually by nurses in the electronic medical record, making it prone to errors and variability. This is in our experience, and if any error is present, we believe it is minimal.
Conclusion
The COVID-19 pandemic has had far-reaching effects on health care worldwide, including neurosurgical care. OR efficiency across the United States generally worsened given the stresses of supply chain issues, staffing shortages, and cancellations. At our institution, we were able to maintain OR efficiency during the known COVID-19 peaks until October 2021. Continually functional neurosurgical ORs are important in preventing delays in care and maintaining a steady revenue in order for hospitals and other health care entities to remain solvent. Further study of OR efficiency is needed for health care systems to prepare for future pandemics and other resource-straining events in order to provide optimal patient care.
Corresponding author: Campbell Liles, MD, Vanderbilt University Medical Center, Department of Neurological Surgery, 1161 21st Ave. South, T4224 Medical Center North, Nashville, TN 37232-2380; [email protected]
Disclosures: None reported.
1. Koester SW, Catapano JS, Ma KL, et al. COVID-19 and neurosurgery consultation call volume at a single large tertiary center with a propensity- adjusted analysis. World Neurosurg. 2021;146:e768-e772. doi:10.1016/j.wneu.2020.11.017
2. Andreata M, Faraldi M, Bucci E, Lombardi G, Zagra L. Operating room efficiency and timing during coronavirus disease 2019 outbreak in a referral orthopaedic hospital in Northern Italy. Int Orthop. 2020;44(12):2499-2504. doi:10.1007/s00264-020-04772-x
3. Dexter F, Abouleish AE, Epstein RH, et al. Use of operating room information system data to predict the impact of reducing turnover times on staffing costs. Anesth Analg. 2003;97(4):1119-1126. doi:10.1213/01.ANE.0000082520.68800.79
4. Zheng NS, Warner JL, Osterman TJ, et al. A retrospective approach to evaluating potential adverse outcomes associated with delay of procedures for cardiovascular and cancer-related diagnoses in the context of COVID-19. J Biomed Inform. 2021;113:103657. doi:10.1016/j.jbi.2020.103657
5. Alcendor DJ. Targeting COVID-19 vaccine hesitancy in rural communities in Tennessee: implications for extending the COVID- 19 pandemic in the South. Vaccines (Basel). 2021;9(11):1279. doi:10.3390/vaccines9111279
6. Perrone G, Giuffrida M, Bellini V, et al. Operating room setup: how to improve health care professionals safety during pandemic COVID- 19: a quality improvement study. J Laparoendosc Adv Surg Tech A. 2021;31(1):85-89. doi:10.1089/lap.2020.0592
7. Iorio-Morin C, Hodaie M, Sarica C, et al. Letter: the risk of COVID-19 infection during neurosurgical procedures: a review of severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) modes of transmission and proposed neurosurgery-specific measures for mitigation. Neurosurgery. 2020;87(2):E178-E185. doi:10.1093/ neuros/nyaa157
8. Gupta P, Muthukumar N, Rajshekhar V, et al. Neurosurgery and neurology practices during the novel COVID-19 pandemic: a consensus statement from India. Neurol India. 2020;68(2):246-254. doi:10.4103/0028-3886.283130
9. Mercer ST, Agarwal R, Dayananda KSS, et al. A comparative study looking at trauma and orthopaedic operating efficiency in the COVID-19 era. Perioper Care Oper Room Manag. 2020;21:100142. doi:10.1016/j.pcorm.2020.100142
10. Rozario N, Rozario D. Can machine learning optimize the efficiency of the operating room in the era of COVID-19? Can J Surg. 2020;63(6):E527-E529. doi:10.1503/cjs.016520
11. Toh KHQ, Barazanchi A, Rajaretnam NS, et al. COVID-19 response by New Zealand general surgical departments in tertiary metropolitan hospitals. ANZ J Surg. 2021;91(7-8):1352-1357. doi:10.1111/ ans.17044
12. Moorthy RK, Rajshekhar V. Impact of COVID-19 pandemic on neurosurgical practice in India: a survey on personal protective equipment usage, testing, and perceptions on disease transmission. Neurol India. 2020;68(5):1133-1138. doi:10.4103/0028- 3886.299173
13. Meneghini RM. Techniques and strategies to optimize efficiencies in the office and operating room: getting through the patient backlog and preserving hospital resources. J Arthroplasty. 2021;36(7S):S49-S51. doi:10.1016/j.arth.2021.03.010
14. Jean WC, Ironside NT, Sack KD, et al. The impact of COVID- 19 on neurosurgeons and the strategy for triaging non-emergent operations: a global neurosurgery study. Acta Neurochir (Wien). 2020;162(6):1229-1240. doi:10.1007/s00701-020- 04342-5
15. Raneri F, Rustemi O, Zambon G, et al. Neurosurgery in times of a pandemic: a survey of neurosurgical services during the COVID-19 outbreak in the Veneto region in Italy. Neurosurg Focus. 2020;49(6):E9. doi:10.3171/2020.9.FOCUS20691
From the Department of Neurological Surgery, Vanderbilt University Medical Center, Nashville, TN (Stefan W. Koester, Puja Jagasia, and Drs. Liles, Dambrino IV, Feldman, and Chambless), and the Department of Anesthesiology, Vanderbilt University Medical Center, Nashville, TN (Drs. Mathews and Tiwari).
ABSTRACT
Background: The COVID-19 pandemic has had broad effects on surgical care, including operating room (OR) staffing, personal protective equipment (PPE) utilization, and newly implemented anti-infective measures. Our aim was to assess neurosurgery OR efficiency before the COVID-19 pandemic, during peak COVID-19, and during current times.
Methods: Institutional perioperative databases at a single, high-volume neurosurgical center were queried for operations performed from December 2019 until October 2021. March 12, 2020, the day that the state of Tennessee declared a state of emergency, was chosen as the onset of the COVID-19 pandemic. The 90-day periods before and after this day were used to define the pre-COVID-19, peak-COVID-19, and post-peak restrictions time periods for comparative analysis. Outcomes included delay in first-start and OR turnover time between neurosurgical cases. Preset threshold times were used in analyses to adjust for normal leniency in OR scheduling (15 minutes for first start and 90 minutes for turnover). Univariate analysis used Wilcoxon rank-sum test for continuous outcomes, while chi-square test and Fisher’s exact test were used for categorical comparisons. Significance was defined as P < .05.
Results: First-start time was analyzed in 426 pre-COVID-19, 357 peak-restrictions, and 2304 post-peak-restrictions cases. The unadjusted mean delay length was found to be significantly different between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes, 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004). The adjusted average delay length and proportion of cases delayed beyond the 15-minute threshold were not significantly different. The proportion of cases that started early, as well as significantly early past a 15-minute threshold, have not been impacted. There was no significant change in turnover time during peak restrictions relative to the pre-COVID-19 period (88 [100] minutes vs 85 [95] minutes), and turnover time has since remained unchanged (83 [87] minutes).
Conclusion: Our center was able to maintain OR efficiency before, during, and after peak restrictions even while instituting advanced infection-control strategies. While there were significant changes, delays were relatively small in magnitude.
Keywords: operating room timing, hospital efficiency, socioeconomics, pandemic.
The COVID-19 pandemic has led to major changes in patient care both from a surgical perspective and in regard to inpatient hospital course. Safety protocols nationwide have been implemented to protect both patients and providers. Some elements of surgical care have drastically changed, including operating room (OR) staffing, personal protective equipment (PPE) utilization, and increased sterilization measures. Furloughs, layoffs, and reassignments due to the focus on nonelective and COVID-19–related cases challenged OR staffing and efficiency. Operating room staff with COVID-19 exposures or COVID-19 infections also caused last-minute changes in staffing. All of these scenarios can cause issues due to actual understaffing or due to staff members being pushed into highly specialized areas, such as neurosurgery, in which they have very little experience. A further obstacle to OR efficiency included policy changes involving PPE utilization, sterilization measures, and supply chain shortages of necessary resources such as PPE.
Neurosurgery in particular has been susceptible to COVID-19–related system-wide changes given operator proximity to the patient’s respiratory passages, frequency of emergent cases, and varying anesthetic needs, as well as the high level of specialization needed to perform neurosurgical care. Previous studies have shown a change in the makeup of neurosurgical patients seeking care, as well as in the acuity of neurological consult of these patients.1 A study in orthopedic surgery by Andreata et al demonstrated worsened OR efficiency, with significantly increased first-start and turnover times.2 In the COVID-19 era, OR quality and safety are crucially important to both patients and providers. Providing this safe and effective care in an efficient manner is important for optimal neurosurgical management in the long term.3 Moreover, the financial burden of implementing new protocols and standards can be compounded by additional financial losses due to reduced OR efficiency.
Methods
To analyze the effect of COVID-19 on neurosurgical OR efficiency, institutional perioperative databases at a single high-volume center were queried for operations performed from December 2019 until October 2021. March 12, 2020, was chosen as the onset of COVID-19 for analytic purposes, as this was the date when the state of Tennessee declared a state of emergency. The 90-day periods before and after this date were used for comparative analysis for pre-COVID-19, peak COVID-19, and post-peak-restrictions time periods. The peak COVID-19 period was defined as the 90-day period following the initial onset of COVID-19 and the surge of cases. For comparison purposes, post-peak COVID-19 was defined as the months following the first peak until October 2021 (approximately 17 months). COVID-19 burden was determined using a COVID-19 single-institution census of confirmed cases by polymerase chain reaction (PCR) for which the average number of cases of COVID-19 during a given month was determined. This number is a scaled trend, and a true number of COVID-19 cases in our hospital was not reported.
Neurosurgical and neuroendovascular cases were included in the analysis. Outcomes included delay in first-start and OR turnover time between neurosurgical cases, defined as the time from the patient leaving the room until the next patient entered the room. Preset threshold times were used in analyses to adjust for normal leniency in OR scheduling (15 minutes for first start and 90 minutes for turnover, which is a standard for our single-institution perioperative center). Statistical analyses, including data aggregation, were performed using R, version 4.0.1 (R Foundation for Statistical Computing). Patients’ demographic and clinical characteristics were analyzed using an independent 2-sample t-test for interval variables and a chi-square test for categorical variables. Significance was defined as P < .05.
Results
First-Start Time
First-start time was analyzed in 426 pre-COVID-19, 357 peak-COVID-19, and 2304 post-peak-COVID-19 cases. The unadjusted mean delay length was significantly different between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes, 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004) (Table 1). 
The adjusted average delay length and proportion of cases delayed beyond the 15-minute threshold were not significantly different, but they have been slightly higher since the onset of COVID-19. The proportion of cases that have started early, as well as significantly early past a 15-minute threshold, have also trended down since the onset of the COVID-19 pandemic, but this difference was again not significant. The temporal relationship of first-start delay, both unadjusted and adjusted, from December 2019 to October 2021 is shown in Figure 1. The trend of increasing delay is loosely associated with the COVID-19 burden experienced by our hospital. The start of COVID-19 as well as both COVID-19 peaks have been associated with increased delays in our hospital.
Turnover Time
Turnover time was assessed in 437 pre-COVID-19, 278 peak-restrictions, and 2411 post-peak-restrictions cases. Turnover time during peak restrictions was not significantly different from pre-COVID-19 (88 [100] vs 85 [95]) and has since remained relatively unchanged (83 [87], P = .78). A similar trend held for comparisons of proportion of cases with turnover time past 90 minutes and average times past the 90-minute threshold (Table 2). The temporal relationship between COVID-19 burden and turnover time, both unadjusted and adjusted, from December 2019 to October 2021 is shown in Figure 2. Both figures demonstrate a slight initial increase in turnover time delay at the start of COVID-19, which stabilized with little variation thereafter.


Discussion
We analyzed the OR efficiency metrics of first-start and turnover time during the 90-day period before COVID-19 (pre-COVID-19), the 90 days following Tennessee declaring a state of emergency (peak COVID-19), and the time following this period (post-COVID-19) for all neurosurgical and neuroendovascular cases at Vanderbilt University Medical Center (VUMC). We found a significant difference in unadjusted mean delay length in first-start time between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes for pre-COVID-19, peak-COVID-19, and post-COVID-19: 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004). No significant increase in turnover time between cases was found between these 3 time periods. Based on metrics from first-start delay and turnover time, our center was able to maintain OR efficiency before, during, and after peak COVID-19.
After the Centers for Disease Control and Prevention released guidelines recommending deferring elective procedures to conserve beds and PPE, VUMC made the decision to suspend all elective surgical procedures from March 18 to April 24, 2020. Prior research conducted during the COVID-19 pandemic has demonstrated more than 400 types of surgical procedures with negatively impacted outcomes when compared to surgical outcomes from the same time frame in 2018 and 2019.4 For more than 20 of these types of procedures, there was a significant association between procedure delay and adverse patient outcomes.4 Testing protocols for patients prior to surgery varied throughout the pandemic based on vaccination status and type of procedure. Before vaccines became widely available, all patients were required to obtain a PCR SARS-CoV-2 test within 48 to 72 hours of the scheduled procedure. If the patient’s procedure was urgent and testing was not feasible, the patient was treated as a SARS-CoV-2–positive patient, which required all health care workers involved in the case to wear gowns, gloves, surgical masks, and eye protection. Testing patients preoperatively likely helped to maintain OR efficiency since not all patients received test results prior to the scheduled procedure, leading to cancellations of cases and therefore more staff available for fewer cases.
After vaccines became widely available to the public, testing requirements for patients preoperatively were relaxed, and only patients who were not fully vaccinated or severely immunocompromised were required to test prior to procedures. However, approximately 40% of the population in Tennessee was fully vaccinated in 2021, which reflects the patient population of VUMC.5 This means that many patients who received care at VUMC were still tested prior to procedures.
Adopting adequate safety protocols was found to be key for OR efficiency during the COVID-19 pandemic since performing surgery increased the risk of infection for each health care worker in the OR.6 VUMC protocols identified procedures that required enhanced safety measures to prevent infection of health care workers and avoid staffing shortages, which would decrease OR efficiency. Protocols mandated that only anesthesia team members were allowed to be in the OR during intubation and extubation of patients, which could be one factor leading to increased delays and decreased efficiency for some institutions. Methods for neurosurgeons to decrease risk of infection in the OR include postponing all nonurgent cases, reappraising the necessity for general anesthesia and endotracheal intubation, considering alternative surgical approaches that avoid the respiratory tract, and limiting the use of aerosol-generating instruments.7,8 VUMC’s success in implementing these protocols likely explains why our center was able to maintain OR efficiency throughout the COVID-19 pandemic.
A study conducted by Andreata et al showed a significantly increased mean first-case delay and a nonsignificant increased turnover time in orthopedic surgeries in Northern Italy when comparing surgeries performed during the COVID-19 pandemic to those performed prior to COVID-19.2 Other studies have indicated a similar trend in decreased OR efficiency during COVID-19 in other areas around the world.9,10 These findings are not consistent with our own findings for neurosurgical and neuroendovascular surgeries at VUMC, and any change at our institution was relatively immaterial. Factors that threatened to change OR efficiency—but did not result in meaningful changes in our institutional experience—include delays due to pending COVID-19 test results, safety procedures such as PPE donning, and planning difficulties to ensure the existence of teams with non-overlapping providers in the case of a surgeon being infected.2,11-13
Globally, many surgery centers halted all elective surgeries during the initial COVID-19 spike to prevent a PPE shortage and mitigate risk of infection of patients and health care workers.8,12,14 However, there is no centralized definition of which neurosurgical procedures are elective, so that decision was made on a surgeon or center level, which could lead to variability in efficiency trends.14 One study on neurosurgical procedures during COVID-19 found a 30% decline in all cases and a 23% decline in emergent procedures, showing that the decrease in volume was not only due to cancellation of elective procedures.15 This decrease in elective and emergent surgeries created a backlog of surgeries as well as a loss in health care revenue, and caused many patients to go without adequate health care.10 Looking forward, it is imperative that surgical centers study trends in OR efficiency from COVID-19 and learn how to better maintain OR efficiency during future pandemic conditions to prevent a backlog of cases, loss of health care revenue, and decreased health care access.
Limitations
Our data are from a single center and therefore may not be representative of experiences of other hospitals due to different populations and different impacts from COVID-19. However, given our center’s high volume and diverse patient population, we believe our analysis highlights important trends in neurosurgery practice. Notably, data for patient and OR timing are digitally generated and are entered manually by nurses in the electronic medical record, making it prone to errors and variability. This is in our experience, and if any error is present, we believe it is minimal.
Conclusion
The COVID-19 pandemic has had far-reaching effects on health care worldwide, including neurosurgical care. OR efficiency across the United States generally worsened given the stresses of supply chain issues, staffing shortages, and cancellations. At our institution, we were able to maintain OR efficiency during the known COVID-19 peaks until October 2021. Continually functional neurosurgical ORs are important in preventing delays in care and maintaining a steady revenue in order for hospitals and other health care entities to remain solvent. Further study of OR efficiency is needed for health care systems to prepare for future pandemics and other resource-straining events in order to provide optimal patient care.
Corresponding author: Campbell Liles, MD, Vanderbilt University Medical Center, Department of Neurological Surgery, 1161 21st Ave. South, T4224 Medical Center North, Nashville, TN 37232-2380; [email protected]
Disclosures: None reported.
From the Department of Neurological Surgery, Vanderbilt University Medical Center, Nashville, TN (Stefan W. Koester, Puja Jagasia, and Drs. Liles, Dambrino IV, Feldman, and Chambless), and the Department of Anesthesiology, Vanderbilt University Medical Center, Nashville, TN (Drs. Mathews and Tiwari).
ABSTRACT
Background: The COVID-19 pandemic has had broad effects on surgical care, including operating room (OR) staffing, personal protective equipment (PPE) utilization, and newly implemented anti-infective measures. Our aim was to assess neurosurgery OR efficiency before the COVID-19 pandemic, during peak COVID-19, and during current times.
Methods: Institutional perioperative databases at a single, high-volume neurosurgical center were queried for operations performed from December 2019 until October 2021. March 12, 2020, the day that the state of Tennessee declared a state of emergency, was chosen as the onset of the COVID-19 pandemic. The 90-day periods before and after this day were used to define the pre-COVID-19, peak-COVID-19, and post-peak restrictions time periods for comparative analysis. Outcomes included delay in first-start and OR turnover time between neurosurgical cases. Preset threshold times were used in analyses to adjust for normal leniency in OR scheduling (15 minutes for first start and 90 minutes for turnover). Univariate analysis used Wilcoxon rank-sum test for continuous outcomes, while chi-square test and Fisher’s exact test were used for categorical comparisons. Significance was defined as P < .05.
Results: First-start time was analyzed in 426 pre-COVID-19, 357 peak-restrictions, and 2304 post-peak-restrictions cases. The unadjusted mean delay length was found to be significantly different between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes, 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004). The adjusted average delay length and proportion of cases delayed beyond the 15-minute threshold were not significantly different. The proportion of cases that started early, as well as significantly early past a 15-minute threshold, have not been impacted. There was no significant change in turnover time during peak restrictions relative to the pre-COVID-19 period (88 [100] minutes vs 85 [95] minutes), and turnover time has since remained unchanged (83 [87] minutes).
Conclusion: Our center was able to maintain OR efficiency before, during, and after peak restrictions even while instituting advanced infection-control strategies. While there were significant changes, delays were relatively small in magnitude.
Keywords: operating room timing, hospital efficiency, socioeconomics, pandemic.
The COVID-19 pandemic has led to major changes in patient care both from a surgical perspective and in regard to inpatient hospital course. Safety protocols nationwide have been implemented to protect both patients and providers. Some elements of surgical care have drastically changed, including operating room (OR) staffing, personal protective equipment (PPE) utilization, and increased sterilization measures. Furloughs, layoffs, and reassignments due to the focus on nonelective and COVID-19–related cases challenged OR staffing and efficiency. Operating room staff with COVID-19 exposures or COVID-19 infections also caused last-minute changes in staffing. All of these scenarios can cause issues due to actual understaffing or due to staff members being pushed into highly specialized areas, such as neurosurgery, in which they have very little experience. A further obstacle to OR efficiency included policy changes involving PPE utilization, sterilization measures, and supply chain shortages of necessary resources such as PPE.
Neurosurgery in particular has been susceptible to COVID-19–related system-wide changes given operator proximity to the patient’s respiratory passages, frequency of emergent cases, and varying anesthetic needs, as well as the high level of specialization needed to perform neurosurgical care. Previous studies have shown a change in the makeup of neurosurgical patients seeking care, as well as in the acuity of neurological consult of these patients.1 A study in orthopedic surgery by Andreata et al demonstrated worsened OR efficiency, with significantly increased first-start and turnover times.2 In the COVID-19 era, OR quality and safety are crucially important to both patients and providers. Providing this safe and effective care in an efficient manner is important for optimal neurosurgical management in the long term.3 Moreover, the financial burden of implementing new protocols and standards can be compounded by additional financial losses due to reduced OR efficiency.
Methods
To analyze the effect of COVID-19 on neurosurgical OR efficiency, institutional perioperative databases at a single high-volume center were queried for operations performed from December 2019 until October 2021. March 12, 2020, was chosen as the onset of COVID-19 for analytic purposes, as this was the date when the state of Tennessee declared a state of emergency. The 90-day periods before and after this date were used for comparative analysis for pre-COVID-19, peak COVID-19, and post-peak-restrictions time periods. The peak COVID-19 period was defined as the 90-day period following the initial onset of COVID-19 and the surge of cases. For comparison purposes, post-peak COVID-19 was defined as the months following the first peak until October 2021 (approximately 17 months). COVID-19 burden was determined using a COVID-19 single-institution census of confirmed cases by polymerase chain reaction (PCR) for which the average number of cases of COVID-19 during a given month was determined. This number is a scaled trend, and a true number of COVID-19 cases in our hospital was not reported.
Neurosurgical and neuroendovascular cases were included in the analysis. Outcomes included delay in first-start and OR turnover time between neurosurgical cases, defined as the time from the patient leaving the room until the next patient entered the room. Preset threshold times were used in analyses to adjust for normal leniency in OR scheduling (15 minutes for first start and 90 minutes for turnover, which is a standard for our single-institution perioperative center). Statistical analyses, including data aggregation, were performed using R, version 4.0.1 (R Foundation for Statistical Computing). Patients’ demographic and clinical characteristics were analyzed using an independent 2-sample t-test for interval variables and a chi-square test for categorical variables. Significance was defined as P < .05.
Results
First-Start Time
First-start time was analyzed in 426 pre-COVID-19, 357 peak-COVID-19, and 2304 post-peak-COVID-19 cases. The unadjusted mean delay length was significantly different between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes, 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004) (Table 1). 
The adjusted average delay length and proportion of cases delayed beyond the 15-minute threshold were not significantly different, but they have been slightly higher since the onset of COVID-19. The proportion of cases that have started early, as well as significantly early past a 15-minute threshold, have also trended down since the onset of the COVID-19 pandemic, but this difference was again not significant. The temporal relationship of first-start delay, both unadjusted and adjusted, from December 2019 to October 2021 is shown in Figure 1. The trend of increasing delay is loosely associated with the COVID-19 burden experienced by our hospital. The start of COVID-19 as well as both COVID-19 peaks have been associated with increased delays in our hospital.
Turnover Time
Turnover time was assessed in 437 pre-COVID-19, 278 peak-restrictions, and 2411 post-peak-restrictions cases. Turnover time during peak restrictions was not significantly different from pre-COVID-19 (88 [100] vs 85 [95]) and has since remained relatively unchanged (83 [87], P = .78). A similar trend held for comparisons of proportion of cases with turnover time past 90 minutes and average times past the 90-minute threshold (Table 2). The temporal relationship between COVID-19 burden and turnover time, both unadjusted and adjusted, from December 2019 to October 2021 is shown in Figure 2. Both figures demonstrate a slight initial increase in turnover time delay at the start of COVID-19, which stabilized with little variation thereafter.


Discussion
We analyzed the OR efficiency metrics of first-start and turnover time during the 90-day period before COVID-19 (pre-COVID-19), the 90 days following Tennessee declaring a state of emergency (peak COVID-19), and the time following this period (post-COVID-19) for all neurosurgical and neuroendovascular cases at Vanderbilt University Medical Center (VUMC). We found a significant difference in unadjusted mean delay length in first-start time between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes for pre-COVID-19, peak-COVID-19, and post-COVID-19: 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004). No significant increase in turnover time between cases was found between these 3 time periods. Based on metrics from first-start delay and turnover time, our center was able to maintain OR efficiency before, during, and after peak COVID-19.
After the Centers for Disease Control and Prevention released guidelines recommending deferring elective procedures to conserve beds and PPE, VUMC made the decision to suspend all elective surgical procedures from March 18 to April 24, 2020. Prior research conducted during the COVID-19 pandemic has demonstrated more than 400 types of surgical procedures with negatively impacted outcomes when compared to surgical outcomes from the same time frame in 2018 and 2019.4 For more than 20 of these types of procedures, there was a significant association between procedure delay and adverse patient outcomes.4 Testing protocols for patients prior to surgery varied throughout the pandemic based on vaccination status and type of procedure. Before vaccines became widely available, all patients were required to obtain a PCR SARS-CoV-2 test within 48 to 72 hours of the scheduled procedure. If the patient’s procedure was urgent and testing was not feasible, the patient was treated as a SARS-CoV-2–positive patient, which required all health care workers involved in the case to wear gowns, gloves, surgical masks, and eye protection. Testing patients preoperatively likely helped to maintain OR efficiency since not all patients received test results prior to the scheduled procedure, leading to cancellations of cases and therefore more staff available for fewer cases.
After vaccines became widely available to the public, testing requirements for patients preoperatively were relaxed, and only patients who were not fully vaccinated or severely immunocompromised were required to test prior to procedures. However, approximately 40% of the population in Tennessee was fully vaccinated in 2021, which reflects the patient population of VUMC.5 This means that many patients who received care at VUMC were still tested prior to procedures.
Adopting adequate safety protocols was found to be key for OR efficiency during the COVID-19 pandemic since performing surgery increased the risk of infection for each health care worker in the OR.6 VUMC protocols identified procedures that required enhanced safety measures to prevent infection of health care workers and avoid staffing shortages, which would decrease OR efficiency. Protocols mandated that only anesthesia team members were allowed to be in the OR during intubation and extubation of patients, which could be one factor leading to increased delays and decreased efficiency for some institutions. Methods for neurosurgeons to decrease risk of infection in the OR include postponing all nonurgent cases, reappraising the necessity for general anesthesia and endotracheal intubation, considering alternative surgical approaches that avoid the respiratory tract, and limiting the use of aerosol-generating instruments.7,8 VUMC’s success in implementing these protocols likely explains why our center was able to maintain OR efficiency throughout the COVID-19 pandemic.
A study conducted by Andreata et al showed a significantly increased mean first-case delay and a nonsignificant increased turnover time in orthopedic surgeries in Northern Italy when comparing surgeries performed during the COVID-19 pandemic to those performed prior to COVID-19.2 Other studies have indicated a similar trend in decreased OR efficiency during COVID-19 in other areas around the world.9,10 These findings are not consistent with our own findings for neurosurgical and neuroendovascular surgeries at VUMC, and any change at our institution was relatively immaterial. Factors that threatened to change OR efficiency—but did not result in meaningful changes in our institutional experience—include delays due to pending COVID-19 test results, safety procedures such as PPE donning, and planning difficulties to ensure the existence of teams with non-overlapping providers in the case of a surgeon being infected.2,11-13
Globally, many surgery centers halted all elective surgeries during the initial COVID-19 spike to prevent a PPE shortage and mitigate risk of infection of patients and health care workers.8,12,14 However, there is no centralized definition of which neurosurgical procedures are elective, so that decision was made on a surgeon or center level, which could lead to variability in efficiency trends.14 One study on neurosurgical procedures during COVID-19 found a 30% decline in all cases and a 23% decline in emergent procedures, showing that the decrease in volume was not only due to cancellation of elective procedures.15 This decrease in elective and emergent surgeries created a backlog of surgeries as well as a loss in health care revenue, and caused many patients to go without adequate health care.10 Looking forward, it is imperative that surgical centers study trends in OR efficiency from COVID-19 and learn how to better maintain OR efficiency during future pandemic conditions to prevent a backlog of cases, loss of health care revenue, and decreased health care access.
Limitations
Our data are from a single center and therefore may not be representative of experiences of other hospitals due to different populations and different impacts from COVID-19. However, given our center’s high volume and diverse patient population, we believe our analysis highlights important trends in neurosurgery practice. Notably, data for patient and OR timing are digitally generated and are entered manually by nurses in the electronic medical record, making it prone to errors and variability. This is in our experience, and if any error is present, we believe it is minimal.
Conclusion
The COVID-19 pandemic has had far-reaching effects on health care worldwide, including neurosurgical care. OR efficiency across the United States generally worsened given the stresses of supply chain issues, staffing shortages, and cancellations. At our institution, we were able to maintain OR efficiency during the known COVID-19 peaks until October 2021. Continually functional neurosurgical ORs are important in preventing delays in care and maintaining a steady revenue in order for hospitals and other health care entities to remain solvent. Further study of OR efficiency is needed for health care systems to prepare for future pandemics and other resource-straining events in order to provide optimal patient care.
Corresponding author: Campbell Liles, MD, Vanderbilt University Medical Center, Department of Neurological Surgery, 1161 21st Ave. South, T4224 Medical Center North, Nashville, TN 37232-2380; [email protected]
Disclosures: None reported.
1. Koester SW, Catapano JS, Ma KL, et al. COVID-19 and neurosurgery consultation call volume at a single large tertiary center with a propensity- adjusted analysis. World Neurosurg. 2021;146:e768-e772. doi:10.1016/j.wneu.2020.11.017
2. Andreata M, Faraldi M, Bucci E, Lombardi G, Zagra L. Operating room efficiency and timing during coronavirus disease 2019 outbreak in a referral orthopaedic hospital in Northern Italy. Int Orthop. 2020;44(12):2499-2504. doi:10.1007/s00264-020-04772-x
3. Dexter F, Abouleish AE, Epstein RH, et al. Use of operating room information system data to predict the impact of reducing turnover times on staffing costs. Anesth Analg. 2003;97(4):1119-1126. doi:10.1213/01.ANE.0000082520.68800.79
4. Zheng NS, Warner JL, Osterman TJ, et al. A retrospective approach to evaluating potential adverse outcomes associated with delay of procedures for cardiovascular and cancer-related diagnoses in the context of COVID-19. J Biomed Inform. 2021;113:103657. doi:10.1016/j.jbi.2020.103657
5. Alcendor DJ. Targeting COVID-19 vaccine hesitancy in rural communities in Tennessee: implications for extending the COVID- 19 pandemic in the South. Vaccines (Basel). 2021;9(11):1279. doi:10.3390/vaccines9111279
6. Perrone G, Giuffrida M, Bellini V, et al. Operating room setup: how to improve health care professionals safety during pandemic COVID- 19: a quality improvement study. J Laparoendosc Adv Surg Tech A. 2021;31(1):85-89. doi:10.1089/lap.2020.0592
7. Iorio-Morin C, Hodaie M, Sarica C, et al. Letter: the risk of COVID-19 infection during neurosurgical procedures: a review of severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) modes of transmission and proposed neurosurgery-specific measures for mitigation. Neurosurgery. 2020;87(2):E178-E185. doi:10.1093/ neuros/nyaa157
8. Gupta P, Muthukumar N, Rajshekhar V, et al. Neurosurgery and neurology practices during the novel COVID-19 pandemic: a consensus statement from India. Neurol India. 2020;68(2):246-254. doi:10.4103/0028-3886.283130
9. Mercer ST, Agarwal R, Dayananda KSS, et al. A comparative study looking at trauma and orthopaedic operating efficiency in the COVID-19 era. Perioper Care Oper Room Manag. 2020;21:100142. doi:10.1016/j.pcorm.2020.100142
10. Rozario N, Rozario D. Can machine learning optimize the efficiency of the operating room in the era of COVID-19? Can J Surg. 2020;63(6):E527-E529. doi:10.1503/cjs.016520
11. Toh KHQ, Barazanchi A, Rajaretnam NS, et al. COVID-19 response by New Zealand general surgical departments in tertiary metropolitan hospitals. ANZ J Surg. 2021;91(7-8):1352-1357. doi:10.1111/ ans.17044
12. Moorthy RK, Rajshekhar V. Impact of COVID-19 pandemic on neurosurgical practice in India: a survey on personal protective equipment usage, testing, and perceptions on disease transmission. Neurol India. 2020;68(5):1133-1138. doi:10.4103/0028- 3886.299173
13. Meneghini RM. Techniques and strategies to optimize efficiencies in the office and operating room: getting through the patient backlog and preserving hospital resources. J Arthroplasty. 2021;36(7S):S49-S51. doi:10.1016/j.arth.2021.03.010
14. Jean WC, Ironside NT, Sack KD, et al. The impact of COVID- 19 on neurosurgeons and the strategy for triaging non-emergent operations: a global neurosurgery study. Acta Neurochir (Wien). 2020;162(6):1229-1240. doi:10.1007/s00701-020- 04342-5
15. Raneri F, Rustemi O, Zambon G, et al. Neurosurgery in times of a pandemic: a survey of neurosurgical services during the COVID-19 outbreak in the Veneto region in Italy. Neurosurg Focus. 2020;49(6):E9. doi:10.3171/2020.9.FOCUS20691
1. Koester SW, Catapano JS, Ma KL, et al. COVID-19 and neurosurgery consultation call volume at a single large tertiary center with a propensity- adjusted analysis. World Neurosurg. 2021;146:e768-e772. doi:10.1016/j.wneu.2020.11.017
2. Andreata M, Faraldi M, Bucci E, Lombardi G, Zagra L. Operating room efficiency and timing during coronavirus disease 2019 outbreak in a referral orthopaedic hospital in Northern Italy. Int Orthop. 2020;44(12):2499-2504. doi:10.1007/s00264-020-04772-x
3. Dexter F, Abouleish AE, Epstein RH, et al. Use of operating room information system data to predict the impact of reducing turnover times on staffing costs. Anesth Analg. 2003;97(4):1119-1126. doi:10.1213/01.ANE.0000082520.68800.79
4. Zheng NS, Warner JL, Osterman TJ, et al. A retrospective approach to evaluating potential adverse outcomes associated with delay of procedures for cardiovascular and cancer-related diagnoses in the context of COVID-19. J Biomed Inform. 2021;113:103657. doi:10.1016/j.jbi.2020.103657
5. Alcendor DJ. Targeting COVID-19 vaccine hesitancy in rural communities in Tennessee: implications for extending the COVID- 19 pandemic in the South. Vaccines (Basel). 2021;9(11):1279. doi:10.3390/vaccines9111279
6. Perrone G, Giuffrida M, Bellini V, et al. Operating room setup: how to improve health care professionals safety during pandemic COVID- 19: a quality improvement study. J Laparoendosc Adv Surg Tech A. 2021;31(1):85-89. doi:10.1089/lap.2020.0592
7. Iorio-Morin C, Hodaie M, Sarica C, et al. Letter: the risk of COVID-19 infection during neurosurgical procedures: a review of severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) modes of transmission and proposed neurosurgery-specific measures for mitigation. Neurosurgery. 2020;87(2):E178-E185. doi:10.1093/ neuros/nyaa157
8. Gupta P, Muthukumar N, Rajshekhar V, et al. Neurosurgery and neurology practices during the novel COVID-19 pandemic: a consensus statement from India. Neurol India. 2020;68(2):246-254. doi:10.4103/0028-3886.283130
9. Mercer ST, Agarwal R, Dayananda KSS, et al. A comparative study looking at trauma and orthopaedic operating efficiency in the COVID-19 era. Perioper Care Oper Room Manag. 2020;21:100142. doi:10.1016/j.pcorm.2020.100142
10. Rozario N, Rozario D. Can machine learning optimize the efficiency of the operating room in the era of COVID-19? Can J Surg. 2020;63(6):E527-E529. doi:10.1503/cjs.016520
11. Toh KHQ, Barazanchi A, Rajaretnam NS, et al. COVID-19 response by New Zealand general surgical departments in tertiary metropolitan hospitals. ANZ J Surg. 2021;91(7-8):1352-1357. doi:10.1111/ ans.17044
12. Moorthy RK, Rajshekhar V. Impact of COVID-19 pandemic on neurosurgical practice in India: a survey on personal protective equipment usage, testing, and perceptions on disease transmission. Neurol India. 2020;68(5):1133-1138. doi:10.4103/0028- 3886.299173
13. Meneghini RM. Techniques and strategies to optimize efficiencies in the office and operating room: getting through the patient backlog and preserving hospital resources. J Arthroplasty. 2021;36(7S):S49-S51. doi:10.1016/j.arth.2021.03.010
14. Jean WC, Ironside NT, Sack KD, et al. The impact of COVID- 19 on neurosurgeons and the strategy for triaging non-emergent operations: a global neurosurgery study. Acta Neurochir (Wien). 2020;162(6):1229-1240. doi:10.1007/s00701-020- 04342-5
15. Raneri F, Rustemi O, Zambon G, et al. Neurosurgery in times of a pandemic: a survey of neurosurgical services during the COVID-19 outbreak in the Veneto region in Italy. Neurosurg Focus. 2020;49(6):E9. doi:10.3171/2020.9.FOCUS20691
The Role of Revascularization and Viability Testing in Patients With Multivessel Coronary Artery Disease and Severely Reduced Ejection Fraction
Study 1 Overview (STICHES Investigators)
Objective: To assess the survival benefit of coronary-artery bypass grafting (CABG) added to guideline-directed medical therapy, compared to optimal medical therapy (OMT) alone, in patients with coronary artery disease, heart failure, and severe left ventricular dysfunction. Design: Multicenter, randomized, prospective study with extended follow-up (median duration of 9.8 years).
Setting and participants: A total of 1212 patients with left ventricular ejection fraction (LVEF) of 35% or less and coronary artery disease were randomized to medical therapy plus CABG or OMT alone at 127 clinical sites in 26 countries.
Main outcome measures: The primary endpoint was death from any cause. Main secondary endpoints were death from cardiovascular causes and a composite outcome of death from any cause or hospitalization for cardiovascular causes.
Main results: There were 359 primary outcome all-cause deaths (58.9%) in the CABG group and 398 (66.1%) in the medical therapy group (hazard ratio [HR], 0.84; 95% CI, 0.73-0.97; P = .02). Death from cardiovascular causes was reported in 247 patients (40.5%) in the CABG group and 297 patients (49.3%) in the medical therapy group (HR, 0.79; 95% CI, 0.66-0.93; P < .01). The composite outcome of death from any cause or hospitalization for cardiovascular causes occurred in 467 patients (76.6%) in the CABG group and 467 patients (87.0%) in the medical therapy group (HR, 0.72; 95% CI, 0.64-0.82; P < .01).
Conclusion: Over a median follow-up of 9.8 years in patients with ischemic cardiomyopathy with severely reduced ejection fraction, the rates of death from any cause, death from cardiovascular causes, and the composite of death from any cause or hospitalization for cardiovascular causes were significantly lower in patients undergoing CABG than in patients receiving medical therapy alone.
Study 2 Overview (REVIVED BCIS Trial Group)
Objective: To assess whether percutaneous coronary intervention (PCI) can improve survival and left ventricular function in patients with severe left ventricular systolic dysfunction as compared to OMT alone.
Design: Multicenter, randomized, prospective study.
Setting and participants: A total of 700 patients with LVEF <35% with severe coronary artery disease amendable to PCI and demonstrable myocardial viability were randomly assigned to either PCI plus optimal medical therapy (PCI group) or OMT alone (OMT group).
Main outcome measures: The primary outcome was death from any cause or hospitalization for heart failure. The main secondary outcomes were LVEF at 6 and 12 months and quality of life (QOL) scores.
Main results: Over a median follow-up of 41 months, the primary outcome was reported in 129 patients (37.2%) in the PCI group and in 134 patients (38.0%) in the OMT group (HR, 0.99; 95% CI, 0.78-1.27; P = .96). The LVEF was similar in the 2 groups at 6 months (mean difference, –1.6 percentage points; 95% CI, –3.7 to 0.5) and at 12 months (mean difference, 0.9 percentage points; 95% CI, –1.7 to 3.4). QOL scores at 6 and 12 months favored the PCI group, but the difference had diminished at 24 months.
Conclusion: In patients with severe ischemic cardiomyopathy, revascularization by PCI in addition to OMT did not result in a lower incidence of death from any cause or hospitalization from heart failure.
Commentary
Coronary artery disease is the most common cause of heart failure with reduced ejection fraction and an important cause of mortality.1 Patients with ischemic cardiomyopathy with reduced ejection fraction are often considered for revascularization in addition to OMT and device therapies. Although there have been multiple retrospective studies and registries suggesting that cardiac outcomes and LVEF improve with revascularization, the number of large-scale prospective studies that assessed this clinical question and randomized patients to revascularization plus OMT compared to OMT alone has been limited.
In the Surgical Treatment for Ischemic Heart Failure (STICH) study,2,3 eligible patients had coronary artery disease amendable to CABG and a LVEF of 35% or less. Patients (N = 1212) were randomly assigned to CABG plus OMT or OMT alone between July 2002 and May 2007. The original study, with a median follow-up of 5 years, did not show survival benefit, but the investigators reported that the primary outcome of death from any cause was significantly lower in the CABG group compared to OMT alone when follow-up of the same study population was extended to 9.8 years (58.9% vs 66.1%, P = .02). The findings from this study led to a class I guideline recommendation of CABG over medical therapy in patients with multivessel disease and low ejection fraction.4
Since the STICH trial was designed, there have been significant improvements in devices and techniques used for PCI, and the procedure is now widely performed in patients with multivessel disease.5 The advantages of PCI over CABG include shorter recovery times and lower risk of immediate complications. In this context, the recently reported Revascularization for Ischemic Ventricular Dysfunction (REVIVED) study assessed clinical outcomes in patients with severe coronary artery disease and reduced ejection fraction by randomizing patients to either PCI with OMT or OMT alone.6 At a median follow-up of 3.5 years, the investigators found no difference in the primary outcome of death from any cause or hospitalization for heart failure (37.2% vs 38.0%; 95% CI, 0.78-1.28; P = .96). Moreover, the degree of LVEF improvement, assessed by follow-up echocardiogram read by the core lab, showed no difference in the degree of LVEF improvement between groups at 6 and 12 months. Finally, although results of the QOL assessment using the Kansas City Cardiomyopathy Questionnaire (KCCQ), a validated, patient-reported, heart-failure-specific QOL scale, favored the PCI group at 6 and 12 months of follow-up, the difference had diminished at 24 months.
The main strength of the REVIVED study was that it targeted a patient population with severe coronary artery disease, including left main disease and severely reduced ejection fraction, that historically have been excluded from large-scale randomized controlled studies evaluating PCI with OMT compared to OMT alone.7 However, there are several points to consider when interpreting the results of this study. First, further details of the PCI procedures are necessary. The REVIVED study recommended revascularization of all territories with viable myocardium; the anatomical revascularization index utilizing the British Cardiovascular Intervention Society (BCIS) Jeopardy Score was 71%. It is important to note that this jeopardy score was operator-reported and the core-lab adjudicated anatomical revascularization rate may be lower. Although viability testing primarily utilizing cardiac magnetic resonance imaging was performed in most patients, correlation between the revascularization territory and the viable segments has yet to be reported. Moreover, procedural details such as use of intravascular ultrasound and physiological testing, known to improve clinical outcome, need to be reported.8,9
Second, there is a high prevalence of ischemic cardiomyopathy, and it is important to note that the patients included in this study were highly selected from daily clinical practice, as evidenced by the prolonged enrollment period (8 years). Individuals were largely stable patients with less complex coronary anatomy as evidenced by the median interval from angiography to randomization of 80 days. Taking into consideration the degree of left ventricular dysfunction for patients included in the trial, only 14% of the patients had left main disease and half of the patients only had 2-vessel disease. The severity of the left main disease also needs to be clarified as it is likely that patients the operator determined to be critical were not enrolled in the study. Furthermore, the standard of care based on the STICH trial is to refer patients with severe multivessel coronary artery disease to CABG, making it more likely that patients with more severe and complex disease were not included in this trial. It is also important to note that this study enrolled patients with stable ischemic heart disease, and the data do not apply to patients presenting with acute coronary syndrome.
Third, although the primary outcome was similar between the groups, the secondary outcome of unplanned revascularization was lower in the PCI group. In addition, the rate of acute myocardial infarction (MI) was similar between the 2 groups, but the rate of spontaneous MI was lower in the PCI group compared to the OMT group (5.2% vs 9.3%) as 40% of MI cases in the PCI group were periprocedural MIs. The correlation between periprocedural MI and long-term outcomes has been modest compared to spontaneous MI. Moreover, with the longer follow-up, the number of spontaneous MI cases is expected to rise while the number of periprocedural MI cases is not. Extending the follow-up period is also important, as the STICH extension trial showed a statistically significant difference at 10-year follow up despite negative results at the time of the original publication.
Fourth, the REVIVED trial randomized a significantly lower number of patients compared to the STICH trial, and the authors reported fewer primary-outcome events than the estimated number needed to achieve the power to assess the primary hypothesis. In addition, significant improvements in medical treatment for heart failure with reduced ejection fraction since the STICH trial make comparison of PCI vs CABG in this patient population unfeasible.
Finally, although severe angina was not an exclusion criterion, two-thirds of the patients enrolled had no angina, and only 2% of the patients had baseline severe angina. This is important to consider when interpreting the results of the patient-reported health status as previous studies have shown that patients with worse angina at baseline derive the largest improvement in their QOL,10,11 and symptom improvement is the main indication for PCI in patients with stable ischemic heart disease.
Applications for Clinical Practice and System Implementation
In patients with severe left ventricular systolic dysfunction and multivessel stable ischemic heart disease who are well compensated and have little or no angina at baseline, OMT alone as an initial strategy may be considered against the addition of PCI after careful risk and benefit discussion. Further details about revascularization and extended follow-up data from the REVIVED trial are necessary.
Practice Points
- Patients with ischemic cardiomyopathy with reduced ejection fraction have been an understudied population in previous studies.
- Further studies are necessary to understand the benefits of revascularization and the role of viability testing in this population.
– Taishi Hirai MD, and Ziad Sayed Ahmad, MD
University of Missouri, Columbia, MO
1. Nowbar AN, Gitto M, Howard JP, et al. Mortality from ischemic heart disease. Circ Cardiovasc Qual Outcomes. 2019;12(6):e005375. doi:10.1161/CIRCOUTCOMES
2. Velazquez EJ, Lee KL, Deja MA, et al; for the STICH Investigators. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364(17):1607-1616. doi:10.1056/NEJMoa1100356
3. Velazquez EJ, Lee KL, Jones RH, et al. Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med. 2016;374(16):1511-1520. doi:10.1056/NEJMoa1602001
4. Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(2):e21-e129. doi:10.1016/j.jacc.2021.09.006
5. Kirtane AJ, Doshi D, Leon MB, et al. Treatment of higher-risk patients with an indication for revascularization: evolution within the field of contemporary percutaneous coronary intervention. Circulation. 2016;134(5):422-431. doi:10.1161/CIRCULATIONAHA
6. Perera D, Clayton T, O’Kane PD, et al. Percutaneous revascularization for ischemic left ventricular dysfunction. N Engl J Med. 2022;387(15):1351-1360. doi:10.1056/NEJMoa2206606
7. Maron DJ, Hochman JS, Reynolds HR, et al. Initial invasive or conservative strategy for stable coronary disease. Circulation. 2020;142(18):1725-1735. doi:10.1161/CIRCULATIONAHA
8. De Bruyne B, Pijls NH, Kalesan B, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367(11):991-1001. doi:10.1056/NEJMoa1205361
9. Zhang J, Gao X, Kan J, et al. Intravascular ultrasound versus angiography-guided drug-eluting stent implantation: The ULTIMATE trial. J Am Coll Cardiol. 2018;72(24):3126-3137. doi:10.1016/j.jacc.2018.09.013
10. Spertus JA, Jones PG, Maron DJ, et al. Health-status outcomes with invasive or conservative care in coronary disease. N Engl J Med. 2020;382(15):1408-1419. doi:10.1056/NEJMoa1916370
11. Hirai T, Grantham JA, Sapontis J, et al. Quality of life changes after chronic total occlusion angioplasty in patients with baseline refractory angina. Circ Cardiovasc Interv. 2019;12:e007558. doi:10.1161/CIRCINTERVENTIONS.118.007558
Study 1 Overview (STICHES Investigators)
Objective: To assess the survival benefit of coronary-artery bypass grafting (CABG) added to guideline-directed medical therapy, compared to optimal medical therapy (OMT) alone, in patients with coronary artery disease, heart failure, and severe left ventricular dysfunction. Design: Multicenter, randomized, prospective study with extended follow-up (median duration of 9.8 years).
Setting and participants: A total of 1212 patients with left ventricular ejection fraction (LVEF) of 35% or less and coronary artery disease were randomized to medical therapy plus CABG or OMT alone at 127 clinical sites in 26 countries.
Main outcome measures: The primary endpoint was death from any cause. Main secondary endpoints were death from cardiovascular causes and a composite outcome of death from any cause or hospitalization for cardiovascular causes.
Main results: There were 359 primary outcome all-cause deaths (58.9%) in the CABG group and 398 (66.1%) in the medical therapy group (hazard ratio [HR], 0.84; 95% CI, 0.73-0.97; P = .02). Death from cardiovascular causes was reported in 247 patients (40.5%) in the CABG group and 297 patients (49.3%) in the medical therapy group (HR, 0.79; 95% CI, 0.66-0.93; P < .01). The composite outcome of death from any cause or hospitalization for cardiovascular causes occurred in 467 patients (76.6%) in the CABG group and 467 patients (87.0%) in the medical therapy group (HR, 0.72; 95% CI, 0.64-0.82; P < .01).
Conclusion: Over a median follow-up of 9.8 years in patients with ischemic cardiomyopathy with severely reduced ejection fraction, the rates of death from any cause, death from cardiovascular causes, and the composite of death from any cause or hospitalization for cardiovascular causes were significantly lower in patients undergoing CABG than in patients receiving medical therapy alone.
Study 2 Overview (REVIVED BCIS Trial Group)
Objective: To assess whether percutaneous coronary intervention (PCI) can improve survival and left ventricular function in patients with severe left ventricular systolic dysfunction as compared to OMT alone.
Design: Multicenter, randomized, prospective study.
Setting and participants: A total of 700 patients with LVEF <35% with severe coronary artery disease amendable to PCI and demonstrable myocardial viability were randomly assigned to either PCI plus optimal medical therapy (PCI group) or OMT alone (OMT group).
Main outcome measures: The primary outcome was death from any cause or hospitalization for heart failure. The main secondary outcomes were LVEF at 6 and 12 months and quality of life (QOL) scores.
Main results: Over a median follow-up of 41 months, the primary outcome was reported in 129 patients (37.2%) in the PCI group and in 134 patients (38.0%) in the OMT group (HR, 0.99; 95% CI, 0.78-1.27; P = .96). The LVEF was similar in the 2 groups at 6 months (mean difference, –1.6 percentage points; 95% CI, –3.7 to 0.5) and at 12 months (mean difference, 0.9 percentage points; 95% CI, –1.7 to 3.4). QOL scores at 6 and 12 months favored the PCI group, but the difference had diminished at 24 months.
Conclusion: In patients with severe ischemic cardiomyopathy, revascularization by PCI in addition to OMT did not result in a lower incidence of death from any cause or hospitalization from heart failure.
Commentary
Coronary artery disease is the most common cause of heart failure with reduced ejection fraction and an important cause of mortality.1 Patients with ischemic cardiomyopathy with reduced ejection fraction are often considered for revascularization in addition to OMT and device therapies. Although there have been multiple retrospective studies and registries suggesting that cardiac outcomes and LVEF improve with revascularization, the number of large-scale prospective studies that assessed this clinical question and randomized patients to revascularization plus OMT compared to OMT alone has been limited.
In the Surgical Treatment for Ischemic Heart Failure (STICH) study,2,3 eligible patients had coronary artery disease amendable to CABG and a LVEF of 35% or less. Patients (N = 1212) were randomly assigned to CABG plus OMT or OMT alone between July 2002 and May 2007. The original study, with a median follow-up of 5 years, did not show survival benefit, but the investigators reported that the primary outcome of death from any cause was significantly lower in the CABG group compared to OMT alone when follow-up of the same study population was extended to 9.8 years (58.9% vs 66.1%, P = .02). The findings from this study led to a class I guideline recommendation of CABG over medical therapy in patients with multivessel disease and low ejection fraction.4
Since the STICH trial was designed, there have been significant improvements in devices and techniques used for PCI, and the procedure is now widely performed in patients with multivessel disease.5 The advantages of PCI over CABG include shorter recovery times and lower risk of immediate complications. In this context, the recently reported Revascularization for Ischemic Ventricular Dysfunction (REVIVED) study assessed clinical outcomes in patients with severe coronary artery disease and reduced ejection fraction by randomizing patients to either PCI with OMT or OMT alone.6 At a median follow-up of 3.5 years, the investigators found no difference in the primary outcome of death from any cause or hospitalization for heart failure (37.2% vs 38.0%; 95% CI, 0.78-1.28; P = .96). Moreover, the degree of LVEF improvement, assessed by follow-up echocardiogram read by the core lab, showed no difference in the degree of LVEF improvement between groups at 6 and 12 months. Finally, although results of the QOL assessment using the Kansas City Cardiomyopathy Questionnaire (KCCQ), a validated, patient-reported, heart-failure-specific QOL scale, favored the PCI group at 6 and 12 months of follow-up, the difference had diminished at 24 months.
The main strength of the REVIVED study was that it targeted a patient population with severe coronary artery disease, including left main disease and severely reduced ejection fraction, that historically have been excluded from large-scale randomized controlled studies evaluating PCI with OMT compared to OMT alone.7 However, there are several points to consider when interpreting the results of this study. First, further details of the PCI procedures are necessary. The REVIVED study recommended revascularization of all territories with viable myocardium; the anatomical revascularization index utilizing the British Cardiovascular Intervention Society (BCIS) Jeopardy Score was 71%. It is important to note that this jeopardy score was operator-reported and the core-lab adjudicated anatomical revascularization rate may be lower. Although viability testing primarily utilizing cardiac magnetic resonance imaging was performed in most patients, correlation between the revascularization territory and the viable segments has yet to be reported. Moreover, procedural details such as use of intravascular ultrasound and physiological testing, known to improve clinical outcome, need to be reported.8,9
Second, there is a high prevalence of ischemic cardiomyopathy, and it is important to note that the patients included in this study were highly selected from daily clinical practice, as evidenced by the prolonged enrollment period (8 years). Individuals were largely stable patients with less complex coronary anatomy as evidenced by the median interval from angiography to randomization of 80 days. Taking into consideration the degree of left ventricular dysfunction for patients included in the trial, only 14% of the patients had left main disease and half of the patients only had 2-vessel disease. The severity of the left main disease also needs to be clarified as it is likely that patients the operator determined to be critical were not enrolled in the study. Furthermore, the standard of care based on the STICH trial is to refer patients with severe multivessel coronary artery disease to CABG, making it more likely that patients with more severe and complex disease were not included in this trial. It is also important to note that this study enrolled patients with stable ischemic heart disease, and the data do not apply to patients presenting with acute coronary syndrome.
Third, although the primary outcome was similar between the groups, the secondary outcome of unplanned revascularization was lower in the PCI group. In addition, the rate of acute myocardial infarction (MI) was similar between the 2 groups, but the rate of spontaneous MI was lower in the PCI group compared to the OMT group (5.2% vs 9.3%) as 40% of MI cases in the PCI group were periprocedural MIs. The correlation between periprocedural MI and long-term outcomes has been modest compared to spontaneous MI. Moreover, with the longer follow-up, the number of spontaneous MI cases is expected to rise while the number of periprocedural MI cases is not. Extending the follow-up period is also important, as the STICH extension trial showed a statistically significant difference at 10-year follow up despite negative results at the time of the original publication.
Fourth, the REVIVED trial randomized a significantly lower number of patients compared to the STICH trial, and the authors reported fewer primary-outcome events than the estimated number needed to achieve the power to assess the primary hypothesis. In addition, significant improvements in medical treatment for heart failure with reduced ejection fraction since the STICH trial make comparison of PCI vs CABG in this patient population unfeasible.
Finally, although severe angina was not an exclusion criterion, two-thirds of the patients enrolled had no angina, and only 2% of the patients had baseline severe angina. This is important to consider when interpreting the results of the patient-reported health status as previous studies have shown that patients with worse angina at baseline derive the largest improvement in their QOL,10,11 and symptom improvement is the main indication for PCI in patients with stable ischemic heart disease.
Applications for Clinical Practice and System Implementation
In patients with severe left ventricular systolic dysfunction and multivessel stable ischemic heart disease who are well compensated and have little or no angina at baseline, OMT alone as an initial strategy may be considered against the addition of PCI after careful risk and benefit discussion. Further details about revascularization and extended follow-up data from the REVIVED trial are necessary.
Practice Points
- Patients with ischemic cardiomyopathy with reduced ejection fraction have been an understudied population in previous studies.
- Further studies are necessary to understand the benefits of revascularization and the role of viability testing in this population.
– Taishi Hirai MD, and Ziad Sayed Ahmad, MD
University of Missouri, Columbia, MO
Study 1 Overview (STICHES Investigators)
Objective: To assess the survival benefit of coronary-artery bypass grafting (CABG) added to guideline-directed medical therapy, compared to optimal medical therapy (OMT) alone, in patients with coronary artery disease, heart failure, and severe left ventricular dysfunction. Design: Multicenter, randomized, prospective study with extended follow-up (median duration of 9.8 years).
Setting and participants: A total of 1212 patients with left ventricular ejection fraction (LVEF) of 35% or less and coronary artery disease were randomized to medical therapy plus CABG or OMT alone at 127 clinical sites in 26 countries.
Main outcome measures: The primary endpoint was death from any cause. Main secondary endpoints were death from cardiovascular causes and a composite outcome of death from any cause or hospitalization for cardiovascular causes.
Main results: There were 359 primary outcome all-cause deaths (58.9%) in the CABG group and 398 (66.1%) in the medical therapy group (hazard ratio [HR], 0.84; 95% CI, 0.73-0.97; P = .02). Death from cardiovascular causes was reported in 247 patients (40.5%) in the CABG group and 297 patients (49.3%) in the medical therapy group (HR, 0.79; 95% CI, 0.66-0.93; P < .01). The composite outcome of death from any cause or hospitalization for cardiovascular causes occurred in 467 patients (76.6%) in the CABG group and 467 patients (87.0%) in the medical therapy group (HR, 0.72; 95% CI, 0.64-0.82; P < .01).
Conclusion: Over a median follow-up of 9.8 years in patients with ischemic cardiomyopathy with severely reduced ejection fraction, the rates of death from any cause, death from cardiovascular causes, and the composite of death from any cause or hospitalization for cardiovascular causes were significantly lower in patients undergoing CABG than in patients receiving medical therapy alone.
Study 2 Overview (REVIVED BCIS Trial Group)
Objective: To assess whether percutaneous coronary intervention (PCI) can improve survival and left ventricular function in patients with severe left ventricular systolic dysfunction as compared to OMT alone.
Design: Multicenter, randomized, prospective study.
Setting and participants: A total of 700 patients with LVEF <35% with severe coronary artery disease amendable to PCI and demonstrable myocardial viability were randomly assigned to either PCI plus optimal medical therapy (PCI group) or OMT alone (OMT group).
Main outcome measures: The primary outcome was death from any cause or hospitalization for heart failure. The main secondary outcomes were LVEF at 6 and 12 months and quality of life (QOL) scores.
Main results: Over a median follow-up of 41 months, the primary outcome was reported in 129 patients (37.2%) in the PCI group and in 134 patients (38.0%) in the OMT group (HR, 0.99; 95% CI, 0.78-1.27; P = .96). The LVEF was similar in the 2 groups at 6 months (mean difference, –1.6 percentage points; 95% CI, –3.7 to 0.5) and at 12 months (mean difference, 0.9 percentage points; 95% CI, –1.7 to 3.4). QOL scores at 6 and 12 months favored the PCI group, but the difference had diminished at 24 months.
Conclusion: In patients with severe ischemic cardiomyopathy, revascularization by PCI in addition to OMT did not result in a lower incidence of death from any cause or hospitalization from heart failure.
Commentary
Coronary artery disease is the most common cause of heart failure with reduced ejection fraction and an important cause of mortality.1 Patients with ischemic cardiomyopathy with reduced ejection fraction are often considered for revascularization in addition to OMT and device therapies. Although there have been multiple retrospective studies and registries suggesting that cardiac outcomes and LVEF improve with revascularization, the number of large-scale prospective studies that assessed this clinical question and randomized patients to revascularization plus OMT compared to OMT alone has been limited.
In the Surgical Treatment for Ischemic Heart Failure (STICH) study,2,3 eligible patients had coronary artery disease amendable to CABG and a LVEF of 35% or less. Patients (N = 1212) were randomly assigned to CABG plus OMT or OMT alone between July 2002 and May 2007. The original study, with a median follow-up of 5 years, did not show survival benefit, but the investigators reported that the primary outcome of death from any cause was significantly lower in the CABG group compared to OMT alone when follow-up of the same study population was extended to 9.8 years (58.9% vs 66.1%, P = .02). The findings from this study led to a class I guideline recommendation of CABG over medical therapy in patients with multivessel disease and low ejection fraction.4
Since the STICH trial was designed, there have been significant improvements in devices and techniques used for PCI, and the procedure is now widely performed in patients with multivessel disease.5 The advantages of PCI over CABG include shorter recovery times and lower risk of immediate complications. In this context, the recently reported Revascularization for Ischemic Ventricular Dysfunction (REVIVED) study assessed clinical outcomes in patients with severe coronary artery disease and reduced ejection fraction by randomizing patients to either PCI with OMT or OMT alone.6 At a median follow-up of 3.5 years, the investigators found no difference in the primary outcome of death from any cause or hospitalization for heart failure (37.2% vs 38.0%; 95% CI, 0.78-1.28; P = .96). Moreover, the degree of LVEF improvement, assessed by follow-up echocardiogram read by the core lab, showed no difference in the degree of LVEF improvement between groups at 6 and 12 months. Finally, although results of the QOL assessment using the Kansas City Cardiomyopathy Questionnaire (KCCQ), a validated, patient-reported, heart-failure-specific QOL scale, favored the PCI group at 6 and 12 months of follow-up, the difference had diminished at 24 months.
The main strength of the REVIVED study was that it targeted a patient population with severe coronary artery disease, including left main disease and severely reduced ejection fraction, that historically have been excluded from large-scale randomized controlled studies evaluating PCI with OMT compared to OMT alone.7 However, there are several points to consider when interpreting the results of this study. First, further details of the PCI procedures are necessary. The REVIVED study recommended revascularization of all territories with viable myocardium; the anatomical revascularization index utilizing the British Cardiovascular Intervention Society (BCIS) Jeopardy Score was 71%. It is important to note that this jeopardy score was operator-reported and the core-lab adjudicated anatomical revascularization rate may be lower. Although viability testing primarily utilizing cardiac magnetic resonance imaging was performed in most patients, correlation between the revascularization territory and the viable segments has yet to be reported. Moreover, procedural details such as use of intravascular ultrasound and physiological testing, known to improve clinical outcome, need to be reported.8,9
Second, there is a high prevalence of ischemic cardiomyopathy, and it is important to note that the patients included in this study were highly selected from daily clinical practice, as evidenced by the prolonged enrollment period (8 years). Individuals were largely stable patients with less complex coronary anatomy as evidenced by the median interval from angiography to randomization of 80 days. Taking into consideration the degree of left ventricular dysfunction for patients included in the trial, only 14% of the patients had left main disease and half of the patients only had 2-vessel disease. The severity of the left main disease also needs to be clarified as it is likely that patients the operator determined to be critical were not enrolled in the study. Furthermore, the standard of care based on the STICH trial is to refer patients with severe multivessel coronary artery disease to CABG, making it more likely that patients with more severe and complex disease were not included in this trial. It is also important to note that this study enrolled patients with stable ischemic heart disease, and the data do not apply to patients presenting with acute coronary syndrome.
Third, although the primary outcome was similar between the groups, the secondary outcome of unplanned revascularization was lower in the PCI group. In addition, the rate of acute myocardial infarction (MI) was similar between the 2 groups, but the rate of spontaneous MI was lower in the PCI group compared to the OMT group (5.2% vs 9.3%) as 40% of MI cases in the PCI group were periprocedural MIs. The correlation between periprocedural MI and long-term outcomes has been modest compared to spontaneous MI. Moreover, with the longer follow-up, the number of spontaneous MI cases is expected to rise while the number of periprocedural MI cases is not. Extending the follow-up period is also important, as the STICH extension trial showed a statistically significant difference at 10-year follow up despite negative results at the time of the original publication.
Fourth, the REVIVED trial randomized a significantly lower number of patients compared to the STICH trial, and the authors reported fewer primary-outcome events than the estimated number needed to achieve the power to assess the primary hypothesis. In addition, significant improvements in medical treatment for heart failure with reduced ejection fraction since the STICH trial make comparison of PCI vs CABG in this patient population unfeasible.
Finally, although severe angina was not an exclusion criterion, two-thirds of the patients enrolled had no angina, and only 2% of the patients had baseline severe angina. This is important to consider when interpreting the results of the patient-reported health status as previous studies have shown that patients with worse angina at baseline derive the largest improvement in their QOL,10,11 and symptom improvement is the main indication for PCI in patients with stable ischemic heart disease.
Applications for Clinical Practice and System Implementation
In patients with severe left ventricular systolic dysfunction and multivessel stable ischemic heart disease who are well compensated and have little or no angina at baseline, OMT alone as an initial strategy may be considered against the addition of PCI after careful risk and benefit discussion. Further details about revascularization and extended follow-up data from the REVIVED trial are necessary.
Practice Points
- Patients with ischemic cardiomyopathy with reduced ejection fraction have been an understudied population in previous studies.
- Further studies are necessary to understand the benefits of revascularization and the role of viability testing in this population.
– Taishi Hirai MD, and Ziad Sayed Ahmad, MD
University of Missouri, Columbia, MO
1. Nowbar AN, Gitto M, Howard JP, et al. Mortality from ischemic heart disease. Circ Cardiovasc Qual Outcomes. 2019;12(6):e005375. doi:10.1161/CIRCOUTCOMES
2. Velazquez EJ, Lee KL, Deja MA, et al; for the STICH Investigators. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364(17):1607-1616. doi:10.1056/NEJMoa1100356
3. Velazquez EJ, Lee KL, Jones RH, et al. Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med. 2016;374(16):1511-1520. doi:10.1056/NEJMoa1602001
4. Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(2):e21-e129. doi:10.1016/j.jacc.2021.09.006
5. Kirtane AJ, Doshi D, Leon MB, et al. Treatment of higher-risk patients with an indication for revascularization: evolution within the field of contemporary percutaneous coronary intervention. Circulation. 2016;134(5):422-431. doi:10.1161/CIRCULATIONAHA
6. Perera D, Clayton T, O’Kane PD, et al. Percutaneous revascularization for ischemic left ventricular dysfunction. N Engl J Med. 2022;387(15):1351-1360. doi:10.1056/NEJMoa2206606
7. Maron DJ, Hochman JS, Reynolds HR, et al. Initial invasive or conservative strategy for stable coronary disease. Circulation. 2020;142(18):1725-1735. doi:10.1161/CIRCULATIONAHA
8. De Bruyne B, Pijls NH, Kalesan B, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367(11):991-1001. doi:10.1056/NEJMoa1205361
9. Zhang J, Gao X, Kan J, et al. Intravascular ultrasound versus angiography-guided drug-eluting stent implantation: The ULTIMATE trial. J Am Coll Cardiol. 2018;72(24):3126-3137. doi:10.1016/j.jacc.2018.09.013
10. Spertus JA, Jones PG, Maron DJ, et al. Health-status outcomes with invasive or conservative care in coronary disease. N Engl J Med. 2020;382(15):1408-1419. doi:10.1056/NEJMoa1916370
11. Hirai T, Grantham JA, Sapontis J, et al. Quality of life changes after chronic total occlusion angioplasty in patients with baseline refractory angina. Circ Cardiovasc Interv. 2019;12:e007558. doi:10.1161/CIRCINTERVENTIONS.118.007558
1. Nowbar AN, Gitto M, Howard JP, et al. Mortality from ischemic heart disease. Circ Cardiovasc Qual Outcomes. 2019;12(6):e005375. doi:10.1161/CIRCOUTCOMES
2. Velazquez EJ, Lee KL, Deja MA, et al; for the STICH Investigators. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364(17):1607-1616. doi:10.1056/NEJMoa1100356
3. Velazquez EJ, Lee KL, Jones RH, et al. Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med. 2016;374(16):1511-1520. doi:10.1056/NEJMoa1602001
4. Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(2):e21-e129. doi:10.1016/j.jacc.2021.09.006
5. Kirtane AJ, Doshi D, Leon MB, et al. Treatment of higher-risk patients with an indication for revascularization: evolution within the field of contemporary percutaneous coronary intervention. Circulation. 2016;134(5):422-431. doi:10.1161/CIRCULATIONAHA
6. Perera D, Clayton T, O’Kane PD, et al. Percutaneous revascularization for ischemic left ventricular dysfunction. N Engl J Med. 2022;387(15):1351-1360. doi:10.1056/NEJMoa2206606
7. Maron DJ, Hochman JS, Reynolds HR, et al. Initial invasive or conservative strategy for stable coronary disease. Circulation. 2020;142(18):1725-1735. doi:10.1161/CIRCULATIONAHA
8. De Bruyne B, Pijls NH, Kalesan B, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367(11):991-1001. doi:10.1056/NEJMoa1205361
9. Zhang J, Gao X, Kan J, et al. Intravascular ultrasound versus angiography-guided drug-eluting stent implantation: The ULTIMATE trial. J Am Coll Cardiol. 2018;72(24):3126-3137. doi:10.1016/j.jacc.2018.09.013
10. Spertus JA, Jones PG, Maron DJ, et al. Health-status outcomes with invasive or conservative care in coronary disease. N Engl J Med. 2020;382(15):1408-1419. doi:10.1056/NEJMoa1916370
11. Hirai T, Grantham JA, Sapontis J, et al. Quality of life changes after chronic total occlusion angioplasty in patients with baseline refractory angina. Circ Cardiovasc Interv. 2019;12:e007558. doi:10.1161/CIRCINTERVENTIONS.118.007558
Effectiveness of Colonoscopy for Colorectal Cancer Screening in Reducing Cancer-Related Mortality: Interpreting the Results From Two Ongoing Randomized Trials
Study 1 Overview (Bretthauer et al)
Objective: To evaluate the impact of screening colonoscopy on colon cancer–related death.
Design: Randomized trial conducted in 4 European countries.
Setting and participants: Presumptively healthy men and women between the ages of 55 and 64 years were selected from population registries in Poland, Norway, Sweden, and the Netherlands between 2009 and 2014. Eligible participants had not previously undergone screening. Patients with a diagnosis of colon cancer before trial entry were excluded.
Intervention: Participants were randomly assigned in a 1:2 ratio to undergo colonoscopy screening by invitation or to no invitation and no screening. Participants were randomized using a computer-generated allocation algorithm. Patients were stratified by age, sex, and municipality.
Main outcome measures: The primary endpoint of the study was risk of colorectal cancer and related death after a median follow-up of 10 to 15 years. The main secondary endpoint was death from any cause.
Main results: The study reported follow-up data from 84,585 participants (89.1% of all participants originally included in the trial). The remaining participants were either excluded or data could not be included due to lack of follow-up data from the usual-care group. Men (50.1%) and women (49.9%) were equally represented. The median age at entry was 59 years. The median follow-up was 10 years. Characteristics were otherwise balanced. Good bowel preparation was reported in 91% of all participants. Cecal intubation was achieved in 96.8% of all participants. The percentage of patients who underwent screening was 42% for the group, but screening rates varied by country (33%-60%). Colorectal cancer was diagnosed at screening in 62 participants (0.5% of screening group). Adenomas were detected in 30.7% of participants; 15 patients had polypectomy-related major bleeding. There were no perforations.
The risk of colorectal cancer at 10 years was 0.98% in the invited-to-screen group and 1.2% in the usual-care group (risk ratio, 0.82; 95% CI, 0.7-0.93). The reported number needed to invite to prevent 1 case of colon cancer in a 10-year period was 455. The risk of colorectal cancer–related death at 10 years was 0.28% in the invited-to-screen group and 0.31% in the usual-care group (risk ratio, 0.9; 95% CI, 0.64-1.16). An adjusted per-protocol analysis was performed to account for the estimated effect of screening if all participants assigned to the screening group underwent screening. In this analysis, the risk of colorectal cancer at 10 years was decreased from 1.22% to 0.84% (risk ratio, 0.69; 95% CI, 0.66-0.83).
Conclusion: Based on the results of this European randomized trial, the risk of colorectal cancer at 10 years was lower among those who were invited to undergo screening.
Study 2 Overview (Forsberg et al)
Objective: To investigate the effect of colorectal cancer screening with once-only colonoscopy or fecal immunochemical testing (FIT) on colorectal cancer mortality and incidence.
Design: Randomized controlled trial in Sweden utilizing a population registry.
Setting and participants: Patients aged 60 years at the time of entry were identified from a population-based registry from the Swedish Tax Agency.
Intervention: Individuals were assigned by an independent statistician to once-only colonoscopy, 2 rounds of FIT 2 years apart, or a control group in which no intervention was performed. Patients were assigned in a 1:6 ratio for colonoscopy vs control and a 1:2 ratio for FIT vs control.
Main outcome measures: The primary endpoint of the trial was colorectal cancer incidence and mortality.
Main results: A total of 278,280 participants were included in the study from March 1, 2014, through December 31, 2020 (31,140 in the colonoscopy group, 60,300 in the FIT group, and 186,840 in the control group). Of those in the colonoscopy group, 35% underwent colonoscopy, and 55% of those in the FIT group participated in testing. Colorectal cancer was detected in 0.16% (49) of people in the colonoscopy group and 0.2% (121) of people in the FIT test group (relative risk, 0.78; 95% CI, 0.56-1.09). The advanced adenoma detection rate was 2.05% in the colonoscopy group and 1.61% in the FIT group (relative risk, 1.27; 95% CI, 1.15-1.41). There were 2 perforations noted in the colonoscopy group and 15 major bleeding events. More right-sided adenomas were detected in the colonoscopy group.
Conclusion: The results of the current study highlight similar detection rates in the colonoscopy and FIT group. Should further follow-up show a benefit in disease-specific mortality, such screening strategies could be translated into population-based screening programs.
Commentary
The first colonoscopy screening recommendations were established in the mid 1990s in the United States, and over the subsequent 2 decades colonoscopy has been the recommended method and main modality for colorectal cancer screening in this country. The advantage of colonoscopy over other screening modalities (sigmoidoscopy and fecal-based testing) is that it can examine the entire large bowel and allow for removal of potential precancerous lesions. However, data to support colonoscopy as a screening modality for colorectal cancer are largely based on cohort studies.1,2 These studies have reported a significant reduction in the incidence of colon cancer. Additionally, colorectal cancer mortality was notably lower in the screened populations. For example, one study among health professionals found a nearly 70% reduction in colorectal cancer mortality in those who underwent at least 1 screening colonoscopy.3
There has been a lack of randomized clinical data to validate the efficacy of colonoscopy screening for reducing colorectal cancer–related deaths. The current study by Bretthauer et al addresses an important need and enhances our understanding of the efficacy of colorectal cancer screening with colonoscopy. In this randomized trial involving more than 84,000 participants from Poland, Norway, Sweden, and the Netherlands, there was a noted 18% decrease in the risk of colorectal cancer over a 10-year period in the intention-to-screen population. The reduction in the risk of death from colorectal cancer was not statistically significant (risk ratio, 0.90; 95% CI, 0.64-1.16). These results are surprising and certainly raise the question as to whether previous studies overestimated the effectiveness of colonoscopy in reducing the risk of colorectal cancer–related deaths. There are several limitations to the Bretthauer et al study, however.
Perhaps the most important limitation is the fact that only 42% of participants in the invited-to-screen cohort underwent screening colonoscopy. Therefore, this raises the question of whether the efficacy noted is simply due to a lack of participation in the screening protocol. In the adjusted per-protocol analysis, colonoscopy was estimated to reduce the risk of colorectal cancer by 31% and the risk of colorectal cancer–related death by around 50%. These findings are more in line with prior published studies regarding the efficacy of colorectal cancer screening. The authors plan to repeat this analysis at 15 years, and it is possible that the risk of colorectal cancer and colorectal cancer–related death can be reduced on subsequent follow-up.
While the results of the Bretthauer et al trial are important, randomized trials that directly compare the effectiveness of different colorectal cancer screening strategies are lacking. The Forsberg et al trial, also an ongoing study, seeks to address this vitally important gap in our current data. The SCREESCO trial is a study that compares the efficacy of colonoscopy with FIT every 2 years or no screening. The currently reported data are preliminary but show a similarly low rate of colonoscopy screening in those invited to do so (35%). This is a similar limitation to that noted in the Bretthauer et al study. Furthermore, there is some question regarding colonoscopy quality in this study, which had a very low reported adenoma detection rate.
While the current studies are important and provide quality randomized data on the effect of colorectal cancer screening, there remain many unanswered questions. Should the results presented by Bretthauer et al represent the current real-world scenario, then colonoscopy screening may not be viewed as an effective screening tool compared to simpler, less-invasive modalities (ie, FIT). Further follow-up from the SCREESCO trial will help shed light on this question. However, there are concerns with this study, including a very low participation rate, which could greatly underestimate the effectiveness of screening. Additional analysis and longer follow-up will be vital to fully understand the benefits of screening colonoscopy. In the meantime, screening remains an important tool for early detection of colorectal cancer and remains a category A recommendation by the United States Preventive Services Task Force.4
Applications for Clinical Practice and System Implementation
Current guidelines continue to strongly recommend screening for colorectal cancer for persons between 45 and 75 years of age (category B recommendation for those aged 45 to 49 years per the United States Preventive Services Task Force). Stool-based tests and direct visualization tests are both endorsed as screening options. Further follow-up from the presented studies is needed to help shed light on the magnitude of benefit of these modalities.
Practice Points
- Current guidelines continue to strongly recommend screening for colon cancer in those aged 45 to 75 years.
- The optimal modality for screening and the impact of screening on cancer-related mortality requires longer- term follow-up from these ongoing studies.
–Daniel Isaac, DO, MS
1. Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for Colorectal Cancer: An Evidence Update for the U.S. Preventive Services Task Force [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2021 May. Report No.: 20-05271-EF-1.
2. Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325(19):1978-1998. doi:10.1001/jama.2021.4417
3. Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369(12):1095-1105. doi:10.1056/NEJMoa1301969
4. U.S. Preventive Services Task Force. Colorectal cancer: screening. Published May 18, 2021. Accessed November 8, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening
Study 1 Overview (Bretthauer et al)
Objective: To evaluate the impact of screening colonoscopy on colon cancer–related death.
Design: Randomized trial conducted in 4 European countries.
Setting and participants: Presumptively healthy men and women between the ages of 55 and 64 years were selected from population registries in Poland, Norway, Sweden, and the Netherlands between 2009 and 2014. Eligible participants had not previously undergone screening. Patients with a diagnosis of colon cancer before trial entry were excluded.
Intervention: Participants were randomly assigned in a 1:2 ratio to undergo colonoscopy screening by invitation or to no invitation and no screening. Participants were randomized using a computer-generated allocation algorithm. Patients were stratified by age, sex, and municipality.
Main outcome measures: The primary endpoint of the study was risk of colorectal cancer and related death after a median follow-up of 10 to 15 years. The main secondary endpoint was death from any cause.
Main results: The study reported follow-up data from 84,585 participants (89.1% of all participants originally included in the trial). The remaining participants were either excluded or data could not be included due to lack of follow-up data from the usual-care group. Men (50.1%) and women (49.9%) were equally represented. The median age at entry was 59 years. The median follow-up was 10 years. Characteristics were otherwise balanced. Good bowel preparation was reported in 91% of all participants. Cecal intubation was achieved in 96.8% of all participants. The percentage of patients who underwent screening was 42% for the group, but screening rates varied by country (33%-60%). Colorectal cancer was diagnosed at screening in 62 participants (0.5% of screening group). Adenomas were detected in 30.7% of participants; 15 patients had polypectomy-related major bleeding. There were no perforations.
The risk of colorectal cancer at 10 years was 0.98% in the invited-to-screen group and 1.2% in the usual-care group (risk ratio, 0.82; 95% CI, 0.7-0.93). The reported number needed to invite to prevent 1 case of colon cancer in a 10-year period was 455. The risk of colorectal cancer–related death at 10 years was 0.28% in the invited-to-screen group and 0.31% in the usual-care group (risk ratio, 0.9; 95% CI, 0.64-1.16). An adjusted per-protocol analysis was performed to account for the estimated effect of screening if all participants assigned to the screening group underwent screening. In this analysis, the risk of colorectal cancer at 10 years was decreased from 1.22% to 0.84% (risk ratio, 0.69; 95% CI, 0.66-0.83).
Conclusion: Based on the results of this European randomized trial, the risk of colorectal cancer at 10 years was lower among those who were invited to undergo screening.
Study 2 Overview (Forsberg et al)
Objective: To investigate the effect of colorectal cancer screening with once-only colonoscopy or fecal immunochemical testing (FIT) on colorectal cancer mortality and incidence.
Design: Randomized controlled trial in Sweden utilizing a population registry.
Setting and participants: Patients aged 60 years at the time of entry were identified from a population-based registry from the Swedish Tax Agency.
Intervention: Individuals were assigned by an independent statistician to once-only colonoscopy, 2 rounds of FIT 2 years apart, or a control group in which no intervention was performed. Patients were assigned in a 1:6 ratio for colonoscopy vs control and a 1:2 ratio for FIT vs control.
Main outcome measures: The primary endpoint of the trial was colorectal cancer incidence and mortality.
Main results: A total of 278,280 participants were included in the study from March 1, 2014, through December 31, 2020 (31,140 in the colonoscopy group, 60,300 in the FIT group, and 186,840 in the control group). Of those in the colonoscopy group, 35% underwent colonoscopy, and 55% of those in the FIT group participated in testing. Colorectal cancer was detected in 0.16% (49) of people in the colonoscopy group and 0.2% (121) of people in the FIT test group (relative risk, 0.78; 95% CI, 0.56-1.09). The advanced adenoma detection rate was 2.05% in the colonoscopy group and 1.61% in the FIT group (relative risk, 1.27; 95% CI, 1.15-1.41). There were 2 perforations noted in the colonoscopy group and 15 major bleeding events. More right-sided adenomas were detected in the colonoscopy group.
Conclusion: The results of the current study highlight similar detection rates in the colonoscopy and FIT group. Should further follow-up show a benefit in disease-specific mortality, such screening strategies could be translated into population-based screening programs.
Commentary
The first colonoscopy screening recommendations were established in the mid 1990s in the United States, and over the subsequent 2 decades colonoscopy has been the recommended method and main modality for colorectal cancer screening in this country. The advantage of colonoscopy over other screening modalities (sigmoidoscopy and fecal-based testing) is that it can examine the entire large bowel and allow for removal of potential precancerous lesions. However, data to support colonoscopy as a screening modality for colorectal cancer are largely based on cohort studies.1,2 These studies have reported a significant reduction in the incidence of colon cancer. Additionally, colorectal cancer mortality was notably lower in the screened populations. For example, one study among health professionals found a nearly 70% reduction in colorectal cancer mortality in those who underwent at least 1 screening colonoscopy.3
There has been a lack of randomized clinical data to validate the efficacy of colonoscopy screening for reducing colorectal cancer–related deaths. The current study by Bretthauer et al addresses an important need and enhances our understanding of the efficacy of colorectal cancer screening with colonoscopy. In this randomized trial involving more than 84,000 participants from Poland, Norway, Sweden, and the Netherlands, there was a noted 18% decrease in the risk of colorectal cancer over a 10-year period in the intention-to-screen population. The reduction in the risk of death from colorectal cancer was not statistically significant (risk ratio, 0.90; 95% CI, 0.64-1.16). These results are surprising and certainly raise the question as to whether previous studies overestimated the effectiveness of colonoscopy in reducing the risk of colorectal cancer–related deaths. There are several limitations to the Bretthauer et al study, however.
Perhaps the most important limitation is the fact that only 42% of participants in the invited-to-screen cohort underwent screening colonoscopy. Therefore, this raises the question of whether the efficacy noted is simply due to a lack of participation in the screening protocol. In the adjusted per-protocol analysis, colonoscopy was estimated to reduce the risk of colorectal cancer by 31% and the risk of colorectal cancer–related death by around 50%. These findings are more in line with prior published studies regarding the efficacy of colorectal cancer screening. The authors plan to repeat this analysis at 15 years, and it is possible that the risk of colorectal cancer and colorectal cancer–related death can be reduced on subsequent follow-up.
While the results of the Bretthauer et al trial are important, randomized trials that directly compare the effectiveness of different colorectal cancer screening strategies are lacking. The Forsberg et al trial, also an ongoing study, seeks to address this vitally important gap in our current data. The SCREESCO trial is a study that compares the efficacy of colonoscopy with FIT every 2 years or no screening. The currently reported data are preliminary but show a similarly low rate of colonoscopy screening in those invited to do so (35%). This is a similar limitation to that noted in the Bretthauer et al study. Furthermore, there is some question regarding colonoscopy quality in this study, which had a very low reported adenoma detection rate.
While the current studies are important and provide quality randomized data on the effect of colorectal cancer screening, there remain many unanswered questions. Should the results presented by Bretthauer et al represent the current real-world scenario, then colonoscopy screening may not be viewed as an effective screening tool compared to simpler, less-invasive modalities (ie, FIT). Further follow-up from the SCREESCO trial will help shed light on this question. However, there are concerns with this study, including a very low participation rate, which could greatly underestimate the effectiveness of screening. Additional analysis and longer follow-up will be vital to fully understand the benefits of screening colonoscopy. In the meantime, screening remains an important tool for early detection of colorectal cancer and remains a category A recommendation by the United States Preventive Services Task Force.4
Applications for Clinical Practice and System Implementation
Current guidelines continue to strongly recommend screening for colorectal cancer for persons between 45 and 75 years of age (category B recommendation for those aged 45 to 49 years per the United States Preventive Services Task Force). Stool-based tests and direct visualization tests are both endorsed as screening options. Further follow-up from the presented studies is needed to help shed light on the magnitude of benefit of these modalities.
Practice Points
- Current guidelines continue to strongly recommend screening for colon cancer in those aged 45 to 75 years.
- The optimal modality for screening and the impact of screening on cancer-related mortality requires longer- term follow-up from these ongoing studies.
–Daniel Isaac, DO, MS
Study 1 Overview (Bretthauer et al)
Objective: To evaluate the impact of screening colonoscopy on colon cancer–related death.
Design: Randomized trial conducted in 4 European countries.
Setting and participants: Presumptively healthy men and women between the ages of 55 and 64 years were selected from population registries in Poland, Norway, Sweden, and the Netherlands between 2009 and 2014. Eligible participants had not previously undergone screening. Patients with a diagnosis of colon cancer before trial entry were excluded.
Intervention: Participants were randomly assigned in a 1:2 ratio to undergo colonoscopy screening by invitation or to no invitation and no screening. Participants were randomized using a computer-generated allocation algorithm. Patients were stratified by age, sex, and municipality.
Main outcome measures: The primary endpoint of the study was risk of colorectal cancer and related death after a median follow-up of 10 to 15 years. The main secondary endpoint was death from any cause.
Main results: The study reported follow-up data from 84,585 participants (89.1% of all participants originally included in the trial). The remaining participants were either excluded or data could not be included due to lack of follow-up data from the usual-care group. Men (50.1%) and women (49.9%) were equally represented. The median age at entry was 59 years. The median follow-up was 10 years. Characteristics were otherwise balanced. Good bowel preparation was reported in 91% of all participants. Cecal intubation was achieved in 96.8% of all participants. The percentage of patients who underwent screening was 42% for the group, but screening rates varied by country (33%-60%). Colorectal cancer was diagnosed at screening in 62 participants (0.5% of screening group). Adenomas were detected in 30.7% of participants; 15 patients had polypectomy-related major bleeding. There were no perforations.
The risk of colorectal cancer at 10 years was 0.98% in the invited-to-screen group and 1.2% in the usual-care group (risk ratio, 0.82; 95% CI, 0.7-0.93). The reported number needed to invite to prevent 1 case of colon cancer in a 10-year period was 455. The risk of colorectal cancer–related death at 10 years was 0.28% in the invited-to-screen group and 0.31% in the usual-care group (risk ratio, 0.9; 95% CI, 0.64-1.16). An adjusted per-protocol analysis was performed to account for the estimated effect of screening if all participants assigned to the screening group underwent screening. In this analysis, the risk of colorectal cancer at 10 years was decreased from 1.22% to 0.84% (risk ratio, 0.69; 95% CI, 0.66-0.83).
Conclusion: Based on the results of this European randomized trial, the risk of colorectal cancer at 10 years was lower among those who were invited to undergo screening.
Study 2 Overview (Forsberg et al)
Objective: To investigate the effect of colorectal cancer screening with once-only colonoscopy or fecal immunochemical testing (FIT) on colorectal cancer mortality and incidence.
Design: Randomized controlled trial in Sweden utilizing a population registry.
Setting and participants: Patients aged 60 years at the time of entry were identified from a population-based registry from the Swedish Tax Agency.
Intervention: Individuals were assigned by an independent statistician to once-only colonoscopy, 2 rounds of FIT 2 years apart, or a control group in which no intervention was performed. Patients were assigned in a 1:6 ratio for colonoscopy vs control and a 1:2 ratio for FIT vs control.
Main outcome measures: The primary endpoint of the trial was colorectal cancer incidence and mortality.
Main results: A total of 278,280 participants were included in the study from March 1, 2014, through December 31, 2020 (31,140 in the colonoscopy group, 60,300 in the FIT group, and 186,840 in the control group). Of those in the colonoscopy group, 35% underwent colonoscopy, and 55% of those in the FIT group participated in testing. Colorectal cancer was detected in 0.16% (49) of people in the colonoscopy group and 0.2% (121) of people in the FIT test group (relative risk, 0.78; 95% CI, 0.56-1.09). The advanced adenoma detection rate was 2.05% in the colonoscopy group and 1.61% in the FIT group (relative risk, 1.27; 95% CI, 1.15-1.41). There were 2 perforations noted in the colonoscopy group and 15 major bleeding events. More right-sided adenomas were detected in the colonoscopy group.
Conclusion: The results of the current study highlight similar detection rates in the colonoscopy and FIT group. Should further follow-up show a benefit in disease-specific mortality, such screening strategies could be translated into population-based screening programs.
Commentary
The first colonoscopy screening recommendations were established in the mid 1990s in the United States, and over the subsequent 2 decades colonoscopy has been the recommended method and main modality for colorectal cancer screening in this country. The advantage of colonoscopy over other screening modalities (sigmoidoscopy and fecal-based testing) is that it can examine the entire large bowel and allow for removal of potential precancerous lesions. However, data to support colonoscopy as a screening modality for colorectal cancer are largely based on cohort studies.1,2 These studies have reported a significant reduction in the incidence of colon cancer. Additionally, colorectal cancer mortality was notably lower in the screened populations. For example, one study among health professionals found a nearly 70% reduction in colorectal cancer mortality in those who underwent at least 1 screening colonoscopy.3
There has been a lack of randomized clinical data to validate the efficacy of colonoscopy screening for reducing colorectal cancer–related deaths. The current study by Bretthauer et al addresses an important need and enhances our understanding of the efficacy of colorectal cancer screening with colonoscopy. In this randomized trial involving more than 84,000 participants from Poland, Norway, Sweden, and the Netherlands, there was a noted 18% decrease in the risk of colorectal cancer over a 10-year period in the intention-to-screen population. The reduction in the risk of death from colorectal cancer was not statistically significant (risk ratio, 0.90; 95% CI, 0.64-1.16). These results are surprising and certainly raise the question as to whether previous studies overestimated the effectiveness of colonoscopy in reducing the risk of colorectal cancer–related deaths. There are several limitations to the Bretthauer et al study, however.
Perhaps the most important limitation is the fact that only 42% of participants in the invited-to-screen cohort underwent screening colonoscopy. Therefore, this raises the question of whether the efficacy noted is simply due to a lack of participation in the screening protocol. In the adjusted per-protocol analysis, colonoscopy was estimated to reduce the risk of colorectal cancer by 31% and the risk of colorectal cancer–related death by around 50%. These findings are more in line with prior published studies regarding the efficacy of colorectal cancer screening. The authors plan to repeat this analysis at 15 years, and it is possible that the risk of colorectal cancer and colorectal cancer–related death can be reduced on subsequent follow-up.
While the results of the Bretthauer et al trial are important, randomized trials that directly compare the effectiveness of different colorectal cancer screening strategies are lacking. The Forsberg et al trial, also an ongoing study, seeks to address this vitally important gap in our current data. The SCREESCO trial is a study that compares the efficacy of colonoscopy with FIT every 2 years or no screening. The currently reported data are preliminary but show a similarly low rate of colonoscopy screening in those invited to do so (35%). This is a similar limitation to that noted in the Bretthauer et al study. Furthermore, there is some question regarding colonoscopy quality in this study, which had a very low reported adenoma detection rate.
While the current studies are important and provide quality randomized data on the effect of colorectal cancer screening, there remain many unanswered questions. Should the results presented by Bretthauer et al represent the current real-world scenario, then colonoscopy screening may not be viewed as an effective screening tool compared to simpler, less-invasive modalities (ie, FIT). Further follow-up from the SCREESCO trial will help shed light on this question. However, there are concerns with this study, including a very low participation rate, which could greatly underestimate the effectiveness of screening. Additional analysis and longer follow-up will be vital to fully understand the benefits of screening colonoscopy. In the meantime, screening remains an important tool for early detection of colorectal cancer and remains a category A recommendation by the United States Preventive Services Task Force.4
Applications for Clinical Practice and System Implementation
Current guidelines continue to strongly recommend screening for colorectal cancer for persons between 45 and 75 years of age (category B recommendation for those aged 45 to 49 years per the United States Preventive Services Task Force). Stool-based tests and direct visualization tests are both endorsed as screening options. Further follow-up from the presented studies is needed to help shed light on the magnitude of benefit of these modalities.
Practice Points
- Current guidelines continue to strongly recommend screening for colon cancer in those aged 45 to 75 years.
- The optimal modality for screening and the impact of screening on cancer-related mortality requires longer- term follow-up from these ongoing studies.
–Daniel Isaac, DO, MS
1. Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for Colorectal Cancer: An Evidence Update for the U.S. Preventive Services Task Force [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2021 May. Report No.: 20-05271-EF-1.
2. Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325(19):1978-1998. doi:10.1001/jama.2021.4417
3. Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369(12):1095-1105. doi:10.1056/NEJMoa1301969
4. U.S. Preventive Services Task Force. Colorectal cancer: screening. Published May 18, 2021. Accessed November 8, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening
1. Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for Colorectal Cancer: An Evidence Update for the U.S. Preventive Services Task Force [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2021 May. Report No.: 20-05271-EF-1.
2. Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325(19):1978-1998. doi:10.1001/jama.2021.4417
3. Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369(12):1095-1105. doi:10.1056/NEJMoa1301969
4. U.S. Preventive Services Task Force. Colorectal cancer: screening. Published May 18, 2021. Accessed November 8, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening
Residents react: Has residency become easier or overly difficult?
Medical residents have cleared many hurdles to get where they are, as detailed in Medscape’s Residents Salary and Debt Report 2022 which explains their challenges with compensation and school loans as well as long hours and problematic personal relationships.
Whereas 72% of residents described themselves as “very satisfied” or “satisfied” with their professional training experience, only 27% felt that highly about how well they’re paid. Satisfaction levels increased somewhat farther into residency, reaching 35% in year 5.
Do residents have it easier today?
If so, is that rite of passage getting any easier? You’ll get different answers from residents and physicians.
Medscape asked respondents whether their journey to residency was made easier once the Step 1 exam was converted to pass-fail, and interviews brought online, because of the COVID-19 pandemic.
Many residents conceded their journey became easier, less stressful, and less expensive under the new Step 1 formats. One respondent said he was freed up to focus more intently on higher-yield academic goals such as research.
Another respondent called the pass/fail change a “total game-changer,” as it lets applicants apply to all specialties while having other qualifications than test scores considered. A resident who took Step 1 before pass/fail was instituted described the “insurmountable stress associated with studying for Step 1 to get the highest score you possibly could.”
But not all residents liked the difficulty in being able to differentiate themselves, beyond med school pedigrees, in the absence of Step 1 scores.
Meanwhile, some doctors posting comments to the Medscape report strongly disagreed with the idea that residency life is getting harder. They depict residency as a rite of passage under the best of circumstances.
“Whatever issues there may be [today’s residents] are still making eight times what I got and, from what I’ve seen, we had a lot more independent responsibilities,” one physician commenter said.
Other doctors were more sympathetic and worried about the future price to be paid for hardships during residency. “Compensation should not be tied to the willingness to sacrifice the most beautiful years of life,” one commentator wrote.
Online interviews: Pros and cons
Many resident respondents celebrated the opportunity to interview for residency programs online. Some who traveled to in-person interviews before the pandemic said they racked up as much as $10,000 in travel costs, adding to their debt loads.
But not everyone was a fan. Other residents sniped that peers can apply to more residencies and “hoard” interviews, making the competition that much harder.
And how useful are online interviews to a prospective resident? “Virtual interviews are terrible for getting a true sense for a program or even the people,” a 1st-year family medicine resident complained. And it’s harder for an applicant “to shine when you’re on Zoom,” a 1st-year internal medicine resident opined.
Whether to report harassment
In survey, respondents were asked whether they ever witnessed sexual abuse, harassment, or misconduct; and if so, what they did about it. Among those who did, many opted to take no action, fearing retaliation or retribution. “I saw a resident made out to be a ‘problem resident’ when reporting it and then ultimately fired,” one respondent recounted.
Other residents said they felt unsure about the protocol, whom to report to, or even what constituted harassment or misconduct. “I didn’t realize [an incident] was harassment until later,” one resident said. Others thought “minor” or “subtle” incidents did not warrant action; “they are typically microaggressions and appear accepted within the culture of the institution.”
Residents’ confusion heightened when the perpetrator was a patient. “I’m not sure what to do about that,” a respondent acknowledged. An emergency medicine resident added, “most of the time … it is the patients who are acting inappropriately, saying inappropriate things, etc. There is no way to file a complaint like that.”
Rewards and challenges for residents
Among the most rewarding parts of residency that respondents described were developing specific skills such as surgical techniques, job security, and “learning a little day by day” in the words of a 1st-year gastroenterology resident.
Others felt gratified by the chances to help patients and families, their teams, and to advance social justice and health equity.
But challenges abound – chiefly money struggles. A 3rd-year psychiatry resident lamented “being financially strapped in the prime of my life from student loans and low wages.”
Stress and emotional fatigue also came up often as major challenges. “Constantly being told to do more, more presentations, more papers, more research, more studying,” a 5th-year neurosurgery resident bemoaned. “Being expected to be at the top of my game despite being sleep-deprived, depressed, and burned out,” a 3rd-year ob.gyn. resident groused.
But some physician commenters urged residents to look for long-term growth behind the challenges. “Yes, it was hard, but the experience was phenomenal, and I am glad I did it,” one doctor said.
A version of this article first appeared on Medscape.com.
Medical residents have cleared many hurdles to get where they are, as detailed in Medscape’s Residents Salary and Debt Report 2022 which explains their challenges with compensation and school loans as well as long hours and problematic personal relationships.
Whereas 72% of residents described themselves as “very satisfied” or “satisfied” with their professional training experience, only 27% felt that highly about how well they’re paid. Satisfaction levels increased somewhat farther into residency, reaching 35% in year 5.
Do residents have it easier today?
If so, is that rite of passage getting any easier? You’ll get different answers from residents and physicians.
Medscape asked respondents whether their journey to residency was made easier once the Step 1 exam was converted to pass-fail, and interviews brought online, because of the COVID-19 pandemic.
Many residents conceded their journey became easier, less stressful, and less expensive under the new Step 1 formats. One respondent said he was freed up to focus more intently on higher-yield academic goals such as research.
Another respondent called the pass/fail change a “total game-changer,” as it lets applicants apply to all specialties while having other qualifications than test scores considered. A resident who took Step 1 before pass/fail was instituted described the “insurmountable stress associated with studying for Step 1 to get the highest score you possibly could.”
But not all residents liked the difficulty in being able to differentiate themselves, beyond med school pedigrees, in the absence of Step 1 scores.
Meanwhile, some doctors posting comments to the Medscape report strongly disagreed with the idea that residency life is getting harder. They depict residency as a rite of passage under the best of circumstances.
“Whatever issues there may be [today’s residents] are still making eight times what I got and, from what I’ve seen, we had a lot more independent responsibilities,” one physician commenter said.
Other doctors were more sympathetic and worried about the future price to be paid for hardships during residency. “Compensation should not be tied to the willingness to sacrifice the most beautiful years of life,” one commentator wrote.
Online interviews: Pros and cons
Many resident respondents celebrated the opportunity to interview for residency programs online. Some who traveled to in-person interviews before the pandemic said they racked up as much as $10,000 in travel costs, adding to their debt loads.
But not everyone was a fan. Other residents sniped that peers can apply to more residencies and “hoard” interviews, making the competition that much harder.
And how useful are online interviews to a prospective resident? “Virtual interviews are terrible for getting a true sense for a program or even the people,” a 1st-year family medicine resident complained. And it’s harder for an applicant “to shine when you’re on Zoom,” a 1st-year internal medicine resident opined.
Whether to report harassment
In survey, respondents were asked whether they ever witnessed sexual abuse, harassment, or misconduct; and if so, what they did about it. Among those who did, many opted to take no action, fearing retaliation or retribution. “I saw a resident made out to be a ‘problem resident’ when reporting it and then ultimately fired,” one respondent recounted.
Other residents said they felt unsure about the protocol, whom to report to, or even what constituted harassment or misconduct. “I didn’t realize [an incident] was harassment until later,” one resident said. Others thought “minor” or “subtle” incidents did not warrant action; “they are typically microaggressions and appear accepted within the culture of the institution.”
Residents’ confusion heightened when the perpetrator was a patient. “I’m not sure what to do about that,” a respondent acknowledged. An emergency medicine resident added, “most of the time … it is the patients who are acting inappropriately, saying inappropriate things, etc. There is no way to file a complaint like that.”
Rewards and challenges for residents
Among the most rewarding parts of residency that respondents described were developing specific skills such as surgical techniques, job security, and “learning a little day by day” in the words of a 1st-year gastroenterology resident.
Others felt gratified by the chances to help patients and families, their teams, and to advance social justice and health equity.
But challenges abound – chiefly money struggles. A 3rd-year psychiatry resident lamented “being financially strapped in the prime of my life from student loans and low wages.”
Stress and emotional fatigue also came up often as major challenges. “Constantly being told to do more, more presentations, more papers, more research, more studying,” a 5th-year neurosurgery resident bemoaned. “Being expected to be at the top of my game despite being sleep-deprived, depressed, and burned out,” a 3rd-year ob.gyn. resident groused.
But some physician commenters urged residents to look for long-term growth behind the challenges. “Yes, it was hard, but the experience was phenomenal, and I am glad I did it,” one doctor said.
A version of this article first appeared on Medscape.com.
Medical residents have cleared many hurdles to get where they are, as detailed in Medscape’s Residents Salary and Debt Report 2022 which explains their challenges with compensation and school loans as well as long hours and problematic personal relationships.
Whereas 72% of residents described themselves as “very satisfied” or “satisfied” with their professional training experience, only 27% felt that highly about how well they’re paid. Satisfaction levels increased somewhat farther into residency, reaching 35% in year 5.
Do residents have it easier today?
If so, is that rite of passage getting any easier? You’ll get different answers from residents and physicians.
Medscape asked respondents whether their journey to residency was made easier once the Step 1 exam was converted to pass-fail, and interviews brought online, because of the COVID-19 pandemic.
Many residents conceded their journey became easier, less stressful, and less expensive under the new Step 1 formats. One respondent said he was freed up to focus more intently on higher-yield academic goals such as research.
Another respondent called the pass/fail change a “total game-changer,” as it lets applicants apply to all specialties while having other qualifications than test scores considered. A resident who took Step 1 before pass/fail was instituted described the “insurmountable stress associated with studying for Step 1 to get the highest score you possibly could.”
But not all residents liked the difficulty in being able to differentiate themselves, beyond med school pedigrees, in the absence of Step 1 scores.
Meanwhile, some doctors posting comments to the Medscape report strongly disagreed with the idea that residency life is getting harder. They depict residency as a rite of passage under the best of circumstances.
“Whatever issues there may be [today’s residents] are still making eight times what I got and, from what I’ve seen, we had a lot more independent responsibilities,” one physician commenter said.
Other doctors were more sympathetic and worried about the future price to be paid for hardships during residency. “Compensation should not be tied to the willingness to sacrifice the most beautiful years of life,” one commentator wrote.
Online interviews: Pros and cons
Many resident respondents celebrated the opportunity to interview for residency programs online. Some who traveled to in-person interviews before the pandemic said they racked up as much as $10,000 in travel costs, adding to their debt loads.
But not everyone was a fan. Other residents sniped that peers can apply to more residencies and “hoard” interviews, making the competition that much harder.
And how useful are online interviews to a prospective resident? “Virtual interviews are terrible for getting a true sense for a program or even the people,” a 1st-year family medicine resident complained. And it’s harder for an applicant “to shine when you’re on Zoom,” a 1st-year internal medicine resident opined.
Whether to report harassment
In survey, respondents were asked whether they ever witnessed sexual abuse, harassment, or misconduct; and if so, what they did about it. Among those who did, many opted to take no action, fearing retaliation or retribution. “I saw a resident made out to be a ‘problem resident’ when reporting it and then ultimately fired,” one respondent recounted.
Other residents said they felt unsure about the protocol, whom to report to, or even what constituted harassment or misconduct. “I didn’t realize [an incident] was harassment until later,” one resident said. Others thought “minor” or “subtle” incidents did not warrant action; “they are typically microaggressions and appear accepted within the culture of the institution.”
Residents’ confusion heightened when the perpetrator was a patient. “I’m not sure what to do about that,” a respondent acknowledged. An emergency medicine resident added, “most of the time … it is the patients who are acting inappropriately, saying inappropriate things, etc. There is no way to file a complaint like that.”
Rewards and challenges for residents
Among the most rewarding parts of residency that respondents described were developing specific skills such as surgical techniques, job security, and “learning a little day by day” in the words of a 1st-year gastroenterology resident.
Others felt gratified by the chances to help patients and families, their teams, and to advance social justice and health equity.
But challenges abound – chiefly money struggles. A 3rd-year psychiatry resident lamented “being financially strapped in the prime of my life from student loans and low wages.”
Stress and emotional fatigue also came up often as major challenges. “Constantly being told to do more, more presentations, more papers, more research, more studying,” a 5th-year neurosurgery resident bemoaned. “Being expected to be at the top of my game despite being sleep-deprived, depressed, and burned out,” a 3rd-year ob.gyn. resident groused.
But some physician commenters urged residents to look for long-term growth behind the challenges. “Yes, it was hard, but the experience was phenomenal, and I am glad I did it,” one doctor said.
A version of this article first appeared on Medscape.com.
A plane crash interrupts a doctor’s vacation
Emergencies happen anywhere, anytime – and sometimes physicians find themselves in situations where they are the only ones who can help. “Is There a Doctor in the House?” is a new series telling these stories.
When the plane crashed, I was asleep. I had arrived the evening before with my wife and three sons at a house on Kezar Lake on the Maine–New Hampshire border. I jumped out of bed and ran downstairs. My kids had been watching a float plane circling and gliding along the lake. It had crashed into the water and flipped upside down. My oldest brother-in-law jumped into his ski boat and we sped out to the scene.
All we can see are the plane’s pontoons. The rest is underwater. A woman has already surfaced, screaming. I dive in.
I find the woman’s husband and 3-year-old son struggling to get free from the plane through the smashed windshield. They manage to get to the surface. The pilot is dead, impaled through the chest by the left wing strut.
The big problem: A little girl, whom I would learn later is named Lauren, remained trapped. The water is murky but I can see her, a 5- or 6-year-old girl with this long hair, strapped in upside down and unconscious.
The mom and I dive down over and over, pulling and ripping at the door. We cannot get it open. Finally, I’m able to bend the door open enough where I can reach in, but I can’t undo the seatbelt. In my mind, I’m debating, should I try and go through the front windshield? I’m getting really tired, I can tell there’s fuel in the water, and I don’t want to drown in the plane. So I pop up to the surface and yell, “Does anyone have a knife?”
My brother-in-law shoots back to shore in the boat, screaming, “Get a knife!” My niece gets in the boat with one. I’m standing on the pontoon, and my niece is in the front of the boat calling, “Uncle Todd! Uncle Todd!” and she throws the knife. It goes way over my head. I can’t even jump for it, it’s so high.
I have to get the knife. So, I dive into the water to try and find it. Somehow, the black knife has landed on the white wing, 4 or 5 feet under the water. Pure luck. It could have sunk down a hundred feet into the lake. I grab the knife and hand it to the mom, Beth. She’s able to cut the seatbelt, and we both pull Lauren to the surface.
I lay her out on the pontoon. She has no pulse and her pupils are fixed and dilated. Her mom is yelling, “She’s dead, isn’t she?” I start CPR. My skin and eyes are burning from the airplane fuel in the water. I get her breathing, and her heart comes back very quickly. Lauren starts to vomit and I’m trying to keep her airway clear. She’s breathing spontaneously and she has a pulse, so I decide it’s time to move her to shore.
We pull the boat up to the dock and Lauren’s now having anoxic seizures. Her brain has been without oxygen, and now she’s getting perfused again. We get her to shore and lay her on the lawn. I’m still doing mouth-to-mouth, but she’s seizing like crazy, and I don’t have any way to control that. Beth is crying and wants to hold her daughter gently while I’m working.
Someone had called 911, and finally this dude shows up with an ambulance, and it’s like something out of World War II. All he has is an oxygen tank, but the mask is old and cracked. It’s too big for Lauren, but it sort of fits me, so I’m sucking in oxygen and blowing it into the girl’s mouth. I’m doing whatever I can, but I don’t have an IV to start. I have no fluids. I got nothing.
As it happens, I’d done my emergency medicine training at Maine Medical Center, so I tell someone to call them and get a Life Flight chopper. We have to drive somewhere where the chopper can land, so we take the ambulance to the parking lot of the closest store called the Wicked Good Store. That’s a common thing in Maine. Everything is “wicked good.”
The whole town is there by that point. The chopper arrives. The ambulance doors pop open and a woman says, “Todd?” And I say, “Heather?”
Heather is an emergency flight nurse whom I’d trained with many years ago. There’s immediate trust. She has all the right equipment. We put in breathing tubes and IVs. We stop Lauren from seizing. The kid is soon stable.
There is only one extra seat in the chopper, so I tell Beth to go. They take off.
Suddenly, I begin to doubt my decision. Lauren had been underwater for 15 minutes at minimum. I know how long that is. Did I do the right thing? Did I resuscitate a brain-dead child? I didn’t think about it at the time, but if that patient had come to me in the emergency department, I’m honestly not sure what I would have done.
So, I go home. And I don’t get a call. The FAA and sheriff arrive to take statements from us. I don’t hear from anyone.
The next day I start calling. No one will tell me anything, so I finally get to one of the pediatric ICU attendings who had trained me. He says Lauren literally woke up and said, “I have to go pee.” And that was it. She was 100% normal. I couldn’t believe it.
Here’s a theory: In kids, there’s something called the glottic reflex. I think her glottic reflex went off as soon as she hit the water, which basically closed her airway. So when she passed out, she could never get enough water in her lungs and still had enough air in there to keep her alive. Later, I got a call from her uncle. He could barely get the words out because he was in tears. He said Lauren was doing beautifully.
Three days later, I drove to Lauren’s house with my wife and kids. I had her read to me. I watched her play on the jungle gym for motor function. All sorts of stuff. She was totally normal.
Beth told us that the night before the accident, her mother had given the women in her family what she called a “miracle bracelet,” a bracelet that is supposed to give you one miracle in your life. Beth said she had the bracelet on her wrist the day of the accident, and now it’s gone. “Saving Lauren’s life was my miracle,” she said.
Funny thing: For 20 years, I ran all the EMS, police, fire, ambulance, in Boulder, Colo., where I live. I wrote all the protocols, and I would never advise any of my paramedics to dive into jet fuel to save someone. That was risky. But at the time, it was totally automatic. I think it taught me not to give up in certain situations, because you really don’t know.
Dr. Dorfman is an emergency medicine physician in Boulder, Colo., and medical director at Cedalion Health.
A version of this article first appeared on Medscape.com.
Emergencies happen anywhere, anytime – and sometimes physicians find themselves in situations where they are the only ones who can help. “Is There a Doctor in the House?” is a new series telling these stories.
When the plane crashed, I was asleep. I had arrived the evening before with my wife and three sons at a house on Kezar Lake on the Maine–New Hampshire border. I jumped out of bed and ran downstairs. My kids had been watching a float plane circling and gliding along the lake. It had crashed into the water and flipped upside down. My oldest brother-in-law jumped into his ski boat and we sped out to the scene.
All we can see are the plane’s pontoons. The rest is underwater. A woman has already surfaced, screaming. I dive in.
I find the woman’s husband and 3-year-old son struggling to get free from the plane through the smashed windshield. They manage to get to the surface. The pilot is dead, impaled through the chest by the left wing strut.
The big problem: A little girl, whom I would learn later is named Lauren, remained trapped. The water is murky but I can see her, a 5- or 6-year-old girl with this long hair, strapped in upside down and unconscious.
The mom and I dive down over and over, pulling and ripping at the door. We cannot get it open. Finally, I’m able to bend the door open enough where I can reach in, but I can’t undo the seatbelt. In my mind, I’m debating, should I try and go through the front windshield? I’m getting really tired, I can tell there’s fuel in the water, and I don’t want to drown in the plane. So I pop up to the surface and yell, “Does anyone have a knife?”
My brother-in-law shoots back to shore in the boat, screaming, “Get a knife!” My niece gets in the boat with one. I’m standing on the pontoon, and my niece is in the front of the boat calling, “Uncle Todd! Uncle Todd!” and she throws the knife. It goes way over my head. I can’t even jump for it, it’s so high.
I have to get the knife. So, I dive into the water to try and find it. Somehow, the black knife has landed on the white wing, 4 or 5 feet under the water. Pure luck. It could have sunk down a hundred feet into the lake. I grab the knife and hand it to the mom, Beth. She’s able to cut the seatbelt, and we both pull Lauren to the surface.
I lay her out on the pontoon. She has no pulse and her pupils are fixed and dilated. Her mom is yelling, “She’s dead, isn’t she?” I start CPR. My skin and eyes are burning from the airplane fuel in the water. I get her breathing, and her heart comes back very quickly. Lauren starts to vomit and I’m trying to keep her airway clear. She’s breathing spontaneously and she has a pulse, so I decide it’s time to move her to shore.
We pull the boat up to the dock and Lauren’s now having anoxic seizures. Her brain has been without oxygen, and now she’s getting perfused again. We get her to shore and lay her on the lawn. I’m still doing mouth-to-mouth, but she’s seizing like crazy, and I don’t have any way to control that. Beth is crying and wants to hold her daughter gently while I’m working.
Someone had called 911, and finally this dude shows up with an ambulance, and it’s like something out of World War II. All he has is an oxygen tank, but the mask is old and cracked. It’s too big for Lauren, but it sort of fits me, so I’m sucking in oxygen and blowing it into the girl’s mouth. I’m doing whatever I can, but I don’t have an IV to start. I have no fluids. I got nothing.
As it happens, I’d done my emergency medicine training at Maine Medical Center, so I tell someone to call them and get a Life Flight chopper. We have to drive somewhere where the chopper can land, so we take the ambulance to the parking lot of the closest store called the Wicked Good Store. That’s a common thing in Maine. Everything is “wicked good.”
The whole town is there by that point. The chopper arrives. The ambulance doors pop open and a woman says, “Todd?” And I say, “Heather?”
Heather is an emergency flight nurse whom I’d trained with many years ago. There’s immediate trust. She has all the right equipment. We put in breathing tubes and IVs. We stop Lauren from seizing. The kid is soon stable.
There is only one extra seat in the chopper, so I tell Beth to go. They take off.
Suddenly, I begin to doubt my decision. Lauren had been underwater for 15 minutes at minimum. I know how long that is. Did I do the right thing? Did I resuscitate a brain-dead child? I didn’t think about it at the time, but if that patient had come to me in the emergency department, I’m honestly not sure what I would have done.
So, I go home. And I don’t get a call. The FAA and sheriff arrive to take statements from us. I don’t hear from anyone.
The next day I start calling. No one will tell me anything, so I finally get to one of the pediatric ICU attendings who had trained me. He says Lauren literally woke up and said, “I have to go pee.” And that was it. She was 100% normal. I couldn’t believe it.
Here’s a theory: In kids, there’s something called the glottic reflex. I think her glottic reflex went off as soon as she hit the water, which basically closed her airway. So when she passed out, she could never get enough water in her lungs and still had enough air in there to keep her alive. Later, I got a call from her uncle. He could barely get the words out because he was in tears. He said Lauren was doing beautifully.
Three days later, I drove to Lauren’s house with my wife and kids. I had her read to me. I watched her play on the jungle gym for motor function. All sorts of stuff. She was totally normal.
Beth told us that the night before the accident, her mother had given the women in her family what she called a “miracle bracelet,” a bracelet that is supposed to give you one miracle in your life. Beth said she had the bracelet on her wrist the day of the accident, and now it’s gone. “Saving Lauren’s life was my miracle,” she said.
Funny thing: For 20 years, I ran all the EMS, police, fire, ambulance, in Boulder, Colo., where I live. I wrote all the protocols, and I would never advise any of my paramedics to dive into jet fuel to save someone. That was risky. But at the time, it was totally automatic. I think it taught me not to give up in certain situations, because you really don’t know.
Dr. Dorfman is an emergency medicine physician in Boulder, Colo., and medical director at Cedalion Health.
A version of this article first appeared on Medscape.com.
Emergencies happen anywhere, anytime – and sometimes physicians find themselves in situations where they are the only ones who can help. “Is There a Doctor in the House?” is a new series telling these stories.
When the plane crashed, I was asleep. I had arrived the evening before with my wife and three sons at a house on Kezar Lake on the Maine–New Hampshire border. I jumped out of bed and ran downstairs. My kids had been watching a float plane circling and gliding along the lake. It had crashed into the water and flipped upside down. My oldest brother-in-law jumped into his ski boat and we sped out to the scene.
All we can see are the plane’s pontoons. The rest is underwater. A woman has already surfaced, screaming. I dive in.
I find the woman’s husband and 3-year-old son struggling to get free from the plane through the smashed windshield. They manage to get to the surface. The pilot is dead, impaled through the chest by the left wing strut.
The big problem: A little girl, whom I would learn later is named Lauren, remained trapped. The water is murky but I can see her, a 5- or 6-year-old girl with this long hair, strapped in upside down and unconscious.
The mom and I dive down over and over, pulling and ripping at the door. We cannot get it open. Finally, I’m able to bend the door open enough where I can reach in, but I can’t undo the seatbelt. In my mind, I’m debating, should I try and go through the front windshield? I’m getting really tired, I can tell there’s fuel in the water, and I don’t want to drown in the plane. So I pop up to the surface and yell, “Does anyone have a knife?”
My brother-in-law shoots back to shore in the boat, screaming, “Get a knife!” My niece gets in the boat with one. I’m standing on the pontoon, and my niece is in the front of the boat calling, “Uncle Todd! Uncle Todd!” and she throws the knife. It goes way over my head. I can’t even jump for it, it’s so high.
I have to get the knife. So, I dive into the water to try and find it. Somehow, the black knife has landed on the white wing, 4 or 5 feet under the water. Pure luck. It could have sunk down a hundred feet into the lake. I grab the knife and hand it to the mom, Beth. She’s able to cut the seatbelt, and we both pull Lauren to the surface.
I lay her out on the pontoon. She has no pulse and her pupils are fixed and dilated. Her mom is yelling, “She’s dead, isn’t she?” I start CPR. My skin and eyes are burning from the airplane fuel in the water. I get her breathing, and her heart comes back very quickly. Lauren starts to vomit and I’m trying to keep her airway clear. She’s breathing spontaneously and she has a pulse, so I decide it’s time to move her to shore.
We pull the boat up to the dock and Lauren’s now having anoxic seizures. Her brain has been without oxygen, and now she’s getting perfused again. We get her to shore and lay her on the lawn. I’m still doing mouth-to-mouth, but she’s seizing like crazy, and I don’t have any way to control that. Beth is crying and wants to hold her daughter gently while I’m working.
Someone had called 911, and finally this dude shows up with an ambulance, and it’s like something out of World War II. All he has is an oxygen tank, but the mask is old and cracked. It’s too big for Lauren, but it sort of fits me, so I’m sucking in oxygen and blowing it into the girl’s mouth. I’m doing whatever I can, but I don’t have an IV to start. I have no fluids. I got nothing.
As it happens, I’d done my emergency medicine training at Maine Medical Center, so I tell someone to call them and get a Life Flight chopper. We have to drive somewhere where the chopper can land, so we take the ambulance to the parking lot of the closest store called the Wicked Good Store. That’s a common thing in Maine. Everything is “wicked good.”
The whole town is there by that point. The chopper arrives. The ambulance doors pop open and a woman says, “Todd?” And I say, “Heather?”
Heather is an emergency flight nurse whom I’d trained with many years ago. There’s immediate trust. She has all the right equipment. We put in breathing tubes and IVs. We stop Lauren from seizing. The kid is soon stable.
There is only one extra seat in the chopper, so I tell Beth to go. They take off.
Suddenly, I begin to doubt my decision. Lauren had been underwater for 15 minutes at minimum. I know how long that is. Did I do the right thing? Did I resuscitate a brain-dead child? I didn’t think about it at the time, but if that patient had come to me in the emergency department, I’m honestly not sure what I would have done.
So, I go home. And I don’t get a call. The FAA and sheriff arrive to take statements from us. I don’t hear from anyone.
The next day I start calling. No one will tell me anything, so I finally get to one of the pediatric ICU attendings who had trained me. He says Lauren literally woke up and said, “I have to go pee.” And that was it. She was 100% normal. I couldn’t believe it.
Here’s a theory: In kids, there’s something called the glottic reflex. I think her glottic reflex went off as soon as she hit the water, which basically closed her airway. So when she passed out, she could never get enough water in her lungs and still had enough air in there to keep her alive. Later, I got a call from her uncle. He could barely get the words out because he was in tears. He said Lauren was doing beautifully.
Three days later, I drove to Lauren’s house with my wife and kids. I had her read to me. I watched her play on the jungle gym for motor function. All sorts of stuff. She was totally normal.
Beth told us that the night before the accident, her mother had given the women in her family what she called a “miracle bracelet,” a bracelet that is supposed to give you one miracle in your life. Beth said she had the bracelet on her wrist the day of the accident, and now it’s gone. “Saving Lauren’s life was my miracle,” she said.
Funny thing: For 20 years, I ran all the EMS, police, fire, ambulance, in Boulder, Colo., where I live. I wrote all the protocols, and I would never advise any of my paramedics to dive into jet fuel to save someone. That was risky. But at the time, it was totally automatic. I think it taught me not to give up in certain situations, because you really don’t know.
Dr. Dorfman is an emergency medicine physician in Boulder, Colo., and medical director at Cedalion Health.
A version of this article first appeared on Medscape.com.
Sick call
They call me and I go.
– William Carlos Williams
I never get sick. I’ve never had the flu. When everyone’s got a cold, I’m somehow immune. The last time I threw up was June 29th, 1980. You see, I work out almost daily, eat vegan, and sleep plenty. I drink gallons of pressed juice and throw down a few high-quality supplements. Yes, I’m that guy: The one who never gets sick. Well, I was anyway.
I am no longer that guy since our little girl became a supersocial little toddler. My undefeated welterweight “never-sick” title has been obliterated by multiple knockouts. One was a wicked adenovirus that broke the no-vomit streak. At one point, I lay on the luxury gray tile bathroom floor hoping to go unconscious to make the nausea stop. I actually called out sick that day. Then with a nasty COVID-despite-vaccine infection. I called out again. Later with a hacking lower respiratory – RSV?! – bug. Called out. All of which our 2-year-old blonde, curly-haired vector transmitted to me with remarkable efficiency.
In fact, That’s saying a lot. Our docs, like most, don’t call out sick.
We physicians have legendary stamina. Compared with other professionals, we are no less likely to become ill but a whopping 80% less likely to call out sick.
Presenteeism is our physician version of Omerta, a code of honor to never give in even at the expense of our, or our family’s, health and well-being. Every medical student is regaled with stories of physicians getting an IV before rounds or finishing clinic after their water broke. Why? In part it’s an indoctrination into this thing of ours we call Medicine: An elitist club that admits only those able to pass O-chem and hold diarrhea. But it is also because our medical system is so brittle that the slightest bend causes it to shatter. When I cancel a clinic, patients who have waited weeks for their spot have to be sent home. And for critical cases or those patients who don’t get the message, my already slammed colleagues have to cram the unlucky ones in between already-scheduled appointments. The guilt induced by inconveniencing our colleagues and our patients is more potent than dry heaves. And so we go. Suck it up. Sip ginger ale. Load up on acetaminophen. Carry on. This harms not only us, but also patients whom we put in the path of transmission. We become terrible 2-year-olds.
Of course, it’s not always easy to tell if you’re sick enough to stay home. But the stigma of calling out is so great that we often show up no matter what symptoms. A recent Medscape survey of physicians found that 85% said they had come to work sick in 2022.
We can do better. Perhaps creating sick-leave protocols could help? For example, if you have a fever above 100.4, have contact with someone positive for influenza, are unable to take POs, etc. then stay home. So might building rolling slack into schedules to accommodate the inevitable physician illness, parenting emergency, or death of an beloved uncle. And if there is one thing artificial intelligence could help us with, it would be smart scheduling. Can’t we build algorithms for anticipating and absorbing these predictable events? I’d take that over an AI skin cancer detector any day. Yet this year we’ll struggle through the cold and flu (and COVID) season again and nothing will have changed.
Our daughter hasn’t had hand, foot, and mouth disease yet. It’s not a question of if, but rather when she, and her mom and I, will get it. I hope it happens on a Friday so that my Monday clinic will be bearable when I show up.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
They call me and I go.
– William Carlos Williams
I never get sick. I’ve never had the flu. When everyone’s got a cold, I’m somehow immune. The last time I threw up was June 29th, 1980. You see, I work out almost daily, eat vegan, and sleep plenty. I drink gallons of pressed juice and throw down a few high-quality supplements. Yes, I’m that guy: The one who never gets sick. Well, I was anyway.
I am no longer that guy since our little girl became a supersocial little toddler. My undefeated welterweight “never-sick” title has been obliterated by multiple knockouts. One was a wicked adenovirus that broke the no-vomit streak. At one point, I lay on the luxury gray tile bathroom floor hoping to go unconscious to make the nausea stop. I actually called out sick that day. Then with a nasty COVID-despite-vaccine infection. I called out again. Later with a hacking lower respiratory – RSV?! – bug. Called out. All of which our 2-year-old blonde, curly-haired vector transmitted to me with remarkable efficiency.
In fact, That’s saying a lot. Our docs, like most, don’t call out sick.
We physicians have legendary stamina. Compared with other professionals, we are no less likely to become ill but a whopping 80% less likely to call out sick.
Presenteeism is our physician version of Omerta, a code of honor to never give in even at the expense of our, or our family’s, health and well-being. Every medical student is regaled with stories of physicians getting an IV before rounds or finishing clinic after their water broke. Why? In part it’s an indoctrination into this thing of ours we call Medicine: An elitist club that admits only those able to pass O-chem and hold diarrhea. But it is also because our medical system is so brittle that the slightest bend causes it to shatter. When I cancel a clinic, patients who have waited weeks for their spot have to be sent home. And for critical cases or those patients who don’t get the message, my already slammed colleagues have to cram the unlucky ones in between already-scheduled appointments. The guilt induced by inconveniencing our colleagues and our patients is more potent than dry heaves. And so we go. Suck it up. Sip ginger ale. Load up on acetaminophen. Carry on. This harms not only us, but also patients whom we put in the path of transmission. We become terrible 2-year-olds.
Of course, it’s not always easy to tell if you’re sick enough to stay home. But the stigma of calling out is so great that we often show up no matter what symptoms. A recent Medscape survey of physicians found that 85% said they had come to work sick in 2022.
We can do better. Perhaps creating sick-leave protocols could help? For example, if you have a fever above 100.4, have contact with someone positive for influenza, are unable to take POs, etc. then stay home. So might building rolling slack into schedules to accommodate the inevitable physician illness, parenting emergency, or death of an beloved uncle. And if there is one thing artificial intelligence could help us with, it would be smart scheduling. Can’t we build algorithms for anticipating and absorbing these predictable events? I’d take that over an AI skin cancer detector any day. Yet this year we’ll struggle through the cold and flu (and COVID) season again and nothing will have changed.
Our daughter hasn’t had hand, foot, and mouth disease yet. It’s not a question of if, but rather when she, and her mom and I, will get it. I hope it happens on a Friday so that my Monday clinic will be bearable when I show up.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
They call me and I go.
– William Carlos Williams
I never get sick. I’ve never had the flu. When everyone’s got a cold, I’m somehow immune. The last time I threw up was June 29th, 1980. You see, I work out almost daily, eat vegan, and sleep plenty. I drink gallons of pressed juice and throw down a few high-quality supplements. Yes, I’m that guy: The one who never gets sick. Well, I was anyway.
I am no longer that guy since our little girl became a supersocial little toddler. My undefeated welterweight “never-sick” title has been obliterated by multiple knockouts. One was a wicked adenovirus that broke the no-vomit streak. At one point, I lay on the luxury gray tile bathroom floor hoping to go unconscious to make the nausea stop. I actually called out sick that day. Then with a nasty COVID-despite-vaccine infection. I called out again. Later with a hacking lower respiratory – RSV?! – bug. Called out. All of which our 2-year-old blonde, curly-haired vector transmitted to me with remarkable efficiency.
In fact, That’s saying a lot. Our docs, like most, don’t call out sick.
We physicians have legendary stamina. Compared with other professionals, we are no less likely to become ill but a whopping 80% less likely to call out sick.
Presenteeism is our physician version of Omerta, a code of honor to never give in even at the expense of our, or our family’s, health and well-being. Every medical student is regaled with stories of physicians getting an IV before rounds or finishing clinic after their water broke. Why? In part it’s an indoctrination into this thing of ours we call Medicine: An elitist club that admits only those able to pass O-chem and hold diarrhea. But it is also because our medical system is so brittle that the slightest bend causes it to shatter. When I cancel a clinic, patients who have waited weeks for their spot have to be sent home. And for critical cases or those patients who don’t get the message, my already slammed colleagues have to cram the unlucky ones in between already-scheduled appointments. The guilt induced by inconveniencing our colleagues and our patients is more potent than dry heaves. And so we go. Suck it up. Sip ginger ale. Load up on acetaminophen. Carry on. This harms not only us, but also patients whom we put in the path of transmission. We become terrible 2-year-olds.
Of course, it’s not always easy to tell if you’re sick enough to stay home. But the stigma of calling out is so great that we often show up no matter what symptoms. A recent Medscape survey of physicians found that 85% said they had come to work sick in 2022.
We can do better. Perhaps creating sick-leave protocols could help? For example, if you have a fever above 100.4, have contact with someone positive for influenza, are unable to take POs, etc. then stay home. So might building rolling slack into schedules to accommodate the inevitable physician illness, parenting emergency, or death of an beloved uncle. And if there is one thing artificial intelligence could help us with, it would be smart scheduling. Can’t we build algorithms for anticipating and absorbing these predictable events? I’d take that over an AI skin cancer detector any day. Yet this year we’ll struggle through the cold and flu (and COVID) season again and nothing will have changed.
Our daughter hasn’t had hand, foot, and mouth disease yet. It’s not a question of if, but rather when she, and her mom and I, will get it. I hope it happens on a Friday so that my Monday clinic will be bearable when I show up.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
Patient harm, not malpractice, top of mind for emergency medicine physicians
according to a study published in JAMA Network Open.
The cross-sectional study was conducted by researchers from Soroka University Medical Center, Israel; the University of Massachusetts, Worcester; Beth Israel Deaconess Medical Center; Harvard Medical School, Boston; and the University of Massachusetts, Amherst.
Online survey responses were collected from 1,222 emergency department attending physicians and advanced practice clinicians (APCs) in acute care hospitals throughout Massachusetts from January to September 2020.
Participants were asked to rank their level of agreement – from “strongly disagree” to “strongly agree” – with two statements: “In my day-to-day practice, I am fearful of making a mistake which results in [1] harm to the patient” (fear of harm) and [2] “being sued” (fear of suit).
The average age of the participants was about 44 years; 54.2% were men, 45.1% were women, and 0.7% were of other gender. Approximately 70% of responses were from MDs or DOs, and the remainder were from nurse practitioners and physician assistants. Participants had between 5 and 19 years of experience (median, 10 years).
The study found that the mean score was greater with regard to fear of harm than to fear of suit, regardless of clinician type, experience, or sex and whether the survey was completed before or after the start of the COVID-19 pandemic. There was no significant difference in mean scores regarding fear of suit before the pandemic and after it.
“Our data show a significantly greater fear of harming a patient than a fear of a malpractice suit,” Linda Isbell, PhD, professor of psychology at the University of Massachusetts, Amherst, who is one of the study’s authors, told this news organization. “There is a genuine concern and fear of harming patients and a desire to provide the best care for the patient’s well-being.”
In general, fear-of-harm and fear-of-suit scores decreased as providers gained experience. Those with less than 5 years of experience reported the highest levels of both.
“Although our data do not specifically provide reasons why age may impact [fear] levels, it is possible that with more practice experience ... providers have a better sense of the likelihood of patient harm and malpractice and how to manage such outcomes should they happen,” says Dr. Isbell. She noted that a longitudinal study is necessary to confirm this hypothesis.
One exception was female APCs, whose fear-of-harm scores remained relatively steady across all experience levels. Among male APCs, fear of causing patient harm decreased among those with 5-14 years of experience but increased slightly at 14-44 years of experience.
While previous research typically focused on fear of malpractice as a significant driver of defensive medicine, such as testing excessively, this study examined providers’ fear of harming patients because of a medical error.
The findings suggest “that fear of harm should be considered with, and may be more consequential than, fear of suit in medical decision-making,” the authors note.
“[F]ear can motivate people to engage in more careful and thorough information processing, which can drive behaviors in systematic ways,” says Dr. Isbell. “It is possible that one’s fear of harming a patient is triggering a high level of vigilance, reflected in the practice of defensive medicine across different types of patients – some of whom may be better off with less testing and referrals.”
Rade B. Vukmir, MD, JD, FACEP, an emergency medicine physician and spokesman for the American College of Emergency Physicians, says defensive medicine is common in the specialty and that it occurs 20%-40% of the time.
“Early in practice, the proverbial worst sin is missing a diagnosis, so that’s where the overtesting mentality comes from,” he says. In addition, “there are cities where you can’t drive a mile without seeing a half dozen legal advertisements. That imposes a cost burden on the system, [adding] roughly 20% to the cost of overall care.”
Emergency medicine providers attempt to minimize testing, but between their role as “America’s safety net” and the difficult circumstances they often face when treating patients, it takes a while to strike a balance, Dr. Vukmir acknowledges.
“There’s a training correlation, which showed up [in this study]; as people got further advanced in training, they felt more comfortable and felt the need to do it less,” says Dr. Vukmir.
The study was funded by a grant from the Agency for Healthcare Research and Quality. Dr. Isbell reports no conflicts of interest. Dr. Vukmir has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a study published in JAMA Network Open.
The cross-sectional study was conducted by researchers from Soroka University Medical Center, Israel; the University of Massachusetts, Worcester; Beth Israel Deaconess Medical Center; Harvard Medical School, Boston; and the University of Massachusetts, Amherst.
Online survey responses were collected from 1,222 emergency department attending physicians and advanced practice clinicians (APCs) in acute care hospitals throughout Massachusetts from January to September 2020.
Participants were asked to rank their level of agreement – from “strongly disagree” to “strongly agree” – with two statements: “In my day-to-day practice, I am fearful of making a mistake which results in [1] harm to the patient” (fear of harm) and [2] “being sued” (fear of suit).
The average age of the participants was about 44 years; 54.2% were men, 45.1% were women, and 0.7% were of other gender. Approximately 70% of responses were from MDs or DOs, and the remainder were from nurse practitioners and physician assistants. Participants had between 5 and 19 years of experience (median, 10 years).
The study found that the mean score was greater with regard to fear of harm than to fear of suit, regardless of clinician type, experience, or sex and whether the survey was completed before or after the start of the COVID-19 pandemic. There was no significant difference in mean scores regarding fear of suit before the pandemic and after it.
“Our data show a significantly greater fear of harming a patient than a fear of a malpractice suit,” Linda Isbell, PhD, professor of psychology at the University of Massachusetts, Amherst, who is one of the study’s authors, told this news organization. “There is a genuine concern and fear of harming patients and a desire to provide the best care for the patient’s well-being.”
In general, fear-of-harm and fear-of-suit scores decreased as providers gained experience. Those with less than 5 years of experience reported the highest levels of both.
“Although our data do not specifically provide reasons why age may impact [fear] levels, it is possible that with more practice experience ... providers have a better sense of the likelihood of patient harm and malpractice and how to manage such outcomes should they happen,” says Dr. Isbell. She noted that a longitudinal study is necessary to confirm this hypothesis.
One exception was female APCs, whose fear-of-harm scores remained relatively steady across all experience levels. Among male APCs, fear of causing patient harm decreased among those with 5-14 years of experience but increased slightly at 14-44 years of experience.
While previous research typically focused on fear of malpractice as a significant driver of defensive medicine, such as testing excessively, this study examined providers’ fear of harming patients because of a medical error.
The findings suggest “that fear of harm should be considered with, and may be more consequential than, fear of suit in medical decision-making,” the authors note.
“[F]ear can motivate people to engage in more careful and thorough information processing, which can drive behaviors in systematic ways,” says Dr. Isbell. “It is possible that one’s fear of harming a patient is triggering a high level of vigilance, reflected in the practice of defensive medicine across different types of patients – some of whom may be better off with less testing and referrals.”
Rade B. Vukmir, MD, JD, FACEP, an emergency medicine physician and spokesman for the American College of Emergency Physicians, says defensive medicine is common in the specialty and that it occurs 20%-40% of the time.
“Early in practice, the proverbial worst sin is missing a diagnosis, so that’s where the overtesting mentality comes from,” he says. In addition, “there are cities where you can’t drive a mile without seeing a half dozen legal advertisements. That imposes a cost burden on the system, [adding] roughly 20% to the cost of overall care.”
Emergency medicine providers attempt to minimize testing, but between their role as “America’s safety net” and the difficult circumstances they often face when treating patients, it takes a while to strike a balance, Dr. Vukmir acknowledges.
“There’s a training correlation, which showed up [in this study]; as people got further advanced in training, they felt more comfortable and felt the need to do it less,” says Dr. Vukmir.
The study was funded by a grant from the Agency for Healthcare Research and Quality. Dr. Isbell reports no conflicts of interest. Dr. Vukmir has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a study published in JAMA Network Open.
The cross-sectional study was conducted by researchers from Soroka University Medical Center, Israel; the University of Massachusetts, Worcester; Beth Israel Deaconess Medical Center; Harvard Medical School, Boston; and the University of Massachusetts, Amherst.
Online survey responses were collected from 1,222 emergency department attending physicians and advanced practice clinicians (APCs) in acute care hospitals throughout Massachusetts from January to September 2020.
Participants were asked to rank their level of agreement – from “strongly disagree” to “strongly agree” – with two statements: “In my day-to-day practice, I am fearful of making a mistake which results in [1] harm to the patient” (fear of harm) and [2] “being sued” (fear of suit).
The average age of the participants was about 44 years; 54.2% were men, 45.1% were women, and 0.7% were of other gender. Approximately 70% of responses were from MDs or DOs, and the remainder were from nurse practitioners and physician assistants. Participants had between 5 and 19 years of experience (median, 10 years).
The study found that the mean score was greater with regard to fear of harm than to fear of suit, regardless of clinician type, experience, or sex and whether the survey was completed before or after the start of the COVID-19 pandemic. There was no significant difference in mean scores regarding fear of suit before the pandemic and after it.
“Our data show a significantly greater fear of harming a patient than a fear of a malpractice suit,” Linda Isbell, PhD, professor of psychology at the University of Massachusetts, Amherst, who is one of the study’s authors, told this news organization. “There is a genuine concern and fear of harming patients and a desire to provide the best care for the patient’s well-being.”
In general, fear-of-harm and fear-of-suit scores decreased as providers gained experience. Those with less than 5 years of experience reported the highest levels of both.
“Although our data do not specifically provide reasons why age may impact [fear] levels, it is possible that with more practice experience ... providers have a better sense of the likelihood of patient harm and malpractice and how to manage such outcomes should they happen,” says Dr. Isbell. She noted that a longitudinal study is necessary to confirm this hypothesis.
One exception was female APCs, whose fear-of-harm scores remained relatively steady across all experience levels. Among male APCs, fear of causing patient harm decreased among those with 5-14 years of experience but increased slightly at 14-44 years of experience.
While previous research typically focused on fear of malpractice as a significant driver of defensive medicine, such as testing excessively, this study examined providers’ fear of harming patients because of a medical error.
The findings suggest “that fear of harm should be considered with, and may be more consequential than, fear of suit in medical decision-making,” the authors note.
“[F]ear can motivate people to engage in more careful and thorough information processing, which can drive behaviors in systematic ways,” says Dr. Isbell. “It is possible that one’s fear of harming a patient is triggering a high level of vigilance, reflected in the practice of defensive medicine across different types of patients – some of whom may be better off with less testing and referrals.”
Rade B. Vukmir, MD, JD, FACEP, an emergency medicine physician and spokesman for the American College of Emergency Physicians, says defensive medicine is common in the specialty and that it occurs 20%-40% of the time.
“Early in practice, the proverbial worst sin is missing a diagnosis, so that’s where the overtesting mentality comes from,” he says. In addition, “there are cities where you can’t drive a mile without seeing a half dozen legal advertisements. That imposes a cost burden on the system, [adding] roughly 20% to the cost of overall care.”
Emergency medicine providers attempt to minimize testing, but between their role as “America’s safety net” and the difficult circumstances they often face when treating patients, it takes a while to strike a balance, Dr. Vukmir acknowledges.
“There’s a training correlation, which showed up [in this study]; as people got further advanced in training, they felt more comfortable and felt the need to do it less,” says Dr. Vukmir.
The study was funded by a grant from the Agency for Healthcare Research and Quality. Dr. Isbell reports no conflicts of interest. Dr. Vukmir has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Is there a doctor on the plane? Tips for providing in-flight assistance
In most cases, passengers on an airline flight are representative of the general population, which means that anyone could have an emergency at any time.
as determined on the basis of in-flight medical emergencies that resulted in calls to a physician-directed medical communications center, said Amy Faith Ho, MD, MPH of Integrative Emergency Services, Dallas–Fort Worth, in a presentation at the annual meeting of the American College of Emergency Physicians.
The study authors reviewed records of 11,920 in-flight medical emergencies between Jan. 1, 2008, and Oct. 31, 2010. The data showed that physician passengers provided medical assistance in nearly half of in-flight emergencies (48.1%) and that flights were diverted because of the emergency in 7.3% of cases.
The majority of the in-flight emergencies involved syncope or presyncope (37.4% of cases), followed by respiratory symptoms (12.1%) and nausea or vomiting (9.5%), according to the study.
When a physician is faced with an in-flight emergency, the medical team includes the physician himself, medical ground control, and the flight attendants, said Dr. Ho. Requirements may vary among airlines, but all flight attendants will be trained in cardiopulmonary resuscitation (CPR) or basic life support, as well as use of automated external defibrillators (AEDs).
Physician call centers (medical ground control) can provide additional assistance remotely, she said.
The in-flight medical bag
Tools in a physician’s in-flight toolbox start with the first-aid kit. Airplanes also have an emergency medical kit (EMK), an oxygen tank, and an AED.
The minimum EMK contents are mandated by the Federal Aviation Administration, said Dr. Ho. The standard equipment includes a stethoscope, a sphygmomanometer, and three sizes of oropharyngeal airways. Other items include self-inflating manual resuscitation devices and CPR masks in thee sizes, alcohol sponges, gloves, adhesive tape, scissors, a tourniquet, as well as saline solution, needles, syringes, and an intravenous administration set consisting of tubing and two Y connectors.
An EMK also should contain the following medications: nonnarcotic analgesic tablets, antihistamine tablets, an injectable antihistamine, atropine, aspirin tablets, a bronchodilator, and epinephrine (both 1:1000; 1 injectable cc and 1:10,000; two injectable cc). Nitroglycerin tablets and 5 cc of 20 mg/mL injectable cardiac lidocaine are part of the mandated kit as well, according to Dr. Ho.
Some airlines carry additional supplies on all their flights, said Dr. Ho. Notably, American Airlines and British Airways carry EpiPens for adults and children, as well as opioid reversal medication (naloxone) and glucose for managing low blood sugar. American Airlines and Delta stock antiemetics, and Delta also carries naloxone. British Airways is unique in stocking additional cardiac medications, both oral and injectable.
How to handle an in-flight emergency
Physicians should always carry a copy of their medical license when traveling for documentation by the airline if they assist in a medical emergency during a flight, Dr. Ho emphasized. “Staff” personnel should be used. These include the flight attendants, medical ground control, and other passengers who might have useful skills, such as nursing, the ability to perform CPR, or therapy/counseling to calm a frightened patient. If needed, “crowdsource additional supplies from passengers,” such as a glucometer or pulse oximeter.
Legal lessons
Physicians are not obligated to assist during an in-flight medical emergency, said Dr. Ho. Legal jurisdiction can vary. In the United States, a bystander who assists in an emergency is generally protected by Good Samaritan laws; for international airlines, the laws may vary; those where the airline is based usually apply.
The Aviation Medical Assistance Act, passed in 1998, protects individuals from being sued for negligence while providing medical assistance, “unless the individual, while rendering such assistance, is guilty of gross negligence of willful misconduct,” Dr. Ho noted. The Aviation Medical Assistance Act also protects the airline itself “if the carrier in good faith believes that the passenger is a medically qualified individual.”
Dr. Ho disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In most cases, passengers on an airline flight are representative of the general population, which means that anyone could have an emergency at any time.
as determined on the basis of in-flight medical emergencies that resulted in calls to a physician-directed medical communications center, said Amy Faith Ho, MD, MPH of Integrative Emergency Services, Dallas–Fort Worth, in a presentation at the annual meeting of the American College of Emergency Physicians.
The study authors reviewed records of 11,920 in-flight medical emergencies between Jan. 1, 2008, and Oct. 31, 2010. The data showed that physician passengers provided medical assistance in nearly half of in-flight emergencies (48.1%) and that flights were diverted because of the emergency in 7.3% of cases.
The majority of the in-flight emergencies involved syncope or presyncope (37.4% of cases), followed by respiratory symptoms (12.1%) and nausea or vomiting (9.5%), according to the study.
When a physician is faced with an in-flight emergency, the medical team includes the physician himself, medical ground control, and the flight attendants, said Dr. Ho. Requirements may vary among airlines, but all flight attendants will be trained in cardiopulmonary resuscitation (CPR) or basic life support, as well as use of automated external defibrillators (AEDs).
Physician call centers (medical ground control) can provide additional assistance remotely, she said.
The in-flight medical bag
Tools in a physician’s in-flight toolbox start with the first-aid kit. Airplanes also have an emergency medical kit (EMK), an oxygen tank, and an AED.
The minimum EMK contents are mandated by the Federal Aviation Administration, said Dr. Ho. The standard equipment includes a stethoscope, a sphygmomanometer, and three sizes of oropharyngeal airways. Other items include self-inflating manual resuscitation devices and CPR masks in thee sizes, alcohol sponges, gloves, adhesive tape, scissors, a tourniquet, as well as saline solution, needles, syringes, and an intravenous administration set consisting of tubing and two Y connectors.
An EMK also should contain the following medications: nonnarcotic analgesic tablets, antihistamine tablets, an injectable antihistamine, atropine, aspirin tablets, a bronchodilator, and epinephrine (both 1:1000; 1 injectable cc and 1:10,000; two injectable cc). Nitroglycerin tablets and 5 cc of 20 mg/mL injectable cardiac lidocaine are part of the mandated kit as well, according to Dr. Ho.
Some airlines carry additional supplies on all their flights, said Dr. Ho. Notably, American Airlines and British Airways carry EpiPens for adults and children, as well as opioid reversal medication (naloxone) and glucose for managing low blood sugar. American Airlines and Delta stock antiemetics, and Delta also carries naloxone. British Airways is unique in stocking additional cardiac medications, both oral and injectable.
How to handle an in-flight emergency
Physicians should always carry a copy of their medical license when traveling for documentation by the airline if they assist in a medical emergency during a flight, Dr. Ho emphasized. “Staff” personnel should be used. These include the flight attendants, medical ground control, and other passengers who might have useful skills, such as nursing, the ability to perform CPR, or therapy/counseling to calm a frightened patient. If needed, “crowdsource additional supplies from passengers,” such as a glucometer or pulse oximeter.
Legal lessons
Physicians are not obligated to assist during an in-flight medical emergency, said Dr. Ho. Legal jurisdiction can vary. In the United States, a bystander who assists in an emergency is generally protected by Good Samaritan laws; for international airlines, the laws may vary; those where the airline is based usually apply.
The Aviation Medical Assistance Act, passed in 1998, protects individuals from being sued for negligence while providing medical assistance, “unless the individual, while rendering such assistance, is guilty of gross negligence of willful misconduct,” Dr. Ho noted. The Aviation Medical Assistance Act also protects the airline itself “if the carrier in good faith believes that the passenger is a medically qualified individual.”
Dr. Ho disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In most cases, passengers on an airline flight are representative of the general population, which means that anyone could have an emergency at any time.
as determined on the basis of in-flight medical emergencies that resulted in calls to a physician-directed medical communications center, said Amy Faith Ho, MD, MPH of Integrative Emergency Services, Dallas–Fort Worth, in a presentation at the annual meeting of the American College of Emergency Physicians.
The study authors reviewed records of 11,920 in-flight medical emergencies between Jan. 1, 2008, and Oct. 31, 2010. The data showed that physician passengers provided medical assistance in nearly half of in-flight emergencies (48.1%) and that flights were diverted because of the emergency in 7.3% of cases.
The majority of the in-flight emergencies involved syncope or presyncope (37.4% of cases), followed by respiratory symptoms (12.1%) and nausea or vomiting (9.5%), according to the study.
When a physician is faced with an in-flight emergency, the medical team includes the physician himself, medical ground control, and the flight attendants, said Dr. Ho. Requirements may vary among airlines, but all flight attendants will be trained in cardiopulmonary resuscitation (CPR) or basic life support, as well as use of automated external defibrillators (AEDs).
Physician call centers (medical ground control) can provide additional assistance remotely, she said.
The in-flight medical bag
Tools in a physician’s in-flight toolbox start with the first-aid kit. Airplanes also have an emergency medical kit (EMK), an oxygen tank, and an AED.
The minimum EMK contents are mandated by the Federal Aviation Administration, said Dr. Ho. The standard equipment includes a stethoscope, a sphygmomanometer, and three sizes of oropharyngeal airways. Other items include self-inflating manual resuscitation devices and CPR masks in thee sizes, alcohol sponges, gloves, adhesive tape, scissors, a tourniquet, as well as saline solution, needles, syringes, and an intravenous administration set consisting of tubing and two Y connectors.
An EMK also should contain the following medications: nonnarcotic analgesic tablets, antihistamine tablets, an injectable antihistamine, atropine, aspirin tablets, a bronchodilator, and epinephrine (both 1:1000; 1 injectable cc and 1:10,000; two injectable cc). Nitroglycerin tablets and 5 cc of 20 mg/mL injectable cardiac lidocaine are part of the mandated kit as well, according to Dr. Ho.
Some airlines carry additional supplies on all their flights, said Dr. Ho. Notably, American Airlines and British Airways carry EpiPens for adults and children, as well as opioid reversal medication (naloxone) and glucose for managing low blood sugar. American Airlines and Delta stock antiemetics, and Delta also carries naloxone. British Airways is unique in stocking additional cardiac medications, both oral and injectable.
How to handle an in-flight emergency
Physicians should always carry a copy of their medical license when traveling for documentation by the airline if they assist in a medical emergency during a flight, Dr. Ho emphasized. “Staff” personnel should be used. These include the flight attendants, medical ground control, and other passengers who might have useful skills, such as nursing, the ability to perform CPR, or therapy/counseling to calm a frightened patient. If needed, “crowdsource additional supplies from passengers,” such as a glucometer or pulse oximeter.
Legal lessons
Physicians are not obligated to assist during an in-flight medical emergency, said Dr. Ho. Legal jurisdiction can vary. In the United States, a bystander who assists in an emergency is generally protected by Good Samaritan laws; for international airlines, the laws may vary; those where the airline is based usually apply.
The Aviation Medical Assistance Act, passed in 1998, protects individuals from being sued for negligence while providing medical assistance, “unless the individual, while rendering such assistance, is guilty of gross negligence of willful misconduct,” Dr. Ho noted. The Aviation Medical Assistance Act also protects the airline itself “if the carrier in good faith believes that the passenger is a medically qualified individual.”
Dr. Ho disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACEP 2022
Starting a podcast
In my last column, I discussed . At this writing (November 2022), more than 600 million blogs are online, compared with about 2 million podcasts, and relatively few of them are run by physicians. With podcasts, you have a better chance of standing out in a crowded online world.
Starting a podcast is not difficult, but there are several steps you need to go through before launching one.
As with blogging, start by outlining a long-range plan. Your general topic will probably be your specialty, but you will need to narrow your focus to a few specific subjects, such as the problems you see most often, or a subspecialty that you concentrate on. You can always expand your topic later, as you get more popular. Choose a name for your podcast, and purchase a domain name that accurately describes it.
You will also need to choose a hosting service. Numerous inexpensive hosting platforms are available, and a simple Google search will find them for you. Many of them provide free learning materials, helpful creative tools, and customer support to get you through the confusing technical aspects. They can also help you choose a music introduction (to add a bit of polish), and help you piece together your audio segments. Buzzsprout, RSS.com, and Podbean get good reviews on many sites. (As always, I have no financial interest in any company or service mentioned herein.)
Hosting services can assist you in creating a template – a framework that you can reuse each time you record an episode – containing your intro and exit music, tracks for your conversations, etc. This will make your podcasts instantly recognizable each time your listeners tune in.
Many podcasting experts recommend recruiting a co-host. This can be an associate within your practice, a friend who practices elsewhere, or perhaps a resident in an academic setting. You will be able to spread the workload of creating, editing, and promoting. Plus, it is much easier to generate interesting content when two people are having a conversation, rather than one person lecturing from a prepared script. You might also consider having multiple co-hosts, either to expand episodes into group discussions, or to take turns working with you in covering different subjects.
How long you make your podcast is entirely up to you. Some consultants recommend specific time frames, such as 5 minutes (because that’s an average attention span), or 28 minutes (because that’s the average driving commute time). There are short podcasts and long ones; whatever works for you is fine, as long as you don’t drift off the topic. Furthermore, no one says they must all be the same length; when you are finished talking, you are done. And no one says you must stick with one subject throughout. Combining several short segments might hold more listeners’ interest and will make it easier to share small clips on social media.
Content guidelines are similar to those for blogs. Give people content that will be of interest or benefit to them. Talk about subjects – medical and otherwise – that are relevant to your practice or are prominent in the news.
As with blogs, try to avoid polarizing political discussions, and while it’s fine to discuss treatments and procedures that you offer, aggressive solicitation tends to make viewers look elsewhere. Keep any medical advice in general terms; don’t portray any specific patients as examples.
When your podcast is ready, your hosting platform will show you how to submit it to iTunes, and how to submit your podcast RSS feed to other podcast directories. As you upload new episodes, your host will automatically update your RSS feed, so that any directory you are listed on will receive the new episode.
Once you are uploaded, you can use your host’s social sharing tools to spread the word. As with blogs, use social media, such as your practice’s Facebook page, to push podcast updates into patients’ feeds and track relevant Twitter hashtags to find online communities that might be interested in your subject matter. You should also find your episode embed code (which your host will have) and place it in a prominent place on your website so patients can listen directly from there.
Transcriptions are another excellent promotional tool. Search engines will “read” your podcasts and list them in searches. Some podcast hosts will do transcribing for a fee, but there are independent transcription services as well.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
In my last column, I discussed . At this writing (November 2022), more than 600 million blogs are online, compared with about 2 million podcasts, and relatively few of them are run by physicians. With podcasts, you have a better chance of standing out in a crowded online world.
Starting a podcast is not difficult, but there are several steps you need to go through before launching one.
As with blogging, start by outlining a long-range plan. Your general topic will probably be your specialty, but you will need to narrow your focus to a few specific subjects, such as the problems you see most often, or a subspecialty that you concentrate on. You can always expand your topic later, as you get more popular. Choose a name for your podcast, and purchase a domain name that accurately describes it.
You will also need to choose a hosting service. Numerous inexpensive hosting platforms are available, and a simple Google search will find them for you. Many of them provide free learning materials, helpful creative tools, and customer support to get you through the confusing technical aspects. They can also help you choose a music introduction (to add a bit of polish), and help you piece together your audio segments. Buzzsprout, RSS.com, and Podbean get good reviews on many sites. (As always, I have no financial interest in any company or service mentioned herein.)
Hosting services can assist you in creating a template – a framework that you can reuse each time you record an episode – containing your intro and exit music, tracks for your conversations, etc. This will make your podcasts instantly recognizable each time your listeners tune in.
Many podcasting experts recommend recruiting a co-host. This can be an associate within your practice, a friend who practices elsewhere, or perhaps a resident in an academic setting. You will be able to spread the workload of creating, editing, and promoting. Plus, it is much easier to generate interesting content when two people are having a conversation, rather than one person lecturing from a prepared script. You might also consider having multiple co-hosts, either to expand episodes into group discussions, or to take turns working with you in covering different subjects.
How long you make your podcast is entirely up to you. Some consultants recommend specific time frames, such as 5 minutes (because that’s an average attention span), or 28 minutes (because that’s the average driving commute time). There are short podcasts and long ones; whatever works for you is fine, as long as you don’t drift off the topic. Furthermore, no one says they must all be the same length; when you are finished talking, you are done. And no one says you must stick with one subject throughout. Combining several short segments might hold more listeners’ interest and will make it easier to share small clips on social media.
Content guidelines are similar to those for blogs. Give people content that will be of interest or benefit to them. Talk about subjects – medical and otherwise – that are relevant to your practice or are prominent in the news.
As with blogs, try to avoid polarizing political discussions, and while it’s fine to discuss treatments and procedures that you offer, aggressive solicitation tends to make viewers look elsewhere. Keep any medical advice in general terms; don’t portray any specific patients as examples.
When your podcast is ready, your hosting platform will show you how to submit it to iTunes, and how to submit your podcast RSS feed to other podcast directories. As you upload new episodes, your host will automatically update your RSS feed, so that any directory you are listed on will receive the new episode.
Once you are uploaded, you can use your host’s social sharing tools to spread the word. As with blogs, use social media, such as your practice’s Facebook page, to push podcast updates into patients’ feeds and track relevant Twitter hashtags to find online communities that might be interested in your subject matter. You should also find your episode embed code (which your host will have) and place it in a prominent place on your website so patients can listen directly from there.
Transcriptions are another excellent promotional tool. Search engines will “read” your podcasts and list them in searches. Some podcast hosts will do transcribing for a fee, but there are independent transcription services as well.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
In my last column, I discussed . At this writing (November 2022), more than 600 million blogs are online, compared with about 2 million podcasts, and relatively few of them are run by physicians. With podcasts, you have a better chance of standing out in a crowded online world.
Starting a podcast is not difficult, but there are several steps you need to go through before launching one.
As with blogging, start by outlining a long-range plan. Your general topic will probably be your specialty, but you will need to narrow your focus to a few specific subjects, such as the problems you see most often, or a subspecialty that you concentrate on. You can always expand your topic later, as you get more popular. Choose a name for your podcast, and purchase a domain name that accurately describes it.
You will also need to choose a hosting service. Numerous inexpensive hosting platforms are available, and a simple Google search will find them for you. Many of them provide free learning materials, helpful creative tools, and customer support to get you through the confusing technical aspects. They can also help you choose a music introduction (to add a bit of polish), and help you piece together your audio segments. Buzzsprout, RSS.com, and Podbean get good reviews on many sites. (As always, I have no financial interest in any company or service mentioned herein.)
Hosting services can assist you in creating a template – a framework that you can reuse each time you record an episode – containing your intro and exit music, tracks for your conversations, etc. This will make your podcasts instantly recognizable each time your listeners tune in.
Many podcasting experts recommend recruiting a co-host. This can be an associate within your practice, a friend who practices elsewhere, or perhaps a resident in an academic setting. You will be able to spread the workload of creating, editing, and promoting. Plus, it is much easier to generate interesting content when two people are having a conversation, rather than one person lecturing from a prepared script. You might also consider having multiple co-hosts, either to expand episodes into group discussions, or to take turns working with you in covering different subjects.
How long you make your podcast is entirely up to you. Some consultants recommend specific time frames, such as 5 minutes (because that’s an average attention span), or 28 minutes (because that’s the average driving commute time). There are short podcasts and long ones; whatever works for you is fine, as long as you don’t drift off the topic. Furthermore, no one says they must all be the same length; when you are finished talking, you are done. And no one says you must stick with one subject throughout. Combining several short segments might hold more listeners’ interest and will make it easier to share small clips on social media.
Content guidelines are similar to those for blogs. Give people content that will be of interest or benefit to them. Talk about subjects – medical and otherwise – that are relevant to your practice or are prominent in the news.
As with blogs, try to avoid polarizing political discussions, and while it’s fine to discuss treatments and procedures that you offer, aggressive solicitation tends to make viewers look elsewhere. Keep any medical advice in general terms; don’t portray any specific patients as examples.
When your podcast is ready, your hosting platform will show you how to submit it to iTunes, and how to submit your podcast RSS feed to other podcast directories. As you upload new episodes, your host will automatically update your RSS feed, so that any directory you are listed on will receive the new episode.
Once you are uploaded, you can use your host’s social sharing tools to spread the word. As with blogs, use social media, such as your practice’s Facebook page, to push podcast updates into patients’ feeds and track relevant Twitter hashtags to find online communities that might be interested in your subject matter. You should also find your episode embed code (which your host will have) and place it in a prominent place on your website so patients can listen directly from there.
Transcriptions are another excellent promotional tool. Search engines will “read” your podcasts and list them in searches. Some podcast hosts will do transcribing for a fee, but there are independent transcription services as well.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
These doctors earn less but say it’s worth it
Earning a huge salary was never a top priority for Sarah Ramer, MD, a nephrologist at the James J. Peters Veterans Affairs Medical Center in New York. That was obvious even when she was still a medical student, since she opted for an extra academic year to get a masters degree in clinical research methods.
After doing a combined internal medicine/pediatric residency, Dr. Ramer completed two fellowships, one in adult nephrology and one in palliative care.
“Every extra year that you spend in training is another year you’re not making a salary as an attending, so by doing 7 years in residency and a fellowship, I was not building my net worth the way some physicians do,” she says.
When Dr. Ramer, now 41, was ready to enter the job market in 2019, she had two offers on the table – one at a large, urban, research-intense medical center that included some clinical work combined with research and that paid $105,000, and one as a clinical nephrologist at a smaller suburban medical center with a $230,000 salary and a $20,000 performance bonus.
She took the first job, because she liked the idea of being ensured of having time to perform research, and she hoped to qualify for a career development grant from the National Institutes of Health.
Over the next few months, Dr. Ramer was diagnosed with cancer and the pandemic began ravaging the country. She considered taking a leave of absence from her job, but since she had only recently started at the job, taking a medical leave would mean she’d get only 50% of her salary, which would have left her with just over $50,000 to cover her mortgage, student loans, and other expenses.
“Financially, it would have been disastrous for me to go on leave at that time,” she says. “Things happen, but that’s something I didn’t consider when I decided to take a very low-paying job.”
Dr. Ramer has completed cancer treatment and has moved on to her current role at the VA medical center, where she is earning less than she would have made at the suburban medical center but more than twice as much as she did at the urban research hospital.
Lifestyle trade-offs
While Dr. Ramer’s salary is nearly four times that of the average American worker, it’s only about 60% of what the average physician earns. That works for Dr. Ramer, who has never put much value in material possessions. She has lived in the same working-class New Jersey neighborhood for more than a decade and drives a 2010 Hyundai Elantra.
“I need a new kitchen and new bathrooms in my apartment,” she says. “I’m still working on that. I have a good cushion, but I need to build up my emergency fund before I start spending money on home renovations.”
Such trade-offs are common among physicians who’ve chosen to work in a rural area, at a Medicaid practice, or in public health.
For Sean Kissel, MD, 30, a family physician in northern Utah, it’s about the lifestyle afforded by his role, which has earned him between $190,000 and $230,000 over the past few years. “I have no on-call shifts,” he says. “So, when I’m done, I’m done. I don’t have to work weekends or holidays, and I have dinner with my kids every night.”
According to the 2022 Medscape physician compensation report, physicians earned an average of $339,000 annually last year. Primary care physicians took home an average of $260,000, compared with $368,000 for specialists. The disparity in physician income was even greater when broken down by specialty. Plastic surgeons earned the most ($576,000), and public health and preventive medicine physicians earned the least ($243,000).
Still, that study found that physician salaries were up across every specialty, ranging from a 1% increase for critical care physicians to a 13% jump for otolaryngologists.
Scaling back
While private-pay physicians tend to make more than peers who work in community or government health clinics, they may have to work longer hours or face pressure to see more patients, which can decrease the quality of care they provide.
A recent study, published in JAMA Health Forum, found that most health systems base physician pay on the number of patients seen. That’s the case for more than 80% of primary care physicians and more than 90% of specialists, according to the study.
Given that landscape, a growing number of physicians are opting for a “lifestyle” practice – accepting lower compensation in order to see a limited number of patients or work only a few days per week, says Stu Schaff, the founder and lead adviser of Contract Medicine, a consulting firm that helps physicians understand, evaluate, and negotiate their employment contracts. Mr. Schaff concedes that most of the doctors who fall into this category are winding down their careers or have a high-earning spouse with a salary that offsets their lower income.
Other physicians move into administrative roles within a hospital or health center. Such positions typically involve seeing fewer patients and may pay less, but they also have more traditional hours, which can be appealing, Mr. Schaff says.
“Those folks might still do patient care 1 or 2 days a week, or even less,” he says. “They’re still physicians. They’re still using their physician expertise, but they’re not practicing at the same level or generating the same level of income as they might in a full-time clinical position.”
Cost of living matters
Dr. Kissel says that while he may take home less than the typical physician, he still makes enough to comfortably cover his expenses, including his student loan payments. Still, when new acquaintances learn he’s a physician, they often assume he’s earning much more.
“People assume most of us make mid-$300,000’s or low $400,000’s, and that’s true for some family doctors, but not for all,” he notes. “I like what I do. I’m in a good place, and we are happy with our life.”
Plus, Dr. Kissel may benefit from living in Utah, where the lower cost of living may allow him to stretch his salary further. Although salaries are typically higher in the most expensive states in the United States, compared with states that have a lower cost of living, those higher salaries aren’t always enough to make up the difference.
A recent WalletHub analysis found that New York, California, and Massachusetts were among the states with the lowest average annual wage when adjusted for the cost of living, while South Dakota, Indiana, and Wisconsin had the highest average wage after the adjustment.
“Even if a physician is in a lower-paying specialty or location, they’re still well-paid relative to the average U.S. citizen,” Mr. Schaff says. “When we talk about specialties that pay less, we’re still talking – if you’re full-time – about a six-figure income.”
Location, location, location
To combat a provider shortage, rural health centers have been increasing the pay doctors receive. Nevertheless, many physicians are opting not to live in a community where they have no connection.
“I think there has to be a tie to the community for a physician to want to be here,” says Scott Crouch, chief executive officer at Ozarks Community Health Center, Bolivar, Missouri. “NHSC [National Health Service Corps] can help some, but it’s not the draw it once was.”
Physicians and dentists who interview at the Ozarks Community Health Center often like the facilities and the area but don’t want to live in a rustic locale. “Most medical schools are in bigger cities,” Mr. Crouch says. “So it’s hard to get them into a rural environment.”
In some cases, physicians opt to go the community health route but choose to work in a city, even if it means they’re going to earn less. That was the case for Kevin King, MD, 33, a general pediatrician at St. John’s Community Health Center, Los Angeles. Dr. King knew he wanted to work in a community health center after completing a residency at a Medicaid clinic.
A rewarding career
“I find my work very rewarding,” Dr. King says. “Working in Medicaid can be difficult, and there are many barriers to care. It’s a lot more work to get things done, but the rewards come from the patients.”
That said, Dr. King adds he wouldn’t have been able to take this job without access to a loan forgiveness program that helps him manage his student-loan debt. “Without that, I’d probably have to find work in a private-pay population, making more money,” he says. “Loan forgiveness allowed me to choose this career path.”
Dr. King says he earns between $150,000 and $200,000 annually. That’s significantly less than the $243,000 median pediatrician salary in Los Angeles, according to Salary.com. Still, Dr. King says he wouldn’t trade his job for a more lucrative one.
“In medicine, there are so many different career paths you can take after residency training,” he says. “Finding one that brings you joy in what you do every day is more valuable than any amount of money.”
A version of this article first appeared on Medscape.com.
Earning a huge salary was never a top priority for Sarah Ramer, MD, a nephrologist at the James J. Peters Veterans Affairs Medical Center in New York. That was obvious even when she was still a medical student, since she opted for an extra academic year to get a masters degree in clinical research methods.
After doing a combined internal medicine/pediatric residency, Dr. Ramer completed two fellowships, one in adult nephrology and one in palliative care.
“Every extra year that you spend in training is another year you’re not making a salary as an attending, so by doing 7 years in residency and a fellowship, I was not building my net worth the way some physicians do,” she says.
When Dr. Ramer, now 41, was ready to enter the job market in 2019, she had two offers on the table – one at a large, urban, research-intense medical center that included some clinical work combined with research and that paid $105,000, and one as a clinical nephrologist at a smaller suburban medical center with a $230,000 salary and a $20,000 performance bonus.
She took the first job, because she liked the idea of being ensured of having time to perform research, and she hoped to qualify for a career development grant from the National Institutes of Health.
Over the next few months, Dr. Ramer was diagnosed with cancer and the pandemic began ravaging the country. She considered taking a leave of absence from her job, but since she had only recently started at the job, taking a medical leave would mean she’d get only 50% of her salary, which would have left her with just over $50,000 to cover her mortgage, student loans, and other expenses.
“Financially, it would have been disastrous for me to go on leave at that time,” she says. “Things happen, but that’s something I didn’t consider when I decided to take a very low-paying job.”
Dr. Ramer has completed cancer treatment and has moved on to her current role at the VA medical center, where she is earning less than she would have made at the suburban medical center but more than twice as much as she did at the urban research hospital.
Lifestyle trade-offs
While Dr. Ramer’s salary is nearly four times that of the average American worker, it’s only about 60% of what the average physician earns. That works for Dr. Ramer, who has never put much value in material possessions. She has lived in the same working-class New Jersey neighborhood for more than a decade and drives a 2010 Hyundai Elantra.
“I need a new kitchen and new bathrooms in my apartment,” she says. “I’m still working on that. I have a good cushion, but I need to build up my emergency fund before I start spending money on home renovations.”
Such trade-offs are common among physicians who’ve chosen to work in a rural area, at a Medicaid practice, or in public health.
For Sean Kissel, MD, 30, a family physician in northern Utah, it’s about the lifestyle afforded by his role, which has earned him between $190,000 and $230,000 over the past few years. “I have no on-call shifts,” he says. “So, when I’m done, I’m done. I don’t have to work weekends or holidays, and I have dinner with my kids every night.”
According to the 2022 Medscape physician compensation report, physicians earned an average of $339,000 annually last year. Primary care physicians took home an average of $260,000, compared with $368,000 for specialists. The disparity in physician income was even greater when broken down by specialty. Plastic surgeons earned the most ($576,000), and public health and preventive medicine physicians earned the least ($243,000).
Still, that study found that physician salaries were up across every specialty, ranging from a 1% increase for critical care physicians to a 13% jump for otolaryngologists.
Scaling back
While private-pay physicians tend to make more than peers who work in community or government health clinics, they may have to work longer hours or face pressure to see more patients, which can decrease the quality of care they provide.
A recent study, published in JAMA Health Forum, found that most health systems base physician pay on the number of patients seen. That’s the case for more than 80% of primary care physicians and more than 90% of specialists, according to the study.
Given that landscape, a growing number of physicians are opting for a “lifestyle” practice – accepting lower compensation in order to see a limited number of patients or work only a few days per week, says Stu Schaff, the founder and lead adviser of Contract Medicine, a consulting firm that helps physicians understand, evaluate, and negotiate their employment contracts. Mr. Schaff concedes that most of the doctors who fall into this category are winding down their careers or have a high-earning spouse with a salary that offsets their lower income.
Other physicians move into administrative roles within a hospital or health center. Such positions typically involve seeing fewer patients and may pay less, but they also have more traditional hours, which can be appealing, Mr. Schaff says.
“Those folks might still do patient care 1 or 2 days a week, or even less,” he says. “They’re still physicians. They’re still using their physician expertise, but they’re not practicing at the same level or generating the same level of income as they might in a full-time clinical position.”
Cost of living matters
Dr. Kissel says that while he may take home less than the typical physician, he still makes enough to comfortably cover his expenses, including his student loan payments. Still, when new acquaintances learn he’s a physician, they often assume he’s earning much more.
“People assume most of us make mid-$300,000’s or low $400,000’s, and that’s true for some family doctors, but not for all,” he notes. “I like what I do. I’m in a good place, and we are happy with our life.”
Plus, Dr. Kissel may benefit from living in Utah, where the lower cost of living may allow him to stretch his salary further. Although salaries are typically higher in the most expensive states in the United States, compared with states that have a lower cost of living, those higher salaries aren’t always enough to make up the difference.
A recent WalletHub analysis found that New York, California, and Massachusetts were among the states with the lowest average annual wage when adjusted for the cost of living, while South Dakota, Indiana, and Wisconsin had the highest average wage after the adjustment.
“Even if a physician is in a lower-paying specialty or location, they’re still well-paid relative to the average U.S. citizen,” Mr. Schaff says. “When we talk about specialties that pay less, we’re still talking – if you’re full-time – about a six-figure income.”
Location, location, location
To combat a provider shortage, rural health centers have been increasing the pay doctors receive. Nevertheless, many physicians are opting not to live in a community where they have no connection.
“I think there has to be a tie to the community for a physician to want to be here,” says Scott Crouch, chief executive officer at Ozarks Community Health Center, Bolivar, Missouri. “NHSC [National Health Service Corps] can help some, but it’s not the draw it once was.”
Physicians and dentists who interview at the Ozarks Community Health Center often like the facilities and the area but don’t want to live in a rustic locale. “Most medical schools are in bigger cities,” Mr. Crouch says. “So it’s hard to get them into a rural environment.”
In some cases, physicians opt to go the community health route but choose to work in a city, even if it means they’re going to earn less. That was the case for Kevin King, MD, 33, a general pediatrician at St. John’s Community Health Center, Los Angeles. Dr. King knew he wanted to work in a community health center after completing a residency at a Medicaid clinic.
A rewarding career
“I find my work very rewarding,” Dr. King says. “Working in Medicaid can be difficult, and there are many barriers to care. It’s a lot more work to get things done, but the rewards come from the patients.”
That said, Dr. King adds he wouldn’t have been able to take this job without access to a loan forgiveness program that helps him manage his student-loan debt. “Without that, I’d probably have to find work in a private-pay population, making more money,” he says. “Loan forgiveness allowed me to choose this career path.”
Dr. King says he earns between $150,000 and $200,000 annually. That’s significantly less than the $243,000 median pediatrician salary in Los Angeles, according to Salary.com. Still, Dr. King says he wouldn’t trade his job for a more lucrative one.
“In medicine, there are so many different career paths you can take after residency training,” he says. “Finding one that brings you joy in what you do every day is more valuable than any amount of money.”
A version of this article first appeared on Medscape.com.
Earning a huge salary was never a top priority for Sarah Ramer, MD, a nephrologist at the James J. Peters Veterans Affairs Medical Center in New York. That was obvious even when she was still a medical student, since she opted for an extra academic year to get a masters degree in clinical research methods.
After doing a combined internal medicine/pediatric residency, Dr. Ramer completed two fellowships, one in adult nephrology and one in palliative care.
“Every extra year that you spend in training is another year you’re not making a salary as an attending, so by doing 7 years in residency and a fellowship, I was not building my net worth the way some physicians do,” she says.
When Dr. Ramer, now 41, was ready to enter the job market in 2019, she had two offers on the table – one at a large, urban, research-intense medical center that included some clinical work combined with research and that paid $105,000, and one as a clinical nephrologist at a smaller suburban medical center with a $230,000 salary and a $20,000 performance bonus.
She took the first job, because she liked the idea of being ensured of having time to perform research, and she hoped to qualify for a career development grant from the National Institutes of Health.
Over the next few months, Dr. Ramer was diagnosed with cancer and the pandemic began ravaging the country. She considered taking a leave of absence from her job, but since she had only recently started at the job, taking a medical leave would mean she’d get only 50% of her salary, which would have left her with just over $50,000 to cover her mortgage, student loans, and other expenses.
“Financially, it would have been disastrous for me to go on leave at that time,” she says. “Things happen, but that’s something I didn’t consider when I decided to take a very low-paying job.”
Dr. Ramer has completed cancer treatment and has moved on to her current role at the VA medical center, where she is earning less than she would have made at the suburban medical center but more than twice as much as she did at the urban research hospital.
Lifestyle trade-offs
While Dr. Ramer’s salary is nearly four times that of the average American worker, it’s only about 60% of what the average physician earns. That works for Dr. Ramer, who has never put much value in material possessions. She has lived in the same working-class New Jersey neighborhood for more than a decade and drives a 2010 Hyundai Elantra.
“I need a new kitchen and new bathrooms in my apartment,” she says. “I’m still working on that. I have a good cushion, but I need to build up my emergency fund before I start spending money on home renovations.”
Such trade-offs are common among physicians who’ve chosen to work in a rural area, at a Medicaid practice, or in public health.
For Sean Kissel, MD, 30, a family physician in northern Utah, it’s about the lifestyle afforded by his role, which has earned him between $190,000 and $230,000 over the past few years. “I have no on-call shifts,” he says. “So, when I’m done, I’m done. I don’t have to work weekends or holidays, and I have dinner with my kids every night.”
According to the 2022 Medscape physician compensation report, physicians earned an average of $339,000 annually last year. Primary care physicians took home an average of $260,000, compared with $368,000 for specialists. The disparity in physician income was even greater when broken down by specialty. Plastic surgeons earned the most ($576,000), and public health and preventive medicine physicians earned the least ($243,000).
Still, that study found that physician salaries were up across every specialty, ranging from a 1% increase for critical care physicians to a 13% jump for otolaryngologists.
Scaling back
While private-pay physicians tend to make more than peers who work in community or government health clinics, they may have to work longer hours or face pressure to see more patients, which can decrease the quality of care they provide.
A recent study, published in JAMA Health Forum, found that most health systems base physician pay on the number of patients seen. That’s the case for more than 80% of primary care physicians and more than 90% of specialists, according to the study.
Given that landscape, a growing number of physicians are opting for a “lifestyle” practice – accepting lower compensation in order to see a limited number of patients or work only a few days per week, says Stu Schaff, the founder and lead adviser of Contract Medicine, a consulting firm that helps physicians understand, evaluate, and negotiate their employment contracts. Mr. Schaff concedes that most of the doctors who fall into this category are winding down their careers or have a high-earning spouse with a salary that offsets their lower income.
Other physicians move into administrative roles within a hospital or health center. Such positions typically involve seeing fewer patients and may pay less, but they also have more traditional hours, which can be appealing, Mr. Schaff says.
“Those folks might still do patient care 1 or 2 days a week, or even less,” he says. “They’re still physicians. They’re still using their physician expertise, but they’re not practicing at the same level or generating the same level of income as they might in a full-time clinical position.”
Cost of living matters
Dr. Kissel says that while he may take home less than the typical physician, he still makes enough to comfortably cover his expenses, including his student loan payments. Still, when new acquaintances learn he’s a physician, they often assume he’s earning much more.
“People assume most of us make mid-$300,000’s or low $400,000’s, and that’s true for some family doctors, but not for all,” he notes. “I like what I do. I’m in a good place, and we are happy with our life.”
Plus, Dr. Kissel may benefit from living in Utah, where the lower cost of living may allow him to stretch his salary further. Although salaries are typically higher in the most expensive states in the United States, compared with states that have a lower cost of living, those higher salaries aren’t always enough to make up the difference.
A recent WalletHub analysis found that New York, California, and Massachusetts were among the states with the lowest average annual wage when adjusted for the cost of living, while South Dakota, Indiana, and Wisconsin had the highest average wage after the adjustment.
“Even if a physician is in a lower-paying specialty or location, they’re still well-paid relative to the average U.S. citizen,” Mr. Schaff says. “When we talk about specialties that pay less, we’re still talking – if you’re full-time – about a six-figure income.”
Location, location, location
To combat a provider shortage, rural health centers have been increasing the pay doctors receive. Nevertheless, many physicians are opting not to live in a community where they have no connection.
“I think there has to be a tie to the community for a physician to want to be here,” says Scott Crouch, chief executive officer at Ozarks Community Health Center, Bolivar, Missouri. “NHSC [National Health Service Corps] can help some, but it’s not the draw it once was.”
Physicians and dentists who interview at the Ozarks Community Health Center often like the facilities and the area but don’t want to live in a rustic locale. “Most medical schools are in bigger cities,” Mr. Crouch says. “So it’s hard to get them into a rural environment.”
In some cases, physicians opt to go the community health route but choose to work in a city, even if it means they’re going to earn less. That was the case for Kevin King, MD, 33, a general pediatrician at St. John’s Community Health Center, Los Angeles. Dr. King knew he wanted to work in a community health center after completing a residency at a Medicaid clinic.
A rewarding career
“I find my work very rewarding,” Dr. King says. “Working in Medicaid can be difficult, and there are many barriers to care. It’s a lot more work to get things done, but the rewards come from the patients.”
That said, Dr. King adds he wouldn’t have been able to take this job without access to a loan forgiveness program that helps him manage his student-loan debt. “Without that, I’d probably have to find work in a private-pay population, making more money,” he says. “Loan forgiveness allowed me to choose this career path.”
Dr. King says he earns between $150,000 and $200,000 annually. That’s significantly less than the $243,000 median pediatrician salary in Los Angeles, according to Salary.com. Still, Dr. King says he wouldn’t trade his job for a more lucrative one.
“In medicine, there are so many different career paths you can take after residency training,” he says. “Finding one that brings you joy in what you do every day is more valuable than any amount of money.”
A version of this article first appeared on Medscape.com.